User login
TCA and punch excision are two options for icepick acne scars
WAIKOLOA, HAWAII – Dermatologists can certainly improve icepick acne scars, but they have to be careful not to make them worse, according to dermatologist Nazanin Saedi, MD, director of the Jefferson Laser Surgery and Cosmetic Dermatology Center at Thomas Jefferson University, Philadelphia.
.
After about three to five TCA treatments, most patients will have a better than 50% improvement, Dr. Saedi said, but the treatment isn’t for darker skin types – Fitzpatrick types V or VI – because of the risk of pigmentation changes. Dr. Saedi uses toothpicks to apply a small amount of 80% TCA to the base of the scar, and waits for the “frost” to appear. It’s important not to reapply the TCA. “A lot of people double dip and ... keep dipping into the scar,” which causes more damage.
For patients with darker skin types, or those who don’t want to go through a series of treatments, punch excision is an option, with nonablative laser treatment a week later when sutures are removed. “Some patients heal beautifully,” but some patients may have a spread scar or a small atrophic scar at the punch site, she noted. Options to treat atrophic scarring after treatment are laser treatments and fillers.
She offered her advice in an interview at the Hawaii Dermatology Seminar, provided by the Global Academy for Medical Education/Skin Disease Education Foundation. It’s important to set realistic expectations, Dr. Saedi said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Dermatologists can certainly improve icepick acne scars, but they have to be careful not to make them worse, according to dermatologist Nazanin Saedi, MD, director of the Jefferson Laser Surgery and Cosmetic Dermatology Center at Thomas Jefferson University, Philadelphia.
.
After about three to five TCA treatments, most patients will have a better than 50% improvement, Dr. Saedi said, but the treatment isn’t for darker skin types – Fitzpatrick types V or VI – because of the risk of pigmentation changes. Dr. Saedi uses toothpicks to apply a small amount of 80% TCA to the base of the scar, and waits for the “frost” to appear. It’s important not to reapply the TCA. “A lot of people double dip and ... keep dipping into the scar,” which causes more damage.
For patients with darker skin types, or those who don’t want to go through a series of treatments, punch excision is an option, with nonablative laser treatment a week later when sutures are removed. “Some patients heal beautifully,” but some patients may have a spread scar or a small atrophic scar at the punch site, she noted. Options to treat atrophic scarring after treatment are laser treatments and fillers.
She offered her advice in an interview at the Hawaii Dermatology Seminar, provided by the Global Academy for Medical Education/Skin Disease Education Foundation. It’s important to set realistic expectations, Dr. Saedi said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Dermatologists can certainly improve icepick acne scars, but they have to be careful not to make them worse, according to dermatologist Nazanin Saedi, MD, director of the Jefferson Laser Surgery and Cosmetic Dermatology Center at Thomas Jefferson University, Philadelphia.
.
After about three to five TCA treatments, most patients will have a better than 50% improvement, Dr. Saedi said, but the treatment isn’t for darker skin types – Fitzpatrick types V or VI – because of the risk of pigmentation changes. Dr. Saedi uses toothpicks to apply a small amount of 80% TCA to the base of the scar, and waits for the “frost” to appear. It’s important not to reapply the TCA. “A lot of people double dip and ... keep dipping into the scar,” which causes more damage.
For patients with darker skin types, or those who don’t want to go through a series of treatments, punch excision is an option, with nonablative laser treatment a week later when sutures are removed. “Some patients heal beautifully,” but some patients may have a spread scar or a small atrophic scar at the punch site, she noted. Options to treat atrophic scarring after treatment are laser treatments and fillers.
She offered her advice in an interview at the Hawaii Dermatology Seminar, provided by the Global Academy for Medical Education/Skin Disease Education Foundation. It’s important to set realistic expectations, Dr. Saedi said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Laser Hair Removal: Survey of the Cutis Editorial Board
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on laser hair removal. Here’s what we found.
Do you perform laser hair removal in your practice?

More than half (58%) of dermatologists perform laser hair removal, while 12% have a PA/NP or aesthetician who performs this procedure on patients. Almost one-third (31%) of respondents do not perform laser hair removal.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Lasers are an important part of dermatology residency training and not a formal part of any other residency program. Therefore, dermatologists are best equipped to treat patients who are interested in removing unwanted hair safely and effectively. Dermatologists should advocate use of both a mask and a vacuum when performing these procedures to protect patients, themselves, residents, and staff from the resulting plume.
Next page: Incidence of treatment
Has the number of patients getting laser hair removal changed over the last 5 years?
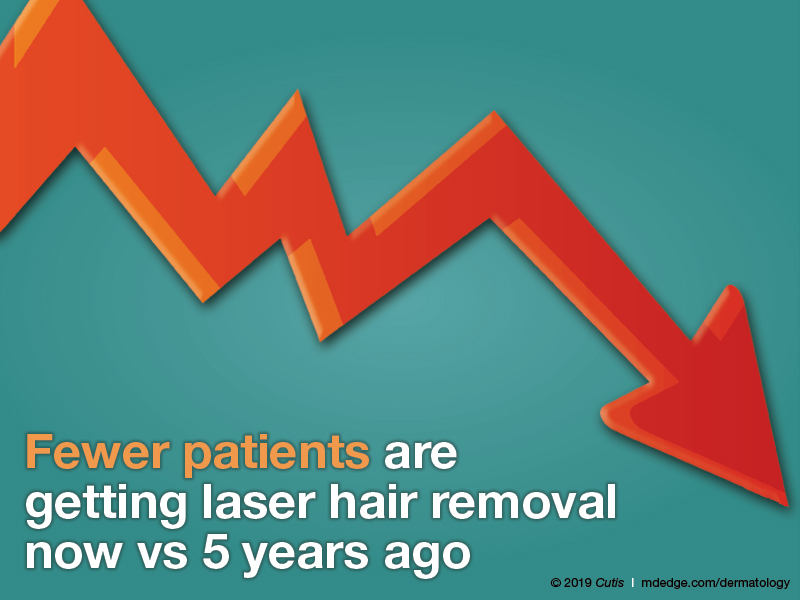
Fewer patients are getting laser hair removal now vs 5 years ago, according to half of dermatologists; 42% reported that roughly the same number of patients are getting it done. Only 8% reported that more patients are getting laser hair removal.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Unfortunately, many patients often undergo hair laser treatments at spas by practitioners with limited laser training with sometimes adverse effects, including burns and scars. Therefore, we have a duty to educate our patients about laser safety and encourage them to seek treatment from a board-certified dermatologist.
Next page: Treatment areas
What area do you treat most often in women?
What area do you treat most often in men?
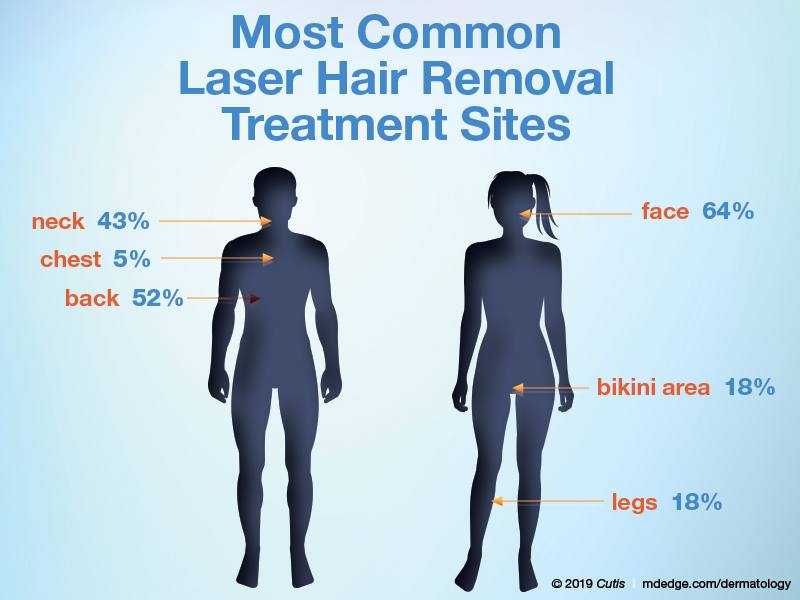
The majority of dermatologists (64%) treat the face most often in women, followed by the bikini area and legs (18% each). In men, half (52%) of dermatologists treat the back most often in men, followed by the neck (43%) and chest (5%).
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Before undergoing laser hair procedures, patients should be counseled that multiple treatments are often necessary, with the goal being reduction in hair density. Some hairs may still remain even after sufficient treatments. Some patients may be more comfortable with a topical numbing agent.
Next page: Lasers for darker skin types
What laser or device do you prefer to use for darker skin types?

Most dermatologists (79%) prefer to use the Nd:YAG 1064-nm laser for laser hair removal in darker skin types; 11% each prefer intense pulsed light or the alexandrite 755-nm laser.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
The alexandrite 755-nm laser can be used safely in lighter skin types, while the Nd:YAG 1064-nm laser is preferred for darker skin types. It is also highly recommended to perform test spots in darker-skinned individuals.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
Laser hair removal appears to be a safe and effective adjunctive therapy for adolescent hidradenitis patients. This has greatly increased the amount of laser hair removal treatments I perform as a pediatric dermatologist over the past 5 years.—Craig Burkhart, MD, MS, MPH (Chapel Hill, North Carolina)
Curbing unrealistic expectations is essential. It isn't magic. You won't have silky smooth, hairless skin after 1 treatment, or 2, or maybe ever. Discoloration, dyspigmentation, and scarring are possible. Making all of that clear in advance—in writing—will preempt 95% of postoperative complaints and angry phone calls.—Joseph Eastern, MD (Belleville, New Jersey)
In some states, laser hair removal is performed in medical spas without any dermatologist supervision. The lasers used in laser hair removal can be very harmful if used by nonphysicians who are not supervised.—Lawrence J. Green, MD (Washington, DC)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from January 7, 2019, to January 29, 2019. A total of 26 usable responses were received.
Georgesen C, Lipner SR. Surgical smoke: risk assessment and mitigation strategies. J Am Acad Dermatol. 2018;79:746-755.
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on laser hair removal. Here’s what we found.
Do you perform laser hair removal in your practice?

More than half (58%) of dermatologists perform laser hair removal, while 12% have a PA/NP or aesthetician who performs this procedure on patients. Almost one-third (31%) of respondents do not perform laser hair removal.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Lasers are an important part of dermatology residency training and not a formal part of any other residency program. Therefore, dermatologists are best equipped to treat patients who are interested in removing unwanted hair safely and effectively. Dermatologists should advocate use of both a mask and a vacuum when performing these procedures to protect patients, themselves, residents, and staff from the resulting plume.
Next page: Incidence of treatment
Has the number of patients getting laser hair removal changed over the last 5 years?

Fewer patients are getting laser hair removal now vs 5 years ago, according to half of dermatologists; 42% reported that roughly the same number of patients are getting it done. Only 8% reported that more patients are getting laser hair removal.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Unfortunately, many patients often undergo hair laser treatments at spas by practitioners with limited laser training with sometimes adverse effects, including burns and scars. Therefore, we have a duty to educate our patients about laser safety and encourage them to seek treatment from a board-certified dermatologist.
Next page: Treatment areas
What area do you treat most often in women?
What area do you treat most often in men?

The majority of dermatologists (64%) treat the face most often in women, followed by the bikini area and legs (18% each). In men, half (52%) of dermatologists treat the back most often in men, followed by the neck (43%) and chest (5%).
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Before undergoing laser hair procedures, patients should be counseled that multiple treatments are often necessary, with the goal being reduction in hair density. Some hairs may still remain even after sufficient treatments. Some patients may be more comfortable with a topical numbing agent.
Next page: Lasers for darker skin types
What laser or device do you prefer to use for darker skin types?

Most dermatologists (79%) prefer to use the Nd:YAG 1064-nm laser for laser hair removal in darker skin types; 11% each prefer intense pulsed light or the alexandrite 755-nm laser.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
The alexandrite 755-nm laser can be used safely in lighter skin types, while the Nd:YAG 1064-nm laser is preferred for darker skin types. It is also highly recommended to perform test spots in darker-skinned individuals.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
Laser hair removal appears to be a safe and effective adjunctive therapy for adolescent hidradenitis patients. This has greatly increased the amount of laser hair removal treatments I perform as a pediatric dermatologist over the past 5 years.—Craig Burkhart, MD, MS, MPH (Chapel Hill, North Carolina)
Curbing unrealistic expectations is essential. It isn't magic. You won't have silky smooth, hairless skin after 1 treatment, or 2, or maybe ever. Discoloration, dyspigmentation, and scarring are possible. Making all of that clear in advance—in writing—will preempt 95% of postoperative complaints and angry phone calls.—Joseph Eastern, MD (Belleville, New Jersey)
In some states, laser hair removal is performed in medical spas without any dermatologist supervision. The lasers used in laser hair removal can be very harmful if used by nonphysicians who are not supervised.—Lawrence J. Green, MD (Washington, DC)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from January 7, 2019, to January 29, 2019. A total of 26 usable responses were received.
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on laser hair removal. Here’s what we found.
Do you perform laser hair removal in your practice?

More than half (58%) of dermatologists perform laser hair removal, while 12% have a PA/NP or aesthetician who performs this procedure on patients. Almost one-third (31%) of respondents do not perform laser hair removal.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Lasers are an important part of dermatology residency training and not a formal part of any other residency program. Therefore, dermatologists are best equipped to treat patients who are interested in removing unwanted hair safely and effectively. Dermatologists should advocate use of both a mask and a vacuum when performing these procedures to protect patients, themselves, residents, and staff from the resulting plume.
Next page: Incidence of treatment
Has the number of patients getting laser hair removal changed over the last 5 years?

Fewer patients are getting laser hair removal now vs 5 years ago, according to half of dermatologists; 42% reported that roughly the same number of patients are getting it done. Only 8% reported that more patients are getting laser hair removal.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Unfortunately, many patients often undergo hair laser treatments at spas by practitioners with limited laser training with sometimes adverse effects, including burns and scars. Therefore, we have a duty to educate our patients about laser safety and encourage them to seek treatment from a board-certified dermatologist.
Next page: Treatment areas
What area do you treat most often in women?
What area do you treat most often in men?

The majority of dermatologists (64%) treat the face most often in women, followed by the bikini area and legs (18% each). In men, half (52%) of dermatologists treat the back most often in men, followed by the neck (43%) and chest (5%).
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Before undergoing laser hair procedures, patients should be counseled that multiple treatments are often necessary, with the goal being reduction in hair density. Some hairs may still remain even after sufficient treatments. Some patients may be more comfortable with a topical numbing agent.
Next page: Lasers for darker skin types
What laser or device do you prefer to use for darker skin types?

