User login
B6 a new approach for depression, anxiety?
Investigators compared supplementation with a 1-month course of vitamin B6 or B12 to supplementation with placebo in almost 500 adults. Results showed that vitamin B6 supplementation was associated with reductions in self-reported anxiety and a trend toward decreased depressive symptoms.
In addition, the vitamin B6 group showed increased levels of gamma-aminobutyric acid (GABA), as indicated by results on a visual test that was administered at the end of the trial. The test results demonstrated subtle changes in participants’ visual performance. The researchers considered this to be consistent with controlled levels of GABA-related brain activity.
However, “before practicing clinicians would recommend taking high doses of vitamin B6, a full-scale clinical trial would have to be carried out to verify the findings, assess any side effects, and find out which types of patients do or don’t benefit,” study investigator David Field, PhD, associate professor, School of Psychological and Clinical Language Sciences, University of Reading (England), told this news organization.
“My relatively small study can only be considered as an initial proof of concept,” Dr. Field said.
The findings were published online in the Journal of Human Psychopharmacology: Clinical and Experimental.
Eat Marmite?
“Recent research has connected mood disorders and some other neuropsychiatric conditions with disturbance in this balance, often in the direction of raised levels of brain activity,” Dr. Field noted.
Vitamin B6 is a coenzyme in the synthesis of GABA, an inhibitory neurotransmitter, from glutamate. Some previous research has suggested that vitamins B6 and B12 have a role in improving mood-related outcomes.
Dr. Field had reviewed a 2017 study of the effects on visual processing of eating Marmite, a type of food spread rich in vitamin B, every day for a few weeks.
“Remarkably, the results of that study suggested that eating Marmite had increased the level of the inhibitory neurotransmitter GABA in the visual part of the brain, damping down the level of neural activity slightly,” he said.
However, Marmite contains other B vitamins and other ingredients that might potentially account for this result, “plus, a lot of people don’t like the taste of Marmite,” Dr. Field noted.
Therefore, he wanted to “find out which individual ingredients were driving the effect, and B6 and B12 were the most plausible candidates.”
He decided to test these vitamins individually and to compare them to placebo. “I added the measures of anxiety and depression that were not in the Marmite study because I reasoned that if GABA levels were altered, this could improve those disorders, because we know that decreased levels of GABA in the brain occur in both of those conditions,” Dr. Field added.
Over the course of 5 years, investigators recruited 478 participants aged 18-58 years (mean age, 23 years; 381 women). Of these, 265 reported having anxiety, and 146 reported having depression.
The study participants were randomly assigned to receive either vitamin B6 (100 mg pyroxidine hydrochloride), vitamin B12 (1,000 mg methylcobalmin), or placebo tablets once daily for a month.
They also completed the Screen for Adult Anxiety Related Disorders (SCAARED) and the Mood and Feelings Questionnaire (MFQ) long version at baseline and following supplementation (“post test”), and they underwent three sensory tests that acted as assays of inhibitory function at post test.
In addition, 307 participants completed the Visual Contrast Sensitivity and Surround Suppression, which “measures the minimum percentage contrast between the lighter and darker regions of a striped pattern that can be detected (called the contrast threshold),” the investigators note.
The contrast threshold was measured with and without a suppressive surround mask that increases the threshold – an effect mediated by GABAergic connections in the visual cortex.
Participants (n = 172) also completed the Binocular Rivalry test and the Tactile Test Battery (n = 180). Both tests are designed to measure responses requiring GABAergic inhibitory activity.
‘Subtle changes’
ANOVA analyses revealed a “highly significant” reduction in anxiety at post test (F[1,173] = 10.03; P = .002; np 2 = .055), driven primarily by reduced anxiety in the B6 group (t[88] = 3.51; P < .001; d = .37). The placebo group also showed some reduction in anxiety, but it was not deemed significant, and the overall interaction itself did not reach significance.
A comparison of the B12 group with the group that received placebo revealed a significant reduction in anxiety at post test (F[1,175] = 4.08; P = .045; np 2 = .023), similarly driven by reduced anxiety in the B12 group (t[89] = 1.84; P = .069; d = .19) – but the interaction was not significant.
Among the B6 group, there was a highly significant reduction in scores on the generalized anxiety disorder and social anxiety subscales of the SCAARED, and there was a trend toward reductions on the other subscales. Among the B12 group, there was a significant reduction only on scores on the separation anxiety subscale. No significant changes were found in the placebo group.
The ANOVA test analysis of the B6 and placebo group data showed “no uniform direction of change” in depression at post test. The researchers found a “tendency” for depression scores to decrease between baseline and post test in the B6 group but to increase in the placebo group – an interaction that “approached” significance (F[1,96] = 3.08; P = .083; np 2 = .031), they report.
The ANOVA analysis of the B12 and placebo group data revealed no significant or trending effects, and the t-test comparing baseline and post-test scores in the B12 group was similarly nonsignificant.
B6 supplementation did change visual contrast thresholds, but only when a suppressive surround was present. There were “no clear effects” of B6 supplementation on other outcome measures, including binocular rivalry reversal rate and the tactile test battery, the investigators note.
“We found that supplementation with B6 produced subtle changes in tests of visual processing in a way that suggested an increase in the level of the inhibitory neurotransmitter GABA,” Dr. Field reported.
Vitamin B6 is a “cofactor for a metabolic pathway in the brain that converts the excitatory neurotransmitter glutamate into the inhibitory/calming GABA,” he said.
“By increasing the quantity of the cofactor, we slightly speed up the rate of this metabolic process, and so you end up with a bit more of the GABA neurotransmitter and a bit less glutamate. The net effect of this is to slightly reduce the amount of activity in the brain,” Dr. Field added.
Most common nutrient deficiency
Carol Johnston, PhD, professor and associate dean for faculty success, College of Health Solutions, Arizona State University, Phoenix, said vitamin B6 is “the most common nutrient deficiency in the United States;” 16% of men and 32% of women are reportedly B6 deficient.
“Young women on birth control are at higher risk for B6 deficiency due to effects of oral contraceptives on B6 metabolism,” whereas vitamin B12 deficiency is more common in older adults, said Dr. Johnston, who was not involved with the study.
The current study’s population mainly consisted of young women, and the interpretation of the data is “limited” because the researchers did not measure blood status for B6 and B12, Dr. Johnston noted. It is possible the sample was low in B6 and that the supplements “improved cognitive measures.”
Because the population was young – no one was older than 60 years – B12 status was likely “adequate in the sample, and supplementation did not have an impact,” she said.
Overall, Dr. Johnston cautioned that it is important to “alert clinicians and the general public about the concerns of overdosing B6.” For example, supplementation at high amounts can cause potentially irreversible sensory neuropathy, she noted.
“The safe upper limit defined by experts is 100 mg per day – the dosage used in this trial. Daily supplementation should not exceed this level,” Dr. Johnston said.
The vitamin tablets used in the study were supplied by Innopure. The investigators and Dr. Johnston have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared supplementation with a 1-month course of vitamin B6 or B12 to supplementation with placebo in almost 500 adults. Results showed that vitamin B6 supplementation was associated with reductions in self-reported anxiety and a trend toward decreased depressive symptoms.
In addition, the vitamin B6 group showed increased levels of gamma-aminobutyric acid (GABA), as indicated by results on a visual test that was administered at the end of the trial. The test results demonstrated subtle changes in participants’ visual performance. The researchers considered this to be consistent with controlled levels of GABA-related brain activity.
However, “before practicing clinicians would recommend taking high doses of vitamin B6, a full-scale clinical trial would have to be carried out to verify the findings, assess any side effects, and find out which types of patients do or don’t benefit,” study investigator David Field, PhD, associate professor, School of Psychological and Clinical Language Sciences, University of Reading (England), told this news organization.
“My relatively small study can only be considered as an initial proof of concept,” Dr. Field said.
The findings were published online in the Journal of Human Psychopharmacology: Clinical and Experimental.
Eat Marmite?
“Recent research has connected mood disorders and some other neuropsychiatric conditions with disturbance in this balance, often in the direction of raised levels of brain activity,” Dr. Field noted.
Vitamin B6 is a coenzyme in the synthesis of GABA, an inhibitory neurotransmitter, from glutamate. Some previous research has suggested that vitamins B6 and B12 have a role in improving mood-related outcomes.
Dr. Field had reviewed a 2017 study of the effects on visual processing of eating Marmite, a type of food spread rich in vitamin B, every day for a few weeks.
“Remarkably, the results of that study suggested that eating Marmite had increased the level of the inhibitory neurotransmitter GABA in the visual part of the brain, damping down the level of neural activity slightly,” he said.
However, Marmite contains other B vitamins and other ingredients that might potentially account for this result, “plus, a lot of people don’t like the taste of Marmite,” Dr. Field noted.
Therefore, he wanted to “find out which individual ingredients were driving the effect, and B6 and B12 were the most plausible candidates.”
He decided to test these vitamins individually and to compare them to placebo. “I added the measures of anxiety and depression that were not in the Marmite study because I reasoned that if GABA levels were altered, this could improve those disorders, because we know that decreased levels of GABA in the brain occur in both of those conditions,” Dr. Field added.
Over the course of 5 years, investigators recruited 478 participants aged 18-58 years (mean age, 23 years; 381 women). Of these, 265 reported having anxiety, and 146 reported having depression.
The study participants were randomly assigned to receive either vitamin B6 (100 mg pyroxidine hydrochloride), vitamin B12 (1,000 mg methylcobalmin), or placebo tablets once daily for a month.
They also completed the Screen for Adult Anxiety Related Disorders (SCAARED) and the Mood and Feelings Questionnaire (MFQ) long version at baseline and following supplementation (“post test”), and they underwent three sensory tests that acted as assays of inhibitory function at post test.
In addition, 307 participants completed the Visual Contrast Sensitivity and Surround Suppression, which “measures the minimum percentage contrast between the lighter and darker regions of a striped pattern that can be detected (called the contrast threshold),” the investigators note.
The contrast threshold was measured with and without a suppressive surround mask that increases the threshold – an effect mediated by GABAergic connections in the visual cortex.
Participants (n = 172) also completed the Binocular Rivalry test and the Tactile Test Battery (n = 180). Both tests are designed to measure responses requiring GABAergic inhibitory activity.
‘Subtle changes’
ANOVA analyses revealed a “highly significant” reduction in anxiety at post test (F[1,173] = 10.03; P = .002; np 2 = .055), driven primarily by reduced anxiety in the B6 group (t[88] = 3.51; P < .001; d = .37). The placebo group also showed some reduction in anxiety, but it was not deemed significant, and the overall interaction itself did not reach significance.
A comparison of the B12 group with the group that received placebo revealed a significant reduction in anxiety at post test (F[1,175] = 4.08; P = .045; np 2 = .023), similarly driven by reduced anxiety in the B12 group (t[89] = 1.84; P = .069; d = .19) – but the interaction was not significant.
Among the B6 group, there was a highly significant reduction in scores on the generalized anxiety disorder and social anxiety subscales of the SCAARED, and there was a trend toward reductions on the other subscales. Among the B12 group, there was a significant reduction only on scores on the separation anxiety subscale. No significant changes were found in the placebo group.
The ANOVA test analysis of the B6 and placebo group data showed “no uniform direction of change” in depression at post test. The researchers found a “tendency” for depression scores to decrease between baseline and post test in the B6 group but to increase in the placebo group – an interaction that “approached” significance (F[1,96] = 3.08; P = .083; np 2 = .031), they report.
The ANOVA analysis of the B12 and placebo group data revealed no significant or trending effects, and the t-test comparing baseline and post-test scores in the B12 group was similarly nonsignificant.
B6 supplementation did change visual contrast thresholds, but only when a suppressive surround was present. There were “no clear effects” of B6 supplementation on other outcome measures, including binocular rivalry reversal rate and the tactile test battery, the investigators note.
“We found that supplementation with B6 produced subtle changes in tests of visual processing in a way that suggested an increase in the level of the inhibitory neurotransmitter GABA,” Dr. Field reported.
Vitamin B6 is a “cofactor for a metabolic pathway in the brain that converts the excitatory neurotransmitter glutamate into the inhibitory/calming GABA,” he said.
“By increasing the quantity of the cofactor, we slightly speed up the rate of this metabolic process, and so you end up with a bit more of the GABA neurotransmitter and a bit less glutamate. The net effect of this is to slightly reduce the amount of activity in the brain,” Dr. Field added.
Most common nutrient deficiency
Carol Johnston, PhD, professor and associate dean for faculty success, College of Health Solutions, Arizona State University, Phoenix, said vitamin B6 is “the most common nutrient deficiency in the United States;” 16% of men and 32% of women are reportedly B6 deficient.
“Young women on birth control are at higher risk for B6 deficiency due to effects of oral contraceptives on B6 metabolism,” whereas vitamin B12 deficiency is more common in older adults, said Dr. Johnston, who was not involved with the study.
The current study’s population mainly consisted of young women, and the interpretation of the data is “limited” because the researchers did not measure blood status for B6 and B12, Dr. Johnston noted. It is possible the sample was low in B6 and that the supplements “improved cognitive measures.”
Because the population was young – no one was older than 60 years – B12 status was likely “adequate in the sample, and supplementation did not have an impact,” she said.
Overall, Dr. Johnston cautioned that it is important to “alert clinicians and the general public about the concerns of overdosing B6.” For example, supplementation at high amounts can cause potentially irreversible sensory neuropathy, she noted.
“The safe upper limit defined by experts is 100 mg per day – the dosage used in this trial. Daily supplementation should not exceed this level,” Dr. Johnston said.
The vitamin tablets used in the study were supplied by Innopure. The investigators and Dr. Johnston have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared supplementation with a 1-month course of vitamin B6 or B12 to supplementation with placebo in almost 500 adults. Results showed that vitamin B6 supplementation was associated with reductions in self-reported anxiety and a trend toward decreased depressive symptoms.
In addition, the vitamin B6 group showed increased levels of gamma-aminobutyric acid (GABA), as indicated by results on a visual test that was administered at the end of the trial. The test results demonstrated subtle changes in participants’ visual performance. The researchers considered this to be consistent with controlled levels of GABA-related brain activity.
However, “before practicing clinicians would recommend taking high doses of vitamin B6, a full-scale clinical trial would have to be carried out to verify the findings, assess any side effects, and find out which types of patients do or don’t benefit,” study investigator David Field, PhD, associate professor, School of Psychological and Clinical Language Sciences, University of Reading (England), told this news organization.
“My relatively small study can only be considered as an initial proof of concept,” Dr. Field said.
The findings were published online in the Journal of Human Psychopharmacology: Clinical and Experimental.
Eat Marmite?
“Recent research has connected mood disorders and some other neuropsychiatric conditions with disturbance in this balance, often in the direction of raised levels of brain activity,” Dr. Field noted.
Vitamin B6 is a coenzyme in the synthesis of GABA, an inhibitory neurotransmitter, from glutamate. Some previous research has suggested that vitamins B6 and B12 have a role in improving mood-related outcomes.
Dr. Field had reviewed a 2017 study of the effects on visual processing of eating Marmite, a type of food spread rich in vitamin B, every day for a few weeks.
“Remarkably, the results of that study suggested that eating Marmite had increased the level of the inhibitory neurotransmitter GABA in the visual part of the brain, damping down the level of neural activity slightly,” he said.
However, Marmite contains other B vitamins and other ingredients that might potentially account for this result, “plus, a lot of people don’t like the taste of Marmite,” Dr. Field noted.
Therefore, he wanted to “find out which individual ingredients were driving the effect, and B6 and B12 were the most plausible candidates.”
He decided to test these vitamins individually and to compare them to placebo. “I added the measures of anxiety and depression that were not in the Marmite study because I reasoned that if GABA levels were altered, this could improve those disorders, because we know that decreased levels of GABA in the brain occur in both of those conditions,” Dr. Field added.
Over the course of 5 years, investigators recruited 478 participants aged 18-58 years (mean age, 23 years; 381 women). Of these, 265 reported having anxiety, and 146 reported having depression.
The study participants were randomly assigned to receive either vitamin B6 (100 mg pyroxidine hydrochloride), vitamin B12 (1,000 mg methylcobalmin), or placebo tablets once daily for a month.
They also completed the Screen for Adult Anxiety Related Disorders (SCAARED) and the Mood and Feelings Questionnaire (MFQ) long version at baseline and following supplementation (“post test”), and they underwent three sensory tests that acted as assays of inhibitory function at post test.
In addition, 307 participants completed the Visual Contrast Sensitivity and Surround Suppression, which “measures the minimum percentage contrast between the lighter and darker regions of a striped pattern that can be detected (called the contrast threshold),” the investigators note.
The contrast threshold was measured with and without a suppressive surround mask that increases the threshold – an effect mediated by GABAergic connections in the visual cortex.
Participants (n = 172) also completed the Binocular Rivalry test and the Tactile Test Battery (n = 180). Both tests are designed to measure responses requiring GABAergic inhibitory activity.
‘Subtle changes’
ANOVA analyses revealed a “highly significant” reduction in anxiety at post test (F[1,173] = 10.03; P = .002; np 2 = .055), driven primarily by reduced anxiety in the B6 group (t[88] = 3.51; P < .001; d = .37). The placebo group also showed some reduction in anxiety, but it was not deemed significant, and the overall interaction itself did not reach significance.
A comparison of the B12 group with the group that received placebo revealed a significant reduction in anxiety at post test (F[1,175] = 4.08; P = .045; np 2 = .023), similarly driven by reduced anxiety in the B12 group (t[89] = 1.84; P = .069; d = .19) – but the interaction was not significant.
Among the B6 group, there was a highly significant reduction in scores on the generalized anxiety disorder and social anxiety subscales of the SCAARED, and there was a trend toward reductions on the other subscales. Among the B12 group, there was a significant reduction only on scores on the separation anxiety subscale. No significant changes were found in the placebo group.
The ANOVA test analysis of the B6 and placebo group data showed “no uniform direction of change” in depression at post test. The researchers found a “tendency” for depression scores to decrease between baseline and post test in the B6 group but to increase in the placebo group – an interaction that “approached” significance (F[1,96] = 3.08; P = .083; np 2 = .031), they report.
The ANOVA analysis of the B12 and placebo group data revealed no significant or trending effects, and the t-test comparing baseline and post-test scores in the B12 group was similarly nonsignificant.
