User login
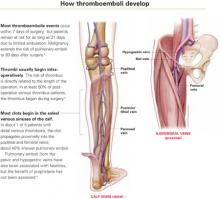
Q Does acute infection raise the risk of venous thromboembolism?
In this study, the incidence ratio for deep venous thrombosis (DVT) following urinary tract infection was 2.10 (95% confidence interval [CI] 1.56–2.82), and for respiratory tract infection, it was 2.86 (95% CI 2.05–3.97).
The incidence ratio for pulmonary embolism (PE) following urinary tract infection was 2.11 (95% CI 1.38–3.23). Although the risk of PE following respiratory tract infection was 11-fold higher, possible misdiagnosis of PE as a respiratory infection precluded reliable estimates of the precise risk.
Details of the study
The study assessed the risk of a first-ever DVT or PE after acute urinary tract infection or acute systemic respiratory infection, excluding pharyngitis and coryza.
Data were from the United Kingdom’s Health Improvement Network, which has complete diagnostic and prescribing information, and covered the years 1987 to 2004, or approximately 20 million person-years.
One strength was use of a self-controlled case series method, which allowed patients to serve as their own controls, thus eliminating variation among individuals in risk factors for venous thromboembolism.
Patients were observed for 12 months after an acute urinary or respiratory tract infection to determine whether a thromboembolic event had occurred. Incidence ratios and confidence intervals were calculated, and the study had adequate power at 5% significance to detect a 4-fold difference during the first 2 weeks after acute infections.
Expert Commentary
The exact mechanism of thrombosis is still unknown, and the possibility of a common pathway not linked to a specific infection is intriguing. Uncovering the mechanism could help us direct therapy to a particular biochemical process.
Virchow proposed his triad of precipitating factors 150 years ago: venous stasis, increased coagulability of the blood, and vessel wall damage.1 It now seems entirely plausible that damage to the vessel wall need not be physical damage, but could include factors, such as inflammation, that affect endothelial function. As the authors noted, “Inflammation is a key determinant of endothelial function in both arteries and veins, and a link between infection and venous thrombosis via endothelial activation has been suggested.” In fact, earlier studies already identified infection as a potential risk factor for venous thromboembolism.2,3
Thromboembolic events occur at a rate of about 0.5 cases per 1,000 person-years and cause considerable morbidity and mortality.
How long to continue prophylaxis?
The study by Smeeth and colleagues should help ObGyns determine the level of prophylaxis appropriate for hospitalized patients. Less clear is whether thromboprophylaxis should be offered to women who have acute infections in an ambulatory setting. Although earlier studies suggested that thromboprophylaxis may be appropriate, I believe the question of whether every patient should receive preventive therapy remains unanswered.
Another unresolved issue: If prophylaxis is initiated, how long should it continue? Because the risk of a thromboembolic event does not return to baseline levels for 1 year, the duration of therapy could be lengthy. At the same time, the risks of anticoagulation are not inconsequential and may increase with extended therapy. As the greatest risk occurs during the first 8 weeks after infection, prophylaxis is most beneficial during this time.
Routine prophylaxis?
Given the data thus far, I do not believe therapy is warranted for every patient with an acute infection. Selective therapy may be justified.
1. Virchow RLK. Thrombosis and Emboli. Matzdorff AC, Bell WR, trans. Canton, Mass: Science History Publications; 1998.
2. Samama MM. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius study. Arch Intern Med. 2000;160:3415-3420.
3. Alikhan R, Cohen AT, Combe S, et al. Risk factors for venous thromboembolism in hospitalized patients with acute medical illness: analysis of the MEDENOX Study. Arch Intern Med. 2004;164:963-968.
In this study, the incidence ratio for deep venous thrombosis (DVT) following urinary tract infection was 2.10 (95% confidence interval [CI] 1.56–2.82), and for respiratory tract infection, it was 2.86 (95% CI 2.05–3.97).
The incidence ratio for pulmonary embolism (PE) following urinary tract infection was 2.11 (95% CI 1.38–3.23). Although the risk of PE following respiratory tract infection was 11-fold higher, possible misdiagnosis of PE as a respiratory infection precluded reliable estimates of the precise risk.
Details of the study
The study assessed the risk of a first-ever DVT or PE after acute urinary tract infection or acute systemic respiratory infection, excluding pharyngitis and coryza.
Data were from the United Kingdom’s Health Improvement Network, which has complete diagnostic and prescribing information, and covered the years 1987 to 2004, or approximately 20 million person-years.
One strength was use of a self-controlled case series method, which allowed patients to serve as their own controls, thus eliminating variation among individuals in risk factors for venous thromboembolism.
Patients were observed for 12 months after an acute urinary or respiratory tract infection to determine whether a thromboembolic event had occurred. Incidence ratios and confidence intervals were calculated, and the study had adequate power at 5% significance to detect a 4-fold difference during the first 2 weeks after acute infections.
Expert Commentary
The exact mechanism of thrombosis is still unknown, and the possibility of a common pathway not linked to a specific infection is intriguing. Uncovering the mechanism could help us direct therapy to a particular biochemical process.
Virchow proposed his triad of precipitating factors 150 years ago: venous stasis, increased coagulability of the blood, and vessel wall damage.1 It now seems entirely plausible that damage to the vessel wall need not be physical damage, but could include factors, such as inflammation, that affect endothelial function. As the authors noted, “Inflammation is a key determinant of endothelial function in both arteries and veins, and a link between infection and venous thrombosis via endothelial activation has been suggested.” In fact, earlier studies already identified infection as a potential risk factor for venous thromboembolism.2,3
Thromboembolic events occur at a rate of about 0.5 cases per 1,000 person-years and cause considerable morbidity and mortality.
How long to continue prophylaxis?
The study by Smeeth and colleagues should help ObGyns determine the level of prophylaxis appropriate for hospitalized patients. Less clear is whether thromboprophylaxis should be offered to women who have acute infections in an ambulatory setting. Although earlier studies suggested that thromboprophylaxis may be appropriate, I believe the question of whether every patient should receive preventive therapy remains unanswered.
Another unresolved issue: If prophylaxis is initiated, how long should it continue? Because the risk of a thromboembolic event does not return to baseline levels for 1 year, the duration of therapy could be lengthy. At the same time, the risks of anticoagulation are not inconsequential and may increase with extended therapy. As the greatest risk occurs during the first 8 weeks after infection, prophylaxis is most beneficial during this time.
Routine prophylaxis?
Given the data thus far, I do not believe therapy is warranted for every patient with an acute infection. Selective therapy may be justified.
In this study, the incidence ratio for deep venous thrombosis (DVT) following urinary tract infection was 2.10 (95% confidence interval [CI] 1.56–2.82), and for respiratory tract infection, it was 2.86 (95% CI 2.05–3.97).
The incidence ratio for pulmonary embolism (PE) following urinary tract infection was 2.11 (95% CI 1.38–3.23). Although the risk of PE following respiratory tract infection was 11-fold higher, possible misdiagnosis of PE as a respiratory infection precluded reliable estimates of the precise risk.
Details of the study
The study assessed the risk of a first-ever DVT or PE after acute urinary tract infection or acute systemic respiratory infection, excluding pharyngitis and coryza.
Data were from the United Kingdom’s Health Improvement Network, which has complete diagnostic and prescribing information, and covered the years 1987 to 2004, or approximately 20 million person-years.
One strength was use of a self-controlled case series method, which allowed patients to serve as their own controls, thus eliminating variation among individuals in risk factors for venous thromboembolism.
Patients were observed for 12 months after an acute urinary or respiratory tract infection to determine whether a thromboembolic event had occurred. Incidence ratios and confidence intervals were calculated, and the study had adequate power at 5% significance to detect a 4-fold difference during the first 2 weeks after acute infections.
Expert Commentary
The exact mechanism of thrombosis is still unknown, and the possibility of a common pathway not linked to a specific infection is intriguing. Uncovering the mechanism could help us direct therapy to a particular biochemical process.
Virchow proposed his triad of precipitating factors 150 years ago: venous stasis, increased coagulability of the blood, and vessel wall damage.1 It now seems entirely plausible that damage to the vessel wall need not be physical damage, but could include factors, such as inflammation, that affect endothelial function. As the authors noted, “Inflammation is a key determinant of endothelial function in both arteries and veins, and a link between infection and venous thrombosis via endothelial activation has been suggested.” In fact, earlier studies already identified infection as a potential risk factor for venous thromboembolism.2,3
Thromboembolic events occur at a rate of about 0.5 cases per 1,000 person-years and cause considerable morbidity and mortality.
How long to continue prophylaxis?
The study by Smeeth and colleagues should help ObGyns determine the level of prophylaxis appropriate for hospitalized patients. Less clear is whether thromboprophylaxis should be offered to women who have acute infections in an ambulatory setting. Although earlier studies suggested that thromboprophylaxis may be appropriate, I believe the question of whether every patient should receive preventive therapy remains unanswered.
Another unresolved issue: If prophylaxis is initiated, how long should it continue? Because the risk of a thromboembolic event does not return to baseline levels for 1 year, the duration of therapy could be lengthy. At the same time, the risks of anticoagulation are not inconsequential and may increase with extended therapy. As the greatest risk occurs during the first 8 weeks after infection, prophylaxis is most beneficial during this time.
Routine prophylaxis?
Given the data thus far, I do not believe therapy is warranted for every patient with an acute infection. Selective therapy may be justified.
1. Virchow RLK. Thrombosis and Emboli. Matzdorff AC, Bell WR, trans. Canton, Mass: Science History Publications; 1998.
2. Samama MM. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius study. Arch Intern Med. 2000;160:3415-3420.
3. Alikhan R, Cohen AT, Combe S, et al. Risk factors for venous thromboembolism in hospitalized patients with acute medical illness: analysis of the MEDENOX Study. Arch Intern Med. 2004;164:963-968.
1. Virchow RLK. Thrombosis and Emboli. Matzdorff AC, Bell WR, trans. Canton, Mass: Science History Publications; 1998.
2. Samama MM. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius study. Arch Intern Med. 2000;160:3415-3420.
3. Alikhan R, Cohen AT, Combe S, et al. Risk factors for venous thromboembolism in hospitalized patients with acute medical illness: analysis of the MEDENOX Study. Arch Intern Med. 2004;164:963-968.
Thromboembolic disease: The case for routine prophylaxis
- Prophylaxis must start before surgery for maximal benefit, since at least 50% of postoperative thromboembolic disease begins intraoperatively.
- A consensus conference found that prophylaxis reduced fatal pulmonary emboli by 75% in 7,000 gynecologic surgery patients.
- Low-dose unfractionated heparin and low molecular weight heparin appear similarly effective in reducing thromboembolic disease in perioperative patients, but it is unclear which form has fewer bleeding complications.
The case is strong for routine prophylaxis against venous thrombosis and pulmonary embolism. The primary reasons: efficacy, ease of use, and safety.
This article reviews the evidence on routine prophylaxis, pros and cons of mechanical and drug therapies (including a comparison of 2 heparins), patient risk factors, and cost-effectiveness. A table (page 32) lists patient characteristics for low, moderate, high, and very high levels of risk, with corresponding appropriate preventive measures.
Routine prophylaxis is a wiser strategy than postevent treatment or surveillance because:
- Thromboembolism is a stealthy adversary, particularly pulmonary embolism. Between 10% and 20% of patients with pulmonary emboli die, often within 30 minutes of the sentinel complaint.
- Even when pulmonary embolus is documented during autopsy, as many as 80% of patients have no antecedent clinical evidence of deep venous thrombosis (DVT)—and no chance of life-saving therapy.2
- Surveillance is the least desirable preventive option due to lack of sensitivity of noninvasive tests for thromboembolic disease.
Scope of thromboembolic disease
Thromboembolism is the leading potentially preventable cause of hospital fatality.1
In postoperative gynecologic surgery patients, pulmonary embolism occurs in 0.1% to 5% of cases, depending on risk.2 Venous thrombosis occurs on average in 15% of postoperative gynecologic surgery patients, with a range of 5% to 29%, depending on patient risk factors and the surgical procedures performed.3 Almost 50% of gynecologic patients undergoing surgery for cancer develop a lower-extremity venous thrombosis if left untreated.4
Overall, thromboembolic disease is linked with some 500,000 hospital admissions every year. Pulmonary embolism, the most serious complication, causes 60,000 to 200,000 deaths annually.
High risk of recurrence. Postthrombotic syndrome is characterized by pain, swelling, and leg ulceration. At least 50% of patients successfully treated for proximal venous thrombosis (thigh) and about 33% treated for distal venous thrombosis (calf) develop postthrombotic syndrome.1 The risk for recurrent thrombosis is high.
Risk factors are listed in TABLE 1. The risk of fatal pulmonary embolism is directly related to age; persons over 60 are at greatest risk. When the patient is obese—particularly when she exceeds 120% of ideal body weight—the risk is even greater due to venous stasis.
TABLE 1
Risk factors for thromboembolism
| Age |
| Estrogen use |
| Extended pelvic surgery |
| Hypercoagulable states |
| Immobility |
| Indwelling central venous catheter(s) |
| Lower-extremity paralysis |
| Malignancy |
| Medical illnesses |
| Chronic pulmonary disease |
| Congestive heart failure |
| Diabetes mellitus |
| Inflammatory bowel disease |
| Nephrotic syndrome |
| Obesity |
| Pregnancy |
| Prior thromboembolic disease |
| Radiation therapy |
| Trauma |
| Varicose veins |
Common hypercoagulable states
Activated protein C resistance, the most common thrombophilia, occurs in 3% to 7% of Caucasians (TABLE 2).7 It is usually associated with factor V Leiden mutation.
Prothrombin gene mutation G20210A, the next most common thrombophilia, occurs in 2% of Caucasians.7
Antiphospholipid antibody syndromes are acquired thrombophilias that may be associated with arterial and venous thrombosis, thrombocytopenia, and complications of pregnancy.8
Hyperhomocystinemia may be congenital or acquired. It is associated with venous thromboembolism and early atherosclerosis with arterial thrombosis.
Thrombosis is more likely with multiple predisposing factors. Most patients with one of the thrombophilia syndromes do not have sentinel thrombotic events unless they are further challenged by environmental risks such as oral contraceptives (OCs), pregnancy, prolonged immobilization, or surgery.9
Risk is markedly enhanced if multiple predisposing factors are present. For example, when factor V Leiden mutation and hyperhomocystinemia coexist, the risk for thrombosis increases 10 to 20 times over that of patients with neither abnormality.10 This combined risk far exceeds the sum of risks for each abnormality alone.
TABLE 2
Common hypercoagulable conditions
| Factor V Leiden mutation |
| Prothrombin gene mutation G20210A |
| Antiphospholipid antibody |
| Lupus anticoagulant |
| Anticardiolipin |
| Protein C deficiency |
| Protein S deficiency |
| Antithrombin III deficiency (Heparin cofactor II) |
| Plasminogen deficiency |
| Hyperhomocystinemia |
| Myeloproliferative disorders |
| Polycythemia |
| Primary thrombocytosis |
When to test for thrombophilias
Although women with thrombophilias are at high risk for thromboembolic disease, it is unclear whether identifying the thrombophilia is more beneficial than universal prophylaxis. If a patient has a personal or family history of thromboembolic disease—especially a patient with Caucasian ancestry—testing is probably warranted. While documentation of the thrombophilia may not help with perioperative management, it may be useful for long-term care.
Testing for factor V Leiden mutation is recommended due to its prevalence. If the results are negative in a patient at risk, test for prothrombin gene mutation G20210A, as well as deficiencies in the naturally occurring inhibitors protein C, protein S, and antithrombin III.
Assess antiphospholipid antibodies in women who have experienced recurrent fetal loss or early pregnancy-induced hypertension.
Continue OCs or hormone therapy
OCs and menopausal hormone replacement therapy produce measurable prothrombotic changes in the clotting system that appear to be directly related to the estrogen content. In theory, discontinuing the OC or hormone replacement therapy preoperatively would allow these changes to return to baseline and help prevent thromboembolic disease.
Although the risk of thromboembolic disease is 0.96% if patients are current users of OCs and 0.5% if they are not, studies have failed to confirm a clinical benefit of discontinuation.11 Further, the patient does not return to baseline for 4 to 6 weeks after ceasing therapy.
The potential risk of thromboembolic events also should be weighed against the risk of conception prior to surgery. We usually do not recommend discontinuation of OCs and hormone therapy before surgery, but give prophylaxis based on risk assessment.
Venous stasis, vessel-wall trauma, and increased blood coagulability—the major contributors to perioperative DVT, known as Virchow’s triad—were identified more than 125 years ago.
Venous stasis. Intraoperatively, venous blood return from the lower extremities is reduced to less than half its normal rate,34 secondary to muscle relaxation during anesthesia, which causes venous dilation and reduced blood-flow velocity. Packing the abdominal contents may further impede blood return from the legs.
Resultant venous stasis causes platelet adhesion to the vein wall, followed by release of a thromboplastin-like substance that may trigger thrombus formation.
Blood flow increases in the immediate postoperative period with return of muscle tone, but remains significantly diminished for 21 days because of immobilization—specifically, lack of the usual pumping action of the leg muscles.
Vessel-wall trauma. Veins are highly likely to be damaged as vessels are skeletonized during major pelvic surgery, especially when malignancy is involved. Tissue injury activates the coagulation cascade by exposing blood to tissue thromboplastin (extrinsic path) and subendothelial collagen in the vessel wall, which activates factor XII (intrinsic path). Both pathways lead to conversion of factor X to its active form, factor Xa. Acting in concert with factor V, calcium, and phospholipids from platelet factor III, factor Xa catalyzes the conversion of prothrombin to thrombin. Thrombin regulates the conversion of fibrinogen to fibrin, the basic building block of a thrombus.
Increased blood coagulability. Clotting factors XI, IX, and VII increase following surgery, as do circulating platelets and platelet aggregation. This enhances coagulability, which persists from 72 to 96 hours after surgery but is usually balanced by the fibrinolytic system. Fibrinolysis is mediated primarily by plasmin, which digests fibrin and fibrinogen and activates factors V and VIII. If the fibrinolytic system is overwhelmed, the clotting system is unimpeded and thrombus formation may accelerate.
Pregnancy increases the risk of thrombosis, in part due to the progressive increase in resistance to activated protein C in the second and third trimesters. Risk is increased eightfold in women with inherited deficiency in any of the naturally occurring anticoagulants—antithrombin III, protein C, or protein S—compared with those with no deficiency.12
Preventive strategies
Two approaches to thromboembolic prophylaxis have been proposed, with prevention of fatal postoperative pulmonary emboli as a clear endpoint:
- Stratify a targeted group into levels of risk; then treat those at higher risk. Unfortunately, efforts to define risk have met with only partial success, due to limited availability of noninvasive screening, screening logistics, and expense. Further, specificity and positive predictive value of screening asymptomatic patients is low.
