User login
AI-Enhanced ECG Used to Predict Hypertension and Associated Risks
TOPLINE:
in addition to traditional markers.
METHODOLOGY:
- Researchers conducted a development and external validation prognostic cohort study in a secondary care setting to identify individuals at risk for incident hypertension.
- They developed AIRE-HTN, which was trained on a derivation cohort from the Beth Israel Deaconess Medical Center in Boston, involving 1,163,401 ECGs from 189,539 patients (mean age, 57.7 years; 52.1% women; 64.5% White individuals).
- External validation was conducted on 65,610 ECGs from a UK-based volunteer cohort, drawn from an equal number of patients (mean age, 65.4 years; 51.5% women; 96.3% White individuals).
- Incident hypertension was evaluated in 19,423 individuals without hypertension from the medical center cohort and in 35,806 individuals without hypertension from the UK cohort.
TAKEAWAY:
- AIRE-HTN predicted incident hypertension with a C-index of 0.70 (95% CI, 0.69-0.71) in both the cohorts. Those in the quartile with the highest AIRE-HTN scores had a fourfold increased risk for incident hypertension (P < .001).
- The model’s predictive accuracy was maintained in individuals without left ventricular hypertrophy and those with normal ECGs and baseline blood pressure, indicating its robustness.
- The model was significantly additive to traditional clinical markers, with a continuous net reclassification index of 0.44 for the medical center cohort and 0.32 for the UK cohort.
- AIRE-HTN was an independent predictor of cardiovascular death (hazard ratio per 1-SD increase in score [HR], 2.24), heart failure (HR, 2.60), myocardial infarction (HR, 3.13), ischemic stroke (HR, 1.23), and chronic kidney disease (HR, 1.89) in outpatients from the medical center cohort (all P < .001), with largely consistent findings in the UK cohort.
IN PRACTICE:
“Results of exploratory and phenotypic analyses suggest the biological plausibility of these findings. Enhanced predictability could influence surveillance programs and primordial prevention,” the authors wrote.
SOURCE:
The study was led by Arunashis Sau, PhD, and Joseph Barker, MRes, National Heart and Lung Institute, Imperial College London, England. It was published online on January 2, 2025, in JAMA Cardiology.
LIMITATIONS:
In one cohort, hypertension was defined using International Classification of Diseases codes, which may lack granularity and not align with contemporary guidelines. The findings were not validated against ambulatory monitoring standards. The performance of the model in different populations and clinical settings remains to be explored.
DISCLOSURES:
The authors acknowledged receiving support from Imperial’s British Heart Foundation Centre for Excellence Award and disclosed receiving support from the British Heart Foundation, the National Institute for Health Research Imperial College Biomedical Research Centre, the EJP RD Research Mobility Fellowship, the Medical Research Council, and the Sir Jules Thorn Charitable Trust. Some authors reported receiving grants, personal fees, advisory fees, or laboratory work fees outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
in addition to traditional markers.
METHODOLOGY:
- Researchers conducted a development and external validation prognostic cohort study in a secondary care setting to identify individuals at risk for incident hypertension.
- They developed AIRE-HTN, which was trained on a derivation cohort from the Beth Israel Deaconess Medical Center in Boston, involving 1,163,401 ECGs from 189,539 patients (mean age, 57.7 years; 52.1% women; 64.5% White individuals).
- External validation was conducted on 65,610 ECGs from a UK-based volunteer cohort, drawn from an equal number of patients (mean age, 65.4 years; 51.5% women; 96.3% White individuals).
- Incident hypertension was evaluated in 19,423 individuals without hypertension from the medical center cohort and in 35,806 individuals without hypertension from the UK cohort.
TAKEAWAY:
- AIRE-HTN predicted incident hypertension with a C-index of 0.70 (95% CI, 0.69-0.71) in both the cohorts. Those in the quartile with the highest AIRE-HTN scores had a fourfold increased risk for incident hypertension (P < .001).
- The model’s predictive accuracy was maintained in individuals without left ventricular hypertrophy and those with normal ECGs and baseline blood pressure, indicating its robustness.
- The model was significantly additive to traditional clinical markers, with a continuous net reclassification index of 0.44 for the medical center cohort and 0.32 for the UK cohort.
- AIRE-HTN was an independent predictor of cardiovascular death (hazard ratio per 1-SD increase in score [HR], 2.24), heart failure (HR, 2.60), myocardial infarction (HR, 3.13), ischemic stroke (HR, 1.23), and chronic kidney disease (HR, 1.89) in outpatients from the medical center cohort (all P < .001), with largely consistent findings in the UK cohort.
IN PRACTICE:
“Results of exploratory and phenotypic analyses suggest the biological plausibility of these findings. Enhanced predictability could influence surveillance programs and primordial prevention,” the authors wrote.
SOURCE:
The study was led by Arunashis Sau, PhD, and Joseph Barker, MRes, National Heart and Lung Institute, Imperial College London, England. It was published online on January 2, 2025, in JAMA Cardiology.
LIMITATIONS:
In one cohort, hypertension was defined using International Classification of Diseases codes, which may lack granularity and not align with contemporary guidelines. The findings were not validated against ambulatory monitoring standards. The performance of the model in different populations and clinical settings remains to be explored.
DISCLOSURES:
The authors acknowledged receiving support from Imperial’s British Heart Foundation Centre for Excellence Award and disclosed receiving support from the British Heart Foundation, the National Institute for Health Research Imperial College Biomedical Research Centre, the EJP RD Research Mobility Fellowship, the Medical Research Council, and the Sir Jules Thorn Charitable Trust. Some authors reported receiving grants, personal fees, advisory fees, or laboratory work fees outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
in addition to traditional markers.
METHODOLOGY:
- Researchers conducted a development and external validation prognostic cohort study in a secondary care setting to identify individuals at risk for incident hypertension.
- They developed AIRE-HTN, which was trained on a derivation cohort from the Beth Israel Deaconess Medical Center in Boston, involving 1,163,401 ECGs from 189,539 patients (mean age, 57.7 years; 52.1% women; 64.5% White individuals).
- External validation was conducted on 65,610 ECGs from a UK-based volunteer cohort, drawn from an equal number of patients (mean age, 65.4 years; 51.5% women; 96.3% White individuals).
- Incident hypertension was evaluated in 19,423 individuals without hypertension from the medical center cohort and in 35,806 individuals without hypertension from the UK cohort.
TAKEAWAY:
- AIRE-HTN predicted incident hypertension with a C-index of 0.70 (95% CI, 0.69-0.71) in both the cohorts. Those in the quartile with the highest AIRE-HTN scores had a fourfold increased risk for incident hypertension (P < .001).
- The model’s predictive accuracy was maintained in individuals without left ventricular hypertrophy and those with normal ECGs and baseline blood pressure, indicating its robustness.
- The model was significantly additive to traditional clinical markers, with a continuous net reclassification index of 0.44 for the medical center cohort and 0.32 for the UK cohort.
- AIRE-HTN was an independent predictor of cardiovascular death (hazard ratio per 1-SD increase in score [HR], 2.24), heart failure (HR, 2.60), myocardial infarction (HR, 3.13), ischemic stroke (HR, 1.23), and chronic kidney disease (HR, 1.89) in outpatients from the medical center cohort (all P < .001), with largely consistent findings in the UK cohort.
IN PRACTICE:
“Results of exploratory and phenotypic analyses suggest the biological plausibility of these findings. Enhanced predictability could influence surveillance programs and primordial prevention,” the authors wrote.
SOURCE:
The study was led by Arunashis Sau, PhD, and Joseph Barker, MRes, National Heart and Lung Institute, Imperial College London, England. It was published online on January 2, 2025, in JAMA Cardiology.
LIMITATIONS:
In one cohort, hypertension was defined using International Classification of Diseases codes, which may lack granularity and not align with contemporary guidelines. The findings were not validated against ambulatory monitoring standards. The performance of the model in different populations and clinical settings remains to be explored.
DISCLOSURES:
The authors acknowledged receiving support from Imperial’s British Heart Foundation Centre for Excellence Award and disclosed receiving support from the British Heart Foundation, the National Institute for Health Research Imperial College Biomedical Research Centre, the EJP RD Research Mobility Fellowship, the Medical Research Council, and the Sir Jules Thorn Charitable Trust. Some authors reported receiving grants, personal fees, advisory fees, or laboratory work fees outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Intensive BP Control May Benefit CKD Patients in Real World
TOPLINE:
The cardiovascular benefits observed with intensive blood pressure (BP) control in patients with hypertension and elevated cardiovascular risk from the Systolic Blood Pressure Intervention Trial (SPRINT) can be largely replicated in real-world settings among patients with chronic kidney disease (CKD), highlighting the advantages of adopting intensive BP targets.
METHODOLOGY:
- Researchers conducted a comparative effectiveness study to determine if the beneficial and adverse effects of intensive vs standard BP control observed in SPRINT were replicable in patients with CKD and hypertension in clinical practice.
- They identified 85,938 patients (mean age, 75.7 years; 95.0% men) and 13,983 patients (mean age, 77.4 years; 38.4% men) from the Veterans Health Administration (VHA) and Kaiser Permanente of Southern California (KPSC) databases, respectively.
- The treatment effect was estimated by combining baseline covariate, treatment, and outcome data of participants from the SPRINT with covariate data from the VHA and KPSC databases.
- The primary outcomes included major cardiovascular events, all-cause death, cognitive impairment, CKD progression, and adverse events at 4 years.
TAKEAWAY:
- Compared with SPRINT participants, those in the VHA and KPSC databases were older, had less prevalent cardiovascular disease, higher albuminuria, and used more statins.
- The benefits of intensive vs standard BP control on major cardiovascular events, all-cause mortality, and certain adverse events (hypotension, syncope, bradycardia, acute kidney injury, and electrolyte abnormality) were transferable from the trial to the VHA and KPSC populations.
- The treatment effect of intensive BP management on CKD progression was transportable to the KPSC population but not to the VHA population. However, the trial’s impact on cognitive outcomes, such as dementia, was not transportable to either the VHA or KPSC populations.
- On the absolute scale, intensive vs standard BP treatment showed greater cardiovascular benefits and fewer safety concerns in the VHA and KPSC populations than in the SPRINT.
IN PRACTICE:
“This example highlights the potential for transportability methods to provide insights that can bridge evidence gaps and inform the application of novel therapies to patients with CKD who are treated in everyday practice,” the authors wrote.
SOURCE:
This study was led by Manjula Kurella Tamura, MD, MPH, Division of Nephrology, Department of Medicine, Stanford University School of Medicine, Palo Alto, California. It was published online on January 7, 2025, in JAMA Network Open.
LIMITATIONS:
Transportability analyses could not account for characteristics that were not well-documented in electronic health records, such as limited life expectancy. The study was conducted before the widespread use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 receptor agonists, and nonsteroidal mineralocorticoid receptor antagonists, making it unclear whether intensive BP treatment would result in similar benefits with current pharmacotherapy regimens. Eligibility for this study was based on BP measurements in routine practice, which tend to be more variable than those collected in research settings.
DISCLOSURES:
This study was supported by grants from the National Institutes of Health. Some authors disclosed serving as a consultant and receiving grants, personal fees, and consulting fees from pharmaceutical companies and other sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
The cardiovascular benefits observed with intensive blood pressure (BP) control in patients with hypertension and elevated cardiovascular risk from the Systolic Blood Pressure Intervention Trial (SPRINT) can be largely replicated in real-world settings among patients with chronic kidney disease (CKD), highlighting the advantages of adopting intensive BP targets.
METHODOLOGY:
- Researchers conducted a comparative effectiveness study to determine if the beneficial and adverse effects of intensive vs standard BP control observed in SPRINT were replicable in patients with CKD and hypertension in clinical practice.
- They identified 85,938 patients (mean age, 75.7 years; 95.0% men) and 13,983 patients (mean age, 77.4 years; 38.4% men) from the Veterans Health Administration (VHA) and Kaiser Permanente of Southern California (KPSC) databases, respectively.
- The treatment effect was estimated by combining baseline covariate, treatment, and outcome data of participants from the SPRINT with covariate data from the VHA and KPSC databases.
- The primary outcomes included major cardiovascular events, all-cause death, cognitive impairment, CKD progression, and adverse events at 4 years.
TAKEAWAY:
- Compared with SPRINT participants, those in the VHA and KPSC databases were older, had less prevalent cardiovascular disease, higher albuminuria, and used more statins.
- The benefits of intensive vs standard BP control on major cardiovascular events, all-cause mortality, and certain adverse events (hypotension, syncope, bradycardia, acute kidney injury, and electrolyte abnormality) were transferable from the trial to the VHA and KPSC populations.
- The treatment effect of intensive BP management on CKD progression was transportable to the KPSC population but not to the VHA population. However, the trial’s impact on cognitive outcomes, such as dementia, was not transportable to either the VHA or KPSC populations.
- On the absolute scale, intensive vs standard BP treatment showed greater cardiovascular benefits and fewer safety concerns in the VHA and KPSC populations than in the SPRINT.
IN PRACTICE:
“This example highlights the potential for transportability methods to provide insights that can bridge evidence gaps and inform the application of novel therapies to patients with CKD who are treated in everyday practice,” the authors wrote.
SOURCE:
This study was led by Manjula Kurella Tamura, MD, MPH, Division of Nephrology, Department of Medicine, Stanford University School of Medicine, Palo Alto, California. It was published online on January 7, 2025, in JAMA Network Open.
LIMITATIONS:
Transportability analyses could not account for characteristics that were not well-documented in electronic health records, such as limited life expectancy. The study was conducted before the widespread use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 receptor agonists, and nonsteroidal mineralocorticoid receptor antagonists, making it unclear whether intensive BP treatment would result in similar benefits with current pharmacotherapy regimens. Eligibility for this study was based on BP measurements in routine practice, which tend to be more variable than those collected in research settings.
DISCLOSURES:
This study was supported by grants from the National Institutes of Health. Some authors disclosed serving as a consultant and receiving grants, personal fees, and consulting fees from pharmaceutical companies and other sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
The cardiovascular benefits observed with intensive blood pressure (BP) control in patients with hypertension and elevated cardiovascular risk from the Systolic Blood Pressure Intervention Trial (SPRINT) can be largely replicated in real-world settings among patients with chronic kidney disease (CKD), highlighting the advantages of adopting intensive BP targets.
METHODOLOGY:
- Researchers conducted a comparative effectiveness study to determine if the beneficial and adverse effects of intensive vs standard BP control observed in SPRINT were replicable in patients with CKD and hypertension in clinical practice.
- They identified 85,938 patients (mean age, 75.7 years; 95.0% men) and 13,983 patients (mean age, 77.4 years; 38.4% men) from the Veterans Health Administration (VHA) and Kaiser Permanente of Southern California (KPSC) databases, respectively.
- The treatment effect was estimated by combining baseline covariate, treatment, and outcome data of participants from the SPRINT with covariate data from the VHA and KPSC databases.
- The primary outcomes included major cardiovascular events, all-cause death, cognitive impairment, CKD progression, and adverse events at 4 years.
TAKEAWAY:
- Compared with SPRINT participants, those in the VHA and KPSC databases were older, had less prevalent cardiovascular disease, higher albuminuria, and used more statins.
- The benefits of intensive vs standard BP control on major cardiovascular events, all-cause mortality, and certain adverse events (hypotension, syncope, bradycardia, acute kidney injury, and electrolyte abnormality) were transferable from the trial to the VHA and KPSC populations.
- The treatment effect of intensive BP management on CKD progression was transportable to the KPSC population but not to the VHA population. However, the trial’s impact on cognitive outcomes, such as dementia, was not transportable to either the VHA or KPSC populations.
- On the absolute scale, intensive vs standard BP treatment showed greater cardiovascular benefits and fewer safety concerns in the VHA and KPSC populations than in the SPRINT.
IN PRACTICE:
“This example highlights the potential for transportability methods to provide insights that can bridge evidence gaps and inform the application of novel therapies to patients with CKD who are treated in everyday practice,” the authors wrote.
SOURCE:
This study was led by Manjula Kurella Tamura, MD, MPH, Division of Nephrology, Department of Medicine, Stanford University School of Medicine, Palo Alto, California. It was published online on January 7, 2025, in JAMA Network Open.
LIMITATIONS:
Transportability analyses could not account for characteristics that were not well-documented in electronic health records, such as limited life expectancy. The study was conducted before the widespread use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 receptor agonists, and nonsteroidal mineralocorticoid receptor antagonists, making it unclear whether intensive BP treatment would result in similar benefits with current pharmacotherapy regimens. Eligibility for this study was based on BP measurements in routine practice, which tend to be more variable than those collected in research settings.
DISCLOSURES:
This study was supported by grants from the National Institutes of Health. Some authors disclosed serving as a consultant and receiving grants, personal fees, and consulting fees from pharmaceutical companies and other sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Pharmacist-Led Deprescribing of Aspirin for Primary Prevention of Cardiovascular Disease Among Geriatric Veterans
Pharmacist-Led Deprescribing of Aspirin for Primary Prevention of Cardiovascular Disease Among Geriatric Veterans
Low-dose aspirin commonly is used for the prevention of cardiovascular disease (CVD) but is associated with an increased risk of major bleeding.1 The use of aspirin for primary prevention is largely extrapolated from clinical trials showing benefit in the secondary prevention of myocardial infarction and ischemic stroke. However, results from the Aspirin in Reducing Events in the Elderly (ASPREE) trial challenged this practice.2 The ASPREE trial, conducted in the United States and Australia from 2010 to 2014, sought to determine whether daily 100 mg aspirin, was superior to placebo in promoting disability-free survival among older adults. Participants were aged ≥ 70 years (≥ 65 years for Hispanic and Black US participants), living in the community, and were free from preexisting CVD, cerebrovascular disease, or any chronic condition likely to limit survival to < 5 years. The study found no significant difference in the primary endpoints of death, dementia, or persistent physical disability, but there was a significantly higher risk of major hemorrhage in the aspirin group (3.8% vs 2.8%; hazard ratio, 1.38; 95% CI, 1.18-1.62; P < .001).
Several medical societies have updated their guideline recommendations for aspirin for primary prevention of CVD. The 2022 United States Public Service Task Force (USPSTF) provides a grade C recommendation (at least moderate certainty that the net benefit is small) to consider low-dose aspirin for the primary prevention of CVD on an individual patient basis for adults aged 40 to 59 years who have a ≥ 10% 10-year CVD risk. For adults aged ≥ 60 years, the USPSTF recommendation is grade D (moderate or high certainty that the practice has no net benefit or that harms outweigh the benefits) for low-dose aspirin use.1,3 The American College of Cardiology and American Heart Association (ACC/AHA) recommend considering low-dose aspirin for primary prevention of atherosclerotic cardiovascular disease (ASCVD) among select adults aged 40 to 70 years at higher CVD risk but not at increased risk of bleeding.4 The American Diabetes Association (ADA) recommends low-dose aspirin for primary prevention of CVD in patients with diabetes and additional risk factors such as family history of premature ASCVD, hypertension, dyslipidemia, smoking, or chronic kidney disease, and who are not at higher risk of bleeding.5 The ADA standards also caution against the use of aspirin as primary prevention in patients aged > 70 years. Low-dose aspirin use is not recommended for the primary prevention of CVD in older adults or adults of any age who are at increased risk of bleeding.
Recent literature using the US Department of Veterans Affairs (VA) Corporate Data Warehouse database confirms 86,555 of 1.8 million veterans aged > 70 years (5%) were taking low-dose aspirin for primary prevention of ASCVD despite guideline recommendations.6 Higher risk of gastrointestinal and other major bleeding from low-dose aspirin has been reported in the literature.1 Major bleeds represent a significant burden to the health care system with an estimated mean $13,093 cost for gastrointestinal bleed hospitalization.7
Considering the large scale aspirin use without appropriate indication within the veteran population, the risk of adverse effects, and the significant cost to patients and the health care system, it is imperative to determine the best approach to efficiently deprescribe aspirin for primary prevention among geriatric patients. Deprescribing refers to the systematic and supervised process of dose reduction or drug discontinuation with the goal of improving health and/or reducing the risk of adverse effects.8 During patient visits, primary care practitioners (PCPs) have opportunities to discontinue aspirin, but these encounters are time-limited and deprescribing might be secondary to more acute primary care needs. The shortage of PCPs is expected to worsen in coming years, which could further reduce their availability to assess inappropriate aspirin use.9
VA clinical pharmacist practitioners (CPPs) serve as medication experts and work autonomously under a broad scope of practice as part of the patient aligned care team.10-12 CPPs can free up time for PCPs and facilitate deprescribing efforts, especially for older adults. One retrospective cohort study conducted at a VA medical center found that CPPs deprescribed more potentially inappropriate medications among individuals aged ≥ 80 years compared with usual care with PCPs (26.8% vs 16.1%; P < .001).12,13 An aspirin deprescribing protocol conducted in 2022 resulted in nearly half of veterans aged ≥ 70 years contacted by phone agreeing to stop aspirin. Although this study supports the role pharmacists can play in reducing aspirin use in accordance with guidelines, the authors acknowledge that their interventions had a mean time of 12 minutes per patient and would require workflow changes.14 The purpose of this study is to evaluate the efficiency of aspirin deprescribing through 2 approaches: direct deprescribing by pharmacists using populationlevel review compared with clinicians following a pharmacist-led education.
Methods
This was a single-center quality improvement cohort study at the Durham VA Health Care System (DVAHCS) in North Carolina. Patients included were aged ≥ 70 years without known ASCVD who received care at any of 3 DVAHCS community-based outpatient clinics and prescribed aspirin. Patient data was obtained using the VIONE (Deprescribing Dashboard called Vital, Important, Optional, Not indicated, and Every medication has a specific indication or diagnosis) dashboard.15 VIONE was developed to identify potentially inappropriate medications (PIMs) that are eligible to deprescribe based on Beers Criteria, Screening Tool of Older Personsf Prescriptions criteria, and common clinical scenarios when clinicians determine the risk outweighs the benefit to continue a specific medication. 16,17 VIONE is used to reduce polypharmacy and improve patient safety, comfort, and medication adherence. Aspirin for patients aged ≥ 70 years without a history of ASCVD is a PIM identified by VIONE. Patients aged ≥ 70 years were chosen as an inclusion criteria in this study to match the ASPREE trial inclusion criteria and age inclusion criteria in the VIONE dashboard for aspirin deprescribing.2 Patient lists were generated for these potentially inappropriate aspirin prescriptions for 3 months before clinician staff education presentations, the day of the presentations, and 3 months after.
