User login
Treating the effects of bruxism with botulinum toxin
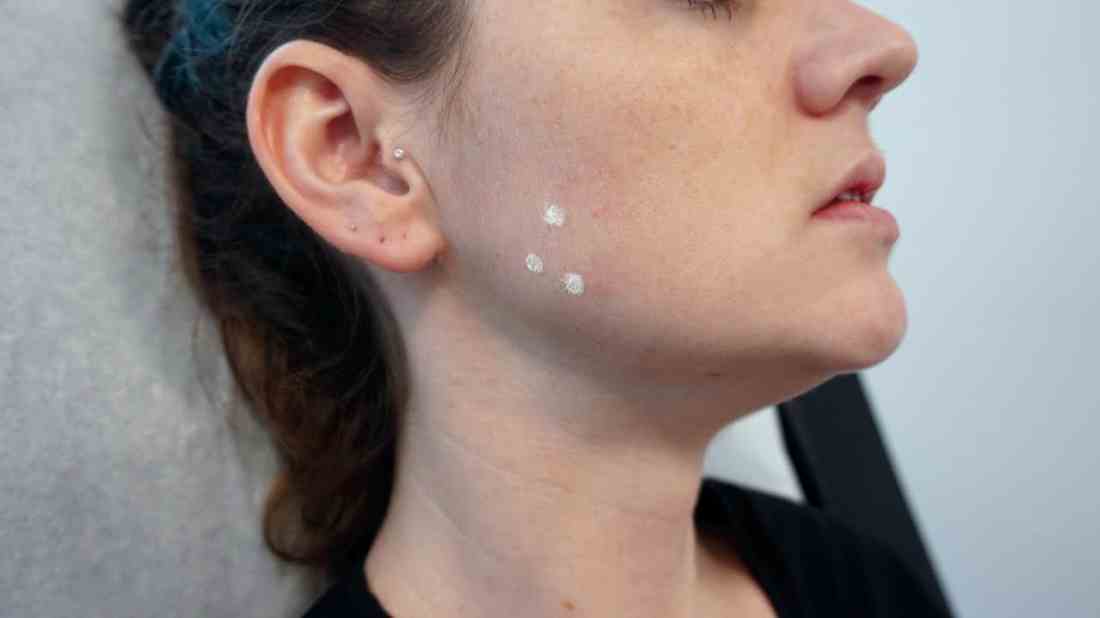
Bruxism is grinding and clenching of the teeth with unconscious contractions of the temporal and masseter muscles while awake or during sleep. Bruxism occurs in 8%-16% of the population and is often an underdiagnosed condition that not only leads to dental problems but also to pain in the teeth, jaw, temporomandibular joint, and neck; headaches; and potentially, to tooth loss.
Although the pathogenesis of bruxism remains unclear, multiple factors, such as physical or psychological stress, malocclusion, sleep disorders and medication side effects, can cause bruxism. Treatment can be difficult given psychogenic and neurogenic components, and bruxism can be resistant to medical and behavioral therapy. There are various treatment options for bruxism, including oral splints; medications, such as muscle relaxants; antidepressants; and botulinum toxin. Multiple studies have shown that botulinum toxin injections into the masseter and temporalis muscles result in relaxation of the muscles and improvement of bruxism and the pain associated with chronic clenching and grinding.
In a recent study by Al-Wayli, 50 subjects who reported nocturnal bruxism were randomized to receive botulinum toxin versus conventional treatment (pharmacotherapy or oral splints). After 3 weeks, 2 months, 6 months, and 1 year, patients who received botulinum toxin had significantly less pain after only one treatment than did the traditional treatment group. Similarly, in a study by Lee et al., subjects randomized to receive botulinum toxin versus a placebo saline injections showed not only decreased pain but also decreased bruxism seen with nocturnal electromyography.
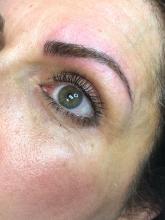
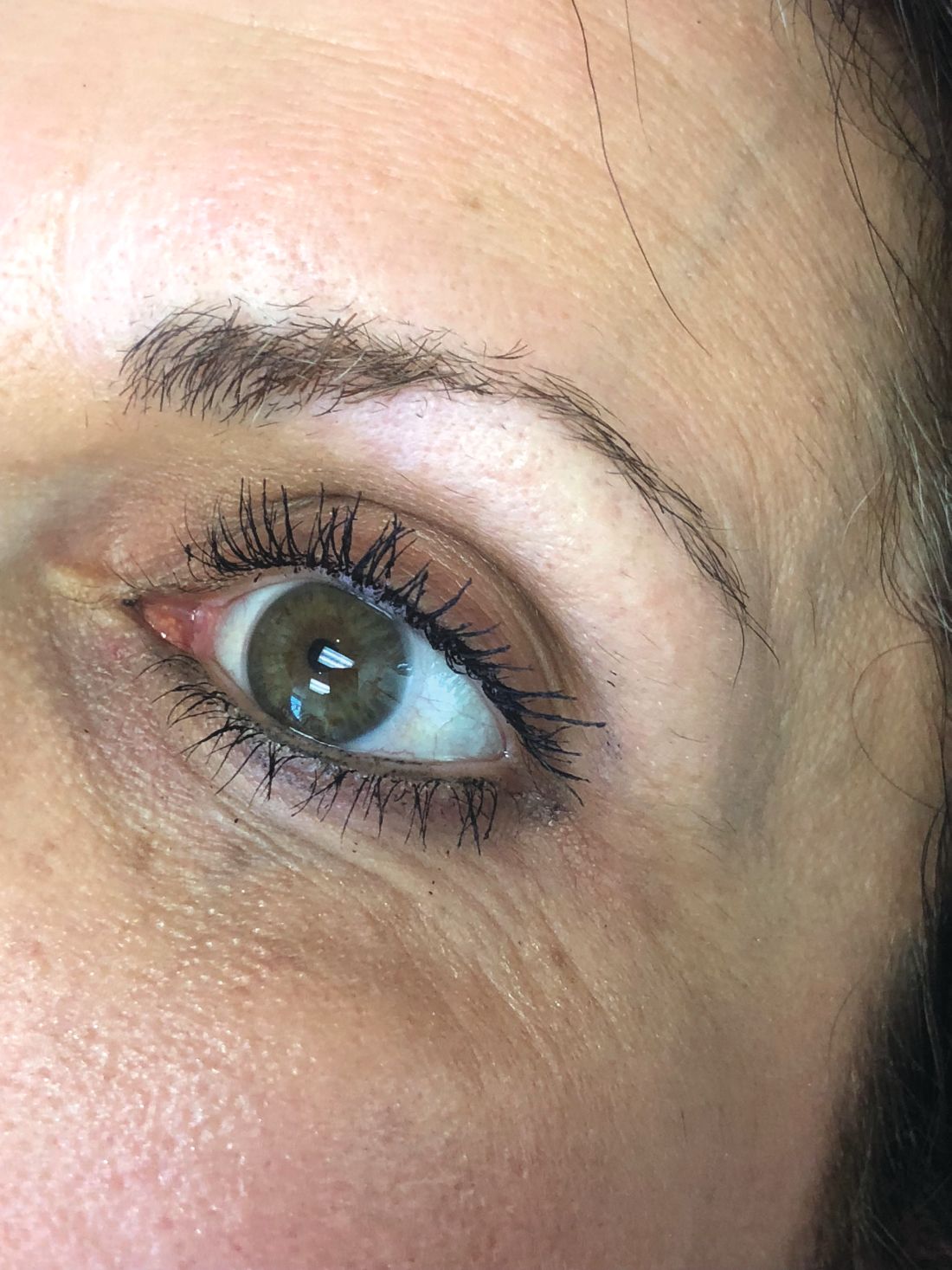
In our clinic, Botulinum toxin when injected into the temporalis and masseter muscles also helps with tension headaches and migraines related to clenching of the jaw. Albeit effective, the dose of botulinum toxin used in the aforementioned studies ranged between 25 U and 40 U of botulinum toxin and were lower than what we have found to be effective. Our patients receive 50 U botulinum toxin in each masseter muscle (100 U total). In a small minority of our patients, the temporalis muscle also needed 15-20 U per side as well. Clinical improvement starts within 3-5 days, and patients can expect to have relaxation of the muscle and decreased pain for 6 months. Side effects include mild swelling and bruising. Rarely, if the injection is not performed properly, the risorius muscle may be paralyzed, leading to an asymmetric smile. In addition, if the botulinum toxin is underdosed, the pain may not completely subside and the patient may report some symptoms returning within a couple of weeks of the initial treatment. Most patients also report thinning of the face and jaw, which is a much anticipated and appreciated result. Masseter hypertrophy with and without bruxism is treated similarly with botulinum toxin to sculpt the lower face.
Bruxism is a growing problem leading to facial pain, headaches, migraines, and significant dental pathology. Traditional treatments have been ineffective at treating the pain and masseter hypertrophy associated with chronic grinding and clenching. Botulinum toxin is a safe, effective, treatment with little downtime or side effects for treating both the neurogenic and muscular components of bruxism.
Dr. Lily Talakoub and Dr. Naissan Wesley and are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to them at [email protected]. They had no relevant disclosures.
References
Al-Wayli H. J Clin Exp Dent. 2017 Jan 1;9(1):e112-e117.
Asutay F et al. Pain Res Manag. 2017;2017:6264146. doi: 10.1155/2017/6264146.
Lee SJ et al. Am J Phys Med Rehabil. 2010 Jan;89(1):16-23.
Santamato A et al. J Chiropr Med. 2010 Sep;9(3):132-7.
Shetty S et al. J Indian Prosthodont Soc. 2010 Sep;10(3):141-8.
Tan EK et al. J Am Dent Assoc. 2000 Feb;131(2):211-6.
Bruxism is grinding and clenching of the teeth with unconscious contractions of the temporal and masseter muscles while awake or during sleep. Bruxism occurs in 8%-16% of the population and is often an underdiagnosed condition that not only leads to dental problems but also to pain in the teeth, jaw, temporomandibular joint, and neck; headaches; and potentially, to tooth loss.
Although the pathogenesis of bruxism remains unclear, multiple factors, such as physical or psychological stress, malocclusion, sleep disorders and medication side effects, can cause bruxism. Treatment can be difficult given psychogenic and neurogenic components, and bruxism can be resistant to medical and behavioral therapy. There are various treatment options for bruxism, including oral splints; medications, such as muscle relaxants; antidepressants; and botulinum toxin. Multiple studies have shown that botulinum toxin injections into the masseter and temporalis muscles result in relaxation of the muscles and improvement of bruxism and the pain associated with chronic clenching and grinding.
In a recent study by Al-Wayli, 50 subjects who reported nocturnal bruxism were randomized to receive botulinum toxin versus conventional treatment (pharmacotherapy or oral splints). After 3 weeks, 2 months, 6 months, and 1 year, patients who received botulinum toxin had significantly less pain after only one treatment than did the traditional treatment group. Similarly, in a study by Lee et al., subjects randomized to receive botulinum toxin versus a placebo saline injections showed not only decreased pain but also decreased bruxism seen with nocturnal electromyography.
In our clinic, Botulinum toxin when injected into the temporalis and masseter muscles also helps with tension headaches and migraines related to clenching of the jaw. Albeit effective, the dose of botulinum toxin used in the aforementioned studies ranged between 25 U and 40 U of botulinum toxin and were lower than what we have found to be effective. Our patients receive 50 U botulinum toxin in each masseter muscle (100 U total). In a small minority of our patients, the temporalis muscle also needed 15-20 U per side as well. Clinical improvement starts within 3-5 days, and patients can expect to have relaxation of the muscle and decreased pain for 6 months. Side effects include mild swelling and bruising. Rarely, if the injection is not performed properly, the risorius muscle may be paralyzed, leading to an asymmetric smile. In addition, if the botulinum toxin is underdosed, the pain may not completely subside and the patient may report some symptoms returning within a couple of weeks of the initial treatment. Most patients also report thinning of the face and jaw, which is a much anticipated and appreciated result. Masseter hypertrophy with and without bruxism is treated similarly with botulinum toxin to sculpt the lower face.
Bruxism is a growing problem leading to facial pain, headaches, migraines, and significant dental pathology. Traditional treatments have been ineffective at treating the pain and masseter hypertrophy associated with chronic grinding and clenching. Botulinum toxin is a safe, effective, treatment with little downtime or side effects for treating both the neurogenic and muscular components of bruxism.
Dr. Lily Talakoub and Dr. Naissan Wesley and are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to them at [email protected]. They had no relevant disclosures.
References
Al-Wayli H. J Clin Exp Dent. 2017 Jan 1;9(1):e112-e117.
Asutay F et al. Pain Res Manag. 2017;2017:6264146. doi: 10.1155/2017/6264146.
Lee SJ et al. Am J Phys Med Rehabil. 2010 Jan;89(1):16-23.
Santamato A et al. J Chiropr Med. 2010 Sep;9(3):132-7.
Shetty S et al. J Indian Prosthodont Soc. 2010 Sep;10(3):141-8.
Tan EK et al. J Am Dent Assoc. 2000 Feb;131(2):211-6.
Bruxism is grinding and clenching of the teeth with unconscious contractions of the temporal and masseter muscles while awake or during sleep. Bruxism occurs in 8%-16% of the population and is often an underdiagnosed condition that not only leads to dental problems but also to pain in the teeth, jaw, temporomandibular joint, and neck; headaches; and potentially, to tooth loss.
Although the pathogenesis of bruxism remains unclear, multiple factors, such as physical or psychological stress, malocclusion, sleep disorders and medication side effects, can cause bruxism. Treatment can be difficult given psychogenic and neurogenic components, and bruxism can be resistant to medical and behavioral therapy. There are various treatment options for bruxism, including oral splints; medications, such as muscle relaxants; antidepressants; and botulinum toxin. Multiple studies have shown that botulinum toxin injections into the masseter and temporalis muscles result in relaxation of the muscles and improvement of bruxism and the pain associated with chronic clenching and grinding.
In a recent study by Al-Wayli, 50 subjects who reported nocturnal bruxism were randomized to receive botulinum toxin versus conventional treatment (pharmacotherapy or oral splints). After 3 weeks, 2 months, 6 months, and 1 year, patients who received botulinum toxin had significantly less pain after only one treatment than did the traditional treatment group. Similarly, in a study by Lee et al., subjects randomized to receive botulinum toxin versus a placebo saline injections showed not only decreased pain but also decreased bruxism seen with nocturnal electromyography.
In our clinic, Botulinum toxin when injected into the temporalis and masseter muscles also helps with tension headaches and migraines related to clenching of the jaw. Albeit effective, the dose of botulinum toxin used in the aforementioned studies ranged between 25 U and 40 U of botulinum toxin and were lower than what we have found to be effective. Our patients receive 50 U botulinum toxin in each masseter muscle (100 U total). In a small minority of our patients, the temporalis muscle also needed 15-20 U per side as well. Clinical improvement starts within 3-5 days, and patients can expect to have relaxation of the muscle and decreased pain for 6 months. Side effects include mild swelling and bruising. Rarely, if the injection is not performed properly, the risorius muscle may be paralyzed, leading to an asymmetric smile. In addition, if the botulinum toxin is underdosed, the pain may not completely subside and the patient may report some symptoms returning within a couple of weeks of the initial treatment. Most patients also report thinning of the face and jaw, which is a much anticipated and appreciated result. Masseter hypertrophy with and without bruxism is treated similarly with botulinum toxin to sculpt the lower face.
Bruxism is a growing problem leading to facial pain, headaches, migraines, and significant dental pathology. Traditional treatments have been ineffective at treating the pain and masseter hypertrophy associated with chronic grinding and clenching. Botulinum toxin is a safe, effective, treatment with little downtime or side effects for treating both the neurogenic and muscular components of bruxism.
Dr. Lily Talakoub and Dr. Naissan Wesley and are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to them at [email protected]. They had no relevant disclosures.
References
Al-Wayli H. J Clin Exp Dent. 2017 Jan 1;9(1):e112-e117.
Asutay F et al. Pain Res Manag. 2017;2017:6264146. doi: 10.1155/2017/6264146.
Lee SJ et al. Am J Phys Med Rehabil. 2010 Jan;89(1):16-23.
Santamato A et al. J Chiropr Med. 2010 Sep;9(3):132-7.
Shetty S et al. J Indian Prosthodont Soc. 2010 Sep;10(3):141-8.
Tan EK et al. J Am Dent Assoc. 2000 Feb;131(2):211-6.
Cosmetic surgery patients want beauty ... and more
but motives involving mental and social well-being and physical health are common as well, according to a prospective national study involving more than 500 patients.
“Cosmetic procedures may also be necessary to correct significant physical disfigurement interfering with work or daily living. Most patients were concerned with how they looked at work and in protecting their physical health, and for some, this motive was the most important. Together, these data add to the growing body of evidence that treatments aimed at improving physical appearance can treat significant physical and psychological illness,” Amanda L. Maisel, of Northwestern University, Chicago, and her associates wrote in JAMA Dermatology.
Among the motives related to appearance, 88.5% of patients said that they “wanted to look better, prettier, or more attractive for themselves,” compared with 64.4% who wanted to look good for others. Patients also were interested in “looking younger or fresher” (83.4%) and having “clear-looking or beautiful skin” (81.4%), the investigators said.
The most common mental or emotional motive was increased self-confidence (69.5%), followed by the desire to “feel happier or better overall or improve quality of life” at 67.2% and to “treat oneself, feel rewarded, or celebrate” at 61.3%. As for social well-being, 56.6% of patients “reported wanting to look good when running into people they knew” and 50.3% reported that they wanted “to feel less self-conscious around others.”
The leading motive involving physical health was “preventing worsening of their condition/symptoms,” which was reported by 53.3% of patients, the investigators reported.
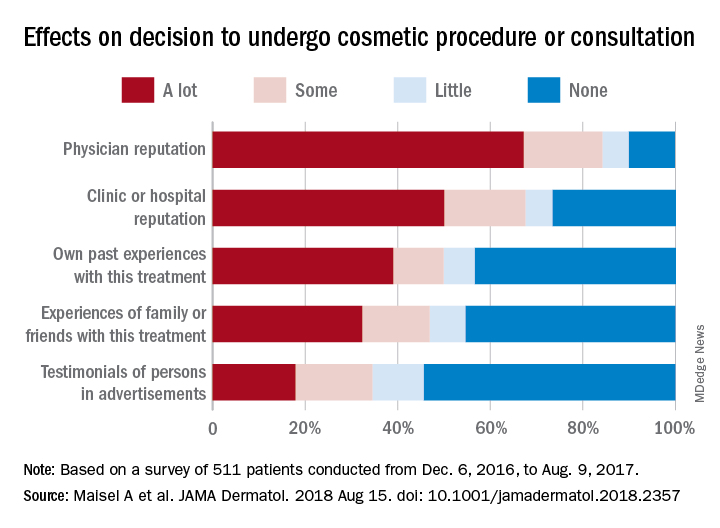
They also examined how reputation and experience influenced patients’ decision to have cosmetic surgery. When asked to what degree a physician’s reputation affected their decision, 67.2% of respondents said a lot, 17% said that it had some effect, 5.7% said it had little effect, and 10% said none. Half of the patients surveyed said the clinic or hospital’s reputation had a lot of influence on their decision, compared with 39% for their own past experiences, 32.2% for experiences of family or friends, and 17.9% for testimonials of persons in advertisements, the researchers said.
The survey was conducted from Dec. 4, 2016, to Aug. 9, 2017, at 2 academic and 11 private dermatology practices. A total of 511 patients were involved, although not all individuals answered every question, so sample sizes varied. The study was supported by a research grant from the American Society for Dermatologic Surgery. The senior investigator reported consulting for Pulse Biosciences that was unrelated to this research and being principal investigator for studies funded in part by Regeneron.
SOURCE: Maisel A et al. JAMA Dermatol. 2018 Aug 15. doi: 10.1001/jamadermatol.2018.2357.
but motives involving mental and social well-being and physical health are common as well, according to a prospective national study involving more than 500 patients.

“Cosmetic procedures may also be necessary to correct significant physical disfigurement interfering with work or daily living. Most patients were concerned with how they looked at work and in protecting their physical health, and for some, this motive was the most important. Together, these data add to the growing body of evidence that treatments aimed at improving physical appearance can treat significant physical and psychological illness,” Amanda L. Maisel, of Northwestern University, Chicago, and her associates wrote in JAMA Dermatology.
Among the motives related to appearance, 88.5% of patients said that they “wanted to look better, prettier, or more attractive for themselves,” compared with 64.4% who wanted to look good for others. Patients also were interested in “looking younger or fresher” (83.4%) and having “clear-looking or beautiful skin” (81.4%), the investigators said.
The most common mental or emotional motive was increased self-confidence (69.5%), followed by the desire to “feel happier or better overall or improve quality of life” at 67.2% and to “treat oneself, feel rewarded, or celebrate” at 61.3%. As for social well-being, 56.6% of patients “reported wanting to look good when running into people they knew” and 50.3% reported that they wanted “to feel less self-conscious around others.”
The leading motive involving physical health was “preventing worsening of their condition/symptoms,” which was reported by 53.3% of patients, the investigators reported.
They also examined how reputation and experience influenced patients’ decision to have cosmetic surgery. When asked to what degree a physician’s reputation affected their decision, 67.2% of respondents said a lot, 17% said that it had some effect, 5.7% said it had little effect, and 10% said none. Half of the patients surveyed said the clinic or hospital’s reputation had a lot of influence on their decision, compared with 39% for their own past experiences, 32.2% for experiences of family or friends, and 17.9% for testimonials of persons in advertisements, the researchers said.
The survey was conducted from Dec. 4, 2016, to Aug. 9, 2017, at 2 academic and 11 private dermatology practices. A total of 511 patients were involved, although not all individuals answered every question, so sample sizes varied. The study was supported by a research grant from the American Society for Dermatologic Surgery. The senior investigator reported consulting for Pulse Biosciences that was unrelated to this research and being principal investigator for studies funded in part by Regeneron.
SOURCE: Maisel A et al. JAMA Dermatol. 2018 Aug 15. doi: 10.1001/jamadermatol.2018.2357.
but motives involving mental and social well-being and physical health are common as well, according to a prospective national study involving more than 500 patients.

“Cosmetic procedures may also be necessary to correct significant physical disfigurement interfering with work or daily living. Most patients were concerned with how they looked at work and in protecting their physical health, and for some, this motive was the most important. Together, these data add to the growing body of evidence that treatments aimed at improving physical appearance can treat significant physical and psychological illness,” Amanda L. Maisel, of Northwestern University, Chicago, and her associates wrote in JAMA Dermatology.
Among the motives related to appearance, 88.5% of patients said that they “wanted to look better, prettier, or more attractive for themselves,” compared with 64.4% who wanted to look good for others. Patients also were interested in “looking younger or fresher” (83.4%) and having “clear-looking or beautiful skin” (81.4%), the investigators said.
The most common mental or emotional motive was increased self-confidence (69.5%), followed by the desire to “feel happier or better overall or improve quality of life” at 67.2% and to “treat oneself, feel rewarded, or celebrate” at 61.3%. As for social well-being, 56.6% of patients “reported wanting to look good when running into people they knew” and 50.3% reported that they wanted “to feel less self-conscious around others.”
The leading motive involving physical health was “preventing worsening of their condition/symptoms,” which was reported by 53.3% of patients, the investigators reported.
They also examined how reputation and experience influenced patients’ decision to have cosmetic surgery. When asked to what degree a physician’s reputation affected their decision, 67.2% of respondents said a lot, 17% said that it had some effect, 5.7% said it had little effect, and 10% said none. Half of the patients surveyed said the clinic or hospital’s reputation had a lot of influence on their decision, compared with 39% for their own past experiences, 32.2% for experiences of family or friends, and 17.9% for testimonials of persons in advertisements, the researchers said.
The survey was conducted from Dec. 4, 2016, to Aug. 9, 2017, at 2 academic and 11 private dermatology practices. A total of 511 patients were involved, although not all individuals answered every question, so sample sizes varied. The study was supported by a research grant from the American Society for Dermatologic Surgery. The senior investigator reported consulting for Pulse Biosciences that was unrelated to this research and being principal investigator for studies funded in part by Regeneron.
SOURCE: Maisel A et al. JAMA Dermatol. 2018 Aug 15. doi: 10.1001/jamadermatol.2018.2357.
FROM JAMA DERMATOLOGY
Laser Scar Management: Focused and High-Intensity Medical Exchange in Vietnam
Over the last decade the treatment of traumatic scars with lasers has emerged as a core component of multidisciplinary management. Military dermatologists have played a fundamental role in this shift by helping to develop new applications for existing technology and promulgate the techniques to reach additional providers and patients. Beyond scar management, the repurposing of adjunctive procedural techniques, such as sweat and hair reduction in amputees, also promises to enhance rehabilitation for many patients.
International engagement is a prominent and highly attractive feature of military practice, and military dermatologists routinely participate in disaster response missions, such as the 2010 Haiti earthquake,1 and ongoing planned operations, such as Pacific Partnership in the Indo-Asia-Pacific region led by the US Navy.2 In this article, I present a military perspective on the emerging niche of trauma dermatology and outline my more than 5 years of experience leveraging these skills to lead a multidisciplinary exchange in restorative medicine and burn scar management in Vietnam.
Trauma Dermatology
Over the course of the last decade, traumatic scar management has emerged as a staple of dermatologic surgery practice in some centers. Dermatologists hold the key to increasing patient access to effective outpatient care for symptomatic traumatic scars and other related issues using devices and techniques initially conceived for cosmetic applications.3 A major impetus for the considerable remodeling in our collective thoughts about traumatic scar management was the emergence of fractional laser technology in the mid-2000s. The remarkable, safe, reproducible, and durable benefits of fractional laser treatment of various scar types have created substantial momentum in recent years. The Naval Medical Center San Diego in California houses 1 of 3 centers of excellence in rehabilitation in the US military. Mastery of minimally invasive procedures to manage scars and other issues associated with trauma for the first time has established dermatologists as important partners in the overall rehabilitative effort.
My perspective on laser scar management has been previously described.4,5 Ablative fractional laser resurfacing is the backbone of rehabilitative scar management.6 Although the literature in this field is still relatively immature, higher-quality studies are accumulating rapidly as the burn and surgical communities adopt the procedure more widely.7-10 A considerable step forward in the dissemination of the procedure occurred recently with the development of category III Current Procedural Terminology (CPT) codes for ablative laser treatment of traumatic scars.11 Category III CPT codes are temporary codes used for emerging procedures that have not yet been deemed medically necessary. Although individual insurance carriers can determine whether to cover these procedures and the corresponding level of reimbursement, regular use is important for ultimate elevation to category I codes by the American Medical Association over a 5-year observation period. The CPT codes 0479T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; first 100 cm2 or part thereof, or 1% of body surface area of infants and children) and 0480T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; each additional 100 cm2, or each additional 1% of body surface area of infants and children, or part thereof [list separately in addition to code for primary procedure]) are examples of these category III codes.11
Nonablative fractional lasers; vascular-specific devices for erythematous scars; and long- and short-pulsed pigment-specific devices for hair and traumatic tattoo treatment, respectively, round out the commonly used laser platforms. For example, laser hair reduction can help improve the fit and comfort of prosthetic devices and has been shown to improve the overall quality of life for amputees.12 Botulinum toxin can be an important component of treatment of excessive sweating induced by occlusive liners in prosthetics, and microwave eccrine ablation is an emerging potential option for longer-lasting sweat reduction in this population.13-15 In addition to providing direct dermatology care and education, having members of the specialty in uniform has been a key to adopting new practical solutions to unsolved problems.
