User login
The Gut Microbiome and Cardiac Arrhythmias
The Gut Microbiome and Cardiac Arrhythmias
The extensive surface of the gastrointestinal tract presents an interface between the human body and its environment. Residing within the intestinal lumen, ingested food and various microorganisms are an essential aspect of this relationship. The trillions of microorganisms, primarily commensal bacteria hosted by the human gut, constitute the human gut microbiome.
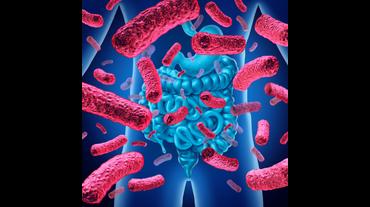
There is growing evidence that the human gut microbiome plays a role in maintaining normal body function and homeostasis.1 Research, such as the National Institute of Health Microbiome Project, is helping to show the impact of gut microorganisms and their negative influence on metabolic diseases and chronic inflammatory disorders.2-5 An imbalance in the microbiota, known as dysbiosis, has been associated with metabolic and cardiovascular diseases (CVD), including hypertension, diabetes mellitus, obesity, and coronary artery disease (CAD). Gut dysbiosis has also been associated with cardiac arrhythmias, including atrial fibrillation (AF) and ventricular arrhythmias (Figure).6-12
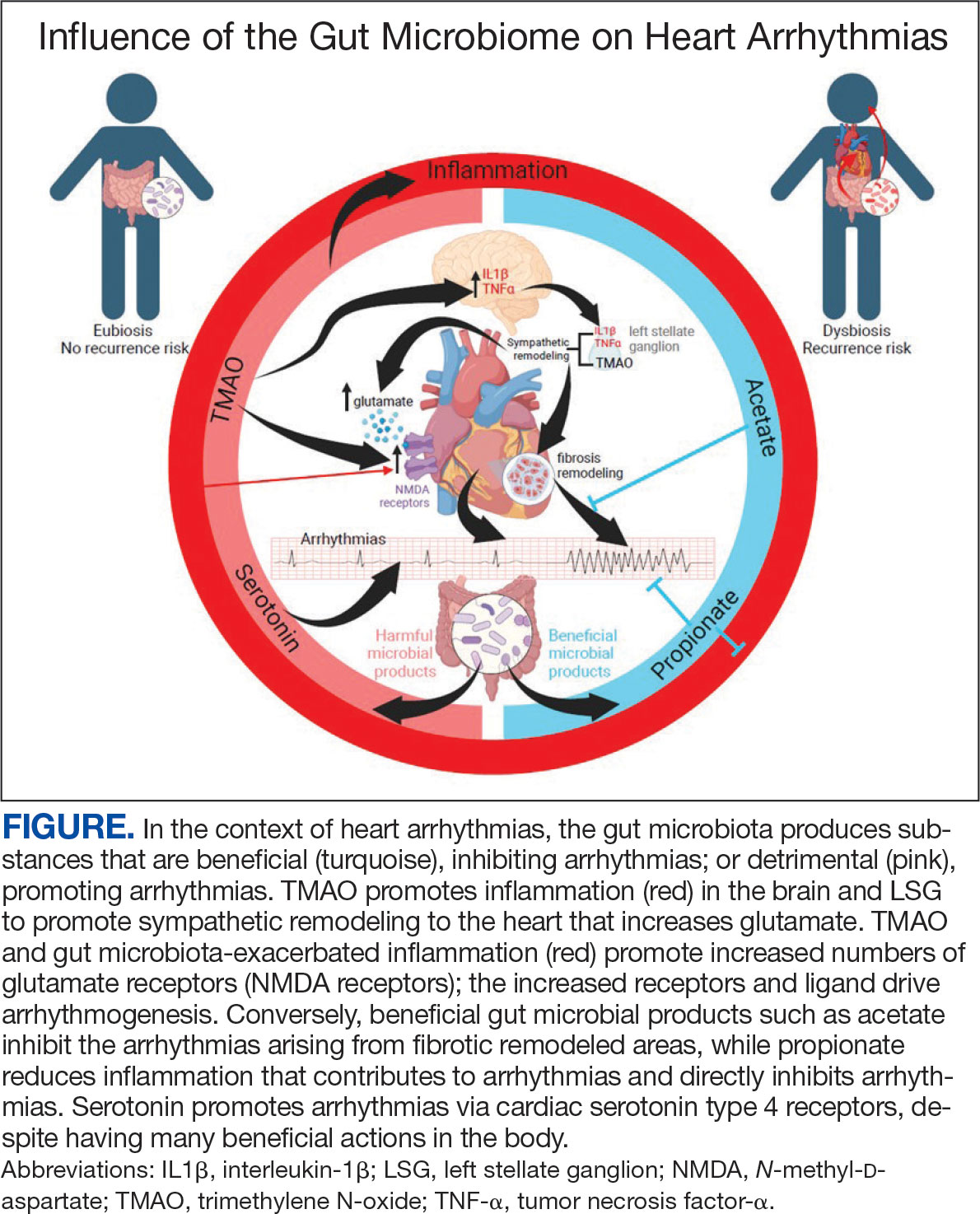
Whether gut dysbiosis is a cause or effect of the human disease process is unclear. While further research is warranted, some evidence of causation has been found. In 2018, Yoshida et al demonstrated an association between patients with CAD who had a significantly lower burden of the gut bacteria species Bacteroides vulgatus and Bacteroides dorei compared to that of patients without CAD. The study found that administration of these Bacteroides species reduced atherosclerotic lesion formation in atherosclerosis-prone mice.13 If altering gut microbial composition can affect the disease process, it may indicate a causative role for gut dysbiosis in disease pathogenesis. Furthermore, this finding also suggests agents may be used to alter the gut microbiome and potentially prevent and treat diseases. An altered gut microbiome may serve as an early marker for human disease, aiding in timely diagnosis and institution of disease-modifying treatments.
This review outlines the broad relationship of the pathways and intermediaries that may be involved in mediating the interaction between the gut microbiome and cardiac arrhythmias based on rapidly increasing evidence. A comprehensive search among PubMed and Google Scholar databases was conducted to find articles relevant to the topic.
Potential Intermediaries
Potential pathways for how the gut microbiome and cardiovascular system interact are subjects of active research. However, recent research may point to potential mechanisms of the association between the systems. The gut microbiome may influence human physiology through 3 principal routes: the autonomic nervous system, inflammatory pathways, and metabolic processes.
Autonomic Nervous System
The concept of bidirectional communication between the gut and central nervous system, known as the microbiota-gut-brain axis, is widely accepted.14 Proposed mediators of this interaction include the vagus nerve, the sympathetic nervous system, and the hypothalamic-pituitary-adrenal axis; cytokines produced by the immune system, tryptophan metabolism, and the production of short-chain fatty acids (SCFAs).15,16
The gut microbiome appears to have a direct impact on the autonomic nervous system, through which it can influence cardiovascular function. Muller et al described how the gut microbiome modulated gut-extrinsic sympathetic neurons and that the depletion of gut microbiota led to activation of both brainstem sensory nuclei and efferent sympathetic premotor glutamatergic neurons.16 Meng et al found that systemic injection of the gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) led to significantly increased activity in the paraventricular nucleus, a hypothalamic structure essential to the central autonomic network. Their study demonstrated that systemic TMAO also led to increased left stellate ganglion (LSG) activity, a known contributor to cardiac sympathetic tone.12
Inflammatory Pathways
Inflammatory responses are another pathway for the gut microbiome to influence the cardiovascular system. SCFAs are a set of gut microbial metabolites produced in the colon by bacterial fermentation and decomposition of resistant starches and dietary fibers.17 These metabolites are increasingly recognized for their role in modulating disease processes, including cardiac disease. Aguilar et al found that the progression of atherosclerosis was slowed in apolipoprotein E (Apo-E) knockout mice by a chow diet supplemented with butyrate, a SCFA, suggesting it is an atheroprotective therapeutic agent. Less adhesion and migration of macrophages, reduced inflammation, improved plaque stability, and lowered atherosclerosis progression.18 Wei et al demonstrated in animal models that direct microinjection of the proinflammatory factors interleukin (IL)-1Β and tumor necrosis factor (TNF)-αdirectly into the subfornical organ increased heart rate, mean blood pressure, and renal sympathetic nerve activity.19
Metabolic Processes
Serotonin (5-HT), a metabolite of tryptophan, is a neurotransmitter that regulates many bodily functions and plays a significant role in the microbiota-brain gut axis.20 Oral ingestion of the bacterial species Bifidobacterium infantis increased plasma tryptophan in rat models.21 Additionally, many other microorganisms, including species of Candida, Streptococcus, Escherichia, and Enterococcus are known to produce 5-HT.22 While a relationship between the gut microbiome and plasma 5-HT has been established, interactions between 5-HT and the cardiovascular system are complex. Research has shown that stimulation of 5-HT1A receptors produces bradycardic and vasopressor effects, while stimulation of the 5-HT2 receptor induces vasoconstriction and tachycardia.23
A high-fiber diet can lower the incidence of hypertension, although the mechanisms are not clear. One potential reason could be alteration in gut bacteria, as a diet high in fiber has been shown to increase the prevalence of acetate-producing bacteria.24
Atherosclerosis
Research investigating the relationship of the gut microbiome with arrhythmias is in its early stages; however, the connection of the gut microbiome and atherosclerosis is more established.25 Contemporary studies have shown various gut microorganisms associated with atherosclerosis.26 Jie et al reported that patients with atherosclerotic cardiovascular disease had increased Enterobacteriaceae loads and oral cavity-associated bacteria with lower levels of butyrate producing bacteria when compared with healthy controls.27 In addition, microbial metabolites such as TMAO appear to promote atherosclerosis by increasing vascular inflammation and platelet reactivity.26 Researchers are investigating the modulation of these associations to help reduce atherosclerotic burden. Kasahara et al found that Roseburia intestinalis could reduce atherosclerotic disease in mice through the production of butyrate.28 Roberts et al established that administration of TMAO inhibitors reduced TMAO levels while reducing thrombus formation without observable toxicity or increased bleeding risk.29
Atrial Arrhythmias
The gut microbiome can also specifically affect cardiac arrhythmogenesis, and multiple studies suggest possible mediators of this interaction. Certain gut microbiome derived metabolites like TMAO may have a role in promoting AF.30 Other gut microbial metabolites like lipopolysaccharides and indoxyl sulfate are implicated in atrial electrical instability.31,32 Microbe-derived free fatty acids such as palmitic acid and adrenic acid can precipitate arrhythmogenesis. 33,34 Preponderances of certain gut bacteria like Ruminococcus, Streptococcus, and Enterococcus, as well as reductions of Faecalibacterium, Alistipes, Oscillibacter, and Bilophila have been detected in patients with AF.8 Tabata et al found that certain clusters of bacterial groups led by Ruminococcus species seem to show higher prevalence in patients with AF, whereas the genus Enterobacter was significantly lower compared with control subjects. That study also noted that gut microbial composition is affected by diet and antacid use.35 Gut microbiome-derived serotonin may be another mediator for AF, which may be related to the fact that 5-HT4 receptors are present in atrial tissue.36
Ventricular Arrhythmias
A critical component to the development of malignant ventricular arrhythmias is an imbalance in autonomic tone; in particular, the overactivation of the sympathetic nervous system.37 Animal models have shown that augmentation of the sympathetic nervous system plays an essential role in the subsequent development of ventricular arrhythmias. 38 Several studies have established the LSG as an important component of the cardiac sympathetic nervous system pathway. 38,39 Ablation of the LSG has been shown to effectively reduce the burden of malignant arrhythmias, further pointing toward the role of excess sympathetic activity.37,39 Stellate ganglion denervation has become an established method for managing life-threatening ventricular arrhythmias.40
Gut metabolites may have significant effects on cardiac sympathetic activity. Meng et al investigated the effect of TMAO on the LSG in animals and its overall effect on the incidence of ventricular arrhythmias under ischemic conditions. To fully explore this interaction, they examined the effect of TMAO on LSG function though 2 mechanisms: local administration of TMAO within the LSG and systemic administration of TMAO leading to activation of the central sympathetic nervous system. In both protocols, left anterior descending coronary artery occlusion was performed after TMAO administration. Injection of TMAO directly into the LSG was found to significantly increase the cardiac sympathetic tone and incidence of ventricular arrhythmias. In the systemic administration control arm, ventricular arrhythmias were also significantly increased.12
Increased inflammatory states appear to correlate with an increase in sympathetic tone and ventricular arrhythmias.12 In an animal study, direct injection of the proinflammatory factor IL-1Β into the LSG not only resulted in increased inflammation, but aggravated cardiac sympathetic remodeling. This led to a decreased effective refractory period and action potential duration, leading to an increased maximal slope of the restitution curve and higher occurrence of ventricular arrhythmias.41 Shi et al demonstrated that paraventricular nucleus microinjection with TNF-α and IL-1Β also enhanced the cardiac sympathetic afferent reflex, showing that these proinflammatory cytokines not only upregulate the inflammatory response, but can also have excitatory effects that stimulate sympathetic activity and have the potential to be proarrhythmic.19,42 Local and systemic administration of the gut microbe-derived TMAO increased the expression of IL-1Β and TNF-α, thus implicating the microbiome as a potential mediator of the inflammatory response and as another potential pathway for increased ventricular arrhythmias.12
The N-methyl-d-aspartate receptor (NMDAR) is found in multiple organs—including the heart—but more specifically in the conducting system and myocardium.43,44 Research has discovered an upregulation of NMDARs in the setting of cardiac sympathetic hyperinnervation in rat models both with healed myocardial necrotic injury and without. The infusion of their ligand, NMDA, provoked ventricular tachycardia and ventricular fibrillation in rat models with sympathetic hyperinnervation and healed myocardial necrotic injury.45 Another study found that NMDAR activation provoked ventricular arrhythmias, but also prolonged repolarization and induced electrical instability.46 Proinflammatory markers have been shown to upregulate the expression of NMDARs; more importantly, NMDAR expression has been shown to be significantly increased in the setting of TMAO administration.12,47,48
5-HT also appears to have a substantial association with ventricular arrhythmias in addition to atrial arrhythmias. el-Mahdy demonstrated in anesthetized rats with acute coronary ligation that systemic doses of 5-HT represented a significant dose-dependent increase in the duration of ventricular tachycardia and ventricular fibrillation, while also increasing the number of ventricular ectopic beats.49 Certain gut microorganisms are known to produce 5-HT, including those in the genera Streptococcus, Escherichia, and Enterococcus.22 Additionally, oral ingestion of the Bifidobacterium infantis increased plasma levels of tryptophan in rat models.21 The gut microbiome may have significant effects on plasma serotonin levels, and thus have the potential to alter the risk for ventricular arrhythmias.
The deleterious effects of the gut microbiome have been documented. However, it appears to have potential protective effects, and several studies point to the possible mechanisms of this beneficial interaction. Propionate is a SCFA microorganism produced by gut microbial fermentation.50 In a rat model study, Zhou et al found that infusion of sodium propionate significantly reduced ventricular arrhythmias during acute myocardial ischemia or burst stimulation, thus confirming cardioprotective effects.50,51
Proposed mechanisms for reduced susceptibility to ventricular arrhythmias with propionate infusion include parasympathetic activation via the gut-brain axis, anti-inflammatory pathways, and improved cardiac electrophysiology instability.50 In addition butyrate has been found to reduce inflammation and myocardial hypertrophy. Jiang et al demonstrated in rats postmyocardial infarction that butyrate promoted expression of anti-inflammatory M2 macrophage markers, decreased expressions of nerve growth factor and norepinephrine, and decreased the density of nerve fibers for growth-associated protein-43 and tyrosine hydroxylase. The cumulative impact of butyrate led to suppression of inflammation and the inhibition of sympathetic neural remodeling, ultimately resulting in improved cardiac function and reduction in ventricular arrhythmias after myocardial infarction.52
Gut bacteria-derived acetate-mediated reduction in cardiac fibrosis may be another mechanism for the effects on ventricular arrhythmias. Cardiac fibrosis and scar are established as the primary substrate for reentrant ventricular arrhythmias seen in various cardiomyopathies.
Future Directions
The microbiome residing in the human gut has a significant impact on cardiac arrhythmias, the details of which remain unknown. A likely bidirectional relationship exists in which the gut microbiome may affect arrhythmogenesis and in turn be affected by cardiac arrhythmias. The mechanisms of action are not well understood, but likely involve the autonomic nervous system, inflammation, and metabolic pathways.
The gut microbiome is a complex collection of heterogenous microorganisms that have dramatic effects on the human body. Additional research is necessary to identify further associations and causations of gut microorganisms with various human body processes, as well as cardiovascular disease. The microbiome has been shown to directly and indirectly influence the development of different disease states, including the cardiovascular system and cardiac arrhythmias. Several pathways have been proposed through which the gut microbiome can potentially affect cardiac arrhythmogenesis. There are likely several mechanisms simultaneously in operation. Understanding the role of human gut microbiome in the genesis of cardiac arrhythmias not only may improve our understanding of arrhythmias, but also may result in novel treatment options. This could potentially lead to the development of therapeutic options and strategies to modulate the gut microbiome to help detect, prevent, and treat cardiac arrhythmias.
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
- Karlsson F, Tremaroli V, Nielsen J, Bäckhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes. 2013;62(10):3341-3349. doi:10.2337/db13-0844
- Danneskiold-Samsøe NB, Dias de Freitas Queiroz Barros H, Santos R, et al. Interplay between food and gut microbiota in health and disease. Food Res Int. 2019;115:23-31. doi:10.1016/j.foodres.2018.07.043
- Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe- derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446-450. doi:10.1038/nature12721
- Integrative HMP (iHMP) Research Network Consortium. The integrative human microbiome project. Nature. 2019;569(7758):641-648. doi:10.1038/s41586-019-1238-8
- Zubcevic J, Richards EM, Yang T, et al. Impaired autonomic nervous system-microbiome circuit in hypertension. Circ Res. 2019;125(1):104-116. doi:10.1161/CIRCRESAHA.119.313965
- Emoto T, Yamashita T, Sasaki N, et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atheroscler Thromb. 2016;23(8):908-921. doi:10.5551/jat.32672
- Zuo K, Li J, Li K, et al. Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. Gigascience. 2019;8(6):giz058. doi:10.1093/gigascience/giz058
- Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. doi:10.1186/s40168-016-0222-x
- Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55-60. doi:10.1038/nature11450
- Chang CJ, Lin CS, Lu CC, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:7489. doi:10.1038/ncomms8489
- Meng G, Zhou X, Wang M, et al. Gut microbederived metabolite trimethylamine N-oxide activates the cardiac autonomic nervous system and facilitates ischemia-induced ventricular arrhythmia via two different pathways. EBioMedicine. 2019;44:656-664. doi:10.1016/j.ebiom.2019.03.066
- Yoshida N, Emoto T, Yamashita T, et al. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation. 2018;138(22):2486-2498. doi:10.1161/CIRCULATIONAHA.118.033714
- Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. Front Neuroendocrinol. 2018;51:80-101. doi:10.1016/j.yfrne.2018.04.002
- Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: how gut microbes shape human behavior. J Psychiatr Res. 2015;63:1-9. doi:10.1016/j.jpsychires.2015.02.021
- Muller PA, Schneeberger M, Matheis F, et al. Microbiota modulate sympathetic neurons via a gutbrain circuit. Nature. 2020;583(7816):441-446. doi:10.1038/s41586-020-2474-7
- Ohira H, Tsutsui W, Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J Atheroscler Thromb. 2017;24(7):660-672. doi:10.5551/jat.RV17006
- Aguilar EC, Leonel AJ, Teixeira LG, et al. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFêB activation. Nutr Metab Cardiovasc Dis. 2014;24(6):606-613. doi:10.1016/j.numecd.2014.01.002
- Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcit - atory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65(5):1126-1133. doi:10.1161/HYPERTENSIONAHA.114.05112
- Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720- 726. doi:10.1016/j.biopsych.2013.05.001
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164-174. doi:10.1016/j.jpsychires.2008.03.009
- Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33(8):574-581. doi:10.1002/bies.201100024
- Yusuf S, Al-Saady N, Camm AJ. 5-hydroxytryptamine and atrial fibrillation: how significant is this piece in the puzzle? J Cardiovasc Electrophysiol. 2003;14(2):209-214. doi:10.1046/j.1540-8167.2003.02381.x
- Marques FZ, Nelson E, Chu PY, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135(10):964-977. doi:10.1161/CIRCULATIONAHA.116.024545
- Björkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185(10):1630-1645. doi:10.1016/j.cell.2022.04.004
- Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(16):2089-2105. doi:10.1016/j.jacc.2019.03.024
- Jie Z, Xia H, Zhong SL, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1):845. doi:10.1038/s41467-017-00900-1
- Kasahara K, Krautkramer KA, Org E, et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol. 2018;3(12):1461- 1471. doi:10.1038/s41564-018-0272-x
- Roberts AB, Gu X, Buffa JA, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24(9):1407-1417. doi:10.1038/s41591-018-0128-1
- Yu L, Meng G, Huang B, et al. A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int J Cardiol. 2018;255:92- 98. doi:10.1016/j.ijcard.2017.11.071
- Okazaki R, Iwasaki YK, Miyauchi Y, et al. Lipopolysaccharide induces atrial arrhythmogenesis via down-regulation of L-type Ca2+ channel genes in rats. Int Heart J. 2009;50(3):353-363. doi:10.1536/ihj.50.353
- Chen WT, Chen YC, Hsieh MH, et al. The uremic toxin indoxyl sulfate increases pulmonary vein and atrial arrhythmogenesis. J Cardiovasc Electrophysiol. 2015;26(2):203- 210. doi:10.1111/jce.12554
- Fretts AM, Mozaffarian D, Siscovick DS, et al. Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc. 2014;3(3):e000889. doi:10.1161/JAHA.114.000889
- Horas HNS, Nishiumi S, Kawano Y, Kobayashi T, Yoshida M, Azuma T. Adrenic acid as an inflammation enhancer in non-alcoholic fatty liver disease. Arch Biochem Biophys. 2017;623-624:64-75. doi:10.1016/j.abb.2017.04.009
- Tabata T, Yamashita T, Hosomi K, et al. Gut microbial composition in patients with atrial fibrillation: effects of diet and drugs. Heart Vessels. 2021;36(1):105-114. doi:10.1007/s00380-020-01669-y
- López-Rodriguez ML, Benhamú B, Morcillo MJ, et al. 5-HT(4) receptor antagonists: structure-affinity relationships and ligand-receptor interactions. Curr Top Med Chem. 2002;2(6):625-641. doi:10.2174/1568026023393769
- Yu L, Zhou L, Cao G, et al. Optogenetic modulation of cardiac sympathetic nerve activity to prevent ventricular arrhythmias. J Am Coll Cardiol. 2017;70(22):2778-2790. doi:10.1016/j.jacc.2017.09.1107
- Schwartz PJ, Vanoli E. Cardiac arrhythmias elicited by interaction between acute myocardial ischemia and sympathetic hyperactivity: a new experimental model for the study of antiarrhythmic drugs. J Cardiovasc Pharmacol. 1981;3(6):1251-1259. doi:10.1097/00005344-198111000-00012
- Puddu PE, Jouve R, Langlet F, Guillen JC, Lanti M, Reale A. Prevention of postischemic ventricular fibrillation late after right or left stellate ganglionectomy in dogs. Circulation. 1988;77(4):935-946. doi:10.1161/01.cir.77.4.935
- Vaseghi M, Gima J, Kanaan C, et al. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and longterm follow-up. Heart Rhythm. 2014;11(3):360-366. doi:10.1016/j.hrthm.2013.11.028
- Wang M, Li S, Zhou X, et al. Increased inflammation promotes ventricular arrhythmia through aggravating left stellate ganglion remodeling in a canine ischemia model. Int J Cardiol. 2017;248:286-293. doi:10.1016/j.ijcard.2017.08.011
- Shi Z, Gan XB, Fan ZD, et al. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf). 2011;203(2):289-297. doi:10.1111/j.1748-1716.2011.02313.x
- Gill S, Veinot J, Kavanagh M, Pulido O. Human heart glutamate receptors - implications for toxicology, food safety, and drug discovery. Toxicol Pathol. 2007;35(3):411-417. doi:10.1080/01926230701230361
- Govoruskina N, Jakovljevic V, Zivkovic V, et al. The role of cardiac N-methyl-D-aspartate receptors in heart conditioning— effects on heart function and oxidative stress. Biomolecules. 2020;10(7):1065. doi:10.3390/biom10071065
- Lü J, Gao X, Gu J, et al. Nerve sprouting contributes to increased severity of ventricular tachyarrhythmias by upregulating iGluRs in rats with healed myocardial necrotic injury. J Mol Neurosci. 2012;48(2):448-455. doi:10.1007/s12031-012-9720-x
- Shi S, Liu T, Li Y, et al. Chronic N-methyl-D-aspartate receptor activation induces cardiac electrical remodeling and increases susceptibility to ventricular arrhythmias. Pacing Clin Electrophysiol. 2014;37(10):1367-1377. doi:10.1111/pace.12430
- Zhang Z, Bassam B, Thomas AG, et al. Maternal inflammation leads to impaired glutamate homeostasis and upregulation of glutamate carboxypeptidase II in activated microglia in the fetal/newborn rabbit brain. Neurobiol Dis. 2016;94:116-128. doi:10.1016/j.nbd.2016.06.010
- Wu LJ, Toyoda H, Zhao MG, et al. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci. 2005;25(48):11107-11116. doi:10.1523/JNEUROSCI.1678-05.2005
- el-Mahdy SA. 5-hydroxytryptamine (serotonin) enhances ventricular arrhythmias induced by acute coronary artery ligation in rats. Res Commun Chem Pathol Pharmacol. 1990;68(3):383-386.
- Zhou M, Li D, Xie K, et al. The short-chain fatty acid propionate improved ventricular electrical remodeling in a rat model with myocardial infarction. Food Funct. 2021;12(24):12580-12593. doi:10.1039/d1fo02040d
- Bartolomaeus H, Balogh A, Yakoub M, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139(11):1407-1421. doi:10.1161/CIRCULATIONAHA.118.036652
- Jiang X, Huang X, Tong Y, Gao H. Butyrate improves cardiac function and sympathetic neural remodeling following myocardial infarction in rats. Can J Physiol Pharmacol. 2020;98(6):391-399. doi:10.1139/cjpp-2019-0531
The extensive surface of the gastrointestinal tract presents an interface between the human body and its environment. Residing within the intestinal lumen, ingested food and various microorganisms are an essential aspect of this relationship. The trillions of microorganisms, primarily commensal bacteria hosted by the human gut, constitute the human gut microbiome.
There is growing evidence that the human gut microbiome plays a role in maintaining normal body function and homeostasis.1 Research, such as the National Institute of Health Microbiome Project, is helping to show the impact of gut microorganisms and their negative influence on metabolic diseases and chronic inflammatory disorders.2-5 An imbalance in the microbiota, known as dysbiosis, has been associated with metabolic and cardiovascular diseases (CVD), including hypertension, diabetes mellitus, obesity, and coronary artery disease (CAD). Gut dysbiosis has also been associated with cardiac arrhythmias, including atrial fibrillation (AF) and ventricular arrhythmias (Figure).6-12

Whether gut dysbiosis is a cause or effect of the human disease process is unclear. While further research is warranted, some evidence of causation has been found. In 2018, Yoshida et al demonstrated an association between patients with CAD who had a significantly lower burden of the gut bacteria species Bacteroides vulgatus and Bacteroides dorei compared to that of patients without CAD. The study found that administration of these Bacteroides species reduced atherosclerotic lesion formation in atherosclerosis-prone mice.13 If altering gut microbial composition can affect the disease process, it may indicate a causative role for gut dysbiosis in disease pathogenesis. Furthermore, this finding also suggests agents may be used to alter the gut microbiome and potentially prevent and treat diseases. An altered gut microbiome may serve as an early marker for human disease, aiding in timely diagnosis and institution of disease-modifying treatments.
This review outlines the broad relationship of the pathways and intermediaries that may be involved in mediating the interaction between the gut microbiome and cardiac arrhythmias based on rapidly increasing evidence. A comprehensive search among PubMed and Google Scholar databases was conducted to find articles relevant to the topic.
Potential Intermediaries
Potential pathways for how the gut microbiome and cardiovascular system interact are subjects of active research. However, recent research may point to potential mechanisms of the association between the systems. The gut microbiome may influence human physiology through 3 principal routes: the autonomic nervous system, inflammatory pathways, and metabolic processes.
Autonomic Nervous System
The concept of bidirectional communication between the gut and central nervous system, known as the microbiota-gut-brain axis, is widely accepted.14 Proposed mediators of this interaction include the vagus nerve, the sympathetic nervous system, and the hypothalamic-pituitary-adrenal axis; cytokines produced by the immune system, tryptophan metabolism, and the production of short-chain fatty acids (SCFAs).15,16
The gut microbiome appears to have a direct impact on the autonomic nervous system, through which it can influence cardiovascular function. Muller et al described how the gut microbiome modulated gut-extrinsic sympathetic neurons and that the depletion of gut microbiota led to activation of both brainstem sensory nuclei and efferent sympathetic premotor glutamatergic neurons.16 Meng et al found that systemic injection of the gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) led to significantly increased activity in the paraventricular nucleus, a hypothalamic structure essential to the central autonomic network. Their study demonstrated that systemic TMAO also led to increased left stellate ganglion (LSG) activity, a known contributor to cardiac sympathetic tone.12
Inflammatory Pathways
Inflammatory responses are another pathway for the gut microbiome to influence the cardiovascular system. SCFAs are a set of gut microbial metabolites produced in the colon by bacterial fermentation and decomposition of resistant starches and dietary fibers.17 These metabolites are increasingly recognized for their role in modulating disease processes, including cardiac disease. Aguilar et al found that the progression of atherosclerosis was slowed in apolipoprotein E (Apo-E) knockout mice by a chow diet supplemented with butyrate, a SCFA, suggesting it is an atheroprotective therapeutic agent. Less adhesion and migration of macrophages, reduced inflammation, improved plaque stability, and lowered atherosclerosis progression.18 Wei et al demonstrated in animal models that direct microinjection of the proinflammatory factors interleukin (IL)-1Β and tumor necrosis factor (TNF)-αdirectly into the subfornical organ increased heart rate, mean blood pressure, and renal sympathetic nerve activity.19
Metabolic Processes
Serotonin (5-HT), a metabolite of tryptophan, is a neurotransmitter that regulates many bodily functions and plays a significant role in the microbiota-brain gut axis.20 Oral ingestion of the bacterial species Bifidobacterium infantis increased plasma tryptophan in rat models.21 Additionally, many other microorganisms, including species of Candida, Streptococcus, Escherichia, and Enterococcus are known to produce 5-HT.22 While a relationship between the gut microbiome and plasma 5-HT has been established, interactions between 5-HT and the cardiovascular system are complex. Research has shown that stimulation of 5-HT1A receptors produces bradycardic and vasopressor effects, while stimulation of the 5-HT2 receptor induces vasoconstriction and tachycardia.23
A high-fiber diet can lower the incidence of hypertension, although the mechanisms are not clear. One potential reason could be alteration in gut bacteria, as a diet high in fiber has been shown to increase the prevalence of acetate-producing bacteria.24
Atherosclerosis
Research investigating the relationship of the gut microbiome with arrhythmias is in its early stages; however, the connection of the gut microbiome and atherosclerosis is more established.25 Contemporary studies have shown various gut microorganisms associated with atherosclerosis.26 Jie et al reported that patients with atherosclerotic cardiovascular disease had increased Enterobacteriaceae loads and oral cavity-associated bacteria with lower levels of butyrate producing bacteria when compared with healthy controls.27 In addition, microbial metabolites such as TMAO appear to promote atherosclerosis by increasing vascular inflammation and platelet reactivity.26 Researchers are investigating the modulation of these associations to help reduce atherosclerotic burden. Kasahara et al found that Roseburia intestinalis could reduce atherosclerotic disease in mice through the production of butyrate.28 Roberts et al established that administration of TMAO inhibitors reduced TMAO levels while reducing thrombus formation without observable toxicity or increased bleeding risk.29
Atrial Arrhythmias
The gut microbiome can also specifically affect cardiac arrhythmogenesis, and multiple studies suggest possible mediators of this interaction. Certain gut microbiome derived metabolites like TMAO may have a role in promoting AF.30 Other gut microbial metabolites like lipopolysaccharides and indoxyl sulfate are implicated in atrial electrical instability.31,32 Microbe-derived free fatty acids such as palmitic acid and adrenic acid can precipitate arrhythmogenesis. 33,34 Preponderances of certain gut bacteria like Ruminococcus, Streptococcus, and Enterococcus, as well as reductions of Faecalibacterium, Alistipes, Oscillibacter, and Bilophila have been detected in patients with AF.8 Tabata et al found that certain clusters of bacterial groups led by Ruminococcus species seem to show higher prevalence in patients with AF, whereas the genus Enterobacter was significantly lower compared with control subjects. That study also noted that gut microbial composition is affected by diet and antacid use.35 Gut microbiome-derived serotonin may be another mediator for AF, which may be related to the fact that 5-HT4 receptors are present in atrial tissue.36
Ventricular Arrhythmias
A critical component to the development of malignant ventricular arrhythmias is an imbalance in autonomic tone; in particular, the overactivation of the sympathetic nervous system.37 Animal models have shown that augmentation of the sympathetic nervous system plays an essential role in the subsequent development of ventricular arrhythmias. 38 Several studies have established the LSG as an important component of the cardiac sympathetic nervous system pathway. 38,39 Ablation of the LSG has been shown to effectively reduce the burden of malignant arrhythmias, further pointing toward the role of excess sympathetic activity.37,39 Stellate ganglion denervation has become an established method for managing life-threatening ventricular arrhythmias.40
Gut metabolites may have significant effects on cardiac sympathetic activity. Meng et al investigated the effect of TMAO on the LSG in animals and its overall effect on the incidence of ventricular arrhythmias under ischemic conditions. To fully explore this interaction, they examined the effect of TMAO on LSG function though 2 mechanisms: local administration of TMAO within the LSG and systemic administration of TMAO leading to activation of the central sympathetic nervous system. In both protocols, left anterior descending coronary artery occlusion was performed after TMAO administration. Injection of TMAO directly into the LSG was found to significantly increase the cardiac sympathetic tone and incidence of ventricular arrhythmias. In the systemic administration control arm, ventricular arrhythmias were also significantly increased.12
Increased inflammatory states appear to correlate with an increase in sympathetic tone and ventricular arrhythmias.12 In an animal study, direct injection of the proinflammatory factor IL-1Β into the LSG not only resulted in increased inflammation, but aggravated cardiac sympathetic remodeling. This led to a decreased effective refractory period and action potential duration, leading to an increased maximal slope of the restitution curve and higher occurrence of ventricular arrhythmias.41 Shi et al demonstrated that paraventricular nucleus microinjection with TNF-α and IL-1Β also enhanced the cardiac sympathetic afferent reflex, showing that these proinflammatory cytokines not only upregulate the inflammatory response, but can also have excitatory effects that stimulate sympathetic activity and have the potential to be proarrhythmic.19,42 Local and systemic administration of the gut microbe-derived TMAO increased the expression of IL-1Β and TNF-α, thus implicating the microbiome as a potential mediator of the inflammatory response and as another potential pathway for increased ventricular arrhythmias.12
The N-methyl-d-aspartate receptor (NMDAR) is found in multiple organs—including the heart—but more specifically in the conducting system and myocardium.43,44 Research has discovered an upregulation of NMDARs in the setting of cardiac sympathetic hyperinnervation in rat models both with healed myocardial necrotic injury and without. The infusion of their ligand, NMDA, provoked ventricular tachycardia and ventricular fibrillation in rat models with sympathetic hyperinnervation and healed myocardial necrotic injury.45 Another study found that NMDAR activation provoked ventricular arrhythmias, but also prolonged repolarization and induced electrical instability.46 Proinflammatory markers have been shown to upregulate the expression of NMDARs; more importantly, NMDAR expression has been shown to be significantly increased in the setting of TMAO administration.12,47,48
5-HT also appears to have a substantial association with ventricular arrhythmias in addition to atrial arrhythmias. el-Mahdy demonstrated in anesthetized rats with acute coronary ligation that systemic doses of 5-HT represented a significant dose-dependent increase in the duration of ventricular tachycardia and ventricular fibrillation, while also increasing the number of ventricular ectopic beats.49 Certain gut microorganisms are known to produce 5-HT, including those in the genera Streptococcus, Escherichia, and Enterococcus.22 Additionally, oral ingestion of the Bifidobacterium infantis increased plasma levels of tryptophan in rat models.21 The gut microbiome may have significant effects on plasma serotonin levels, and thus have the potential to alter the risk for ventricular arrhythmias.
