User login
- Inspect the pelvic cavity prior to beginning adhesiolysis because the peritoneum becomes more opaque as the surgery progresses, obscuring anatomical definition.
- Grasp adhesions from the side contralateral to where you plan to make the first cut.
- Divide superficial adhesions first to ensure unimpeded access to deeper ones in case bleeding occurs.
- Coagulate thick or vascular adhesions with bipolar coagulation prior to division.
- Cut adhesions at both ends and remove the tissue rather than just dividing it.
Although more than 50% of all women who undergo pelvic or abdominal surgery will develop adhesions, the condition garners limited attention in the literature and from physicians. And, yet, the problems—infertility, chronic pelvic pain, and bowel obstruction, to name a few—with which patients present to Ob/Gyns every day often can be attributed to pelvic adhesions. In short, the quality of life of a significant number of women is affected by this condition, warranting a detailed look at the laparoscopic technique of adhesiolysis.
Assessing etiology
Any injury to the peritoneum has the potential to cause a pelvic adhesion, including endometriosis, pelvic inflammatory disease (PID), infection with a sexually transmitted disease, bleeding, and prior surgery. When the peritoneum is injured, the superficial mesothelial cells are disrupted allowing leakage of fibrinogen and cells into the pelvic cavity. Fibrinogen is then converted to fibrin, which covers all injured surfaces. When excess fibrin is present, it binds one organ to another. During normal healing, plasmin (which is formed from plasminogen) breaks down these fibrin bridges. But when plasmin production is inhibited, the bridges between organs remain intact. Within 7 to 10 days after the initial injury, mesothelial cells infiltrate the fibrin bridges, causing adhesions to become more solid.
Consider the presence of adhesions in patients who present with pelvic pain or infertility, especially if they have had prior pelvic or abdominal surgery.
Salpingitis following chlamydia or gonorrhea is another common etiology of pelvic adhesions. The spread of the infection through the fallopian tubes and into the peritoneal cavity sets the stage for the condition, as do ulcerative colitis, appendicitis, and diverticulitis.
Endometriosis is one of the most common etiologies because peritoneal lesions activate a chronic extensive inflammatory process, leading to dense adhesions. In fact, endometriosis-related adhesions are so common that the American Society for Reproductive Medicine (ASRM) assigns more points to adhesions for the staging of endometriosis than it does to the disease itself.1-2
Making the diagnosis
After reviewing the patient’s medical history, perform a physical exam. In some patients, an area of tenderness may be encountered when one palpates a prior incision, indicating that a loop of bowel may be adhered to this area. In addition, in women with adhesions, the uterus often is fixed either to the posterior pelvis or to the anterior abdominal wall, and the adnexa may be thickened and fixed to the uterus or pelvic sidewall. On rectal exam, the anterior wall of the rectum may be adhered to the uterus, or the rectum may be pulled to one side of the pelvis. Radiologic studies, with the exception of the hysterosalpingogram, do not usually aid in the diagnosis of pelvic adhesions.
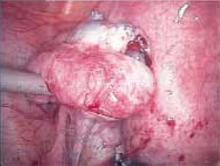
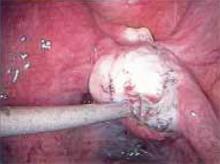
Figure 1
Carefully inspect the pelvic cavity after placing the trocars. In this case, the woman was found to have adhesions on the left ovary.
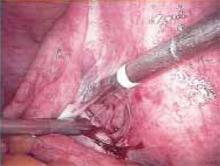
Figure 2
Grasp the adhesion from the side contralateral to where you plan to make the first cut. Use laparoscopic scissors to divide and remove the tissue.
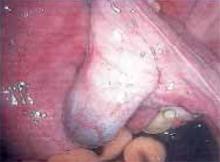
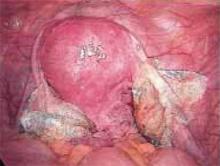
Figure 3
After completing adhesiolysis on the left ovary, check the contralateral organ. In this case, adhesions were found on the distal right tube and ovary.
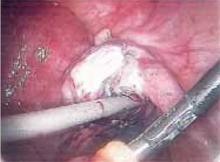
Figure 4
Begin by excising superficial adhesions. This ensures unimpeded access to deeper adhesions in case bleeding occurs.
Figure 5
After all adhesions have been resected and removed, stop all bleeding and lavishly irrigate the pelvis with saline.
Figure 6
Just prior to closure, place oxidized regenerated cellulose (ORC) around both ovaries to help prevent recurrent adhesions.
Preparing the patient
After making the diagnosis and determining that surgery is the optimal treatment (see “Patient selection”), consider the approach. With rare exception, the procedure of choice is laparoscopy and is the method I prefer for adhesiolysis. Regardless of the route, prep the bowel either via an enema or an oral solution, as it decreases distention, making the operation safer and easier. In addition, evacuate the stomach contents via an oral gastric tube.
