User login
Whole body cryotherapy
.
WBC has been purported to manage pain, reduce inflammation, and speed up recovery after injury, as well as relieve sore muscles after exercise, aid in weight loss, and improve mood. There have also been claims that it can treat acne, eczema, and psoriasis – even multiple sclerosis, fibromyalgia, and rheumatoid arthritis. It has been used as a beauty aid to reduce pore size and reduce wrinkles. Its popularity has exploded as centers are advertised on discount sites such as Groupon and Living Social, and it is now available as classes in the popular exercise app ClassPass as “Whole Body Cryotherapy” or a “CryoBeauty Facial.”
Despite these claims and popularity, research studies have yet to prove that WBC can deliver any of these benefits. The American Academy of Dermatology has released a statement for consumers that does not support its use. Because of the lack of research, WBC has not been cleared by the Food and Drug Administration for treating any medical indication.
Its popularity has to stem from somewhere. In many cultures, cold therapy has been used for health benefits for centuries. For example, Turkish, Russian, Finnish, Roman, and Chinese spas offer cold baths at 50 degrees Fahrenheit after heat therapy (saunas, baths) as a form of hydrotherapy to alter circulation for health benefits, with the goal of releasing toxins with heat, then closing pores and bringing the circulation back to the body’s core with cold therapy. Cold ice baths and ice packs are used by athletes routinely after games, practices, and injuries to reduce inflammation. How is WBC different?
With WBC, a person who is nearly nude enters a cold chamber of minus 200 degrees Fahrenheit, in sessions that typically last 2-4 minutes.
While the majority of those who engage in WBC have not had complications, the AAD statement refers to a Finnish study that found that 16% of individuals exposed to WBC had mild frostbite. In 2011, U.S. sprinter and Olympic gold medalist Justin Gatlin developed frostbite on both feet after a WBC session. Additional WBC-related complications that have been reported include a frozen limb (a frozen arm in a woman in Dallas in 2013, after a 3-minute session, manifesting as painful swelling, blisters, and third-degree burns – a more severe type of frostbite), and cold panniculitis (JAAD Case Rep. 2018;4:344-5). Others include eye injuries, temporary loss of memory, and even death due to suffocation, reported in 2015, of a staff member at a cryotherapy center outside of Las Vegas who went into a tank alone after hours when no one else was around.
Cryotherapy, when delivered to specific areas of the skin by a dermatologist, is a useful low-risk treatment. Postinflammatory pigment alteration can occur, but there has been great success in using the treatment locally for warts, actinic keratoses, and other benign skin growths, when it is done done by trained professionals. Granted, while localized cryotherapy to treat a skin growth is not the same as whole body cryotherapy, the same types of complication risks should be considered, including postinflammatory pigment alteration, particularly in skin of color, as cryotherapy can be toxic to melanocytes.
Before it is completely discounted, if it makes the person feel good or better, perhaps if the patient and practitioner are aware of the risks and how to identify and manage them, cold therapy could be useful. I once had a patient who described great relief with WBC after a Fraxel laser treatment, when her face felt like it was “on fire” despite refrigerated topical Biafine, cold air, and ice packs. As with most treatments, if someone feels better, they often look better.
While medical or aesthetic benefits of WBC have not been proved and WBC has definite risks, if the procedure is done in an appropriate and responsible way, perhaps the benefit could outweigh the informed risks for some patients. Claims should not be advertised until they are proven, so that patients are not misinformed. The same is true of chemical peels, microneedling, hyperbaric oxygen, and vitamin drips, which are provided over the counter in nonmedical settings for health and beauty uses. Medical history should be taken into account with WBC by the facility and the practitioner, including history of blood clots, smoking, vasculitis, Raynaud’s disease, autoimmune conditions, neuropathy, and prior history of frostbite. Perhaps these should be contraindications to WBC and mechanisms should be in place to manage complications should they occur. Better regulation of WBC is needed so that the procedure can be done effectively and safely.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
.
WBC has been purported to manage pain, reduce inflammation, and speed up recovery after injury, as well as relieve sore muscles after exercise, aid in weight loss, and improve mood. There have also been claims that it can treat acne, eczema, and psoriasis – even multiple sclerosis, fibromyalgia, and rheumatoid arthritis. It has been used as a beauty aid to reduce pore size and reduce wrinkles. Its popularity has exploded as centers are advertised on discount sites such as Groupon and Living Social, and it is now available as classes in the popular exercise app ClassPass as “Whole Body Cryotherapy” or a “CryoBeauty Facial.”
Despite these claims and popularity, research studies have yet to prove that WBC can deliver any of these benefits. The American Academy of Dermatology has released a statement for consumers that does not support its use. Because of the lack of research, WBC has not been cleared by the Food and Drug Administration for treating any medical indication.
Its popularity has to stem from somewhere. In many cultures, cold therapy has been used for health benefits for centuries. For example, Turkish, Russian, Finnish, Roman, and Chinese spas offer cold baths at 50 degrees Fahrenheit after heat therapy (saunas, baths) as a form of hydrotherapy to alter circulation for health benefits, with the goal of releasing toxins with heat, then closing pores and bringing the circulation back to the body’s core with cold therapy. Cold ice baths and ice packs are used by athletes routinely after games, practices, and injuries to reduce inflammation. How is WBC different?
With WBC, a person who is nearly nude enters a cold chamber of minus 200 degrees Fahrenheit, in sessions that typically last 2-4 minutes.
While the majority of those who engage in WBC have not had complications, the AAD statement refers to a Finnish study that found that 16% of individuals exposed to WBC had mild frostbite. In 2011, U.S. sprinter and Olympic gold medalist Justin Gatlin developed frostbite on both feet after a WBC session. Additional WBC-related complications that have been reported include a frozen limb (a frozen arm in a woman in Dallas in 2013, after a 3-minute session, manifesting as painful swelling, blisters, and third-degree burns – a more severe type of frostbite), and cold panniculitis (JAAD Case Rep. 2018;4:344-5). Others include eye injuries, temporary loss of memory, and even death due to suffocation, reported in 2015, of a staff member at a cryotherapy center outside of Las Vegas who went into a tank alone after hours when no one else was around.
Cryotherapy, when delivered to specific areas of the skin by a dermatologist, is a useful low-risk treatment. Postinflammatory pigment alteration can occur, but there has been great success in using the treatment locally for warts, actinic keratoses, and other benign skin growths, when it is done done by trained professionals. Granted, while localized cryotherapy to treat a skin growth is not the same as whole body cryotherapy, the same types of complication risks should be considered, including postinflammatory pigment alteration, particularly in skin of color, as cryotherapy can be toxic to melanocytes.
Before it is completely discounted, if it makes the person feel good or better, perhaps if the patient and practitioner are aware of the risks and how to identify and manage them, cold therapy could be useful. I once had a patient who described great relief with WBC after a Fraxel laser treatment, when her face felt like it was “on fire” despite refrigerated topical Biafine, cold air, and ice packs. As with most treatments, if someone feels better, they often look better.
While medical or aesthetic benefits of WBC have not been proved and WBC has definite risks, if the procedure is done in an appropriate and responsible way, perhaps the benefit could outweigh the informed risks for some patients. Claims should not be advertised until they are proven, so that patients are not misinformed. The same is true of chemical peels, microneedling, hyperbaric oxygen, and vitamin drips, which are provided over the counter in nonmedical settings for health and beauty uses. Medical history should be taken into account with WBC by the facility and the practitioner, including history of blood clots, smoking, vasculitis, Raynaud’s disease, autoimmune conditions, neuropathy, and prior history of frostbite. Perhaps these should be contraindications to WBC and mechanisms should be in place to manage complications should they occur. Better regulation of WBC is needed so that the procedure can be done effectively and safely.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
.
WBC has been purported to manage pain, reduce inflammation, and speed up recovery after injury, as well as relieve sore muscles after exercise, aid in weight loss, and improve mood. There have also been claims that it can treat acne, eczema, and psoriasis – even multiple sclerosis, fibromyalgia, and rheumatoid arthritis. It has been used as a beauty aid to reduce pore size and reduce wrinkles. Its popularity has exploded as centers are advertised on discount sites such as Groupon and Living Social, and it is now available as classes in the popular exercise app ClassPass as “Whole Body Cryotherapy” or a “CryoBeauty Facial.”
Despite these claims and popularity, research studies have yet to prove that WBC can deliver any of these benefits. The American Academy of Dermatology has released a statement for consumers that does not support its use. Because of the lack of research, WBC has not been cleared by the Food and Drug Administration for treating any medical indication.
Its popularity has to stem from somewhere. In many cultures, cold therapy has been used for health benefits for centuries. For example, Turkish, Russian, Finnish, Roman, and Chinese spas offer cold baths at 50 degrees Fahrenheit after heat therapy (saunas, baths) as a form of hydrotherapy to alter circulation for health benefits, with the goal of releasing toxins with heat, then closing pores and bringing the circulation back to the body’s core with cold therapy. Cold ice baths and ice packs are used by athletes routinely after games, practices, and injuries to reduce inflammation. How is WBC different?
With WBC, a person who is nearly nude enters a cold chamber of minus 200 degrees Fahrenheit, in sessions that typically last 2-4 minutes.
While the majority of those who engage in WBC have not had complications, the AAD statement refers to a Finnish study that found that 16% of individuals exposed to WBC had mild frostbite. In 2011, U.S. sprinter and Olympic gold medalist Justin Gatlin developed frostbite on both feet after a WBC session. Additional WBC-related complications that have been reported include a frozen limb (a frozen arm in a woman in Dallas in 2013, after a 3-minute session, manifesting as painful swelling, blisters, and third-degree burns – a more severe type of frostbite), and cold panniculitis (JAAD Case Rep. 2018;4:344-5). Others include eye injuries, temporary loss of memory, and even death due to suffocation, reported in 2015, of a staff member at a cryotherapy center outside of Las Vegas who went into a tank alone after hours when no one else was around.
Cryotherapy, when delivered to specific areas of the skin by a dermatologist, is a useful low-risk treatment. Postinflammatory pigment alteration can occur, but there has been great success in using the treatment locally for warts, actinic keratoses, and other benign skin growths, when it is done done by trained professionals. Granted, while localized cryotherapy to treat a skin growth is not the same as whole body cryotherapy, the same types of complication risks should be considered, including postinflammatory pigment alteration, particularly in skin of color, as cryotherapy can be toxic to melanocytes.
Before it is completely discounted, if it makes the person feel good or better, perhaps if the patient and practitioner are aware of the risks and how to identify and manage them, cold therapy could be useful. I once had a patient who described great relief with WBC after a Fraxel laser treatment, when her face felt like it was “on fire” despite refrigerated topical Biafine, cold air, and ice packs. As with most treatments, if someone feels better, they often look better.
While medical or aesthetic benefits of WBC have not been proved and WBC has definite risks, if the procedure is done in an appropriate and responsible way, perhaps the benefit could outweigh the informed risks for some patients. Claims should not be advertised until they are proven, so that patients are not misinformed. The same is true of chemical peels, microneedling, hyperbaric oxygen, and vitamin drips, which are provided over the counter in nonmedical settings for health and beauty uses. Medical history should be taken into account with WBC by the facility and the practitioner, including history of blood clots, smoking, vasculitis, Raynaud’s disease, autoimmune conditions, neuropathy, and prior history of frostbite. Perhaps these should be contraindications to WBC and mechanisms should be in place to manage complications should they occur. Better regulation of WBC is needed so that the procedure can be done effectively and safely.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Laser treatment tips for pigmented lesions
SAN DIEGO –
Victor Ross, MD, turns to the Q-switched alexandrite laser as his device of choice for most pigmented lesions. “I also use the Q-switched 1,064 nm Nd:YAG and sometimes use the Q-switched 532 nm Nd:YAG, particularly for lighter-skinned patients with lighter lesions,” he said at the annual Masters of Aesthetics Symposium.
Compared with long-pulsed devices, the Q-switched 532 nm neodymium:YAG laser is better for one-time pigment reduction and better for treating lighter pigmented spots, yet it’s associated with a higher risk of postinflammatory hyperpigmentation and short-term crusting. “The Q-switched 532 nm Nd:YAG laser will even treat very tight lentigines, but vascular effects tend to cause an immediate bright red color and more postinflammatory hyperpigmentation,” said Dr. Ross, director of laser and cosmetic dermatology at the Scripps Clinic in San Diego. He cautioned that the Q-switched 532 nm Nd:YAG laser may cause prolonged redness on the legs and arms of some patients. “This laser is best reserved for lighter skinned patients with very light lentigines – the brisk purpura can prove distasteful short term for cosmetic patients,” he said. “For darker lentigines, I prefer the IPL [intense pulse light], KTP [potassium titanyl phosphate] laser, or Q-switched alexandrite lasers.”
Meanwhile, treating pigmented lesions treated with long-pulse IPL, KTP, and pulsed dye lasers show less risk of postinflammatory hyperpigmentation and better coverage rates. However, they are sensitive to background color and are less likely to achieve complete one-time removal. The first treatment works the best because the “low hanging fruit” (darker lesions) will do well, he said.
For clinicians looking to improve their skills in treating pigmented lesions with lasers, Dr. Ross recommended using a skin meter such as Cynosure’s Skintel Melanin Reader, which measures the real-time pigment of skin. “You measure the pigment, and it gives you a reading,” he said. “It gives you a recommended setting based on the hand piece and the pulse duration.”
Melasma remains a difficult condition to treat with laser and light. In fact, Dr. Ross joked that he wouldn’t mind if the words “He cured melasma” graced his tombstone one day. “I have been treating melasma patients for 29 years now, and I’m not closer to a cure than when I started out,” he said. “I’ve tried lots of things. In my defense, I’ve made more people better than worse.”
His approach to treating melasma is to begin with a KTP laser or a gentle IPL if discrete lesions or telangiectasia are present. Next, he applies hydroquinone followed by a series of treatment sessions with the Q-switched Nd:YAG laser or a conservative fractional laser. “This tends to induce remission, but is associated with a high rate of relapse,” he said.
Dr. Ross disclosed having research and financial ties to numerous pharmaceutical and device companies.
SAN DIEGO –
Victor Ross, MD, turns to the Q-switched alexandrite laser as his device of choice for most pigmented lesions. “I also use the Q-switched 1,064 nm Nd:YAG and sometimes use the Q-switched 532 nm Nd:YAG, particularly for lighter-skinned patients with lighter lesions,” he said at the annual Masters of Aesthetics Symposium.
Compared with long-pulsed devices, the Q-switched 532 nm neodymium:YAG laser is better for one-time pigment reduction and better for treating lighter pigmented spots, yet it’s associated with a higher risk of postinflammatory hyperpigmentation and short-term crusting. “The Q-switched 532 nm Nd:YAG laser will even treat very tight lentigines, but vascular effects tend to cause an immediate bright red color and more postinflammatory hyperpigmentation,” said Dr. Ross, director of laser and cosmetic dermatology at the Scripps Clinic in San Diego. He cautioned that the Q-switched 532 nm Nd:YAG laser may cause prolonged redness on the legs and arms of some patients. “This laser is best reserved for lighter skinned patients with very light lentigines – the brisk purpura can prove distasteful short term for cosmetic patients,” he said. “For darker lentigines, I prefer the IPL [intense pulse light], KTP [potassium titanyl phosphate] laser, or Q-switched alexandrite lasers.”
Meanwhile, treating pigmented lesions treated with long-pulse IPL, KTP, and pulsed dye lasers show less risk of postinflammatory hyperpigmentation and better coverage rates. However, they are sensitive to background color and are less likely to achieve complete one-time removal. The first treatment works the best because the “low hanging fruit” (darker lesions) will do well, he said.
For clinicians looking to improve their skills in treating pigmented lesions with lasers, Dr. Ross recommended using a skin meter such as Cynosure’s Skintel Melanin Reader, which measures the real-time pigment of skin. “You measure the pigment, and it gives you a reading,” he said. “It gives you a recommended setting based on the hand piece and the pulse duration.”
Melasma remains a difficult condition to treat with laser and light. In fact, Dr. Ross joked that he wouldn’t mind if the words “He cured melasma” graced his tombstone one day. “I have been treating melasma patients for 29 years now, and I’m not closer to a cure than when I started out,” he said. “I’ve tried lots of things. In my defense, I’ve made more people better than worse.”
His approach to treating melasma is to begin with a KTP laser or a gentle IPL if discrete lesions or telangiectasia are present. Next, he applies hydroquinone followed by a series of treatment sessions with the Q-switched Nd:YAG laser or a conservative fractional laser. “This tends to induce remission, but is associated with a high rate of relapse,” he said.
Dr. Ross disclosed having research and financial ties to numerous pharmaceutical and device companies.
SAN DIEGO –
Victor Ross, MD, turns to the Q-switched alexandrite laser as his device of choice for most pigmented lesions. “I also use the Q-switched 1,064 nm Nd:YAG and sometimes use the Q-switched 532 nm Nd:YAG, particularly for lighter-skinned patients with lighter lesions,” he said at the annual Masters of Aesthetics Symposium.
Compared with long-pulsed devices, the Q-switched 532 nm neodymium:YAG laser is better for one-time pigment reduction and better for treating lighter pigmented spots, yet it’s associated with a higher risk of postinflammatory hyperpigmentation and short-term crusting. “The Q-switched 532 nm Nd:YAG laser will even treat very tight lentigines, but vascular effects tend to cause an immediate bright red color and more postinflammatory hyperpigmentation,” said Dr. Ross, director of laser and cosmetic dermatology at the Scripps Clinic in San Diego. He cautioned that the Q-switched 532 nm Nd:YAG laser may cause prolonged redness on the legs and arms of some patients. “This laser is best reserved for lighter skinned patients with very light lentigines – the brisk purpura can prove distasteful short term for cosmetic patients,” he said. “For darker lentigines, I prefer the IPL [intense pulse light], KTP [potassium titanyl phosphate] laser, or Q-switched alexandrite lasers.”
Meanwhile, treating pigmented lesions treated with long-pulse IPL, KTP, and pulsed dye lasers show less risk of postinflammatory hyperpigmentation and better coverage rates. However, they are sensitive to background color and are less likely to achieve complete one-time removal. The first treatment works the best because the “low hanging fruit” (darker lesions) will do well, he said.
For clinicians looking to improve their skills in treating pigmented lesions with lasers, Dr. Ross recommended using a skin meter such as Cynosure’s Skintel Melanin Reader, which measures the real-time pigment of skin. “You measure the pigment, and it gives you a reading,” he said. “It gives you a recommended setting based on the hand piece and the pulse duration.”
Melasma remains a difficult condition to treat with laser and light. In fact, Dr. Ross joked that he wouldn’t mind if the words “He cured melasma” graced his tombstone one day. “I have been treating melasma patients for 29 years now, and I’m not closer to a cure than when I started out,” he said. “I’ve tried lots of things. In my defense, I’ve made more people better than worse.”
His approach to treating melasma is to begin with a KTP laser or a gentle IPL if discrete lesions or telangiectasia are present. Next, he applies hydroquinone followed by a series of treatment sessions with the Q-switched Nd:YAG laser or a conservative fractional laser. “This tends to induce remission, but is associated with a high rate of relapse,” he said.
Dr. Ross disclosed having research and financial ties to numerous pharmaceutical and device companies.
EXPERT ANALYSIS FROM MOAS 2018
Sunscreens: Survey of the Cutis Editorial Board
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on sunscreens. Here’s what we found.
What sun protection factor (SPF) do you recommend for the majority of your patients?

Fifty percent of dermatologists we surveyed recommend SPF 30. SPF 50 was recommended by 26%, SPF 50+ by 21%, and SPF 15 by only 2%.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Half of our Editorial Board recommends sunscreen with SPF 30, with many recommending SPF 50 or higher. This trend toward sunscreens with higher SPF is consistent with a survey-based study with 97% of dermatologists stating they were comfortable recommending sunscreens with an SPF of 50 or higher and 83.3% stating that they believe that high SPF sunscreens provide an additional margin of safety (Farberg et al). These trends are supported by a randomized, double-blind, split-face clinical trial in which participants applied either SPF 50+ or SPF 100+ sunscreen after exposure to natural sunlight. The results showed that SPF 100+ sunscreen was remarkably more effective in protecting against sunburn than SPF 50+ sunscreen in actual use conditions (Williams et al).
Next page: Spray sunscreens
Which patient populations do you feel may benefit from spray sunscreens?