The deleterious effects of the gut microbiome have been documented. However, it appears to have potential protective effects, and several studies point to the possible mechanisms of this beneficial interaction. Propionate is a SCFA microorganism produced by gut microbial fermentation.50 In a rat model study, Zhou et al found that infusion of sodium propionate significantly reduced ventricular arrhythmias during acute myocardial ischemia or burst stimulation, thus confirming cardioprotective effects.50,51
Proposed mechanisms for reduced susceptibility to ventricular arrhythmias with propionate infusion include parasympathetic activation via the gut-brain axis, anti-inflammatory pathways, and improved cardiac electrophysiology instability.50 In addition butyrate has been found to reduce inflammation and myocardial hypertrophy. Jiang et al demonstrated in rats postmyocardial infarction that butyrate promoted expression of anti-inflammatory M2 macrophage markers, decreased expressions of nerve growth factor and norepinephrine, and decreased the density of nerve fibers for growth-associated protein-43 and tyrosine hydroxylase. The cumulative impact of butyrate led to suppression of inflammation and the inhibition of sympathetic neural remodeling, ultimately resulting in improved cardiac function and reduction in ventricular arrhythmias after myocardial infarction.52
Gut bacteria-derived acetate-mediated reduction in cardiac fibrosis may be another mechanism for the effects on ventricular arrhythmias. Cardiac fibrosis and scar are established as the primary substrate for reentrant ventricular arrhythmias seen in various cardiomyopathies.
Future Directions
The microbiome residing in the human gut has a significant impact on cardiac arrhythmias, the details of which remain unknown. A likely bidirectional relationship exists in which the gut microbiome may affect arrhythmogenesis and in turn be affected by cardiac arrhythmias. The mechanisms of action are not well understood, but likely involve the autonomic nervous system, inflammation, and metabolic pathways.
The gut microbiome is a complex collection of heterogenous microorganisms that have dramatic effects on the human body. Additional research is necessary to identify further associations and causations of gut microorganisms with various human body processes, as well as cardiovascular disease. The microbiome has been shown to directly and indirectly influence the development of different disease states, including the cardiovascular system and cardiac arrhythmias. Several pathways have been proposed through which the gut microbiome can potentially affect cardiac arrhythmogenesis. There are likely several mechanisms simultaneously in operation. Understanding the role of human gut microbiome in the genesis of cardiac arrhythmias not only may improve our understanding of arrhythmias, but also may result in novel treatment options. This could potentially lead to the development of therapeutic options and strategies to modulate the gut microbiome to help detect, prevent, and treat cardiac arrhythmias.
The extensive surface of the gastrointestinal tract presents an interface between the human body and its environment. Residing within the intestinal lumen, ingested food and various microorganisms are an essential aspect of this relationship. The trillions of microorganisms, primarily commensal bacteria hosted by the human gut, constitute the human gut microbiome.
There is growing evidence that the human gut microbiome plays a role in maintaining normal body function and homeostasis.1 Research, such as the National Institute of Health Microbiome Project, is helping to show the impact of gut microorganisms and their negative influence on metabolic diseases and chronic inflammatory disorders.2-5 An imbalance in the microbiota, known as dysbiosis, has been associated with metabolic and cardiovascular diseases (CVD), including hypertension, diabetes mellitus, obesity, and coronary artery disease (CAD). Gut dysbiosis has also been associated with cardiac arrhythmias, including atrial fibrillation (AF) and ventricular arrhythmias (Figure).6-12

Whether gut dysbiosis is a cause or effect of the human disease process is unclear. While further research is warranted, some evidence of causation has been found. In 2018, Yoshida et al demonstrated an association between patients with CAD who had a significantly lower burden of the gut bacteria species Bacteroides vulgatus and Bacteroides dorei compared to that of patients without CAD. The study found that administration of these Bacteroides species reduced atherosclerotic lesion formation in atherosclerosis-prone mice.13 If altering gut microbial composition can affect the disease process, it may indicate a causative role for gut dysbiosis in disease pathogenesis. Furthermore, this finding also suggests agents may be used to alter the gut microbiome and potentially prevent and treat diseases. An altered gut microbiome may serve as an early marker for human disease, aiding in timely diagnosis and institution of disease-modifying treatments.
This review outlines the broad relationship of the pathways and intermediaries that may be involved in mediating the interaction between the gut microbiome and cardiac arrhythmias based on rapidly increasing evidence. A comprehensive search among PubMed and Google Scholar databases was conducted to find articles relevant to the topic.
Potential Intermediaries
Potential pathways for how the gut microbiome and cardiovascular system interact are subjects of active research. However, recent research may point to potential mechanisms of the association between the systems. The gut microbiome may influence human physiology through 3 principal routes: the autonomic nervous system, inflammatory pathways, and metabolic processes.
Autonomic Nervous System
The concept of bidirectional communication between the gut and central nervous system, known as the microbiota-gut-brain axis, is widely accepted.14 Proposed mediators of this interaction include the vagus nerve, the sympathetic nervous system, and the hypothalamic-pituitary-adrenal axis; cytokines produced by the immune system, tryptophan metabolism, and the production of short-chain fatty acids (SCFAs).15,16
The gut microbiome appears to have a direct impact on the autonomic nervous system, through which it can influence cardiovascular function. Muller et al described how the gut microbiome modulated gut-extrinsic sympathetic neurons and that the depletion of gut microbiota led to activation of both brainstem sensory nuclei and efferent sympathetic premotor glutamatergic neurons.16 Meng et al found that systemic injection of the gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) led to significantly increased activity in the paraventricular nucleus, a hypothalamic structure essential to the central autonomic network. Their study demonstrated that systemic TMAO also led to increased left stellate ganglion (LSG) activity, a known contributor to cardiac sympathetic tone.12
Inflammatory Pathways
Inflammatory responses are another pathway for the gut microbiome to influence the cardiovascular system. SCFAs are a set of gut microbial metabolites produced in the colon by bacterial fermentation and decomposition of resistant starches and dietary fibers.17 These metabolites are increasingly recognized for their role in modulating disease processes, including cardiac disease. Aguilar et al found that the progression of atherosclerosis was slowed in apolipoprotein E (Apo-E) knockout mice by a chow diet supplemented with butyrate, a SCFA, suggesting it is an atheroprotective therapeutic agent. Less adhesion and migration of macrophages, reduced inflammation, improved plaque stability, and lowered atherosclerosis progression.18 Wei et al demonstrated in animal models that direct microinjection of the proinflammatory factors interleukin (IL)-1Β and tumor necrosis factor (TNF)-αdirectly into the subfornical organ increased heart rate, mean blood pressure, and renal sympathetic nerve activity.19
Metabolic Processes
Serotonin (5-HT), a metabolite of tryptophan, is a neurotransmitter that regulates many bodily functions and plays a significant role in the microbiota-brain gut axis.20 Oral ingestion of the bacterial species Bifidobacterium infantis increased plasma tryptophan in rat models.21 Additionally, many other microorganisms, including species of Candida, Streptococcus, Escherichia, and Enterococcus are known to produce 5-HT.22 While a relationship between the gut microbiome and plasma 5-HT has been established, interactions between 5-HT and the cardiovascular system are complex. Research has shown that stimulation of 5-HT1A receptors produces bradycardic and vasopressor effects, while stimulation of the 5-HT2 receptor induces vasoconstriction and tachycardia.23
A high-fiber diet can lower the incidence of hypertension, although the mechanisms are not clear. One potential reason could be alteration in gut bacteria, as a diet high in fiber has been shown to increase the prevalence of acetate-producing bacteria.24
Atherosclerosis
Research investigating the relationship of the gut microbiome with arrhythmias is in its early stages; however, the connection of the gut microbiome and atherosclerosis is more established.25 Contemporary studies have shown various gut microorganisms associated with atherosclerosis.26 Jie et al reported that patients with atherosclerotic cardiovascular disease had increased Enterobacteriaceae loads and oral cavity-associated bacteria with lower levels of butyrate producing bacteria when compared with healthy controls.27 In addition, microbial metabolites such as TMAO appear to promote atherosclerosis by increasing vascular inflammation and platelet reactivity.26 Researchers are investigating the modulation of these associations to help reduce atherosclerotic burden. Kasahara et al found that Roseburia intestinalis could reduce atherosclerotic disease in mice through the production of butyrate.28 Roberts et al established that administration of TMAO inhibitors reduced TMAO levels while reducing thrombus formation without observable toxicity or increased bleeding risk.29
Atrial Arrhythmias
The gut microbiome can also specifically affect cardiac arrhythmogenesis, and multiple studies suggest possible mediators of this interaction. Certain gut microbiome derived metabolites like TMAO may have a role in promoting AF.30 Other gut microbial metabolites like lipopolysaccharides and indoxyl sulfate are implicated in atrial electrical instability.31,32 Microbe-derived free fatty acids such as palmitic acid and adrenic acid can precipitate arrhythmogenesis. 33,34 Preponderances of certain gut bacteria like Ruminococcus, Streptococcus, and Enterococcus, as well as reductions of Faecalibacterium, Alistipes, Oscillibacter, and Bilophila have been detected in patients with AF.8 Tabata et al found that certain clusters of bacterial groups led by Ruminococcus species seem to show higher prevalence in patients with AF, whereas the genus Enterobacter was significantly lower compared with control subjects. That study also noted that gut microbial composition is affected by diet and antacid use.35 Gut microbiome-derived serotonin may be another mediator for AF, which may be related to the fact that 5-HT4 receptors are present in atrial tissue.36
Ventricular Arrhythmias
A critical component to the development of malignant ventricular arrhythmias is an imbalance in autonomic tone; in particular, the overactivation of the sympathetic nervous system.37 Animal models have shown that augmentation of the sympathetic nervous system plays an essential role in the subsequent development of ventricular arrhythmias. 38 Several studies have established the LSG as an important component of the cardiac sympathetic nervous system pathway. 38,39 Ablation of the LSG has been shown to effectively reduce the burden of malignant arrhythmias, further pointing toward the role of excess sympathetic activity.37,39 Stellate ganglion denervation has become an established method for managing life-threatening ventricular arrhythmias.40
Gut metabolites may have significant effects on cardiac sympathetic activity. Meng et al investigated the effect of TMAO on the LSG in animals and its overall effect on the incidence of ventricular arrhythmias under ischemic conditions. To fully explore this interaction, they examined the effect of TMAO on LSG function though 2 mechanisms: local administration of TMAO within the LSG and systemic administration of TMAO leading to activation of the central sympathetic nervous system. In both protocols, left anterior descending coronary artery occlusion was performed after TMAO administration. Injection of TMAO directly into the LSG was found to significantly increase the cardiac sympathetic tone and incidence of ventricular arrhythmias. In the systemic administration control arm, ventricular arrhythmias were also significantly increased.12
Increased inflammatory states appear to correlate with an increase in sympathetic tone and ventricular arrhythmias.12 In an animal study, direct injection of the proinflammatory factor IL-1Β into the LSG not only resulted in increased inflammation, but aggravated cardiac sympathetic remodeling. This led to a decreased effective refractory period and action potential duration, leading to an increased maximal slope of the restitution curve and higher occurrence of ventricular arrhythmias.41 Shi et al demonstrated that paraventricular nucleus microinjection with TNF-α and IL-1Β also enhanced the cardiac sympathetic afferent reflex, showing that these proinflammatory cytokines not only upregulate the inflammatory response, but can also have excitatory effects that stimulate sympathetic activity and have the potential to be proarrhythmic.19,42 Local and systemic administration of the gut microbe-derived TMAO increased the expression of IL-1Β and TNF-α, thus implicating the microbiome as a potential mediator of the inflammatory response and as another potential pathway for increased ventricular arrhythmias.12
The N-methyl-d-aspartate receptor (NMDAR) is found in multiple organs—including the heart—but more specifically in the conducting system and myocardium.43,44 Research has discovered an upregulation of NMDARs in the setting of cardiac sympathetic hyperinnervation in rat models both with healed myocardial necrotic injury and without. The infusion of their ligand, NMDA, provoked ventricular tachycardia and ventricular fibrillation in rat models with sympathetic hyperinnervation and healed myocardial necrotic injury.45 Another study found that NMDAR activation provoked ventricular arrhythmias, but also prolonged repolarization and induced electrical instability.46 Proinflammatory markers have been shown to upregulate the expression of NMDARs; more importantly, NMDAR expression has been shown to be significantly increased in the setting of TMAO administration.12,47,48
5-HT also appears to have a substantial association with ventricular arrhythmias in addition to atrial arrhythmias. el-Mahdy demonstrated in anesthetized rats with acute coronary ligation that systemic doses of 5-HT represented a significant dose-dependent increase in the duration of ventricular tachycardia and ventricular fibrillation, while also increasing the number of ventricular ectopic beats.49 Certain gut microorganisms are known to produce 5-HT, including those in the genera Streptococcus, Escherichia, and Enterococcus.22 Additionally, oral ingestion of the Bifidobacterium infantis increased plasma levels of tryptophan in rat models.21 The gut microbiome may have significant effects on plasma serotonin levels, and thus have the potential to alter the risk for ventricular arrhythmias.
The deleterious effects of the gut microbiome have been documented. However, it appears to have potential protective effects, and several studies point to the possible mechanisms of this beneficial interaction. Propionate is a SCFA microorganism produced by gut microbial fermentation.50 In a rat model study, Zhou et al found that infusion of sodium propionate significantly reduced ventricular arrhythmias during acute myocardial ischemia or burst stimulation, thus confirming cardioprotective effects.50,51
Proposed mechanisms for reduced susceptibility to ventricular arrhythmias with propionate infusion include parasympathetic activation via the gut-brain axis, anti-inflammatory pathways, and improved cardiac electrophysiology instability.50 In addition butyrate has been found to reduce inflammation and myocardial hypertrophy. Jiang et al demonstrated in rats postmyocardial infarction that butyrate promoted expression of anti-inflammatory M2 macrophage markers, decreased expressions of nerve growth factor and norepinephrine, and decreased the density of nerve fibers for growth-associated protein-43 and tyrosine hydroxylase. The cumulative impact of butyrate led to suppression of inflammation and the inhibition of sympathetic neural remodeling, ultimately resulting in improved cardiac function and reduction in ventricular arrhythmias after myocardial infarction.52
Gut bacteria-derived acetate-mediated reduction in cardiac fibrosis may be another mechanism for the effects on ventricular arrhythmias. Cardiac fibrosis and scar are established as the primary substrate for reentrant ventricular arrhythmias seen in various cardiomyopathies.
Future Directions
The microbiome residing in the human gut has a significant impact on cardiac arrhythmias, the details of which remain unknown. A likely bidirectional relationship exists in which the gut microbiome may affect arrhythmogenesis and in turn be affected by cardiac arrhythmias. The mechanisms of action are not well understood, but likely involve the autonomic nervous system, inflammation, and metabolic pathways.
The gut microbiome is a complex collection of heterogenous microorganisms that have dramatic effects on the human body. Additional research is necessary to identify further associations and causations of gut microorganisms with various human body processes, as well as cardiovascular disease. The microbiome has been shown to directly and indirectly influence the development of different disease states, including the cardiovascular system and cardiac arrhythmias. Several pathways have been proposed through which the gut microbiome can potentially affect cardiac arrhythmogenesis. There are likely several mechanisms simultaneously in operation. Understanding the role of human gut microbiome in the genesis of cardiac arrhythmias not only may improve our understanding of arrhythmias, but also may result in novel treatment options. This could potentially lead to the development of therapeutic options and strategies to modulate the gut microbiome to help detect, prevent, and treat cardiac arrhythmias.
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
- Karlsson F, Tremaroli V, Nielsen J, Bäckhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes. 2013;62(10):3341-3349. doi:10.2337/db13-0844
- Danneskiold-Samsøe NB, Dias de Freitas Queiroz Barros H, Santos R, et al. Interplay between food and gut microbiota in health and disease. Food Res Int. 2019;115:23-31. doi:10.1016/j.foodres.2018.07.043
- Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe- derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446-450. doi:10.1038/nature12721
- Integrative HMP (iHMP) Research Network Consortium. The integrative human microbiome project. Nature. 2019;569(7758):641-648. doi:10.1038/s41586-019-1238-8
- Zubcevic J, Richards EM, Yang T, et al. Impaired autonomic nervous system-microbiome circuit in hypertension. Circ Res. 2019;125(1):104-116. doi:10.1161/CIRCRESAHA.119.313965
- Emoto T, Yamashita T, Sasaki N, et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atheroscler Thromb. 2016;23(8):908-921. doi:10.5551/jat.32672
- Zuo K, Li J, Li K, et al. Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. Gigascience. 2019;8(6):giz058. doi:10.1093/gigascience/giz058
- Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. doi:10.1186/s40168-016-0222-x
- Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55-60. doi:10.1038/nature11450
- Chang CJ, Lin CS, Lu CC, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:7489. doi:10.1038/ncomms8489
- Meng G, Zhou X, Wang M, et al. Gut microbederived metabolite trimethylamine N-oxide activates the cardiac autonomic nervous system and facilitates ischemia-induced ventricular arrhythmia via two different pathways. EBioMedicine. 2019;44:656-664. doi:10.1016/j.ebiom.2019.03.066
- Yoshida N, Emoto T, Yamashita T, et al. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation. 2018;138(22):2486-2498. doi:10.1161/CIRCULATIONAHA.118.033714
- Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. Front Neuroendocrinol. 2018;51:80-101. doi:10.1016/j.yfrne.2018.04.002
- Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: how gut microbes shape human behavior. J Psychiatr Res. 2015;63:1-9. doi:10.1016/j.jpsychires.2015.02.021
- Muller PA, Schneeberger M, Matheis F, et al. Microbiota modulate sympathetic neurons via a gutbrain circuit. Nature. 2020;583(7816):441-446. doi:10.1038/s41586-020-2474-7
- Ohira H, Tsutsui W, Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J Atheroscler Thromb. 2017;24(7):660-672. doi:10.5551/jat.RV17006
- Aguilar EC, Leonel AJ, Teixeira LG, et al. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFêB activation. Nutr Metab Cardiovasc Dis. 2014;24(6):606-613. doi:10.1016/j.numecd.2014.01.002
- Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcit - atory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65(5):1126-1133. doi:10.1161/HYPERTENSIONAHA.114.05112
- Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720- 726. doi:10.1016/j.biopsych.2013.05.001
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164-174. doi:10.1016/j.jpsychires.2008.03.009
- Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33(8):574-581. doi:10.1002/bies.201100024
- Yusuf S, Al-Saady N, Camm AJ. 5-hydroxytryptamine and atrial fibrillation: how significant is this piece in the puzzle? J Cardiovasc Electrophysiol. 2003;14(2):209-214. doi:10.1046/j.1540-8167.2003.02381.x
- Marques FZ, Nelson E, Chu PY, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135(10):964-977. doi:10.1161/CIRCULATIONAHA.116.024545
- Björkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185(10):1630-1645. doi:10.1016/j.cell.2022.04.004
- Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(16):2089-2105. doi:10.1016/j.jacc.2019.03.024
- Jie Z, Xia H, Zhong SL, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1):845. doi:10.1038/s41467-017-00900-1
- Kasahara K, Krautkramer KA, Org E, et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol. 2018;3(12):1461- 1471. doi:10.1038/s41564-018-0272-x
- Roberts AB, Gu X, Buffa JA, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24(9):1407-1417. doi:10.1038/s41591-018-0128-1
- Yu L, Meng G, Huang B, et al. A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int J Cardiol. 2018;255:92- 98. doi:10.1016/j.ijcard.2017.11.071
- Okazaki R, Iwasaki YK, Miyauchi Y, et al. Lipopolysaccharide induces atrial arrhythmogenesis via down-regulation of L-type Ca2+ channel genes in rats. Int Heart J. 2009;50(3):353-363. doi:10.1536/ihj.50.353
- Chen WT, Chen YC, Hsieh MH, et al. The uremic toxin indoxyl sulfate increases pulmonary vein and atrial arrhythmogenesis. J Cardiovasc Electrophysiol. 2015;26(2):203- 210. doi:10.1111/jce.12554
- Fretts AM, Mozaffarian D, Siscovick DS, et al. Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc. 2014;3(3):e000889. doi:10.1161/JAHA.114.000889
- Horas HNS, Nishiumi S, Kawano Y, Kobayashi T, Yoshida M, Azuma T. Adrenic acid as an inflammation enhancer in non-alcoholic fatty liver disease. Arch Biochem Biophys. 2017;623-624:64-75. doi:10.1016/j.abb.2017.04.009
- Tabata T, Yamashita T, Hosomi K, et al. Gut microbial composition in patients with atrial fibrillation: effects of diet and drugs. Heart Vessels. 2021;36(1):105-114. doi:10.1007/s00380-020-01669-y
- López-Rodriguez ML, Benhamú B, Morcillo MJ, et al. 5-HT(4) receptor antagonists: structure-affinity relationships and ligand-receptor interactions. Curr Top Med Chem. 2002;2(6):625-641. doi:10.2174/1568026023393769
- Yu L, Zhou L, Cao G, et al. Optogenetic modulation of cardiac sympathetic nerve activity to prevent ventricular arrhythmias. J Am Coll Cardiol. 2017;70(22):2778-2790. doi:10.1016/j.jacc.2017.09.1107
- Schwartz PJ, Vanoli E. Cardiac arrhythmias elicited by interaction between acute myocardial ischemia and sympathetic hyperactivity: a new experimental model for the study of antiarrhythmic drugs. J Cardiovasc Pharmacol. 1981;3(6):1251-1259. doi:10.1097/00005344-198111000-00012
- Puddu PE, Jouve R, Langlet F, Guillen JC, Lanti M, Reale A. Prevention of postischemic ventricular fibrillation late after right or left stellate ganglionectomy in dogs. Circulation. 1988;77(4):935-946. doi:10.1161/01.cir.77.4.935
- Vaseghi M, Gima J, Kanaan C, et al. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and longterm follow-up. Heart Rhythm. 2014;11(3):360-366. doi:10.1016/j.hrthm.2013.11.028
- Wang M, Li S, Zhou X, et al. Increased inflammation promotes ventricular arrhythmia through aggravating left stellate ganglion remodeling in a canine ischemia model. Int J Cardiol. 2017;248:286-293. doi:10.1016/j.ijcard.2017.08.011
- Shi Z, Gan XB, Fan ZD, et al. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf). 2011;203(2):289-297. doi:10.1111/j.1748-1716.2011.02313.x
- Gill S, Veinot J, Kavanagh M, Pulido O. Human heart glutamate receptors - implications for toxicology, food safety, and drug discovery. Toxicol Pathol. 2007;35(3):411-417. doi:10.1080/01926230701230361
- Govoruskina N, Jakovljevic V, Zivkovic V, et al. The role of cardiac N-methyl-D-aspartate receptors in heart conditioning— effects on heart function and oxidative stress. Biomolecules. 2020;10(7):1065. doi:10.3390/biom10071065
- Lü J, Gao X, Gu J, et al. Nerve sprouting contributes to increased severity of ventricular tachyarrhythmias by upregulating iGluRs in rats with healed myocardial necrotic injury. J Mol Neurosci. 2012;48(2):448-455. doi:10.1007/s12031-012-9720-x
- Shi S, Liu T, Li Y, et al. Chronic N-methyl-D-aspartate receptor activation induces cardiac electrical remodeling and increases susceptibility to ventricular arrhythmias. Pacing Clin Electrophysiol. 2014;37(10):1367-1377. doi:10.1111/pace.12430
- Zhang Z, Bassam B, Thomas AG, et al. Maternal inflammation leads to impaired glutamate homeostasis and upregulation of glutamate carboxypeptidase II in activated microglia in the fetal/newborn rabbit brain. Neurobiol Dis. 2016;94:116-128. doi:10.1016/j.nbd.2016.06.010
- Wu LJ, Toyoda H, Zhao MG, et al. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci. 2005;25(48):11107-11116. doi:10.1523/JNEUROSCI.1678-05.2005
- el-Mahdy SA. 5-hydroxytryptamine (serotonin) enhances ventricular arrhythmias induced by acute coronary artery ligation in rats. Res Commun Chem Pathol Pharmacol. 1990;68(3):383-386.
- Zhou M, Li D, Xie K, et al. The short-chain fatty acid propionate improved ventricular electrical remodeling in a rat model with myocardial infarction. Food Funct. 2021;12(24):12580-12593. doi:10.1039/d1fo02040d
- Bartolomaeus H, Balogh A, Yakoub M, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139(11):1407-1421. doi:10.1161/CIRCULATIONAHA.118.036652
- Jiang X, Huang X, Tong Y, Gao H. Butyrate improves cardiac function and sympathetic neural remodeling following myocardial infarction in rats. Can J Physiol Pharmacol. 2020;98(6):391-399. doi:10.1139/cjpp-2019-0531
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
- Karlsson F, Tremaroli V, Nielsen J, Bäckhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes. 2013;62(10):3341-3349. doi:10.2337/db13-0844
- Danneskiold-Samsøe NB, Dias de Freitas Queiroz Barros H, Santos R, et al. Interplay between food and gut microbiota in health and disease. Food Res Int. 2019;115:23-31. doi:10.1016/j.foodres.2018.07.043
- Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe- derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446-450. doi:10.1038/nature12721
- Integrative HMP (iHMP) Research Network Consortium. The integrative human microbiome project. Nature. 2019;569(7758):641-648. doi:10.1038/s41586-019-1238-8
- Zubcevic J, Richards EM, Yang T, et al. Impaired autonomic nervous system-microbiome circuit in hypertension. Circ Res. 2019;125(1):104-116. doi:10.1161/CIRCRESAHA.119.313965
- Emoto T, Yamashita T, Sasaki N, et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atheroscler Thromb. 2016;23(8):908-921. doi:10.5551/jat.32672
- Zuo K, Li J, Li K, et al. Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. Gigascience. 2019;8(6):giz058. doi:10.1093/gigascience/giz058
- Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. doi:10.1186/s40168-016-0222-x
- Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55-60. doi:10.1038/nature11450
- Chang CJ, Lin CS, Lu CC, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:7489. doi:10.1038/ncomms8489
- Meng G, Zhou X, Wang M, et al. Gut microbederived metabolite trimethylamine N-oxide activates the cardiac autonomic nervous system and facilitates ischemia-induced ventricular arrhythmia via two different pathways. EBioMedicine. 2019;44:656-664. doi:10.1016/j.ebiom.2019.03.066
- Yoshida N, Emoto T, Yamashita T, et al. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation. 2018;138(22):2486-2498. doi:10.1161/CIRCULATIONAHA.118.033714
- Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. Front Neuroendocrinol. 2018;51:80-101. doi:10.1016/j.yfrne.2018.04.002
- Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: how gut microbes shape human behavior. J Psychiatr Res. 2015;63:1-9. doi:10.1016/j.jpsychires.2015.02.021
- Muller PA, Schneeberger M, Matheis F, et al. Microbiota modulate sympathetic neurons via a gutbrain circuit. Nature. 2020;583(7816):441-446. doi:10.1038/s41586-020-2474-7
- Ohira H, Tsutsui W, Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J Atheroscler Thromb. 2017;24(7):660-672. doi:10.5551/jat.RV17006
- Aguilar EC, Leonel AJ, Teixeira LG, et al. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFêB activation. Nutr Metab Cardiovasc Dis. 2014;24(6):606-613. doi:10.1016/j.numecd.2014.01.002
- Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcit - atory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65(5):1126-1133. doi:10.1161/HYPERTENSIONAHA.114.05112
- Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720- 726. doi:10.1016/j.biopsych.2013.05.001
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164-174. doi:10.1016/j.jpsychires.2008.03.009
- Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33(8):574-581. doi:10.1002/bies.201100024
- Yusuf S, Al-Saady N, Camm AJ. 5-hydroxytryptamine and atrial fibrillation: how significant is this piece in the puzzle? J Cardiovasc Electrophysiol. 2003;14(2):209-214. doi:10.1046/j.1540-8167.2003.02381.x
- Marques FZ, Nelson E, Chu PY, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135(10):964-977. doi:10.1161/CIRCULATIONAHA.116.024545
- Björkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185(10):1630-1645. doi:10.1016/j.cell.2022.04.004
- Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(16):2089-2105. doi:10.1016/j.jacc.2019.03.024
- Jie Z, Xia H, Zhong SL, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1):845. doi:10.1038/s41467-017-00900-1
- Kasahara K, Krautkramer KA, Org E, et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol. 2018;3(12):1461- 1471. doi:10.1038/s41564-018-0272-x
- Roberts AB, Gu X, Buffa JA, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24(9):1407-1417. doi:10.1038/s41591-018-0128-1
- Yu L, Meng G, Huang B, et al. A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int J Cardiol. 2018;255:92- 98. doi:10.1016/j.ijcard.2017.11.071
- Okazaki R, Iwasaki YK, Miyauchi Y, et al. Lipopolysaccharide induces atrial arrhythmogenesis via down-regulation of L-type Ca2+ channel genes in rats. Int Heart J. 2009;50(3):353-363. doi:10.1536/ihj.50.353
- Chen WT, Chen YC, Hsieh MH, et al. The uremic toxin indoxyl sulfate increases pulmonary vein and atrial arrhythmogenesis. J Cardiovasc Electrophysiol. 2015;26(2):203- 210. doi:10.1111/jce.12554
- Fretts AM, Mozaffarian D, Siscovick DS, et al. Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc. 2014;3(3):e000889. doi:10.1161/JAHA.114.000889
- Horas HNS, Nishiumi S, Kawano Y, Kobayashi T, Yoshida M, Azuma T. Adrenic acid as an inflammation enhancer in non-alcoholic fatty liver disease. Arch Biochem Biophys. 2017;623-624:64-75. doi:10.1016/j.abb.2017.04.009
- Tabata T, Yamashita T, Hosomi K, et al. Gut microbial composition in patients with atrial fibrillation: effects of diet and drugs. Heart Vessels. 2021;36(1):105-114. doi:10.1007/s00380-020-01669-y
- López-Rodriguez ML, Benhamú B, Morcillo MJ, et al. 5-HT(4) receptor antagonists: structure-affinity relationships and ligand-receptor interactions. Curr Top Med Chem. 2002;2(6):625-641. doi:10.2174/1568026023393769
- Yu L, Zhou L, Cao G, et al. Optogenetic modulation of cardiac sympathetic nerve activity to prevent ventricular arrhythmias. J Am Coll Cardiol. 2017;70(22):2778-2790. doi:10.1016/j.jacc.2017.09.1107
- Schwartz PJ, Vanoli E. Cardiac arrhythmias elicited by interaction between acute myocardial ischemia and sympathetic hyperactivity: a new experimental model for the study of antiarrhythmic drugs. J Cardiovasc Pharmacol. 1981;3(6):1251-1259. doi:10.1097/00005344-198111000-00012
- Puddu PE, Jouve R, Langlet F, Guillen JC, Lanti M, Reale A. Prevention of postischemic ventricular fibrillation late after right or left stellate ganglionectomy in dogs. Circulation. 1988;77(4):935-946. doi:10.1161/01.cir.77.4.935
- Vaseghi M, Gima J, Kanaan C, et al. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and longterm follow-up. Heart Rhythm. 2014;11(3):360-366. doi:10.1016/j.hrthm.2013.11.028
- Wang M, Li S, Zhou X, et al. Increased inflammation promotes ventricular arrhythmia through aggravating left stellate ganglion remodeling in a canine ischemia model. Int J Cardiol. 2017;248:286-293. doi:10.1016/j.ijcard.2017.08.011
- Shi Z, Gan XB, Fan ZD, et al. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf). 2011;203(2):289-297. doi:10.1111/j.1748-1716.2011.02313.x
- Gill S, Veinot J, Kavanagh M, Pulido O. Human heart glutamate receptors - implications for toxicology, food safety, and drug discovery. Toxicol Pathol. 2007;35(3):411-417. doi:10.1080/01926230701230361
- Govoruskina N, Jakovljevic V, Zivkovic V, et al. The role of cardiac N-methyl-D-aspartate receptors in heart conditioning— effects on heart function and oxidative stress. Biomolecules. 2020;10(7):1065. doi:10.3390/biom10071065
- Lü J, Gao X, Gu J, et al. Nerve sprouting contributes to increased severity of ventricular tachyarrhythmias by upregulating iGluRs in rats with healed myocardial necrotic injury. J Mol Neurosci. 2012;48(2):448-455. doi:10.1007/s12031-012-9720-x
- Shi S, Liu T, Li Y, et al. Chronic N-methyl-D-aspartate receptor activation induces cardiac electrical remodeling and increases susceptibility to ventricular arrhythmias. Pacing Clin Electrophysiol. 2014;37(10):1367-1377. doi:10.1111/pace.12430
- Zhang Z, Bassam B, Thomas AG, et al. Maternal inflammation leads to impaired glutamate homeostasis and upregulation of glutamate carboxypeptidase II in activated microglia in the fetal/newborn rabbit brain. Neurobiol Dis. 2016;94:116-128. doi:10.1016/j.nbd.2016.06.010
- Wu LJ, Toyoda H, Zhao MG, et al. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci. 2005;25(48):11107-11116. doi:10.1523/JNEUROSCI.1678-05.2005
- el-Mahdy SA. 5-hydroxytryptamine (serotonin) enhances ventricular arrhythmias induced by acute coronary artery ligation in rats. Res Commun Chem Pathol Pharmacol. 1990;68(3):383-386.
