User login
- Prophylaxis must start before surgery for maximal benefit, since at least 50% of postoperative thromboembolic disease begins intraoperatively.
- A consensus conference found that prophylaxis reduced fatal pulmonary emboli by 75% in 7,000 gynecologic surgery patients.
- Low-dose unfractionated heparin and low molecular weight heparin appear similarly effective in reducing thromboembolic disease in perioperative patients, but it is unclear which form has fewer bleeding complications.
The case is strong for routine prophylaxis against venous thrombosis and pulmonary embolism. The primary reasons: efficacy, ease of use, and safety.
This article reviews the evidence on routine prophylaxis, pros and cons of mechanical and drug therapies (including a comparison of 2 heparins), patient risk factors, and cost-effectiveness. A table (page 32) lists patient characteristics for low, moderate, high, and very high levels of risk, with corresponding appropriate preventive measures.
Routine prophylaxis is a wiser strategy than postevent treatment or surveillance because:
- Thromboembolism is a stealthy adversary, particularly pulmonary embolism. Between 10% and 20% of patients with pulmonary emboli die, often within 30 minutes of the sentinel complaint.
- Even when pulmonary embolus is documented during autopsy, as many as 80% of patients have no antecedent clinical evidence of deep venous thrombosis (DVT)—and no chance of life-saving therapy.2
- Surveillance is the least desirable preventive option due to lack of sensitivity of noninvasive tests for thromboembolic disease.
Scope of thromboembolic disease
Thromboembolism is the leading potentially preventable cause of hospital fatality.1
In postoperative gynecologic surgery patients, pulmonary embolism occurs in 0.1% to 5% of cases, depending on risk.2 Venous thrombosis occurs on average in 15% of postoperative gynecologic surgery patients, with a range of 5% to 29%, depending on patient risk factors and the surgical procedures performed.3 Almost 50% of gynecologic patients undergoing surgery for cancer develop a lower-extremity venous thrombosis if left untreated.4
Overall, thromboembolic disease is linked with some 500,000 hospital admissions every year. Pulmonary embolism, the most serious complication, causes 60,000 to 200,000 deaths annually.
High risk of recurrence. Postthrombotic syndrome is characterized by pain, swelling, and leg ulceration. At least 50% of patients successfully treated for proximal venous thrombosis (thigh) and about 33% treated for distal venous thrombosis (calf) develop postthrombotic syndrome.1 The risk for recurrent thrombosis is high.
Risk factors are listed in TABLE 1. The risk of fatal pulmonary embolism is directly related to age; persons over 60 are at greatest risk. When the patient is obese—particularly when she exceeds 120% of ideal body weight—the risk is even greater due to venous stasis.
TABLE 1
Risk factors for thromboembolism
| Age |
| Estrogen use |
| Extended pelvic surgery |
| Hypercoagulable states |
| Immobility |
| Indwelling central venous catheter(s) |
| Lower-extremity paralysis |
| Malignancy |
| Medical illnesses |
| Chronic pulmonary disease |
| Congestive heart failure |
| Diabetes mellitus |
| Inflammatory bowel disease |
| Nephrotic syndrome |
| Obesity |
| Pregnancy |
| Prior thromboembolic disease |
| Radiation therapy |
| Trauma |
| Varicose veins |
Common hypercoagulable states
Activated protein C resistance, the most common thrombophilia, occurs in 3% to 7% of Caucasians (TABLE 2).7 It is usually associated with factor V Leiden mutation.
Prothrombin gene mutation G20210A, the next most common thrombophilia, occurs in 2% of Caucasians.7
Antiphospholipid antibody syndromes are acquired thrombophilias that may be associated with arterial and venous thrombosis, thrombocytopenia, and complications of pregnancy.8
Hyperhomocystinemia may be congenital or acquired. It is associated with venous thromboembolism and early atherosclerosis with arterial thrombosis.
Thrombosis is more likely with multiple predisposing factors. Most patients with one of the thrombophilia syndromes do not have sentinel thrombotic events unless they are further challenged by environmental risks such as oral contraceptives (OCs), pregnancy, prolonged immobilization, or surgery.9
Risk is markedly enhanced if multiple predisposing factors are present. For example, when factor V Leiden mutation and hyperhomocystinemia coexist, the risk for thrombosis increases 10 to 20 times over that of patients with neither abnormality.10 This combined risk far exceeds the sum of risks for each abnormality alone.
TABLE 2
Common hypercoagulable conditions
| Factor V Leiden mutation |
| Prothrombin gene mutation G20210A |
| Antiphospholipid antibody |
| Lupus anticoagulant |
| Anticardiolipin |
| Protein C deficiency |
| Protein S deficiency |
| Antithrombin III deficiency (Heparin cofactor II) |
| Plasminogen deficiency |
| Hyperhomocystinemia |
| Myeloproliferative disorders |
| Polycythemia |
| Primary thrombocytosis |
When to test for thrombophilias
Although women with thrombophilias are at high risk for thromboembolic disease, it is unclear whether identifying the thrombophilia is more beneficial than universal prophylaxis. If a patient has a personal or family history of thromboembolic disease—especially a patient with Caucasian ancestry—testing is probably warranted. While documentation of the thrombophilia may not help with perioperative management, it may be useful for long-term care.
Testing for factor V Leiden mutation is recommended due to its prevalence. If the results are negative in a patient at risk, test for prothrombin gene mutation G20210A, as well as deficiencies in the naturally occurring inhibitors protein C, protein S, and antithrombin III.
Assess antiphospholipid antibodies in women who have experienced recurrent fetal loss or early pregnancy-induced hypertension.
Continue OCs or hormone therapy
OCs and menopausal hormone replacement therapy produce measurable prothrombotic changes in the clotting system that appear to be directly related to the estrogen content. In theory, discontinuing the OC or hormone replacement therapy preoperatively would allow these changes to return to baseline and help prevent thromboembolic disease.
Although the risk of thromboembolic disease is 0.96% if patients are current users of OCs and 0.5% if they are not, studies have failed to confirm a clinical benefit of discontinuation.11 Further, the patient does not return to baseline for 4 to 6 weeks after ceasing therapy.
The potential risk of thromboembolic events also should be weighed against the risk of conception prior to surgery. We usually do not recommend discontinuation of OCs and hormone therapy before surgery, but give prophylaxis based on risk assessment.
Venous stasis, vessel-wall trauma, and increased blood coagulability—the major contributors to perioperative DVT, known as Virchow’s triad—were identified more than 125 years ago.
Venous stasis. Intraoperatively, venous blood return from the lower extremities is reduced to less than half its normal rate,34 secondary to muscle relaxation during anesthesia, which causes venous dilation and reduced blood-flow velocity. Packing the abdominal contents may further impede blood return from the legs.
Resultant venous stasis causes platelet adhesion to the vein wall, followed by release of a thromboplastin-like substance that may trigger thrombus formation.
Blood flow increases in the immediate postoperative period with return of muscle tone, but remains significantly diminished for 21 days because of immobilization—specifically, lack of the usual pumping action of the leg muscles.
Vessel-wall trauma. Veins are highly likely to be damaged as vessels are skeletonized during major pelvic surgery, especially when malignancy is involved. Tissue injury activates the coagulation cascade by exposing blood to tissue thromboplastin (extrinsic path) and subendothelial collagen in the vessel wall, which activates factor XII (intrinsic path). Both pathways lead to conversion of factor X to its active form, factor Xa. Acting in concert with factor V, calcium, and phospholipids from platelet factor III, factor Xa catalyzes the conversion of prothrombin to thrombin. Thrombin regulates the conversion of fibrinogen to fibrin, the basic building block of a thrombus.
Increased blood coagulability. Clotting factors XI, IX, and VII increase following surgery, as do circulating platelets and platelet aggregation. This enhances coagulability, which persists from 72 to 96 hours after surgery but is usually balanced by the fibrinolytic system. Fibrinolysis is mediated primarily by plasmin, which digests fibrin and fibrinogen and activates factors V and VIII. If the fibrinolytic system is overwhelmed, the clotting system is unimpeded and thrombus formation may accelerate.
Pregnancy increases the risk of thrombosis, in part due to the progressive increase in resistance to activated protein C in the second and third trimesters. Risk is increased eightfold in women with inherited deficiency in any of the naturally occurring anticoagulants—antithrombin III, protein C, or protein S—compared with those with no deficiency.12
Preventive strategies
Two approaches to thromboembolic prophylaxis have been proposed, with prevention of fatal postoperative pulmonary emboli as a clear endpoint:
- Stratify a targeted group into levels of risk; then treat those at higher risk. Unfortunately, efforts to define risk have met with only partial success, due to limited availability of noninvasive screening, screening logistics, and expense. Further, specificity and positive predictive value of screening asymptomatic patients is low.
- Use prophylaxis in all patients in the targeted group, regardless of risk (TABLES 3 AND 4). This strategy seems effective. For example, in 2001, the Sixth American College of Chest Physicians Consensus Conference—the most recent of the consensus conferences—evaluated the risks of pulmonary embolus in 7,000 gynecologic surgery patients enrolled in prospective studies. Routine prophylaxis reduced fatal pulmonary emboli by 75%.13
The difficulty of defining patients at highest risk makes the concept of universal prophylaxis for a targeted group an attractive option unless a specific contraindication is identified.
TABLE 3
Risk stratification and prophylactic regimens
| LEVEL OF RISK | PATIENT CHARACTERISTICS | RECOMMENDED REGIMEN |
|---|---|---|
| Low | Less than 40 years of age | No specific recommendation for therapy |
| Undergoing uncomplicated minor surgery | Adequate hydration | |
| Requires less than 30 minutes of anesthesia | Aggressive early ambulation | |
| No additional risk factors | ||
| Moderate | Undergoing minor surgery with additional risk factors | Graduated compression stockings or SPCDs or |
| 40 to 60 years of age, undergoing minor surgery, and no additional risk factors | LDUH 5,000 U every 12 hours or | |
| Less than 40 years of age, undergoing major surgery, and no additional risk factors | LMWH (20 mg) or 2,500 U antifactor Xa once daily | |
| High | More than 60 years of age, undergoing minor surgery or with additional risk factors | LDUH 5,000 U every 8–12 hours or LMWH (40 mg) or 5,000 U antifactor Xa once daily |
| More than 40 years of age undergoing major surgery or with additional risk factors | SPCDs may be added | |
| Very high | More than 60 years of age, undergoing major surgery, with other risk factors such as cancer, prior venous thromboembolism, molecular hypercoagulable state, major trauma, spinal cord injury, or lower-extremity paralysis | Graduated compression stockings or SPCDs and LDUH 5,000 U every 8 hours or |
| Other risk factors such as cancer, prior venous thromboembolism, molecular hypercoagulable state, major trauma, spinal cord injury, or lower-extremity paralysis | LMWH (40 mg) or 5,000 U antifactor Xa once daily | |
| LDUH = low-dose unfractionated heparin; LMWH = low molecular weight heparin; SPCD = sequential pneumatic compression device | ||
| Note: Because perioperative prophylaxis is still evolving, these suggestions should not be considered inviolable. | ||
| Source: Modified from Geerts WH, et al.13 | ||
TABLE 4
Incidence of thromboembolic events based on level of risk
| SITE OF THROMBOSIS | RISK LEVEL (%) | |||
|---|---|---|---|---|
| Low | Moderate | High | Very high | |
| Calf veins (distal) | 2 | 10–35 | 30–60 | 63.5 |
| Iliofemoral veins (proximal) | 0.4 | 2–8 | 5–10 | 10–20 |
| Pulmonary emboli | 0.2 | 1–2 | 2–4 | 4–10 |
| Fatal pulmonary emboli | 0.002 | 0.1–0.5 | 0.4–1 | 0.2–5 |
| Source: Modified from Geerts WH, et al.13 | ||||
Drug and mechanical therapies
Strategies for preventing thromboembolic disease attempt to mitigate the impact of venous stasis, endothelial injury, and hypercoagulable states. Traditionally, early ambulation, adequate hydration, and elevation of the lower extremities have been used, because they are simple and inexpensive interventions.
