User login
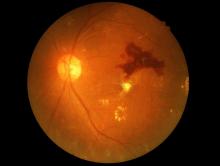
Eye check important before starting semaglutide for diabetes
A small potential increased risk of retinopathy worsening at 1 year with injected semaglutide (Ozempic, Novo Nordisk), a glucagon-like peptide 1 (GLP-1) agonist approved for type 2 diabetes, doesn’t outweigh the drug’s cardiovascular benefits but does highlight the need for baseline ophthalmologic evaluation before initiating treatment and ongoing retinal monitoring, researchers say.
That conclusion was based on data from a meta-analysis of the seven major cardiovascular outcomes trials of GLP-1 agonists currently on the market.
The findings were recently published in Diabetes & Metabolic Syndrome: Clinical Research & Reviews, by Stewart G. Albert, MD, and colleagues.
Concerns about retinopathy worsening with the GLP-1 agonist drug class first arose from the SUSTAIN-6 cardiovascular outcomes trial for injectable semaglutide, although a subsequent analysis of data from that trial appeared to suggest the problem is likely due to rapid glucose-lowering in already vulnerable patients rather than a drug-specific effect. This effect had been previously reported, most notably in the landmark Diabetes Control and Complications Trial.
In this new meta-analysis, “we showed that with improvements in A1c there were correlations with decreases in the rate of cardiovascular events but increases in the rate of retinopathy,” Dr. Albert, of St. Louis University, told this news organization.
“As a class of drugs, we did not find an increased rate of retinopathy. The effect of GLP-1 agonists on retinopathy did not appear to be due to an immediate direct toxic effect of the drug. The worsening of the rate of retinopathy was seen with semaglutide after 1 year of therapy and when there was a decrease in A1c of 1%,” he explained.
He noted that because the increased risk was seen primarily among those who already had retinopathy at baseline, “it would seem prudent to know the level of retinopathy either before or plan for close ophthalmologic monitoring around the time of drug initiation ... We routinely evaluate patients with known type 2 diabetes mellitus at yearly intervals for retinopathy. From our data, we saw worsening at 1 year of drug exposure, but we do not know the exact time when the changes occurred during that year.”
The Ozempic label advises that “patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy” but doesn’t specifically mention baseline assessment at the time of drug initiation.
No increase in retinopathy risk for GLP-1 agonist class overall
The seven trials in the meta-analysis comprised 56,004 participants, with baseline retinopathy prevalence ranging from 9% to 31%.
For the GLP-1 agonist class overall, there was no significant increase in the relative rate (RR) of retinopathy (RR, 1.09; P = .36), while there were significant reductions in relative rates of major adverse cardiac events, overall deaths, and cardiovascular deaths (all P < .001 or P = .001).
The increased retinopathy risk was seen only in the subcutaneous semaglutide group (RR, 1.73; P = .02).
The overall number needed to harm was 1,000 and the number to treat was 77. For semaglutide, those values were 77 and 43, respectively.
There was a significant correlation between a decrease in major adverse cardiac events and a decrease in A1c (P = .014), while for retinopathy, the risk increased with improved A1c (P = .076).
Semaglutide subanalysis finds increased retinopathy worsening
Dr. Albert and colleagues conducted a separate subanalysis of 11 studies of semaglutide that enrolled 11,894 patients, of which 6 studies (n = 5,610) were of oral semaglutide (Rybelsus) and 5 studies were of subcutaneous semaglutide (Ozempic; n = 6,284).
In the subanalysis, there was an overall increase in relative rates of new or worsening retinopathy (RR, 1.218; P = .049).
The change in relative rate of retinopathy was predominantly found for subcutaneous semaglutide given for longer than 1 year (RR, 1.559; P = .022) and decreases in A1c of more than 1.0% (RR, 1.590; P = .016). No such differences were seen with oral semaglutide.
A further evaluation of the data without the SUSTAIN 6 trial showed no effect on retinopathy but the analysis lacked power.
Dr. Albert told this news organization: “We did not find an immediate toxic effect of any drug. However, we cannot rule out that there was a cumulative effect of the dose over longer times.”
No disclosures were given.
A version of this article first appeared on Medscape.com.
A small potential increased risk of retinopathy worsening at 1 year with injected semaglutide (Ozempic, Novo Nordisk), a glucagon-like peptide 1 (GLP-1) agonist approved for type 2 diabetes, doesn’t outweigh the drug’s cardiovascular benefits but does highlight the need for baseline ophthalmologic evaluation before initiating treatment and ongoing retinal monitoring, researchers say.
That conclusion was based on data from a meta-analysis of the seven major cardiovascular outcomes trials of GLP-1 agonists currently on the market.
The findings were recently published in Diabetes & Metabolic Syndrome: Clinical Research & Reviews, by Stewart G. Albert, MD, and colleagues.
Concerns about retinopathy worsening with the GLP-1 agonist drug class first arose from the SUSTAIN-6 cardiovascular outcomes trial for injectable semaglutide, although a subsequent analysis of data from that trial appeared to suggest the problem is likely due to rapid glucose-lowering in already vulnerable patients rather than a drug-specific effect. This effect had been previously reported, most notably in the landmark Diabetes Control and Complications Trial.
In this new meta-analysis, “we showed that with improvements in A1c there were correlations with decreases in the rate of cardiovascular events but increases in the rate of retinopathy,” Dr. Albert, of St. Louis University, told this news organization.
“As a class of drugs, we did not find an increased rate of retinopathy. The effect of GLP-1 agonists on retinopathy did not appear to be due to an immediate direct toxic effect of the drug. The worsening of the rate of retinopathy was seen with semaglutide after 1 year of therapy and when there was a decrease in A1c of 1%,” he explained.
He noted that because the increased risk was seen primarily among those who already had retinopathy at baseline, “it would seem prudent to know the level of retinopathy either before or plan for close ophthalmologic monitoring around the time of drug initiation ... We routinely evaluate patients with known type 2 diabetes mellitus at yearly intervals for retinopathy. From our data, we saw worsening at 1 year of drug exposure, but we do not know the exact time when the changes occurred during that year.”
The Ozempic label advises that “patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy” but doesn’t specifically mention baseline assessment at the time of drug initiation.
No increase in retinopathy risk for GLP-1 agonist class overall
The seven trials in the meta-analysis comprised 56,004 participants, with baseline retinopathy prevalence ranging from 9% to 31%.
For the GLP-1 agonist class overall, there was no significant increase in the relative rate (RR) of retinopathy (RR, 1.09; P = .36), while there were significant reductions in relative rates of major adverse cardiac events, overall deaths, and cardiovascular deaths (all P < .001 or P = .001).
The increased retinopathy risk was seen only in the subcutaneous semaglutide group (RR, 1.73; P = .02).
The overall number needed to harm was 1,000 and the number to treat was 77. For semaglutide, those values were 77 and 43, respectively.
There was a significant correlation between a decrease in major adverse cardiac events and a decrease in A1c (P = .014), while for retinopathy, the risk increased with improved A1c (P = .076).
Semaglutide subanalysis finds increased retinopathy worsening
Dr. Albert and colleagues conducted a separate subanalysis of 11 studies of semaglutide that enrolled 11,894 patients, of which 6 studies (n = 5,610) were of oral semaglutide (Rybelsus) and 5 studies were of subcutaneous semaglutide (Ozempic; n = 6,284).
In the subanalysis, there was an overall increase in relative rates of new or worsening retinopathy (RR, 1.218; P = .049).
The change in relative rate of retinopathy was predominantly found for subcutaneous semaglutide given for longer than 1 year (RR, 1.559; P = .022) and decreases in A1c of more than 1.0% (RR, 1.590; P = .016). No such differences were seen with oral semaglutide.
A further evaluation of the data without the SUSTAIN 6 trial showed no effect on retinopathy but the analysis lacked power.
Dr. Albert told this news organization: “We did not find an immediate toxic effect of any drug. However, we cannot rule out that there was a cumulative effect of the dose over longer times.”
No disclosures were given.
A version of this article first appeared on Medscape.com.
A small potential increased risk of retinopathy worsening at 1 year with injected semaglutide (Ozempic, Novo Nordisk), a glucagon-like peptide 1 (GLP-1) agonist approved for type 2 diabetes, doesn’t outweigh the drug’s cardiovascular benefits but does highlight the need for baseline ophthalmologic evaluation before initiating treatment and ongoing retinal monitoring, researchers say.
