User login
Early dexmedetomidine did not reduce 90-day mortality in ICU patients on mechanical ventilation
Dexmedetomidine fell short for reducing 90-day mortality as the primary sedative for patients on mechanical ventilation, according to results of the randomized, controlled, open-label SPICE III trial, which was presented at the annual meeting of the American Thoracic Society and simultaneously published in the New England Journal of Medicine.
“Among patients undergoing mechanical ventilation in the ICU, those who received early dexmedetomidine for sedation had a rate of death at 90 days similar to that in the usual-care group and required supplemental sedatives to achieve the prescribed level of sedation,” Yahya Shehabi, PhD, of Monash University in Clayton, Australia, and colleagues wrote.
The study was conducted in 74 ICUs in eight countries. Researchers randomly assigned 4,000 patients who were critically ill, had received ventilation for less than 12 hours, and were likely to require mechanical ventilation for at least the next day to either dexmedetomidine or usual care (propofol, midazolam, or another sedative). The sedation goal was a Richmond Agitation and Sedation Scale (RASS) score of –2 (lightly sedated) to +1 (restless), and was assessed every 4 hours. Intravenous dexmedetomidine was administered at 1 mcg/kg of body weight per hour without a loading dose and adjusted to a maximum dose of 1.5 mcg/kg per hour to achieve a RASS score in the target range. Use was continued as clinically required for up to 28 days.
The modified intention-to-treat analysis included 3,904 patients. The 90-day mortality rate was 29.1% (556 of 1,948 patients) for patients who received dexmedetomidine and 29.1% (569 of 1,956 patients) for those who received usual care. There was no significant difference for patients with suspected or proven sepsis at randomization and those without sepsis. Mortality did not vary based on country, cause of death, or discharge destination.
Dr. Shehabi and colleagues noted that, for 2 days after randomization, patients who received dexmedetomidine were also given propofol (64% of patients), midazolam (3%), or both (7%) as supplemental sedation. In the control group, 60% of the patients received propofol, 12% received midazolam, and 20% received both. About 80% of patients in both groups received fentanyl. The use of multiple agents may reflect sedation requirements during the acute phase of critical illness.
With regard to adverse events, the patients receiving dexmedetomidine more commonly experienced bradycardia and hypotension than the usual-care group.
SPICE III was funded in part by a grant from the National Health and Medical Research Council of Australia and the National Heart Institute of Malaysia. Dr. Shehabi reports grants from the National Health and Medical Research Council of Australia, nonfinancial and other support from Pfizer, and nonfinancial and other support from Orion Pharma.
SOURCE: Shehabi Y et al. N Eng J Med. 2019 May 19. doi: 10.1056/NEJMoa1904710.
Dexmedetomidine fell short for reducing 90-day mortality as the primary sedative for patients on mechanical ventilation, according to results of the randomized, controlled, open-label SPICE III trial, which was presented at the annual meeting of the American Thoracic Society and simultaneously published in the New England Journal of Medicine.
“Among patients undergoing mechanical ventilation in the ICU, those who received early dexmedetomidine for sedation had a rate of death at 90 days similar to that in the usual-care group and required supplemental sedatives to achieve the prescribed level of sedation,” Yahya Shehabi, PhD, of Monash University in Clayton, Australia, and colleagues wrote.
The study was conducted in 74 ICUs in eight countries. Researchers randomly assigned 4,000 patients who were critically ill, had received ventilation for less than 12 hours, and were likely to require mechanical ventilation for at least the next day to either dexmedetomidine or usual care (propofol, midazolam, or another sedative). The sedation goal was a Richmond Agitation and Sedation Scale (RASS) score of –2 (lightly sedated) to +1 (restless), and was assessed every 4 hours. Intravenous dexmedetomidine was administered at 1 mcg/kg of body weight per hour without a loading dose and adjusted to a maximum dose of 1.5 mcg/kg per hour to achieve a RASS score in the target range. Use was continued as clinically required for up to 28 days.
The modified intention-to-treat analysis included 3,904 patients. The 90-day mortality rate was 29.1% (556 of 1,948 patients) for patients who received dexmedetomidine and 29.1% (569 of 1,956 patients) for those who received usual care. There was no significant difference for patients with suspected or proven sepsis at randomization and those without sepsis. Mortality did not vary based on country, cause of death, or discharge destination.
Dr. Shehabi and colleagues noted that, for 2 days after randomization, patients who received dexmedetomidine were also given propofol (64% of patients), midazolam (3%), or both (7%) as supplemental sedation. In the control group, 60% of the patients received propofol, 12% received midazolam, and 20% received both. About 80% of patients in both groups received fentanyl. The use of multiple agents may reflect sedation requirements during the acute phase of critical illness.
With regard to adverse events, the patients receiving dexmedetomidine more commonly experienced bradycardia and hypotension than the usual-care group.
SPICE III was funded in part by a grant from the National Health and Medical Research Council of Australia and the National Heart Institute of Malaysia. Dr. Shehabi reports grants from the National Health and Medical Research Council of Australia, nonfinancial and other support from Pfizer, and nonfinancial and other support from Orion Pharma.
SOURCE: Shehabi Y et al. N Eng J Med. 2019 May 19. doi: 10.1056/NEJMoa1904710.
Dexmedetomidine fell short for reducing 90-day mortality as the primary sedative for patients on mechanical ventilation, according to results of the randomized, controlled, open-label SPICE III trial, which was presented at the annual meeting of the American Thoracic Society and simultaneously published in the New England Journal of Medicine.
“Among patients undergoing mechanical ventilation in the ICU, those who received early dexmedetomidine for sedation had a rate of death at 90 days similar to that in the usual-care group and required supplemental sedatives to achieve the prescribed level of sedation,” Yahya Shehabi, PhD, of Monash University in Clayton, Australia, and colleagues wrote.
The study was conducted in 74 ICUs in eight countries. Researchers randomly assigned 4,000 patients who were critically ill, had received ventilation for less than 12 hours, and were likely to require mechanical ventilation for at least the next day to either dexmedetomidine or usual care (propofol, midazolam, or another sedative). The sedation goal was a Richmond Agitation and Sedation Scale (RASS) score of –2 (lightly sedated) to +1 (restless), and was assessed every 4 hours. Intravenous dexmedetomidine was administered at 1 mcg/kg of body weight per hour without a loading dose and adjusted to a maximum dose of 1.5 mcg/kg per hour to achieve a RASS score in the target range. Use was continued as clinically required for up to 28 days.
The modified intention-to-treat analysis included 3,904 patients. The 90-day mortality rate was 29.1% (556 of 1,948 patients) for patients who received dexmedetomidine and 29.1% (569 of 1,956 patients) for those who received usual care. There was no significant difference for patients with suspected or proven sepsis at randomization and those without sepsis. Mortality did not vary based on country, cause of death, or discharge destination.
Dr. Shehabi and colleagues noted that, for 2 days after randomization, patients who received dexmedetomidine were also given propofol (64% of patients), midazolam (3%), or both (7%) as supplemental sedation. In the control group, 60% of the patients received propofol, 12% received midazolam, and 20% received both. About 80% of patients in both groups received fentanyl. The use of multiple agents may reflect sedation requirements during the acute phase of critical illness.
With regard to adverse events, the patients receiving dexmedetomidine more commonly experienced bradycardia and hypotension than the usual-care group.
SPICE III was funded in part by a grant from the National Health and Medical Research Council of Australia and the National Heart Institute of Malaysia. Dr. Shehabi reports grants from the National Health and Medical Research Council of Australia, nonfinancial and other support from Pfizer, and nonfinancial and other support from Orion Pharma.
SOURCE: Shehabi Y et al. N Eng J Med. 2019 May 19. doi: 10.1056/NEJMoa1904710.
FROM ATS 2019
Key clinical point: Patients receiving dexmedetomidine for sedation had a comparable 90-day mortality rate with patients who received usual care.
Major finding: The mortality rate at 90 days was 29.1% (556 of 1,948 patients) for patients who received dexmedetomidine and 29.1% (569 of 1,956 patients) for those who received usual care.
Study details: The study was conducted in 74 ICUs in 8 countries. Researchers randomly assigned 4,000 patients who were critically ill, had received ventilation for less than 12 hours, and were likely to require mechanical ventilation for at least the next day to either dexmedetomidine or usual care (propofol, midazolam, or another sedative).
Disclosures: The study was funded in part by a grant from the National Health and Medical Research Council of Australia and the National Heart Institute of Malaysia. Dr. Shehabi reports grants from The National Health and Medical Research Council of Australia, nonfinancial and other support from Pfizer, and nonfinancial and other support from Orion Pharma.Source: Shehabi Y et al. N Eng J Med. 2019 May 19. doi: 10.1056/NEJMoa1904710.
AFib on the rise in end-stage COPD patients hospitalized for exacerbations
Atrial fibrillation is being seen with increasing frequency in patients admitted to U.S. hospitals for exacerbations of end-stage chronic obstructive pulmonary disease, based on a retrospective analysis of data from the Nationwide Inpatient Sample.
The prevalence of atrial fibrillation (AFib) among patients with end-stage chronic obstructive pulmonary disease (COPD) on home oxygen who were admitted with COPD exacerbations increased from 12.9% in 2003 to 21.3% in 2014, according to Xiaochun Xiao of the department of health statistics at Second Military Medical University in Shanghai and colleagues.
Additionally, “we found that comorbid [AFib] was associated with an increased risk of the need for mechanical ventilation, especially invasive mechanical ventilation. Moreover, comorbid [AFib] was associated with adverse clinical outcomes, including increased in-hospital death, acute respiratory failure, acute kidney injury, sepsis, and stroke,” the researchers wrote in the study published in the journal CHEST.
