User login
Bacterial contamination behind most cosmetics recalls
Most of the 313 cosmetic and personal care products recalled from 2002 to 2016 had problems with bacterial contamination, according to data obtained from the Food and Drug Administration.
, said Timothy M. Janetos, MD, and his associates at Northwestern University in Chicago. Bacterial contamination was by far the most common reason – 76% of the recalls over that period (11 class I, 217 class II, and 9 class II) – with unapproved ingredients and labeling problems well behind at 6%.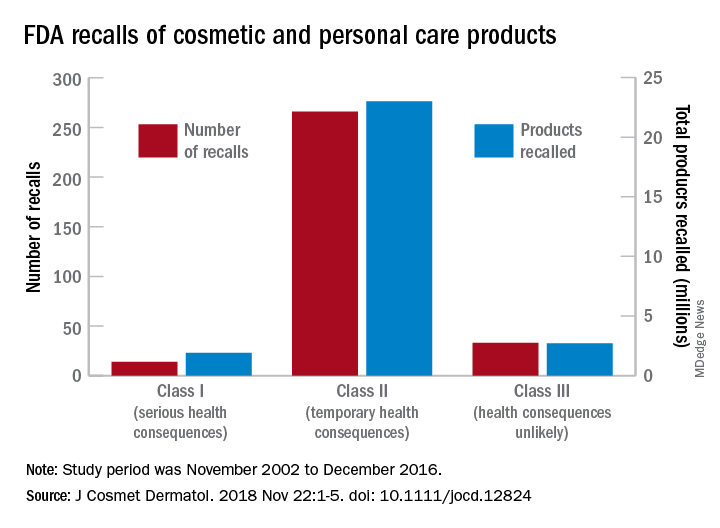
Recalls are classified by the FDA according to risk to patient safety: Class I means there is “reasonable probability of causing serious adverse health outcomes or death,” class II defines the risk as “temporary or reversible,” and class III recalls are “unlikely to cause an adverse health consequence,” they explained.
“While the number of total recalls per year was low in the context of the industry’s size and the ubiquity of cosmetic use by consumers (median: 17/year), these events involved millions of products distributed worldwide,” Dr. Janetos and his associates wrote. The class I recalls covered over 1.9 million products in distribution, the class II recalls accounted for 23 million products, and class II recalls involved over 2.7 million products.
Baby products were the category most likely to be affected, accounting for 76 (24%) of all recalls, the investigators said, with 30 involving one manufacturer of cleansing kits intended for hospital use. All but 3 of the 76 recalls resulted from bacterial contamination.
“The FDA currently has no authority to order a cosmetics manufacturer to recall a product,” they wrote, and “inspectors are only capable of inspecting 0.3% of foreign-imported products yearly,” so underreporting of such problems is likely. “Dermatologists are often the first to encounter [adverse events] related to cosmetic products and can help strengthen public safety by actively reporting these events and advocating for recalls,” said Dr. Janetos and his associates, who did not declare any conflicts of interest.
Information on reporting cosmetic-related complaints to the FDA is available on the FDA website at: https://www.fda.gov/Cosmetics/ComplianceEnforcement/AdverseEventReporting/default.htm.
[email protected]
SOURCE: Janetos TM et al. J Cosmet Dermatol. 2018 Nov 22:1-5. doi: 10.1111/jocd.12824.
Most of the 313 cosmetic and personal care products recalled from 2002 to 2016 had problems with bacterial contamination, according to data obtained from the Food and Drug Administration.
, said Timothy M. Janetos, MD, and his associates at Northwestern University in Chicago. Bacterial contamination was by far the most common reason – 76% of the recalls over that period (11 class I, 217 class II, and 9 class II) – with unapproved ingredients and labeling problems well behind at 6%.
Recalls are classified by the FDA according to risk to patient safety: Class I means there is “reasonable probability of causing serious adverse health outcomes or death,” class II defines the risk as “temporary or reversible,” and class III recalls are “unlikely to cause an adverse health consequence,” they explained.
“While the number of total recalls per year was low in the context of the industry’s size and the ubiquity of cosmetic use by consumers (median: 17/year), these events involved millions of products distributed worldwide,” Dr. Janetos and his associates wrote. The class I recalls covered over 1.9 million products in distribution, the class II recalls accounted for 23 million products, and class II recalls involved over 2.7 million products.
Baby products were the category most likely to be affected, accounting for 76 (24%) of all recalls, the investigators said, with 30 involving one manufacturer of cleansing kits intended for hospital use. All but 3 of the 76 recalls resulted from bacterial contamination.
“The FDA currently has no authority to order a cosmetics manufacturer to recall a product,” they wrote, and “inspectors are only capable of inspecting 0.3% of foreign-imported products yearly,” so underreporting of such problems is likely. “Dermatologists are often the first to encounter [adverse events] related to cosmetic products and can help strengthen public safety by actively reporting these events and advocating for recalls,” said Dr. Janetos and his associates, who did not declare any conflicts of interest.
Information on reporting cosmetic-related complaints to the FDA is available on the FDA website at: https://www.fda.gov/Cosmetics/ComplianceEnforcement/AdverseEventReporting/default.htm.
[email protected]
SOURCE: Janetos TM et al. J Cosmet Dermatol. 2018 Nov 22:1-5. doi: 10.1111/jocd.12824.
Most of the 313 cosmetic and personal care products recalled from 2002 to 2016 had problems with bacterial contamination, according to data obtained from the Food and Drug Administration.
, said Timothy M. Janetos, MD, and his associates at Northwestern University in Chicago. Bacterial contamination was by far the most common reason – 76% of the recalls over that period (11 class I, 217 class II, and 9 class II) – with unapproved ingredients and labeling problems well behind at 6%.
Recalls are classified by the FDA according to risk to patient safety: Class I means there is “reasonable probability of causing serious adverse health outcomes or death,” class II defines the risk as “temporary or reversible,” and class III recalls are “unlikely to cause an adverse health consequence,” they explained.
“While the number of total recalls per year was low in the context of the industry’s size and the ubiquity of cosmetic use by consumers (median: 17/year), these events involved millions of products distributed worldwide,” Dr. Janetos and his associates wrote. The class I recalls covered over 1.9 million products in distribution, the class II recalls accounted for 23 million products, and class II recalls involved over 2.7 million products.
Baby products were the category most likely to be affected, accounting for 76 (24%) of all recalls, the investigators said, with 30 involving one manufacturer of cleansing kits intended for hospital use. All but 3 of the 76 recalls resulted from bacterial contamination.
“The FDA currently has no authority to order a cosmetics manufacturer to recall a product,” they wrote, and “inspectors are only capable of inspecting 0.3% of foreign-imported products yearly,” so underreporting of such problems is likely. “Dermatologists are often the first to encounter [adverse events] related to cosmetic products and can help strengthen public safety by actively reporting these events and advocating for recalls,” said Dr. Janetos and his associates, who did not declare any conflicts of interest.
Information on reporting cosmetic-related complaints to the FDA is available on the FDA website at: https://www.fda.gov/Cosmetics/ComplianceEnforcement/AdverseEventReporting/default.htm.
[email protected]
SOURCE: Janetos TM et al. J Cosmet Dermatol. 2018 Nov 22:1-5. doi: 10.1111/jocd.12824.
FROM THE JOURNAL OF COSMETIC DERMATOLOGY
Nail Care: Survey of the Cutis Editorial Board
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on nail care. Here’s what we found.
Do you routinely perform diagnostic testing before treating for onychomycosis?
Ninety-five percent of dermatologists perform diagnostic testing before treating onychomycosis. Of them, nearly two-thirds only test before treating with systemic antifungals, while one-third test before starting systemic or topical antifungals.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
A laboratory diagnosis of onychomycosis is an absolute necessity before treating for onychomycosis, and the vast majority of our board members are testing routinely. Diagnosis should ideally be performed before initiating both oral and topical therapy. Failure to do so may lead to incorrect treatment with progression of disease and missed diagnoses of malignancy (Lipner and Scher, 2016; Lipner and Scher, 2016).
Next page: Nail fungus
What diagnostic tests do you use to confirm the presence of a nail fungus?
More than 70% of respondents use histopathology or fungal culture to confirm the presence of a nail fungus. Direct microscopy is used by 38% and only 5% use polymerase chain reaction.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Options for diagnosis are potassium hydroxide preparation with microscopy, fungal culture, or nail plate clipping with histopathology. Polymerase chain reaction is another option that is available and covered by many insurance plans. Many of our board members use histopathology and fungal culture more often than other methods. Histopathology is advantageous for its high sensitivity and capacity to detect other nail diseases, such as nail psoriasis. A disadvantage is that the identity and viability of the infecting organism cannot be determined. While fungal culture can detect both identity and viability, the organism may take several weeks to grow and there is a high false-negative rate (Lipner and Scher, 2018 [Part 1]).
Next page: Laboratory monitoring with terbinafine
Almost half (48%) of dermatologists monitor laboratory test results in onychomycosis patients taking terbinafine at both baseline and during therapy. Twenty-three percent monitor at baseline only; 14% at baseline and after therapy; 5% at baseline, during therapy, and after therapy; and 10% don’t monitor at all.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Almost half of board members perform laboratory monitoring for patients taking terbinafine, which was reasonable prior to any published data on blood count and liver function tests in patients taking this drug. However, a new study on this topic should make us reconsider our practices. This study analyzed the rate of laboratory test abnormalities in 4985 patients taking terbinafine or griseofulvin for dermatophyte infections. Elevated alanine aminotransferase, aspartate aminotransferase, anemia, lymphopenia, and neutropenia were uncommon and similar to the baseline rates. Therefore, routine interval laboratory monitoring may be unnecessary in healthy patients taking oral terbinafine for onychomycosis (Stolmeier et al).
Next page: Biotin recommendations
Do you routinely recommend biotin to your patients?
Approximately half (52%) of dermatologists do not recommend biotin to their patients. However, 29% do recommend it for hair and nail disorders.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Biotin is an essential cofactor for mammalian carboxylase enzymes that are involved in important metabolic pathways in humans. Biotin supplementation is likely unnecessary for most individuals, as biotin intake is likely sufficient in a Western diet. There are limited data on biotin supplementation to treat dermatologic conditions, especially in patients with normal biotin levels. In addition, a recent warning issued by the US Food and Drug Administration reported that consumption of biotin may interfere with laboratory tests. Therefore, biotin should not be routinely recommended to patients without sufficient evidence that it would benefit their condition (Lipner, 2018).
Next page: Medication for onychomycosis
Which medication(s) do you prescribe most often for onychomycosis?
The top medications prescribed by dermatologists for onychomycosis were oral terbinafine (62%) and topical ciclopirox (52%), followed by oral fluconazole (29%), topical efinaconazole (24%), oral itraconazole (14%), and topical tavaborole (5%).

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Oral terbinafine is most frequently prescribed by our board, likely because it has the best efficacy, dosing regimen, and minimal potential for systemic side effects or drug-drug interactions. Efficacy with ciclopirox lacquer is quite low and the medication is difficult to apply. For toenail onychomycosis, application is daily with weekly clipping and removal and monthly debridement. Patients who are not candidates for terbinafine would likely benefit more from oral itraconazole, oral fluconazole (off label), efinaconazole, or tavaborole (Lipner and Scher, 2018 [Part II]).
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
As with care in general in dermatology, the care and treatment of healthy nails and especially diseased nails is multifaceted and is optimized by using combination therapy. The combination of an oral antifungal and a topical that treats the local area and also provides protection and a healthy environment for nail growth and repair is ideal.—Fran E. Cook-Bolden, MD (New York, New York)
It’s important to culture for fungus before starting treatment. It seems many cultures turn out to be nondermatophytes, and terbinafine is not the best treatment.—Lawrence J. Green, MD (Washington, DC)
Nails do care. Diagnoses should be confirmed.—Richard K. Scher, MD (New York, New York)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from October 22, 2018, to November 14, 2018. A total of 21 usable responses were received.
- Lipner SR. Rethinking biotin therapy for hair, nail, and skin disorders. J Am Acad Dermatol. 2018;78:1236-1238.
- Lipner SR, Scher RK. Confirmatory testing for onychomycosis. JAMA Dermatol. 2016 Jul 1;152:847.
- Lipner SR, Scher RK. Onychomycosis–a small step for quality of care. Curr Med Res Opin. 2016;32:865-867.
- Lipner SR, Scher RK. Part I: onychomycosis: clinical overview and diagnosis [published online June 27, 2018]. J Am Acad Dermatol. pii:S0190-9622(18)32188-1.
- Lipner SR, Scher RK. Part II: onychomycosis: treatment and prevention of recurrence [published online June 27, 2018]. J Am Acad Dermatol. pii: S0190-9622(18)32187-X.)
- Stolmeier DA, Stratman HB, McIntee TJ, et al. Utility of laboratory test result monitoring in patients taking oral terbinafine or griseofulvin for dermatophyte infections [published online October 17, 2018]. JAMA Dermatol. doi:10.1001/jamadermatol.2018.3578.
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on nail care. Here’s what we found.
Do you routinely perform diagnostic testing before treating for onychomycosis?
Ninety-five percent of dermatologists perform diagnostic testing before treating onychomycosis. Of them, nearly two-thirds only test before treating with systemic antifungals, while one-third test before starting systemic or topical antifungals.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
A laboratory diagnosis of onychomycosis is an absolute necessity before treating for onychomycosis, and the vast majority of our board members are testing routinely. Diagnosis should ideally be performed before initiating both oral and topical therapy. Failure to do so may lead to incorrect treatment with progression of disease and missed diagnoses of malignancy (Lipner and Scher, 2016; Lipner and Scher, 2016).
Next page: Nail fungus
What diagnostic tests do you use to confirm the presence of a nail fungus?
More than 70% of respondents use histopathology or fungal culture to confirm the presence of a nail fungus. Direct microscopy is used by 38% and only 5% use polymerase chain reaction.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Options for diagnosis are potassium hydroxide preparation with microscopy, fungal culture, or nail plate clipping with histopathology. Polymerase chain reaction is another option that is available and covered by many insurance plans. Many of our board members use histopathology and fungal culture more often than other methods. Histopathology is advantageous for its high sensitivity and capacity to detect other nail diseases, such as nail psoriasis. A disadvantage is that the identity and viability of the infecting organism cannot be determined. While fungal culture can detect both identity and viability, the organism may take several weeks to grow and there is a high false-negative rate (Lipner and Scher, 2018 [Part 1]).
Next page: Laboratory monitoring with terbinafine
Almost half (48%) of dermatologists monitor laboratory test results in onychomycosis patients taking terbinafine at both baseline and during therapy. Twenty-three percent monitor at baseline only; 14% at baseline and after therapy; 5% at baseline, during therapy, and after therapy; and 10% don’t monitor at all.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Almost half of board members perform laboratory monitoring for patients taking terbinafine, which was reasonable prior to any published data on blood count and liver function tests in patients taking this drug. However, a new study on this topic should make us reconsider our practices. This study analyzed the rate of laboratory test abnormalities in 4985 patients taking terbinafine or griseofulvin for dermatophyte infections. Elevated alanine aminotransferase, aspartate aminotransferase, anemia, lymphopenia, and neutropenia were uncommon and similar to the baseline rates. Therefore, routine interval laboratory monitoring may be unnecessary in healthy patients taking oral terbinafine for onychomycosis (Stolmeier et al).
Next page: Biotin recommendations
Do you routinely recommend biotin to your patients?
