User login
Treating stress urinary incontinence with suburethral slings
- Suburethral sling procedures are effective in treating patients with urethral hypermobility, intrinsic sphincter deficiency, low-pressure urethras, and increased intra-abdominal pressure.
- Autologous slings may be a better choice in cases of severe urogenital atrophy, previous radiation, or extensive scarring from previous repairs.
- For both the tension-free vaginal tape and SPARC slings, mark the suprapubic region 1 cm above and 1 cm lateral to the pubic symphsis on the left and right sides and inject 20 cc of a 1:1 mixture of local anesthetic and normal saline into the marked regions.
- Once the trocars are in place, fill the bladder with 250 cc of water and perform a cough stress test to confirm continence.
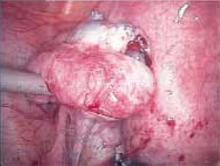
When the suburethral sling was first described in 1907 by von Giordano, it entailed placing autologous tissue underneath the bladder neck and suspending it superiorly. Complications including urethral erosion, infection, bleeding, and fistula formation led many surgeons to use it sparingly.
Fast forward to the 21st century: Synthetic materials and new techniques were introduced, simplifying the sling procedures and raising the long-term success rates to 84%.1 As a result, slings now stand at the forefront of stress urinary incontinence (SUI) treatment. Among advances are the tension-free vaginal tape (TVT) sling (Gynecare, a division of Ethicon Inc., Somerville, NJ) and the SPARC sling (American Medical Systems, Inc., Minnetonka, Minn). The former, approved in the U.S. in 1998, calls for another look due to of the recent publication of a Cochrane review of outcomes studies, while the latter, approved by the FDA in August 2001, is the newest technique deserving examination. Clearly, with 83,010 incontinence procedures performed in the U.S. in 1999,2 a detailed look at the suburethral sling is warranted. Here, we review materials, indications, techniques, complications, and outcomes.
Materials
The choice of material—either organic or synthetic—depends on several factors: availability, cost, patient and surgeon preference, and clinical variables. (TABLE 1) outlines the advantages and disadvantages of each material type. Organic slings include autologous tissues (rectus fascia and fascia lata graft), and allografts or xenografts (cadaveric fascia lata graft, human dermal graft, or porcine small intestine and dermal graft). Synthetic slings are made of polyethylene terephthalate, expanded polytetrafluoroethylene, and polypropylene.
While sling procedures utilizing organic materials do have their benefits, synthetic slings, particularly the polypropylene mesh used in TVT and SPARC, have proven to be a stable material unlikely to deteriorate with time. Further, increased collagen metabolism around this synthetic sling promotes an ingrowth of tissue through the mesh.
TABLE 1
Slings: advantages and disadvantages of various materials
| SLING MATERIAL | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| Autologous tissues (rectus fascia, fascia lata, or vaginal wall) |
|
|
| Allografts (cadaveric fascia lata or dermis) |
|
|
| Xenografts (porcine dermis or small intestine) | ||
| Synthetic mesh (polyethylene terephthalate, expanded polytetrafluoroethylene, or polypropylene) |
|
|
Indications
Suburethral sling procedures are typically used for the treatment of genuine stress urinary incontinence (GSUI), in which the urethra becomes either hypermobile and unstable or its intrinsic sphincter becomes incompetent. In fact, slings are technically easier to place in patients with anatomic urethrovesical junction hypermobility compared to those with fixed urethras. Several authors also have suggested the sling’s advantage in patients with low-pressure urethras.3
Use urodynamic criteria to diagnose intrinsic sphincter deficiency (ISD), which is defined as a Valsalva leak point pressure of less than 60 cm water or maximal urethral closure pressure of less than 20 cm water. (Bear in mind, however, that these cut-off criteria are controversial.4,5)
Also, consider slings in patients with recurrent GSUI, inherited collagen deficiency, and increased abdominal pressure (e.g., women with chronic obstructive pulmonary disease, obesity, or high-impact physical activity). The sling also can be used as an adjunct to other transvaginal surgeries (e.g., hysterectomy or prolapse repair).
Autologous slings may be a better choice than synthetic slings in cases of severe urogenital atrophy, previous radiation, or extensive scarring from previous repairs. In these instances, the patient may be at-risk for postoperative vaginal necrosis or erosion.6 Due to their biocompatibility, autologous slings are more likely to heal over a vaginal erosion and less likely to infect or erode into the urethra. In any event, urogenital atrophy should be treated with local estrogen preoperatively to prevent some of these complications.
Technique
Conventionally, suburethral slings were placed via a combined vaginal and abdominal approach into the retropubic space of Retzius. Alternatively, the procedure could be performed abdominally by creating a suburethral tunnel via pelvic incisions, but this is the most difficult route.
Most recently, technological advances have simplified the vaginal approach, which utilizes minimal suburethral dissection and small suprapubic incisions. This technique is subdivided into “bottom-up” and “top-down” approaches. In the bottom-up TVT, the sling is inserted into a vaginal incision and threaded up through the patient’s pelvis, exiting from a small suprapubic incision. The topdown SPARC entails a reverse approach, starting from a suprapubic incision and exiting from a vaginal incision. New modifications allow for an abdominal TVT approach, as well, which we describe in detail in a later section.
Surgeons who are familiar with traditional needle suspensions may be more comfortable with the top-down approach. The need for concomitant surgery (e.g., hysterectomy or prolapse repair) not only determines the type of incontinence procedure, but also dictates the approach.
Preparing the patient. Place the patient under regional or local anesthesia with sedation so that an intraoperative cough stress test can be performed. Then administer an intravenous dose of a broad-spectrum antibiotic. Insert a 16 to 18 French Foley catheter into the urethra. Mark the suprapubic region 1 cm above and 1 cm lateral to the pubic symphsis on the left and right sides of the patient. Inject approximately 20 cc of a 1:1 mixture of local anesthetic and normal saline into the marked areas. We typically use 60 cc of 0.25% bupivicaine with epinephrine, diluted 1:1 with 60 cc of normal saline. After administering the local anesthetic suprapubically, inject a similar solution into the anterior vaginal wall suburethrally in the midline and laterally toward the retropubic tunnels.
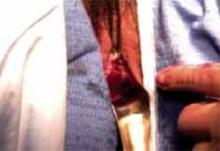
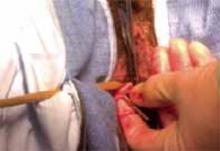
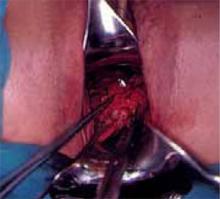
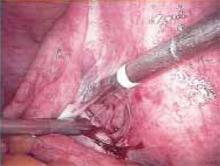
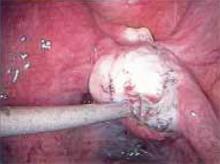
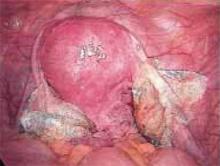
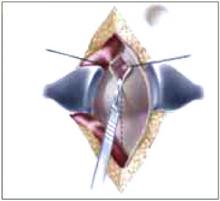
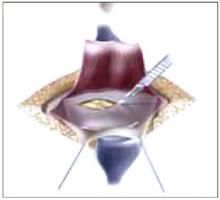
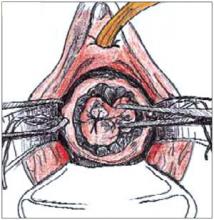
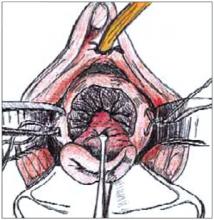
Making the incisions. Both the TVT and SPARC techniques utilize the same type and location of incisions. As such, make a 0.5-cm incision into the abdominal skin on each side of the midline, approximately 1 cm lateral to midline and 1 cm above the pubic symphsis. Next, make a 1.5- to 2-cm vertical incision in the vaginal mucosa, starting 1.5 cm from the urethral meatus (FIGURE 1). Use Metzenbaum scissors to dissect the vaginal mucosa from the pubocervical fascia sub- and para-urethrally on both sides (FIGURE 2). Insert a Foley catheter guide (similar to the Lowsley retractor) into the catheter and deviate it to the ipsilateral side, thereby retracting the bladder neck to the contralateral side. Proceed with the placement of either the TVT or SPARC sling.
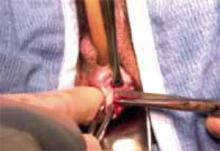
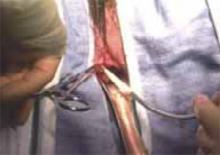
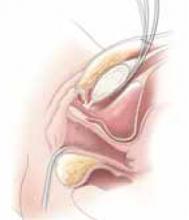
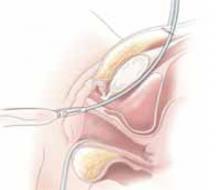
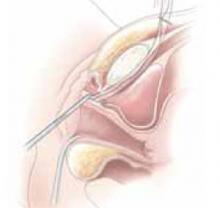
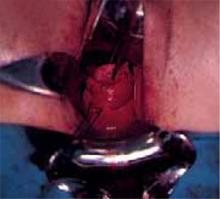
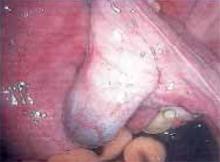
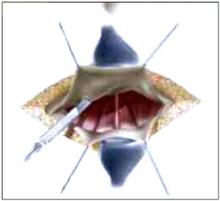
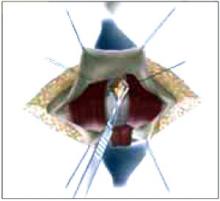
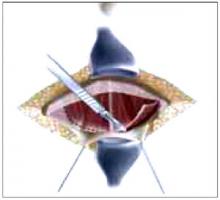
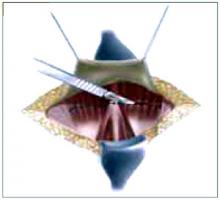
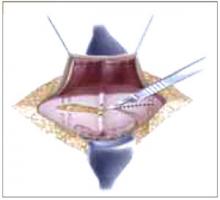
Placing the TVT sling. Attach the TVT introducer to the curved needle trocar on 1 end of the polypropylene sling. Insert the trocar with the tape attached into the vaginal incision and push through the retropubic space, keeping the trocar in close contact with the posterior surface of the pubic bone (FIGURES 3 and 4). Continue pushing the trocar through the urogenital diaphragm until its tip comes through the suprapubic incision on the ipsilateral side (FIGURE 5). It is important to not deviate too laterally, medially, or cephalad during trocar insertion to prevent vessel, bladder, or bowel injury. Perform a cystoscopy to rule out cystotomy. Place the second trocar in a similar manner on the opposite side. After both trocars have been pulled through their respective incisions, perform a tension test.
Placing the SPARC/abdominal TVT sling. Guide the abdominal needles through the previously marked suprapubic incision and the patient’s retropubic cavity (keeping the needle behind the pubic bone), to a finger placed in the vaginal incision (FIGURE 6). Snap the abdominal needle guides with the attached polypropylene mesh to the sling connectors (FIGURE 7). Bring the abdominal needles through the suprapubic incisions. Perform a tension test. As with the TVT sling, perform a cystoscopy after each needle placement to rule out cystotomy. Then pass the sling through the tunnel.
Testing for continence. Once the sling is in place, fill the bladder with 250 cc of water and perform a cough stress test. Adjust sling tension by pulling up on both sling arms until only a few drops of leakage are noted. It is important not to secure the sling too tightly as this may lead to urinary retention, detrusor instability, or urethral erosion. We prefer placing a hemostat between the sling tape and the urethra to avoid over tightening.
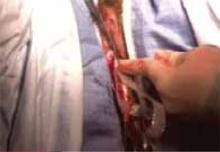
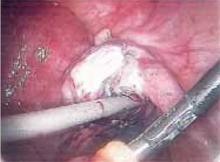
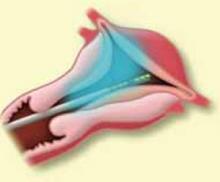
Suspending the sling arms. Remove the plastic sheaths after tension adjustment and cut the sling flush with the skin (FIGURE 8). Compared to the conventional bone-anchored slings, the newer tension-free sling devices are not anchored but instead suspended through the retropubic space. At first, the sling is held in place by friction from the opposing tissues. Over time, collagen formation fixes the mesh more strongly within the suburethral and paravaginal tissues.
Finally, close the suprapubic and vaginal incisions with absorbable sutures.
Placing autologous or allogenic slings. Fashion the graft, typically 2 cm wide and 10 to 12 cm long, with permanent sutures at the edges. Make a 1-cm incision into the suprapubic rectus fascia. Use either a Stamey-type needle trocar or uterine packing forceps and guide the instrument “top down” from the retropubic incision to the vaginal tunnel. The tunnel is made directly into the retropubic space from the vaginal incision. Bring the sling arms up on each side. Attach the arms to the rectus fascia and tie them down once cystoscopy and the tension test are complete.
The sling also can be performed with bone anchors placed through the vaginal incision into the pubic bone. Placement requires vaginal dissection into the retropubic space with no suprapubic incision. Once anchored, the sutures are then passed through the chosen graft materials and tied down. Bear in mind that anchoring into the periosteum of the pubic bone may cause severe osteomyelitis or osteitis pubis, though the actual incidence is unknown.7
FIGURE 1 Surgical steps for tension-free vaginal tape (TVT)
Place a Sims speculum into the vagina and make a vertical incision 1.5 cm from the external urethra meatus.
FIGURE 2
Use Metzenbaum scissors to dissect the vaginal mucosa from the underlying fascia bilaterally. Insert a Foley catheter with guide.
FIGURE 3
Place TVT trocars through the vaginal incision. Place abdominal guides suprapubically and attach them to the TVT trocar tip.
FIGURE 4
Push the tape through the retropubic space, keeping the trocar in close contact with the posterior surface of the pubic bone.
FIGURE 5
Continue pushing the trocar through the urogenital diaphragm until its tip comes through the suprapubic incision on the ipsilateral side.
FIGURE 6 Surgical steps for SPARC
Guide the needle down the posterior side of the pubic bone, keeping the needle tip in contact with the pubic bone.
FIGURE 7
Snap the needle guide and sling onto the sling connectors and pull through the suprapubic incision.
FIGURE 8
Perform a tension test. Remove the plastic sheaths and cut the sling flush with the skin.
Complications
Intraoperative and immediate postoperative complications include bladder perforation, vaginal or retropubic bleeding, wound or urinary tract infection (UTI), and short-term urinary retention. Possible long-term problems include urethral or vaginal erosion, mesh infection, prolonged voiding dysfunction, fistula formation, or de novo urge incontinence (TABLE 2).
Specifically, the TVT sling, which has been placed in more than 50,000 women in the U.S. and 200,000 worldwide, carries the potential for significant vascular injury and bowel perforation. In addition, the Food and Drug Administration (FDA) reported 4 deaths (2 from unrecognized bowel injuries, 1 from retropubic bleeding in a patient with a bleeding disorder, and 1 from a heart attack more than 1 week after an incontinence repair procedure complicated by a vascular injury); 168 device malfunctions (mostly tape or sheath detachment from the trocar); and 128 other injuries, including bowel perforations and major vascular injuries to the obturator, external iliac, femoral, or inferior epigastrics (TABLE 3).8 In a review of 1,455 TVT sling cases at 38 hospitals, Kuuva and Nilsson9 found bladder perforation in 3.8% of the patients and retropubic hematoma in 1.9%, along with 1 case of vesicovaginal fistula, 1 obturator nerve injury, and 1 epigastric vessel injury.
According to the FDA, there have been 2 complications reported with the SPARC sling system. Both involved vaginal erosion subsequently repaired by oversewing the vaginal mucosa. One of these complications occurred in a woman undergoing her fourth vaginal procedure who was therefore deemed to have “poor tissue.”8
TABLE 2
Suburethral sling complications1
| COMPLICATION | 1,715 AUTOLOGOUS | 1,515 SYNTHETIC |
|---|---|---|
| Vaginal erosion | 1 (.0001%) | 10 (.007%) |
| Urethral erosion | 5 (.003%) | 27 (.02%) |
| Fistula | 6 (.003%) | 4 (.002%) |
| Wound sinus | 3 (.002%) | 11 (.007%) |
| Wound infection | 11 (.006%) | 15 (.009%) |
| Seroma | 6 (.003%) | 1 (.0007%) |
TABLE 3
TVT complications in 200,000 procedures worldwide
| COMPLICATION | U.S. | WORLD | TOTAL |
|---|---|---|---|
| Vascular injury | 3 | 25 | 28 |
| Vaginal mesh exposure | 15 | 2 | 17 |
| Urethral erosion | 8 | 0 | 8 |
| Bowel perforation | 4 | 6 | 10 |
| Nerve injury | 1 | 0 | 1 |
Outcomes studies
Unfortunately, most of the published clinical studies on the surgical management of stress urinary incontinence suffer from inadequate follow-up and sample size, unclear patient selection criteria, and poor postoperative documentation, especially with respect to quality of life. However, multiple studies to assess the effectiveness and safety of TVT slings have been published. The following is an outline of these preliminary yet important findings.
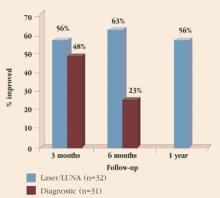
In 2002, the Cochrane Database evaluated 7 randomized and quasi-randomized trials of suburethral slings for the treatment of urinary incontinence.10 Of 682 women evaluated, 457 had some type of suburethral sling procedure. Four trials compared slings to retropubic urethropexies, 1 compared slings to Stamey needle suspensions, and 2 compared the use of different sling materials. The results indicated that the data were insufficient to suggest that slings were more effective than other incontinence procedures or that slings were associated with fewer postoperative complications. While TVT slings did provide similar cure rates as open retropubic urethropexy, research is still lacking with respect to other types of slings. More studies comparing TVT slings to traditional pubovaginal slings also are needed before the 2 can be deemed equivalent.
In Sweden and Finland, where the TVT procedure was developed,11 85 patients who had undergone the procedure were evaluated at 48 to 70 months. Of those, 84.7% were completely cured of stress incontinence, 10.6% had significantly improved symptoms, and 4.7% were regarded as failures.
A recent well-designed, multicenter, randomized, prospective trial in the U.K. and Ireland compared 146 open Burch colposuspensions to 170 TVTs. Similar cure rates (57% and 66%, respectively) were reported.12 Although these rates are low compared to the Nordic nonrandomized TVT studies mentioned, the U.K./Ireland outcome criteria were particularly stringent and included a negative cystometrogram for stress incontinence and negative pad test. These differences in reported success rates highlight the importance of clearly defining objective outcomes criteria from randomized trials.
Nonetheless, the U.K./Ireland study showed that TVT is less invasive than the Burch procedure and is associated with shorter recovery periods and greater cost savings. Follow-up on complications (bladder perforation and hematoma in TVTs and incisional hernia formation in Burch colposuspensions) will be the most crucial aspect of this study.13
Clearly, the question of whether a Burch retropubic urethropexy or a suburethral sling procedure is better for SUI needs to be further investigated. Weber and Walters sought to answer this question by developing a decision analytical model (without the aid of randomized, controlled trials) and discovered similar cure rates.14 However, there were higher rates of urinary retention and detrusor instability associated with the traditional pubovaginal sling. But, most importantly, sensitivity analyses proved that if the rate of permanent urinary retention after a sling procedure was less than 9%—as in most sling series—the overall effectiveness of slings was higher than that of the Burch.
Conclusion
The suburethral sling procedure has undergone many modifications since its first description nearly a century ago. As such, Ob/Gyns need to familiarize themselves with the current options. Typically, we perform up to 6 suburethral sling procedures per month. Of those, 50% are referrals from failed incontinence procedures. Recently, we have made the switch from using autologous slings to tension-free type slings due to ease and good outcomes. While more data from randomized, prospective, multicenter trials are needed to determine the best approach for individual patients, surgeons should become comfortable with the technique that works best for them.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Leach GE, Dmochowski RR, Appell RA, et al. Female Stress Urinary Incontinence Clinical Guidelines Panel summary report on surgical management of female stress urinary incontinence. J Urol. 1997;875-880.
2. Nihira MA, Schaffer JI. Surgical procedures for stress urinary incontinence in 1999. Presented at: 28th Annual Scientific Meeting of the Society of Gynecologic Surgeons; March 6, 2002; Dallas, Texas.
3. Kobashi KC, Leach GE. Stress urinary incontinence. Curr Opin Urol. 1999;9:285-290.
4. Bowen LW, Sand PK, Ostergard DR, Franti CE. Unsuccessful Burch retropubic urethropexy: a case-controlled urodynamic study. Am J Obstet Gynecol. 1989;160:452-458.
5. Bump RC, Coates KW, Cundiff GW, Harris RL, Weidner AC. Diagnosing intrinsic sphincteric deficiency: Comparing urethral closure pressure, urethral axis, and Valsalva leak point pressures. Am J Obstet Gynecol. 1997;177:303-310.
6. Nichols DH, Randal CL. Operations for urinary stress incontinence. In: Vaginal Surgery. 4th ed. Baltimore, Md: Williams & Wilkins; 1996;402-415.
7. Rackley RR, Abdelmalak JB, Madjar S, Yanilmaz A, Appell RA, Tchetgen MB. Bone anchor infections in female pelvic reconstructive procedures: a literature review of series and case reports. J Urol. 2001;165:1975-1978.
8. Food and Drug Administration manufacturer and user facility device experience database. Available at: www.fda.gov/cdrh/maude.html. Accessed November 12, 2002.
9. Kuuva N, Nilsson CG. A nationwide analysis of complications associated with the tension-free vaginal tape (TVT) procedure. Neurourol Urodyn. 2000;19:394.-
10. Bezerra CA, Bruschini H. Suburethral sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2001;(3):CD001754.-
11. Nilsson CG, Kuuva N, Falconer C, Rezapour M, Ulmsten U. Long-term results of the tension-free vaginal tape procedure (TVT) for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(suppl 2):S5-S8.
12. Ward KL, Hilton P, Browning J. A randomized trial of colposuspension and tension-free vaginal tape (TVT) for primary genuine stress incontinence. Neurourol Urodyn. 2000;19:386.-
13. Ward K, Hilton P. Prospective multicenter randomized trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ. 2002;325:67.-
14. Weber A, Walters M. Burch procedure compared with sling for stress urinary incontinence: a decision analysis. Obstet Gynecol. 2000;96:867-873.
- Suburethral sling procedures are effective in treating patients with urethral hypermobility, intrinsic sphincter deficiency, low-pressure urethras, and increased intra-abdominal pressure.
- Autologous slings may be a better choice in cases of severe urogenital atrophy, previous radiation, or extensive scarring from previous repairs.
- For both the tension-free vaginal tape and SPARC slings, mark the suprapubic region 1 cm above and 1 cm lateral to the pubic symphsis on the left and right sides and inject 20 cc of a 1:1 mixture of local anesthetic and normal saline into the marked regions.
- Once the trocars are in place, fill the bladder with 250 cc of water and perform a cough stress test to confirm continence.
When the suburethral sling was first described in 1907 by von Giordano, it entailed placing autologous tissue underneath the bladder neck and suspending it superiorly. Complications including urethral erosion, infection, bleeding, and fistula formation led many surgeons to use it sparingly.
Fast forward to the 21st century: Synthetic materials and new techniques were introduced, simplifying the sling procedures and raising the long-term success rates to 84%.1 As a result, slings now stand at the forefront of stress urinary incontinence (SUI) treatment. Among advances are the tension-free vaginal tape (TVT) sling (Gynecare, a division of Ethicon Inc., Somerville, NJ) and the SPARC sling (American Medical Systems, Inc., Minnetonka, Minn). The former, approved in the U.S. in 1998, calls for another look due to of the recent publication of a Cochrane review of outcomes studies, while the latter, approved by the FDA in August 2001, is the newest technique deserving examination. Clearly, with 83,010 incontinence procedures performed in the U.S. in 1999,2 a detailed look at the suburethral sling is warranted. Here, we review materials, indications, techniques, complications, and outcomes.
Materials
The choice of material—either organic or synthetic—depends on several factors: availability, cost, patient and surgeon preference, and clinical variables. (TABLE 1) outlines the advantages and disadvantages of each material type. Organic slings include autologous tissues (rectus fascia and fascia lata graft), and allografts or xenografts (cadaveric fascia lata graft, human dermal graft, or porcine small intestine and dermal graft). Synthetic slings are made of polyethylene terephthalate, expanded polytetrafluoroethylene, and polypropylene.
While sling procedures utilizing organic materials do have their benefits, synthetic slings, particularly the polypropylene mesh used in TVT and SPARC, have proven to be a stable material unlikely to deteriorate with time. Further, increased collagen metabolism around this synthetic sling promotes an ingrowth of tissue through the mesh.
TABLE 1
Slings: advantages and disadvantages of various materials
| SLING MATERIAL | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| Autologous tissues (rectus fascia, fascia lata, or vaginal wall) |
|
|
| Allografts (cadaveric fascia lata or dermis) |
|
|
| Xenografts (porcine dermis or small intestine) | ||
| Synthetic mesh (polyethylene terephthalate, expanded polytetrafluoroethylene, or polypropylene) |
|
|
Indications
Suburethral sling procedures are typically used for the treatment of genuine stress urinary incontinence (GSUI), in which the urethra becomes either hypermobile and unstable or its intrinsic sphincter becomes incompetent. In fact, slings are technically easier to place in patients with anatomic urethrovesical junction hypermobility compared to those with fixed urethras. Several authors also have suggested the sling’s advantage in patients with low-pressure urethras.3
Use urodynamic criteria to diagnose intrinsic sphincter deficiency (ISD), which is defined as a Valsalva leak point pressure of less than 60 cm water or maximal urethral closure pressure of less than 20 cm water. (Bear in mind, however, that these cut-off criteria are controversial.4,5)
Also, consider slings in patients with recurrent GSUI, inherited collagen deficiency, and increased abdominal pressure (e.g., women with chronic obstructive pulmonary disease, obesity, or high-impact physical activity). The sling also can be used as an adjunct to other transvaginal surgeries (e.g., hysterectomy or prolapse repair).
Autologous slings may be a better choice than synthetic slings in cases of severe urogenital atrophy, previous radiation, or extensive scarring from previous repairs. In these instances, the patient may be at-risk for postoperative vaginal necrosis or erosion.6 Due to their biocompatibility, autologous slings are more likely to heal over a vaginal erosion and less likely to infect or erode into the urethra. In any event, urogenital atrophy should be treated with local estrogen preoperatively to prevent some of these complications.
Technique
Conventionally, suburethral slings were placed via a combined vaginal and abdominal approach into the retropubic space of Retzius. Alternatively, the procedure could be performed abdominally by creating a suburethral tunnel via pelvic incisions, but this is the most difficult route.
Most recently, technological advances have simplified the vaginal approach, which utilizes minimal suburethral dissection and small suprapubic incisions. This technique is subdivided into “bottom-up” and “top-down” approaches. In the bottom-up TVT, the sling is inserted into a vaginal incision and threaded up through the patient’s pelvis, exiting from a small suprapubic incision. The topdown SPARC entails a reverse approach, starting from a suprapubic incision and exiting from a vaginal incision. New modifications allow for an abdominal TVT approach, as well, which we describe in detail in a later section.
Surgeons who are familiar with traditional needle suspensions may be more comfortable with the top-down approach. The need for concomitant surgery (e.g., hysterectomy or prolapse repair) not only determines the type of incontinence procedure, but also dictates the approach.
Preparing the patient. Place the patient under regional or local anesthesia with sedation so that an intraoperative cough stress test can be performed. Then administer an intravenous dose of a broad-spectrum antibiotic. Insert a 16 to 18 French Foley catheter into the urethra. Mark the suprapubic region 1 cm above and 1 cm lateral to the pubic symphsis on the left and right sides of the patient. Inject approximately 20 cc of a 1:1 mixture of local anesthetic and normal saline into the marked areas. We typically use 60 cc of 0.25% bupivicaine with epinephrine, diluted 1:1 with 60 cc of normal saline. After administering the local anesthetic suprapubically, inject a similar solution into the anterior vaginal wall suburethrally in the midline and laterally toward the retropubic tunnels.
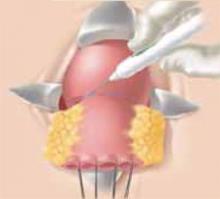
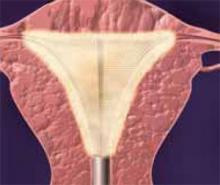
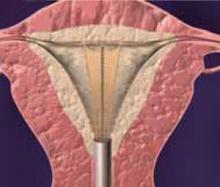
Making the incisions. Both the TVT and SPARC techniques utilize the same type and location of incisions. As such, make a 0.5-cm incision into the abdominal skin on each side of the midline, approximately 1 cm lateral to midline and 1 cm above the pubic symphsis. Next, make a 1.5- to 2-cm vertical incision in the vaginal mucosa, starting 1.5 cm from the urethral meatus (FIGURE 1). Use Metzenbaum scissors to dissect the vaginal mucosa from the pubocervical fascia sub- and para-urethrally on both sides (FIGURE 2). Insert a Foley catheter guide (similar to the Lowsley retractor) into the catheter and deviate it to the ipsilateral side, thereby retracting the bladder neck to the contralateral side. Proceed with the placement of either the TVT or SPARC sling.
Placing the TVT sling. Attach the TVT introducer to the curved needle trocar on 1 end of the polypropylene sling. Insert the trocar with the tape attached into the vaginal incision and push through the retropubic space, keeping the trocar in close contact with the posterior surface of the pubic bone (FIGURES 3 and 4). Continue pushing the trocar through the urogenital diaphragm until its tip comes through the suprapubic incision on the ipsilateral side (FIGURE 5). It is important to not deviate too laterally, medially, or cephalad during trocar insertion to prevent vessel, bladder, or bowel injury. Perform a cystoscopy to rule out cystotomy. Place the second trocar in a similar manner on the opposite side. After both trocars have been pulled through their respective incisions, perform a tension test.
Placing the SPARC/abdominal TVT sling. Guide the abdominal needles through the previously marked suprapubic incision and the patient’s retropubic cavity (keeping the needle behind the pubic bone), to a finger placed in the vaginal incision (FIGURE 6). Snap the abdominal needle guides with the attached polypropylene mesh to the sling connectors (FIGURE 7). Bring the abdominal needles through the suprapubic incisions. Perform a tension test. As with the TVT sling, perform a cystoscopy after each needle placement to rule out cystotomy. Then pass the sling through the tunnel.
Testing for continence. Once the sling is in place, fill the bladder with 250 cc of water and perform a cough stress test. Adjust sling tension by pulling up on both sling arms until only a few drops of leakage are noted. It is important not to secure the sling too tightly as this may lead to urinary retention, detrusor instability, or urethral erosion. We prefer placing a hemostat between the sling tape and the urethra to avoid over tightening.
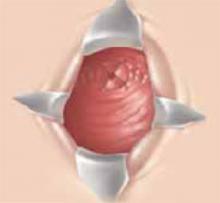
Suspending the sling arms. Remove the plastic sheaths after tension adjustment and cut the sling flush with the skin (FIGURE 8). Compared to the conventional bone-anchored slings, the newer tension-free sling devices are not anchored but instead suspended through the retropubic space. At first, the sling is held in place by friction from the opposing tissues. Over time, collagen formation fixes the mesh more strongly within the suburethral and paravaginal tissues.
Finally, close the suprapubic and vaginal incisions with absorbable sutures.
Placing autologous or allogenic slings. Fashion the graft, typically 2 cm wide and 10 to 12 cm long, with permanent sutures at the edges. Make a 1-cm incision into the suprapubic rectus fascia. Use either a Stamey-type needle trocar or uterine packing forceps and guide the instrument “top down” from the retropubic incision to the vaginal tunnel. The tunnel is made directly into the retropubic space from the vaginal incision. Bring the sling arms up on each side. Attach the arms to the rectus fascia and tie them down once cystoscopy and the tension test are complete.
The sling also can be performed with bone anchors placed through the vaginal incision into the pubic bone. Placement requires vaginal dissection into the retropubic space with no suprapubic incision. Once anchored, the sutures are then passed through the chosen graft materials and tied down. Bear in mind that anchoring into the periosteum of the pubic bone may cause severe osteomyelitis or osteitis pubis, though the actual incidence is unknown.7
FIGURE 1 Surgical steps for tension-free vaginal tape (TVT)
Place a Sims speculum into the vagina and make a vertical incision 1.5 cm from the external urethra meatus.
FIGURE 2
Use Metzenbaum scissors to dissect the vaginal mucosa from the underlying fascia bilaterally. Insert a Foley catheter with guide.
FIGURE 3
Place TVT trocars through the vaginal incision. Place abdominal guides suprapubically and attach them to the TVT trocar tip.
FIGURE 4
Push the tape through the retropubic space, keeping the trocar in close contact with the posterior surface of the pubic bone.
FIGURE 5
Continue pushing the trocar through the urogenital diaphragm until its tip comes through the suprapubic incision on the ipsilateral side.
FIGURE 6 Surgical steps for SPARC
Guide the needle down the posterior side of the pubic bone, keeping the needle tip in contact with the pubic bone.
FIGURE 7
Snap the needle guide and sling onto the sling connectors and pull through the suprapubic incision.
FIGURE 8
Perform a tension test. Remove the plastic sheaths and cut the sling flush with the skin.
Complications
Intraoperative and immediate postoperative complications include bladder perforation, vaginal or retropubic bleeding, wound or urinary tract infection (UTI), and short-term urinary retention. Possible long-term problems include urethral or vaginal erosion, mesh infection, prolonged voiding dysfunction, fistula formation, or de novo urge incontinence (TABLE 2).
Specifically, the TVT sling, which has been placed in more than 50,000 women in the U.S. and 200,000 worldwide, carries the potential for significant vascular injury and bowel perforation. In addition, the Food and Drug Administration (FDA) reported 4 deaths (2 from unrecognized bowel injuries, 1 from retropubic bleeding in a patient with a bleeding disorder, and 1 from a heart attack more than 1 week after an incontinence repair procedure complicated by a vascular injury); 168 device malfunctions (mostly tape or sheath detachment from the trocar); and 128 other injuries, including bowel perforations and major vascular injuries to the obturator, external iliac, femoral, or inferior epigastrics (TABLE 3).8 In a review of 1,455 TVT sling cases at 38 hospitals, Kuuva and Nilsson9 found bladder perforation in 3.8% of the patients and retropubic hematoma in 1.9%, along with 1 case of vesicovaginal fistula, 1 obturator nerve injury, and 1 epigastric vessel injury.
According to the FDA, there have been 2 complications reported with the SPARC sling system. Both involved vaginal erosion subsequently repaired by oversewing the vaginal mucosa. One of these complications occurred in a woman undergoing her fourth vaginal procedure who was therefore deemed to have “poor tissue.”8
TABLE 2
Suburethral sling complications1
| COMPLICATION | 1,715 AUTOLOGOUS | 1,515 SYNTHETIC |
|---|---|---|
| Vaginal erosion | 1 (.0001%) | 10 (.007%) |
| Urethral erosion | 5 (.003%) | 27 (.02%) |
| Fistula | 6 (.003%) | 4 (.002%) |
| Wound sinus | 3 (.002%) | 11 (.007%) |
| Wound infection | 11 (.006%) | 15 (.009%) |
| Seroma | 6 (.003%) | 1 (.0007%) |
TABLE 3
TVT complications in 200,000 procedures worldwide
| COMPLICATION | U.S. | WORLD | TOTAL |
|---|---|---|---|
| Vascular injury | 3 | 25 | 28 |
| Vaginal mesh exposure | 15 | 2 | 17 |
| Urethral erosion | 8 | 0 | 8 |
| Bowel perforation | 4 | 6 | 10 |
| Nerve injury | 1 | 0 | 1 |
Outcomes studies
Unfortunately, most of the published clinical studies on the surgical management of stress urinary incontinence suffer from inadequate follow-up and sample size, unclear patient selection criteria, and poor postoperative documentation, especially with respect to quality of life. However, multiple studies to assess the effectiveness and safety of TVT slings have been published. The following is an outline of these preliminary yet important findings.
In 2002, the Cochrane Database evaluated 7 randomized and quasi-randomized trials of suburethral slings for the treatment of urinary incontinence.10 Of 682 women evaluated, 457 had some type of suburethral sling procedure. Four trials compared slings to retropubic urethropexies, 1 compared slings to Stamey needle suspensions, and 2 compared the use of different sling materials. The results indicated that the data were insufficient to suggest that slings were more effective than other incontinence procedures or that slings were associated with fewer postoperative complications. While TVT slings did provide similar cure rates as open retropubic urethropexy, research is still lacking with respect to other types of slings. More studies comparing TVT slings to traditional pubovaginal slings also are needed before the 2 can be deemed equivalent.
In Sweden and Finland, where the TVT procedure was developed,11 85 patients who had undergone the procedure were evaluated at 48 to 70 months. Of those, 84.7% were completely cured of stress incontinence, 10.6% had significantly improved symptoms, and 4.7% were regarded as failures.
A recent well-designed, multicenter, randomized, prospective trial in the U.K. and Ireland compared 146 open Burch colposuspensions to 170 TVTs. Similar cure rates (57% and 66%, respectively) were reported.12 Although these rates are low compared to the Nordic nonrandomized TVT studies mentioned, the U.K./Ireland outcome criteria were particularly stringent and included a negative cystometrogram for stress incontinence and negative pad test. These differences in reported success rates highlight the importance of clearly defining objective outcomes criteria from randomized trials.
Nonetheless, the U.K./Ireland study showed that TVT is less invasive than the Burch procedure and is associated with shorter recovery periods and greater cost savings. Follow-up on complications (bladder perforation and hematoma in TVTs and incisional hernia formation in Burch colposuspensions) will be the most crucial aspect of this study.13
Clearly, the question of whether a Burch retropubic urethropexy or a suburethral sling procedure is better for SUI needs to be further investigated. Weber and Walters sought to answer this question by developing a decision analytical model (without the aid of randomized, controlled trials) and discovered similar cure rates.14 However, there were higher rates of urinary retention and detrusor instability associated with the traditional pubovaginal sling. But, most importantly, sensitivity analyses proved that if the rate of permanent urinary retention after a sling procedure was less than 9%—as in most sling series—the overall effectiveness of slings was higher than that of the Burch.
Conclusion
The suburethral sling procedure has undergone many modifications since its first description nearly a century ago. As such, Ob/Gyns need to familiarize themselves with the current options. Typically, we perform up to 6 suburethral sling procedures per month. Of those, 50% are referrals from failed incontinence procedures. Recently, we have made the switch from using autologous slings to tension-free type slings due to ease and good outcomes. While more data from randomized, prospective, multicenter trials are needed to determine the best approach for individual patients, surgeons should become comfortable with the technique that works best for them.
The authors report no financial relationship with any companies whose products are mentioned in this article.
- Suburethral sling procedures are effective in treating patients with urethral hypermobility, intrinsic sphincter deficiency, low-pressure urethras, and increased intra-abdominal pressure.
- Autologous slings may be a better choice in cases of severe urogenital atrophy, previous radiation, or extensive scarring from previous repairs.
- For both the tension-free vaginal tape and SPARC slings, mark the suprapubic region 1 cm above and 1 cm lateral to the pubic symphsis on the left and right sides and inject 20 cc of a 1:1 mixture of local anesthetic and normal saline into the marked regions.
- Once the trocars are in place, fill the bladder with 250 cc of water and perform a cough stress test to confirm continence.
When the suburethral sling was first described in 1907 by von Giordano, it entailed placing autologous tissue underneath the bladder neck and suspending it superiorly. Complications including urethral erosion, infection, bleeding, and fistula formation led many surgeons to use it sparingly.
Fast forward to the 21st century: Synthetic materials and new techniques were introduced, simplifying the sling procedures and raising the long-term success rates to 84%.1 As a result, slings now stand at the forefront of stress urinary incontinence (SUI) treatment. Among advances are the tension-free vaginal tape (TVT) sling (Gynecare, a division of Ethicon Inc., Somerville, NJ) and the SPARC sling (American Medical Systems, Inc., Minnetonka, Minn). The former, approved in the U.S. in 1998, calls for another look due to of the recent publication of a Cochrane review of outcomes studies, while the latter, approved by the FDA in August 2001, is the newest technique deserving examination. Clearly, with 83,010 incontinence procedures performed in the U.S. in 1999,2 a detailed look at the suburethral sling is warranted. Here, we review materials, indications, techniques, complications, and outcomes.
Materials
The choice of material—either organic or synthetic—depends on several factors: availability, cost, patient and surgeon preference, and clinical variables. (TABLE 1) outlines the advantages and disadvantages of each material type. Organic slings include autologous tissues (rectus fascia and fascia lata graft), and allografts or xenografts (cadaveric fascia lata graft, human dermal graft, or porcine small intestine and dermal graft). Synthetic slings are made of polyethylene terephthalate, expanded polytetrafluoroethylene, and polypropylene.
While sling procedures utilizing organic materials do have their benefits, synthetic slings, particularly the polypropylene mesh used in TVT and SPARC, have proven to be a stable material unlikely to deteriorate with time. Further, increased collagen metabolism around this synthetic sling promotes an ingrowth of tissue through the mesh.
TABLE 1
Slings: advantages and disadvantages of various materials
| SLING MATERIAL | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| Autologous tissues (rectus fascia, fascia lata, or vaginal wall) |
|
|
| Allografts (cadaveric fascia lata or dermis) |
|
|
| Xenografts (porcine dermis or small intestine) | ||
| Synthetic mesh (polyethylene terephthalate, expanded polytetrafluoroethylene, or polypropylene) |
|
|
Indications
Suburethral sling procedures are typically used for the treatment of genuine stress urinary incontinence (GSUI), in which the urethra becomes either hypermobile and unstable or its intrinsic sphincter becomes incompetent. In fact, slings are technically easier to place in patients with anatomic urethrovesical junction hypermobility compared to those with fixed urethras. Several authors also have suggested the sling’s advantage in patients with low-pressure urethras.3
Use urodynamic criteria to diagnose intrinsic sphincter deficiency (ISD), which is defined as a Valsalva leak point pressure of less than 60 cm water or maximal urethral closure pressure of less than 20 cm water. (Bear in mind, however, that these cut-off criteria are controversial.4,5)
Also, consider slings in patients with recurrent GSUI, inherited collagen deficiency, and increased abdominal pressure (e.g., women with chronic obstructive pulmonary disease, obesity, or high-impact physical activity). The sling also can be used as an adjunct to other transvaginal surgeries (e.g., hysterectomy or prolapse repair).
Autologous slings may be a better choice than synthetic slings in cases of severe urogenital atrophy, previous radiation, or extensive scarring from previous repairs. In these instances, the patient may be at-risk for postoperative vaginal necrosis or erosion.6 Due to their biocompatibility, autologous slings are more likely to heal over a vaginal erosion and less likely to infect or erode into the urethra. In any event, urogenital atrophy should be treated with local estrogen preoperatively to prevent some of these complications.
Technique
Conventionally, suburethral slings were placed via a combined vaginal and abdominal approach into the retropubic space of Retzius. Alternatively, the procedure could be performed abdominally by creating a suburethral tunnel via pelvic incisions, but this is the most difficult route.
Most recently, technological advances have simplified the vaginal approach, which utilizes minimal suburethral dissection and small suprapubic incisions. This technique is subdivided into “bottom-up” and “top-down” approaches. In the bottom-up TVT, the sling is inserted into a vaginal incision and threaded up through the patient’s pelvis, exiting from a small suprapubic incision. The topdown SPARC entails a reverse approach, starting from a suprapubic incision and exiting from a vaginal incision. New modifications allow for an abdominal TVT approach, as well, which we describe in detail in a later section.
Surgeons who are familiar with traditional needle suspensions may be more comfortable with the top-down approach. The need for concomitant surgery (e.g., hysterectomy or prolapse repair) not only determines the type of incontinence procedure, but also dictates the approach.
Preparing the patient. Place the patient under regional or local anesthesia with sedation so that an intraoperative cough stress test can be performed. Then administer an intravenous dose of a broad-spectrum antibiotic. Insert a 16 to 18 French Foley catheter into the urethra. Mark the suprapubic region 1 cm above and 1 cm lateral to the pubic symphsis on the left and right sides of the patient. Inject approximately 20 cc of a 1:1 mixture of local anesthetic and normal saline into the marked areas. We typically use 60 cc of 0.25% bupivicaine with epinephrine, diluted 1:1 with 60 cc of normal saline. After administering the local anesthetic suprapubically, inject a similar solution into the anterior vaginal wall suburethrally in the midline and laterally toward the retropubic tunnels.
Making the incisions. Both the TVT and SPARC techniques utilize the same type and location of incisions. As such, make a 0.5-cm incision into the abdominal skin on each side of the midline, approximately 1 cm lateral to midline and 1 cm above the pubic symphsis. Next, make a 1.5- to 2-cm vertical incision in the vaginal mucosa, starting 1.5 cm from the urethral meatus (FIGURE 1). Use Metzenbaum scissors to dissect the vaginal mucosa from the pubocervical fascia sub- and para-urethrally on both sides (FIGURE 2). Insert a Foley catheter guide (similar to the Lowsley retractor) into the catheter and deviate it to the ipsilateral side, thereby retracting the bladder neck to the contralateral side. Proceed with the placement of either the TVT or SPARC sling.
Placing the TVT sling. Attach the TVT introducer to the curved needle trocar on 1 end of the polypropylene sling. Insert the trocar with the tape attached into the vaginal incision and push through the retropubic space, keeping the trocar in close contact with the posterior surface of the pubic bone (FIGURES 3 and 4). Continue pushing the trocar through the urogenital diaphragm until its tip comes through the suprapubic incision on the ipsilateral side (FIGURE 5). It is important to not deviate too laterally, medially, or cephalad during trocar insertion to prevent vessel, bladder, or bowel injury. Perform a cystoscopy to rule out cystotomy. Place the second trocar in a similar manner on the opposite side. After both trocars have been pulled through their respective incisions, perform a tension test.
Placing the SPARC/abdominal TVT sling. Guide the abdominal needles through the previously marked suprapubic incision and the patient’s retropubic cavity (keeping the needle behind the pubic bone), to a finger placed in the vaginal incision (FIGURE 6). Snap the abdominal needle guides with the attached polypropylene mesh to the sling connectors (FIGURE 7). Bring the abdominal needles through the suprapubic incisions. Perform a tension test. As with the TVT sling, perform a cystoscopy after each needle placement to rule out cystotomy. Then pass the sling through the tunnel.
Testing for continence. Once the sling is in place, fill the bladder with 250 cc of water and perform a cough stress test. Adjust sling tension by pulling up on both sling arms until only a few drops of leakage are noted. It is important not to secure the sling too tightly as this may lead to urinary retention, detrusor instability, or urethral erosion. We prefer placing a hemostat between the sling tape and the urethra to avoid over tightening.
Suspending the sling arms. Remove the plastic sheaths after tension adjustment and cut the sling flush with the skin (FIGURE 8). Compared to the conventional bone-anchored slings, the newer tension-free sling devices are not anchored but instead suspended through the retropubic space. At first, the sling is held in place by friction from the opposing tissues. Over time, collagen formation fixes the mesh more strongly within the suburethral and paravaginal tissues.
Finally, close the suprapubic and vaginal incisions with absorbable sutures.
Placing autologous or allogenic slings. Fashion the graft, typically 2 cm wide and 10 to 12 cm long, with permanent sutures at the edges. Make a 1-cm incision into the suprapubic rectus fascia. Use either a Stamey-type needle trocar or uterine packing forceps and guide the instrument “top down” from the retropubic incision to the vaginal tunnel. The tunnel is made directly into the retropubic space from the vaginal incision. Bring the sling arms up on each side. Attach the arms to the rectus fascia and tie them down once cystoscopy and the tension test are complete.
The sling also can be performed with bone anchors placed through the vaginal incision into the pubic bone. Placement requires vaginal dissection into the retropubic space with no suprapubic incision. Once anchored, the sutures are then passed through the chosen graft materials and tied down. Bear in mind that anchoring into the periosteum of the pubic bone may cause severe osteomyelitis or osteitis pubis, though the actual incidence is unknown.7
FIGURE 1 Surgical steps for tension-free vaginal tape (TVT)
Place a Sims speculum into the vagina and make a vertical incision 1.5 cm from the external urethra meatus.
FIGURE 2
Use Metzenbaum scissors to dissect the vaginal mucosa from the underlying fascia bilaterally. Insert a Foley catheter with guide.
FIGURE 3
Place TVT trocars through the vaginal incision. Place abdominal guides suprapubically and attach them to the TVT trocar tip.
FIGURE 4
Push the tape through the retropubic space, keeping the trocar in close contact with the posterior surface of the pubic bone.
FIGURE 5
Continue pushing the trocar through the urogenital diaphragm until its tip comes through the suprapubic incision on the ipsilateral side.
FIGURE 6 Surgical steps for SPARC
Guide the needle down the posterior side of the pubic bone, keeping the needle tip in contact with the pubic bone.
FIGURE 7
Snap the needle guide and sling onto the sling connectors and pull through the suprapubic incision.
FIGURE 8
Perform a tension test. Remove the plastic sheaths and cut the sling flush with the skin.
Complications
Intraoperative and immediate postoperative complications include bladder perforation, vaginal or retropubic bleeding, wound or urinary tract infection (UTI), and short-term urinary retention. Possible long-term problems include urethral or vaginal erosion, mesh infection, prolonged voiding dysfunction, fistula formation, or de novo urge incontinence (TABLE 2).
Specifically, the TVT sling, which has been placed in more than 50,000 women in the U.S. and 200,000 worldwide, carries the potential for significant vascular injury and bowel perforation. In addition, the Food and Drug Administration (FDA) reported 4 deaths (2 from unrecognized bowel injuries, 1 from retropubic bleeding in a patient with a bleeding disorder, and 1 from a heart attack more than 1 week after an incontinence repair procedure complicated by a vascular injury); 168 device malfunctions (mostly tape or sheath detachment from the trocar); and 128 other injuries, including bowel perforations and major vascular injuries to the obturator, external iliac, femoral, or inferior epigastrics (TABLE 3).8 In a review of 1,455 TVT sling cases at 38 hospitals, Kuuva and Nilsson9 found bladder perforation in 3.8% of the patients and retropubic hematoma in 1.9%, along with 1 case of vesicovaginal fistula, 1 obturator nerve injury, and 1 epigastric vessel injury.
According to the FDA, there have been 2 complications reported with the SPARC sling system. Both involved vaginal erosion subsequently repaired by oversewing the vaginal mucosa. One of these complications occurred in a woman undergoing her fourth vaginal procedure who was therefore deemed to have “poor tissue.”8
TABLE 2
Suburethral sling complications1
| COMPLICATION | 1,715 AUTOLOGOUS | 1,515 SYNTHETIC |
|---|---|---|
| Vaginal erosion | 1 (.0001%) | 10 (.007%) |
| Urethral erosion | 5 (.003%) | 27 (.02%) |
| Fistula | 6 (.003%) | 4 (.002%) |
| Wound sinus | 3 (.002%) | 11 (.007%) |
| Wound infection | 11 (.006%) | 15 (.009%) |
| Seroma | 6 (.003%) | 1 (.0007%) |
TABLE 3
TVT complications in 200,000 procedures worldwide
| COMPLICATION | U.S. | WORLD | TOTAL |
|---|---|---|---|
| Vascular injury | 3 | 25 | 28 |
| Vaginal mesh exposure | 15 | 2 | 17 |
| Urethral erosion | 8 | 0 | 8 |
| Bowel perforation | 4 | 6 | 10 |
| Nerve injury | 1 | 0 | 1 |
Outcomes studies
Unfortunately, most of the published clinical studies on the surgical management of stress urinary incontinence suffer from inadequate follow-up and sample size, unclear patient selection criteria, and poor postoperative documentation, especially with respect to quality of life. However, multiple studies to assess the effectiveness and safety of TVT slings have been published. The following is an outline of these preliminary yet important findings.
In 2002, the Cochrane Database evaluated 7 randomized and quasi-randomized trials of suburethral slings for the treatment of urinary incontinence.10 Of 682 women evaluated, 457 had some type of suburethral sling procedure. Four trials compared slings to retropubic urethropexies, 1 compared slings to Stamey needle suspensions, and 2 compared the use of different sling materials. The results indicated that the data were insufficient to suggest that slings were more effective than other incontinence procedures or that slings were associated with fewer postoperative complications. While TVT slings did provide similar cure rates as open retropubic urethropexy, research is still lacking with respect to other types of slings. More studies comparing TVT slings to traditional pubovaginal slings also are needed before the 2 can be deemed equivalent.
In Sweden and Finland, where the TVT procedure was developed,11 85 patients who had undergone the procedure were evaluated at 48 to 70 months. Of those, 84.7% were completely cured of stress incontinence, 10.6% had significantly improved symptoms, and 4.7% were regarded as failures.
A recent well-designed, multicenter, randomized, prospective trial in the U.K. and Ireland compared 146 open Burch colposuspensions to 170 TVTs. Similar cure rates (57% and 66%, respectively) were reported.12 Although these rates are low compared to the Nordic nonrandomized TVT studies mentioned, the U.K./Ireland outcome criteria were particularly stringent and included a negative cystometrogram for stress incontinence and negative pad test. These differences in reported success rates highlight the importance of clearly defining objective outcomes criteria from randomized trials.
Nonetheless, the U.K./Ireland study showed that TVT is less invasive than the Burch procedure and is associated with shorter recovery periods and greater cost savings. Follow-up on complications (bladder perforation and hematoma in TVTs and incisional hernia formation in Burch colposuspensions) will be the most crucial aspect of this study.13
Clearly, the question of whether a Burch retropubic urethropexy or a suburethral sling procedure is better for SUI needs to be further investigated. Weber and Walters sought to answer this question by developing a decision analytical model (without the aid of randomized, controlled trials) and discovered similar cure rates.14 However, there were higher rates of urinary retention and detrusor instability associated with the traditional pubovaginal sling. But, most importantly, sensitivity analyses proved that if the rate of permanent urinary retention after a sling procedure was less than 9%—as in most sling series—the overall effectiveness of slings was higher than that of the Burch.