Pacific Partnership
Pacific Partnership is the largest annual multinational humanitarian assistance and disaster preparedness mission in the Indo-Asia-Pacific region.16 It was started in 2006 following the tsunami that devastated parts of South and Southeast Asia in 2004. The recently concluded Pacific Partnership 2018 marked the 13th iteration of the annual mission led by the US Navy in collaboration with other partner nations, which in 2018 included Japan, Australia, Canada, the United Kingdom, France, Singapore, Korea, and Peru, as well as nongovernmental organizations and international governmental agencies. Host nation mission locations vary somewhat from year to year, but 2018 included visits of the hospital ship USNS Mercy and more than 800 personnel to Indonesia, Malaysia, Sri Lanka, and Vietnam. Medical/dental, engineering, and veterinary teams join with their counterparts in each host nation to conduct civic action projects, community health exchanges, medical care, and disaster response training activities.16
Rehabilitation As a Vehicle for Medical Exchange
Since approximately 2012 there has been an evolving paradigm in Pacific Partnership from an emphasis on maximizing direct patient care in changing locations to one focused on building lasting partnerships through subject matter expert exchange. Multidisciplinary scar management, including surgical and laser scar revision and physical and occupational therapy, is a very promising model for engagement. Potential advantages of this type of exchange include the following: developing nations have relatively high rates of burns and other forms of trauma as well as uneven access to acute and ongoing rehabilitative care; patients often are otherwise healthy and young; results are frequently profound and readily demonstrable; and it is a skill set that has become highly developed in the military system. Just as dermatologists are illustrating their utility in trauma rehabilitation at home, these procedural skills provide fertile ground for exchange overseas.
The Overseas Humanitarian Assistance Shared Information System is an online platform that allows users to apply for grants under the Asia-Pacific Regional Initiative. In 2013, I started the Burn Scar Treatment/Restorative Medicine exchange with a grant under this program. A multidisciplinary team representing the specialties of dermatology, hand surgery, plastic surgery, physical medicine and rehabilitation, and pulmonary critical care participated in the 2013 Asia Pacific Burn Congress hosted by the National Institute of Burns (NIB) in Hanoi, Vietnam, and then followed up with didactics and patient care alongside Vietnamese physicians in the management of disfiguring and debilitating scars from burns and other trauma. This pilot project consisted of three 2- to 3-week phases: 2 at the NIB in Hanoi and 1 with a delegation from the NIB visiting the Naval Medical Center San Diego. When initial project funds expired in 2014, the exchange was absorbed into Pacific Partnership 2014, which began a string of 4 consecutive annual Pacific Partnership engagements at Da Nang General Hospital in Vietnam. The 2 most recent exchanges, including the exchange associated with Pacific Partnership 2018, have taken place at Khanh Hoa General Hospital in Nha Trang, Vietnam. During this time the team has grown to include physical and occupational therapists as well as a wound care nurse.
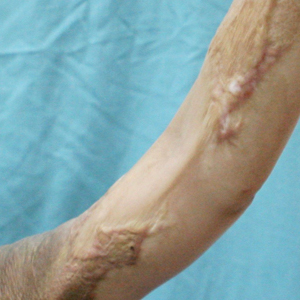
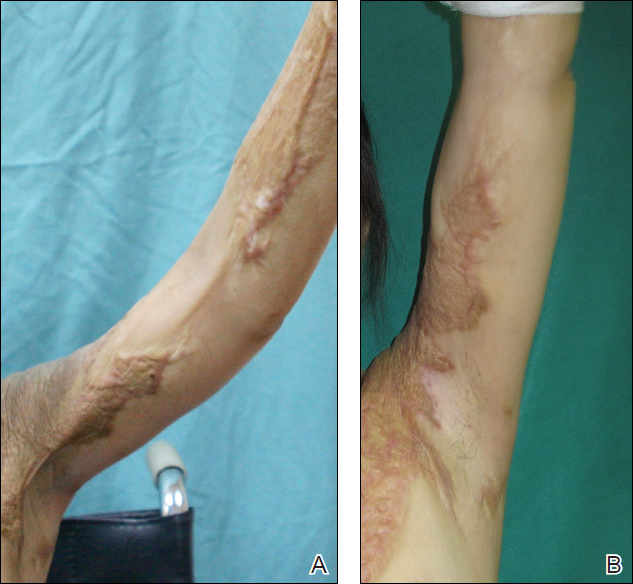
The Burn Scar Treatment/Restorative Medicine exchange consists of side-by-side laser and surgical scar revision performed with our Vietnamese hosts in their own hospital. Our Vietnamese partners perform a large volume of reconstructive surgeries in their usual practice, so it truly has been a bilateral exchange incorporating some advanced technology and techniques with an emphasis on longitudinal multidisciplinary care. Importantly, the procedures are supplemented with preoperative and postoperative care as well as instruction provided by physical and occupational therapy and wound care professionals working alongside host nation support staff. Because the areas of involvement often are extensive and a patient may only be seen once in this setting, laser and surgical procedures often are performed concurrently in the host nation operating room. Anesthesia support is provided by the host nation. Basic consumable surgical supplies (eg, sutures, gloves, marking pens, staplers) are supplemented with mission funds. Special adjuncts for the most severe contractures have included negative pressure wound therapy and a collagen-based bilayer matrix wound dressing. Laser treatments have been performed on the vast majority of patients with an ablative fractional CO2 laser and laser-assisted delivery of corticosteroid in hypertrophic areas. Of note, use of the laser has been provided to our hosts by the manufacturer for each of the 7 iterations of the exchange, and the wound dressing manufacturer also has donated some of their product to the exchange through the nongovernmental organization Project Hope for 2 missions. To date, more than 300 patients have safely received life-changing treatment during the exchange, with some receiving multiple treatments (Figure). Although multiple treatments over time are ideal, even a single treatment session can result in considerable and lasting improvements in function and symptoms.17 The hospital ship USNS Mercy has the same laser technology and has brought advanced scar treatment techniques to the far corners of the Pacific.
Measuring overall success—treatment and international relations—in this setting can be challenging. On an individual patient level, the benefits of restoring the ability to walk and work as well as reducing pain and itching are manifest and transformative for both the patient and family; however, aggregating this information into high-quality outcome data is difficult given the heterogeneous nature of traumatic injuries, which is compounded in the setting of international engagement where the intersection between patient and visiting provider may be singular or difficult to predict, funding is limited, language frequently is a barrier, and documentation, privacy, and medical research guidelines may be unfamiliar or contradictory. The cumulative impact of these types of exchanges on the relationship between nations also is critical but difficult to measure. It is common sense that deepening personal and professional relationships in the medical setting over time can increase trust and mutual understanding, perhaps setting the stage for broader engagement in other more sensitive areas. Trust and understanding are rather nebulous concepts, but earlier this year marked the first visit of an American aircraft carrier to Da Nang since 1975, following 4 consecutive annual Pacific Partnership missions in the same city, which does carry the patina of successful engagement on a systemic level.
Final Thoughts
Based on my personal experience, I provide the following tips for building a successful, focused, long-term medical exchange.
- Leverage your strengths and respect the strengths and style of practice of your hosts. A mind-set of exchange and not simply humanitarian care will be more successful. Your hosts are experts in a style of practice adapted to their surroundings and introducing new techniques that are grounded in the local practice patterns are more likely to be perpetuated.
- Collaboration with nongovernmental organizations and industry can be extremely helpful. Military and governmental organizations often are limited in funding, in the ways they can spend available funding, and in the receipt of donations. Appropriate coordination with civilian entities can elevate the exchange considerably by adding expertise and available assets as well as broadening the overall impact.
- Engage the support staff as well as the physicians. You will leverage contact with families and enhance care over the long-term.
- The benefits of multiple interactions over time are manifest, for both the patients and the participants. Personal and professional relationships are intertwined and naturally mature over time. Go for singles and doubles first before swinging for the fences.
- Multidisciplinary work overseas informs and enhances collaboration at home.
- Adding regional experts in international research and assessment to these specialized medical teams may better capture the impact of future exchanges of any flavor.
- The model of creating a focused exchange with independent funding followed by incorporation of successful concepts into larger missions seems to be a worthy and reproducible approach for future projects of any variety.
- Galeckas K. Dermatology aboard the USNS Comfort: disaster relief operations in Haiti after the 2010 earthquake. Dermatol Clin. 2011;29:15-19.
- Satter EK. The role of the dermatologist on military humanitarian missions. Cutis. 2010;85:85-89.
- Miletta NR, Donelan MB, Hivnor CM. Management of trauma and burn scars; the dermatologist's role in expanding patient access to care. Cutis. 2017;100:18-20.
- Shumaker PR. Laser treatment of traumatic scars: a military perspective. Semin Cutan Med Surg. 2015;34:17-23.
- Shumaker PR, Beachkofsky T, Basnett A, et al. A military perspective. In: Krakowski AC, Shumaker PR, eds. The Scar Book: Formation, Mitigation, Rehabilitation and Prevention. Philadelphia, PA: Wolters Kluwer; 2017:327-338.
- Anderson RR, Donelan MB, Greeson E, et al. Consensus report: laser treatment of traumatic scars with an emphasis on ablative fractional resurfacing. JAMA Dermatol. 2014;150:187-193.
- Hultman CS, Friedstat JS, Edkins RE, et al. Laser resurfacing and remodeling of hypertrophic burn scars: the results of a large, prospective, before and after cohort study, with long-term follow-up. Ann Surg. 2014;260:519-532.
- Blome-Eberwein S, Gogal C, Weiss MJ, et al. Prospective evaluation of fractional CO2 laser treatment of mature burn scars. J Burn Care Res. 2016;37:379-387.
- Issler-Fisher AC, Fisher OM, Smialkowski AO, et al. Ablative fractional CO2 laser for burn scar reconstruction: an extensive subjective and objective short-term outcome analysis of a prospective treatment cohort. Burns. 2017;43:573-582.
- Zuccaro J, Zlolkowski N, Fish J. A systematic review of the effectiveness of laser therapy for hypertrophic burn scars. Clin Plast Surg. 2017;44:767-779.
- Miller A. CPT 2018: What's new, part 2. American Academy of Dermatology website. https://www.aad.org/dw/monthly/2018/january/cpt-2018-whats-new-part-2. Accessed July 24, 2018.
- Miletta NR, Kim S, Lezanski-Gujda A, et al. Improving health-related quality of life in wounded warriors: the promising benefits of laser hair removal to the residual limb-prosthetic interface. Dermatol Surg. 2016;42:1182-1187.
- Gratrix M, Hivnor C. Botulinum toxin for hyperhidrosis in patients with prosthetic limbs. Arch Dermatol. 2010;146:1314-1315.
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- USNS Mercy deploys in support of Pacific Partnership 2018 [news release]. Washington, DC: US Department of Defense; February 26, 2018. https://www.defense.gov/News/Article/Article/1450292/usns-mercy-deploys-in-support-of-pacific-partnership-2018/. Accessed July 11, 2018.
- Burns C, Basnett A, Valentine J, et al. Ablative fractional resurfacing: a powerful tool to help restore form and function during international medical exchange. Lasers Surg Med. 2017;49:471-474.
Over the last decade the treatment of traumatic scars with lasers has emerged as a core component of multidisciplinary management. Military dermatologists have played a fundamental role in this shift by helping to develop new applications for existing technology and promulgate the techniques to reach additional providers and patients. Beyond scar management, the repurposing of adjunctive procedural techniques, such as sweat and hair reduction in amputees, also promises to enhance rehabilitation for many patients.
International engagement is a prominent and highly attractive feature of military practice, and military dermatologists routinely participate in disaster response missions, such as the 2010 Haiti earthquake,1 and ongoing planned operations, such as Pacific Partnership in the Indo-Asia-Pacific region led by the US Navy.2 In this article, I present a military perspective on the emerging niche of trauma dermatology and outline my more than 5 years of experience leveraging these skills to lead a multidisciplinary exchange in restorative medicine and burn scar management in Vietnam.
Trauma Dermatology
Over the course of the last decade, traumatic scar management has emerged as a staple of dermatologic surgery practice in some centers. Dermatologists hold the key to increasing patient access to effective outpatient care for symptomatic traumatic scars and other related issues using devices and techniques initially conceived for cosmetic applications.3 A major impetus for the considerable remodeling in our collective thoughts about traumatic scar management was the emergence of fractional laser technology in the mid-2000s. The remarkable, safe, reproducible, and durable benefits of fractional laser treatment of various scar types have created substantial momentum in recent years. The Naval Medical Center San Diego in California houses 1 of 3 centers of excellence in rehabilitation in the US military. Mastery of minimally invasive procedures to manage scars and other issues associated with trauma for the first time has established dermatologists as important partners in the overall rehabilitative effort.
My perspective on laser scar management has been previously described.4,5 Ablative fractional laser resurfacing is the backbone of rehabilitative scar management.6 Although the literature in this field is still relatively immature, higher-quality studies are accumulating rapidly as the burn and surgical communities adopt the procedure more widely.7-10 A considerable step forward in the dissemination of the procedure occurred recently with the development of category III Current Procedural Terminology (CPT) codes for ablative laser treatment of traumatic scars.11 Category III CPT codes are temporary codes used for emerging procedures that have not yet been deemed medically necessary. Although individual insurance carriers can determine whether to cover these procedures and the corresponding level of reimbursement, regular use is important for ultimate elevation to category I codes by the American Medical Association over a 5-year observation period. The CPT codes 0479T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; first 100 cm2 or part thereof, or 1% of body surface area of infants and children) and 0480T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; each additional 100 cm2, or each additional 1% of body surface area of infants and children, or part thereof [list separately in addition to code for primary procedure]) are examples of these category III codes.11
Nonablative fractional lasers; vascular-specific devices for erythematous scars; and long- and short-pulsed pigment-specific devices for hair and traumatic tattoo treatment, respectively, round out the commonly used laser platforms. For example, laser hair reduction can help improve the fit and comfort of prosthetic devices and has been shown to improve the overall quality of life for amputees.12 Botulinum toxin can be an important component of treatment of excessive sweating induced by occlusive liners in prosthetics, and microwave eccrine ablation is an emerging potential option for longer-lasting sweat reduction in this population.13-15 In addition to providing direct dermatology care and education, having members of the specialty in uniform has been a key to adopting new practical solutions to unsolved problems.
Pacific Partnership
Pacific Partnership is the largest annual multinational humanitarian assistance and disaster preparedness mission in the Indo-Asia-Pacific region.16 It was started in 2006 following the tsunami that devastated parts of South and Southeast Asia in 2004. The recently concluded Pacific Partnership 2018 marked the 13th iteration of the annual mission led by the US Navy in collaboration with other partner nations, which in 2018 included Japan, Australia, Canada, the United Kingdom, France, Singapore, Korea, and Peru, as well as nongovernmental organizations and international governmental agencies. Host nation mission locations vary somewhat from year to year, but 2018 included visits of the hospital ship USNS Mercy and more than 800 personnel to Indonesia, Malaysia, Sri Lanka, and Vietnam. Medical/dental, engineering, and veterinary teams join with their counterparts in each host nation to conduct civic action projects, community health exchanges, medical care, and disaster response training activities.16
Rehabilitation As a Vehicle for Medical Exchange
Since approximately 2012 there has been an evolving paradigm in Pacific Partnership from an emphasis on maximizing direct patient care in changing locations to one focused on building lasting partnerships through subject matter expert exchange. Multidisciplinary scar management, including surgical and laser scar revision and physical and occupational therapy, is a very promising model for engagement. Potential advantages of this type of exchange include the following: developing nations have relatively high rates of burns and other forms of trauma as well as uneven access to acute and ongoing rehabilitative care; patients often are otherwise healthy and young; results are frequently profound and readily demonstrable; and it is a skill set that has become highly developed in the military system. Just as dermatologists are illustrating their utility in trauma rehabilitation at home, these procedural skills provide fertile ground for exchange overseas.
The Overseas Humanitarian Assistance Shared Information System is an online platform that allows users to apply for grants under the Asia-Pacific Regional Initiative. In 2013, I started the Burn Scar Treatment/Restorative Medicine exchange with a grant under this program. A multidisciplinary team representing the specialties of dermatology, hand surgery, plastic surgery, physical medicine and rehabilitation, and pulmonary critical care participated in the 2013 Asia Pacific Burn Congress hosted by the National Institute of Burns (NIB) in Hanoi, Vietnam, and then followed up with didactics and patient care alongside Vietnamese physicians in the management of disfiguring and debilitating scars from burns and other trauma. This pilot project consisted of three 2- to 3-week phases: 2 at the NIB in Hanoi and 1 with a delegation from the NIB visiting the Naval Medical Center San Diego. When initial project funds expired in 2014, the exchange was absorbed into Pacific Partnership 2014, which began a string of 4 consecutive annual Pacific Partnership engagements at Da Nang General Hospital in Vietnam. The 2 most recent exchanges, including the exchange associated with Pacific Partnership 2018, have taken place at Khanh Hoa General Hospital in Nha Trang, Vietnam. During this time the team has grown to include physical and occupational therapists as well as a wound care nurse.
The Burn Scar Treatment/Restorative Medicine exchange consists of side-by-side laser and surgical scar revision performed with our Vietnamese hosts in their own hospital. Our Vietnamese partners perform a large volume of reconstructive surgeries in their usual practice, so it truly has been a bilateral exchange incorporating some advanced technology and techniques with an emphasis on longitudinal multidisciplinary care. Importantly, the procedures are supplemented with preoperative and postoperative care as well as instruction provided by physical and occupational therapy and wound care professionals working alongside host nation support staff. Because the areas of involvement often are extensive and a patient may only be seen once in this setting, laser and surgical procedures often are performed concurrently in the host nation operating room. Anesthesia support is provided by the host nation. Basic consumable surgical supplies (eg, sutures, gloves, marking pens, staplers) are supplemented with mission funds. Special adjuncts for the most severe contractures have included negative pressure wound therapy and a collagen-based bilayer matrix wound dressing. Laser treatments have been performed on the vast majority of patients with an ablative fractional CO2 laser and laser-assisted delivery of corticosteroid in hypertrophic areas. Of note, use of the laser has been provided to our hosts by the manufacturer for each of the 7 iterations of the exchange, and the wound dressing manufacturer also has donated some of their product to the exchange through the nongovernmental organization Project Hope for 2 missions. To date, more than 300 patients have safely received life-changing treatment during the exchange, with some receiving multiple treatments (Figure). Although multiple treatments over time are ideal, even a single treatment session can result in considerable and lasting improvements in function and symptoms.17 The hospital ship USNS Mercy has the same laser technology and has brought advanced scar treatment techniques to the far corners of the Pacific.

Measuring overall success—treatment and international relations—in this setting can be challenging. On an individual patient level, the benefits of restoring the ability to walk and work as well as reducing pain and itching are manifest and transformative for both the patient and family; however, aggregating this information into high-quality outcome data is difficult given the heterogeneous nature of traumatic injuries, which is compounded in the setting of international engagement where the intersection between patient and visiting provider may be singular or difficult to predict, funding is limited, language frequently is a barrier, and documentation, privacy, and medical research guidelines may be unfamiliar or contradictory. The cumulative impact of these types of exchanges on the relationship between nations also is critical but difficult to measure. It is common sense that deepening personal and professional relationships in the medical setting over time can increase trust and mutual understanding, perhaps setting the stage for broader engagement in other more sensitive areas. Trust and understanding are rather nebulous concepts, but earlier this year marked the first visit of an American aircraft carrier to Da Nang since 1975, following 4 consecutive annual Pacific Partnership missions in the same city, which does carry the patina of successful engagement on a systemic level.
Final Thoughts
Based on my personal experience, I provide the following tips for building a successful, focused, long-term medical exchange.
- Leverage your strengths and respect the strengths and style of practice of your hosts. A mind-set of exchange and not simply humanitarian care will be more successful. Your hosts are experts in a style of practice adapted to their surroundings and introducing new techniques that are grounded in the local practice patterns are more likely to be perpetuated.
- Collaboration with nongovernmental organizations and industry can be extremely helpful. Military and governmental organizations often are limited in funding, in the ways they can spend available funding, and in the receipt of donations. Appropriate coordination with civilian entities can elevate the exchange considerably by adding expertise and available assets as well as broadening the overall impact.
- Engage the support staff as well as the physicians. You will leverage contact with families and enhance care over the long-term.
- The benefits of multiple interactions over time are manifest, for both the patients and the participants. Personal and professional relationships are intertwined and naturally mature over time. Go for singles and doubles first before swinging for the fences.
- Multidisciplinary work overseas informs and enhances collaboration at home.
- Adding regional experts in international research and assessment to these specialized medical teams may better capture the impact of future exchanges of any flavor.
- The model of creating a focused exchange with independent funding followed by incorporation of successful concepts into larger missions seems to be a worthy and reproducible approach for future projects of any variety.
Over the last decade the treatment of traumatic scars with lasers has emerged as a core component of multidisciplinary management. Military dermatologists have played a fundamental role in this shift by helping to develop new applications for existing technology and promulgate the techniques to reach additional providers and patients. Beyond scar management, the repurposing of adjunctive procedural techniques, such as sweat and hair reduction in amputees, also promises to enhance rehabilitation for many patients.
International engagement is a prominent and highly attractive feature of military practice, and military dermatologists routinely participate in disaster response missions, such as the 2010 Haiti earthquake,1 and ongoing planned operations, such as Pacific Partnership in the Indo-Asia-Pacific region led by the US Navy.2 In this article, I present a military perspective on the emerging niche of trauma dermatology and outline my more than 5 years of experience leveraging these skills to lead a multidisciplinary exchange in restorative medicine and burn scar management in Vietnam.
Trauma Dermatology
Over the course of the last decade, traumatic scar management has emerged as a staple of dermatologic surgery practice in some centers. Dermatologists hold the key to increasing patient access to effective outpatient care for symptomatic traumatic scars and other related issues using devices and techniques initially conceived for cosmetic applications.3 A major impetus for the considerable remodeling in our collective thoughts about traumatic scar management was the emergence of fractional laser technology in the mid-2000s. The remarkable, safe, reproducible, and durable benefits of fractional laser treatment of various scar types have created substantial momentum in recent years. The Naval Medical Center San Diego in California houses 1 of 3 centers of excellence in rehabilitation in the US military. Mastery of minimally invasive procedures to manage scars and other issues associated with trauma for the first time has established dermatologists as important partners in the overall rehabilitative effort.
My perspective on laser scar management has been previously described.4,5 Ablative fractional laser resurfacing is the backbone of rehabilitative scar management.6 Although the literature in this field is still relatively immature, higher-quality studies are accumulating rapidly as the burn and surgical communities adopt the procedure more widely.7-10 A considerable step forward in the dissemination of the procedure occurred recently with the development of category III Current Procedural Terminology (CPT) codes for ablative laser treatment of traumatic scars.11 Category III CPT codes are temporary codes used for emerging procedures that have not yet been deemed medically necessary. Although individual insurance carriers can determine whether to cover these procedures and the corresponding level of reimbursement, regular use is important for ultimate elevation to category I codes by the American Medical Association over a 5-year observation period. The CPT codes 0479T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; first 100 cm2 or part thereof, or 1% of body surface area of infants and children) and 0480T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; each additional 100 cm2, or each additional 1% of body surface area of infants and children, or part thereof [list separately in addition to code for primary procedure]) are examples of these category III codes.11
Nonablative fractional lasers; vascular-specific devices for erythematous scars; and long- and short-pulsed pigment-specific devices for hair and traumatic tattoo treatment, respectively, round out the commonly used laser platforms. For example, laser hair reduction can help improve the fit and comfort of prosthetic devices and has been shown to improve the overall quality of life for amputees.12 Botulinum toxin can be an important component of treatment of excessive sweating induced by occlusive liners in prosthetics, and microwave eccrine ablation is an emerging potential option for longer-lasting sweat reduction in this population.13-15 In addition to providing direct dermatology care and education, having members of the specialty in uniform has been a key to adopting new practical solutions to unsolved problems.
Pacific Partnership
Pacific Partnership is the largest annual multinational humanitarian assistance and disaster preparedness mission in the Indo-Asia-Pacific region.16 It was started in 2006 following the tsunami that devastated parts of South and Southeast Asia in 2004. The recently concluded Pacific Partnership 2018 marked the 13th iteration of the annual mission led by the US Navy in collaboration with other partner nations, which in 2018 included Japan, Australia, Canada, the United Kingdom, France, Singapore, Korea, and Peru, as well as nongovernmental organizations and international governmental agencies. Host nation mission locations vary somewhat from year to year, but 2018 included visits of the hospital ship USNS Mercy and more than 800 personnel to Indonesia, Malaysia, Sri Lanka, and Vietnam. Medical/dental, engineering, and veterinary teams join with their counterparts in each host nation to conduct civic action projects, community health exchanges, medical care, and disaster response training activities.16
Rehabilitation As a Vehicle for Medical Exchange
Since approximately 2012 there has been an evolving paradigm in Pacific Partnership from an emphasis on maximizing direct patient care in changing locations to one focused on building lasting partnerships through subject matter expert exchange. Multidisciplinary scar management, including surgical and laser scar revision and physical and occupational therapy, is a very promising model for engagement. Potential advantages of this type of exchange include the following: developing nations have relatively high rates of burns and other forms of trauma as well as uneven access to acute and ongoing rehabilitative care; patients often are otherwise healthy and young; results are frequently profound and readily demonstrable; and it is a skill set that has become highly developed in the military system. Just as dermatologists are illustrating their utility in trauma rehabilitation at home, these procedural skills provide fertile ground for exchange overseas.
The Overseas Humanitarian Assistance Shared Information System is an online platform that allows users to apply for grants under the Asia-Pacific Regional Initiative. In 2013, I started the Burn Scar Treatment/Restorative Medicine exchange with a grant under this program. A multidisciplinary team representing the specialties of dermatology, hand surgery, plastic surgery, physical medicine and rehabilitation, and pulmonary critical care participated in the 2013 Asia Pacific Burn Congress hosted by the National Institute of Burns (NIB) in Hanoi, Vietnam, and then followed up with didactics and patient care alongside Vietnamese physicians in the management of disfiguring and debilitating scars from burns and other trauma. This pilot project consisted of three 2- to 3-week phases: 2 at the NIB in Hanoi and 1 with a delegation from the NIB visiting the Naval Medical Center San Diego. When initial project funds expired in 2014, the exchange was absorbed into Pacific Partnership 2014, which began a string of 4 consecutive annual Pacific Partnership engagements at Da Nang General Hospital in Vietnam. The 2 most recent exchanges, including the exchange associated with Pacific Partnership 2018, have taken place at Khanh Hoa General Hospital in Nha Trang, Vietnam. During this time the team has grown to include physical and occupational therapists as well as a wound care nurse.
The Burn Scar Treatment/Restorative Medicine exchange consists of side-by-side laser and surgical scar revision performed with our Vietnamese hosts in their own hospital. Our Vietnamese partners perform a large volume of reconstructive surgeries in their usual practice, so it truly has been a bilateral exchange incorporating some advanced technology and techniques with an emphasis on longitudinal multidisciplinary care. Importantly, the procedures are supplemented with preoperative and postoperative care as well as instruction provided by physical and occupational therapy and wound care professionals working alongside host nation support staff. Because the areas of involvement often are extensive and a patient may only be seen once in this setting, laser and surgical procedures often are performed concurrently in the host nation operating room. Anesthesia support is provided by the host nation. Basic consumable surgical supplies (eg, sutures, gloves, marking pens, staplers) are supplemented with mission funds. Special adjuncts for the most severe contractures have included negative pressure wound therapy and a collagen-based bilayer matrix wound dressing. Laser treatments have been performed on the vast majority of patients with an ablative fractional CO2 laser and laser-assisted delivery of corticosteroid in hypertrophic areas. Of note, use of the laser has been provided to our hosts by the manufacturer for each of the 7 iterations of the exchange, and the wound dressing manufacturer also has donated some of their product to the exchange through the nongovernmental organization Project Hope for 2 missions. To date, more than 300 patients have safely received life-changing treatment during the exchange, with some receiving multiple treatments (Figure). Although multiple treatments over time are ideal, even a single treatment session can result in considerable and lasting improvements in function and symptoms.17 The hospital ship USNS Mercy has the same laser technology and has brought advanced scar treatment techniques to the far corners of the Pacific.