- Zhou M, Li D, Xie K, et al. The short-chain fatty acid propionate improved ventricular electrical remodeling in a rat model with myocardial infarction. Food Funct. 2021;12(24):12580-12593. doi:10.1039/d1fo02040d
- Bartolomaeus H, Balogh A, Yakoub M, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139(11):1407-1421. doi:10.1161/CIRCULATIONAHA.118.036652
- Jiang X, Huang X, Tong Y, Gao H. Butyrate improves cardiac function and sympathetic neural remodeling following myocardial infarction in rats. Can J Physiol Pharmacol. 2020;98(6):391-399. doi:10.1139/cjpp-2019-0531
The Gut Microbiome and Cardiac Arrhythmias
The Gut Microbiome and Cardiac Arrhythmias
Safety and Efficacy of Ezetimibe in Patients With and Without Chronic Kidney Disease at a Pharmacist-Managed Clinic
Statins are widely used to reduce low-density lipoprotein (LDL) and non-high-density lipoprotein (HDL) levels for the prevention of atherosclerotic cardiovascular disease (ASCVD).1 However, despite maximally tolerated statin therapy, many patients may not reach their LDL and non-HDL goals. Some patients may experience adverse events (AEs), particularly muscle-related AEs, which can limit the use of these medications.
The 2022 American College of Cardiology (ACC) expert consensus pathway recommends a goal LDL of < 55 mg/dL in very high-risk patients, defined as those with a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions.2 Major ASCVD events include acute coronary syndrome within 12 months, history of myocardial infarction (MI) or ischemic stroke, and symptomatic peripheral arterial disease (ie, claudication with ankle-brachial index < 0.85 or previous revascularization or amputation). Factors for being considered high risk include age > 65 years, heterozygous familial hypercholesterolemia, history of prior coronary artery bypass surgery or percutaneous coronary intervention outside the major ASCVD events, diabetes, hypertension, chronic kidney disease (CKD) (estimated glomerular filtration rate [eGFR] 15-59 mL/min/1.73 m2), current smoking, persistently elevated LDL cholesterol (LDL-C) levels despite maximally tolerated statin therapy and ezetimibe, and history of congestive heart failure.2 For these patients, statin therapy alone may not achieve LDL goal.
The ACC recommends ezetimibe as the initial nonstatin therapy in patients who are not at their goal LDL.2 Ezetimibe works by inhibiting Niemann-Pick C1-Like 1 protein, which causes reduced cholesterol absorption in the small intestine.2,3 Previous studies have shown the benefit of ezetimibe for LDL reduction and ASCVD prevention.4-7 The 2015 IMPROVE-IT study found the combination of simvastatin and ezetimibe resulted in a significantly lower risk of cardiovascular events than simvastatin monotherapy. IMPROVE-IT also reported a further clinical benefit when lower LDL targets (ie, < 55 mg/dL) are achieved, which aligns with the expert consensus pathway recommendations for a lower LDL goal for very high-risk patients.2,5
The RACING trial found that treatment with a moderate-intensity statin and ezetimibe was noninferior to treatment with a high-intensity statin for the primary outcome of occurrence of cardiovascular death, major cardiovascular events, or nonfatal stroke within 3 years. The combination of moderate-intensity statin and ezetimibe achieved lower LDL-C levels and lower incidence of drug intolerance compared to high intensity statin monotherapy.6 The SHARP-CKD study assessed major atherosclerotic events in patients with CKD who had no history of MI or coronary revascularization. The study found that lowering LDL-C with the combination of simvastatin plus ezetimibe safely reduces the risk of major atherosclerotic events in a wide range of patients with CKD.7
Lastly, the 2019 EWTOPIA 75 study found that ezetimibe noted a statistically significant reduction in the incidence of the composite of sudden cardiac death, MI, coronary revascularization, or stroke compared to placebo. Ezetimibe showed benefits in preventing ASCVD events independently of statin therapy.8 These clinical trials provided evidence for the efficacy of ezetimibe for secondary or primary prevention of ASCVD, patients with CKD, and patients who are not at their LDL goal despite maximally tolerated statin therapy.
Reductions in LDL levels with ezetimibe are reported to be 15% to 19% for monotherapy and 13% to 25% when used in combination with a statin.4 Given that the ACC now recommends lower LDL goals, patients may need additional lowering despite taking maximally tolerated statin therapy.2 Additionally, the package insert for ezetimibe reports increased area under the curve (AUC) values of ezetimibe and its metabolites in patients with severe renal disease. It is anticipated that ezetimibe may show an increased reduction of LDL and non-HDL, but there may also be an increased risk for muscle-related AEs.3
This quality-assurance quality improvement project investigated the use of ezetimibe in patients with CKD to determine whether there is further LDL and non-HDL reduction in this patient population. It sought to determine the LDL and non-HDL percentage reduction in patients with and without CKD at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) and whether there is an increased risk for muscle-related AEs. Determining the percentage reduction of LDL and non-HDL within this population can help increase use of ezetimibe in patients not at their LDL or non-HDL goal or for those patients unable to tolerate statin therapy.
Methods
This single-center retrospective chart review investigated patients prescribed ezetimibe by a patient aligned care team (PACT) pharmacist at WBVAMC between September 1, 2021, and September 1, 2023. This project was determined to be nonresearch by the Veterans Integrated Service Network 4 multisite institutional review board. Patients were excluded from the review if they started taking ezetimibe outside of the prespecified time frame, if ezetimibe was initiated by a non-WBVAMC PACT pharmacist, or if there was no follow-up lipid panel obtained within 6 months of initiation of ezetimibe.
The primary outcomes were to determine the percentage mean change in LDL and non-HDL reduction and the incidence of muscle-related AEs after initiation of ezetimibe in patients without CKD. The secondary outcomes were to determine the percentage mean change in LDL and non-HDL levels and the incidence of muscle-related AEs after initiation of ezetimibe in patients with CKD. For this study, CKD was defined as a patient having an eGFR 15 to 60 ml/min/1.73 m2. Non-HDL is the combination of LDL-C and very LDL-C and represents all potentially atherogenic particles. The 2022 Expert Consensus Pathway included non-HDL goals in addition to LDL goals.2 Non-HDL cholesterol levels can be used for patients with elevated triglycerides where LDL levels may not be as accurate. To account for instances of elevated triglycerides, this study assessed changes in both LDL and non-HDL levels.
Data were collected from the US Department of Veterans Affairs (VA) Computerized Patient Record System (CPRS) and recorded in a spreadsheet. Collected data included age, sex, race, concomitant cholesterol-lowering medications (statin, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitor, bempedoic acid, fish oil, niacin, bile acid sequestrants, and fibrates), baseline lipid panel, lipid panel within 6 months of ezetimibe initiation, and eGFR level. If the patient’s LDL or non-HDL levels worsened on the follow-up lipid panel, their baseline LDL and non-HDL levels were used to calculate the percentage reduction; thus, the percentage reduction would be 0%. This strategy was used in prior research, notably the IMPROVE-IT and SHARP-CKD trials.
Ezetimibe 5 mg once daily was used in this study based on a 2008 VA study that evaluated the use of ezetimibe 5 mg vs ezetimibe 10 mg and the percentage reduction of LDL with each dose. The study found no significant difference between the 5 mg and 10 mg dose.9 Most patients included in this study received the 5 mg dose.
Results
This retrospective chart review consisted of 173 patients, 137 (79.2%) without CKD and 36 (20.8%) with CKD at baseline. The mean age was 69.6 years, 155 (89.6%) patients were male, and 18 (10.4%) were female. There were 164 concomitant medications, including 115 patients prescribed a statin and 38 patients prescribed fish oil (Table 1).
Patients without CKD had mean reductions in LDL levels of 23.5% and non-HDL levels of 21.7% (Figure). Patients who had an increase in LDL and non-HDL levels were excluded to control for potential confounding factors such as dietary changes, discontinuation of ezetimibe therapy, nonadherence to ezetimibe, and medication changes that impacted follow-up laboratory tests such as discontinuation of a statin. Fifteen patients experienced an increase in LDL or non-HDL levels. After excluding these patients, those without CKD had a mean reduction in LDL levels of 28.0% and non-HDL levels of 25.5%. Nineteen (13.9%) patients without CKD experienced a muscle-related AE (Table 2). One patient discontinued ezetimibe and statin use following a Lyme disease diagnosis due to concerns over potential muscle-related AEs.
Patients with CKD had a mean reduction in LDL and non-HDL levels of 27.0% and 24.8%, respectively. Patients with an increase in LDL or non-HDL levels were also excluded to help control for potential confounding factors. After excluding 4 patients with increased LDL and non-HDL levels, the mean reduction in LDL and non-HDL levels was 30.5% and 27.5%, respectively. Five (13.9%) patients with CKD experienced muscle-related AEs thought to be due to ezetimibe. Other AEs (eg, urticaria, diarrhea, reflux, dizziness, headache, upset stomach) were reported that led to discontinuation of ezetimibe, but only muscle-related AEs were analyzed.
Discussion
This retrospective chart review found larger reductions in LDL and non-HDL levels for patients with CKD than reported in the literature.4 Based on the findings that indicate a greater cholesterol reduction with ezetimibe, the results suggest an underutilization of ezetimibe in clinical practice, which may be due to clinicians favoring statin therapy and overlooking ezetimibe as a viable option based on recommendation in earlier guidelines. The 2022 guidelines transitioned from a statin focus to a focus on LDL targets and goals.2
According to the ACC, there is evidence to support a direct relationship between LDL-C levels, atherosclerosis progression, and ASCVD event risk.2 Absolute LDL-C level reduction is directly associated with ASCVD risk reduction which supports the LDL hypothesis. There appears to be no specific LDL-C level below which benefit ceases.2 This suggests that lower LDL-C targets (< 55 mg/dL) should be used when clinically indicated. Many patients are either unable to reach their goal LDL levels with statin monotherapy or are unable to tolerate statin therapy at higher doses, which may require additional pharmacotherapy to reach goal LDL-C. The ACC expert consensus pathway recommends ezetimibe as the initial add-on treatment to statins.2 The RACING trial showed the benefit of adding ezetimibe to a moderate-intensity statin vs increasing to a high-intensity statin dose. This trial found patients had lower LDL levels and lower rates of intolerances, which further supports ezetimibe use.6
This quality improvement project assessed LDL and non-HDL level reduction in patients with CKD. As anticipated, there was greater reduction in LDL and non-HDL levels seen in patients with CKD. The SHARP-CKD trial also found reductions in LDL levels with ezetimibe in patients with CKD.7 Given the reduction in LDL and non-HDL levels with ezetimibe in patients with or without CKD, add-on therapy of ezetimibe should be recommended for patients who do not achieve their LDL goals with statin therapy or for patients who intolerant to statin therapy.
The ezetimibe package insert reports myalgias incidence to be < 5% in patients and research has shown up to a 20% incidence of muscle-related AEs with statin therapy.3,10 Based on the package information reporting increased AUC values of ezetimibe and its metabolites in patients with severe renal disease, it was anticipated there may be an increased risk of muscle-related AEs in patients with CKD.3 However, this study found the same incidence of muscle-related AEs in patients with and without CKD. Previous research on statin-intolerant patients found the incidence of muscle-related AEs with ezetimibe to be 23.0% and 28.8%.11,12 This increased incidence of muscle-related AEs may be the result of including patients with a history of statin intolerance. Collectively, data from clinical trials and this study indicate that patients with prior intolerances to statins appear to have a higher likelihood of developing a muscle-related AEs with ezetimibe.11,12 Clinicians and patients should be educated on the potential for these AEs and be aware that the likelihood may be greater if there is a history of statin intolerance. To our knowledge, this was the first study to evaluate muscle-related AEs with ezetimibe in patients with and without CKD.
Limitations
This retrospective chart review was performed over a prespecified period and only patients initiated on ezetimibe by a PACT pharmacist were included. This study did not assess the percentage of LDL reduction in patients on concomitant statins vs those who were not on concomitant statins. The study only included 173 patients. Additionally, the study was primarily composed of White men and may not be representative of other populations. In addition, veterans may not be representative of the general population given their high comorbidity burden and other exposures. Some reported muscle-related AEs associated with ezetimibe may be attributed to the nocebo effect.
Conclusions
The results of this retrospective chart review suggest there may be a larger mean reduction in LDL and non-HDL levels seen with ezetimibe therapy than reported within the literature. There was a larger mean reduction in LDL and non-HDL levels in patients with CKD than in patients without CKD. Additionally, there were the same rates of muscle-related AEs with ezetimibe therapy in patients with and without CKD. The rates of muscle-related AEs with ezetimibe therapy were higher than reported in the medication’s package insert, but lower than reported in literature that included statin-intolerant patients. These results indicate there may be a benefit to an increase in use of ezetimibe in clinical practice due to its increased effectiveness and safety in patients with and without CKD. Ultimately, this can help patients achieve their LDL goals as recommended by ACC clinical practice guidelines.
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-e350. doi:10.1016/j.jacc.2018.11.003
Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80(14):1366-1418. doi:10.1016/j.jacc.2022.07.006
US Food and Drug Administration. Ezetimibe. 2007. Accessed April 1, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021445s019lbl.pdf
Singh A, Cho LS. Nonstatin therapy to reduce low-density lipoprotein cholesterol and improve cardiovascular outcomes. Cleve Clin J Med. 2024;91(1):53-63. doi:10.3949/ccjm.91a.23058
Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. doi:10.1056/NEJMoa1410489
Kim B, Hong S, Lee Y, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400(10349):380-390. doi:10.1016/S0140-6736(22)00916-3
Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181-2192. doi:10.1016/S0140-6736(11)60739-3
Ouchi Y, Sasaki J, Arai H, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (EWTOPIA 75): a randomized, controlled trial. Circulation. 2019;140:992-1003. doi:10.1161/CIRCULATIONAHA.118.039415
Baruch L, Gupta B, Lieberman-Blum SS, Agarwal S, Eng C. Ezetimibe 5 and 10 mg for lowering LDL-C: potential billion-dollar savings with improved tolerability. Am J Manag Care. 2008;14(10):637-641. https://www.ajmc.com/view/oct08-3644p637-641
Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608
Statins are widely used to reduce low-density lipoprotein (LDL) and non-high-density lipoprotein (HDL) levels for the prevention of atherosclerotic cardiovascular disease (ASCVD).1 However, despite maximally tolerated statin therapy, many patients may not reach their LDL and non-HDL goals. Some patients may experience adverse events (AEs), particularly muscle-related AEs, which can limit the use of these medications.
The 2022 American College of Cardiology (ACC) expert consensus pathway recommends a goal LDL of < 55 mg/dL in very high-risk patients, defined as those with a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions.2 Major ASCVD events include acute coronary syndrome within 12 months, history of myocardial infarction (MI) or ischemic stroke, and symptomatic peripheral arterial disease (ie, claudication with ankle-brachial index < 0.85 or previous revascularization or amputation). Factors for being considered high risk include age > 65 years, heterozygous familial hypercholesterolemia, history of prior coronary artery bypass surgery or percutaneous coronary intervention outside the major ASCVD events, diabetes, hypertension, chronic kidney disease (CKD) (estimated glomerular filtration rate [eGFR] 15-59 mL/min/1.73 m2), current smoking, persistently elevated LDL cholesterol (LDL-C) levels despite maximally tolerated statin therapy and ezetimibe, and history of congestive heart failure.2 For these patients, statin therapy alone may not achieve LDL goal.
The ACC recommends ezetimibe as the initial nonstatin therapy in patients who are not at their goal LDL.2 Ezetimibe works by inhibiting Niemann-Pick C1-Like 1 protein, which causes reduced cholesterol absorption in the small intestine.2,3 Previous studies have shown the benefit of ezetimibe for LDL reduction and ASCVD prevention.4-7 The 2015 IMPROVE-IT study found the combination of simvastatin and ezetimibe resulted in a significantly lower risk of cardiovascular events than simvastatin monotherapy. IMPROVE-IT also reported a further clinical benefit when lower LDL targets (ie, < 55 mg/dL) are achieved, which aligns with the expert consensus pathway recommendations for a lower LDL goal for very high-risk patients.2,5
The RACING trial found that treatment with a moderate-intensity statin and ezetimibe was noninferior to treatment with a high-intensity statin for the primary outcome of occurrence of cardiovascular death, major cardiovascular events, or nonfatal stroke within 3 years. The combination of moderate-intensity statin and ezetimibe achieved lower LDL-C levels and lower incidence of drug intolerance compared to high intensity statin monotherapy.6 The SHARP-CKD study assessed major atherosclerotic events in patients with CKD who had no history of MI or coronary revascularization. The study found that lowering LDL-C with the combination of simvastatin plus ezetimibe safely reduces the risk of major atherosclerotic events in a wide range of patients with CKD.7
Lastly, the 2019 EWTOPIA 75 study found that ezetimibe noted a statistically significant reduction in the incidence of the composite of sudden cardiac death, MI, coronary revascularization, or stroke compared to placebo. Ezetimibe showed benefits in preventing ASCVD events independently of statin therapy.8 These clinical trials provided evidence for the efficacy of ezetimibe for secondary or primary prevention of ASCVD, patients with CKD, and patients who are not at their LDL goal despite maximally tolerated statin therapy.
Reductions in LDL levels with ezetimibe are reported to be 15% to 19% for monotherapy and 13% to 25% when used in combination with a statin.4 Given that the ACC now recommends lower LDL goals, patients may need additional lowering despite taking maximally tolerated statin therapy.2 Additionally, the package insert for ezetimibe reports increased area under the curve (AUC) values of ezetimibe and its metabolites in patients with severe renal disease. It is anticipated that ezetimibe may show an increased reduction of LDL and non-HDL, but there may also be an increased risk for muscle-related AEs.3
This quality-assurance quality improvement project investigated the use of ezetimibe in patients with CKD to determine whether there is further LDL and non-HDL reduction in this patient population. It sought to determine the LDL and non-HDL percentage reduction in patients with and without CKD at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) and whether there is an increased risk for muscle-related AEs. Determining the percentage reduction of LDL and non-HDL within this population can help increase use of ezetimibe in patients not at their LDL or non-HDL goal or for those patients unable to tolerate statin therapy.
Methods
This single-center retrospective chart review investigated patients prescribed ezetimibe by a patient aligned care team (PACT) pharmacist at WBVAMC between September 1, 2021, and September 1, 2023. This project was determined to be nonresearch by the Veterans Integrated Service Network 4 multisite institutional review board. Patients were excluded from the review if they started taking ezetimibe outside of the prespecified time frame, if ezetimibe was initiated by a non-WBVAMC PACT pharmacist, or if there was no follow-up lipid panel obtained within 6 months of initiation of ezetimibe.
The primary outcomes were to determine the percentage mean change in LDL and non-HDL reduction and the incidence of muscle-related AEs after initiation of ezetimibe in patients without CKD. The secondary outcomes were to determine the percentage mean change in LDL and non-HDL levels and the incidence of muscle-related AEs after initiation of ezetimibe in patients with CKD. For this study, CKD was defined as a patient having an eGFR 15 to 60 ml/min/1.73 m2. Non-HDL is the combination of LDL-C and very LDL-C and represents all potentially atherogenic particles. The 2022 Expert Consensus Pathway included non-HDL goals in addition to LDL goals.2 Non-HDL cholesterol levels can be used for patients with elevated triglycerides where LDL levels may not be as accurate. To account for instances of elevated triglycerides, this study assessed changes in both LDL and non-HDL levels.
Data were collected from the US Department of Veterans Affairs (VA) Computerized Patient Record System (CPRS) and recorded in a spreadsheet. Collected data included age, sex, race, concomitant cholesterol-lowering medications (statin, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitor, bempedoic acid, fish oil, niacin, bile acid sequestrants, and fibrates), baseline lipid panel, lipid panel within 6 months of ezetimibe initiation, and eGFR level. If the patient’s LDL or non-HDL levels worsened on the follow-up lipid panel, their baseline LDL and non-HDL levels were used to calculate the percentage reduction; thus, the percentage reduction would be 0%. This strategy was used in prior research, notably the IMPROVE-IT and SHARP-CKD trials.
Ezetimibe 5 mg once daily was used in this study based on a 2008 VA study that evaluated the use of ezetimibe 5 mg vs ezetimibe 10 mg and the percentage reduction of LDL with each dose. The study found no significant difference between the 5 mg and 10 mg dose.9 Most patients included in this study received the 5 mg dose.
Results
This retrospective chart review consisted of 173 patients, 137 (79.2%) without CKD and 36 (20.8%) with CKD at baseline. The mean age was 69.6 years, 155 (89.6%) patients were male, and 18 (10.4%) were female. There were 164 concomitant medications, including 115 patients prescribed a statin and 38 patients prescribed fish oil (Table 1).
Patients without CKD had mean reductions in LDL levels of 23.5% and non-HDL levels of 21.7% (Figure). Patients who had an increase in LDL and non-HDL levels were excluded to control for potential confounding factors such as dietary changes, discontinuation of ezetimibe therapy, nonadherence to ezetimibe, and medication changes that impacted follow-up laboratory tests such as discontinuation of a statin. Fifteen patients experienced an increase in LDL or non-HDL levels. After excluding these patients, those without CKD had a mean reduction in LDL levels of 28.0% and non-HDL levels of 25.5%. Nineteen (13.9%) patients without CKD experienced a muscle-related AE (Table 2). One patient discontinued ezetimibe and statin use following a Lyme disease diagnosis due to concerns over potential muscle-related AEs.
Patients with CKD had a mean reduction in LDL and non-HDL levels of 27.0% and 24.8%, respectively. Patients with an increase in LDL or non-HDL levels were also excluded to help control for potential confounding factors. After excluding 4 patients with increased LDL and non-HDL levels, the mean reduction in LDL and non-HDL levels was 30.5% and 27.5%, respectively. Five (13.9%) patients with CKD experienced muscle-related AEs thought to be due to ezetimibe. Other AEs (eg, urticaria, diarrhea, reflux, dizziness, headache, upset stomach) were reported that led to discontinuation of ezetimibe, but only muscle-related AEs were analyzed.
Discussion
This retrospective chart review found larger reductions in LDL and non-HDL levels for patients with CKD than reported in the literature.4 Based on the findings that indicate a greater cholesterol reduction with ezetimibe, the results suggest an underutilization of ezetimibe in clinical practice, which may be due to clinicians favoring statin therapy and overlooking ezetimibe as a viable option based on recommendation in earlier guidelines. The 2022 guidelines transitioned from a statin focus to a focus on LDL targets and goals.2
According to the ACC, there is evidence to support a direct relationship between LDL-C levels, atherosclerosis progression, and ASCVD event risk.2 Absolute LDL-C level reduction is directly associated with ASCVD risk reduction which supports the LDL hypothesis. There appears to be no specific LDL-C level below which benefit ceases.2 This suggests that lower LDL-C targets (< 55 mg/dL) should be used when clinically indicated. Many patients are either unable to reach their goal LDL levels with statin monotherapy or are unable to tolerate statin therapy at higher doses, which may require additional pharmacotherapy to reach goal LDL-C. The ACC expert consensus pathway recommends ezetimibe as the initial add-on treatment to statins.2 The RACING trial showed the benefit of adding ezetimibe to a moderate-intensity statin vs increasing to a high-intensity statin dose. This trial found patients had lower LDL levels and lower rates of intolerances, which further supports ezetimibe use.6
This quality improvement project assessed LDL and non-HDL level reduction in patients with CKD. As anticipated, there was greater reduction in LDL and non-HDL levels seen in patients with CKD. The SHARP-CKD trial also found reductions in LDL levels with ezetimibe in patients with CKD.7 Given the reduction in LDL and non-HDL levels with ezetimibe in patients with or without CKD, add-on therapy of ezetimibe should be recommended for patients who do not achieve their LDL goals with statin therapy or for patients who intolerant to statin therapy.
The ezetimibe package insert reports myalgias incidence to be < 5% in patients and research has shown up to a 20% incidence of muscle-related AEs with statin therapy.3,10 Based on the package information reporting increased AUC values of ezetimibe and its metabolites in patients with severe renal disease, it was anticipated there may be an increased risk of muscle-related AEs in patients with CKD.3 However, this study found the same incidence of muscle-related AEs in patients with and without CKD. Previous research on statin-intolerant patients found the incidence of muscle-related AEs with ezetimibe to be 23.0% and 28.8%.11,12 This increased incidence of muscle-related AEs may be the result of including patients with a history of statin intolerance. Collectively, data from clinical trials and this study indicate that patients with prior intolerances to statins appear to have a higher likelihood of developing a muscle-related AEs with ezetimibe.11,12 Clinicians and patients should be educated on the potential for these AEs and be aware that the likelihood may be greater if there is a history of statin intolerance. To our knowledge, this was the first study to evaluate muscle-related AEs with ezetimibe in patients with and without CKD.
Limitations
This retrospective chart review was performed over a prespecified period and only patients initiated on ezetimibe by a PACT pharmacist were included. This study did not assess the percentage of LDL reduction in patients on concomitant statins vs those who were not on concomitant statins. The study only included 173 patients. Additionally, the study was primarily composed of White men and may not be representative of other populations. In addition, veterans may not be representative of the general population given their high comorbidity burden and other exposures. Some reported muscle-related AEs associated with ezetimibe may be attributed to the nocebo effect.
Conclusions
The results of this retrospective chart review suggest there may be a larger mean reduction in LDL and non-HDL levels seen with ezetimibe therapy than reported within the literature. There was a larger mean reduction in LDL and non-HDL levels in patients with CKD than in patients without CKD. Additionally, there were the same rates of muscle-related AEs with ezetimibe therapy in patients with and without CKD. The rates of muscle-related AEs with ezetimibe therapy were higher than reported in the medication’s package insert, but lower than reported in literature that included statin-intolerant patients. These results indicate there may be a benefit to an increase in use of ezetimibe in clinical practice due to its increased effectiveness and safety in patients with and without CKD. Ultimately, this can help patients achieve their LDL goals as recommended by ACC clinical practice guidelines.
Statins are widely used to reduce low-density lipoprotein (LDL) and non-high-density lipoprotein (HDL) levels for the prevention of atherosclerotic cardiovascular disease (ASCVD).1 However, despite maximally tolerated statin therapy, many patients may not reach their LDL and non-HDL goals. Some patients may experience adverse events (AEs), particularly muscle-related AEs, which can limit the use of these medications.
The 2022 American College of Cardiology (ACC) expert consensus pathway recommends a goal LDL of < 55 mg/dL in very high-risk patients, defined as those with a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions.2 Major ASCVD events include acute coronary syndrome within 12 months, history of myocardial infarction (MI) or ischemic stroke, and symptomatic peripheral arterial disease (ie, claudication with ankle-brachial index < 0.85 or previous revascularization or amputation). Factors for being considered high risk include age > 65 years, heterozygous familial hypercholesterolemia, history of prior coronary artery bypass surgery or percutaneous coronary intervention outside the major ASCVD events, diabetes, hypertension, chronic kidney disease (CKD) (estimated glomerular filtration rate [eGFR] 15-59 mL/min/1.73 m2), current smoking, persistently elevated LDL cholesterol (LDL-C) levels despite maximally tolerated statin therapy and ezetimibe, and history of congestive heart failure.2 For these patients, statin therapy alone may not achieve LDL goal.
The ACC recommends ezetimibe as the initial nonstatin therapy in patients who are not at their goal LDL.2 Ezetimibe works by inhibiting Niemann-Pick C1-Like 1 protein, which causes reduced cholesterol absorption in the small intestine.2,3 Previous studies have shown the benefit of ezetimibe for LDL reduction and ASCVD prevention.4-7 The 2015 IMPROVE-IT study found the combination of simvastatin and ezetimibe resulted in a significantly lower risk of cardiovascular events than simvastatin monotherapy. IMPROVE-IT also reported a further clinical benefit when lower LDL targets (ie, < 55 mg/dL) are achieved, which aligns with the expert consensus pathway recommendations for a lower LDL goal for very high-risk patients.2,5
The RACING trial found that treatment with a moderate-intensity statin and ezetimibe was noninferior to treatment with a high-intensity statin for the primary outcome of occurrence of cardiovascular death, major cardiovascular events, or nonfatal stroke within 3 years. The combination of moderate-intensity statin and ezetimibe achieved lower LDL-C levels and lower incidence of drug intolerance compared to high intensity statin monotherapy.6 The SHARP-CKD study assessed major atherosclerotic events in patients with CKD who had no history of MI or coronary revascularization. The study found that lowering LDL-C with the combination of simvastatin plus ezetimibe safely reduces the risk of major atherosclerotic events in a wide range of patients with CKD.7
Lastly, the 2019 EWTOPIA 75 study found that ezetimibe noted a statistically significant reduction in the incidence of the composite of sudden cardiac death, MI, coronary revascularization, or stroke compared to placebo. Ezetimibe showed benefits in preventing ASCVD events independently of statin therapy.8 These clinical trials provided evidence for the efficacy of ezetimibe for secondary or primary prevention of ASCVD, patients with CKD, and patients who are not at their LDL goal despite maximally tolerated statin therapy.
Reductions in LDL levels with ezetimibe are reported to be 15% to 19% for monotherapy and 13% to 25% when used in combination with a statin.4 Given that the ACC now recommends lower LDL goals, patients may need additional lowering despite taking maximally tolerated statin therapy.2 Additionally, the package insert for ezetimibe reports increased area under the curve (AUC) values of ezetimibe and its metabolites in patients with severe renal disease. It is anticipated that ezetimibe may show an increased reduction of LDL and non-HDL, but there may also be an increased risk for muscle-related AEs.3
This quality-assurance quality improvement project investigated the use of ezetimibe in patients with CKD to determine whether there is further LDL and non-HDL reduction in this patient population. It sought to determine the LDL and non-HDL percentage reduction in patients with and without CKD at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) and whether there is an increased risk for muscle-related AEs. Determining the percentage reduction of LDL and non-HDL within this population can help increase use of ezetimibe in patients not at their LDL or non-HDL goal or for those patients unable to tolerate statin therapy.
Methods
This single-center retrospective chart review investigated patients prescribed ezetimibe by a patient aligned care team (PACT) pharmacist at WBVAMC between September 1, 2021, and September 1, 2023. This project was determined to be nonresearch by the Veterans Integrated Service Network 4 multisite institutional review board. Patients were excluded from the review if they started taking ezetimibe outside of the prespecified time frame, if ezetimibe was initiated by a non-WBVAMC PACT pharmacist, or if there was no follow-up lipid panel obtained within 6 months of initiation of ezetimibe.
The primary outcomes were to determine the percentage mean change in LDL and non-HDL reduction and the incidence of muscle-related AEs after initiation of ezetimibe in patients without CKD. The secondary outcomes were to determine the percentage mean change in LDL and non-HDL levels and the incidence of muscle-related AEs after initiation of ezetimibe in patients with CKD. For this study, CKD was defined as a patient having an eGFR 15 to 60 ml/min/1.73 m2. Non-HDL is the combination of LDL-C and very LDL-C and represents all potentially atherogenic particles. The 2022 Expert Consensus Pathway included non-HDL goals in addition to LDL goals.2 Non-HDL cholesterol levels can be used for patients with elevated triglycerides where LDL levels may not be as accurate. To account for instances of elevated triglycerides, this study assessed changes in both LDL and non-HDL levels.
Data were collected from the US Department of Veterans Affairs (VA) Computerized Patient Record System (CPRS) and recorded in a spreadsheet. Collected data included age, sex, race, concomitant cholesterol-lowering medications (statin, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitor, bempedoic acid, fish oil, niacin, bile acid sequestrants, and fibrates), baseline lipid panel, lipid panel within 6 months of ezetimibe initiation, and eGFR level. If the patient’s LDL or non-HDL levels worsened on the follow-up lipid panel, their baseline LDL and non-HDL levels were used to calculate the percentage reduction; thus, the percentage reduction would be 0%. This strategy was used in prior research, notably the IMPROVE-IT and SHARP-CKD trials.
Ezetimibe 5 mg once daily was used in this study based on a 2008 VA study that evaluated the use of ezetimibe 5 mg vs ezetimibe 10 mg and the percentage reduction of LDL with each dose. The study found no significant difference between the 5 mg and 10 mg dose.9 Most patients included in this study received the 5 mg dose.
Results
This retrospective chart review consisted of 173 patients, 137 (79.2%) without CKD and 36 (20.8%) with CKD at baseline. The mean age was 69.6 years, 155 (89.6%) patients were male, and 18 (10.4%) were female. There were 164 concomitant medications, including 115 patients prescribed a statin and 38 patients prescribed fish oil (Table 1).
Patients without CKD had mean reductions in LDL levels of 23.5% and non-HDL levels of 21.7% (Figure). Patients who had an increase in LDL and non-HDL levels were excluded to control for potential confounding factors such as dietary changes, discontinuation of ezetimibe therapy, nonadherence to ezetimibe, and medication changes that impacted follow-up laboratory tests such as discontinuation of a statin. Fifteen patients experienced an increase in LDL or non-HDL levels. After excluding these patients, those without CKD had a mean reduction in LDL levels of 28.0% and non-HDL levels of 25.5%. Nineteen (13.9%) patients without CKD experienced a muscle-related AE (Table 2). One patient discontinued ezetimibe and statin use following a Lyme disease diagnosis due to concerns over potential muscle-related AEs.