Conclusion
The suburethral sling procedure has undergone many modifications since its first description nearly a century ago. As such, Ob/Gyns need to familiarize themselves with the current options. Typically, we perform up to 6 suburethral sling procedures per month. Of those, 50% are referrals from failed incontinence procedures. Recently, we have made the switch from using autologous slings to tension-free type slings due to ease and good outcomes. While more data from randomized, prospective, multicenter trials are needed to determine the best approach for individual patients, surgeons should become comfortable with the technique that works best for them.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Leach GE, Dmochowski RR, Appell RA, et al. Female Stress Urinary Incontinence Clinical Guidelines Panel summary report on surgical management of female stress urinary incontinence. J Urol. 1997;875-880.
2. Nihira MA, Schaffer JI. Surgical procedures for stress urinary incontinence in 1999. Presented at: 28th Annual Scientific Meeting of the Society of Gynecologic Surgeons; March 6, 2002; Dallas, Texas.
3. Kobashi KC, Leach GE. Stress urinary incontinence. Curr Opin Urol. 1999;9:285-290.
4. Bowen LW, Sand PK, Ostergard DR, Franti CE. Unsuccessful Burch retropubic urethropexy: a case-controlled urodynamic study. Am J Obstet Gynecol. 1989;160:452-458.
5. Bump RC, Coates KW, Cundiff GW, Harris RL, Weidner AC. Diagnosing intrinsic sphincteric deficiency: Comparing urethral closure pressure, urethral axis, and Valsalva leak point pressures. Am J Obstet Gynecol. 1997;177:303-310.
6. Nichols DH, Randal CL. Operations for urinary stress incontinence. In: Vaginal Surgery. 4th ed. Baltimore, Md: Williams & Wilkins; 1996;402-415.
7. Rackley RR, Abdelmalak JB, Madjar S, Yanilmaz A, Appell RA, Tchetgen MB. Bone anchor infections in female pelvic reconstructive procedures: a literature review of series and case reports. J Urol. 2001;165:1975-1978.
8. Food and Drug Administration manufacturer and user facility device experience database. Available at: www.fda.gov/cdrh/maude.html. Accessed November 12, 2002.
9. Kuuva N, Nilsson CG. A nationwide analysis of complications associated with the tension-free vaginal tape (TVT) procedure. Neurourol Urodyn. 2000;19:394.-
10. Bezerra CA, Bruschini H. Suburethral sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2001;(3):CD001754.-
11. Nilsson CG, Kuuva N, Falconer C, Rezapour M, Ulmsten U. Long-term results of the tension-free vaginal tape procedure (TVT) for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(suppl 2):S5-S8.
12. Ward KL, Hilton P, Browning J. A randomized trial of colposuspension and tension-free vaginal tape (TVT) for primary genuine stress incontinence. Neurourol Urodyn. 2000;19:386.-
13. Ward K, Hilton P. Prospective multicenter randomized trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ. 2002;325:67.-
14. Weber A, Walters M. Burch procedure compared with sling for stress urinary incontinence: a decision analysis. Obstet Gynecol. 2000;96:867-873.
1. Leach GE, Dmochowski RR, Appell RA, et al. Female Stress Urinary Incontinence Clinical Guidelines Panel summary report on surgical management of female stress urinary incontinence. J Urol. 1997;875-880.
2. Nihira MA, Schaffer JI. Surgical procedures for stress urinary incontinence in 1999. Presented at: 28th Annual Scientific Meeting of the Society of Gynecologic Surgeons; March 6, 2002; Dallas, Texas.
3. Kobashi KC, Leach GE. Stress urinary incontinence. Curr Opin Urol. 1999;9:285-290.
4. Bowen LW, Sand PK, Ostergard DR, Franti CE. Unsuccessful Burch retropubic urethropexy: a case-controlled urodynamic study. Am J Obstet Gynecol. 1989;160:452-458.
5. Bump RC, Coates KW, Cundiff GW, Harris RL, Weidner AC. Diagnosing intrinsic sphincteric deficiency: Comparing urethral closure pressure, urethral axis, and Valsalva leak point pressures. Am J Obstet Gynecol. 1997;177:303-310.
6. Nichols DH, Randal CL. Operations for urinary stress incontinence. In: Vaginal Surgery. 4th ed. Baltimore, Md: Williams & Wilkins; 1996;402-415.
7. Rackley RR, Abdelmalak JB, Madjar S, Yanilmaz A, Appell RA, Tchetgen MB. Bone anchor infections in female pelvic reconstructive procedures: a literature review of series and case reports. J Urol. 2001;165:1975-1978.
8. Food and Drug Administration manufacturer and user facility device experience database. Available at: www.fda.gov/cdrh/maude.html. Accessed November 12, 2002.
9. Kuuva N, Nilsson CG. A nationwide analysis of complications associated with the tension-free vaginal tape (TVT) procedure. Neurourol Urodyn. 2000;19:394.-
10. Bezerra CA, Bruschini H. Suburethral sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2001;(3):CD001754.-
11. Nilsson CG, Kuuva N, Falconer C, Rezapour M, Ulmsten U. Long-term results of the tension-free vaginal tape procedure (TVT) for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(suppl 2):S5-S8.
12. Ward KL, Hilton P, Browning J. A randomized trial of colposuspension and tension-free vaginal tape (TVT) for primary genuine stress incontinence. Neurourol Urodyn. 2000;19:386.-
13. Ward K, Hilton P. Prospective multicenter randomized trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ. 2002;325:67.-
14. Weber A, Walters M. Burch procedure compared with sling for stress urinary incontinence: a decision analysis. Obstet Gynecol. 2000;96:867-873.
Pearls on cesarean
Even with its recent decline in occurrence, cesarean delivery remains the most frequently performed surgical procedure in the United States, accounting for approximately 21.5% of all deliveries. Here, obstetricians from across the country share pearls on various aspects of cesarean birth.
The difficult cesarean. When a difficult cesarean is predicted and the threat of obstetrical hemorrhage is imminent, I recommend that clinicians position the patient in low Allen stirrups, using a drape that provides vaginal access. This allows for proper elevation of the fetal head and better assessment of potential blood loss. Make sure a hysterectomy instrument tray is nearby and an experienced gynecologic scrub nurse is available. These steps should eliminate occult hemorrhage and allow for rapid response to any changes in the patient’s clinical status. —Richard Hill, MD, Kansas City, Mo
Cranial dystocia. A cesarean is often required when labor-arrest disorders occur. Cranial dystocia is sometimes found in these cases, making for a difficult delivery. When manual extraction of the fetal head fails, the surgeon typically has an assistant place a hand in the vagina to elevate and disengage the head. In these cases, I instead suggest surgeons remove their delivering hand from the uterine incision, cup the fetal head outside the lower uterine segment and bladder, and then pull upward. This will break the vacuum that spontaneously occurs, liberating the fetal head and allowing for successful cesarean delivery. —Peter Napolitano, MD, Tacoma, Wash
Intact membranes. When a patient with unruptured membranes presents for a cesarean, avoid breaching the amniotic sac sharply or with a clamp. Instead, first approach the uterine muscle sharply through the outer layers, then bluntly dissect the remaining fibers with your fingertip, allowing the amniotic sac to bulge out through the incision. Complete the uterine incision sharply with bandage scissors prior to rupturing the membranes, then open the bulging membranes either bluntly or with an Allis clamp. These steps ensure that the fetus will not experience trauma from sharp surgical objects. —Scott Resnick, MD, Taos, NM
Lowering the cesarean rate. To reduce the number of cesarean deliveries, try inducing labor of the unscarred uterus as follows:
- 8 AM: Insert 50 μg of misoprostol deep in the vagina.
- Noon: If the cervix is dilated, rupture membranes regardless of the Bishop score. Labor will almost invariably ensue within 1 to 2 hours. If the cervix is still closed at this time, insert another dose of misoprostol.
- Noon to 5 PM: Observe for cervical dilation. If this does not occur, augment labor with oxytocin.
Some patients will achieve vaginal delivery by 5 PM, and most will deliver by midnight.
With this technique, selecting patients for induction prior to their due date and before macrosomia or fetal stress develop is key to successfully keeping the cesarean rate low. —Susan Vicente, MD, Kailua, Hawaii
Even with its recent decline in occurrence, cesarean delivery remains the most frequently performed surgical procedure in the United States, accounting for approximately 21.5% of all deliveries. Here, obstetricians from across the country share pearls on various aspects of cesarean birth.
The difficult cesarean. When a difficult cesarean is predicted and the threat of obstetrical hemorrhage is imminent, I recommend that clinicians position the patient in low Allen stirrups, using a drape that provides vaginal access. This allows for proper elevation of the fetal head and better assessment of potential blood loss. Make sure a hysterectomy instrument tray is nearby and an experienced gynecologic scrub nurse is available. These steps should eliminate occult hemorrhage and allow for rapid response to any changes in the patient’s clinical status. —Richard Hill, MD, Kansas City, Mo
Cranial dystocia. A cesarean is often required when labor-arrest disorders occur. Cranial dystocia is sometimes found in these cases, making for a difficult delivery. When manual extraction of the fetal head fails, the surgeon typically has an assistant place a hand in the vagina to elevate and disengage the head. In these cases, I instead suggest surgeons remove their delivering hand from the uterine incision, cup the fetal head outside the lower uterine segment and bladder, and then pull upward. This will break the vacuum that spontaneously occurs, liberating the fetal head and allowing for successful cesarean delivery. —Peter Napolitano, MD, Tacoma, Wash
Intact membranes. When a patient with unruptured membranes presents for a cesarean, avoid breaching the amniotic sac sharply or with a clamp. Instead, first approach the uterine muscle sharply through the outer layers, then bluntly dissect the remaining fibers with your fingertip, allowing the amniotic sac to bulge out through the incision. Complete the uterine incision sharply with bandage scissors prior to rupturing the membranes, then open the bulging membranes either bluntly or with an Allis clamp. These steps ensure that the fetus will not experience trauma from sharp surgical objects. —Scott Resnick, MD, Taos, NM
Lowering the cesarean rate. To reduce the number of cesarean deliveries, try inducing labor of the unscarred uterus as follows:
- 8 AM: Insert 50 μg of misoprostol deep in the vagina.
- Noon: If the cervix is dilated, rupture membranes regardless of the Bishop score. Labor will almost invariably ensue within 1 to 2 hours. If the cervix is still closed at this time, insert another dose of misoprostol.
- Noon to 5 PM: Observe for cervical dilation. If this does not occur, augment labor with oxytocin.
Some patients will achieve vaginal delivery by 5 PM, and most will deliver by midnight.
With this technique, selecting patients for induction prior to their due date and before macrosomia or fetal stress develop is key to successfully keeping the cesarean rate low. —Susan Vicente, MD, Kailua, Hawaii
Even with its recent decline in occurrence, cesarean delivery remains the most frequently performed surgical procedure in the United States, accounting for approximately 21.5% of all deliveries. Here, obstetricians from across the country share pearls on various aspects of cesarean birth.
The difficult cesarean. When a difficult cesarean is predicted and the threat of obstetrical hemorrhage is imminent, I recommend that clinicians position the patient in low Allen stirrups, using a drape that provides vaginal access. This allows for proper elevation of the fetal head and better assessment of potential blood loss. Make sure a hysterectomy instrument tray is nearby and an experienced gynecologic scrub nurse is available. These steps should eliminate occult hemorrhage and allow for rapid response to any changes in the patient’s clinical status. —Richard Hill, MD, Kansas City, Mo
Cranial dystocia. A cesarean is often required when labor-arrest disorders occur. Cranial dystocia is sometimes found in these cases, making for a difficult delivery. When manual extraction of the fetal head fails, the surgeon typically has an assistant place a hand in the vagina to elevate and disengage the head. In these cases, I instead suggest surgeons remove their delivering hand from the uterine incision, cup the fetal head outside the lower uterine segment and bladder, and then pull upward. This will break the vacuum that spontaneously occurs, liberating the fetal head and allowing for successful cesarean delivery. —Peter Napolitano, MD, Tacoma, Wash
Intact membranes. When a patient with unruptured membranes presents for a cesarean, avoid breaching the amniotic sac sharply or with a clamp. Instead, first approach the uterine muscle sharply through the outer layers, then bluntly dissect the remaining fibers with your fingertip, allowing the amniotic sac to bulge out through the incision. Complete the uterine incision sharply with bandage scissors prior to rupturing the membranes, then open the bulging membranes either bluntly or with an Allis clamp. These steps ensure that the fetus will not experience trauma from sharp surgical objects. —Scott Resnick, MD, Taos, NM
Lowering the cesarean rate. To reduce the number of cesarean deliveries, try inducing labor of the unscarred uterus as follows:
- 8 AM: Insert 50 μg of misoprostol deep in the vagina.
- Noon: If the cervix is dilated, rupture membranes regardless of the Bishop score. Labor will almost invariably ensue within 1 to 2 hours. If the cervix is still closed at this time, insert another dose of misoprostol.
- Noon to 5 PM: Observe for cervical dilation. If this does not occur, augment labor with oxytocin.
Some patients will achieve vaginal delivery by 5 PM, and most will deliver by midnight.
With this technique, selecting patients for induction prior to their due date and before macrosomia or fetal stress develop is key to successfully keeping the cesarean rate low. —Susan Vicente, MD, Kailua, Hawaii
Excisional biopsy for CIN
- In most cases, loop electrosurgical excision procedure (LEEP) and cold-knife conization (CKC) result in equivalent success rates and margin status.
- CKC is preferred in cases of adenocarcinoma in situ or squamous microinvasion.
- Conservative follow-up is generally possible in adenocarcinoma in situ and squamous microinvasion when margins are negative.
- Colposcopy, endocervical curettage, and biopsies should be part of the follow-up strategy for patients with positive margins.
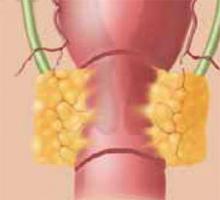
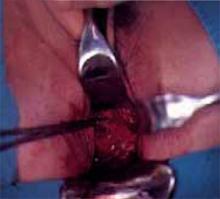
When cervical intraepithelial neoplasia (CIN) requires treatment, loop electrosurgical excision procedure (LEEP) is the most frequently used modality, although cold-knife conization (CKC) of the cervical transformation zone still is preferred in select cases. Since excisional techniques are used with CKC, margin status is known and clinical decisions may be based on this information.
This article reviews current indications for excisional biopsy and presents evidence to direct management and follow-up of patients with positive and negative margins.
Selecting a technique
Several large randomized and prospective studies have demonstrated that LEEP is similar in efficacy to CKC and may even remove less of the normal cervical stroma.1-3 In general, LEEP is used to excise high-grade and recurrent squamous dysplasia, as it is similar to CKC in its rates of incomplete excision and residual disease.1,4 However, when adenocarcinoma in situ (AIS) is present, CKC is preferred for diagnosis and treatment, since it results in a lower incidence of involved margins and a lower recurrence rate.3,5-7
CKC also is preferred when histologic confirmation of the margin status is crucial, such as when invasion with squamous lesions is suspected. This is because thermal artifacts that may result from LEEP will interfere with interpretation, further complicating treatment planning.1,4,8,9 In cases of microinvasion, the ability to confirm margins makes conservative treatment possible; if the depth of invasion cannot be determined from the specimen, radical surgery may be necessary.
In the hands of an experienced clinician, however, thermal artifact from the LEEP technique generally is not a significant problem. Series reporting high rates of uninterpretable margins have been attributed to operator inexperience.10 In general, thermal artifact is reduced by limiting the number of sections taken. In ideal cases, only a single-piece specimen is obtained, similar to that achieved using CKC.8,10,11 Newer loops, such as the cone biopsy excision loop, may decrease the number of sections and further improve margin interpretation.8
I use CKC in cases of suspected squamous invasion and in the evaluation of glandular lesions, but feel that in other cases LEEP is efficacious and quicker.
Identifying residual disease
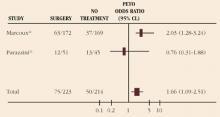
When both the endocervical margin and endocervical curettage (ECC) are positive for squamous dysplasia at the time of excision, there is an increased risk of residual disease (TABLE 1).12-14 However, it is not clear whether ECC alone is an independent predictor of residual disease. Although 1 study suggests an increased risk of invasive cancer in women over 50 years of age with a positive ECC at the time of conization, other series have failed to demonstrate a difference in treatment failure rates based on ECC.15-17 Thus, the utility of ECC in directing further therapy at the time of excisional biopsy is unclear. I generally do not perform an ECC with LEEP for squamous dysplasia.
In AIS, a positive ECC is a strong predictor of residual disease, while a negative ECC is of limited significance.18 I am more likely to perform an ECC with glandular lesions. If it is negative, close follow-up still is indicated. If it is positive, repeat excision may be necessary.
TABLE 1
Residual disease and margin status: squamous dysplasia
| AUTHOR | PROPORTION WITH RESIDUAL DISEASE | |||
|---|---|---|---|---|
| NEGATIVE MARGINS | POSITIVE MARGINS | |||
| NUMBER | % | NUMBER | % | |
| SQUAMOUS LESIONS | ||||
| Bertelsen et al, 199923 | 44/485 | 9 | 29/76 | 38.2 |
| Moore et al, 199524 | 170/523 | 32.5 | 37/91 | 40.7 |
| Lopes et al, 199325 | 0/176 | 0 | 11/131 | 8.4 |
| Murdoch et al, 199226 | 7/405 | 1.7 | 22/160 | 13.8 |
| Andersen et al, 199019 | 0/411 | 0 | 6/469 | 1.3 |
| TOTALS | 221/2,000 | 11 | 105/927 | 11.3 |
| SQUAMOUS MICROINVASION | ||||
| Gurgel et al, 199727 | 6/74 | 8.1 | 45/76 | 59.2 |
| Roman et al, 199722 | 1/30 | 3.3 | 7/50 | 14 |
| TOTALS | 7/104 | 6.7 | 52/126 | 41.3 |
Follow-up of squamous lesions
Considerable clinical uncertainty remains over the relative strengths of cytologic, colposcopic, and histologic evaluation for residual or recurrent disease following excisional biopsy. Conservative management includes Papanicolaou smears alone or in combination with ECC and/or colposcopy.
Negative margins. If margins are negative, the success rate of excisional biopsy is high (90%-100%), and careful observation is the preferred follow-up. Repeat cytologic testing will identify the majority of patients with residual high-grade disease. A prospective randomized trial of cytologic surveillance showed that the detection rate was only 1.9% higher for histology.19 Although 1 report suggests that colposcopy can expedite the diagnosis of recurrent dysplasia, it is unclear from this report whether colposcopy identified significant high-grade dysplasia that cytology missed.20
Positive margins. In most series involving CIN with positive margins, treatment success rates do not differ significantly between patients who undergo repeat surgical procedures and those who have close follow-up. However, endocervical margin involvement appears to carry a higher treatment failure rate than ectocervical margin involvement. Data are conflicting on the proper method of conservative follow-up. Some authors propose only frequent cytologic testing, while others recommend that colposcopy be performed.19-21
For patients with positive margins, I perform both cytology and colposcopy in 4 to 6 months. If an endocervical margin was positive, I also perform an ECC. If this evaluation is negative, I repeat cytologic testing every 6 months until 3 consecutive Pap smears are normal and satisfactory. In cases of recurrent high-grade dysplasia with positive margins, hysterectomy may be indicated, depending on the patient’s age and desire for continued fertility.14,21
Squamous microinvasion
There is a significant risk (50%-80%) of residual disease or true invasion when margins or an ECC are positive and microinvasion is present.2,5-7 In these cases, a repeat excisional biopsy should be performed to determine the true extent of disease prior to deciding on definitive therapy.22 As previously mentioned, CKC is preferred to limit artifact that could obscure interpretation. If the patient has completed her childbearing, hysterectomy remains the standard treatment for microinvasive squamous cell carcinoma. When the woman wishes to preserve fertility, conservative follow-up appears to be safe if the final pathologic specimen has negative margins. A follow-up protocol including cytologic, colposcopic, and ECC monitoring in the first post-conization visit is recommended.2,6
Glandular lesions
AIS with involved margins requires further surgery due to the possibility of residual AIS or invasion (TABLES 2 and 3).2,5-7 In these cases, CKC is preferable to LEEP.3,5-7 Hysterectomy remains the standard therapy for AIS. Conservative management is an option if fertility is desired and margin status is negative. Patients should be informed that persistent disease or recurrence is possible and that there is a risk of invasive disease.15
Counseling, thorough documentation, and second surgical and pathologic opinions may be helpful in conservative management. Colposcopy, ECC, and cytology are indicated at the first follow-up visit, with cytology repeated every 6 months until 4 consecutive Paps are normal. More frequent colposcopy and liberal use of ECC also may be considered.
TABLE 2
Residual disease and margin status: adenocarcinoma in situ
| AUTHOR | PROPORTION WITH RESIDUAL DISEASE | |||
|---|---|---|---|---|
| NEGATIVE MARGINS | POSITIVE MARGINS | |||
| NUMBER | % | NUMBER | % | |
| Azodi et al, 199928 | 5/16 | 31.3 | 9/16 | 56.3 |
| Goldstein et al, 199828 | 13/43 | 30.2 | 8/18 | 44.4 |
| Denehy et al, 19976 | 2/7 | 28.6 | 7/10 | 70 |
| Widrich et al, 19965 | 0/3 | 0 | 9/14 | 64.3 |
| Wolf et al, 199629 | 7/21 | 33.3 | 10/19 | 52.6 |
| TOTAL | 27/90 | 30 | 43/77 | 55.8 |
TABLE 3
Follow-up recommendations
| Pathology | Margin status | Recommendation |
|---|---|---|
| High-grade squamous lesion | Negative |
|
| Positive, endocervical |
| |
| Positive, ectocervical |
| |
| Squamous microinvasion | Negative, fertility desired |
|
| Negative, no desire for fertility | Hysterectomy | |
| Positive | CKC | |
| Adenocarcinoma in situ | Negative, fertility desired |
|
| Negative, no desire for fertility | Hysterectomy | |
| Positive | CKC | |
| CKC = cold-knife conization; ECC = endocervical curettage | ||
When further excision is necessary
As noted earlier, repeat excision is necessary in cases of squamous microinvasion or AIS with positive margins. CKC is generally preferred to allow for optimal pathologic interpretation. For squamous intraepithelial lesions, excisional biopsy with close follow-up has a significant cure rate, and hysterectomy usually is not indicated. However, hysterectomy still should be considered part of the treatment continuum for CIN, particularly for patients who have completed childbearing.
Conclusion
Although the risk of recurrence is correlated with a patient’s margin status in cases of squamous dysplasia, conservative follow-up is possible and has a high success rate. Cytology is sufficient surveillance for cases involving negative margins. When margins are positive, colposcopy and ECC also may be useful. Microinvasive lesions with positive margins require further surgical evaluation to determine treatment. For glandular lesions, CKC is preferred for both diagnosis and treatment.
Dr. Dunton reports no affiliation or financial arrangement with any of the companies that manufacture drugs or devices in any of the product classes mentioned in this article.
1. Mathevet P, Dargent D, Roy M, Beau G. A randomized prospective study comparing three techniques of conization: cold knife, laser, and LEEP. Gynecol Oncol. 1994;54(2):175-179.
2. Duggan BD, Felix JC, Muderspach LI, et al. Cold-knife conization versus conization by the loop electrosurgical excision procedure: a randomized, prospective study. Am J Obstet Gynecol. 1999;180:276-282.
3. Girardi F, Heydarfadi M, Koroschetz F, et al. Cold-knife conization versus loop excision: histopathologic and clinical results of a randomized trial. Gynecol Oncol. 1994;55:368-370.
4. Gold M, Dunton CJ, Murray J, et al. Loop electrocautery excisional procedure: therapeutic effectiveness as an ablation and a conization equivalent. Gynecol Oncol. 1996;61:241-244.
5. Widrich T, Kennedy AW, Myers TM, et al. Adenocarcinoma in situ of the uterine cervix: management and outcome. Gynecol Oncol. 1996;61:304-308.
6. Denehy TR, Gregori CA, Breen JL. Endocervical curettage, cone margins, and residual adenocarcinoma in situ of the cervix. Obstet Gynecol. 1997;90:1-6.
7. Muntz HG, Bell DA, Lage JM, et al. Adenocarcinoma in situ of the uterine cervix. Obstet Gynecol. 1992;80:935-939.
8. Naumann RW, Bell MC, Alvarez RD, et al. LLETZ is an acceptable alternative to diagnostic cold-knife conization. Gynecol Oncol. 1994;55:224-228.
9. Eddy GL, Spiegel GW, Creasman WT. Adverse effect of electrosurgical loop excision on assignment of FIGO stage in cervical cancer: report of two cases. Gynecol Oncol. 1994;55:313-317.
10. Montz FJ, Holschneider CH, Thompson LDR. Large-loop excision of the transformation zone: effect on the pathologic interpretation of resection margins. Obstet Gynecol. 1993;81:976-982.
11. Gardeil F, Barry-Walsh C, Prendiville W, et al. Persistent intraepithelial neoplasia after excision for cervical intraepithelial neoplasia grade III. Obstet Gynecol. 1987;89:419-422.
12. Felix JC, Muderspach LI, Duggan BD, Roman LD. The significance of positive margins in loop electrosurgical cone biopsies. Obstet Gynecol. 1994;84:996-1000.
13. Kobak WH, Roman LD, Felix JC, et al. The role of endocervical curettage at cervical conization for high-grade dysplasia. Obstet Gynecol. 1995;85:197-201.
14. Husseinzadeh N, Shbara I, Wessler T. Predictive value of cone margins and post-cone endocervical curettage with residual disease in subsequent hysterectomy. Gynecol Oncol. 1989;33:198-200.
15. Poyner EA, Barakat RR, Hoskins WJ. Management and follow-up of patients with adenocarcinoma in situ of the uterine cervix. Gynecol Oncol. 1995;57:158-164.
16. Frauchiger WL, De Frias DVS, Cajulis RS, Yu GH. The immediate postconization endocervical smear: evaluation of its utility in the detection of residual dysplasia. Acta Cytol. 1998;42:1139-1143.
17. Wright TC, Gagnon S, Richart RM, Ferenczy A. Treatment of cervical intraepithelial neoplasia using the loop electrosurgical excision procedure. Obstet Gynecol. 1992;79:173-178.
18. Goldstein NS, Mani A. The status and distance of cone biopsy margins as a predictor of excision adequacy for endocervical adenocarcinoma in situ. Am J Clin Pathol. 1998;109:727-732.
19. Andersen ES, Nielsen K, Larsen G. Laser conization: follow-up in patients with cervical intraepithelial neoplasia in the cone margin. Gynecol Oncol. 1990;39:328-331.
20. Paraskevaidis E, Jandial L, Mann EMF, Fisher PM. Pattern of treatment failure following laser for cervical intraepithelial neoplasia: implications for follow-up protocol. Obstet Gynecol. 1991;78:80-83.
21. Lapaquette TK, Dinh TV, Hannigan EV, et al. Management of patients with positive margins after cervical conization. Obstet Gynecol. 1993;82:440-443.
22. Roman LD, Felix JC, Muderspach LI, et al. Risk of residual invasive disease in women with microinvasive squamous cancer in a conization specimen. Obstet Gynecol. 1997;90:759-764.
23. Bertelsen B, Tande T, Sandvei, Hartveit F. Laser conization of cervical intraepithelial neoplasia grade 3. Acta Obstet Gynecol Scand. 1999;78:54-59.
24. Moore BC, Higgins RV, Laurent SL, et al. Predictive factors from cold knife conization for residual cervical intraepithelial neoplasia in subsequent hysterectomy. Am J Obstet Gynecol. 1995;173:361-368.
25. Lopes A, Morgan P, Murdoch J, et al. The case for conservative management of “incomplete excision” of CIN after laser conization. Gynecol Oncol. 1993;49:247-249.
26. Murdoch JB, Morgan PR, Lopes A, Monaghan JM. Histological incomplete excision of CIN after large loop excision of the transformation zone (LLETZ) merits careful follow up, not retreatment. Br J Obstet Gynaecol. 1992;99:990-993.
27. Gurgel MSC, Bedone AJ, Andrade LA, et al. Microinvasive carcinoma of the uterine cervix: histological findings on cone specimens related to residual neoplasia on hysterectomy. Gynecol Oncol. 1997;65:437-440.
28. Azodi M, Chambers SK, Rutherford TJ, et al. Adenocarcinoma in situ of the cervix: management and outcome. Gynecol Oncol. 1999;73:348-353.
29. Wolf JK, Levenback C, Malpica A, et al. Adenocarcinoma in situ of the cervix: significance of cone biopsy margins. Obstet Gynecol. 1996;88:82-86.
- In most cases, loop electrosurgical excision procedure (LEEP) and cold-knife conization (CKC) result in equivalent success rates and margin status.
- CKC is preferred in cases of adenocarcinoma in situ or squamous microinvasion.
- Conservative follow-up is generally possible in adenocarcinoma in situ and squamous microinvasion when margins are negative.
- Colposcopy, endocervical curettage, and biopsies should be part of the follow-up strategy for patients with positive margins.
When cervical intraepithelial neoplasia (CIN) requires treatment, loop electrosurgical excision procedure (LEEP) is the most frequently used modality, although cold-knife conization (CKC) of the cervical transformation zone still is preferred in select cases. Since excisional techniques are used with CKC, margin status is known and clinical decisions may be based on this information.
This article reviews current indications for excisional biopsy and presents evidence to direct management and follow-up of patients with positive and negative margins.
Selecting a technique
Several large randomized and prospective studies have demonstrated that LEEP is similar in efficacy to CKC and may even remove less of the normal cervical stroma.1-3 In general, LEEP is used to excise high-grade and recurrent squamous dysplasia, as it is similar to CKC in its rates of incomplete excision and residual disease.1,4 However, when adenocarcinoma in situ (AIS) is present, CKC is preferred for diagnosis and treatment, since it results in a lower incidence of involved margins and a lower recurrence rate.3,5-7
CKC also is preferred when histologic confirmation of the margin status is crucial, such as when invasion with squamous lesions is suspected. This is because thermal artifacts that may result from LEEP will interfere with interpretation, further complicating treatment planning.1,4,8,9 In cases of microinvasion, the ability to confirm margins makes conservative treatment possible; if the depth of invasion cannot be determined from the specimen, radical surgery may be necessary.
In the hands of an experienced clinician, however, thermal artifact from the LEEP technique generally is not a significant problem. Series reporting high rates of uninterpretable margins have been attributed to operator inexperience.10 In general, thermal artifact is reduced by limiting the number of sections taken. In ideal cases, only a single-piece specimen is obtained, similar to that achieved using CKC.8,10,11 Newer loops, such as the cone biopsy excision loop, may decrease the number of sections and further improve margin interpretation.8
I use CKC in cases of suspected squamous invasion and in the evaluation of glandular lesions, but feel that in other cases LEEP is efficacious and quicker.
Identifying residual disease
When both the endocervical margin and endocervical curettage (ECC) are positive for squamous dysplasia at the time of excision, there is an increased risk of residual disease (TABLE 1).12-14 However, it is not clear whether ECC alone is an independent predictor of residual disease. Although 1 study suggests an increased risk of invasive cancer in women over 50 years of age with a positive ECC at the time of conization, other series have failed to demonstrate a difference in treatment failure rates based on ECC.15-17 Thus, the utility of ECC in directing further therapy at the time of excisional biopsy is unclear. I generally do not perform an ECC with LEEP for squamous dysplasia.
In AIS, a positive ECC is a strong predictor of residual disease, while a negative ECC is of limited significance.18 I am more likely to perform an ECC with glandular lesions. If it is negative, close follow-up still is indicated. If it is positive, repeat excision may be necessary.
TABLE 1
Residual disease and margin status: squamous dysplasia
| AUTHOR | PROPORTION WITH RESIDUAL DISEASE | |||
|---|---|---|---|---|
| NEGATIVE MARGINS | POSITIVE MARGINS | |||
| NUMBER | % | NUMBER | % | |
| SQUAMOUS LESIONS | ||||
| Bertelsen et al, 199923 | 44/485 | 9 | 29/76 | 38.2 |
| Moore et al, 199524 | 170/523 | 32.5 | 37/91 | 40.7 |
| Lopes et al, 199325 | 0/176 | 0 | 11/131 | 8.4 |
| Murdoch et al, 199226 | 7/405 | 1.7 | 22/160 | 13.8 |
| Andersen et al, 199019 | 0/411 | 0 | 6/469 | 1.3 |
| TOTALS | 221/2,000 | 11 | 105/927 | 11.3 |
| SQUAMOUS MICROINVASION | ||||
| Gurgel et al, 199727 | 6/74 | 8.1 | 45/76 | 59.2 |
| Roman et al, 199722 | 1/30 | 3.3 | 7/50 | 14 |
| TOTALS | 7/104 | 6.7 | 52/126 | 41.3 |
Follow-up of squamous lesions
Considerable clinical uncertainty remains over the relative strengths of cytologic, colposcopic, and histologic evaluation for residual or recurrent disease following excisional biopsy. Conservative management includes Papanicolaou smears alone or in combination with ECC and/or colposcopy.
Negative margins. If margins are negative, the success rate of excisional biopsy is high (90%-100%), and careful observation is the preferred follow-up. Repeat cytologic testing will identify the majority of patients with residual high-grade disease. A prospective randomized trial of cytologic surveillance showed that the detection rate was only 1.9% higher for histology.19 Although 1 report suggests that colposcopy can expedite the diagnosis of recurrent dysplasia, it is unclear from this report whether colposcopy identified significant high-grade dysplasia that cytology missed.20
Positive margins. In most series involving CIN with positive margins, treatment success rates do not differ significantly between patients who undergo repeat surgical procedures and those who have close follow-up. However, endocervical margin involvement appears to carry a higher treatment failure rate than ectocervical margin involvement. Data are conflicting on the proper method of conservative follow-up. Some authors propose only frequent cytologic testing, while others recommend that colposcopy be performed.19-21
For patients with positive margins, I perform both cytology and colposcopy in 4 to 6 months. If an endocervical margin was positive, I also perform an ECC. If this evaluation is negative, I repeat cytologic testing every 6 months until 3 consecutive Pap smears are normal and satisfactory. In cases of recurrent high-grade dysplasia with positive margins, hysterectomy may be indicated, depending on the patient’s age and desire for continued fertility.14,21
Squamous microinvasion
There is a significant risk (50%-80%) of residual disease or true invasion when margins or an ECC are positive and microinvasion is present.2,5-7 In these cases, a repeat excisional biopsy should be performed to determine the true extent of disease prior to deciding on definitive therapy.22 As previously mentioned, CKC is preferred to limit artifact that could obscure interpretation. If the patient has completed her childbearing, hysterectomy remains the standard treatment for microinvasive squamous cell carcinoma. When the woman wishes to preserve fertility, conservative follow-up appears to be safe if the final pathologic specimen has negative margins. A follow-up protocol including cytologic, colposcopic, and ECC monitoring in the first post-conization visit is recommended.2,6
Glandular lesions
AIS with involved margins requires further surgery due to the possibility of residual AIS or invasion (TABLES 2 and 3).2,5-7 In these cases, CKC is preferable to LEEP.3,5-7 Hysterectomy remains the standard therapy for AIS. Conservative management is an option if fertility is desired and margin status is negative. Patients should be informed that persistent disease or recurrence is possible and that there is a risk of invasive disease.15
Counseling, thorough documentation, and second surgical and pathologic opinions may be helpful in conservative management. Colposcopy, ECC, and cytology are indicated at the first follow-up visit, with cytology repeated every 6 months until 4 consecutive Paps are normal. More frequent colposcopy and liberal use of ECC also may be considered.
TABLE 2
Residual disease and margin status: adenocarcinoma in situ
| AUTHOR | PROPORTION WITH RESIDUAL DISEASE | |||
|---|---|---|---|---|
| NEGATIVE MARGINS | POSITIVE MARGINS | |||
| NUMBER | % | NUMBER | % | |
| Azodi et al, 199928 | 5/16 | 31.3 | 9/16 | 56.3 |
| Goldstein et al, 199828 | 13/43 | 30.2 | 8/18 | 44.4 |
| Denehy et al, 19976 | 2/7 | 28.6 | 7/10 | 70 |
| Widrich et al, 19965 | 0/3 | 0 | 9/14 | 64.3 |
| Wolf et al, 199629 | 7/21 | 33.3 | 10/19 | 52.6 |
| TOTAL | 27/90 | 30 | 43/77 | 55.8 |
TABLE 3
Follow-up recommendations
| Pathology | Margin status | Recommendation |
|---|---|---|
| High-grade squamous lesion | Negative |
|
| Positive, endocervical |
| |
| Positive, ectocervical |
| |
| Squamous microinvasion | Negative, fertility desired |
|
| Negative, no desire for fertility | Hysterectomy | |
| Positive | CKC | |
| Adenocarcinoma in situ | Negative, fertility desired |
|
| Negative, no desire for fertility | Hysterectomy | |
| Positive | CKC | |
| CKC = cold-knife conization; ECC = endocervical curettage | ||
When further excision is necessary
As noted earlier, repeat excision is necessary in cases of squamous microinvasion or AIS with positive margins. CKC is generally preferred to allow for optimal pathologic interpretation. For squamous intraepithelial lesions, excisional biopsy with close follow-up has a significant cure rate, and hysterectomy usually is not indicated. However, hysterectomy still should be considered part of the treatment continuum for CIN, particularly for patients who have completed childbearing.
Conclusion
Although the risk of recurrence is correlated with a patient’s margin status in cases of squamous dysplasia, conservative follow-up is possible and has a high success rate. Cytology is sufficient surveillance for cases involving negative margins. When margins are positive, colposcopy and ECC also may be useful. Microinvasive lesions with positive margins require further surgical evaluation to determine treatment. For glandular lesions, CKC is preferred for both diagnosis and treatment.
Dr. Dunton reports no affiliation or financial arrangement with any of the companies that manufacture drugs or devices in any of the product classes mentioned in this article.
- In most cases, loop electrosurgical excision procedure (LEEP) and cold-knife conization (CKC) result in equivalent success rates and margin status.
- CKC is preferred in cases of adenocarcinoma in situ or squamous microinvasion.
- Conservative follow-up is generally possible in adenocarcinoma in situ and squamous microinvasion when margins are negative.
- Colposcopy, endocervical curettage, and biopsies should be part of the follow-up strategy for patients with positive margins.
When cervical intraepithelial neoplasia (CIN) requires treatment, loop electrosurgical excision procedure (LEEP) is the most frequently used modality, although cold-knife conization (CKC) of the cervical transformation zone still is preferred in select cases. Since excisional techniques are used with CKC, margin status is known and clinical decisions may be based on this information.
This article reviews current indications for excisional biopsy and presents evidence to direct management and follow-up of patients with positive and negative margins.
Selecting a technique
Several large randomized and prospective studies have demonstrated that LEEP is similar in efficacy to CKC and may even remove less of the normal cervical stroma.1-3 In general, LEEP is used to excise high-grade and recurrent squamous dysplasia, as it is similar to CKC in its rates of incomplete excision and residual disease.1,4 However, when adenocarcinoma in situ (AIS) is present, CKC is preferred for diagnosis and treatment, since it results in a lower incidence of involved margins and a lower recurrence rate.3,5-7
CKC also is preferred when histologic confirmation of the margin status is crucial, such as when invasion with squamous lesions is suspected. This is because thermal artifacts that may result from LEEP will interfere with interpretation, further complicating treatment planning.1,4,8,9 In cases of microinvasion, the ability to confirm margins makes conservative treatment possible; if the depth of invasion cannot be determined from the specimen, radical surgery may be necessary.
In the hands of an experienced clinician, however, thermal artifact from the LEEP technique generally is not a significant problem. Series reporting high rates of uninterpretable margins have been attributed to operator inexperience.10 In general, thermal artifact is reduced by limiting the number of sections taken. In ideal cases, only a single-piece specimen is obtained, similar to that achieved using CKC.8,10,11 Newer loops, such as the cone biopsy excision loop, may decrease the number of sections and further improve margin interpretation.8
I use CKC in cases of suspected squamous invasion and in the evaluation of glandular lesions, but feel that in other cases LEEP is efficacious and quicker.
Identifying residual disease
When both the endocervical margin and endocervical curettage (ECC) are positive for squamous dysplasia at the time of excision, there is an increased risk of residual disease (TABLE 1).12-14 However, it is not clear whether ECC alone is an independent predictor of residual disease. Although 1 study suggests an increased risk of invasive cancer in women over 50 years of age with a positive ECC at the time of conization, other series have failed to demonstrate a difference in treatment failure rates based on ECC.15-17 Thus, the utility of ECC in directing further therapy at the time of excisional biopsy is unclear. I generally do not perform an ECC with LEEP for squamous dysplasia.
In AIS, a positive ECC is a strong predictor of residual disease, while a negative ECC is of limited significance.18 I am more likely to perform an ECC with glandular lesions. If it is negative, close follow-up still is indicated. If it is positive, repeat excision may be necessary.
TABLE 1
Residual disease and margin status: squamous dysplasia
| AUTHOR | PROPORTION WITH RESIDUAL DISEASE | |||
|---|---|---|---|---|
| NEGATIVE MARGINS | POSITIVE MARGINS | |||
| NUMBER | % | NUMBER | % | |
| SQUAMOUS LESIONS | ||||
| Bertelsen et al, 199923 | 44/485 | 9 | 29/76 | 38.2 |
| Moore et al, 199524 | 170/523 | 32.5 | 37/91 | 40.7 |
| Lopes et al, 199325 | 0/176 | 0 | 11/131 | 8.4 |
| Murdoch et al, 199226 | 7/405 | 1.7 | 22/160 | 13.8 |
| Andersen et al, 199019 | 0/411 | 0 | 6/469 | 1.3 |
| TOTALS | 221/2,000 | 11 | 105/927 | 11.3 |
| SQUAMOUS MICROINVASION | ||||
| Gurgel et al, 199727 | 6/74 | 8.1 | 45/76 | 59.2 |
| Roman et al, 199722 | 1/30 | 3.3 | 7/50 | 14 |
| TOTALS | 7/104 | 6.7 | 52/126 | 41.3 |
Follow-up of squamous lesions
Considerable clinical uncertainty remains over the relative strengths of cytologic, colposcopic, and histologic evaluation for residual or recurrent disease following excisional biopsy. Conservative management includes Papanicolaou smears alone or in combination with ECC and/or colposcopy.
Negative margins. If margins are negative, the success rate of excisional biopsy is high (90%-100%), and careful observation is the preferred follow-up. Repeat cytologic testing will identify the majority of patients with residual high-grade disease. A prospective randomized trial of cytologic surveillance showed that the detection rate was only 1.9% higher for histology.19 Although 1 report suggests that colposcopy can expedite the diagnosis of recurrent dysplasia, it is unclear from this report whether colposcopy identified significant high-grade dysplasia that cytology missed.20
Positive margins. In most series involving CIN with positive margins, treatment success rates do not differ significantly between patients who undergo repeat surgical procedures and those who have close follow-up. However, endocervical margin involvement appears to carry a higher treatment failure rate than ectocervical margin involvement. Data are conflicting on the proper method of conservative follow-up. Some authors propose only frequent cytologic testing, while others recommend that colposcopy be performed.19-21
For patients with positive margins, I perform both cytology and colposcopy in 4 to 6 months. If an endocervical margin was positive, I also perform an ECC. If this evaluation is negative, I repeat cytologic testing every 6 months until 3 consecutive Pap smears are normal and satisfactory. In cases of recurrent high-grade dysplasia with positive margins, hysterectomy may be indicated, depending on the patient’s age and desire for continued fertility.14,21
Squamous microinvasion
There is a significant risk (50%-80%) of residual disease or true invasion when margins or an ECC are positive and microinvasion is present.2,5-7 In these cases, a repeat excisional biopsy should be performed to determine the true extent of disease prior to deciding on definitive therapy.22 As previously mentioned, CKC is preferred to limit artifact that could obscure interpretation. If the patient has completed her childbearing, hysterectomy remains the standard treatment for microinvasive squamous cell carcinoma. When the woman wishes to preserve fertility, conservative follow-up appears to be safe if the final pathologic specimen has negative margins. A follow-up protocol including cytologic, colposcopic, and ECC monitoring in the first post-conization visit is recommended.2,6
Glandular lesions
AIS with involved margins requires further surgery due to the possibility of residual AIS or invasion (TABLES 2 and 3).2,5-7 In these cases, CKC is preferable to LEEP.3,5-7 Hysterectomy remains the standard therapy for AIS. Conservative management is an option if fertility is desired and margin status is negative. Patients should be informed that persistent disease or recurrence is possible and that there is a risk of invasive disease.15
Counseling, thorough documentation, and second surgical and pathologic opinions may be helpful in conservative management. Colposcopy, ECC, and cytology are indicated at the first follow-up visit, with cytology repeated every 6 months until 4 consecutive Paps are normal. More frequent colposcopy and liberal use of ECC also may be considered.
TABLE 2
Residual disease and margin status: adenocarcinoma in situ
| AUTHOR | PROPORTION WITH RESIDUAL DISEASE | |||
|---|---|---|---|---|
| NEGATIVE MARGINS | POSITIVE MARGINS | |||
| NUMBER | % | NUMBER | % | |
| Azodi et al, 199928 | 5/16 | 31.3 | 9/16 | 56.3 |
| Goldstein et al, 199828 | 13/43 | 30.2 | 8/18 | 44.4 |
| Denehy et al, 19976 | 2/7 | 28.6 | 7/10 | 70 |
| Widrich et al, 19965 | 0/3 | 0 | 9/14 | 64.3 |
| Wolf et al, 199629 | 7/21 | 33.3 | 10/19 | 52.6 |
| TOTAL | 27/90 | 30 | 43/77 | 55.8 |
TABLE 3
Follow-up recommendations
| Pathology | Margin status | Recommendation |
|---|---|---|
| High-grade squamous lesion | Negative |
|
| Positive, endocervical |
| |
| Positive, ectocervical |
| |
| Squamous microinvasion | Negative, fertility desired |
|
| Negative, no desire for fertility | Hysterectomy | |
| Positive | CKC | |
| Adenocarcinoma in situ | Negative, fertility desired |
|
| Negative, no desire for fertility | Hysterectomy | |
| Positive | CKC | |
| CKC = cold-knife conization; ECC = endocervical curettage | ||
When further excision is necessary
As noted earlier, repeat excision is necessary in cases of squamous microinvasion or AIS with positive margins. CKC is generally preferred to allow for optimal pathologic interpretation. For squamous intraepithelial lesions, excisional biopsy with close follow-up has a significant cure rate, and hysterectomy usually is not indicated. However, hysterectomy still should be considered part of the treatment continuum for CIN, particularly for patients who have completed childbearing.
Conclusion
Although the risk of recurrence is correlated with a patient’s margin status in cases of squamous dysplasia, conservative follow-up is possible and has a high success rate. Cytology is sufficient surveillance for cases involving negative margins. When margins are positive, colposcopy and ECC also may be useful. Microinvasive lesions with positive margins require further surgical evaluation to determine treatment. For glandular lesions, CKC is preferred for both diagnosis and treatment.
Dr. Dunton reports no affiliation or financial arrangement with any of the companies that manufacture drugs or devices in any of the product classes mentioned in this article.
1. Mathevet P, Dargent D, Roy M, Beau G. A randomized prospective study comparing three techniques of conization: cold knife, laser, and LEEP. Gynecol Oncol. 1994;54(2):175-179.
2. Duggan BD, Felix JC, Muderspach LI, et al. Cold-knife conization versus conization by the loop electrosurgical excision procedure: a randomized, prospective study. Am J Obstet Gynecol. 1999;180:276-282.
3. Girardi F, Heydarfadi M, Koroschetz F, et al. Cold-knife conization versus loop excision: histopathologic and clinical results of a randomized trial. Gynecol Oncol. 1994;55:368-370.
4. Gold M, Dunton CJ, Murray J, et al. Loop electrocautery excisional procedure: therapeutic effectiveness as an ablation and a conization equivalent. Gynecol Oncol. 1996;61:241-244.
5. Widrich T, Kennedy AW, Myers TM, et al. Adenocarcinoma in situ of the uterine cervix: management and outcome. Gynecol Oncol. 1996;61:304-308.
6. Denehy TR, Gregori CA, Breen JL. Endocervical curettage, cone margins, and residual adenocarcinoma in situ of the cervix. Obstet Gynecol. 1997;90:1-6.
7. Muntz HG, Bell DA, Lage JM, et al. Adenocarcinoma in situ of the uterine cervix. Obstet Gynecol. 1992;80:935-939.
8. Naumann RW, Bell MC, Alvarez RD, et al. LLETZ is an acceptable alternative to diagnostic cold-knife conization. Gynecol Oncol. 1994;55:224-228.
9. Eddy GL, Spiegel GW, Creasman WT. Adverse effect of electrosurgical loop excision on assignment of FIGO stage in cervical cancer: report of two cases. Gynecol Oncol. 1994;55:313-317.
10. Montz FJ, Holschneider CH, Thompson LDR. Large-loop excision of the transformation zone: effect on the pathologic interpretation of resection margins. Obstet Gynecol. 1993;81:976-982.
11. Gardeil F, Barry-Walsh C, Prendiville W, et al. Persistent intraepithelial neoplasia after excision for cervical intraepithelial neoplasia grade III. Obstet Gynecol. 1987;89:419-422.
12. Felix JC, Muderspach LI, Duggan BD, Roman LD. The significance of positive margins in loop electrosurgical cone biopsies. Obstet Gynecol. 1994;84:996-1000.
13. Kobak WH, Roman LD, Felix JC, et al. The role of endocervical curettage at cervical conization for high-grade dysplasia. Obstet Gynecol. 1995;85:197-201.
14. Husseinzadeh N, Shbara I, Wessler T. Predictive value of cone margins and post-cone endocervical curettage with residual disease in subsequent hysterectomy. Gynecol Oncol. 1989;33:198-200.
15. Poyner EA, Barakat RR, Hoskins WJ. Management and follow-up of patients with adenocarcinoma in situ of the uterine cervix. Gynecol Oncol. 1995;57:158-164.
16. Frauchiger WL, De Frias DVS, Cajulis RS, Yu GH. The immediate postconization endocervical smear: evaluation of its utility in the detection of residual dysplasia. Acta Cytol. 1998;42:1139-1143.
17. Wright TC, Gagnon S, Richart RM, Ferenczy A. Treatment of cervical intraepithelial neoplasia using the loop electrosurgical excision procedure. Obstet Gynecol. 1992;79:173-178.
18. Goldstein NS, Mani A. The status and distance of cone biopsy margins as a predictor of excision adequacy for endocervical adenocarcinoma in situ. Am J Clin Pathol. 1998;109:727-732.
19. Andersen ES, Nielsen K, Larsen G. Laser conization: follow-up in patients with cervical intraepithelial neoplasia in the cone margin. Gynecol Oncol. 1990;39:328-331.
20. Paraskevaidis E, Jandial L, Mann EMF, Fisher PM. Pattern of treatment failure following laser for cervical intraepithelial neoplasia: implications for follow-up protocol. Obstet Gynecol. 1991;78:80-83.
21. Lapaquette TK, Dinh TV, Hannigan EV, et al. Management of patients with positive margins after cervical conization. Obstet Gynecol. 1993;82:440-443.
22. Roman LD, Felix JC, Muderspach LI, et al. Risk of residual invasive disease in women with microinvasive squamous cancer in a conization specimen. Obstet Gynecol. 1997;90:759-764.
23. Bertelsen B, Tande T, Sandvei, Hartveit F. Laser conization of cervical intraepithelial neoplasia grade 3. Acta Obstet Gynecol Scand. 1999;78:54-59.
24. Moore BC, Higgins RV, Laurent SL, et al. Predictive factors from cold knife conization for residual cervical intraepithelial neoplasia in subsequent hysterectomy. Am J Obstet Gynecol. 1995;173:361-368.
25. Lopes A, Morgan P, Murdoch J, et al. The case for conservative management of “incomplete excision” of CIN after laser conization. Gynecol Oncol. 1993;49:247-249.
26. Murdoch JB, Morgan PR, Lopes A, Monaghan JM. Histological incomplete excision of CIN after large loop excision of the transformation zone (LLETZ) merits careful follow up, not retreatment. Br J Obstet Gynaecol. 1992;99:990-993.
27. Gurgel MSC, Bedone AJ, Andrade LA, et al. Microinvasive carcinoma of the uterine cervix: histological findings on cone specimens related to residual neoplasia on hysterectomy. Gynecol Oncol. 1997;65:437-440.
28. Azodi M, Chambers SK, Rutherford TJ, et al. Adenocarcinoma in situ of the cervix: management and outcome. Gynecol Oncol. 1999;73:348-353.
29. Wolf JK, Levenback C, Malpica A, et al. Adenocarcinoma in situ of the cervix: significance of cone biopsy margins. Obstet Gynecol. 1996;88:82-86.
1. Mathevet P, Dargent D, Roy M, Beau G. A randomized prospective study comparing three techniques of conization: cold knife, laser, and LEEP. Gynecol Oncol. 1994;54(2):175-179.
2. Duggan BD, Felix JC, Muderspach LI, et al. Cold-knife conization versus conization by the loop electrosurgical excision procedure: a randomized, prospective study. Am J Obstet Gynecol. 1999;180:276-282.
3. Girardi F, Heydarfadi M, Koroschetz F, et al. Cold-knife conization versus loop excision: histopathologic and clinical results of a randomized trial. Gynecol Oncol. 1994;55:368-370.
4. Gold M, Dunton CJ, Murray J, et al. Loop electrocautery excisional procedure: therapeutic effectiveness as an ablation and a conization equivalent. Gynecol Oncol. 1996;61:241-244.
5. Widrich T, Kennedy AW, Myers TM, et al. Adenocarcinoma in situ of the uterine cervix: management and outcome. Gynecol Oncol. 1996;61:304-308.
6. Denehy TR, Gregori CA, Breen JL. Endocervical curettage, cone margins, and residual adenocarcinoma in situ of the cervix. Obstet Gynecol. 1997;90:1-6.
7. Muntz HG, Bell DA, Lage JM, et al. Adenocarcinoma in situ of the uterine cervix. Obstet Gynecol. 1992;80:935-939.
8. Naumann RW, Bell MC, Alvarez RD, et al. LLETZ is an acceptable alternative to diagnostic cold-knife conization. Gynecol Oncol. 1994;55:224-228.
9. Eddy GL, Spiegel GW, Creasman WT. Adverse effect of electrosurgical loop excision on assignment of FIGO stage in cervical cancer: report of two cases. Gynecol Oncol. 1994;55:313-317.