More recently, pharmacologic and mechanical therapies have proven effective in reducing the incidence of DVT and fatal pulmonary emboli. Prophylactic measures commonly used today are:
- graduated compression stockings
- external pneumatic leg or foot compression devices
- low-dose unfractionated heparin
- low molecular weight heparin
MECHANICAL THERAPIESGraduated compression stockings
Compression stockings are one of the earliest methods of preventing perioperative thrombosis. Compression is greatest at the toe and gradually diminishes toward the thigh. When Belcaro14 studied the risk of recurrent venous thrombosis in nonsurgical hospitalized patients, thrombosis recurred in 40% of patients with no therapy, but only 9.4% of patients wearing graduated compression stockings (GCS). Adding oral antiplatelet therapy lowered the risk to 2%. However, GCS were not superior to any other method of preventing recurrent DVT.
As for surgical patients, a single study15 demonstrated protection against DVT when compared with no GCS in elective gynecologic surgery patients.
Today, GCS are usually a perioperative adjunct to other preventive methods, to provide added protection.
Proper fitting by trained personnel is vital; otherwise, a tourniquet effect may cause venous stasis and reduce benefit.
Sequential pneumatic compression devices
Like GCS, these devices decrease the caliber of veins by simple compression. They also increase blood flow velocity and stimulate the endogenous fibrinolytic system.
Enhanced fibrinolytic activity due to intermittent compression occurs even if the device is used on only 1 lower extremity, or on an upper extremity. Patient and nursing-staff compliance may affect efficacy. Sequential pneumatic compression devices (SPCDs) may be thought inconvenient, impeding nursing functions. Some patients may find the repetitive inflation-deflation cycles annoying.
These devices must be activated prior to surgery and continued for a minimum of 24 hours. Some studies suggest that SPCDs be used for 5 days in high-risk situations.16
Calf- and thigh-length devices have similar effects. Rare complications include peroneal nerve injury and compartment syndrome.
What the data show. In an analysis of 4 trials comparing SPCDs with no therapy, thromboembolic disease occurred in only 2% of patients who wore SPCDs but in 20% of those who did not.17 Compared with low-dose unfractionated heparin, no difference in the rate of DVT was seen.18 However, risk of transfusion and retroperitoneal drainage volume increased in the heparin group.
Contraindications include active or suspected DVT, congestive heart failure, known pulmonary embolus, and leg injuries.19
Combined therapy may be best for high-risk women. A study20 of women who developed thromboemboli despite appropriate treatment with SPCDs found the women more likely to be older than 60 years and/or to have cancer or history of thromboembolic disease or hypertension. This high-risk group may benefit from combined therapy.
Foot compression devices resemble booties and mimic the plantar compression that occurs during walking. They increase blood-flow velocity, stimulate the endogenous fibrinolytic system, and have the same indications as SPCDs. However, they are only moderately effective in reducing venous thrombosis.21 Potential drawbacks are that the devices must be removed when the patient ambulates and replaced when she returns to bed.
DRUG THERAPIESLow-dose unfractionated heparin
Heparin alters the molecular configuration of antithrombin III, making it 1,000 to 4,000 times more potent as an inhibitor of thrombin formation, which in turn limits conversion of fibrinogen to fibrin. This prolongs the activated partial thromboplastin time (aPTT) commonly used to monitor patients receiving full anticoagulation therapy.
A naturally occurring mucopolysaccharide with a molecular weight ranging from 3,000 to 30,000 daltons, heparin is richly concentrated in mast cells. Its anticoagulant properties relate primarily to interaction with antithrombin III (also known as heparin cofactor II), factor IXa, factor Xa, factor XIa, factor XIIa, and platelet aggregation.22
Heparin also inhibits the effects of factor Xa on the coagulation cascade and limits platelet aggregation.23
Half-life is 1 hour for intravenous heparin and about 3 hours for subcutaneous heparin.
Use in pregnancy. Low-dose unfractionated heparin (LDUH) does not cross the placenta and is safe to use during pregnancy.
What the data show. Randomized clinical trials conducted prior to 1988 showed venous thrombosis decreased by 70% and pulmonary embolus by 50% in patients treated with LDUH, compared with those receiving no therapy.24
Dosing options. LDUH typically is given as a 5,000-U dose 2 hours before surgery. The single preoperative dose seems to be as effective as multiple preoperative doses.25 Postoperative therapy is instituted 8 to 12 hours after surgery; heparin is given every 8 to 12 hours until the patient is fully ambulatory.
Patients having gynecologic surgery for benign conditions benefit from the twice-daily regimen, and those undergoing gynecologic oncology surgery or other high-risk procedures seem to benefit from thrice-daily dosing.26
These regimens also significantly reduced DVT and fatal pulmonary emboli in general surgery patients.27 The LDUH regimen is now used to judge the efficacy of other prophylactic measures.
Low molecular weight heparin
This form of heparin acts primarily by inhibiting factor Xa, which is higher in the coagulation cascade than antithrombin. Thus, low molecular weight heparin (LMWH) is more efficient than unfractionated heparin.
LMWH has a molecular weight of 3,000 to 6,000 daltons and is produced by concentrating the low molecular component of heparin. Because the molecular configuration of antithrombin III is not altered by LMWH, thrombin conversion is minimally inhibited and aPTT is not appreciably affected.
Half-life is approximately 4 hours, by any route of administration. The longer half-life provides a longer dosing interval. Bioavailability is more consistent than that of LDUH, approaching 90% to 95%. The excellent bioavailability allows dosing to be based on lean body mass. Less heparin-induced thrombocytopenia is another plus.
Use in pregnancy. LMWH does not cross the placenta and is safe to use during pregnancy.
What the data show. No randomized trials have compared LMWH therapy with no therapy, with efficacy assessed by venography or fibrinogen uptake. An uncontrolled series28 of 2,030 patients did show that LMWH reduced the incidence of thromboembolic disease.
A randomized controlled trial29 comparing 2,500 U and 5,000 U daily of dalteparin (an LMWH) found greater efficacy in the higher-dose group (6.6% versus 12.7% incidence of DVT), but bleeding complications also were higher (4.7% versus 2.7%). In a subgroup of patients with malignancy, the high-dose therapy remained superior in preventing DVT, and the bleeding risk was equal.
Dosing options. Once a day dosing is normally adequate for prophylaxis; twice-daily dosing is needed for therapy. Enoxaparin is an LMWH that is readily available in the United States and commonly prescribed when use of LMWH is desired. For prophylaxis, it can be given in daily doses of 20 mg, 30 mg, 40 mg, or 60 mg. None of these doses has proven superior to the others. The typical regimen for moderate risk is 20 mg per day; for high risk, 40 mg per day. Enoxaparin appears to convey the same degree of protection as 5,000 units of LDUH every 8 hours.
2 heparins compared
Randomized controlled trials comparing LDUH and LMWH have found them to be similarly effective. In addition, within the LMWH class, all compounds appear to have similar benefits. However, it remains unclear which form of heparin is associated with fewer bleeding complications.
In theory, LMWH would be associated with an increase in these complications due to its longer half-life and increased bioavailability.30 However, studies have not consistently identified excess bleeding in any group.31 Fortunately, excess postoperative bleeding requiring transfusion is uncommon.
Complications of heparin
Heparin-induced thrombocytopenia is a recognized complication, seen in as many as 20% of LDUH patients. The diagnosis is made when the platelet count falls below the lower limits of normal or when the platelet count falls by 50% but remains in the normal range. Two forms of heparin-induced thrombocytopenia have been described.
• Type 1 thrombocytopenia is initially mild, rarely dropping below 100,000 platelets per milliliter. Platelet count monitoring is important, but therapy usually can continue, since the platelet count returns to normal even with continued use. Type 1 thrombocytopenia appears to directly result from platelet activation and is not immune-mediated.
• Type 2 thrombocytopenia occurs 7 to 14 days after starting therapy. Platelet counts frequently drop to 20,000 per milliliter. Type 2 is an immune response caused by antibodies to the heparin-platelet factor 4 complex. Patients are at risk for venous and arterial thrombosis, but often the diagnosis is made only after a complicating thrombotic event.
The risk of type 2 thrombocytopenia appears to be related to the molecular weight of the compound being administered, its dose, and the duration of therapy. Therefore, unfractionated heparin—if given in full anticoagulation doses and beyond 14 days—seems to have the greatest potential for this complication.
Paradoxically, these patients also are at risk for severe hemorrhage. Because mortality and morbidity are high, immediate withdrawal of all forms of heparin—including LMWH—is mandatory.32 Catastrophic hemorrhage or thrombosis may be the first sign. Often a fall in platelet concentration precedes serious complications. Thus, platelet concentration should be monitored at least every 2 days.
Hematoma with conduction anesthesia. Use of major conduction anesthesia in patients who also need LMWH, LDUH, or oral anticoagulants is controversial. LMWH may pose a risk of hematoma if initiated preoperatively, intraoperatively, or within 3 hours of surgery in patients who have a continuous epidural. Hematoma formation seems to occur immediately following catheter withdrawal.
The risk of hematoma appears to be lower with single-dose spinal or single-dose epidural anesthesia. While the incidence of epidural or spinal hematoma is not known, some cases have been associated with long-term neurologic sequelae, including permanent paralysis.