B6 supplementation did change visual contrast thresholds, but only when a suppressive surround was present. There were “no clear effects” of B6 supplementation on other outcome measures, including binocular rivalry reversal rate and the tactile test battery, the investigators note.
“We found that supplementation with B6 produced subtle changes in tests of visual processing in a way that suggested an increase in the level of the inhibitory neurotransmitter GABA,” Dr. Field reported.
Vitamin B6 is a “cofactor for a metabolic pathway in the brain that converts the excitatory neurotransmitter glutamate into the inhibitory/calming GABA,” he said.
“By increasing the quantity of the cofactor, we slightly speed up the rate of this metabolic process, and so you end up with a bit more of the GABA neurotransmitter and a bit less glutamate. The net effect of this is to slightly reduce the amount of activity in the brain,” Dr. Field added.
Most common nutrient deficiency
Carol Johnston, PhD, professor and associate dean for faculty success, College of Health Solutions, Arizona State University, Phoenix, said vitamin B6 is “the most common nutrient deficiency in the United States;” 16% of men and 32% of women are reportedly B6 deficient.
“Young women on birth control are at higher risk for B6 deficiency due to effects of oral contraceptives on B6 metabolism,” whereas vitamin B12 deficiency is more common in older adults, said Dr. Johnston, who was not involved with the study.
The current study’s population mainly consisted of young women, and the interpretation of the data is “limited” because the researchers did not measure blood status for B6 and B12, Dr. Johnston noted. It is possible the sample was low in B6 and that the supplements “improved cognitive measures.”
Because the population was young – no one was older than 60 years – B12 status was likely “adequate in the sample, and supplementation did not have an impact,” she said.
Overall, Dr. Johnston cautioned that it is important to “alert clinicians and the general public about the concerns of overdosing B6.” For example, supplementation at high amounts can cause potentially irreversible sensory neuropathy, she noted.
“The safe upper limit defined by experts is 100 mg per day – the dosage used in this trial. Daily supplementation should not exceed this level,” Dr. Johnston said.
The vitamin tablets used in the study were supplied by Innopure. The investigators and Dr. Johnston have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Node-negative triple-negative breast cancer prognosis lies within stromal lymphocytes
and may be suitable candidates for reduced intensity pre- or postoperative chemotherapy, according to a team of European investigators.
Among 441 women in a Dutch cancer registry who were younger than 40 when they were diagnosed with node-negative TNBC and had not undergone systemic therapy, those who had 75% or more TILs in the intratumoral stromal area had a 15-year cumulative incidence of distant metastases or death of just 2.1%, and every 10% increase in sTILs was associated with a 19% decrease in the risk of death.
In contrast, the 15-year cumulative incidence of distant metastases was 38.4% for women with stromal TIL scores of less than 30%, according to researchers writing in the Journal of Clinical Oncology.
“These data could be used as a starting point for designing a randomized controlled chemotherapy de-escalation trial. The current study confirms the importance of sTILs as a valuable addition to the set of standard prognostic factors in patients with TNBC,” wrote the researchers, who were led by Sabine C. Linn, MD, of the Netherlands Cancer Institute, Amsterdam.
Markers for immune response
Stromal TILs, a mixture of mononuclear immune cells, have been shown in previous studies to be prognostic for outcomes in patients with early-stage TNBC treated either with or without neoadjuvant or adjuvant chemotherapy.
For example, investigators cited a study published in JCO in 2014, that showed among women with TNBC enrolled in the phase 3 ECOG 2197 clinical trial and the related ECOG 119 clinical trial, after a nearly 11-year follow-up, higher sTIL scores were associated with significantly better prognosis with every 10% increase translating into a 14% reduction in the risk of recurrence or death (P = .02).
“The prognostic importance of sTILs is, however, unexplored in patients diagnosed under age 40 years, let alone in the subgroup of systemic therapy–naive patients,” Dr. Linn and colleagues wrote.
Retrospective study
To see whether the prognostic value of sTILs was as strong among young, systemic therapy–naive women, the investigators conducted a retrospective study of women enrolled in the Netherlands Cancer Registry who were diagnosed with node-negative TNBC from 1989 to 2000. The patients selected had undergone only locoregional treatment, including axillary node dissection, but had not received any systemic therapy.
Pathologists reviewed samples, with TILs reported for the stromal compartment. The samples were grouped by sTIL score categories of high (75% or greater), intermediate (30% to less than 75%), or low (less than 30%). The investigators looked at overall survival (OS) and distant metastasis-free survival (DMFS) stratified by sTIL scores,
During a median follow-up of 15 years, 107 women died or developed distant metastases, and 78 experienced a second primary cancer.
The results were as noted, with patients in the highest category of sTILs having very low rates of either death or distant metastases during follow-up.
“We confirm the prognostic value of sTILs in young patients with early-stage N0 TNBC who are systemic therapy naive by taking advantage of a prospectively collected population-based cohort. Increasing sTILs are significantly associated with improved OS and DMFS. Patients with high sTILs (> 75%) had an excellent 10-year overall survival and a very low 10-year incidence of distant metastasis or death.
The study was supported by grants from The Netherlands Organization for Health Research and Development, A Sister’s Hope, De Vrienden van UMC Utrecht, Agilent Technologies, the Dutch Cancer Society, and Breast Cancer Research Foundation. Dr. Linn reported consulting with and receiving compensation from Daiichi Sankyo, as well as receiving research funding from Genentech/Roche, AstraZeneca, Bristol-Myers Squibb, Tesaro, Merck, Immunomedics, Eurocept Pharmaceuticals, Agendia, and Novartis.
and may be suitable candidates for reduced intensity pre- or postoperative chemotherapy, according to a team of European investigators.
Among 441 women in a Dutch cancer registry who were younger than 40 when they were diagnosed with node-negative TNBC and had not undergone systemic therapy, those who had 75% or more TILs in the intratumoral stromal area had a 15-year cumulative incidence of distant metastases or death of just 2.1%, and every 10% increase in sTILs was associated with a 19% decrease in the risk of death.
In contrast, the 15-year cumulative incidence of distant metastases was 38.4% for women with stromal TIL scores of less than 30%, according to researchers writing in the Journal of Clinical Oncology.
“These data could be used as a starting point for designing a randomized controlled chemotherapy de-escalation trial. The current study confirms the importance of sTILs as a valuable addition to the set of standard prognostic factors in patients with TNBC,” wrote the researchers, who were led by Sabine C. Linn, MD, of the Netherlands Cancer Institute, Amsterdam.
Markers for immune response
Stromal TILs, a mixture of mononuclear immune cells, have been shown in previous studies to be prognostic for outcomes in patients with early-stage TNBC treated either with or without neoadjuvant or adjuvant chemotherapy.
For example, investigators cited a study published in JCO in 2014, that showed among women with TNBC enrolled in the phase 3 ECOG 2197 clinical trial and the related ECOG 119 clinical trial, after a nearly 11-year follow-up, higher sTIL scores were associated with significantly better prognosis with every 10% increase translating into a 14% reduction in the risk of recurrence or death (P = .02).
“The prognostic importance of sTILs is, however, unexplored in patients diagnosed under age 40 years, let alone in the subgroup of systemic therapy–naive patients,” Dr. Linn and colleagues wrote.
Retrospective study
To see whether the prognostic value of sTILs was as strong among young, systemic therapy–naive women, the investigators conducted a retrospective study of women enrolled in the Netherlands Cancer Registry who were diagnosed with node-negative TNBC from 1989 to 2000. The patients selected had undergone only locoregional treatment, including axillary node dissection, but had not received any systemic therapy.
Pathologists reviewed samples, with TILs reported for the stromal compartment. The samples were grouped by sTIL score categories of high (75% or greater), intermediate (30% to less than 75%), or low (less than 30%). The investigators looked at overall survival (OS) and distant metastasis-free survival (DMFS) stratified by sTIL scores,
During a median follow-up of 15 years, 107 women died or developed distant metastases, and 78 experienced a second primary cancer.
The results were as noted, with patients in the highest category of sTILs having very low rates of either death or distant metastases during follow-up.
“We confirm the prognostic value of sTILs in young patients with early-stage N0 TNBC who are systemic therapy naive by taking advantage of a prospectively collected population-based cohort. Increasing sTILs are significantly associated with improved OS and DMFS. Patients with high sTILs (> 75%) had an excellent 10-year overall survival and a very low 10-year incidence of distant metastasis or death.
The study was supported by grants from The Netherlands Organization for Health Research and Development, A Sister’s Hope, De Vrienden van UMC Utrecht, Agilent Technologies, the Dutch Cancer Society, and Breast Cancer Research Foundation. Dr. Linn reported consulting with and receiving compensation from Daiichi Sankyo, as well as receiving research funding from Genentech/Roche, AstraZeneca, Bristol-Myers Squibb, Tesaro, Merck, Immunomedics, Eurocept Pharmaceuticals, Agendia, and Novartis.
and may be suitable candidates for reduced intensity pre- or postoperative chemotherapy, according to a team of European investigators.
Among 441 women in a Dutch cancer registry who were younger than 40 when they were diagnosed with node-negative TNBC and had not undergone systemic therapy, those who had 75% or more TILs in the intratumoral stromal area had a 15-year cumulative incidence of distant metastases or death of just 2.1%, and every 10% increase in sTILs was associated with a 19% decrease in the risk of death.
In contrast, the 15-year cumulative incidence of distant metastases was 38.4% for women with stromal TIL scores of less than 30%, according to researchers writing in the Journal of Clinical Oncology.
“These data could be used as a starting point for designing a randomized controlled chemotherapy de-escalation trial. The current study confirms the importance of sTILs as a valuable addition to the set of standard prognostic factors in patients with TNBC,” wrote the researchers, who were led by Sabine C. Linn, MD, of the Netherlands Cancer Institute, Amsterdam.
Markers for immune response
Stromal TILs, a mixture of mononuclear immune cells, have been shown in previous studies to be prognostic for outcomes in patients with early-stage TNBC treated either with or without neoadjuvant or adjuvant chemotherapy.
For example, investigators cited a study published in JCO in 2014, that showed among women with TNBC enrolled in the phase 3 ECOG 2197 clinical trial and the related ECOG 119 clinical trial, after a nearly 11-year follow-up, higher sTIL scores were associated with significantly better prognosis with every 10% increase translating into a 14% reduction in the risk of recurrence or death (P = .02).
“The prognostic importance of sTILs is, however, unexplored in patients diagnosed under age 40 years, let alone in the subgroup of systemic therapy–naive patients,” Dr. Linn and colleagues wrote.
Retrospective study
To see whether the prognostic value of sTILs was as strong among young, systemic therapy–naive women, the investigators conducted a retrospective study of women enrolled in the Netherlands Cancer Registry who were diagnosed with node-negative TNBC from 1989 to 2000. The patients selected had undergone only locoregional treatment, including axillary node dissection, but had not received any systemic therapy.
Pathologists reviewed samples, with TILs reported for the stromal compartment. The samples were grouped by sTIL score categories of high (75% or greater), intermediate (30% to less than 75%), or low (less than 30%). The investigators looked at overall survival (OS) and distant metastasis-free survival (DMFS) stratified by sTIL scores,
During a median follow-up of 15 years, 107 women died or developed distant metastases, and 78 experienced a second primary cancer.
The results were as noted, with patients in the highest category of sTILs having very low rates of either death or distant metastases during follow-up.
“We confirm the prognostic value of sTILs in young patients with early-stage N0 TNBC who are systemic therapy naive by taking advantage of a prospectively collected population-based cohort. Increasing sTILs are significantly associated with improved OS and DMFS. Patients with high sTILs (> 75%) had an excellent 10-year overall survival and a very low 10-year incidence of distant metastasis or death.
The study was supported by grants from The Netherlands Organization for Health Research and Development, A Sister’s Hope, De Vrienden van UMC Utrecht, Agilent Technologies, the Dutch Cancer Society, and Breast Cancer Research Foundation. Dr. Linn reported consulting with and receiving compensation from Daiichi Sankyo, as well as receiving research funding from Genentech/Roche, AstraZeneca, Bristol-Myers Squibb, Tesaro, Merck, Immunomedics, Eurocept Pharmaceuticals, Agendia, and Novartis.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Novel guidance informs plasma biomarker use for Alzheimer’s disease
SAN DIEGO – The organization has previously published recommendations for use of amyloid positron emission tomography (PET) and cerebrospinal fluid (CSF) biomarkers for Alzheimer’s disease.
The recommendations were the subject of a presentation at the 2022 Alzheimer’s Association International Conference and were published online in Alzheimer’s & Dementia.
During his presentation, Oskar Hansson, MD, PhD, stressed that the document describes recommendations, not criteria, for use of blood-based biomarkers. He suggested that the recommendations will need to be updated within 9-12 months, and that criteria for blood-based biomarkers use could come within 2 years.
The new recommendations reflect the recent acceleration of progress in the field, according to Wiesje M. van der Flier, PhD, who moderated the session. “It’s just growing so quickly. I think within 5 years the whole field will have transformed. By starting to use them in specialized memory clinics first, but then also local memory clinics, and then finally, I think that they may also transform primary care,” said Dr. van der Flier, who is a professor of neurology at Amsterdam University Medical Center.
Guidance for clinical trials and memory clinics
The guidelines were created in part because blood-based biomarkers for Alzheimer’s disease have become increasingly available, and there has been a call from the community for guidance, according to Dr. Hansson. There is also a hazard that widespread adoption could interfere with the field itself, especially if physicians don’t understand how to interpret the results. That’s a particularly acute problem since Alzheimer’s disease pathology can precede symptoms. “It’s important to have some guidance about regulating their use so we don’t get the problem that they are misused and get a bad reputation,” said Dr. Hansson in an interview.
The current recommendations are for use in clinical trials to identify patients likely to have Alzheimer’s disease, as well as in memory clinics, though “we’re still a bit cautious. We still need to confirm it with other biomarkers. The reason for that is we still don’t know how these will perform in the clinical reality. So it’s a bit trying it out. You can start using these blood biomarkers to some degree,” said Dr. Hansson.
However, he offered the caveat that plasma-based biomarkers should only be used while confirming that the blood-based biomarkers agree with CSF tests, ideally more than 90% of the time. “If suddenly only 60% of the plasma biomarkers agree with CSF, you have a problem and you need to stop,” said Dr. Hansson.
The authors recommend that blood-based biomarkers be used in clinical trials to help select patients and identify healthy controls. Dr. Hansson said that there is not enough evidence that blood-based biomarkers have sufficient positive predictive value to be used as the sole criteria for clinical trial admission. However, they could also be used to inform decision-making in adaptive clinical trials.
Specifically, plasma Abeta42/Abeta40 and P-tau assays using established thresholds can be used in clinical studies first-screening step for clinical trials, though they should be confirmed by PET or CSF in those with abnormal blood biomarker levels. The biomarkers could also be used in non–Alzheimer’s disease clinical trials to exclude patients with probable Alzheimer’s disease copathology.
In memory clinics, the authors recommend that BBMs be used only in patients who are symptomatic and, when possible, should be confirmed by PET or CSF.
More work to be done
Dr. Hansson noted that 50%-70% of patients with Alzheimer’s disease are misdiagnosed in primary care, showing a clear need for biomarkers that could improve diagnosis. However, he stressed that blood-based biomarkers are not yet ready for use in that setting.
Still, they could eventually become a boon. “The majority of patients now do not get any biomarker support to diagnosis. They do not have access to amyloid PET or [CSF] biomarkers, but when the blood-based biomarkers are good enough, that means that biomarker support for an Alzheimer’s diagnosis [will be] available to many patients … across the globe,” said Dr. van der Flier.
There are numerous research efforts underway to validate blood-based biomarkers in more diverse groups of patients. That’s because the retrospective studies typically used to identify and validate biomarkers tend to recruit carefully selected patients, with clearly defined cases and good CSF characterization, according to Charlotte Teunissen, PhD, who is also a coauthor of the guidelines and professor of neuropsychiatry at Amsterdam University Medical Center. “Now we want to go one step further to go real-life practice, and there are several initiatives,” she said.
Dr. Hansson, Dr. Tenuissen, and Dr. van der Flier have no relevant financial disclosures.
SAN DIEGO – The organization has previously published recommendations for use of amyloid positron emission tomography (PET) and cerebrospinal fluid (CSF) biomarkers for Alzheimer’s disease.
The recommendations were the subject of a presentation at the 2022 Alzheimer’s Association International Conference and were published online in Alzheimer’s & Dementia.
During his presentation, Oskar Hansson, MD, PhD, stressed that the document describes recommendations, not criteria, for use of blood-based biomarkers. He suggested that the recommendations will need to be updated within 9-12 months, and that criteria for blood-based biomarkers use could come within 2 years.
The new recommendations reflect the recent acceleration of progress in the field, according to Wiesje M. van der Flier, PhD, who moderated the session. “It’s just growing so quickly. I think within 5 years the whole field will have transformed. By starting to use them in specialized memory clinics first, but then also local memory clinics, and then finally, I think that they may also transform primary care,” said Dr. van der Flier, who is a professor of neurology at Amsterdam University Medical Center.
Guidance for clinical trials and memory clinics
The guidelines were created in part because blood-based biomarkers for Alzheimer’s disease have become increasingly available, and there has been a call from the community for guidance, according to Dr. Hansson. There is also a hazard that widespread adoption could interfere with the field itself, especially if physicians don’t understand how to interpret the results. That’s a particularly acute problem since Alzheimer’s disease pathology can precede symptoms. “It’s important to have some guidance about regulating their use so we don’t get the problem that they are misused and get a bad reputation,” said Dr. Hansson in an interview.
The current recommendations are for use in clinical trials to identify patients likely to have Alzheimer’s disease, as well as in memory clinics, though “we’re still a bit cautious. We still need to confirm it with other biomarkers. The reason for that is we still don’t know how these will perform in the clinical reality. So it’s a bit trying it out. You can start using these blood biomarkers to some degree,” said Dr. Hansson.
However, he offered the caveat that plasma-based biomarkers should only be used while confirming that the blood-based biomarkers agree with CSF tests, ideally more than 90% of the time. “If suddenly only 60% of the plasma biomarkers agree with CSF, you have a problem and you need to stop,” said Dr. Hansson.
The authors recommend that blood-based biomarkers be used in clinical trials to help select patients and identify healthy controls. Dr. Hansson said that there is not enough evidence that blood-based biomarkers have sufficient positive predictive value to be used as the sole criteria for clinical trial admission. However, they could also be used to inform decision-making in adaptive clinical trials.