Patients included in the study were aged at least 18 years, were diagnosed with end-stage COPD and on home oxygen, and were hospitalized because of a COPD-related exacerbation. Based on 1,345,270 weighted hospital admissions of adults with end-stage COPD on home oxygen who met the inclusion criteria for the study, 18.2% (244,488 admissions) of patients had AFib, and the prevalence of AFib in COPD patients increased over time from 2003 (12.9%) to 2014 (21.3%; P less than .0001).
Patients with AFib, compared with patients without AFib, were older (75.5 years vs. 69.6 years; P less than .0001) and more likely to be male (50.7% vs. 59.1%; P less than .0001) and white (80.9% vs. 74.4%; P less than .0001). Patients with AFib also had higher stroke risk reflected in higher CHA2DS2-VASc scores (3.26 vs. 2.45; P less than .0001), and higher likelihood of in-hospital mortality and readmission reflected in Elixhauser scores greater than or equal to 4 (51.2% vs. 35.6%).
In addition, the prevalence of AFib increased with increasing income. Larger hospitals in terms of bed size, urban environment, and Medicare insurance status also were associated with a higher AFib prevalence.
AFib was associated with an increased cost of $1,415 and an increased length of stay of 0.6 days after adjustment for potential confounders. AFib also predicted risk for several adverse events, including stroke (odds ratio, 1.80; in-hospital death, [OR, 1.54]), invasive mechanical ventilation (OR, 1.37), sepsis (OR, 1.23), noninvasive mechanical ventilation (OR, 1.14), acute kidney injury (OR, 1.09), and acute respiratory failure (OR, 1.09).
The researchers noted the database could have potentially overinflated AFib prevalence, as they could not differentiate index admissions and readmissions. The database also does not contain information about secondary diagnoses codes present on admission, which could make it difficult to identify adverse events that occurred during hospitalization.
“Our findings should prompt further efforts to identify the reasons for increased [AFib] prevalence and provide better management strategies for end-stage COPD patients comorbid with [AFib],” the researchers concluded.
This study was funded by a grant from the Fourth Round of the Shanghai 3-year Action Plan on Public Health Discipline and Talent Program. The authors reported no relevant conflict of interest.
SOURCE: Xiao X et al. CHEST. 2019 Jan 23. doi: 10.1016/j.chest.2018.12.021.
Atrial fibrillation is being seen with increasing frequency in patients admitted to U.S. hospitals for exacerbations of end-stage chronic obstructive pulmonary disease, based on a retrospective analysis of data from the Nationwide Inpatient Sample.
The prevalence of atrial fibrillation (AFib) among patients with end-stage chronic obstructive pulmonary disease (COPD) on home oxygen who were admitted with COPD exacerbations increased from 12.9% in 2003 to 21.3% in 2014, according to Xiaochun Xiao of the department of health statistics at Second Military Medical University in Shanghai and colleagues.
Additionally, “we found that comorbid [AFib] was associated with an increased risk of the need for mechanical ventilation, especially invasive mechanical ventilation. Moreover, comorbid [AFib] was associated with adverse clinical outcomes, including increased in-hospital death, acute respiratory failure, acute kidney injury, sepsis, and stroke,” the researchers wrote in the study published in the journal CHEST.
Patients included in the study were aged at least 18 years, were diagnosed with end-stage COPD and on home oxygen, and were hospitalized because of a COPD-related exacerbation. Based on 1,345,270 weighted hospital admissions of adults with end-stage COPD on home oxygen who met the inclusion criteria for the study, 18.2% (244,488 admissions) of patients had AFib, and the prevalence of AFib in COPD patients increased over time from 2003 (12.9%) to 2014 (21.3%; P less than .0001).
Patients with AFib, compared with patients without AFib, were older (75.5 years vs. 69.6 years; P less than .0001) and more likely to be male (50.7% vs. 59.1%; P less than .0001) and white (80.9% vs. 74.4%; P less than .0001). Patients with AFib also had higher stroke risk reflected in higher CHA2DS2-VASc scores (3.26 vs. 2.45; P less than .0001), and higher likelihood of in-hospital mortality and readmission reflected in Elixhauser scores greater than or equal to 4 (51.2% vs. 35.6%).
In addition, the prevalence of AFib increased with increasing income. Larger hospitals in terms of bed size, urban environment, and Medicare insurance status also were associated with a higher AFib prevalence.
AFib was associated with an increased cost of $1,415 and an increased length of stay of 0.6 days after adjustment for potential confounders. AFib also predicted risk for several adverse events, including stroke (odds ratio, 1.80; in-hospital death, [OR, 1.54]), invasive mechanical ventilation (OR, 1.37), sepsis (OR, 1.23), noninvasive mechanical ventilation (OR, 1.14), acute kidney injury (OR, 1.09), and acute respiratory failure (OR, 1.09).
The researchers noted the database could have potentially overinflated AFib prevalence, as they could not differentiate index admissions and readmissions. The database also does not contain information about secondary diagnoses codes present on admission, which could make it difficult to identify adverse events that occurred during hospitalization.
“Our findings should prompt further efforts to identify the reasons for increased [AFib] prevalence and provide better management strategies for end-stage COPD patients comorbid with [AFib],” the researchers concluded.
This study was funded by a grant from the Fourth Round of the Shanghai 3-year Action Plan on Public Health Discipline and Talent Program. The authors reported no relevant conflict of interest.
SOURCE: Xiao X et al. CHEST. 2019 Jan 23. doi: 10.1016/j.chest.2018.12.021.
Atrial fibrillation is being seen with increasing frequency in patients admitted to U.S. hospitals for exacerbations of end-stage chronic obstructive pulmonary disease, based on a retrospective analysis of data from the Nationwide Inpatient Sample.
The prevalence of atrial fibrillation (AFib) among patients with end-stage chronic obstructive pulmonary disease (COPD) on home oxygen who were admitted with COPD exacerbations increased from 12.9% in 2003 to 21.3% in 2014, according to Xiaochun Xiao of the department of health statistics at Second Military Medical University in Shanghai and colleagues.
Additionally, “we found that comorbid [AFib] was associated with an increased risk of the need for mechanical ventilation, especially invasive mechanical ventilation. Moreover, comorbid [AFib] was associated with adverse clinical outcomes, including increased in-hospital death, acute respiratory failure, acute kidney injury, sepsis, and stroke,” the researchers wrote in the study published in the journal CHEST.
Patients included in the study were aged at least 18 years, were diagnosed with end-stage COPD and on home oxygen, and were hospitalized because of a COPD-related exacerbation. Based on 1,345,270 weighted hospital admissions of adults with end-stage COPD on home oxygen who met the inclusion criteria for the study, 18.2% (244,488 admissions) of patients had AFib, and the prevalence of AFib in COPD patients increased over time from 2003 (12.9%) to 2014 (21.3%; P less than .0001).
Patients with AFib, compared with patients without AFib, were older (75.5 years vs. 69.6 years; P less than .0001) and more likely to be male (50.7% vs. 59.1%; P less than .0001) and white (80.9% vs. 74.4%; P less than .0001). Patients with AFib also had higher stroke risk reflected in higher CHA2DS2-VASc scores (3.26 vs. 2.45; P less than .0001), and higher likelihood of in-hospital mortality and readmission reflected in Elixhauser scores greater than or equal to 4 (51.2% vs. 35.6%).
In addition, the prevalence of AFib increased with increasing income. Larger hospitals in terms of bed size, urban environment, and Medicare insurance status also were associated with a higher AFib prevalence.
AFib was associated with an increased cost of $1,415 and an increased length of stay of 0.6 days after adjustment for potential confounders. AFib also predicted risk for several adverse events, including stroke (odds ratio, 1.80; in-hospital death, [OR, 1.54]), invasive mechanical ventilation (OR, 1.37), sepsis (OR, 1.23), noninvasive mechanical ventilation (OR, 1.14), acute kidney injury (OR, 1.09), and acute respiratory failure (OR, 1.09).
The researchers noted the database could have potentially overinflated AFib prevalence, as they could not differentiate index admissions and readmissions. The database also does not contain information about secondary diagnoses codes present on admission, which could make it difficult to identify adverse events that occurred during hospitalization.
“Our findings should prompt further efforts to identify the reasons for increased [AFib] prevalence and provide better management strategies for end-stage COPD patients comorbid with [AFib],” the researchers concluded.
This study was funded by a grant from the Fourth Round of the Shanghai 3-year Action Plan on Public Health Discipline and Talent Program. The authors reported no relevant conflict of interest.
SOURCE: Xiao X et al. CHEST. 2019 Jan 23. doi: 10.1016/j.chest.2018.12.021.
FROM CHEST
Key clinical point: Comorbid atrial fibrillation was associated with an increased risk of the need for mechanical ventilation, especially invasive mechanical ventilation, and of adverse outcomes including in-hospital death, acute respiratory failure, acute kidney injury, sepsis, and stroke.
Major finding: The prevalence of atrial fibrillation with end-stage chronic obstructive pulmonary disease increased over time from 2003 (12.9%) to 2014 (21.3%). Study details: A retrospective analysis based on 1,345,270 weighted hospital admissions of adults with end-stage chronic obstructive pulmonary disease on home oxygen from the Nationwide Impatient Sample during 2003-2014.
Disclosures: The study was funded by a grant from the Fourth Round of the Shanghai 3-Year Action Plan on Public Health Discipline and Talent Program. The authors reported no conflicts of interest.
Source: Xiao X et al. CHEST. 2019 Jan 23. doi: 10.1016/j.chest.2018.12.021.
Weekly ciprofloxacin as effective as daily norfloxacin in prevention of SBP
Clinical question: Does ciprofloxacin administered once weekly prevent spontaneous bacterial peritonitis (SBP) as effectively as daily norfloxacin?
Background: Studies have shown that daily administration of norfloxacin is effective for primary prophylaxis as well as secondary prevention of SBP in patients with cirrhosis and ascites. Prior studies have demonstrated efficacy of weekly ciprofloxacin, but no previous studies have compared the two antibiotics.
Study design: Investigator initiated open-label randomized, controlled trial.
Setting: Seven tertiary hospitals in South Korea.