10. Montz FJ, Holschneider CH, Thompson LDR. Large-loop excision of the transformation zone: effect on the pathologic interpretation of resection margins. Obstet Gynecol. 1993;81:976-982.
11. Gardeil F, Barry-Walsh C, Prendiville W, et al. Persistent intraepithelial neoplasia after excision for cervical intraepithelial neoplasia grade III. Obstet Gynecol. 1987;89:419-422.
12. Felix JC, Muderspach LI, Duggan BD, Roman LD. The significance of positive margins in loop electrosurgical cone biopsies. Obstet Gynecol. 1994;84:996-1000.
13. Kobak WH, Roman LD, Felix JC, et al. The role of endocervical curettage at cervical conization for high-grade dysplasia. Obstet Gynecol. 1995;85:197-201.
14. Husseinzadeh N, Shbara I, Wessler T. Predictive value of cone margins and post-cone endocervical curettage with residual disease in subsequent hysterectomy. Gynecol Oncol. 1989;33:198-200.
15. Poyner EA, Barakat RR, Hoskins WJ. Management and follow-up of patients with adenocarcinoma in situ of the uterine cervix. Gynecol Oncol. 1995;57:158-164.
16. Frauchiger WL, De Frias DVS, Cajulis RS, Yu GH. The immediate postconization endocervical smear: evaluation of its utility in the detection of residual dysplasia. Acta Cytol. 1998;42:1139-1143.
17. Wright TC, Gagnon S, Richart RM, Ferenczy A. Treatment of cervical intraepithelial neoplasia using the loop electrosurgical excision procedure. Obstet Gynecol. 1992;79:173-178.
18. Goldstein NS, Mani A. The status and distance of cone biopsy margins as a predictor of excision adequacy for endocervical adenocarcinoma in situ. Am J Clin Pathol. 1998;109:727-732.
19. Andersen ES, Nielsen K, Larsen G. Laser conization: follow-up in patients with cervical intraepithelial neoplasia in the cone margin. Gynecol Oncol. 1990;39:328-331.
20. Paraskevaidis E, Jandial L, Mann EMF, Fisher PM. Pattern of treatment failure following laser for cervical intraepithelial neoplasia: implications for follow-up protocol. Obstet Gynecol. 1991;78:80-83.
21. Lapaquette TK, Dinh TV, Hannigan EV, et al. Management of patients with positive margins after cervical conization. Obstet Gynecol. 1993;82:440-443.
22. Roman LD, Felix JC, Muderspach LI, et al. Risk of residual invasive disease in women with microinvasive squamous cancer in a conization specimen. Obstet Gynecol. 1997;90:759-764.
23. Bertelsen B, Tande T, Sandvei, Hartveit F. Laser conization of cervical intraepithelial neoplasia grade 3. Acta Obstet Gynecol Scand. 1999;78:54-59.
24. Moore BC, Higgins RV, Laurent SL, et al. Predictive factors from cold knife conization for residual cervical intraepithelial neoplasia in subsequent hysterectomy. Am J Obstet Gynecol. 1995;173:361-368.
25. Lopes A, Morgan P, Murdoch J, et al. The case for conservative management of “incomplete excision” of CIN after laser conization. Gynecol Oncol. 1993;49:247-249.
26. Murdoch JB, Morgan PR, Lopes A, Monaghan JM. Histological incomplete excision of CIN after large loop excision of the transformation zone (LLETZ) merits careful follow up, not retreatment. Br J Obstet Gynaecol. 1992;99:990-993.
27. Gurgel MSC, Bedone AJ, Andrade LA, et al. Microinvasive carcinoma of the uterine cervix: histological findings on cone specimens related to residual neoplasia on hysterectomy. Gynecol Oncol. 1997;65:437-440.
28. Azodi M, Chambers SK, Rutherford TJ, et al. Adenocarcinoma in situ of the cervix: management and outcome. Gynecol Oncol. 1999;73:348-353.
29. Wolf JK, Levenback C, Malpica A, et al. Adenocarcinoma in situ of the cervix: significance of cone biopsy margins. Obstet Gynecol. 1996;88:82-86.
Determining the best route for hysterectomy
- Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma.
- Use a combination of uterine morcellation techniques to accomplish a vaginal hysterectomy, as researchers have found morcellation of an enlarged uterus to be safer than removing it abdominally.
- For laparoscopic-assisted vaginal hysterectomy, use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles to help minimize costs.
- Seek alternatives to abdominal hysterectomy given its less favorable outcome in terms of morbidity and recovery.
While hysterectomy is one of the most frequently performed operations in gynecology, how to perform it—abdominally, vaginally, or laparoscopically—is less evident. Numerous studies have been published in an attempt to shed some light on this controversy.
Individualize the approach for each patient rather than rely on a dogmatic assignment of technique.
Prior to the introduction of the laparoscopic-assisted vaginal hysterectomy (LAVH) by Reich et al in 1989,1 several large studies were published that compared the abdominal and vaginal routes for hysterectomy. The largest was the Collaborative Review of Sterilization (CREST) study conducted by the Centers for Disease Control (CDC).2 This report included 1,856 women aged 15 to 44 who underwent non-emergency, non-radical hysterectomies at 9 institutions between 1978 and 1981. Fewer complications were associated with vaginal hysterectomy (VH) than abdominal hysterectomy (AH) (Table 1).
Now, several trials have included LAVH in the comparison of hysterectomy routes. In the most comprehensive study to date, Johns et al reviewed 2,563 hysterectomies performed for nonmalignant indications by 37 private gynecologists from a single institution.3 The researchers found that bowel, bladder, and ureteral injuries were uncommon, and the rates of each were similar among LAVH, abdominal hysterectomy, and vaginal hysterectomy (Table 2). In addition, a review of the literature between 1989 and 1995 revealed that LAVH is associated with a shorter hospital stay, decreased recovery time, and less analgesia compared with AH.4
However, since most of the data on route for hysterectomy are from retrospective and uncontrolled trials, one must interpret the findings carefully. For example, many studies do not control for additional procedures performed at the time of hysterectomy (e.g., enterocele, rectocele, and cystocele repairs). In addition, information on how researchers categorized unsuccessful attempts at VH or LAVH—which then had to be converted to AH—often is excluded. Also, physicians usually select the technique based on personal preference, practice style, and traditional dogma such as uterine size rather than a standard protocol.5,6 Therefore, the increased incidence of postoperative morbidity associated with AH is difficult to decipher. Is it due to the increased number of obese and nulliparous women undergoing AH, the surgeon’s experience, pelvic pathology or operative indication, or is it related to the actual opening of the abdomen and intraperitoneal manipulation? Most likely, it is a combination of these factors.
Overall, hysterectomy is a relatively safe procedure with a mortality rate of 1 to 2 per 1,000.7 Morbidity, however, remains high. Fortunately, most complications are minor and easily remedied with little clinical consequence. Since certain aspects of postoperative morbidity are related to the route for hysterectomy, the surgeon must individualize the approach for each patient and not rely on a dogmatic assignment of technique. Here, we will review the patient selection for and provide pearls on abdominal, vaginal, and laparoscopic-assisted vaginal hysterectomy, as well as look at the advantages and disadvantages of each method.
TABLE 1
CREST*study results
| VH | AH | |
|---|---|---|
| Mean age (yrs) | 34.4 | 35.8 |
| Nulliparous (%) | 1.4 | 13.3 |
| Prior cesarean section (%) | 4.8 | 10.1 |
| Obese** (%) | 38.0 | 44.7 |
| Febrile morbidity (%) | 15.3 | 32.3 |
| Required transfusion (%) | 8.3 | 15.4 |
| Death (%) | 0.2 | 0.1 |
| *The Collaborative Review of Sterilization | ||
| **Greater than 120% ideal body weight | ||
| VH=vaginal hysterectomy | ||
| AH=abdominal hysterectomy | ||
| Source: Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848. | ||
TABLE 2
Complications at hysterectomy
| AH | VH | LAVH | |
|---|---|---|---|
| Operating time (minutes) | 82 | 63 | 102 |
| Uterine weight (grams) | 216 | 113 | 129 |
| Febrile morbidity (%) | 9.1 | 3.2 | 2.0 |
| Required transfusion (%) | 2.5 | 1.0 | 0.06 |
| Bowel, bladder, or ureteral injury (%) | 1.0 | 0.9 | 1.1 |
| Death (%) | 0 | 0.2 | 0 |
| AH=abdominal hysterectomy | |||
| VH=vaginal hysterectomy | |||
| LAVH=laparoscopic-assisted vaginal hysterectomy | |||
| Source: Johns DA, et al. The medical and economic impact of laparoscopically assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-1719. | |||
Abdominal hysterectomy
Patient selection. Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma. Severe pelvic adhesive disease from documented severe endometriosis, salpingitis, or significant adnexal pathology and a considerably enlarged uterus also are best approached abdominally (Figure 1). In addition, use the abdominal route for obstetric emergencies such as postpartum hemorrhage.
Technique. First, determine the type of incision based on the following factors: Which one will allow completion of the procedure in a safe and efficient manner, minimize complications, expedite recovery, and offer a favorable cosmetic result? A vertical midline incision is the most adaptable to unsuspected pathology, especially if found in the upper abdomen. It is the quickest to perform and is associated with the least blood loss, an important advantage when operating emergently on an anemic or hemorrhaging patient. However, bear in mind that vertical incisions that have been extended into the upper abdomen are accompanied by increased postoperative pulmonary morbidity, pain, and risk of hernia formation.8,9
Transverse incisions such as the commonly used Pfannenstiel, Cherney, and Maylard compromise upper abdominal exposure but are more cosmetically appealing than vertical incisions. Each of these involves a lower abdominal transverse skin incision and a transverse fascial incision. With the Pfannenstiel technique, the rectus fascia is separated from the rectus muscles, and the muscles are split vertically in the midline, allowing access to the peritoneal cavity. This is the quickest transverse incision to make but provides the least exposure.
If a Pfannenstiel incision has been made and additional lateral pelvic exposure is needed, convert the incision to a Cherney. Both the Cherney and Maylard incisions provide additional exposure to the pelvis, especially laterally, which is advantageous when complex pathology is limited to the pelvis. The Cherney incision involves dividing the rectus muscles from the pubic symphysis, whereas the Maylard incision entails horizontal transection of the rectus muscles at the level of the fascial incision. With the Maylard technique, the rectus fascia is not separated from the underlying muscles as it is in the Cherney incision. If upper abdominal exposure becomes necessary, all of these incisions can be extended upward from their lateral margin, creating a “J.” Alternatively, a second incision can be made in the upper abdomen.
Another option is mini-laparotomy (an incision less than 6 cm). Hoffman and Lynch reported success with this technique for hysterectomy in select patients.10 Most of the procedures were completed with minimal use of retractors by exteriorizing the uterus via one of the aforementioned incisions. They found this approach to be safe and effective in nonobese women in whom a vaginal approach was precluded due to anatomy.
Use the mini-laparotomy approach when there is a suspicious adnexal mass that can be removed through a small abdominal incision.11 In addition, a large benign-appearing cystic adnexal mass can be drained, and the procedure then completed through this incision. Proceed cautiously, however, since an occasional unanticipated carcinoma will be encountered, and the act of drainage will result in the need for chemotherapy in a woman with otherwise early-stage disease. If cancer is suspected, consider a vertical minilaparotomy, which allows easy extension into the upper abdomen should surgical staging or debulking become necessary.
Advantages and disadvantages. The abdominal approach offers the best exposure of the pelvic and upper abdominal cavity but is associated with a high rate of complications, including fever and excessive blood loss. We surmise that postoperative recovery with a mini-laparotomy is likely to be improved compared with the traditional abdominal technique.
Figure 1
Opt for abdominal hysterectomy when a considerably enlarged uterus due to numerous fibroids is encountered.
Vaginal hysterectomy
Patient selection. Choose the vaginal route when pelvic support defects are present (Figure 2), but bear in mind that the course of the ureter changes with worsening degrees of uterine prolapse. We have found the ureter to be palpable in the bladder pillar in most women undergoing VH for prolapse, which helps avoid intraoperative ureteral injury.
The ovaries can be safely removed through the vagina in a large percentage of patients.
Obese, elderly, and otherwise medically debilitated patients also will benefit from the vaginal approach. Avoiding an abdominal wound in these women has obvious advantages. Also, vaginal surgery is associated with a reduction in postoperative pulmonary complications when compared with AH.6,12
Vaginal accessibility inevitably influences the decision to proceed vaginally with a hysterectomy. An inadequate bony pelvis is reason to forgo a VH. Orthopedic conditions and muscular contractures of the lower extremities, which prevent safe positioning, also inhibit this approach. Some surgeons also consider prior abdominal or pelvic surgery a reason to avoid vaginal hysterectomy, while others find this route a means of eluding potential adhesions.
We are strong advocates of vaginal surgery and do not consider nulliparity, lack of uterine descent, or prior abdominopelvic surgery to be strict contraindications for VH. A Schuchardt incision may overcome a small introitus, and a mobile uterus often will descend as the uterosacral and cardinal ligaments are divided.
In women with a prior cesarean delivery, it may be easier to enter the vesicouterine space by approaching it from the less-scarred vaginal side. In a study of more than 200 patients with a previous cesarean who underwent VH, Sheth and Malpani found no increase in complications and concluded that VH is the route of choice in this patient population.13 When unsuspected pelvic adhesions are encountered, carefully dissect them transvaginally and complete the procedure.
Besides accessibility, uterine size must be considered. However, size alone should present a dilemma in only about 15% of patients since most hysterectomy specimens are 12 weeks’ gestational size or smaller.14
Technique. Large uteri can be removed vaginally, depending on the surgeon’s technical ability and experience. A familiarity with the various methods of morcellation—hemisection, posterior fundal morcellation, intramyometrial coring, and myomectomy—is mandatory. It often is beneficial to use a combination of these techniques to accomplish the procedure vaginally, as opposed to a single means of morcellation. With any morcellation procedure, maintain a midline orientation to avoid dissection into the broad ligament. Continued caudal traction also is helpful in eliminating excessive blood loss during this prolonged procedure. Do not morcellate a uterus when endometrial carcinoma is suspected.
In a prospective observational study comparing vaginal morcellation to AH, Hoffman et al15 found the former to be safer. Other researchers also have demonstrated the procedure’s safety in retrospective reports16-18 and as subgroups in other series.14 Alternatively, preoperative treatment with a gonadotropin-releasing hor-Surgical Techniques mone (GnRH) agonist can reduce uterine size and result in a technically less challenging VH in some women.19,20
Also, whether or not the ovaries will be resected may influence the decision to perform a VH. While some surgeons believe that oophorectomy precludes the vaginal approach, the ovaries can be safely removed through the vagina in a large percentage of patients.21-23 In fact, Sheth24 and Kovac14 have published 95% and 97% success rates in 2 separate series in which vaginal oophorectomy was attempted in 740 and 142 women, respectively. However, do not attempt adnexal removal without adequate transvaginal access and visibility, as it increases the risk of hemorrhage and ureteral injury. In these cases, LAVH or mini-laparotomy AH may be preferable if oophorectomy is mandatory, i.e., in women who are predisposed to ovarian malignancies because of genetic mutations. However, when patients are appropriately selected, there are essentially no disadvantages to VH.
Advantages and disadvantages.VH has many advantages over AH. Minimal intraperitoneal manipulation and the avoidance of an abdominal wound lead to a shorter hospital stay and decreased recovery time, which is coupled with less cost. Patients also find the lack of an abdominal scar to be an attractive feature of VH. A disadvantage is reduced operative exposure, making it difficult to manipulate pelvic pathology and resect the adnexa.
Figure 2
Choose the vaginal route when pelvic support defects are present, but bear in mind that the course of the ureter changes due to prolapse.
Laparoscopic-assisted vaginal hysterectomy
Patient selection. When in doubt about which approach to use, turn to LAVH. The laparoscope allows the surgeon to thoroughly assess intra-abdominal pathology, which aids in the appropriate selection of a hysterectomy route. For example, if cancer is suspected based on the visual inspection, opt for an AH. On the other hand, if only extensive adhesions are noted, laparoscopic adhesiolysis can be performed to allow the hysterectomy to be completed vaginally. As previously noted, LAVH is appropriate in patients undergoing hysterectomy with prophylactic oophorectomy due to genetic or familial risk factors.
Technique. Bear in mind that the laparoscope does not permit all patients to undergo a VH. In fact, about 10% of attempted LAVHs will be unsuccessful.25,26 Some authors have reported failure during the laparoscopic portion,25,26 while others have attributed the lack of success to the vaginal portion of the procedure.27 Thus, it is important to be skilled at both laparoscopic and vaginal surgery in order to be successful with an LAVH.
LAVH requires three to five 5-to 10-mm abdominal incisions for port sites. An umbilical port is commonly used for the camera, and the remaining ports are used for operating. We typically use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles, which helps minimize costs.3 Other alternatives include the use of endoscopic staplers, the Harmonic Scalpel (Ethicon Endo-Surgery, a Johnson & Johnson company, Cincinnati, Ohio), and suture ligatures with extra-or intracorporeal knot tying. Whichever instrument is used, familiarize yourself with all available equipment given the occasional malfunction encountered during laparoscopy.
Advantages and disadvantages. LAVH requires special equipment and expertise beyond that needed for an abdominal or vaginal hysterectomy. Additionally, this procedure increases operative time, cost, and morbidity (depending on the surgeon’s experience),4,27,28 while the postoperative recovery is similar to that of VH.
Although we use the laparoscope infrequently and believe there is little need to perform excessive laparoscopy at the time of routine hysterectomy, we do not wish to understate the role of the laparoscope. Its use in specific instances can certainly avoid a laparotomy or help determine when an AH is more appropriate.
Conclusion
Emergent situations and patients with excessively enlarged uteri, significant pelvic pathology, or cancer are obvious candidates for AH. On the other hand, VH is frequently chosen for the small uterus in a multiparous woman with a large pelvis and no prior pelvic inflammatory disease or surgery. The dilemma arises when determining the approach for those patients with moderately enlarged uteri or presumptive risk factors for serious pelvic disease.
Kovac reported a standard protocol for selecting the route for hysterectomy. Uterine size, other pelvic pathology (endometriosis, adnexal disease, chronic pain, etc.), and uterine and adnexal accessibility (bony architecture, uterine support, and vaginal diameter) were each considered in the decision-making. A simplification of Kovac’s guidelines applicable to women undergoing hysterectomy for benign indications is summarized in Figure 3. Using these guidelines, Kovac reported a 99% (608/611) success rate for women assigned to VH or LAVH. The laparoscope was deemed necessary in only 19% of those assigned to the LAVH group, and ultimately only 9 patients required AH, yielding a 1:68 ratio for AH to VH.14
Gynecologists should seek alternatives to AH given its less favorable outcome in terms of morbidity and recovery. However, the surgeon who is competent with AH better serves the patient by performing the procedure via this route than by attempting an alternative procedure without the necessary proficiency. In other words, pelvic surgeons must be cognizant of their abilities and practice within that realm. Ultimately, the final selection of hysterectomy route should be based on the surgeon’s experience, the indication for surgery, and the patient’s anatomy.
FIGURE 3Guidelines to determine route of hysterectomy.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Reich H, DeCaprio J, McGlynn F. Laparoscopic hysterectomy. J Gynecol Surg. 1989;5:213-216.
2. Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848.
3. Johns DA, Carrera B, Jones J, DeLeon F, Vincent R, et al. The medical and economic impact of laparoscopic-assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-719.
4. Meikle SF, Nugent EW, Orleans M. Complication and recovery for laparoscopic-assisted vaginal hysterectomy compared with abdominal and vaginal hysterectomy. Obstet Gynecol. 1997;89:304-311.
5. Kovac SR, Christie SJ, Bindbeutel GA. Abdominal versus vaginal hysterectomy: a statistical model for determining physician decision making and patient outcomes. Med Decis Making. 1991;11:19-28.
6. Dorsey JH, Steinberg EP, Holtz PM. Clinical indications for hysterectomy route: patient characteristics or physician preference. Am J Obstet Gynecol. 1995;173:1452-1460.
7. Wingo PA, Huezo CM, Rubin GL, Ory HW, Peterson HB. The mortality risk associated with hysterectomy. Am J Obstet Gynecol. 1985;152:803-808.
8. Tollefson DG, Russell KP. The transverse incision in pelvic surgery. Am J Obstet Gynecol. 1954;68:410-422.
9. Helmkamp BF. Abdominal wound dehiscence. Am J Obstet Gynecol. 1977;128:803-807.
10. Hoffman MS, Lynch CM. Minilaparotomy hysterectomy. Am J Obstet Gynecol. 1998;179:316-320.
11. Flynn M, Niloff JM. Minilaparotomy for the ambulatory management of ovarian cysts. Am J Obstet Gynecol. 1995;173:1727-1730.
12. White SC, Wartel LJ, Wade ME. Comparison of abdominal and vaginal hysterectomies. Obstet Gynecol. 1971;37:530-537.
13. Sheth SS, Malpani AN. Vaginal hysterectomy following previous cesarean section. Int J Gynaecol Obstet. 1995;50:65-69.
14. Kovac SR. Guidelines to determine route of hysterectomy. Obstet Gynecol. 1995;85:18-23.
15. Hoffman MS, DeCesare S, Kalter C. Abdominal hysterectomy versus transvaginal morcellation for the removal of enlarged uteri. Am J Obstet Gynecol. 1994;171:309-315.
16. Mazdisnian F, Kurzel RB, Coe S, Bosuk M, Montz F. Vaginal hysterectomy by uterine morcellation: an efficient, non-morbid procedure. Obstet Gynecol. 1995;86:60-64.
17. Kudo R, Yamauchi O, Okazaki T, Sagac S, Ito E, Hashimoto M. Vaginal hysterectomy without ligation of the ligaments of the cervix uteri. Surg Gynecol Obstet. 1990;70:299-305.
18. Kovac SR. Intramyometrial coring as an adjunct to vaginal hysterectomy. Obstet Gynecol. 1986;67:131-136.
19. Stovall TG, Summit RL, Washburn SA, Ling FW. Gonadotropin-releasing hormone agonist use before hysterectomy. Am J Obstet Gynecol. 1994;170:1744-1748.
20. Crosignani PG, Vercellini P, Meschia M, Oldani S, Bramante T. GnRH agonists before surgery for uterine leiomyomas. J Reprod Med. 1996;41:415-421.
21. Hoffman MS. Transvaginal removal of ovaries with endoloop sutures at the time of transvaginal hysterectomy. Am J Obstet Gynecol. 1991;165:407-408.
22. Sheth SS. Ovarian clamp for oophorectomy during vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;2(4):S76-S77.
23. Hefni MA, Davies AE. Vaginal endoscopic oophorectomy with vaginal hysterectomy: a simple minimal access surgery technique. Br J Obstet Gynaecol. 1997;104:621-622.
24. Sheth SS. The place of oophorectomy at vaginal hysterectomy. Br J Obstet Gynaecol. 1991;98:662-666.
25. Cristoforoni PM, Palmieri A, Gerbaldo D, Montz FJ. Frequency and cause of aborted laparoscopic-assisted vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;3:33-37.
26. Summitt RL, Stovall TG, Steege JF, Lipscomb GH. A multicenter randomized comparison of laparoscopic-assisted vaginal hysterectomy and abdominal hysterectomy in abdominal hysterectomy candidates. Obstet Gynecol. 1998;92:321-326.
27. Dorsey JH, Holtz PM, Griffiths RI, McGrath MM, Steinberg EP. Costs and charges associated with three alternative techniques of hysterectomy. N Engl J Med. 1996;335:476-482.
28. Summitt RL, Stovall TG, Lipscomb GH, Ling FW. Randomized comparison of laparoscopic-assisted vaginal hysterectomy with standard vaginal hysterectomy in an outpatient setting. Obstet Gynecol. 1992;80:895-901.
- Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma.
- Use a combination of uterine morcellation techniques to accomplish a vaginal hysterectomy, as researchers have found morcellation of an enlarged uterus to be safer than removing it abdominally.
- For laparoscopic-assisted vaginal hysterectomy, use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles to help minimize costs.
- Seek alternatives to abdominal hysterectomy given its less favorable outcome in terms of morbidity and recovery.
While hysterectomy is one of the most frequently performed operations in gynecology, how to perform it—abdominally, vaginally, or laparoscopically—is less evident. Numerous studies have been published in an attempt to shed some light on this controversy.
Individualize the approach for each patient rather than rely on a dogmatic assignment of technique.
Prior to the introduction of the laparoscopic-assisted vaginal hysterectomy (LAVH) by Reich et al in 1989,1 several large studies were published that compared the abdominal and vaginal routes for hysterectomy. The largest was the Collaborative Review of Sterilization (CREST) study conducted by the Centers for Disease Control (CDC).2 This report included 1,856 women aged 15 to 44 who underwent non-emergency, non-radical hysterectomies at 9 institutions between 1978 and 1981. Fewer complications were associated with vaginal hysterectomy (VH) than abdominal hysterectomy (AH) (Table 1).
Now, several trials have included LAVH in the comparison of hysterectomy routes. In the most comprehensive study to date, Johns et al reviewed 2,563 hysterectomies performed for nonmalignant indications by 37 private gynecologists from a single institution.3 The researchers found that bowel, bladder, and ureteral injuries were uncommon, and the rates of each were similar among LAVH, abdominal hysterectomy, and vaginal hysterectomy (Table 2). In addition, a review of the literature between 1989 and 1995 revealed that LAVH is associated with a shorter hospital stay, decreased recovery time, and less analgesia compared with AH.4
However, since most of the data on route for hysterectomy are from retrospective and uncontrolled trials, one must interpret the findings carefully. For example, many studies do not control for additional procedures performed at the time of hysterectomy (e.g., enterocele, rectocele, and cystocele repairs). In addition, information on how researchers categorized unsuccessful attempts at VH or LAVH—which then had to be converted to AH—often is excluded. Also, physicians usually select the technique based on personal preference, practice style, and traditional dogma such as uterine size rather than a standard protocol.5,6 Therefore, the increased incidence of postoperative morbidity associated with AH is difficult to decipher. Is it due to the increased number of obese and nulliparous women undergoing AH, the surgeon’s experience, pelvic pathology or operative indication, or is it related to the actual opening of the abdomen and intraperitoneal manipulation? Most likely, it is a combination of these factors.
Overall, hysterectomy is a relatively safe procedure with a mortality rate of 1 to 2 per 1,000.7 Morbidity, however, remains high. Fortunately, most complications are minor and easily remedied with little clinical consequence. Since certain aspects of postoperative morbidity are related to the route for hysterectomy, the surgeon must individualize the approach for each patient and not rely on a dogmatic assignment of technique. Here, we will review the patient selection for and provide pearls on abdominal, vaginal, and laparoscopic-assisted vaginal hysterectomy, as well as look at the advantages and disadvantages of each method.
TABLE 1
CREST*study results
| VH | AH | |
|---|---|---|
| Mean age (yrs) | 34.4 | 35.8 |
| Nulliparous (%) | 1.4 | 13.3 |
| Prior cesarean section (%) | 4.8 | 10.1 |
| Obese** (%) | 38.0 | 44.7 |
| Febrile morbidity (%) | 15.3 | 32.3 |
| Required transfusion (%) | 8.3 | 15.4 |
| Death (%) | 0.2 | 0.1 |
| *The Collaborative Review of Sterilization | ||
| **Greater than 120% ideal body weight | ||
| VH=vaginal hysterectomy | ||
| AH=abdominal hysterectomy | ||
| Source: Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848. | ||
TABLE 2
Complications at hysterectomy
| AH | VH | LAVH | |
|---|---|---|---|
| Operating time (minutes) | 82 | 63 | 102 |
| Uterine weight (grams) | 216 | 113 | 129 |
| Febrile morbidity (%) | 9.1 | 3.2 | 2.0 |
| Required transfusion (%) | 2.5 | 1.0 | 0.06 |
| Bowel, bladder, or ureteral injury (%) | 1.0 | 0.9 | 1.1 |
| Death (%) | 0 | 0.2 | 0 |
| AH=abdominal hysterectomy | |||
| VH=vaginal hysterectomy | |||
| LAVH=laparoscopic-assisted vaginal hysterectomy | |||
| Source: Johns DA, et al. The medical and economic impact of laparoscopically assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-1719. | |||
Abdominal hysterectomy
Patient selection. Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma. Severe pelvic adhesive disease from documented severe endometriosis, salpingitis, or significant adnexal pathology and a considerably enlarged uterus also are best approached abdominally (Figure 1). In addition, use the abdominal route for obstetric emergencies such as postpartum hemorrhage.
Technique. First, determine the type of incision based on the following factors: Which one will allow completion of the procedure in a safe and efficient manner, minimize complications, expedite recovery, and offer a favorable cosmetic result? A vertical midline incision is the most adaptable to unsuspected pathology, especially if found in the upper abdomen. It is the quickest to perform and is associated with the least blood loss, an important advantage when operating emergently on an anemic or hemorrhaging patient. However, bear in mind that vertical incisions that have been extended into the upper abdomen are accompanied by increased postoperative pulmonary morbidity, pain, and risk of hernia formation.8,9
Transverse incisions such as the commonly used Pfannenstiel, Cherney, and Maylard compromise upper abdominal exposure but are more cosmetically appealing than vertical incisions. Each of these involves a lower abdominal transverse skin incision and a transverse fascial incision. With the Pfannenstiel technique, the rectus fascia is separated from the rectus muscles, and the muscles are split vertically in the midline, allowing access to the peritoneal cavity. This is the quickest transverse incision to make but provides the least exposure.
If a Pfannenstiel incision has been made and additional lateral pelvic exposure is needed, convert the incision to a Cherney. Both the Cherney and Maylard incisions provide additional exposure to the pelvis, especially laterally, which is advantageous when complex pathology is limited to the pelvis. The Cherney incision involves dividing the rectus muscles from the pubic symphysis, whereas the Maylard incision entails horizontal transection of the rectus muscles at the level of the fascial incision. With the Maylard technique, the rectus fascia is not separated from the underlying muscles as it is in the Cherney incision. If upper abdominal exposure becomes necessary, all of these incisions can be extended upward from their lateral margin, creating a “J.” Alternatively, a second incision can be made in the upper abdomen.
Another option is mini-laparotomy (an incision less than 6 cm). Hoffman and Lynch reported success with this technique for hysterectomy in select patients.10 Most of the procedures were completed with minimal use of retractors by exteriorizing the uterus via one of the aforementioned incisions. They found this approach to be safe and effective in nonobese women in whom a vaginal approach was precluded due to anatomy.
Use the mini-laparotomy approach when there is a suspicious adnexal mass that can be removed through a small abdominal incision.11 In addition, a large benign-appearing cystic adnexal mass can be drained, and the procedure then completed through this incision. Proceed cautiously, however, since an occasional unanticipated carcinoma will be encountered, and the act of drainage will result in the need for chemotherapy in a woman with otherwise early-stage disease. If cancer is suspected, consider a vertical minilaparotomy, which allows easy extension into the upper abdomen should surgical staging or debulking become necessary.
Advantages and disadvantages. The abdominal approach offers the best exposure of the pelvic and upper abdominal cavity but is associated with a high rate of complications, including fever and excessive blood loss. We surmise that postoperative recovery with a mini-laparotomy is likely to be improved compared with the traditional abdominal technique.
Figure 1
Opt for abdominal hysterectomy when a considerably enlarged uterus due to numerous fibroids is encountered.
Vaginal hysterectomy
Patient selection. Choose the vaginal route when pelvic support defects are present (Figure 2), but bear in mind that the course of the ureter changes with worsening degrees of uterine prolapse. We have found the ureter to be palpable in the bladder pillar in most women undergoing VH for prolapse, which helps avoid intraoperative ureteral injury.
The ovaries can be safely removed through the vagina in a large percentage of patients.
Obese, elderly, and otherwise medically debilitated patients also will benefit from the vaginal approach. Avoiding an abdominal wound in these women has obvious advantages. Also, vaginal surgery is associated with a reduction in postoperative pulmonary complications when compared with AH.6,12
Vaginal accessibility inevitably influences the decision to proceed vaginally with a hysterectomy. An inadequate bony pelvis is reason to forgo a VH. Orthopedic conditions and muscular contractures of the lower extremities, which prevent safe positioning, also inhibit this approach. Some surgeons also consider prior abdominal or pelvic surgery a reason to avoid vaginal hysterectomy, while others find this route a means of eluding potential adhesions.
We are strong advocates of vaginal surgery and do not consider nulliparity, lack of uterine descent, or prior abdominopelvic surgery to be strict contraindications for VH. A Schuchardt incision may overcome a small introitus, and a mobile uterus often will descend as the uterosacral and cardinal ligaments are divided.
In women with a prior cesarean delivery, it may be easier to enter the vesicouterine space by approaching it from the less-scarred vaginal side. In a study of more than 200 patients with a previous cesarean who underwent VH, Sheth and Malpani found no increase in complications and concluded that VH is the route of choice in this patient population.13 When unsuspected pelvic adhesions are encountered, carefully dissect them transvaginally and complete the procedure.
Besides accessibility, uterine size must be considered. However, size alone should present a dilemma in only about 15% of patients since most hysterectomy specimens are 12 weeks’ gestational size or smaller.14
Technique. Large uteri can be removed vaginally, depending on the surgeon’s technical ability and experience. A familiarity with the various methods of morcellation—hemisection, posterior fundal morcellation, intramyometrial coring, and myomectomy—is mandatory. It often is beneficial to use a combination of these techniques to accomplish the procedure vaginally, as opposed to a single means of morcellation. With any morcellation procedure, maintain a midline orientation to avoid dissection into the broad ligament. Continued caudal traction also is helpful in eliminating excessive blood loss during this prolonged procedure. Do not morcellate a uterus when endometrial carcinoma is suspected.
In a prospective observational study comparing vaginal morcellation to AH, Hoffman et al15 found the former to be safer. Other researchers also have demonstrated the procedure’s safety in retrospective reports16-18 and as subgroups in other series.14 Alternatively, preoperative treatment with a gonadotropin-releasing hor-Surgical Techniques mone (GnRH) agonist can reduce uterine size and result in a technically less challenging VH in some women.19,20
Also, whether or not the ovaries will be resected may influence the decision to perform a VH. While some surgeons believe that oophorectomy precludes the vaginal approach, the ovaries can be safely removed through the vagina in a large percentage of patients.21-23 In fact, Sheth24 and Kovac14 have published 95% and 97% success rates in 2 separate series in which vaginal oophorectomy was attempted in 740 and 142 women, respectively. However, do not attempt adnexal removal without adequate transvaginal access and visibility, as it increases the risk of hemorrhage and ureteral injury. In these cases, LAVH or mini-laparotomy AH may be preferable if oophorectomy is mandatory, i.e., in women who are predisposed to ovarian malignancies because of genetic mutations. However, when patients are appropriately selected, there are essentially no disadvantages to VH.
Advantages and disadvantages.VH has many advantages over AH. Minimal intraperitoneal manipulation and the avoidance of an abdominal wound lead to a shorter hospital stay and decreased recovery time, which is coupled with less cost. Patients also find the lack of an abdominal scar to be an attractive feature of VH. A disadvantage is reduced operative exposure, making it difficult to manipulate pelvic pathology and resect the adnexa.
Figure 2
Choose the vaginal route when pelvic support defects are present, but bear in mind that the course of the ureter changes due to prolapse.
Laparoscopic-assisted vaginal hysterectomy
Patient selection. When in doubt about which approach to use, turn to LAVH. The laparoscope allows the surgeon to thoroughly assess intra-abdominal pathology, which aids in the appropriate selection of a hysterectomy route. For example, if cancer is suspected based on the visual inspection, opt for an AH. On the other hand, if only extensive adhesions are noted, laparoscopic adhesiolysis can be performed to allow the hysterectomy to be completed vaginally. As previously noted, LAVH is appropriate in patients undergoing hysterectomy with prophylactic oophorectomy due to genetic or familial risk factors.
Technique. Bear in mind that the laparoscope does not permit all patients to undergo a VH. In fact, about 10% of attempted LAVHs will be unsuccessful.25,26 Some authors have reported failure during the laparoscopic portion,25,26 while others have attributed the lack of success to the vaginal portion of the procedure.27 Thus, it is important to be skilled at both laparoscopic and vaginal surgery in order to be successful with an LAVH.
LAVH requires three to five 5-to 10-mm abdominal incisions for port sites. An umbilical port is commonly used for the camera, and the remaining ports are used for operating. We typically use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles, which helps minimize costs.3 Other alternatives include the use of endoscopic staplers, the Harmonic Scalpel (Ethicon Endo-Surgery, a Johnson & Johnson company, Cincinnati, Ohio), and suture ligatures with extra-or intracorporeal knot tying. Whichever instrument is used, familiarize yourself with all available equipment given the occasional malfunction encountered during laparoscopy.
Advantages and disadvantages. LAVH requires special equipment and expertise beyond that needed for an abdominal or vaginal hysterectomy. Additionally, this procedure increases operative time, cost, and morbidity (depending on the surgeon’s experience),4,27,28 while the postoperative recovery is similar to that of VH.
Although we use the laparoscope infrequently and believe there is little need to perform excessive laparoscopy at the time of routine hysterectomy, we do not wish to understate the role of the laparoscope. Its use in specific instances can certainly avoid a laparotomy or help determine when an AH is more appropriate.
Conclusion
Emergent situations and patients with excessively enlarged uteri, significant pelvic pathology, or cancer are obvious candidates for AH. On the other hand, VH is frequently chosen for the small uterus in a multiparous woman with a large pelvis and no prior pelvic inflammatory disease or surgery. The dilemma arises when determining the approach for those patients with moderately enlarged uteri or presumptive risk factors for serious pelvic disease.
Kovac reported a standard protocol for selecting the route for hysterectomy. Uterine size, other pelvic pathology (endometriosis, adnexal disease, chronic pain, etc.), and uterine and adnexal accessibility (bony architecture, uterine support, and vaginal diameter) were each considered in the decision-making. A simplification of Kovac’s guidelines applicable to women undergoing hysterectomy for benign indications is summarized in Figure 3. Using these guidelines, Kovac reported a 99% (608/611) success rate for women assigned to VH or LAVH. The laparoscope was deemed necessary in only 19% of those assigned to the LAVH group, and ultimately only 9 patients required AH, yielding a 1:68 ratio for AH to VH.14
Gynecologists should seek alternatives to AH given its less favorable outcome in terms of morbidity and recovery. However, the surgeon who is competent with AH better serves the patient by performing the procedure via this route than by attempting an alternative procedure without the necessary proficiency. In other words, pelvic surgeons must be cognizant of their abilities and practice within that realm. Ultimately, the final selection of hysterectomy route should be based on the surgeon’s experience, the indication for surgery, and the patient’s anatomy.
FIGURE 3Guidelines to determine route of hysterectomy.
The authors report no financial relationship with any companies whose products are mentioned in this article.
- Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma.
- Use a combination of uterine morcellation techniques to accomplish a vaginal hysterectomy, as researchers have found morcellation of an enlarged uterus to be safer than removing it abdominally.
- For laparoscopic-assisted vaginal hysterectomy, use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles to help minimize costs.
- Seek alternatives to abdominal hysterectomy given its less favorable outcome in terms of morbidity and recovery.
While hysterectomy is one of the most frequently performed operations in gynecology, how to perform it—abdominally, vaginally, or laparoscopically—is less evident. Numerous studies have been published in an attempt to shed some light on this controversy.
Individualize the approach for each patient rather than rely on a dogmatic assignment of technique.
Prior to the introduction of the laparoscopic-assisted vaginal hysterectomy (LAVH) by Reich et al in 1989,1 several large studies were published that compared the abdominal and vaginal routes for hysterectomy. The largest was the Collaborative Review of Sterilization (CREST) study conducted by the Centers for Disease Control (CDC).2 This report included 1,856 women aged 15 to 44 who underwent non-emergency, non-radical hysterectomies at 9 institutions between 1978 and 1981. Fewer complications were associated with vaginal hysterectomy (VH) than abdominal hysterectomy (AH) (Table 1).
Now, several trials have included LAVH in the comparison of hysterectomy routes. In the most comprehensive study to date, Johns et al reviewed 2,563 hysterectomies performed for nonmalignant indications by 37 private gynecologists from a single institution.3 The researchers found that bowel, bladder, and ureteral injuries were uncommon, and the rates of each were similar among LAVH, abdominal hysterectomy, and vaginal hysterectomy (Table 2). In addition, a review of the literature between 1989 and 1995 revealed that LAVH is associated with a shorter hospital stay, decreased recovery time, and less analgesia compared with AH.4
However, since most of the data on route for hysterectomy are from retrospective and uncontrolled trials, one must interpret the findings carefully. For example, many studies do not control for additional procedures performed at the time of hysterectomy (e.g., enterocele, rectocele, and cystocele repairs). In addition, information on how researchers categorized unsuccessful attempts at VH or LAVH—which then had to be converted to AH—often is excluded. Also, physicians usually select the technique based on personal preference, practice style, and traditional dogma such as uterine size rather than a standard protocol.5,6 Therefore, the increased incidence of postoperative morbidity associated with AH is difficult to decipher. Is it due to the increased number of obese and nulliparous women undergoing AH, the surgeon’s experience, pelvic pathology or operative indication, or is it related to the actual opening of the abdomen and intraperitoneal manipulation? Most likely, it is a combination of these factors.
Overall, hysterectomy is a relatively safe procedure with a mortality rate of 1 to 2 per 1,000.7 Morbidity, however, remains high. Fortunately, most complications are minor and easily remedied with little clinical consequence. Since certain aspects of postoperative morbidity are related to the route for hysterectomy, the surgeon must individualize the approach for each patient and not rely on a dogmatic assignment of technique. Here, we will review the patient selection for and provide pearls on abdominal, vaginal, and laparoscopic-assisted vaginal hysterectomy, as well as look at the advantages and disadvantages of each method.
TABLE 1
CREST*study results
| VH | AH | |
|---|---|---|
| Mean age (yrs) | 34.4 | 35.8 |
| Nulliparous (%) | 1.4 | 13.3 |
| Prior cesarean section (%) | 4.8 | 10.1 |
| Obese** (%) | 38.0 | 44.7 |
| Febrile morbidity (%) | 15.3 | 32.3 |
| Required transfusion (%) | 8.3 | 15.4 |
| Death (%) | 0.2 | 0.1 |
| *The Collaborative Review of Sterilization | ||
| **Greater than 120% ideal body weight | ||
| VH=vaginal hysterectomy | ||
| AH=abdominal hysterectomy | ||
| Source: Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848. | ||
TABLE 2
Complications at hysterectomy
| AH | VH | LAVH | |
|---|---|---|---|
| Operating time (minutes) | 82 | 63 | 102 |
| Uterine weight (grams) | 216 | 113 | 129 |
| Febrile morbidity (%) | 9.1 | 3.2 | 2.0 |
| Required transfusion (%) | 2.5 | 1.0 | 0.06 |
| Bowel, bladder, or ureteral injury (%) | 1.0 | 0.9 | 1.1 |
| Death (%) | 0 | 0.2 | 0 |
| AH=abdominal hysterectomy | |||
| VH=vaginal hysterectomy | |||
| LAVH=laparoscopic-assisted vaginal hysterectomy | |||
| Source: Johns DA, et al. The medical and economic impact of laparoscopically assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-1719. | |||
Abdominal hysterectomy
Patient selection. Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma. Severe pelvic adhesive disease from documented severe endometriosis, salpingitis, or significant adnexal pathology and a considerably enlarged uterus also are best approached abdominally (Figure 1). In addition, use the abdominal route for obstetric emergencies such as postpartum hemorrhage.
Technique. First, determine the type of incision based on the following factors: Which one will allow completion of the procedure in a safe and efficient manner, minimize complications, expedite recovery, and offer a favorable cosmetic result? A vertical midline incision is the most adaptable to unsuspected pathology, especially if found in the upper abdomen. It is the quickest to perform and is associated with the least blood loss, an important advantage when operating emergently on an anemic or hemorrhaging patient. However, bear in mind that vertical incisions that have been extended into the upper abdomen are accompanied by increased postoperative pulmonary morbidity, pain, and risk of hernia formation.8,9
Transverse incisions such as the commonly used Pfannenstiel, Cherney, and Maylard compromise upper abdominal exposure but are more cosmetically appealing than vertical incisions. Each of these involves a lower abdominal transverse skin incision and a transverse fascial incision. With the Pfannenstiel technique, the rectus fascia is separated from the rectus muscles, and the muscles are split vertically in the midline, allowing access to the peritoneal cavity. This is the quickest transverse incision to make but provides the least exposure.
If a Pfannenstiel incision has been made and additional lateral pelvic exposure is needed, convert the incision to a Cherney. Both the Cherney and Maylard incisions provide additional exposure to the pelvis, especially laterally, which is advantageous when complex pathology is limited to the pelvis. The Cherney incision involves dividing the rectus muscles from the pubic symphysis, whereas the Maylard incision entails horizontal transection of the rectus muscles at the level of the fascial incision. With the Maylard technique, the rectus fascia is not separated from the underlying muscles as it is in the Cherney incision. If upper abdominal exposure becomes necessary, all of these incisions can be extended upward from their lateral margin, creating a “J.” Alternatively, a second incision can be made in the upper abdomen.
Another option is mini-laparotomy (an incision less than 6 cm). Hoffman and Lynch reported success with this technique for hysterectomy in select patients.10 Most of the procedures were completed with minimal use of retractors by exteriorizing the uterus via one of the aforementioned incisions. They found this approach to be safe and effective in nonobese women in whom a vaginal approach was precluded due to anatomy.
Use the mini-laparotomy approach when there is a suspicious adnexal mass that can be removed through a small abdominal incision.11 In addition, a large benign-appearing cystic adnexal mass can be drained, and the procedure then completed through this incision. Proceed cautiously, however, since an occasional unanticipated carcinoma will be encountered, and the act of drainage will result in the need for chemotherapy in a woman with otherwise early-stage disease. If cancer is suspected, consider a vertical minilaparotomy, which allows easy extension into the upper abdomen should surgical staging or debulking become necessary.
Advantages and disadvantages. The abdominal approach offers the best exposure of the pelvic and upper abdominal cavity but is associated with a high rate of complications, including fever and excessive blood loss. We surmise that postoperative recovery with a mini-laparotomy is likely to be improved compared with the traditional abdominal technique.
Figure 1
Opt for abdominal hysterectomy when a considerably enlarged uterus due to numerous fibroids is encountered.
Vaginal hysterectomy
Patient selection. Choose the vaginal route when pelvic support defects are present (Figure 2), but bear in mind that the course of the ureter changes with worsening degrees of uterine prolapse. We have found the ureter to be palpable in the bladder pillar in most women undergoing VH for prolapse, which helps avoid intraoperative ureteral injury.
The ovaries can be safely removed through the vagina in a large percentage of patients.
Obese, elderly, and otherwise medically debilitated patients also will benefit from the vaginal approach. Avoiding an abdominal wound in these women has obvious advantages. Also, vaginal surgery is associated with a reduction in postoperative pulmonary complications when compared with AH.6,12
Vaginal accessibility inevitably influences the decision to proceed vaginally with a hysterectomy. An inadequate bony pelvis is reason to forgo a VH. Orthopedic conditions and muscular contractures of the lower extremities, which prevent safe positioning, also inhibit this approach. Some surgeons also consider prior abdominal or pelvic surgery a reason to avoid vaginal hysterectomy, while others find this route a means of eluding potential adhesions.
We are strong advocates of vaginal surgery and do not consider nulliparity, lack of uterine descent, or prior abdominopelvic surgery to be strict contraindications for VH. A Schuchardt incision may overcome a small introitus, and a mobile uterus often will descend as the uterosacral and cardinal ligaments are divided.
In women with a prior cesarean delivery, it may be easier to enter the vesicouterine space by approaching it from the less-scarred vaginal side. In a study of more than 200 patients with a previous cesarean who underwent VH, Sheth and Malpani found no increase in complications and concluded that VH is the route of choice in this patient population.13 When unsuspected pelvic adhesions are encountered, carefully dissect them transvaginally and complete the procedure.
Besides accessibility, uterine size must be considered. However, size alone should present a dilemma in only about 15% of patients since most hysterectomy specimens are 12 weeks’ gestational size or smaller.14
Technique. Large uteri can be removed vaginally, depending on the surgeon’s technical ability and experience. A familiarity with the various methods of morcellation—hemisection, posterior fundal morcellation, intramyometrial coring, and myomectomy—is mandatory. It often is beneficial to use a combination of these techniques to accomplish the procedure vaginally, as opposed to a single means of morcellation. With any morcellation procedure, maintain a midline orientation to avoid dissection into the broad ligament. Continued caudal traction also is helpful in eliminating excessive blood loss during this prolonged procedure. Do not morcellate a uterus when endometrial carcinoma is suspected.
In a prospective observational study comparing vaginal morcellation to AH, Hoffman et al15 found the former to be safer. Other researchers also have demonstrated the procedure’s safety in retrospective reports16-18 and as subgroups in other series.14 Alternatively, preoperative treatment with a gonadotropin-releasing hor-Surgical Techniques mone (GnRH) agonist can reduce uterine size and result in a technically less challenging VH in some women.19,20
Also, whether or not the ovaries will be resected may influence the decision to perform a VH. While some surgeons believe that oophorectomy precludes the vaginal approach, the ovaries can be safely removed through the vagina in a large percentage of patients.21-23 In fact, Sheth24 and Kovac14 have published 95% and 97% success rates in 2 separate series in which vaginal oophorectomy was attempted in 740 and 142 women, respectively. However, do not attempt adnexal removal without adequate transvaginal access and visibility, as it increases the risk of hemorrhage and ureteral injury. In these cases, LAVH or mini-laparotomy AH may be preferable if oophorectomy is mandatory, i.e., in women who are predisposed to ovarian malignancies because of genetic mutations. However, when patients are appropriately selected, there are essentially no disadvantages to VH.
Advantages and disadvantages.VH has many advantages over AH. Minimal intraperitoneal manipulation and the avoidance of an abdominal wound lead to a shorter hospital stay and decreased recovery time, which is coupled with less cost. Patients also find the lack of an abdominal scar to be an attractive feature of VH. A disadvantage is reduced operative exposure, making it difficult to manipulate pelvic pathology and resect the adnexa.
Figure 2
Choose the vaginal route when pelvic support defects are present, but bear in mind that the course of the ureter changes due to prolapse.
Laparoscopic-assisted vaginal hysterectomy
Patient selection. When in doubt about which approach to use, turn to LAVH. The laparoscope allows the surgeon to thoroughly assess intra-abdominal pathology, which aids in the appropriate selection of a hysterectomy route. For example, if cancer is suspected based on the visual inspection, opt for an AH. On the other hand, if only extensive adhesions are noted, laparoscopic adhesiolysis can be performed to allow the hysterectomy to be completed vaginally. As previously noted, LAVH is appropriate in patients undergoing hysterectomy with prophylactic oophorectomy due to genetic or familial risk factors.
Technique. Bear in mind that the laparoscope does not permit all patients to undergo a VH. In fact, about 10% of attempted LAVHs will be unsuccessful.25,26 Some authors have reported failure during the laparoscopic portion,25,26 while others have attributed the lack of success to the vaginal portion of the procedure.27 Thus, it is important to be skilled at both laparoscopic and vaginal surgery in order to be successful with an LAVH.
LAVH requires three to five 5-to 10-mm abdominal incisions for port sites. An umbilical port is commonly used for the camera, and the remaining ports are used for operating. We typically use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles, which helps minimize costs.3 Other alternatives include the use of endoscopic staplers, the Harmonic Scalpel (Ethicon Endo-Surgery, a Johnson & Johnson company, Cincinnati, Ohio), and suture ligatures with extra-or intracorporeal knot tying. Whichever instrument is used, familiarize yourself with all available equipment given the occasional malfunction encountered during laparoscopy.
Advantages and disadvantages. LAVH requires special equipment and expertise beyond that needed for an abdominal or vaginal hysterectomy. Additionally, this procedure increases operative time, cost, and morbidity (depending on the surgeon’s experience),4,27,28 while the postoperative recovery is similar to that of VH.
Although we use the laparoscope infrequently and believe there is little need to perform excessive laparoscopy at the time of routine hysterectomy, we do not wish to understate the role of the laparoscope. Its use in specific instances can certainly avoid a laparotomy or help determine when an AH is more appropriate.
Conclusion
Emergent situations and patients with excessively enlarged uteri, significant pelvic pathology, or cancer are obvious candidates for AH. On the other hand, VH is frequently chosen for the small uterus in a multiparous woman with a large pelvis and no prior pelvic inflammatory disease or surgery. The dilemma arises when determining the approach for those patients with moderately enlarged uteri or presumptive risk factors for serious pelvic disease.
Kovac reported a standard protocol for selecting the route for hysterectomy. Uterine size, other pelvic pathology (endometriosis, adnexal disease, chronic pain, etc.), and uterine and adnexal accessibility (bony architecture, uterine support, and vaginal diameter) were each considered in the decision-making. A simplification of Kovac’s guidelines applicable to women undergoing hysterectomy for benign indications is summarized in Figure 3. Using these guidelines, Kovac reported a 99% (608/611) success rate for women assigned to VH or LAVH. The laparoscope was deemed necessary in only 19% of those assigned to the LAVH group, and ultimately only 9 patients required AH, yielding a 1:68 ratio for AH to VH.14
Gynecologists should seek alternatives to AH given its less favorable outcome in terms of morbidity and recovery. However, the surgeon who is competent with AH better serves the patient by performing the procedure via this route than by attempting an alternative procedure without the necessary proficiency. In other words, pelvic surgeons must be cognizant of their abilities and practice within that realm. Ultimately, the final selection of hysterectomy route should be based on the surgeon’s experience, the indication for surgery, and the patient’s anatomy.
FIGURE 3Guidelines to determine route of hysterectomy.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Reich H, DeCaprio J, McGlynn F. Laparoscopic hysterectomy. J Gynecol Surg. 1989;5:213-216.
2. Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848.
3. Johns DA, Carrera B, Jones J, DeLeon F, Vincent R, et al. The medical and economic impact of laparoscopic-assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-719.
4. Meikle SF, Nugent EW, Orleans M. Complication and recovery for laparoscopic-assisted vaginal hysterectomy compared with abdominal and vaginal hysterectomy. Obstet Gynecol. 1997;89:304-311.