The primary endpoint was the number of veterans with aspirin deprescribed directly by 2 pharmacists over 12 weeks, divided by total patient care time spent, compared with the change in number of veterans with aspirin deprescribed by any DVAHCS physician, nurse practitioner, physician assistant, or CPP over 12 weeks, divided by the total pharmacist time spent on PCP education. Secondary endpoints were the number of aspirin orders discontinued by pharmacists and CPPs, the number of aspirin orders discontinued 12 weeks before pharmacist-led education compared with the number of aspirin orders discontinued 12 weeks after CPP-led education, average and median pharmacist time spent per patient encounter, and time of direct patient encounters vs time spent on PCP education.
Pharmacists reviewed each patient who met the inclusion criteria from the list generated by VIONE on December 1, 2022, for aspirin appropriateness according to the ACC/AHA and USPSTF guidelines, with the goal to discontinue aspirin for primary prevention of ASCVD and no other indications.1,4 Pharmacists documented their visits using VIONE methodology in the Computerized Patient Record System (CPRS) using a polypharmacy review note. CPPs contacted patients who were taking aspirin for primary prevention by unscheduled telephone call to assess for aspirin adherence, undocumented history of ASCVD, cardiovascular risk factors, and history of bleeding. Aspirin was discontinued if patients met guideline criteria recommendations and agreed to discontinuation. Risk-benefit discussions were completed when patients without known ASCVD were considered high risk because the ACC/AHA guidelines mention there is insufficient evidence of safety and efficacy of aspirin for primary prevention for patients with other known ASCVD risk factors (eg, strong family history of premature myocardial infarction, inability to achieve lipid, blood pressure, or glucose targets, or significant elevation in coronary artery calcium score).
High risk was defined as family history of premature ASCVD (in a male first-degree relative aged < 55 years or a female first-degree relative aged < 65 years), most recent blood pressure or 2 blood pressure results in the last 12 months > 160/100 mm Hg, recent hemoglobin A1c > 9%, and/or low-density lipoprotein > 190 mg/dL or not prescribed an indicated statin.3 Aspirin was continued or discontinued according to patient preference after the personalized risk-benefit discussion.
For patients with a clinical indication for aspirin use other than ASCVD (eg, atrial fibrillation not on anticoagulation, venous thromboembolism prophylaxis, carotid artery disease), CPPs documented their assessment and when appropriate deferred to the PCP for consideration of stopping aspirin. For patients with undocumented ASCVD, CPPs added their ASCVD history to their problem list and aspirin was continued. PCPs were notified by alert when aspirin was discontinued and when patients could not be reached by telephone.
presented a review of recent guideline updates and supporting literature at 2 online staff meetings. The education sessions lasted about 10 minutes and were presented to PCPs across 3 community-based outpatient clinics. An estimated 40 minutes were spent creating the PowerPoint education materials, seeking feedback, making edits, and answering questions or emails from PCPs after the presentation. During the presentation, pharmacists encouraged PCPs to discontinue aspirin (active VA prescriptions and reported over-the-counter use) for primary prevention of ASCVD in patients aged ≥ 70 years during their upcoming appointments and consider risk factors recommended by the ACC/AHA guidelines when applicable. PCPs were notified that CPPs planned to start a population review for discontinuing active VA aspirin prescriptions on December 1, 2022. The primary endpoint and secondary endpoints were analyzed using descriptive statistics. All data were analyzed using Microsoft Excel.
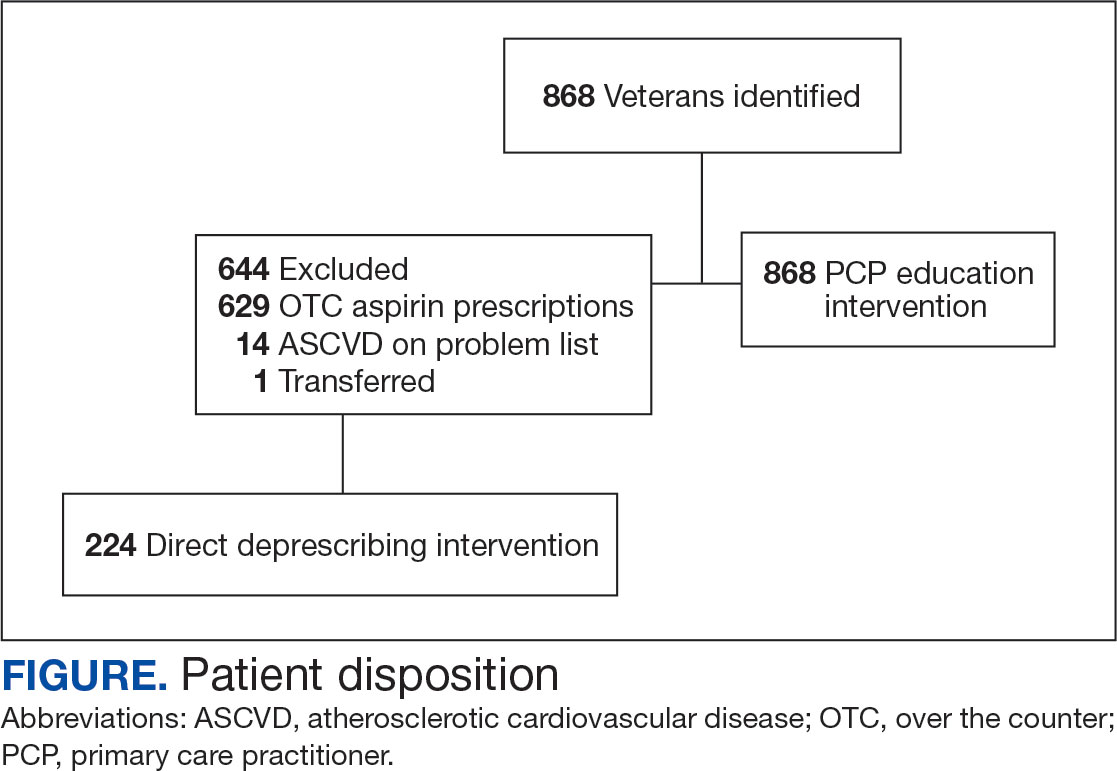
Results
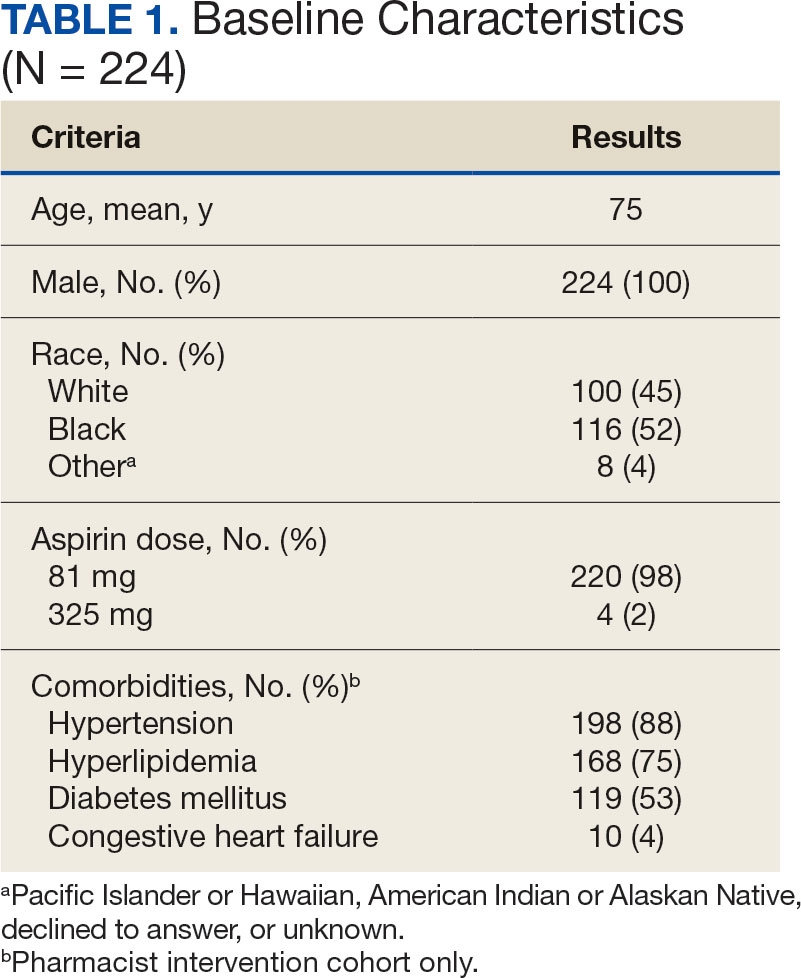
A total of 868 patients aged ≥ 70 years with active prescriptions for aspirin were identified on December 1, 2022. After applying inclusion and exclusion criteria for the pharmacist population review, 224 patients were included for cohort final analysis (Figure). All 868 patients were eligible for the CPP intervention. Primary reasons for exclusion from the CPP population included over-thecounter aspirin and a history of ASCVD in the patient’s problem list. All patients were male, with a mean (SD) age of 75 (4.4) years (Table 1). Most patients were prescribed aspirin, 81 mg daily (n = 220; 98%).
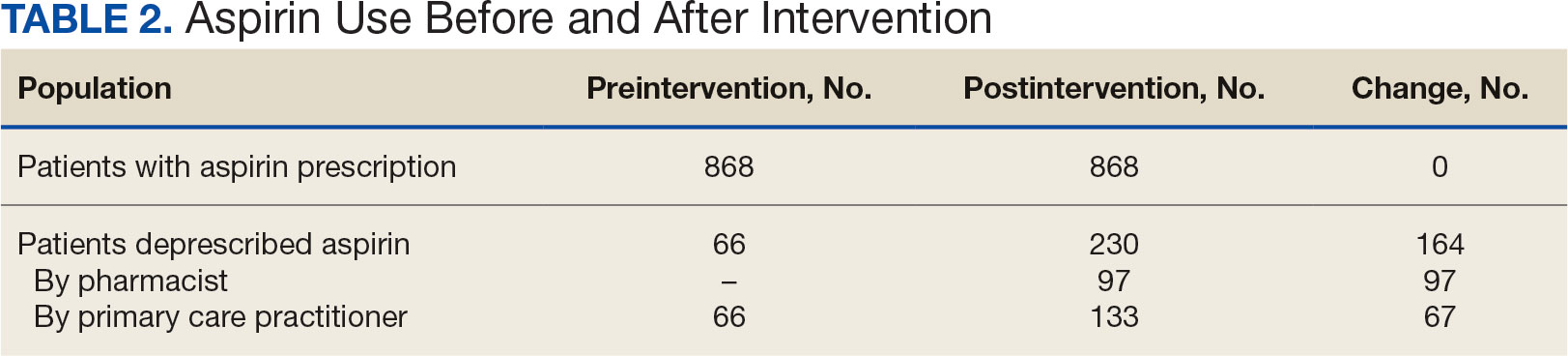
The direct CPP deprescribing intervention resulted in 2 aspirin prescriptions discontinued per hour of pharmacist time and 67 aspirin prescriptions discontinued per hour of pharmacist time via the PCP education intervention. CPPs discontinued 66 aspirin orders in the 12 weeks before the PCP education sessions. A total of 230 aspirin prescriptions were discontinued in the 12 weeks following the PCP education sessions, with 97 discontinued directly by CPPs and 133 discontinued by PCPs. The PCP education session yielded an additional 67 discontinued aspirin orders compared with the 12 weeks before the education sessions (Table 2).
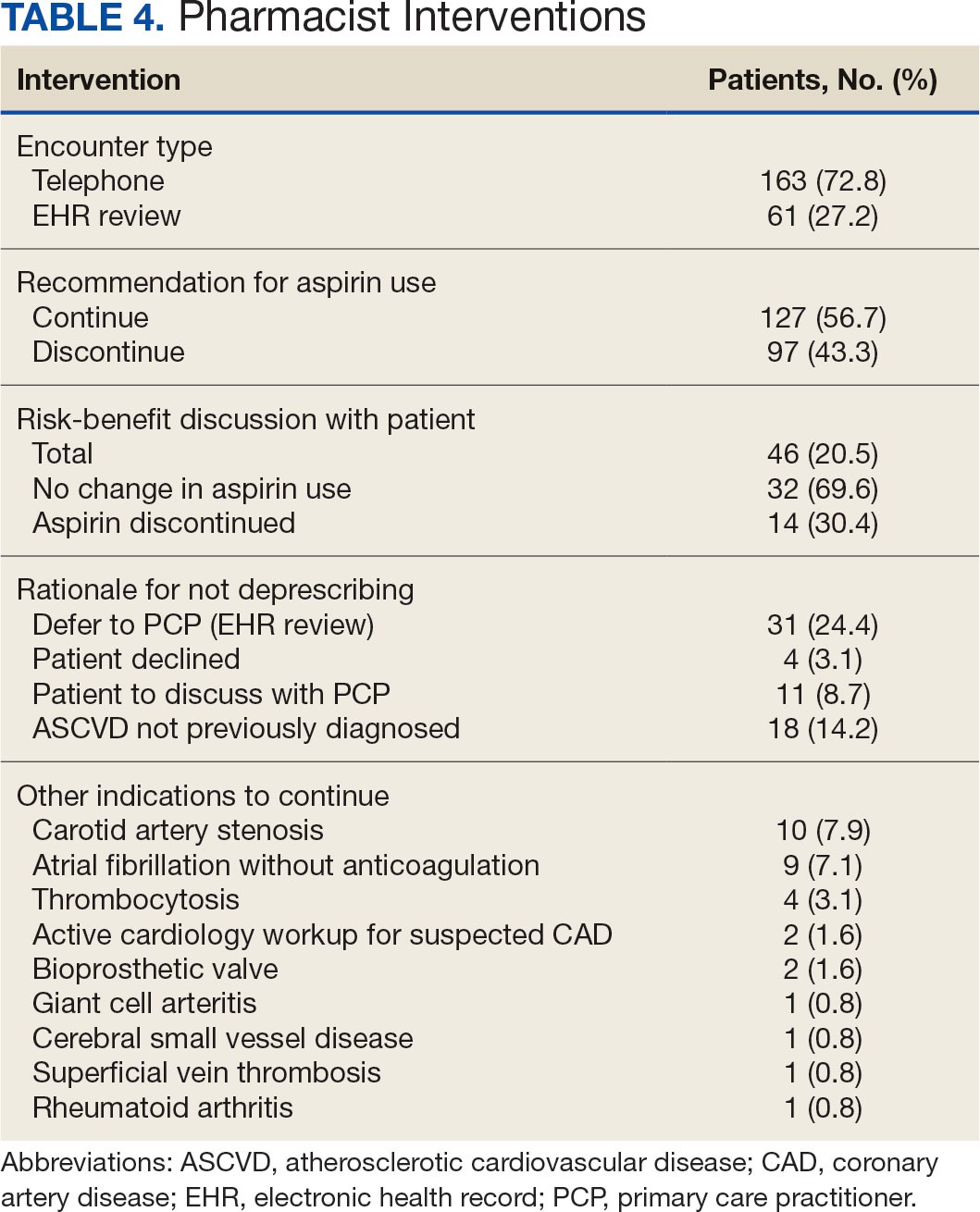
The CPP direct deprescribing intervention took about 48.3 hours, accounting for health record review and time interacting with patients. The PCP education intervention took about 60 minutes, which included time for preparing and delivering education materials (Table 3). CPP deprescribing encounter types, interventions, and related subcategories, and other identified indications to continue aspirin are listed in Table 4.

Discussion
Compared with direct deprescribing by pharmacists, the PCP education intervention was more efficient based on number of aspirin orders discontinued by pharmacist time. PCPs discontinued twice as many aspirin prescriptions in the 12 weeks after pharmacist-led education compared with the 12 weeks before.
Patients were primarily contacted by telephone (73%) for deprescribing. Among the 163 patients reached by phone and encouraged to discontinue aspirin, 97 patients (60%) accepted the recommendation, which was similar to the acceptance rates found in the literature (48% to 55%).14,18 Although many veterans continued taking aspirin (78%), most had indications for its continued use, such as a history of ASCVD, atrial fibrillation without anticoagulation, and carotid artery stenosis, and complex comorbidities that required further discussion with their PCP. Less common uses for aspirin were identified through CPRS review or patient reports included cerebral small vessel disease without history of ASCVD, subclavian artery stenosis, thrombocytosis, bioprosthetic valve replacement, giant cell arteritis, rheumatoid arthritis, and prevention of second eye involvement of ischemic optic neuropathy.
to describe the benefit of clinical pharmacy services for deprescribing aspirin for primary prevention of ASCVD through PCP education. Previously published literature has assessed alternative ways to identify or discontinue PIMs—including aspirin—among geriatric patients. One study evaluated the use of marking inappropriate aspirin prescriptions in the electronic health database, leading to a significant reduction in incidence of inappropriate aspirin prescribing; however, it did not assess changes in discontinuation rates of existing aspirin prescriptions.19 The previous VA pharmacist aspirin deprescribing protocol demonstrated pharmacists’ aptitude at discontinuing aspirin for primary prevention but only used direct patient contact and did not compare efficiency with other methods, including PCP education.14
This quality improvement project contributes new data to the existing literature to support the use of clinical pharmacists to discontinue aspirin for primary prevention and suggests a strong role for pharmacists as educators on clinical guidelines, in addition to their roles directly deprescribing PIMs in clinical practice. This study is further strengthened by its use of VIONE, which previously has demonstrated effectiveness in deprescribing a variety of PIMs in primary care settings.20
Despite using VIONE for generating a list of patients eligible for deprescription, our CPRS review found that this list was frequently inaccurate. For example, a small portion of patients were on the VIONE generated list indicating they had no ASCVD history, but had transient ischemic attack listed in their problem lists. Patient problem lists often were missing documented ASCVD history that was revealed by patient interview or CPRS review. It is possible that patients interviewed might have omitted relevant ASCVD history because of low health literacy, conditions affecting memory, or use of health care services outside the VA system.
There were several instances of aspirin used for other non-ASCVD indications, such as primary stroke prevention in atrial fibrillation. The ACC/AHA atrial fibrillation guidelines previously provided a Class IIb recommendation (benefit is greater than risk but additional studies are needed) for considering no antithrombic therapy or treatment with oral anticoagulant or aspirin for nonvalvular atrial fibrillation with CHA2DS2-VASc (Congestive heart failure, Hypertension, Age [> 65 y, 1 point; > 75 y, 2 points], Diabetes, previous Stroke/transient ischemic attack [2 points]) score of 1.21 The ACC/ AHA guidelines were updated in 2023 to recommend against antiplatelet therapy as an alternative to anticoagulation for reducing cardioembolic stroke risk among patients with atrial fibrillation with no indication for antiplatelet therapy because of risk of harm.22 If a patient has no risk factors for stroke, aspirin is not recommended to prevent thromboembolic events because of a lack of benefit. Interventions from this quality improvement study were completed before the 2023 atrial fibrillation guideline was published and therefore in this study aspirin was not discontinued when used for atrial fibrillation. Aspirin use for atrial fibrillation might benefit from similar discontinuation efforts analyzed within this study. Beyond atrial fibrillation, major guidelines do not comment on the use of aspirin for any other indications in the absence of clinical ASCVD.
Limitations
This study is limited by the lack of clinical consensus for complex patients and demonstrates the importance of individualized patient assessment when considering discontinuing aspirin. Because of the project’s relatively short intervention period, aspirin deprescribing rates could decrease over time and repeated education efforts might be necessary to see lasting impact. Health care professionals from services outside of primary care also might have discontinued aspirin during the study period unrelated to the education and these discontinued aspirin prescriptions could contribute to the higher rate observed among PCPs. This study included a specific population cohort of male, US veterans and might not reflect other populations where these interventions could be implemented.
The measurement of time spent by pharmacists and PCPs is an additional limitation. Although it is expected that PCPs attempt to discontinue aspirin during their existing patient care appointments, the time spent during visits was not measured or documented. Direct deprescribing by pharmacist CPRS review required a significant amount of time and could be a barrier to successful intervention by CPPs in patient aligned care teams.
To reduce the time pharmacists spent completing CPRS reviews, an aspirin deprescribing clinical reminder tool could be used to assess use and appropriate indication quickly during any primary care visit led by a PCP or CPP. In addition, it is recommended that pharmacists regularly educate health care professionals on guideline recommendations for aspirin use among geriatric patients. Future studies of the incidence of major cardiovascular events after aspirin deprescribing among geriatric patients and a longitudinal cost/benefit analysis could support these initiatives.
Conclusions
In this study, pharmacists successfully deprescribed inappropriate medications, such as aspirin. However, pharmacist-led PCP education is more efficient compared with direct deprescribing using a population-level review. PCP education requires less time and could allow ambulatory care pharmacists to spend more time on other direct patient care interventions to improve quality and access to care in primary care clinics. This study’s results further support the role of pharmacists in deprescribing PIMs for older adults and the use of a deprescribing tool, such as VIONE, in a primary care setting.