Specifically, plasma Abeta42/Abeta40 and P-tau assays using established thresholds can be used in clinical studies first-screening step for clinical trials, though they should be confirmed by PET or CSF in those with abnormal blood biomarker levels. The biomarkers could also be used in non–Alzheimer’s disease clinical trials to exclude patients with probable Alzheimer’s disease copathology.
In memory clinics, the authors recommend that BBMs be used only in patients who are symptomatic and, when possible, should be confirmed by PET or CSF.
More work to be done
Dr. Hansson noted that 50%-70% of patients with Alzheimer’s disease are misdiagnosed in primary care, showing a clear need for biomarkers that could improve diagnosis. However, he stressed that blood-based biomarkers are not yet ready for use in that setting.
Still, they could eventually become a boon. “The majority of patients now do not get any biomarker support to diagnosis. They do not have access to amyloid PET or [CSF] biomarkers, but when the blood-based biomarkers are good enough, that means that biomarker support for an Alzheimer’s diagnosis [will be] available to many patients … across the globe,” said Dr. van der Flier.
There are numerous research efforts underway to validate blood-based biomarkers in more diverse groups of patients. That’s because the retrospective studies typically used to identify and validate biomarkers tend to recruit carefully selected patients, with clearly defined cases and good CSF characterization, according to Charlotte Teunissen, PhD, who is also a coauthor of the guidelines and professor of neuropsychiatry at Amsterdam University Medical Center. “Now we want to go one step further to go real-life practice, and there are several initiatives,” she said.
Dr. Hansson, Dr. Tenuissen, and Dr. van der Flier have no relevant financial disclosures.
SAN DIEGO – The organization has previously published recommendations for use of amyloid positron emission tomography (PET) and cerebrospinal fluid (CSF) biomarkers for Alzheimer’s disease.
The recommendations were the subject of a presentation at the 2022 Alzheimer’s Association International Conference and were published online in Alzheimer’s & Dementia.
During his presentation, Oskar Hansson, MD, PhD, stressed that the document describes recommendations, not criteria, for use of blood-based biomarkers. He suggested that the recommendations will need to be updated within 9-12 months, and that criteria for blood-based biomarkers use could come within 2 years.
The new recommendations reflect the recent acceleration of progress in the field, according to Wiesje M. van der Flier, PhD, who moderated the session. “It’s just growing so quickly. I think within 5 years the whole field will have transformed. By starting to use them in specialized memory clinics first, but then also local memory clinics, and then finally, I think that they may also transform primary care,” said Dr. van der Flier, who is a professor of neurology at Amsterdam University Medical Center.
Guidance for clinical trials and memory clinics
The guidelines were created in part because blood-based biomarkers for Alzheimer’s disease have become increasingly available, and there has been a call from the community for guidance, according to Dr. Hansson. There is also a hazard that widespread adoption could interfere with the field itself, especially if physicians don’t understand how to interpret the results. That’s a particularly acute problem since Alzheimer’s disease pathology can precede symptoms. “It’s important to have some guidance about regulating their use so we don’t get the problem that they are misused and get a bad reputation,” said Dr. Hansson in an interview.
The current recommendations are for use in clinical trials to identify patients likely to have Alzheimer’s disease, as well as in memory clinics, though “we’re still a bit cautious. We still need to confirm it with other biomarkers. The reason for that is we still don’t know how these will perform in the clinical reality. So it’s a bit trying it out. You can start using these blood biomarkers to some degree,” said Dr. Hansson.
However, he offered the caveat that plasma-based biomarkers should only be used while confirming that the blood-based biomarkers agree with CSF tests, ideally more than 90% of the time. “If suddenly only 60% of the plasma biomarkers agree with CSF, you have a problem and you need to stop,” said Dr. Hansson.
The authors recommend that blood-based biomarkers be used in clinical trials to help select patients and identify healthy controls. Dr. Hansson said that there is not enough evidence that blood-based biomarkers have sufficient positive predictive value to be used as the sole criteria for clinical trial admission. However, they could also be used to inform decision-making in adaptive clinical trials.
Specifically, plasma Abeta42/Abeta40 and P-tau assays using established thresholds can be used in clinical studies first-screening step for clinical trials, though they should be confirmed by PET or CSF in those with abnormal blood biomarker levels. The biomarkers could also be used in non–Alzheimer’s disease clinical trials to exclude patients with probable Alzheimer’s disease copathology.
In memory clinics, the authors recommend that BBMs be used only in patients who are symptomatic and, when possible, should be confirmed by PET or CSF.
More work to be done
Dr. Hansson noted that 50%-70% of patients with Alzheimer’s disease are misdiagnosed in primary care, showing a clear need for biomarkers that could improve diagnosis. However, he stressed that blood-based biomarkers are not yet ready for use in that setting.
Still, they could eventually become a boon. “The majority of patients now do not get any biomarker support to diagnosis. They do not have access to amyloid PET or [CSF] biomarkers, but when the blood-based biomarkers are good enough, that means that biomarker support for an Alzheimer’s diagnosis [will be] available to many patients … across the globe,” said Dr. van der Flier.
There are numerous research efforts underway to validate blood-based biomarkers in more diverse groups of patients. That’s because the retrospective studies typically used to identify and validate biomarkers tend to recruit carefully selected patients, with clearly defined cases and good CSF characterization, according to Charlotte Teunissen, PhD, who is also a coauthor of the guidelines and professor of neuropsychiatry at Amsterdam University Medical Center. “Now we want to go one step further to go real-life practice, and there are several initiatives,” she said.
Dr. Hansson, Dr. Tenuissen, and Dr. van der Flier have no relevant financial disclosures.
FROM AAIC 2022
ICU stays linked to a doubling of dementia risk
compared with older adults who have never stayed in the ICU, new research suggests.
“ICU hospitalization may be an underrecognized risk factor for dementia in older adults,” Bryan D. James, PhD, epidemiologist with Rush Alzheimer’s Disease Center, Chicago, said in an interview.
“Health care providers caring for older patients who have experienced a hospitalization for critical illness should be prepared to assess and monitor their patients’ cognitive status as part of their long-term care plan,” Dr. James added.
The findings were presented at the Alzheimer’s Association International Conference.
Hidden risk factor?
ICU hospitalization as a result of critical illness has been linked to subsequent cognitive impairment in older patients. However, how ICU hospitalization relates to the long-term risk of developing Alzheimer’s and other age-related dementias is unknown.
“Given the high rate of ICU hospitalization in older persons, especially during the COVID-19 pandemic, it is critical to explore this relationship, Dr. James said.
The Rush team assessed the impact of an ICU stay on dementia risk in 3,822 older adults (mean age, 77 years) without known dementia at baseline participating in five diverse epidemiologic cohorts.
Participants were checked annually for development of Alzheimer’s and all-type dementia using standardized cognitive assessments.
Over an average of 7.8 years, 1,991 (52%) adults had at least one ICU stay; 1,031 (27%) had an ICU stay before study enrollment; and 961 (25%) had an ICU stay during the study period.
In models adjusted for age, sex, education, and race, ICU hospitalization was associated with 63% higher risk of Alzheimer’s dementia (hazard ratio, 1.63; 95% confidence interval, 1.41-1.88) and 71% higher risk of all-type dementia (HR, 1.71; 95% CI, 1.48-1.97).
In models further adjusted for other health factors such as vascular risk factors and disease, other chronic medical conditions and functional disabilities, the association was even stronger: ICU hospitalization was associated with roughly double the risk of Alzheimer’s dementia (HR 2.10; 95% CI, 1.66-2.65) and all-type dementia (HR, 2.20; 95% CI, 1.75-2.77).
Dr. James said in an interview that it remains unclear why an ICU stay may raise the dementia risk.
“This study was not designed to assess the causes of the higher risk of dementia in persons who had ICU hospitalizations. However, researchers have looked into a number of factors that could account for this increased risk,” he explained.
One is critical illness itself that leads to hospitalization, which could result in damage to the brain; for example, severe COVID-19 has been shown to directly harm the brain, Dr. James said.
He also noted that specific events experienced during ICU stay have been shown to increase risk for cognitive impairment, including infection and severe sepsis, acute dialysis, neurologic dysfunction and delirium, and sedation.
Important work
Commenting on the study, Heather Snyder, PhD, vice president of medical & scientific relations at the Alzheimer’s Association, said what’s interesting about the study is that it looks at individuals in the ICU, regardless of the cause.
“The study shows that having some type of health issue that results in some type of ICU stay is associated with an increased risk of declining cognition,” Dr. Snyder said.
“That’s really important,” she said, “especially given the increase in individuals, particularly those 60 and older, who did experience an ICU stay over the last couple of years and understanding how that might impact their long-term risk related to Alzheimer’s and other changes in memory.”
“If an individual has been in the ICU, that should be part of the conversation with their physician or health care provider,” Dr. Snyder advised.
The study was funded by the National Institute on Aging. Dr. James and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
compared with older adults who have never stayed in the ICU, new research suggests.
“ICU hospitalization may be an underrecognized risk factor for dementia in older adults,” Bryan D. James, PhD, epidemiologist with Rush Alzheimer’s Disease Center, Chicago, said in an interview.
“Health care providers caring for older patients who have experienced a hospitalization for critical illness should be prepared to assess and monitor their patients’ cognitive status as part of their long-term care plan,” Dr. James added.
The findings were presented at the Alzheimer’s Association International Conference.
Hidden risk factor?
ICU hospitalization as a result of critical illness has been linked to subsequent cognitive impairment in older patients. However, how ICU hospitalization relates to the long-term risk of developing Alzheimer’s and other age-related dementias is unknown.
“Given the high rate of ICU hospitalization in older persons, especially during the COVID-19 pandemic, it is critical to explore this relationship, Dr. James said.
The Rush team assessed the impact of an ICU stay on dementia risk in 3,822 older adults (mean age, 77 years) without known dementia at baseline participating in five diverse epidemiologic cohorts.
Participants were checked annually for development of Alzheimer’s and all-type dementia using standardized cognitive assessments.
Over an average of 7.8 years, 1,991 (52%) adults had at least one ICU stay; 1,031 (27%) had an ICU stay before study enrollment; and 961 (25%) had an ICU stay during the study period.
In models adjusted for age, sex, education, and race, ICU hospitalization was associated with 63% higher risk of Alzheimer’s dementia (hazard ratio, 1.63; 95% confidence interval, 1.41-1.88) and 71% higher risk of all-type dementia (HR, 1.71; 95% CI, 1.48-1.97).
In models further adjusted for other health factors such as vascular risk factors and disease, other chronic medical conditions and functional disabilities, the association was even stronger: ICU hospitalization was associated with roughly double the risk of Alzheimer’s dementia (HR 2.10; 95% CI, 1.66-2.65) and all-type dementia (HR, 2.20; 95% CI, 1.75-2.77).
Dr. James said in an interview that it remains unclear why an ICU stay may raise the dementia risk.
“This study was not designed to assess the causes of the higher risk of dementia in persons who had ICU hospitalizations. However, researchers have looked into a number of factors that could account for this increased risk,” he explained.
One is critical illness itself that leads to hospitalization, which could result in damage to the brain; for example, severe COVID-19 has been shown to directly harm the brain, Dr. James said.
He also noted that specific events experienced during ICU stay have been shown to increase risk for cognitive impairment, including infection and severe sepsis, acute dialysis, neurologic dysfunction and delirium, and sedation.
Important work
Commenting on the study, Heather Snyder, PhD, vice president of medical & scientific relations at the Alzheimer’s Association, said what’s interesting about the study is that it looks at individuals in the ICU, regardless of the cause.
“The study shows that having some type of health issue that results in some type of ICU stay is associated with an increased risk of declining cognition,” Dr. Snyder said.
“That’s really important,” she said, “especially given the increase in individuals, particularly those 60 and older, who did experience an ICU stay over the last couple of years and understanding how that might impact their long-term risk related to Alzheimer’s and other changes in memory.”
“If an individual has been in the ICU, that should be part of the conversation with their physician or health care provider,” Dr. Snyder advised.
The study was funded by the National Institute on Aging. Dr. James and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
compared with older adults who have never stayed in the ICU, new research suggests.
“ICU hospitalization may be an underrecognized risk factor for dementia in older adults,” Bryan D. James, PhD, epidemiologist with Rush Alzheimer’s Disease Center, Chicago, said in an interview.
“Health care providers caring for older patients who have experienced a hospitalization for critical illness should be prepared to assess and monitor their patients’ cognitive status as part of their long-term care plan,” Dr. James added.
The findings were presented at the Alzheimer’s Association International Conference.
Hidden risk factor?
ICU hospitalization as a result of critical illness has been linked to subsequent cognitive impairment in older patients. However, how ICU hospitalization relates to the long-term risk of developing Alzheimer’s and other age-related dementias is unknown.
“Given the high rate of ICU hospitalization in older persons, especially during the COVID-19 pandemic, it is critical to explore this relationship, Dr. James said.
The Rush team assessed the impact of an ICU stay on dementia risk in 3,822 older adults (mean age, 77 years) without known dementia at baseline participating in five diverse epidemiologic cohorts.
Participants were checked annually for development of Alzheimer’s and all-type dementia using standardized cognitive assessments.
Over an average of 7.8 years, 1,991 (52%) adults had at least one ICU stay; 1,031 (27%) had an ICU stay before study enrollment; and 961 (25%) had an ICU stay during the study period.
In models adjusted for age, sex, education, and race, ICU hospitalization was associated with 63% higher risk of Alzheimer’s dementia (hazard ratio, 1.63; 95% confidence interval, 1.41-1.88) and 71% higher risk of all-type dementia (HR, 1.71; 95% CI, 1.48-1.97).
In models further adjusted for other health factors such as vascular risk factors and disease, other chronic medical conditions and functional disabilities, the association was even stronger: ICU hospitalization was associated with roughly double the risk of Alzheimer’s dementia (HR 2.10; 95% CI, 1.66-2.65) and all-type dementia (HR, 2.20; 95% CI, 1.75-2.77).
Dr. James said in an interview that it remains unclear why an ICU stay may raise the dementia risk.
“This study was not designed to assess the causes of the higher risk of dementia in persons who had ICU hospitalizations. However, researchers have looked into a number of factors that could account for this increased risk,” he explained.
One is critical illness itself that leads to hospitalization, which could result in damage to the brain; for example, severe COVID-19 has been shown to directly harm the brain, Dr. James said.
He also noted that specific events experienced during ICU stay have been shown to increase risk for cognitive impairment, including infection and severe sepsis, acute dialysis, neurologic dysfunction and delirium, and sedation.
Important work
Commenting on the study, Heather Snyder, PhD, vice president of medical & scientific relations at the Alzheimer’s Association, said what’s interesting about the study is that it looks at individuals in the ICU, regardless of the cause.
“The study shows that having some type of health issue that results in some type of ICU stay is associated with an increased risk of declining cognition,” Dr. Snyder said.
“That’s really important,” she said, “especially given the increase in individuals, particularly those 60 and older, who did experience an ICU stay over the last couple of years and understanding how that might impact their long-term risk related to Alzheimer’s and other changes in memory.”
“If an individual has been in the ICU, that should be part of the conversation with their physician or health care provider,” Dr. Snyder advised.
The study was funded by the National Institute on Aging. Dr. James and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAIC 2022
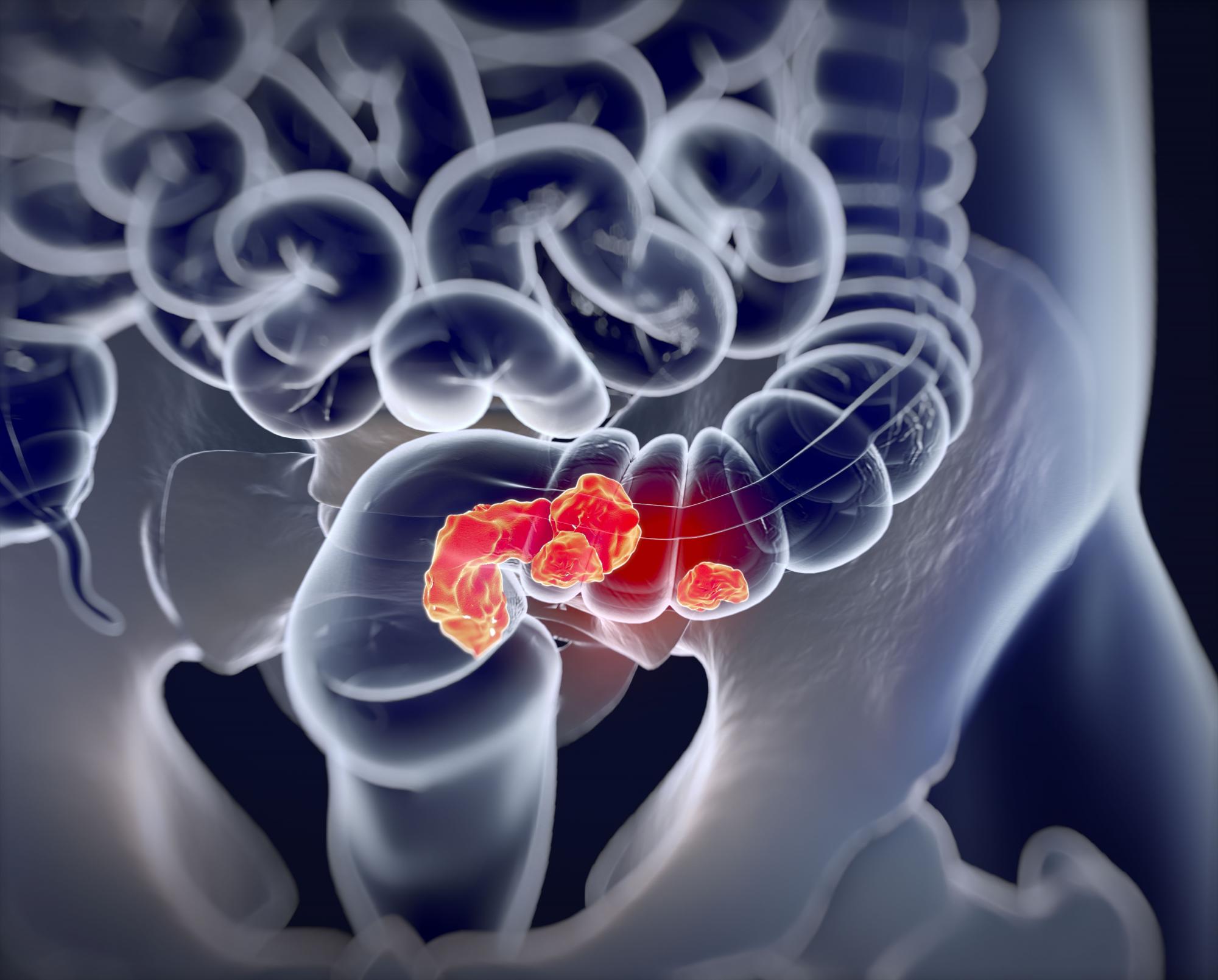
Higher ADR continues to show ‘strong, consistent’ link with lower interval CRC
Higher adenoma detection rates (ADR) during colonoscopies were associated with lower rates of interim colorectal cancer (CRC), and the relationship held true along a broad range of ADR values, according to a retrospective study.