Responding to this concern, the US Food and Drug Administration issued a 1997 advisory noting the risk of spinal hematoma in patients receiving enoxaparin plus conduction anesthesia or lumbar spinal puncture. Most anesthesiologists significantly restrict the use of conduction anesthesia in patients requiring heparin prophylaxis.33
Cost-effectiveness
The cost of perioperative prophylaxis has been compared with the cost of immediate therapy for thromboembolic disease and long-term therapy for postthrombotic syndrome. Across the board, universal prophylaxis with pharmacotherapy or mechanical devices in patients undergoing abdominal surgery is less expensive than no prophylaxis, based on a reduced incidence of DVT. Pneumatic compression seems to be more cost-effective than pharmacotherapy.34
Because LDUH is substantially less expensive than LMWH in the United States, it has a better cost profile when pharmacotherapy is warranted. In Europe, where the cost of heparin compounds is not an issue, LMWH has a slight advantage.
The authors report no financial relationships relevant to this article.
1. Dismuke SE, Wagner EH. Pulmonary embolism as a cause of death. The changing mortality in hospitalized patients. JAMA. 1986;255:2039-2042.
2. Farquharson DI, Orr JW, Jr. Prophylaxis against thromboembolism in gynecologic patients. J Reprod Med. 1984;29:845-862.
3. Bergqvist D. Prolonged prophylaxis against postoperative venous thromboembolism. Haemostasis. 1996;26(suppl 4):379-387.
4. Clarke-Pearson DL, DeLong ER, Synan IS, Coleman RE, Creasman WT. Variables associated with postoperative deep venous thrombosis: a prospective study of 411 gynecology patients and creation of a prognostic model. Obstet Gynecol. 1987;69:146-150.
5. Kemble JVH. Incidence of deep vein thrombosis. Br J Hosp Med. 1971;6:721-726.
6. Spritzer CE, Evans AC, Kay HH. Magnetic resonance imaging of deep venous thrombosis in pregnant women with lower extremity edema. Obstet Gynecol. 1995;85:603-607.
7. Florell SR, Rodgers GM. Inherited thrombotic disorders: an update. Am J Hematol. 1997;54:53-60.
8. Shapiro GA. Antiphospholipid syndrome in obstetrics and gynecology. Semin Thromb Hemost. 1994;20:64-70.
9. De Stafano V, Leone G, Mastrangelo S, et al. Clinical manifestations and management of inherited thrombophilia: retrospective analysis and follow-up after diagnosis in 238 patients with congenital deficiency of antithrombin III, protein C, protein S. S Throm Haemost. 1994;72:352-358.
10. Ridker PM, Hennekens CH, Selhub J, et al. Interrelation of hyperhomocyst(e)inemia, factor V Leiden, and risk of future venous thromboembolism. Circulation. 1997;95:1777-1782.
11. Vessey M, Jewell D, Smith A, Yeates D, McPherson K. Chronic inflammatory bowel disease, cigarette smoking, and use of oral contraceptives: findings in a large cohort study of women of childbearing age. Br Med J (Clin Res Ed). 1986;292:1101-1103.
12. Walker MC, Garner PR, Keely EJ, et al. Changes in activated protein C resistance during normal pregnancy. Am J Obstet Gynecol. 1997;177:162-169.
13. Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest. 2001;119(suppl 1):132S-175S.
14. Belcaro G, Laurora G, Cesarone MR, et al. Prophylaxis of recurrent deep venous thrombosis. A randomized, prospective study using indobufen and graduated elastic compression stockings. Angiology. 1993;44:695-699.
15. Clarke-Pearson DL, Synan IS, Dodge R, et al. A randomized trial of low-dose heparin and intermittent pneumatic calf compression for the prevention of deep venous thrombosis after gynecologic oncology surgery. Am J Obstet Gynecol. 1993;168:1146-1153.
16. Handoll HH, Farrar MJ, McBirnie J, et al. Prophylaxis using heparin, low molecular weight heparin and physical methods against deep vein thrombosis and pulmonary embolism in hip fracture. The Cochrane Library, Issue I. 1998. Evidence-based medicine: 146.
17. Clarke-Pearson DL, Synan IS, Hinshaw WM, et al. Prevention of postoperative venous thromboembolism by external pneumatic calf compression in patients with gynecologic malignancy. Obstet Gynecol. 1984;63(1):92-98.
18. Lachman EA, Rouk JL, et al. Complications associated with intermittent pneumatic compression. Arch Phys Med Rehab. 1992;73:482-485.
19. Clarke-Pearson DL, Dodge RK, Synan I, et al. Venous thromboembolism prophylaxis: patients at high risk to fail intermittent pneumatic compression. Obstet Gynecol. 101;2003:157-163.
20. Wilson NV, Das SK, Kakkar VV, et al. Thrombo-embolic prophylaxis in total knee replacement. Evaluation of the A-V Impulse System. J Bone Joint Surg Br. 1992;74:50-52.
21. Rosenberg RD, Damus PS. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973;248:6490-6505.
22. Scurr JH, Coleridge-Smith PD, Hasty JH. Regimen for improved effectiveness of intermittent pneumatic compression in deep venous thrombosis prophylaxis. Surgery. 1987;102:816-820.
23. Collins R, Scrimgeour A, Yusuf S, et al. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162-1173.
24. Hull RD, Pineo GF, Stein PD, et al. Timing of initial administration of low-molecular-weight heparin prophylaxis against deep vein thrombosis in patients following elective hip arthroplasty: a systematic review. Arch Intern Med. 2001;161:1952-1960.
25. Clarke-Pearson DL, DeLong ER, Synan IS, et al. A controlled trial of two low dose heparin regimens for the prevention of postoperative deep vein thrombosis. Obstet Gynecol. 1990;75:684-689.
26. Kakkar VV, Murray WJ. Efficacy and safety of low-molecular-weight heparin (CY216) in preventing postoperative venous thrombo-embolism: a co-operative study. Br J Surg. 1985;72:786-791.
27. Ward B, Pradhan S. Comparison of low molecular weight heparin (Fragmin) with sodium heparin for prophylaxis against postoperative thrombosis in women undergoing major gynaecological surgery. Aust N Z J Obstet Gynaecol. 1998;38:91-92.
28. Bergqvist D, Burmark US, Flordal PA, et al. Low molecular weight heparin started before surgery as prophylaxis against deep vein thrombosis: 2500 versus 5000 XaI units in 2070 patients. Br J Surg. 1995;82:496-501.
29. Bratt G, Tornebohm E, Widlund L, et al. Low molecular weight heparin (KABI 2165, Fragmin): pharmacokinetics after intravenous and subcutaneous administration in human volunteers. Thromb Res. 1986;42:613-620.
30. Kakkar VV, Boedki O, Boneau B, et al. Efficacy and safety profile of a low-molecular weight heparin and standard unfractionated heparin for prophylaxis of postoperative venous thromboembolism: European multicenter trial. World J Surg. 1997;21:2-8.
31. Warkentin TE. Heparin-induced thrombocytopenia: pathogenesis and management. Br J Haematol. 2003;121:535-555.
32. Wysowski DK, Talarico L, Bacsanyi J, et al. Spinal and epidural hematoma and low-molecular-weight heparin. N Engl J Med. 1998;338:1774-1775.
33. Maxwell GL, Myers ER, Clarke-Pearson DL. Cost-effectiveness of deep venous thrombosis prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 2000;95:206-214.
34. Doran FSA. Prevention of deep vein thrombosis. Br J Hosp Med. 1971;6:773-779.
- Prophylaxis must start before surgery for maximal benefit, since at least 50% of postoperative thromboembolic disease begins intraoperatively.
- A consensus conference found that prophylaxis reduced fatal pulmonary emboli by 75% in 7,000 gynecologic surgery patients.
- Low-dose unfractionated heparin and low molecular weight heparin appear similarly effective in reducing thromboembolic disease in perioperative patients, but it is unclear which form has fewer bleeding complications.
The case is strong for routine prophylaxis against venous thrombosis and pulmonary embolism. The primary reasons: efficacy, ease of use, and safety.
This article reviews the evidence on routine prophylaxis, pros and cons of mechanical and drug therapies (including a comparison of 2 heparins), patient risk factors, and cost-effectiveness. A table (page 32) lists patient characteristics for low, moderate, high, and very high levels of risk, with corresponding appropriate preventive measures.
Routine prophylaxis is a wiser strategy than postevent treatment or surveillance because:
- Thromboembolism is a stealthy adversary, particularly pulmonary embolism. Between 10% and 20% of patients with pulmonary emboli die, often within 30 minutes of the sentinel complaint.
- Even when pulmonary embolus is documented during autopsy, as many as 80% of patients have no antecedent clinical evidence of deep venous thrombosis (DVT)—and no chance of life-saving therapy.2
- Surveillance is the least desirable preventive option due to lack of sensitivity of noninvasive tests for thromboembolic disease.
Scope of thromboembolic disease
Thromboembolism is the leading potentially preventable cause of hospital fatality.1
In postoperative gynecologic surgery patients, pulmonary embolism occurs in 0.1% to 5% of cases, depending on risk.2 Venous thrombosis occurs on average in 15% of postoperative gynecologic surgery patients, with a range of 5% to 29%, depending on patient risk factors and the surgical procedures performed.3 Almost 50% of gynecologic patients undergoing surgery for cancer develop a lower-extremity venous thrombosis if left untreated.4
Overall, thromboembolic disease is linked with some 500,000 hospital admissions every year. Pulmonary embolism, the most serious complication, causes 60,000 to 200,000 deaths annually.
High risk of recurrence. Postthrombotic syndrome is characterized by pain, swelling, and leg ulceration. At least 50% of patients successfully treated for proximal venous thrombosis (thigh) and about 33% treated for distal venous thrombosis (calf) develop postthrombotic syndrome.1 The risk for recurrent thrombosis is high.
Risk factors are listed in TABLE 1. The risk of fatal pulmonary embolism is directly related to age; persons over 60 are at greatest risk. When the patient is obese—particularly when she exceeds 120% of ideal body weight—the risk is even greater due to venous stasis.
TABLE 1
Risk factors for thromboembolism
| Age |
| Estrogen use |
| Extended pelvic surgery |
| Hypercoagulable states |
| Immobility |
| Indwelling central venous catheter(s) |
| Lower-extremity paralysis |
| Malignancy |
| Medical illnesses |
| Chronic pulmonary disease |
| Congestive heart failure |
| Diabetes mellitus |
| Inflammatory bowel disease |
| Nephrotic syndrome |
| Obesity |
| Pregnancy |
| Prior thromboembolic disease |
| Radiation therapy |
| Trauma |
| Varicose veins |
Common hypercoagulable states
Activated protein C resistance, the most common thrombophilia, occurs in 3% to 7% of Caucasians (TABLE 2).7 It is usually associated with factor V Leiden mutation.
Prothrombin gene mutation G20210A, the next most common thrombophilia, occurs in 2% of Caucasians.7
Antiphospholipid antibody syndromes are acquired thrombophilias that may be associated with arterial and venous thrombosis, thrombocytopenia, and complications of pregnancy.8
Hyperhomocystinemia may be congenital or acquired. It is associated with venous thromboembolism and early atherosclerosis with arterial thrombosis.