- US Preventive Services Task Force; Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force recommendation statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
- McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379(16):1519-1528. doi:10.1056/NEJMoa1803955
- Barry MJ, Wolff TA, Pbert L, et al. Putting evidence into practice: an update on the US Preventive Services Task Force methods for developing recommendations for preventive services. Ann Fam Med. 2023;21(2):165-171. doi:10.1370/afm.2946
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/ AHA Guideline on the Primary Prevention of Cardiovascular Disease: A report of the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: Standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S179-S218. doi:10.2337/dc24-S010
- Ong SY, Chui P, Bhargava A, Justice A, Hauser RG. Estimating aspirin overuse for primary prevention of atherosclerotic cardiovascular disease (from a nationwide healthcare system). Am J Cardiol. 2020;137:25-30. doi:10.1016/j.amjcard.2020.09.042
- Weiss AJ, Jiang HJ. Overview of clinical conditions with frequent and costly hospital readmissions by payer, 2018. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); July 20, 2021.
- Krishnaswami A, Steinman MA, Goyal P, et al. Deprescribing in older adults with cardiovascular disease. J Am Coll Cardiol. 2019;73(20):2584-2595. doi:10.1016/j.jacc.2019.03.467
- Association of American Medical Colleges. The complexities of physician supply and demand: projections from 2019 to 2034. Accessed March 17, 2024. https://www.aamc.org/media/54681/download
- US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1108.07(1): General pharmacy service requirements. November 28, 2022. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=10045
- US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1108.11(3): Clinical pharmacy services. July 1, 2015. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3120
- US Department of Veterans Affairs. Clinical pharmacist practitioner (CPP) to improve access to and quality of care August 2021. August 2021. Accessed May 19, 2023. https://www.pbm.va.gov/PBM/CPPO/Documents/ExternalFactSheet_OptimizingtheCPPToImproveAccess_508.pdf
- Ammerman CA, Simpkins BA, Warman N, Downs TN. Potentially inappropriate medications in older adults: Deprescribing with a clinical pharmacist. J Am Geriatr Soc. 2019;67(1):115-118. doi:10.1111/jgs.15623
- Rothbauer K, Siodlak M, Dreischmeier E, Ranola TS, Welch L. Evaluation of a pharmacist-driven ambulatory aspirin deprescribing protocol. Fed Pract. 2022;39(suppl 5):S37- S41a. doi:10.12788/fp.0294
- US Department of Veterans Affairs. VIONE changes the way VA handles prescriptions. January 25, 2020. Accessed May 21, 2023. https://news.va.gov/70709/vione-changes-way-va-handles-prescriptions/
- 2023 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052- 2081. doi:10.1111/jgs.18372
- O’Mahony D, Cherubini A, Guiteras AR, et al. STOPP/ START criteria for potentially inappropriate prescribing in older people: version 3. Eur Geriatr Med. 2023;14(4):625- 632. doi:10.1007/s41999-023-00777-y
- Draeger C, Lodhi F, Geissinger N, Larson T, Griesbach S. Interdisciplinary deprescribing of aspirin through prescriber education and provision of patient-specific recommendations. WMJ. 2022;121(3):220-225
- de Lusignan S, Hinton W, Seidu S, et al. Dashboards to reduce inappropriate prescribing of metformin and aspirin: A quality assurance programme in a primary care sentinel network. Prim Care Diabetes. 2021;15(6):1075-1079. doi:10.1016/j.pcd.2021.06.003
- Nelson MW, Downs TN, Puglisi GM, Simpkins BA, Collier AS. Use of a deprescribing tool in an interdisciplinary primary-care patient-aligned care team. Sr Care Pharm. 2022;37(1):34-43. doi:10.4140/TCP.n.2022.34
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199-e267. doi:10.1161/CIR.0000000000000041
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/ AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines Circulation. 2024;149(1):e1- e156. doi:10.1161/CIR.0000000000001193
Low-dose aspirin commonly is used for the prevention of cardiovascular disease (CVD) but is associated with an increased risk of major bleeding.1 The use of aspirin for primary prevention is largely extrapolated from clinical trials showing benefit in the secondary prevention of myocardial infarction and ischemic stroke. However, results from the Aspirin in Reducing Events in the Elderly (ASPREE) trial challenged this practice.2 The ASPREE trial, conducted in the United States and Australia from 2010 to 2014, sought to determine whether daily 100 mg aspirin, was superior to placebo in promoting disability-free survival among older adults. Participants were aged ≥ 70 years (≥ 65 years for Hispanic and Black US participants), living in the community, and were free from preexisting CVD, cerebrovascular disease, or any chronic condition likely to limit survival to < 5 years. The study found no significant difference in the primary endpoints of death, dementia, or persistent physical disability, but there was a significantly higher risk of major hemorrhage in the aspirin group (3.8% vs 2.8%; hazard ratio, 1.38; 95% CI, 1.18-1.62; P < .001).
Several medical societies have updated their guideline recommendations for aspirin for primary prevention of CVD. The 2022 United States Public Service Task Force (USPSTF) provides a grade C recommendation (at least moderate certainty that the net benefit is small) to consider low-dose aspirin for the primary prevention of CVD on an individual patient basis for adults aged 40 to 59 years who have a ≥ 10% 10-year CVD risk. For adults aged ≥ 60 years, the USPSTF recommendation is grade D (moderate or high certainty that the practice has no net benefit or that harms outweigh the benefits) for low-dose aspirin use.1,3 The American College of Cardiology and American Heart Association (ACC/AHA) recommend considering low-dose aspirin for primary prevention of atherosclerotic cardiovascular disease (ASCVD) among select adults aged 40 to 70 years at higher CVD risk but not at increased risk of bleeding.4 The American Diabetes Association (ADA) recommends low-dose aspirin for primary prevention of CVD in patients with diabetes and additional risk factors such as family history of premature ASCVD, hypertension, dyslipidemia, smoking, or chronic kidney disease, and who are not at higher risk of bleeding.5 The ADA standards also caution against the use of aspirin as primary prevention in patients aged > 70 years. Low-dose aspirin use is not recommended for the primary prevention of CVD in older adults or adults of any age who are at increased risk of bleeding.
Recent literature using the US Department of Veterans Affairs (VA) Corporate Data Warehouse database confirms 86,555 of 1.8 million veterans aged > 70 years (5%) were taking low-dose aspirin for primary prevention of ASCVD despite guideline recommendations.6 Higher risk of gastrointestinal and other major bleeding from low-dose aspirin has been reported in the literature.1 Major bleeds represent a significant burden to the health care system with an estimated mean $13,093 cost for gastrointestinal bleed hospitalization.7
Considering the large scale aspirin use without appropriate indication within the veteran population, the risk of adverse effects, and the significant cost to patients and the health care system, it is imperative to determine the best approach to efficiently deprescribe aspirin for primary prevention among geriatric patients. Deprescribing refers to the systematic and supervised process of dose reduction or drug discontinuation with the goal of improving health and/or reducing the risk of adverse effects.8 During patient visits, primary care practitioners (PCPs) have opportunities to discontinue aspirin, but these encounters are time-limited and deprescribing might be secondary to more acute primary care needs. The shortage of PCPs is expected to worsen in coming years, which could further reduce their availability to assess inappropriate aspirin use.9
VA clinical pharmacist practitioners (CPPs) serve as medication experts and work autonomously under a broad scope of practice as part of the patient aligned care team.10-12 CPPs can free up time for PCPs and facilitate deprescribing efforts, especially for older adults. One retrospective cohort study conducted at a VA medical center found that CPPs deprescribed more potentially inappropriate medications among individuals aged ≥ 80 years compared with usual care with PCPs (26.8% vs 16.1%; P < .001).12,13 An aspirin deprescribing protocol conducted in 2022 resulted in nearly half of veterans aged ≥ 70 years contacted by phone agreeing to stop aspirin. Although this study supports the role pharmacists can play in reducing aspirin use in accordance with guidelines, the authors acknowledge that their interventions had a mean time of 12 minutes per patient and would require workflow changes.14 The purpose of this study is to evaluate the efficiency of aspirin deprescribing through 2 approaches: direct deprescribing by pharmacists using populationlevel review compared with clinicians following a pharmacist-led education.
Methods
This was a single-center quality improvement cohort study at the Durham VA Health Care System (DVAHCS) in North Carolina. Patients included were aged ≥ 70 years without known ASCVD who received care at any of 3 DVAHCS community-based outpatient clinics and prescribed aspirin. Patient data was obtained using the VIONE (Deprescribing Dashboard called Vital, Important, Optional, Not indicated, and Every medication has a specific indication or diagnosis) dashboard.15 VIONE was developed to identify potentially inappropriate medications (PIMs) that are eligible to deprescribe based on Beers Criteria, Screening Tool of Older Personsf Prescriptions criteria, and common clinical scenarios when clinicians determine the risk outweighs the benefit to continue a specific medication. 16,17 VIONE is used to reduce polypharmacy and improve patient safety, comfort, and medication adherence. Aspirin for patients aged ≥ 70 years without a history of ASCVD is a PIM identified by VIONE. Patients aged ≥ 70 years were chosen as an inclusion criteria in this study to match the ASPREE trial inclusion criteria and age inclusion criteria in the VIONE dashboard for aspirin deprescribing.2 Patient lists were generated for these potentially inappropriate aspirin prescriptions for 3 months before clinician staff education presentations, the day of the presentations, and 3 months after.
The primary endpoint was the number of veterans with aspirin deprescribed directly by 2 pharmacists over 12 weeks, divided by total patient care time spent, compared with the change in number of veterans with aspirin deprescribed by any DVAHCS physician, nurse practitioner, physician assistant, or CPP over 12 weeks, divided by the total pharmacist time spent on PCP education. Secondary endpoints were the number of aspirin orders discontinued by pharmacists and CPPs, the number of aspirin orders discontinued 12 weeks before pharmacist-led education compared with the number of aspirin orders discontinued 12 weeks after CPP-led education, average and median pharmacist time spent per patient encounter, and time of direct patient encounters vs time spent on PCP education.
Pharmacists reviewed each patient who met the inclusion criteria from the list generated by VIONE on December 1, 2022, for aspirin appropriateness according to the ACC/AHA and USPSTF guidelines, with the goal to discontinue aspirin for primary prevention of ASCVD and no other indications.1,4 Pharmacists documented their visits using VIONE methodology in the Computerized Patient Record System (CPRS) using a polypharmacy review note. CPPs contacted patients who were taking aspirin for primary prevention by unscheduled telephone call to assess for aspirin adherence, undocumented history of ASCVD, cardiovascular risk factors, and history of bleeding. Aspirin was discontinued if patients met guideline criteria recommendations and agreed to discontinuation. Risk-benefit discussions were completed when patients without known ASCVD were considered high risk because the ACC/AHA guidelines mention there is insufficient evidence of safety and efficacy of aspirin for primary prevention for patients with other known ASCVD risk factors (eg, strong family history of premature myocardial infarction, inability to achieve lipid, blood pressure, or glucose targets, or significant elevation in coronary artery calcium score).
High risk was defined as family history of premature ASCVD (in a male first-degree relative aged < 55 years or a female first-degree relative aged < 65 years), most recent blood pressure or 2 blood pressure results in the last 12 months > 160/100 mm Hg, recent hemoglobin A1c > 9%, and/or low-density lipoprotein > 190 mg/dL or not prescribed an indicated statin.3 Aspirin was continued or discontinued according to patient preference after the personalized risk-benefit discussion.
For patients with a clinical indication for aspirin use other than ASCVD (eg, atrial fibrillation not on anticoagulation, venous thromboembolism prophylaxis, carotid artery disease), CPPs documented their assessment and when appropriate deferred to the PCP for consideration of stopping aspirin. For patients with undocumented ASCVD, CPPs added their ASCVD history to their problem list and aspirin was continued. PCPs were notified by alert when aspirin was discontinued and when patients could not be reached by telephone.
presented a review of recent guideline updates and supporting literature at 2 online staff meetings. The education sessions lasted about 10 minutes and were presented to PCPs across 3 community-based outpatient clinics. An estimated 40 minutes were spent creating the PowerPoint education materials, seeking feedback, making edits, and answering questions or emails from PCPs after the presentation. During the presentation, pharmacists encouraged PCPs to discontinue aspirin (active VA prescriptions and reported over-the-counter use) for primary prevention of ASCVD in patients aged ≥ 70 years during their upcoming appointments and consider risk factors recommended by the ACC/AHA guidelines when applicable. PCPs were notified that CPPs planned to start a population review for discontinuing active VA aspirin prescriptions on December 1, 2022. The primary endpoint and secondary endpoints were analyzed using descriptive statistics. All data were analyzed using Microsoft Excel.

Results
A total of 868 patients aged ≥ 70 years with active prescriptions for aspirin were identified on December 1, 2022. After applying inclusion and exclusion criteria for the pharmacist population review, 224 patients were included for cohort final analysis (Figure). All 868 patients were eligible for the CPP intervention. Primary reasons for exclusion from the CPP population included over-thecounter aspirin and a history of ASCVD in the patient’s problem list. All patients were male, with a mean (SD) age of 75 (4.4) years (Table 1). Most patients were prescribed aspirin, 81 mg daily (n = 220; 98%).

The direct CPP deprescribing intervention resulted in 2 aspirin prescriptions discontinued per hour of pharmacist time and 67 aspirin prescriptions discontinued per hour of pharmacist time via the PCP education intervention. CPPs discontinued 66 aspirin orders in the 12 weeks before the PCP education sessions. A total of 230 aspirin prescriptions were discontinued in the 12 weeks following the PCP education sessions, with 97 discontinued directly by CPPs and 133 discontinued by PCPs. The PCP education session yielded an additional 67 discontinued aspirin orders compared with the 12 weeks before the education sessions (Table 2).

The CPP direct deprescribing intervention took about 48.3 hours, accounting for health record review and time interacting with patients. The PCP education intervention took about 60 minutes, which included time for preparing and delivering education materials (Table 3). CPP deprescribing encounter types, interventions, and related subcategories, and other identified indications to continue aspirin are listed in Table 4.


Discussion
Compared with direct deprescribing by pharmacists, the PCP education intervention was more efficient based on number of aspirin orders discontinued by pharmacist time. PCPs discontinued twice as many aspirin prescriptions in the 12 weeks after pharmacist-led education compared with the 12 weeks before.
Patients were primarily contacted by telephone (73%) for deprescribing. Among the 163 patients reached by phone and encouraged to discontinue aspirin, 97 patients (60%) accepted the recommendation, which was similar to the acceptance rates found in the literature (48% to 55%).14,18 Although many veterans continued taking aspirin (78%), most had indications for its continued use, such as a history of ASCVD, atrial fibrillation without anticoagulation, and carotid artery stenosis, and complex comorbidities that required further discussion with their PCP. Less common uses for aspirin were identified through CPRS review or patient reports included cerebral small vessel disease without history of ASCVD, subclavian artery stenosis, thrombocytosis, bioprosthetic valve replacement, giant cell arteritis, rheumatoid arthritis, and prevention of second eye involvement of ischemic optic neuropathy.
to describe the benefit of clinical pharmacy services for deprescribing aspirin for primary prevention of ASCVD through PCP education. Previously published literature has assessed alternative ways to identify or discontinue PIMs—including aspirin—among geriatric patients. One study evaluated the use of marking inappropriate aspirin prescriptions in the electronic health database, leading to a significant reduction in incidence of inappropriate aspirin prescribing; however, it did not assess changes in discontinuation rates of existing aspirin prescriptions.19 The previous VA pharmacist aspirin deprescribing protocol demonstrated pharmacists’ aptitude at discontinuing aspirin for primary prevention but only used direct patient contact and did not compare efficiency with other methods, including PCP education.14
This quality improvement project contributes new data to the existing literature to support the use of clinical pharmacists to discontinue aspirin for primary prevention and suggests a strong role for pharmacists as educators on clinical guidelines, in addition to their roles directly deprescribing PIMs in clinical practice. This study is further strengthened by its use of VIONE, which previously has demonstrated effectiveness in deprescribing a variety of PIMs in primary care settings.20
Despite using VIONE for generating a list of patients eligible for deprescription, our CPRS review found that this list was frequently inaccurate. For example, a small portion of patients were on the VIONE generated list indicating they had no ASCVD history, but had transient ischemic attack listed in their problem lists. Patient problem lists often were missing documented ASCVD history that was revealed by patient interview or CPRS review. It is possible that patients interviewed might have omitted relevant ASCVD history because of low health literacy, conditions affecting memory, or use of health care services outside the VA system.
There were several instances of aspirin used for other non-ASCVD indications, such as primary stroke prevention in atrial fibrillation. The ACC/AHA atrial fibrillation guidelines previously provided a Class IIb recommendation (benefit is greater than risk but additional studies are needed) for considering no antithrombic therapy or treatment with oral anticoagulant or aspirin for nonvalvular atrial fibrillation with CHA2DS2-VASc (Congestive heart failure, Hypertension, Age [> 65 y, 1 point; > 75 y, 2 points], Diabetes, previous Stroke/transient ischemic attack [2 points]) score of 1.21 The ACC/ AHA guidelines were updated in 2023 to recommend against antiplatelet therapy as an alternative to anticoagulation for reducing cardioembolic stroke risk among patients with atrial fibrillation with no indication for antiplatelet therapy because of risk of harm.22 If a patient has no risk factors for stroke, aspirin is not recommended to prevent thromboembolic events because of a lack of benefit. Interventions from this quality improvement study were completed before the 2023 atrial fibrillation guideline was published and therefore in this study aspirin was not discontinued when used for atrial fibrillation. Aspirin use for atrial fibrillation might benefit from similar discontinuation efforts analyzed within this study. Beyond atrial fibrillation, major guidelines do not comment on the use of aspirin for any other indications in the absence of clinical ASCVD.
Limitations
This study is limited by the lack of clinical consensus for complex patients and demonstrates the importance of individualized patient assessment when considering discontinuing aspirin. Because of the project’s relatively short intervention period, aspirin deprescribing rates could decrease over time and repeated education efforts might be necessary to see lasting impact. Health care professionals from services outside of primary care also might have discontinued aspirin during the study period unrelated to the education and these discontinued aspirin prescriptions could contribute to the higher rate observed among PCPs. This study included a specific population cohort of male, US veterans and might not reflect other populations where these interventions could be implemented.
The measurement of time spent by pharmacists and PCPs is an additional limitation. Although it is expected that PCPs attempt to discontinue aspirin during their existing patient care appointments, the time spent during visits was not measured or documented. Direct deprescribing by pharmacist CPRS review required a significant amount of time and could be a barrier to successful intervention by CPPs in patient aligned care teams.
To reduce the time pharmacists spent completing CPRS reviews, an aspirin deprescribing clinical reminder tool could be used to assess use and appropriate indication quickly during any primary care visit led by a PCP or CPP. In addition, it is recommended that pharmacists regularly educate health care professionals on guideline recommendations for aspirin use among geriatric patients. Future studies of the incidence of major cardiovascular events after aspirin deprescribing among geriatric patients and a longitudinal cost/benefit analysis could support these initiatives.
Conclusions
In this study, pharmacists successfully deprescribed inappropriate medications, such as aspirin. However, pharmacist-led PCP education is more efficient compared with direct deprescribing using a population-level review. PCP education requires less time and could allow ambulatory care pharmacists to spend more time on other direct patient care interventions to improve quality and access to care in primary care clinics. This study’s results further support the role of pharmacists in deprescribing PIMs for older adults and the use of a deprescribing tool, such as VIONE, in a primary care setting.
Low-dose aspirin commonly is used for the prevention of cardiovascular disease (CVD) but is associated with an increased risk of major bleeding.1 The use of aspirin for primary prevention is largely extrapolated from clinical trials showing benefit in the secondary prevention of myocardial infarction and ischemic stroke. However, results from the Aspirin in Reducing Events in the Elderly (ASPREE) trial challenged this practice.2 The ASPREE trial, conducted in the United States and Australia from 2010 to 2014, sought to determine whether daily 100 mg aspirin, was superior to placebo in promoting disability-free survival among older adults. Participants were aged ≥ 70 years (≥ 65 years for Hispanic and Black US participants), living in the community, and were free from preexisting CVD, cerebrovascular disease, or any chronic condition likely to limit survival to < 5 years. The study found no significant difference in the primary endpoints of death, dementia, or persistent physical disability, but there was a significantly higher risk of major hemorrhage in the aspirin group (3.8% vs 2.8%; hazard ratio, 1.38; 95% CI, 1.18-1.62; P < .001).
Several medical societies have updated their guideline recommendations for aspirin for primary prevention of CVD. The 2022 United States Public Service Task Force (USPSTF) provides a grade C recommendation (at least moderate certainty that the net benefit is small) to consider low-dose aspirin for the primary prevention of CVD on an individual patient basis for adults aged 40 to 59 years who have a ≥ 10% 10-year CVD risk. For adults aged ≥ 60 years, the USPSTF recommendation is grade D (moderate or high certainty that the practice has no net benefit or that harms outweigh the benefits) for low-dose aspirin use.1,3 The American College of Cardiology and American Heart Association (ACC/AHA) recommend considering low-dose aspirin for primary prevention of atherosclerotic cardiovascular disease (ASCVD) among select adults aged 40 to 70 years at higher CVD risk but not at increased risk of bleeding.4 The American Diabetes Association (ADA) recommends low-dose aspirin for primary prevention of CVD in patients with diabetes and additional risk factors such as family history of premature ASCVD, hypertension, dyslipidemia, smoking, or chronic kidney disease, and who are not at higher risk of bleeding.5 The ADA standards also caution against the use of aspirin as primary prevention in patients aged > 70 years. Low-dose aspirin use is not recommended for the primary prevention of CVD in older adults or adults of any age who are at increased risk of bleeding.