That conclusion was based on data from a meta-analysis of the seven major cardiovascular outcomes trials of GLP-1 agonists currently on the market.
The findings were recently published in Diabetes & Metabolic Syndrome: Clinical Research & Reviews, by Stewart G. Albert, MD, and colleagues.
Concerns about retinopathy worsening with the GLP-1 agonist drug class first arose from the SUSTAIN-6 cardiovascular outcomes trial for injectable semaglutide, although a subsequent analysis of data from that trial appeared to suggest the problem is likely due to rapid glucose-lowering in already vulnerable patients rather than a drug-specific effect. This effect had been previously reported, most notably in the landmark Diabetes Control and Complications Trial.
In this new meta-analysis, “we showed that with improvements in A1c there were correlations with decreases in the rate of cardiovascular events but increases in the rate of retinopathy,” Dr. Albert, of St. Louis University, told this news organization.
“As a class of drugs, we did not find an increased rate of retinopathy. The effect of GLP-1 agonists on retinopathy did not appear to be due to an immediate direct toxic effect of the drug. The worsening of the rate of retinopathy was seen with semaglutide after 1 year of therapy and when there was a decrease in A1c of 1%,” he explained.
He noted that because the increased risk was seen primarily among those who already had retinopathy at baseline, “it would seem prudent to know the level of retinopathy either before or plan for close ophthalmologic monitoring around the time of drug initiation ... We routinely evaluate patients with known type 2 diabetes mellitus at yearly intervals for retinopathy. From our data, we saw worsening at 1 year of drug exposure, but we do not know the exact time when the changes occurred during that year.”
The Ozempic label advises that “patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy” but doesn’t specifically mention baseline assessment at the time of drug initiation.
No increase in retinopathy risk for GLP-1 agonist class overall
The seven trials in the meta-analysis comprised 56,004 participants, with baseline retinopathy prevalence ranging from 9% to 31%.
For the GLP-1 agonist class overall, there was no significant increase in the relative rate (RR) of retinopathy (RR, 1.09; P = .36), while there were significant reductions in relative rates of major adverse cardiac events, overall deaths, and cardiovascular deaths (all P < .001 or P = .001).
The increased retinopathy risk was seen only in the subcutaneous semaglutide group (RR, 1.73; P = .02).
The overall number needed to harm was 1,000 and the number to treat was 77. For semaglutide, those values were 77 and 43, respectively.
There was a significant correlation between a decrease in major adverse cardiac events and a decrease in A1c (P = .014), while for retinopathy, the risk increased with improved A1c (P = .076).
Semaglutide subanalysis finds increased retinopathy worsening
Dr. Albert and colleagues conducted a separate subanalysis of 11 studies of semaglutide that enrolled 11,894 patients, of which 6 studies (n = 5,610) were of oral semaglutide (Rybelsus) and 5 studies were of subcutaneous semaglutide (Ozempic; n = 6,284).
In the subanalysis, there was an overall increase in relative rates of new or worsening retinopathy (RR, 1.218; P = .049).
The change in relative rate of retinopathy was predominantly found for subcutaneous semaglutide given for longer than 1 year (RR, 1.559; P = .022) and decreases in A1c of more than 1.0% (RR, 1.590; P = .016). No such differences were seen with oral semaglutide.
A further evaluation of the data without the SUSTAIN 6 trial showed no effect on retinopathy but the analysis lacked power.
Dr. Albert told this news organization: “We did not find an immediate toxic effect of any drug. However, we cannot rule out that there was a cumulative effect of the dose over longer times.”
No disclosures were given.
A version of this article first appeared on Medscape.com.
FROM DIABETES & METABOLIC SYNDROME: CLINICAL RESEARCH & REVIEWS
EMR screening in emergency department tags undiagnosed diabetes
A diabetes screening program built into an electronic medical records system identified diabetes or prediabetes in 52% of individuals flagged for abnormal hemoglobin A1c, based on data from more than 2,000 adults.
“Despite the best efforts of clinicians, researchers, and educators, the number of patients living with undiagnosed diabetes is still rising and is currently at approximately 8.5 million, and the number of people unaware of their prediabetes is approximately 77 million,” lead investigator Kristie K. Danielson, PhD, said in an interview. Screening for diabetes is critical to start treatment early, to potentially reverse prediabetes, and to prevent the long-term complications of diabetes and reduced life expectancy.
In a pilot study published in JAMA Network Open, Dr. Danielson and colleagues reviewed data from 8,441 adults who visited a single emergency department in Chicago during February–April 2021.
The EMR at the hospital contained a built-in best practice alert (BPA) that flagged patients as being at risk for type 2 diabetes based the American Diabetes Association recommendations; the identification algorithm included age 45 years and older, or those aged 18-44 years with a body mass index of 25 kg/m2 or higher, no previous history of diabetes, and no A1c measure in the last 3 years, according to the EMR.
A total of 8,441 adult patients visited the ED during the study period; 2,576 triggered BPA tests, and 2,074 had A1c results for review. Among the patients with A1c results, 52% had elevated values of 5.7% or higher. Of these, a total of 758 individuals were identified with prediabetes (A1c, 5.7%-6.4%), 265 with diabetes (A1c, 6.5%-9.9%), and 62 with severe diabetes (A1c, 10% or higher).
After testing, 352 patients with elevated A1c were contacted by the researchers. The mean age of this group was 52.2 years, 54.5% were women, and nearly two-thirds (64.8%) were non-Hispanic Black. The median income of those contacted was in the 44th percentile, and 50% had public insurance.
Most of those contacted (264 patients) were not aware of a previous diagnosis of prediabetes or diabetes; the remaining 88 had a previous diagnosis, but only 51 self-reported receiving treatment, the researchers noted.
Although the screening program successfully identified a significant number of previously undiagnosed individuals with diabetes, prediabetes, or poorly controlled diabetes, its feasibility in routine practice requires further study, the researchers wrote.
The findings were limited by several factors including the identification of patients previously diagnosed with diabetes but who were not being treated, and the potential bias toward individuals of higher socioeconomic status, the researchers noted. However, the results support further exploration of the program as a way to identify undiagnosed diabetes, especially in underserved populations.
Diabetes in underserved groups goes undetected
“We were surprised by the sheer number of people newly diagnosed with diabetes or prediabetes,” which was far greater than expected, commented Dr. Danielson of the University of Illinois at Chicago. “Clearly, we tapped into a new population that has not often been seen by primary care providers or endocrinologists, as is often the case for underserved and vulnerable individuals who visit the emergency department as a first line for health care.”
The screening alert system is straightforward to build into an existing EMR, with technical support, Dr. Danielson said. “In theory, it should be able to be incorporated into other clinical centers and emergency departments. One of the current limitations that we are seeing is that the EMR is still flagging some people already diagnosed with diabetes to be screened for diabetes.” However, “because of this, we also see this as an opportunity to identify and reach out to those with diabetes who are still underserved and not receiving the appropriate diabetes care they need.”
The study results have broader public health implications, Dr. Danielson added. “We have identified a new, large population of people with diabetes who need medical care and diabetes education. This will further add to the burden of health care and costs, and it raises the ethical question of screening and not having full resources readily available to help.
“In my opinion, the study sheds light on a significant issue that will hopefully help drive change at both a health systems and public health level locally and nationally,” she added.
“One of the significant research gaps that has emerged now is how to link these new patients to health care and diabetes education at our institution after they leave the emergency department,” said Dr. Danielson. Diabetes screening in the ED setting is “a very novel area for health system scientists, social workers, and others to now come to the table and collaborate on next steps to help our patients.”
The study was initiated by the investigators, but was supported by a grant from Novo Nordisk to two coauthors. Dr. Danielson also disclosed grant funding from Novo Nordisk during the conduct of the study.
A diabetes screening program built into an electronic medical records system identified diabetes or prediabetes in 52% of individuals flagged for abnormal hemoglobin A1c, based on data from more than 2,000 adults.
“Despite the best efforts of clinicians, researchers, and educators, the number of patients living with undiagnosed diabetes is still rising and is currently at approximately 8.5 million, and the number of people unaware of their prediabetes is approximately 77 million,” lead investigator Kristie K. Danielson, PhD, said in an interview. Screening for diabetes is critical to start treatment early, to potentially reverse prediabetes, and to prevent the long-term complications of diabetes and reduced life expectancy.
In a pilot study published in JAMA Network Open, Dr. Danielson and colleagues reviewed data from 8,441 adults who visited a single emergency department in Chicago during February–April 2021.
