User login
Hypopigmentation
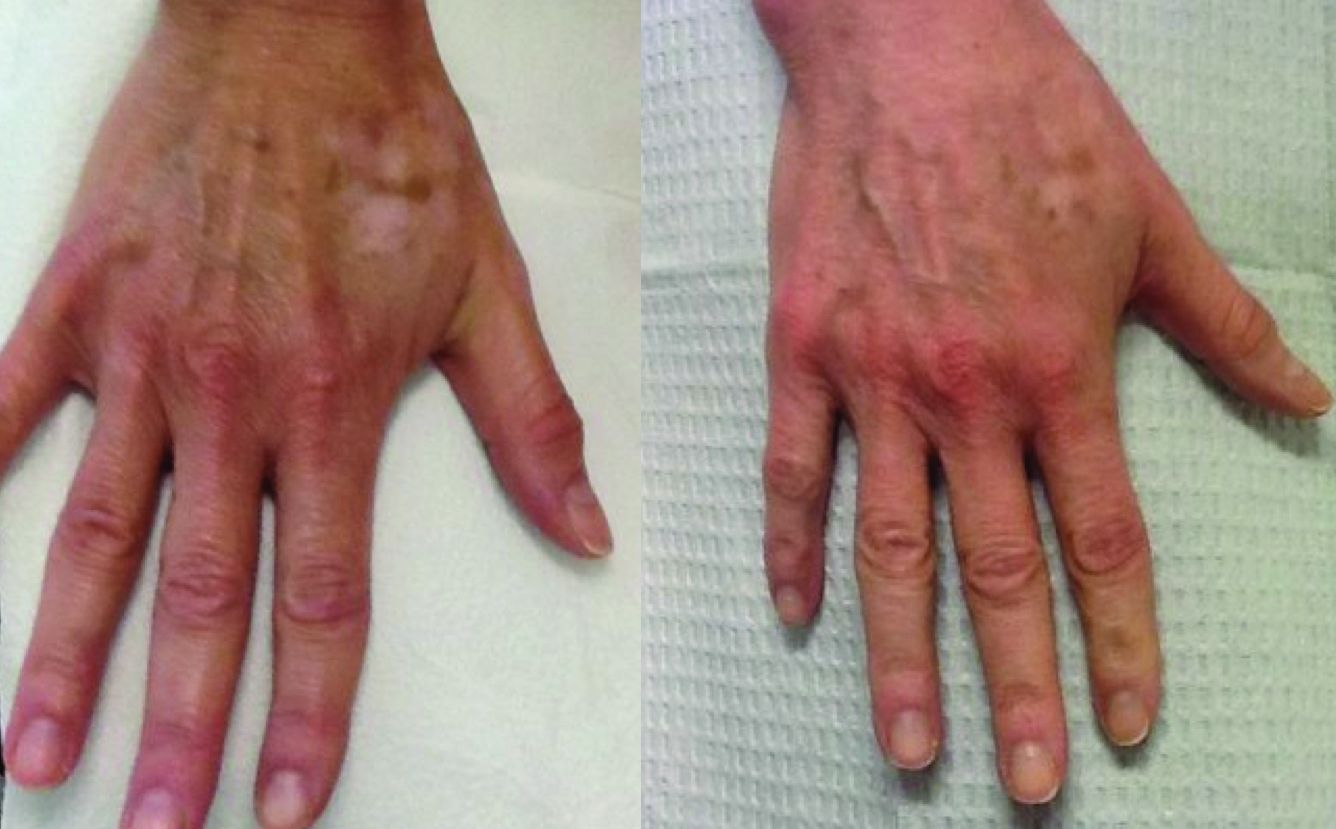
Hypopigmentation or depigmentation of the skin is very challenging to treat. The loss of melanin in the skin is often a frustrating problem, resulting from acne, burn scars, vitiligo, topically applied chemicals, or cryotherapy. To date, there is no universally accepted treatment that restores skin pigmentation. In our clinic, the induction of trauma to the skin via a series of microneedling or subcision treatments has shown promise in increasing the pigmentation of skin with localized hypo- or depigmented patches.
to improve the appearance of fine lines, acne scars, photoaging, stretch marks, enlarged pores, and other cosmetic issues characterized by loss of collagen or altered collagen remodeling. The skin-needling technique involves fine sterile needles 0.1 mm–2.5 mm in length that repeatedly pierce the stratum corneum producing microscopic “holes” in the dermis. These microscopic wounds lead to the release of growth factors stimulating the formation of new collagen, elastin, and neovascularization in the dermis. Similar to microneedling, subcision uses repeat trauma to the dermis and subcutis through the insertion and repeat movement of a needle.
In our practice, patients presenting with hypopigmentation of the skin from a variety of causes have been treated with a series of five subcision or microneedling procedures, resulting in rapid repigmentation of the skin with minimal to no side effects. Trauma to the skin causes regenerative mechanisms and wound healing. The release of cytokines that induce neoangiogenesis, neocollagenesis, and the deposition of hemosiderin from dermal bleeding induce the activation of melanocytes and stimulate skin pigmentation.
Subcision and microneedling are safe, effective, in-office procedures with vast indications that now can be applied to depigmented and hypopigmented skin. Patients have little to no downtime and results are permanent.
Dr. Talakoub and Dr. Wesley are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Dermatol Surg. 1995 Jun;21(6):543-9.
Aesthet Plast Surg. 1997 Jan-Feb;21(1):48-51.
Oral Maxillofac Surg Clin North Am. 2005 Feb;17(1):51-63.
Plast Reconstr Surg. 2008 Apr;121(4):1421-9.
Clin Dermatol. 2008 Mar-Apr;26(2):192-9.Plast Reconstr Surg. 2008 Nov;122(5):1553-63.
J Dermatolog Treat. 2012 Apr;23(2):144-52.
J Cutan Aesthet Surg. 2009 Jan;2(1):26-30.
J Cutan Aesthet Surg. 2009 Jul;2(2):110-1.
J Cosmet Dermatol. 2014 Sep;13(3):180-7.J Am Acad Dermatol. 2016 Nov;75(5):e195-e197.
Hypopigmentation or depigmentation of the skin is very challenging to treat. The loss of melanin in the skin is often a frustrating problem, resulting from acne, burn scars, vitiligo, topically applied chemicals, or cryotherapy. To date, there is no universally accepted treatment that restores skin pigmentation. In our clinic, the induction of trauma to the skin via a series of microneedling or subcision treatments has shown promise in increasing the pigmentation of skin with localized hypo- or depigmented patches.

to improve the appearance of fine lines, acne scars, photoaging, stretch marks, enlarged pores, and other cosmetic issues characterized by loss of collagen or altered collagen remodeling. The skin-needling technique involves fine sterile needles 0.1 mm–2.5 mm in length that repeatedly pierce the stratum corneum producing microscopic “holes” in the dermis. These microscopic wounds lead to the release of growth factors stimulating the formation of new collagen, elastin, and neovascularization in the dermis. Similar to microneedling, subcision uses repeat trauma to the dermis and subcutis through the insertion and repeat movement of a needle.
In our practice, patients presenting with hypopigmentation of the skin from a variety of causes have been treated with a series of five subcision or microneedling procedures, resulting in rapid repigmentation of the skin with minimal to no side effects. Trauma to the skin causes regenerative mechanisms and wound healing. The release of cytokines that induce neoangiogenesis, neocollagenesis, and the deposition of hemosiderin from dermal bleeding induce the activation of melanocytes and stimulate skin pigmentation.
Subcision and microneedling are safe, effective, in-office procedures with vast indications that now can be applied to depigmented and hypopigmented skin. Patients have little to no downtime and results are permanent.
Dr. Talakoub and Dr. Wesley are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Dermatol Surg. 1995 Jun;21(6):543-9.
Aesthet Plast Surg. 1997 Jan-Feb;21(1):48-51.
Oral Maxillofac Surg Clin North Am. 2005 Feb;17(1):51-63.
Plast Reconstr Surg. 2008 Apr;121(4):1421-9.
Clin Dermatol. 2008 Mar-Apr;26(2):192-9.Plast Reconstr Surg. 2008 Nov;122(5):1553-63.
J Dermatolog Treat. 2012 Apr;23(2):144-52.
J Cutan Aesthet Surg. 2009 Jan;2(1):26-30.
J Cutan Aesthet Surg. 2009 Jul;2(2):110-1.
J Cosmet Dermatol. 2014 Sep;13(3):180-7.J Am Acad Dermatol. 2016 Nov;75(5):e195-e197.
Hypopigmentation or depigmentation of the skin is very challenging to treat. The loss of melanin in the skin is often a frustrating problem, resulting from acne, burn scars, vitiligo, topically applied chemicals, or cryotherapy. To date, there is no universally accepted treatment that restores skin pigmentation. In our clinic, the induction of trauma to the skin via a series of microneedling or subcision treatments has shown promise in increasing the pigmentation of skin with localized hypo- or depigmented patches.

to improve the appearance of fine lines, acne scars, photoaging, stretch marks, enlarged pores, and other cosmetic issues characterized by loss of collagen or altered collagen remodeling. The skin-needling technique involves fine sterile needles 0.1 mm–2.5 mm in length that repeatedly pierce the stratum corneum producing microscopic “holes” in the dermis. These microscopic wounds lead to the release of growth factors stimulating the formation of new collagen, elastin, and neovascularization in the dermis. Similar to microneedling, subcision uses repeat trauma to the dermis and subcutis through the insertion and repeat movement of a needle.
In our practice, patients presenting with hypopigmentation of the skin from a variety of causes have been treated with a series of five subcision or microneedling procedures, resulting in rapid repigmentation of the skin with minimal to no side effects. Trauma to the skin causes regenerative mechanisms and wound healing. The release of cytokines that induce neoangiogenesis, neocollagenesis, and the deposition of hemosiderin from dermal bleeding induce the activation of melanocytes and stimulate skin pigmentation.
Subcision and microneedling are safe, effective, in-office procedures with vast indications that now can be applied to depigmented and hypopigmented skin. Patients have little to no downtime and results are permanent.
Dr. Talakoub and Dr. Wesley are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Dermatol Surg. 1995 Jun;21(6):543-9.
Aesthet Plast Surg. 1997 Jan-Feb;21(1):48-51.
Oral Maxillofac Surg Clin North Am. 2005 Feb;17(1):51-63.
Plast Reconstr Surg. 2008 Apr;121(4):1421-9.
Clin Dermatol. 2008 Mar-Apr;26(2):192-9.Plast Reconstr Surg. 2008 Nov;122(5):1553-63.
J Dermatolog Treat. 2012 Apr;23(2):144-52.
J Cutan Aesthet Surg. 2009 Jan;2(1):26-30.
J Cutan Aesthet Surg. 2009 Jul;2(2):110-1.
J Cosmet Dermatol. 2014 Sep;13(3):180-7.J Am Acad Dermatol. 2016 Nov;75(5):e195-e197.
Pediatric Skin Care: Survey of the Cutis Editorial Board
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on pediatric skin care. Here’s what we found.
Do you recommend sunscreen in babies younger than 6 months?
More than half (64%) of dermatologists we surveyed do not recommend using sunscreens in babies younger than 6 months; they should stay out of the sun. They recommended sun-protective clothing, hats, and sunglasses in this age group.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Babies younger than 6 months are still developing barrier functionality and have a higher surface area to body weight ratio compared to older children and adults. There is decreased UV light barrier protection and increased risk for systemic drug absorption. Therefore, it is best to avoid sunscreen in babies younger than 6 months. Instead, I recommend avoiding sunlight during peak hours, keeping babies in the shade, and dressing them in sun-protective clothing and hats.
Next page: Bathing and eczema
What advice do you give parents/guardians on bathing for babies with eczema?
Two-thirds of dermatologists (68%) indicated that moisturizers should be applied after bathing babies with eczema. More than half (64%) suggested using fragrance-free cleansers. Results varied on the frequency of bathing; 32% said bathe once daily for short periods with warm water, and 41% suggested to reduce bathing to a few nights a week.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Dry skin is a characteristic feature of atopic dermatitis. We now know that skin barrier dysfunction, such as filaggrin deficiency, contributes to the pathophysiology in some patients. In addition, a paucity of stratum corneum and intercellular lipids increases transepidermal water loss. Therefore, emollients containing humectants to augment stratum corneum hydration and occludents to reduce evaporation are extremely helpful in maintenance treatment of atopic dermatitis.
Next page: Nonsteroidal agents
Do you prescribe nonsteroidal agents for children younger than 2 years?
Almost two-thirds (64%) of dermatologists do prescribe nonsteroidal agents for children younger than 2 years.
If yes, which nonsteroidal agents do you prescribe for children?
Of those dermatologists who indicated that they do prescribe nonsteroidal agents for children younger than 2 years, almost half (45%) prescribe pimecrolimus, while 36% prescribe crisaborole or tacrolimus.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
We have limited options for US Food and Drug Administration (FDA)–approved therapies for atopic dermatitis in children younger than 2 years. The risks of adverse effects also are higher in younger children compared to older children and adults, particularly hypothalamic-pituitary-adrenal suppression with corticosteroids. Pimecrolimus was the most common nonsteroidal prescribed in this group. It should be noted that pimecrolimus is not FDA approved for use in children younger than 2 years; however, 2 phase 3 studies were conducted with 436 infants aged 3 months to 23 months and they demonstrated safety and efficacy.
Next page: Procedures in adolescents
Which procedures do you perform on adolescents?
Forty-one percent of dermatologists surveyed indicated that they do not perform procedures on adolescents. Of those that do, 32% use laser hair removal or chemical peels in adolescents, while only 23% each use light therapy for acne or onabotulinumtoxinA for hyperhidrosis. Pulsed dye laser use was reported in only 18% of dermatologists.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
The results of this survey are a reflection of the relatively recent trends in dermatology training. Now that there is board certification in pediatric dermatology, many dermatologists now refer to pediatric dermatologists for children who need or want procedures.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
Keep the regimen as simple as possible and make sure your skin care advice is culturally relevant.—Craig Burkhart, MD (Chapel Hill, North Carolina)
Please, avoid direct sun exposure as much as possible by wearing protective garments and finding shades in the outside.—Jisun Cha, MD (New Brunswick, New Jersey)
Good habits start early. Teach children how to care for their skin and they will carry that practice with them over the course of their lifetime, and hopefully pass it on.—James Q. Del Rosso, DO (Las Vegas, Nevada)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from September 13, 2018, to October 4, 2018. A total of 22 usable responses were received.
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on pediatric skin care. Here’s what we found.
Do you recommend sunscreen in babies younger than 6 months?
More than half (64%) of dermatologists we surveyed do not recommend using sunscreens in babies younger than 6 months; they should stay out of the sun. They recommended sun-protective clothing, hats, and sunglasses in this age group.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Babies younger than 6 months are still developing barrier functionality and have a higher surface area to body weight ratio compared to older children and adults. There is decreased UV light barrier protection and increased risk for systemic drug absorption. Therefore, it is best to avoid sunscreen in babies younger than 6 months. Instead, I recommend avoiding sunlight during peak hours, keeping babies in the shade, and dressing them in sun-protective clothing and hats.
Next page: Bathing and eczema
What advice do you give parents/guardians on bathing for babies with eczema?
Two-thirds of dermatologists (68%) indicated that moisturizers should be applied after bathing babies with eczema. More than half (64%) suggested using fragrance-free cleansers. Results varied on the frequency of bathing; 32% said bathe once daily for short periods with warm water, and 41% suggested to reduce bathing to a few nights a week.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Dry skin is a characteristic feature of atopic dermatitis. We now know that skin barrier dysfunction, such as filaggrin deficiency, contributes to the pathophysiology in some patients. In addition, a paucity of stratum corneum and intercellular lipids increases transepidermal water loss. Therefore, emollients containing humectants to augment stratum corneum hydration and occludents to reduce evaporation are extremely helpful in maintenance treatment of atopic dermatitis.
Next page: Nonsteroidal agents
Do you prescribe nonsteroidal agents for children younger than 2 years?
Almost two-thirds (64%) of dermatologists do prescribe nonsteroidal agents for children younger than 2 years.
If yes, which nonsteroidal agents do you prescribe for children?
Of those dermatologists who indicated that they do prescribe nonsteroidal agents for children younger than 2 years, almost half (45%) prescribe pimecrolimus, while 36% prescribe crisaborole or tacrolimus.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
We have limited options for US Food and Drug Administration (FDA)–approved therapies for atopic dermatitis in children younger than 2 years. The risks of adverse effects also are higher in younger children compared to older children and adults, particularly hypothalamic-pituitary-adrenal suppression with corticosteroids. Pimecrolimus was the most common nonsteroidal prescribed in this group. It should be noted that pimecrolimus is not FDA approved for use in children younger than 2 years; however, 2 phase 3 studies were conducted with 436 infants aged 3 months to 23 months and they demonstrated safety and efficacy.
Next page: Procedures in adolescents
Which procedures do you perform on adolescents?
Forty-one percent of dermatologists surveyed indicated that they do not perform procedures on adolescents. Of those that do, 32% use laser hair removal or chemical peels in adolescents, while only 23% each use light therapy for acne or onabotulinumtoxinA for hyperhidrosis. Pulsed dye laser use was reported in only 18% of dermatologists.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
The results of this survey are a reflection of the relatively recent trends in dermatology training. Now that there is board certification in pediatric dermatology, many dermatologists now refer to pediatric dermatologists for children who need or want procedures.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
Keep the regimen as simple as possible and make sure your skin care advice is culturally relevant.—Craig Burkhart, MD (Chapel Hill, North Carolina)
Please, avoid direct sun exposure as much as possible by wearing protective garments and finding shades in the outside.—Jisun Cha, MD (New Brunswick, New Jersey)
Good habits start early. Teach children how to care for their skin and they will carry that practice with them over the course of their lifetime, and hopefully pass it on.—James Q. Del Rosso, DO (Las Vegas, Nevada)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from September 13, 2018, to October 4, 2018. A total of 22 usable responses were received.
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on pediatric skin care. Here’s what we found.
Do you recommend sunscreen in babies younger than 6 months?
More than half (64%) of dermatologists we surveyed do not recommend using sunscreens in babies younger than 6 months; they should stay out of the sun. They recommended sun-protective clothing, hats, and sunglasses in this age group.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Babies younger than 6 months are still developing barrier functionality and have a higher surface area to body weight ratio compared to older children and adults. There is decreased UV light barrier protection and increased risk for systemic drug absorption. Therefore, it is best to avoid sunscreen in babies younger than 6 months. Instead, I recommend avoiding sunlight during peak hours, keeping babies in the shade, and dressing them in sun-protective clothing and hats.
Next page: Bathing and eczema
What advice do you give parents/guardians on bathing for babies with eczema?
Two-thirds of dermatologists (68%) indicated that moisturizers should be applied after bathing babies with eczema. More than half (64%) suggested using fragrance-free cleansers. Results varied on the frequency of bathing; 32% said bathe once daily for short periods with warm water, and 41% suggested to reduce bathing to a few nights a week.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Dry skin is a characteristic feature of atopic dermatitis. We now know that skin barrier dysfunction, such as filaggrin deficiency, contributes to the pathophysiology in some patients. In addition, a paucity of stratum corneum and intercellular lipids increases transepidermal water loss. Therefore, emollients containing humectants to augment stratum corneum hydration and occludents to reduce evaporation are extremely helpful in maintenance treatment of atopic dermatitis.
Next page: Nonsteroidal agents
Do you prescribe nonsteroidal agents for children younger than 2 years?
Almost two-thirds (64%) of dermatologists do prescribe nonsteroidal agents for children younger than 2 years.
If yes, which nonsteroidal agents do you prescribe for children?
Of those dermatologists who indicated that they do prescribe nonsteroidal agents for children younger than 2 years, almost half (45%) prescribe pimecrolimus, while 36% prescribe crisaborole or tacrolimus.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
We have limited options for US Food and Drug Administration (FDA)–approved therapies for atopic dermatitis in children younger than 2 years. The risks of adverse effects also are higher in younger children compared to older children and adults, particularly hypothalamic-pituitary-adrenal suppression with corticosteroids. Pimecrolimus was the most common nonsteroidal prescribed in this group. It should be noted that pimecrolimus is not FDA approved for use in children younger than 2 years; however, 2 phase 3 studies were conducted with 436 infants aged 3 months to 23 months and they demonstrated safety and efficacy.
Next page: Procedures in adolescents
Which procedures do you perform on adolescents?