Most dermatologists (79%) prefer to use the Nd:YAG 1064-nm laser for laser hair removal in darker skin types; 11% each prefer intense pulsed light or the alexandrite 755-nm laser.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
The alexandrite 755-nm laser can be used safely in lighter skin types, while the Nd:YAG 1064-nm laser is preferred for darker skin types. It is also highly recommended to perform test spots in darker-skinned individuals.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
Laser hair removal appears to be a safe and effective adjunctive therapy for adolescent hidradenitis patients. This has greatly increased the amount of laser hair removal treatments I perform as a pediatric dermatologist over the past 5 years.—Craig Burkhart, MD, MS, MPH (Chapel Hill, North Carolina)
Curbing unrealistic expectations is essential. It isn't magic. You won't have silky smooth, hairless skin after 1 treatment, or 2, or maybe ever. Discoloration, dyspigmentation, and scarring are possible. Making all of that clear in advance—in writing—will preempt 95% of postoperative complaints and angry phone calls.—Joseph Eastern, MD (Belleville, New Jersey)
In some states, laser hair removal is performed in medical spas without any dermatologist supervision. The lasers used in laser hair removal can be very harmful if used by nonphysicians who are not supervised.—Lawrence J. Green, MD (Washington, DC)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from January 7, 2019, to January 29, 2019. A total of 26 usable responses were received.
Georgesen C, Lipner SR. Surgical smoke: risk assessment and mitigation strategies. J Am Acad Dermatol. 2018;79:746-755.
Georgesen C, Lipner SR. Surgical smoke: risk assessment and mitigation strategies. J Am Acad Dermatol. 2018;79:746-755.
Probiotic, prebiotic, and postbiotic skin care
A decade ago, I gave my senior expert talk at the University of California, San Francisco, department of dermatology on skin care and brought up the controversial topic that sterile or clean skin is bad. At the time, I initiated the conversation on the Today, I not only preach this message to my patients, but I also practice the “less-is-more” philosophy every day. It is my hope that this brief summary of the skin microbiome and the importance of skin bacteria will affect the development of the next generation of skin care products.
The normal human skin is a microbiome colonized by 10,000-1,000,000 bacteria units/cm2 that prevent the growth of pathogenic organisms and maintain the immunity of the skin. The diversity and type of skin bacteria (that is, Staphylococcus or Propionibacterium acnes), as well as their concentration, varies by person, body location, and environment. Symbiotic with bacteria on the skin are yeasts, such as Malassezia, and parasites, such as Demodex. When the composition and diversity of microorganisms are disrupted, the skin can no longer protect its barrier functions, leading to pathogenic bacterial infections, altered skin pH, decreased production of antimicrobial peptides, and increased inflammation. The microbiome also serves to shield the skin from environmental stressors, such as free radicals, UV radiation, and pollution.
What can lead to disruption of our skin is hygiene. Over-washing; stripping of the skin with lathering cleaner; overexfoliation; long, hot showers; and the use of products with antibacterial properties have increased over the last 50 years, and so has skin disease. The removal of these microorganisms, either by overcleansing or with antibiotic use, disrupts the microflora and leads to pH-imbalanced and inflamed skin. Our microflora contains prebiotics, probiotics, and postbiotics. Prebiotics are the “fertilizer” or “food,” so to speak, that encourages these essential microorganisms to grow; probiotics are the microorganisms themselves; and postbiotics are the chemical byproducts of bacteria, such as antimicrobial peptides and fragments of dead bacterial cells that remain on the skin.
Skin care tailored to our unique microbiome is in its infancy. On the frontier of microflora-rich skin care are organisms like Bifidobacterium longum, which increases the skin’s resistance to temperature and product-related irritation. Streptococcus thermophilus has been shown to increase the production of ceramides in the skin, which could help atopic dermatitis. Lactobacillus paracasei has been shown to inhibit the neuropeptide substance P, which increases inflammation and oil production. Enterococcus faecalis, Streptococcus salivarius, and Lactobacillus plantarum have all been shown to decrease Propionibacterium acnes. Bacillus coagulans and Bifidobacterium breve have been shown to decrease free radicals and protect against UV rays.
Probiotic, prebiotic, and postbiotic skin care, however, does have its challenges. Probiotics are live bacteria, and thus need refrigeration. These products are also not intended for use in anyone who is immunosuppressed or neutropenic. Another complexity in the development of probiotic, prebiotic, and postbiotic skin care is that each person may have a different need in terms of their skin microflora and that microflora is inherently different in different body parts. Furthermore, people with skin inflammation may require a different concentration or population of that flora.
In 2007, the National Institutes of Health initiated the Human Microbiome Project, and in 2016, the White House announced the creation of a new National Microbiome Initiative (NMI). Through this research, the identification and importance of our gut bacteria has led to a vast increase in development and near obsession with probiotic supplements, foods, and drinks (examples include Kombucha tea, kimchi, miso, and Kefir). Although oral consumption of prebiotics and probiotics may prove to be helpful, the skin does have its own unique flora and will benefit from targeted skin care. In the meantime, fostering the skins’s microflora is as important or more important than the replacement of it. My recommendations include using “microflora friendly” products that are lather-free, cream- or oil-based cleansers with acidic pH’s, and moisturizing heavily and consistently. I recommend staying away from antibacterial wipes, antibacterial soaps, and sanitizers.
Fostering this bacterial rich environment will help maintain your skin integrity. Squeaky clean skin is damaged skin.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Al-Ghazzewi F et al. Benef Microbes. 2014 Jun 1;5(2):99-107.
Baquerizo Nole K et al. J Am Acad Dermatol. 2014 Oct;71(4):814-21.
Chen Y et al. J Am Acad Dermatol. 2013 Jul;69(1):143-55.e3.
Grice E et al. Nat Rev Microbiol. 2011 Apr;9(4):244-53.
Kong H et al. J Invest Dermatol. 2012 Mar;132(3, part 2):933-9.
Hutkins R et al. Curr Opin Biotechnol. 2016 Feb;37:1-7.
Kober MM et al. Int J Womens Dermatol. 2015 Apr 6;1(2):85-9.
Maquire M. et al. Arch Dermatol Res. 2017 Aug;309(6):411-421.
Sugimoto S. et al. Photodermatol. Photoimmunol. Photomed. 2012 Dec;28(6): 312-9.
A decade ago, I gave my senior expert talk at the University of California, San Francisco, department of dermatology on skin care and brought up the controversial topic that sterile or clean skin is bad. At the time, I initiated the conversation on the Today, I not only preach this message to my patients, but I also practice the “less-is-more” philosophy every day. It is my hope that this brief summary of the skin microbiome and the importance of skin bacteria will affect the development of the next generation of skin care products.
The normal human skin is a microbiome colonized by 10,000-1,000,000 bacteria units/cm2 that prevent the growth of pathogenic organisms and maintain the immunity of the skin. The diversity and type of skin bacteria (that is, Staphylococcus or Propionibacterium acnes), as well as their concentration, varies by person, body location, and environment. Symbiotic with bacteria on the skin are yeasts, such as Malassezia, and parasites, such as Demodex. When the composition and diversity of microorganisms are disrupted, the skin can no longer protect its barrier functions, leading to pathogenic bacterial infections, altered skin pH, decreased production of antimicrobial peptides, and increased inflammation. The microbiome also serves to shield the skin from environmental stressors, such as free radicals, UV radiation, and pollution.
What can lead to disruption of our skin is hygiene. Over-washing; stripping of the skin with lathering cleaner; overexfoliation; long, hot showers; and the use of products with antibacterial properties have increased over the last 50 years, and so has skin disease. The removal of these microorganisms, either by overcleansing or with antibiotic use, disrupts the microflora and leads to pH-imbalanced and inflamed skin. Our microflora contains prebiotics, probiotics, and postbiotics. Prebiotics are the “fertilizer” or “food,” so to speak, that encourages these essential microorganisms to grow; probiotics are the microorganisms themselves; and postbiotics are the chemical byproducts of bacteria, such as antimicrobial peptides and fragments of dead bacterial cells that remain on the skin.
Skin care tailored to our unique microbiome is in its infancy. On the frontier of microflora-rich skin care are organisms like Bifidobacterium longum, which increases the skin’s resistance to temperature and product-related irritation. Streptococcus thermophilus has been shown to increase the production of ceramides in the skin, which could help atopic dermatitis. Lactobacillus paracasei has been shown to inhibit the neuropeptide substance P, which increases inflammation and oil production. Enterococcus faecalis, Streptococcus salivarius, and Lactobacillus plantarum have all been shown to decrease Propionibacterium acnes. Bacillus coagulans and Bifidobacterium breve have been shown to decrease free radicals and protect against UV rays.
Probiotic, prebiotic, and postbiotic skin care, however, does have its challenges. Probiotics are live bacteria, and thus need refrigeration. These products are also not intended for use in anyone who is immunosuppressed or neutropenic. Another complexity in the development of probiotic, prebiotic, and postbiotic skin care is that each person may have a different need in terms of their skin microflora and that microflora is inherently different in different body parts. Furthermore, people with skin inflammation may require a different concentration or population of that flora.
In 2007, the National Institutes of Health initiated the Human Microbiome Project, and in 2016, the White House announced the creation of a new National Microbiome Initiative (NMI). Through this research, the identification and importance of our gut bacteria has led to a vast increase in development and near obsession with probiotic supplements, foods, and drinks (examples include Kombucha tea, kimchi, miso, and Kefir). Although oral consumption of prebiotics and probiotics may prove to be helpful, the skin does have its own unique flora and will benefit from targeted skin care. In the meantime, fostering the skins’s microflora is as important or more important than the replacement of it. My recommendations include using “microflora friendly” products that are lather-free, cream- or oil-based cleansers with acidic pH’s, and moisturizing heavily and consistently. I recommend staying away from antibacterial wipes, antibacterial soaps, and sanitizers.
Fostering this bacterial rich environment will help maintain your skin integrity. Squeaky clean skin is damaged skin.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Al-Ghazzewi F et al. Benef Microbes. 2014 Jun 1;5(2):99-107.
Baquerizo Nole K et al. J Am Acad Dermatol. 2014 Oct;71(4):814-21.
Chen Y et al. J Am Acad Dermatol. 2013 Jul;69(1):143-55.e3.
Grice E et al. Nat Rev Microbiol. 2011 Apr;9(4):244-53.
Kong H et al. J Invest Dermatol. 2012 Mar;132(3, part 2):933-9.
Hutkins R et al. Curr Opin Biotechnol. 2016 Feb;37:1-7.
Kober MM et al. Int J Womens Dermatol. 2015 Apr 6;1(2):85-9.
Maquire M. et al. Arch Dermatol Res. 2017 Aug;309(6):411-421.
Sugimoto S. et al. Photodermatol. Photoimmunol. Photomed. 2012 Dec;28(6): 312-9.
A decade ago, I gave my senior expert talk at the University of California, San Francisco, department of dermatology on skin care and brought up the controversial topic that sterile or clean skin is bad. At the time, I initiated the conversation on the Today, I not only preach this message to my patients, but I also practice the “less-is-more” philosophy every day. It is my hope that this brief summary of the skin microbiome and the importance of skin bacteria will affect the development of the next generation of skin care products.
The normal human skin is a microbiome colonized by 10,000-1,000,000 bacteria units/cm2 that prevent the growth of pathogenic organisms and maintain the immunity of the skin. The diversity and type of skin bacteria (that is, Staphylococcus or Propionibacterium acnes), as well as their concentration, varies by person, body location, and environment. Symbiotic with bacteria on the skin are yeasts, such as Malassezia, and parasites, such as Demodex. When the composition and diversity of microorganisms are disrupted, the skin can no longer protect its barrier functions, leading to pathogenic bacterial infections, altered skin pH, decreased production of antimicrobial peptides, and increased inflammation. The microbiome also serves to shield the skin from environmental stressors, such as free radicals, UV radiation, and pollution.
What can lead to disruption of our skin is hygiene. Over-washing; stripping of the skin with lathering cleaner; overexfoliation; long, hot showers; and the use of products with antibacterial properties have increased over the last 50 years, and so has skin disease. The removal of these microorganisms, either by overcleansing or with antibiotic use, disrupts the microflora and leads to pH-imbalanced and inflamed skin. Our microflora contains prebiotics, probiotics, and postbiotics. Prebiotics are the “fertilizer” or “food,” so to speak, that encourages these essential microorganisms to grow; probiotics are the microorganisms themselves; and postbiotics are the chemical byproducts of bacteria, such as antimicrobial peptides and fragments of dead bacterial cells that remain on the skin.
Skin care tailored to our unique microbiome is in its infancy. On the frontier of microflora-rich skin care are organisms like Bifidobacterium longum, which increases the skin’s resistance to temperature and product-related irritation. Streptococcus thermophilus has been shown to increase the production of ceramides in the skin, which could help atopic dermatitis. Lactobacillus paracasei has been shown to inhibit the neuropeptide substance P, which increases inflammation and oil production. Enterococcus faecalis, Streptococcus salivarius, and Lactobacillus plantarum have all been shown to decrease Propionibacterium acnes. Bacillus coagulans and Bifidobacterium breve have been shown to decrease free radicals and protect against UV rays.
Probiotic, prebiotic, and postbiotic skin care, however, does have its challenges. Probiotics are live bacteria, and thus need refrigeration. These products are also not intended for use in anyone who is immunosuppressed or neutropenic. Another complexity in the development of probiotic, prebiotic, and postbiotic skin care is that each person may have a different need in terms of their skin microflora and that microflora is inherently different in different body parts. Furthermore, people with skin inflammation may require a different concentration or population of that flora.
In 2007, the National Institutes of Health initiated the Human Microbiome Project, and in 2016, the White House announced the creation of a new National Microbiome Initiative (NMI). Through this research, the identification and importance of our gut bacteria has led to a vast increase in development and near obsession with probiotic supplements, foods, and drinks (examples include Kombucha tea, kimchi, miso, and Kefir). Although oral consumption of prebiotics and probiotics may prove to be helpful, the skin does have its own unique flora and will benefit from targeted skin care. In the meantime, fostering the skins’s microflora is as important or more important than the replacement of it. My recommendations include using “microflora friendly” products that are lather-free, cream- or oil-based cleansers with acidic pH’s, and moisturizing heavily and consistently. I recommend staying away from antibacterial wipes, antibacterial soaps, and sanitizers.
Fostering this bacterial rich environment will help maintain your skin integrity. Squeaky clean skin is damaged skin.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Al-Ghazzewi F et al. Benef Microbes. 2014 Jun 1;5(2):99-107.
Baquerizo Nole K et al. J Am Acad Dermatol. 2014 Oct;71(4):814-21.
Chen Y et al. J Am Acad Dermatol. 2013 Jul;69(1):143-55.e3.
Grice E et al. Nat Rev Microbiol. 2011 Apr;9(4):244-53.
Kong H et al. J Invest Dermatol. 2012 Mar;132(3, part 2):933-9.
Hutkins R et al. Curr Opin Biotechnol. 2016 Feb;37:1-7.
Kober MM et al. Int J Womens Dermatol. 2015 Apr 6;1(2):85-9.
Maquire M. et al. Arch Dermatol Res. 2017 Aug;309(6):411-421.
Sugimoto S. et al. Photodermatol. Photoimmunol. Photomed. 2012 Dec;28(6): 312-9.
