User login
Neuropathy drives hypoglycemia cluelessness in T1D
Researchers published the study covered in this summary on researchsquare.com as a preprint that has not yet been peer reviewed.
Key takeaways
- In Japanese adults with type 1 diabetes insulin-pump treatment (continuous subcutaneous insulin infusion) and higher problem-solving perception appear protective against impaired awareness of hypoglycemia (IAH), while diabetic peripheral neuropathy (DPN) is associated with increased risk.
- Diabetes distress and fear of hypoglycemia are common in people with IAH.
Why this matters
- Adults with type 1 diabetes and IAH have a reduced ability to perceive hypoglycemic symptoms and are at risk of severe hypoglycemic events because they are unable to take immediate corrective action.
- This is the first study to identify protective factors and risk factors of IAH in Japanese adults with type 1 diabetes.
- People with IAH may plan to loosen tight glucose management and intentionally omit insulin injection to prevent severe hypoglycemia.
- The information in this report may help improve the management of people with problematic hypoglycemia, the authors suggested. Treatment with an insulin pump and structured education aimed at improving problem-solving skills may be useful interventions for adults with type 1 diabetes and IAH, they suggested.
Study design
- The study involved a cross-sectional analysis of 288 Japanese adults with type 1 diabetes who averaged 50 years old, had diabetes for an average of about 18 years, had an average hemoglobin A1c at baseline of 7.7%, and included about 37% men and 63% women.
- The cohort included 55 people with IAH (19%) and 233 with no impairment of their hypoglycemia awareness, based on their score on the .
Key results
- DPN was significantly more prevalent in the IAH group than in the control group (12.0% vs. 26.5%). A logistic regression analysis showed that the odds ratio for DPN was 2.63-fold higher among people with IAH, compared with those without IAH, but there were no differences in other complications or by HbA1c levels.
- Treatment with continuous subcutaneous insulin therapy (an insulin pump) was significantly less prevalent in the IAH group, compared with those without IAH (23.6% vs 39.5%), with an adjusted odds ratio of 0.48. The two subgroups showed no differences in use of continuous glucose monitoring, used by 56% of the people in each of the two subgroups.
- The two subgroups showed no differences in their healthy lifestyle score, sleep debt, or rates of excessive drinking.
- Mean autonomic symptom scores for both sweating and shaking were significantly reduced in the IAH group, but no between-group differences appeared for palpations or hunger.
- All mean neuroglycopenic symptom scores were significantly lower in those without IAH, including confusion and speech difficulty.
- Scores for measures of diabetes distress and for the worry component of the fear of hypoglycemia were significantly higher in the IAH group, but there were no differences in other psychological measures.
- Higher were significantly associated with decreased IAH risk with a calculated odds ratio of 0.54, but other aspects of hypoglycemia problem-solving such as detection control, goal setting, and strategy evaluation showed no significant links.
Limitations
- The study used a cross-sectional design, which is not suited to making causal inferences.
- The authors characterized DPN as either present or absent. They did not evaluate or analyze the severity of peripheral neuropathy.
- The authors evaluated diabetic cardiac autonomic neuropathy (DCAN) by a person’s coefficient of variation of R-R intervals, and definitive diagnosis of DCAN required at least two positive results on a cardiac autonomic test. More vigorous evaluation using a more definitive assessment of DCAN is needed to relate DCAN and IAH status.
Disclosures
- The study received no commercial funding.
- The authors have disclosed no relevant financial relationships.
This is a summary of a preprint research study, “Protective and risk factors of impaired awareness of hypoglycemia in patients with type 1 diabetes: a cross- sectional analysis of baseline data from the PR-IAH study,” written by researchers at several hospitals in Japan, all affiliated with the National Hospital Organization, on Research Square. The study has not yet been peer reviewed. The full text of the study can be found on researchsquare.com.
A version of this article first appeared on Medscape.com.
Researchers published the study covered in this summary on researchsquare.com as a preprint that has not yet been peer reviewed.
Key takeaways
- In Japanese adults with type 1 diabetes insulin-pump treatment (continuous subcutaneous insulin infusion) and higher problem-solving perception appear protective against impaired awareness of hypoglycemia (IAH), while diabetic peripheral neuropathy (DPN) is associated with increased risk.
- Diabetes distress and fear of hypoglycemia are common in people with IAH.
Why this matters
- Adults with type 1 diabetes and IAH have a reduced ability to perceive hypoglycemic symptoms and are at risk of severe hypoglycemic events because they are unable to take immediate corrective action.
- This is the first study to identify protective factors and risk factors of IAH in Japanese adults with type 1 diabetes.
- People with IAH may plan to loosen tight glucose management and intentionally omit insulin injection to prevent severe hypoglycemia.
- The information in this report may help improve the management of people with problematic hypoglycemia, the authors suggested. Treatment with an insulin pump and structured education aimed at improving problem-solving skills may be useful interventions for adults with type 1 diabetes and IAH, they suggested.
Study design
- The study involved a cross-sectional analysis of 288 Japanese adults with type 1 diabetes who averaged 50 years old, had diabetes for an average of about 18 years, had an average hemoglobin A1c at baseline of 7.7%, and included about 37% men and 63% women.
- The cohort included 55 people with IAH (19%) and 233 with no impairment of their hypoglycemia awareness, based on their score on the .
Key results
- DPN was significantly more prevalent in the IAH group than in the control group (12.0% vs. 26.5%). A logistic regression analysis showed that the odds ratio for DPN was 2.63-fold higher among people with IAH, compared with those without IAH, but there were no differences in other complications or by HbA1c levels.
- Treatment with continuous subcutaneous insulin therapy (an insulin pump) was significantly less prevalent in the IAH group, compared with those without IAH (23.6% vs 39.5%), with an adjusted odds ratio of 0.48. The two subgroups showed no differences in use of continuous glucose monitoring, used by 56% of the people in each of the two subgroups.
- The two subgroups showed no differences in their healthy lifestyle score, sleep debt, or rates of excessive drinking.
- Mean autonomic symptom scores for both sweating and shaking were significantly reduced in the IAH group, but no between-group differences appeared for palpations or hunger.
- All mean neuroglycopenic symptom scores were significantly lower in those without IAH, including confusion and speech difficulty.
- Scores for measures of diabetes distress and for the worry component of the fear of hypoglycemia were significantly higher in the IAH group, but there were no differences in other psychological measures.
- Higher were significantly associated with decreased IAH risk with a calculated odds ratio of 0.54, but other aspects of hypoglycemia problem-solving such as detection control, goal setting, and strategy evaluation showed no significant links.
Limitations
- The study used a cross-sectional design, which is not suited to making causal inferences.
- The authors characterized DPN as either present or absent. They did not evaluate or analyze the severity of peripheral neuropathy.
- The authors evaluated diabetic cardiac autonomic neuropathy (DCAN) by a person’s coefficient of variation of R-R intervals, and definitive diagnosis of DCAN required at least two positive results on a cardiac autonomic test. More vigorous evaluation using a more definitive assessment of DCAN is needed to relate DCAN and IAH status.
Disclosures
- The study received no commercial funding.
- The authors have disclosed no relevant financial relationships.
This is a summary of a preprint research study, “Protective and risk factors of impaired awareness of hypoglycemia in patients with type 1 diabetes: a cross- sectional analysis of baseline data from the PR-IAH study,” written by researchers at several hospitals in Japan, all affiliated with the National Hospital Organization, on Research Square. The study has not yet been peer reviewed. The full text of the study can be found on researchsquare.com.
A version of this article first appeared on Medscape.com.
Researchers published the study covered in this summary on researchsquare.com as a preprint that has not yet been peer reviewed.
Key takeaways
- In Japanese adults with type 1 diabetes insulin-pump treatment (continuous subcutaneous insulin infusion) and higher problem-solving perception appear protective against impaired awareness of hypoglycemia (IAH), while diabetic peripheral neuropathy (DPN) is associated with increased risk.
- Diabetes distress and fear of hypoglycemia are common in people with IAH.
Why this matters
- Adults with type 1 diabetes and IAH have a reduced ability to perceive hypoglycemic symptoms and are at risk of severe hypoglycemic events because they are unable to take immediate corrective action.
- This is the first study to identify protective factors and risk factors of IAH in Japanese adults with type 1 diabetes.
- People with IAH may plan to loosen tight glucose management and intentionally omit insulin injection to prevent severe hypoglycemia.
- The information in this report may help improve the management of people with problematic hypoglycemia, the authors suggested. Treatment with an insulin pump and structured education aimed at improving problem-solving skills may be useful interventions for adults with type 1 diabetes and IAH, they suggested.
Study design
- The study involved a cross-sectional analysis of 288 Japanese adults with type 1 diabetes who averaged 50 years old, had diabetes for an average of about 18 years, had an average hemoglobin A1c at baseline of 7.7%, and included about 37% men and 63% women.
- The cohort included 55 people with IAH (19%) and 233 with no impairment of their hypoglycemia awareness, based on their score on the .
Key results
- DPN was significantly more prevalent in the IAH group than in the control group (12.0% vs. 26.5%). A logistic regression analysis showed that the odds ratio for DPN was 2.63-fold higher among people with IAH, compared with those without IAH, but there were no differences in other complications or by HbA1c levels.
- Treatment with continuous subcutaneous insulin therapy (an insulin pump) was significantly less prevalent in the IAH group, compared with those without IAH (23.6% vs 39.5%), with an adjusted odds ratio of 0.48. The two subgroups showed no differences in use of continuous glucose monitoring, used by 56% of the people in each of the two subgroups.
- The two subgroups showed no differences in their healthy lifestyle score, sleep debt, or rates of excessive drinking.
- Mean autonomic symptom scores for both sweating and shaking were significantly reduced in the IAH group, but no between-group differences appeared for palpations or hunger.
- All mean neuroglycopenic symptom scores were significantly lower in those without IAH, including confusion and speech difficulty.
- Scores for measures of diabetes distress and for the worry component of the fear of hypoglycemia were significantly higher in the IAH group, but there were no differences in other psychological measures.
- Higher were significantly associated with decreased IAH risk with a calculated odds ratio of 0.54, but other aspects of hypoglycemia problem-solving such as detection control, goal setting, and strategy evaluation showed no significant links.
Limitations
- The study used a cross-sectional design, which is not suited to making causal inferences.
- The authors characterized DPN as either present or absent. They did not evaluate or analyze the severity of peripheral neuropathy.
- The authors evaluated diabetic cardiac autonomic neuropathy (DCAN) by a person’s coefficient of variation of R-R intervals, and definitive diagnosis of DCAN required at least two positive results on a cardiac autonomic test. More vigorous evaluation using a more definitive assessment of DCAN is needed to relate DCAN and IAH status.
Disclosures
- The study received no commercial funding.
- The authors have disclosed no relevant financial relationships.
This is a summary of a preprint research study, “Protective and risk factors of impaired awareness of hypoglycemia in patients with type 1 diabetes: a cross- sectional analysis of baseline data from the PR-IAH study,” written by researchers at several hospitals in Japan, all affiliated with the National Hospital Organization, on Research Square. The study has not yet been peer reviewed. The full text of the study can be found on researchsquare.com.
A version of this article first appeared on Medscape.com.
Onset and awareness of hypertension varies by race, ethnicity
Black and Hispanic adults are diagnosed with hypertension at a significantly younger age than are white adults, and they also are more likely than Whites to be unaware of undiagnosed high blood pressure, based on national survey data collected from 2011 to 2020.
“Earlier hypertension onset in Black and Hispanic adults may contribute to racial and ethnic CVD disparities,” Xiaoning Huang, PhD, and associates wrote in JAMA Cardiology, also noting that “lower hypertension awareness among racial and ethnic minoritized groups suggests potential for underestimating differences in age at onset.”
Overall mean age at diagnosis was 46 years for the overall study sample of 9,627 participants in the National Health and Nutrition Examination Surveys over the 10 years covered in the analysis. Black adults, with a median age of 42 years, and Hispanic adults (median, 43 years) were significantly younger at diagnosis than White adults, who had a median age of 47 years, the investigators reported.
“Earlier age at hypertension onset may mean greater cumulative exposure to high blood pressure across the life course, which is associated with increased risk of [cardiovascular disease] and may contribute to racial disparities in hypertension-related outcomes,” said Dr. Huang and associates at Northwestern University, Chicago.
The increased cumulative exposure can be seen when age at diagnosis is stratified “across the life course.” Black/Hispanic adults were significantly more likely than White/Asian adults to be diagnosed at or before 30 years of age, and that difference continued to at least age 50 years, the investigators said.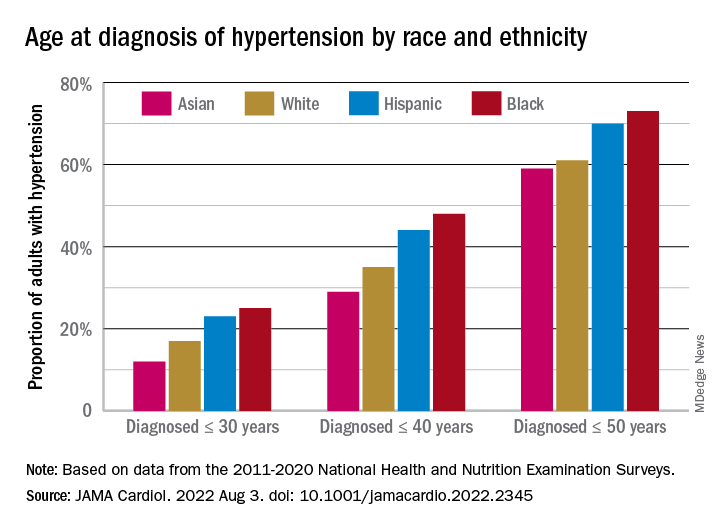
Many adults unaware of their hypertension
There was a somewhat different trend among those in the study population who reported BP at or above 140/90 mm Hg but did not report a hypertension diagnosis. Black, Hispanic, and Asian adults all were significantly more likely than White adults to be unaware of their hypertension, the survey data showed.
Overall, 18% of those who did not report a hypertension diagnosis had a BP of 140/90 mm Hg or higher and 38% had a BP of 130/80 mm Hg or more. Broken down by race and ethnicity, 16% and 36% of Whites reporting no hypertension had BPs of 140/90 and 130/80 mm Hg, respectively; those proportions were 21% and 42% for Hispanics, 24% and 44% for Asians, and 28% and 51% for Blacks, with all of the differences between Whites and the others significant, the research team reported.
One investigator is an associate editor for JAMA Cardiology and reported receiving grants from the American Heart Association and the National Institutes of Health during the conduct of the study. None of the other investigators reported any conflicts.
Black and Hispanic adults are diagnosed with hypertension at a significantly younger age than are white adults, and they also are more likely than Whites to be unaware of undiagnosed high blood pressure, based on national survey data collected from 2011 to 2020.
“Earlier hypertension onset in Black and Hispanic adults may contribute to racial and ethnic CVD disparities,” Xiaoning Huang, PhD, and associates wrote in JAMA Cardiology, also noting that “lower hypertension awareness among racial and ethnic minoritized groups suggests potential for underestimating differences in age at onset.”
Overall mean age at diagnosis was 46 years for the overall study sample of 9,627 participants in the National Health and Nutrition Examination Surveys over the 10 years covered in the analysis. Black adults, with a median age of 42 years, and Hispanic adults (median, 43 years) were significantly younger at diagnosis than White adults, who had a median age of 47 years, the investigators reported.
“Earlier age at hypertension onset may mean greater cumulative exposure to high blood pressure across the life course, which is associated with increased risk of [cardiovascular disease] and may contribute to racial disparities in hypertension-related outcomes,” said Dr. Huang and associates at Northwestern University, Chicago.
The increased cumulative exposure can be seen when age at diagnosis is stratified “across the life course.” Black/Hispanic adults were significantly more likely than White/Asian adults to be diagnosed at or before 30 years of age, and that difference continued to at least age 50 years, the investigators said.
Many adults unaware of their hypertension
There was a somewhat different trend among those in the study population who reported BP at or above 140/90 mm Hg but did not report a hypertension diagnosis. Black, Hispanic, and Asian adults all were significantly more likely than White adults to be unaware of their hypertension, the survey data showed.
Overall, 18% of those who did not report a hypertension diagnosis had a BP of 140/90 mm Hg or higher and 38% had a BP of 130/80 mm Hg or more. Broken down by race and ethnicity, 16% and 36% of Whites reporting no hypertension had BPs of 140/90 and 130/80 mm Hg, respectively; those proportions were 21% and 42% for Hispanics, 24% and 44% for Asians, and 28% and 51% for Blacks, with all of the differences between Whites and the others significant, the research team reported.
One investigator is an associate editor for JAMA Cardiology and reported receiving grants from the American Heart Association and the National Institutes of Health during the conduct of the study. None of the other investigators reported any conflicts.