Thrombosis is more likely with multiple predisposing factors. Most patients with one of the thrombophilia syndromes do not have sentinel thrombotic events unless they are further challenged by environmental risks such as oral contraceptives (OCs), pregnancy, prolonged immobilization, or surgery.9
Risk is markedly enhanced if multiple predisposing factors are present. For example, when factor V Leiden mutation and hyperhomocystinemia coexist, the risk for thrombosis increases 10 to 20 times over that of patients with neither abnormality.10 This combined risk far exceeds the sum of risks for each abnormality alone.
TABLE 2
Common hypercoagulable conditions
| Factor V Leiden mutation |
| Prothrombin gene mutation G20210A |
| Antiphospholipid antibody |
| Lupus anticoagulant |
| Anticardiolipin |
| Protein C deficiency |
| Protein S deficiency |
| Antithrombin III deficiency (Heparin cofactor II) |
| Plasminogen deficiency |
| Hyperhomocystinemia |
| Myeloproliferative disorders |
| Polycythemia |
| Primary thrombocytosis |
When to test for thrombophilias
Although women with thrombophilias are at high risk for thromboembolic disease, it is unclear whether identifying the thrombophilia is more beneficial than universal prophylaxis. If a patient has a personal or family history of thromboembolic disease—especially a patient with Caucasian ancestry—testing is probably warranted. While documentation of the thrombophilia may not help with perioperative management, it may be useful for long-term care.
Testing for factor V Leiden mutation is recommended due to its prevalence. If the results are negative in a patient at risk, test for prothrombin gene mutation G20210A, as well as deficiencies in the naturally occurring inhibitors protein C, protein S, and antithrombin III.
Assess antiphospholipid antibodies in women who have experienced recurrent fetal loss or early pregnancy-induced hypertension.
Continue OCs or hormone therapy
OCs and menopausal hormone replacement therapy produce measurable prothrombotic changes in the clotting system that appear to be directly related to the estrogen content. In theory, discontinuing the OC or hormone replacement therapy preoperatively would allow these changes to return to baseline and help prevent thromboembolic disease.
Although the risk of thromboembolic disease is 0.96% if patients are current users of OCs and 0.5% if they are not, studies have failed to confirm a clinical benefit of discontinuation.11 Further, the patient does not return to baseline for 4 to 6 weeks after ceasing therapy.
The potential risk of thromboembolic events also should be weighed against the risk of conception prior to surgery. We usually do not recommend discontinuation of OCs and hormone therapy before surgery, but give prophylaxis based on risk assessment.
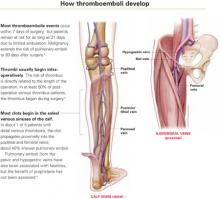
Venous stasis, vessel-wall trauma, and increased blood coagulability—the major contributors to perioperative DVT, known as Virchow’s triad—were identified more than 125 years ago.
Venous stasis. Intraoperatively, venous blood return from the lower extremities is reduced to less than half its normal rate,34 secondary to muscle relaxation during anesthesia, which causes venous dilation and reduced blood-flow velocity. Packing the abdominal contents may further impede blood return from the legs.
Resultant venous stasis causes platelet adhesion to the vein wall, followed by release of a thromboplastin-like substance that may trigger thrombus formation.
Blood flow increases in the immediate postoperative period with return of muscle tone, but remains significantly diminished for 21 days because of immobilization—specifically, lack of the usual pumping action of the leg muscles.
Vessel-wall trauma. Veins are highly likely to be damaged as vessels are skeletonized during major pelvic surgery, especially when malignancy is involved. Tissue injury activates the coagulation cascade by exposing blood to tissue thromboplastin (extrinsic path) and subendothelial collagen in the vessel wall, which activates factor XII (intrinsic path). Both pathways lead to conversion of factor X to its active form, factor Xa. Acting in concert with factor V, calcium, and phospholipids from platelet factor III, factor Xa catalyzes the conversion of prothrombin to thrombin. Thrombin regulates the conversion of fibrinogen to fibrin, the basic building block of a thrombus.
Increased blood coagulability. Clotting factors XI, IX, and VII increase following surgery, as do circulating platelets and platelet aggregation. This enhances coagulability, which persists from 72 to 96 hours after surgery but is usually balanced by the fibrinolytic system. Fibrinolysis is mediated primarily by plasmin, which digests fibrin and fibrinogen and activates factors V and VIII. If the fibrinolytic system is overwhelmed, the clotting system is unimpeded and thrombus formation may accelerate.
Pregnancy increases the risk of thrombosis, in part due to the progressive increase in resistance to activated protein C in the second and third trimesters. Risk is increased eightfold in women with inherited deficiency in any of the naturally occurring anticoagulants—antithrombin III, protein C, or protein S—compared with those with no deficiency.12
Preventive strategies
Two approaches to thromboembolic prophylaxis have been proposed, with prevention of fatal postoperative pulmonary emboli as a clear endpoint:
- Stratify a targeted group into levels of risk; then treat those at higher risk. Unfortunately, efforts to define risk have met with only partial success, due to limited availability of noninvasive screening, screening logistics, and expense. Further, specificity and positive predictive value of screening asymptomatic patients is low.
- Use prophylaxis in all patients in the targeted group, regardless of risk (TABLES 3 AND 4). This strategy seems effective. For example, in 2001, the Sixth American College of Chest Physicians Consensus Conference—the most recent of the consensus conferences—evaluated the risks of pulmonary embolus in 7,000 gynecologic surgery patients enrolled in prospective studies. Routine prophylaxis reduced fatal pulmonary emboli by 75%.13
The difficulty of defining patients at highest risk makes the concept of universal prophylaxis for a targeted group an attractive option unless a specific contraindication is identified.
TABLE 3
Risk stratification and prophylactic regimens
| LEVEL OF RISK | PATIENT CHARACTERISTICS | RECOMMENDED REGIMEN |
|---|---|---|
| Low | Less than 40 years of age | No specific recommendation for therapy |
| Undergoing uncomplicated minor surgery | Adequate hydration | |
| Requires less than 30 minutes of anesthesia | Aggressive early ambulation | |
| No additional risk factors | ||
| Moderate | Undergoing minor surgery with additional risk factors | Graduated compression stockings or SPCDs or |
| 40 to 60 years of age, undergoing minor surgery, and no additional risk factors | LDUH 5,000 U every 12 hours or | |
| Less than 40 years of age, undergoing major surgery, and no additional risk factors | LMWH (20 mg) or 2,500 U antifactor Xa once daily | |
| High | More than 60 years of age, undergoing minor surgery or with additional risk factors | LDUH 5,000 U every 8–12 hours or LMWH (40 mg) or 5,000 U antifactor Xa once daily |
| More than 40 years of age undergoing major surgery or with additional risk factors | SPCDs may be added | |
| Very high | More than 60 years of age, undergoing major surgery, with other risk factors such as cancer, prior venous thromboembolism, molecular hypercoagulable state, major trauma, spinal cord injury, or lower-extremity paralysis | Graduated compression stockings or SPCDs and LDUH 5,000 U every 8 hours or |
| Other risk factors such as cancer, prior venous thromboembolism, molecular hypercoagulable state, major trauma, spinal cord injury, or lower-extremity paralysis | LMWH (40 mg) or 5,000 U antifactor Xa once daily | |
| LDUH = low-dose unfractionated heparin; LMWH = low molecular weight heparin; SPCD = sequential pneumatic compression device | ||
| Note: Because perioperative prophylaxis is still evolving, these suggestions should not be considered inviolable. | ||
| Source: Modified from Geerts WH, et al.13 | ||
TABLE 4
Incidence of thromboembolic events based on level of risk
| SITE OF THROMBOSIS | RISK LEVEL (%) | |||
|---|---|---|---|---|
| Low | Moderate | High | Very high | |
| Calf veins (distal) | 2 | 10–35 | 30–60 | 63.5 |
| Iliofemoral veins (proximal) | 0.4 | 2–8 | 5–10 | 10–20 |
| Pulmonary emboli | 0.2 | 1–2 | 2–4 | 4–10 |
| Fatal pulmonary emboli | 0.002 | 0.1–0.5 | 0.4–1 | 0.2–5 |
| Source: Modified from Geerts WH, et al.13 | ||||
Drug and mechanical therapies
Strategies for preventing thromboembolic disease attempt to mitigate the impact of venous stasis, endothelial injury, and hypercoagulable states. Traditionally, early ambulation, adequate hydration, and elevation of the lower extremities have been used, because they are simple and inexpensive interventions.
More recently, pharmacologic and mechanical therapies have proven effective in reducing the incidence of DVT and fatal pulmonary emboli. Prophylactic measures commonly used today are:
- graduated compression stockings
- external pneumatic leg or foot compression devices
- low-dose unfractionated heparin
- low molecular weight heparin
MECHANICAL THERAPIESGraduated compression stockings
Compression stockings are one of the earliest methods of preventing perioperative thrombosis. Compression is greatest at the toe and gradually diminishes toward the thigh. When Belcaro14 studied the risk of recurrent venous thrombosis in nonsurgical hospitalized patients, thrombosis recurred in 40% of patients with no therapy, but only 9.4% of patients wearing graduated compression stockings (GCS). Adding oral antiplatelet therapy lowered the risk to 2%. However, GCS were not superior to any other method of preventing recurrent DVT.
As for surgical patients, a single study15 demonstrated protection against DVT when compared with no GCS in elective gynecologic surgery patients.
Today, GCS are usually a perioperative adjunct to other preventive methods, to provide added protection.
Proper fitting by trained personnel is vital; otherwise, a tourniquet effect may cause venous stasis and reduce benefit.
Sequential pneumatic compression devices
Like GCS, these devices decrease the caliber of veins by simple compression. They also increase blood flow velocity and stimulate the endogenous fibrinolytic system.
Enhanced fibrinolytic activity due to intermittent compression occurs even if the device is used on only 1 lower extremity, or on an upper extremity. Patient and nursing-staff compliance may affect efficacy. Sequential pneumatic compression devices (SPCDs) may be thought inconvenient, impeding nursing functions. Some patients may find the repetitive inflation-deflation cycles annoying.
These devices must be activated prior to surgery and continued for a minimum of 24 hours. Some studies suggest that SPCDs be used for 5 days in high-risk situations.16
Calf- and thigh-length devices have similar effects. Rare complications include peroneal nerve injury and compartment syndrome.
What the data show. In an analysis of 4 trials comparing SPCDs with no therapy, thromboembolic disease occurred in only 2% of patients who wore SPCDs but in 20% of those who did not.17 Compared with low-dose unfractionated heparin, no difference in the rate of DVT was seen.18 However, risk of transfusion and retroperitoneal drainage volume increased in the heparin group.