FDA: 246 new reports on breast implant-associated lymphoma
The Food and Drug Administration has identified 457 unique cases of breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) and 9 related deaths since 2010, and received 246 new medical device reports (MDRs) regarding BIA-ALCL between September 2017 and September 2018, according to an update from the agency’s Center for Devices and Radiological Health.

That brings the total number of reports to 660; however, that number reflects duplicative cases, Binita Ashar, MD, a general surgeon and the director of the division of surgical devices at the center, said in a statement.
“These types of increases in the MDRs are to be expected and may include past cases that were not previously reported to the FDA,” Dr. Ashar said, addressing the high number of new reports. “The increased number of MDRs contributes to our evolving understanding of BIA-ALCL and represents a more thorough and comprehensive analysis.”
BIA-ALCL is a type of non-Hodgkin lymphoma and a known risk from breast implants that was first communicated by the FDA in 2011. Regular updates have been provided with respect to related medical device reports, cases, deaths, and known risks.
“We hope that this information prompts providers and patients to have important, informed conversations about breast implants and the risk of BIA-ALCL. At the same time, we remain committed to working in partnership with all stakeholders to continue to study, understand, and provide updates about this important public health issue,” Dr. Ashar said.
To that end, the center also issued a Letter to Health Care Providers to “encourage those who regularly treat patients, including primary care physicians and gynecologists, to learn about BIA-ALCL in patients with breast implants.”
Patients and providers are encouraged to file MDRs with the FDA via MedWatch, the FDA Safety Information and Adverse Event Reporting program, she said.
The Food and Drug Administration has identified 457 unique cases of breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) and 9 related deaths since 2010, and received 246 new medical device reports (MDRs) regarding BIA-ALCL between September 2017 and September 2018, according to an update from the agency’s Center for Devices and Radiological Health.

That brings the total number of reports to 660; however, that number reflects duplicative cases, Binita Ashar, MD, a general surgeon and the director of the division of surgical devices at the center, said in a statement.
“These types of increases in the MDRs are to be expected and may include past cases that were not previously reported to the FDA,” Dr. Ashar said, addressing the high number of new reports. “The increased number of MDRs contributes to our evolving understanding of BIA-ALCL and represents a more thorough and comprehensive analysis.”
BIA-ALCL is a type of non-Hodgkin lymphoma and a known risk from breast implants that was first communicated by the FDA in 2011. Regular updates have been provided with respect to related medical device reports, cases, deaths, and known risks.
“We hope that this information prompts providers and patients to have important, informed conversations about breast implants and the risk of BIA-ALCL. At the same time, we remain committed to working in partnership with all stakeholders to continue to study, understand, and provide updates about this important public health issue,” Dr. Ashar said.
To that end, the center also issued a Letter to Health Care Providers to “encourage those who regularly treat patients, including primary care physicians and gynecologists, to learn about BIA-ALCL in patients with breast implants.”
Patients and providers are encouraged to file MDRs with the FDA via MedWatch, the FDA Safety Information and Adverse Event Reporting program, she said.
The Food and Drug Administration has identified 457 unique cases of breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) and 9 related deaths since 2010, and received 246 new medical device reports (MDRs) regarding BIA-ALCL between September 2017 and September 2018, according to an update from the agency’s Center for Devices and Radiological Health.

That brings the total number of reports to 660; however, that number reflects duplicative cases, Binita Ashar, MD, a general surgeon and the director of the division of surgical devices at the center, said in a statement.
“These types of increases in the MDRs are to be expected and may include past cases that were not previously reported to the FDA,” Dr. Ashar said, addressing the high number of new reports. “The increased number of MDRs contributes to our evolving understanding of BIA-ALCL and represents a more thorough and comprehensive analysis.”
BIA-ALCL is a type of non-Hodgkin lymphoma and a known risk from breast implants that was first communicated by the FDA in 2011. Regular updates have been provided with respect to related medical device reports, cases, deaths, and known risks.
“We hope that this information prompts providers and patients to have important, informed conversations about breast implants and the risk of BIA-ALCL. At the same time, we remain committed to working in partnership with all stakeholders to continue to study, understand, and provide updates about this important public health issue,” Dr. Ashar said.
To that end, the center also issued a Letter to Health Care Providers to “encourage those who regularly treat patients, including primary care physicians and gynecologists, to learn about BIA-ALCL in patients with breast implants.”
Patients and providers are encouraged to file MDRs with the FDA via MedWatch, the FDA Safety Information and Adverse Event Reporting program, she said.
Melatonin update, Part 1
Found in various plant and animal species, including humans, melatonin (N-acetyl-5-methoxytryptamine) is best known for its daily fluctuations in circulating levels that regulate circadian rhythms. But this ancient serotonin derivative, stimulated by beta-adrenergic receptors, is the primary neuroendocrine product of the pineal gland (discovered as such in 1917) in humans and a dynamic compound with diverse roles in human health levels of which decrease with age.1,2 Over the last quarter of a century, we have arrived at a much greater understanding of the varied biological functions of this highly lipophilic hormone, which is now recognized as the strongest endogenous antioxidant, particularly potent against hydroxyl radicals, the most harmful of reactive oxygen species, and known to protect mitochondria and DNA from direct oxidative harm.2-4 Directly or via its circadian impact, melatonin also affects skin as well as core body temperature.1 This column is a . Next month’s column will address some more of the activities of this dynamic hormone while concentrating on the interaction of melatonin and ultraviolet radiation.
Early studies
In the mid-1990s, Bangha et al. performed several studies in healthy human volunteers that demonstrated that topically applied melatonin suppressed UVB-induced erythema (with one study showing pre- and posttreatment as effective and a subsequent one showing only pretreatment as effective), and also found that melatonin appears to have the potential to accumulate in the stratum corneum with extended release into the blood system through cutaneous delivery.5-7
A randomized, double-blind study by Dreher et al. in 12 healthy adults (6 women and 6 men, all white, aged 29-49 years) considered the short-term photoprotective effects of topically applied vitamin C, vitamin E, and melatonin, alone or in combination, 30 minutes after UV exposure. A dose-dependent photoprotective effect was associated with melatonin, and photoprotective properties were enhanced when melatonin in was combined with vitamins C and E.8
The following year, Dreher et al. evaluated the short-term photoprotective effects of the same compounds in a randomized, double-blind, placebo-controlled human study. Each antioxidant was topically applied alone or in combination after UV exposure in a single application (immediately or 30 minutes after UV exposure) or in multiple applications 30 minutes, 1 hour, and 2 hours after UV exposure (totaling three applications). Interestingly, no photoprotective effects were seen. The researchers concluded that given the speed of cutaneous damage from UV radiation, antioxidants likely must be delivered at the appropriate site in sufficient doses at the outset of and during active oxidative harm.9
In 2004, Fischer et al. conducted a clinical study of 15 healthy volunteers to test the skin penetration activity of melatonin 0.01% in a cream and 0.01% and 0.03% in a solution. During a 24-hour period, researchers obtained blood samples for melatonin measurement prior to application at 9 a.m. as well as 1, 4, 8, and 24 hours after application. Preapplication serum melatonin levels ranged from 0.6 to 15.9 pg/mL. The mean serum value 24 hours later after application of the 0.01% melatonin cream was 9.0 pg/mL. For the 0.01% solution group, the mean melatonin level was 12.7 pg/mL 24 hours after application. Melatonin levels also substantially rose just 1 and 8 hours later in the 0.03% solution group, with cumulative melatonin measured as 7.1 pg/mL in the 0.01% cream group, 8.6 pg/mL in the 0.01% solution participants, and 15.7 pg/mL in the 0.03% group. The investigators concluded that as a strong lipophilic compound melatonin penetrates the skin with serum blood levels increasing in a dose- and galenic-dependent manner without prompting spikes above the physiological range.10
Wound healing and atopic dermatitis
In 2006, Sener et al. reported that topically applied and systemically administered melatonin was successful as a pressure ulcer treatment in rats.11 Four years later, in a study using a chronic wound model in rats with pinealectomy that suppressed basal melatonin, Ozler et al. found that systemic and topical melatonin treatment were equally effective in imparting wound healing effects.12
A study in mice conducted by Kim et al. at around the same time showed that topically applied melatonin, by reducing total IgE in serum and interleukin-4 and interferon-gamma production by activated CD4(+) T cells, inhibits atopic dermatitis–like skin lesion development engendered by 2,4-dinitrofluorobenzene (DNFB) treatment in NC/Nga mice.13
More recently, Abbaszadeh et al. have suggested that melatonin has the potential to enhance the therapeutic ratio in radiation oncology, and to be more effective at reducing skin damage in this setting when used in optimal and non-toxic doses.2
Pigmentation disorders
Melatonin and serotonin are thought to have potential to ameliorate or attenuate the spread of vitiligo.1 In addition, melatonin appears to have potential in the realm of hyperpigmentation treatment. Investigators have found that the combination of topical melatonin 5% and a daily dose of 3 g of oral melatonin over 120 days significantly reduces Melasma Area Severity Index scores in comparison to placebo; the improvement is attributed primarily to the use of topical melatonin.14,15
Androgenetic alopecia
In 2018, Hatem et al. designed nanostructured lipid carriers to better deliver melatonin in antioxidant oils to treat androgenic alopecia. They found that the carriers achieved a sustained release of 6 hours and raised the skin deposition of melatonin 4.5-fold in the stratum corneum, 7-fold in the epidermis, and 6.8-fold in the dermis compared with a melatonin solution. The nanostructured lipid carriers also improved on clinical results, compared to the melatonin formula, by increasing hair density and thickness and reducing hair loss in patients with androgenic alopecia.16
Conclusion
Studies in humans have shown that through systemic administration and, particularly, topical application. Demonstrated to be safe and effective, topically applied melatonin appears to warrant serious consideration as a skin-protective, anti-aging tool in the dermatologic armamentarium.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Slominski AT et al. J Invest Dermatol. 2018 Mar;138(3):490-9.
2. Abbaszadeh A et al. J Biomed Phys Eng. 2017 Jun;7(2):127-136.
3. Fischer T et al. Hautarzt. 1999 Jan;50(1):5-11.
4. Scheuer C. Dan Med J. 2017 Jun;64(6). pii:B5358.
5. Bangha E et al. Arch Dermatol Res. 1996 Aug;288(9):522-6.
6. Bangha E et al. Dermatology. 1997;195(3):248-52.
7. Bangha E et al. Skin Pharmacol. 1997;10(5-6):298-302.
8. Dreher F et al. Br J Dermatol. 1998 Aug;139(2):332-9.
9. Dreher F et al. Dermatology. 1999;198(1):52-5.
10. Fischer TW et al. Skin Pharmacol Physiol. 2004 Jul-Aug;17(4):190-4.
11. Sener G et al. J Pineal Res. 2006 Apr;40(3):280-7.
12. Ozler M et al. Scand J Clin Lab Invest. 2010 Oct;70(6):447-52.
13. Kim TH et al. J Pineal Res. 2009 Nov;47(4):324-9.
14. Juhasz MLW et al. J Cosmet Dermatol. 2018 Dec;17(6):1144-57.
15. Hamadi SA, Mohammed MM, Aljaf AN, et al. The role of topical and oral melatonin in management of melasma patients. J Arab Univ Basic Appl Sci. 2009;8:30‐42.
16. Hatem S et al. Expert Opin Drug Deliv. 2018 Oct;15(10):927-35.
Found in various plant and animal species, including humans, melatonin (N-acetyl-5-methoxytryptamine) is best known for its daily fluctuations in circulating levels that regulate circadian rhythms. But this ancient serotonin derivative, stimulated by beta-adrenergic receptors, is the primary neuroendocrine product of the pineal gland (discovered as such in 1917) in humans and a dynamic compound with diverse roles in human health levels of which decrease with age.1,2 Over the last quarter of a century, we have arrived at a much greater understanding of the varied biological functions of this highly lipophilic hormone, which is now recognized as the strongest endogenous antioxidant, particularly potent against hydroxyl radicals, the most harmful of reactive oxygen species, and known to protect mitochondria and DNA from direct oxidative harm.2-4 Directly or via its circadian impact, melatonin also affects skin as well as core body temperature.1 This column is a . Next month’s column will address some more of the activities of this dynamic hormone while concentrating on the interaction of melatonin and ultraviolet radiation.
Early studies
In the mid-1990s, Bangha et al. performed several studies in healthy human volunteers that demonstrated that topically applied melatonin suppressed UVB-induced erythema (with one study showing pre- and posttreatment as effective and a subsequent one showing only pretreatment as effective), and also found that melatonin appears to have the potential to accumulate in the stratum corneum with extended release into the blood system through cutaneous delivery.5-7
A randomized, double-blind study by Dreher et al. in 12 healthy adults (6 women and 6 men, all white, aged 29-49 years) considered the short-term photoprotective effects of topically applied vitamin C, vitamin E, and melatonin, alone or in combination, 30 minutes after UV exposure. A dose-dependent photoprotective effect was associated with melatonin, and photoprotective properties were enhanced when melatonin in was combined with vitamins C and E.8
The following year, Dreher et al. evaluated the short-term photoprotective effects of the same compounds in a randomized, double-blind, placebo-controlled human study. Each antioxidant was topically applied alone or in combination after UV exposure in a single application (immediately or 30 minutes after UV exposure) or in multiple applications 30 minutes, 1 hour, and 2 hours after UV exposure (totaling three applications). Interestingly, no photoprotective effects were seen. The researchers concluded that given the speed of cutaneous damage from UV radiation, antioxidants likely must be delivered at the appropriate site in sufficient doses at the outset of and during active oxidative harm.9
In 2004, Fischer et al. conducted a clinical study of 15 healthy volunteers to test the skin penetration activity of melatonin 0.01% in a cream and 0.01% and 0.03% in a solution. During a 24-hour period, researchers obtained blood samples for melatonin measurement prior to application at 9 a.m. as well as 1, 4, 8, and 24 hours after application. Preapplication serum melatonin levels ranged from 0.6 to 15.9 pg/mL. The mean serum value 24 hours later after application of the 0.01% melatonin cream was 9.0 pg/mL. For the 0.01% solution group, the mean melatonin level was 12.7 pg/mL 24 hours after application. Melatonin levels also substantially rose just 1 and 8 hours later in the 0.03% solution group, with cumulative melatonin measured as 7.1 pg/mL in the 0.01% cream group, 8.6 pg/mL in the 0.01% solution participants, and 15.7 pg/mL in the 0.03% group. The investigators concluded that as a strong lipophilic compound melatonin penetrates the skin with serum blood levels increasing in a dose- and galenic-dependent manner without prompting spikes above the physiological range.10
Wound healing and atopic dermatitis
In 2006, Sener et al. reported that topically applied and systemically administered melatonin was successful as a pressure ulcer treatment in rats.11 Four years later, in a study using a chronic wound model in rats with pinealectomy that suppressed basal melatonin, Ozler et al. found that systemic and topical melatonin treatment were equally effective in imparting wound healing effects.12
A study in mice conducted by Kim et al. at around the same time showed that topically applied melatonin, by reducing total IgE in serum and interleukin-4 and interferon-gamma production by activated CD4(+) T cells, inhibits atopic dermatitis–like skin lesion development engendered by 2,4-dinitrofluorobenzene (DNFB) treatment in NC/Nga mice.13
More recently, Abbaszadeh et al. have suggested that melatonin has the potential to enhance the therapeutic ratio in radiation oncology, and to be more effective at reducing skin damage in this setting when used in optimal and non-toxic doses.2
Pigmentation disorders
Melatonin and serotonin are thought to have potential to ameliorate or attenuate the spread of vitiligo.1 In addition, melatonin appears to have potential in the realm of hyperpigmentation treatment. Investigators have found that the combination of topical melatonin 5% and a daily dose of 3 g of oral melatonin over 120 days significantly reduces Melasma Area Severity Index scores in comparison to placebo; the improvement is attributed primarily to the use of topical melatonin.14,15
Androgenetic alopecia
In 2018, Hatem et al. designed nanostructured lipid carriers to better deliver melatonin in antioxidant oils to treat androgenic alopecia. They found that the carriers achieved a sustained release of 6 hours and raised the skin deposition of melatonin 4.5-fold in the stratum corneum, 7-fold in the epidermis, and 6.8-fold in the dermis compared with a melatonin solution. The nanostructured lipid carriers also improved on clinical results, compared to the melatonin formula, by increasing hair density and thickness and reducing hair loss in patients with androgenic alopecia.16
Conclusion
Studies in humans have shown that through systemic administration and, particularly, topical application. Demonstrated to be safe and effective, topically applied melatonin appears to warrant serious consideration as a skin-protective, anti-aging tool in the dermatologic armamentarium.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Slominski AT et al. J Invest Dermatol. 2018 Mar;138(3):490-9.