Patients with CKD had a mean reduction in LDL and non-HDL levels of 27.0% and 24.8%, respectively. Patients with an increase in LDL or non-HDL levels were also excluded to help control for potential confounding factors. After excluding 4 patients with increased LDL and non-HDL levels, the mean reduction in LDL and non-HDL levels was 30.5% and 27.5%, respectively. Five (13.9%) patients with CKD experienced muscle-related AEs thought to be due to ezetimibe. Other AEs (eg, urticaria, diarrhea, reflux, dizziness, headache, upset stomach) were reported that led to discontinuation of ezetimibe, but only muscle-related AEs were analyzed.
Discussion
This retrospective chart review found larger reductions in LDL and non-HDL levels for patients with CKD than reported in the literature.4 Based on the findings that indicate a greater cholesterol reduction with ezetimibe, the results suggest an underutilization of ezetimibe in clinical practice, which may be due to clinicians favoring statin therapy and overlooking ezetimibe as a viable option based on recommendation in earlier guidelines. The 2022 guidelines transitioned from a statin focus to a focus on LDL targets and goals.2
According to the ACC, there is evidence to support a direct relationship between LDL-C levels, atherosclerosis progression, and ASCVD event risk.2 Absolute LDL-C level reduction is directly associated with ASCVD risk reduction which supports the LDL hypothesis. There appears to be no specific LDL-C level below which benefit ceases.2 This suggests that lower LDL-C targets (< 55 mg/dL) should be used when clinically indicated. Many patients are either unable to reach their goal LDL levels with statin monotherapy or are unable to tolerate statin therapy at higher doses, which may require additional pharmacotherapy to reach goal LDL-C. The ACC expert consensus pathway recommends ezetimibe as the initial add-on treatment to statins.2 The RACING trial showed the benefit of adding ezetimibe to a moderate-intensity statin vs increasing to a high-intensity statin dose. This trial found patients had lower LDL levels and lower rates of intolerances, which further supports ezetimibe use.6
This quality improvement project assessed LDL and non-HDL level reduction in patients with CKD. As anticipated, there was greater reduction in LDL and non-HDL levels seen in patients with CKD. The SHARP-CKD trial also found reductions in LDL levels with ezetimibe in patients with CKD.7 Given the reduction in LDL and non-HDL levels with ezetimibe in patients with or without CKD, add-on therapy of ezetimibe should be recommended for patients who do not achieve their LDL goals with statin therapy or for patients who intolerant to statin therapy.
The ezetimibe package insert reports myalgias incidence to be < 5% in patients and research has shown up to a 20% incidence of muscle-related AEs with statin therapy.3,10 Based on the package information reporting increased AUC values of ezetimibe and its metabolites in patients with severe renal disease, it was anticipated there may be an increased risk of muscle-related AEs in patients with CKD.3 However, this study found the same incidence of muscle-related AEs in patients with and without CKD. Previous research on statin-intolerant patients found the incidence of muscle-related AEs with ezetimibe to be 23.0% and 28.8%.11,12 This increased incidence of muscle-related AEs may be the result of including patients with a history of statin intolerance. Collectively, data from clinical trials and this study indicate that patients with prior intolerances to statins appear to have a higher likelihood of developing a muscle-related AEs with ezetimibe.11,12 Clinicians and patients should be educated on the potential for these AEs and be aware that the likelihood may be greater if there is a history of statin intolerance. To our knowledge, this was the first study to evaluate muscle-related AEs with ezetimibe in patients with and without CKD.
Limitations
This retrospective chart review was performed over a prespecified period and only patients initiated on ezetimibe by a PACT pharmacist were included. This study did not assess the percentage of LDL reduction in patients on concomitant statins vs those who were not on concomitant statins. The study only included 173 patients. Additionally, the study was primarily composed of White men and may not be representative of other populations. In addition, veterans may not be representative of the general population given their high comorbidity burden and other exposures. Some reported muscle-related AEs associated with ezetimibe may be attributed to the nocebo effect.
Conclusions
The results of this retrospective chart review suggest there may be a larger mean reduction in LDL and non-HDL levels seen with ezetimibe therapy than reported within the literature. There was a larger mean reduction in LDL and non-HDL levels in patients with CKD than in patients without CKD. Additionally, there were the same rates of muscle-related AEs with ezetimibe therapy in patients with and without CKD. The rates of muscle-related AEs with ezetimibe therapy were higher than reported in the medication’s package insert, but lower than reported in literature that included statin-intolerant patients. These results indicate there may be a benefit to an increase in use of ezetimibe in clinical practice due to its increased effectiveness and safety in patients with and without CKD. Ultimately, this can help patients achieve their LDL goals as recommended by ACC clinical practice guidelines.
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-e350. doi:10.1016/j.jacc.2018.11.003
Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80(14):1366-1418. doi:10.1016/j.jacc.2022.07.006
US Food and Drug Administration. Ezetimibe. 2007. Accessed April 1, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021445s019lbl.pdf
Singh A, Cho LS. Nonstatin therapy to reduce low-density lipoprotein cholesterol and improve cardiovascular outcomes. Cleve Clin J Med. 2024;91(1):53-63. doi:10.3949/ccjm.91a.23058
Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. doi:10.1056/NEJMoa1410489
Kim B, Hong S, Lee Y, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400(10349):380-390. doi:10.1016/S0140-6736(22)00916-3
Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181-2192. doi:10.1016/S0140-6736(11)60739-3
Ouchi Y, Sasaki J, Arai H, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (EWTOPIA 75): a randomized, controlled trial. Circulation. 2019;140:992-1003. doi:10.1161/CIRCULATIONAHA.118.039415
Baruch L, Gupta B, Lieberman-Blum SS, Agarwal S, Eng C. Ezetimibe 5 and 10 mg for lowering LDL-C: potential billion-dollar savings with improved tolerability. Am J Manag Care. 2008;14(10):637-641. https://www.ajmc.com/view/oct08-3644p637-641
Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-e350. doi:10.1016/j.jacc.2018.11.003
Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80(14):1366-1418. doi:10.1016/j.jacc.2022.07.006
US Food and Drug Administration. Ezetimibe. 2007. Accessed April 1, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021445s019lbl.pdf
Singh A, Cho LS. Nonstatin therapy to reduce low-density lipoprotein cholesterol and improve cardiovascular outcomes. Cleve Clin J Med. 2024;91(1):53-63. doi:10.3949/ccjm.91a.23058
Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. doi:10.1056/NEJMoa1410489
Kim B, Hong S, Lee Y, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400(10349):380-390. doi:10.1016/S0140-6736(22)00916-3
Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181-2192. doi:10.1016/S0140-6736(11)60739-3
Ouchi Y, Sasaki J, Arai H, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (EWTOPIA 75): a randomized, controlled trial. Circulation. 2019;140:992-1003. doi:10.1161/CIRCULATIONAHA.118.039415
Baruch L, Gupta B, Lieberman-Blum SS, Agarwal S, Eng C. Ezetimibe 5 and 10 mg for lowering LDL-C: potential billion-dollar savings with improved tolerability. Am J Manag Care. 2008;14(10):637-641. https://www.ajmc.com/view/oct08-3644p637-641
Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608
Importance of Recognizing Hypertrophic Cardiomyopathy in the Preoperative Clinic
Importance of Recognizing Hypertrophic Cardiomyopathy in the Preoperative Clinic
Hypertrophic cardiomyopathy (HCM) is a relatively common inherited condition characterized by abnormal asymmetric left ventricular (LV) thickening. This can lead to LV outflow tract (LVOT) obstruction, which has important implications for anesthesia management. This article describes a case of previously undiagnosed HCM discovered during a preoperative physical examination prior to a routine surveillance colonoscopy.
CASE PRESENTATION
A 55-year-old Army veteran with a history of a sessile serrated colon adenoma presented to the preadmission testing clinic prior to planned surveillance colonoscopy under monitored anesthesia care. His medical history included untreated severe obstructive sleep apnea (53 apnea-hypopnea index score), diet-controlled hypertension, prediabetes (6.3% hemoglobin A1c), hypogonadism, and obesity (41 body mass index). Medications included semaglutide 1.7 mg injected subcutaneously weekly and testosterone 200 mg injected intramuscularly every 2 weeks, as well as lisinopril-hydrochlorothiazide 10 to 12.5 mg daily, which had recently been discontinued because his blood pressure had improved with a low-sodium diet.
A review of systems was unremarkable except for progressive weight gain. The patient had no family history of sudden cardiac death. On physical examination, the patient’s blood pressure was 119/81 mm Hg, pulse was 86 beats/min, and respiratory rate was 18 breaths/min. The patient was clinically euvolemic, with no jugular venous distention or peripheral edema, and his lungs were clear to auscultation. There was, however, a soft, nonradiating grade 2/6 systolic murmur that had not been previously documented. The murmur decreased substantially with the Valsalva maneuver, with no change in hand grip.
Laboratory studies revealed hemoglobin and renal function were within the reference range. A routine 12-lead electrocardiogram (ECG) was unremarkable. A transthoracic echocardiogram revealed moderate pulmonary hypertension (59 mm Hg right ventricular systolic pressure), asymmetric LV hypertrophy (2.1 cm septal thickness), and severe LVOT obstruction (131.8 mm Hg gradient). Severe systolic anterior motion of the mitral valve was also present. The LV ejection fraction was 60% to 65%, with normal cavity size and systolic function. These findings were consistent with severe hypertrophic obstructive cardiomyopathy (HOCM). Upon more detailed questioning, the patient reported that over the previous 5 years he had experienced gradually decreasing exercise tolerance and mild dyspnea on exertion, particularly in hot weather, which he attributed to weight gain. He also reported a presyncopal episode the previous month while working in his garage in hot weather for a prolonged period of time.
The patient’s elective colonoscopy was canceled, and he was referred to cardiology. While awaiting cardiac consultation, he was instructed to maintain good hydration and avoid any heavy physical activity beyond walking. He was told not to resume his use of lisinopril-hydrochlorothiazide. A screening 7-day Holter monitor showed no ventricular or supraventricular ectopy. After cardiology consultation, the patient was referred to a HCM specialty clinic, where a cardiac magnetic resonance imaging confirmed severe asymmetric hypertrophy with resting obstruction (Figures 1-4). Treatment options were discussed with the patient, and he underwent a trial with the Β—blocker metoprolol 50 mg daily, which he could not tolerate. Verapamil extended-release 180 mg orally once daily was then initiated; however, his dyspnea persisted. He was amenable to surgical therapy and underwent septal myectomy, with 12 g of septal myocardium removed. He did well postoperatively, with a follow-up echocardiogram showing normal LV systolic function and no LVOT gradient detectable at rest or with Valsalva maneuver. His fatigue and exertional dyspnea significantly improved. Once the patient underwent septal myectomy and was determined to have no detectable LVOT gradient, he was approved for colonoscopy which has been scheduled but not completed.
DISCUSSION
Once thought rare, HCM is now considered to be a relatively common inherited disorder, occurring in about 1 in 500 persons, with some suggesting that the actual prevalence is closer to 1 in 200 persons.1,2 Most often caused by mutations in ≥ 1 of 11 genes responsible for encoding cardiac sarcomere proteins, HCM is characterized by abnormal LV thickening without chamber enlargement in the absence of any identifiable cause, such as aortic valve stenosis or uncontrolled hypertension. The hypertrophy is often asymmetric, and in cases of asymmetric septal hypertrophy, dynamic LVOT obstruction can occur (known as HOCM). The condition is inherited in an autosomal dominant pattern with variable expression and is associated with myocardial fiber disarray, which can occur years before symptom onset.3 This myocardial disarray can lead to remodeling and an increased wall-to-lumen ratio of the coronary arteries, resulting in impaired coronary reserve.
Depending on the degree of LVOT obstruction, patients with HCM may be classified as nonobstructive, labile, or obstructive at rest. Patients without obstruction have an outflow gradient ≤ 30 mm Hg that is not provoked with Valsalva maneuver, administration of amyl nitrite, or exercise treadmill testing.3 Patients classified as labile do not have LVOT obstruction at rest, but obstruction may be induced by provocative measures. Finally, about one-third of patients with HCM will have LVOT gradients of > 30 mm Hg at rest. These patients are at increased risk for progression to symptomatic heart failure and may be candidates for surgical myectomy or catheter-based alcohol septal ablation.4 The patient in this case had a resting LVOT gradient of 131.8 mm Hg on echocardiography. The magnitude of this gradient placed the patient at a significantly higher risk of ventricular dysrhythmias and sudden cardiac death.5
Wall thickness also has prognostic implications. 6 Although any area of the myocardium can be affected, the septum is involved in about 90% cases. In their series of 48 patients followed over 6.5 years, Spirito et al found that the risk of sudden death in patients with HCM increased as wall thickness increased. For patients with a wall thickness of < 15 mm, the risk of death was 0 per 1000 person-years; however, this increased to 18.2 per 1000 person-years for patients with a wall thickness of > 30 mm.7
While many patients with HCM are asymptomatic, others may report dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, chest pain, palpitations, presyncope/ syncope, postural lightheadedness, fatigue, or edema. Symptomatology, however, is quite variable and does not necessarily correlate with the degree of outflow obstruction. Surprisingly, some patients with significant LVOT may have minimal symptoms, such as the patient in this case, while others with a lesser degree of LVOT obstruction may be very symptomatic.3,4
Physical examination of a patient with HCM may be normal or may reveal nonspecific findings such as a fourth heart sound or a systolic murmur. In general, physical examination abnormalities are related to LVOT obstruction. Those patients without significant outflow obstruction may have a normal cardiac examination. While patients with HCM may have a variety of systolic murmurs, the 2 most common are those related to outflow tract obstruction and mitral regurgitation caused by systolic anterior motion of the mitral valve.4 The systolic murmur associated with significant LVOT obstruction has been described as a harsh, crescendo-decrescendo type that begins just after S1 and is heard best at the apex and lower left sternal border.4 It may radiate to the axilla and base but not generally into the neck. The murmur usually increases with Valsalva maneuver and decreases with handgrip or going from a standing to a sitting/ squatting position. The initial examination of the patient in this case was not suggestive of HOCM, as confirmed by 2 practitioners (a cardiologist and an internist), each with > 30 years of clinical experience. This may have been related to the patient’s hydration status at the time, with Valsalva maneuver increasing obstruction to the point of reduced flow.
About 90% of patients with HCM will have abnormalities on ECG, most commonly LV hypertrophy with a strain pattern. Other ECG findings include: (1) prominent abnormal Q waves, particularly in the inferior (II, III, and aVF) and lateral leads (I, aVL, and V4-V6), reflecting depolarization of a hypertrophied septum; (2) left axis deviation; (3) deeply inverted T waves in leads V2 through V4; and (4) P wave abnormalities indicative of left atrial (LA) or biatrial enlargement. 8 It is notable that the patient in this case had a normal ECG, given that a minority of patients with HCM have been shown to have a normal ECG.9
Echocardiography plays an important role in diagnosing HCM. Diagnostic criteria include the presence of asymmetric hypertrophy (most commonly with anterior septal involvement), systolic anterior motion of the mitral valve, a nondilated LV cavity, septal immobility, and premature closure of the aortic valve. LV thickness is measured at both the septum and free wall; values ≥ 15 mm, with a septal-to-free wall thickness ratio of ≥ 1.3, are suggestive of HCM. Asymmetric LV hypertrophy can also be seen in other segments besides the septum, such as the apex.10
HCM/HOCM is the most common cause of sudden cardiac death in young people. The condition also contributes to significant functional morbidity due to heart failure and increases the risk of atrial fibrillation and subsequent stroke. Treatments tend to focus on symptom relief and slowing disease progression and include the use of medications such as Β—blockers, nondihydropyridine calcium channel blockers, and the myosin inhibitor mavacamten.11 Select patients, such as those with severe LVOT obstruction and symptoms despite treatment with Β—blockers or nondihydropyridine calcium channel blockers, may be offered septal myectomy or catheter-based alcohol septal ablation, coupled with insertion of an implantable cardiac defibrillator to prevent sudden cardiac death in patients at high arrhythmic risk.1,12
Patients with HCM, particularly those with LVOT obstruction, pose distinct challenges to the anesthesiologist because they are highly sensitive to decreases in preload and afterload. These patients frequently experience adverse perioperative events such as myocardial ischemia, systemic hypotension, and supraventricular or ventricular arrhythmias. Acute congestive heart failure may also occur, presumably due to concomitant diastolic dysfunction. Patients with previously unrecognized HCM are of particular concern, as they may manifest unexpected and sudden hypotension with the induction of anesthesia. There may then be a paradoxical response to vasoactive drugs and anesthetic agents, which accentuate LVOT obstruction. In these circumstances, undiagnosed HCM should be considered, and intraoperative rescue transesophageal echocardiography be performed.13 Once the diagnosis is confirmed, efforts should be made to reduce myocardial contractility and sympathetic discharge (eg, with Β—blockers), increase afterload (eg, with α1 agonists), and improve preload with adequate hydration. Proper resuscitation of hypotensive patients with HCM requires a thorough understanding of disease pathology, as effective interventions may seem to be counterintuitive. Inotropic agents such as epinephrine are contraindicated in HCM because increased inotropy and chronotropy worsen LVOT obstruction. Volume status is often tenuous; while adequate preload is important, overly aggressive fluid resuscitation may promote heart failure. It is important to keep in mind that even patients without resting LVOT obstruction may develop dynamic obstruction with anesthesia induction due to sudden reductions in preload and afterload. It is also important to note that the degree of LV hypertrophy is directly correlated with arrhythmic sudden death. Those patients with LV wall thickness ≥ 30 mm are at increased risk for potentially lethal tachyarrhythmias in the operating room.14
These considerations reinforce the need for proper preoperative identification of patients with HCM. Heightened awareness is key, given the fact that HCM is relatively common and tends to be underdiagnosed in the general population. These patients are generally young, otherwise healthy, and often undergo minor operative procedures in outpatient settings. It is incumbent upon the preoperative evaluator to take a thorough medical history and perform a careful physical examination. Clues to the diagnosis include exertional dyspnea, fatigue, angina, syncope/presyncope, or a family history of sudden cardiac death or HCM. A systolic ejection murmur, particularly one that increases with standing or Valsalva maneuver, and decreases with squatting or handgrip may also raise clinical suspicion. These patients should undergo a full cardiac evaluation, including echocardiography.
CONCLUSIONS
HCM is a common condition that is important to diagnose in the preoperative clinic. Failure to do so can lead to catastrophic complications during induction of anesthesia due to the sudden reduction in preload and afterload, which may cause a significant increase in LVOT obstruction. A high index of suspicion is essential, as clinical diagnosis can be challenging. The physical examination may be deceiving and symptoms are often subtle and nonspecific. It is imperative to alert the anesthesiologist before surgery so the complex hemodynamic management of patients with HOCM can be appropriately managed.
- Cheng Z, Fang T, Huang J, Guo Y, Alam M, Qian H. Hypertrophic cardiomyopathy: from phenotype and pathogenesis to treatment. Front Cardiovasc Med. 2021;8:722340. doi:10.3389/fcvm.2021.722340
- Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249-1254. doi:10.1016/j.jacc.2015.01.019
- Hensley N, Dietrich J, Nyhan D, Mitter N, Yee MS, Brady M. Hypertrophic cardiomyopathy: a review. Anesth Analg. 2015;120(3):554-569. doi:10.1213/ ANE.0000000000000538
- Maron BJ, Desai MY, Nishimura RA, et al. Diagnosis and evaluation of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(4):372–389. doi:10.1016/j.jacc.2021.12.002
- Jorda P, Garcia-Alvarez A. Hypertrophic cardiomyopathy: sudden cardiac death risk stratification in adults. Glob Cardiol Sci Pract. 2018;3(25). doi:10.21542/gcsp.2018.25
- Wigle ED, Sasson Z, Henderson MA, et al. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis. 1985;28(1):1-83. doi:10.1016/0033-0620(85)90024-6
- Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342(24):1778–1785. doi:10.1056/ NEJM200006153422403
- Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy Lancet. 2017;389(10075):1253-1267. doi:10.1016/S0140-6736(16)31321-6
- Rowin EJ, Maron BJ, Appelbaum E, et al. Significance of false negative electrocardiograms in preparticipation screening of athletes for hypertrophic cardiomyopathy. Am J Cardiol. 2012;110(7):1027-1032. doi:10.1016/j. amjcard.2012.05.035
- Losi MA, Nistri S, Galderisi M et al. Echocardiography in patients with hypertrophic cardiomyopathy: usefulness of old and new techniques in the diagnosis and pathophysiological assessment. Cardiovasc Ultrasound. 2010;8(7). doi:10.1186/1476-7120-8-7
- Tian Z, Li L, Li X, et al. Effect of mavacamten on chinese patients with symptomatic obstructive hypertrophic cardiomyopathy: the EXPLORER-CN randomized clinical trial. JAMA Cardiol. 2023;8(10):957-965. doi:10.1001/ jamacardio.2023.3030
- Fang J, Liu Y, Zhu Y, et al. First-in-human transapical beating-heart septal myectomy in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2023;82(7):575-586. doi:10.1016/j.jacc.2023.05.052
- Jain P, Patel PA, Fabbro M 2nd. Hypertrophic cardiomyopathy and left ventricular outflow tract obstruction: expecting the unexpected. J Cardiothorac Vasc Anesth. 2018;32(1):467-477. doi:10.1053/j.jvca.2017.04.054
- Poliac LC, Barron ME, Maron BJ. Hypertrophic cardiomyopathy. Anesthesiology. 2006;104(1):183-192. doi:10.1097/00000542-200601000-00025
Hypertrophic cardiomyopathy (HCM) is a relatively common inherited condition characterized by abnormal asymmetric left ventricular (LV) thickening. This can lead to LV outflow tract (LVOT) obstruction, which has important implications for anesthesia management. This article describes a case of previously undiagnosed HCM discovered during a preoperative physical examination prior to a routine surveillance colonoscopy.
CASE PRESENTATION
A 55-year-old Army veteran with a history of a sessile serrated colon adenoma presented to the preadmission testing clinic prior to planned surveillance colonoscopy under monitored anesthesia care. His medical history included untreated severe obstructive sleep apnea (53 apnea-hypopnea index score), diet-controlled hypertension, prediabetes (6.3% hemoglobin A1c), hypogonadism, and obesity (41 body mass index). Medications included semaglutide 1.7 mg injected subcutaneously weekly and testosterone 200 mg injected intramuscularly every 2 weeks, as well as lisinopril-hydrochlorothiazide 10 to 12.5 mg daily, which had recently been discontinued because his blood pressure had improved with a low-sodium diet.
A review of systems was unremarkable except for progressive weight gain. The patient had no family history of sudden cardiac death. On physical examination, the patient’s blood pressure was 119/81 mm Hg, pulse was 86 beats/min, and respiratory rate was 18 breaths/min. The patient was clinically euvolemic, with no jugular venous distention or peripheral edema, and his lungs were clear to auscultation. There was, however, a soft, nonradiating grade 2/6 systolic murmur that had not been previously documented. The murmur decreased substantially with the Valsalva maneuver, with no change in hand grip.
Laboratory studies revealed hemoglobin and renal function were within the reference range. A routine 12-lead electrocardiogram (ECG) was unremarkable. A transthoracic echocardiogram revealed moderate pulmonary hypertension (59 mm Hg right ventricular systolic pressure), asymmetric LV hypertrophy (2.1 cm septal thickness), and severe LVOT obstruction (131.8 mm Hg gradient). Severe systolic anterior motion of the mitral valve was also present. The LV ejection fraction was 60% to 65%, with normal cavity size and systolic function. These findings were consistent with severe hypertrophic obstructive cardiomyopathy (HOCM). Upon more detailed questioning, the patient reported that over the previous 5 years he had experienced gradually decreasing exercise tolerance and mild dyspnea on exertion, particularly in hot weather, which he attributed to weight gain. He also reported a presyncopal episode the previous month while working in his garage in hot weather for a prolonged period of time.
The patient’s elective colonoscopy was canceled, and he was referred to cardiology. While awaiting cardiac consultation, he was instructed to maintain good hydration and avoid any heavy physical activity beyond walking. He was told not to resume his use of lisinopril-hydrochlorothiazide. A screening 7-day Holter monitor showed no ventricular or supraventricular ectopy. After cardiology consultation, the patient was referred to a HCM specialty clinic, where a cardiac magnetic resonance imaging confirmed severe asymmetric hypertrophy with resting obstruction (Figures 1-4). Treatment options were discussed with the patient, and he underwent a trial with the Β—blocker metoprolol 50 mg daily, which he could not tolerate. Verapamil extended-release 180 mg orally once daily was then initiated; however, his dyspnea persisted. He was amenable to surgical therapy and underwent septal myectomy, with 12 g of septal myocardium removed. He did well postoperatively, with a follow-up echocardiogram showing normal LV systolic function and no LVOT gradient detectable at rest or with Valsalva maneuver. His fatigue and exertional dyspnea significantly improved. Once the patient underwent septal myectomy and was determined to have no detectable LVOT gradient, he was approved for colonoscopy which has been scheduled but not completed.




DISCUSSION
Once thought rare, HCM is now considered to be a relatively common inherited disorder, occurring in about 1 in 500 persons, with some suggesting that the actual prevalence is closer to 1 in 200 persons.1,2 Most often caused by mutations in ≥ 1 of 11 genes responsible for encoding cardiac sarcomere proteins, HCM is characterized by abnormal LV thickening without chamber enlargement in the absence of any identifiable cause, such as aortic valve stenosis or uncontrolled hypertension. The hypertrophy is often asymmetric, and in cases of asymmetric septal hypertrophy, dynamic LVOT obstruction can occur (known as HOCM). The condition is inherited in an autosomal dominant pattern with variable expression and is associated with myocardial fiber disarray, which can occur years before symptom onset.3 This myocardial disarray can lead to remodeling and an increased wall-to-lumen ratio of the coronary arteries, resulting in impaired coronary reserve.
Depending on the degree of LVOT obstruction, patients with HCM may be classified as nonobstructive, labile, or obstructive at rest. Patients without obstruction have an outflow gradient ≤ 30 mm Hg that is not provoked with Valsalva maneuver, administration of amyl nitrite, or exercise treadmill testing.3 Patients classified as labile do not have LVOT obstruction at rest, but obstruction may be induced by provocative measures. Finally, about one-third of patients with HCM will have LVOT gradients of > 30 mm Hg at rest. These patients are at increased risk for progression to symptomatic heart failure and may be candidates for surgical myectomy or catheter-based alcohol septal ablation.4 The patient in this case had a resting LVOT gradient of 131.8 mm Hg on echocardiography. The magnitude of this gradient placed the patient at a significantly higher risk of ventricular dysrhythmias and sudden cardiac death.5
Wall thickness also has prognostic implications. 6 Although any area of the myocardium can be affected, the septum is involved in about 90% cases. In their series of 48 patients followed over 6.5 years, Spirito et al found that the risk of sudden death in patients with HCM increased as wall thickness increased. For patients with a wall thickness of < 15 mm, the risk of death was 0 per 1000 person-years; however, this increased to 18.2 per 1000 person-years for patients with a wall thickness of > 30 mm.7
While many patients with HCM are asymptomatic, others may report dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, chest pain, palpitations, presyncope/ syncope, postural lightheadedness, fatigue, or edema. Symptomatology, however, is quite variable and does not necessarily correlate with the degree of outflow obstruction. Surprisingly, some patients with significant LVOT may have minimal symptoms, such as the patient in this case, while others with a lesser degree of LVOT obstruction may be very symptomatic.3,4
Physical examination of a patient with HCM may be normal or may reveal nonspecific findings such as a fourth heart sound or a systolic murmur. In general, physical examination abnormalities are related to LVOT obstruction. Those patients without significant outflow obstruction may have a normal cardiac examination. While patients with HCM may have a variety of systolic murmurs, the 2 most common are those related to outflow tract obstruction and mitral regurgitation caused by systolic anterior motion of the mitral valve.4 The systolic murmur associated with significant LVOT obstruction has been described as a harsh, crescendo-decrescendo type that begins just after S1 and is heard best at the apex and lower left sternal border.4 It may radiate to the axilla and base but not generally into the neck. The murmur usually increases with Valsalva maneuver and decreases with handgrip or going from a standing to a sitting/ squatting position. The initial examination of the patient in this case was not suggestive of HOCM, as confirmed by 2 practitioners (a cardiologist and an internist), each with > 30 years of clinical experience. This may have been related to the patient’s hydration status at the time, with Valsalva maneuver increasing obstruction to the point of reduced flow.
About 90% of patients with HCM will have abnormalities on ECG, most commonly LV hypertrophy with a strain pattern. Other ECG findings include: (1) prominent abnormal Q waves, particularly in the inferior (II, III, and aVF) and lateral leads (I, aVL, and V4-V6), reflecting depolarization of a hypertrophied septum; (2) left axis deviation; (3) deeply inverted T waves in leads V2 through V4; and (4) P wave abnormalities indicative of left atrial (LA) or biatrial enlargement. 8 It is notable that the patient in this case had a normal ECG, given that a minority of patients with HCM have been shown to have a normal ECG.9
Echocardiography plays an important role in diagnosing HCM. Diagnostic criteria include the presence of asymmetric hypertrophy (most commonly with anterior septal involvement), systolic anterior motion of the mitral valve, a nondilated LV cavity, septal immobility, and premature closure of the aortic valve. LV thickness is measured at both the septum and free wall; values ≥ 15 mm, with a septal-to-free wall thickness ratio of ≥ 1.3, are suggestive of HCM. Asymmetric LV hypertrophy can also be seen in other segments besides the septum, such as the apex.10
HCM/HOCM is the most common cause of sudden cardiac death in young people. The condition also contributes to significant functional morbidity due to heart failure and increases the risk of atrial fibrillation and subsequent stroke. Treatments tend to focus on symptom relief and slowing disease progression and include the use of medications such as Β—blockers, nondihydropyridine calcium channel blockers, and the myosin inhibitor mavacamten.11 Select patients, such as those with severe LVOT obstruction and symptoms despite treatment with Β—blockers or nondihydropyridine calcium channel blockers, may be offered septal myectomy or catheter-based alcohol septal ablation, coupled with insertion of an implantable cardiac defibrillator to prevent sudden cardiac death in patients at high arrhythmic risk.1,12
Patients with HCM, particularly those with LVOT obstruction, pose distinct challenges to the anesthesiologist because they are highly sensitive to decreases in preload and afterload. These patients frequently experience adverse perioperative events such as myocardial ischemia, systemic hypotension, and supraventricular or ventricular arrhythmias. Acute congestive heart failure may also occur, presumably due to concomitant diastolic dysfunction. Patients with previously unrecognized HCM are of particular concern, as they may manifest unexpected and sudden hypotension with the induction of anesthesia. There may then be a paradoxical response to vasoactive drugs and anesthetic agents, which accentuate LVOT obstruction. In these circumstances, undiagnosed HCM should be considered, and intraoperative rescue transesophageal echocardiography be performed.13 Once the diagnosis is confirmed, efforts should be made to reduce myocardial contractility and sympathetic discharge (eg, with Β—blockers), increase afterload (eg, with α1 agonists), and improve preload with adequate hydration. Proper resuscitation of hypotensive patients with HCM requires a thorough understanding of disease pathology, as effective interventions may seem to be counterintuitive. Inotropic agents such as epinephrine are contraindicated in HCM because increased inotropy and chronotropy worsen LVOT obstruction. Volume status is often tenuous; while adequate preload is important, overly aggressive fluid resuscitation may promote heart failure. It is important to keep in mind that even patients without resting LVOT obstruction may develop dynamic obstruction with anesthesia induction due to sudden reductions in preload and afterload. It is also important to note that the degree of LV hypertrophy is directly correlated with arrhythmic sudden death. Those patients with LV wall thickness ≥ 30 mm are at increased risk for potentially lethal tachyarrhythmias in the operating room.14
These considerations reinforce the need for proper preoperative identification of patients with HCM. Heightened awareness is key, given the fact that HCM is relatively common and tends to be underdiagnosed in the general population. These patients are generally young, otherwise healthy, and often undergo minor operative procedures in outpatient settings. It is incumbent upon the preoperative evaluator to take a thorough medical history and perform a careful physical examination. Clues to the diagnosis include exertional dyspnea, fatigue, angina, syncope/presyncope, or a family history of sudden cardiac death or HCM. A systolic ejection murmur, particularly one that increases with standing or Valsalva maneuver, and decreases with squatting or handgrip may also raise clinical suspicion. These patients should undergo a full cardiac evaluation, including echocardiography.
CONCLUSIONS
HCM is a common condition that is important to diagnose in the preoperative clinic. Failure to do so can lead to catastrophic complications during induction of anesthesia due to the sudden reduction in preload and afterload, which may cause a significant increase in LVOT obstruction. A high index of suspicion is essential, as clinical diagnosis can be challenging. The physical examination may be deceiving and symptoms are often subtle and nonspecific. It is imperative to alert the anesthesiologist before surgery so the complex hemodynamic management of patients with HOCM can be appropriately managed.
Hypertrophic cardiomyopathy (HCM) is a relatively common inherited condition characterized by abnormal asymmetric left ventricular (LV) thickening. This can lead to LV outflow tract (LVOT) obstruction, which has important implications for anesthesia management. This article describes a case of previously undiagnosed HCM discovered during a preoperative physical examination prior to a routine surveillance colonoscopy.
CASE PRESENTATION
A 55-year-old Army veteran with a history of a sessile serrated colon adenoma presented to the preadmission testing clinic prior to planned surveillance colonoscopy under monitored anesthesia care. His medical history included untreated severe obstructive sleep apnea (53 apnea-hypopnea index score), diet-controlled hypertension, prediabetes (6.3% hemoglobin A1c), hypogonadism, and obesity (41 body mass index). Medications included semaglutide 1.7 mg injected subcutaneously weekly and testosterone 200 mg injected intramuscularly every 2 weeks, as well as lisinopril-hydrochlorothiazide 10 to 12.5 mg daily, which had recently been discontinued because his blood pressure had improved with a low-sodium diet.