- Use prophylaxis in all patients in the targeted group, regardless of risk (TABLES 3 AND 4). This strategy seems effective. For example, in 2001, the Sixth American College of Chest Physicians Consensus Conference—the most recent of the consensus conferences—evaluated the risks of pulmonary embolus in 7,000 gynecologic surgery patients enrolled in prospective studies. Routine prophylaxis reduced fatal pulmonary emboli by 75%.13
The difficulty of defining patients at highest risk makes the concept of universal prophylaxis for a targeted group an attractive option unless a specific contraindication is identified.
TABLE 3
Risk stratification and prophylactic regimens
| LEVEL OF RISK | PATIENT CHARACTERISTICS | RECOMMENDED REGIMEN |
|---|---|---|
| Low | Less than 40 years of age | No specific recommendation for therapy |
| Undergoing uncomplicated minor surgery | Adequate hydration | |
| Requires less than 30 minutes of anesthesia | Aggressive early ambulation | |
| No additional risk factors | ||
| Moderate | Undergoing minor surgery with additional risk factors | Graduated compression stockings or SPCDs or |
| 40 to 60 years of age, undergoing minor surgery, and no additional risk factors | LDUH 5,000 U every 12 hours or | |
| Less than 40 years of age, undergoing major surgery, and no additional risk factors | LMWH (20 mg) or 2,500 U antifactor Xa once daily | |
| High | More than 60 years of age, undergoing minor surgery or with additional risk factors | LDUH 5,000 U every 8–12 hours or LMWH (40 mg) or 5,000 U antifactor Xa once daily |
| More than 40 years of age undergoing major surgery or with additional risk factors | SPCDs may be added | |
| Very high | More than 60 years of age, undergoing major surgery, with other risk factors such as cancer, prior venous thromboembolism, molecular hypercoagulable state, major trauma, spinal cord injury, or lower-extremity paralysis | Graduated compression stockings or SPCDs and LDUH 5,000 U every 8 hours or |
| Other risk factors such as cancer, prior venous thromboembolism, molecular hypercoagulable state, major trauma, spinal cord injury, or lower-extremity paralysis | LMWH (40 mg) or 5,000 U antifactor Xa once daily | |
| LDUH = low-dose unfractionated heparin; LMWH = low molecular weight heparin; SPCD = sequential pneumatic compression device | ||
| Note: Because perioperative prophylaxis is still evolving, these suggestions should not be considered inviolable. | ||
| Source: Modified from Geerts WH, et al.13 | ||
TABLE 4
Incidence of thromboembolic events based on level of risk
| SITE OF THROMBOSIS | RISK LEVEL (%) | |||
|---|---|---|---|---|
| Low | Moderate | High | Very high | |
| Calf veins (distal) | 2 | 10–35 | 30–60 | 63.5 |
| Iliofemoral veins (proximal) | 0.4 | 2–8 | 5–10 | 10–20 |
| Pulmonary emboli | 0.2 | 1–2 | 2–4 | 4–10 |
| Fatal pulmonary emboli | 0.002 | 0.1–0.5 | 0.4–1 | 0.2–5 |
| Source: Modified from Geerts WH, et al.13 | ||||
Drug and mechanical therapies
Strategies for preventing thromboembolic disease attempt to mitigate the impact of venous stasis, endothelial injury, and hypercoagulable states. Traditionally, early ambulation, adequate hydration, and elevation of the lower extremities have been used, because they are simple and inexpensive interventions.
More recently, pharmacologic and mechanical therapies have proven effective in reducing the incidence of DVT and fatal pulmonary emboli. Prophylactic measures commonly used today are:
- graduated compression stockings
- external pneumatic leg or foot compression devices
- low-dose unfractionated heparin
- low molecular weight heparin
MECHANICAL THERAPIESGraduated compression stockings
Compression stockings are one of the earliest methods of preventing perioperative thrombosis. Compression is greatest at the toe and gradually diminishes toward the thigh. When Belcaro14 studied the risk of recurrent venous thrombosis in nonsurgical hospitalized patients, thrombosis recurred in 40% of patients with no therapy, but only 9.4% of patients wearing graduated compression stockings (GCS). Adding oral antiplatelet therapy lowered the risk to 2%. However, GCS were not superior to any other method of preventing recurrent DVT.
As for surgical patients, a single study15 demonstrated protection against DVT when compared with no GCS in elective gynecologic surgery patients.
Today, GCS are usually a perioperative adjunct to other preventive methods, to provide added protection.
Proper fitting by trained personnel is vital; otherwise, a tourniquet effect may cause venous stasis and reduce benefit.
Sequential pneumatic compression devices
Like GCS, these devices decrease the caliber of veins by simple compression. They also increase blood flow velocity and stimulate the endogenous fibrinolytic system.
Enhanced fibrinolytic activity due to intermittent compression occurs even if the device is used on only 1 lower extremity, or on an upper extremity. Patient and nursing-staff compliance may affect efficacy. Sequential pneumatic compression devices (SPCDs) may be thought inconvenient, impeding nursing functions. Some patients may find the repetitive inflation-deflation cycles annoying.
These devices must be activated prior to surgery and continued for a minimum of 24 hours. Some studies suggest that SPCDs be used for 5 days in high-risk situations.16
Calf- and thigh-length devices have similar effects. Rare complications include peroneal nerve injury and compartment syndrome.
What the data show. In an analysis of 4 trials comparing SPCDs with no therapy, thromboembolic disease occurred in only 2% of patients who wore SPCDs but in 20% of those who did not.17 Compared with low-dose unfractionated heparin, no difference in the rate of DVT was seen.18 However, risk of transfusion and retroperitoneal drainage volume increased in the heparin group.
Contraindications include active or suspected DVT, congestive heart failure, known pulmonary embolus, and leg injuries.19
Combined therapy may be best for high-risk women. A study20 of women who developed thromboemboli despite appropriate treatment with SPCDs found the women more likely to be older than 60 years and/or to have cancer or history of thromboembolic disease or hypertension. This high-risk group may benefit from combined therapy.
Foot compression devices resemble booties and mimic the plantar compression that occurs during walking. They increase blood-flow velocity, stimulate the endogenous fibrinolytic system, and have the same indications as SPCDs. However, they are only moderately effective in reducing venous thrombosis.21 Potential drawbacks are that the devices must be removed when the patient ambulates and replaced when she returns to bed.
DRUG THERAPIESLow-dose unfractionated heparin
Heparin alters the molecular configuration of antithrombin III, making it 1,000 to 4,000 times more potent as an inhibitor of thrombin formation, which in turn limits conversion of fibrinogen to fibrin. This prolongs the activated partial thromboplastin time (aPTT) commonly used to monitor patients receiving full anticoagulation therapy.
A naturally occurring mucopolysaccharide with a molecular weight ranging from 3,000 to 30,000 daltons, heparin is richly concentrated in mast cells. Its anticoagulant properties relate primarily to interaction with antithrombin III (also known as heparin cofactor II), factor IXa, factor Xa, factor XIa, factor XIIa, and platelet aggregation.22
Heparin also inhibits the effects of factor Xa on the coagulation cascade and limits platelet aggregation.23
Half-life is 1 hour for intravenous heparin and about 3 hours for subcutaneous heparin.
Use in pregnancy. Low-dose unfractionated heparin (LDUH) does not cross the placenta and is safe to use during pregnancy.
What the data show. Randomized clinical trials conducted prior to 1988 showed venous thrombosis decreased by 70% and pulmonary embolus by 50% in patients treated with LDUH, compared with those receiving no therapy.24
Dosing options. LDUH typically is given as a 5,000-U dose 2 hours before surgery. The single preoperative dose seems to be as effective as multiple preoperative doses.25 Postoperative therapy is instituted 8 to 12 hours after surgery; heparin is given every 8 to 12 hours until the patient is fully ambulatory.
Patients having gynecologic surgery for benign conditions benefit from the twice-daily regimen, and those undergoing gynecologic oncology surgery or other high-risk procedures seem to benefit from thrice-daily dosing.26
These regimens also significantly reduced DVT and fatal pulmonary emboli in general surgery patients.27 The LDUH regimen is now used to judge the efficacy of other prophylactic measures.
Low molecular weight heparin
This form of heparin acts primarily by inhibiting factor Xa, which is higher in the coagulation cascade than antithrombin. Thus, low molecular weight heparin (LMWH) is more efficient than unfractionated heparin.
LMWH has a molecular weight of 3,000 to 6,000 daltons and is produced by concentrating the low molecular component of heparin. Because the molecular configuration of antithrombin III is not altered by LMWH, thrombin conversion is minimally inhibited and aPTT is not appreciably affected.
Half-life is approximately 4 hours, by any route of administration. The longer half-life provides a longer dosing interval. Bioavailability is more consistent than that of LDUH, approaching 90% to 95%. The excellent bioavailability allows dosing to be based on lean body mass. Less heparin-induced thrombocytopenia is another plus.
Use in pregnancy. LMWH does not cross the placenta and is safe to use during pregnancy.
What the data show. No randomized trials have compared LMWH therapy with no therapy, with efficacy assessed by venography or fibrinogen uptake. An uncontrolled series28 of 2,030 patients did show that LMWH reduced the incidence of thromboembolic disease.
A randomized controlled trial29 comparing 2,500 U and 5,000 U daily of dalteparin (an LMWH) found greater efficacy in the higher-dose group (6.6% versus 12.7% incidence of DVT), but bleeding complications also were higher (4.7% versus 2.7%). In a subgroup of patients with malignancy, the high-dose therapy remained superior in preventing DVT, and the bleeding risk was equal.
Dosing options. Once a day dosing is normally adequate for prophylaxis; twice-daily dosing is needed for therapy. Enoxaparin is an LMWH that is readily available in the United States and commonly prescribed when use of LMWH is desired. For prophylaxis, it can be given in daily doses of 20 mg, 30 mg, 40 mg, or 60 mg. None of these doses has proven superior to the others. The typical regimen for moderate risk is 20 mg per day; for high risk, 40 mg per day. Enoxaparin appears to convey the same degree of protection as 5,000 units of LDUH every 8 hours.
2 heparins compared
Randomized controlled trials comparing LDUH and LMWH have found them to be similarly effective. In addition, within the LMWH class, all compounds appear to have similar benefits. However, it remains unclear which form of heparin is associated with fewer bleeding complications.
In theory, LMWH would be associated with an increase in these complications due to its longer half-life and increased bioavailability.30 However, studies have not consistently identified excess bleeding in any group.31 Fortunately, excess postoperative bleeding requiring transfusion is uncommon.
Complications of heparin
Heparin-induced thrombocytopenia is a recognized complication, seen in as many as 20% of LDUH patients. The diagnosis is made when the platelet count falls below the lower limits of normal or when the platelet count falls by 50% but remains in the normal range. Two forms of heparin-induced thrombocytopenia have been described.
• Type 1 thrombocytopenia is initially mild, rarely dropping below 100,000 platelets per milliliter. Platelet count monitoring is important, but therapy usually can continue, since the platelet count returns to normal even with continued use. Type 1 thrombocytopenia appears to directly result from platelet activation and is not immune-mediated.
• Type 2 thrombocytopenia occurs 7 to 14 days after starting therapy. Platelet counts frequently drop to 20,000 per milliliter. Type 2 is an immune response caused by antibodies to the heparin-platelet factor 4 complex. Patients are at risk for venous and arterial thrombosis, but often the diagnosis is made only after a complicating thrombotic event.
The risk of type 2 thrombocytopenia appears to be related to the molecular weight of the compound being administered, its dose, and the duration of therapy. Therefore, unfractionated heparin—if given in full anticoagulation doses and beyond 14 days—seems to have the greatest potential for this complication.
Paradoxically, these patients also are at risk for severe hemorrhage. Because mortality and morbidity are high, immediate withdrawal of all forms of heparin—including LMWH—is mandatory.32 Catastrophic hemorrhage or thrombosis may be the first sign. Often a fall in platelet concentration precedes serious complications. Thus, platelet concentration should be monitored at least every 2 days.
Hematoma with conduction anesthesia. Use of major conduction anesthesia in patients who also need LMWH, LDUH, or oral anticoagulants is controversial. LMWH may pose a risk of hematoma if initiated preoperatively, intraoperatively, or within 3 hours of surgery in patients who have a continuous epidural. Hematoma formation seems to occur immediately following catheter withdrawal.
The risk of hematoma appears to be lower with single-dose spinal or single-dose epidural anesthesia. While the incidence of epidural or spinal hematoma is not known, some cases have been associated with long-term neurologic sequelae, including permanent paralysis.
Responding to this concern, the US Food and Drug Administration issued a 1997 advisory noting the risk of spinal hematoma in patients receiving enoxaparin plus conduction anesthesia or lumbar spinal puncture. Most anesthesiologists significantly restrict the use of conduction anesthesia in patients requiring heparin prophylaxis.33
Cost-effectiveness
The cost of perioperative prophylaxis has been compared with the cost of immediate therapy for thromboembolic disease and long-term therapy for postthrombotic syndrome. Across the board, universal prophylaxis with pharmacotherapy or mechanical devices in patients undergoing abdominal surgery is less expensive than no prophylaxis, based on a reduced incidence of DVT. Pneumatic compression seems to be more cost-effective than pharmacotherapy.34
Because LDUH is substantially less expensive than LMWH in the United States, it has a better cost profile when pharmacotherapy is warranted. In Europe, where the cost of heparin compounds is not an issue, LMWH has a slight advantage.
The authors report no financial relationships relevant to this article.
1. Dismuke SE, Wagner EH. Pulmonary embolism as a cause of death. The changing mortality in hospitalized patients. JAMA. 1986;255:2039-2042.
2. Farquharson DI, Orr JW, Jr. Prophylaxis against thromboembolism in gynecologic patients. J Reprod Med. 1984;29:845-862.
3. Bergqvist D. Prolonged prophylaxis against postoperative venous thromboembolism. Haemostasis. 1996;26(suppl 4):379-387.
4. Clarke-Pearson DL, DeLong ER, Synan IS, Coleman RE, Creasman WT. Variables associated with postoperative deep venous thrombosis: a prospective study of 411 gynecology patients and creation of a prognostic model. Obstet Gynecol. 1987;69:146-150.
5. Kemble JVH. Incidence of deep vein thrombosis. Br J Hosp Med. 1971;6:721-726.
6. Spritzer CE, Evans AC, Kay HH. Magnetic resonance imaging of deep venous thrombosis in pregnant women with lower extremity edema. Obstet Gynecol. 1995;85:603-607.
7. Florell SR, Rodgers GM. Inherited thrombotic disorders: an update. Am J Hematol. 1997;54:53-60.
8. Shapiro GA. Antiphospholipid syndrome in obstetrics and gynecology. Semin Thromb Hemost. 1994;20:64-70.
9. De Stafano V, Leone G, Mastrangelo S, et al. Clinical manifestations and management of inherited thrombophilia: retrospective analysis and follow-up after diagnosis in 238 patients with congenital deficiency of antithrombin III, protein C, protein S. S Throm Haemost. 1994;72:352-358.
10. Ridker PM, Hennekens CH, Selhub J, et al. Interrelation of hyperhomocyst(e)inemia, factor V Leiden, and risk of future venous thromboembolism. Circulation. 1997;95:1777-1782.
11. Vessey M, Jewell D, Smith A, Yeates D, McPherson K. Chronic inflammatory bowel disease, cigarette smoking, and use of oral contraceptives: findings in a large cohort study of women of childbearing age. Br Med J (Clin Res Ed). 1986;292:1101-1103.
12. Walker MC, Garner PR, Keely EJ, et al. Changes in activated protein C resistance during normal pregnancy. Am J Obstet Gynecol. 1997;177:162-169.
13. Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest. 2001;119(suppl 1):132S-175S.
14. Belcaro G, Laurora G, Cesarone MR, et al. Prophylaxis of recurrent deep venous thrombosis. A randomized, prospective study using indobufen and graduated elastic compression stockings. Angiology. 1993;44:695-699.
15. Clarke-Pearson DL, Synan IS, Dodge R, et al. A randomized trial of low-dose heparin and intermittent pneumatic calf compression for the prevention of deep venous thrombosis after gynecologic oncology surgery. Am J Obstet Gynecol. 1993;168:1146-1153.
16. Handoll HH, Farrar MJ, McBirnie J, et al. Prophylaxis using heparin, low molecular weight heparin and physical methods against deep vein thrombosis and pulmonary embolism in hip fracture. The Cochrane Library, Issue I. 1998. Evidence-based medicine: 146.
17. Clarke-Pearson DL, Synan IS, Hinshaw WM, et al. Prevention of postoperative venous thromboembolism by external pneumatic calf compression in patients with gynecologic malignancy. Obstet Gynecol. 1984;63(1):92-98.
18. Lachman EA, Rouk JL, et al. Complications associated with intermittent pneumatic compression. Arch Phys Med Rehab. 1992;73:482-485.
19. Clarke-Pearson DL, Dodge RK, Synan I, et al. Venous thromboembolism prophylaxis: patients at high risk to fail intermittent pneumatic compression. Obstet Gynecol. 101;2003:157-163.
20. Wilson NV, Das SK, Kakkar VV, et al. Thrombo-embolic prophylaxis in total knee replacement. Evaluation of the A-V Impulse System. J Bone Joint Surg Br. 1992;74:50-52.
21. Rosenberg RD, Damus PS. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973;248:6490-6505.
22. Scurr JH, Coleridge-Smith PD, Hasty JH. Regimen for improved effectiveness of intermittent pneumatic compression in deep venous thrombosis prophylaxis. Surgery. 1987;102:816-820.
23. Collins R, Scrimgeour A, Yusuf S, et al. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162-1173.
24. Hull RD, Pineo GF, Stein PD, et al. Timing of initial administration of low-molecular-weight heparin prophylaxis against deep vein thrombosis in patients following elective hip arthroplasty: a systematic review. Arch Intern Med. 2001;161:1952-1960.
25. Clarke-Pearson DL, DeLong ER, Synan IS, et al. A controlled trial of two low dose heparin regimens for the prevention of postoperative deep vein thrombosis. Obstet Gynecol. 1990;75:684-689.
26. Kakkar VV, Murray WJ. Efficacy and safety of low-molecular-weight heparin (CY216) in preventing postoperative venous thrombo-embolism: a co-operative study. Br J Surg. 1985;72:786-791.