2. Abbaszadeh A et al. J Biomed Phys Eng. 2017 Jun;7(2):127-136.
3. Fischer T et al. Hautarzt. 1999 Jan;50(1):5-11.
4. Scheuer C. Dan Med J. 2017 Jun;64(6). pii:B5358.
5. Bangha E et al. Arch Dermatol Res. 1996 Aug;288(9):522-6.
6. Bangha E et al. Dermatology. 1997;195(3):248-52.
7. Bangha E et al. Skin Pharmacol. 1997;10(5-6):298-302.
8. Dreher F et al. Br J Dermatol. 1998 Aug;139(2):332-9.
9. Dreher F et al. Dermatology. 1999;198(1):52-5.
10. Fischer TW et al. Skin Pharmacol Physiol. 2004 Jul-Aug;17(4):190-4.
11. Sener G et al. J Pineal Res. 2006 Apr;40(3):280-7.
12. Ozler M et al. Scand J Clin Lab Invest. 2010 Oct;70(6):447-52.
13. Kim TH et al. J Pineal Res. 2009 Nov;47(4):324-9.
14. Juhasz MLW et al. J Cosmet Dermatol. 2018 Dec;17(6):1144-57.
15. Hamadi SA, Mohammed MM, Aljaf AN, et al. The role of topical and oral melatonin in management of melasma patients. J Arab Univ Basic Appl Sci. 2009;8:30‐42.
16. Hatem S et al. Expert Opin Drug Deliv. 2018 Oct;15(10):927-35.
Found in various plant and animal species, including humans, melatonin (N-acetyl-5-methoxytryptamine) is best known for its daily fluctuations in circulating levels that regulate circadian rhythms. But this ancient serotonin derivative, stimulated by beta-adrenergic receptors, is the primary neuroendocrine product of the pineal gland (discovered as such in 1917) in humans and a dynamic compound with diverse roles in human health levels of which decrease with age.1,2 Over the last quarter of a century, we have arrived at a much greater understanding of the varied biological functions of this highly lipophilic hormone, which is now recognized as the strongest endogenous antioxidant, particularly potent against hydroxyl radicals, the most harmful of reactive oxygen species, and known to protect mitochondria and DNA from direct oxidative harm.2-4 Directly or via its circadian impact, melatonin also affects skin as well as core body temperature.1 This column is a . Next month’s column will address some more of the activities of this dynamic hormone while concentrating on the interaction of melatonin and ultraviolet radiation.
Early studies
In the mid-1990s, Bangha et al. performed several studies in healthy human volunteers that demonstrated that topically applied melatonin suppressed UVB-induced erythema (with one study showing pre- and posttreatment as effective and a subsequent one showing only pretreatment as effective), and also found that melatonin appears to have the potential to accumulate in the stratum corneum with extended release into the blood system through cutaneous delivery.5-7
A randomized, double-blind study by Dreher et al. in 12 healthy adults (6 women and 6 men, all white, aged 29-49 years) considered the short-term photoprotective effects of topically applied vitamin C, vitamin E, and melatonin, alone or in combination, 30 minutes after UV exposure. A dose-dependent photoprotective effect was associated with melatonin, and photoprotective properties were enhanced when melatonin in was combined with vitamins C and E.8
The following year, Dreher et al. evaluated the short-term photoprotective effects of the same compounds in a randomized, double-blind, placebo-controlled human study. Each antioxidant was topically applied alone or in combination after UV exposure in a single application (immediately or 30 minutes after UV exposure) or in multiple applications 30 minutes, 1 hour, and 2 hours after UV exposure (totaling three applications). Interestingly, no photoprotective effects were seen. The researchers concluded that given the speed of cutaneous damage from UV radiation, antioxidants likely must be delivered at the appropriate site in sufficient doses at the outset of and during active oxidative harm.9
In 2004, Fischer et al. conducted a clinical study of 15 healthy volunteers to test the skin penetration activity of melatonin 0.01% in a cream and 0.01% and 0.03% in a solution. During a 24-hour period, researchers obtained blood samples for melatonin measurement prior to application at 9 a.m. as well as 1, 4, 8, and 24 hours after application. Preapplication serum melatonin levels ranged from 0.6 to 15.9 pg/mL. The mean serum value 24 hours later after application of the 0.01% melatonin cream was 9.0 pg/mL. For the 0.01% solution group, the mean melatonin level was 12.7 pg/mL 24 hours after application. Melatonin levels also substantially rose just 1 and 8 hours later in the 0.03% solution group, with cumulative melatonin measured as 7.1 pg/mL in the 0.01% cream group, 8.6 pg/mL in the 0.01% solution participants, and 15.7 pg/mL in the 0.03% group. The investigators concluded that as a strong lipophilic compound melatonin penetrates the skin with serum blood levels increasing in a dose- and galenic-dependent manner without prompting spikes above the physiological range.10
Wound healing and atopic dermatitis
In 2006, Sener et al. reported that topically applied and systemically administered melatonin was successful as a pressure ulcer treatment in rats.11 Four years later, in a study using a chronic wound model in rats with pinealectomy that suppressed basal melatonin, Ozler et al. found that systemic and topical melatonin treatment were equally effective in imparting wound healing effects.12
A study in mice conducted by Kim et al. at around the same time showed that topically applied melatonin, by reducing total IgE in serum and interleukin-4 and interferon-gamma production by activated CD4(+) T cells, inhibits atopic dermatitis–like skin lesion development engendered by 2,4-dinitrofluorobenzene (DNFB) treatment in NC/Nga mice.13
More recently, Abbaszadeh et al. have suggested that melatonin has the potential to enhance the therapeutic ratio in radiation oncology, and to be more effective at reducing skin damage in this setting when used in optimal and non-toxic doses.2
Pigmentation disorders
Melatonin and serotonin are thought to have potential to ameliorate or attenuate the spread of vitiligo.1 In addition, melatonin appears to have potential in the realm of hyperpigmentation treatment. Investigators have found that the combination of topical melatonin 5% and a daily dose of 3 g of oral melatonin over 120 days significantly reduces Melasma Area Severity Index scores in comparison to placebo; the improvement is attributed primarily to the use of topical melatonin.14,15
Androgenetic alopecia
In 2018, Hatem et al. designed nanostructured lipid carriers to better deliver melatonin in antioxidant oils to treat androgenic alopecia. They found that the carriers achieved a sustained release of 6 hours and raised the skin deposition of melatonin 4.5-fold in the stratum corneum, 7-fold in the epidermis, and 6.8-fold in the dermis compared with a melatonin solution. The nanostructured lipid carriers also improved on clinical results, compared to the melatonin formula, by increasing hair density and thickness and reducing hair loss in patients with androgenic alopecia.16
Conclusion
Studies in humans have shown that through systemic administration and, particularly, topical application. Demonstrated to be safe and effective, topically applied melatonin appears to warrant serious consideration as a skin-protective, anti-aging tool in the dermatologic armamentarium.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Slominski AT et al. J Invest Dermatol. 2018 Mar;138(3):490-9.
2. Abbaszadeh A et al. J Biomed Phys Eng. 2017 Jun;7(2):127-136.
3. Fischer T et al. Hautarzt. 1999 Jan;50(1):5-11.
4. Scheuer C. Dan Med J. 2017 Jun;64(6). pii:B5358.
5. Bangha E et al. Arch Dermatol Res. 1996 Aug;288(9):522-6.
6. Bangha E et al. Dermatology. 1997;195(3):248-52.
7. Bangha E et al. Skin Pharmacol. 1997;10(5-6):298-302.
8. Dreher F et al. Br J Dermatol. 1998 Aug;139(2):332-9.
9. Dreher F et al. Dermatology. 1999;198(1):52-5.
10. Fischer TW et al. Skin Pharmacol Physiol. 2004 Jul-Aug;17(4):190-4.
11. Sener G et al. J Pineal Res. 2006 Apr;40(3):280-7.
12. Ozler M et al. Scand J Clin Lab Invest. 2010 Oct;70(6):447-52.
13. Kim TH et al. J Pineal Res. 2009 Nov;47(4):324-9.
14. Juhasz MLW et al. J Cosmet Dermatol. 2018 Dec;17(6):1144-57.
15. Hamadi SA, Mohammed MM, Aljaf AN, et al. The role of topical and oral melatonin in management of melasma patients. J Arab Univ Basic Appl Sci. 2009;8:30‐42.
16. Hatem S et al. Expert Opin Drug Deliv. 2018 Oct;15(10):927-35.
Winter exfoliation: A multicultural approach
Winter or postwinter exfoliation may seem counterintuitive to some patients because skin is often more dry because of cold weather and dry heat from heaters in the home, car, and workplace. Some patients even admit to using emollients less frequently in the winter because they are too cold to do it after bathing or are covering more of their body. But winter exfoliation can be an important method for improving skin hydration by aiding skin cell turnover, removing surface flaky skin, and enhancing penetration of moisturizers and active ingredients applied afterward. Here we explore exfoliation techniques used in various cultures around the world.
Ancient Egypt: Egyptians are credited with the first exfoliation techniques. Mechanical exfoliation was practiced in ancient Egypt via pumice stones, as well as alabaster particles, and scrubs made from sand or plants, such as aloe vera. (Although the subject is beyond the scope of this article, the first use of chemical exfoliation, using sour milk, which contains lactic acid, has been credited to ancient Egypt.)
Iran: Most traditional Iranian households are familiar with kiseh and sefidab, used for exfoliation as often as once a week. Kiseh is a special loofah-like exfoliating mitt, often hand woven. Sefidab is a whitish ball that looks like a dense piece of chalk made from animal fats and natural minerals that is rubbed on the kiseh, which is then rubbed on the skin. Exfoliation results as the sefidab and top layers of skin come off in gray white rolls, which are then rinsed off. The dead skin left on the mitt is known as “chairk.” Archaeological excavations have provided evidence that sefidab may have been used in Persian cosmetics as long ago as 2000 BC–4500 BC, as part of Zoroastrian traditions.
Korea: Koreans have long been known for practicing skin exfoliation. Here in Los Angeles, especially in Koreatown, many Korean spas or bathhouses, known as jjimjilbang, can be found; these provide various therapies, particularly “detoxification” in hot tubs, saunas (many with different stones and crystal minerals for healing properties), computer rooms, restaurants, theater rooms. They also provide body scrubs, or seshin: A soak in the hot tub for at least 30 minutes is recommended, followed by a hot water rinse and a scrub by a “ddemiri” (a scrub practitioner), who intensely scrubs the skin from head to toe using a roughened cloth. Going into a hot room or sauna is recommended after the scrub for relaxation, with the belief that the sweat won’t be blocked by dirty or clogged pores. Scrubs in jjimjilbang are recommended as often as once per week.
Indigenous people of the Americas and Caribbean: Sea salt is used commonly as an exfoliant among people from Caribbean countries and those of indigenous ancestry in the Americas (North America, including Hawaii, and Central and South America). Finer-grained sea salt is commonly found in the showers of my friends of Afro-Caribbean and indigenous American descent. While sugar is less coarse and easy to wash off in warm water, finer-grained sea salt provides more friction but is not as rough as coarse sea salt. Fine sea salt, because it is less coarse, can also be used on the face, if used carefully. While the effect of topical salt on skin microbes is unknown, cutaneous sodium storage has been found to strengthen the antimicrobial barrier function and boost macrophage host defense (Cell Metab. 2015 Mar 3;21[3]:493-501). Additionally, it has been noted that some Native Americans used dried corncobs for exfoliation. The people of the Comanche tribe would use sand from the bottom of a river bed to scrub the skin (similarly, Polynesian people have been known to use crushed sea shells for this purpose).
India (Ayurveda): Garshana is a dry brushing technique performed in Ayurvedic medicine. Dry brushing may be performed with a bristle brush or with slightly roughened silk gloves. The motion of dry brushing is intended to stimulate lymphatic drainage for elimination of toxins from the body. Circular strokes are used on the stomach and joints (shoulders, elbows, knees, wrists, hips, and ankles), and long sweeping strokes are used on the arms and legs. It is recommended for the morning, upon awakening and before a shower, because it is a stimulating practice. Sometimes oils, specific to an individual’s “dosha” (constitutional type or energy as defined by Ayurveda) – are applied afterward in a similar head-to-toe motion as a self-massage called Abhyanga.
Japan: Shaving, particularly facial shaving, is frequently done not just among men in Japan, but also among women who have shaved their faces and skin for years as a method of exfoliation for skin rejuvenation. In the United States, facial shaving among women has evolved to a method of exfoliation called “dermaplaning,” which involves dry shaving hairs (including facial vellus hairs) as well as top layers of stratum corneum. The procedure uses of a 25-centimeter (10-inch) scalpel, which curves into a sharp point. Potential risks include irritation from friction, as well as folliculitis.
France: It is not certain whether “gommage” originated in France, but in French, it means “to erase” because the rubbing action is similar to erasing a word. In gommage, a paste is applied to the skin and allowed to dry slightly while gentle enzymes digest dead skin cells on the surface; then it is rubbed off, taking skin cells with it. Most of what comes off is the product itself, but this may include some skin cells. One commonly used enzyme in gommage is papain, derived from the papaya fruit. Gommage was popular with facials before stronger chemical exfoliants like alpha-hydroxy acids became widely available commercially.
West Africa (Ghana, Nigeria): A long mesh body exfoliator, much like a tightly woven fishing net made of nylon, is common in Ghanaian and Nigerian households. The textured washcloth typically stretches up to 3 times the size of a regular washcloth, making it easy to scrub hard-to-reach places like the back.