Synopsis: The investigators enrolled 124 patients aged 20-75 with cirrhosis and ascites, ascitic cell count less than 250/mm3, and either ascitic protein less than 1.5g/dL or a history of spontaneous bacterial peritonitis. The patients were randomized to receive norfloxacin 400 mg daily or ciprofloxacin 750 mg weekly, with routine visits during the 12-month study period.
The primary end point of SBP prevention rates at 1 year were 92.7% (51/55) in the norfloxacin group and 96.5% (55/57) in the ciprofloxacin group (P = .712), which met criteria for noninferiority. Other outcomes included no difference in rates of liver transplantation, infectious complications, hepatorenal syndrome, hepatic encephalopathy, variceal bleeding, and hepatocellular carcinoma. A subgroup analysis of patients at higher risk of developing SBP showed 87% prevention rates for the norfloxacin group and 94% for the ciprofloxacin group, although this result was not statistically significant.
The major limitation of this study is that it was not double blinded, so patients were aware of which medication they were taking. Additionally, almost 10% of the cohort was lost to follow-up, but this was accounted for in the sample-size calculation.
Bottom line: Once weekly administration of ciprofloxacin is not inferior to daily norfloxacin for the prevention of SBP in patients with cirrhosis and low ascitic protein levels and may provide a more cost-effective therapy with greater patient compliance.
Citation: Yim HJ et al. Daily norfloxacin vs weekly ciprofloxacin to prevent spontaneous bacterial peritonitis: A randomized controlled trial. Am J Gastroenterol. 2018 Aug;113:1167-76.
Dr. Angeli is an assistant professor in the division of hospital medicine, University of New Mexico.
Clinical question: Does ciprofloxacin administered once weekly prevent spontaneous bacterial peritonitis (SBP) as effectively as daily norfloxacin?
Background: Studies have shown that daily administration of norfloxacin is effective for primary prophylaxis as well as secondary prevention of SBP in patients with cirrhosis and ascites. Prior studies have demonstrated efficacy of weekly ciprofloxacin, but no previous studies have compared the two antibiotics.
Study design: Investigator initiated open-label randomized, controlled trial.
Setting: Seven tertiary hospitals in South Korea.
Synopsis: The investigators enrolled 124 patients aged 20-75 with cirrhosis and ascites, ascitic cell count less than 250/mm3, and either ascitic protein less than 1.5g/dL or a history of spontaneous bacterial peritonitis. The patients were randomized to receive norfloxacin 400 mg daily or ciprofloxacin 750 mg weekly, with routine visits during the 12-month study period.
The primary end point of SBP prevention rates at 1 year were 92.7% (51/55) in the norfloxacin group and 96.5% (55/57) in the ciprofloxacin group (P = .712), which met criteria for noninferiority. Other outcomes included no difference in rates of liver transplantation, infectious complications, hepatorenal syndrome, hepatic encephalopathy, variceal bleeding, and hepatocellular carcinoma. A subgroup analysis of patients at higher risk of developing SBP showed 87% prevention rates for the norfloxacin group and 94% for the ciprofloxacin group, although this result was not statistically significant.
The major limitation of this study is that it was not double blinded, so patients were aware of which medication they were taking. Additionally, almost 10% of the cohort was lost to follow-up, but this was accounted for in the sample-size calculation.
Bottom line: Once weekly administration of ciprofloxacin is not inferior to daily norfloxacin for the prevention of SBP in patients with cirrhosis and low ascitic protein levels and may provide a more cost-effective therapy with greater patient compliance.
Citation: Yim HJ et al. Daily norfloxacin vs weekly ciprofloxacin to prevent spontaneous bacterial peritonitis: A randomized controlled trial. Am J Gastroenterol. 2018 Aug;113:1167-76.
Dr. Angeli is an assistant professor in the division of hospital medicine, University of New Mexico.
Clinical question: Does ciprofloxacin administered once weekly prevent spontaneous bacterial peritonitis (SBP) as effectively as daily norfloxacin?
Background: Studies have shown that daily administration of norfloxacin is effective for primary prophylaxis as well as secondary prevention of SBP in patients with cirrhosis and ascites. Prior studies have demonstrated efficacy of weekly ciprofloxacin, but no previous studies have compared the two antibiotics.
Study design: Investigator initiated open-label randomized, controlled trial.
Setting: Seven tertiary hospitals in South Korea.
Synopsis: The investigators enrolled 124 patients aged 20-75 with cirrhosis and ascites, ascitic cell count less than 250/mm3, and either ascitic protein less than 1.5g/dL or a history of spontaneous bacterial peritonitis. The patients were randomized to receive norfloxacin 400 mg daily or ciprofloxacin 750 mg weekly, with routine visits during the 12-month study period.
The primary end point of SBP prevention rates at 1 year were 92.7% (51/55) in the norfloxacin group and 96.5% (55/57) in the ciprofloxacin group (P = .712), which met criteria for noninferiority. Other outcomes included no difference in rates of liver transplantation, infectious complications, hepatorenal syndrome, hepatic encephalopathy, variceal bleeding, and hepatocellular carcinoma. A subgroup analysis of patients at higher risk of developing SBP showed 87% prevention rates for the norfloxacin group and 94% for the ciprofloxacin group, although this result was not statistically significant.
The major limitation of this study is that it was not double blinded, so patients were aware of which medication they were taking. Additionally, almost 10% of the cohort was lost to follow-up, but this was accounted for in the sample-size calculation.
Bottom line: Once weekly administration of ciprofloxacin is not inferior to daily norfloxacin for the prevention of SBP in patients with cirrhosis and low ascitic protein levels and may provide a more cost-effective therapy with greater patient compliance.
Citation: Yim HJ et al. Daily norfloxacin vs weekly ciprofloxacin to prevent spontaneous bacterial peritonitis: A randomized controlled trial. Am J Gastroenterol. 2018 Aug;113:1167-76.
Dr. Angeli is an assistant professor in the division of hospital medicine, University of New Mexico.
Add magnesium to treatment of AF with rapid ventricular response
Background: Most large studies of magnesium sulfate for assistance with rate control in AF occurred in the postoperative setting. This study compared rate control in the ED using magnesium sulfate at high (9 g) and low (4.5 g) doses vs. placebo in combination with usual treatment with atrioventricular nodal-blocking agents.
Study design: Double-blind, prospective, randomized, controlled trial.
Setting: Three tertiary Tunisian EDs.
Synopsis: This trial in Tunisian EDs enrolled 450 patients who presented with AF with rapid ventricular response and were divided into three groups: placebo, low-dose magnesium, and high-dose magnesium. Each patient’s trial medication was given as a 100-cc infusion. Patients were then treated with AV nodal-blocking agents at the discretion of the ED physician. The primary outcome was 20% reduction in rate or heart rate of less than 90 beats per minute. Notable exclusion criteria included hypotension, altered consciousness, decompensated heart failure, MI, and renal failure.
Rate control was achieved at 4 hours in 64% of patients with low-dose magnesium, 59% with high-dose magnesium, and 43% with placebo. At 24 hours, reduction in rate was controlled for 97% of patients on the low dose, 94% on the high dose, and 83% on placebo. Adverse events were mostly flushing, which occurred more frequently with the high dose than the low dose. Major limitations of the study included a lack of statistical assessment regarding baseline similarity between the two groups and that generalizability was limited by a preference for digoxin as the AV nodal agent.
Bottom line: This trial demonstrated that 4.5 g of magnesium sulfate was a useful addition to AV nodal blockers in achieving faster rate control for atrial fibrillation with rapid ventricular response in selected ED patients.
Citation: Bouida W et al. Low-dose magnesium sulfate versus high dose in the early management of rapid atrial fibrillation: Randomized controlled double blind study. Acad Emerg Med. 2018 Jul 19. doi: 10.1111/acem.13522.
Dr. Scott is an assistant professor in the division of hospital medicine, University of New Mexico.
Background: Most large studies of magnesium sulfate for assistance with rate control in AF occurred in the postoperative setting. This study compared rate control in the ED using magnesium sulfate at high (9 g) and low (4.5 g) doses vs. placebo in combination with usual treatment with atrioventricular nodal-blocking agents.
Study design: Double-blind, prospective, randomized, controlled trial.
Setting: Three tertiary Tunisian EDs.
Synopsis: This trial in Tunisian EDs enrolled 450 patients who presented with AF with rapid ventricular response and were divided into three groups: placebo, low-dose magnesium, and high-dose magnesium. Each patient’s trial medication was given as a 100-cc infusion. Patients were then treated with AV nodal-blocking agents at the discretion of the ED physician. The primary outcome was 20% reduction in rate or heart rate of less than 90 beats per minute. Notable exclusion criteria included hypotension, altered consciousness, decompensated heart failure, MI, and renal failure.
Rate control was achieved at 4 hours in 64% of patients with low-dose magnesium, 59% with high-dose magnesium, and 43% with placebo. At 24 hours, reduction in rate was controlled for 97% of patients on the low dose, 94% on the high dose, and 83% on placebo. Adverse events were mostly flushing, which occurred more frequently with the high dose than the low dose. Major limitations of the study included a lack of statistical assessment regarding baseline similarity between the two groups and that generalizability was limited by a preference for digoxin as the AV nodal agent.
Bottom line: This trial demonstrated that 4.5 g of magnesium sulfate was a useful addition to AV nodal blockers in achieving faster rate control for atrial fibrillation with rapid ventricular response in selected ED patients.
Citation: Bouida W et al. Low-dose magnesium sulfate versus high dose in the early management of rapid atrial fibrillation: Randomized controlled double blind study. Acad Emerg Med. 2018 Jul 19. doi: 10.1111/acem.13522.
Dr. Scott is an assistant professor in the division of hospital medicine, University of New Mexico.
Background: Most large studies of magnesium sulfate for assistance with rate control in AF occurred in the postoperative setting. This study compared rate control in the ED using magnesium sulfate at high (9 g) and low (4.5 g) doses vs. placebo in combination with usual treatment with atrioventricular nodal-blocking agents.