Forty-one percent of dermatologists surveyed indicated that they do not perform procedures on adolescents. Of those that do, 32% use laser hair removal or chemical peels in adolescents, while only 23% each use light therapy for acne or onabotulinumtoxinA for hyperhidrosis. Pulsed dye laser use was reported in only 18% of dermatologists.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
The results of this survey are a reflection of the relatively recent trends in dermatology training. Now that there is board certification in pediatric dermatology, many dermatologists now refer to pediatric dermatologists for children who need or want procedures.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
Keep the regimen as simple as possible and make sure your skin care advice is culturally relevant.—Craig Burkhart, MD (Chapel Hill, North Carolina)
Please, avoid direct sun exposure as much as possible by wearing protective garments and finding shades in the outside.—Jisun Cha, MD (New Brunswick, New Jersey)
Good habits start early. Teach children how to care for their skin and they will carry that practice with them over the course of their lifetime, and hopefully pass it on.—James Q. Del Rosso, DO (Las Vegas, Nevada)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from September 13, 2018, to October 4, 2018. A total of 22 usable responses were received.
FDA expands CoolSculpting indication to submandibular area
FDA clearance of the expanded indication was based on results of a 22-week study in which patients achieved a mean 33% reduction in fat layer thickness after two treatments. In addition, 85% of patients reported satisfaction with their treatment in that and two other studies. The data were provided in Allergan’s press release announcing the clearance for the cryolipolysis device.
Adverse events associated with the CoolSculpting treatment include temporary redness, swelling, blanching, bruising, firmness, tingling, stinging, tenderness, cramping, aching, itching, and skin sensitivity. People with cryoglobulinemia, cold agglutinin disease, or paroxysmal cold hemoglobinuria should not receive CoolSculpting treatments, according to the company.
The FDA also expanded the CoolSculpting indication to people with a body mass index up to 46.2 kg/m2 when treating the submental and submandibular areas.
FDA clearance of the expanded indication was based on results of a 22-week study in which patients achieved a mean 33% reduction in fat layer thickness after two treatments. In addition, 85% of patients reported satisfaction with their treatment in that and two other studies. The data were provided in Allergan’s press release announcing the clearance for the cryolipolysis device.
Adverse events associated with the CoolSculpting treatment include temporary redness, swelling, blanching, bruising, firmness, tingling, stinging, tenderness, cramping, aching, itching, and skin sensitivity. People with cryoglobulinemia, cold agglutinin disease, or paroxysmal cold hemoglobinuria should not receive CoolSculpting treatments, according to the company.
The FDA also expanded the CoolSculpting indication to people with a body mass index up to 46.2 kg/m2 when treating the submental and submandibular areas.
FDA clearance of the expanded indication was based on results of a 22-week study in which patients achieved a mean 33% reduction in fat layer thickness after two treatments. In addition, 85% of patients reported satisfaction with their treatment in that and two other studies. The data were provided in Allergan’s press release announcing the clearance for the cryolipolysis device.
Adverse events associated with the CoolSculpting treatment include temporary redness, swelling, blanching, bruising, firmness, tingling, stinging, tenderness, cramping, aching, itching, and skin sensitivity. People with cryoglobulinemia, cold agglutinin disease, or paroxysmal cold hemoglobinuria should not receive CoolSculpting treatments, according to the company.
The FDA also expanded the CoolSculpting indication to people with a body mass index up to 46.2 kg/m2 when treating the submental and submandibular areas.
Platelet-rich plasma injections yield substantial improvement in androgenetic alopecia
Autologous treatment with injected (AGA) after three monthly treatments, in a study that compared two treatment regimens.
PRP is gaining popularity because of its efficacy in stimulating fibroblast proliferation, triggering the production of collagen and elastin, and boosting the quantity and quality of the extracellular matrix, noted the investigators, Amelia K. Hausauer, MD, in private practice in Campbell, Calif., and Derek H. Jones, MD, in private practice in Los Angeles. Both are also with the department of dermatology at the University of California, Los Angeles.
They undertook this study to determine the optimal number and timing of treatments in patients with AGA, comparing two different injection protocols over a 6-month period. The study evaluated 40 healthy men (30) and women (10), whose mean age was 44 years, with AGA stages Norwood-Hamilton II-V (in men) and Ludwig I2-II1 (in women), recruited from a private practice in Los Angeles between November 2016 and January 2017. They were randomly assigned to one of two treatment groups: three monthly sessions followed by a fourth injection 3 months later (group 1), or two treatments, one at baseline and the second 3 months later (group 2). One of the men dropped out for reasons unrelated to the treatment.
Those with clinically stable effects of Food and Drug Administration–approved AGA treatments for 12 months were permitted to participate while continuing those treatments (topical minoxidil and/or oral finasteride), since PRP is often coadministered with other therapies. But additional products, devices, or medications used for hair regrowth were not allowed. The washout period for antiandrogen therapies was 90 days.
At 3 months, the mean increase in hair counts was significant in the first group only, but at 6 months, both groups experienced significant increases in hair count (P less than .001). However, those in the first group had superior results at 6 months, with a mean 30% increase in hair counts from baseline, compared with a 7% increase in the second group (P less than .001).
Both groups had significant increases in the mean hair shaft caliber at 3 and 6 months.
Overall, 82% of participants who completed treatment reported being satisfied or highly satisfied, and 72% expressed interest in continuing treatment after the study period; almost two-thirds considered the procedure “tolerable.”
While the authors stipulated that they did not undertake the study primarily to predict treatment response to PRP, they uncovered some significant trends that they said warranted further evaluation, including the finding that those who had experienced hair loss for less than 5-6 years were more likely to have rapid and pronounced treatment response.
Their overall findings correlated with those of previous studies supporting the increase in density of hair or hair numbers, but the existing literature draws from studies that have been open label or unblinded, which makes it difficult to evaluate them head to head. The novel, subdermal injection technique used in the study “allows for fewer, more widely spaced injection points than the traditional nappage procedure ... because PRP can diffuse further once in the deeper, subgaleal space,” they wrote. The investigators noted similar response between men and women, which is important given sparse data on the efficacy of PRP in women.
Weaknesses of the study included its small sample size and short follow-up period, the authors noted. Longer-duration studies have reported relapse between 3 and 12 months.
This study is the first of its kind to directly compare efficacy rates of two injection protocols, the authors wrote, cautioning that future studies are necessary to “fine-tune preparation methods, determine optimal maintenance schedule(s), and parse out clinical predictors of efficacy.”
Eclipse Aesthetics (the manufacturer of the PRP preparation kits) provided funding for this study, but the authors acknowledged no significant interest with commercial supporters.
SOURCE: Hausauer A et al. Dermatol Surg. 2018 Sep;44(9):1191-200.
Autologous treatment with injected (AGA) after three monthly treatments, in a study that compared two treatment regimens.
PRP is gaining popularity because of its efficacy in stimulating fibroblast proliferation, triggering the production of collagen and elastin, and boosting the quantity and quality of the extracellular matrix, noted the investigators, Amelia K. Hausauer, MD, in private practice in Campbell, Calif., and Derek H. Jones, MD, in private practice in Los Angeles. Both are also with the department of dermatology at the University of California, Los Angeles.
They undertook this study to determine the optimal number and timing of treatments in patients with AGA, comparing two different injection protocols over a 6-month period. The study evaluated 40 healthy men (30) and women (10), whose mean age was 44 years, with AGA stages Norwood-Hamilton II-V (in men) and Ludwig I2-II1 (in women), recruited from a private practice in Los Angeles between November 2016 and January 2017. They were randomly assigned to one of two treatment groups: three monthly sessions followed by a fourth injection 3 months later (group 1), or two treatments, one at baseline and the second 3 months later (group 2). One of the men dropped out for reasons unrelated to the treatment.
Those with clinically stable effects of Food and Drug Administration–approved AGA treatments for 12 months were permitted to participate while continuing those treatments (topical minoxidil and/or oral finasteride), since PRP is often coadministered with other therapies. But additional products, devices, or medications used for hair regrowth were not allowed. The washout period for antiandrogen therapies was 90 days.
At 3 months, the mean increase in hair counts was significant in the first group only, but at 6 months, both groups experienced significant increases in hair count (P less than .001). However, those in the first group had superior results at 6 months, with a mean 30% increase in hair counts from baseline, compared with a 7% increase in the second group (P less than .001).
Both groups had significant increases in the mean hair shaft caliber at 3 and 6 months.
Overall, 82% of participants who completed treatment reported being satisfied or highly satisfied, and 72% expressed interest in continuing treatment after the study period; almost two-thirds considered the procedure “tolerable.”
While the authors stipulated that they did not undertake the study primarily to predict treatment response to PRP, they uncovered some significant trends that they said warranted further evaluation, including the finding that those who had experienced hair loss for less than 5-6 years were more likely to have rapid and pronounced treatment response.
Their overall findings correlated with those of previous studies supporting the increase in density of hair or hair numbers, but the existing literature draws from studies that have been open label or unblinded, which makes it difficult to evaluate them head to head. The novel, subdermal injection technique used in the study “allows for fewer, more widely spaced injection points than the traditional nappage procedure ... because PRP can diffuse further once in the deeper, subgaleal space,” they wrote. The investigators noted similar response between men and women, which is important given sparse data on the efficacy of PRP in women.
Weaknesses of the study included its small sample size and short follow-up period, the authors noted. Longer-duration studies have reported relapse between 3 and 12 months.
This study is the first of its kind to directly compare efficacy rates of two injection protocols, the authors wrote, cautioning that future studies are necessary to “fine-tune preparation methods, determine optimal maintenance schedule(s), and parse out clinical predictors of efficacy.”
Eclipse Aesthetics (the manufacturer of the PRP preparation kits) provided funding for this study, but the authors acknowledged no significant interest with commercial supporters.
SOURCE: Hausauer A et al. Dermatol Surg. 2018 Sep;44(9):1191-200.
Autologous treatment with injected (AGA) after three monthly treatments, in a study that compared two treatment regimens.
PRP is gaining popularity because of its efficacy in stimulating fibroblast proliferation, triggering the production of collagen and elastin, and boosting the quantity and quality of the extracellular matrix, noted the investigators, Amelia K. Hausauer, MD, in private practice in Campbell, Calif., and Derek H. Jones, MD, in private practice in Los Angeles. Both are also with the department of dermatology at the University of California, Los Angeles.
They undertook this study to determine the optimal number and timing of treatments in patients with AGA, comparing two different injection protocols over a 6-month period. The study evaluated 40 healthy men (30) and women (10), whose mean age was 44 years, with AGA stages Norwood-Hamilton II-V (in men) and Ludwig I2-II1 (in women), recruited from a private practice in Los Angeles between November 2016 and January 2017. They were randomly assigned to one of two treatment groups: three monthly sessions followed by a fourth injection 3 months later (group 1), or two treatments, one at baseline and the second 3 months later (group 2). One of the men dropped out for reasons unrelated to the treatment.
Those with clinically stable effects of Food and Drug Administration–approved AGA treatments for 12 months were permitted to participate while continuing those treatments (topical minoxidil and/or oral finasteride), since PRP is often coadministered with other therapies. But additional products, devices, or medications used for hair regrowth were not allowed. The washout period for antiandrogen therapies was 90 days.
At 3 months, the mean increase in hair counts was significant in the first group only, but at 6 months, both groups experienced significant increases in hair count (P less than .001). However, those in the first group had superior results at 6 months, with a mean 30% increase in hair counts from baseline, compared with a 7% increase in the second group (P less than .001).
Both groups had significant increases in the mean hair shaft caliber at 3 and 6 months.
Overall, 82% of participants who completed treatment reported being satisfied or highly satisfied, and 72% expressed interest in continuing treatment after the study period; almost two-thirds considered the procedure “tolerable.”
While the authors stipulated that they did not undertake the study primarily to predict treatment response to PRP, they uncovered some significant trends that they said warranted further evaluation, including the finding that those who had experienced hair loss for less than 5-6 years were more likely to have rapid and pronounced treatment response.
Their overall findings correlated with those of previous studies supporting the increase in density of hair or hair numbers, but the existing literature draws from studies that have been open label or unblinded, which makes it difficult to evaluate them head to head. The novel, subdermal injection technique used in the study “allows for fewer, more widely spaced injection points than the traditional nappage procedure ... because PRP can diffuse further once in the deeper, subgaleal space,” they wrote. The investigators noted similar response between men and women, which is important given sparse data on the efficacy of PRP in women.
Weaknesses of the study included its small sample size and short follow-up period, the authors noted. Longer-duration studies have reported relapse between 3 and 12 months.
This study is the first of its kind to directly compare efficacy rates of two injection protocols, the authors wrote, cautioning that future studies are necessary to “fine-tune preparation methods, determine optimal maintenance schedule(s), and parse out clinical predictors of efficacy.”
Eclipse Aesthetics (the manufacturer of the PRP preparation kits) provided funding for this study, but the authors acknowledged no significant interest with commercial supporters.
SOURCE: Hausauer A et al. Dermatol Surg. 2018 Sep;44(9):1191-200.
FROM DERMATOLOGIC SURGERY
Key clinical point: Starting off with monthly PRP injections may yield more hair growth than a protocol that uses less frequently administered injections.
Major finding: Of the patients who completed treatment, 82% were satisfied with the results.
Study details: A prospective, randomized trial comparing two early-phase treatment protocols in 40 patients.
Disclosures: Eclipse Aesthetics provided funding for this study; the authors said they had no significant interest with commercial supporters.
Source: Hausauer A et al. Dermatol Surg. 2018 Sep;44(9):1191-200.
Noninvasive Vaginal Rejuvenation
Vaginal rejuvenation encompasses a group of procedures that alter the vaginal anatomy to improve cosmesis or achieve more pleasurable sexual intercourse. External vaginal procedures are defined as those performed on the female genitalia outside of the vaginal introitus, with major structures including the labia majora, mons pubis, labia minora, clitoral hood, clitoral glans, and vaginal vestibule. Internal vaginal procedures are defined as those performed within the vagina, extending from the vaginal introitus to the cervix.
The prevalence of elective vaginal rejuvenation procedures has increased in recent years, a trend that may be attributed to greater exposure through the media, including reality television and pornography. In a survey of 482 women undergoing labiaplasty, nearly all had heard about rejuvenation procedures within the last 2.2 years, and 78% had received their information through the media.1 Additionally, genital self-image can have a considerable effect on a woman’s sexual behavior and relationships. Genital dissatisfaction has been associated with decreased sexual activity, whereas positive genital self-image correlates with increased sexual desire and less sexual distress or depression.2,3
Currently, the 2 primary applications of noninvasive vaginal rejuvenation are vaginal laxity and genitourinary syndrome of menopause (GSM). Vaginal laxity occurs in premenopausal or postmenopausal women and is caused by aging, childbearing, or hormonal imbalances. These factors can lead to decreased friction within the vagina during intercourse, which in turn can decrease sexual pleasure. Genitourinary syndrome of menopause, previously known as vulvovaginal atrophy, encompasses genital (eg, dryness, burning, irritation), sexual (eg, lack of lubrication, discomfort or pain, impaired function), and urinary (eg, urgency, dysuria, recurrent urinary tract infections) symptoms of menopause.4
Noninvasive procedures are designed to apply ablative or nonablative energy to the vaginal mucosa to tighten a lax upper vagina, also known as a wide vagina.5 A wide vagina has been defined as a widened vaginal diameter that interferes with sexual function and sensation.6 Decreased sexual sensation also may result from fibrosis or scarring of the vaginal mucosa after prior vaginal surgery, episiotomy, or tears during childbirth.7 The objective of rejuvenation procedures to treat the vaginal mucosa is to create increased frictional forces that may lead to increased sexual sensation.8 Although there are numerous reports of heightened sexual satisfaction after reduction of the vaginal diameter, a formal link between sexual pleasure and vaginal laxity has yet to be established.8,9 At present, there are no US Food and Drug Administration (FDA)–approved energy-based devices to treat urinary incontinence or sexual function, and the FDA recently issued an alert cautioning patients on the current lack of safety and efficacy regulations.10
In this article we review the safety and efficacy data behind lasers and radiofrequency (RF) devices used in noninvasive vaginal rejuvenation procedures.
Lasers
CO2 Laser
The infrared CO2 laser utilizes 10,600-nm energy to target and vaporize water molecules within the target tissue. This thermal heating extends to the dermal collagen, which stimulates inflammatory pathways and neocollagenesis.11 The depth of penetration ranges from 20 to 125 μm.12 Zerbinati et al13 demonstrated the histologic and ultrastructural effects of a fractional CO2 laser on atrophic vaginal mucosa. Comparing pretreatment and posttreatment mucosal biopsies in 5 postmenopausal women, the investigators found that fractional CO2 laser treatment caused increased epithelial thickness, vascularity, and fibroblast activity, which led to augmented synthesis of collagen and ground substance proteins.13
New devices seek to translate these histologic improvements to the aesthetic appearance and function of female genitalia. The MonaLisa Touch (Cynosure), a new fractional CO2 laser specifically designed for treatment of the vaginal mucosa, uses dermal optical thermolysis (DOT) therapy to apply energy in a noncontinuous mode at 200-μm dots. Salvatore et al14 examined the use of this device in a noncontrolled study of 50 patients with GSM, with each patient undergoing 3 treatment sessions at monthly intervals. Intravaginal treatments were performed at the following settings: DOT (microablative zone) power of 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack parameter of 1 to 3. The investigators used the Vaginal Health Index (VHI) to objectively assess vaginal elasticity, secretions, pH, mucosa integrity, and moisture. Total VHI scores significantly improved between baseline and 1 month following the final treatment (mean score [SD], 13.1 [2.5] vs 23.1 [1.9]; P<.0001). There were no significant adverse events, and 84% of patients reported being satisfied with their outcome; however, the study lacked a comparison or control group, raising the possibility of placebo effect.14
Other noncontrolled series have corroborated the benefits of CO2 laser in GSM patients.15,16 In one of the largest studies to date, Filippini et al17 reviewed the outcomes of 386 menopausal women treated for GSM. Patients underwent 3 intravaginal laser sessions with the MonaLisa Touch. Intravaginal treatments were performed at a DOT power of 40 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 2. For the vulva, the DOT power was reduced to 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 1. Two months after the final treatment session, patients completed a nonvalidated questionnaire about their symptoms, with improved dryness reported in 60% of patients, improved burning in 56%, improved dyspareunia in 49%, improved itch in 56%, improved soreness in 73%, and improved vaginal introitus pain in 49%. Although most patients did not experience discomfort with the procedure, a minority noted a burning sensation (11%), bother with handpiece movement (6%), or vulvar pain (5%).17
Recently, Cruz et al18 performed one of the first randomized, double-blind, placebo-controlled trials comparing fractional CO2 laser therapy, topical estrogen therapy, and the combination of both treatments in patients with GSM. Forty-five women were included in the study, and validated assessments were performed at baseline and weeks 8 and 20. Intravaginal treatments were performed at a DOT power of 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 2. Importantly, the study incorporated placebo laser treatments (with the power adjusted to 0.0 W) in the topical estrogen group, thereby decreasing result bias. There was a significant increase in VHI scores from baseline to week 8 (P<.05) and week 20 (P<.01) in all study arms. At week 20, the laser group and laser plus estrogen group showed significant improvements in reported dyspareunia, burning, and dryness, whereas the estrogen arm only reported improvements in dryness (all values P<.05).18
Erbium-Doped YAG Laser
The erbium-doped YAG (Er:YAG) laser is an ablative laser emitting light at 2940 nm. This wavelength provides an absorption coefficient for water 16 times greater than the CO2 laser, leading to decreased penetration depth of 1 to 3 μm and reduced damage to the surrounding tissues.19,20 As such, the Er:YAG laser results in milder postoperative discomfort and faster overall healing times.21
In a noncontrolled study of vaginal relaxation syndrome, Lee22 used an Er:YAG laser fitted with Petit Lady (Lutronic) 90° and 360° vaginal scanning scopes. Thirty patients were divided into 2 groups and were treated with 4 sessions at weekly intervals. In group A, the first 2 sessions were performed with the 360° scope, and the last 2 sessions with the 90° scope in multiple micropulse mode (3 multishots; pulse width of 250 μs; 1.7 J delivered per shot). Group B was treated with the 90° scope in all 4 sessions in multiple micropulse mode (same parameters as group A), and during the last 2 sessions patients were additionally treated with 2 passes per session with the 360° scope (long-pulsed mode; pulse width of 1000 μs; 3.7 J delivered per shot). Perineometer measurements taken 2 months after the final treatment showed that the combined patient population experienced significant increases in both maximal vaginal pressure (P<.01) and average vaginal pressure (P<.05). Roughly 76% of patients’ partners noted improved vaginal tightening, and 70% of patients reported being satisfied with their treatment outcome. Histologic specimens taken at baseline and 2 months postprocedure showed evidence of thicker and more cellular epithelia along with more compact lamina propria with denser connective tissue. The sessions were well tolerated, with patients reporting a nonpainful heating sensation in the vagina during treatment. Three patients from the combined patient population experienced a mild burning sensation and vaginal ecchymoses, which lasted 24 to 48 hours following treatment and resolved spontaneously. There was no control group and no reports of major or long-term adverse events.22
Investigations also have shown the benefit of Er:YAG in the treatment of GSM.23,24 In a study by Gambacciani et al,24 patients treated with the Er:YAG laser FotonaSmooth (Fotona) every 30 days for 3 months reported significant improvements in vaginal dryness and dyspareunia (P<.01), which lasted up to 6 months posttreatment, though there was no placebo group comparator. Similar results were seen by Gaspar et al23 using 3 treatments at 3-week intervals, with results sustained up to 18 months after the final session.