Indications. Consider for adhesiolysis any patient with chronic pelvic pain for more than 6 months or infertility for more than 1 year, after ruling out other potential etiologies. Patients who complain of localized pelvic pain are more likely to have adhesions than those with diffuse symptoms. But adhesions may be found even in women with diffuse pain. Although this pathology cannot always be incriminated as the cause of the pain, adhesiolysis often leads to a reduction of symptoms.1-3
Contraindications. An abdominal mass large enough to preclude trocar insertion, bowel obstruction, or diaphragmatic hernia are absolute contraindications. One caveat: While previous bowel resection and adhesiolysis are not contraindications to laparoscopy, both increase the risk of bowel perforation on trocar insertion. Proceed with caution or consider laparotomy.
REFERENCES
1. Malik E, Berg C, Meyhofer-Malik A, et al. Subjective evaluation of the therapeutic value of laparoscopic adhesiolysis: a retrospective analysis. Surg Endosc. 2000;14(1):79-81.
2. Nezhat FR, Crystal RA, Nezhat CH, et al. Laparoscopic adhesiolysis and relief of chronic pelvic pain. JSLS. 2000;4(4):281-285.
3. Lavonius M, Gullichsen R, Laine S, et al. Laparoscopy for chronic pelvic pain. Surg Laparosc Endosc. 1999;9(10):42-44.
Placing the trocars
Correct trocar placement facilitates any laparoscopic procedure, especially when dense adhesions are present. First determine the insertion sites, then select the type of entry technique and the length of the trocars.
Insertion sites. In most cases, the primary trocar is placed at the umbilicus. However, in a laparoscopy being performed for adhesions or in patients who have had an umbilical hernia operation or a vertical abdominal incision, place the primary trocar in the left upper quadrant. The reason: This region usually is spared from the formation of dense adhesions; therefore, entry can be performed safely by placing the trocar in the midclavicular line just below the lowest rib.
From that position, examine the abdomen for bowel adhesions to the anterior abdominal wall. If present, excise these adhesions prior to placing a trocar at the umbilical site. (It often is unnecessary to move the camera to the umbilical port, as the view with modern optical equipment from the left upper quadrant is more than adequate.)
Further, surgery can be eased by spacing the trocars as widely apart as possible and placing them on the same side of the patient.
Entry techniques. An alternative to direct trocar placement in highrisk patients is to use the open trocar insertion technique. First, make an abdominal incision and then insert the trocar through it. While some believe that this method reduces the risk of bowel perforation, the survey data from the American Association of Gynecologic Laparoscopists (AAGL) have not supported this theory.3
Trocar length. To determine the proper length, consider the thickness of the patient’s abdominal wall and the distance from the trocar to the adhesion. Trocars that are too long prevent the grasper jaws and scissors from opening, while those that are too short keep slipping back into the retroperitoneal space.
After all the trocars have been placed, carefully inspect the pelvic cavity because the peritoneum becomes more opaque as surgery progresses, obscuring anatomical definition.
Divide and conquer
To excise the adhesion, grasp it from the side contralateral to where you plan to make the first cut. Traction on both the organ and the adhesion is essential to establish the correct tissue planes, which in turn ensures adequate and safe excision of the adhesion. Because the bowel is the organ commonly involved in the adhesive process, there are many graspers available for atraumatic manipulation, including the Duval and Pennington forceps. Avoiding injury to the bowel is paramount to the procedure, as trauma will cause more adhesions, worsening the patient’s condition.
After grasping the adhesion, select a method by which to divide and remove it. This selection is based on the surgeon’s experience, the location of the adhesions, and the organs involved. In most cases, laparoscopic scissors are the instrument of choice. However, opt for electrosurgery (unipolar and bipolar), carbon dioxide laser, or ultrasonic energy (see “Tools for excision”) when energy is needed as a secondary source of hemostasis.
Begin by excising superficial adhesions. This ensures unimpeded access to deeper adhesions in case bleeding occurs. In electrosurgery, slowly pass the probe near the adhesion, allowing the electrons to arc from the instrument tip to the tissue. Proceed with extreme care when using unipolar energy near vital structures because electrons travel the path of least resistance. For example, if there is an adhesion between a loop of bowel and the uterus, always resect the adhesion from the bowel first. The reason: If you remove the adhesion from the uterus first, electrons would flow toward the bowel while you excise the adhesion from this organ. This is because it is the only path remaining for the electrons to egress, as you already have severed the adhesion from the uterus. The end result: thermal damage to the bowel.
When extremely thick or vascular adhesions are encountered, coagulate them prior to division as follows: First, achieve hemostasis using bipolar forceps. This instrument allows the precise application of energy to the tissue because electrons flow from one side of the forceps to the other, rather than through the body as with unipolar electrosurgery. Apply the energy until the tissue is desiccated and the high resistance stops electron flow. Second, divide the tissue using laparoscopic scissors. Bipolar electrosurgery also should be used when coagulation is needed near vital structures such as great vessels, the bowel, bladder, and ureters.
A new type of electrosurgical generator seals vessel walls rather than coagulating them (LigaSure; Valleylab, Boulder, Colo). This results in a much higher vessel burst strength than either unipolar or bipolar electrosurgery, allowing the surgeon to close vessels up to 7 mm in diameter prior to division of the vascular adhesion.