Study design: Double-blind, prospective, randomized, controlled trial.
Setting: Three tertiary Tunisian EDs.
Synopsis: This trial in Tunisian EDs enrolled 450 patients who presented with AF with rapid ventricular response and were divided into three groups: placebo, low-dose magnesium, and high-dose magnesium. Each patient’s trial medication was given as a 100-cc infusion. Patients were then treated with AV nodal-blocking agents at the discretion of the ED physician. The primary outcome was 20% reduction in rate or heart rate of less than 90 beats per minute. Notable exclusion criteria included hypotension, altered consciousness, decompensated heart failure, MI, and renal failure.
Rate control was achieved at 4 hours in 64% of patients with low-dose magnesium, 59% with high-dose magnesium, and 43% with placebo. At 24 hours, reduction in rate was controlled for 97% of patients on the low dose, 94% on the high dose, and 83% on placebo. Adverse events were mostly flushing, which occurred more frequently with the high dose than the low dose. Major limitations of the study included a lack of statistical assessment regarding baseline similarity between the two groups and that generalizability was limited by a preference for digoxin as the AV nodal agent.
Bottom line: This trial demonstrated that 4.5 g of magnesium sulfate was a useful addition to AV nodal blockers in achieving faster rate control for atrial fibrillation with rapid ventricular response in selected ED patients.
Citation: Bouida W et al. Low-dose magnesium sulfate versus high dose in the early management of rapid atrial fibrillation: Randomized controlled double blind study. Acad Emerg Med. 2018 Jul 19. doi: 10.1111/acem.13522.
Dr. Scott is an assistant professor in the division of hospital medicine, University of New Mexico.
New recommendations on TB screening for health care workers
U.S. health care personnel no longer need to undergo routine tuberculosis testing in the absence of known exposure, according to new screening guidelines from the National Tuberculosis Controllers Association and CDC.
The revised guidelines on tuberculosis screening, testing, and treatment of U.S. health care personnel, published in Morbidity and Mortality Weekly Report, are the first update since 2005. The new recommendations reflect a reduction in concern about U.S. health care personnel’s risk of occupational exposure to latent and active tuberculosis infection.
Lynn E. Sosa, MD, from the Connecticut Department of Public Health and National Tuberculosis Controllers Association, and coauthors wrote that rates of tuberculosis infection in the United States have declined by 73% since 1991, from 10.4/100,000 population in 1991 to 2.8/100,000 in 2017. This has been matched by similar declines among health care workers, which the authors said raised questions about the cost-effectiveness of the previously recommended routine serial occupational testing.
“In addition, a recent retrospective cohort study of approximately 40,000 health care personnel at a tertiary U.S. medical center in a low TB-incidence state found an extremely low rate of TST conversion (0.3%) during 1998-2014, with a limited proportion attributable to occupational exposure,” they wrote.
The new guidelines recommend health care personnel undergo baseline or preplacement tuberculosis testing with an interferon-gamma release assay (IGRA) or a tuberculin skin test (TST), as well as individual risk assessment and symptom evaluation.
The individual risk assessment considers whether the person has lived in a country with a high tuberculosis rate, whether they are immunosuppressed, or whether they have had close contact with someone with infectious tuberculosis.
This risk assessment can help decide how to interpret an initial positive test result, the authors said.
“For example, health care personnel with a positive test who are asymptomatic, unlikely to be infected with M. [Mycobacterium] tuberculosis, and at low risk for progression on the basis of their risk assessment should have a second test (either an IGRA or a TST) as recommended in the 2017 TB diagnostic guidelines of the American Thoracic Society, Infectious Diseases Society of America, and CDC,” they wrote. “In this example, the health care personnel should be considered infected with M. tuberculosis only if both the first and second tests are positive.”
After that baseline testing, personnel do not need to undergo routine serial testing except in the case of known exposure or ongoing transmission. The guideline authors suggested serial screening might be considered for health care workers whose work puts them at greater risk – for example, pulmonologists or respiratory therapists – or for those working in settings in which transmission has happened in the past.
For personnel with latent tuberculosis infection, the guidelines recommend “encouragement of treatment” unless it is contraindicated, and annual symptom screening in those not undergoing treatment.
The guideline committee also advocated for annual tuberculosis education for all health care workers.
The new recommendations were based on a systematic review of 36 studies of tuberculosis screening and testing among health care personnel, 16 of which were performed in the United States, and all but two of which were conducted in a hospital setting.
The authors stressed that recommendations from the 2005 CDC guidelines – which do not pertain to health care personnel screening, testing, treatment and education – remain unchanged.
One author declared personal fees from the National Tuberculosis Controllers Association during the conduct of the study. Two others reported unrelated grants and personal fees from private industry. No other conflicts of interest were disclosed.
SOURCE: Sosa L et al. MMWR. 2019;68:439-43.
U.S. health care personnel no longer need to undergo routine tuberculosis testing in the absence of known exposure, according to new screening guidelines from the National Tuberculosis Controllers Association and CDC.
The revised guidelines on tuberculosis screening, testing, and treatment of U.S. health care personnel, published in Morbidity and Mortality Weekly Report, are the first update since 2005. The new recommendations reflect a reduction in concern about U.S. health care personnel’s risk of occupational exposure to latent and active tuberculosis infection.
Lynn E. Sosa, MD, from the Connecticut Department of Public Health and National Tuberculosis Controllers Association, and coauthors wrote that rates of tuberculosis infection in the United States have declined by 73% since 1991, from 10.4/100,000 population in 1991 to 2.8/100,000 in 2017. This has been matched by similar declines among health care workers, which the authors said raised questions about the cost-effectiveness of the previously recommended routine serial occupational testing.
“In addition, a recent retrospective cohort study of approximately 40,000 health care personnel at a tertiary U.S. medical center in a low TB-incidence state found an extremely low rate of TST conversion (0.3%) during 1998-2014, with a limited proportion attributable to occupational exposure,” they wrote.
The new guidelines recommend health care personnel undergo baseline or preplacement tuberculosis testing with an interferon-gamma release assay (IGRA) or a tuberculin skin test (TST), as well as individual risk assessment and symptom evaluation.
The individual risk assessment considers whether the person has lived in a country with a high tuberculosis rate, whether they are immunosuppressed, or whether they have had close contact with someone with infectious tuberculosis.
This risk assessment can help decide how to interpret an initial positive test result, the authors said.
“For example, health care personnel with a positive test who are asymptomatic, unlikely to be infected with M. [Mycobacterium] tuberculosis, and at low risk for progression on the basis of their risk assessment should have a second test (either an IGRA or a TST) as recommended in the 2017 TB diagnostic guidelines of the American Thoracic Society, Infectious Diseases Society of America, and CDC,” they wrote. “In this example, the health care personnel should be considered infected with M. tuberculosis only if both the first and second tests are positive.”
After that baseline testing, personnel do not need to undergo routine serial testing except in the case of known exposure or ongoing transmission. The guideline authors suggested serial screening might be considered for health care workers whose work puts them at greater risk – for example, pulmonologists or respiratory therapists – or for those working in settings in which transmission has happened in the past.
For personnel with latent tuberculosis infection, the guidelines recommend “encouragement of treatment” unless it is contraindicated, and annual symptom screening in those not undergoing treatment.
The guideline committee also advocated for annual tuberculosis education for all health care workers.
The new recommendations were based on a systematic review of 36 studies of tuberculosis screening and testing among health care personnel, 16 of which were performed in the United States, and all but two of which were conducted in a hospital setting.
The authors stressed that recommendations from the 2005 CDC guidelines – which do not pertain to health care personnel screening, testing, treatment and education – remain unchanged.
One author declared personal fees from the National Tuberculosis Controllers Association during the conduct of the study. Two others reported unrelated grants and personal fees from private industry. No other conflicts of interest were disclosed.
SOURCE: Sosa L et al. MMWR. 2019;68:439-43.
U.S. health care personnel no longer need to undergo routine tuberculosis testing in the absence of known exposure, according to new screening guidelines from the National Tuberculosis Controllers Association and CDC.
The revised guidelines on tuberculosis screening, testing, and treatment of U.S. health care personnel, published in Morbidity and Mortality Weekly Report, are the first update since 2005. The new recommendations reflect a reduction in concern about U.S. health care personnel’s risk of occupational exposure to latent and active tuberculosis infection.
Lynn E. Sosa, MD, from the Connecticut Department of Public Health and National Tuberculosis Controllers Association, and coauthors wrote that rates of tuberculosis infection in the United States have declined by 73% since 1991, from 10.4/100,000 population in 1991 to 2.8/100,000 in 2017. This has been matched by similar declines among health care workers, which the authors said raised questions about the cost-effectiveness of the previously recommended routine serial occupational testing.
“In addition, a recent retrospective cohort study of approximately 40,000 health care personnel at a tertiary U.S. medical center in a low TB-incidence state found an extremely low rate of TST conversion (0.3%) during 1998-2014, with a limited proportion attributable to occupational exposure,” they wrote.
The new guidelines recommend health care personnel undergo baseline or preplacement tuberculosis testing with an interferon-gamma release assay (IGRA) or a tuberculin skin test (TST), as well as individual risk assessment and symptom evaluation.
The individual risk assessment considers whether the person has lived in a country with a high tuberculosis rate, whether they are immunosuppressed, or whether they have had close contact with someone with infectious tuberculosis.
This risk assessment can help decide how to interpret an initial positive test result, the authors said.
“For example, health care personnel with a positive test who are asymptomatic, unlikely to be infected with M. [Mycobacterium] tuberculosis, and at low risk for progression on the basis of their risk assessment should have a second test (either an IGRA or a TST) as recommended in the 2017 TB diagnostic guidelines of the American Thoracic Society, Infectious Diseases Society of America, and CDC,” they wrote. “In this example, the health care personnel should be considered infected with M. tuberculosis only if both the first and second tests are positive.”