Radiofrequency Devices
Radiofrequency devices emit focused electromagnetic waves that heat underlying tissues without targeting melanin. The release of thermal energy induces collagen contraction, neocollagenesis, and neovascularization, all of which aid in restoring the elasticity and moisture of the vaginal mucosa.25 Devices also may be equipped with cooling probes and reverse-heating gradients to protect the surface mucosa while deeper tissues are heated.
Millheiser et al26 performed a noncontrolled pilot study in 24 women with vaginal laxity using the Viveve System (Viveve), a cryogen-cooled monopolar RF device. Participants underwent a single 30-minute session (energy ranging from 75–90 J/cm2) during which the mucosal surface of the vaginal introitus (excluding the urethra) was treated with pulses at 0.5-cm overlapping intervals. Follow-up assessments were completed at 1, 3, and 6 months posttreatment. Self-reported vaginal tightness improved in 67% of participants at 1-month posttreatment and in 87% of participants at 6 months posttreatment (P<.001). There were no adverse events reported.26 Sekiguchi et al27 reported similar benefits lasting up to 12 months after a single 26-minute session at 90 J/cm2.
A prospective, randomized, placebo-controlled clinical trial using the Viveve system was recently completed by Krychman et al.28 Participants (N=186) were randomized to receive a single session of active treatment (90 J/cm2) or placebo treatment (1 J/cm2). In both groups, the vaginal introitus was treated with pulses at 0.5 cm in overlapping intervals, with the entire area (excluding the urethra) treated 5 times up to a total of 110 pulses. The primary end point was the proportion of randomized participants reporting no vaginal laxity at 6 months postin-tervention, which was assessed using the Vaginal Laxity Questionnaire. A grade of no vaginal laxity was achieved by 43.5% of participants in the active treatment group and 19.6% of participants in the sham group (P=.002). Overall numbers of treatment-emergent adverse events were comparable between the 2 groups, with the most commonly reported being vaginal discharge (2.6% in the active treatment group vs 3.5% in the sham group). There were no serious adverse events reported in the active treatment group.28
ThermiVa (ThermiGen, LLC), a unipolar RF device, was evaluated by Alinsod29 in the treatment of orgasmic dysfunction. The noncontrolled study included 25 women with self-reported difficulty achieving orgasm during intercourse, each of whom underwent 3 treatment sessions at 1-month intervals. Of the 25 enrolled women, 19 (76%) reported an average reduction in time to orgasm of at least 50%. All anorgasmic patients (n=10) at baseline reported renewed ability to achieve orgasms. Two (8%) patients failed to achieve a significant benefit from the treatments. Of note, the study did not include a control group, and specific data on the durability of beneficial effects was lacking.29
The Ultra Femme 360 (BLT Industries Inc), a monopolar RF device, was evaluated by Lalji and Lozanova30 in a noncontrolled study of 27 women with mild to moderate vaginal laxity and urinary incontinence. Participants underwent 3 treatment sessions at weekly intervals. Vaginal laxity was assessed by a subjective vulvovaginal laxity questionnaire, and data were collected before the first treatment and at 1-month follow-up. All 27 participants reported improvements in vaginal laxity, with the average grade (SD) increasing from very loose (2.19 [1.08]) to moderately tight (5.74 [0.76]; P<.05) on the questionnaire’s 7-point scale. The trial did not include a control group.30
Conclusion
With growing patient interest in vaginal rejuvenation, clinicians are increasingly incorporating a variety of procedures into their practice. Although long-term data on the safety and efficacy of these treatments has yet to be established, current evidence indicates that fractional ablative lasers and RF devices can improve vaginal laxity, sexual sensation, and symptoms of GSM.
To date, major complications have not been reported, but the FDA has advocated caution until regulatory approval is achieved.10 Concerns exist over the limited number of robust clinical trials as well as the prevalence of advertising campaigns that promise wide-ranging improvements without sufficient evidence. Definitive statements on medical or cosmetic indications will undoubtedly require more thorough investigation. At this time, the safety profile of these devices appears to be favorable, and high rates of patient satisfaction have been reported. As such, noninvasive vaginal rejuvenation procedures may represent a valuable addition to the cosmetic landscape.
- Koning M, Zeijlmans IA, Bouman TK, et al. Female attitudes regarding labia minora appearance and reduction with consideration of media influence. Aesthet Surg J. 2009;29:65-71.
- Rowen TS, Gaither TW, Shindel AW, et al. Characteristics of genital dissatisfaction among a nationally representative sample of U.S. women. J Sex Med. 2018;15:698-704.
- Berman L, Berman J, Miles M, et al. Genital self-image as a component of sexual health: relationship between genital self-image, female sexual function, and quality of life measures. J Sex Marital Ther. 2003;29(suppl 1):11-21.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Goodman MP, Placik OJ, Benson RH 3rd, et al. A large multicenter outcome study of female genital plastic surgery. J Sex Med. 2010;7(4 pt 1):1565-1577.
- Ostrzenski A. Vaginal rugation rejuvenation (restoration): a new surgical technique for an acquired sensation of wide/smooth vagina. Gynecol Obstet Invest. 2012;73:48-52.
- Singh A, Swift S, Khullar V, et al. Laser vaginal rejuvenation: not ready for prime time. Int Urogynecol J. 2015;26:163-164.
- Iglesia CB, Yurteri-Kaplan L, Alinsod R. Female genital cosmetic surgery: a review of techniques and outcomes. Int Urogynecol J. 2013;24:1997-2009.
- Dobbeleir JM, Landuyt KV, Monstrey SJ. Aesthetic surgery of the female genitalia. Semin Plast Surg. 2011;25:130-141.
- US Food and Drug Administration. FDA warns against use of energy-based devices to perform vaginal ‘rejuvenation’ or vaginal cosmetic procedures: FDA safety communication. July 30, 2018. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed September 10, 2018.
- Patil UA, Dhami LD. Overview of lasers. Indian J Plast Surg. 2008;41(suppl):S101-S113.
- Qureshi AA, Tenenbaum MM, Myckatyn TM. Nonsurgical vulvovaginal rejuvenation with radiofrequency and laser devices: a literature review and comprehensive update for aesthetic surgeons. Aesthet Surg J. 2018;38:302-311.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30:429-436.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17:363-369.
- Eder SE. Early effect of fractional CO2 laser treatment in post-menopausal women with vaginal atrophy. Laser Ther. 2018;27:41-47.
- Perino A, Calligaro A, Forlani F, et al. Vulvo-vaginal atrophy: a new treatment modality using thermo-ablative fractional CO2 laser. Maturitas. 2015;80:296-301.
- Filippini M, Del Duca E, Negosanti F, et al. Fractional CO2 laser: from skin rejuvenation to vulvo-vaginal reshaping. Photomed Laser Surg. 2017;35:171-175.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28.
- Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116.
- Kaushik SB, Alexis AF. Nonablative fractional laser resurfacing in skin of color: evidence-based review. J Clin Aesthet Dermatol. 2017;10:51-67.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. Fractional laser skin resurfacing. J Drugs Dermatol. 2012;11:1274-1287.
- Lee MS. Treatment of vaginal relaxation syndrome with an erbium:YAG laser using 90 degrees and 360 degrees scanning scopes: a pilot study & short-term results. Laser Ther. 2014;23:129-138.
- Gaspar A, Brandi H, Gomez V, et al. Efficacy of erbium:YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg Med. 2017;49:160-168.
- Gambacciani M, Levancini M, Cervigni M. Vaginal erbium laser: the second-generation thermotherapy for the genitourinary syndrome of menopause. Climacteric. 2015;18:757-763.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. 2017;49:137-159.
- Millheiser LS, Pauls RN, Herbst SJ, et al. Radiofrequency treatment of vaginal laxity after vaginal delivery: nonsurgical vaginal tightening. J Sex Med. 2010;7:3088-3095.
- Sekiguchi Y, Utsugisawa Y, Azekosi Y, et al. Laxity of the vaginal introitus after childbirth: nonsurgical outpatient procedure for vaginal tissue restoration and improved sexual satisfaction using low-energy radiofrequency thermal therapy. J Womens Health (Larchmt). 2013;22:775-781.
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14:215-225.
- Alinsod RM. Transcutaneous temperature controlled radiofrequency for orgasmic dysfunction. Lasers Surg Med. 2016;48:641-645.
- Lalji S, Lozanova P. Evaluation of the safety and efficacy of a monopolar nonablative radiofrequency device for the improvement of vulvo-vaginal laxity and urinary incontinence. J Cosmet Dermatol. 2017;16:230-234.
Vaginal rejuvenation encompasses a group of procedures that alter the vaginal anatomy to improve cosmesis or achieve more pleasurable sexual intercourse. External vaginal procedures are defined as those performed on the female genitalia outside of the vaginal introitus, with major structures including the labia majora, mons pubis, labia minora, clitoral hood, clitoral glans, and vaginal vestibule. Internal vaginal procedures are defined as those performed within the vagina, extending from the vaginal introitus to the cervix.
The prevalence of elective vaginal rejuvenation procedures has increased in recent years, a trend that may be attributed to greater exposure through the media, including reality television and pornography. In a survey of 482 women undergoing labiaplasty, nearly all had heard about rejuvenation procedures within the last 2.2 years, and 78% had received their information through the media.1 Additionally, genital self-image can have a considerable effect on a woman’s sexual behavior and relationships. Genital dissatisfaction has been associated with decreased sexual activity, whereas positive genital self-image correlates with increased sexual desire and less sexual distress or depression.2,3
Currently, the 2 primary applications of noninvasive vaginal rejuvenation are vaginal laxity and genitourinary syndrome of menopause (GSM). Vaginal laxity occurs in premenopausal or postmenopausal women and is caused by aging, childbearing, or hormonal imbalances. These factors can lead to decreased friction within the vagina during intercourse, which in turn can decrease sexual pleasure. Genitourinary syndrome of menopause, previously known as vulvovaginal atrophy, encompasses genital (eg, dryness, burning, irritation), sexual (eg, lack of lubrication, discomfort or pain, impaired function), and urinary (eg, urgency, dysuria, recurrent urinary tract infections) symptoms of menopause.4
Noninvasive procedures are designed to apply ablative or nonablative energy to the vaginal mucosa to tighten a lax upper vagina, also known as a wide vagina.5 A wide vagina has been defined as a widened vaginal diameter that interferes with sexual function and sensation.6 Decreased sexual sensation also may result from fibrosis or scarring of the vaginal mucosa after prior vaginal surgery, episiotomy, or tears during childbirth.7 The objective of rejuvenation procedures to treat the vaginal mucosa is to create increased frictional forces that may lead to increased sexual sensation.8 Although there are numerous reports of heightened sexual satisfaction after reduction of the vaginal diameter, a formal link between sexual pleasure and vaginal laxity has yet to be established.8,9 At present, there are no US Food and Drug Administration (FDA)–approved energy-based devices to treat urinary incontinence or sexual function, and the FDA recently issued an alert cautioning patients on the current lack of safety and efficacy regulations.10
In this article we review the safety and efficacy data behind lasers and radiofrequency (RF) devices used in noninvasive vaginal rejuvenation procedures.
Lasers
CO2 Laser
The infrared CO2 laser utilizes 10,600-nm energy to target and vaporize water molecules within the target tissue. This thermal heating extends to the dermal collagen, which stimulates inflammatory pathways and neocollagenesis.11 The depth of penetration ranges from 20 to 125 μm.12 Zerbinati et al13 demonstrated the histologic and ultrastructural effects of a fractional CO2 laser on atrophic vaginal mucosa. Comparing pretreatment and posttreatment mucosal biopsies in 5 postmenopausal women, the investigators found that fractional CO2 laser treatment caused increased epithelial thickness, vascularity, and fibroblast activity, which led to augmented synthesis of collagen and ground substance proteins.13
New devices seek to translate these histologic improvements to the aesthetic appearance and function of female genitalia. The MonaLisa Touch (Cynosure), a new fractional CO2 laser specifically designed for treatment of the vaginal mucosa, uses dermal optical thermolysis (DOT) therapy to apply energy in a noncontinuous mode at 200-μm dots. Salvatore et al14 examined the use of this device in a noncontrolled study of 50 patients with GSM, with each patient undergoing 3 treatment sessions at monthly intervals. Intravaginal treatments were performed at the following settings: DOT (microablative zone) power of 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack parameter of 1 to 3. The investigators used the Vaginal Health Index (VHI) to objectively assess vaginal elasticity, secretions, pH, mucosa integrity, and moisture. Total VHI scores significantly improved between baseline and 1 month following the final treatment (mean score [SD], 13.1 [2.5] vs 23.1 [1.9]; P<.0001). There were no significant adverse events, and 84% of patients reported being satisfied with their outcome; however, the study lacked a comparison or control group, raising the possibility of placebo effect.14
Other noncontrolled series have corroborated the benefits of CO2 laser in GSM patients.15,16 In one of the largest studies to date, Filippini et al17 reviewed the outcomes of 386 menopausal women treated for GSM. Patients underwent 3 intravaginal laser sessions with the MonaLisa Touch. Intravaginal treatments were performed at a DOT power of 40 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 2. For the vulva, the DOT power was reduced to 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 1. Two months after the final treatment session, patients completed a nonvalidated questionnaire about their symptoms, with improved dryness reported in 60% of patients, improved burning in 56%, improved dyspareunia in 49%, improved itch in 56%, improved soreness in 73%, and improved vaginal introitus pain in 49%. Although most patients did not experience discomfort with the procedure, a minority noted a burning sensation (11%), bother with handpiece movement (6%), or vulvar pain (5%).17
Recently, Cruz et al18 performed one of the first randomized, double-blind, placebo-controlled trials comparing fractional CO2 laser therapy, topical estrogen therapy, and the combination of both treatments in patients with GSM. Forty-five women were included in the study, and validated assessments were performed at baseline and weeks 8 and 20. Intravaginal treatments were performed at a DOT power of 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 2. Importantly, the study incorporated placebo laser treatments (with the power adjusted to 0.0 W) in the topical estrogen group, thereby decreasing result bias. There was a significant increase in VHI scores from baseline to week 8 (P<.05) and week 20 (P<.01) in all study arms. At week 20, the laser group and laser plus estrogen group showed significant improvements in reported dyspareunia, burning, and dryness, whereas the estrogen arm only reported improvements in dryness (all values P<.05).18
Erbium-Doped YAG Laser
The erbium-doped YAG (Er:YAG) laser is an ablative laser emitting light at 2940 nm. This wavelength provides an absorption coefficient for water 16 times greater than the CO2 laser, leading to decreased penetration depth of 1 to 3 μm and reduced damage to the surrounding tissues.19,20 As such, the Er:YAG laser results in milder postoperative discomfort and faster overall healing times.21
In a noncontrolled study of vaginal relaxation syndrome, Lee22 used an Er:YAG laser fitted with Petit Lady (Lutronic) 90° and 360° vaginal scanning scopes. Thirty patients were divided into 2 groups and were treated with 4 sessions at weekly intervals. In group A, the first 2 sessions were performed with the 360° scope, and the last 2 sessions with the 90° scope in multiple micropulse mode (3 multishots; pulse width of 250 μs; 1.7 J delivered per shot). Group B was treated with the 90° scope in all 4 sessions in multiple micropulse mode (same parameters as group A), and during the last 2 sessions patients were additionally treated with 2 passes per session with the 360° scope (long-pulsed mode; pulse width of 1000 μs; 3.7 J delivered per shot). Perineometer measurements taken 2 months after the final treatment showed that the combined patient population experienced significant increases in both maximal vaginal pressure (P<.01) and average vaginal pressure (P<.05). Roughly 76% of patients’ partners noted improved vaginal tightening, and 70% of patients reported being satisfied with their treatment outcome. Histologic specimens taken at baseline and 2 months postprocedure showed evidence of thicker and more cellular epithelia along with more compact lamina propria with denser connective tissue. The sessions were well tolerated, with patients reporting a nonpainful heating sensation in the vagina during treatment. Three patients from the combined patient population experienced a mild burning sensation and vaginal ecchymoses, which lasted 24 to 48 hours following treatment and resolved spontaneously. There was no control group and no reports of major or long-term adverse events.22
Investigations also have shown the benefit of Er:YAG in the treatment of GSM.23,24 In a study by Gambacciani et al,24 patients treated with the Er:YAG laser FotonaSmooth (Fotona) every 30 days for 3 months reported significant improvements in vaginal dryness and dyspareunia (P<.01), which lasted up to 6 months posttreatment, though there was no placebo group comparator. Similar results were seen by Gaspar et al23 using 3 treatments at 3-week intervals, with results sustained up to 18 months after the final session.
Radiofrequency Devices
Radiofrequency devices emit focused electromagnetic waves that heat underlying tissues without targeting melanin. The release of thermal energy induces collagen contraction, neocollagenesis, and neovascularization, all of which aid in restoring the elasticity and moisture of the vaginal mucosa.25 Devices also may be equipped with cooling probes and reverse-heating gradients to protect the surface mucosa while deeper tissues are heated.
Millheiser et al26 performed a noncontrolled pilot study in 24 women with vaginal laxity using the Viveve System (Viveve), a cryogen-cooled monopolar RF device. Participants underwent a single 30-minute session (energy ranging from 75–90 J/cm2) during which the mucosal surface of the vaginal introitus (excluding the urethra) was treated with pulses at 0.5-cm overlapping intervals. Follow-up assessments were completed at 1, 3, and 6 months posttreatment. Self-reported vaginal tightness improved in 67% of participants at 1-month posttreatment and in 87% of participants at 6 months posttreatment (P<.001). There were no adverse events reported.26 Sekiguchi et al27 reported similar benefits lasting up to 12 months after a single 26-minute session at 90 J/cm2.
A prospective, randomized, placebo-controlled clinical trial using the Viveve system was recently completed by Krychman et al.28 Participants (N=186) were randomized to receive a single session of active treatment (90 J/cm2) or placebo treatment (1 J/cm2). In both groups, the vaginal introitus was treated with pulses at 0.5 cm in overlapping intervals, with the entire area (excluding the urethra) treated 5 times up to a total of 110 pulses. The primary end point was the proportion of randomized participants reporting no vaginal laxity at 6 months postin-tervention, which was assessed using the Vaginal Laxity Questionnaire. A grade of no vaginal laxity was achieved by 43.5% of participants in the active treatment group and 19.6% of participants in the sham group (P=.002). Overall numbers of treatment-emergent adverse events were comparable between the 2 groups, with the most commonly reported being vaginal discharge (2.6% in the active treatment group vs 3.5% in the sham group). There were no serious adverse events reported in the active treatment group.28
ThermiVa (ThermiGen, LLC), a unipolar RF device, was evaluated by Alinsod29 in the treatment of orgasmic dysfunction. The noncontrolled study included 25 women with self-reported difficulty achieving orgasm during intercourse, each of whom underwent 3 treatment sessions at 1-month intervals. Of the 25 enrolled women, 19 (76%) reported an average reduction in time to orgasm of at least 50%. All anorgasmic patients (n=10) at baseline reported renewed ability to achieve orgasms. Two (8%) patients failed to achieve a significant benefit from the treatments. Of note, the study did not include a control group, and specific data on the durability of beneficial effects was lacking.29
The Ultra Femme 360 (BLT Industries Inc), a monopolar RF device, was evaluated by Lalji and Lozanova30 in a noncontrolled study of 27 women with mild to moderate vaginal laxity and urinary incontinence. Participants underwent 3 treatment sessions at weekly intervals. Vaginal laxity was assessed by a subjective vulvovaginal laxity questionnaire, and data were collected before the first treatment and at 1-month follow-up. All 27 participants reported improvements in vaginal laxity, with the average grade (SD) increasing from very loose (2.19 [1.08]) to moderately tight (5.74 [0.76]; P<.05) on the questionnaire’s 7-point scale. The trial did not include a control group.30
Conclusion
With growing patient interest in vaginal rejuvenation, clinicians are increasingly incorporating a variety of procedures into their practice. Although long-term data on the safety and efficacy of these treatments has yet to be established, current evidence indicates that fractional ablative lasers and RF devices can improve vaginal laxity, sexual sensation, and symptoms of GSM.
To date, major complications have not been reported, but the FDA has advocated caution until regulatory approval is achieved.10 Concerns exist over the limited number of robust clinical trials as well as the prevalence of advertising campaigns that promise wide-ranging improvements without sufficient evidence. Definitive statements on medical or cosmetic indications will undoubtedly require more thorough investigation. At this time, the safety profile of these devices appears to be favorable, and high rates of patient satisfaction have been reported. As such, noninvasive vaginal rejuvenation procedures may represent a valuable addition to the cosmetic landscape.