After all adhesions have been resected and removed, stop all bleeding and lavishly irrigate the pelvis with saline prior to closure. If extensive adhesions have been resected from the rectal wall, assess this organ’s integrity by filling the pelvis with saline and placing air in the rectum with either a sigmoidoscope or 30-cc Foley catheter.
Laparoscopic scissors. These are inexpensive, create no peripheral damage, give direct mechanical feedback, and are familiar to all physicians. No smoke or plume is created to obstruct vision. However, because hemostasis cannot be achieved with scissors, they are inappropriate for vascular adhesions. In addition, they cannot be used in areas of the pelvis that can be seen but not reached. Instead, opt for one of the cutting methods described here.
Electrosurgery. The efficiency of electrosurgery depends on the power, shape of the probe tip, and waveform. The higher the power and the finer the tip, the faster the probe will resect the adhesion, thereby minimizing thermal damage. Pure cutting currents also reduce thermal damage but provide less hemostasis than blended or pure coagulation currents.
In general, the thickness, vascularity, and water content of the adhesions will dictate the equipment and settings used. (There are no standard wattage settings, but surgeons typically use 25 to 125 watts.)
Carbon dioxide laser. This instrument allows surgeons to cut with hemostasis without coming into direct contact with the tissue. There are an infinite number of settings, wavelengths, and spot sizes available, making this method of resection extremely precise. However, the laser is costly, cumbersome, and produces a lot of smoke.
Ultrasonic energy. The ultrasonic blade and forceps create hemostasis using vibrational energy to coagulate tissue. Pass the blade through the tissue, slowly separating it while achieving hemostasis. Or, use the forceps to resect vascular adhesions, grasping and coagulating the tissue first and then using the scissors to divide the adhesion.
The advantages: minimal thermal spread, tissue-contact feedback, and essentially no smoke. However, the disadvantages are that direct tissue contact is necessary, traction is vital, the coagulation process is slow, and the disposable equipment is expensive.—Stephen Cohen, MD
Preventing adhesion recurrence
Unfortunately, approximately 85% of patients who undergo surgery for pelvic adhesions will develop recurrent adhesions. Available products that attempt to minimize this effect include oxidized regenerated cellulose (ORC) (Interceed; Ethicon Inc, Somerville, NJ), hyaluronic acid with carboxymethylcellulose (HAL-F) (Seprafilm; Genzyme Biosurgery, Cambridge, Mass), and expanded polytetrafluoroethylene (ePTFE) (Gore-Tex; W.L. Gore & Assocs, Flagstaff, Ariz). However, these products can only be applied to small surface areas, and only ORC can easily be used laparoscopically. As a result, they have achieved limited success.
Some additional progress has been made with the recent FDA approval of hyaluronate gel (Intergel; Gynecare, a division of Ethicon Inc, Somerville, NJ), which coats the entire peritoneal surface and all the intra-abdominal organs.4 Surgeons instill 300 cc of the gel through a trocar into the abdominal cavity just prior to closure, and the body completely absorbs it over time.
The author reports no financial relationship with any companies whose products are mentioned in this article.
1. Porpora MG, et al. Correlation between endometriosis and pelvic pain. J Am Assoc Gynecol Laparosc. 1999;6(4):429-434.
2. Buttram VC, et al. Pelvic Adhesions American Fertility Society. Fertil Steril. 1998;49:6.-
3. Levy BS, Hulka JF, Peterson HB, Phillips JM. Operative laparoscopy: American Association of Gynecologic Laparoscopists. 1993 Membership Survey. J Am Assoc Gynecol Laparosc. 1994;1:301.-
4. Johns DB, Keyport GM, Hoehler F, et al. Reduction of postsurgical adhesions with Intergel adhesion prevention solution: a multicenter study of safety and efficacy after conservative gynecologic surgery. Fertil Steril. 2001;76(3):595-604.
- Inspect the pelvic cavity prior to beginning adhesiolysis because the peritoneum becomes more opaque as the surgery progresses, obscuring anatomical definition.
- Grasp adhesions from the side contralateral to where you plan to make the first cut.
- Divide superficial adhesions first to ensure unimpeded access to deeper ones in case bleeding occurs.
- Coagulate thick or vascular adhesions with bipolar coagulation prior to division.
- Cut adhesions at both ends and remove the tissue rather than just dividing it.
Although more than 50% of all women who undergo pelvic or abdominal surgery will develop adhesions, the condition garners limited attention in the literature and from physicians. And, yet, the problems—infertility, chronic pelvic pain, and bowel obstruction, to name a few—with which patients present to Ob/Gyns every day often can be attributed to pelvic adhesions. In short, the quality of life of a significant number of women is affected by this condition, warranting a detailed look at the laparoscopic technique of adhesiolysis.