The EMR at the hospital contained a built-in best practice alert (BPA) that flagged patients as being at risk for type 2 diabetes based the American Diabetes Association recommendations; the identification algorithm included age 45 years and older, or those aged 18-44 years with a body mass index of 25 kg/m2 or higher, no previous history of diabetes, and no A1c measure in the last 3 years, according to the EMR.
A total of 8,441 adult patients visited the ED during the study period; 2,576 triggered BPA tests, and 2,074 had A1c results for review. Among the patients with A1c results, 52% had elevated values of 5.7% or higher. Of these, a total of 758 individuals were identified with prediabetes (A1c, 5.7%-6.4%), 265 with diabetes (A1c, 6.5%-9.9%), and 62 with severe diabetes (A1c, 10% or higher).
After testing, 352 patients with elevated A1c were contacted by the researchers. The mean age of this group was 52.2 years, 54.5% were women, and nearly two-thirds (64.8%) were non-Hispanic Black. The median income of those contacted was in the 44th percentile, and 50% had public insurance.
Most of those contacted (264 patients) were not aware of a previous diagnosis of prediabetes or diabetes; the remaining 88 had a previous diagnosis, but only 51 self-reported receiving treatment, the researchers noted.
Although the screening program successfully identified a significant number of previously undiagnosed individuals with diabetes, prediabetes, or poorly controlled diabetes, its feasibility in routine practice requires further study, the researchers wrote.
The findings were limited by several factors including the identification of patients previously diagnosed with diabetes but who were not being treated, and the potential bias toward individuals of higher socioeconomic status, the researchers noted. However, the results support further exploration of the program as a way to identify undiagnosed diabetes, especially in underserved populations.
Diabetes in underserved groups goes undetected
“We were surprised by the sheer number of people newly diagnosed with diabetes or prediabetes,” which was far greater than expected, commented Dr. Danielson of the University of Illinois at Chicago. “Clearly, we tapped into a new population that has not often been seen by primary care providers or endocrinologists, as is often the case for underserved and vulnerable individuals who visit the emergency department as a first line for health care.”
The screening alert system is straightforward to build into an existing EMR, with technical support, Dr. Danielson said. “In theory, it should be able to be incorporated into other clinical centers and emergency departments. One of the current limitations that we are seeing is that the EMR is still flagging some people already diagnosed with diabetes to be screened for diabetes.” However, “because of this, we also see this as an opportunity to identify and reach out to those with diabetes who are still underserved and not receiving the appropriate diabetes care they need.”
The study results have broader public health implications, Dr. Danielson added. “We have identified a new, large population of people with diabetes who need medical care and diabetes education. This will further add to the burden of health care and costs, and it raises the ethical question of screening and not having full resources readily available to help.
“In my opinion, the study sheds light on a significant issue that will hopefully help drive change at both a health systems and public health level locally and nationally,” she added.
“One of the significant research gaps that has emerged now is how to link these new patients to health care and diabetes education at our institution after they leave the emergency department,” said Dr. Danielson. Diabetes screening in the ED setting is “a very novel area for health system scientists, social workers, and others to now come to the table and collaborate on next steps to help our patients.”
The study was initiated by the investigators, but was supported by a grant from Novo Nordisk to two coauthors. Dr. Danielson also disclosed grant funding from Novo Nordisk during the conduct of the study.
A diabetes screening program built into an electronic medical records system identified diabetes or prediabetes in 52% of individuals flagged for abnormal hemoglobin A1c, based on data from more than 2,000 adults.
“Despite the best efforts of clinicians, researchers, and educators, the number of patients living with undiagnosed diabetes is still rising and is currently at approximately 8.5 million, and the number of people unaware of their prediabetes is approximately 77 million,” lead investigator Kristie K. Danielson, PhD, said in an interview. Screening for diabetes is critical to start treatment early, to potentially reverse prediabetes, and to prevent the long-term complications of diabetes and reduced life expectancy.
In a pilot study published in JAMA Network Open, Dr. Danielson and colleagues reviewed data from 8,441 adults who visited a single emergency department in Chicago during February–April 2021.
The EMR at the hospital contained a built-in best practice alert (BPA) that flagged patients as being at risk for type 2 diabetes based the American Diabetes Association recommendations; the identification algorithm included age 45 years and older, or those aged 18-44 years with a body mass index of 25 kg/m2 or higher, no previous history of diabetes, and no A1c measure in the last 3 years, according to the EMR.
A total of 8,441 adult patients visited the ED during the study period; 2,576 triggered BPA tests, and 2,074 had A1c results for review. Among the patients with A1c results, 52% had elevated values of 5.7% or higher. Of these, a total of 758 individuals were identified with prediabetes (A1c, 5.7%-6.4%), 265 with diabetes (A1c, 6.5%-9.9%), and 62 with severe diabetes (A1c, 10% or higher).
After testing, 352 patients with elevated A1c were contacted by the researchers. The mean age of this group was 52.2 years, 54.5% were women, and nearly two-thirds (64.8%) were non-Hispanic Black. The median income of those contacted was in the 44th percentile, and 50% had public insurance.
Most of those contacted (264 patients) were not aware of a previous diagnosis of prediabetes or diabetes; the remaining 88 had a previous diagnosis, but only 51 self-reported receiving treatment, the researchers noted.
Although the screening program successfully identified a significant number of previously undiagnosed individuals with diabetes, prediabetes, or poorly controlled diabetes, its feasibility in routine practice requires further study, the researchers wrote.
The findings were limited by several factors including the identification of patients previously diagnosed with diabetes but who were not being treated, and the potential bias toward individuals of higher socioeconomic status, the researchers noted. However, the results support further exploration of the program as a way to identify undiagnosed diabetes, especially in underserved populations.
Diabetes in underserved groups goes undetected
“We were surprised by the sheer number of people newly diagnosed with diabetes or prediabetes,” which was far greater than expected, commented Dr. Danielson of the University of Illinois at Chicago. “Clearly, we tapped into a new population that has not often been seen by primary care providers or endocrinologists, as is often the case for underserved and vulnerable individuals who visit the emergency department as a first line for health care.”
The screening alert system is straightforward to build into an existing EMR, with technical support, Dr. Danielson said. “In theory, it should be able to be incorporated into other clinical centers and emergency departments. One of the current limitations that we are seeing is that the EMR is still flagging some people already diagnosed with diabetes to be screened for diabetes.” However, “because of this, we also see this as an opportunity to identify and reach out to those with diabetes who are still underserved and not receiving the appropriate diabetes care they need.”
The study results have broader public health implications, Dr. Danielson added. “We have identified a new, large population of people with diabetes who need medical care and diabetes education. This will further add to the burden of health care and costs, and it raises the ethical question of screening and not having full resources readily available to help.
“In my opinion, the study sheds light on a significant issue that will hopefully help drive change at both a health systems and public health level locally and nationally,” she added.
“One of the significant research gaps that has emerged now is how to link these new patients to health care and diabetes education at our institution after they leave the emergency department,” said Dr. Danielson. Diabetes screening in the ED setting is “a very novel area for health system scientists, social workers, and others to now come to the table and collaborate on next steps to help our patients.”
The study was initiated by the investigators, but was supported by a grant from Novo Nordisk to two coauthors. Dr. Danielson also disclosed grant funding from Novo Nordisk during the conduct of the study.
FROM JAMA NETWORK OPEN
Despite ongoing challenges, experts are optimistic about the future of MS therapy
Prior to 1993, a multiple sclerosis (MS) diagnosis could often mean an abbreviated lifespan marked by progressive disability and loss of function. That changed when the Food and Drug Administration approved interferon beta-1b (Betaseron) in 1993, which revolutionized MS therapy and gave hope to the entire MS community.
"The most surprising thing about MS management over the last 30 years is that we’ve been able to treat MS – especially relapsing MS,” said Fred D. Lublin, MD, professor of neurology and director of the Corinne Goldsmith Dickinson Center for Multiple Sclerosis in Mount Sinai in New York. “The approval of interferon was a major therapeutic advancement because it was the first treatment for what was an untreatable disease.”
Mark Gudesblatt, MD, medical director of the Comprehensive MS Care Center of South Shore Neurologic Associates in Patchogue, N.Y., agrees.