The new study, published online in JAMA, examined ADRs and rates of interim colorectal cancer among patients in California and Washington State between 2011 and 2017. The authors found a 3% reduction in risk for each additional 1% value of ADR. The reduction in risk held true even at high ADRs.
“It basically reaffirms what we’ve believed for the longest time, and other research work has documented – that interim cancers are higher in association with lower adenoma detection rates. The higher you can get that adenoma detection rate, the more we’re going to be able to lower the [rate of] cancers that develop within 3 years of a colonoscopy,” said Lawrence Kosinski, MD, who was asked to comment on the study.
The study included 735,396 patients with a median age of 61.4 years. Among these patients, 852,624 negative colonoscopies were performed by 383 eligible physicians. Participating physicians had to perform at least 25 screening colonoscopies and 100 total colonoscopies per year. After 2.4 million person-years of follow-up, the researchers observed 619 postcolonoscopy colorectal cancers and 36 related deaths over a median follow-up of 3.25 years.
There was an association between each 1% increase in ADR and a reduced probability of postcolonoscopy CRC (hazard ratio [HR], 0.97; 95% confidence interval [CI], 0.96-0.98) and mortality from postcolonoscopy CRC (HR, 0.95; 95% CI, 0.92-0.99).
The median ADR was 28.3%. There was an association between ADR above the median versus below the median and a reduced risk of postcolonoscopy CRC with 1.79 cases versus 3.10 cases per 10,000 person-years, respectively (absolute difference in 7-year risk, –12.2 per 10,000 negative colonoscopies; HR, 0.61; 95% CI, 0.52-0.73). There was a similar reduction in risk of postcolonoscopy CRC-related mortality (0.05 versus 0.22 per 10,000 person-years; absolute difference in 7-year risk, –1.2 per 10,000 negative colonoscopies; HR, 0.26; 95% CI, 0.11-0.65).
These findings may be limited in generalizability to physicians with lower procedure volumes or to populations with different adenoma prevalence.
“Given the strong, consistent associations of higher adenoma detection rates with colonoscopy effectiveness for reducing colorectal cancer incidence and mortality, the current results support more research to identify reliable and readily adoptable methods for increasing adenoma detection rates among physicians with lower values across diverse settings,” the researchers wrote.
The improvement over a broad range of ADRs, along with other recent findings, suggests that there may need to be updates to the use of ADRs as a quality metric, according to an accompanying editorial by Douglas K. Rex, MD, of the division of gastroenterology/hepatology at Indiana University, Indianapolis. For example, it’s possible that ADRs could be measured by averaging values from screening, diagnostic, and surveillance colonoscopy. The editorialist suggested that, if improvements in interim cancer rates continue as ADRs approach 50%, the current view of ADRs, as a minimally acceptable standard, may require reconsideration. Instead, it may be appropriate to continue with a minimum threshold, but add a much higher, aspirational target. Dr. Rex also suggested that highly-variable detection of sessile serrated lesions could be excluded from ADRs in order to reduce variability.
Factors to consider
The study is useful, but it doesn’t address the disparity in adenoma detection that exists between individual doctors, according to Dr. Kosinski, founder and chief medical officer of SonarMD and previously director of a large gastroenterology clinic. “Even if you look at doctors who do a minimum of 250 screening colonoscopies in a year, there’s still variability. There was even a study published in 2014 showing ADRs anywhere from 7.4% to 52.5%. The bell curve is broad,” he said.
As patients age, they have a higher frequency of polyps appearing on the right side of the colon, and those polyps are flatter and more easily missed than polyps on the left side. “The variation in ADR is higher on the right side of the colon than it is on the left. Doctors have to really do a very good job of examining that right side of the colon so that they don’t miss the flat polyps,” said Dr. Kosinski.
To improve ADRs, Dr. Kosinski emphasized the need to take the required time out to complete a procedure, despite the tight schedules often faced by ambulatory centers. “It’s the time you take coming out of the colon that’s critical. You owe it to the patient,” he said.
And if a patient hasn’t prepped well enough, it’s better to send the patient home without the procedure than to conduct a poor-quality screening. “If you can’t see the mucosal surface, you can’t tell the patient that they have a negative colonoscopy. If you have to do more cleaning during the procedure, then do more cleaning during the procedure. If you have to cancel the procedure and bring the patient back, it’s better to do that than it is to do an incomplete colonoscopy,” said Dr. Kosinski.
He also stressed the need to make sure that the patient is properly sedated and comfortable “so that you can do the job you’re supposed to do,” he said.
Some authors disclosed relationships with Amgen and the National Cancer Institute. Dr. Rex disclosed relationships with Olympus, Boston Scientific, Aries, and others, all outside the submitted work.
Higher adenoma detection rates (ADR) during colonoscopies were associated with lower rates of interim colorectal cancer (CRC), and the relationship held true along a broad range of ADR values, according to a retrospective study.
The new study, published online in JAMA, examined ADRs and rates of interim colorectal cancer among patients in California and Washington State between 2011 and 2017. The authors found a 3% reduction in risk for each additional 1% value of ADR. The reduction in risk held true even at high ADRs.
“It basically reaffirms what we’ve believed for the longest time, and other research work has documented – that interim cancers are higher in association with lower adenoma detection rates. The higher you can get that adenoma detection rate, the more we’re going to be able to lower the [rate of] cancers that develop within 3 years of a colonoscopy,” said Lawrence Kosinski, MD, who was asked to comment on the study.
The study included 735,396 patients with a median age of 61.4 years. Among these patients, 852,624 negative colonoscopies were performed by 383 eligible physicians. Participating physicians had to perform at least 25 screening colonoscopies and 100 total colonoscopies per year. After 2.4 million person-years of follow-up, the researchers observed 619 postcolonoscopy colorectal cancers and 36 related deaths over a median follow-up of 3.25 years.
There was an association between each 1% increase in ADR and a reduced probability of postcolonoscopy CRC (hazard ratio [HR], 0.97; 95% confidence interval [CI], 0.96-0.98) and mortality from postcolonoscopy CRC (HR, 0.95; 95% CI, 0.92-0.99).
The median ADR was 28.3%. There was an association between ADR above the median versus below the median and a reduced risk of postcolonoscopy CRC with 1.79 cases versus 3.10 cases per 10,000 person-years, respectively (absolute difference in 7-year risk, –12.2 per 10,000 negative colonoscopies; HR, 0.61; 95% CI, 0.52-0.73). There was a similar reduction in risk of postcolonoscopy CRC-related mortality (0.05 versus 0.22 per 10,000 person-years; absolute difference in 7-year risk, –1.2 per 10,000 negative colonoscopies; HR, 0.26; 95% CI, 0.11-0.65).
These findings may be limited in generalizability to physicians with lower procedure volumes or to populations with different adenoma prevalence.
“Given the strong, consistent associations of higher adenoma detection rates with colonoscopy effectiveness for reducing colorectal cancer incidence and mortality, the current results support more research to identify reliable and readily adoptable methods for increasing adenoma detection rates among physicians with lower values across diverse settings,” the researchers wrote.
The improvement over a broad range of ADRs, along with other recent findings, suggests that there may need to be updates to the use of ADRs as a quality metric, according to an accompanying editorial by Douglas K. Rex, MD, of the division of gastroenterology/hepatology at Indiana University, Indianapolis. For example, it’s possible that ADRs could be measured by averaging values from screening, diagnostic, and surveillance colonoscopy. The editorialist suggested that, if improvements in interim cancer rates continue as ADRs approach 50%, the current view of ADRs, as a minimally acceptable standard, may require reconsideration. Instead, it may be appropriate to continue with a minimum threshold, but add a much higher, aspirational target. Dr. Rex also suggested that highly-variable detection of sessile serrated lesions could be excluded from ADRs in order to reduce variability.
Factors to consider
The study is useful, but it doesn’t address the disparity in adenoma detection that exists between individual doctors, according to Dr. Kosinski, founder and chief medical officer of SonarMD and previously director of a large gastroenterology clinic. “Even if you look at doctors who do a minimum of 250 screening colonoscopies in a year, there’s still variability. There was even a study published in 2014 showing ADRs anywhere from 7.4% to 52.5%. The bell curve is broad,” he said.
As patients age, they have a higher frequency of polyps appearing on the right side of the colon, and those polyps are flatter and more easily missed than polyps on the left side. “The variation in ADR is higher on the right side of the colon than it is on the left. Doctors have to really do a very good job of examining that right side of the colon so that they don’t miss the flat polyps,” said Dr. Kosinski.
To improve ADRs, Dr. Kosinski emphasized the need to take the required time out to complete a procedure, despite the tight schedules often faced by ambulatory centers. “It’s the time you take coming out of the colon that’s critical. You owe it to the patient,” he said.
And if a patient hasn’t prepped well enough, it’s better to send the patient home without the procedure than to conduct a poor-quality screening. “If you can’t see the mucosal surface, you can’t tell the patient that they have a negative colonoscopy. If you have to do more cleaning during the procedure, then do more cleaning during the procedure. If you have to cancel the procedure and bring the patient back, it’s better to do that than it is to do an incomplete colonoscopy,” said Dr. Kosinski.
He also stressed the need to make sure that the patient is properly sedated and comfortable “so that you can do the job you’re supposed to do,” he said.
Some authors disclosed relationships with Amgen and the National Cancer Institute. Dr. Rex disclosed relationships with Olympus, Boston Scientific, Aries, and others, all outside the submitted work.
Higher adenoma detection rates (ADR) during colonoscopies were associated with lower rates of interim colorectal cancer (CRC), and the relationship held true along a broad range of ADR values, according to a retrospective study.
The new study, published online in JAMA, examined ADRs and rates of interim colorectal cancer among patients in California and Washington State between 2011 and 2017. The authors found a 3% reduction in risk for each additional 1% value of ADR. The reduction in risk held true even at high ADRs.
“It basically reaffirms what we’ve believed for the longest time, and other research work has documented – that interim cancers are higher in association with lower adenoma detection rates. The higher you can get that adenoma detection rate, the more we’re going to be able to lower the [rate of] cancers that develop within 3 years of a colonoscopy,” said Lawrence Kosinski, MD, who was asked to comment on the study.
The study included 735,396 patients with a median age of 61.4 years. Among these patients, 852,624 negative colonoscopies were performed by 383 eligible physicians. Participating physicians had to perform at least 25 screening colonoscopies and 100 total colonoscopies per year. After 2.4 million person-years of follow-up, the researchers observed 619 postcolonoscopy colorectal cancers and 36 related deaths over a median follow-up of 3.25 years.
There was an association between each 1% increase in ADR and a reduced probability of postcolonoscopy CRC (hazard ratio [HR], 0.97; 95% confidence interval [CI], 0.96-0.98) and mortality from postcolonoscopy CRC (HR, 0.95; 95% CI, 0.92-0.99).
The median ADR was 28.3%. There was an association between ADR above the median versus below the median and a reduced risk of postcolonoscopy CRC with 1.79 cases versus 3.10 cases per 10,000 person-years, respectively (absolute difference in 7-year risk, –12.2 per 10,000 negative colonoscopies; HR, 0.61; 95% CI, 0.52-0.73). There was a similar reduction in risk of postcolonoscopy CRC-related mortality (0.05 versus 0.22 per 10,000 person-years; absolute difference in 7-year risk, –1.2 per 10,000 negative colonoscopies; HR, 0.26; 95% CI, 0.11-0.65).
These findings may be limited in generalizability to physicians with lower procedure volumes or to populations with different adenoma prevalence.
“Given the strong, consistent associations of higher adenoma detection rates with colonoscopy effectiveness for reducing colorectal cancer incidence and mortality, the current results support more research to identify reliable and readily adoptable methods for increasing adenoma detection rates among physicians with lower values across diverse settings,” the researchers wrote.
The improvement over a broad range of ADRs, along with other recent findings, suggests that there may need to be updates to the use of ADRs as a quality metric, according to an accompanying editorial by Douglas K. Rex, MD, of the division of gastroenterology/hepatology at Indiana University, Indianapolis. For example, it’s possible that ADRs could be measured by averaging values from screening, diagnostic, and surveillance colonoscopy. The editorialist suggested that, if improvements in interim cancer rates continue as ADRs approach 50%, the current view of ADRs, as a minimally acceptable standard, may require reconsideration. Instead, it may be appropriate to continue with a minimum threshold, but add a much higher, aspirational target. Dr. Rex also suggested that highly-variable detection of sessile serrated lesions could be excluded from ADRs in order to reduce variability.
Factors to consider
The study is useful, but it doesn’t address the disparity in adenoma detection that exists between individual doctors, according to Dr. Kosinski, founder and chief medical officer of SonarMD and previously director of a large gastroenterology clinic. “Even if you look at doctors who do a minimum of 250 screening colonoscopies in a year, there’s still variability. There was even a study published in 2014 showing ADRs anywhere from 7.4% to 52.5%. The bell curve is broad,” he said.
As patients age, they have a higher frequency of polyps appearing on the right side of the colon, and those polyps are flatter and more easily missed than polyps on the left side. “The variation in ADR is higher on the right side of the colon than it is on the left. Doctors have to really do a very good job of examining that right side of the colon so that they don’t miss the flat polyps,” said Dr. Kosinski.
To improve ADRs, Dr. Kosinski emphasized the need to take the required time out to complete a procedure, despite the tight schedules often faced by ambulatory centers. “It’s the time you take coming out of the colon that’s critical. You owe it to the patient,” he said.
And if a patient hasn’t prepped well enough, it’s better to send the patient home without the procedure than to conduct a poor-quality screening. “If you can’t see the mucosal surface, you can’t tell the patient that they have a negative colonoscopy. If you have to do more cleaning during the procedure, then do more cleaning during the procedure. If you have to cancel the procedure and bring the patient back, it’s better to do that than it is to do an incomplete colonoscopy,” said Dr. Kosinski.
He also stressed the need to make sure that the patient is properly sedated and comfortable “so that you can do the job you’re supposed to do,” he said.
Some authors disclosed relationships with Amgen and the National Cancer Institute. Dr. Rex disclosed relationships with Olympus, Boston Scientific, Aries, and others, all outside the submitted work.
FROM JAMA
Malpractice lawyer gloats at win, then puts foot in mouth
During the closing arguments in a $10 million malpractice trial, attorney Robert McKenna III told jurors the claims against his client, a gastroenterologist, were baseless and equivalent to “extortion.” The patient’s family blamed the gastroenterologist for their father’s death, alleging the doctor perforated his colon during insertion of a feeding tube.
“I take pride in what I do, and I’ve got to tell you, in the 30 years I have been doing this, I have never seen a more insulting, factually devoid presentation in my entire career,” Mr. McKenna said, according to court transcripts. “On the strength of this evidence, they want you to award them $10 million. Welcome to America. Welcome to the personal injury machine, the personal injury industrial complex.”
After less than 30 minutes of deliberation, jurors returned a 12-0 verdict in favor of the physician.
However, Mr. McKenna, from Huntington Beach, Calif., described the case very differently to his staff in a celebration video, which he never expected to become public.
In the video, posted on Twitter and Instagram, Mr. McKenna bragged about how his legal team convinced jurors to doubt the patient’s official cause of death. He said the lawsuit involved a guy “that was probably negligently killed, but we kind of made it look like other people did it.”
“We actually had a death certificate that said he died the very way the plaintiff said he died, and we had to say, ‘No, you really shouldn’t believe what that death certificate says, or the coroner from the Orange County coroner’s office ... who says that it’s right,’” Mr. McKenna said in the video.
The 26-minute verdict was the fastest he’s ever received, Mr. McKenna says in the video, encouraging his partner to ring the firm’s victory bell.
“Overcoming all of those hurdles, we managed to sock three lawyers in the face,” Mr. McKenna said, referring to the plaintiffs’ lawyers.
The video of Mr. McKenna’s remarks is now in wide circulation after having been posted to online attorney forums, Instagram, where it’s been viewed more than 8,000 times, and Twitter, where views have reached over 3,000.
Jorge Ledezma, an Orange County, Calif., attorney who represented the patient’s family in the case, said the remarks make it appear as if Mr. McKenna tricked the jury.
“It was a drastic change from the comments he made to the jury during his closing arguments,” Mr. Ledezma said. “But the video is more important for what he doesn’t say. He doesn’t say his client did everything properly. He doesn’t say our case didn’t have any merit. He doesn’t say his client was a good doctor. Clearly, what he told the jury and what he believes are the exact opposite of each other.”
Mr. McKenna did not return multiple messages seeking comment for this story. In a statement to the LA Times, Mr. McKenna said his remarks were “intended purely as an internal briefing to our staff, using shorthand phrases which might understandably cause confusion for a lay audience unfamiliar with the case at hand, and the law in general.”
“I have expressed my apologies to my client, opposing counsel, and both the medical and legal communities,” Mr. McKenna said in the statement to the LA Times. “However, nothing about my remarks should call into question our very transparent trial strategy or the jury’s verdict in favor of my client.”
What happened to the patient?
Enrique Garcia Sanchez, 49, arrived at the critical care unit at South Coast Global Medical Center in Santa Ana, Calif., on Nov. 5, 2017, complaining of abdominal pain. He was diagnosed with acute pancreatitis, acute hypokalemia, and alcohol abuse, and transferred to the ICU, according to the family’s legal complaint.