Recent literature using the US Department of Veterans Affairs (VA) Corporate Data Warehouse database confirms 86,555 of 1.8 million veterans aged > 70 years (5%) were taking low-dose aspirin for primary prevention of ASCVD despite guideline recommendations.6 Higher risk of gastrointestinal and other major bleeding from low-dose aspirin has been reported in the literature.1 Major bleeds represent a significant burden to the health care system with an estimated mean $13,093 cost for gastrointestinal bleed hospitalization.7
Considering the large scale aspirin use without appropriate indication within the veteran population, the risk of adverse effects, and the significant cost to patients and the health care system, it is imperative to determine the best approach to efficiently deprescribe aspirin for primary prevention among geriatric patients. Deprescribing refers to the systematic and supervised process of dose reduction or drug discontinuation with the goal of improving health and/or reducing the risk of adverse effects.8 During patient visits, primary care practitioners (PCPs) have opportunities to discontinue aspirin, but these encounters are time-limited and deprescribing might be secondary to more acute primary care needs. The shortage of PCPs is expected to worsen in coming years, which could further reduce their availability to assess inappropriate aspirin use.9
VA clinical pharmacist practitioners (CPPs) serve as medication experts and work autonomously under a broad scope of practice as part of the patient aligned care team.10-12 CPPs can free up time for PCPs and facilitate deprescribing efforts, especially for older adults. One retrospective cohort study conducted at a VA medical center found that CPPs deprescribed more potentially inappropriate medications among individuals aged ≥ 80 years compared with usual care with PCPs (26.8% vs 16.1%; P < .001).12,13 An aspirin deprescribing protocol conducted in 2022 resulted in nearly half of veterans aged ≥ 70 years contacted by phone agreeing to stop aspirin. Although this study supports the role pharmacists can play in reducing aspirin use in accordance with guidelines, the authors acknowledge that their interventions had a mean time of 12 minutes per patient and would require workflow changes.14 The purpose of this study is to evaluate the efficiency of aspirin deprescribing through 2 approaches: direct deprescribing by pharmacists using populationlevel review compared with clinicians following a pharmacist-led education.
Methods
This was a single-center quality improvement cohort study at the Durham VA Health Care System (DVAHCS) in North Carolina. Patients included were aged ≥ 70 years without known ASCVD who received care at any of 3 DVAHCS community-based outpatient clinics and prescribed aspirin. Patient data was obtained using the VIONE (Deprescribing Dashboard called Vital, Important, Optional, Not indicated, and Every medication has a specific indication or diagnosis) dashboard.15 VIONE was developed to identify potentially inappropriate medications (PIMs) that are eligible to deprescribe based on Beers Criteria, Screening Tool of Older Personsf Prescriptions criteria, and common clinical scenarios when clinicians determine the risk outweighs the benefit to continue a specific medication. 16,17 VIONE is used to reduce polypharmacy and improve patient safety, comfort, and medication adherence. Aspirin for patients aged ≥ 70 years without a history of ASCVD is a PIM identified by VIONE. Patients aged ≥ 70 years were chosen as an inclusion criteria in this study to match the ASPREE trial inclusion criteria and age inclusion criteria in the VIONE dashboard for aspirin deprescribing.2 Patient lists were generated for these potentially inappropriate aspirin prescriptions for 3 months before clinician staff education presentations, the day of the presentations, and 3 months after.
The primary endpoint was the number of veterans with aspirin deprescribed directly by 2 pharmacists over 12 weeks, divided by total patient care time spent, compared with the change in number of veterans with aspirin deprescribed by any DVAHCS physician, nurse practitioner, physician assistant, or CPP over 12 weeks, divided by the total pharmacist time spent on PCP education. Secondary endpoints were the number of aspirin orders discontinued by pharmacists and CPPs, the number of aspirin orders discontinued 12 weeks before pharmacist-led education compared with the number of aspirin orders discontinued 12 weeks after CPP-led education, average and median pharmacist time spent per patient encounter, and time of direct patient encounters vs time spent on PCP education.
Pharmacists reviewed each patient who met the inclusion criteria from the list generated by VIONE on December 1, 2022, for aspirin appropriateness according to the ACC/AHA and USPSTF guidelines, with the goal to discontinue aspirin for primary prevention of ASCVD and no other indications.1,4 Pharmacists documented their visits using VIONE methodology in the Computerized Patient Record System (CPRS) using a polypharmacy review note. CPPs contacted patients who were taking aspirin for primary prevention by unscheduled telephone call to assess for aspirin adherence, undocumented history of ASCVD, cardiovascular risk factors, and history of bleeding. Aspirin was discontinued if patients met guideline criteria recommendations and agreed to discontinuation. Risk-benefit discussions were completed when patients without known ASCVD were considered high risk because the ACC/AHA guidelines mention there is insufficient evidence of safety and efficacy of aspirin for primary prevention for patients with other known ASCVD risk factors (eg, strong family history of premature myocardial infarction, inability to achieve lipid, blood pressure, or glucose targets, or significant elevation in coronary artery calcium score).
High risk was defined as family history of premature ASCVD (in a male first-degree relative aged < 55 years or a female first-degree relative aged < 65 years), most recent blood pressure or 2 blood pressure results in the last 12 months > 160/100 mm Hg, recent hemoglobin A1c > 9%, and/or low-density lipoprotein > 190 mg/dL or not prescribed an indicated statin.3 Aspirin was continued or discontinued according to patient preference after the personalized risk-benefit discussion.
For patients with a clinical indication for aspirin use other than ASCVD (eg, atrial fibrillation not on anticoagulation, venous thromboembolism prophylaxis, carotid artery disease), CPPs documented their assessment and when appropriate deferred to the PCP for consideration of stopping aspirin. For patients with undocumented ASCVD, CPPs added their ASCVD history to their problem list and aspirin was continued. PCPs were notified by alert when aspirin was discontinued and when patients could not be reached by telephone.
presented a review of recent guideline updates and supporting literature at 2 online staff meetings. The education sessions lasted about 10 minutes and were presented to PCPs across 3 community-based outpatient clinics. An estimated 40 minutes were spent creating the PowerPoint education materials, seeking feedback, making edits, and answering questions or emails from PCPs after the presentation. During the presentation, pharmacists encouraged PCPs to discontinue aspirin (active VA prescriptions and reported over-the-counter use) for primary prevention of ASCVD in patients aged ≥ 70 years during their upcoming appointments and consider risk factors recommended by the ACC/AHA guidelines when applicable. PCPs were notified that CPPs planned to start a population review for discontinuing active VA aspirin prescriptions on December 1, 2022. The primary endpoint and secondary endpoints were analyzed using descriptive statistics. All data were analyzed using Microsoft Excel.

Results
A total of 868 patients aged ≥ 70 years with active prescriptions for aspirin were identified on December 1, 2022. After applying inclusion and exclusion criteria for the pharmacist population review, 224 patients were included for cohort final analysis (Figure). All 868 patients were eligible for the CPP intervention. Primary reasons for exclusion from the CPP population included over-thecounter aspirin and a history of ASCVD in the patient’s problem list. All patients were male, with a mean (SD) age of 75 (4.4) years (Table 1). Most patients were prescribed aspirin, 81 mg daily (n = 220; 98%).

The direct CPP deprescribing intervention resulted in 2 aspirin prescriptions discontinued per hour of pharmacist time and 67 aspirin prescriptions discontinued per hour of pharmacist time via the PCP education intervention. CPPs discontinued 66 aspirin orders in the 12 weeks before the PCP education sessions. A total of 230 aspirin prescriptions were discontinued in the 12 weeks following the PCP education sessions, with 97 discontinued directly by CPPs and 133 discontinued by PCPs. The PCP education session yielded an additional 67 discontinued aspirin orders compared with the 12 weeks before the education sessions (Table 2).

The CPP direct deprescribing intervention took about 48.3 hours, accounting for health record review and time interacting with patients. The PCP education intervention took about 60 minutes, which included time for preparing and delivering education materials (Table 3). CPP deprescribing encounter types, interventions, and related subcategories, and other identified indications to continue aspirin are listed in Table 4.


Discussion
Compared with direct deprescribing by pharmacists, the PCP education intervention was more efficient based on number of aspirin orders discontinued by pharmacist time. PCPs discontinued twice as many aspirin prescriptions in the 12 weeks after pharmacist-led education compared with the 12 weeks before.
Patients were primarily contacted by telephone (73%) for deprescribing. Among the 163 patients reached by phone and encouraged to discontinue aspirin, 97 patients (60%) accepted the recommendation, which was similar to the acceptance rates found in the literature (48% to 55%).14,18 Although many veterans continued taking aspirin (78%), most had indications for its continued use, such as a history of ASCVD, atrial fibrillation without anticoagulation, and carotid artery stenosis, and complex comorbidities that required further discussion with their PCP. Less common uses for aspirin were identified through CPRS review or patient reports included cerebral small vessel disease without history of ASCVD, subclavian artery stenosis, thrombocytosis, bioprosthetic valve replacement, giant cell arteritis, rheumatoid arthritis, and prevention of second eye involvement of ischemic optic neuropathy.
to describe the benefit of clinical pharmacy services for deprescribing aspirin for primary prevention of ASCVD through PCP education. Previously published literature has assessed alternative ways to identify or discontinue PIMs—including aspirin—among geriatric patients. One study evaluated the use of marking inappropriate aspirin prescriptions in the electronic health database, leading to a significant reduction in incidence of inappropriate aspirin prescribing; however, it did not assess changes in discontinuation rates of existing aspirin prescriptions.19 The previous VA pharmacist aspirin deprescribing protocol demonstrated pharmacists’ aptitude at discontinuing aspirin for primary prevention but only used direct patient contact and did not compare efficiency with other methods, including PCP education.14
This quality improvement project contributes new data to the existing literature to support the use of clinical pharmacists to discontinue aspirin for primary prevention and suggests a strong role for pharmacists as educators on clinical guidelines, in addition to their roles directly deprescribing PIMs in clinical practice. This study is further strengthened by its use of VIONE, which previously has demonstrated effectiveness in deprescribing a variety of PIMs in primary care settings.20
Despite using VIONE for generating a list of patients eligible for deprescription, our CPRS review found that this list was frequently inaccurate. For example, a small portion of patients were on the VIONE generated list indicating they had no ASCVD history, but had transient ischemic attack listed in their problem lists. Patient problem lists often were missing documented ASCVD history that was revealed by patient interview or CPRS review. It is possible that patients interviewed might have omitted relevant ASCVD history because of low health literacy, conditions affecting memory, or use of health care services outside the VA system.
There were several instances of aspirin used for other non-ASCVD indications, such as primary stroke prevention in atrial fibrillation. The ACC/AHA atrial fibrillation guidelines previously provided a Class IIb recommendation (benefit is greater than risk but additional studies are needed) for considering no antithrombic therapy or treatment with oral anticoagulant or aspirin for nonvalvular atrial fibrillation with CHA2DS2-VASc (Congestive heart failure, Hypertension, Age [> 65 y, 1 point; > 75 y, 2 points], Diabetes, previous Stroke/transient ischemic attack [2 points]) score of 1.21 The ACC/ AHA guidelines were updated in 2023 to recommend against antiplatelet therapy as an alternative to anticoagulation for reducing cardioembolic stroke risk among patients with atrial fibrillation with no indication for antiplatelet therapy because of risk of harm.22 If a patient has no risk factors for stroke, aspirin is not recommended to prevent thromboembolic events because of a lack of benefit. Interventions from this quality improvement study were completed before the 2023 atrial fibrillation guideline was published and therefore in this study aspirin was not discontinued when used for atrial fibrillation. Aspirin use for atrial fibrillation might benefit from similar discontinuation efforts analyzed within this study. Beyond atrial fibrillation, major guidelines do not comment on the use of aspirin for any other indications in the absence of clinical ASCVD.
Limitations
This study is limited by the lack of clinical consensus for complex patients and demonstrates the importance of individualized patient assessment when considering discontinuing aspirin. Because of the project’s relatively short intervention period, aspirin deprescribing rates could decrease over time and repeated education efforts might be necessary to see lasting impact. Health care professionals from services outside of primary care also might have discontinued aspirin during the study period unrelated to the education and these discontinued aspirin prescriptions could contribute to the higher rate observed among PCPs. This study included a specific population cohort of male, US veterans and might not reflect other populations where these interventions could be implemented.
The measurement of time spent by pharmacists and PCPs is an additional limitation. Although it is expected that PCPs attempt to discontinue aspirin during their existing patient care appointments, the time spent during visits was not measured or documented. Direct deprescribing by pharmacist CPRS review required a significant amount of time and could be a barrier to successful intervention by CPPs in patient aligned care teams.
To reduce the time pharmacists spent completing CPRS reviews, an aspirin deprescribing clinical reminder tool could be used to assess use and appropriate indication quickly during any primary care visit led by a PCP or CPP. In addition, it is recommended that pharmacists regularly educate health care professionals on guideline recommendations for aspirin use among geriatric patients. Future studies of the incidence of major cardiovascular events after aspirin deprescribing among geriatric patients and a longitudinal cost/benefit analysis could support these initiatives.
Conclusions
In this study, pharmacists successfully deprescribed inappropriate medications, such as aspirin. However, pharmacist-led PCP education is more efficient compared with direct deprescribing using a population-level review. PCP education requires less time and could allow ambulatory care pharmacists to spend more time on other direct patient care interventions to improve quality and access to care in primary care clinics. This study’s results further support the role of pharmacists in deprescribing PIMs for older adults and the use of a deprescribing tool, such as VIONE, in a primary care setting.
- US Preventive Services Task Force; Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force recommendation statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
- McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379(16):1519-1528. doi:10.1056/NEJMoa1803955
- Barry MJ, Wolff TA, Pbert L, et al. Putting evidence into practice: an update on the US Preventive Services Task Force methods for developing recommendations for preventive services. Ann Fam Med. 2023;21(2):165-171. doi:10.1370/afm.2946
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/ AHA Guideline on the Primary Prevention of Cardiovascular Disease: A report of the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: Standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S179-S218. doi:10.2337/dc24-S010
- Ong SY, Chui P, Bhargava A, Justice A, Hauser RG. Estimating aspirin overuse for primary prevention of atherosclerotic cardiovascular disease (from a nationwide healthcare system). Am J Cardiol. 2020;137:25-30. doi:10.1016/j.amjcard.2020.09.042
- Weiss AJ, Jiang HJ. Overview of clinical conditions with frequent and costly hospital readmissions by payer, 2018. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); July 20, 2021.
- Krishnaswami A, Steinman MA, Goyal P, et al. Deprescribing in older adults with cardiovascular disease. J Am Coll Cardiol. 2019;73(20):2584-2595. doi:10.1016/j.jacc.2019.03.467
- Association of American Medical Colleges. The complexities of physician supply and demand: projections from 2019 to 2034. Accessed March 17, 2024. https://www.aamc.org/media/54681/download
- US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1108.07(1): General pharmacy service requirements. November 28, 2022. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=10045
- US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1108.11(3): Clinical pharmacy services. July 1, 2015. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3120
- US Department of Veterans Affairs. Clinical pharmacist practitioner (CPP) to improve access to and quality of care August 2021. August 2021. Accessed May 19, 2023. https://www.pbm.va.gov/PBM/CPPO/Documents/ExternalFactSheet_OptimizingtheCPPToImproveAccess_508.pdf
- Ammerman CA, Simpkins BA, Warman N, Downs TN. Potentially inappropriate medications in older adults: Deprescribing with a clinical pharmacist. J Am Geriatr Soc. 2019;67(1):115-118. doi:10.1111/jgs.15623
- Rothbauer K, Siodlak M, Dreischmeier E, Ranola TS, Welch L. Evaluation of a pharmacist-driven ambulatory aspirin deprescribing protocol. Fed Pract. 2022;39(suppl 5):S37- S41a. doi:10.12788/fp.0294
- US Department of Veterans Affairs. VIONE changes the way VA handles prescriptions. January 25, 2020. Accessed May 21, 2023. https://news.va.gov/70709/vione-changes-way-va-handles-prescriptions/
- 2023 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052- 2081. doi:10.1111/jgs.18372
- O’Mahony D, Cherubini A, Guiteras AR, et al. STOPP/ START criteria for potentially inappropriate prescribing in older people: version 3. Eur Geriatr Med. 2023;14(4):625- 632. doi:10.1007/s41999-023-00777-y
- Draeger C, Lodhi F, Geissinger N, Larson T, Griesbach S. Interdisciplinary deprescribing of aspirin through prescriber education and provision of patient-specific recommendations. WMJ. 2022;121(3):220-225
- de Lusignan S, Hinton W, Seidu S, et al. Dashboards to reduce inappropriate prescribing of metformin and aspirin: A quality assurance programme in a primary care sentinel network. Prim Care Diabetes. 2021;15(6):1075-1079. doi:10.1016/j.pcd.2021.06.003
- Nelson MW, Downs TN, Puglisi GM, Simpkins BA, Collier AS. Use of a deprescribing tool in an interdisciplinary primary-care patient-aligned care team. Sr Care Pharm. 2022;37(1):34-43. doi:10.4140/TCP.n.2022.34
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199-e267. doi:10.1161/CIR.0000000000000041
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/ AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines Circulation. 2024;149(1):e1- e156. doi:10.1161/CIR.0000000000001193
- US Preventive Services Task Force; Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force recommendation statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
- McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379(16):1519-1528. doi:10.1056/NEJMoa1803955
- Barry MJ, Wolff TA, Pbert L, et al. Putting evidence into practice: an update on the US Preventive Services Task Force methods for developing recommendations for preventive services. Ann Fam Med. 2023;21(2):165-171. doi:10.1370/afm.2946
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/ AHA Guideline on the Primary Prevention of Cardiovascular Disease: A report of the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: Standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S179-S218. doi:10.2337/dc24-S010
- Ong SY, Chui P, Bhargava A, Justice A, Hauser RG. Estimating aspirin overuse for primary prevention of atherosclerotic cardiovascular disease (from a nationwide healthcare system). Am J Cardiol. 2020;137:25-30. doi:10.1016/j.amjcard.2020.09.042
- Weiss AJ, Jiang HJ. Overview of clinical conditions with frequent and costly hospital readmissions by payer, 2018. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); July 20, 2021.
- Krishnaswami A, Steinman MA, Goyal P, et al. Deprescribing in older adults with cardiovascular disease. J Am Coll Cardiol. 2019;73(20):2584-2595. doi:10.1016/j.jacc.2019.03.467
- Association of American Medical Colleges. The complexities of physician supply and demand: projections from 2019 to 2034. Accessed March 17, 2024. https://www.aamc.org/media/54681/download
- US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1108.07(1): General pharmacy service requirements. November 28, 2022. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=10045
- US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1108.11(3): Clinical pharmacy services. July 1, 2015. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3120
- US Department of Veterans Affairs. Clinical pharmacist practitioner (CPP) to improve access to and quality of care August 2021. August 2021. Accessed May 19, 2023. https://www.pbm.va.gov/PBM/CPPO/Documents/ExternalFactSheet_OptimizingtheCPPToImproveAccess_508.pdf
- Ammerman CA, Simpkins BA, Warman N, Downs TN. Potentially inappropriate medications in older adults: Deprescribing with a clinical pharmacist. J Am Geriatr Soc. 2019;67(1):115-118. doi:10.1111/jgs.15623
- Rothbauer K, Siodlak M, Dreischmeier E, Ranola TS, Welch L. Evaluation of a pharmacist-driven ambulatory aspirin deprescribing protocol. Fed Pract. 2022;39(suppl 5):S37- S41a. doi:10.12788/fp.0294
- US Department of Veterans Affairs. VIONE changes the way VA handles prescriptions. January 25, 2020. Accessed May 21, 2023. https://news.va.gov/70709/vione-changes-way-va-handles-prescriptions/
- 2023 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052- 2081. doi:10.1111/jgs.18372
- O’Mahony D, Cherubini A, Guiteras AR, et al. STOPP/ START criteria for potentially inappropriate prescribing in older people: version 3. Eur Geriatr Med. 2023;14(4):625- 632. doi:10.1007/s41999-023-00777-y
- Draeger C, Lodhi F, Geissinger N, Larson T, Griesbach S. Interdisciplinary deprescribing of aspirin through prescriber education and provision of patient-specific recommendations. WMJ. 2022;121(3):220-225
- de Lusignan S, Hinton W, Seidu S, et al. Dashboards to reduce inappropriate prescribing of metformin and aspirin: A quality assurance programme in a primary care sentinel network. Prim Care Diabetes. 2021;15(6):1075-1079. doi:10.1016/j.pcd.2021.06.003
- Nelson MW, Downs TN, Puglisi GM, Simpkins BA, Collier AS. Use of a deprescribing tool in an interdisciplinary primary-care patient-aligned care team. Sr Care Pharm. 2022;37(1):34-43. doi:10.4140/TCP.n.2022.34
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199-e267. doi:10.1161/CIR.0000000000000041
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/ AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines Circulation. 2024;149(1):e1- e156. doi:10.1161/CIR.0000000000001193
Pharmacist-Led Deprescribing of Aspirin for Primary Prevention of Cardiovascular Disease Among Geriatric Veterans
Pharmacist-Led Deprescribing of Aspirin for Primary Prevention of Cardiovascular Disease Among Geriatric Veterans
Cardiac Risks of Newer Psoriasis Biologics vs. TNF Inhibitors Compared
TOPLINE:
The newer biologics — .
METHODOLOGY:
- In a retrospective cohort study, researchers conducted an emulated target trial analysis using data of 32,098 biologic-naive patients with psoriasis or PsA who were treated with one of the newer biologics (infliximab, adalimumab, etanercept, certolizumab pegol, secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, and tildrakizumab) from the TriNetX Research Network between 2014 and 2022.
- Patients received TNF inhibitors (n = 20,314), IL-17 inhibitors (n = 5073), IL-12/23 inhibitors (n = 3573), or IL-23 inhibitors (n = 3138).
- A propensity-matched analysis compared each class of newer biologics with TNF inhibitors, adjusting for demographics, comorbidities, and medication use.
- The primary outcomes were major adverse cardiovascular events (MACE; myocardial infarction and stroke) or venous thromboembolic events (VTE).
TAKEAWAY:
- Compared with patients who received TNF inhibitors, the risk for MACE was not significantly different between patients who received IL-17 inhibitors (incidence rate ratio [IRR], 1.14; 95% CI, 0.86-1.52), IL-12/23 inhibitors (IRR, 1.24; 95% CI, 0.84-1.78), or IL-23 inhibitors (IRR, 0.93; 95% CI, 0.61-1.38)
- The VTE risk was also not significantly different between patients who received IL-17 inhibitors (IRR, 1.12; 95% CI, 0.63-2.08), IL-12/23 inhibitors (IRR, 1.51; 95% CI, 0.73-3.19), or IL-23 inhibitors (IRR, 1.42; 95% CI, 0.64-3.25) compared with those who received TNF inhibitors.