Two-thirds of dermatologists indicated that spray sunscreens may benefit patients traveling alone. Men with bald spots also may benefit (62%), as well as athletes, children, and older patients (57% each).
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
As dermatologists, we tell our patients that the best sunscreens are ones that are used consistently. Spray sunscreens are likely as effective as lotions (Ou-Yang et al). There has been a clear trend in consumer purchasing of spray sunscreens from 2011 to 2016 (Teplitz et al). Spray sunscreens may benefit those traveling alone, particularly for hard-to-reach areas.
Next page: Supplemental vitamin D
In patients who apply sunscreen regularly, do you recommend supplemental vitamin D3?

More than half (53%) of dermatologists recommend supplemental vitamin D3.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Because use of photoprotection results in decreased vitamin D levels in most individuals, it is good practice to recommend vitamin D supplementation in patients who are applying sunscreen regularly (Bogaczewicz et al).
Next page: Sunscreen compliance
What is the most often heard reason(s) for not using sunscreen in your patients?

Nearly three-quarters (72%) of dermatologists reported that patients do not use sunscreen because of cosmetic acceptance. Almost one-third (31%) said their patients prefer “natural” products. Price was a factor for 26%. Fewer dermatologists indicated risk of environmental damage (14%), allergy (12%), cancer induction (5%), and hormonal alteration (5%) were reasons patients are not compliant.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Cosmetic acceptance is paramount for patient compliance for sunscreen application. These results from our Editorial Board echo a study on sunscreen product performance and other determinants of consumer preferences, which cited “cosmetic elegance” as an important factor in choosing sunscreens (Xu et al). Dermatologists must stress to patients to find a sunscreen that they find acceptable in terms of vehicle and price to increase compliance.
Next page: Sunscreens in pregnant women
What sunscreens do you recommend to pregnant women and children?

Most dermatologists (86%) recommend physical blockers “chemical-free” only sunscreens to pregnant women and children.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
While absorption of sunscreen by human embryos is likely negligible, because there is limited data on sunscreen effects in embryos and children, it is reasonable to recommend physical blockers for pregnant women and children.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
As a dermatologist married to a pediatrician, I try to get my kids to embrace sun-protection strategies. For the little ones it’s hard, but as they have gotten older and been exposed to more derm journals sitting around with pretty graphic pictures, they seem to get on board, even when away at summer camp on their own. If only our patients knew what our kids do.—Joel L. Cohen, MD (Denver, Colorado)
The most important factor in getting patient compliance with sunscreen usage is “cosmetic acceptance.” If they or their children or their spouse don’t like the feel, they won’t use it.—Vincent A. DeLeo, MD (Los Angeles, California)
Not using photoprotection with sunscreen is like crossing a busy road without looking both ways first.—James Q. Del Rosso, DO (Las Vegas, Nevada)
I do not recommend spray sunscreens. At least half of the spray seems to go in the air rather than on the skin. And people often do not rub the spray into their skin well enough. Lotions are better!—Lawrence J. Green, MD (Washington, DC)
The most important factor in sunscreen is not SPF; educate patients on the important role vehicle and sweating play in the length of sun protection.—Orit Markowitz, MD (New York, New York)
Reapplying sunscreen in the appropriate amount is key to blocking the danger rays of the sun.—Vineet Mishra, MD (San Antonio, Texas)
A good sunscreen is the one you put on properly. Regardless of the formulation, make sure you apply the sunscreen evenly to all exposed skin and reapply according to directions on the container. Remember, a regular white T-shirt has minimal SPF 4-5. Either wear sun-protective clothing or wear sunscreen underneath!—Larisa Ravitskiy, MD (Gahanna, Ohio)
Sun protection and sunscreen application go hand-in-hand. We can still enjoy the outdoors without getting excessive UV exposure.—Anthony M. Rossi, MD (New York, New York)
Sunscreens are only part of sun protection. Make sure to reapply them regularly, try to avoid direct sun between about 10 AM and 2 PM if possible, and wear a hat with a wide brim (not a baseball cap, which, after all, is designed for catching baseballs, not sun protection).—Robert I. Rudolph, MD (Wyomissing, Pennsylvania)
Sunscreens keep you younger looking longer!—Richard K. Scher, MD (New York, New York)
The dentist says only floss the teeth you want to keep. I tell patients to only sun block the skin they want to keep.—Daniel M. Siegel, MD, MS (Brooklyn, New York)
The best sunscreen is the one that is used! If it's too greasy or drying, smells bad or stings, it won't be used. Stick to the one YOU like, but at least SPF 30 or better.—Stephen P. Stone, MD, (Springfield, Illinois)
Sunscreen can be a meaningful part of your sun-protection regimen used in conjunction with sun-protective clothing, sun safe behaviors, and a diet rich in natural antioxidants.—Michelle Tarbox, MD (Lubbock, Texas)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from August 2, 2018, to September 2, 2018. A total of 42 usable responses were received.
Bogaczewicz J, Karczmarewicz E, Pludowski P, et al. Requirement for vitamin D supplementation in patients using photoprotection: variations in vitamin D levels and bone formation markers. Int J Dermatol. 2016;55:e176-e183.
Farberg AS, Glazer AM, Rigel AC, et al. Dermatologists’ perceptions, recommendations, and use of sunscreen. JAMA Dermatol. 2017;153:99-101.
Ou-Yang H, Stanfield J, Cole C, et al. High-SPF sunscreens (SPF ≥ 70) may provide ultraviolet protection above minimal recommended levels by adequately compensating for lower sunscreen user application amounts. J Am Acad Dermatol. 2012;67:1220-1227.
Teplitz RW, Glazer AM, Svoboda RM, et al. Trends in US sunscreen formulations: impact of increasing spray usage. J Am Acad Dermatol. 2018;78:187-189.
Williams JD, Maitra P, Atillasoy E, et al. SPF 100+ sunscreen is more protective against sunburn than SPF 50+ in actual use: Results of a randomized, double-blind, split-face, natural sunlight exposure clinical trial. J Am Acad Dermatol. 2018;78:902.e2-910.e2.
Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on sunscreens. Here’s what we found.
What sun protection factor (SPF) do you recommend for the majority of your patients?

Fifty percent of dermatologists we surveyed recommend SPF 30. SPF 50 was recommended by 26%, SPF 50+ by 21%, and SPF 15 by only 2%.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Half of our Editorial Board recommends sunscreen with SPF 30, with many recommending SPF 50 or higher. This trend toward sunscreens with higher SPF is consistent with a survey-based study with 97% of dermatologists stating they were comfortable recommending sunscreens with an SPF of 50 or higher and 83.3% stating that they believe that high SPF sunscreens provide an additional margin of safety (Farberg et al). These trends are supported by a randomized, double-blind, split-face clinical trial in which participants applied either SPF 50+ or SPF 100+ sunscreen after exposure to natural sunlight. The results showed that SPF 100+ sunscreen was remarkably more effective in protecting against sunburn than SPF 50+ sunscreen in actual use conditions (Williams et al).
Next page: Spray sunscreens
Which patient populations do you feel may benefit from spray sunscreens?

Two-thirds of dermatologists indicated that spray sunscreens may benefit patients traveling alone. Men with bald spots also may benefit (62%), as well as athletes, children, and older patients (57% each).
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
As dermatologists, we tell our patients that the best sunscreens are ones that are used consistently. Spray sunscreens are likely as effective as lotions (Ou-Yang et al). There has been a clear trend in consumer purchasing of spray sunscreens from 2011 to 2016 (Teplitz et al). Spray sunscreens may benefit those traveling alone, particularly for hard-to-reach areas.
Next page: Supplemental vitamin D
In patients who apply sunscreen regularly, do you recommend supplemental vitamin D3?

More than half (53%) of dermatologists recommend supplemental vitamin D3.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Because use of photoprotection results in decreased vitamin D levels in most individuals, it is good practice to recommend vitamin D supplementation in patients who are applying sunscreen regularly (Bogaczewicz et al).
Next page: Sunscreen compliance
What is the most often heard reason(s) for not using sunscreen in your patients?

Nearly three-quarters (72%) of dermatologists reported that patients do not use sunscreen because of cosmetic acceptance. Almost one-third (31%) said their patients prefer “natural” products. Price was a factor for 26%. Fewer dermatologists indicated risk of environmental damage (14%), allergy (12%), cancer induction (5%), and hormonal alteration (5%) were reasons patients are not compliant.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Cosmetic acceptance is paramount for patient compliance for sunscreen application. These results from our Editorial Board echo a study on sunscreen product performance and other determinants of consumer preferences, which cited “cosmetic elegance” as an important factor in choosing sunscreens (Xu et al). Dermatologists must stress to patients to find a sunscreen that they find acceptable in terms of vehicle and price to increase compliance.
Next page: Sunscreens in pregnant women
What sunscreens do you recommend to pregnant women and children?

Most dermatologists (86%) recommend physical blockers “chemical-free” only sunscreens to pregnant women and children.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
While absorption of sunscreen by human embryos is likely negligible, because there is limited data on sunscreen effects in embryos and children, it is reasonable to recommend physical blockers for pregnant women and children.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
As a dermatologist married to a pediatrician, I try to get my kids to embrace sun-protection strategies. For the little ones it’s hard, but as they have gotten older and been exposed to more derm journals sitting around with pretty graphic pictures, they seem to get on board, even when away at summer camp on their own. If only our patients knew what our kids do.—Joel L. Cohen, MD (Denver, Colorado)
The most important factor in getting patient compliance with sunscreen usage is “cosmetic acceptance.” If they or their children or their spouse don’t like the feel, they won’t use it.—Vincent A. DeLeo, MD (Los Angeles, California)
Not using photoprotection with sunscreen is like crossing a busy road without looking both ways first.—James Q. Del Rosso, DO (Las Vegas, Nevada)
I do not recommend spray sunscreens. At least half of the spray seems to go in the air rather than on the skin. And people often do not rub the spray into their skin well enough. Lotions are better!—Lawrence J. Green, MD (Washington, DC)
The most important factor in sunscreen is not SPF; educate patients on the important role vehicle and sweating play in the length of sun protection.—Orit Markowitz, MD (New York, New York)
Reapplying sunscreen in the appropriate amount is key to blocking the danger rays of the sun.—Vineet Mishra, MD (San Antonio, Texas)
A good sunscreen is the one you put on properly. Regardless of the formulation, make sure you apply the sunscreen evenly to all exposed skin and reapply according to directions on the container. Remember, a regular white T-shirt has minimal SPF 4-5. Either wear sun-protective clothing or wear sunscreen underneath!—Larisa Ravitskiy, MD (Gahanna, Ohio)
Sun protection and sunscreen application go hand-in-hand. We can still enjoy the outdoors without getting excessive UV exposure.—Anthony M. Rossi, MD (New York, New York)
Sunscreens are only part of sun protection. Make sure to reapply them regularly, try to avoid direct sun between about 10 AM and 2 PM if possible, and wear a hat with a wide brim (not a baseball cap, which, after all, is designed for catching baseballs, not sun protection).—Robert I. Rudolph, MD (Wyomissing, Pennsylvania)
Sunscreens keep you younger looking longer!—Richard K. Scher, MD (New York, New York)
The dentist says only floss the teeth you want to keep. I tell patients to only sun block the skin they want to keep.—Daniel M. Siegel, MD, MS (Brooklyn, New York)
The best sunscreen is the one that is used! If it's too greasy or drying, smells bad or stings, it won't be used. Stick to the one YOU like, but at least SPF 30 or better.—Stephen P. Stone, MD, (Springfield, Illinois)
Sunscreen can be a meaningful part of your sun-protection regimen used in conjunction with sun-protective clothing, sun safe behaviors, and a diet rich in natural antioxidants.—Michelle Tarbox, MD (Lubbock, Texas)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from August 2, 2018, to September 2, 2018. A total of 42 usable responses were received.
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on sunscreens. Here’s what we found.
What sun protection factor (SPF) do you recommend for the majority of your patients?

Fifty percent of dermatologists we surveyed recommend SPF 30. SPF 50 was recommended by 26%, SPF 50+ by 21%, and SPF 15 by only 2%.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Half of our Editorial Board recommends sunscreen with SPF 30, with many recommending SPF 50 or higher. This trend toward sunscreens with higher SPF is consistent with a survey-based study with 97% of dermatologists stating they were comfortable recommending sunscreens with an SPF of 50 or higher and 83.3% stating that they believe that high SPF sunscreens provide an additional margin of safety (Farberg et al). These trends are supported by a randomized, double-blind, split-face clinical trial in which participants applied either SPF 50+ or SPF 100+ sunscreen after exposure to natural sunlight. The results showed that SPF 100+ sunscreen was remarkably more effective in protecting against sunburn than SPF 50+ sunscreen in actual use conditions (Williams et al).
Next page: Spray sunscreens
Which patient populations do you feel may benefit from spray sunscreens?

Two-thirds of dermatologists indicated that spray sunscreens may benefit patients traveling alone. Men with bald spots also may benefit (62%), as well as athletes, children, and older patients (57% each).
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
As dermatologists, we tell our patients that the best sunscreens are ones that are used consistently. Spray sunscreens are likely as effective as lotions (Ou-Yang et al). There has been a clear trend in consumer purchasing of spray sunscreens from 2011 to 2016 (Teplitz et al). Spray sunscreens may benefit those traveling alone, particularly for hard-to-reach areas.
Next page: Supplemental vitamin D
In patients who apply sunscreen regularly, do you recommend supplemental vitamin D3?

More than half (53%) of dermatologists recommend supplemental vitamin D3.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Because use of photoprotection results in decreased vitamin D levels in most individuals, it is good practice to recommend vitamin D supplementation in patients who are applying sunscreen regularly (Bogaczewicz et al).
Next page: Sunscreen compliance
What is the most often heard reason(s) for not using sunscreen in your patients?

Nearly three-quarters (72%) of dermatologists reported that patients do not use sunscreen because of cosmetic acceptance. Almost one-third (31%) said their patients prefer “natural” products. Price was a factor for 26%. Fewer dermatologists indicated risk of environmental damage (14%), allergy (12%), cancer induction (5%), and hormonal alteration (5%) were reasons patients are not compliant.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Cosmetic acceptance is paramount for patient compliance for sunscreen application. These results from our Editorial Board echo a study on sunscreen product performance and other determinants of consumer preferences, which cited “cosmetic elegance” as an important factor in choosing sunscreens (Xu et al). Dermatologists must stress to patients to find a sunscreen that they find acceptable in terms of vehicle and price to increase compliance.
Next page: Sunscreens in pregnant women
What sunscreens do you recommend to pregnant women and children?