Vaginal rejuvenation encompasses a group of procedures that alter the vaginal anatomy to improve cosmesis or achieve more pleasurable sexual intercourse. External vaginal procedures are defined as those performed on the female genitalia outside of the vaginal introitus, with major structures including the labia majora, mons pubis, labia minora, clitoral hood, clitoral glans, and vaginal vestibule. Internal vaginal procedures are defined as those performed within the vagina, extending from the vaginal introitus to the cervix.
The prevalence of elective vaginal rejuvenation procedures has increased in recent years, a trend that may be attributed to greater exposure through the media, including reality television and pornography. In a survey of 482 women undergoing labiaplasty, nearly all had heard about rejuvenation procedures within the last 2.2 years, and 78% had received their information through the media.1 Additionally, genital self-image can have a considerable effect on a woman’s sexual behavior and relationships. Genital dissatisfaction has been associated with decreased sexual activity, whereas positive genital self-image correlates with increased sexual desire and less sexual distress or depression.2,3
Currently, the 2 primary applications of noninvasive vaginal rejuvenation are vaginal laxity and genitourinary syndrome of menopause (GSM). Vaginal laxity occurs in premenopausal or postmenopausal women and is caused by aging, childbearing, or hormonal imbalances. These factors can lead to decreased friction within the vagina during intercourse, which in turn can decrease sexual pleasure. Genitourinary syndrome of menopause, previously known as vulvovaginal atrophy, encompasses genital (eg, dryness, burning, irritation), sexual (eg, lack of lubrication, discomfort or pain, impaired function), and urinary (eg, urgency, dysuria, recurrent urinary tract infections) symptoms of menopause.4
Noninvasive procedures are designed to apply ablative or nonablative energy to the vaginal mucosa to tighten a lax upper vagina, also known as a wide vagina.5 A wide vagina has been defined as a widened vaginal diameter that interferes with sexual function and sensation.6 Decreased sexual sensation also may result from fibrosis or scarring of the vaginal mucosa after prior vaginal surgery, episiotomy, or tears during childbirth.7 The objective of rejuvenation procedures to treat the vaginal mucosa is to create increased frictional forces that may lead to increased sexual sensation.8 Although there are numerous reports of heightened sexual satisfaction after reduction of the vaginal diameter, a formal link between sexual pleasure and vaginal laxity has yet to be established.8,9 At present, there are no US Food and Drug Administration (FDA)–approved energy-based devices to treat urinary incontinence or sexual function, and the FDA recently issued an alert cautioning patients on the current lack of safety and efficacy regulations.10
In this article we review the safety and efficacy data behind lasers and radiofrequency (RF) devices used in noninvasive vaginal rejuvenation procedures.
Lasers
CO2 Laser
The infrared CO2 laser utilizes 10,600-nm energy to target and vaporize water molecules within the target tissue. This thermal heating extends to the dermal collagen, which stimulates inflammatory pathways and neocollagenesis.11 The depth of penetration ranges from 20 to 125 μm.12 Zerbinati et al13 demonstrated the histologic and ultrastructural effects of a fractional CO2 laser on atrophic vaginal mucosa. Comparing pretreatment and posttreatment mucosal biopsies in 5 postmenopausal women, the investigators found that fractional CO2 laser treatment caused increased epithelial thickness, vascularity, and fibroblast activity, which led to augmented synthesis of collagen and ground substance proteins.13
New devices seek to translate these histologic improvements to the aesthetic appearance and function of female genitalia. The MonaLisa Touch (Cynosure), a new fractional CO2 laser specifically designed for treatment of the vaginal mucosa, uses dermal optical thermolysis (DOT) therapy to apply energy in a noncontinuous mode at 200-μm dots. Salvatore et al14 examined the use of this device in a noncontrolled study of 50 patients with GSM, with each patient undergoing 3 treatment sessions at monthly intervals. Intravaginal treatments were performed at the following settings: DOT (microablative zone) power of 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack parameter of 1 to 3. The investigators used the Vaginal Health Index (VHI) to objectively assess vaginal elasticity, secretions, pH, mucosa integrity, and moisture. Total VHI scores significantly improved between baseline and 1 month following the final treatment (mean score [SD], 13.1 [2.5] vs 23.1 [1.9]; P<.0001). There were no significant adverse events, and 84% of patients reported being satisfied with their outcome; however, the study lacked a comparison or control group, raising the possibility of placebo effect.14
Other noncontrolled series have corroborated the benefits of CO2 laser in GSM patients.15,16 In one of the largest studies to date, Filippini et al17 reviewed the outcomes of 386 menopausal women treated for GSM. Patients underwent 3 intravaginal laser sessions with the MonaLisa Touch. Intravaginal treatments were performed at a DOT power of 40 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 2. For the vulva, the DOT power was reduced to 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 1. Two months after the final treatment session, patients completed a nonvalidated questionnaire about their symptoms, with improved dryness reported in 60% of patients, improved burning in 56%, improved dyspareunia in 49%, improved itch in 56%, improved soreness in 73%, and improved vaginal introitus pain in 49%. Although most patients did not experience discomfort with the procedure, a minority noted a burning sensation (11%), bother with handpiece movement (6%), or vulvar pain (5%).17
Recently, Cruz et al18 performed one of the first randomized, double-blind, placebo-controlled trials comparing fractional CO2 laser therapy, topical estrogen therapy, and the combination of both treatments in patients with GSM. Forty-five women were included in the study, and validated assessments were performed at baseline and weeks 8 and 20. Intravaginal treatments were performed at a DOT power of 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 2. Importantly, the study incorporated placebo laser treatments (with the power adjusted to 0.0 W) in the topical estrogen group, thereby decreasing result bias. There was a significant increase in VHI scores from baseline to week 8 (P<.05) and week 20 (P<.01) in all study arms. At week 20, the laser group and laser plus estrogen group showed significant improvements in reported dyspareunia, burning, and dryness, whereas the estrogen arm only reported improvements in dryness (all values P<.05).18
Erbium-Doped YAG Laser
The erbium-doped YAG (Er:YAG) laser is an ablative laser emitting light at 2940 nm. This wavelength provides an absorption coefficient for water 16 times greater than the CO2 laser, leading to decreased penetration depth of 1 to 3 μm and reduced damage to the surrounding tissues.19,20 As such, the Er:YAG laser results in milder postoperative discomfort and faster overall healing times.21
In a noncontrolled study of vaginal relaxation syndrome, Lee22 used an Er:YAG laser fitted with Petit Lady (Lutronic) 90° and 360° vaginal scanning scopes. Thirty patients were divided into 2 groups and were treated with 4 sessions at weekly intervals. In group A, the first 2 sessions were performed with the 360° scope, and the last 2 sessions with the 90° scope in multiple micropulse mode (3 multishots; pulse width of 250 μs; 1.7 J delivered per shot). Group B was treated with the 90° scope in all 4 sessions in multiple micropulse mode (same parameters as group A), and during the last 2 sessions patients were additionally treated with 2 passes per session with the 360° scope (long-pulsed mode; pulse width of 1000 μs; 3.7 J delivered per shot). Perineometer measurements taken 2 months after the final treatment showed that the combined patient population experienced significant increases in both maximal vaginal pressure (P<.01) and average vaginal pressure (P<.05). Roughly 76% of patients’ partners noted improved vaginal tightening, and 70% of patients reported being satisfied with their treatment outcome. Histologic specimens taken at baseline and 2 months postprocedure showed evidence of thicker and more cellular epithelia along with more compact lamina propria with denser connective tissue. The sessions were well tolerated, with patients reporting a nonpainful heating sensation in the vagina during treatment. Three patients from the combined patient population experienced a mild burning sensation and vaginal ecchymoses, which lasted 24 to 48 hours following treatment and resolved spontaneously. There was no control group and no reports of major or long-term adverse events.22
Investigations also have shown the benefit of Er:YAG in the treatment of GSM.23,24 In a study by Gambacciani et al,24 patients treated with the Er:YAG laser FotonaSmooth (Fotona) every 30 days for 3 months reported significant improvements in vaginal dryness and dyspareunia (P<.01), which lasted up to 6 months posttreatment, though there was no placebo group comparator. Similar results were seen by Gaspar et al23 using 3 treatments at 3-week intervals, with results sustained up to 18 months after the final session.
Radiofrequency Devices
Radiofrequency devices emit focused electromagnetic waves that heat underlying tissues without targeting melanin. The release of thermal energy induces collagen contraction, neocollagenesis, and neovascularization, all of which aid in restoring the elasticity and moisture of the vaginal mucosa.25 Devices also may be equipped with cooling probes and reverse-heating gradients to protect the surface mucosa while deeper tissues are heated.
Millheiser et al26 performed a noncontrolled pilot study in 24 women with vaginal laxity using the Viveve System (Viveve), a cryogen-cooled monopolar RF device. Participants underwent a single 30-minute session (energy ranging from 75–90 J/cm2) during which the mucosal surface of the vaginal introitus (excluding the urethra) was treated with pulses at 0.5-cm overlapping intervals. Follow-up assessments were completed at 1, 3, and 6 months posttreatment. Self-reported vaginal tightness improved in 67% of participants at 1-month posttreatment and in 87% of participants at 6 months posttreatment (P<.001). There were no adverse events reported.26 Sekiguchi et al27 reported similar benefits lasting up to 12 months after a single 26-minute session at 90 J/cm2.
A prospective, randomized, placebo-controlled clinical trial using the Viveve system was recently completed by Krychman et al.28 Participants (N=186) were randomized to receive a single session of active treatment (90 J/cm2) or placebo treatment (1 J/cm2). In both groups, the vaginal introitus was treated with pulses at 0.5 cm in overlapping intervals, with the entire area (excluding the urethra) treated 5 times up to a total of 110 pulses. The primary end point was the proportion of randomized participants reporting no vaginal laxity at 6 months postin-tervention, which was assessed using the Vaginal Laxity Questionnaire. A grade of no vaginal laxity was achieved by 43.5% of participants in the active treatment group and 19.6% of participants in the sham group (P=.002). Overall numbers of treatment-emergent adverse events were comparable between the 2 groups, with the most commonly reported being vaginal discharge (2.6% in the active treatment group vs 3.5% in the sham group). There were no serious adverse events reported in the active treatment group.28
ThermiVa (ThermiGen, LLC), a unipolar RF device, was evaluated by Alinsod29 in the treatment of orgasmic dysfunction. The noncontrolled study included 25 women with self-reported difficulty achieving orgasm during intercourse, each of whom underwent 3 treatment sessions at 1-month intervals. Of the 25 enrolled women, 19 (76%) reported an average reduction in time to orgasm of at least 50%. All anorgasmic patients (n=10) at baseline reported renewed ability to achieve orgasms. Two (8%) patients failed to achieve a significant benefit from the treatments. Of note, the study did not include a control group, and specific data on the durability of beneficial effects was lacking.29
The Ultra Femme 360 (BLT Industries Inc), a monopolar RF device, was evaluated by Lalji and Lozanova30 in a noncontrolled study of 27 women with mild to moderate vaginal laxity and urinary incontinence. Participants underwent 3 treatment sessions at weekly intervals. Vaginal laxity was assessed by a subjective vulvovaginal laxity questionnaire, and data were collected before the first treatment and at 1-month follow-up. All 27 participants reported improvements in vaginal laxity, with the average grade (SD) increasing from very loose (2.19 [1.08]) to moderately tight (5.74 [0.76]; P<.05) on the questionnaire’s 7-point scale. The trial did not include a control group.30
Conclusion
With growing patient interest in vaginal rejuvenation, clinicians are increasingly incorporating a variety of procedures into their practice. Although long-term data on the safety and efficacy of these treatments has yet to be established, current evidence indicates that fractional ablative lasers and RF devices can improve vaginal laxity, sexual sensation, and symptoms of GSM.
To date, major complications have not been reported, but the FDA has advocated caution until regulatory approval is achieved.10 Concerns exist over the limited number of robust clinical trials as well as the prevalence of advertising campaigns that promise wide-ranging improvements without sufficient evidence. Definitive statements on medical or cosmetic indications will undoubtedly require more thorough investigation. At this time, the safety profile of these devices appears to be favorable, and high rates of patient satisfaction have been reported. As such, noninvasive vaginal rejuvenation procedures may represent a valuable addition to the cosmetic landscape.
- Koning M, Zeijlmans IA, Bouman TK, et al. Female attitudes regarding labia minora appearance and reduction with consideration of media influence. Aesthet Surg J. 2009;29:65-71.
- Rowen TS, Gaither TW, Shindel AW, et al. Characteristics of genital dissatisfaction among a nationally representative sample of U.S. women. J Sex Med. 2018;15:698-704.
- Berman L, Berman J, Miles M, et al. Genital self-image as a component of sexual health: relationship between genital self-image, female sexual function, and quality of life measures. J Sex Marital Ther. 2003;29(suppl 1):11-21.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Goodman MP, Placik OJ, Benson RH 3rd, et al. A large multicenter outcome study of female genital plastic surgery. J Sex Med. 2010;7(4 pt 1):1565-1577.
- Ostrzenski A. Vaginal rugation rejuvenation (restoration): a new surgical technique for an acquired sensation of wide/smooth vagina. Gynecol Obstet Invest. 2012;73:48-52.
- Singh A, Swift S, Khullar V, et al. Laser vaginal rejuvenation: not ready for prime time. Int Urogynecol J. 2015;26:163-164.
- Iglesia CB, Yurteri-Kaplan L, Alinsod R. Female genital cosmetic surgery: a review of techniques and outcomes. Int Urogynecol J. 2013;24:1997-2009.
- Dobbeleir JM, Landuyt KV, Monstrey SJ. Aesthetic surgery of the female genitalia. Semin Plast Surg. 2011;25:130-141.
- US Food and Drug Administration. FDA warns against use of energy-based devices to perform vaginal ‘rejuvenation’ or vaginal cosmetic procedures: FDA safety communication. July 30, 2018. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed September 10, 2018.
- Patil UA, Dhami LD. Overview of lasers. Indian J Plast Surg. 2008;41(suppl):S101-S113.
- Qureshi AA, Tenenbaum MM, Myckatyn TM. Nonsurgical vulvovaginal rejuvenation with radiofrequency and laser devices: a literature review and comprehensive update for aesthetic surgeons. Aesthet Surg J. 2018;38:302-311.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30:429-436.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17:363-369.
- Eder SE. Early effect of fractional CO2 laser treatment in post-menopausal women with vaginal atrophy. Laser Ther. 2018;27:41-47.
- Perino A, Calligaro A, Forlani F, et al. Vulvo-vaginal atrophy: a new treatment modality using thermo-ablative fractional CO2 laser. Maturitas. 2015;80:296-301.
- Filippini M, Del Duca E, Negosanti F, et al. Fractional CO2 laser: from skin rejuvenation to vulvo-vaginal reshaping. Photomed Laser Surg. 2017;35:171-175.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28.
- Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116.
- Kaushik SB, Alexis AF. Nonablative fractional laser resurfacing in skin of color: evidence-based review. J Clin Aesthet Dermatol. 2017;10:51-67.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. Fractional laser skin resurfacing. J Drugs Dermatol. 2012;11:1274-1287.
- Lee MS. Treatment of vaginal relaxation syndrome with an erbium:YAG laser using 90 degrees and 360 degrees scanning scopes: a pilot study & short-term results. Laser Ther. 2014;23:129-138.
- Gaspar A, Brandi H, Gomez V, et al. Efficacy of erbium:YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg Med. 2017;49:160-168.
- Gambacciani M, Levancini M, Cervigni M. Vaginal erbium laser: the second-generation thermotherapy for the genitourinary syndrome of menopause. Climacteric. 2015;18:757-763.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. 2017;49:137-159.
- Millheiser LS, Pauls RN, Herbst SJ, et al. Radiofrequency treatment of vaginal laxity after vaginal delivery: nonsurgical vaginal tightening. J Sex Med. 2010;7:3088-3095.
- Sekiguchi Y, Utsugisawa Y, Azekosi Y, et al. Laxity of the vaginal introitus after childbirth: nonsurgical outpatient procedure for vaginal tissue restoration and improved sexual satisfaction using low-energy radiofrequency thermal therapy. J Womens Health (Larchmt). 2013;22:775-781.
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14:215-225.
- Alinsod RM. Transcutaneous temperature controlled radiofrequency for orgasmic dysfunction. Lasers Surg Med. 2016;48:641-645.
- Lalji S, Lozanova P. Evaluation of the safety and efficacy of a monopolar nonablative radiofrequency device for the improvement of vulvo-vaginal laxity and urinary incontinence. J Cosmet Dermatol. 2017;16:230-234.
- Koning M, Zeijlmans IA, Bouman TK, et al. Female attitudes regarding labia minora appearance and reduction with consideration of media influence. Aesthet Surg J. 2009;29:65-71.
- Rowen TS, Gaither TW, Shindel AW, et al. Characteristics of genital dissatisfaction among a nationally representative sample of U.S. women. J Sex Med. 2018;15:698-704.
- Berman L, Berman J, Miles M, et al. Genital self-image as a component of sexual health: relationship between genital self-image, female sexual function, and quality of life measures. J Sex Marital Ther. 2003;29(suppl 1):11-21.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Goodman MP, Placik OJ, Benson RH 3rd, et al. A large multicenter outcome study of female genital plastic surgery. J Sex Med. 2010;7(4 pt 1):1565-1577.
- Ostrzenski A. Vaginal rugation rejuvenation (restoration): a new surgical technique for an acquired sensation of wide/smooth vagina. Gynecol Obstet Invest. 2012;73:48-52.
- Singh A, Swift S, Khullar V, et al. Laser vaginal rejuvenation: not ready for prime time. Int Urogynecol J. 2015;26:163-164.
- Iglesia CB, Yurteri-Kaplan L, Alinsod R. Female genital cosmetic surgery: a review of techniques and outcomes. Int Urogynecol J. 2013;24:1997-2009.
- Dobbeleir JM, Landuyt KV, Monstrey SJ. Aesthetic surgery of the female genitalia. Semin Plast Surg. 2011;25:130-141.
- US Food and Drug Administration. FDA warns against use of energy-based devices to perform vaginal ‘rejuvenation’ or vaginal cosmetic procedures: FDA safety communication. July 30, 2018. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed September 10, 2018.
- Patil UA, Dhami LD. Overview of lasers. Indian J Plast Surg. 2008;41(suppl):S101-S113.
- Qureshi AA, Tenenbaum MM, Myckatyn TM. Nonsurgical vulvovaginal rejuvenation with radiofrequency and laser devices: a literature review and comprehensive update for aesthetic surgeons. Aesthet Surg J. 2018;38:302-311.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30:429-436.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17:363-369.
- Eder SE. Early effect of fractional CO2 laser treatment in post-menopausal women with vaginal atrophy. Laser Ther. 2018;27:41-47.
- Perino A, Calligaro A, Forlani F, et al. Vulvo-vaginal atrophy: a new treatment modality using thermo-ablative fractional CO2 laser. Maturitas. 2015;80:296-301.
- Filippini M, Del Duca E, Negosanti F, et al. Fractional CO2 laser: from skin rejuvenation to vulvo-vaginal reshaping. Photomed Laser Surg. 2017;35:171-175.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28.
- Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116.
- Kaushik SB, Alexis AF. Nonablative fractional laser resurfacing in skin of color: evidence-based review. J Clin Aesthet Dermatol. 2017;10:51-67.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. Fractional laser skin resurfacing. J Drugs Dermatol. 2012;11:1274-1287.
- Lee MS. Treatment of vaginal relaxation syndrome with an erbium:YAG laser using 90 degrees and 360 degrees scanning scopes: a pilot study & short-term results. Laser Ther. 2014;23:129-138.
- Gaspar A, Brandi H, Gomez V, et al. Efficacy of erbium:YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg Med. 2017;49:160-168.
- Gambacciani M, Levancini M, Cervigni M. Vaginal erbium laser: the second-generation thermotherapy for the genitourinary syndrome of menopause. Climacteric. 2015;18:757-763.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. 2017;49:137-159.
- Millheiser LS, Pauls RN, Herbst SJ, et al. Radiofrequency treatment of vaginal laxity after vaginal delivery: nonsurgical vaginal tightening. J Sex Med. 2010;7:3088-3095.
- Sekiguchi Y, Utsugisawa Y, Azekosi Y, et al. Laxity of the vaginal introitus after childbirth: nonsurgical outpatient procedure for vaginal tissue restoration and improved sexual satisfaction using low-energy radiofrequency thermal therapy. J Womens Health (Larchmt). 2013;22:775-781.
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14:215-225.
- Alinsod RM. Transcutaneous temperature controlled radiofrequency for orgasmic dysfunction. Lasers Surg Med. 2016;48:641-645.
- Lalji S, Lozanova P. Evaluation of the safety and efficacy of a monopolar nonablative radiofrequency device for the improvement of vulvo-vaginal laxity and urinary incontinence. J Cosmet Dermatol. 2017;16:230-234.
