User login
Nurses questions answered: Could you face repercussions for your actions?
Nurses are the most trusted profession for one reason. They care.
Nurses are passionate about patient interactions, quality, and giving optimal support, often to the detriment of self-care. Many do not hesitate to voice concerns in an atmosphere that produces anxiety, whether it be regarding supplies, documentation, or staffing. As a result,
In October, three nurses at Ascension Saint Joseph in Joliet, Ill., were escorted off the premises of a hospital emergency room when they began an understaffed shift. They were removed from work by hospital security and then suspended for 1 week. It was a decision that was incomprehensible, because the emergency room faced an overwhelming influx of patients – 46 that evening alone – and only four nurses instead of the more than 10 approved staffing were on duty. Why were they suspended?
Hospital officials have been quiet in responding to their alarmed community as well as in answering the Illinois Nurses Association, who criticized the hospital’s response. It has been suggested that the nurses had been intensely vocal about staffing for several weeks and the hospital might have wanted to silence their voices.
In my opinion, this could be considered a professional repercussion of the post-pandemic work environment. Though the nurses were reinstated after the week expired, nursing organizations believed that the actions of their employer were too harsh.
There was a similar response by employers after a string of large strikes of California nurses earlier this year. For example, a walkout by nurses at Stanford Health Care and Lucile Packard Children’s Hospital resulted in the hospitals withholding wages during the strike period and stating they might withhold health coverage from striking workers.
“Our sincere hope is that an agreement can be reached promptly so that nurses don’t lose additional pay, don’t risk losing the subsidy for employer-paid health benefits, and can return to patient care,” the hospital newsletter StanfordPackardVoice.com reported before the strike began in April. “Nurses who choose to go out on strike will not paid for missed shifts and cannot use PTO, ESL, or Education Hours.”
Nurse: Could a similar repercussion be in my work future?
Nurses may take to picket lines or contact administrators (for example, Human Resources) for multiple reasons, but the most common issues are related to staffing, scheduling, mandatory overtime, or equipment required to do their job: safety or lifting equipment and broken or missing tools for monitoring patients. The lack of hospital security services to assist with violent or threatening patients has also become a concern.
Goodman: The inability to provide safe care is a common fear of all nurses, one that was exacerbated by health care workers leaving during the pandemic, primarily from nursing homes. Although overall safety has improved, that may not be the case in smaller, rural institutions. Staffing for all shifts may also be erratic as the country faces an uphill winter battle with influenza, respiratory syncytial virus, and newer COVID variants.
Report to your supervisor: First, be familiar with your institution’s policy regarding chain of command. Know where to take a complaint when staffing seems unsafe. Contact your immediate supervisor as soon as the situation has been assessed. They might be able to shift resources to your area or find coverage to help. In addition, keep accurate notes related to your actions.
I covered a night shift where I was directly responsible for the care of 13 subacute medical-surgical patients (new admissions and postoperative patients). Patients kept arriving with no regard for the load that was present. One of the patients was completely unhappy with her pain regimen and kept calling for assistance, as is often the case.
While I was doing my best to assess arrivals, another nurse contacted a supervisor. The next thing that happened was an on-site visit by hospital administrators (unusual!) who asked to see my assignment sheet. I had been hesitant to share the list, fearing recrimination from intermediate leadership (this was not my home unit). But it led to an immediate change in staffing. The ordeal ended amicably, but not all do. Thereafter, no nurse was expected to care for more than eight patients on the night shift.
Notify proper authorities: Nurses may believe contacting the Occupational Safety and Health Administration (OSHA) might be helpful; however, OSHA may not have jurisdiction over the hospital, as the Saint Joseph nurses discovered. Working without safety equipment or with reduced supplies (for example, automatic blood pressure cuffs, oxygen saturation monitors, isolation gear) may appear to be a federal complaint, but it depends where the nurse is employed. The hospital in Joliet was covered by the Illinois Department of Public Health.
Federal law entitles you to work in a safe place. Contacting OSHA for direction should not lead to recrimination for nurses. Although OSHA has been overwhelmed with complaints since the onset of the pandemic, their website directs nurses. For example, a whistleblower complaint can be filed up to 30 days after an incident of worker retaliation.
If you are a member of a nursing union, follow union guidelines related to your actions. Thousands of nurses went on strike in the past 2 years. Most remained employed and returned to work with negotiations complete. As far as the nurses in Massachusetts, the state does not have mandatory staffing ratios – most do not – which complicated contract negotiations. At this time, California is the only state that has mandatory nurse-patient ratios written into law.
It is also important to know state law and to be cognizant of nursing organizations within your geographic area. Staying connected means staying informed and having nursing resources.
Respond rationally: An additional reminder for nurses is not to react to a tense situation impulsively. Leaving an assignment unfinished or walking off the job is never a good idea. (A scheduled strike organized by union leaders is different). Leaving work is viewed by institutions as job abandonment and can be grounds for dismissal. Most states are currently “at will” employers, meaning hospitals can terminate nurses without due process.
Above all, know that nurses worry about providing safe practice and avoiding recrimination. Only one of these should be in your future.
Ms. Goodman has no disclosures.
A version of this article first appeared on Medscape.com.
Nurses are the most trusted profession for one reason. They care.
Nurses are passionate about patient interactions, quality, and giving optimal support, often to the detriment of self-care. Many do not hesitate to voice concerns in an atmosphere that produces anxiety, whether it be regarding supplies, documentation, or staffing. As a result,
In October, three nurses at Ascension Saint Joseph in Joliet, Ill., were escorted off the premises of a hospital emergency room when they began an understaffed shift. They were removed from work by hospital security and then suspended for 1 week. It was a decision that was incomprehensible, because the emergency room faced an overwhelming influx of patients – 46 that evening alone – and only four nurses instead of the more than 10 approved staffing were on duty. Why were they suspended?
Hospital officials have been quiet in responding to their alarmed community as well as in answering the Illinois Nurses Association, who criticized the hospital’s response. It has been suggested that the nurses had been intensely vocal about staffing for several weeks and the hospital might have wanted to silence their voices.
In my opinion, this could be considered a professional repercussion of the post-pandemic work environment. Though the nurses were reinstated after the week expired, nursing organizations believed that the actions of their employer were too harsh.
There was a similar response by employers after a string of large strikes of California nurses earlier this year. For example, a walkout by nurses at Stanford Health Care and Lucile Packard Children’s Hospital resulted in the hospitals withholding wages during the strike period and stating they might withhold health coverage from striking workers.
“Our sincere hope is that an agreement can be reached promptly so that nurses don’t lose additional pay, don’t risk losing the subsidy for employer-paid health benefits, and can return to patient care,” the hospital newsletter StanfordPackardVoice.com reported before the strike began in April. “Nurses who choose to go out on strike will not paid for missed shifts and cannot use PTO, ESL, or Education Hours.”
Nurse: Could a similar repercussion be in my work future?
Nurses may take to picket lines or contact administrators (for example, Human Resources) for multiple reasons, but the most common issues are related to staffing, scheduling, mandatory overtime, or equipment required to do their job: safety or lifting equipment and broken or missing tools for monitoring patients. The lack of hospital security services to assist with violent or threatening patients has also become a concern.
Goodman: The inability to provide safe care is a common fear of all nurses, one that was exacerbated by health care workers leaving during the pandemic, primarily from nursing homes. Although overall safety has improved, that may not be the case in smaller, rural institutions. Staffing for all shifts may also be erratic as the country faces an uphill winter battle with influenza, respiratory syncytial virus, and newer COVID variants.
Report to your supervisor: First, be familiar with your institution’s policy regarding chain of command. Know where to take a complaint when staffing seems unsafe. Contact your immediate supervisor as soon as the situation has been assessed. They might be able to shift resources to your area or find coverage to help. In addition, keep accurate notes related to your actions.
I covered a night shift where I was directly responsible for the care of 13 subacute medical-surgical patients (new admissions and postoperative patients). Patients kept arriving with no regard for the load that was present. One of the patients was completely unhappy with her pain regimen and kept calling for assistance, as is often the case.
While I was doing my best to assess arrivals, another nurse contacted a supervisor. The next thing that happened was an on-site visit by hospital administrators (unusual!) who asked to see my assignment sheet. I had been hesitant to share the list, fearing recrimination from intermediate leadership (this was not my home unit). But it led to an immediate change in staffing. The ordeal ended amicably, but not all do. Thereafter, no nurse was expected to care for more than eight patients on the night shift.
Notify proper authorities: Nurses may believe contacting the Occupational Safety and Health Administration (OSHA) might be helpful; however, OSHA may not have jurisdiction over the hospital, as the Saint Joseph nurses discovered. Working without safety equipment or with reduced supplies (for example, automatic blood pressure cuffs, oxygen saturation monitors, isolation gear) may appear to be a federal complaint, but it depends where the nurse is employed. The hospital in Joliet was covered by the Illinois Department of Public Health.
Federal law entitles you to work in a safe place. Contacting OSHA for direction should not lead to recrimination for nurses. Although OSHA has been overwhelmed with complaints since the onset of the pandemic, their website directs nurses. For example, a whistleblower complaint can be filed up to 30 days after an incident of worker retaliation.
If you are a member of a nursing union, follow union guidelines related to your actions. Thousands of nurses went on strike in the past 2 years. Most remained employed and returned to work with negotiations complete. As far as the nurses in Massachusetts, the state does not have mandatory staffing ratios – most do not – which complicated contract negotiations. At this time, California is the only state that has mandatory nurse-patient ratios written into law.
It is also important to know state law and to be cognizant of nursing organizations within your geographic area. Staying connected means staying informed and having nursing resources.
Respond rationally: An additional reminder for nurses is not to react to a tense situation impulsively. Leaving an assignment unfinished or walking off the job is never a good idea. (A scheduled strike organized by union leaders is different). Leaving work is viewed by institutions as job abandonment and can be grounds for dismissal. Most states are currently “at will” employers, meaning hospitals can terminate nurses without due process.
Above all, know that nurses worry about providing safe practice and avoiding recrimination. Only one of these should be in your future.
Ms. Goodman has no disclosures.
A version of this article first appeared on Medscape.com.
Nurses are the most trusted profession for one reason. They care.
Nurses are passionate about patient interactions, quality, and giving optimal support, often to the detriment of self-care. Many do not hesitate to voice concerns in an atmosphere that produces anxiety, whether it be regarding supplies, documentation, or staffing. As a result,
In October, three nurses at Ascension Saint Joseph in Joliet, Ill., were escorted off the premises of a hospital emergency room when they began an understaffed shift. They were removed from work by hospital security and then suspended for 1 week. It was a decision that was incomprehensible, because the emergency room faced an overwhelming influx of patients – 46 that evening alone – and only four nurses instead of the more than 10 approved staffing were on duty. Why were they suspended?
Hospital officials have been quiet in responding to their alarmed community as well as in answering the Illinois Nurses Association, who criticized the hospital’s response. It has been suggested that the nurses had been intensely vocal about staffing for several weeks and the hospital might have wanted to silence their voices.
In my opinion, this could be considered a professional repercussion of the post-pandemic work environment. Though the nurses were reinstated after the week expired, nursing organizations believed that the actions of their employer were too harsh.
There was a similar response by employers after a string of large strikes of California nurses earlier this year. For example, a walkout by nurses at Stanford Health Care and Lucile Packard Children’s Hospital resulted in the hospitals withholding wages during the strike period and stating they might withhold health coverage from striking workers.
“Our sincere hope is that an agreement can be reached promptly so that nurses don’t lose additional pay, don’t risk losing the subsidy for employer-paid health benefits, and can return to patient care,” the hospital newsletter StanfordPackardVoice.com reported before the strike began in April. “Nurses who choose to go out on strike will not paid for missed shifts and cannot use PTO, ESL, or Education Hours.”
Nurse: Could a similar repercussion be in my work future?
Nurses may take to picket lines or contact administrators (for example, Human Resources) for multiple reasons, but the most common issues are related to staffing, scheduling, mandatory overtime, or equipment required to do their job: safety or lifting equipment and broken or missing tools for monitoring patients. The lack of hospital security services to assist with violent or threatening patients has also become a concern.
Goodman: The inability to provide safe care is a common fear of all nurses, one that was exacerbated by health care workers leaving during the pandemic, primarily from nursing homes. Although overall safety has improved, that may not be the case in smaller, rural institutions. Staffing for all shifts may also be erratic as the country faces an uphill winter battle with influenza, respiratory syncytial virus, and newer COVID variants.
Report to your supervisor: First, be familiar with your institution’s policy regarding chain of command. Know where to take a complaint when staffing seems unsafe. Contact your immediate supervisor as soon as the situation has been assessed. They might be able to shift resources to your area or find coverage to help. In addition, keep accurate notes related to your actions.
I covered a night shift where I was directly responsible for the care of 13 subacute medical-surgical patients (new admissions and postoperative patients). Patients kept arriving with no regard for the load that was present. One of the patients was completely unhappy with her pain regimen and kept calling for assistance, as is often the case.
While I was doing my best to assess arrivals, another nurse contacted a supervisor. The next thing that happened was an on-site visit by hospital administrators (unusual!) who asked to see my assignment sheet. I had been hesitant to share the list, fearing recrimination from intermediate leadership (this was not my home unit). But it led to an immediate change in staffing. The ordeal ended amicably, but not all do. Thereafter, no nurse was expected to care for more than eight patients on the night shift.
Notify proper authorities: Nurses may believe contacting the Occupational Safety and Health Administration (OSHA) might be helpful; however, OSHA may not have jurisdiction over the hospital, as the Saint Joseph nurses discovered. Working without safety equipment or with reduced supplies (for example, automatic blood pressure cuffs, oxygen saturation monitors, isolation gear) may appear to be a federal complaint, but it depends where the nurse is employed. The hospital in Joliet was covered by the Illinois Department of Public Health.
Federal law entitles you to work in a safe place. Contacting OSHA for direction should not lead to recrimination for nurses. Although OSHA has been overwhelmed with complaints since the onset of the pandemic, their website directs nurses. For example, a whistleblower complaint can be filed up to 30 days after an incident of worker retaliation.
If you are a member of a nursing union, follow union guidelines related to your actions. Thousands of nurses went on strike in the past 2 years. Most remained employed and returned to work with negotiations complete. As far as the nurses in Massachusetts, the state does not have mandatory staffing ratios – most do not – which complicated contract negotiations. At this time, California is the only state that has mandatory nurse-patient ratios written into law.
It is also important to know state law and to be cognizant of nursing organizations within your geographic area. Staying connected means staying informed and having nursing resources.
Respond rationally: An additional reminder for nurses is not to react to a tense situation impulsively. Leaving an assignment unfinished or walking off the job is never a good idea. (A scheduled strike organized by union leaders is different). Leaving work is viewed by institutions as job abandonment and can be grounds for dismissal. Most states are currently “at will” employers, meaning hospitals can terminate nurses without due process.
Above all, know that nurses worry about providing safe practice and avoiding recrimination. Only one of these should be in your future.
Ms. Goodman has no disclosures.
A version of this article first appeared on Medscape.com.
Stroke management: A 30-year retrospective
In 1993, managing patients with stroke had long remained an elusive and somewhat intimidating task for the neurological world. Previous efforts to treat the condition had produced more frustration than success, leaving clinicians and patients alike in despair for a solution. However, some successes in treating coronary thrombosis during that era rejuvenated researchers’ efforts to crack the code. An international team of researchers had studied a Streptococcus derivative (streptokinase) and others had begun to study a natural substance termed tissue plasminogen activator (tPA) as thrombolytic agents to lyse coronary clots and to treat pulmonary embolism. The adverse event of excessive bleeding found in Australian studies done on streptokinase intervention in patients with stroke prompted researchers to contemplate use of tPA in stroke management.
A group of German, Japanese, and American investigators began to research thrombolysis in acute stroke patients during the mid-1980s.
“What was unique is that patients had a CT scan followed by a catheter angiogram,” said Louis Caplan, MD, a senior member of the division of cerebrovascular disease at Beth Israel Deaconess Medical Center, Boston, professor of neurology at Harvard Medical School, Boston, and founder of the Harvard Stroke Registry at Beth Israel Deaconess Medical Center.
“If they had a blocked vessel, they got the drug, delivered either intravenously or intra-arterially.”
The process involved keeping the catheter open after drug administration to determine whether the vessel had opened or remained occluded. The researchers learned which blocked vessels opened when the drug was given intravenously and which required direct introduction of the drug into the clots.
A group of investigators in the United States funded by the National Institute of Neurological Disease and Stroke then performed a randomized therapeutic trial of intravenous tPA given within 90 minutes and 180 minutes after stroke symptom onset. The study was reported in the New England Journal of Medicine. Soon thereafter, in 1995, the Food and Drug Administration approved the use of tPA following the inclusion and exclusion rules used in the NINDS trial.
After the FDA approved tPA in 1995, stroke management was never the same.
tPA was just one factor in optimizing stroke management
Despite the major therapeutic breakthrough with tPA’s approval, it took the clinics, hospitals, and other acute care systems a while to catch up. “Neurologists and hospitals weren’t ready for acute stroke intervention and proper stroke management in the mid-90s,” Dr. Caplan recalled. “At the time, stroke wasn’t at the forefront of treatment, general neurologists weren’t trained, and there weren’t enough stroke neurologists.”
The preparation and training deficit was further exacerbated by low reimbursement for services. As a result, only about 5% of patients who were eligible for acute stroke management were treated with tPA.
According to Dr. Caplan, during the next 15-20 years, the accumulation of stroke data from MRI and CT vascular imaging clarified further which patients, with what extent of infarction, with which blocked vessels, would be good candidates for treatment.
More patients received interventional treatment using catheters directed into the area of clotting in attempt to remove the blockages. In addition, information regarding intervention at different periods (10-16 hours, up to 24 hours) and conditions (for example, patients with varying degrees of disability, infarct) were tested.
Eventually, hospitals became more attuned to emergency stroke treatment. More neurologists became trained, more stroke centers emerged, and clinicians enjoyed the benefit of technological advancements that allowed them to explore perfusion.
While decentralized care enhances outcomes in stroke management, more progress is needed
As of early 2023, stroke is one of the leading emergency diagnoses, and patients have access to primary and secondary stroke centers that are sprinkled throughout the United States. As impressive as the feat may seem, health care systems still have major strides to make to truly optimize therapy and outcomes in this patient population.
For example, location and access remain important issues. Secondary centers are typically located in large, metropolitan areas. While an urban location makes a primary center geographically more accessible to a larger patient population, traffic frequently hinders door-to-door access.
In the case of rural centers, distance can retard access, but they also face the challenges of how to route patients – especially patients who require more specialized care offered by secondary centers. Fortunately, primary centers have some ways to help better support their patients.
“One thing that happened is that primary centers made agreements with secondary centers via telemedicine to determine whether patients should be treated at the primary center or whether they should be routed to the higher-level center. These arrangements were termed ‘spoke and wheel,’ ” Dr. Caplan told this publication.
However, not all patients who are candidates for transport to a secondary center are able to be transported. In such cases, primary centers can use telemedicine to collaborate with secondary centers for support.
