User login
- The relative value of surgery in the treatment of endometriosis—when compared with medical therapy for pain relief or assisted reproduction for fertility enhancement—has yet to be adequately evaluated.
- Although surgery appears to enhance fertility for all stages of the disease, the effect is marginal with early-stage disease.
- Laparoscopy produces excellent results and should be the method of choice for the surgical management of endometriosis.
- Endometriomas are best treated by removal rather than simple drainage and coagulation.
Surgery traditionally has been a mainstay in the treatment of endometriosis, one of the most common and debilitating diseases in benign gynecology. Surgeons were the first to attack the disease, and surgery remained the primary therapy through the 1970s. Only recently, with the development of drugs to combat endometriosis and techniques to circumvent the pelvic damage associated with the disease, has surgery begun to take a back seat to other therapeutic approaches.
The magnification afforded by laparoscopy frequently facilitates a more precise technique.
This review discusses the various surgical techniques available, including their advantages and disadvantages. An evidence-based review of the therapeutic value of surgery also is provided, along with recommendations for the application of surgery to different presentations of the disease.
One difficulty in reviewing the treatment of endometriosis lies in the heterogeneity of the disease itself. There are many physical manifestations of endometriosis, ranging from superficial peritoneal implants to deep, nodular lesions. Pelvic adhesions with fibrosis and distortion also are common, as are ovarian cysts (endometriomas) and involvement of organs such as the bladder and bowel. This wide range of presentations results in a variety of symptoms, with pelvic/abdominal pain and infertility being the most prominent. Although I will attempt to subdivide the data among these many “forms” of endometriosis, I would like to emphasize that, to some degree, the disease is unique in each woman. Of necessity, the recommendations here will be painted with broad strokes. Nevertheless, it is my belief that such generalizations retain their value in the quest to optimize therapy for individual patients.
Surgical methods
Conservative versus definitive surgery. When treating endometriosis, the surgeon is confronted with a number of technical considerations. The first is whether conservative or definitive surgery is advisable.
If a conservative approach is taken, the physician should assess the desired method of access, the method of treating implants, and the type of surgery done for endometriomas. The surgeon also should assess the need for ancillary procedures such as lysis of adhesions, appendectomy, and nerve interruption.
In conservative surgery, the patient’s fertility is preserved, while definitive surgery generally involves removal of the ovaries, a hysterectomy, or a combination of the 2 procedures. Definitive surgery is thought to be more effective over time, but must be reserved for patients whose fertility or continued endocrine function is deemed less important than the relief of pain.
Unfortunately, hysterectomy and ooph-orectomy do not guarantee the relief of pain. A study from Johns Hopkins suggests that the incidence of persistent or recurrent pain following hysterectomy and bilateral oophorectomy is 10%1. One explanation for persistence/recurrence may be the presence of ovarian remnants, a common finding among such patients. One method of checking for postoperative remnants is checking serum FSH levels.
Method of access. When conservative surgery is desired, the surgeon must select a method of access. Although laparotomy traditionally has been used, most surgeons performing extensive surgery for endometriosis now favor a laparoscopic approach. There are several reasons for this. First, laparoscopy is less invasive, with a much more rapid recovery time. In addition, a laparoscopic procedure costs less than major surgery. Finally, the magnification afforded by laparoscopy frequently facilitates a more precise technique.
There are limited data comparing laparoscopic surgery to laparotomy for the conservative treatment of endometriosis, and all derive from observational cohort studies.2-5 Despite the relatively poor quality of these studies, the evidence suggests little difference in pain relief via major or minor surgery. When the desired outcome is enhanced fertility, 3-year cumulative life-table pregnancy rates for the 2 techniques are equivalent.6
Destruction of implants. The surgical destruction of endometriotic lesions can be accomplished in a variety of ways: excision, vaporization, and fulguration/desiccation. Excision is generally thought to be the most complete of these techniques. It can be performed with a variety of instruments, ranging from the laser to monopolar needles to scissors. The technique is straightforward: The lesion margins are identified by close inspection of the peritoneum, and the cutting instrument is used to outline the area to be excised. The implant then is lifted with atraumatic forceps and separated from the underlying normal tissue by careful dissection. This procedure may be simple or extremely difficult, depending upon the location, thickness, and size of the implant. Many surgeons favor hydrodissection, a technique of irrigating under pressure the tissue near the lesion, in an attempt to separate normal tissue from abnormal. However, 2 dangers exist. First, fluid below the peritoneum frequently distorts anatomy, making a difficult dissection even more challenging. Second, structures fibrotically adherent to endometriosis will not separate and can be damaged if care is not taken to ensure their safety during excision of the lesion.
Implants may be vaporized using high-power-density energy over a short time. This induces a rapid increase in water temperature, resulting in vaporization and tissue destruction. If carbonization can be avoided, this method is very precise. It requires a focused, extremely high-power density, such as that achieved with the superpulse or ultra-pulse CO2laser.
Coagulation occurs with lower energies and results in lower temperatures at the tissue level. At 60° to 80°C, there is a loss of intracellular water and coagulation of protein, resulting in cell destruction. Because the depth of penetration is not always predictable, this technique is considerably less precise than vaporization. In addition, while lasers are a rapid means of performing this method, bipolar electrical current or even monopolar techniques can be used.
No randomized comparisons of these approaches have been conducted. Only 1 retrospective, comparative trial exists. Winkel and Bray reported the results of a 24-month follow-up of 240 women with endometriosis and pelvic pain who underwent surgical treatment in the form of excision alone, laser coagulation alone, or laser coagulation plus medical therapy.7 Twelve months after surgery, 96% of excision patients were pain-free compared with 69% of those undergoing coagulation. At 2 years, the corresponding figures were 69% and 23%, respectively. While this seems to indicate that excision is superior to coagulation, the retrospective study design makes such a conclusion suggestive at best. For example, excision may have been used in easier, less risky situations, whereas in difficult cases, only coagulation may have been performed. The resulting success rates would thus reflect the amount of disease discovered, not the type of surgery. To truly clarify this issue, a randomized trial is needed.
Only 40% of patients surgically treated for endometriosis experience pain relief as a direct result of surgery.
As mentioned earlier, numerous weapons have been employed to destroy endometriosis lesions: the CO2, KTP, Nd:YAG, and argon lasers; ultrasonic shears; monopolar electrical energy; and bipolar electrical energy. No comparisons of the efficacy of these instruments have been conducted.