Practice Points
- Noninvasive vaginal rejuvenation represents a growing area of cosmetic dermatology.
- Radiofrequency and ablative laser devices have demonstrated promising results in treating vaginal laxity and genitourinary syndrome of menopause, but US Food and Drug Administration approval has yet to be obtained.
Clove
Cloves (Syzygium aromaticum, also known as Eugenia caryophyllata) are the aromatic flower buds of a tree in the Myrtaceae family native to Indonesia. The essential oil of clove is known to exhibit antioxidant, anti-inflammatory, antimicrobial, antifungal, antiviral, anticancer, cytotoxic, insect repellent, and anesthetic activities.1,2 It is used topically in herbal medicine to alleviate pain and facilitate healing,3 and has been used in traditional medicine to confer analgesic, anti-inflammatory, antimicrobial, antiviral, and antiseptic activity.4 Cloves also are used in fragrances and for food flavoring.2
The two main constituents of clove oil are eugenol (78%) and beta-caryophyllene (13%). Although clove oil and its primary components are generally recognized as safe, a 2006 in vitro study by Prashar et al. found that clove oil and eugenol displayed cytotoxicity toward human fibroblasts and endothelial cells. Clove oil, in concentrations as low as 0.03%, was noted for being exceedingly cytotoxic, with up to 73% of this effect ascribed to eugenol, with beta-caryophyllene displaying no toxicity.3 In addition to beta-caryophyllene and the phenylpropanoid eugenol, other important constituents of clove essential oil are the phenylpropanoids carvacrol, thymol, and cinnamaldehyde.2
Topical applications and human studies
constituent, eugenol.5 It also has been used as a penetration enhancer in various forms of topical products, including creams, ointments, gels, and patches.6
Palmar hyperhidrosis
In 2017, Ibrahim et al. treated 45 patients with palmar hyperhidrosis with clove oil 45% in liposome, with 20 patients in a control group treated with 0.9% saline solution. Subjects were assessed by gravimetry testing and hyperhidrosis disease severity scale to determine the impact of clove oil on decreasing the sweating rate in patients with idiopathic palmar hyperhidrosis. Gravimetry testing revealed that the sweating rate decreased significantly in the clove oil group but that there was no significant improvement in the placebo group. The investigators concluded that twice-daily topical application of 45% clove oil in liposome for 2 weeks showed promise in significantly reducing palmar sweating.5
Pruritus
That same year Ibrahim et al. evaluated the effects of topically applied clove oil in treating 50 patients with chronic pruritus due to hepatic, renal, or diabetic origin. The investigators divided the subjects into two groups of 25, with the first directed to hydrate their skin before applying topical clove oil twice daily for 2 weeks. The second group was instructed to apply topical petrolatum by hand on the same schedule. Using the 5-D itch scale, researchers noted a significant improvement in all parameters in the patients using clove oil and no such improvements in the petrolatum group. They concluded that particularly for patients whose topical or systemic treatments are not well tolerated or are contraindicated.7
Anal fissure
In 2007, Elwakeel et al. evaluated the use of a clove oil 1% cream for the treatment of chronic anal fissure as opposed to the traditional treatment of stool softeners and lignocaine cream 5% in a single-blind randomized comparative trial over 6 weeks. Healing was observed in 60% of the 30 patients in the clove oil group and in 12% of the 25 patients in the control group at the 3-month follow-up visit. The researchers concluded that topically applied clove oil cream yielded significant benefits in the treatment of chronic anal fissures.8
More recently, Nelson et al. conducted a literature survey to evaluate the efficacy and morbidity of nonsurgical treatments for anal fissures from 1966 to August 2010. Clove oil was among 17 agents used in the 77 cited studies. While no medical therapies were found to display the efficacy of surgical sphincterotomy (or, fortunately, linked to the risk of incontinence), clove oil was identified as one of the “newer” agents demonstrating promise.9
Musculoskeletal pain
Clove oil is included among several herbal ingredients (i.e., eucalyptus oil, gaultheria oil, turpentine oil, menthol, and camphor) associated with analgesic and anti-inflammatory properties that are used in the topical spray Eezpain. Nawaz et al. showed in a prospective pilot study with 20 male and female subjects that the polyherbal formulation was efficacious in relieving mild to moderate knee and wrist joint pain.10
Laboratory studies
Just over a decade ago, Chaieb et al. assessed the antioxidant characteristics of the essential oil of clove, finding that it displayed a robust radical scavenging capacity against 2,2-diphenyl-1-picrylhydrazyl in comparison to the synthetic antioxidant tert-butylated hydroxytoluene. It also showed potent antifungal activity against 53 test strains of human pathogenic yeasts. The authors noted that clove oil is a readily available source of natural antioxidants and is a worthy ingredient in pharmaceutical products.11
Anti-inflammatory activity
In 2017, Han and Parker studied the biological activity of four concentrations of a commercially available clove essential oil product on 17 protein biomarkers important in inflammation in a model of human skin disease. They found that the 0.011% concentration of the oil enacted strong antiproliferative effects on human dermal skin fibroblasts, and significantly suppressed multiple proinflammatory biomarkers as well as tissue remodeling protein molecules. The investigators also observed that essential clove oil significantly influenced global gene expression and signaling pathways involved in inflammation, tissue remodeling, and cancer processes. They concluded that their results indicate anti-inflammatory, anticancer, and tissue-remodeling properties of clove essential oil, and its main active ingredient eugenol, in human dermal fibroblasts.1
UVB protection
Recently, Patwardhan and Bhatt assessed the capacity of flavonoids from clove buds to protect human dermal fibroblasts from UVB exposure. They found that the flavonoid-enriched fraction of clove demonstrated significant potential, as it mitigated the effects of UVB radiation, and delivered protection via the nuclear factor E2-related factor 2-antioxidant response pathway. The flavonoid-enriched clove fraction, they concluded, warrants consideration as a topically applied cutaneous protectant against the effects of UVB exposure.4
Antiviral and immunomodulatory activity
Based on their earlier work showing the antiviral activity of clove bud oil against Pseudomonas aeruginosa PAO1, Haripriyan et al. reported this year that clove bud oil affects pseudomonal proteases (elastase A, elastase B, protease IV, and alkaline protease), attenuating significant viral mechanisms of this noted human disease agent while bolstering host immunomodulatory functions. They concluded that their results suggest the viability of clove bud oil as a topical treatment for infections resistant to antibiotics.12
Acne
In 2017, Owen et al. developed a topical preparation incorporating clove bud, rosewood, and litsea essential oils that compared favorably with the topical antibiotics Dalacin T and Stiemycin in controlling acne vulgaris-linked bacteria. Specifically, the herbal formulation exhibited synergistic activity against Propionibacterium acnes, although not to Staphylococcus epidermidis, and its antimicrobial activity exceeded or equated to that of the tested antibiotics. The investigators suggested that the polyherbal preparation may serve as an option for treating acne-linked bacteria.13
Scabies
In a study 2 years ago to ascertain the efficacy of 10 essential oils against Sarcoptes scabiei, Fang et al. conducted contact bioassays and fumigation bioassays using clove, palmarosa, geranium, tea tree, lavender, Manuka, bitter orange, eucalyptus, Japanese cedar, and cade oil. In the contact bioassays, clove oil 1%, the most effective of the oils, eliminated the mites within 20 minutes. In the fumigation bioassay, clove was second to tea tree oil in efficacy. The investigators concluded that clove, tea tree, palmarosa, and eucalyptus oils demonstrate potential in pest control and for treating scabies infections in humans or animals.14
Conclusion
Clove oil is an active ingredient in various topical treatments. While not typically a first-line therapy, it shows promise for a wider range of applications. Research continues to determine the extent to which this botanical agent can reach into the dermatologic armamentarium and, more importantly, how effective it can be in treating cutaneous disorders.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
References
1. Pharm Biol. 2017 Dec;55(1):1619-22.
2. Phytother Res. 2007 Jun;21(6):501-6.
3. Cell Prolif. 2006 Aug;39(4):241-8.
4. Pharmacogn Mag. 2015 Oct;11(Suppl 3):S397-406.
5. J Cosmet Dermatol. 2017 Dec 28. doi: 10.1111/jocd.12471.
6. Curr Drug Deliv. 2012 Mar;9(2):219-30.
7. J Cosmet Dermatol. 2017 Dec;16(4):508-11.
8. Colorectal Dis. 2007 Jul;9(6):549-52
9. Cochrane Database Syst Rev. 2012 Feb 15;(2):CD003431.
10. Pak J Pharm Sci. 2015 Jan;28(1):43-7.
11. Mycoses. 2007 Sep;50(5):403-6.
12. Sci Rep. 2018 Feb 21;8(1):3437.
13. Phytother Res. 2017 Mar;31(3):410-7.
14. Parasit Vectors. 2016 Nov 22;9(1):594.
Cloves (Syzygium aromaticum, also known as Eugenia caryophyllata) are the aromatic flower buds of a tree in the Myrtaceae family native to Indonesia. The essential oil of clove is known to exhibit antioxidant, anti-inflammatory, antimicrobial, antifungal, antiviral, anticancer, cytotoxic, insect repellent, and anesthetic activities.1,2 It is used topically in herbal medicine to alleviate pain and facilitate healing,3 and has been used in traditional medicine to confer analgesic, anti-inflammatory, antimicrobial, antiviral, and antiseptic activity.4 Cloves also are used in fragrances and for food flavoring.2
The two main constituents of clove oil are eugenol (78%) and beta-caryophyllene (13%). Although clove oil and its primary components are generally recognized as safe, a 2006 in vitro study by Prashar et al. found that clove oil and eugenol displayed cytotoxicity toward human fibroblasts and endothelial cells. Clove oil, in concentrations as low as 0.03%, was noted for being exceedingly cytotoxic, with up to 73% of this effect ascribed to eugenol, with beta-caryophyllene displaying no toxicity.3 In addition to beta-caryophyllene and the phenylpropanoid eugenol, other important constituents of clove essential oil are the phenylpropanoids carvacrol, thymol, and cinnamaldehyde.2
Topical applications and human studies
constituent, eugenol.5 It also has been used as a penetration enhancer in various forms of topical products, including creams, ointments, gels, and patches.6
Palmar hyperhidrosis
In 2017, Ibrahim et al. treated 45 patients with palmar hyperhidrosis with clove oil 45% in liposome, with 20 patients in a control group treated with 0.9% saline solution. Subjects were assessed by gravimetry testing and hyperhidrosis disease severity scale to determine the impact of clove oil on decreasing the sweating rate in patients with idiopathic palmar hyperhidrosis. Gravimetry testing revealed that the sweating rate decreased significantly in the clove oil group but that there was no significant improvement in the placebo group. The investigators concluded that twice-daily topical application of 45% clove oil in liposome for 2 weeks showed promise in significantly reducing palmar sweating.5
Pruritus
That same year Ibrahim et al. evaluated the effects of topically applied clove oil in treating 50 patients with chronic pruritus due to hepatic, renal, or diabetic origin. The investigators divided the subjects into two groups of 25, with the first directed to hydrate their skin before applying topical clove oil twice daily for 2 weeks. The second group was instructed to apply topical petrolatum by hand on the same schedule. Using the 5-D itch scale, researchers noted a significant improvement in all parameters in the patients using clove oil and no such improvements in the petrolatum group. They concluded that particularly for patients whose topical or systemic treatments are not well tolerated or are contraindicated.7
Anal fissure
In 2007, Elwakeel et al. evaluated the use of a clove oil 1% cream for the treatment of chronic anal fissure as opposed to the traditional treatment of stool softeners and lignocaine cream 5% in a single-blind randomized comparative trial over 6 weeks. Healing was observed in 60% of the 30 patients in the clove oil group and in 12% of the 25 patients in the control group at the 3-month follow-up visit. The researchers concluded that topically applied clove oil cream yielded significant benefits in the treatment of chronic anal fissures.8
More recently, Nelson et al. conducted a literature survey to evaluate the efficacy and morbidity of nonsurgical treatments for anal fissures from 1966 to August 2010. Clove oil was among 17 agents used in the 77 cited studies. While no medical therapies were found to display the efficacy of surgical sphincterotomy (or, fortunately, linked to the risk of incontinence), clove oil was identified as one of the “newer” agents demonstrating promise.9
Musculoskeletal pain
Clove oil is included among several herbal ingredients (i.e., eucalyptus oil, gaultheria oil, turpentine oil, menthol, and camphor) associated with analgesic and anti-inflammatory properties that are used in the topical spray Eezpain. Nawaz et al. showed in a prospective pilot study with 20 male and female subjects that the polyherbal formulation was efficacious in relieving mild to moderate knee and wrist joint pain.10
Laboratory studies
Just over a decade ago, Chaieb et al. assessed the antioxidant characteristics of the essential oil of clove, finding that it displayed a robust radical scavenging capacity against 2,2-diphenyl-1-picrylhydrazyl in comparison to the synthetic antioxidant tert-butylated hydroxytoluene. It also showed potent antifungal activity against 53 test strains of human pathogenic yeasts. The authors noted that clove oil is a readily available source of natural antioxidants and is a worthy ingredient in pharmaceutical products.11
Anti-inflammatory activity
In 2017, Han and Parker studied the biological activity of four concentrations of a commercially available clove essential oil product on 17 protein biomarkers important in inflammation in a model of human skin disease. They found that the 0.011% concentration of the oil enacted strong antiproliferative effects on human dermal skin fibroblasts, and significantly suppressed multiple proinflammatory biomarkers as well as tissue remodeling protein molecules. The investigators also observed that essential clove oil significantly influenced global gene expression and signaling pathways involved in inflammation, tissue remodeling, and cancer processes. They concluded that their results indicate anti-inflammatory, anticancer, and tissue-remodeling properties of clove essential oil, and its main active ingredient eugenol, in human dermal fibroblasts.1
UVB protection
Recently, Patwardhan and Bhatt assessed the capacity of flavonoids from clove buds to protect human dermal fibroblasts from UVB exposure. They found that the flavonoid-enriched fraction of clove demonstrated significant potential, as it mitigated the effects of UVB radiation, and delivered protection via the nuclear factor E2-related factor 2-antioxidant response pathway. The flavonoid-enriched clove fraction, they concluded, warrants consideration as a topically applied cutaneous protectant against the effects of UVB exposure.4
Antiviral and immunomodulatory activity
Based on their earlier work showing the antiviral activity of clove bud oil against Pseudomonas aeruginosa PAO1, Haripriyan et al. reported this year that clove bud oil affects pseudomonal proteases (elastase A, elastase B, protease IV, and alkaline protease), attenuating significant viral mechanisms of this noted human disease agent while bolstering host immunomodulatory functions. They concluded that their results suggest the viability of clove bud oil as a topical treatment for infections resistant to antibiotics.12
Acne
In 2017, Owen et al. developed a topical preparation incorporating clove bud, rosewood, and litsea essential oils that compared favorably with the topical antibiotics Dalacin T and Stiemycin in controlling acne vulgaris-linked bacteria. Specifically, the herbal formulation exhibited synergistic activity against Propionibacterium acnes, although not to Staphylococcus epidermidis, and its antimicrobial activity exceeded or equated to that of the tested antibiotics. The investigators suggested that the polyherbal preparation may serve as an option for treating acne-linked bacteria.13
Scabies
In a study 2 years ago to ascertain the efficacy of 10 essential oils against Sarcoptes scabiei, Fang et al. conducted contact bioassays and fumigation bioassays using clove, palmarosa, geranium, tea tree, lavender, Manuka, bitter orange, eucalyptus, Japanese cedar, and cade oil. In the contact bioassays, clove oil 1%, the most effective of the oils, eliminated the mites within 20 minutes. In the fumigation bioassay, clove was second to tea tree oil in efficacy. The investigators concluded that clove, tea tree, palmarosa, and eucalyptus oils demonstrate potential in pest control and for treating scabies infections in humans or animals.14
Conclusion
Clove oil is an active ingredient in various topical treatments. While not typically a first-line therapy, it shows promise for a wider range of applications. Research continues to determine the extent to which this botanical agent can reach into the dermatologic armamentarium and, more importantly, how effective it can be in treating cutaneous disorders.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
References
1. Pharm Biol. 2017 Dec;55(1):1619-22.
2. Phytother Res. 2007 Jun;21(6):501-6.
3. Cell Prolif. 2006 Aug;39(4):241-8.
4. Pharmacogn Mag. 2015 Oct;11(Suppl 3):S397-406.
5. J Cosmet Dermatol. 2017 Dec 28. doi: 10.1111/jocd.12471.
6. Curr Drug Deliv. 2012 Mar;9(2):219-30.
7. J Cosmet Dermatol. 2017 Dec;16(4):508-11.
8. Colorectal Dis. 2007 Jul;9(6):549-52
9. Cochrane Database Syst Rev. 2012 Feb 15;(2):CD003431.
10. Pak J Pharm Sci. 2015 Jan;28(1):43-7.
11. Mycoses. 2007 Sep;50(5):403-6.
12. Sci Rep. 2018 Feb 21;8(1):3437.
13. Phytother Res. 2017 Mar;31(3):410-7.
14. Parasit Vectors. 2016 Nov 22;9(1):594.
Cloves (Syzygium aromaticum, also known as Eugenia caryophyllata) are the aromatic flower buds of a tree in the Myrtaceae family native to Indonesia. The essential oil of clove is known to exhibit antioxidant, anti-inflammatory, antimicrobial, antifungal, antiviral, anticancer, cytotoxic, insect repellent, and anesthetic activities.1,2 It is used topically in herbal medicine to alleviate pain and facilitate healing,3 and has been used in traditional medicine to confer analgesic, anti-inflammatory, antimicrobial, antiviral, and antiseptic activity.4 Cloves also are used in fragrances and for food flavoring.2
The two main constituents of clove oil are eugenol (78%) and beta-caryophyllene (13%). Although clove oil and its primary components are generally recognized as safe, a 2006 in vitro study by Prashar et al. found that clove oil and eugenol displayed cytotoxicity toward human fibroblasts and endothelial cells. Clove oil, in concentrations as low as 0.03%, was noted for being exceedingly cytotoxic, with up to 73% of this effect ascribed to eugenol, with beta-caryophyllene displaying no toxicity.3 In addition to beta-caryophyllene and the phenylpropanoid eugenol, other important constituents of clove essential oil are the phenylpropanoids carvacrol, thymol, and cinnamaldehyde.2
Topical applications and human studies
constituent, eugenol.5 It also has been used as a penetration enhancer in various forms of topical products, including creams, ointments, gels, and patches.6
Palmar hyperhidrosis
In 2017, Ibrahim et al. treated 45 patients with palmar hyperhidrosis with clove oil 45% in liposome, with 20 patients in a control group treated with 0.9% saline solution. Subjects were assessed by gravimetry testing and hyperhidrosis disease severity scale to determine the impact of clove oil on decreasing the sweating rate in patients with idiopathic palmar hyperhidrosis. Gravimetry testing revealed that the sweating rate decreased significantly in the clove oil group but that there was no significant improvement in the placebo group. The investigators concluded that twice-daily topical application of 45% clove oil in liposome for 2 weeks showed promise in significantly reducing palmar sweating.5
Pruritus
That same year Ibrahim et al. evaluated the effects of topically applied clove oil in treating 50 patients with chronic pruritus due to hepatic, renal, or diabetic origin. The investigators divided the subjects into two groups of 25, with the first directed to hydrate their skin before applying topical clove oil twice daily for 2 weeks. The second group was instructed to apply topical petrolatum by hand on the same schedule. Using the 5-D itch scale, researchers noted a significant improvement in all parameters in the patients using clove oil and no such improvements in the petrolatum group. They concluded that particularly for patients whose topical or systemic treatments are not well tolerated or are contraindicated.7
Anal fissure
In 2007, Elwakeel et al. evaluated the use of a clove oil 1% cream for the treatment of chronic anal fissure as opposed to the traditional treatment of stool softeners and lignocaine cream 5% in a single-blind randomized comparative trial over 6 weeks. Healing was observed in 60% of the 30 patients in the clove oil group and in 12% of the 25 patients in the control group at the 3-month follow-up visit. The researchers concluded that topically applied clove oil cream yielded significant benefits in the treatment of chronic anal fissures.8
More recently, Nelson et al. conducted a literature survey to evaluate the efficacy and morbidity of nonsurgical treatments for anal fissures from 1966 to August 2010. Clove oil was among 17 agents used in the 77 cited studies. While no medical therapies were found to display the efficacy of surgical sphincterotomy (or, fortunately, linked to the risk of incontinence), clove oil was identified as one of the “newer” agents demonstrating promise.9
Musculoskeletal pain
Clove oil is included among several herbal ingredients (i.e., eucalyptus oil, gaultheria oil, turpentine oil, menthol, and camphor) associated with analgesic and anti-inflammatory properties that are used in the topical spray Eezpain. Nawaz et al. showed in a prospective pilot study with 20 male and female subjects that the polyherbal formulation was efficacious in relieving mild to moderate knee and wrist joint pain.10
Laboratory studies
Just over a decade ago, Chaieb et al. assessed the antioxidant characteristics of the essential oil of clove, finding that it displayed a robust radical scavenging capacity against 2,2-diphenyl-1-picrylhydrazyl in comparison to the synthetic antioxidant tert-butylated hydroxytoluene. It also showed potent antifungal activity against 53 test strains of human pathogenic yeasts. The authors noted that clove oil is a readily available source of natural antioxidants and is a worthy ingredient in pharmaceutical products.11
Anti-inflammatory activity
In 2017, Han and Parker studied the biological activity of four concentrations of a commercially available clove essential oil product on 17 protein biomarkers important in inflammation in a model of human skin disease. They found that the 0.011% concentration of the oil enacted strong antiproliferative effects on human dermal skin fibroblasts, and significantly suppressed multiple proinflammatory biomarkers as well as tissue remodeling protein molecules. The investigators also observed that essential clove oil significantly influenced global gene expression and signaling pathways involved in inflammation, tissue remodeling, and cancer processes. They concluded that their results indicate anti-inflammatory, anticancer, and tissue-remodeling properties of clove essential oil, and its main active ingredient eugenol, in human dermal fibroblasts.1
UVB protection
Recently, Patwardhan and Bhatt assessed the capacity of flavonoids from clove buds to protect human dermal fibroblasts from UVB exposure. They found that the flavonoid-enriched fraction of clove demonstrated significant potential, as it mitigated the effects of UVB radiation, and delivered protection via the nuclear factor E2-related factor 2-antioxidant response pathway. The flavonoid-enriched clove fraction, they concluded, warrants consideration as a topically applied cutaneous protectant against the effects of UVB exposure.4
Antiviral and immunomodulatory activity
Based on their earlier work showing the antiviral activity of clove bud oil against Pseudomonas aeruginosa PAO1, Haripriyan et al. reported this year that clove bud oil affects pseudomonal proteases (elastase A, elastase B, protease IV, and alkaline protease), attenuating significant viral mechanisms of this noted human disease agent while bolstering host immunomodulatory functions. They concluded that their results suggest the viability of clove bud oil as a topical treatment for infections resistant to antibiotics.12
Acne
In 2017, Owen et al. developed a topical preparation incorporating clove bud, rosewood, and litsea essential oils that compared favorably with the topical antibiotics Dalacin T and Stiemycin in controlling acne vulgaris-linked bacteria. Specifically, the herbal formulation exhibited synergistic activity against Propionibacterium acnes, although not to Staphylococcus epidermidis, and its antimicrobial activity exceeded or equated to that of the tested antibiotics. The investigators suggested that the polyherbal preparation may serve as an option for treating acne-linked bacteria.13
Scabies
In a study 2 years ago to ascertain the efficacy of 10 essential oils against Sarcoptes scabiei, Fang et al. conducted contact bioassays and fumigation bioassays using clove, palmarosa, geranium, tea tree, lavender, Manuka, bitter orange, eucalyptus, Japanese cedar, and cade oil. In the contact bioassays, clove oil 1%, the most effective of the oils, eliminated the mites within 20 minutes. In the fumigation bioassay, clove was second to tea tree oil in efficacy. The investigators concluded that clove, tea tree, palmarosa, and eucalyptus oils demonstrate potential in pest control and for treating scabies infections in humans or animals.14
Conclusion
Clove oil is an active ingredient in various topical treatments. While not typically a first-line therapy, it shows promise for a wider range of applications. Research continues to determine the extent to which this botanical agent can reach into the dermatologic armamentarium and, more importantly, how effective it can be in treating cutaneous disorders.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