Most dermatologists (86%) recommend physical blockers “chemical-free” only sunscreens to pregnant women and children.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
While absorption of sunscreen by human embryos is likely negligible, because there is limited data on sunscreen effects in embryos and children, it is reasonable to recommend physical blockers for pregnant women and children.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
As a dermatologist married to a pediatrician, I try to get my kids to embrace sun-protection strategies. For the little ones it’s hard, but as they have gotten older and been exposed to more derm journals sitting around with pretty graphic pictures, they seem to get on board, even when away at summer camp on their own. If only our patients knew what our kids do.—Joel L. Cohen, MD (Denver, Colorado)
The most important factor in getting patient compliance with sunscreen usage is “cosmetic acceptance.” If they or their children or their spouse don’t like the feel, they won’t use it.—Vincent A. DeLeo, MD (Los Angeles, California)
Not using photoprotection with sunscreen is like crossing a busy road without looking both ways first.—James Q. Del Rosso, DO (Las Vegas, Nevada)
I do not recommend spray sunscreens. At least half of the spray seems to go in the air rather than on the skin. And people often do not rub the spray into their skin well enough. Lotions are better!—Lawrence J. Green, MD (Washington, DC)
The most important factor in sunscreen is not SPF; educate patients on the important role vehicle and sweating play in the length of sun protection.—Orit Markowitz, MD (New York, New York)
Reapplying sunscreen in the appropriate amount is key to blocking the danger rays of the sun.—Vineet Mishra, MD (San Antonio, Texas)
A good sunscreen is the one you put on properly. Regardless of the formulation, make sure you apply the sunscreen evenly to all exposed skin and reapply according to directions on the container. Remember, a regular white T-shirt has minimal SPF 4-5. Either wear sun-protective clothing or wear sunscreen underneath!—Larisa Ravitskiy, MD (Gahanna, Ohio)
Sun protection and sunscreen application go hand-in-hand. We can still enjoy the outdoors without getting excessive UV exposure.—Anthony M. Rossi, MD (New York, New York)
Sunscreens are only part of sun protection. Make sure to reapply them regularly, try to avoid direct sun between about 10 AM and 2 PM if possible, and wear a hat with a wide brim (not a baseball cap, which, after all, is designed for catching baseballs, not sun protection).—Robert I. Rudolph, MD (Wyomissing, Pennsylvania)
Sunscreens keep you younger looking longer!—Richard K. Scher, MD (New York, New York)
The dentist says only floss the teeth you want to keep. I tell patients to only sun block the skin they want to keep.—Daniel M. Siegel, MD, MS (Brooklyn, New York)
The best sunscreen is the one that is used! If it's too greasy or drying, smells bad or stings, it won't be used. Stick to the one YOU like, but at least SPF 30 or better.—Stephen P. Stone, MD, (Springfield, Illinois)
Sunscreen can be a meaningful part of your sun-protection regimen used in conjunction with sun-protective clothing, sun safe behaviors, and a diet rich in natural antioxidants.—Michelle Tarbox, MD (Lubbock, Texas)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from August 2, 2018, to September 2, 2018. A total of 42 usable responses were received.
Bogaczewicz J, Karczmarewicz E, Pludowski P, et al. Requirement for vitamin D supplementation in patients using photoprotection: variations in vitamin D levels and bone formation markers. Int J Dermatol. 2016;55:e176-e183.
Farberg AS, Glazer AM, Rigel AC, et al. Dermatologists’ perceptions, recommendations, and use of sunscreen. JAMA Dermatol. 2017;153:99-101.
Ou-Yang H, Stanfield J, Cole C, et al. High-SPF sunscreens (SPF ≥ 70) may provide ultraviolet protection above minimal recommended levels by adequately compensating for lower sunscreen user application amounts. J Am Acad Dermatol. 2012;67:1220-1227.
Teplitz RW, Glazer AM, Svoboda RM, et al. Trends in US sunscreen formulations: impact of increasing spray usage. J Am Acad Dermatol. 2018;78:187-189.
Williams JD, Maitra P, Atillasoy E, et al. SPF 100+ sunscreen is more protective against sunburn than SPF 50+ in actual use: Results of a randomized, double-blind, split-face, natural sunlight exposure clinical trial. J Am Acad Dermatol. 2018;78:902.e2-910.e2.
Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
Bogaczewicz J, Karczmarewicz E, Pludowski P, et al. Requirement for vitamin D supplementation in patients using photoprotection: variations in vitamin D levels and bone formation markers. Int J Dermatol. 2016;55:e176-e183.
Farberg AS, Glazer AM, Rigel AC, et al. Dermatologists’ perceptions, recommendations, and use of sunscreen. JAMA Dermatol. 2017;153:99-101.
Ou-Yang H, Stanfield J, Cole C, et al. High-SPF sunscreens (SPF ≥ 70) may provide ultraviolet protection above minimal recommended levels by adequately compensating for lower sunscreen user application amounts. J Am Acad Dermatol. 2012;67:1220-1227.
Teplitz RW, Glazer AM, Svoboda RM, et al. Trends in US sunscreen formulations: impact of increasing spray usage. J Am Acad Dermatol. 2018;78:187-189.
Williams JD, Maitra P, Atillasoy E, et al. SPF 100+ sunscreen is more protective against sunburn than SPF 50+ in actual use: Results of a randomized, double-blind, split-face, natural sunlight exposure clinical trial. J Am Acad Dermatol. 2018;78:902.e2-910.e2.
Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
Noninvasive fat removal devices continue to gain popularity
SAN DIEGO – Noninvasive fat removal, such as laser treatment and cryolipolysis, is here to stay.
That’s what Mathew M. Avram, MD, JD, told attendees at the annual Masters of Aesthetics Symposium.
“It does not compare to liposuction, but many patients prefer these devices because there’s less downtime,” he said. “They don’t want pain. They don’t want time away from work.”
In the decade or so since the inception of the noninvasive body contouring field, noninvasive and minimally invasive devices have become far more popular than traditional liposuction, said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital in Boston. “It’s not even close. Among American Society for Dermatologic Surgery [ASDS] members, for every 1 liposuction, there are over 10 noninvasive body sculpting treatments.”
There were 408,000 body-sculpting procedures performed in 2017. More than half of those (208,000) were cryolipolysis, followed by use of radiofrequency (89,000), deoxycholic acid (46,000), laser lipolysis (25,000), “other” procedures (25,000), and tumescent liposuction (15,000), according to data from the ASDS.
“Each treatment has improved in efficacy with time,” Dr. Avram said.
Devices currently cleared by the Food and Drug Administration for the noninvasive removal of fat include ultrasound, lasers, low-level laser therapy, cryolipolysis, and radiofrequency. One of the most common is low-level laser therapy.
“There are several different devices on the market, but there’s no good histology to confirm mechanism of action, so you question what the efficacy is,” said Dr. Avram, who is also the immediate past president of the American Society for Laser Medicine and Surgery.
SculpSure
In the traditional laser domain, the one most commonly used for fat removal is SculpSure, an FDA-cleared hyperthermic 1,060-nm diode laser. It features four flat, nonsuction applicators, a contact cooling system, and it enables the user to treat more than one area in a 25-minute session. There is minimal absorption by melanin.
“You shouldn’t treat over tattoos, however, because if there’s black ink, the tattoo will absorb the wavelength,” Dr. Avram said. “It’s safe in all skin types and there are a variety of different configurations you can use to treat the desired area.”
Initially there is a 4-minute warm-up cycle to achieve the target temperature. Over the next 21 minutes, 1,060-nm energy is delivered via proprietary modulation, alternating between heat and cooling.
“There is definitely some pain with this device,” he said. “Whether or not it’s a relevant endpoint is not known at this point. But typically, if you’re destroying fat with heat there should be some pain. It’s relieved by a period of cooling. A submental fat treatment is now available. I have not personally used it, but it’s the same technology.”
CoolSculpting
Another popular technology is cryolipolysis (CoolSculpting), which was developed at Massachusetts General Hospital by R. Rox Anderson, MD, and Dieter Manstein, MD, PhD.
It’s FDA cleared for noninvasive fat removal and there have been more than 6 million cryolipolysis treatments performed around the world. The purported mechanism of action is selective crystallization of lipids and fat cells at temperatures below freezing. An inflammatory process results in fat reduction over 2-4 months.
“When it first started, cryolipolysis was designed to treat local areas like love handles in males,” Dr. Avram said. “Over time, applicators have been designed to treat different areas, most recently, one for the posterior upper arms and above the knees. There is now a larger, faster CoolSculpting applicator which results in a 35-minute treatment. It’s a little larger, there’s a little less pain, a lower temperature, and it’s a little bit more effective. This has been helpful in our practice in terms of getting more treatment cycles in a visit.”
Postprocedurally, massage may improve clinical results by mobilizing lipid crystals created from treatment.
Extracorporeal shock wave therapy
Another modality to consider is extracorporeal shock wave therapy, which is the application of mechanically generated external sound waves. “It’s not the same as ultrasound or focused ultrasound, and it’s FDA cleared for the treatment of cellulite,” Dr. Avram said.
EmSculpt
A newer innovation, known as High Intensity Focused Electromagnetic (HIFEM) technology (EmSculpt), induces 20,000 forced muscle contractions per session, which leads to supramaximal contractions that can’t be achieved through normal voluntary muscle action.
“The idea is that you’re getting hypertrophy of the muscle to get volumetric growth,” he said. “There’s believed to be a cascaded apoptotic effect, inducing apoptosis and fat disruption.”
Dr. Avram added that HIFEM is nonionizing, nonradiating, nonthermal, and it does not affect sensory nerves. “It’s designed to only stimulate motor neurons. Time will tell in terms of what the ultimate results are with that device.”
truSculpt
Some clinicians are using monopolar radiofrequency with truSculpt, the proprietary delivery of deep radiofrequency energy. This device increases fat temperature between 6 and 10 degrees Celsius with dual frequency at 1 and 2 MHz.
Published data show that 45 degrees Celsius sustained for 3 minutes resulted in a 60% loss of adipose tissue viability (Lasers Surg Med. 2009;41:745-50; Lasers Surg Med. 2010;42:361-70).
“The heat delivery induces cell apoptosis, leading to the removal of those cells by natural healing processes,” said Dr. Avram, who added that he has not used the device. “We need more clinical data to assess this as well.”
Selective photothermolysis
Another technology being evaluated for fat removal is selective photothermolysis, a concept developed at Massachusetts General Hospital in 1983. It extends the theory of selective photothermolysis to target the lipids that make up subcutaneous fat.
“The theory is that you must select a wavelength well absorbed by the target chromophore with a pulse duration shorter than the target’s thermal relaxation time,” Dr. Avram explained. “This produces selective, localized heating with focal destruction of the target with minimal damage to the surrounding tissue. It requires a deeply penetrating wavelength.”
Lipids are a tempting target “because they heat quickly and easily and they do not lose their heat easily to surrounding structures. You want to target fat selectively and confine thermal damage effectively,” he said.
Nearly 10 years ago, Dr. Avram and his associates evaluated the effects of noninvasive 1,210-nm laser exposure on the adipose tissue of 24 patients with skin types 1-5 (Laser Surg Med. 2009;41:401-7).
“The laser pulses were painful, which limited the efficacy,” he said.
The contact cooling device failed in some subjects, and two patients had bulla, but no scarring. Histologic evidence of laser-induced fat damage was observed in 89% of test sites at 4-7 weeks, but dermal damage was also seen.
“This was the first study to show histologic evidence of laser-induced damage to subcutaneous fat,” Dr. Avram said.
Development of selective photothermolysis technology fell off the wayside after the Great Recession of 2008, but it is still being evaluated at Massachusetts General Hospital and other centers.
To optimize the technology, Dr. Avram said that longer pulse durations may target larger volumes of fat. “Cooling is essential to protect the epidermis, as well as to control pain.”
Injectables
Injectables provide a new, minimally invasive means to achieve noninvasive fat removal, Dr. Avram noted.
“Many injectables of questionable efficacy and safety had been available internationally for years,” he said, but none had FDA clearance until 2015, when the agency gave ATX-101 (Kybella) the nod.
ATX-101 is a nonanimal-derived formulation of deoxycholic acid that causes preferential adipocytolysis. Data from a phase 3 trial presented at the 2014 American Society of Plastic Surgery and the American Society of Aesthetic Plastic Surgery meetings showed a statistically significant reduction in submental fat among subjects who received ATX-101, compared with placebo. It requires an average of 2-4 treatments.
“In my experience, it tends not to require that many, but based on MRI, as well as clinician and patient-reported outcomes, there are significant improvements in the visual impact of chin fat,” Dr. Avram said.
Most adverse events are mild to moderate in severity, primarily bruising, pain, and a sensation of numbness to the anesthesia. “They decrease in incidence and severity over successive treatments, and they infrequently lead to discontinuation of treatment,” he said.
For submental fat, clinicians can combine cryolipolysis and deoxycholic acid. “Here, the idea is to assess the amount of fat targeted for treatment,” Dr. Avram said. “If the fat fills the cryolipolysis cup, use cryolipolysis alone. If the fat does not fill the cup, inject deoxycholic acid for a more targeted treatment. If there is residual fat after cryolipolysis, consider treating more focally with deoxycholic acid.”
Both treatments can produce temporary marginal mandibular nerve injury. “It’s not common, but that’s something to include in your consent forms,” he said.
Dr. Avram reported that he has received consulting fees from Allergan, Merz Pharma, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis Biosystems, Invasix, and Zalea.
[email protected]
SAN DIEGO – Noninvasive fat removal, such as laser treatment and cryolipolysis, is here to stay.
That’s what Mathew M. Avram, MD, JD, told attendees at the annual Masters of Aesthetics Symposium.
“It does not compare to liposuction, but many patients prefer these devices because there’s less downtime,” he said. “They don’t want pain. They don’t want time away from work.”
In the decade or so since the inception of the noninvasive body contouring field, noninvasive and minimally invasive devices have become far more popular than traditional liposuction, said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital in Boston. “It’s not even close. Among American Society for Dermatologic Surgery [ASDS] members, for every 1 liposuction, there are over 10 noninvasive body sculpting treatments.”
There were 408,000 body-sculpting procedures performed in 2017. More than half of those (208,000) were cryolipolysis, followed by use of radiofrequency (89,000), deoxycholic acid (46,000), laser lipolysis (25,000), “other” procedures (25,000), and tumescent liposuction (15,000), according to data from the ASDS.
“Each treatment has improved in efficacy with time,” Dr. Avram said.
Devices currently cleared by the Food and Drug Administration for the noninvasive removal of fat include ultrasound, lasers, low-level laser therapy, cryolipolysis, and radiofrequency. One of the most common is low-level laser therapy.
“There are several different devices on the market, but there’s no good histology to confirm mechanism of action, so you question what the efficacy is,” said Dr. Avram, who is also the immediate past president of the American Society for Laser Medicine and Surgery.
SculpSure
In the traditional laser domain, the one most commonly used for fat removal is SculpSure, an FDA-cleared hyperthermic 1,060-nm diode laser. It features four flat, nonsuction applicators, a contact cooling system, and it enables the user to treat more than one area in a 25-minute session. There is minimal absorption by melanin.
“You shouldn’t treat over tattoos, however, because if there’s black ink, the tattoo will absorb the wavelength,” Dr. Avram said. “It’s safe in all skin types and there are a variety of different configurations you can use to treat the desired area.”
Initially there is a 4-minute warm-up cycle to achieve the target temperature. Over the next 21 minutes, 1,060-nm energy is delivered via proprietary modulation, alternating between heat and cooling.
“There is definitely some pain with this device,” he said. “Whether or not it’s a relevant endpoint is not known at this point. But typically, if you’re destroying fat with heat there should be some pain. It’s relieved by a period of cooling. A submental fat treatment is now available. I have not personally used it, but it’s the same technology.”
CoolSculpting
Another popular technology is cryolipolysis (CoolSculpting), which was developed at Massachusetts General Hospital by R. Rox Anderson, MD, and Dieter Manstein, MD, PhD.
It’s FDA cleared for noninvasive fat removal and there have been more than 6 million cryolipolysis treatments performed around the world. The purported mechanism of action is selective crystallization of lipids and fat cells at temperatures below freezing. An inflammatory process results in fat reduction over 2-4 months.
“When it first started, cryolipolysis was designed to treat local areas like love handles in males,” Dr. Avram said. “Over time, applicators have been designed to treat different areas, most recently, one for the posterior upper arms and above the knees. There is now a larger, faster CoolSculpting applicator which results in a 35-minute treatment. It’s a little larger, there’s a little less pain, a lower temperature, and it’s a little bit more effective. This has been helpful in our practice in terms of getting more treatment cycles in a visit.”
Postprocedurally, massage may improve clinical results by mobilizing lipid crystals created from treatment.
Extracorporeal shock wave therapy
Another modality to consider is extracorporeal shock wave therapy, which is the application of mechanically generated external sound waves. “It’s not the same as ultrasound or focused ultrasound, and it’s FDA cleared for the treatment of cellulite,” Dr. Avram said.
EmSculpt
A newer innovation, known as High Intensity Focused Electromagnetic (HIFEM) technology (EmSculpt), induces 20,000 forced muscle contractions per session, which leads to supramaximal contractions that can’t be achieved through normal voluntary muscle action.
“The idea is that you’re getting hypertrophy of the muscle to get volumetric growth,” he said. “There’s believed to be a cascaded apoptotic effect, inducing apoptosis and fat disruption.”
Dr. Avram added that HIFEM is nonionizing, nonradiating, nonthermal, and it does not affect sensory nerves. “It’s designed to only stimulate motor neurons. Time will tell in terms of what the ultimate results are with that device.”
truSculpt
Some clinicians are using monopolar radiofrequency with truSculpt, the proprietary delivery of deep radiofrequency energy. This device increases fat temperature between 6 and 10 degrees Celsius with dual frequency at 1 and 2 MHz.
Published data show that 45 degrees Celsius sustained for 3 minutes resulted in a 60% loss of adipose tissue viability (Lasers Surg Med. 2009;41:745-50; Lasers Surg Med. 2010;42:361-70).
“The heat delivery induces cell apoptosis, leading to the removal of those cells by natural healing processes,” said Dr. Avram, who added that he has not used the device. “We need more clinical data to assess this as well.”
Selective photothermolysis
Another technology being evaluated for fat removal is selective photothermolysis, a concept developed at Massachusetts General Hospital in 1983. It extends the theory of selective photothermolysis to target the lipids that make up subcutaneous fat.
“The theory is that you must select a wavelength well absorbed by the target chromophore with a pulse duration shorter than the target’s thermal relaxation time,” Dr. Avram explained. “This produces selective, localized heating with focal destruction of the target with minimal damage to the surrounding tissue. It requires a deeply penetrating wavelength.”
Lipids are a tempting target “because they heat quickly and easily and they do not lose their heat easily to surrounding structures. You want to target fat selectively and confine thermal damage effectively,” he said.
Nearly 10 years ago, Dr. Avram and his associates evaluated the effects of noninvasive 1,210-nm laser exposure on the adipose tissue of 24 patients with skin types 1-5 (Laser Surg Med. 2009;41:401-7).
“The laser pulses were painful, which limited the efficacy,” he said.
The contact cooling device failed in some subjects, and two patients had bulla, but no scarring. Histologic evidence of laser-induced fat damage was observed in 89% of test sites at 4-7 weeks, but dermal damage was also seen.
“This was the first study to show histologic evidence of laser-induced damage to subcutaneous fat,” Dr. Avram said.
Development of selective photothermolysis technology fell off the wayside after the Great Recession of 2008, but it is still being evaluated at Massachusetts General Hospital and other centers.
To optimize the technology, Dr. Avram said that longer pulse durations may target larger volumes of fat. “Cooling is essential to protect the epidermis, as well as to control pain.”
Injectables
Injectables provide a new, minimally invasive means to achieve noninvasive fat removal, Dr. Avram noted.
“Many injectables of questionable efficacy and safety had been available internationally for years,” he said, but none had FDA clearance until 2015, when the agency gave ATX-101 (Kybella) the nod.
ATX-101 is a nonanimal-derived formulation of deoxycholic acid that causes preferential adipocytolysis. Data from a phase 3 trial presented at the 2014 American Society of Plastic Surgery and the American Society of Aesthetic Plastic Surgery meetings showed a statistically significant reduction in submental fat among subjects who received ATX-101, compared with placebo. It requires an average of 2-4 treatments.
“In my experience, it tends not to require that many, but based on MRI, as well as clinician and patient-reported outcomes, there are significant improvements in the visual impact of chin fat,” Dr. Avram said.
Most adverse events are mild to moderate in severity, primarily bruising, pain, and a sensation of numbness to the anesthesia. “They decrease in incidence and severity over successive treatments, and they infrequently lead to discontinuation of treatment,” he said.
For submental fat, clinicians can combine cryolipolysis and deoxycholic acid. “Here, the idea is to assess the amount of fat targeted for treatment,” Dr. Avram said. “If the fat fills the cryolipolysis cup, use cryolipolysis alone. If the fat does not fill the cup, inject deoxycholic acid for a more targeted treatment. If there is residual fat after cryolipolysis, consider treating more focally with deoxycholic acid.”
Both treatments can produce temporary marginal mandibular nerve injury. “It’s not common, but that’s something to include in your consent forms,” he said.
Dr. Avram reported that he has received consulting fees from Allergan, Merz Pharma, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis Biosystems, Invasix, and Zalea.
[email protected]
SAN DIEGO – Noninvasive fat removal, such as laser treatment and cryolipolysis, is here to stay.
That’s what Mathew M. Avram, MD, JD, told attendees at the annual Masters of Aesthetics Symposium.
“It does not compare to liposuction, but many patients prefer these devices because there’s less downtime,” he said. “They don’t want pain. They don’t want time away from work.”
In the decade or so since the inception of the noninvasive body contouring field, noninvasive and minimally invasive devices have become far more popular than traditional liposuction, said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital in Boston. “It’s not even close. Among American Society for Dermatologic Surgery [ASDS] members, for every 1 liposuction, there are over 10 noninvasive body sculpting treatments.”
There were 408,000 body-sculpting procedures performed in 2017. More than half of those (208,000) were cryolipolysis, followed by use of radiofrequency (89,000), deoxycholic acid (46,000), laser lipolysis (25,000), “other” procedures (25,000), and tumescent liposuction (15,000), according to data from the ASDS.
“Each treatment has improved in efficacy with time,” Dr. Avram said.
Devices currently cleared by the Food and Drug Administration for the noninvasive removal of fat include ultrasound, lasers, low-level laser therapy, cryolipolysis, and radiofrequency. One of the most common is low-level laser therapy.
“There are several different devices on the market, but there’s no good histology to confirm mechanism of action, so you question what the efficacy is,” said Dr. Avram, who is also the immediate past president of the American Society for Laser Medicine and Surgery.
SculpSure
In the traditional laser domain, the one most commonly used for fat removal is SculpSure, an FDA-cleared hyperthermic 1,060-nm diode laser. It features four flat, nonsuction applicators, a contact cooling system, and it enables the user to treat more than one area in a 25-minute session. There is minimal absorption by melanin.
“You shouldn’t treat over tattoos, however, because if there’s black ink, the tattoo will absorb the wavelength,” Dr. Avram said. “It’s safe in all skin types and there are a variety of different configurations you can use to treat the desired area.”
Initially there is a 4-minute warm-up cycle to achieve the target temperature. Over the next 21 minutes, 1,060-nm energy is delivered via proprietary modulation, alternating between heat and cooling.
“There is definitely some pain with this device,” he said. “Whether or not it’s a relevant endpoint is not known at this point. But typically, if you’re destroying fat with heat there should be some pain. It’s relieved by a period of cooling. A submental fat treatment is now available. I have not personally used it, but it’s the same technology.”
CoolSculpting
Another popular technology is cryolipolysis (CoolSculpting), which was developed at Massachusetts General Hospital by R. Rox Anderson, MD, and Dieter Manstein, MD, PhD.
It’s FDA cleared for noninvasive fat removal and there have been more than 6 million cryolipolysis treatments performed around the world. The purported mechanism of action is selective crystallization of lipids and fat cells at temperatures below freezing. An inflammatory process results in fat reduction over 2-4 months.
“When it first started, cryolipolysis was designed to treat local areas like love handles in males,” Dr. Avram said. “Over time, applicators have been designed to treat different areas, most recently, one for the posterior upper arms and above the knees. There is now a larger, faster CoolSculpting applicator which results in a 35-minute treatment. It’s a little larger, there’s a little less pain, a lower temperature, and it’s a little bit more effective. This has been helpful in our practice in terms of getting more treatment cycles in a visit.”
Postprocedurally, massage may improve clinical results by mobilizing lipid crystals created from treatment.
Extracorporeal shock wave therapy
Another modality to consider is extracorporeal shock wave therapy, which is the application of mechanically generated external sound waves. “It’s not the same as ultrasound or focused ultrasound, and it’s FDA cleared for the treatment of cellulite,” Dr. Avram said.
EmSculpt
A newer innovation, known as High Intensity Focused Electromagnetic (HIFEM) technology (EmSculpt), induces 20,000 forced muscle contractions per session, which leads to supramaximal contractions that can’t be achieved through normal voluntary muscle action.
“The idea is that you’re getting hypertrophy of the muscle to get volumetric growth,” he said. “There’s believed to be a cascaded apoptotic effect, inducing apoptosis and fat disruption.”
Dr. Avram added that HIFEM is nonionizing, nonradiating, nonthermal, and it does not affect sensory nerves. “It’s designed to only stimulate motor neurons. Time will tell in terms of what the ultimate results are with that device.”
truSculpt
Some clinicians are using monopolar radiofrequency with truSculpt, the proprietary delivery of deep radiofrequency energy. This device increases fat temperature between 6 and 10 degrees Celsius with dual frequency at 1 and 2 MHz.
Published data show that 45 degrees Celsius sustained for 3 minutes resulted in a 60% loss of adipose tissue viability (Lasers Surg Med. 2009;41:745-50; Lasers Surg Med. 2010;42:361-70).
“The heat delivery induces cell apoptosis, leading to the removal of those cells by natural healing processes,” said Dr. Avram, who added that he has not used the device. “We need more clinical data to assess this as well.”
Selective photothermolysis
Another technology being evaluated for fat removal is selective photothermolysis, a concept developed at Massachusetts General Hospital in 1983. It extends the theory of selective photothermolysis to target the lipids that make up subcutaneous fat.
“The theory is that you must select a wavelength well absorbed by the target chromophore with a pulse duration shorter than the target’s thermal relaxation time,” Dr. Avram explained. “This produces selective, localized heating with focal destruction of the target with minimal damage to the surrounding tissue. It requires a deeply penetrating wavelength.”
Lipids are a tempting target “because they heat quickly and easily and they do not lose their heat easily to surrounding structures. You want to target fat selectively and confine thermal damage effectively,” he said.
Nearly 10 years ago, Dr. Avram and his associates evaluated the effects of noninvasive 1,210-nm laser exposure on the adipose tissue of 24 patients with skin types 1-5 (Laser Surg Med. 2009;41:401-7).
“The laser pulses were painful, which limited the efficacy,” he said.
The contact cooling device failed in some subjects, and two patients had bulla, but no scarring. Histologic evidence of laser-induced fat damage was observed in 89% of test sites at 4-7 weeks, but dermal damage was also seen.
“This was the first study to show histologic evidence of laser-induced damage to subcutaneous fat,” Dr. Avram said.
Development of selective photothermolysis technology fell off the wayside after the Great Recession of 2008, but it is still being evaluated at Massachusetts General Hospital and other centers.
To optimize the technology, Dr. Avram said that longer pulse durations may target larger volumes of fat. “Cooling is essential to protect the epidermis, as well as to control pain.”
Injectables