“For people with MS, it’s an extraordinarily lucky and amazingly optimistic time,” he said. “Before interferon beta-1b, MS was called ‘the crippler of young adults’ because more than 50% of these people would require a walker 10 years after diagnosis, and a large number of young and middle-age patients with MS were residing in nursing homes.”
According to Dr. Lublin, the emergence of the immunomodulating therapies placed MS at the leading edge of neurotherapeutics. Interferon beta-1b laid the foundation for new therapies such as another interferon (interferon beta-1a; Avonex), glatiramer acetate (Copaxone), and many other effective therapies with different mechanisms of action. Since the emergence of the first therapy, more than 20 oral and infusion agents with moderate to high efficacy have come to market for relapsing MS.
Treatment options, treatment challenges
Dr. Gudesblatt points out that having numerous therapies from which to choose is both a blessing and a problem.
“The good news is that there are so many options for treating relapsing MS today,” he said. “The bad news is there are so many options. Like doctors who are treating high blood pressure, doctors managing patients with MS often struggle to determine which medication is best for individual patients.”
Despite the promise of vastly better outcomes and prolonged lifespan, MS therapy still faces its share of challenges, including effective therapies for progressive MS and reparative-restorative therapies.
“Choice in route of administration and timing of administration allow for larger and broader discussions to try to meet patients’ needs,” Dr. Lublin said. “We’ve been extremely successful at treating relapses, but not as successful in treating progressive disease.”
The unclear mechanism of pathogenesis amplifies the challenges clinicians face in successful management of patients with MS. For example, experts agree that the therapies for progressive MS have only proven moderately effective at best. The paucity of therapies available for progressive MS and the limitations of the current therapies further limit the outcomes.
Looking ahead
Experts expressed optimistic views about the future of MS therapy as a whole. From Dr. Lublin’s perspective, the MS community stands to gain valuable insights from emerging research focused on treating progressive disease along with new testing to understand the underlying mechanism of progressive disease. Enhanced understanding of the underlying pathogenesis of progressive MS coupled with the ability to diagnose MS – such as improved MRI techniques – have facilitated this process.
Among the therapies with novel mechanisms of action in the pipeline include agents that generate myelin sheath repair. Another potential therapeutic class on the horizon, known as TPK inhibitors, addresses the smoldering of the disease. With these and other therapeutic advances, Dr. Lublin hopes to see better control of progressive disease.
An agenda for the future
In addition, barriers such as access to care, cost, insurance coverage, and tolerance remain ongoing stressors that will likely continue weighing on the MS community and its stakeholders into the future.
Dr. Gudesblatt concluded that advancing MS outcomes in the future hinges on several additional factors.
“We need medicines that are better for relapse and progression; medicines that are better tolerated and safer; and better medicine to address the underlying disease as well as its symptoms. But we also need to appreciate, recognize, and address cognitive impairment along the MS continuum and develop effective reparative options,” he said.
Regardless, he emphasized that these “amazing advancements” in MS therapy have renewed hope that research may identify and expand effective treatments for multiple other neurologic conditions such as muscular dystrophies, neurodegenerative and genetic disorders, movement disorders, and dysautonomia-related diseases. Like MS, all of these conditions have limited therapies, some of which have minimal efficacy. But none of these other disorders has disease-modifying therapies currently available.
‘A beacon of hope’
“MS is the beacon of hope for multiple disease states because it’s cracked the door wide open,” Dr. Gudesblatt said. Relapse no longer gauges the prognosis of today’s MS patient – a prognosis both experts think will only continue to improve with forthcoming innovations.
While the challenges for MS still exist, the bright future that lies ahead may eventually eclipse them.
Prior to 1993, a multiple sclerosis (MS) diagnosis could often mean an abbreviated lifespan marked by progressive disability and loss of function. That changed when the Food and Drug Administration approved interferon beta-1b (Betaseron) in 1993, which revolutionized MS therapy and gave hope to the entire MS community.
"The most surprising thing about MS management over the last 30 years is that we’ve been able to treat MS – especially relapsing MS,” said Fred D. Lublin, MD, professor of neurology and director of the Corinne Goldsmith Dickinson Center for Multiple Sclerosis in Mount Sinai in New York. “The approval of interferon was a major therapeutic advancement because it was the first treatment for what was an untreatable disease.”
Mark Gudesblatt, MD, medical director of the Comprehensive MS Care Center of South Shore Neurologic Associates in Patchogue, N.Y., agrees.
“For people with MS, it’s an extraordinarily lucky and amazingly optimistic time,” he said. “Before interferon beta-1b, MS was called ‘the crippler of young adults’ because more than 50% of these people would require a walker 10 years after diagnosis, and a large number of young and middle-age patients with MS were residing in nursing homes.”
According to Dr. Lublin, the emergence of the immunomodulating therapies placed MS at the leading edge of neurotherapeutics. Interferon beta-1b laid the foundation for new therapies such as another interferon (interferon beta-1a; Avonex), glatiramer acetate (Copaxone), and many other effective therapies with different mechanisms of action. Since the emergence of the first therapy, more than 20 oral and infusion agents with moderate to high efficacy have come to market for relapsing MS.
Treatment options, treatment challenges
Dr. Gudesblatt points out that having numerous therapies from which to choose is both a blessing and a problem.
“The good news is that there are so many options for treating relapsing MS today,” he said. “The bad news is there are so many options. Like doctors who are treating high blood pressure, doctors managing patients with MS often struggle to determine which medication is best for individual patients.”
Despite the promise of vastly better outcomes and prolonged lifespan, MS therapy still faces its share of challenges, including effective therapies for progressive MS and reparative-restorative therapies.
“Choice in route of administration and timing of administration allow for larger and broader discussions to try to meet patients’ needs,” Dr. Lublin said. “We’ve been extremely successful at treating relapses, but not as successful in treating progressive disease.”
The unclear mechanism of pathogenesis amplifies the challenges clinicians face in successful management of patients with MS. For example, experts agree that the therapies for progressive MS have only proven moderately effective at best. The paucity of therapies available for progressive MS and the limitations of the current therapies further limit the outcomes.
Looking ahead
Experts expressed optimistic views about the future of MS therapy as a whole. From Dr. Lublin’s perspective, the MS community stands to gain valuable insights from emerging research focused on treating progressive disease along with new testing to understand the underlying mechanism of progressive disease. Enhanced understanding of the underlying pathogenesis of progressive MS coupled with the ability to diagnose MS – such as improved MRI techniques – have facilitated this process.
Among the therapies with novel mechanisms of action in the pipeline include agents that generate myelin sheath repair. Another potential therapeutic class on the horizon, known as TPK inhibitors, addresses the smoldering of the disease. With these and other therapeutic advances, Dr. Lublin hopes to see better control of progressive disease.
An agenda for the future
In addition, barriers such as access to care, cost, insurance coverage, and tolerance remain ongoing stressors that will likely continue weighing on the MS community and its stakeholders into the future.
Dr. Gudesblatt concluded that advancing MS outcomes in the future hinges on several additional factors.
“We need medicines that are better for relapse and progression; medicines that are better tolerated and safer; and better medicine to address the underlying disease as well as its symptoms. But we also need to appreciate, recognize, and address cognitive impairment along the MS continuum and develop effective reparative options,” he said.
Regardless, he emphasized that these “amazing advancements” in MS therapy have renewed hope that research may identify and expand effective treatments for multiple other neurologic conditions such as muscular dystrophies, neurodegenerative and genetic disorders, movement disorders, and dysautonomia-related diseases. Like MS, all of these conditions have limited therapies, some of which have minimal efficacy. But none of these other disorders has disease-modifying therapies currently available.
‘A beacon of hope’
“MS is the beacon of hope for multiple disease states because it’s cracked the door wide open,” Dr. Gudesblatt said. Relapse no longer gauges the prognosis of today’s MS patient – a prognosis both experts think will only continue to improve with forthcoming innovations.
While the challenges for MS still exist, the bright future that lies ahead may eventually eclipse them.
Prior to 1993, a multiple sclerosis (MS) diagnosis could often mean an abbreviated lifespan marked by progressive disability and loss of function. That changed when the Food and Drug Administration approved interferon beta-1b (Betaseron) in 1993, which revolutionized MS therapy and gave hope to the entire MS community.
"The most surprising thing about MS management over the last 30 years is that we’ve been able to treat MS – especially relapsing MS,” said Fred D. Lublin, MD, professor of neurology and director of the Corinne Goldsmith Dickinson Center for Multiple Sclerosis in Mount Sinai in New York. “The approval of interferon was a major therapeutic advancement because it was the first treatment for what was an untreatable disease.”