Approximately half (52%) of dermatologists do not recommend biotin to their patients. However, 29% do recommend it for hair and nail disorders.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Biotin is an essential cofactor for mammalian carboxylase enzymes that are involved in important metabolic pathways in humans. Biotin supplementation is likely unnecessary for most individuals, as biotin intake is likely sufficient in a Western diet. There are limited data on biotin supplementation to treat dermatologic conditions, especially in patients with normal biotin levels. In addition, a recent warning issued by the US Food and Drug Administration reported that consumption of biotin may interfere with laboratory tests. Therefore, biotin should not be routinely recommended to patients without sufficient evidence that it would benefit their condition (Lipner, 2018).
Next page: Medication for onychomycosis
Which medication(s) do you prescribe most often for onychomycosis?
The top medications prescribed by dermatologists for onychomycosis were oral terbinafine (62%) and topical ciclopirox (52%), followed by oral fluconazole (29%), topical efinaconazole (24%), oral itraconazole (14%), and topical tavaborole (5%).

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Oral terbinafine is most frequently prescribed by our board, likely because it has the best efficacy, dosing regimen, and minimal potential for systemic side effects or drug-drug interactions. Efficacy with ciclopirox lacquer is quite low and the medication is difficult to apply. For toenail onychomycosis, application is daily with weekly clipping and removal and monthly debridement. Patients who are not candidates for terbinafine would likely benefit more from oral itraconazole, oral fluconazole (off label), efinaconazole, or tavaborole (Lipner and Scher, 2018 [Part II]).
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
As with care in general in dermatology, the care and treatment of healthy nails and especially diseased nails is multifaceted and is optimized by using combination therapy. The combination of an oral antifungal and a topical that treats the local area and also provides protection and a healthy environment for nail growth and repair is ideal.—Fran E. Cook-Bolden, MD (New York, New York)
It’s important to culture for fungus before starting treatment. It seems many cultures turn out to be nondermatophytes, and terbinafine is not the best treatment.—Lawrence J. Green, MD (Washington, DC)
Nails do care. Diagnoses should be confirmed.—Richard K. Scher, MD (New York, New York)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from October 22, 2018, to November 14, 2018. A total of 21 usable responses were received.
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on nail care. Here’s what we found.
Do you routinely perform diagnostic testing before treating for onychomycosis?
Ninety-five percent of dermatologists perform diagnostic testing before treating onychomycosis. Of them, nearly two-thirds only test before treating with systemic antifungals, while one-third test before starting systemic or topical antifungals.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
A laboratory diagnosis of onychomycosis is an absolute necessity before treating for onychomycosis, and the vast majority of our board members are testing routinely. Diagnosis should ideally be performed before initiating both oral and topical therapy. Failure to do so may lead to incorrect treatment with progression of disease and missed diagnoses of malignancy (Lipner and Scher, 2016; Lipner and Scher, 2016).
Next page: Nail fungus
What diagnostic tests do you use to confirm the presence of a nail fungus?
More than 70% of respondents use histopathology or fungal culture to confirm the presence of a nail fungus. Direct microscopy is used by 38% and only 5% use polymerase chain reaction.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Options for diagnosis are potassium hydroxide preparation with microscopy, fungal culture, or nail plate clipping with histopathology. Polymerase chain reaction is another option that is available and covered by many insurance plans. Many of our board members use histopathology and fungal culture more often than other methods. Histopathology is advantageous for its high sensitivity and capacity to detect other nail diseases, such as nail psoriasis. A disadvantage is that the identity and viability of the infecting organism cannot be determined. While fungal culture can detect both identity and viability, the organism may take several weeks to grow and there is a high false-negative rate (Lipner and Scher, 2018 [Part 1]).
Next page: Laboratory monitoring with terbinafine
Almost half (48%) of dermatologists monitor laboratory test results in onychomycosis patients taking terbinafine at both baseline and during therapy. Twenty-three percent monitor at baseline only; 14% at baseline and after therapy; 5% at baseline, during therapy, and after therapy; and 10% don’t monitor at all.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Almost half of board members perform laboratory monitoring for patients taking terbinafine, which was reasonable prior to any published data on blood count and liver function tests in patients taking this drug. However, a new study on this topic should make us reconsider our practices. This study analyzed the rate of laboratory test abnormalities in 4985 patients taking terbinafine or griseofulvin for dermatophyte infections. Elevated alanine aminotransferase, aspartate aminotransferase, anemia, lymphopenia, and neutropenia were uncommon and similar to the baseline rates. Therefore, routine interval laboratory monitoring may be unnecessary in healthy patients taking oral terbinafine for onychomycosis (Stolmeier et al).
Next page: Biotin recommendations
Do you routinely recommend biotin to your patients?
Approximately half (52%) of dermatologists do not recommend biotin to their patients. However, 29% do recommend it for hair and nail disorders.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Biotin is an essential cofactor for mammalian carboxylase enzymes that are involved in important metabolic pathways in humans. Biotin supplementation is likely unnecessary for most individuals, as biotin intake is likely sufficient in a Western diet. There are limited data on biotin supplementation to treat dermatologic conditions, especially in patients with normal biotin levels. In addition, a recent warning issued by the US Food and Drug Administration reported that consumption of biotin may interfere with laboratory tests. Therefore, biotin should not be routinely recommended to patients without sufficient evidence that it would benefit their condition (Lipner, 2018).
Next page: Medication for onychomycosis
Which medication(s) do you prescribe most often for onychomycosis?
The top medications prescribed by dermatologists for onychomycosis were oral terbinafine (62%) and topical ciclopirox (52%), followed by oral fluconazole (29%), topical efinaconazole (24%), oral itraconazole (14%), and topical tavaborole (5%).

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Oral terbinafine is most frequently prescribed by our board, likely because it has the best efficacy, dosing regimen, and minimal potential for systemic side effects or drug-drug interactions. Efficacy with ciclopirox lacquer is quite low and the medication is difficult to apply. For toenail onychomycosis, application is daily with weekly clipping and removal and monthly debridement. Patients who are not candidates for terbinafine would likely benefit more from oral itraconazole, oral fluconazole (off label), efinaconazole, or tavaborole (Lipner and Scher, 2018 [Part II]).
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
As with care in general in dermatology, the care and treatment of healthy nails and especially diseased nails is multifaceted and is optimized by using combination therapy. The combination of an oral antifungal and a topical that treats the local area and also provides protection and a healthy environment for nail growth and repair is ideal.—Fran E. Cook-Bolden, MD (New York, New York)
It’s important to culture for fungus before starting treatment. It seems many cultures turn out to be nondermatophytes, and terbinafine is not the best treatment.—Lawrence J. Green, MD (Washington, DC)
Nails do care. Diagnoses should be confirmed.—Richard K. Scher, MD (New York, New York)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from October 22, 2018, to November 14, 2018. A total of 21 usable responses were received.
- Lipner SR. Rethinking biotin therapy for hair, nail, and skin disorders. J Am Acad Dermatol. 2018;78:1236-1238.
- Lipner SR, Scher RK. Confirmatory testing for onychomycosis. JAMA Dermatol. 2016 Jul 1;152:847.
- Lipner SR, Scher RK. Onychomycosis–a small step for quality of care. Curr Med Res Opin. 2016;32:865-867.
- Lipner SR, Scher RK. Part I: onychomycosis: clinical overview and diagnosis [published online June 27, 2018]. J Am Acad Dermatol. pii:S0190-9622(18)32188-1.
- Lipner SR, Scher RK. Part II: onychomycosis: treatment and prevention of recurrence [published online June 27, 2018]. J Am Acad Dermatol. pii: S0190-9622(18)32187-X.)
- Stolmeier DA, Stratman HB, McIntee TJ, et al. Utility of laboratory test result monitoring in patients taking oral terbinafine or griseofulvin for dermatophyte infections [published online October 17, 2018]. JAMA Dermatol. doi:10.1001/jamadermatol.2018.3578.
- Lipner SR. Rethinking biotin therapy for hair, nail, and skin disorders. J Am Acad Dermatol. 2018;78:1236-1238.
- Lipner SR, Scher RK. Confirmatory testing for onychomycosis. JAMA Dermatol. 2016 Jul 1;152:847.
- Lipner SR, Scher RK. Onychomycosis–a small step for quality of care. Curr Med Res Opin. 2016;32:865-867.
- Lipner SR, Scher RK. Part I: onychomycosis: clinical overview and diagnosis [published online June 27, 2018]. J Am Acad Dermatol. pii:S0190-9622(18)32188-1.
- Lipner SR, Scher RK. Part II: onychomycosis: treatment and prevention of recurrence [published online June 27, 2018]. J Am Acad Dermatol. pii: S0190-9622(18)32187-X.)
- Stolmeier DA, Stratman HB, McIntee TJ, et al. Utility of laboratory test result monitoring in patients taking oral terbinafine or griseofulvin for dermatophyte infections [published online October 17, 2018]. JAMA Dermatol. doi:10.1001/jamadermatol.2018.3578.
Topical retinoid found effective as microneedling for acne scars
according to a new study.
In a prospective, randomized, split-face study of adults with postacne scarring, both treatments resulted in similar efficacy after 6 months, reported T.P. Afra, MD, and associates from the department of dermatology, venereology, and leprology at the Postgraduate Institute of Medical Education and Research in Chandigarh, India. While the clinical usefulness of microneedling as a procedure for postacne scarring is well established, research exploring the effectiveness of topical therapies for acne scarring that could be used at home is lacking. “A home-based topical treatment with a comparable efficacy to microneedling and that is well tolerated would be a useful addition in the armamentarium of acne scar management,” they wrote in the study, published in JAMA Facial Plastic Surgery.
The study included 34 patients, aged 18-30 years, with grade 2-4 facial atrophic acne scars at their initial visit to the research team’s skin clinic. One side of each participants face was randomized to receive microneedling treatment for four sessions over 3 months (using a dermaroller with 1.5-mm needles). Topical tazarotene gel 0.1%, a retinoid approved by the Food and Drug Administration as a treatment for mild to moderate facial acne, was applied to the other side of their face once a night during the same time. Almost 81% were skin phototypes IV, the rest were type III or V. Patients followed up every month for 3 months, then at 6 months.
Changes in acne scar severity from baseline, the primary outcome, were assessed using Goodman and Baron quantitative and qualitative scores and a subjective dermatologist score. Patient satisfaction measured with a Patient Global Assessment (PGA) score and adverse events were secondary outcomes.
In 31 patients (91.2%), overall improvements from baseline to the 6-month visit in quantitative acne scar severity scores for both treatments were seen, with significant improvements from baseline to 6 months: A median improvement of 3 on the sides of the face treated with microneedling and a median improvement of 2.5 on the sides of the face treated with tazarotene (between-group comparison, P = .42). The qualitative acne scar severity score did not significantly improve with either treatment, the investigators noted.
The median improvement in the independent dermatologist score was also comparable for both methods at 3 and 6 months.
At 6 months, improvement in the mean PGA score was “slightly but significantly superior” for the microneedling treatment, compared with that for tazarotene (mean of 5.86 vs. 5.76, respectively; P less than .001), with both falling into the “satisfactory” range for the PGA, the investigators wrote. They also noted a positive correlation between previous exposure to oral isotretinoin and patient satisfaction.
“Although collagen accumulation has been considered a drawback of isotretinoin therapy owing to the development of hypertrophic scars, the better atrophic acne scar outcomes observed for both the present treatment groups in patients with a history of isotretinoin treatment indicates that the collagen accumulation in this case may actually be beneficial,” they wrote.
The topical retinoid was well tolerated by participants, with less than a third reporting dryness and scaling, and adverse effects associated with microneedling were described as “minimal.”
“The use of a modality such as tazarotene that prevents acne flares while addressing acne scarring is a practical addition to clinical practice,” the investigators concluded. “Tazarotene gel 0.1% would be a useful alternative to microneedling in the management of atrophic acne scars. Such a home-based medical management option for acne scarring may decrease physician dependence and health care expenditures for patients with postacne scarring.”
The study authors noted that, as collagen remodeling is a continuous process lasting more than 1 year, a limitation of their study was its short-follow-up of 6 months. However, a strength of the study was its use of validated acne scar severity scoring tools as well as patient and physician assessment of scar improvement in the outcome assessments.
The authors had no disclosures to report.
SOURCE: Afra TP et al. JAMA Facial Plast Surg. 2018 Nov 15. doi: 10.1001/jamafacial.2018.1404.
according to a new study.
In a prospective, randomized, split-face study of adults with postacne scarring, both treatments resulted in similar efficacy after 6 months, reported T.P. Afra, MD, and associates from the department of dermatology, venereology, and leprology at the Postgraduate Institute of Medical Education and Research in Chandigarh, India. While the clinical usefulness of microneedling as a procedure for postacne scarring is well established, research exploring the effectiveness of topical therapies for acne scarring that could be used at home is lacking. “A home-based topical treatment with a comparable efficacy to microneedling and that is well tolerated would be a useful addition in the armamentarium of acne scar management,” they wrote in the study, published in JAMA Facial Plastic Surgery.
The study included 34 patients, aged 18-30 years, with grade 2-4 facial atrophic acne scars at their initial visit to the research team’s skin clinic. One side of each participants face was randomized to receive microneedling treatment for four sessions over 3 months (using a dermaroller with 1.5-mm needles). Topical tazarotene gel 0.1%, a retinoid approved by the Food and Drug Administration as a treatment for mild to moderate facial acne, was applied to the other side of their face once a night during the same time. Almost 81% were skin phototypes IV, the rest were type III or V. Patients followed up every month for 3 months, then at 6 months.
Changes in acne scar severity from baseline, the primary outcome, were assessed using Goodman and Baron quantitative and qualitative scores and a subjective dermatologist score. Patient satisfaction measured with a Patient Global Assessment (PGA) score and adverse events were secondary outcomes.
In 31 patients (91.2%), overall improvements from baseline to the 6-month visit in quantitative acne scar severity scores for both treatments were seen, with significant improvements from baseline to 6 months: A median improvement of 3 on the sides of the face treated with microneedling and a median improvement of 2.5 on the sides of the face treated with tazarotene (between-group comparison, P = .42). The qualitative acne scar severity score did not significantly improve with either treatment, the investigators noted.
The median improvement in the independent dermatologist score was also comparable for both methods at 3 and 6 months.
At 6 months, improvement in the mean PGA score was “slightly but significantly superior” for the microneedling treatment, compared with that for tazarotene (mean of 5.86 vs. 5.76, respectively; P less than .001), with both falling into the “satisfactory” range for the PGA, the investigators wrote. They also noted a positive correlation between previous exposure to oral isotretinoin and patient satisfaction.
“Although collagen accumulation has been considered a drawback of isotretinoin therapy owing to the development of hypertrophic scars, the better atrophic acne scar outcomes observed for both the present treatment groups in patients with a history of isotretinoin treatment indicates that the collagen accumulation in this case may actually be beneficial,” they wrote.
The topical retinoid was well tolerated by participants, with less than a third reporting dryness and scaling, and adverse effects associated with microneedling were described as “minimal.”
“The use of a modality such as tazarotene that prevents acne flares while addressing acne scarring is a practical addition to clinical practice,” the investigators concluded. “Tazarotene gel 0.1% would be a useful alternative to microneedling in the management of atrophic acne scars. Such a home-based medical management option for acne scarring may decrease physician dependence and health care expenditures for patients with postacne scarring.”
The study authors noted that, as collagen remodeling is a continuous process lasting more than 1 year, a limitation of their study was its short-follow-up of 6 months. However, a strength of the study was its use of validated acne scar severity scoring tools as well as patient and physician assessment of scar improvement in the outcome assessments.
The authors had no disclosures to report.
SOURCE: Afra TP et al. JAMA Facial Plast Surg. 2018 Nov 15. doi: 10.1001/jamafacial.2018.1404.
according to a new study.