Black and Hispanic adults are diagnosed with hypertension at a significantly younger age than are white adults, and they also are more likely than Whites to be unaware of undiagnosed high blood pressure, based on national survey data collected from 2011 to 2020.
“Earlier hypertension onset in Black and Hispanic adults may contribute to racial and ethnic CVD disparities,” Xiaoning Huang, PhD, and associates wrote in JAMA Cardiology, also noting that “lower hypertension awareness among racial and ethnic minoritized groups suggests potential for underestimating differences in age at onset.”
Overall mean age at diagnosis was 46 years for the overall study sample of 9,627 participants in the National Health and Nutrition Examination Surveys over the 10 years covered in the analysis. Black adults, with a median age of 42 years, and Hispanic adults (median, 43 years) were significantly younger at diagnosis than White adults, who had a median age of 47 years, the investigators reported.
“Earlier age at hypertension onset may mean greater cumulative exposure to high blood pressure across the life course, which is associated with increased risk of [cardiovascular disease] and may contribute to racial disparities in hypertension-related outcomes,” said Dr. Huang and associates at Northwestern University, Chicago.
The increased cumulative exposure can be seen when age at diagnosis is stratified “across the life course.” Black/Hispanic adults were significantly more likely than White/Asian adults to be diagnosed at or before 30 years of age, and that difference continued to at least age 50 years, the investigators said.
Many adults unaware of their hypertension
There was a somewhat different trend among those in the study population who reported BP at or above 140/90 mm Hg but did not report a hypertension diagnosis. Black, Hispanic, and Asian adults all were significantly more likely than White adults to be unaware of their hypertension, the survey data showed.
Overall, 18% of those who did not report a hypertension diagnosis had a BP of 140/90 mm Hg or higher and 38% had a BP of 130/80 mm Hg or more. Broken down by race and ethnicity, 16% and 36% of Whites reporting no hypertension had BPs of 140/90 and 130/80 mm Hg, respectively; those proportions were 21% and 42% for Hispanics, 24% and 44% for Asians, and 28% and 51% for Blacks, with all of the differences between Whites and the others significant, the research team reported.
One investigator is an associate editor for JAMA Cardiology and reported receiving grants from the American Heart Association and the National Institutes of Health during the conduct of the study. None of the other investigators reported any conflicts.
FROM JAMA CARDIOLOGY
Antibiotic-resistant bacteria emerging in community settings
A new study from the Centers for Disease Control and Prevention found that
Traditionally, CRE has been thought of as a nosocomial infection, acquired in a hospital or other health care facility (nursing home, long-term acute care hospital, dialysis center, etc.). This is the first population-level study to show otherwise, with fully 10% of the CRE isolates found to be community acquired.
CREs are a group of multidrug-resistant bacteria considered an urgent health threat by the CDC because they can rapidly spread between patients, especially those who are most seriously ill and vulnerable, and because they are so difficult to treat. These patients often require treatment with toxic antibiotics, such as colistin, and carry a high mortality rate – up to 50% in some studies.
Overall, 30% of CREs carry a carbapenemase – an enzyme that can make them resistant to carbapenem antibiotics. The genes for this are readily transferable between bacteria and help account for their spread in hospitals.
But in this study, published in the American Journal of Infection Control, of the 12 isolates that underwent whole-genome sequencing, 42% of the CA-CRE isolates carried the carbapenemase gene. Lead author Sandra Bulens, MPH, a health scientist in the CDC’s division of health care quality promotion, said in an interview, “The findings highlight the potential for CP-CRE to move from health care settings into the community. The fact that 5 of the 12 isolates harbored a carbapenemase gene introduces new challenges for controlling spread of CP-CRE.”
CDC researchers analyzed data from eight U.S. metropolitan areas between 2012 and 2015 as part of the CDC’s Emerging Infections Program (EIP) health care–associated infections – community interface activity, which conducts surveillance for CRE and other drug-resistant gram-negative bacteria. Cases of CA-CRE were compared with HCA-CRE, with 1499 cases in 1,194 case-patients being analyzed. Though Klebsiella pneumoniae was the most common isolate, there were some differences between metropolitan areas.
The incidence of CRE cases per 100,000 population was 2.96 (95% confidence interval, 2.81-3.11) overall and 0.29 (95% CI, 0.25-0.25) for CA-CRE. Most CA-CRE cases were in White persons (73%) and women (84%). Urine cultures were the source of 98% of all CA-CRE cases, compared with 86% of HCA-CRE cases (P < .001). Though small numbers, the numbers of patients with CA-CRE without apparent underlying medical condition (n = 51; 37%) was greater when compared with patients with HCA-CRE (n = 36; 3%; P < .001).
Asked for independent comment, Lance Price, PhD, of George Washington University and the founding director of GW’s Antibiotic Resistance Action Center, Washington, said, “what’s striking about these data is that: ‘Who is the front line, at least in the United States for CRE?’ It’s women, older women. ... At some point, we have to frame drug resistance as a women’s health issue.”
Dr. Price noted that the 10% of patients with CA-CRE acquired it in the community. “I would argue that probably none of them had any idea, because there’s this silent community epidemic,” he said. “It’s asymptomatic carriage and transmission in the community. Somebody can be this walking reservoir of these really dangerous bacteria and have no idea.”
This is an increasingly serious problem for women, Dr. Price said, because, “with a community-acquired bladder infection, you’re going to call your doctor or go to an urgent care, and they’re not going to test you. They’re going to guess what you have, and they’re going to prescribe an antibiotic, and that antibiotic is going to fail. So then your bladder infection continues, and then you wait a few more days, and you start to get flank pain and kidney infection. ... If you start getting a fever, they might admit you. They are going to start treating you immediately, and they might miss it because you’ve got this organism that’s resistant to all the best antibiotics. ... The gateway to the blood is the UTI.”
Because of such empiric treatment and increasing resistance, the risk for treatment failure is quite high, especially for older women. Ms. Bulens, however, said that, “[although] 10% of CRE were in persons without health care risk factors, the proportion of all UTIs in this population that are CRE is going to be very, very small.”
This study involved cultures from 2012 to 2015. Before the pandemic, from 2012 to 2017, U.S. deaths from antibiotic resistance fell by 18% overall and by 30% in hospitals.
But in the first year of the COVID-19 pandemic, there was a 15% increase in infections and deaths from antibiotic-resistant (AMR), hospital-acquired bacteria. In 2020, 29,400 patients died from AMR infections. There was a 78% increase in carbapenem-resistant Acinetobacter baumannii health care–associated infections, a 35% increase in carbapenem-resistant Enterobacterales, and 32% increases in both multidrug-resistant Pseudomonas aeruginosa and extended-spectrum beta-lactamase–producing Enterobacterales. Aside from gram-negative bacteria, methicillin-resistant Staphylococcus aureus rose 13%, and Candida auris rose 60%. But owing to limited surveillance, recent sound figures are lacking.
Ms. Bulens and Dr. Price reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study from the Centers for Disease Control and Prevention found that
Traditionally, CRE has been thought of as a nosocomial infection, acquired in a hospital or other health care facility (nursing home, long-term acute care hospital, dialysis center, etc.). This is the first population-level study to show otherwise, with fully 10% of the CRE isolates found to be community acquired.
CREs are a group of multidrug-resistant bacteria considered an urgent health threat by the CDC because they can rapidly spread between patients, especially those who are most seriously ill and vulnerable, and because they are so difficult to treat. These patients often require treatment with toxic antibiotics, such as colistin, and carry a high mortality rate – up to 50% in some studies.
Overall, 30% of CREs carry a carbapenemase – an enzyme that can make them resistant to carbapenem antibiotics. The genes for this are readily transferable between bacteria and help account for their spread in hospitals.
But in this study, published in the American Journal of Infection Control, of the 12 isolates that underwent whole-genome sequencing, 42% of the CA-CRE isolates carried the carbapenemase gene. Lead author Sandra Bulens, MPH, a health scientist in the CDC’s division of health care quality promotion, said in an interview, “The findings highlight the potential for CP-CRE to move from health care settings into the community. The fact that 5 of the 12 isolates harbored a carbapenemase gene introduces new challenges for controlling spread of CP-CRE.”
CDC researchers analyzed data from eight U.S. metropolitan areas between 2012 and 2015 as part of the CDC’s Emerging Infections Program (EIP) health care–associated infections – community interface activity, which conducts surveillance for CRE and other drug-resistant gram-negative bacteria. Cases of CA-CRE were compared with HCA-CRE, with 1499 cases in 1,194 case-patients being analyzed. Though Klebsiella pneumoniae was the most common isolate, there were some differences between metropolitan areas.
The incidence of CRE cases per 100,000 population was 2.96 (95% confidence interval, 2.81-3.11) overall and 0.29 (95% CI, 0.25-0.25) for CA-CRE. Most CA-CRE cases were in White persons (73%) and women (84%). Urine cultures were the source of 98% of all CA-CRE cases, compared with 86% of HCA-CRE cases (P < .001). Though small numbers, the numbers of patients with CA-CRE without apparent underlying medical condition (n = 51; 37%) was greater when compared with patients with HCA-CRE (n = 36; 3%; P < .001).
Asked for independent comment, Lance Price, PhD, of George Washington University and the founding director of GW’s Antibiotic Resistance Action Center, Washington, said, “what’s striking about these data is that: ‘Who is the front line, at least in the United States for CRE?’ It’s women, older women. ... At some point, we have to frame drug resistance as a women’s health issue.”
Dr. Price noted that the 10% of patients with CA-CRE acquired it in the community. “I would argue that probably none of them had any idea, because there’s this silent community epidemic,” he said. “It’s asymptomatic carriage and transmission in the community. Somebody can be this walking reservoir of these really dangerous bacteria and have no idea.”
This is an increasingly serious problem for women, Dr. Price said, because, “with a community-acquired bladder infection, you’re going to call your doctor or go to an urgent care, and they’re not going to test you. They’re going to guess what you have, and they’re going to prescribe an antibiotic, and that antibiotic is going to fail. So then your bladder infection continues, and then you wait a few more days, and you start to get flank pain and kidney infection. ... If you start getting a fever, they might admit you. They are going to start treating you immediately, and they might miss it because you’ve got this organism that’s resistant to all the best antibiotics. ... The gateway to the blood is the UTI.”
Because of such empiric treatment and increasing resistance, the risk for treatment failure is quite high, especially for older women. Ms. Bulens, however, said that, “[although] 10% of CRE were in persons without health care risk factors, the proportion of all UTIs in this population that are CRE is going to be very, very small.”
This study involved cultures from 2012 to 2015. Before the pandemic, from 2012 to 2017, U.S. deaths from antibiotic resistance fell by 18% overall and by 30% in hospitals.
But in the first year of the COVID-19 pandemic, there was a 15% increase in infections and deaths from antibiotic-resistant (AMR), hospital-acquired bacteria. In 2020, 29,400 patients died from AMR infections. There was a 78% increase in carbapenem-resistant Acinetobacter baumannii health care–associated infections, a 35% increase in carbapenem-resistant Enterobacterales, and 32% increases in both multidrug-resistant Pseudomonas aeruginosa and extended-spectrum beta-lactamase–producing Enterobacterales. Aside from gram-negative bacteria, methicillin-resistant Staphylococcus aureus rose 13%, and Candida auris rose 60%. But owing to limited surveillance, recent sound figures are lacking.
Ms. Bulens and Dr. Price reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study from the Centers for Disease Control and Prevention found that
Traditionally, CRE has been thought of as a nosocomial infection, acquired in a hospital or other health care facility (nursing home, long-term acute care hospital, dialysis center, etc.). This is the first population-level study to show otherwise, with fully 10% of the CRE isolates found to be community acquired.
CREs are a group of multidrug-resistant bacteria considered an urgent health threat by the CDC because they can rapidly spread between patients, especially those who are most seriously ill and vulnerable, and because they are so difficult to treat. These patients often require treatment with toxic antibiotics, such as colistin, and carry a high mortality rate – up to 50% in some studies.
Overall, 30% of CREs carry a carbapenemase – an enzyme that can make them resistant to carbapenem antibiotics. The genes for this are readily transferable between bacteria and help account for their spread in hospitals.
But in this study, published in the American Journal of Infection Control, of the 12 isolates that underwent whole-genome sequencing, 42% of the CA-CRE isolates carried the carbapenemase gene. Lead author Sandra Bulens, MPH, a health scientist in the CDC’s division of health care quality promotion, said in an interview, “The findings highlight the potential for CP-CRE to move from health care settings into the community. The fact that 5 of the 12 isolates harbored a carbapenemase gene introduces new challenges for controlling spread of CP-CRE.”
CDC researchers analyzed data from eight U.S. metropolitan areas between 2012 and 2015 as part of the CDC’s Emerging Infections Program (EIP) health care–associated infections – community interface activity, which conducts surveillance for CRE and other drug-resistant gram-negative bacteria. Cases of CA-CRE were compared with HCA-CRE, with 1499 cases in 1,194 case-patients being analyzed. Though Klebsiella pneumoniae was the most common isolate, there were some differences between metropolitan areas.
The incidence of CRE cases per 100,000 population was 2.96 (95% confidence interval, 2.81-3.11) overall and 0.29 (95% CI, 0.25-0.25) for CA-CRE. Most CA-CRE cases were in White persons (73%) and women (84%). Urine cultures were the source of 98% of all CA-CRE cases, compared with 86% of HCA-CRE cases (P < .001). Though small numbers, the numbers of patients with CA-CRE without apparent underlying medical condition (n = 51; 37%) was greater when compared with patients with HCA-CRE (n = 36; 3%; P < .001).
Asked for independent comment, Lance Price, PhD, of George Washington University and the founding director of GW’s Antibiotic Resistance Action Center, Washington, said, “what’s striking about these data is that: ‘Who is the front line, at least in the United States for CRE?’ It’s women, older women. ... At some point, we have to frame drug resistance as a women’s health issue.”
Dr. Price noted that the 10% of patients with CA-CRE acquired it in the community. “I would argue that probably none of them had any idea, because there’s this silent community epidemic,” he said. “It’s asymptomatic carriage and transmission in the community. Somebody can be this walking reservoir of these really dangerous bacteria and have no idea.”
This is an increasingly serious problem for women, Dr. Price said, because, “with a community-acquired bladder infection, you’re going to call your doctor or go to an urgent care, and they’re not going to test you. They’re going to guess what you have, and they’re going to prescribe an antibiotic, and that antibiotic is going to fail. So then your bladder infection continues, and then you wait a few more days, and you start to get flank pain and kidney infection. ... If you start getting a fever, they might admit you. They are going to start treating you immediately, and they might miss it because you’ve got this organism that’s resistant to all the best antibiotics. ... The gateway to the blood is the UTI.”
Because of such empiric treatment and increasing resistance, the risk for treatment failure is quite high, especially for older women. Ms. Bulens, however, said that, “[although] 10% of CRE were in persons without health care risk factors, the proportion of all UTIs in this population that are CRE is going to be very, very small.”
This study involved cultures from 2012 to 2015. Before the pandemic, from 2012 to 2017, U.S. deaths from antibiotic resistance fell by 18% overall and by 30% in hospitals.
But in the first year of the COVID-19 pandemic, there was a 15% increase in infections and deaths from antibiotic-resistant (AMR), hospital-acquired bacteria. In 2020, 29,400 patients died from AMR infections. There was a 78% increase in carbapenem-resistant Acinetobacter baumannii health care–associated infections, a 35% increase in carbapenem-resistant Enterobacterales, and 32% increases in both multidrug-resistant Pseudomonas aeruginosa and extended-spectrum beta-lactamase–producing Enterobacterales. Aside from gram-negative bacteria, methicillin-resistant Staphylococcus aureus rose 13%, and Candida auris rose 60%. But owing to limited surveillance, recent sound figures are lacking.
Ms. Bulens and Dr. Price reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF INFECTION CONTROL
‘Self-boosting’ vaccines could be immunizations of the future
Most vaccines don’t come as one-shot deals. A series of boosters is needed to step up immunity to COVID-19, tetanus, and other infectious threats over time.
But what if you could receive just one shot that boosts itself whenever you need a bump in protection?
Researchers at the Massachusetts Institute of Technology (MIT) have developed microparticles that could be used to create self-boosting vaccines that deliver their contents at carefully set time points. In a new study published in the journal Science Advances, the scientists describe how they tune the particles to release the goods at the right time and offer insights on how they can keep the particles stable until then.
How self-boosting vaccines could work
The team developed tiny particles that look like coffee cups – except instead of your favorite brew, they’re filled with vaccine.
“You can put the lid on, and then inject it into the body, and once the lid breaks, whatever is in there is released,” says study author Ana Jaklenec, PhD, a research scientist at MIT’s Koch Institute for Integrative Cancer Research.