Logistics aside, perhaps today’s greatest challenge for clinicians is ensuring their patients and families receive education to increase their awareness of stroke centers as an important option for treatment and outcome optimization. Many patients and their loved ones do not realize that these centers exist or how to utilize them if and when the time comes.
Right now, some cities have stroke ambulances staffed with physicians to treat patients in the field. This decentralized model helps address access burdens such as door-to-needle delays and transportation while improving survival and recovery. Dr. Caplan said these services are available in Munich, and in a few select U.S. cities such as Cleveland and Houston, which helped pioneer the concept.
Better access in the future?
Looking ahead, Dr. Caplan seems optimistic about how stroke management will continue to evolve. Many cities will have stroke ambulances to provide on-site care, while stroke institutions will improve their cross-collaborative efforts to support their patient populations.
At the crux of cross-collaboration lies enhanced communication between peripheral and urban hospitals.
“Peripheral and urban hospitals and state organizations will engage in smoother integration to figure out when to take patient to the bigger hospitals,” Dr. Caplan said. “I also believe we will see greater emphasis on rehabilitation and recovery.”
As promising as the future looks, only time will tell.
In 1993, managing patients with stroke had long remained an elusive and somewhat intimidating task for the neurological world. Previous efforts to treat the condition had produced more frustration than success, leaving clinicians and patients alike in despair for a solution. However, some successes in treating coronary thrombosis during that era rejuvenated researchers’ efforts to crack the code. An international team of researchers had studied a Streptococcus derivative (streptokinase) and others had begun to study a natural substance termed tissue plasminogen activator (tPA) as thrombolytic agents to lyse coronary clots and to treat pulmonary embolism. The adverse event of excessive bleeding found in Australian studies done on streptokinase intervention in patients with stroke prompted researchers to contemplate use of tPA in stroke management.
A group of German, Japanese, and American investigators began to research thrombolysis in acute stroke patients during the mid-1980s.
“What was unique is that patients had a CT scan followed by a catheter angiogram,” said Louis Caplan, MD, a senior member of the division of cerebrovascular disease at Beth Israel Deaconess Medical Center, Boston, professor of neurology at Harvard Medical School, Boston, and founder of the Harvard Stroke Registry at Beth Israel Deaconess Medical Center.
“If they had a blocked vessel, they got the drug, delivered either intravenously or intra-arterially.”
The process involved keeping the catheter open after drug administration to determine whether the vessel had opened or remained occluded. The researchers learned which blocked vessels opened when the drug was given intravenously and which required direct introduction of the drug into the clots.
A group of investigators in the United States funded by the National Institute of Neurological Disease and Stroke then performed a randomized therapeutic trial of intravenous tPA given within 90 minutes and 180 minutes after stroke symptom onset. The study was reported in the New England Journal of Medicine. Soon thereafter, in 1995, the Food and Drug Administration approved the use of tPA following the inclusion and exclusion rules used in the NINDS trial.
After the FDA approved tPA in 1995, stroke management was never the same.
tPA was just one factor in optimizing stroke management
Despite the major therapeutic breakthrough with tPA’s approval, it took the clinics, hospitals, and other acute care systems a while to catch up. “Neurologists and hospitals weren’t ready for acute stroke intervention and proper stroke management in the mid-90s,” Dr. Caplan recalled. “At the time, stroke wasn’t at the forefront of treatment, general neurologists weren’t trained, and there weren’t enough stroke neurologists.”
The preparation and training deficit was further exacerbated by low reimbursement for services. As a result, only about 5% of patients who were eligible for acute stroke management were treated with tPA.
According to Dr. Caplan, during the next 15-20 years, the accumulation of stroke data from MRI and CT vascular imaging clarified further which patients, with what extent of infarction, with which blocked vessels, would be good candidates for treatment.
More patients received interventional treatment using catheters directed into the area of clotting in attempt to remove the blockages. In addition, information regarding intervention at different periods (10-16 hours, up to 24 hours) and conditions (for example, patients with varying degrees of disability, infarct) were tested.
Eventually, hospitals became more attuned to emergency stroke treatment. More neurologists became trained, more stroke centers emerged, and clinicians enjoyed the benefit of technological advancements that allowed them to explore perfusion.
While decentralized care enhances outcomes in stroke management, more progress is needed
As of early 2023, stroke is one of the leading emergency diagnoses, and patients have access to primary and secondary stroke centers that are sprinkled throughout the United States. As impressive as the feat may seem, health care systems still have major strides to make to truly optimize therapy and outcomes in this patient population.
For example, location and access remain important issues. Secondary centers are typically located in large, metropolitan areas. While an urban location makes a primary center geographically more accessible to a larger patient population, traffic frequently hinders door-to-door access.
In the case of rural centers, distance can retard access, but they also face the challenges of how to route patients – especially patients who require more specialized care offered by secondary centers. Fortunately, primary centers have some ways to help better support their patients.
“One thing that happened is that primary centers made agreements with secondary centers via telemedicine to determine whether patients should be treated at the primary center or whether they should be routed to the higher-level center. These arrangements were termed ‘spoke and wheel,’ ” Dr. Caplan told this publication.
However, not all patients who are candidates for transport to a secondary center are able to be transported. In such cases, primary centers can use telemedicine to collaborate with secondary centers for support.
Logistics aside, perhaps today’s greatest challenge for clinicians is ensuring their patients and families receive education to increase their awareness of stroke centers as an important option for treatment and outcome optimization. Many patients and their loved ones do not realize that these centers exist or how to utilize them if and when the time comes.
Right now, some cities have stroke ambulances staffed with physicians to treat patients in the field. This decentralized model helps address access burdens such as door-to-needle delays and transportation while improving survival and recovery. Dr. Caplan said these services are available in Munich, and in a few select U.S. cities such as Cleveland and Houston, which helped pioneer the concept.
Better access in the future?
Looking ahead, Dr. Caplan seems optimistic about how stroke management will continue to evolve. Many cities will have stroke ambulances to provide on-site care, while stroke institutions will improve their cross-collaborative efforts to support their patient populations.
At the crux of cross-collaboration lies enhanced communication between peripheral and urban hospitals.
“Peripheral and urban hospitals and state organizations will engage in smoother integration to figure out when to take patient to the bigger hospitals,” Dr. Caplan said. “I also believe we will see greater emphasis on rehabilitation and recovery.”
As promising as the future looks, only time will tell.
In 1993, managing patients with stroke had long remained an elusive and somewhat intimidating task for the neurological world. Previous efforts to treat the condition had produced more frustration than success, leaving clinicians and patients alike in despair for a solution. However, some successes in treating coronary thrombosis during that era rejuvenated researchers’ efforts to crack the code. An international team of researchers had studied a Streptococcus derivative (streptokinase) and others had begun to study a natural substance termed tissue plasminogen activator (tPA) as thrombolytic agents to lyse coronary clots and to treat pulmonary embolism. The adverse event of excessive bleeding found in Australian studies done on streptokinase intervention in patients with stroke prompted researchers to contemplate use of tPA in stroke management.
A group of German, Japanese, and American investigators began to research thrombolysis in acute stroke patients during the mid-1980s.
“What was unique is that patients had a CT scan followed by a catheter angiogram,” said Louis Caplan, MD, a senior member of the division of cerebrovascular disease at Beth Israel Deaconess Medical Center, Boston, professor of neurology at Harvard Medical School, Boston, and founder of the Harvard Stroke Registry at Beth Israel Deaconess Medical Center.
“If they had a blocked vessel, they got the drug, delivered either intravenously or intra-arterially.”
The process involved keeping the catheter open after drug administration to determine whether the vessel had opened or remained occluded. The researchers learned which blocked vessels opened when the drug was given intravenously and which required direct introduction of the drug into the clots.
A group of investigators in the United States funded by the National Institute of Neurological Disease and Stroke then performed a randomized therapeutic trial of intravenous tPA given within 90 minutes and 180 minutes after stroke symptom onset. The study was reported in the New England Journal of Medicine. Soon thereafter, in 1995, the Food and Drug Administration approved the use of tPA following the inclusion and exclusion rules used in the NINDS trial.
After the FDA approved tPA in 1995, stroke management was never the same.
tPA was just one factor in optimizing stroke management
Despite the major therapeutic breakthrough with tPA’s approval, it took the clinics, hospitals, and other acute care systems a while to catch up. “Neurologists and hospitals weren’t ready for acute stroke intervention and proper stroke management in the mid-90s,” Dr. Caplan recalled. “At the time, stroke wasn’t at the forefront of treatment, general neurologists weren’t trained, and there weren’t enough stroke neurologists.”
The preparation and training deficit was further exacerbated by low reimbursement for services. As a result, only about 5% of patients who were eligible for acute stroke management were treated with tPA.
According to Dr. Caplan, during the next 15-20 years, the accumulation of stroke data from MRI and CT vascular imaging clarified further which patients, with what extent of infarction, with which blocked vessels, would be good candidates for treatment.
More patients received interventional treatment using catheters directed into the area of clotting in attempt to remove the blockages. In addition, information regarding intervention at different periods (10-16 hours, up to 24 hours) and conditions (for example, patients with varying degrees of disability, infarct) were tested.
Eventually, hospitals became more attuned to emergency stroke treatment. More neurologists became trained, more stroke centers emerged, and clinicians enjoyed the benefit of technological advancements that allowed them to explore perfusion.
While decentralized care enhances outcomes in stroke management, more progress is needed
As of early 2023, stroke is one of the leading emergency diagnoses, and patients have access to primary and secondary stroke centers that are sprinkled throughout the United States. As impressive as the feat may seem, health care systems still have major strides to make to truly optimize therapy and outcomes in this patient population.
For example, location and access remain important issues. Secondary centers are typically located in large, metropolitan areas. While an urban location makes a primary center geographically more accessible to a larger patient population, traffic frequently hinders door-to-door access.
In the case of rural centers, distance can retard access, but they also face the challenges of how to route patients – especially patients who require more specialized care offered by secondary centers. Fortunately, primary centers have some ways to help better support their patients.
“One thing that happened is that primary centers made agreements with secondary centers via telemedicine to determine whether patients should be treated at the primary center or whether they should be routed to the higher-level center. These arrangements were termed ‘spoke and wheel,’ ” Dr. Caplan told this publication.
However, not all patients who are candidates for transport to a secondary center are able to be transported. In such cases, primary centers can use telemedicine to collaborate with secondary centers for support.
Logistics aside, perhaps today’s greatest challenge for clinicians is ensuring their patients and families receive education to increase their awareness of stroke centers as an important option for treatment and outcome optimization. Many patients and their loved ones do not realize that these centers exist or how to utilize them if and when the time comes.
Right now, some cities have stroke ambulances staffed with physicians to treat patients in the field. This decentralized model helps address access burdens such as door-to-needle delays and transportation while improving survival and recovery. Dr. Caplan said these services are available in Munich, and in a few select U.S. cities such as Cleveland and Houston, which helped pioneer the concept.
Better access in the future?
Looking ahead, Dr. Caplan seems optimistic about how stroke management will continue to evolve. Many cities will have stroke ambulances to provide on-site care, while stroke institutions will improve their cross-collaborative efforts to support their patient populations.
At the crux of cross-collaboration lies enhanced communication between peripheral and urban hospitals.
“Peripheral and urban hospitals and state organizations will engage in smoother integration to figure out when to take patient to the bigger hospitals,” Dr. Caplan said. “I also believe we will see greater emphasis on rehabilitation and recovery.”
As promising as the future looks, only time will tell.
Eliminating the language of blame in lung cancer
“Do you smoke?” I asked the patient.
“Yes, and I got what I deserved,” he answered, clearly upset.
I ignored his reaction and continued with the exam, but in retrospect, I should have explained why doctors ask patients this question.
It was not my intention to be rude or blame the patient for his lung cancer diagnosis. Doctors ask patients if they smoke because a smoking history can change the type of treatment and it can be associated with other conditions that may interfere with treatment. It can also determine whether smoking cessation assistance should be offered to the patient. It is crucial that we as doctors know a patient’s medical history, but how we approach sensitive issues may determine if we even get the information we need. In this case, I didn’t explain why I asked the patient if he smoked. Had I taken the time to explain why I needed to know if and how long he smoked and that I was not blaming him for his lung cancer diagnosis, we may have had a more mutually respectful and beneficial relationship.
Almost all of my patients with lung cancer have been asked at one time or another – by a health care provider, friends, or acquaintances – “Do you smoke?” Whether or not they smoked, patients with lung cancer feel the weight of moral judgment being cast upon them by society.
It is common for people who smoke and who go on to develop lung cancer to be weighed down by guilt associated with their diagnosis. Patients with lung cancer face stigma-associated hurdles based on the “I did it to myself” mindset. This societal stigma is not without harm as it can result in emotional responses of guilt and self-blame. This internalized stigma may lead to psychosocial distress and decreased interactions with family, friends, and health care providers. The guilt may drive a patient to forgo lung cancer screening, minimize symptoms, delay seeking treatment, and not advocate for themselves with their physician. Some patients even decide to forgo all treatment.
What about patients who never smoked? They too feel tinged with blame. Many of these patients feel called upon to defend themselves by proclaiming loudly that they have never smoked.
Blame and shame also divides the lung cancer community, resulting in less advocacy. It may also impact research dollars for lung cancer. According to the Lung Cancer Research Foundation, “Despite being the leading cause of cancer mortality, lung cancer receives far less research funding than any other cancer.” By comparison, women with breast cancer are showered with far more resources, supportive services, fundraising events, and certainly more lobbying.
By making unintentional hurtful statements and using judgmental or denigrating language, the lung cancer community may unconsciously be playing a role in perpetuating stigmas associated with lung cancer. That kind of language can come across as blame.
The International Association for the Study of Lung Cancer has developed a language guide to help reduce stigma associated with lung cancer. The aim is to reduce and replace traditional medical language during our patient interactions, presentations, and publications with language that is more empathic and nonjudgmental.
For example, replace the term “cancer patient” with the term “the patient with cancer.” The patient is a person who happens to have been diagnosed with lung cancer, they are not “cancer.” Patients can be very sensitive to language and may misinterpret language that doctors commonly use. Language such as “the patient failed treatment” may be interpreted by patients as a personal failure. In reality, the treatment failed the patient, instead of the other way around. Instead, shift the blame from the patient to the cancer. Adopt terms like “the tumor did not respond to treatment.” Or, “the cancer progressed” instead of “the patient progressed.”
Language around smoking is particularly stigmatizing because it categorizes a person by a behavior. As health care providers, we should consider removing the term “smoker” from our interactions with patients and instead, use “patient who smokes” or ”patient with a smoking history.” Other ways health care providers can reduce stigma triggered by assessing smoking status include using supportive communication skills, providing a rationale for asking smoking related questions, offering help and tobacco cessation and other resources, and displaying empathic behavior, such as maintaining eye contact and a nonjudgmental body position orientated toward the patient.
Many of these common medical phrases were developed to enable efficient communication among health care professionals. Times have changed and patients should not be defined by an illness. They are people first. In addition to improving patient interactions in clinic, using nonjudgmental language whenever possible in presentations and publications is also extremely important, as patients are living longer and getting more involved in research and advocacy.
“Words have energy and power with the ability to help, to heal, to hinder, to hurt, to harm, to humiliate, and to humble,” says Yehuda Berg, author and codirector of the Kabbalah Centre International in Los Angeles.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
“Do you smoke?” I asked the patient.
“Yes, and I got what I deserved,” he answered, clearly upset.
I ignored his reaction and continued with the exam, but in retrospect, I should have explained why doctors ask patients this question.
It was not my intention to be rude or blame the patient for his lung cancer diagnosis. Doctors ask patients if they smoke because a smoking history can change the type of treatment and it can be associated with other conditions that may interfere with treatment. It can also determine whether smoking cessation assistance should be offered to the patient. It is crucial that we as doctors know a patient’s medical history, but how we approach sensitive issues may determine if we even get the information we need. In this case, I didn’t explain why I asked the patient if he smoked. Had I taken the time to explain why I needed to know if and how long he smoked and that I was not blaming him for his lung cancer diagnosis, we may have had a more mutually respectful and beneficial relationship.
Almost all of my patients with lung cancer have been asked at one time or another – by a health care provider, friends, or acquaintances – “Do you smoke?” Whether or not they smoked, patients with lung cancer feel the weight of moral judgment being cast upon them by society.
It is common for people who smoke and who go on to develop lung cancer to be weighed down by guilt associated with their diagnosis. Patients with lung cancer face stigma-associated hurdles based on the “I did it to myself” mindset. This societal stigma is not without harm as it can result in emotional responses of guilt and self-blame. This internalized stigma may lead to psychosocial distress and decreased interactions with family, friends, and health care providers. The guilt may drive a patient to forgo lung cancer screening, minimize symptoms, delay seeking treatment, and not advocate for themselves with their physician. Some patients even decide to forgo all treatment.
What about patients who never smoked? They too feel tinged with blame. Many of these patients feel called upon to defend themselves by proclaiming loudly that they have never smoked.
Blame and shame also divides the lung cancer community, resulting in less advocacy. It may also impact research dollars for lung cancer. According to the Lung Cancer Research Foundation, “Despite being the leading cause of cancer mortality, lung cancer receives far less research funding than any other cancer.” By comparison, women with breast cancer are showered with far more resources, supportive services, fundraising events, and certainly more lobbying.
By making unintentional hurtful statements and using judgmental or denigrating language, the lung cancer community may unconsciously be playing a role in perpetuating stigmas associated with lung cancer. That kind of language can come across as blame.
The International Association for the Study of Lung Cancer has developed a language guide to help reduce stigma associated with lung cancer. The aim is to reduce and replace traditional medical language during our patient interactions, presentations, and publications with language that is more empathic and nonjudgmental.
For example, replace the term “cancer patient” with the term “the patient with cancer.” The patient is a person who happens to have been diagnosed with lung cancer, they are not “cancer.” Patients can be very sensitive to language and may misinterpret language that doctors commonly use. Language such as “the patient failed treatment” may be interpreted by patients as a personal failure. In reality, the treatment failed the patient, instead of the other way around. Instead, shift the blame from the patient to the cancer. Adopt terms like “the tumor did not respond to treatment.” Or, “the cancer progressed” instead of “the patient progressed.”
Language around smoking is particularly stigmatizing because it categorizes a person by a behavior. As health care providers, we should consider removing the term “smoker” from our interactions with patients and instead, use “patient who smokes” or ”patient with a smoking history.” Other ways health care providers can reduce stigma triggered by assessing smoking status include using supportive communication skills, providing a rationale for asking smoking related questions, offering help and tobacco cessation and other resources, and displaying empathic behavior, such as maintaining eye contact and a nonjudgmental body position orientated toward the patient.
Many of these common medical phrases were developed to enable efficient communication among health care professionals. Times have changed and patients should not be defined by an illness. They are people first. In addition to improving patient interactions in clinic, using nonjudgmental language whenever possible in presentations and publications is also extremely important, as patients are living longer and getting more involved in research and advocacy.
“Words have energy and power with the ability to help, to heal, to hinder, to hurt, to harm, to humiliate, and to humble,” says Yehuda Berg, author and codirector of the Kabbalah Centre International in Los Angeles.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
“Do you smoke?” I asked the patient.