In a prospective, randomized, split-face study of adults with postacne scarring, both treatments resulted in similar efficacy after 6 months, reported T.P. Afra, MD, and associates from the department of dermatology, venereology, and leprology at the Postgraduate Institute of Medical Education and Research in Chandigarh, India. While the clinical usefulness of microneedling as a procedure for postacne scarring is well established, research exploring the effectiveness of topical therapies for acne scarring that could be used at home is lacking. “A home-based topical treatment with a comparable efficacy to microneedling and that is well tolerated would be a useful addition in the armamentarium of acne scar management,” they wrote in the study, published in JAMA Facial Plastic Surgery.
The study included 34 patients, aged 18-30 years, with grade 2-4 facial atrophic acne scars at their initial visit to the research team’s skin clinic. One side of each participants face was randomized to receive microneedling treatment for four sessions over 3 months (using a dermaroller with 1.5-mm needles). Topical tazarotene gel 0.1%, a retinoid approved by the Food and Drug Administration as a treatment for mild to moderate facial acne, was applied to the other side of their face once a night during the same time. Almost 81% were skin phototypes IV, the rest were type III or V. Patients followed up every month for 3 months, then at 6 months.
Changes in acne scar severity from baseline, the primary outcome, were assessed using Goodman and Baron quantitative and qualitative scores and a subjective dermatologist score. Patient satisfaction measured with a Patient Global Assessment (PGA) score and adverse events were secondary outcomes.
In 31 patients (91.2%), overall improvements from baseline to the 6-month visit in quantitative acne scar severity scores for both treatments were seen, with significant improvements from baseline to 6 months: A median improvement of 3 on the sides of the face treated with microneedling and a median improvement of 2.5 on the sides of the face treated with tazarotene (between-group comparison, P = .42). The qualitative acne scar severity score did not significantly improve with either treatment, the investigators noted.
The median improvement in the independent dermatologist score was also comparable for both methods at 3 and 6 months.
At 6 months, improvement in the mean PGA score was “slightly but significantly superior” for the microneedling treatment, compared with that for tazarotene (mean of 5.86 vs. 5.76, respectively; P less than .001), with both falling into the “satisfactory” range for the PGA, the investigators wrote. They also noted a positive correlation between previous exposure to oral isotretinoin and patient satisfaction.
“Although collagen accumulation has been considered a drawback of isotretinoin therapy owing to the development of hypertrophic scars, the better atrophic acne scar outcomes observed for both the present treatment groups in patients with a history of isotretinoin treatment indicates that the collagen accumulation in this case may actually be beneficial,” they wrote.
The topical retinoid was well tolerated by participants, with less than a third reporting dryness and scaling, and adverse effects associated with microneedling were described as “minimal.”
“The use of a modality such as tazarotene that prevents acne flares while addressing acne scarring is a practical addition to clinical practice,” the investigators concluded. “Tazarotene gel 0.1% would be a useful alternative to microneedling in the management of atrophic acne scars. Such a home-based medical management option for acne scarring may decrease physician dependence and health care expenditures for patients with postacne scarring.”
The study authors noted that, as collagen remodeling is a continuous process lasting more than 1 year, a limitation of their study was its short-follow-up of 6 months. However, a strength of the study was its use of validated acne scar severity scoring tools as well as patient and physician assessment of scar improvement in the outcome assessments.
The authors had no disclosures to report.
SOURCE: Afra TP et al. JAMA Facial Plast Surg. 2018 Nov 15. doi: 10.1001/jamafacial.2018.1404.
FROM JAMA FACIAL PLASTIC SURGERY
Key clinical point: The topical retinoid tazarotene could be a home-based option for treating atrophic acne scarring.
Major finding: Improvements in acne scarring were similar with microneedling and nightly applications of tazarotene gel 0.1% after 6 months.
Study details: A prospective, observer-blinded, split-face, randomized, clinical trial involving 34 patients with grade 2-4 facial atrophic postacne scars.
Disclosures: The authors had no disclosures to report.
Source: Afra TP et al. JAMA Facial Plast Surg. 2018 Nov 15. doi: 10.1001/jamafacial.2018.1404.
Integrative dermatology
In October of this year, the , and practitioners of Ayurvedic, Naturopathic, and traditional Chinese medicine (TCM), in one place. This was the first time in the United States that practitioners from these different areas of medicine were brought together to discuss and learn different approaches to skin care and treatment of dermatologic diseases.
Of all the medical specialties, it is presumed that dermatology is the most inherently holistic. By examining the hair, skin, and nails, we are able to diagnose internal organ diseases such as liver failure (jaundice, veins on stomach), thyroid disease (madarosis), sarcoidosis, and infectious diseases (cutaneous manifestations of HIV), diabetes (acanthosis nigricans, tripe palm), polycystic ovary syndrome (acne, hirsutism), and porphyria, just to name a few. We are also able to treat cutaneous conditions, such as psoriasis, with biologic medications, treatment that in turn, also benefits internal manifestations such as joint, cardiovascular, and metabolic disease. In TCM and Ayurveda, the skin, hair, body type, and tongue can also be analyzed to diagnose and treat disease.
Salves and skin care routines that would be considered natural or holistic have been “prescribed” by Western dermatologists with an MD license for many years. Most medicines initially come from nature, and it is only in the past century, with the boom in the pharmaceutical industry and development of synthetic prescription medications, that people have forgotten this. Some of this boom has been needed to treat enormous populations, as natural resources can be scarce, and in some cases, only an extract of the plant may be needed for treatment, where other elements may be ineffective or even harmful.
Domeboro solution, Epsom salt soaks, and wet to dry soaks are used to draw out and treat infections. Bleach baths are often used to decrease bacterial load and calm inflammation when treating eczema. In Mohs surgery, Fredrick Mohs initially used a zinc chloride paste on nonmelanoma skin cancers in between stages, before frozen section processing and cosmetic reconstruction made Mohs what it is today. In the days of Hippocrates, food was medicine. If you were “red in the face” your blood was deemed too acidic and alkaline-forming foods or “cold foods” were given. This has now again come full circle with rosacea and evidence supporting a link between disease flares or improvement related to foods and the gut microbiome.
On a photography trip to Wyoming, I learned how Native Americans in the United States wiped the white powder from the bark of aspen trees on their skin and used it as sunscreen. In Mongolia, I learned how fat from a sheep’s bottom was used in beauty skin care routines. It is from native and nomadic people that we can often learn how effective natural methods can be used, especially in cases where the treatment regimens may not be written down. With Ayurveda and TCM, we are lucky that textbooks thousands of years old and professors and schools are available to educate us about these ancient practices.
The rediscovery of ancient treatments through the study of ethnobotany, Ayurveda, and TCM has been fascinating, as most of these approaches focus not just on the skin, but on treating the patient as a whole, inside and out (often depending on the discipline treating mind, body, and spirit), with the effects ultimately benefiting the skin. With the many advances in Western medicine over the past 2,000 years, starting with Hippocrates, it will be interesting to see how we, in the field of dermatology, can still learn from and potentially integrate medicine that originated 3,000-5,000 plus years ago in Ayurveda and 2,000-plus years ago in TCM that is still practiced today. In the future, we hope to have more columns about these specialties and how they are used in skin and beauty.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
In October of this year, the , and practitioners of Ayurvedic, Naturopathic, and traditional Chinese medicine (TCM), in one place. This was the first time in the United States that practitioners from these different areas of medicine were brought together to discuss and learn different approaches to skin care and treatment of dermatologic diseases.
Of all the medical specialties, it is presumed that dermatology is the most inherently holistic. By examining the hair, skin, and nails, we are able to diagnose internal organ diseases such as liver failure (jaundice, veins on stomach), thyroid disease (madarosis), sarcoidosis, and infectious diseases (cutaneous manifestations of HIV), diabetes (acanthosis nigricans, tripe palm), polycystic ovary syndrome (acne, hirsutism), and porphyria, just to name a few. We are also able to treat cutaneous conditions, such as psoriasis, with biologic medications, treatment that in turn, also benefits internal manifestations such as joint, cardiovascular, and metabolic disease. In TCM and Ayurveda, the skin, hair, body type, and tongue can also be analyzed to diagnose and treat disease.
Salves and skin care routines that would be considered natural or holistic have been “prescribed” by Western dermatologists with an MD license for many years. Most medicines initially come from nature, and it is only in the past century, with the boom in the pharmaceutical industry and development of synthetic prescription medications, that people have forgotten this. Some of this boom has been needed to treat enormous populations, as natural resources can be scarce, and in some cases, only an extract of the plant may be needed for treatment, where other elements may be ineffective or even harmful.
Domeboro solution, Epsom salt soaks, and wet to dry soaks are used to draw out and treat infections. Bleach baths are often used to decrease bacterial load and calm inflammation when treating eczema. In Mohs surgery, Fredrick Mohs initially used a zinc chloride paste on nonmelanoma skin cancers in between stages, before frozen section processing and cosmetic reconstruction made Mohs what it is today. In the days of Hippocrates, food was medicine. If you were “red in the face” your blood was deemed too acidic and alkaline-forming foods or “cold foods” were given. This has now again come full circle with rosacea and evidence supporting a link between disease flares or improvement related to foods and the gut microbiome.
On a photography trip to Wyoming, I learned how Native Americans in the United States wiped the white powder from the bark of aspen trees on their skin and used it as sunscreen. In Mongolia, I learned how fat from a sheep’s bottom was used in beauty skin care routines. It is from native and nomadic people that we can often learn how effective natural methods can be used, especially in cases where the treatment regimens may not be written down. With Ayurveda and TCM, we are lucky that textbooks thousands of years old and professors and schools are available to educate us about these ancient practices.
The rediscovery of ancient treatments through the study of ethnobotany, Ayurveda, and TCM has been fascinating, as most of these approaches focus not just on the skin, but on treating the patient as a whole, inside and out (often depending on the discipline treating mind, body, and spirit), with the effects ultimately benefiting the skin. With the many advances in Western medicine over the past 2,000 years, starting with Hippocrates, it will be interesting to see how we, in the field of dermatology, can still learn from and potentially integrate medicine that originated 3,000-5,000 plus years ago in Ayurveda and 2,000-plus years ago in TCM that is still practiced today. In the future, we hope to have more columns about these specialties and how they are used in skin and beauty.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
In October of this year, the , and practitioners of Ayurvedic, Naturopathic, and traditional Chinese medicine (TCM), in one place. This was the first time in the United States that practitioners from these different areas of medicine were brought together to discuss and learn different approaches to skin care and treatment of dermatologic diseases.
Of all the medical specialties, it is presumed that dermatology is the most inherently holistic. By examining the hair, skin, and nails, we are able to diagnose internal organ diseases such as liver failure (jaundice, veins on stomach), thyroid disease (madarosis), sarcoidosis, and infectious diseases (cutaneous manifestations of HIV), diabetes (acanthosis nigricans, tripe palm), polycystic ovary syndrome (acne, hirsutism), and porphyria, just to name a few. We are also able to treat cutaneous conditions, such as psoriasis, with biologic medications, treatment that in turn, also benefits internal manifestations such as joint, cardiovascular, and metabolic disease. In TCM and Ayurveda, the skin, hair, body type, and tongue can also be analyzed to diagnose and treat disease.
Salves and skin care routines that would be considered natural or holistic have been “prescribed” by Western dermatologists with an MD license for many years. Most medicines initially come from nature, and it is only in the past century, with the boom in the pharmaceutical industry and development of synthetic prescription medications, that people have forgotten this. Some of this boom has been needed to treat enormous populations, as natural resources can be scarce, and in some cases, only an extract of the plant may be needed for treatment, where other elements may be ineffective or even harmful.
Domeboro solution, Epsom salt soaks, and wet to dry soaks are used to draw out and treat infections. Bleach baths are often used to decrease bacterial load and calm inflammation when treating eczema. In Mohs surgery, Fredrick Mohs initially used a zinc chloride paste on nonmelanoma skin cancers in between stages, before frozen section processing and cosmetic reconstruction made Mohs what it is today. In the days of Hippocrates, food was medicine. If you were “red in the face” your blood was deemed too acidic and alkaline-forming foods or “cold foods” were given. This has now again come full circle with rosacea and evidence supporting a link between disease flares or improvement related to foods and the gut microbiome.
On a photography trip to Wyoming, I learned how Native Americans in the United States wiped the white powder from the bark of aspen trees on their skin and used it as sunscreen. In Mongolia, I learned how fat from a sheep’s bottom was used in beauty skin care routines. It is from native and nomadic people that we can often learn how effective natural methods can be used, especially in cases where the treatment regimens may not be written down. With Ayurveda and TCM, we are lucky that textbooks thousands of years old and professors and schools are available to educate us about these ancient practices.
The rediscovery of ancient treatments through the study of ethnobotany, Ayurveda, and TCM has been fascinating, as most of these approaches focus not just on the skin, but on treating the patient as a whole, inside and out (often depending on the discipline treating mind, body, and spirit), with the effects ultimately benefiting the skin. With the many advances in Western medicine over the past 2,000 years, starting with Hippocrates, it will be interesting to see how we, in the field of dermatology, can still learn from and potentially integrate medicine that originated 3,000-5,000 plus years ago in Ayurveda and 2,000-plus years ago in TCM that is still practiced today. In the future, we hope to have more columns about these specialties and how they are used in skin and beauty.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
The role of the skin microbiome in skin care
It may not seem intuitive, but to understand some of the new skin care claims, you need to know a bit about the gut microbiome and its role in skin health. The The gut and skin play a balancing act between beneficial, neutral, and harmful flora that are interrelated with the innate and adaptive immune systems.1 The skin and gut seem to be intertwined and express several comorbidities.2 In this column, the focus is on the cutaneous microbiome’s role in skin health. To understand the cosmeceutical claims about pre- and probiotics, you first need to familiarize yourself with skin microbiome science. The skin-gut nexus will be discussed in next month’s column, which will address the role of the skin microbiome in skin diseases.
Why is the microbiome such a hot topic?
Genetic sequencing has spurred advances in the study of the microbiome and has provided intriguing clues that the gut and skin microbiome have influences on each other. Sequencing assays that focus on bacterial 16S ribosomal RNA genes have been used by investigators to distinguish and describe the wide variety of resident and transient microorganisms on the skin and elucidate their roles in skin health and disease.1 Genomic sequencing has identified species in the skin and gut that were not found previously by cultivating microbial isolates.3,4 Advances in technologies such as whole-genome shotgun sequencing, metagenomics, and functional metabolomics will further contribute to our understanding of the effects of the skin microbiome on skin health and skin type. Of course, many supplement and cosmeceutical companies have jumped on this bandwagon prematurely and claim that their products increase “good bacteria while diminishing bad bacteria.” While there are interesting data that have emerged, we still cannot say which bacteria are “good” and ‘bad” as far as the skin is concerned – with a few exceptions that we have known all along. For example, Cutibacterium acnes and Staphylococcus aureus still remain in the undesirable category. (P. acnes has been renamed and now is officially referred to as C. acnes.) While it is premature to recommend probiotic– or prebiotic–containing cosmeceuticals, your patients will ask you about them. New studies about rosacea and the microbiome have generated a lot of patient questions in my practice, so I am writing several blogs about how to answer patient questions, which can be found at STSFranchise.com/blog. I’m also educating consumers on Facebook and Instagram @skintypesolutions so that they will not be taken advantage of by the too early “pseudoscience.” So now that you have heard that it is too early to recommend pre- and probiotic skin care to target skin issues, let’s look at the science that does exist.
Terminology
- Microbiome: Microbes that live in a particular environment or biome.
- Microbiota: The collection of living microbes that live in or on an environment. This term includes the microorganisms only and not the characteristics of their environment.
- Prebiotics: A nondigestible food ingredient that promotes the growth of microorganisms in the intestines. These can promote the growth of beneficial or harmful microorganisms. Think of them as a type of “fertilizer” for the microbiome.