- Subgroup analyses for psoriasis or psoriatic arthritis alone confirmed consistent findings.
- Patients with preexisting hyperlipidemia and diabetes mellitus showed lower risks for MACE and VTE with newer biologics compared with TNF inhibitors.
IN PRACTICE:
“No significant MACE and VTE risk differences were detected in patients with psoriasis or PsA between those receiving IL-17, IL-12/23, and IL-23 inhibitors and those with TNF inhibitors,” the authors concluded. These findings, they added “can be considered by physicians and patients when making treatment decisions” and also provide “evidence for future pharmacovigilance studies.”
SOURCE:
The study was led by Tai-Li Chen, MD, of the Department of Dermatology, Taipei Veterans General Hospital in Taipei, Taiwan. It was published online on December 27, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Study limitations included potential residual confounding factors, lack of information on disease severity, and inclusion of predominantly White individuals.
DISCLOSURES:
The study received support from Taipei Veterans General Hospital and Ministry of Science and Technology, Taiwan. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The newer biologics — .
METHODOLOGY:
- In a retrospective cohort study, researchers conducted an emulated target trial analysis using data of 32,098 biologic-naive patients with psoriasis or PsA who were treated with one of the newer biologics (infliximab, adalimumab, etanercept, certolizumab pegol, secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, and tildrakizumab) from the TriNetX Research Network between 2014 and 2022.
- Patients received TNF inhibitors (n = 20,314), IL-17 inhibitors (n = 5073), IL-12/23 inhibitors (n = 3573), or IL-23 inhibitors (n = 3138).
- A propensity-matched analysis compared each class of newer biologics with TNF inhibitors, adjusting for demographics, comorbidities, and medication use.
- The primary outcomes were major adverse cardiovascular events (MACE; myocardial infarction and stroke) or venous thromboembolic events (VTE).
TAKEAWAY:
- Compared with patients who received TNF inhibitors, the risk for MACE was not significantly different between patients who received IL-17 inhibitors (incidence rate ratio [IRR], 1.14; 95% CI, 0.86-1.52), IL-12/23 inhibitors (IRR, 1.24; 95% CI, 0.84-1.78), or IL-23 inhibitors (IRR, 0.93; 95% CI, 0.61-1.38)
- The VTE risk was also not significantly different between patients who received IL-17 inhibitors (IRR, 1.12; 95% CI, 0.63-2.08), IL-12/23 inhibitors (IRR, 1.51; 95% CI, 0.73-3.19), or IL-23 inhibitors (IRR, 1.42; 95% CI, 0.64-3.25) compared with those who received TNF inhibitors.
- Subgroup analyses for psoriasis or psoriatic arthritis alone confirmed consistent findings.
- Patients with preexisting hyperlipidemia and diabetes mellitus showed lower risks for MACE and VTE with newer biologics compared with TNF inhibitors.
IN PRACTICE:
“No significant MACE and VTE risk differences were detected in patients with psoriasis or PsA between those receiving IL-17, IL-12/23, and IL-23 inhibitors and those with TNF inhibitors,” the authors concluded. These findings, they added “can be considered by physicians and patients when making treatment decisions” and also provide “evidence for future pharmacovigilance studies.”
SOURCE:
The study was led by Tai-Li Chen, MD, of the Department of Dermatology, Taipei Veterans General Hospital in Taipei, Taiwan. It was published online on December 27, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Study limitations included potential residual confounding factors, lack of information on disease severity, and inclusion of predominantly White individuals.
DISCLOSURES:
The study received support from Taipei Veterans General Hospital and Ministry of Science and Technology, Taiwan. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The newer biologics — .
METHODOLOGY:
- In a retrospective cohort study, researchers conducted an emulated target trial analysis using data of 32,098 biologic-naive patients with psoriasis or PsA who were treated with one of the newer biologics (infliximab, adalimumab, etanercept, certolizumab pegol, secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, and tildrakizumab) from the TriNetX Research Network between 2014 and 2022.
- Patients received TNF inhibitors (n = 20,314), IL-17 inhibitors (n = 5073), IL-12/23 inhibitors (n = 3573), or IL-23 inhibitors (n = 3138).
- A propensity-matched analysis compared each class of newer biologics with TNF inhibitors, adjusting for demographics, comorbidities, and medication use.
- The primary outcomes were major adverse cardiovascular events (MACE; myocardial infarction and stroke) or venous thromboembolic events (VTE).
TAKEAWAY:
- Compared with patients who received TNF inhibitors, the risk for MACE was not significantly different between patients who received IL-17 inhibitors (incidence rate ratio [IRR], 1.14; 95% CI, 0.86-1.52), IL-12/23 inhibitors (IRR, 1.24; 95% CI, 0.84-1.78), or IL-23 inhibitors (IRR, 0.93; 95% CI, 0.61-1.38)
- The VTE risk was also not significantly different between patients who received IL-17 inhibitors (IRR, 1.12; 95% CI, 0.63-2.08), IL-12/23 inhibitors (IRR, 1.51; 95% CI, 0.73-3.19), or IL-23 inhibitors (IRR, 1.42; 95% CI, 0.64-3.25) compared with those who received TNF inhibitors.
- Subgroup analyses for psoriasis or psoriatic arthritis alone confirmed consistent findings.
- Patients with preexisting hyperlipidemia and diabetes mellitus showed lower risks for MACE and VTE with newer biologics compared with TNF inhibitors.
IN PRACTICE:
“No significant MACE and VTE risk differences were detected in patients with psoriasis or PsA between those receiving IL-17, IL-12/23, and IL-23 inhibitors and those with TNF inhibitors,” the authors concluded. These findings, they added “can be considered by physicians and patients when making treatment decisions” and also provide “evidence for future pharmacovigilance studies.”
SOURCE:
The study was led by Tai-Li Chen, MD, of the Department of Dermatology, Taipei Veterans General Hospital in Taipei, Taiwan. It was published online on December 27, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Study limitations included potential residual confounding factors, lack of information on disease severity, and inclusion of predominantly White individuals.
DISCLOSURES:
The study received support from Taipei Veterans General Hospital and Ministry of Science and Technology, Taiwan. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
On Second Thought: Making Sense of Blood Pressure Guidelines — What Happened in the 1930s Should Stay There
This transcript has been edited for clarity.
Blood pressure. If you’re a primary care provider trying to do right by your patients, you might be understandably confused by the current mishmash of guidelines with different blood pressure targets. But as chaotic as things are, at least it’s not the 1930s, when you might hear John Hay give a lecture to the British Medical Association and say, “The greatest danger to a man with high blood pressure lies in its discovery, because then some fool is certain to try and reduce it.”
Yeah, he said that. But what happened in the 1930s stays in the 1930s. And now we can at least agree that we should be treating high blood pressure. But what’s the goal we should be aiming for? This is On Second Thought.
We’ve come a long way since FDR was recording blood pressures of 200 and his doctor prescribed him barbiturates and massage therapy.
That s#$# don’t fly no more. Over the past hundred years, we have become much more aggressive in treating blood pressure. Remember the Oslo study? It defined mild hypertension as a blood pressure between 150 and 180 mm Hg. Now, those numbers send people screaming to the emergency room. So, let’s acknowledge that things are substantially better than they once were. Let’s agree on that and we can start to heal this nation again.
Before we get into the numbers, when we’re treating blood pressure, let’s make a few points about measuring it. Obviously, to treat something, you have to measure it properly. Two recent trials have illustrated that these details matter a lot.
The Cuff(SZ) randomized crossover trial — and it took me a minute to realize that Cuff(SZ) meant cuff size, so bravo, Ishigami et al — showed that picking the wrong cuff size could affect BP measurements by 4.5 points if you were one size off. If you were two sizes too small, you overestimated BP by almost 20 points.
Add on here another recent study, the ARMS crossover randomized clinical trial, looking at how arm position affected BP measures. If the arm was resting on your lap or hanging by your side, that overestimated blood pressure by 4 and 6.5 points. So sometimes you have to remember the fundamentals: cuff size, arm position — it might make the difference between increasing or maintaining the patient’s meds.
But on to the main show. What numbers should we be aiming for? We no longer live in the “BP 200, the president’s going to have a stroke” world of the 1940s, and even a BP of 150 is considered quite high these days. Studies like the MRC trial, INVEST, and SPRINT have pushed BP targets ever lower. SPRINT, in particular, randomized patients to a blood pressure target under 120 systolic vs under 140 systolic, and the under-120 arm won out with fewer cardiovascular events and lower all-cause mortality.
Pretty definitive slam dunk. But the more intensive treatment came with more hypotension, syncope, and kidney injury, because there is no free lunch in medicine. And ditto with BPROAD, just published in The New England Journal of Medicine and presented at the American Heart Association annual meeting. A diabetic population randomized to 120 vs 140 as a BP target showed that more aggressive treatment was better.
Fewer cardiovascular events, like stroke, but no mortality difference, and more hypotension. So a cardiovascular benefit at the cost of more side effects. Now, like all cardiologists, my motto is “Save the heart and screw the kidney.” But if you do care about the other organs in this meat sack that we call a human body, the question you need to wrestle with is, how much do you value cardiovascular protection vs how willing are you to tolerate side effects?
Hypotension may not sound dangerous, but gravity is an unforgiving mistress. If you painstakingly compile the summary of the various BP guidelines for easy perusal, you would notice something critical: One, I have too much free time on my hands; two, the disagreements are not really all that profound.
Arguing about 120 vs 130 vs 140 is not the same as saying, “Drugs schmugs; a good massage will fix what ails you, and here are some addictive sleeping pills for good measure.” Physicians from the 1930s were a little sketchy. So much of this controversy is about how you define high-risk patients and what are the age cutoffs.
Basically, the cardiovascular guidelines say, “Treat them all and let God sort it out” because they care about cardiovascular events and are concerned about cardiovascular endpoints. Whereas general practice guidelines put more emphasis on potential side effects and admittedly tend to treat a not so high-risk population, so they have laxer targets.
A 2014 analysis from the Blood Pressure Lowering Treatment Trialists’ Collaboration [The Lancet] had a good mathematical way of explaining this problem. Now, lowering blood pressure is obviously a good thing. That prevents heart attacks, strokes, kidney failure, and all that. Please don’t let hypertension denialism become a thing.
Let’s start with the basics. Treating high blood pressure led to a 15% to 18% decrease in cardiovascular events, pretty consistently across all risk categories, and other analyses have found that every 5-point decrease in blood pressure gives you about a 10% decrease in major cardiovascular events on the relative-risk scale.
While the benefits are pretty consistent across all groups, that difference in baseline risk translates into different absolute benefits. In the Lancet paper, when the population was divided into four different groups based on their cardiovascular risk, the absolute risk reduction in the lowest-risk group was 14 fewer cardiovascular events if you treat 1000 patients for 5 years.
With each higher-risk group, it was 20 fewer, 24 fewer, and 38 fewer. At the lowest-risk group, the number needed to treat was 71, 50, 42, and 26 fewer cardiovascular events with 5 years of treatment.
And herein lies the secret to the disagreement: If you have a high-risk patient, there is a big benefit to bringing that blood pressure down from 135 to 130. Whereas for a low-risk patient, it probably doesn’t matter as much. And the cardiovascular benefits are going to be offset by the side effects and the risks for hypotension.
Of course, there’s a simple solution to this dilemma: Just speak to the patient in front of you. Treat high blood pressure, and if your patient’s blood pressure drops or they get dizzy or have fainting spells, then just ease up on the meds. It’s not rocket science; it’s just cardiology.
Arguing about five millimeters of mercury of blood pressure is probably less important from the public health perspective than the fact that tens of millions of people in the United States are unaware that they have hypertension, and even those diagnosed are being inadequately treated.
So, let’s all do better as a medical community. Nobody should have untreated hypertension in this day and age. It’s not the 1930s.
Dr Labos, Cardiologist, Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Blood pressure. If you’re a primary care provider trying to do right by your patients, you might be understandably confused by the current mishmash of guidelines with different blood pressure targets. But as chaotic as things are, at least it’s not the 1930s, when you might hear John Hay give a lecture to the British Medical Association and say, “The greatest danger to a man with high blood pressure lies in its discovery, because then some fool is certain to try and reduce it.”
Yeah, he said that. But what happened in the 1930s stays in the 1930s. And now we can at least agree that we should be treating high blood pressure. But what’s the goal we should be aiming for? This is On Second Thought.
We’ve come a long way since FDR was recording blood pressures of 200 and his doctor prescribed him barbiturates and massage therapy.
That s#$# don’t fly no more. Over the past hundred years, we have become much more aggressive in treating blood pressure. Remember the Oslo study? It defined mild hypertension as a blood pressure between 150 and 180 mm Hg. Now, those numbers send people screaming to the emergency room. So, let’s acknowledge that things are substantially better than they once were. Let’s agree on that and we can start to heal this nation again.
Before we get into the numbers, when we’re treating blood pressure, let’s make a few points about measuring it. Obviously, to treat something, you have to measure it properly. Two recent trials have illustrated that these details matter a lot.
The Cuff(SZ) randomized crossover trial — and it took me a minute to realize that Cuff(SZ) meant cuff size, so bravo, Ishigami et al — showed that picking the wrong cuff size could affect BP measurements by 4.5 points if you were one size off. If you were two sizes too small, you overestimated BP by almost 20 points.
Add on here another recent study, the ARMS crossover randomized clinical trial, looking at how arm position affected BP measures. If the arm was resting on your lap or hanging by your side, that overestimated blood pressure by 4 and 6.5 points. So sometimes you have to remember the fundamentals: cuff size, arm position — it might make the difference between increasing or maintaining the patient’s meds.
But on to the main show. What numbers should we be aiming for? We no longer live in the “BP 200, the president’s going to have a stroke” world of the 1940s, and even a BP of 150 is considered quite high these days. Studies like the MRC trial, INVEST, and SPRINT have pushed BP targets ever lower. SPRINT, in particular, randomized patients to a blood pressure target under 120 systolic vs under 140 systolic, and the under-120 arm won out with fewer cardiovascular events and lower all-cause mortality.
Pretty definitive slam dunk. But the more intensive treatment came with more hypotension, syncope, and kidney injury, because there is no free lunch in medicine. And ditto with BPROAD, just published in The New England Journal of Medicine and presented at the American Heart Association annual meeting. A diabetic population randomized to 120 vs 140 as a BP target showed that more aggressive treatment was better.
Fewer cardiovascular events, like stroke, but no mortality difference, and more hypotension. So a cardiovascular benefit at the cost of more side effects. Now, like all cardiologists, my motto is “Save the heart and screw the kidney.” But if you do care about the other organs in this meat sack that we call a human body, the question you need to wrestle with is, how much do you value cardiovascular protection vs how willing are you to tolerate side effects?
Hypotension may not sound dangerous, but gravity is an unforgiving mistress. If you painstakingly compile the summary of the various BP guidelines for easy perusal, you would notice something critical: One, I have too much free time on my hands; two, the disagreements are not really all that profound.
Arguing about 120 vs 130 vs 140 is not the same as saying, “Drugs schmugs; a good massage will fix what ails you, and here are some addictive sleeping pills for good measure.” Physicians from the 1930s were a little sketchy. So much of this controversy is about how you define high-risk patients and what are the age cutoffs.
Basically, the cardiovascular guidelines say, “Treat them all and let God sort it out” because they care about cardiovascular events and are concerned about cardiovascular endpoints. Whereas general practice guidelines put more emphasis on potential side effects and admittedly tend to treat a not so high-risk population, so they have laxer targets.
A 2014 analysis from the Blood Pressure Lowering Treatment Trialists’ Collaboration [The Lancet] had a good mathematical way of explaining this problem. Now, lowering blood pressure is obviously a good thing. That prevents heart attacks, strokes, kidney failure, and all that. Please don’t let hypertension denialism become a thing.
Let’s start with the basics. Treating high blood pressure led to a 15% to 18% decrease in cardiovascular events, pretty consistently across all risk categories, and other analyses have found that every 5-point decrease in blood pressure gives you about a 10% decrease in major cardiovascular events on the relative-risk scale.
While the benefits are pretty consistent across all groups, that difference in baseline risk translates into different absolute benefits. In the Lancet paper, when the population was divided into four different groups based on their cardiovascular risk, the absolute risk reduction in the lowest-risk group was 14 fewer cardiovascular events if you treat 1000 patients for 5 years.
With each higher-risk group, it was 20 fewer, 24 fewer, and 38 fewer. At the lowest-risk group, the number needed to treat was 71, 50, 42, and 26 fewer cardiovascular events with 5 years of treatment.
And herein lies the secret to the disagreement: If you have a high-risk patient, there is a big benefit to bringing that blood pressure down from 135 to 130. Whereas for a low-risk patient, it probably doesn’t matter as much. And the cardiovascular benefits are going to be offset by the side effects and the risks for hypotension.
Of course, there’s a simple solution to this dilemma: Just speak to the patient in front of you. Treat high blood pressure, and if your patient’s blood pressure drops or they get dizzy or have fainting spells, then just ease up on the meds. It’s not rocket science; it’s just cardiology.
Arguing about five millimeters of mercury of blood pressure is probably less important from the public health perspective than the fact that tens of millions of people in the United States are unaware that they have hypertension, and even those diagnosed are being inadequately treated.
So, let’s all do better as a medical community. Nobody should have untreated hypertension in this day and age. It’s not the 1930s.
Dr Labos, Cardiologist, Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Blood pressure. If you’re a primary care provider trying to do right by your patients, you might be understandably confused by the current mishmash of guidelines with different blood pressure targets. But as chaotic as things are, at least it’s not the 1930s, when you might hear John Hay give a lecture to the British Medical Association and say, “The greatest danger to a man with high blood pressure lies in its discovery, because then some fool is certain to try and reduce it.”
Yeah, he said that. But what happened in the 1930s stays in the 1930s. And now we can at least agree that we should be treating high blood pressure. But what’s the goal we should be aiming for? This is On Second Thought.
We’ve come a long way since FDR was recording blood pressures of 200 and his doctor prescribed him barbiturates and massage therapy.
That s#$# don’t fly no more. Over the past hundred years, we have become much more aggressive in treating blood pressure. Remember the Oslo study? It defined mild hypertension as a blood pressure between 150 and 180 mm Hg. Now, those numbers send people screaming to the emergency room. So, let’s acknowledge that things are substantially better than they once were. Let’s agree on that and we can start to heal this nation again.
Before we get into the numbers, when we’re treating blood pressure, let’s make a few points about measuring it. Obviously, to treat something, you have to measure it properly. Two recent trials have illustrated that these details matter a lot.
The Cuff(SZ) randomized crossover trial — and it took me a minute to realize that Cuff(SZ) meant cuff size, so bravo, Ishigami et al — showed that picking the wrong cuff size could affect BP measurements by 4.5 points if you were one size off. If you were two sizes too small, you overestimated BP by almost 20 points.
Add on here another recent study, the ARMS crossover randomized clinical trial, looking at how arm position affected BP measures. If the arm was resting on your lap or hanging by your side, that overestimated blood pressure by 4 and 6.5 points. So sometimes you have to remember the fundamentals: cuff size, arm position — it might make the difference between increasing or maintaining the patient’s meds.
But on to the main show. What numbers should we be aiming for? We no longer live in the “BP 200, the president’s going to have a stroke” world of the 1940s, and even a BP of 150 is considered quite high these days. Studies like the MRC trial, INVEST, and SPRINT have pushed BP targets ever lower. SPRINT, in particular, randomized patients to a blood pressure target under 120 systolic vs under 140 systolic, and the under-120 arm won out with fewer cardiovascular events and lower all-cause mortality.
Pretty definitive slam dunk. But the more intensive treatment came with more hypotension, syncope, and kidney injury, because there is no free lunch in medicine. And ditto with BPROAD, just published in The New England Journal of Medicine and presented at the American Heart Association annual meeting. A diabetic population randomized to 120 vs 140 as a BP target showed that more aggressive treatment was better.
Fewer cardiovascular events, like stroke, but no mortality difference, and more hypotension. So a cardiovascular benefit at the cost of more side effects. Now, like all cardiologists, my motto is “Save the heart and screw the kidney.” But if you do care about the other organs in this meat sack that we call a human body, the question you need to wrestle with is, how much do you value cardiovascular protection vs how willing are you to tolerate side effects?
Hypotension may not sound dangerous, but gravity is an unforgiving mistress. If you painstakingly compile the summary of the various BP guidelines for easy perusal, you would notice something critical: One, I have too much free time on my hands; two, the disagreements are not really all that profound.
Arguing about 120 vs 130 vs 140 is not the same as saying, “Drugs schmugs; a good massage will fix what ails you, and here are some addictive sleeping pills for good measure.” Physicians from the 1930s were a little sketchy. So much of this controversy is about how you define high-risk patients and what are the age cutoffs.
Basically, the cardiovascular guidelines say, “Treat them all and let God sort it out” because they care about cardiovascular events and are concerned about cardiovascular endpoints. Whereas general practice guidelines put more emphasis on potential side effects and admittedly tend to treat a not so high-risk population, so they have laxer targets.
A 2014 analysis from the Blood Pressure Lowering Treatment Trialists’ Collaboration [The Lancet] had a good mathematical way of explaining this problem. Now, lowering blood pressure is obviously a good thing. That prevents heart attacks, strokes, kidney failure, and all that. Please don’t let hypertension denialism become a thing.
Let’s start with the basics. Treating high blood pressure led to a 15% to 18% decrease in cardiovascular events, pretty consistently across all risk categories, and other analyses have found that every 5-point decrease in blood pressure gives you about a 10% decrease in major cardiovascular events on the relative-risk scale.