Measuring overall success—treatment and international relations—in this setting can be challenging. On an individual patient level, the benefits of restoring the ability to walk and work as well as reducing pain and itching are manifest and transformative for both the patient and family; however, aggregating this information into high-quality outcome data is difficult given the heterogeneous nature of traumatic injuries, which is compounded in the setting of international engagement where the intersection between patient and visiting provider may be singular or difficult to predict, funding is limited, language frequently is a barrier, and documentation, privacy, and medical research guidelines may be unfamiliar or contradictory. The cumulative impact of these types of exchanges on the relationship between nations also is critical but difficult to measure. It is common sense that deepening personal and professional relationships in the medical setting over time can increase trust and mutual understanding, perhaps setting the stage for broader engagement in other more sensitive areas. Trust and understanding are rather nebulous concepts, but earlier this year marked the first visit of an American aircraft carrier to Da Nang since 1975, following 4 consecutive annual Pacific Partnership missions in the same city, which does carry the patina of successful engagement on a systemic level.
Final Thoughts
Based on my personal experience, I provide the following tips for building a successful, focused, long-term medical exchange.
- Leverage your strengths and respect the strengths and style of practice of your hosts. A mind-set of exchange and not simply humanitarian care will be more successful. Your hosts are experts in a style of practice adapted to their surroundings and introducing new techniques that are grounded in the local practice patterns are more likely to be perpetuated.
- Collaboration with nongovernmental organizations and industry can be extremely helpful. Military and governmental organizations often are limited in funding, in the ways they can spend available funding, and in the receipt of donations. Appropriate coordination with civilian entities can elevate the exchange considerably by adding expertise and available assets as well as broadening the overall impact.
- Engage the support staff as well as the physicians. You will leverage contact with families and enhance care over the long-term.
- The benefits of multiple interactions over time are manifest, for both the patients and the participants. Personal and professional relationships are intertwined and naturally mature over time. Go for singles and doubles first before swinging for the fences.
- Multidisciplinary work overseas informs and enhances collaboration at home.
- Adding regional experts in international research and assessment to these specialized medical teams may better capture the impact of future exchanges of any flavor.
- The model of creating a focused exchange with independent funding followed by incorporation of successful concepts into larger missions seems to be a worthy and reproducible approach for future projects of any variety.
- Galeckas K. Dermatology aboard the USNS Comfort: disaster relief operations in Haiti after the 2010 earthquake. Dermatol Clin. 2011;29:15-19.
- Satter EK. The role of the dermatologist on military humanitarian missions. Cutis. 2010;85:85-89.
- Miletta NR, Donelan MB, Hivnor CM. Management of trauma and burn scars; the dermatologist's role in expanding patient access to care. Cutis. 2017;100:18-20.
- Shumaker PR. Laser treatment of traumatic scars: a military perspective. Semin Cutan Med Surg. 2015;34:17-23.
- Shumaker PR, Beachkofsky T, Basnett A, et al. A military perspective. In: Krakowski AC, Shumaker PR, eds. The Scar Book: Formation, Mitigation, Rehabilitation and Prevention. Philadelphia, PA: Wolters Kluwer; 2017:327-338.
- Anderson RR, Donelan MB, Greeson E, et al. Consensus report: laser treatment of traumatic scars with an emphasis on ablative fractional resurfacing. JAMA Dermatol. 2014;150:187-193.
- Hultman CS, Friedstat JS, Edkins RE, et al. Laser resurfacing and remodeling of hypertrophic burn scars: the results of a large, prospective, before and after cohort study, with long-term follow-up. Ann Surg. 2014;260:519-532.
- Blome-Eberwein S, Gogal C, Weiss MJ, et al. Prospective evaluation of fractional CO2 laser treatment of mature burn scars. J Burn Care Res. 2016;37:379-387.
- Issler-Fisher AC, Fisher OM, Smialkowski AO, et al. Ablative fractional CO2 laser for burn scar reconstruction: an extensive subjective and objective short-term outcome analysis of a prospective treatment cohort. Burns. 2017;43:573-582.
- Zuccaro J, Zlolkowski N, Fish J. A systematic review of the effectiveness of laser therapy for hypertrophic burn scars. Clin Plast Surg. 2017;44:767-779.
- Miller A. CPT 2018: What's new, part 2. American Academy of Dermatology website. https://www.aad.org/dw/monthly/2018/january/cpt-2018-whats-new-part-2. Accessed July 24, 2018.
- Miletta NR, Kim S, Lezanski-Gujda A, et al. Improving health-related quality of life in wounded warriors: the promising benefits of laser hair removal to the residual limb-prosthetic interface. Dermatol Surg. 2016;42:1182-1187.
- Gratrix M, Hivnor C. Botulinum toxin for hyperhidrosis in patients with prosthetic limbs. Arch Dermatol. 2010;146:1314-1315.
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- USNS Mercy deploys in support of Pacific Partnership 2018 [news release]. Washington, DC: US Department of Defense; February 26, 2018. https://www.defense.gov/News/Article/Article/1450292/usns-mercy-deploys-in-support-of-pacific-partnership-2018/. Accessed July 11, 2018.
- Burns C, Basnett A, Valentine J, et al. Ablative fractional resurfacing: a powerful tool to help restore form and function during international medical exchange. Lasers Surg Med. 2017;49:471-474.
- Galeckas K. Dermatology aboard the USNS Comfort: disaster relief operations in Haiti after the 2010 earthquake. Dermatol Clin. 2011;29:15-19.
- Satter EK. The role of the dermatologist on military humanitarian missions. Cutis. 2010;85:85-89.
- Miletta NR, Donelan MB, Hivnor CM. Management of trauma and burn scars; the dermatologist's role in expanding patient access to care. Cutis. 2017;100:18-20.
- Shumaker PR. Laser treatment of traumatic scars: a military perspective. Semin Cutan Med Surg. 2015;34:17-23.
- Shumaker PR, Beachkofsky T, Basnett A, et al. A military perspective. In: Krakowski AC, Shumaker PR, eds. The Scar Book: Formation, Mitigation, Rehabilitation and Prevention. Philadelphia, PA: Wolters Kluwer; 2017:327-338.
- Anderson RR, Donelan MB, Greeson E, et al. Consensus report: laser treatment of traumatic scars with an emphasis on ablative fractional resurfacing. JAMA Dermatol. 2014;150:187-193.
- Hultman CS, Friedstat JS, Edkins RE, et al. Laser resurfacing and remodeling of hypertrophic burn scars: the results of a large, prospective, before and after cohort study, with long-term follow-up. Ann Surg. 2014;260:519-532.
- Blome-Eberwein S, Gogal C, Weiss MJ, et al. Prospective evaluation of fractional CO2 laser treatment of mature burn scars. J Burn Care Res. 2016;37:379-387.
- Issler-Fisher AC, Fisher OM, Smialkowski AO, et al. Ablative fractional CO2 laser for burn scar reconstruction: an extensive subjective and objective short-term outcome analysis of a prospective treatment cohort. Burns. 2017;43:573-582.
- Zuccaro J, Zlolkowski N, Fish J. A systematic review of the effectiveness of laser therapy for hypertrophic burn scars. Clin Plast Surg. 2017;44:767-779.
- Miller A. CPT 2018: What's new, part 2. American Academy of Dermatology website. https://www.aad.org/dw/monthly/2018/january/cpt-2018-whats-new-part-2. Accessed July 24, 2018.
- Miletta NR, Kim S, Lezanski-Gujda A, et al. Improving health-related quality of life in wounded warriors: the promising benefits of laser hair removal to the residual limb-prosthetic interface. Dermatol Surg. 2016;42:1182-1187.
- Gratrix M, Hivnor C. Botulinum toxin for hyperhidrosis in patients with prosthetic limbs. Arch Dermatol. 2010;146:1314-1315.
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- USNS Mercy deploys in support of Pacific Partnership 2018 [news release]. Washington, DC: US Department of Defense; February 26, 2018. https://www.defense.gov/News/Article/Article/1450292/usns-mercy-deploys-in-support-of-pacific-partnership-2018/. Accessed July 11, 2018.
- Burns C, Basnett A, Valentine J, et al. Ablative fractional resurfacing: a powerful tool to help restore form and function during international medical exchange. Lasers Surg Med. 2017;49:471-474.
Five common pitfalls of retailing skin care
Others believe that providing patients with the correct skin care product recommendations for their skin’s needs is a crucial step to improving outcomes and educating patients.
There is a wide range of challenges related to skin care retail that many physicians face. I will be running a course on Skin Care Retail at the American Society for Dermatologic Surgery meeting in October in Scottsdale, Ariz., if you want to learn more or share your opinions. I have surveyed plastic surgeons and dermatologists via LinkedIn about what they believe are some of the biggest pitfalls to retailing skin care. Here, I will share some of their insights and suggestions for overcoming these obstacles.
1. Patients are more knowledgeable about skin care than ever before
Facing an increasing number of over-the-counter skin care products available, as well as buzzwords like “organic ingredients” and “vegan,” patients are now bombarded with information from a variety of different sources. Because of this, patients come to the doctor with preconceived ideas that can affect compliance if their specific needs and beliefs are not properly addressed.
For New York plastic surgeon Sonita M. Sadio, MD, this is one of the reasons why she chooses not to sell skin care in her office.
“My practice is highly consultative, and ongoing skin care recommendations are a significant part of what I do to optimize patient outcomes,” Dr. Sadio said. “Patients are well-educated about skin care today. They know their ingredients and insist on clean formulations, free of certain ingredients, such as ‘cruelty-free’ and ‘vegan.’ Others feel deprived if they are not using an expensive product in elegant packaging. Still, others insist on drugstore favorites or ‘eco’ offerings and have their own sense of what that means. My job is to optimize the clinical outcome while also meeting these patients needs to ensure compliance.”
Not all doctors have the time, knowledge or desire to personally design each patient’s skin care regimen. Many delegate this to the staff. However, it is impossible to ensure that your staff matches patients to the proper products unless they have had extensive training on both skin care products and how to match them to the patient’s skin issues.
2. Patients are wary when the doctors sells only one product brand
Many studies have shown that, although consumers desire a choice when making purchases, they get overwhelmed if they are presented with too many options. One study showed that it is optimal to carry at least 3 brands of products. For this reason, limiting the skin care you sell to one brand or doing your own private label is not optimal.
New York dermatologist Rebecca Tamez, MD, pointed out the same problem when selling practice-specific skin care. “At my previous job, we sold skin care products directly to patients. I had no issues selling products that were readily available in drugstores or online (such as Vanicream and EltaMD). We usually sold these around the same cost as the drugstore or Amazon. However, it was harder to sell the practice-specific skin care line. I feel patients were more wary of these products.”
3. Doctors do not want to feel like salespeople
If you have read my Dermatology News columns in the past, you may know that I think it is unethical for dermatologists to not offer specific skin care advice to their patients. If patients do not get ethical and scientific recommendations from us, they will follow the advice of a friend or salesperson or purchase based on often inflated marketing claims.
Dermatologists often tell me: “I am not a cosmetic dermatologist so I do not sell skin care.” I feel strongly that general dermatologists should be giving specific written skin care recommendations for their patients too. Acne, rosacea, melasma, eczema, psoriasis, keratosis pilaris, and many other conditions will improve faster with an efficacious skin care regimen, assuming the patient is compliant with the instructions. Retailing skin care improves compliance by eliminating a few barriers to beginning the skin care regimen. I believe that the mindset of dermatologists needs to change: It is not about selling products to patients, it is about educating them on what to use and offering the products out of convenience and the desire to improve compliance.
Meadowbrook, Pa., dermatologist Michael A. Tomeo, MD, explained an obstacle faced by many dermatologists:
“I suspect, like many of my colleagues,” said Dr. Tomeo, “that I am held back in terms of salesmanship, having been trained in the traditional way. Physicians of my generation were taught to be ethical and professional and to focus on academic and clinical excellence, and salesmanship and advertising one’s services were frowned upon. It takes time to reset one’s former proclivities. Cosmeceuticals and nutraceuticals are revolutionizing the skin care world, and as experts in all things skin, we need to be well informed and offer our patients safe, effective, and cutting-edge treatments.”
4. Providers are concerned about product costs and time constraints
Providing excellent patient care and improving outcomes is at the forefront of our business, but financial concerns and time constraints prevent some doctors from offering skin care to their patients.
Rochester Hills, Mich., plastic surgeon Richard Hainer, MD, has found that “skin care is often too complex with too many products and is not very profitable.” For those reasons, Dr. Hainer has chosen not to retail skin care in his practice.
Nampa, Idaho, dermatologist Ryan S. Owsley, MD, explained that “the required minimum purchases by some of the product lines can leave the practice with expired product if it is not selling a particular line well. Cost can also be an issue for some patients in the area we are located.”
As a burn survivor and burn surgeon, Mark McDonough, MD, from Orlando “has a long history with skin care and rejuvenation. I did have a private label skin care line, including a moisturizer, a hydroquinone product, a retinol cream, and a sunscreen,” Dr. McDonough said. “However, and regrettably, I have not kept up with marketing and promotion, with most of my energy invested in trauma and disease survivors through a book, a blog, and my platform through my website.”
Doing your own product line is costly and spending the time and resources to promote it is not always possible. Buying the minimum order of products is often expensive, and you will not be able to sell them without a proven methodology in place. New products enter the market frequently, and it is expensive to always carry the latest technologies because new minimum orders must be met with each new brand that you add.
5. Selling skin care requires ongoing education
Properly recommending and retailing skin care involves physician, staff, and patient education. Unfortunately, most practices rely on training from the cosmeceutical sales reps who obviously have a brand bias. There is minimal unbiased “brand agnostic” skin care training for dermatologists and their staff. In fact, the AAD meeting has only a few skin care lectures in the program. Plastic surgeon Gaurav Bharti, MD, of Charlotte, N.C., explained that “motivating staff to help with retail skin care can be challenging. The first step is to get the staff familiar with the products with open discussions with the representatives. The next step has been to have the staff actually use the products and believe in them. Once they believe in the product, we have used an incentivization model that’s simple, transparent, and predictable.”
We are all too busy to spend adequate time with our patients, so it is critical that our staff be able to properly recommended skin care for us. We have to ensure that our staff is taking an ethical and scientific approach to skin care retail rather than a financial one. Rigorous staff training on how to match skin care products to skin type is the key to improving outcomes with skin care recommendations.
In a similar sense, Cincinnati plastic surgeon Richard Williams, MD, commented that “aestheticians often succumb to the desires of our patients to carry too many products in inventory, for which they do not have enough knowledge of the product’s benefits. This can be a very frustrating challenge.”
Conclusion
Although there are many obstacles to retailing skin care in your medical practice, the benefits that it provides to both your patients (improved outcomes) and your practice (increased profitability) far outweigh the challenges. I solved these pitfalls in my own practice by developing a standardized staff training program and skin care diagnostic software that is now used by over 100 medical practices. If you want to start retaining skin care, my advice is develop a training plan and a methodology for the recommendation and patient education process before you spend a lot of money on the required minimum product order. Feel free to contact me for advice. Alternatively, if you already do a great job of retailing skin care and want to provide tips to include in my American Society for Dermatologic Surgery course, contact me on LinkedIn or [email protected]. You can also find blogs I have written on skin care retail advice at STSFranchise.com.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also wrote a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems.
Others believe that providing patients with the correct skin care product recommendations for their skin’s needs is a crucial step to improving outcomes and educating patients.
There is a wide range of challenges related to skin care retail that many physicians face. I will be running a course on Skin Care Retail at the American Society for Dermatologic Surgery meeting in October in Scottsdale, Ariz., if you want to learn more or share your opinions. I have surveyed plastic surgeons and dermatologists via LinkedIn about what they believe are some of the biggest pitfalls to retailing skin care. Here, I will share some of their insights and suggestions for overcoming these obstacles.
1. Patients are more knowledgeable about skin care than ever before
Facing an increasing number of over-the-counter skin care products available, as well as buzzwords like “organic ingredients” and “vegan,” patients are now bombarded with information from a variety of different sources. Because of this, patients come to the doctor with preconceived ideas that can affect compliance if their specific needs and beliefs are not properly addressed.
For New York plastic surgeon Sonita M. Sadio, MD, this is one of the reasons why she chooses not to sell skin care in her office.
“My practice is highly consultative, and ongoing skin care recommendations are a significant part of what I do to optimize patient outcomes,” Dr. Sadio said. “Patients are well-educated about skin care today. They know their ingredients and insist on clean formulations, free of certain ingredients, such as ‘cruelty-free’ and ‘vegan.’ Others feel deprived if they are not using an expensive product in elegant packaging. Still, others insist on drugstore favorites or ‘eco’ offerings and have their own sense of what that means. My job is to optimize the clinical outcome while also meeting these patients needs to ensure compliance.”
Not all doctors have the time, knowledge or desire to personally design each patient’s skin care regimen. Many delegate this to the staff. However, it is impossible to ensure that your staff matches patients to the proper products unless they have had extensive training on both skin care products and how to match them to the patient’s skin issues.
2. Patients are wary when the doctors sells only one product brand
Many studies have shown that, although consumers desire a choice when making purchases, they get overwhelmed if they are presented with too many options. One study showed that it is optimal to carry at least 3 brands of products. For this reason, limiting the skin care you sell to one brand or doing your own private label is not optimal.
New York dermatologist Rebecca Tamez, MD, pointed out the same problem when selling practice-specific skin care. “At my previous job, we sold skin care products directly to patients. I had no issues selling products that were readily available in drugstores or online (such as Vanicream and EltaMD). We usually sold these around the same cost as the drugstore or Amazon. However, it was harder to sell the practice-specific skin care line. I feel patients were more wary of these products.”
3. Doctors do not want to feel like salespeople
If you have read my Dermatology News columns in the past, you may know that I think it is unethical for dermatologists to not offer specific skin care advice to their patients. If patients do not get ethical and scientific recommendations from us, they will follow the advice of a friend or salesperson or purchase based on often inflated marketing claims.
Dermatologists often tell me: “I am not a cosmetic dermatologist so I do not sell skin care.” I feel strongly that general dermatologists should be giving specific written skin care recommendations for their patients too. Acne, rosacea, melasma, eczema, psoriasis, keratosis pilaris, and many other conditions will improve faster with an efficacious skin care regimen, assuming the patient is compliant with the instructions. Retailing skin care improves compliance by eliminating a few barriers to beginning the skin care regimen. I believe that the mindset of dermatologists needs to change: It is not about selling products to patients, it is about educating them on what to use and offering the products out of convenience and the desire to improve compliance.
Meadowbrook, Pa., dermatologist Michael A. Tomeo, MD, explained an obstacle faced by many dermatologists:
“I suspect, like many of my colleagues,” said Dr. Tomeo, “that I am held back in terms of salesmanship, having been trained in the traditional way. Physicians of my generation were taught to be ethical and professional and to focus on academic and clinical excellence, and salesmanship and advertising one’s services were frowned upon. It takes time to reset one’s former proclivities. Cosmeceuticals and nutraceuticals are revolutionizing the skin care world, and as experts in all things skin, we need to be well informed and offer our patients safe, effective, and cutting-edge treatments.”
4. Providers are concerned about product costs and time constraints
Providing excellent patient care and improving outcomes is at the forefront of our business, but financial concerns and time constraints prevent some doctors from offering skin care to their patients.
Rochester Hills, Mich., plastic surgeon Richard Hainer, MD, has found that “skin care is often too complex with too many products and is not very profitable.” For those reasons, Dr. Hainer has chosen not to retail skin care in his practice.
Nampa, Idaho, dermatologist Ryan S. Owsley, MD, explained that “the required minimum purchases by some of the product lines can leave the practice with expired product if it is not selling a particular line well. Cost can also be an issue for some patients in the area we are located.”
As a burn survivor and burn surgeon, Mark McDonough, MD, from Orlando “has a long history with skin care and rejuvenation. I did have a private label skin care line, including a moisturizer, a hydroquinone product, a retinol cream, and a sunscreen,” Dr. McDonough said. “However, and regrettably, I have not kept up with marketing and promotion, with most of my energy invested in trauma and disease survivors through a book, a blog, and my platform through my website.”
Doing your own product line is costly and spending the time and resources to promote it is not always possible. Buying the minimum order of products is often expensive, and you will not be able to sell them without a proven methodology in place. New products enter the market frequently, and it is expensive to always carry the latest technologies because new minimum orders must be met with each new brand that you add.
5. Selling skin care requires ongoing education
Properly recommending and retailing skin care involves physician, staff, and patient education. Unfortunately, most practices rely on training from the cosmeceutical sales reps who obviously have a brand bias. There is minimal unbiased “brand agnostic” skin care training for dermatologists and their staff. In fact, the AAD meeting has only a few skin care lectures in the program. Plastic surgeon Gaurav Bharti, MD, of Charlotte, N.C., explained that “motivating staff to help with retail skin care can be challenging. The first step is to get the staff familiar with the products with open discussions with the representatives. The next step has been to have the staff actually use the products and believe in them. Once they believe in the product, we have used an incentivization model that’s simple, transparent, and predictable.”
We are all too busy to spend adequate time with our patients, so it is critical that our staff be able to properly recommended skin care for us. We have to ensure that our staff is taking an ethical and scientific approach to skin care retail rather than a financial one. Rigorous staff training on how to match skin care products to skin type is the key to improving outcomes with skin care recommendations.
In a similar sense, Cincinnati plastic surgeon Richard Williams, MD, commented that “aestheticians often succumb to the desires of our patients to carry too many products in inventory, for which they do not have enough knowledge of the product’s benefits. This can be a very frustrating challenge.”
Conclusion
Although there are many obstacles to retailing skin care in your medical practice, the benefits that it provides to both your patients (improved outcomes) and your practice (increased profitability) far outweigh the challenges. I solved these pitfalls in my own practice by developing a standardized staff training program and skin care diagnostic software that is now used by over 100 medical practices. If you want to start retaining skin care, my advice is develop a training plan and a methodology for the recommendation and patient education process before you spend a lot of money on the required minimum product order. Feel free to contact me for advice. Alternatively, if you already do a great job of retailing skin care and want to provide tips to include in my American Society for Dermatologic Surgery course, contact me on LinkedIn or [email protected]. You can also find blogs I have written on skin care retail advice at STSFranchise.com.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also wrote a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems.
Others believe that providing patients with the correct skin care product recommendations for their skin’s needs is a crucial step to improving outcomes and educating patients.
There is a wide range of challenges related to skin care retail that many physicians face. I will be running a course on Skin Care Retail at the American Society for Dermatologic Surgery meeting in October in Scottsdale, Ariz., if you want to learn more or share your opinions. I have surveyed plastic surgeons and dermatologists via LinkedIn about what they believe are some of the biggest pitfalls to retailing skin care. Here, I will share some of their insights and suggestions for overcoming these obstacles.
1. Patients are more knowledgeable about skin care than ever before
Facing an increasing number of over-the-counter skin care products available, as well as buzzwords like “organic ingredients” and “vegan,” patients are now bombarded with information from a variety of different sources. Because of this, patients come to the doctor with preconceived ideas that can affect compliance if their specific needs and beliefs are not properly addressed.
For New York plastic surgeon Sonita M. Sadio, MD, this is one of the reasons why she chooses not to sell skin care in her office.
“My practice is highly consultative, and ongoing skin care recommendations are a significant part of what I do to optimize patient outcomes,” Dr. Sadio said. “Patients are well-educated about skin care today. They know their ingredients and insist on clean formulations, free of certain ingredients, such as ‘cruelty-free’ and ‘vegan.’ Others feel deprived if they are not using an expensive product in elegant packaging. Still, others insist on drugstore favorites or ‘eco’ offerings and have their own sense of what that means. My job is to optimize the clinical outcome while also meeting these patients needs to ensure compliance.”
Not all doctors have the time, knowledge or desire to personally design each patient’s skin care regimen. Many delegate this to the staff. However, it is impossible to ensure that your staff matches patients to the proper products unless they have had extensive training on both skin care products and how to match them to the patient’s skin issues.
2. Patients are wary when the doctors sells only one product brand
Many studies have shown that, although consumers desire a choice when making purchases, they get overwhelmed if they are presented with too many options. One study showed that it is optimal to carry at least 3 brands of products. For this reason, limiting the skin care you sell to one brand or doing your own private label is not optimal.
New York dermatologist Rebecca Tamez, MD, pointed out the same problem when selling practice-specific skin care. “At my previous job, we sold skin care products directly to patients. I had no issues selling products that were readily available in drugstores or online (such as Vanicream and EltaMD). We usually sold these around the same cost as the drugstore or Amazon. However, it was harder to sell the practice-specific skin care line. I feel patients were more wary of these products.”
3. Doctors do not want to feel like salespeople
If you have read my Dermatology News columns in the past, you may know that I think it is unethical for dermatologists to not offer specific skin care advice to their patients. If patients do not get ethical and scientific recommendations from us, they will follow the advice of a friend or salesperson or purchase based on often inflated marketing claims.
Dermatologists often tell me: “I am not a cosmetic dermatologist so I do not sell skin care.” I feel strongly that general dermatologists should be giving specific written skin care recommendations for their patients too. Acne, rosacea, melasma, eczema, psoriasis, keratosis pilaris, and many other conditions will improve faster with an efficacious skin care regimen, assuming the patient is compliant with the instructions. Retailing skin care improves compliance by eliminating a few barriers to beginning the skin care regimen. I believe that the mindset of dermatologists needs to change: It is not about selling products to patients, it is about educating them on what to use and offering the products out of convenience and the desire to improve compliance.
Meadowbrook, Pa., dermatologist Michael A. Tomeo, MD, explained an obstacle faced by many dermatologists:
“I suspect, like many of my colleagues,” said Dr. Tomeo, “that I am held back in terms of salesmanship, having been trained in the traditional way. Physicians of my generation were taught to be ethical and professional and to focus on academic and clinical excellence, and salesmanship and advertising one’s services were frowned upon. It takes time to reset one’s former proclivities. Cosmeceuticals and nutraceuticals are revolutionizing the skin care world, and as experts in all things skin, we need to be well informed and offer our patients safe, effective, and cutting-edge treatments.”
4. Providers are concerned about product costs and time constraints
Providing excellent patient care and improving outcomes is at the forefront of our business, but financial concerns and time constraints prevent some doctors from offering skin care to their patients.
Rochester Hills, Mich., plastic surgeon Richard Hainer, MD, has found that “skin care is often too complex with too many products and is not very profitable.” For those reasons, Dr. Hainer has chosen not to retail skin care in his practice.
Nampa, Idaho, dermatologist Ryan S. Owsley, MD, explained that “the required minimum purchases by some of the product lines can leave the practice with expired product if it is not selling a particular line well. Cost can also be an issue for some patients in the area we are located.”
As a burn survivor and burn surgeon, Mark McDonough, MD, from Orlando “has a long history with skin care and rejuvenation. I did have a private label skin care line, including a moisturizer, a hydroquinone product, a retinol cream, and a sunscreen,” Dr. McDonough said. “However, and regrettably, I have not kept up with marketing and promotion, with most of my energy invested in trauma and disease survivors through a book, a blog, and my platform through my website.”