Injectables provide a new, minimally invasive means to achieve noninvasive fat removal, Dr. Avram noted.
“Many injectables of questionable efficacy and safety had been available internationally for years,” he said, but none had FDA clearance until 2015, when the agency gave ATX-101 (Kybella) the nod.
ATX-101 is a nonanimal-derived formulation of deoxycholic acid that causes preferential adipocytolysis. Data from a phase 3 trial presented at the 2014 American Society of Plastic Surgery and the American Society of Aesthetic Plastic Surgery meetings showed a statistically significant reduction in submental fat among subjects who received ATX-101, compared with placebo. It requires an average of 2-4 treatments.
“In my experience, it tends not to require that many, but based on MRI, as well as clinician and patient-reported outcomes, there are significant improvements in the visual impact of chin fat,” Dr. Avram said.
Most adverse events are mild to moderate in severity, primarily bruising, pain, and a sensation of numbness to the anesthesia. “They decrease in incidence and severity over successive treatments, and they infrequently lead to discontinuation of treatment,” he said.
For submental fat, clinicians can combine cryolipolysis and deoxycholic acid. “Here, the idea is to assess the amount of fat targeted for treatment,” Dr. Avram said. “If the fat fills the cryolipolysis cup, use cryolipolysis alone. If the fat does not fill the cup, inject deoxycholic acid for a more targeted treatment. If there is residual fat after cryolipolysis, consider treating more focally with deoxycholic acid.”
Both treatments can produce temporary marginal mandibular nerve injury. “It’s not common, but that’s something to include in your consent forms,” he said.
Dr. Avram reported that he has received consulting fees from Allergan, Merz Pharma, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis Biosystems, Invasix, and Zalea.
[email protected]
EXPERT ANALYSIS FROM MOAS 2018
Many devices optimal for treating vascular skin lesions
SAN DIEGO – According to J. Stuart Nelson, MD, PhD,
“You’re going to be using wavelengths of light generally in the green and yellow portion of the spectrum,” Dr. Nelson, professor of surgery and biomedical engineering at the Beckman Laser Institute and Medical Clinic at the University of California, Irvine, said at the annual Masters of Aesthetics Symposium. “Blue light is highly absorbed by hemoglobin but unfortunately, blue light is highly scattered by human skin, so it won’t penetrate deep enough into the dermis. So primarily, we’re targeting hemoglobin using green and yellow light sources.”
The second principle is to match the pulse width with the vessel size, while the third is to give sufficient energy to irreversibly injure vessels based on selective photothermolysis.
Next, he advised clinicians to ask themselves three questions: What is the vessel size? “The larger the vessel, the longer the thermal relaxation time,” he said. What is the vessel depth? Deeper vessels require longer wavelengths of light and larger spot sizes. What is the patient’s skin phototype? Darker skin contains more epidermal melanin and requires extra caution during treatment.
Dr. Nelson listed seven optimal devices for the treatment of vascular skin lesions: intense pulsed light (IPL) with wavelengths of 515-1,200 nm and pulse durations of 1-10 ms, pulsed green light with a wavelength of 532 nm and pulse durations of 1-50 ms, pulsed dye yellow light with wavelengths of 585-600 nm and pulse durations of 0.5-40 ms, pulsed dye plus Nd:YAG with wavelengths of 595 and 1,064 nm and pulse durations of 0.5-40 ms, alexandrite laser with a wavelength of 755 nm and pulse durations of 0.25-100 ms, diode laser with a wavelength of 940 nm and pulse durations of 5-100 ms, and the pulsed Nd:YAG laser with a wavelength of 1,064 nm and pulse durations of 0.25-100 ms.
“You can get good results with every one of these devices,” Dr. Nelson said. “What you need to do is pick one and become what R. Rox Anderson, MD, calls an ‘endpointologist,’ so you can understand the clinical endpoints. Do not use a cookbook approach by trying to memorize treatment settings.”
Pulsed dye lasers with a wavelength of 585-600 nm have been the standard of care for years, he said, and is the treatment of choice for port wine stains in infants and young children. Upsides include the ability to treat large areas quickly and the ability to use two to three separate passes. It also induces diminution in diffuse redness and telangiectasia. Drawbacks include its potential to cause purpura when short pulse durations are used, it requires several treatments, it can be painful, and it causes considerable edema and erythema.
Millisecond green lasers at a wavelength of 532 nm are also effective for treating vascular skin lesions. “The nice thing about these devices is that you can focus them down to very small spots, so you can literally trace out individual blood vessels,” Dr. Nelson said. Other upsides include the fact that it can be performed without producing purpura, only transient erythema if few areas are treated. Drawbacks are that it’s moderately painful and may cause considerable edema. It also causes significant melanin absorption so is not advised for use in tanned and darker-skinned individuals. For all patients, contact cooling must be assured.
IPL, meanwhile, “can be very useful for treating not only vascular lesions, but also concurrently pigmented lesions such as poikiloderma of Civatte,” Dr. Nelson said. Potential drawbacks to IPL therapy are that the spectrum of light emitted and the pulse duration characteristics vary between devices and multiple treatments are required.
Finally, in the millisecond domain, the pulsed alexandrite 755-nm and Nd:YAG 1,064-nm lasers “are very good when trying to target something very deep in the skin like a vein,” he said. “But when you’re using those devices, you’re coagulating a large volume of tissue, so you need to be very careful about the amount of heat that you’re generating deep in the skin.”
When consulting with patients who have rosacea or telangiectasia, Dr. Nelson tells them multiple treatments will be required. “These are chronic conditions, and they may need ongoing maintenance treatments. The nice thing about all these procedures you’re doing for rosacea and telangiectasia is that they can be combined with all of your FDA [Food and Drug Administration]-approved topical and oral treatment protocols. All of these drugs you have at your disposal to medically treat rosacea can be all used concurrently with your laser treatment. When you see a patient you need to emphasize to them: ‘I’m not treating your rosacea with the laser. I’m treating a symptom of your rosacea with the laser.’ ”
Dr. Nelson closed his presentation by offering basic principles for success, the first being do no harm. “That’s the single most important thing you want to remember. No one will get mad if the blood vessel’s still there, but they’ll get very mad if something bad happens. You also want to underpromise and overdeliver. I always tell patients it’s going to require two to four treatments. When in doubt, don’t treat or undertreat. You can always treat again.”
If you’re concerned, perform a test spot. “There’s nothing wrong with that, particularly in a patient where you’re not sure what the outcome will be,” he said. “Check for any unusual skin reaction and for potential success of the procedure. Finally, don’t treat patients who are tanned.”
Dr. Nelson reported having intellectual property rights with Syneron Candela.
SAN DIEGO – According to J. Stuart Nelson, MD, PhD,
“You’re going to be using wavelengths of light generally in the green and yellow portion of the spectrum,” Dr. Nelson, professor of surgery and biomedical engineering at the Beckman Laser Institute and Medical Clinic at the University of California, Irvine, said at the annual Masters of Aesthetics Symposium. “Blue light is highly absorbed by hemoglobin but unfortunately, blue light is highly scattered by human skin, so it won’t penetrate deep enough into the dermis. So primarily, we’re targeting hemoglobin using green and yellow light sources.”
The second principle is to match the pulse width with the vessel size, while the third is to give sufficient energy to irreversibly injure vessels based on selective photothermolysis.
Next, he advised clinicians to ask themselves three questions: What is the vessel size? “The larger the vessel, the longer the thermal relaxation time,” he said. What is the vessel depth? Deeper vessels require longer wavelengths of light and larger spot sizes. What is the patient’s skin phototype? Darker skin contains more epidermal melanin and requires extra caution during treatment.
Dr. Nelson listed seven optimal devices for the treatment of vascular skin lesions: intense pulsed light (IPL) with wavelengths of 515-1,200 nm and pulse durations of 1-10 ms, pulsed green light with a wavelength of 532 nm and pulse durations of 1-50 ms, pulsed dye yellow light with wavelengths of 585-600 nm and pulse durations of 0.5-40 ms, pulsed dye plus Nd:YAG with wavelengths of 595 and 1,064 nm and pulse durations of 0.5-40 ms, alexandrite laser with a wavelength of 755 nm and pulse durations of 0.25-100 ms, diode laser with a wavelength of 940 nm and pulse durations of 5-100 ms, and the pulsed Nd:YAG laser with a wavelength of 1,064 nm and pulse durations of 0.25-100 ms.
“You can get good results with every one of these devices,” Dr. Nelson said. “What you need to do is pick one and become what R. Rox Anderson, MD, calls an ‘endpointologist,’ so you can understand the clinical endpoints. Do not use a cookbook approach by trying to memorize treatment settings.”
Pulsed dye lasers with a wavelength of 585-600 nm have been the standard of care for years, he said, and is the treatment of choice for port wine stains in infants and young children. Upsides include the ability to treat large areas quickly and the ability to use two to three separate passes. It also induces diminution in diffuse redness and telangiectasia. Drawbacks include its potential to cause purpura when short pulse durations are used, it requires several treatments, it can be painful, and it causes considerable edema and erythema.
Millisecond green lasers at a wavelength of 532 nm are also effective for treating vascular skin lesions. “The nice thing about these devices is that you can focus them down to very small spots, so you can literally trace out individual blood vessels,” Dr. Nelson said. Other upsides include the fact that it can be performed without producing purpura, only transient erythema if few areas are treated. Drawbacks are that it’s moderately painful and may cause considerable edema. It also causes significant melanin absorption so is not advised for use in tanned and darker-skinned individuals. For all patients, contact cooling must be assured.
IPL, meanwhile, “can be very useful for treating not only vascular lesions, but also concurrently pigmented lesions such as poikiloderma of Civatte,” Dr. Nelson said. Potential drawbacks to IPL therapy are that the spectrum of light emitted and the pulse duration characteristics vary between devices and multiple treatments are required.
Finally, in the millisecond domain, the pulsed alexandrite 755-nm and Nd:YAG 1,064-nm lasers “are very good when trying to target something very deep in the skin like a vein,” he said. “But when you’re using those devices, you’re coagulating a large volume of tissue, so you need to be very careful about the amount of heat that you’re generating deep in the skin.”
When consulting with patients who have rosacea or telangiectasia, Dr. Nelson tells them multiple treatments will be required. “These are chronic conditions, and they may need ongoing maintenance treatments. The nice thing about all these procedures you’re doing for rosacea and telangiectasia is that they can be combined with all of your FDA [Food and Drug Administration]-approved topical and oral treatment protocols. All of these drugs you have at your disposal to medically treat rosacea can be all used concurrently with your laser treatment. When you see a patient you need to emphasize to them: ‘I’m not treating your rosacea with the laser. I’m treating a symptom of your rosacea with the laser.’ ”
Dr. Nelson closed his presentation by offering basic principles for success, the first being do no harm. “That’s the single most important thing you want to remember. No one will get mad if the blood vessel’s still there, but they’ll get very mad if something bad happens. You also want to underpromise and overdeliver. I always tell patients it’s going to require two to four treatments. When in doubt, don’t treat or undertreat. You can always treat again.”
If you’re concerned, perform a test spot. “There’s nothing wrong with that, particularly in a patient where you’re not sure what the outcome will be,” he said. “Check for any unusual skin reaction and for potential success of the procedure. Finally, don’t treat patients who are tanned.”
Dr. Nelson reported having intellectual property rights with Syneron Candela.
SAN DIEGO – According to J. Stuart Nelson, MD, PhD,
“You’re going to be using wavelengths of light generally in the green and yellow portion of the spectrum,” Dr. Nelson, professor of surgery and biomedical engineering at the Beckman Laser Institute and Medical Clinic at the University of California, Irvine, said at the annual Masters of Aesthetics Symposium. “Blue light is highly absorbed by hemoglobin but unfortunately, blue light is highly scattered by human skin, so it won’t penetrate deep enough into the dermis. So primarily, we’re targeting hemoglobin using green and yellow light sources.”
The second principle is to match the pulse width with the vessel size, while the third is to give sufficient energy to irreversibly injure vessels based on selective photothermolysis.
Next, he advised clinicians to ask themselves three questions: What is the vessel size? “The larger the vessel, the longer the thermal relaxation time,” he said. What is the vessel depth? Deeper vessels require longer wavelengths of light and larger spot sizes. What is the patient’s skin phototype? Darker skin contains more epidermal melanin and requires extra caution during treatment.
Dr. Nelson listed seven optimal devices for the treatment of vascular skin lesions: intense pulsed light (IPL) with wavelengths of 515-1,200 nm and pulse durations of 1-10 ms, pulsed green light with a wavelength of 532 nm and pulse durations of 1-50 ms, pulsed dye yellow light with wavelengths of 585-600 nm and pulse durations of 0.5-40 ms, pulsed dye plus Nd:YAG with wavelengths of 595 and 1,064 nm and pulse durations of 0.5-40 ms, alexandrite laser with a wavelength of 755 nm and pulse durations of 0.25-100 ms, diode laser with a wavelength of 940 nm and pulse durations of 5-100 ms, and the pulsed Nd:YAG laser with a wavelength of 1,064 nm and pulse durations of 0.25-100 ms.
“You can get good results with every one of these devices,” Dr. Nelson said. “What you need to do is pick one and become what R. Rox Anderson, MD, calls an ‘endpointologist,’ so you can understand the clinical endpoints. Do not use a cookbook approach by trying to memorize treatment settings.”
Pulsed dye lasers with a wavelength of 585-600 nm have been the standard of care for years, he said, and is the treatment of choice for port wine stains in infants and young children. Upsides include the ability to treat large areas quickly and the ability to use two to three separate passes. It also induces diminution in diffuse redness and telangiectasia. Drawbacks include its potential to cause purpura when short pulse durations are used, it requires several treatments, it can be painful, and it causes considerable edema and erythema.
Millisecond green lasers at a wavelength of 532 nm are also effective for treating vascular skin lesions. “The nice thing about these devices is that you can focus them down to very small spots, so you can literally trace out individual blood vessels,” Dr. Nelson said. Other upsides include the fact that it can be performed without producing purpura, only transient erythema if few areas are treated. Drawbacks are that it’s moderately painful and may cause considerable edema. It also causes significant melanin absorption so is not advised for use in tanned and darker-skinned individuals. For all patients, contact cooling must be assured.
IPL, meanwhile, “can be very useful for treating not only vascular lesions, but also concurrently pigmented lesions such as poikiloderma of Civatte,” Dr. Nelson said. Potential drawbacks to IPL therapy are that the spectrum of light emitted and the pulse duration characteristics vary between devices and multiple treatments are required.
Finally, in the millisecond domain, the pulsed alexandrite 755-nm and Nd:YAG 1,064-nm lasers “are very good when trying to target something very deep in the skin like a vein,” he said. “But when you’re using those devices, you’re coagulating a large volume of tissue, so you need to be very careful about the amount of heat that you’re generating deep in the skin.”
When consulting with patients who have rosacea or telangiectasia, Dr. Nelson tells them multiple treatments will be required. “These are chronic conditions, and they may need ongoing maintenance treatments. The nice thing about all these procedures you’re doing for rosacea and telangiectasia is that they can be combined with all of your FDA [Food and Drug Administration]-approved topical and oral treatment protocols. All of these drugs you have at your disposal to medically treat rosacea can be all used concurrently with your laser treatment. When you see a patient you need to emphasize to them: ‘I’m not treating your rosacea with the laser. I’m treating a symptom of your rosacea with the laser.’ ”
Dr. Nelson closed his presentation by offering basic principles for success, the first being do no harm. “That’s the single most important thing you want to remember. No one will get mad if the blood vessel’s still there, but they’ll get very mad if something bad happens. You also want to underpromise and overdeliver. I always tell patients it’s going to require two to four treatments. When in doubt, don’t treat or undertreat. You can always treat again.”
If you’re concerned, perform a test spot. “There’s nothing wrong with that, particularly in a patient where you’re not sure what the outcome will be,” he said. “Check for any unusual skin reaction and for potential success of the procedure. Finally, don’t treat patients who are tanned.”
Dr. Nelson reported having intellectual property rights with Syneron Candela.
EXPERT ANALYSIS FROM MOAS 2018
When treating the lower face and neck, ‘don’t forget the platysma muscle’
SAN DIEGO – Of all the data points featured in a 2017 survey on cosmetic dermatologic procedures, one stands out to Jean Carruthers, MD: Among the 7,322 consumers surveyed, about 70% cited the lower face and submental contour as a significant cosmetic and social concern.
“That is quite a key statistic,” she said at the annual Masters of Aesthetics Symposium. The Consumer Survey on Cosmetic Dermatologic Procedures was conducted by the American Society for Dermatologic Surgery.
Dr. Carruthers, who, with her husband, Alastair Carruthers, MD, pioneered the cosmetic use of onabotulinumtoxinA (Botox), said that the world’s great sculptors “think that there is a difference between the classical faces of males and females, and we all know instinctually what those changes are, with a square face and larger jawline in males and a smooth oval, heart-shaped face in women,” she said.
“But what about the neck?” She cited an article by Greg J. Goodman, MD, and colleagues, which defined an ideal neck as the distinct inferior mandibular border from mentum to angle with no jowl overhang (Dermatol Surg 2016;42:S260-2). It includes subhyoid depression, which visually enhances the impression that the neck is thin and long; visible thyroid cartilage bulge; a visible anterior border of the sternocleidomastoid muscle, distinct in its entire course from the mastoid to the sternum; and a cervicomental angle between 105 and 120 degrees. An angle greater than 120 degrees appears as a double chin or heavy neck, according to the authors.
In the past, clinicians used to think of the lower face and neck as two separate cosmetic units, but now they are considered one cosmetic unit, said Dr. Carruthers, of the University of British Columbia, Vancouver. “Don’t forget the platysma muscle,” she added. “The platysma is a lower facial muscle of expression and it affects all the other muscles. It interdigitates with the depressor anguli oris, with orbicularis oris, and depressor labii inferioris muscles, and it goes backwards into the masseter muscle.”
She and two Brazilian investigators published a retrospective analysis of 161 patients treated by a Botox injection pattern encompassing the facial platysma components, aiming to block the lower face as a whole complex (Dermatol Surg. 2017;43[8]:1042-9). According to the article, results included “frontal and lateral enhancement of lower facial contour, relaxation of high horizontal lines located just below the lateral mandibular border, and lower deep vertical smile lines present lateral to the oral commissures and melomental folds.”
Fillers and tightening the face and neck envelope without surgery takes maintenance, Dr. Carruthers said. She also noted that deoxycholic acid works on jowls as well as submental fat. Noninvasive combinations for lower face and neck include neuromodulators and deep and superficial fillers, cryolipolysis and deoxycholic acid, energy-based devices, and microneedling. “I think that these are fantastic combinations, and they don’t have to all be done at the same time on the same day,” Dr. Carruthers said. “I’m very impressed with cryolipolysis as the unheralded skin tightener. I looked at our first 464 patients and saw tremendous skin tightening.”
Of all the injectable products on the market, she said that neuromodulators “have set a new gold standard for all aesthetic treatments. It’s the most powerful primary aesthetic modulator and enhances the result of everything else that you do.”
Dr. Carruthers disclosed that she is a consultant to and has received research support from Allergan, Alphaeon, Bonti, Merz, Revance, and Zeltiq.
SAN DIEGO – Of all the data points featured in a 2017 survey on cosmetic dermatologic procedures, one stands out to Jean Carruthers, MD: Among the 7,322 consumers surveyed, about 70% cited the lower face and submental contour as a significant cosmetic and social concern.
“That is quite a key statistic,” she said at the annual Masters of Aesthetics Symposium. The Consumer Survey on Cosmetic Dermatologic Procedures was conducted by the American Society for Dermatologic Surgery.
Dr. Carruthers, who, with her husband, Alastair Carruthers, MD, pioneered the cosmetic use of onabotulinumtoxinA (Botox), said that the world’s great sculptors “think that there is a difference between the classical faces of males and females, and we all know instinctually what those changes are, with a square face and larger jawline in males and a smooth oval, heart-shaped face in women,” she said.
“But what about the neck?” She cited an article by Greg J. Goodman, MD, and colleagues, which defined an ideal neck as the distinct inferior mandibular border from mentum to angle with no jowl overhang (Dermatol Surg 2016;42:S260-2). It includes subhyoid depression, which visually enhances the impression that the neck is thin and long; visible thyroid cartilage bulge; a visible anterior border of the sternocleidomastoid muscle, distinct in its entire course from the mastoid to the sternum; and a cervicomental angle between 105 and 120 degrees. An angle greater than 120 degrees appears as a double chin or heavy neck, according to the authors.
In the past, clinicians used to think of the lower face and neck as two separate cosmetic units, but now they are considered one cosmetic unit, said Dr. Carruthers, of the University of British Columbia, Vancouver. “Don’t forget the platysma muscle,” she added. “The platysma is a lower facial muscle of expression and it affects all the other muscles. It interdigitates with the depressor anguli oris, with orbicularis oris, and depressor labii inferioris muscles, and it goes backwards into the masseter muscle.”
She and two Brazilian investigators published a retrospective analysis of 161 patients treated by a Botox injection pattern encompassing the facial platysma components, aiming to block the lower face as a whole complex (Dermatol Surg. 2017;43[8]:1042-9). According to the article, results included “frontal and lateral enhancement of lower facial contour, relaxation of high horizontal lines located just below the lateral mandibular border, and lower deep vertical smile lines present lateral to the oral commissures and melomental folds.”
Fillers and tightening the face and neck envelope without surgery takes maintenance, Dr. Carruthers said. She also noted that deoxycholic acid works on jowls as well as submental fat. Noninvasive combinations for lower face and neck include neuromodulators and deep and superficial fillers, cryolipolysis and deoxycholic acid, energy-based devices, and microneedling. “I think that these are fantastic combinations, and they don’t have to all be done at the same time on the same day,” Dr. Carruthers said. “I’m very impressed with cryolipolysis as the unheralded skin tightener. I looked at our first 464 patients and saw tremendous skin tightening.”
Of all the injectable products on the market, she said that neuromodulators “have set a new gold standard for all aesthetic treatments. It’s the most powerful primary aesthetic modulator and enhances the result of everything else that you do.”
Dr. Carruthers disclosed that she is a consultant to and has received research support from Allergan, Alphaeon, Bonti, Merz, Revance, and Zeltiq.
SAN DIEGO – Of all the data points featured in a 2017 survey on cosmetic dermatologic procedures, one stands out to Jean Carruthers, MD: Among the 7,322 consumers surveyed, about 70% cited the lower face and submental contour as a significant cosmetic and social concern.
“That is quite a key statistic,” she said at the annual Masters of Aesthetics Symposium. The Consumer Survey on Cosmetic Dermatologic Procedures was conducted by the American Society for Dermatologic Surgery.
Dr. Carruthers, who, with her husband, Alastair Carruthers, MD, pioneered the cosmetic use of onabotulinumtoxinA (Botox), said that the world’s great sculptors “think that there is a difference between the classical faces of males and females, and we all know instinctually what those changes are, with a square face and larger jawline in males and a smooth oval, heart-shaped face in women,” she said.
“But what about the neck?” She cited an article by Greg J. Goodman, MD, and colleagues, which defined an ideal neck as the distinct inferior mandibular border from mentum to angle with no jowl overhang (Dermatol Surg 2016;42:S260-2). It includes subhyoid depression, which visually enhances the impression that the neck is thin and long; visible thyroid cartilage bulge; a visible anterior border of the sternocleidomastoid muscle, distinct in its entire course from the mastoid to the sternum; and a cervicomental angle between 105 and 120 degrees. An angle greater than 120 degrees appears as a double chin or heavy neck, according to the authors.
In the past, clinicians used to think of the lower face and neck as two separate cosmetic units, but now they are considered one cosmetic unit, said Dr. Carruthers, of the University of British Columbia, Vancouver. “Don’t forget the platysma muscle,” she added. “The platysma is a lower facial muscle of expression and it affects all the other muscles. It interdigitates with the depressor anguli oris, with orbicularis oris, and depressor labii inferioris muscles, and it goes backwards into the masseter muscle.”
She and two Brazilian investigators published a retrospective analysis of 161 patients treated by a Botox injection pattern encompassing the facial platysma components, aiming to block the lower face as a whole complex (Dermatol Surg. 2017;43[8]:1042-9). According to the article, results included “frontal and lateral enhancement of lower facial contour, relaxation of high horizontal lines located just below the lateral mandibular border, and lower deep vertical smile lines present lateral to the oral commissures and melomental folds.”
Fillers and tightening the face and neck envelope without surgery takes maintenance, Dr. Carruthers said. She also noted that deoxycholic acid works on jowls as well as submental fat. Noninvasive combinations for lower face and neck include neuromodulators and deep and superficial fillers, cryolipolysis and deoxycholic acid, energy-based devices, and microneedling. “I think that these are fantastic combinations, and they don’t have to all be done at the same time on the same day,” Dr. Carruthers said. “I’m very impressed with cryolipolysis as the unheralded skin tightener. I looked at our first 464 patients and saw tremendous skin tightening.”
Of all the injectable products on the market, she said that neuromodulators “have set a new gold standard for all aesthetic treatments. It’s the most powerful primary aesthetic modulator and enhances the result of everything else that you do.”
Dr. Carruthers disclosed that she is a consultant to and has received research support from Allergan, Alphaeon, Bonti, Merz, Revance, and Zeltiq.
AT MOAS 2018
Social media: ‘The more you do, the easier it gets’
SAN DIEGO – , the first question to ask yourself is why.
“Why are you doing this, and why does it matter?” she asked attendees at the annual Masters of Aesthetics Symposium. “If you know why, then you can answer a lot of questions.”
Dr. Day of the department of dermatology at New York University, said there are at least four quadrants of personal branding. The first is personal (who you are, where you live, family, hobbies, interests, education), and the second is professional (where you work, training, important skills and experience, and what sets you apart). The last two are thought leadership and legacy, “which is not your image,” she said. “It’s what you do for others; it’s the teaching you do from day to day.”
She noted that 74% of people look to social media networks for advice on buying decisions, and 40% of people purchased an item based on seeing it promoted by an influencer via Instagram or Twitter. This extends to aesthetics as well. “We need to become the influencers,” Dr. Day said. However, social media “is not all always about you; it’s the message, so knowing your medium is important. Each medium has its own velocity, its own type of follower, its own best practice to attract the best amount of viewers. Consistency is important. You have to reach your target generation.”
According to Dr. Day, Millennials (those aged 20-35 years) spend an average of 8 hours per day online; 70% use Facebook, 63% use YouTube, and 43% want brands to reach them via e-mail. “They’re a little more concerned about their financial future than other generations,” she said. Meanwhile, 76% of the demographic Generation X (those aged 36-49) access some form of social media. They have an annual buying power of $200 million, and 68% make decisions based on reviews like those on Yelp. “Reviews matter,” she said. “It’s only the people who are unhappy with you who are happy to go out and talk about it. People who are happy with you are going to need more encouragement to write a review.” On average, baby boomers (those aged 50-65 years) spend 27 hours per week online, and nearly 16% spend at least 11 hours on Facebook. Nearly half (48%) rely on credit cards for their purchases, and 13% use LinkedIn.