Treating endometriomas. Ovarian cysts are common in the endometriosis patient, and the method by which they are surgically treated may be vital to the outcome. The goals of treating ovarian endometriomas are removing all ectopic endometrium in the ovary, reducing ovarian trauma, preserving follicles, and minimizing postoperative adhesion formation.8
Two types of endometriomas are recognized. The least common is contained entirely within the ovary. More widespread is an inverted anterior ovarian cortex with adhesions and implants on the surface, which is frequently adherent to the broad ligament. The latter type represents more than 90% of ovarian endometriomas.9
Before operating, the ovaries should be freed of all adhesions. The endometrioma may open spontaneously during this process; if not, incision and drainage are warranted. At this point, the cyst wall may be stripped, excised, or drained as indicated. Stripping involves separating the cyst wall from the ovary and slowly peeling them apart. In the Putman-Redwine technique, the opening to the endometrioma is circumscribed with a laser or electrosurgery, followed by dissection down to the cyst wall. The cyst wall and ovary then are separated sharply and bluntly until the cyst wall is removed, frequently intact. Excision also can be accomplished in a manner similar to a wedge resection. However, while removal of the endometrioma is invariably complete with this method, the potential for adhesion formation is higher.10
The vaporization or coagulation of cyst walls also has been described. A randomized trial comparing removal of the cyst wall versus fenestration and aspiration of cyst contents clearly demonstrates improved results with removal, whether the desired outcome is pain relief, fertility, or a lower rate of reoperation.11
The value of closing the ovary after endometrioma removal has been greatly debated, with no consensus among surgeons. Data suggest more adhesions form with suturing of the ovary than without closure, but it is unclear whether this applies to all sizes of defects.12
Ancillary procedures. Adhesiolysis is an important step in the restoration of normal pelvic and abdominal anatomy. While simple lysis of adhesions is adequate if they were formed following infection, most experts believe a more complete approach is required for the endometriosis-induced adhesion. This is because of the relatively high incidence of endometriosis present within the adhesion itself. Thus, removal of the adhesion by lysing both boundaries of the scar tissue and connecting structures is preferable. The instrumentation is of little consequence as long as precision and hemostasis are maintained.
To minimize adhesion reformation, adhesion-prevention adjuvants should be used. Four are presently available, including Interceed (Ethicon Inc, Somerville, NJ), a cellulose based barrier; Seprafilm (Genzyme Corp, Cambridge, Mass), a hyaluronidase-impregnated barrier; Preclude (W.L. Gore and Associates Inc, Newark, Del), a non-absorbable barrier composed of the proprietary Gore-Tex; and Intergel (Lifecore Biomedical Inc, Chaska, Minn), a thick solution placed within the peritoneal cavity that provides widespread adhesion prophylaxis. All adjuvants currently available have been associated with decreased adhesion reformation in randomized clinical trials.13 They can be applied either laparoscopically or abdominally.
Appendectomy. Endometriosis of the appendix has been described in 17% of patients with bowel involvement.14 In all endometriosis surgeries, the appendix should be carefully inspected and removed if it appears abnormal. In patients who experience endometriosis-associated pelvic pain, it may be desirable to electively remove the appendix at the time of laparoscopy. This will prevent future confusion. Otherwise, emergency room physicians would have to differentiate the symptoms of chronic pelvic/abdominal pain from those of appendicitis in the event the patient presents with a complaint of pelvic pain.
Nerve interruption procedures. Two surgical procedures were designed to help reduce pain transmission in the endometriosis patient: uterosacral nerve ablation/resection and presacral neurectomy. Both involve interruption of the major efferent nerve fibers from the uterus, thus diminishing uterine and central pelvic pain. These procedures can be performed via laparoscopy or laparotomy. Unfortunately, neither has been shown to provide pain relief beyond that achieved with surgery directed against the disease itself.15-18
Outcomes
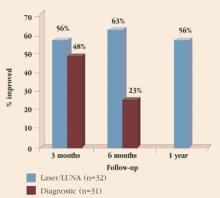
Pain relief. Although there have been many investigations of the effect of endometriosis surgery on pelvic pain, the vast majority are of low quality. No randomized, controlled trials (RCTs) have compared surgery to medical therapy, and only 1 has investigated surgery versus sham surgery. Sutton and colleagues assessed the efficacy of laser laparoscopic surgery in the treatment of pain associated with minimal, mild, or moderate endometriosis.19 They found no difference in pain at 3 months follow-up. However, by 6 months, a clear-cut advantage was seen for surgery (Figure 1). Pain relief also was maintained for at least 1 year in patients undergoing laser surgery. These patients underwent ablation of endometriosis and of the uterosacral nerve (although, as noted earlier, the latter procedure provides no additional pain relief).
Most disconcerting is the finding that only 40% of patients surgically treated for endometriosis experience pain relief as a direct result of the surgery. (The number of patients who need to be treated to reduce pain in 1 patient is 2.5.)
It remains unclear whether excision of the disease will produce better results than coagulation with the CO2 laser. Nor have the duration of pain relief and rate of reoperation been properly examined. Thus, although many surgeons feel quite strongly about the value of surgery to treat endometriosis-associated pain, there are few data to support their clinical impression of efficacy.
Fertility enhancement. Conservative surgery has been used extensively in an attempt to enhance fertility. Unfortunately, most studies on the subject are uncontrolled and of poor quality. In women who have early-stage disease, surgical therapy has resulted in pregnancy rates of 40% to 75%; in severe disease, the rates are lower, ranging from 20% to 50%.20
A meta-analysis encompassing studies from 1982 through 1994 showed that either no treatment or surgery alone is superior to medical treatment for early-stage endometriosis-associated infertility.6
A separate meta-analysis of 25 RCTs and cohort studies examined the same issue.21 It found a possible treatment benefit from laparoscopic conservative surgery, but concluded that medical treatment of the disease was ineffective.
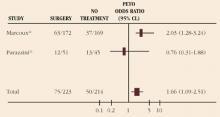
A multicenter randomized trial (ENDOCAN) assessed the value of surgery for early-stage endometriosis in the infertile patient. In this study, 341 infertile women with minimal or mild endometriosis were randomized to diagnostic laparoscopy or resection/ablation of disease.22 They then were followed for 36 weeks. The pregnancy rates were 31% for the treated group and 18% for the diagnostic group, demonstrating a clear advantage to surgery. The data also imply that to achieve 1 additional pregnancy in 9 months, 7.7 women must be surgically treated.
Adding confusion, a second randomized trial—this one from Italy—failed to demonstrate an improvement in fertility among those treated for early-stage endometriosis.23 Nevertheless, when the studies are combined into meta-analysis, surgical treatment of early-stage endometriosis appears to provide a significant improvement in pregnancy rates (odds ratio [OR], 1.66; 95% confidence limits [CL], 1.09-2.51) (Figure 2).
The surgical treatment of endometriosis probably enhances fertility when the disease is advanced, although no high-quality studies specifically address this issue. For early-stage disease, the data are conflicting but suggest that a small effect may exist. Further studies are needed to assess the value of surgery for this disorder.
FIGURE 1 Rate of pain relief with operative versus diagnostic laparoscopy
Adapted from: Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, or moderate endometriosis. Fertil Steril. 1994;62:696.
FIGURE 2 Pregnancy rate with surgical treatment versus diagnostic laparoscopy for early-stage endometriosis
Conclusion
The role of surgery in the treatment of endometriosis is controversial. The procedure can be highly demanding technically and may involve a number of ancillary procedures besides destruction of lesions. Many questions remain unanswered. For example, we do not know the best surgical technique or ancillary procedures, or the best instruments for these procedures. While there appears to be value in treating pain and infertility, that value may be far less than anticipated. Moreover, the relative value of surgery in the treatment of endometriosis—when compared with medical therapy for pain relief or assisted reproduction for fertility enhancement—has yet to be adequately evaluated. Given this level of uncertainty and confusion, surgery should be undertaken with caution and restraint.