- Probiotics: Living microorganisms that can provide beneficial qualities when used orally or topically. What probiotics are not? Microbes naturally found in your body and on your skin; microbes that are no longer alive; fermented foods that contain an unknown amount of bacteria.
Skin surface area
Richard Gallo, MD, a dermatologist from the University of California, San Diego, who is a leader in the microbiome field of study, says that estimates of the cutaneous microbiome’s impact on human health via skin have failed to acknowledge the inner follicular surface, thus drastically undervaluing the potential of the cutaneous microbiome to influence systemic health.5 He suggests that the surface area of skin has been miscalculated as measuring 2 m2 because it is considered a flat surface. This ignores the plethora of hair follicles and sweat ducts that significantly broaden the epithelial surface to measure closer to 25 m2 and underscores that the expansive skin microbiome is much larger than previously recognized.5 Taking the hair follicle surface area into account, the skin has vast space to harbor various organisms and microbiome environments. What our patients use on their skin certainly influences these environments. The key is trying to figure out how to manipulate the microbiome to our patient’s advantage.
Microbes have environmental preferences
Different microbial species thrive on particular regions of the diverse topography of the expansive surface area and choose their preferred environments from among sebaceous or nonsebaceous, hairy or smooth, moist or dry, and creased or noncreased areas.6,7 Other host factors that affect which microorganisms colonize the skin include hair follicle thickness, age, sex, diet (especially high fat and sugar intake), climate, occupation, and personal hygiene.7-10 Gene sequencing has revealed that these variations are partially because of factors such as ultraviolet exposure, pH, and temperature.4,6,11 For example, C. acnes has been found to be more prevalent in highly sebaceous sites on the head and upper torso.4 In general, Propionibacteriaceae (Cutibacterium) prefer sebaceous areas, whereas Corynebacteriaceae and Staphylococcaceae prevail in moist regions, such as the navel or axilla. Dry areas host the widest diversity of microbes, including Corynebacterium, Staphylococcus, and Streptococcus species.1,7,12
Impact of sebum and skin hydration on microbiome
In 2016, Mukherjee et al. measured sebum and hydration from the forehead and cheeks of 30 healthy female volunteers in a study that tested the hypothesis that differences in sebum and hydration levels in specific facial areas account for interindividual variation in facial skin microbiome. They found that the most significant predictor of microbiome composition was cheek sebum level, followed by forehead hydration level, while cheek hydration and forehead sebum levels were not predictive. The prevalence of Actinobacteria/Propionibacterium rose, while microbiome diversity diminished with an increase in cheek sebum, with such trends reversed in relation to forehead hydration. The investigators concluded that site-specific sebum and water levels impact the nature and diversity of the facial skin microbiome.13
Lability of the cutaneous microbiome
The skin microbiome changes during various times of life. For example, in puberty, more lipophilic species such as Propionibacteriaceae and Cornebacteriaceae predominate, while prior to puberty there is a preponderance of Firmicutes, Bacteroidetes, and Proteobacteria.4,14 However, in the absence of lifestyle changes, cutaneous microbial communities have been found through longitudinal studies to be relatively stable over a 2-year period.6 A person’s skin microbiome is subject to influence from an adjacent skin microbiome, such as between cohabiting couples or the influence of breastfeeding mothers.15 It is never too early to consider the role of the microbiome in health and disease. For example, infant microbiomes play a role in eczema and the atopic march.16 For this reason, those of us who treat children need to be familiar with studies that have demonstrate how the cutaneous microbiome is affected by childbirth delivery method, breastfeeding, the mother’s diet antibiotic use during pregnancy and breastfeeding.4,17
Microbiome effects on skin function
The skin barrier, a bilayer lipid-laden membrane that surrounds keratinocytes and prevents transepidermal water loss, is affected by resident microbial communities and has been shown by research to be influenced by the volume and diversity of such microbes.18 Organisms on the skin’s surface play an important role in communicating with and educating the cutaneous arm of the immune system.19 In 2017, Maguire and Maguire reviewed recent studies of the gut and skin microbiomes and suggested that Nitrobacter, Lactobacillus, and Bifidobacterium can improve skin health and could be useful bacterial adjuvants in a probiotic and prebiotic strategy in homeostatic renormalization when skin health is compromised.20Nitrobacter has displayed antifungal activity against dermatophytes and Staphylococcus; Lactobacillus has exhibited anti-inflammatory effects and was shown to improve adult acne in a small study; Bifidobacterium combined with Lactobacillus lowered the incidence of atopic eczema in early childhood; and Bifidobacterium and the prebiotic galacto-oligosaccharide prevented hydration level losses in the stratum corneum among other beneficial effects in a double-blind, placebo-controlled, randomized trial.20
Microbiome diversity is key
Microbes interact, collaborate, and oppose one another while exerting influence and being affected by the host. Effective communication among the innate and adaptive parts of the immune system, epithelial cells, and cutaneous microbiota is essential for optimal functioning of the skin.6,7 Studies on subjects with atopic dermatitis showed a strong association between decreased diversity and increased disease severity. This suggests that a diverse microbiome is associated with skin health.21 For this reason, use of pre- and probiotics for skin issues is discouraged at this time. If we replace the normal diverse flora with one organism, we do not yet know the consequences. It is much more likely that successful treatments in the future will contain a diverse group of organisms.
Cosmeceutical effects on the skin microbiome
Cleansing and use of emollients certainly affect the skin biome, but we do not yet know to what extent. A study that looked at the effects of emollients on infants with atopic dermatitis showed that the emollient group has a lower skin pH and a more diverse microbiome.22 In a 2016 study on the impact of acute treatment with topical skin cleansers on the cutaneous microbiome, investigators evaluated multiple common skin cleansers in the washing of human forearms. Group A Streptococcus growth was reduced after washing with soaps infused with such antimicrobial compounds as benzalkonium chloride or triclocarban. The researchers stipulated that much more research is necessary to ascertain the effects of chronic washing as well as the that role skin care products may play in skin homeostasis or dysbiosis in some individuals.23
In a 2017 analysis of the effects of cosmetics on the skin microbiome of facial cheeks with high- and low-hydration levels over 4 weeks, Lee et al. found that bacterial diversity was higher in the low-hydration group, with increases in both observed after the use of cosmetics. The high-hydration group showed a greater supply of Propionibacterium. Cosmetic use was found not to have caused a shift in bacterial communities in the low-hydration group.24
Conclusion
We are in the early stages as we strive to learn more about the microbiome to leverage such knowledge to improve skin health. In the meantime, there is not enough evidence to suggest the use of any oral or topical prebiotics or probiotics to improve skin health. In fact, we may be causing harm by lessening diversity. The New York Times recently published an article called “The Problem with Probiotics” that referenced a JAMA Internal Medicine article entitled “Probiotic Safety – No Guarantees.”25 I recommend that you read those. Next month, I will look more closely at microbiome research pertaining to skin disease.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
References
1. Dréno B et al. J Eur Acad Dermatol Venereol. 2016 Dec;30(12):2038-47.
2. O’Neill CA et al. Bioessays. 2016 Nov;38(11):1167-76.
3. Kong HH. Trends Mol Med. 2011 Jun;17(6):320-8.
4. Kong HH et al. J Invest Dermatol. 2017 May;137(5):e119-22.
5. Gallo RL. J Invest Dermatol. 2017 Jun;137(6):1213-4.
6. Byrd AL et al. Nat Rev Microbiol. 2018 Mar;16(3):143-55.
7. Grice EA et al. Nat Rev Microbiol. 2011 Apr;9(4):244-53.
8. Rodrigues Hoffmann A. Vet Dermatol. 2017 Feb;28(1):60-e15.
9. Moestrup KS et al. J Invest Dermatol. 2018 May;138(5):1225-8.
10. Prescott SL et al. World Allergy Organ J. 2017 Aug 22;10(1):29.
11. Costello EK et al. Science. 2009 Dec 18;326(5960):1694-7.
12. Zeeuwen PL et al. Genome Biol. 2012 Nov 15;13(11):R101.
13. Mukherjee S et al. Sci Rep. 2016 Oct 27;6:36062.
14. Oh J et al. Genome Med. 2012 Oct 10;4(10):77.
15. Ross AA et al. mSystems. 2017 Jul 20;2(4).
16. Blázquez AB et al. Transl Res. 2017 Jan;179:199-203.
17. Rock R et al. Open Forum Infect Dis. 2017 Oct;4(1):S232.
18. Baldwin HE et al. J Drugs Dermatol. 2017 Jan 1;16(1):12-8.
19. Byrd AL et al. Nat Rev Microbiol. 2018 Mar;16(3):143-55.
20. Maguire M et al. Arch Dermatol Res. 2017 Aug;309(6):411-21.
21. Kong HH et al. Genome Res. 2012 May;22(5):850-9.
22. Glatz M et al. PLoS One. 2018 Feb 28;13(2):e0192443.
23. Two AM et al. J Invest Dermatol. 2016 Oct;136(10):1950-4.
24. Lee HJ et al. MicrobiologyOpen. 2018 Apr;7(2):e00557. doi: 10.1002/mbo3.557.
25. Cohen PA. JAMA Intern Med. 2018 Sep 17. doi: 10.1001/jamainternmed.2018.5403.
It may not seem intuitive, but to understand some of the new skin care claims, you need to know a bit about the gut microbiome and its role in skin health. The The gut and skin play a balancing act between beneficial, neutral, and harmful flora that are interrelated with the innate and adaptive immune systems.1 The skin and gut seem to be intertwined and express several comorbidities.2 In this column, the focus is on the cutaneous microbiome’s role in skin health. To understand the cosmeceutical claims about pre- and probiotics, you first need to familiarize yourself with skin microbiome science. The skin-gut nexus will be discussed in next month’s column, which will address the role of the skin microbiome in skin diseases.
Why is the microbiome such a hot topic?
Genetic sequencing has spurred advances in the study of the microbiome and has provided intriguing clues that the gut and skin microbiome have influences on each other. Sequencing assays that focus on bacterial 16S ribosomal RNA genes have been used by investigators to distinguish and describe the wide variety of resident and transient microorganisms on the skin and elucidate their roles in skin health and disease.1 Genomic sequencing has identified species in the skin and gut that were not found previously by cultivating microbial isolates.3,4 Advances in technologies such as whole-genome shotgun sequencing, metagenomics, and functional metabolomics will further contribute to our understanding of the effects of the skin microbiome on skin health and skin type. Of course, many supplement and cosmeceutical companies have jumped on this bandwagon prematurely and claim that their products increase “good bacteria while diminishing bad bacteria.” While there are interesting data that have emerged, we still cannot say which bacteria are “good” and ‘bad” as far as the skin is concerned – with a few exceptions that we have known all along. For example, Cutibacterium acnes and Staphylococcus aureus still remain in the undesirable category. (P. acnes has been renamed and now is officially referred to as C. acnes.) While it is premature to recommend probiotic– or prebiotic–containing cosmeceuticals, your patients will ask you about them. New studies about rosacea and the microbiome have generated a lot of patient questions in my practice, so I am writing several blogs about how to answer patient questions, which can be found at STSFranchise.com/blog. I’m also educating consumers on Facebook and Instagram @skintypesolutions so that they will not be taken advantage of by the too early “pseudoscience.” So now that you have heard that it is too early to recommend pre- and probiotic skin care to target skin issues, let’s look at the science that does exist.
Terminology
- Microbiome: Microbes that live in a particular environment or biome.
- Microbiota: The collection of living microbes that live in or on an environment. This term includes the microorganisms only and not the characteristics of their environment.
- Prebiotics: A nondigestible food ingredient that promotes the growth of microorganisms in the intestines. These can promote the growth of beneficial or harmful microorganisms. Think of them as a type of “fertilizer” for the microbiome.
- Probiotics: Living microorganisms that can provide beneficial qualities when used orally or topically. What probiotics are not? Microbes naturally found in your body and on your skin; microbes that are no longer alive; fermented foods that contain an unknown amount of bacteria.
Skin surface area
Richard Gallo, MD, a dermatologist from the University of California, San Diego, who is a leader in the microbiome field of study, says that estimates of the cutaneous microbiome’s impact on human health via skin have failed to acknowledge the inner follicular surface, thus drastically undervaluing the potential of the cutaneous microbiome to influence systemic health.5 He suggests that the surface area of skin has been miscalculated as measuring 2 m2 because it is considered a flat surface. This ignores the plethora of hair follicles and sweat ducts that significantly broaden the epithelial surface to measure closer to 25 m2 and underscores that the expansive skin microbiome is much larger than previously recognized.5 Taking the hair follicle surface area into account, the skin has vast space to harbor various organisms and microbiome environments. What our patients use on their skin certainly influences these environments. The key is trying to figure out how to manipulate the microbiome to our patient’s advantage.
Microbes have environmental preferences
Different microbial species thrive on particular regions of the diverse topography of the expansive surface area and choose their preferred environments from among sebaceous or nonsebaceous, hairy or smooth, moist or dry, and creased or noncreased areas.6,7 Other host factors that affect which microorganisms colonize the skin include hair follicle thickness, age, sex, diet (especially high fat and sugar intake), climate, occupation, and personal hygiene.7-10 Gene sequencing has revealed that these variations are partially because of factors such as ultraviolet exposure, pH, and temperature.4,6,11 For example, C. acnes has been found to be more prevalent in highly sebaceous sites on the head and upper torso.4 In general, Propionibacteriaceae (Cutibacterium) prefer sebaceous areas, whereas Corynebacteriaceae and Staphylococcaceae prevail in moist regions, such as the navel or axilla. Dry areas host the widest diversity of microbes, including Corynebacterium, Staphylococcus, and Streptococcus species.1,7,12
Impact of sebum and skin hydration on microbiome
In 2016, Mukherjee et al. measured sebum and hydration from the forehead and cheeks of 30 healthy female volunteers in a study that tested the hypothesis that differences in sebum and hydration levels in specific facial areas account for interindividual variation in facial skin microbiome. They found that the most significant predictor of microbiome composition was cheek sebum level, followed by forehead hydration level, while cheek hydration and forehead sebum levels were not predictive. The prevalence of Actinobacteria/Propionibacterium rose, while microbiome diversity diminished with an increase in cheek sebum, with such trends reversed in relation to forehead hydration. The investigators concluded that site-specific sebum and water levels impact the nature and diversity of the facial skin microbiome.13
Lability of the cutaneous microbiome
The skin microbiome changes during various times of life. For example, in puberty, more lipophilic species such as Propionibacteriaceae and Cornebacteriaceae predominate, while prior to puberty there is a preponderance of Firmicutes, Bacteroidetes, and Proteobacteria.4,14 However, in the absence of lifestyle changes, cutaneous microbial communities have been found through longitudinal studies to be relatively stable over a 2-year period.6 A person’s skin microbiome is subject to influence from an adjacent skin microbiome, such as between cohabiting couples or the influence of breastfeeding mothers.15 It is never too early to consider the role of the microbiome in health and disease. For example, infant microbiomes play a role in eczema and the atopic march.16 For this reason, those of us who treat children need to be familiar with studies that have demonstrate how the cutaneous microbiome is affected by childbirth delivery method, breastfeeding, the mother’s diet antibiotic use during pregnancy and breastfeeding.4,17
Microbiome effects on skin function
The skin barrier, a bilayer lipid-laden membrane that surrounds keratinocytes and prevents transepidermal water loss, is affected by resident microbial communities and has been shown by research to be influenced by the volume and diversity of such microbes.18 Organisms on the skin’s surface play an important role in communicating with and educating the cutaneous arm of the immune system.19 In 2017, Maguire and Maguire reviewed recent studies of the gut and skin microbiomes and suggested that Nitrobacter, Lactobacillus, and Bifidobacterium can improve skin health and could be useful bacterial adjuvants in a probiotic and prebiotic strategy in homeostatic renormalization when skin health is compromised.20Nitrobacter has displayed antifungal activity against dermatophytes and Staphylococcus; Lactobacillus has exhibited anti-inflammatory effects and was shown to improve adult acne in a small study; Bifidobacterium combined with Lactobacillus lowered the incidence of atopic eczema in early childhood; and Bifidobacterium and the prebiotic galacto-oligosaccharide prevented hydration level losses in the stratum corneum among other beneficial effects in a double-blind, placebo-controlled, randomized trial.20
Microbiome diversity is key
Microbes interact, collaborate, and oppose one another while exerting influence and being affected by the host. Effective communication among the innate and adaptive parts of the immune system, epithelial cells, and cutaneous microbiota is essential for optimal functioning of the skin.6,7 Studies on subjects with atopic dermatitis showed a strong association between decreased diversity and increased disease severity. This suggests that a diverse microbiome is associated with skin health.21 For this reason, use of pre- and probiotics for skin issues is discouraged at this time. If we replace the normal diverse flora with one organism, we do not yet know the consequences. It is much more likely that successful treatments in the future will contain a diverse group of organisms.