Achieving success in social media takes patience, perseverance, and authenticity. “The more you do, the easier it gets,” said Dr. Day, who earned a master’s degree in journalism from NYU. “I have a group of people who tend to like my posts consistently, comment consistently, share consistently, and I respond consistently. That’s how you build relationships. When somebody posts, respond.”
Video content is being consumed online more than ever before, but Dr. Day emphasized that such visual content should “have a purpose and be directed to your specific audience – not just making video for the sake of it.” She recommends being selective when choosing your content, and where you post it. “Don’t stretch your brand over multiple [social media] channels if your team can’t support it.” If you thrive in a multichannel environment, BuzzBundle integrates Facebook, Twitter, Google+, LinkedIn, blogs, forums, and Q&A sites for posting with a purpose and a converged strategy across all sites.
“As with any smart strategy, make sure you are tracking your progress,” she said, suggesting AddThis analytics as one way to track how, where, and by whom your content is being shared.
Dr. Day reported having no relevant financial disclosures.
SAN DIEGO – , the first question to ask yourself is why.
“Why are you doing this, and why does it matter?” she asked attendees at the annual Masters of Aesthetics Symposium. “If you know why, then you can answer a lot of questions.”
Dr. Day of the department of dermatology at New York University, said there are at least four quadrants of personal branding. The first is personal (who you are, where you live, family, hobbies, interests, education), and the second is professional (where you work, training, important skills and experience, and what sets you apart). The last two are thought leadership and legacy, “which is not your image,” she said. “It’s what you do for others; it’s the teaching you do from day to day.”
She noted that 74% of people look to social media networks for advice on buying decisions, and 40% of people purchased an item based on seeing it promoted by an influencer via Instagram or Twitter. This extends to aesthetics as well. “We need to become the influencers,” Dr. Day said. However, social media “is not all always about you; it’s the message, so knowing your medium is important. Each medium has its own velocity, its own type of follower, its own best practice to attract the best amount of viewers. Consistency is important. You have to reach your target generation.”
According to Dr. Day, Millennials (those aged 20-35 years) spend an average of 8 hours per day online; 70% use Facebook, 63% use YouTube, and 43% want brands to reach them via e-mail. “They’re a little more concerned about their financial future than other generations,” she said. Meanwhile, 76% of the demographic Generation X (those aged 36-49) access some form of social media. They have an annual buying power of $200 million, and 68% make decisions based on reviews like those on Yelp. “Reviews matter,” she said. “It’s only the people who are unhappy with you who are happy to go out and talk about it. People who are happy with you are going to need more encouragement to write a review.” On average, baby boomers (those aged 50-65 years) spend 27 hours per week online, and nearly 16% spend at least 11 hours on Facebook. Nearly half (48%) rely on credit cards for their purchases, and 13% use LinkedIn.
Achieving success in social media takes patience, perseverance, and authenticity. “The more you do, the easier it gets,” said Dr. Day, who earned a master’s degree in journalism from NYU. “I have a group of people who tend to like my posts consistently, comment consistently, share consistently, and I respond consistently. That’s how you build relationships. When somebody posts, respond.”
Video content is being consumed online more than ever before, but Dr. Day emphasized that such visual content should “have a purpose and be directed to your specific audience – not just making video for the sake of it.” She recommends being selective when choosing your content, and where you post it. “Don’t stretch your brand over multiple [social media] channels if your team can’t support it.” If you thrive in a multichannel environment, BuzzBundle integrates Facebook, Twitter, Google+, LinkedIn, blogs, forums, and Q&A sites for posting with a purpose and a converged strategy across all sites.
“As with any smart strategy, make sure you are tracking your progress,” she said, suggesting AddThis analytics as one way to track how, where, and by whom your content is being shared.
Dr. Day reported having no relevant financial disclosures.
SAN DIEGO – , the first question to ask yourself is why.
“Why are you doing this, and why does it matter?” she asked attendees at the annual Masters of Aesthetics Symposium. “If you know why, then you can answer a lot of questions.”
Dr. Day of the department of dermatology at New York University, said there are at least four quadrants of personal branding. The first is personal (who you are, where you live, family, hobbies, interests, education), and the second is professional (where you work, training, important skills and experience, and what sets you apart). The last two are thought leadership and legacy, “which is not your image,” she said. “It’s what you do for others; it’s the teaching you do from day to day.”
She noted that 74% of people look to social media networks for advice on buying decisions, and 40% of people purchased an item based on seeing it promoted by an influencer via Instagram or Twitter. This extends to aesthetics as well. “We need to become the influencers,” Dr. Day said. However, social media “is not all always about you; it’s the message, so knowing your medium is important. Each medium has its own velocity, its own type of follower, its own best practice to attract the best amount of viewers. Consistency is important. You have to reach your target generation.”
According to Dr. Day, Millennials (those aged 20-35 years) spend an average of 8 hours per day online; 70% use Facebook, 63% use YouTube, and 43% want brands to reach them via e-mail. “They’re a little more concerned about their financial future than other generations,” she said. Meanwhile, 76% of the demographic Generation X (those aged 36-49) access some form of social media. They have an annual buying power of $200 million, and 68% make decisions based on reviews like those on Yelp. “Reviews matter,” she said. “It’s only the people who are unhappy with you who are happy to go out and talk about it. People who are happy with you are going to need more encouragement to write a review.” On average, baby boomers (those aged 50-65 years) spend 27 hours per week online, and nearly 16% spend at least 11 hours on Facebook. Nearly half (48%) rely on credit cards for their purchases, and 13% use LinkedIn.
Achieving success in social media takes patience, perseverance, and authenticity. “The more you do, the easier it gets,” said Dr. Day, who earned a master’s degree in journalism from NYU. “I have a group of people who tend to like my posts consistently, comment consistently, share consistently, and I respond consistently. That’s how you build relationships. When somebody posts, respond.”
Video content is being consumed online more than ever before, but Dr. Day emphasized that such visual content should “have a purpose and be directed to your specific audience – not just making video for the sake of it.” She recommends being selective when choosing your content, and where you post it. “Don’t stretch your brand over multiple [social media] channels if your team can’t support it.” If you thrive in a multichannel environment, BuzzBundle integrates Facebook, Twitter, Google+, LinkedIn, blogs, forums, and Q&A sites for posting with a purpose and a converged strategy across all sites.
“As with any smart strategy, make sure you are tracking your progress,” she said, suggesting AddThis analytics as one way to track how, where, and by whom your content is being shared.
Dr. Day reported having no relevant financial disclosures.
AT MOAS 2018
Tribulus terrestris
A member of the Zygophyllaceae family, Tribulus terrestris, also known as Gokshura, Gokharu, or puncture vine, is an annual herb; its aerial parts, roots, and fruits have been used in traditional medicine for anti-inflammatory, diuretic, tonic, antimicrobial, and aphrodisiac purposes for thousands of years in China, India, Pakistan, and Sudan.1-3 In modern times, the health benefits of T. terrestris have been attributed to the constituent saponins, flavonoids, alkaloids, lignins, amides, and glycosides that have been isolated and found as bioactive compounds in the plant.2-4
In an ethnobotanical survey of medicinal plants used in Nepal that was conducted in 2010 and 2011, Singh et al. found that T. terrestris was one of the 66 plant species important in the region. They also reported that it is one of the threatened species requiring conservation efforts.5 Although T. terrestris has long had a reputation for aphrodisiac qualities, critical reviews of the literature have undermined this historical reputation.1,6 Nevertheless, the botanical agent is used most often to treat infertility and loss of libido.4 More germane to the dermatologic realm, T. terrestris is thought to exhibit antioxidant, anticarcinogenic, and immunomodulatory potential, among other health benefits.4
Skin lightening activity
In a study published in 2002, Deng et al. evaluated the effects of a decoction of T. terrestris on tyrosinase activity and melanogenesis on cultured human melanocytes. They found that the amount of melanin increased when the decoction was administered in higher concentrations (optimally 1.5 mg/mL) but the effects were reversed at lower concentrations (0.5 mg/mL). Similarly, tyrosinase activity was facilitated by high concentrations of the decoction (optimally 100 mg/mL) and hindered at low concentrations (10 mg/mL). The investigators concluded that T. terrestris showed intriguing potential for use as a skin lightening agent that warranted further study.7
A mouse study performed by Yang et al. in 2006 revealed that T. terrestris extract administered orally to C57BL/6J mice resulted in a significantly higher expression of melanocyte-stimulating hormone in the hair follicles of treated mice (75%), compared with that in the control group (18.75%). The researchers concluded that T. terrestris galvanizes tyrosinase activity and fosters melanocyte increase, melanin production, and the epidermal movement of dormant melanocytes.8
Anticancer activity
Kumar et al. showed in 2006 that the aqueous extracts of T. terrestris roots and fruits displayed chemopreventive activity in male Swiss albino mice. Specifically, oral administration of T. terrestris before, during, and after papillomagenesis induced by 7, 12-Dimethylbenz(a)anthracene (DMBA) resulted in significant decreases in tumor incidence, tumor burden, and cumulative number of papillomas, as well as a significant increase in average latent period as compared with the control group treated with DMBA and croton oil.9
The next year, Neychev et al. published a study on the effects of T. terrestris–derived saponins on normal human skin fibroblasts with a focus on anticancer activities. The researchers noted that the botanical engendered a dose-dependent reduction in [3H]-thymidine incorporation into the DNA of treated fibroblasts, which was not the case for untreated controls. This and several other metrics suggested that T. terrestris poses much less toxicity to normal human skin fibroblasts than multiple previously explored cancer lines by virtue of the up-regulation and down-regulation of polyamine homeostasis, hampering proliferation, and apoptosis induction.10
In 2012, Sisto et al. investigated the effects of T. terrestris–derived saponins on apoptosis in normal human keratinocytes exposed to UVB, as well as their antitumoral activity. They found that the saponins blunted UVB-induced apoptosis in normal human keratinocytes and did not render malignant keratinocytes more resistant to UVB in squamous cell carcinomas. The investigators concluded that their findings suggest a preventive capacity of T. terrestris against UVB-induced damage and carcinogenesis.11
Conclusion
As is the case with numerous botanical agents used for health purposes, where there’s smoke, there’s fire. That is, T. terrestris has warranted investigation for its applicability in the modern health armamentarium. I hope that conservation efforts for this plant will prevail, as much more research is necessary to determine whether it can become useful in the dermatologic realm.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers,“The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems.
References
1. Qureshi A et al. J Diet Suppl. 2014 Mar;11(1):64-79.
2. Zhu W et al. Chem Cent J. 2017 Jul 11;11(1):60.
3. Chhatre S et al. Pharmacogn Rev. 2014 Jan;8(15):45-51
4. Shahid M et al. J Biol Regul Homeost Agents. 2016 Jul-Sep;30(3):785-8.
5. Singh AG et al. J Ethnobiol Ethnomed. 2012 May 16;8:19.
6. Neychev V et al. J Ethnopharmacol. 2016 Feb 17;179:345-55.
7. Deng Y et al. Di Yi Jun Yi Da Xue Xue Bao. 2002 Nov;22(11):1017-9.
8. Yang L et al. Nan Fang Yi Ke Da Xue Xue Bao. 2006 Dec;26(12):1777-9.
9. Kumar M et al. Asian Pac J Cancer Prev. 2006 Apr-Jun;7(2):289-94.
10. Neychev VK et al. Exp Biol Med (Maywood). 2007 Jan;232(1):126-33.
11. Sisto M et al. J Photochem Photobiol B. 2012 Dec 5;117:193-201.
A member of the Zygophyllaceae family, Tribulus terrestris, also known as Gokshura, Gokharu, or puncture vine, is an annual herb; its aerial parts, roots, and fruits have been used in traditional medicine for anti-inflammatory, diuretic, tonic, antimicrobial, and aphrodisiac purposes for thousands of years in China, India, Pakistan, and Sudan.1-3 In modern times, the health benefits of T. terrestris have been attributed to the constituent saponins, flavonoids, alkaloids, lignins, amides, and glycosides that have been isolated and found as bioactive compounds in the plant.2-4
In an ethnobotanical survey of medicinal plants used in Nepal that was conducted in 2010 and 2011, Singh et al. found that T. terrestris was one of the 66 plant species important in the region. They also reported that it is one of the threatened species requiring conservation efforts.5 Although T. terrestris has long had a reputation for aphrodisiac qualities, critical reviews of the literature have undermined this historical reputation.1,6 Nevertheless, the botanical agent is used most often to treat infertility and loss of libido.4 More germane to the dermatologic realm, T. terrestris is thought to exhibit antioxidant, anticarcinogenic, and immunomodulatory potential, among other health benefits.4
Skin lightening activity
In a study published in 2002, Deng et al. evaluated the effects of a decoction of T. terrestris on tyrosinase activity and melanogenesis on cultured human melanocytes. They found that the amount of melanin increased when the decoction was administered in higher concentrations (optimally 1.5 mg/mL) but the effects were reversed at lower concentrations (0.5 mg/mL). Similarly, tyrosinase activity was facilitated by high concentrations of the decoction (optimally 100 mg/mL) and hindered at low concentrations (10 mg/mL). The investigators concluded that T. terrestris showed intriguing potential for use as a skin lightening agent that warranted further study.7
A mouse study performed by Yang et al. in 2006 revealed that T. terrestris extract administered orally to C57BL/6J mice resulted in a significantly higher expression of melanocyte-stimulating hormone in the hair follicles of treated mice (75%), compared with that in the control group (18.75%). The researchers concluded that T. terrestris galvanizes tyrosinase activity and fosters melanocyte increase, melanin production, and the epidermal movement of dormant melanocytes.8
Anticancer activity
Kumar et al. showed in 2006 that the aqueous extracts of T. terrestris roots and fruits displayed chemopreventive activity in male Swiss albino mice. Specifically, oral administration of T. terrestris before, during, and after papillomagenesis induced by 7, 12-Dimethylbenz(a)anthracene (DMBA) resulted in significant decreases in tumor incidence, tumor burden, and cumulative number of papillomas, as well as a significant increase in average latent period as compared with the control group treated with DMBA and croton oil.9
The next year, Neychev et al. published a study on the effects of T. terrestris–derived saponins on normal human skin fibroblasts with a focus on anticancer activities. The researchers noted that the botanical engendered a dose-dependent reduction in [3H]-thymidine incorporation into the DNA of treated fibroblasts, which was not the case for untreated controls. This and several other metrics suggested that T. terrestris poses much less toxicity to normal human skin fibroblasts than multiple previously explored cancer lines by virtue of the up-regulation and down-regulation of polyamine homeostasis, hampering proliferation, and apoptosis induction.10
In 2012, Sisto et al. investigated the effects of T. terrestris–derived saponins on apoptosis in normal human keratinocytes exposed to UVB, as well as their antitumoral activity. They found that the saponins blunted UVB-induced apoptosis in normal human keratinocytes and did not render malignant keratinocytes more resistant to UVB in squamous cell carcinomas. The investigators concluded that their findings suggest a preventive capacity of T. terrestris against UVB-induced damage and carcinogenesis.11
Conclusion
As is the case with numerous botanical agents used for health purposes, where there’s smoke, there’s fire. That is, T. terrestris has warranted investigation for its applicability in the modern health armamentarium. I hope that conservation efforts for this plant will prevail, as much more research is necessary to determine whether it can become useful in the dermatologic realm.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers,“The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems.
References
1. Qureshi A et al. J Diet Suppl. 2014 Mar;11(1):64-79.
2. Zhu W et al. Chem Cent J. 2017 Jul 11;11(1):60.
3. Chhatre S et al. Pharmacogn Rev. 2014 Jan;8(15):45-51
4. Shahid M et al. J Biol Regul Homeost Agents. 2016 Jul-Sep;30(3):785-8.
5. Singh AG et al. J Ethnobiol Ethnomed. 2012 May 16;8:19.
6. Neychev V et al. J Ethnopharmacol. 2016 Feb 17;179:345-55.
7. Deng Y et al. Di Yi Jun Yi Da Xue Xue Bao. 2002 Nov;22(11):1017-9.
8. Yang L et al. Nan Fang Yi Ke Da Xue Xue Bao. 2006 Dec;26(12):1777-9.
9. Kumar M et al. Asian Pac J Cancer Prev. 2006 Apr-Jun;7(2):289-94.
10. Neychev VK et al. Exp Biol Med (Maywood). 2007 Jan;232(1):126-33.
11. Sisto M et al. J Photochem Photobiol B. 2012 Dec 5;117:193-201.
A member of the Zygophyllaceae family, Tribulus terrestris, also known as Gokshura, Gokharu, or puncture vine, is an annual herb; its aerial parts, roots, and fruits have been used in traditional medicine for anti-inflammatory, diuretic, tonic, antimicrobial, and aphrodisiac purposes for thousands of years in China, India, Pakistan, and Sudan.1-3 In modern times, the health benefits of T. terrestris have been attributed to the constituent saponins, flavonoids, alkaloids, lignins, amides, and glycosides that have been isolated and found as bioactive compounds in the plant.2-4
In an ethnobotanical survey of medicinal plants used in Nepal that was conducted in 2010 and 2011, Singh et al. found that T. terrestris was one of the 66 plant species important in the region. They also reported that it is one of the threatened species requiring conservation efforts.5 Although T. terrestris has long had a reputation for aphrodisiac qualities, critical reviews of the literature have undermined this historical reputation.1,6 Nevertheless, the botanical agent is used most often to treat infertility and loss of libido.4 More germane to the dermatologic realm, T. terrestris is thought to exhibit antioxidant, anticarcinogenic, and immunomodulatory potential, among other health benefits.4
Skin lightening activity
In a study published in 2002, Deng et al. evaluated the effects of a decoction of T. terrestris on tyrosinase activity and melanogenesis on cultured human melanocytes. They found that the amount of melanin increased when the decoction was administered in higher concentrations (optimally 1.5 mg/mL) but the effects were reversed at lower concentrations (0.5 mg/mL). Similarly, tyrosinase activity was facilitated by high concentrations of the decoction (optimally 100 mg/mL) and hindered at low concentrations (10 mg/mL). The investigators concluded that T. terrestris showed intriguing potential for use as a skin lightening agent that warranted further study.7
A mouse study performed by Yang et al. in 2006 revealed that T. terrestris extract administered orally to C57BL/6J mice resulted in a significantly higher expression of melanocyte-stimulating hormone in the hair follicles of treated mice (75%), compared with that in the control group (18.75%). The researchers concluded that T. terrestris galvanizes tyrosinase activity and fosters melanocyte increase, melanin production, and the epidermal movement of dormant melanocytes.8
Anticancer activity
Kumar et al. showed in 2006 that the aqueous extracts of T. terrestris roots and fruits displayed chemopreventive activity in male Swiss albino mice. Specifically, oral administration of T. terrestris before, during, and after papillomagenesis induced by 7, 12-Dimethylbenz(a)anthracene (DMBA) resulted in significant decreases in tumor incidence, tumor burden, and cumulative number of papillomas, as well as a significant increase in average latent period as compared with the control group treated with DMBA and croton oil.9
The next year, Neychev et al. published a study on the effects of T. terrestris–derived saponins on normal human skin fibroblasts with a focus on anticancer activities. The researchers noted that the botanical engendered a dose-dependent reduction in [3H]-thymidine incorporation into the DNA of treated fibroblasts, which was not the case for untreated controls. This and several other metrics suggested that T. terrestris poses much less toxicity to normal human skin fibroblasts than multiple previously explored cancer lines by virtue of the up-regulation and down-regulation of polyamine homeostasis, hampering proliferation, and apoptosis induction.10
In 2012, Sisto et al. investigated the effects of T. terrestris–derived saponins on apoptosis in normal human keratinocytes exposed to UVB, as well as their antitumoral activity. They found that the saponins blunted UVB-induced apoptosis in normal human keratinocytes and did not render malignant keratinocytes more resistant to UVB in squamous cell carcinomas. The investigators concluded that their findings suggest a preventive capacity of T. terrestris against UVB-induced damage and carcinogenesis.11
Conclusion
As is the case with numerous botanical agents used for health purposes, where there’s smoke, there’s fire. That is, T. terrestris has warranted investigation for its applicability in the modern health armamentarium. I hope that conservation efforts for this plant will prevail, as much more research is necessary to determine whether it can become useful in the dermatologic realm.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers,“The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems.
References
1. Qureshi A et al. J Diet Suppl. 2014 Mar;11(1):64-79.
2. Zhu W et al. Chem Cent J. 2017 Jul 11;11(1):60.
3. Chhatre S et al. Pharmacogn Rev. 2014 Jan;8(15):45-51
4. Shahid M et al. J Biol Regul Homeost Agents. 2016 Jul-Sep;30(3):785-8.
5. Singh AG et al. J Ethnobiol Ethnomed. 2012 May 16;8:19.
6. Neychev V et al. J Ethnopharmacol. 2016 Feb 17;179:345-55.
7. Deng Y et al. Di Yi Jun Yi Da Xue Xue Bao. 2002 Nov;22(11):1017-9.
8. Yang L et al. Nan Fang Yi Ke Da Xue Xue Bao. 2006 Dec;26(12):1777-9.
9. Kumar M et al. Asian Pac J Cancer Prev. 2006 Apr-Jun;7(2):289-94.
10. Neychev VK et al. Exp Biol Med (Maywood). 2007 Jan;232(1):126-33.
11. Sisto M et al. J Photochem Photobiol B. 2012 Dec 5;117:193-201.
Laser tattoo removal techniques continue to be refined
SAN DIEGO –
“A picosecond is to a second as 1 second is to 37,000 years,” Mathew M. Avram, MD, JD, said at the annual Masters of Aesthetics Symposium. “That’s equivalent to the total energy of the city of San Diego for 300-750 trillionths of a second.”
According to Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, picosecond lasers produce extreme cavitation and cell rupture, with a desired clinical endpoint of immediate dermal whitening of tattooed skin. The process causes transdermal elimination of the tattoo ink. Some of the ink flows into the lymphatic system, while the rest undergoes rephagocytosis by dermal scavenger cells.
Commercially available picosecond lasers include devices with wavelengths of 532 nm, 755 nm, and 1,064 nm that deliver energy in a range of 300-750 picoseconds. Nd:YAG lasers work best for red and black ink, while alexandrite lasers work best for green and blue ink. In Dr. Avram’s experience, picosecond lasers are generally more effective for tattoo removal, compared with nanosecond lasers. “There is some nonselective targeting of other pigments, and they’re particularly effective for faded tattoos, but the devices are more expensive,” he said.
Dr. Avram, who is also the faculty director for laser and cosmetic dermatology training at Harvard Medical School, also in Boston, advises against promising a certain number of laser treatments during initial patient consultations. “You will regret it,” he said. “Tattoos are notoriously unpredictable in how they respond. I often hear people say they get rid of these in three to five treatments. That isn’t my experience with these lasers. Often, all you’re going to be able to do is get significant clearing rather than tattoo removal. Professional tattoos are the most difficult to treat because they are the deepest and they have the most amount of ink.”
On the other hand, amateur tattoos, traumatic tattoos, and radiation tattoos require far fewer treatments. “The color is important,” he said. “Multicolored tattoos, regardless of the colors, are always going to be more difficult to clear than a single-color tattoo.” Black and dark-blue tattoos respond best to laser light; light-blue and green also respond well. Red responds well, while purple can be challenging. Yellow and orange do not respond very well, but they do respond partially.
According to a trial that analyzed variables influencing the outcome of tattoos treated by Q-switched lasers, 47% were cleared after 10 sessions, while 75% were cleared after 15 sessions (Arch Dermatol 2012;148[12]:1364-9). “It’s very important to message to your patients how many treatments this might take, because there is going to be an annuity of patients who are unhappy because they have to keep coming back,” said Dr. Avram, who is the immediate past president of the American Society for Laser Medicine and Surgery. Skin type and pigmentation also affect treatment outcomes. “For darker skin types or tanned individuals, hyper- or hypopigmentation is a greater concern than in patients with lighter skin types,” he said. “A test spot may be beneficial. The 1,064-nm Q-switched Nd:YAG laser is least likely to affect skin pigment; it’s safest for skin types IV-VI . This is great if it’s a black tattoo. But if it’s a green, blue, or red tattoo, you have a problem because you’re not going to target it very effectively.”
Some degree of posttreatment hypopigmentation is likely to occur, regardless of skin type. “Let patients know this is going to happen, but over time, this usually resolves, because you’re not destroying the melanocytes, unless you’re going too strong,” Dr. Avram said. “It may take a few months. It may take a year or 2, but the pigment should recur.”
He emphasized that the key variable during laser treatment of tattoos is the clinical endpoint, not the energy setting of the device. “What you want to see is immediate whitening of the treated area,” he said. “With the 1,064-nm Nd:YAG, you may get a little pinpoint bleeding in addition to whitening. Do not memorize treatment settings. Many Q-switched lasers are not externally calibrated. Thus, energy levels may change day to day or before and after servicing [of the device]. Trust your eyes; trust your clinical skills.” If you see epidermal disruption and bleeding during treatment, you’re probably being too aggressive. If that happens, “decrease your fluence,” he recommended. “You also want to decrease fluences when treating tattoos that are placed over other tattoos.”
Another rule of thumb is to use larger spot sizes during treatment sessions. “The larger the spot size, the more efficient the energy is going to get more deeply, and less is going to be at the dermal-epidermal junction,” Dr. Avram said. “So you’re going to get less hypopigmentation and less hyperpigmentation. Follow your endpoints and you are less likely to get pigmentation changes.”
Posttreatment care typically includes the application of topical petroleum jelly and a Telfa dressing. “Wait about a week to heal, counsel patients to keep out of the sun, and avoid friction to the treated area during healing,” he said. Patients can be rescheduled for retreatment 6-8 weeks later.
Common adverse events during laser treatment of tattoos include erythema, blistering, hyperpigmentation, hypopigmentation, and scarring, which occurs in about 5% of cases. Less common adverse events include allergic reaction, darkening of cosmetic tattoos, immune reaction, and chrysiasis, which is a dark-blue pigmentation caused by Q-switched laser treatment in patients with a history of gold salt ingestion. “Any history of gold salt ingestion will produce this characteristic finding, even if they took it when they were 5 years old and they come to you when they’re 85,” Dr. Avram said. “All of our intake forms include a question about this, and before I treat patients I always ask if they have a history of gold ingestion, because it’s very difficult to treat.”
Surgical excision may be an alternative for smaller tattoos. “Another option is ablative fractional resurfacing as a solo treatment or in combination with the Q-switched or picosecond laser, which has better efficacy,” he said. “The ablative fractional laser also may help with fibrosis after multiple treatments in a recalcitrant tattoo.” He noted that cosmetic tattoos such as lip liner and blush tattoos might darken because of oxidation of ferric oxide or titanium oxide pigment. The best approach to such cases is to perform an inconspicuous test spot prior to treatment.
Clinicians continue to explore the optimal interval between treatments. For example, the “R20” method consists of four consecutive treatment passes separated by 20 minutes. The initial study found that this approach led to better outcomes, compared with conventional, single-pass laser treatment (J Am Acad Dermatol 2012;66[2]:271-7). A follow-up study by Dr. Avram and his colleagues contradicted these findings, while another follow-up study was supportive.
Another technology playing a role in such repeat treatments is a perfluorodecalin-infused silicone patch, which is placed over the treatment area. According to Dr. Avram, the FDA-cleared patch helps reduce scatter during treatment and likely improves efficacy. It also allows for performing consecutive repeat laser treatments at the same visit. In one study, 11 of 17 patients had more rapid clearance on the side treated with the perfluorodecalin patch, compared with the side treated without the patch (Laser Surg Med 2015;47[8]:613-8).
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea.
[email protected]
SAN DIEGO –