Dr. Olive reports that he is a consultant to TAP Pharmaceuticals. He also serves on the company’s speakers’ bureau.
1. Hickman TN, Namnoum AB, Hinton EL, Zacur HA, Rock JA. Timing of estrogen replacement therapy following hysterectomy with oophorectomy for endometriosis. Obstet Gynecol. 1998;91:673-6772
2. Crosignani PG, Vercellini P, Biffignandi F, Constantini W, Cortesi I, Imparato E. Laparoscopy versus laparotomy in conservative surgical treatment for severe endometriosis. Fertil Steril. 1996;66:706-711.
3. Busacca M, Fedele L, Bianchi S, et al. Surgical treatment of recurrent endometriosis: laparotomy versus laparoscopy. Hum Reprod. 1998;13:2271-2274.
4. Catalano GF, Marana R, Caruana P, Muzii L, Mancuso S. Laparoscopy versus microsurgery by laparotomy for excision of ovarian cysts in patients with moderate or severe endometriosis. J Am Assoc Gynecol Laparosc. 1996;3:267-270.
5. Bateman BG, Kolp LA, Mills S. Endoscopic versus laparotomy management of endometriomas. Fertil Steril. 1994;62:690-695.
6. Adamson GD, Pasta DJ. Surgical treatment of endometriosis-associated infertility: meta-analysis compared with survival analysis. Am J Obstet Gynecol. 1994;171:1488.-
7. Winkel CA, Bray M. Treatment of women with endometriosis using excision alone, ablation alone, or ablation in combination with leuprolide acetate. Proceedings of the Fourth World CongresS on Endometriosis, Yokahama, Japan, 1996:55.
8. Guarnaccia MM, Silverberg K, Olive DL. Endometriosis and adenomyosis. In: Copeland LJ, ed. Textbook of Gynecology. Philadelphia: W.B. Saunders; 2000: 687.
9. Hughesdon PE. The structure of endometrial cysts of the ovary. J Obstet Gynaecol British Empire. 1957;44:481-487.
10. Fayes JA, Vogel MF. Comparison of different treatment methods of endometriomas by laparoscopy. Obstet Gynecol. 1991;78:660-665.
11. Beretta P, Franchi M, Ghezzi F. Randomized clinical trial of two laparoscopic treatments of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70:1176-1180.
12. Meyer WR, Grainger DA, DeCherney AH, Lachs MS, Diamond MP. Ovarian surgery on the rabbit. Effect of cortex closure on adhesion formation and ovarian function. J Reprod Med. 1991;36:639-643.
13. Farquhar C, Vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility (Cochrane Review). The Cochrane Library. Issue 1. Oxford: Update Software; 2000.
14. Franklin RR, Grunert GM. Extragenital endometriosis. In: Endometriosis: Advanced Management and Surgical Techniques. New York: Springer-Verlag; 1995;128.-
15. Dover RW, Pooley P, Haines P, Sutton CJG. Prospective, randomized, double-blind controlled trial of laparoscopic laser uterine nerve ablation in the treatment of pelvic pain associated with endometriosis [unpublished].
16. Vercellini P, Aimi G, Busacca M, Uglietti A, Viganali M, Crosignani PG. Laparoscopic uterosacral ligament resection for dysmenorrhea associated with endometriosis. Results of a randomized controlled trial. Fertil Steril. 1997;Oct(Suppl):S3 [abstract].
17. Candiani GB, Fedele L, Vercellini P, Bianchi S, DiNola G. Presacral neurectomy for the treatment of pelvic pain associated with endometriosis: a controlled study. Am J Obstet Gynecol. 1992;167:100-103.
18. Tjaden B, Schlaff WD, Kimball A, Rock JA. The efficacy of presacral neurectomy for the relief of midline dysmenorrhea. Obstet Gynecol. 1990;76:89.-
19. Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, or moderate endometriosis. Fertil Steril. 1994;62:696.-
20. Olive DL. Conservative surgery. In: Schenken RS, ed. Endometriosis: Contemporary Concepts in Clinical Management. Philadelphia: J.B. Lippincott; 1989;213.-
21. Hughes EG, Fedorkow DM, Collins JA. A quantitative overview of controlled trials in endometriosis associated infertility. Fertil Steril. 1993;59:963.-
22. Marcoux S, Maheux R, Berube S. Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group in Endometriosis. N Engl J Med. 1997;337:217-222.
23. Gruppo Italiano per lo Studio dell Endometriosis. Ablation of lesions or no treatment in minimal-mild endometriosis in infertile women: a randomized trial. Hum Reprod. 1999;14:1332-1334.
- The relative value of surgery in the treatment of endometriosis—when compared with medical therapy for pain relief or assisted reproduction for fertility enhancement—has yet to be adequately evaluated.
- Although surgery appears to enhance fertility for all stages of the disease, the effect is marginal with early-stage disease.
- Laparoscopy produces excellent results and should be the method of choice for the surgical management of endometriosis.
- Endometriomas are best treated by removal rather than simple drainage and coagulation.
Surgery traditionally has been a mainstay in the treatment of endometriosis, one of the most common and debilitating diseases in benign gynecology. Surgeons were the first to attack the disease, and surgery remained the primary therapy through the 1970s. Only recently, with the development of drugs to combat endometriosis and techniques to circumvent the pelvic damage associated with the disease, has surgery begun to take a back seat to other therapeutic approaches.
The magnification afforded by laparoscopy frequently facilitates a more precise technique.
This review discusses the various surgical techniques available, including their advantages and disadvantages. An evidence-based review of the therapeutic value of surgery also is provided, along with recommendations for the application of surgery to different presentations of the disease.
One difficulty in reviewing the treatment of endometriosis lies in the heterogeneity of the disease itself. There are many physical manifestations of endometriosis, ranging from superficial peritoneal implants to deep, nodular lesions. Pelvic adhesions with fibrosis and distortion also are common, as are ovarian cysts (endometriomas) and involvement of organs such as the bladder and bowel. This wide range of presentations results in a variety of symptoms, with pelvic/abdominal pain and infertility being the most prominent. Although I will attempt to subdivide the data among these many “forms” of endometriosis, I would like to emphasize that, to some degree, the disease is unique in each woman. Of necessity, the recommendations here will be painted with broad strokes. Nevertheless, it is my belief that such generalizations retain their value in the quest to optimize therapy for individual patients.
Surgical methods
Conservative versus definitive surgery. When treating endometriosis, the surgeon is confronted with a number of technical considerations. The first is whether conservative or definitive surgery is advisable.
If a conservative approach is taken, the physician should assess the desired method of access, the method of treating implants, and the type of surgery done for endometriomas. The surgeon also should assess the need for ancillary procedures such as lysis of adhesions, appendectomy, and nerve interruption.