27. Ward B, Pradhan S. Comparison of low molecular weight heparin (Fragmin) with sodium heparin for prophylaxis against postoperative thrombosis in women undergoing major gynaecological surgery. Aust N Z J Obstet Gynaecol. 1998;38:91-92.
28. Bergqvist D, Burmark US, Flordal PA, et al. Low molecular weight heparin started before surgery as prophylaxis against deep vein thrombosis: 2500 versus 5000 XaI units in 2070 patients. Br J Surg. 1995;82:496-501.
29. Bratt G, Tornebohm E, Widlund L, et al. Low molecular weight heparin (KABI 2165, Fragmin): pharmacokinetics after intravenous and subcutaneous administration in human volunteers. Thromb Res. 1986;42:613-620.
30. Kakkar VV, Boedki O, Boneau B, et al. Efficacy and safety profile of a low-molecular weight heparin and standard unfractionated heparin for prophylaxis of postoperative venous thromboembolism: European multicenter trial. World J Surg. 1997;21:2-8.
31. Warkentin TE. Heparin-induced thrombocytopenia: pathogenesis and management. Br J Haematol. 2003;121:535-555.
32. Wysowski DK, Talarico L, Bacsanyi J, et al. Spinal and epidural hematoma and low-molecular-weight heparin. N Engl J Med. 1998;338:1774-1775.
33. Maxwell GL, Myers ER, Clarke-Pearson DL. Cost-effectiveness of deep venous thrombosis prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 2000;95:206-214.
34. Doran FSA. Prevention of deep vein thrombosis. Br J Hosp Med. 1971;6:773-779.
- Prophylaxis must start before surgery for maximal benefit, since at least 50% of postoperative thromboembolic disease begins intraoperatively.
- A consensus conference found that prophylaxis reduced fatal pulmonary emboli by 75% in 7,000 gynecologic surgery patients.
- Low-dose unfractionated heparin and low molecular weight heparin appear similarly effective in reducing thromboembolic disease in perioperative patients, but it is unclear which form has fewer bleeding complications.
The case is strong for routine prophylaxis against venous thrombosis and pulmonary embolism. The primary reasons: efficacy, ease of use, and safety.
This article reviews the evidence on routine prophylaxis, pros and cons of mechanical and drug therapies (including a comparison of 2 heparins), patient risk factors, and cost-effectiveness. A table (page 32) lists patient characteristics for low, moderate, high, and very high levels of risk, with corresponding appropriate preventive measures.
Routine prophylaxis is a wiser strategy than postevent treatment or surveillance because:
- Thromboembolism is a stealthy adversary, particularly pulmonary embolism. Between 10% and 20% of patients with pulmonary emboli die, often within 30 minutes of the sentinel complaint.
- Even when pulmonary embolus is documented during autopsy, as many as 80% of patients have no antecedent clinical evidence of deep venous thrombosis (DVT)—and no chance of life-saving therapy.2
- Surveillance is the least desirable preventive option due to lack of sensitivity of noninvasive tests for thromboembolic disease.
Scope of thromboembolic disease
Thromboembolism is the leading potentially preventable cause of hospital fatality.1
In postoperative gynecologic surgery patients, pulmonary embolism occurs in 0.1% to 5% of cases, depending on risk.2 Venous thrombosis occurs on average in 15% of postoperative gynecologic surgery patients, with a range of 5% to 29%, depending on patient risk factors and the surgical procedures performed.3 Almost 50% of gynecologic patients undergoing surgery for cancer develop a lower-extremity venous thrombosis if left untreated.4
Overall, thromboembolic disease is linked with some 500,000 hospital admissions every year. Pulmonary embolism, the most serious complication, causes 60,000 to 200,000 deaths annually.
High risk of recurrence. Postthrombotic syndrome is characterized by pain, swelling, and leg ulceration. At least 50% of patients successfully treated for proximal venous thrombosis (thigh) and about 33% treated for distal venous thrombosis (calf) develop postthrombotic syndrome.1 The risk for recurrent thrombosis is high.
Risk factors are listed in TABLE 1. The risk of fatal pulmonary embolism is directly related to age; persons over 60 are at greatest risk. When the patient is obese—particularly when she exceeds 120% of ideal body weight—the risk is even greater due to venous stasis.
TABLE 1
Risk factors for thromboembolism
| Age |
| Estrogen use |
| Extended pelvic surgery |
| Hypercoagulable states |
| Immobility |
| Indwelling central venous catheter(s) |
| Lower-extremity paralysis |
| Malignancy |
| Medical illnesses |
| Chronic pulmonary disease |
| Congestive heart failure |
| Diabetes mellitus |
| Inflammatory bowel disease |
| Nephrotic syndrome |
| Obesity |
| Pregnancy |
| Prior thromboembolic disease |
| Radiation therapy |
| Trauma |
| Varicose veins |
Common hypercoagulable states
Activated protein C resistance, the most common thrombophilia, occurs in 3% to 7% of Caucasians (TABLE 2).7 It is usually associated with factor V Leiden mutation.
Prothrombin gene mutation G20210A, the next most common thrombophilia, occurs in 2% of Caucasians.7
Antiphospholipid antibody syndromes are acquired thrombophilias that may be associated with arterial and venous thrombosis, thrombocytopenia, and complications of pregnancy.8
Hyperhomocystinemia may be congenital or acquired. It is associated with venous thromboembolism and early atherosclerosis with arterial thrombosis.
Thrombosis is more likely with multiple predisposing factors. Most patients with one of the thrombophilia syndromes do not have sentinel thrombotic events unless they are further challenged by environmental risks such as oral contraceptives (OCs), pregnancy, prolonged immobilization, or surgery.9
Risk is markedly enhanced if multiple predisposing factors are present. For example, when factor V Leiden mutation and hyperhomocystinemia coexist, the risk for thrombosis increases 10 to 20 times over that of patients with neither abnormality.10 This combined risk far exceeds the sum of risks for each abnormality alone.
TABLE 2
Common hypercoagulable conditions
| Factor V Leiden mutation |
| Prothrombin gene mutation G20210A |
| Antiphospholipid antibody |
| Lupus anticoagulant |
| Anticardiolipin |
| Protein C deficiency |
| Protein S deficiency |
| Antithrombin III deficiency (Heparin cofactor II) |
| Plasminogen deficiency |
| Hyperhomocystinemia |
| Myeloproliferative disorders |
| Polycythemia |
| Primary thrombocytosis |
When to test for thrombophilias
Although women with thrombophilias are at high risk for thromboembolic disease, it is unclear whether identifying the thrombophilia is more beneficial than universal prophylaxis. If a patient has a personal or family history of thromboembolic disease—especially a patient with Caucasian ancestry—testing is probably warranted. While documentation of the thrombophilia may not help with perioperative management, it may be useful for long-term care.
Testing for factor V Leiden mutation is recommended due to its prevalence. If the results are negative in a patient at risk, test for prothrombin gene mutation G20210A, as well as deficiencies in the naturally occurring inhibitors protein C, protein S, and antithrombin III.
Assess antiphospholipid antibodies in women who have experienced recurrent fetal loss or early pregnancy-induced hypertension.
Continue OCs or hormone therapy
OCs and menopausal hormone replacement therapy produce measurable prothrombotic changes in the clotting system that appear to be directly related to the estrogen content. In theory, discontinuing the OC or hormone replacement therapy preoperatively would allow these changes to return to baseline and help prevent thromboembolic disease.
Although the risk of thromboembolic disease is 0.96% if patients are current users of OCs and 0.5% if they are not, studies have failed to confirm a clinical benefit of discontinuation.11 Further, the patient does not return to baseline for 4 to 6 weeks after ceasing therapy.
The potential risk of thromboembolic events also should be weighed against the risk of conception prior to surgery. We usually do not recommend discontinuation of OCs and hormone therapy before surgery, but give prophylaxis based on risk assessment.
Venous stasis, vessel-wall trauma, and increased blood coagulability—the major contributors to perioperative DVT, known as Virchow’s triad—were identified more than 125 years ago.
Venous stasis. Intraoperatively, venous blood return from the lower extremities is reduced to less than half its normal rate,34 secondary to muscle relaxation during anesthesia, which causes venous dilation and reduced blood-flow velocity. Packing the abdominal contents may further impede blood return from the legs.
Resultant venous stasis causes platelet adhesion to the vein wall, followed by release of a thromboplastin-like substance that may trigger thrombus formation.
Blood flow increases in the immediate postoperative period with return of muscle tone, but remains significantly diminished for 21 days because of immobilization—specifically, lack of the usual pumping action of the leg muscles.
Vessel-wall trauma. Veins are highly likely to be damaged as vessels are skeletonized during major pelvic surgery, especially when malignancy is involved. Tissue injury activates the coagulation cascade by exposing blood to tissue thromboplastin (extrinsic path) and subendothelial collagen in the vessel wall, which activates factor XII (intrinsic path). Both pathways lead to conversion of factor X to its active form, factor Xa. Acting in concert with factor V, calcium, and phospholipids from platelet factor III, factor Xa catalyzes the conversion of prothrombin to thrombin. Thrombin regulates the conversion of fibrinogen to fibrin, the basic building block of a thrombus.
Increased blood coagulability. Clotting factors XI, IX, and VII increase following surgery, as do circulating platelets and platelet aggregation. This enhances coagulability, which persists from 72 to 96 hours after surgery but is usually balanced by the fibrinolytic system. Fibrinolysis is mediated primarily by plasmin, which digests fibrin and fibrinogen and activates factors V and VIII. If the fibrinolytic system is overwhelmed, the clotting system is unimpeded and thrombus formation may accelerate.
Pregnancy increases the risk of thrombosis, in part due to the progressive increase in resistance to activated protein C in the second and third trimesters. Risk is increased eightfold in women with inherited deficiency in any of the naturally occurring anticoagulants—antithrombin III, protein C, or protein S—compared with those with no deficiency.12
Preventive strategies
Two approaches to thromboembolic prophylaxis have been proposed, with prevention of fatal postoperative pulmonary emboli as a clear endpoint:
- Stratify a targeted group into levels of risk; then treat those at higher risk. Unfortunately, efforts to define risk have met with only partial success, due to limited availability of noninvasive screening, screening logistics, and expense. Further, specificity and positive predictive value of screening asymptomatic patients is low.
- Use prophylaxis in all patients in the targeted group, regardless of risk (TABLES 3 AND 4). This strategy seems effective. For example, in 2001, the Sixth American College of Chest Physicians Consensus Conference—the most recent of the consensus conferences—evaluated the risks of pulmonary embolus in 7,000 gynecologic surgery patients enrolled in prospective studies. Routine prophylaxis reduced fatal pulmonary emboli by 75%.13
The difficulty of defining patients at highest risk makes the concept of universal prophylaxis for a targeted group an attractive option unless a specific contraindication is identified.
TABLE 3
Risk stratification and prophylactic regimens
| LEVEL OF RISK | PATIENT CHARACTERISTICS | RECOMMENDED REGIMEN |
|---|---|---|
| Low | Less than 40 years of age | No specific recommendation for therapy |
| Undergoing uncomplicated minor surgery | Adequate hydration | |
| Requires less than 30 minutes of anesthesia | Aggressive early ambulation | |
| No additional risk factors | ||
| Moderate | Undergoing minor surgery with additional risk factors | Graduated compression stockings or SPCDs or |
| 40 to 60 years of age, undergoing minor surgery, and no additional risk factors | LDUH 5,000 U every 12 hours or | |
| Less than 40 years of age, undergoing major surgery, and no additional risk factors | LMWH (20 mg) or 2,500 U antifactor Xa once daily | |
| High | More than 60 years of age, undergoing minor surgery or with additional risk factors | LDUH 5,000 U every 8–12 hours or LMWH (40 mg) or 5,000 U antifactor Xa once daily |
| More than 40 years of age undergoing major surgery or with additional risk factors | SPCDs may be added | |
| Very high | More than 60 years of age, undergoing major surgery, with other risk factors such as cancer, prior venous thromboembolism, molecular hypercoagulable state, major trauma, spinal cord injury, or lower-extremity paralysis | Graduated compression stockings or SPCDs and LDUH 5,000 U every 8 hours or |
| Other risk factors such as cancer, prior venous thromboembolism, molecular hypercoagulable state, major trauma, spinal cord injury, or lower-extremity paralysis | LMWH (40 mg) or 5,000 U antifactor Xa once daily | |
| LDUH = low-dose unfractionated heparin; LMWH = low molecular weight heparin; SPCD = sequential pneumatic compression device | ||
| Note: Because perioperative prophylaxis is still evolving, these suggestions should not be considered inviolable. | ||
| Source: Modified from Geerts WH, et al.13 | ||
TABLE 4
Incidence of thromboembolic events based on level of risk
| SITE OF THROMBOSIS | RISK LEVEL (%) | |||
|---|---|---|---|---|
| Low | Moderate | High | Very high | |
| Calf veins (distal) | 2 | 10–35 | 30–60 | 63.5 |
| Iliofemoral veins (proximal) | 0.4 | 2–8 | 5–10 | 10–20 |
| Pulmonary emboli | 0.2 | 1–2 | 2–4 | 4–10 |
| Fatal pulmonary emboli | 0.002 | 0.1–0.5 | 0.4–1 | 0.2–5 |
| Source: Modified from Geerts WH, et al.13 | ||||
Drug and mechanical therapies
Strategies for preventing thromboembolic disease attempt to mitigate the impact of venous stasis, endothelial injury, and hypercoagulable states. Traditionally, early ambulation, adequate hydration, and elevation of the lower extremities have been used, because they are simple and inexpensive interventions.
More recently, pharmacologic and mechanical therapies have proven effective in reducing the incidence of DVT and fatal pulmonary emboli. Prophylactic measures commonly used today are:
- graduated compression stockings
- external pneumatic leg or foot compression devices
- low-dose unfractionated heparin
- low molecular weight heparin
MECHANICAL THERAPIESGraduated compression stockings
Compression stockings are one of the earliest methods of preventing perioperative thrombosis. Compression is greatest at the toe and gradually diminishes toward the thigh. When Belcaro14 studied the risk of recurrent venous thrombosis in nonsurgical hospitalized patients, thrombosis recurred in 40% of patients with no therapy, but only 9.4% of patients wearing graduated compression stockings (GCS). Adding oral antiplatelet therapy lowered the risk to 2%. However, GCS were not superior to any other method of preventing recurrent DVT.
As for surgical patients, a single study15 demonstrated protection against DVT when compared with no GCS in elective gynecologic surgery patients.
Today, GCS are usually a perioperative adjunct to other preventive methods, to provide added protection.
Proper fitting by trained personnel is vital; otherwise, a tourniquet effect may cause venous stasis and reduce benefit.
Sequential pneumatic compression devices
Like GCS, these devices decrease the caliber of veins by simple compression. They also increase blood flow velocity and stimulate the endogenous fibrinolytic system.
Enhanced fibrinolytic activity due to intermittent compression occurs even if the device is used on only 1 lower extremity, or on an upper extremity. Patient and nursing-staff compliance may affect efficacy. Sequential pneumatic compression devices (SPCDs) may be thought inconvenient, impeding nursing functions. Some patients may find the repetitive inflation-deflation cycles annoying.
These devices must be activated prior to surgery and continued for a minimum of 24 hours. Some studies suggest that SPCDs be used for 5 days in high-risk situations.16
Calf- and thigh-length devices have similar effects. Rare complications include peroneal nerve injury and compartment syndrome.
What the data show. In an analysis of 4 trials comparing SPCDs with no therapy, thromboembolic disease occurred in only 2% of patients who wore SPCDs but in 20% of those who did not.17 Compared with low-dose unfractionated heparin, no difference in the rate of DVT was seen.18 However, risk of transfusion and retroperitoneal drainage volume increased in the heparin group.
Contraindications include active or suspected DVT, congestive heart failure, known pulmonary embolus, and leg injuries.19
Combined therapy may be best for high-risk women. A study20 of women who developed thromboemboli despite appropriate treatment with SPCDs found the women more likely to be older than 60 years and/or to have cancer or history of thromboembolic disease or hypertension. This high-risk group may benefit from combined therapy.
Foot compression devices resemble booties and mimic the plantar compression that occurs during walking. They increase blood-flow velocity, stimulate the endogenous fibrinolytic system, and have the same indications as SPCDs. However, they are only moderately effective in reducing venous thrombosis.21 Potential drawbacks are that the devices must be removed when the patient ambulates and replaced when she returns to bed.
DRUG THERAPIESLow-dose unfractionated heparin
Heparin alters the molecular configuration of antithrombin III, making it 1,000 to 4,000 times more potent as an inhibitor of thrombin formation, which in turn limits conversion of fibrinogen to fibrin. This prolongs the activated partial thromboplastin time (aPTT) commonly used to monitor patients receiving full anticoagulation therapy.
A naturally occurring mucopolysaccharide with a molecular weight ranging from 3,000 to 30,000 daltons, heparin is richly concentrated in mast cells. Its anticoagulant properties relate primarily to interaction with antithrombin III (also known as heparin cofactor II), factor IXa, factor Xa, factor XIa, factor XIIa, and platelet aggregation.22
Heparin also inhibits the effects of factor Xa on the coagulation cascade and limits platelet aggregation.23
Half-life is 1 hour for intravenous heparin and about 3 hours for subcutaneous heparin.
Use in pregnancy. Low-dose unfractionated heparin (LDUH) does not cross the placenta and is safe to use during pregnancy.
What the data show. Randomized clinical trials conducted prior to 1988 showed venous thrombosis decreased by 70% and pulmonary embolus by 50% in patients treated with LDUH, compared with those receiving no therapy.24
Dosing options. LDUH typically is given as a 5,000-U dose 2 hours before surgery. The single preoperative dose seems to be as effective as multiple preoperative doses.25 Postoperative therapy is instituted 8 to 12 hours after surgery; heparin is given every 8 to 12 hours until the patient is fully ambulatory.
Patients having gynecologic surgery for benign conditions benefit from the twice-daily regimen, and those undergoing gynecologic oncology surgery or other high-risk procedures seem to benefit from thrice-daily dosing.26
These regimens also significantly reduced DVT and fatal pulmonary emboli in general surgery patients.27 The LDUH regimen is now used to judge the efficacy of other prophylactic measures.
Low molecular weight heparin
This form of heparin acts primarily by inhibiting factor Xa, which is higher in the coagulation cascade than antithrombin. Thus, low molecular weight heparin (LMWH) is more efficient than unfractionated heparin.
LMWH has a molecular weight of 3,000 to 6,000 daltons and is produced by concentrating the low molecular component of heparin. Because the molecular configuration of antithrombin III is not altered by LMWH, thrombin conversion is minimally inhibited and aPTT is not appreciably affected.