A review of systems was unremarkable except for progressive weight gain. The patient had no family history of sudden cardiac death. On physical examination, the patient’s blood pressure was 119/81 mm Hg, pulse was 86 beats/min, and respiratory rate was 18 breaths/min. The patient was clinically euvolemic, with no jugular venous distention or peripheral edema, and his lungs were clear to auscultation. There was, however, a soft, nonradiating grade 2/6 systolic murmur that had not been previously documented. The murmur decreased substantially with the Valsalva maneuver, with no change in hand grip.
Laboratory studies revealed hemoglobin and renal function were within the reference range. A routine 12-lead electrocardiogram (ECG) was unremarkable. A transthoracic echocardiogram revealed moderate pulmonary hypertension (59 mm Hg right ventricular systolic pressure), asymmetric LV hypertrophy (2.1 cm septal thickness), and severe LVOT obstruction (131.8 mm Hg gradient). Severe systolic anterior motion of the mitral valve was also present. The LV ejection fraction was 60% to 65%, with normal cavity size and systolic function. These findings were consistent with severe hypertrophic obstructive cardiomyopathy (HOCM). Upon more detailed questioning, the patient reported that over the previous 5 years he had experienced gradually decreasing exercise tolerance and mild dyspnea on exertion, particularly in hot weather, which he attributed to weight gain. He also reported a presyncopal episode the previous month while working in his garage in hot weather for a prolonged period of time.
The patient’s elective colonoscopy was canceled, and he was referred to cardiology. While awaiting cardiac consultation, he was instructed to maintain good hydration and avoid any heavy physical activity beyond walking. He was told not to resume his use of lisinopril-hydrochlorothiazide. A screening 7-day Holter monitor showed no ventricular or supraventricular ectopy. After cardiology consultation, the patient was referred to a HCM specialty clinic, where a cardiac magnetic resonance imaging confirmed severe asymmetric hypertrophy with resting obstruction (Figures 1-4). Treatment options were discussed with the patient, and he underwent a trial with the Β—blocker metoprolol 50 mg daily, which he could not tolerate. Verapamil extended-release 180 mg orally once daily was then initiated; however, his dyspnea persisted. He was amenable to surgical therapy and underwent septal myectomy, with 12 g of septal myocardium removed. He did well postoperatively, with a follow-up echocardiogram showing normal LV systolic function and no LVOT gradient detectable at rest or with Valsalva maneuver. His fatigue and exertional dyspnea significantly improved. Once the patient underwent septal myectomy and was determined to have no detectable LVOT gradient, he was approved for colonoscopy which has been scheduled but not completed.




DISCUSSION
Once thought rare, HCM is now considered to be a relatively common inherited disorder, occurring in about 1 in 500 persons, with some suggesting that the actual prevalence is closer to 1 in 200 persons.1,2 Most often caused by mutations in ≥ 1 of 11 genes responsible for encoding cardiac sarcomere proteins, HCM is characterized by abnormal LV thickening without chamber enlargement in the absence of any identifiable cause, such as aortic valve stenosis or uncontrolled hypertension. The hypertrophy is often asymmetric, and in cases of asymmetric septal hypertrophy, dynamic LVOT obstruction can occur (known as HOCM). The condition is inherited in an autosomal dominant pattern with variable expression and is associated with myocardial fiber disarray, which can occur years before symptom onset.3 This myocardial disarray can lead to remodeling and an increased wall-to-lumen ratio of the coronary arteries, resulting in impaired coronary reserve.
Depending on the degree of LVOT obstruction, patients with HCM may be classified as nonobstructive, labile, or obstructive at rest. Patients without obstruction have an outflow gradient ≤ 30 mm Hg that is not provoked with Valsalva maneuver, administration of amyl nitrite, or exercise treadmill testing.3 Patients classified as labile do not have LVOT obstruction at rest, but obstruction may be induced by provocative measures. Finally, about one-third of patients with HCM will have LVOT gradients of > 30 mm Hg at rest. These patients are at increased risk for progression to symptomatic heart failure and may be candidates for surgical myectomy or catheter-based alcohol septal ablation.4 The patient in this case had a resting LVOT gradient of 131.8 mm Hg on echocardiography. The magnitude of this gradient placed the patient at a significantly higher risk of ventricular dysrhythmias and sudden cardiac death.5
Wall thickness also has prognostic implications. 6 Although any area of the myocardium can be affected, the septum is involved in about 90% cases. In their series of 48 patients followed over 6.5 years, Spirito et al found that the risk of sudden death in patients with HCM increased as wall thickness increased. For patients with a wall thickness of < 15 mm, the risk of death was 0 per 1000 person-years; however, this increased to 18.2 per 1000 person-years for patients with a wall thickness of > 30 mm.7
While many patients with HCM are asymptomatic, others may report dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, chest pain, palpitations, presyncope/ syncope, postural lightheadedness, fatigue, or edema. Symptomatology, however, is quite variable and does not necessarily correlate with the degree of outflow obstruction. Surprisingly, some patients with significant LVOT may have minimal symptoms, such as the patient in this case, while others with a lesser degree of LVOT obstruction may be very symptomatic.3,4
Physical examination of a patient with HCM may be normal or may reveal nonspecific findings such as a fourth heart sound or a systolic murmur. In general, physical examination abnormalities are related to LVOT obstruction. Those patients without significant outflow obstruction may have a normal cardiac examination. While patients with HCM may have a variety of systolic murmurs, the 2 most common are those related to outflow tract obstruction and mitral regurgitation caused by systolic anterior motion of the mitral valve.4 The systolic murmur associated with significant LVOT obstruction has been described as a harsh, crescendo-decrescendo type that begins just after S1 and is heard best at the apex and lower left sternal border.4 It may radiate to the axilla and base but not generally into the neck. The murmur usually increases with Valsalva maneuver and decreases with handgrip or going from a standing to a sitting/ squatting position. The initial examination of the patient in this case was not suggestive of HOCM, as confirmed by 2 practitioners (a cardiologist and an internist), each with > 30 years of clinical experience. This may have been related to the patient’s hydration status at the time, with Valsalva maneuver increasing obstruction to the point of reduced flow.
About 90% of patients with HCM will have abnormalities on ECG, most commonly LV hypertrophy with a strain pattern. Other ECG findings include: (1) prominent abnormal Q waves, particularly in the inferior (II, III, and aVF) and lateral leads (I, aVL, and V4-V6), reflecting depolarization of a hypertrophied septum; (2) left axis deviation; (3) deeply inverted T waves in leads V2 through V4; and (4) P wave abnormalities indicative of left atrial (LA) or biatrial enlargement. 8 It is notable that the patient in this case had a normal ECG, given that a minority of patients with HCM have been shown to have a normal ECG.9
Echocardiography plays an important role in diagnosing HCM. Diagnostic criteria include the presence of asymmetric hypertrophy (most commonly with anterior septal involvement), systolic anterior motion of the mitral valve, a nondilated LV cavity, septal immobility, and premature closure of the aortic valve. LV thickness is measured at both the septum and free wall; values ≥ 15 mm, with a septal-to-free wall thickness ratio of ≥ 1.3, are suggestive of HCM. Asymmetric LV hypertrophy can also be seen in other segments besides the septum, such as the apex.10
HCM/HOCM is the most common cause of sudden cardiac death in young people. The condition also contributes to significant functional morbidity due to heart failure and increases the risk of atrial fibrillation and subsequent stroke. Treatments tend to focus on symptom relief and slowing disease progression and include the use of medications such as Β—blockers, nondihydropyridine calcium channel blockers, and the myosin inhibitor mavacamten.11 Select patients, such as those with severe LVOT obstruction and symptoms despite treatment with Β—blockers or nondihydropyridine calcium channel blockers, may be offered septal myectomy or catheter-based alcohol septal ablation, coupled with insertion of an implantable cardiac defibrillator to prevent sudden cardiac death in patients at high arrhythmic risk.1,12
Patients with HCM, particularly those with LVOT obstruction, pose distinct challenges to the anesthesiologist because they are highly sensitive to decreases in preload and afterload. These patients frequently experience adverse perioperative events such as myocardial ischemia, systemic hypotension, and supraventricular or ventricular arrhythmias. Acute congestive heart failure may also occur, presumably due to concomitant diastolic dysfunction. Patients with previously unrecognized HCM are of particular concern, as they may manifest unexpected and sudden hypotension with the induction of anesthesia. There may then be a paradoxical response to vasoactive drugs and anesthetic agents, which accentuate LVOT obstruction. In these circumstances, undiagnosed HCM should be considered, and intraoperative rescue transesophageal echocardiography be performed.13 Once the diagnosis is confirmed, efforts should be made to reduce myocardial contractility and sympathetic discharge (eg, with Β—blockers), increase afterload (eg, with α1 agonists), and improve preload with adequate hydration. Proper resuscitation of hypotensive patients with HCM requires a thorough understanding of disease pathology, as effective interventions may seem to be counterintuitive. Inotropic agents such as epinephrine are contraindicated in HCM because increased inotropy and chronotropy worsen LVOT obstruction. Volume status is often tenuous; while adequate preload is important, overly aggressive fluid resuscitation may promote heart failure. It is important to keep in mind that even patients without resting LVOT obstruction may develop dynamic obstruction with anesthesia induction due to sudden reductions in preload and afterload. It is also important to note that the degree of LV hypertrophy is directly correlated with arrhythmic sudden death. Those patients with LV wall thickness ≥ 30 mm are at increased risk for potentially lethal tachyarrhythmias in the operating room.14
These considerations reinforce the need for proper preoperative identification of patients with HCM. Heightened awareness is key, given the fact that HCM is relatively common and tends to be underdiagnosed in the general population. These patients are generally young, otherwise healthy, and often undergo minor operative procedures in outpatient settings. It is incumbent upon the preoperative evaluator to take a thorough medical history and perform a careful physical examination. Clues to the diagnosis include exertional dyspnea, fatigue, angina, syncope/presyncope, or a family history of sudden cardiac death or HCM. A systolic ejection murmur, particularly one that increases with standing or Valsalva maneuver, and decreases with squatting or handgrip may also raise clinical suspicion. These patients should undergo a full cardiac evaluation, including echocardiography.
CONCLUSIONS
HCM is a common condition that is important to diagnose in the preoperative clinic. Failure to do so can lead to catastrophic complications during induction of anesthesia due to the sudden reduction in preload and afterload, which may cause a significant increase in LVOT obstruction. A high index of suspicion is essential, as clinical diagnosis can be challenging. The physical examination may be deceiving and symptoms are often subtle and nonspecific. It is imperative to alert the anesthesiologist before surgery so the complex hemodynamic management of patients with HOCM can be appropriately managed.
- Cheng Z, Fang T, Huang J, Guo Y, Alam M, Qian H. Hypertrophic cardiomyopathy: from phenotype and pathogenesis to treatment. Front Cardiovasc Med. 2021;8:722340. doi:10.3389/fcvm.2021.722340
- Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249-1254. doi:10.1016/j.jacc.2015.01.019
- Hensley N, Dietrich J, Nyhan D, Mitter N, Yee MS, Brady M. Hypertrophic cardiomyopathy: a review. Anesth Analg. 2015;120(3):554-569. doi:10.1213/ ANE.0000000000000538
- Maron BJ, Desai MY, Nishimura RA, et al. Diagnosis and evaluation of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(4):372–389. doi:10.1016/j.jacc.2021.12.002
- Jorda P, Garcia-Alvarez A. Hypertrophic cardiomyopathy: sudden cardiac death risk stratification in adults. Glob Cardiol Sci Pract. 2018;3(25). doi:10.21542/gcsp.2018.25
- Wigle ED, Sasson Z, Henderson MA, et al. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis. 1985;28(1):1-83. doi:10.1016/0033-0620(85)90024-6
- Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342(24):1778–1785. doi:10.1056/ NEJM200006153422403
- Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy Lancet. 2017;389(10075):1253-1267. doi:10.1016/S0140-6736(16)31321-6
- Rowin EJ, Maron BJ, Appelbaum E, et al. Significance of false negative electrocardiograms in preparticipation screening of athletes for hypertrophic cardiomyopathy. Am J Cardiol. 2012;110(7):1027-1032. doi:10.1016/j. amjcard.2012.05.035
- Losi MA, Nistri S, Galderisi M et al. Echocardiography in patients with hypertrophic cardiomyopathy: usefulness of old and new techniques in the diagnosis and pathophysiological assessment. Cardiovasc Ultrasound. 2010;8(7). doi:10.1186/1476-7120-8-7
- Tian Z, Li L, Li X, et al. Effect of mavacamten on chinese patients with symptomatic obstructive hypertrophic cardiomyopathy: the EXPLORER-CN randomized clinical trial. JAMA Cardiol. 2023;8(10):957-965. doi:10.1001/ jamacardio.2023.3030
- Fang J, Liu Y, Zhu Y, et al. First-in-human transapical beating-heart septal myectomy in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2023;82(7):575-586. doi:10.1016/j.jacc.2023.05.052
- Jain P, Patel PA, Fabbro M 2nd. Hypertrophic cardiomyopathy and left ventricular outflow tract obstruction: expecting the unexpected. J Cardiothorac Vasc Anesth. 2018;32(1):467-477. doi:10.1053/j.jvca.2017.04.054
- Poliac LC, Barron ME, Maron BJ. Hypertrophic cardiomyopathy. Anesthesiology. 2006;104(1):183-192. doi:10.1097/00000542-200601000-00025
- Cheng Z, Fang T, Huang J, Guo Y, Alam M, Qian H. Hypertrophic cardiomyopathy: from phenotype and pathogenesis to treatment. Front Cardiovasc Med. 2021;8:722340. doi:10.3389/fcvm.2021.722340
- Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249-1254. doi:10.1016/j.jacc.2015.01.019
- Hensley N, Dietrich J, Nyhan D, Mitter N, Yee MS, Brady M. Hypertrophic cardiomyopathy: a review. Anesth Analg. 2015;120(3):554-569. doi:10.1213/ ANE.0000000000000538
- Maron BJ, Desai MY, Nishimura RA, et al. Diagnosis and evaluation of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(4):372–389. doi:10.1016/j.jacc.2021.12.002
- Jorda P, Garcia-Alvarez A. Hypertrophic cardiomyopathy: sudden cardiac death risk stratification in adults. Glob Cardiol Sci Pract. 2018;3(25). doi:10.21542/gcsp.2018.25
- Wigle ED, Sasson Z, Henderson MA, et al. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis. 1985;28(1):1-83. doi:10.1016/0033-0620(85)90024-6
- Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342(24):1778–1785. doi:10.1056/ NEJM200006153422403
- Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy Lancet. 2017;389(10075):1253-1267. doi:10.1016/S0140-6736(16)31321-6
- Rowin EJ, Maron BJ, Appelbaum E, et al. Significance of false negative electrocardiograms in preparticipation screening of athletes for hypertrophic cardiomyopathy. Am J Cardiol. 2012;110(7):1027-1032. doi:10.1016/j. amjcard.2012.05.035
- Losi MA, Nistri S, Galderisi M et al. Echocardiography in patients with hypertrophic cardiomyopathy: usefulness of old and new techniques in the diagnosis and pathophysiological assessment. Cardiovasc Ultrasound. 2010;8(7). doi:10.1186/1476-7120-8-7
- Tian Z, Li L, Li X, et al. Effect of mavacamten on chinese patients with symptomatic obstructive hypertrophic cardiomyopathy: the EXPLORER-CN randomized clinical trial. JAMA Cardiol. 2023;8(10):957-965. doi:10.1001/ jamacardio.2023.3030
- Fang J, Liu Y, Zhu Y, et al. First-in-human transapical beating-heart septal myectomy in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2023;82(7):575-586. doi:10.1016/j.jacc.2023.05.052
- Jain P, Patel PA, Fabbro M 2nd. Hypertrophic cardiomyopathy and left ventricular outflow tract obstruction: expecting the unexpected. J Cardiothorac Vasc Anesth. 2018;32(1):467-477. doi:10.1053/j.jvca.2017.04.054
- Poliac LC, Barron ME, Maron BJ. Hypertrophic cardiomyopathy. Anesthesiology. 2006;104(1):183-192. doi:10.1097/00000542-200601000-00025
Importance of Recognizing Hypertrophic Cardiomyopathy in the Preoperative Clinic
Importance of Recognizing Hypertrophic Cardiomyopathy in the Preoperative Clinic
The Heart Matters: Women Veterans, Cardiovascular Disease, and PTSD
The Heart Matters: Women Veterans, Cardiovascular Disease, and PTSD
If I can stop one heart from breaking, I shall not live in vain.
Emily Dickinson1
The celebration of Valentine’s Day has made the association of hearts with the month of February almost automatic. There is, though, another commemoration of hearts in the second month of the year with special significance for federal practice: American Heart Month. President Lyndon B. Johnson proclaimed February as American Heart Month in 1964 to raise awareness of the enormous human and economic cost of cardiovascular diseases (CVD) that impact many Americans in their prime.
The Centers for Disease Control and Prevention estimates that 1 in 5 deaths in the United States is due to CVD, which includes coronary artery disease, heart failure, heart attack, and stroke.2 American Heart Month aims to increase public attention to heart disease prevention and promote research to develop better diagnostic treatment methods for the leading cause of death in most populations.
Forty years after this proclamation, the American Heart Association launched Go Red for Women. On the first Friday of American Heart Month, Americans are encouraged to wear red to draw attention to CVD as the leading cause of death among women as well as men.2,3 A 2024 report from the American Heart Institute and McKinsey Health Institute attributed at least one-third of the overall health care disparities between men and women to inequities in CVD care. These detrimental differences in the management of heart disease in women encompass both diagnostic misadventures and failure to promptly employ effective therapeutics. CVD morbidity and mortality data for Black women are even higher due to multiple and overlapping social determinants of health.4
Higher rates of hypertension, hyperlipidemia, and smoking in women veterans compared with civilians have resulted in an increased risk of heart disease and a 26% higher rate of CVD-related mortality. One in 10 women enrolled in US Department of Veterans Affairs (VA) health care has CVD. Research shows that these women are less likely compared to male veterans to receive counseling about exercise or to be prescribed medications such as statins, even when evidence-based treatment guidelines are followed. The increased rates of heart disease and its complications in women veterans are in part due to risk factors related to military service such as posttraumatic stress disorder (PTSD) and depression, which exceed the rates of nonveteran women.5
The heart has a long association with psychological health. For millennia, philosophers and physicians alike believed the heart was the center of the self and the locus of sentience. Even William Harvey, whose discovery of the circulation of blood earned him the title of the father of cardiology, viewed the heart as the life force.6 The heart has been explicitly linked to American military trauma since the Civil War era diagnosis of Soldier’s Heart. More recently, mutual genetic vulnerabilities to PTSD and CVD have been posited.7 Indeed, research with male combat veterans helped establish the association.
Until recently, there has been a dearth of research to establish the same connection between CVD and PTSD in women veterans, who have elevated rates of PTSD in part due to higher rates of homelessness and military sexual trauma.5 Due in large part to the work of a group of VA and US Department of Defense (DoD) researchers, this is starting to change. A research group conducted a retrospective longitudinal study using electronic health record data from nearly 400,000 women veterans to determine the propensity scores of associations between a PTSD diagnosis and the incidence of heart disease over nearly 5 years. The hazard ratio (HR) for the incidence of CVD in women with trauma was 1.44 (compared with matched controls) and even higher in younger women (HR, 1.72).8 Researchers also compared CVD mortality in civilian and veteran women and found a concerning trend: not only were mortality rates higher in veterans, but they also did not benefit from an overall improved trend in deaths from heart disease over the past 20 years.9
Two years later, the same VA/DoD research group conducted additional analysis on the dataset used in the prior study to examine potential mechanisms underlying the epidemiological link between CVD and PTSD in women veterans. Women with and without PTSD were matched on age and traditional CVD risk factor parameters. The findings demonstrated an association of PTSD with higher risks of diabetes, hypertension, hyperlipidemia, and smoking. However, these traditional risk factors only accounted for one-fourth of the total association. About 34% of the risk was attributed to depression, anxiety, and substance use disorders, as well as obesity and neuroendocrine disorders. This leaves slightly more than half of the elevated risk of CVD unexplained.10
This research, along with other studies, have identified several mechanisms elucidating the link. Promising translational research may lead to new diagnostic techniques or improved treatment modalities for CVD in women. The most established etiology is that veterans with PTSD have a higher prevalence of multiple CVD risk factors, including smoking, substance use disorders, obesity, poor diet, sleep disorders, depression, and inactivity. There is also increased recognition that PTSD involves neuroendocrine dysfunction in the stress-response that triggers a cascade of metabolic responses (eg, chronic inflammation) that contribute to the onset and progression of heart disease.11
This burgeoning scientific work on CVD and its close association with PTSD and the role of both traditional and nontraditional risk factors can inform VA efforts to educate frontline VA and DoD clinicians, leading to better care for women veterans. Whether a practitioner provides primary, specialty, or mental health care, this new knowledge can inform efforts to optimize prevention and treatment for both PTSD and CVD. For example, the VA/DoD researchers recommend prescribing antidepressants that are less likely to cause or worsen hypertension and to employ psychotherapies known to reduce the harmful CVD effects of increased stress acting through the hypothalamic-pituitary axis. These studies empower VA clinicians to realize Emily Dickinson’s aspiration to prevent trauma and reduce damage to both the psyche and the soma. The health of every veteran’s heart and mind matters, as does every effort of federal practitioners to protect and heal it.
- Dickinson E. The Complete Poems of Emily Dickinson. Back Bay Books; 1976.
- Centers for Disease Control. Heart disease facts. Updated October 24, 2024. Accessed January 27, 2025. https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html
- American Heart Association. Historical timeline of the American Heart Association. Accessed January 27, 2025. https:// www.heart.org/-/media/files/about-us/history/history-of-the-american-heart-association.pdf
- McKinsey Health Institute in Collaboration with the American Heart Association. The state of US women’s heart health: a path to improved health and financial outcomes. June 2024. Accessed January 27, 2025. https://www.goredforwomen.org/-/media/GRFW-Files/About-Heart-Disease-in-Women/The-state-of-US-womens-heart-health-report.pdf?sc_lang=en
- Han JK, Yano EM, Watson KE, Ebrahimi R. Cardiovascular Care in women veterans. Circulation. 2019;139(8):1102-1109. doi:10.1161/CIRCULATIONAHA.118.037748
- Conrad LI, Neve M, Nutton V, Porter R, Wear A. The Western Medical Tradition: 800 BC to AD 1800. Cambridge University Press; 1995:335-338.
- Bremner JD, Wittbrodt MT, Shah AJ, et al. Confederates in the attic: posttraumatic stress disorder, cardiovascular disease, and the return of soldier’s heart. J Nerv Ment Dis. 2020;208(3):171-180. doi:10.1097/NMD.0000000000001100
- Ebrahimi R, Lynch KE, Beckham JC, et al. Association of posttraumatic stress disorder and incident ischemic heart disease in women veterans. JAMA Cardiol. 2021;6(6):642-651. doi:10.1001/jamacardio.2021.0227
- Ebrahimi R, Yano EM, Alvarez CA, et al. Trends in cardiovascular disease mortality in US women veterans vs civilians. JAMA Netw Open. 2023;6(10):e2340242. doi:10.1001/jamanetworkopen.2023.40242
- Ebrahimi R, Dennis PA, Shroyer ALW, et al. Pathways linking post-traumatic stress disorder to incident ischemic heart disease in women: call to action. JACC Adv. 2023;3(1):100744. doi:10.1016/j.jacadv.2023.100744
- Arenson M, Cohen B. Posttraumatic Stress Disorder and Cardiovascular Disease. National Center for PTSD. PTSD Res Q. 2017;28(1):1-3. Accessed January 27, 2025. https://www.ptsd.va.gov/publications/rq_docs/V28N1.pdf
If I can stop one heart from breaking, I shall not live in vain.
Emily Dickinson1
The celebration of Valentine’s Day has made the association of hearts with the month of February almost automatic. There is, though, another commemoration of hearts in the second month of the year with special significance for federal practice: American Heart Month. President Lyndon B. Johnson proclaimed February as American Heart Month in 1964 to raise awareness of the enormous human and economic cost of cardiovascular diseases (CVD) that impact many Americans in their prime.
The Centers for Disease Control and Prevention estimates that 1 in 5 deaths in the United States is due to CVD, which includes coronary artery disease, heart failure, heart attack, and stroke.2 American Heart Month aims to increase public attention to heart disease prevention and promote research to develop better diagnostic treatment methods for the leading cause of death in most populations.
Forty years after this proclamation, the American Heart Association launched Go Red for Women. On the first Friday of American Heart Month, Americans are encouraged to wear red to draw attention to CVD as the leading cause of death among women as well as men.2,3 A 2024 report from the American Heart Institute and McKinsey Health Institute attributed at least one-third of the overall health care disparities between men and women to inequities in CVD care. These detrimental differences in the management of heart disease in women encompass both diagnostic misadventures and failure to promptly employ effective therapeutics. CVD morbidity and mortality data for Black women are even higher due to multiple and overlapping social determinants of health.4
Higher rates of hypertension, hyperlipidemia, and smoking in women veterans compared with civilians have resulted in an increased risk of heart disease and a 26% higher rate of CVD-related mortality. One in 10 women enrolled in US Department of Veterans Affairs (VA) health care has CVD. Research shows that these women are less likely compared to male veterans to receive counseling about exercise or to be prescribed medications such as statins, even when evidence-based treatment guidelines are followed. The increased rates of heart disease and its complications in women veterans are in part due to risk factors related to military service such as posttraumatic stress disorder (PTSD) and depression, which exceed the rates of nonveteran women.5
The heart has a long association with psychological health. For millennia, philosophers and physicians alike believed the heart was the center of the self and the locus of sentience. Even William Harvey, whose discovery of the circulation of blood earned him the title of the father of cardiology, viewed the heart as the life force.6 The heart has been explicitly linked to American military trauma since the Civil War era diagnosis of Soldier’s Heart. More recently, mutual genetic vulnerabilities to PTSD and CVD have been posited.7 Indeed, research with male combat veterans helped establish the association.
Until recently, there has been a dearth of research to establish the same connection between CVD and PTSD in women veterans, who have elevated rates of PTSD in part due to higher rates of homelessness and military sexual trauma.5 Due in large part to the work of a group of VA and US Department of Defense (DoD) researchers, this is starting to change. A research group conducted a retrospective longitudinal study using electronic health record data from nearly 400,000 women veterans to determine the propensity scores of associations between a PTSD diagnosis and the incidence of heart disease over nearly 5 years. The hazard ratio (HR) for the incidence of CVD in women with trauma was 1.44 (compared with matched controls) and even higher in younger women (HR, 1.72).8 Researchers also compared CVD mortality in civilian and veteran women and found a concerning trend: not only were mortality rates higher in veterans, but they also did not benefit from an overall improved trend in deaths from heart disease over the past 20 years.9
Two years later, the same VA/DoD research group conducted additional analysis on the dataset used in the prior study to examine potential mechanisms underlying the epidemiological link between CVD and PTSD in women veterans. Women with and without PTSD were matched on age and traditional CVD risk factor parameters. The findings demonstrated an association of PTSD with higher risks of diabetes, hypertension, hyperlipidemia, and smoking. However, these traditional risk factors only accounted for one-fourth of the total association. About 34% of the risk was attributed to depression, anxiety, and substance use disorders, as well as obesity and neuroendocrine disorders. This leaves slightly more than half of the elevated risk of CVD unexplained.10
This research, along with other studies, have identified several mechanisms elucidating the link. Promising translational research may lead to new diagnostic techniques or improved treatment modalities for CVD in women. The most established etiology is that veterans with PTSD have a higher prevalence of multiple CVD risk factors, including smoking, substance use disorders, obesity, poor diet, sleep disorders, depression, and inactivity. There is also increased recognition that PTSD involves neuroendocrine dysfunction in the stress-response that triggers a cascade of metabolic responses (eg, chronic inflammation) that contribute to the onset and progression of heart disease.11
This burgeoning scientific work on CVD and its close association with PTSD and the role of both traditional and nontraditional risk factors can inform VA efforts to educate frontline VA and DoD clinicians, leading to better care for women veterans. Whether a practitioner provides primary, specialty, or mental health care, this new knowledge can inform efforts to optimize prevention and treatment for both PTSD and CVD. For example, the VA/DoD researchers recommend prescribing antidepressants that are less likely to cause or worsen hypertension and to employ psychotherapies known to reduce the harmful CVD effects of increased stress acting through the hypothalamic-pituitary axis. These studies empower VA clinicians to realize Emily Dickinson’s aspiration to prevent trauma and reduce damage to both the psyche and the soma. The health of every veteran’s heart and mind matters, as does every effort of federal practitioners to protect and heal it.
If I can stop one heart from breaking, I shall not live in vain.
Emily Dickinson1
The celebration of Valentine’s Day has made the association of hearts with the month of February almost automatic. There is, though, another commemoration of hearts in the second month of the year with special significance for federal practice: American Heart Month. President Lyndon B. Johnson proclaimed February as American Heart Month in 1964 to raise awareness of the enormous human and economic cost of cardiovascular diseases (CVD) that impact many Americans in their prime.
The Centers for Disease Control and Prevention estimates that 1 in 5 deaths in the United States is due to CVD, which includes coronary artery disease, heart failure, heart attack, and stroke.2 American Heart Month aims to increase public attention to heart disease prevention and promote research to develop better diagnostic treatment methods for the leading cause of death in most populations.
Forty years after this proclamation, the American Heart Association launched Go Red for Women. On the first Friday of American Heart Month, Americans are encouraged to wear red to draw attention to CVD as the leading cause of death among women as well as men.2,3 A 2024 report from the American Heart Institute and McKinsey Health Institute attributed at least one-third of the overall health care disparities between men and women to inequities in CVD care. These detrimental differences in the management of heart disease in women encompass both diagnostic misadventures and failure to promptly employ effective therapeutics. CVD morbidity and mortality data for Black women are even higher due to multiple and overlapping social determinants of health.4
Higher rates of hypertension, hyperlipidemia, and smoking in women veterans compared with civilians have resulted in an increased risk of heart disease and a 26% higher rate of CVD-related mortality. One in 10 women enrolled in US Department of Veterans Affairs (VA) health care has CVD. Research shows that these women are less likely compared to male veterans to receive counseling about exercise or to be prescribed medications such as statins, even when evidence-based treatment guidelines are followed. The increased rates of heart disease and its complications in women veterans are in part due to risk factors related to military service such as posttraumatic stress disorder (PTSD) and depression, which exceed the rates of nonveteran women.5
The heart has a long association with psychological health. For millennia, philosophers and physicians alike believed the heart was the center of the self and the locus of sentience. Even William Harvey, whose discovery of the circulation of blood earned him the title of the father of cardiology, viewed the heart as the life force.6 The heart has been explicitly linked to American military trauma since the Civil War era diagnosis of Soldier’s Heart. More recently, mutual genetic vulnerabilities to PTSD and CVD have been posited.7 Indeed, research with male combat veterans helped establish the association.
Until recently, there has been a dearth of research to establish the same connection between CVD and PTSD in women veterans, who have elevated rates of PTSD in part due to higher rates of homelessness and military sexual trauma.5 Due in large part to the work of a group of VA and US Department of Defense (DoD) researchers, this is starting to change. A research group conducted a retrospective longitudinal study using electronic health record data from nearly 400,000 women veterans to determine the propensity scores of associations between a PTSD diagnosis and the incidence of heart disease over nearly 5 years. The hazard ratio (HR) for the incidence of CVD in women with trauma was 1.44 (compared with matched controls) and even higher in younger women (HR, 1.72).8 Researchers also compared CVD mortality in civilian and veteran women and found a concerning trend: not only were mortality rates higher in veterans, but they also did not benefit from an overall improved trend in deaths from heart disease over the past 20 years.9
Two years later, the same VA/DoD research group conducted additional analysis on the dataset used in the prior study to examine potential mechanisms underlying the epidemiological link between CVD and PTSD in women veterans. Women with and without PTSD were matched on age and traditional CVD risk factor parameters. The findings demonstrated an association of PTSD with higher risks of diabetes, hypertension, hyperlipidemia, and smoking. However, these traditional risk factors only accounted for one-fourth of the total association. About 34% of the risk was attributed to depression, anxiety, and substance use disorders, as well as obesity and neuroendocrine disorders. This leaves slightly more than half of the elevated risk of CVD unexplained.10
This research, along with other studies, have identified several mechanisms elucidating the link. Promising translational research may lead to new diagnostic techniques or improved treatment modalities for CVD in women. The most established etiology is that veterans with PTSD have a higher prevalence of multiple CVD risk factors, including smoking, substance use disorders, obesity, poor diet, sleep disorders, depression, and inactivity. There is also increased recognition that PTSD involves neuroendocrine dysfunction in the stress-response that triggers a cascade of metabolic responses (eg, chronic inflammation) that contribute to the onset and progression of heart disease.11
This burgeoning scientific work on CVD and its close association with PTSD and the role of both traditional and nontraditional risk factors can inform VA efforts to educate frontline VA and DoD clinicians, leading to better care for women veterans. Whether a practitioner provides primary, specialty, or mental health care, this new knowledge can inform efforts to optimize prevention and treatment for both PTSD and CVD. For example, the VA/DoD researchers recommend prescribing antidepressants that are less likely to cause or worsen hypertension and to employ psychotherapies known to reduce the harmful CVD effects of increased stress acting through the hypothalamic-pituitary axis. These studies empower VA clinicians to realize Emily Dickinson’s aspiration to prevent trauma and reduce damage to both the psyche and the soma. The health of every veteran’s heart and mind matters, as does every effort of federal practitioners to protect and heal it.
- Dickinson E. The Complete Poems of Emily Dickinson. Back Bay Books; 1976.
- Centers for Disease Control. Heart disease facts. Updated October 24, 2024. Accessed January 27, 2025. https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html
- American Heart Association. Historical timeline of the American Heart Association. Accessed January 27, 2025. https:// www.heart.org/-/media/files/about-us/history/history-of-the-american-heart-association.pdf
- McKinsey Health Institute in Collaboration with the American Heart Association. The state of US women’s heart health: a path to improved health and financial outcomes. June 2024. Accessed January 27, 2025. https://www.goredforwomen.org/-/media/GRFW-Files/About-Heart-Disease-in-Women/The-state-of-US-womens-heart-health-report.pdf?sc_lang=en
- Han JK, Yano EM, Watson KE, Ebrahimi R. Cardiovascular Care in women veterans. Circulation. 2019;139(8):1102-1109. doi:10.1161/CIRCULATIONAHA.118.037748
- Conrad LI, Neve M, Nutton V, Porter R, Wear A. The Western Medical Tradition: 800 BC to AD 1800. Cambridge University Press; 1995:335-338.