To make the tiny cups, the researchers use various polymers already used in medical applications, such as dissolvable stitches. Then they fill the cups with vaccine material that is dried and combined with sugars and other stabilizers.
The particles can be made in various shapes and fine-tuned using polymers with different properties. Some polymers last longer in the body than others, so their choice helps determine how long everything will stay stable under the skin after the injection and when the particles will release their cargo. It could be days or months after the injection.
One challenge is that as the particles open, the environment around them becomes more acidic. The team is working on ways to curb that acidity to make the vaccine material more stable.
“We have ongoing research that has produced some really, really exciting results about their stability and [shows] that you’re able to maintain really sensitive vaccines, stable for a good period of time,” says study author Morteza Sarmadi, PhD, a research specialist at the Koch Institute.
The potential public health impact
This research, funded by the Bill & Melinda Gates Foundation, started with the developing world in mind.
“The intent was actually helping people in the developing world, because a lot of times, people don’t come back for a second injection,” says study author Robert Langer, ScD, the David H. Koch Institute professor at MIT.
But a one-shot plan could benefit the developed world, too. One reason is that self-boosting vaccines could help those who get one achieve higher antibody responses than they would with just one dose. That could mean more protection for the person and the population, because as people develop stronger immunity, germs may have less of a chance to evolve and spread.
Take the COVID-19 pandemic, for example. Only 67% of Americans are fully vaccinated, and most people eligible for first and second boosters haven’t gotten them. New variants, such as the recent Omicron ones, continue to emerge and infect.
“I think those variants would have had a lot less chance to come about if everybody that had gotten vaccinated the first time got repeat injections, which they didn’t,” says Dr. Langer.
Self-boosting vaccines could also benefit infants, children who fear shots, and older adults who have a hard time getting health care.
Also, because the vaccine material is encapsulated and its release can be staggered, this technology might help people receive multiple vaccines at the same time that must now be given separately.
What comes next
The team is testing self-boosting polio and hepatitis vaccines in non-human primates. A small trial in healthy humans might follow within the next few years.
“We think that there’s really high potential for this technology, and we hope it can be developed and get to the human phase very soon,” says Dr. Jaklenec.
In smaller animal models, they are exploring the potential of self-boosting mRNA vaccines. They’re also working with scientists who are studying HIV vaccines.
“There has been some recent progress where very complex regimens seem to be working, but they’re not practical,” says Dr. Jaklenec. “And so, this is where this particular technology could be useful, because you have to prime and boost with different things, and this allows you to do that.”
This system could also extend beyond vaccines and be used to deliver cancer therapies, hormones, and biologics in a shot.
Through new work with researchers at Georgia Tech University, the team will study the potential of giving self-boosting vaccines through 3D-printed microneedles. These vaccines, which would stick on your skin like a bandage, could be self-administered and deployed globally in response to local outbreaks.
A version of this article first appeared on WebMD.com.
Most vaccines don’t come as one-shot deals. A series of boosters is needed to step up immunity to COVID-19, tetanus, and other infectious threats over time.
But what if you could receive just one shot that boosts itself whenever you need a bump in protection?
Researchers at the Massachusetts Institute of Technology (MIT) have developed microparticles that could be used to create self-boosting vaccines that deliver their contents at carefully set time points. In a new study published in the journal Science Advances, the scientists describe how they tune the particles to release the goods at the right time and offer insights on how they can keep the particles stable until then.
How self-boosting vaccines could work
The team developed tiny particles that look like coffee cups – except instead of your favorite brew, they’re filled with vaccine.
“You can put the lid on, and then inject it into the body, and once the lid breaks, whatever is in there is released,” says study author Ana Jaklenec, PhD, a research scientist at MIT’s Koch Institute for Integrative Cancer Research.
To make the tiny cups, the researchers use various polymers already used in medical applications, such as dissolvable stitches. Then they fill the cups with vaccine material that is dried and combined with sugars and other stabilizers.
The particles can be made in various shapes and fine-tuned using polymers with different properties. Some polymers last longer in the body than others, so their choice helps determine how long everything will stay stable under the skin after the injection and when the particles will release their cargo. It could be days or months after the injection.
One challenge is that as the particles open, the environment around them becomes more acidic. The team is working on ways to curb that acidity to make the vaccine material more stable.
“We have ongoing research that has produced some really, really exciting results about their stability and [shows] that you’re able to maintain really sensitive vaccines, stable for a good period of time,” says study author Morteza Sarmadi, PhD, a research specialist at the Koch Institute.
The potential public health impact
This research, funded by the Bill & Melinda Gates Foundation, started with the developing world in mind.
“The intent was actually helping people in the developing world, because a lot of times, people don’t come back for a second injection,” says study author Robert Langer, ScD, the David H. Koch Institute professor at MIT.
But a one-shot plan could benefit the developed world, too. One reason is that self-boosting vaccines could help those who get one achieve higher antibody responses than they would with just one dose. That could mean more protection for the person and the population, because as people develop stronger immunity, germs may have less of a chance to evolve and spread.
Take the COVID-19 pandemic, for example. Only 67% of Americans are fully vaccinated, and most people eligible for first and second boosters haven’t gotten them. New variants, such as the recent Omicron ones, continue to emerge and infect.
“I think those variants would have had a lot less chance to come about if everybody that had gotten vaccinated the first time got repeat injections, which they didn’t,” says Dr. Langer.
Self-boosting vaccines could also benefit infants, children who fear shots, and older adults who have a hard time getting health care.
Also, because the vaccine material is encapsulated and its release can be staggered, this technology might help people receive multiple vaccines at the same time that must now be given separately.
What comes next
The team is testing self-boosting polio and hepatitis vaccines in non-human primates. A small trial in healthy humans might follow within the next few years.
“We think that there’s really high potential for this technology, and we hope it can be developed and get to the human phase very soon,” says Dr. Jaklenec.
In smaller animal models, they are exploring the potential of self-boosting mRNA vaccines. They’re also working with scientists who are studying HIV vaccines.
“There has been some recent progress where very complex regimens seem to be working, but they’re not practical,” says Dr. Jaklenec. “And so, this is where this particular technology could be useful, because you have to prime and boost with different things, and this allows you to do that.”
This system could also extend beyond vaccines and be used to deliver cancer therapies, hormones, and biologics in a shot.
Through new work with researchers at Georgia Tech University, the team will study the potential of giving self-boosting vaccines through 3D-printed microneedles. These vaccines, which would stick on your skin like a bandage, could be self-administered and deployed globally in response to local outbreaks.
A version of this article first appeared on WebMD.com.
Most vaccines don’t come as one-shot deals. A series of boosters is needed to step up immunity to COVID-19, tetanus, and other infectious threats over time.
But what if you could receive just one shot that boosts itself whenever you need a bump in protection?
Researchers at the Massachusetts Institute of Technology (MIT) have developed microparticles that could be used to create self-boosting vaccines that deliver their contents at carefully set time points. In a new study published in the journal Science Advances, the scientists describe how they tune the particles to release the goods at the right time and offer insights on how they can keep the particles stable until then.
How self-boosting vaccines could work
The team developed tiny particles that look like coffee cups – except instead of your favorite brew, they’re filled with vaccine.
“You can put the lid on, and then inject it into the body, and once the lid breaks, whatever is in there is released,” says study author Ana Jaklenec, PhD, a research scientist at MIT’s Koch Institute for Integrative Cancer Research.
To make the tiny cups, the researchers use various polymers already used in medical applications, such as dissolvable stitches. Then they fill the cups with vaccine material that is dried and combined with sugars and other stabilizers.
The particles can be made in various shapes and fine-tuned using polymers with different properties. Some polymers last longer in the body than others, so their choice helps determine how long everything will stay stable under the skin after the injection and when the particles will release their cargo. It could be days or months after the injection.
One challenge is that as the particles open, the environment around them becomes more acidic. The team is working on ways to curb that acidity to make the vaccine material more stable.
“We have ongoing research that has produced some really, really exciting results about their stability and [shows] that you’re able to maintain really sensitive vaccines, stable for a good period of time,” says study author Morteza Sarmadi, PhD, a research specialist at the Koch Institute.
The potential public health impact
This research, funded by the Bill & Melinda Gates Foundation, started with the developing world in mind.
“The intent was actually helping people in the developing world, because a lot of times, people don’t come back for a second injection,” says study author Robert Langer, ScD, the David H. Koch Institute professor at MIT.
But a one-shot plan could benefit the developed world, too. One reason is that self-boosting vaccines could help those who get one achieve higher antibody responses than they would with just one dose. That could mean more protection for the person and the population, because as people develop stronger immunity, germs may have less of a chance to evolve and spread.
Take the COVID-19 pandemic, for example. Only 67% of Americans are fully vaccinated, and most people eligible for first and second boosters haven’t gotten them. New variants, such as the recent Omicron ones, continue to emerge and infect.
“I think those variants would have had a lot less chance to come about if everybody that had gotten vaccinated the first time got repeat injections, which they didn’t,” says Dr. Langer.
Self-boosting vaccines could also benefit infants, children who fear shots, and older adults who have a hard time getting health care.
Also, because the vaccine material is encapsulated and its release can be staggered, this technology might help people receive multiple vaccines at the same time that must now be given separately.
What comes next
The team is testing self-boosting polio and hepatitis vaccines in non-human primates. A small trial in healthy humans might follow within the next few years.
“We think that there’s really high potential for this technology, and we hope it can be developed and get to the human phase very soon,” says Dr. Jaklenec.
In smaller animal models, they are exploring the potential of self-boosting mRNA vaccines. They’re also working with scientists who are studying HIV vaccines.
“There has been some recent progress where very complex regimens seem to be working, but they’re not practical,” says Dr. Jaklenec. “And so, this is where this particular technology could be useful, because you have to prime and boost with different things, and this allows you to do that.”
This system could also extend beyond vaccines and be used to deliver cancer therapies, hormones, and biologics in a shot.
Through new work with researchers at Georgia Tech University, the team will study the potential of giving self-boosting vaccines through 3D-printed microneedles. These vaccines, which would stick on your skin like a bandage, could be self-administered and deployed globally in response to local outbreaks.
A version of this article first appeared on WebMD.com.
FROM SCIENCE ADVANCES
One in eight COVID patients likely to develop long COVID: Large study
a large study published in The Lancet indicates.
The researchers determined that percentage by comparing long-term symptoms in people infected by SARS-CoV-2 with similar symptoms in uninfected people over the same time period.
Among the group of infected study participants in the Netherlands, 21.4% had at least one new or severely increased symptom 3-5 months after infection compared with before infection. When that group of 21.4% was compared with 8.7% of uninfected people in the same study, the researchers were able to calculate a prevalence 12.7% with long COVID.
“This finding shows that post–COVID-19 condition is an urgent problem with a mounting human toll,” the study authors wrote.
The research design was novel, two editorialists said in an accompanying commentary.
Christopher Brightling, PhD, and Rachael Evans, MBChB, PhD, of the Institute for Lung Health, University of Leicester (England), noted: “This is a major advance on prior long COVID prevalence estimates as it includes a matched uninfected group and accounts for symptoms before COVID-19 infection.”
Symptoms that persist
The Lancet study found that 3-5 months after COVID (compared with before COVID) and compared with the non-COVID comparison group, the symptoms that persist were chest pain, breathing difficulties, pain when breathing, muscle pain, loss of taste and/or smell, tingling extremities, lump in throat, feeling hot and cold alternately, heavy limbs, and tiredness.
The authors noted that symptoms such as brain fog were found to be relevant to long COVID after the data collection period for this paper and were not included in this research.
Researcher Aranka V. Ballering, MSc, PhD candidate, said in an interview that the researchers found fever is a symptom that is clearly present during the acute phase of the disease and it peaks the day of the COVID-19 diagnosis, but also wears off.
Loss of taste and smell, however, rapidly increases in severity when COVID-19 is diagnosed, but also persists and is still present 3-5 months after COVID.
Ms. Ballering, with the department of psychiatry at the University of Groningen (the Netherlands), said she was surprised by the sex difference made evident in their research: “Women showed more severe persistent symptoms than men.”
Closer to a clearer definition
The authors said their findings also pinpoint symptoms that bring us closer to a better definition of long COVID, which has many different definitions globally.
“These symptoms have the highest discriminative ability to distinguish between post–COVID-19 condition and non–COVID-19–related symptoms,” they wrote.
Researchers collected data by asking participants in the northern Netherlands, who were part of the population-based Lifelines COVID-19 study, to regularly complete digital questionnaires on 23 symptoms commonly associated with long COVID. The questionnaire was sent out 24 times to the same people between March 2020 and August 2021. At that time, people had the Alpha or earlier variants.
Participants were considered COVID-19 positive if they had either a positive test or a doctor’s diagnosis of COVID-19.
Of 76,422 study participants, the 5.5% (4,231) who had COVID were matched to 8,462 controls. Researchers accounted for sex, age, and time of completing questionnaires.
Effect of hospitalization, vaccination unclear
Ms. Ballering said it’s unclear from this data whether vaccination or whether a person was hospitalized would change the prevalence of persistent symptoms.
Because of the period when the data were collected, “the vast majority of our study population was not fully vaccinated,” she said.
However, she pointed to recent research that shows that immunization against COVID is only partially effective against persistent somatic symptoms after COVID.
Also, only 5% of men and 2.5% of women in the study were hospitalized as a result of COVID-19, so the findings can’t easily be generalized to hospitalized patients.
The Lifelines study was an add-on study to the multidisciplinary, prospective, population-based, observational Dutch Lifelines cohort study examining 167,729 people in the Netherlands. Almost all were White, a limitation of the study, and 58% were female. Average age was 54.
The editorialists also noted additional limitations of the study were that this research “did not fully consider the impact on mental health” and was conducted in one region in the Netherlands.
Janko Nikolich-Žugich, MD, PhD, director of the Aegis Consortium for Pandemic-Free Future and head of the immunobiology department at University of Arizona, Tucson, said in an interview that he agreed with the editorialists that a primary benefit of this study is that it corrected for symptoms people had before COVID, something other studies have not been able to do.
However, he cautioned about generalizing the results for the United States and other countries because of the lack of diversity in the study population with regard to education level, socioeconomic factors, and race. He pointed out that access issues are also different in the Netherlands, which has universal health care.
He said brain fog as a symptom of long COVID is of high interest and will be important to include in future studies that are able to extend the study period.
The work was funded by ZonMw; the Dutch Ministry of Health, Welfare, and Sport; Dutch Ministry of Economic Affairs; University Medical Center Groningen, University of Groningen; and the provinces of Drenthe, Friesland, and Groningen. The study authors and Dr. Nikolich-Žugich have reported no relevant financial relationships. Dr. Brightling has received consultancy and or grants paid to his institution from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis, Chiesi, Genentech, Roche, Sanofi, Regeneron, Mologic, and 4DPharma for asthma and chronic obstructive pulmonary disease research. Dr. Evans has received consultancy fees from AstraZeneca on the topic of long COVID and from GlaxoSmithKline on digital health, and speaker’s fees from Boehringer Ingelheim on long COVID.
A version of this article first appeared on Medscape.com.
a large study published in The Lancet indicates.
The researchers determined that percentage by comparing long-term symptoms in people infected by SARS-CoV-2 with similar symptoms in uninfected people over the same time period.
Among the group of infected study participants in the Netherlands, 21.4% had at least one new or severely increased symptom 3-5 months after infection compared with before infection. When that group of 21.4% was compared with 8.7% of uninfected people in the same study, the researchers were able to calculate a prevalence 12.7% with long COVID.
“This finding shows that post–COVID-19 condition is an urgent problem with a mounting human toll,” the study authors wrote.
The research design was novel, two editorialists said in an accompanying commentary.
Christopher Brightling, PhD, and Rachael Evans, MBChB, PhD, of the Institute for Lung Health, University of Leicester (England), noted: “This is a major advance on prior long COVID prevalence estimates as it includes a matched uninfected group and accounts for symptoms before COVID-19 infection.”
Symptoms that persist
The Lancet study found that 3-5 months after COVID (compared with before COVID) and compared with the non-COVID comparison group, the symptoms that persist were chest pain, breathing difficulties, pain when breathing, muscle pain, loss of taste and/or smell, tingling extremities, lump in throat, feeling hot and cold alternately, heavy limbs, and tiredness.
The authors noted that symptoms such as brain fog were found to be relevant to long COVID after the data collection period for this paper and were not included in this research.
Researcher Aranka V. Ballering, MSc, PhD candidate, said in an interview that the researchers found fever is a symptom that is clearly present during the acute phase of the disease and it peaks the day of the COVID-19 diagnosis, but also wears off.
Loss of taste and smell, however, rapidly increases in severity when COVID-19 is diagnosed, but also persists and is still present 3-5 months after COVID.
Ms. Ballering, with the department of psychiatry at the University of Groningen (the Netherlands), said she was surprised by the sex difference made evident in their research: “Women showed more severe persistent symptoms than men.”