Half-life is approximately 4 hours, by any route of administration. The longer half-life provides a longer dosing interval. Bioavailability is more consistent than that of LDUH, approaching 90% to 95%. The excellent bioavailability allows dosing to be based on lean body mass. Less heparin-induced thrombocytopenia is another plus.
Use in pregnancy. LMWH does not cross the placenta and is safe to use during pregnancy.
What the data show. No randomized trials have compared LMWH therapy with no therapy, with efficacy assessed by venography or fibrinogen uptake. An uncontrolled series28 of 2,030 patients did show that LMWH reduced the incidence of thromboembolic disease.
A randomized controlled trial29 comparing 2,500 U and 5,000 U daily of dalteparin (an LMWH) found greater efficacy in the higher-dose group (6.6% versus 12.7% incidence of DVT), but bleeding complications also were higher (4.7% versus 2.7%). In a subgroup of patients with malignancy, the high-dose therapy remained superior in preventing DVT, and the bleeding risk was equal.
Dosing options. Once a day dosing is normally adequate for prophylaxis; twice-daily dosing is needed for therapy. Enoxaparin is an LMWH that is readily available in the United States and commonly prescribed when use of LMWH is desired. For prophylaxis, it can be given in daily doses of 20 mg, 30 mg, 40 mg, or 60 mg. None of these doses has proven superior to the others. The typical regimen for moderate risk is 20 mg per day; for high risk, 40 mg per day. Enoxaparin appears to convey the same degree of protection as 5,000 units of LDUH every 8 hours.
2 heparins compared
Randomized controlled trials comparing LDUH and LMWH have found them to be similarly effective. In addition, within the LMWH class, all compounds appear to have similar benefits. However, it remains unclear which form of heparin is associated with fewer bleeding complications.
In theory, LMWH would be associated with an increase in these complications due to its longer half-life and increased bioavailability.30 However, studies have not consistently identified excess bleeding in any group.31 Fortunately, excess postoperative bleeding requiring transfusion is uncommon.
Complications of heparin
Heparin-induced thrombocytopenia is a recognized complication, seen in as many as 20% of LDUH patients. The diagnosis is made when the platelet count falls below the lower limits of normal or when the platelet count falls by 50% but remains in the normal range. Two forms of heparin-induced thrombocytopenia have been described.
• Type 1 thrombocytopenia is initially mild, rarely dropping below 100,000 platelets per milliliter. Platelet count monitoring is important, but therapy usually can continue, since the platelet count returns to normal even with continued use. Type 1 thrombocytopenia appears to directly result from platelet activation and is not immune-mediated.
• Type 2 thrombocytopenia occurs 7 to 14 days after starting therapy. Platelet counts frequently drop to 20,000 per milliliter. Type 2 is an immune response caused by antibodies to the heparin-platelet factor 4 complex. Patients are at risk for venous and arterial thrombosis, but often the diagnosis is made only after a complicating thrombotic event.
The risk of type 2 thrombocytopenia appears to be related to the molecular weight of the compound being administered, its dose, and the duration of therapy. Therefore, unfractionated heparin—if given in full anticoagulation doses and beyond 14 days—seems to have the greatest potential for this complication.
Paradoxically, these patients also are at risk for severe hemorrhage. Because mortality and morbidity are high, immediate withdrawal of all forms of heparin—including LMWH—is mandatory.32 Catastrophic hemorrhage or thrombosis may be the first sign. Often a fall in platelet concentration precedes serious complications. Thus, platelet concentration should be monitored at least every 2 days.
Hematoma with conduction anesthesia. Use of major conduction anesthesia in patients who also need LMWH, LDUH, or oral anticoagulants is controversial. LMWH may pose a risk of hematoma if initiated preoperatively, intraoperatively, or within 3 hours of surgery in patients who have a continuous epidural. Hematoma formation seems to occur immediately following catheter withdrawal.
The risk of hematoma appears to be lower with single-dose spinal or single-dose epidural anesthesia. While the incidence of epidural or spinal hematoma is not known, some cases have been associated with long-term neurologic sequelae, including permanent paralysis.
Responding to this concern, the US Food and Drug Administration issued a 1997 advisory noting the risk of spinal hematoma in patients receiving enoxaparin plus conduction anesthesia or lumbar spinal puncture. Most anesthesiologists significantly restrict the use of conduction anesthesia in patients requiring heparin prophylaxis.33
Cost-effectiveness
The cost of perioperative prophylaxis has been compared with the cost of immediate therapy for thromboembolic disease and long-term therapy for postthrombotic syndrome. Across the board, universal prophylaxis with pharmacotherapy or mechanical devices in patients undergoing abdominal surgery is less expensive than no prophylaxis, based on a reduced incidence of DVT. Pneumatic compression seems to be more cost-effective than pharmacotherapy.34
Because LDUH is substantially less expensive than LMWH in the United States, it has a better cost profile when pharmacotherapy is warranted. In Europe, where the cost of heparin compounds is not an issue, LMWH has a slight advantage.
The authors report no financial relationships relevant to this article.
- Prophylaxis must start before surgery for maximal benefit, since at least 50% of postoperative thromboembolic disease begins intraoperatively.
- A consensus conference found that prophylaxis reduced fatal pulmonary emboli by 75% in 7,000 gynecologic surgery patients.
- Low-dose unfractionated heparin and low molecular weight heparin appear similarly effective in reducing thromboembolic disease in perioperative patients, but it is unclear which form has fewer bleeding complications.
The case is strong for routine prophylaxis against venous thrombosis and pulmonary embolism. The primary reasons: efficacy, ease of use, and safety.
This article reviews the evidence on routine prophylaxis, pros and cons of mechanical and drug therapies (including a comparison of 2 heparins), patient risk factors, and cost-effectiveness. A table (page 32) lists patient characteristics for low, moderate, high, and very high levels of risk, with corresponding appropriate preventive measures.
Routine prophylaxis is a wiser strategy than postevent treatment or surveillance because:
- Thromboembolism is a stealthy adversary, particularly pulmonary embolism. Between 10% and 20% of patients with pulmonary emboli die, often within 30 minutes of the sentinel complaint.
- Even when pulmonary embolus is documented during autopsy, as many as 80% of patients have no antecedent clinical evidence of deep venous thrombosis (DVT)—and no chance of life-saving therapy.2
- Surveillance is the least desirable preventive option due to lack of sensitivity of noninvasive tests for thromboembolic disease.
Scope of thromboembolic disease
Thromboembolism is the leading potentially preventable cause of hospital fatality.1
In postoperative gynecologic surgery patients, pulmonary embolism occurs in 0.1% to 5% of cases, depending on risk.2 Venous thrombosis occurs on average in 15% of postoperative gynecologic surgery patients, with a range of 5% to 29%, depending on patient risk factors and the surgical procedures performed.3 Almost 50% of gynecologic patients undergoing surgery for cancer develop a lower-extremity venous thrombosis if left untreated.4
Overall, thromboembolic disease is linked with some 500,000 hospital admissions every year. Pulmonary embolism, the most serious complication, causes 60,000 to 200,000 deaths annually.
High risk of recurrence. Postthrombotic syndrome is characterized by pain, swelling, and leg ulceration. At least 50% of patients successfully treated for proximal venous thrombosis (thigh) and about 33% treated for distal venous thrombosis (calf) develop postthrombotic syndrome.1 The risk for recurrent thrombosis is high.
Risk factors are listed in TABLE 1. The risk of fatal pulmonary embolism is directly related to age; persons over 60 are at greatest risk. When the patient is obese—particularly when she exceeds 120% of ideal body weight—the risk is even greater due to venous stasis.
TABLE 1
Risk factors for thromboembolism
| Age |
| Estrogen use |
| Extended pelvic surgery |
| Hypercoagulable states |
| Immobility |
| Indwelling central venous catheter(s) |
| Lower-extremity paralysis |
| Malignancy |
| Medical illnesses |
| Chronic pulmonary disease |
| Congestive heart failure |
| Diabetes mellitus |
| Inflammatory bowel disease |
| Nephrotic syndrome |
| Obesity |
| Pregnancy |
| Prior thromboembolic disease |
| Radiation therapy |
| Trauma |
| Varicose veins |
Common hypercoagulable states
Activated protein C resistance, the most common thrombophilia, occurs in 3% to 7% of Caucasians (TABLE 2).7 It is usually associated with factor V Leiden mutation.
Prothrombin gene mutation G20210A, the next most common thrombophilia, occurs in 2% of Caucasians.7
Antiphospholipid antibody syndromes are acquired thrombophilias that may be associated with arterial and venous thrombosis, thrombocytopenia, and complications of pregnancy.8
Hyperhomocystinemia may be congenital or acquired. It is associated with venous thromboembolism and early atherosclerosis with arterial thrombosis.
Thrombosis is more likely with multiple predisposing factors. Most patients with one of the thrombophilia syndromes do not have sentinel thrombotic events unless they are further challenged by environmental risks such as oral contraceptives (OCs), pregnancy, prolonged immobilization, or surgery.9
Risk is markedly enhanced if multiple predisposing factors are present. For example, when factor V Leiden mutation and hyperhomocystinemia coexist, the risk for thrombosis increases 10 to 20 times over that of patients with neither abnormality.10 This combined risk far exceeds the sum of risks for each abnormality alone.
TABLE 2
Common hypercoagulable conditions
| Factor V Leiden mutation |
| Prothrombin gene mutation G20210A |
| Antiphospholipid antibody |
| Lupus anticoagulant |
| Anticardiolipin |
| Protein C deficiency |
| Protein S deficiency |
| Antithrombin III deficiency (Heparin cofactor II) |
| Plasminogen deficiency |
| Hyperhomocystinemia |
| Myeloproliferative disorders |
| Polycythemia |
| Primary thrombocytosis |
When to test for thrombophilias
Although women with thrombophilias are at high risk for thromboembolic disease, it is unclear whether identifying the thrombophilia is more beneficial than universal prophylaxis. If a patient has a personal or family history of thromboembolic disease—especially a patient with Caucasian ancestry—testing is probably warranted. While documentation of the thrombophilia may not help with perioperative management, it may be useful for long-term care.
Testing for factor V Leiden mutation is recommended due to its prevalence. If the results are negative in a patient at risk, test for prothrombin gene mutation G20210A, as well as deficiencies in the naturally occurring inhibitors protein C, protein S, and antithrombin III.
Assess antiphospholipid antibodies in women who have experienced recurrent fetal loss or early pregnancy-induced hypertension.
Continue OCs or hormone therapy
OCs and menopausal hormone replacement therapy produce measurable prothrombotic changes in the clotting system that appear to be directly related to the estrogen content. In theory, discontinuing the OC or hormone replacement therapy preoperatively would allow these changes to return to baseline and help prevent thromboembolic disease.
Although the risk of thromboembolic disease is 0.96% if patients are current users of OCs and 0.5% if they are not, studies have failed to confirm a clinical benefit of discontinuation.11 Further, the patient does not return to baseline for 4 to 6 weeks after ceasing therapy.
The potential risk of thromboembolic events also should be weighed against the risk of conception prior to surgery. We usually do not recommend discontinuation of OCs and hormone therapy before surgery, but give prophylaxis based on risk assessment.
Venous stasis, vessel-wall trauma, and increased blood coagulability—the major contributors to perioperative DVT, known as Virchow’s triad—were identified more than 125 years ago.
Venous stasis. Intraoperatively, venous blood return from the lower extremities is reduced to less than half its normal rate,34 secondary to muscle relaxation during anesthesia, which causes venous dilation and reduced blood-flow velocity. Packing the abdominal contents may further impede blood return from the legs.
Resultant venous stasis causes platelet adhesion to the vein wall, followed by release of a thromboplastin-like substance that may trigger thrombus formation.
Blood flow increases in the immediate postoperative period with return of muscle tone, but remains significantly diminished for 21 days because of immobilization—specifically, lack of the usual pumping action of the leg muscles.
Vessel-wall trauma. Veins are highly likely to be damaged as vessels are skeletonized during major pelvic surgery, especially when malignancy is involved. Tissue injury activates the coagulation cascade by exposing blood to tissue thromboplastin (extrinsic path) and subendothelial collagen in the vessel wall, which activates factor XII (intrinsic path). Both pathways lead to conversion of factor X to its active form, factor Xa. Acting in concert with factor V, calcium, and phospholipids from platelet factor III, factor Xa catalyzes the conversion of prothrombin to thrombin. Thrombin regulates the conversion of fibrinogen to fibrin, the basic building block of a thrombus.
Increased blood coagulability. Clotting factors XI, IX, and VII increase following surgery, as do circulating platelets and platelet aggregation. This enhances coagulability, which persists from 72 to 96 hours after surgery but is usually balanced by the fibrinolytic system. Fibrinolysis is mediated primarily by plasmin, which digests fibrin and fibrinogen and activates factors V and VIII. If the fibrinolytic system is overwhelmed, the clotting system is unimpeded and thrombus formation may accelerate.
Pregnancy increases the risk of thrombosis, in part due to the progressive increase in resistance to activated protein C in the second and third trimesters. Risk is increased eightfold in women with inherited deficiency in any of the naturally occurring anticoagulants—antithrombin III, protein C, or protein S—compared with those with no deficiency.12
Preventive strategies
Two approaches to thromboembolic prophylaxis have been proposed, with prevention of fatal postoperative pulmonary emboli as a clear endpoint:
- Stratify a targeted group into levels of risk; then treat those at higher risk. Unfortunately, efforts to define risk have met with only partial success, due to limited availability of noninvasive screening, screening logistics, and expense. Further, specificity and positive predictive value of screening asymptomatic patients is low.
- Use prophylaxis in all patients in the targeted group, regardless of risk (TABLES 3 AND 4). This strategy seems effective. For example, in 2001, the Sixth American College of Chest Physicians Consensus Conference—the most recent of the consensus conferences—evaluated the risks of pulmonary embolus in 7,000 gynecologic surgery patients enrolled in prospective studies. Routine prophylaxis reduced fatal pulmonary emboli by 75%.13
The difficulty of defining patients at highest risk makes the concept of universal prophylaxis for a targeted group an attractive option unless a specific contraindication is identified.
TABLE 3
Risk stratification and prophylactic regimens
| LEVEL OF RISK | PATIENT CHARACTERISTICS | RECOMMENDED REGIMEN |
|---|---|---|
| Low | Less than 40 years of age | No specific recommendation for therapy |
| Undergoing uncomplicated minor surgery | Adequate hydration | |
| Requires less than 30 minutes of anesthesia | Aggressive early ambulation | |
| No additional risk factors | ||
| Moderate | Undergoing minor surgery with additional risk factors | Graduated compression stockings or SPCDs or |
| 40 to 60 years of age, undergoing minor surgery, and no additional risk factors | LDUH 5,000 U every 12 hours or | |
| Less than 40 years of age, undergoing major surgery, and no additional risk factors | LMWH (20 mg) or 2,500 U antifactor Xa once daily | |
| High | More than 60 years of age, undergoing minor surgery or with additional risk factors | LDUH 5,000 U every 8–12 hours or LMWH (40 mg) or 5,000 U antifactor Xa once daily |
| More than 40 years of age undergoing major surgery or with additional risk factors | SPCDs may be added | |
| Very high | More than 60 years of age, undergoing major surgery, with other risk factors such as cancer, prior venous thromboembolism, molecular hypercoagulable state, major trauma, spinal cord injury, or lower-extremity paralysis | Graduated compression stockings or SPCDs and LDUH 5,000 U every 8 hours or |
| Other risk factors such as cancer, prior venous thromboembolism, molecular hypercoagulable state, major trauma, spinal cord injury, or lower-extremity paralysis | LMWH (40 mg) or 5,000 U antifactor Xa once daily | |
| LDUH = low-dose unfractionated heparin; LMWH = low molecular weight heparin; SPCD = sequential pneumatic compression device | ||
| Note: Because perioperative prophylaxis is still evolving, these suggestions should not be considered inviolable. | ||
| Source: Modified from Geerts WH, et al.13 | ||
TABLE 4
Incidence of thromboembolic events based on level of risk
| SITE OF THROMBOSIS | RISK LEVEL (%) | |||
|---|---|---|---|---|
| Low | Moderate | High | Very high | |
| Calf veins (distal) | 2 | 10–35 | 30–60 | 63.5 |
| Iliofemoral veins (proximal) | 0.4 | 2–8 | 5–10 | 10–20 |
| Pulmonary emboli | 0.2 | 1–2 | 2–4 | 4–10 |
| Fatal pulmonary emboli | 0.002 | 0.1–0.5 | 0.4–1 | 0.2–5 |
| Source: Modified from Geerts WH, et al.13 | ||||
Drug and mechanical therapies
Strategies for preventing thromboembolic disease attempt to mitigate the impact of venous stasis, endothelial injury, and hypercoagulable states. Traditionally, early ambulation, adequate hydration, and elevation of the lower extremities have been used, because they are simple and inexpensive interventions.
More recently, pharmacologic and mechanical therapies have proven effective in reducing the incidence of DVT and fatal pulmonary emboli. Prophylactic measures commonly used today are:
- graduated compression stockings
- external pneumatic leg or foot compression devices
- low-dose unfractionated heparin
- low molecular weight heparin
MECHANICAL THERAPIESGraduated compression stockings
Compression stockings are one of the earliest methods of preventing perioperative thrombosis. Compression is greatest at the toe and gradually diminishes toward the thigh. When Belcaro14 studied the risk of recurrent venous thrombosis in nonsurgical hospitalized patients, thrombosis recurred in 40% of patients with no therapy, but only 9.4% of patients wearing graduated compression stockings (GCS). Adding oral antiplatelet therapy lowered the risk to 2%. However, GCS were not superior to any other method of preventing recurrent DVT.
As for surgical patients, a single study15 demonstrated protection against DVT when compared with no GCS in elective gynecologic surgery patients.
Today, GCS are usually a perioperative adjunct to other preventive methods, to provide added protection.
Proper fitting by trained personnel is vital; otherwise, a tourniquet effect may cause venous stasis and reduce benefit.
Sequential pneumatic compression devices
Like GCS, these devices decrease the caliber of veins by simple compression. They also increase blood flow velocity and stimulate the endogenous fibrinolytic system.
Enhanced fibrinolytic activity due to intermittent compression occurs even if the device is used on only 1 lower extremity, or on an upper extremity. Patient and nursing-staff compliance may affect efficacy. Sequential pneumatic compression devices (SPCDs) may be thought inconvenient, impeding nursing functions. Some patients may find the repetitive inflation-deflation cycles annoying.
These devices must be activated prior to surgery and continued for a minimum of 24 hours. Some studies suggest that SPCDs be used for 5 days in high-risk situations.16
Calf- and thigh-length devices have similar effects. Rare complications include peroneal nerve injury and compartment syndrome.