While the benefits are pretty consistent across all groups, that difference in baseline risk translates into different absolute benefits. In the Lancet paper, when the population was divided into four different groups based on their cardiovascular risk, the absolute risk reduction in the lowest-risk group was 14 fewer cardiovascular events if you treat 1000 patients for 5 years.
With each higher-risk group, it was 20 fewer, 24 fewer, and 38 fewer. At the lowest-risk group, the number needed to treat was 71, 50, 42, and 26 fewer cardiovascular events with 5 years of treatment.
And herein lies the secret to the disagreement: If you have a high-risk patient, there is a big benefit to bringing that blood pressure down from 135 to 130. Whereas for a low-risk patient, it probably doesn’t matter as much. And the cardiovascular benefits are going to be offset by the side effects and the risks for hypotension.
Of course, there’s a simple solution to this dilemma: Just speak to the patient in front of you. Treat high blood pressure, and if your patient’s blood pressure drops or they get dizzy or have fainting spells, then just ease up on the meds. It’s not rocket science; it’s just cardiology.
Arguing about five millimeters of mercury of blood pressure is probably less important from the public health perspective than the fact that tens of millions of people in the United States are unaware that they have hypertension, and even those diagnosed are being inadequately treated.
So, let’s all do better as a medical community. Nobody should have untreated hypertension in this day and age. It’s not the 1930s.
Dr Labos, Cardiologist, Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Exercising Longer May Boost Weight Loss, Meta-Analysis Shows
TOPLINE:
Aerobic exercise shows a linear relationship with weight loss, with 30 minutes of weekly exercise linked to reduced body weight, waist circumference, and body fat in adults who were overweight or had obesity.
METHODOLOGY:
- Researchers conducted a meta-analysis of randomized clinical trials to investigate the association of varying intensities and durations of aerobic exercise with adiposity measures in adults with obesity or who were overweight.
- Overall, 116 randomized clinical trials that spanned across North America, Asia, Europe, Australia, South America, and Africa and involved 6880 adults (mean age, 46 years; 61% women) were included.
- The trials were required to have intervention durations of at least 8 weeks; all trials used supervised aerobic exercise, such as walking or running, while the control groups remained sedentary or continued usual activities.
- The intensity of exercise was defined as: Light (40%-55% maximum heart rate), moderate (55%-70% maximum heart rate), and vigorous (70%-90% maximum heart rate).
- The primary outcomes were body weight changes and adverse events; the secondary outcomes included changes in waist circumference, quality-of-life scores, and reduction in medications like antihypertensives.
TAKEAWAY:
- Every 30 minutes of aerobic exercise per week was also associated with lower waist circumference (mean difference, −0.56 cm; 95% CI, –0.67 to –0.45), body fat percentage (mean difference, –0.37%; 95% CI, –0.43 to –0.31), and body fat mass (mean difference, –0.20 kg; 95% CI, –0.32 to –0.08), along with reduced visceral and subcutaneous adipose tissue.
- A dose-response meta-analysis revealed that body fat percentage improved most significantly with 150 minutes of aerobic exercise per week, while body weight and waist circumference decreased linearly with increasing duration of aerobic exercise at 300 min/wk at different intensities.
- Adverse events with aerobic exercise were mostly mild or moderate musculoskeletal symptoms.
IN PRACTICE:
“Point-specific estimates for different aerobic exercise duration and intensity can help patients and healthcare professionals select the optimal aerobic exercise duration and intensity according to their weight loss goals,” the authors wrote.
SOURCE:
The study was led by Ahmad Jayedi, PhD, of the Department of Epidemiology and Biostatistics in the School of Public Health at the Imperial College London in England. It was published online on December 26, 2024, in JAMA Network Open.
LIMITATIONS:
High heterogeneity was present in the data. Only one trial included measures of health-related quality of life, and two studies included measures of medication use. Dietary habits and smoking status of participants were not included in studies, so any potential effects were not risk adjusted for.
DISCLOSURES:
No funding sources were reported. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Aerobic exercise shows a linear relationship with weight loss, with 30 minutes of weekly exercise linked to reduced body weight, waist circumference, and body fat in adults who were overweight or had obesity.
METHODOLOGY:
- Researchers conducted a meta-analysis of randomized clinical trials to investigate the association of varying intensities and durations of aerobic exercise with adiposity measures in adults with obesity or who were overweight.
- Overall, 116 randomized clinical trials that spanned across North America, Asia, Europe, Australia, South America, and Africa and involved 6880 adults (mean age, 46 years; 61% women) were included.
- The trials were required to have intervention durations of at least 8 weeks; all trials used supervised aerobic exercise, such as walking or running, while the control groups remained sedentary or continued usual activities.
- The intensity of exercise was defined as: Light (40%-55% maximum heart rate), moderate (55%-70% maximum heart rate), and vigorous (70%-90% maximum heart rate).
- The primary outcomes were body weight changes and adverse events; the secondary outcomes included changes in waist circumference, quality-of-life scores, and reduction in medications like antihypertensives.
TAKEAWAY:
- Every 30 minutes of aerobic exercise per week was also associated with lower waist circumference (mean difference, −0.56 cm; 95% CI, –0.67 to –0.45), body fat percentage (mean difference, –0.37%; 95% CI, –0.43 to –0.31), and body fat mass (mean difference, –0.20 kg; 95% CI, –0.32 to –0.08), along with reduced visceral and subcutaneous adipose tissue.
- A dose-response meta-analysis revealed that body fat percentage improved most significantly with 150 minutes of aerobic exercise per week, while body weight and waist circumference decreased linearly with increasing duration of aerobic exercise at 300 min/wk at different intensities.
- Adverse events with aerobic exercise were mostly mild or moderate musculoskeletal symptoms.
IN PRACTICE:
“Point-specific estimates for different aerobic exercise duration and intensity can help patients and healthcare professionals select the optimal aerobic exercise duration and intensity according to their weight loss goals,” the authors wrote.
SOURCE:
The study was led by Ahmad Jayedi, PhD, of the Department of Epidemiology and Biostatistics in the School of Public Health at the Imperial College London in England. It was published online on December 26, 2024, in JAMA Network Open.
LIMITATIONS:
High heterogeneity was present in the data. Only one trial included measures of health-related quality of life, and two studies included measures of medication use. Dietary habits and smoking status of participants were not included in studies, so any potential effects were not risk adjusted for.
DISCLOSURES:
No funding sources were reported. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Aerobic exercise shows a linear relationship with weight loss, with 30 minutes of weekly exercise linked to reduced body weight, waist circumference, and body fat in adults who were overweight or had obesity.
METHODOLOGY:
- Researchers conducted a meta-analysis of randomized clinical trials to investigate the association of varying intensities and durations of aerobic exercise with adiposity measures in adults with obesity or who were overweight.
- Overall, 116 randomized clinical trials that spanned across North America, Asia, Europe, Australia, South America, and Africa and involved 6880 adults (mean age, 46 years; 61% women) were included.
- The trials were required to have intervention durations of at least 8 weeks; all trials used supervised aerobic exercise, such as walking or running, while the control groups remained sedentary or continued usual activities.
- The intensity of exercise was defined as: Light (40%-55% maximum heart rate), moderate (55%-70% maximum heart rate), and vigorous (70%-90% maximum heart rate).
- The primary outcomes were body weight changes and adverse events; the secondary outcomes included changes in waist circumference, quality-of-life scores, and reduction in medications like antihypertensives.
TAKEAWAY:
- Every 30 minutes of aerobic exercise per week was also associated with lower waist circumference (mean difference, −0.56 cm; 95% CI, –0.67 to –0.45), body fat percentage (mean difference, –0.37%; 95% CI, –0.43 to –0.31), and body fat mass (mean difference, –0.20 kg; 95% CI, –0.32 to –0.08), along with reduced visceral and subcutaneous adipose tissue.
- A dose-response meta-analysis revealed that body fat percentage improved most significantly with 150 minutes of aerobic exercise per week, while body weight and waist circumference decreased linearly with increasing duration of aerobic exercise at 300 min/wk at different intensities.
- Adverse events with aerobic exercise were mostly mild or moderate musculoskeletal symptoms.
IN PRACTICE:
“Point-specific estimates for different aerobic exercise duration and intensity can help patients and healthcare professionals select the optimal aerobic exercise duration and intensity according to their weight loss goals,” the authors wrote.
SOURCE:
The study was led by Ahmad Jayedi, PhD, of the Department of Epidemiology and Biostatistics in the School of Public Health at the Imperial College London in England. It was published online on December 26, 2024, in JAMA Network Open.
LIMITATIONS:
High heterogeneity was present in the data. Only one trial included measures of health-related quality of life, and two studies included measures of medication use. Dietary habits and smoking status of participants were not included in studies, so any potential effects were not risk adjusted for.
DISCLOSURES:
No funding sources were reported. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Proteins in Plasma Linked to MI, Especially for Women
Forty-five circulating proteins in plasma are linked to the risk for myocardial infarction (MI), showed a new study that confirms some known associations and identifies new ones. Several proteins are associated with MI in women but not men, and some proteins linked with MI in both men and women are more strongly associated with MI in women.
“We hope that our study will shed light on pathways in MI,” said principal author Olga Titova, PhD, an epidemiologist at Uppsala University in Uppsala, Sweden. The work was published in the European Heart Journal.
Martha Gulati, MD, a cardiologist and associate director of the Barbra Streisand Women’s Heart Center at Cedars-Sinai Medical Center in Los Angeles and coauthor of an accompanying editorial, said the novel discovery of different patterns between men and women makes this an exciting study. The findings “highlight that sex differences in disease phenotype begin at the molecular level,” she said.
Titova and her team analyzed thousands of patients in two databases — one in Sweden (11,751 patients), the other in the United Kingdom (51,613 patients) — to discover proteins in the patients who went on to have an MI. Using one database to discover biomarkers and a second to replicate the findings is a common approach, said Titova.
Casting a Wide Net to Catch Proteins
The two databases “make findings more generalizable, allow us to confirm robust associations, and help minimize the risk of false positives.” The two databases mean researchers are more confident that the findings can be applied across populations, Titova added.
A total of 44 proteins were associated with later MI in both databases, adjusted for common MI risk factors as well as such factors as education, diet, physical activity, and alcohol intake, Titova explained. An additional protein was included from the first database that was unavailable in the second. Some of the proteins have been found in other studies, and this study confirms the link. Others were new, and a few appear to protect patients from MI.
“Most of the proteins are related to or involved in inflammation and atherosclerosis,” said Titova.
This is the first study to cast such a wide net, Titova pointed out. While several proteins have previously been linked to MI, most earlier studies have focused on specific proteins in populations that already have coronary artery disease or have involved cohorts of men only.
But she stresses that this study poses more questions than it answers. More research is needed to determine how proteins are involved in pathways leading to MI. The study found that some proteins may be mediators of general cardiovascular disease risk, whereas others are involved in mechanisms specifically linked to MI. Many proteins are involved in atherosclerosis, thrombosis, inflammation, immune system–related pathways, injury and tissue repair, coagulation, bone homeostasis, and iron metabolism.
“At this point, some [proteins] appear to be causal, some seem to be an association,” said Titova. It remains to be determined “which are on the causal path, which are potential biomarkers, which are going to shed light on the mechanisms” of MI.
The study took a step toward determining which proteins might be involved in causing MI through an analysis of some protein levels determined by genetics. This found three proteins linked to a higher risk for MI and three linked to a lower risk.
It’s Different for Women
Thirteen of the proteins were linked with later MI in women, either exclusively or more strongly than in men. Many of these associations were replicated in the second database, showing an alignment across populations.
Titova said the reason for the sex difference remains a mystery. “We have to go to the molecular level. It could be a consequence of risk factors affecting the sexes differently or different biology” between men and women.
Gulati, who specializes in women’s heart health, explained, “We know inflammation is much more prevalent in women and is the pathway to cardiovascular disease.” She points out that noncardiac inflammatory diseases are also more prevalent in women. Other biomarkers for inflammation, such as C-reactive protein, are higher in women than in men. She thinks the underlying mechanisms could involve “how we [women] make our proteins and how we respond to hormones.”
By identifying proteins linked to MI in women, the study helps to fill an important gap in our knowledge. “I can’t tell you how many papers don’t even look at sex differences. If we don’t look, we won’t know there are differences,” Gulati said. “In much of our cardiac research, women are underrepresented.”
The findings of this trial and others like it may lead to new approaches to prevention and treatment, Titova and Gulati agreed. Several proteins found in this study that may have a causal link with MI are already targets of drug development, they added.
Titova said other proteins may be useful in the future as biomarkers that indicate a need for preventive action.
Gulati asked, “If we can show some of the proteins are involved in the inflammatory response — if they are causal and we can prevent them upfront — can we reduce the chance of MI?” She and Titova said the many questions remaining should prove a rewarding avenue for research.
A version of this article appeared on Medscape.com.
Forty-five circulating proteins in plasma are linked to the risk for myocardial infarction (MI), showed a new study that confirms some known associations and identifies new ones. Several proteins are associated with MI in women but not men, and some proteins linked with MI in both men and women are more strongly associated with MI in women.
“We hope that our study will shed light on pathways in MI,” said principal author Olga Titova, PhD, an epidemiologist at Uppsala University in Uppsala, Sweden. The work was published in the European Heart Journal.
Martha Gulati, MD, a cardiologist and associate director of the Barbra Streisand Women’s Heart Center at Cedars-Sinai Medical Center in Los Angeles and coauthor of an accompanying editorial, said the novel discovery of different patterns between men and women makes this an exciting study. The findings “highlight that sex differences in disease phenotype begin at the molecular level,” she said.
Titova and her team analyzed thousands of patients in two databases — one in Sweden (11,751 patients), the other in the United Kingdom (51,613 patients) — to discover proteins in the patients who went on to have an MI. Using one database to discover biomarkers and a second to replicate the findings is a common approach, said Titova.
Casting a Wide Net to Catch Proteins
The two databases “make findings more generalizable, allow us to confirm robust associations, and help minimize the risk of false positives.” The two databases mean researchers are more confident that the findings can be applied across populations, Titova added.
A total of 44 proteins were associated with later MI in both databases, adjusted for common MI risk factors as well as such factors as education, diet, physical activity, and alcohol intake, Titova explained. An additional protein was included from the first database that was unavailable in the second. Some of the proteins have been found in other studies, and this study confirms the link. Others were new, and a few appear to protect patients from MI.
“Most of the proteins are related to or involved in inflammation and atherosclerosis,” said Titova.
This is the first study to cast such a wide net, Titova pointed out. While several proteins have previously been linked to MI, most earlier studies have focused on specific proteins in populations that already have coronary artery disease or have involved cohorts of men only.
But she stresses that this study poses more questions than it answers. More research is needed to determine how proteins are involved in pathways leading to MI. The study found that some proteins may be mediators of general cardiovascular disease risk, whereas others are involved in mechanisms specifically linked to MI. Many proteins are involved in atherosclerosis, thrombosis, inflammation, immune system–related pathways, injury and tissue repair, coagulation, bone homeostasis, and iron metabolism.
“At this point, some [proteins] appear to be causal, some seem to be an association,” said Titova. It remains to be determined “which are on the causal path, which are potential biomarkers, which are going to shed light on the mechanisms” of MI.
The study took a step toward determining which proteins might be involved in causing MI through an analysis of some protein levels determined by genetics. This found three proteins linked to a higher risk for MI and three linked to a lower risk.
It’s Different for Women
Thirteen of the proteins were linked with later MI in women, either exclusively or more strongly than in men. Many of these associations were replicated in the second database, showing an alignment across populations.
Titova said the reason for the sex difference remains a mystery. “We have to go to the molecular level. It could be a consequence of risk factors affecting the sexes differently or different biology” between men and women.
Gulati, who specializes in women’s heart health, explained, “We know inflammation is much more prevalent in women and is the pathway to cardiovascular disease.” She points out that noncardiac inflammatory diseases are also more prevalent in women. Other biomarkers for inflammation, such as C-reactive protein, are higher in women than in men. She thinks the underlying mechanisms could involve “how we [women] make our proteins and how we respond to hormones.”
By identifying proteins linked to MI in women, the study helps to fill an important gap in our knowledge. “I can’t tell you how many papers don’t even look at sex differences. If we don’t look, we won’t know there are differences,” Gulati said. “In much of our cardiac research, women are underrepresented.”
The findings of this trial and others like it may lead to new approaches to prevention and treatment, Titova and Gulati agreed. Several proteins found in this study that may have a causal link with MI are already targets of drug development, they added.
Titova said other proteins may be useful in the future as biomarkers that indicate a need for preventive action.
Gulati asked, “If we can show some of the proteins are involved in the inflammatory response — if they are causal and we can prevent them upfront — can we reduce the chance of MI?” She and Titova said the many questions remaining should prove a rewarding avenue for research.
A version of this article appeared on Medscape.com.
Forty-five circulating proteins in plasma are linked to the risk for myocardial infarction (MI), showed a new study that confirms some known associations and identifies new ones. Several proteins are associated with MI in women but not men, and some proteins linked with MI in both men and women are more strongly associated with MI in women.
“We hope that our study will shed light on pathways in MI,” said principal author Olga Titova, PhD, an epidemiologist at Uppsala University in Uppsala, Sweden. The work was published in the European Heart Journal.
Martha Gulati, MD, a cardiologist and associate director of the Barbra Streisand Women’s Heart Center at Cedars-Sinai Medical Center in Los Angeles and coauthor of an accompanying editorial, said the novel discovery of different patterns between men and women makes this an exciting study. The findings “highlight that sex differences in disease phenotype begin at the molecular level,” she said.
Titova and her team analyzed thousands of patients in two databases — one in Sweden (11,751 patients), the other in the United Kingdom (51,613 patients) — to discover proteins in the patients who went on to have an MI. Using one database to discover biomarkers and a second to replicate the findings is a common approach, said Titova.
Casting a Wide Net to Catch Proteins
The two databases “make findings more generalizable, allow us to confirm robust associations, and help minimize the risk of false positives.” The two databases mean researchers are more confident that the findings can be applied across populations, Titova added.
A total of 44 proteins were associated with later MI in both databases, adjusted for common MI risk factors as well as such factors as education, diet, physical activity, and alcohol intake, Titova explained. An additional protein was included from the first database that was unavailable in the second. Some of the proteins have been found in other studies, and this study confirms the link. Others were new, and a few appear to protect patients from MI.
“Most of the proteins are related to or involved in inflammation and atherosclerosis,” said Titova.
This is the first study to cast such a wide net, Titova pointed out. While several proteins have previously been linked to MI, most earlier studies have focused on specific proteins in populations that already have coronary artery disease or have involved cohorts of men only.
But she stresses that this study poses more questions than it answers. More research is needed to determine how proteins are involved in pathways leading to MI. The study found that some proteins may be mediators of general cardiovascular disease risk, whereas others are involved in mechanisms specifically linked to MI. Many proteins are involved in atherosclerosis, thrombosis, inflammation, immune system–related pathways, injury and tissue repair, coagulation, bone homeostasis, and iron metabolism.
“At this point, some [proteins] appear to be causal, some seem to be an association,” said Titova. It remains to be determined “which are on the causal path, which are potential biomarkers, which are going to shed light on the mechanisms” of MI.
The study took a step toward determining which proteins might be involved in causing MI through an analysis of some protein levels determined by genetics. This found three proteins linked to a higher risk for MI and three linked to a lower risk.
It’s Different for Women
Thirteen of the proteins were linked with later MI in women, either exclusively or more strongly than in men. Many of these associations were replicated in the second database, showing an alignment across populations.
Titova said the reason for the sex difference remains a mystery. “We have to go to the molecular level. It could be a consequence of risk factors affecting the sexes differently or different biology” between men and women.
Gulati, who specializes in women’s heart health, explained, “We know inflammation is much more prevalent in women and is the pathway to cardiovascular disease.” She points out that noncardiac inflammatory diseases are also more prevalent in women. Other biomarkers for inflammation, such as C-reactive protein, are higher in women than in men. She thinks the underlying mechanisms could involve “how we [women] make our proteins and how we respond to hormones.”
By identifying proteins linked to MI in women, the study helps to fill an important gap in our knowledge. “I can’t tell you how many papers don’t even look at sex differences. If we don’t look, we won’t know there are differences,” Gulati said. “In much of our cardiac research, women are underrepresented.”
The findings of this trial and others like it may lead to new approaches to prevention and treatment, Titova and Gulati agreed. Several proteins found in this study that may have a causal link with MI are already targets of drug development, they added.
Titova said other proteins may be useful in the future as biomarkers that indicate a need for preventive action.
Gulati asked, “If we can show some of the proteins are involved in the inflammatory response — if they are causal and we can prevent them upfront — can we reduce the chance of MI?” She and Titova said the many questions remaining should prove a rewarding avenue for research.
A version of this article appeared on Medscape.com.
Do We Need Cardiovascular Risk Equations to Guide Statin Use?
An individual’s estimated risk of having a heart attack or stroke in the next 10 years is widely used to guide preventative medication prescriptions with statins or antihypertensive drugs in those who have not yet had such an event.
To estimate that risk, doctors use equations that include different risk factors, such as age, cholesterol levels, and blood pressure. The current equations, known as the pooled cohort equations, are considered to be outdated as they were developed in 2013 based on population data from the 1960s and 70s. A new set of risk equations — known as the PREVENT equations — were developed by the American Heart Association (AHA) in 2023, and are based on a more contemporary population. It is anticipated that AHA will recommend these new risk equations be used in clinical practice in the next primary prevention guidelines.
But could these new risk equations do more harm than good?
Two recent studies found that applying the PREVENT risk equations to the US population results in a much lower overall level of risk compared with the pooled cohort equations. And, if the current threshold for starting statin treatment — which is an estimated 7.5% risk of having a heart attack or stroke in the next 10 years — is kept the same, this would result in many fewer patients being eligible for statin treatment.
As cardiovascular risk is also used to guide antihypertensive treatment, the new risk equations would also result in fewer people with borderline high blood pressure being eligible for those medications.