Worldwide: Around the world in places where coffee beans are native, including Kenya and other parts of Africa, the Middle East, South America, Australia, and Hawaii, coffee beans are used as a skin exfoliant. Coffee grounds can however, should be used cautiously in showers as they can coagulate in water and clog drains and pipes. One tradition in Kenya is to crush and rub coffee beans on the skin with a piece of sugarcane to remove top layers of skin. Often too harsh to use directly, coffee grounds in cosmetic formulations are often mixed with oils or shea butter to create a smoother texture.
May this list grow as we continue to learn from the skin care techniques practiced in different cultures around the world.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Winter or postwinter exfoliation may seem counterintuitive to some patients because skin is often more dry because of cold weather and dry heat from heaters in the home, car, and workplace. Some patients even admit to using emollients less frequently in the winter because they are too cold to do it after bathing or are covering more of their body. But winter exfoliation can be an important method for improving skin hydration by aiding skin cell turnover, removing surface flaky skin, and enhancing penetration of moisturizers and active ingredients applied afterward. Here we explore exfoliation techniques used in various cultures around the world.
Ancient Egypt: Egyptians are credited with the first exfoliation techniques. Mechanical exfoliation was practiced in ancient Egypt via pumice stones, as well as alabaster particles, and scrubs made from sand or plants, such as aloe vera. (Although the subject is beyond the scope of this article, the first use of chemical exfoliation, using sour milk, which contains lactic acid, has been credited to ancient Egypt.)
Iran: Most traditional Iranian households are familiar with kiseh and sefidab, used for exfoliation as often as once a week. Kiseh is a special loofah-like exfoliating mitt, often hand woven. Sefidab is a whitish ball that looks like a dense piece of chalk made from animal fats and natural minerals that is rubbed on the kiseh, which is then rubbed on the skin. Exfoliation results as the sefidab and top layers of skin come off in gray white rolls, which are then rinsed off. The dead skin left on the mitt is known as “chairk.” Archaeological excavations have provided evidence that sefidab may have been used in Persian cosmetics as long ago as 2000 BC–4500 BC, as part of Zoroastrian traditions.
Korea: Koreans have long been known for practicing skin exfoliation. Here in Los Angeles, especially in Koreatown, many Korean spas or bathhouses, known as jjimjilbang, can be found; these provide various therapies, particularly “detoxification” in hot tubs, saunas (many with different stones and crystal minerals for healing properties), computer rooms, restaurants, theater rooms. They also provide body scrubs, or seshin: A soak in the hot tub for at least 30 minutes is recommended, followed by a hot water rinse and a scrub by a “ddemiri” (a scrub practitioner), who intensely scrubs the skin from head to toe using a roughened cloth. Going into a hot room or sauna is recommended after the scrub for relaxation, with the belief that the sweat won’t be blocked by dirty or clogged pores. Scrubs in jjimjilbang are recommended as often as once per week.
Indigenous people of the Americas and Caribbean: Sea salt is used commonly as an exfoliant among people from Caribbean countries and those of indigenous ancestry in the Americas (North America, including Hawaii, and Central and South America). Finer-grained sea salt is commonly found in the showers of my friends of Afro-Caribbean and indigenous American descent. While sugar is less coarse and easy to wash off in warm water, finer-grained sea salt provides more friction but is not as rough as coarse sea salt. Fine sea salt, because it is less coarse, can also be used on the face, if used carefully. While the effect of topical salt on skin microbes is unknown, cutaneous sodium storage has been found to strengthen the antimicrobial barrier function and boost macrophage host defense (Cell Metab. 2015 Mar 3;21[3]:493-501). Additionally, it has been noted that some Native Americans used dried corncobs for exfoliation. The people of the Comanche tribe would use sand from the bottom of a river bed to scrub the skin (similarly, Polynesian people have been known to use crushed sea shells for this purpose).
India (Ayurveda): Garshana is a dry brushing technique performed in Ayurvedic medicine. Dry brushing may be performed with a bristle brush or with slightly roughened silk gloves. The motion of dry brushing is intended to stimulate lymphatic drainage for elimination of toxins from the body. Circular strokes are used on the stomach and joints (shoulders, elbows, knees, wrists, hips, and ankles), and long sweeping strokes are used on the arms and legs. It is recommended for the morning, upon awakening and before a shower, because it is a stimulating practice. Sometimes oils, specific to an individual’s “dosha” (constitutional type or energy as defined by Ayurveda) – are applied afterward in a similar head-to-toe motion as a self-massage called Abhyanga.
Japan: Shaving, particularly facial shaving, is frequently done not just among men in Japan, but also among women who have shaved their faces and skin for years as a method of exfoliation for skin rejuvenation. In the United States, facial shaving among women has evolved to a method of exfoliation called “dermaplaning,” which involves dry shaving hairs (including facial vellus hairs) as well as top layers of stratum corneum. The procedure uses of a 25-centimeter (10-inch) scalpel, which curves into a sharp point. Potential risks include irritation from friction, as well as folliculitis.
France: It is not certain whether “gommage” originated in France, but in French, it means “to erase” because the rubbing action is similar to erasing a word. In gommage, a paste is applied to the skin and allowed to dry slightly while gentle enzymes digest dead skin cells on the surface; then it is rubbed off, taking skin cells with it. Most of what comes off is the product itself, but this may include some skin cells. One commonly used enzyme in gommage is papain, derived from the papaya fruit. Gommage was popular with facials before stronger chemical exfoliants like alpha-hydroxy acids became widely available commercially.
West Africa (Ghana, Nigeria): A long mesh body exfoliator, much like a tightly woven fishing net made of nylon, is common in Ghanaian and Nigerian households. The textured washcloth typically stretches up to 3 times the size of a regular washcloth, making it easy to scrub hard-to-reach places like the back.
Worldwide: Around the world in places where coffee beans are native, including Kenya and other parts of Africa, the Middle East, South America, Australia, and Hawaii, coffee beans are used as a skin exfoliant. Coffee grounds can however, should be used cautiously in showers as they can coagulate in water and clog drains and pipes. One tradition in Kenya is to crush and rub coffee beans on the skin with a piece of sugarcane to remove top layers of skin. Often too harsh to use directly, coffee grounds in cosmetic formulations are often mixed with oils or shea butter to create a smoother texture.
May this list grow as we continue to learn from the skin care techniques practiced in different cultures around the world.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Winter or postwinter exfoliation may seem counterintuitive to some patients because skin is often more dry because of cold weather and dry heat from heaters in the home, car, and workplace. Some patients even admit to using emollients less frequently in the winter because they are too cold to do it after bathing or are covering more of their body. But winter exfoliation can be an important method for improving skin hydration by aiding skin cell turnover, removing surface flaky skin, and enhancing penetration of moisturizers and active ingredients applied afterward. Here we explore exfoliation techniques used in various cultures around the world.
Ancient Egypt: Egyptians are credited with the first exfoliation techniques. Mechanical exfoliation was practiced in ancient Egypt via pumice stones, as well as alabaster particles, and scrubs made from sand or plants, such as aloe vera. (Although the subject is beyond the scope of this article, the first use of chemical exfoliation, using sour milk, which contains lactic acid, has been credited to ancient Egypt.)
Iran: Most traditional Iranian households are familiar with kiseh and sefidab, used for exfoliation as often as once a week. Kiseh is a special loofah-like exfoliating mitt, often hand woven. Sefidab is a whitish ball that looks like a dense piece of chalk made from animal fats and natural minerals that is rubbed on the kiseh, which is then rubbed on the skin. Exfoliation results as the sefidab and top layers of skin come off in gray white rolls, which are then rinsed off. The dead skin left on the mitt is known as “chairk.” Archaeological excavations have provided evidence that sefidab may have been used in Persian cosmetics as long ago as 2000 BC–4500 BC, as part of Zoroastrian traditions.
Korea: Koreans have long been known for practicing skin exfoliation. Here in Los Angeles, especially in Koreatown, many Korean spas or bathhouses, known as jjimjilbang, can be found; these provide various therapies, particularly “detoxification” in hot tubs, saunas (many with different stones and crystal minerals for healing properties), computer rooms, restaurants, theater rooms. They also provide body scrubs, or seshin: A soak in the hot tub for at least 30 minutes is recommended, followed by a hot water rinse and a scrub by a “ddemiri” (a scrub practitioner), who intensely scrubs the skin from head to toe using a roughened cloth. Going into a hot room or sauna is recommended after the scrub for relaxation, with the belief that the sweat won’t be blocked by dirty or clogged pores. Scrubs in jjimjilbang are recommended as often as once per week.
Indigenous people of the Americas and Caribbean: Sea salt is used commonly as an exfoliant among people from Caribbean countries and those of indigenous ancestry in the Americas (North America, including Hawaii, and Central and South America). Finer-grained sea salt is commonly found in the showers of my friends of Afro-Caribbean and indigenous American descent. While sugar is less coarse and easy to wash off in warm water, finer-grained sea salt provides more friction but is not as rough as coarse sea salt. Fine sea salt, because it is less coarse, can also be used on the face, if used carefully. While the effect of topical salt on skin microbes is unknown, cutaneous sodium storage has been found to strengthen the antimicrobial barrier function and boost macrophage host defense (Cell Metab. 2015 Mar 3;21[3]:493-501). Additionally, it has been noted that some Native Americans used dried corncobs for exfoliation. The people of the Comanche tribe would use sand from the bottom of a river bed to scrub the skin (similarly, Polynesian people have been known to use crushed sea shells for this purpose).
India (Ayurveda): Garshana is a dry brushing technique performed in Ayurvedic medicine. Dry brushing may be performed with a bristle brush or with slightly roughened silk gloves. The motion of dry brushing is intended to stimulate lymphatic drainage for elimination of toxins from the body. Circular strokes are used on the stomach and joints (shoulders, elbows, knees, wrists, hips, and ankles), and long sweeping strokes are used on the arms and legs. It is recommended for the morning, upon awakening and before a shower, because it is a stimulating practice. Sometimes oils, specific to an individual’s “dosha” (constitutional type or energy as defined by Ayurveda) – are applied afterward in a similar head-to-toe motion as a self-massage called Abhyanga.
Japan: Shaving, particularly facial shaving, is frequently done not just among men in Japan, but also among women who have shaved their faces and skin for years as a method of exfoliation for skin rejuvenation. In the United States, facial shaving among women has evolved to a method of exfoliation called “dermaplaning,” which involves dry shaving hairs (including facial vellus hairs) as well as top layers of stratum corneum. The procedure uses of a 25-centimeter (10-inch) scalpel, which curves into a sharp point. Potential risks include irritation from friction, as well as folliculitis.
France: It is not certain whether “gommage” originated in France, but in French, it means “to erase” because the rubbing action is similar to erasing a word. In gommage, a paste is applied to the skin and allowed to dry slightly while gentle enzymes digest dead skin cells on the surface; then it is rubbed off, taking skin cells with it. Most of what comes off is the product itself, but this may include some skin cells. One commonly used enzyme in gommage is papain, derived from the papaya fruit. Gommage was popular with facials before stronger chemical exfoliants like alpha-hydroxy acids became widely available commercially.
West Africa (Ghana, Nigeria): A long mesh body exfoliator, much like a tightly woven fishing net made of nylon, is common in Ghanaian and Nigerian households. The textured washcloth typically stretches up to 3 times the size of a regular washcloth, making it easy to scrub hard-to-reach places like the back.
Worldwide: Around the world in places where coffee beans are native, including Kenya and other parts of Africa, the Middle East, South America, Australia, and Hawaii, coffee beans are used as a skin exfoliant. Coffee grounds can however, should be used cautiously in showers as they can coagulate in water and clog drains and pipes. One tradition in Kenya is to crush and rub coffee beans on the skin with a piece of sugarcane to remove top layers of skin. Often too harsh to use directly, coffee grounds in cosmetic formulations are often mixed with oils or shea butter to create a smoother texture.
May this list grow as we continue to learn from the skin care techniques practiced in different cultures around the world.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Fine-tuning the male aesthetic consultation includes consideration of gender-specific wrinkle pattern
ORLANDO – The , a dermatologist in private practice in Arlington, Va. And he may not even be sure what he’s looking for.
However, he probably knows what he doesn’t like about his appearance. When men are questioned, the three areas they are most concerned about is their hairline, their eyes, and their jawline, said Dr. Keaney, speaking at the Orlando Dermatology Aesthetic and Clinical Conference.
It’s important to evaluate men differently, not just for anatomic differences from women, but also for behavioral and psychological factors unique to men as aesthetic patients, he noted.
Anatomic differences are significant and fundamentally shape treatment decisions, Dr. Keaney said. Broadly speaking, “the male face is traditionally more square, with a prominent supraorbital ridge.” Rather than emphasizing breadth across the cheeks, as in rejuvenation for women, a strong, youthful male face will have a square jaw, appropriate width across the cheeks, and a strong brow line with an arch that is flatter than what women seek.
Male cosmetic goals can be complicated by the fact that men “age poorly” and tend to look older than their stated age, he continued, referring to a study that found that men appear about one-third year older than their age, and women about a half year younger. A higher rate of smoking and excess ultraviolet light exposure among men may contribute to this discrepancy, he said.
Overall, men see steady atrophy of facial soft tissue throughout adulthood, in contrast to women who see a more rapid decline that starts at menopause. This makes sense in the context of the slow drop in circulating testosterone that men see beginning at about age 30, at which time men can expect a decrease of about 1% per year, he noted.
A common opener from men, said Dr. Keaney, is “’I look tired.’ When I hear that, I’m thinking about the eyes.” Men are more likely to develop tear troughs and a sunken appearance because of their larger orbital cavities and smaller orbital fat pads, so periocular fillers are often a treatment tool to consider.
Around mens’ eyes, “it’s not just the presence, but the pattern of wrinkles that guides treatment,” he noted. At the lateral canthi, both at rest and with maximum smile, the predominant wrinkle pattern in crows’ feet is the lower fan. The cheek elevator musculature, including the zygomaticus major, are more involved in animation around the eyes with smiling, which is important because reaching for botulinum toxin alone is probably not going to give the patient his desired effect, he added.
Other differences in the male wrinkle pattern can be found on the brow. The “U” contraction pattern between the brows is more common in men than women, at least partly because men have greater involvement of the procerus muscle, Dr. Keaney said.
Taking all of this into account, caution is the watchword when using botulinum toxin for the glabellar and brow region. “Beware of eyebrow ptosis: avoid the frontalis in patients with eyebrow ptosis,” he said. Treating the corrugators can also be an unwise move, since toxin can diffuse to the inferior fibers of the frontalis muscle, he added.
For men with jawline concerns, consider a combination approach, Dr. Keaney said. The lower face and neck can be rejuvenated by a redraping solution, such as skin tightening with high frequency ultrasound or radiofrequency ablation.
Sometimes, a clean, tight sweep of jawline can be restored with dermal fillers to the lower face, along with a noninvasive fat reduction approach, said Dr. Keaney.