Contraindications include active or suspected DVT, congestive heart failure, known pulmonary embolus, and leg injuries.19
Combined therapy may be best for high-risk women. A study20 of women who developed thromboemboli despite appropriate treatment with SPCDs found the women more likely to be older than 60 years and/or to have cancer or history of thromboembolic disease or hypertension. This high-risk group may benefit from combined therapy.
Foot compression devices resemble booties and mimic the plantar compression that occurs during walking. They increase blood-flow velocity, stimulate the endogenous fibrinolytic system, and have the same indications as SPCDs. However, they are only moderately effective in reducing venous thrombosis.21 Potential drawbacks are that the devices must be removed when the patient ambulates and replaced when she returns to bed.
DRUG THERAPIESLow-dose unfractionated heparin
Heparin alters the molecular configuration of antithrombin III, making it 1,000 to 4,000 times more potent as an inhibitor of thrombin formation, which in turn limits conversion of fibrinogen to fibrin. This prolongs the activated partial thromboplastin time (aPTT) commonly used to monitor patients receiving full anticoagulation therapy.
A naturally occurring mucopolysaccharide with a molecular weight ranging from 3,000 to 30,000 daltons, heparin is richly concentrated in mast cells. Its anticoagulant properties relate primarily to interaction with antithrombin III (also known as heparin cofactor II), factor IXa, factor Xa, factor XIa, factor XIIa, and platelet aggregation.22
Heparin also inhibits the effects of factor Xa on the coagulation cascade and limits platelet aggregation.23
Half-life is 1 hour for intravenous heparin and about 3 hours for subcutaneous heparin.
Use in pregnancy. Low-dose unfractionated heparin (LDUH) does not cross the placenta and is safe to use during pregnancy.
What the data show. Randomized clinical trials conducted prior to 1988 showed venous thrombosis decreased by 70% and pulmonary embolus by 50% in patients treated with LDUH, compared with those receiving no therapy.24
Dosing options. LDUH typically is given as a 5,000-U dose 2 hours before surgery. The single preoperative dose seems to be as effective as multiple preoperative doses.25 Postoperative therapy is instituted 8 to 12 hours after surgery; heparin is given every 8 to 12 hours until the patient is fully ambulatory.
Patients having gynecologic surgery for benign conditions benefit from the twice-daily regimen, and those undergoing gynecologic oncology surgery or other high-risk procedures seem to benefit from thrice-daily dosing.26
These regimens also significantly reduced DVT and fatal pulmonary emboli in general surgery patients.27 The LDUH regimen is now used to judge the efficacy of other prophylactic measures.
Low molecular weight heparin
This form of heparin acts primarily by inhibiting factor Xa, which is higher in the coagulation cascade than antithrombin. Thus, low molecular weight heparin (LMWH) is more efficient than unfractionated heparin.
LMWH has a molecular weight of 3,000 to 6,000 daltons and is produced by concentrating the low molecular component of heparin. Because the molecular configuration of antithrombin III is not altered by LMWH, thrombin conversion is minimally inhibited and aPTT is not appreciably affected.
Half-life is approximately 4 hours, by any route of administration. The longer half-life provides a longer dosing interval. Bioavailability is more consistent than that of LDUH, approaching 90% to 95%. The excellent bioavailability allows dosing to be based on lean body mass. Less heparin-induced thrombocytopenia is another plus.
Use in pregnancy. LMWH does not cross the placenta and is safe to use during pregnancy.
What the data show. No randomized trials have compared LMWH therapy with no therapy, with efficacy assessed by venography or fibrinogen uptake. An uncontrolled series28 of 2,030 patients did show that LMWH reduced the incidence of thromboembolic disease.
A randomized controlled trial29 comparing 2,500 U and 5,000 U daily of dalteparin (an LMWH) found greater efficacy in the higher-dose group (6.6% versus 12.7% incidence of DVT), but bleeding complications also were higher (4.7% versus 2.7%). In a subgroup of patients with malignancy, the high-dose therapy remained superior in preventing DVT, and the bleeding risk was equal.
Dosing options. Once a day dosing is normally adequate for prophylaxis; twice-daily dosing is needed for therapy. Enoxaparin is an LMWH that is readily available in the United States and commonly prescribed when use of LMWH is desired. For prophylaxis, it can be given in daily doses of 20 mg, 30 mg, 40 mg, or 60 mg. None of these doses has proven superior to the others. The typical regimen for moderate risk is 20 mg per day; for high risk, 40 mg per day. Enoxaparin appears to convey the same degree of protection as 5,000 units of LDUH every 8 hours.
2 heparins compared
Randomized controlled trials comparing LDUH and LMWH have found them to be similarly effective. In addition, within the LMWH class, all compounds appear to have similar benefits. However, it remains unclear which form of heparin is associated with fewer bleeding complications.
In theory, LMWH would be associated with an increase in these complications due to its longer half-life and increased bioavailability.30 However, studies have not consistently identified excess bleeding in any group.31 Fortunately, excess postoperative bleeding requiring transfusion is uncommon.
Complications of heparin
Heparin-induced thrombocytopenia is a recognized complication, seen in as many as 20% of LDUH patients. The diagnosis is made when the platelet count falls below the lower limits of normal or when the platelet count falls by 50% but remains in the normal range. Two forms of heparin-induced thrombocytopenia have been described.
• Type 1 thrombocytopenia is initially mild, rarely dropping below 100,000 platelets per milliliter. Platelet count monitoring is important, but therapy usually can continue, since the platelet count returns to normal even with continued use. Type 1 thrombocytopenia appears to directly result from platelet activation and is not immune-mediated.
• Type 2 thrombocytopenia occurs 7 to 14 days after starting therapy. Platelet counts frequently drop to 20,000 per milliliter. Type 2 is an immune response caused by antibodies to the heparin-platelet factor 4 complex. Patients are at risk for venous and arterial thrombosis, but often the diagnosis is made only after a complicating thrombotic event.
The risk of type 2 thrombocytopenia appears to be related to the molecular weight of the compound being administered, its dose, and the duration of therapy. Therefore, unfractionated heparin—if given in full anticoagulation doses and beyond 14 days—seems to have the greatest potential for this complication.
Paradoxically, these patients also are at risk for severe hemorrhage. Because mortality and morbidity are high, immediate withdrawal of all forms of heparin—including LMWH—is mandatory.32 Catastrophic hemorrhage or thrombosis may be the first sign. Often a fall in platelet concentration precedes serious complications. Thus, platelet concentration should be monitored at least every 2 days.
Hematoma with conduction anesthesia. Use of major conduction anesthesia in patients who also need LMWH, LDUH, or oral anticoagulants is controversial. LMWH may pose a risk of hematoma if initiated preoperatively, intraoperatively, or within 3 hours of surgery in patients who have a continuous epidural. Hematoma formation seems to occur immediately following catheter withdrawal.
The risk of hematoma appears to be lower with single-dose spinal or single-dose epidural anesthesia. While the incidence of epidural or spinal hematoma is not known, some cases have been associated with long-term neurologic sequelae, including permanent paralysis.
Responding to this concern, the US Food and Drug Administration issued a 1997 advisory noting the risk of spinal hematoma in patients receiving enoxaparin plus conduction anesthesia or lumbar spinal puncture. Most anesthesiologists significantly restrict the use of conduction anesthesia in patients requiring heparin prophylaxis.33
Cost-effectiveness
The cost of perioperative prophylaxis has been compared with the cost of immediate therapy for thromboembolic disease and long-term therapy for postthrombotic syndrome. Across the board, universal prophylaxis with pharmacotherapy or mechanical devices in patients undergoing abdominal surgery is less expensive than no prophylaxis, based on a reduced incidence of DVT. Pneumatic compression seems to be more cost-effective than pharmacotherapy.34
Because LDUH is substantially less expensive than LMWH in the United States, it has a better cost profile when pharmacotherapy is warranted. In Europe, where the cost of heparin compounds is not an issue, LMWH has a slight advantage.
The authors report no financial relationships relevant to this article.
- Prophylaxis must start before surgery for maximal benefit, since at least 50% of postoperative thromboembolic disease begins intraoperatively.
- A consensus conference found that prophylaxis reduced fatal pulmonary emboli by 75% in 7,000 gynecologic surgery patients.
- Low-dose unfractionated heparin and low molecular weight heparin appear similarly effective in reducing thromboembolic disease in perioperative patients, but it is unclear which form has fewer bleeding complications.
The case is strong for routine prophylaxis against venous thrombosis and pulmonary embolism. The primary reasons: efficacy, ease of use, and safety.
This article reviews the evidence on routine prophylaxis, pros and cons of mechanical and drug therapies (including a comparison of 2 heparins), patient risk factors, and cost-effectiveness. A table (page 32) lists patient characteristics for low, moderate, high, and very high levels of risk, with corresponding appropriate preventive measures.
Routine prophylaxis is a wiser strategy than postevent treatment or surveillance because:
- Thromboembolism is a stealthy adversary, particularly pulmonary embolism. Between 10% and 20% of patients with pulmonary emboli die, often within 30 minutes of the sentinel complaint.
- Even when pulmonary embolus is documented during autopsy, as many as 80% of patients have no antecedent clinical evidence of deep venous thrombosis (DVT)—and no chance of life-saving therapy.2
- Surveillance is the least desirable preventive option due to lack of sensitivity of noninvasive tests for thromboembolic disease.
Scope of thromboembolic disease
Thromboembolism is the leading potentially preventable cause of hospital fatality.1
In postoperative gynecologic surgery patients, pulmonary embolism occurs in 0.1% to 5% of cases, depending on risk.2 Venous thrombosis occurs on average in 15% of postoperative gynecologic surgery patients, with a range of 5% to 29%, depending on patient risk factors and the surgical procedures performed.3 Almost 50% of gynecologic patients undergoing surgery for cancer develop a lower-extremity venous thrombosis if left untreated.4
Overall, thromboembolic disease is linked with some 500,000 hospital admissions every year. Pulmonary embolism, the most serious complication, causes 60,000 to 200,000 deaths annually.
High risk of recurrence. Postthrombotic syndrome is characterized by pain, swelling, and leg ulceration. At least 50% of patients successfully treated for proximal venous thrombosis (thigh) and about 33% treated for distal venous thrombosis (calf) develop postthrombotic syndrome.1 The risk for recurrent thrombosis is high.
Risk factors are listed in TABLE 1. The risk of fatal pulmonary embolism is directly related to age; persons over 60 are at greatest risk. When the patient is obese—particularly when she exceeds 120% of ideal body weight—the risk is even greater due to venous stasis.