References
1. Pharm Biol. 2017 Dec;55(1):1619-22.
2. Phytother Res. 2007 Jun;21(6):501-6.
3. Cell Prolif. 2006 Aug;39(4):241-8.
4. Pharmacogn Mag. 2015 Oct;11(Suppl 3):S397-406.
5. J Cosmet Dermatol. 2017 Dec 28. doi: 10.1111/jocd.12471.
6. Curr Drug Deliv. 2012 Mar;9(2):219-30.
7. J Cosmet Dermatol. 2017 Dec;16(4):508-11.
8. Colorectal Dis. 2007 Jul;9(6):549-52
9. Cochrane Database Syst Rev. 2012 Feb 15;(2):CD003431.
10. Pak J Pharm Sci. 2015 Jan;28(1):43-7.
11. Mycoses. 2007 Sep;50(5):403-6.
12. Sci Rep. 2018 Feb 21;8(1):3437.
13. Phytother Res. 2017 Mar;31(3):410-7.
14. Parasit Vectors. 2016 Nov 22;9(1):594.
Laser tattoo removal plume ‘probably safer’ than laser hair removal plume
SAN DIEGO – results from a novel study demonstrated.
“The laser plume is known to contain possible hazards,” Yakir Levin, MD, PhD, said at the annual Masters of Aesthetics Symposium. “Intact human papillomavirus DNA has been demonstrated in the CO2 laser plume of common wart treatments,” he noted, and transmission of bovine papillomavirus has been shown in a bovine model of CO2 laser treatment (Arch Dermatol 2002;138[10]:1303-7). “In addition, aerosolized human cells have been demonstrated in laser tattoo removal.”
In a more recent study, Gary S. Chuang, MD, and his colleagues demonstrated hazards in the laser hair removal plume (JAMA Dermatol 2016;152[12]:1320-6). “These include ultrafine particles that become lodged in pulmonary alveoli and cause long-term respiratory problems, as well as volatile organic compounds, which can be carcinogens and environmental toxins,” said Dr. Levin of the Massachusetts General Hospital department of dermatology and the Wellman Center for Photomedicine, both in Boston. “They showed that this can be improved but not cured by proper use of a smoke evacuator; they also emphasized the importance of wearing a mask.”
Dr. Levin and his colleagues chose to study laser tattoo removal plume because more than 40 million Americans have tattoos, especially younger adults. In addition, 17% regret having their tattoo and 11% are undergoing or have undergone tattoo removal procedures. In what is believed to be the first study of its kind, the researchers performed a study in ex vivo pig skin and in humans undergoing routine laser tattoo removal. They measured the concentration of nanoparticles as well as the presence of heavy metals, volatile organic compounds, and airborne bacteria.
For the swine study, the excised pig skin was tattooed with several differently colored inks. Dr. Levin and his colleagues found that the concentration of airborne nanoparticles measured during laser tattoo removal was elevated and varied with different inks and different lasers used. Fine metals were measured in mcg/m3 air and were below safe occupational exposure limits. The same effect was seen for volatile organic compounds.
Next, the researchers analyzed the laser plume in humans undergoing removal of blue, black, and multicolored tattoos. “Here, the results were a little bit different,” Dr. Levin said. “Airborne particle concentrations were higher in the dermatologist’s breathing zone and near the tattoo removal site than in the remainder of the room or outside of the room. However, concentrations were 30 times lower for human skin than for pig skin. That’s because the pig study was somewhat artificial in that the tattoos were done when the pig was dead.”
Metals were detected in the plume in the human study, but they were all below occupational exposure limits. The same effect was seen for volatile organic compounds.
Dr. Levin said that airborne nanoparticle concentrations for laser tattoo removal of ex vivo tattooed swine skin were comparable to those reported for hair removal, while airborne nanoparticle concentrations for laser removal of in vivo human skin were much lower than those reported for laser hair removal. “So it’s probably true that the potential health hazards from laser tattoo removal are lower than for laser hair removal, but we did not study viral particles or the presence of viable human cells in the plume,” he said.
Current methods to limit laser plume exposure include suction of the plume with a smoke evacuator, use of a barrier device placed over the skin, and wearing a face mask constructed to filter nanoparticles, such as an N95 mask.
Other safety issues to consider
Dr. Levin discussed additional safety considerations in performing laser treatments.
“We want to protect the epidermis from injury during the laser exposure, which is currently done with spray cooling, air cooling, and/or contact cooling,” he said. “We want to limit the pain experienced by patients throughout the laser treatment before and after the brief laser exposure. This is often accomplished with the use of ice packs or air cooling. We also want to avoid double pulsing and skip areas. This can sometimes but not always be achieved by paying close attention to clinical endpoints.”
He and his associates are currently developing a device to accomplish all of those safety goals with a multilayer approach. “One of the layers would be a hydrogel, which serves to protect the epidermis and to provide pain relief throughout the laser treatment,” he said. “Above that layer is an indicator layer that is not aqueous, and on top of that is a fine layer of particles. The idea is, if you’re looking at this from above, when you fire the laser, you would see a change of color or some other indicator to show you exactly where you fired the laser. Finally, the multilayer patch also serves to obstruct the laser plume.”
Dr. Levin acknowledged research support from the American Society for Dermatologic Surgery’s Fredric S. Brandt, MD, Innovations in Aesthetics Fellowship Fund and assistance from the Health Hazard Evaluation Program of National Institute for Occupational Safety and Health.
SAN DIEGO – results from a novel study demonstrated.
“The laser plume is known to contain possible hazards,” Yakir Levin, MD, PhD, said at the annual Masters of Aesthetics Symposium. “Intact human papillomavirus DNA has been demonstrated in the CO2 laser plume of common wart treatments,” he noted, and transmission of bovine papillomavirus has been shown in a bovine model of CO2 laser treatment (Arch Dermatol 2002;138[10]:1303-7). “In addition, aerosolized human cells have been demonstrated in laser tattoo removal.”
In a more recent study, Gary S. Chuang, MD, and his colleagues demonstrated hazards in the laser hair removal plume (JAMA Dermatol 2016;152[12]:1320-6). “These include ultrafine particles that become lodged in pulmonary alveoli and cause long-term respiratory problems, as well as volatile organic compounds, which can be carcinogens and environmental toxins,” said Dr. Levin of the Massachusetts General Hospital department of dermatology and the Wellman Center for Photomedicine, both in Boston. “They showed that this can be improved but not cured by proper use of a smoke evacuator; they also emphasized the importance of wearing a mask.”
Dr. Levin and his colleagues chose to study laser tattoo removal plume because more than 40 million Americans have tattoos, especially younger adults. In addition, 17% regret having their tattoo and 11% are undergoing or have undergone tattoo removal procedures. In what is believed to be the first study of its kind, the researchers performed a study in ex vivo pig skin and in humans undergoing routine laser tattoo removal. They measured the concentration of nanoparticles as well as the presence of heavy metals, volatile organic compounds, and airborne bacteria.
For the swine study, the excised pig skin was tattooed with several differently colored inks. Dr. Levin and his colleagues found that the concentration of airborne nanoparticles measured during laser tattoo removal was elevated and varied with different inks and different lasers used. Fine metals were measured in mcg/m3 air and were below safe occupational exposure limits. The same effect was seen for volatile organic compounds.
Next, the researchers analyzed the laser plume in humans undergoing removal of blue, black, and multicolored tattoos. “Here, the results were a little bit different,” Dr. Levin said. “Airborne particle concentrations were higher in the dermatologist’s breathing zone and near the tattoo removal site than in the remainder of the room or outside of the room. However, concentrations were 30 times lower for human skin than for pig skin. That’s because the pig study was somewhat artificial in that the tattoos were done when the pig was dead.”
Metals were detected in the plume in the human study, but they were all below occupational exposure limits. The same effect was seen for volatile organic compounds.
Dr. Levin said that airborne nanoparticle concentrations for laser tattoo removal of ex vivo tattooed swine skin were comparable to those reported for hair removal, while airborne nanoparticle concentrations for laser removal of in vivo human skin were much lower than those reported for laser hair removal. “So it’s probably true that the potential health hazards from laser tattoo removal are lower than for laser hair removal, but we did not study viral particles or the presence of viable human cells in the plume,” he said.
Current methods to limit laser plume exposure include suction of the plume with a smoke evacuator, use of a barrier device placed over the skin, and wearing a face mask constructed to filter nanoparticles, such as an N95 mask.
Other safety issues to consider
Dr. Levin discussed additional safety considerations in performing laser treatments.
“We want to protect the epidermis from injury during the laser exposure, which is currently done with spray cooling, air cooling, and/or contact cooling,” he said. “We want to limit the pain experienced by patients throughout the laser treatment before and after the brief laser exposure. This is often accomplished with the use of ice packs or air cooling. We also want to avoid double pulsing and skip areas. This can sometimes but not always be achieved by paying close attention to clinical endpoints.”
He and his associates are currently developing a device to accomplish all of those safety goals with a multilayer approach. “One of the layers would be a hydrogel, which serves to protect the epidermis and to provide pain relief throughout the laser treatment,” he said. “Above that layer is an indicator layer that is not aqueous, and on top of that is a fine layer of particles. The idea is, if you’re looking at this from above, when you fire the laser, you would see a change of color or some other indicator to show you exactly where you fired the laser. Finally, the multilayer patch also serves to obstruct the laser plume.”
Dr. Levin acknowledged research support from the American Society for Dermatologic Surgery’s Fredric S. Brandt, MD, Innovations in Aesthetics Fellowship Fund and assistance from the Health Hazard Evaluation Program of National Institute for Occupational Safety and Health.
SAN DIEGO – results from a novel study demonstrated.
“The laser plume is known to contain possible hazards,” Yakir Levin, MD, PhD, said at the annual Masters of Aesthetics Symposium. “Intact human papillomavirus DNA has been demonstrated in the CO2 laser plume of common wart treatments,” he noted, and transmission of bovine papillomavirus has been shown in a bovine model of CO2 laser treatment (Arch Dermatol 2002;138[10]:1303-7). “In addition, aerosolized human cells have been demonstrated in laser tattoo removal.”
In a more recent study, Gary S. Chuang, MD, and his colleagues demonstrated hazards in the laser hair removal plume (JAMA Dermatol 2016;152[12]:1320-6). “These include ultrafine particles that become lodged in pulmonary alveoli and cause long-term respiratory problems, as well as volatile organic compounds, which can be carcinogens and environmental toxins,” said Dr. Levin of the Massachusetts General Hospital department of dermatology and the Wellman Center for Photomedicine, both in Boston. “They showed that this can be improved but not cured by proper use of a smoke evacuator; they also emphasized the importance of wearing a mask.”
Dr. Levin and his colleagues chose to study laser tattoo removal plume because more than 40 million Americans have tattoos, especially younger adults. In addition, 17% regret having their tattoo and 11% are undergoing or have undergone tattoo removal procedures. In what is believed to be the first study of its kind, the researchers performed a study in ex vivo pig skin and in humans undergoing routine laser tattoo removal. They measured the concentration of nanoparticles as well as the presence of heavy metals, volatile organic compounds, and airborne bacteria.
For the swine study, the excised pig skin was tattooed with several differently colored inks. Dr. Levin and his colleagues found that the concentration of airborne nanoparticles measured during laser tattoo removal was elevated and varied with different inks and different lasers used. Fine metals were measured in mcg/m3 air and were below safe occupational exposure limits. The same effect was seen for volatile organic compounds.
Next, the researchers analyzed the laser plume in humans undergoing removal of blue, black, and multicolored tattoos. “Here, the results were a little bit different,” Dr. Levin said. “Airborne particle concentrations were higher in the dermatologist’s breathing zone and near the tattoo removal site than in the remainder of the room or outside of the room. However, concentrations were 30 times lower for human skin than for pig skin. That’s because the pig study was somewhat artificial in that the tattoos were done when the pig was dead.”
Metals were detected in the plume in the human study, but they were all below occupational exposure limits. The same effect was seen for volatile organic compounds.
Dr. Levin said that airborne nanoparticle concentrations for laser tattoo removal of ex vivo tattooed swine skin were comparable to those reported for hair removal, while airborne nanoparticle concentrations for laser removal of in vivo human skin were much lower than those reported for laser hair removal. “So it’s probably true that the potential health hazards from laser tattoo removal are lower than for laser hair removal, but we did not study viral particles or the presence of viable human cells in the plume,” he said.
Current methods to limit laser plume exposure include suction of the plume with a smoke evacuator, use of a barrier device placed over the skin, and wearing a face mask constructed to filter nanoparticles, such as an N95 mask.
Other safety issues to consider
Dr. Levin discussed additional safety considerations in performing laser treatments.
“We want to protect the epidermis from injury during the laser exposure, which is currently done with spray cooling, air cooling, and/or contact cooling,” he said. “We want to limit the pain experienced by patients throughout the laser treatment before and after the brief laser exposure. This is often accomplished with the use of ice packs or air cooling. We also want to avoid double pulsing and skip areas. This can sometimes but not always be achieved by paying close attention to clinical endpoints.”
He and his associates are currently developing a device to accomplish all of those safety goals with a multilayer approach. “One of the layers would be a hydrogel, which serves to protect the epidermis and to provide pain relief throughout the laser treatment,” he said. “Above that layer is an indicator layer that is not aqueous, and on top of that is a fine layer of particles. The idea is, if you’re looking at this from above, when you fire the laser, you would see a change of color or some other indicator to show you exactly where you fired the laser. Finally, the multilayer patch also serves to obstruct the laser plume.”
Dr. Levin acknowledged research support from the American Society for Dermatologic Surgery’s Fredric S. Brandt, MD, Innovations in Aesthetics Fellowship Fund and assistance from the Health Hazard Evaluation Program of National Institute for Occupational Safety and Health.
REPORTING FROM MOAS 2018
Expert shares laser hair removal clinical pearls
SAN DIEGO – annually than for other applications, Kristen M. Kelly, MD, said at the annual Masters of Aesthetics Symposium.
Clinicians perform an estimated 445,000 laser hair removal procedures each year, trailing those performed for wrinkles (561,000), facial redness (598,000), and sun damage (610,000), according to the 2017 American Society for Dermatologic Surgery Procedures Survey. However, hair removal has been found to be the most common procedure resulting in litigation (JAMA Dermatol. 2013;149[2]:188-93), “which is what we want to avoid,” Dr. Kelly said. “We want to learn how to do it in the best way possible.”
Dr. Kelly, professor of dermatology and surgery at the University of California, Irvine, pointed out that clinicians are targeting melanin during laser hair removal. “This is important because it means that gray, white, blonde, and in some cases, red hair are not going to respond very well. In order to reach stem cells in the hair follicle, you can’t use superficial wavelengths, so we end up using the 755-nm alexandrite laser, 810-nm diode laser, or the 1064-nm long-pulsed Nd:YAG laser for the most part in order to get our result. You can also use intense pulsed light at 590-1200 nm.”
The pulse duration should be on the order of the thermal relaxation time (TRT) of the target. She defined thermal relaxation time as the duration required for the heat generated by absorbed light energy within the chromophore to dissipate to 50% of its value immediately after laser exposure.
“We want that heat to be absorbed by the melanin in the hair,” Dr. Kelly said. “Then we want that heat to radiate out to the hair follicle and the stem cells, which is going to prevent the hair from coming back in the future.” Epidermal cooling via cryogen spray cooling, contact cooling, or air-cooling allows clinicians to use higher fluences, allows for the use of safe treatment of darker skin types, and decreases treatment discomfort.
Prior to performing laser hair removal on the perioral area, Dr. Kelly provides a prophylactic antiviral medication for patients with a history of herpetic infection to suppress recurrence. She also advises patients to remove sunless tanner or other products from the skin surface, and she shaves or clips hair close to the surface gently, avoiding abrasion or surface damage. Most of the time she does not use a topical anesthetic. “You always want to make sure the laser is functioning properly by checking the laser’s cooling components, etc.,” she said. “I always tell patients, ‘8-10 treatments is not uncommon. You’re going to have fewer hairs and thinner hairs, but it doesn’t mean that you’re going to have zero hairs in this area for the rest of your life.’ Set the expectation correctly.”
Patients should wear protective goggles if being treated in areas other than the face. “If being treated on the face, make sure they’re wearing appropriate protective eyewear such as laser safe eye shields. Those performing the treatment should wear surgical masks and use a smoke evacuator, as there are multiple known carcinogens and environmental toxins in the plume generated by laser hair removal,” she said (JAMA Dermatol. 2016;152[12]:1320-6).
Factors to consider in choosing treatment settings include hair color, the patient’s skin color, hair thickness, hair density, and body location. For example, the genital area may be more pigmented in some patients, and also may be more sensitive. “If you have thicker and darker hair it’s going to absorb more energy, so you have to adjust your settings,” Dr. Kelly said. She also avoids treating acutely tanned patients. “What you don’t want is for a tanned patient have a complication. I’d rather have them wait 2 or 3 weeks and come back. Sometimes it frustrates them a little, but it is safer.”
At the start of each procedure, Dr. Kelly delivers a couple of pulses then asks patients how uncomfortable they are on a scale from 1 to 10. “It often will vary,” she said. “Even if I’ve seen a patient for 10 treatments, some days it will hurt a little more than others. If the patient says it hurts a lot more than it normally does, you need to stop and think. That tells you that something is not right. They might be too tanned, or perhaps the [device] settings are wrong or the laser’s not functioning properly. Monitor the skin response. It takes time to see the final skin response, so wait before adjusting the energy. You want to see mild redness or mild follicular response. People report a little bit of a burning sensation.”