In conservative surgery, the patient’s fertility is preserved, while definitive surgery generally involves removal of the ovaries, a hysterectomy, or a combination of the 2 procedures. Definitive surgery is thought to be more effective over time, but must be reserved for patients whose fertility or continued endocrine function is deemed less important than the relief of pain.
Unfortunately, hysterectomy and ooph-orectomy do not guarantee the relief of pain. A study from Johns Hopkins suggests that the incidence of persistent or recurrent pain following hysterectomy and bilateral oophorectomy is 10%1. One explanation for persistence/recurrence may be the presence of ovarian remnants, a common finding among such patients. One method of checking for postoperative remnants is checking serum FSH levels.
Method of access. When conservative surgery is desired, the surgeon must select a method of access. Although laparotomy traditionally has been used, most surgeons performing extensive surgery for endometriosis now favor a laparoscopic approach. There are several reasons for this. First, laparoscopy is less invasive, with a much more rapid recovery time. In addition, a laparoscopic procedure costs less than major surgery. Finally, the magnification afforded by laparoscopy frequently facilitates a more precise technique.
There are limited data comparing laparoscopic surgery to laparotomy for the conservative treatment of endometriosis, and all derive from observational cohort studies.2-5 Despite the relatively poor quality of these studies, the evidence suggests little difference in pain relief via major or minor surgery. When the desired outcome is enhanced fertility, 3-year cumulative life-table pregnancy rates for the 2 techniques are equivalent.6
Destruction of implants. The surgical destruction of endometriotic lesions can be accomplished in a variety of ways: excision, vaporization, and fulguration/desiccation. Excision is generally thought to be the most complete of these techniques. It can be performed with a variety of instruments, ranging from the laser to monopolar needles to scissors. The technique is straightforward: The lesion margins are identified by close inspection of the peritoneum, and the cutting instrument is used to outline the area to be excised. The implant then is lifted with atraumatic forceps and separated from the underlying normal tissue by careful dissection. This procedure may be simple or extremely difficult, depending upon the location, thickness, and size of the implant. Many surgeons favor hydrodissection, a technique of irrigating under pressure the tissue near the lesion, in an attempt to separate normal tissue from abnormal. However, 2 dangers exist. First, fluid below the peritoneum frequently distorts anatomy, making a difficult dissection even more challenging. Second, structures fibrotically adherent to endometriosis will not separate and can be damaged if care is not taken to ensure their safety during excision of the lesion.
Implants may be vaporized using high-power-density energy over a short time. This induces a rapid increase in water temperature, resulting in vaporization and tissue destruction. If carbonization can be avoided, this method is very precise. It requires a focused, extremely high-power density, such as that achieved with the superpulse or ultra-pulse CO2laser.
Coagulation occurs with lower energies and results in lower temperatures at the tissue level. At 60° to 80°C, there is a loss of intracellular water and coagulation of protein, resulting in cell destruction. Because the depth of penetration is not always predictable, this technique is considerably less precise than vaporization. In addition, while lasers are a rapid means of performing this method, bipolar electrical current or even monopolar techniques can be used.
No randomized comparisons of these approaches have been conducted. Only 1 retrospective, comparative trial exists. Winkel and Bray reported the results of a 24-month follow-up of 240 women with endometriosis and pelvic pain who underwent surgical treatment in the form of excision alone, laser coagulation alone, or laser coagulation plus medical therapy.7 Twelve months after surgery, 96% of excision patients were pain-free compared with 69% of those undergoing coagulation. At 2 years, the corresponding figures were 69% and 23%, respectively. While this seems to indicate that excision is superior to coagulation, the retrospective study design makes such a conclusion suggestive at best. For example, excision may have been used in easier, less risky situations, whereas in difficult cases, only coagulation may have been performed. The resulting success rates would thus reflect the amount of disease discovered, not the type of surgery. To truly clarify this issue, a randomized trial is needed.
Only 40% of patients surgically treated for endometriosis experience pain relief as a direct result of surgery.
As mentioned earlier, numerous weapons have been employed to destroy endometriosis lesions: the CO2, KTP, Nd:YAG, and argon lasers; ultrasonic shears; monopolar electrical energy; and bipolar electrical energy. No comparisons of the efficacy of these instruments have been conducted.
Treating endometriomas. Ovarian cysts are common in the endometriosis patient, and the method by which they are surgically treated may be vital to the outcome. The goals of treating ovarian endometriomas are removing all ectopic endometrium in the ovary, reducing ovarian trauma, preserving follicles, and minimizing postoperative adhesion formation.8
Two types of endometriomas are recognized. The least common is contained entirely within the ovary. More widespread is an inverted anterior ovarian cortex with adhesions and implants on the surface, which is frequently adherent to the broad ligament. The latter type represents more than 90% of ovarian endometriomas.9
Before operating, the ovaries should be freed of all adhesions. The endometrioma may open spontaneously during this process; if not, incision and drainage are warranted. At this point, the cyst wall may be stripped, excised, or drained as indicated. Stripping involves separating the cyst wall from the ovary and slowly peeling them apart. In the Putman-Redwine technique, the opening to the endometrioma is circumscribed with a laser or electrosurgery, followed by dissection down to the cyst wall. The cyst wall and ovary then are separated sharply and bluntly until the cyst wall is removed, frequently intact. Excision also can be accomplished in a manner similar to a wedge resection. However, while removal of the endometrioma is invariably complete with this method, the potential for adhesion formation is higher.10
The vaporization or coagulation of cyst walls also has been described. A randomized trial comparing removal of the cyst wall versus fenestration and aspiration of cyst contents clearly demonstrates improved results with removal, whether the desired outcome is pain relief, fertility, or a lower rate of reoperation.11
The value of closing the ovary after endometrioma removal has been greatly debated, with no consensus among surgeons. Data suggest more adhesions form with suturing of the ovary than without closure, but it is unclear whether this applies to all sizes of defects.12
Ancillary procedures. Adhesiolysis is an important step in the restoration of normal pelvic and abdominal anatomy. While simple lysis of adhesions is adequate if they were formed following infection, most experts believe a more complete approach is required for the endometriosis-induced adhesion. This is because of the relatively high incidence of endometriosis present within the adhesion itself. Thus, removal of the adhesion by lysing both boundaries of the scar tissue and connecting structures is preferable. The instrumentation is of little consequence as long as precision and hemostasis are maintained.
To minimize adhesion reformation, adhesion-prevention adjuvants should be used. Four are presently available, including Interceed (Ethicon Inc, Somerville, NJ), a cellulose based barrier; Seprafilm (Genzyme Corp, Cambridge, Mass), a hyaluronidase-impregnated barrier; Preclude (W.L. Gore and Associates Inc, Newark, Del), a non-absorbable barrier composed of the proprietary Gore-Tex; and Intergel (Lifecore Biomedical Inc, Chaska, Minn), a thick solution placed within the peritoneal cavity that provides widespread adhesion prophylaxis. All adjuvants currently available have been associated with decreased adhesion reformation in randomized clinical trials.13 They can be applied either laparoscopically or abdominally.