5. Kovac SR, Christie SJ, Bindbeutel GA. Abdominal versus vaginal hysterectomy: a statistical model for determining physician decision making and patient outcomes. Med Decis Making. 1991;11:19-28.
6. Dorsey JH, Steinberg EP, Holtz PM. Clinical indications for hysterectomy route: patient characteristics or physician preference. Am J Obstet Gynecol. 1995;173:1452-1460.
7. Wingo PA, Huezo CM, Rubin GL, Ory HW, Peterson HB. The mortality risk associated with hysterectomy. Am J Obstet Gynecol. 1985;152:803-808.
8. Tollefson DG, Russell KP. The transverse incision in pelvic surgery. Am J Obstet Gynecol. 1954;68:410-422.
9. Helmkamp BF. Abdominal wound dehiscence. Am J Obstet Gynecol. 1977;128:803-807.
10. Hoffman MS, Lynch CM. Minilaparotomy hysterectomy. Am J Obstet Gynecol. 1998;179:316-320.
11. Flynn M, Niloff JM. Minilaparotomy for the ambulatory management of ovarian cysts. Am J Obstet Gynecol. 1995;173:1727-1730.
12. White SC, Wartel LJ, Wade ME. Comparison of abdominal and vaginal hysterectomies. Obstet Gynecol. 1971;37:530-537.
13. Sheth SS, Malpani AN. Vaginal hysterectomy following previous cesarean section. Int J Gynaecol Obstet. 1995;50:65-69.
14. Kovac SR. Guidelines to determine route of hysterectomy. Obstet Gynecol. 1995;85:18-23.
15. Hoffman MS, DeCesare S, Kalter C. Abdominal hysterectomy versus transvaginal morcellation for the removal of enlarged uteri. Am J Obstet Gynecol. 1994;171:309-315.
16. Mazdisnian F, Kurzel RB, Coe S, Bosuk M, Montz F. Vaginal hysterectomy by uterine morcellation: an efficient, non-morbid procedure. Obstet Gynecol. 1995;86:60-64.
17. Kudo R, Yamauchi O, Okazaki T, Sagac S, Ito E, Hashimoto M. Vaginal hysterectomy without ligation of the ligaments of the cervix uteri. Surg Gynecol Obstet. 1990;70:299-305.
18. Kovac SR. Intramyometrial coring as an adjunct to vaginal hysterectomy. Obstet Gynecol. 1986;67:131-136.
19. Stovall TG, Summit RL, Washburn SA, Ling FW. Gonadotropin-releasing hormone agonist use before hysterectomy. Am J Obstet Gynecol. 1994;170:1744-1748.
20. Crosignani PG, Vercellini P, Meschia M, Oldani S, Bramante T. GnRH agonists before surgery for uterine leiomyomas. J Reprod Med. 1996;41:415-421.
21. Hoffman MS. Transvaginal removal of ovaries with endoloop sutures at the time of transvaginal hysterectomy. Am J Obstet Gynecol. 1991;165:407-408.
22. Sheth SS. Ovarian clamp for oophorectomy during vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;2(4):S76-S77.
23. Hefni MA, Davies AE. Vaginal endoscopic oophorectomy with vaginal hysterectomy: a simple minimal access surgery technique. Br J Obstet Gynaecol. 1997;104:621-622.
24. Sheth SS. The place of oophorectomy at vaginal hysterectomy. Br J Obstet Gynaecol. 1991;98:662-666.
25. Cristoforoni PM, Palmieri A, Gerbaldo D, Montz FJ. Frequency and cause of aborted laparoscopic-assisted vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;3:33-37.
26. Summitt RL, Stovall TG, Steege JF, Lipscomb GH. A multicenter randomized comparison of laparoscopic-assisted vaginal hysterectomy and abdominal hysterectomy in abdominal hysterectomy candidates. Obstet Gynecol. 1998;92:321-326.
27. Dorsey JH, Holtz PM, Griffiths RI, McGrath MM, Steinberg EP. Costs and charges associated with three alternative techniques of hysterectomy. N Engl J Med. 1996;335:476-482.
28. Summitt RL, Stovall TG, Lipscomb GH, Ling FW. Randomized comparison of laparoscopic-assisted vaginal hysterectomy with standard vaginal hysterectomy in an outpatient setting. Obstet Gynecol. 1992;80:895-901.
1. Reich H, DeCaprio J, McGlynn F. Laparoscopic hysterectomy. J Gynecol Surg. 1989;5:213-216.
2. Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848.
3. Johns DA, Carrera B, Jones J, DeLeon F, Vincent R, et al. The medical and economic impact of laparoscopic-assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-719.
4. Meikle SF, Nugent EW, Orleans M. Complication and recovery for laparoscopic-assisted vaginal hysterectomy compared with abdominal and vaginal hysterectomy. Obstet Gynecol. 1997;89:304-311.
5. Kovac SR, Christie SJ, Bindbeutel GA. Abdominal versus vaginal hysterectomy: a statistical model for determining physician decision making and patient outcomes. Med Decis Making. 1991;11:19-28.
6. Dorsey JH, Steinberg EP, Holtz PM. Clinical indications for hysterectomy route: patient characteristics or physician preference. Am J Obstet Gynecol. 1995;173:1452-1460.
7. Wingo PA, Huezo CM, Rubin GL, Ory HW, Peterson HB. The mortality risk associated with hysterectomy. Am J Obstet Gynecol. 1985;152:803-808.
8. Tollefson DG, Russell KP. The transverse incision in pelvic surgery. Am J Obstet Gynecol. 1954;68:410-422.
9. Helmkamp BF. Abdominal wound dehiscence. Am J Obstet Gynecol. 1977;128:803-807.
10. Hoffman MS, Lynch CM. Minilaparotomy hysterectomy. Am J Obstet Gynecol. 1998;179:316-320.
11. Flynn M, Niloff JM. Minilaparotomy for the ambulatory management of ovarian cysts. Am J Obstet Gynecol. 1995;173:1727-1730.
12. White SC, Wartel LJ, Wade ME. Comparison of abdominal and vaginal hysterectomies. Obstet Gynecol. 1971;37:530-537.
13. Sheth SS, Malpani AN. Vaginal hysterectomy following previous cesarean section. Int J Gynaecol Obstet. 1995;50:65-69.
14. Kovac SR. Guidelines to determine route of hysterectomy. Obstet Gynecol. 1995;85:18-23.
15. Hoffman MS, DeCesare S, Kalter C. Abdominal hysterectomy versus transvaginal morcellation for the removal of enlarged uteri. Am J Obstet Gynecol. 1994;171:309-315.
16. Mazdisnian F, Kurzel RB, Coe S, Bosuk M, Montz F. Vaginal hysterectomy by uterine morcellation: an efficient, non-morbid procedure. Obstet Gynecol. 1995;86:60-64.
17. Kudo R, Yamauchi O, Okazaki T, Sagac S, Ito E, Hashimoto M. Vaginal hysterectomy without ligation of the ligaments of the cervix uteri. Surg Gynecol Obstet. 1990;70:299-305.
18. Kovac SR. Intramyometrial coring as an adjunct to vaginal hysterectomy. Obstet Gynecol. 1986;67:131-136.
19. Stovall TG, Summit RL, Washburn SA, Ling FW. Gonadotropin-releasing hormone agonist use before hysterectomy. Am J Obstet Gynecol. 1994;170:1744-1748.
20. Crosignani PG, Vercellini P, Meschia M, Oldani S, Bramante T. GnRH agonists before surgery for uterine leiomyomas. J Reprod Med. 1996;41:415-421.
21. Hoffman MS. Transvaginal removal of ovaries with endoloop sutures at the time of transvaginal hysterectomy. Am J Obstet Gynecol. 1991;165:407-408.
22. Sheth SS. Ovarian clamp for oophorectomy during vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;2(4):S76-S77.
23. Hefni MA, Davies AE. Vaginal endoscopic oophorectomy with vaginal hysterectomy: a simple minimal access surgery technique. Br J Obstet Gynaecol. 1997;104:621-622.
24. Sheth SS. The place of oophorectomy at vaginal hysterectomy. Br J Obstet Gynaecol. 1991;98:662-666.
25. Cristoforoni PM, Palmieri A, Gerbaldo D, Montz FJ. Frequency and cause of aborted laparoscopic-assisted vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;3:33-37.
26. Summitt RL, Stovall TG, Steege JF, Lipscomb GH. A multicenter randomized comparison of laparoscopic-assisted vaginal hysterectomy and abdominal hysterectomy in abdominal hysterectomy candidates. Obstet Gynecol. 1998;92:321-326.
27. Dorsey JH, Holtz PM, Griffiths RI, McGrath MM, Steinberg EP. Costs and charges associated with three alternative techniques of hysterectomy. N Engl J Med. 1996;335:476-482.
28. Summitt RL, Stovall TG, Lipscomb GH, Ling FW. Randomized comparison of laparoscopic-assisted vaginal hysterectomy with standard vaginal hysterectomy in an outpatient setting. Obstet Gynecol. 1992;80:895-901.
Endometriosis: does surgery make a difference?
- The relative value of surgery in the treatment of endometriosis—when compared with medical therapy for pain relief or assisted reproduction for fertility enhancement—has yet to be adequately evaluated.
- Although surgery appears to enhance fertility for all stages of the disease, the effect is marginal with early-stage disease.
- Laparoscopy produces excellent results and should be the method of choice for the surgical management of endometriosis.
- Endometriomas are best treated by removal rather than simple drainage and coagulation.
Surgery traditionally has been a mainstay in the treatment of endometriosis, one of the most common and debilitating diseases in benign gynecology. Surgeons were the first to attack the disease, and surgery remained the primary therapy through the 1970s. Only recently, with the development of drugs to combat endometriosis and techniques to circumvent the pelvic damage associated with the disease, has surgery begun to take a back seat to other therapeutic approaches.
The magnification afforded by laparoscopy frequently facilitates a more precise technique.
This review discusses the various surgical techniques available, including their advantages and disadvantages. An evidence-based review of the therapeutic value of surgery also is provided, along with recommendations for the application of surgery to different presentations of the disease.
One difficulty in reviewing the treatment of endometriosis lies in the heterogeneity of the disease itself. There are many physical manifestations of endometriosis, ranging from superficial peritoneal implants to deep, nodular lesions. Pelvic adhesions with fibrosis and distortion also are common, as are ovarian cysts (endometriomas) and involvement of organs such as the bladder and bowel. This wide range of presentations results in a variety of symptoms, with pelvic/abdominal pain and infertility being the most prominent. Although I will attempt to subdivide the data among these many “forms” of endometriosis, I would like to emphasize that, to some degree, the disease is unique in each woman. Of necessity, the recommendations here will be painted with broad strokes. Nevertheless, it is my belief that such generalizations retain their value in the quest to optimize therapy for individual patients.
Surgical methods
Conservative versus definitive surgery. When treating endometriosis, the surgeon is confronted with a number of technical considerations. The first is whether conservative or definitive surgery is advisable.
If a conservative approach is taken, the physician should assess the desired method of access, the method of treating implants, and the type of surgery done for endometriomas. The surgeon also should assess the need for ancillary procedures such as lysis of adhesions, appendectomy, and nerve interruption.
In conservative surgery, the patient’s fertility is preserved, while definitive surgery generally involves removal of the ovaries, a hysterectomy, or a combination of the 2 procedures. Definitive surgery is thought to be more effective over time, but must be reserved for patients whose fertility or continued endocrine function is deemed less important than the relief of pain.
Unfortunately, hysterectomy and ooph-orectomy do not guarantee the relief of pain. A study from Johns Hopkins suggests that the incidence of persistent or recurrent pain following hysterectomy and bilateral oophorectomy is 10%1. One explanation for persistence/recurrence may be the presence of ovarian remnants, a common finding among such patients. One method of checking for postoperative remnants is checking serum FSH levels.
Method of access. When conservative surgery is desired, the surgeon must select a method of access. Although laparotomy traditionally has been used, most surgeons performing extensive surgery for endometriosis now favor a laparoscopic approach. There are several reasons for this. First, laparoscopy is less invasive, with a much more rapid recovery time. In addition, a laparoscopic procedure costs less than major surgery. Finally, the magnification afforded by laparoscopy frequently facilitates a more precise technique.
There are limited data comparing laparoscopic surgery to laparotomy for the conservative treatment of endometriosis, and all derive from observational cohort studies.2-5 Despite the relatively poor quality of these studies, the evidence suggests little difference in pain relief via major or minor surgery. When the desired outcome is enhanced fertility, 3-year cumulative life-table pregnancy rates for the 2 techniques are equivalent.6
Destruction of implants. The surgical destruction of endometriotic lesions can be accomplished in a variety of ways: excision, vaporization, and fulguration/desiccation. Excision is generally thought to be the most complete of these techniques. It can be performed with a variety of instruments, ranging from the laser to monopolar needles to scissors. The technique is straightforward: The lesion margins are identified by close inspection of the peritoneum, and the cutting instrument is used to outline the area to be excised. The implant then is lifted with atraumatic forceps and separated from the underlying normal tissue by careful dissection. This procedure may be simple or extremely difficult, depending upon the location, thickness, and size of the implant. Many surgeons favor hydrodissection, a technique of irrigating under pressure the tissue near the lesion, in an attempt to separate normal tissue from abnormal. However, 2 dangers exist. First, fluid below the peritoneum frequently distorts anatomy, making a difficult dissection even more challenging. Second, structures fibrotically adherent to endometriosis will not separate and can be damaged if care is not taken to ensure their safety during excision of the lesion.
Implants may be vaporized using high-power-density energy over a short time. This induces a rapid increase in water temperature, resulting in vaporization and tissue destruction. If carbonization can be avoided, this method is very precise. It requires a focused, extremely high-power density, such as that achieved with the superpulse or ultra-pulse CO2laser.
Coagulation occurs with lower energies and results in lower temperatures at the tissue level. At 60° to 80°C, there is a loss of intracellular water and coagulation of protein, resulting in cell destruction. Because the depth of penetration is not always predictable, this technique is considerably less precise than vaporization. In addition, while lasers are a rapid means of performing this method, bipolar electrical current or even monopolar techniques can be used.
No randomized comparisons of these approaches have been conducted. Only 1 retrospective, comparative trial exists. Winkel and Bray reported the results of a 24-month follow-up of 240 women with endometriosis and pelvic pain who underwent surgical treatment in the form of excision alone, laser coagulation alone, or laser coagulation plus medical therapy.7 Twelve months after surgery, 96% of excision patients were pain-free compared with 69% of those undergoing coagulation. At 2 years, the corresponding figures were 69% and 23%, respectively. While this seems to indicate that excision is superior to coagulation, the retrospective study design makes such a conclusion suggestive at best. For example, excision may have been used in easier, less risky situations, whereas in difficult cases, only coagulation may have been performed. The resulting success rates would thus reflect the amount of disease discovered, not the type of surgery. To truly clarify this issue, a randomized trial is needed.
Only 40% of patients surgically treated for endometriosis experience pain relief as a direct result of surgery.
As mentioned earlier, numerous weapons have been employed to destroy endometriosis lesions: the CO2, KTP, Nd:YAG, and argon lasers; ultrasonic shears; monopolar electrical energy; and bipolar electrical energy. No comparisons of the efficacy of these instruments have been conducted.
Treating endometriomas. Ovarian cysts are common in the endometriosis patient, and the method by which they are surgically treated may be vital to the outcome. The goals of treating ovarian endometriomas are removing all ectopic endometrium in the ovary, reducing ovarian trauma, preserving follicles, and minimizing postoperative adhesion formation.8
Two types of endometriomas are recognized. The least common is contained entirely within the ovary. More widespread is an inverted anterior ovarian cortex with adhesions and implants on the surface, which is frequently adherent to the broad ligament. The latter type represents more than 90% of ovarian endometriomas.9
Before operating, the ovaries should be freed of all adhesions. The endometrioma may open spontaneously during this process; if not, incision and drainage are warranted. At this point, the cyst wall may be stripped, excised, or drained as indicated. Stripping involves separating the cyst wall from the ovary and slowly peeling them apart. In the Putman-Redwine technique, the opening to the endometrioma is circumscribed with a laser or electrosurgery, followed by dissection down to the cyst wall. The cyst wall and ovary then are separated sharply and bluntly until the cyst wall is removed, frequently intact. Excision also can be accomplished in a manner similar to a wedge resection. However, while removal of the endometrioma is invariably complete with this method, the potential for adhesion formation is higher.10
The vaporization or coagulation of cyst walls also has been described. A randomized trial comparing removal of the cyst wall versus fenestration and aspiration of cyst contents clearly demonstrates improved results with removal, whether the desired outcome is pain relief, fertility, or a lower rate of reoperation.11
The value of closing the ovary after endometrioma removal has been greatly debated, with no consensus among surgeons. Data suggest more adhesions form with suturing of the ovary than without closure, but it is unclear whether this applies to all sizes of defects.12
Ancillary procedures. Adhesiolysis is an important step in the restoration of normal pelvic and abdominal anatomy. While simple lysis of adhesions is adequate if they were formed following infection, most experts believe a more complete approach is required for the endometriosis-induced adhesion. This is because of the relatively high incidence of endometriosis present within the adhesion itself. Thus, removal of the adhesion by lysing both boundaries of the scar tissue and connecting structures is preferable. The instrumentation is of little consequence as long as precision and hemostasis are maintained.
To minimize adhesion reformation, adhesion-prevention adjuvants should be used. Four are presently available, including Interceed (Ethicon Inc, Somerville, NJ), a cellulose based barrier; Seprafilm (Genzyme Corp, Cambridge, Mass), a hyaluronidase-impregnated barrier; Preclude (W.L. Gore and Associates Inc, Newark, Del), a non-absorbable barrier composed of the proprietary Gore-Tex; and Intergel (Lifecore Biomedical Inc, Chaska, Minn), a thick solution placed within the peritoneal cavity that provides widespread adhesion prophylaxis. All adjuvants currently available have been associated with decreased adhesion reformation in randomized clinical trials.13 They can be applied either laparoscopically or abdominally.
Appendectomy. Endometriosis of the appendix has been described in 17% of patients with bowel involvement.14 In all endometriosis surgeries, the appendix should be carefully inspected and removed if it appears abnormal. In patients who experience endometriosis-associated pelvic pain, it may be desirable to electively remove the appendix at the time of laparoscopy. This will prevent future confusion. Otherwise, emergency room physicians would have to differentiate the symptoms of chronic pelvic/abdominal pain from those of appendicitis in the event the patient presents with a complaint of pelvic pain.
Nerve interruption procedures. Two surgical procedures were designed to help reduce pain transmission in the endometriosis patient: uterosacral nerve ablation/resection and presacral neurectomy. Both involve interruption of the major efferent nerve fibers from the uterus, thus diminishing uterine and central pelvic pain. These procedures can be performed via laparoscopy or laparotomy. Unfortunately, neither has been shown to provide pain relief beyond that achieved with surgery directed against the disease itself.15-18
Outcomes
Pain relief. Although there have been many investigations of the effect of endometriosis surgery on pelvic pain, the vast majority are of low quality. No randomized, controlled trials (RCTs) have compared surgery to medical therapy, and only 1 has investigated surgery versus sham surgery. Sutton and colleagues assessed the efficacy of laser laparoscopic surgery in the treatment of pain associated with minimal, mild, or moderate endometriosis.19 They found no difference in pain at 3 months follow-up. However, by 6 months, a clear-cut advantage was seen for surgery (Figure 1). Pain relief also was maintained for at least 1 year in patients undergoing laser surgery. These patients underwent ablation of endometriosis and of the uterosacral nerve (although, as noted earlier, the latter procedure provides no additional pain relief).
Most disconcerting is the finding that only 40% of patients surgically treated for endometriosis experience pain relief as a direct result of the surgery. (The number of patients who need to be treated to reduce pain in 1 patient is 2.5.)
It remains unclear whether excision of the disease will produce better results than coagulation with the CO2 laser. Nor have the duration of pain relief and rate of reoperation been properly examined. Thus, although many surgeons feel quite strongly about the value of surgery to treat endometriosis-associated pain, there are few data to support their clinical impression of efficacy.
Fertility enhancement. Conservative surgery has been used extensively in an attempt to enhance fertility. Unfortunately, most studies on the subject are uncontrolled and of poor quality. In women who have early-stage disease, surgical therapy has resulted in pregnancy rates of 40% to 75%; in severe disease, the rates are lower, ranging from 20% to 50%.20
A meta-analysis encompassing studies from 1982 through 1994 showed that either no treatment or surgery alone is superior to medical treatment for early-stage endometriosis-associated infertility.6
A separate meta-analysis of 25 RCTs and cohort studies examined the same issue.21 It found a possible treatment benefit from laparoscopic conservative surgery, but concluded that medical treatment of the disease was ineffective.
A multicenter randomized trial (ENDOCAN) assessed the value of surgery for early-stage endometriosis in the infertile patient. In this study, 341 infertile women with minimal or mild endometriosis were randomized to diagnostic laparoscopy or resection/ablation of disease.22 They then were followed for 36 weeks. The pregnancy rates were 31% for the treated group and 18% for the diagnostic group, demonstrating a clear advantage to surgery. The data also imply that to achieve 1 additional pregnancy in 9 months, 7.7 women must be surgically treated.
Adding confusion, a second randomized trial—this one from Italy—failed to demonstrate an improvement in fertility among those treated for early-stage endometriosis.23 Nevertheless, when the studies are combined into meta-analysis, surgical treatment of early-stage endometriosis appears to provide a significant improvement in pregnancy rates (odds ratio [OR], 1.66; 95% confidence limits [CL], 1.09-2.51) (Figure 2).
The surgical treatment of endometriosis probably enhances fertility when the disease is advanced, although no high-quality studies specifically address this issue. For early-stage disease, the data are conflicting but suggest that a small effect may exist. Further studies are needed to assess the value of surgery for this disorder.
FIGURE 1 Rate of pain relief with operative versus diagnostic laparoscopy
Adapted from: Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, or moderate endometriosis. Fertil Steril. 1994;62:696.
FIGURE 2 Pregnancy rate with surgical treatment versus diagnostic laparoscopy for early-stage endometriosis
Conclusion
The role of surgery in the treatment of endometriosis is controversial. The procedure can be highly demanding technically and may involve a number of ancillary procedures besides destruction of lesions. Many questions remain unanswered. For example, we do not know the best surgical technique or ancillary procedures, or the best instruments for these procedures. While there appears to be value in treating pain and infertility, that value may be far less than anticipated. Moreover, the relative value of surgery in the treatment of endometriosis—when compared with medical therapy for pain relief or assisted reproduction for fertility enhancement—has yet to be adequately evaluated. Given this level of uncertainty and confusion, surgery should be undertaken with caution and restraint.
Dr. Olive reports that he is a consultant to TAP Pharmaceuticals. He also serves on the company’s speakers’ bureau.
1. Hickman TN, Namnoum AB, Hinton EL, Zacur HA, Rock JA. Timing of estrogen replacement therapy following hysterectomy with oophorectomy for endometriosis. Obstet Gynecol. 1998;91:673-6772
2. Crosignani PG, Vercellini P, Biffignandi F, Constantini W, Cortesi I, Imparato E. Laparoscopy versus laparotomy in conservative surgical treatment for severe endometriosis. Fertil Steril. 1996;66:706-711.
3. Busacca M, Fedele L, Bianchi S, et al. Surgical treatment of recurrent endometriosis: laparotomy versus laparoscopy. Hum Reprod. 1998;13:2271-2274.
4. Catalano GF, Marana R, Caruana P, Muzii L, Mancuso S. Laparoscopy versus microsurgery by laparotomy for excision of ovarian cysts in patients with moderate or severe endometriosis. J Am Assoc Gynecol Laparosc. 1996;3:267-270.
5. Bateman BG, Kolp LA, Mills S. Endoscopic versus laparotomy management of endometriomas. Fertil Steril. 1994;62:690-695.
6. Adamson GD, Pasta DJ. Surgical treatment of endometriosis-associated infertility: meta-analysis compared with survival analysis. Am J Obstet Gynecol. 1994;171:1488.-
7. Winkel CA, Bray M. Treatment of women with endometriosis using excision alone, ablation alone, or ablation in combination with leuprolide acetate. Proceedings of the Fourth World CongresS on Endometriosis, Yokahama, Japan, 1996:55.
8. Guarnaccia MM, Silverberg K, Olive DL. Endometriosis and adenomyosis. In: Copeland LJ, ed. Textbook of Gynecology. Philadelphia: W.B. Saunders; 2000: 687.
9. Hughesdon PE. The structure of endometrial cysts of the ovary. J Obstet Gynaecol British Empire. 1957;44:481-487.
10. Fayes JA, Vogel MF. Comparison of different treatment methods of endometriomas by laparoscopy. Obstet Gynecol. 1991;78:660-665.
11. Beretta P, Franchi M, Ghezzi F. Randomized clinical trial of two laparoscopic treatments of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70:1176-1180.
12. Meyer WR, Grainger DA, DeCherney AH, Lachs MS, Diamond MP. Ovarian surgery on the rabbit. Effect of cortex closure on adhesion formation and ovarian function. J Reprod Med. 1991;36:639-643.
13. Farquhar C, Vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility (Cochrane Review). The Cochrane Library. Issue 1. Oxford: Update Software; 2000.
14. Franklin RR, Grunert GM. Extragenital endometriosis. In: Endometriosis: Advanced Management and Surgical Techniques. New York: Springer-Verlag; 1995;128.-
15. Dover RW, Pooley P, Haines P, Sutton CJG. Prospective, randomized, double-blind controlled trial of laparoscopic laser uterine nerve ablation in the treatment of pelvic pain associated with endometriosis [unpublished].
16. Vercellini P, Aimi G, Busacca M, Uglietti A, Viganali M, Crosignani PG. Laparoscopic uterosacral ligament resection for dysmenorrhea associated with endometriosis. Results of a randomized controlled trial. Fertil Steril. 1997;Oct(Suppl):S3 [abstract].
17. Candiani GB, Fedele L, Vercellini P, Bianchi S, DiNola G. Presacral neurectomy for the treatment of pelvic pain associated with endometriosis: a controlled study. Am J Obstet Gynecol. 1992;167:100-103.
18. Tjaden B, Schlaff WD, Kimball A, Rock JA. The efficacy of presacral neurectomy for the relief of midline dysmenorrhea. Obstet Gynecol. 1990;76:89.-
19. Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, or moderate endometriosis. Fertil Steril. 1994;62:696.-
20. Olive DL. Conservative surgery. In: Schenken RS, ed. Endometriosis: Contemporary Concepts in Clinical Management. Philadelphia: J.B. Lippincott; 1989;213.-
21. Hughes EG, Fedorkow DM, Collins JA. A quantitative overview of controlled trials in endometriosis associated infertility. Fertil Steril. 1993;59:963.-
22. Marcoux S, Maheux R, Berube S. Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group in Endometriosis. N Engl J Med. 1997;337:217-222.
23. Gruppo Italiano per lo Studio dell Endometriosis. Ablation of lesions or no treatment in minimal-mild endometriosis in infertile women: a randomized trial. Hum Reprod. 1999;14:1332-1334.
- The relative value of surgery in the treatment of endometriosis—when compared with medical therapy for pain relief or assisted reproduction for fertility enhancement—has yet to be adequately evaluated.
- Although surgery appears to enhance fertility for all stages of the disease, the effect is marginal with early-stage disease.
- Laparoscopy produces excellent results and should be the method of choice for the surgical management of endometriosis.
- Endometriomas are best treated by removal rather than simple drainage and coagulation.
Surgery traditionally has been a mainstay in the treatment of endometriosis, one of the most common and debilitating diseases in benign gynecology. Surgeons were the first to attack the disease, and surgery remained the primary therapy through the 1970s. Only recently, with the development of drugs to combat endometriosis and techniques to circumvent the pelvic damage associated with the disease, has surgery begun to take a back seat to other therapeutic approaches.
The magnification afforded by laparoscopy frequently facilitates a more precise technique.
This review discusses the various surgical techniques available, including their advantages and disadvantages. An evidence-based review of the therapeutic value of surgery also is provided, along with recommendations for the application of surgery to different presentations of the disease.
One difficulty in reviewing the treatment of endometriosis lies in the heterogeneity of the disease itself. There are many physical manifestations of endometriosis, ranging from superficial peritoneal implants to deep, nodular lesions. Pelvic adhesions with fibrosis and distortion also are common, as are ovarian cysts (endometriomas) and involvement of organs such as the bladder and bowel. This wide range of presentations results in a variety of symptoms, with pelvic/abdominal pain and infertility being the most prominent. Although I will attempt to subdivide the data among these many “forms” of endometriosis, I would like to emphasize that, to some degree, the disease is unique in each woman. Of necessity, the recommendations here will be painted with broad strokes. Nevertheless, it is my belief that such generalizations retain their value in the quest to optimize therapy for individual patients.
Surgical methods
Conservative versus definitive surgery. When treating endometriosis, the surgeon is confronted with a number of technical considerations. The first is whether conservative or definitive surgery is advisable.
If a conservative approach is taken, the physician should assess the desired method of access, the method of treating implants, and the type of surgery done for endometriomas. The surgeon also should assess the need for ancillary procedures such as lysis of adhesions, appendectomy, and nerve interruption.
In conservative surgery, the patient’s fertility is preserved, while definitive surgery generally involves removal of the ovaries, a hysterectomy, or a combination of the 2 procedures. Definitive surgery is thought to be more effective over time, but must be reserved for patients whose fertility or continued endocrine function is deemed less important than the relief of pain.
Unfortunately, hysterectomy and ooph-orectomy do not guarantee the relief of pain. A study from Johns Hopkins suggests that the incidence of persistent or recurrent pain following hysterectomy and bilateral oophorectomy is 10%1. One explanation for persistence/recurrence may be the presence of ovarian remnants, a common finding among such patients. One method of checking for postoperative remnants is checking serum FSH levels.
Method of access. When conservative surgery is desired, the surgeon must select a method of access. Although laparotomy traditionally has been used, most surgeons performing extensive surgery for endometriosis now favor a laparoscopic approach. There are several reasons for this. First, laparoscopy is less invasive, with a much more rapid recovery time. In addition, a laparoscopic procedure costs less than major surgery. Finally, the magnification afforded by laparoscopy frequently facilitates a more precise technique.
There are limited data comparing laparoscopic surgery to laparotomy for the conservative treatment of endometriosis, and all derive from observational cohort studies.2-5 Despite the relatively poor quality of these studies, the evidence suggests little difference in pain relief via major or minor surgery. When the desired outcome is enhanced fertility, 3-year cumulative life-table pregnancy rates for the 2 techniques are equivalent.6
Destruction of implants. The surgical destruction of endometriotic lesions can be accomplished in a variety of ways: excision, vaporization, and fulguration/desiccation. Excision is generally thought to be the most complete of these techniques. It can be performed with a variety of instruments, ranging from the laser to monopolar needles to scissors. The technique is straightforward: The lesion margins are identified by close inspection of the peritoneum, and the cutting instrument is used to outline the area to be excised. The implant then is lifted with atraumatic forceps and separated from the underlying normal tissue by careful dissection. This procedure may be simple or extremely difficult, depending upon the location, thickness, and size of the implant. Many surgeons favor hydrodissection, a technique of irrigating under pressure the tissue near the lesion, in an attempt to separate normal tissue from abnormal. However, 2 dangers exist. First, fluid below the peritoneum frequently distorts anatomy, making a difficult dissection even more challenging. Second, structures fibrotically adherent to endometriosis will not separate and can be damaged if care is not taken to ensure their safety during excision of the lesion.
Implants may be vaporized using high-power-density energy over a short time. This induces a rapid increase in water temperature, resulting in vaporization and tissue destruction. If carbonization can be avoided, this method is very precise. It requires a focused, extremely high-power density, such as that achieved with the superpulse or ultra-pulse CO2laser.
Coagulation occurs with lower energies and results in lower temperatures at the tissue level. At 60° to 80°C, there is a loss of intracellular water and coagulation of protein, resulting in cell destruction. Because the depth of penetration is not always predictable, this technique is considerably less precise than vaporization. In addition, while lasers are a rapid means of performing this method, bipolar electrical current or even monopolar techniques can be used.
No randomized comparisons of these approaches have been conducted. Only 1 retrospective, comparative trial exists. Winkel and Bray reported the results of a 24-month follow-up of 240 women with endometriosis and pelvic pain who underwent surgical treatment in the form of excision alone, laser coagulation alone, or laser coagulation plus medical therapy.7 Twelve months after surgery, 96% of excision patients were pain-free compared with 69% of those undergoing coagulation. At 2 years, the corresponding figures were 69% and 23%, respectively. While this seems to indicate that excision is superior to coagulation, the retrospective study design makes such a conclusion suggestive at best. For example, excision may have been used in easier, less risky situations, whereas in difficult cases, only coagulation may have been performed. The resulting success rates would thus reflect the amount of disease discovered, not the type of surgery. To truly clarify this issue, a randomized trial is needed.
Only 40% of patients surgically treated for endometriosis experience pain relief as a direct result of surgery.
As mentioned earlier, numerous weapons have been employed to destroy endometriosis lesions: the CO2, KTP, Nd:YAG, and argon lasers; ultrasonic shears; monopolar electrical energy; and bipolar electrical energy. No comparisons of the efficacy of these instruments have been conducted.
Treating endometriomas. Ovarian cysts are common in the endometriosis patient, and the method by which they are surgically treated may be vital to the outcome. The goals of treating ovarian endometriomas are removing all ectopic endometrium in the ovary, reducing ovarian trauma, preserving follicles, and minimizing postoperative adhesion formation.8
Two types of endometriomas are recognized. The least common is contained entirely within the ovary. More widespread is an inverted anterior ovarian cortex with adhesions and implants on the surface, which is frequently adherent to the broad ligament. The latter type represents more than 90% of ovarian endometriomas.9
Before operating, the ovaries should be freed of all adhesions. The endometrioma may open spontaneously during this process; if not, incision and drainage are warranted. At this point, the cyst wall may be stripped, excised, or drained as indicated. Stripping involves separating the cyst wall from the ovary and slowly peeling them apart. In the Putman-Redwine technique, the opening to the endometrioma is circumscribed with a laser or electrosurgery, followed by dissection down to the cyst wall. The cyst wall and ovary then are separated sharply and bluntly until the cyst wall is removed, frequently intact. Excision also can be accomplished in a manner similar to a wedge resection. However, while removal of the endometrioma is invariably complete with this method, the potential for adhesion formation is higher.10
The vaporization or coagulation of cyst walls also has been described. A randomized trial comparing removal of the cyst wall versus fenestration and aspiration of cyst contents clearly demonstrates improved results with removal, whether the desired outcome is pain relief, fertility, or a lower rate of reoperation.11
The value of closing the ovary after endometrioma removal has been greatly debated, with no consensus among surgeons. Data suggest more adhesions form with suturing of the ovary than without closure, but it is unclear whether this applies to all sizes of defects.12
Ancillary procedures. Adhesiolysis is an important step in the restoration of normal pelvic and abdominal anatomy. While simple lysis of adhesions is adequate if they were formed following infection, most experts believe a more complete approach is required for the endometriosis-induced adhesion. This is because of the relatively high incidence of endometriosis present within the adhesion itself. Thus, removal of the adhesion by lysing both boundaries of the scar tissue and connecting structures is preferable. The instrumentation is of little consequence as long as precision and hemostasis are maintained.
To minimize adhesion reformation, adhesion-prevention adjuvants should be used. Four are presently available, including Interceed (Ethicon Inc, Somerville, NJ), a cellulose based barrier; Seprafilm (Genzyme Corp, Cambridge, Mass), a hyaluronidase-impregnated barrier; Preclude (W.L. Gore and Associates Inc, Newark, Del), a non-absorbable barrier composed of the proprietary Gore-Tex; and Intergel (Lifecore Biomedical Inc, Chaska, Minn), a thick solution placed within the peritoneal cavity that provides widespread adhesion prophylaxis. All adjuvants currently available have been associated with decreased adhesion reformation in randomized clinical trials.13 They can be applied either laparoscopically or abdominally.
Appendectomy. Endometriosis of the appendix has been described in 17% of patients with bowel involvement.14 In all endometriosis surgeries, the appendix should be carefully inspected and removed if it appears abnormal. In patients who experience endometriosis-associated pelvic pain, it may be desirable to electively remove the appendix at the time of laparoscopy. This will prevent future confusion. Otherwise, emergency room physicians would have to differentiate the symptoms of chronic pelvic/abdominal pain from those of appendicitis in the event the patient presents with a complaint of pelvic pain.
Nerve interruption procedures. Two surgical procedures were designed to help reduce pain transmission in the endometriosis patient: uterosacral nerve ablation/resection and presacral neurectomy. Both involve interruption of the major efferent nerve fibers from the uterus, thus diminishing uterine and central pelvic pain. These procedures can be performed via laparoscopy or laparotomy. Unfortunately, neither has been shown to provide pain relief beyond that achieved with surgery directed against the disease itself.15-18
Outcomes
Pain relief. Although there have been many investigations of the effect of endometriosis surgery on pelvic pain, the vast majority are of low quality. No randomized, controlled trials (RCTs) have compared surgery to medical therapy, and only 1 has investigated surgery versus sham surgery. Sutton and colleagues assessed the efficacy of laser laparoscopic surgery in the treatment of pain associated with minimal, mild, or moderate endometriosis.19 They found no difference in pain at 3 months follow-up. However, by 6 months, a clear-cut advantage was seen for surgery (Figure 1). Pain relief also was maintained for at least 1 year in patients undergoing laser surgery. These patients underwent ablation of endometriosis and of the uterosacral nerve (although, as noted earlier, the latter procedure provides no additional pain relief).
Most disconcerting is the finding that only 40% of patients surgically treated for endometriosis experience pain relief as a direct result of the surgery. (The number of patients who need to be treated to reduce pain in 1 patient is 2.5.)
It remains unclear whether excision of the disease will produce better results than coagulation with the CO2 laser. Nor have the duration of pain relief and rate of reoperation been properly examined. Thus, although many surgeons feel quite strongly about the value of surgery to treat endometriosis-associated pain, there are few data to support their clinical impression of efficacy.
Fertility enhancement. Conservative surgery has been used extensively in an attempt to enhance fertility. Unfortunately, most studies on the subject are uncontrolled and of poor quality. In women who have early-stage disease, surgical therapy has resulted in pregnancy rates of 40% to 75%; in severe disease, the rates are lower, ranging from 20% to 50%.20
A meta-analysis encompassing studies from 1982 through 1994 showed that either no treatment or surgery alone is superior to medical treatment for early-stage endometriosis-associated infertility.6
A separate meta-analysis of 25 RCTs and cohort studies examined the same issue.21 It found a possible treatment benefit from laparoscopic conservative surgery, but concluded that medical treatment of the disease was ineffective.
A multicenter randomized trial (ENDOCAN) assessed the value of surgery for early-stage endometriosis in the infertile patient. In this study, 341 infertile women with minimal or mild endometriosis were randomized to diagnostic laparoscopy or resection/ablation of disease.22 They then were followed for 36 weeks. The pregnancy rates were 31% for the treated group and 18% for the diagnostic group, demonstrating a clear advantage to surgery. The data also imply that to achieve 1 additional pregnancy in 9 months, 7.7 women must be surgically treated.
Adding confusion, a second randomized trial—this one from Italy—failed to demonstrate an improvement in fertility among those treated for early-stage endometriosis.23 Nevertheless, when the studies are combined into meta-analysis, surgical treatment of early-stage endometriosis appears to provide a significant improvement in pregnancy rates (odds ratio [OR], 1.66; 95% confidence limits [CL], 1.09-2.51) (Figure 2).
The surgical treatment of endometriosis probably enhances fertility when the disease is advanced, although no high-quality studies specifically address this issue. For early-stage disease, the data are conflicting but suggest that a small effect may exist. Further studies are needed to assess the value of surgery for this disorder.
FIGURE 1 Rate of pain relief with operative versus diagnostic laparoscopy
Adapted from: Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, or moderate endometriosis. Fertil Steril. 1994;62:696.
FIGURE 2 Pregnancy rate with surgical treatment versus diagnostic laparoscopy for early-stage endometriosis
Conclusion
The role of surgery in the treatment of endometriosis is controversial. The procedure can be highly demanding technically and may involve a number of ancillary procedures besides destruction of lesions. Many questions remain unanswered. For example, we do not know the best surgical technique or ancillary procedures, or the best instruments for these procedures. While there appears to be value in treating pain and infertility, that value may be far less than anticipated. Moreover, the relative value of surgery in the treatment of endometriosis—when compared with medical therapy for pain relief or assisted reproduction for fertility enhancement—has yet to be adequately evaluated. Given this level of uncertainty and confusion, surgery should be undertaken with caution and restraint.
Dr. Olive reports that he is a consultant to TAP Pharmaceuticals. He also serves on the company’s speakers’ bureau.
- The relative value of surgery in the treatment of endometriosis—when compared with medical therapy for pain relief or assisted reproduction for fertility enhancement—has yet to be adequately evaluated.
- Although surgery appears to enhance fertility for all stages of the disease, the effect is marginal with early-stage disease.
- Laparoscopy produces excellent results and should be the method of choice for the surgical management of endometriosis.
- Endometriomas are best treated by removal rather than simple drainage and coagulation.
Surgery traditionally has been a mainstay in the treatment of endometriosis, one of the most common and debilitating diseases in benign gynecology. Surgeons were the first to attack the disease, and surgery remained the primary therapy through the 1970s. Only recently, with the development of drugs to combat endometriosis and techniques to circumvent the pelvic damage associated with the disease, has surgery begun to take a back seat to other therapeutic approaches.
The magnification afforded by laparoscopy frequently facilitates a more precise technique.
This review discusses the various surgical techniques available, including their advantages and disadvantages. An evidence-based review of the therapeutic value of surgery also is provided, along with recommendations for the application of surgery to different presentations of the disease.
One difficulty in reviewing the treatment of endometriosis lies in the heterogeneity of the disease itself. There are many physical manifestations of endometriosis, ranging from superficial peritoneal implants to deep, nodular lesions. Pelvic adhesions with fibrosis and distortion also are common, as are ovarian cysts (endometriomas) and involvement of organs such as the bladder and bowel. This wide range of presentations results in a variety of symptoms, with pelvic/abdominal pain and infertility being the most prominent. Although I will attempt to subdivide the data among these many “forms” of endometriosis, I would like to emphasize that, to some degree, the disease is unique in each woman. Of necessity, the recommendations here will be painted with broad strokes. Nevertheless, it is my belief that such generalizations retain their value in the quest to optimize therapy for individual patients.
Surgical methods
Conservative versus definitive surgery. When treating endometriosis, the surgeon is confronted with a number of technical considerations. The first is whether conservative or definitive surgery is advisable.
If a conservative approach is taken, the physician should assess the desired method of access, the method of treating implants, and the type of surgery done for endometriomas. The surgeon also should assess the need for ancillary procedures such as lysis of adhesions, appendectomy, and nerve interruption.
In conservative surgery, the patient’s fertility is preserved, while definitive surgery generally involves removal of the ovaries, a hysterectomy, or a combination of the 2 procedures. Definitive surgery is thought to be more effective over time, but must be reserved for patients whose fertility or continued endocrine function is deemed less important than the relief of pain.
Unfortunately, hysterectomy and ooph-orectomy do not guarantee the relief of pain. A study from Johns Hopkins suggests that the incidence of persistent or recurrent pain following hysterectomy and bilateral oophorectomy is 10%1. One explanation for persistence/recurrence may be the presence of ovarian remnants, a common finding among such patients. One method of checking for postoperative remnants is checking serum FSH levels.
Method of access. When conservative surgery is desired, the surgeon must select a method of access. Although laparotomy traditionally has been used, most surgeons performing extensive surgery for endometriosis now favor a laparoscopic approach. There are several reasons for this. First, laparoscopy is less invasive, with a much more rapid recovery time. In addition, a laparoscopic procedure costs less than major surgery. Finally, the magnification afforded by laparoscopy frequently facilitates a more precise technique.
There are limited data comparing laparoscopic surgery to laparotomy for the conservative treatment of endometriosis, and all derive from observational cohort studies.2-5 Despite the relatively poor quality of these studies, the evidence suggests little difference in pain relief via major or minor surgery. When the desired outcome is enhanced fertility, 3-year cumulative life-table pregnancy rates for the 2 techniques are equivalent.6
Destruction of implants. The surgical destruction of endometriotic lesions can be accomplished in a variety of ways: excision, vaporization, and fulguration/desiccation. Excision is generally thought to be the most complete of these techniques. It can be performed with a variety of instruments, ranging from the laser to monopolar needles to scissors. The technique is straightforward: The lesion margins are identified by close inspection of the peritoneum, and the cutting instrument is used to outline the area to be excised. The implant then is lifted with atraumatic forceps and separated from the underlying normal tissue by careful dissection. This procedure may be simple or extremely difficult, depending upon the location, thickness, and size of the implant. Many surgeons favor hydrodissection, a technique of irrigating under pressure the tissue near the lesion, in an attempt to separate normal tissue from abnormal. However, 2 dangers exist. First, fluid below the peritoneum frequently distorts anatomy, making a difficult dissection even more challenging. Second, structures fibrotically adherent to endometriosis will not separate and can be damaged if care is not taken to ensure their safety during excision of the lesion.
Implants may be vaporized using high-power-density energy over a short time. This induces a rapid increase in water temperature, resulting in vaporization and tissue destruction. If carbonization can be avoided, this method is very precise. It requires a focused, extremely high-power density, such as that achieved with the superpulse or ultra-pulse CO2laser.
Coagulation occurs with lower energies and results in lower temperatures at the tissue level. At 60° to 80°C, there is a loss of intracellular water and coagulation of protein, resulting in cell destruction. Because the depth of penetration is not always predictable, this technique is considerably less precise than vaporization. In addition, while lasers are a rapid means of performing this method, bipolar electrical current or even monopolar techniques can be used.
No randomized comparisons of these approaches have been conducted. Only 1 retrospective, comparative trial exists. Winkel and Bray reported the results of a 24-month follow-up of 240 women with endometriosis and pelvic pain who underwent surgical treatment in the form of excision alone, laser coagulation alone, or laser coagulation plus medical therapy.7 Twelve months after surgery, 96% of excision patients were pain-free compared with 69% of those undergoing coagulation. At 2 years, the corresponding figures were 69% and 23%, respectively. While this seems to indicate that excision is superior to coagulation, the retrospective study design makes such a conclusion suggestive at best. For example, excision may have been used in easier, less risky situations, whereas in difficult cases, only coagulation may have been performed. The resulting success rates would thus reflect the amount of disease discovered, not the type of surgery. To truly clarify this issue, a randomized trial is needed.
Only 40% of patients surgically treated for endometriosis experience pain relief as a direct result of surgery.
As mentioned earlier, numerous weapons have been employed to destroy endometriosis lesions: the CO2, KTP, Nd:YAG, and argon lasers; ultrasonic shears; monopolar electrical energy; and bipolar electrical energy. No comparisons of the efficacy of these instruments have been conducted.
Treating endometriomas. Ovarian cysts are common in the endometriosis patient, and the method by which they are surgically treated may be vital to the outcome. The goals of treating ovarian endometriomas are removing all ectopic endometrium in the ovary, reducing ovarian trauma, preserving follicles, and minimizing postoperative adhesion formation.8
Two types of endometriomas are recognized. The least common is contained entirely within the ovary. More widespread is an inverted anterior ovarian cortex with adhesions and implants on the surface, which is frequently adherent to the broad ligament. The latter type represents more than 90% of ovarian endometriomas.9
Before operating, the ovaries should be freed of all adhesions. The endometrioma may open spontaneously during this process; if not, incision and drainage are warranted. At this point, the cyst wall may be stripped, excised, or drained as indicated. Stripping involves separating the cyst wall from the ovary and slowly peeling them apart. In the Putman-Redwine technique, the opening to the endometrioma is circumscribed with a laser or electrosurgery, followed by dissection down to the cyst wall. The cyst wall and ovary then are separated sharply and bluntly until the cyst wall is removed, frequently intact. Excision also can be accomplished in a manner similar to a wedge resection. However, while removal of the endometrioma is invariably complete with this method, the potential for adhesion formation is higher.10
The vaporization or coagulation of cyst walls also has been described. A randomized trial comparing removal of the cyst wall versus fenestration and aspiration of cyst contents clearly demonstrates improved results with removal, whether the desired outcome is pain relief, fertility, or a lower rate of reoperation.11
The value of closing the ovary after endometrioma removal has been greatly debated, with no consensus among surgeons. Data suggest more adhesions form with suturing of the ovary than without closure, but it is unclear whether this applies to all sizes of defects.12
Ancillary procedures. Adhesiolysis is an important step in the restoration of normal pelvic and abdominal anatomy. While simple lysis of adhesions is adequate if they were formed following infection, most experts believe a more complete approach is required for the endometriosis-induced adhesion. This is because of the relatively high incidence of endometriosis present within the adhesion itself. Thus, removal of the adhesion by lysing both boundaries of the scar tissue and connecting structures is preferable. The instrumentation is of little consequence as long as precision and hemostasis are maintained.
To minimize adhesion reformation, adhesion-prevention adjuvants should be used. Four are presently available, including Interceed (Ethicon Inc, Somerville, NJ), a cellulose based barrier; Seprafilm (Genzyme Corp, Cambridge, Mass), a hyaluronidase-impregnated barrier; Preclude (W.L. Gore and Associates Inc, Newark, Del), a non-absorbable barrier composed of the proprietary Gore-Tex; and Intergel (Lifecore Biomedical Inc, Chaska, Minn), a thick solution placed within the peritoneal cavity that provides widespread adhesion prophylaxis. All adjuvants currently available have been associated with decreased adhesion reformation in randomized clinical trials.13 They can be applied either laparoscopically or abdominally.
Appendectomy. Endometriosis of the appendix has been described in 17% of patients with bowel involvement.14 In all endometriosis surgeries, the appendix should be carefully inspected and removed if it appears abnormal. In patients who experience endometriosis-associated pelvic pain, it may be desirable to electively remove the appendix at the time of laparoscopy. This will prevent future confusion. Otherwise, emergency room physicians would have to differentiate the symptoms of chronic pelvic/abdominal pain from those of appendicitis in the event the patient presents with a complaint of pelvic pain.
Nerve interruption procedures. Two surgical procedures were designed to help reduce pain transmission in the endometriosis patient: uterosacral nerve ablation/resection and presacral neurectomy. Both involve interruption of the major efferent nerve fibers from the uterus, thus diminishing uterine and central pelvic pain. These procedures can be performed via laparoscopy or laparotomy. Unfortunately, neither has been shown to provide pain relief beyond that achieved with surgery directed against the disease itself.15-18
Outcomes
Pain relief. Although there have been many investigations of the effect of endometriosis surgery on pelvic pain, the vast majority are of low quality. No randomized, controlled trials (RCTs) have compared surgery to medical therapy, and only 1 has investigated surgery versus sham surgery. Sutton and colleagues assessed the efficacy of laser laparoscopic surgery in the treatment of pain associated with minimal, mild, or moderate endometriosis.19 They found no difference in pain at 3 months follow-up. However, by 6 months, a clear-cut advantage was seen for surgery (Figure 1). Pain relief also was maintained for at least 1 year in patients undergoing laser surgery. These patients underwent ablation of endometriosis and of the uterosacral nerve (although, as noted earlier, the latter procedure provides no additional pain relief).
Most disconcerting is the finding that only 40% of patients surgically treated for endometriosis experience pain relief as a direct result of the surgery. (The number of patients who need to be treated to reduce pain in 1 patient is 2.5.)
It remains unclear whether excision of the disease will produce better results than coagulation with the CO2 laser. Nor have the duration of pain relief and rate of reoperation been properly examined. Thus, although many surgeons feel quite strongly about the value of surgery to treat endometriosis-associated pain, there are few data to support their clinical impression of efficacy.
Fertility enhancement. Conservative surgery has been used extensively in an attempt to enhance fertility. Unfortunately, most studies on the subject are uncontrolled and of poor quality. In women who have early-stage disease, surgical therapy has resulted in pregnancy rates of 40% to 75%; in severe disease, the rates are lower, ranging from 20% to 50%.20
A meta-analysis encompassing studies from 1982 through 1994 showed that either no treatment or surgery alone is superior to medical treatment for early-stage endometriosis-associated infertility.6
A separate meta-analysis of 25 RCTs and cohort studies examined the same issue.21 It found a possible treatment benefit from laparoscopic conservative surgery, but concluded that medical treatment of the disease was ineffective.
A multicenter randomized trial (ENDOCAN) assessed the value of surgery for early-stage endometriosis in the infertile patient. In this study, 341 infertile women with minimal or mild endometriosis were randomized to diagnostic laparoscopy or resection/ablation of disease.22 They then were followed for 36 weeks. The pregnancy rates were 31% for the treated group and 18% for the diagnostic group, demonstrating a clear advantage to surgery. The data also imply that to achieve 1 additional pregnancy in 9 months, 7.7 women must be surgically treated.
Adding confusion, a second randomized trial—this one from Italy—failed to demonstrate an improvement in fertility among those treated for early-stage endometriosis.23 Nevertheless, when the studies are combined into meta-analysis, surgical treatment of early-stage endometriosis appears to provide a significant improvement in pregnancy rates (odds ratio [OR], 1.66; 95% confidence limits [CL], 1.09-2.51) (Figure 2).
The surgical treatment of endometriosis probably enhances fertility when the disease is advanced, although no high-quality studies specifically address this issue. For early-stage disease, the data are conflicting but suggest that a small effect may exist. Further studies are needed to assess the value of surgery for this disorder.
FIGURE 1 Rate of pain relief with operative versus diagnostic laparoscopy
Adapted from: Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, or moderate endometriosis. Fertil Steril. 1994;62:696.
FIGURE 2 Pregnancy rate with surgical treatment versus diagnostic laparoscopy for early-stage endometriosis
Conclusion
The role of surgery in the treatment of endometriosis is controversial. The procedure can be highly demanding technically and may involve a number of ancillary procedures besides destruction of lesions. Many questions remain unanswered. For example, we do not know the best surgical technique or ancillary procedures, or the best instruments for these procedures. While there appears to be value in treating pain and infertility, that value may be far less than anticipated. Moreover, the relative value of surgery in the treatment of endometriosis—when compared with medical therapy for pain relief or assisted reproduction for fertility enhancement—has yet to be adequately evaluated. Given this level of uncertainty and confusion, surgery should be undertaken with caution and restraint.