Cosmeceutical effects on the skin microbiome
Cleansing and use of emollients certainly affect the skin biome, but we do not yet know to what extent. A study that looked at the effects of emollients on infants with atopic dermatitis showed that the emollient group has a lower skin pH and a more diverse microbiome.22 In a 2016 study on the impact of acute treatment with topical skin cleansers on the cutaneous microbiome, investigators evaluated multiple common skin cleansers in the washing of human forearms. Group A Streptococcus growth was reduced after washing with soaps infused with such antimicrobial compounds as benzalkonium chloride or triclocarban. The researchers stipulated that much more research is necessary to ascertain the effects of chronic washing as well as the that role skin care products may play in skin homeostasis or dysbiosis in some individuals.23
In a 2017 analysis of the effects of cosmetics on the skin microbiome of facial cheeks with high- and low-hydration levels over 4 weeks, Lee et al. found that bacterial diversity was higher in the low-hydration group, with increases in both observed after the use of cosmetics. The high-hydration group showed a greater supply of Propionibacterium. Cosmetic use was found not to have caused a shift in bacterial communities in the low-hydration group.24
Conclusion
We are in the early stages as we strive to learn more about the microbiome to leverage such knowledge to improve skin health. In the meantime, there is not enough evidence to suggest the use of any oral or topical prebiotics or probiotics to improve skin health. In fact, we may be causing harm by lessening diversity. The New York Times recently published an article called “The Problem with Probiotics” that referenced a JAMA Internal Medicine article entitled “Probiotic Safety – No Guarantees.”25 I recommend that you read those. Next month, I will look more closely at microbiome research pertaining to skin disease.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
References
1. Dréno B et al. J Eur Acad Dermatol Venereol. 2016 Dec;30(12):2038-47.
2. O’Neill CA et al. Bioessays. 2016 Nov;38(11):1167-76.
3. Kong HH. Trends Mol Med. 2011 Jun;17(6):320-8.
4. Kong HH et al. J Invest Dermatol. 2017 May;137(5):e119-22.
5. Gallo RL. J Invest Dermatol. 2017 Jun;137(6):1213-4.
6. Byrd AL et al. Nat Rev Microbiol. 2018 Mar;16(3):143-55.
7. Grice EA et al. Nat Rev Microbiol. 2011 Apr;9(4):244-53.
8. Rodrigues Hoffmann A. Vet Dermatol. 2017 Feb;28(1):60-e15.
9. Moestrup KS et al. J Invest Dermatol. 2018 May;138(5):1225-8.
10. Prescott SL et al. World Allergy Organ J. 2017 Aug 22;10(1):29.
11. Costello EK et al. Science. 2009 Dec 18;326(5960):1694-7.
12. Zeeuwen PL et al. Genome Biol. 2012 Nov 15;13(11):R101.
13. Mukherjee S et al. Sci Rep. 2016 Oct 27;6:36062.
14. Oh J et al. Genome Med. 2012 Oct 10;4(10):77.
15. Ross AA et al. mSystems. 2017 Jul 20;2(4).
16. Blázquez AB et al. Transl Res. 2017 Jan;179:199-203.
17. Rock R et al. Open Forum Infect Dis. 2017 Oct;4(1):S232.
18. Baldwin HE et al. J Drugs Dermatol. 2017 Jan 1;16(1):12-8.
19. Byrd AL et al. Nat Rev Microbiol. 2018 Mar;16(3):143-55.
20. Maguire M et al. Arch Dermatol Res. 2017 Aug;309(6):411-21.
21. Kong HH et al. Genome Res. 2012 May;22(5):850-9.
22. Glatz M et al. PLoS One. 2018 Feb 28;13(2):e0192443.
23. Two AM et al. J Invest Dermatol. 2016 Oct;136(10):1950-4.
24. Lee HJ et al. MicrobiologyOpen. 2018 Apr;7(2):e00557. doi: 10.1002/mbo3.557.
25. Cohen PA. JAMA Intern Med. 2018 Sep 17. doi: 10.1001/jamainternmed.2018.5403.
It may not seem intuitive, but to understand some of the new skin care claims, you need to know a bit about the gut microbiome and its role in skin health. The The gut and skin play a balancing act between beneficial, neutral, and harmful flora that are interrelated with the innate and adaptive immune systems.1 The skin and gut seem to be intertwined and express several comorbidities.2 In this column, the focus is on the cutaneous microbiome’s role in skin health. To understand the cosmeceutical claims about pre- and probiotics, you first need to familiarize yourself with skin microbiome science. The skin-gut nexus will be discussed in next month’s column, which will address the role of the skin microbiome in skin diseases.
Why is the microbiome such a hot topic?
Genetic sequencing has spurred advances in the study of the microbiome and has provided intriguing clues that the gut and skin microbiome have influences on each other. Sequencing assays that focus on bacterial 16S ribosomal RNA genes have been used by investigators to distinguish and describe the wide variety of resident and transient microorganisms on the skin and elucidate their roles in skin health and disease.1 Genomic sequencing has identified species in the skin and gut that were not found previously by cultivating microbial isolates.3,4 Advances in technologies such as whole-genome shotgun sequencing, metagenomics, and functional metabolomics will further contribute to our understanding of the effects of the skin microbiome on skin health and skin type. Of course, many supplement and cosmeceutical companies have jumped on this bandwagon prematurely and claim that their products increase “good bacteria while diminishing bad bacteria.” While there are interesting data that have emerged, we still cannot say which bacteria are “good” and ‘bad” as far as the skin is concerned – with a few exceptions that we have known all along. For example, Cutibacterium acnes and Staphylococcus aureus still remain in the undesirable category. (P. acnes has been renamed and now is officially referred to as C. acnes.) While it is premature to recommend probiotic– or prebiotic–containing cosmeceuticals, your patients will ask you about them. New studies about rosacea and the microbiome have generated a lot of patient questions in my practice, so I am writing several blogs about how to answer patient questions, which can be found at STSFranchise.com/blog. I’m also educating consumers on Facebook and Instagram @skintypesolutions so that they will not be taken advantage of by the too early “pseudoscience.” So now that you have heard that it is too early to recommend pre- and probiotic skin care to target skin issues, let’s look at the science that does exist.
Terminology
- Microbiome: Microbes that live in a particular environment or biome.
- Microbiota: The collection of living microbes that live in or on an environment. This term includes the microorganisms only and not the characteristics of their environment.
- Prebiotics: A nondigestible food ingredient that promotes the growth of microorganisms in the intestines. These can promote the growth of beneficial or harmful microorganisms. Think of them as a type of “fertilizer” for the microbiome.
- Probiotics: Living microorganisms that can provide beneficial qualities when used orally or topically. What probiotics are not? Microbes naturally found in your body and on your skin; microbes that are no longer alive; fermented foods that contain an unknown amount of bacteria.
Skin surface area
Richard Gallo, MD, a dermatologist from the University of California, San Diego, who is a leader in the microbiome field of study, says that estimates of the cutaneous microbiome’s impact on human health via skin have failed to acknowledge the inner follicular surface, thus drastically undervaluing the potential of the cutaneous microbiome to influence systemic health.5 He suggests that the surface area of skin has been miscalculated as measuring 2 m2 because it is considered a flat surface. This ignores the plethora of hair follicles and sweat ducts that significantly broaden the epithelial surface to measure closer to 25 m2 and underscores that the expansive skin microbiome is much larger than previously recognized.5 Taking the hair follicle surface area into account, the skin has vast space to harbor various organisms and microbiome environments. What our patients use on their skin certainly influences these environments. The key is trying to figure out how to manipulate the microbiome to our patient’s advantage.
Microbes have environmental preferences
Different microbial species thrive on particular regions of the diverse topography of the expansive surface area and choose their preferred environments from among sebaceous or nonsebaceous, hairy or smooth, moist or dry, and creased or noncreased areas.6,7 Other host factors that affect which microorganisms colonize the skin include hair follicle thickness, age, sex, diet (especially high fat and sugar intake), climate, occupation, and personal hygiene.7-10 Gene sequencing has revealed that these variations are partially because of factors such as ultraviolet exposure, pH, and temperature.4,6,11 For example, C. acnes has been found to be more prevalent in highly sebaceous sites on the head and upper torso.4 In general, Propionibacteriaceae (Cutibacterium) prefer sebaceous areas, whereas Corynebacteriaceae and Staphylococcaceae prevail in moist regions, such as the navel or axilla. Dry areas host the widest diversity of microbes, including Corynebacterium, Staphylococcus, and Streptococcus species.1,7,12
Impact of sebum and skin hydration on microbiome
In 2016, Mukherjee et al. measured sebum and hydration from the forehead and cheeks of 30 healthy female volunteers in a study that tested the hypothesis that differences in sebum and hydration levels in specific facial areas account for interindividual variation in facial skin microbiome. They found that the most significant predictor of microbiome composition was cheek sebum level, followed by forehead hydration level, while cheek hydration and forehead sebum levels were not predictive. The prevalence of Actinobacteria/Propionibacterium rose, while microbiome diversity diminished with an increase in cheek sebum, with such trends reversed in relation to forehead hydration. The investigators concluded that site-specific sebum and water levels impact the nature and diversity of the facial skin microbiome.13
Lability of the cutaneous microbiome
The skin microbiome changes during various times of life. For example, in puberty, more lipophilic species such as Propionibacteriaceae and Cornebacteriaceae predominate, while prior to puberty there is a preponderance of Firmicutes, Bacteroidetes, and Proteobacteria.4,14 However, in the absence of lifestyle changes, cutaneous microbial communities have been found through longitudinal studies to be relatively stable over a 2-year period.6 A person’s skin microbiome is subject to influence from an adjacent skin microbiome, such as between cohabiting couples or the influence of breastfeeding mothers.15 It is never too early to consider the role of the microbiome in health and disease. For example, infant microbiomes play a role in eczema and the atopic march.16 For this reason, those of us who treat children need to be familiar with studies that have demonstrate how the cutaneous microbiome is affected by childbirth delivery method, breastfeeding, the mother’s diet antibiotic use during pregnancy and breastfeeding.4,17
Microbiome effects on skin function
The skin barrier, a bilayer lipid-laden membrane that surrounds keratinocytes and prevents transepidermal water loss, is affected by resident microbial communities and has been shown by research to be influenced by the volume and diversity of such microbes.18 Organisms on the skin’s surface play an important role in communicating with and educating the cutaneous arm of the immune system.19 In 2017, Maguire and Maguire reviewed recent studies of the gut and skin microbiomes and suggested that Nitrobacter, Lactobacillus, and Bifidobacterium can improve skin health and could be useful bacterial adjuvants in a probiotic and prebiotic strategy in homeostatic renormalization when skin health is compromised.20Nitrobacter has displayed antifungal activity against dermatophytes and Staphylococcus; Lactobacillus has exhibited anti-inflammatory effects and was shown to improve adult acne in a small study; Bifidobacterium combined with Lactobacillus lowered the incidence of atopic eczema in early childhood; and Bifidobacterium and the prebiotic galacto-oligosaccharide prevented hydration level losses in the stratum corneum among other beneficial effects in a double-blind, placebo-controlled, randomized trial.20
Microbiome diversity is key
Microbes interact, collaborate, and oppose one another while exerting influence and being affected by the host. Effective communication among the innate and adaptive parts of the immune system, epithelial cells, and cutaneous microbiota is essential for optimal functioning of the skin.6,7 Studies on subjects with atopic dermatitis showed a strong association between decreased diversity and increased disease severity. This suggests that a diverse microbiome is associated with skin health.21 For this reason, use of pre- and probiotics for skin issues is discouraged at this time. If we replace the normal diverse flora with one organism, we do not yet know the consequences. It is much more likely that successful treatments in the future will contain a diverse group of organisms.
Cosmeceutical effects on the skin microbiome
Cleansing and use of emollients certainly affect the skin biome, but we do not yet know to what extent. A study that looked at the effects of emollients on infants with atopic dermatitis showed that the emollient group has a lower skin pH and a more diverse microbiome.22 In a 2016 study on the impact of acute treatment with topical skin cleansers on the cutaneous microbiome, investigators evaluated multiple common skin cleansers in the washing of human forearms. Group A Streptococcus growth was reduced after washing with soaps infused with such antimicrobial compounds as benzalkonium chloride or triclocarban. The researchers stipulated that much more research is necessary to ascertain the effects of chronic washing as well as the that role skin care products may play in skin homeostasis or dysbiosis in some individuals.23
In a 2017 analysis of the effects of cosmetics on the skin microbiome of facial cheeks with high- and low-hydration levels over 4 weeks, Lee et al. found that bacterial diversity was higher in the low-hydration group, with increases in both observed after the use of cosmetics. The high-hydration group showed a greater supply of Propionibacterium. Cosmetic use was found not to have caused a shift in bacterial communities in the low-hydration group.24
Conclusion
We are in the early stages as we strive to learn more about the microbiome to leverage such knowledge to improve skin health. In the meantime, there is not enough evidence to suggest the use of any oral or topical prebiotics or probiotics to improve skin health. In fact, we may be causing harm by lessening diversity. The New York Times recently published an article called “The Problem with Probiotics” that referenced a JAMA Internal Medicine article entitled “Probiotic Safety – No Guarantees.”25 I recommend that you read those. Next month, I will look more closely at microbiome research pertaining to skin disease.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
References
1. Dréno B et al. J Eur Acad Dermatol Venereol. 2016 Dec;30(12):2038-47.
2. O’Neill CA et al. Bioessays. 2016 Nov;38(11):1167-76.
3. Kong HH. Trends Mol Med. 2011 Jun;17(6):320-8.
4. Kong HH et al. J Invest Dermatol. 2017 May;137(5):e119-22.
5. Gallo RL. J Invest Dermatol. 2017 Jun;137(6):1213-4.
6. Byrd AL et al. Nat Rev Microbiol. 2018 Mar;16(3):143-55.
7. Grice EA et al. Nat Rev Microbiol. 2011 Apr;9(4):244-53.
8. Rodrigues Hoffmann A. Vet Dermatol. 2017 Feb;28(1):60-e15.
9. Moestrup KS et al. J Invest Dermatol. 2018 May;138(5):1225-8.
10. Prescott SL et al. World Allergy Organ J. 2017 Aug 22;10(1):29.
11. Costello EK et al. Science. 2009 Dec 18;326(5960):1694-7.
12. Zeeuwen PL et al. Genome Biol. 2012 Nov 15;13(11):R101.
13. Mukherjee S et al. Sci Rep. 2016 Oct 27;6:36062.
14. Oh J et al. Genome Med. 2012 Oct 10;4(10):77.
15. Ross AA et al. mSystems. 2017 Jul 20;2(4).
16. Blázquez AB et al. Transl Res. 2017 Jan;179:199-203.
17. Rock R et al. Open Forum Infect Dis. 2017 Oct;4(1):S232.
18. Baldwin HE et al. J Drugs Dermatol. 2017 Jan 1;16(1):12-8.
19. Byrd AL et al. Nat Rev Microbiol. 2018 Mar;16(3):143-55.
20. Maguire M et al. Arch Dermatol Res. 2017 Aug;309(6):411-21.
21. Kong HH et al. Genome Res. 2012 May;22(5):850-9.
22. Glatz M et al. PLoS One. 2018 Feb 28;13(2):e0192443.
23. Two AM et al. J Invest Dermatol. 2016 Oct;136(10):1950-4.
24. Lee HJ et al. MicrobiologyOpen. 2018 Apr;7(2):e00557. doi: 10.1002/mbo3.557.
25. Cohen PA. JAMA Intern Med. 2018 Sep 17. doi: 10.1001/jamainternmed.2018.5403.
Facial exercises hastened the effects of botulinum toxin in small study
Performing of the injections by 1 day, a small, randomized study has shown.