Closer to a clearer definition
The authors said their findings also pinpoint symptoms that bring us closer to a better definition of long COVID, which has many different definitions globally.
“These symptoms have the highest discriminative ability to distinguish between post–COVID-19 condition and non–COVID-19–related symptoms,” they wrote.
Researchers collected data by asking participants in the northern Netherlands, who were part of the population-based Lifelines COVID-19 study, to regularly complete digital questionnaires on 23 symptoms commonly associated with long COVID. The questionnaire was sent out 24 times to the same people between March 2020 and August 2021. At that time, people had the Alpha or earlier variants.
Participants were considered COVID-19 positive if they had either a positive test or a doctor’s diagnosis of COVID-19.
Of 76,422 study participants, the 5.5% (4,231) who had COVID were matched to 8,462 controls. Researchers accounted for sex, age, and time of completing questionnaires.
Effect of hospitalization, vaccination unclear
Ms. Ballering said it’s unclear from this data whether vaccination or whether a person was hospitalized would change the prevalence of persistent symptoms.
Because of the period when the data were collected, “the vast majority of our study population was not fully vaccinated,” she said.
However, she pointed to recent research that shows that immunization against COVID is only partially effective against persistent somatic symptoms after COVID.
Also, only 5% of men and 2.5% of women in the study were hospitalized as a result of COVID-19, so the findings can’t easily be generalized to hospitalized patients.
The Lifelines study was an add-on study to the multidisciplinary, prospective, population-based, observational Dutch Lifelines cohort study examining 167,729 people in the Netherlands. Almost all were White, a limitation of the study, and 58% were female. Average age was 54.
The editorialists also noted additional limitations of the study were that this research “did not fully consider the impact on mental health” and was conducted in one region in the Netherlands.
Janko Nikolich-Žugich, MD, PhD, director of the Aegis Consortium for Pandemic-Free Future and head of the immunobiology department at University of Arizona, Tucson, said in an interview that he agreed with the editorialists that a primary benefit of this study is that it corrected for symptoms people had before COVID, something other studies have not been able to do.
However, he cautioned about generalizing the results for the United States and other countries because of the lack of diversity in the study population with regard to education level, socioeconomic factors, and race. He pointed out that access issues are also different in the Netherlands, which has universal health care.
He said brain fog as a symptom of long COVID is of high interest and will be important to include in future studies that are able to extend the study period.
The work was funded by ZonMw; the Dutch Ministry of Health, Welfare, and Sport; Dutch Ministry of Economic Affairs; University Medical Center Groningen, University of Groningen; and the provinces of Drenthe, Friesland, and Groningen. The study authors and Dr. Nikolich-Žugich have reported no relevant financial relationships. Dr. Brightling has received consultancy and or grants paid to his institution from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis, Chiesi, Genentech, Roche, Sanofi, Regeneron, Mologic, and 4DPharma for asthma and chronic obstructive pulmonary disease research. Dr. Evans has received consultancy fees from AstraZeneca on the topic of long COVID and from GlaxoSmithKline on digital health, and speaker’s fees from Boehringer Ingelheim on long COVID.
A version of this article first appeared on Medscape.com.
a large study published in The Lancet indicates.
The researchers determined that percentage by comparing long-term symptoms in people infected by SARS-CoV-2 with similar symptoms in uninfected people over the same time period.
Among the group of infected study participants in the Netherlands, 21.4% had at least one new or severely increased symptom 3-5 months after infection compared with before infection. When that group of 21.4% was compared with 8.7% of uninfected people in the same study, the researchers were able to calculate a prevalence 12.7% with long COVID.
“This finding shows that post–COVID-19 condition is an urgent problem with a mounting human toll,” the study authors wrote.
The research design was novel, two editorialists said in an accompanying commentary.
Christopher Brightling, PhD, and Rachael Evans, MBChB, PhD, of the Institute for Lung Health, University of Leicester (England), noted: “This is a major advance on prior long COVID prevalence estimates as it includes a matched uninfected group and accounts for symptoms before COVID-19 infection.”
Symptoms that persist
The Lancet study found that 3-5 months after COVID (compared with before COVID) and compared with the non-COVID comparison group, the symptoms that persist were chest pain, breathing difficulties, pain when breathing, muscle pain, loss of taste and/or smell, tingling extremities, lump in throat, feeling hot and cold alternately, heavy limbs, and tiredness.
The authors noted that symptoms such as brain fog were found to be relevant to long COVID after the data collection period for this paper and were not included in this research.
Researcher Aranka V. Ballering, MSc, PhD candidate, said in an interview that the researchers found fever is a symptom that is clearly present during the acute phase of the disease and it peaks the day of the COVID-19 diagnosis, but also wears off.
Loss of taste and smell, however, rapidly increases in severity when COVID-19 is diagnosed, but also persists and is still present 3-5 months after COVID.
Ms. Ballering, with the department of psychiatry at the University of Groningen (the Netherlands), said she was surprised by the sex difference made evident in their research: “Women showed more severe persistent symptoms than men.”
Closer to a clearer definition
The authors said their findings also pinpoint symptoms that bring us closer to a better definition of long COVID, which has many different definitions globally.
“These symptoms have the highest discriminative ability to distinguish between post–COVID-19 condition and non–COVID-19–related symptoms,” they wrote.
Researchers collected data by asking participants in the northern Netherlands, who were part of the population-based Lifelines COVID-19 study, to regularly complete digital questionnaires on 23 symptoms commonly associated with long COVID. The questionnaire was sent out 24 times to the same people between March 2020 and August 2021. At that time, people had the Alpha or earlier variants.
Participants were considered COVID-19 positive if they had either a positive test or a doctor’s diagnosis of COVID-19.
Of 76,422 study participants, the 5.5% (4,231) who had COVID were matched to 8,462 controls. Researchers accounted for sex, age, and time of completing questionnaires.
Effect of hospitalization, vaccination unclear
Ms. Ballering said it’s unclear from this data whether vaccination or whether a person was hospitalized would change the prevalence of persistent symptoms.
Because of the period when the data were collected, “the vast majority of our study population was not fully vaccinated,” she said.
However, she pointed to recent research that shows that immunization against COVID is only partially effective against persistent somatic symptoms after COVID.
Also, only 5% of men and 2.5% of women in the study were hospitalized as a result of COVID-19, so the findings can’t easily be generalized to hospitalized patients.
The Lifelines study was an add-on study to the multidisciplinary, prospective, population-based, observational Dutch Lifelines cohort study examining 167,729 people in the Netherlands. Almost all were White, a limitation of the study, and 58% were female. Average age was 54.
The editorialists also noted additional limitations of the study were that this research “did not fully consider the impact on mental health” and was conducted in one region in the Netherlands.
Janko Nikolich-Žugich, MD, PhD, director of the Aegis Consortium for Pandemic-Free Future and head of the immunobiology department at University of Arizona, Tucson, said in an interview that he agreed with the editorialists that a primary benefit of this study is that it corrected for symptoms people had before COVID, something other studies have not been able to do.
However, he cautioned about generalizing the results for the United States and other countries because of the lack of diversity in the study population with regard to education level, socioeconomic factors, and race. He pointed out that access issues are also different in the Netherlands, which has universal health care.
He said brain fog as a symptom of long COVID is of high interest and will be important to include in future studies that are able to extend the study period.
The work was funded by ZonMw; the Dutch Ministry of Health, Welfare, and Sport; Dutch Ministry of Economic Affairs; University Medical Center Groningen, University of Groningen; and the provinces of Drenthe, Friesland, and Groningen. The study authors and Dr. Nikolich-Žugich have reported no relevant financial relationships. Dr. Brightling has received consultancy and or grants paid to his institution from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis, Chiesi, Genentech, Roche, Sanofi, Regeneron, Mologic, and 4DPharma for asthma and chronic obstructive pulmonary disease research. Dr. Evans has received consultancy fees from AstraZeneca on the topic of long COVID and from GlaxoSmithKline on digital health, and speaker’s fees from Boehringer Ingelheim on long COVID.
A version of this article first appeared on Medscape.com.
FROM THE LANCET
Doctor faces apparent retaliation after alleging data manipulation in published trial
A rheumatologist was suspended from a professional society and his license to practice medicine was threatened after he raised concerns about data manipulation in a published study for which he recruited patients, according to documents seen by Retraction Watch.
The study, “Added Value of Anti-CD74 Autoantibodies in Axial SpondyloArthritis in a Population With Low HLA-B27 Prevalence,” was published in Frontiers in Immunology in 2019 and has been cited 13 times, according to Clarivate’s Web of Science. In its acknowledgments, it listed Fouad Fayad, PhD, a rheumatologist at the University of Saint Joseph and Hotel-Dieu de France University Medical Center in Beirut, as one of the researchers who recruited patients for the trial.
Dr. Fayad alleged that the researchers tested patient samples multiple times and used a mix of old and new values in their analysis. After he reported his concerns to the journal and then the university, which both concluded that they could not confirm or refute his allegations, he has faced apparent retaliation, including the suspension of his membership in the Lebanese Society of Rheumatology.
In comments to Retraction Watch, the corresponding author for the study noted that the two investigations did not find data manipulation, and said the issue was “based on a background of personal and professional conflicts.”
In an April video recorded with Nassim Nicholas Taleb, PhD, a former quant trader and retired distinguished professor of finance and risk engineering at New York University’s Tandon School of Engineering, Dr. Fayad explained that he was originally an author on the paper, but after expressing concerns about the methodology to the other authors, they didn’t respond to him and his name was dropped from the author list without warning or explanation.
Dr. Taleb also detailed the issues with the study, showing graphs that indicate “very poor correlation” between the old and new test results from participant samples.
In October 2019, Dr. Fayad contacted Frontiers in Immunology with his concerns. But the journal’s investigation was inconclusive, and a staffer on the research integrity team told him in July 2020 to contact his institution to investigate, according to emails seen by Retraction Watch.
Dr. Fayad did so, but the University of Saint Joseph “rushed an incomplete investigation,” he said. It began in September of 2021 and concluded 2 months later that the investigation committee could not confirm or disprove Dr. Fayad’s allegations of data manipulation, according to a copy of the report seen by Retraction Watch. He said that their statistical reviewer did not receive all of the relevant documents, although he had provided them to the university.
A university official sent the findings from the investigation to the Lebanese Order of Physicians – Beirut, which decided to suspend Dr. Fayad’s membership in the Lebanese Society of Rheumatology. It’s “needless to explain the damage resulting from this suspension,” Dr. Fayad said.
The Beirut organization wrote to the Lebanese Order of Physicians – Tripoli, the body with which Dr. Fayad’s license is registered, informing them of the decision. In a copy of the letter seen by Retraction Watch, the Beirut organization cited the university investigation finding Dr. Fayad’s allegations to be invalid, as well as a letter in which he alleged mismanagement of the rheumatology society, as reasons for the decision, and referred the matter to the Tripoli organization for further investigation.
Dr. Fayad told us that the letter asking the Tripoli organization to investigate him could have led to the suspension of his license to practice medicine:
“My license is registered with the Lebanese Order of Physicians – Tripoli. So legally speaking, it is only Tripoli organization that can suspend my license/permit to practice. Beirut Organization has tried to summon me to their investigation committee, but my license (being registered in Tripoli Organization) does not fall under Beirut’s jurisdiction; in other words Beirut Organization violated the law; they can not approach me directly, they have to go through the Tripoli Organization.
“As such, and since Beirut organization could not suspend my license (as they did for my membership in the Lebanese Society of Rheumatology) they sent the letter to Tripoli organization asking them to investigate the matter and take necessary disciplinary action. This was a threat to suspending my license to practice medicine. Should Tripoli Organization have used the [University of Saint Joseph] letter and investigation report without conducting their own international investigation, my permit to practice would have been suspended.”
The Lebanese Order of Physicians – Tripoli conducted its own investigation and confirmed “the existence of manipulation in the study data and failure to respect the data integrity,” according to an official translation of the investigation report seen by Retraction Watch. The Lebanese Order of Physicians – Tripoli decided after its investigation that Dr. Fayad’s suspension from the rheumatology society was invalid.
The lead author of the study in question, Nelly R. Ziade of Saint Joseph University and Hotel-Dieu de France Hospital in Beirut, told Retraction Watch that the investigation by the Lebanese Order of Physicians – Tripoli “cannot be considered as final or official” and that she was “never approached, interviewed, or asked to provide any documents related to this complaint.”
She continued: “I will always be available to give any scientific clarification requested by the Order of Physicians in Beirut where a serious investigation giving equal voice to both parties is currently conducted.
“Kindly note that the concerned journal has already conducted an internal investigation where both parties provided all documents and it was concluded that there was no scientific foundation for the accusations.
“Again, a similar investigation was conducted by the Saint-Joseph University in Beirut (where myself and the other party work). Both parties presented study documents to a committee including the president of the IRB, the vice president of the University, the medical director of the University Hospital, experts in musculoskeletal system and biostatistics. In brief, the case against the authors was dismissed, no data manipulation was found and the colleague from Tripoli also was submitted to University sanctions. The report of the University can be shared with you should you need it.
“I’m afraid that this issue is based on a background of personal and professional conflicts.”
Dr. Fayad added: “The beauty of science is that the truth will always prevail and cannot be obscured for long time.”
A version of this article first appeared on RetractionWatch.com.
A rheumatologist was suspended from a professional society and his license to practice medicine was threatened after he raised concerns about data manipulation in a published study for which he recruited patients, according to documents seen by Retraction Watch.
The study, “Added Value of Anti-CD74 Autoantibodies in Axial SpondyloArthritis in a Population With Low HLA-B27 Prevalence,” was published in Frontiers in Immunology in 2019 and has been cited 13 times, according to Clarivate’s Web of Science. In its acknowledgments, it listed Fouad Fayad, PhD, a rheumatologist at the University of Saint Joseph and Hotel-Dieu de France University Medical Center in Beirut, as one of the researchers who recruited patients for the trial.
Dr. Fayad alleged that the researchers tested patient samples multiple times and used a mix of old and new values in their analysis. After he reported his concerns to the journal and then the university, which both concluded that they could not confirm or refute his allegations, he has faced apparent retaliation, including the suspension of his membership in the Lebanese Society of Rheumatology.
In comments to Retraction Watch, the corresponding author for the study noted that the two investigations did not find data manipulation, and said the issue was “based on a background of personal and professional conflicts.”
In an April video recorded with Nassim Nicholas Taleb, PhD, a former quant trader and retired distinguished professor of finance and risk engineering at New York University’s Tandon School of Engineering, Dr. Fayad explained that he was originally an author on the paper, but after expressing concerns about the methodology to the other authors, they didn’t respond to him and his name was dropped from the author list without warning or explanation.
Dr. Taleb also detailed the issues with the study, showing graphs that indicate “very poor correlation” between the old and new test results from participant samples.
In October 2019, Dr. Fayad contacted Frontiers in Immunology with his concerns. But the journal’s investigation was inconclusive, and a staffer on the research integrity team told him in July 2020 to contact his institution to investigate, according to emails seen by Retraction Watch.
Dr. Fayad did so, but the University of Saint Joseph “rushed an incomplete investigation,” he said. It began in September of 2021 and concluded 2 months later that the investigation committee could not confirm or disprove Dr. Fayad’s allegations of data manipulation, according to a copy of the report seen by Retraction Watch. He said that their statistical reviewer did not receive all of the relevant documents, although he had provided them to the university.
A university official sent the findings from the investigation to the Lebanese Order of Physicians – Beirut, which decided to suspend Dr. Fayad’s membership in the Lebanese Society of Rheumatology. It’s “needless to explain the damage resulting from this suspension,” Dr. Fayad said.
The Beirut organization wrote to the Lebanese Order of Physicians – Tripoli, the body with which Dr. Fayad’s license is registered, informing them of the decision. In a copy of the letter seen by Retraction Watch, the Beirut organization cited the university investigation finding Dr. Fayad’s allegations to be invalid, as well as a letter in which he alleged mismanagement of the rheumatology society, as reasons for the decision, and referred the matter to the Tripoli organization for further investigation.
Dr. Fayad told us that the letter asking the Tripoli organization to investigate him could have led to the suspension of his license to practice medicine:
“My license is registered with the Lebanese Order of Physicians – Tripoli. So legally speaking, it is only Tripoli organization that can suspend my license/permit to practice. Beirut Organization has tried to summon me to their investigation committee, but my license (being registered in Tripoli Organization) does not fall under Beirut’s jurisdiction; in other words Beirut Organization violated the law; they can not approach me directly, they have to go through the Tripoli Organization.
“As such, and since Beirut organization could not suspend my license (as they did for my membership in the Lebanese Society of Rheumatology) they sent the letter to Tripoli organization asking them to investigate the matter and take necessary disciplinary action. This was a threat to suspending my license to practice medicine. Should Tripoli Organization have used the [University of Saint Joseph] letter and investigation report without conducting their own international investigation, my permit to practice would have been suspended.”