Appendectomy. Endometriosis of the appendix has been described in 17% of patients with bowel involvement.14 In all endometriosis surgeries, the appendix should be carefully inspected and removed if it appears abnormal. In patients who experience endometriosis-associated pelvic pain, it may be desirable to electively remove the appendix at the time of laparoscopy. This will prevent future confusion. Otherwise, emergency room physicians would have to differentiate the symptoms of chronic pelvic/abdominal pain from those of appendicitis in the event the patient presents with a complaint of pelvic pain.
Nerve interruption procedures. Two surgical procedures were designed to help reduce pain transmission in the endometriosis patient: uterosacral nerve ablation/resection and presacral neurectomy. Both involve interruption of the major efferent nerve fibers from the uterus, thus diminishing uterine and central pelvic pain. These procedures can be performed via laparoscopy or laparotomy. Unfortunately, neither has been shown to provide pain relief beyond that achieved with surgery directed against the disease itself.15-18
Outcomes
Pain relief. Although there have been many investigations of the effect of endometriosis surgery on pelvic pain, the vast majority are of low quality. No randomized, controlled trials (RCTs) have compared surgery to medical therapy, and only 1 has investigated surgery versus sham surgery. Sutton and colleagues assessed the efficacy of laser laparoscopic surgery in the treatment of pain associated with minimal, mild, or moderate endometriosis.19 They found no difference in pain at 3 months follow-up. However, by 6 months, a clear-cut advantage was seen for surgery (Figure 1). Pain relief also was maintained for at least 1 year in patients undergoing laser surgery. These patients underwent ablation of endometriosis and of the uterosacral nerve (although, as noted earlier, the latter procedure provides no additional pain relief).
Most disconcerting is the finding that only 40% of patients surgically treated for endometriosis experience pain relief as a direct result of the surgery. (The number of patients who need to be treated to reduce pain in 1 patient is 2.5.)
It remains unclear whether excision of the disease will produce better results than coagulation with the CO2 laser. Nor have the duration of pain relief and rate of reoperation been properly examined. Thus, although many surgeons feel quite strongly about the value of surgery to treat endometriosis-associated pain, there are few data to support their clinical impression of efficacy.
Fertility enhancement. Conservative surgery has been used extensively in an attempt to enhance fertility. Unfortunately, most studies on the subject are uncontrolled and of poor quality. In women who have early-stage disease, surgical therapy has resulted in pregnancy rates of 40% to 75%; in severe disease, the rates are lower, ranging from 20% to 50%.20
A meta-analysis encompassing studies from 1982 through 1994 showed that either no treatment or surgery alone is superior to medical treatment for early-stage endometriosis-associated infertility.6
A separate meta-analysis of 25 RCTs and cohort studies examined the same issue.21 It found a possible treatment benefit from laparoscopic conservative surgery, but concluded that medical treatment of the disease was ineffective.
A multicenter randomized trial (ENDOCAN) assessed the value of surgery for early-stage endometriosis in the infertile patient. In this study, 341 infertile women with minimal or mild endometriosis were randomized to diagnostic laparoscopy or resection/ablation of disease.22 They then were followed for 36 weeks. The pregnancy rates were 31% for the treated group and 18% for the diagnostic group, demonstrating a clear advantage to surgery. The data also imply that to achieve 1 additional pregnancy in 9 months, 7.7 women must be surgically treated.
Adding confusion, a second randomized trial—this one from Italy—failed to demonstrate an improvement in fertility among those treated for early-stage endometriosis.23 Nevertheless, when the studies are combined into meta-analysis, surgical treatment of early-stage endometriosis appears to provide a significant improvement in pregnancy rates (odds ratio [OR], 1.66; 95% confidence limits [CL], 1.09-2.51) (Figure 2).
The surgical treatment of endometriosis probably enhances fertility when the disease is advanced, although no high-quality studies specifically address this issue. For early-stage disease, the data are conflicting but suggest that a small effect may exist. Further studies are needed to assess the value of surgery for this disorder.
FIGURE 1 Rate of pain relief with operative versus diagnostic laparoscopy
Adapted from: Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, or moderate endometriosis. Fertil Steril. 1994;62:696.
FIGURE 2 Pregnancy rate with surgical treatment versus diagnostic laparoscopy for early-stage endometriosis
Conclusion
The role of surgery in the treatment of endometriosis is controversial. The procedure can be highly demanding technically and may involve a number of ancillary procedures besides destruction of lesions. Many questions remain unanswered. For example, we do not know the best surgical technique or ancillary procedures, or the best instruments for these procedures. While there appears to be value in treating pain and infertility, that value may be far less than anticipated. Moreover, the relative value of surgery in the treatment of endometriosis—when compared with medical therapy for pain relief or assisted reproduction for fertility enhancement—has yet to be adequately evaluated. Given this level of uncertainty and confusion, surgery should be undertaken with caution and restraint.
Dr. Olive reports that he is a consultant to TAP Pharmaceuticals. He also serves on the company’s speakers’ bureau.
- The relative value of surgery in the treatment of endometriosis—when compared with medical therapy for pain relief or assisted reproduction for fertility enhancement—has yet to be adequately evaluated.
- Although surgery appears to enhance fertility for all stages of the disease, the effect is marginal with early-stage disease.
- Laparoscopy produces excellent results and should be the method of choice for the surgical management of endometriosis.
- Endometriomas are best treated by removal rather than simple drainage and coagulation.
Surgery traditionally has been a mainstay in the treatment of endometriosis, one of the most common and debilitating diseases in benign gynecology. Surgeons were the first to attack the disease, and surgery remained the primary therapy through the 1970s. Only recently, with the development of drugs to combat endometriosis and techniques to circumvent the pelvic damage associated with the disease, has surgery begun to take a back seat to other therapeutic approaches.
The magnification afforded by laparoscopy frequently facilitates a more precise technique.
This review discusses the various surgical techniques available, including their advantages and disadvantages. An evidence-based review of the therapeutic value of surgery also is provided, along with recommendations for the application of surgery to different presentations of the disease.
One difficulty in reviewing the treatment of endometriosis lies in the heterogeneity of the disease itself. There are many physical manifestations of endometriosis, ranging from superficial peritoneal implants to deep, nodular lesions. Pelvic adhesions with fibrosis and distortion also are common, as are ovarian cysts (endometriomas) and involvement of organs such as the bladder and bowel. This wide range of presentations results in a variety of symptoms, with pelvic/abdominal pain and infertility being the most prominent. Although I will attempt to subdivide the data among these many “forms” of endometriosis, I would like to emphasize that, to some degree, the disease is unique in each woman. Of necessity, the recommendations here will be painted with broad strokes. Nevertheless, it is my belief that such generalizations retain their value in the quest to optimize therapy for individual patients.
Surgical methods
Conservative versus definitive surgery. When treating endometriosis, the surgeon is confronted with a number of technical considerations. The first is whether conservative or definitive surgery is advisable.