Doing your own product line is costly and spending the time and resources to promote it is not always possible. Buying the minimum order of products is often expensive, and you will not be able to sell them without a proven methodology in place. New products enter the market frequently, and it is expensive to always carry the latest technologies because new minimum orders must be met with each new brand that you add.
5. Selling skin care requires ongoing education
Properly recommending and retailing skin care involves physician, staff, and patient education. Unfortunately, most practices rely on training from the cosmeceutical sales reps who obviously have a brand bias. There is minimal unbiased “brand agnostic” skin care training for dermatologists and their staff. In fact, the AAD meeting has only a few skin care lectures in the program. Plastic surgeon Gaurav Bharti, MD, of Charlotte, N.C., explained that “motivating staff to help with retail skin care can be challenging. The first step is to get the staff familiar with the products with open discussions with the representatives. The next step has been to have the staff actually use the products and believe in them. Once they believe in the product, we have used an incentivization model that’s simple, transparent, and predictable.”
We are all too busy to spend adequate time with our patients, so it is critical that our staff be able to properly recommended skin care for us. We have to ensure that our staff is taking an ethical and scientific approach to skin care retail rather than a financial one. Rigorous staff training on how to match skin care products to skin type is the key to improving outcomes with skin care recommendations.
In a similar sense, Cincinnati plastic surgeon Richard Williams, MD, commented that “aestheticians often succumb to the desires of our patients to carry too many products in inventory, for which they do not have enough knowledge of the product’s benefits. This can be a very frustrating challenge.”
Conclusion
Although there are many obstacles to retailing skin care in your medical practice, the benefits that it provides to both your patients (improved outcomes) and your practice (increased profitability) far outweigh the challenges. I solved these pitfalls in my own practice by developing a standardized staff training program and skin care diagnostic software that is now used by over 100 medical practices. If you want to start retaining skin care, my advice is develop a training plan and a methodology for the recommendation and patient education process before you spend a lot of money on the required minimum product order. Feel free to contact me for advice. Alternatively, if you already do a great job of retailing skin care and want to provide tips to include in my American Society for Dermatologic Surgery course, contact me on LinkedIn or [email protected]. You can also find blogs I have written on skin care retail advice at STSFranchise.com.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also wrote a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems.
Fish pedicures
A letter published in JAMA Dermatology describes an otherwise healthy woman in her 20s who experienced nail abnormalities some months after having a fish pedicure. Onychomadesis, or transverse splitting of the nail plate, occurs when the nail matrix has arrested in producing the nail plate. It can be thought of as more severe form of Beau’s lines, in which the nail itself actually breaks and separates from the proximal nail plate and eventually sheds.
Fish pedicures have a long-standing history in Mediterranean and Middle Eastern cultures for aiding such skin conditions as psoriasis and helping to remove scaly skin. The Garra rufa fish are nonmigratory freshwater fish native to the Persian Gulf and Eastern Mediterranean. Suction allows them to attach to rocks and eat plankton. These “doctor fish,” as they are nicknamed, when placed in a warm bath of 25°C to 30°C, will also eat human skin when starved of their natural food source. As the JAMA Dermatology letter mentions, this was demonstrated in a study in Kangal, Turkey, where Garra rufa fish were used to improve psoriasis by feeding on psoriasis plaques but not normal skin. After 3 weeks of therapy with Garra rufa in 67 patients, there was a 72% reduction in the Psoriasis Area and Severity Index (PASI) score from baseline (Evid Based Complement Alternat Med. 2006 Dec;3[4]:483-8).
Popular in the United States and Europe about a decade ago, fish pedicures have now been banned in 10 U.S. states and in some parts of Europe. While the trend in the United States has waned, fish pedicures have recently become more popular in vacation destinations, such as the Caribbean. The inherent concern of fish pedicures is risk of infection as the same fish are used successively and cannot be adequately sanitized between people.
Two cases of staphylococcus infections and one of Mycobacterium marinum have been reported after fish pedicures. Whether these infections were caused by the fish or the water source, however, remains to be determined. If the fish were transmitting infections, it seems that more infections would likely have been reported, considering the widespread popularity in the past. I, like Antonella Tosti, MD, who commented in a CNN report on the JAMA Dermatology case, also doubt that the fish pedicure alone caused onychomadesis in this woman. In order for onychomadesis to occur, there would have had to have been significant trauma to all 10 nails at the matrix. Would the fish been able to have caused the same amount of trauma to all 10 nails in one setting? While it is possible, I believe a more likely explanation would be an alternate endogenous or exogenous source.
Traditional medicine has been used to enhance beauty and cure ailments for thousands of years before the advent of modern medicine as demonstrated by the Kangal study. Before discounting fish pedicures completely, perhaps some thought should also be given to how this practice affects wildlife and the fish. The CNN report refers to a 2011 investigation by the U.K.’s Fish Health Inspectorate, which “found a bacterial outbreak among thousands of these fish, which had been transported from Indonesia to the United Kingdom pedicure spas. Fish were found with bulging eyes, many hemorrhaging around the gills and mouth. The culprit was found to be a streptococcal bacteria, a strain that is associated with fish like tilapia, according to David Verner-Jeffreys, a senior microbiologist at the Centre for Environment, Fisheries and Aquaculture Science in the U.K.”
Whether or not these fish would pose any risk to humans is unknown, but certainly, this practice adversely affects the welfare of the fish and their environment. The overharvesting of these fish has led the Turkish government to introduce legal protections for the country’s Garra rufa in an attempt to combat overfishing and exploitation.
Perhaps fish pedicures solely for aesthetic reasons should not be practiced because of the potential infection risk – as well as the harm (to both humans and fish) and overharvesting of the fish. If used properly, these fish, however, could be an aid in treating certain skin pathologies.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
A letter published in JAMA Dermatology describes an otherwise healthy woman in her 20s who experienced nail abnormalities some months after having a fish pedicure. Onychomadesis, or transverse splitting of the nail plate, occurs when the nail matrix has arrested in producing the nail plate. It can be thought of as more severe form of Beau’s lines, in which the nail itself actually breaks and separates from the proximal nail plate and eventually sheds.
Fish pedicures have a long-standing history in Mediterranean and Middle Eastern cultures for aiding such skin conditions as psoriasis and helping to remove scaly skin. The Garra rufa fish are nonmigratory freshwater fish native to the Persian Gulf and Eastern Mediterranean. Suction allows them to attach to rocks and eat plankton. These “doctor fish,” as they are nicknamed, when placed in a warm bath of 25°C to 30°C, will also eat human skin when starved of their natural food source. As the JAMA Dermatology letter mentions, this was demonstrated in a study in Kangal, Turkey, where Garra rufa fish were used to improve psoriasis by feeding on psoriasis plaques but not normal skin. After 3 weeks of therapy with Garra rufa in 67 patients, there was a 72% reduction in the Psoriasis Area and Severity Index (PASI) score from baseline (Evid Based Complement Alternat Med. 2006 Dec;3[4]:483-8).
Popular in the United States and Europe about a decade ago, fish pedicures have now been banned in 10 U.S. states and in some parts of Europe. While the trend in the United States has waned, fish pedicures have recently become more popular in vacation destinations, such as the Caribbean. The inherent concern of fish pedicures is risk of infection as the same fish are used successively and cannot be adequately sanitized between people.
Two cases of staphylococcus infections and one of Mycobacterium marinum have been reported after fish pedicures. Whether these infections were caused by the fish or the water source, however, remains to be determined. If the fish were transmitting infections, it seems that more infections would likely have been reported, considering the widespread popularity in the past. I, like Antonella Tosti, MD, who commented in a CNN report on the JAMA Dermatology case, also doubt that the fish pedicure alone caused onychomadesis in this woman. In order for onychomadesis to occur, there would have had to have been significant trauma to all 10 nails at the matrix. Would the fish been able to have caused the same amount of trauma to all 10 nails in one setting? While it is possible, I believe a more likely explanation would be an alternate endogenous or exogenous source.
Traditional medicine has been used to enhance beauty and cure ailments for thousands of years before the advent of modern medicine as demonstrated by the Kangal study. Before discounting fish pedicures completely, perhaps some thought should also be given to how this practice affects wildlife and the fish. The CNN report refers to a 2011 investigation by the U.K.’s Fish Health Inspectorate, which “found a bacterial outbreak among thousands of these fish, which had been transported from Indonesia to the United Kingdom pedicure spas. Fish were found with bulging eyes, many hemorrhaging around the gills and mouth. The culprit was found to be a streptococcal bacteria, a strain that is associated with fish like tilapia, according to David Verner-Jeffreys, a senior microbiologist at the Centre for Environment, Fisheries and Aquaculture Science in the U.K.”
Whether or not these fish would pose any risk to humans is unknown, but certainly, this practice adversely affects the welfare of the fish and their environment. The overharvesting of these fish has led the Turkish government to introduce legal protections for the country’s Garra rufa in an attempt to combat overfishing and exploitation.
Perhaps fish pedicures solely for aesthetic reasons should not be practiced because of the potential infection risk – as well as the harm (to both humans and fish) and overharvesting of the fish. If used properly, these fish, however, could be an aid in treating certain skin pathologies.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
A letter published in JAMA Dermatology describes an otherwise healthy woman in her 20s who experienced nail abnormalities some months after having a fish pedicure. Onychomadesis, or transverse splitting of the nail plate, occurs when the nail matrix has arrested in producing the nail plate. It can be thought of as more severe form of Beau’s lines, in which the nail itself actually breaks and separates from the proximal nail plate and eventually sheds.
Fish pedicures have a long-standing history in Mediterranean and Middle Eastern cultures for aiding such skin conditions as psoriasis and helping to remove scaly skin. The Garra rufa fish are nonmigratory freshwater fish native to the Persian Gulf and Eastern Mediterranean. Suction allows them to attach to rocks and eat plankton. These “doctor fish,” as they are nicknamed, when placed in a warm bath of 25°C to 30°C, will also eat human skin when starved of their natural food source. As the JAMA Dermatology letter mentions, this was demonstrated in a study in Kangal, Turkey, where Garra rufa fish were used to improve psoriasis by feeding on psoriasis plaques but not normal skin. After 3 weeks of therapy with Garra rufa in 67 patients, there was a 72% reduction in the Psoriasis Area and Severity Index (PASI) score from baseline (Evid Based Complement Alternat Med. 2006 Dec;3[4]:483-8).
Popular in the United States and Europe about a decade ago, fish pedicures have now been banned in 10 U.S. states and in some parts of Europe. While the trend in the United States has waned, fish pedicures have recently become more popular in vacation destinations, such as the Caribbean. The inherent concern of fish pedicures is risk of infection as the same fish are used successively and cannot be adequately sanitized between people.
Two cases of staphylococcus infections and one of Mycobacterium marinum have been reported after fish pedicures. Whether these infections were caused by the fish or the water source, however, remains to be determined. If the fish were transmitting infections, it seems that more infections would likely have been reported, considering the widespread popularity in the past. I, like Antonella Tosti, MD, who commented in a CNN report on the JAMA Dermatology case, also doubt that the fish pedicure alone caused onychomadesis in this woman. In order for onychomadesis to occur, there would have had to have been significant trauma to all 10 nails at the matrix. Would the fish been able to have caused the same amount of trauma to all 10 nails in one setting? While it is possible, I believe a more likely explanation would be an alternate endogenous or exogenous source.
Traditional medicine has been used to enhance beauty and cure ailments for thousands of years before the advent of modern medicine as demonstrated by the Kangal study. Before discounting fish pedicures completely, perhaps some thought should also be given to how this practice affects wildlife and the fish. The CNN report refers to a 2011 investigation by the U.K.’s Fish Health Inspectorate, which “found a bacterial outbreak among thousands of these fish, which had been transported from Indonesia to the United Kingdom pedicure spas. Fish were found with bulging eyes, many hemorrhaging around the gills and mouth. The culprit was found to be a streptococcal bacteria, a strain that is associated with fish like tilapia, according to David Verner-Jeffreys, a senior microbiologist at the Centre for Environment, Fisheries and Aquaculture Science in the U.K.”
Whether or not these fish would pose any risk to humans is unknown, but certainly, this practice adversely affects the welfare of the fish and their environment. The overharvesting of these fish has led the Turkish government to introduce legal protections for the country’s Garra rufa in an attempt to combat overfishing and exploitation.
Perhaps fish pedicures solely for aesthetic reasons should not be practiced because of the potential infection risk – as well as the harm (to both humans and fish) and overharvesting of the fish. If used properly, these fish, however, could be an aid in treating certain skin pathologies.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Facial and Orbital Asymmetry in Oculofacial Surgery Patients
Facial symmetry plays a role in attractiveness, but a small degree of asymmetry is normal and more common than symmetry. Mild asymmetry has been noted in the general population, even in the absence of pathology such as trauma or craniosynostosis.1,2 Asymmetry may be static or dynamic and is thought to arise from a multitude of developmental factors, including skeletal, neurologic, and soft tissue changes, as well as photoaging.3-5 Cosmetic and reconstructive surgical procedures strive to achieve facial symmetry. Patients often are unaware of their preexisting facial asymmetry.6 Anecdotally, we have found patients tend to be more cognizant of preexisting facial asymmetry following a notable change in facial appearance (eg, surgery). In counseling patients who are considering reconstructive or cosmetic surgery, it is beneficial to identify any preexisting facial asymmetries and discuss if they are within normal limits. The current literature, however, lacks thresholds for what is considered normal in many cases. In this study, we reviewed 100 faces without unilateral or orbital pathology or diplopia to describe the occurrence of facial asymmetries, including larger hemiface and hemiface with greater excursion of motion upon smiling (interpreted to signify stronger seventh cranial nerve), hemiface with more rhytides at rest, higher globe, higher earlobe, and higher lip.
Methods
One hundred oculofacial surgery patients without unilateral or orbital pathology or diplopia were included in this retrospective evaluation of static and dynamic facial asymmetry via facial photography (100 participants). Three graders were provided standard frontal and frontal smiling photographs with overlying facial grids to aid in assessing larger hemiface and hemiface with stronger seventh cranial nerve, which was judged in smiling photographs by assessing the excursion and the vector of motion; more rhytides at rest; higher globe; higher earlobe; and higher lip. Difference in globe height was measured relative to interpupillary distance (IPD) and recorded as the ratio of difference in globe height to IPD. The data were analyzed to see if there were any correlations among the 6 variables. This study was approved by the Duke University Health System (Durham, North Carolina) institutional review board.
Results
One hundred photographs were analyzed including 82 women aged 42 to 85 years and 18 men aged 22 to 88 years (overall average age, 61.64 years). The average difference in globe height was 1.2% of IPD; the maximum was 4.4% of IPD. The difference in globe height was verified by 3 graders via 2 different methods. Fifty-four patients were found to have a larger right hemiface, 36 had a larger left hemiface, and 10 had symmetrically sized hemifaces. Nearly half of patients were judged to have greater seventh cranial nerve action on the left (n=47), approximately one-quarter had greater action on the right (n=28), and another quarter were judged to have equal action (n=25). Most patients had static facial asymmetry; 72 had rhytides more pronounced on one hemiface compared to the other, 79 with a difference in globe height, and 68 with a difference in lip height. In approximately 40% of photographs, the graders were unable to judge earlobe height difference; therefore, this data was not analyzed. There was no correlation among the 6 variables.
Discussion
Facial asymmetry has long been a topic of interest in the plastic and reconstructive surgery fields. Ercan et al7 used statistical shape analysis to study facial asymmetry in young healthy subjects and found the left hemiface to be larger than the right hemiface in both sexes. Smith4 evaluated facial asymmetry in healthy college students and found the left hemiface to be larger in males and the right hemiface to be larger in females. Our group was predominantly female, but we found the right hemiface to be larger in both females and males, similar to the findings of Lepich et al.8
We also found that most patients had static and dynamic facial asymmetry despite no known unilateral pathology. The present literature lacks normative values to help determine what degree of asymmetry should be considered pathologic. Vertical orbital dystopia is defined as an inequality in the horizontal levels of the whole orbits.9 It has been hypothesized that most vertical dystopia is caused by congenital malformations, but no threshold has been set for the difference in height that qualifies as dystopia.10 Regarding the difference we found in globe height relative to IPD, if one takes the mean IPD of 63.36 mm (based on a study of 3976 American adults aged 17–51 years)11 and makes the assumption that our patients have this IPD, then one can extrapolate that on average there was a difference of 0.76 mm between the 2 globe heights. Likewise, nearly all patients (n=96) had less than a 2-mm difference (21 had symmetric globe heights, 46 had a difference in globe height of <1 mm, and 29 had a difference of >1 mm and <2 mm). Four patients had a difference greater than 2 mm, with the largest difference being 2.75 mm. A limitation of this retrospective study is the need to extrapolate these distances, as our patients were not photographed with rulers.
Hafezi et al12 looked at the facial asymmetry in patients without history of trauma or nasal fracture who were seeking rhinoplasty. They noted vertical orbital dystopia in this patient population, but the degree of dystopia was not quantified.12 We believe our data highlight the importance of counseling patients about preexisting facial asymmetry with normative values in mind. Patients may be dissatisfied by new or preexisting asymmetry following surgery, even if such asymmetries are less objectively apparent than in the patient’s preoperative appearance. Even when patients are already acutely aware of their facial asymmetries, they should learn that facial asymmetries, to varying degrees, are natural and not necessarily unattractive. In fact, a 2006
- Wang TT, Wessels L, Hussain G, et al. Discriminative thresholds in facial asymmetry: a review of the literature. Aesthet Surg J. 2017;37:375-385.
- Zaidel DW, Cohen JA. The face, beauty, and symmetry: perceiving asymmetry in beautiful faces. Int J Neurosci. 2005;115:1165-1173.
- Rossi M, Ribeiro E, Smith R. Craniofacial asymmetry in development: an anatomical study. Angle Orthod. 2003;73:381-385.
- Smith WM. Hemispheric and facial asymmetry: gender differences. Laterality. 2000;5:251-258.
- Gordon JR, Brieva JC. Images in clinical medicine. unilateral dermatoheliosis. N Engl J Med. 2012;366:e25.
- Macdonald KI, Mendez AI, Hart RD, et al. Eyelid and brow asymmetry in patients evaluated for upper lid blepharoplasty. J Otolaryngol Head Neck Surg. 2014;43:36.
- Ercan I, Ozdemir ST, Etoz A, et al. Facial asymmetry in young healthy subjects evaluated by statistical shape analysis. J Anat. 2008;213:663-669.
- Lepich T, Dabek J, Witkowska M, et al. Female and male orbit asymmetry: digital analysis. Adv Clin Exp Med. 2017;26:69-76.
- Tan ST, Ashworth G, Czypionka S, et al. Vertical orbital dystopia. Plast Reconstr Surg. 1996;97:1349-1361.
- De Ponte FS, Fadda T, Rinna C, et al. Early and late surgical treatment of orbital dystopia in craniofacial malformation. J Craniofac Surg. 1997;8:17-22.
- Dodgson NA. Variation and extrema of human interpupillary distance. Proc Int Soc Opt Eng. 2004;5291:36-46.
- Hafezi F, Naghibzadeh B, Nouhi A, et al. Asymmetric facial growth and deviated nose: a new concept. Ann Plast Surg. 2010;64:47-51.
- Ing E, Safarpour A, Ing T, et al. Ocular adnexal asymmetry in models: a magazine photograph analysis. Can J Ophthalmol. 2006;41:175-182.
Facial symmetry plays a role in attractiveness, but a small degree of asymmetry is normal and more common than symmetry. Mild asymmetry has been noted in the general population, even in the absence of pathology such as trauma or craniosynostosis.1,2 Asymmetry may be static or dynamic and is thought to arise from a multitude of developmental factors, including skeletal, neurologic, and soft tissue changes, as well as photoaging.3-5 Cosmetic and reconstructive surgical procedures strive to achieve facial symmetry. Patients often are unaware of their preexisting facial asymmetry.6 Anecdotally, we have found patients tend to be more cognizant of preexisting facial asymmetry following a notable change in facial appearance (eg, surgery). In counseling patients who are considering reconstructive or cosmetic surgery, it is beneficial to identify any preexisting facial asymmetries and discuss if they are within normal limits. The current literature, however, lacks thresholds for what is considered normal in many cases. In this study, we reviewed 100 faces without unilateral or orbital pathology or diplopia to describe the occurrence of facial asymmetries, including larger hemiface and hemiface with greater excursion of motion upon smiling (interpreted to signify stronger seventh cranial nerve), hemiface with more rhytides at rest, higher globe, higher earlobe, and higher lip.
Methods
One hundred oculofacial surgery patients without unilateral or orbital pathology or diplopia were included in this retrospective evaluation of static and dynamic facial asymmetry via facial photography (100 participants). Three graders were provided standard frontal and frontal smiling photographs with overlying facial grids to aid in assessing larger hemiface and hemiface with stronger seventh cranial nerve, which was judged in smiling photographs by assessing the excursion and the vector of motion; more rhytides at rest; higher globe; higher earlobe; and higher lip. Difference in globe height was measured relative to interpupillary distance (IPD) and recorded as the ratio of difference in globe height to IPD. The data were analyzed to see if there were any correlations among the 6 variables. This study was approved by the Duke University Health System (Durham, North Carolina) institutional review board.
Results
One hundred photographs were analyzed including 82 women aged 42 to 85 years and 18 men aged 22 to 88 years (overall average age, 61.64 years). The average difference in globe height was 1.2% of IPD; the maximum was 4.4% of IPD. The difference in globe height was verified by 3 graders via 2 different methods. Fifty-four patients were found to have a larger right hemiface, 36 had a larger left hemiface, and 10 had symmetrically sized hemifaces. Nearly half of patients were judged to have greater seventh cranial nerve action on the left (n=47), approximately one-quarter had greater action on the right (n=28), and another quarter were judged to have equal action (n=25). Most patients had static facial asymmetry; 72 had rhytides more pronounced on one hemiface compared to the other, 79 with a difference in globe height, and 68 with a difference in lip height. In approximately 40% of photographs, the graders were unable to judge earlobe height difference; therefore, this data was not analyzed. There was no correlation among the 6 variables.
Discussion
Facial asymmetry has long been a topic of interest in the plastic and reconstructive surgery fields. Ercan et al7 used statistical shape analysis to study facial asymmetry in young healthy subjects and found the left hemiface to be larger than the right hemiface in both sexes. Smith4 evaluated facial asymmetry in healthy college students and found the left hemiface to be larger in males and the right hemiface to be larger in females. Our group was predominantly female, but we found the right hemiface to be larger in both females and males, similar to the findings of Lepich et al.8
We also found that most patients had static and dynamic facial asymmetry despite no known unilateral pathology. The present literature lacks normative values to help determine what degree of asymmetry should be considered pathologic. Vertical orbital dystopia is defined as an inequality in the horizontal levels of the whole orbits.9 It has been hypothesized that most vertical dystopia is caused by congenital malformations, but no threshold has been set for the difference in height that qualifies as dystopia.10 Regarding the difference we found in globe height relative to IPD, if one takes the mean IPD of 63.36 mm (based on a study of 3976 American adults aged 17–51 years)11 and makes the assumption that our patients have this IPD, then one can extrapolate that on average there was a difference of 0.76 mm between the 2 globe heights. Likewise, nearly all patients (n=96) had less than a 2-mm difference (21 had symmetric globe heights, 46 had a difference in globe height of <1 mm, and 29 had a difference of >1 mm and <2 mm). Four patients had a difference greater than 2 mm, with the largest difference being 2.75 mm. A limitation of this retrospective study is the need to extrapolate these distances, as our patients were not photographed with rulers.
Hafezi et al12 looked at the facial asymmetry in patients without history of trauma or nasal fracture who were seeking rhinoplasty. They noted vertical orbital dystopia in this patient population, but the degree of dystopia was not quantified.12 We believe our data highlight the importance of counseling patients about preexisting facial asymmetry with normative values in mind. Patients may be dissatisfied by new or preexisting asymmetry following surgery, even if such asymmetries are less objectively apparent than in the patient’s preoperative appearance. Even when patients are already acutely aware of their facial asymmetries, they should learn that facial asymmetries, to varying degrees, are natural and not necessarily unattractive. In fact, a 2006
Facial symmetry plays a role in attractiveness, but a small degree of asymmetry is normal and more common than symmetry. Mild asymmetry has been noted in the general population, even in the absence of pathology such as trauma or craniosynostosis.1,2 Asymmetry may be static or dynamic and is thought to arise from a multitude of developmental factors, including skeletal, neurologic, and soft tissue changes, as well as photoaging.3-5 Cosmetic and reconstructive surgical procedures strive to achieve facial symmetry. Patients often are unaware of their preexisting facial asymmetry.6 Anecdotally, we have found patients tend to be more cognizant of preexisting facial asymmetry following a notable change in facial appearance (eg, surgery). In counseling patients who are considering reconstructive or cosmetic surgery, it is beneficial to identify any preexisting facial asymmetries and discuss if they are within normal limits. The current literature, however, lacks thresholds for what is considered normal in many cases. In this study, we reviewed 100 faces without unilateral or orbital pathology or diplopia to describe the occurrence of facial asymmetries, including larger hemiface and hemiface with greater excursion of motion upon smiling (interpreted to signify stronger seventh cranial nerve), hemiface with more rhytides at rest, higher globe, higher earlobe, and higher lip.
Methods
One hundred oculofacial surgery patients without unilateral or orbital pathology or diplopia were included in this retrospective evaluation of static and dynamic facial asymmetry via facial photography (100 participants). Three graders were provided standard frontal and frontal smiling photographs with overlying facial grids to aid in assessing larger hemiface and hemiface with stronger seventh cranial nerve, which was judged in smiling photographs by assessing the excursion and the vector of motion; more rhytides at rest; higher globe; higher earlobe; and higher lip. Difference in globe height was measured relative to interpupillary distance (IPD) and recorded as the ratio of difference in globe height to IPD. The data were analyzed to see if there were any correlations among the 6 variables. This study was approved by the Duke University Health System (Durham, North Carolina) institutional review board.
Results
One hundred photographs were analyzed including 82 women aged 42 to 85 years and 18 men aged 22 to 88 years (overall average age, 61.64 years). The average difference in globe height was 1.2% of IPD; the maximum was 4.4% of IPD. The difference in globe height was verified by 3 graders via 2 different methods. Fifty-four patients were found to have a larger right hemiface, 36 had a larger left hemiface, and 10 had symmetrically sized hemifaces. Nearly half of patients were judged to have greater seventh cranial nerve action on the left (n=47), approximately one-quarter had greater action on the right (n=28), and another quarter were judged to have equal action (n=25). Most patients had static facial asymmetry; 72 had rhytides more pronounced on one hemiface compared to the other, 79 with a difference in globe height, and 68 with a difference in lip height. In approximately 40% of photographs, the graders were unable to judge earlobe height difference; therefore, this data was not analyzed. There was no correlation among the 6 variables.
Discussion
Facial asymmetry has long been a topic of interest in the plastic and reconstructive surgery fields. Ercan et al7 used statistical shape analysis to study facial asymmetry in young healthy subjects and found the left hemiface to be larger than the right hemiface in both sexes. Smith4 evaluated facial asymmetry in healthy college students and found the left hemiface to be larger in males and the right hemiface to be larger in females. Our group was predominantly female, but we found the right hemiface to be larger in both females and males, similar to the findings of Lepich et al.8
We also found that most patients had static and dynamic facial asymmetry despite no known unilateral pathology. The present literature lacks normative values to help determine what degree of asymmetry should be considered pathologic. Vertical orbital dystopia is defined as an inequality in the horizontal levels of the whole orbits.9 It has been hypothesized that most vertical dystopia is caused by congenital malformations, but no threshold has been set for the difference in height that qualifies as dystopia.10 Regarding the difference we found in globe height relative to IPD, if one takes the mean IPD of 63.36 mm (based on a study of 3976 American adults aged 17–51 years)11 and makes the assumption that our patients have this IPD, then one can extrapolate that on average there was a difference of 0.76 mm between the 2 globe heights. Likewise, nearly all patients (n=96) had less than a 2-mm difference (21 had symmetric globe heights, 46 had a difference in globe height of <1 mm, and 29 had a difference of >1 mm and <2 mm). Four patients had a difference greater than 2 mm, with the largest difference being 2.75 mm. A limitation of this retrospective study is the need to extrapolate these distances, as our patients were not photographed with rulers.