- Bremner JD, Wittbrodt MT, Shah AJ, et al. Confederates in the attic: posttraumatic stress disorder, cardiovascular disease, and the return of soldier’s heart. J Nerv Ment Dis. 2020;208(3):171-180. doi:10.1097/NMD.0000000000001100
- Ebrahimi R, Lynch KE, Beckham JC, et al. Association of posttraumatic stress disorder and incident ischemic heart disease in women veterans. JAMA Cardiol. 2021;6(6):642-651. doi:10.1001/jamacardio.2021.0227
- Ebrahimi R, Yano EM, Alvarez CA, et al. Trends in cardiovascular disease mortality in US women veterans vs civilians. JAMA Netw Open. 2023;6(10):e2340242. doi:10.1001/jamanetworkopen.2023.40242
- Ebrahimi R, Dennis PA, Shroyer ALW, et al. Pathways linking post-traumatic stress disorder to incident ischemic heart disease in women: call to action. JACC Adv. 2023;3(1):100744. doi:10.1016/j.jacadv.2023.100744
- Arenson M, Cohen B. Posttraumatic Stress Disorder and Cardiovascular Disease. National Center for PTSD. PTSD Res Q. 2017;28(1):1-3. Accessed January 27, 2025. https://www.ptsd.va.gov/publications/rq_docs/V28N1.pdf
- Dickinson E. The Complete Poems of Emily Dickinson. Back Bay Books; 1976.
- Centers for Disease Control. Heart disease facts. Updated October 24, 2024. Accessed January 27, 2025. https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html
- American Heart Association. Historical timeline of the American Heart Association. Accessed January 27, 2025. https:// www.heart.org/-/media/files/about-us/history/history-of-the-american-heart-association.pdf
- McKinsey Health Institute in Collaboration with the American Heart Association. The state of US women’s heart health: a path to improved health and financial outcomes. June 2024. Accessed January 27, 2025. https://www.goredforwomen.org/-/media/GRFW-Files/About-Heart-Disease-in-Women/The-state-of-US-womens-heart-health-report.pdf?sc_lang=en
- Han JK, Yano EM, Watson KE, Ebrahimi R. Cardiovascular Care in women veterans. Circulation. 2019;139(8):1102-1109. doi:10.1161/CIRCULATIONAHA.118.037748
- Conrad LI, Neve M, Nutton V, Porter R, Wear A. The Western Medical Tradition: 800 BC to AD 1800. Cambridge University Press; 1995:335-338.
- Bremner JD, Wittbrodt MT, Shah AJ, et al. Confederates in the attic: posttraumatic stress disorder, cardiovascular disease, and the return of soldier’s heart. J Nerv Ment Dis. 2020;208(3):171-180. doi:10.1097/NMD.0000000000001100
- Ebrahimi R, Lynch KE, Beckham JC, et al. Association of posttraumatic stress disorder and incident ischemic heart disease in women veterans. JAMA Cardiol. 2021;6(6):642-651. doi:10.1001/jamacardio.2021.0227
- Ebrahimi R, Yano EM, Alvarez CA, et al. Trends in cardiovascular disease mortality in US women veterans vs civilians. JAMA Netw Open. 2023;6(10):e2340242. doi:10.1001/jamanetworkopen.2023.40242
- Ebrahimi R, Dennis PA, Shroyer ALW, et al. Pathways linking post-traumatic stress disorder to incident ischemic heart disease in women: call to action. JACC Adv. 2023;3(1):100744. doi:10.1016/j.jacadv.2023.100744
- Arenson M, Cohen B. Posttraumatic Stress Disorder and Cardiovascular Disease. National Center for PTSD. PTSD Res Q. 2017;28(1):1-3. Accessed January 27, 2025. https://www.ptsd.va.gov/publications/rq_docs/V28N1.pdf
The Heart Matters: Women Veterans, Cardiovascular Disease, and PTSD
The Heart Matters: Women Veterans, Cardiovascular Disease, and PTSD
Losing Your Mind Trying to Understand the BP-Dementia Link
You could be forgiven if you are confused about how blood pressure (BP) affects dementia. First, you read an article extolling the benefits of BP lowering, then a study about how stopping antihypertensives slows cognitive decline in nursing home residents. It’s enough to make you lose your mind.
The Brain Benefits of BP Lowering
It should be stated unequivocally that you should absolutely treat high BP. It may have once been acceptable to state, “The greatest danger to a man with high blood pressure lies in its discovery, because then some fool is certain to try and reduce it.” But those dark days are long behind us.
In these divided times, at least we can agree that we should treat high BP. The cardiovascular (CV) benefits, in and of themselves, justify the decision. But BP’s relationship with dementia is more complex. There are different types of dementia even though we tend to lump them all into one category. Vascular dementia is driven by the same pathophysiology and risk factors as cardiac disease. It’s intuitive that treating hypertension, diabetes, hypercholesterolemia, and smoking will decrease the risk for stroke and limit the damage to the brain that we see with repeated vascular insults. For Alzheimer’s disease, high BP and other CV risk factors seem to increase the risk even if the mechanism is not fully elucidated.
Estimates suggest that if we could lower the prevalence of hypertension by 25%, there would be 160,000 fewer cases of Alzheimer’s disease. But the data are not as robust as one might hope. A 2021 Cochrane review found that hypertension treatment slowed cognitive decline, but the quality of the evidence was low. Short duration of follow-up, dropouts, crossovers, and other problems with the data precluded any certainty. What’s more, hypertension in midlife is associated with cognitive decline and dementia, but its impact in those over age 70 is less clear. Later in life, or once cognitive impairment has already developed, it may be too late for BP lowering to have any impact.
Potential Harms of Lowering BP
All this needs to be weighed against the potential harms of treating hypertension. I will reiterate that hypertension should be treated and treated aggressively for the prevention of CV events. But overtreatment, especially in older patients, is associated with hypotension, falls, and syncope. Older patients are also at risk for polypharmacy and drug-drug interactions.
A Korean nationwide survey showed a U-shaped association between BP and Alzheimer’s disease risk in adults (mean age, 67 years), with both high and low BPs associated with a higher risk for Alzheimer’s disease. Though not all studies agree. A post hoc analysis of SPRINT MIND did not find any negative impact of intensive BP lowering on cognitive outcomes or cerebral perfusion in older adults (mean age, 68 years). But it didn’t do much good either. Given the heterogeneity of the data, doubts remain on whether aggressive BP lowering might be detrimental in older patients with comorbidities and preexisting dementia. The obvious corollary then is whether deprescribing hypertensive medications could be beneficial.
A recent publication in JAMA Internal Medicine attempted to address this very question. The cohort study used data from Veterans Affairs nursing home residents (mean age, 78 years) to emulate a randomized trial on deprescribing antihypertensives and cognitive decline. Many of the residents’ cognitive scores worsened over the course of follow-up; however, the decline was less pronounced in the deprescribing group (10% vs 12%). The same group did a similar analysis looking at CV outcomes and found no increased risk for heart attack or stroke with deprescribing BP medications. Taken together, these nursing home data suggest that deprescribing may help slow cognitive decline without the expected trade-off of increased CV events.
Deprescribing, Yes or No?
However, randomized data would obviously be preferable, and these are in short supply. One such trial, the DANTE study, found no benefit to deprescribing in terms of cognition in adults aged 75 years or older with mild cognitive impairment. The study follow-up was only 16 weeks, however, which is hardly enough time to demonstrate any effect, positive or negative. The most that can be said is that it didn’t cause many short-term adverse events.
Perhaps the best conclusion to draw from this somewhat underwhelming collection of data is that lowering high BP is important, but less important the closer we get to the end of life. Hypotension is obviously bad, and overly aggressive BP lowering is going to lead to negative outcomes in older adults because gravity is an unforgiving mistress.
Deprescribing antihypertensives in older adults is probably not going to cause major negative outcomes, but whether it will do much good in nonhypotensive patients is debatable. The bigger problem is the millions of people with undiagnosed or undertreated hypertension. We would probably have less dementia if we treated hypertension when it does the most good: as a primary-prevention strategy in midlife.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal, Quebec, Canada. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
You could be forgiven if you are confused about how blood pressure (BP) affects dementia. First, you read an article extolling the benefits of BP lowering, then a study about how stopping antihypertensives slows cognitive decline in nursing home residents. It’s enough to make you lose your mind.
The Brain Benefits of BP Lowering
It should be stated unequivocally that you should absolutely treat high BP. It may have once been acceptable to state, “The greatest danger to a man with high blood pressure lies in its discovery, because then some fool is certain to try and reduce it.” But those dark days are long behind us.
In these divided times, at least we can agree that we should treat high BP. The cardiovascular (CV) benefits, in and of themselves, justify the decision. But BP’s relationship with dementia is more complex. There are different types of dementia even though we tend to lump them all into one category. Vascular dementia is driven by the same pathophysiology and risk factors as cardiac disease. It’s intuitive that treating hypertension, diabetes, hypercholesterolemia, and smoking will decrease the risk for stroke and limit the damage to the brain that we see with repeated vascular insults. For Alzheimer’s disease, high BP and other CV risk factors seem to increase the risk even if the mechanism is not fully elucidated.
Estimates suggest that if we could lower the prevalence of hypertension by 25%, there would be 160,000 fewer cases of Alzheimer’s disease. But the data are not as robust as one might hope. A 2021 Cochrane review found that hypertension treatment slowed cognitive decline, but the quality of the evidence was low. Short duration of follow-up, dropouts, crossovers, and other problems with the data precluded any certainty. What’s more, hypertension in midlife is associated with cognitive decline and dementia, but its impact in those over age 70 is less clear. Later in life, or once cognitive impairment has already developed, it may be too late for BP lowering to have any impact.
Potential Harms of Lowering BP
All this needs to be weighed against the potential harms of treating hypertension. I will reiterate that hypertension should be treated and treated aggressively for the prevention of CV events. But overtreatment, especially in older patients, is associated with hypotension, falls, and syncope. Older patients are also at risk for polypharmacy and drug-drug interactions.
A Korean nationwide survey showed a U-shaped association between BP and Alzheimer’s disease risk in adults (mean age, 67 years), with both high and low BPs associated with a higher risk for Alzheimer’s disease. Though not all studies agree. A post hoc analysis of SPRINT MIND did not find any negative impact of intensive BP lowering on cognitive outcomes or cerebral perfusion in older adults (mean age, 68 years). But it didn’t do much good either. Given the heterogeneity of the data, doubts remain on whether aggressive BP lowering might be detrimental in older patients with comorbidities and preexisting dementia. The obvious corollary then is whether deprescribing hypertensive medications could be beneficial.
A recent publication in JAMA Internal Medicine attempted to address this very question. The cohort study used data from Veterans Affairs nursing home residents (mean age, 78 years) to emulate a randomized trial on deprescribing antihypertensives and cognitive decline. Many of the residents’ cognitive scores worsened over the course of follow-up; however, the decline was less pronounced in the deprescribing group (10% vs 12%). The same group did a similar analysis looking at CV outcomes and found no increased risk for heart attack or stroke with deprescribing BP medications. Taken together, these nursing home data suggest that deprescribing may help slow cognitive decline without the expected trade-off of increased CV events.
Deprescribing, Yes or No?
However, randomized data would obviously be preferable, and these are in short supply. One such trial, the DANTE study, found no benefit to deprescribing in terms of cognition in adults aged 75 years or older with mild cognitive impairment. The study follow-up was only 16 weeks, however, which is hardly enough time to demonstrate any effect, positive or negative. The most that can be said is that it didn’t cause many short-term adverse events.
Perhaps the best conclusion to draw from this somewhat underwhelming collection of data is that lowering high BP is important, but less important the closer we get to the end of life. Hypotension is obviously bad, and overly aggressive BP lowering is going to lead to negative outcomes in older adults because gravity is an unforgiving mistress.
Deprescribing antihypertensives in older adults is probably not going to cause major negative outcomes, but whether it will do much good in nonhypotensive patients is debatable. The bigger problem is the millions of people with undiagnosed or undertreated hypertension. We would probably have less dementia if we treated hypertension when it does the most good: as a primary-prevention strategy in midlife.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal, Quebec, Canada. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
You could be forgiven if you are confused about how blood pressure (BP) affects dementia. First, you read an article extolling the benefits of BP lowering, then a study about how stopping antihypertensives slows cognitive decline in nursing home residents. It’s enough to make you lose your mind.
The Brain Benefits of BP Lowering
It should be stated unequivocally that you should absolutely treat high BP. It may have once been acceptable to state, “The greatest danger to a man with high blood pressure lies in its discovery, because then some fool is certain to try and reduce it.” But those dark days are long behind us.
In these divided times, at least we can agree that we should treat high BP. The cardiovascular (CV) benefits, in and of themselves, justify the decision. But BP’s relationship with dementia is more complex. There are different types of dementia even though we tend to lump them all into one category. Vascular dementia is driven by the same pathophysiology and risk factors as cardiac disease. It’s intuitive that treating hypertension, diabetes, hypercholesterolemia, and smoking will decrease the risk for stroke and limit the damage to the brain that we see with repeated vascular insults. For Alzheimer’s disease, high BP and other CV risk factors seem to increase the risk even if the mechanism is not fully elucidated.
Estimates suggest that if we could lower the prevalence of hypertension by 25%, there would be 160,000 fewer cases of Alzheimer’s disease. But the data are not as robust as one might hope. A 2021 Cochrane review found that hypertension treatment slowed cognitive decline, but the quality of the evidence was low. Short duration of follow-up, dropouts, crossovers, and other problems with the data precluded any certainty. What’s more, hypertension in midlife is associated with cognitive decline and dementia, but its impact in those over age 70 is less clear. Later in life, or once cognitive impairment has already developed, it may be too late for BP lowering to have any impact.
Potential Harms of Lowering BP
All this needs to be weighed against the potential harms of treating hypertension. I will reiterate that hypertension should be treated and treated aggressively for the prevention of CV events. But overtreatment, especially in older patients, is associated with hypotension, falls, and syncope. Older patients are also at risk for polypharmacy and drug-drug interactions.
A Korean nationwide survey showed a U-shaped association between BP and Alzheimer’s disease risk in adults (mean age, 67 years), with both high and low BPs associated with a higher risk for Alzheimer’s disease. Though not all studies agree. A post hoc analysis of SPRINT MIND did not find any negative impact of intensive BP lowering on cognitive outcomes or cerebral perfusion in older adults (mean age, 68 years). But it didn’t do much good either. Given the heterogeneity of the data, doubts remain on whether aggressive BP lowering might be detrimental in older patients with comorbidities and preexisting dementia. The obvious corollary then is whether deprescribing hypertensive medications could be beneficial.
A recent publication in JAMA Internal Medicine attempted to address this very question. The cohort study used data from Veterans Affairs nursing home residents (mean age, 78 years) to emulate a randomized trial on deprescribing antihypertensives and cognitive decline. Many of the residents’ cognitive scores worsened over the course of follow-up; however, the decline was less pronounced in the deprescribing group (10% vs 12%). The same group did a similar analysis looking at CV outcomes and found no increased risk for heart attack or stroke with deprescribing BP medications. Taken together, these nursing home data suggest that deprescribing may help slow cognitive decline without the expected trade-off of increased CV events.
Deprescribing, Yes or No?
However, randomized data would obviously be preferable, and these are in short supply. One such trial, the DANTE study, found no benefit to deprescribing in terms of cognition in adults aged 75 years or older with mild cognitive impairment. The study follow-up was only 16 weeks, however, which is hardly enough time to demonstrate any effect, positive or negative. The most that can be said is that it didn’t cause many short-term adverse events.
Perhaps the best conclusion to draw from this somewhat underwhelming collection of data is that lowering high BP is important, but less important the closer we get to the end of life. Hypotension is obviously bad, and overly aggressive BP lowering is going to lead to negative outcomes in older adults because gravity is an unforgiving mistress.
Deprescribing antihypertensives in older adults is probably not going to cause major negative outcomes, but whether it will do much good in nonhypotensive patients is debatable. The bigger problem is the millions of people with undiagnosed or undertreated hypertension. We would probably have less dementia if we treated hypertension when it does the most good: as a primary-prevention strategy in midlife.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal, Quebec, Canada. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Most Kids With COVID-Linked MIS-C Recover by 6 Months
Children who were severely ill with multisystem inflammatory syndrome in children (MIS-C) related to COVID-19 infection appear to show excellent cardiovascular and noncardiovascular outcomes by 6 months, according to data published in JAMA Pediatrics.
MIS-C is a life-threatening complication of COVID-19 infection and data on outcomes are limited, wrote the authors, led by Dongngan T. Truong, MD, MSSI, with Children’s Healthcare of Atlanta Cardiology, Emory University School of Medicine in Atlanta, Georgia. These 6-month results are from the Long-Term Outcomes After the Multisystem Inflammatory Syndrome in Children (MUSIC) study, sponsored by the National Heart, Lung, and Blood Institute.
Researchers found in this cohort study of 1204 participants that by 6 months after hospital discharge, 99% had normalization of left ventricular systolic function, and 92.3% had normalized coronary artery dimensions. More than 95% reported being more than 90% back to baseline health.
Patient-Reported Outcomes Measurement Information Systems (PROMIS) Global Health scores were at least equivalent to prepandemic population normative values. PROMIS Global Health parent/guardian proxy median T scores for fatigue, global health, and pain interference improved significantly from 2 weeks to 6 months: fatigue, 56.1 vs 48.9; global health, 48.8 vs 51.3; pain interference, 53.0 vs 43.3 (P < .001).
The most common symptoms reported at 2 weeks were fatigue (15.9%) and low stamina/energy (9.2%); both decreased to 3.4% and 3.3%, respectively, by 6 months. The most common cardiovascular symptom at 2 weeks was palpitations (1.5%), which decreased to 0.6%.
Chest Pain Increased Over Time
Reports of chest pain, however, reportedly increased over time, with 1.3% reporting chest pain at rest at 2 weeks and 2.2% at 6 months. Although gastrointestinal symptoms were common during the acute MIS-C, only 5.3% of respondents reported those symptoms at 2 weeks.
Children in the cohort had a median age of 9 years, and 60% were men. They self-identified with the following races and ethnicities: American Indian or Alaska Native (0.1%), Asian (3.3%), Black (27.0%), Hawaiian Native or Other Pacific Islander (0.2%), Hispanic or Latino (26.9%), multiracial (2.7%), White (31.2%), other (1.0%), and unknown or refused to specify (7.6%). Authors wrote that the cohort was followed-up to 2 years after illness onset and long-term results are not yet known.
Time to Exhale
David J. Goldberg, MD, with the Cardiac Center, Children’s Hospital of Philadelphia, Pennsylvania, and colleagues, wrote in an accompanying editorial that “the decreased frequency of the disease along (with) the reassuring reports on midterm outcomes can allow the pediatric community a moment of collective exhale.”
The editorialists note that of those who initially presented with myocardial dysfunction, all but one patient evaluated had a normal ejection fraction at follow-up. Energy, sleep, appetite, cognition, and mood also normalized by midterm.
“The results of the MUSIC study add to the emerging midterm outcomes data suggesting a near-complete cardiovascular recovery in the overwhelming majority of patients who develop MIS-C,” Goldberg and colleagues wrote. “Despite initial concerns, driven by the severity of acute presentation at diagnosis and longer-term questions that remain (for example, does coronary microvascular dysfunction persist even after normalization of coronary artery z score?), these data suggest an encouraging outlook for the long-term health of affected children.”
The Centers for Disease Control and Prevention and other agencies have reported a declining overall incidence of MIS-C and highlighted the protective value of vaccination.
The editorialists add, however, that while the drop in MIS-C cases is encouraging, cases are still reported, especially amid high viral activity periods, “and nearly half of affected children continue to require intensive care in the acute phase of illness.”
Truong reported grants from the National Institutes of Health and serving as coprincipal investigator for Pfizer for research on COVID-19 vaccine-associated myocarditis funded by Pfizer and occurring through the framework of the National Heart, Lung, and Blood Institute’s Pediatric Heart Network outside the submitted work. One coauthor reported grants from Pfizer and Boston Scientific outside the submitted work. One coauthor reported receiving grants from Additional Ventures Foundation outside the submitted work. One coauthor reported receiving consultant fees from Amryt Pharma, Chiesi, Esperion, and Ultragenyx outside the submitted work. A coauthor reported receiving consultant fees from Larimar Therapeutics for mitochondrial therapies outside the submitted work. One coauthor reported being an employee of Takeda Pharmaceuticals since July 2023. One editorialist reported grants from Childhood Arthritis and Rheumatology Research Alliance and the Arthritis Foundation, Academy Health, and the Gordon and Betty Moore Foundation during the conduct of the study.
A version of this article first appeared on Medscape.com.
Children who were severely ill with multisystem inflammatory syndrome in children (MIS-C) related to COVID-19 infection appear to show excellent cardiovascular and noncardiovascular outcomes by 6 months, according to data published in JAMA Pediatrics.
MIS-C is a life-threatening complication of COVID-19 infection and data on outcomes are limited, wrote the authors, led by Dongngan T. Truong, MD, MSSI, with Children’s Healthcare of Atlanta Cardiology, Emory University School of Medicine in Atlanta, Georgia. These 6-month results are from the Long-Term Outcomes After the Multisystem Inflammatory Syndrome in Children (MUSIC) study, sponsored by the National Heart, Lung, and Blood Institute.
Researchers found in this cohort study of 1204 participants that by 6 months after hospital discharge, 99% had normalization of left ventricular systolic function, and 92.3% had normalized coronary artery dimensions. More than 95% reported being more than 90% back to baseline health.
Patient-Reported Outcomes Measurement Information Systems (PROMIS) Global Health scores were at least equivalent to prepandemic population normative values. PROMIS Global Health parent/guardian proxy median T scores for fatigue, global health, and pain interference improved significantly from 2 weeks to 6 months: fatigue, 56.1 vs 48.9; global health, 48.8 vs 51.3; pain interference, 53.0 vs 43.3 (P < .001).
The most common symptoms reported at 2 weeks were fatigue (15.9%) and low stamina/energy (9.2%); both decreased to 3.4% and 3.3%, respectively, by 6 months. The most common cardiovascular symptom at 2 weeks was palpitations (1.5%), which decreased to 0.6%.
Chest Pain Increased Over Time
Reports of chest pain, however, reportedly increased over time, with 1.3% reporting chest pain at rest at 2 weeks and 2.2% at 6 months. Although gastrointestinal symptoms were common during the acute MIS-C, only 5.3% of respondents reported those symptoms at 2 weeks.
Children in the cohort had a median age of 9 years, and 60% were men. They self-identified with the following races and ethnicities: American Indian or Alaska Native (0.1%), Asian (3.3%), Black (27.0%), Hawaiian Native or Other Pacific Islander (0.2%), Hispanic or Latino (26.9%), multiracial (2.7%), White (31.2%), other (1.0%), and unknown or refused to specify (7.6%). Authors wrote that the cohort was followed-up to 2 years after illness onset and long-term results are not yet known.
Time to Exhale
David J. Goldberg, MD, with the Cardiac Center, Children’s Hospital of Philadelphia, Pennsylvania, and colleagues, wrote in an accompanying editorial that “the decreased frequency of the disease along (with) the reassuring reports on midterm outcomes can allow the pediatric community a moment of collective exhale.”
The editorialists note that of those who initially presented with myocardial dysfunction, all but one patient evaluated had a normal ejection fraction at follow-up. Energy, sleep, appetite, cognition, and mood also normalized by midterm.
“The results of the MUSIC study add to the emerging midterm outcomes data suggesting a near-complete cardiovascular recovery in the overwhelming majority of patients who develop MIS-C,” Goldberg and colleagues wrote. “Despite initial concerns, driven by the severity of acute presentation at diagnosis and longer-term questions that remain (for example, does coronary microvascular dysfunction persist even after normalization of coronary artery z score?), these data suggest an encouraging outlook for the long-term health of affected children.”
The Centers for Disease Control and Prevention and other agencies have reported a declining overall incidence of MIS-C and highlighted the protective value of vaccination.
The editorialists add, however, that while the drop in MIS-C cases is encouraging, cases are still reported, especially amid high viral activity periods, “and nearly half of affected children continue to require intensive care in the acute phase of illness.”
Truong reported grants from the National Institutes of Health and serving as coprincipal investigator for Pfizer for research on COVID-19 vaccine-associated myocarditis funded by Pfizer and occurring through the framework of the National Heart, Lung, and Blood Institute’s Pediatric Heart Network outside the submitted work. One coauthor reported grants from Pfizer and Boston Scientific outside the submitted work. One coauthor reported receiving grants from Additional Ventures Foundation outside the submitted work. One coauthor reported receiving consultant fees from Amryt Pharma, Chiesi, Esperion, and Ultragenyx outside the submitted work. A coauthor reported receiving consultant fees from Larimar Therapeutics for mitochondrial therapies outside the submitted work. One coauthor reported being an employee of Takeda Pharmaceuticals since July 2023. One editorialist reported grants from Childhood Arthritis and Rheumatology Research Alliance and the Arthritis Foundation, Academy Health, and the Gordon and Betty Moore Foundation during the conduct of the study.
A version of this article first appeared on Medscape.com.
Children who were severely ill with multisystem inflammatory syndrome in children (MIS-C) related to COVID-19 infection appear to show excellent cardiovascular and noncardiovascular outcomes by 6 months, according to data published in JAMA Pediatrics.
MIS-C is a life-threatening complication of COVID-19 infection and data on outcomes are limited, wrote the authors, led by Dongngan T. Truong, MD, MSSI, with Children’s Healthcare of Atlanta Cardiology, Emory University School of Medicine in Atlanta, Georgia. These 6-month results are from the Long-Term Outcomes After the Multisystem Inflammatory Syndrome in Children (MUSIC) study, sponsored by the National Heart, Lung, and Blood Institute.
Researchers found in this cohort study of 1204 participants that by 6 months after hospital discharge, 99% had normalization of left ventricular systolic function, and 92.3% had normalized coronary artery dimensions. More than 95% reported being more than 90% back to baseline health.
Patient-Reported Outcomes Measurement Information Systems (PROMIS) Global Health scores were at least equivalent to prepandemic population normative values. PROMIS Global Health parent/guardian proxy median T scores for fatigue, global health, and pain interference improved significantly from 2 weeks to 6 months: fatigue, 56.1 vs 48.9; global health, 48.8 vs 51.3; pain interference, 53.0 vs 43.3 (P < .001).
The most common symptoms reported at 2 weeks were fatigue (15.9%) and low stamina/energy (9.2%); both decreased to 3.4% and 3.3%, respectively, by 6 months. The most common cardiovascular symptom at 2 weeks was palpitations (1.5%), which decreased to 0.6%.
Chest Pain Increased Over Time
Reports of chest pain, however, reportedly increased over time, with 1.3% reporting chest pain at rest at 2 weeks and 2.2% at 6 months. Although gastrointestinal symptoms were common during the acute MIS-C, only 5.3% of respondents reported those symptoms at 2 weeks.
Children in the cohort had a median age of 9 years, and 60% were men. They self-identified with the following races and ethnicities: American Indian or Alaska Native (0.1%), Asian (3.3%), Black (27.0%), Hawaiian Native or Other Pacific Islander (0.2%), Hispanic or Latino (26.9%), multiracial (2.7%), White (31.2%), other (1.0%), and unknown or refused to specify (7.6%). Authors wrote that the cohort was followed-up to 2 years after illness onset and long-term results are not yet known.
Time to Exhale
David J. Goldberg, MD, with the Cardiac Center, Children’s Hospital of Philadelphia, Pennsylvania, and colleagues, wrote in an accompanying editorial that “the decreased frequency of the disease along (with) the reassuring reports on midterm outcomes can allow the pediatric community a moment of collective exhale.”
The editorialists note that of those who initially presented with myocardial dysfunction, all but one patient evaluated had a normal ejection fraction at follow-up. Energy, sleep, appetite, cognition, and mood also normalized by midterm.
“The results of the MUSIC study add to the emerging midterm outcomes data suggesting a near-complete cardiovascular recovery in the overwhelming majority of patients who develop MIS-C,” Goldberg and colleagues wrote. “Despite initial concerns, driven by the severity of acute presentation at diagnosis and longer-term questions that remain (for example, does coronary microvascular dysfunction persist even after normalization of coronary artery z score?), these data suggest an encouraging outlook for the long-term health of affected children.”
The Centers for Disease Control and Prevention and other agencies have reported a declining overall incidence of MIS-C and highlighted the protective value of vaccination.
The editorialists add, however, that while the drop in MIS-C cases is encouraging, cases are still reported, especially amid high viral activity periods, “and nearly half of affected children continue to require intensive care in the acute phase of illness.”
Truong reported grants from the National Institutes of Health and serving as coprincipal investigator for Pfizer for research on COVID-19 vaccine-associated myocarditis funded by Pfizer and occurring through the framework of the National Heart, Lung, and Blood Institute’s Pediatric Heart Network outside the submitted work. One coauthor reported grants from Pfizer and Boston Scientific outside the submitted work. One coauthor reported receiving grants from Additional Ventures Foundation outside the submitted work. One coauthor reported receiving consultant fees from Amryt Pharma, Chiesi, Esperion, and Ultragenyx outside the submitted work. A coauthor reported receiving consultant fees from Larimar Therapeutics for mitochondrial therapies outside the submitted work. One coauthor reported being an employee of Takeda Pharmaceuticals since July 2023. One editorialist reported grants from Childhood Arthritis and Rheumatology Research Alliance and the Arthritis Foundation, Academy Health, and the Gordon and Betty Moore Foundation during the conduct of the study.
A version of this article first appeared on Medscape.com.
FROM JAMA PEDIATRICS
Both High and Low HDL Levels Linked to Increased Risk for Age-Related Macular Degeneration
TOPLINE:
This study also identified a potential novel single-nucleotide polymorphism linked to an elevated risk for the retina condition.
METHODOLOGY:
- Researchers conducted a cross-sectional retrospective analysis using data from the All of Us research program to assess the association between lipoprotein and the risk for AMD.
- They analyzed data from 2328 patients with AMD (mean age, 75.5 years; 46.7% women; 84.2% White individuals) and 5028 matched controls (mean age, 75.6 years; 52.5% women; 82.9% White individuals).
- Data were extracted for smoking status, history of hyperlipidemia, use of statins (categorized as hepatically and non-hepatically metabolized), and laboratory values for total triglyceride, low-density lipoprotein (LDL), and HDL levels.
- Data for single-nucleotide polymorphisms associated with the dysregulation of LDL and HDL metabolism were extracted using the PLINK toolkit.
TAKEAWAY:
- Both high and low HDL levels were associated with an increased risk for AMD (adjusted odds ratio [aOR], 1.28 for both; both P < .001), whereas low and high levels of triglyceride and LDL did not demonstrate a statistically significant association with the risk for AMD.
- A history of smoking and statin use showed significant associations with an increased risk for AMD (aOR, 1.30 and 1.36, respectively; both P < .001).
- Single-nucleotide polymorphisms in the genes associated with HDL metabolism, ABCA1 and LIPC, were negatively associated with the risk for AMD (aOR, 0.88; P = .04 and aOR, 0.86; P = .001, respectively).
- Lipoprotein(a) or Lp(a) was identified as a novel single nucleotide polymorphism linked to an increased risk for AMD (aOR, 1.37; P = .007).
IN PRACTICE:
“Despite conflicting evidence regarding the relationship with elevated HDL and AMD risk, this is to our knowledge the first time a U-shaped relationship with low and high HDL and AMD has been described. In fact, the presence of a U-shaped relationship may explain inconsistency in prior analyses comparing mean HDL levels in AMD and control populations,” the study authors wrote.
SOURCE:
The study was led by Jimmy S. Chen, MD, of the Viterbi Family Department of Ophthalmology and Shiley Eye Institute at the University of California, San Diego. It was published online on January 3, 2025, in Ophthalmology.
LIMITATIONS:
The study was limited by the retrospective collection and analysis of data. The use of billing codes for diagnosis extraction may have introduced documentation inaccuracies. The subgroup analysis by severity of AMD was not performed.
DISCLOSURES:
One of the authors was funded by grants from the National Eye Institute (NEI), Research to Prevent Blindness Career Development Award, Robert Machemer MD and International Retinal Research Foundation, and the UC San Diego Academic Senate. Another author reported receiving a grant from the National Heart, Lung, and Blood Institute, while a third author received funding from the National Institutes of Health (NIH), NEI, and Research to Prevent Blindness. The All of Us Research Program was supported by grants from the NIH and other sources. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
This study also identified a potential novel single-nucleotide polymorphism linked to an elevated risk for the retina condition.
METHODOLOGY:
- Researchers conducted a cross-sectional retrospective analysis using data from the All of Us research program to assess the association between lipoprotein and the risk for AMD.
- They analyzed data from 2328 patients with AMD (mean age, 75.5 years; 46.7% women; 84.2% White individuals) and 5028 matched controls (mean age, 75.6 years; 52.5% women; 82.9% White individuals).
- Data were extracted for smoking status, history of hyperlipidemia, use of statins (categorized as hepatically and non-hepatically metabolized), and laboratory values for total triglyceride, low-density lipoprotein (LDL), and HDL levels.
- Data for single-nucleotide polymorphisms associated with the dysregulation of LDL and HDL metabolism were extracted using the PLINK toolkit.
TAKEAWAY:
- Both high and low HDL levels were associated with an increased risk for AMD (adjusted odds ratio [aOR], 1.28 for both; both P < .001), whereas low and high levels of triglyceride and LDL did not demonstrate a statistically significant association with the risk for AMD.
- A history of smoking and statin use showed significant associations with an increased risk for AMD (aOR, 1.30 and 1.36, respectively; both P < .001).
- Single-nucleotide polymorphisms in the genes associated with HDL metabolism, ABCA1 and LIPC, were negatively associated with the risk for AMD (aOR, 0.88; P = .04 and aOR, 0.86; P = .001, respectively).
- Lipoprotein(a) or Lp(a) was identified as a novel single nucleotide polymorphism linked to an increased risk for AMD (aOR, 1.37; P = .007).