Mark Gudesblatt, MD, medical director of the Comprehensive MS Care Center of South Shore Neurologic Associates in Patchogue, N.Y., agrees.
“For people with MS, it’s an extraordinarily lucky and amazingly optimistic time,” he said. “Before interferon beta-1b, MS was called ‘the crippler of young adults’ because more than 50% of these people would require a walker 10 years after diagnosis, and a large number of young and middle-age patients with MS were residing in nursing homes.”
According to Dr. Lublin, the emergence of the immunomodulating therapies placed MS at the leading edge of neurotherapeutics. Interferon beta-1b laid the foundation for new therapies such as another interferon (interferon beta-1a; Avonex), glatiramer acetate (Copaxone), and many other effective therapies with different mechanisms of action. Since the emergence of the first therapy, more than 20 oral and infusion agents with moderate to high efficacy have come to market for relapsing MS.
Treatment options, treatment challenges
Dr. Gudesblatt points out that having numerous therapies from which to choose is both a blessing and a problem.
“The good news is that there are so many options for treating relapsing MS today,” he said. “The bad news is there are so many options. Like doctors who are treating high blood pressure, doctors managing patients with MS often struggle to determine which medication is best for individual patients.”
Despite the promise of vastly better outcomes and prolonged lifespan, MS therapy still faces its share of challenges, including effective therapies for progressive MS and reparative-restorative therapies.
“Choice in route of administration and timing of administration allow for larger and broader discussions to try to meet patients’ needs,” Dr. Lublin said. “We’ve been extremely successful at treating relapses, but not as successful in treating progressive disease.”
The unclear mechanism of pathogenesis amplifies the challenges clinicians face in successful management of patients with MS. For example, experts agree that the therapies for progressive MS have only proven moderately effective at best. The paucity of therapies available for progressive MS and the limitations of the current therapies further limit the outcomes.
Looking ahead
Experts expressed optimistic views about the future of MS therapy as a whole. From Dr. Lublin’s perspective, the MS community stands to gain valuable insights from emerging research focused on treating progressive disease along with new testing to understand the underlying mechanism of progressive disease. Enhanced understanding of the underlying pathogenesis of progressive MS coupled with the ability to diagnose MS – such as improved MRI techniques – have facilitated this process.
Among the therapies with novel mechanisms of action in the pipeline include agents that generate myelin sheath repair. Another potential therapeutic class on the horizon, known as TPK inhibitors, addresses the smoldering of the disease. With these and other therapeutic advances, Dr. Lublin hopes to see better control of progressive disease.
An agenda for the future
In addition, barriers such as access to care, cost, insurance coverage, and tolerance remain ongoing stressors that will likely continue weighing on the MS community and its stakeholders into the future.
Dr. Gudesblatt concluded that advancing MS outcomes in the future hinges on several additional factors.
“We need medicines that are better for relapse and progression; medicines that are better tolerated and safer; and better medicine to address the underlying disease as well as its symptoms. But we also need to appreciate, recognize, and address cognitive impairment along the MS continuum and develop effective reparative options,” he said.
Regardless, he emphasized that these “amazing advancements” in MS therapy have renewed hope that research may identify and expand effective treatments for multiple other neurologic conditions such as muscular dystrophies, neurodegenerative and genetic disorders, movement disorders, and dysautonomia-related diseases. Like MS, all of these conditions have limited therapies, some of which have minimal efficacy. But none of these other disorders has disease-modifying therapies currently available.
‘A beacon of hope’
“MS is the beacon of hope for multiple disease states because it’s cracked the door wide open,” Dr. Gudesblatt said. Relapse no longer gauges the prognosis of today’s MS patient – a prognosis both experts think will only continue to improve with forthcoming innovations.
While the challenges for MS still exist, the bright future that lies ahead may eventually eclipse them.
FDA wants annual COVID boosters, just like annual flu shots
The U.S. Food and Drug Administration is suggesting a single annual shot. The formulation would be selected in June targeting the most threatening COVID-19 strains, and then people could get a shot in the fall when people begin spending more time indoors and exposure increases.
Some people, such as those who are older or immunocompromised, may need more than one dose.
A national advisory committee is expected to vote on the proposal at a meeting Jan. 26.
People in the United States have been much less likely to get an updated COVID-19 booster shot, compared with widespread uptake of the primary vaccine series. In its proposal, the FDA indicated it hoped a single annual shot would overcome challenges created by the complexity of the process – both in messaging and administration – attributed to that low booster rate. Nine in 10 people age 12 or older got the primary vaccine series in the United States, but only 15% got the latest booster shot for COVID-19.
About half of children and adults in the U.S. get an annual flu shot, according to Centers for Disease Control and Prevention data.
The FDA also wants to move to a single COVID-19 vaccine formulation that would be used for primary vaccine series and for booster shots.
COVID-19 cases, hospitalizations, and deaths are trending downward, according to the data tracker from the New York Times. Cases are down 28%, with 47,290 tallied daily. Hospitalizations are down 22%, with 37,474 daily. Deaths are down 4%, with an average of 489 per day as of Jan. 22.
A version of this article originally appeared on WebMD.com.
The U.S. Food and Drug Administration is suggesting a single annual shot. The formulation would be selected in June targeting the most threatening COVID-19 strains, and then people could get a shot in the fall when people begin spending more time indoors and exposure increases.
Some people, such as those who are older or immunocompromised, may need more than one dose.
A national advisory committee is expected to vote on the proposal at a meeting Jan. 26.
People in the United States have been much less likely to get an updated COVID-19 booster shot, compared with widespread uptake of the primary vaccine series. In its proposal, the FDA indicated it hoped a single annual shot would overcome challenges created by the complexity of the process – both in messaging and administration – attributed to that low booster rate. Nine in 10 people age 12 or older got the primary vaccine series in the United States, but only 15% got the latest booster shot for COVID-19.
About half of children and adults in the U.S. get an annual flu shot, according to Centers for Disease Control and Prevention data.
The FDA also wants to move to a single COVID-19 vaccine formulation that would be used for primary vaccine series and for booster shots.
COVID-19 cases, hospitalizations, and deaths are trending downward, according to the data tracker from the New York Times. Cases are down 28%, with 47,290 tallied daily. Hospitalizations are down 22%, with 37,474 daily. Deaths are down 4%, with an average of 489 per day as of Jan. 22.
A version of this article originally appeared on WebMD.com.
The U.S. Food and Drug Administration is suggesting a single annual shot. The formulation would be selected in June targeting the most threatening COVID-19 strains, and then people could get a shot in the fall when people begin spending more time indoors and exposure increases.
Some people, such as those who are older or immunocompromised, may need more than one dose.
A national advisory committee is expected to vote on the proposal at a meeting Jan. 26.
People in the United States have been much less likely to get an updated COVID-19 booster shot, compared with widespread uptake of the primary vaccine series. In its proposal, the FDA indicated it hoped a single annual shot would overcome challenges created by the complexity of the process – both in messaging and administration – attributed to that low booster rate. Nine in 10 people age 12 or older got the primary vaccine series in the United States, but only 15% got the latest booster shot for COVID-19.
About half of children and adults in the U.S. get an annual flu shot, according to Centers for Disease Control and Prevention data.
The FDA also wants to move to a single COVID-19 vaccine formulation that would be used for primary vaccine series and for booster shots.
COVID-19 cases, hospitalizations, and deaths are trending downward, according to the data tracker from the New York Times. Cases are down 28%, with 47,290 tallied daily. Hospitalizations are down 22%, with 37,474 daily. Deaths are down 4%, with an average of 489 per day as of Jan. 22.
A version of this article originally appeared on WebMD.com.
High-deductible health plans detrimental for those with diabetes
Individuals with diabetes who are forced to switch to high-deductible health plans have more episodes of severe hypo- and hyperglycemia compared with those on conventional insurance plans, according to a new study.
Previous studies have shown that people with diabetes who are enrolled in high-deductible health plans (HDHPs) have an increased financial burden, lower medication adherence, and more low-severity emergency department visits, and they delay care for cardiovascular conditions.
But no study has looked at the plans’ impact on acute diabetes complications and glycemic control, wrote the authors in JAMA Network Open.