What the data show. In an analysis of 4 trials comparing SPCDs with no therapy, thromboembolic disease occurred in only 2% of patients who wore SPCDs but in 20% of those who did not.17 Compared with low-dose unfractionated heparin, no difference in the rate of DVT was seen.18 However, risk of transfusion and retroperitoneal drainage volume increased in the heparin group.
Contraindications include active or suspected DVT, congestive heart failure, known pulmonary embolus, and leg injuries.19
Combined therapy may be best for high-risk women. A study20 of women who developed thromboemboli despite appropriate treatment with SPCDs found the women more likely to be older than 60 years and/or to have cancer or history of thromboembolic disease or hypertension. This high-risk group may benefit from combined therapy.
Foot compression devices resemble booties and mimic the plantar compression that occurs during walking. They increase blood-flow velocity, stimulate the endogenous fibrinolytic system, and have the same indications as SPCDs. However, they are only moderately effective in reducing venous thrombosis.21 Potential drawbacks are that the devices must be removed when the patient ambulates and replaced when she returns to bed.
DRUG THERAPIESLow-dose unfractionated heparin
Heparin alters the molecular configuration of antithrombin III, making it 1,000 to 4,000 times more potent as an inhibitor of thrombin formation, which in turn limits conversion of fibrinogen to fibrin. This prolongs the activated partial thromboplastin time (aPTT) commonly used to monitor patients receiving full anticoagulation therapy.
A naturally occurring mucopolysaccharide with a molecular weight ranging from 3,000 to 30,000 daltons, heparin is richly concentrated in mast cells. Its anticoagulant properties relate primarily to interaction with antithrombin III (also known as heparin cofactor II), factor IXa, factor Xa, factor XIa, factor XIIa, and platelet aggregation.22
Heparin also inhibits the effects of factor Xa on the coagulation cascade and limits platelet aggregation.23
Half-life is 1 hour for intravenous heparin and about 3 hours for subcutaneous heparin.
Use in pregnancy. Low-dose unfractionated heparin (LDUH) does not cross the placenta and is safe to use during pregnancy.
What the data show. Randomized clinical trials conducted prior to 1988 showed venous thrombosis decreased by 70% and pulmonary embolus by 50% in patients treated with LDUH, compared with those receiving no therapy.24
Dosing options. LDUH typically is given as a 5,000-U dose 2 hours before surgery. The single preoperative dose seems to be as effective as multiple preoperative doses.25 Postoperative therapy is instituted 8 to 12 hours after surgery; heparin is given every 8 to 12 hours until the patient is fully ambulatory.
Patients having gynecologic surgery for benign conditions benefit from the twice-daily regimen, and those undergoing gynecologic oncology surgery or other high-risk procedures seem to benefit from thrice-daily dosing.26
These regimens also significantly reduced DVT and fatal pulmonary emboli in general surgery patients.27 The LDUH regimen is now used to judge the efficacy of other prophylactic measures.
Low molecular weight heparin
This form of heparin acts primarily by inhibiting factor Xa, which is higher in the coagulation cascade than antithrombin. Thus, low molecular weight heparin (LMWH) is more efficient than unfractionated heparin.
LMWH has a molecular weight of 3,000 to 6,000 daltons and is produced by concentrating the low molecular component of heparin. Because the molecular configuration of antithrombin III is not altered by LMWH, thrombin conversion is minimally inhibited and aPTT is not appreciably affected.
Half-life is approximately 4 hours, by any route of administration. The longer half-life provides a longer dosing interval. Bioavailability is more consistent than that of LDUH, approaching 90% to 95%. The excellent bioavailability allows dosing to be based on lean body mass. Less heparin-induced thrombocytopenia is another plus.
Use in pregnancy. LMWH does not cross the placenta and is safe to use during pregnancy.
What the data show. No randomized trials have compared LMWH therapy with no therapy, with efficacy assessed by venography or fibrinogen uptake. An uncontrolled series28 of 2,030 patients did show that LMWH reduced the incidence of thromboembolic disease.
A randomized controlled trial29 comparing 2,500 U and 5,000 U daily of dalteparin (an LMWH) found greater efficacy in the higher-dose group (6.6% versus 12.7% incidence of DVT), but bleeding complications also were higher (4.7% versus 2.7%). In a subgroup of patients with malignancy, the high-dose therapy remained superior in preventing DVT, and the bleeding risk was equal.
Dosing options. Once a day dosing is normally adequate for prophylaxis; twice-daily dosing is needed for therapy. Enoxaparin is an LMWH that is readily available in the United States and commonly prescribed when use of LMWH is desired. For prophylaxis, it can be given in daily doses of 20 mg, 30 mg, 40 mg, or 60 mg. None of these doses has proven superior to the others. The typical regimen for moderate risk is 20 mg per day; for high risk, 40 mg per day. Enoxaparin appears to convey the same degree of protection as 5,000 units of LDUH every 8 hours.
2 heparins compared
Randomized controlled trials comparing LDUH and LMWH have found them to be similarly effective. In addition, within the LMWH class, all compounds appear to have similar benefits. However, it remains unclear which form of heparin is associated with fewer bleeding complications.
In theory, LMWH would be associated with an increase in these complications due to its longer half-life and increased bioavailability.30 However, studies have not consistently identified excess bleeding in any group.31 Fortunately, excess postoperative bleeding requiring transfusion is uncommon.
Complications of heparin
Heparin-induced thrombocytopenia is a recognized complication, seen in as many as 20% of LDUH patients. The diagnosis is made when the platelet count falls below the lower limits of normal or when the platelet count falls by 50% but remains in the normal range. Two forms of heparin-induced thrombocytopenia have been described.
• Type 1 thrombocytopenia is initially mild, rarely dropping below 100,000 platelets per milliliter. Platelet count monitoring is important, but therapy usually can continue, since the platelet count returns to normal even with continued use. Type 1 thrombocytopenia appears to directly result from platelet activation and is not immune-mediated.
• Type 2 thrombocytopenia occurs 7 to 14 days after starting therapy. Platelet counts frequently drop to 20,000 per milliliter. Type 2 is an immune response caused by antibodies to the heparin-platelet factor 4 complex. Patients are at risk for venous and arterial thrombosis, but often the diagnosis is made only after a complicating thrombotic event.
The risk of type 2 thrombocytopenia appears to be related to the molecular weight of the compound being administered, its dose, and the duration of therapy. Therefore, unfractionated heparin—if given in full anticoagulation doses and beyond 14 days—seems to have the greatest potential for this complication.
Paradoxically, these patients also are at risk for severe hemorrhage. Because mortality and morbidity are high, immediate withdrawal of all forms of heparin—including LMWH—is mandatory.32 Catastrophic hemorrhage or thrombosis may be the first sign. Often a fall in platelet concentration precedes serious complications. Thus, platelet concentration should be monitored at least every 2 days.
Hematoma with conduction anesthesia. Use of major conduction anesthesia in patients who also need LMWH, LDUH, or oral anticoagulants is controversial. LMWH may pose a risk of hematoma if initiated preoperatively, intraoperatively, or within 3 hours of surgery in patients who have a continuous epidural. Hematoma formation seems to occur immediately following catheter withdrawal.
The risk of hematoma appears to be lower with single-dose spinal or single-dose epidural anesthesia. While the incidence of epidural or spinal hematoma is not known, some cases have been associated with long-term neurologic sequelae, including permanent paralysis.
Responding to this concern, the US Food and Drug Administration issued a 1997 advisory noting the risk of spinal hematoma in patients receiving enoxaparin plus conduction anesthesia or lumbar spinal puncture. Most anesthesiologists significantly restrict the use of conduction anesthesia in patients requiring heparin prophylaxis.33
Cost-effectiveness
The cost of perioperative prophylaxis has been compared with the cost of immediate therapy for thromboembolic disease and long-term therapy for postthrombotic syndrome. Across the board, universal prophylaxis with pharmacotherapy or mechanical devices in patients undergoing abdominal surgery is less expensive than no prophylaxis, based on a reduced incidence of DVT. Pneumatic compression seems to be more cost-effective than pharmacotherapy.34
Because LDUH is substantially less expensive than LMWH in the United States, it has a better cost profile when pharmacotherapy is warranted. In Europe, where the cost of heparin compounds is not an issue, LMWH has a slight advantage.
The authors report no financial relationships relevant to this article.
1. Dismuke SE, Wagner EH. Pulmonary embolism as a cause of death. The changing mortality in hospitalized patients. JAMA. 1986;255:2039-2042.
2. Farquharson DI, Orr JW, Jr. Prophylaxis against thromboembolism in gynecologic patients. J Reprod Med. 1984;29:845-862.
3. Bergqvist D. Prolonged prophylaxis against postoperative venous thromboembolism. Haemostasis. 1996;26(suppl 4):379-387.
4. Clarke-Pearson DL, DeLong ER, Synan IS, Coleman RE, Creasman WT. Variables associated with postoperative deep venous thrombosis: a prospective study of 411 gynecology patients and creation of a prognostic model. Obstet Gynecol. 1987;69:146-150.
5. Kemble JVH. Incidence of deep vein thrombosis. Br J Hosp Med. 1971;6:721-726.
6. Spritzer CE, Evans AC, Kay HH. Magnetic resonance imaging of deep venous thrombosis in pregnant women with lower extremity edema. Obstet Gynecol. 1995;85:603-607.
7. Florell SR, Rodgers GM. Inherited thrombotic disorders: an update. Am J Hematol. 1997;54:53-60.
8. Shapiro GA. Antiphospholipid syndrome in obstetrics and gynecology. Semin Thromb Hemost. 1994;20:64-70.
9. De Stafano V, Leone G, Mastrangelo S, et al. Clinical manifestations and management of inherited thrombophilia: retrospective analysis and follow-up after diagnosis in 238 patients with congenital deficiency of antithrombin III, protein C, protein S. S Throm Haemost. 1994;72:352-358.
10. Ridker PM, Hennekens CH, Selhub J, et al. Interrelation of hyperhomocyst(e)inemia, factor V Leiden, and risk of future venous thromboembolism. Circulation. 1997;95:1777-1782.
11. Vessey M, Jewell D, Smith A, Yeates D, McPherson K. Chronic inflammatory bowel disease, cigarette smoking, and use of oral contraceptives: findings in a large cohort study of women of childbearing age. Br Med J (Clin Res Ed). 1986;292:1101-1103.
12. Walker MC, Garner PR, Keely EJ, et al. Changes in activated protein C resistance during normal pregnancy. Am J Obstet Gynecol. 1997;177:162-169.
13. Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest. 2001;119(suppl 1):132S-175S.
14. Belcaro G, Laurora G, Cesarone MR, et al. Prophylaxis of recurrent deep venous thrombosis. A randomized, prospective study using indobufen and graduated elastic compression stockings. Angiology. 1993;44:695-699.
15. Clarke-Pearson DL, Synan IS, Dodge R, et al. A randomized trial of low-dose heparin and intermittent pneumatic calf compression for the prevention of deep venous thrombosis after gynecologic oncology surgery. Am J Obstet Gynecol. 1993;168:1146-1153.
16. Handoll HH, Farrar MJ, McBirnie J, et al. Prophylaxis using heparin, low molecular weight heparin and physical methods against deep vein thrombosis and pulmonary embolism in hip fracture. The Cochrane Library, Issue I. 1998. Evidence-based medicine: 146.
17. Clarke-Pearson DL, Synan IS, Hinshaw WM, et al. Prevention of postoperative venous thromboembolism by external pneumatic calf compression in patients with gynecologic malignancy. Obstet Gynecol. 1984;63(1):92-98.
18. Lachman EA, Rouk JL, et al. Complications associated with intermittent pneumatic compression. Arch Phys Med Rehab. 1992;73:482-485.
19. Clarke-Pearson DL, Dodge RK, Synan I, et al. Venous thromboembolism prophylaxis: patients at high risk to fail intermittent pneumatic compression. Obstet Gynecol. 101;2003:157-163.
20. Wilson NV, Das SK, Kakkar VV, et al. Thrombo-embolic prophylaxis in total knee replacement. Evaluation of the A-V Impulse System. J Bone Joint Surg Br. 1992;74:50-52.
21. Rosenberg RD, Damus PS. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973;248:6490-6505.
22. Scurr JH, Coleridge-Smith PD, Hasty JH. Regimen for improved effectiveness of intermittent pneumatic compression in deep venous thrombosis prophylaxis. Surgery. 1987;102:816-820.
23. Collins R, Scrimgeour A, Yusuf S, et al. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162-1173.
24. Hull RD, Pineo GF, Stein PD, et al. Timing of initial administration of low-molecular-weight heparin prophylaxis against deep vein thrombosis in patients following elective hip arthroplasty: a systematic review. Arch Intern Med. 2001;161:1952-1960.
25. Clarke-Pearson DL, DeLong ER, Synan IS, et al. A controlled trial of two low dose heparin regimens for the prevention of postoperative deep vein thrombosis. Obstet Gynecol. 1990;75:684-689.
26. Kakkar VV, Murray WJ. Efficacy and safety of low-molecular-weight heparin (CY216) in preventing postoperative venous thrombo-embolism: a co-operative study. Br J Surg. 1985;72:786-791.
27. Ward B, Pradhan S. Comparison of low molecular weight heparin (Fragmin) with sodium heparin for prophylaxis against postoperative thrombosis in women undergoing major gynaecological surgery. Aust N Z J Obstet Gynaecol. 1998;38:91-92.
28. Bergqvist D, Burmark US, Flordal PA, et al. Low molecular weight heparin started before surgery as prophylaxis against deep vein thrombosis: 2500 versus 5000 XaI units in 2070 patients. Br J Surg. 1995;82:496-501.
29. Bratt G, Tornebohm E, Widlund L, et al. Low molecular weight heparin (KABI 2165, Fragmin): pharmacokinetics after intravenous and subcutaneous administration in human volunteers. Thromb Res. 1986;42:613-620.
30. Kakkar VV, Boedki O, Boneau B, et al. Efficacy and safety profile of a low-molecular weight heparin and standard unfractionated heparin for prophylaxis of postoperative venous thromboembolism: European multicenter trial. World J Surg. 1997;21:2-8.
31. Warkentin TE. Heparin-induced thrombocytopenia: pathogenesis and management. Br J Haematol. 2003;121:535-555.
32. Wysowski DK, Talarico L, Bacsanyi J, et al. Spinal and epidural hematoma and low-molecular-weight heparin. N Engl J Med. 1998;338:1774-1775.
33. Maxwell GL, Myers ER, Clarke-Pearson DL. Cost-effectiveness of deep venous thrombosis prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 2000;95:206-214.
34. Doran FSA. Prevention of deep vein thrombosis. Br J Hosp Med. 1971;6:773-779.
1. Dismuke SE, Wagner EH. Pulmonary embolism as a cause of death. The changing mortality in hospitalized patients. JAMA. 1986;255:2039-2042.
2. Farquharson DI, Orr JW, Jr. Prophylaxis against thromboembolism in gynecologic patients. J Reprod Med. 1984;29:845-862.
3. Bergqvist D. Prolonged prophylaxis against postoperative venous thromboembolism. Haemostasis. 1996;26(suppl 4):379-387.
4. Clarke-Pearson DL, DeLong ER, Synan IS, Coleman RE, Creasman WT. Variables associated with postoperative deep venous thrombosis: a prospective study of 411 gynecology patients and creation of a prognostic model. Obstet Gynecol. 1987;69:146-150.
5. Kemble JVH. Incidence of deep vein thrombosis. Br J Hosp Med. 1971;6:721-726.
6. Spritzer CE, Evans AC, Kay HH. Magnetic resonance imaging of deep venous thrombosis in pregnant women with lower extremity edema. Obstet Gynecol. 1995;85:603-607.
7. Florell SR, Rodgers GM. Inherited thrombotic disorders: an update. Am J Hematol. 1997;54:53-60.
8. Shapiro GA. Antiphospholipid syndrome in obstetrics and gynecology. Semin Thromb Hemost. 1994;20:64-70.
9. De Stafano V, Leone G, Mastrangelo S, et al. Clinical manifestations and management of inherited thrombophilia: retrospective analysis and follow-up after diagnosis in 238 patients with congenital deficiency of antithrombin III, protein C, protein S. S Throm Haemost. 1994;72:352-358.
10. Ridker PM, Hennekens CH, Selhub J, et al. Interrelation of hyperhomocyst(e)inemia, factor V Leiden, and risk of future venous thromboembolism. Circulation. 1997;95:1777-1782.
11. Vessey M, Jewell D, Smith A, Yeates D, McPherson K. Chronic inflammatory bowel disease, cigarette smoking, and use of oral contraceptives: findings in a large cohort study of women of childbearing age. Br Med J (Clin Res Ed). 1986;292:1101-1103.
12. Walker MC, Garner PR, Keely EJ, et al. Changes in activated protein C resistance during normal pregnancy. Am J Obstet Gynecol. 1997;177:162-169.
13. Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest. 2001;119(suppl 1):132S-175S.
14. Belcaro G, Laurora G, Cesarone MR, et al. Prophylaxis of recurrent deep venous thrombosis. A randomized, prospective study using indobufen and graduated elastic compression stockings. Angiology. 1993;44:695-699.
15. Clarke-Pearson DL, Synan IS, Dodge R, et al. A randomized trial of low-dose heparin and intermittent pneumatic calf compression for the prevention of deep venous thrombosis after gynecologic oncology surgery. Am J Obstet Gynecol. 1993;168:1146-1153.
16. Handoll HH, Farrar MJ, McBirnie J, et al. Prophylaxis using heparin, low molecular weight heparin and physical methods against deep vein thrombosis and pulmonary embolism in hip fracture. The Cochrane Library, Issue I. 1998. Evidence-based medicine: 146.
17. Clarke-Pearson DL, Synan IS, Hinshaw WM, et al. Prevention of postoperative venous thromboembolism by external pneumatic calf compression in patients with gynecologic malignancy. Obstet Gynecol. 1984;63(1):92-98.
18. Lachman EA, Rouk JL, et al. Complications associated with intermittent pneumatic compression. Arch Phys Med Rehab. 1992;73:482-485.
19. Clarke-Pearson DL, Dodge RK, Synan I, et al. Venous thromboembolism prophylaxis: patients at high risk to fail intermittent pneumatic compression. Obstet Gynecol. 101;2003:157-163.
20. Wilson NV, Das SK, Kakkar VV, et al. Thrombo-embolic prophylaxis in total knee replacement. Evaluation of the A-V Impulse System. J Bone Joint Surg Br. 1992;74:50-52.