After that baseline testing, personnel do not need to undergo routine serial testing except in the case of known exposure or ongoing transmission. The guideline authors suggested serial screening might be considered for health care workers whose work puts them at greater risk – for example, pulmonologists or respiratory therapists – or for those working in settings in which transmission has happened in the past.
For personnel with latent tuberculosis infection, the guidelines recommend “encouragement of treatment” unless it is contraindicated, and annual symptom screening in those not undergoing treatment.
The guideline committee also advocated for annual tuberculosis education for all health care workers.
The new recommendations were based on a systematic review of 36 studies of tuberculosis screening and testing among health care personnel, 16 of which were performed in the United States, and all but two of which were conducted in a hospital setting.
The authors stressed that recommendations from the 2005 CDC guidelines – which do not pertain to health care personnel screening, testing, treatment and education – remain unchanged.
One author declared personal fees from the National Tuberculosis Controllers Association during the conduct of the study. Two others reported unrelated grants and personal fees from private industry. No other conflicts of interest were disclosed.
SOURCE: Sosa L et al. MMWR. 2019;68:439-43.
FROM MMWR
Survey: Physicians predict increase in measles deaths
by real-time market insights technology firm InCrowd.
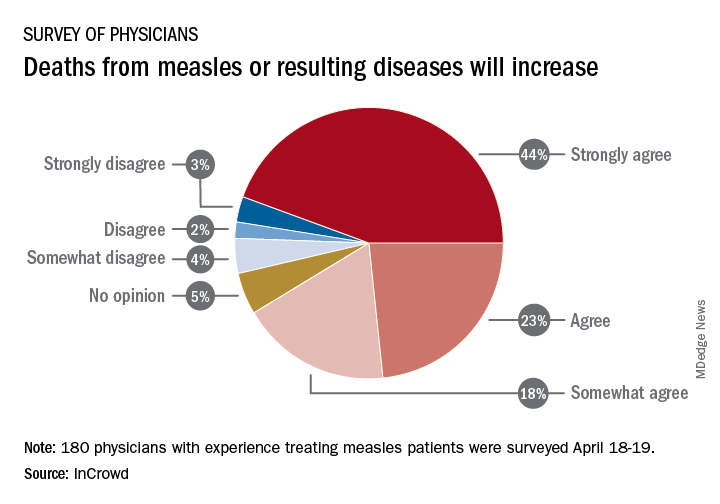
Among the 180 physicians with experience treating measles, 23% agreed and 44% said that they strongly agreed with the statement that measles deaths would increase, and another 18% said that they somewhat agreed. Only 9% expressed some level of disagreement, InCrowd said.
Most of those respondents also believe that summer travel will increase measles outbreaks (29% agreed and 30% strongly agreed) and that more communities will adopt requirements for measles vaccinations (26% and 36%). A majority also said that education about vaccinations will improve (26% agreed and 29% strongly agreed), but almost half of the physicians surveyed also expect vaccination misinformation to get worse (29% and 19%), InCrowd reported.
“With 44% of respondents predicting a high likelihood that deaths caused by measles will increase, the data show the imperative for physicians and patients to keep up the dialogue. … We have a long way to go before declaring victory,” said Diane Hayes, PhD, president and cofounder of InCrowd.
The InCrowd 5-minute microsurvey was conducted on April 18-19, 2019, and included 455 primary care physicians, of whom 40% said that they have treated or knew of colleagues in their facility or community who have treated patients with measles. Of those 180 respondents, 89 were pediatricians and 91 were in other primary care specialties.
by real-time market insights technology firm InCrowd.

Among the 180 physicians with experience treating measles, 23% agreed and 44% said that they strongly agreed with the statement that measles deaths would increase, and another 18% said that they somewhat agreed. Only 9% expressed some level of disagreement, InCrowd said.
Most of those respondents also believe that summer travel will increase measles outbreaks (29% agreed and 30% strongly agreed) and that more communities will adopt requirements for measles vaccinations (26% and 36%). A majority also said that education about vaccinations will improve (26% agreed and 29% strongly agreed), but almost half of the physicians surveyed also expect vaccination misinformation to get worse (29% and 19%), InCrowd reported.
“With 44% of respondents predicting a high likelihood that deaths caused by measles will increase, the data show the imperative for physicians and patients to keep up the dialogue. … We have a long way to go before declaring victory,” said Diane Hayes, PhD, president and cofounder of InCrowd.
The InCrowd 5-minute microsurvey was conducted on April 18-19, 2019, and included 455 primary care physicians, of whom 40% said that they have treated or knew of colleagues in their facility or community who have treated patients with measles. Of those 180 respondents, 89 were pediatricians and 91 were in other primary care specialties.
by real-time market insights technology firm InCrowd.

Among the 180 physicians with experience treating measles, 23% agreed and 44% said that they strongly agreed with the statement that measles deaths would increase, and another 18% said that they somewhat agreed. Only 9% expressed some level of disagreement, InCrowd said.
Most of those respondents also believe that summer travel will increase measles outbreaks (29% agreed and 30% strongly agreed) and that more communities will adopt requirements for measles vaccinations (26% and 36%). A majority also said that education about vaccinations will improve (26% agreed and 29% strongly agreed), but almost half of the physicians surveyed also expect vaccination misinformation to get worse (29% and 19%), InCrowd reported.
“With 44% of respondents predicting a high likelihood that deaths caused by measles will increase, the data show the imperative for physicians and patients to keep up the dialogue. … We have a long way to go before declaring victory,” said Diane Hayes, PhD, president and cofounder of InCrowd.
The InCrowd 5-minute microsurvey was conducted on April 18-19, 2019, and included 455 primary care physicians, of whom 40% said that they have treated or knew of colleagues in their facility or community who have treated patients with measles. Of those 180 respondents, 89 were pediatricians and 91 were in other primary care specialties.
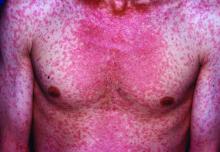
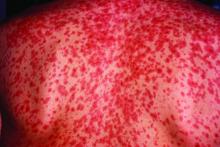
Measles complications in the U.S. unchanged in posteradication era
CHICAGO – An evaluation of the measles threat in the modern era gives no indication that the risk of complications or death is any different than it was before a vaccine became available, according to an analysis of inpatient complications between 2002 and 2013.
In 2000, measles was declared eliminated in the United States, but for those who have been infected since that time, the risk of serious complications and death has not diminished, noted Raj Chovatiya, MD, PhD, in a session at the annual meeting of the Society for Investigative Dermatology.
By eliminated, the Centers of Disease Control and Prevention – which reported 86 confirmed cases of measles in 2000 – was referring to a technical definition of no new endemic or continuous transmissions in the previous 12 months. It was expected that a modest number of cases of this reportable disease would continue to accrue for an infection that remains common elsewhere in the world.
“Worldwide there are about 20 million cases of measles annually with an estimated 100,000 deaths attributed to this cause,” said Dr. Chovatiya, who is a dermatology resident at Northwestern University, Chicago.
In the United States, posteradication infection rates remained at low levels for several years but were already rising from 2002 to 2013, when Dr. Chovatiya and his coinvestigators sought to describe the incidence, associations, comorbidities, and outcomes of hospitalizations for measles. Toward the end of the period the researchers were examining the incidence rates climbed more steeply.
“So far this year, 764 CDC cases of measles [were] reported. That is the most we have seen in the U.S. since 1994,” Dr. Chovatiya said.
Based on his analysis of hospitalizations from 2002 to 2013, the threat of these outbreaks is no different then that before the disease was declared eliminated or before a vaccine became available.
The cross-sectional study was conducted with data from the Nationwide Inpatient Sample, an all-payer database that is considered to be a representative of national trends.
Characteristic of measles, the majority of the 582 hospitalizations evaluated over this period occurred in children aged between 1 and 9 years. The proportion of patients with preexisting chronic comorbid conditions was low. Rather, “most were pretty healthy” prior to admission, according to Dr. Chovatiya, who said that the majority of admissions were from an emergency department.
Measles, which targets epithelial cells and depresses the immune system, is a potentially serious disease because of its ability to produce complications in essentially every organ of the body, including the lungs, kidneys, blood, and central nervous system. Consistent with past studies, the most common complication in this series was pneumonia, observed in 20% of patients. The list of other serious complications identified in this study period, including encephalitis and acute renal failure, was long.
“We observed death in 4.3% of our 582 cases, or about 25 cases,” reported Dr. Chovatiya. He indicated that this is a high percentage among a population composed largely of children who were well before hospitalization.
The mortality rate from measles was numerically but not statistically higher than that of overall hospital admissions during this period, but an admission for measles was associated with significantly longer average length of stay (3.7 vs. 3.5 days) and slightly but significantly higher direct costs ($18,907 vs. $18,474).
“I want to point out that these are just direct inpatient costs,” Dr. Chovatiya said. Extrapolating from published data about indirect expenses, he said that the total health cost burden “is absolutely staggering.”
Previous studies have suggested that about 25% of patients with measles require hospitalization and 1 in every 1,000 patients will die. The data collected by Dr. Chovatiya support these often-cited figures, indicating that they remain unchanged in the modern era.
particularly insufficient penetration of vaccination in many communities.
The vaccine “is inexpensive, extremely effective, and lifesaving,” said Dr. Chovatiya, making the point that all of the morbidity, mortality, and costs he described are largely avoidable.
Attempting to provide perspective of the measles threat and the impact of the vaccine, Dr. Chovatiya cited a hypothetical calculation that 732,000 deaths from measles would have been expected in the United States among the pool of children born between 1994 and 2013 had no vaccine been offered. Again, most of these deaths would have occurred in otherwise healthy children.
Dr. Chovatiya reported no potential conflicts of interest.
CHICAGO – An evaluation of the measles threat in the modern era gives no indication that the risk of complications or death is any different than it was before a vaccine became available, according to an analysis of inpatient complications between 2002 and 2013.