“A picosecond is to a second as 1 second is to 37,000 years,” Mathew M. Avram, MD, JD, said at the annual Masters of Aesthetics Symposium. “That’s equivalent to the total energy of the city of San Diego for 300-750 trillionths of a second.”
According to Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, picosecond lasers produce extreme cavitation and cell rupture, with a desired clinical endpoint of immediate dermal whitening of tattooed skin. The process causes transdermal elimination of the tattoo ink. Some of the ink flows into the lymphatic system, while the rest undergoes rephagocytosis by dermal scavenger cells.
Commercially available picosecond lasers include devices with wavelengths of 532 nm, 755 nm, and 1,064 nm that deliver energy in a range of 300-750 picoseconds. Nd:YAG lasers work best for red and black ink, while alexandrite lasers work best for green and blue ink. In Dr. Avram’s experience, picosecond lasers are generally more effective for tattoo removal, compared with nanosecond lasers. “There is some nonselective targeting of other pigments, and they’re particularly effective for faded tattoos, but the devices are more expensive,” he said.
Dr. Avram, who is also the faculty director for laser and cosmetic dermatology training at Harvard Medical School, also in Boston, advises against promising a certain number of laser treatments during initial patient consultations. “You will regret it,” he said. “Tattoos are notoriously unpredictable in how they respond. I often hear people say they get rid of these in three to five treatments. That isn’t my experience with these lasers. Often, all you’re going to be able to do is get significant clearing rather than tattoo removal. Professional tattoos are the most difficult to treat because they are the deepest and they have the most amount of ink.”
On the other hand, amateur tattoos, traumatic tattoos, and radiation tattoos require far fewer treatments. “The color is important,” he said. “Multicolored tattoos, regardless of the colors, are always going to be more difficult to clear than a single-color tattoo.” Black and dark-blue tattoos respond best to laser light; light-blue and green also respond well. Red responds well, while purple can be challenging. Yellow and orange do not respond very well, but they do respond partially.
According to a trial that analyzed variables influencing the outcome of tattoos treated by Q-switched lasers, 47% were cleared after 10 sessions, while 75% were cleared after 15 sessions (Arch Dermatol 2012;148[12]:1364-9). “It’s very important to message to your patients how many treatments this might take, because there is going to be an annuity of patients who are unhappy because they have to keep coming back,” said Dr. Avram, who is the immediate past president of the American Society for Laser Medicine and Surgery. Skin type and pigmentation also affect treatment outcomes. “For darker skin types or tanned individuals, hyper- or hypopigmentation is a greater concern than in patients with lighter skin types,” he said. “A test spot may be beneficial. The 1,064-nm Q-switched Nd:YAG laser is least likely to affect skin pigment; it’s safest for skin types IV-VI . This is great if it’s a black tattoo. But if it’s a green, blue, or red tattoo, you have a problem because you’re not going to target it very effectively.”
Some degree of posttreatment hypopigmentation is likely to occur, regardless of skin type. “Let patients know this is going to happen, but over time, this usually resolves, because you’re not destroying the melanocytes, unless you’re going too strong,” Dr. Avram said. “It may take a few months. It may take a year or 2, but the pigment should recur.”
He emphasized that the key variable during laser treatment of tattoos is the clinical endpoint, not the energy setting of the device. “What you want to see is immediate whitening of the treated area,” he said. “With the 1,064-nm Nd:YAG, you may get a little pinpoint bleeding in addition to whitening. Do not memorize treatment settings. Many Q-switched lasers are not externally calibrated. Thus, energy levels may change day to day or before and after servicing [of the device]. Trust your eyes; trust your clinical skills.” If you see epidermal disruption and bleeding during treatment, you’re probably being too aggressive. If that happens, “decrease your fluence,” he recommended. “You also want to decrease fluences when treating tattoos that are placed over other tattoos.”
Another rule of thumb is to use larger spot sizes during treatment sessions. “The larger the spot size, the more efficient the energy is going to get more deeply, and less is going to be at the dermal-epidermal junction,” Dr. Avram said. “So you’re going to get less hypopigmentation and less hyperpigmentation. Follow your endpoints and you are less likely to get pigmentation changes.”
Posttreatment care typically includes the application of topical petroleum jelly and a Telfa dressing. “Wait about a week to heal, counsel patients to keep out of the sun, and avoid friction to the treated area during healing,” he said. Patients can be rescheduled for retreatment 6-8 weeks later.
Common adverse events during laser treatment of tattoos include erythema, blistering, hyperpigmentation, hypopigmentation, and scarring, which occurs in about 5% of cases. Less common adverse events include allergic reaction, darkening of cosmetic tattoos, immune reaction, and chrysiasis, which is a dark-blue pigmentation caused by Q-switched laser treatment in patients with a history of gold salt ingestion. “Any history of gold salt ingestion will produce this characteristic finding, even if they took it when they were 5 years old and they come to you when they’re 85,” Dr. Avram said. “All of our intake forms include a question about this, and before I treat patients I always ask if they have a history of gold ingestion, because it’s very difficult to treat.”
Surgical excision may be an alternative for smaller tattoos. “Another option is ablative fractional resurfacing as a solo treatment or in combination with the Q-switched or picosecond laser, which has better efficacy,” he said. “The ablative fractional laser also may help with fibrosis after multiple treatments in a recalcitrant tattoo.” He noted that cosmetic tattoos such as lip liner and blush tattoos might darken because of oxidation of ferric oxide or titanium oxide pigment. The best approach to such cases is to perform an inconspicuous test spot prior to treatment.
Clinicians continue to explore the optimal interval between treatments. For example, the “R20” method consists of four consecutive treatment passes separated by 20 minutes. The initial study found that this approach led to better outcomes, compared with conventional, single-pass laser treatment (J Am Acad Dermatol 2012;66[2]:271-7). A follow-up study by Dr. Avram and his colleagues contradicted these findings, while another follow-up study was supportive.
Another technology playing a role in such repeat treatments is a perfluorodecalin-infused silicone patch, which is placed over the treatment area. According to Dr. Avram, the FDA-cleared patch helps reduce scatter during treatment and likely improves efficacy. It also allows for performing consecutive repeat laser treatments at the same visit. In one study, 11 of 17 patients had more rapid clearance on the side treated with the perfluorodecalin patch, compared with the side treated without the patch (Laser Surg Med 2015;47[8]:613-8).
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea.
[email protected]
SAN DIEGO –