Mr. Sanchez had a positive D-Dimer test, indicating a probable blood clot, and he appeared to be experiencing septic shock caused by pancreatitis, according to the complaint. By Nov. 17, Mr. Sanchez was suffering from respiratory failure and severe hypoxemia, and as a result, he was sedated. In addition, his abdomen was described as distended with decreased bowel sounds, according to court documents.
On. Nov. 18, a gastrointestinal specialist was consulted because of Mr. Sanchez’s prolonged intubation and oropharyngeal dysphagia, according to the lawsuit. On Nov. 21, air was leaking from Mr. Sanchez’s breathing tube with diffuse infiltration noted on the right side, and pneumonia.
Mr. Sanchez was eventually unable to swallow, and the gastroenterologist inserted a percutaneous endoscopic gastrostomy (PEG) tube, according to court records.
Mr. Sanchez’s condition worsened, and he developed respiratory distress, hypotension, and weakness during dialysis. On Dec. 9, 2017, physicians noted he had a bacterial infection, and he was later intubated on vent support because of progressive respiratory failure. Additionally, an internist reported that “fecal material” was observed per the PEG tube. Mr. Sanchez’s white blood cell count continued to rise, and his condition deteriorated. Mr. Sanchez died on Dec. 31, 2017.
A death certificate concluded that Mr. Sanchez died from complications of a PEG tube that perforated his colon, according to Mr. Ledezma. The plaintiffs’ legal team argued the gastroenterologist breached the standard of care by failing to ensure the tube was placed properly and failing to remedy the error after leakage was noted.
“Mr. Garcia died because of a misplaced PEG tube that perforated the colon, resulting in peritonitis and sepsis,” attorney Jose Robles said during his closing arguments. “Mr. Garcia had ascites, a contraindication for PEG tube placement. He had ileus, a contraindication for PEG tube placement. The standard of care requires that [the gastroenterologist] conduct a proper workup to confirm that a PEG tube placement can be done appropriately and safely.”
Mr. McKenna argued the gastroenterologist was not at fault for the patient’s death, and that complications from his pancreatitis ultimately killed him. During the trial, physicians who cared for Mr. Sanchez testified the patient had a less than 50% chance of survival.
“What he had was end-stage catastrophic [pancreatitis] that was affecting his organ system and aspiration pneumonia that made it impossible for him to try to breathe on his own,” Mr. McKenna said during closing arguments. “The man ... had a catastrophic injury that ate most of his pancreas. That is not a survivable event.”
Attorney faces backlash from legal community
Since his celebratory remarks were posted online, Mr. McKenna has faced much backlash, particularly from the legal community.
@mgvolada tweeted, “As an attorney I am revolted and I hope sanctions follow ... this is why people hate attorneys.”
@stevewieland, who identified himself as a trial lawyer, wrote he would not feel good about winning such a case.
“No wonder we get no love from the public,” he tweeted.
“Let’s see how the Court of Appeals thinks about your braggadocio and how this makes lawyers appear to the public,” tweeted @Stephen60134955, a self-identified attorney.
Mr. McKenna’s license remains active and in good standing with no disciplinary actions, according to the State Bar of California website.
Mr. Ledezma has filed a motion for a new trial, and a hearing on the motion is scheduled for Aug. 4, 2022. The motion was filed primarily because of issues during the trial, what Mr. Ledezma described as “inflammatory closing arguments,” and in small part, Mr. McKenna’s video remarks, he said.
If the motion is denied, the plaintiffs will move forward with an appeal, he said.
A version of this article first appeared on Medscape.com.
During the closing arguments in a $10 million malpractice trial, attorney Robert McKenna III told jurors the claims against his client, a gastroenterologist, were baseless and equivalent to “extortion.” The patient’s family blamed the gastroenterologist for their father’s death, alleging the doctor perforated his colon during insertion of a feeding tube.
“I take pride in what I do, and I’ve got to tell you, in the 30 years I have been doing this, I have never seen a more insulting, factually devoid presentation in my entire career,” Mr. McKenna said, according to court transcripts. “On the strength of this evidence, they want you to award them $10 million. Welcome to America. Welcome to the personal injury machine, the personal injury industrial complex.”
After less than 30 minutes of deliberation, jurors returned a 12-0 verdict in favor of the physician.
However, Mr. McKenna, from Huntington Beach, Calif., described the case very differently to his staff in a celebration video, which he never expected to become public.
In the video, posted on Twitter and Instagram, Mr. McKenna bragged about how his legal team convinced jurors to doubt the patient’s official cause of death. He said the lawsuit involved a guy “that was probably negligently killed, but we kind of made it look like other people did it.”
“We actually had a death certificate that said he died the very way the plaintiff said he died, and we had to say, ‘No, you really shouldn’t believe what that death certificate says, or the coroner from the Orange County coroner’s office ... who says that it’s right,’” Mr. McKenna said in the video.
The 26-minute verdict was the fastest he’s ever received, Mr. McKenna says in the video, encouraging his partner to ring the firm’s victory bell.
“Overcoming all of those hurdles, we managed to sock three lawyers in the face,” Mr. McKenna said, referring to the plaintiffs’ lawyers.
The video of Mr. McKenna’s remarks is now in wide circulation after having been posted to online attorney forums, Instagram, where it’s been viewed more than 8,000 times, and Twitter, where views have reached over 3,000.
Jorge Ledezma, an Orange County, Calif., attorney who represented the patient’s family in the case, said the remarks make it appear as if Mr. McKenna tricked the jury.
“It was a drastic change from the comments he made to the jury during his closing arguments,” Mr. Ledezma said. “But the video is more important for what he doesn’t say. He doesn’t say his client did everything properly. He doesn’t say our case didn’t have any merit. He doesn’t say his client was a good doctor. Clearly, what he told the jury and what he believes are the exact opposite of each other.”
Mr. McKenna did not return multiple messages seeking comment for this story. In a statement to the LA Times, Mr. McKenna said his remarks were “intended purely as an internal briefing to our staff, using shorthand phrases which might understandably cause confusion for a lay audience unfamiliar with the case at hand, and the law in general.”
“I have expressed my apologies to my client, opposing counsel, and both the medical and legal communities,” Mr. McKenna said in the statement to the LA Times. “However, nothing about my remarks should call into question our very transparent trial strategy or the jury’s verdict in favor of my client.”
What happened to the patient?
Enrique Garcia Sanchez, 49, arrived at the critical care unit at South Coast Global Medical Center in Santa Ana, Calif., on Nov. 5, 2017, complaining of abdominal pain. He was diagnosed with acute pancreatitis, acute hypokalemia, and alcohol abuse, and transferred to the ICU, according to the family’s legal complaint.
Mr. Sanchez had a positive D-Dimer test, indicating a probable blood clot, and he appeared to be experiencing septic shock caused by pancreatitis, according to the complaint. By Nov. 17, Mr. Sanchez was suffering from respiratory failure and severe hypoxemia, and as a result, he was sedated. In addition, his abdomen was described as distended with decreased bowel sounds, according to court documents.
On. Nov. 18, a gastrointestinal specialist was consulted because of Mr. Sanchez’s prolonged intubation and oropharyngeal dysphagia, according to the lawsuit. On Nov. 21, air was leaking from Mr. Sanchez’s breathing tube with diffuse infiltration noted on the right side, and pneumonia.
Mr. Sanchez was eventually unable to swallow, and the gastroenterologist inserted a percutaneous endoscopic gastrostomy (PEG) tube, according to court records.
Mr. Sanchez’s condition worsened, and he developed respiratory distress, hypotension, and weakness during dialysis. On Dec. 9, 2017, physicians noted he had a bacterial infection, and he was later intubated on vent support because of progressive respiratory failure. Additionally, an internist reported that “fecal material” was observed per the PEG tube. Mr. Sanchez’s white blood cell count continued to rise, and his condition deteriorated. Mr. Sanchez died on Dec. 31, 2017.
A death certificate concluded that Mr. Sanchez died from complications of a PEG tube that perforated his colon, according to Mr. Ledezma. The plaintiffs’ legal team argued the gastroenterologist breached the standard of care by failing to ensure the tube was placed properly and failing to remedy the error after leakage was noted.
“Mr. Garcia died because of a misplaced PEG tube that perforated the colon, resulting in peritonitis and sepsis,” attorney Jose Robles said during his closing arguments. “Mr. Garcia had ascites, a contraindication for PEG tube placement. He had ileus, a contraindication for PEG tube placement. The standard of care requires that [the gastroenterologist] conduct a proper workup to confirm that a PEG tube placement can be done appropriately and safely.”
Mr. McKenna argued the gastroenterologist was not at fault for the patient’s death, and that complications from his pancreatitis ultimately killed him. During the trial, physicians who cared for Mr. Sanchez testified the patient had a less than 50% chance of survival.
“What he had was end-stage catastrophic [pancreatitis] that was affecting his organ system and aspiration pneumonia that made it impossible for him to try to breathe on his own,” Mr. McKenna said during closing arguments. “The man ... had a catastrophic injury that ate most of his pancreas. That is not a survivable event.”
Attorney faces backlash from legal community
Since his celebratory remarks were posted online, Mr. McKenna has faced much backlash, particularly from the legal community.
@mgvolada tweeted, “As an attorney I am revolted and I hope sanctions follow ... this is why people hate attorneys.”
@stevewieland, who identified himself as a trial lawyer, wrote he would not feel good about winning such a case.
“No wonder we get no love from the public,” he tweeted.
“Let’s see how the Court of Appeals thinks about your braggadocio and how this makes lawyers appear to the public,” tweeted @Stephen60134955, a self-identified attorney.
Mr. McKenna’s license remains active and in good standing with no disciplinary actions, according to the State Bar of California website.
Mr. Ledezma has filed a motion for a new trial, and a hearing on the motion is scheduled for Aug. 4, 2022. The motion was filed primarily because of issues during the trial, what Mr. Ledezma described as “inflammatory closing arguments,” and in small part, Mr. McKenna’s video remarks, he said.
If the motion is denied, the plaintiffs will move forward with an appeal, he said.
A version of this article first appeared on Medscape.com.
During the closing arguments in a $10 million malpractice trial, attorney Robert McKenna III told jurors the claims against his client, a gastroenterologist, were baseless and equivalent to “extortion.” The patient’s family blamed the gastroenterologist for their father’s death, alleging the doctor perforated his colon during insertion of a feeding tube.
“I take pride in what I do, and I’ve got to tell you, in the 30 years I have been doing this, I have never seen a more insulting, factually devoid presentation in my entire career,” Mr. McKenna said, according to court transcripts. “On the strength of this evidence, they want you to award them $10 million. Welcome to America. Welcome to the personal injury machine, the personal injury industrial complex.”
After less than 30 minutes of deliberation, jurors returned a 12-0 verdict in favor of the physician.
However, Mr. McKenna, from Huntington Beach, Calif., described the case very differently to his staff in a celebration video, which he never expected to become public.
In the video, posted on Twitter and Instagram, Mr. McKenna bragged about how his legal team convinced jurors to doubt the patient’s official cause of death. He said the lawsuit involved a guy “that was probably negligently killed, but we kind of made it look like other people did it.”
“We actually had a death certificate that said he died the very way the plaintiff said he died, and we had to say, ‘No, you really shouldn’t believe what that death certificate says, or the coroner from the Orange County coroner’s office ... who says that it’s right,’” Mr. McKenna said in the video.
The 26-minute verdict was the fastest he’s ever received, Mr. McKenna says in the video, encouraging his partner to ring the firm’s victory bell.
“Overcoming all of those hurdles, we managed to sock three lawyers in the face,” Mr. McKenna said, referring to the plaintiffs’ lawyers.
The video of Mr. McKenna’s remarks is now in wide circulation after having been posted to online attorney forums, Instagram, where it’s been viewed more than 8,000 times, and Twitter, where views have reached over 3,000.
Jorge Ledezma, an Orange County, Calif., attorney who represented the patient’s family in the case, said the remarks make it appear as if Mr. McKenna tricked the jury.
“It was a drastic change from the comments he made to the jury during his closing arguments,” Mr. Ledezma said. “But the video is more important for what he doesn’t say. He doesn’t say his client did everything properly. He doesn’t say our case didn’t have any merit. He doesn’t say his client was a good doctor. Clearly, what he told the jury and what he believes are the exact opposite of each other.”
Mr. McKenna did not return multiple messages seeking comment for this story. In a statement to the LA Times, Mr. McKenna said his remarks were “intended purely as an internal briefing to our staff, using shorthand phrases which might understandably cause confusion for a lay audience unfamiliar with the case at hand, and the law in general.”
“I have expressed my apologies to my client, opposing counsel, and both the medical and legal communities,” Mr. McKenna said in the statement to the LA Times. “However, nothing about my remarks should call into question our very transparent trial strategy or the jury’s verdict in favor of my client.”
What happened to the patient?
Enrique Garcia Sanchez, 49, arrived at the critical care unit at South Coast Global Medical Center in Santa Ana, Calif., on Nov. 5, 2017, complaining of abdominal pain. He was diagnosed with acute pancreatitis, acute hypokalemia, and alcohol abuse, and transferred to the ICU, according to the family’s legal complaint.
Mr. Sanchez had a positive D-Dimer test, indicating a probable blood clot, and he appeared to be experiencing septic shock caused by pancreatitis, according to the complaint. By Nov. 17, Mr. Sanchez was suffering from respiratory failure and severe hypoxemia, and as a result, he was sedated. In addition, his abdomen was described as distended with decreased bowel sounds, according to court documents.
On. Nov. 18, a gastrointestinal specialist was consulted because of Mr. Sanchez’s prolonged intubation and oropharyngeal dysphagia, according to the lawsuit. On Nov. 21, air was leaking from Mr. Sanchez’s breathing tube with diffuse infiltration noted on the right side, and pneumonia.
Mr. Sanchez was eventually unable to swallow, and the gastroenterologist inserted a percutaneous endoscopic gastrostomy (PEG) tube, according to court records.
Mr. Sanchez’s condition worsened, and he developed respiratory distress, hypotension, and weakness during dialysis. On Dec. 9, 2017, physicians noted he had a bacterial infection, and he was later intubated on vent support because of progressive respiratory failure. Additionally, an internist reported that “fecal material” was observed per the PEG tube. Mr. Sanchez’s white blood cell count continued to rise, and his condition deteriorated. Mr. Sanchez died on Dec. 31, 2017.
A death certificate concluded that Mr. Sanchez died from complications of a PEG tube that perforated his colon, according to Mr. Ledezma. The plaintiffs’ legal team argued the gastroenterologist breached the standard of care by failing to ensure the tube was placed properly and failing to remedy the error after leakage was noted.
“Mr. Garcia died because of a misplaced PEG tube that perforated the colon, resulting in peritonitis and sepsis,” attorney Jose Robles said during his closing arguments. “Mr. Garcia had ascites, a contraindication for PEG tube placement. He had ileus, a contraindication for PEG tube placement. The standard of care requires that [the gastroenterologist] conduct a proper workup to confirm that a PEG tube placement can be done appropriately and safely.”
Mr. McKenna argued the gastroenterologist was not at fault for the patient’s death, and that complications from his pancreatitis ultimately killed him. During the trial, physicians who cared for Mr. Sanchez testified the patient had a less than 50% chance of survival.
“What he had was end-stage catastrophic [pancreatitis] that was affecting his organ system and aspiration pneumonia that made it impossible for him to try to breathe on his own,” Mr. McKenna said during closing arguments. “The man ... had a catastrophic injury that ate most of his pancreas. That is not a survivable event.”
Attorney faces backlash from legal community
Since his celebratory remarks were posted online, Mr. McKenna has faced much backlash, particularly from the legal community.
@mgvolada tweeted, “As an attorney I am revolted and I hope sanctions follow ... this is why people hate attorneys.”
@stevewieland, who identified himself as a trial lawyer, wrote he would not feel good about winning such a case.
“No wonder we get no love from the public,” he tweeted.
“Let’s see how the Court of Appeals thinks about your braggadocio and how this makes lawyers appear to the public,” tweeted @Stephen60134955, a self-identified attorney.
Mr. McKenna’s license remains active and in good standing with no disciplinary actions, according to the State Bar of California website.
Mr. Ledezma has filed a motion for a new trial, and a hearing on the motion is scheduled for Aug. 4, 2022. The motion was filed primarily because of issues during the trial, what Mr. Ledezma described as “inflammatory closing arguments,” and in small part, Mr. McKenna’s video remarks, he said.
If the motion is denied, the plaintiffs will move forward with an appeal, he said.
A version of this article first appeared on Medscape.com.
Ezetimibe plus statin: Attractive bypass to high-dose monotherapy
More patients with established atherosclerotic cardiovascular disease (ASCVD) achieved a low-density lipoprotein (LDL) cholesterol of less than 70 mg/dL, and fewer discontinued treatment with ezetimibe plus a moderate-dose statin, than did those on high-intensity statin monotherapy, a noninferiority trial shows.
While it’s now established that drug combinations can achieve better efficacy with lower risks than high-dose monotherapy, the study is the first to show the benefits of the strategy for ASCVD in a randomized trial with long-term follow-up.
The primary endpoint – 3-year composite of cardiovascular death, major cardiovascular events, or nonfatal stroke – occurred in about 9% of patients in each group, showing non-inferiority.
Furthermore, the authors suggest that ezetimibe combination therapy be considered earlier in the treatment of those at high risk of adverse events, rather than doubling the statin dose.
The study was published online in The Lancet.
Less intolerance, less discontinuations
The open-label study, dubbed RACING, randomized 3,780 patients with ASCVD to receive moderate-intensity rosuvastatin 10 mg plus ezetimibe 10 mg or high-intensity 20 mg rosuvastatin monotherapy. Participants’ average age was 64 and 75% were men.
The primary endpoint occurred in 9.1% of patients in the combination therapy group and 9.9% in the high-intensity monotherapy group. The absolute between-group difference was −0.78% (90% confidence interval [CI], −2.39 to 0.83), well below the 2% noninferiority margin.
In the combination therapy group, LDL cholesterol concentrations of less than 70 mg/dL were achieved in 73% of patients at 1 year, 75% at 2 years, and 72% at 3 years. By contrast, in the monotherapy group, the lower concentrations were seen in 55% at 1 year, 60% at 2 years, and 58% at 3 years.