In 2000, measles was declared eliminated in the United States, but for those who have been infected since that time, the risk of serious complications and death has not diminished, noted Raj Chovatiya, MD, PhD, in a session at the annual meeting of the Society for Investigative Dermatology.
By eliminated, the Centers of Disease Control and Prevention – which reported 86 confirmed cases of measles in 2000 – was referring to a technical definition of no new endemic or continuous transmissions in the previous 12 months. It was expected that a modest number of cases of this reportable disease would continue to accrue for an infection that remains common elsewhere in the world.
“Worldwide there are about 20 million cases of measles annually with an estimated 100,000 deaths attributed to this cause,” said Dr. Chovatiya, who is a dermatology resident at Northwestern University, Chicago.
In the United States, posteradication infection rates remained at low levels for several years but were already rising from 2002 to 2013, when Dr. Chovatiya and his coinvestigators sought to describe the incidence, associations, comorbidities, and outcomes of hospitalizations for measles. Toward the end of the period the researchers were examining the incidence rates climbed more steeply.
“So far this year, 764 CDC cases of measles [were] reported. That is the most we have seen in the U.S. since 1994,” Dr. Chovatiya said.
Based on his analysis of hospitalizations from 2002 to 2013, the threat of these outbreaks is no different then that before the disease was declared eliminated or before a vaccine became available.
The cross-sectional study was conducted with data from the Nationwide Inpatient Sample, an all-payer database that is considered to be a representative of national trends.
Characteristic of measles, the majority of the 582 hospitalizations evaluated over this period occurred in children aged between 1 and 9 years. The proportion of patients with preexisting chronic comorbid conditions was low. Rather, “most were pretty healthy” prior to admission, according to Dr. Chovatiya, who said that the majority of admissions were from an emergency department.
Measles, which targets epithelial cells and depresses the immune system, is a potentially serious disease because of its ability to produce complications in essentially every organ of the body, including the lungs, kidneys, blood, and central nervous system. Consistent with past studies, the most common complication in this series was pneumonia, observed in 20% of patients. The list of other serious complications identified in this study period, including encephalitis and acute renal failure, was long.
“We observed death in 4.3% of our 582 cases, or about 25 cases,” reported Dr. Chovatiya. He indicated that this is a high percentage among a population composed largely of children who were well before hospitalization.
The mortality rate from measles was numerically but not statistically higher than that of overall hospital admissions during this period, but an admission for measles was associated with significantly longer average length of stay (3.7 vs. 3.5 days) and slightly but significantly higher direct costs ($18,907 vs. $18,474).
“I want to point out that these are just direct inpatient costs,” Dr. Chovatiya said. Extrapolating from published data about indirect expenses, he said that the total health cost burden “is absolutely staggering.”
Previous studies have suggested that about 25% of patients with measles require hospitalization and 1 in every 1,000 patients will die. The data collected by Dr. Chovatiya support these often-cited figures, indicating that they remain unchanged in the modern era.
particularly insufficient penetration of vaccination in many communities.
The vaccine “is inexpensive, extremely effective, and lifesaving,” said Dr. Chovatiya, making the point that all of the morbidity, mortality, and costs he described are largely avoidable.
Attempting to provide perspective of the measles threat and the impact of the vaccine, Dr. Chovatiya cited a hypothetical calculation that 732,000 deaths from measles would have been expected in the United States among the pool of children born between 1994 and 2013 had no vaccine been offered. Again, most of these deaths would have occurred in otherwise healthy children.
Dr. Chovatiya reported no potential conflicts of interest.
CHICAGO – An evaluation of the measles threat in the modern era gives no indication that the risk of complications or death is any different than it was before a vaccine became available, according to an analysis of inpatient complications between 2002 and 2013.
In 2000, measles was declared eliminated in the United States, but for those who have been infected since that time, the risk of serious complications and death has not diminished, noted Raj Chovatiya, MD, PhD, in a session at the annual meeting of the Society for Investigative Dermatology.
By eliminated, the Centers of Disease Control and Prevention – which reported 86 confirmed cases of measles in 2000 – was referring to a technical definition of no new endemic or continuous transmissions in the previous 12 months. It was expected that a modest number of cases of this reportable disease would continue to accrue for an infection that remains common elsewhere in the world.
“Worldwide there are about 20 million cases of measles annually with an estimated 100,000 deaths attributed to this cause,” said Dr. Chovatiya, who is a dermatology resident at Northwestern University, Chicago.
In the United States, posteradication infection rates remained at low levels for several years but were already rising from 2002 to 2013, when Dr. Chovatiya and his coinvestigators sought to describe the incidence, associations, comorbidities, and outcomes of hospitalizations for measles. Toward the end of the period the researchers were examining the incidence rates climbed more steeply.
“So far this year, 764 CDC cases of measles [were] reported. That is the most we have seen in the U.S. since 1994,” Dr. Chovatiya said.
Based on his analysis of hospitalizations from 2002 to 2013, the threat of these outbreaks is no different then that before the disease was declared eliminated or before a vaccine became available.
The cross-sectional study was conducted with data from the Nationwide Inpatient Sample, an all-payer database that is considered to be a representative of national trends.
Characteristic of measles, the majority of the 582 hospitalizations evaluated over this period occurred in children aged between 1 and 9 years. The proportion of patients with preexisting chronic comorbid conditions was low. Rather, “most were pretty healthy” prior to admission, according to Dr. Chovatiya, who said that the majority of admissions were from an emergency department.
Measles, which targets epithelial cells and depresses the immune system, is a potentially serious disease because of its ability to produce complications in essentially every organ of the body, including the lungs, kidneys, blood, and central nervous system. Consistent with past studies, the most common complication in this series was pneumonia, observed in 20% of patients. The list of other serious complications identified in this study period, including encephalitis and acute renal failure, was long.
“We observed death in 4.3% of our 582 cases, or about 25 cases,” reported Dr. Chovatiya. He indicated that this is a high percentage among a population composed largely of children who were well before hospitalization.
The mortality rate from measles was numerically but not statistically higher than that of overall hospital admissions during this period, but an admission for measles was associated with significantly longer average length of stay (3.7 vs. 3.5 days) and slightly but significantly higher direct costs ($18,907 vs. $18,474).
“I want to point out that these are just direct inpatient costs,” Dr. Chovatiya said. Extrapolating from published data about indirect expenses, he said that the total health cost burden “is absolutely staggering.”
Previous studies have suggested that about 25% of patients with measles require hospitalization and 1 in every 1,000 patients will die. The data collected by Dr. Chovatiya support these often-cited figures, indicating that they remain unchanged in the modern era.
particularly insufficient penetration of vaccination in many communities.
The vaccine “is inexpensive, extremely effective, and lifesaving,” said Dr. Chovatiya, making the point that all of the morbidity, mortality, and costs he described are largely avoidable.
Attempting to provide perspective of the measles threat and the impact of the vaccine, Dr. Chovatiya cited a hypothetical calculation that 732,000 deaths from measles would have been expected in the United States among the pool of children born between 1994 and 2013 had no vaccine been offered. Again, most of these deaths would have occurred in otherwise healthy children.
Dr. Chovatiya reported no potential conflicts of interest.
REPORTING FROM SID 2019
Aspirin shows little benefit for primary prevention of vascular disease in diabetes
Background: Multiple large, randomized, controlled trials and meta-analyses that used aspirin as primary prevention for vascular events showed decreased vascular events, but a significant counterbalanced risk of bleeding. Since diabetes carries a higher risk of vascular events, this study examines aspirin for primary prevention of vascular events in diabetic patients.
Study design: Large, randomized, controlled trial.
Setting: British registry-based study.
Synopsis: This is a 9-year randomized, controlled trial that included 15,480 British patients with diabetes without known vascular disease who were randomized to receive a 100-mg aspirin daily or placebo. Participants in each group were closely matched patients with diabetes who were recruited using registry data and were aged 40 years and older with no alternative strong indication for aspirin.
Overall, aspirin provided no difference in mortality but showed an absolute 1.3% decrease in first vascular events or revascularization procedures with an absolute 1.1% increase in first occurrence of major bleeding event. Approximately 60% of the bleeding events were gastrointestinal or “other” urinary/nose bleeding, and there was no statistically significant increase in intracranial hemorrhage, hemorrhagic stroke, or vision-threatening eye bleeding. Vascular events were defined as transient ischemic attack (TIA), nonfatal MI, nonfatal ischemic stroke, or vascular death excluding intracranial hemorrhage. The major limitation of this study is that it had a composite of endpoints of different clinical significance. Furthermore, TIA as a major vascular event was added after the study began to increase statistical power, and when it is excluded, the difference for vascular events is not statistically significant.
Bottom line: Aspirin when used in primary prevention of vascular events in diabetes provides no improvement in mortality, and the benefit of prevention of vascular events must be weighed against the risks of bleeding.
Citation: The ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in diabetes. N Eng J Med. 2018 Oct 18;379(16):1529-39.
Dr. Scott is an assistant professor in the division of hospital medicine, University of New Mexico.
Background: Multiple large, randomized, controlled trials and meta-analyses that used aspirin as primary prevention for vascular events showed decreased vascular events, but a significant counterbalanced risk of bleeding. Since diabetes carries a higher risk of vascular events, this study examines aspirin for primary prevention of vascular events in diabetic patients.
Study design: Large, randomized, controlled trial.
Setting: British registry-based study.
Synopsis: This is a 9-year randomized, controlled trial that included 15,480 British patients with diabetes without known vascular disease who were randomized to receive a 100-mg aspirin daily or placebo. Participants in each group were closely matched patients with diabetes who were recruited using registry data and were aged 40 years and older with no alternative strong indication for aspirin.