IN PRACTICE:
“Despite conflicting evidence regarding the relationship with elevated HDL and AMD risk, this is to our knowledge the first time a U-shaped relationship with low and high HDL and AMD has been described. In fact, the presence of a U-shaped relationship may explain inconsistency in prior analyses comparing mean HDL levels in AMD and control populations,” the study authors wrote.
SOURCE:
The study was led by Jimmy S. Chen, MD, of the Viterbi Family Department of Ophthalmology and Shiley Eye Institute at the University of California, San Diego. It was published online on January 3, 2025, in Ophthalmology.
LIMITATIONS:
The study was limited by the retrospective collection and analysis of data. The use of billing codes for diagnosis extraction may have introduced documentation inaccuracies. The subgroup analysis by severity of AMD was not performed.
DISCLOSURES:
One of the authors was funded by grants from the National Eye Institute (NEI), Research to Prevent Blindness Career Development Award, Robert Machemer MD and International Retinal Research Foundation, and the UC San Diego Academic Senate. Another author reported receiving a grant from the National Heart, Lung, and Blood Institute, while a third author received funding from the National Institutes of Health (NIH), NEI, and Research to Prevent Blindness. The All of Us Research Program was supported by grants from the NIH and other sources. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
This study also identified a potential novel single-nucleotide polymorphism linked to an elevated risk for the retina condition.
METHODOLOGY:
- Researchers conducted a cross-sectional retrospective analysis using data from the All of Us research program to assess the association between lipoprotein and the risk for AMD.
- They analyzed data from 2328 patients with AMD (mean age, 75.5 years; 46.7% women; 84.2% White individuals) and 5028 matched controls (mean age, 75.6 years; 52.5% women; 82.9% White individuals).
- Data were extracted for smoking status, history of hyperlipidemia, use of statins (categorized as hepatically and non-hepatically metabolized), and laboratory values for total triglyceride, low-density lipoprotein (LDL), and HDL levels.
- Data for single-nucleotide polymorphisms associated with the dysregulation of LDL and HDL metabolism were extracted using the PLINK toolkit.
TAKEAWAY:
- Both high and low HDL levels were associated with an increased risk for AMD (adjusted odds ratio [aOR], 1.28 for both; both P < .001), whereas low and high levels of triglyceride and LDL did not demonstrate a statistically significant association with the risk for AMD.
- A history of smoking and statin use showed significant associations with an increased risk for AMD (aOR, 1.30 and 1.36, respectively; both P < .001).
- Single-nucleotide polymorphisms in the genes associated with HDL metabolism, ABCA1 and LIPC, were negatively associated with the risk for AMD (aOR, 0.88; P = .04 and aOR, 0.86; P = .001, respectively).
- Lipoprotein(a) or Lp(a) was identified as a novel single nucleotide polymorphism linked to an increased risk for AMD (aOR, 1.37; P = .007).
IN PRACTICE:
“Despite conflicting evidence regarding the relationship with elevated HDL and AMD risk, this is to our knowledge the first time a U-shaped relationship with low and high HDL and AMD has been described. In fact, the presence of a U-shaped relationship may explain inconsistency in prior analyses comparing mean HDL levels in AMD and control populations,” the study authors wrote.
SOURCE:
The study was led by Jimmy S. Chen, MD, of the Viterbi Family Department of Ophthalmology and Shiley Eye Institute at the University of California, San Diego. It was published online on January 3, 2025, in Ophthalmology.
LIMITATIONS:
The study was limited by the retrospective collection and analysis of data. The use of billing codes for diagnosis extraction may have introduced documentation inaccuracies. The subgroup analysis by severity of AMD was not performed.
DISCLOSURES:
One of the authors was funded by grants from the National Eye Institute (NEI), Research to Prevent Blindness Career Development Award, Robert Machemer MD and International Retinal Research Foundation, and the UC San Diego Academic Senate. Another author reported receiving a grant from the National Heart, Lung, and Blood Institute, while a third author received funding from the National Institutes of Health (NIH), NEI, and Research to Prevent Blindness. The All of Us Research Program was supported by grants from the NIH and other sources. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Red Wine May Not Be a Health Tonic, But Is It a Cancer Risk?
Earlier this month, US surgeon general Vivek Murthy, MD, issued an advisory, calling for alcoholic beverages to carry a warning label about cancer risk. The advisory flagged alcohol as the third leading preventable cause of cancer in the United States, after tobacco and obesity, and highlighted people’s limited awareness about the relationship between alcohol and cancer risk.
But, when it comes to cancer risk, are all types of alcohol created equal?
For many years, red wine seemed to be an outlier, with studies indicating that, in moderation, it might even be good for you. Red wine has anti-inflammatory and antioxidant properties — most notably, it contains the antioxidant resveratrol. Starting in the 1990s, research began to hint that the compound might protect against heart disease, aging, and cancer, though much of this work was done in animals or test tubes.
The idea that red wine carries health benefits, however, has been called into question more recently. A recent meta-analysis, for instance, suggests that many previous studies touting the health benefits of more moderate drinking were likely biased, potentially leading to “misleading positive health associations.” And one recent study found that alcohol consumption, largely red wine and beer, at all levels was linked to an increased risk for cardiovascular disease.
Although wine’s health halo is dwindling, there might be an exception: Cancer risk.
Overall, research shows that even light to moderate drinking increases the risk for at least seven types of cancer, but when focusing on red wine, in particular, that risk calculus can look different.
“It’s very complicated and nuanced,” said Timothy Rebbeck, PhD, professor of cancer prevention, Harvard T.H. Chan School of Public Health, Boston. “And ‘complicated and nuanced’ doesn’t work very well in public health messages.”
The Knowns About Alcohol and Cancer Risk
Some things about the relationship between alcohol and cancer risk are crystal clear. “There’s no question that alcohol is a group 1 carcinogen,” Rebbeck said. “Alcohol can cause cancer.”
Groups including the International Agency for Research on Cancer (IARC) and American Cancer Society agree that alcohol use is an established cause of seven types of cancer: Those of the oral cavity, larynx, pharynx, esophagus (squamous cell carcinoma), liver (hepatocellular carcinoma), breast, and colon/rectum. Heavy drinking — at least 8 standard drinks a week for women and 15 for men — and binge drinking — 4 or more drinks in 2 hours for women and 5 or more for men — only amplify that risk. (A “standard” drink has 14 g of alcohol, which translates to a 5-oz glass of wine.)
“We’re most concerned about high-risk drinking — more than 2 drinks a day — and/or binge drinking,” said Noelle LoConte, MD, of the Division of Hematology, Medical Oncology and Palliative Care, University of Wisconsin School of Medicine and Public Health, Madison, who authored a 2018 statement on alcohol and cancer risk from the American Society of Clinical Oncology (ASCO).
Compared with not drinking, heavy drinking is linked with a roughly fivefold increase in the risk for oral cavity, pharyngeal, and esophageal cancers, and a 61% increase in the risk for breast cancer, according to LoConte and colleagues.
Things get murkier when it comes to moderate drinking — defined as up to 1 standard drink per day for women and 2 per day for men. There is evidence, LoConte said, that moderate drinking is associated with increased cancer risks, though the magnitude is generally much less than heavier drinking.
Cancer type also matters. One analysis found that the risk for breast cancer increased with even light to moderate alcohol consumption. Compared with no drinking, light to moderate drinking has also been linked to increased risks for oral cavity, pharynx, larynx, and esophageal cancers.
As for whether the type of alcoholic beverage matters, LoConte said, there’s no clear physiological reason that wine would be less risky than beer or liquor. Research indicates that ethanol is the problematic ingredient: Once ingested, it’s metabolized into acetaldehyde, a DNA-damaging substance that’s considered a probable human carcinogen. Ethanol can also alter circulating levels of estrogens and androgens, LoConte said, which is thought to drive its association with breast cancer risk.
“It likely doesn’t matter how you choose to get your ethanol,” she said. “It’s a question of volume.”
Hints That Wine Is an Outlier
Still, some studies suggest that how people ingest ethanol could make a difference.
A study published in August in JAMA Network Open is a case in point. The study found that, among older adults, light to heavy drinkers had an increased risk of dying from cancer, compared with occasional drinkers (though the increased risk among light to moderate drinkers occurred only among people who also had chronic health conditions, such as diabetes or high blood pressure, or were of lower socioeconomic status).
Wine drinkers fared differently. Most notably, drinkers who “preferred” wine — consuming over 80% of total ethanol from wine — or those who drank only with meals showed a small reduction in their risk for cancer mortality and all-cause mortality (hazard ratio [HR], 0.94 for both). The small protective association was somewhat stronger among people who reported both patterns (HR, 0.88), especially if they were of lower socioeconomic status (HR, 0.79).
The findings are in line with other research suggesting that wine drinkers may be outliers when it comes to cancer risk. A 2023 meta-analysis of 26 observational studies, for instance, found no association between wine consumption and any cancer type, with the caveat that there was «substantial» heterogeneity among the studies.
This heterogeneity caveat speaks to the inherent limitations of observational research, said Tim Stockwell, PhD, of the Canadian Institute for Substance Use Research, University of Victoria in British Columbia, Canada.
“Individual studies of alcohol and cancer risk do find differences by type of drink, or patterns of drinking,” Stockwell said. “But it’s so hard to unpack the confounding that goes along with the type of person who’s a wine drinker or a beer drinker or a spirit drinker. The beverage of choice seems to come with a lot of baggage.”
Compared with people who favor beer or liquor, he noted, wine aficionados are typically higher-income, exercise more often, smoke less, and have different diets, for example. The “best” studies, Rebbeck said, try to adjust for those differences, but it’s challenging.
The authors of the 2023 meta-analysis noted that “many components in wine could have anticarcinogenic effects” that theoretically could counter the ill effects of ethanol. Besides resveratrol, which is mainly found in red wine, the list includes anthocyanins, quercetin, and tannins. However, the authors also acknowledged that they couldn’t account for whether other lifestyle habits might explain why wine drinkers, overall, showed no increased cancer risks and sometimes lower risks.
Still, groups such as the IARC and ASCO hold that there is no known “safe” level, or type, of alcohol when it comes to cancer.
In the latest Canadian guidelines on alcohol use, the scientific panel calculated that people who have 6 drinks a week throughout adulthood (whatever the source of the alcohol) could shave 11 weeks from their life expectancy, on average, said Stockwell, who was on the guideline panel. Compare that with heavy drinking, where 4 drinks a day could rob the average person of 2 or 3 years. “If you’re drinking a lot, you could get huge benefits from cutting down,” Stockwell explained. “If you’re a moderate drinker, the benefits would obviously be less.”
Stockwell said that choices around drinking and breast cancer risk, specifically, can be “tough.” Unlike many of the other alcohol-associated cancers, he noted, breast cancer is common — so even small relative risk increases may be concerning. Based on a 2020 meta-analysis of 22 cohort studies, the risk for breast cancer rises by about 10%, on average, for every 10 g of alcohol a woman drinks per day. This study also found no evidence that wine is any different from other types of alcohol.
In real life, the calculus around wine consumption and cancer risk will probably vary widely from person to person, Rebbeck said. One woman with a family history of breast cancer might decide that having wine with dinner isn’t worth it. Another with the same family history might see that glass of wine as a stress reliever and opt to focus on other ways to reduce her breast cancer risk — by exercising and maintaining a healthy weight, for example.
“The bottom line is, in human studies, the data on light to moderate drinking and cancer are limited and messy, and you can’t draw firm conclusions from them,” Rebbeck said. “It probably raises risk in some people, but we don’t know who those people are. And the risk increases are relatively small.”
A Conversation Few Are Having
Even with many studies highlighting the connection between alcohol consumption and cancer risk, most people remain unaware about this risk.
A 2023 study by the National Cancer Institute found that only a minority of US adults knew that drinking alcohol is linked to increased cancer risk, and they were much less likely to say that was true of wine: Only 20% did, vs 31% who said that liquor can boost cancer risk. Meanwhile, 10% believed that wine helps prevent cancer. Other studies show that even among cancer survivors and patients undergoing active cancer treatment, many drink — often heavily.
“What we know right now is, physicians almost never talk about this,” LoConte said.
That could be due to time constraints, according to Rebbeck, or clinicians’ perceptions that the subject is too complicated and/or their own confusion about the data. There could also be some “cognitive dissonance” at play, LoConte noted, because many doctors drink alcohol.
It’s critical, she said, that conversations about drinking habits become “normalized,” and that should include informing patients that alcohol use is associated with certain cancers. Again, LoConte said, it’s high-risk drinking that’s most concerning and where reducing intake could have the biggest impact on cancer risk and other health outcomes.
“From a cancer prevention standpoint, it’s probably best not to drink,” she said. “But people don’t make choices based solely on cancer risk. We don’t want to come out with recommendations saying no one should drink. I don’t think the data support that, and people would buck against that advice.”
Rebbeck made a similar point. Even if there’s uncertainty about the risks for a daily glass of wine, he said, people can use that information to make decisions. “Everybody’s preferences and choices are going to be different,” Rebbeck said. “And that’s all we can really do.”
A version of this article appeared on Medscape.com.
Earlier this month, US surgeon general Vivek Murthy, MD, issued an advisory, calling for alcoholic beverages to carry a warning label about cancer risk. The advisory flagged alcohol as the third leading preventable cause of cancer in the United States, after tobacco and obesity, and highlighted people’s limited awareness about the relationship between alcohol and cancer risk.
But, when it comes to cancer risk, are all types of alcohol created equal?
For many years, red wine seemed to be an outlier, with studies indicating that, in moderation, it might even be good for you. Red wine has anti-inflammatory and antioxidant properties — most notably, it contains the antioxidant resveratrol. Starting in the 1990s, research began to hint that the compound might protect against heart disease, aging, and cancer, though much of this work was done in animals or test tubes.
The idea that red wine carries health benefits, however, has been called into question more recently. A recent meta-analysis, for instance, suggests that many previous studies touting the health benefits of more moderate drinking were likely biased, potentially leading to “misleading positive health associations.” And one recent study found that alcohol consumption, largely red wine and beer, at all levels was linked to an increased risk for cardiovascular disease.
Although wine’s health halo is dwindling, there might be an exception: Cancer risk.
Overall, research shows that even light to moderate drinking increases the risk for at least seven types of cancer, but when focusing on red wine, in particular, that risk calculus can look different.
“It’s very complicated and nuanced,” said Timothy Rebbeck, PhD, professor of cancer prevention, Harvard T.H. Chan School of Public Health, Boston. “And ‘complicated and nuanced’ doesn’t work very well in public health messages.”
The Knowns About Alcohol and Cancer Risk
Some things about the relationship between alcohol and cancer risk are crystal clear. “There’s no question that alcohol is a group 1 carcinogen,” Rebbeck said. “Alcohol can cause cancer.”
Groups including the International Agency for Research on Cancer (IARC) and American Cancer Society agree that alcohol use is an established cause of seven types of cancer: Those of the oral cavity, larynx, pharynx, esophagus (squamous cell carcinoma), liver (hepatocellular carcinoma), breast, and colon/rectum. Heavy drinking — at least 8 standard drinks a week for women and 15 for men — and binge drinking — 4 or more drinks in 2 hours for women and 5 or more for men — only amplify that risk. (A “standard” drink has 14 g of alcohol, which translates to a 5-oz glass of wine.)
“We’re most concerned about high-risk drinking — more than 2 drinks a day — and/or binge drinking,” said Noelle LoConte, MD, of the Division of Hematology, Medical Oncology and Palliative Care, University of Wisconsin School of Medicine and Public Health, Madison, who authored a 2018 statement on alcohol and cancer risk from the American Society of Clinical Oncology (ASCO).
Compared with not drinking, heavy drinking is linked with a roughly fivefold increase in the risk for oral cavity, pharyngeal, and esophageal cancers, and a 61% increase in the risk for breast cancer, according to LoConte and colleagues.
Things get murkier when it comes to moderate drinking — defined as up to 1 standard drink per day for women and 2 per day for men. There is evidence, LoConte said, that moderate drinking is associated with increased cancer risks, though the magnitude is generally much less than heavier drinking.
Cancer type also matters. One analysis found that the risk for breast cancer increased with even light to moderate alcohol consumption. Compared with no drinking, light to moderate drinking has also been linked to increased risks for oral cavity, pharynx, larynx, and esophageal cancers.
As for whether the type of alcoholic beverage matters, LoConte said, there’s no clear physiological reason that wine would be less risky than beer or liquor. Research indicates that ethanol is the problematic ingredient: Once ingested, it’s metabolized into acetaldehyde, a DNA-damaging substance that’s considered a probable human carcinogen. Ethanol can also alter circulating levels of estrogens and androgens, LoConte said, which is thought to drive its association with breast cancer risk.
“It likely doesn’t matter how you choose to get your ethanol,” she said. “It’s a question of volume.”
Hints That Wine Is an Outlier
Still, some studies suggest that how people ingest ethanol could make a difference.
A study published in August in JAMA Network Open is a case in point. The study found that, among older adults, light to heavy drinkers had an increased risk of dying from cancer, compared with occasional drinkers (though the increased risk among light to moderate drinkers occurred only among people who also had chronic health conditions, such as diabetes or high blood pressure, or were of lower socioeconomic status).
Wine drinkers fared differently. Most notably, drinkers who “preferred” wine — consuming over 80% of total ethanol from wine — or those who drank only with meals showed a small reduction in their risk for cancer mortality and all-cause mortality (hazard ratio [HR], 0.94 for both). The small protective association was somewhat stronger among people who reported both patterns (HR, 0.88), especially if they were of lower socioeconomic status (HR, 0.79).
The findings are in line with other research suggesting that wine drinkers may be outliers when it comes to cancer risk. A 2023 meta-analysis of 26 observational studies, for instance, found no association between wine consumption and any cancer type, with the caveat that there was «substantial» heterogeneity among the studies.
This heterogeneity caveat speaks to the inherent limitations of observational research, said Tim Stockwell, PhD, of the Canadian Institute for Substance Use Research, University of Victoria in British Columbia, Canada.
“Individual studies of alcohol and cancer risk do find differences by type of drink, or patterns of drinking,” Stockwell said. “But it’s so hard to unpack the confounding that goes along with the type of person who’s a wine drinker or a beer drinker or a spirit drinker. The beverage of choice seems to come with a lot of baggage.”
Compared with people who favor beer or liquor, he noted, wine aficionados are typically higher-income, exercise more often, smoke less, and have different diets, for example. The “best” studies, Rebbeck said, try to adjust for those differences, but it’s challenging.
The authors of the 2023 meta-analysis noted that “many components in wine could have anticarcinogenic effects” that theoretically could counter the ill effects of ethanol. Besides resveratrol, which is mainly found in red wine, the list includes anthocyanins, quercetin, and tannins. However, the authors also acknowledged that they couldn’t account for whether other lifestyle habits might explain why wine drinkers, overall, showed no increased cancer risks and sometimes lower risks.
Still, groups such as the IARC and ASCO hold that there is no known “safe” level, or type, of alcohol when it comes to cancer.
In the latest Canadian guidelines on alcohol use, the scientific panel calculated that people who have 6 drinks a week throughout adulthood (whatever the source of the alcohol) could shave 11 weeks from their life expectancy, on average, said Stockwell, who was on the guideline panel. Compare that with heavy drinking, where 4 drinks a day could rob the average person of 2 or 3 years. “If you’re drinking a lot, you could get huge benefits from cutting down,” Stockwell explained. “If you’re a moderate drinker, the benefits would obviously be less.”
Stockwell said that choices around drinking and breast cancer risk, specifically, can be “tough.” Unlike many of the other alcohol-associated cancers, he noted, breast cancer is common — so even small relative risk increases may be concerning. Based on a 2020 meta-analysis of 22 cohort studies, the risk for breast cancer rises by about 10%, on average, for every 10 g of alcohol a woman drinks per day. This study also found no evidence that wine is any different from other types of alcohol.
In real life, the calculus around wine consumption and cancer risk will probably vary widely from person to person, Rebbeck said. One woman with a family history of breast cancer might decide that having wine with dinner isn’t worth it. Another with the same family history might see that glass of wine as a stress reliever and opt to focus on other ways to reduce her breast cancer risk — by exercising and maintaining a healthy weight, for example.
“The bottom line is, in human studies, the data on light to moderate drinking and cancer are limited and messy, and you can’t draw firm conclusions from them,” Rebbeck said. “It probably raises risk in some people, but we don’t know who those people are. And the risk increases are relatively small.”
A Conversation Few Are Having
Even with many studies highlighting the connection between alcohol consumption and cancer risk, most people remain unaware about this risk.
A 2023 study by the National Cancer Institute found that only a minority of US adults knew that drinking alcohol is linked to increased cancer risk, and they were much less likely to say that was true of wine: Only 20% did, vs 31% who said that liquor can boost cancer risk. Meanwhile, 10% believed that wine helps prevent cancer. Other studies show that even among cancer survivors and patients undergoing active cancer treatment, many drink — often heavily.
“What we know right now is, physicians almost never talk about this,” LoConte said.
That could be due to time constraints, according to Rebbeck, or clinicians’ perceptions that the subject is too complicated and/or their own confusion about the data. There could also be some “cognitive dissonance” at play, LoConte noted, because many doctors drink alcohol.
It’s critical, she said, that conversations about drinking habits become “normalized,” and that should include informing patients that alcohol use is associated with certain cancers. Again, LoConte said, it’s high-risk drinking that’s most concerning and where reducing intake could have the biggest impact on cancer risk and other health outcomes.
“From a cancer prevention standpoint, it’s probably best not to drink,” she said. “But people don’t make choices based solely on cancer risk. We don’t want to come out with recommendations saying no one should drink. I don’t think the data support that, and people would buck against that advice.”
Rebbeck made a similar point. Even if there’s uncertainty about the risks for a daily glass of wine, he said, people can use that information to make decisions. “Everybody’s preferences and choices are going to be different,” Rebbeck said. “And that’s all we can really do.”
A version of this article appeared on Medscape.com.
Earlier this month, US surgeon general Vivek Murthy, MD, issued an advisory, calling for alcoholic beverages to carry a warning label about cancer risk. The advisory flagged alcohol as the third leading preventable cause of cancer in the United States, after tobacco and obesity, and highlighted people’s limited awareness about the relationship between alcohol and cancer risk.
But, when it comes to cancer risk, are all types of alcohol created equal?
For many years, red wine seemed to be an outlier, with studies indicating that, in moderation, it might even be good for you. Red wine has anti-inflammatory and antioxidant properties — most notably, it contains the antioxidant resveratrol. Starting in the 1990s, research began to hint that the compound might protect against heart disease, aging, and cancer, though much of this work was done in animals or test tubes.
The idea that red wine carries health benefits, however, has been called into question more recently. A recent meta-analysis, for instance, suggests that many previous studies touting the health benefits of more moderate drinking were likely biased, potentially leading to “misleading positive health associations.” And one recent study found that alcohol consumption, largely red wine and beer, at all levels was linked to an increased risk for cardiovascular disease.
Although wine’s health halo is dwindling, there might be an exception: Cancer risk.
Overall, research shows that even light to moderate drinking increases the risk for at least seven types of cancer, but when focusing on red wine, in particular, that risk calculus can look different.
“It’s very complicated and nuanced,” said Timothy Rebbeck, PhD, professor of cancer prevention, Harvard T.H. Chan School of Public Health, Boston. “And ‘complicated and nuanced’ doesn’t work very well in public health messages.”
The Knowns About Alcohol and Cancer Risk
Some things about the relationship between alcohol and cancer risk are crystal clear. “There’s no question that alcohol is a group 1 carcinogen,” Rebbeck said. “Alcohol can cause cancer.”
Groups including the International Agency for Research on Cancer (IARC) and American Cancer Society agree that alcohol use is an established cause of seven types of cancer: Those of the oral cavity, larynx, pharynx, esophagus (squamous cell carcinoma), liver (hepatocellular carcinoma), breast, and colon/rectum. Heavy drinking — at least 8 standard drinks a week for women and 15 for men — and binge drinking — 4 or more drinks in 2 hours for women and 5 or more for men — only amplify that risk. (A “standard” drink has 14 g of alcohol, which translates to a 5-oz glass of wine.)
“We’re most concerned about high-risk drinking — more than 2 drinks a day — and/or binge drinking,” said Noelle LoConte, MD, of the Division of Hematology, Medical Oncology and Palliative Care, University of Wisconsin School of Medicine and Public Health, Madison, who authored a 2018 statement on alcohol and cancer risk from the American Society of Clinical Oncology (ASCO).
Compared with not drinking, heavy drinking is linked with a roughly fivefold increase in the risk for oral cavity, pharyngeal, and esophageal cancers, and a 61% increase in the risk for breast cancer, according to LoConte and colleagues.
Things get murkier when it comes to moderate drinking — defined as up to 1 standard drink per day for women and 2 per day for men. There is evidence, LoConte said, that moderate drinking is associated with increased cancer risks, though the magnitude is generally much less than heavier drinking.
Cancer type also matters. One analysis found that the risk for breast cancer increased with even light to moderate alcohol consumption. Compared with no drinking, light to moderate drinking has also been linked to increased risks for oral cavity, pharynx, larynx, and esophageal cancers.
As for whether the type of alcoholic beverage matters, LoConte said, there’s no clear physiological reason that wine would be less risky than beer or liquor. Research indicates that ethanol is the problematic ingredient: Once ingested, it’s metabolized into acetaldehyde, a DNA-damaging substance that’s considered a probable human carcinogen. Ethanol can also alter circulating levels of estrogens and androgens, LoConte said, which is thought to drive its association with breast cancer risk.
“It likely doesn’t matter how you choose to get your ethanol,” she said. “It’s a question of volume.”
Hints That Wine Is an Outlier
Still, some studies suggest that how people ingest ethanol could make a difference.
A study published in August in JAMA Network Open is a case in point. The study found that, among older adults, light to heavy drinkers had an increased risk of dying from cancer, compared with occasional drinkers (though the increased risk among light to moderate drinkers occurred only among people who also had chronic health conditions, such as diabetes or high blood pressure, or were of lower socioeconomic status).
Wine drinkers fared differently. Most notably, drinkers who “preferred” wine — consuming over 80% of total ethanol from wine — or those who drank only with meals showed a small reduction in their risk for cancer mortality and all-cause mortality (hazard ratio [HR], 0.94 for both). The small protective association was somewhat stronger among people who reported both patterns (HR, 0.88), especially if they were of lower socioeconomic status (HR, 0.79).
The findings are in line with other research suggesting that wine drinkers may be outliers when it comes to cancer risk. A 2023 meta-analysis of 26 observational studies, for instance, found no association between wine consumption and any cancer type, with the caveat that there was «substantial» heterogeneity among the studies.
This heterogeneity caveat speaks to the inherent limitations of observational research, said Tim Stockwell, PhD, of the Canadian Institute for Substance Use Research, University of Victoria in British Columbia, Canada.
“Individual studies of alcohol and cancer risk do find differences by type of drink, or patterns of drinking,” Stockwell said. “But it’s so hard to unpack the confounding that goes along with the type of person who’s a wine drinker or a beer drinker or a spirit drinker. The beverage of choice seems to come with a lot of baggage.”
Compared with people who favor beer or liquor, he noted, wine aficionados are typically higher-income, exercise more often, smoke less, and have different diets, for example. The “best” studies, Rebbeck said, try to adjust for those differences, but it’s challenging.
The authors of the 2023 meta-analysis noted that “many components in wine could have anticarcinogenic effects” that theoretically could counter the ill effects of ethanol. Besides resveratrol, which is mainly found in red wine, the list includes anthocyanins, quercetin, and tannins. However, the authors also acknowledged that they couldn’t account for whether other lifestyle habits might explain why wine drinkers, overall, showed no increased cancer risks and sometimes lower risks.
Still, groups such as the IARC and ASCO hold that there is no known “safe” level, or type, of alcohol when it comes to cancer.
In the latest Canadian guidelines on alcohol use, the scientific panel calculated that people who have 6 drinks a week throughout adulthood (whatever the source of the alcohol) could shave 11 weeks from their life expectancy, on average, said Stockwell, who was on the guideline panel. Compare that with heavy drinking, where 4 drinks a day could rob the average person of 2 or 3 years. “If you’re drinking a lot, you could get huge benefits from cutting down,” Stockwell explained. “If you’re a moderate drinker, the benefits would obviously be less.”
Stockwell said that choices around drinking and breast cancer risk, specifically, can be “tough.” Unlike many of the other alcohol-associated cancers, he noted, breast cancer is common — so even small relative risk increases may be concerning. Based on a 2020 meta-analysis of 22 cohort studies, the risk for breast cancer rises by about 10%, on average, for every 10 g of alcohol a woman drinks per day. This study also found no evidence that wine is any different from other types of alcohol.
In real life, the calculus around wine consumption and cancer risk will probably vary widely from person to person, Rebbeck said. One woman with a family history of breast cancer might decide that having wine with dinner isn’t worth it. Another with the same family history might see that glass of wine as a stress reliever and opt to focus on other ways to reduce her breast cancer risk — by exercising and maintaining a healthy weight, for example.
“The bottom line is, in human studies, the data on light to moderate drinking and cancer are limited and messy, and you can’t draw firm conclusions from them,” Rebbeck said. “It probably raises risk in some people, but we don’t know who those people are. And the risk increases are relatively small.”
A Conversation Few Are Having
Even with many studies highlighting the connection between alcohol consumption and cancer risk, most people remain unaware about this risk.
A 2023 study by the National Cancer Institute found that only a minority of US adults knew that drinking alcohol is linked to increased cancer risk, and they were much less likely to say that was true of wine: Only 20% did, vs 31% who said that liquor can boost cancer risk. Meanwhile, 10% believed that wine helps prevent cancer. Other studies show that even among cancer survivors and patients undergoing active cancer treatment, many drink — often heavily.
“What we know right now is, physicians almost never talk about this,” LoConte said.
That could be due to time constraints, according to Rebbeck, or clinicians’ perceptions that the subject is too complicated and/or their own confusion about the data. There could also be some “cognitive dissonance” at play, LoConte noted, because many doctors drink alcohol.
It’s critical, she said, that conversations about drinking habits become “normalized,” and that should include informing patients that alcohol use is associated with certain cancers. Again, LoConte said, it’s high-risk drinking that’s most concerning and where reducing intake could have the biggest impact on cancer risk and other health outcomes.
“From a cancer prevention standpoint, it’s probably best not to drink,” she said. “But people don’t make choices based solely on cancer risk. We don’t want to come out with recommendations saying no one should drink. I don’t think the data support that, and people would buck against that advice.”
Rebbeck made a similar point. Even if there’s uncertainty about the risks for a daily glass of wine, he said, people can use that information to make decisions. “Everybody’s preferences and choices are going to be different,” Rebbeck said. “And that’s all we can really do.”
A version of this article appeared on Medscape.com.
Nutrition, Drugs, or Bariatric Surgery: What’s the Best Approach for Sustained Weight Loss?
Given that more than 100 million US adults have obesity, including 22 million with severe obesity, physicians regularly see patients with the condition in their practices.
Fortunately, doctors have more tools than ever to help their patients. But the question remains: Which method is the safest and most effective? Is it diet and lifestyle changes, one of the recently approved anti-obesity medications (AOMs), bariatric surgery, or a combination approach?
There are no head-to-head trials comparing these three approaches, said Vanita Rahman, MD, clinic director of the Barnard Medical Center, Washington, DC, at the International Conference on Nutrition in Medicine, sponsored by the Physicians Committee for Responsible Medicine.
Instead, doctors must evaluate the merits and drawbacks of each intervention and decide with their patients which treatment is best for them, she told Medscape Medical News. When she sees patients, Rahman shares the pertinent research with them, so they are able to make an informed choice.
Looking at the Options
In her presentation at the conference, Rahman summarized the guidelines issued by the American Heart Association/American College of Cardiology/The Obesity Society for Management of Overweight and Obesity in Adults and the American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines For Medical Care of Patients with Obesity, including lifestyle changes, AOMs, and bariatric surgery (Table 1).

As shown, the current clinical guidelines offer recommendations that consider such factors as the patient’s BMI and presence of one or more comorbidities. Generally, they begin with lifestyle changes for people with overweight, the possibility of an AOM for those with obesity, and bariatric surgery as an option for those with severe obesity-related complications.
“In obesity, we traditionally thought the process was ‘either-or’ — either lifestyle or surgery or medication — and somehow lifestyle is better,” Sheethal Reddy, PhD, a psychologist at the Bariatric Center at Emory University Hospital Midtown, Atlanta, told Medscape Medical News.
Now physicians often use a combination of methods, but lifestyle is foundational to all of them, she said.
“If you don’t make lifestyle changes, none of the approaches will ultimately be effective,” said Reddy, who also is an assistant professor in the Division of General and GI Surgery at Emory School of Medicine, Atlanta.
Lifestyle changes don’t just involve diet and nutrition but include physical exercise.
“Being sedentary affects everything — sleep quality, appetite regulation, and metabolism. Without sufficient exercise, the body isn’t functioning well enough to have a healthy metabolism,” Reddy said.
How Durable Are the Interventions?
Although bariatric surgery has demonstrated effectiveness in helping patients lose weight, many of them regain some or most of it, Rahman said.
A systematic review and meta-analysis found weight regain in 49% of patients who underwent bariatric surgery patients, with the highest prevalence after Roux-en-Y gastric bypass.
Another study of approximately 45,000 patients who underwent bariatric surgery found differences not only in the percentage of total weight loss among Roux-en-Y gastric bypass, sleeve gastrectomy, and adjustable gastric band procedures but also in how much of that weight stayed off between 1 and 5 years following the procedure (Table 2).

Weight regain also is a risk with AOMs, if they’re discontinued.
The STEP 1 trial tested the effectiveness of semaglutide — a glucagon-like peptide 1 (GLP-1) receptor agonist — as an adjunct to lifestyle intervention for weight loss in patients with obesity or with overweight and at least one comorbidity but not diabetes. Mean weight loss with semaglutide was 17.3% but that figure dropped 11.6 percentage points after treatment was discontinued.
Other studies also have found that patients regain weight after GLP-1 discontinuation.
Tirzepatide, a GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) combination, has shown efficacy with weight reduction, but patients experienced some weight regain upon discontinuation. In one study, patients experienced a mean weight loss of 20.9% after 36 weeks of tirzepatide. In the study’s subsequent 52-week double-blind, placebo-controlled period, patients who stopped taking the medication experienced a weight regain of 14%, whereas those who remained on the medication lost an additional 5.5% of weight.