21. Rosenberg RD, Damus PS. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973;248:6490-6505.
22. Scurr JH, Coleridge-Smith PD, Hasty JH. Regimen for improved effectiveness of intermittent pneumatic compression in deep venous thrombosis prophylaxis. Surgery. 1987;102:816-820.
23. Collins R, Scrimgeour A, Yusuf S, et al. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162-1173.
24. Hull RD, Pineo GF, Stein PD, et al. Timing of initial administration of low-molecular-weight heparin prophylaxis against deep vein thrombosis in patients following elective hip arthroplasty: a systematic review. Arch Intern Med. 2001;161:1952-1960.
25. Clarke-Pearson DL, DeLong ER, Synan IS, et al. A controlled trial of two low dose heparin regimens for the prevention of postoperative deep vein thrombosis. Obstet Gynecol. 1990;75:684-689.
26. Kakkar VV, Murray WJ. Efficacy and safety of low-molecular-weight heparin (CY216) in preventing postoperative venous thrombo-embolism: a co-operative study. Br J Surg. 1985;72:786-791.
27. Ward B, Pradhan S. Comparison of low molecular weight heparin (Fragmin) with sodium heparin for prophylaxis against postoperative thrombosis in women undergoing major gynaecological surgery. Aust N Z J Obstet Gynaecol. 1998;38:91-92.
28. Bergqvist D, Burmark US, Flordal PA, et al. Low molecular weight heparin started before surgery as prophylaxis against deep vein thrombosis: 2500 versus 5000 XaI units in 2070 patients. Br J Surg. 1995;82:496-501.
29. Bratt G, Tornebohm E, Widlund L, et al. Low molecular weight heparin (KABI 2165, Fragmin): pharmacokinetics after intravenous and subcutaneous administration in human volunteers. Thromb Res. 1986;42:613-620.
30. Kakkar VV, Boedki O, Boneau B, et al. Efficacy and safety profile of a low-molecular weight heparin and standard unfractionated heparin for prophylaxis of postoperative venous thromboembolism: European multicenter trial. World J Surg. 1997;21:2-8.
31. Warkentin TE. Heparin-induced thrombocytopenia: pathogenesis and management. Br J Haematol. 2003;121:535-555.
32. Wysowski DK, Talarico L, Bacsanyi J, et al. Spinal and epidural hematoma and low-molecular-weight heparin. N Engl J Med. 1998;338:1774-1775.
33. Maxwell GL, Myers ER, Clarke-Pearson DL. Cost-effectiveness of deep venous thrombosis prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 2000;95:206-214.
34. Doran FSA. Prevention of deep vein thrombosis. Br J Hosp Med. 1971;6:773-779.
Incision decisions: which ones for which procedures?
- The incision should be considered as a second surgical procedure, which temporarily interferes with normal abdominal wall function.
- The midline incision provides excellent exposure to all areas of the abdomen and retroperitoneum, which can be accessed with minimal risk of significant vascular or nerve injury.
- Transverse incisions create less tension on the opposing skin edges because the incision follows Langer’s lines. The incidence of incisional hernias and wound dehiscence has been reported to be lower, but these studies are not randomized.
An abdominal incision often is given little thought other than as an access site through which a surgical procedure is performed. In reality, the incision is a second surgical procedure, which interferes—at least temporarily—with normal abdominal wall function.
While most physicians concur that the essential elements of a well-planned incision include adequate access to anticipated pathology, extensibility, and security of closure, many may not consider preservation of abdominal wall function as a key factor in their decision-making. Additional considerations include certainty of diagnosis, speed of entry, body habitus, presence of previous scars, potential for problems with hemostasis, and cosmetic outcome. These factors are the key determinants of whether the incision will be longitudinal (midline or paramedian) or transverse (Pfannenstiel’s, Cherney’s, or Maylard’s). For most gynecologic procedures confined to the pelvis, either option may be considered. The exceptions are patients with uncertain diagnoses or when access to the upper abdomen is indicated.
Regardless of the type of incision selected, the skin should be incised with a single, clean stroke of a sharp scalpel. However, when it comes to dissecting the underlying subcutaneous tissues, the debate continues over whether a scalpel or electrosurgery is best. While I recently have switched to the latter, here is a look at what the data say: Johnson and Serpell demonstrated that electrosurgery is associated with faster hemostasis, with no difference in the incidence of wound infection.1 Similarly, a recent randomized trial by Kearns et al found electro-surgery causes less blood loss and does not increase the risk of wound infections or fascial dehiscence.2 In contrast, a large prospective study by Cruse et al suggested that the use of diathermy is associated with twice the wound infection rate.3
This controversy also involves patients with gynecologic malignancies. Kolb et al found that electrosurgery was an independent risk factor for wound complications following surgery for ovarian cancer.4 However, Franchi and colleagues reported no difference in the rate of wound complications between scalpel and diathermy in patients who underwent mid-line abdominal incisions for the treatment of uterine cancer.5
Use the midline when the diagnosis and the extent of surgery are uncertain.
The inconsistencies in the data may reflect differences in electrosurgical technique. Non-modulated (cutting) current concentrates energy, vaporizing the tissue with little heat injury to surrounding areas. Conversely, modulated (coagulating) current coagulates the tissue with heat-producing char over a large area, and tissue injury often extends beyond the char. This effect is magnified if the electrode comes in direct contact with the tissue. Use the arc, rather than direct contact, to prevent excessive devitalization of tissue.
This article will review the techniques for, as well as the rationale and disadvantages of, common incisions—both longitudinal and transverse—to help the gynecologic surgeon minimize morbidity and maximize outcomes.
Longitudinal incisions
The longitudinal incisions that will be reviewed here are the midline (median) and paramedian. Classically, it was thought that longitudinal incisions were at greater risk of dehiscence than transverse incisions.6 However, it is difficult to make legitimate comparisons since longitudinal incisions are more likely to be performed in cases of hemorrhage, trauma, sepsis, multiorgan disease, previous surgery, previous radiation therapy, and malignancy—all of which increase the likelihood of postoperative complications. Furthermore, prospective and randomized studies have shown little, if any, difference in the incidence of dehiscence and hernias between longitudinal and transverse incisions.6-8
MidlineTechnique. Initiate the midline as a low abdominal incision (approximately 2 cm above the pubic symphysis), cutting along the linea alba. To extend the incision, if necessary, continue the dissection to the left of the umbilicus to avoid the ligamentum teres. Open the peritoneum at the cephalad pole of the incision (Figure 1). Expand this cut slightly off midline to avoid the urachus yet adequately expose the peritoneal cavity (Figure 2).
Rationale. While this incision can be used for any surgical procedure, it is especially appropriate when the diagnosis is uncertain and the exact procedure or extent of surgery is unclear. It is an excellent choice when access to the upper abdomen may be necessary, e.g., patients with gynecologic malignancies who may need assessment of the diaphragm, liver biopsy, para-aortic node biopsy, omentectomy, or debulking procedures. Patients with benign gynecologic conditions also may benefit from a midline incision. For example, when pelvic anatomy is distorted, as with severe endometriosis or sepsis, recognizable anatomy may be found only above the pelvic brim.
A midline incision allows the quickest entry, which is especially important for an unstable or seriously ill patient. Exposure is excellent, as all areas of the abdomen and retroperitoneum can be accessed with minimal risk of significant vascular or nerve injury. This is because only terminal branches of the abdominal wall blood vessels and nerves are located at the linea alba. In addition, because deep tissue planes are not opened, this incision may be ideal for patients who are anticoagulated, have enlarged epigastric vessels that are more susceptible to injury, or have an intraabdominal infection.
Transverse incisions help reduce the rate of wound dehiscence.
Disadvantages. Two potential problems are the higher rate of hernia formation and wound dehiscence, which may be due to constant lateral tension, compared with transverse incisions. In addition, coughing, retching, and straining may exacerbate the lateral tension. Proper suture selection can reduce the incidence of complications. As previously mentioned, the reality of these disadvantages is subject to debate; further studies are needed to determine the true risk.
From a cosmetic standpoint, the midline scar often is prominent because the incision transects Langer’s lines. Hence, it cannot be concealed by lingerie or swimwear.
FIGURE 1 Midline incision
After incising the linea alba and separating the muscles in the midline, open the peritoneum at the cephalad pole of the incision.
FIGURE 2 Midline incision
Expand this cut slightly off midline to avoid the urachus yet adequately expose the peritoneal cavity.
ParamedianTechnique. Place this incision 2 to 5 cm to the left or right of the midline, depending on the indication for surgery. After dissecting the skin and subcutaneous layers, incise the anterior rectus sheath vertically over the rectus abdominis muscle (Figure 3) and dissect it from the medial muscle edge. Retract the muscle laterally to expose the posterior rectus sheath and then incise the sheath and the peritoneum vertically to expose the peritoneal cavity (Figure 4). Alternatively, a more lateral paramedian incision can be placed over the rectus abdominis muscle. In this case, however, the rectus muscle should be separated vertically, instead of retracted laterally, to expose the posterior rectus sheath.
Rationale. Paramedian incisions provide excellent exposure of the pelvis, excluding the upper abdomen. Therefore, consider this technique when the procedure will be confined to the ipsilateral pelvis, e.g., rightor left-side lymph node biopsies and exposure of the sigmoid colon on the left. Also, a paramedian incision at the level of the umbilicus can be used for a cesarean delivery or a hysterectomy.
This incision is the best choice when performing pelvic surgery on morbidly obese patients. By placing the incision low on the abdomen, the large panniculus can be retracted over the mons pubis and thighs, providing excellent exposure of the pelvis.
Disadvantages. Some researchers have reported that muscle-splitting lateral paramedian incisions have a lower incidence of incisional hernias compared with midline incisions.7,9 However, they take longer to perform and restrict access to the contralateral pelvis. In addition, the risk of vascular injury and hematomas is increased, especially in the lower pole where branches of the epigastric arteries penetrate the muscle.
With regard to nerve injury, terminal nerves are cut, resulting in paralysis of the inner portion of the rectus abdominis muscle. This paralysis can be permanent, as the muscle medial to the vertical separation is involved. However, if only the medial third of the muscle is denervated, the paralysis rarely limits function.
FIGURE 3 Paramedian incision
Place the incision 2 to 5 cm to the left or right of the midline. Incise the anterior rectus sheath vertically over the rectus abdominis muscle.
FIGURE 4 Paramedian incision
Retract the muscle laterally to expose the posterior sheath; then incise the sheath and the peritoneum vertically to expose the pelvic cavity.
Transverse incisions
Transverse incisions such as Pfannenstiel’s (muscle separating), Cherney’s (tendon detaching), and Maylard’s (muscle cutting) were developed to reduce the incidence of incisional hernias and wound dehiscence. Their success lies in the fact that they cause less tension on the opposing wound edges because the incisions follow Langer’s lines, unlike longitudinal incisions. Their placement also allows for a better cosmetic outcome. Because they can be placed in the pubic hairline or in a natural skin crease, they are easily concealed by lingerie or swimwear. However, the incision should not be placed in a deep skin fold of a large panniculus where maceration of the skin can increase the risk of infection.
The main disadvantages of transverse incisions are limited exposure of the upper abdomen and limited extensibility. Further, because more tissue planes are opened and more vessels are encountered, these incisions increase the risk of hematomas and infection.
Pfannenstiel’sTechnique. Incise the skin and subcutaneous tissues 2 to 5 cm above the pubic symphysis.10 Then dissect the rectus sheath transversely, separating it sharply from the rectus muscle at the linea alba and retracting it superiorly toward the umbilicus and inferiorly toward the pubic symphysis (Figure 5). Divide the rectus abdominis muscle along the midline raphe and retract it laterally, exposing the transversalis fascia and the posterior rectus sheath. Incise these layers and the peritoneum vertically to expose the peritoneal cavity (Figure 6).
To minimize retraction of the rectus abdominus muscle during Maylard’s, do not separate it from the anterior rectus sheath.
Rationale. Since exposure is limited and the incision can be only minimally extended, this incision is appropriate for procedures that are limited to the pelvis, e.g., abdominal hysterectomy, cesarean delivery, retropubic urethropexy, and paravaginal defect repairs. (Extension of the incision can be achieved by modifying it to Cherney’s incision.)
Disadvantages. The fact that the incision severs multiple tissue planes is an advantage as well as a disadvantage. Postoperative dehiscence and incisional hernias are rare because the closed wound has a high tensile strength. However, the incidence of inguinal hernias may increase when the incision is placed close to the external inguinal ring,11 and very low incisions may increase the risk of femoral nerve injury.12 The ilioinguinal and iliohypogastric nerves may be damaged, especially if the rectus incision is extended far laterally. Most commonly, they are trapped in the suture during closure, producing postoperative pain. Incising multiple layers also slows entry and increases the risk of seromas, hematomas, and wound infections.
This approach is contraindicated when time is of the essence, e.g., hemorrhage, or in the face of an abdominal infection, e.g., sepsis.
FIGURE 5 Pfannenstiel’s incision
After incising the skin and subcutaneous tissues, dissect the rectus sheath transversely, separating it sharply from the rectus muscle.
FIGURE 6 Pfannenstiel’s incision
Divide and retract the rectus abdominus muscle and then incise the tranversalis fascia and posterior rectus sheath vertically.
Cherney’sTechnique. Dissect the skin and subcutaneous tissue 2 to 3 cm above the pubic symphysis, which is lower than most Pfannenstiel’s incisions. Then incise the anterior rectus sheath transversely and dissect it from the rectus abdominis muscle superiorly and inferiorly. Using blunt dissection, separate the rectus and pyramidalis muscles from the underlying bladder and adventitial tissue. Then incise transversely the tendons of the rectus and pyramidalis muscles 0.5 cm above the pubic symphysis (Figure 7). (This segment of tendon facilitates the reattachment of the muscles at closure.) Retract the muscles and tendons cephalad to expose the retropubic space. Incise the peritoneum transversely to expose the peritoneal cavity (Figure 8).
Rationale. Cherney’s incision provides excellent access to the retropubic space of Retzius, making it a good choice for retropubic urethropexy and paravaginal repair. Exposure is the main advantage to Cherney’s incision. The reason: It is a modification of Pfannenstiel’s incision and, as such, provides excellent lateral exposure because it is placed much lower on the abdominal wall.
Disadvantages. The benefits and risks of this incision are similar to those of Pfannenstiel’s incision since the same tissue planes are opened. However, it is more time consuming due to the dissection required to separate the muscles and tendons from the underlying tissues.
In addition, the deep inferior epigastric arteries could be injured as a result of the lateral dissection. Lastly, reattachment of the tendons is tedious.
FIGURE 7 Cherney’s incision
Incise transversely the tendons of the rectus and pyramidalis muscles 0.5 cm above the pubic symphysis.
FIGURE 8 Cherney’s incision
Retract the muscles and tendons cephalad and incise the peritoneum transversely to expose the peritoneal cavity.
Maylard’sTechnique. After transecting the skin and subcutaneous layers in a plane at the level of the anterior superior iliac spine, incise the rectus sheath transversely and extend the incision through the aponeuroses of the abdominal muscles to about 2 to 3 cm medial to the anterior iliac crest. Then dissect the rectus abdominis muscle transversely with a scalpel, electrocautery, or surgical stapler (Figure 9). To minimize retraction of this muscle, do not separate it from the anterior rectus sheath. (Alternatively, many surgeons recommend that the cut edge of the muscle be secured to the anterior sheath with mattress sutures to prevent retraction and reduce blood loss.)
Prior to transecting the peritoneum, isolate, ligate, and divide the deep inferior epigastric vessels. (Patients with significant aortoiliac atherosclerosis or aortic coarctation develop considerable collateral circulation through the epigastric vessels for perfusion of the lower extremities. Ligating these vessels may cause claudication and even lifethreatening ischemia. Therefore, assess iliac flow before ligating and transecting the arteries.) Then transect the transversalis fascia and peritoneum transversely, allowing access to the peritoneal cavity (Figure 10).
Rationale. Many oncologists use this approach for pelvic lymphadenectomy and staging procedures because the exposure to the lateral pelvis is excellent. The reason: This transverse incision cuts through all layers of the abdominal wall at the level of the anterior iliac spine. It also may be appropriate when pelvic pathology extends to the sidewall, e.g., endometriosis.
It is advisable to place a drain prior to closing Maylard’s.
Physicians should consider using this approach more than they currently are, especially when Pfannenstiel’s incision cannot provide satisfactory exposure or a longitudinal incision is thought to be the only alternative. The decision to use Maylard’s incision should be planned during the preoperative assessment, as Pfannenstiel’s incision should not be modified to Maylard’s intraoperatively.
Disadvantages. Although Maylard’s incision improves access to the upper abdomen compared to other transverse incisions, exposure is still limited. In addition, trauma to the deep epigastric arteries may result in considerable hemorrhage. And since hematomas commonly infiltrate the retroperitoneal space, large quantities of blood already may be lost before the hemorrhage is clinically apparent. Finally, oozing from the cut edge of the muscles may result in significant fluid collection, increasing the risk of infection. Therefore, it is advisable to place a drain prior to closure.
Like other transverse incisions, the wound parallels the nerves, providing a measure of protection. However, the rectus incision approaches the anterior iliac spine, where both the ilioinguinal and iliohypogastric nerves lie. Both may be damaged during the incision and can become entrapped during closure. Additionally, the femoral nerve, the lateral femoral cutaneous nerve, and the genitofemoral nerve are easily compressed by the lateral blades of the retractors because of the extreme lateral positions of the incision’s poles. Injury to these nerves can result in paresthesia, pain, and paralysis of the leg muscles.
FIGURE 9 Maylard’s incision
After incising the aponeuroses of the abdominal muscles medial to the anterior iliac crest, dissect the rectus abdominus muscle transversely.
FIGURE 10 Maylard’s incision
Isolate, ligate, and divide the epigastric vessels. Then incise the transversalis fascia and peritoneum transversely to access the peritoneal cavity.
In summary
While the midline and Pfannenstiel’s incisions are an integral part of the gynecologic surgeon’s armamentarium, the paramedian and Maylard’s incisions should be added to the repertoire because these incisions can greatly enhance exposure. The paramedian shares the advantages of the midline incision but also allows better access to the ipsilateral pelvis. Furthermore, although Maylard’s incision takes longer to perform than other techniques, the excellent exposure—both laterally and superiorly—makes it worthwhile.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Johnson CD, Serpell JW. Wound infection after abdominal incision with scalpel or diathermy. Br J Surg. 1990;77:626-627.
2. Cruse PJE, Ford R. The epidemiology of wound infection: a 10-year prospective study of 62,939 wounds. Surg Clin North Am. 1980;60:27.-
3. Kearns SR, Connolly EM, McNally S, McNamara DA, Deasy J. Randomized clinical trial of diathermy versus scalpel incision in elective midline laparotomy. Br J Surg. 2001;88(1):41-44.