The Lebanese Order of Physicians – Tripoli conducted its own investigation and confirmed “the existence of manipulation in the study data and failure to respect the data integrity,” according to an official translation of the investigation report seen by Retraction Watch. The Lebanese Order of Physicians – Tripoli decided after its investigation that Dr. Fayad’s suspension from the rheumatology society was invalid.
The lead author of the study in question, Nelly R. Ziade of Saint Joseph University and Hotel-Dieu de France Hospital in Beirut, told Retraction Watch that the investigation by the Lebanese Order of Physicians – Tripoli “cannot be considered as final or official” and that she was “never approached, interviewed, or asked to provide any documents related to this complaint.”
She continued: “I will always be available to give any scientific clarification requested by the Order of Physicians in Beirut where a serious investigation giving equal voice to both parties is currently conducted.
“Kindly note that the concerned journal has already conducted an internal investigation where both parties provided all documents and it was concluded that there was no scientific foundation for the accusations.
“Again, a similar investigation was conducted by the Saint-Joseph University in Beirut (where myself and the other party work). Both parties presented study documents to a committee including the president of the IRB, the vice president of the University, the medical director of the University Hospital, experts in musculoskeletal system and biostatistics. In brief, the case against the authors was dismissed, no data manipulation was found and the colleague from Tripoli also was submitted to University sanctions. The report of the University can be shared with you should you need it.
“I’m afraid that this issue is based on a background of personal and professional conflicts.”
Dr. Fayad added: “The beauty of science is that the truth will always prevail and cannot be obscured for long time.”
A version of this article first appeared on RetractionWatch.com.
A rheumatologist was suspended from a professional society and his license to practice medicine was threatened after he raised concerns about data manipulation in a published study for which he recruited patients, according to documents seen by Retraction Watch.
The study, “Added Value of Anti-CD74 Autoantibodies in Axial SpondyloArthritis in a Population With Low HLA-B27 Prevalence,” was published in Frontiers in Immunology in 2019 and has been cited 13 times, according to Clarivate’s Web of Science. In its acknowledgments, it listed Fouad Fayad, PhD, a rheumatologist at the University of Saint Joseph and Hotel-Dieu de France University Medical Center in Beirut, as one of the researchers who recruited patients for the trial.
Dr. Fayad alleged that the researchers tested patient samples multiple times and used a mix of old and new values in their analysis. After he reported his concerns to the journal and then the university, which both concluded that they could not confirm or refute his allegations, he has faced apparent retaliation, including the suspension of his membership in the Lebanese Society of Rheumatology.
In comments to Retraction Watch, the corresponding author for the study noted that the two investigations did not find data manipulation, and said the issue was “based on a background of personal and professional conflicts.”
In an April video recorded with Nassim Nicholas Taleb, PhD, a former quant trader and retired distinguished professor of finance and risk engineering at New York University’s Tandon School of Engineering, Dr. Fayad explained that he was originally an author on the paper, but after expressing concerns about the methodology to the other authors, they didn’t respond to him and his name was dropped from the author list without warning or explanation.
Dr. Taleb also detailed the issues with the study, showing graphs that indicate “very poor correlation” between the old and new test results from participant samples.
In October 2019, Dr. Fayad contacted Frontiers in Immunology with his concerns. But the journal’s investigation was inconclusive, and a staffer on the research integrity team told him in July 2020 to contact his institution to investigate, according to emails seen by Retraction Watch.
Dr. Fayad did so, but the University of Saint Joseph “rushed an incomplete investigation,” he said. It began in September of 2021 and concluded 2 months later that the investigation committee could not confirm or disprove Dr. Fayad’s allegations of data manipulation, according to a copy of the report seen by Retraction Watch. He said that their statistical reviewer did not receive all of the relevant documents, although he had provided them to the university.
A university official sent the findings from the investigation to the Lebanese Order of Physicians – Beirut, which decided to suspend Dr. Fayad’s membership in the Lebanese Society of Rheumatology. It’s “needless to explain the damage resulting from this suspension,” Dr. Fayad said.
The Beirut organization wrote to the Lebanese Order of Physicians – Tripoli, the body with which Dr. Fayad’s license is registered, informing them of the decision. In a copy of the letter seen by Retraction Watch, the Beirut organization cited the university investigation finding Dr. Fayad’s allegations to be invalid, as well as a letter in which he alleged mismanagement of the rheumatology society, as reasons for the decision, and referred the matter to the Tripoli organization for further investigation.
Dr. Fayad told us that the letter asking the Tripoli organization to investigate him could have led to the suspension of his license to practice medicine:
“My license is registered with the Lebanese Order of Physicians – Tripoli. So legally speaking, it is only Tripoli organization that can suspend my license/permit to practice. Beirut Organization has tried to summon me to their investigation committee, but my license (being registered in Tripoli Organization) does not fall under Beirut’s jurisdiction; in other words Beirut Organization violated the law; they can not approach me directly, they have to go through the Tripoli Organization.
“As such, and since Beirut organization could not suspend my license (as they did for my membership in the Lebanese Society of Rheumatology) they sent the letter to Tripoli organization asking them to investigate the matter and take necessary disciplinary action. This was a threat to suspending my license to practice medicine. Should Tripoli Organization have used the [University of Saint Joseph] letter and investigation report without conducting their own international investigation, my permit to practice would have been suspended.”
The Lebanese Order of Physicians – Tripoli conducted its own investigation and confirmed “the existence of manipulation in the study data and failure to respect the data integrity,” according to an official translation of the investigation report seen by Retraction Watch. The Lebanese Order of Physicians – Tripoli decided after its investigation that Dr. Fayad’s suspension from the rheumatology society was invalid.
The lead author of the study in question, Nelly R. Ziade of Saint Joseph University and Hotel-Dieu de France Hospital in Beirut, told Retraction Watch that the investigation by the Lebanese Order of Physicians – Tripoli “cannot be considered as final or official” and that she was “never approached, interviewed, or asked to provide any documents related to this complaint.”
She continued: “I will always be available to give any scientific clarification requested by the Order of Physicians in Beirut where a serious investigation giving equal voice to both parties is currently conducted.
“Kindly note that the concerned journal has already conducted an internal investigation where both parties provided all documents and it was concluded that there was no scientific foundation for the accusations.
“Again, a similar investigation was conducted by the Saint-Joseph University in Beirut (where myself and the other party work). Both parties presented study documents to a committee including the president of the IRB, the vice president of the University, the medical director of the University Hospital, experts in musculoskeletal system and biostatistics. In brief, the case against the authors was dismissed, no data manipulation was found and the colleague from Tripoli also was submitted to University sanctions. The report of the University can be shared with you should you need it.
“I’m afraid that this issue is based on a background of personal and professional conflicts.”
Dr. Fayad added: “The beauty of science is that the truth will always prevail and cannot be obscured for long time.”
A version of this article first appeared on RetractionWatch.com.
Haven’t had COVID yet? Wanna bet?
We all have friends or relatives who, somehow, have managed to avoid catching COVID-19, which has infected more than 91.5 million Americans. You may even be one of the lucky ones yourself.
But health experts are saying: Not so fast. because they didn’t have symptoms or had mild cases they mistook for a cold or allergies.
The upshot: These silent COVID-19 cases reflect a hidden side of the pandemic that may be helping to drive new surges and viral variants.
Still, infectious disease experts say there is little doubt that some people have indeed managed to avoid COVID-19 infection altogether, and they are trying to understand why.
Several recent studies have suggested certain genetic and immune system traits may better protect this group of people against the coronavirus, making them less likely than others to be infected or seriously sickened. Researchers around the world are now studying these seemingly super-immune people for clues to what makes them so special, with an eye toward better vaccines, treatments, and prevention strategies.
Infectious disease specialists say both types of cases – those unknowingly infected by COVID-19 and people who’ve avoided the virus altogether – matter greatly to public health, more than 2 years into the pandemic.
“It’s definitely true that some people have had COVID and don’t realize it,” says Stephen Kissler, PhD, an infectious disease researcher with the Harvard T.H. Chan School of Public Health, Boston. “It is potentially good news if there’s more immunity in the population than we realize.”
But he says that being able to identify genetic and other factors that may offer some people protection against COVID-19 is an “exciting prospect” that could help find out who’s most at risk and improve efforts to get the pandemic under control.
Some studies have found a person’s genetic profile, past exposure to other COVID-like viruses, allergies, and even drugs they take for other conditions may all provide some defense – even for people who have not been vaccinated, don’t use masks, or don’t practice social distancing.
A person’s medical history and genetics may help decide their risk from new diseases, meaning “we may be able to help identify people who are at especially high risk from infection,” Dr. Kissler says. “That knowledge could help those people better shield themselves from infection and get quicker access to treatment and vaccines, if necessary. … We don’t yet know, but studies are ongoing for these things.”
Amesh Adalja, MD, an infectious disease specialist with the Johns Hopkins Center for Health Security, Baltimore, agrees that emerging research on people who’ve avoided infection offers the chance of new public health strategies to combat COVID-19.
“I’m sure there is some subset of people who are [COVID] negative,” he says. “So what explains that phenomenon, especially if that person was out there getting significant exposures?”
Have you had COVID without knowing it?
In a media briefing late last month, White House COVID-19 Response Coordinator Ashish Jha, MD, said more than 70% of the U.S. population has had the virus, according to the latest CDC data. That’s up from 33.5% in December.
But the actual number of people in the U.S. who have been infected with SARS-CoV-2, the scientific name for the virus that causes COVID-19, is likely to be much higher due to cases without symptoms that are unreported, experts say.
Since the early days of the pandemic, researchers have tried to put a number on these hidden cases, but that figure has been evolving and a clear consensus has not emerged.
In September 2020, a study published in the Annals of Internal Medicine said “approximately 40% to 45% of those infected with SARS-CoV-2 will remain asymptomatic.”
A follow-up analysis of 95 studies, published last December, reached similar findings, estimating that more than 40% of COVID-19 infections didn’t come with symptoms.
To get a better handle on the issue, CDC officials have been working with the American Red Cross and other blood banks to track COVID-19 antibodies – proteins your body makes after exposure to the virus to fight off an infection – in donors who said they have never had COVID-19.
While that joint effort is still ongoing, early findings say the number of donors with antibodies from COVID-19 infection increased in blood donors from 3.5% in July 2020 to at least 20.2% in May 2021. Since then, those percentages have soared, in part due to the introduction of vaccines, which also make the body produce COVID-19 antibodies.
The most current findings show that 83.3% of donors have combined COVID infection– and vaccine-induced antibodies in their blood. Those findings are based on 1.4 million blood donations.
Health experts say all of these studies are strong evidence that many COVID-19 cases continue to go undetected. In fact, the University of Washington Institute for Health Metrics and Evaluation estimates that only 7% of positive COVID-19 cases in the U.S. are being detected. That means case rates are actually 14.5 times higher than the official count of 131,000 new COVID infections each day, according to the Centers for Disease Control and Prevention, which reports the virus is still killing about 440 Americans daily.
So, why is all this important, in terms of public health?
Experts say people are more likely to be cautious if they know COVID-19 cases are high where they live, work, and play. On the other hand, if they believe case rates in their communities are lower than they actually are, they may be less likely to get vaccinated and boosted, wear masks indoors, avoid crowded indoor spaces, and take other precautions to fend off infection.
How do some avoid infection altogether?
In addition to tracking cases that go unreported and don’t have symptoms, infectious disease experts have also been trying to figure out why some people have managed to avoid getting the highly contagious virus.
Several leading lines of research have produced promising early results – suggesting that a person’s genetic makeup, past exposure to less-lethal coronaviruses, allergies, and even certain drugs they take for other conditions may all provide at least some protection against COVID.
“Our study showed that there are many human genes – hundreds of genes – that can impact SARS-CoV-2 infection,” says Neville Sanjana, PhD, a geneticist at New York University and the New York Genome Center who co-led the study. “With a better understanding of host genetic factors, we can find new kinds of therapies that target these host factors to block infection.”
In addition, he says several studies show some drugs that regulate genes, such as the breast cancer drug tamoxifen, also appear to knock down COVID-19 risk. He suggests such drugs, already approved by the Food and Drug Administration, might be “repurposed” to target the virus.
Studies in other countries show that patients taking tamoxifen before the pandemic were protected against severe COVID-19, Dr. Sanjana says. “That was a really cool thing, highlighting the power of harnessing host genetics. The virus critically depends on our genes to complete key parts of its life cycle.”
The NYU research findings echo other studies that have been published in recent months.
In July, a team of researchers led by the National Cancer Institute identified a genetic factor that appears to determine how severe an infection will be. In a study involving 3,000 people, they found that two gene changes, or mutations, that decrease the expression of a gene called OAS1 boosted the risk of hospitalization from COVID-19. OAS1 is part of the immune system’s response to viral infections.
As a result, developing a genetic therapy designed to increase the OAS1 gene’s expression might reduce the risk of severe disease.
“It’s very natural to get infected once you are exposed. There’s no magic bullet for that. But after you get infected, how you’re going to respond to this infection, that’s what is going to be affected by your genetic variants,” said Ludmila Prokunina-Olsson, PhD, the study’s lead researcher and chief of the National Cancer Institute’s Laboratory of Translational Genomics, Bethesda, Md., in an interview with NBC News.
Benjamin tenOever, PhD, a New York University virologist who co-authored the 2020 research, says the new genetic research is promising, but he believes it’s unlikely scientists will be able to identify a single gene responsible for actually preventing a COVID-19 infection.
“On the flip side, we have identified many genes that makes the disease worse,” he says.
T cells ‘remember’ past viral infections
As Dr. tenOever and Dr. Sanjana suggest, another intriguing line of research has found that prior viral infections may prime the body’s immune system to fight COVID-19.
Four other common coronaviruses – aside from SARS-CoV-2 – infect people worldwide, typically causing mild to moderate upper respiratory illnesses like the common cold, says Alessandro Sette, PhD, an infectious disease expert and vaccine researcher with the La Jolla (Calif.) Institute for Immunology.
In a recent study published in Science, he and his team found past infection with these other coronaviruses may give some protection against SARS-CoV-2.
T cells – white blood cells that act like immunological ninjas to ferret out and fight infections – appear to maintain a kind of “biological memory” of coronaviruses they have seen before and can mount an attack on similar pathogens, such SARS-CoV-2, Dr. Sette says.
The new work builds on a prior research he helped lead that found 40%-60% of people never exposed to SARS-CoV-2 had T cells that reacted to the virus – with their immune systems recognizing fragments of a virus they had never seen before.
Dr. Sette says his research shows that people whose T cells have this “preexisting memory” of past coronavirus exposures also tend to respond better to vaccination for reasons not yet well understood.
“The question is, at which point will there be enough immunity from vaccination, repeated infections from other coronaviruses, but also some of the variants of the SARS-CoV-2 … where infections become less frequent? We’re not there yet,” he says.
In addition to these exciting genetic and T-cell findings, other research has suggested low-grade inflammation from allergies – a key part of the body’s immune response to foreign substances – may also give some people an extra leg up, in terms of avoiding COVID infection.
Last May, a study of 1,400 households published in The Journal of Allergy and Clinical Immunology found that having a food allergy cut the risk of COVID-19 infection in half.
The researchers said it’s unclear why allergies may reduce the risk of infection, but they noted that people with food allergies express fewer ACE2 receptors on the surface of their airway cells, making it harder for the virus to enter cells.
The big picture: Prevention still your best bet
So, what’s the takeaway from all of this emerging research?
New York University’s Dr. tenOever says that while genes, T cells and allergies may offer some protection against COVID, tried-and-true precautions – vaccination, wearing masks, avoiding crowded indoor spaces, and social distancing – are likely to provide a greater defense.
He believes these precautions are likely why he and his family have never contracted COVID-19.
“I was tested weekly, as were my kids at school,” he says. “We definitely never got COVID, despite the fact that we live in New York City and I worked in a hospital every single day of the pandemic.”
Ziyad Al-Aly, MD, an infectious disease specialist and director of clinical epidemiology at Washington University in St. Louis, agrees that the new research on COVID-19 is intriguing but won’t likely result in practical changes in the approach to fighting the virus in the near term.
“Getting a deeper understanding of potential genetic factors or other characteristics – that could really help us understand why the virus just comes and goes without any ill effects in some people, and in other people it produces really serious disease,” he says. “That will really help us eventually to design better vaccines to prevent it or reduce severity or even [treat] people who get severe disease.”
In the meantime, Dr. Al-Aly says, “it’s still best to do everything you can to avoid infection in the first place – even if you’re vaccinated or previously infected, you should really try to avoid reinfection.”
That means sit outside if you can when visiting a restaurant. Wear a mask on a plane, even though it’s not required. And get vaccinated and boosted.