Postprocedure, she routinely applies a topical steroid such as betamethasone or clobetasol immediately after treatment. “That calms down the inflammatory response. If I see an area that I think is going to blister, I will apply the steroid multiple times until I see that response go away.” She asks patients about their pain level and typically applies ice to the treated area for 10 minutes before they leave the office, and they head home with after-care instructions, including a phone number where Dr. Kelly can be reached if patients have concerns. “I call my patients in the evening to ask how they’re doing, and if they have any questions.”
Dr. Kelly does not do laser hair removal inside the eye orbit or between the eyebrows, “because even with eye shields, you can have a problem,” she said. A potential outcome to be aware of is paradoxical hypertrichosis, or increased hair growth after laser hair removal estimated to occur in 0.6%-10% of patients. She discusses this with patients when consenting them. “Some people say this occurs in darker skin types, while others say it happens in people with thicker hair or thinner hair. Some of it occurs on the edges of the treatment area.” The treatment for paradoxical hypertrichosis is to continue laser hair removal and “try to push the energy just a little bit, but be cautious. It can be a difficult problem to resolve.”
Dr. Kelly has noticed a recent uptick at her practice in gender transition patients seeking hair removal procedures. “Removing facial and chest hair may be desirable, and may be covered by the patient’s health insurance,” she said. “Multiple treatments are required. A thick beard area needs to be approached cautiously.”
She referred to future advances in laser hair removal that may involve the use of topical agents to improve outcomes. For example, Sienna Biopharmaceuticals has developed Topical Photoparticle Therapy which, according to its website, uses “silver particles to absorb laser light and convert the light energy into heat to facilitate local tissue injury ... in the case of unwanted light or mixed pigment hair, which can’t be removed with lasers alone, [this process] targets the hair follicle.”
Dr. Kelly disclosed that she is an advisory consultant to Syneron Candela and Allergan. In addition, Allergan has provided drug products to Dr. Kelly for research purposes, while Solta Medical and ThermiRF have provided her with donated light sources for research or clinical use. Dr. Kelly has also received funding from the Sturge-Weber Foundation, the American Society for Laser Medicine and Surgery, and the National Institutes of Health.
[email protected]
SAN DIEGO – annually than for other applications, Kristen M. Kelly, MD, said at the annual Masters of Aesthetics Symposium.
Clinicians perform an estimated 445,000 laser hair removal procedures each year, trailing those performed for wrinkles (561,000), facial redness (598,000), and sun damage (610,000), according to the 2017 American Society for Dermatologic Surgery Procedures Survey. However, hair removal has been found to be the most common procedure resulting in litigation (JAMA Dermatol. 2013;149[2]:188-93), “which is what we want to avoid,” Dr. Kelly said. “We want to learn how to do it in the best way possible.”
Dr. Kelly, professor of dermatology and surgery at the University of California, Irvine, pointed out that clinicians are targeting melanin during laser hair removal. “This is important because it means that gray, white, blonde, and in some cases, red hair are not going to respond very well. In order to reach stem cells in the hair follicle, you can’t use superficial wavelengths, so we end up using the 755-nm alexandrite laser, 810-nm diode laser, or the 1064-nm long-pulsed Nd:YAG laser for the most part in order to get our result. You can also use intense pulsed light at 590-1200 nm.”
The pulse duration should be on the order of the thermal relaxation time (TRT) of the target. She defined thermal relaxation time as the duration required for the heat generated by absorbed light energy within the chromophore to dissipate to 50% of its value immediately after laser exposure.
“We want that heat to be absorbed by the melanin in the hair,” Dr. Kelly said. “Then we want that heat to radiate out to the hair follicle and the stem cells, which is going to prevent the hair from coming back in the future.” Epidermal cooling via cryogen spray cooling, contact cooling, or air-cooling allows clinicians to use higher fluences, allows for the use of safe treatment of darker skin types, and decreases treatment discomfort.
Prior to performing laser hair removal on the perioral area, Dr. Kelly provides a prophylactic antiviral medication for patients with a history of herpetic infection to suppress recurrence. She also advises patients to remove sunless tanner or other products from the skin surface, and she shaves or clips hair close to the surface gently, avoiding abrasion or surface damage. Most of the time she does not use a topical anesthetic. “You always want to make sure the laser is functioning properly by checking the laser’s cooling components, etc.,” she said. “I always tell patients, ‘8-10 treatments is not uncommon. You’re going to have fewer hairs and thinner hairs, but it doesn’t mean that you’re going to have zero hairs in this area for the rest of your life.’ Set the expectation correctly.”
Patients should wear protective goggles if being treated in areas other than the face. “If being treated on the face, make sure they’re wearing appropriate protective eyewear such as laser safe eye shields. Those performing the treatment should wear surgical masks and use a smoke evacuator, as there are multiple known carcinogens and environmental toxins in the plume generated by laser hair removal,” she said (JAMA Dermatol. 2016;152[12]:1320-6).
Factors to consider in choosing treatment settings include hair color, the patient’s skin color, hair thickness, hair density, and body location. For example, the genital area may be more pigmented in some patients, and also may be more sensitive. “If you have thicker and darker hair it’s going to absorb more energy, so you have to adjust your settings,” Dr. Kelly said. She also avoids treating acutely tanned patients. “What you don’t want is for a tanned patient have a complication. I’d rather have them wait 2 or 3 weeks and come back. Sometimes it frustrates them a little, but it is safer.”
At the start of each procedure, Dr. Kelly delivers a couple of pulses then asks patients how uncomfortable they are on a scale from 1 to 10. “It often will vary,” she said. “Even if I’ve seen a patient for 10 treatments, some days it will hurt a little more than others. If the patient says it hurts a lot more than it normally does, you need to stop and think. That tells you that something is not right. They might be too tanned, or perhaps the [device] settings are wrong or the laser’s not functioning properly. Monitor the skin response. It takes time to see the final skin response, so wait before adjusting the energy. You want to see mild redness or mild follicular response. People report a little bit of a burning sensation.”
Postprocedure, she routinely applies a topical steroid such as betamethasone or clobetasol immediately after treatment. “That calms down the inflammatory response. If I see an area that I think is going to blister, I will apply the steroid multiple times until I see that response go away.” She asks patients about their pain level and typically applies ice to the treated area for 10 minutes before they leave the office, and they head home with after-care instructions, including a phone number where Dr. Kelly can be reached if patients have concerns. “I call my patients in the evening to ask how they’re doing, and if they have any questions.”
Dr. Kelly does not do laser hair removal inside the eye orbit or between the eyebrows, “because even with eye shields, you can have a problem,” she said. A potential outcome to be aware of is paradoxical hypertrichosis, or increased hair growth after laser hair removal estimated to occur in 0.6%-10% of patients. She discusses this with patients when consenting them. “Some people say this occurs in darker skin types, while others say it happens in people with thicker hair or thinner hair. Some of it occurs on the edges of the treatment area.” The treatment for paradoxical hypertrichosis is to continue laser hair removal and “try to push the energy just a little bit, but be cautious. It can be a difficult problem to resolve.”
Dr. Kelly has noticed a recent uptick at her practice in gender transition patients seeking hair removal procedures. “Removing facial and chest hair may be desirable, and may be covered by the patient’s health insurance,” she said. “Multiple treatments are required. A thick beard area needs to be approached cautiously.”
She referred to future advances in laser hair removal that may involve the use of topical agents to improve outcomes. For example, Sienna Biopharmaceuticals has developed Topical Photoparticle Therapy which, according to its website, uses “silver particles to absorb laser light and convert the light energy into heat to facilitate local tissue injury ... in the case of unwanted light or mixed pigment hair, which can’t be removed with lasers alone, [this process] targets the hair follicle.”
Dr. Kelly disclosed that she is an advisory consultant to Syneron Candela and Allergan. In addition, Allergan has provided drug products to Dr. Kelly for research purposes, while Solta Medical and ThermiRF have provided her with donated light sources for research or clinical use. Dr. Kelly has also received funding from the Sturge-Weber Foundation, the American Society for Laser Medicine and Surgery, and the National Institutes of Health.
[email protected]
SAN DIEGO – annually than for other applications, Kristen M. Kelly, MD, said at the annual Masters of Aesthetics Symposium.
Clinicians perform an estimated 445,000 laser hair removal procedures each year, trailing those performed for wrinkles (561,000), facial redness (598,000), and sun damage (610,000), according to the 2017 American Society for Dermatologic Surgery Procedures Survey. However, hair removal has been found to be the most common procedure resulting in litigation (JAMA Dermatol. 2013;149[2]:188-93), “which is what we want to avoid,” Dr. Kelly said. “We want to learn how to do it in the best way possible.”
Dr. Kelly, professor of dermatology and surgery at the University of California, Irvine, pointed out that clinicians are targeting melanin during laser hair removal. “This is important because it means that gray, white, blonde, and in some cases, red hair are not going to respond very well. In order to reach stem cells in the hair follicle, you can’t use superficial wavelengths, so we end up using the 755-nm alexandrite laser, 810-nm diode laser, or the 1064-nm long-pulsed Nd:YAG laser for the most part in order to get our result. You can also use intense pulsed light at 590-1200 nm.”
The pulse duration should be on the order of the thermal relaxation time (TRT) of the target. She defined thermal relaxation time as the duration required for the heat generated by absorbed light energy within the chromophore to dissipate to 50% of its value immediately after laser exposure.
“We want that heat to be absorbed by the melanin in the hair,” Dr. Kelly said. “Then we want that heat to radiate out to the hair follicle and the stem cells, which is going to prevent the hair from coming back in the future.” Epidermal cooling via cryogen spray cooling, contact cooling, or air-cooling allows clinicians to use higher fluences, allows for the use of safe treatment of darker skin types, and decreases treatment discomfort.
Prior to performing laser hair removal on the perioral area, Dr. Kelly provides a prophylactic antiviral medication for patients with a history of herpetic infection to suppress recurrence. She also advises patients to remove sunless tanner or other products from the skin surface, and she shaves or clips hair close to the surface gently, avoiding abrasion or surface damage. Most of the time she does not use a topical anesthetic. “You always want to make sure the laser is functioning properly by checking the laser’s cooling components, etc.,” she said. “I always tell patients, ‘8-10 treatments is not uncommon. You’re going to have fewer hairs and thinner hairs, but it doesn’t mean that you’re going to have zero hairs in this area for the rest of your life.’ Set the expectation correctly.”
Patients should wear protective goggles if being treated in areas other than the face. “If being treated on the face, make sure they’re wearing appropriate protective eyewear such as laser safe eye shields. Those performing the treatment should wear surgical masks and use a smoke evacuator, as there are multiple known carcinogens and environmental toxins in the plume generated by laser hair removal,” she said (JAMA Dermatol. 2016;152[12]:1320-6).
Factors to consider in choosing treatment settings include hair color, the patient’s skin color, hair thickness, hair density, and body location. For example, the genital area may be more pigmented in some patients, and also may be more sensitive. “If you have thicker and darker hair it’s going to absorb more energy, so you have to adjust your settings,” Dr. Kelly said. She also avoids treating acutely tanned patients. “What you don’t want is for a tanned patient have a complication. I’d rather have them wait 2 or 3 weeks and come back. Sometimes it frustrates them a little, but it is safer.”
At the start of each procedure, Dr. Kelly delivers a couple of pulses then asks patients how uncomfortable they are on a scale from 1 to 10. “It often will vary,” she said. “Even if I’ve seen a patient for 10 treatments, some days it will hurt a little more than others. If the patient says it hurts a lot more than it normally does, you need to stop and think. That tells you that something is not right. They might be too tanned, or perhaps the [device] settings are wrong or the laser’s not functioning properly. Monitor the skin response. It takes time to see the final skin response, so wait before adjusting the energy. You want to see mild redness or mild follicular response. People report a little bit of a burning sensation.”
Postprocedure, she routinely applies a topical steroid such as betamethasone or clobetasol immediately after treatment. “That calms down the inflammatory response. If I see an area that I think is going to blister, I will apply the steroid multiple times until I see that response go away.” She asks patients about their pain level and typically applies ice to the treated area for 10 minutes before they leave the office, and they head home with after-care instructions, including a phone number where Dr. Kelly can be reached if patients have concerns. “I call my patients in the evening to ask how they’re doing, and if they have any questions.”
Dr. Kelly does not do laser hair removal inside the eye orbit or between the eyebrows, “because even with eye shields, you can have a problem,” she said. A potential outcome to be aware of is paradoxical hypertrichosis, or increased hair growth after laser hair removal estimated to occur in 0.6%-10% of patients. She discusses this with patients when consenting them. “Some people say this occurs in darker skin types, while others say it happens in people with thicker hair or thinner hair. Some of it occurs on the edges of the treatment area.” The treatment for paradoxical hypertrichosis is to continue laser hair removal and “try to push the energy just a little bit, but be cautious. It can be a difficult problem to resolve.”
Dr. Kelly has noticed a recent uptick at her practice in gender transition patients seeking hair removal procedures. “Removing facial and chest hair may be desirable, and may be covered by the patient’s health insurance,” she said. “Multiple treatments are required. A thick beard area needs to be approached cautiously.”
She referred to future advances in laser hair removal that may involve the use of topical agents to improve outcomes. For example, Sienna Biopharmaceuticals has developed Topical Photoparticle Therapy which, according to its website, uses “silver particles to absorb laser light and convert the light energy into heat to facilitate local tissue injury ... in the case of unwanted light or mixed pigment hair, which can’t be removed with lasers alone, [this process] targets the hair follicle.”
Dr. Kelly disclosed that she is an advisory consultant to Syneron Candela and Allergan. In addition, Allergan has provided drug products to Dr. Kelly for research purposes, while Solta Medical and ThermiRF have provided her with donated light sources for research or clinical use. Dr. Kelly has also received funding from the Sturge-Weber Foundation, the American Society for Laser Medicine and Surgery, and the National Institutes of Health.
[email protected]
EXPERT ANALYSIS FROM MOAS 2018
Expert shares his approach for aesthetic treatments of ethnic skin
SAN DIEGO – The United States is more .
“Unfortunately, one’s ethnic background is not always clear,” Ashish C. Bhatia, MD, said at the annual Masters of Aesthetics Symposium. “There’s a lot more blending, a lot more difficulty figuring out what people’s ethnicity is and how their skin is going to respond to different treatments.”
When consulting with patients, Dr. Bhatia, director of dermatologic and cosmetic surgery at Oak Dermatology, outside of Chicago, determines their Fitzpatrick skin type and asks about their heritage. “Some people who are adopted don’t know their heritage,” he said. “We also ask about their history of keloids, hypertrophic scars, postinflammatory hyperpigmentation or postinflammatory erythema.”
He also makes it a point to ask patients about blemishes. “If they get pimples, how long do the marks last, and what do they look like?” is one question he asks patients. “That dialogue gives you a lot of useful information. If they get hyperpigmentation, that’s one thing. But many times, people just get postinflammatory erythema, which is often a lot easier to treat.”
In his clinical experience, challenges and risks of performing aesthetic procedures on ethnic skin include postinflammatory hyperpigmentation and hypopigmentation, depigmentation, keloids, and hypertrophic scars. He explained that patients with darker skin types have bigger melanin granules and melanosomes, and more of them are deposited into keratinocytes.
“There’s a complex interaction that occurs between the melanocytes and the keratinocytes, where they phagocytize the ends and take up the melanosomes,” said Dr. Bhatia, also of the department of dermatology at Northwestern University, Chicago. “There’s an opportunity to block tyrosinase to prevent the production of melanin, but once the melanin is produced, what we really worry about is where that melanin ends up. It may end up in the epidermis in the form of light brown pigment. You can see this enhanced if you look at it with a Wood’s light. But if it ends up in the dermis, you don’t get enhancement and clinically it’s more of a bluish-gray pigment. The deeper melanin is much more difficult to treat. Often the postinflammatory hyperpigmentation people get is a combination of these two.”
To reduce the risks of hyperpigmentation and hypopigmentation from procedures, he generally advises against the use of intense pulsed light (IPL), fractional ablative lasers, shorter-wavelength lasers, and cryotherapy. “It’s not to say you can’t use them, but you have to be very careful,” he said. For clinicians with less experience treating skin of color, he recommends using procedures that spare the epidermis and the dermal/epidermal junction altogether. “This includes lasers with longer wavelengths such as the 1,064-nm Nd:YAG, always using generous cooling to avoid injury or trauma to the dermal/epidermal junction and using longer pulse durations.”
Nonlaser procedures to consider using for darker-skinned patients include superficial chemical peels, radiofrequency (RF) microneedling with semi-insulated needles, microfocused ultrasound, and noninvasive RF procedures that spare or cool the epidermis. “You should never be afraid to do a test spot,” he said. Adjunctive therapies to consider using include hydroquinone and other tyrosinase-receptor blockers, and preprocedural preparatory formulas. “Always advise sun protection and avoidance and administer HSV [herpes simplex virus] prophylaxis as indicated,” he added. “Ample evidence exists to show that currently available fillers and neuromodulators are safe to use in darker skin types. The issue here is more with postinflammatory hyperpigmentation, which can occur from needle punctures, so small-gauge needles and linear threading versus serial puncture is preferred.”
Adjunctive postprocedure preventative therapies include hydroquinone and other tyrosinase-receptor blockers; high-potency topical steroids such as clobetasol twice a day for 3 days and tapering to once a day for 3 days before halting; and postprocedural recovery/healing formulas.
Dr. Bhatia said that hypopigmentation can be treated with UV therapy, with bimatoprost topically combined with needling or low-density fractional lasers, as well as with epidermal microsuction grafting such as the CelluTome Epidermal Harvesting System. Hyperpigmentation can be treated with tyrosinase inhibitors, retinoids, chemical peels, and with microdermal/dermal infusion.
As for cosmeceuticals, sunscreens are necessary for keeping skin tone even. Retinoids are also helpful for maintenance, “but irritation can lead to postinflammatory hyperpigmentation, so go slow,” he said. Hyperpigmentation can be treated with hydroquinone, azelaic acid (Finacea), as well as many other preparations.
“Don’t be afraid to treat these patients,” Dr. Bhatia concluded. “As you get more used to performing procedures on people with darker skin types, you discover the limits of what you can and can’t do. But in general, we can do a lot to make these patients happy.”
Dr. Bhatia reported having research and financial ties to numerous pharmaceutical and device companies.
[email protected]
SAN DIEGO – The United States is more .
“Unfortunately, one’s ethnic background is not always clear,” Ashish C. Bhatia, MD, said at the annual Masters of Aesthetics Symposium. “There’s a lot more blending, a lot more difficulty figuring out what people’s ethnicity is and how their skin is going to respond to different treatments.”
When consulting with patients, Dr. Bhatia, director of dermatologic and cosmetic surgery at Oak Dermatology, outside of Chicago, determines their Fitzpatrick skin type and asks about their heritage. “Some people who are adopted don’t know their heritage,” he said. “We also ask about their history of keloids, hypertrophic scars, postinflammatory hyperpigmentation or postinflammatory erythema.”
He also makes it a point to ask patients about blemishes. “If they get pimples, how long do the marks last, and what do they look like?” is one question he asks patients. “That dialogue gives you a lot of useful information. If they get hyperpigmentation, that’s one thing. But many times, people just get postinflammatory erythema, which is often a lot easier to treat.”
In his clinical experience, challenges and risks of performing aesthetic procedures on ethnic skin include postinflammatory hyperpigmentation and hypopigmentation, depigmentation, keloids, and hypertrophic scars. He explained that patients with darker skin types have bigger melanin granules and melanosomes, and more of them are deposited into keratinocytes.
“There’s a complex interaction that occurs between the melanocytes and the keratinocytes, where they phagocytize the ends and take up the melanosomes,” said Dr. Bhatia, also of the department of dermatology at Northwestern University, Chicago. “There’s an opportunity to block tyrosinase to prevent the production of melanin, but once the melanin is produced, what we really worry about is where that melanin ends up. It may end up in the epidermis in the form of light brown pigment. You can see this enhanced if you look at it with a Wood’s light. But if it ends up in the dermis, you don’t get enhancement and clinically it’s more of a bluish-gray pigment. The deeper melanin is much more difficult to treat. Often the postinflammatory hyperpigmentation people get is a combination of these two.”
To reduce the risks of hyperpigmentation and hypopigmentation from procedures, he generally advises against the use of intense pulsed light (IPL), fractional ablative lasers, shorter-wavelength lasers, and cryotherapy. “It’s not to say you can’t use them, but you have to be very careful,” he said. For clinicians with less experience treating skin of color, he recommends using procedures that spare the epidermis and the dermal/epidermal junction altogether. “This includes lasers with longer wavelengths such as the 1,064-nm Nd:YAG, always using generous cooling to avoid injury or trauma to the dermal/epidermal junction and using longer pulse durations.”