This has raised concerns in the medical community, where there is a widespread view that many more people would benefit from primary prevention treatment, and that anything that may cause fewer people to receive these medications would be harmful.
“I believe the new equations more accurately predict the risk of the current US population, but we need to be aware of what effect that may have on use of statins,” said Tim Anderson, MD, who studies healthcare delivery at the University of Pittsburgh in Pennsylvania and is lead author of one of the studies evaluating the equations.
Anderson told this news organization that the pooled cohort equations have long been viewed as problematic.
“Because these equations were based on cohorts from the 1960s and 70s, it is believed they overestimate the current population’s risk of MI and stroke as the burden of disease has shifted in the intervening 50-60 years,” he said.
Current Equations Overestimate Risk
The new equations are based on more recent, representative, and diverse cohorts that capture a wider spectrum of the population in terms of race, ethnicity, and socioeconomic status. They also include factors that are now known to be relevant to cardiovascular risk, such as chronic kidney disease.
Anderson compared how the two sets of equations estimated risk of cardiovascular disease in the next 10 years in the US population using the NHANES survey — a large nationally representative survey conducted between 2017 and 2020.
He found that the pooled cohort equations estimated the population average 10-year risk of cardiovascular disease to be about 8%, but the PREVENT equations estimated it at just over 4%.
“The new equations estimate that the middle-aged US population have almost half the level of risk of MI and stroke over next 10 years compared with the equations used currently. So, we will substantially change risk estimates if the new equations are introduced into practice,” Anderson said.
The study found that, if the PREVENT equations are adopted in the next set of primary prevention guidelines and the current threshold of a 7.5% risk of having an MI or stroke in the next 10 years is maintained as the starting point for statin treatment, then 17.3 million adults who were previously recommended primary prevention statin therapy would no longer be eligible.
A second, similar study, conducted by a different team of US researchers, estimated that using PREVENT would decrease the number of US adults receiving or recommended for statin therapy by 14.3 million and antihypertensive therapy by 2.62 million.
The researchers, led by James A. Diao, MD, from Harvard Medical School, Boston, Massachusetts, also suggested that over 10 years, reductions in treatment eligibility could result in an estimated 107,000 additional MI or stroke events.
Anderson points out that using the new equations would not affect the highest-risk patients. “They are still going to be high risk whichever equations are used. If you smoke a pack of cigarettes a day, have very high blood pressure or cholesterol and are older, then you are high risk. That part hasn’t changed. These people will qualify for statin treatment many times over with both sets of guidelines,” he said.
Rather it will be the large population at moderate risk of cardiovascular disease that will be affected, with far fewer of these individuals likely to get statins.
“If you are on the fence about whether to take a statin or not and you’re currently just on the threshold where they might be recommended then these new equations could mean that you’ll be less likely to be offered them,” he said. “Using the new equations may result in a delay of a couple of years to have that conversation.”
A Red Flag
Steve Nissen, MD, a cardiologist at the Cleveland Clinic in Ohio, is not a fan of cardiovascular risk equations in general. He points out that less than half of those currently eligible for statins are actually treated. And he believes the studies suggesting fewer people will be eligible with the new risk equations raise a red flag on whether they should be used.
“Anything that may result in fewer people being treated is a huge problem,” he told this news organization. “We have abundant evidence that we should be treating more people, not fewer people. Every study we have done has shown benefit with statins.”
The risk calculators were initially developed to limit use of statins and other medications to high-risk patients, he said, but now that we know more about safety of these drugs, it’s clear that the risks are almost nonexistent.
“We really need something else to guide the prescription of statins,” said Nissen.
Nissen suggests the risk calculators and guidelines have resulted in undertreatment of the population because they lack nuance and put too much emphasis on age. We should be more interested in reducing the lifetime risk of cardiovascular events, he said. “Calculators don’t do a good job of that. Their time horizons are too short. Young people with a family history of cardiovascular disease may have a low 10-year risk on a risk calculator but their lifetime risk is elevated, and as such, they should be considered for statin treatment. We need to find a more nuanced approach to understanding the lifetime risk of individuals,” he said.
Nissen said risk calculators can be useful in high-risk patients to help demonstrate their need for treatment. “I can show them the calculator and that they have a 20% chance of an event — that can help convince them to take a statin.”
But at the lower end of the risk scale, “all it does is keep patients who should be getting treatment from having that treatment.”
Nissen said changing the risk calculator won’t affect how he treats patients. “I use judgment to decide who to treat based on scientific literature and the patient in front of me. We will engage in a discussion and make a shared decision on what is the best course of action. Calculators will never be a substitute for medical judgment,” he said.
Equations Don’t Decide
Sadiya Khan, MD, a cardiologist at Northwestern University, Evanston, Illinois, and lead author of the PREVENT equations, told this news organization that it is important to put this discussion into context.
“The two recent papers do a good job of describing differences in predictive risk between the two sets of equations but that’s where they stop,” she said. “The translation from that to the decision on who should or should not be on statins or other medications is a step too far.”
Clinical guidelines will need to be updated to take the PREVENT equations into account, as Khan argued in a JAMA editorial. So it is not clear whether the current 7.5% 10-year risk figure will remain the threshold to start treatment. Khan anticipates the guidelines committee will have to re-evaluate that threshold.
“The 7.5% risk threshold was advised in the 2013 guidelines, based on what we knew then about the balance between benefit and harm and with the knowledge that the risk equations overestimated risk,” she said. “We now have a lot more data on the safety of statin therapy. We see this frequently in preventive care. Treatments often becomes more widespread in time and use expands into lower-risk patients.”
She also pointed out that the current primary prevention guidelines encourage consideration of other factors, not just predictive risk scores, when thinking about starting statins, including very high LDL cholesterol, family history, and apo B and Lp(a) levels.
“The recommendation on who would qualify for statin therapy is not based on one number,” she said. “It is based on many considerations, including both qualitative and quantitative factors, and discussions between the patient and the doctor. It is not a straightforward yes or no based on a 7.5% risk threshold.”
The equations, she said, should only be viewed as the first step in the process, and she said she agrees with Nissen that when applying the equations, doctors need to use additional data from each individual patient to make a judgment. “Equations do not decide who gets treated. Clinical practice guidelines do that.”
Khan also agreed with Nissen that more effort is needed to identify longer term cardiovascular risk in younger people, and so the PREVENT equations include 30-year risk estimates.
“I totally agree that we need to start earlier in having these prevention conversations. The PREVENT model starts at age 30 which is 10 years earlier than the pooled cohort equations and they add a 30-year time horizon as well as the 10-year period for these discussions on predicted risk estimates,” she said. “We need to make sure we are not missing risk in young adults just because we are waiting for them to get into some arbitrary age category.”
Khan says she believes that, used correctly, the new equations will not limit access to statins or other cardiovascular treatments. “Because they are a more accurate reflection of risk in the contemporary population, the new PREVENT equations should identify the correct patients to be treated, within the confines of knowing that no risk prediction equation is perfect,” she said. “And if everything else is considered as well, not just the numbers in the risk equations, it shouldn’t result in fewer patients being treated.”
Anderson reported receiving grants from the American Heart Association, the American College of Cardiology, and the US Deprescribing Research Network. Nissen is leading a development program for a nonprescription low dose of rosuvastatin. He is also involved in trials of a new cholesterol lowering drug, obicetrapib, and on trials on drugs that lower Lp(a). Khan reported receiving grants from the American Heart Association and National Heart, Lung, and Blood Institute.
A version of this article first appeared on Medscape.com.
An individual’s estimated risk of having a heart attack or stroke in the next 10 years is widely used to guide preventative medication prescriptions with statins or antihypertensive drugs in those who have not yet had such an event.
To estimate that risk, doctors use equations that include different risk factors, such as age, cholesterol levels, and blood pressure. The current equations, known as the pooled cohort equations, are considered to be outdated as they were developed in 2013 based on population data from the 1960s and 70s. A new set of risk equations — known as the PREVENT equations — were developed by the American Heart Association (AHA) in 2023, and are based on a more contemporary population. It is anticipated that AHA will recommend these new risk equations be used in clinical practice in the next primary prevention guidelines.
But could these new risk equations do more harm than good?
Two recent studies found that applying the PREVENT risk equations to the US population results in a much lower overall level of risk compared with the pooled cohort equations. And, if the current threshold for starting statin treatment — which is an estimated 7.5% risk of having a heart attack or stroke in the next 10 years — is kept the same, this would result in many fewer patients being eligible for statin treatment.
As cardiovascular risk is also used to guide antihypertensive treatment, the new risk equations would also result in fewer people with borderline high blood pressure being eligible for those medications.
This has raised concerns in the medical community, where there is a widespread view that many more people would benefit from primary prevention treatment, and that anything that may cause fewer people to receive these medications would be harmful.
“I believe the new equations more accurately predict the risk of the current US population, but we need to be aware of what effect that may have on use of statins,” said Tim Anderson, MD, who studies healthcare delivery at the University of Pittsburgh in Pennsylvania and is lead author of one of the studies evaluating the equations.
Anderson told this news organization that the pooled cohort equations have long been viewed as problematic.
“Because these equations were based on cohorts from the 1960s and 70s, it is believed they overestimate the current population’s risk of MI and stroke as the burden of disease has shifted in the intervening 50-60 years,” he said.
Current Equations Overestimate Risk
The new equations are based on more recent, representative, and diverse cohorts that capture a wider spectrum of the population in terms of race, ethnicity, and socioeconomic status. They also include factors that are now known to be relevant to cardiovascular risk, such as chronic kidney disease.
Anderson compared how the two sets of equations estimated risk of cardiovascular disease in the next 10 years in the US population using the NHANES survey — a large nationally representative survey conducted between 2017 and 2020.
He found that the pooled cohort equations estimated the population average 10-year risk of cardiovascular disease to be about 8%, but the PREVENT equations estimated it at just over 4%.
“The new equations estimate that the middle-aged US population have almost half the level of risk of MI and stroke over next 10 years compared with the equations used currently. So, we will substantially change risk estimates if the new equations are introduced into practice,” Anderson said.
The study found that, if the PREVENT equations are adopted in the next set of primary prevention guidelines and the current threshold of a 7.5% risk of having an MI or stroke in the next 10 years is maintained as the starting point for statin treatment, then 17.3 million adults who were previously recommended primary prevention statin therapy would no longer be eligible.
A second, similar study, conducted by a different team of US researchers, estimated that using PREVENT would decrease the number of US adults receiving or recommended for statin therapy by 14.3 million and antihypertensive therapy by 2.62 million.
The researchers, led by James A. Diao, MD, from Harvard Medical School, Boston, Massachusetts, also suggested that over 10 years, reductions in treatment eligibility could result in an estimated 107,000 additional MI or stroke events.
Anderson points out that using the new equations would not affect the highest-risk patients. “They are still going to be high risk whichever equations are used. If you smoke a pack of cigarettes a day, have very high blood pressure or cholesterol and are older, then you are high risk. That part hasn’t changed. These people will qualify for statin treatment many times over with both sets of guidelines,” he said.
Rather it will be the large population at moderate risk of cardiovascular disease that will be affected, with far fewer of these individuals likely to get statins.
“If you are on the fence about whether to take a statin or not and you’re currently just on the threshold where they might be recommended then these new equations could mean that you’ll be less likely to be offered them,” he said. “Using the new equations may result in a delay of a couple of years to have that conversation.”
A Red Flag
Steve Nissen, MD, a cardiologist at the Cleveland Clinic in Ohio, is not a fan of cardiovascular risk equations in general. He points out that less than half of those currently eligible for statins are actually treated. And he believes the studies suggesting fewer people will be eligible with the new risk equations raise a red flag on whether they should be used.
“Anything that may result in fewer people being treated is a huge problem,” he told this news organization. “We have abundant evidence that we should be treating more people, not fewer people. Every study we have done has shown benefit with statins.”
The risk calculators were initially developed to limit use of statins and other medications to high-risk patients, he said, but now that we know more about safety of these drugs, it’s clear that the risks are almost nonexistent.
“We really need something else to guide the prescription of statins,” said Nissen.
Nissen suggests the risk calculators and guidelines have resulted in undertreatment of the population because they lack nuance and put too much emphasis on age. We should be more interested in reducing the lifetime risk of cardiovascular events, he said. “Calculators don’t do a good job of that. Their time horizons are too short. Young people with a family history of cardiovascular disease may have a low 10-year risk on a risk calculator but their lifetime risk is elevated, and as such, they should be considered for statin treatment. We need to find a more nuanced approach to understanding the lifetime risk of individuals,” he said.
Nissen said risk calculators can be useful in high-risk patients to help demonstrate their need for treatment. “I can show them the calculator and that they have a 20% chance of an event — that can help convince them to take a statin.”
But at the lower end of the risk scale, “all it does is keep patients who should be getting treatment from having that treatment.”
Nissen said changing the risk calculator won’t affect how he treats patients. “I use judgment to decide who to treat based on scientific literature and the patient in front of me. We will engage in a discussion and make a shared decision on what is the best course of action. Calculators will never be a substitute for medical judgment,” he said.
Equations Don’t Decide
Sadiya Khan, MD, a cardiologist at Northwestern University, Evanston, Illinois, and lead author of the PREVENT equations, told this news organization that it is important to put this discussion into context.
“The two recent papers do a good job of describing differences in predictive risk between the two sets of equations but that’s where they stop,” she said. “The translation from that to the decision on who should or should not be on statins or other medications is a step too far.”
Clinical guidelines will need to be updated to take the PREVENT equations into account, as Khan argued in a JAMA editorial. So it is not clear whether the current 7.5% 10-year risk figure will remain the threshold to start treatment. Khan anticipates the guidelines committee will have to re-evaluate that threshold.
“The 7.5% risk threshold was advised in the 2013 guidelines, based on what we knew then about the balance between benefit and harm and with the knowledge that the risk equations overestimated risk,” she said. “We now have a lot more data on the safety of statin therapy. We see this frequently in preventive care. Treatments often becomes more widespread in time and use expands into lower-risk patients.”
She also pointed out that the current primary prevention guidelines encourage consideration of other factors, not just predictive risk scores, when thinking about starting statins, including very high LDL cholesterol, family history, and apo B and Lp(a) levels.
“The recommendation on who would qualify for statin therapy is not based on one number,” she said. “It is based on many considerations, including both qualitative and quantitative factors, and discussions between the patient and the doctor. It is not a straightforward yes or no based on a 7.5% risk threshold.”
The equations, she said, should only be viewed as the first step in the process, and she said she agrees with Nissen that when applying the equations, doctors need to use additional data from each individual patient to make a judgment. “Equations do not decide who gets treated. Clinical practice guidelines do that.”
Khan also agreed with Nissen that more effort is needed to identify longer term cardiovascular risk in younger people, and so the PREVENT equations include 30-year risk estimates.
“I totally agree that we need to start earlier in having these prevention conversations. The PREVENT model starts at age 30 which is 10 years earlier than the pooled cohort equations and they add a 30-year time horizon as well as the 10-year period for these discussions on predicted risk estimates,” she said. “We need to make sure we are not missing risk in young adults just because we are waiting for them to get into some arbitrary age category.”
Khan says she believes that, used correctly, the new equations will not limit access to statins or other cardiovascular treatments. “Because they are a more accurate reflection of risk in the contemporary population, the new PREVENT equations should identify the correct patients to be treated, within the confines of knowing that no risk prediction equation is perfect,” she said. “And if everything else is considered as well, not just the numbers in the risk equations, it shouldn’t result in fewer patients being treated.”
Anderson reported receiving grants from the American Heart Association, the American College of Cardiology, and the US Deprescribing Research Network. Nissen is leading a development program for a nonprescription low dose of rosuvastatin. He is also involved in trials of a new cholesterol lowering drug, obicetrapib, and on trials on drugs that lower Lp(a). Khan reported receiving grants from the American Heart Association and National Heart, Lung, and Blood Institute.
A version of this article first appeared on Medscape.com.
An individual’s estimated risk of having a heart attack or stroke in the next 10 years is widely used to guide preventative medication prescriptions with statins or antihypertensive drugs in those who have not yet had such an event.
To estimate that risk, doctors use equations that include different risk factors, such as age, cholesterol levels, and blood pressure. The current equations, known as the pooled cohort equations, are considered to be outdated as they were developed in 2013 based on population data from the 1960s and 70s. A new set of risk equations — known as the PREVENT equations — were developed by the American Heart Association (AHA) in 2023, and are based on a more contemporary population. It is anticipated that AHA will recommend these new risk equations be used in clinical practice in the next primary prevention guidelines.
But could these new risk equations do more harm than good?
Two recent studies found that applying the PREVENT risk equations to the US population results in a much lower overall level of risk compared with the pooled cohort equations. And, if the current threshold for starting statin treatment — which is an estimated 7.5% risk of having a heart attack or stroke in the next 10 years — is kept the same, this would result in many fewer patients being eligible for statin treatment.
As cardiovascular risk is also used to guide antihypertensive treatment, the new risk equations would also result in fewer people with borderline high blood pressure being eligible for those medications.
This has raised concerns in the medical community, where there is a widespread view that many more people would benefit from primary prevention treatment, and that anything that may cause fewer people to receive these medications would be harmful.
“I believe the new equations more accurately predict the risk of the current US population, but we need to be aware of what effect that may have on use of statins,” said Tim Anderson, MD, who studies healthcare delivery at the University of Pittsburgh in Pennsylvania and is lead author of one of the studies evaluating the equations.
Anderson told this news organization that the pooled cohort equations have long been viewed as problematic.
“Because these equations were based on cohorts from the 1960s and 70s, it is believed they overestimate the current population’s risk of MI and stroke as the burden of disease has shifted in the intervening 50-60 years,” he said.
Current Equations Overestimate Risk
The new equations are based on more recent, representative, and diverse cohorts that capture a wider spectrum of the population in terms of race, ethnicity, and socioeconomic status. They also include factors that are now known to be relevant to cardiovascular risk, such as chronic kidney disease.
Anderson compared how the two sets of equations estimated risk of cardiovascular disease in the next 10 years in the US population using the NHANES survey — a large nationally representative survey conducted between 2017 and 2020.
He found that the pooled cohort equations estimated the population average 10-year risk of cardiovascular disease to be about 8%, but the PREVENT equations estimated it at just over 4%.
“The new equations estimate that the middle-aged US population have almost half the level of risk of MI and stroke over next 10 years compared with the equations used currently. So, we will substantially change risk estimates if the new equations are introduced into practice,” Anderson said.
The study found that, if the PREVENT equations are adopted in the next set of primary prevention guidelines and the current threshold of a 7.5% risk of having an MI or stroke in the next 10 years is maintained as the starting point for statin treatment, then 17.3 million adults who were previously recommended primary prevention statin therapy would no longer be eligible.
A second, similar study, conducted by a different team of US researchers, estimated that using PREVENT would decrease the number of US adults receiving or recommended for statin therapy by 14.3 million and antihypertensive therapy by 2.62 million.
The researchers, led by James A. Diao, MD, from Harvard Medical School, Boston, Massachusetts, also suggested that over 10 years, reductions in treatment eligibility could result in an estimated 107,000 additional MI or stroke events.
Anderson points out that using the new equations would not affect the highest-risk patients. “They are still going to be high risk whichever equations are used. If you smoke a pack of cigarettes a day, have very high blood pressure or cholesterol and are older, then you are high risk. That part hasn’t changed. These people will qualify for statin treatment many times over with both sets of guidelines,” he said.
Rather it will be the large population at moderate risk of cardiovascular disease that will be affected, with far fewer of these individuals likely to get statins.
“If you are on the fence about whether to take a statin or not and you’re currently just on the threshold where they might be recommended then these new equations could mean that you’ll be less likely to be offered them,” he said. “Using the new equations may result in a delay of a couple of years to have that conversation.”
A Red Flag
Steve Nissen, MD, a cardiologist at the Cleveland Clinic in Ohio, is not a fan of cardiovascular risk equations in general. He points out that less than half of those currently eligible for statins are actually treated. And he believes the studies suggesting fewer people will be eligible with the new risk equations raise a red flag on whether they should be used.
“Anything that may result in fewer people being treated is a huge problem,” he told this news organization. “We have abundant evidence that we should be treating more people, not fewer people. Every study we have done has shown benefit with statins.”
The risk calculators were initially developed to limit use of statins and other medications to high-risk patients, he said, but now that we know more about safety of these drugs, it’s clear that the risks are almost nonexistent.
“We really need something else to guide the prescription of statins,” said Nissen.
Nissen suggests the risk calculators and guidelines have resulted in undertreatment of the population because they lack nuance and put too much emphasis on age. We should be more interested in reducing the lifetime risk of cardiovascular events, he said. “Calculators don’t do a good job of that. Their time horizons are too short. Young people with a family history of cardiovascular disease may have a low 10-year risk on a risk calculator but their lifetime risk is elevated, and as such, they should be considered for statin treatment. We need to find a more nuanced approach to understanding the lifetime risk of individuals,” he said.
Nissen said risk calculators can be useful in high-risk patients to help demonstrate their need for treatment. “I can show them the calculator and that they have a 20% chance of an event — that can help convince them to take a statin.”
But at the lower end of the risk scale, “all it does is keep patients who should be getting treatment from having that treatment.”
Nissen said changing the risk calculator won’t affect how he treats patients. “I use judgment to decide who to treat based on scientific literature and the patient in front of me. We will engage in a discussion and make a shared decision on what is the best course of action. Calculators will never be a substitute for medical judgment,” he said.
Equations Don’t Decide
Sadiya Khan, MD, a cardiologist at Northwestern University, Evanston, Illinois, and lead author of the PREVENT equations, told this news organization that it is important to put this discussion into context.
“The two recent papers do a good job of describing differences in predictive risk between the two sets of equations but that’s where they stop,” she said. “The translation from that to the decision on who should or should not be on statins or other medications is a step too far.”
Clinical guidelines will need to be updated to take the PREVENT equations into account, as Khan argued in a JAMA editorial. So it is not clear whether the current 7.5% 10-year risk figure will remain the threshold to start treatment. Khan anticipates the guidelines committee will have to re-evaluate that threshold.