Further, a post hoc analysis showed LDL concentrations of less than 55 mg/dL at 1, 2, and 3 years in 42%, 45%, and 42% of patients in the combination therapy group versus 25%, 29%, and 25% of those in the high-intensity statin monotherapy group.
Eighty-eight patients (4.8%) on combination therapy discontinued medication or received a dose reduction, versus 150 patients (8.2%) on monotherapy.
Rates of myonecrosis were similar in the combination therapy and high-intensity statin groups (11 vs. 13), whereas myalgia was more common with high-intensity statins (29 vs. 17). The open-label design could have led to bias in reporting of patient symptoms, the authors noted. All clinical events, however, were adjudicated by an independent committee masked to treatment assignment.
There might be “some level of difference” when extending the findings to other populations because the trial involved only Koreans, coauthor Myeong-Ki Hong, MD, Yonsei University, Seoul, South Korea, acknowledged in response to a query from this news organization. He thinks the findings can be applied broadly nonetheless, and his team is currently investigating whether certain patients might benefit more than others from the combination.
Various options for patients
“The field of hypertension changed [its] guidelines almost 20 years ago to consider the initial use of combination therapy in hard-to-treat patients,” Christie Mitchell Ballantyne, MD, Baylor College of Medicine, Houston, said in an interview. He coauthored an accompanying editorial with Baylor colleague Layla A. Abushamat, MD.
“We now have enough evidence of the efficacy and safety of combination therapy to consider early initiation of this approach in patients with challenging lipid disorders who are at increased risk of ASCVD events,” affirmed Dr. Ballantyne.
“This study reinforces important principles in the management and secondary prevention of cardiovascular disease, namely that LDL reduction and associated risk reduction can be achieved in various ways,” said Daniel Muñoz, MD, MPA, executive medical director of the Vanderbilt Heart & Vascular Institute, Vanderbilt University Medical Center, Nashville, Tenn.
However, he noted, “The high-intensity statin dose used as a comparator in this study was rosuvastatin 20 mg. In clinical practice, we often target maximally aggressive reduction of LDL via higher doses – that is, rosuvastatin 40 mg or atorvastatin 80 mg.”
The bottom line, said Dr. Muñoz, who was not involved in the study: “There are different ways to achieve LDL-lowering and associated risk reduction in patients with CVD. For patients who warrant but might not tolerate high-intensity statin therapy, this study supports the use of a moderate-intensity statin in combination with ezetimibe.”
The study was funded by Hanmi Pharmaceutical, Seoul, South Korea. One study coauthor received an institutional research grant from the company. No other authors reported relevant financial relationships, nor did Dr. Ballantyne, Dr. Abushamat, or Dr. Muñoz.
A version of this article first appeared on Medscape.com.
More patients with established atherosclerotic cardiovascular disease (ASCVD) achieved a low-density lipoprotein (LDL) cholesterol of less than 70 mg/dL, and fewer discontinued treatment with ezetimibe plus a moderate-dose statin, than did those on high-intensity statin monotherapy, a noninferiority trial shows.
While it’s now established that drug combinations can achieve better efficacy with lower risks than high-dose monotherapy, the study is the first to show the benefits of the strategy for ASCVD in a randomized trial with long-term follow-up.
The primary endpoint – 3-year composite of cardiovascular death, major cardiovascular events, or nonfatal stroke – occurred in about 9% of patients in each group, showing non-inferiority.
Furthermore, the authors suggest that ezetimibe combination therapy be considered earlier in the treatment of those at high risk of adverse events, rather than doubling the statin dose.
The study was published online in The Lancet.
Less intolerance, less discontinuations
The open-label study, dubbed RACING, randomized 3,780 patients with ASCVD to receive moderate-intensity rosuvastatin 10 mg plus ezetimibe 10 mg or high-intensity 20 mg rosuvastatin monotherapy. Participants’ average age was 64 and 75% were men.
The primary endpoint occurred in 9.1% of patients in the combination therapy group and 9.9% in the high-intensity monotherapy group. The absolute between-group difference was −0.78% (90% confidence interval [CI], −2.39 to 0.83), well below the 2% noninferiority margin.
In the combination therapy group, LDL cholesterol concentrations of less than 70 mg/dL were achieved in 73% of patients at 1 year, 75% at 2 years, and 72% at 3 years. By contrast, in the monotherapy group, the lower concentrations were seen in 55% at 1 year, 60% at 2 years, and 58% at 3 years.
Further, a post hoc analysis showed LDL concentrations of less than 55 mg/dL at 1, 2, and 3 years in 42%, 45%, and 42% of patients in the combination therapy group versus 25%, 29%, and 25% of those in the high-intensity statin monotherapy group.
Eighty-eight patients (4.8%) on combination therapy discontinued medication or received a dose reduction, versus 150 patients (8.2%) on monotherapy.
Rates of myonecrosis were similar in the combination therapy and high-intensity statin groups (11 vs. 13), whereas myalgia was more common with high-intensity statins (29 vs. 17). The open-label design could have led to bias in reporting of patient symptoms, the authors noted. All clinical events, however, were adjudicated by an independent committee masked to treatment assignment.
There might be “some level of difference” when extending the findings to other populations because the trial involved only Koreans, coauthor Myeong-Ki Hong, MD, Yonsei University, Seoul, South Korea, acknowledged in response to a query from this news organization. He thinks the findings can be applied broadly nonetheless, and his team is currently investigating whether certain patients might benefit more than others from the combination.
Various options for patients
“The field of hypertension changed [its] guidelines almost 20 years ago to consider the initial use of combination therapy in hard-to-treat patients,” Christie Mitchell Ballantyne, MD, Baylor College of Medicine, Houston, said in an interview. He coauthored an accompanying editorial with Baylor colleague Layla A. Abushamat, MD.
“We now have enough evidence of the efficacy and safety of combination therapy to consider early initiation of this approach in patients with challenging lipid disorders who are at increased risk of ASCVD events,” affirmed Dr. Ballantyne.
“This study reinforces important principles in the management and secondary prevention of cardiovascular disease, namely that LDL reduction and associated risk reduction can be achieved in various ways,” said Daniel Muñoz, MD, MPA, executive medical director of the Vanderbilt Heart & Vascular Institute, Vanderbilt University Medical Center, Nashville, Tenn.
However, he noted, “The high-intensity statin dose used as a comparator in this study was rosuvastatin 20 mg. In clinical practice, we often target maximally aggressive reduction of LDL via higher doses – that is, rosuvastatin 40 mg or atorvastatin 80 mg.”
The bottom line, said Dr. Muñoz, who was not involved in the study: “There are different ways to achieve LDL-lowering and associated risk reduction in patients with CVD. For patients who warrant but might not tolerate high-intensity statin therapy, this study supports the use of a moderate-intensity statin in combination with ezetimibe.”
The study was funded by Hanmi Pharmaceutical, Seoul, South Korea. One study coauthor received an institutional research grant from the company. No other authors reported relevant financial relationships, nor did Dr. Ballantyne, Dr. Abushamat, or Dr. Muñoz.
A version of this article first appeared on Medscape.com.
More patients with established atherosclerotic cardiovascular disease (ASCVD) achieved a low-density lipoprotein (LDL) cholesterol of less than 70 mg/dL, and fewer discontinued treatment with ezetimibe plus a moderate-dose statin, than did those on high-intensity statin monotherapy, a noninferiority trial shows.
While it’s now established that drug combinations can achieve better efficacy with lower risks than high-dose monotherapy, the study is the first to show the benefits of the strategy for ASCVD in a randomized trial with long-term follow-up.
The primary endpoint – 3-year composite of cardiovascular death, major cardiovascular events, or nonfatal stroke – occurred in about 9% of patients in each group, showing non-inferiority.
Furthermore, the authors suggest that ezetimibe combination therapy be considered earlier in the treatment of those at high risk of adverse events, rather than doubling the statin dose.
The study was published online in The Lancet.
Less intolerance, less discontinuations
The open-label study, dubbed RACING, randomized 3,780 patients with ASCVD to receive moderate-intensity rosuvastatin 10 mg plus ezetimibe 10 mg or high-intensity 20 mg rosuvastatin monotherapy. Participants’ average age was 64 and 75% were men.
The primary endpoint occurred in 9.1% of patients in the combination therapy group and 9.9% in the high-intensity monotherapy group. The absolute between-group difference was −0.78% (90% confidence interval [CI], −2.39 to 0.83), well below the 2% noninferiority margin.
In the combination therapy group, LDL cholesterol concentrations of less than 70 mg/dL were achieved in 73% of patients at 1 year, 75% at 2 years, and 72% at 3 years. By contrast, in the monotherapy group, the lower concentrations were seen in 55% at 1 year, 60% at 2 years, and 58% at 3 years.
Further, a post hoc analysis showed LDL concentrations of less than 55 mg/dL at 1, 2, and 3 years in 42%, 45%, and 42% of patients in the combination therapy group versus 25%, 29%, and 25% of those in the high-intensity statin monotherapy group.
Eighty-eight patients (4.8%) on combination therapy discontinued medication or received a dose reduction, versus 150 patients (8.2%) on monotherapy.
Rates of myonecrosis were similar in the combination therapy and high-intensity statin groups (11 vs. 13), whereas myalgia was more common with high-intensity statins (29 vs. 17). The open-label design could have led to bias in reporting of patient symptoms, the authors noted. All clinical events, however, were adjudicated by an independent committee masked to treatment assignment.
There might be “some level of difference” when extending the findings to other populations because the trial involved only Koreans, coauthor Myeong-Ki Hong, MD, Yonsei University, Seoul, South Korea, acknowledged in response to a query from this news organization. He thinks the findings can be applied broadly nonetheless, and his team is currently investigating whether certain patients might benefit more than others from the combination.
Various options for patients
“The field of hypertension changed [its] guidelines almost 20 years ago to consider the initial use of combination therapy in hard-to-treat patients,” Christie Mitchell Ballantyne, MD, Baylor College of Medicine, Houston, said in an interview. He coauthored an accompanying editorial with Baylor colleague Layla A. Abushamat, MD.
“We now have enough evidence of the efficacy and safety of combination therapy to consider early initiation of this approach in patients with challenging lipid disorders who are at increased risk of ASCVD events,” affirmed Dr. Ballantyne.
“This study reinforces important principles in the management and secondary prevention of cardiovascular disease, namely that LDL reduction and associated risk reduction can be achieved in various ways,” said Daniel Muñoz, MD, MPA, executive medical director of the Vanderbilt Heart & Vascular Institute, Vanderbilt University Medical Center, Nashville, Tenn.
However, he noted, “The high-intensity statin dose used as a comparator in this study was rosuvastatin 20 mg. In clinical practice, we often target maximally aggressive reduction of LDL via higher doses – that is, rosuvastatin 40 mg or atorvastatin 80 mg.”
The bottom line, said Dr. Muñoz, who was not involved in the study: “There are different ways to achieve LDL-lowering and associated risk reduction in patients with CVD. For patients who warrant but might not tolerate high-intensity statin therapy, this study supports the use of a moderate-intensity statin in combination with ezetimibe.”
The study was funded by Hanmi Pharmaceutical, Seoul, South Korea. One study coauthor received an institutional research grant from the company. No other authors reported relevant financial relationships, nor did Dr. Ballantyne, Dr. Abushamat, or Dr. Muñoz.
A version of this article first appeared on Medscape.com.
Scientists aim to combat COVID with a shot in the nose
Scientists seeking to stay ahead of an evolving SARS-Cov-2 virus are looking at new strategies, including developing intranasal vaccines, according to speakers at a conference on July 26.
inviting researchers to provide a public update on efforts to try to keep ahead of SARS-CoV-2.
Scientists and federal officials are looking to build on the successes seen in developing the original crop of COVID vaccines, which were authorized for use in the United States less than a year after the pandemic took hold.
But emerging variants are eroding these gains. For months now, officials at the Centers for Disease Control and Prevention and Food and Drug Administration have been keeping an eye on how the level of effectiveness of COVID vaccines has waned during the rise of the Omicron strain. And there’s continual concern about how SARS-CoV-2 might evolve over time.
“Our vaccines are terrific,” Ashish K. Jha, MD, the White House’s COVID-19 response coordinator, said at the summit. “[But] we have to do better.”
Among the approaches being considered are vaccines that would be applied intranasally, with the idea that this might be able to boost the immune response to SARS-CoV-2.
At the summit, Akiko Iwasaki, PhD, of Yale University, New Haven, Conn., said the intranasal approach might be helpful in preventing transmission as well as reducing the burden of illness for those who are infected with SARS-CoV-2.
“We’re stopping the virus from spreading right at the border,” Dr. Iwasaki said at the summit. “This is akin to putting a guard outside of the house in order to patrol for invaders compared to putting the guards in the hallway of the building in the hope that they capture the invader.”
Dr. Iwasaki is one of the founders of Xanadu Bio, a private company created last year to focus on ways to kill SARS-CoV-2 in the nasosinus before it spreads deeper into the respiratory tract. In an editorial in Science Immunology, Dr. Iwasaki and Eric J. Topol, MD, director of the Scripps Research Translational Institute, urged greater federal investment in this approach to fighting SARS-CoV-2. (Dr. Topol is editor-in-chief of Medscape.)
Titled “Operation Nasal Vaccine – Lightning speed to counter COVID-19,” their editorial noted the “unprecedented success” seen in the rapid development of the first two mRNA shots. Dr. Iwasaki and Dr. Topol noted that these victories had been “fueled by the $10 billion governmental investment in Operation Warp Speed.
“During the first year of the pandemic, meaningful evolution of the virus was slow-paced, without any functional consequences, but since that time we have seen a succession of important variants of concern, with increasing transmissibility and immune evasion, culminating in the Omicron lineages,” wrote Dr. Iwasaki and Dr. Topol.
Recent developments have “spotlighted the possibility of nasal vaccines, with their allure for achieving mucosal immunity, complementing, and likely bolstering the circulating immunity achieved via intramuscular shots,” they added.
An early setback
Scientists at the National Institutes of Health and the Biomedical Advanced Research and Development Authority (BARDA) have for some time been looking to vet an array of next-generation vaccine concepts, including ones that trigger mucosal immunity, the Washington Post reported in April.
At the summit on July 26, several participants, including Dr. Jha, stressed the role that public-private partnerships were key to the rapid development of the initial COVID vaccines. They said continued U.S. government support will be needed to make advances in this field.
One of the presenters, Biao He, PhD, founder and president of CyanVac and Blue Lake Biotechnology, spoke of the federal support that his efforts have received over the years to develop intranasal vaccines. His Georgia-based firm already has an experimental intranasal vaccine candidate, CVXGA1-001, in phase 1 testing (NCT04954287).
The CVXGA-001 builds on technology already used in a veterinary product, an intranasal vaccine long used to prevent kennel cough in dogs, he said at the summit.
The emerging field of experimental intranasal COVID vaccines already has had at least one setback.
The biotech firm Altimmune in June 2021 announced that it would discontinue development of its experimental intranasal AdCOVID vaccine following disappointing phase 1 results. The vaccine appeared to be well tolerated in the test, but the immunogenicity data demonstrated lower than expected results in healthy volunteers, especially in light of the responses seen to already cleared vaccines, Altimmune said in a release.
In the statement, Scot Roberts, PhD, chief scientific officer at Altimmune, noted that the study participants lacked immunity from prior infection or vaccination. “We believe that prior immunity in humans may be important for a robust immune response to intranasal dosing with AdCOVID,” he said.
At the summit, Marty Moore, PhD, cofounder and chief scientific officer for Redwood City, Calif.–based Meissa Vaccines, noted the challenges that remain ahead for intranasal COVID vaccines, while also highlighting what he sees as the potential of this approach.
Meissa also has advanced an experimental intranasal COVID vaccine as far as phase 1 testing (NCT04798001).
“No one here today can tell you that mucosal COVID vaccines work. We’re not there yet. We need clinical efficacy data to answer that question,” Dr. Moore said.
But there’s a potential for a “knockout blow to COVID, a transmission-blocking vaccine” from the intranasal approach, he said.
“The virus is mutating faster than our ability to manage vaccines and not enough people are getting boosters. These injectable vaccines do a great job of preventing severe disease, but they do little to prevent infection” from spreading, Dr. Moore said.
A version of this article first appeared on Medscape.com.
Scientists seeking to stay ahead of an evolving SARS-Cov-2 virus are looking at new strategies, including developing intranasal vaccines, according to speakers at a conference on July 26.
inviting researchers to provide a public update on efforts to try to keep ahead of SARS-CoV-2.
Scientists and federal officials are looking to build on the successes seen in developing the original crop of COVID vaccines, which were authorized for use in the United States less than a year after the pandemic took hold.
But emerging variants are eroding these gains. For months now, officials at the Centers for Disease Control and Prevention and Food and Drug Administration have been keeping an eye on how the level of effectiveness of COVID vaccines has waned during the rise of the Omicron strain. And there’s continual concern about how SARS-CoV-2 might evolve over time.
“Our vaccines are terrific,” Ashish K. Jha, MD, the White House’s COVID-19 response coordinator, said at the summit. “[But] we have to do better.”
Among the approaches being considered are vaccines that would be applied intranasally, with the idea that this might be able to boost the immune response to SARS-CoV-2.
At the summit, Akiko Iwasaki, PhD, of Yale University, New Haven, Conn., said the intranasal approach might be helpful in preventing transmission as well as reducing the burden of illness for those who are infected with SARS-CoV-2.
“We’re stopping the virus from spreading right at the border,” Dr. Iwasaki said at the summit. “This is akin to putting a guard outside of the house in order to patrol for invaders compared to putting the guards in the hallway of the building in the hope that they capture the invader.”
Dr. Iwasaki is one of the founders of Xanadu Bio, a private company created last year to focus on ways to kill SARS-CoV-2 in the nasosinus before it spreads deeper into the respiratory tract. In an editorial in Science Immunology, Dr. Iwasaki and Eric J. Topol, MD, director of the Scripps Research Translational Institute, urged greater federal investment in this approach to fighting SARS-CoV-2. (Dr. Topol is editor-in-chief of Medscape.)
Titled “Operation Nasal Vaccine – Lightning speed to counter COVID-19,” their editorial noted the “unprecedented success” seen in the rapid development of the first two mRNA shots. Dr. Iwasaki and Dr. Topol noted that these victories had been “fueled by the $10 billion governmental investment in Operation Warp Speed.