“Yes, and I got what I deserved,” he answered, clearly upset.
I ignored his reaction and continued with the exam, but in retrospect, I should have explained why doctors ask patients this question.
It was not my intention to be rude or blame the patient for his lung cancer diagnosis. Doctors ask patients if they smoke because a smoking history can change the type of treatment and it can be associated with other conditions that may interfere with treatment. It can also determine whether smoking cessation assistance should be offered to the patient. It is crucial that we as doctors know a patient’s medical history, but how we approach sensitive issues may determine if we even get the information we need. In this case, I didn’t explain why I asked the patient if he smoked. Had I taken the time to explain why I needed to know if and how long he smoked and that I was not blaming him for his lung cancer diagnosis, we may have had a more mutually respectful and beneficial relationship.
Almost all of my patients with lung cancer have been asked at one time or another – by a health care provider, friends, or acquaintances – “Do you smoke?” Whether or not they smoked, patients with lung cancer feel the weight of moral judgment being cast upon them by society.
It is common for people who smoke and who go on to develop lung cancer to be weighed down by guilt associated with their diagnosis. Patients with lung cancer face stigma-associated hurdles based on the “I did it to myself” mindset. This societal stigma is not without harm as it can result in emotional responses of guilt and self-blame. This internalized stigma may lead to psychosocial distress and decreased interactions with family, friends, and health care providers. The guilt may drive a patient to forgo lung cancer screening, minimize symptoms, delay seeking treatment, and not advocate for themselves with their physician. Some patients even decide to forgo all treatment.
What about patients who never smoked? They too feel tinged with blame. Many of these patients feel called upon to defend themselves by proclaiming loudly that they have never smoked.
Blame and shame also divides the lung cancer community, resulting in less advocacy. It may also impact research dollars for lung cancer. According to the Lung Cancer Research Foundation, “Despite being the leading cause of cancer mortality, lung cancer receives far less research funding than any other cancer.” By comparison, women with breast cancer are showered with far more resources, supportive services, fundraising events, and certainly more lobbying.
By making unintentional hurtful statements and using judgmental or denigrating language, the lung cancer community may unconsciously be playing a role in perpetuating stigmas associated with lung cancer. That kind of language can come across as blame.
The International Association for the Study of Lung Cancer has developed a language guide to help reduce stigma associated with lung cancer. The aim is to reduce and replace traditional medical language during our patient interactions, presentations, and publications with language that is more empathic and nonjudgmental.
For example, replace the term “cancer patient” with the term “the patient with cancer.” The patient is a person who happens to have been diagnosed with lung cancer, they are not “cancer.” Patients can be very sensitive to language and may misinterpret language that doctors commonly use. Language such as “the patient failed treatment” may be interpreted by patients as a personal failure. In reality, the treatment failed the patient, instead of the other way around. Instead, shift the blame from the patient to the cancer. Adopt terms like “the tumor did not respond to treatment.” Or, “the cancer progressed” instead of “the patient progressed.”
Language around smoking is particularly stigmatizing because it categorizes a person by a behavior. As health care providers, we should consider removing the term “smoker” from our interactions with patients and instead, use “patient who smokes” or ”patient with a smoking history.” Other ways health care providers can reduce stigma triggered by assessing smoking status include using supportive communication skills, providing a rationale for asking smoking related questions, offering help and tobacco cessation and other resources, and displaying empathic behavior, such as maintaining eye contact and a nonjudgmental body position orientated toward the patient.
Many of these common medical phrases were developed to enable efficient communication among health care professionals. Times have changed and patients should not be defined by an illness. They are people first. In addition to improving patient interactions in clinic, using nonjudgmental language whenever possible in presentations and publications is also extremely important, as patients are living longer and getting more involved in research and advocacy.
“Words have energy and power with the ability to help, to heal, to hinder, to hurt, to harm, to humiliate, and to humble,” says Yehuda Berg, author and codirector of the Kabbalah Centre International in Los Angeles.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
Immunity debt and the tripledemic
Respiratory syncytial virus (RSV) and influenza cases are surging to record numbers this winter in the wake of the COVID-19 pandemic when children were sheltering in the home, receiving virtual education, masking, and hand sanitizing, and when other precautionary health measures were in place.
RSV and flu illness in children now have hospital emergency rooms and pediatric ICUs and wards over capacity. As these respiratory infections increase and variants of SARS-CoV-2 come to dominate, we may expect the full impact of a tripledemic (RSV + flu + SARS-CoV-2).
It has been estimated that RSV causes 33 million lower respiratory infections and 3.6 million hospitalizations annually worldwide in children younger than 5 years old (Lancet. 2022 May 19. doi: 10.1016/S0140-6736(22)00478-0). RSV is typically a seasonal respiratory infection occurring in late fall through early winter, when it gives way to dominance by flu. Thus, we have experienced an out-of-season surge in RSV since it began in early fall 2022, and it persists. A likely explanation for the early and persisting surge in RSV is immunity debt (Infect Dis Now. 2021 Aug. doi: 10.1016/j.idnow.2021.05.004).
Immunity debt is an unintended consequence of prevention of infections that occurred because of public health measures to prevent spread of SARS-CoV-2 infections. The COVID-19 lockdown undoubtedly saved many lives. However, while we were sheltering from SARS-CoV-2 infections, we also were avoiding other infections, especially other respiratory infections such as RSV and flu.
Our group studied this in community-based pediatric practices in Rochester, N.Y. Physician-diagnosed, medically attended infectious disease illness visits were assessed in two child cohorts, age 6-36 months from March 15 to Dec. 31, 2020 (the pandemic period), compared with the same months in 2019 (prepandemic). One hundred forty-four children were included in the pandemic cohort and 215 in the prepandemic cohort. Visits for bronchiolitis were 7.4-fold lower (P = .04), acute otitis media 3.7-fold lower (P < .0001), viral upper respiratory infections (URI) 3.8-fold lower (P < .0001), and croup 27.5-fold lower (P < .0001) in the pandemic than the prepandemic cohort (Front Pediatr. 2021 Sep 13. doi: 10.3389/fped.2021.72248).
The significant reduction in respiratory illness during the COVID-19 epidemic we and others observed resulted in a large pool of children who did not experience RSV or flu infections for an entire year or more. Herd immunity dropped. The susceptible child population increased, including children older than typically seen. We had an immunity debt that had to be repaid, and the repayment is occurring now.
As a consequence of the surge in RSV, interest in prevention has gained more attention. In 1966, tragically, two infant deaths and hospitalization of 80% of the participating infants occurred during a clinical trial of an experimental candidate RSV vaccine, which contained an inactivated version of the entire virus. The severe side effect was later found to be caused by both an antibody and a T-cell problem. The antibody produced in response to the inactivated whole virus didn’t have very good functional activity at blocking or neutralizing the virus. That led to deposition of immune complexes and activation of complement that damaged the airways. The vaccine also triggered a T-cell response with inflammatory cytokine release that added to airway obstruction and lack of clearance of the virus. RSV vaccine development was halted and the bar for further studies was raised very high to ensure safety of any future RSV vaccines. Now, 55 years later, two RSV vaccines and a new preventive monoclonal antibody are nearing licensure.
GlaxoSmithKline (GSK) and Pfizer are in phase 3 clinical trials of a safer RSV vaccine that contains only the RSV surface protein known as protein F. Protein F changes its structure when the virus infects and fuses with human respiratory epithelial cells. The GSK and Pfizer vaccines use a molecular strategy developed at the National Institutes of Health to lock protein F into its original, prefusion configuration. A similar strategy was used by Pfizer/BioNTech and Moderna in their design of mRNA vaccines to the SARS-CoV-2 spike surface protein.
A vaccine with the F protein in its prefusion form takes care of the antibody problem that caused the severe side-effects from the 1966 version of inactivated whole virus vaccine because it induces very high-efficiency, high-potency antibodies that neutralize the RSV. The T-cell response is not as well understood and that is why studies are being done in adults first and then moving to young infants.
The new RSV vaccines are being developed for use in adults over age 60, adults with comorbidities, maternal immunization, and infants. Encouraging results were recently reported by GSK and Pfizer from adult trials. In an interim analysis, Pfizer also recently reported that maternal immunization in the late second or third trimester with their vaccine had an efficacy of 82% within a newborn’s first 90 days of life against severe lower respiratory tract illness. At age 6 months, the efficacy was sustained at 69%. So far, both the GSK and Pfizer RSV vaccines have shown a favorable safety profile.
Another strategy in the RSV prevention field has been a monoclonal antibody. Palivizumab (Synagis, AstraZeneca) is used to prevent severe RSV infections in prematurely born and other infants who are at higher risk of mortality and severe morbidity. Soon there will likely be another monoclonal antibody, called nirsevimab (Beyfortus, AstraZeneca and Sanofi). It is approved in Europe but not yet approved in the United States as I prepare this column. Nirsevimab may be even better than palivizumab – based on phase 3 trial data – and a single injection lasts through an entire normal RSV season while palivizumab requires monthly injections.
Similar to the situation with RSV, the flu season started earlier than usual in fall 2022 and has been picking up steam, likely also because of immunity debt. The WHO estimates that annual epidemics of influenza cause 1 billion infections, 3 million to 5 million severe cases, and 300,000-500,000 deaths. Seasonal flu vaccines provide modest protection. Current flu vaccine formulations consist of the hemagglutinin (H) and neuraminidase (N) proteins but those proteins change sufficiently (called antigenic drift) such that production of the vaccines based on a best guess each year often is not correct for the influenza A or influenza B strains that circulate in a given year (antigenic mismatch).
Public health authorities have long worried about a major change in the composition of the H and N proteins of the influenza virus (called antigenic shift). Preparedness and response to the COVID-19 pandemic was based on preparedness and response to an anticipated influenza pandemic similar to the 1918 flu pandemic. For flu, new “universal” vaccines are in development. Among the candidate vaccines are mRNA vaccines, building on the success of the SARS-CoV-2 mRNA vaccines (Science. 2022 Nov 24. doi: 10.1126/science.abm0271).
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
Respiratory syncytial virus (RSV) and influenza cases are surging to record numbers this winter in the wake of the COVID-19 pandemic when children were sheltering in the home, receiving virtual education, masking, and hand sanitizing, and when other precautionary health measures were in place.
RSV and flu illness in children now have hospital emergency rooms and pediatric ICUs and wards over capacity. As these respiratory infections increase and variants of SARS-CoV-2 come to dominate, we may expect the full impact of a tripledemic (RSV + flu + SARS-CoV-2).
It has been estimated that RSV causes 33 million lower respiratory infections and 3.6 million hospitalizations annually worldwide in children younger than 5 years old (Lancet. 2022 May 19. doi: 10.1016/S0140-6736(22)00478-0). RSV is typically a seasonal respiratory infection occurring in late fall through early winter, when it gives way to dominance by flu. Thus, we have experienced an out-of-season surge in RSV since it began in early fall 2022, and it persists. A likely explanation for the early and persisting surge in RSV is immunity debt (Infect Dis Now. 2021 Aug. doi: 10.1016/j.idnow.2021.05.004).
Immunity debt is an unintended consequence of prevention of infections that occurred because of public health measures to prevent spread of SARS-CoV-2 infections. The COVID-19 lockdown undoubtedly saved many lives. However, while we were sheltering from SARS-CoV-2 infections, we also were avoiding other infections, especially other respiratory infections such as RSV and flu.
Our group studied this in community-based pediatric practices in Rochester, N.Y. Physician-diagnosed, medically attended infectious disease illness visits were assessed in two child cohorts, age 6-36 months from March 15 to Dec. 31, 2020 (the pandemic period), compared with the same months in 2019 (prepandemic). One hundred forty-four children were included in the pandemic cohort and 215 in the prepandemic cohort. Visits for bronchiolitis were 7.4-fold lower (P = .04), acute otitis media 3.7-fold lower (P < .0001), viral upper respiratory infections (URI) 3.8-fold lower (P < .0001), and croup 27.5-fold lower (P < .0001) in the pandemic than the prepandemic cohort (Front Pediatr. 2021 Sep 13. doi: 10.3389/fped.2021.72248).
The significant reduction in respiratory illness during the COVID-19 epidemic we and others observed resulted in a large pool of children who did not experience RSV or flu infections for an entire year or more. Herd immunity dropped. The susceptible child population increased, including children older than typically seen. We had an immunity debt that had to be repaid, and the repayment is occurring now.
As a consequence of the surge in RSV, interest in prevention has gained more attention. In 1966, tragically, two infant deaths and hospitalization of 80% of the participating infants occurred during a clinical trial of an experimental candidate RSV vaccine, which contained an inactivated version of the entire virus. The severe side effect was later found to be caused by both an antibody and a T-cell problem. The antibody produced in response to the inactivated whole virus didn’t have very good functional activity at blocking or neutralizing the virus. That led to deposition of immune complexes and activation of complement that damaged the airways. The vaccine also triggered a T-cell response with inflammatory cytokine release that added to airway obstruction and lack of clearance of the virus. RSV vaccine development was halted and the bar for further studies was raised very high to ensure safety of any future RSV vaccines. Now, 55 years later, two RSV vaccines and a new preventive monoclonal antibody are nearing licensure.
GlaxoSmithKline (GSK) and Pfizer are in phase 3 clinical trials of a safer RSV vaccine that contains only the RSV surface protein known as protein F. Protein F changes its structure when the virus infects and fuses with human respiratory epithelial cells. The GSK and Pfizer vaccines use a molecular strategy developed at the National Institutes of Health to lock protein F into its original, prefusion configuration. A similar strategy was used by Pfizer/BioNTech and Moderna in their design of mRNA vaccines to the SARS-CoV-2 spike surface protein.
A vaccine with the F protein in its prefusion form takes care of the antibody problem that caused the severe side-effects from the 1966 version of inactivated whole virus vaccine because it induces very high-efficiency, high-potency antibodies that neutralize the RSV. The T-cell response is not as well understood and that is why studies are being done in adults first and then moving to young infants.
The new RSV vaccines are being developed for use in adults over age 60, adults with comorbidities, maternal immunization, and infants. Encouraging results were recently reported by GSK and Pfizer from adult trials. In an interim analysis, Pfizer also recently reported that maternal immunization in the late second or third trimester with their vaccine had an efficacy of 82% within a newborn’s first 90 days of life against severe lower respiratory tract illness. At age 6 months, the efficacy was sustained at 69%. So far, both the GSK and Pfizer RSV vaccines have shown a favorable safety profile.
Another strategy in the RSV prevention field has been a monoclonal antibody. Palivizumab (Synagis, AstraZeneca) is used to prevent severe RSV infections in prematurely born and other infants who are at higher risk of mortality and severe morbidity. Soon there will likely be another monoclonal antibody, called nirsevimab (Beyfortus, AstraZeneca and Sanofi). It is approved in Europe but not yet approved in the United States as I prepare this column. Nirsevimab may be even better than palivizumab – based on phase 3 trial data – and a single injection lasts through an entire normal RSV season while palivizumab requires monthly injections.
Similar to the situation with RSV, the flu season started earlier than usual in fall 2022 and has been picking up steam, likely also because of immunity debt. The WHO estimates that annual epidemics of influenza cause 1 billion infections, 3 million to 5 million severe cases, and 300,000-500,000 deaths. Seasonal flu vaccines provide modest protection. Current flu vaccine formulations consist of the hemagglutinin (H) and neuraminidase (N) proteins but those proteins change sufficiently (called antigenic drift) such that production of the vaccines based on a best guess each year often is not correct for the influenza A or influenza B strains that circulate in a given year (antigenic mismatch).
Public health authorities have long worried about a major change in the composition of the H and N proteins of the influenza virus (called antigenic shift). Preparedness and response to the COVID-19 pandemic was based on preparedness and response to an anticipated influenza pandemic similar to the 1918 flu pandemic. For flu, new “universal” vaccines are in development. Among the candidate vaccines are mRNA vaccines, building on the success of the SARS-CoV-2 mRNA vaccines (Science. 2022 Nov 24. doi: 10.1126/science.abm0271).
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
Respiratory syncytial virus (RSV) and influenza cases are surging to record numbers this winter in the wake of the COVID-19 pandemic when children were sheltering in the home, receiving virtual education, masking, and hand sanitizing, and when other precautionary health measures were in place.
RSV and flu illness in children now have hospital emergency rooms and pediatric ICUs and wards over capacity. As these respiratory infections increase and variants of SARS-CoV-2 come to dominate, we may expect the full impact of a tripledemic (RSV + flu + SARS-CoV-2).
It has been estimated that RSV causes 33 million lower respiratory infections and 3.6 million hospitalizations annually worldwide in children younger than 5 years old (Lancet. 2022 May 19. doi: 10.1016/S0140-6736(22)00478-0). RSV is typically a seasonal respiratory infection occurring in late fall through early winter, when it gives way to dominance by flu. Thus, we have experienced an out-of-season surge in RSV since it began in early fall 2022, and it persists. A likely explanation for the early and persisting surge in RSV is immunity debt (Infect Dis Now. 2021 Aug. doi: 10.1016/j.idnow.2021.05.004).
Immunity debt is an unintended consequence of prevention of infections that occurred because of public health measures to prevent spread of SARS-CoV-2 infections. The COVID-19 lockdown undoubtedly saved many lives. However, while we were sheltering from SARS-CoV-2 infections, we also were avoiding other infections, especially other respiratory infections such as RSV and flu.
Our group studied this in community-based pediatric practices in Rochester, N.Y. Physician-diagnosed, medically attended infectious disease illness visits were assessed in two child cohorts, age 6-36 months from March 15 to Dec. 31, 2020 (the pandemic period), compared with the same months in 2019 (prepandemic). One hundred forty-four children were included in the pandemic cohort and 215 in the prepandemic cohort. Visits for bronchiolitis were 7.4-fold lower (P = .04), acute otitis media 3.7-fold lower (P < .0001), viral upper respiratory infections (URI) 3.8-fold lower (P < .0001), and croup 27.5-fold lower (P < .0001) in the pandemic than the prepandemic cohort (Front Pediatr. 2021 Sep 13. doi: 10.3389/fped.2021.72248).
The significant reduction in respiratory illness during the COVID-19 epidemic we and others observed resulted in a large pool of children who did not experience RSV or flu infections for an entire year or more. Herd immunity dropped. The susceptible child population increased, including children older than typically seen. We had an immunity debt that had to be repaid, and the repayment is occurring now.
As a consequence of the surge in RSV, interest in prevention has gained more attention. In 1966, tragically, two infant deaths and hospitalization of 80% of the participating infants occurred during a clinical trial of an experimental candidate RSV vaccine, which contained an inactivated version of the entire virus. The severe side effect was later found to be caused by both an antibody and a T-cell problem. The antibody produced in response to the inactivated whole virus didn’t have very good functional activity at blocking or neutralizing the virus. That led to deposition of immune complexes and activation of complement that damaged the airways. The vaccine also triggered a T-cell response with inflammatory cytokine release that added to airway obstruction and lack of clearance of the virus. RSV vaccine development was halted and the bar for further studies was raised very high to ensure safety of any future RSV vaccines. Now, 55 years later, two RSV vaccines and a new preventive monoclonal antibody are nearing licensure.