TABLE 1
Risk factors for thromboembolism
| Age |
| Estrogen use |
| Extended pelvic surgery |
| Hypercoagulable states |
| Immobility |
| Indwelling central venous catheter(s) |
| Lower-extremity paralysis |
| Malignancy |
| Medical illnesses |
| Chronic pulmonary disease |
| Congestive heart failure |
| Diabetes mellitus |
| Inflammatory bowel disease |
| Nephrotic syndrome |
| Obesity |
| Pregnancy |
| Prior thromboembolic disease |
| Radiation therapy |
| Trauma |
| Varicose veins |
Common hypercoagulable states
Activated protein C resistance, the most common thrombophilia, occurs in 3% to 7% of Caucasians (TABLE 2).7 It is usually associated with factor V Leiden mutation.
Prothrombin gene mutation G20210A, the next most common thrombophilia, occurs in 2% of Caucasians.7
Antiphospholipid antibody syndromes are acquired thrombophilias that may be associated with arterial and venous thrombosis, thrombocytopenia, and complications of pregnancy.8
Hyperhomocystinemia may be congenital or acquired. It is associated with venous thromboembolism and early atherosclerosis with arterial thrombosis.
Thrombosis is more likely with multiple predisposing factors. Most patients with one of the thrombophilia syndromes do not have sentinel thrombotic events unless they are further challenged by environmental risks such as oral contraceptives (OCs), pregnancy, prolonged immobilization, or surgery.9
Risk is markedly enhanced if multiple predisposing factors are present. For example, when factor V Leiden mutation and hyperhomocystinemia coexist, the risk for thrombosis increases 10 to 20 times over that of patients with neither abnormality.10 This combined risk far exceeds the sum of risks for each abnormality alone.
TABLE 2
Common hypercoagulable conditions
| Factor V Leiden mutation |
| Prothrombin gene mutation G20210A |
| Antiphospholipid antibody |
| Lupus anticoagulant |
| Anticardiolipin |
| Protein C deficiency |
| Protein S deficiency |
| Antithrombin III deficiency (Heparin cofactor II) |
| Plasminogen deficiency |
| Hyperhomocystinemia |
| Myeloproliferative disorders |
| Polycythemia |
| Primary thrombocytosis |
When to test for thrombophilias
Although women with thrombophilias are at high risk for thromboembolic disease, it is unclear whether identifying the thrombophilia is more beneficial than universal prophylaxis. If a patient has a personal or family history of thromboembolic disease—especially a patient with Caucasian ancestry—testing is probably warranted. While documentation of the thrombophilia may not help with perioperative management, it may be useful for long-term care.
Testing for factor V Leiden mutation is recommended due to its prevalence. If the results are negative in a patient at risk, test for prothrombin gene mutation G20210A, as well as deficiencies in the naturally occurring inhibitors protein C, protein S, and antithrombin III.
Assess antiphospholipid antibodies in women who have experienced recurrent fetal loss or early pregnancy-induced hypertension.
Continue OCs or hormone therapy
OCs and menopausal hormone replacement therapy produce measurable prothrombotic changes in the clotting system that appear to be directly related to the estrogen content. In theory, discontinuing the OC or hormone replacement therapy preoperatively would allow these changes to return to baseline and help prevent thromboembolic disease.
Although the risk of thromboembolic disease is 0.96% if patients are current users of OCs and 0.5% if they are not, studies have failed to confirm a clinical benefit of discontinuation.11 Further, the patient does not return to baseline for 4 to 6 weeks after ceasing therapy.
The potential risk of thromboembolic events also should be weighed against the risk of conception prior to surgery. We usually do not recommend discontinuation of OCs and hormone therapy before surgery, but give prophylaxis based on risk assessment.
Venous stasis, vessel-wall trauma, and increased blood coagulability—the major contributors to perioperative DVT, known as Virchow’s triad—were identified more than 125 years ago.
Venous stasis. Intraoperatively, venous blood return from the lower extremities is reduced to less than half its normal rate,34 secondary to muscle relaxation during anesthesia, which causes venous dilation and reduced blood-flow velocity. Packing the abdominal contents may further impede blood return from the legs.
Resultant venous stasis causes platelet adhesion to the vein wall, followed by release of a thromboplastin-like substance that may trigger thrombus formation.
Blood flow increases in the immediate postoperative period with return of muscle tone, but remains significantly diminished for 21 days because of immobilization—specifically, lack of the usual pumping action of the leg muscles.
Vessel-wall trauma. Veins are highly likely to be damaged as vessels are skeletonized during major pelvic surgery, especially when malignancy is involved. Tissue injury activates the coagulation cascade by exposing blood to tissue thromboplastin (extrinsic path) and subendothelial collagen in the vessel wall, which activates factor XII (intrinsic path). Both pathways lead to conversion of factor X to its active form, factor Xa. Acting in concert with factor V, calcium, and phospholipids from platelet factor III, factor Xa catalyzes the conversion of prothrombin to thrombin. Thrombin regulates the conversion of fibrinogen to fibrin, the basic building block of a thrombus.
Increased blood coagulability. Clotting factors XI, IX, and VII increase following surgery, as do circulating platelets and platelet aggregation. This enhances coagulability, which persists from 72 to 96 hours after surgery but is usually balanced by the fibrinolytic system. Fibrinolysis is mediated primarily by plasmin, which digests fibrin and fibrinogen and activates factors V and VIII. If the fibrinolytic system is overwhelmed, the clotting system is unimpeded and thrombus formation may accelerate.
Pregnancy increases the risk of thrombosis, in part due to the progressive increase in resistance to activated protein C in the second and third trimesters. Risk is increased eightfold in women with inherited deficiency in any of the naturally occurring anticoagulants—antithrombin III, protein C, or protein S—compared with those with no deficiency.12
Preventive strategies
Two approaches to thromboembolic prophylaxis have been proposed, with prevention of fatal postoperative pulmonary emboli as a clear endpoint:
- Stratify a targeted group into levels of risk; then treat those at higher risk. Unfortunately, efforts to define risk have met with only partial success, due to limited availability of noninvasive screening, screening logistics, and expense. Further, specificity and positive predictive value of screening asymptomatic patients is low.
- Use prophylaxis in all patients in the targeted group, regardless of risk (TABLES 3 AND 4). This strategy seems effective. For example, in 2001, the Sixth American College of Chest Physicians Consensus Conference—the most recent of the consensus conferences—evaluated the risks of pulmonary embolus in 7,000 gynecologic surgery patients enrolled in prospective studies. Routine prophylaxis reduced fatal pulmonary emboli by 75%.13
The difficulty of defining patients at highest risk makes the concept of universal prophylaxis for a targeted group an attractive option unless a specific contraindication is identified.
TABLE 3
Risk stratification and prophylactic regimens
| LEVEL OF RISK | PATIENT CHARACTERISTICS | RECOMMENDED REGIMEN |
|---|---|---|
| Low | Less than 40 years of age | No specific recommendation for therapy |
| Undergoing uncomplicated minor surgery | Adequate hydration | |
| Requires less than 30 minutes of anesthesia | Aggressive early ambulation | |
| No additional risk factors | ||
| Moderate | Undergoing minor surgery with additional risk factors | Graduated compression stockings or SPCDs or |
| 40 to 60 years of age, undergoing minor surgery, and no additional risk factors | LDUH 5,000 U every 12 hours or | |
| Less than 40 years of age, undergoing major surgery, and no additional risk factors | LMWH (20 mg) or 2,500 U antifactor Xa once daily | |
| High | More than 60 years of age, undergoing minor surgery or with additional risk factors | LDUH 5,000 U every 8–12 hours or LMWH (40 mg) or 5,000 U antifactor Xa once daily |
| More than 40 years of age undergoing major surgery or with additional risk factors | SPCDs may be added | |
| Very high | More than 60 years of age, undergoing major surgery, with other risk factors such as cancer, prior venous thromboembolism, molecular hypercoagulable state, major trauma, spinal cord injury, or lower-extremity paralysis | Graduated compression stockings or SPCDs and LDUH 5,000 U every 8 hours or |
| Other risk factors such as cancer, prior venous thromboembolism, molecular hypercoagulable state, major trauma, spinal cord injury, or lower-extremity paralysis | LMWH (40 mg) or 5,000 U antifactor Xa once daily | |
| LDUH = low-dose unfractionated heparin; LMWH = low molecular weight heparin; SPCD = sequential pneumatic compression device | ||
| Note: Because perioperative prophylaxis is still evolving, these suggestions should not be considered inviolable. | ||
| Source: Modified from Geerts WH, et al.13 | ||
TABLE 4
Incidence of thromboembolic events based on level of risk
| SITE OF THROMBOSIS | RISK LEVEL (%) | |||
|---|---|---|---|---|
| Low | Moderate | High | Very high | |
| Calf veins (distal) | 2 | 10–35 | 30–60 | 63.5 |
| Iliofemoral veins (proximal) | 0.4 | 2–8 | 5–10 | 10–20 |
| Pulmonary emboli | 0.2 | 1–2 | 2–4 | 4–10 |
| Fatal pulmonary emboli | 0.002 | 0.1–0.5 | 0.4–1 | 0.2–5 |
| Source: Modified from Geerts WH, et al.13 | ||||
Drug and mechanical therapies
Strategies for preventing thromboembolic disease attempt to mitigate the impact of venous stasis, endothelial injury, and hypercoagulable states. Traditionally, early ambulation, adequate hydration, and elevation of the lower extremities have been used, because they are simple and inexpensive interventions.
More recently, pharmacologic and mechanical therapies have proven effective in reducing the incidence of DVT and fatal pulmonary emboli. Prophylactic measures commonly used today are:
- graduated compression stockings
- external pneumatic leg or foot compression devices
- low-dose unfractionated heparin
- low molecular weight heparin
MECHANICAL THERAPIESGraduated compression stockings
Compression stockings are one of the earliest methods of preventing perioperative thrombosis. Compression is greatest at the toe and gradually diminishes toward the thigh. When Belcaro14 studied the risk of recurrent venous thrombosis in nonsurgical hospitalized patients, thrombosis recurred in 40% of patients with no therapy, but only 9.4% of patients wearing graduated compression stockings (GCS). Adding oral antiplatelet therapy lowered the risk to 2%. However, GCS were not superior to any other method of preventing recurrent DVT.
As for surgical patients, a single study15 demonstrated protection against DVT when compared with no GCS in elective gynecologic surgery patients.
Today, GCS are usually a perioperative adjunct to other preventive methods, to provide added protection.
Proper fitting by trained personnel is vital; otherwise, a tourniquet effect may cause venous stasis and reduce benefit.
Sequential pneumatic compression devices
Like GCS, these devices decrease the caliber of veins by simple compression. They also increase blood flow velocity and stimulate the endogenous fibrinolytic system.
Enhanced fibrinolytic activity due to intermittent compression occurs even if the device is used on only 1 lower extremity, or on an upper extremity. Patient and nursing-staff compliance may affect efficacy. Sequential pneumatic compression devices (SPCDs) may be thought inconvenient, impeding nursing functions. Some patients may find the repetitive inflation-deflation cycles annoying.