Dr. Olive reports that he is a consultant to TAP Pharmaceuticals. He also serves on the company’s speakers’ bureau.
1. Hickman TN, Namnoum AB, Hinton EL, Zacur HA, Rock JA. Timing of estrogen replacement therapy following hysterectomy with oophorectomy for endometriosis. Obstet Gynecol. 1998;91:673-6772
2. Crosignani PG, Vercellini P, Biffignandi F, Constantini W, Cortesi I, Imparato E. Laparoscopy versus laparotomy in conservative surgical treatment for severe endometriosis. Fertil Steril. 1996;66:706-711.
3. Busacca M, Fedele L, Bianchi S, et al. Surgical treatment of recurrent endometriosis: laparotomy versus laparoscopy. Hum Reprod. 1998;13:2271-2274.
4. Catalano GF, Marana R, Caruana P, Muzii L, Mancuso S. Laparoscopy versus microsurgery by laparotomy for excision of ovarian cysts in patients with moderate or severe endometriosis. J Am Assoc Gynecol Laparosc. 1996;3:267-270.
5. Bateman BG, Kolp LA, Mills S. Endoscopic versus laparotomy management of endometriomas. Fertil Steril. 1994;62:690-695.
6. Adamson GD, Pasta DJ. Surgical treatment of endometriosis-associated infertility: meta-analysis compared with survival analysis. Am J Obstet Gynecol. 1994;171:1488.-
7. Winkel CA, Bray M. Treatment of women with endometriosis using excision alone, ablation alone, or ablation in combination with leuprolide acetate. Proceedings of the Fourth World CongresS on Endometriosis, Yokahama, Japan, 1996:55.
8. Guarnaccia MM, Silverberg K, Olive DL. Endometriosis and adenomyosis. In: Copeland LJ, ed. Textbook of Gynecology. Philadelphia: W.B. Saunders; 2000: 687.
9. Hughesdon PE. The structure of endometrial cysts of the ovary. J Obstet Gynaecol British Empire. 1957;44:481-487.
10. Fayes JA, Vogel MF. Comparison of different treatment methods of endometriomas by laparoscopy. Obstet Gynecol. 1991;78:660-665.
11. Beretta P, Franchi M, Ghezzi F. Randomized clinical trial of two laparoscopic treatments of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70:1176-1180.
12. Meyer WR, Grainger DA, DeCherney AH, Lachs MS, Diamond MP. Ovarian surgery on the rabbit. Effect of cortex closure on adhesion formation and ovarian function. J Reprod Med. 1991;36:639-643.
13. Farquhar C, Vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility (Cochrane Review). The Cochrane Library. Issue 1. Oxford: Update Software; 2000.
14. Franklin RR, Grunert GM. Extragenital endometriosis. In: Endometriosis: Advanced Management and Surgical Techniques. New York: Springer-Verlag; 1995;128.-
15. Dover RW, Pooley P, Haines P, Sutton CJG. Prospective, randomized, double-blind controlled trial of laparoscopic laser uterine nerve ablation in the treatment of pelvic pain associated with endometriosis [unpublished].
16. Vercellini P, Aimi G, Busacca M, Uglietti A, Viganali M, Crosignani PG. Laparoscopic uterosacral ligament resection for dysmenorrhea associated with endometriosis. Results of a randomized controlled trial. Fertil Steril. 1997;Oct(Suppl):S3 [abstract].
17. Candiani GB, Fedele L, Vercellini P, Bianchi S, DiNola G. Presacral neurectomy for the treatment of pelvic pain associated with endometriosis: a controlled study. Am J Obstet Gynecol. 1992;167:100-103.
18. Tjaden B, Schlaff WD, Kimball A, Rock JA. The efficacy of presacral neurectomy for the relief of midline dysmenorrhea. Obstet Gynecol. 1990;76:89.-
19. Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, or moderate endometriosis. Fertil Steril. 1994;62:696.-
20. Olive DL. Conservative surgery. In: Schenken RS, ed. Endometriosis: Contemporary Concepts in Clinical Management. Philadelphia: J.B. Lippincott; 1989;213.-
21. Hughes EG, Fedorkow DM, Collins JA. A quantitative overview of controlled trials in endometriosis associated infertility. Fertil Steril. 1993;59:963.-
22. Marcoux S, Maheux R, Berube S. Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group in Endometriosis. N Engl J Med. 1997;337:217-222.
23. Gruppo Italiano per lo Studio dell Endometriosis. Ablation of lesions or no treatment in minimal-mild endometriosis in infertile women: a randomized trial. Hum Reprod. 1999;14:1332-1334.
1. Hickman TN, Namnoum AB, Hinton EL, Zacur HA, Rock JA. Timing of estrogen replacement therapy following hysterectomy with oophorectomy for endometriosis. Obstet Gynecol. 1998;91:673-6772
2. Crosignani PG, Vercellini P, Biffignandi F, Constantini W, Cortesi I, Imparato E. Laparoscopy versus laparotomy in conservative surgical treatment for severe endometriosis. Fertil Steril. 1996;66:706-711.
3. Busacca M, Fedele L, Bianchi S, et al. Surgical treatment of recurrent endometriosis: laparotomy versus laparoscopy. Hum Reprod. 1998;13:2271-2274.
4. Catalano GF, Marana R, Caruana P, Muzii L, Mancuso S. Laparoscopy versus microsurgery by laparotomy for excision of ovarian cysts in patients with moderate or severe endometriosis. J Am Assoc Gynecol Laparosc. 1996;3:267-270.
5. Bateman BG, Kolp LA, Mills S. Endoscopic versus laparotomy management of endometriomas. Fertil Steril. 1994;62:690-695.
6. Adamson GD, Pasta DJ. Surgical treatment of endometriosis-associated infertility: meta-analysis compared with survival analysis. Am J Obstet Gynecol. 1994;171:1488.-
7. Winkel CA, Bray M. Treatment of women with endometriosis using excision alone, ablation alone, or ablation in combination with leuprolide acetate. Proceedings of the Fourth World CongresS on Endometriosis, Yokahama, Japan, 1996:55.
8. Guarnaccia MM, Silverberg K, Olive DL. Endometriosis and adenomyosis. In: Copeland LJ, ed. Textbook of Gynecology. Philadelphia: W.B. Saunders; 2000: 687.
9. Hughesdon PE. The structure of endometrial cysts of the ovary. J Obstet Gynaecol British Empire. 1957;44:481-487.
10. Fayes JA, Vogel MF. Comparison of different treatment methods of endometriomas by laparoscopy. Obstet Gynecol. 1991;78:660-665.
11. Beretta P, Franchi M, Ghezzi F. Randomized clinical trial of two laparoscopic treatments of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70:1176-1180.
12. Meyer WR, Grainger DA, DeCherney AH, Lachs MS, Diamond MP. Ovarian surgery on the rabbit. Effect of cortex closure on adhesion formation and ovarian function. J Reprod Med. 1991;36:639-643.
13. Farquhar C, Vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility (Cochrane Review). The Cochrane Library. Issue 1. Oxford: Update Software; 2000.
14. Franklin RR, Grunert GM. Extragenital endometriosis. In: Endometriosis: Advanced Management and Surgical Techniques. New York: Springer-Verlag; 1995;128.-
15. Dover RW, Pooley P, Haines P, Sutton CJG. Prospective, randomized, double-blind controlled trial of laparoscopic laser uterine nerve ablation in the treatment of pelvic pain associated with endometriosis [unpublished].
16. Vercellini P, Aimi G, Busacca M, Uglietti A, Viganali M, Crosignani PG. Laparoscopic uterosacral ligament resection for dysmenorrhea associated with endometriosis. Results of a randomized controlled trial. Fertil Steril. 1997;Oct(Suppl):S3 [abstract].
17. Candiani GB, Fedele L, Vercellini P, Bianchi S, DiNola G. Presacral neurectomy for the treatment of pelvic pain associated with endometriosis: a controlled study. Am J Obstet Gynecol. 1992;167:100-103.
18. Tjaden B, Schlaff WD, Kimball A, Rock JA. The efficacy of presacral neurectomy for the relief of midline dysmenorrhea. Obstet Gynecol. 1990;76:89.-
19. Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, or moderate endometriosis. Fertil Steril. 1994;62:696.-
20. Olive DL. Conservative surgery. In: Schenken RS, ed. Endometriosis: Contemporary Concepts in Clinical Management. Philadelphia: J.B. Lippincott; 1989;213.-
21. Hughes EG, Fedorkow DM, Collins JA. A quantitative overview of controlled trials in endometriosis associated infertility. Fertil Steril. 1993;59:963.-
22. Marcoux S, Maheux R, Berube S. Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group in Endometriosis. N Engl J Med. 1997;337:217-222.
23. Gruppo Italiano per lo Studio dell Endometriosis. Ablation of lesions or no treatment in minimal-mild endometriosis in infertile women: a randomized trial. Hum Reprod. 1999;14:1332-1334.
Cervical Cancer Patients: Need Not Forgo Fertility
- Lesions should be smaller than 2 cm to achieve the most favorable outcomes. Masses up to 3 cm can be resected, but the recurrence rate is higher, especially if the tumor has invaded the lymph-vascular space.
- Resect the cervix 0.5 to 1 cm distal to the isthmus. If the proximal incision margin has cleared the cancer, close the peritoneum and suture the vaginal mucosa to the cervical stump.
- The rate of second-trimester loss is elevated as a result of subclinical chorioamnionitis due to exposure of the membranes to vaginal flora. The Saling procedure has been shown to prevent ascending infections.
- The majority of women who have undergone radical vaginal trachelectomy successfully conceive, and most pregnancies result in a live birth.
While the mean age at which cervical cancer is diagnosed is 51, approximately 10% to 15% of women will develop cervical cancer in their reproductive years.1 Traditionally, early-stage cervical cancers in young women have been treated by radical hysterectomy, resulting in permanent sterility. Now, these patients have an alternative: radical vaginal trachelectomy. This entails the excision of the majority of the cervix and parametria, with preservation of the uppermost portion of the cervix and uterus, allowing the possibility of future childbearing (Figure 1).
The rate of second trimester loss is elevated in radical vaginal trachelectomy patients.
A conservative approach for the treatment of early-stage cervical cancer was first proposed approximately 20 years ago by Aburel, a Romanian gynecologist.2 He reported his experience with the abdominal “subfundic radical hysterectomy.” However, subsequent successful pregnancies did not occur, and the procedure was abandoned. In 1987, the French gynecologist Dargent began performing radical vaginal trachelectomy in concert with laparoscopic pelvic lymphadenectomy. He published his initial results in 1994.3 Thereafter, researchers in Quebec,4 Toronto,5 and London6 have reported their experiences with this procedure. Currently, almost 200 cases are reported in the literature. After describing patient selection and the technique, I will review the data regarding complications and oncologic and reproductive outcomes.
Figure 1
The shaded area indicates the portion of the cervix and upper vagina that will be resected during the procedure.
Patient selection
Although the potential benefit—preservation of fertility—is profound, the percentage of patients with cervical cancer who are candidates for radical vaginal trachelectomy is relatively small. The indications are the presence of an invasive cervical cancer (stage Ia2, Ib1, or IIa), the desire for future childbearing, no involvement of the upper endocervical canal, and no evidence of metastases on preoperative chest radiography and physical exam. Lesions 2 cm or smaller are ideal. While masses up to 3 cm can be resected, the recurrence rate is higher (similar to that after radical hysterectomy). Further, the absence of lymph-vascular space involvement (LVSI) is preferred, but its presence is not an absolute contraindication to the surgery.
Technique
Prior to the radical vaginal trachelectomy, perform a pelvic lymphadenectomy either transperitoneally or retroperitoneally, depending on the surgeon’s preference. If immediate histopathologic analysis of the lymph nodes is negative for metastases, proceed with the trachelectomy.
With the patient in the dorsal lithotomy position, grasp the vagina approximately 3 cm from the cervix and inject it with lidocaine. Then, using a scalpel, incise the vagina at the 12 o’clock position. After developing the vaginal cuff, suture it over the tumor using an absorbable suture in a figure-of-eight stitch, which provides traction to help identify proper surgical planes. Using the technique for a vaginal hysterectomy, create the vesicovaginal space. Then grasp the vagina at the 9 o’clock and 11 o’clock positions and sharply develop the paravesical space. Access to this space is located at approximately the 10 o’clock position. Repeat the same procedure on the contralateral side. (In this case, grasp the vagina at the 3 o’clock and 1 o’clock positions; access to this space is located at the 2 o’clock position.)
In the hands of an experienced surgeon, the complication rate is comparable to that of radical hysterectomy.
Place retractors in the paravesical spaces and retract the bladder anteriorly. The bladder pillars, which contain the ureters and are located in between the vesicovaginal and paravesical spaces, are now fully exposed (Figure 2). Palpate the ureter in each bladder pillar, then sharply divide each pillar, using cautery for hemostasis as needed. The ureters are now visible, as are the descending branches of the uterine artery.
At this point, the posterior cul-de-sac and the pararectal spaces can be accessed. Divide the uterosacral and cardinal ligaments 2 cm from the cervix (Figure 3). Retract the ureters and clamp the descending branch of the uterine artery. Finally, resect the cervix 0.5 to 1 cm distal to the isthmus and send the specimen to pathology (Figure 4). If the proximal (deep) incision margin has cleared the cancer, close the peritoneum and place a cerclage using a strong nonabsorbable suture (Figure 5). I prefer a double cerclage of No. 1 polyester or polypropylene. Then suture the vaginal mucosa to the cervical stump, not to the mucosa, to help prevent postoperative stenosis (Figure 6).
Prior to discharge, attempt a bladder challenge (usually postoperative day 2). In the rare instance the woman is unable to void, discharge the patient and repeat the bladder challenge in 1 week.
Figure 2
After dividing the bladder pillar, located between the vesicovaginal and paravesical spaces, identify the ureter (the tubular structure at 12 o’clock).
Figure 3
Access the posterior cul-de-sac and the pararectal spaces, and then divide the uterosacral and cardinal ligaments 2 cm from the cervix.
Figure 4
After retracting the ureters and clamping the descending branch of the uterine artery, resect the cervix 0.5 to 1 cm distal to the isthmus.
Figure 5
If the proximal incision margin has cleared the cancer, close the peritoneum and place a cerclage using a strong nonabsorbable suture.
Figure 6
Finally, suture the vaginal mucosa to the cervical stump, not to the mucosa, to help prevent postoperative stenosis.
Complications
The rate of morbidity associated with this procedure varies from 13% to 25%.3-5 (In the hands of an experienced surgeon, the complication rate is comparable to that of radical hysterectomy.) Dargent et al reported complications in 7 of 47 women (1 required reoperation for sidewall hemorrhage, 5 had postoperative hematomas, and 1 had prolonged urinary retention).3 Similarly, Roy and Plante reported 2 external iliac artery injuries, 1 conversion to laparotomy for hemostasis, and 1 cystotomy among 30 patients who underwent the procedure.4 Covens et al encountered complications in 8 of 32 women, including 6 cystotomies, 1 enterotomy, and 1 external iliac artery injury.5 It is important to note that the vascular injuries most commonly occurred during the lymphadenectomy. To date, no operative mortalities have been reported in association with radical trachelectomy.
Oncologic outcome
Roy recently summarized the oncologic outcome of women undergoing radical vaginal trachelectomy for early-stage cervical cancer at medical centers in Lyon, Quebec, and Toronto.7 This data, as well as that from London and the Women’s Hospital in Los Angeles, are shown in Table 1. (Women who underwent radical trachelectomy followed by hysterectomy due to lack of lesion clearance are excluded.)
Among the almost 200 cases, the cancer recurrence rate is low, and viable pregnancies are clearly possible.
Seven of 197 women (3.5%) had positive lymph nodes discovered postoperatively. However, all of them have remained disease-free. (Most of the women received pelvic radiotherapy postoperatively.) To date, there have been 6 recurrences (3%) reported, 2 of which have been in women with neuroendocrine tumors (a histology associated with an especially poor prognosis). Excluding the 2 neuroendocrine tumors, the recurrence rate drops to 2.1%. All of the 4 remaining women had negative lymph nodes and LVSI, and 2 had lesions larger than 2 cm (Table 2).
Of the 92 women whose cervical cancers did not invade the lymph-vascular space, none experienced a recurrence. Similarly, smaller tumors were associated with a low recurrence rate; in women with lesions 2 cm or smaller, only 1.6% had a recurrence. Women with lesions larger than 2 cm or with LVSI had a somewhat higher recurrence rate (10.5% and 11.1%, respectively), as would be expected based on our knowledge of recurrence risk after radical hysterectomy (Table 3).8,9
There are no prospective randomized trials that have analyzed oncologic outcome in women undergoing radical vaginal trachelectomy as compared to radical hysterectomy. Clearly, such a trial would not be feasible. However, the results of a 1999 retrospective case-control study are promising. In this trial, Covens compared the outcome of 32 women who underwent radical vaginal trachelectomy (all of whom had negative nodes and none of whom received radiation) to that of 30 women who underwent radical hystererctomy (matched for age, tumor size, histology, depth of invasion, and presence of LVSI).5 A second, unmatched, control group consisted of all women who had undergone radical hysterectomy for tumors 2 cm or smaller, had negative nodes, and had not received postoperative pelvic radiation. After a median follow-up of 23.4 months for the study patients, 46.5 months for the matched control patients, and 50.3 months for the unmatched control group, the 2-year actuarial recurrence-free survival was 95%, 97%, and 100%, respectively. These differences were not statistically significant.
TABLE 1
Oncologic outcome
| LYON N=71 | QUEBEC N=43 | TORONTO N=53 | LOSANGELES N=20 | LONDON N=9 | TOTAL N=196 | |
|---|---|---|---|---|---|---|
| Follow-up (mean) | 60 | 36 | 30 | 18.5 | 2 | - |
| Positive nodes | 2 | 1 | 1 | 0 | 3 | 7 (3.5%) |
| Recurrence | 3* | 2* | 1 | 0 | 0 | 6† (3%) |
| *One patient in each had a neuroendocrine tumor | ||||||
| †Recurrence rate is 2.1%, if women with neuroendocrine tumors are excluded. | ||||||
TABLE 2
Recurrences after radical vaginal trachelectomy*
| SIZE | HISTOLOGY | LN STATUS | LVSI | SITE | STATUS |
|---|---|---|---|---|---|
| 2 cm | Adenocarcinoma | Negative | Yes | Aortic LN | DOD |
| 2.5 cm | Squamous | Negative | Yes | Pelvic LN | NED |
| 3 cm | Squamous | Negative | Yes | Pelvis | DOD |
| <2 cm | Adenocarcinoma | Negative | Yes | Pelvis | DOD |
| LN=lymph node | |||||
| LVSI=lymph-vascular space involvement | |||||
| DOD=dead of disease | |||||
| NED=no evidence of disease | |||||
| *Neuroendocrine tumors excluded | |||||
TABLE 3
Site recurrence by size and LVSI*
| UTERUS | PELVIS | DISTANT | TOTAL | |
|---|---|---|---|---|
| Size | ||||
| ≤2 cm (n=125) | 0 | 1 (0.8%) | 1 (0.8%) | 2 (1.6%) |
| >2 cm (n=19) | 0 | 2 (10.5%) | 0 | 2 (10.5%) |
| LVSI | ||||
| No (n=92) | 0 | 0 | 0 | 0 |
| Yes (n=36) | 0 | 3 (8.3%) | 1 (2.8%) | 4 (11.1%) |
| LVSI=lymph-vascular space involvement | ||||
| *Neuroendocrine tumors excluded | ||||
Reproductive outcome
Most researchers have reported that the majority of women attempting to conceive have been successful, and that most pregnancies result in a live birth (Table 4). The rate of second-trimester loss is clearly elevated in women who have undergone radical vaginal trachelectomy. The likely reason: the development of subclinical chorioamnionitis due to an inadequate mucus plug and exposure of the membranes to vaginal flora. To combat this problem, Dargent began performing the Saling procedure at 14 weeks’ gestation.2 This entails approximating the vaginal mucosa over the cervix to provide a barrier to ascending infections. Since performing this technique, Dargent has not observed any cases of second-trimester loss. To date, this technique has not been adopted by other medical centers.
TABLE 4
Reproductive outcome
| LYON N=71 | QUEBEC N=43 | TORONTO N=53 | LOS ANGELES N=20 | LONDON N=9 | TOTAL N=196 | |
|---|---|---|---|---|---|---|
| No. pregnancies | 44 (27)† | 15 (11) | 9 (7)† | 3 (3)† | 7 (4) | 78 |
| Live births | 23 | 8 | 5 | 3‡ | 3 | 42 |
| Neonatal deaths | 0 | 1 | 0 | 0 | 0 | 1 |
| First-trimester loss | 6 | 3 | 2 | 0 | 3 | 14 |
| TAB | 3 | 0 | 0 | 0 | 0 | 3 |
| Ectopic | 1 | 0 | 0 | 0 | 0 | 1 |
| Second-trimester loss | 8 | 2 | 0 | 1 | 1 | 12 |
| Pregnancy ongoing | 3 | 1 | 2 | 0 | 0 | 6 |
| Numbers in parentheses represent number of women who were able to achieve a pregnancy | ||||||
| *5 pregnancies at the time of RVT | ||||||
| †1 pregnancy at the time of RVT | ||||||
| ‡1 set of twins | ||||||
| TAB=therapeutic abortion | ||||||
Conclusion
Based on the available data, the recurrence rate for women with squamous and adenocarcinomas appears to be low and comparable to that observed in women undergoing radical hysterectomy. Women with lesions larger than 2 cm or whose tumors have invaded the lymph-vascular space have a higher rate of recurrence, though not necessarily higher than if they had undergone radical hysterectomy.8,9
However, this data must be interpreted with caution. The majority of recurrences reported have been located in the pelvis, raising the concern that the parametrial resection at the time of radical trachelectomy may be inadequate. Yet, recurrences after radical hysterectomy also tend to be local rather than distant in non-irradiated patients.9,10 Also, the follow-up for women undergoing radical trachelectomy has been relatively short, and the number of women with lesions larger than 2 cm who have undergone this procedure is small. Thus, it is difficult to draw conclusions about the safety and efficacy of radical vaginal trachelectomy in this group.
Nonetheless, this technique appears to be a reasonable option for women with early-stage cervical cancer who desire future fertility. Among the almost 200 cases reported, the cancer recurrence rate is low, and viable pregnancies are clearly possible. However, longer follow-up is desirable. To meet this need, centers performing the procedure are forming a registry to better track both oncologic and reproductive outcomes. Further, more research is needed as to whether the Saling procedure will improve reproductive outcome and whether women with lesions larger than 2 cm and/or tumors invading the lymph-vascular space are at greater risk of recurrence with radical trachelectomy than if they had undergone radical hysterectomy.
At present, I offer radical vaginal trachelectomy to reproductive-age women with lesions 2 cm or smaller who are extremely desirous of future fertility. If the lesion is larger than 2 cm but is distal so that clearance appears feasible, I inform patients that there is limited data available regarding outcome, especially if LVSI is present.
The author reports no financial relationship with any companies whose products are mentioned in this article.
1. Van der Vange N, Woverling G, Ketting B, et al. The prognosis of cervical cancer associated with pregnancy: a matched cohort study. Obstet Gynecol. 1995;85:1022-1026.
2. Aburel E. Colpohistorectomia largita subfundica. In: Sirbu P, ed. Chirurgica Gynecologica. Bucharest, Romania: Editura Medicala Pub; 1981;714-721.
3. Dargent D, Martin X, Sacchetoni A, Mathevet P. Laparoscopic vaginal radical trachelectomy. Cancer. 2000;88:1877-1882.
4. Roy M, Plante M. Pregnancies after radical vaginal trachelectomy for early-stage cervical cancer. Am J Obstet Gynecol. 1998;179:1491-1496.
5. Covens A, Shaw P, Murphy J, DePetrillo D, Lickrish G, LaFramboise S, Rosen B. Is radical trachelectomy a safe alternative to radical hysterectomy for patients with stage IA-B carcinoma of the cervix? Cancer. 1999;86:2273-2279.
6. Shepherd JH, Crawford RAF, Oram DH. Radical trachelectomy: a way to preserve fertility in the treatment of early cervical cancer. Br J Obstet Gynaecol. 1998;105:912-916.
7. Roy M. Vaginal radical trachelectomy in early-stage cervical cancer. Presented at: Advanced Laparoscopic and Vaginal Surgery in Gynecologic Oncology; June 5-6, 2000; University of Southern California, Los Angeles, Calif,
8. Delgado G, Bundy B, Zaino R, Bernd-Uwe S, Creasman WT, Major F. Prospective surgical-pathological study of diseasefree interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1990;38:352-357.
9. Hopkins MP, Morley GW. Radical hysterectomy versus radiation therapy for stage IB squamous cell cancer of the cervix. Cancer. 1991;68:272-277.
10. Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group study. Gynecol Oncol. 1999;73:177-183.
- Lesions should be smaller than 2 cm to achieve the most favorable outcomes. Masses up to 3 cm can be resected, but the recurrence rate is higher, especially if the tumor has invaded the lymph-vascular space.
- Resect the cervix 0.5 to 1 cm distal to the isthmus. If the proximal incision margin has cleared the cancer, close the peritoneum and suture the vaginal mucosa to the cervical stump.
- The rate of second-trimester loss is elevated as a result of subclinical chorioamnionitis due to exposure of the membranes to vaginal flora. The Saling procedure has been shown to prevent ascending infections.
- The majority of women who have undergone radical vaginal trachelectomy successfully conceive, and most pregnancies result in a live birth.
While the mean age at which cervical cancer is diagnosed is 51, approximately 10% to 15% of women will develop cervical cancer in their reproductive years.1 Traditionally, early-stage cervical cancers in young women have been treated by radical hysterectomy, resulting in permanent sterility. Now, these patients have an alternative: radical vaginal trachelectomy. This entails the excision of the majority of the cervix and parametria, with preservation of the uppermost portion of the cervix and uterus, allowing the possibility of future childbearing (Figure 1).
The rate of second trimester loss is elevated in radical vaginal trachelectomy patients.
A conservative approach for the treatment of early-stage cervical cancer was first proposed approximately 20 years ago by Aburel, a Romanian gynecologist.2 He reported his experience with the abdominal “subfundic radical hysterectomy.” However, subsequent successful pregnancies did not occur, and the procedure was abandoned. In 1987, the French gynecologist Dargent began performing radical vaginal trachelectomy in concert with laparoscopic pelvic lymphadenectomy. He published his initial results in 1994.3 Thereafter, researchers in Quebec,4 Toronto,5 and London6 have reported their experiences with this procedure. Currently, almost 200 cases are reported in the literature. After describing patient selection and the technique, I will review the data regarding complications and oncologic and reproductive outcomes.
Figure 1
The shaded area indicates the portion of the cervix and upper vagina that will be resected during the procedure.
Patient selection
Although the potential benefit—preservation of fertility—is profound, the percentage of patients with cervical cancer who are candidates for radical vaginal trachelectomy is relatively small. The indications are the presence of an invasive cervical cancer (stage Ia2, Ib1, or IIa), the desire for future childbearing, no involvement of the upper endocervical canal, and no evidence of metastases on preoperative chest radiography and physical exam. Lesions 2 cm or smaller are ideal. While masses up to 3 cm can be resected, the recurrence rate is higher (similar to that after radical hysterectomy). Further, the absence of lymph-vascular space involvement (LVSI) is preferred, but its presence is not an absolute contraindication to the surgery.
Technique
Prior to the radical vaginal trachelectomy, perform a pelvic lymphadenectomy either transperitoneally or retroperitoneally, depending on the surgeon’s preference. If immediate histopathologic analysis of the lymph nodes is negative for metastases, proceed with the trachelectomy.
With the patient in the dorsal lithotomy position, grasp the vagina approximately 3 cm from the cervix and inject it with lidocaine. Then, using a scalpel, incise the vagina at the 12 o’clock position. After developing the vaginal cuff, suture it over the tumor using an absorbable suture in a figure-of-eight stitch, which provides traction to help identify proper surgical planes. Using the technique for a vaginal hysterectomy, create the vesicovaginal space. Then grasp the vagina at the 9 o’clock and 11 o’clock positions and sharply develop the paravesical space. Access to this space is located at approximately the 10 o’clock position. Repeat the same procedure on the contralateral side. (In this case, grasp the vagina at the 3 o’clock and 1 o’clock positions; access to this space is located at the 2 o’clock position.)
In the hands of an experienced surgeon, the complication rate is comparable to that of radical hysterectomy.
Place retractors in the paravesical spaces and retract the bladder anteriorly. The bladder pillars, which contain the ureters and are located in between the vesicovaginal and paravesical spaces, are now fully exposed (Figure 2). Palpate the ureter in each bladder pillar, then sharply divide each pillar, using cautery for hemostasis as needed. The ureters are now visible, as are the descending branches of the uterine artery.
At this point, the posterior cul-de-sac and the pararectal spaces can be accessed. Divide the uterosacral and cardinal ligaments 2 cm from the cervix (Figure 3). Retract the ureters and clamp the descending branch of the uterine artery. Finally, resect the cervix 0.5 to 1 cm distal to the isthmus and send the specimen to pathology (Figure 4). If the proximal (deep) incision margin has cleared the cancer, close the peritoneum and place a cerclage using a strong nonabsorbable suture (Figure 5). I prefer a double cerclage of No. 1 polyester or polypropylene. Then suture the vaginal mucosa to the cervical stump, not to the mucosa, to help prevent postoperative stenosis (Figure 6).
Prior to discharge, attempt a bladder challenge (usually postoperative day 2). In the rare instance the woman is unable to void, discharge the patient and repeat the bladder challenge in 1 week.
Figure 2
After dividing the bladder pillar, located between the vesicovaginal and paravesical spaces, identify the ureter (the tubular structure at 12 o’clock).
Figure 3
Access the posterior cul-de-sac and the pararectal spaces, and then divide the uterosacral and cardinal ligaments 2 cm from the cervix.
Figure 4
After retracting the ureters and clamping the descending branch of the uterine artery, resect the cervix 0.5 to 1 cm distal to the isthmus.
Figure 5
If the proximal incision margin has cleared the cancer, close the peritoneum and place a cerclage using a strong nonabsorbable suture.
Figure 6
Finally, suture the vaginal mucosa to the cervical stump, not to the mucosa, to help prevent postoperative stenosis.
Complications
The rate of morbidity associated with this procedure varies from 13% to 25%.3-5 (In the hands of an experienced surgeon, the complication rate is comparable to that of radical hysterectomy.) Dargent et al reported complications in 7 of 47 women (1 required reoperation for sidewall hemorrhage, 5 had postoperative hematomas, and 1 had prolonged urinary retention).3 Similarly, Roy and Plante reported 2 external iliac artery injuries, 1 conversion to laparotomy for hemostasis, and 1 cystotomy among 30 patients who underwent the procedure.4 Covens et al encountered complications in 8 of 32 women, including 6 cystotomies, 1 enterotomy, and 1 external iliac artery injury.5 It is important to note that the vascular injuries most commonly occurred during the lymphadenectomy. To date, no operative mortalities have been reported in association with radical trachelectomy.
Oncologic outcome
Roy recently summarized the oncologic outcome of women undergoing radical vaginal trachelectomy for early-stage cervical cancer at medical centers in Lyon, Quebec, and Toronto.7 This data, as well as that from London and the Women’s Hospital in Los Angeles, are shown in Table 1. (Women who underwent radical trachelectomy followed by hysterectomy due to lack of lesion clearance are excluded.)
Among the almost 200 cases, the cancer recurrence rate is low, and viable pregnancies are clearly possible.
Seven of 197 women (3.5%) had positive lymph nodes discovered postoperatively. However, all of them have remained disease-free. (Most of the women received pelvic radiotherapy postoperatively.) To date, there have been 6 recurrences (3%) reported, 2 of which have been in women with neuroendocrine tumors (a histology associated with an especially poor prognosis). Excluding the 2 neuroendocrine tumors, the recurrence rate drops to 2.1%. All of the 4 remaining women had negative lymph nodes and LVSI, and 2 had lesions larger than 2 cm (Table 2).
Of the 92 women whose cervical cancers did not invade the lymph-vascular space, none experienced a recurrence. Similarly, smaller tumors were associated with a low recurrence rate; in women with lesions 2 cm or smaller, only 1.6% had a recurrence. Women with lesions larger than 2 cm or with LVSI had a somewhat higher recurrence rate (10.5% and 11.1%, respectively), as would be expected based on our knowledge of recurrence risk after radical hysterectomy (Table 3).8,9
There are no prospective randomized trials that have analyzed oncologic outcome in women undergoing radical vaginal trachelectomy as compared to radical hysterectomy. Clearly, such a trial would not be feasible. However, the results of a 1999 retrospective case-control study are promising. In this trial, Covens compared the outcome of 32 women who underwent radical vaginal trachelectomy (all of whom had negative nodes and none of whom received radiation) to that of 30 women who underwent radical hystererctomy (matched for age, tumor size, histology, depth of invasion, and presence of LVSI).5 A second, unmatched, control group consisted of all women who had undergone radical hysterectomy for tumors 2 cm or smaller, had negative nodes, and had not received postoperative pelvic radiation. After a median follow-up of 23.4 months for the study patients, 46.5 months for the matched control patients, and 50.3 months for the unmatched control group, the 2-year actuarial recurrence-free survival was 95%, 97%, and 100%, respectively. These differences were not statistically significant.
TABLE 1
Oncologic outcome
| LYON N=71 | QUEBEC N=43 | TORONTO N=53 | LOSANGELES N=20 | LONDON N=9 | TOTAL N=196 | |
|---|---|---|---|---|---|---|
| Follow-up (mean) | 60 | 36 | 30 | 18.5 | 2 | - |
| Positive nodes | 2 | 1 | 1 | 0 | 3 | 7 (3.5%) |
| Recurrence | 3* | 2* | 1 | 0 | 0 | 6† (3%) |
| *One patient in each had a neuroendocrine tumor | ||||||
| †Recurrence rate is 2.1%, if women with neuroendocrine tumors are excluded. | ||||||
TABLE 2
Recurrences after radical vaginal trachelectomy*
| SIZE | HISTOLOGY | LN STATUS | LVSI | SITE | STATUS |
|---|---|---|---|---|---|
| 2 cm | Adenocarcinoma | Negative | Yes | Aortic LN | DOD |
| 2.5 cm | Squamous | Negative | Yes | Pelvic LN | NED |
| 3 cm | Squamous | Negative | Yes | Pelvis | DOD |
| <2 cm | Adenocarcinoma | Negative | Yes | Pelvis | DOD |
| LN=lymph node | |||||
| LVSI=lymph-vascular space involvement | |||||
| DOD=dead of disease | |||||
| NED=no evidence of disease | |||||
| *Neuroendocrine tumors excluded | |||||
TABLE 3
Site recurrence by size and LVSI*
| UTERUS | PELVIS | DISTANT | TOTAL | |
|---|---|---|---|---|
| Size | ||||
| ≤2 cm (n=125) | 0 | 1 (0.8%) | 1 (0.8%) | 2 (1.6%) |
| >2 cm (n=19) | 0 | 2 (10.5%) | 0 | 2 (10.5%) |
| LVSI | ||||
| No (n=92) | 0 | 0 | 0 | 0 |
| Yes (n=36) | 0 | 3 (8.3%) | 1 (2.8%) | 4 (11.1%) |
| LVSI=lymph-vascular space involvement | ||||
| *Neuroendocrine tumors excluded | ||||
Reproductive outcome
Most researchers have reported that the majority of women attempting to conceive have been successful, and that most pregnancies result in a live birth (Table 4). The rate of second-trimester loss is clearly elevated in women who have undergone radical vaginal trachelectomy. The likely reason: the development of subclinical chorioamnionitis due to an inadequate mucus plug and exposure of the membranes to vaginal flora. To combat this problem, Dargent began performing the Saling procedure at 14 weeks’ gestation.2 This entails approximating the vaginal mucosa over the cervix to provide a barrier to ascending infections. Since performing this technique, Dargent has not observed any cases of second-trimester loss. To date, this technique has not been adopted by other medical centers.
TABLE 4
Reproductive outcome
| LYON N=71 | QUEBEC N=43 | TORONTO N=53 | LOS ANGELES N=20 | LONDON N=9 | TOTAL N=196 | |
|---|---|---|---|---|---|---|
| No. pregnancies | 44 (27)† | 15 (11) | 9 (7)† | 3 (3)† | 7 (4) | 78 |
| Live births | 23 | 8 | 5 | 3‡ | 3 | 42 |
| Neonatal deaths | 0 | 1 | 0 | 0 | 0 | 1 |
| First-trimester loss | 6 | 3 | 2 | 0 | 3 | 14 |
| TAB | 3 | 0 | 0 | 0 | 0 | 3 |
| Ectopic | 1 | 0 | 0 | 0 | 0 | 1 |
| Second-trimester loss | 8 | 2 | 0 | 1 | 1 | 12 |
| Pregnancy ongoing | 3 | 1 | 2 | 0 | 0 | 6 |
| Numbers in parentheses represent number of women who were able to achieve a pregnancy | ||||||
| *5 pregnancies at the time of RVT | ||||||
| †1 pregnancy at the time of RVT | ||||||
| ‡1 set of twins | ||||||
| TAB=therapeutic abortion | ||||||
Conclusion
Based on the available data, the recurrence rate for women with squamous and adenocarcinomas appears to be low and comparable to that observed in women undergoing radical hysterectomy. Women with lesions larger than 2 cm or whose tumors have invaded the lymph-vascular space have a higher rate of recurrence, though not necessarily higher than if they had undergone radical hysterectomy.8,9
However, this data must be interpreted with caution. The majority of recurrences reported have been located in the pelvis, raising the concern that the parametrial resection at the time of radical trachelectomy may be inadequate. Yet, recurrences after radical hysterectomy also tend to be local rather than distant in non-irradiated patients.9,10 Also, the follow-up for women undergoing radical trachelectomy has been relatively short, and the number of women with lesions larger than 2 cm who have undergone this procedure is small. Thus, it is difficult to draw conclusions about the safety and efficacy of radical vaginal trachelectomy in this group.
Nonetheless, this technique appears to be a reasonable option for women with early-stage cervical cancer who desire future fertility. Among the almost 200 cases reported, the cancer recurrence rate is low, and viable pregnancies are clearly possible. However, longer follow-up is desirable. To meet this need, centers performing the procedure are forming a registry to better track both oncologic and reproductive outcomes. Further, more research is needed as to whether the Saling procedure will improve reproductive outcome and whether women with lesions larger than 2 cm and/or tumors invading the lymph-vascular space are at greater risk of recurrence with radical trachelectomy than if they had undergone radical hysterectomy.
At present, I offer radical vaginal trachelectomy to reproductive-age women with lesions 2 cm or smaller who are extremely desirous of future fertility. If the lesion is larger than 2 cm but is distal so that clearance appears feasible, I inform patients that there is limited data available regarding outcome, especially if LVSI is present.
The author reports no financial relationship with any companies whose products are mentioned in this article.
- Lesions should be smaller than 2 cm to achieve the most favorable outcomes. Masses up to 3 cm can be resected, but the recurrence rate is higher, especially if the tumor has invaded the lymph-vascular space.
- Resect the cervix 0.5 to 1 cm distal to the isthmus. If the proximal incision margin has cleared the cancer, close the peritoneum and suture the vaginal mucosa to the cervical stump.
- The rate of second-trimester loss is elevated as a result of subclinical chorioamnionitis due to exposure of the membranes to vaginal flora. The Saling procedure has been shown to prevent ascending infections.
- The majority of women who have undergone radical vaginal trachelectomy successfully conceive, and most pregnancies result in a live birth.
While the mean age at which cervical cancer is diagnosed is 51, approximately 10% to 15% of women will develop cervical cancer in their reproductive years.1 Traditionally, early-stage cervical cancers in young women have been treated by radical hysterectomy, resulting in permanent sterility. Now, these patients have an alternative: radical vaginal trachelectomy. This entails the excision of the majority of the cervix and parametria, with preservation of the uppermost portion of the cervix and uterus, allowing the possibility of future childbearing (Figure 1).
The rate of second trimester loss is elevated in radical vaginal trachelectomy patients.
A conservative approach for the treatment of early-stage cervical cancer was first proposed approximately 20 years ago by Aburel, a Romanian gynecologist.2 He reported his experience with the abdominal “subfundic radical hysterectomy.” However, subsequent successful pregnancies did not occur, and the procedure was abandoned. In 1987, the French gynecologist Dargent began performing radical vaginal trachelectomy in concert with laparoscopic pelvic lymphadenectomy. He published his initial results in 1994.3 Thereafter, researchers in Quebec,4 Toronto,5 and London6 have reported their experiences with this procedure. Currently, almost 200 cases are reported in the literature. After describing patient selection and the technique, I will review the data regarding complications and oncologic and reproductive outcomes.
Figure 1
The shaded area indicates the portion of the cervix and upper vagina that will be resected during the procedure.
Patient selection
Although the potential benefit—preservation of fertility—is profound, the percentage of patients with cervical cancer who are candidates for radical vaginal trachelectomy is relatively small. The indications are the presence of an invasive cervical cancer (stage Ia2, Ib1, or IIa), the desire for future childbearing, no involvement of the upper endocervical canal, and no evidence of metastases on preoperative chest radiography and physical exam. Lesions 2 cm or smaller are ideal. While masses up to 3 cm can be resected, the recurrence rate is higher (similar to that after radical hysterectomy). Further, the absence of lymph-vascular space involvement (LVSI) is preferred, but its presence is not an absolute contraindication to the surgery.
Technique
Prior to the radical vaginal trachelectomy, perform a pelvic lymphadenectomy either transperitoneally or retroperitoneally, depending on the surgeon’s preference. If immediate histopathologic analysis of the lymph nodes is negative for metastases, proceed with the trachelectomy.
With the patient in the dorsal lithotomy position, grasp the vagina approximately 3 cm from the cervix and inject it with lidocaine. Then, using a scalpel, incise the vagina at the 12 o’clock position. After developing the vaginal cuff, suture it over the tumor using an absorbable suture in a figure-of-eight stitch, which provides traction to help identify proper surgical planes. Using the technique for a vaginal hysterectomy, create the vesicovaginal space. Then grasp the vagina at the 9 o’clock and 11 o’clock positions and sharply develop the paravesical space. Access to this space is located at approximately the 10 o’clock position. Repeat the same procedure on the contralateral side. (In this case, grasp the vagina at the 3 o’clock and 1 o’clock positions; access to this space is located at the 2 o’clock position.)
In the hands of an experienced surgeon, the complication rate is comparable to that of radical hysterectomy.
Place retractors in the paravesical spaces and retract the bladder anteriorly. The bladder pillars, which contain the ureters and are located in between the vesicovaginal and paravesical spaces, are now fully exposed (Figure 2). Palpate the ureter in each bladder pillar, then sharply divide each pillar, using cautery for hemostasis as needed. The ureters are now visible, as are the descending branches of the uterine artery.
At this point, the posterior cul-de-sac and the pararectal spaces can be accessed. Divide the uterosacral and cardinal ligaments 2 cm from the cervix (Figure 3). Retract the ureters and clamp the descending branch of the uterine artery. Finally, resect the cervix 0.5 to 1 cm distal to the isthmus and send the specimen to pathology (Figure 4). If the proximal (deep) incision margin has cleared the cancer, close the peritoneum and place a cerclage using a strong nonabsorbable suture (Figure 5). I prefer a double cerclage of No. 1 polyester or polypropylene. Then suture the vaginal mucosa to the cervical stump, not to the mucosa, to help prevent postoperative stenosis (Figure 6).
Prior to discharge, attempt a bladder challenge (usually postoperative day 2). In the rare instance the woman is unable to void, discharge the patient and repeat the bladder challenge in 1 week.
Figure 2
After dividing the bladder pillar, located between the vesicovaginal and paravesical spaces, identify the ureter (the tubular structure at 12 o’clock).
Figure 3
Access the posterior cul-de-sac and the pararectal spaces, and then divide the uterosacral and cardinal ligaments 2 cm from the cervix.
Figure 4
After retracting the ureters and clamping the descending branch of the uterine artery, resect the cervix 0.5 to 1 cm distal to the isthmus.
Figure 5
If the proximal incision margin has cleared the cancer, close the peritoneum and place a cerclage using a strong nonabsorbable suture.
Figure 6
Finally, suture the vaginal mucosa to the cervical stump, not to the mucosa, to help prevent postoperative stenosis.
Complications
The rate of morbidity associated with this procedure varies from 13% to 25%.3-5 (In the hands of an experienced surgeon, the complication rate is comparable to that of radical hysterectomy.) Dargent et al reported complications in 7 of 47 women (1 required reoperation for sidewall hemorrhage, 5 had postoperative hematomas, and 1 had prolonged urinary retention).3 Similarly, Roy and Plante reported 2 external iliac artery injuries, 1 conversion to laparotomy for hemostasis, and 1 cystotomy among 30 patients who underwent the procedure.4 Covens et al encountered complications in 8 of 32 women, including 6 cystotomies, 1 enterotomy, and 1 external iliac artery injury.5 It is important to note that the vascular injuries most commonly occurred during the lymphadenectomy. To date, no operative mortalities have been reported in association with radical trachelectomy.
Oncologic outcome
Roy recently summarized the oncologic outcome of women undergoing radical vaginal trachelectomy for early-stage cervical cancer at medical centers in Lyon, Quebec, and Toronto.7 This data, as well as that from London and the Women’s Hospital in Los Angeles, are shown in Table 1. (Women who underwent radical trachelectomy followed by hysterectomy due to lack of lesion clearance are excluded.)
Among the almost 200 cases, the cancer recurrence rate is low, and viable pregnancies are clearly possible.
Seven of 197 women (3.5%) had positive lymph nodes discovered postoperatively. However, all of them have remained disease-free. (Most of the women received pelvic radiotherapy postoperatively.) To date, there have been 6 recurrences (3%) reported, 2 of which have been in women with neuroendocrine tumors (a histology associated with an especially poor prognosis). Excluding the 2 neuroendocrine tumors, the recurrence rate drops to 2.1%. All of the 4 remaining women had negative lymph nodes and LVSI, and 2 had lesions larger than 2 cm (Table 2).
Of the 92 women whose cervical cancers did not invade the lymph-vascular space, none experienced a recurrence. Similarly, smaller tumors were associated with a low recurrence rate; in women with lesions 2 cm or smaller, only 1.6% had a recurrence. Women with lesions larger than 2 cm or with LVSI had a somewhat higher recurrence rate (10.5% and 11.1%, respectively), as would be expected based on our knowledge of recurrence risk after radical hysterectomy (Table 3).8,9
There are no prospective randomized trials that have analyzed oncologic outcome in women undergoing radical vaginal trachelectomy as compared to radical hysterectomy. Clearly, such a trial would not be feasible. However, the results of a 1999 retrospective case-control study are promising. In this trial, Covens compared the outcome of 32 women who underwent radical vaginal trachelectomy (all of whom had negative nodes and none of whom received radiation) to that of 30 women who underwent radical hystererctomy (matched for age, tumor size, histology, depth of invasion, and presence of LVSI).5 A second, unmatched, control group consisted of all women who had undergone radical hysterectomy for tumors 2 cm or smaller, had negative nodes, and had not received postoperative pelvic radiation. After a median follow-up of 23.4 months for the study patients, 46.5 months for the matched control patients, and 50.3 months for the unmatched control group, the 2-year actuarial recurrence-free survival was 95%, 97%, and 100%, respectively. These differences were not statistically significant.
TABLE 1
Oncologic outcome
| LYON N=71 | QUEBEC N=43 | TORONTO N=53 | LOSANGELES N=20 | LONDON N=9 | TOTAL N=196 | |
|---|---|---|---|---|---|---|
| Follow-up (mean) | 60 | 36 | 30 | 18.5 | 2 | - |
| Positive nodes | 2 | 1 | 1 | 0 | 3 | 7 (3.5%) |
| Recurrence | 3* | 2* | 1 | 0 | 0 | 6† (3%) |
| *One patient in each had a neuroendocrine tumor | ||||||
| †Recurrence rate is 2.1%, if women with neuroendocrine tumors are excluded. | ||||||
TABLE 2
Recurrences after radical vaginal trachelectomy*
| SIZE | HISTOLOGY | LN STATUS | LVSI | SITE | STATUS |
|---|---|---|---|---|---|
| 2 cm | Adenocarcinoma | Negative | Yes | Aortic LN | DOD |
| 2.5 cm | Squamous | Negative | Yes | Pelvic LN | NED |
| 3 cm | Squamous | Negative | Yes | Pelvis | DOD |
| <2 cm | Adenocarcinoma | Negative | Yes | Pelvis | DOD |
| LN=lymph node | |||||
| LVSI=lymph-vascular space involvement | |||||
| DOD=dead of disease | |||||
| NED=no evidence of disease | |||||
| *Neuroendocrine tumors excluded | |||||
TABLE 3
Site recurrence by size and LVSI*
| UTERUS | PELVIS | DISTANT | TOTAL | |
|---|---|---|---|---|
| Size | ||||
| ≤2 cm (n=125) | 0 | 1 (0.8%) | 1 (0.8%) | 2 (1.6%) |
| >2 cm (n=19) | 0 | 2 (10.5%) | 0 | 2 (10.5%) |
| LVSI | ||||
| No (n=92) | 0 | 0 | 0 | 0 |
| Yes (n=36) | 0 | 3 (8.3%) | 1 (2.8%) | 4 (11.1%) |
| LVSI=lymph-vascular space involvement | ||||
| *Neuroendocrine tumors excluded | ||||
Reproductive outcome
Most researchers have reported that the majority of women attempting to conceive have been successful, and that most pregnancies result in a live birth (Table 4). The rate of second-trimester loss is clearly elevated in women who have undergone radical vaginal trachelectomy. The likely reason: the development of subclinical chorioamnionitis due to an inadequate mucus plug and exposure of the membranes to vaginal flora. To combat this problem, Dargent began performing the Saling procedure at 14 weeks’ gestation.2 This entails approximating the vaginal mucosa over the cervix to provide a barrier to ascending infections. Since performing this technique, Dargent has not observed any cases of second-trimester loss. To date, this technique has not been adopted by other medical centers.
TABLE 4
Reproductive outcome
| LYON N=71 | QUEBEC N=43 | TORONTO N=53 | LOS ANGELES N=20 | LONDON N=9 | TOTAL N=196 | |
|---|---|---|---|---|---|---|
| No. pregnancies | 44 (27)† | 15 (11) | 9 (7)† | 3 (3)† | 7 (4) | 78 |
| Live births | 23 | 8 | 5 | 3‡ | 3 | 42 |
| Neonatal deaths | 0 | 1 | 0 | 0 | 0 | 1 |
| First-trimester loss | 6 | 3 | 2 | 0 | 3 | 14 |
| TAB | 3 | 0 | 0 | 0 | 0 | 3 |
| Ectopic | 1 | 0 | 0 | 0 | 0 | 1 |
| Second-trimester loss | 8 | 2 | 0 | 1 | 1 | 12 |
| Pregnancy ongoing | 3 | 1 | 2 | 0 | 0 | 6 |
| Numbers in parentheses represent number of women who were able to achieve a pregnancy | ||||||
| *5 pregnancies at the time of RVT | ||||||
| †1 pregnancy at the time of RVT | ||||||
| ‡1 set of twins | ||||||
| TAB=therapeutic abortion | ||||||
Conclusion
Based on the available data, the recurrence rate for women with squamous and adenocarcinomas appears to be low and comparable to that observed in women undergoing radical hysterectomy. Women with lesions larger than 2 cm or whose tumors have invaded the lymph-vascular space have a higher rate of recurrence, though not necessarily higher than if they had undergone radical hysterectomy.8,9
However, this data must be interpreted with caution. The majority of recurrences reported have been located in the pelvis, raising the concern that the parametrial resection at the time of radical trachelectomy may be inadequate. Yet, recurrences after radical hysterectomy also tend to be local rather than distant in non-irradiated patients.9,10 Also, the follow-up for women undergoing radical trachelectomy has been relatively short, and the number of women with lesions larger than 2 cm who have undergone this procedure is small. Thus, it is difficult to draw conclusions about the safety and efficacy of radical vaginal trachelectomy in this group.
Nonetheless, this technique appears to be a reasonable option for women with early-stage cervical cancer who desire future fertility. Among the almost 200 cases reported, the cancer recurrence rate is low, and viable pregnancies are clearly possible. However, longer follow-up is desirable. To meet this need, centers performing the procedure are forming a registry to better track both oncologic and reproductive outcomes. Further, more research is needed as to whether the Saling procedure will improve reproductive outcome and whether women with lesions larger than 2 cm and/or tumors invading the lymph-vascular space are at greater risk of recurrence with radical trachelectomy than if they had undergone radical hysterectomy.
At present, I offer radical vaginal trachelectomy to reproductive-age women with lesions 2 cm or smaller who are extremely desirous of future fertility. If the lesion is larger than 2 cm but is distal so that clearance appears feasible, I inform patients that there is limited data available regarding outcome, especially if LVSI is present.
The author reports no financial relationship with any companies whose products are mentioned in this article.
1. Van der Vange N, Woverling G, Ketting B, et al. The prognosis of cervical cancer associated with pregnancy: a matched cohort study. Obstet Gynecol. 1995;85:1022-1026.
2. Aburel E. Colpohistorectomia largita subfundica. In: Sirbu P, ed. Chirurgica Gynecologica. Bucharest, Romania: Editura Medicala Pub; 1981;714-721.
3. Dargent D, Martin X, Sacchetoni A, Mathevet P. Laparoscopic vaginal radical trachelectomy. Cancer. 2000;88:1877-1882.
4. Roy M, Plante M. Pregnancies after radical vaginal trachelectomy for early-stage cervical cancer. Am J Obstet Gynecol. 1998;179:1491-1496.
5. Covens A, Shaw P, Murphy J, DePetrillo D, Lickrish G, LaFramboise S, Rosen B. Is radical trachelectomy a safe alternative to radical hysterectomy for patients with stage IA-B carcinoma of the cervix? Cancer. 1999;86:2273-2279.
6. Shepherd JH, Crawford RAF, Oram DH. Radical trachelectomy: a way to preserve fertility in the treatment of early cervical cancer. Br J Obstet Gynaecol. 1998;105:912-916.