The study addressed the lack of data regarding whether exercising treated muscles for several hours after injections helped “to enhance uptake” of botulinum toxin, said Murad Alam, MD, chief of cutaneous and aesthetic surgery and a professor of dermatology at Northwestern University, Chicago, and his coauthors.
“The results of this study suggest that a postinjection facial exercise regimen is a safe and effective method for achieving an earlier onset of clinical effect of botulinum toxin injections,” he and his coauthors concluded. The results were reported in the Journal of the American Academy of Dermatology.
The study enrolled 22 women, aged 27-66 years (mean age, 47 years) who received botulinum toxin injections for forehead and glabella dynamic rhytids. Following the injections, half of the women did the exercises for 4 hours and the other half avoided facial contractions for 4 hours. Exercises included raised motions of the forehead and scowls – such as knitting the brows – in three sets of 40 repetitions separated by 10 minutes, according to a Northwestern University press release. At 7 months, the women came back for treatment, and switched groups.
Two blinded dermatologists rated photos of forehead and glabella dynamic creases at baseline and on days 1,2,3,4,7, and 14 with the 5-point Carruthers’ Forehead Lines Grading Scale and the 4-point Gladys study group rating scale for glabellar frown lines. The women also assessed their own dynamic creases using a 7-point Subject Self-Evaluation Improvement Scale.
By day 3, ratings by dermatologists and patients of glabellar and forehead wrinkles were statistically significantly better for patients who had performed the exercises after the injections. When facial exercises followed the injections, women said they saw noticeable glabellar improvement by day 2 or 3, compared with day 3 or 4 among those who did not do facial exercises after the injections (P = .02).
“A significant advantage in the exercise group was detectable as early as day 3, at which point patients’ self-evaluation wrinkle scores increased by approximately twice as much in exercisers compared to non-exercisers,” the study authors noted.
But by 2 weeks, the effects of treatment were similar in both groups, and the effects of treatment lasted for a similar period of time with or without exercises.
“Expediting the time to noticeable benefit, even by one day, may be clinically significant for some patients,” the authors wrote. Exercises could be recommended only to those patients who need faster results “to avoid needless inconvenience,” they added.
The study was supported by research funds from the department of dermatology at Northwestern University. The authors had no relevant disclosures.
SOURCE: Alam M et al. J Am Acad Dermatol. 2018. doi: 10.1016/j.jaad.2018.10.013.
Performing of the injections by 1 day, a small, randomized study has shown.
The study addressed the lack of data regarding whether exercising treated muscles for several hours after injections helped “to enhance uptake” of botulinum toxin, said Murad Alam, MD, chief of cutaneous and aesthetic surgery and a professor of dermatology at Northwestern University, Chicago, and his coauthors.
“The results of this study suggest that a postinjection facial exercise regimen is a safe and effective method for achieving an earlier onset of clinical effect of botulinum toxin injections,” he and his coauthors concluded. The results were reported in the Journal of the American Academy of Dermatology.
The study enrolled 22 women, aged 27-66 years (mean age, 47 years) who received botulinum toxin injections for forehead and glabella dynamic rhytids. Following the injections, half of the women did the exercises for 4 hours and the other half avoided facial contractions for 4 hours. Exercises included raised motions of the forehead and scowls – such as knitting the brows – in three sets of 40 repetitions separated by 10 minutes, according to a Northwestern University press release. At 7 months, the women came back for treatment, and switched groups.
Two blinded dermatologists rated photos of forehead and glabella dynamic creases at baseline and on days 1,2,3,4,7, and 14 with the 5-point Carruthers’ Forehead Lines Grading Scale and the 4-point Gladys study group rating scale for glabellar frown lines. The women also assessed their own dynamic creases using a 7-point Subject Self-Evaluation Improvement Scale.
By day 3, ratings by dermatologists and patients of glabellar and forehead wrinkles were statistically significantly better for patients who had performed the exercises after the injections. When facial exercises followed the injections, women said they saw noticeable glabellar improvement by day 2 or 3, compared with day 3 or 4 among those who did not do facial exercises after the injections (P = .02).
“A significant advantage in the exercise group was detectable as early as day 3, at which point patients’ self-evaluation wrinkle scores increased by approximately twice as much in exercisers compared to non-exercisers,” the study authors noted.
But by 2 weeks, the effects of treatment were similar in both groups, and the effects of treatment lasted for a similar period of time with or without exercises.
“Expediting the time to noticeable benefit, even by one day, may be clinically significant for some patients,” the authors wrote. Exercises could be recommended only to those patients who need faster results “to avoid needless inconvenience,” they added.
The study was supported by research funds from the department of dermatology at Northwestern University. The authors had no relevant disclosures.
SOURCE: Alam M et al. J Am Acad Dermatol. 2018. doi: 10.1016/j.jaad.2018.10.013.
Performing of the injections by 1 day, a small, randomized study has shown.
The study addressed the lack of data regarding whether exercising treated muscles for several hours after injections helped “to enhance uptake” of botulinum toxin, said Murad Alam, MD, chief of cutaneous and aesthetic surgery and a professor of dermatology at Northwestern University, Chicago, and his coauthors.
“The results of this study suggest that a postinjection facial exercise regimen is a safe and effective method for achieving an earlier onset of clinical effect of botulinum toxin injections,” he and his coauthors concluded. The results were reported in the Journal of the American Academy of Dermatology.
The study enrolled 22 women, aged 27-66 years (mean age, 47 years) who received botulinum toxin injections for forehead and glabella dynamic rhytids. Following the injections, half of the women did the exercises for 4 hours and the other half avoided facial contractions for 4 hours. Exercises included raised motions of the forehead and scowls – such as knitting the brows – in three sets of 40 repetitions separated by 10 minutes, according to a Northwestern University press release. At 7 months, the women came back for treatment, and switched groups.
Two blinded dermatologists rated photos of forehead and glabella dynamic creases at baseline and on days 1,2,3,4,7, and 14 with the 5-point Carruthers’ Forehead Lines Grading Scale and the 4-point Gladys study group rating scale for glabellar frown lines. The women also assessed their own dynamic creases using a 7-point Subject Self-Evaluation Improvement Scale.
By day 3, ratings by dermatologists and patients of glabellar and forehead wrinkles were statistically significantly better for patients who had performed the exercises after the injections. When facial exercises followed the injections, women said they saw noticeable glabellar improvement by day 2 or 3, compared with day 3 or 4 among those who did not do facial exercises after the injections (P = .02).
“A significant advantage in the exercise group was detectable as early as day 3, at which point patients’ self-evaluation wrinkle scores increased by approximately twice as much in exercisers compared to non-exercisers,” the study authors noted.
But by 2 weeks, the effects of treatment were similar in both groups, and the effects of treatment lasted for a similar period of time with or without exercises.
“Expediting the time to noticeable benefit, even by one day, may be clinically significant for some patients,” the authors wrote. Exercises could be recommended only to those patients who need faster results “to avoid needless inconvenience,” they added.
The study was supported by research funds from the department of dermatology at Northwestern University. The authors had no relevant disclosures.
SOURCE: Alam M et al. J Am Acad Dermatol. 2018. doi: 10.1016/j.jaad.2018.10.013.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Recommending facial muscle exercises after botulinum toxin injections to the forehead is now evidence based.
Major finding: Posttreatment facial exercise after botulinum toxin injections reduced the appearance of forehead wrinkles one day earlier.
Study details: A randomized, crossover clinical trial of 22 women treated with botulinum toxin for dynamic rhytids of the forehead and glabella.
Disclosures: The study was supported by research funds from the department of dermatology at Northwestern University. The authors had no relevant disclosures.
Source: Alam M et al. J Am Acad Dermatol. 2018. doi: 10.1016/j.jaad.2018.10.013.
Taurine
Taurine, also known as 2-aminoethanesulfonic acid, is a naturally occurring beta-amino acid (which has a sulphonic acid group instead of carboxylic acid, differentiating it from other amino acids) yielded by methionine and cysteine metabolism in the liver.1,2 An important free beta-amino acid in mammals, it is often the free amino acid present in the greatest concentrations in several cell types in humans.1,2 Dietary intake of taurine also plays an important role in maintaining the body’s taurine levels because of mammals’ limited ability to synthesize it.1
Notably in terms of dermatologic treatment options, the combination product taurine bromamine is known to impart antioxidant, anti-inflammatory, and antibacterial activities.3 And taurine itself is associated with antioxidant, anti-inflammatory, antifibrotic, and immunomodulatory characteristics,1,4 and is noted for conferring antiaging benefits.5
Acne and other inflammatory conditions
The use of .6,7
In response to the problem of evolving antibiotic resistance, Marcinkiewicz reported in 2009 on the then-new therapeutic option of topical taurine bromamine for the treatment of inflammatory skin disorders such as acne. The author pointed out that Propionibacterium acnes is particularly sensitive to taurine bromamine, with the substance now known to suppress H2O2 production by activated neutrophils, likely contributing to moderating the severity and lowering the number of inflammatory acne lesions. In a 6-week double-blind pilot clinical study, Marcinkiewicz and his team compared the efficacy of 0.5% taurine bromamine cream with 1% clindamycin gel in 40 patients with mild to moderate acne. Treatments, which were randomly assigned, occurred twice daily through the study. Amelioration of acne symptoms was comparable in the two groups, with more than 90% of patients improving clinically and experiencing similar decreases in acne lesions (65% in the taurine bromamine group and 68% in the clindamycin group). Marcinkiewicz concluded that these results indicate the viability of taurine bromamine as an option for inflammatory acne therapy, particularly for patients who have shown antibiotic resistance.3
Wide-ranging protection potential
In 2003, Janeke et al. conducted analyses that showed that taurine accumulation defended cultured human keratinocytes from osmotically- and UV-induced apoptosis, suggesting the importance of taurine as an epidermal osmolyte necessary for maintaining keratinocyte hydration in a dry environment.2
Three years later, Collin et al. demonstrated the dynamic protective effects of taurine on the human hair follicle in an in vitro study in which taurine promoted hair survival and protected against TGF-beta1-induced damage.1
Taurine has also been found to stabilize and protect the catalytic activity of the hemoprotein cytochrome P450 3A4, which is a key enzyme responsible for metabolizing various endogenous as well as foreign substances, including drugs.8
Penetration enhancement
In 2016, Mueller et al. studied the effects of urea and taurine as hydrophilic penetration enhancers on stratum corneum lipid models as both substances are known to exert such effects. With inconclusive results as to the roots of such activity, they speculated that both entities enhance penetration through the introduction of copious water into the corneocytes, resulting from the robust water-binding capacity of urea and the consequent osmotic pressure related to taurine.9
Possible skin whitening and anti-aging roles and other promising lab results
Based on their previous work demonstrating that azelaic acid, a saturated dicarboxylic acid found naturally in wheat, rye, and barley, suppressed melanogenesis, Yu and Kim investigated the antimelanogenic activity of azelaic acid and taurine in B16F10 mouse melanoma cells in 2010. They found that the combination of the two substances exhibited a greater inhibitory effect in melanocytes than azelaic acid alone, with melanin production and tyrosinase activity suppressed without inducing cytotoxicity. The investigators concluded the combination of azelaic acid and taurine may be an effective approach for treating hyperpigmentation.10
In 2015, Ito et al. investigated the possible anti-aging role of taurine using a taurine transporter knockout mouse model. They noted that aging-related disorders affecting the skin, heart, skeletal muscle, and liver and resulting in a shorter lifespan have been correlated with tissue taurine depletion. The researchers proposed that proper protein folding allows endogenous taurine to perform as an antiaging molecule.5
Also in 2015, Kim et al. investigated potential mechanisms of the antiproliferative activity of taurine on murine B16F10 melanoma cells via the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and neutral red assays and microscopic analysis. They found that taurine prevented cell proliferation and engendered apoptosis in B16F10 cells, concluding that taurine may have a role to play as a chemotherapeutic agent for skin cancer.11
In 2014, Ashkani-Esfahani et al. studied the impact of taurine on cutaneous leishmaniasis wounds in a mouse model. Investigators induced 18 mice with wounds using L. major promastigotes, and divided them into a taurine injection group, taurine gel group, and no treatment group, performing treatments every 24 hours over 21 days. The taurine treatment groups exhibited significantly greater numerical fibroblast density, collagen bundle volume density, and vessel length densities compared with the nontreatment group. The taurine injection group displayed higher fibroblast numerical density than did the taurine gel group. The researchers concluded that taurine has the capacity to enhance wound healing and tissue regeneration but showed no direct anti-leishmaniasis effect.4
Conclusion
Taurine has been found over the last few decades to impart salutary effects for human health. This beta-amino acid that occurs naturally in humans and other mammals also appears to hold promising potential in the dermatologic realm, particularly for its anti-inflammatory and antioxidant effects. More research is needed to ascertain just how pivotal this compound can be for skin health.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Int J Cosmet Sci. 2006 Aug;28(4):289-98.