GlaxoSmithKline (GSK) and Pfizer are in phase 3 clinical trials of a safer RSV vaccine that contains only the RSV surface protein known as protein F. Protein F changes its structure when the virus infects and fuses with human respiratory epithelial cells. The GSK and Pfizer vaccines use a molecular strategy developed at the National Institutes of Health to lock protein F into its original, prefusion configuration. A similar strategy was used by Pfizer/BioNTech and Moderna in their design of mRNA vaccines to the SARS-CoV-2 spike surface protein.
A vaccine with the F protein in its prefusion form takes care of the antibody problem that caused the severe side-effects from the 1966 version of inactivated whole virus vaccine because it induces very high-efficiency, high-potency antibodies that neutralize the RSV. The T-cell response is not as well understood and that is why studies are being done in adults first and then moving to young infants.
The new RSV vaccines are being developed for use in adults over age 60, adults with comorbidities, maternal immunization, and infants. Encouraging results were recently reported by GSK and Pfizer from adult trials. In an interim analysis, Pfizer also recently reported that maternal immunization in the late second or third trimester with their vaccine had an efficacy of 82% within a newborn’s first 90 days of life against severe lower respiratory tract illness. At age 6 months, the efficacy was sustained at 69%. So far, both the GSK and Pfizer RSV vaccines have shown a favorable safety profile.
Another strategy in the RSV prevention field has been a monoclonal antibody. Palivizumab (Synagis, AstraZeneca) is used to prevent severe RSV infections in prematurely born and other infants who are at higher risk of mortality and severe morbidity. Soon there will likely be another monoclonal antibody, called nirsevimab (Beyfortus, AstraZeneca and Sanofi). It is approved in Europe but not yet approved in the United States as I prepare this column. Nirsevimab may be even better than palivizumab – based on phase 3 trial data – and a single injection lasts through an entire normal RSV season while palivizumab requires monthly injections.
Similar to the situation with RSV, the flu season started earlier than usual in fall 2022 and has been picking up steam, likely also because of immunity debt. The WHO estimates that annual epidemics of influenza cause 1 billion infections, 3 million to 5 million severe cases, and 300,000-500,000 deaths. Seasonal flu vaccines provide modest protection. Current flu vaccine formulations consist of the hemagglutinin (H) and neuraminidase (N) proteins but those proteins change sufficiently (called antigenic drift) such that production of the vaccines based on a best guess each year often is not correct for the influenza A or influenza B strains that circulate in a given year (antigenic mismatch).
Public health authorities have long worried about a major change in the composition of the H and N proteins of the influenza virus (called antigenic shift). Preparedness and response to the COVID-19 pandemic was based on preparedness and response to an anticipated influenza pandemic similar to the 1918 flu pandemic. For flu, new “universal” vaccines are in development. Among the candidate vaccines are mRNA vaccines, building on the success of the SARS-CoV-2 mRNA vaccines (Science. 2022 Nov 24. doi: 10.1126/science.abm0271).
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
Diagnosed too late
It had only been 3 weeks since I first met this patient. She presented with an advanced case of colon cancer, but instead of treatment,
Within the course of 2 weeks I saw another new patient, but this time with pancreatic cancer that metastasized to the liver. “When can we start treatment?” he asked. Like my female patient with colon cancer, he was diagnosed too late as he was already in an incurable stage. He was shocked to learn that his condition was in stage 4, that achieving remission would be difficult and a cure, not likely. Certainly, standard of care treatments and clinical trials offered him hope, but they were unlikely to change the outcome.
We take a course in this – that is, in giving bad news, but every doctor has his or her own approach. Some are so uncomfortable with the talk, they choose avoidance and adopt the “look like you gotta go approach.” Or, the doctor may schedule another treatment or another test with the intention of avoiding end-of-life discussions. Other doctors opt for straight talk: “I think you should get your affairs in order. You’ve got 3 months to live.” These are extreme behaviors I wouldn’t recommend.
In my practice, I sit with my patients and explain the diagnosis. After discussing all options and the advanced stage and diagnosis, it ultimately comes down to “Win or lose, I will be here to take care of you.” Sometimes there is therapy that may help, but either way, the patient understands that death is a real possibility.
I find that people just want to know if there is hope. A different treatment regimen or a clinical trial may (or may not) extend their life. And while we cannot predict outcomes, we can give them hope. You can’t shut down hope. True for some people the cup is always half empty, but most people want to live and are optimistic no matter how small the chances are.
These conversations are very difficult. I don’t like them, but then I don’t avoid them either. Fortunately, patients don’t usually come to my office for the first visit presenting with advanced disease. In the cases I described above, one patient had been experiencing unexplained weight loss, but didn’t share it with a physician. And, for the patient with pancreatic cancer, other than some discomfort in the last couple of weeks, the disease was not associated with other symptoms. But the absence of symptoms should not in any way rule out a malignant disease. A diagnosis should be based on a complete evaluation of signs and symptoms followed by testing.
We’ve got to be able to take the time to listen to our patients during these encounters. We may not spend as much time as we should because we’re so busy now and we’re slaves to EMRs. It helps if we take more time to probe symptoms a little longer, especially in the primary care setting.
It is possible for a patient with cancer to be asymptomatic up until the later stages of the disease. A study published in ESMO Open in 2020 found that fewer than half of patients with stage 4 non–small cell lung cancer have only one or two symptoms at diagnosis regardless of whether the patient was a smoker. In this study only 33% of patients reported having a cough and 25% had chest pain.
A study presented in October at the United European Gastroenterology Week found that of 600 pancreatic cancer cases, 46 of these were not detected by CT or MRI conducted 3-18 months prior to diagnosis. Of the 46 cases, 26% were not picked up by the radiologist and the rest were largely as a result of imaging changes over time. Radiology techniques are good, but they cannot pick up lesions that are too small. And some lesions, particularly in pancreatic cancer, can grow and metastasize rather quickly.
When a patient is diagnosed with advanced disease, it is most often simply because of the nature of the disease. But sometimes patients put off scheduling a doctor visit because of fear of the potential for bad news or fear of the doctor belittling their symptoms. Some tell me they were “just hoping the symptoms would disappear.” Waiting too long to see a doctor is never a good idea because timing is crucial. In many cases, there is a small window of opportunity to treat disease if remission is to be achieved.
Dr. Henry is a practicing clinical oncologist with PennMedicine in Philadelphia where he also serves as Vice Chair of the Department of Medicine at Pennsylvania Hospital.
This article was updated 12/7/22.
It had only been 3 weeks since I first met this patient. She presented with an advanced case of colon cancer, but instead of treatment,
Within the course of 2 weeks I saw another new patient, but this time with pancreatic cancer that metastasized to the liver. “When can we start treatment?” he asked. Like my female patient with colon cancer, he was diagnosed too late as he was already in an incurable stage. He was shocked to learn that his condition was in stage 4, that achieving remission would be difficult and a cure, not likely. Certainly, standard of care treatments and clinical trials offered him hope, but they were unlikely to change the outcome.
We take a course in this – that is, in giving bad news, but every doctor has his or her own approach. Some are so uncomfortable with the talk, they choose avoidance and adopt the “look like you gotta go approach.” Or, the doctor may schedule another treatment or another test with the intention of avoiding end-of-life discussions. Other doctors opt for straight talk: “I think you should get your affairs in order. You’ve got 3 months to live.” These are extreme behaviors I wouldn’t recommend.
In my practice, I sit with my patients and explain the diagnosis. After discussing all options and the advanced stage and diagnosis, it ultimately comes down to “Win or lose, I will be here to take care of you.” Sometimes there is therapy that may help, but either way, the patient understands that death is a real possibility.
I find that people just want to know if there is hope. A different treatment regimen or a clinical trial may (or may not) extend their life. And while we cannot predict outcomes, we can give them hope. You can’t shut down hope. True for some people the cup is always half empty, but most people want to live and are optimistic no matter how small the chances are.
These conversations are very difficult. I don’t like them, but then I don’t avoid them either. Fortunately, patients don’t usually come to my office for the first visit presenting with advanced disease. In the cases I described above, one patient had been experiencing unexplained weight loss, but didn’t share it with a physician. And, for the patient with pancreatic cancer, other than some discomfort in the last couple of weeks, the disease was not associated with other symptoms. But the absence of symptoms should not in any way rule out a malignant disease. A diagnosis should be based on a complete evaluation of signs and symptoms followed by testing.
We’ve got to be able to take the time to listen to our patients during these encounters. We may not spend as much time as we should because we’re so busy now and we’re slaves to EMRs. It helps if we take more time to probe symptoms a little longer, especially in the primary care setting.
It is possible for a patient with cancer to be asymptomatic up until the later stages of the disease. A study published in ESMO Open in 2020 found that fewer than half of patients with stage 4 non–small cell lung cancer have only one or two symptoms at diagnosis regardless of whether the patient was a smoker. In this study only 33% of patients reported having a cough and 25% had chest pain.
A study presented in October at the United European Gastroenterology Week found that of 600 pancreatic cancer cases, 46 of these were not detected by CT or MRI conducted 3-18 months prior to diagnosis. Of the 46 cases, 26% were not picked up by the radiologist and the rest were largely as a result of imaging changes over time. Radiology techniques are good, but they cannot pick up lesions that are too small. And some lesions, particularly in pancreatic cancer, can grow and metastasize rather quickly.
When a patient is diagnosed with advanced disease, it is most often simply because of the nature of the disease. But sometimes patients put off scheduling a doctor visit because of fear of the potential for bad news or fear of the doctor belittling their symptoms. Some tell me they were “just hoping the symptoms would disappear.” Waiting too long to see a doctor is never a good idea because timing is crucial. In many cases, there is a small window of opportunity to treat disease if remission is to be achieved.
Dr. Henry is a practicing clinical oncologist with PennMedicine in Philadelphia where he also serves as Vice Chair of the Department of Medicine at Pennsylvania Hospital.
This article was updated 12/7/22.
It had only been 3 weeks since I first met this patient. She presented with an advanced case of colon cancer, but instead of treatment,
Within the course of 2 weeks I saw another new patient, but this time with pancreatic cancer that metastasized to the liver. “When can we start treatment?” he asked. Like my female patient with colon cancer, he was diagnosed too late as he was already in an incurable stage. He was shocked to learn that his condition was in stage 4, that achieving remission would be difficult and a cure, not likely. Certainly, standard of care treatments and clinical trials offered him hope, but they were unlikely to change the outcome.
We take a course in this – that is, in giving bad news, but every doctor has his or her own approach. Some are so uncomfortable with the talk, they choose avoidance and adopt the “look like you gotta go approach.” Or, the doctor may schedule another treatment or another test with the intention of avoiding end-of-life discussions. Other doctors opt for straight talk: “I think you should get your affairs in order. You’ve got 3 months to live.” These are extreme behaviors I wouldn’t recommend.
In my practice, I sit with my patients and explain the diagnosis. After discussing all options and the advanced stage and diagnosis, it ultimately comes down to “Win or lose, I will be here to take care of you.” Sometimes there is therapy that may help, but either way, the patient understands that death is a real possibility.
I find that people just want to know if there is hope. A different treatment regimen or a clinical trial may (or may not) extend their life. And while we cannot predict outcomes, we can give them hope. You can’t shut down hope. True for some people the cup is always half empty, but most people want to live and are optimistic no matter how small the chances are.
These conversations are very difficult. I don’t like them, but then I don’t avoid them either. Fortunately, patients don’t usually come to my office for the first visit presenting with advanced disease. In the cases I described above, one patient had been experiencing unexplained weight loss, but didn’t share it with a physician. And, for the patient with pancreatic cancer, other than some discomfort in the last couple of weeks, the disease was not associated with other symptoms. But the absence of symptoms should not in any way rule out a malignant disease. A diagnosis should be based on a complete evaluation of signs and symptoms followed by testing.
We’ve got to be able to take the time to listen to our patients during these encounters. We may not spend as much time as we should because we’re so busy now and we’re slaves to EMRs. It helps if we take more time to probe symptoms a little longer, especially in the primary care setting.
It is possible for a patient with cancer to be asymptomatic up until the later stages of the disease. A study published in ESMO Open in 2020 found that fewer than half of patients with stage 4 non–small cell lung cancer have only one or two symptoms at diagnosis regardless of whether the patient was a smoker. In this study only 33% of patients reported having a cough and 25% had chest pain.
A study presented in October at the United European Gastroenterology Week found that of 600 pancreatic cancer cases, 46 of these were not detected by CT or MRI conducted 3-18 months prior to diagnosis. Of the 46 cases, 26% were not picked up by the radiologist and the rest were largely as a result of imaging changes over time. Radiology techniques are good, but they cannot pick up lesions that are too small. And some lesions, particularly in pancreatic cancer, can grow and metastasize rather quickly.
When a patient is diagnosed with advanced disease, it is most often simply because of the nature of the disease. But sometimes patients put off scheduling a doctor visit because of fear of the potential for bad news or fear of the doctor belittling their symptoms. Some tell me they were “just hoping the symptoms would disappear.” Waiting too long to see a doctor is never a good idea because timing is crucial. In many cases, there is a small window of opportunity to treat disease if remission is to be achieved.
Dr. Henry is a practicing clinical oncologist with PennMedicine in Philadelphia where he also serves as Vice Chair of the Department of Medicine at Pennsylvania Hospital.
This article was updated 12/7/22.
Medically speaking, 2022 was the best year yet for children
Headlines from earlier in the fall were grim: Thanks to the COVID-19 pandemic, life expectancy in the United States has fallen for 2 years running. Last year, according to health officials, the average American newborn could hope to reach 76.1 years, down from 79 years in 2019.
So far, so bad. But the headlines don’t tell the full story, which is much less dire. In fact, 2022 is the best year in human history for a child to arrive on Earth.
For a child born this year, in a developed country, into a family with access to good health care, the odds of living into the 22nd century are almost 50%. One in three will live to be 100. Those estimates reflect only incremental progress in medicine and public health, with COVID-19 baked in. They don’t account for biotechnologies beckoning to take control of the cell cycle and aging itself – which could make the outlook much brighter.
For some perspective, consider that a century ago, life expectancy for an American neonate was about 60 years. That 1922 figure was itself nothing short of miraculous, representing a 25% jump since 1901 – a leap that far outstrips the first 2 decades of the current century, during which life expectancy rose by just 2.5 years.
A gain of 2.5 years over 2 decades might not sound impressive, even without COVID-19 causing life expectancy in this country and abroad to sag. But during the pandemic, exciting new technologies that could drive gains in lifespan and healthspan, even bigger than those seen in the early 20th century, have moved closer to clinical reality. Think Star Trek-ish technologies like human hibernation, universal blood, mRNA therapy able to reprogram immune cells to hunt malignancies and fibrotic tissue, even head transplantation.
How long that last one will take to reach a clinic near you is hard to predict, but advances in the needed technology to anastomose cephalic and somatic portions of the spinal cord are mind-boggling. All this means that, from a medical standpoint, the future for babies born in the early 2020s looks dazzlingly bright.
Those sunny rays of optimism likely have failed to pierce the gloom of public discourse. Between “breakthrough infections,” “long COVID,” “Paxlovid rebound,” vaccine-induced myopericarditis, the current respiratory syncytial virus (RSV) outbreak, school shootings, climate change, and the youth mental health crisis, news headlines are undoubtedly frightful.
RSV: What’s old is new again
For the youngest children, the RSV outbreak is currently the scariest story. With social interactions returning toward a pre-COVID state, RSV has rebounded with a vengeance. In many places, pediatric wards are close to, at, or even beyond capacity. With no antiviral treatment for RSV, no licensed vaccine quite yet, and passive immunization (intravenous palivizumab) reserved for children at greatest risk (those under age 6 months and born preterm 35 weeks or earlier), the situation does have the feel of the first year of COVID-19, when treatments were similarly limited.
But let’s keep some perspective. RSV has always been a devastating infection. Prior to COVID-19, in the United States alone RSV killed 100-300 children below age 5 and 6,000-10,000 adults above age 65. The toll has always been worse on the international level. In 2019, 3.6 million people around the world were hospitalized for RSV infections, mostly the very old and the very young. Among causes of death below the age of 5, RSV ranks second only to malaria.
Postvaccine myopericarditis, a favorite concern of the vaccine hesitant, is a real phenomenon in young males. But generally, the condition has a subclinical to mild manifestation and fully resolves within 2 weeks.
Vaccines on the horizon
Monkeypox also was putting a damper on health news in recent months. Yet outreach efforts and selective vaccination and other precautions based on risk stratification appear to have calmed the outbreak. That’s good news, as is the fact that the struggle against malaria may be about to change. After decades of trying, we now have a malaria vaccine with what appears to be 80% efficacy against the infection. The same goes for RSV; finally, not one but two RSV vaccines are showing promise in late-stage clinical trials.
To be sure, for many young people, the times don’t seem so wonderful. The rate of teen suicide is alarming – yet it remains well below that seen in the 1990s. Are social media to blame, or is it something more complex?
If COVID-19 has taught us anything, it’s that development of vaccines and treatments need not take a decade or more. Operation Warp Speed may have seemed like a marketing gimmick and political grandstanding, but you can’t argue with the results.
Keep that perspective in mind to appreciate the moment – which I believe is coming soon – when the same type of intramuscular injection that we now use to trigger immunity against SARS-CoV-2 hits clinics, only this time as a way to cure cancer. Or when you read the stories of young victims of firearm violence who would have died but are rapidly cooled and kept hibernating for hours, so that their wounds can be repaired. And although you may not see that head transplant, one of these new babies might see it, or even might perform the procedure.
Dr. Warmflash is a freelance health and science writer living in Portland, Ore. His recent book, Moon: An Illustrated History: From Ancient Myths to the Colonies of Tomorrow, tells the story of the moon’s role in a plethora of historical events, from the origin of life to early calendar systems, the emergence of science and technology, and the dawn of the Space Age. He reported having no relevant financial disclosures. A version of this article first appeared on Medscape.com.
Headlines from earlier in the fall were grim: Thanks to the COVID-19 pandemic, life expectancy in the United States has fallen for 2 years running. Last year, according to health officials, the average American newborn could hope to reach 76.1 years, down from 79 years in 2019.
So far, so bad. But the headlines don’t tell the full story, which is much less dire. In fact, 2022 is the best year in human history for a child to arrive on Earth.
For a child born this year, in a developed country, into a family with access to good health care, the odds of living into the 22nd century are almost 50%. One in three will live to be 100. Those estimates reflect only incremental progress in medicine and public health, with COVID-19 baked in. They don’t account for biotechnologies beckoning to take control of the cell cycle and aging itself – which could make the outlook much brighter.
For some perspective, consider that a century ago, life expectancy for an American neonate was about 60 years. That 1922 figure was itself nothing short of miraculous, representing a 25% jump since 1901 – a leap that far outstrips the first 2 decades of the current century, during which life expectancy rose by just 2.5 years.
A gain of 2.5 years over 2 decades might not sound impressive, even without COVID-19 causing life expectancy in this country and abroad to sag. But during the pandemic, exciting new technologies that could drive gains in lifespan and healthspan, even bigger than those seen in the early 20th century, have moved closer to clinical reality. Think Star Trek-ish technologies like human hibernation, universal blood, mRNA therapy able to reprogram immune cells to hunt malignancies and fibrotic tissue, even head transplantation.