“During the first year of the pandemic, meaningful evolution of the virus was slow-paced, without any functional consequences, but since that time we have seen a succession of important variants of concern, with increasing transmissibility and immune evasion, culminating in the Omicron lineages,” wrote Dr. Iwasaki and Dr. Topol.
Recent developments have “spotlighted the possibility of nasal vaccines, with their allure for achieving mucosal immunity, complementing, and likely bolstering the circulating immunity achieved via intramuscular shots,” they added.
An early setback
Scientists at the National Institutes of Health and the Biomedical Advanced Research and Development Authority (BARDA) have for some time been looking to vet an array of next-generation vaccine concepts, including ones that trigger mucosal immunity, the Washington Post reported in April.
At the summit on July 26, several participants, including Dr. Jha, stressed the role that public-private partnerships were key to the rapid development of the initial COVID vaccines. They said continued U.S. government support will be needed to make advances in this field.
One of the presenters, Biao He, PhD, founder and president of CyanVac and Blue Lake Biotechnology, spoke of the federal support that his efforts have received over the years to develop intranasal vaccines. His Georgia-based firm already has an experimental intranasal vaccine candidate, CVXGA1-001, in phase 1 testing (NCT04954287).
The CVXGA-001 builds on technology already used in a veterinary product, an intranasal vaccine long used to prevent kennel cough in dogs, he said at the summit.
The emerging field of experimental intranasal COVID vaccines already has had at least one setback.
The biotech firm Altimmune in June 2021 announced that it would discontinue development of its experimental intranasal AdCOVID vaccine following disappointing phase 1 results. The vaccine appeared to be well tolerated in the test, but the immunogenicity data demonstrated lower than expected results in healthy volunteers, especially in light of the responses seen to already cleared vaccines, Altimmune said in a release.
In the statement, Scot Roberts, PhD, chief scientific officer at Altimmune, noted that the study participants lacked immunity from prior infection or vaccination. “We believe that prior immunity in humans may be important for a robust immune response to intranasal dosing with AdCOVID,” he said.
At the summit, Marty Moore, PhD, cofounder and chief scientific officer for Redwood City, Calif.–based Meissa Vaccines, noted the challenges that remain ahead for intranasal COVID vaccines, while also highlighting what he sees as the potential of this approach.
Meissa also has advanced an experimental intranasal COVID vaccine as far as phase 1 testing (NCT04798001).
“No one here today can tell you that mucosal COVID vaccines work. We’re not there yet. We need clinical efficacy data to answer that question,” Dr. Moore said.
But there’s a potential for a “knockout blow to COVID, a transmission-blocking vaccine” from the intranasal approach, he said.
“The virus is mutating faster than our ability to manage vaccines and not enough people are getting boosters. These injectable vaccines do a great job of preventing severe disease, but they do little to prevent infection” from spreading, Dr. Moore said.
A version of this article first appeared on Medscape.com.
Scientists seeking to stay ahead of an evolving SARS-Cov-2 virus are looking at new strategies, including developing intranasal vaccines, according to speakers at a conference on July 26.
inviting researchers to provide a public update on efforts to try to keep ahead of SARS-CoV-2.
Scientists and federal officials are looking to build on the successes seen in developing the original crop of COVID vaccines, which were authorized for use in the United States less than a year after the pandemic took hold.
But emerging variants are eroding these gains. For months now, officials at the Centers for Disease Control and Prevention and Food and Drug Administration have been keeping an eye on how the level of effectiveness of COVID vaccines has waned during the rise of the Omicron strain. And there’s continual concern about how SARS-CoV-2 might evolve over time.
“Our vaccines are terrific,” Ashish K. Jha, MD, the White House’s COVID-19 response coordinator, said at the summit. “[But] we have to do better.”
Among the approaches being considered are vaccines that would be applied intranasally, with the idea that this might be able to boost the immune response to SARS-CoV-2.
At the summit, Akiko Iwasaki, PhD, of Yale University, New Haven, Conn., said the intranasal approach might be helpful in preventing transmission as well as reducing the burden of illness for those who are infected with SARS-CoV-2.
“We’re stopping the virus from spreading right at the border,” Dr. Iwasaki said at the summit. “This is akin to putting a guard outside of the house in order to patrol for invaders compared to putting the guards in the hallway of the building in the hope that they capture the invader.”
Dr. Iwasaki is one of the founders of Xanadu Bio, a private company created last year to focus on ways to kill SARS-CoV-2 in the nasosinus before it spreads deeper into the respiratory tract. In an editorial in Science Immunology, Dr. Iwasaki and Eric J. Topol, MD, director of the Scripps Research Translational Institute, urged greater federal investment in this approach to fighting SARS-CoV-2. (Dr. Topol is editor-in-chief of Medscape.)
Titled “Operation Nasal Vaccine – Lightning speed to counter COVID-19,” their editorial noted the “unprecedented success” seen in the rapid development of the first two mRNA shots. Dr. Iwasaki and Dr. Topol noted that these victories had been “fueled by the $10 billion governmental investment in Operation Warp Speed.
“During the first year of the pandemic, meaningful evolution of the virus was slow-paced, without any functional consequences, but since that time we have seen a succession of important variants of concern, with increasing transmissibility and immune evasion, culminating in the Omicron lineages,” wrote Dr. Iwasaki and Dr. Topol.
Recent developments have “spotlighted the possibility of nasal vaccines, with their allure for achieving mucosal immunity, complementing, and likely bolstering the circulating immunity achieved via intramuscular shots,” they added.
An early setback
Scientists at the National Institutes of Health and the Biomedical Advanced Research and Development Authority (BARDA) have for some time been looking to vet an array of next-generation vaccine concepts, including ones that trigger mucosal immunity, the Washington Post reported in April.
At the summit on July 26, several participants, including Dr. Jha, stressed the role that public-private partnerships were key to the rapid development of the initial COVID vaccines. They said continued U.S. government support will be needed to make advances in this field.
One of the presenters, Biao He, PhD, founder and president of CyanVac and Blue Lake Biotechnology, spoke of the federal support that his efforts have received over the years to develop intranasal vaccines. His Georgia-based firm already has an experimental intranasal vaccine candidate, CVXGA1-001, in phase 1 testing (NCT04954287).
The CVXGA-001 builds on technology already used in a veterinary product, an intranasal vaccine long used to prevent kennel cough in dogs, he said at the summit.
The emerging field of experimental intranasal COVID vaccines already has had at least one setback.
The biotech firm Altimmune in June 2021 announced that it would discontinue development of its experimental intranasal AdCOVID vaccine following disappointing phase 1 results. The vaccine appeared to be well tolerated in the test, but the immunogenicity data demonstrated lower than expected results in healthy volunteers, especially in light of the responses seen to already cleared vaccines, Altimmune said in a release.
In the statement, Scot Roberts, PhD, chief scientific officer at Altimmune, noted that the study participants lacked immunity from prior infection or vaccination. “We believe that prior immunity in humans may be important for a robust immune response to intranasal dosing with AdCOVID,” he said.
At the summit, Marty Moore, PhD, cofounder and chief scientific officer for Redwood City, Calif.–based Meissa Vaccines, noted the challenges that remain ahead for intranasal COVID vaccines, while also highlighting what he sees as the potential of this approach.
Meissa also has advanced an experimental intranasal COVID vaccine as far as phase 1 testing (NCT04798001).
“No one here today can tell you that mucosal COVID vaccines work. We’re not there yet. We need clinical efficacy data to answer that question,” Dr. Moore said.
But there’s a potential for a “knockout blow to COVID, a transmission-blocking vaccine” from the intranasal approach, he said.
“The virus is mutating faster than our ability to manage vaccines and not enough people are getting boosters. These injectable vaccines do a great job of preventing severe disease, but they do little to prevent infection” from spreading, Dr. Moore said.
A version of this article first appeared on Medscape.com.
Sugary drinks, rather than artificially sweetened beverages or juices, show link to IBD
Drinks sweetened with sugar – but not natural juices or drinks sweetened artificially – were linked to a higher risk of inflammatory bowel disease (IBD) in people who drank more than one a day in a study of more than 120,000 people.
IBD has previously been linked with high consumption of sugar, but population-based evidence has been inconclusive, and natural juices have not been studied, write lead author Tian Fu, with the department of gastroenterology, the Third Xiangya Hospital of Central South University in Changsha, China, and colleagues.
Their study compared the associations of sugar-sweetened drinks, artificially sweetened beverages, and natural juices (including pure fruit or vegetable juices) with IBD risk.
“As one of the major sources of free sugar, beverages have been related to inflammation-related health outcomes but received less attention in the field of IBD,” the authors wrote.
, especially Crohn’s disease (CD), but further studies are needed to confirm these findings and explore the underlying mechanism,” they write.
The study was published online in Alimentary Pharmacology and Therapeutics.
Link significant for Crohn’s disease but not ulcerative colitis
The researchers used data from 121,490 participants in the UK Biobank who did not have IBD at trial recruitment in 2006-2010. The average age of the participants was 56 years, and almost all (96.9%) were White. The researchers studied their intake of beverages with 24-hour diet recalls from 2009 to 2012.
Participants were sorted into three groups according to the consumption of each beverage: 0 unit (glasses, cans, or 250 mL/cartons) per day (reference group), 0-1 unit per day, and more than 1 unit per day.
While most (66.3%) did not drink any sugar-sweetened beverages, participants who reported drinking more than 1 unit per day were more likely to have a higher body mass index and consume higher amounts of total energy and sugar.
During an average follow-up of about 10 years, the investigators documented 510 incident IBD cases: 143 cases of CD and 367 cases of ulcerative colitis (UC).
Compared to people who did not drink sugar-sweetened beverages, those who drank at least 1 unit per day had a significantly higher IBD risk (hazard ratio, 1.51; 95% confidence interval, 1.11-2.05), but the trend was statistically nonsignificant.
The association was significant for CD (HR, 2.05; 95% CI, 1.22,3.46) but not for UC (HR, 1.31; 95% CI, 0.89-1.92). The positive association between sugar-sweetened drinks and risk of CD, but not UC, was in line with previous studies showing that dietary patterns were more associated with CD risk, the authors noted.
They also highlighted that there was no positive link between artificially sweetened beverages, natural juices, or total sugar intake and IBD risk. They noted that the inflammatory role of artificial sweeteners is still being debated.
Additionally, the effect of natural sugar in juices may be counteracted by fiber and bioactive compounds in the juices, the authors wrote.
A limitation of the study is that at baseline, all participants in the UK Biobank were older than 40 years, so the researchers could not examine any links with younger-onset IBD.
Additionally, the self-reported questionnaires are subject to recall bias, though the survey has been validated.
Study adds to previous evidence
Hasan Zaki, PhD, an assistant professor with the department of pathology at University of Texas Southwestern Medical Center, Dallas, said in an interview that the size of this population-based study adds evidence that simple sugar can increase the risk for IBD. Dr. Zaki studies the relationship of inflammatory disorders and diet and was not involved in the study.
“This study is very strong evidence of the association between high sugar and IBD,” he said. He noted that more studies are needed because there are few studies in this area and results have varied.
His lab conducted work on the subject previously in mice. In a 2020 study, they found that a high-sugar diet helped promote IBD development and gut microbiota dysfunction.
Dr. Zaki pointed out that, among people in the United Kingdom and those in the United States, diets, demographics, and IBD incidence are similar, a fact that may make the findings more generalizable.
However, studies comparing the categories of sweetened drinks should be conducted in a U.S. population to assess the results in a diverse group to see whether ethnicity plays a role, because almost all of the people in the UK group were White, he said.
Also important, Dr. Zaki said, will be follow-up studies of the link between sweet drinks and IBD in U.S. children, among whom consumption is particularly high and the IBD incidence is rising. One study showed the prevalence increased 133% from 2007 to 2016.
The results of this study should help gastroenterologists counsel patients on an ideal diet to avoid IBD or reduce IBD severity, he said.
The study was supported by the National Natural Science Foundation of China and Key Project of Research and Development Plan of Hunan Province. The study authors and Dr. Zaki report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Drinks sweetened with sugar – but not natural juices or drinks sweetened artificially – were linked to a higher risk of inflammatory bowel disease (IBD) in people who drank more than one a day in a study of more than 120,000 people.
IBD has previously been linked with high consumption of sugar, but population-based evidence has been inconclusive, and natural juices have not been studied, write lead author Tian Fu, with the department of gastroenterology, the Third Xiangya Hospital of Central South University in Changsha, China, and colleagues.
Their study compared the associations of sugar-sweetened drinks, artificially sweetened beverages, and natural juices (including pure fruit or vegetable juices) with IBD risk.
“As one of the major sources of free sugar, beverages have been related to inflammation-related health outcomes but received less attention in the field of IBD,” the authors wrote.
, especially Crohn’s disease (CD), but further studies are needed to confirm these findings and explore the underlying mechanism,” they write.
The study was published online in Alimentary Pharmacology and Therapeutics.
Link significant for Crohn’s disease but not ulcerative colitis
The researchers used data from 121,490 participants in the UK Biobank who did not have IBD at trial recruitment in 2006-2010. The average age of the participants was 56 years, and almost all (96.9%) were White. The researchers studied their intake of beverages with 24-hour diet recalls from 2009 to 2012.
Participants were sorted into three groups according to the consumption of each beverage: 0 unit (glasses, cans, or 250 mL/cartons) per day (reference group), 0-1 unit per day, and more than 1 unit per day.
While most (66.3%) did not drink any sugar-sweetened beverages, participants who reported drinking more than 1 unit per day were more likely to have a higher body mass index and consume higher amounts of total energy and sugar.
During an average follow-up of about 10 years, the investigators documented 510 incident IBD cases: 143 cases of CD and 367 cases of ulcerative colitis (UC).
Compared to people who did not drink sugar-sweetened beverages, those who drank at least 1 unit per day had a significantly higher IBD risk (hazard ratio, 1.51; 95% confidence interval, 1.11-2.05), but the trend was statistically nonsignificant.
The association was significant for CD (HR, 2.05; 95% CI, 1.22,3.46) but not for UC (HR, 1.31; 95% CI, 0.89-1.92). The positive association between sugar-sweetened drinks and risk of CD, but not UC, was in line with previous studies showing that dietary patterns were more associated with CD risk, the authors noted.
They also highlighted that there was no positive link between artificially sweetened beverages, natural juices, or total sugar intake and IBD risk. They noted that the inflammatory role of artificial sweeteners is still being debated.
Additionally, the effect of natural sugar in juices may be counteracted by fiber and bioactive compounds in the juices, the authors wrote.
A limitation of the study is that at baseline, all participants in the UK Biobank were older than 40 years, so the researchers could not examine any links with younger-onset IBD.
Additionally, the self-reported questionnaires are subject to recall bias, though the survey has been validated.
Study adds to previous evidence
Hasan Zaki, PhD, an assistant professor with the department of pathology at University of Texas Southwestern Medical Center, Dallas, said in an interview that the size of this population-based study adds evidence that simple sugar can increase the risk for IBD. Dr. Zaki studies the relationship of inflammatory disorders and diet and was not involved in the study.
“This study is very strong evidence of the association between high sugar and IBD,” he said. He noted that more studies are needed because there are few studies in this area and results have varied.
His lab conducted work on the subject previously in mice. In a 2020 study, they found that a high-sugar diet helped promote IBD development and gut microbiota dysfunction.
Dr. Zaki pointed out that, among people in the United Kingdom and those in the United States, diets, demographics, and IBD incidence are similar, a fact that may make the findings more generalizable.
However, studies comparing the categories of sweetened drinks should be conducted in a U.S. population to assess the results in a diverse group to see whether ethnicity plays a role, because almost all of the people in the UK group were White, he said.
Also important, Dr. Zaki said, will be follow-up studies of the link between sweet drinks and IBD in U.S. children, among whom consumption is particularly high and the IBD incidence is rising. One study showed the prevalence increased 133% from 2007 to 2016.
The results of this study should help gastroenterologists counsel patients on an ideal diet to avoid IBD or reduce IBD severity, he said.
The study was supported by the National Natural Science Foundation of China and Key Project of Research and Development Plan of Hunan Province. The study authors and Dr. Zaki report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Drinks sweetened with sugar – but not natural juices or drinks sweetened artificially – were linked to a higher risk of inflammatory bowel disease (IBD) in people who drank more than one a day in a study of more than 120,000 people.
IBD has previously been linked with high consumption of sugar, but population-based evidence has been inconclusive, and natural juices have not been studied, write lead author Tian Fu, with the department of gastroenterology, the Third Xiangya Hospital of Central South University in Changsha, China, and colleagues.
Their study compared the associations of sugar-sweetened drinks, artificially sweetened beverages, and natural juices (including pure fruit or vegetable juices) with IBD risk.
“As one of the major sources of free sugar, beverages have been related to inflammation-related health outcomes but received less attention in the field of IBD,” the authors wrote.
, especially Crohn’s disease (CD), but further studies are needed to confirm these findings and explore the underlying mechanism,” they write.
The study was published online in Alimentary Pharmacology and Therapeutics.
Link significant for Crohn’s disease but not ulcerative colitis
The researchers used data from 121,490 participants in the UK Biobank who did not have IBD at trial recruitment in 2006-2010. The average age of the participants was 56 years, and almost all (96.9%) were White. The researchers studied their intake of beverages with 24-hour diet recalls from 2009 to 2012.
Participants were sorted into three groups according to the consumption of each beverage: 0 unit (glasses, cans, or 250 mL/cartons) per day (reference group), 0-1 unit per day, and more than 1 unit per day.
While most (66.3%) did not drink any sugar-sweetened beverages, participants who reported drinking more than 1 unit per day were more likely to have a higher body mass index and consume higher amounts of total energy and sugar.
During an average follow-up of about 10 years, the investigators documented 510 incident IBD cases: 143 cases of CD and 367 cases of ulcerative colitis (UC).
Compared to people who did not drink sugar-sweetened beverages, those who drank at least 1 unit per day had a significantly higher IBD risk (hazard ratio, 1.51; 95% confidence interval, 1.11-2.05), but the trend was statistically nonsignificant.
The association was significant for CD (HR, 2.05; 95% CI, 1.22,3.46) but not for UC (HR, 1.31; 95% CI, 0.89-1.92). The positive association between sugar-sweetened drinks and risk of CD, but not UC, was in line with previous studies showing that dietary patterns were more associated with CD risk, the authors noted.