Knowing men’s unique anatomy and aging patterns is only half the battle, though. “How you communicate with men and evaluate them has to take into account that you might be seeing a sweaty, nervous, treatment-naive patient,” at the initial consultation, said Dr. Keaney. “Do men care? Yes, but men display a different set of motivations.”
Plan for all of this to take time. “The initial consultation tends to be longer” for male patients, who are “less savvy” about cosmetic procedures than women, he pointed out.
A clear discussion of side effects is critical when discussing treatment options with men. “If they get a side effect, you’ll never see them again – and you won’t ever hear about it,” he added.
Though overall, women have been shown to be more sensitive to pain, in cosmetic procedures, “men are less tolerant of pain.” Plan for this, set reasonable expectations but be proactive about pain control, and still expect a need for some hand-holding, said Dr. Keaney.
“Men do not like surprises,” he added. Clearly define what expected side effects may be, what they’ll look like, and what downtime the patient should expect.
For Dr. Keaney’s part, he’s found that the best way to retain men in his cosmetic practice is to “start with a home run treatment to earn trust.” Men often appreciate a slow and steady approach to addressing concerns. “Make sure to connect the treatment plan to the primary cosmetic concern.”
Dr. Keaney reported that he has relationships with multiple pharmaceutical and cosmetic companies.
ORLANDO – The , a dermatologist in private practice in Arlington, Va. And he may not even be sure what he’s looking for.
However, he probably knows what he doesn’t like about his appearance. When men are questioned, the three areas they are most concerned about is their hairline, their eyes, and their jawline, said Dr. Keaney, speaking at the Orlando Dermatology Aesthetic and Clinical Conference.
It’s important to evaluate men differently, not just for anatomic differences from women, but also for behavioral and psychological factors unique to men as aesthetic patients, he noted.
Anatomic differences are significant and fundamentally shape treatment decisions, Dr. Keaney said. Broadly speaking, “the male face is traditionally more square, with a prominent supraorbital ridge.” Rather than emphasizing breadth across the cheeks, as in rejuvenation for women, a strong, youthful male face will have a square jaw, appropriate width across the cheeks, and a strong brow line with an arch that is flatter than what women seek.
Male cosmetic goals can be complicated by the fact that men “age poorly” and tend to look older than their stated age, he continued, referring to a study that found that men appear about one-third year older than their age, and women about a half year younger. A higher rate of smoking and excess ultraviolet light exposure among men may contribute to this discrepancy, he said.
Overall, men see steady atrophy of facial soft tissue throughout adulthood, in contrast to women who see a more rapid decline that starts at menopause. This makes sense in the context of the slow drop in circulating testosterone that men see beginning at about age 30, at which time men can expect a decrease of about 1% per year, he noted.
A common opener from men, said Dr. Keaney, is “’I look tired.’ When I hear that, I’m thinking about the eyes.” Men are more likely to develop tear troughs and a sunken appearance because of their larger orbital cavities and smaller orbital fat pads, so periocular fillers are often a treatment tool to consider.
Around mens’ eyes, “it’s not just the presence, but the pattern of wrinkles that guides treatment,” he noted. At the lateral canthi, both at rest and with maximum smile, the predominant wrinkle pattern in crows’ feet is the lower fan. The cheek elevator musculature, including the zygomaticus major, are more involved in animation around the eyes with smiling, which is important because reaching for botulinum toxin alone is probably not going to give the patient his desired effect, he added.
Other differences in the male wrinkle pattern can be found on the brow. The “U” contraction pattern between the brows is more common in men than women, at least partly because men have greater involvement of the procerus muscle, Dr. Keaney said.
Taking all of this into account, caution is the watchword when using botulinum toxin for the glabellar and brow region. “Beware of eyebrow ptosis: avoid the frontalis in patients with eyebrow ptosis,” he said. Treating the corrugators can also be an unwise move, since toxin can diffuse to the inferior fibers of the frontalis muscle, he added.
For men with jawline concerns, consider a combination approach, Dr. Keaney said. The lower face and neck can be rejuvenated by a redraping solution, such as skin tightening with high frequency ultrasound or radiofrequency ablation.
Sometimes, a clean, tight sweep of jawline can be restored with dermal fillers to the lower face, along with a noninvasive fat reduction approach, said Dr. Keaney.
Knowing men’s unique anatomy and aging patterns is only half the battle, though. “How you communicate with men and evaluate them has to take into account that you might be seeing a sweaty, nervous, treatment-naive patient,” at the initial consultation, said Dr. Keaney. “Do men care? Yes, but men display a different set of motivations.”
Plan for all of this to take time. “The initial consultation tends to be longer” for male patients, who are “less savvy” about cosmetic procedures than women, he pointed out.
A clear discussion of side effects is critical when discussing treatment options with men. “If they get a side effect, you’ll never see them again – and you won’t ever hear about it,” he added.
Though overall, women have been shown to be more sensitive to pain, in cosmetic procedures, “men are less tolerant of pain.” Plan for this, set reasonable expectations but be proactive about pain control, and still expect a need for some hand-holding, said Dr. Keaney.
“Men do not like surprises,” he added. Clearly define what expected side effects may be, what they’ll look like, and what downtime the patient should expect.
For Dr. Keaney’s part, he’s found that the best way to retain men in his cosmetic practice is to “start with a home run treatment to earn trust.” Men often appreciate a slow and steady approach to addressing concerns. “Make sure to connect the treatment plan to the primary cosmetic concern.”
Dr. Keaney reported that he has relationships with multiple pharmaceutical and cosmetic companies.
ORLANDO – The , a dermatologist in private practice in Arlington, Va. And he may not even be sure what he’s looking for.
However, he probably knows what he doesn’t like about his appearance. When men are questioned, the three areas they are most concerned about is their hairline, their eyes, and their jawline, said Dr. Keaney, speaking at the Orlando Dermatology Aesthetic and Clinical Conference.
It’s important to evaluate men differently, not just for anatomic differences from women, but also for behavioral and psychological factors unique to men as aesthetic patients, he noted.
Anatomic differences are significant and fundamentally shape treatment decisions, Dr. Keaney said. Broadly speaking, “the male face is traditionally more square, with a prominent supraorbital ridge.” Rather than emphasizing breadth across the cheeks, as in rejuvenation for women, a strong, youthful male face will have a square jaw, appropriate width across the cheeks, and a strong brow line with an arch that is flatter than what women seek.
Male cosmetic goals can be complicated by the fact that men “age poorly” and tend to look older than their stated age, he continued, referring to a study that found that men appear about one-third year older than their age, and women about a half year younger. A higher rate of smoking and excess ultraviolet light exposure among men may contribute to this discrepancy, he said.
Overall, men see steady atrophy of facial soft tissue throughout adulthood, in contrast to women who see a more rapid decline that starts at menopause. This makes sense in the context of the slow drop in circulating testosterone that men see beginning at about age 30, at which time men can expect a decrease of about 1% per year, he noted.
A common opener from men, said Dr. Keaney, is “’I look tired.’ When I hear that, I’m thinking about the eyes.” Men are more likely to develop tear troughs and a sunken appearance because of their larger orbital cavities and smaller orbital fat pads, so periocular fillers are often a treatment tool to consider.
Around mens’ eyes, “it’s not just the presence, but the pattern of wrinkles that guides treatment,” he noted. At the lateral canthi, both at rest and with maximum smile, the predominant wrinkle pattern in crows’ feet is the lower fan. The cheek elevator musculature, including the zygomaticus major, are more involved in animation around the eyes with smiling, which is important because reaching for botulinum toxin alone is probably not going to give the patient his desired effect, he added.
Other differences in the male wrinkle pattern can be found on the brow. The “U” contraction pattern between the brows is more common in men than women, at least partly because men have greater involvement of the procerus muscle, Dr. Keaney said.
Taking all of this into account, caution is the watchword when using botulinum toxin for the glabellar and brow region. “Beware of eyebrow ptosis: avoid the frontalis in patients with eyebrow ptosis,” he said. Treating the corrugators can also be an unwise move, since toxin can diffuse to the inferior fibers of the frontalis muscle, he added.
For men with jawline concerns, consider a combination approach, Dr. Keaney said. The lower face and neck can be rejuvenated by a redraping solution, such as skin tightening with high frequency ultrasound or radiofrequency ablation.
Sometimes, a clean, tight sweep of jawline can be restored with dermal fillers to the lower face, along with a noninvasive fat reduction approach, said Dr. Keaney.
Knowing men’s unique anatomy and aging patterns is only half the battle, though. “How you communicate with men and evaluate them has to take into account that you might be seeing a sweaty, nervous, treatment-naive patient,” at the initial consultation, said Dr. Keaney. “Do men care? Yes, but men display a different set of motivations.”
Plan for all of this to take time. “The initial consultation tends to be longer” for male patients, who are “less savvy” about cosmetic procedures than women, he pointed out.
A clear discussion of side effects is critical when discussing treatment options with men. “If they get a side effect, you’ll never see them again – and you won’t ever hear about it,” he added.
Though overall, women have been shown to be more sensitive to pain, in cosmetic procedures, “men are less tolerant of pain.” Plan for this, set reasonable expectations but be proactive about pain control, and still expect a need for some hand-holding, said Dr. Keaney.
“Men do not like surprises,” he added. Clearly define what expected side effects may be, what they’ll look like, and what downtime the patient should expect.
For Dr. Keaney’s part, he’s found that the best way to retain men in his cosmetic practice is to “start with a home run treatment to earn trust.” Men often appreciate a slow and steady approach to addressing concerns. “Make sure to connect the treatment plan to the primary cosmetic concern.”
Dr. Keaney reported that he has relationships with multiple pharmaceutical and cosmetic companies.
EXPERT ANALYSIS FROM ODAC 2019
Small White Spots on the Lips
The Diagnosis: Fordyce Granules
Fordyce granules are prevalent benign anatomic variations that occur in approximately 80% of the population.1 The spots usually present as multiple (usually >10) 1- to 2-mm, painless, yellow-white papules in a symmetric bilateral distribution. They are normal superficial sebaceous glands seen on mucosal surfaces including the oral mucosa, lips, and genitalia. The papules are asymptomatic, and patients often are unaware of their presence. They can appear at any age and can last for months to years. No treatment is indicated, and patients need only reassurance.1
There are several differential diagnoses.2 Granular cell tumors present as solitary, yellowish or pink, slightly indurated, nonmobile, firm masses that usually measure less than 2 cm in diameter and can be associated with local paresthesia. The oral cavity is the second most common site after the skin and usually involves the dorsum of the tongue; however, granular cell tumors also may develop in the substance of the buccal mucosa, lips, or floor of the mouth. On histopathology, the neoplasm is composed of cells with granular cytoplasm that is of neural origin. Granular cell tumors are slow growing and may be present for months. The mean age of onset is in the fourth decade, and females are more likely to be affected. Excisional biopsy is diagnostic and curative.2
Mucoceles of the mouth are solitary, bluish clear, fluctuant, dome-shaped, well-demarcated nodules that usually appear on the lower lip.3 They are caused by rupture of a salivary gland duct due to minor trauma. Mucin is excreted into the surrounding soft tissues, leading to abrupt nontender swelling over the next several weeks. If they originate deeper within the lip they may appear normal in color. Most range from 1 to 2 mm in diameter but can grow to up to several centimeters in size. Other affected sites may include the ventral tongue, posterior buccal mucosa, or soft palate. Excisional biopsy and conservative surgical excision are recommended for diagnosis and management, respectively.3
Oral leukoplakia is a sharply demarcated, white, mucosal plaque that represents either epithelial dysplasia, carcinoma in situ, invasive carcinoma, or hyperkeratosis of unknown etiology. It is a clinical diagnosis of exclusion. The patient may present with a hoarse voice and history of tobacco use. The risk for malignant transformation to squamous cell carcinoma varies from 0% to 20% over the course of 30
years.4 The lesions occur on any mucosal surface, cannot be rubbed off, and usually are asymptomatic.5 The ventral tongue, floor of the mouth, and soft palate are associated with epithelial dysplasia and invasive carcinoma more often than other mucosal sites. There are 2 main types of leukoplakia: localized (unilateral plaque) and proliferative. Because of the risk for cancer, biopsy always is indicated and should be taken from different areas of the lesion (ie, red, verrucous, or nodular areas) if the lesion is nonhomogeneous. Treatment involves excision in the setting of dysplasia or invasive carcinoma. Photodynamic therapy has been shown to reduce the size of oral leukoplakia lesions and is being studied as an alternative therapy.5
Herpes simplex virus type 1 is a common infection of the oral mucosa that classically causes multiple vesicular lesions with an inflammatory erythematous base.6 The lesions are painful and may last for 10 to 14 days. Patients also may develop systemic symptoms such as fever and malaise. Once primary infection with herpes simplex virus has occurred, the virus lives in a latent state in ganglion neurons and can reactivate.6
- Massmanian A, Sorni Valls G, Vera Sempere FJ. Fordyce spots on the glans penis. Br J Dermatol. 1995;133:498-500.
- Lerman M, Freedman PD. Nonneural granular cell tumor of the oral cavity: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:382-384.
- Oka M, Nishioka E, Miyachi R, et al. Case of superficial mucocele of the lower lip. J Dermatol. 2007;34:754-756.
- Lodi G, Sardella A, Bez C, et al. Interventions for treating oral leukoplakia. Cochrane Database Syst Rev. 2006:CD001829.
- Selvam NP, Sadaksharam J, Singaravelu G, et al. Treatment of oral leukoplakia with photodynamic therapy: a pilot study. J Cancer Res Ther. 2015;11:464-467.
- Klein RS. Clinical manifestations and diagnosis of herpes simplex virus type 1 infection. UpToDate website. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-herpes-simplex-virus-type-1-infection.