How long that last one will take to reach a clinic near you is hard to predict, but advances in the needed technology to anastomose cephalic and somatic portions of the spinal cord are mind-boggling. All this means that, from a medical standpoint, the future for babies born in the early 2020s looks dazzlingly bright.
Those sunny rays of optimism likely have failed to pierce the gloom of public discourse. Between “breakthrough infections,” “long COVID,” “Paxlovid rebound,” vaccine-induced myopericarditis, the current respiratory syncytial virus (RSV) outbreak, school shootings, climate change, and the youth mental health crisis, news headlines are undoubtedly frightful.
RSV: What’s old is new again
For the youngest children, the RSV outbreak is currently the scariest story. With social interactions returning toward a pre-COVID state, RSV has rebounded with a vengeance. In many places, pediatric wards are close to, at, or even beyond capacity. With no antiviral treatment for RSV, no licensed vaccine quite yet, and passive immunization (intravenous palivizumab) reserved for children at greatest risk (those under age 6 months and born preterm 35 weeks or earlier), the situation does have the feel of the first year of COVID-19, when treatments were similarly limited.
But let’s keep some perspective. RSV has always been a devastating infection. Prior to COVID-19, in the United States alone RSV killed 100-300 children below age 5 and 6,000-10,000 adults above age 65. The toll has always been worse on the international level. In 2019, 3.6 million people around the world were hospitalized for RSV infections, mostly the very old and the very young. Among causes of death below the age of 5, RSV ranks second only to malaria.
Postvaccine myopericarditis, a favorite concern of the vaccine hesitant, is a real phenomenon in young males. But generally, the condition has a subclinical to mild manifestation and fully resolves within 2 weeks.
Vaccines on the horizon
Monkeypox also was putting a damper on health news in recent months. Yet outreach efforts and selective vaccination and other precautions based on risk stratification appear to have calmed the outbreak. That’s good news, as is the fact that the struggle against malaria may be about to change. After decades of trying, we now have a malaria vaccine with what appears to be 80% efficacy against the infection. The same goes for RSV; finally, not one but two RSV vaccines are showing promise in late-stage clinical trials.
To be sure, for many young people, the times don’t seem so wonderful. The rate of teen suicide is alarming – yet it remains well below that seen in the 1990s. Are social media to blame, or is it something more complex?
If COVID-19 has taught us anything, it’s that development of vaccines and treatments need not take a decade or more. Operation Warp Speed may have seemed like a marketing gimmick and political grandstanding, but you can’t argue with the results.
Keep that perspective in mind to appreciate the moment – which I believe is coming soon – when the same type of intramuscular injection that we now use to trigger immunity against SARS-CoV-2 hits clinics, only this time as a way to cure cancer. Or when you read the stories of young victims of firearm violence who would have died but are rapidly cooled and kept hibernating for hours, so that their wounds can be repaired. And although you may not see that head transplant, one of these new babies might see it, or even might perform the procedure.
Dr. Warmflash is a freelance health and science writer living in Portland, Ore. His recent book, Moon: An Illustrated History: From Ancient Myths to the Colonies of Tomorrow, tells the story of the moon’s role in a plethora of historical events, from the origin of life to early calendar systems, the emergence of science and technology, and the dawn of the Space Age. He reported having no relevant financial disclosures. A version of this article first appeared on Medscape.com.
Headlines from earlier in the fall were grim: Thanks to the COVID-19 pandemic, life expectancy in the United States has fallen for 2 years running. Last year, according to health officials, the average American newborn could hope to reach 76.1 years, down from 79 years in 2019.
So far, so bad. But the headlines don’t tell the full story, which is much less dire. In fact, 2022 is the best year in human history for a child to arrive on Earth.
For a child born this year, in a developed country, into a family with access to good health care, the odds of living into the 22nd century are almost 50%. One in three will live to be 100. Those estimates reflect only incremental progress in medicine and public health, with COVID-19 baked in. They don’t account for biotechnologies beckoning to take control of the cell cycle and aging itself – which could make the outlook much brighter.
For some perspective, consider that a century ago, life expectancy for an American neonate was about 60 years. That 1922 figure was itself nothing short of miraculous, representing a 25% jump since 1901 – a leap that far outstrips the first 2 decades of the current century, during which life expectancy rose by just 2.5 years.
A gain of 2.5 years over 2 decades might not sound impressive, even without COVID-19 causing life expectancy in this country and abroad to sag. But during the pandemic, exciting new technologies that could drive gains in lifespan and healthspan, even bigger than those seen in the early 20th century, have moved closer to clinical reality. Think Star Trek-ish technologies like human hibernation, universal blood, mRNA therapy able to reprogram immune cells to hunt malignancies and fibrotic tissue, even head transplantation.
How long that last one will take to reach a clinic near you is hard to predict, but advances in the needed technology to anastomose cephalic and somatic portions of the spinal cord are mind-boggling. All this means that, from a medical standpoint, the future for babies born in the early 2020s looks dazzlingly bright.
Those sunny rays of optimism likely have failed to pierce the gloom of public discourse. Between “breakthrough infections,” “long COVID,” “Paxlovid rebound,” vaccine-induced myopericarditis, the current respiratory syncytial virus (RSV) outbreak, school shootings, climate change, and the youth mental health crisis, news headlines are undoubtedly frightful.
RSV: What’s old is new again
For the youngest children, the RSV outbreak is currently the scariest story. With social interactions returning toward a pre-COVID state, RSV has rebounded with a vengeance. In many places, pediatric wards are close to, at, or even beyond capacity. With no antiviral treatment for RSV, no licensed vaccine quite yet, and passive immunization (intravenous palivizumab) reserved for children at greatest risk (those under age 6 months and born preterm 35 weeks or earlier), the situation does have the feel of the first year of COVID-19, when treatments were similarly limited.
But let’s keep some perspective. RSV has always been a devastating infection. Prior to COVID-19, in the United States alone RSV killed 100-300 children below age 5 and 6,000-10,000 adults above age 65. The toll has always been worse on the international level. In 2019, 3.6 million people around the world were hospitalized for RSV infections, mostly the very old and the very young. Among causes of death below the age of 5, RSV ranks second only to malaria.
Postvaccine myopericarditis, a favorite concern of the vaccine hesitant, is a real phenomenon in young males. But generally, the condition has a subclinical to mild manifestation and fully resolves within 2 weeks.
Vaccines on the horizon
Monkeypox also was putting a damper on health news in recent months. Yet outreach efforts and selective vaccination and other precautions based on risk stratification appear to have calmed the outbreak. That’s good news, as is the fact that the struggle against malaria may be about to change. After decades of trying, we now have a malaria vaccine with what appears to be 80% efficacy against the infection. The same goes for RSV; finally, not one but two RSV vaccines are showing promise in late-stage clinical trials.
To be sure, for many young people, the times don’t seem so wonderful. The rate of teen suicide is alarming – yet it remains well below that seen in the 1990s. Are social media to blame, or is it something more complex?
If COVID-19 has taught us anything, it’s that development of vaccines and treatments need not take a decade or more. Operation Warp Speed may have seemed like a marketing gimmick and political grandstanding, but you can’t argue with the results.
Keep that perspective in mind to appreciate the moment – which I believe is coming soon – when the same type of intramuscular injection that we now use to trigger immunity against SARS-CoV-2 hits clinics, only this time as a way to cure cancer. Or when you read the stories of young victims of firearm violence who would have died but are rapidly cooled and kept hibernating for hours, so that their wounds can be repaired. And although you may not see that head transplant, one of these new babies might see it, or even might perform the procedure.
Dr. Warmflash is a freelance health and science writer living in Portland, Ore. His recent book, Moon: An Illustrated History: From Ancient Myths to the Colonies of Tomorrow, tells the story of the moon’s role in a plethora of historical events, from the origin of life to early calendar systems, the emergence of science and technology, and the dawn of the Space Age. He reported having no relevant financial disclosures. A version of this article first appeared on Medscape.com.
Visualization can improve sports performance
Over the past 30 years, Dr. Richard W. Cohen has used visualization techniques to help world class tennis players and recreational tennis players become the best they could be.
Visualization should be used in two ways to help players improve. First, to improve technique, after every practice session I have the player think about one shot they did not do well technically, and I have them, in vivo, shadow the shot on the court correctly before they leave the court. That night I tell the player to put themselves in a quiet, relaxed place and, in vitro, visualize themselves hitting the shot the correct way.
Almost always, the next day the players tell me they are hitting that one shot better and are motivated to again think about the one shot that was not technically correct and repeat the in vivo technique with similar great results.
The second way I use visualization for tennis players is to decrease their anxiety before matches. It is important to have some preparatory anxiety to perform optimally but having excessive anxiety will decrease performance. To alleviate excessive anxiety before matches, I have players watch their opponents hit the day before the match for at least 5 minutes to see their strengths and weaknesses. Then, the night before the match, I have them visualize how they will play a big point utilizing their strength into their opponent’s weakness. This rehearsal using imagery the night before a big match will decrease a player’s excessive anxiety and allow them to achieve their best effort in the match.
An example of this is if their opponent has a weak backhand that they can only slice. They visualize hitting wide to their forehand to get into their weak backhand and see themselves going forward and putting away a volley. Visualization used in these two ways helps improve stroke mechanics and match results in players of all levels. These visualization techniques can also be extended to other sports and to help improve life habits.
For example, Dr. Susan A. Cohen has seen that many patients have a decline in their dental health because of fear of going to the dentist to receive the treatment they need. Visualization techniques decrease the patient’s anxiety by rehearsing the possible traumatic events of the dental visit – e.g., the injection of anesthesia before the dental procedure. Visualization of calmness with systematic desensitization has helped decrease anxiety in patients.
In 20 years of clinical experience as a dentist, Dr. Cohen has seen how these techniques have increased compliance in her dental patients. She has also noted that visualizing the results of having a healthy mouth with improved appearance and function leads to an overall willingness to visit the dentist regularly and enjoy the dental experience. These examples demonstrate how visualization can enhance sports performance, quality of life, and overall health.
Dr. Richard W. Cohen is a psychiatrist who has been in private practice for over 40 years and is on the editorial advisory board for Clinical Psychiatry News. He has won 18 USTA national tennis championships. Dr. Susan A. Cohen has practiced dentistry for over 20 years. The Cohens, who are married, are based in Philadelphia.
Over the past 30 years, Dr. Richard W. Cohen has used visualization techniques to help world class tennis players and recreational tennis players become the best they could be.
Visualization should be used in two ways to help players improve. First, to improve technique, after every practice session I have the player think about one shot they did not do well technically, and I have them, in vivo, shadow the shot on the court correctly before they leave the court. That night I tell the player to put themselves in a quiet, relaxed place and, in vitro, visualize themselves hitting the shot the correct way.
Almost always, the next day the players tell me they are hitting that one shot better and are motivated to again think about the one shot that was not technically correct and repeat the in vivo technique with similar great results.
The second way I use visualization for tennis players is to decrease their anxiety before matches. It is important to have some preparatory anxiety to perform optimally but having excessive anxiety will decrease performance. To alleviate excessive anxiety before matches, I have players watch their opponents hit the day before the match for at least 5 minutes to see their strengths and weaknesses. Then, the night before the match, I have them visualize how they will play a big point utilizing their strength into their opponent’s weakness. This rehearsal using imagery the night before a big match will decrease a player’s excessive anxiety and allow them to achieve their best effort in the match.
An example of this is if their opponent has a weak backhand that they can only slice. They visualize hitting wide to their forehand to get into their weak backhand and see themselves going forward and putting away a volley. Visualization used in these two ways helps improve stroke mechanics and match results in players of all levels. These visualization techniques can also be extended to other sports and to help improve life habits.
For example, Dr. Susan A. Cohen has seen that many patients have a decline in their dental health because of fear of going to the dentist to receive the treatment they need. Visualization techniques decrease the patient’s anxiety by rehearsing the possible traumatic events of the dental visit – e.g., the injection of anesthesia before the dental procedure. Visualization of calmness with systematic desensitization has helped decrease anxiety in patients.
In 20 years of clinical experience as a dentist, Dr. Cohen has seen how these techniques have increased compliance in her dental patients. She has also noted that visualizing the results of having a healthy mouth with improved appearance and function leads to an overall willingness to visit the dentist regularly and enjoy the dental experience. These examples demonstrate how visualization can enhance sports performance, quality of life, and overall health.
Dr. Richard W. Cohen is a psychiatrist who has been in private practice for over 40 years and is on the editorial advisory board for Clinical Psychiatry News. He has won 18 USTA national tennis championships. Dr. Susan A. Cohen has practiced dentistry for over 20 years. The Cohens, who are married, are based in Philadelphia.
Over the past 30 years, Dr. Richard W. Cohen has used visualization techniques to help world class tennis players and recreational tennis players become the best they could be.
Visualization should be used in two ways to help players improve. First, to improve technique, after every practice session I have the player think about one shot they did not do well technically, and I have them, in vivo, shadow the shot on the court correctly before they leave the court. That night I tell the player to put themselves in a quiet, relaxed place and, in vitro, visualize themselves hitting the shot the correct way.
Almost always, the next day the players tell me they are hitting that one shot better and are motivated to again think about the one shot that was not technically correct and repeat the in vivo technique with similar great results.
The second way I use visualization for tennis players is to decrease their anxiety before matches. It is important to have some preparatory anxiety to perform optimally but having excessive anxiety will decrease performance. To alleviate excessive anxiety before matches, I have players watch their opponents hit the day before the match for at least 5 minutes to see their strengths and weaknesses. Then, the night before the match, I have them visualize how they will play a big point utilizing their strength into their opponent’s weakness. This rehearsal using imagery the night before a big match will decrease a player’s excessive anxiety and allow them to achieve their best effort in the match.
An example of this is if their opponent has a weak backhand that they can only slice. They visualize hitting wide to their forehand to get into their weak backhand and see themselves going forward and putting away a volley. Visualization used in these two ways helps improve stroke mechanics and match results in players of all levels. These visualization techniques can also be extended to other sports and to help improve life habits.
For example, Dr. Susan A. Cohen has seen that many patients have a decline in their dental health because of fear of going to the dentist to receive the treatment they need. Visualization techniques decrease the patient’s anxiety by rehearsing the possible traumatic events of the dental visit – e.g., the injection of anesthesia before the dental procedure. Visualization of calmness with systematic desensitization has helped decrease anxiety in patients.
In 20 years of clinical experience as a dentist, Dr. Cohen has seen how these techniques have increased compliance in her dental patients. She has also noted that visualizing the results of having a healthy mouth with improved appearance and function leads to an overall willingness to visit the dentist regularly and enjoy the dental experience. These examples demonstrate how visualization can enhance sports performance, quality of life, and overall health.
Dr. Richard W. Cohen is a psychiatrist who has been in private practice for over 40 years and is on the editorial advisory board for Clinical Psychiatry News. He has won 18 USTA national tennis championships. Dr. Susan A. Cohen has practiced dentistry for over 20 years. The Cohens, who are married, are based in Philadelphia.
New guidelines say pediatricians should screen for anxiety: Now what?
Recently the U.S. Preventive Services Task Force issued a formal recommendation that adolescents and children as young as 8 should be screened for anxiety.1 The advice was based on a review of the research that concluded that anxiety disorders were common in youth (prevalence around 8%), screening was not overly burdensome or dangerous, and treatments were available and effective.
While pediatricians fully appreciate how common clinically significant anxiety is and its impact on the lives of youth, the reception for the recommendations have been mixed. Some are concerned that it could lead to the overprescribing of medications. Arguably, the biggest pushback, however, relates to the question of what to do when a child screens positive in a time when finding an available child and adolescent psychiatrist or other type of pediatric mental health professional can feel next to impossible. The hope of this article is to fill in some of those gaps.
Screening for anxiety disorders
The recommendations suggest using a rating scale as part of the screen but doesn’t dictate which one. A common instrument that has been employed is the Screen for Child Anxiety and Related Disorders, which is a freely available 41-item instrument that has versions for youth self-report and parent-report. A shorter 7-item rating scale, the General Anxiety Disorder–7, and the even shorter GAD-2 (the first two questions of the GAD-7), are also popular but focus, as the name applies, on general anxiety disorder and not related conditions such as social or separation anxiety that can have some different symptoms. These instruments can be given to patients and families in the waiting room or administered with the help of a nurse, physician, or embedded mental health professional. The recommendations do not include specific guidance on how often the screening should be done but repeated screenings are likely important at some interval.
Confirming the diagnosis
Of course, a screening isn’t a formal diagnosis. The American Academy of Pediatrics has expressed the view that the initial diagnosis and treatment for anxiety disorders is well within a pediatrician’s scope of practice, which means further steps are likely required beyond a referral. Fortunately, going from a positive screen to an initial diagnosis does not have to overly laborious and can focus on reviewing the DSM-5 criteria for key anxiety disorders while also ensuring that there isn’t a nonpsychiatric cause driving the symptoms, such as the often cited but rarely seen pheochromocytoma. More common rule-outs include medication-induced anxiety or substance use, excessive caffeine intake, and cardiac arrhythmias. Assessing for current and past trauma or specific causes of the anxiety such as bullying are also important.
It is important to note that it is the rule rather than the exception that youth with clinical levels of anxiety will frequently endorse a number of criteria that span multiple diagnoses including generalized anxiety disorder, social anxiety disorder, and separation anxiety disorder.2 Spending a lot of effort to narrow things down to a single anxiety diagnosis often is unnecessary, as both pharmacologic and nonpharmacologic treatments don’t change all that much between individual diagnoses.
Explaining the diagnosis
In general, I’m a strong proponent of trying to explain any behavioral diagnoses that you make to kids in a way that is accurate but nonstigmatizing. When it comes to anxiety, one parallel I often draw is to our immune system, which most youth understand at least in basic terms. Both our immune system and our anxiety networks are natural and important; as a species, we wouldn’t have lasted long without them. Both are built to assess and respond to threats. Problems can arise, however, if the response is too strong relative to the threat or the response is activated when it doesn’t need to be. Treatment is directed not at ridding ourselves of anxiety but at helping regulate it so it works for us and not against us. Spending a few minutes going through a discussion like this can be very helpful, and perhaps more so than some dry summary of DSM-5 criteria.
Starting treatment
It is important to note that best practice recommendations when it comes to the treatment of anxiety disorder in youth do not suggest medications as the only type of treatment and often urge clinicians to try nonpharmacological interventions first.3 A specific type of psychotherapy called cognitive-behavioral therapy has the strongest scientific support as an effective treatment for anxiety but other modalities, including parenting guidance, can be helpful as well. Consequently, a referral to a good psychotherapist is paramount. For many kids, the key to overcoming anxiety is exposure: which means confronting anxiety slowly, with support, and with specific skills.
If there is a traumatic source of the anxiety, addressing that as much as possible is obviously critical and could involve working with the family or school. For some kids, this may involve frightening things they are seeing online or through other media. Finally, some health promotion activities such as exercise or mindfulness can also be quite useful.