“A picosecond is to a second as 1 second is to 37,000 years,” Mathew M. Avram, MD, JD, said at the annual Masters of Aesthetics Symposium. “That’s equivalent to the total energy of the city of San Diego for 300-750 trillionths of a second.”
According to Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, picosecond lasers produce extreme cavitation and cell rupture, with a desired clinical endpoint of immediate dermal whitening of tattooed skin. The process causes transdermal elimination of the tattoo ink. Some of the ink flows into the lymphatic system, while the rest undergoes rephagocytosis by dermal scavenger cells.
Commercially available picosecond lasers include devices with wavelengths of 532 nm, 755 nm, and 1,064 nm that deliver energy in a range of 300-750 picoseconds. Nd:YAG lasers work best for red and black ink, while alexandrite lasers work best for green and blue ink. In Dr. Avram’s experience, picosecond lasers are generally more effective for tattoo removal, compared with nanosecond lasers. “There is some nonselective targeting of other pigments, and they’re particularly effective for faded tattoos, but the devices are more expensive,” he said.
Dr. Avram, who is also the faculty director for laser and cosmetic dermatology training at Harvard Medical School, also in Boston, advises against promising a certain number of laser treatments during initial patient consultations. “You will regret it,” he said. “Tattoos are notoriously unpredictable in how they respond. I often hear people say they get rid of these in three to five treatments. That isn’t my experience with these lasers. Often, all you’re going to be able to do is get significant clearing rather than tattoo removal. Professional tattoos are the most difficult to treat because they are the deepest and they have the most amount of ink.”
On the other hand, amateur tattoos, traumatic tattoos, and radiation tattoos require far fewer treatments. “The color is important,” he said. “Multicolored tattoos, regardless of the colors, are always going to be more difficult to clear than a single-color tattoo.” Black and dark-blue tattoos respond best to laser light; light-blue and green also respond well. Red responds well, while purple can be challenging. Yellow and orange do not respond very well, but they do respond partially.
According to a trial that analyzed variables influencing the outcome of tattoos treated by Q-switched lasers, 47% were cleared after 10 sessions, while 75% were cleared after 15 sessions (Arch Dermatol 2012;148[12]:1364-9). “It’s very important to message to your patients how many treatments this might take, because there is going to be an annuity of patients who are unhappy because they have to keep coming back,” said Dr. Avram, who is the immediate past president of the American Society for Laser Medicine and Surgery. Skin type and pigmentation also affect treatment outcomes. “For darker skin types or tanned individuals, hyper- or hypopigmentation is a greater concern than in patients with lighter skin types,” he said. “A test spot may be beneficial. The 1,064-nm Q-switched Nd:YAG laser is least likely to affect skin pigment; it’s safest for skin types IV-VI . This is great if it’s a black tattoo. But if it’s a green, blue, or red tattoo, you have a problem because you’re not going to target it very effectively.”
Some degree of posttreatment hypopigmentation is likely to occur, regardless of skin type. “Let patients know this is going to happen, but over time, this usually resolves, because you’re not destroying the melanocytes, unless you’re going too strong,” Dr. Avram said. “It may take a few months. It may take a year or 2, but the pigment should recur.”
He emphasized that the key variable during laser treatment of tattoos is the clinical endpoint, not the energy setting of the device. “What you want to see is immediate whitening of the treated area,” he said. “With the 1,064-nm Nd:YAG, you may get a little pinpoint bleeding in addition to whitening. Do not memorize treatment settings. Many Q-switched lasers are not externally calibrated. Thus, energy levels may change day to day or before and after servicing [of the device]. Trust your eyes; trust your clinical skills.” If you see epidermal disruption and bleeding during treatment, you’re probably being too aggressive. If that happens, “decrease your fluence,” he recommended. “You also want to decrease fluences when treating tattoos that are placed over other tattoos.”
Another rule of thumb is to use larger spot sizes during treatment sessions. “The larger the spot size, the more efficient the energy is going to get more deeply, and less is going to be at the dermal-epidermal junction,” Dr. Avram said. “So you’re going to get less hypopigmentation and less hyperpigmentation. Follow your endpoints and you are less likely to get pigmentation changes.”
Posttreatment care typically includes the application of topical petroleum jelly and a Telfa dressing. “Wait about a week to heal, counsel patients to keep out of the sun, and avoid friction to the treated area during healing,” he said. Patients can be rescheduled for retreatment 6-8 weeks later.
Common adverse events during laser treatment of tattoos include erythema, blistering, hyperpigmentation, hypopigmentation, and scarring, which occurs in about 5% of cases. Less common adverse events include allergic reaction, darkening of cosmetic tattoos, immune reaction, and chrysiasis, which is a dark-blue pigmentation caused by Q-switched laser treatment in patients with a history of gold salt ingestion. “Any history of gold salt ingestion will produce this characteristic finding, even if they took it when they were 5 years old and they come to you when they’re 85,” Dr. Avram said. “All of our intake forms include a question about this, and before I treat patients I always ask if they have a history of gold ingestion, because it’s very difficult to treat.”
Surgical excision may be an alternative for smaller tattoos. “Another option is ablative fractional resurfacing as a solo treatment or in combination with the Q-switched or picosecond laser, which has better efficacy,” he said. “The ablative fractional laser also may help with fibrosis after multiple treatments in a recalcitrant tattoo.” He noted that cosmetic tattoos such as lip liner and blush tattoos might darken because of oxidation of ferric oxide or titanium oxide pigment. The best approach to such cases is to perform an inconspicuous test spot prior to treatment.
Clinicians continue to explore the optimal interval between treatments. For example, the “R20” method consists of four consecutive treatment passes separated by 20 minutes. The initial study found that this approach led to better outcomes, compared with conventional, single-pass laser treatment (J Am Acad Dermatol 2012;66[2]:271-7). A follow-up study by Dr. Avram and his colleagues contradicted these findings, while another follow-up study was supportive.
Another technology playing a role in such repeat treatments is a perfluorodecalin-infused silicone patch, which is placed over the treatment area. According to Dr. Avram, the FDA-cleared patch helps reduce scatter during treatment and likely improves efficacy. It also allows for performing consecutive repeat laser treatments at the same visit. In one study, 11 of 17 patients had more rapid clearance on the side treated with the perfluorodecalin patch, compared with the side treated without the patch (Laser Surg Med 2015;47[8]:613-8).
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea.
[email protected]
AT MOAS 2018
Learning Curves: Historical Trends of FDA-Reported Adverse Events for Dermal Fillers
Dermal fillers are considered Class III medical devices by the US Food and Drug Administration (FDA).1 Reports of adverse events (AEs) for medical devices are made public by the FDA to allow for transparent postmarketing surveillance.2The AE trends extracted from these historical data may help distinguish between expected learning curves of new dermal fillers versus unsafe products that may require FDA intervention. Considering that aesthetic treatments are not medically necessary, a low risk profile is paramount and determining what constitutes normal learning curves is important for impartial assessment of AEs as new fillers come on the market. The concept of a 3-year learning curve can be an important tool for safety monitoring going forward, creating a bar for quality that could trigger increased surveillance if a product fails to meet an expected arc of diminished AEs over time. This study serves to evaluate historical AE data and to establish learning curves for FDA-approved dermal fillers.
Methods
We searched the OpenFDA Device Adverse Event Report Browser (http://openfda.shinyapps.io/devicereports/) for reported AEs within the FDA product code LMH (Implant, Dermal, For Aesthetic Use) that were received from January 1, 1983, to December 31, 2017. For each reported AE, information related to the date of the reported event and the device brand name were recorded. Devices implicated in each AE were classified based on primary composition according to the following 5 categories: collagen, hyaluronic acid (HA), hydroxylapatite, poly-L-lactic acid (PLLA), and polymethyl methacrylate (PMMA). Inaccurate entries of reported AEs or those intended for nonaesthetic use were excluded from the study. A total of 8530 AEs were included in the study. To normalize the data, we obtained annual reports for the number of procedures performed by filler type from the American Society of Plastic Surgeons (ASPS) cosmetic procedure trends. 3 We calculated the annual AE rates for each approved filler by dividing the number of AEs by the number of procedures performed that year.
Results
The trends of different filler types depicting the number of procedures performed over time are shown in Figure 1. Data from the ASPS dated back to 2005; therefore, the number of procedures performed prior to that were extrapolated with knowledge of products’ approval dates and market share, indicated by a dotted line. To determine AE rates for each year, we divided the number of AEs by the number of reported procedures for each filler type. The AE rates are displayed graphically in Figures 2 and 3 with superimposed FDA approval dates for each filler.4
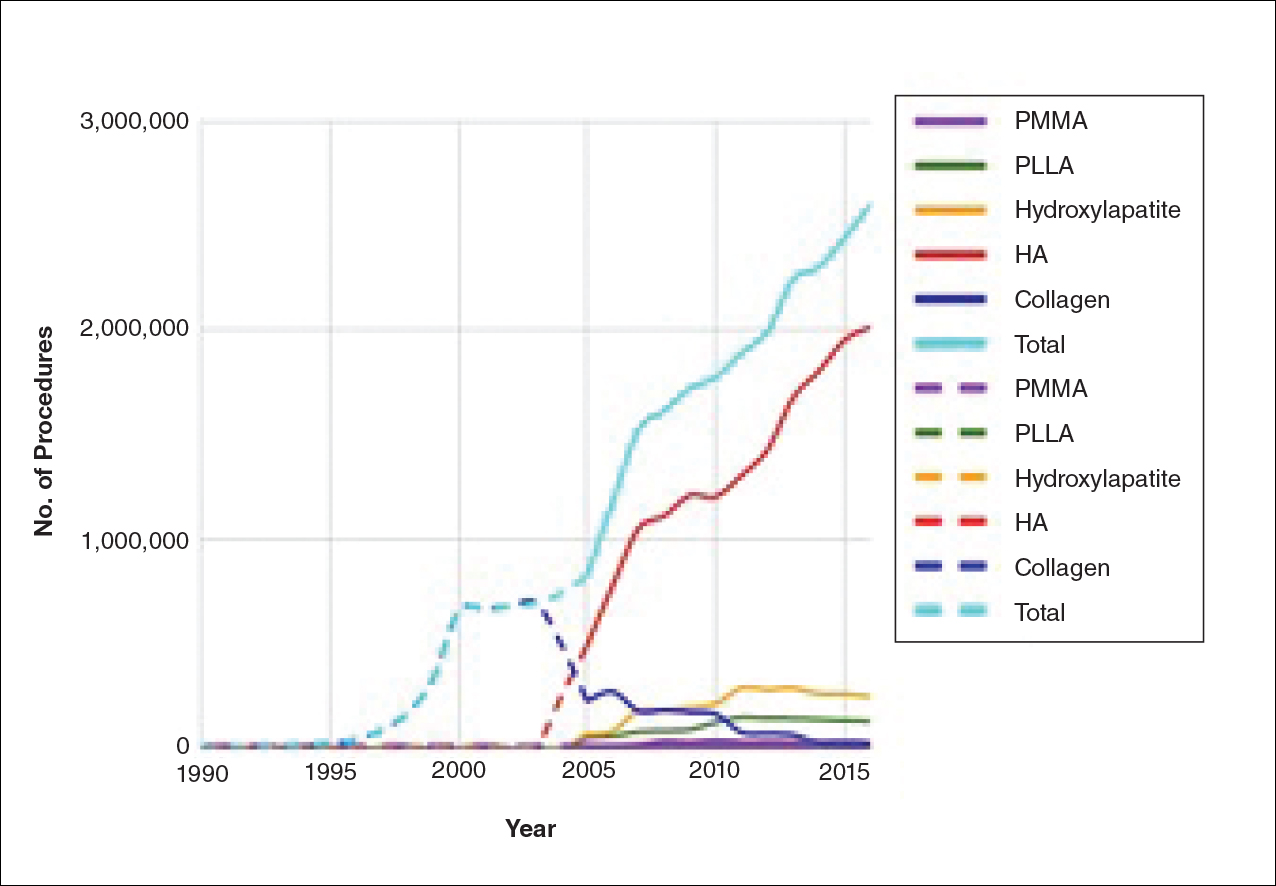
Two major peaks in reported AE rates for all fillers were noted in the late 1990s and late 2000s, mostly associated with collagen and PLLA fillers, respectively (Figure 2). Overall, there has been a low rate of AEs associated with HA fillers since their initial approval in the early 2000s.
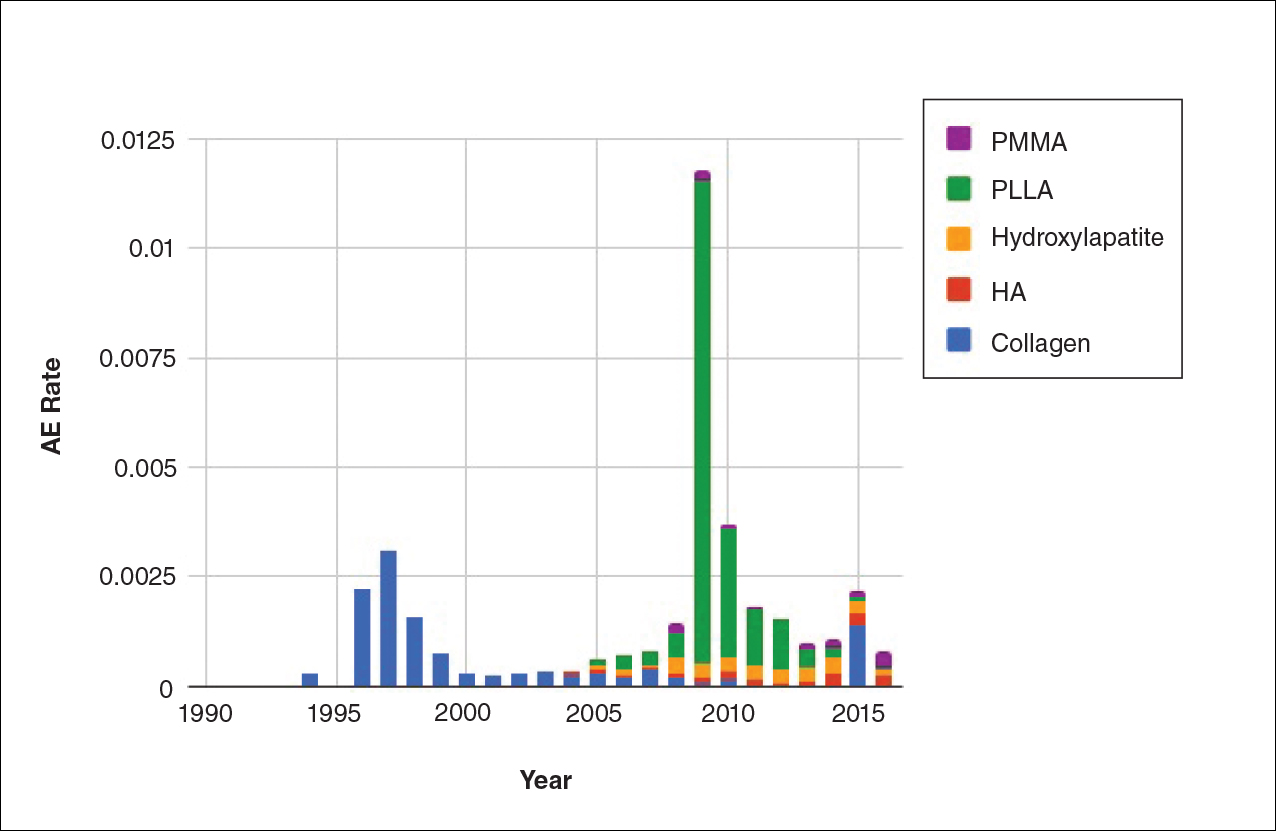
Individual filler AE rates also were analyzed. Hyaluronic acid fillers were associated with an extremely low rate of AEs, ranging from 1 to 4 AEs per 10,000 procedures (Figure 3A). This low AE rate profile underscores the safety of HA fillers, which has spurred their popularity. Adverseevent rates for collagen fillers spiked in the mid- to late 1990s and resolved over the course of the next 3 years (Figure 3B). Hydroxylapatite fillers had a rather uniform AE rate with an early indication of a drop-off after 2015 (Figure 3C). Poly-L-lactic acid fillers showed the steepest learning curve, with a peak of 1 AE per 100 procedures after they were approved in 2008 (Figure 3D); however, there is a comparable 3-year resolution of AE rates. Adverse events for PMMA fillers did not show specific resolution, meaning that they did not follow the 3-year arc that was seen for the other dermal fillers reported in the data set (Figure 3E).
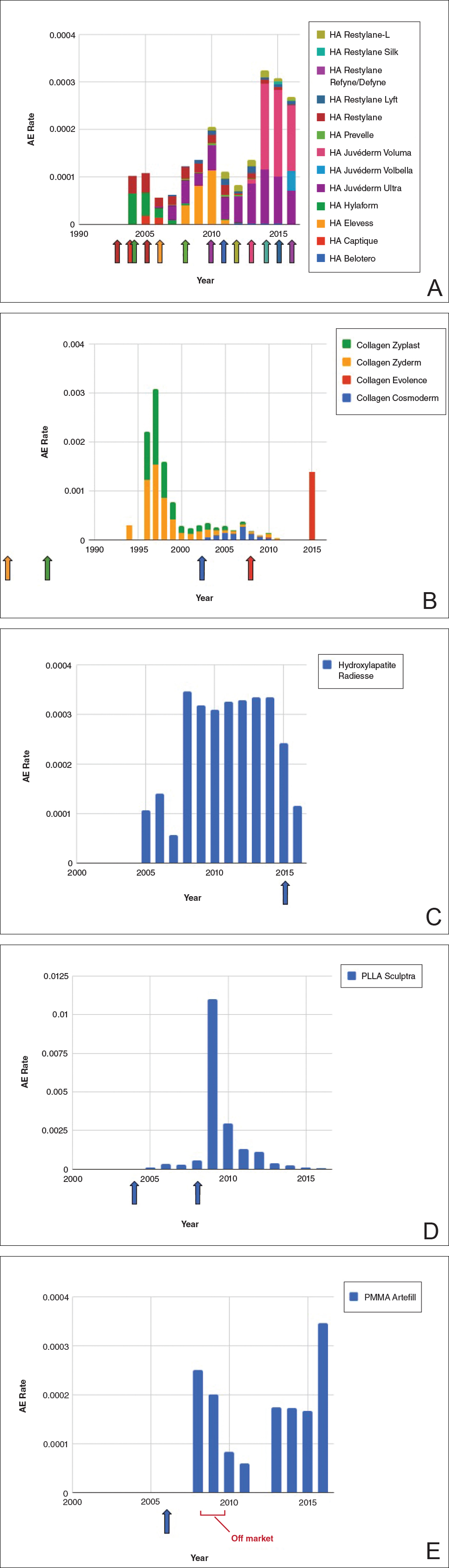
Comment
Our study is unique in that it analyzes reported AE data over a 34-year period for injectable dermal fillers. To our knowledge, this novel method of calculating AE rates across dermal fillers and for individual products is the first of its kind that facilitates usage-normalized comparison of different filler types.
All OpenFDA data are self-reported and therefore have inherent limitations. Anyone can enter information on AEs in this system, including both patients and health care providers, so the quality of the input may be variable. However, this output is the only representation we have for nearly 35 years of AE history for this burgeoning category of popular aesthetic treatments. Another study limitation is that not everyone may know that reporting an AE in the OpenFDA is an option; therefore, we may be missing a portion of AEs due to underreporting. Underreporting may be especially at play in the years before the Internet was prevalent for residential use since access to the Internet would be required to report an AR on the website. However, examining the available data provides an important window into valuable information on complications that have occurred and have been reported for FDA-approved dermal fillers.
An additional challenge in constructing this study was assessing the total number of injectable dermal filler treatments being performed annually across filler types for normalization of the data. Although the absolute numbers of filler use as captured by the ASPS are smaller than the true total filler use across all injectors, the relative use of different filler products will be similar across all specialties because it reflects product popularity. Annual surveys on aesthetic procedures also are conducted by the American Society for Dermatologic Surgery and the American Association for Facial Plastic and Reconstructive Surgery, but neither one captures the relative usage of different filler types. Because individual filler companies do not publish their annual sales numbers by product, the ASPS data give us the best gauge of relative use of fillers by product type given the available information. We conclude that the comparison of AE rates would remain the same even if we had data for total annual filler use across specialties.
Our graphical depiction of the data clearly demonstrates the low AE profile of HA fillers, which is in line with the general consensus of their safety that has contributed to their vast popularity; however, this study represents the first time usage-normalized AE rates are compared to other filler compositions. Hyaluronic acid fillers have the unique feature of being able to be dissolved with the hyaluronidase enzyme, which can limit adverse event potential as compared to other ingredient classes of filler types and may be reflected in their low overall AE profile. The AE rate spike and resolution for collagen fillers represent what we refer to as a “normal learning curve” based on our analysis of the data set as a whole, suggesting an appropriate time course of increased familiarity with the product without inherent issues with the product itself. Multiple sequential anatomic site indications were approved for hydroxylapatite fillers from 2006 through 2015, which may have yielded overlapping learning curves for each approval, resulting in a rather uniform AE rate. The early drop-off in AE rates after the 2015 anatomic site approval may represent the beginning of a normal learning curve, and continued surveillance of AE rates would be of value to confirm this trend. We saw a similar 3-year learning curve for PLLA fillers as the curve for collagen fillers, suggesting a normal learning curve and no out-of-line safety issues. Polymethylmethacrylate fillers were approved in 2006 and were taken off the market for a period in the late 2000s, explaining the drop-off. Once they were back on the market, we do not see a typical learning curve for PMMA, which may warrant surveillance for safety by both clinicians and the FDA.
Conclusion
Our study represents a novel method of evaluating the safety of medical devices, specifically aesthetic fillers. We showed that every AE rate curve for different filler types tells a story. Reactions to AEs for new fillers should be placed in the context of whether they seem to be following the established learning curve.
- Dermal fillers (soft tissue fillers). US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ucm2007470.htm. Updated May 31, 2018. Accessed June 29, 2018.
- Kass-Hout TA, Xu Z, Mohebbi M, et al. OpenFDA: an innovative platform providing access to a wealth of FDA’s publicly available data. J Am Med Inform Assoc. 2016;23:596-600.
- Plastic surgery statistics. American Society of Plastic Surgeons website. https://www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-full-report-2017.pdf. Accessed June 28, 2018.
- Dermal fillers approved by the Center for Devices and Radiological Health. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CosmeticDevices/WrinkleFillers/ucm227749.htm. Accessed June 28, 2018. Updated March 19, 2018.
Dermal fillers are considered Class III medical devices by the US Food and Drug Administration (FDA).1 Reports of adverse events (AEs) for medical devices are made public by the FDA to allow for transparent postmarketing surveillance.2The AE trends extracted from these historical data may help distinguish between expected learning curves of new dermal fillers versus unsafe products that may require FDA intervention. Considering that aesthetic treatments are not medically necessary, a low risk profile is paramount and determining what constitutes normal learning curves is important for impartial assessment of AEs as new fillers come on the market. The concept of a 3-year learning curve can be an important tool for safety monitoring going forward, creating a bar for quality that could trigger increased surveillance if a product fails to meet an expected arc of diminished AEs over time. This study serves to evaluate historical AE data and to establish learning curves for FDA-approved dermal fillers.
Methods
We searched the OpenFDA Device Adverse Event Report Browser (http://openfda.shinyapps.io/devicereports/) for reported AEs within the FDA product code LMH (Implant, Dermal, For Aesthetic Use) that were received from January 1, 1983, to December 31, 2017. For each reported AE, information related to the date of the reported event and the device brand name were recorded. Devices implicated in each AE were classified based on primary composition according to the following 5 categories: collagen, hyaluronic acid (HA), hydroxylapatite, poly-L-lactic acid (PLLA), and polymethyl methacrylate (PMMA). Inaccurate entries of reported AEs or those intended for nonaesthetic use were excluded from the study. A total of 8530 AEs were included in the study. To normalize the data, we obtained annual reports for the number of procedures performed by filler type from the American Society of Plastic Surgeons (ASPS) cosmetic procedure trends. 3 We calculated the annual AE rates for each approved filler by dividing the number of AEs by the number of procedures performed that year.
Results
The trends of different filler types depicting the number of procedures performed over time are shown in Figure 1. Data from the ASPS dated back to 2005; therefore, the number of procedures performed prior to that were extrapolated with knowledge of products’ approval dates and market share, indicated by a dotted line. To determine AE rates for each year, we divided the number of AEs by the number of reported procedures for each filler type. The AE rates are displayed graphically in Figures 2 and 3 with superimposed FDA approval dates for each filler.4