Hafezi et al12 looked at the facial asymmetry in patients without history of trauma or nasal fracture who were seeking rhinoplasty. They noted vertical orbital dystopia in this patient population, but the degree of dystopia was not quantified.12 We believe our data highlight the importance of counseling patients about preexisting facial asymmetry with normative values in mind. Patients may be dissatisfied by new or preexisting asymmetry following surgery, even if such asymmetries are less objectively apparent than in the patient’s preoperative appearance. Even when patients are already acutely aware of their facial asymmetries, they should learn that facial asymmetries, to varying degrees, are natural and not necessarily unattractive. In fact, a 2006
- Wang TT, Wessels L, Hussain G, et al. Discriminative thresholds in facial asymmetry: a review of the literature. Aesthet Surg J. 2017;37:375-385.
- Zaidel DW, Cohen JA. The face, beauty, and symmetry: perceiving asymmetry in beautiful faces. Int J Neurosci. 2005;115:1165-1173.
- Rossi M, Ribeiro E, Smith R. Craniofacial asymmetry in development: an anatomical study. Angle Orthod. 2003;73:381-385.
- Smith WM. Hemispheric and facial asymmetry: gender differences. Laterality. 2000;5:251-258.
- Gordon JR, Brieva JC. Images in clinical medicine. unilateral dermatoheliosis. N Engl J Med. 2012;366:e25.
- Macdonald KI, Mendez AI, Hart RD, et al. Eyelid and brow asymmetry in patients evaluated for upper lid blepharoplasty. J Otolaryngol Head Neck Surg. 2014;43:36.
- Ercan I, Ozdemir ST, Etoz A, et al. Facial asymmetry in young healthy subjects evaluated by statistical shape analysis. J Anat. 2008;213:663-669.
- Lepich T, Dabek J, Witkowska M, et al. Female and male orbit asymmetry: digital analysis. Adv Clin Exp Med. 2017;26:69-76.
- Tan ST, Ashworth G, Czypionka S, et al. Vertical orbital dystopia. Plast Reconstr Surg. 1996;97:1349-1361.
- De Ponte FS, Fadda T, Rinna C, et al. Early and late surgical treatment of orbital dystopia in craniofacial malformation. J Craniofac Surg. 1997;8:17-22.
- Dodgson NA. Variation and extrema of human interpupillary distance. Proc Int Soc Opt Eng. 2004;5291:36-46.
- Hafezi F, Naghibzadeh B, Nouhi A, et al. Asymmetric facial growth and deviated nose: a new concept. Ann Plast Surg. 2010;64:47-51.
- Ing E, Safarpour A, Ing T, et al. Ocular adnexal asymmetry in models: a magazine photograph analysis. Can J Ophthalmol. 2006;41:175-182.
- Wang TT, Wessels L, Hussain G, et al. Discriminative thresholds in facial asymmetry: a review of the literature. Aesthet Surg J. 2017;37:375-385.
- Zaidel DW, Cohen JA. The face, beauty, and symmetry: perceiving asymmetry in beautiful faces. Int J Neurosci. 2005;115:1165-1173.
- Rossi M, Ribeiro E, Smith R. Craniofacial asymmetry in development: an anatomical study. Angle Orthod. 2003;73:381-385.
- Smith WM. Hemispheric and facial asymmetry: gender differences. Laterality. 2000;5:251-258.
- Gordon JR, Brieva JC. Images in clinical medicine. unilateral dermatoheliosis. N Engl J Med. 2012;366:e25.
- Macdonald KI, Mendez AI, Hart RD, et al. Eyelid and brow asymmetry in patients evaluated for upper lid blepharoplasty. J Otolaryngol Head Neck Surg. 2014;43:36.
- Ercan I, Ozdemir ST, Etoz A, et al. Facial asymmetry in young healthy subjects evaluated by statistical shape analysis. J Anat. 2008;213:663-669.
- Lepich T, Dabek J, Witkowska M, et al. Female and male orbit asymmetry: digital analysis. Adv Clin Exp Med. 2017;26:69-76.
- Tan ST, Ashworth G, Czypionka S, et al. Vertical orbital dystopia. Plast Reconstr Surg. 1996;97:1349-1361.
- De Ponte FS, Fadda T, Rinna C, et al. Early and late surgical treatment of orbital dystopia in craniofacial malformation. J Craniofac Surg. 1997;8:17-22.
- Dodgson NA. Variation and extrema of human interpupillary distance. Proc Int Soc Opt Eng. 2004;5291:36-46.
- Hafezi F, Naghibzadeh B, Nouhi A, et al. Asymmetric facial growth and deviated nose: a new concept. Ann Plast Surg. 2010;64:47-51.
- Ing E, Safarpour A, Ing T, et al. Ocular adnexal asymmetry in models: a magazine photograph analysis. Can J Ophthalmol. 2006;41:175-182.
Resident Pearl
- A small degree of asymmetry is normal and more common than perfect symmetry.
Update on Acne Scar Treatment
Acne vulgaris is prevalent in the general population, with 40 to 50 million affected individuals in the United States.1 Severe inflammation and injury can lead to disfiguring scarring, which has a considerable impact on quality of life.2 Numerous therapeutic options for acne scarring are available, including microneedling with and without platelet-rich plasma (PRP), lasers, chemical peels, and dermal fillers, with different modalities suited to individual patients and scar characteristics. This article reviews updates in treatment options for acne scarring.
Microneedling
Microneedling, also known as percutaneous collagen induction or collagen induction therapy, has been utilized for more than 2 decades.3 Dermatologic indications for microneedling include skin rejuvenation,4-6 atrophic acne scarring,7-9 and androgenic alopecia.10,11 Microneedling also has been used to enhance skin penetration of topically applied drugs.12-15 Fernandes16 described percutaneous collagen induction as the skin’s natural response to injury. Microneedling creates small wounds as fine needles puncture the epidermis and dermis, resulting in a cascade of growth factors that lead to tissue proliferation, regeneration, and a collagen remodeling phase that can last for several months.8,16
Microneedling has gained popularity in the treatment of acne scarring.7 Alam et al9 conducted a split-face randomized clinical trial (RCT) to evaluate acne scarring after 3 microneedling sessions performed at 2-week intervals. Twenty participants with acne scarring on both sides of the face were enrolled in the study and one side of the face was randomized for treatment. Participants had at least two 5×5-cm areas of acne scarring graded as 2 (moderately atrophic scars) to 4 (hyperplastic or papular scars) on the quantitative Global Acne Scarring Classification system. A roller device with a 1.0-mm depth was used on participants with fine, less sebaceous skin and a 2.0-mm device for all others. Two blinded investigators assessed acne scars at baseline and at 3 and 6 months after treatment. Scar improvement was measured using the quantitative Goodman and Baron scale, which provides a score according to type and number of scars.17 Mean scar scores were significantly reduced at 6 months compared to baseline on the treatment side (P=.03) but not the control side. Participants experienced minimal pain associated with microneedling therapy, rated 1.08 of 10, and adverse effects were limited to mild transient erythema and edema.9 Several other clinical trials have demonstrated clinical improvements with microneedling.18-20
The benefits of microneedling also have been observed on a histologic level. One group of investigators explored the effects of microneedling on dermal collagen in the treatment of various atrophic acne scars in 10 participants.7 After 6 treatment sessions performed at 2-week intervals, dermal collagen was assessed via punch biopsy. A roller device with a needle depth of 1.5 mm was used for all patients. At 1 month after treatment compared to baseline, mean (SD) levels of type I collagen were significantly increased (67.1% [4.2%] vs 70.4% [5.4%]; P=.01) as well as at 3 months after treatment compared to baseline for type III collagen (61.4% [3.6%] vs 74.3% [7.4%]; P=.01), type VII collagen (15.2% [2.1%] vs 21.3% [1.2%]; P=.03), and newly synthesized collagen (14.5% [5.8%] vs 19.5% [3.2%]; P=.02). Total elastin levels were significantly decreased at 3 months after treatment compared to baseline (51.3% [6.7%] vs 46.9% [4.3%]; P=.04). Adverse effects were limited to transient erythema and edema.7
Microneedling With Platelet-Rich Plasma
Microneedling has been combined with platelet-rich plasma (PRP) in the treatment of atrophic acne scars.21 In addition to inducing new collagen synthesis, microneedling aids in the absorption of PRP, an autologous concentrate of platelets that is obtained through peripheral venipuncture. The concentrate is centrifuged into 3 layers: (1) platelet-poor plasma, (2) PRP, and (3) erythrocytes.22 Platelet-rich plasma contains growth factors such as platelet-derived growth factor, transforming growth factor (TGF), and vascular endothelial growth factor, as well as cell adhesion molecules.22,23 The application of PRP is thought to result in upregulated protein synthesis, greater collagen remodeling, and accelerated wound healing.21
Several studies have shown that the addition of PRP to microneedling can improve treatment outcome (Table 1).24-27 Severity of acne scarring can be improved, such as reduced scar depth, by using both modalities synergistically (Figure).24 Asif et al26 compared microneedling with PRP to microneedling with distilled water in the treatment of 50 patients with atrophic acne scars graded 2 to 4 (mild to severe acne scarring) on the Goodman’s Qualitative classification and equal Goodman’s Qualitative and Quantitative scores on both halves of the face.17,28 The right side of the face was treated with a 1.5-mm microneedling roller with intradermal and topical PRP, while the left side was treated with distilled water (placebo) delivered intradermally. Patients underwent 3 treatment sessions at 1-month intervals. The area treated with microneedling and PRP showed a 62.20% improvement from baseline after 3 treatments, while the placebo-treated area showed a 45.84% improvement on the Goodman and Baron quantitative scale.26
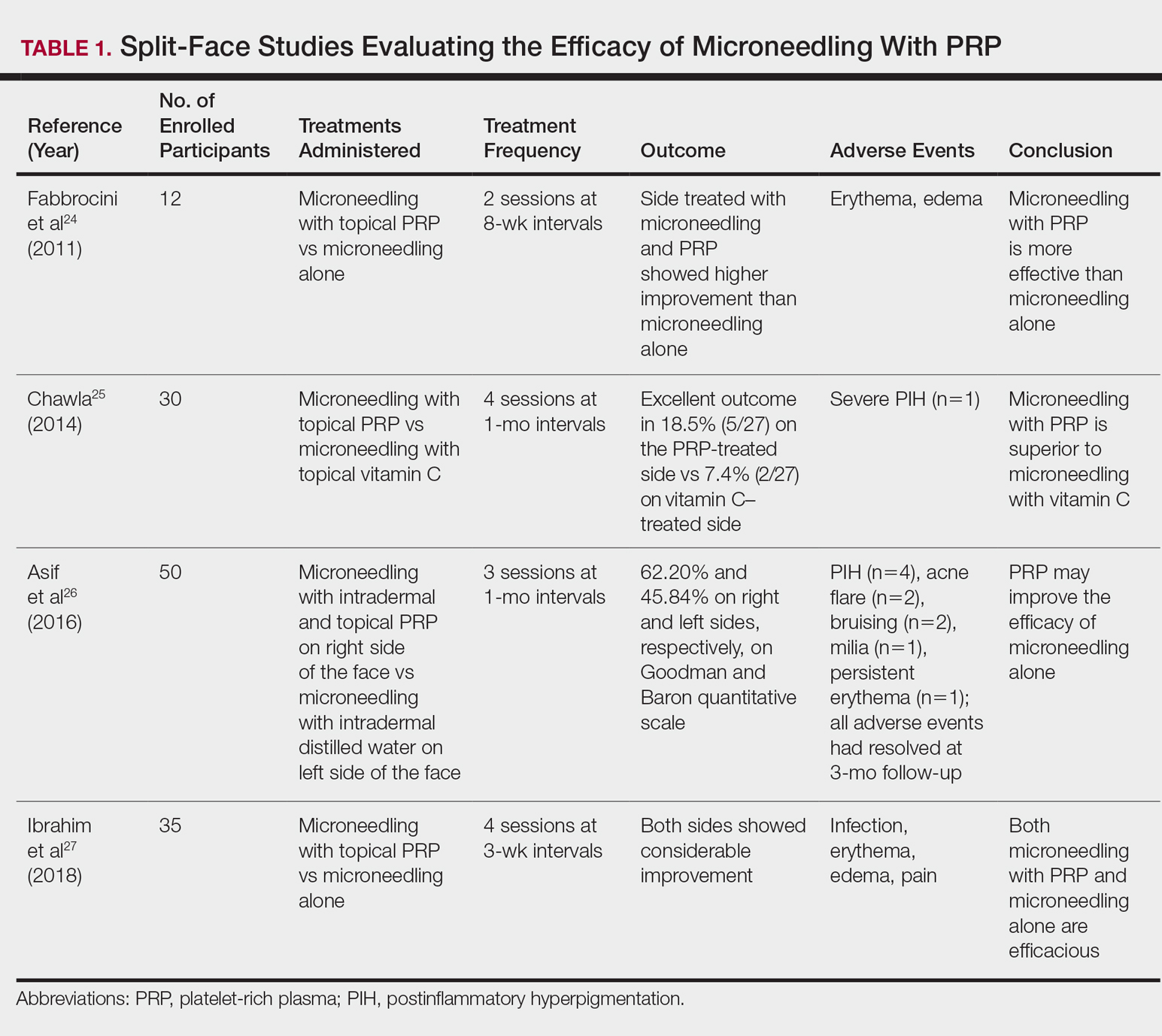
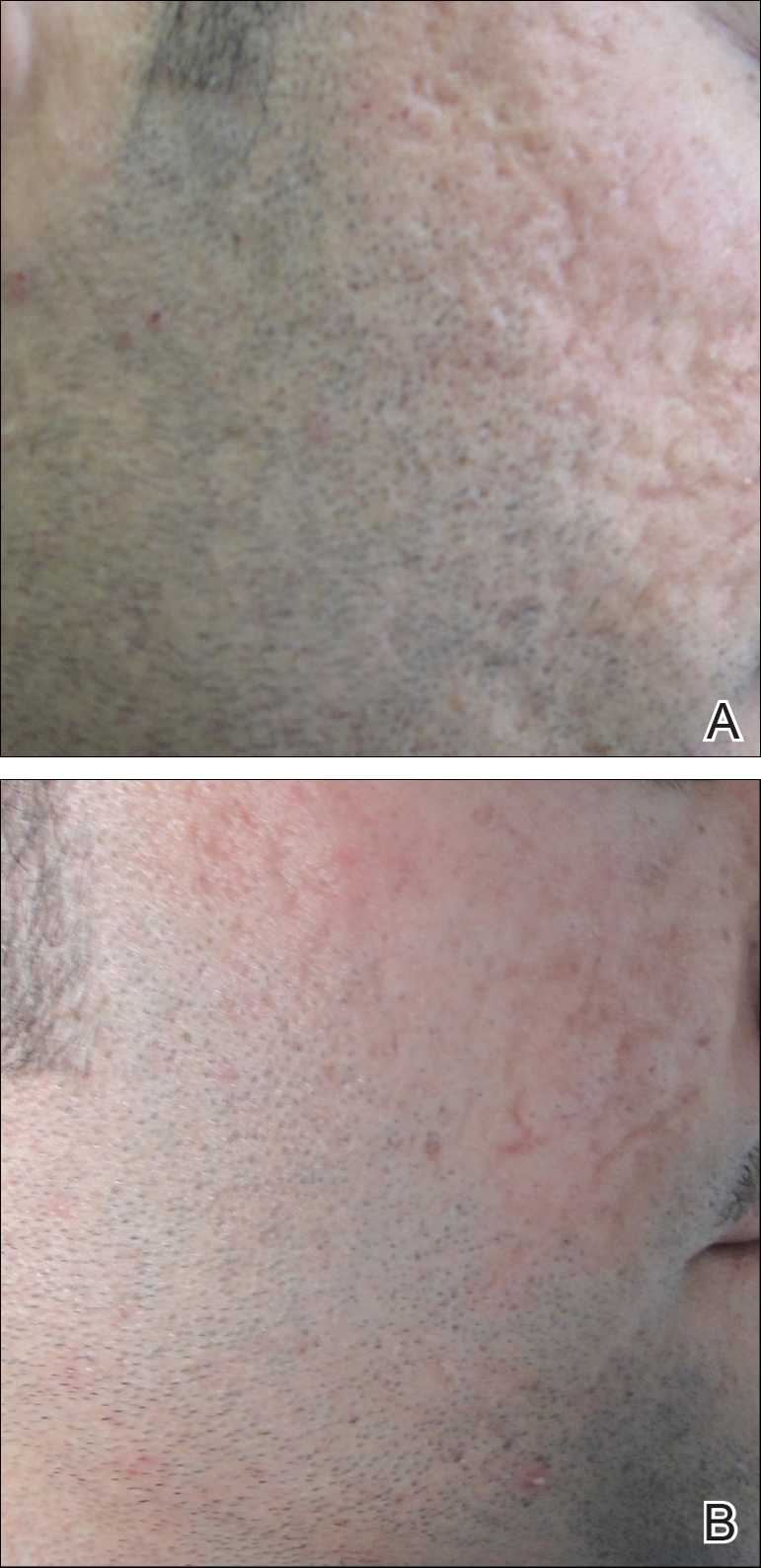
Chawla25 compared microneedling with topical PRP to microneedling with topical vitamin C in a split-face study of 30 participants with atrophic acne scarring graded 2 to 4 on the Goodman and Baron scale. A 1.5-mm roller device was used. Patients underwent 4 treatment sessions at 1-month intervals, and treatment efficacy was evaluated using the qualitative Goodman and Baron scale.28 Participants experienced positive outcomes overall with both treatments. Notably, 18.5% (5/27) on the microneedling with PRP side demonstrated excellent response compared to 7.4% (2/27) on the microneedling with vitamin C side.25
Laser Treatment
Laser skin resurfacing has shown to be efficacious in the treatment of both acne vulgaris and acne scarring. Various lasers have been utilized, including nonfractional CO2 and erbium-doped:YAG (Er:YAG) lasers, as well as ablative fractional lasers (AFLs) and nonablative fractional lasers (NAFLs).29
One retrospective study of 58 patients compared the use of 2 resurfacing lasers—10,600-nm nonfractional CO2 and 2940-nm Er:YAG—and 2 fractional lasers—1550-nm NAFL and 10,600-nm AFL—in the treatment of atrophic acne scars.29 A retrospective photographic analysis was performed by 6 blinded dermatologists to evaluate clinical improvement on a scale of 0 (no improvement) to 10 (excellent improvement). The mean improvement scores of the CO2, Er:YAG, AFL, and NAFL groups were 6.0, 5.8, 2.2, and 5.2, respectively, and the mean number of treatments was 1.6, 1.1, 4.0, and 3.4, respectively. Thus, patients in the fractional laser groups required more treatments; however, those in the resurfacing laser groups had longer recovery times, pain, erythema, and postinflammatory hyperpigmentation. The investigators concluded that 3 consecutive AFL treatments could be as effective as a single resurfacing treatment with lower risk for complications.29
A split-face RCT compared the use of the fractional Er:YAG laser on one side of the face to microneedling with a 2.0-mm needle on the other side for treatment of atrophic acne scars.30 Thirty patients underwent 5 treatments at 1-month intervals. At 3-month follow-up, the areas treated with the Er:YAG laser showed 70% improvement from baseline compared to 30% improvement in the areas treated with microneedling (P<.001). Histologically, the Er:YAG laser showed a higher increase in dermal collagen than microneedling (P<.001). Furthermore, the Er:YAG laser yielded significantly lower pain scores (P<.001); however, patients reported higher rates of erythema, swelling, superficial crusting, and total downtime.30
Lasers With PRP
More recent studies have examined the use of laser therapy in addition to PRP for the treatment of acne scars (Table 2).31-34 Abdel Aal et al33 examined the use of the ablative fractional CO2 laser with and without intradermal PRP in a split-face study of 30 participants with various types of acne scarring (ie, boxcar, ice pick, and rolling scars). Participants underwent 2 treatments at 4-week intervals. Evaluations were performed by 2 blinded dermatologists 6 months after the final laser treatment using the qualitative Goodman and Baron scale.28 Both treatments yielded improvement in scarring, but the PRP-treated side showed shorter durations of postprocedure erythema (P=.0052) as well as higher patient satisfaction scores (P<.001) than laser therapy alone.33
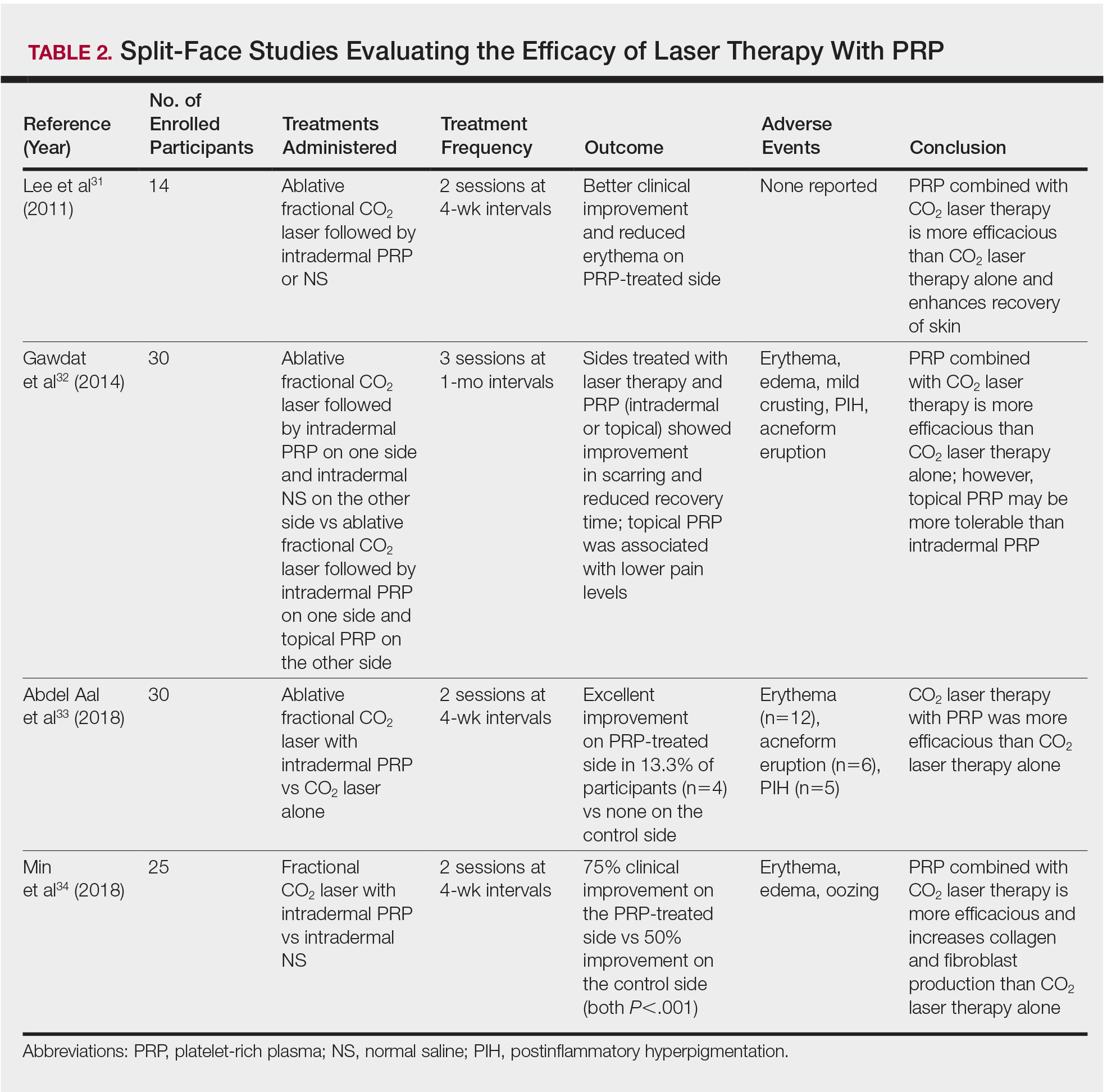
In another split-face study, Gawdat et al32 examined combination treatment with the ablative fractional CO2 laser and PRP in 30 participants with atrophic acne scars graded 2 to 4 on the qualitative Goodman and Baron scale.28 Participants were randomized to 2 different treatment groups: In group 1, half of the face was treated with the fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and intradermal saline. In group 2, half of the face was treated with fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and topical PRP. All patients underwent 3 treatment sessions at 1-month intervals with assessment occurring a 6-month follow-up using the qualitative Goodman and Baron Scale.28 In all participants, areas treated with the combined laser and PRP showed significant improvement in scarring (P=.03) and reduced recovery time (P=.02) compared to areas treated with laser therapy only. Patients receiving intradermal or topical PRP showed no statistically significant differences in improvement of scarring or recovery time; however, areas treated with topical PRP had significantly lower pain levels (P=.005).32
Lee et al31 conducted a split-face study of 14 patients with moderate to severe acne scarring treated with an ablative fractional CO2 laser followed by intradermal PRP or intradermal normal saline injections. Patients underwent 2 treatment sessions at 4-week intervals. Photographs taken at baseline and 4 months posttreatment were evaluated by 2 blinded dermatologists for clinical improvement using a quartile grading system. Erythema was assessed using a skin color measuring device. A blinded dermatologist assessed patients for adverse events. At 4-month follow-up, mean (SD) clinical improvement on the side receiving intradermal PRP was significantly better than the control side (2.7 [0.7] vs 2.3 [0.5]; P=.03). Erythema on posttreatment day 4 was significantly less on the side treated with PRP (P=.01). No adverse events were reported.31
Another split-face study compared the use of intradermal PRP to intradermal normal saline following fractional CO2 laser treatment.34 Twenty-five participants with moderate to severe acne scars completed 2 treatment sessions at 4-week intervals. Additionally, skin biopsies were collected to evaluate collagen production using immunohistochemistry, quantitative polymerase chain reaction, and western blot techniques. Experimental fibroblasts and keratinocytes were isolated and cultured. The cultures were irradiated with a fractional CO2 laser and treated with PRP or platelet-poor plasma. Cultures were evaluated at 30 minutes, 24 hours, and 48 hours. Participants reported 75% improvement of acne scarring from baseline in the side treated with PRP compared to 50% improvement of acne scarring from baseline in the control group (P<.001). On days 7 and 84, participants reported greater improvement on the side treated with PRP (P=.03 and P=.02, respectively). On day 28, skin biopsy evaluation yielded higher levels of TGF-β1 (P=.02), TGF-β3 (P=.004), c-myc (P=.004), type I collagen (P=.03), and type III collagen (P=.03) on the PRP-treated side compared to the control side. Transforming growth factor β increases collagen and fibroblast production, while c-myc leads to cell cycle progression.35-37 Similarly, TGF-β1, TGF-β3, types I andIII collagen, and p-Akt were increased in all cultures treated with PRP and platelet-poor plasma in a dose-dependent manner.34 p-Akt is thought to regulate wound healing38; however, PRP-treated keratinocytes yielded increased epidermal growth factor receptor and decreased keratin-16 at 48 hours, which suggests PRP plays a role in increasing epithelization and reducing laser-induced keratinocyte damage.39 Adverse effects (eg, erythema, edema, oozing) were less frequent in the PRP-treated side.34
Chemical Peels
Chemical peels are widely used in the treatment of acne scarring.40 Peels improve scarring through destruction of the epidermal and/or dermal layers, leading to skin exfoliation, rejuvenation, and remodeling. Superficial peeling agents, which extend to the dermoepidermal junction, include resorcinol, tretinoin, glycolic acid, lactic acid, salicylic acid, and trichloroacetic acid (TCA) 10% to 35%.41 Medium-depth peeling agents extend to the upper reticular dermis and include phenol, TCA 35% to 50%, and Jessner solution (resorcinol, lactic acid, and salicylic acid in ethanol) followed by TCA 35%.41 Finally, the effects of deep peeling agents reach the mid reticular dermis and include the Baker-Gordon or Litton phenol formulas.41 Deep peels are associated with higher rates of adverse outcomes including infection, dyschromia, and scarring.41,42
An RCT was performed to evaluate the use of a deep phenol 60% peel compared to microneedling with a 1.5-mm roller device plus a TCA 20% peel in the treatment of atrophic acne scars.43 Twenty-four patients were randomly and evenly assigned to both treatment groups. The phenol group underwent a single treatment session, while the microneedling plus TCA group underwent 4 treatment sessions at 6-week intervals. Both groups were instructed to use daily topical tretinoin and hydroquinone 2% in the 2 weeks prior to treatment. Posttreatment results were evaluated using a quartile grading scale. Scarring improved from baseline by 75.12% (P<.001) in the phenol group and 69.43% (P<.001) in the microneedling plus TCA group, with no significant difference between groups. Adverse effects in the phenol group included erythema and hyperpigmentation, while adverse events in the microneedling plus TCA group included transient pain, edema, erythema, and desquamation.43
Another study compared the use of a TCA 15% peel with microneedling to PRP with microneedling and microneedling alone in the treatment of atrophic acne scars.44 Twenty-four patients were randomly assigned to the 3 treatment groups (8 to each group) and underwent 6 treatment sessions with 2-week intervals. A roller device with a 1.5-mm needle was used for microneedling. Microneedling plus TCA and microneedling plus PRP were significantly more effective than microneedling alone (P=.011 and P=.015, respectively); however, the TCA 15% peel with microneedling resulted in the largest increase in epidermal thickening. The investigators concluded that combined use of a TCA 15% peel and microneedling was the most effective in treating atrophic acne scarring.44
Dermal Fillers
Dermal or subcutaneous fillers are used to increase volume in depressed scars and stimulate the skin’s natural production.45 Tissue augmentation methods commonly are used for larger rolling acne scars. Options for filler materials include autologous fat, bovine, or human collagen derivatives; hyaluronic acid; and polymethyl methacrylate microspheres with collagen.45 Newer fillers are formulated with lidocaine to decrease pain associated with the procedure.46 Hyaluronic acid fillers provide natural volume correction and have limited potential to elicit an immune response due to their derivation from bacterial fermentation. Fillers using polymethyl methacrylate microspheres with collagen are permanent and effective, which may lead to reduced patient costs; however, they often are not a first choice for treatment.45,46 Furthermore, if dermal fillers consist of bovine collagen, it is necessary to perform skin testing for allergy prior to use. Autologous fat transfer also has become popular for treatment of acne scarring, especially because there is no risk of allergic reaction, as the patient’s own fat is used for correction.46 However, this method requires a high degree of skill, and results are unpredictable, generally lasting from 6 months to several years.