They also highlighted that there was no positive link between artificially sweetened beverages, natural juices, or total sugar intake and IBD risk. They noted that the inflammatory role of artificial sweeteners is still being debated.
Additionally, the effect of natural sugar in juices may be counteracted by fiber and bioactive compounds in the juices, the authors wrote.
A limitation of the study is that at baseline, all participants in the UK Biobank were older than 40 years, so the researchers could not examine any links with younger-onset IBD.
Additionally, the self-reported questionnaires are subject to recall bias, though the survey has been validated.
Study adds to previous evidence
Hasan Zaki, PhD, an assistant professor with the department of pathology at University of Texas Southwestern Medical Center, Dallas, said in an interview that the size of this population-based study adds evidence that simple sugar can increase the risk for IBD. Dr. Zaki studies the relationship of inflammatory disorders and diet and was not involved in the study.
“This study is very strong evidence of the association between high sugar and IBD,” he said. He noted that more studies are needed because there are few studies in this area and results have varied.
His lab conducted work on the subject previously in mice. In a 2020 study, they found that a high-sugar diet helped promote IBD development and gut microbiota dysfunction.
Dr. Zaki pointed out that, among people in the United Kingdom and those in the United States, diets, demographics, and IBD incidence are similar, a fact that may make the findings more generalizable.
However, studies comparing the categories of sweetened drinks should be conducted in a U.S. population to assess the results in a diverse group to see whether ethnicity plays a role, because almost all of the people in the UK group were White, he said.
Also important, Dr. Zaki said, will be follow-up studies of the link between sweet drinks and IBD in U.S. children, among whom consumption is particularly high and the IBD incidence is rising. One study showed the prevalence increased 133% from 2007 to 2016.
The results of this study should help gastroenterologists counsel patients on an ideal diet to avoid IBD or reduce IBD severity, he said.
The study was supported by the National Natural Science Foundation of China and Key Project of Research and Development Plan of Hunan Province. The study authors and Dr. Zaki report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALIMENTARY PHARMACOLOGY AND THERAPEUTICS
VA foster program helps older vets manage COVID challenges
Susan Snead used to live in an apartment complex for older adults. The complex had a nice dayroom, and neighbors would knock on her door every now and then to check in.
But despite not being lonely, Ms. Snead, 89, did live alone in downtown Charleston, S.C. Eventually, that became dangerous.
“I fell a few times,” she says. “I had to call somebody to come and get me up.”
Sometimes help would come from the apartment complex’s office. Sometimes it came with a police escort.
Over time, needing to make those calls became a burden. Making and keeping appointments with her doctor, something she had to do regularly, as she has diabetes, got harder, too.
“It kind of wore me out,” she says. “Like you’re going up a hill.”
As she was beginning to accept she could no longer live alone, Ms. Snead, an Air Force veteran, learned about a program run by the Department of Veterans Affairs called Medical Foster Home.
Caregivers help aging veterans with activities of daily living like bathing, cooking, making and getting to appointments, getting dressed, and taking daily medication.
Caregivers can take care of up to three residents in their home at a time. While most residents are veterans, caregivers sometimes care for non-veteran residents, such as a veteran’s spouse or a caregiver’s family member.
Veterans typically pay about $1,500 to $3,000 out-of-pocket per month for the service, depending on location.
According to the VA, the concept of medical foster homes has been around since 1999, when VA hospitals across the country began reaching out to people willing to provide live-in care for veterans. The option is led by local VA hospitals, which approve caregivers and provide administrative services. There are now 517 medical foster homes, the VA says.
Much like other residential care facilities, medical foster homes get regular inspections for safety, nutrition, and more.
In 2019, Ms. Snead signed up for the program. She expected to be cared for, but she found a sense of family with her caregiver, Wilhelmina Brown, and another veteran in the home.
Ms. Brown started taking care of people – but not necessarily veterans – in 1997 when her grandmother was unable to care for herself, she says.
“My grandmama carried me to church every Sunday, she carried me to the beach – everywhere she went, she took me with her,” Ms. Brown says. As her grandmother got older, “I said, ‘I’m going to take care of her in my home.’ ”
Caring for others must come from the heart, Ms. Brown says.
She cooks her residents’ meals three times a day with dietary restrictions in mind, washes their dishes, does their laundry, remembers birthdays, and plans little parties.
“That’s my family,” Ms. Brown says.
In 2020, the COVID-19 pandemic upended the world – but at the same time, it highlighted the advantages of the medical foster home model.
Home-based primary care keeps veterans out of nursing homes – something that became particularly important as COVID-19 hit nursing homes and long-term care facilities.
Caregivers in the system were also able to help veterans, often living in rural areas, pivot and adapt to telehealth during a time of crisis.
One study, published in the journal Geriatrics, set out to identify how medical foster homes were able to deliver safe, effective health care during the early stages of the pandemic.
Researchers interviewed 37 VA care providers at 16 rural medical foster home programs across the country. The interviews took place between December 2020 and February 2021. They found medical foster home caregivers, coordinators, and health care providers communicated to move office visits to the home, helped veterans navigate telehealth, advocated to get veterans vaccinated in-home, and relied on each other to fight social isolation.
Caregivers also adapted quickly to telehealth, according to Leah Haverhals, PhD, a health research scientist and communications director for the Seattle-Denver Center of Innovation for Veteran Centered and Value Driven Care, who led the study.
Most veterans in the foster home program are older and find new technology difficult to use.
Caregivers, coordinators, and health care providers were largely new to the technology, too.
While the study found that most veterans and caregivers preferred in-person care, they were able to work together to make the best of telehealth.
“That speaks to the nature of the care being given, being able to pivot in a crisis like that,” Dr. Haverhals says.
If caregivers didn’t already have computers or telehealth-compatible devices, the VA provided iPads that would connect to the internet using cellular signals. According to the study, this helped to overcome connectivity issues that may have caused problems in rural areas.
Ms. Snead says Ms. Brown helped a lot with her telehealth calls.
“If we had to do things over the phone or with video, she was able to set that up to work with the person on the other end. She knows a lot about that stuff – about computers and things like that,” Ms. Snead says, adding that she hadn’t worked with computers since retirement in 1998.
Telehealth helped health care providers identify infections and quickly prescribe antibiotics to veterans in rural areas and provide other care that was more safely delivered in private homes.
“The findings from our study highlighted that when working together for the common goal of keeping vulnerable populations like veterans in MFHs [medical foster homes] safe during times of crisis, adaptation and collaboration facilitated the ongoing provision of high-quality care,” Dr. Haverhals’s group wrote. “Such collaboration has been shown to be critical in recent research in the United States on supporting older adults during the pandemic.”
Cari Levy, MD, PhD, a professor at the University of Colorado at Denver, Aurora, and a co-author of the study, specializes in palliative and telenursing home care for the VA.
Dr. Levy, who has worked for the VA for about 20 years, says how medical foster homes provided care during the pandemic carries lessons for civilian clinics. One of the most important lessons, she says, is that medical professionals will need to provide more care where people are, especially in populations that are too sick to get to the clinic.
“For years, there was all this hope that telehealth would expand,” but it took a pandemic to authorize approval from federal agencies to explode, she says. “I shudder to think what would have happened if we didn’t have telehealth. Fortunately, it was the right time to be able to flip a switch.”
Crisis aside, Dr. Levy says her dream would be for health care providers to do more home-based care. The model allows people to preserve the relational aspects of medicine, which can counteract a lot of the moral injury and burnout in the field, she says, adding:
“I see this as the kind of medicine many people intended to do when they got into medicine.”
A version of this article first appeared on WebMD.com.
Susan Snead used to live in an apartment complex for older adults. The complex had a nice dayroom, and neighbors would knock on her door every now and then to check in.
But despite not being lonely, Ms. Snead, 89, did live alone in downtown Charleston, S.C. Eventually, that became dangerous.
“I fell a few times,” she says. “I had to call somebody to come and get me up.”
Sometimes help would come from the apartment complex’s office. Sometimes it came with a police escort.
Over time, needing to make those calls became a burden. Making and keeping appointments with her doctor, something she had to do regularly, as she has diabetes, got harder, too.
“It kind of wore me out,” she says. “Like you’re going up a hill.”
As she was beginning to accept she could no longer live alone, Ms. Snead, an Air Force veteran, learned about a program run by the Department of Veterans Affairs called Medical Foster Home.
Caregivers help aging veterans with activities of daily living like bathing, cooking, making and getting to appointments, getting dressed, and taking daily medication.
Caregivers can take care of up to three residents in their home at a time. While most residents are veterans, caregivers sometimes care for non-veteran residents, such as a veteran’s spouse or a caregiver’s family member.
Veterans typically pay about $1,500 to $3,000 out-of-pocket per month for the service, depending on location.
According to the VA, the concept of medical foster homes has been around since 1999, when VA hospitals across the country began reaching out to people willing to provide live-in care for veterans. The option is led by local VA hospitals, which approve caregivers and provide administrative services. There are now 517 medical foster homes, the VA says.
Much like other residential care facilities, medical foster homes get regular inspections for safety, nutrition, and more.
In 2019, Ms. Snead signed up for the program. She expected to be cared for, but she found a sense of family with her caregiver, Wilhelmina Brown, and another veteran in the home.
Ms. Brown started taking care of people – but not necessarily veterans – in 1997 when her grandmother was unable to care for herself, she says.
“My grandmama carried me to church every Sunday, she carried me to the beach – everywhere she went, she took me with her,” Ms. Brown says. As her grandmother got older, “I said, ‘I’m going to take care of her in my home.’ ”
Caring for others must come from the heart, Ms. Brown says.
She cooks her residents’ meals three times a day with dietary restrictions in mind, washes their dishes, does their laundry, remembers birthdays, and plans little parties.
“That’s my family,” Ms. Brown says.
In 2020, the COVID-19 pandemic upended the world – but at the same time, it highlighted the advantages of the medical foster home model.
Home-based primary care keeps veterans out of nursing homes – something that became particularly important as COVID-19 hit nursing homes and long-term care facilities.
Caregivers in the system were also able to help veterans, often living in rural areas, pivot and adapt to telehealth during a time of crisis.
One study, published in the journal Geriatrics, set out to identify how medical foster homes were able to deliver safe, effective health care during the early stages of the pandemic.
Researchers interviewed 37 VA care providers at 16 rural medical foster home programs across the country. The interviews took place between December 2020 and February 2021. They found medical foster home caregivers, coordinators, and health care providers communicated to move office visits to the home, helped veterans navigate telehealth, advocated to get veterans vaccinated in-home, and relied on each other to fight social isolation.
Caregivers also adapted quickly to telehealth, according to Leah Haverhals, PhD, a health research scientist and communications director for the Seattle-Denver Center of Innovation for Veteran Centered and Value Driven Care, who led the study.
Most veterans in the foster home program are older and find new technology difficult to use.
Caregivers, coordinators, and health care providers were largely new to the technology, too.
While the study found that most veterans and caregivers preferred in-person care, they were able to work together to make the best of telehealth.
“That speaks to the nature of the care being given, being able to pivot in a crisis like that,” Dr. Haverhals says.
If caregivers didn’t already have computers or telehealth-compatible devices, the VA provided iPads that would connect to the internet using cellular signals. According to the study, this helped to overcome connectivity issues that may have caused problems in rural areas.
Ms. Snead says Ms. Brown helped a lot with her telehealth calls.
“If we had to do things over the phone or with video, she was able to set that up to work with the person on the other end. She knows a lot about that stuff – about computers and things like that,” Ms. Snead says, adding that she hadn’t worked with computers since retirement in 1998.
Telehealth helped health care providers identify infections and quickly prescribe antibiotics to veterans in rural areas and provide other care that was more safely delivered in private homes.
“The findings from our study highlighted that when working together for the common goal of keeping vulnerable populations like veterans in MFHs [medical foster homes] safe during times of crisis, adaptation and collaboration facilitated the ongoing provision of high-quality care,” Dr. Haverhals’s group wrote. “Such collaboration has been shown to be critical in recent research in the United States on supporting older adults during the pandemic.”
Cari Levy, MD, PhD, a professor at the University of Colorado at Denver, Aurora, and a co-author of the study, specializes in palliative and telenursing home care for the VA.
Dr. Levy, who has worked for the VA for about 20 years, says how medical foster homes provided care during the pandemic carries lessons for civilian clinics. One of the most important lessons, she says, is that medical professionals will need to provide more care where people are, especially in populations that are too sick to get to the clinic.
“For years, there was all this hope that telehealth would expand,” but it took a pandemic to authorize approval from federal agencies to explode, she says. “I shudder to think what would have happened if we didn’t have telehealth. Fortunately, it was the right time to be able to flip a switch.”
Crisis aside, Dr. Levy says her dream would be for health care providers to do more home-based care. The model allows people to preserve the relational aspects of medicine, which can counteract a lot of the moral injury and burnout in the field, she says, adding:
“I see this as the kind of medicine many people intended to do when they got into medicine.”
A version of this article first appeared on WebMD.com.
Susan Snead used to live in an apartment complex for older adults. The complex had a nice dayroom, and neighbors would knock on her door every now and then to check in.
But despite not being lonely, Ms. Snead, 89, did live alone in downtown Charleston, S.C. Eventually, that became dangerous.
“I fell a few times,” she says. “I had to call somebody to come and get me up.”
Sometimes help would come from the apartment complex’s office. Sometimes it came with a police escort.
Over time, needing to make those calls became a burden. Making and keeping appointments with her doctor, something she had to do regularly, as she has diabetes, got harder, too.
“It kind of wore me out,” she says. “Like you’re going up a hill.”
As she was beginning to accept she could no longer live alone, Ms. Snead, an Air Force veteran, learned about a program run by the Department of Veterans Affairs called Medical Foster Home.
Caregivers help aging veterans with activities of daily living like bathing, cooking, making and getting to appointments, getting dressed, and taking daily medication.
Caregivers can take care of up to three residents in their home at a time. While most residents are veterans, caregivers sometimes care for non-veteran residents, such as a veteran’s spouse or a caregiver’s family member.
Veterans typically pay about $1,500 to $3,000 out-of-pocket per month for the service, depending on location.
According to the VA, the concept of medical foster homes has been around since 1999, when VA hospitals across the country began reaching out to people willing to provide live-in care for veterans. The option is led by local VA hospitals, which approve caregivers and provide administrative services. There are now 517 medical foster homes, the VA says.
Much like other residential care facilities, medical foster homes get regular inspections for safety, nutrition, and more.
In 2019, Ms. Snead signed up for the program. She expected to be cared for, but she found a sense of family with her caregiver, Wilhelmina Brown, and another veteran in the home.
Ms. Brown started taking care of people – but not necessarily veterans – in 1997 when her grandmother was unable to care for herself, she says.
“My grandmama carried me to church every Sunday, she carried me to the beach – everywhere she went, she took me with her,” Ms. Brown says. As her grandmother got older, “I said, ‘I’m going to take care of her in my home.’ ”
Caring for others must come from the heart, Ms. Brown says.
She cooks her residents’ meals three times a day with dietary restrictions in mind, washes their dishes, does their laundry, remembers birthdays, and plans little parties.
“That’s my family,” Ms. Brown says.
In 2020, the COVID-19 pandemic upended the world – but at the same time, it highlighted the advantages of the medical foster home model.
Home-based primary care keeps veterans out of nursing homes – something that became particularly important as COVID-19 hit nursing homes and long-term care facilities.
Caregivers in the system were also able to help veterans, often living in rural areas, pivot and adapt to telehealth during a time of crisis.
One study, published in the journal Geriatrics, set out to identify how medical foster homes were able to deliver safe, effective health care during the early stages of the pandemic.
Researchers interviewed 37 VA care providers at 16 rural medical foster home programs across the country. The interviews took place between December 2020 and February 2021. They found medical foster home caregivers, coordinators, and health care providers communicated to move office visits to the home, helped veterans navigate telehealth, advocated to get veterans vaccinated in-home, and relied on each other to fight social isolation.
Caregivers also adapted quickly to telehealth, according to Leah Haverhals, PhD, a health research scientist and communications director for the Seattle-Denver Center of Innovation for Veteran Centered and Value Driven Care, who led the study.
Most veterans in the foster home program are older and find new technology difficult to use.
Caregivers, coordinators, and health care providers were largely new to the technology, too.
While the study found that most veterans and caregivers preferred in-person care, they were able to work together to make the best of telehealth.
“That speaks to the nature of the care being given, being able to pivot in a crisis like that,” Dr. Haverhals says.
If caregivers didn’t already have computers or telehealth-compatible devices, the VA provided iPads that would connect to the internet using cellular signals. According to the study, this helped to overcome connectivity issues that may have caused problems in rural areas.
Ms. Snead says Ms. Brown helped a lot with her telehealth calls.
“If we had to do things over the phone or with video, she was able to set that up to work with the person on the other end. She knows a lot about that stuff – about computers and things like that,” Ms. Snead says, adding that she hadn’t worked with computers since retirement in 1998.
Telehealth helped health care providers identify infections and quickly prescribe antibiotics to veterans in rural areas and provide other care that was more safely delivered in private homes.
“The findings from our study highlighted that when working together for the common goal of keeping vulnerable populations like veterans in MFHs [medical foster homes] safe during times of crisis, adaptation and collaboration facilitated the ongoing provision of high-quality care,” Dr. Haverhals’s group wrote. “Such collaboration has been shown to be critical in recent research in the United States on supporting older adults during the pandemic.”
Cari Levy, MD, PhD, a professor at the University of Colorado at Denver, Aurora, and a co-author of the study, specializes in palliative and telenursing home care for the VA.
Dr. Levy, who has worked for the VA for about 20 years, says how medical foster homes provided care during the pandemic carries lessons for civilian clinics. One of the most important lessons, she says, is that medical professionals will need to provide more care where people are, especially in populations that are too sick to get to the clinic.
“For years, there was all this hope that telehealth would expand,” but it took a pandemic to authorize approval from federal agencies to explode, she says. “I shudder to think what would have happened if we didn’t have telehealth. Fortunately, it was the right time to be able to flip a switch.”
Crisis aside, Dr. Levy says her dream would be for health care providers to do more home-based care. The model allows people to preserve the relational aspects of medicine, which can counteract a lot of the moral injury and burnout in the field, she says, adding:
“I see this as the kind of medicine many people intended to do when they got into medicine.”
A version of this article first appeared on WebMD.com.