The Diagnosis: Fordyce Granules
Fordyce granules are prevalent benign anatomic variations that occur in approximately 80% of the population.1 The spots usually present as multiple (usually >10) 1- to 2-mm, painless, yellow-white papules in a symmetric bilateral distribution. They are normal superficial sebaceous glands seen on mucosal surfaces including the oral mucosa, lips, and genitalia. The papules are asymptomatic, and patients often are unaware of their presence. They can appear at any age and can last for months to years. No treatment is indicated, and patients need only reassurance.1
There are several differential diagnoses.2 Granular cell tumors present as solitary, yellowish or pink, slightly indurated, nonmobile, firm masses that usually measure less than 2 cm in diameter and can be associated with local paresthesia. The oral cavity is the second most common site after the skin and usually involves the dorsum of the tongue; however, granular cell tumors also may develop in the substance of the buccal mucosa, lips, or floor of the mouth. On histopathology, the neoplasm is composed of cells with granular cytoplasm that is of neural origin. Granular cell tumors are slow growing and may be present for months. The mean age of onset is in the fourth decade, and females are more likely to be affected. Excisional biopsy is diagnostic and curative.2
Mucoceles of the mouth are solitary, bluish clear, fluctuant, dome-shaped, well-demarcated nodules that usually appear on the lower lip.3 They are caused by rupture of a salivary gland duct due to minor trauma. Mucin is excreted into the surrounding soft tissues, leading to abrupt nontender swelling over the next several weeks. If they originate deeper within the lip they may appear normal in color. Most range from 1 to 2 mm in diameter but can grow to up to several centimeters in size. Other affected sites may include the ventral tongue, posterior buccal mucosa, or soft palate. Excisional biopsy and conservative surgical excision are recommended for diagnosis and management, respectively.3
Oral leukoplakia is a sharply demarcated, white, mucosal plaque that represents either epithelial dysplasia, carcinoma in situ, invasive carcinoma, or hyperkeratosis of unknown etiology. It is a clinical diagnosis of exclusion. The patient may present with a hoarse voice and history of tobacco use. The risk for malignant transformation to squamous cell carcinoma varies from 0% to 20% over the course of 30
years.4 The lesions occur on any mucosal surface, cannot be rubbed off, and usually are asymptomatic.5 The ventral tongue, floor of the mouth, and soft palate are associated with epithelial dysplasia and invasive carcinoma more often than other mucosal sites. There are 2 main types of leukoplakia: localized (unilateral plaque) and proliferative. Because of the risk for cancer, biopsy always is indicated and should be taken from different areas of the lesion (ie, red, verrucous, or nodular areas) if the lesion is nonhomogeneous. Treatment involves excision in the setting of dysplasia or invasive carcinoma. Photodynamic therapy has been shown to reduce the size of oral leukoplakia lesions and is being studied as an alternative therapy.5
Herpes simplex virus type 1 is a common infection of the oral mucosa that classically causes multiple vesicular lesions with an inflammatory erythematous base.6 The lesions are painful and may last for 10 to 14 days. Patients also may develop systemic symptoms such as fever and malaise. Once primary infection with herpes simplex virus has occurred, the virus lives in a latent state in ganglion neurons and can reactivate.6
The Diagnosis: Fordyce Granules
Fordyce granules are prevalent benign anatomic variations that occur in approximately 80% of the population.1 The spots usually present as multiple (usually >10) 1- to 2-mm, painless, yellow-white papules in a symmetric bilateral distribution. They are normal superficial sebaceous glands seen on mucosal surfaces including the oral mucosa, lips, and genitalia. The papules are asymptomatic, and patients often are unaware of their presence. They can appear at any age and can last for months to years. No treatment is indicated, and patients need only reassurance.1
There are several differential diagnoses.2 Granular cell tumors present as solitary, yellowish or pink, slightly indurated, nonmobile, firm masses that usually measure less than 2 cm in diameter and can be associated with local paresthesia. The oral cavity is the second most common site after the skin and usually involves the dorsum of the tongue; however, granular cell tumors also may develop in the substance of the buccal mucosa, lips, or floor of the mouth. On histopathology, the neoplasm is composed of cells with granular cytoplasm that is of neural origin. Granular cell tumors are slow growing and may be present for months. The mean age of onset is in the fourth decade, and females are more likely to be affected. Excisional biopsy is diagnostic and curative.2
Mucoceles of the mouth are solitary, bluish clear, fluctuant, dome-shaped, well-demarcated nodules that usually appear on the lower lip.3 They are caused by rupture of a salivary gland duct due to minor trauma. Mucin is excreted into the surrounding soft tissues, leading to abrupt nontender swelling over the next several weeks. If they originate deeper within the lip they may appear normal in color. Most range from 1 to 2 mm in diameter but can grow to up to several centimeters in size. Other affected sites may include the ventral tongue, posterior buccal mucosa, or soft palate. Excisional biopsy and conservative surgical excision are recommended for diagnosis and management, respectively.3
Oral leukoplakia is a sharply demarcated, white, mucosal plaque that represents either epithelial dysplasia, carcinoma in situ, invasive carcinoma, or hyperkeratosis of unknown etiology. It is a clinical diagnosis of exclusion. The patient may present with a hoarse voice and history of tobacco use. The risk for malignant transformation to squamous cell carcinoma varies from 0% to 20% over the course of 30
years.4 The lesions occur on any mucosal surface, cannot be rubbed off, and usually are asymptomatic.5 The ventral tongue, floor of the mouth, and soft palate are associated with epithelial dysplasia and invasive carcinoma more often than other mucosal sites. There are 2 main types of leukoplakia: localized (unilateral plaque) and proliferative. Because of the risk for cancer, biopsy always is indicated and should be taken from different areas of the lesion (ie, red, verrucous, or nodular areas) if the lesion is nonhomogeneous. Treatment involves excision in the setting of dysplasia or invasive carcinoma. Photodynamic therapy has been shown to reduce the size of oral leukoplakia lesions and is being studied as an alternative therapy.5
Herpes simplex virus type 1 is a common infection of the oral mucosa that classically causes multiple vesicular lesions with an inflammatory erythematous base.6 The lesions are painful and may last for 10 to 14 days. Patients also may develop systemic symptoms such as fever and malaise. Once primary infection with herpes simplex virus has occurred, the virus lives in a latent state in ganglion neurons and can reactivate.6
- Massmanian A, Sorni Valls G, Vera Sempere FJ. Fordyce spots on the glans penis. Br J Dermatol. 1995;133:498-500.
- Lerman M, Freedman PD. Nonneural granular cell tumor of the oral cavity: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:382-384.
- Oka M, Nishioka E, Miyachi R, et al. Case of superficial mucocele of the lower lip. J Dermatol. 2007;34:754-756.
- Lodi G, Sardella A, Bez C, et al. Interventions for treating oral leukoplakia. Cochrane Database Syst Rev. 2006:CD001829.
- Selvam NP, Sadaksharam J, Singaravelu G, et al. Treatment of oral leukoplakia with photodynamic therapy: a pilot study. J Cancer Res Ther. 2015;11:464-467.
- Klein RS. Clinical manifestations and diagnosis of herpes simplex virus type 1 infection. UpToDate website. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-herpes-simplex-virus-type-1-infection.
- Massmanian A, Sorni Valls G, Vera Sempere FJ. Fordyce spots on the glans penis. Br J Dermatol. 1995;133:498-500.
- Lerman M, Freedman PD. Nonneural granular cell tumor of the oral cavity: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:382-384.
- Oka M, Nishioka E, Miyachi R, et al. Case of superficial mucocele of the lower lip. J Dermatol. 2007;34:754-756.
- Lodi G, Sardella A, Bez C, et al. Interventions for treating oral leukoplakia. Cochrane Database Syst Rev. 2006:CD001829.
- Selvam NP, Sadaksharam J, Singaravelu G, et al. Treatment of oral leukoplakia with photodynamic therapy: a pilot study. J Cancer Res Ther. 2015;11:464-467.
- Klein RS. Clinical manifestations and diagnosis of herpes simplex virus type 1 infection. UpToDate website. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-herpes-simplex-virus-type-1-infection.
Platelet-rich plasma: Is your practice ready?
ORLANDO – Platelet-rich plasma offers much for patients and dermatologists: It’s low-risk, has a low cost of entry, and usefully augments other medications and procedures for androgenetic alopecia and facial rejuvenation.
But there’s work to be done in standardizing its use and really understanding where, when, and for whom platelet-rich plasma (PRP) will be best used, said Dierdre Hooper, MD, a dermatologist in private practice in New Orleans.
As far back as the 1970s, PRP was used as a transfusion product, with use expanding during the following decade. “It’s really the ‘everywhere’ product,’” said Dr. Hooper, speaking at the Aesthetic, Surgical, and Clinical Dermatology Conference (ODAC).
Over the course of the past four decades, PRP has been explored for musculoskeletal healing, in gynecology, urology, cardiac surgery, ophthalmology, and for plastic surgery. “Initial skepticism has given way as some evidence is building,” said Dr. Hooper.
PRP, considered a biologic product, is produced by centrifuging a donor venipuncture. Among the pros of using PRP in a clinical practice, said Dr. Hooper, is the fact that numerous clinical studies do show benefit. The risk is low, as is the cost, and downtime is brief. All of these contribute to attractiveness to patients, who also like the idea of an all-natural product with an autologous source.
But consensus is lacking about some key aspects of utilization, including the best mode of preparation and optimal treatment schedule. Outcomes can be unpredictable, making it tough to say how cost-effective the regimen will be for a particular patient. “The ‘cons’ just come down to no consensus,” said Dr. Hooper.
Some of the basic science makes a compelling case for PRP: Activated platelets have secretory granules. These modify the pericellular milieu through release of a variety of growth factors by secretory granules. “We all were taught back in the day that platelets adhere to promote clotting, but they do a lot more than that – when the platelet is activated, it releases growth factors,” said Dr. Hooper. “Big picture? Think: This is how we heal.”
After blood collection, the sample is centrifuged. The goal of centrifuging is to achieve a platelet concentration of 1 to 1.5 million platelets per mL, or four to six times the platelet concentration seen in whole blood. In practice, there are variations in the mode of preparation, and in an individual’s platelet level at the time of venipuncture, said Dr. Hooper, so it’s hard to know what the platelet “dose” is from PRP.
After centrifuging, the sample will be stratified into a bottom portion, consisting primarily of red blood cells, a middle portion that’s the PRP, and a top portion that is platelet-poor plasma. Dr. Hooper draws up and saves the platelet- poor plasma as well, since it probably also contains some growth factors. She’ll save that for application or injection after a PRP treatment for some patients.
Dermatology presents a host of uses for PRP. In addition to application after microneedling or resurfacing and injectable aesthetic uses, PRP can also be used to treat melasma, acne scarring, and androgenetic alopecia.
The strongest data for PRP currently are for androgenetic alopecia, said Dr. Hooper, because that’s where most of the work has been done to date. Growth factors in PRP can target the dermal papillae, shortening the anagen phase. “You will improve the anagen:telogen ratio and increase hair density and thickness,” she said.
“When you talk to your hair loss patients, there are drawbacks” with home therapy such as minoxidil and finasteride, said Dr. Hooper. “Compliance is an issue. I’m a firm believer in combination treatment for hair loss.” Studies have shown increased hair thickness and moderately decreased hair loss with PRP. Anecdotally, said Dr. Hooper, hair becomes coarser, feeling fuller and thicker; one study found that about a quarter of patients reported this effect.
Through experience, Dr. Hooper’s learned some pearls for using PRP for androgenetic alopecia. Her male patients appreciate the use of a chilling device to help with pain, especially since Dr. Hooper uses a triple-needle syringe to stamp the scalp as she injects the PRP. Depending on how her patients are tolerating the procedure, she’ll follow up by injecting some platelet-poor plasma as well.
An additional pearl? “Have your patients bring a baseball cap.” Between procedure preparation, some oozing of PRP, and bleeding from injection sites, men don’t leave as well-coiffed as when they entered, she said.
Dr. Hooper has patients return four times over the course of 6 months for androgenetic alopecia, with repeat treatments about every 6 months thereafter.
Several studies have looked at using intradermal PRP for facial rejuvenation, with largely positive results. “Once again, we see consistent efficacy with no side effects,” said Dr. Hooper. She will use PRP either intradermally or topically after microneedling or fractional ablative laser resurfacing.
If it’s being used topically, Dr. Hooper will simply wipe the PRP on after the resurfacing treatment. For microneedling, “As we finish one zone, we topically apply the PRP and move on,” she said, adding that she instructs the patient not to wash her face until bedtime.
“I like injectable delivery for PRP as well,” said Dr. Hooper. She will often use it for crepey skin under the eyes as an add-on to other treatments, she said.
Her patients report that one major upside of post-resurfacing PRP is that they feel they recover more quickly. “Less erythema and less recovery time – that’s something that’s always helpful,” said Dr. Hooper. She uses the same treatment schedule for rejuvenation as for alopecia.
Some studies have shown promise for injected PRP for striae, said Dr. Hooper. She has just begun using injected PRP for striae in her practice and is encouraged by early results she’s seeing. It’s easier for patients than using multiple at-home treatments: “I think it’s just an option, they can pop in 3 times over the next few months” for some added benefit, she said.
Scanning the audience, Dr. Hooper said, “I see a lot of younger faces out there. I would challenge you to do the studies” to build evidence-based protocols for PRP in dermatology, since lack of consensus still hinders both adoption and high-quality research.
Dr. Hooper reported multiple financial relationships with pharmaceutical and cosmetic companies.
SOURCE: Hooper, D. ODAC 2018.
ORLANDO – Platelet-rich plasma offers much for patients and dermatologists: It’s low-risk, has a low cost of entry, and usefully augments other medications and procedures for androgenetic alopecia and facial rejuvenation.
But there’s work to be done in standardizing its use and really understanding where, when, and for whom platelet-rich plasma (PRP) will be best used, said Dierdre Hooper, MD, a dermatologist in private practice in New Orleans.
As far back as the 1970s, PRP was used as a transfusion product, with use expanding during the following decade. “It’s really the ‘everywhere’ product,’” said Dr. Hooper, speaking at the Aesthetic, Surgical, and Clinical Dermatology Conference (ODAC).
Over the course of the past four decades, PRP has been explored for musculoskeletal healing, in gynecology, urology, cardiac surgery, ophthalmology, and for plastic surgery. “Initial skepticism has given way as some evidence is building,” said Dr. Hooper.
PRP, considered a biologic product, is produced by centrifuging a donor venipuncture. Among the pros of using PRP in a clinical practice, said Dr. Hooper, is the fact that numerous clinical studies do show benefit. The risk is low, as is the cost, and downtime is brief. All of these contribute to attractiveness to patients, who also like the idea of an all-natural product with an autologous source.
But consensus is lacking about some key aspects of utilization, including the best mode of preparation and optimal treatment schedule. Outcomes can be unpredictable, making it tough to say how cost-effective the regimen will be for a particular patient. “The ‘cons’ just come down to no consensus,” said Dr. Hooper.
Some of the basic science makes a compelling case for PRP: Activated platelets have secretory granules. These modify the pericellular milieu through release of a variety of growth factors by secretory granules. “We all were taught back in the day that platelets adhere to promote clotting, but they do a lot more than that – when the platelet is activated, it releases growth factors,” said Dr. Hooper. “Big picture? Think: This is how we heal.”
After blood collection, the sample is centrifuged. The goal of centrifuging is to achieve a platelet concentration of 1 to 1.5 million platelets per mL, or four to six times the platelet concentration seen in whole blood. In practice, there are variations in the mode of preparation, and in an individual’s platelet level at the time of venipuncture, said Dr. Hooper, so it’s hard to know what the platelet “dose” is from PRP.
After centrifuging, the sample will be stratified into a bottom portion, consisting primarily of red blood cells, a middle portion that’s the PRP, and a top portion that is platelet-poor plasma. Dr. Hooper draws up and saves the platelet- poor plasma as well, since it probably also contains some growth factors. She’ll save that for application or injection after a PRP treatment for some patients.
Dermatology presents a host of uses for PRP. In addition to application after microneedling or resurfacing and injectable aesthetic uses, PRP can also be used to treat melasma, acne scarring, and androgenetic alopecia.
The strongest data for PRP currently are for androgenetic alopecia, said Dr. Hooper, because that’s where most of the work has been done to date. Growth factors in PRP can target the dermal papillae, shortening the anagen phase. “You will improve the anagen:telogen ratio and increase hair density and thickness,” she said.