Therapies on the horizon include autologous cell therapy. A multicenter, double-blinded, placebo-controlled RCT examined the use of an autologous fibroblast filler in the treatment of bilateral, depressed, and distensible acne scars that were graded as moderate to severe.47 Autologous fat fibroblasts were harvested from full-thickness postauricular punch biopsies. In this split-face study, 99 participants were treated with an intradermal autologous fibroblast filler on one cheek and a protein-free cell-culture medium on the contralateral cheek. Participants received an average of 5.9 mL of both autologous fat fibroblasts and cell-culture medium over 3 treatment sessions at 2-week intervals. The autologous fat fibroblasts were associated with greater improvement compared to cell-culture medium based on participant (43% vs 18%), evaluator (59% vs 42%), and independent photographic viewer’s assessment.47
Conclusion
Acne scarring is a burden affecting millions of Americans. It often has a negative impact on quality of life and can lead to low self-esteem in patients. Numerous trials have indicated that microneedling is beneficial in the treatment of acne scarring, and emerging evidence indicates that the addition of PRP provides measurable benefits. Similarly, the addition of PRP to laser therapy may reduce recovery time as well as the commonly associated adverse events of erythema and pain. Chemical peels provide the advantage of being easily and efficiently performed in the office setting. Finally, the wide range of available dermal fillers can be tailored to treat specific types of acne scars. Autologous dermal fillers recently have been used and show promising benefits. It is important to consider desired outcome, cost, and adverse events when discussing therapeutic options for acne scarring with patients. The numerous therapeutic options warrant further research and well-designed RCTs to ensure optimal patient outcomes.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2, pt 3):S34-S37.
- Yazici K, Baz K, Yazici AE, et al. Disease-specific quality of life is associated with anxiety and depression in patients with acne. J Eur Acad Dermatol Venereol. 2004;18:435-439.
- Orentreich DS, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatol Surg. 1995;21:543-549.
- Fabbrocini G, De Padova M, De Vita V, et al. Periorbital wrinkles treatment using collagen induction therapy. Surg Cosmet Dermatol. 2009;1:106-111.
- Fabbrocini G, De Vita V, Pastore F, et al. Collagen induction therapy for the treatment of upper lip wrinkles. J Dermatol Treat. 2012;23:144-152.
- Fabbrocini G, De Vita V, Di Costanzo L, et al. Skin needling in the treatment of the aging neck. Skinmed. 2011;9:347-351.
- El-Domyati M, Barakat M, Awad S, et al. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8:36-42.
- Fabbrocini G, Fardella N, Monfrecola A, et al. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34:874-879.
- Alam M, Han S, Pongprutthipan M, et al. Efficacy of a needling device for the treatment of acne scars: a randomized clinical trial. JAMA Dermatol. 2014;150:844-849.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
- Dhurat R, Mathapati S. Response to microneedling treatment in men with androgenetic alopecia who failed to respond to conventional therapy. Indian J Dermatol. 2015;60:260-263.
- Fabbrocini G, De Vita V, Fardella N, et al. Skin needling to enhance depigmenting serum penetration in the treatment of melasma [published online April 7, 2011]. Plast Surg Int. 2011;2011:158241.
- Bariya SH, Gohel MC, Mehta TA, et al. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol. 2012;64:11-29.
- Fabbrocini G, De Vita V, Izzo R, et al. The use of skin needling for the delivery of a eutectic mixture of local anesthetics. G Ital Dermatol Venereol. 2014;149:581-585.
- De Vita V. How to choose among the multiple options to enhance the penetration of topically applied methyl aminolevulinate prior to photodynamic therapy [published online February 22, 2018]. Photodiagnosis Photodyn Ther. doi:10.1016/j.pdpdt.2018.02.014.
- Fernandes D. Minimally invasive percutaneous collagen induction. Oral Maxillofac Surg Clin North Am. 2005;17:51-63.
- Goodman GJ, Baron JA. Postacne scarring—a quantitative global scarring grading system. J Cosmet Dermatol. 2006;5:48-52.
- Majid I. Microneedling therapy in atrophic facial scars: an objective assessment. J Cutan Aesthet Surg. 2009;2:26-30.
- Dogra S, Yadav S, Sarangal R. Microneedling for acne scars in Asian skin type: an effective low cost treatment modality. J Cosmet Dermatol. 2014;13:180-187.
- Fabbrocini G, De Vita V, Monfrecola A, et al. Percutaneous collagen induction: an effective and safe treatment for post-acne scarring in different skin phototypes. J Dermatol Treat. 2014;25:147-152.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
- Wang HL, Avila G. Platelet rich plasma: myth or reality? Eur J Dent. 2007;1:192-194.
- Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489-496.
- Fabbrocini G, De Vita V, Pastore F, et al. Combined use of skin needling and platelet-rich plasma in acne scarring treatment. Cosmet Dermatol. 2011;24:177-183.
- Chawla S. Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg. 2014;7:209-212.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Ibrahim MK, Ibrahim SM, Salem AM. Skin microneedling plus platelet-rich plasma versus skin microneedling alone in the treatment of atrophic post acne scars: a split face comparative study. J Dermatolog Treat. 2018;29:281-286.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458-1466.
- You H, Kim D, Yoon E, et al. Comparison of four different lasers for acne scars: resurfacing and fractional lasers. J Plast Reconstr Aesthet Surg. 2016;69:E87-E95.
- Osman MA, Shokeir HA, Fawzy MM. Fractional erbium-doped yttrium aluminum garnet laser versus microneedling in treatment of atrophic acne scars: a randomized split-face clinical study. Dermatol Surg. 2017;43(suppl 1):S47-S56.
- Lee JW, Kim BJ, Kim MN, et al. The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: a simultaneous split-face trial. Dermatol Surg. 2011;37:931-938.
- Gawdat HI, Hegazy RA, Fawzy MM, et al. Autologous platelet rich plasma: topical versus intradermal after fractional ablative carbon dioxide laser treatment of atrophic acne scars. Dermatol Surg. 2014;40:152-161.
- Abdel Aal AM, Ibrahim IM, Sami NA, et al. Evaluation of autologous platelet rich plasma plus ablative carbon dioxide fractional laser in the treatment of acne scars. J Cosmet Laser Ther. 2018;20:106-113.
- Min S, Yoon JY, Park SY, et al. Combination of platelet rich plasma in fractional carbon dioxide laser treatment increased clinical efficacy of for acne scar by enhancement of collagen production and modulation of laser-induced inflammation. Lasers Surg Med. 2018;50:302-310.
- Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986;83:4167-4171.
- Schmidt EV. The role of c-myc in cellular growth control. Oncogene. 1999;18:2988-2996.
- Varga J, Rosenbloom J, Jimenez SA. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J. 1987;247:597-604.
- Chen J, Somanath PR, Razorenova O, et al. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188-1196.
- Repertinger SK, Campagnaro E, Fuhrman J, et al. EGFR enhances early healing after cutaneous incisional wounding. J Invest Dermatol. 2004;123:982-989.
- Landau M. Chemical peels. Clin Dermatol. 2008;26:200-208.
- Drake LA, Dinehart SM, Goltz RW, et al. Guidelines of care for chemical peeling. J Am Acad Dermatol. 1995;33:497-503.
- Meaike JD, Agrawal N, Chang D, et al. Noninvasive facial rejuvenation. part 3: physician-directed-lasers, chemical peels, and other noninvasive modalities. Semin Plast Surg. 2016;30:143-150.
- Leheta TM, Abdel Hay RM, El Garem YF. Deep peeling using phenol versus percutaneous collagen induction combined with trichloroacetic acid 20% in atrophic post-acne scars; a randomized controlled trial.J Dermatol Treat. 2014;25:130-136.
- El-Domyati M, Abdel-Wahab H, Hossam A. Microneedling combined with platelet-rich plasma or trichloroacetic acid peeling for management of acne scarring: a split-face clinical and histologic comparison.J Cosmet Dermatol. 2018;17:73-83.
- Hession MT, Graber EM. Atrophic acne scarring: a review of treatment options. J Clin Aesthet Dermatol. 2015;8:50-58.
- Dayan SH, Bassichis BA. Facial dermal fillers: selection of appropriate products and techniques. Aesthet Surg J. 2008;28:335-347.
- Munavalli GS, Smith S, Maslowski JM, et al. Successful treatment of depressed, distensible acne scars using autologous fibroblasts: a multi-site, prospective, double blind, placebo-controlled clinical trial. Dermatol Surg. 2013;39:1226-1236.
Acne vulgaris is prevalent in the general population, with 40 to 50 million affected individuals in the United States.1 Severe inflammation and injury can lead to disfiguring scarring, which has a considerable impact on quality of life.2 Numerous therapeutic options for acne scarring are available, including microneedling with and without platelet-rich plasma (PRP), lasers, chemical peels, and dermal fillers, with different modalities suited to individual patients and scar characteristics. This article reviews updates in treatment options for acne scarring.
Microneedling
Microneedling, also known as percutaneous collagen induction or collagen induction therapy, has been utilized for more than 2 decades.3 Dermatologic indications for microneedling include skin rejuvenation,4-6 atrophic acne scarring,7-9 and androgenic alopecia.10,11 Microneedling also has been used to enhance skin penetration of topically applied drugs.12-15 Fernandes16 described percutaneous collagen induction as the skin’s natural response to injury. Microneedling creates small wounds as fine needles puncture the epidermis and dermis, resulting in a cascade of growth factors that lead to tissue proliferation, regeneration, and a collagen remodeling phase that can last for several months.8,16
Microneedling has gained popularity in the treatment of acne scarring.7 Alam et al9 conducted a split-face randomized clinical trial (RCT) to evaluate acne scarring after 3 microneedling sessions performed at 2-week intervals. Twenty participants with acne scarring on both sides of the face were enrolled in the study and one side of the face was randomized for treatment. Participants had at least two 5×5-cm areas of acne scarring graded as 2 (moderately atrophic scars) to 4 (hyperplastic or papular scars) on the quantitative Global Acne Scarring Classification system. A roller device with a 1.0-mm depth was used on participants with fine, less sebaceous skin and a 2.0-mm device for all others. Two blinded investigators assessed acne scars at baseline and at 3 and 6 months after treatment. Scar improvement was measured using the quantitative Goodman and Baron scale, which provides a score according to type and number of scars.17 Mean scar scores were significantly reduced at 6 months compared to baseline on the treatment side (P=.03) but not the control side. Participants experienced minimal pain associated with microneedling therapy, rated 1.08 of 10, and adverse effects were limited to mild transient erythema and edema.9 Several other clinical trials have demonstrated clinical improvements with microneedling.18-20
The benefits of microneedling also have been observed on a histologic level. One group of investigators explored the effects of microneedling on dermal collagen in the treatment of various atrophic acne scars in 10 participants.7 After 6 treatment sessions performed at 2-week intervals, dermal collagen was assessed via punch biopsy. A roller device with a needle depth of 1.5 mm was used for all patients. At 1 month after treatment compared to baseline, mean (SD) levels of type I collagen were significantly increased (67.1% [4.2%] vs 70.4% [5.4%]; P=.01) as well as at 3 months after treatment compared to baseline for type III collagen (61.4% [3.6%] vs 74.3% [7.4%]; P=.01), type VII collagen (15.2% [2.1%] vs 21.3% [1.2%]; P=.03), and newly synthesized collagen (14.5% [5.8%] vs 19.5% [3.2%]; P=.02). Total elastin levels were significantly decreased at 3 months after treatment compared to baseline (51.3% [6.7%] vs 46.9% [4.3%]; P=.04). Adverse effects were limited to transient erythema and edema.7
Microneedling With Platelet-Rich Plasma
Microneedling has been combined with platelet-rich plasma (PRP) in the treatment of atrophic acne scars.21 In addition to inducing new collagen synthesis, microneedling aids in the absorption of PRP, an autologous concentrate of platelets that is obtained through peripheral venipuncture. The concentrate is centrifuged into 3 layers: (1) platelet-poor plasma, (2) PRP, and (3) erythrocytes.22 Platelet-rich plasma contains growth factors such as platelet-derived growth factor, transforming growth factor (TGF), and vascular endothelial growth factor, as well as cell adhesion molecules.22,23 The application of PRP is thought to result in upregulated protein synthesis, greater collagen remodeling, and accelerated wound healing.21
Several studies have shown that the addition of PRP to microneedling can improve treatment outcome (Table 1).24-27 Severity of acne scarring can be improved, such as reduced scar depth, by using both modalities synergistically (Figure).24 Asif et al26 compared microneedling with PRP to microneedling with distilled water in the treatment of 50 patients with atrophic acne scars graded 2 to 4 (mild to severe acne scarring) on the Goodman’s Qualitative classification and equal Goodman’s Qualitative and Quantitative scores on both halves of the face.17,28 The right side of the face was treated with a 1.5-mm microneedling roller with intradermal and topical PRP, while the left side was treated with distilled water (placebo) delivered intradermally. Patients underwent 3 treatment sessions at 1-month intervals. The area treated with microneedling and PRP showed a 62.20% improvement from baseline after 3 treatments, while the placebo-treated area showed a 45.84% improvement on the Goodman and Baron quantitative scale.26


Chawla25 compared microneedling with topical PRP to microneedling with topical vitamin C in a split-face study of 30 participants with atrophic acne scarring graded 2 to 4 on the Goodman and Baron scale. A 1.5-mm roller device was used. Patients underwent 4 treatment sessions at 1-month intervals, and treatment efficacy was evaluated using the qualitative Goodman and Baron scale.28 Participants experienced positive outcomes overall with both treatments. Notably, 18.5% (5/27) on the microneedling with PRP side demonstrated excellent response compared to 7.4% (2/27) on the microneedling with vitamin C side.25
Laser Treatment
Laser skin resurfacing has shown to be efficacious in the treatment of both acne vulgaris and acne scarring. Various lasers have been utilized, including nonfractional CO2 and erbium-doped:YAG (Er:YAG) lasers, as well as ablative fractional lasers (AFLs) and nonablative fractional lasers (NAFLs).29
One retrospective study of 58 patients compared the use of 2 resurfacing lasers—10,600-nm nonfractional CO2 and 2940-nm Er:YAG—and 2 fractional lasers—1550-nm NAFL and 10,600-nm AFL—in the treatment of atrophic acne scars.29 A retrospective photographic analysis was performed by 6 blinded dermatologists to evaluate clinical improvement on a scale of 0 (no improvement) to 10 (excellent improvement). The mean improvement scores of the CO2, Er:YAG, AFL, and NAFL groups were 6.0, 5.8, 2.2, and 5.2, respectively, and the mean number of treatments was 1.6, 1.1, 4.0, and 3.4, respectively. Thus, patients in the fractional laser groups required more treatments; however, those in the resurfacing laser groups had longer recovery times, pain, erythema, and postinflammatory hyperpigmentation. The investigators concluded that 3 consecutive AFL treatments could be as effective as a single resurfacing treatment with lower risk for complications.29
A split-face RCT compared the use of the fractional Er:YAG laser on one side of the face to microneedling with a 2.0-mm needle on the other side for treatment of atrophic acne scars.30 Thirty patients underwent 5 treatments at 1-month intervals. At 3-month follow-up, the areas treated with the Er:YAG laser showed 70% improvement from baseline compared to 30% improvement in the areas treated with microneedling (P<.001). Histologically, the Er:YAG laser showed a higher increase in dermal collagen than microneedling (P<.001). Furthermore, the Er:YAG laser yielded significantly lower pain scores (P<.001); however, patients reported higher rates of erythema, swelling, superficial crusting, and total downtime.30
Lasers With PRP
More recent studies have examined the use of laser therapy in addition to PRP for the treatment of acne scars (Table 2).31-34 Abdel Aal et al33 examined the use of the ablative fractional CO2 laser with and without intradermal PRP in a split-face study of 30 participants with various types of acne scarring (ie, boxcar, ice pick, and rolling scars). Participants underwent 2 treatments at 4-week intervals. Evaluations were performed by 2 blinded dermatologists 6 months after the final laser treatment using the qualitative Goodman and Baron scale.28 Both treatments yielded improvement in scarring, but the PRP-treated side showed shorter durations of postprocedure erythema (P=.0052) as well as higher patient satisfaction scores (P<.001) than laser therapy alone.33

In another split-face study, Gawdat et al32 examined combination treatment with the ablative fractional CO2 laser and PRP in 30 participants with atrophic acne scars graded 2 to 4 on the qualitative Goodman and Baron scale.28 Participants were randomized to 2 different treatment groups: In group 1, half of the face was treated with the fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and intradermal saline. In group 2, half of the face was treated with fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and topical PRP. All patients underwent 3 treatment sessions at 1-month intervals with assessment occurring a 6-month follow-up using the qualitative Goodman and Baron Scale.28 In all participants, areas treated with the combined laser and PRP showed significant improvement in scarring (P=.03) and reduced recovery time (P=.02) compared to areas treated with laser therapy only. Patients receiving intradermal or topical PRP showed no statistically significant differences in improvement of scarring or recovery time; however, areas treated with topical PRP had significantly lower pain levels (P=.005).32
Lee et al31 conducted a split-face study of 14 patients with moderate to severe acne scarring treated with an ablative fractional CO2 laser followed by intradermal PRP or intradermal normal saline injections. Patients underwent 2 treatment sessions at 4-week intervals. Photographs taken at baseline and 4 months posttreatment were evaluated by 2 blinded dermatologists for clinical improvement using a quartile grading system. Erythema was assessed using a skin color measuring device. A blinded dermatologist assessed patients for adverse events. At 4-month follow-up, mean (SD) clinical improvement on the side receiving intradermal PRP was significantly better than the control side (2.7 [0.7] vs 2.3 [0.5]; P=.03). Erythema on posttreatment day 4 was significantly less on the side treated with PRP (P=.01). No adverse events were reported.31
Another split-face study compared the use of intradermal PRP to intradermal normal saline following fractional CO2 laser treatment.34 Twenty-five participants with moderate to severe acne scars completed 2 treatment sessions at 4-week intervals. Additionally, skin biopsies were collected to evaluate collagen production using immunohistochemistry, quantitative polymerase chain reaction, and western blot techniques. Experimental fibroblasts and keratinocytes were isolated and cultured. The cultures were irradiated with a fractional CO2 laser and treated with PRP or platelet-poor plasma. Cultures were evaluated at 30 minutes, 24 hours, and 48 hours. Participants reported 75% improvement of acne scarring from baseline in the side treated with PRP compared to 50% improvement of acne scarring from baseline in the control group (P<.001). On days 7 and 84, participants reported greater improvement on the side treated with PRP (P=.03 and P=.02, respectively). On day 28, skin biopsy evaluation yielded higher levels of TGF-β1 (P=.02), TGF-β3 (P=.004), c-myc (P=.004), type I collagen (P=.03), and type III collagen (P=.03) on the PRP-treated side compared to the control side. Transforming growth factor β increases collagen and fibroblast production, while c-myc leads to cell cycle progression.35-37 Similarly, TGF-β1, TGF-β3, types I andIII collagen, and p-Akt were increased in all cultures treated with PRP and platelet-poor plasma in a dose-dependent manner.34 p-Akt is thought to regulate wound healing38; however, PRP-treated keratinocytes yielded increased epidermal growth factor receptor and decreased keratin-16 at 48 hours, which suggests PRP plays a role in increasing epithelization and reducing laser-induced keratinocyte damage.39 Adverse effects (eg, erythema, edema, oozing) were less frequent in the PRP-treated side.34
Chemical Peels
Chemical peels are widely used in the treatment of acne scarring.40 Peels improve scarring through destruction of the epidermal and/or dermal layers, leading to skin exfoliation, rejuvenation, and remodeling. Superficial peeling agents, which extend to the dermoepidermal junction, include resorcinol, tretinoin, glycolic acid, lactic acid, salicylic acid, and trichloroacetic acid (TCA) 10% to 35%.41 Medium-depth peeling agents extend to the upper reticular dermis and include phenol, TCA 35% to 50%, and Jessner solution (resorcinol, lactic acid, and salicylic acid in ethanol) followed by TCA 35%.41 Finally, the effects of deep peeling agents reach the mid reticular dermis and include the Baker-Gordon or Litton phenol formulas.41 Deep peels are associated with higher rates of adverse outcomes including infection, dyschromia, and scarring.41,42
An RCT was performed to evaluate the use of a deep phenol 60% peel compared to microneedling with a 1.5-mm roller device plus a TCA 20% peel in the treatment of atrophic acne scars.43 Twenty-four patients were randomly and evenly assigned to both treatment groups. The phenol group underwent a single treatment session, while the microneedling plus TCA group underwent 4 treatment sessions at 6-week intervals. Both groups were instructed to use daily topical tretinoin and hydroquinone 2% in the 2 weeks prior to treatment. Posttreatment results were evaluated using a quartile grading scale. Scarring improved from baseline by 75.12% (P<.001) in the phenol group and 69.43% (P<.001) in the microneedling plus TCA group, with no significant difference between groups. Adverse effects in the phenol group included erythema and hyperpigmentation, while adverse events in the microneedling plus TCA group included transient pain, edema, erythema, and desquamation.43
Another study compared the use of a TCA 15% peel with microneedling to PRP with microneedling and microneedling alone in the treatment of atrophic acne scars.44 Twenty-four patients were randomly assigned to the 3 treatment groups (8 to each group) and underwent 6 treatment sessions with 2-week intervals. A roller device with a 1.5-mm needle was used for microneedling. Microneedling plus TCA and microneedling plus PRP were significantly more effective than microneedling alone (P=.011 and P=.015, respectively); however, the TCA 15% peel with microneedling resulted in the largest increase in epidermal thickening. The investigators concluded that combined use of a TCA 15% peel and microneedling was the most effective in treating atrophic acne scarring.44
Dermal Fillers
Dermal or subcutaneous fillers are used to increase volume in depressed scars and stimulate the skin’s natural production.45 Tissue augmentation methods commonly are used for larger rolling acne scars. Options for filler materials include autologous fat, bovine, or human collagen derivatives; hyaluronic acid; and polymethyl methacrylate microspheres with collagen.45 Newer fillers are formulated with lidocaine to decrease pain associated with the procedure.46 Hyaluronic acid fillers provide natural volume correction and have limited potential to elicit an immune response due to their derivation from bacterial fermentation. Fillers using polymethyl methacrylate microspheres with collagen are permanent and effective, which may lead to reduced patient costs; however, they often are not a first choice for treatment.45,46 Furthermore, if dermal fillers consist of bovine collagen, it is necessary to perform skin testing for allergy prior to use. Autologous fat transfer also has become popular for treatment of acne scarring, especially because there is no risk of allergic reaction, as the patient’s own fat is used for correction.46 However, this method requires a high degree of skill, and results are unpredictable, generally lasting from 6 months to several years.
Therapies on the horizon include autologous cell therapy. A multicenter, double-blinded, placebo-controlled RCT examined the use of an autologous fibroblast filler in the treatment of bilateral, depressed, and distensible acne scars that were graded as moderate to severe.47 Autologous fat fibroblasts were harvested from full-thickness postauricular punch biopsies. In this split-face study, 99 participants were treated with an intradermal autologous fibroblast filler on one cheek and a protein-free cell-culture medium on the contralateral cheek. Participants received an average of 5.9 mL of both autologous fat fibroblasts and cell-culture medium over 3 treatment sessions at 2-week intervals. The autologous fat fibroblasts were associated with greater improvement compared to cell-culture medium based on participant (43% vs 18%), evaluator (59% vs 42%), and independent photographic viewer’s assessment.47
Conclusion
Acne scarring is a burden affecting millions of Americans. It often has a negative impact on quality of life and can lead to low self-esteem in patients. Numerous trials have indicated that microneedling is beneficial in the treatment of acne scarring, and emerging evidence indicates that the addition of PRP provides measurable benefits. Similarly, the addition of PRP to laser therapy may reduce recovery time as well as the commonly associated adverse events of erythema and pain. Chemical peels provide the advantage of being easily and efficiently performed in the office setting. Finally, the wide range of available dermal fillers can be tailored to treat specific types of acne scars. Autologous dermal fillers recently have been used and show promising benefits. It is important to consider desired outcome, cost, and adverse events when discussing therapeutic options for acne scarring with patients. The numerous therapeutic options warrant further research and well-designed RCTs to ensure optimal patient outcomes.