“The 7.5% risk threshold was advised in the 2013 guidelines, based on what we knew then about the balance between benefit and harm and with the knowledge that the risk equations overestimated risk,” she said. “We now have a lot more data on the safety of statin therapy. We see this frequently in preventive care. Treatments often becomes more widespread in time and use expands into lower-risk patients.”
She also pointed out that the current primary prevention guidelines encourage consideration of other factors, not just predictive risk scores, when thinking about starting statins, including very high LDL cholesterol, family history, and apo B and Lp(a) levels.
“The recommendation on who would qualify for statin therapy is not based on one number,” she said. “It is based on many considerations, including both qualitative and quantitative factors, and discussions between the patient and the doctor. It is not a straightforward yes or no based on a 7.5% risk threshold.”
The equations, she said, should only be viewed as the first step in the process, and she said she agrees with Nissen that when applying the equations, doctors need to use additional data from each individual patient to make a judgment. “Equations do not decide who gets treated. Clinical practice guidelines do that.”
Khan also agreed with Nissen that more effort is needed to identify longer term cardiovascular risk in younger people, and so the PREVENT equations include 30-year risk estimates.
“I totally agree that we need to start earlier in having these prevention conversations. The PREVENT model starts at age 30 which is 10 years earlier than the pooled cohort equations and they add a 30-year time horizon as well as the 10-year period for these discussions on predicted risk estimates,” she said. “We need to make sure we are not missing risk in young adults just because we are waiting for them to get into some arbitrary age category.”
Khan says she believes that, used correctly, the new equations will not limit access to statins or other cardiovascular treatments. “Because they are a more accurate reflection of risk in the contemporary population, the new PREVENT equations should identify the correct patients to be treated, within the confines of knowing that no risk prediction equation is perfect,” she said. “And if everything else is considered as well, not just the numbers in the risk equations, it shouldn’t result in fewer patients being treated.”
Anderson reported receiving grants from the American Heart Association, the American College of Cardiology, and the US Deprescribing Research Network. Nissen is leading a development program for a nonprescription low dose of rosuvastatin. He is also involved in trials of a new cholesterol lowering drug, obicetrapib, and on trials on drugs that lower Lp(a). Khan reported receiving grants from the American Heart Association and National Heart, Lung, and Blood Institute.
A version of this article first appeared on Medscape.com.
Plant-Based Food Prioritized Over Meat in Dietary Guidelines Report
The scientific report that offers evidence-based guidance for the next iteration of the Dietary Guidelines for Americans has been submitted to federal agencies, and the document — which already has generated controversy because of its emphasis on plant-based foods — is now open for public comment.
“We saw something over and over again — when you look at a population level, diets for which the predominant composition was plants performed better when it came to health outcomes,” advisory committee member Cheryl Anderson, PhD, MPH, who is a professor and dean of the Herbert Wertheim School of Public Health and Human Longevity Science at the University of California, San Diego, said in an interview. “There’s a pretty consistent body of literature showing benefits of fruits, vegetables, and legumes and reductions in salt, added sugars, and saturated fats.”
Clinicians should read and comment on the report, said Anderson.
“Commenting sends the right signal that they are interested in what’s needed for nutrition education,” she said. “It will also activate a conversation with the people who are writing the guidelines.”
Instructions for submitting comments online through February 10, 2025, and for participating in the oral comment meeting on January 16, 2025, are posted online.
The Department of Agriculture (USDA) and the Department of Health & Human Services will use the report as a key resource, alongside the public comments and agency input, as they jointly develop the Dietary Guidelines for Americans, 2025-2030.
Meat Given a Back Seat
Overall, the advisory committee defined a “healthy dietary pattern” as one that is “higher in vegetables, fruits, legumes (ie, beans, peas, lentils), nuts, whole grains, fish/seafood, and vegetable oils higher in unsaturated fat — and lower in red and processed meats, sugar-sweetened foods and beverages, refined grains, and saturated fat.”
The report emphasizes “plain drinking water” as the primary beverage for people to consume and states that sugar-sweetened beverage consumption should be limited.
It recommends limiting total saturated fat intake to less than 10% of daily calories and replacing it with unsaturated fat, particularly polyunsaturated fats.
Notably, the report advocates increasing the consumption of beans, peas, and lentils and decreasing starchy vegetables (such as potatoes), as well as reducing total protein foods by reducing meat, poultry, and eggs. This recommendation and the report’s broad emphasis on plant-based foods have drawn criticism, mainly from the food industry.
Also likely to be controversial are the recommendations to move beans, peas, and lentils from the vegetable group to the protein group and the proposed reorganization of the order of the protein foods group to list beans, peas, and lentils first, followed by nuts, seeds, and soy products; then seafood; and finally meats, poultry, and eggs.
Gastroenterologists and dietitians should support the emphasis on plant-based protein sources, water for hydration, and the importance of personalized nutrition plans, including culturally diverse and ethnic food options, said Stephanie Gold, MD, assistant professor of medicine at the Icahn School of Medicine at Mount Sinai and a gastroenterologist at Mount Sinai Hospital, both in New York City.
“The newly proposed 2025 Dietary Guidelines are approaching a Mediterranean-style diet by focusing on plant-based protein sources while limiting red meat and saturated fats, as well as added sugar. By including these legumes in the protein category (not only as a starchy vegetable), the proposed guideline recognizes both the health benefits and sustainability of plant-based proteins,” Gold said in an interview.
Although the report recognizes “the potential negative impact and the varying definitions of ultra-processed foods, it does not provide concrete recommendations regarding intake, and perhaps, this could be an area of focus going forward,” she added.
Anderson noted that the science around ultra-processed food is “underdeveloped.” However, the definition of a healthy diet “has never suggested that we have foods that are extremely processed in it.”
“Right now, there’s a lot of interest in ultra-processed foods and what they mean for health, but the science is going to need to catch up with that interest,” Anderson said.
What’s Next
The release of the scientific report is part of a five-step process to develop the new guidelines that included input from the public during the report’s development. According to the USDA, the advisory committee received approximately 9900 public comments, more than any other previous committee.
Once the new dietary guidelines are complete, Anderson said, “clinicians have an opportunity to really lean into a science-based framework to talk about overall health concerns and reducing the burden of diet-related illnesses with their patients.”
Meanwhile, they can voice their approval or concerns about the scientific report.
Anderson and Gold reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The scientific report that offers evidence-based guidance for the next iteration of the Dietary Guidelines for Americans has been submitted to federal agencies, and the document — which already has generated controversy because of its emphasis on plant-based foods — is now open for public comment.
“We saw something over and over again — when you look at a population level, diets for which the predominant composition was plants performed better when it came to health outcomes,” advisory committee member Cheryl Anderson, PhD, MPH, who is a professor and dean of the Herbert Wertheim School of Public Health and Human Longevity Science at the University of California, San Diego, said in an interview. “There’s a pretty consistent body of literature showing benefits of fruits, vegetables, and legumes and reductions in salt, added sugars, and saturated fats.”
Clinicians should read and comment on the report, said Anderson.
“Commenting sends the right signal that they are interested in what’s needed for nutrition education,” she said. “It will also activate a conversation with the people who are writing the guidelines.”
Instructions for submitting comments online through February 10, 2025, and for participating in the oral comment meeting on January 16, 2025, are posted online.
The Department of Agriculture (USDA) and the Department of Health & Human Services will use the report as a key resource, alongside the public comments and agency input, as they jointly develop the Dietary Guidelines for Americans, 2025-2030.
Meat Given a Back Seat
Overall, the advisory committee defined a “healthy dietary pattern” as one that is “higher in vegetables, fruits, legumes (ie, beans, peas, lentils), nuts, whole grains, fish/seafood, and vegetable oils higher in unsaturated fat — and lower in red and processed meats, sugar-sweetened foods and beverages, refined grains, and saturated fat.”
The report emphasizes “plain drinking water” as the primary beverage for people to consume and states that sugar-sweetened beverage consumption should be limited.
It recommends limiting total saturated fat intake to less than 10% of daily calories and replacing it with unsaturated fat, particularly polyunsaturated fats.
Notably, the report advocates increasing the consumption of beans, peas, and lentils and decreasing starchy vegetables (such as potatoes), as well as reducing total protein foods by reducing meat, poultry, and eggs. This recommendation and the report’s broad emphasis on plant-based foods have drawn criticism, mainly from the food industry.
Also likely to be controversial are the recommendations to move beans, peas, and lentils from the vegetable group to the protein group and the proposed reorganization of the order of the protein foods group to list beans, peas, and lentils first, followed by nuts, seeds, and soy products; then seafood; and finally meats, poultry, and eggs.
Gastroenterologists and dietitians should support the emphasis on plant-based protein sources, water for hydration, and the importance of personalized nutrition plans, including culturally diverse and ethnic food options, said Stephanie Gold, MD, assistant professor of medicine at the Icahn School of Medicine at Mount Sinai and a gastroenterologist at Mount Sinai Hospital, both in New York City.
“The newly proposed 2025 Dietary Guidelines are approaching a Mediterranean-style diet by focusing on plant-based protein sources while limiting red meat and saturated fats, as well as added sugar. By including these legumes in the protein category (not only as a starchy vegetable), the proposed guideline recognizes both the health benefits and sustainability of plant-based proteins,” Gold said in an interview.
Although the report recognizes “the potential negative impact and the varying definitions of ultra-processed foods, it does not provide concrete recommendations regarding intake, and perhaps, this could be an area of focus going forward,” she added.
Anderson noted that the science around ultra-processed food is “underdeveloped.” However, the definition of a healthy diet “has never suggested that we have foods that are extremely processed in it.”
“Right now, there’s a lot of interest in ultra-processed foods and what they mean for health, but the science is going to need to catch up with that interest,” Anderson said.
What’s Next
The release of the scientific report is part of a five-step process to develop the new guidelines that included input from the public during the report’s development. According to the USDA, the advisory committee received approximately 9900 public comments, more than any other previous committee.
Once the new dietary guidelines are complete, Anderson said, “clinicians have an opportunity to really lean into a science-based framework to talk about overall health concerns and reducing the burden of diet-related illnesses with their patients.”
Meanwhile, they can voice their approval or concerns about the scientific report.
Anderson and Gold reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The scientific report that offers evidence-based guidance for the next iteration of the Dietary Guidelines for Americans has been submitted to federal agencies, and the document — which already has generated controversy because of its emphasis on plant-based foods — is now open for public comment.
“We saw something over and over again — when you look at a population level, diets for which the predominant composition was plants performed better when it came to health outcomes,” advisory committee member Cheryl Anderson, PhD, MPH, who is a professor and dean of the Herbert Wertheim School of Public Health and Human Longevity Science at the University of California, San Diego, said in an interview. “There’s a pretty consistent body of literature showing benefits of fruits, vegetables, and legumes and reductions in salt, added sugars, and saturated fats.”
Clinicians should read and comment on the report, said Anderson.
“Commenting sends the right signal that they are interested in what’s needed for nutrition education,” she said. “It will also activate a conversation with the people who are writing the guidelines.”
Instructions for submitting comments online through February 10, 2025, and for participating in the oral comment meeting on January 16, 2025, are posted online.
The Department of Agriculture (USDA) and the Department of Health & Human Services will use the report as a key resource, alongside the public comments and agency input, as they jointly develop the Dietary Guidelines for Americans, 2025-2030.
Meat Given a Back Seat
Overall, the advisory committee defined a “healthy dietary pattern” as one that is “higher in vegetables, fruits, legumes (ie, beans, peas, lentils), nuts, whole grains, fish/seafood, and vegetable oils higher in unsaturated fat — and lower in red and processed meats, sugar-sweetened foods and beverages, refined grains, and saturated fat.”
The report emphasizes “plain drinking water” as the primary beverage for people to consume and states that sugar-sweetened beverage consumption should be limited.
It recommends limiting total saturated fat intake to less than 10% of daily calories and replacing it with unsaturated fat, particularly polyunsaturated fats.
Notably, the report advocates increasing the consumption of beans, peas, and lentils and decreasing starchy vegetables (such as potatoes), as well as reducing total protein foods by reducing meat, poultry, and eggs. This recommendation and the report’s broad emphasis on plant-based foods have drawn criticism, mainly from the food industry.
Also likely to be controversial are the recommendations to move beans, peas, and lentils from the vegetable group to the protein group and the proposed reorganization of the order of the protein foods group to list beans, peas, and lentils first, followed by nuts, seeds, and soy products; then seafood; and finally meats, poultry, and eggs.
Gastroenterologists and dietitians should support the emphasis on plant-based protein sources, water for hydration, and the importance of personalized nutrition plans, including culturally diverse and ethnic food options, said Stephanie Gold, MD, assistant professor of medicine at the Icahn School of Medicine at Mount Sinai and a gastroenterologist at Mount Sinai Hospital, both in New York City.
“The newly proposed 2025 Dietary Guidelines are approaching a Mediterranean-style diet by focusing on plant-based protein sources while limiting red meat and saturated fats, as well as added sugar. By including these legumes in the protein category (not only as a starchy vegetable), the proposed guideline recognizes both the health benefits and sustainability of plant-based proteins,” Gold said in an interview.
Although the report recognizes “the potential negative impact and the varying definitions of ultra-processed foods, it does not provide concrete recommendations regarding intake, and perhaps, this could be an area of focus going forward,” she added.
Anderson noted that the science around ultra-processed food is “underdeveloped.” However, the definition of a healthy diet “has never suggested that we have foods that are extremely processed in it.”
“Right now, there’s a lot of interest in ultra-processed foods and what they mean for health, but the science is going to need to catch up with that interest,” Anderson said.
What’s Next
The release of the scientific report is part of a five-step process to develop the new guidelines that included input from the public during the report’s development. According to the USDA, the advisory committee received approximately 9900 public comments, more than any other previous committee.
Once the new dietary guidelines are complete, Anderson said, “clinicians have an opportunity to really lean into a science-based framework to talk about overall health concerns and reducing the burden of diet-related illnesses with their patients.”
Meanwhile, they can voice their approval or concerns about the scientific report.
Anderson and Gold reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Potassium Nitrate Fails to Boost Exercise Capacity in Patients With Heart Failure With Preserved Ejection Fraction
TOPLINE:
METHODOLOGY:
- This multicenter crossover trial, conducted across three centers in the United States, assessed the effect of administering KNO3 on exercise capacity and quality of life.
- It included 84 patients with symptomatic HFpEF (median age, 68 years; 69% women; 76% White) who had a left ventricular ejection fraction over 50% and elevated intracardiac pressures. Participants had obesity (mean body mass index, 36.22), with a high prevalence of hypertension, diabetes, and obstructive sleep apnea.
- Patients were randomly assigned to receive either 6 mmol KNO3 first (n = 41) or 6 mmol potassium chloride (KCl) first (n = 43) three times daily for 6 weeks, with a 1-week washout period in between.
- At the end of each intervention phase, a test of incremental cardiopulmonary exercise was conducted using a supine cycle ergometer.
- Primary endpoints were the difference in peak oxygen uptake and total work performed during the exercise test; secondary endpoints included quality of life, left ventricular systolic and diastolic function, exercise systemic vasodilatory reserve, and parameters related to pulsatile arterial load.
TAKEAWAY:
- The administration of KNO3 vs KCl increased the levels of serum metabolites of nitric oxide significantly after 6 weeks (418.44 vs 40.11 μM; P < .001).
- Peak oxygen uptake or the total work performed did not improve significantly with the administration of KNO3, compared with KCl. Quality of life also did not improve with the administration of KNO3.
- Mean arterial pressure at peak exercise was significantly lower after the administration of KNO3 than after KCl (122.5 vs 127.6 mm Hg; P = .04), but the vasodilatory reserve and resting and orthostatic blood pressure did not differ.
- Adverse events were mostly minor, with gastrointestinal issues being the most common side effects reported.
IN PRACTICE:
“In this randomized crossover trial, chronic KNO3 administration did not improve exercise capacity or quality of life, as compared with KCl among participants with HFpEF,” the authors of the study wrote.
SOURCE:
The study was led by Payman Zamani, MD, MTR, of the Perelman School of Medicine at the University of Pennsylvania, Philadelphia. It was published online on December 18, 2024, in JAMA Cardiology.
LIMITATIONS:
The potential activation of compensatory mechanisms by the chronic inorganic nitrate administration may have neutralized the short-term benefits. Various abnormalities in oxygen transport may be present simultaneously in patients with HFpEF, suggesting a combination of interventions may be required to improve exercise capacity.
DISCLOSURES:
This trial was supported by the National Heart, Lung, and Blood Institute. The study was supported by the National Center for Advancing Translational Sciences and National Institutes of Health. Some authors reported receiving grants, personal fees, and consulting fees and having patents from various pharmaceutical and medical device companies and institutes. One author reported having full-time employment with a healthcare company.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- This multicenter crossover trial, conducted across three centers in the United States, assessed the effect of administering KNO3 on exercise capacity and quality of life.
- It included 84 patients with symptomatic HFpEF (median age, 68 years; 69% women; 76% White) who had a left ventricular ejection fraction over 50% and elevated intracardiac pressures. Participants had obesity (mean body mass index, 36.22), with a high prevalence of hypertension, diabetes, and obstructive sleep apnea.
- Patients were randomly assigned to receive either 6 mmol KNO3 first (n = 41) or 6 mmol potassium chloride (KCl) first (n = 43) three times daily for 6 weeks, with a 1-week washout period in between.
- At the end of each intervention phase, a test of incremental cardiopulmonary exercise was conducted using a supine cycle ergometer.
- Primary endpoints were the difference in peak oxygen uptake and total work performed during the exercise test; secondary endpoints included quality of life, left ventricular systolic and diastolic function, exercise systemic vasodilatory reserve, and parameters related to pulsatile arterial load.
TAKEAWAY:
- The administration of KNO3 vs KCl increased the levels of serum metabolites of nitric oxide significantly after 6 weeks (418.44 vs 40.11 μM; P < .001).
- Peak oxygen uptake or the total work performed did not improve significantly with the administration of KNO3, compared with KCl. Quality of life also did not improve with the administration of KNO3.
- Mean arterial pressure at peak exercise was significantly lower after the administration of KNO3 than after KCl (122.5 vs 127.6 mm Hg; P = .04), but the vasodilatory reserve and resting and orthostatic blood pressure did not differ.
- Adverse events were mostly minor, with gastrointestinal issues being the most common side effects reported.
IN PRACTICE:
“In this randomized crossover trial, chronic KNO3 administration did not improve exercise capacity or quality of life, as compared with KCl among participants with HFpEF,” the authors of the study wrote.
SOURCE:
The study was led by Payman Zamani, MD, MTR, of the Perelman School of Medicine at the University of Pennsylvania, Philadelphia. It was published online on December 18, 2024, in JAMA Cardiology.
LIMITATIONS:
The potential activation of compensatory mechanisms by the chronic inorganic nitrate administration may have neutralized the short-term benefits. Various abnormalities in oxygen transport may be present simultaneously in patients with HFpEF, suggesting a combination of interventions may be required to improve exercise capacity.
DISCLOSURES:
This trial was supported by the National Heart, Lung, and Blood Institute. The study was supported by the National Center for Advancing Translational Sciences and National Institutes of Health. Some authors reported receiving grants, personal fees, and consulting fees and having patents from various pharmaceutical and medical device companies and institutes. One author reported having full-time employment with a healthcare company.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- This multicenter crossover trial, conducted across three centers in the United States, assessed the effect of administering KNO3 on exercise capacity and quality of life.
- It included 84 patients with symptomatic HFpEF (median age, 68 years; 69% women; 76% White) who had a left ventricular ejection fraction over 50% and elevated intracardiac pressures. Participants had obesity (mean body mass index, 36.22), with a high prevalence of hypertension, diabetes, and obstructive sleep apnea.
- Patients were randomly assigned to receive either 6 mmol KNO3 first (n = 41) or 6 mmol potassium chloride (KCl) first (n = 43) three times daily for 6 weeks, with a 1-week washout period in between.
- At the end of each intervention phase, a test of incremental cardiopulmonary exercise was conducted using a supine cycle ergometer.
- Primary endpoints were the difference in peak oxygen uptake and total work performed during the exercise test; secondary endpoints included quality of life, left ventricular systolic and diastolic function, exercise systemic vasodilatory reserve, and parameters related to pulsatile arterial load.
TAKEAWAY:
- The administration of KNO3 vs KCl increased the levels of serum metabolites of nitric oxide significantly after 6 weeks (418.44 vs 40.11 μM; P < .001).
- Peak oxygen uptake or the total work performed did not improve significantly with the administration of KNO3, compared with KCl. Quality of life also did not improve with the administration of KNO3.
- Mean arterial pressure at peak exercise was significantly lower after the administration of KNO3 than after KCl (122.5 vs 127.6 mm Hg; P = .04), but the vasodilatory reserve and resting and orthostatic blood pressure did not differ.
- Adverse events were mostly minor, with gastrointestinal issues being the most common side effects reported.
IN PRACTICE:
“In this randomized crossover trial, chronic KNO3 administration did not improve exercise capacity or quality of life, as compared with KCl among participants with HFpEF,” the authors of the study wrote.
SOURCE:
The study was led by Payman Zamani, MD, MTR, of the Perelman School of Medicine at the University of Pennsylvania, Philadelphia. It was published online on December 18, 2024, in JAMA Cardiology.
LIMITATIONS:
The potential activation of compensatory mechanisms by the chronic inorganic nitrate administration may have neutralized the short-term benefits. Various abnormalities in oxygen transport may be present simultaneously in patients with HFpEF, suggesting a combination of interventions may be required to improve exercise capacity.
DISCLOSURES:
This trial was supported by the National Heart, Lung, and Blood Institute. The study was supported by the National Center for Advancing Translational Sciences and National Institutes of Health. Some authors reported receiving grants, personal fees, and consulting fees and having patents from various pharmaceutical and medical device companies and institutes. One author reported having full-time employment with a healthcare company.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.