Nonlaser procedures to consider using for darker-skinned patients include superficial chemical peels, radiofrequency (RF) microneedling with semi-insulated needles, microfocused ultrasound, and noninvasive RF procedures that spare or cool the epidermis. “You should never be afraid to do a test spot,” he said. Adjunctive therapies to consider using include hydroquinone and other tyrosinase-receptor blockers, and preprocedural preparatory formulas. “Always advise sun protection and avoidance and administer HSV [herpes simplex virus] prophylaxis as indicated,” he added. “Ample evidence exists to show that currently available fillers and neuromodulators are safe to use in darker skin types. The issue here is more with postinflammatory hyperpigmentation, which can occur from needle punctures, so small-gauge needles and linear threading versus serial puncture is preferred.”
Adjunctive postprocedure preventative therapies include hydroquinone and other tyrosinase-receptor blockers; high-potency topical steroids such as clobetasol twice a day for 3 days and tapering to once a day for 3 days before halting; and postprocedural recovery/healing formulas.
Dr. Bhatia said that hypopigmentation can be treated with UV therapy, with bimatoprost topically combined with needling or low-density fractional lasers, as well as with epidermal microsuction grafting such as the CelluTome Epidermal Harvesting System. Hyperpigmentation can be treated with tyrosinase inhibitors, retinoids, chemical peels, and with microdermal/dermal infusion.
As for cosmeceuticals, sunscreens are necessary for keeping skin tone even. Retinoids are also helpful for maintenance, “but irritation can lead to postinflammatory hyperpigmentation, so go slow,” he said. Hyperpigmentation can be treated with hydroquinone, azelaic acid (Finacea), as well as many other preparations.
“Don’t be afraid to treat these patients,” Dr. Bhatia concluded. “As you get more used to performing procedures on people with darker skin types, you discover the limits of what you can and can’t do. But in general, we can do a lot to make these patients happy.”
Dr. Bhatia reported having research and financial ties to numerous pharmaceutical and device companies.
[email protected]
SAN DIEGO – The United States is more .
“Unfortunately, one’s ethnic background is not always clear,” Ashish C. Bhatia, MD, said at the annual Masters of Aesthetics Symposium. “There’s a lot more blending, a lot more difficulty figuring out what people’s ethnicity is and how their skin is going to respond to different treatments.”
When consulting with patients, Dr. Bhatia, director of dermatologic and cosmetic surgery at Oak Dermatology, outside of Chicago, determines their Fitzpatrick skin type and asks about their heritage. “Some people who are adopted don’t know their heritage,” he said. “We also ask about their history of keloids, hypertrophic scars, postinflammatory hyperpigmentation or postinflammatory erythema.”
He also makes it a point to ask patients about blemishes. “If they get pimples, how long do the marks last, and what do they look like?” is one question he asks patients. “That dialogue gives you a lot of useful information. If they get hyperpigmentation, that’s one thing. But many times, people just get postinflammatory erythema, which is often a lot easier to treat.”
In his clinical experience, challenges and risks of performing aesthetic procedures on ethnic skin include postinflammatory hyperpigmentation and hypopigmentation, depigmentation, keloids, and hypertrophic scars. He explained that patients with darker skin types have bigger melanin granules and melanosomes, and more of them are deposited into keratinocytes.
“There’s a complex interaction that occurs between the melanocytes and the keratinocytes, where they phagocytize the ends and take up the melanosomes,” said Dr. Bhatia, also of the department of dermatology at Northwestern University, Chicago. “There’s an opportunity to block tyrosinase to prevent the production of melanin, but once the melanin is produced, what we really worry about is where that melanin ends up. It may end up in the epidermis in the form of light brown pigment. You can see this enhanced if you look at it with a Wood’s light. But if it ends up in the dermis, you don’t get enhancement and clinically it’s more of a bluish-gray pigment. The deeper melanin is much more difficult to treat. Often the postinflammatory hyperpigmentation people get is a combination of these two.”
To reduce the risks of hyperpigmentation and hypopigmentation from procedures, he generally advises against the use of intense pulsed light (IPL), fractional ablative lasers, shorter-wavelength lasers, and cryotherapy. “It’s not to say you can’t use them, but you have to be very careful,” he said. For clinicians with less experience treating skin of color, he recommends using procedures that spare the epidermis and the dermal/epidermal junction altogether. “This includes lasers with longer wavelengths such as the 1,064-nm Nd:YAG, always using generous cooling to avoid injury or trauma to the dermal/epidermal junction and using longer pulse durations.”
Nonlaser procedures to consider using for darker-skinned patients include superficial chemical peels, radiofrequency (RF) microneedling with semi-insulated needles, microfocused ultrasound, and noninvasive RF procedures that spare or cool the epidermis. “You should never be afraid to do a test spot,” he said. Adjunctive therapies to consider using include hydroquinone and other tyrosinase-receptor blockers, and preprocedural preparatory formulas. “Always advise sun protection and avoidance and administer HSV [herpes simplex virus] prophylaxis as indicated,” he added. “Ample evidence exists to show that currently available fillers and neuromodulators are safe to use in darker skin types. The issue here is more with postinflammatory hyperpigmentation, which can occur from needle punctures, so small-gauge needles and linear threading versus serial puncture is preferred.”
Adjunctive postprocedure preventative therapies include hydroquinone and other tyrosinase-receptor blockers; high-potency topical steroids such as clobetasol twice a day for 3 days and tapering to once a day for 3 days before halting; and postprocedural recovery/healing formulas.
Dr. Bhatia said that hypopigmentation can be treated with UV therapy, with bimatoprost topically combined with needling or low-density fractional lasers, as well as with epidermal microsuction grafting such as the CelluTome Epidermal Harvesting System. Hyperpigmentation can be treated with tyrosinase inhibitors, retinoids, chemical peels, and with microdermal/dermal infusion.
As for cosmeceuticals, sunscreens are necessary for keeping skin tone even. Retinoids are also helpful for maintenance, “but irritation can lead to postinflammatory hyperpigmentation, so go slow,” he said. Hyperpigmentation can be treated with hydroquinone, azelaic acid (Finacea), as well as many other preparations.
“Don’t be afraid to treat these patients,” Dr. Bhatia concluded. “As you get more used to performing procedures on people with darker skin types, you discover the limits of what you can and can’t do. But in general, we can do a lot to make these patients happy.”
Dr. Bhatia reported having research and financial ties to numerous pharmaceutical and device companies.
[email protected]
REPORTING FROM MOAS 2018
Ablative fractional lasers treat scars like ‘a magic wand’
SAN DIEGO – .
“I tell patients it’s like boiling water in a tea kettle and watching the vapor form,” Dr. Waibel, a dermatologist with the Miami Dermatology and Laser Institute, said at the annual Masters of Aesthetics Symposium. “You literally ‘steam off’ their bad scar and the human body will heal that wound to almost normal skin. It’s the closest thing we have to a magic wand.”
In the not-too-distant past, dermatologists “were treating scars just to make them look better,” she said. However, thanks to groundbreaking work by clinicians at Naval Medical Center San Diego, the use of ablative fractional lasers to treat scars was found to improve range of motion in patients, as well as their pain and pruritus. “It represents a major innovation that heals in ways not previously possible,” said Dr. Waibel, who is also chief of dermatology at Baptist Hospital in Miami. “We’re not just healing the scar; we’re healing the skin back to its physiological normal place. A lot of these patients suffer quite a bit.”
Dr. Waibel likened her scar treatment approach to a three-course meal. Lesion color drives her choice of what device to use as an “appetizer” treatment. Most scars are either red (erythematous), brown (hyperpigmented), or white (hypopigmented). Though every scar is unique and individually evaluated for treatment, typically she uses pulsed dye laser, intense pulsed light, or broadband light therapy to treat erythematous/early scars; nonablative fractional lasers to treat atrophic scars, and the thulium or 1,470-nm laser to treat hyperpigmented scars. The “main course” device in her practice is an ablative fractional erbium or CO2 laser.
“Once I treat the scar three to five times, I might switch to a nonablative laser, but I’m really an ablative fractional user,” Dr. Waibel said. “Dessert” can be whatever adjunctive therapies you need, she continued. This may include triamcinolone acetonide, 5-fluorouracil, poly-l-lactic acid, hyaluronidase, Z-plasty, punch biopsies, shave biopsies, compression, chemical reconstruction of skin scars (CROSS), and subcision.
For erythematous surgical and trauma scars, she uses a combination of pulsed dye laser and ablative fractional laser. “Same day, same treatment; one after each other,” she said. She favors using intense pulsed light for donor sites because it has filters that address both melanin and hemosiderin, superiority for scar erythema, and deeper penetration with greater speed to treat large surface areas.
One recent advance in the vascular arena is the new 595-nm pulsed dye laser by Candela, known as the VBeam Prima. It features increased energy, a 15-nm spot size, a zoom hand piece, once-a-day calibration, and contact cooling, which may be better for pigmented and possibly microvascular structures. The device is cleared for treating conditions like rosacea, acne, spider veins, port-wine stains, wrinkles, warts and stretch marks, as well as photoaging and benign pigmented lesions.
Dr. Waibel’s go-to device for treating a hypertrophic, hyperpigmented surgical scar is a 1927-nm or 1470-nm nonablative fractional laser, followed by a fractional ablative laser and injection of 1-2 ccs of 5-fluorouracil only to elevated areas. Hypopigmented scars are “by far the toughest to treat,” she said. However, she has a formula for these, too, and recently conducted a trial comparing the efficacy of nonablative fractional laser, ablative fractional laser, and ablative fractional laser followed by laser-assisted delivery of bimatoprost (Latisse) to treat hypopigmentation.
Surgical scars get better on their own in many cases, but sometimes early intervention is warranted. “Most surgeons will tell patients, ‘Wait a year. What you have [in terms of scar formation] is what you have,’” Dr. Waibel said. “If a surgical scar becomes hypertrophic, it does so within a month of surgery. I don’t prophylactically treat surgical scars unless the patient has had multiple surgeries in the same location with trouble healing. But if it’s been 6 months to a year, or if the patient is developing hypertrophic scars, then I will treat.”
Acne scars are challenging, because patients want to look good right away. “With deep scars, it takes several treatments to see good improvements,” she said. “I tell all my acne scar patients it takes a year [to get good results].”
Most burn patients require three to six treatment sessions, “but sometimes you get remarkable improvement sooner,” she said. “That’s due to the patient’s healing.” She and her associates recently completed an unpublished study that examined early intervention of fractional ablative laser versus control in 20 subjects with acute burn injuries who ranged in age from 18 to 80 years. The subjects underwent treatment with an ablative fractional CO2 laser within 3 months of sustaining the burn injury, leaving an untreated control area for comparison. According to Dr. Waibel, 100% of the blinded physician evaluators graded the laser-treated area correctly, compared with the control area. In addition, a significant improvement in all points of the Manchester Scar Scale was observed in the laser-treated area. “The earlier you treat burn and trauma patients, the easier it is to get them back to normal,” she said.
Dr. Waibel disclosed that she has conducted clinical research for Aquavit, Cytrellis, Lumenis, Lutronic, Michelson Diagnostics, RegenX, Sciton, Sebacia, and Syneron/Candela. She is also a consultant for RegenX, Strata, and Syneron/Candela and is a member of the advisory board for Dominion Technologies, Sciton, and Sebacia.
SAN DIEGO – .
“I tell patients it’s like boiling water in a tea kettle and watching the vapor form,” Dr. Waibel, a dermatologist with the Miami Dermatology and Laser Institute, said at the annual Masters of Aesthetics Symposium. “You literally ‘steam off’ their bad scar and the human body will heal that wound to almost normal skin. It’s the closest thing we have to a magic wand.”
In the not-too-distant past, dermatologists “were treating scars just to make them look better,” she said. However, thanks to groundbreaking work by clinicians at Naval Medical Center San Diego, the use of ablative fractional lasers to treat scars was found to improve range of motion in patients, as well as their pain and pruritus. “It represents a major innovation that heals in ways not previously possible,” said Dr. Waibel, who is also chief of dermatology at Baptist Hospital in Miami. “We’re not just healing the scar; we’re healing the skin back to its physiological normal place. A lot of these patients suffer quite a bit.”
Dr. Waibel likened her scar treatment approach to a three-course meal. Lesion color drives her choice of what device to use as an “appetizer” treatment. Most scars are either red (erythematous), brown (hyperpigmented), or white (hypopigmented). Though every scar is unique and individually evaluated for treatment, typically she uses pulsed dye laser, intense pulsed light, or broadband light therapy to treat erythematous/early scars; nonablative fractional lasers to treat atrophic scars, and the thulium or 1,470-nm laser to treat hyperpigmented scars. The “main course” device in her practice is an ablative fractional erbium or CO2 laser.
“Once I treat the scar three to five times, I might switch to a nonablative laser, but I’m really an ablative fractional user,” Dr. Waibel said. “Dessert” can be whatever adjunctive therapies you need, she continued. This may include triamcinolone acetonide, 5-fluorouracil, poly-l-lactic acid, hyaluronidase, Z-plasty, punch biopsies, shave biopsies, compression, chemical reconstruction of skin scars (CROSS), and subcision.
For erythematous surgical and trauma scars, she uses a combination of pulsed dye laser and ablative fractional laser. “Same day, same treatment; one after each other,” she said. She favors using intense pulsed light for donor sites because it has filters that address both melanin and hemosiderin, superiority for scar erythema, and deeper penetration with greater speed to treat large surface areas.
One recent advance in the vascular arena is the new 595-nm pulsed dye laser by Candela, known as the VBeam Prima. It features increased energy, a 15-nm spot size, a zoom hand piece, once-a-day calibration, and contact cooling, which may be better for pigmented and possibly microvascular structures. The device is cleared for treating conditions like rosacea, acne, spider veins, port-wine stains, wrinkles, warts and stretch marks, as well as photoaging and benign pigmented lesions.
Dr. Waibel’s go-to device for treating a hypertrophic, hyperpigmented surgical scar is a 1927-nm or 1470-nm nonablative fractional laser, followed by a fractional ablative laser and injection of 1-2 ccs of 5-fluorouracil only to elevated areas. Hypopigmented scars are “by far the toughest to treat,” she said. However, she has a formula for these, too, and recently conducted a trial comparing the efficacy of nonablative fractional laser, ablative fractional laser, and ablative fractional laser followed by laser-assisted delivery of bimatoprost (Latisse) to treat hypopigmentation.
Surgical scars get better on their own in many cases, but sometimes early intervention is warranted. “Most surgeons will tell patients, ‘Wait a year. What you have [in terms of scar formation] is what you have,’” Dr. Waibel said. “If a surgical scar becomes hypertrophic, it does so within a month of surgery. I don’t prophylactically treat surgical scars unless the patient has had multiple surgeries in the same location with trouble healing. But if it’s been 6 months to a year, or if the patient is developing hypertrophic scars, then I will treat.”
Acne scars are challenging, because patients want to look good right away. “With deep scars, it takes several treatments to see good improvements,” she said. “I tell all my acne scar patients it takes a year [to get good results].”
Most burn patients require three to six treatment sessions, “but sometimes you get remarkable improvement sooner,” she said. “That’s due to the patient’s healing.” She and her associates recently completed an unpublished study that examined early intervention of fractional ablative laser versus control in 20 subjects with acute burn injuries who ranged in age from 18 to 80 years. The subjects underwent treatment with an ablative fractional CO2 laser within 3 months of sustaining the burn injury, leaving an untreated control area for comparison. According to Dr. Waibel, 100% of the blinded physician evaluators graded the laser-treated area correctly, compared with the control area. In addition, a significant improvement in all points of the Manchester Scar Scale was observed in the laser-treated area. “The earlier you treat burn and trauma patients, the easier it is to get them back to normal,” she said.
Dr. Waibel disclosed that she has conducted clinical research for Aquavit, Cytrellis, Lumenis, Lutronic, Michelson Diagnostics, RegenX, Sciton, Sebacia, and Syneron/Candela. She is also a consultant for RegenX, Strata, and Syneron/Candela and is a member of the advisory board for Dominion Technologies, Sciton, and Sebacia.
SAN DIEGO – .
“I tell patients it’s like boiling water in a tea kettle and watching the vapor form,” Dr. Waibel, a dermatologist with the Miami Dermatology and Laser Institute, said at the annual Masters of Aesthetics Symposium. “You literally ‘steam off’ their bad scar and the human body will heal that wound to almost normal skin. It’s the closest thing we have to a magic wand.”
In the not-too-distant past, dermatologists “were treating scars just to make them look better,” she said. However, thanks to groundbreaking work by clinicians at Naval Medical Center San Diego, the use of ablative fractional lasers to treat scars was found to improve range of motion in patients, as well as their pain and pruritus. “It represents a major innovation that heals in ways not previously possible,” said Dr. Waibel, who is also chief of dermatology at Baptist Hospital in Miami. “We’re not just healing the scar; we’re healing the skin back to its physiological normal place. A lot of these patients suffer quite a bit.”
Dr. Waibel likened her scar treatment approach to a three-course meal. Lesion color drives her choice of what device to use as an “appetizer” treatment. Most scars are either red (erythematous), brown (hyperpigmented), or white (hypopigmented). Though every scar is unique and individually evaluated for treatment, typically she uses pulsed dye laser, intense pulsed light, or broadband light therapy to treat erythematous/early scars; nonablative fractional lasers to treat atrophic scars, and the thulium or 1,470-nm laser to treat hyperpigmented scars. The “main course” device in her practice is an ablative fractional erbium or CO2 laser.
“Once I treat the scar three to five times, I might switch to a nonablative laser, but I’m really an ablative fractional user,” Dr. Waibel said. “Dessert” can be whatever adjunctive therapies you need, she continued. This may include triamcinolone acetonide, 5-fluorouracil, poly-l-lactic acid, hyaluronidase, Z-plasty, punch biopsies, shave biopsies, compression, chemical reconstruction of skin scars (CROSS), and subcision.
For erythematous surgical and trauma scars, she uses a combination of pulsed dye laser and ablative fractional laser. “Same day, same treatment; one after each other,” she said. She favors using intense pulsed light for donor sites because it has filters that address both melanin and hemosiderin, superiority for scar erythema, and deeper penetration with greater speed to treat large surface areas.
One recent advance in the vascular arena is the new 595-nm pulsed dye laser by Candela, known as the VBeam Prima. It features increased energy, a 15-nm spot size, a zoom hand piece, once-a-day calibration, and contact cooling, which may be better for pigmented and possibly microvascular structures. The device is cleared for treating conditions like rosacea, acne, spider veins, port-wine stains, wrinkles, warts and stretch marks, as well as photoaging and benign pigmented lesions.
Dr. Waibel’s go-to device for treating a hypertrophic, hyperpigmented surgical scar is a 1927-nm or 1470-nm nonablative fractional laser, followed by a fractional ablative laser and injection of 1-2 ccs of 5-fluorouracil only to elevated areas. Hypopigmented scars are “by far the toughest to treat,” she said. However, she has a formula for these, too, and recently conducted a trial comparing the efficacy of nonablative fractional laser, ablative fractional laser, and ablative fractional laser followed by laser-assisted delivery of bimatoprost (Latisse) to treat hypopigmentation.
Surgical scars get better on their own in many cases, but sometimes early intervention is warranted. “Most surgeons will tell patients, ‘Wait a year. What you have [in terms of scar formation] is what you have,’” Dr. Waibel said. “If a surgical scar becomes hypertrophic, it does so within a month of surgery. I don’t prophylactically treat surgical scars unless the patient has had multiple surgeries in the same location with trouble healing. But if it’s been 6 months to a year, or if the patient is developing hypertrophic scars, then I will treat.”
Acne scars are challenging, because patients want to look good right away. “With deep scars, it takes several treatments to see good improvements,” she said. “I tell all my acne scar patients it takes a year [to get good results].”
Most burn patients require three to six treatment sessions, “but sometimes you get remarkable improvement sooner,” she said. “That’s due to the patient’s healing.” She and her associates recently completed an unpublished study that examined early intervention of fractional ablative laser versus control in 20 subjects with acute burn injuries who ranged in age from 18 to 80 years. The subjects underwent treatment with an ablative fractional CO2 laser within 3 months of sustaining the burn injury, leaving an untreated control area for comparison. According to Dr. Waibel, 100% of the blinded physician evaluators graded the laser-treated area correctly, compared with the control area. In addition, a significant improvement in all points of the Manchester Scar Scale was observed in the laser-treated area. “The earlier you treat burn and trauma patients, the easier it is to get them back to normal,” she said.
Dr. Waibel disclosed that she has conducted clinical research for Aquavit, Cytrellis, Lumenis, Lutronic, Michelson Diagnostics, RegenX, Sciton, Sebacia, and Syneron/Candela. She is also a consultant for RegenX, Strata, and Syneron/Candela and is a member of the advisory board for Dominion Technologies, Sciton, and Sebacia.
REPORTING FROM MOAS 2018