GLP-1 and GLP-1/GIP medications do not address the factors that contribute to overweight and obesity, Rahman said. “They simply suppress the appetite; therefore, weight gain occurs after stopping them.”
Patients may stop taking anti-obesity drugs for a variety of reasons, including side effects. Rahman noted that the common side effects include nausea, vomiting, and constipation, whereas rare side effects include gastroparesis, gallbladder and biliary disease, thyroid cancer, and suicidal thoughts. GLP-1 and GLP-1/GIP medications also carry a risk for non-arteritic anterior ischemic optic neuropathy, she said.
Moreover, health insurance does not always cover these medications, which likely affects patient access to the drugs and compliance rates.
“Given the side effects and frequent lack of insurance coverage, significant questions remain about long-term safety and feasibility of these agents,” Rahman said.
What About Nutritional Approaches?
The lifestyle interventions in the semaglutide and tirzepatide studies included 500 kcal/d deficit diets, which is difficult for people to maintain, noted Rahman, who is the author of the book Simply Plant Based: Fabulous Food for a Healthy Life.
Additionally, bariatric surgery has been associated with long-term micronutrient deficiencies, including deficiencies in vitamins A, D, E, K, B1, and B12, as well as folate, iron, zinc, copper, selenium, and calcium, she said.
The best approach to food from a patient compliance standpoint and to avoid nutrient deficiencies is a whole-food, plant-based diet, Rahman said. She advocates this nutritional approach, along with physical activity, for patients regardless of whether they’ve selected lifestyle intervention alone or combined with an AOM or bariatric surgery to address obesity.
Rahman cited a 5-year heart disease study comparing an intensive lifestyle program involving a vegetarian diet, aerobic exercise, stress management training, smoking cessation, and group psychosocial support to treatment as usual. Patients in the lifestyle group lost 10.9 kg at 1 year and sustained weight loss of 5.8 kg at 5 years, whereas weight in the control group remained relatively unchanged from baseline.
She also pointed to the findings of a study of patients with obesity or with overweight and at least one comorbidity that compared standard care with a low-fat, whole-food, plant-based diet with vitamin B12 supplementation. At 6 months, mean BMI reduction was greater in the intervention group than the standard care group (−4.4 vs −0.4).
In her practice, Rahman has seen the benefits of a whole-food, plant-based diet for patients with obesity.
If people are committed to this type of dietary approach and are given the tools and resources to do it effectively, “their thinking changes, their taste buds change, and they grow to enjoy this new way of eating,” she said. “They see results, and it’s a lifestyle that can be sustained long-term.”
Addressing Drivers of Weight Gain
Patients also need help addressing the various factors that may contribute to overweight and obesity, including overconsumption of ultra-processed foods, substandard nutritional quality of restaurant foods, increasing portion sizes, distraction during eating, emotional eating, late-night eating, and cultural/traditional values surrounding food, Rahman noted.
Supatra Tovar, PsyD, RD, a clinical psychologist with a practice in Pasadena, California, agreed that identifying the reasons for weight gain is critical for treatment.
“If you’re not addressing underlying issues, such as a person’s relationship with food, behaviors around food, the tendency to mindlessly eat or emotionally eat or eat to seek comfort, the person’s weight problems won’t ultimately be fully solved by any of the three approaches — dieting, medications, or bariatric surgery,” she said.
Some of her patients “engage in extreme dieting and deprivation, and many who use medications or have had bariatric surgery hardly eat and often develop nutritional deficiencies,” said Tovar, author of the book Deprogram Diet Culture: Rethink Your Relationship with Food, Heal Your Mind, and Live a Diet-Free Life.
The key to healthy and sustained weight loss is to “become attuned to the body’s signals, learn how to honor hunger, stop eating when satisfied, and eat more healthful foods, such as fruits and vegetables, whole grains, lean proteins — especially plant-based proteins — and the body gives signals that this is what it wants,” she said.
Tovar doesn’t give her clients a specific diet or set of portions.
“I teach them to listen to their bodies,” she said. “They’ve lost significant amounts of weight and continued to keep it off because they’ve done this kind of work.”
When Lifestyle Changes Aren’t Enough
For many patients, lifestyle interventions are insufficient to address the degree of overweight and obesity and common comorbidities, said W. Timothy Garvey, MD, associate director and professor, Department of Nutrition Sciences, School of Health Professions, University of Alabama at Birmingham.
“Of course, nutritional approaches are very important, not only for weight but also for general health-related reasons,” said Garvey, lead author of the 2016 American Association of Clinical Endocrinologists obesity guidelines. “We’ve seen that the Mediterranean and some plant-based diets can prevent progression from prediabetes to diabetes and improve other parameters that reflect metabolic health.”
However, it’s “not common that patients can follow these diets, lose weight, and keep it off,” Garvey cautioned. Up to 50% of weight that’s lost through lifestyle changes is typically regained by 1-year follow-up, with almost all remaining lost weight subsequently regained in the majority of individuals because the person “has to fight against pathophysiological process that drive weight regain,” he noted.
Weight-loss medications can address these pathophysiologic processes by “addressing interactions of satiety hormones with feeding centers in the brain, suppressing the appetite, and making it easier for patients to adhere to a reduced-calorie diet.”
Garvey views the weight-loss medications in the same light as drugs for diabetes and hypertension, in that people need to keep taking them to sustain the benefit.
There’s still a role for bariatric surgery because not everyone can tolerate the AOMs or achieve sufficient weight loss.
“Patients with very high BMI who have trouble ambulating might benefit from a combination of bariatric surgery and medication,” Garvey said.
While some side effects are associated with AOMs, being an “alarmist” about them can be detrimental to patients, he warned.
Rahman and Tovar are authors of books about weight loss. Reddy reported no relevant financial relationships. Garvey is a consultant on advisory boards for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Pfizer, Fractyl Health, Alnylam Pharmaceuticals, Inogen, Zealand, Allurion, Carmot/Roche, Terns Pharmaceuticals, Neurocrine, Keros Therapeutics, and Regeneron. He is the site principal investigator for multi-centered clinical trials sponsored by his university and funded by Novo Nordisk, Eli Lilly, Epitomee, Neurovalens, and Pfizer. He serves as a consultant on the advisory board for the nonprofit Milken Foundation and is a member of the Data Monitoring Committee for phase 3 clinical trials conducted by Boehringer-Ingelheim and Eli Lilly.
A version of this article first appeared on Medscape.com.
Given that more than 100 million US adults have obesity, including 22 million with severe obesity, physicians regularly see patients with the condition in their practices.
Fortunately, doctors have more tools than ever to help their patients. But the question remains: Which method is the safest and most effective? Is it diet and lifestyle changes, one of the recently approved anti-obesity medications (AOMs), bariatric surgery, or a combination approach?
There are no head-to-head trials comparing these three approaches, said Vanita Rahman, MD, clinic director of the Barnard Medical Center, Washington, DC, at the International Conference on Nutrition in Medicine, sponsored by the Physicians Committee for Responsible Medicine.
Instead, doctors must evaluate the merits and drawbacks of each intervention and decide with their patients which treatment is best for them, she told Medscape Medical News. When she sees patients, Rahman shares the pertinent research with them, so they are able to make an informed choice.
Looking at the Options
In her presentation at the conference, Rahman summarized the guidelines issued by the American Heart Association/American College of Cardiology/The Obesity Society for Management of Overweight and Obesity in Adults and the American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines For Medical Care of Patients with Obesity, including lifestyle changes, AOMs, and bariatric surgery (Table 1).

As shown, the current clinical guidelines offer recommendations that consider such factors as the patient’s BMI and presence of one or more comorbidities. Generally, they begin with lifestyle changes for people with overweight, the possibility of an AOM for those with obesity, and bariatric surgery as an option for those with severe obesity-related complications.
“In obesity, we traditionally thought the process was ‘either-or’ — either lifestyle or surgery or medication — and somehow lifestyle is better,” Sheethal Reddy, PhD, a psychologist at the Bariatric Center at Emory University Hospital Midtown, Atlanta, told Medscape Medical News.
Now physicians often use a combination of methods, but lifestyle is foundational to all of them, she said.
“If you don’t make lifestyle changes, none of the approaches will ultimately be effective,” said Reddy, who also is an assistant professor in the Division of General and GI Surgery at Emory School of Medicine, Atlanta.
Lifestyle changes don’t just involve diet and nutrition but include physical exercise.
“Being sedentary affects everything — sleep quality, appetite regulation, and metabolism. Without sufficient exercise, the body isn’t functioning well enough to have a healthy metabolism,” Reddy said.
How Durable Are the Interventions?
Although bariatric surgery has demonstrated effectiveness in helping patients lose weight, many of them regain some or most of it, Rahman said.
A systematic review and meta-analysis found weight regain in 49% of patients who underwent bariatric surgery patients, with the highest prevalence after Roux-en-Y gastric bypass.
Another study of approximately 45,000 patients who underwent bariatric surgery found differences not only in the percentage of total weight loss among Roux-en-Y gastric bypass, sleeve gastrectomy, and adjustable gastric band procedures but also in how much of that weight stayed off between 1 and 5 years following the procedure (Table 2).

Weight regain also is a risk with AOMs, if they’re discontinued.
The STEP 1 trial tested the effectiveness of semaglutide — a glucagon-like peptide 1 (GLP-1) receptor agonist — as an adjunct to lifestyle intervention for weight loss in patients with obesity or with overweight and at least one comorbidity but not diabetes. Mean weight loss with semaglutide was 17.3% but that figure dropped 11.6 percentage points after treatment was discontinued.
Other studies also have found that patients regain weight after GLP-1 discontinuation.
Tirzepatide, a GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) combination, has shown efficacy with weight reduction, but patients experienced some weight regain upon discontinuation. In one study, patients experienced a mean weight loss of 20.9% after 36 weeks of tirzepatide. In the study’s subsequent 52-week double-blind, placebo-controlled period, patients who stopped taking the medication experienced a weight regain of 14%, whereas those who remained on the medication lost an additional 5.5% of weight.
GLP-1 and GLP-1/GIP medications do not address the factors that contribute to overweight and obesity, Rahman said. “They simply suppress the appetite; therefore, weight gain occurs after stopping them.”
Patients may stop taking anti-obesity drugs for a variety of reasons, including side effects. Rahman noted that the common side effects include nausea, vomiting, and constipation, whereas rare side effects include gastroparesis, gallbladder and biliary disease, thyroid cancer, and suicidal thoughts. GLP-1 and GLP-1/GIP medications also carry a risk for non-arteritic anterior ischemic optic neuropathy, she said.
Moreover, health insurance does not always cover these medications, which likely affects patient access to the drugs and compliance rates.
“Given the side effects and frequent lack of insurance coverage, significant questions remain about long-term safety and feasibility of these agents,” Rahman said.
What About Nutritional Approaches?
The lifestyle interventions in the semaglutide and tirzepatide studies included 500 kcal/d deficit diets, which is difficult for people to maintain, noted Rahman, who is the author of the book Simply Plant Based: Fabulous Food for a Healthy Life.
Additionally, bariatric surgery has been associated with long-term micronutrient deficiencies, including deficiencies in vitamins A, D, E, K, B1, and B12, as well as folate, iron, zinc, copper, selenium, and calcium, she said.
The best approach to food from a patient compliance standpoint and to avoid nutrient deficiencies is a whole-food, plant-based diet, Rahman said. She advocates this nutritional approach, along with physical activity, for patients regardless of whether they’ve selected lifestyle intervention alone or combined with an AOM or bariatric surgery to address obesity.
Rahman cited a 5-year heart disease study comparing an intensive lifestyle program involving a vegetarian diet, aerobic exercise, stress management training, smoking cessation, and group psychosocial support to treatment as usual. Patients in the lifestyle group lost 10.9 kg at 1 year and sustained weight loss of 5.8 kg at 5 years, whereas weight in the control group remained relatively unchanged from baseline.
She also pointed to the findings of a study of patients with obesity or with overweight and at least one comorbidity that compared standard care with a low-fat, whole-food, plant-based diet with vitamin B12 supplementation. At 6 months, mean BMI reduction was greater in the intervention group than the standard care group (−4.4 vs −0.4).
In her practice, Rahman has seen the benefits of a whole-food, plant-based diet for patients with obesity.
If people are committed to this type of dietary approach and are given the tools and resources to do it effectively, “their thinking changes, their taste buds change, and they grow to enjoy this new way of eating,” she said. “They see results, and it’s a lifestyle that can be sustained long-term.”
Addressing Drivers of Weight Gain
Patients also need help addressing the various factors that may contribute to overweight and obesity, including overconsumption of ultra-processed foods, substandard nutritional quality of restaurant foods, increasing portion sizes, distraction during eating, emotional eating, late-night eating, and cultural/traditional values surrounding food, Rahman noted.
Supatra Tovar, PsyD, RD, a clinical psychologist with a practice in Pasadena, California, agreed that identifying the reasons for weight gain is critical for treatment.
“If you’re not addressing underlying issues, such as a person’s relationship with food, behaviors around food, the tendency to mindlessly eat or emotionally eat or eat to seek comfort, the person’s weight problems won’t ultimately be fully solved by any of the three approaches — dieting, medications, or bariatric surgery,” she said.
Some of her patients “engage in extreme dieting and deprivation, and many who use medications or have had bariatric surgery hardly eat and often develop nutritional deficiencies,” said Tovar, author of the book Deprogram Diet Culture: Rethink Your Relationship with Food, Heal Your Mind, and Live a Diet-Free Life.
The key to healthy and sustained weight loss is to “become attuned to the body’s signals, learn how to honor hunger, stop eating when satisfied, and eat more healthful foods, such as fruits and vegetables, whole grains, lean proteins — especially plant-based proteins — and the body gives signals that this is what it wants,” she said.
Tovar doesn’t give her clients a specific diet or set of portions.
“I teach them to listen to their bodies,” she said. “They’ve lost significant amounts of weight and continued to keep it off because they’ve done this kind of work.”
When Lifestyle Changes Aren’t Enough
For many patients, lifestyle interventions are insufficient to address the degree of overweight and obesity and common comorbidities, said W. Timothy Garvey, MD, associate director and professor, Department of Nutrition Sciences, School of Health Professions, University of Alabama at Birmingham.
“Of course, nutritional approaches are very important, not only for weight but also for general health-related reasons,” said Garvey, lead author of the 2016 American Association of Clinical Endocrinologists obesity guidelines. “We’ve seen that the Mediterranean and some plant-based diets can prevent progression from prediabetes to diabetes and improve other parameters that reflect metabolic health.”
However, it’s “not common that patients can follow these diets, lose weight, and keep it off,” Garvey cautioned. Up to 50% of weight that’s lost through lifestyle changes is typically regained by 1-year follow-up, with almost all remaining lost weight subsequently regained in the majority of individuals because the person “has to fight against pathophysiological process that drive weight regain,” he noted.
Weight-loss medications can address these pathophysiologic processes by “addressing interactions of satiety hormones with feeding centers in the brain, suppressing the appetite, and making it easier for patients to adhere to a reduced-calorie diet.”
Garvey views the weight-loss medications in the same light as drugs for diabetes and hypertension, in that people need to keep taking them to sustain the benefit.
There’s still a role for bariatric surgery because not everyone can tolerate the AOMs or achieve sufficient weight loss.
“Patients with very high BMI who have trouble ambulating might benefit from a combination of bariatric surgery and medication,” Garvey said.
While some side effects are associated with AOMs, being an “alarmist” about them can be detrimental to patients, he warned.
Rahman and Tovar are authors of books about weight loss. Reddy reported no relevant financial relationships. Garvey is a consultant on advisory boards for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Pfizer, Fractyl Health, Alnylam Pharmaceuticals, Inogen, Zealand, Allurion, Carmot/Roche, Terns Pharmaceuticals, Neurocrine, Keros Therapeutics, and Regeneron. He is the site principal investigator for multi-centered clinical trials sponsored by his university and funded by Novo Nordisk, Eli Lilly, Epitomee, Neurovalens, and Pfizer. He serves as a consultant on the advisory board for the nonprofit Milken Foundation and is a member of the Data Monitoring Committee for phase 3 clinical trials conducted by Boehringer-Ingelheim and Eli Lilly.
A version of this article first appeared on Medscape.com.
Given that more than 100 million US adults have obesity, including 22 million with severe obesity, physicians regularly see patients with the condition in their practices.
Fortunately, doctors have more tools than ever to help their patients. But the question remains: Which method is the safest and most effective? Is it diet and lifestyle changes, one of the recently approved anti-obesity medications (AOMs), bariatric surgery, or a combination approach?
There are no head-to-head trials comparing these three approaches, said Vanita Rahman, MD, clinic director of the Barnard Medical Center, Washington, DC, at the International Conference on Nutrition in Medicine, sponsored by the Physicians Committee for Responsible Medicine.
Instead, doctors must evaluate the merits and drawbacks of each intervention and decide with their patients which treatment is best for them, she told Medscape Medical News. When she sees patients, Rahman shares the pertinent research with them, so they are able to make an informed choice.
Looking at the Options
In her presentation at the conference, Rahman summarized the guidelines issued by the American Heart Association/American College of Cardiology/The Obesity Society for Management of Overweight and Obesity in Adults and the American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines For Medical Care of Patients with Obesity, including lifestyle changes, AOMs, and bariatric surgery (Table 1).

As shown, the current clinical guidelines offer recommendations that consider such factors as the patient’s BMI and presence of one or more comorbidities. Generally, they begin with lifestyle changes for people with overweight, the possibility of an AOM for those with obesity, and bariatric surgery as an option for those with severe obesity-related complications.
“In obesity, we traditionally thought the process was ‘either-or’ — either lifestyle or surgery or medication — and somehow lifestyle is better,” Sheethal Reddy, PhD, a psychologist at the Bariatric Center at Emory University Hospital Midtown, Atlanta, told Medscape Medical News.
Now physicians often use a combination of methods, but lifestyle is foundational to all of them, she said.
“If you don’t make lifestyle changes, none of the approaches will ultimately be effective,” said Reddy, who also is an assistant professor in the Division of General and GI Surgery at Emory School of Medicine, Atlanta.
Lifestyle changes don’t just involve diet and nutrition but include physical exercise.
“Being sedentary affects everything — sleep quality, appetite regulation, and metabolism. Without sufficient exercise, the body isn’t functioning well enough to have a healthy metabolism,” Reddy said.
How Durable Are the Interventions?
Although bariatric surgery has demonstrated effectiveness in helping patients lose weight, many of them regain some or most of it, Rahman said.
A systematic review and meta-analysis found weight regain in 49% of patients who underwent bariatric surgery patients, with the highest prevalence after Roux-en-Y gastric bypass.
Another study of approximately 45,000 patients who underwent bariatric surgery found differences not only in the percentage of total weight loss among Roux-en-Y gastric bypass, sleeve gastrectomy, and adjustable gastric band procedures but also in how much of that weight stayed off between 1 and 5 years following the procedure (Table 2).

Weight regain also is a risk with AOMs, if they’re discontinued.
The STEP 1 trial tested the effectiveness of semaglutide — a glucagon-like peptide 1 (GLP-1) receptor agonist — as an adjunct to lifestyle intervention for weight loss in patients with obesity or with overweight and at least one comorbidity but not diabetes. Mean weight loss with semaglutide was 17.3% but that figure dropped 11.6 percentage points after treatment was discontinued.
Other studies also have found that patients regain weight after GLP-1 discontinuation.
Tirzepatide, a GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) combination, has shown efficacy with weight reduction, but patients experienced some weight regain upon discontinuation. In one study, patients experienced a mean weight loss of 20.9% after 36 weeks of tirzepatide. In the study’s subsequent 52-week double-blind, placebo-controlled period, patients who stopped taking the medication experienced a weight regain of 14%, whereas those who remained on the medication lost an additional 5.5% of weight.
GLP-1 and GLP-1/GIP medications do not address the factors that contribute to overweight and obesity, Rahman said. “They simply suppress the appetite; therefore, weight gain occurs after stopping them.”
Patients may stop taking anti-obesity drugs for a variety of reasons, including side effects. Rahman noted that the common side effects include nausea, vomiting, and constipation, whereas rare side effects include gastroparesis, gallbladder and biliary disease, thyroid cancer, and suicidal thoughts. GLP-1 and GLP-1/GIP medications also carry a risk for non-arteritic anterior ischemic optic neuropathy, she said.
Moreover, health insurance does not always cover these medications, which likely affects patient access to the drugs and compliance rates.
“Given the side effects and frequent lack of insurance coverage, significant questions remain about long-term safety and feasibility of these agents,” Rahman said.
What About Nutritional Approaches?
The lifestyle interventions in the semaglutide and tirzepatide studies included 500 kcal/d deficit diets, which is difficult for people to maintain, noted Rahman, who is the author of the book Simply Plant Based: Fabulous Food for a Healthy Life.
Additionally, bariatric surgery has been associated with long-term micronutrient deficiencies, including deficiencies in vitamins A, D, E, K, B1, and B12, as well as folate, iron, zinc, copper, selenium, and calcium, she said.
The best approach to food from a patient compliance standpoint and to avoid nutrient deficiencies is a whole-food, plant-based diet, Rahman said. She advocates this nutritional approach, along with physical activity, for patients regardless of whether they’ve selected lifestyle intervention alone or combined with an AOM or bariatric surgery to address obesity.
Rahman cited a 5-year heart disease study comparing an intensive lifestyle program involving a vegetarian diet, aerobic exercise, stress management training, smoking cessation, and group psychosocial support to treatment as usual. Patients in the lifestyle group lost 10.9 kg at 1 year and sustained weight loss of 5.8 kg at 5 years, whereas weight in the control group remained relatively unchanged from baseline.
She also pointed to the findings of a study of patients with obesity or with overweight and at least one comorbidity that compared standard care with a low-fat, whole-food, plant-based diet with vitamin B12 supplementation. At 6 months, mean BMI reduction was greater in the intervention group than the standard care group (−4.4 vs −0.4).
In her practice, Rahman has seen the benefits of a whole-food, plant-based diet for patients with obesity.
If people are committed to this type of dietary approach and are given the tools and resources to do it effectively, “their thinking changes, their taste buds change, and they grow to enjoy this new way of eating,” she said. “They see results, and it’s a lifestyle that can be sustained long-term.”
Addressing Drivers of Weight Gain
Patients also need help addressing the various factors that may contribute to overweight and obesity, including overconsumption of ultra-processed foods, substandard nutritional quality of restaurant foods, increasing portion sizes, distraction during eating, emotional eating, late-night eating, and cultural/traditional values surrounding food, Rahman noted.
Supatra Tovar, PsyD, RD, a clinical psychologist with a practice in Pasadena, California, agreed that identifying the reasons for weight gain is critical for treatment.
“If you’re not addressing underlying issues, such as a person’s relationship with food, behaviors around food, the tendency to mindlessly eat or emotionally eat or eat to seek comfort, the person’s weight problems won’t ultimately be fully solved by any of the three approaches — dieting, medications, or bariatric surgery,” she said.
Some of her patients “engage in extreme dieting and deprivation, and many who use medications or have had bariatric surgery hardly eat and often develop nutritional deficiencies,” said Tovar, author of the book Deprogram Diet Culture: Rethink Your Relationship with Food, Heal Your Mind, and Live a Diet-Free Life.
The key to healthy and sustained weight loss is to “become attuned to the body’s signals, learn how to honor hunger, stop eating when satisfied, and eat more healthful foods, such as fruits and vegetables, whole grains, lean proteins — especially plant-based proteins — and the body gives signals that this is what it wants,” she said.
Tovar doesn’t give her clients a specific diet or set of portions.
“I teach them to listen to their bodies,” she said. “They’ve lost significant amounts of weight and continued to keep it off because they’ve done this kind of work.”
When Lifestyle Changes Aren’t Enough
For many patients, lifestyle interventions are insufficient to address the degree of overweight and obesity and common comorbidities, said W. Timothy Garvey, MD, associate director and professor, Department of Nutrition Sciences, School of Health Professions, University of Alabama at Birmingham.
“Of course, nutritional approaches are very important, not only for weight but also for general health-related reasons,” said Garvey, lead author of the 2016 American Association of Clinical Endocrinologists obesity guidelines. “We’ve seen that the Mediterranean and some plant-based diets can prevent progression from prediabetes to diabetes and improve other parameters that reflect metabolic health.”
However, it’s “not common that patients can follow these diets, lose weight, and keep it off,” Garvey cautioned. Up to 50% of weight that’s lost through lifestyle changes is typically regained by 1-year follow-up, with almost all remaining lost weight subsequently regained in the majority of individuals because the person “has to fight against pathophysiological process that drive weight regain,” he noted.
Weight-loss medications can address these pathophysiologic processes by “addressing interactions of satiety hormones with feeding centers in the brain, suppressing the appetite, and making it easier for patients to adhere to a reduced-calorie diet.”
Garvey views the weight-loss medications in the same light as drugs for diabetes and hypertension, in that people need to keep taking them to sustain the benefit.
There’s still a role for bariatric surgery because not everyone can tolerate the AOMs or achieve sufficient weight loss.
“Patients with very high BMI who have trouble ambulating might benefit from a combination of bariatric surgery and medication,” Garvey said.
While some side effects are associated with AOMs, being an “alarmist” about them can be detrimental to patients, he warned.
Rahman and Tovar are authors of books about weight loss. Reddy reported no relevant financial relationships. Garvey is a consultant on advisory boards for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Pfizer, Fractyl Health, Alnylam Pharmaceuticals, Inogen, Zealand, Allurion, Carmot/Roche, Terns Pharmaceuticals, Neurocrine, Keros Therapeutics, and Regeneron. He is the site principal investigator for multi-centered clinical trials sponsored by his university and funded by Novo Nordisk, Eli Lilly, Epitomee, Neurovalens, and Pfizer. He serves as a consultant on the advisory board for the nonprofit Milken Foundation and is a member of the Data Monitoring Committee for phase 3 clinical trials conducted by Boehringer-Ingelheim and Eli Lilly.
A version of this article first appeared on Medscape.com.
How Does End of Life Impact Diabetes Care?
TOPLINE:
Among older adults with type 2 diabetes (T2D), the use of antidiabetes medications declined in the last year before death, with notable shifts from metformin and sulfonylureas toward insulin therapy.
METHODOLOGY:
Current recommendations emphasize a more liberal approach to glycemic control in people with a high burden of comorbidities and shorter life expectancy, but little is known about the changes and discontinuation patterns of diabetes medications among older adults near the end of life.
.
All medication classes available during the study period were considered, including short-acting and long-acting insulins, metformin, sulfonylureas, dipeptidyl peptidase-4 inhibitors, glucagon-like peptide 1 (GLP-1) receptor agonists, sodium-glucose cotransporter-2 (SGLT2) inhibitors, and other medications.
Analysis included temporal trends in prescribing antidiabetes medications, stratified by frailty using a validated claims-based frailty index, with scores ≥ 0.30 indicating higher frailty.
Antidiabetes medication fills were assessed within 1 year before death, examining changes across three time periods: 12 to 8 months, 8 to 4 months, and 4 to 0 months before death.
TAKEAWAY:
The proportion of older patients receiving antidiabetes medications increased slightly from 71.4% in 2015 to 72.9% in 2019, with metformin showing the largest increase from 40.7% to 46.5% (standardized mean difference [SMD], −0.12) and sulfonylureas showing the largest decrease from 37.0% to 31.8% (SMD, 0.11).
The use of newer diabetes medications with cardiovascular benefits, such as GLP-1 receptor agonists and SGLT2 inhibitors, remained less common but showed increasing trends over time.
The use of any antidiabetes medication decreased from 66.1% in the 9 to 12 months before death to 60.8% in the last 4 months of life (P < .01), primarily due to the reduced use of metformin and sulfonylureas.
The use of both short-acting and long-acting insulin agents increased toward the end of life (from 28.0% to 32.9% and from 41.2% to 43.9%, respectively; both P < .001) , particularly among frailer individuals.
IN PRACTICE:
“[The study] findings underscore important implications for diabetes management in patients nearing the end of life. With ~70% of patients with T2D using at least one antidiabetes medication, there is a need to consider further de-escalation or deprescribing in this vulnerable population,” the authors wrote.
SOURCE:
The study was led by Alexander Kutz, MD, MPH, MSc, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, and was published online in Diabetes Care.
LIMITATIONS:
The study lacked details on the reasons for changes in medication patterns, making it unclear whether these changes were due to clinical guidelines or to reduce adverse events. Moreover, the study could not capture transitions or substitutions between medications, information on the dosage data, and causes of death.
DISCLOSURES:
This study was supported by the Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School. Some authors reported receiving personal fees or research grants from the National Institutes of Health and other institutions and a few pharmaceutical companies. One author reported acting as a principal investigator and receiving a research grant from Boehringer-Ingelheim, unrelated to the work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Among older adults with type 2 diabetes (T2D), the use of antidiabetes medications declined in the last year before death, with notable shifts from metformin and sulfonylureas toward insulin therapy.
METHODOLOGY:
Current recommendations emphasize a more liberal approach to glycemic control in people with a high burden of comorbidities and shorter life expectancy, but little is known about the changes and discontinuation patterns of diabetes medications among older adults near the end of life.
.
All medication classes available during the study period were considered, including short-acting and long-acting insulins, metformin, sulfonylureas, dipeptidyl peptidase-4 inhibitors, glucagon-like peptide 1 (GLP-1) receptor agonists, sodium-glucose cotransporter-2 (SGLT2) inhibitors, and other medications.
Analysis included temporal trends in prescribing antidiabetes medications, stratified by frailty using a validated claims-based frailty index, with scores ≥ 0.30 indicating higher frailty.
Antidiabetes medication fills were assessed within 1 year before death, examining changes across three time periods: 12 to 8 months, 8 to 4 months, and 4 to 0 months before death.
TAKEAWAY:
The proportion of older patients receiving antidiabetes medications increased slightly from 71.4% in 2015 to 72.9% in 2019, with metformin showing the largest increase from 40.7% to 46.5% (standardized mean difference [SMD], −0.12) and sulfonylureas showing the largest decrease from 37.0% to 31.8% (SMD, 0.11).
The use of newer diabetes medications with cardiovascular benefits, such as GLP-1 receptor agonists and SGLT2 inhibitors, remained less common but showed increasing trends over time.
The use of any antidiabetes medication decreased from 66.1% in the 9 to 12 months before death to 60.8% in the last 4 months of life (P < .01), primarily due to the reduced use of metformin and sulfonylureas.
The use of both short-acting and long-acting insulin agents increased toward the end of life (from 28.0% to 32.9% and from 41.2% to 43.9%, respectively; both P < .001) , particularly among frailer individuals.
IN PRACTICE:
“[The study] findings underscore important implications for diabetes management in patients nearing the end of life. With ~70% of patients with T2D using at least one antidiabetes medication, there is a need to consider further de-escalation or deprescribing in this vulnerable population,” the authors wrote.
SOURCE:
The study was led by Alexander Kutz, MD, MPH, MSc, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, and was published online in Diabetes Care.
LIMITATIONS:
The study lacked details on the reasons for changes in medication patterns, making it unclear whether these changes were due to clinical guidelines or to reduce adverse events. Moreover, the study could not capture transitions or substitutions between medications, information on the dosage data, and causes of death.
DISCLOSURES:
This study was supported by the Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School. Some authors reported receiving personal fees or research grants from the National Institutes of Health and other institutions and a few pharmaceutical companies. One author reported acting as a principal investigator and receiving a research grant from Boehringer-Ingelheim, unrelated to the work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Among older adults with type 2 diabetes (T2D), the use of antidiabetes medications declined in the last year before death, with notable shifts from metformin and sulfonylureas toward insulin therapy.
METHODOLOGY:
Current recommendations emphasize a more liberal approach to glycemic control in people with a high burden of comorbidities and shorter life expectancy, but little is known about the changes and discontinuation patterns of diabetes medications among older adults near the end of life.
.
All medication classes available during the study period were considered, including short-acting and long-acting insulins, metformin, sulfonylureas, dipeptidyl peptidase-4 inhibitors, glucagon-like peptide 1 (GLP-1) receptor agonists, sodium-glucose cotransporter-2 (SGLT2) inhibitors, and other medications.
Analysis included temporal trends in prescribing antidiabetes medications, stratified by frailty using a validated claims-based frailty index, with scores ≥ 0.30 indicating higher frailty.
Antidiabetes medication fills were assessed within 1 year before death, examining changes across three time periods: 12 to 8 months, 8 to 4 months, and 4 to 0 months before death.
TAKEAWAY:
The proportion of older patients receiving antidiabetes medications increased slightly from 71.4% in 2015 to 72.9% in 2019, with metformin showing the largest increase from 40.7% to 46.5% (standardized mean difference [SMD], −0.12) and sulfonylureas showing the largest decrease from 37.0% to 31.8% (SMD, 0.11).
The use of newer diabetes medications with cardiovascular benefits, such as GLP-1 receptor agonists and SGLT2 inhibitors, remained less common but showed increasing trends over time.
The use of any antidiabetes medication decreased from 66.1% in the 9 to 12 months before death to 60.8% in the last 4 months of life (P < .01), primarily due to the reduced use of metformin and sulfonylureas.
The use of both short-acting and long-acting insulin agents increased toward the end of life (from 28.0% to 32.9% and from 41.2% to 43.9%, respectively; both P < .001) , particularly among frailer individuals.
IN PRACTICE:
“[The study] findings underscore important implications for diabetes management in patients nearing the end of life. With ~70% of patients with T2D using at least one antidiabetes medication, there is a need to consider further de-escalation or deprescribing in this vulnerable population,” the authors wrote.
SOURCE:
The study was led by Alexander Kutz, MD, MPH, MSc, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, and was published online in Diabetes Care.
LIMITATIONS:
The study lacked details on the reasons for changes in medication patterns, making it unclear whether these changes were due to clinical guidelines or to reduce adverse events. Moreover, the study could not capture transitions or substitutions between medications, information on the dosage data, and causes of death.
DISCLOSURES:
This study was supported by the Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School. Some authors reported receiving personal fees or research grants from the National Institutes of Health and other institutions and a few pharmaceutical companies. One author reported acting as a principal investigator and receiving a research grant from Boehringer-Ingelheim, unrelated to the work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.