Assessing etiology
Any injury to the peritoneum has the potential to cause a pelvic adhesion, including endometriosis, pelvic inflammatory disease (PID), infection with a sexually transmitted disease, bleeding, and prior surgery. When the peritoneum is injured, the superficial mesothelial cells are disrupted allowing leakage of fibrinogen and cells into the pelvic cavity. Fibrinogen is then converted to fibrin, which covers all injured surfaces. When excess fibrin is present, it binds one organ to another. During normal healing, plasmin (which is formed from plasminogen) breaks down these fibrin bridges. But when plasmin production is inhibited, the bridges between organs remain intact. Within 7 to 10 days after the initial injury, mesothelial cells infiltrate the fibrin bridges, causing adhesions to become more solid.
Consider the presence of adhesions in patients who present with pelvic pain or infertility, especially if they have had prior pelvic or abdominal surgery.
Salpingitis following chlamydia or gonorrhea is another common etiology of pelvic adhesions. The spread of the infection through the fallopian tubes and into the peritoneal cavity sets the stage for the condition, as do ulcerative colitis, appendicitis, and diverticulitis.
Endometriosis is one of the most common etiologies because peritoneal lesions activate a chronic extensive inflammatory process, leading to dense adhesions. In fact, endometriosis-related adhesions are so common that the American Society for Reproductive Medicine (ASRM) assigns more points to adhesions for the staging of endometriosis than it does to the disease itself.1-2
Making the diagnosis
After reviewing the patient’s medical history, perform a physical exam. In some patients, an area of tenderness may be encountered when one palpates a prior incision, indicating that a loop of bowel may be adhered to this area. In addition, in women with adhesions, the uterus often is fixed either to the posterior pelvis or to the anterior abdominal wall, and the adnexa may be thickened and fixed to the uterus or pelvic sidewall. On rectal exam, the anterior wall of the rectum may be adhered to the uterus, or the rectum may be pulled to one side of the pelvis. Radiologic studies, with the exception of the hysterosalpingogram, do not usually aid in the diagnosis of pelvic adhesions.
Figure 1
Carefully inspect the pelvic cavity after placing the trocars. In this case, the woman was found to have adhesions on the left ovary.
Figure 2
Grasp the adhesion from the side contralateral to where you plan to make the first cut. Use laparoscopic scissors to divide and remove the tissue.
Figure 3
After completing adhesiolysis on the left ovary, check the contralateral organ. In this case, adhesions were found on the distal right tube and ovary.
Figure 4
Begin by excising superficial adhesions. This ensures unimpeded access to deeper adhesions in case bleeding occurs.
Figure 5
After all adhesions have been resected and removed, stop all bleeding and lavishly irrigate the pelvis with saline.
Figure 6
Just prior to closure, place oxidized regenerated cellulose (ORC) around both ovaries to help prevent recurrent adhesions.
Preparing the patient
After making the diagnosis and determining that surgery is the optimal treatment (see “Patient selection”), consider the approach. With rare exception, the procedure of choice is laparoscopy and is the method I prefer for adhesiolysis. Regardless of the route, prep the bowel either via an enema or an oral solution, as it decreases distention, making the operation safer and easier. In addition, evacuate the stomach contents via an oral gastric tube.
Indications. Consider for adhesiolysis any patient with chronic pelvic pain for more than 6 months or infertility for more than 1 year, after ruling out other potential etiologies. Patients who complain of localized pelvic pain are more likely to have adhesions than those with diffuse symptoms. But adhesions may be found even in women with diffuse pain. Although this pathology cannot always be incriminated as the cause of the pain, adhesiolysis often leads to a reduction of symptoms.1-3
Contraindications. An abdominal mass large enough to preclude trocar insertion, bowel obstruction, or diaphragmatic hernia are absolute contraindications. One caveat: While previous bowel resection and adhesiolysis are not contraindications to laparoscopy, both increase the risk of bowel perforation on trocar insertion. Proceed with caution or consider laparotomy.
REFERENCES
1. Malik E, Berg C, Meyhofer-Malik A, et al. Subjective evaluation of the therapeutic value of laparoscopic adhesiolysis: a retrospective analysis. Surg Endosc. 2000;14(1):79-81.
2. Nezhat FR, Crystal RA, Nezhat CH, et al. Laparoscopic adhesiolysis and relief of chronic pelvic pain. JSLS. 2000;4(4):281-285.
3. Lavonius M, Gullichsen R, Laine S, et al. Laparoscopy for chronic pelvic pain. Surg Laparosc Endosc. 1999;9(10):42-44.
Placing the trocars
Correct trocar placement facilitates any laparoscopic procedure, especially when dense adhesions are present. First determine the insertion sites, then select the type of entry technique and the length of the trocars.
Insertion sites. In most cases, the primary trocar is placed at the umbilicus. However, in a laparoscopy being performed for adhesions or in patients who have had an umbilical hernia operation or a vertical abdominal incision, place the primary trocar in the left upper quadrant. The reason: This region usually is spared from the formation of dense adhesions; therefore, entry can be performed safely by placing the trocar in the midclavicular line just below the lowest rib.
From that position, examine the abdomen for bowel adhesions to the anterior abdominal wall. If present, excise these adhesions prior to placing a trocar at the umbilical site. (It often is unnecessary to move the camera to the umbilical port, as the view with modern optical equipment from the left upper quadrant is more than adequate.)