Acne vulgaris is prevalent in the general population, with 40 to 50 million affected individuals in the United States.1 Severe inflammation and injury can lead to disfiguring scarring, which has a considerable impact on quality of life.2 Numerous therapeutic options for acne scarring are available, including microneedling with and without platelet-rich plasma (PRP), lasers, chemical peels, and dermal fillers, with different modalities suited to individual patients and scar characteristics. This article reviews updates in treatment options for acne scarring.
Microneedling
Microneedling, also known as percutaneous collagen induction or collagen induction therapy, has been utilized for more than 2 decades.3 Dermatologic indications for microneedling include skin rejuvenation,4-6 atrophic acne scarring,7-9 and androgenic alopecia.10,11 Microneedling also has been used to enhance skin penetration of topically applied drugs.12-15 Fernandes16 described percutaneous collagen induction as the skin’s natural response to injury. Microneedling creates small wounds as fine needles puncture the epidermis and dermis, resulting in a cascade of growth factors that lead to tissue proliferation, regeneration, and a collagen remodeling phase that can last for several months.8,16
Microneedling has gained popularity in the treatment of acne scarring.7 Alam et al9 conducted a split-face randomized clinical trial (RCT) to evaluate acne scarring after 3 microneedling sessions performed at 2-week intervals. Twenty participants with acne scarring on both sides of the face were enrolled in the study and one side of the face was randomized for treatment. Participants had at least two 5×5-cm areas of acne scarring graded as 2 (moderately atrophic scars) to 4 (hyperplastic or papular scars) on the quantitative Global Acne Scarring Classification system. A roller device with a 1.0-mm depth was used on participants with fine, less sebaceous skin and a 2.0-mm device for all others. Two blinded investigators assessed acne scars at baseline and at 3 and 6 months after treatment. Scar improvement was measured using the quantitative Goodman and Baron scale, which provides a score according to type and number of scars.17 Mean scar scores were significantly reduced at 6 months compared to baseline on the treatment side (P=.03) but not the control side. Participants experienced minimal pain associated with microneedling therapy, rated 1.08 of 10, and adverse effects were limited to mild transient erythema and edema.9 Several other clinical trials have demonstrated clinical improvements with microneedling.18-20
The benefits of microneedling also have been observed on a histologic level. One group of investigators explored the effects of microneedling on dermal collagen in the treatment of various atrophic acne scars in 10 participants.7 After 6 treatment sessions performed at 2-week intervals, dermal collagen was assessed via punch biopsy. A roller device with a needle depth of 1.5 mm was used for all patients. At 1 month after treatment compared to baseline, mean (SD) levels of type I collagen were significantly increased (67.1% [4.2%] vs 70.4% [5.4%]; P=.01) as well as at 3 months after treatment compared to baseline for type III collagen (61.4% [3.6%] vs 74.3% [7.4%]; P=.01), type VII collagen (15.2% [2.1%] vs 21.3% [1.2%]; P=.03), and newly synthesized collagen (14.5% [5.8%] vs 19.5% [3.2%]; P=.02). Total elastin levels were significantly decreased at 3 months after treatment compared to baseline (51.3% [6.7%] vs 46.9% [4.3%]; P=.04). Adverse effects were limited to transient erythema and edema.7
Microneedling With Platelet-Rich Plasma
Microneedling has been combined with platelet-rich plasma (PRP) in the treatment of atrophic acne scars.21 In addition to inducing new collagen synthesis, microneedling aids in the absorption of PRP, an autologous concentrate of platelets that is obtained through peripheral venipuncture. The concentrate is centrifuged into 3 layers: (1) platelet-poor plasma, (2) PRP, and (3) erythrocytes.22 Platelet-rich plasma contains growth factors such as platelet-derived growth factor, transforming growth factor (TGF), and vascular endothelial growth factor, as well as cell adhesion molecules.22,23 The application of PRP is thought to result in upregulated protein synthesis, greater collagen remodeling, and accelerated wound healing.21
Several studies have shown that the addition of PRP to microneedling can improve treatment outcome (Table 1).24-27 Severity of acne scarring can be improved, such as reduced scar depth, by using both modalities synergistically (Figure).24 Asif et al26 compared microneedling with PRP to microneedling with distilled water in the treatment of 50 patients with atrophic acne scars graded 2 to 4 (mild to severe acne scarring) on the Goodman’s Qualitative classification and equal Goodman’s Qualitative and Quantitative scores on both halves of the face.17,28 The right side of the face was treated with a 1.5-mm microneedling roller with intradermal and topical PRP, while the left side was treated with distilled water (placebo) delivered intradermally. Patients underwent 3 treatment sessions at 1-month intervals. The area treated with microneedling and PRP showed a 62.20% improvement from baseline after 3 treatments, while the placebo-treated area showed a 45.84% improvement on the Goodman and Baron quantitative scale.26


Chawla25 compared microneedling with topical PRP to microneedling with topical vitamin C in a split-face study of 30 participants with atrophic acne scarring graded 2 to 4 on the Goodman and Baron scale. A 1.5-mm roller device was used. Patients underwent 4 treatment sessions at 1-month intervals, and treatment efficacy was evaluated using the qualitative Goodman and Baron scale.28 Participants experienced positive outcomes overall with both treatments. Notably, 18.5% (5/27) on the microneedling with PRP side demonstrated excellent response compared to 7.4% (2/27) on the microneedling with vitamin C side.25
Laser Treatment
Laser skin resurfacing has shown to be efficacious in the treatment of both acne vulgaris and acne scarring. Various lasers have been utilized, including nonfractional CO2 and erbium-doped:YAG (Er:YAG) lasers, as well as ablative fractional lasers (AFLs) and nonablative fractional lasers (NAFLs).29
One retrospective study of 58 patients compared the use of 2 resurfacing lasers—10,600-nm nonfractional CO2 and 2940-nm Er:YAG—and 2 fractional lasers—1550-nm NAFL and 10,600-nm AFL—in the treatment of atrophic acne scars.29 A retrospective photographic analysis was performed by 6 blinded dermatologists to evaluate clinical improvement on a scale of 0 (no improvement) to 10 (excellent improvement). The mean improvement scores of the CO2, Er:YAG, AFL, and NAFL groups were 6.0, 5.8, 2.2, and 5.2, respectively, and the mean number of treatments was 1.6, 1.1, 4.0, and 3.4, respectively. Thus, patients in the fractional laser groups required more treatments; however, those in the resurfacing laser groups had longer recovery times, pain, erythema, and postinflammatory hyperpigmentation. The investigators concluded that 3 consecutive AFL treatments could be as effective as a single resurfacing treatment with lower risk for complications.29
A split-face RCT compared the use of the fractional Er:YAG laser on one side of the face to microneedling with a 2.0-mm needle on the other side for treatment of atrophic acne scars.30 Thirty patients underwent 5 treatments at 1-month intervals. At 3-month follow-up, the areas treated with the Er:YAG laser showed 70% improvement from baseline compared to 30% improvement in the areas treated with microneedling (P<.001). Histologically, the Er:YAG laser showed a higher increase in dermal collagen than microneedling (P<.001). Furthermore, the Er:YAG laser yielded significantly lower pain scores (P<.001); however, patients reported higher rates of erythema, swelling, superficial crusting, and total downtime.30
Lasers With PRP
More recent studies have examined the use of laser therapy in addition to PRP for the treatment of acne scars (Table 2).31-34 Abdel Aal et al33 examined the use of the ablative fractional CO2 laser with and without intradermal PRP in a split-face study of 30 participants with various types of acne scarring (ie, boxcar, ice pick, and rolling scars). Participants underwent 2 treatments at 4-week intervals. Evaluations were performed by 2 blinded dermatologists 6 months after the final laser treatment using the qualitative Goodman and Baron scale.28 Both treatments yielded improvement in scarring, but the PRP-treated side showed shorter durations of postprocedure erythema (P=.0052) as well as higher patient satisfaction scores (P<.001) than laser therapy alone.33

In another split-face study, Gawdat et al32 examined combination treatment with the ablative fractional CO2 laser and PRP in 30 participants with atrophic acne scars graded 2 to 4 on the qualitative Goodman and Baron scale.28 Participants were randomized to 2 different treatment groups: In group 1, half of the face was treated with the fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and intradermal saline. In group 2, half of the face was treated with fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and topical PRP. All patients underwent 3 treatment sessions at 1-month intervals with assessment occurring a 6-month follow-up using the qualitative Goodman and Baron Scale.28 In all participants, areas treated with the combined laser and PRP showed significant improvement in scarring (P=.03) and reduced recovery time (P=.02) compared to areas treated with laser therapy only. Patients receiving intradermal or topical PRP showed no statistically significant differences in improvement of scarring or recovery time; however, areas treated with topical PRP had significantly lower pain levels (P=.005).32
Lee et al31 conducted a split-face study of 14 patients with moderate to severe acne scarring treated with an ablative fractional CO2 laser followed by intradermal PRP or intradermal normal saline injections. Patients underwent 2 treatment sessions at 4-week intervals. Photographs taken at baseline and 4 months posttreatment were evaluated by 2 blinded dermatologists for clinical improvement using a quartile grading system. Erythema was assessed using a skin color measuring device. A blinded dermatologist assessed patients for adverse events. At 4-month follow-up, mean (SD) clinical improvement on the side receiving intradermal PRP was significantly better than the control side (2.7 [0.7] vs 2.3 [0.5]; P=.03). Erythema on posttreatment day 4 was significantly less on the side treated with PRP (P=.01). No adverse events were reported.31
Another split-face study compared the use of intradermal PRP to intradermal normal saline following fractional CO2 laser treatment.34 Twenty-five participants with moderate to severe acne scars completed 2 treatment sessions at 4-week intervals. Additionally, skin biopsies were collected to evaluate collagen production using immunohistochemistry, quantitative polymerase chain reaction, and western blot techniques. Experimental fibroblasts and keratinocytes were isolated and cultured. The cultures were irradiated with a fractional CO2 laser and treated with PRP or platelet-poor plasma. Cultures were evaluated at 30 minutes, 24 hours, and 48 hours. Participants reported 75% improvement of acne scarring from baseline in the side treated with PRP compared to 50% improvement of acne scarring from baseline in the control group (P<.001). On days 7 and 84, participants reported greater improvement on the side treated with PRP (P=.03 and P=.02, respectively). On day 28, skin biopsy evaluation yielded higher levels of TGF-β1 (P=.02), TGF-β3 (P=.004), c-myc (P=.004), type I collagen (P=.03), and type III collagen (P=.03) on the PRP-treated side compared to the control side. Transforming growth factor β increases collagen and fibroblast production, while c-myc leads to cell cycle progression.35-37 Similarly, TGF-β1, TGF-β3, types I andIII collagen, and p-Akt were increased in all cultures treated with PRP and platelet-poor plasma in a dose-dependent manner.34 p-Akt is thought to regulate wound healing38; however, PRP-treated keratinocytes yielded increased epidermal growth factor receptor and decreased keratin-16 at 48 hours, which suggests PRP plays a role in increasing epithelization and reducing laser-induced keratinocyte damage.39 Adverse effects (eg, erythema, edema, oozing) were less frequent in the PRP-treated side.34
Chemical Peels
Chemical peels are widely used in the treatment of acne scarring.40 Peels improve scarring through destruction of the epidermal and/or dermal layers, leading to skin exfoliation, rejuvenation, and remodeling. Superficial peeling agents, which extend to the dermoepidermal junction, include resorcinol, tretinoin, glycolic acid, lactic acid, salicylic acid, and trichloroacetic acid (TCA) 10% to 35%.41 Medium-depth peeling agents extend to the upper reticular dermis and include phenol, TCA 35% to 50%, and Jessner solution (resorcinol, lactic acid, and salicylic acid in ethanol) followed by TCA 35%.41 Finally, the effects of deep peeling agents reach the mid reticular dermis and include the Baker-Gordon or Litton phenol formulas.41 Deep peels are associated with higher rates of adverse outcomes including infection, dyschromia, and scarring.41,42
An RCT was performed to evaluate the use of a deep phenol 60% peel compared to microneedling with a 1.5-mm roller device plus a TCA 20% peel in the treatment of atrophic acne scars.43 Twenty-four patients were randomly and evenly assigned to both treatment groups. The phenol group underwent a single treatment session, while the microneedling plus TCA group underwent 4 treatment sessions at 6-week intervals. Both groups were instructed to use daily topical tretinoin and hydroquinone 2% in the 2 weeks prior to treatment. Posttreatment results were evaluated using a quartile grading scale. Scarring improved from baseline by 75.12% (P<.001) in the phenol group and 69.43% (P<.001) in the microneedling plus TCA group, with no significant difference between groups. Adverse effects in the phenol group included erythema and hyperpigmentation, while adverse events in the microneedling plus TCA group included transient pain, edema, erythema, and desquamation.43
Another study compared the use of a TCA 15% peel with microneedling to PRP with microneedling and microneedling alone in the treatment of atrophic acne scars.44 Twenty-four patients were randomly assigned to the 3 treatment groups (8 to each group) and underwent 6 treatment sessions with 2-week intervals. A roller device with a 1.5-mm needle was used for microneedling. Microneedling plus TCA and microneedling plus PRP were significantly more effective than microneedling alone (P=.011 and P=.015, respectively); however, the TCA 15% peel with microneedling resulted in the largest increase in epidermal thickening. The investigators concluded that combined use of a TCA 15% peel and microneedling was the most effective in treating atrophic acne scarring.44
Dermal Fillers
Dermal or subcutaneous fillers are used to increase volume in depressed scars and stimulate the skin’s natural production.45 Tissue augmentation methods commonly are used for larger rolling acne scars. Options for filler materials include autologous fat, bovine, or human collagen derivatives; hyaluronic acid; and polymethyl methacrylate microspheres with collagen.45 Newer fillers are formulated with lidocaine to decrease pain associated with the procedure.46 Hyaluronic acid fillers provide natural volume correction and have limited potential to elicit an immune response due to their derivation from bacterial fermentation. Fillers using polymethyl methacrylate microspheres with collagen are permanent and effective, which may lead to reduced patient costs; however, they often are not a first choice for treatment.45,46 Furthermore, if dermal fillers consist of bovine collagen, it is necessary to perform skin testing for allergy prior to use. Autologous fat transfer also has become popular for treatment of acne scarring, especially because there is no risk of allergic reaction, as the patient’s own fat is used for correction.46 However, this method requires a high degree of skill, and results are unpredictable, generally lasting from 6 months to several years.
Therapies on the horizon include autologous cell therapy. A multicenter, double-blinded, placebo-controlled RCT examined the use of an autologous fibroblast filler in the treatment of bilateral, depressed, and distensible acne scars that were graded as moderate to severe.47 Autologous fat fibroblasts were harvested from full-thickness postauricular punch biopsies. In this split-face study, 99 participants were treated with an intradermal autologous fibroblast filler on one cheek and a protein-free cell-culture medium on the contralateral cheek. Participants received an average of 5.9 mL of both autologous fat fibroblasts and cell-culture medium over 3 treatment sessions at 2-week intervals. The autologous fat fibroblasts were associated with greater improvement compared to cell-culture medium based on participant (43% vs 18%), evaluator (59% vs 42%), and independent photographic viewer’s assessment.47
Conclusion
Acne scarring is a burden affecting millions of Americans. It often has a negative impact on quality of life and can lead to low self-esteem in patients. Numerous trials have indicated that microneedling is beneficial in the treatment of acne scarring, and emerging evidence indicates that the addition of PRP provides measurable benefits. Similarly, the addition of PRP to laser therapy may reduce recovery time as well as the commonly associated adverse events of erythema and pain. Chemical peels provide the advantage of being easily and efficiently performed in the office setting. Finally, the wide range of available dermal fillers can be tailored to treat specific types of acne scars. Autologous dermal fillers recently have been used and show promising benefits. It is important to consider desired outcome, cost, and adverse events when discussing therapeutic options for acne scarring with patients. The numerous therapeutic options warrant further research and well-designed RCTs to ensure optimal patient outcomes.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2, pt 3):S34-S37.
- Yazici K, Baz K, Yazici AE, et al. Disease-specific quality of life is associated with anxiety and depression in patients with acne. J Eur Acad Dermatol Venereol. 2004;18:435-439.
- Orentreich DS, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatol Surg. 1995;21:543-549.
- Fabbrocini G, De Padova M, De Vita V, et al. Periorbital wrinkles treatment using collagen induction therapy. Surg Cosmet Dermatol. 2009;1:106-111.
- Fabbrocini G, De Vita V, Pastore F, et al. Collagen induction therapy for the treatment of upper lip wrinkles. J Dermatol Treat. 2012;23:144-152.
- Fabbrocini G, De Vita V, Di Costanzo L, et al. Skin needling in the treatment of the aging neck. Skinmed. 2011;9:347-351.
- El-Domyati M, Barakat M, Awad S, et al. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8:36-42.
- Fabbrocini G, Fardella N, Monfrecola A, et al. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34:874-879.
- Alam M, Han S, Pongprutthipan M, et al. Efficacy of a needling device for the treatment of acne scars: a randomized clinical trial. JAMA Dermatol. 2014;150:844-849.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
- Dhurat R, Mathapati S. Response to microneedling treatment in men with androgenetic alopecia who failed to respond to conventional therapy. Indian J Dermatol. 2015;60:260-263.
- Fabbrocini G, De Vita V, Fardella N, et al. Skin needling to enhance depigmenting serum penetration in the treatment of melasma [published online April 7, 2011]. Plast Surg Int. 2011;2011:158241.
- Bariya SH, Gohel MC, Mehta TA, et al. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol. 2012;64:11-29.
- Fabbrocini G, De Vita V, Izzo R, et al. The use of skin needling for the delivery of a eutectic mixture of local anesthetics. G Ital Dermatol Venereol. 2014;149:581-585.
- De Vita V. How to choose among the multiple options to enhance the penetration of topically applied methyl aminolevulinate prior to photodynamic therapy [published online February 22, 2018]. Photodiagnosis Photodyn Ther. doi:10.1016/j.pdpdt.2018.02.014.
- Fernandes D. Minimally invasive percutaneous collagen induction. Oral Maxillofac Surg Clin North Am. 2005;17:51-63.
- Goodman GJ, Baron JA. Postacne scarring—a quantitative global scarring grading system. J Cosmet Dermatol. 2006;5:48-52.
- Majid I. Microneedling therapy in atrophic facial scars: an objective assessment. J Cutan Aesthet Surg. 2009;2:26-30.
- Dogra S, Yadav S, Sarangal R. Microneedling for acne scars in Asian skin type: an effective low cost treatment modality. J Cosmet Dermatol. 2014;13:180-187.
- Fabbrocini G, De Vita V, Monfrecola A, et al. Percutaneous collagen induction: an effective and safe treatment for post-acne scarring in different skin phototypes. J Dermatol Treat. 2014;25:147-152.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
- Wang HL, Avila G. Platelet rich plasma: myth or reality? Eur J Dent. 2007;1:192-194.
- Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489-496.
- Fabbrocini G, De Vita V, Pastore F, et al. Combined use of skin needling and platelet-rich plasma in acne scarring treatment. Cosmet Dermatol. 2011;24:177-183.
- Chawla S. Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg. 2014;7:209-212.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Ibrahim MK, Ibrahim SM, Salem AM. Skin microneedling plus platelet-rich plasma versus skin microneedling alone in the treatment of atrophic post acne scars: a split face comparative study. J Dermatolog Treat. 2018;29:281-286.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458-1466.
- You H, Kim D, Yoon E, et al. Comparison of four different lasers for acne scars: resurfacing and fractional lasers. J Plast Reconstr Aesthet Surg. 2016;69:E87-E95.
- Osman MA, Shokeir HA, Fawzy MM. Fractional erbium-doped yttrium aluminum garnet laser versus microneedling in treatment of atrophic acne scars: a randomized split-face clinical study. Dermatol Surg. 2017;43(suppl 1):S47-S56.
- Lee JW, Kim BJ, Kim MN, et al. The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: a simultaneous split-face trial. Dermatol Surg. 2011;37:931-938.
- Gawdat HI, Hegazy RA, Fawzy MM, et al. Autologous platelet rich plasma: topical versus intradermal after fractional ablative carbon dioxide laser treatment of atrophic acne scars. Dermatol Surg. 2014;40:152-161.
- Abdel Aal AM, Ibrahim IM, Sami NA, et al. Evaluation of autologous platelet rich plasma plus ablative carbon dioxide fractional laser in the treatment of acne scars. J Cosmet Laser Ther. 2018;20:106-113.
- Min S, Yoon JY, Park SY, et al. Combination of platelet rich plasma in fractional carbon dioxide laser treatment increased clinical efficacy of for acne scar by enhancement of collagen production and modulation of laser-induced inflammation. Lasers Surg Med. 2018;50:302-310.
- Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986;83:4167-4171.
- Schmidt EV. The role of c-myc in cellular growth control. Oncogene. 1999;18:2988-2996.
- Varga J, Rosenbloom J, Jimenez SA. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J. 1987;247:597-604.
- Chen J, Somanath PR, Razorenova O, et al. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188-1196.
- Repertinger SK, Campagnaro E, Fuhrman J, et al. EGFR enhances early healing after cutaneous incisional wounding. J Invest Dermatol. 2004;123:982-989.
- Landau M. Chemical peels. Clin Dermatol. 2008;26:200-208.
- Drake LA, Dinehart SM, Goltz RW, et al. Guidelines of care for chemical peeling. J Am Acad Dermatol. 1995;33:497-503.
- Meaike JD, Agrawal N, Chang D, et al. Noninvasive facial rejuvenation. part 3: physician-directed-lasers, chemical peels, and other noninvasive modalities. Semin Plast Surg. 2016;30:143-150.
- Leheta TM, Abdel Hay RM, El Garem YF. Deep peeling using phenol versus percutaneous collagen induction combined with trichloroacetic acid 20% in atrophic post-acne scars; a randomized controlled trial.J Dermatol Treat. 2014;25:130-136.
- El-Domyati M, Abdel-Wahab H, Hossam A. Microneedling combined with platelet-rich plasma or trichloroacetic acid peeling for management of acne scarring: a split-face clinical and histologic comparison.J Cosmet Dermatol. 2018;17:73-83.
- Hession MT, Graber EM. Atrophic acne scarring: a review of treatment options. J Clin Aesthet Dermatol. 2015;8:50-58.
- Dayan SH, Bassichis BA. Facial dermal fillers: selection of appropriate products and techniques. Aesthet Surg J. 2008;28:335-347.
- Munavalli GS, Smith S, Maslowski JM, et al. Successful treatment of depressed, distensible acne scars using autologous fibroblasts: a multi-site, prospective, double blind, placebo-controlled clinical trial. Dermatol Surg. 2013;39:1226-1236.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2, pt 3):S34-S37.
- Yazici K, Baz K, Yazici AE, et al. Disease-specific quality of life is associated with anxiety and depression in patients with acne. J Eur Acad Dermatol Venereol. 2004;18:435-439.
- Orentreich DS, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatol Surg. 1995;21:543-549.
- Fabbrocini G, De Padova M, De Vita V, et al. Periorbital wrinkles treatment using collagen induction therapy. Surg Cosmet Dermatol. 2009;1:106-111.
- Fabbrocini G, De Vita V, Pastore F, et al. Collagen induction therapy for the treatment of upper lip wrinkles. J Dermatol Treat. 2012;23:144-152.
- Fabbrocini G, De Vita V, Di Costanzo L, et al. Skin needling in the treatment of the aging neck. Skinmed. 2011;9:347-351.
- El-Domyati M, Barakat M, Awad S, et al. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8:36-42.
- Fabbrocini G, Fardella N, Monfrecola A, et al. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34:874-879.
- Alam M, Han S, Pongprutthipan M, et al. Efficacy of a needling device for the treatment of acne scars: a randomized clinical trial. JAMA Dermatol. 2014;150:844-849.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
- Dhurat R, Mathapati S. Response to microneedling treatment in men with androgenetic alopecia who failed to respond to conventional therapy. Indian J Dermatol. 2015;60:260-263.
- Fabbrocini G, De Vita V, Fardella N, et al. Skin needling to enhance depigmenting serum penetration in the treatment of melasma [published online April 7, 2011]. Plast Surg Int. 2011;2011:158241.
- Bariya SH, Gohel MC, Mehta TA, et al. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol. 2012;64:11-29.
- Fabbrocini G, De Vita V, Izzo R, et al. The use of skin needling for the delivery of a eutectic mixture of local anesthetics. G Ital Dermatol Venereol. 2014;149:581-585.
- De Vita V. How to choose among the multiple options to enhance the penetration of topically applied methyl aminolevulinate prior to photodynamic therapy [published online February 22, 2018]. Photodiagnosis Photodyn Ther. doi:10.1016/j.pdpdt.2018.02.014.
- Fernandes D. Minimally invasive percutaneous collagen induction. Oral Maxillofac Surg Clin North Am. 2005;17:51-63.
- Goodman GJ, Baron JA. Postacne scarring—a quantitative global scarring grading system. J Cosmet Dermatol. 2006;5:48-52.
- Majid I. Microneedling therapy in atrophic facial scars: an objective assessment. J Cutan Aesthet Surg. 2009;2:26-30.
- Dogra S, Yadav S, Sarangal R. Microneedling for acne scars in Asian skin type: an effective low cost treatment modality. J Cosmet Dermatol. 2014;13:180-187.
- Fabbrocini G, De Vita V, Monfrecola A, et al. Percutaneous collagen induction: an effective and safe treatment for post-acne scarring in different skin phototypes. J Dermatol Treat. 2014;25:147-152.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
- Wang HL, Avila G. Platelet rich plasma: myth or reality? Eur J Dent. 2007;1:192-194.
- Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489-496.
- Fabbrocini G, De Vita V, Pastore F, et al. Combined use of skin needling and platelet-rich plasma in acne scarring treatment. Cosmet Dermatol. 2011;24:177-183.
- Chawla S. Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg. 2014;7:209-212.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Ibrahim MK, Ibrahim SM, Salem AM. Skin microneedling plus platelet-rich plasma versus skin microneedling alone in the treatment of atrophic post acne scars: a split face comparative study. J Dermatolog Treat. 2018;29:281-286.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458-1466.
- You H, Kim D, Yoon E, et al. Comparison of four different lasers for acne scars: resurfacing and fractional lasers. J Plast Reconstr Aesthet Surg. 2016;69:E87-E95.
- Osman MA, Shokeir HA, Fawzy MM. Fractional erbium-doped yttrium aluminum garnet laser versus microneedling in treatment of atrophic acne scars: a randomized split-face clinical study. Dermatol Surg. 2017;43(suppl 1):S47-S56.
- Lee JW, Kim BJ, Kim MN, et al. The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: a simultaneous split-face trial. Dermatol Surg. 2011;37:931-938.
- Gawdat HI, Hegazy RA, Fawzy MM, et al. Autologous platelet rich plasma: topical versus intradermal after fractional ablative carbon dioxide laser treatment of atrophic acne scars. Dermatol Surg. 2014;40:152-161.
- Abdel Aal AM, Ibrahim IM, Sami NA, et al. Evaluation of autologous platelet rich plasma plus ablative carbon dioxide fractional laser in the treatment of acne scars. J Cosmet Laser Ther. 2018;20:106-113.
- Min S, Yoon JY, Park SY, et al. Combination of platelet rich plasma in fractional carbon dioxide laser treatment increased clinical efficacy of for acne scar by enhancement of collagen production and modulation of laser-induced inflammation. Lasers Surg Med. 2018;50:302-310.
- Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986;83:4167-4171.
- Schmidt EV. The role of c-myc in cellular growth control. Oncogene. 1999;18:2988-2996.
- Varga J, Rosenbloom J, Jimenez SA. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J. 1987;247:597-604.
- Chen J, Somanath PR, Razorenova O, et al. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188-1196.