Despite the fact that SSRIs are referred to as antidepressants, there is increasing appreciation that these medications are useful for anxiety, perhaps even more so than for mood. While only one medication, duloxetine, has Food and Drug Administration approval to treat anxiety in children as young as 7, there is good evidence to support the use of many of the most common SSRIs in treating clinical anxiety. Buspirone, beta-blockers, and antihistamine medications like hydroxyzine also can have their place in treatment, while benzodiazepines and antipsychotic medications are generally best avoided for anxious youth, especially in the primary care setting. A short but helpful medication guide with regard to pediatric anxiety has been published by the American Academy of Child and Adolescent Psychiatry.4
Conclusions
Clinical levels of anxiety in children and adolescents are both common and quite treatable, which has prompted new recommendations that primary care clinicians screen for them starting at age 8. While this recommendation may at first seem like yet one more task to fit in, following the guidance can be accomplished with the help of short screening tools and a managed multimodal approach to treatment.
Dr. Rettew is a child and adolescent psychiatrist with Lane County Behavioral Health in Eugene, Ore., and Oregon Health & Science University, Portland. You can follow him on Twitter and Facebook @PediPsych.
References
1. U.S. Preventive Services Task Force. JAMA. 2022;328(14):1438-44.
2. Strawn JR. Curr Psychiatry. 2012;11(9):16-21.
3. Walter HJ et al. J Am Acad Child Adolesc Psychiatry. 2020;59(10):1107-24.
4. Anxiety Disorders: Parents’ Medication Guide Workgroup. “Anxiety disorders: Parents’ medication guide.” Washington D.C.: American Academy of Child & Adolescent Psychiatry, 2020.
Recently the U.S. Preventive Services Task Force issued a formal recommendation that adolescents and children as young as 8 should be screened for anxiety.1 The advice was based on a review of the research that concluded that anxiety disorders were common in youth (prevalence around 8%), screening was not overly burdensome or dangerous, and treatments were available and effective.
While pediatricians fully appreciate how common clinically significant anxiety is and its impact on the lives of youth, the reception for the recommendations have been mixed. Some are concerned that it could lead to the overprescribing of medications. Arguably, the biggest pushback, however, relates to the question of what to do when a child screens positive in a time when finding an available child and adolescent psychiatrist or other type of pediatric mental health professional can feel next to impossible. The hope of this article is to fill in some of those gaps.
Screening for anxiety disorders
The recommendations suggest using a rating scale as part of the screen but doesn’t dictate which one. A common instrument that has been employed is the Screen for Child Anxiety and Related Disorders, which is a freely available 41-item instrument that has versions for youth self-report and parent-report. A shorter 7-item rating scale, the General Anxiety Disorder–7, and the even shorter GAD-2 (the first two questions of the GAD-7), are also popular but focus, as the name applies, on general anxiety disorder and not related conditions such as social or separation anxiety that can have some different symptoms. These instruments can be given to patients and families in the waiting room or administered with the help of a nurse, physician, or embedded mental health professional. The recommendations do not include specific guidance on how often the screening should be done but repeated screenings are likely important at some interval.
Confirming the diagnosis
Of course, a screening isn’t a formal diagnosis. The American Academy of Pediatrics has expressed the view that the initial diagnosis and treatment for anxiety disorders is well within a pediatrician’s scope of practice, which means further steps are likely required beyond a referral. Fortunately, going from a positive screen to an initial diagnosis does not have to overly laborious and can focus on reviewing the DSM-5 criteria for key anxiety disorders while also ensuring that there isn’t a nonpsychiatric cause driving the symptoms, such as the often cited but rarely seen pheochromocytoma. More common rule-outs include medication-induced anxiety or substance use, excessive caffeine intake, and cardiac arrhythmias. Assessing for current and past trauma or specific causes of the anxiety such as bullying are also important.
It is important to note that it is the rule rather than the exception that youth with clinical levels of anxiety will frequently endorse a number of criteria that span multiple diagnoses including generalized anxiety disorder, social anxiety disorder, and separation anxiety disorder.2 Spending a lot of effort to narrow things down to a single anxiety diagnosis often is unnecessary, as both pharmacologic and nonpharmacologic treatments don’t change all that much between individual diagnoses.
Explaining the diagnosis
In general, I’m a strong proponent of trying to explain any behavioral diagnoses that you make to kids in a way that is accurate but nonstigmatizing. When it comes to anxiety, one parallel I often draw is to our immune system, which most youth understand at least in basic terms. Both our immune system and our anxiety networks are natural and important; as a species, we wouldn’t have lasted long without them. Both are built to assess and respond to threats. Problems can arise, however, if the response is too strong relative to the threat or the response is activated when it doesn’t need to be. Treatment is directed not at ridding ourselves of anxiety but at helping regulate it so it works for us and not against us. Spending a few minutes going through a discussion like this can be very helpful, and perhaps more so than some dry summary of DSM-5 criteria.
Starting treatment
It is important to note that best practice recommendations when it comes to the treatment of anxiety disorder in youth do not suggest medications as the only type of treatment and often urge clinicians to try nonpharmacological interventions first.3 A specific type of psychotherapy called cognitive-behavioral therapy has the strongest scientific support as an effective treatment for anxiety but other modalities, including parenting guidance, can be helpful as well. Consequently, a referral to a good psychotherapist is paramount. For many kids, the key to overcoming anxiety is exposure: which means confronting anxiety slowly, with support, and with specific skills.
If there is a traumatic source of the anxiety, addressing that as much as possible is obviously critical and could involve working with the family or school. For some kids, this may involve frightening things they are seeing online or through other media. Finally, some health promotion activities such as exercise or mindfulness can also be quite useful.
Despite the fact that SSRIs are referred to as antidepressants, there is increasing appreciation that these medications are useful for anxiety, perhaps even more so than for mood. While only one medication, duloxetine, has Food and Drug Administration approval to treat anxiety in children as young as 7, there is good evidence to support the use of many of the most common SSRIs in treating clinical anxiety. Buspirone, beta-blockers, and antihistamine medications like hydroxyzine also can have their place in treatment, while benzodiazepines and antipsychotic medications are generally best avoided for anxious youth, especially in the primary care setting. A short but helpful medication guide with regard to pediatric anxiety has been published by the American Academy of Child and Adolescent Psychiatry.4
Conclusions
Clinical levels of anxiety in children and adolescents are both common and quite treatable, which has prompted new recommendations that primary care clinicians screen for them starting at age 8. While this recommendation may at first seem like yet one more task to fit in, following the guidance can be accomplished with the help of short screening tools and a managed multimodal approach to treatment.
Dr. Rettew is a child and adolescent psychiatrist with Lane County Behavioral Health in Eugene, Ore., and Oregon Health & Science University, Portland. You can follow him on Twitter and Facebook @PediPsych.
References
1. U.S. Preventive Services Task Force. JAMA. 2022;328(14):1438-44.
2. Strawn JR. Curr Psychiatry. 2012;11(9):16-21.
3. Walter HJ et al. J Am Acad Child Adolesc Psychiatry. 2020;59(10):1107-24.
4. Anxiety Disorders: Parents’ Medication Guide Workgroup. “Anxiety disorders: Parents’ medication guide.” Washington D.C.: American Academy of Child & Adolescent Psychiatry, 2020.
Recently the U.S. Preventive Services Task Force issued a formal recommendation that adolescents and children as young as 8 should be screened for anxiety.1 The advice was based on a review of the research that concluded that anxiety disorders were common in youth (prevalence around 8%), screening was not overly burdensome or dangerous, and treatments were available and effective.
While pediatricians fully appreciate how common clinically significant anxiety is and its impact on the lives of youth, the reception for the recommendations have been mixed. Some are concerned that it could lead to the overprescribing of medications. Arguably, the biggest pushback, however, relates to the question of what to do when a child screens positive in a time when finding an available child and adolescent psychiatrist or other type of pediatric mental health professional can feel next to impossible. The hope of this article is to fill in some of those gaps.
Screening for anxiety disorders
The recommendations suggest using a rating scale as part of the screen but doesn’t dictate which one. A common instrument that has been employed is the Screen for Child Anxiety and Related Disorders, which is a freely available 41-item instrument that has versions for youth self-report and parent-report. A shorter 7-item rating scale, the General Anxiety Disorder–7, and the even shorter GAD-2 (the first two questions of the GAD-7), are also popular but focus, as the name applies, on general anxiety disorder and not related conditions such as social or separation anxiety that can have some different symptoms. These instruments can be given to patients and families in the waiting room or administered with the help of a nurse, physician, or embedded mental health professional. The recommendations do not include specific guidance on how often the screening should be done but repeated screenings are likely important at some interval.
Confirming the diagnosis
Of course, a screening isn’t a formal diagnosis. The American Academy of Pediatrics has expressed the view that the initial diagnosis and treatment for anxiety disorders is well within a pediatrician’s scope of practice, which means further steps are likely required beyond a referral. Fortunately, going from a positive screen to an initial diagnosis does not have to overly laborious and can focus on reviewing the DSM-5 criteria for key anxiety disorders while also ensuring that there isn’t a nonpsychiatric cause driving the symptoms, such as the often cited but rarely seen pheochromocytoma. More common rule-outs include medication-induced anxiety or substance use, excessive caffeine intake, and cardiac arrhythmias. Assessing for current and past trauma or specific causes of the anxiety such as bullying are also important.
It is important to note that it is the rule rather than the exception that youth with clinical levels of anxiety will frequently endorse a number of criteria that span multiple diagnoses including generalized anxiety disorder, social anxiety disorder, and separation anxiety disorder.2 Spending a lot of effort to narrow things down to a single anxiety diagnosis often is unnecessary, as both pharmacologic and nonpharmacologic treatments don’t change all that much between individual diagnoses.
Explaining the diagnosis
In general, I’m a strong proponent of trying to explain any behavioral diagnoses that you make to kids in a way that is accurate but nonstigmatizing. When it comes to anxiety, one parallel I often draw is to our immune system, which most youth understand at least in basic terms. Both our immune system and our anxiety networks are natural and important; as a species, we wouldn’t have lasted long without them. Both are built to assess and respond to threats. Problems can arise, however, if the response is too strong relative to the threat or the response is activated when it doesn’t need to be. Treatment is directed not at ridding ourselves of anxiety but at helping regulate it so it works for us and not against us. Spending a few minutes going through a discussion like this can be very helpful, and perhaps more so than some dry summary of DSM-5 criteria.
Starting treatment
It is important to note that best practice recommendations when it comes to the treatment of anxiety disorder in youth do not suggest medications as the only type of treatment and often urge clinicians to try nonpharmacological interventions first.3 A specific type of psychotherapy called cognitive-behavioral therapy has the strongest scientific support as an effective treatment for anxiety but other modalities, including parenting guidance, can be helpful as well. Consequently, a referral to a good psychotherapist is paramount. For many kids, the key to overcoming anxiety is exposure: which means confronting anxiety slowly, with support, and with specific skills.
If there is a traumatic source of the anxiety, addressing that as much as possible is obviously critical and could involve working with the family or school. For some kids, this may involve frightening things they are seeing online or through other media. Finally, some health promotion activities such as exercise or mindfulness can also be quite useful.
Despite the fact that SSRIs are referred to as antidepressants, there is increasing appreciation that these medications are useful for anxiety, perhaps even more so than for mood. While only one medication, duloxetine, has Food and Drug Administration approval to treat anxiety in children as young as 7, there is good evidence to support the use of many of the most common SSRIs in treating clinical anxiety. Buspirone, beta-blockers, and antihistamine medications like hydroxyzine also can have their place in treatment, while benzodiazepines and antipsychotic medications are generally best avoided for anxious youth, especially in the primary care setting. A short but helpful medication guide with regard to pediatric anxiety has been published by the American Academy of Child and Adolescent Psychiatry.4
Conclusions
Clinical levels of anxiety in children and adolescents are both common and quite treatable, which has prompted new recommendations that primary care clinicians screen for them starting at age 8. While this recommendation may at first seem like yet one more task to fit in, following the guidance can be accomplished with the help of short screening tools and a managed multimodal approach to treatment.
Dr. Rettew is a child and adolescent psychiatrist with Lane County Behavioral Health in Eugene, Ore., and Oregon Health & Science University, Portland. You can follow him on Twitter and Facebook @PediPsych.
References
1. U.S. Preventive Services Task Force. JAMA. 2022;328(14):1438-44.
2. Strawn JR. Curr Psychiatry. 2012;11(9):16-21.
3. Walter HJ et al. J Am Acad Child Adolesc Psychiatry. 2020;59(10):1107-24.
4. Anxiety Disorders: Parents’ Medication Guide Workgroup. “Anxiety disorders: Parents’ medication guide.” Washington D.C.: American Academy of Child & Adolescent Psychiatry, 2020.
Optimal psychiatric treatment: Target the brain and avoid the body
Pharmacotherapy for psychiatric disorders is a mixed blessing. The advent of psychotropic medications since the 1950s (antipsychotics, antidepressants, anxiolytics, mood stabilizers) has revolutionized the treatment of serious psychiatric brain disorders, allowing certain patients to be discharged to the community after a lifetime of institutionalization.
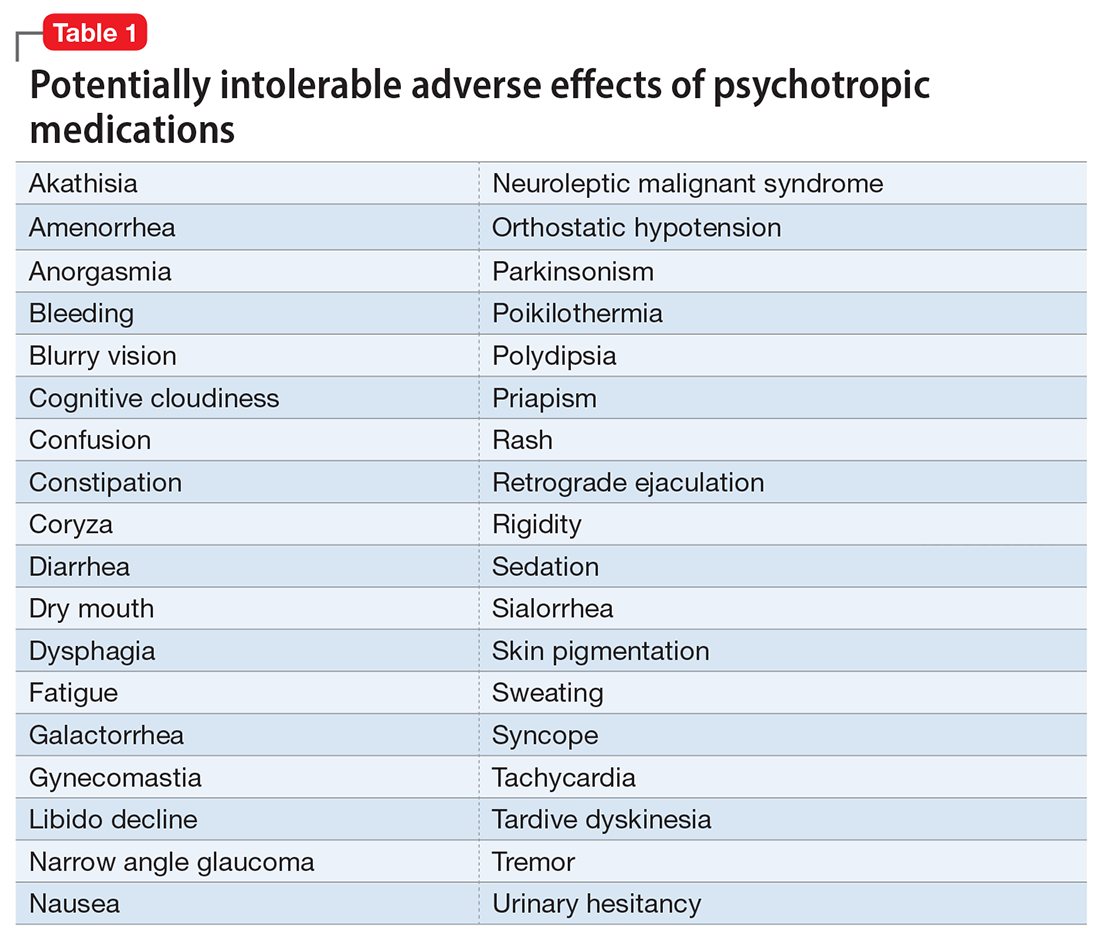
However, like all medications, psychotropic agents are often associated with various potentially intolerable symptoms (Table 1) or safety complications (Table 2) because they interact with every organ in the body besides their intended target, the brain, and its neurochemical circuitry.
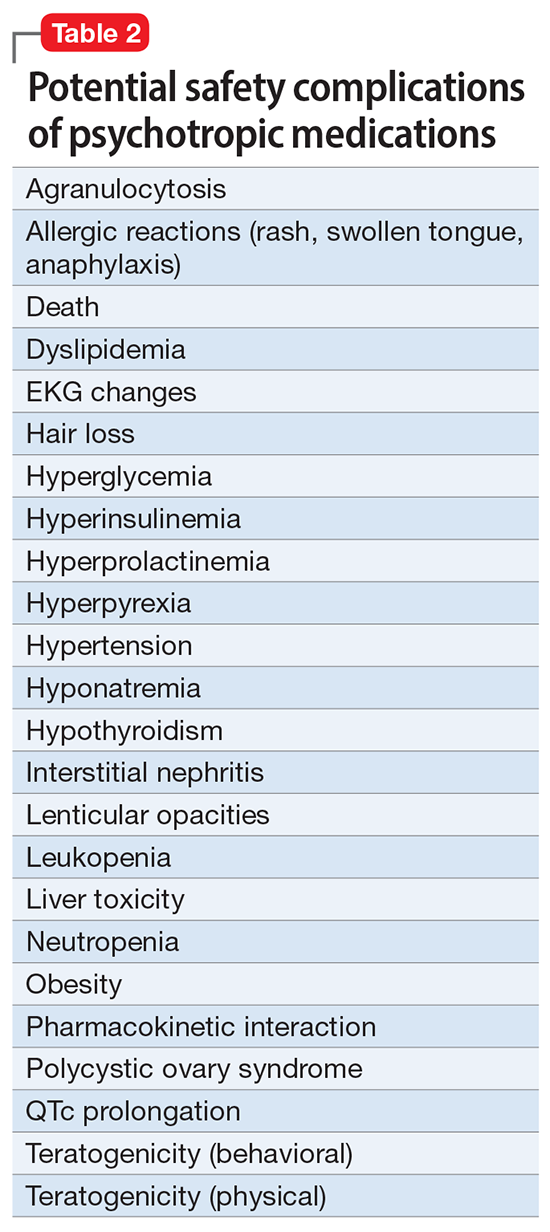
Imagine if we could treat our psychiatric patients while bypassing the body and achieve response, remission, and ultimately recovery without any systemic adverse effects. Adherence would dramatically improve, our patients’ quality of life would be enhanced, and the overall effectiveness (defined as the complex package of efficacy, safety, and tolerability) would be superior to current pharmacotherapies. This is important because most psychiatric medications must be taken daily for years, even a lifetime, to avoid a relapse of the illness. Psychiatrists frequently must manage adverse effects or switch the patient to a different medication if a tolerability or safety issue emerges, which is very common in psychiatric practice. A significant part of psychopharmacologic management includes ordering various laboratory tests to monitor adverse reactions in major organs, especially the liver, kidney, and heart. Additionally, psychiatric physicians must be constantly cognizant of medications prescribed by other clinicians for comorbid medical conditions to successfully navigate the turbulent seas of pharmacokinetic interactions.