4. Kolb BA, Buller RE, Connoer JP, et al. Effects of early postoperative chemotherapy on wound healing. Obstet Gynecol. 1992;79:988-992.
5. Franchi M, Ghezzi F, Bendetti-Panici PL, et al. A multicenter collaborative study on the use of cold scalpel and electrocautery for midline abdominal incision. Am J Surg. 2001;181(2):128-132.
6. Hendrix SL, Schimp V, Martin J. The legendary superior strength of the Pfannenstiel incision: a myth? Am J Obstet Gynecol. 2000;182(6):1446-1451.
7. Ellis H, Coleridge-Smith PD, Joyce AD. Abdominal incisions—vertical or transverse? Postgrad Med J. 1984;60:407-410.
8. Sanders RJ, DiClementi D. Principles of abdominal wound closure. II. Prevention of wound dehiscence. Arch Surg. 1977;112:1188.-
9. Donaldson DR, Hegarty JH, Brennan TG, et al. The lateral paramedian incision-experience with 850 cases. Br J Surg. 1982;69:630.-
10. Greenall MJ, et al. Midline or transverse laparotomy? A random controlled clinical trial. Part I: Influence on healing. Br J Surg. 1980;7:188.-
11. Pfannenstiel J. Ueber die Vortheile des suprasymphysaren Fascienquerschnitts für die gynakologischen Koliotomien zugleich ein Beitrag zu der Indikationsstellung der Operationswege. Samml Klin Vortr (Leipzig). 1900;268:1735.-
12. Griffiths DA. A reappraisal of the Pfannenstiel incision. Br J Urol. 1976;48:469.-
- The incision should be considered as a second surgical procedure, which temporarily interferes with normal abdominal wall function.
- The midline incision provides excellent exposure to all areas of the abdomen and retroperitoneum, which can be accessed with minimal risk of significant vascular or nerve injury.
- Transverse incisions create less tension on the opposing skin edges because the incision follows Langer’s lines. The incidence of incisional hernias and wound dehiscence has been reported to be lower, but these studies are not randomized.
An abdominal incision often is given little thought other than as an access site through which a surgical procedure is performed. In reality, the incision is a second surgical procedure, which interferes—at least temporarily—with normal abdominal wall function.
While most physicians concur that the essential elements of a well-planned incision include adequate access to anticipated pathology, extensibility, and security of closure, many may not consider preservation of abdominal wall function as a key factor in their decision-making. Additional considerations include certainty of diagnosis, speed of entry, body habitus, presence of previous scars, potential for problems with hemostasis, and cosmetic outcome. These factors are the key determinants of whether the incision will be longitudinal (midline or paramedian) or transverse (Pfannenstiel’s, Cherney’s, or Maylard’s). For most gynecologic procedures confined to the pelvis, either option may be considered. The exceptions are patients with uncertain diagnoses or when access to the upper abdomen is indicated.
Regardless of the type of incision selected, the skin should be incised with a single, clean stroke of a sharp scalpel. However, when it comes to dissecting the underlying subcutaneous tissues, the debate continues over whether a scalpel or electrosurgery is best. While I recently have switched to the latter, here is a look at what the data say: Johnson and Serpell demonstrated that electrosurgery is associated with faster hemostasis, with no difference in the incidence of wound infection.1 Similarly, a recent randomized trial by Kearns et al found electro-surgery causes less blood loss and does not increase the risk of wound infections or fascial dehiscence.2 In contrast, a large prospective study by Cruse et al suggested that the use of diathermy is associated with twice the wound infection rate.3
This controversy also involves patients with gynecologic malignancies. Kolb et al found that electrosurgery was an independent risk factor for wound complications following surgery for ovarian cancer.4 However, Franchi and colleagues reported no difference in the rate of wound complications between scalpel and diathermy in patients who underwent mid-line abdominal incisions for the treatment of uterine cancer.5
Use the midline when the diagnosis and the extent of surgery are uncertain.
The inconsistencies in the data may reflect differences in electrosurgical technique. Non-modulated (cutting) current concentrates energy, vaporizing the tissue with little heat injury to surrounding areas. Conversely, modulated (coagulating) current coagulates the tissue with heat-producing char over a large area, and tissue injury often extends beyond the char. This effect is magnified if the electrode comes in direct contact with the tissue. Use the arc, rather than direct contact, to prevent excessive devitalization of tissue.
This article will review the techniques for, as well as the rationale and disadvantages of, common incisions—both longitudinal and transverse—to help the gynecologic surgeon minimize morbidity and maximize outcomes.
Longitudinal incisions
The longitudinal incisions that will be reviewed here are the midline (median) and paramedian. Classically, it was thought that longitudinal incisions were at greater risk of dehiscence than transverse incisions.6 However, it is difficult to make legitimate comparisons since longitudinal incisions are more likely to be performed in cases of hemorrhage, trauma, sepsis, multiorgan disease, previous surgery, previous radiation therapy, and malignancy—all of which increase the likelihood of postoperative complications. Furthermore, prospective and randomized studies have shown little, if any, difference in the incidence of dehiscence and hernias between longitudinal and transverse incisions.6-8
MidlineTechnique. Initiate the midline as a low abdominal incision (approximately 2 cm above the pubic symphysis), cutting along the linea alba. To extend the incision, if necessary, continue the dissection to the left of the umbilicus to avoid the ligamentum teres. Open the peritoneum at the cephalad pole of the incision (Figure 1). Expand this cut slightly off midline to avoid the urachus yet adequately expose the peritoneal cavity (Figure 2).
Rationale. While this incision can be used for any surgical procedure, it is especially appropriate when the diagnosis is uncertain and the exact procedure or extent of surgery is unclear. It is an excellent choice when access to the upper abdomen may be necessary, e.g., patients with gynecologic malignancies who may need assessment of the diaphragm, liver biopsy, para-aortic node biopsy, omentectomy, or debulking procedures. Patients with benign gynecologic conditions also may benefit from a midline incision. For example, when pelvic anatomy is distorted, as with severe endometriosis or sepsis, recognizable anatomy may be found only above the pelvic brim.
A midline incision allows the quickest entry, which is especially important for an unstable or seriously ill patient. Exposure is excellent, as all areas of the abdomen and retroperitoneum can be accessed with minimal risk of significant vascular or nerve injury. This is because only terminal branches of the abdominal wall blood vessels and nerves are located at the linea alba. In addition, because deep tissue planes are not opened, this incision may be ideal for patients who are anticoagulated, have enlarged epigastric vessels that are more susceptible to injury, or have an intraabdominal infection.
Transverse incisions help reduce the rate of wound dehiscence.
Disadvantages. Two potential problems are the higher rate of hernia formation and wound dehiscence, which may be due to constant lateral tension, compared with transverse incisions. In addition, coughing, retching, and straining may exacerbate the lateral tension. Proper suture selection can reduce the incidence of complications. As previously mentioned, the reality of these disadvantages is subject to debate; further studies are needed to determine the true risk.
From a cosmetic standpoint, the midline scar often is prominent because the incision transects Langer’s lines. Hence, it cannot be concealed by lingerie or swimwear.
FIGURE 1 Midline incision
After incising the linea alba and separating the muscles in the midline, open the peritoneum at the cephalad pole of the incision.
FIGURE 2 Midline incision
Expand this cut slightly off midline to avoid the urachus yet adequately expose the peritoneal cavity.
ParamedianTechnique. Place this incision 2 to 5 cm to the left or right of the midline, depending on the indication for surgery. After dissecting the skin and subcutaneous layers, incise the anterior rectus sheath vertically over the rectus abdominis muscle (Figure 3) and dissect it from the medial muscle edge. Retract the muscle laterally to expose the posterior rectus sheath and then incise the sheath and the peritoneum vertically to expose the peritoneal cavity (Figure 4). Alternatively, a more lateral paramedian incision can be placed over the rectus abdominis muscle. In this case, however, the rectus muscle should be separated vertically, instead of retracted laterally, to expose the posterior rectus sheath.
Rationale. Paramedian incisions provide excellent exposure of the pelvis, excluding the upper abdomen. Therefore, consider this technique when the procedure will be confined to the ipsilateral pelvis, e.g., rightor left-side lymph node biopsies and exposure of the sigmoid colon on the left. Also, a paramedian incision at the level of the umbilicus can be used for a cesarean delivery or a hysterectomy.
This incision is the best choice when performing pelvic surgery on morbidly obese patients. By placing the incision low on the abdomen, the large panniculus can be retracted over the mons pubis and thighs, providing excellent exposure of the pelvis.
Disadvantages. Some researchers have reported that muscle-splitting lateral paramedian incisions have a lower incidence of incisional hernias compared with midline incisions.7,9 However, they take longer to perform and restrict access to the contralateral pelvis. In addition, the risk of vascular injury and hematomas is increased, especially in the lower pole where branches of the epigastric arteries penetrate the muscle.
With regard to nerve injury, terminal nerves are cut, resulting in paralysis of the inner portion of the rectus abdominis muscle. This paralysis can be permanent, as the muscle medial to the vertical separation is involved. However, if only the medial third of the muscle is denervated, the paralysis rarely limits function.
FIGURE 3 Paramedian incision
Place the incision 2 to 5 cm to the left or right of the midline. Incise the anterior rectus sheath vertically over the rectus abdominis muscle.
FIGURE 4 Paramedian incision
Retract the muscle laterally to expose the posterior sheath; then incise the sheath and the peritoneum vertically to expose the pelvic cavity.
Transverse incisions
Transverse incisions such as Pfannenstiel’s (muscle separating), Cherney’s (tendon detaching), and Maylard’s (muscle cutting) were developed to reduce the incidence of incisional hernias and wound dehiscence. Their success lies in the fact that they cause less tension on the opposing wound edges because the incisions follow Langer’s lines, unlike longitudinal incisions. Their placement also allows for a better cosmetic outcome. Because they can be placed in the pubic hairline or in a natural skin crease, they are easily concealed by lingerie or swimwear. However, the incision should not be placed in a deep skin fold of a large panniculus where maceration of the skin can increase the risk of infection.
The main disadvantages of transverse incisions are limited exposure of the upper abdomen and limited extensibility. Further, because more tissue planes are opened and more vessels are encountered, these incisions increase the risk of hematomas and infection.
Pfannenstiel’sTechnique. Incise the skin and subcutaneous tissues 2 to 5 cm above the pubic symphysis.10 Then dissect the rectus sheath transversely, separating it sharply from the rectus muscle at the linea alba and retracting it superiorly toward the umbilicus and inferiorly toward the pubic symphysis (Figure 5). Divide the rectus abdominis muscle along the midline raphe and retract it laterally, exposing the transversalis fascia and the posterior rectus sheath. Incise these layers and the peritoneum vertically to expose the peritoneal cavity (Figure 6).
To minimize retraction of the rectus abdominus muscle during Maylard’s, do not separate it from the anterior rectus sheath.
Rationale. Since exposure is limited and the incision can be only minimally extended, this incision is appropriate for procedures that are limited to the pelvis, e.g., abdominal hysterectomy, cesarean delivery, retropubic urethropexy, and paravaginal defect repairs. (Extension of the incision can be achieved by modifying it to Cherney’s incision.)
Disadvantages. The fact that the incision severs multiple tissue planes is an advantage as well as a disadvantage. Postoperative dehiscence and incisional hernias are rare because the closed wound has a high tensile strength. However, the incidence of inguinal hernias may increase when the incision is placed close to the external inguinal ring,11 and very low incisions may increase the risk of femoral nerve injury.12 The ilioinguinal and iliohypogastric nerves may be damaged, especially if the rectus incision is extended far laterally. Most commonly, they are trapped in the suture during closure, producing postoperative pain. Incising multiple layers also slows entry and increases the risk of seromas, hematomas, and wound infections.
This approach is contraindicated when time is of the essence, e.g., hemorrhage, or in the face of an abdominal infection, e.g., sepsis.
FIGURE 5 Pfannenstiel’s incision
After incising the skin and subcutaneous tissues, dissect the rectus sheath transversely, separating it sharply from the rectus muscle.
FIGURE 6 Pfannenstiel’s incision
Divide and retract the rectus abdominus muscle and then incise the tranversalis fascia and posterior rectus sheath vertically.
Cherney’sTechnique. Dissect the skin and subcutaneous tissue 2 to 3 cm above the pubic symphysis, which is lower than most Pfannenstiel’s incisions. Then incise the anterior rectus sheath transversely and dissect it from the rectus abdominis muscle superiorly and inferiorly. Using blunt dissection, separate the rectus and pyramidalis muscles from the underlying bladder and adventitial tissue. Then incise transversely the tendons of the rectus and pyramidalis muscles 0.5 cm above the pubic symphysis (Figure 7). (This segment of tendon facilitates the reattachment of the muscles at closure.) Retract the muscles and tendons cephalad to expose the retropubic space. Incise the peritoneum transversely to expose the peritoneal cavity (Figure 8).
Rationale. Cherney’s incision provides excellent access to the retropubic space of Retzius, making it a good choice for retropubic urethropexy and paravaginal repair. Exposure is the main advantage to Cherney’s incision. The reason: It is a modification of Pfannenstiel’s incision and, as such, provides excellent lateral exposure because it is placed much lower on the abdominal wall.
Disadvantages. The benefits and risks of this incision are similar to those of Pfannenstiel’s incision since the same tissue planes are opened. However, it is more time consuming due to the dissection required to separate the muscles and tendons from the underlying tissues.
In addition, the deep inferior epigastric arteries could be injured as a result of the lateral dissection. Lastly, reattachment of the tendons is tedious.
FIGURE 7 Cherney’s incision
Incise transversely the tendons of the rectus and pyramidalis muscles 0.5 cm above the pubic symphysis.
FIGURE 8 Cherney’s incision
Retract the muscles and tendons cephalad and incise the peritoneum transversely to expose the peritoneal cavity.
Maylard’sTechnique. After transecting the skin and subcutaneous layers in a plane at the level of the anterior superior iliac spine, incise the rectus sheath transversely and extend the incision through the aponeuroses of the abdominal muscles to about 2 to 3 cm medial to the anterior iliac crest. Then dissect the rectus abdominis muscle transversely with a scalpel, electrocautery, or surgical stapler (Figure 9). To minimize retraction of this muscle, do not separate it from the anterior rectus sheath. (Alternatively, many surgeons recommend that the cut edge of the muscle be secured to the anterior sheath with mattress sutures to prevent retraction and reduce blood loss.)
Prior to transecting the peritoneum, isolate, ligate, and divide the deep inferior epigastric vessels. (Patients with significant aortoiliac atherosclerosis or aortic coarctation develop considerable collateral circulation through the epigastric vessels for perfusion of the lower extremities. Ligating these vessels may cause claudication and even lifethreatening ischemia. Therefore, assess iliac flow before ligating and transecting the arteries.) Then transect the transversalis fascia and peritoneum transversely, allowing access to the peritoneal cavity (Figure 10).
Rationale. Many oncologists use this approach for pelvic lymphadenectomy and staging procedures because the exposure to the lateral pelvis is excellent. The reason: This transverse incision cuts through all layers of the abdominal wall at the level of the anterior iliac spine. It also may be appropriate when pelvic pathology extends to the sidewall, e.g., endometriosis.
It is advisable to place a drain prior to closing Maylard’s.
Physicians should consider using this approach more than they currently are, especially when Pfannenstiel’s incision cannot provide satisfactory exposure or a longitudinal incision is thought to be the only alternative. The decision to use Maylard’s incision should be planned during the preoperative assessment, as Pfannenstiel’s incision should not be modified to Maylard’s intraoperatively.
Disadvantages. Although Maylard’s incision improves access to the upper abdomen compared to other transverse incisions, exposure is still limited. In addition, trauma to the deep epigastric arteries may result in considerable hemorrhage. And since hematomas commonly infiltrate the retroperitoneal space, large quantities of blood already may be lost before the hemorrhage is clinically apparent. Finally, oozing from the cut edge of the muscles may result in significant fluid collection, increasing the risk of infection. Therefore, it is advisable to place a drain prior to closure.
Like other transverse incisions, the wound parallels the nerves, providing a measure of protection. However, the rectus incision approaches the anterior iliac spine, where both the ilioinguinal and iliohypogastric nerves lie. Both may be damaged during the incision and can become entrapped during closure. Additionally, the femoral nerve, the lateral femoral cutaneous nerve, and the genitofemoral nerve are easily compressed by the lateral blades of the retractors because of the extreme lateral positions of the incision’s poles. Injury to these nerves can result in paresthesia, pain, and paralysis of the leg muscles.
FIGURE 9 Maylard’s incision
After incising the aponeuroses of the abdominal muscles medial to the anterior iliac crest, dissect the rectus abdominus muscle transversely.
FIGURE 10 Maylard’s incision
Isolate, ligate, and divide the epigastric vessels. Then incise the transversalis fascia and peritoneum transversely to access the peritoneal cavity.
In summary
While the midline and Pfannenstiel’s incisions are an integral part of the gynecologic surgeon’s armamentarium, the paramedian and Maylard’s incisions should be added to the repertoire because these incisions can greatly enhance exposure. The paramedian shares the advantages of the midline incision but also allows better access to the ipsilateral pelvis. Furthermore, although Maylard’s incision takes longer to perform than other techniques, the excellent exposure—both laterally and superiorly—makes it worthwhile.
The authors report no financial relationship with any companies whose products are mentioned in this article.
- The incision should be considered as a second surgical procedure, which temporarily interferes with normal abdominal wall function.
- The midline incision provides excellent exposure to all areas of the abdomen and retroperitoneum, which can be accessed with minimal risk of significant vascular or nerve injury.
- Transverse incisions create less tension on the opposing skin edges because the incision follows Langer’s lines. The incidence of incisional hernias and wound dehiscence has been reported to be lower, but these studies are not randomized.
An abdominal incision often is given little thought other than as an access site through which a surgical procedure is performed. In reality, the incision is a second surgical procedure, which interferes—at least temporarily—with normal abdominal wall function.
While most physicians concur that the essential elements of a well-planned incision include adequate access to anticipated pathology, extensibility, and security of closure, many may not consider preservation of abdominal wall function as a key factor in their decision-making. Additional considerations include certainty of diagnosis, speed of entry, body habitus, presence of previous scars, potential for problems with hemostasis, and cosmetic outcome. These factors are the key determinants of whether the incision will be longitudinal (midline or paramedian) or transverse (Pfannenstiel’s, Cherney’s, or Maylard’s). For most gynecologic procedures confined to the pelvis, either option may be considered. The exceptions are patients with uncertain diagnoses or when access to the upper abdomen is indicated.