2. J Invest Dermatol. 2003 Aug;121(2):354-61.
3. Pol Arch Med Wewn. 2009 Oct;119(10):673-6.
4. Adv Biomed Res. 2014 Oct 7;3:204.
5. Adv Exp Med Biol. 2015;803:481-7.
6. Am J Clin Dermatol. 2012 Dec 1;13(6):357-64.
7. Eur J Dermatol. 2008 Jul-Aug;18(4):433-9.
8. Biochemistry (Mosc). 2015 Mar;80(3):366-73.
9. Biochim Biophys Acta. 2016 Sep;1858(9):2006-18.
10. J Biomed Sci. 2010 Aug 24;17 Suppl 1:S45.
11. Adv Exp Med Biol. 2015;803:167-77.
Taurine, also known as 2-aminoethanesulfonic acid, is a naturally occurring beta-amino acid (which has a sulphonic acid group instead of carboxylic acid, differentiating it from other amino acids) yielded by methionine and cysteine metabolism in the liver.1,2 An important free beta-amino acid in mammals, it is often the free amino acid present in the greatest concentrations in several cell types in humans.1,2 Dietary intake of taurine also plays an important role in maintaining the body’s taurine levels because of mammals’ limited ability to synthesize it.1
Notably in terms of dermatologic treatment options, the combination product taurine bromamine is known to impart antioxidant, anti-inflammatory, and antibacterial activities.3 And taurine itself is associated with antioxidant, anti-inflammatory, antifibrotic, and immunomodulatory characteristics,1,4 and is noted for conferring antiaging benefits.5
Acne and other inflammatory conditions
The use of .6,7
In response to the problem of evolving antibiotic resistance, Marcinkiewicz reported in 2009 on the then-new therapeutic option of topical taurine bromamine for the treatment of inflammatory skin disorders such as acne. The author pointed out that Propionibacterium acnes is particularly sensitive to taurine bromamine, with the substance now known to suppress H2O2 production by activated neutrophils, likely contributing to moderating the severity and lowering the number of inflammatory acne lesions. In a 6-week double-blind pilot clinical study, Marcinkiewicz and his team compared the efficacy of 0.5% taurine bromamine cream with 1% clindamycin gel in 40 patients with mild to moderate acne. Treatments, which were randomly assigned, occurred twice daily through the study. Amelioration of acne symptoms was comparable in the two groups, with more than 90% of patients improving clinically and experiencing similar decreases in acne lesions (65% in the taurine bromamine group and 68% in the clindamycin group). Marcinkiewicz concluded that these results indicate the viability of taurine bromamine as an option for inflammatory acne therapy, particularly for patients who have shown antibiotic resistance.3
Wide-ranging protection potential
In 2003, Janeke et al. conducted analyses that showed that taurine accumulation defended cultured human keratinocytes from osmotically- and UV-induced apoptosis, suggesting the importance of taurine as an epidermal osmolyte necessary for maintaining keratinocyte hydration in a dry environment.2
Three years later, Collin et al. demonstrated the dynamic protective effects of taurine on the human hair follicle in an in vitro study in which taurine promoted hair survival and protected against TGF-beta1-induced damage.1
Taurine has also been found to stabilize and protect the catalytic activity of the hemoprotein cytochrome P450 3A4, which is a key enzyme responsible for metabolizing various endogenous as well as foreign substances, including drugs.8
Penetration enhancement
In 2016, Mueller et al. studied the effects of urea and taurine as hydrophilic penetration enhancers on stratum corneum lipid models as both substances are known to exert such effects. With inconclusive results as to the roots of such activity, they speculated that both entities enhance penetration through the introduction of copious water into the corneocytes, resulting from the robust water-binding capacity of urea and the consequent osmotic pressure related to taurine.9
Possible skin whitening and anti-aging roles and other promising lab results
Based on their previous work demonstrating that azelaic acid, a saturated dicarboxylic acid found naturally in wheat, rye, and barley, suppressed melanogenesis, Yu and Kim investigated the antimelanogenic activity of azelaic acid and taurine in B16F10 mouse melanoma cells in 2010. They found that the combination of the two substances exhibited a greater inhibitory effect in melanocytes than azelaic acid alone, with melanin production and tyrosinase activity suppressed without inducing cytotoxicity. The investigators concluded the combination of azelaic acid and taurine may be an effective approach for treating hyperpigmentation.10
In 2015, Ito et al. investigated the possible anti-aging role of taurine using a taurine transporter knockout mouse model. They noted that aging-related disorders affecting the skin, heart, skeletal muscle, and liver and resulting in a shorter lifespan have been correlated with tissue taurine depletion. The researchers proposed that proper protein folding allows endogenous taurine to perform as an antiaging molecule.5
Also in 2015, Kim et al. investigated potential mechanisms of the antiproliferative activity of taurine on murine B16F10 melanoma cells via the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and neutral red assays and microscopic analysis. They found that taurine prevented cell proliferation and engendered apoptosis in B16F10 cells, concluding that taurine may have a role to play as a chemotherapeutic agent for skin cancer.11
In 2014, Ashkani-Esfahani et al. studied the impact of taurine on cutaneous leishmaniasis wounds in a mouse model. Investigators induced 18 mice with wounds using L. major promastigotes, and divided them into a taurine injection group, taurine gel group, and no treatment group, performing treatments every 24 hours over 21 days. The taurine treatment groups exhibited significantly greater numerical fibroblast density, collagen bundle volume density, and vessel length densities compared with the nontreatment group. The taurine injection group displayed higher fibroblast numerical density than did the taurine gel group. The researchers concluded that taurine has the capacity to enhance wound healing and tissue regeneration but showed no direct anti-leishmaniasis effect.4
Conclusion
Taurine has been found over the last few decades to impart salutary effects for human health. This beta-amino acid that occurs naturally in humans and other mammals also appears to hold promising potential in the dermatologic realm, particularly for its anti-inflammatory and antioxidant effects. More research is needed to ascertain just how pivotal this compound can be for skin health.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Int J Cosmet Sci. 2006 Aug;28(4):289-98.
2. J Invest Dermatol. 2003 Aug;121(2):354-61.
3. Pol Arch Med Wewn. 2009 Oct;119(10):673-6.
4. Adv Biomed Res. 2014 Oct 7;3:204.
5. Adv Exp Med Biol. 2015;803:481-7.
6. Am J Clin Dermatol. 2012 Dec 1;13(6):357-64.
7. Eur J Dermatol. 2008 Jul-Aug;18(4):433-9.
8. Biochemistry (Mosc). 2015 Mar;80(3):366-73.
9. Biochim Biophys Acta. 2016 Sep;1858(9):2006-18.
10. J Biomed Sci. 2010 Aug 24;17 Suppl 1:S45.
11. Adv Exp Med Biol. 2015;803:167-77.
Taurine, also known as 2-aminoethanesulfonic acid, is a naturally occurring beta-amino acid (which has a sulphonic acid group instead of carboxylic acid, differentiating it from other amino acids) yielded by methionine and cysteine metabolism in the liver.1,2 An important free beta-amino acid in mammals, it is often the free amino acid present in the greatest concentrations in several cell types in humans.1,2 Dietary intake of taurine also plays an important role in maintaining the body’s taurine levels because of mammals’ limited ability to synthesize it.1
Notably in terms of dermatologic treatment options, the combination product taurine bromamine is known to impart antioxidant, anti-inflammatory, and antibacterial activities.3 And taurine itself is associated with antioxidant, anti-inflammatory, antifibrotic, and immunomodulatory characteristics,1,4 and is noted for conferring antiaging benefits.5
Acne and other inflammatory conditions
The use of .6,7
In response to the problem of evolving antibiotic resistance, Marcinkiewicz reported in 2009 on the then-new therapeutic option of topical taurine bromamine for the treatment of inflammatory skin disorders such as acne. The author pointed out that Propionibacterium acnes is particularly sensitive to taurine bromamine, with the substance now known to suppress H2O2 production by activated neutrophils, likely contributing to moderating the severity and lowering the number of inflammatory acne lesions. In a 6-week double-blind pilot clinical study, Marcinkiewicz and his team compared the efficacy of 0.5% taurine bromamine cream with 1% clindamycin gel in 40 patients with mild to moderate acne. Treatments, which were randomly assigned, occurred twice daily through the study. Amelioration of acne symptoms was comparable in the two groups, with more than 90% of patients improving clinically and experiencing similar decreases in acne lesions (65% in the taurine bromamine group and 68% in the clindamycin group). Marcinkiewicz concluded that these results indicate the viability of taurine bromamine as an option for inflammatory acne therapy, particularly for patients who have shown antibiotic resistance.3
Wide-ranging protection potential
In 2003, Janeke et al. conducted analyses that showed that taurine accumulation defended cultured human keratinocytes from osmotically- and UV-induced apoptosis, suggesting the importance of taurine as an epidermal osmolyte necessary for maintaining keratinocyte hydration in a dry environment.2
Three years later, Collin et al. demonstrated the dynamic protective effects of taurine on the human hair follicle in an in vitro study in which taurine promoted hair survival and protected against TGF-beta1-induced damage.1
Taurine has also been found to stabilize and protect the catalytic activity of the hemoprotein cytochrome P450 3A4, which is a key enzyme responsible for metabolizing various endogenous as well as foreign substances, including drugs.8
Penetration enhancement
In 2016, Mueller et al. studied the effects of urea and taurine as hydrophilic penetration enhancers on stratum corneum lipid models as both substances are known to exert such effects. With inconclusive results as to the roots of such activity, they speculated that both entities enhance penetration through the introduction of copious water into the corneocytes, resulting from the robust water-binding capacity of urea and the consequent osmotic pressure related to taurine.9
Possible skin whitening and anti-aging roles and other promising lab results
Based on their previous work demonstrating that azelaic acid, a saturated dicarboxylic acid found naturally in wheat, rye, and barley, suppressed melanogenesis, Yu and Kim investigated the antimelanogenic activity of azelaic acid and taurine in B16F10 mouse melanoma cells in 2010. They found that the combination of the two substances exhibited a greater inhibitory effect in melanocytes than azelaic acid alone, with melanin production and tyrosinase activity suppressed without inducing cytotoxicity. The investigators concluded the combination of azelaic acid and taurine may be an effective approach for treating hyperpigmentation.10
In 2015, Ito et al. investigated the possible anti-aging role of taurine using a taurine transporter knockout mouse model. They noted that aging-related disorders affecting the skin, heart, skeletal muscle, and liver and resulting in a shorter lifespan have been correlated with tissue taurine depletion. The researchers proposed that proper protein folding allows endogenous taurine to perform as an antiaging molecule.5
Also in 2015, Kim et al. investigated potential mechanisms of the antiproliferative activity of taurine on murine B16F10 melanoma cells via the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and neutral red assays and microscopic analysis. They found that taurine prevented cell proliferation and engendered apoptosis in B16F10 cells, concluding that taurine may have a role to play as a chemotherapeutic agent for skin cancer.11
In 2014, Ashkani-Esfahani et al. studied the impact of taurine on cutaneous leishmaniasis wounds in a mouse model. Investigators induced 18 mice with wounds using L. major promastigotes, and divided them into a taurine injection group, taurine gel group, and no treatment group, performing treatments every 24 hours over 21 days. The taurine treatment groups exhibited significantly greater numerical fibroblast density, collagen bundle volume density, and vessel length densities compared with the nontreatment group. The taurine injection group displayed higher fibroblast numerical density than did the taurine gel group. The researchers concluded that taurine has the capacity to enhance wound healing and tissue regeneration but showed no direct anti-leishmaniasis effect.4
Conclusion
Taurine has been found over the last few decades to impart salutary effects for human health. This beta-amino acid that occurs naturally in humans and other mammals also appears to hold promising potential in the dermatologic realm, particularly for its anti-inflammatory and antioxidant effects. More research is needed to ascertain just how pivotal this compound can be for skin health.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Int J Cosmet Sci. 2006 Aug;28(4):289-98.
2. J Invest Dermatol. 2003 Aug;121(2):354-61.
3. Pol Arch Med Wewn. 2009 Oct;119(10):673-6.
4. Adv Biomed Res. 2014 Oct 7;3:204.
5. Adv Exp Med Biol. 2015;803:481-7.
6. Am J Clin Dermatol. 2012 Dec 1;13(6):357-64.
7. Eur J Dermatol. 2008 Jul-Aug;18(4):433-9.
8. Biochemistry (Mosc). 2015 Mar;80(3):366-73.
9. Biochim Biophys Acta. 2016 Sep;1858(9):2006-18.
10. J Biomed Sci. 2010 Aug 24;17 Suppl 1:S45.
11. Adv Exp Med Biol. 2015;803:167-77.
Hypopigmentation
Hypopigmentation or depigmentation of the skin is very challenging to treat. The loss of melanin in the skin is often a frustrating problem, resulting from acne, burn scars, vitiligo, topically applied chemicals, or cryotherapy. To date, there is no universally accepted treatment that restores skin pigmentation. In our clinic, the induction of trauma to the skin via a series of microneedling or subcision treatments has shown promise in increasing the pigmentation of skin with localized hypo- or depigmented patches.
to improve the appearance of fine lines, acne scars, photoaging, stretch marks, enlarged pores, and other cosmetic issues characterized by loss of collagen or altered collagen remodeling. The skin-needling technique involves fine sterile needles 0.1 mm–2.5 mm in length that repeatedly pierce the stratum corneum producing microscopic “holes” in the dermis. These microscopic wounds lead to the release of growth factors stimulating the formation of new collagen, elastin, and neovascularization in the dermis. Similar to microneedling, subcision uses repeat trauma to the dermis and subcutis through the insertion and repeat movement of a needle.
In our practice, patients presenting with hypopigmentation of the skin from a variety of causes have been treated with a series of five subcision or microneedling procedures, resulting in rapid repigmentation of the skin with minimal to no side effects. Trauma to the skin causes regenerative mechanisms and wound healing. The release of cytokines that induce neoangiogenesis, neocollagenesis, and the deposition of hemosiderin from dermal bleeding induce the activation of melanocytes and stimulate skin pigmentation.
Subcision and microneedling are safe, effective, in-office procedures with vast indications that now can be applied to depigmented and hypopigmented skin. Patients have little to no downtime and results are permanent.
Dr. Talakoub and Dr. Wesley are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Dermatol Surg. 1995 Jun;21(6):543-9.
Aesthet Plast Surg. 1997 Jan-Feb;21(1):48-51.
Oral Maxillofac Surg Clin North Am. 2005 Feb;17(1):51-63.
Plast Reconstr Surg. 2008 Apr;121(4):1421-9.
Clin Dermatol. 2008 Mar-Apr;26(2):192-9.Plast Reconstr Surg. 2008 Nov;122(5):1553-63.
J Dermatolog Treat. 2012 Apr;23(2):144-52.
J Cutan Aesthet Surg. 2009 Jan;2(1):26-30.
J Cutan Aesthet Surg. 2009 Jul;2(2):110-1.
J Cosmet Dermatol. 2014 Sep;13(3):180-7.J Am Acad Dermatol. 2016 Nov;75(5):e195-e197.
Hypopigmentation or depigmentation of the skin is very challenging to treat. The loss of melanin in the skin is often a frustrating problem, resulting from acne, burn scars, vitiligo, topically applied chemicals, or cryotherapy. To date, there is no universally accepted treatment that restores skin pigmentation. In our clinic, the induction of trauma to the skin via a series of microneedling or subcision treatments has shown promise in increasing the pigmentation of skin with localized hypo- or depigmented patches.

to improve the appearance of fine lines, acne scars, photoaging, stretch marks, enlarged pores, and other cosmetic issues characterized by loss of collagen or altered collagen remodeling. The skin-needling technique involves fine sterile needles 0.1 mm–2.5 mm in length that repeatedly pierce the stratum corneum producing microscopic “holes” in the dermis. These microscopic wounds lead to the release of growth factors stimulating the formation of new collagen, elastin, and neovascularization in the dermis. Similar to microneedling, subcision uses repeat trauma to the dermis and subcutis through the insertion and repeat movement of a needle.
In our practice, patients presenting with hypopigmentation of the skin from a variety of causes have been treated with a series of five subcision or microneedling procedures, resulting in rapid repigmentation of the skin with minimal to no side effects. Trauma to the skin causes regenerative mechanisms and wound healing. The release of cytokines that induce neoangiogenesis, neocollagenesis, and the deposition of hemosiderin from dermal bleeding induce the activation of melanocytes and stimulate skin pigmentation.
Subcision and microneedling are safe, effective, in-office procedures with vast indications that now can be applied to depigmented and hypopigmented skin. Patients have little to no downtime and results are permanent.
Dr. Talakoub and Dr. Wesley are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Dermatol Surg. 1995 Jun;21(6):543-9.
Aesthet Plast Surg. 1997 Jan-Feb;21(1):48-51.
Oral Maxillofac Surg Clin North Am. 2005 Feb;17(1):51-63.
Plast Reconstr Surg. 2008 Apr;121(4):1421-9.
Clin Dermatol. 2008 Mar-Apr;26(2):192-9.Plast Reconstr Surg. 2008 Nov;122(5):1553-63.
J Dermatolog Treat. 2012 Apr;23(2):144-52.
J Cutan Aesthet Surg. 2009 Jan;2(1):26-30.