7. Roy M. Vaginal radical trachelectomy in early-stage cervical cancer. Presented at: Advanced Laparoscopic and Vaginal Surgery in Gynecologic Oncology; June 5-6, 2000; University of Southern California, Los Angeles, Calif,
8. Delgado G, Bundy B, Zaino R, Bernd-Uwe S, Creasman WT, Major F. Prospective surgical-pathological study of diseasefree interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1990;38:352-357.
9. Hopkins MP, Morley GW. Radical hysterectomy versus radiation therapy for stage IB squamous cell cancer of the cervix. Cancer. 1991;68:272-277.
10. Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group study. Gynecol Oncol. 1999;73:177-183.
1. Van der Vange N, Woverling G, Ketting B, et al. The prognosis of cervical cancer associated with pregnancy: a matched cohort study. Obstet Gynecol. 1995;85:1022-1026.
2. Aburel E. Colpohistorectomia largita subfundica. In: Sirbu P, ed. Chirurgica Gynecologica. Bucharest, Romania: Editura Medicala Pub; 1981;714-721.
3. Dargent D, Martin X, Sacchetoni A, Mathevet P. Laparoscopic vaginal radical trachelectomy. Cancer. 2000;88:1877-1882.
4. Roy M, Plante M. Pregnancies after radical vaginal trachelectomy for early-stage cervical cancer. Am J Obstet Gynecol. 1998;179:1491-1496.
5. Covens A, Shaw P, Murphy J, DePetrillo D, Lickrish G, LaFramboise S, Rosen B. Is radical trachelectomy a safe alternative to radical hysterectomy for patients with stage IA-B carcinoma of the cervix? Cancer. 1999;86:2273-2279.
6. Shepherd JH, Crawford RAF, Oram DH. Radical trachelectomy: a way to preserve fertility in the treatment of early cervical cancer. Br J Obstet Gynaecol. 1998;105:912-916.
7. Roy M. Vaginal radical trachelectomy in early-stage cervical cancer. Presented at: Advanced Laparoscopic and Vaginal Surgery in Gynecologic Oncology; June 5-6, 2000; University of Southern California, Los Angeles, Calif,
8. Delgado G, Bundy B, Zaino R, Bernd-Uwe S, Creasman WT, Major F. Prospective surgical-pathological study of diseasefree interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1990;38:352-357.
9. Hopkins MP, Morley GW. Radical hysterectomy versus radiation therapy for stage IB squamous cell cancer of the cervix. Cancer. 1991;68:272-277.
10. Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group study. Gynecol Oncol. 1999;73:177-183.
Laparoscopic adhesiolysis
- Inspect the pelvic cavity prior to beginning adhesiolysis because the peritoneum becomes more opaque as the surgery progresses, obscuring anatomical definition.
- Grasp adhesions from the side contralateral to where you plan to make the first cut.
- Divide superficial adhesions first to ensure unimpeded access to deeper ones in case bleeding occurs.
- Coagulate thick or vascular adhesions with bipolar coagulation prior to division.
- Cut adhesions at both ends and remove the tissue rather than just dividing it.
Although more than 50% of all women who undergo pelvic or abdominal surgery will develop adhesions, the condition garners limited attention in the literature and from physicians. And, yet, the problems—infertility, chronic pelvic pain, and bowel obstruction, to name a few—with which patients present to Ob/Gyns every day often can be attributed to pelvic adhesions. In short, the quality of life of a significant number of women is affected by this condition, warranting a detailed look at the laparoscopic technique of adhesiolysis.
Assessing etiology
Any injury to the peritoneum has the potential to cause a pelvic adhesion, including endometriosis, pelvic inflammatory disease (PID), infection with a sexually transmitted disease, bleeding, and prior surgery. When the peritoneum is injured, the superficial mesothelial cells are disrupted allowing leakage of fibrinogen and cells into the pelvic cavity. Fibrinogen is then converted to fibrin, which covers all injured surfaces. When excess fibrin is present, it binds one organ to another. During normal healing, plasmin (which is formed from plasminogen) breaks down these fibrin bridges. But when plasmin production is inhibited, the bridges between organs remain intact. Within 7 to 10 days after the initial injury, mesothelial cells infiltrate the fibrin bridges, causing adhesions to become more solid.
Consider the presence of adhesions in patients who present with pelvic pain or infertility, especially if they have had prior pelvic or abdominal surgery.
Salpingitis following chlamydia or gonorrhea is another common etiology of pelvic adhesions. The spread of the infection through the fallopian tubes and into the peritoneal cavity sets the stage for the condition, as do ulcerative colitis, appendicitis, and diverticulitis.
Endometriosis is one of the most common etiologies because peritoneal lesions activate a chronic extensive inflammatory process, leading to dense adhesions. In fact, endometriosis-related adhesions are so common that the American Society for Reproductive Medicine (ASRM) assigns more points to adhesions for the staging of endometriosis than it does to the disease itself.1-2
Making the diagnosis
After reviewing the patient’s medical history, perform a physical exam. In some patients, an area of tenderness may be encountered when one palpates a prior incision, indicating that a loop of bowel may be adhered to this area. In addition, in women with adhesions, the uterus often is fixed either to the posterior pelvis or to the anterior abdominal wall, and the adnexa may be thickened and fixed to the uterus or pelvic sidewall. On rectal exam, the anterior wall of the rectum may be adhered to the uterus, or the rectum may be pulled to one side of the pelvis. Radiologic studies, with the exception of the hysterosalpingogram, do not usually aid in the diagnosis of pelvic adhesions.
Figure 1
Carefully inspect the pelvic cavity after placing the trocars. In this case, the woman was found to have adhesions on the left ovary.
Figure 2
Grasp the adhesion from the side contralateral to where you plan to make the first cut. Use laparoscopic scissors to divide and remove the tissue.
Figure 3
After completing adhesiolysis on the left ovary, check the contralateral organ. In this case, adhesions were found on the distal right tube and ovary.
Figure 4
Begin by excising superficial adhesions. This ensures unimpeded access to deeper adhesions in case bleeding occurs.
Figure 5
After all adhesions have been resected and removed, stop all bleeding and lavishly irrigate the pelvis with saline.
Figure 6
Just prior to closure, place oxidized regenerated cellulose (ORC) around both ovaries to help prevent recurrent adhesions.
Preparing the patient
After making the diagnosis and determining that surgery is the optimal treatment (see “Patient selection”), consider the approach. With rare exception, the procedure of choice is laparoscopy and is the method I prefer for adhesiolysis. Regardless of the route, prep the bowel either via an enema or an oral solution, as it decreases distention, making the operation safer and easier. In addition, evacuate the stomach contents via an oral gastric tube.
Indications. Consider for adhesiolysis any patient with chronic pelvic pain for more than 6 months or infertility for more than 1 year, after ruling out other potential etiologies. Patients who complain of localized pelvic pain are more likely to have adhesions than those with diffuse symptoms. But adhesions may be found even in women with diffuse pain. Although this pathology cannot always be incriminated as the cause of the pain, adhesiolysis often leads to a reduction of symptoms.1-3
Contraindications. An abdominal mass large enough to preclude trocar insertion, bowel obstruction, or diaphragmatic hernia are absolute contraindications. One caveat: While previous bowel resection and adhesiolysis are not contraindications to laparoscopy, both increase the risk of bowel perforation on trocar insertion. Proceed with caution or consider laparotomy.
REFERENCES
1. Malik E, Berg C, Meyhofer-Malik A, et al. Subjective evaluation of the therapeutic value of laparoscopic adhesiolysis: a retrospective analysis. Surg Endosc. 2000;14(1):79-81.
2. Nezhat FR, Crystal RA, Nezhat CH, et al. Laparoscopic adhesiolysis and relief of chronic pelvic pain. JSLS. 2000;4(4):281-285.
3. Lavonius M, Gullichsen R, Laine S, et al. Laparoscopy for chronic pelvic pain. Surg Laparosc Endosc. 1999;9(10):42-44.
Placing the trocars
Correct trocar placement facilitates any laparoscopic procedure, especially when dense adhesions are present. First determine the insertion sites, then select the type of entry technique and the length of the trocars.
Insertion sites. In most cases, the primary trocar is placed at the umbilicus. However, in a laparoscopy being performed for adhesions or in patients who have had an umbilical hernia operation or a vertical abdominal incision, place the primary trocar in the left upper quadrant. The reason: This region usually is spared from the formation of dense adhesions; therefore, entry can be performed safely by placing the trocar in the midclavicular line just below the lowest rib.
From that position, examine the abdomen for bowel adhesions to the anterior abdominal wall. If present, excise these adhesions prior to placing a trocar at the umbilical site. (It often is unnecessary to move the camera to the umbilical port, as the view with modern optical equipment from the left upper quadrant is more than adequate.)
Further, surgery can be eased by spacing the trocars as widely apart as possible and placing them on the same side of the patient.
Entry techniques. An alternative to direct trocar placement in highrisk patients is to use the open trocar insertion technique. First, make an abdominal incision and then insert the trocar through it. While some believe that this method reduces the risk of bowel perforation, the survey data from the American Association of Gynecologic Laparoscopists (AAGL) have not supported this theory.3
Trocar length. To determine the proper length, consider the thickness of the patient’s abdominal wall and the distance from the trocar to the adhesion. Trocars that are too long prevent the grasper jaws and scissors from opening, while those that are too short keep slipping back into the retroperitoneal space.
After all the trocars have been placed, carefully inspect the pelvic cavity because the peritoneum becomes more opaque as surgery progresses, obscuring anatomical definition.
Divide and conquer
To excise the adhesion, grasp it from the side contralateral to where you plan to make the first cut. Traction on both the organ and the adhesion is essential to establish the correct tissue planes, which in turn ensures adequate and safe excision of the adhesion. Because the bowel is the organ commonly involved in the adhesive process, there are many graspers available for atraumatic manipulation, including the Duval and Pennington forceps. Avoiding injury to the bowel is paramount to the procedure, as trauma will cause more adhesions, worsening the patient’s condition.
After grasping the adhesion, select a method by which to divide and remove it. This selection is based on the surgeon’s experience, the location of the adhesions, and the organs involved. In most cases, laparoscopic scissors are the instrument of choice. However, opt for electrosurgery (unipolar and bipolar), carbon dioxide laser, or ultrasonic energy (see “Tools for excision”) when energy is needed as a secondary source of hemostasis.
Begin by excising superficial adhesions. This ensures unimpeded access to deeper adhesions in case bleeding occurs. In electrosurgery, slowly pass the probe near the adhesion, allowing the electrons to arc from the instrument tip to the tissue. Proceed with extreme care when using unipolar energy near vital structures because electrons travel the path of least resistance. For example, if there is an adhesion between a loop of bowel and the uterus, always resect the adhesion from the bowel first. The reason: If you remove the adhesion from the uterus first, electrons would flow toward the bowel while you excise the adhesion from this organ. This is because it is the only path remaining for the electrons to egress, as you already have severed the adhesion from the uterus. The end result: thermal damage to the bowel.
When extremely thick or vascular adhesions are encountered, coagulate them prior to division as follows: First, achieve hemostasis using bipolar forceps. This instrument allows the precise application of energy to the tissue because electrons flow from one side of the forceps to the other, rather than through the body as with unipolar electrosurgery. Apply the energy until the tissue is desiccated and the high resistance stops electron flow. Second, divide the tissue using laparoscopic scissors. Bipolar electrosurgery also should be used when coagulation is needed near vital structures such as great vessels, the bowel, bladder, and ureters.
A new type of electrosurgical generator seals vessel walls rather than coagulating them (LigaSure; Valleylab, Boulder, Colo). This results in a much higher vessel burst strength than either unipolar or bipolar electrosurgery, allowing the surgeon to close vessels up to 7 mm in diameter prior to division of the vascular adhesion.
After all adhesions have been resected and removed, stop all bleeding and lavishly irrigate the pelvis with saline prior to closure. If extensive adhesions have been resected from the rectal wall, assess this organ’s integrity by filling the pelvis with saline and placing air in the rectum with either a sigmoidoscope or 30-cc Foley catheter.
Laparoscopic scissors. These are inexpensive, create no peripheral damage, give direct mechanical feedback, and are familiar to all physicians. No smoke or plume is created to obstruct vision. However, because hemostasis cannot be achieved with scissors, they are inappropriate for vascular adhesions. In addition, they cannot be used in areas of the pelvis that can be seen but not reached. Instead, opt for one of the cutting methods described here.
Electrosurgery. The efficiency of electrosurgery depends on the power, shape of the probe tip, and waveform. The higher the power and the finer the tip, the faster the probe will resect the adhesion, thereby minimizing thermal damage. Pure cutting currents also reduce thermal damage but provide less hemostasis than blended or pure coagulation currents.
In general, the thickness, vascularity, and water content of the adhesions will dictate the equipment and settings used. (There are no standard wattage settings, but surgeons typically use 25 to 125 watts.)
Carbon dioxide laser. This instrument allows surgeons to cut with hemostasis without coming into direct contact with the tissue. There are an infinite number of settings, wavelengths, and spot sizes available, making this method of resection extremely precise. However, the laser is costly, cumbersome, and produces a lot of smoke.
Ultrasonic energy. The ultrasonic blade and forceps create hemostasis using vibrational energy to coagulate tissue. Pass the blade through the tissue, slowly separating it while achieving hemostasis. Or, use the forceps to resect vascular adhesions, grasping and coagulating the tissue first and then using the scissors to divide the adhesion.
The advantages: minimal thermal spread, tissue-contact feedback, and essentially no smoke. However, the disadvantages are that direct tissue contact is necessary, traction is vital, the coagulation process is slow, and the disposable equipment is expensive.—Stephen Cohen, MD
Preventing adhesion recurrence
Unfortunately, approximately 85% of patients who undergo surgery for pelvic adhesions will develop recurrent adhesions. Available products that attempt to minimize this effect include oxidized regenerated cellulose (ORC) (Interceed; Ethicon Inc, Somerville, NJ), hyaluronic acid with carboxymethylcellulose (HAL-F) (Seprafilm; Genzyme Biosurgery, Cambridge, Mass), and expanded polytetrafluoroethylene (ePTFE) (Gore-Tex; W.L. Gore & Assocs, Flagstaff, Ariz). However, these products can only be applied to small surface areas, and only ORC can easily be used laparoscopically. As a result, they have achieved limited success.
Some additional progress has been made with the recent FDA approval of hyaluronate gel (Intergel; Gynecare, a division of Ethicon Inc, Somerville, NJ), which coats the entire peritoneal surface and all the intra-abdominal organs.4 Surgeons instill 300 cc of the gel through a trocar into the abdominal cavity just prior to closure, and the body completely absorbs it over time.
The author reports no financial relationship with any companies whose products are mentioned in this article.
1. Porpora MG, et al. Correlation between endometriosis and pelvic pain. J Am Assoc Gynecol Laparosc. 1999;6(4):429-434.
2. Buttram VC, et al. Pelvic Adhesions American Fertility Society. Fertil Steril. 1998;49:6.-
3. Levy BS, Hulka JF, Peterson HB, Phillips JM. Operative laparoscopy: American Association of Gynecologic Laparoscopists. 1993 Membership Survey. J Am Assoc Gynecol Laparosc. 1994;1:301.-
4. Johns DB, Keyport GM, Hoehler F, et al. Reduction of postsurgical adhesions with Intergel adhesion prevention solution: a multicenter study of safety and efficacy after conservative gynecologic surgery. Fertil Steril. 2001;76(3):595-604.
- Inspect the pelvic cavity prior to beginning adhesiolysis because the peritoneum becomes more opaque as the surgery progresses, obscuring anatomical definition.
- Grasp adhesions from the side contralateral to where you plan to make the first cut.
- Divide superficial adhesions first to ensure unimpeded access to deeper ones in case bleeding occurs.
- Coagulate thick or vascular adhesions with bipolar coagulation prior to division.
- Cut adhesions at both ends and remove the tissue rather than just dividing it.
Although more than 50% of all women who undergo pelvic or abdominal surgery will develop adhesions, the condition garners limited attention in the literature and from physicians. And, yet, the problems—infertility, chronic pelvic pain, and bowel obstruction, to name a few—with which patients present to Ob/Gyns every day often can be attributed to pelvic adhesions. In short, the quality of life of a significant number of women is affected by this condition, warranting a detailed look at the laparoscopic technique of adhesiolysis.
Assessing etiology
Any injury to the peritoneum has the potential to cause a pelvic adhesion, including endometriosis, pelvic inflammatory disease (PID), infection with a sexually transmitted disease, bleeding, and prior surgery. When the peritoneum is injured, the superficial mesothelial cells are disrupted allowing leakage of fibrinogen and cells into the pelvic cavity. Fibrinogen is then converted to fibrin, which covers all injured surfaces. When excess fibrin is present, it binds one organ to another. During normal healing, plasmin (which is formed from plasminogen) breaks down these fibrin bridges. But when plasmin production is inhibited, the bridges between organs remain intact. Within 7 to 10 days after the initial injury, mesothelial cells infiltrate the fibrin bridges, causing adhesions to become more solid.
Consider the presence of adhesions in patients who present with pelvic pain or infertility, especially if they have had prior pelvic or abdominal surgery.
Salpingitis following chlamydia or gonorrhea is another common etiology of pelvic adhesions. The spread of the infection through the fallopian tubes and into the peritoneal cavity sets the stage for the condition, as do ulcerative colitis, appendicitis, and diverticulitis.
Endometriosis is one of the most common etiologies because peritoneal lesions activate a chronic extensive inflammatory process, leading to dense adhesions. In fact, endometriosis-related adhesions are so common that the American Society for Reproductive Medicine (ASRM) assigns more points to adhesions for the staging of endometriosis than it does to the disease itself.1-2
Making the diagnosis
After reviewing the patient’s medical history, perform a physical exam. In some patients, an area of tenderness may be encountered when one palpates a prior incision, indicating that a loop of bowel may be adhered to this area. In addition, in women with adhesions, the uterus often is fixed either to the posterior pelvis or to the anterior abdominal wall, and the adnexa may be thickened and fixed to the uterus or pelvic sidewall. On rectal exam, the anterior wall of the rectum may be adhered to the uterus, or the rectum may be pulled to one side of the pelvis. Radiologic studies, with the exception of the hysterosalpingogram, do not usually aid in the diagnosis of pelvic adhesions.
Figure 1
Carefully inspect the pelvic cavity after placing the trocars. In this case, the woman was found to have adhesions on the left ovary.
Figure 2
Grasp the adhesion from the side contralateral to where you plan to make the first cut. Use laparoscopic scissors to divide and remove the tissue.
Figure 3
After completing adhesiolysis on the left ovary, check the contralateral organ. In this case, adhesions were found on the distal right tube and ovary.
Figure 4
Begin by excising superficial adhesions. This ensures unimpeded access to deeper adhesions in case bleeding occurs.
Figure 5
After all adhesions have been resected and removed, stop all bleeding and lavishly irrigate the pelvis with saline.
Figure 6
Just prior to closure, place oxidized regenerated cellulose (ORC) around both ovaries to help prevent recurrent adhesions.
Preparing the patient
After making the diagnosis and determining that surgery is the optimal treatment (see “Patient selection”), consider the approach. With rare exception, the procedure of choice is laparoscopy and is the method I prefer for adhesiolysis. Regardless of the route, prep the bowel either via an enema or an oral solution, as it decreases distention, making the operation safer and easier. In addition, evacuate the stomach contents via an oral gastric tube.
Indications. Consider for adhesiolysis any patient with chronic pelvic pain for more than 6 months or infertility for more than 1 year, after ruling out other potential etiologies. Patients who complain of localized pelvic pain are more likely to have adhesions than those with diffuse symptoms. But adhesions may be found even in women with diffuse pain. Although this pathology cannot always be incriminated as the cause of the pain, adhesiolysis often leads to a reduction of symptoms.1-3
Contraindications. An abdominal mass large enough to preclude trocar insertion, bowel obstruction, or diaphragmatic hernia are absolute contraindications. One caveat: While previous bowel resection and adhesiolysis are not contraindications to laparoscopy, both increase the risk of bowel perforation on trocar insertion. Proceed with caution or consider laparotomy.
REFERENCES
1. Malik E, Berg C, Meyhofer-Malik A, et al. Subjective evaluation of the therapeutic value of laparoscopic adhesiolysis: a retrospective analysis. Surg Endosc. 2000;14(1):79-81.
2. Nezhat FR, Crystal RA, Nezhat CH, et al. Laparoscopic adhesiolysis and relief of chronic pelvic pain. JSLS. 2000;4(4):281-285.
3. Lavonius M, Gullichsen R, Laine S, et al. Laparoscopy for chronic pelvic pain. Surg Laparosc Endosc. 1999;9(10):42-44.
Placing the trocars
Correct trocar placement facilitates any laparoscopic procedure, especially when dense adhesions are present. First determine the insertion sites, then select the type of entry technique and the length of the trocars.
Insertion sites. In most cases, the primary trocar is placed at the umbilicus. However, in a laparoscopy being performed for adhesions or in patients who have had an umbilical hernia operation or a vertical abdominal incision, place the primary trocar in the left upper quadrant. The reason: This region usually is spared from the formation of dense adhesions; therefore, entry can be performed safely by placing the trocar in the midclavicular line just below the lowest rib.
From that position, examine the abdomen for bowel adhesions to the anterior abdominal wall. If present, excise these adhesions prior to placing a trocar at the umbilical site. (It often is unnecessary to move the camera to the umbilical port, as the view with modern optical equipment from the left upper quadrant is more than adequate.)
Further, surgery can be eased by spacing the trocars as widely apart as possible and placing them on the same side of the patient.
Entry techniques. An alternative to direct trocar placement in highrisk patients is to use the open trocar insertion technique. First, make an abdominal incision and then insert the trocar through it. While some believe that this method reduces the risk of bowel perforation, the survey data from the American Association of Gynecologic Laparoscopists (AAGL) have not supported this theory.3
Trocar length. To determine the proper length, consider the thickness of the patient’s abdominal wall and the distance from the trocar to the adhesion. Trocars that are too long prevent the grasper jaws and scissors from opening, while those that are too short keep slipping back into the retroperitoneal space.
After all the trocars have been placed, carefully inspect the pelvic cavity because the peritoneum becomes more opaque as surgery progresses, obscuring anatomical definition.
Divide and conquer
To excise the adhesion, grasp it from the side contralateral to where you plan to make the first cut. Traction on both the organ and the adhesion is essential to establish the correct tissue planes, which in turn ensures adequate and safe excision of the adhesion. Because the bowel is the organ commonly involved in the adhesive process, there are many graspers available for atraumatic manipulation, including the Duval and Pennington forceps. Avoiding injury to the bowel is paramount to the procedure, as trauma will cause more adhesions, worsening the patient’s condition.
After grasping the adhesion, select a method by which to divide and remove it. This selection is based on the surgeon’s experience, the location of the adhesions, and the organs involved. In most cases, laparoscopic scissors are the instrument of choice. However, opt for electrosurgery (unipolar and bipolar), carbon dioxide laser, or ultrasonic energy (see “Tools for excision”) when energy is needed as a secondary source of hemostasis.
Begin by excising superficial adhesions. This ensures unimpeded access to deeper adhesions in case bleeding occurs. In electrosurgery, slowly pass the probe near the adhesion, allowing the electrons to arc from the instrument tip to the tissue. Proceed with extreme care when using unipolar energy near vital structures because electrons travel the path of least resistance. For example, if there is an adhesion between a loop of bowel and the uterus, always resect the adhesion from the bowel first. The reason: If you remove the adhesion from the uterus first, electrons would flow toward the bowel while you excise the adhesion from this organ. This is because it is the only path remaining for the electrons to egress, as you already have severed the adhesion from the uterus. The end result: thermal damage to the bowel.
When extremely thick or vascular adhesions are encountered, coagulate them prior to division as follows: First, achieve hemostasis using bipolar forceps. This instrument allows the precise application of energy to the tissue because electrons flow from one side of the forceps to the other, rather than through the body as with unipolar electrosurgery. Apply the energy until the tissue is desiccated and the high resistance stops electron flow. Second, divide the tissue using laparoscopic scissors. Bipolar electrosurgery also should be used when coagulation is needed near vital structures such as great vessels, the bowel, bladder, and ureters.
A new type of electrosurgical generator seals vessel walls rather than coagulating them (LigaSure; Valleylab, Boulder, Colo). This results in a much higher vessel burst strength than either unipolar or bipolar electrosurgery, allowing the surgeon to close vessels up to 7 mm in diameter prior to division of the vascular adhesion.
After all adhesions have been resected and removed, stop all bleeding and lavishly irrigate the pelvis with saline prior to closure. If extensive adhesions have been resected from the rectal wall, assess this organ’s integrity by filling the pelvis with saline and placing air in the rectum with either a sigmoidoscope or 30-cc Foley catheter.
Laparoscopic scissors. These are inexpensive, create no peripheral damage, give direct mechanical feedback, and are familiar to all physicians. No smoke or plume is created to obstruct vision. However, because hemostasis cannot be achieved with scissors, they are inappropriate for vascular adhesions. In addition, they cannot be used in areas of the pelvis that can be seen but not reached. Instead, opt for one of the cutting methods described here.
Electrosurgery. The efficiency of electrosurgery depends on the power, shape of the probe tip, and waveform. The higher the power and the finer the tip, the faster the probe will resect the adhesion, thereby minimizing thermal damage. Pure cutting currents also reduce thermal damage but provide less hemostasis than blended or pure coagulation currents.
In general, the thickness, vascularity, and water content of the adhesions will dictate the equipment and settings used. (There are no standard wattage settings, but surgeons typically use 25 to 125 watts.)
Carbon dioxide laser. This instrument allows surgeons to cut with hemostasis without coming into direct contact with the tissue. There are an infinite number of settings, wavelengths, and spot sizes available, making this method of resection extremely precise. However, the laser is costly, cumbersome, and produces a lot of smoke.
Ultrasonic energy. The ultrasonic blade and forceps create hemostasis using vibrational energy to coagulate tissue. Pass the blade through the tissue, slowly separating it while achieving hemostasis. Or, use the forceps to resect vascular adhesions, grasping and coagulating the tissue first and then using the scissors to divide the adhesion.
The advantages: minimal thermal spread, tissue-contact feedback, and essentially no smoke. However, the disadvantages are that direct tissue contact is necessary, traction is vital, the coagulation process is slow, and the disposable equipment is expensive.—Stephen Cohen, MD
Preventing adhesion recurrence
Unfortunately, approximately 85% of patients who undergo surgery for pelvic adhesions will develop recurrent adhesions. Available products that attempt to minimize this effect include oxidized regenerated cellulose (ORC) (Interceed; Ethicon Inc, Somerville, NJ), hyaluronic acid with carboxymethylcellulose (HAL-F) (Seprafilm; Genzyme Biosurgery, Cambridge, Mass), and expanded polytetrafluoroethylene (ePTFE) (Gore-Tex; W.L. Gore & Assocs, Flagstaff, Ariz). However, these products can only be applied to small surface areas, and only ORC can easily be used laparoscopically. As a result, they have achieved limited success.
Some additional progress has been made with the recent FDA approval of hyaluronate gel (Intergel; Gynecare, a division of Ethicon Inc, Somerville, NJ), which coats the entire peritoneal surface and all the intra-abdominal organs.4 Surgeons instill 300 cc of the gel through a trocar into the abdominal cavity just prior to closure, and the body completely absorbs it over time.
The author reports no financial relationship with any companies whose products are mentioned in this article.
- Inspect the pelvic cavity prior to beginning adhesiolysis because the peritoneum becomes more opaque as the surgery progresses, obscuring anatomical definition.
- Grasp adhesions from the side contralateral to where you plan to make the first cut.
- Divide superficial adhesions first to ensure unimpeded access to deeper ones in case bleeding occurs.
- Coagulate thick or vascular adhesions with bipolar coagulation prior to division.
- Cut adhesions at both ends and remove the tissue rather than just dividing it.
Although more than 50% of all women who undergo pelvic or abdominal surgery will develop adhesions, the condition garners limited attention in the literature and from physicians. And, yet, the problems—infertility, chronic pelvic pain, and bowel obstruction, to name a few—with which patients present to Ob/Gyns every day often can be attributed to pelvic adhesions. In short, the quality of life of a significant number of women is affected by this condition, warranting a detailed look at the laparoscopic technique of adhesiolysis.
Assessing etiology
Any injury to the peritoneum has the potential to cause a pelvic adhesion, including endometriosis, pelvic inflammatory disease (PID), infection with a sexually transmitted disease, bleeding, and prior surgery. When the peritoneum is injured, the superficial mesothelial cells are disrupted allowing leakage of fibrinogen and cells into the pelvic cavity. Fibrinogen is then converted to fibrin, which covers all injured surfaces. When excess fibrin is present, it binds one organ to another. During normal healing, plasmin (which is formed from plasminogen) breaks down these fibrin bridges. But when plasmin production is inhibited, the bridges between organs remain intact. Within 7 to 10 days after the initial injury, mesothelial cells infiltrate the fibrin bridges, causing adhesions to become more solid.
Consider the presence of adhesions in patients who present with pelvic pain or infertility, especially if they have had prior pelvic or abdominal surgery.
Salpingitis following chlamydia or gonorrhea is another common etiology of pelvic adhesions. The spread of the infection through the fallopian tubes and into the peritoneal cavity sets the stage for the condition, as do ulcerative colitis, appendicitis, and diverticulitis.
Endometriosis is one of the most common etiologies because peritoneal lesions activate a chronic extensive inflammatory process, leading to dense adhesions. In fact, endometriosis-related adhesions are so common that the American Society for Reproductive Medicine (ASRM) assigns more points to adhesions for the staging of endometriosis than it does to the disease itself.1-2
Making the diagnosis
After reviewing the patient’s medical history, perform a physical exam. In some patients, an area of tenderness may be encountered when one palpates a prior incision, indicating that a loop of bowel may be adhered to this area. In addition, in women with adhesions, the uterus often is fixed either to the posterior pelvis or to the anterior abdominal wall, and the adnexa may be thickened and fixed to the uterus or pelvic sidewall. On rectal exam, the anterior wall of the rectum may be adhered to the uterus, or the rectum may be pulled to one side of the pelvis. Radiologic studies, with the exception of the hysterosalpingogram, do not usually aid in the diagnosis of pelvic adhesions.
Figure 1
Carefully inspect the pelvic cavity after placing the trocars. In this case, the woman was found to have adhesions on the left ovary.
Figure 2
Grasp the adhesion from the side contralateral to where you plan to make the first cut. Use laparoscopic scissors to divide and remove the tissue.
Figure 3
After completing adhesiolysis on the left ovary, check the contralateral organ. In this case, adhesions were found on the distal right tube and ovary.
Figure 4
Begin by excising superficial adhesions. This ensures unimpeded access to deeper adhesions in case bleeding occurs.
Figure 5
After all adhesions have been resected and removed, stop all bleeding and lavishly irrigate the pelvis with saline.
Figure 6
Just prior to closure, place oxidized regenerated cellulose (ORC) around both ovaries to help prevent recurrent adhesions.
Preparing the patient
After making the diagnosis and determining that surgery is the optimal treatment (see “Patient selection”), consider the approach. With rare exception, the procedure of choice is laparoscopy and is the method I prefer for adhesiolysis. Regardless of the route, prep the bowel either via an enema or an oral solution, as it decreases distention, making the operation safer and easier. In addition, evacuate the stomach contents via an oral gastric tube.
Indications. Consider for adhesiolysis any patient with chronic pelvic pain for more than 6 months or infertility for more than 1 year, after ruling out other potential etiologies. Patients who complain of localized pelvic pain are more likely to have adhesions than those with diffuse symptoms. But adhesions may be found even in women with diffuse pain. Although this pathology cannot always be incriminated as the cause of the pain, adhesiolysis often leads to a reduction of symptoms.1-3
Contraindications. An abdominal mass large enough to preclude trocar insertion, bowel obstruction, or diaphragmatic hernia are absolute contraindications. One caveat: While previous bowel resection and adhesiolysis are not contraindications to laparoscopy, both increase the risk of bowel perforation on trocar insertion. Proceed with caution or consider laparotomy.
REFERENCES
1. Malik E, Berg C, Meyhofer-Malik A, et al. Subjective evaluation of the therapeutic value of laparoscopic adhesiolysis: a retrospective analysis. Surg Endosc. 2000;14(1):79-81.
2. Nezhat FR, Crystal RA, Nezhat CH, et al. Laparoscopic adhesiolysis and relief of chronic pelvic pain. JSLS. 2000;4(4):281-285.
3. Lavonius M, Gullichsen R, Laine S, et al. Laparoscopy for chronic pelvic pain. Surg Laparosc Endosc. 1999;9(10):42-44.
Placing the trocars
Correct trocar placement facilitates any laparoscopic procedure, especially when dense adhesions are present. First determine the insertion sites, then select the type of entry technique and the length of the trocars.
Insertion sites. In most cases, the primary trocar is placed at the umbilicus. However, in a laparoscopy being performed for adhesions or in patients who have had an umbilical hernia operation or a vertical abdominal incision, place the primary trocar in the left upper quadrant. The reason: This region usually is spared from the formation of dense adhesions; therefore, entry can be performed safely by placing the trocar in the midclavicular line just below the lowest rib.
From that position, examine the abdomen for bowel adhesions to the anterior abdominal wall. If present, excise these adhesions prior to placing a trocar at the umbilical site. (It often is unnecessary to move the camera to the umbilical port, as the view with modern optical equipment from the left upper quadrant is more than adequate.)
Further, surgery can be eased by spacing the trocars as widely apart as possible and placing them on the same side of the patient.
Entry techniques. An alternative to direct trocar placement in highrisk patients is to use the open trocar insertion technique. First, make an abdominal incision and then insert the trocar through it. While some believe that this method reduces the risk of bowel perforation, the survey data from the American Association of Gynecologic Laparoscopists (AAGL) have not supported this theory.3
Trocar length. To determine the proper length, consider the thickness of the patient’s abdominal wall and the distance from the trocar to the adhesion. Trocars that are too long prevent the grasper jaws and scissors from opening, while those that are too short keep slipping back into the retroperitoneal space.
After all the trocars have been placed, carefully inspect the pelvic cavity because the peritoneum becomes more opaque as surgery progresses, obscuring anatomical definition.
Divide and conquer
To excise the adhesion, grasp it from the side contralateral to where you plan to make the first cut. Traction on both the organ and the adhesion is essential to establish the correct tissue planes, which in turn ensures adequate and safe excision of the adhesion. Because the bowel is the organ commonly involved in the adhesive process, there are many graspers available for atraumatic manipulation, including the Duval and Pennington forceps. Avoiding injury to the bowel is paramount to the procedure, as trauma will cause more adhesions, worsening the patient’s condition.
After grasping the adhesion, select a method by which to divide and remove it. This selection is based on the surgeon’s experience, the location of the adhesions, and the organs involved. In most cases, laparoscopic scissors are the instrument of choice. However, opt for electrosurgery (unipolar and bipolar), carbon dioxide laser, or ultrasonic energy (see “Tools for excision”) when energy is needed as a secondary source of hemostasis.
Begin by excising superficial adhesions. This ensures unimpeded access to deeper adhesions in case bleeding occurs. In electrosurgery, slowly pass the probe near the adhesion, allowing the electrons to arc from the instrument tip to the tissue. Proceed with extreme care when using unipolar energy near vital structures because electrons travel the path of least resistance. For example, if there is an adhesion between a loop of bowel and the uterus, always resect the adhesion from the bowel first. The reason: If you remove the adhesion from the uterus first, electrons would flow toward the bowel while you excise the adhesion from this organ. This is because it is the only path remaining for the electrons to egress, as you already have severed the adhesion from the uterus. The end result: thermal damage to the bowel.
When extremely thick or vascular adhesions are encountered, coagulate them prior to division as follows: First, achieve hemostasis using bipolar forceps. This instrument allows the precise application of energy to the tissue because electrons flow from one side of the forceps to the other, rather than through the body as with unipolar electrosurgery. Apply the energy until the tissue is desiccated and the high resistance stops electron flow. Second, divide the tissue using laparoscopic scissors. Bipolar electrosurgery also should be used when coagulation is needed near vital structures such as great vessels, the bowel, bladder, and ureters.
A new type of electrosurgical generator seals vessel walls rather than coagulating them (LigaSure; Valleylab, Boulder, Colo). This results in a much higher vessel burst strength than either unipolar or bipolar electrosurgery, allowing the surgeon to close vessels up to 7 mm in diameter prior to division of the vascular adhesion.
After all adhesions have been resected and removed, stop all bleeding and lavishly irrigate the pelvis with saline prior to closure. If extensive adhesions have been resected from the rectal wall, assess this organ’s integrity by filling the pelvis with saline and placing air in the rectum with either a sigmoidoscope or 30-cc Foley catheter.
Laparoscopic scissors. These are inexpensive, create no peripheral damage, give direct mechanical feedback, and are familiar to all physicians. No smoke or plume is created to obstruct vision. However, because hemostasis cannot be achieved with scissors, they are inappropriate for vascular adhesions. In addition, they cannot be used in areas of the pelvis that can be seen but not reached. Instead, opt for one of the cutting methods described here.
Electrosurgery. The efficiency of electrosurgery depends on the power, shape of the probe tip, and waveform. The higher the power and the finer the tip, the faster the probe will resect the adhesion, thereby minimizing thermal damage. Pure cutting currents also reduce thermal damage but provide less hemostasis than blended or pure coagulation currents.
In general, the thickness, vascularity, and water content of the adhesions will dictate the equipment and settings used. (There are no standard wattage settings, but surgeons typically use 25 to 125 watts.)
Carbon dioxide laser. This instrument allows surgeons to cut with hemostasis without coming into direct contact with the tissue. There are an infinite number of settings, wavelengths, and spot sizes available, making this method of resection extremely precise. However, the laser is costly, cumbersome, and produces a lot of smoke.
Ultrasonic energy. The ultrasonic blade and forceps create hemostasis using vibrational energy to coagulate tissue. Pass the blade through the tissue, slowly separating it while achieving hemostasis. Or, use the forceps to resect vascular adhesions, grasping and coagulating the tissue first and then using the scissors to divide the adhesion.
The advantages: minimal thermal spread, tissue-contact feedback, and essentially no smoke. However, the disadvantages are that direct tissue contact is necessary, traction is vital, the coagulation process is slow, and the disposable equipment is expensive.—Stephen Cohen, MD
Preventing adhesion recurrence
Unfortunately, approximately 85% of patients who undergo surgery for pelvic adhesions will develop recurrent adhesions. Available products that attempt to minimize this effect include oxidized regenerated cellulose (ORC) (Interceed; Ethicon Inc, Somerville, NJ), hyaluronic acid with carboxymethylcellulose (HAL-F) (Seprafilm; Genzyme Biosurgery, Cambridge, Mass), and expanded polytetrafluoroethylene (ePTFE) (Gore-Tex; W.L. Gore & Assocs, Flagstaff, Ariz). However, these products can only be applied to small surface areas, and only ORC can easily be used laparoscopically. As a result, they have achieved limited success.
Some additional progress has been made with the recent FDA approval of hyaluronate gel (Intergel; Gynecare, a division of Ethicon Inc, Somerville, NJ), which coats the entire peritoneal surface and all the intra-abdominal organs.4 Surgeons instill 300 cc of the gel through a trocar into the abdominal cavity just prior to closure, and the body completely absorbs it over time.
The author reports no financial relationship with any companies whose products are mentioned in this article.
1. Porpora MG, et al. Correlation between endometriosis and pelvic pain. J Am Assoc Gynecol Laparosc. 1999;6(4):429-434.
2. Buttram VC, et al. Pelvic Adhesions American Fertility Society. Fertil Steril. 1998;49:6.-
3. Levy BS, Hulka JF, Peterson HB, Phillips JM. Operative laparoscopy: American Association of Gynecologic Laparoscopists. 1993 Membership Survey. J Am Assoc Gynecol Laparosc. 1994;1:301.-
4. Johns DB, Keyport GM, Hoehler F, et al. Reduction of postsurgical adhesions with Intergel adhesion prevention solution: a multicenter study of safety and efficacy after conservative gynecologic surgery. Fertil Steril. 2001;76(3):595-604.
1. Porpora MG, et al. Correlation between endometriosis and pelvic pain. J Am Assoc Gynecol Laparosc. 1999;6(4):429-434.
2. Buttram VC, et al. Pelvic Adhesions American Fertility Society. Fertil Steril. 1998;49:6.-
3. Levy BS, Hulka JF, Peterson HB, Phillips JM. Operative laparoscopy: American Association of Gynecologic Laparoscopists. 1993 Membership Survey. J Am Assoc Gynecol Laparosc. 1994;1:301.-
4. Johns DB, Keyport GM, Hoehler F, et al. Reduction of postsurgical adhesions with Intergel adhesion prevention solution: a multicenter study of safety and efficacy after conservative gynecologic surgery. Fertil Steril. 2001;76(3):595-604.
Incision decisions: which ones for which procedures?
- The incision should be considered as a second surgical procedure, which temporarily interferes with normal abdominal wall function.
- The midline incision provides excellent exposure to all areas of the abdomen and retroperitoneum, which can be accessed with minimal risk of significant vascular or nerve injury.
- Transverse incisions create less tension on the opposing skin edges because the incision follows Langer’s lines. The incidence of incisional hernias and wound dehiscence has been reported to be lower, but these studies are not randomized.
An abdominal incision often is given little thought other than as an access site through which a surgical procedure is performed. In reality, the incision is a second surgical procedure, which interferes—at least temporarily—with normal abdominal wall function.
While most physicians concur that the essential elements of a well-planned incision include adequate access to anticipated pathology, extensibility, and security of closure, many may not consider preservation of abdominal wall function as a key factor in their decision-making. Additional considerations include certainty of diagnosis, speed of entry, body habitus, presence of previous scars, potential for problems with hemostasis, and cosmetic outcome. These factors are the key determinants of whether the incision will be longitudinal (midline or paramedian) or transverse (Pfannenstiel’s, Cherney’s, or Maylard’s). For most gynecologic procedures confined to the pelvis, either option may be considered. The exceptions are patients with uncertain diagnoses or when access to the upper abdomen is indicated.
Regardless of the type of incision selected, the skin should be incised with a single, clean stroke of a sharp scalpel. However, when it comes to dissecting the underlying subcutaneous tissues, the debate continues over whether a scalpel or electrosurgery is best. While I recently have switched to the latter, here is a look at what the data say: Johnson and Serpell demonstrated that electrosurgery is associated with faster hemostasis, with no difference in the incidence of wound infection.1 Similarly, a recent randomized trial by Kearns et al found electro-surgery causes less blood loss and does not increase the risk of wound infections or fascial dehiscence.2 In contrast, a large prospective study by Cruse et al suggested that the use of diathermy is associated with twice the wound infection rate.3
This controversy also involves patients with gynecologic malignancies. Kolb et al found that electrosurgery was an independent risk factor for wound complications following surgery for ovarian cancer.4 However, Franchi and colleagues reported no difference in the rate of wound complications between scalpel and diathermy in patients who underwent mid-line abdominal incisions for the treatment of uterine cancer.5
Use the midline when the diagnosis and the extent of surgery are uncertain.
The inconsistencies in the data may reflect differences in electrosurgical technique. Non-modulated (cutting) current concentrates energy, vaporizing the tissue with little heat injury to surrounding areas. Conversely, modulated (coagulating) current coagulates the tissue with heat-producing char over a large area, and tissue injury often extends beyond the char. This effect is magnified if the electrode comes in direct contact with the tissue. Use the arc, rather than direct contact, to prevent excessive devitalization of tissue.
This article will review the techniques for, as well as the rationale and disadvantages of, common incisions—both longitudinal and transverse—to help the gynecologic surgeon minimize morbidity and maximize outcomes.
Longitudinal incisions
The longitudinal incisions that will be reviewed here are the midline (median) and paramedian. Classically, it was thought that longitudinal incisions were at greater risk of dehiscence than transverse incisions.6 However, it is difficult to make legitimate comparisons since longitudinal incisions are more likely to be performed in cases of hemorrhage, trauma, sepsis, multiorgan disease, previous surgery, previous radiation therapy, and malignancy—all of which increase the likelihood of postoperative complications. Furthermore, prospective and randomized studies have shown little, if any, difference in the incidence of dehiscence and hernias between longitudinal and transverse incisions.6-8
MidlineTechnique. Initiate the midline as a low abdominal incision (approximately 2 cm above the pubic symphysis), cutting along the linea alba. To extend the incision, if necessary, continue the dissection to the left of the umbilicus to avoid the ligamentum teres. Open the peritoneum at the cephalad pole of the incision (Figure 1). Expand this cut slightly off midline to avoid the urachus yet adequately expose the peritoneal cavity (Figure 2).
Rationale. While this incision can be used for any surgical procedure, it is especially appropriate when the diagnosis is uncertain and the exact procedure or extent of surgery is unclear. It is an excellent choice when access to the upper abdomen may be necessary, e.g., patients with gynecologic malignancies who may need assessment of the diaphragm, liver biopsy, para-aortic node biopsy, omentectomy, or debulking procedures. Patients with benign gynecologic conditions also may benefit from a midline incision. For example, when pelvic anatomy is distorted, as with severe endometriosis or sepsis, recognizable anatomy may be found only above the pelvic brim.
A midline incision allows the quickest entry, which is especially important for an unstable or seriously ill patient. Exposure is excellent, as all areas of the abdomen and retroperitoneum can be accessed with minimal risk of significant vascular or nerve injury. This is because only terminal branches of the abdominal wall blood vessels and nerves are located at the linea alba. In addition, because deep tissue planes are not opened, this incision may be ideal for patients who are anticoagulated, have enlarged epigastric vessels that are more susceptible to injury, or have an intraabdominal infection.
Transverse incisions help reduce the rate of wound dehiscence.
Disadvantages. Two potential problems are the higher rate of hernia formation and wound dehiscence, which may be due to constant lateral tension, compared with transverse incisions. In addition, coughing, retching, and straining may exacerbate the lateral tension. Proper suture selection can reduce the incidence of complications. As previously mentioned, the reality of these disadvantages is subject to debate; further studies are needed to determine the true risk.
From a cosmetic standpoint, the midline scar often is prominent because the incision transects Langer’s lines. Hence, it cannot be concealed by lingerie or swimwear.
FIGURE 1 Midline incision
After incising the linea alba and separating the muscles in the midline, open the peritoneum at the cephalad pole of the incision.
FIGURE 2 Midline incision
Expand this cut slightly off midline to avoid the urachus yet adequately expose the peritoneal cavity.
ParamedianTechnique. Place this incision 2 to 5 cm to the left or right of the midline, depending on the indication for surgery. After dissecting the skin and subcutaneous layers, incise the anterior rectus sheath vertically over the rectus abdominis muscle (Figure 3) and dissect it from the medial muscle edge. Retract the muscle laterally to expose the posterior rectus sheath and then incise the sheath and the peritoneum vertically to expose the peritoneal cavity (Figure 4). Alternatively, a more lateral paramedian incision can be placed over the rectus abdominis muscle. In this case, however, the rectus muscle should be separated vertically, instead of retracted laterally, to expose the posterior rectus sheath.
Rationale. Paramedian incisions provide excellent exposure of the pelvis, excluding the upper abdomen. Therefore, consider this technique when the procedure will be confined to the ipsilateral pelvis, e.g., rightor left-side lymph node biopsies and exposure of the sigmoid colon on the left. Also, a paramedian incision at the level of the umbilicus can be used for a cesarean delivery or a hysterectomy.
This incision is the best choice when performing pelvic surgery on morbidly obese patients. By placing the incision low on the abdomen, the large panniculus can be retracted over the mons pubis and thighs, providing excellent exposure of the pelvis.
Disadvantages. Some researchers have reported that muscle-splitting lateral paramedian incisions have a lower incidence of incisional hernias compared with midline incisions.7,9 However, they take longer to perform and restrict access to the contralateral pelvis. In addition, the risk of vascular injury and hematomas is increased, especially in the lower pole where branches of the epigastric arteries penetrate the muscle.
With regard to nerve injury, terminal nerves are cut, resulting in paralysis of the inner portion of the rectus abdominis muscle. This paralysis can be permanent, as the muscle medial to the vertical separation is involved. However, if only the medial third of the muscle is denervated, the paralysis rarely limits function.
FIGURE 3 Paramedian incision
Place the incision 2 to 5 cm to the left or right of the midline. Incise the anterior rectus sheath vertically over the rectus abdominis muscle.
FIGURE 4 Paramedian incision
Retract the muscle laterally to expose the posterior sheath; then incise the sheath and the peritoneum vertically to expose the pelvic cavity.
Transverse incisions
Transverse incisions such as Pfannenstiel’s (muscle separating), Cherney’s (tendon detaching), and Maylard’s (muscle cutting) were developed to reduce the incidence of incisional hernias and wound dehiscence. Their success lies in the fact that they cause less tension on the opposing wound edges because the incisions follow Langer’s lines, unlike longitudinal incisions. Their placement also allows for a better cosmetic outcome. Because they can be placed in the pubic hairline or in a natural skin crease, they are easily concealed by lingerie or swimwear. However, the incision should not be placed in a deep skin fold of a large panniculus where maceration of the skin can increase the risk of infection.
The main disadvantages of transverse incisions are limited exposure of the upper abdomen and limited extensibility. Further, because more tissue planes are opened and more vessels are encountered, these incisions increase the risk of hematomas and infection.
Pfannenstiel’sTechnique. Incise the skin and subcutaneous tissues 2 to 5 cm above the pubic symphysis.10 Then dissect the rectus sheath transversely, separating it sharply from the rectus muscle at the linea alba and retracting it superiorly toward the umbilicus and inferiorly toward the pubic symphysis (Figure 5). Divide the rectus abdominis muscle along the midline raphe and retract it laterally, exposing the transversalis fascia and the posterior rectus sheath. Incise these layers and the peritoneum vertically to expose the peritoneal cavity (Figure 6).
To minimize retraction of the rectus abdominus muscle during Maylard’s, do not separate it from the anterior rectus sheath.
Rationale. Since exposure is limited and the incision can be only minimally extended, this incision is appropriate for procedures that are limited to the pelvis, e.g., abdominal hysterectomy, cesarean delivery, retropubic urethropexy, and paravaginal defect repairs. (Extension of the incision can be achieved by modifying it to Cherney’s incision.)
Disadvantages. The fact that the incision severs multiple tissue planes is an advantage as well as a disadvantage. Postoperative dehiscence and incisional hernias are rare because the closed wound has a high tensile strength. However, the incidence of inguinal hernias may increase when the incision is placed close to the external inguinal ring,11 and very low incisions may increase the risk of femoral nerve injury.12 The ilioinguinal and iliohypogastric nerves may be damaged, especially if the rectus incision is extended far laterally. Most commonly, they are trapped in the suture during closure, producing postoperative pain. Incising multiple layers also slows entry and increases the risk of seromas, hematomas, and wound infections.
This approach is contraindicated when time is of the essence, e.g., hemorrhage, or in the face of an abdominal infection, e.g., sepsis.
FIGURE 5 Pfannenstiel’s incision
After incising the skin and subcutaneous tissues, dissect the rectus sheath transversely, separating it sharply from the rectus muscle.
FIGURE 6 Pfannenstiel’s incision
Divide and retract the rectus abdominus muscle and then incise the tranversalis fascia and posterior rectus sheath vertically.
Cherney’sTechnique. Dissect the skin and subcutaneous tissue 2 to 3 cm above the pubic symphysis, which is lower than most Pfannenstiel’s incisions. Then incise the anterior rectus sheath transversely and dissect it from the rectus abdominis muscle superiorly and inferiorly. Using blunt dissection, separate the rectus and pyramidalis muscles from the underlying bladder and adventitial tissue. Then incise transversely the tendons of the rectus and pyramidalis muscles 0.5 cm above the pubic symphysis (Figure 7). (This segment of tendon facilitates the reattachment of the muscles at closure.) Retract the muscles and tendons cephalad to expose the retropubic space. Incise the peritoneum transversely to expose the peritoneal cavity (Figure 8).
Rationale. Cherney’s incision provides excellent access to the retropubic space of Retzius, making it a good choice for retropubic urethropexy and paravaginal repair. Exposure is the main advantage to Cherney’s incision. The reason: It is a modification of Pfannenstiel’s incision and, as such, provides excellent lateral exposure because it is placed much lower on the abdominal wall.
Disadvantages. The benefits and risks of this incision are similar to those of Pfannenstiel’s incision since the same tissue planes are opened. However, it is more time consuming due to the dissection required to separate the muscles and tendons from the underlying tissues.
In addition, the deep inferior epigastric arteries could be injured as a result of the lateral dissection. Lastly, reattachment of the tendons is tedious.
FIGURE 7 Cherney’s incision
Incise transversely the tendons of the rectus and pyramidalis muscles 0.5 cm above the pubic symphysis.
FIGURE 8 Cherney’s incision
Retract the muscles and tendons cephalad and incise the peritoneum transversely to expose the peritoneal cavity.
Maylard’sTechnique. After transecting the skin and subcutaneous layers in a plane at the level of the anterior superior iliac spine, incise the rectus sheath transversely and extend the incision through the aponeuroses of the abdominal muscles to about 2 to 3 cm medial to the anterior iliac crest. Then dissect the rectus abdominis muscle transversely with a scalpel, electrocautery, or surgical stapler (Figure 9). To minimize retraction of this muscle, do not separate it from the anterior rectus sheath. (Alternatively, many surgeons recommend that the cut edge of the muscle be secured to the anterior sheath with mattress sutures to prevent retraction and reduce blood loss.)
Prior to transecting the peritoneum, isolate, ligate, and divide the deep inferior epigastric vessels. (Patients with significant aortoiliac atherosclerosis or aortic coarctation develop considerable collateral circulation through the epigastric vessels for perfusion of the lower extremities. Ligating these vessels may cause claudication and even lifethreatening ischemia. Therefore, assess iliac flow before ligating and transecting the arteries.) Then transect the transversalis fascia and peritoneum transversely, allowing access to the peritoneal cavity (Figure 10).
Rationale. Many oncologists use this approach for pelvic lymphadenectomy and staging procedures because the exposure to the lateral pelvis is excellent. The reason: This transverse incision cuts through all layers of the abdominal wall at the level of the anterior iliac spine. It also may be appropriate when pelvic pathology extends to the sidewall, e.g., endometriosis.
It is advisable to place a drain prior to closing Maylard’s.