- Repertinger SK, Campagnaro E, Fuhrman J, et al. EGFR enhances early healing after cutaneous incisional wounding. J Invest Dermatol. 2004;123:982-989.
- Landau M. Chemical peels. Clin Dermatol. 2008;26:200-208.
- Drake LA, Dinehart SM, Goltz RW, et al. Guidelines of care for chemical peeling. J Am Acad Dermatol. 1995;33:497-503.
- Meaike JD, Agrawal N, Chang D, et al. Noninvasive facial rejuvenation. part 3: physician-directed-lasers, chemical peels, and other noninvasive modalities. Semin Plast Surg. 2016;30:143-150.
- Leheta TM, Abdel Hay RM, El Garem YF. Deep peeling using phenol versus percutaneous collagen induction combined with trichloroacetic acid 20% in atrophic post-acne scars; a randomized controlled trial.J Dermatol Treat. 2014;25:130-136.
- El-Domyati M, Abdel-Wahab H, Hossam A. Microneedling combined with platelet-rich plasma or trichloroacetic acid peeling for management of acne scarring: a split-face clinical and histologic comparison.J Cosmet Dermatol. 2018;17:73-83.
- Hession MT, Graber EM. Atrophic acne scarring: a review of treatment options. J Clin Aesthet Dermatol. 2015;8:50-58.
- Dayan SH, Bassichis BA. Facial dermal fillers: selection of appropriate products and techniques. Aesthet Surg J. 2008;28:335-347.
- Munavalli GS, Smith S, Maslowski JM, et al. Successful treatment of depressed, distensible acne scars using autologous fibroblasts: a multi-site, prospective, double blind, placebo-controlled clinical trial. Dermatol Surg. 2013;39:1226-1236.
Practice Points
- Acne scarring affects millions of Americans and can lead to poor psychological sequelae such as low self-esteem.
- Multiple modalities for acne scarring treatment exist including microneedling, lasers, chemical peels, and dermal fillers.
- Consider patient-desired outcome, cost, and adverse events when choosing a specific treatment modality.
Buckwheat Extract
Native to North and East Asia, This highly adaptable plant – the most common species of which are Fagopyrum esculentum (common buckwheat or sweet buckwheat), and F. tataricum (which grows in more mountainous regions) – has acclimated to cultivation in North America, as well.1 Increasingly popular as a healthy grain option, buckwheat flour has been touted for beneficial effects on diabetes, obesity, hypertension, hypercholesterolemia, and constipation.1 It has also gained attention for its association with some allergic reactions.
Wound Healing
In 2008, van den Berg et al. performed an in vitro investigation of the antioxidant and anti-inflammatory qualities of buckwheat honey for consideration in wound healing. American buckwheat honey from New York was found to be the source of the most salient activities, with such properties attributed to its abundant phenolic components. The researchers suggested that these phenols might impart antibacterial activity, while the low pH and high free acid content of the buckwheat honey could contribute to healing wounds.4
Antioxidant Activity
The antioxidant capacity, along with other traits, characterizing the sprouts of common buckwheat (F. esculentum) and tartary buckwheat (F. tataricum) was evaluated by Liu et al. in 2008. Rutin is the main flavonoid found in both species, with fivefold higher levels identified in tartary buckwheat in this study. Ethanol extracts of tartary buckwheat also exhibited greater free radical scavenging activity and superoxide scavenging activity, compared with common buckwheat. Both buckwheat species displayed antioxidant activity on human hepatoma HepG2 cells, with tartary buckwheat more effective in diminishing cellular oxidative stress, which the authors attributed to its greater rutin and quercetin levels.5
Zhou et al. studied the protective effects of buckwheat honey on hydroxyl radical-induced DNA damage in 2012, finding that all studied honeys more effectively protected DNA in non–site specific rather than site-specific systems.6
Photoprotection
In a 2005 screening of 47 antioxidant substances and study of their effects on UV-induced lipid peroxidation, Trommer and Neubert reported that buckwheat extract significantly lowered radiation levels, as did extracts of St. John’s Wort, melissa, and sage. They concluded that their in vitro findings supported the inclusion of such ingredients in photoprotective cosmetic formulations or sunscreens pending the results of in vivo experiments with these compounds.7
In 2006, Hinneburg et al. evaluated the antioxidant and photoprotective activity of a buckwheat herb extract, also comparing its photoprotective characteristics to those of a commercial UV absorber. In an assay with 1,1-diphenyl-2-picryl-hydrazyl radical (DPPH), buckwheat extract exhibited significantly more antioxidant activity than did pure rutin, with buckwheat observed to more effectively block UV-induced peroxidation of linoleic acid as compared with rutin and the commercial UV absorber. The researchers concluded that including antioxidants such as buckwheat extract in photoprotective formulations may serve to maximize skin protection in such products.8
Buckwheat Sensitivity
Conclusion
Because it is a popular component in many diets around the world, especially Japan, Korea, Russia, and Poland, as well as other Asian and European countries, South Africa, Australia, and North America,4 it is reasonable to expect that we’ll see more research on buckwheat. For now, there are indications to suggest that more investigations are warranted to determine whether this botanical agent will have a meaningful role in the dermatologic armamentarium.
References
1. Li SQ et al. Crit Rev Food Sci Nutr. 2001 Sep;41(6):451-64.
2. Dattner AM. Dermatol Ther. 2003;16(2):106-13.
3. Hinneburg I et al. J Agric Food Chem. 2005 Jan 12;53(1):3-7.
4. van den Berg AJ et al. J Wound Care. 2008 Apr;17(4):172-4, 176-8.
5. Liu CL et al. J Agric Food Chem. 2008 Jan 9;56(1):173-8.
6. Zhou J et al. Food Chem Toxicol. 2012 Aug;50(8):2766-73.
7. Trommer H et al. J Pharm Pharm Sci. 2005 Sep 15;8(3):494-506.
8. Hinneburg I et al. Pharmazie. 2006 Mar;61(3):237-40.
9. Geiselhart S et al. Clin Exp Allergy. 2018 Feb;48(2):217-24.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also authored a New York Times Best Seller for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance Therapeutics. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
Native to North and East Asia, This highly adaptable plant – the most common species of which are Fagopyrum esculentum (common buckwheat or sweet buckwheat), and F. tataricum (which grows in more mountainous regions) – has acclimated to cultivation in North America, as well.1 Increasingly popular as a healthy grain option, buckwheat flour has been touted for beneficial effects on diabetes, obesity, hypertension, hypercholesterolemia, and constipation.1 It has also gained attention for its association with some allergic reactions.
Wound Healing
In 2008, van den Berg et al. performed an in vitro investigation of the antioxidant and anti-inflammatory qualities of buckwheat honey for consideration in wound healing. American buckwheat honey from New York was found to be the source of the most salient activities, with such properties attributed to its abundant phenolic components. The researchers suggested that these phenols might impart antibacterial activity, while the low pH and high free acid content of the buckwheat honey could contribute to healing wounds.4
Antioxidant Activity
The antioxidant capacity, along with other traits, characterizing the sprouts of common buckwheat (F. esculentum) and tartary buckwheat (F. tataricum) was evaluated by Liu et al. in 2008. Rutin is the main flavonoid found in both species, with fivefold higher levels identified in tartary buckwheat in this study. Ethanol extracts of tartary buckwheat also exhibited greater free radical scavenging activity and superoxide scavenging activity, compared with common buckwheat. Both buckwheat species displayed antioxidant activity on human hepatoma HepG2 cells, with tartary buckwheat more effective in diminishing cellular oxidative stress, which the authors attributed to its greater rutin and quercetin levels.5
Zhou et al. studied the protective effects of buckwheat honey on hydroxyl radical-induced DNA damage in 2012, finding that all studied honeys more effectively protected DNA in non–site specific rather than site-specific systems.6
Photoprotection
In a 2005 screening of 47 antioxidant substances and study of their effects on UV-induced lipid peroxidation, Trommer and Neubert reported that buckwheat extract significantly lowered radiation levels, as did extracts of St. John’s Wort, melissa, and sage. They concluded that their in vitro findings supported the inclusion of such ingredients in photoprotective cosmetic formulations or sunscreens pending the results of in vivo experiments with these compounds.7
In 2006, Hinneburg et al. evaluated the antioxidant and photoprotective activity of a buckwheat herb extract, also comparing its photoprotective characteristics to those of a commercial UV absorber. In an assay with 1,1-diphenyl-2-picryl-hydrazyl radical (DPPH), buckwheat extract exhibited significantly more antioxidant activity than did pure rutin, with buckwheat observed to more effectively block UV-induced peroxidation of linoleic acid as compared with rutin and the commercial UV absorber. The researchers concluded that including antioxidants such as buckwheat extract in photoprotective formulations may serve to maximize skin protection in such products.8
Buckwheat Sensitivity
Conclusion
Because it is a popular component in many diets around the world, especially Japan, Korea, Russia, and Poland, as well as other Asian and European countries, South Africa, Australia, and North America,4 it is reasonable to expect that we’ll see more research on buckwheat. For now, there are indications to suggest that more investigations are warranted to determine whether this botanical agent will have a meaningful role in the dermatologic armamentarium.
References
1. Li SQ et al. Crit Rev Food Sci Nutr. 2001 Sep;41(6):451-64.
2. Dattner AM. Dermatol Ther. 2003;16(2):106-13.
3. Hinneburg I et al. J Agric Food Chem. 2005 Jan 12;53(1):3-7.
4. van den Berg AJ et al. J Wound Care. 2008 Apr;17(4):172-4, 176-8.
5. Liu CL et al. J Agric Food Chem. 2008 Jan 9;56(1):173-8.
6. Zhou J et al. Food Chem Toxicol. 2012 Aug;50(8):2766-73.
7. Trommer H et al. J Pharm Pharm Sci. 2005 Sep 15;8(3):494-506.
8. Hinneburg I et al. Pharmazie. 2006 Mar;61(3):237-40.
9. Geiselhart S et al. Clin Exp Allergy. 2018 Feb;48(2):217-24.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also authored a New York Times Best Seller for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance Therapeutics. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
Native to North and East Asia, This highly adaptable plant – the most common species of which are Fagopyrum esculentum (common buckwheat or sweet buckwheat), and F. tataricum (which grows in more mountainous regions) – has acclimated to cultivation in North America, as well.1 Increasingly popular as a healthy grain option, buckwheat flour has been touted for beneficial effects on diabetes, obesity, hypertension, hypercholesterolemia, and constipation.1 It has also gained attention for its association with some allergic reactions.
Wound Healing
In 2008, van den Berg et al. performed an in vitro investigation of the antioxidant and anti-inflammatory qualities of buckwheat honey for consideration in wound healing. American buckwheat honey from New York was found to be the source of the most salient activities, with such properties attributed to its abundant phenolic components. The researchers suggested that these phenols might impart antibacterial activity, while the low pH and high free acid content of the buckwheat honey could contribute to healing wounds.4
Antioxidant Activity
The antioxidant capacity, along with other traits, characterizing the sprouts of common buckwheat (F. esculentum) and tartary buckwheat (F. tataricum) was evaluated by Liu et al. in 2008. Rutin is the main flavonoid found in both species, with fivefold higher levels identified in tartary buckwheat in this study. Ethanol extracts of tartary buckwheat also exhibited greater free radical scavenging activity and superoxide scavenging activity, compared with common buckwheat. Both buckwheat species displayed antioxidant activity on human hepatoma HepG2 cells, with tartary buckwheat more effective in diminishing cellular oxidative stress, which the authors attributed to its greater rutin and quercetin levels.5
Zhou et al. studied the protective effects of buckwheat honey on hydroxyl radical-induced DNA damage in 2012, finding that all studied honeys more effectively protected DNA in non–site specific rather than site-specific systems.6
Photoprotection
In a 2005 screening of 47 antioxidant substances and study of their effects on UV-induced lipid peroxidation, Trommer and Neubert reported that buckwheat extract significantly lowered radiation levels, as did extracts of St. John’s Wort, melissa, and sage. They concluded that their in vitro findings supported the inclusion of such ingredients in photoprotective cosmetic formulations or sunscreens pending the results of in vivo experiments with these compounds.7
In 2006, Hinneburg et al. evaluated the antioxidant and photoprotective activity of a buckwheat herb extract, also comparing its photoprotective characteristics to those of a commercial UV absorber. In an assay with 1,1-diphenyl-2-picryl-hydrazyl radical (DPPH), buckwheat extract exhibited significantly more antioxidant activity than did pure rutin, with buckwheat observed to more effectively block UV-induced peroxidation of linoleic acid as compared with rutin and the commercial UV absorber. The researchers concluded that including antioxidants such as buckwheat extract in photoprotective formulations may serve to maximize skin protection in such products.8
Buckwheat Sensitivity
Conclusion
Because it is a popular component in many diets around the world, especially Japan, Korea, Russia, and Poland, as well as other Asian and European countries, South Africa, Australia, and North America,4 it is reasonable to expect that we’ll see more research on buckwheat. For now, there are indications to suggest that more investigations are warranted to determine whether this botanical agent will have a meaningful role in the dermatologic armamentarium.
References
1. Li SQ et al. Crit Rev Food Sci Nutr. 2001 Sep;41(6):451-64.
2. Dattner AM. Dermatol Ther. 2003;16(2):106-13.
3. Hinneburg I et al. J Agric Food Chem. 2005 Jan 12;53(1):3-7.
4. van den Berg AJ et al. J Wound Care. 2008 Apr;17(4):172-4, 176-8.
5. Liu CL et al. J Agric Food Chem. 2008 Jan 9;56(1):173-8.
6. Zhou J et al. Food Chem Toxicol. 2012 Aug;50(8):2766-73.
7. Trommer H et al. J Pharm Pharm Sci. 2005 Sep 15;8(3):494-506.
8. Hinneburg I et al. Pharmazie. 2006 Mar;61(3):237-40.
9. Geiselhart S et al. Clin Exp Allergy. 2018 Feb;48(2):217-24.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also authored a New York Times Best Seller for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance Therapeutics. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
The magic of microblading
The use of permanent cosmetics dates back thousands of years in history. and has rapidly become one of the most popular cosmetic procedures in the United States. However, it has not completely replaced traditional eyebrow micropigmentation techniques: Many people may not be candidates for microblading because of how the pigment is manually deposited in the skin through tiny “tears” in the skin with this procedure.
The use of microblading has increased exponentially since 2015, as reflected by the millions of searches on popular social media sites. With the increase in the popularity and volume of tattoo artists performing these procedures, there has also been an increase in side effects and complications from microblading provided by poorly trained and unlicensed “artists,” a problem facilitated by the absence of adequate training requirements and/or regulatory oversight in many states.
Microblading is a revolutionary technique that can transform the lives of patients with hypotrichosis of the eyebrows, trichotillomania, eyebrow loss due to internal disease (such as thyroid disease), chemotherapy-induced eyebrow loss, or alopecia – or simply those seeking it for cosmetic improvement. The art of shaping the eyebrow depends on the natural growth of the brow (if any), facial symmetry, and meticulous measurement and mapping of the brow position based on facial landmarks and bone structure. The color of pigment selection is based on Fitzpatrick skin type and skin color undertones.
While dermatologists usually do not perform microblading, we may see patients with these complications. Practitioners treating patients who have had eyebrow microblading should also be aware of how to prevent premature fading of the eyebrow tattoo pigment. Tattooed eyebrows should be covered with petroleum jelly prior to the use of alpha hydroxy acids, vitamin C, chemical peels, hydroquinone, or retinols because these preparations can fade the pigment rapidly even if applied far from the microblading site. Any UV exposure, heat (such as steam from a facial), LED light exposure, or radio frequency can fade the pigment and exacerbate postinflammatory hyperpigmentation. Patients who have a history of hypertrophic scarring or keloids or are using isotretinoin concurrently should avoid microblading entirely. Resurfacing lasers and intense pulsed-light lasers should be used with caution as these aesthetic procedures will cause fading of the eyebrow pigment even if applied at a considerable distance from the eyebrow. Microbladed eyebrows should be covered with 20% zinc oxide paste prior to the use of any intense pulsed-light or resurfacing lasers.
The pigment used in eyebrow colors also may be composed of a mixture of iron oxide pigments, which should not be removed with traditional Q-switched lasers, with which not only is there potential for the pigment to darken but also postinflammatory hyper- or hypopigmentation to occur as well. Hairs can be singed, and the light absorbed by the pigment chromophore in the hair follicle can permanently damage the follicle, leading to hair loss in the area.
Despite the absolute precision and aggressive safety precautions needed for microblading, there are wide state-to-state variations in training and regulatory oversight. Infectious diseases, poor treatment outcomes, and unsterile conditions are just a few of the horrific consequences of unlicensed and untrained tattoo artists. Regulations should be imposed in every state to protect consumers and prevent serious medical complications related to microblading.
Like other cosmetic treatments, cheaper is never better.
Dr. Talakoub and Dr. Wesley and are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected]. This column was written with the help and professional expertise of Emily Joy, a cosmetic tattoo artist and the founder of Dollistic in McLean.
The use of permanent cosmetics dates back thousands of years in history. and has rapidly become one of the most popular cosmetic procedures in the United States. However, it has not completely replaced traditional eyebrow micropigmentation techniques: Many people may not be candidates for microblading because of how the pigment is manually deposited in the skin through tiny “tears” in the skin with this procedure.
The use of microblading has increased exponentially since 2015, as reflected by the millions of searches on popular social media sites. With the increase in the popularity and volume of tattoo artists performing these procedures, there has also been an increase in side effects and complications from microblading provided by poorly trained and unlicensed “artists,” a problem facilitated by the absence of adequate training requirements and/or regulatory oversight in many states.
Microblading is a revolutionary technique that can transform the lives of patients with hypotrichosis of the eyebrows, trichotillomania, eyebrow loss due to internal disease (such as thyroid disease), chemotherapy-induced eyebrow loss, or alopecia – or simply those seeking it for cosmetic improvement. The art of shaping the eyebrow depends on the natural growth of the brow (if any), facial symmetry, and meticulous measurement and mapping of the brow position based on facial landmarks and bone structure. The color of pigment selection is based on Fitzpatrick skin type and skin color undertones.
While dermatologists usually do not perform microblading, we may see patients with these complications. Practitioners treating patients who have had eyebrow microblading should also be aware of how to prevent premature fading of the eyebrow tattoo pigment. Tattooed eyebrows should be covered with petroleum jelly prior to the use of alpha hydroxy acids, vitamin C, chemical peels, hydroquinone, or retinols because these preparations can fade the pigment rapidly even if applied far from the microblading site. Any UV exposure, heat (such as steam from a facial), LED light exposure, or radio frequency can fade the pigment and exacerbate postinflammatory hyperpigmentation. Patients who have a history of hypertrophic scarring or keloids or are using isotretinoin concurrently should avoid microblading entirely. Resurfacing lasers and intense pulsed-light lasers should be used with caution as these aesthetic procedures will cause fading of the eyebrow pigment even if applied at a considerable distance from the eyebrow. Microbladed eyebrows should be covered with 20% zinc oxide paste prior to the use of any intense pulsed-light or resurfacing lasers.
The pigment used in eyebrow colors also may be composed of a mixture of iron oxide pigments, which should not be removed with traditional Q-switched lasers, with which not only is there potential for the pigment to darken but also postinflammatory hyper- or hypopigmentation to occur as well. Hairs can be singed, and the light absorbed by the pigment chromophore in the hair follicle can permanently damage the follicle, leading to hair loss in the area.
Despite the absolute precision and aggressive safety precautions needed for microblading, there are wide state-to-state variations in training and regulatory oversight. Infectious diseases, poor treatment outcomes, and unsterile conditions are just a few of the horrific consequences of unlicensed and untrained tattoo artists. Regulations should be imposed in every state to protect consumers and prevent serious medical complications related to microblading.
Like other cosmetic treatments, cheaper is never better.
Dr. Talakoub and Dr. Wesley and are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected]. This column was written with the help and professional expertise of Emily Joy, a cosmetic tattoo artist and the founder of Dollistic in McLean.
The use of permanent cosmetics dates back thousands of years in history. and has rapidly become one of the most popular cosmetic procedures in the United States. However, it has not completely replaced traditional eyebrow micropigmentation techniques: Many people may not be candidates for microblading because of how the pigment is manually deposited in the skin through tiny “tears” in the skin with this procedure.
The use of microblading has increased exponentially since 2015, as reflected by the millions of searches on popular social media sites. With the increase in the popularity and volume of tattoo artists performing these procedures, there has also been an increase in side effects and complications from microblading provided by poorly trained and unlicensed “artists,” a problem facilitated by the absence of adequate training requirements and/or regulatory oversight in many states.
Microblading is a revolutionary technique that can transform the lives of patients with hypotrichosis of the eyebrows, trichotillomania, eyebrow loss due to internal disease (such as thyroid disease), chemotherapy-induced eyebrow loss, or alopecia – or simply those seeking it for cosmetic improvement. The art of shaping the eyebrow depends on the natural growth of the brow (if any), facial symmetry, and meticulous measurement and mapping of the brow position based on facial landmarks and bone structure. The color of pigment selection is based on Fitzpatrick skin type and skin color undertones.
While dermatologists usually do not perform microblading, we may see patients with these complications. Practitioners treating patients who have had eyebrow microblading should also be aware of how to prevent premature fading of the eyebrow tattoo pigment. Tattooed eyebrows should be covered with petroleum jelly prior to the use of alpha hydroxy acids, vitamin C, chemical peels, hydroquinone, or retinols because these preparations can fade the pigment rapidly even if applied far from the microblading site. Any UV exposure, heat (such as steam from a facial), LED light exposure, or radio frequency can fade the pigment and exacerbate postinflammatory hyperpigmentation. Patients who have a history of hypertrophic scarring or keloids or are using isotretinoin concurrently should avoid microblading entirely. Resurfacing lasers and intense pulsed-light lasers should be used with caution as these aesthetic procedures will cause fading of the eyebrow pigment even if applied at a considerable distance from the eyebrow. Microbladed eyebrows should be covered with 20% zinc oxide paste prior to the use of any intense pulsed-light or resurfacing lasers.
The pigment used in eyebrow colors also may be composed of a mixture of iron oxide pigments, which should not be removed with traditional Q-switched lasers, with which not only is there potential for the pigment to darken but also postinflammatory hyper- or hypopigmentation to occur as well. Hairs can be singed, and the light absorbed by the pigment chromophore in the hair follicle can permanently damage the follicle, leading to hair loss in the area.
Despite the absolute precision and aggressive safety precautions needed for microblading, there are wide state-to-state variations in training and regulatory oversight. Infectious diseases, poor treatment outcomes, and unsterile conditions are just a few of the horrific consequences of unlicensed and untrained tattoo artists. Regulations should be imposed in every state to protect consumers and prevent serious medical complications related to microblading.
Like other cosmetic treatments, cheaper is never better.
Dr. Talakoub and Dr. Wesley and are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected]. This column was written with the help and professional expertise of Emily Joy, a cosmetic tattoo artist and the founder of Dollistic in McLean.
Cosmetic procedures show continued growth
and two declining, according to the American Society of Plastic Surgeons.
The gainers were the three most popular procedures: OnabotulinumtoxinA injections topped the list with 7.23 million anatomic sites injected – an increase of 2% over 2016 – followed by injection of soft tissue fillers with 2.69 million procedures (up 3%) and chemical peels with 1.38 million procedures (an increase of 1%), the ASPS said in its 2017 Plastic Surgery Statistics Report.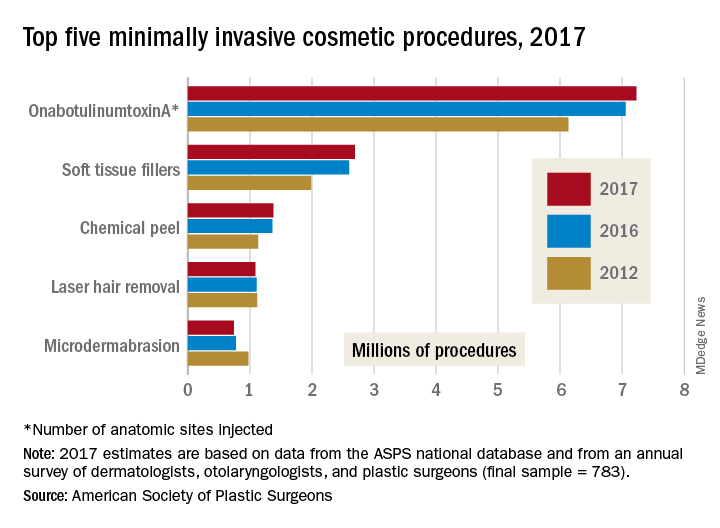
The two decliners among the top five were laser hair removal, which dropped 2% to 1.09 million procedures, and microdermabrasion, which continued a long-term decline by falling 4% to 740,000 procedures in 2017, the ASPS reported.
The minimally invasive cosmetic sector as a whole was up by 2% last year, bringing the number of total procedures to 15.7 million. Cosmetic surgical procedures were up by 1% from 2016 to 2017, reaching a total of 1.79 million. The five most popular cosmetic surgeries were breast augmentation (300,000 performed), liposuction (246,000), rhinoplasty (219,000), blepharoplasty (210,000), and abdominoplasty (130,000), according to the ASPS Tracking Operations and Outcomes for Plastic Surgeons database and an annual survey of board-certified dermatologists, otolaryngologists, and plastic surgeons (final sample = 783).
and two declining, according to the American Society of Plastic Surgeons.
The gainers were the three most popular procedures: OnabotulinumtoxinA injections topped the list with 7.23 million anatomic sites injected – an increase of 2% over 2016 – followed by injection of soft tissue fillers with 2.69 million procedures (up 3%) and chemical peels with 1.38 million procedures (an increase of 1%), the ASPS said in its 2017 Plastic Surgery Statistics Report.
The two decliners among the top five were laser hair removal, which dropped 2% to 1.09 million procedures, and microdermabrasion, which continued a long-term decline by falling 4% to 740,000 procedures in 2017, the ASPS reported.
The minimally invasive cosmetic sector as a whole was up by 2% last year, bringing the number of total procedures to 15.7 million. Cosmetic surgical procedures were up by 1% from 2016 to 2017, reaching a total of 1.79 million. The five most popular cosmetic surgeries were breast augmentation (300,000 performed), liposuction (246,000), rhinoplasty (219,000), blepharoplasty (210,000), and abdominoplasty (130,000), according to the ASPS Tracking Operations and Outcomes for Plastic Surgeons database and an annual survey of board-certified dermatologists, otolaryngologists, and plastic surgeons (final sample = 783).
and two declining, according to the American Society of Plastic Surgeons.
The gainers were the three most popular procedures: OnabotulinumtoxinA injections topped the list with 7.23 million anatomic sites injected – an increase of 2% over 2016 – followed by injection of soft tissue fillers with 2.69 million procedures (up 3%) and chemical peels with 1.38 million procedures (an increase of 1%), the ASPS said in its 2017 Plastic Surgery Statistics Report.
The two decliners among the top five were laser hair removal, which dropped 2% to 1.09 million procedures, and microdermabrasion, which continued a long-term decline by falling 4% to 740,000 procedures in 2017, the ASPS reported.
The minimally invasive cosmetic sector as a whole was up by 2% last year, bringing the number of total procedures to 15.7 million. Cosmetic surgical procedures were up by 1% from 2016 to 2017, reaching a total of 1.79 million. The five most popular cosmetic surgeries were breast augmentation (300,000 performed), liposuction (246,000), rhinoplasty (219,000), blepharoplasty (210,000), and abdominoplasty (130,000), according to the ASPS Tracking Operations and Outcomes for Plastic Surgeons database and an annual survey of board-certified dermatologists, otolaryngologists, and plastic surgeons (final sample = 783).