I am sure you have noticed that whenever you watch a direct-to-consumer commercial for any medication, 90% of the advertisement is a background voice listing the various tolerability and safety complications of the medication as required by the FDA. Interestingly, these ads frequently contain colorful scenery and joyful clips, which I suspect are cleverly designed to distract the audience from focusing on the list of adverse effects.
Benefits of nonpharmacologic treatments
No wonder I am a fan of psychotherapy, a well-established psychiatric treatment modality that completely avoids body tissues. It directly targets the brain without needlessly interacting with any other organ. Psychotherapy’s many benefits (improving insight, enhancing adherence, improving self-esteem, reducing risky behaviors, guiding stress management and coping skills, modifying unhealthy beliefs, and ultimately relieving symptoms such as anxiety and depression) are achieved without any somatic adverse effects! Psychotherapy has also been shown to induce neuroplasticity and reduce inflammatory biomarkers.1 Unlike FDA-approved medications, psychotherapy does not include a “package insert,” 10 to 20 pages (in small print) that mostly focus on warnings, precautions, and sundry physical adverse effects. Even the dosing of psychotherapy is left entirely up to the treating clinician!
Although I have had many gratifying results with pharmacotherapy in my practice, especially in combination with psychotherapy,2 I also have observed excellent outcomes with nonpharmacologic approaches, especially neuromodulation therapies. The best antidepressant I have ever used since my residency training days is electroconvulsive therapy (ECT). My experience is consistent with a large meta-analysis3showing a huge effect size (Cohen d = .91) in contrast to the usual effect size of .3 to .5 for standard antidepressants (except IV ketamine). A recent study showed ECT is even better than the vaunted rapid-acting ketamine,4 which is further evidence of its remarkable efficacy in depression. Neuroimaging studies report that ECT rapidly increases the volume of the hippocampus,5,6 which shrinks in size in patients with unipolar or bipolar depression.
Neuromodulation may very well be the future of psychiatric therapeutics. It targets the brain and avoids the body, thus achieving efficacy with minimal systemic tolerability (ie, patient complaints) (Table 1) or safety (abnormal laboratory test results) issues (Table 2). This sounds ideal, and it is arguably an optimal approach to repairing the brain and healing the mind.
Continue to: ECT is the oldest...
ECT is the oldest neuromodulation technique (developed almost 100 years ago and significantly refined since then). Newer FDA-approved neuromodulation therapies include repetitive transcranial magnetic stimulation (rTMS), which was approved for depression in 2013, obsessive-compulsive disorder (OCD) in 2018, smoking cessation in 2020, and anxious depression in 2021.7 Vagus nerve stimulation (VNS) is used for drug-resistant epilepsy and was later approved for treatment-resistant depression,8,9 but some studies report it can be helpful for fear and anxiety in autism spectrum disorder10 and primary insomnia.11
There are many other neuromodulation therapies in development12 that have not yet been FDA approved (Table 3). The most prominent of these is deep brain stimulation (DBS), which is approved for Parkinson disease and has been reported in many studies to improve treatment-resistant depression13,14 and OCD.15 Another promising neuromodulation therapy is transcranial direct current stimulation (tDCS), which has promising results in schizophrenia16 similar to ECT’s effects in treatment-resistant schizophrenia.17
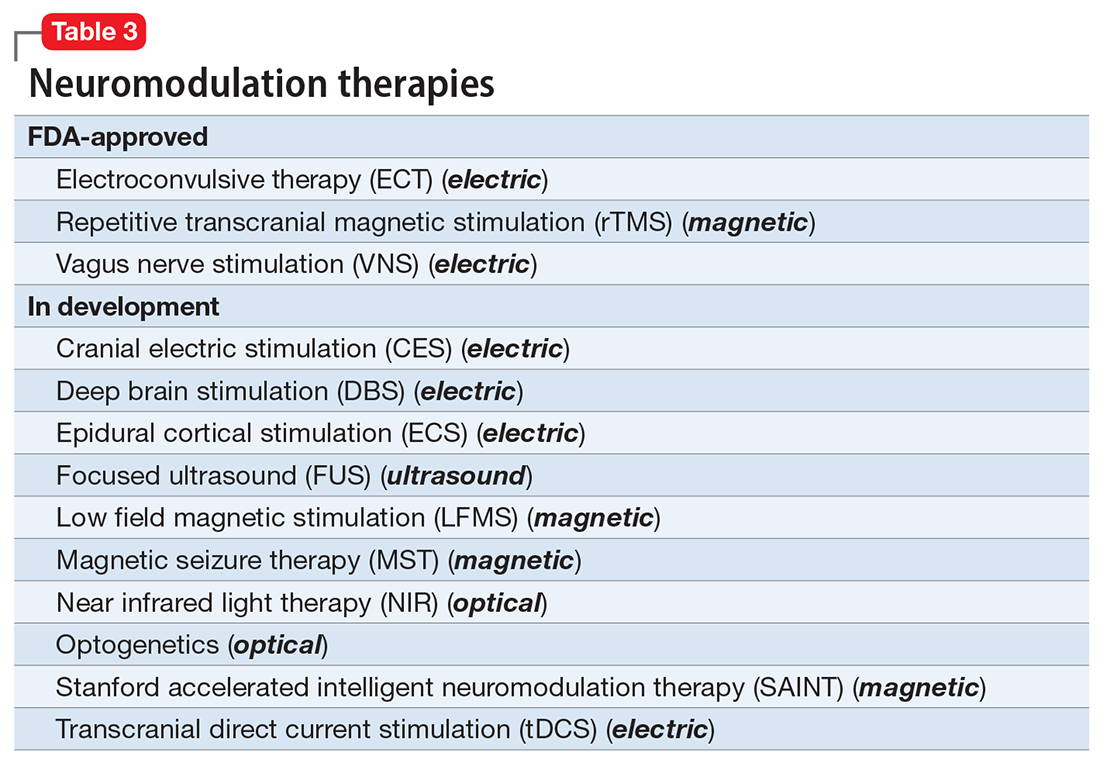
A particularly exciting neuromodulation approach published by Stanford University researchers is Stanford accelerated intelligent neuromodulation therapy (SAINT),18 which uses intermittent theta-burst stimulation (iTBS) daily for 5 days, targeted at the subgenual anterior cingulate gyrus (Brodman area 25). Remarkably, efficacy was rapid, with a very high remission rate (absence of symptoms) in approximately 90% of patients with severe depression.18
The future is bright for neuromodulation therapies, and for a good reason. Why send a chemical agent to every cell and organ in the body when the brain can be targeted directly? As psychiatric neuroscience advances to a point where we can localize the abnormal neurologic circuit in a specific brain region for each psychiatric disorder, it will be possible to treat almost all psychiatric disorders without burdening patients with the intolerable symptoms or safety adverse effects of medications. Psychiatrists should modulate their perspective about the future of psychiatric treatments. And finally, I propose that psychotherapy should be reclassified as a “verbal neuromodulation” technique.
1. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
2. Nasrallah HA. Bipolar disorder: clinical questions beg for answers. Current Psychiatry. 2006;5(12):11-12.
3. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
4. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022:e223352. doi:10.1001/jamapsychiatry.2022.3352
5. Nuninga JO, Mandl RCW, Boks MP, et al. Volume increase in the dentate gyrus after electroconvulsive therapy in depressed patients as measured with 7T. Mol Psychiatry. 2020;25(7):1559-1568.
6. Joshi SH, Espinoza RT, Pirnia T, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
7. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715.
8. Hilz MJ. Transcutaneous vagus nerve stimulation - a brief introduction and overview. Auton Neurosci. 2022;243:103038. doi:10.1016/j.autneu.2022.103038
9. Pigato G, Rosson S, Bresolin N, et al. Vagus nerve stimulation in treatment-resistant depression: a case series of long-term follow-up. J ECT. 2022. doi:10.1097/YCT.0000000000000869
10. Shivaswamy T, Souza RR, Engineer CT, et al. Vagus nerve stimulation as a treatment for fear and anxiety in individuals with autism spectrum disorder. J Psychiatr Brain Sci. 2022;7(4):e220007. doi:10.20900/jpbs.20220007
11. Wu Y, Song L, Wang X, et al. Transcutaneous vagus nerve stimulation could improve the effective rate on the quality of sleep in the treatment of primary insomnia: a randomized control trial. Brain Sci. 2022;12(10):1296. doi:10.3390/brainsci12101296
12. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
13. Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660.
14. Choi KS, Mayberg H. Connectomic DBS in major depression. In: Horn A, ed. Connectomic Deep Brain Stimulation. Academic Press; 2022:433-447.
15. Cruz S, Gutiérrez-Rojas L, González-Domenech P, et al. Deep brain stimulation in obsessive-compulsive disorder: results from meta-analysis. Psychiatry Res. 2022;317:114869. doi:10.1016/j.psychres.2022.114869
16. Lisoni J, Baldacci G, Nibbio G, et al. Effects of bilateral, bipolar-nonbalanced, frontal transcranial direct current stimulation (tDCS) on negative symptoms and neurocognition in a sample of patients living with schizophrenia: results of a randomized double-blind sham-controlled trial. J Psychiatr Res. 2022;155:430-442.
17. Sinclair DJ, Zhao S, Qi F, et al. Electroconvulsive therapy for treatment-resistant schizophrenia. Cochrane Database Syst Rev. 2019;3(3):CD011847. doi:10.1002/14651858.CD011847.pub2
18. Cole EJ, Stimpson KH, Bentzley BS, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020;177(8):716-726.
Pharmacotherapy for psychiatric disorders is a mixed blessing. The advent of psychotropic medications since the 1950s (antipsychotics, antidepressants, anxiolytics, mood stabilizers) has revolutionized the treatment of serious psychiatric brain disorders, allowing certain patients to be discharged to the community after a lifetime of institutionalization.

However, like all medications, psychotropic agents are often associated with various potentially intolerable symptoms (Table 1) or safety complications (Table 2) because they interact with every organ in the body besides their intended target, the brain, and its neurochemical circuitry.

Imagine if we could treat our psychiatric patients while bypassing the body and achieve response, remission, and ultimately recovery without any systemic adverse effects. Adherence would dramatically improve, our patients’ quality of life would be enhanced, and the overall effectiveness (defined as the complex package of efficacy, safety, and tolerability) would be superior to current pharmacotherapies. This is important because most psychiatric medications must be taken daily for years, even a lifetime, to avoid a relapse of the illness. Psychiatrists frequently must manage adverse effects or switch the patient to a different medication if a tolerability or safety issue emerges, which is very common in psychiatric practice. A significant part of psychopharmacologic management includes ordering various laboratory tests to monitor adverse reactions in major organs, especially the liver, kidney, and heart. Additionally, psychiatric physicians must be constantly cognizant of medications prescribed by other clinicians for comorbid medical conditions to successfully navigate the turbulent seas of pharmacokinetic interactions.
I am sure you have noticed that whenever you watch a direct-to-consumer commercial for any medication, 90% of the advertisement is a background voice listing the various tolerability and safety complications of the medication as required by the FDA. Interestingly, these ads frequently contain colorful scenery and joyful clips, which I suspect are cleverly designed to distract the audience from focusing on the list of adverse effects.
Benefits of nonpharmacologic treatments
No wonder I am a fan of psychotherapy, a well-established psychiatric treatment modality that completely avoids body tissues. It directly targets the brain without needlessly interacting with any other organ. Psychotherapy’s many benefits (improving insight, enhancing adherence, improving self-esteem, reducing risky behaviors, guiding stress management and coping skills, modifying unhealthy beliefs, and ultimately relieving symptoms such as anxiety and depression) are achieved without any somatic adverse effects! Psychotherapy has also been shown to induce neuroplasticity and reduce inflammatory biomarkers.1 Unlike FDA-approved medications, psychotherapy does not include a “package insert,” 10 to 20 pages (in small print) that mostly focus on warnings, precautions, and sundry physical adverse effects. Even the dosing of psychotherapy is left entirely up to the treating clinician!
Although I have had many gratifying results with pharmacotherapy in my practice, especially in combination with psychotherapy,2 I also have observed excellent outcomes with nonpharmacologic approaches, especially neuromodulation therapies. The best antidepressant I have ever used since my residency training days is electroconvulsive therapy (ECT). My experience is consistent with a large meta-analysis3showing a huge effect size (Cohen d = .91) in contrast to the usual effect size of .3 to .5 for standard antidepressants (except IV ketamine). A recent study showed ECT is even better than the vaunted rapid-acting ketamine,4 which is further evidence of its remarkable efficacy in depression. Neuroimaging studies report that ECT rapidly increases the volume of the hippocampus,5,6 which shrinks in size in patients with unipolar or bipolar depression.
Neuromodulation may very well be the future of psychiatric therapeutics. It targets the brain and avoids the body, thus achieving efficacy with minimal systemic tolerability (ie, patient complaints) (Table 1) or safety (abnormal laboratory test results) issues (Table 2). This sounds ideal, and it is arguably an optimal approach to repairing the brain and healing the mind.
Continue to: ECT is the oldest...
ECT is the oldest neuromodulation technique (developed almost 100 years ago and significantly refined since then). Newer FDA-approved neuromodulation therapies include repetitive transcranial magnetic stimulation (rTMS), which was approved for depression in 2013, obsessive-compulsive disorder (OCD) in 2018, smoking cessation in 2020, and anxious depression in 2021.7 Vagus nerve stimulation (VNS) is used for drug-resistant epilepsy and was later approved for treatment-resistant depression,8,9 but some studies report it can be helpful for fear and anxiety in autism spectrum disorder10 and primary insomnia.11
There are many other neuromodulation therapies in development12 that have not yet been FDA approved (Table 3). The most prominent of these is deep brain stimulation (DBS), which is approved for Parkinson disease and has been reported in many studies to improve treatment-resistant depression13,14 and OCD.15 Another promising neuromodulation therapy is transcranial direct current stimulation (tDCS), which has promising results in schizophrenia16 similar to ECT’s effects in treatment-resistant schizophrenia.17

A particularly exciting neuromodulation approach published by Stanford University researchers is Stanford accelerated intelligent neuromodulation therapy (SAINT),18 which uses intermittent theta-burst stimulation (iTBS) daily for 5 days, targeted at the subgenual anterior cingulate gyrus (Brodman area 25). Remarkably, efficacy was rapid, with a very high remission rate (absence of symptoms) in approximately 90% of patients with severe depression.18
The future is bright for neuromodulation therapies, and for a good reason. Why send a chemical agent to every cell and organ in the body when the brain can be targeted directly? As psychiatric neuroscience advances to a point where we can localize the abnormal neurologic circuit in a specific brain region for each psychiatric disorder, it will be possible to treat almost all psychiatric disorders without burdening patients with the intolerable symptoms or safety adverse effects of medications. Psychiatrists should modulate their perspective about the future of psychiatric treatments. And finally, I propose that psychotherapy should be reclassified as a “verbal neuromodulation” technique.
Pharmacotherapy for psychiatric disorders is a mixed blessing. The advent of psychotropic medications since the 1950s (antipsychotics, antidepressants, anxiolytics, mood stabilizers) has revolutionized the treatment of serious psychiatric brain disorders, allowing certain patients to be discharged to the community after a lifetime of institutionalization.

However, like all medications, psychotropic agents are often associated with various potentially intolerable symptoms (Table 1) or safety complications (Table 2) because they interact with every organ in the body besides their intended target, the brain, and its neurochemical circuitry.

Imagine if we could treat our psychiatric patients while bypassing the body and achieve response, remission, and ultimately recovery without any systemic adverse effects. Adherence would dramatically improve, our patients’ quality of life would be enhanced, and the overall effectiveness (defined as the complex package of efficacy, safety, and tolerability) would be superior to current pharmacotherapies. This is important because most psychiatric medications must be taken daily for years, even a lifetime, to avoid a relapse of the illness. Psychiatrists frequently must manage adverse effects or switch the patient to a different medication if a tolerability or safety issue emerges, which is very common in psychiatric practice. A significant part of psychopharmacologic management includes ordering various laboratory tests to monitor adverse reactions in major organs, especially the liver, kidney, and heart. Additionally, psychiatric physicians must be constantly cognizant of medications prescribed by other clinicians for comorbid medical conditions to successfully navigate the turbulent seas of pharmacokinetic interactions.
I am sure you have noticed that whenever you watch a direct-to-consumer commercial for any medication, 90% of the advertisement is a background voice listing the various tolerability and safety complications of the medication as required by the FDA. Interestingly, these ads frequently contain colorful scenery and joyful clips, which I suspect are cleverly designed to distract the audience from focusing on the list of adverse effects.
Benefits of nonpharmacologic treatments
No wonder I am a fan of psychotherapy, a well-established psychiatric treatment modality that completely avoids body tissues. It directly targets the brain without needlessly interacting with any other organ. Psychotherapy’s many benefits (improving insight, enhancing adherence, improving self-esteem, reducing risky behaviors, guiding stress management and coping skills, modifying unhealthy beliefs, and ultimately relieving symptoms such as anxiety and depression) are achieved without any somatic adverse effects! Psychotherapy has also been shown to induce neuroplasticity and reduce inflammatory biomarkers.1 Unlike FDA-approved medications, psychotherapy does not include a “package insert,” 10 to 20 pages (in small print) that mostly focus on warnings, precautions, and sundry physical adverse effects. Even the dosing of psychotherapy is left entirely up to the treating clinician!
Although I have had many gratifying results with pharmacotherapy in my practice, especially in combination with psychotherapy,2 I also have observed excellent outcomes with nonpharmacologic approaches, especially neuromodulation therapies. The best antidepressant I have ever used since my residency training days is electroconvulsive therapy (ECT). My experience is consistent with a large meta-analysis3showing a huge effect size (Cohen d = .91) in contrast to the usual effect size of .3 to .5 for standard antidepressants (except IV ketamine). A recent study showed ECT is even better than the vaunted rapid-acting ketamine,4 which is further evidence of its remarkable efficacy in depression. Neuroimaging studies report that ECT rapidly increases the volume of the hippocampus,5,6 which shrinks in size in patients with unipolar or bipolar depression.
Neuromodulation may very well be the future of psychiatric therapeutics. It targets the brain and avoids the body, thus achieving efficacy with minimal systemic tolerability (ie, patient complaints) (Table 1) or safety (abnormal laboratory test results) issues (Table 2). This sounds ideal, and it is arguably an optimal approach to repairing the brain and healing the mind.
Continue to: ECT is the oldest...