“In the future, there may be more tools to address this pandemic, but that’s really the best advice for now,” Dr. Al-Aly says.
A version of this article first appeared on WebMD.com.
We all have friends or relatives who, somehow, have managed to avoid catching COVID-19, which has infected more than 91.5 million Americans. You may even be one of the lucky ones yourself.
But health experts are saying: Not so fast. because they didn’t have symptoms or had mild cases they mistook for a cold or allergies.
The upshot: These silent COVID-19 cases reflect a hidden side of the pandemic that may be helping to drive new surges and viral variants.
Still, infectious disease experts say there is little doubt that some people have indeed managed to avoid COVID-19 infection altogether, and they are trying to understand why.
Several recent studies have suggested certain genetic and immune system traits may better protect this group of people against the coronavirus, making them less likely than others to be infected or seriously sickened. Researchers around the world are now studying these seemingly super-immune people for clues to what makes them so special, with an eye toward better vaccines, treatments, and prevention strategies.
Infectious disease specialists say both types of cases – those unknowingly infected by COVID-19 and people who’ve avoided the virus altogether – matter greatly to public health, more than 2 years into the pandemic.
“It’s definitely true that some people have had COVID and don’t realize it,” says Stephen Kissler, PhD, an infectious disease researcher with the Harvard T.H. Chan School of Public Health, Boston. “It is potentially good news if there’s more immunity in the population than we realize.”
But he says that being able to identify genetic and other factors that may offer some people protection against COVID-19 is an “exciting prospect” that could help find out who’s most at risk and improve efforts to get the pandemic under control.
Some studies have found a person’s genetic profile, past exposure to other COVID-like viruses, allergies, and even drugs they take for other conditions may all provide some defense – even for people who have not been vaccinated, don’t use masks, or don’t practice social distancing.
A person’s medical history and genetics may help decide their risk from new diseases, meaning “we may be able to help identify people who are at especially high risk from infection,” Dr. Kissler says. “That knowledge could help those people better shield themselves from infection and get quicker access to treatment and vaccines, if necessary. … We don’t yet know, but studies are ongoing for these things.”
Amesh Adalja, MD, an infectious disease specialist with the Johns Hopkins Center for Health Security, Baltimore, agrees that emerging research on people who’ve avoided infection offers the chance of new public health strategies to combat COVID-19.
“I’m sure there is some subset of people who are [COVID] negative,” he says. “So what explains that phenomenon, especially if that person was out there getting significant exposures?”
Have you had COVID without knowing it?
In a media briefing late last month, White House COVID-19 Response Coordinator Ashish Jha, MD, said more than 70% of the U.S. population has had the virus, according to the latest CDC data. That’s up from 33.5% in December.
But the actual number of people in the U.S. who have been infected with SARS-CoV-2, the scientific name for the virus that causes COVID-19, is likely to be much higher due to cases without symptoms that are unreported, experts say.
Since the early days of the pandemic, researchers have tried to put a number on these hidden cases, but that figure has been evolving and a clear consensus has not emerged.
In September 2020, a study published in the Annals of Internal Medicine said “approximately 40% to 45% of those infected with SARS-CoV-2 will remain asymptomatic.”
A follow-up analysis of 95 studies, published last December, reached similar findings, estimating that more than 40% of COVID-19 infections didn’t come with symptoms.
To get a better handle on the issue, CDC officials have been working with the American Red Cross and other blood banks to track COVID-19 antibodies – proteins your body makes after exposure to the virus to fight off an infection – in donors who said they have never had COVID-19.
While that joint effort is still ongoing, early findings say the number of donors with antibodies from COVID-19 infection increased in blood donors from 3.5% in July 2020 to at least 20.2% in May 2021. Since then, those percentages have soared, in part due to the introduction of vaccines, which also make the body produce COVID-19 antibodies.
The most current findings show that 83.3% of donors have combined COVID infection– and vaccine-induced antibodies in their blood. Those findings are based on 1.4 million blood donations.
Health experts say all of these studies are strong evidence that many COVID-19 cases continue to go undetected. In fact, the University of Washington Institute for Health Metrics and Evaluation estimates that only 7% of positive COVID-19 cases in the U.S. are being detected. That means case rates are actually 14.5 times higher than the official count of 131,000 new COVID infections each day, according to the Centers for Disease Control and Prevention, which reports the virus is still killing about 440 Americans daily.
So, why is all this important, in terms of public health?
Experts say people are more likely to be cautious if they know COVID-19 cases are high where they live, work, and play. On the other hand, if they believe case rates in their communities are lower than they actually are, they may be less likely to get vaccinated and boosted, wear masks indoors, avoid crowded indoor spaces, and take other precautions to fend off infection.
How do some avoid infection altogether?
In addition to tracking cases that go unreported and don’t have symptoms, infectious disease experts have also been trying to figure out why some people have managed to avoid getting the highly contagious virus.
Several leading lines of research have produced promising early results – suggesting that a person’s genetic makeup, past exposure to less-lethal coronaviruses, allergies, and even certain drugs they take for other conditions may all provide at least some protection against COVID.
“Our study showed that there are many human genes – hundreds of genes – that can impact SARS-CoV-2 infection,” says Neville Sanjana, PhD, a geneticist at New York University and the New York Genome Center who co-led the study. “With a better understanding of host genetic factors, we can find new kinds of therapies that target these host factors to block infection.”
In addition, he says several studies show some drugs that regulate genes, such as the breast cancer drug tamoxifen, also appear to knock down COVID-19 risk. He suggests such drugs, already approved by the Food and Drug Administration, might be “repurposed” to target the virus.
Studies in other countries show that patients taking tamoxifen before the pandemic were protected against severe COVID-19, Dr. Sanjana says. “That was a really cool thing, highlighting the power of harnessing host genetics. The virus critically depends on our genes to complete key parts of its life cycle.”
The NYU research findings echo other studies that have been published in recent months.
In July, a team of researchers led by the National Cancer Institute identified a genetic factor that appears to determine how severe an infection will be. In a study involving 3,000 people, they found that two gene changes, or mutations, that decrease the expression of a gene called OAS1 boosted the risk of hospitalization from COVID-19. OAS1 is part of the immune system’s response to viral infections.
As a result, developing a genetic therapy designed to increase the OAS1 gene’s expression might reduce the risk of severe disease.
“It’s very natural to get infected once you are exposed. There’s no magic bullet for that. But after you get infected, how you’re going to respond to this infection, that’s what is going to be affected by your genetic variants,” said Ludmila Prokunina-Olsson, PhD, the study’s lead researcher and chief of the National Cancer Institute’s Laboratory of Translational Genomics, Bethesda, Md., in an interview with NBC News.
Benjamin tenOever, PhD, a New York University virologist who co-authored the 2020 research, says the new genetic research is promising, but he believes it’s unlikely scientists will be able to identify a single gene responsible for actually preventing a COVID-19 infection.
“On the flip side, we have identified many genes that makes the disease worse,” he says.
T cells ‘remember’ past viral infections
As Dr. tenOever and Dr. Sanjana suggest, another intriguing line of research has found that prior viral infections may prime the body’s immune system to fight COVID-19.
Four other common coronaviruses – aside from SARS-CoV-2 – infect people worldwide, typically causing mild to moderate upper respiratory illnesses like the common cold, says Alessandro Sette, PhD, an infectious disease expert and vaccine researcher with the La Jolla (Calif.) Institute for Immunology.
In a recent study published in Science, he and his team found past infection with these other coronaviruses may give some protection against SARS-CoV-2.
T cells – white blood cells that act like immunological ninjas to ferret out and fight infections – appear to maintain a kind of “biological memory” of coronaviruses they have seen before and can mount an attack on similar pathogens, such SARS-CoV-2, Dr. Sette says.
The new work builds on a prior research he helped lead that found 40%-60% of people never exposed to SARS-CoV-2 had T cells that reacted to the virus – with their immune systems recognizing fragments of a virus they had never seen before.
Dr. Sette says his research shows that people whose T cells have this “preexisting memory” of past coronavirus exposures also tend to respond better to vaccination for reasons not yet well understood.
“The question is, at which point will there be enough immunity from vaccination, repeated infections from other coronaviruses, but also some of the variants of the SARS-CoV-2 … where infections become less frequent? We’re not there yet,” he says.
In addition to these exciting genetic and T-cell findings, other research has suggested low-grade inflammation from allergies – a key part of the body’s immune response to foreign substances – may also give some people an extra leg up, in terms of avoiding COVID infection.
Last May, a study of 1,400 households published in The Journal of Allergy and Clinical Immunology found that having a food allergy cut the risk of COVID-19 infection in half.
The researchers said it’s unclear why allergies may reduce the risk of infection, but they noted that people with food allergies express fewer ACE2 receptors on the surface of their airway cells, making it harder for the virus to enter cells.
The big picture: Prevention still your best bet
So, what’s the takeaway from all of this emerging research?
New York University’s Dr. tenOever says that while genes, T cells and allergies may offer some protection against COVID, tried-and-true precautions – vaccination, wearing masks, avoiding crowded indoor spaces, and social distancing – are likely to provide a greater defense.
He believes these precautions are likely why he and his family have never contracted COVID-19.
“I was tested weekly, as were my kids at school,” he says. “We definitely never got COVID, despite the fact that we live in New York City and I worked in a hospital every single day of the pandemic.”
Ziyad Al-Aly, MD, an infectious disease specialist and director of clinical epidemiology at Washington University in St. Louis, agrees that the new research on COVID-19 is intriguing but won’t likely result in practical changes in the approach to fighting the virus in the near term.
“Getting a deeper understanding of potential genetic factors or other characteristics – that could really help us understand why the virus just comes and goes without any ill effects in some people, and in other people it produces really serious disease,” he says. “That will really help us eventually to design better vaccines to prevent it or reduce severity or even [treat] people who get severe disease.”
In the meantime, Dr. Al-Aly says, “it’s still best to do everything you can to avoid infection in the first place – even if you’re vaccinated or previously infected, you should really try to avoid reinfection.”
That means sit outside if you can when visiting a restaurant. Wear a mask on a plane, even though it’s not required. And get vaccinated and boosted.
“In the future, there may be more tools to address this pandemic, but that’s really the best advice for now,” Dr. Al-Aly says.
A version of this article first appeared on WebMD.com.
We all have friends or relatives who, somehow, have managed to avoid catching COVID-19, which has infected more than 91.5 million Americans. You may even be one of the lucky ones yourself.
But health experts are saying: Not so fast. because they didn’t have symptoms or had mild cases they mistook for a cold or allergies.
The upshot: These silent COVID-19 cases reflect a hidden side of the pandemic that may be helping to drive new surges and viral variants.
Still, infectious disease experts say there is little doubt that some people have indeed managed to avoid COVID-19 infection altogether, and they are trying to understand why.
Several recent studies have suggested certain genetic and immune system traits may better protect this group of people against the coronavirus, making them less likely than others to be infected or seriously sickened. Researchers around the world are now studying these seemingly super-immune people for clues to what makes them so special, with an eye toward better vaccines, treatments, and prevention strategies.
Infectious disease specialists say both types of cases – those unknowingly infected by COVID-19 and people who’ve avoided the virus altogether – matter greatly to public health, more than 2 years into the pandemic.
“It’s definitely true that some people have had COVID and don’t realize it,” says Stephen Kissler, PhD, an infectious disease researcher with the Harvard T.H. Chan School of Public Health, Boston. “It is potentially good news if there’s more immunity in the population than we realize.”
But he says that being able to identify genetic and other factors that may offer some people protection against COVID-19 is an “exciting prospect” that could help find out who’s most at risk and improve efforts to get the pandemic under control.
Some studies have found a person’s genetic profile, past exposure to other COVID-like viruses, allergies, and even drugs they take for other conditions may all provide some defense – even for people who have not been vaccinated, don’t use masks, or don’t practice social distancing.
A person’s medical history and genetics may help decide their risk from new diseases, meaning “we may be able to help identify people who are at especially high risk from infection,” Dr. Kissler says. “That knowledge could help those people better shield themselves from infection and get quicker access to treatment and vaccines, if necessary. … We don’t yet know, but studies are ongoing for these things.”
Amesh Adalja, MD, an infectious disease specialist with the Johns Hopkins Center for Health Security, Baltimore, agrees that emerging research on people who’ve avoided infection offers the chance of new public health strategies to combat COVID-19.
“I’m sure there is some subset of people who are [COVID] negative,” he says. “So what explains that phenomenon, especially if that person was out there getting significant exposures?”
Have you had COVID without knowing it?
In a media briefing late last month, White House COVID-19 Response Coordinator Ashish Jha, MD, said more than 70% of the U.S. population has had the virus, according to the latest CDC data. That’s up from 33.5% in December.
But the actual number of people in the U.S. who have been infected with SARS-CoV-2, the scientific name for the virus that causes COVID-19, is likely to be much higher due to cases without symptoms that are unreported, experts say.
Since the early days of the pandemic, researchers have tried to put a number on these hidden cases, but that figure has been evolving and a clear consensus has not emerged.
In September 2020, a study published in the Annals of Internal Medicine said “approximately 40% to 45% of those infected with SARS-CoV-2 will remain asymptomatic.”
A follow-up analysis of 95 studies, published last December, reached similar findings, estimating that more than 40% of COVID-19 infections didn’t come with symptoms.
To get a better handle on the issue, CDC officials have been working with the American Red Cross and other blood banks to track COVID-19 antibodies – proteins your body makes after exposure to the virus to fight off an infection – in donors who said they have never had COVID-19.
While that joint effort is still ongoing, early findings say the number of donors with antibodies from COVID-19 infection increased in blood donors from 3.5% in July 2020 to at least 20.2% in May 2021. Since then, those percentages have soared, in part due to the introduction of vaccines, which also make the body produce COVID-19 antibodies.
The most current findings show that 83.3% of donors have combined COVID infection– and vaccine-induced antibodies in their blood. Those findings are based on 1.4 million blood donations.
Health experts say all of these studies are strong evidence that many COVID-19 cases continue to go undetected. In fact, the University of Washington Institute for Health Metrics and Evaluation estimates that only 7% of positive COVID-19 cases in the U.S. are being detected. That means case rates are actually 14.5 times higher than the official count of 131,000 new COVID infections each day, according to the Centers for Disease Control and Prevention, which reports the virus is still killing about 440 Americans daily.
So, why is all this important, in terms of public health?
Experts say people are more likely to be cautious if they know COVID-19 cases are high where they live, work, and play. On the other hand, if they believe case rates in their communities are lower than they actually are, they may be less likely to get vaccinated and boosted, wear masks indoors, avoid crowded indoor spaces, and take other precautions to fend off infection.
How do some avoid infection altogether?
In addition to tracking cases that go unreported and don’t have symptoms, infectious disease experts have also been trying to figure out why some people have managed to avoid getting the highly contagious virus.
Several leading lines of research have produced promising early results – suggesting that a person’s genetic makeup, past exposure to less-lethal coronaviruses, allergies, and even certain drugs they take for other conditions may all provide at least some protection against COVID.
“Our study showed that there are many human genes – hundreds of genes – that can impact SARS-CoV-2 infection,” says Neville Sanjana, PhD, a geneticist at New York University and the New York Genome Center who co-led the study. “With a better understanding of host genetic factors, we can find new kinds of therapies that target these host factors to block infection.”
In addition, he says several studies show some drugs that regulate genes, such as the breast cancer drug tamoxifen, also appear to knock down COVID-19 risk. He suggests such drugs, already approved by the Food and Drug Administration, might be “repurposed” to target the virus.
Studies in other countries show that patients taking tamoxifen before the pandemic were protected against severe COVID-19, Dr. Sanjana says. “That was a really cool thing, highlighting the power of harnessing host genetics. The virus critically depends on our genes to complete key parts of its life cycle.”
The NYU research findings echo other studies that have been published in recent months.
In July, a team of researchers led by the National Cancer Institute identified a genetic factor that appears to determine how severe an infection will be. In a study involving 3,000 people, they found that two gene changes, or mutations, that decrease the expression of a gene called OAS1 boosted the risk of hospitalization from COVID-19. OAS1 is part of the immune system’s response to viral infections.
As a result, developing a genetic therapy designed to increase the OAS1 gene’s expression might reduce the risk of severe disease.
“It’s very natural to get infected once you are exposed. There’s no magic bullet for that. But after you get infected, how you’re going to respond to this infection, that’s what is going to be affected by your genetic variants,” said Ludmila Prokunina-Olsson, PhD, the study’s lead researcher and chief of the National Cancer Institute’s Laboratory of Translational Genomics, Bethesda, Md., in an interview with NBC News.
Benjamin tenOever, PhD, a New York University virologist who co-authored the 2020 research, says the new genetic research is promising, but he believes it’s unlikely scientists will be able to identify a single gene responsible for actually preventing a COVID-19 infection.