Further, surgery can be eased by spacing the trocars as widely apart as possible and placing them on the same side of the patient.
Entry techniques. An alternative to direct trocar placement in highrisk patients is to use the open trocar insertion technique. First, make an abdominal incision and then insert the trocar through it. While some believe that this method reduces the risk of bowel perforation, the survey data from the American Association of Gynecologic Laparoscopists (AAGL) have not supported this theory.3
Trocar length. To determine the proper length, consider the thickness of the patient’s abdominal wall and the distance from the trocar to the adhesion. Trocars that are too long prevent the grasper jaws and scissors from opening, while those that are too short keep slipping back into the retroperitoneal space.
After all the trocars have been placed, carefully inspect the pelvic cavity because the peritoneum becomes more opaque as surgery progresses, obscuring anatomical definition.
Divide and conquer
To excise the adhesion, grasp it from the side contralateral to where you plan to make the first cut. Traction on both the organ and the adhesion is essential to establish the correct tissue planes, which in turn ensures adequate and safe excision of the adhesion. Because the bowel is the organ commonly involved in the adhesive process, there are many graspers available for atraumatic manipulation, including the Duval and Pennington forceps. Avoiding injury to the bowel is paramount to the procedure, as trauma will cause more adhesions, worsening the patient’s condition.
After grasping the adhesion, select a method by which to divide and remove it. This selection is based on the surgeon’s experience, the location of the adhesions, and the organs involved. In most cases, laparoscopic scissors are the instrument of choice. However, opt for electrosurgery (unipolar and bipolar), carbon dioxide laser, or ultrasonic energy (see “Tools for excision”) when energy is needed as a secondary source of hemostasis.
Begin by excising superficial adhesions. This ensures unimpeded access to deeper adhesions in case bleeding occurs. In electrosurgery, slowly pass the probe near the adhesion, allowing the electrons to arc from the instrument tip to the tissue. Proceed with extreme care when using unipolar energy near vital structures because electrons travel the path of least resistance. For example, if there is an adhesion between a loop of bowel and the uterus, always resect the adhesion from the bowel first. The reason: If you remove the adhesion from the uterus first, electrons would flow toward the bowel while you excise the adhesion from this organ. This is because it is the only path remaining for the electrons to egress, as you already have severed the adhesion from the uterus. The end result: thermal damage to the bowel.
When extremely thick or vascular adhesions are encountered, coagulate them prior to division as follows: First, achieve hemostasis using bipolar forceps. This instrument allows the precise application of energy to the tissue because electrons flow from one side of the forceps to the other, rather than through the body as with unipolar electrosurgery. Apply the energy until the tissue is desiccated and the high resistance stops electron flow. Second, divide the tissue using laparoscopic scissors. Bipolar electrosurgery also should be used when coagulation is needed near vital structures such as great vessels, the bowel, bladder, and ureters.
A new type of electrosurgical generator seals vessel walls rather than coagulating them (LigaSure; Valleylab, Boulder, Colo). This results in a much higher vessel burst strength than either unipolar or bipolar electrosurgery, allowing the surgeon to close vessels up to 7 mm in diameter prior to division of the vascular adhesion.
After all adhesions have been resected and removed, stop all bleeding and lavishly irrigate the pelvis with saline prior to closure. If extensive adhesions have been resected from the rectal wall, assess this organ’s integrity by filling the pelvis with saline and placing air in the rectum with either a sigmoidoscope or 30-cc Foley catheter.
Laparoscopic scissors. These are inexpensive, create no peripheral damage, give direct mechanical feedback, and are familiar to all physicians. No smoke or plume is created to obstruct vision. However, because hemostasis cannot be achieved with scissors, they are inappropriate for vascular adhesions. In addition, they cannot be used in areas of the pelvis that can be seen but not reached. Instead, opt for one of the cutting methods described here.
Electrosurgery. The efficiency of electrosurgery depends on the power, shape of the probe tip, and waveform. The higher the power and the finer the tip, the faster the probe will resect the adhesion, thereby minimizing thermal damage. Pure cutting currents also reduce thermal damage but provide less hemostasis than blended or pure coagulation currents.
In general, the thickness, vascularity, and water content of the adhesions will dictate the equipment and settings used. (There are no standard wattage settings, but surgeons typically use 25 to 125 watts.)
Carbon dioxide laser. This instrument allows surgeons to cut with hemostasis without coming into direct contact with the tissue. There are an infinite number of settings, wavelengths, and spot sizes available, making this method of resection extremely precise. However, the laser is costly, cumbersome, and produces a lot of smoke.
Ultrasonic energy. The ultrasonic blade and forceps create hemostasis using vibrational energy to coagulate tissue. Pass the blade through the tissue, slowly separating it while achieving hemostasis. Or, use the forceps to resect vascular adhesions, grasping and coagulating the tissue first and then using the scissors to divide the adhesion.