They found evidence that the high-dollar plans were associated with increased odds of severe hypoglycemic and hyperglycemic events, and that the risk increased with each successive year of enrollment. Low-income individuals, Blacks, and Hispanics were disproportionately more impacted, noted senior author Rozalina G. McCoy, MD, Mayo Clinic, Rochester, Minn., and colleagues.
Overall, “enrollees may be rationing or forgoing necessary care, which is detrimental to their health and ultimately increases the morbidity, mortality, and costs associated with diabetes,” they concluded.
A systematic review of eight studies published in Endocrine Practice in 2021 backs up this latest finding. That analysis reported enrollees in HDHPs often forgo routine care and monitoring, and that they have lower medication adherence, leading to an increase in total health care expenditures for emergency department visits, hospitalizations, and preventable complications.
Increased frequency of hypoglycemia is detrimental
The new study published in JAMA Network Open was based on data for adults enrolled in private insurance programs from 2010 to 2018. Researchers analyzed medical and pharmacy claims data contained in a large health insurance claims database, comparing adults with diabetes who had been in an HDHP for at least 1 year (and after a year of being in a conventional plan), with those who were in a conventional plan.
They identified 42,326 individuals who had been switched from a conventional plan to an HDHP. Of those, 7,375 (17.4%) were Black, 5,740 (13.6%) were Hispanic, 26,572 (62.8%) were non-Hispanic White, and 6,880 (16.3%) had a household income below $40,000 a year.
Baseline characteristics of the 202,729 people in conventional plans were similar to those in the HDHP group.
The median deductible for individuals in the HDHP group was $1,500 and for families it was $3,000, compared with $350 and $800, respectively, for those in conventional plans.
The odds of having any severe hypoglycemic event were significantly higher in the HDHP group (odds ratio [OR], 1.11; P < .001). Each year of HDHP enrollment increased the odds of a hypoglycemia-related ED or hospital visit by 2% (OR, 1.02; P = .04).
Aware that only a small number of severe hypoglycemic events, as well as an unknown number of such events, result in an emergency department visit or hospitalization, and that “the decision to seek ED or hospital care may be influenced by health plan assignment,” the authors also looked at office visits where severe, or any, hypoglycemia or hyperglycemia was coded or documented.
The proportion of HDHP enrollees where hypoglycemia was coded was 14% higher than for conventional plan enrollees (OR, 1.14; P < .001), with each year of the high-dollar plan enrollment increasing these odds by 6% (OR, 1.06; P < .001).
The tally of hypoglycemic events is an underestimate because HDHP enrollees might forgo ambulatory care for cost-related reasons, wrote the authors. Hypoglycemia might also be treated at home. But that is not necessarily a positive, they noted.
“The increased frequency of severe hypoglycemia – no matter where managed and discussed – is a sign of detrimental effects of HDHP enrollment for people living with diabetes.”
They found that individuals of racial and ethnic minorities were less likely than were White patients to have an increase in hypoglycemia-related office visits, which suggests that those patients were deferring care, wrote Dr. McCoy and colleagues.
Switching to an HDHP was associated with a significant increase in the odds of having at least one hyperglycemia-related ED or hospital visit per year (OR, 1.25; P < .001). Each successive year in the plan increased these odds by 5% (OR, 1.05; P = .02). However, the authors found no increase in hyperglycemia-related office visits.
“Because severe dysglycemic events may be prevented with optimal glycemic management, the increase in the frequency of their occurrence suggests important gaps in access to and implementation of diabetes therapy,” wrote the authors.
They noted that people with diabetes already face high out-of-pocket expenses. A high-deductible plan might make care even less affordable, they wrote.
“Individuals may be forced to ration medications, glucose-monitoring supplies, diabetes self-management education, food, and other essential cares to the detriment of their health,” they noted.
The authors added that because the study was observational, they could not delve into the root causes of the glycemic events or whether, for instance, any HDHP enrollees also had health savings accounts (HSAs) that might help defray costs.
They suggested that employers offer a wide variety of health plans, or if they are offering only a high-deductible plan that they be more transparent about potential costs. “Previous studies have shown that enrollees are not fully aware of the details within their health plans and may be focusing on reducing the cost of monthly premiums – not overall care – when choosing health plans.”
The authors said employers should find ways to fund HSAs for people with low incomes – those who appear to be most vulnerable to the effects of HDHPs.
A study published in JAMA Internal Medicine in 2017 found that low-income and HSA-eligible individuals with diabetes switched to an HDHP had major increases in emergency department visits for preventable acute diabetes complications.
The study was funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the Mayo Clinic K2R Research Award, and the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery. Dr. McCoy has reported receiving grants from the NIDDK, AARP, and the Patient-Centered Outcomes Research Institute, and personal fees from Emmi for the development of patient education materials about diabetes outside the submitted work.
A version of this article first appeared on Medscape.com.
Individuals with diabetes who are forced to switch to high-deductible health plans have more episodes of severe hypo- and hyperglycemia compared with those on conventional insurance plans, according to a new study.
Previous studies have shown that people with diabetes who are enrolled in high-deductible health plans (HDHPs) have an increased financial burden, lower medication adherence, and more low-severity emergency department visits, and they delay care for cardiovascular conditions.
But no study has looked at the plans’ impact on acute diabetes complications and glycemic control, wrote the authors in JAMA Network Open.
They found evidence that the high-dollar plans were associated with increased odds of severe hypoglycemic and hyperglycemic events, and that the risk increased with each successive year of enrollment. Low-income individuals, Blacks, and Hispanics were disproportionately more impacted, noted senior author Rozalina G. McCoy, MD, Mayo Clinic, Rochester, Minn., and colleagues.
Overall, “enrollees may be rationing or forgoing necessary care, which is detrimental to their health and ultimately increases the morbidity, mortality, and costs associated with diabetes,” they concluded.
A systematic review of eight studies published in Endocrine Practice in 2021 backs up this latest finding. That analysis reported enrollees in HDHPs often forgo routine care and monitoring, and that they have lower medication adherence, leading to an increase in total health care expenditures for emergency department visits, hospitalizations, and preventable complications.
Increased frequency of hypoglycemia is detrimental
The new study published in JAMA Network Open was based on data for adults enrolled in private insurance programs from 2010 to 2018. Researchers analyzed medical and pharmacy claims data contained in a large health insurance claims database, comparing adults with diabetes who had been in an HDHP for at least 1 year (and after a year of being in a conventional plan), with those who were in a conventional plan.
They identified 42,326 individuals who had been switched from a conventional plan to an HDHP. Of those, 7,375 (17.4%) were Black, 5,740 (13.6%) were Hispanic, 26,572 (62.8%) were non-Hispanic White, and 6,880 (16.3%) had a household income below $40,000 a year.
Baseline characteristics of the 202,729 people in conventional plans were similar to those in the HDHP group.
The median deductible for individuals in the HDHP group was $1,500 and for families it was $3,000, compared with $350 and $800, respectively, for those in conventional plans.
The odds of having any severe hypoglycemic event were significantly higher in the HDHP group (odds ratio [OR], 1.11; P < .001). Each year of HDHP enrollment increased the odds of a hypoglycemia-related ED or hospital visit by 2% (OR, 1.02; P = .04).
Aware that only a small number of severe hypoglycemic events, as well as an unknown number of such events, result in an emergency department visit or hospitalization, and that “the decision to seek ED or hospital care may be influenced by health plan assignment,” the authors also looked at office visits where severe, or any, hypoglycemia or hyperglycemia was coded or documented.
The proportion of HDHP enrollees where hypoglycemia was coded was 14% higher than for conventional plan enrollees (OR, 1.14; P < .001), with each year of the high-dollar plan enrollment increasing these odds by 6% (OR, 1.06; P < .001).
The tally of hypoglycemic events is an underestimate because HDHP enrollees might forgo ambulatory care for cost-related reasons, wrote the authors. Hypoglycemia might also be treated at home. But that is not necessarily a positive, they noted.
“The increased frequency of severe hypoglycemia – no matter where managed and discussed – is a sign of detrimental effects of HDHP enrollment for people living with diabetes.”
They found that individuals of racial and ethnic minorities were less likely than were White patients to have an increase in hypoglycemia-related office visits, which suggests that those patients were deferring care, wrote Dr. McCoy and colleagues.
Switching to an HDHP was associated with a significant increase in the odds of having at least one hyperglycemia-related ED or hospital visit per year (OR, 1.25; P < .001). Each successive year in the plan increased these odds by 5% (OR, 1.05; P = .02). However, the authors found no increase in hyperglycemia-related office visits.