If a conservative approach is taken, the physician should assess the desired method of access, the method of treating implants, and the type of surgery done for endometriomas. The surgeon also should assess the need for ancillary procedures such as lysis of adhesions, appendectomy, and nerve interruption.
In conservative surgery, the patient’s fertility is preserved, while definitive surgery generally involves removal of the ovaries, a hysterectomy, or a combination of the 2 procedures. Definitive surgery is thought to be more effective over time, but must be reserved for patients whose fertility or continued endocrine function is deemed less important than the relief of pain.
Unfortunately, hysterectomy and ooph-orectomy do not guarantee the relief of pain. A study from Johns Hopkins suggests that the incidence of persistent or recurrent pain following hysterectomy and bilateral oophorectomy is 10%1. One explanation for persistence/recurrence may be the presence of ovarian remnants, a common finding among such patients. One method of checking for postoperative remnants is checking serum FSH levels.
Method of access. When conservative surgery is desired, the surgeon must select a method of access. Although laparotomy traditionally has been used, most surgeons performing extensive surgery for endometriosis now favor a laparoscopic approach. There are several reasons for this. First, laparoscopy is less invasive, with a much more rapid recovery time. In addition, a laparoscopic procedure costs less than major surgery. Finally, the magnification afforded by laparoscopy frequently facilitates a more precise technique.
There are limited data comparing laparoscopic surgery to laparotomy for the conservative treatment of endometriosis, and all derive from observational cohort studies.2-5 Despite the relatively poor quality of these studies, the evidence suggests little difference in pain relief via major or minor surgery. When the desired outcome is enhanced fertility, 3-year cumulative life-table pregnancy rates for the 2 techniques are equivalent.6
Destruction of implants. The surgical destruction of endometriotic lesions can be accomplished in a variety of ways: excision, vaporization, and fulguration/desiccation. Excision is generally thought to be the most complete of these techniques. It can be performed with a variety of instruments, ranging from the laser to monopolar needles to scissors. The technique is straightforward: The lesion margins are identified by close inspection of the peritoneum, and the cutting instrument is used to outline the area to be excised. The implant then is lifted with atraumatic forceps and separated from the underlying normal tissue by careful dissection. This procedure may be simple or extremely difficult, depending upon the location, thickness, and size of the implant. Many surgeons favor hydrodissection, a technique of irrigating under pressure the tissue near the lesion, in an attempt to separate normal tissue from abnormal. However, 2 dangers exist. First, fluid below the peritoneum frequently distorts anatomy, making a difficult dissection even more challenging. Second, structures fibrotically adherent to endometriosis will not separate and can be damaged if care is not taken to ensure their safety during excision of the lesion.
Implants may be vaporized using high-power-density energy over a short time. This induces a rapid increase in water temperature, resulting in vaporization and tissue destruction. If carbonization can be avoided, this method is very precise. It requires a focused, extremely high-power density, such as that achieved with the superpulse or ultra-pulse CO2laser.
Coagulation occurs with lower energies and results in lower temperatures at the tissue level. At 60° to 80°C, there is a loss of intracellular water and coagulation of protein, resulting in cell destruction. Because the depth of penetration is not always predictable, this technique is considerably less precise than vaporization. In addition, while lasers are a rapid means of performing this method, bipolar electrical current or even monopolar techniques can be used.
No randomized comparisons of these approaches have been conducted. Only 1 retrospective, comparative trial exists. Winkel and Bray reported the results of a 24-month follow-up of 240 women with endometriosis and pelvic pain who underwent surgical treatment in the form of excision alone, laser coagulation alone, or laser coagulation plus medical therapy.7 Twelve months after surgery, 96% of excision patients were pain-free compared with 69% of those undergoing coagulation. At 2 years, the corresponding figures were 69% and 23%, respectively. While this seems to indicate that excision is superior to coagulation, the retrospective study design makes such a conclusion suggestive at best. For example, excision may have been used in easier, less risky situations, whereas in difficult cases, only coagulation may have been performed. The resulting success rates would thus reflect the amount of disease discovered, not the type of surgery. To truly clarify this issue, a randomized trial is needed.
Only 40% of patients surgically treated for endometriosis experience pain relief as a direct result of surgery.
As mentioned earlier, numerous weapons have been employed to destroy endometriosis lesions: the CO2, KTP, Nd:YAG, and argon lasers; ultrasonic shears; monopolar electrical energy; and bipolar electrical energy. No comparisons of the efficacy of these instruments have been conducted.
Treating endometriomas. Ovarian cysts are common in the endometriosis patient, and the method by which they are surgically treated may be vital to the outcome. The goals of treating ovarian endometriomas are removing all ectopic endometrium in the ovary, reducing ovarian trauma, preserving follicles, and minimizing postoperative adhesion formation.8
Two types of endometriomas are recognized. The least common is contained entirely within the ovary. More widespread is an inverted anterior ovarian cortex with adhesions and implants on the surface, which is frequently adherent to the broad ligament. The latter type represents more than 90% of ovarian endometriomas.9
Before operating, the ovaries should be freed of all adhesions. The endometrioma may open spontaneously during this process; if not, incision and drainage are warranted. At this point, the cyst wall may be stripped, excised, or drained as indicated. Stripping involves separating the cyst wall from the ovary and slowly peeling them apart. In the Putman-Redwine technique, the opening to the endometrioma is circumscribed with a laser or electrosurgery, followed by dissection down to the cyst wall. The cyst wall and ovary then are separated sharply and bluntly until the cyst wall is removed, frequently intact. Excision also can be accomplished in a manner similar to a wedge resection. However, while removal of the endometrioma is invariably complete with this method, the potential for adhesion formation is higher.10
The vaporization or coagulation of cyst walls also has been described. A randomized trial comparing removal of the cyst wall versus fenestration and aspiration of cyst contents clearly demonstrates improved results with removal, whether the desired outcome is pain relief, fertility, or a lower rate of reoperation.11
The value of closing the ovary after endometrioma removal has been greatly debated, with no consensus among surgeons. Data suggest more adhesions form with suturing of the ovary than without closure, but it is unclear whether this applies to all sizes of defects.12
Ancillary procedures. Adhesiolysis is an important step in the restoration of normal pelvic and abdominal anatomy. While simple lysis of adhesions is adequate if they were formed following infection, most experts believe a more complete approach is required for the endometriosis-induced adhesion. This is because of the relatively high incidence of endometriosis present within the adhesion itself. Thus, removal of the adhesion by lysing both boundaries of the scar tissue and connecting structures is preferable. The instrumentation is of little consequence as long as precision and hemostasis are maintained.
To minimize adhesion reformation, adhesion-prevention adjuvants should be used. Four are presently available, including Interceed (Ethicon Inc, Somerville, NJ), a cellulose based barrier; Seprafilm (Genzyme Corp, Cambridge, Mass), a hyaluronidase-impregnated barrier; Preclude (W.L. Gore and Associates Inc, Newark, Del), a non-absorbable barrier composed of the proprietary Gore-Tex; and Intergel (Lifecore Biomedical Inc, Chaska, Minn), a thick solution placed within the peritoneal cavity that provides widespread adhesion prophylaxis. All adjuvants currently available have been associated with decreased adhesion reformation in randomized clinical trials.13 They can be applied either laparoscopically or abdominally.