Overall, aspirin provided no difference in mortality but showed an absolute 1.3% decrease in first vascular events or revascularization procedures with an absolute 1.1% increase in first occurrence of major bleeding event. Approximately 60% of the bleeding events were gastrointestinal or “other” urinary/nose bleeding, and there was no statistically significant increase in intracranial hemorrhage, hemorrhagic stroke, or vision-threatening eye bleeding. Vascular events were defined as transient ischemic attack (TIA), nonfatal MI, nonfatal ischemic stroke, or vascular death excluding intracranial hemorrhage. The major limitation of this study is that it had a composite of endpoints of different clinical significance. Furthermore, TIA as a major vascular event was added after the study began to increase statistical power, and when it is excluded, the difference for vascular events is not statistically significant.
Bottom line: Aspirin when used in primary prevention of vascular events in diabetes provides no improvement in mortality, and the benefit of prevention of vascular events must be weighed against the risks of bleeding.
Citation: The ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in diabetes. N Eng J Med. 2018 Oct 18;379(16):1529-39.
Dr. Scott is an assistant professor in the division of hospital medicine, University of New Mexico.
Background: Multiple large, randomized, controlled trials and meta-analyses that used aspirin as primary prevention for vascular events showed decreased vascular events, but a significant counterbalanced risk of bleeding. Since diabetes carries a higher risk of vascular events, this study examines aspirin for primary prevention of vascular events in diabetic patients.
Study design: Large, randomized, controlled trial.
Setting: British registry-based study.
Synopsis: This is a 9-year randomized, controlled trial that included 15,480 British patients with diabetes without known vascular disease who were randomized to receive a 100-mg aspirin daily or placebo. Participants in each group were closely matched patients with diabetes who were recruited using registry data and were aged 40 years and older with no alternative strong indication for aspirin.
Overall, aspirin provided no difference in mortality but showed an absolute 1.3% decrease in first vascular events or revascularization procedures with an absolute 1.1% increase in first occurrence of major bleeding event. Approximately 60% of the bleeding events were gastrointestinal or “other” urinary/nose bleeding, and there was no statistically significant increase in intracranial hemorrhage, hemorrhagic stroke, or vision-threatening eye bleeding. Vascular events were defined as transient ischemic attack (TIA), nonfatal MI, nonfatal ischemic stroke, or vascular death excluding intracranial hemorrhage. The major limitation of this study is that it had a composite of endpoints of different clinical significance. Furthermore, TIA as a major vascular event was added after the study began to increase statistical power, and when it is excluded, the difference for vascular events is not statistically significant.
Bottom line: Aspirin when used in primary prevention of vascular events in diabetes provides no improvement in mortality, and the benefit of prevention of vascular events must be weighed against the risks of bleeding.
Citation: The ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in diabetes. N Eng J Med. 2018 Oct 18;379(16):1529-39.
Dr. Scott is an assistant professor in the division of hospital medicine, University of New Mexico.
Comorbid depression worsens many pediatric hospital outcomes
according to a study in the Journal of Affective Disorders.
The investigators led by Mayowa Olusunmade, MD, MPH, of New Jersey Medical School, Newark, found that, compared with those among nondepressed pediatric patients, hospitalization costs were $2,961 higher (P less than .001), length of stay was 0.89 days longer (P less than .001), and odds of death as an outcome while hospitalized was 1.77 times higher (P = .013) among depressed pediatric patients. On the other hand, depressed patients had 0.3 fewer procedures (P less than .001) than nondepressed patients.
This analysis is based on 17,073 pairs of patients with and without depression that were created through one-to-one propensity score matching. The investigators drew these pairs from an estimated 937,971 patients in the Kids’ Inpatient Database for 2012 who were identified as being aged 6 years and older and having any of the 10 of the most common diagnoses other than affective disorders. The investigators then determined which children among those identified had comorbid depression (2.9%) and which did not (97.1%) to create the propensity score–matched pairs.
One limitation in this study is that the mean age was 17.5 years because depression diagnosis is more atypical among younger patients such that adolescents were disproportionately represented.
The study did not receive funding, and the authors declared there are no conflicts of interest.
SOURCE: Olusunmade M et al. J Affect Disord. 2019 Mar 27. doi: 10.1016/j.jad.2019.03.073.
according to a study in the Journal of Affective Disorders.
The investigators led by Mayowa Olusunmade, MD, MPH, of New Jersey Medical School, Newark, found that, compared with those among nondepressed pediatric patients, hospitalization costs were $2,961 higher (P less than .001), length of stay was 0.89 days longer (P less than .001), and odds of death as an outcome while hospitalized was 1.77 times higher (P = .013) among depressed pediatric patients. On the other hand, depressed patients had 0.3 fewer procedures (P less than .001) than nondepressed patients.
This analysis is based on 17,073 pairs of patients with and without depression that were created through one-to-one propensity score matching. The investigators drew these pairs from an estimated 937,971 patients in the Kids’ Inpatient Database for 2012 who were identified as being aged 6 years and older and having any of the 10 of the most common diagnoses other than affective disorders. The investigators then determined which children among those identified had comorbid depression (2.9%) and which did not (97.1%) to create the propensity score–matched pairs.
One limitation in this study is that the mean age was 17.5 years because depression diagnosis is more atypical among younger patients such that adolescents were disproportionately represented.
The study did not receive funding, and the authors declared there are no conflicts of interest.
SOURCE: Olusunmade M et al. J Affect Disord. 2019 Mar 27. doi: 10.1016/j.jad.2019.03.073.
according to a study in the Journal of Affective Disorders.
The investigators led by Mayowa Olusunmade, MD, MPH, of New Jersey Medical School, Newark, found that, compared with those among nondepressed pediatric patients, hospitalization costs were $2,961 higher (P less than .001), length of stay was 0.89 days longer (P less than .001), and odds of death as an outcome while hospitalized was 1.77 times higher (P = .013) among depressed pediatric patients. On the other hand, depressed patients had 0.3 fewer procedures (P less than .001) than nondepressed patients.
This analysis is based on 17,073 pairs of patients with and without depression that were created through one-to-one propensity score matching. The investigators drew these pairs from an estimated 937,971 patients in the Kids’ Inpatient Database for 2012 who were identified as being aged 6 years and older and having any of the 10 of the most common diagnoses other than affective disorders. The investigators then determined which children among those identified had comorbid depression (2.9%) and which did not (97.1%) to create the propensity score–matched pairs.
One limitation in this study is that the mean age was 17.5 years because depression diagnosis is more atypical among younger patients such that adolescents were disproportionately represented.
The study did not receive funding, and the authors declared there are no conflicts of interest.
SOURCE: Olusunmade M et al. J Affect Disord. 2019 Mar 27. doi: 10.1016/j.jad.2019.03.073.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Severe OSA increases cardiovascular risk after surgery
Unrecognized severe obstructive sleep apnea is a risk factor for cardiovascular complications after major noncardiac surgery, according to a study published in JAMA.
The researchers state that perioperative mismanagement of obstructive sleep apnea can lead to serious medical consequences. “General anesthetics, sedatives, and postoperative analgesics are potent respiratory depressants that relax the upper airway dilator muscles and impair ventilatory response to hypoxemia and hypercapnia. Each of these events exacerbates [obstructive sleep apnea] and may predispose patients to postoperative cardiovascular complications,” said researchers who conducted the The Postoperative vascular complications in unrecognised Obstructive Sleep apnoea (POSA) study (NCT01494181).
They undertook a prospective observational cohort study involving 1,218 patients undergoing major noncardiac surgery, who were already considered at high risk of postoperative cardiovascular events – having, for example, a history of coronary artery disease, stroke, diabetes, or renal impairment. However, none had a prior diagnosis of obstructive sleep apnea.
Preoperative sleep monitoring revealed that two-thirds of the cohort had unrecognized and untreated obstructive sleep apnea, including 11.2% with severe obstructive sleep apnea.
At 30 days after surgery, patients with obstructive sleep apnea had a 49% higher risk of the primary outcome of myocardial injury, cardiac death, heart failure, thromboembolism, atrial fibrillation, or stroke, compared with those without obstructive sleep apnea.
However, this association was largely due to a significant 2.23-fold higher risk among patients with severe obstructive sleep apnea, while those with only moderate or mild sleep apnea did not show a significant increased risk of cardiovascular complications.
Patients in this study with severe obstructive sleep apnea had a 13-fold higher risk of cardiac death, 80% higher risk of myocardial injury, more than sixfold higher risk of heart failure, and nearly fourfold higher risk of atrial fibrillation.
Researchers also saw an association between obstructive sleep apnea and increased risk of infective outcomes, unplanned tracheal intubation, postoperative lung ventilation, and readmission to the ICU.
The majority of patients received nocturnal oximetry monitoring during their first 3 nights after surgery. This revealed that patients without obstructive sleep apnea had significant increases in oxygen desaturation index during their first night after surgery, while those with sleep apnea did not return to their baseline oxygen desaturation index until the third night after surgery.
“Despite a substantial decrease in ODI [oxygen desaturation index] with oxygen therapy in patients with OSA during the first 3 postoperative nights, supplemental oxygen did not modify the association between OSA and postoperative cardiovascular event,” wrote Matthew T.V. Chan, MD, of Chinese University of Hong Kong, Prince of Wales Hospital, and coauthors.
Given that the events were associated with longer durations of severe oxyhemoglobin desaturation, more aggressive interventions such as positive airway pressure or oral appliances may be required, they noted.
“However, high-level evidence demonstrating the effect of these measures on perioperative outcomes is lacking [and] further clinical trials are now required to test if additional monitoring or alternative interventions would reduce the risk,” they wrote.
The study was supported by the Health and Medical Research Fund (Hong Kong), National Healthcare Group–Khoo Teck Puat Hospital, University Health Network Foundation, University of Malaya, Malaysian Society of Anaesthesiologists, Auckland Medical Research Foundation, and ResMed. One author declared grants from private industry and a patent pending on an obstructive sleep apnea risk questionnaire used in the study.