The advantages: minimal thermal spread, tissue-contact feedback, and essentially no smoke. However, the disadvantages are that direct tissue contact is necessary, traction is vital, the coagulation process is slow, and the disposable equipment is expensive.—Stephen Cohen, MD
Preventing adhesion recurrence
Unfortunately, approximately 85% of patients who undergo surgery for pelvic adhesions will develop recurrent adhesions. Available products that attempt to minimize this effect include oxidized regenerated cellulose (ORC) (Interceed; Ethicon Inc, Somerville, NJ), hyaluronic acid with carboxymethylcellulose (HAL-F) (Seprafilm; Genzyme Biosurgery, Cambridge, Mass), and expanded polytetrafluoroethylene (ePTFE) (Gore-Tex; W.L. Gore & Assocs, Flagstaff, Ariz). However, these products can only be applied to small surface areas, and only ORC can easily be used laparoscopically. As a result, they have achieved limited success.
Some additional progress has been made with the recent FDA approval of hyaluronate gel (Intergel; Gynecare, a division of Ethicon Inc, Somerville, NJ), which coats the entire peritoneal surface and all the intra-abdominal organs.4 Surgeons instill 300 cc of the gel through a trocar into the abdominal cavity just prior to closure, and the body completely absorbs it over time.
The author reports no financial relationship with any companies whose products are mentioned in this article.
- Inspect the pelvic cavity prior to beginning adhesiolysis because the peritoneum becomes more opaque as the surgery progresses, obscuring anatomical definition.
- Grasp adhesions from the side contralateral to where you plan to make the first cut.
- Divide superficial adhesions first to ensure unimpeded access to deeper ones in case bleeding occurs.
- Coagulate thick or vascular adhesions with bipolar coagulation prior to division.
- Cut adhesions at both ends and remove the tissue rather than just dividing it.
Although more than 50% of all women who undergo pelvic or abdominal surgery will develop adhesions, the condition garners limited attention in the literature and from physicians. And, yet, the problems—infertility, chronic pelvic pain, and bowel obstruction, to name a few—with which patients present to Ob/Gyns every day often can be attributed to pelvic adhesions. In short, the quality of life of a significant number of women is affected by this condition, warranting a detailed look at the laparoscopic technique of adhesiolysis.
Assessing etiology
Any injury to the peritoneum has the potential to cause a pelvic adhesion, including endometriosis, pelvic inflammatory disease (PID), infection with a sexually transmitted disease, bleeding, and prior surgery. When the peritoneum is injured, the superficial mesothelial cells are disrupted allowing leakage of fibrinogen and cells into the pelvic cavity. Fibrinogen is then converted to fibrin, which covers all injured surfaces. When excess fibrin is present, it binds one organ to another. During normal healing, plasmin (which is formed from plasminogen) breaks down these fibrin bridges. But when plasmin production is inhibited, the bridges between organs remain intact. Within 7 to 10 days after the initial injury, mesothelial cells infiltrate the fibrin bridges, causing adhesions to become more solid.
Consider the presence of adhesions in patients who present with pelvic pain or infertility, especially if they have had prior pelvic or abdominal surgery.
Salpingitis following chlamydia or gonorrhea is another common etiology of pelvic adhesions. The spread of the infection through the fallopian tubes and into the peritoneal cavity sets the stage for the condition, as do ulcerative colitis, appendicitis, and diverticulitis.
Endometriosis is one of the most common etiologies because peritoneal lesions activate a chronic extensive inflammatory process, leading to dense adhesions. In fact, endometriosis-related adhesions are so common that the American Society for Reproductive Medicine (ASRM) assigns more points to adhesions for the staging of endometriosis than it does to the disease itself.1-2
Making the diagnosis
After reviewing the patient’s medical history, perform a physical exam. In some patients, an area of tenderness may be encountered when one palpates a prior incision, indicating that a loop of bowel may be adhered to this area. In addition, in women with adhesions, the uterus often is fixed either to the posterior pelvis or to the anterior abdominal wall, and the adnexa may be thickened and fixed to the uterus or pelvic sidewall. On rectal exam, the anterior wall of the rectum may be adhered to the uterus, or the rectum may be pulled to one side of the pelvis. Radiologic studies, with the exception of the hysterosalpingogram, do not usually aid in the diagnosis of pelvic adhesions.
Figure 1
Carefully inspect the pelvic cavity after placing the trocars. In this case, the woman was found to have adhesions on the left ovary.
Figure 2
Grasp the adhesion from the side contralateral to where you plan to make the first cut. Use laparoscopic scissors to divide and remove the tissue.
Figure 3
After completing adhesiolysis on the left ovary, check the contralateral organ. In this case, adhesions were found on the distal right tube and ovary.
Figure 4
Begin by excising superficial adhesions. This ensures unimpeded access to deeper adhesions in case bleeding occurs.
Figure 5
After all adhesions have been resected and removed, stop all bleeding and lavishly irrigate the pelvis with saline.
Figure 6
Just prior to closure, place oxidized regenerated cellulose (ORC) around both ovaries to help prevent recurrent adhesions.