Appendectomy. Endometriosis of the appendix has been described in 17% of patients with bowel involvement.14 In all endometriosis surgeries, the appendix should be carefully inspected and removed if it appears abnormal. In patients who experience endometriosis-associated pelvic pain, it may be desirable to electively remove the appendix at the time of laparoscopy. This will prevent future confusion. Otherwise, emergency room physicians would have to differentiate the symptoms of chronic pelvic/abdominal pain from those of appendicitis in the event the patient presents with a complaint of pelvic pain.
Nerve interruption procedures. Two surgical procedures were designed to help reduce pain transmission in the endometriosis patient: uterosacral nerve ablation/resection and presacral neurectomy. Both involve interruption of the major efferent nerve fibers from the uterus, thus diminishing uterine and central pelvic pain. These procedures can be performed via laparoscopy or laparotomy. Unfortunately, neither has been shown to provide pain relief beyond that achieved with surgery directed against the disease itself.15-18
Outcomes
Pain relief. Although there have been many investigations of the effect of endometriosis surgery on pelvic pain, the vast majority are of low quality. No randomized, controlled trials (RCTs) have compared surgery to medical therapy, and only 1 has investigated surgery versus sham surgery. Sutton and colleagues assessed the efficacy of laser laparoscopic surgery in the treatment of pain associated with minimal, mild, or moderate endometriosis.19 They found no difference in pain at 3 months follow-up. However, by 6 months, a clear-cut advantage was seen for surgery (Figure 1). Pain relief also was maintained for at least 1 year in patients undergoing laser surgery. These patients underwent ablation of endometriosis and of the uterosacral nerve (although, as noted earlier, the latter procedure provides no additional pain relief).
Most disconcerting is the finding that only 40% of patients surgically treated for endometriosis experience pain relief as a direct result of the surgery. (The number of patients who need to be treated to reduce pain in 1 patient is 2.5.)
It remains unclear whether excision of the disease will produce better results than coagulation with the CO2 laser. Nor have the duration of pain relief and rate of reoperation been properly examined. Thus, although many surgeons feel quite strongly about the value of surgery to treat endometriosis-associated pain, there are few data to support their clinical impression of efficacy.
Fertility enhancement. Conservative surgery has been used extensively in an attempt to enhance fertility. Unfortunately, most studies on the subject are uncontrolled and of poor quality. In women who have early-stage disease, surgical therapy has resulted in pregnancy rates of 40% to 75%; in severe disease, the rates are lower, ranging from 20% to 50%.20
A meta-analysis encompassing studies from 1982 through 1994 showed that either no treatment or surgery alone is superior to medical treatment for early-stage endometriosis-associated infertility.6
A separate meta-analysis of 25 RCTs and cohort studies examined the same issue.21 It found a possible treatment benefit from laparoscopic conservative surgery, but concluded that medical treatment of the disease was ineffective.
A multicenter randomized trial (ENDOCAN) assessed the value of surgery for early-stage endometriosis in the infertile patient. In this study, 341 infertile women with minimal or mild endometriosis were randomized to diagnostic laparoscopy or resection/ablation of disease.22 They then were followed for 36 weeks. The pregnancy rates were 31% for the treated group and 18% for the diagnostic group, demonstrating a clear advantage to surgery. The data also imply that to achieve 1 additional pregnancy in 9 months, 7.7 women must be surgically treated.
Adding confusion, a second randomized trial—this one from Italy—failed to demonstrate an improvement in fertility among those treated for early-stage endometriosis.23 Nevertheless, when the studies are combined into meta-analysis, surgical treatment of early-stage endometriosis appears to provide a significant improvement in pregnancy rates (odds ratio [OR], 1.66; 95% confidence limits [CL], 1.09-2.51) (Figure 2).
The surgical treatment of endometriosis probably enhances fertility when the disease is advanced, although no high-quality studies specifically address this issue. For early-stage disease, the data are conflicting but suggest that a small effect may exist. Further studies are needed to assess the value of surgery for this disorder.
FIGURE 1 Rate of pain relief with operative versus diagnostic laparoscopy
Adapted from: Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, or moderate endometriosis. Fertil Steril. 1994;62:696.
FIGURE 2 Pregnancy rate with surgical treatment versus diagnostic laparoscopy for early-stage endometriosis
Conclusion
The role of surgery in the treatment of endometriosis is controversial. The procedure can be highly demanding technically and may involve a number of ancillary procedures besides destruction of lesions. Many questions remain unanswered. For example, we do not know the best surgical technique or ancillary procedures, or the best instruments for these procedures. While there appears to be value in treating pain and infertility, that value may be far less than anticipated. Moreover, the relative value of surgery in the treatment of endometriosis—when compared with medical therapy for pain relief or assisted reproduction for fertility enhancement—has yet to be adequately evaluated. Given this level of uncertainty and confusion, surgery should be undertaken with caution and restraint.
Dr. Olive reports that he is a consultant to TAP Pharmaceuticals. He also serves on the company’s speakers’ bureau.
1. Hickman TN, Namnoum AB, Hinton EL, Zacur HA, Rock JA. Timing of estrogen replacement therapy following hysterectomy with oophorectomy for endometriosis. Obstet Gynecol. 1998;91:673-6772
2. Crosignani PG, Vercellini P, Biffignandi F, Constantini W, Cortesi I, Imparato E. Laparoscopy versus laparotomy in conservative surgical treatment for severe endometriosis. Fertil Steril. 1996;66:706-711.
3. Busacca M, Fedele L, Bianchi S, et al. Surgical treatment of recurrent endometriosis: laparotomy versus laparoscopy. Hum Reprod. 1998;13:2271-2274.
4. Catalano GF, Marana R, Caruana P, Muzii L, Mancuso S. Laparoscopy versus microsurgery by laparotomy for excision of ovarian cysts in patients with moderate or severe endometriosis. J Am Assoc Gynecol Laparosc. 1996;3:267-270.
5. Bateman BG, Kolp LA, Mills S. Endoscopic versus laparotomy management of endometriomas. Fertil Steril. 1994;62:690-695.
6. Adamson GD, Pasta DJ. Surgical treatment of endometriosis-associated infertility: meta-analysis compared with survival analysis. Am J Obstet Gynecol. 1994;171:1488.-
7. Winkel CA, Bray M. Treatment of women with endometriosis using excision alone, ablation alone, or ablation in combination with leuprolide acetate. Proceedings of the Fourth World CongresS on Endometriosis, Yokahama, Japan, 1996:55.
8. Guarnaccia MM, Silverberg K, Olive DL. Endometriosis and adenomyosis. In: Copeland LJ, ed. Textbook of Gynecology. Philadelphia: W.B. Saunders; 2000: 687.