SOURCE: Chan M et al. JAMA 2019;321[18]:1788-98. doi: 10.1001/jama.2019.4783.
This study is large, prospective, and rigorous and adds important new information to the puzzle of the impact of sleep apnea on postoperative risk, Dennis Auckley, MD, and Stavros Memtsoudis, MD, wrote in an editorial accompanying this study. The study focused on predetermined clinically significant and measurable events, used standardized and objective sleep apnea testing, and attempted to control for many of the confounders that might have influenced outcomes.
The results suggest that obstructive sleep apnea should be recognized as a major perioperative risk factor, and it should receive the same attention and optimization efforts as comorbidities such as diabetes.
Dr. Auckley is from the division of pulmonary, critical care and sleep medicine at MetroHealth Medical Center, Case Western Reserve University, Cleveland, and Dr. Memtsoudis is clinical professor of anesthesiology at Cornell University, New York. These comments are adapted from an editorial (JAMA 2019;231[18]:1775-6). Both declared board and executive positions with the Society of Anesthesia and Sleep Medicine. Dr. Auckley declared research funding from Medtronic, and Dr. Memtsoudis declared personal fees from Teikoku and Sandoz.
This study is large, prospective, and rigorous and adds important new information to the puzzle of the impact of sleep apnea on postoperative risk, Dennis Auckley, MD, and Stavros Memtsoudis, MD, wrote in an editorial accompanying this study. The study focused on predetermined clinically significant and measurable events, used standardized and objective sleep apnea testing, and attempted to control for many of the confounders that might have influenced outcomes.
The results suggest that obstructive sleep apnea should be recognized as a major perioperative risk factor, and it should receive the same attention and optimization efforts as comorbidities such as diabetes.
Dr. Auckley is from the division of pulmonary, critical care and sleep medicine at MetroHealth Medical Center, Case Western Reserve University, Cleveland, and Dr. Memtsoudis is clinical professor of anesthesiology at Cornell University, New York. These comments are adapted from an editorial (JAMA 2019;231[18]:1775-6). Both declared board and executive positions with the Society of Anesthesia and Sleep Medicine. Dr. Auckley declared research funding from Medtronic, and Dr. Memtsoudis declared personal fees from Teikoku and Sandoz.
This study is large, prospective, and rigorous and adds important new information to the puzzle of the impact of sleep apnea on postoperative risk, Dennis Auckley, MD, and Stavros Memtsoudis, MD, wrote in an editorial accompanying this study. The study focused on predetermined clinically significant and measurable events, used standardized and objective sleep apnea testing, and attempted to control for many of the confounders that might have influenced outcomes.
The results suggest that obstructive sleep apnea should be recognized as a major perioperative risk factor, and it should receive the same attention and optimization efforts as comorbidities such as diabetes.
Dr. Auckley is from the division of pulmonary, critical care and sleep medicine at MetroHealth Medical Center, Case Western Reserve University, Cleveland, and Dr. Memtsoudis is clinical professor of anesthesiology at Cornell University, New York. These comments are adapted from an editorial (JAMA 2019;231[18]:1775-6). Both declared board and executive positions with the Society of Anesthesia and Sleep Medicine. Dr. Auckley declared research funding from Medtronic, and Dr. Memtsoudis declared personal fees from Teikoku and Sandoz.
Unrecognized severe obstructive sleep apnea is a risk factor for cardiovascular complications after major noncardiac surgery, according to a study published in JAMA.
The researchers state that perioperative mismanagement of obstructive sleep apnea can lead to serious medical consequences. “General anesthetics, sedatives, and postoperative analgesics are potent respiratory depressants that relax the upper airway dilator muscles and impair ventilatory response to hypoxemia and hypercapnia. Each of these events exacerbates [obstructive sleep apnea] and may predispose patients to postoperative cardiovascular complications,” said researchers who conducted the The Postoperative vascular complications in unrecognised Obstructive Sleep apnoea (POSA) study (NCT01494181).
They undertook a prospective observational cohort study involving 1,218 patients undergoing major noncardiac surgery, who were already considered at high risk of postoperative cardiovascular events – having, for example, a history of coronary artery disease, stroke, diabetes, or renal impairment. However, none had a prior diagnosis of obstructive sleep apnea.
Preoperative sleep monitoring revealed that two-thirds of the cohort had unrecognized and untreated obstructive sleep apnea, including 11.2% with severe obstructive sleep apnea.
At 30 days after surgery, patients with obstructive sleep apnea had a 49% higher risk of the primary outcome of myocardial injury, cardiac death, heart failure, thromboembolism, atrial fibrillation, or stroke, compared with those without obstructive sleep apnea.
However, this association was largely due to a significant 2.23-fold higher risk among patients with severe obstructive sleep apnea, while those with only moderate or mild sleep apnea did not show a significant increased risk of cardiovascular complications.
Patients in this study with severe obstructive sleep apnea had a 13-fold higher risk of cardiac death, 80% higher risk of myocardial injury, more than sixfold higher risk of heart failure, and nearly fourfold higher risk of atrial fibrillation.
Researchers also saw an association between obstructive sleep apnea and increased risk of infective outcomes, unplanned tracheal intubation, postoperative lung ventilation, and readmission to the ICU.
The majority of patients received nocturnal oximetry monitoring during their first 3 nights after surgery. This revealed that patients without obstructive sleep apnea had significant increases in oxygen desaturation index during their first night after surgery, while those with sleep apnea did not return to their baseline oxygen desaturation index until the third night after surgery.
“Despite a substantial decrease in ODI [oxygen desaturation index] with oxygen therapy in patients with OSA during the first 3 postoperative nights, supplemental oxygen did not modify the association between OSA and postoperative cardiovascular event,” wrote Matthew T.V. Chan, MD, of Chinese University of Hong Kong, Prince of Wales Hospital, and coauthors.
Given that the events were associated with longer durations of severe oxyhemoglobin desaturation, more aggressive interventions such as positive airway pressure or oral appliances may be required, they noted.
“However, high-level evidence demonstrating the effect of these measures on perioperative outcomes is lacking [and] further clinical trials are now required to test if additional monitoring or alternative interventions would reduce the risk,” they wrote.
The study was supported by the Health and Medical Research Fund (Hong Kong), National Healthcare Group–Khoo Teck Puat Hospital, University Health Network Foundation, University of Malaya, Malaysian Society of Anaesthesiologists, Auckland Medical Research Foundation, and ResMed. One author declared grants from private industry and a patent pending on an obstructive sleep apnea risk questionnaire used in the study.
SOURCE: Chan M et al. JAMA 2019;321[18]:1788-98. doi: 10.1001/jama.2019.4783.
Unrecognized severe obstructive sleep apnea is a risk factor for cardiovascular complications after major noncardiac surgery, according to a study published in JAMA.
The researchers state that perioperative mismanagement of obstructive sleep apnea can lead to serious medical consequences. “General anesthetics, sedatives, and postoperative analgesics are potent respiratory depressants that relax the upper airway dilator muscles and impair ventilatory response to hypoxemia and hypercapnia. Each of these events exacerbates [obstructive sleep apnea] and may predispose patients to postoperative cardiovascular complications,” said researchers who conducted the The Postoperative vascular complications in unrecognised Obstructive Sleep apnoea (POSA) study (NCT01494181).
They undertook a prospective observational cohort study involving 1,218 patients undergoing major noncardiac surgery, who were already considered at high risk of postoperative cardiovascular events – having, for example, a history of coronary artery disease, stroke, diabetes, or renal impairment. However, none had a prior diagnosis of obstructive sleep apnea.
Preoperative sleep monitoring revealed that two-thirds of the cohort had unrecognized and untreated obstructive sleep apnea, including 11.2% with severe obstructive sleep apnea.
At 30 days after surgery, patients with obstructive sleep apnea had a 49% higher risk of the primary outcome of myocardial injury, cardiac death, heart failure, thromboembolism, atrial fibrillation, or stroke, compared with those without obstructive sleep apnea.
However, this association was largely due to a significant 2.23-fold higher risk among patients with severe obstructive sleep apnea, while those with only moderate or mild sleep apnea did not show a significant increased risk of cardiovascular complications.
Patients in this study with severe obstructive sleep apnea had a 13-fold higher risk of cardiac death, 80% higher risk of myocardial injury, more than sixfold higher risk of heart failure, and nearly fourfold higher risk of atrial fibrillation.
Researchers also saw an association between obstructive sleep apnea and increased risk of infective outcomes, unplanned tracheal intubation, postoperative lung ventilation, and readmission to the ICU.
The majority of patients received nocturnal oximetry monitoring during their first 3 nights after surgery. This revealed that patients without obstructive sleep apnea had significant increases in oxygen desaturation index during their first night after surgery, while those with sleep apnea did not return to their baseline oxygen desaturation index until the third night after surgery.
“Despite a substantial decrease in ODI [oxygen desaturation index] with oxygen therapy in patients with OSA during the first 3 postoperative nights, supplemental oxygen did not modify the association between OSA and postoperative cardiovascular event,” wrote Matthew T.V. Chan, MD, of Chinese University of Hong Kong, Prince of Wales Hospital, and coauthors.
Given that the events were associated with longer durations of severe oxyhemoglobin desaturation, more aggressive interventions such as positive airway pressure or oral appliances may be required, they noted.
“However, high-level evidence demonstrating the effect of these measures on perioperative outcomes is lacking [and] further clinical trials are now required to test if additional monitoring or alternative interventions would reduce the risk,” they wrote.
The study was supported by the Health and Medical Research Fund (Hong Kong), National Healthcare Group–Khoo Teck Puat Hospital, University Health Network Foundation, University of Malaya, Malaysian Society of Anaesthesiologists, Auckland Medical Research Foundation, and ResMed. One author declared grants from private industry and a patent pending on an obstructive sleep apnea risk questionnaire used in the study.
SOURCE: Chan M et al. JAMA 2019;321[18]:1788-98. doi: 10.1001/jama.2019.4783.
FROM JAMA