“On the flip side, we have identified many genes that makes the disease worse,” he says.
T cells ‘remember’ past viral infections
As Dr. tenOever and Dr. Sanjana suggest, another intriguing line of research has found that prior viral infections may prime the body’s immune system to fight COVID-19.
Four other common coronaviruses – aside from SARS-CoV-2 – infect people worldwide, typically causing mild to moderate upper respiratory illnesses like the common cold, says Alessandro Sette, PhD, an infectious disease expert and vaccine researcher with the La Jolla (Calif.) Institute for Immunology.
In a recent study published in Science, he and his team found past infection with these other coronaviruses may give some protection against SARS-CoV-2.
T cells – white blood cells that act like immunological ninjas to ferret out and fight infections – appear to maintain a kind of “biological memory” of coronaviruses they have seen before and can mount an attack on similar pathogens, such SARS-CoV-2, Dr. Sette says.
The new work builds on a prior research he helped lead that found 40%-60% of people never exposed to SARS-CoV-2 had T cells that reacted to the virus – with their immune systems recognizing fragments of a virus they had never seen before.
Dr. Sette says his research shows that people whose T cells have this “preexisting memory” of past coronavirus exposures also tend to respond better to vaccination for reasons not yet well understood.
“The question is, at which point will there be enough immunity from vaccination, repeated infections from other coronaviruses, but also some of the variants of the SARS-CoV-2 … where infections become less frequent? We’re not there yet,” he says.
In addition to these exciting genetic and T-cell findings, other research has suggested low-grade inflammation from allergies – a key part of the body’s immune response to foreign substances – may also give some people an extra leg up, in terms of avoiding COVID infection.
Last May, a study of 1,400 households published in The Journal of Allergy and Clinical Immunology found that having a food allergy cut the risk of COVID-19 infection in half.
The researchers said it’s unclear why allergies may reduce the risk of infection, but they noted that people with food allergies express fewer ACE2 receptors on the surface of their airway cells, making it harder for the virus to enter cells.
The big picture: Prevention still your best bet
So, what’s the takeaway from all of this emerging research?
New York University’s Dr. tenOever says that while genes, T cells and allergies may offer some protection against COVID, tried-and-true precautions – vaccination, wearing masks, avoiding crowded indoor spaces, and social distancing – are likely to provide a greater defense.
He believes these precautions are likely why he and his family have never contracted COVID-19.
“I was tested weekly, as were my kids at school,” he says. “We definitely never got COVID, despite the fact that we live in New York City and I worked in a hospital every single day of the pandemic.”
Ziyad Al-Aly, MD, an infectious disease specialist and director of clinical epidemiology at Washington University in St. Louis, agrees that the new research on COVID-19 is intriguing but won’t likely result in practical changes in the approach to fighting the virus in the near term.
“Getting a deeper understanding of potential genetic factors or other characteristics – that could really help us understand why the virus just comes and goes without any ill effects in some people, and in other people it produces really serious disease,” he says. “That will really help us eventually to design better vaccines to prevent it or reduce severity or even [treat] people who get severe disease.”
In the meantime, Dr. Al-Aly says, “it’s still best to do everything you can to avoid infection in the first place – even if you’re vaccinated or previously infected, you should really try to avoid reinfection.”
That means sit outside if you can when visiting a restaurant. Wear a mask on a plane, even though it’s not required. And get vaccinated and boosted.
“In the future, there may be more tools to address this pandemic, but that’s really the best advice for now,” Dr. Al-Aly says.
A version of this article first appeared on WebMD.com.
One in four NSCLC patients respond poorly to COVID-19 vaccine
according to a new study.
The study was published in the Journal of Clinical Oncology.
“Booster vaccination increased binding and neutralizing antibody titers to Omicron, but antibody titers declined after 3 months. These data highlight the concern for patients with cancer given the rapid spread of SARS-CoV-2 Omicron variant,” wrote the authors, who were led by Rafi Ahmed, PhD, Emory University, Atlanta.
Researchers found that 18% had no detectable antibody at all and active treatment type had no association with vaccine response.
Researchers examined antibody titers among 82 NSCLC patients and 53 healthy volunteers. They collected blood samples longitudinally for analysis. While most patients had binding and neutralizing antibody titers that were comparable with healthy volunteers, 25% had poor responses, which led to six- to sevenfold lower titers than healthy controls. There was no association between worse vaccine responses and history of programmed death–1 monotherapy, chemotherapy, or both in combination. Receipt of a booster vaccine improved binding and neutralizing antibody titers to both the wild type and the Omicron variant, but 2-4 months after the booster there was a five- to sevenfold decrease in neutralizing titers to both the wild type and Omicron variant.
“This study indicates both the need to monitor our patients with lung cancer for response to COVID-19 mRNA vaccines, identify the nonresponders for follow-up and further attempts at immunization, and continue collecting and analyzing clinicodemographic information and biospecimens from our patients,” wrote the authors of an accompanying editorial.
Although the findings reveal potential concerns, the good news is that most patients NSCLC patients do respond normally to COVID-19 vaccination, said John D. Minna, MD, University of Texas Southwestern Medical Center, Dallas, lead author of the editorial.
He offered some advice to physicians. “You can test your patients using currently available [Clinical Laboratory Improvement Amendments]–approved lab tests to determine what their antibody titers are. This should be done after boosting since titers will go down after time. We know that if a patient has lung cancer and they do get infected with SARS-CoV-2 that potentially they could develop serious COVID-19 disease. Besides giving antiviral treatment, it is important that they be closely monitored for symptoms of progression so if they need to be hospitalized it can be done in a prudent manner,” said Dr. Minna, who is director of the Hamon Center for Therapeutic Oncology Research at the University of Texas Southwestern Medical Center.
No clinical details have emerged that might predict which patients have an insufficient response to vaccination. “When we started these studies, a lot of us thought that anyone who did not develop a good antibody response would be weak or sicker. For example, [patients with] late-stage disease, or having had a lot of therapy, or perhaps immune checkpoint blockade. However, none of these things are correlated. The main advice we are giving our lung cancer patients are to get vaccinated, get boosted (double boosted), and just do the smart thing to protect yourself from exposure,” he said.
For example, when traveling on a plane, patients should wear a mask. They should also avoid large indoor events. He also recommended that, following vaccination and boosters, patients seek out CLIA-certified tests to get their titer checked.
“Upon any COVID infection, even if their titer is at or above 80%, patients should see their physician to consider treatment with Paxlovid (nirmatrelvir/ritonavir), which has emergency use authorization. For patients with a lower titer, it’s important to seek out a physician and consider Paxlovid and possibly antibody therapy. But these are individual decisions to be made with your doctor,” Dr. Minna said.
The next important research question is what happens to T-cell immune response following vaccination. “We know that a good cellular immune response is also important in preventing infection and severe infection, but we don’t yet know which persons (with or without cancer) have good T-cell responses. This information will also likely impact what we tell our patients and will add to the antibody data,” he said.
Studies are ongoing to determine specific T-cell responses to mRNA vaccines, and how well those T-cell responses respond to SARS-CoV-2 infection in the laboratory.
according to a new study.
The study was published in the Journal of Clinical Oncology.
“Booster vaccination increased binding and neutralizing antibody titers to Omicron, but antibody titers declined after 3 months. These data highlight the concern for patients with cancer given the rapid spread of SARS-CoV-2 Omicron variant,” wrote the authors, who were led by Rafi Ahmed, PhD, Emory University, Atlanta.
Researchers found that 18% had no detectable antibody at all and active treatment type had no association with vaccine response.
Researchers examined antibody titers among 82 NSCLC patients and 53 healthy volunteers. They collected blood samples longitudinally for analysis. While most patients had binding and neutralizing antibody titers that were comparable with healthy volunteers, 25% had poor responses, which led to six- to sevenfold lower titers than healthy controls. There was no association between worse vaccine responses and history of programmed death–1 monotherapy, chemotherapy, or both in combination. Receipt of a booster vaccine improved binding and neutralizing antibody titers to both the wild type and the Omicron variant, but 2-4 months after the booster there was a five- to sevenfold decrease in neutralizing titers to both the wild type and Omicron variant.
“This study indicates both the need to monitor our patients with lung cancer for response to COVID-19 mRNA vaccines, identify the nonresponders for follow-up and further attempts at immunization, and continue collecting and analyzing clinicodemographic information and biospecimens from our patients,” wrote the authors of an accompanying editorial.
Although the findings reveal potential concerns, the good news is that most patients NSCLC patients do respond normally to COVID-19 vaccination, said John D. Minna, MD, University of Texas Southwestern Medical Center, Dallas, lead author of the editorial.
He offered some advice to physicians. “You can test your patients using currently available [Clinical Laboratory Improvement Amendments]–approved lab tests to determine what their antibody titers are. This should be done after boosting since titers will go down after time. We know that if a patient has lung cancer and they do get infected with SARS-CoV-2 that potentially they could develop serious COVID-19 disease. Besides giving antiviral treatment, it is important that they be closely monitored for symptoms of progression so if they need to be hospitalized it can be done in a prudent manner,” said Dr. Minna, who is director of the Hamon Center for Therapeutic Oncology Research at the University of Texas Southwestern Medical Center.
No clinical details have emerged that might predict which patients have an insufficient response to vaccination. “When we started these studies, a lot of us thought that anyone who did not develop a good antibody response would be weak or sicker. For example, [patients with] late-stage disease, or having had a lot of therapy, or perhaps immune checkpoint blockade. However, none of these things are correlated. The main advice we are giving our lung cancer patients are to get vaccinated, get boosted (double boosted), and just do the smart thing to protect yourself from exposure,” he said.
For example, when traveling on a plane, patients should wear a mask. They should also avoid large indoor events. He also recommended that, following vaccination and boosters, patients seek out CLIA-certified tests to get their titer checked.
“Upon any COVID infection, even if their titer is at or above 80%, patients should see their physician to consider treatment with Paxlovid (nirmatrelvir/ritonavir), which has emergency use authorization. For patients with a lower titer, it’s important to seek out a physician and consider Paxlovid and possibly antibody therapy. But these are individual decisions to be made with your doctor,” Dr. Minna said.
The next important research question is what happens to T-cell immune response following vaccination. “We know that a good cellular immune response is also important in preventing infection and severe infection, but we don’t yet know which persons (with or without cancer) have good T-cell responses. This information will also likely impact what we tell our patients and will add to the antibody data,” he said.
Studies are ongoing to determine specific T-cell responses to mRNA vaccines, and how well those T-cell responses respond to SARS-CoV-2 infection in the laboratory.
according to a new study.
The study was published in the Journal of Clinical Oncology.
“Booster vaccination increased binding and neutralizing antibody titers to Omicron, but antibody titers declined after 3 months. These data highlight the concern for patients with cancer given the rapid spread of SARS-CoV-2 Omicron variant,” wrote the authors, who were led by Rafi Ahmed, PhD, Emory University, Atlanta.
Researchers found that 18% had no detectable antibody at all and active treatment type had no association with vaccine response.
Researchers examined antibody titers among 82 NSCLC patients and 53 healthy volunteers. They collected blood samples longitudinally for analysis. While most patients had binding and neutralizing antibody titers that were comparable with healthy volunteers, 25% had poor responses, which led to six- to sevenfold lower titers than healthy controls. There was no association between worse vaccine responses and history of programmed death–1 monotherapy, chemotherapy, or both in combination. Receipt of a booster vaccine improved binding and neutralizing antibody titers to both the wild type and the Omicron variant, but 2-4 months after the booster there was a five- to sevenfold decrease in neutralizing titers to both the wild type and Omicron variant.
“This study indicates both the need to monitor our patients with lung cancer for response to COVID-19 mRNA vaccines, identify the nonresponders for follow-up and further attempts at immunization, and continue collecting and analyzing clinicodemographic information and biospecimens from our patients,” wrote the authors of an accompanying editorial.
Although the findings reveal potential concerns, the good news is that most patients NSCLC patients do respond normally to COVID-19 vaccination, said John D. Minna, MD, University of Texas Southwestern Medical Center, Dallas, lead author of the editorial.
He offered some advice to physicians. “You can test your patients using currently available [Clinical Laboratory Improvement Amendments]–approved lab tests to determine what their antibody titers are. This should be done after boosting since titers will go down after time. We know that if a patient has lung cancer and they do get infected with SARS-CoV-2 that potentially they could develop serious COVID-19 disease. Besides giving antiviral treatment, it is important that they be closely monitored for symptoms of progression so if they need to be hospitalized it can be done in a prudent manner,” said Dr. Minna, who is director of the Hamon Center for Therapeutic Oncology Research at the University of Texas Southwestern Medical Center.
No clinical details have emerged that might predict which patients have an insufficient response to vaccination. “When we started these studies, a lot of us thought that anyone who did not develop a good antibody response would be weak or sicker. For example, [patients with] late-stage disease, or having had a lot of therapy, or perhaps immune checkpoint blockade. However, none of these things are correlated. The main advice we are giving our lung cancer patients are to get vaccinated, get boosted (double boosted), and just do the smart thing to protect yourself from exposure,” he said.
For example, when traveling on a plane, patients should wear a mask. They should also avoid large indoor events. He also recommended that, following vaccination and boosters, patients seek out CLIA-certified tests to get their titer checked.
“Upon any COVID infection, even if their titer is at or above 80%, patients should see their physician to consider treatment with Paxlovid (nirmatrelvir/ritonavir), which has emergency use authorization. For patients with a lower titer, it’s important to seek out a physician and consider Paxlovid and possibly antibody therapy. But these are individual decisions to be made with your doctor,” Dr. Minna said.
The next important research question is what happens to T-cell immune response following vaccination. “We know that a good cellular immune response is also important in preventing infection and severe infection, but we don’t yet know which persons (with or without cancer) have good T-cell responses. This information will also likely impact what we tell our patients and will add to the antibody data,” he said.
Studies are ongoing to determine specific T-cell responses to mRNA vaccines, and how well those T-cell responses respond to SARS-CoV-2 infection in the laboratory.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
What the Supreme Court Ruling on Abortion Means for Service Members
After the US Supreme Court overturned Roe v Wade in June, Gilbert R. Cisneros Jr., Under Secretary of Defense for Personnel and Readiness, released a memo on “Ensuring Access to Essential Women’s Health Care Services for Service Members, Dependents, Beneficiaries, and Department of Defense Civilian Employees.” In the memo, Cisneros clarified the US Department of Defense (DoD) policies and emphasized, “There will be no interruption to this care.”
Covered abortions—instances where the life of the mother would be endangered if the fetus were carried to term, or when the pregnancy is the result of rape or incest—are still covered. Health care professionals will continue to follow this policy and military medical facilities leadership will implement measures to ensure continued access to care.
The implications of the Supreme Court decision are complicated, Cisneros said. “It is the Department of Justice’s longstanding position that States generally may not impose criminal or civil liability on federal employees who perform their duties in a manner authorized by federal law,” the memo continues. “We will work with the Department of Justice to ensure access to counsel for such civilian employees and Service members if needed and as appropriate.”
The decision also does not affect the DoD’s existing leave policies, which authorize active-duty service members to travel as necessary to receive abortion care. The travel may be government-funded official travel for a covered abortion, or for all other cases, may be undertaken as regular leave at the service member’s expense. DoD civilian employees may continue to use sick leave or other forms of leave to care for themselves or their family members. Sick leave may be used to cover travel to obtain any type of medical treatment.
The Court’s decision “will have significant implications,” Cisneros wrote, adding, “As Secretary Austin has made clear, nothing is more important than the health and well-being of our Service members, the civilian workforce, and DoD families, and we are committed to taking care of all our people and ensuring that the entire Force remains ready and resilient.”
After the US Supreme Court overturned Roe v Wade in June, Gilbert R. Cisneros Jr., Under Secretary of Defense for Personnel and Readiness, released a memo on “Ensuring Access to Essential Women’s Health Care Services for Service Members, Dependents, Beneficiaries, and Department of Defense Civilian Employees.” In the memo, Cisneros clarified the US Department of Defense (DoD) policies and emphasized, “There will be no interruption to this care.”
Covered abortions—instances where the life of the mother would be endangered if the fetus were carried to term, or when the pregnancy is the result of rape or incest—are still covered. Health care professionals will continue to follow this policy and military medical facilities leadership will implement measures to ensure continued access to care.
The implications of the Supreme Court decision are complicated, Cisneros said. “It is the Department of Justice’s longstanding position that States generally may not impose criminal or civil liability on federal employees who perform their duties in a manner authorized by federal law,” the memo continues. “We will work with the Department of Justice to ensure access to counsel for such civilian employees and Service members if needed and as appropriate.”