ECT is the oldest neuromodulation technique (developed almost 100 years ago and significantly refined since then). Newer FDA-approved neuromodulation therapies include repetitive transcranial magnetic stimulation (rTMS), which was approved for depression in 2013, obsessive-compulsive disorder (OCD) in 2018, smoking cessation in 2020, and anxious depression in 2021.7 Vagus nerve stimulation (VNS) is used for drug-resistant epilepsy and was later approved for treatment-resistant depression,8,9 but some studies report it can be helpful for fear and anxiety in autism spectrum disorder10 and primary insomnia.11
There are many other neuromodulation therapies in development12 that have not yet been FDA approved (Table 3). The most prominent of these is deep brain stimulation (DBS), which is approved for Parkinson disease and has been reported in many studies to improve treatment-resistant depression13,14 and OCD.15 Another promising neuromodulation therapy is transcranial direct current stimulation (tDCS), which has promising results in schizophrenia16 similar to ECT’s effects in treatment-resistant schizophrenia.17

A particularly exciting neuromodulation approach published by Stanford University researchers is Stanford accelerated intelligent neuromodulation therapy (SAINT),18 which uses intermittent theta-burst stimulation (iTBS) daily for 5 days, targeted at the subgenual anterior cingulate gyrus (Brodman area 25). Remarkably, efficacy was rapid, with a very high remission rate (absence of symptoms) in approximately 90% of patients with severe depression.18
The future is bright for neuromodulation therapies, and for a good reason. Why send a chemical agent to every cell and organ in the body when the brain can be targeted directly? As psychiatric neuroscience advances to a point where we can localize the abnormal neurologic circuit in a specific brain region for each psychiatric disorder, it will be possible to treat almost all psychiatric disorders without burdening patients with the intolerable symptoms or safety adverse effects of medications. Psychiatrists should modulate their perspective about the future of psychiatric treatments. And finally, I propose that psychotherapy should be reclassified as a “verbal neuromodulation” technique.
1. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
2. Nasrallah HA. Bipolar disorder: clinical questions beg for answers. Current Psychiatry. 2006;5(12):11-12.
3. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
4. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022:e223352. doi:10.1001/jamapsychiatry.2022.3352
5. Nuninga JO, Mandl RCW, Boks MP, et al. Volume increase in the dentate gyrus after electroconvulsive therapy in depressed patients as measured with 7T. Mol Psychiatry. 2020;25(7):1559-1568.
6. Joshi SH, Espinoza RT, Pirnia T, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
7. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715.
8. Hilz MJ. Transcutaneous vagus nerve stimulation - a brief introduction and overview. Auton Neurosci. 2022;243:103038. doi:10.1016/j.autneu.2022.103038
9. Pigato G, Rosson S, Bresolin N, et al. Vagus nerve stimulation in treatment-resistant depression: a case series of long-term follow-up. J ECT. 2022. doi:10.1097/YCT.0000000000000869
10. Shivaswamy T, Souza RR, Engineer CT, et al. Vagus nerve stimulation as a treatment for fear and anxiety in individuals with autism spectrum disorder. J Psychiatr Brain Sci. 2022;7(4):e220007. doi:10.20900/jpbs.20220007
11. Wu Y, Song L, Wang X, et al. Transcutaneous vagus nerve stimulation could improve the effective rate on the quality of sleep in the treatment of primary insomnia: a randomized control trial. Brain Sci. 2022;12(10):1296. doi:10.3390/brainsci12101296
12. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
13. Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660.
14. Choi KS, Mayberg H. Connectomic DBS in major depression. In: Horn A, ed. Connectomic Deep Brain Stimulation. Academic Press; 2022:433-447.
15. Cruz S, Gutiérrez-Rojas L, González-Domenech P, et al. Deep brain stimulation in obsessive-compulsive disorder: results from meta-analysis. Psychiatry Res. 2022;317:114869. doi:10.1016/j.psychres.2022.114869
16. Lisoni J, Baldacci G, Nibbio G, et al. Effects of bilateral, bipolar-nonbalanced, frontal transcranial direct current stimulation (tDCS) on negative symptoms and neurocognition in a sample of patients living with schizophrenia: results of a randomized double-blind sham-controlled trial. J Psychiatr Res. 2022;155:430-442.
17. Sinclair DJ, Zhao S, Qi F, et al. Electroconvulsive therapy for treatment-resistant schizophrenia. Cochrane Database Syst Rev. 2019;3(3):CD011847. doi:10.1002/14651858.CD011847.pub2
18. Cole EJ, Stimpson KH, Bentzley BS, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020;177(8):716-726.
1. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
2. Nasrallah HA. Bipolar disorder: clinical questions beg for answers. Current Psychiatry. 2006;5(12):11-12.
3. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
4. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022:e223352. doi:10.1001/jamapsychiatry.2022.3352
5. Nuninga JO, Mandl RCW, Boks MP, et al. Volume increase in the dentate gyrus after electroconvulsive therapy in depressed patients as measured with 7T. Mol Psychiatry. 2020;25(7):1559-1568.
6. Joshi SH, Espinoza RT, Pirnia T, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
7. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715.
8. Hilz MJ. Transcutaneous vagus nerve stimulation - a brief introduction and overview. Auton Neurosci. 2022;243:103038. doi:10.1016/j.autneu.2022.103038
9. Pigato G, Rosson S, Bresolin N, et al. Vagus nerve stimulation in treatment-resistant depression: a case series of long-term follow-up. J ECT. 2022. doi:10.1097/YCT.0000000000000869
10. Shivaswamy T, Souza RR, Engineer CT, et al. Vagus nerve stimulation as a treatment for fear and anxiety in individuals with autism spectrum disorder. J Psychiatr Brain Sci. 2022;7(4):e220007. doi:10.20900/jpbs.20220007
11. Wu Y, Song L, Wang X, et al. Transcutaneous vagus nerve stimulation could improve the effective rate on the quality of sleep in the treatment of primary insomnia: a randomized control trial. Brain Sci. 2022;12(10):1296. doi:10.3390/brainsci12101296
12. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
13. Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660.
14. Choi KS, Mayberg H. Connectomic DBS in major depression. In: Horn A, ed. Connectomic Deep Brain Stimulation. Academic Press; 2022:433-447.
15. Cruz S, Gutiérrez-Rojas L, González-Domenech P, et al. Deep brain stimulation in obsessive-compulsive disorder: results from meta-analysis. Psychiatry Res. 2022;317:114869. doi:10.1016/j.psychres.2022.114869
16. Lisoni J, Baldacci G, Nibbio G, et al. Effects of bilateral, bipolar-nonbalanced, frontal transcranial direct current stimulation (tDCS) on negative symptoms and neurocognition in a sample of patients living with schizophrenia: results of a randomized double-blind sham-controlled trial. J Psychiatr Res. 2022;155:430-442.
17. Sinclair DJ, Zhao S, Qi F, et al. Electroconvulsive therapy for treatment-resistant schizophrenia. Cochrane Database Syst Rev. 2019;3(3):CD011847. doi:10.1002/14651858.CD011847.pub2
18. Cole EJ, Stimpson KH, Bentzley BS, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020;177(8):716-726.
The surprising failure of vitamin D in deficient kids
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
My basic gripe is that you’ve got all these observational studies linking lower levels of vitamin D to everything from dementia to falls to cancer to COVID infection, and then you do a big randomized trial of supplementation and don’t see an effect.
And the explanation is that vitamin D is not necessarily the thing causing these bad outcomes; it’s a bystander – a canary in the coal mine. Your vitamin D level is a marker of your lifestyle; it’s higher in people who eat healthier foods, who exercise, and who spend more time out in the sun.
And yet ... if you were to ask me whether supplementing vitamin D in children with vitamin D deficiency would help them grow better and be healthier, I probably would have been on board for the idea.
And, it looks like, I would have been wrong.
Yes, it’s another negative randomized trial of vitamin D supplementation to add to the seemingly ever-growing body of literature suggesting that your money is better spent on a day at the park rather than buying D3 from your local GNC.
We are talking about this study, appearing in JAMA Pediatrics.
Briefly, 8,851 children from around Ulaanbaatar, Mongolia, were randomized to receive 14,000 international units of vitamin D3 or placebo every week for 3 years.
Before we get into the results of the study, I need to point out that this part of Mongolia has a high rate of vitamin D deficiency. Beyond that, a prior observational study by these authors had shown that lower vitamin D levels were linked to the risk of acquiring latent tuberculosis infection in this area. Other studies have linked vitamin D deficiency with poorer growth metrics in children. Given the global scourge that is TB (around 2 million deaths a year) and childhood malnutrition (around 10% of children around the world), vitamin D supplementation is incredibly attractive as a public health intervention. It is relatively low on side effects and, importantly, it is cheap – and thus scalable.
Back to the study. These kids had pretty poor vitamin D levels at baseline; 95% of them were deficient, based on the accepted standard of levels less than 20 ng/mL. Over 30% were severely deficient, with levels less than 10 ng/mL.
The initial purpose of this study was to see if supplementation would prevent TB, but that analysis, which was published a few months ago, was negative. Vitamin D levels went up dramatically in the intervention group – they were taking their pills – but there was no difference in the rate of latent TB infection, active TB, other respiratory infections, or even serum interferon gamma levels.
Nothing.
But to be fair, the TB seroconversion rate was lower than expected, potentially leading to an underpowered study.
Which brings us to the just-published analysis which moves away from infectious disease to something where vitamin D should have some stronger footing: growth.
Would the kids who were randomized to vitamin D, those same kids who got their vitamin D levels into the normal range over 3 years of supplementation, grow more or grow better than the kids who didn’t?
And, unfortunately, the answer is still no.
At the end of follow-up, height z scores were not different between the groups. BMI z scores were not different between the groups. Pubertal development was not different between the groups. This was true not only overall, but across various subgroups, including analyses of those kids who had vitamin D levels less than 10 ng/mL to start with.
So, what’s going on? There are two very broad possibilities we can endorse. First, there’s the idea that vitamin D supplementation simply doesn’t do much for health. This is supported, now, by a long string of large clinical trials that show no effect across a variety of disease states and predisease states. In other words, the observational data linking low vitamin D to bad outcomes is correlation, not causation.
Or we can take the tack of some vitamin D apologists and decide that this trial just got it wrong. Perhaps the dose wasn’t given correctly, or 3 years isn’t long enough to see a real difference, or the growth metrics were wrong, or vitamin D needs to be given alongside something else to really work and so on. This is fine; no study is perfect and there is always something to criticize, believe me. But we need to be careful not to fall into the baby-and-bathwater fallacy. Just because we think a study could have done something better, or differently, doesn’t mean we can ignore all the results. And as each new randomized trial of vitamin D supplementation comes out, it’s getting harder and harder to believe that these trialists keep getting their methods wrong. Maybe they are just testing something that doesn’t work.
What to do? Well, it should be obvious. If low vitamin D levels are linked to TB rates and poor growth but supplementation doesn’t fix the problem, then we have to fix what is upstream of the problem. We need to boost vitamin D levels not through supplements, but through nutrition, exercise, activity, and getting outside. That’s a randomized trial you can sign me up for any day.
Dr. Wilson is associate professor, department of medicine, Yale University, New Haven, Conn. He reported no relevant conflicts of interest.
A version of this video transcript first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
My basic gripe is that you’ve got all these observational studies linking lower levels of vitamin D to everything from dementia to falls to cancer to COVID infection, and then you do a big randomized trial of supplementation and don’t see an effect.
And the explanation is that vitamin D is not necessarily the thing causing these bad outcomes; it’s a bystander – a canary in the coal mine. Your vitamin D level is a marker of your lifestyle; it’s higher in people who eat healthier foods, who exercise, and who spend more time out in the sun.
And yet ... if you were to ask me whether supplementing vitamin D in children with vitamin D deficiency would help them grow better and be healthier, I probably would have been on board for the idea.
And, it looks like, I would have been wrong.
Yes, it’s another negative randomized trial of vitamin D supplementation to add to the seemingly ever-growing body of literature suggesting that your money is better spent on a day at the park rather than buying D3 from your local GNC.
We are talking about this study, appearing in JAMA Pediatrics.
Briefly, 8,851 children from around Ulaanbaatar, Mongolia, were randomized to receive 14,000 international units of vitamin D3 or placebo every week for 3 years.
Before we get into the results of the study, I need to point out that this part of Mongolia has a high rate of vitamin D deficiency. Beyond that, a prior observational study by these authors had shown that lower vitamin D levels were linked to the risk of acquiring latent tuberculosis infection in this area. Other studies have linked vitamin D deficiency with poorer growth metrics in children. Given the global scourge that is TB (around 2 million deaths a year) and childhood malnutrition (around 10% of children around the world), vitamin D supplementation is incredibly attractive as a public health intervention. It is relatively low on side effects and, importantly, it is cheap – and thus scalable.
Back to the study. These kids had pretty poor vitamin D levels at baseline; 95% of them were deficient, based on the accepted standard of levels less than 20 ng/mL. Over 30% were severely deficient, with levels less than 10 ng/mL.
The initial purpose of this study was to see if supplementation would prevent TB, but that analysis, which was published a few months ago, was negative. Vitamin D levels went up dramatically in the intervention group – they were taking their pills – but there was no difference in the rate of latent TB infection, active TB, other respiratory infections, or even serum interferon gamma levels.
Nothing.
But to be fair, the TB seroconversion rate was lower than expected, potentially leading to an underpowered study.
Which brings us to the just-published analysis which moves away from infectious disease to something where vitamin D should have some stronger footing: growth.
Would the kids who were randomized to vitamin D, those same kids who got their vitamin D levels into the normal range over 3 years of supplementation, grow more or grow better than the kids who didn’t?
And, unfortunately, the answer is still no.
At the end of follow-up, height z scores were not different between the groups. BMI z scores were not different between the groups. Pubertal development was not different between the groups. This was true not only overall, but across various subgroups, including analyses of those kids who had vitamin D levels less than 10 ng/mL to start with.
So, what’s going on? There are two very broad possibilities we can endorse. First, there’s the idea that vitamin D supplementation simply doesn’t do much for health. This is supported, now, by a long string of large clinical trials that show no effect across a variety of disease states and predisease states. In other words, the observational data linking low vitamin D to bad outcomes is correlation, not causation.
Or we can take the tack of some vitamin D apologists and decide that this trial just got it wrong. Perhaps the dose wasn’t given correctly, or 3 years isn’t long enough to see a real difference, or the growth metrics were wrong, or vitamin D needs to be given alongside something else to really work and so on. This is fine; no study is perfect and there is always something to criticize, believe me. But we need to be careful not to fall into the baby-and-bathwater fallacy. Just because we think a study could have done something better, or differently, doesn’t mean we can ignore all the results. And as each new randomized trial of vitamin D supplementation comes out, it’s getting harder and harder to believe that these trialists keep getting their methods wrong. Maybe they are just testing something that doesn’t work.
What to do? Well, it should be obvious. If low vitamin D levels are linked to TB rates and poor growth but supplementation doesn’t fix the problem, then we have to fix what is upstream of the problem. We need to boost vitamin D levels not through supplements, but through nutrition, exercise, activity, and getting outside. That’s a randomized trial you can sign me up for any day.
Dr. Wilson is associate professor, department of medicine, Yale University, New Haven, Conn. He reported no relevant conflicts of interest.
A version of this video transcript first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
My basic gripe is that you’ve got all these observational studies linking lower levels of vitamin D to everything from dementia to falls to cancer to COVID infection, and then you do a big randomized trial of supplementation and don’t see an effect.
And the explanation is that vitamin D is not necessarily the thing causing these bad outcomes; it’s a bystander – a canary in the coal mine. Your vitamin D level is a marker of your lifestyle; it’s higher in people who eat healthier foods, who exercise, and who spend more time out in the sun.
And yet ... if you were to ask me whether supplementing vitamin D in children with vitamin D deficiency would help them grow better and be healthier, I probably would have been on board for the idea.
And, it looks like, I would have been wrong.
Yes, it’s another negative randomized trial of vitamin D supplementation to add to the seemingly ever-growing body of literature suggesting that your money is better spent on a day at the park rather than buying D3 from your local GNC.
We are talking about this study, appearing in JAMA Pediatrics.
Briefly, 8,851 children from around Ulaanbaatar, Mongolia, were randomized to receive 14,000 international units of vitamin D3 or placebo every week for 3 years.
Before we get into the results of the study, I need to point out that this part of Mongolia has a high rate of vitamin D deficiency. Beyond that, a prior observational study by these authors had shown that lower vitamin D levels were linked to the risk of acquiring latent tuberculosis infection in this area. Other studies have linked vitamin D deficiency with poorer growth metrics in children. Given the global scourge that is TB (around 2 million deaths a year) and childhood malnutrition (around 10% of children around the world), vitamin D supplementation is incredibly attractive as a public health intervention. It is relatively low on side effects and, importantly, it is cheap – and thus scalable.
Back to the study. These kids had pretty poor vitamin D levels at baseline; 95% of them were deficient, based on the accepted standard of levels less than 20 ng/mL. Over 30% were severely deficient, with levels less than 10 ng/mL.
The initial purpose of this study was to see if supplementation would prevent TB, but that analysis, which was published a few months ago, was negative. Vitamin D levels went up dramatically in the intervention group – they were taking their pills – but there was no difference in the rate of latent TB infection, active TB, other respiratory infections, or even serum interferon gamma levels.
Nothing.
But to be fair, the TB seroconversion rate was lower than expected, potentially leading to an underpowered study.
Which brings us to the just-published analysis which moves away from infectious disease to something where vitamin D should have some stronger footing: growth.
Would the kids who were randomized to vitamin D, those same kids who got their vitamin D levels into the normal range over 3 years of supplementation, grow more or grow better than the kids who didn’t?
And, unfortunately, the answer is still no.
At the end of follow-up, height z scores were not different between the groups. BMI z scores were not different between the groups. Pubertal development was not different between the groups. This was true not only overall, but across various subgroups, including analyses of those kids who had vitamin D levels less than 10 ng/mL to start with.
So, what’s going on? There are two very broad possibilities we can endorse. First, there’s the idea that vitamin D supplementation simply doesn’t do much for health. This is supported, now, by a long string of large clinical trials that show no effect across a variety of disease states and predisease states. In other words, the observational data linking low vitamin D to bad outcomes is correlation, not causation.
Or we can take the tack of some vitamin D apologists and decide that this trial just got it wrong. Perhaps the dose wasn’t given correctly, or 3 years isn’t long enough to see a real difference, or the growth metrics were wrong, or vitamin D needs to be given alongside something else to really work and so on. This is fine; no study is perfect and there is always something to criticize, believe me. But we need to be careful not to fall into the baby-and-bathwater fallacy. Just because we think a study could have done something better, or differently, doesn’t mean we can ignore all the results. And as each new randomized trial of vitamin D supplementation comes out, it’s getting harder and harder to believe that these trialists keep getting their methods wrong. Maybe they are just testing something that doesn’t work.
What to do? Well, it should be obvious. If low vitamin D levels are linked to TB rates and poor growth but supplementation doesn’t fix the problem, then we have to fix what is upstream of the problem. We need to boost vitamin D levels not through supplements, but through nutrition, exercise, activity, and getting outside. That’s a randomized trial you can sign me up for any day.
Dr. Wilson is associate professor, department of medicine, Yale University, New Haven, Conn. He reported no relevant conflicts of interest.
A version of this video transcript first appeared on Medscape.com.