“Because severe dysglycemic events may be prevented with optimal glycemic management, the increase in the frequency of their occurrence suggests important gaps in access to and implementation of diabetes therapy,” wrote the authors.
They noted that people with diabetes already face high out-of-pocket expenses. A high-deductible plan might make care even less affordable, they wrote.
“Individuals may be forced to ration medications, glucose-monitoring supplies, diabetes self-management education, food, and other essential cares to the detriment of their health,” they noted.
The authors added that because the study was observational, they could not delve into the root causes of the glycemic events or whether, for instance, any HDHP enrollees also had health savings accounts (HSAs) that might help defray costs.
They suggested that employers offer a wide variety of health plans, or if they are offering only a high-deductible plan that they be more transparent about potential costs. “Previous studies have shown that enrollees are not fully aware of the details within their health plans and may be focusing on reducing the cost of monthly premiums – not overall care – when choosing health plans.”
The authors said employers should find ways to fund HSAs for people with low incomes – those who appear to be most vulnerable to the effects of HDHPs.
A study published in JAMA Internal Medicine in 2017 found that low-income and HSA-eligible individuals with diabetes switched to an HDHP had major increases in emergency department visits for preventable acute diabetes complications.
The study was funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the Mayo Clinic K2R Research Award, and the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery. Dr. McCoy has reported receiving grants from the NIDDK, AARP, and the Patient-Centered Outcomes Research Institute, and personal fees from Emmi for the development of patient education materials about diabetes outside the submitted work.
A version of this article first appeared on Medscape.com.
Individuals with diabetes who are forced to switch to high-deductible health plans have more episodes of severe hypo- and hyperglycemia compared with those on conventional insurance plans, according to a new study.
Previous studies have shown that people with diabetes who are enrolled in high-deductible health plans (HDHPs) have an increased financial burden, lower medication adherence, and more low-severity emergency department visits, and they delay care for cardiovascular conditions.
But no study has looked at the plans’ impact on acute diabetes complications and glycemic control, wrote the authors in JAMA Network Open.
They found evidence that the high-dollar plans were associated with increased odds of severe hypoglycemic and hyperglycemic events, and that the risk increased with each successive year of enrollment. Low-income individuals, Blacks, and Hispanics were disproportionately more impacted, noted senior author Rozalina G. McCoy, MD, Mayo Clinic, Rochester, Minn., and colleagues.
Overall, “enrollees may be rationing or forgoing necessary care, which is detrimental to their health and ultimately increases the morbidity, mortality, and costs associated with diabetes,” they concluded.
A systematic review of eight studies published in Endocrine Practice in 2021 backs up this latest finding. That analysis reported enrollees in HDHPs often forgo routine care and monitoring, and that they have lower medication adherence, leading to an increase in total health care expenditures for emergency department visits, hospitalizations, and preventable complications.
Increased frequency of hypoglycemia is detrimental
The new study published in JAMA Network Open was based on data for adults enrolled in private insurance programs from 2010 to 2018. Researchers analyzed medical and pharmacy claims data contained in a large health insurance claims database, comparing adults with diabetes who had been in an HDHP for at least 1 year (and after a year of being in a conventional plan), with those who were in a conventional plan.
They identified 42,326 individuals who had been switched from a conventional plan to an HDHP. Of those, 7,375 (17.4%) were Black, 5,740 (13.6%) were Hispanic, 26,572 (62.8%) were non-Hispanic White, and 6,880 (16.3%) had a household income below $40,000 a year.
Baseline characteristics of the 202,729 people in conventional plans were similar to those in the HDHP group.
The median deductible for individuals in the HDHP group was $1,500 and for families it was $3,000, compared with $350 and $800, respectively, for those in conventional plans.
The odds of having any severe hypoglycemic event were significantly higher in the HDHP group (odds ratio [OR], 1.11; P < .001). Each year of HDHP enrollment increased the odds of a hypoglycemia-related ED or hospital visit by 2% (OR, 1.02; P = .04).
Aware that only a small number of severe hypoglycemic events, as well as an unknown number of such events, result in an emergency department visit or hospitalization, and that “the decision to seek ED or hospital care may be influenced by health plan assignment,” the authors also looked at office visits where severe, or any, hypoglycemia or hyperglycemia was coded or documented.
The proportion of HDHP enrollees where hypoglycemia was coded was 14% higher than for conventional plan enrollees (OR, 1.14; P < .001), with each year of the high-dollar plan enrollment increasing these odds by 6% (OR, 1.06; P < .001).
The tally of hypoglycemic events is an underestimate because HDHP enrollees might forgo ambulatory care for cost-related reasons, wrote the authors. Hypoglycemia might also be treated at home. But that is not necessarily a positive, they noted.
“The increased frequency of severe hypoglycemia – no matter where managed and discussed – is a sign of detrimental effects of HDHP enrollment for people living with diabetes.”
They found that individuals of racial and ethnic minorities were less likely than were White patients to have an increase in hypoglycemia-related office visits, which suggests that those patients were deferring care, wrote Dr. McCoy and colleagues.
Switching to an HDHP was associated with a significant increase in the odds of having at least one hyperglycemia-related ED or hospital visit per year (OR, 1.25; P < .001). Each successive year in the plan increased these odds by 5% (OR, 1.05; P = .02). However, the authors found no increase in hyperglycemia-related office visits.
“Because severe dysglycemic events may be prevented with optimal glycemic management, the increase in the frequency of their occurrence suggests important gaps in access to and implementation of diabetes therapy,” wrote the authors.
They noted that people with diabetes already face high out-of-pocket expenses. A high-deductible plan might make care even less affordable, they wrote.
“Individuals may be forced to ration medications, glucose-monitoring supplies, diabetes self-management education, food, and other essential cares to the detriment of their health,” they noted.
The authors added that because the study was observational, they could not delve into the root causes of the glycemic events or whether, for instance, any HDHP enrollees also had health savings accounts (HSAs) that might help defray costs.
They suggested that employers offer a wide variety of health plans, or if they are offering only a high-deductible plan that they be more transparent about potential costs. “Previous studies have shown that enrollees are not fully aware of the details within their health plans and may be focusing on reducing the cost of monthly premiums – not overall care – when choosing health plans.”
The authors said employers should find ways to fund HSAs for people with low incomes – those who appear to be most vulnerable to the effects of HDHPs.
A study published in JAMA Internal Medicine in 2017 found that low-income and HSA-eligible individuals with diabetes switched to an HDHP had major increases in emergency department visits for preventable acute diabetes complications.
The study was funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the Mayo Clinic K2R Research Award, and the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery. Dr. McCoy has reported receiving grants from the NIDDK, AARP, and the Patient-Centered Outcomes Research Institute, and personal fees from Emmi for the development of patient education materials about diabetes outside the submitted work.
A version of this article first appeared on Medscape.com.
FDA approves new type 2 diabetes drug bexagliflozin
The U.S. Food and Drug Administration has approved bexagliflozin (Brenzavvy, TheracosBio) for the treatment of adults with type 2 diabetes.
The once-daily 20-mg oral sodium-glucose cotransporter 2 (SGLT2) inhibitor is indicated as an adjunct to diet and exercise to improve glycemic control for those with type 2 diabetes, but not type 1 diabetes. It can be used in adults with an estimated glomerular filtration rate (eGFR) > 30 mL/min per 1.73 m2.
Approval was based on results from 23 clinical trials with more than 5,000 participants, including more than 300 patients with stage 3 kidney disease (eGFR < 60 and > 30 mL/min per 1.73 m2).
In the phase 3 studies, bexagliflozin significantly reduced hemoglobin A1c and fasting blood glucose at 24 weeks as monotherapy or as add-on to metformin and other glucose-lowering drugs and combinations. It also produced modest reductions in body weight and systolic blood pressure.
In the phase 3 Bexagliflozin Efficacy and Safety Trial (BEST) cardiovascular outcomes trial, the drug met its efficacy and safety objectives in patients at high cardiovascular risk. Noninferiority was demonstrated for the composite outcome of cardiovascular death, myocardial infarction, stroke, or unstable angina.
“As a class of drugs, SGLT2 inhibitors have shown tremendous benefit in treating adults with type 2 diabetes,” said Mason Freeman, MD, director of the Translational Research Center at Massachusetts General Hospital, Boston, in a press release from TheracosBio.