The decision also does not affect the DoD’s existing leave policies, which authorize active-duty service members to travel as necessary to receive abortion care. The travel may be government-funded official travel for a covered abortion, or for all other cases, may be undertaken as regular leave at the service member’s expense. DoD civilian employees may continue to use sick leave or other forms of leave to care for themselves or their family members. Sick leave may be used to cover travel to obtain any type of medical treatment.
The Court’s decision “will have significant implications,” Cisneros wrote, adding, “As Secretary Austin has made clear, nothing is more important than the health and well-being of our Service members, the civilian workforce, and DoD families, and we are committed to taking care of all our people and ensuring that the entire Force remains ready and resilient.”
After the US Supreme Court overturned Roe v Wade in June, Gilbert R. Cisneros Jr., Under Secretary of Defense for Personnel and Readiness, released a memo on “Ensuring Access to Essential Women’s Health Care Services for Service Members, Dependents, Beneficiaries, and Department of Defense Civilian Employees.” In the memo, Cisneros clarified the US Department of Defense (DoD) policies and emphasized, “There will be no interruption to this care.”
Covered abortions—instances where the life of the mother would be endangered if the fetus were carried to term, or when the pregnancy is the result of rape or incest—are still covered. Health care professionals will continue to follow this policy and military medical facilities leadership will implement measures to ensure continued access to care.
The implications of the Supreme Court decision are complicated, Cisneros said. “It is the Department of Justice’s longstanding position that States generally may not impose criminal or civil liability on federal employees who perform their duties in a manner authorized by federal law,” the memo continues. “We will work with the Department of Justice to ensure access to counsel for such civilian employees and Service members if needed and as appropriate.”
The decision also does not affect the DoD’s existing leave policies, which authorize active-duty service members to travel as necessary to receive abortion care. The travel may be government-funded official travel for a covered abortion, or for all other cases, may be undertaken as regular leave at the service member’s expense. DoD civilian employees may continue to use sick leave or other forms of leave to care for themselves or their family members. Sick leave may be used to cover travel to obtain any type of medical treatment.
The Court’s decision “will have significant implications,” Cisneros wrote, adding, “As Secretary Austin has made clear, nothing is more important than the health and well-being of our Service members, the civilian workforce, and DoD families, and we are committed to taking care of all our people and ensuring that the entire Force remains ready and resilient.”
Shereef Elnahal Confirmed to Fill Long-empty VA Health Post
After a 5-year search, the US Senate in a 66-23 vote, confirmed a new US Department of Veterans Affairs (VA) Under Secretary for Health, filling a role that has been without permanent leadership since 2017. Shereef Elnahal, MD, takes over from Steve Lieberman, MD, who has been serving the role in an acting capacity since July 2021.
Elnahal’s nomination had been in limbo since May after Sen. Rick Scott (R-FL) blocked an attempt to fast-track his confirmation, which was led by Sen. John Tester (D-MT) who chairs the Senate Committee on Veterans’ Affairs. Scott, who had no specific objection to Elnahal, argued that President Joseph Biden’s nominees haven’t been qualified. The debate turned acrimonious, with Tester accusing Scott of “turning his back on America’s veterans.” He called Scott’s objection as “obstruction at the worst, because this stops our veterans from getting the health care that they need.”
Tester urged his colleagues to support Elnahal’s confirmation, stressing the importance of filling the position. “Dr. Shereef Elnahal has an impressive record of leading health care systems and agencies and has shown a strong commitment to serving millions of veterans and hardworking employees at VA. Now more than ever,” Tester said, “the Department needs permanent, qualified leadership to guide the nation’s largest integrated health care system in the right direction.”
In a statement, Rep. Mike Bost (R-IL), ranking member of the US House of Representatives Committee on Veterans’ Affairs, agreed, saying, “Dr. Elnahal’s position is a vitally important one, particularly as the VA health care system prepares to care for millions more toxic-exposed veterans under the PACT [Promise to Address Comprehensive Toxics] Act and the new electronic health record rollout continues to disappoint. Dr. Elnahal has his work cut out for him, and I look forward to working with him to ensure that veterans get the health care they have earned when they need it and where they want it, without having to wait too long or travel too far.”
Elnahal is in fact considered well qualified for the job. He was New Jersey’s 21st health commissioner, confirmed unanimously by the New Jersey Senate. During his nearly 2 years in that position, he led with what has become a signature move for him—increasing transparent access to information—by expanding the New Jersey Health Information Network, an interoperability platform that allows for electronic exchange of patient health information among health care providers.
Most recently, as president and CEO of University Hospital in Newark, New Jersey, he oversaw improvements in care quality and patient safety. He also established a partnership to provide supportive housing to patients experiencing homelessness, a hospital-based violence intervention program that has served as a national model, and a program that deploys trusted chaplains as community health workers. Notably, he led the hospital through the COVID-19 crisis; the hospital has served as a model for urban hospital and regional response efforts. Elnahal also set up one of the first COVID-19 vaccination sites in New Jersey.
Moreover, he’s not actually a newcomer to the VA. He served as Assistant Deputy Under Secretary for Health for Quality, Safety, and Value from 2016 through 2018, where he oversaw national policies around quality of care for the Veterans Health Administration (VHA).
During that earlier tenure, he was at the forefront of making VA care more transparent and responsive. Among other things, he cofounded the VHA Innovation Ecosystem, a program that fosters the spread of innovation and best practices. On his watch the VA also launched accesstocare.gov, which provides public access to performance, wait time, and other data. The rationale, Elnahal said in a 2018 interview with Federal Practitioner, was simple: “If we provide veterans with an easy-to-use tool that lets them see data on wait times and quality, they’ll be able to make informed decisions about where and when they receive their health care.” The site allows users to compare quality of care provided by VA medical centers with that of local private hospitals. For instance, they can see if a local VA facility’s wait time is better, worse, or the same as the regional average of private sector clinics.
In his drive to harness smart, sustainable ideas for improving veteran care, Elnahal also helmed the VA Diffusion of Excellence (VADOE) program, whose Shark Tank Competition gives a platform to employees “passionate about solving some of the toughest challenges across VHA.” The innovative winners have included VIONE, a medication deprescribing program, and the β-Lactam Allergy Assessment, an initiative to clarify which patients are truly allergic to BL antibiotics, reduce the incidence of multidrug-resistant infections, and reduce hospital length of stay. Both programs are being replicated across multiple facilities.
“We really empower and recognize the frontline employees who not only contribute the best practices but who replicate them,” Elnahal told Federal Practitioner in 2016. “Essentially, we give them a systemwide leadership role… This is part of many different initiatives that are trying to recognize and elevate the great work that physicians do and really improve their morale and reduce burnout.”
As Rep. Bost suggested, Elnahal now has even more work cut out for him. At this new starting gate, Elnahal says a top priority is improving recruiting and retention for clinical care positions. “The sacred healthcare mission of VA simply cannot be fulfilled without having people to do it, talented healthcare professionals who put the mission above all else.”
In a LinkedIn post, Elnahal thanked President Biden and VA Secretary McDonough for their confidence in him, and the US Senate for confirming him in a bipartisan vote. But “[m]ost of all,” he said, “my gratitude goes to Veterans, families, caregivers, and survivors…. Beyond thrilled and eager to get to work for them in a health system with more than 300,000 heroes. Onward to an incredible journey!”
After a 5-year search, the US Senate in a 66-23 vote, confirmed a new US Department of Veterans Affairs (VA) Under Secretary for Health, filling a role that has been without permanent leadership since 2017. Shereef Elnahal, MD, takes over from Steve Lieberman, MD, who has been serving the role in an acting capacity since July 2021.
Elnahal’s nomination had been in limbo since May after Sen. Rick Scott (R-FL) blocked an attempt to fast-track his confirmation, which was led by Sen. John Tester (D-MT) who chairs the Senate Committee on Veterans’ Affairs. Scott, who had no specific objection to Elnahal, argued that President Joseph Biden’s nominees haven’t been qualified. The debate turned acrimonious, with Tester accusing Scott of “turning his back on America’s veterans.” He called Scott’s objection as “obstruction at the worst, because this stops our veterans from getting the health care that they need.”
Tester urged his colleagues to support Elnahal’s confirmation, stressing the importance of filling the position. “Dr. Shereef Elnahal has an impressive record of leading health care systems and agencies and has shown a strong commitment to serving millions of veterans and hardworking employees at VA. Now more than ever,” Tester said, “the Department needs permanent, qualified leadership to guide the nation’s largest integrated health care system in the right direction.”
In a statement, Rep. Mike Bost (R-IL), ranking member of the US House of Representatives Committee on Veterans’ Affairs, agreed, saying, “Dr. Elnahal’s position is a vitally important one, particularly as the VA health care system prepares to care for millions more toxic-exposed veterans under the PACT [Promise to Address Comprehensive Toxics] Act and the new electronic health record rollout continues to disappoint. Dr. Elnahal has his work cut out for him, and I look forward to working with him to ensure that veterans get the health care they have earned when they need it and where they want it, without having to wait too long or travel too far.”
Elnahal is in fact considered well qualified for the job. He was New Jersey’s 21st health commissioner, confirmed unanimously by the New Jersey Senate. During his nearly 2 years in that position, he led with what has become a signature move for him—increasing transparent access to information—by expanding the New Jersey Health Information Network, an interoperability platform that allows for electronic exchange of patient health information among health care providers.
Most recently, as president and CEO of University Hospital in Newark, New Jersey, he oversaw improvements in care quality and patient safety. He also established a partnership to provide supportive housing to patients experiencing homelessness, a hospital-based violence intervention program that has served as a national model, and a program that deploys trusted chaplains as community health workers. Notably, he led the hospital through the COVID-19 crisis; the hospital has served as a model for urban hospital and regional response efforts. Elnahal also set up one of the first COVID-19 vaccination sites in New Jersey.
Moreover, he’s not actually a newcomer to the VA. He served as Assistant Deputy Under Secretary for Health for Quality, Safety, and Value from 2016 through 2018, where he oversaw national policies around quality of care for the Veterans Health Administration (VHA).
During that earlier tenure, he was at the forefront of making VA care more transparent and responsive. Among other things, he cofounded the VHA Innovation Ecosystem, a program that fosters the spread of innovation and best practices. On his watch the VA also launched accesstocare.gov, which provides public access to performance, wait time, and other data. The rationale, Elnahal said in a 2018 interview with Federal Practitioner, was simple: “If we provide veterans with an easy-to-use tool that lets them see data on wait times and quality, they’ll be able to make informed decisions about where and when they receive their health care.” The site allows users to compare quality of care provided by VA medical centers with that of local private hospitals. For instance, they can see if a local VA facility’s wait time is better, worse, or the same as the regional average of private sector clinics.
In his drive to harness smart, sustainable ideas for improving veteran care, Elnahal also helmed the VA Diffusion of Excellence (VADOE) program, whose Shark Tank Competition gives a platform to employees “passionate about solving some of the toughest challenges across VHA.” The innovative winners have included VIONE, a medication deprescribing program, and the β-Lactam Allergy Assessment, an initiative to clarify which patients are truly allergic to BL antibiotics, reduce the incidence of multidrug-resistant infections, and reduce hospital length of stay. Both programs are being replicated across multiple facilities.
“We really empower and recognize the frontline employees who not only contribute the best practices but who replicate them,” Elnahal told Federal Practitioner in 2016. “Essentially, we give them a systemwide leadership role… This is part of many different initiatives that are trying to recognize and elevate the great work that physicians do and really improve their morale and reduce burnout.”
As Rep. Bost suggested, Elnahal now has even more work cut out for him. At this new starting gate, Elnahal says a top priority is improving recruiting and retention for clinical care positions. “The sacred healthcare mission of VA simply cannot be fulfilled without having people to do it, talented healthcare professionals who put the mission above all else.”
In a LinkedIn post, Elnahal thanked President Biden and VA Secretary McDonough for their confidence in him, and the US Senate for confirming him in a bipartisan vote. But “[m]ost of all,” he said, “my gratitude goes to Veterans, families, caregivers, and survivors…. Beyond thrilled and eager to get to work for them in a health system with more than 300,000 heroes. Onward to an incredible journey!”
After a 5-year search, the US Senate in a 66-23 vote, confirmed a new US Department of Veterans Affairs (VA) Under Secretary for Health, filling a role that has been without permanent leadership since 2017. Shereef Elnahal, MD, takes over from Steve Lieberman, MD, who has been serving the role in an acting capacity since July 2021.
Elnahal’s nomination had been in limbo since May after Sen. Rick Scott (R-FL) blocked an attempt to fast-track his confirmation, which was led by Sen. John Tester (D-MT) who chairs the Senate Committee on Veterans’ Affairs. Scott, who had no specific objection to Elnahal, argued that President Joseph Biden’s nominees haven’t been qualified. The debate turned acrimonious, with Tester accusing Scott of “turning his back on America’s veterans.” He called Scott’s objection as “obstruction at the worst, because this stops our veterans from getting the health care that they need.”
Tester urged his colleagues to support Elnahal’s confirmation, stressing the importance of filling the position. “Dr. Shereef Elnahal has an impressive record of leading health care systems and agencies and has shown a strong commitment to serving millions of veterans and hardworking employees at VA. Now more than ever,” Tester said, “the Department needs permanent, qualified leadership to guide the nation’s largest integrated health care system in the right direction.”
In a statement, Rep. Mike Bost (R-IL), ranking member of the US House of Representatives Committee on Veterans’ Affairs, agreed, saying, “Dr. Elnahal’s position is a vitally important one, particularly as the VA health care system prepares to care for millions more toxic-exposed veterans under the PACT [Promise to Address Comprehensive Toxics] Act and the new electronic health record rollout continues to disappoint. Dr. Elnahal has his work cut out for him, and I look forward to working with him to ensure that veterans get the health care they have earned when they need it and where they want it, without having to wait too long or travel too far.”
Elnahal is in fact considered well qualified for the job. He was New Jersey’s 21st health commissioner, confirmed unanimously by the New Jersey Senate. During his nearly 2 years in that position, he led with what has become a signature move for him—increasing transparent access to information—by expanding the New Jersey Health Information Network, an interoperability platform that allows for electronic exchange of patient health information among health care providers.
Most recently, as president and CEO of University Hospital in Newark, New Jersey, he oversaw improvements in care quality and patient safety. He also established a partnership to provide supportive housing to patients experiencing homelessness, a hospital-based violence intervention program that has served as a national model, and a program that deploys trusted chaplains as community health workers. Notably, he led the hospital through the COVID-19 crisis; the hospital has served as a model for urban hospital and regional response efforts. Elnahal also set up one of the first COVID-19 vaccination sites in New Jersey.
Moreover, he’s not actually a newcomer to the VA. He served as Assistant Deputy Under Secretary for Health for Quality, Safety, and Value from 2016 through 2018, where he oversaw national policies around quality of care for the Veterans Health Administration (VHA).
During that earlier tenure, he was at the forefront of making VA care more transparent and responsive. Among other things, he cofounded the VHA Innovation Ecosystem, a program that fosters the spread of innovation and best practices. On his watch the VA also launched accesstocare.gov, which provides public access to performance, wait time, and other data. The rationale, Elnahal said in a 2018 interview with Federal Practitioner, was simple: “If we provide veterans with an easy-to-use tool that lets them see data on wait times and quality, they’ll be able to make informed decisions about where and when they receive their health care.” The site allows users to compare quality of care provided by VA medical centers with that of local private hospitals. For instance, they can see if a local VA facility’s wait time is better, worse, or the same as the regional average of private sector clinics.
In his drive to harness smart, sustainable ideas for improving veteran care, Elnahal also helmed the VA Diffusion of Excellence (VADOE) program, whose Shark Tank Competition gives a platform to employees “passionate about solving some of the toughest challenges across VHA.” The innovative winners have included VIONE, a medication deprescribing program, and the β-Lactam Allergy Assessment, an initiative to clarify which patients are truly allergic to BL antibiotics, reduce the incidence of multidrug-resistant infections, and reduce hospital length of stay. Both programs are being replicated across multiple facilities.
“We really empower and recognize the frontline employees who not only contribute the best practices but who replicate them,” Elnahal told Federal Practitioner in 2016. “Essentially, we give them a systemwide leadership role… This is part of many different initiatives that are trying to recognize and elevate the great work that physicians do and really improve their morale and reduce burnout.”
As Rep. Bost suggested, Elnahal now has even more work cut out for him. At this new starting gate, Elnahal says a top priority is improving recruiting and retention for clinical care positions. “The sacred healthcare mission of VA simply cannot be fulfilled without having people to do it, talented healthcare professionals who put the mission above all else.”
In a LinkedIn post, Elnahal thanked President Biden and VA Secretary McDonough for their confidence in him, and the US Senate for confirming him in a bipartisan vote. But “[m]ost of all,” he said, “my gratitude goes to Veterans, families, caregivers, and survivors…. Beyond thrilled and eager to get to work for them in a health system with more than 300,000 heroes. Onward to an incredible journey!”