“When you talk to your hair loss patients, there are drawbacks” with home therapy such as minoxidil and finasteride, said Dr. Hooper. “Compliance is an issue. I’m a firm believer in combination treatment for hair loss.” Studies have shown increased hair thickness and moderately decreased hair loss with PRP. Anecdotally, said Dr. Hooper, hair becomes coarser, feeling fuller and thicker; one study found that about a quarter of patients reported this effect.
Through experience, Dr. Hooper’s learned some pearls for using PRP for androgenetic alopecia. Her male patients appreciate the use of a chilling device to help with pain, especially since Dr. Hooper uses a triple-needle syringe to stamp the scalp as she injects the PRP. Depending on how her patients are tolerating the procedure, she’ll follow up by injecting some platelet-poor plasma as well.
An additional pearl? “Have your patients bring a baseball cap.” Between procedure preparation, some oozing of PRP, and bleeding from injection sites, men don’t leave as well-coiffed as when they entered, she said.
Dr. Hooper has patients return four times over the course of 6 months for androgenetic alopecia, with repeat treatments about every 6 months thereafter.
Several studies have looked at using intradermal PRP for facial rejuvenation, with largely positive results. “Once again, we see consistent efficacy with no side effects,” said Dr. Hooper. She will use PRP either intradermally or topically after microneedling or fractional ablative laser resurfacing.
If it’s being used topically, Dr. Hooper will simply wipe the PRP on after the resurfacing treatment. For microneedling, “As we finish one zone, we topically apply the PRP and move on,” she said, adding that she instructs the patient not to wash her face until bedtime.
“I like injectable delivery for PRP as well,” said Dr. Hooper. She will often use it for crepey skin under the eyes as an add-on to other treatments, she said.
Her patients report that one major upside of post-resurfacing PRP is that they feel they recover more quickly. “Less erythema and less recovery time – that’s something that’s always helpful,” said Dr. Hooper. She uses the same treatment schedule for rejuvenation as for alopecia.
Some studies have shown promise for injected PRP for striae, said Dr. Hooper. She has just begun using injected PRP for striae in her practice and is encouraged by early results she’s seeing. It’s easier for patients than using multiple at-home treatments: “I think it’s just an option, they can pop in 3 times over the next few months” for some added benefit, she said.
Scanning the audience, Dr. Hooper said, “I see a lot of younger faces out there. I would challenge you to do the studies” to build evidence-based protocols for PRP in dermatology, since lack of consensus still hinders both adoption and high-quality research.
Dr. Hooper reported multiple financial relationships with pharmaceutical and cosmetic companies.
SOURCE: Hooper, D. ODAC 2018.
ORLANDO – Platelet-rich plasma offers much for patients and dermatologists: It’s low-risk, has a low cost of entry, and usefully augments other medications and procedures for androgenetic alopecia and facial rejuvenation.
But there’s work to be done in standardizing its use and really understanding where, when, and for whom platelet-rich plasma (PRP) will be best used, said Dierdre Hooper, MD, a dermatologist in private practice in New Orleans.
As far back as the 1970s, PRP was used as a transfusion product, with use expanding during the following decade. “It’s really the ‘everywhere’ product,’” said Dr. Hooper, speaking at the Aesthetic, Surgical, and Clinical Dermatology Conference (ODAC).
Over the course of the past four decades, PRP has been explored for musculoskeletal healing, in gynecology, urology, cardiac surgery, ophthalmology, and for plastic surgery. “Initial skepticism has given way as some evidence is building,” said Dr. Hooper.
PRP, considered a biologic product, is produced by centrifuging a donor venipuncture. Among the pros of using PRP in a clinical practice, said Dr. Hooper, is the fact that numerous clinical studies do show benefit. The risk is low, as is the cost, and downtime is brief. All of these contribute to attractiveness to patients, who also like the idea of an all-natural product with an autologous source.
But consensus is lacking about some key aspects of utilization, including the best mode of preparation and optimal treatment schedule. Outcomes can be unpredictable, making it tough to say how cost-effective the regimen will be for a particular patient. “The ‘cons’ just come down to no consensus,” said Dr. Hooper.
Some of the basic science makes a compelling case for PRP: Activated platelets have secretory granules. These modify the pericellular milieu through release of a variety of growth factors by secretory granules. “We all were taught back in the day that platelets adhere to promote clotting, but they do a lot more than that – when the platelet is activated, it releases growth factors,” said Dr. Hooper. “Big picture? Think: This is how we heal.”
After blood collection, the sample is centrifuged. The goal of centrifuging is to achieve a platelet concentration of 1 to 1.5 million platelets per mL, or four to six times the platelet concentration seen in whole blood. In practice, there are variations in the mode of preparation, and in an individual’s platelet level at the time of venipuncture, said Dr. Hooper, so it’s hard to know what the platelet “dose” is from PRP.
After centrifuging, the sample will be stratified into a bottom portion, consisting primarily of red blood cells, a middle portion that’s the PRP, and a top portion that is platelet-poor plasma. Dr. Hooper draws up and saves the platelet- poor plasma as well, since it probably also contains some growth factors. She’ll save that for application or injection after a PRP treatment for some patients.
Dermatology presents a host of uses for PRP. In addition to application after microneedling or resurfacing and injectable aesthetic uses, PRP can also be used to treat melasma, acne scarring, and androgenetic alopecia.
The strongest data for PRP currently are for androgenetic alopecia, said Dr. Hooper, because that’s where most of the work has been done to date. Growth factors in PRP can target the dermal papillae, shortening the anagen phase. “You will improve the anagen:telogen ratio and increase hair density and thickness,” she said.
“When you talk to your hair loss patients, there are drawbacks” with home therapy such as minoxidil and finasteride, said Dr. Hooper. “Compliance is an issue. I’m a firm believer in combination treatment for hair loss.” Studies have shown increased hair thickness and moderately decreased hair loss with PRP. Anecdotally, said Dr. Hooper, hair becomes coarser, feeling fuller and thicker; one study found that about a quarter of patients reported this effect.
Through experience, Dr. Hooper’s learned some pearls for using PRP for androgenetic alopecia. Her male patients appreciate the use of a chilling device to help with pain, especially since Dr. Hooper uses a triple-needle syringe to stamp the scalp as she injects the PRP. Depending on how her patients are tolerating the procedure, she’ll follow up by injecting some platelet-poor plasma as well.
An additional pearl? “Have your patients bring a baseball cap.” Between procedure preparation, some oozing of PRP, and bleeding from injection sites, men don’t leave as well-coiffed as when they entered, she said.
Dr. Hooper has patients return four times over the course of 6 months for androgenetic alopecia, with repeat treatments about every 6 months thereafter.
Several studies have looked at using intradermal PRP for facial rejuvenation, with largely positive results. “Once again, we see consistent efficacy with no side effects,” said Dr. Hooper. She will use PRP either intradermally or topically after microneedling or fractional ablative laser resurfacing.
If it’s being used topically, Dr. Hooper will simply wipe the PRP on after the resurfacing treatment. For microneedling, “As we finish one zone, we topically apply the PRP and move on,” she said, adding that she instructs the patient not to wash her face until bedtime.
“I like injectable delivery for PRP as well,” said Dr. Hooper. She will often use it for crepey skin under the eyes as an add-on to other treatments, she said.
Her patients report that one major upside of post-resurfacing PRP is that they feel they recover more quickly. “Less erythema and less recovery time – that’s something that’s always helpful,” said Dr. Hooper. She uses the same treatment schedule for rejuvenation as for alopecia.
Some studies have shown promise for injected PRP for striae, said Dr. Hooper. She has just begun using injected PRP for striae in her practice and is encouraged by early results she’s seeing. It’s easier for patients than using multiple at-home treatments: “I think it’s just an option, they can pop in 3 times over the next few months” for some added benefit, she said.
Scanning the audience, Dr. Hooper said, “I see a lot of younger faces out there. I would challenge you to do the studies” to build evidence-based protocols for PRP in dermatology, since lack of consensus still hinders both adoption and high-quality research.
Dr. Hooper reported multiple financial relationships with pharmaceutical and cosmetic companies.
SOURCE: Hooper, D. ODAC 2018.
EXPERT ANALYSIS FROM ODAC 2019
Brazilian study finds oral tranexamic acid effective for facial melasma
of patients from a public dermatology clinic in Brazil.
“Our study was one of the few to compare the use of oral TA isolated against a control group using a placebo and to evaluate the results by four different methods,” wrote lead author Mariana Morais Tavares Colferai, MD, of the Universidade de Mogi das Cruzes (Brazil), and her coauthors. The study was published online in the Journal of Cosmetic Dermatology.TA is a plasmin inhibitor, first described as a treatment for melasma in 1979. It is approved in the United States for treating menorrhagia.
In the randomized, double-blind, controlled trial of 47 patients with facial melasma – 37 completed the study – participants were assigned to one of two groups: Group A received 250 mg of tranexamic acid twice daily (n = 20) while group B received a placebo twice daily (n = 17). All patients were advised to use sunscreen. Before treatment and after 12 weeks, the researchers evaluated patients with four methods: the Melasma Area Severity Index (MASI), photographic records, patient evaluation with questionnaire (MELASQoL), and colorimetry assessed via a colorimeter.
Per evaluation of all tests, melasma improved in 50% of patients in group A, compared with 5.9% of patients in group B (P less than .005). Group A saw improvements in the mean MASI score (20.9 at pretreatment vs. 10.8 after treatment, P less than .001), mean MELASQoL value (55.4 vs. 38.2, P less than .001), and colorimetry (55.0 vs. 56.1, with higher values indicating lighter pigmentation, P = .033).
The most common side effects among those who received TA were gastrointestinal effects, such as nausea and diarrhea (5%); changes in menstrual flow (10%); and headache (14%). No serious side effects were reported.
The authors acknowledged several potential limitations in their study, including a lack of intermediate evaluations between pretreatment and 12 weeks. They also noted that the photographs were dichotomously classified as either “yes for improvement” or “no for the lack of improvement or worsening,” which may “limit the accuracy of our results” for that particular method.
The tranexamic acid and placebo for the study was provided by U.SK Dermatology/Brazil. No conflicts of interest were reported.
SOURCE: Colferai MMT et al. J Cosmet Dermatol. 2018 Dec 9. doi: 10.1111/jocd.12830.
of patients from a public dermatology clinic in Brazil.
“Our study was one of the few to compare the use of oral TA isolated against a control group using a placebo and to evaluate the results by four different methods,” wrote lead author Mariana Morais Tavares Colferai, MD, of the Universidade de Mogi das Cruzes (Brazil), and her coauthors. The study was published online in the Journal of Cosmetic Dermatology.TA is a plasmin inhibitor, first described as a treatment for melasma in 1979. It is approved in the United States for treating menorrhagia.
In the randomized, double-blind, controlled trial of 47 patients with facial melasma – 37 completed the study – participants were assigned to one of two groups: Group A received 250 mg of tranexamic acid twice daily (n = 20) while group B received a placebo twice daily (n = 17). All patients were advised to use sunscreen. Before treatment and after 12 weeks, the researchers evaluated patients with four methods: the Melasma Area Severity Index (MASI), photographic records, patient evaluation with questionnaire (MELASQoL), and colorimetry assessed via a colorimeter.
Per evaluation of all tests, melasma improved in 50% of patients in group A, compared with 5.9% of patients in group B (P less than .005). Group A saw improvements in the mean MASI score (20.9 at pretreatment vs. 10.8 after treatment, P less than .001), mean MELASQoL value (55.4 vs. 38.2, P less than .001), and colorimetry (55.0 vs. 56.1, with higher values indicating lighter pigmentation, P = .033).
The most common side effects among those who received TA were gastrointestinal effects, such as nausea and diarrhea (5%); changes in menstrual flow (10%); and headache (14%). No serious side effects were reported.
The authors acknowledged several potential limitations in their study, including a lack of intermediate evaluations between pretreatment and 12 weeks. They also noted that the photographs were dichotomously classified as either “yes for improvement” or “no for the lack of improvement or worsening,” which may “limit the accuracy of our results” for that particular method.
The tranexamic acid and placebo for the study was provided by U.SK Dermatology/Brazil. No conflicts of interest were reported.
SOURCE: Colferai MMT et al. J Cosmet Dermatol. 2018 Dec 9. doi: 10.1111/jocd.12830.
of patients from a public dermatology clinic in Brazil.
“Our study was one of the few to compare the use of oral TA isolated against a control group using a placebo and to evaluate the results by four different methods,” wrote lead author Mariana Morais Tavares Colferai, MD, of the Universidade de Mogi das Cruzes (Brazil), and her coauthors. The study was published online in the Journal of Cosmetic Dermatology.TA is a plasmin inhibitor, first described as a treatment for melasma in 1979. It is approved in the United States for treating menorrhagia.
In the randomized, double-blind, controlled trial of 47 patients with facial melasma – 37 completed the study – participants were assigned to one of two groups: Group A received 250 mg of tranexamic acid twice daily (n = 20) while group B received a placebo twice daily (n = 17). All patients were advised to use sunscreen. Before treatment and after 12 weeks, the researchers evaluated patients with four methods: the Melasma Area Severity Index (MASI), photographic records, patient evaluation with questionnaire (MELASQoL), and colorimetry assessed via a colorimeter.
Per evaluation of all tests, melasma improved in 50% of patients in group A, compared with 5.9% of patients in group B (P less than .005). Group A saw improvements in the mean MASI score (20.9 at pretreatment vs. 10.8 after treatment, P less than .001), mean MELASQoL value (55.4 vs. 38.2, P less than .001), and colorimetry (55.0 vs. 56.1, with higher values indicating lighter pigmentation, P = .033).
The most common side effects among those who received TA were gastrointestinal effects, such as nausea and diarrhea (5%); changes in menstrual flow (10%); and headache (14%). No serious side effects were reported.
The authors acknowledged several potential limitations in their study, including a lack of intermediate evaluations between pretreatment and 12 weeks. They also noted that the photographs were dichotomously classified as either “yes for improvement” or “no for the lack of improvement or worsening,” which may “limit the accuracy of our results” for that particular method.
The tranexamic acid and placebo for the study was provided by U.SK Dermatology/Brazil. No conflicts of interest were reported.
SOURCE: Colferai MMT et al. J Cosmet Dermatol. 2018 Dec 9. doi: 10.1111/jocd.12830.
FROM THE JOURNAL OF COSMETIC DERMATOLOGY
Key clinical point: After 12 weeks and according to four methods of evaluation, oral tranexamic acid was effective in 50% of patients with melasma.
Major finding: Melasma improved in 50% of patients who received oral tranexamic acid, compared with 5.9% of patients who received placebo (P less than .005).
Study details: A randomized, double-blind, controlled trial of 37 patients who received oral tranexamic acid or placebo twice a day for 12 weeks.
Disclosures: The tranexamic acid and placebo were provided by U.SK Dermatology/Brazil. No conflicts of interest were reported.
Source: Colferai MMT et al. J Cosmet Dermatol. 2018 Dec 9. doi: 10.1111/jocd.12830.