Preparing the patient
After making the diagnosis and determining that surgery is the optimal treatment (see “Patient selection”), consider the approach. With rare exception, the procedure of choice is laparoscopy and is the method I prefer for adhesiolysis. Regardless of the route, prep the bowel either via an enema or an oral solution, as it decreases distention, making the operation safer and easier. In addition, evacuate the stomach contents via an oral gastric tube.
Indications. Consider for adhesiolysis any patient with chronic pelvic pain for more than 6 months or infertility for more than 1 year, after ruling out other potential etiologies. Patients who complain of localized pelvic pain are more likely to have adhesions than those with diffuse symptoms. But adhesions may be found even in women with diffuse pain. Although this pathology cannot always be incriminated as the cause of the pain, adhesiolysis often leads to a reduction of symptoms.1-3
Contraindications. An abdominal mass large enough to preclude trocar insertion, bowel obstruction, or diaphragmatic hernia are absolute contraindications. One caveat: While previous bowel resection and adhesiolysis are not contraindications to laparoscopy, both increase the risk of bowel perforation on trocar insertion. Proceed with caution or consider laparotomy.
REFERENCES
1. Malik E, Berg C, Meyhofer-Malik A, et al. Subjective evaluation of the therapeutic value of laparoscopic adhesiolysis: a retrospective analysis. Surg Endosc. 2000;14(1):79-81.
2. Nezhat FR, Crystal RA, Nezhat CH, et al. Laparoscopic adhesiolysis and relief of chronic pelvic pain. JSLS. 2000;4(4):281-285.
3. Lavonius M, Gullichsen R, Laine S, et al. Laparoscopy for chronic pelvic pain. Surg Laparosc Endosc. 1999;9(10):42-44.
Placing the trocars
Correct trocar placement facilitates any laparoscopic procedure, especially when dense adhesions are present. First determine the insertion sites, then select the type of entry technique and the length of the trocars.
Insertion sites. In most cases, the primary trocar is placed at the umbilicus. However, in a laparoscopy being performed for adhesions or in patients who have had an umbilical hernia operation or a vertical abdominal incision, place the primary trocar in the left upper quadrant. The reason: This region usually is spared from the formation of dense adhesions; therefore, entry can be performed safely by placing the trocar in the midclavicular line just below the lowest rib.
From that position, examine the abdomen for bowel adhesions to the anterior abdominal wall. If present, excise these adhesions prior to placing a trocar at the umbilical site. (It often is unnecessary to move the camera to the umbilical port, as the view with modern optical equipment from the left upper quadrant is more than adequate.)
Further, surgery can be eased by spacing the trocars as widely apart as possible and placing them on the same side of the patient.
Entry techniques. An alternative to direct trocar placement in highrisk patients is to use the open trocar insertion technique. First, make an abdominal incision and then insert the trocar through it. While some believe that this method reduces the risk of bowel perforation, the survey data from the American Association of Gynecologic Laparoscopists (AAGL) have not supported this theory.3
Trocar length. To determine the proper length, consider the thickness of the patient’s abdominal wall and the distance from the trocar to the adhesion. Trocars that are too long prevent the grasper jaws and scissors from opening, while those that are too short keep slipping back into the retroperitoneal space.
After all the trocars have been placed, carefully inspect the pelvic cavity because the peritoneum becomes more opaque as surgery progresses, obscuring anatomical definition.
Divide and conquer
To excise the adhesion, grasp it from the side contralateral to where you plan to make the first cut. Traction on both the organ and the adhesion is essential to establish the correct tissue planes, which in turn ensures adequate and safe excision of the adhesion. Because the bowel is the organ commonly involved in the adhesive process, there are many graspers available for atraumatic manipulation, including the Duval and Pennington forceps. Avoiding injury to the bowel is paramount to the procedure, as trauma will cause more adhesions, worsening the patient’s condition.
After grasping the adhesion, select a method by which to divide and remove it. This selection is based on the surgeon’s experience, the location of the adhesions, and the organs involved. In most cases, laparoscopic scissors are the instrument of choice. However, opt for electrosurgery (unipolar and bipolar), carbon dioxide laser, or ultrasonic energy (see “Tools for excision”) when energy is needed as a secondary source of hemostasis.
Begin by excising superficial adhesions. This ensures unimpeded access to deeper adhesions in case bleeding occurs. In electrosurgery, slowly pass the probe near the adhesion, allowing the electrons to arc from the instrument tip to the tissue. Proceed with extreme care when using unipolar energy near vital structures because electrons travel the path of least resistance. For example, if there is an adhesion between a loop of bowel and the uterus, always resect the adhesion from the bowel first. The reason: If you remove the adhesion from the uterus first, electrons would flow toward the bowel while you excise the adhesion from this organ. This is because it is the only path remaining for the electrons to egress, as you already have severed the adhesion from the uterus. The end result: thermal damage to the bowel.
When extremely thick or vascular adhesions are encountered, coagulate them prior to division as follows: First, achieve hemostasis using bipolar forceps. This instrument allows the precise application of energy to the tissue because electrons flow from one side of the forceps to the other, rather than through the body as with unipolar electrosurgery. Apply the energy until the tissue is desiccated and the high resistance stops electron flow. Second, divide the tissue using laparoscopic scissors. Bipolar electrosurgery also should be used when coagulation is needed near vital structures such as great vessels, the bowel, bladder, and ureters.