Regardless of the type of incision selected, the skin should be incised with a single, clean stroke of a sharp scalpel. However, when it comes to dissecting the underlying subcutaneous tissues, the debate continues over whether a scalpel or electrosurgery is best. While I recently have switched to the latter, here is a look at what the data say: Johnson and Serpell demonstrated that electrosurgery is associated with faster hemostasis, with no difference in the incidence of wound infection.1 Similarly, a recent randomized trial by Kearns et al found electro-surgery causes less blood loss and does not increase the risk of wound infections or fascial dehiscence.2 In contrast, a large prospective study by Cruse et al suggested that the use of diathermy is associated with twice the wound infection rate.3
This controversy also involves patients with gynecologic malignancies. Kolb et al found that electrosurgery was an independent risk factor for wound complications following surgery for ovarian cancer.4 However, Franchi and colleagues reported no difference in the rate of wound complications between scalpel and diathermy in patients who underwent mid-line abdominal incisions for the treatment of uterine cancer.5
Use the midline when the diagnosis and the extent of surgery are uncertain.
The inconsistencies in the data may reflect differences in electrosurgical technique. Non-modulated (cutting) current concentrates energy, vaporizing the tissue with little heat injury to surrounding areas. Conversely, modulated (coagulating) current coagulates the tissue with heat-producing char over a large area, and tissue injury often extends beyond the char. This effect is magnified if the electrode comes in direct contact with the tissue. Use the arc, rather than direct contact, to prevent excessive devitalization of tissue.
This article will review the techniques for, as well as the rationale and disadvantages of, common incisions—both longitudinal and transverse—to help the gynecologic surgeon minimize morbidity and maximize outcomes.
Longitudinal incisions
The longitudinal incisions that will be reviewed here are the midline (median) and paramedian. Classically, it was thought that longitudinal incisions were at greater risk of dehiscence than transverse incisions.6 However, it is difficult to make legitimate comparisons since longitudinal incisions are more likely to be performed in cases of hemorrhage, trauma, sepsis, multiorgan disease, previous surgery, previous radiation therapy, and malignancy—all of which increase the likelihood of postoperative complications. Furthermore, prospective and randomized studies have shown little, if any, difference in the incidence of dehiscence and hernias between longitudinal and transverse incisions.6-8
MidlineTechnique. Initiate the midline as a low abdominal incision (approximately 2 cm above the pubic symphysis), cutting along the linea alba. To extend the incision, if necessary, continue the dissection to the left of the umbilicus to avoid the ligamentum teres. Open the peritoneum at the cephalad pole of the incision (Figure 1). Expand this cut slightly off midline to avoid the urachus yet adequately expose the peritoneal cavity (Figure 2).
Rationale. While this incision can be used for any surgical procedure, it is especially appropriate when the diagnosis is uncertain and the exact procedure or extent of surgery is unclear. It is an excellent choice when access to the upper abdomen may be necessary, e.g., patients with gynecologic malignancies who may need assessment of the diaphragm, liver biopsy, para-aortic node biopsy, omentectomy, or debulking procedures. Patients with benign gynecologic conditions also may benefit from a midline incision. For example, when pelvic anatomy is distorted, as with severe endometriosis or sepsis, recognizable anatomy may be found only above the pelvic brim.
A midline incision allows the quickest entry, which is especially important for an unstable or seriously ill patient. Exposure is excellent, as all areas of the abdomen and retroperitoneum can be accessed with minimal risk of significant vascular or nerve injury. This is because only terminal branches of the abdominal wall blood vessels and nerves are located at the linea alba. In addition, because deep tissue planes are not opened, this incision may be ideal for patients who are anticoagulated, have enlarged epigastric vessels that are more susceptible to injury, or have an intraabdominal infection.
Transverse incisions help reduce the rate of wound dehiscence.
Disadvantages. Two potential problems are the higher rate of hernia formation and wound dehiscence, which may be due to constant lateral tension, compared with transverse incisions. In addition, coughing, retching, and straining may exacerbate the lateral tension. Proper suture selection can reduce the incidence of complications. As previously mentioned, the reality of these disadvantages is subject to debate; further studies are needed to determine the true risk.
From a cosmetic standpoint, the midline scar often is prominent because the incision transects Langer’s lines. Hence, it cannot be concealed by lingerie or swimwear.
FIGURE 1 Midline incision
After incising the linea alba and separating the muscles in the midline, open the peritoneum at the cephalad pole of the incision.
FIGURE 2 Midline incision
Expand this cut slightly off midline to avoid the urachus yet adequately expose the peritoneal cavity.
ParamedianTechnique. Place this incision 2 to 5 cm to the left or right of the midline, depending on the indication for surgery. After dissecting the skin and subcutaneous layers, incise the anterior rectus sheath vertically over the rectus abdominis muscle (Figure 3) and dissect it from the medial muscle edge. Retract the muscle laterally to expose the posterior rectus sheath and then incise the sheath and the peritoneum vertically to expose the peritoneal cavity (Figure 4). Alternatively, a more lateral paramedian incision can be placed over the rectus abdominis muscle. In this case, however, the rectus muscle should be separated vertically, instead of retracted laterally, to expose the posterior rectus sheath.
Rationale. Paramedian incisions provide excellent exposure of the pelvis, excluding the upper abdomen. Therefore, consider this technique when the procedure will be confined to the ipsilateral pelvis, e.g., rightor left-side lymph node biopsies and exposure of the sigmoid colon on the left. Also, a paramedian incision at the level of the umbilicus can be used for a cesarean delivery or a hysterectomy.
This incision is the best choice when performing pelvic surgery on morbidly obese patients. By placing the incision low on the abdomen, the large panniculus can be retracted over the mons pubis and thighs, providing excellent exposure of the pelvis.
Disadvantages. Some researchers have reported that muscle-splitting lateral paramedian incisions have a lower incidence of incisional hernias compared with midline incisions.7,9 However, they take longer to perform and restrict access to the contralateral pelvis. In addition, the risk of vascular injury and hematomas is increased, especially in the lower pole where branches of the epigastric arteries penetrate the muscle.
With regard to nerve injury, terminal nerves are cut, resulting in paralysis of the inner portion of the rectus abdominis muscle. This paralysis can be permanent, as the muscle medial to the vertical separation is involved. However, if only the medial third of the muscle is denervated, the paralysis rarely limits function.
FIGURE 3 Paramedian incision
Place the incision 2 to 5 cm to the left or right of the midline. Incise the anterior rectus sheath vertically over the rectus abdominis muscle.
FIGURE 4 Paramedian incision
Retract the muscle laterally to expose the posterior sheath; then incise the sheath and the peritoneum vertically to expose the pelvic cavity.
Transverse incisions
Transverse incisions such as Pfannenstiel’s (muscle separating), Cherney’s (tendon detaching), and Maylard’s (muscle cutting) were developed to reduce the incidence of incisional hernias and wound dehiscence. Their success lies in the fact that they cause less tension on the opposing wound edges because the incisions follow Langer’s lines, unlike longitudinal incisions. Their placement also allows for a better cosmetic outcome. Because they can be placed in the pubic hairline or in a natural skin crease, they are easily concealed by lingerie or swimwear. However, the incision should not be placed in a deep skin fold of a large panniculus where maceration of the skin can increase the risk of infection.
The main disadvantages of transverse incisions are limited exposure of the upper abdomen and limited extensibility. Further, because more tissue planes are opened and more vessels are encountered, these incisions increase the risk of hematomas and infection.
Pfannenstiel’sTechnique. Incise the skin and subcutaneous tissues 2 to 5 cm above the pubic symphysis.10 Then dissect the rectus sheath transversely, separating it sharply from the rectus muscle at the linea alba and retracting it superiorly toward the umbilicus and inferiorly toward the pubic symphysis (Figure 5). Divide the rectus abdominis muscle along the midline raphe and retract it laterally, exposing the transversalis fascia and the posterior rectus sheath. Incise these layers and the peritoneum vertically to expose the peritoneal cavity (Figure 6).
To minimize retraction of the rectus abdominus muscle during Maylard’s, do not separate it from the anterior rectus sheath.
Rationale. Since exposure is limited and the incision can be only minimally extended, this incision is appropriate for procedures that are limited to the pelvis, e.g., abdominal hysterectomy, cesarean delivery, retropubic urethropexy, and paravaginal defect repairs. (Extension of the incision can be achieved by modifying it to Cherney’s incision.)
Disadvantages. The fact that the incision severs multiple tissue planes is an advantage as well as a disadvantage. Postoperative dehiscence and incisional hernias are rare because the closed wound has a high tensile strength. However, the incidence of inguinal hernias may increase when the incision is placed close to the external inguinal ring,11 and very low incisions may increase the risk of femoral nerve injury.12 The ilioinguinal and iliohypogastric nerves may be damaged, especially if the rectus incision is extended far laterally. Most commonly, they are trapped in the suture during closure, producing postoperative pain. Incising multiple layers also slows entry and increases the risk of seromas, hematomas, and wound infections.
This approach is contraindicated when time is of the essence, e.g., hemorrhage, or in the face of an abdominal infection, e.g., sepsis.
FIGURE 5 Pfannenstiel’s incision
After incising the skin and subcutaneous tissues, dissect the rectus sheath transversely, separating it sharply from the rectus muscle.
FIGURE 6 Pfannenstiel’s incision
Divide and retract the rectus abdominus muscle and then incise the tranversalis fascia and posterior rectus sheath vertically.
Cherney’sTechnique. Dissect the skin and subcutaneous tissue 2 to 3 cm above the pubic symphysis, which is lower than most Pfannenstiel’s incisions. Then incise the anterior rectus sheath transversely and dissect it from the rectus abdominis muscle superiorly and inferiorly. Using blunt dissection, separate the rectus and pyramidalis muscles from the underlying bladder and adventitial tissue. Then incise transversely the tendons of the rectus and pyramidalis muscles 0.5 cm above the pubic symphysis (Figure 7). (This segment of tendon facilitates the reattachment of the muscles at closure.) Retract the muscles and tendons cephalad to expose the retropubic space. Incise the peritoneum transversely to expose the peritoneal cavity (Figure 8).
Rationale. Cherney’s incision provides excellent access to the retropubic space of Retzius, making it a good choice for retropubic urethropexy and paravaginal repair. Exposure is the main advantage to Cherney’s incision. The reason: It is a modification of Pfannenstiel’s incision and, as such, provides excellent lateral exposure because it is placed much lower on the abdominal wall.
Disadvantages. The benefits and risks of this incision are similar to those of Pfannenstiel’s incision since the same tissue planes are opened. However, it is more time consuming due to the dissection required to separate the muscles and tendons from the underlying tissues.
In addition, the deep inferior epigastric arteries could be injured as a result of the lateral dissection. Lastly, reattachment of the tendons is tedious.
FIGURE 7 Cherney’s incision
Incise transversely the tendons of the rectus and pyramidalis muscles 0.5 cm above the pubic symphysis.
FIGURE 8 Cherney’s incision
Retract the muscles and tendons cephalad and incise the peritoneum transversely to expose the peritoneal cavity.
Maylard’sTechnique. After transecting the skin and subcutaneous layers in a plane at the level of the anterior superior iliac spine, incise the rectus sheath transversely and extend the incision through the aponeuroses of the abdominal muscles to about 2 to 3 cm medial to the anterior iliac crest. Then dissect the rectus abdominis muscle transversely with a scalpel, electrocautery, or surgical stapler (Figure 9). To minimize retraction of this muscle, do not separate it from the anterior rectus sheath. (Alternatively, many surgeons recommend that the cut edge of the muscle be secured to the anterior sheath with mattress sutures to prevent retraction and reduce blood loss.)
Prior to transecting the peritoneum, isolate, ligate, and divide the deep inferior epigastric vessels. (Patients with significant aortoiliac atherosclerosis or aortic coarctation develop considerable collateral circulation through the epigastric vessels for perfusion of the lower extremities. Ligating these vessels may cause claudication and even lifethreatening ischemia. Therefore, assess iliac flow before ligating and transecting the arteries.) Then transect the transversalis fascia and peritoneum transversely, allowing access to the peritoneal cavity (Figure 10).
Rationale. Many oncologists use this approach for pelvic lymphadenectomy and staging procedures because the exposure to the lateral pelvis is excellent. The reason: This transverse incision cuts through all layers of the abdominal wall at the level of the anterior iliac spine. It also may be appropriate when pelvic pathology extends to the sidewall, e.g., endometriosis.
It is advisable to place a drain prior to closing Maylard’s.
Physicians should consider using this approach more than they currently are, especially when Pfannenstiel’s incision cannot provide satisfactory exposure or a longitudinal incision is thought to be the only alternative. The decision to use Maylard’s incision should be planned during the preoperative assessment, as Pfannenstiel’s incision should not be modified to Maylard’s intraoperatively.
Disadvantages. Although Maylard’s incision improves access to the upper abdomen compared to other transverse incisions, exposure is still limited. In addition, trauma to the deep epigastric arteries may result in considerable hemorrhage. And since hematomas commonly infiltrate the retroperitoneal space, large quantities of blood already may be lost before the hemorrhage is clinically apparent. Finally, oozing from the cut edge of the muscles may result in significant fluid collection, increasing the risk of infection. Therefore, it is advisable to place a drain prior to closure.
Like other transverse incisions, the wound parallels the nerves, providing a measure of protection. However, the rectus incision approaches the anterior iliac spine, where both the ilioinguinal and iliohypogastric nerves lie. Both may be damaged during the incision and can become entrapped during closure. Additionally, the femoral nerve, the lateral femoral cutaneous nerve, and the genitofemoral nerve are easily compressed by the lateral blades of the retractors because of the extreme lateral positions of the incision’s poles. Injury to these nerves can result in paresthesia, pain, and paralysis of the leg muscles.
FIGURE 9 Maylard’s incision
After incising the aponeuroses of the abdominal muscles medial to the anterior iliac crest, dissect the rectus abdominus muscle transversely.
FIGURE 10 Maylard’s incision
Isolate, ligate, and divide the epigastric vessels. Then incise the transversalis fascia and peritoneum transversely to access the peritoneal cavity.
In summary
While the midline and Pfannenstiel’s incisions are an integral part of the gynecologic surgeon’s armamentarium, the paramedian and Maylard’s incisions should be added to the repertoire because these incisions can greatly enhance exposure. The paramedian shares the advantages of the midline incision but also allows better access to the ipsilateral pelvis. Furthermore, although Maylard’s incision takes longer to perform than other techniques, the excellent exposure—both laterally and superiorly—makes it worthwhile.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Johnson CD, Serpell JW. Wound infection after abdominal incision with scalpel or diathermy. Br J Surg. 1990;77:626-627.
2. Cruse PJE, Ford R. The epidemiology of wound infection: a 10-year prospective study of 62,939 wounds. Surg Clin North Am. 1980;60:27.-
3. Kearns SR, Connolly EM, McNally S, McNamara DA, Deasy J. Randomized clinical trial of diathermy versus scalpel incision in elective midline laparotomy. Br J Surg. 2001;88(1):41-44.
4. Kolb BA, Buller RE, Connoer JP, et al. Effects of early postoperative chemotherapy on wound healing. Obstet Gynecol. 1992;79:988-992.
5. Franchi M, Ghezzi F, Bendetti-Panici PL, et al. A multicenter collaborative study on the use of cold scalpel and electrocautery for midline abdominal incision. Am J Surg. 2001;181(2):128-132.
6. Hendrix SL, Schimp V, Martin J. The legendary superior strength of the Pfannenstiel incision: a myth? Am J Obstet Gynecol. 2000;182(6):1446-1451.
7. Ellis H, Coleridge-Smith PD, Joyce AD. Abdominal incisions—vertical or transverse? Postgrad Med J. 1984;60:407-410.
8. Sanders RJ, DiClementi D. Principles of abdominal wound closure. II. Prevention of wound dehiscence. Arch Surg. 1977;112:1188.-
9. Donaldson DR, Hegarty JH, Brennan TG, et al. The lateral paramedian incision-experience with 850 cases. Br J Surg. 1982;69:630.-
10. Greenall MJ, et al. Midline or transverse laparotomy? A random controlled clinical trial. Part I: Influence on healing. Br J Surg. 1980;7:188.-
11. Pfannenstiel J. Ueber die Vortheile des suprasymphysaren Fascienquerschnitts für die gynakologischen Koliotomien zugleich ein Beitrag zu der Indikationsstellung der Operationswege. Samml Klin Vortr (Leipzig). 1900;268:1735.-
12. Griffiths DA. A reappraisal of the Pfannenstiel incision. Br J Urol. 1976;48:469.-
1. Johnson CD, Serpell JW. Wound infection after abdominal incision with scalpel or diathermy. Br J Surg. 1990;77:626-627.
2. Cruse PJE, Ford R. The epidemiology of wound infection: a 10-year prospective study of 62,939 wounds. Surg Clin North Am. 1980;60:27.-
3. Kearns SR, Connolly EM, McNally S, McNamara DA, Deasy J. Randomized clinical trial of diathermy versus scalpel incision in elective midline laparotomy. Br J Surg. 2001;88(1):41-44.
4. Kolb BA, Buller RE, Connoer JP, et al. Effects of early postoperative chemotherapy on wound healing. Obstet Gynecol. 1992;79:988-992.
5. Franchi M, Ghezzi F, Bendetti-Panici PL, et al. A multicenter collaborative study on the use of cold scalpel and electrocautery for midline abdominal incision. Am J Surg. 2001;181(2):128-132.
6. Hendrix SL, Schimp V, Martin J. The legendary superior strength of the Pfannenstiel incision: a myth? Am J Obstet Gynecol. 2000;182(6):1446-1451.
7. Ellis H, Coleridge-Smith PD, Joyce AD. Abdominal incisions—vertical or transverse? Postgrad Med J. 1984;60:407-410.
8. Sanders RJ, DiClementi D. Principles of abdominal wound closure. II. Prevention of wound dehiscence. Arch Surg. 1977;112:1188.-
9. Donaldson DR, Hegarty JH, Brennan TG, et al. The lateral paramedian incision-experience with 850 cases. Br J Surg. 1982;69:630.-
10. Greenall MJ, et al. Midline or transverse laparotomy? A random controlled clinical trial. Part I: Influence on healing. Br J Surg. 1980;7:188.-
11. Pfannenstiel J. Ueber die Vortheile des suprasymphysaren Fascienquerschnitts für die gynakologischen Koliotomien zugleich ein Beitrag zu der Indikationsstellung der Operationswege. Samml Klin Vortr (Leipzig). 1900;268:1735.-
12. Griffiths DA. A reappraisal of the Pfannenstiel incision. Br J Urol. 1976;48:469.-