J Cutan Aesthet Surg. 2009 Jul;2(2):110-1.
J Cosmet Dermatol. 2014 Sep;13(3):180-7.J Am Acad Dermatol. 2016 Nov;75(5):e195-e197.
Hypopigmentation or depigmentation of the skin is very challenging to treat. The loss of melanin in the skin is often a frustrating problem, resulting from acne, burn scars, vitiligo, topically applied chemicals, or cryotherapy. To date, there is no universally accepted treatment that restores skin pigmentation. In our clinic, the induction of trauma to the skin via a series of microneedling or subcision treatments has shown promise in increasing the pigmentation of skin with localized hypo- or depigmented patches.

to improve the appearance of fine lines, acne scars, photoaging, stretch marks, enlarged pores, and other cosmetic issues characterized by loss of collagen or altered collagen remodeling. The skin-needling technique involves fine sterile needles 0.1 mm–2.5 mm in length that repeatedly pierce the stratum corneum producing microscopic “holes” in the dermis. These microscopic wounds lead to the release of growth factors stimulating the formation of new collagen, elastin, and neovascularization in the dermis. Similar to microneedling, subcision uses repeat trauma to the dermis and subcutis through the insertion and repeat movement of a needle.
In our practice, patients presenting with hypopigmentation of the skin from a variety of causes have been treated with a series of five subcision or microneedling procedures, resulting in rapid repigmentation of the skin with minimal to no side effects. Trauma to the skin causes regenerative mechanisms and wound healing. The release of cytokines that induce neoangiogenesis, neocollagenesis, and the deposition of hemosiderin from dermal bleeding induce the activation of melanocytes and stimulate skin pigmentation.
Subcision and microneedling are safe, effective, in-office procedures with vast indications that now can be applied to depigmented and hypopigmented skin. Patients have little to no downtime and results are permanent.
Dr. Talakoub and Dr. Wesley are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Dermatol Surg. 1995 Jun;21(6):543-9.
Aesthet Plast Surg. 1997 Jan-Feb;21(1):48-51.
Oral Maxillofac Surg Clin North Am. 2005 Feb;17(1):51-63.
Plast Reconstr Surg. 2008 Apr;121(4):1421-9.
Clin Dermatol. 2008 Mar-Apr;26(2):192-9.Plast Reconstr Surg. 2008 Nov;122(5):1553-63.
J Dermatolog Treat. 2012 Apr;23(2):144-52.
J Cutan Aesthet Surg. 2009 Jan;2(1):26-30.
J Cutan Aesthet Surg. 2009 Jul;2(2):110-1.
J Cosmet Dermatol. 2014 Sep;13(3):180-7.J Am Acad Dermatol. 2016 Nov;75(5):e195-e197.
Pediatric Skin Care: Survey of the Cutis Editorial Board
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on pediatric skin care. Here’s what we found.
Do you recommend sunscreen in babies younger than 6 months?
More than half (64%) of dermatologists we surveyed do not recommend using sunscreens in babies younger than 6 months; they should stay out of the sun. They recommended sun-protective clothing, hats, and sunglasses in this age group.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Babies younger than 6 months are still developing barrier functionality and have a higher surface area to body weight ratio compared to older children and adults. There is decreased UV light barrier protection and increased risk for systemic drug absorption. Therefore, it is best to avoid sunscreen in babies younger than 6 months. Instead, I recommend avoiding sunlight during peak hours, keeping babies in the shade, and dressing them in sun-protective clothing and hats.
Next page: Bathing and eczema
What advice do you give parents/guardians on bathing for babies with eczema?
Two-thirds of dermatologists (68%) indicated that moisturizers should be applied after bathing babies with eczema. More than half (64%) suggested using fragrance-free cleansers. Results varied on the frequency of bathing; 32% said bathe once daily for short periods with warm water, and 41% suggested to reduce bathing to a few nights a week.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Dry skin is a characteristic feature of atopic dermatitis. We now know that skin barrier dysfunction, such as filaggrin deficiency, contributes to the pathophysiology in some patients. In addition, a paucity of stratum corneum and intercellular lipids increases transepidermal water loss. Therefore, emollients containing humectants to augment stratum corneum hydration and occludents to reduce evaporation are extremely helpful in maintenance treatment of atopic dermatitis.
Next page: Nonsteroidal agents
Do you prescribe nonsteroidal agents for children younger than 2 years?
Almost two-thirds (64%) of dermatologists do prescribe nonsteroidal agents for children younger than 2 years.
If yes, which nonsteroidal agents do you prescribe for children?
Of those dermatologists who indicated that they do prescribe nonsteroidal agents for children younger than 2 years, almost half (45%) prescribe pimecrolimus, while 36% prescribe crisaborole or tacrolimus.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
We have limited options for US Food and Drug Administration (FDA)–approved therapies for atopic dermatitis in children younger than 2 years. The risks of adverse effects also are higher in younger children compared to older children and adults, particularly hypothalamic-pituitary-adrenal suppression with corticosteroids. Pimecrolimus was the most common nonsteroidal prescribed in this group. It should be noted that pimecrolimus is not FDA approved for use in children younger than 2 years; however, 2 phase 3 studies were conducted with 436 infants aged 3 months to 23 months and they demonstrated safety and efficacy.
Next page: Procedures in adolescents
Which procedures do you perform on adolescents?
Forty-one percent of dermatologists surveyed indicated that they do not perform procedures on adolescents. Of those that do, 32% use laser hair removal or chemical peels in adolescents, while only 23% each use light therapy for acne or onabotulinumtoxinA for hyperhidrosis. Pulsed dye laser use was reported in only 18% of dermatologists.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
The results of this survey are a reflection of the relatively recent trends in dermatology training. Now that there is board certification in pediatric dermatology, many dermatologists now refer to pediatric dermatologists for children who need or want procedures.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
Keep the regimen as simple as possible and make sure your skin care advice is culturally relevant.—Craig Burkhart, MD (Chapel Hill, North Carolina)
Please, avoid direct sun exposure as much as possible by wearing protective garments and finding shades in the outside.—Jisun Cha, MD (New Brunswick, New Jersey)
Good habits start early. Teach children how to care for their skin and they will carry that practice with them over the course of their lifetime, and hopefully pass it on.—James Q. Del Rosso, DO (Las Vegas, Nevada)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from September 13, 2018, to October 4, 2018. A total of 22 usable responses were received.
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on pediatric skin care. Here’s what we found.
Do you recommend sunscreen in babies younger than 6 months?
More than half (64%) of dermatologists we surveyed do not recommend using sunscreens in babies younger than 6 months; they should stay out of the sun. They recommended sun-protective clothing, hats, and sunglasses in this age group.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Babies younger than 6 months are still developing barrier functionality and have a higher surface area to body weight ratio compared to older children and adults. There is decreased UV light barrier protection and increased risk for systemic drug absorption. Therefore, it is best to avoid sunscreen in babies younger than 6 months. Instead, I recommend avoiding sunlight during peak hours, keeping babies in the shade, and dressing them in sun-protective clothing and hats.
Next page: Bathing and eczema
What advice do you give parents/guardians on bathing for babies with eczema?
Two-thirds of dermatologists (68%) indicated that moisturizers should be applied after bathing babies with eczema. More than half (64%) suggested using fragrance-free cleansers. Results varied on the frequency of bathing; 32% said bathe once daily for short periods with warm water, and 41% suggested to reduce bathing to a few nights a week.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Dry skin is a characteristic feature of atopic dermatitis. We now know that skin barrier dysfunction, such as filaggrin deficiency, contributes to the pathophysiology in some patients. In addition, a paucity of stratum corneum and intercellular lipids increases transepidermal water loss. Therefore, emollients containing humectants to augment stratum corneum hydration and occludents to reduce evaporation are extremely helpful in maintenance treatment of atopic dermatitis.
Next page: Nonsteroidal agents
Do you prescribe nonsteroidal agents for children younger than 2 years?
Almost two-thirds (64%) of dermatologists do prescribe nonsteroidal agents for children younger than 2 years.
If yes, which nonsteroidal agents do you prescribe for children?
Of those dermatologists who indicated that they do prescribe nonsteroidal agents for children younger than 2 years, almost half (45%) prescribe pimecrolimus, while 36% prescribe crisaborole or tacrolimus.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
We have limited options for US Food and Drug Administration (FDA)–approved therapies for atopic dermatitis in children younger than 2 years. The risks of adverse effects also are higher in younger children compared to older children and adults, particularly hypothalamic-pituitary-adrenal suppression with corticosteroids. Pimecrolimus was the most common nonsteroidal prescribed in this group. It should be noted that pimecrolimus is not FDA approved for use in children younger than 2 years; however, 2 phase 3 studies were conducted with 436 infants aged 3 months to 23 months and they demonstrated safety and efficacy.
Next page: Procedures in adolescents
Which procedures do you perform on adolescents?
Forty-one percent of dermatologists surveyed indicated that they do not perform procedures on adolescents. Of those that do, 32% use laser hair removal or chemical peels in adolescents, while only 23% each use light therapy for acne or onabotulinumtoxinA for hyperhidrosis. Pulsed dye laser use was reported in only 18% of dermatologists.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
The results of this survey are a reflection of the relatively recent trends in dermatology training. Now that there is board certification in pediatric dermatology, many dermatologists now refer to pediatric dermatologists for children who need or want procedures.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
Keep the regimen as simple as possible and make sure your skin care advice is culturally relevant.—Craig Burkhart, MD (Chapel Hill, North Carolina)
Please, avoid direct sun exposure as much as possible by wearing protective garments and finding shades in the outside.—Jisun Cha, MD (New Brunswick, New Jersey)
Good habits start early. Teach children how to care for their skin and they will carry that practice with them over the course of their lifetime, and hopefully pass it on.—James Q. Del Rosso, DO (Las Vegas, Nevada)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from September 13, 2018, to October 4, 2018. A total of 22 usable responses were received.
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on pediatric skin care. Here’s what we found.
Do you recommend sunscreen in babies younger than 6 months?
More than half (64%) of dermatologists we surveyed do not recommend using sunscreens in babies younger than 6 months; they should stay out of the sun. They recommended sun-protective clothing, hats, and sunglasses in this age group.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Babies younger than 6 months are still developing barrier functionality and have a higher surface area to body weight ratio compared to older children and adults. There is decreased UV light barrier protection and increased risk for systemic drug absorption. Therefore, it is best to avoid sunscreen in babies younger than 6 months. Instead, I recommend avoiding sunlight during peak hours, keeping babies in the shade, and dressing them in sun-protective clothing and hats.
Next page: Bathing and eczema
What advice do you give parents/guardians on bathing for babies with eczema?
Two-thirds of dermatologists (68%) indicated that moisturizers should be applied after bathing babies with eczema. More than half (64%) suggested using fragrance-free cleansers. Results varied on the frequency of bathing; 32% said bathe once daily for short periods with warm water, and 41% suggested to reduce bathing to a few nights a week.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Dry skin is a characteristic feature of atopic dermatitis. We now know that skin barrier dysfunction, such as filaggrin deficiency, contributes to the pathophysiology in some patients. In addition, a paucity of stratum corneum and intercellular lipids increases transepidermal water loss. Therefore, emollients containing humectants to augment stratum corneum hydration and occludents to reduce evaporation are extremely helpful in maintenance treatment of atopic dermatitis.
Next page: Nonsteroidal agents
Do you prescribe nonsteroidal agents for children younger than 2 years?
Almost two-thirds (64%) of dermatologists do prescribe nonsteroidal agents for children younger than 2 years.
If yes, which nonsteroidal agents do you prescribe for children?
Of those dermatologists who indicated that they do prescribe nonsteroidal agents for children younger than 2 years, almost half (45%) prescribe pimecrolimus, while 36% prescribe crisaborole or tacrolimus.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
We have limited options for US Food and Drug Administration (FDA)–approved therapies for atopic dermatitis in children younger than 2 years. The risks of adverse effects also are higher in younger children compared to older children and adults, particularly hypothalamic-pituitary-adrenal suppression with corticosteroids. Pimecrolimus was the most common nonsteroidal prescribed in this group. It should be noted that pimecrolimus is not FDA approved for use in children younger than 2 years; however, 2 phase 3 studies were conducted with 436 infants aged 3 months to 23 months and they demonstrated safety and efficacy.
Next page: Procedures in adolescents
Which procedures do you perform on adolescents?
Forty-one percent of dermatologists surveyed indicated that they do not perform procedures on adolescents. Of those that do, 32% use laser hair removal or chemical peels in adolescents, while only 23% each use light therapy for acne or onabotulinumtoxinA for hyperhidrosis. Pulsed dye laser use was reported in only 18% of dermatologists.

Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
The results of this survey are a reflection of the relatively recent trends in dermatology training. Now that there is board certification in pediatric dermatology, many dermatologists now refer to pediatric dermatologists for children who need or want procedures.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
Keep the regimen as simple as possible and make sure your skin care advice is culturally relevant.—Craig Burkhart, MD (Chapel Hill, North Carolina)
Please, avoid direct sun exposure as much as possible by wearing protective garments and finding shades in the outside.—Jisun Cha, MD (New Brunswick, New Jersey)
Good habits start early. Teach children how to care for their skin and they will carry that practice with them over the course of their lifetime, and hopefully pass it on.—James Q. Del Rosso, DO (Las Vegas, Nevada)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from September 13, 2018, to October 4, 2018. A total of 22 usable responses were received.
FDA expands CoolSculpting indication to submandibular area
FDA clearance of the expanded indication was based on results of a 22-week study in which patients achieved a mean 33% reduction in fat layer thickness after two treatments. In addition, 85% of patients reported satisfaction with their treatment in that and two other studies. The data were provided in Allergan’s press release announcing the clearance for the cryolipolysis device.
Adverse events associated with the CoolSculpting treatment include temporary redness, swelling, blanching, bruising, firmness, tingling, stinging, tenderness, cramping, aching, itching, and skin sensitivity. People with cryoglobulinemia, cold agglutinin disease, or paroxysmal cold hemoglobinuria should not receive CoolSculpting treatments, according to the company.
The FDA also expanded the CoolSculpting indication to people with a body mass index up to 46.2 kg/m2 when treating the submental and submandibular areas.
FDA clearance of the expanded indication was based on results of a 22-week study in which patients achieved a mean 33% reduction in fat layer thickness after two treatments. In addition, 85% of patients reported satisfaction with their treatment in that and two other studies. The data were provided in Allergan’s press release announcing the clearance for the cryolipolysis device.
Adverse events associated with the CoolSculpting treatment include temporary redness, swelling, blanching, bruising, firmness, tingling, stinging, tenderness, cramping, aching, itching, and skin sensitivity. People with cryoglobulinemia, cold agglutinin disease, or paroxysmal cold hemoglobinuria should not receive CoolSculpting treatments, according to the company.
The FDA also expanded the CoolSculpting indication to people with a body mass index up to 46.2 kg/m2 when treating the submental and submandibular areas.
FDA clearance of the expanded indication was based on results of a 22-week study in which patients achieved a mean 33% reduction in fat layer thickness after two treatments. In addition, 85% of patients reported satisfaction with their treatment in that and two other studies. The data were provided in Allergan’s press release announcing the clearance for the cryolipolysis device.
Adverse events associated with the CoolSculpting treatment include temporary redness, swelling, blanching, bruising, firmness, tingling, stinging, tenderness, cramping, aching, itching, and skin sensitivity. People with cryoglobulinemia, cold agglutinin disease, or paroxysmal cold hemoglobinuria should not receive CoolSculpting treatments, according to the company.
The FDA also expanded the CoolSculpting indication to people with a body mass index up to 46.2 kg/m2 when treating the submental and submandibular areas.