A new type of electrosurgical generator seals vessel walls rather than coagulating them (LigaSure; Valleylab, Boulder, Colo). This results in a much higher vessel burst strength than either unipolar or bipolar electrosurgery, allowing the surgeon to close vessels up to 7 mm in diameter prior to division of the vascular adhesion.
After all adhesions have been resected and removed, stop all bleeding and lavishly irrigate the pelvis with saline prior to closure. If extensive adhesions have been resected from the rectal wall, assess this organ’s integrity by filling the pelvis with saline and placing air in the rectum with either a sigmoidoscope or 30-cc Foley catheter.
Laparoscopic scissors. These are inexpensive, create no peripheral damage, give direct mechanical feedback, and are familiar to all physicians. No smoke or plume is created to obstruct vision. However, because hemostasis cannot be achieved with scissors, they are inappropriate for vascular adhesions. In addition, they cannot be used in areas of the pelvis that can be seen but not reached. Instead, opt for one of the cutting methods described here.
Electrosurgery. The efficiency of electrosurgery depends on the power, shape of the probe tip, and waveform. The higher the power and the finer the tip, the faster the probe will resect the adhesion, thereby minimizing thermal damage. Pure cutting currents also reduce thermal damage but provide less hemostasis than blended or pure coagulation currents.
In general, the thickness, vascularity, and water content of the adhesions will dictate the equipment and settings used. (There are no standard wattage settings, but surgeons typically use 25 to 125 watts.)
Carbon dioxide laser. This instrument allows surgeons to cut with hemostasis without coming into direct contact with the tissue. There are an infinite number of settings, wavelengths, and spot sizes available, making this method of resection extremely precise. However, the laser is costly, cumbersome, and produces a lot of smoke.
Ultrasonic energy. The ultrasonic blade and forceps create hemostasis using vibrational energy to coagulate tissue. Pass the blade through the tissue, slowly separating it while achieving hemostasis. Or, use the forceps to resect vascular adhesions, grasping and coagulating the tissue first and then using the scissors to divide the adhesion.
The advantages: minimal thermal spread, tissue-contact feedback, and essentially no smoke. However, the disadvantages are that direct tissue contact is necessary, traction is vital, the coagulation process is slow, and the disposable equipment is expensive.—Stephen Cohen, MD
Preventing adhesion recurrence
Unfortunately, approximately 85% of patients who undergo surgery for pelvic adhesions will develop recurrent adhesions. Available products that attempt to minimize this effect include oxidized regenerated cellulose (ORC) (Interceed; Ethicon Inc, Somerville, NJ), hyaluronic acid with carboxymethylcellulose (HAL-F) (Seprafilm; Genzyme Biosurgery, Cambridge, Mass), and expanded polytetrafluoroethylene (ePTFE) (Gore-Tex; W.L. Gore & Assocs, Flagstaff, Ariz). However, these products can only be applied to small surface areas, and only ORC can easily be used laparoscopically. As a result, they have achieved limited success.
Some additional progress has been made with the recent FDA approval of hyaluronate gel (Intergel; Gynecare, a division of Ethicon Inc, Somerville, NJ), which coats the entire peritoneal surface and all the intra-abdominal organs.4 Surgeons instill 300 cc of the gel through a trocar into the abdominal cavity just prior to closure, and the body completely absorbs it over time.
The author reports no financial relationship with any companies whose products are mentioned in this article.
1. Porpora MG, et al. Correlation between endometriosis and pelvic pain. J Am Assoc Gynecol Laparosc. 1999;6(4):429-434.
2. Buttram VC, et al. Pelvic Adhesions American Fertility Society. Fertil Steril. 1998;49:6.-
3. Levy BS, Hulka JF, Peterson HB, Phillips JM. Operative laparoscopy: American Association of Gynecologic Laparoscopists. 1993 Membership Survey. J Am Assoc Gynecol Laparosc. 1994;1:301.-
4. Johns DB, Keyport GM, Hoehler F, et al. Reduction of postsurgical adhesions with Intergel adhesion prevention solution: a multicenter study of safety and efficacy after conservative gynecologic surgery. Fertil Steril. 2001;76(3):595-604.
1. Porpora MG, et al. Correlation between endometriosis and pelvic pain. J Am Assoc Gynecol Laparosc. 1999;6(4):429-434.
2. Buttram VC, et al. Pelvic Adhesions American Fertility Society. Fertil Steril. 1998;49:6.-
3. Levy BS, Hulka JF, Peterson HB, Phillips JM. Operative laparoscopy: American Association of Gynecologic Laparoscopists. 1993 Membership Survey. J Am Assoc Gynecol Laparosc. 1994;1:301.-
4. Johns DB, Keyport GM, Hoehler F, et al. Reduction of postsurgical adhesions with Intergel adhesion prevention solution: a multicenter study of safety and efficacy after conservative gynecologic surgery. Fertil Steril. 2001;76(3):595-604.