9. Hughesdon PE. The structure of endometrial cysts of the ovary. J Obstet Gynaecol British Empire. 1957;44:481-487.
10. Fayes JA, Vogel MF. Comparison of different treatment methods of endometriomas by laparoscopy. Obstet Gynecol. 1991;78:660-665.
11. Beretta P, Franchi M, Ghezzi F. Randomized clinical trial of two laparoscopic treatments of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70:1176-1180.
12. Meyer WR, Grainger DA, DeCherney AH, Lachs MS, Diamond MP. Ovarian surgery on the rabbit. Effect of cortex closure on adhesion formation and ovarian function. J Reprod Med. 1991;36:639-643.
13. Farquhar C, Vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility (Cochrane Review). The Cochrane Library. Issue 1. Oxford: Update Software; 2000.
14. Franklin RR, Grunert GM. Extragenital endometriosis. In: Endometriosis: Advanced Management and Surgical Techniques. New York: Springer-Verlag; 1995;128.-
15. Dover RW, Pooley P, Haines P, Sutton CJG. Prospective, randomized, double-blind controlled trial of laparoscopic laser uterine nerve ablation in the treatment of pelvic pain associated with endometriosis [unpublished].
16. Vercellini P, Aimi G, Busacca M, Uglietti A, Viganali M, Crosignani PG. Laparoscopic uterosacral ligament resection for dysmenorrhea associated with endometriosis. Results of a randomized controlled trial. Fertil Steril. 1997;Oct(Suppl):S3 [abstract].
17. Candiani GB, Fedele L, Vercellini P, Bianchi S, DiNola G. Presacral neurectomy for the treatment of pelvic pain associated with endometriosis: a controlled study. Am J Obstet Gynecol. 1992;167:100-103.
18. Tjaden B, Schlaff WD, Kimball A, Rock JA. The efficacy of presacral neurectomy for the relief of midline dysmenorrhea. Obstet Gynecol. 1990;76:89.-
19. Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, or moderate endometriosis. Fertil Steril. 1994;62:696.-
20. Olive DL. Conservative surgery. In: Schenken RS, ed. Endometriosis: Contemporary Concepts in Clinical Management. Philadelphia: J.B. Lippincott; 1989;213.-
21. Hughes EG, Fedorkow DM, Collins JA. A quantitative overview of controlled trials in endometriosis associated infertility. Fertil Steril. 1993;59:963.-
22. Marcoux S, Maheux R, Berube S. Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group in Endometriosis. N Engl J Med. 1997;337:217-222.
23. Gruppo Italiano per lo Studio dell Endometriosis. Ablation of lesions or no treatment in minimal-mild endometriosis in infertile women: a randomized trial. Hum Reprod. 1999;14:1332-1334.
1. Hickman TN, Namnoum AB, Hinton EL, Zacur HA, Rock JA. Timing of estrogen replacement therapy following hysterectomy with oophorectomy for endometriosis. Obstet Gynecol. 1998;91:673-6772
2. Crosignani PG, Vercellini P, Biffignandi F, Constantini W, Cortesi I, Imparato E. Laparoscopy versus laparotomy in conservative surgical treatment for severe endometriosis. Fertil Steril. 1996;66:706-711.
3. Busacca M, Fedele L, Bianchi S, et al. Surgical treatment of recurrent endometriosis: laparotomy versus laparoscopy. Hum Reprod. 1998;13:2271-2274.
4. Catalano GF, Marana R, Caruana P, Muzii L, Mancuso S. Laparoscopy versus microsurgery by laparotomy for excision of ovarian cysts in patients with moderate or severe endometriosis. J Am Assoc Gynecol Laparosc. 1996;3:267-270.
5. Bateman BG, Kolp LA, Mills S. Endoscopic versus laparotomy management of endometriomas. Fertil Steril. 1994;62:690-695.
6. Adamson GD, Pasta DJ. Surgical treatment of endometriosis-associated infertility: meta-analysis compared with survival analysis. Am J Obstet Gynecol. 1994;171:1488.-
7. Winkel CA, Bray M. Treatment of women with endometriosis using excision alone, ablation alone, or ablation in combination with leuprolide acetate. Proceedings of the Fourth World CongresS on Endometriosis, Yokahama, Japan, 1996:55.
8. Guarnaccia MM, Silverberg K, Olive DL. Endometriosis and adenomyosis. In: Copeland LJ, ed. Textbook of Gynecology. Philadelphia: W.B. Saunders; 2000: 687.
9. Hughesdon PE. The structure of endometrial cysts of the ovary. J Obstet Gynaecol British Empire. 1957;44:481-487.
10. Fayes JA, Vogel MF. Comparison of different treatment methods of endometriomas by laparoscopy. Obstet Gynecol. 1991;78:660-665.
11. Beretta P, Franchi M, Ghezzi F. Randomized clinical trial of two laparoscopic treatments of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70:1176-1180.
12. Meyer WR, Grainger DA, DeCherney AH, Lachs MS, Diamond MP. Ovarian surgery on the rabbit. Effect of cortex closure on adhesion formation and ovarian function. J Reprod Med. 1991;36:639-643.
13. Farquhar C, Vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility (Cochrane Review). The Cochrane Library. Issue 1. Oxford: Update Software; 2000.
14. Franklin RR, Grunert GM. Extragenital endometriosis. In: Endometriosis: Advanced Management and Surgical Techniques. New York: Springer-Verlag; 1995;128.-
15. Dover RW, Pooley P, Haines P, Sutton CJG. Prospective, randomized, double-blind controlled trial of laparoscopic laser uterine nerve ablation in the treatment of pelvic pain associated with endometriosis [unpublished].
16. Vercellini P, Aimi G, Busacca M, Uglietti A, Viganali M, Crosignani PG. Laparoscopic uterosacral ligament resection for dysmenorrhea associated with endometriosis. Results of a randomized controlled trial. Fertil Steril. 1997;Oct(Suppl):S3 [abstract].
17. Candiani GB, Fedele L, Vercellini P, Bianchi S, DiNola G. Presacral neurectomy for the treatment of pelvic pain associated with endometriosis: a controlled study. Am J Obstet Gynecol. 1992;167:100-103.
18. Tjaden B, Schlaff WD, Kimball A, Rock JA. The efficacy of presacral neurectomy for the relief of midline dysmenorrhea. Obstet Gynecol. 1990;76:89.-
19. Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, or moderate endometriosis. Fertil Steril. 1994;62:696.-
20. Olive DL. Conservative surgery. In: Schenken RS, ed. Endometriosis: Contemporary Concepts in Clinical Management. Philadelphia: J.B. Lippincott; 1989;213.-
21. Hughes EG, Fedorkow DM, Collins JA. A quantitative overview of controlled trials in endometriosis associated infertility. Fertil Steril. 1993;59:963.-
22. Marcoux S, Maheux R, Berube S. Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group in Endometriosis. N Engl J Med. 1997;337:217-222.
23. Gruppo Italiano per lo Studio dell Endometriosis. Ablation of lesions or no treatment in minimal-mild endometriosis in infertile women: a randomized trial. Hum Reprod. 1999;14:1332-1334.