Physicians should consider using this approach more than they currently are, especially when Pfannenstiel’s incision cannot provide satisfactory exposure or a longitudinal incision is thought to be the only alternative. The decision to use Maylard’s incision should be planned during the preoperative assessment, as Pfannenstiel’s incision should not be modified to Maylard’s intraoperatively.
Disadvantages. Although Maylard’s incision improves access to the upper abdomen compared to other transverse incisions, exposure is still limited. In addition, trauma to the deep epigastric arteries may result in considerable hemorrhage. And since hematomas commonly infiltrate the retroperitoneal space, large quantities of blood already may be lost before the hemorrhage is clinically apparent. Finally, oozing from the cut edge of the muscles may result in significant fluid collection, increasing the risk of infection. Therefore, it is advisable to place a drain prior to closure.
Like other transverse incisions, the wound parallels the nerves, providing a measure of protection. However, the rectus incision approaches the anterior iliac spine, where both the ilioinguinal and iliohypogastric nerves lie. Both may be damaged during the incision and can become entrapped during closure. Additionally, the femoral nerve, the lateral femoral cutaneous nerve, and the genitofemoral nerve are easily compressed by the lateral blades of the retractors because of the extreme lateral positions of the incision’s poles. Injury to these nerves can result in paresthesia, pain, and paralysis of the leg muscles.
FIGURE 9 Maylard’s incision
After incising the aponeuroses of the abdominal muscles medial to the anterior iliac crest, dissect the rectus abdominus muscle transversely.
FIGURE 10 Maylard’s incision
Isolate, ligate, and divide the epigastric vessels. Then incise the transversalis fascia and peritoneum transversely to access the peritoneal cavity.
In summary
While the midline and Pfannenstiel’s incisions are an integral part of the gynecologic surgeon’s armamentarium, the paramedian and Maylard’s incisions should be added to the repertoire because these incisions can greatly enhance exposure. The paramedian shares the advantages of the midline incision but also allows better access to the ipsilateral pelvis. Furthermore, although Maylard’s incision takes longer to perform than other techniques, the excellent exposure—both laterally and superiorly—makes it worthwhile.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Johnson CD, Serpell JW. Wound infection after abdominal incision with scalpel or diathermy. Br J Surg. 1990;77:626-627.
2. Cruse PJE, Ford R. The epidemiology of wound infection: a 10-year prospective study of 62,939 wounds. Surg Clin North Am. 1980;60:27.-
3. Kearns SR, Connolly EM, McNally S, McNamara DA, Deasy J. Randomized clinical trial of diathermy versus scalpel incision in elective midline laparotomy. Br J Surg. 2001;88(1):41-44.
4. Kolb BA, Buller RE, Connoer JP, et al. Effects of early postoperative chemotherapy on wound healing. Obstet Gynecol. 1992;79:988-992.
5. Franchi M, Ghezzi F, Bendetti-Panici PL, et al. A multicenter collaborative study on the use of cold scalpel and electrocautery for midline abdominal incision. Am J Surg. 2001;181(2):128-132.
6. Hendrix SL, Schimp V, Martin J. The legendary superior strength of the Pfannenstiel incision: a myth? Am J Obstet Gynecol. 2000;182(6):1446-1451.
7. Ellis H, Coleridge-Smith PD, Joyce AD. Abdominal incisions—vertical or transverse? Postgrad Med J. 1984;60:407-410.
8. Sanders RJ, DiClementi D. Principles of abdominal wound closure. II. Prevention of wound dehiscence. Arch Surg. 1977;112:1188.-
9. Donaldson DR, Hegarty JH, Brennan TG, et al. The lateral paramedian incision-experience with 850 cases. Br J Surg. 1982;69:630.-
10. Greenall MJ, et al. Midline or transverse laparotomy? A random controlled clinical trial. Part I: Influence on healing. Br J Surg. 1980;7:188.-
11. Pfannenstiel J. Ueber die Vortheile des suprasymphysaren Fascienquerschnitts für die gynakologischen Koliotomien zugleich ein Beitrag zu der Indikationsstellung der Operationswege. Samml Klin Vortr (Leipzig). 1900;268:1735.-
12. Griffiths DA. A reappraisal of the Pfannenstiel incision. Br J Urol. 1976;48:469.-
- The incision should be considered as a second surgical procedure, which temporarily interferes with normal abdominal wall function.
- The midline incision provides excellent exposure to all areas of the abdomen and retroperitoneum, which can be accessed with minimal risk of significant vascular or nerve injury.
- Transverse incisions create less tension on the opposing skin edges because the incision follows Langer’s lines. The incidence of incisional hernias and wound dehiscence has been reported to be lower, but these studies are not randomized.
An abdominal incision often is given little thought other than as an access site through which a surgical procedure is performed. In reality, the incision is a second surgical procedure, which interferes—at least temporarily—with normal abdominal wall function.
While most physicians concur that the essential elements of a well-planned incision include adequate access to anticipated pathology, extensibility, and security of closure, many may not consider preservation of abdominal wall function as a key factor in their decision-making. Additional considerations include certainty of diagnosis, speed of entry, body habitus, presence of previous scars, potential for problems with hemostasis, and cosmetic outcome. These factors are the key determinants of whether the incision will be longitudinal (midline or paramedian) or transverse (Pfannenstiel’s, Cherney’s, or Maylard’s). For most gynecologic procedures confined to the pelvis, either option may be considered. The exceptions are patients with uncertain diagnoses or when access to the upper abdomen is indicated.
Regardless of the type of incision selected, the skin should be incised with a single, clean stroke of a sharp scalpel. However, when it comes to dissecting the underlying subcutaneous tissues, the debate continues over whether a scalpel or electrosurgery is best. While I recently have switched to the latter, here is a look at what the data say: Johnson and Serpell demonstrated that electrosurgery is associated with faster hemostasis, with no difference in the incidence of wound infection.1 Similarly, a recent randomized trial by Kearns et al found electro-surgery causes less blood loss and does not increase the risk of wound infections or fascial dehiscence.2 In contrast, a large prospective study by Cruse et al suggested that the use of diathermy is associated with twice the wound infection rate.3
This controversy also involves patients with gynecologic malignancies. Kolb et al found that electrosurgery was an independent risk factor for wound complications following surgery for ovarian cancer.4 However, Franchi and colleagues reported no difference in the rate of wound complications between scalpel and diathermy in patients who underwent mid-line abdominal incisions for the treatment of uterine cancer.5
Use the midline when the diagnosis and the extent of surgery are uncertain.
The inconsistencies in the data may reflect differences in electrosurgical technique. Non-modulated (cutting) current concentrates energy, vaporizing the tissue with little heat injury to surrounding areas. Conversely, modulated (coagulating) current coagulates the tissue with heat-producing char over a large area, and tissue injury often extends beyond the char. This effect is magnified if the electrode comes in direct contact with the tissue. Use the arc, rather than direct contact, to prevent excessive devitalization of tissue.
This article will review the techniques for, as well as the rationale and disadvantages of, common incisions—both longitudinal and transverse—to help the gynecologic surgeon minimize morbidity and maximize outcomes.
Longitudinal incisions
The longitudinal incisions that will be reviewed here are the midline (median) and paramedian. Classically, it was thought that longitudinal incisions were at greater risk of dehiscence than transverse incisions.6 However, it is difficult to make legitimate comparisons since longitudinal incisions are more likely to be performed in cases of hemorrhage, trauma, sepsis, multiorgan disease, previous surgery, previous radiation therapy, and malignancy—all of which increase the likelihood of postoperative complications. Furthermore, prospective and randomized studies have shown little, if any, difference in the incidence of dehiscence and hernias between longitudinal and transverse incisions.6-8
MidlineTechnique. Initiate the midline as a low abdominal incision (approximately 2 cm above the pubic symphysis), cutting along the linea alba. To extend the incision, if necessary, continue the dissection to the left of the umbilicus to avoid the ligamentum teres. Open the peritoneum at the cephalad pole of the incision (Figure 1). Expand this cut slightly off midline to avoid the urachus yet adequately expose the peritoneal cavity (Figure 2).
Rationale. While this incision can be used for any surgical procedure, it is especially appropriate when the diagnosis is uncertain and the exact procedure or extent of surgery is unclear. It is an excellent choice when access to the upper abdomen may be necessary, e.g., patients with gynecologic malignancies who may need assessment of the diaphragm, liver biopsy, para-aortic node biopsy, omentectomy, or debulking procedures. Patients with benign gynecologic conditions also may benefit from a midline incision. For example, when pelvic anatomy is distorted, as with severe endometriosis or sepsis, recognizable anatomy may be found only above the pelvic brim.
A midline incision allows the quickest entry, which is especially important for an unstable or seriously ill patient. Exposure is excellent, as all areas of the abdomen and retroperitoneum can be accessed with minimal risk of significant vascular or nerve injury. This is because only terminal branches of the abdominal wall blood vessels and nerves are located at the linea alba. In addition, because deep tissue planes are not opened, this incision may be ideal for patients who are anticoagulated, have enlarged epigastric vessels that are more susceptible to injury, or have an intraabdominal infection.
Transverse incisions help reduce the rate of wound dehiscence.
Disadvantages. Two potential problems are the higher rate of hernia formation and wound dehiscence, which may be due to constant lateral tension, compared with transverse incisions. In addition, coughing, retching, and straining may exacerbate the lateral tension. Proper suture selection can reduce the incidence of complications. As previously mentioned, the reality of these disadvantages is subject to debate; further studies are needed to determine the true risk.
From a cosmetic standpoint, the midline scar often is prominent because the incision transects Langer’s lines. Hence, it cannot be concealed by lingerie or swimwear.
FIGURE 1 Midline incision
After incising the linea alba and separating the muscles in the midline, open the peritoneum at the cephalad pole of the incision.
FIGURE 2 Midline incision
Expand this cut slightly off midline to avoid the urachus yet adequately expose the peritoneal cavity.
ParamedianTechnique. Place this incision 2 to 5 cm to the left or right of the midline, depending on the indication for surgery. After dissecting the skin and subcutaneous layers, incise the anterior rectus sheath vertically over the rectus abdominis muscle (Figure 3) and dissect it from the medial muscle edge. Retract the muscle laterally to expose the posterior rectus sheath and then incise the sheath and the peritoneum vertically to expose the peritoneal cavity (Figure 4). Alternatively, a more lateral paramedian incision can be placed over the rectus abdominis muscle. In this case, however, the rectus muscle should be separated vertically, instead of retracted laterally, to expose the posterior rectus sheath.
Rationale. Paramedian incisions provide excellent exposure of the pelvis, excluding the upper abdomen. Therefore, consider this technique when the procedure will be confined to the ipsilateral pelvis, e.g., rightor left-side lymph node biopsies and exposure of the sigmoid colon on the left. Also, a paramedian incision at the level of the umbilicus can be used for a cesarean delivery or a hysterectomy.
This incision is the best choice when performing pelvic surgery on morbidly obese patients. By placing the incision low on the abdomen, the large panniculus can be retracted over the mons pubis and thighs, providing excellent exposure of the pelvis.
Disadvantages. Some researchers have reported that muscle-splitting lateral paramedian incisions have a lower incidence of incisional hernias compared with midline incisions.7,9 However, they take longer to perform and restrict access to the contralateral pelvis. In addition, the risk of vascular injury and hematomas is increased, especially in the lower pole where branches of the epigastric arteries penetrate the muscle.
With regard to nerve injury, terminal nerves are cut, resulting in paralysis of the inner portion of the rectus abdominis muscle. This paralysis can be permanent, as the muscle medial to the vertical separation is involved. However, if only the medial third of the muscle is denervated, the paralysis rarely limits function.
FIGURE 3 Paramedian incision
Place the incision 2 to 5 cm to the left or right of the midline. Incise the anterior rectus sheath vertically over the rectus abdominis muscle.
FIGURE 4 Paramedian incision
Retract the muscle laterally to expose the posterior sheath; then incise the sheath and the peritoneum vertically to expose the pelvic cavity.
Transverse incisions
Transverse incisions such as Pfannenstiel’s (muscle separating), Cherney’s (tendon detaching), and Maylard’s (muscle cutting) were developed to reduce the incidence of incisional hernias and wound dehiscence. Their success lies in the fact that they cause less tension on the opposing wound edges because the incisions follow Langer’s lines, unlike longitudinal incisions. Their placement also allows for a better cosmetic outcome. Because they can be placed in the pubic hairline or in a natural skin crease, they are easily concealed by lingerie or swimwear. However, the incision should not be placed in a deep skin fold of a large panniculus where maceration of the skin can increase the risk of infection.
The main disadvantages of transverse incisions are limited exposure of the upper abdomen and limited extensibility. Further, because more tissue planes are opened and more vessels are encountered, these incisions increase the risk of hematomas and infection.
Pfannenstiel’sTechnique. Incise the skin and subcutaneous tissues 2 to 5 cm above the pubic symphysis.10 Then dissect the rectus sheath transversely, separating it sharply from the rectus muscle at the linea alba and retracting it superiorly toward the umbilicus and inferiorly toward the pubic symphysis (Figure 5). Divide the rectus abdominis muscle along the midline raphe and retract it laterally, exposing the transversalis fascia and the posterior rectus sheath. Incise these layers and the peritoneum vertically to expose the peritoneal cavity (Figure 6).
To minimize retraction of the rectus abdominus muscle during Maylard’s, do not separate it from the anterior rectus sheath.
Rationale. Since exposure is limited and the incision can be only minimally extended, this incision is appropriate for procedures that are limited to the pelvis, e.g., abdominal hysterectomy, cesarean delivery, retropubic urethropexy, and paravaginal defect repairs. (Extension of the incision can be achieved by modifying it to Cherney’s incision.)
Disadvantages. The fact that the incision severs multiple tissue planes is an advantage as well as a disadvantage. Postoperative dehiscence and incisional hernias are rare because the closed wound has a high tensile strength. However, the incidence of inguinal hernias may increase when the incision is placed close to the external inguinal ring,11 and very low incisions may increase the risk of femoral nerve injury.12 The ilioinguinal and iliohypogastric nerves may be damaged, especially if the rectus incision is extended far laterally. Most commonly, they are trapped in the suture during closure, producing postoperative pain. Incising multiple layers also slows entry and increases the risk of seromas, hematomas, and wound infections.
This approach is contraindicated when time is of the essence, e.g., hemorrhage, or in the face of an abdominal infection, e.g., sepsis.
FIGURE 5 Pfannenstiel’s incision
After incising the skin and subcutaneous tissues, dissect the rectus sheath transversely, separating it sharply from the rectus muscle.
FIGURE 6 Pfannenstiel’s incision
Divide and retract the rectus abdominus muscle and then incise the tranversalis fascia and posterior rectus sheath vertically.
Cherney’sTechnique. Dissect the skin and subcutaneous tissue 2 to 3 cm above the pubic symphysis, which is lower than most Pfannenstiel’s incisions. Then incise the anterior rectus sheath transversely and dissect it from the rectus abdominis muscle superiorly and inferiorly. Using blunt dissection, separate the rectus and pyramidalis muscles from the underlying bladder and adventitial tissue. Then incise transversely the tendons of the rectus and pyramidalis muscles 0.5 cm above the pubic symphysis (Figure 7). (This segment of tendon facilitates the reattachment of the muscles at closure.) Retract the muscles and tendons cephalad to expose the retropubic space. Incise the peritoneum transversely to expose the peritoneal cavity (Figure 8).
Rationale. Cherney’s incision provides excellent access to the retropubic space of Retzius, making it a good choice for retropubic urethropexy and paravaginal repair. Exposure is the main advantage to Cherney’s incision. The reason: It is a modification of Pfannenstiel’s incision and, as such, provides excellent lateral exposure because it is placed much lower on the abdominal wall.
Disadvantages. The benefits and risks of this incision are similar to those of Pfannenstiel’s incision since the same tissue planes are opened. However, it is more time consuming due to the dissection required to separate the muscles and tendons from the underlying tissues.
In addition, the deep inferior epigastric arteries could be injured as a result of the lateral dissection. Lastly, reattachment of the tendons is tedious.
FIGURE 7 Cherney’s incision
Incise transversely the tendons of the rectus and pyramidalis muscles 0.5 cm above the pubic symphysis.
FIGURE 8 Cherney’s incision
Retract the muscles and tendons cephalad and incise the peritoneum transversely to expose the peritoneal cavity.
Maylard’sTechnique. After transecting the skin and subcutaneous layers in a plane at the level of the anterior superior iliac spine, incise the rectus sheath transversely and extend the incision through the aponeuroses of the abdominal muscles to about 2 to 3 cm medial to the anterior iliac crest. Then dissect the rectus abdominis muscle transversely with a scalpel, electrocautery, or surgical stapler (Figure 9). To minimize retraction of this muscle, do not separate it from the anterior rectus sheath. (Alternatively, many surgeons recommend that the cut edge of the muscle be secured to the anterior sheath with mattress sutures to prevent retraction and reduce blood loss.)
Prior to transecting the peritoneum, isolate, ligate, and divide the deep inferior epigastric vessels. (Patients with significant aortoiliac atherosclerosis or aortic coarctation develop considerable collateral circulation through the epigastric vessels for perfusion of the lower extremities. Ligating these vessels may cause claudication and even lifethreatening ischemia. Therefore, assess iliac flow before ligating and transecting the arteries.) Then transect the transversalis fascia and peritoneum transversely, allowing access to the peritoneal cavity (Figure 10).
Rationale. Many oncologists use this approach for pelvic lymphadenectomy and staging procedures because the exposure to the lateral pelvis is excellent. The reason: This transverse incision cuts through all layers of the abdominal wall at the level of the anterior iliac spine. It also may be appropriate when pelvic pathology extends to the sidewall, e.g., endometriosis.
It is advisable to place a drain prior to closing Maylard’s.
Physicians should consider using this approach more than they currently are, especially when Pfannenstiel’s incision cannot provide satisfactory exposure or a longitudinal incision is thought to be the only alternative. The decision to use Maylard’s incision should be planned during the preoperative assessment, as Pfannenstiel’s incision should not be modified to Maylard’s intraoperatively.
Disadvantages. Although Maylard’s incision improves access to the upper abdomen compared to other transverse incisions, exposure is still limited. In addition, trauma to the deep epigastric arteries may result in considerable hemorrhage. And since hematomas commonly infiltrate the retroperitoneal space, large quantities of blood already may be lost before the hemorrhage is clinically apparent. Finally, oozing from the cut edge of the muscles may result in significant fluid collection, increasing the risk of infection. Therefore, it is advisable to place a drain prior to closure.
Like other transverse incisions, the wound parallels the nerves, providing a measure of protection. However, the rectus incision approaches the anterior iliac spine, where both the ilioinguinal and iliohypogastric nerves lie. Both may be damaged during the incision and can become entrapped during closure. Additionally, the femoral nerve, the lateral femoral cutaneous nerve, and the genitofemoral nerve are easily compressed by the lateral blades of the retractors because of the extreme lateral positions of the incision’s poles. Injury to these nerves can result in paresthesia, pain, and paralysis of the leg muscles.
FIGURE 9 Maylard’s incision
After incising the aponeuroses of the abdominal muscles medial to the anterior iliac crest, dissect the rectus abdominus muscle transversely.
FIGURE 10 Maylard’s incision
Isolate, ligate, and divide the epigastric vessels. Then incise the transversalis fascia and peritoneum transversely to access the peritoneal cavity.
In summary
While the midline and Pfannenstiel’s incisions are an integral part of the gynecologic surgeon’s armamentarium, the paramedian and Maylard’s incisions should be added to the repertoire because these incisions can greatly enhance exposure. The paramedian shares the advantages of the midline incision but also allows better access to the ipsilateral pelvis. Furthermore, although Maylard’s incision takes longer to perform than other techniques, the excellent exposure—both laterally and superiorly—makes it worthwhile.
The authors report no financial relationship with any companies whose products are mentioned in this article.
- The incision should be considered as a second surgical procedure, which temporarily interferes with normal abdominal wall function.
- The midline incision provides excellent exposure to all areas of the abdomen and retroperitoneum, which can be accessed with minimal risk of significant vascular or nerve injury.
- Transverse incisions create less tension on the opposing skin edges because the incision follows Langer’s lines. The incidence of incisional hernias and wound dehiscence has been reported to be lower, but these studies are not randomized.
An abdominal incision often is given little thought other than as an access site through which a surgical procedure is performed. In reality, the incision is a second surgical procedure, which interferes—at least temporarily—with normal abdominal wall function.
While most physicians concur that the essential elements of a well-planned incision include adequate access to anticipated pathology, extensibility, and security of closure, many may not consider preservation of abdominal wall function as a key factor in their decision-making. Additional considerations include certainty of diagnosis, speed of entry, body habitus, presence of previous scars, potential for problems with hemostasis, and cosmetic outcome. These factors are the key determinants of whether the incision will be longitudinal (midline or paramedian) or transverse (Pfannenstiel’s, Cherney’s, or Maylard’s). For most gynecologic procedures confined to the pelvis, either option may be considered. The exceptions are patients with uncertain diagnoses or when access to the upper abdomen is indicated.
Regardless of the type of incision selected, the skin should be incised with a single, clean stroke of a sharp scalpel. However, when it comes to dissecting the underlying subcutaneous tissues, the debate continues over whether a scalpel or electrosurgery is best. While I recently have switched to the latter, here is a look at what the data say: Johnson and Serpell demonstrated that electrosurgery is associated with faster hemostasis, with no difference in the incidence of wound infection.1 Similarly, a recent randomized trial by Kearns et al found electro-surgery causes less blood loss and does not increase the risk of wound infections or fascial dehiscence.2 In contrast, a large prospective study by Cruse et al suggested that the use of diathermy is associated with twice the wound infection rate.3
This controversy also involves patients with gynecologic malignancies. Kolb et al found that electrosurgery was an independent risk factor for wound complications following surgery for ovarian cancer.4 However, Franchi and colleagues reported no difference in the rate of wound complications between scalpel and diathermy in patients who underwent mid-line abdominal incisions for the treatment of uterine cancer.5
Use the midline when the diagnosis and the extent of surgery are uncertain.
The inconsistencies in the data may reflect differences in electrosurgical technique. Non-modulated (cutting) current concentrates energy, vaporizing the tissue with little heat injury to surrounding areas. Conversely, modulated (coagulating) current coagulates the tissue with heat-producing char over a large area, and tissue injury often extends beyond the char. This effect is magnified if the electrode comes in direct contact with the tissue. Use the arc, rather than direct contact, to prevent excessive devitalization of tissue.
This article will review the techniques for, as well as the rationale and disadvantages of, common incisions—both longitudinal and transverse—to help the gynecologic surgeon minimize morbidity and maximize outcomes.
Longitudinal incisions
The longitudinal incisions that will be reviewed here are the midline (median) and paramedian. Classically, it was thought that longitudinal incisions were at greater risk of dehiscence than transverse incisions.6 However, it is difficult to make legitimate comparisons since longitudinal incisions are more likely to be performed in cases of hemorrhage, trauma, sepsis, multiorgan disease, previous surgery, previous radiation therapy, and malignancy—all of which increase the likelihood of postoperative complications. Furthermore, prospective and randomized studies have shown little, if any, difference in the incidence of dehiscence and hernias between longitudinal and transverse incisions.6-8
MidlineTechnique. Initiate the midline as a low abdominal incision (approximately 2 cm above the pubic symphysis), cutting along the linea alba. To extend the incision, if necessary, continue the dissection to the left of the umbilicus to avoid the ligamentum teres. Open the peritoneum at the cephalad pole of the incision (Figure 1). Expand this cut slightly off midline to avoid the urachus yet adequately expose the peritoneal cavity (Figure 2).
Rationale. While this incision can be used for any surgical procedure, it is especially appropriate when the diagnosis is uncertain and the exact procedure or extent of surgery is unclear. It is an excellent choice when access to the upper abdomen may be necessary, e.g., patients with gynecologic malignancies who may need assessment of the diaphragm, liver biopsy, para-aortic node biopsy, omentectomy, or debulking procedures. Patients with benign gynecologic conditions also may benefit from a midline incision. For example, when pelvic anatomy is distorted, as with severe endometriosis or sepsis, recognizable anatomy may be found only above the pelvic brim.
A midline incision allows the quickest entry, which is especially important for an unstable or seriously ill patient. Exposure is excellent, as all areas of the abdomen and retroperitoneum can be accessed with minimal risk of significant vascular or nerve injury. This is because only terminal branches of the abdominal wall blood vessels and nerves are located at the linea alba. In addition, because deep tissue planes are not opened, this incision may be ideal for patients who are anticoagulated, have enlarged epigastric vessels that are more susceptible to injury, or have an intraabdominal infection.
Transverse incisions help reduce the rate of wound dehiscence.
Disadvantages. Two potential problems are the higher rate of hernia formation and wound dehiscence, which may be due to constant lateral tension, compared with transverse incisions. In addition, coughing, retching, and straining may exacerbate the lateral tension. Proper suture selection can reduce the incidence of complications. As previously mentioned, the reality of these disadvantages is subject to debate; further studies are needed to determine the true risk.
From a cosmetic standpoint, the midline scar often is prominent because the incision transects Langer’s lines. Hence, it cannot be concealed by lingerie or swimwear.
FIGURE 1 Midline incision
After incising the linea alba and separating the muscles in the midline, open the peritoneum at the cephalad pole of the incision.
FIGURE 2 Midline incision
Expand this cut slightly off midline to avoid the urachus yet adequately expose the peritoneal cavity.
ParamedianTechnique. Place this incision 2 to 5 cm to the left or right of the midline, depending on the indication for surgery. After dissecting the skin and subcutaneous layers, incise the anterior rectus sheath vertically over the rectus abdominis muscle (Figure 3) and dissect it from the medial muscle edge. Retract the muscle laterally to expose the posterior rectus sheath and then incise the sheath and the peritoneum vertically to expose the peritoneal cavity (Figure 4). Alternatively, a more lateral paramedian incision can be placed over the rectus abdominis muscle. In this case, however, the rectus muscle should be separated vertically, instead of retracted laterally, to expose the posterior rectus sheath.
Rationale. Paramedian incisions provide excellent exposure of the pelvis, excluding the upper abdomen. Therefore, consider this technique when the procedure will be confined to the ipsilateral pelvis, e.g., rightor left-side lymph node biopsies and exposure of the sigmoid colon on the left. Also, a paramedian incision at the level of the umbilicus can be used for a cesarean delivery or a hysterectomy.
This incision is the best choice when performing pelvic surgery on morbidly obese patients. By placing the incision low on the abdomen, the large panniculus can be retracted over the mons pubis and thighs, providing excellent exposure of the pelvis.
Disadvantages. Some researchers have reported that muscle-splitting lateral paramedian incisions have a lower incidence of incisional hernias compared with midline incisions.7,9 However, they take longer to perform and restrict access to the contralateral pelvis. In addition, the risk of vascular injury and hematomas is increased, especially in the lower pole where branches of the epigastric arteries penetrate the muscle.
With regard to nerve injury, terminal nerves are cut, resulting in paralysis of the inner portion of the rectus abdominis muscle. This paralysis can be permanent, as the muscle medial to the vertical separation is involved. However, if only the medial third of the muscle is denervated, the paralysis rarely limits function.
FIGURE 3 Paramedian incision
Place the incision 2 to 5 cm to the left or right of the midline. Incise the anterior rectus sheath vertically over the rectus abdominis muscle.
FIGURE 4 Paramedian incision
Retract the muscle laterally to expose the posterior sheath; then incise the sheath and the peritoneum vertically to expose the pelvic cavity.
Transverse incisions
Transverse incisions such as Pfannenstiel’s (muscle separating), Cherney’s (tendon detaching), and Maylard’s (muscle cutting) were developed to reduce the incidence of incisional hernias and wound dehiscence. Their success lies in the fact that they cause less tension on the opposing wound edges because the incisions follow Langer’s lines, unlike longitudinal incisions. Their placement also allows for a better cosmetic outcome. Because they can be placed in the pubic hairline or in a natural skin crease, they are easily concealed by lingerie or swimwear. However, the incision should not be placed in a deep skin fold of a large panniculus where maceration of the skin can increase the risk of infection.
The main disadvantages of transverse incisions are limited exposure of the upper abdomen and limited extensibility. Further, because more tissue planes are opened and more vessels are encountered, these incisions increase the risk of hematomas and infection.
Pfannenstiel’sTechnique. Incise the skin and subcutaneous tissues 2 to 5 cm above the pubic symphysis.10 Then dissect the rectus sheath transversely, separating it sharply from the rectus muscle at the linea alba and retracting it superiorly toward the umbilicus and inferiorly toward the pubic symphysis (Figure 5). Divide the rectus abdominis muscle along the midline raphe and retract it laterally, exposing the transversalis fascia and the posterior rectus sheath. Incise these layers and the peritoneum vertically to expose the peritoneal cavity (Figure 6).
To minimize retraction of the rectus abdominus muscle during Maylard’s, do not separate it from the anterior rectus sheath.
Rationale. Since exposure is limited and the incision can be only minimally extended, this incision is appropriate for procedures that are limited to the pelvis, e.g., abdominal hysterectomy, cesarean delivery, retropubic urethropexy, and paravaginal defect repairs. (Extension of the incision can be achieved by modifying it to Cherney’s incision.)
Disadvantages. The fact that the incision severs multiple tissue planes is an advantage as well as a disadvantage. Postoperative dehiscence and incisional hernias are rare because the closed wound has a high tensile strength. However, the incidence of inguinal hernias may increase when the incision is placed close to the external inguinal ring,11 and very low incisions may increase the risk of femoral nerve injury.12 The ilioinguinal and iliohypogastric nerves may be damaged, especially if the rectus incision is extended far laterally. Most commonly, they are trapped in the suture during closure, producing postoperative pain. Incising multiple layers also slows entry and increases the risk of seromas, hematomas, and wound infections.
This approach is contraindicated when time is of the essence, e.g., hemorrhage, or in the face of an abdominal infection, e.g., sepsis.
FIGURE 5 Pfannenstiel’s incision
After incising the skin and subcutaneous tissues, dissect the rectus sheath transversely, separating it sharply from the rectus muscle.
FIGURE 6 Pfannenstiel’s incision
Divide and retract the rectus abdominus muscle and then incise the tranversalis fascia and posterior rectus sheath vertically.
Cherney’sTechnique. Dissect the skin and subcutaneous tissue 2 to 3 cm above the pubic symphysis, which is lower than most Pfannenstiel’s incisions. Then incise the anterior rectus sheath transversely and dissect it from the rectus abdominis muscle superiorly and inferiorly. Using blunt dissection, separate the rectus and pyramidalis muscles from the underlying bladder and adventitial tissue. Then incise transversely the tendons of the rectus and pyramidalis muscles 0.5 cm above the pubic symphysis (Figure 7). (This segment of tendon facilitates the reattachment of the muscles at closure.) Retract the muscles and tendons cephalad to expose the retropubic space. Incise the peritoneum transversely to expose the peritoneal cavity (Figure 8).
Rationale. Cherney’s incision provides excellent access to the retropubic space of Retzius, making it a good choice for retropubic urethropexy and paravaginal repair. Exposure is the main advantage to Cherney’s incision. The reason: It is a modification of Pfannenstiel’s incision and, as such, provides excellent lateral exposure because it is placed much lower on the abdominal wall.
Disadvantages. The benefits and risks of this incision are similar to those of Pfannenstiel’s incision since the same tissue planes are opened. However, it is more time consuming due to the dissection required to separate the muscles and tendons from the underlying tissues.
In addition, the deep inferior epigastric arteries could be injured as a result of the lateral dissection. Lastly, reattachment of the tendons is tedious.
FIGURE 7 Cherney’s incision
Incise transversely the tendons of the rectus and pyramidalis muscles 0.5 cm above the pubic symphysis.
FIGURE 8 Cherney’s incision
Retract the muscles and tendons cephalad and incise the peritoneum transversely to expose the peritoneal cavity.
Maylard’sTechnique. After transecting the skin and subcutaneous layers in a plane at the level of the anterior superior iliac spine, incise the rectus sheath transversely and extend the incision through the aponeuroses of the abdominal muscles to about 2 to 3 cm medial to the anterior iliac crest. Then dissect the rectus abdominis muscle transversely with a scalpel, electrocautery, or surgical stapler (Figure 9). To minimize retraction of this muscle, do not separate it from the anterior rectus sheath. (Alternatively, many surgeons recommend that the cut edge of the muscle be secured to the anterior sheath with mattress sutures to prevent retraction and reduce blood loss.)
Prior to transecting the peritoneum, isolate, ligate, and divide the deep inferior epigastric vessels. (Patients with significant aortoiliac atherosclerosis or aortic coarctation develop considerable collateral circulation through the epigastric vessels for perfusion of the lower extremities. Ligating these vessels may cause claudication and even lifethreatening ischemia. Therefore, assess iliac flow before ligating and transecting the arteries.) Then transect the transversalis fascia and peritoneum transversely, allowing access to the peritoneal cavity (Figure 10).
Rationale. Many oncologists use this approach for pelvic lymphadenectomy and staging procedures because the exposure to the lateral pelvis is excellent. The reason: This transverse incision cuts through all layers of the abdominal wall at the level of the anterior iliac spine. It also may be appropriate when pelvic pathology extends to the sidewall, e.g., endometriosis.
It is advisable to place a drain prior to closing Maylard’s.
Physicians should consider using this approach more than they currently are, especially when Pfannenstiel’s incision cannot provide satisfactory exposure or a longitudinal incision is thought to be the only alternative. The decision to use Maylard’s incision should be planned during the preoperative assessment, as Pfannenstiel’s incision should not be modified to Maylard’s intraoperatively.
Disadvantages. Although Maylard’s incision improves access to the upper abdomen compared to other transverse incisions, exposure is still limited. In addition, trauma to the deep epigastric arteries may result in considerable hemorrhage. And since hematomas commonly infiltrate the retroperitoneal space, large quantities of blood already may be lost before the hemorrhage is clinically apparent. Finally, oozing from the cut edge of the muscles may result in significant fluid collection, increasing the risk of infection. Therefore, it is advisable to place a drain prior to closure.
Like other transverse incisions, the wound parallels the nerves, providing a measure of protection. However, the rectus incision approaches the anterior iliac spine, where both the ilioinguinal and iliohypogastric nerves lie. Both may be damaged during the incision and can become entrapped during closure. Additionally, the femoral nerve, the lateral femoral cutaneous nerve, and the genitofemoral nerve are easily compressed by the lateral blades of the retractors because of the extreme lateral positions of the incision’s poles. Injury to these nerves can result in paresthesia, pain, and paralysis of the leg muscles.
FIGURE 9 Maylard’s incision
After incising the aponeuroses of the abdominal muscles medial to the anterior iliac crest, dissect the rectus abdominus muscle transversely.
FIGURE 10 Maylard’s incision
Isolate, ligate, and divide the epigastric vessels. Then incise the transversalis fascia and peritoneum transversely to access the peritoneal cavity.
In summary
While the midline and Pfannenstiel’s incisions are an integral part of the gynecologic surgeon’s armamentarium, the paramedian and Maylard’s incisions should be added to the repertoire because these incisions can greatly enhance exposure. The paramedian shares the advantages of the midline incision but also allows better access to the ipsilateral pelvis. Furthermore, although Maylard’s incision takes longer to perform than other techniques, the excellent exposure—both laterally and superiorly—makes it worthwhile.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Johnson CD, Serpell JW. Wound infection after abdominal incision with scalpel or diathermy. Br J Surg. 1990;77:626-627.
2. Cruse PJE, Ford R. The epidemiology of wound infection: a 10-year prospective study of 62,939 wounds. Surg Clin North Am. 1980;60:27.-
3. Kearns SR, Connolly EM, McNally S, McNamara DA, Deasy J. Randomized clinical trial of diathermy versus scalpel incision in elective midline laparotomy. Br J Surg. 2001;88(1):41-44.
4. Kolb BA, Buller RE, Connoer JP, et al. Effects of early postoperative chemotherapy on wound healing. Obstet Gynecol. 1992;79:988-992.
5. Franchi M, Ghezzi F, Bendetti-Panici PL, et al. A multicenter collaborative study on the use of cold scalpel and electrocautery for midline abdominal incision. Am J Surg. 2001;181(2):128-132.
6. Hendrix SL, Schimp V, Martin J. The legendary superior strength of the Pfannenstiel incision: a myth? Am J Obstet Gynecol. 2000;182(6):1446-1451.
7. Ellis H, Coleridge-Smith PD, Joyce AD. Abdominal incisions—vertical or transverse? Postgrad Med J. 1984;60:407-410.
8. Sanders RJ, DiClementi D. Principles of abdominal wound closure. II. Prevention of wound dehiscence. Arch Surg. 1977;112:1188.-
9. Donaldson DR, Hegarty JH, Brennan TG, et al. The lateral paramedian incision-experience with 850 cases. Br J Surg. 1982;69:630.-
10. Greenall MJ, et al. Midline or transverse laparotomy? A random controlled clinical trial. Part I: Influence on healing. Br J Surg. 1980;7:188.-
11. Pfannenstiel J. Ueber die Vortheile des suprasymphysaren Fascienquerschnitts für die gynakologischen Koliotomien zugleich ein Beitrag zu der Indikationsstellung der Operationswege. Samml Klin Vortr (Leipzig). 1900;268:1735.-
12. Griffiths DA. A reappraisal of the Pfannenstiel incision. Br J Urol. 1976;48:469.-
1. Johnson CD, Serpell JW. Wound infection after abdominal incision with scalpel or diathermy. Br J Surg. 1990;77:626-627.
2. Cruse PJE, Ford R. The epidemiology of wound infection: a 10-year prospective study of 62,939 wounds. Surg Clin North Am. 1980;60:27.-
3. Kearns SR, Connolly EM, McNally S, McNamara DA, Deasy J. Randomized clinical trial of diathermy versus scalpel incision in elective midline laparotomy. Br J Surg. 2001;88(1):41-44.
4. Kolb BA, Buller RE, Connoer JP, et al. Effects of early postoperative chemotherapy on wound healing. Obstet Gynecol. 1992;79:988-992.
5. Franchi M, Ghezzi F, Bendetti-Panici PL, et al. A multicenter collaborative study on the use of cold scalpel and electrocautery for midline abdominal incision. Am J Surg. 2001;181(2):128-132.
6. Hendrix SL, Schimp V, Martin J. The legendary superior strength of the Pfannenstiel incision: a myth? Am J Obstet Gynecol. 2000;182(6):1446-1451.
7. Ellis H, Coleridge-Smith PD, Joyce AD. Abdominal incisions—vertical or transverse? Postgrad Med J. 1984;60:407-410.
8. Sanders RJ, DiClementi D. Principles of abdominal wound closure. II. Prevention of wound dehiscence. Arch Surg. 1977;112:1188.-
9. Donaldson DR, Hegarty JH, Brennan TG, et al. The lateral paramedian incision-experience with 850 cases. Br J Surg. 1982;69:630.-
10. Greenall MJ, et al. Midline or transverse laparotomy? A random controlled clinical trial. Part I: Influence on healing. Br J Surg. 1980;7:188.-
11. Pfannenstiel J. Ueber die Vortheile des suprasymphysaren Fascienquerschnitts für die gynakologischen Koliotomien zugleich ein Beitrag zu der Indikationsstellung der Operationswege. Samml Klin Vortr (Leipzig). 1900;268:1735.-
12. Griffiths DA. A reappraisal of the Pfannenstiel incision. Br J Urol. 1976;48:469.-
Endometrial ablation: a look at the newest global procedures
- Global ablation methods involve destroying the endometrium using specialized devices that do not require an operative hysteroscope or resectoscope.
- The endometrium should be destroyed or resected to the level of the basalis, which is approximately 4 to 6 mm deep.
- In cryoablation, freezing the tissue causes less pain than the heat energy associated with other ablation devices. The procedure typically takes 10 to 20 minutes.
- Not only is bipolar desiccation quick and simple, no endometrial pretreatment is required because the system allows for consistent depths of ablation regardless of endometrial thickness.
- The advantage of hydrothermal ablation is that the circulating hot saline solution contacts the entire endometrial surface regardless of the shape or size of the cavity.
Traditionally, physicians have preferred hysterectomy for the treatment of abnormal uterine bleeding not related to endometrial cancer, representing about 20% of the 590,000 hysterectomie performed annually in the United States.1 The advent of standard endometrial ablation, e.g., surgical resection and rollerball desiccation, offered women less radical alternatives to hysterectomy. However, these “classic” methods are considered by some physicians to be technically difficult because they require the use of an operative hysteroscope—and all of its attendant risks. As a result, many Ob/Gyns continued to opt for hysterectomy when given a choice.
Now there are simpler alternatives: global ablation methods, including the newest—cryoablation, bipolar desiccation, and hydrothermal ablation. These procedures involve destroying the endometrium using specialized devices that do not require an operative hysteroscope or resectoscope. The advantage of these methods is that they are simple, rapid procedures that are easier to perform than standard endometrial ablation. Many surgeons who were uncomfortable utilizing the standard resectoscope can offer this treatment option to women with menorrhagia. In fact, it generally takes clinicians only 5 to 10 procedures to become proficient in endometrial ablation.
While global ablation is not intended to replace hysterectomy—the definitive treatment for most uterine pathology2—it gives patients a choice. Women who want permanent cessation of menses can choose hys-terectomy. Those who want to preserve the uterus or desire an outpatient procedure with minimal morbidity may opt for endometrial ablation. Regardless of the method they choose, patients who participate in the deci-sion-making process are more likely to be satisfied with their outcome.
The thinner the endometrial lining, the more likely ablation will be successful.
Part of this process includes an informed-consent discussion with the patient, including a review of each technique, its risks and complications, and outcomes. The long-term consequences of endometrial ablation remain unknown. The reason: Follow-up data beyond 24 months are minimal. And another question remains unanswered: How many women who have undergone endometrial ablation will develop endometrial carcinoma without symptomatic bleeding? There has been at least 1 report of this phenomenon occurring in a woman who ultimately underwent a hysterectomy for other reasons.3 Other case reports have noted uterine bleeding as the presenting symptom of endometrial carcinoma in women who had undergone ablation.4-6 The bottom line: The risk of asymptomatic endometrial cancer appears to be small in properly selected patients, i.e.,women without a history of endometrial hyperplasia or carcinoma (Table 1).
TABLE 1
Patient selection
INDICATIONS
|
CONTRAINDICATIONS
|
Preparation
Prior to performing any endometrial ablation, obtain an endometrial sample to rule out premalignant or malignant disease. Also, assess the endometrial cavity using either office hysteroscopy or sonohysterography to exclude the possibility of submucous myomata or polyps, which can be treated with simple resection. These imaging techniques also can reveal abnormally shaped uterine cavities, thus eliminating certain women as candidates for global techniques such as balloon ablation.
For all methods, destroy or resect the endometrium to the level of the basalis, which is approximately 4 to 6 mm deep, depending upon the stage of the menstrual cycle or cycle suppression. It is reasonable to assume that the thinner the endometrial lining, the more likely ablation will be successful. Several methods of producing endometrial atrophy have been used, including dilatation and curettage (D&C) and hormonal suppression with medroxyprogesterone acetate, oral contraceptives (OCs), danocrine, or gonadotropin releasing hormone ago-nists (GnRH-a). I prefer to use leuprolide acetate (7.5 mg for 1 dose) 4 to 5 weeks prior to performing cryoablation or hydrothermal ablation. (Pretreatment of the endometrial lining is not necessary with bipolar desiccation.)
Many patients who undergo endometrial ablation via either standard or global techniques can tolerate the procedure well with local anesthesia and intravenous sedation. This is especially true for global procedures because they are of short duration, i.e., about 5 to 20 minutes, depending on the method. Much of the pain results from cervical dilation and manipulation, as well as trauma to the endometrium. Therefore, the global ablation techniques that utilize the smallest probe (5.5 mm or smaller) and freeze the uterus, i.e., cryoablation, require the least anesthesia.
After the patient has been properly selected and prepared, proceed with one the global techniques—cryoablation, bipolar desiccation, or hydrothermal ablation—outlined in this article.
Cryoablation
Technique. Dilate the cervix and insert a 5.5-mm cryoprobe into the uterine cavity. Cool the probe to less than-80 degrees Celsius by liquid differential gas exchange, described by Joule-Thompson, utilizing the cooling unit (Her Option; CryoGen, San Diego, Calif). An elliptical ice ball approximately 3.5 by 5 cm will then form around the probe.
Using abdominal ultrasound guidance, follow the edge of the ice ball into the uterine muscle at 1 cornu. (While the edge of the ice ball reaches 0 degrees Celsius, which is nondestructive to surrounding tissue, the endometrium is permanently destroyed approximately 1.5 cm from the edge of the ice ball, where a temperature of-20 degrees Celsius is reached.) Before the outer edge of the ice ball approaches the serosa of the uterus, stop the procedure (Figure 1). Heat the probe to body temperature and remove it from the cornu. Repeat the process in the contralateral cornu (Figure 2) and, in large uteri (greater than 10 cm sound), in the lower uterine segment (Figure 3). Each freeze cycle takes about 5 to 6 minutes.
FIGURE 1
Using ultrasound guidance, follow the edge of the ice ball into the uterine muscle at 1 cornu. Stop before the edge of the ice ball approaches the serosa.
FIGURE 2
Heat the probe to body temperature, remove it from the first cornu, and repeat the process in the contralateral cornu.
FIGURE 3
For large uteri (greater than 10 cm sound), perform another freeze cycle in the lower uterine segment. Each cycle takes about 5 to 6 minutes.The number of ice balls needed to destroy the entire uterine cavity and the length of time required to perform the procedure depend on the size of the cavity. In general, 2 to 3 ice balls are sufficient, and the entire procedure takes 10 to 20 minutes.
In a recent randomized multicenter study, researchers investigated whether a freeze cycle longer than recommended would result in better outcomes. At 12-month follow-up, 7 (64%) of the 12 patients who underwent treatments of 5 to 7 minutes rather than 4 minutes were amenorrheic/spotting, with a 91% success rate.7 Larger studies are underway to confirm these data. Further, repeat treatment can contribute to better outcomes, especially when the patient receives leuprolide acetate prior to the procedure.8
Advantages. The primary advantage of cryoab-lation is that it is not a totally blind procedure. The visual feedback provided by the ultrasound facilitates complete ablation of the entire uterine cavity, regardless of size. Further, while the hysteroscope used in standard ablation allows the physician to see only the destruction of the surface epithelium, ultrasound enables the surgeon to visualize the depth of treatment during cryoablation.
Another advantage: Freezing tissue causes less pain (cryoanesthesia) than the heat energy associated with other ablation devices.
Disadvantages. While ultrasound visualization has its benefits, it also can complicate matters because accurate imaging and interpretation require a certain level of skill. For Ob/Gyns not experienced in ultrasound, there is a potential for poor positioning of the probe, which could lead to bowel and bladder injuries. Further, physicians can mistakenly place the cryoprobe back into the treated cornu instead of the contralateral side, leading to ablation of the uterine serosa. Lastly, the technology involved in this procedure increases the cost of the equipment and disposable instruments.
What the evidence shows. According to an unpublished study conducted by Cryogen, 54% of 222 patients experienced amenorrhea or staining only at 6-month follow-up. Ninety-three percent of the patients had a Patient Bleeding Assessment Card (PBAC) score of less than 75, and 75% of the patients had a greater than 90% reduction in their PBAC scores. (A score of greater than 150 was needed to enter the study; the score is reached by assigning a value to the amount of staining on a validated pad and adding these points based on the number of pads used per cycle.)
Cost. The disposable cryoprobes are $1,250 per case, and the unit costs $29,995. Since most ablation procedures currently are performed in the operating room, ancillary costs of the techniques are similar.
Bipolar desiccation
Technique. After dilating the cervix, insert the 8-mm NovaSure System (Novacept, Palo Alto, Calif) catheter into the uterus. To rule out auterine perforation prior to beginning the procedure, insert carbon dioxide (CO2) into the cavity (Figure 4).The machine will not turn on if a seal of CO2 has not been created, unless the surgeon overrides the system.
Then expand the 3-dimensional gold-plated bipolar mesh electrode, which imitates the shape of the uterine cavity, out of the catheter. Gently push the probe toward the fundus so that the mesh electrode is accurately positioned within the uterine cavity (Figure 5). Once the electrode is activated with up to 180 W of bipolar power, gentle suction brings the endometrium into close contact with the mesh (Figure 6). This suction also removes debris and allows for more complete desiccation.
The entire bipolar desiccation procedure takes less than 5 minutes to perform.
The system shuts down automatically when complete desiccation (calculated at 50 ohms of resistance) has occurred. The average treatment time is just over 1 minute, and the average depth of ablation is 4 to 5 mm.9 Once ablation is complete, retract the mesh electrode and remove the catheter (Figure 7). The entire procedure takes less than 5 minutes.
Advantages. Not only is the technique quick and simple, no endometrial pretreatment is required because the system allows for consistent depths of ablation regardless of endometrial thickness.
Disadvantages. There are limited data on the 1-year success rates for this completely blind procedure. In addition, the uterus needs to be of normal shape to successfully ablate the endometrium.
What the evidence shows. Unpublished data from randomized, controlled clinical trials comparing bipolar desiccation with balloon ablation have demonstrated that the former achieves higher amenorrhea and success (a return to normal menstrual bleeding) rates than the latter. Further, patients treated with bipolar desiccation experienced less pain, both intra-operatively and postoperatively, than women who underwent balloon ablation.
Cost. Each disposable probe costs $850, and the bipolar controller lists for $15,000.
FIGURE 4
Dilate the cervix and insert the 8-mm catheter. To rule out a uterine perforation prior to beginning the procedure, place carbon dioxide in the cavity.
FIGURE 5
Expand the bipolar mesh electrode. Gently push the probe toward the fundus to accurately position the electrode within the uterine cavity.
FIGURE 6
Activate the electrode with up to 180 W of bipolar power. A gentle suction brings the endometrium into close contact with the mesh.
FIGURE 7
The system shuts down automatically when complete desiccation has occurred. Retract the bipolar mesh electrode and remove the catheter.
Hydrothermal ablation/circulating heated saline
Technique. Begin by dilating the cervix and inserting the sheath, which includes an 8-mm hysteroscope (Hydro ThermAblator; BEI Medical Systems, Teterboro, NJ), into the uter-ine cavity. Using gravity rather than a pump, introduce saline solution through the sheath into the uterus. As the saline circulates, gradually heat it until the solution reaches and maintains 90 degrees Celsius for 10 minutes, completely destroying the endometrium. (The total time from insertion of the sheath to completion of the procedure is 17 minutes.)
A safety feature detects whether any fluid has escaped from the closed system; a shutoff mechanism activates if more than 10 cc of fluid are lost. The system is controlled by software, so even physicians who are inexperienced with hysteroscopy can easily perform this technique.
Advantages. The primary advantage of hydrothermal ablation (HTA) is that the circulating hot saline solution contacts the entire endometrial surface regardless of the size or shape of the uterine cavity. In addition, since the procedure is performed under direct visualization, the surgeon can ensure that the entire cavity has been thoroughly ablated.
Disadvantages. One drawback is that it can be a painful procedure because the hysteroscope is large, necessitating wide dilation of the cervix, and hot water stimulates pain. Further, there have been unpublished reports of vaginal burns when the cervix created a poor seal around the sheath.
What the evidence shows. Based on their research of 276 women (187 women in the HTA group and 89 patients in the rollerball group), investigators have found that HTA has a treatment success rate of 77% compared to 82% for rollerball ablation. (The treatment success rate is defined as a PBAC score of 75 or lower; eumenorrhea is a score of less than 100.) Amenorrhea rates at 12-month follow-up were 40% and 51%, respectively. The researchers concluded that HTA offers an advantage over rollerball ablation in that it reduces anesthesia requirements and eliminates the problem of hypotonic fluid absorption.10
Cost. Disposable hysteroscopic tubing is $625 per case. The machine costs $10,900 plus $395 for the heater canister.
The author reports no financial relationship with any companies whose products are mentioned in this article.
1. Carlson KJ, Nichols DH, Schiff I. Indications for hysterectomy. N Engl J Med. 1993;Mar 25 328(12):856-860.
2. Carlson KJ, Miller BA, Fowler FJ, Jr. The Maine Women’s Health Study: I. Outcomes of hysterectomy. Obstet Gynecol. 1994;83-556.
3. Margolis MT, et al. Asymptomatic endometrial carcinoma after endometrial ablation. Int J Gynaecol Obstet. 1995;51-255.
4. Gimpelson RJ. Not so benign endometrial hyperplasia: endometrial cancer after endometrial ablation. J Am Assoc Gynecol Laparosc. 1997;4-507.
5. Horowitz IR, Copas PR, Aaronoff, et al. Endometrial adenocarcinoma following endometrial ablation for postmenopausal bleeding. Gynecol Oncol. 1995;56:460.-
6. Valle RF, Baggish MS. Endometrial carcinoma after endometrial ablation: high-risk factors predicting its occurrence. Am J Obstet Gynecol. 1998;179:569.-
7. Heppard M, et al. Data on 222 patients from a multicenter study using Cryogen First Option uterine cryoablation therapy in women with abnormal uterine bleeding. Obstet Gynecol. 2001;97(4 Suppl 1):S21.-
8. Sanders B. Preliminary outcomes of longer freeze cycles in the treatment of women with abnormal uterine bleeding using Cryogen First Option uterine cryoablation therapy. Obstet Gynecol. 2001;97(4 Suppl 1):S14.-
9. Cooper JM, Erickson ML. Global endometrial ablation technologies. Obstet Gynecol Clin North Am. 2000;27-385.
10. Corson SL. A multicenter evaluation of endometrial ablation by Hydro ThermAblator and rollerball for treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2001;8(3):359-367.
- Global ablation methods involve destroying the endometrium using specialized devices that do not require an operative hysteroscope or resectoscope.
- The endometrium should be destroyed or resected to the level of the basalis, which is approximately 4 to 6 mm deep.
- In cryoablation, freezing the tissue causes less pain than the heat energy associated with other ablation devices. The procedure typically takes 10 to 20 minutes.
- Not only is bipolar desiccation quick and simple, no endometrial pretreatment is required because the system allows for consistent depths of ablation regardless of endometrial thickness.
- The advantage of hydrothermal ablation is that the circulating hot saline solution contacts the entire endometrial surface regardless of the shape or size of the cavity.