These devices must be activated prior to surgery and continued for a minimum of 24 hours. Some studies suggest that SPCDs be used for 5 days in high-risk situations.16
Calf- and thigh-length devices have similar effects. Rare complications include peroneal nerve injury and compartment syndrome.
What the data show. In an analysis of 4 trials comparing SPCDs with no therapy, thromboembolic disease occurred in only 2% of patients who wore SPCDs but in 20% of those who did not.17 Compared with low-dose unfractionated heparin, no difference in the rate of DVT was seen.18 However, risk of transfusion and retroperitoneal drainage volume increased in the heparin group.
Contraindications include active or suspected DVT, congestive heart failure, known pulmonary embolus, and leg injuries.19
Combined therapy may be best for high-risk women. A study20 of women who developed thromboemboli despite appropriate treatment with SPCDs found the women more likely to be older than 60 years and/or to have cancer or history of thromboembolic disease or hypertension. This high-risk group may benefit from combined therapy.
Foot compression devices resemble booties and mimic the plantar compression that occurs during walking. They increase blood-flow velocity, stimulate the endogenous fibrinolytic system, and have the same indications as SPCDs. However, they are only moderately effective in reducing venous thrombosis.21 Potential drawbacks are that the devices must be removed when the patient ambulates and replaced when she returns to bed.
DRUG THERAPIESLow-dose unfractionated heparin
Heparin alters the molecular configuration of antithrombin III, making it 1,000 to 4,000 times more potent as an inhibitor of thrombin formation, which in turn limits conversion of fibrinogen to fibrin. This prolongs the activated partial thromboplastin time (aPTT) commonly used to monitor patients receiving full anticoagulation therapy.
A naturally occurring mucopolysaccharide with a molecular weight ranging from 3,000 to 30,000 daltons, heparin is richly concentrated in mast cells. Its anticoagulant properties relate primarily to interaction with antithrombin III (also known as heparin cofactor II), factor IXa, factor Xa, factor XIa, factor XIIa, and platelet aggregation.22
Heparin also inhibits the effects of factor Xa on the coagulation cascade and limits platelet aggregation.23
Half-life is 1 hour for intravenous heparin and about 3 hours for subcutaneous heparin.
Use in pregnancy. Low-dose unfractionated heparin (LDUH) does not cross the placenta and is safe to use during pregnancy.
What the data show. Randomized clinical trials conducted prior to 1988 showed venous thrombosis decreased by 70% and pulmonary embolus by 50% in patients treated with LDUH, compared with those receiving no therapy.24
Dosing options. LDUH typically is given as a 5,000-U dose 2 hours before surgery. The single preoperative dose seems to be as effective as multiple preoperative doses.25 Postoperative therapy is instituted 8 to 12 hours after surgery; heparin is given every 8 to 12 hours until the patient is fully ambulatory.
Patients having gynecologic surgery for benign conditions benefit from the twice-daily regimen, and those undergoing gynecologic oncology surgery or other high-risk procedures seem to benefit from thrice-daily dosing.26
These regimens also significantly reduced DVT and fatal pulmonary emboli in general surgery patients.27 The LDUH regimen is now used to judge the efficacy of other prophylactic measures.
Low molecular weight heparin
This form of heparin acts primarily by inhibiting factor Xa, which is higher in the coagulation cascade than antithrombin. Thus, low molecular weight heparin (LMWH) is more efficient than unfractionated heparin.
LMWH has a molecular weight of 3,000 to 6,000 daltons and is produced by concentrating the low molecular component of heparin. Because the molecular configuration of antithrombin III is not altered by LMWH, thrombin conversion is minimally inhibited and aPTT is not appreciably affected.
Half-life is approximately 4 hours, by any route of administration. The longer half-life provides a longer dosing interval. Bioavailability is more consistent than that of LDUH, approaching 90% to 95%. The excellent bioavailability allows dosing to be based on lean body mass. Less heparin-induced thrombocytopenia is another plus.
Use in pregnancy. LMWH does not cross the placenta and is safe to use during pregnancy.
What the data show. No randomized trials have compared LMWH therapy with no therapy, with efficacy assessed by venography or fibrinogen uptake. An uncontrolled series28 of 2,030 patients did show that LMWH reduced the incidence of thromboembolic disease.
A randomized controlled trial29 comparing 2,500 U and 5,000 U daily of dalteparin (an LMWH) found greater efficacy in the higher-dose group (6.6% versus 12.7% incidence of DVT), but bleeding complications also were higher (4.7% versus 2.7%). In a subgroup of patients with malignancy, the high-dose therapy remained superior in preventing DVT, and the bleeding risk was equal.
Dosing options. Once a day dosing is normally adequate for prophylaxis; twice-daily dosing is needed for therapy. Enoxaparin is an LMWH that is readily available in the United States and commonly prescribed when use of LMWH is desired. For prophylaxis, it can be given in daily doses of 20 mg, 30 mg, 40 mg, or 60 mg. None of these doses has proven superior to the others. The typical regimen for moderate risk is 20 mg per day; for high risk, 40 mg per day. Enoxaparin appears to convey the same degree of protection as 5,000 units of LDUH every 8 hours.
2 heparins compared
Randomized controlled trials comparing LDUH and LMWH have found them to be similarly effective. In addition, within the LMWH class, all compounds appear to have similar benefits. However, it remains unclear which form of heparin is associated with fewer bleeding complications.
In theory, LMWH would be associated with an increase in these complications due to its longer half-life and increased bioavailability.30 However, studies have not consistently identified excess bleeding in any group.31 Fortunately, excess postoperative bleeding requiring transfusion is uncommon.
Complications of heparin
Heparin-induced thrombocytopenia is a recognized complication, seen in as many as 20% of LDUH patients. The diagnosis is made when the platelet count falls below the lower limits of normal or when the platelet count falls by 50% but remains in the normal range. Two forms of heparin-induced thrombocytopenia have been described.
• Type 1 thrombocytopenia is initially mild, rarely dropping below 100,000 platelets per milliliter. Platelet count monitoring is important, but therapy usually can continue, since the platelet count returns to normal even with continued use. Type 1 thrombocytopenia appears to directly result from platelet activation and is not immune-mediated.
• Type 2 thrombocytopenia occurs 7 to 14 days after starting therapy. Platelet counts frequently drop to 20,000 per milliliter. Type 2 is an immune response caused by antibodies to the heparin-platelet factor 4 complex. Patients are at risk for venous and arterial thrombosis, but often the diagnosis is made only after a complicating thrombotic event.
The risk of type 2 thrombocytopenia appears to be related to the molecular weight of the compound being administered, its dose, and the duration of therapy. Therefore, unfractionated heparin—if given in full anticoagulation doses and beyond 14 days—seems to have the greatest potential for this complication.
Paradoxically, these patients also are at risk for severe hemorrhage. Because mortality and morbidity are high, immediate withdrawal of all forms of heparin—including LMWH—is mandatory.32 Catastrophic hemorrhage or thrombosis may be the first sign. Often a fall in platelet concentration precedes serious complications. Thus, platelet concentration should be monitored at least every 2 days.
Hematoma with conduction anesthesia. Use of major conduction anesthesia in patients who also need LMWH, LDUH, or oral anticoagulants is controversial. LMWH may pose a risk of hematoma if initiated preoperatively, intraoperatively, or within 3 hours of surgery in patients who have a continuous epidural. Hematoma formation seems to occur immediately following catheter withdrawal.
The risk of hematoma appears to be lower with single-dose spinal or single-dose epidural anesthesia. While the incidence of epidural or spinal hematoma is not known, some cases have been associated with long-term neurologic sequelae, including permanent paralysis.
Responding to this concern, the US Food and Drug Administration issued a 1997 advisory noting the risk of spinal hematoma in patients receiving enoxaparin plus conduction anesthesia or lumbar spinal puncture. Most anesthesiologists significantly restrict the use of conduction anesthesia in patients requiring heparin prophylaxis.33
Cost-effectiveness
The cost of perioperative prophylaxis has been compared with the cost of immediate therapy for thromboembolic disease and long-term therapy for postthrombotic syndrome. Across the board, universal prophylaxis with pharmacotherapy or mechanical devices in patients undergoing abdominal surgery is less expensive than no prophylaxis, based on a reduced incidence of DVT. Pneumatic compression seems to be more cost-effective than pharmacotherapy.34
Because LDUH is substantially less expensive than LMWH in the United States, it has a better cost profile when pharmacotherapy is warranted. In Europe, where the cost of heparin compounds is not an issue, LMWH has a slight advantage.
The authors report no financial relationships relevant to this article.
1. Dismuke SE, Wagner EH. Pulmonary embolism as a cause of death. The changing mortality in hospitalized patients. JAMA. 1986;255:2039-2042.
2. Farquharson DI, Orr JW, Jr. Prophylaxis against thromboembolism in gynecologic patients. J Reprod Med. 1984;29:845-862.
3. Bergqvist D. Prolonged prophylaxis against postoperative venous thromboembolism. Haemostasis. 1996;26(suppl 4):379-387.
4. Clarke-Pearson DL, DeLong ER, Synan IS, Coleman RE, Creasman WT. Variables associated with postoperative deep venous thrombosis: a prospective study of 411 gynecology patients and creation of a prognostic model. Obstet Gynecol. 1987;69:146-150.
5. Kemble JVH. Incidence of deep vein thrombosis. Br J Hosp Med. 1971;6:721-726.
6. Spritzer CE, Evans AC, Kay HH. Magnetic resonance imaging of deep venous thrombosis in pregnant women with lower extremity edema. Obstet Gynecol. 1995;85:603-607.
7. Florell SR, Rodgers GM. Inherited thrombotic disorders: an update. Am J Hematol. 1997;54:53-60.
8. Shapiro GA. Antiphospholipid syndrome in obstetrics and gynecology. Semin Thromb Hemost. 1994;20:64-70.
9. De Stafano V, Leone G, Mastrangelo S, et al. Clinical manifestations and management of inherited thrombophilia: retrospective analysis and follow-up after diagnosis in 238 patients with congenital deficiency of antithrombin III, protein C, protein S. S Throm Haemost. 1994;72:352-358.
10. Ridker PM, Hennekens CH, Selhub J, et al. Interrelation of hyperhomocyst(e)inemia, factor V Leiden, and risk of future venous thromboembolism. Circulation. 1997;95:1777-1782.
11. Vessey M, Jewell D, Smith A, Yeates D, McPherson K. Chronic inflammatory bowel disease, cigarette smoking, and use of oral contraceptives: findings in a large cohort study of women of childbearing age. Br Med J (Clin Res Ed). 1986;292:1101-1103.
12. Walker MC, Garner PR, Keely EJ, et al. Changes in activated protein C resistance during normal pregnancy. Am J Obstet Gynecol. 1997;177:162-169.
13. Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest. 2001;119(suppl 1):132S-175S.
14. Belcaro G, Laurora G, Cesarone MR, et al. Prophylaxis of recurrent deep venous thrombosis. A randomized, prospective study using indobufen and graduated elastic compression stockings. Angiology. 1993;44:695-699.
15. Clarke-Pearson DL, Synan IS, Dodge R, et al. A randomized trial of low-dose heparin and intermittent pneumatic calf compression for the prevention of deep venous thrombosis after gynecologic oncology surgery. Am J Obstet Gynecol. 1993;168:1146-1153.
16. Handoll HH, Farrar MJ, McBirnie J, et al. Prophylaxis using heparin, low molecular weight heparin and physical methods against deep vein thrombosis and pulmonary embolism in hip fracture. The Cochrane Library, Issue I. 1998. Evidence-based medicine: 146.
17. Clarke-Pearson DL, Synan IS, Hinshaw WM, et al. Prevention of postoperative venous thromboembolism by external pneumatic calf compression in patients with gynecologic malignancy. Obstet Gynecol. 1984;63(1):92-98.
18. Lachman EA, Rouk JL, et al. Complications associated with intermittent pneumatic compression. Arch Phys Med Rehab. 1992;73:482-485.
19. Clarke-Pearson DL, Dodge RK, Synan I, et al. Venous thromboembolism prophylaxis: patients at high risk to fail intermittent pneumatic compression. Obstet Gynecol. 101;2003:157-163.
20. Wilson NV, Das SK, Kakkar VV, et al. Thrombo-embolic prophylaxis in total knee replacement. Evaluation of the A-V Impulse System. J Bone Joint Surg Br. 1992;74:50-52.
21. Rosenberg RD, Damus PS. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973;248:6490-6505.
22. Scurr JH, Coleridge-Smith PD, Hasty JH. Regimen for improved effectiveness of intermittent pneumatic compression in deep venous thrombosis prophylaxis. Surgery. 1987;102:816-820.
23. Collins R, Scrimgeour A, Yusuf S, et al. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162-1173.
24. Hull RD, Pineo GF, Stein PD, et al. Timing of initial administration of low-molecular-weight heparin prophylaxis against deep vein thrombosis in patients following elective hip arthroplasty: a systematic review. Arch Intern Med. 2001;161:1952-1960.
25. Clarke-Pearson DL, DeLong ER, Synan IS, et al. A controlled trial of two low dose heparin regimens for the prevention of postoperative deep vein thrombosis. Obstet Gynecol. 1990;75:684-689.
26. Kakkar VV, Murray WJ. Efficacy and safety of low-molecular-weight heparin (CY216) in preventing postoperative venous thrombo-embolism: a co-operative study. Br J Surg. 1985;72:786-791.
27. Ward B, Pradhan S. Comparison of low molecular weight heparin (Fragmin) with sodium heparin for prophylaxis against postoperative thrombosis in women undergoing major gynaecological surgery. Aust N Z J Obstet Gynaecol. 1998;38:91-92.
28. Bergqvist D, Burmark US, Flordal PA, et al. Low molecular weight heparin started before surgery as prophylaxis against deep vein thrombosis: 2500 versus 5000 XaI units in 2070 patients. Br J Surg. 1995;82:496-501.
29. Bratt G, Tornebohm E, Widlund L, et al. Low molecular weight heparin (KABI 2165, Fragmin): pharmacokinetics after intravenous and subcutaneous administration in human volunteers. Thromb Res. 1986;42:613-620.
30. Kakkar VV, Boedki O, Boneau B, et al. Efficacy and safety profile of a low-molecular weight heparin and standard unfractionated heparin for prophylaxis of postoperative venous thromboembolism: European multicenter trial. World J Surg. 1997;21:2-8.
31. Warkentin TE. Heparin-induced thrombocytopenia: pathogenesis and management. Br J Haematol. 2003;121:535-555.
32. Wysowski DK, Talarico L, Bacsanyi J, et al. Spinal and epidural hematoma and low-molecular-weight heparin. N Engl J Med. 1998;338:1774-1775.
33. Maxwell GL, Myers ER, Clarke-Pearson DL. Cost-effectiveness of deep venous thrombosis prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 2000;95:206-214.
34. Doran FSA. Prevention of deep vein thrombosis. Br J Hosp Med. 1971;6:773-779.
1. Dismuke SE, Wagner EH. Pulmonary embolism as a cause of death. The changing mortality in hospitalized patients. JAMA. 1986;255:2039-2042.
2. Farquharson DI, Orr JW, Jr. Prophylaxis against thromboembolism in gynecologic patients. J Reprod Med. 1984;29:845-862.
3. Bergqvist D. Prolonged prophylaxis against postoperative venous thromboembolism. Haemostasis. 1996;26(suppl 4):379-387.
4. Clarke-Pearson DL, DeLong ER, Synan IS, Coleman RE, Creasman WT. Variables associated with postoperative deep venous thrombosis: a prospective study of 411 gynecology patients and creation of a prognostic model. Obstet Gynecol. 1987;69:146-150.
5. Kemble JVH. Incidence of deep vein thrombosis. Br J Hosp Med. 1971;6:721-726.
6. Spritzer CE, Evans AC, Kay HH. Magnetic resonance imaging of deep venous thrombosis in pregnant women with lower extremity edema. Obstet Gynecol. 1995;85:603-607.
7. Florell SR, Rodgers GM. Inherited thrombotic disorders: an update. Am J Hematol. 1997;54:53-60.
8. Shapiro GA. Antiphospholipid syndrome in obstetrics and gynecology. Semin Thromb Hemost. 1994;20:64-70.
9. De Stafano V, Leone G, Mastrangelo S, et al. Clinical manifestations and management of inherited thrombophilia: retrospective analysis and follow-up after diagnosis in 238 patients with congenital deficiency of antithrombin III, protein C, protein S. S Throm Haemost. 1994;72:352-358.
10. Ridker PM, Hennekens CH, Selhub J, et al. Interrelation of hyperhomocyst(e)inemia, factor V Leiden, and risk of future venous thromboembolism. Circulation. 1997;95:1777-1782.
11. Vessey M, Jewell D, Smith A, Yeates D, McPherson K. Chronic inflammatory bowel disease, cigarette smoking, and use of oral contraceptives: findings in a large cohort study of women of childbearing age. Br Med J (Clin Res Ed). 1986;292:1101-1103.
12. Walker MC, Garner PR, Keely EJ, et al. Changes in activated protein C resistance during normal pregnancy. Am J Obstet Gynecol. 1997;177:162-169.
13. Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest. 2001;119(suppl 1):132S-175S.
14. Belcaro G, Laurora G, Cesarone MR, et al. Prophylaxis of recurrent deep venous thrombosis. A randomized, prospective study using indobufen and graduated elastic compression stockings. Angiology. 1993;44:695-699.
15. Clarke-Pearson DL, Synan IS, Dodge R, et al. A randomized trial of low-dose heparin and intermittent pneumatic calf compression for the prevention of deep venous thrombosis after gynecologic oncology surgery. Am J Obstet Gynecol. 1993;168:1146-1153.
16. Handoll HH, Farrar MJ, McBirnie J, et al. Prophylaxis using heparin, low molecular weight heparin and physical methods against deep vein thrombosis and pulmonary embolism in hip fracture. The Cochrane Library, Issue I. 1998. Evidence-based medicine: 146.
17. Clarke-Pearson DL, Synan IS, Hinshaw WM, et al. Prevention of postoperative venous thromboembolism by external pneumatic calf compression in patients with gynecologic malignancy. Obstet Gynecol. 1984;63(1):92-98.
18. Lachman EA, Rouk JL, et al. Complications associated with intermittent pneumatic compression. Arch Phys Med Rehab. 1992;73:482-485.
19. Clarke-Pearson DL, Dodge RK, Synan I, et al. Venous thromboembolism prophylaxis: patients at high risk to fail intermittent pneumatic compression. Obstet Gynecol. 101;2003:157-163.
20. Wilson NV, Das SK, Kakkar VV, et al. Thrombo-embolic prophylaxis in total knee replacement. Evaluation of the A-V Impulse System. J Bone Joint Surg Br. 1992;74:50-52.
21. Rosenberg RD, Damus PS. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973;248:6490-6505.
22. Scurr JH, Coleridge-Smith PD, Hasty JH. Regimen for improved effectiveness of intermittent pneumatic compression in deep venous thrombosis prophylaxis. Surgery. 1987;102:816-820.
23. Collins R, Scrimgeour A, Yusuf S, et al. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162-1173.
24. Hull RD, Pineo GF, Stein PD, et al. Timing of initial administration of low-molecular-weight heparin prophylaxis against deep vein thrombosis in patients following elective hip arthroplasty: a systematic review. Arch Intern Med. 2001;161:1952-1960.
25. Clarke-Pearson DL, DeLong ER, Synan IS, et al. A controlled trial of two low dose heparin regimens for the prevention of postoperative deep vein thrombosis. Obstet Gynecol. 1990;75:684-689.
26. Kakkar VV, Murray WJ. Efficacy and safety of low-molecular-weight heparin (CY216) in preventing postoperative venous thrombo-embolism: a co-operative study. Br J Surg. 1985;72:786-791.
27. Ward B, Pradhan S. Comparison of low molecular weight heparin (Fragmin) with sodium heparin for prophylaxis against postoperative thrombosis in women undergoing major gynaecological surgery. Aust N Z J Obstet Gynaecol. 1998;38:91-92.
28. Bergqvist D, Burmark US, Flordal PA, et al. Low molecular weight heparin started before surgery as prophylaxis against deep vein thrombosis: 2500 versus 5000 XaI units in 2070 patients. Br J Surg. 1995;82:496-501.
29. Bratt G, Tornebohm E, Widlund L, et al. Low molecular weight heparin (KABI 2165, Fragmin): pharmacokinetics after intravenous and subcutaneous administration in human volunteers. Thromb Res. 1986;42:613-620.
30. Kakkar VV, Boedki O, Boneau B, et al. Efficacy and safety profile of a low-molecular weight heparin and standard unfractionated heparin for prophylaxis of postoperative venous thromboembolism: European multicenter trial. World J Surg. 1997;21:2-8.
31. Warkentin TE. Heparin-induced thrombocytopenia: pathogenesis and management. Br J Haematol. 2003;121:535-555.
32. Wysowski DK, Talarico L, Bacsanyi J, et al. Spinal and epidural hematoma and low-molecular-weight heparin. N Engl J Med. 1998;338:1774-1775.
33. Maxwell GL, Myers ER, Clarke-Pearson DL. Cost-effectiveness of deep venous thrombosis prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 2000;95:206-214.
34. Doran FSA. Prevention of deep vein thrombosis. Br J Hosp Med. 1971;6:773-779.