Two major peaks in reported AE rates for all fillers were noted in the late 1990s and late 2000s, mostly associated with collagen and PLLA fillers, respectively (Figure 2). Overall, there has been a low rate of AEs associated with HA fillers since their initial approval in the early 2000s.

Individual filler AE rates also were analyzed. Hyaluronic acid fillers were associated with an extremely low rate of AEs, ranging from 1 to 4 AEs per 10,000 procedures (Figure 3A). This low AE rate profile underscores the safety of HA fillers, which has spurred their popularity. Adverseevent rates for collagen fillers spiked in the mid- to late 1990s and resolved over the course of the next 3 years (Figure 3B). Hydroxylapatite fillers had a rather uniform AE rate with an early indication of a drop-off after 2015 (Figure 3C). Poly-L-lactic acid fillers showed the steepest learning curve, with a peak of 1 AE per 100 procedures after they were approved in 2008 (Figure 3D); however, there is a comparable 3-year resolution of AE rates. Adverse events for PMMA fillers did not show specific resolution, meaning that they did not follow the 3-year arc that was seen for the other dermal fillers reported in the data set (Figure 3E).

Comment
Our study is unique in that it analyzes reported AE data over a 34-year period for injectable dermal fillers. To our knowledge, this novel method of calculating AE rates across dermal fillers and for individual products is the first of its kind that facilitates usage-normalized comparison of different filler types.
All OpenFDA data are self-reported and therefore have inherent limitations. Anyone can enter information on AEs in this system, including both patients and health care providers, so the quality of the input may be variable. However, this output is the only representation we have for nearly 35 years of AE history for this burgeoning category of popular aesthetic treatments. Another study limitation is that not everyone may know that reporting an AE in the OpenFDA is an option; therefore, we may be missing a portion of AEs due to underreporting. Underreporting may be especially at play in the years before the Internet was prevalent for residential use since access to the Internet would be required to report an AR on the website. However, examining the available data provides an important window into valuable information on complications that have occurred and have been reported for FDA-approved dermal fillers.
An additional challenge in constructing this study was assessing the total number of injectable dermal filler treatments being performed annually across filler types for normalization of the data. Although the absolute numbers of filler use as captured by the ASPS are smaller than the true total filler use across all injectors, the relative use of different filler products will be similar across all specialties because it reflects product popularity. Annual surveys on aesthetic procedures also are conducted by the American Society for Dermatologic Surgery and the American Association for Facial Plastic and Reconstructive Surgery, but neither one captures the relative usage of different filler types. Because individual filler companies do not publish their annual sales numbers by product, the ASPS data give us the best gauge of relative use of fillers by product type given the available information. We conclude that the comparison of AE rates would remain the same even if we had data for total annual filler use across specialties.
Our graphical depiction of the data clearly demonstrates the low AE profile of HA fillers, which is in line with the general consensus of their safety that has contributed to their vast popularity; however, this study represents the first time usage-normalized AE rates are compared to other filler compositions. Hyaluronic acid fillers have the unique feature of being able to be dissolved with the hyaluronidase enzyme, which can limit adverse event potential as compared to other ingredient classes of filler types and may be reflected in their low overall AE profile. The AE rate spike and resolution for collagen fillers represent what we refer to as a “normal learning curve” based on our analysis of the data set as a whole, suggesting an appropriate time course of increased familiarity with the product without inherent issues with the product itself. Multiple sequential anatomic site indications were approved for hydroxylapatite fillers from 2006 through 2015, which may have yielded overlapping learning curves for each approval, resulting in a rather uniform AE rate. The early drop-off in AE rates after the 2015 anatomic site approval may represent the beginning of a normal learning curve, and continued surveillance of AE rates would be of value to confirm this trend. We saw a similar 3-year learning curve for PLLA fillers as the curve for collagen fillers, suggesting a normal learning curve and no out-of-line safety issues. Polymethylmethacrylate fillers were approved in 2006 and were taken off the market for a period in the late 2000s, explaining the drop-off. Once they were back on the market, we do not see a typical learning curve for PMMA, which may warrant surveillance for safety by both clinicians and the FDA.
Conclusion
Our study represents a novel method of evaluating the safety of medical devices, specifically aesthetic fillers. We showed that every AE rate curve for different filler types tells a story. Reactions to AEs for new fillers should be placed in the context of whether they seem to be following the established learning curve.
Dermal fillers are considered Class III medical devices by the US Food and Drug Administration (FDA).1 Reports of adverse events (AEs) for medical devices are made public by the FDA to allow for transparent postmarketing surveillance.2The AE trends extracted from these historical data may help distinguish between expected learning curves of new dermal fillers versus unsafe products that may require FDA intervention. Considering that aesthetic treatments are not medically necessary, a low risk profile is paramount and determining what constitutes normal learning curves is important for impartial assessment of AEs as new fillers come on the market. The concept of a 3-year learning curve can be an important tool for safety monitoring going forward, creating a bar for quality that could trigger increased surveillance if a product fails to meet an expected arc of diminished AEs over time. This study serves to evaluate historical AE data and to establish learning curves for FDA-approved dermal fillers.
Methods
We searched the OpenFDA Device Adverse Event Report Browser (http://openfda.shinyapps.io/devicereports/) for reported AEs within the FDA product code LMH (Implant, Dermal, For Aesthetic Use) that were received from January 1, 1983, to December 31, 2017. For each reported AE, information related to the date of the reported event and the device brand name were recorded. Devices implicated in each AE were classified based on primary composition according to the following 5 categories: collagen, hyaluronic acid (HA), hydroxylapatite, poly-L-lactic acid (PLLA), and polymethyl methacrylate (PMMA). Inaccurate entries of reported AEs or those intended for nonaesthetic use were excluded from the study. A total of 8530 AEs were included in the study. To normalize the data, we obtained annual reports for the number of procedures performed by filler type from the American Society of Plastic Surgeons (ASPS) cosmetic procedure trends. 3 We calculated the annual AE rates for each approved filler by dividing the number of AEs by the number of procedures performed that year.
Results
The trends of different filler types depicting the number of procedures performed over time are shown in Figure 1. Data from the ASPS dated back to 2005; therefore, the number of procedures performed prior to that were extrapolated with knowledge of products’ approval dates and market share, indicated by a dotted line. To determine AE rates for each year, we divided the number of AEs by the number of reported procedures for each filler type. The AE rates are displayed graphically in Figures 2 and 3 with superimposed FDA approval dates for each filler.4

Two major peaks in reported AE rates for all fillers were noted in the late 1990s and late 2000s, mostly associated with collagen and PLLA fillers, respectively (Figure 2). Overall, there has been a low rate of AEs associated with HA fillers since their initial approval in the early 2000s.

Individual filler AE rates also were analyzed. Hyaluronic acid fillers were associated with an extremely low rate of AEs, ranging from 1 to 4 AEs per 10,000 procedures (Figure 3A). This low AE rate profile underscores the safety of HA fillers, which has spurred their popularity. Adverseevent rates for collagen fillers spiked in the mid- to late 1990s and resolved over the course of the next 3 years (Figure 3B). Hydroxylapatite fillers had a rather uniform AE rate with an early indication of a drop-off after 2015 (Figure 3C). Poly-L-lactic acid fillers showed the steepest learning curve, with a peak of 1 AE per 100 procedures after they were approved in 2008 (Figure 3D); however, there is a comparable 3-year resolution of AE rates. Adverse events for PMMA fillers did not show specific resolution, meaning that they did not follow the 3-year arc that was seen for the other dermal fillers reported in the data set (Figure 3E).

Comment
Our study is unique in that it analyzes reported AE data over a 34-year period for injectable dermal fillers. To our knowledge, this novel method of calculating AE rates across dermal fillers and for individual products is the first of its kind that facilitates usage-normalized comparison of different filler types.
All OpenFDA data are self-reported and therefore have inherent limitations. Anyone can enter information on AEs in this system, including both patients and health care providers, so the quality of the input may be variable. However, this output is the only representation we have for nearly 35 years of AE history for this burgeoning category of popular aesthetic treatments. Another study limitation is that not everyone may know that reporting an AE in the OpenFDA is an option; therefore, we may be missing a portion of AEs due to underreporting. Underreporting may be especially at play in the years before the Internet was prevalent for residential use since access to the Internet would be required to report an AR on the website. However, examining the available data provides an important window into valuable information on complications that have occurred and have been reported for FDA-approved dermal fillers.
An additional challenge in constructing this study was assessing the total number of injectable dermal filler treatments being performed annually across filler types for normalization of the data. Although the absolute numbers of filler use as captured by the ASPS are smaller than the true total filler use across all injectors, the relative use of different filler products will be similar across all specialties because it reflects product popularity. Annual surveys on aesthetic procedures also are conducted by the American Society for Dermatologic Surgery and the American Association for Facial Plastic and Reconstructive Surgery, but neither one captures the relative usage of different filler types. Because individual filler companies do not publish their annual sales numbers by product, the ASPS data give us the best gauge of relative use of fillers by product type given the available information. We conclude that the comparison of AE rates would remain the same even if we had data for total annual filler use across specialties.
Our graphical depiction of the data clearly demonstrates the low AE profile of HA fillers, which is in line with the general consensus of their safety that has contributed to their vast popularity; however, this study represents the first time usage-normalized AE rates are compared to other filler compositions. Hyaluronic acid fillers have the unique feature of being able to be dissolved with the hyaluronidase enzyme, which can limit adverse event potential as compared to other ingredient classes of filler types and may be reflected in their low overall AE profile. The AE rate spike and resolution for collagen fillers represent what we refer to as a “normal learning curve” based on our analysis of the data set as a whole, suggesting an appropriate time course of increased familiarity with the product without inherent issues with the product itself. Multiple sequential anatomic site indications were approved for hydroxylapatite fillers from 2006 through 2015, which may have yielded overlapping learning curves for each approval, resulting in a rather uniform AE rate. The early drop-off in AE rates after the 2015 anatomic site approval may represent the beginning of a normal learning curve, and continued surveillance of AE rates would be of value to confirm this trend. We saw a similar 3-year learning curve for PLLA fillers as the curve for collagen fillers, suggesting a normal learning curve and no out-of-line safety issues. Polymethylmethacrylate fillers were approved in 2006 and were taken off the market for a period in the late 2000s, explaining the drop-off. Once they were back on the market, we do not see a typical learning curve for PMMA, which may warrant surveillance for safety by both clinicians and the FDA.
Conclusion
Our study represents a novel method of evaluating the safety of medical devices, specifically aesthetic fillers. We showed that every AE rate curve for different filler types tells a story. Reactions to AEs for new fillers should be placed in the context of whether they seem to be following the established learning curve.
- Dermal fillers (soft tissue fillers). US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ucm2007470.htm. Updated May 31, 2018. Accessed June 29, 2018.
- Kass-Hout TA, Xu Z, Mohebbi M, et al. OpenFDA: an innovative platform providing access to a wealth of FDA’s publicly available data. J Am Med Inform Assoc. 2016;23:596-600.
- Plastic surgery statistics. American Society of Plastic Surgeons website. https://www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-full-report-2017.pdf. Accessed June 28, 2018.
- Dermal fillers approved by the Center for Devices and Radiological Health. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CosmeticDevices/WrinkleFillers/ucm227749.htm. Accessed June 28, 2018. Updated March 19, 2018.
- Dermal fillers (soft tissue fillers). US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ucm2007470.htm. Updated May 31, 2018. Accessed June 29, 2018.
- Kass-Hout TA, Xu Z, Mohebbi M, et al. OpenFDA: an innovative platform providing access to a wealth of FDA’s publicly available data. J Am Med Inform Assoc. 2016;23:596-600.
- Plastic surgery statistics. American Society of Plastic Surgeons website. https://www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-full-report-2017.pdf. Accessed June 28, 2018.
- Dermal fillers approved by the Center for Devices and Radiological Health. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CosmeticDevices/WrinkleFillers/ucm227749.htm. Accessed June 28, 2018. Updated March 19, 2018.
Resident Pearl
- The US Food and Drug Administration’s (FDA) adverse event database, OpenFDA, provides extensive information regarding safety for a variety of cosmetic devices. Injectable dermal fillers are classified as a medical device by the FDA; therefore, safety studies can be performed using this publicly available database.
