Traditionally, physicians have preferred hysterectomy for the treatment of abnormal uterine bleeding not related to endometrial cancer, representing about 20% of the 590,000 hysterectomie performed annually in the United States.1 The advent of standard endometrial ablation, e.g., surgical resection and rollerball desiccation, offered women less radical alternatives to hysterectomy. However, these “classic” methods are considered by some physicians to be technically difficult because they require the use of an operative hysteroscope—and all of its attendant risks. As a result, many Ob/Gyns continued to opt for hysterectomy when given a choice.
Now there are simpler alternatives: global ablation methods, including the newest—cryoablation, bipolar desiccation, and hydrothermal ablation. These procedures involve destroying the endometrium using specialized devices that do not require an operative hysteroscope or resectoscope. The advantage of these methods is that they are simple, rapid procedures that are easier to perform than standard endometrial ablation. Many surgeons who were uncomfortable utilizing the standard resectoscope can offer this treatment option to women with menorrhagia. In fact, it generally takes clinicians only 5 to 10 procedures to become proficient in endometrial ablation.
While global ablation is not intended to replace hysterectomy—the definitive treatment for most uterine pathology2—it gives patients a choice. Women who want permanent cessation of menses can choose hys-terectomy. Those who want to preserve the uterus or desire an outpatient procedure with minimal morbidity may opt for endometrial ablation. Regardless of the method they choose, patients who participate in the deci-sion-making process are more likely to be satisfied with their outcome.
The thinner the endometrial lining, the more likely ablation will be successful.
Part of this process includes an informed-consent discussion with the patient, including a review of each technique, its risks and complications, and outcomes. The long-term consequences of endometrial ablation remain unknown. The reason: Follow-up data beyond 24 months are minimal. And another question remains unanswered: How many women who have undergone endometrial ablation will develop endometrial carcinoma without symptomatic bleeding? There has been at least 1 report of this phenomenon occurring in a woman who ultimately underwent a hysterectomy for other reasons.3 Other case reports have noted uterine bleeding as the presenting symptom of endometrial carcinoma in women who had undergone ablation.4-6 The bottom line: The risk of asymptomatic endometrial cancer appears to be small in properly selected patients, i.e.,women without a history of endometrial hyperplasia or carcinoma (Table 1).
TABLE 1
Patient selection
INDICATIONS
|
CONTRAINDICATIONS
|
Preparation
Prior to performing any endometrial ablation, obtain an endometrial sample to rule out premalignant or malignant disease. Also, assess the endometrial cavity using either office hysteroscopy or sonohysterography to exclude the possibility of submucous myomata or polyps, which can be treated with simple resection. These imaging techniques also can reveal abnormally shaped uterine cavities, thus eliminating certain women as candidates for global techniques such as balloon ablation.
For all methods, destroy or resect the endometrium to the level of the basalis, which is approximately 4 to 6 mm deep, depending upon the stage of the menstrual cycle or cycle suppression. It is reasonable to assume that the thinner the endometrial lining, the more likely ablation will be successful. Several methods of producing endometrial atrophy have been used, including dilatation and curettage (D&C) and hormonal suppression with medroxyprogesterone acetate, oral contraceptives (OCs), danocrine, or gonadotropin releasing hormone ago-nists (GnRH-a). I prefer to use leuprolide acetate (7.5 mg for 1 dose) 4 to 5 weeks prior to performing cryoablation or hydrothermal ablation. (Pretreatment of the endometrial lining is not necessary with bipolar desiccation.)
Many patients who undergo endometrial ablation via either standard or global techniques can tolerate the procedure well with local anesthesia and intravenous sedation. This is especially true for global procedures because they are of short duration, i.e., about 5 to 20 minutes, depending on the method. Much of the pain results from cervical dilation and manipulation, as well as trauma to the endometrium. Therefore, the global ablation techniques that utilize the smallest probe (5.5 mm or smaller) and freeze the uterus, i.e., cryoablation, require the least anesthesia.
After the patient has been properly selected and prepared, proceed with one the global techniques—cryoablation, bipolar desiccation, or hydrothermal ablation—outlined in this article.
Cryoablation
Technique. Dilate the cervix and insert a 5.5-mm cryoprobe into the uterine cavity. Cool the probe to less than-80 degrees Celsius by liquid differential gas exchange, described by Joule-Thompson, utilizing the cooling unit (Her Option; CryoGen, San Diego, Calif). An elliptical ice ball approximately 3.5 by 5 cm will then form around the probe.
Using abdominal ultrasound guidance, follow the edge of the ice ball into the uterine muscle at 1 cornu. (While the edge of the ice ball reaches 0 degrees Celsius, which is nondestructive to surrounding tissue, the endometrium is permanently destroyed approximately 1.5 cm from the edge of the ice ball, where a temperature of-20 degrees Celsius is reached.) Before the outer edge of the ice ball approaches the serosa of the uterus, stop the procedure (Figure 1). Heat the probe to body temperature and remove it from the cornu. Repeat the process in the contralateral cornu (Figure 2) and, in large uteri (greater than 10 cm sound), in the lower uterine segment (Figure 3). Each freeze cycle takes about 5 to 6 minutes.
FIGURE 1
Using ultrasound guidance, follow the edge of the ice ball into the uterine muscle at 1 cornu. Stop before the edge of the ice ball approaches the serosa.
FIGURE 2
Heat the probe to body temperature, remove it from the first cornu, and repeat the process in the contralateral cornu.
FIGURE 3
For large uteri (greater than 10 cm sound), perform another freeze cycle in the lower uterine segment. Each cycle takes about 5 to 6 minutes.The number of ice balls needed to destroy the entire uterine cavity and the length of time required to perform the procedure depend on the size of the cavity. In general, 2 to 3 ice balls are sufficient, and the entire procedure takes 10 to 20 minutes.
In a recent randomized multicenter study, researchers investigated whether a freeze cycle longer than recommended would result in better outcomes. At 12-month follow-up, 7 (64%) of the 12 patients who underwent treatments of 5 to 7 minutes rather than 4 minutes were amenorrheic/spotting, with a 91% success rate.7 Larger studies are underway to confirm these data. Further, repeat treatment can contribute to better outcomes, especially when the patient receives leuprolide acetate prior to the procedure.8
Advantages. The primary advantage of cryoab-lation is that it is not a totally blind procedure. The visual feedback provided by the ultrasound facilitates complete ablation of the entire uterine cavity, regardless of size. Further, while the hysteroscope used in standard ablation allows the physician to see only the destruction of the surface epithelium, ultrasound enables the surgeon to visualize the depth of treatment during cryoablation.
Another advantage: Freezing tissue causes less pain (cryoanesthesia) than the heat energy associated with other ablation devices.
Disadvantages. While ultrasound visualization has its benefits, it also can complicate matters because accurate imaging and interpretation require a certain level of skill. For Ob/Gyns not experienced in ultrasound, there is a potential for poor positioning of the probe, which could lead to bowel and bladder injuries. Further, physicians can mistakenly place the cryoprobe back into the treated cornu instead of the contralateral side, leading to ablation of the uterine serosa. Lastly, the technology involved in this procedure increases the cost of the equipment and disposable instruments.
What the evidence shows. According to an unpublished study conducted by Cryogen, 54% of 222 patients experienced amenorrhea or staining only at 6-month follow-up. Ninety-three percent of the patients had a Patient Bleeding Assessment Card (PBAC) score of less than 75, and 75% of the patients had a greater than 90% reduction in their PBAC scores. (A score of greater than 150 was needed to enter the study; the score is reached by assigning a value to the amount of staining on a validated pad and adding these points based on the number of pads used per cycle.)
Cost. The disposable cryoprobes are $1,250 per case, and the unit costs $29,995. Since most ablation procedures currently are performed in the operating room, ancillary costs of the techniques are similar.
Bipolar desiccation
Technique. After dilating the cervix, insert the 8-mm NovaSure System (Novacept, Palo Alto, Calif) catheter into the uterus. To rule out auterine perforation prior to beginning the procedure, insert carbon dioxide (CO2) into the cavity (Figure 4).The machine will not turn on if a seal of CO2 has not been created, unless the surgeon overrides the system.
Then expand the 3-dimensional gold-plated bipolar mesh electrode, which imitates the shape of the uterine cavity, out of the catheter. Gently push the probe toward the fundus so that the mesh electrode is accurately positioned within the uterine cavity (Figure 5). Once the electrode is activated with up to 180 W of bipolar power, gentle suction brings the endometrium into close contact with the mesh (Figure 6). This suction also removes debris and allows for more complete desiccation.
The entire bipolar desiccation procedure takes less than 5 minutes to perform.
The system shuts down automatically when complete desiccation (calculated at 50 ohms of resistance) has occurred. The average treatment time is just over 1 minute, and the average depth of ablation is 4 to 5 mm.9 Once ablation is complete, retract the mesh electrode and remove the catheter (Figure 7). The entire procedure takes less than 5 minutes.
Advantages. Not only is the technique quick and simple, no endometrial pretreatment is required because the system allows for consistent depths of ablation regardless of endometrial thickness.
Disadvantages. There are limited data on the 1-year success rates for this completely blind procedure. In addition, the uterus needs to be of normal shape to successfully ablate the endometrium.
What the evidence shows. Unpublished data from randomized, controlled clinical trials comparing bipolar desiccation with balloon ablation have demonstrated that the former achieves higher amenorrhea and success (a return to normal menstrual bleeding) rates than the latter. Further, patients treated with bipolar desiccation experienced less pain, both intra-operatively and postoperatively, than women who underwent balloon ablation.
Cost. Each disposable probe costs $850, and the bipolar controller lists for $15,000.
FIGURE 4
Dilate the cervix and insert the 8-mm catheter. To rule out a uterine perforation prior to beginning the procedure, place carbon dioxide in the cavity.
FIGURE 5
Expand the bipolar mesh electrode. Gently push the probe toward the fundus to accurately position the electrode within the uterine cavity.
FIGURE 6
Activate the electrode with up to 180 W of bipolar power. A gentle suction brings the endometrium into close contact with the mesh.
FIGURE 7
The system shuts down automatically when complete desiccation has occurred. Retract the bipolar mesh electrode and remove the catheter.
Hydrothermal ablation/circulating heated saline
Technique. Begin by dilating the cervix and inserting the sheath, which includes an 8-mm hysteroscope (Hydro ThermAblator; BEI Medical Systems, Teterboro, NJ), into the uter-ine cavity. Using gravity rather than a pump, introduce saline solution through the sheath into the uterus. As the saline circulates, gradually heat it until the solution reaches and maintains 90 degrees Celsius for 10 minutes, completely destroying the endometrium. (The total time from insertion of the sheath to completion of the procedure is 17 minutes.)
A safety feature detects whether any fluid has escaped from the closed system; a shutoff mechanism activates if more than 10 cc of fluid are lost. The system is controlled by software, so even physicians who are inexperienced with hysteroscopy can easily perform this technique.
Advantages. The primary advantage of hydrothermal ablation (HTA) is that the circulating hot saline solution contacts the entire endometrial surface regardless of the size or shape of the uterine cavity. In addition, since the procedure is performed under direct visualization, the surgeon can ensure that the entire cavity has been thoroughly ablated.
Disadvantages. One drawback is that it can be a painful procedure because the hysteroscope is large, necessitating wide dilation of the cervix, and hot water stimulates pain. Further, there have been unpublished reports of vaginal burns when the cervix created a poor seal around the sheath.
What the evidence shows. Based on their research of 276 women (187 women in the HTA group and 89 patients in the rollerball group), investigators have found that HTA has a treatment success rate of 77% compared to 82% for rollerball ablation. (The treatment success rate is defined as a PBAC score of 75 or lower; eumenorrhea is a score of less than 100.) Amenorrhea rates at 12-month follow-up were 40% and 51%, respectively. The researchers concluded that HTA offers an advantage over rollerball ablation in that it reduces anesthesia requirements and eliminates the problem of hypotonic fluid absorption.10
Cost. Disposable hysteroscopic tubing is $625 per case. The machine costs $10,900 plus $395 for the heater canister.
The author reports no financial relationship with any companies whose products are mentioned in this article.
- Global ablation methods involve destroying the endometrium using specialized devices that do not require an operative hysteroscope or resectoscope.
- The endometrium should be destroyed or resected to the level of the basalis, which is approximately 4 to 6 mm deep.
- In cryoablation, freezing the tissue causes less pain than the heat energy associated with other ablation devices. The procedure typically takes 10 to 20 minutes.
- Not only is bipolar desiccation quick and simple, no endometrial pretreatment is required because the system allows for consistent depths of ablation regardless of endometrial thickness.
- The advantage of hydrothermal ablation is that the circulating hot saline solution contacts the entire endometrial surface regardless of the shape or size of the cavity.
Traditionally, physicians have preferred hysterectomy for the treatment of abnormal uterine bleeding not related to endometrial cancer, representing about 20% of the 590,000 hysterectomie performed annually in the United States.1 The advent of standard endometrial ablation, e.g., surgical resection and rollerball desiccation, offered women less radical alternatives to hysterectomy. However, these “classic” methods are considered by some physicians to be technically difficult because they require the use of an operative hysteroscope—and all of its attendant risks. As a result, many Ob/Gyns continued to opt for hysterectomy when given a choice.
Now there are simpler alternatives: global ablation methods, including the newest—cryoablation, bipolar desiccation, and hydrothermal ablation. These procedures involve destroying the endometrium using specialized devices that do not require an operative hysteroscope or resectoscope. The advantage of these methods is that they are simple, rapid procedures that are easier to perform than standard endometrial ablation. Many surgeons who were uncomfortable utilizing the standard resectoscope can offer this treatment option to women with menorrhagia. In fact, it generally takes clinicians only 5 to 10 procedures to become proficient in endometrial ablation.
While global ablation is not intended to replace hysterectomy—the definitive treatment for most uterine pathology2—it gives patients a choice. Women who want permanent cessation of menses can choose hys-terectomy. Those who want to preserve the uterus or desire an outpatient procedure with minimal morbidity may opt for endometrial ablation. Regardless of the method they choose, patients who participate in the deci-sion-making process are more likely to be satisfied with their outcome.
The thinner the endometrial lining, the more likely ablation will be successful.
Part of this process includes an informed-consent discussion with the patient, including a review of each technique, its risks and complications, and outcomes. The long-term consequences of endometrial ablation remain unknown. The reason: Follow-up data beyond 24 months are minimal. And another question remains unanswered: How many women who have undergone endometrial ablation will develop endometrial carcinoma without symptomatic bleeding? There has been at least 1 report of this phenomenon occurring in a woman who ultimately underwent a hysterectomy for other reasons.3 Other case reports have noted uterine bleeding as the presenting symptom of endometrial carcinoma in women who had undergone ablation.4-6 The bottom line: The risk of asymptomatic endometrial cancer appears to be small in properly selected patients, i.e.,women without a history of endometrial hyperplasia or carcinoma (Table 1).
TABLE 1
Patient selection
INDICATIONS
|
CONTRAINDICATIONS
|
Preparation
Prior to performing any endometrial ablation, obtain an endometrial sample to rule out premalignant or malignant disease. Also, assess the endometrial cavity using either office hysteroscopy or sonohysterography to exclude the possibility of submucous myomata or polyps, which can be treated with simple resection. These imaging techniques also can reveal abnormally shaped uterine cavities, thus eliminating certain women as candidates for global techniques such as balloon ablation.
For all methods, destroy or resect the endometrium to the level of the basalis, which is approximately 4 to 6 mm deep, depending upon the stage of the menstrual cycle or cycle suppression. It is reasonable to assume that the thinner the endometrial lining, the more likely ablation will be successful. Several methods of producing endometrial atrophy have been used, including dilatation and curettage (D&C) and hormonal suppression with medroxyprogesterone acetate, oral contraceptives (OCs), danocrine, or gonadotropin releasing hormone ago-nists (GnRH-a). I prefer to use leuprolide acetate (7.5 mg for 1 dose) 4 to 5 weeks prior to performing cryoablation or hydrothermal ablation. (Pretreatment of the endometrial lining is not necessary with bipolar desiccation.)
Many patients who undergo endometrial ablation via either standard or global techniques can tolerate the procedure well with local anesthesia and intravenous sedation. This is especially true for global procedures because they are of short duration, i.e., about 5 to 20 minutes, depending on the method. Much of the pain results from cervical dilation and manipulation, as well as trauma to the endometrium. Therefore, the global ablation techniques that utilize the smallest probe (5.5 mm or smaller) and freeze the uterus, i.e., cryoablation, require the least anesthesia.
After the patient has been properly selected and prepared, proceed with one the global techniques—cryoablation, bipolar desiccation, or hydrothermal ablation—outlined in this article.
Cryoablation
Technique. Dilate the cervix and insert a 5.5-mm cryoprobe into the uterine cavity. Cool the probe to less than-80 degrees Celsius by liquid differential gas exchange, described by Joule-Thompson, utilizing the cooling unit (Her Option; CryoGen, San Diego, Calif). An elliptical ice ball approximately 3.5 by 5 cm will then form around the probe.
Using abdominal ultrasound guidance, follow the edge of the ice ball into the uterine muscle at 1 cornu. (While the edge of the ice ball reaches 0 degrees Celsius, which is nondestructive to surrounding tissue, the endometrium is permanently destroyed approximately 1.5 cm from the edge of the ice ball, where a temperature of-20 degrees Celsius is reached.) Before the outer edge of the ice ball approaches the serosa of the uterus, stop the procedure (Figure 1). Heat the probe to body temperature and remove it from the cornu. Repeat the process in the contralateral cornu (Figure 2) and, in large uteri (greater than 10 cm sound), in the lower uterine segment (Figure 3). Each freeze cycle takes about 5 to 6 minutes.
FIGURE 1
Using ultrasound guidance, follow the edge of the ice ball into the uterine muscle at 1 cornu. Stop before the edge of the ice ball approaches the serosa.
FIGURE 2
Heat the probe to body temperature, remove it from the first cornu, and repeat the process in the contralateral cornu.
FIGURE 3
For large uteri (greater than 10 cm sound), perform another freeze cycle in the lower uterine segment. Each cycle takes about 5 to 6 minutes.The number of ice balls needed to destroy the entire uterine cavity and the length of time required to perform the procedure depend on the size of the cavity. In general, 2 to 3 ice balls are sufficient, and the entire procedure takes 10 to 20 minutes.
In a recent randomized multicenter study, researchers investigated whether a freeze cycle longer than recommended would result in better outcomes. At 12-month follow-up, 7 (64%) of the 12 patients who underwent treatments of 5 to 7 minutes rather than 4 minutes were amenorrheic/spotting, with a 91% success rate.7 Larger studies are underway to confirm these data. Further, repeat treatment can contribute to better outcomes, especially when the patient receives leuprolide acetate prior to the procedure.8
Advantages. The primary advantage of cryoab-lation is that it is not a totally blind procedure. The visual feedback provided by the ultrasound facilitates complete ablation of the entire uterine cavity, regardless of size. Further, while the hysteroscope used in standard ablation allows the physician to see only the destruction of the surface epithelium, ultrasound enables the surgeon to visualize the depth of treatment during cryoablation.
Another advantage: Freezing tissue causes less pain (cryoanesthesia) than the heat energy associated with other ablation devices.
Disadvantages. While ultrasound visualization has its benefits, it also can complicate matters because accurate imaging and interpretation require a certain level of skill. For Ob/Gyns not experienced in ultrasound, there is a potential for poor positioning of the probe, which could lead to bowel and bladder injuries. Further, physicians can mistakenly place the cryoprobe back into the treated cornu instead of the contralateral side, leading to ablation of the uterine serosa. Lastly, the technology involved in this procedure increases the cost of the equipment and disposable instruments.
What the evidence shows. According to an unpublished study conducted by Cryogen, 54% of 222 patients experienced amenorrhea or staining only at 6-month follow-up. Ninety-three percent of the patients had a Patient Bleeding Assessment Card (PBAC) score of less than 75, and 75% of the patients had a greater than 90% reduction in their PBAC scores. (A score of greater than 150 was needed to enter the study; the score is reached by assigning a value to the amount of staining on a validated pad and adding these points based on the number of pads used per cycle.)
Cost. The disposable cryoprobes are $1,250 per case, and the unit costs $29,995. Since most ablation procedures currently are performed in the operating room, ancillary costs of the techniques are similar.
Bipolar desiccation
Technique. After dilating the cervix, insert the 8-mm NovaSure System (Novacept, Palo Alto, Calif) catheter into the uterus. To rule out auterine perforation prior to beginning the procedure, insert carbon dioxide (CO2) into the cavity (Figure 4).The machine will not turn on if a seal of CO2 has not been created, unless the surgeon overrides the system.
Then expand the 3-dimensional gold-plated bipolar mesh electrode, which imitates the shape of the uterine cavity, out of the catheter. Gently push the probe toward the fundus so that the mesh electrode is accurately positioned within the uterine cavity (Figure 5). Once the electrode is activated with up to 180 W of bipolar power, gentle suction brings the endometrium into close contact with the mesh (Figure 6). This suction also removes debris and allows for more complete desiccation.
The entire bipolar desiccation procedure takes less than 5 minutes to perform.
The system shuts down automatically when complete desiccation (calculated at 50 ohms of resistance) has occurred. The average treatment time is just over 1 minute, and the average depth of ablation is 4 to 5 mm.9 Once ablation is complete, retract the mesh electrode and remove the catheter (Figure 7). The entire procedure takes less than 5 minutes.
Advantages. Not only is the technique quick and simple, no endometrial pretreatment is required because the system allows for consistent depths of ablation regardless of endometrial thickness.
Disadvantages. There are limited data on the 1-year success rates for this completely blind procedure. In addition, the uterus needs to be of normal shape to successfully ablate the endometrium.
What the evidence shows. Unpublished data from randomized, controlled clinical trials comparing bipolar desiccation with balloon ablation have demonstrated that the former achieves higher amenorrhea and success (a return to normal menstrual bleeding) rates than the latter. Further, patients treated with bipolar desiccation experienced less pain, both intra-operatively and postoperatively, than women who underwent balloon ablation.
Cost. Each disposable probe costs $850, and the bipolar controller lists for $15,000.
FIGURE 4
Dilate the cervix and insert the 8-mm catheter. To rule out a uterine perforation prior to beginning the procedure, place carbon dioxide in the cavity.
FIGURE 5
Expand the bipolar mesh electrode. Gently push the probe toward the fundus to accurately position the electrode within the uterine cavity.
FIGURE 6
Activate the electrode with up to 180 W of bipolar power. A gentle suction brings the endometrium into close contact with the mesh.
FIGURE 7
The system shuts down automatically when complete desiccation has occurred. Retract the bipolar mesh electrode and remove the catheter.
Hydrothermal ablation/circulating heated saline
Technique. Begin by dilating the cervix and inserting the sheath, which includes an 8-mm hysteroscope (Hydro ThermAblator; BEI Medical Systems, Teterboro, NJ), into the uter-ine cavity. Using gravity rather than a pump, introduce saline solution through the sheath into the uterus. As the saline circulates, gradually heat it until the solution reaches and maintains 90 degrees Celsius for 10 minutes, completely destroying the endometrium. (The total time from insertion of the sheath to completion of the procedure is 17 minutes.)
A safety feature detects whether any fluid has escaped from the closed system; a shutoff mechanism activates if more than 10 cc of fluid are lost. The system is controlled by software, so even physicians who are inexperienced with hysteroscopy can easily perform this technique.
Advantages. The primary advantage of hydrothermal ablation (HTA) is that the circulating hot saline solution contacts the entire endometrial surface regardless of the size or shape of the uterine cavity. In addition, since the procedure is performed under direct visualization, the surgeon can ensure that the entire cavity has been thoroughly ablated.
Disadvantages. One drawback is that it can be a painful procedure because the hysteroscope is large, necessitating wide dilation of the cervix, and hot water stimulates pain. Further, there have been unpublished reports of vaginal burns when the cervix created a poor seal around the sheath.
What the evidence shows. Based on their research of 276 women (187 women in the HTA group and 89 patients in the rollerball group), investigators have found that HTA has a treatment success rate of 77% compared to 82% for rollerball ablation. (The treatment success rate is defined as a PBAC score of 75 or lower; eumenorrhea is a score of less than 100.) Amenorrhea rates at 12-month follow-up were 40% and 51%, respectively. The researchers concluded that HTA offers an advantage over rollerball ablation in that it reduces anesthesia requirements and eliminates the problem of hypotonic fluid absorption.10
Cost. Disposable hysteroscopic tubing is $625 per case. The machine costs $10,900 plus $395 for the heater canister.
The author reports no financial relationship with any companies whose products are mentioned in this article.
1. Carlson KJ, Nichols DH, Schiff I. Indications for hysterectomy. N Engl J Med. 1993;Mar 25 328(12):856-860.
2. Carlson KJ, Miller BA, Fowler FJ, Jr. The Maine Women’s Health Study: I. Outcomes of hysterectomy. Obstet Gynecol. 1994;83-556.
3. Margolis MT, et al. Asymptomatic endometrial carcinoma after endometrial ablation. Int J Gynaecol Obstet. 1995;51-255.
4. Gimpelson RJ. Not so benign endometrial hyperplasia: endometrial cancer after endometrial ablation. J Am Assoc Gynecol Laparosc. 1997;4-507.
5. Horowitz IR, Copas PR, Aaronoff, et al. Endometrial adenocarcinoma following endometrial ablation for postmenopausal bleeding. Gynecol Oncol. 1995;56:460.-
6. Valle RF, Baggish MS. Endometrial carcinoma after endometrial ablation: high-risk factors predicting its occurrence. Am J Obstet Gynecol. 1998;179:569.-
7. Heppard M, et al. Data on 222 patients from a multicenter study using Cryogen First Option uterine cryoablation therapy in women with abnormal uterine bleeding. Obstet Gynecol. 2001;97(4 Suppl 1):S21.-
8. Sanders B. Preliminary outcomes of longer freeze cycles in the treatment of women with abnormal uterine bleeding using Cryogen First Option uterine cryoablation therapy. Obstet Gynecol. 2001;97(4 Suppl 1):S14.-
9. Cooper JM, Erickson ML. Global endometrial ablation technologies. Obstet Gynecol Clin North Am. 2000;27-385.
10. Corson SL. A multicenter evaluation of endometrial ablation by Hydro ThermAblator and rollerball for treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2001;8(3):359-367.
1. Carlson KJ, Nichols DH, Schiff I. Indications for hysterectomy. N Engl J Med. 1993;Mar 25 328(12):856-860.
2. Carlson KJ, Miller BA, Fowler FJ, Jr. The Maine Women’s Health Study: I. Outcomes of hysterectomy. Obstet Gynecol. 1994;83-556.
3. Margolis MT, et al. Asymptomatic endometrial carcinoma after endometrial ablation. Int J Gynaecol Obstet. 1995;51-255.
4. Gimpelson RJ. Not so benign endometrial hyperplasia: endometrial cancer after endometrial ablation. J Am Assoc Gynecol Laparosc. 1997;4-507.
5. Horowitz IR, Copas PR, Aaronoff, et al. Endometrial adenocarcinoma following endometrial ablation for postmenopausal bleeding. Gynecol Oncol. 1995;56:460.-
6. Valle RF, Baggish MS. Endometrial carcinoma after endometrial ablation: high-risk factors predicting its occurrence. Am J Obstet Gynecol. 1998;179:569.-
7. Heppard M, et al. Data on 222 patients from a multicenter study using Cryogen First Option uterine cryoablation therapy in women with abnormal uterine bleeding. Obstet Gynecol. 2001;97(4 Suppl 1):S21.-
8. Sanders B. Preliminary outcomes of longer freeze cycles in the treatment of women with abnormal uterine bleeding using Cryogen First Option uterine cryoablation therapy. Obstet Gynecol. 2001;97(4 Suppl 1):S14.-
9. Cooper JM, Erickson ML. Global endometrial ablation technologies. Obstet Gynecol Clin North Am. 2000;27-385.
10. Corson SL. A multicenter evaluation of endometrial ablation by Hydro ThermAblator and rollerball for treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2001;8(3):359-367.
Cone biopsy: perfecting the procedure
- Cone biopsy typically includes the removal of the entire squamocolumnar junction of the cervix, generally agreed to be the site of origin of squamous cell carcinoma.
- Inject a premixed solution of 2% xylocaine and epinephrine in a concentration of 1:200,000 into the cervical stroma at 12 o’clock outside the intended margin.
- For a “cold-knife” cone, use a #11 surgical blade to begin a circular incision starting at 12 o’clock on the face of the cervix.
Cone biopsy of the cervix has been used for more than a century to rule out the presence of invasive carcinoma in women with squamous intraepithelial lesions (SIL). And while less invasive techniques such as colposcopy and loop electrosurgical excision procedures (LEEP) have reduced the need for diagnostic conization dramatically, cervical cone biopsy becomes necessary when these techniques prove inadequate.
Indications
When routine screening reveals abnormal cervical cytology such as atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesions (LGSIL), and high-grade squamous intraepithelial lesions (HGSIL), colposcopy and directed biopsy often are indicated. They help the physician rule out the presence of invasive carcinoma and determine the grade and distribution of the intraepithelial lesion. Currently, only HGSIL is considered premalignant and requires aggressive treatment via cone biopsy.1 Additional indications for the procedure are listed in Table 1.
Remove a single specimen that includes the entire transformation zone.
From a diagnostic standpoint, cone biopsy should be performed when the endocervical curettage is positive for dysplasia because it is difficult to grade the severity of dysplasia on the basis of the scant tissue fragments obtained by curettage. From a therapeutic standpoint, lesions that involve the endocervical canal are less likely to be adequately treated by destructive techniques such as cryotherapy. Thus, for most women, cone biopsy of the cervix is both diagnostic and therapeutic.
TABLE 1
Indications for cervical conization
|
Preparing for cone biopsy
Cone biopsy involves the surgical excision of a wedge-shaped portion of the ecto- and endocervix, including the removal of the entire squamocolumnar junction (SC junction) of the cervix, generally agreed to be the site of origin of squamous cell carcinoma of the cervix. The procedure may be performed using a scalpel, electroexcision (LEEP or fine-needle electrode), or CO2 laser. The choice between performing “cold-knife” versus “hot-knife” conization is largely one of personal preference and depends on surgical experience, the size/severity of the lesion, and the desires of the patient. In most cases, I prefer to use electrocautery because of its technical simplicity and the ability to operate with only a local anesthetic. It is particularly appropriate for patients with an obvious ectocervical lesion and in young, nulliparous women in whom I am trying to minimize the amount of healthy cervical tissue removed.
The use of colposcopy, either preoperatively or intraoperatively, allows precise evaluation of the amount of cervical tissue needed to be removed and reduces the incidence of positive margins. The geometry, i.e., width and depth, of the cone specimen will vary from patient to patient, depending on the size and location of the dysplastic lesion, as well as the location of the SC junction. Specifically, the width of the cone (ectocervical portion) is determined by the size of the transformation zone and size and location of any ectocervical lesions. The depth of the cone (endocervical portion) is determined by the location of the SC junction, the presence or absence of endocervi-cal disease, or the suspicion of a glandular lesion. When a discrete intraepithelial lesion has not been identified, it is critical to rule out a significant endocervical lesion. Therefore, the amount of tissue I plan to remove is based on the following 2 factors:
- Location of the SC junction. The more endocervical the SC junction is, the more likely the presence of a lesion higher in the canal. However, squamous intraepithelial lesions rarely extend higher than 2 cm into the canal.2
- Endocervical gland involvement. SIL frequently involves the endocervical glands, often to a depth of 5 mm or less. Several investigators have suggested that excision of endocervical glands to a depth of 7 to 10 mm produces acceptable cure rates.3
Based on these 2 principles, the endocer-vical portion of the cone should be 20 mm wide (10 mm on either side of the canal) and no more than 2 cm deep.
Technique
Traditionally, the first step in performing a cone biopsy is to place sutures lateral to the cervix to decrease bleeding (Figure 1). While many surgeons believe that these sutures have a beneficial effect on hemosta-sis, others have not found them to be necessary.4 And although cone biopsy typically is performed under regional or general anesthesia, I have found that IV sedation and injection of a local anesthetic are adequate for most cone biopsies.
Begin the procedure by placing either a posterior weighted speculum (for a cold-knife cone) or an insulated speculum (for electrocautery) into the vagina. Then inject a premixed solution of 2% xylocaine and epi-nephrine in a concentration of 1:200,000 into the cervical stroma at 12 o’clock outside the intended margin of the cone biopsy. Then grasp this tissue with a single-tooth tenacu-lum. Inject 5 to 10 cc of the solution para-cervically at 3 o’clock and again at 9 o’clock. I typically use a total of 20 to 30 cc to maintain hemostasis. Alternatively, the anesthetic can be infiltrated into the subepithelial stroma circumferentially around the ectocervix.
To perform a cold-knife cone, use a #11 surgical blade to begin a circular incision starting at 12 o’clock on the face of the cervix, angling the tip of the blade toward the endocervical canal (Figure 2). Use a uterine sound to mark a depth of 2 cm within the endo-cervical canal, typically the most cephalad margin of the cone. The goal is to remove a single specimen that includes the entire transformation zone and distal endocervical canal (Figure 3). After removing the cone, perform an endocervical curettage to collect an additional specimen. Apply light cautery to the edges of the cervical bed, as well as any bleeding points (Figure 4). Place 1 interrupted suture (I prefer 2-0 polyglactin) in each quadrant of the face of the cervix, starting from outside the margin of the cone and exiting near the cervical os. Then insert the suture near the cervical os and exit outside the margin of the cone. Tying these sut ures everts the cervical os to a degree and keeps the transformation zone closer to the external surface of the cervix, making it easier to find when performing future colposcopies.
Electroexcision with a fine-needle electrode is performed with a blend-1 waveform using 18 watts of power.5 Perform the excision using the “cut” button on the bovie handpiece and then use the leading edge of the electrical arc to incise the tissue. (It is best to use the arc, i.e., visible spark, to do the cutting rather than the tip of the instrument, which may “stick” to surrounding tissues.) Make a full circle to a depth of about 7 mm to outline the ectocervical cone margins. Then “pull down” the stroma on the edge of the specimen with a skin hook to continue the dissection parallel to the long axis of the endocervical canal to a depth of 1.5 to 2.0 cm. I excise the “base” of the cone with scissors to minimize any cautery effect at the endocervical margin. Achieve hemostasis by electrofulgurating the bleeding vessels using 30 to 40 watts of power in the coagulation mode. Monsel solution or paste then can be applied as needed. This technique is similar to CO2 laser conization, only it is easier to learn and quicker to perform.
FIGURE 1
Begin the cone biopsy by placing lateral sutures at the cervicovaginal junction to decrease bleeding.
FIGURE 2
Use a #11 surgical blade to make the circular incision, angling the tip of the blade toward the endocervical canal.
FIGURE 3
Grasp the specimen, including the entire transformation zone and distal endocervical canal, with an Allis clamp.
FIGURE 4
Complete the cone excision by cutting across endocervix. Apply light cautery to the edges of the cervical bed.
Follow-up
Postoperatively, patients should undergo cytologic screening every 4 months for 2 years to identify persistent or recurrent disease. Patients with positive cone biopsy margins face the highest risk of persistent or recurrent cervical intraepithelial neoplasia (CIN). Although there is considerable variation, studies generally have reported a 30% incidence of positive margins. The incidence of recurrent dysplasia when the margins are positive has been reported to be as high as 50%.6,7
In women with positive margins, colposcopy and cervical and endocervical cytology are an important part of the follow-up. I perform an endocervical curettage, in addition to a Pap smear, at each interval visit during the first year postoperatively and perform colposcopy at the 6- and 12- month visits. After 2 years of follow-up with normal results, cytologic screening should be performed annually, per routine care.
The author reports no financial relationship with any companies whose products are mentioned in this article.
1. Koutsky LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to the papillomavirus. N Engl J Med. 1992;327:1272-1278.
2. Burke L. Evolution of therapeutic approaches to cervical intraepithelial neoplasia. J Lower Genital Tract Disease. 1997;1:267-273.
3. Burke L, Covell L, Antonioli D. Carbon dioxide laser therapy of cervical intraepithelial neoplasia: factors determining success rates. Lasers Surg Med. 1980;1:113-122.
4. Gilbert L, Saunders NJ, Stringer R, Sharp F. Haemostasis and cold knife biopsy: a prospective randomized trial comparing a suture versus nonsuture technique. Obstet Gynecol. 1989;74:640-643.
5. Ferenczy A. Electroconization of the cervix with a fine-needle electrode. Obstet Gynecol. 1994;84:152-159.
6. AbdulKarim F, Nunez C. Cervical intraepithelial neoplasia after conization:a study of 522 consecutive cervical cones. Obstet Gynecol. 1985;65:77-81.
7. Lubicz S, Ezekwche C, Allen A, Schiffer M. Significance of cone biopsy margins in the management of patients with cervical neoplasia. J Reprod Med. 1984;29:178-184.
- Cone biopsy typically includes the removal of the entire squamocolumnar junction of the cervix, generally agreed to be the site of origin of squamous cell carcinoma.
- Inject a premixed solution of 2% xylocaine and epinephrine in a concentration of 1:200,000 into the cervical stroma at 12 o’clock outside the intended margin.
- For a “cold-knife” cone, use a #11 surgical blade to begin a circular incision starting at 12 o’clock on the face of the cervix.
Cone biopsy of the cervix has been used for more than a century to rule out the presence of invasive carcinoma in women with squamous intraepithelial lesions (SIL). And while less invasive techniques such as colposcopy and loop electrosurgical excision procedures (LEEP) have reduced the need for diagnostic conization dramatically, cervical cone biopsy becomes necessary when these techniques prove inadequate.
Indications
When routine screening reveals abnormal cervical cytology such as atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesions (LGSIL), and high-grade squamous intraepithelial lesions (HGSIL), colposcopy and directed biopsy often are indicated. They help the physician rule out the presence of invasive carcinoma and determine the grade and distribution of the intraepithelial lesion. Currently, only HGSIL is considered premalignant and requires aggressive treatment via cone biopsy.1 Additional indications for the procedure are listed in Table 1.
Remove a single specimen that includes the entire transformation zone.
From a diagnostic standpoint, cone biopsy should be performed when the endocervical curettage is positive for dysplasia because it is difficult to grade the severity of dysplasia on the basis of the scant tissue fragments obtained by curettage. From a therapeutic standpoint, lesions that involve the endocervical canal are less likely to be adequately treated by destructive techniques such as cryotherapy. Thus, for most women, cone biopsy of the cervix is both diagnostic and therapeutic.
TABLE 1
Indications for cervical conization
|
Preparing for cone biopsy
Cone biopsy involves the surgical excision of a wedge-shaped portion of the ecto- and endocervix, including the removal of the entire squamocolumnar junction (SC junction) of the cervix, generally agreed to be the site of origin of squamous cell carcinoma of the cervix. The procedure may be performed using a scalpel, electroexcision (LEEP or fine-needle electrode), or CO2 laser. The choice between performing “cold-knife” versus “hot-knife” conization is largely one of personal preference and depends on surgical experience, the size/severity of the lesion, and the desires of the patient. In most cases, I prefer to use electrocautery because of its technical simplicity and the ability to operate with only a local anesthetic. It is particularly appropriate for patients with an obvious ectocervical lesion and in young, nulliparous women in whom I am trying to minimize the amount of healthy cervical tissue removed.
The use of colposcopy, either preoperatively or intraoperatively, allows precise evaluation of the amount of cervical tissue needed to be removed and reduces the incidence of positive margins. The geometry, i.e., width and depth, of the cone specimen will vary from patient to patient, depending on the size and location of the dysplastic lesion, as well as the location of the SC junction. Specifically, the width of the cone (ectocervical portion) is determined by the size of the transformation zone and size and location of any ectocervical lesions. The depth of the cone (endocervical portion) is determined by the location of the SC junction, the presence or absence of endocervi-cal disease, or the suspicion of a glandular lesion. When a discrete intraepithelial lesion has not been identified, it is critical to rule out a significant endocervical lesion. Therefore, the amount of tissue I plan to remove is based on the following 2 factors:
- Location of the SC junction. The more endocervical the SC junction is, the more likely the presence of a lesion higher in the canal. However, squamous intraepithelial lesions rarely extend higher than 2 cm into the canal.2
- Endocervical gland involvement. SIL frequently involves the endocervical glands, often to a depth of 5 mm or less. Several investigators have suggested that excision of endocervical glands to a depth of 7 to 10 mm produces acceptable cure rates.3
Based on these 2 principles, the endocer-vical portion of the cone should be 20 mm wide (10 mm on either side of the canal) and no more than 2 cm deep.
Technique
Traditionally, the first step in performing a cone biopsy is to place sutures lateral to the cervix to decrease bleeding (Figure 1). While many surgeons believe that these sutures have a beneficial effect on hemosta-sis, others have not found them to be necessary.4 And although cone biopsy typically is performed under regional or general anesthesia, I have found that IV sedation and injection of a local anesthetic are adequate for most cone biopsies.
Begin the procedure by placing either a posterior weighted speculum (for a cold-knife cone) or an insulated speculum (for electrocautery) into the vagina. Then inject a premixed solution of 2% xylocaine and epi-nephrine in a concentration of 1:200,000 into the cervical stroma at 12 o’clock outside the intended margin of the cone biopsy. Then grasp this tissue with a single-tooth tenacu-lum. Inject 5 to 10 cc of the solution para-cervically at 3 o’clock and again at 9 o’clock. I typically use a total of 20 to 30 cc to maintain hemostasis. Alternatively, the anesthetic can be infiltrated into the subepithelial stroma circumferentially around the ectocervix.
To perform a cold-knife cone, use a #11 surgical blade to begin a circular incision starting at 12 o’clock on the face of the cervix, angling the tip of the blade toward the endocervical canal (Figure 2). Use a uterine sound to mark a depth of 2 cm within the endo-cervical canal, typically the most cephalad margin of the cone. The goal is to remove a single specimen that includes the entire transformation zone and distal endocervical canal (Figure 3). After removing the cone, perform an endocervical curettage to collect an additional specimen. Apply light cautery to the edges of the cervical bed, as well as any bleeding points (Figure 4). Place 1 interrupted suture (I prefer 2-0 polyglactin) in each quadrant of the face of the cervix, starting from outside the margin of the cone and exiting near the cervical os. Then insert the suture near the cervical os and exit outside the margin of the cone. Tying these sut ures everts the cervical os to a degree and keeps the transformation zone closer to the external surface of the cervix, making it easier to find when performing future colposcopies.
Electroexcision with a fine-needle electrode is performed with a blend-1 waveform using 18 watts of power.5 Perform the excision using the “cut” button on the bovie handpiece and then use the leading edge of the electrical arc to incise the tissue. (It is best to use the arc, i.e., visible spark, to do the cutting rather than the tip of the instrument, which may “stick” to surrounding tissues.) Make a full circle to a depth of about 7 mm to outline the ectocervical cone margins. Then “pull down” the stroma on the edge of the specimen with a skin hook to continue the dissection parallel to the long axis of the endocervical canal to a depth of 1.5 to 2.0 cm. I excise the “base” of the cone with scissors to minimize any cautery effect at the endocervical margin. Achieve hemostasis by electrofulgurating the bleeding vessels using 30 to 40 watts of power in the coagulation mode. Monsel solution or paste then can be applied as needed. This technique is similar to CO2 laser conization, only it is easier to learn and quicker to perform.
FIGURE 1
Begin the cone biopsy by placing lateral sutures at the cervicovaginal junction to decrease bleeding.
FIGURE 2
Use a #11 surgical blade to make the circular incision, angling the tip of the blade toward the endocervical canal.
FIGURE 3
Grasp the specimen, including the entire transformation zone and distal endocervical canal, with an Allis clamp.
FIGURE 4
Complete the cone excision by cutting across endocervix. Apply light cautery to the edges of the cervical bed.
Follow-up
Postoperatively, patients should undergo cytologic screening every 4 months for 2 years to identify persistent or recurrent disease. Patients with positive cone biopsy margins face the highest risk of persistent or recurrent cervical intraepithelial neoplasia (CIN). Although there is considerable variation, studies generally have reported a 30% incidence of positive margins. The incidence of recurrent dysplasia when the margins are positive has been reported to be as high as 50%.6,7
In women with positive margins, colposcopy and cervical and endocervical cytology are an important part of the follow-up. I perform an endocervical curettage, in addition to a Pap smear, at each interval visit during the first year postoperatively and perform colposcopy at the 6- and 12- month visits. After 2 years of follow-up with normal results, cytologic screening should be performed annually, per routine care.
The author reports no financial relationship with any companies whose products are mentioned in this article.
- Cone biopsy typically includes the removal of the entire squamocolumnar junction of the cervix, generally agreed to be the site of origin of squamous cell carcinoma.
- Inject a premixed solution of 2% xylocaine and epinephrine in a concentration of 1:200,000 into the cervical stroma at 12 o’clock outside the intended margin.
- For a “cold-knife” cone, use a #11 surgical blade to begin a circular incision starting at 12 o’clock on the face of the cervix.
Cone biopsy of the cervix has been used for more than a century to rule out the presence of invasive carcinoma in women with squamous intraepithelial lesions (SIL). And while less invasive techniques such as colposcopy and loop electrosurgical excision procedures (LEEP) have reduced the need for diagnostic conization dramatically, cervical cone biopsy becomes necessary when these techniques prove inadequate.
Indications
When routine screening reveals abnormal cervical cytology such as atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesions (LGSIL), and high-grade squamous intraepithelial lesions (HGSIL), colposcopy and directed biopsy often are indicated. They help the physician rule out the presence of invasive carcinoma and determine the grade and distribution of the intraepithelial lesion. Currently, only HGSIL is considered premalignant and requires aggressive treatment via cone biopsy.1 Additional indications for the procedure are listed in Table 1.
Remove a single specimen that includes the entire transformation zone.
From a diagnostic standpoint, cone biopsy should be performed when the endocervical curettage is positive for dysplasia because it is difficult to grade the severity of dysplasia on the basis of the scant tissue fragments obtained by curettage. From a therapeutic standpoint, lesions that involve the endocervical canal are less likely to be adequately treated by destructive techniques such as cryotherapy. Thus, for most women, cone biopsy of the cervix is both diagnostic and therapeutic.
TABLE 1
Indications for cervical conization
|
Preparing for cone biopsy
Cone biopsy involves the surgical excision of a wedge-shaped portion of the ecto- and endocervix, including the removal of the entire squamocolumnar junction (SC junction) of the cervix, generally agreed to be the site of origin of squamous cell carcinoma of the cervix. The procedure may be performed using a scalpel, electroexcision (LEEP or fine-needle electrode), or CO2 laser. The choice between performing “cold-knife” versus “hot-knife” conization is largely one of personal preference and depends on surgical experience, the size/severity of the lesion, and the desires of the patient. In most cases, I prefer to use electrocautery because of its technical simplicity and the ability to operate with only a local anesthetic. It is particularly appropriate for patients with an obvious ectocervical lesion and in young, nulliparous women in whom I am trying to minimize the amount of healthy cervical tissue removed.
The use of colposcopy, either preoperatively or intraoperatively, allows precise evaluation of the amount of cervical tissue needed to be removed and reduces the incidence of positive margins. The geometry, i.e., width and depth, of the cone specimen will vary from patient to patient, depending on the size and location of the dysplastic lesion, as well as the location of the SC junction. Specifically, the width of the cone (ectocervical portion) is determined by the size of the transformation zone and size and location of any ectocervical lesions. The depth of the cone (endocervical portion) is determined by the location of the SC junction, the presence or absence of endocervi-cal disease, or the suspicion of a glandular lesion. When a discrete intraepithelial lesion has not been identified, it is critical to rule out a significant endocervical lesion. Therefore, the amount of tissue I plan to remove is based on the following 2 factors:
- Location of the SC junction. The more endocervical the SC junction is, the more likely the presence of a lesion higher in the canal. However, squamous intraepithelial lesions rarely extend higher than 2 cm into the canal.2
- Endocervical gland involvement. SIL frequently involves the endocervical glands, often to a depth of 5 mm or less. Several investigators have suggested that excision of endocervical glands to a depth of 7 to 10 mm produces acceptable cure rates.3
Based on these 2 principles, the endocer-vical portion of the cone should be 20 mm wide (10 mm on either side of the canal) and no more than 2 cm deep.
Technique
Traditionally, the first step in performing a cone biopsy is to place sutures lateral to the cervix to decrease bleeding (Figure 1). While many surgeons believe that these sutures have a beneficial effect on hemosta-sis, others have not found them to be necessary.4 And although cone biopsy typically is performed under regional or general anesthesia, I have found that IV sedation and injection of a local anesthetic are adequate for most cone biopsies.
Begin the procedure by placing either a posterior weighted speculum (for a cold-knife cone) or an insulated speculum (for electrocautery) into the vagina. Then inject a premixed solution of 2% xylocaine and epi-nephrine in a concentration of 1:200,000 into the cervical stroma at 12 o’clock outside the intended margin of the cone biopsy. Then grasp this tissue with a single-tooth tenacu-lum. Inject 5 to 10 cc of the solution para-cervically at 3 o’clock and again at 9 o’clock. I typically use a total of 20 to 30 cc to maintain hemostasis. Alternatively, the anesthetic can be infiltrated into the subepithelial stroma circumferentially around the ectocervix.
To perform a cold-knife cone, use a #11 surgical blade to begin a circular incision starting at 12 o’clock on the face of the cervix, angling the tip of the blade toward the endocervical canal (Figure 2). Use a uterine sound to mark a depth of 2 cm within the endo-cervical canal, typically the most cephalad margin of the cone. The goal is to remove a single specimen that includes the entire transformation zone and distal endocervical canal (Figure 3). After removing the cone, perform an endocervical curettage to collect an additional specimen. Apply light cautery to the edges of the cervical bed, as well as any bleeding points (Figure 4). Place 1 interrupted suture (I prefer 2-0 polyglactin) in each quadrant of the face of the cervix, starting from outside the margin of the cone and exiting near the cervical os. Then insert the suture near the cervical os and exit outside the margin of the cone. Tying these sut ures everts the cervical os to a degree and keeps the transformation zone closer to the external surface of the cervix, making it easier to find when performing future colposcopies.
Electroexcision with a fine-needle electrode is performed with a blend-1 waveform using 18 watts of power.5 Perform the excision using the “cut” button on the bovie handpiece and then use the leading edge of the electrical arc to incise the tissue. (It is best to use the arc, i.e., visible spark, to do the cutting rather than the tip of the instrument, which may “stick” to surrounding tissues.) Make a full circle to a depth of about 7 mm to outline the ectocervical cone margins. Then “pull down” the stroma on the edge of the specimen with a skin hook to continue the dissection parallel to the long axis of the endocervical canal to a depth of 1.5 to 2.0 cm. I excise the “base” of the cone with scissors to minimize any cautery effect at the endocervical margin. Achieve hemostasis by electrofulgurating the bleeding vessels using 30 to 40 watts of power in the coagulation mode. Monsel solution or paste then can be applied as needed. This technique is similar to CO2 laser conization, only it is easier to learn and quicker to perform.
FIGURE 1
Begin the cone biopsy by placing lateral sutures at the cervicovaginal junction to decrease bleeding.
FIGURE 2
Use a #11 surgical blade to make the circular incision, angling the tip of the blade toward the endocervical canal.
FIGURE 3
Grasp the specimen, including the entire transformation zone and distal endocervical canal, with an Allis clamp.
FIGURE 4
Complete the cone excision by cutting across endocervix. Apply light cautery to the edges of the cervical bed.
Follow-up
Postoperatively, patients should undergo cytologic screening every 4 months for 2 years to identify persistent or recurrent disease. Patients with positive cone biopsy margins face the highest risk of persistent or recurrent cervical intraepithelial neoplasia (CIN). Although there is considerable variation, studies generally have reported a 30% incidence of positive margins. The incidence of recurrent dysplasia when the margins are positive has been reported to be as high as 50%.6,7
In women with positive margins, colposcopy and cervical and endocervical cytology are an important part of the follow-up. I perform an endocervical curettage, in addition to a Pap smear, at each interval visit during the first year postoperatively and perform colposcopy at the 6- and 12- month visits. After 2 years of follow-up with normal results, cytologic screening should be performed annually, per routine care.
The author reports no financial relationship with any companies whose products are mentioned in this article.
1. Koutsky LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to the papillomavirus. N Engl J Med. 1992;327:1272-1278.
2. Burke L. Evolution of therapeutic approaches to cervical intraepithelial neoplasia. J Lower Genital Tract Disease. 1997;1:267-273.
3. Burke L, Covell L, Antonioli D. Carbon dioxide laser therapy of cervical intraepithelial neoplasia: factors determining success rates. Lasers Surg Med. 1980;1:113-122.
4. Gilbert L, Saunders NJ, Stringer R, Sharp F. Haemostasis and cold knife biopsy: a prospective randomized trial comparing a suture versus nonsuture technique. Obstet Gynecol. 1989;74:640-643.
5. Ferenczy A. Electroconization of the cervix with a fine-needle electrode. Obstet Gynecol. 1994;84:152-159.
6. AbdulKarim F, Nunez C. Cervical intraepithelial neoplasia after conization:a study of 522 consecutive cervical cones. Obstet Gynecol. 1985;65:77-81.
7. Lubicz S, Ezekwche C, Allen A, Schiffer M. Significance of cone biopsy margins in the management of patients with cervical neoplasia. J Reprod Med. 1984;29:178-184.
1. Koutsky LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to the papillomavirus. N Engl J Med. 1992;327:1272-1278.
2. Burke L. Evolution of therapeutic approaches to cervical intraepithelial neoplasia. J Lower Genital Tract Disease. 1997;1:267-273.
3. Burke L, Covell L, Antonioli D. Carbon dioxide laser therapy of cervical intraepithelial neoplasia: factors determining success rates. Lasers Surg Med. 1980;1:113-122.
4. Gilbert L, Saunders NJ, Stringer R, Sharp F. Haemostasis and cold knife biopsy: a prospective randomized trial comparing a suture versus nonsuture technique. Obstet Gynecol. 1989;74:640-643.
5. Ferenczy A. Electroconization of the cervix with a fine-needle electrode. Obstet Gynecol. 1994;84:152-159.
6. AbdulKarim F, Nunez C. Cervical intraepithelial neoplasia after conization:a study of 522 consecutive cervical cones. Obstet Gynecol. 1985;65:77-81.
7. Lubicz S, Ezekwche C, Allen A, Schiffer M. Significance of cone biopsy margins in the management of patients with cervical neoplasia. J Reprod Med. 1984;29:178-184.