“Being involved in all of the clinical trials for Brenzavvy, I am greatly impressed with the efficacy of the drug in reducing blood glucose levels and I believe it is an important addition to the SGLT2 inhibitor class of drugs.”
As with other SGLT2 inhibitors, adverse events seen in the trials include ketoacidosis, lower limb amputation, volume depletion, urosepsis, pyelonephritis, Fournier’s gangrene, genital mycotic infections, and hypoglycemia when used with insulin or insulin secretagogues.
Bexagliflozin joins an already crowded field of SGLT2 inhibitors, some of which have been approved for additional cardiovascular and kidney indications.
Of interest, bexagliflozin was approved by the FDA for diabetes in cats in December 2022, as the first oral new animal drug to improve glycemic control in otherwise healthy cats with diabetes not previously treated with insulin.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved bexagliflozin (Brenzavvy, TheracosBio) for the treatment of adults with type 2 diabetes.
The once-daily 20-mg oral sodium-glucose cotransporter 2 (SGLT2) inhibitor is indicated as an adjunct to diet and exercise to improve glycemic control for those with type 2 diabetes, but not type 1 diabetes. It can be used in adults with an estimated glomerular filtration rate (eGFR) > 30 mL/min per 1.73 m2.
Approval was based on results from 23 clinical trials with more than 5,000 participants, including more than 300 patients with stage 3 kidney disease (eGFR < 60 and > 30 mL/min per 1.73 m2).
In the phase 3 studies, bexagliflozin significantly reduced hemoglobin A1c and fasting blood glucose at 24 weeks as monotherapy or as add-on to metformin and other glucose-lowering drugs and combinations. It also produced modest reductions in body weight and systolic blood pressure.
In the phase 3 Bexagliflozin Efficacy and Safety Trial (BEST) cardiovascular outcomes trial, the drug met its efficacy and safety objectives in patients at high cardiovascular risk. Noninferiority was demonstrated for the composite outcome of cardiovascular death, myocardial infarction, stroke, or unstable angina.
“As a class of drugs, SGLT2 inhibitors have shown tremendous benefit in treating adults with type 2 diabetes,” said Mason Freeman, MD, director of the Translational Research Center at Massachusetts General Hospital, Boston, in a press release from TheracosBio.
“Being involved in all of the clinical trials for Brenzavvy, I am greatly impressed with the efficacy of the drug in reducing blood glucose levels and I believe it is an important addition to the SGLT2 inhibitor class of drugs.”
As with other SGLT2 inhibitors, adverse events seen in the trials include ketoacidosis, lower limb amputation, volume depletion, urosepsis, pyelonephritis, Fournier’s gangrene, genital mycotic infections, and hypoglycemia when used with insulin or insulin secretagogues.
Bexagliflozin joins an already crowded field of SGLT2 inhibitors, some of which have been approved for additional cardiovascular and kidney indications.
Of interest, bexagliflozin was approved by the FDA for diabetes in cats in December 2022, as the first oral new animal drug to improve glycemic control in otherwise healthy cats with diabetes not previously treated with insulin.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved bexagliflozin (Brenzavvy, TheracosBio) for the treatment of adults with type 2 diabetes.
The once-daily 20-mg oral sodium-glucose cotransporter 2 (SGLT2) inhibitor is indicated as an adjunct to diet and exercise to improve glycemic control for those with type 2 diabetes, but not type 1 diabetes. It can be used in adults with an estimated glomerular filtration rate (eGFR) > 30 mL/min per 1.73 m2.
Approval was based on results from 23 clinical trials with more than 5,000 participants, including more than 300 patients with stage 3 kidney disease (eGFR < 60 and > 30 mL/min per 1.73 m2).
In the phase 3 studies, bexagliflozin significantly reduced hemoglobin A1c and fasting blood glucose at 24 weeks as monotherapy or as add-on to metformin and other glucose-lowering drugs and combinations. It also produced modest reductions in body weight and systolic blood pressure.
In the phase 3 Bexagliflozin Efficacy and Safety Trial (BEST) cardiovascular outcomes trial, the drug met its efficacy and safety objectives in patients at high cardiovascular risk. Noninferiority was demonstrated for the composite outcome of cardiovascular death, myocardial infarction, stroke, or unstable angina.
“As a class of drugs, SGLT2 inhibitors have shown tremendous benefit in treating adults with type 2 diabetes,” said Mason Freeman, MD, director of the Translational Research Center at Massachusetts General Hospital, Boston, in a press release from TheracosBio.
“Being involved in all of the clinical trials for Brenzavvy, I am greatly impressed with the efficacy of the drug in reducing blood glucose levels and I believe it is an important addition to the SGLT2 inhibitor class of drugs.”
As with other SGLT2 inhibitors, adverse events seen in the trials include ketoacidosis, lower limb amputation, volume depletion, urosepsis, pyelonephritis, Fournier’s gangrene, genital mycotic infections, and hypoglycemia when used with insulin or insulin secretagogues.
Bexagliflozin joins an already crowded field of SGLT2 inhibitors, some of which have been approved for additional cardiovascular and kidney indications.
Of interest, bexagliflozin was approved by the FDA for diabetes in cats in December 2022, as the first oral new animal drug to improve glycemic control in otherwise healthy cats with diabetes not previously treated with insulin.
A version of this article first appeared on Medscape.com.
Doctors’ happiness has not rebounded as pandemic drags on
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
Is it time for yet another COVID booster? It’s complicated
For some people who have received a two-dose primary series and all the recommended boosters, that could mean a sixth shot since COVID-19 vaccines became available. But is even that enough (or too much)?
At this point, no one knows for sure, but new guidance may be on the docket.
On Jan. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee is meeting. On the agenda is discussion about plans for future vaccinations for COVID-19.The committee, made up of external advisers, evaluates data on vaccines and other products for the agency.
According to the FDA announcement, after the meeting, “the FDA will consider whether to recommend adjustments to the current authorizations and approvals, and the FDA will consider the most efficient and transparent process to use for selection of strains for inclusion in the primary and booster vaccines.”
From there, the CDC will take up the issue and decide on recommendations.
The issue is important, as more than 550 Americans a day are still dying from COVID-19, as of the week ending Jan. 13, the CDC reported. That’s up from 346 a day for the week ending Dec. 28.
Yet, uptake of the newest vaccine, the bivalent booster, has been slow. As of Jan. 11, just 15.9% of the population 5 years and up has gotten it; for those most vulnerable to COVID19 – those 65 and up – the number is just 39%.
COVID vaccines, 2023 and beyond
Meanwhile, infectious disease experts have widely differing views on what the vaccination landscape of 2023 and beyond should look like. Among the areas of disagreement are how effective the bivalent vaccine is, which people most need another shot, and what type of vaccine is best.
“I think we probably will need another booster,” says Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine, and codirector of the Center for Vaccine Development at Texas Children’s Hospital in Houston. “The question is, what is it going to be? Is it going to be the same bivalent that we just got, or will it be a new bivalent or even a trivalent?”
The trivalent booster, he suggested, might include something more protective against XBB.1.5.
The bivalent booster gives “broadened immunity” that is improved from the original booster shots, says Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, WebMD’s sister site for health professionals.
In his publication Ground Truths, Dr. Topol on Jan. 11 explained how new data caused him to reverse his previously skeptical view of how the FDA authorized the bivalent vaccine in September without data on how it affected humans at the time.
Paul Offit, MD, director of the Vaccine Education Center and a professor of pediatrics at the Children’s Hospital of Philadelphia, is a member of the FDA advisory committee for vaccines. He still takes a dimmer view of more bivalent booster vaccines, at least as a blanket recommendation.
While he acknowledges that boosters can help some groups – such as older adults, people with multiple health conditions, and those with compromised immune systems – he opposes a recommendation that’s population-wide.
“People who fall into those three groups do benefit,” he says, “but the recommendation is everyone over 6 months get the bivalent, and what I’m asking is, ‘Where is the data that a healthy 12-year-old boy needs a booster to stay out of the hospital?’ ”
Evolving research
“We are trying to understand how to stay one step ahead rather than several steps behind [the virus],“ says Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Among the key questions: How well can a vaccine work against a single subvariant, when no one can say for sure what the next predominant subvariant will be?
Much more research has become available recently about the bivalent vaccine and its effectiveness, Dr. Osterholm says. “The bivalent vaccine is working as well as we could have expected,” he says, especially in high-risk people and in those over age 65. “The challenge we have is, what does that mean going forward?”
In his review, Dr. Topol concludes: “There is now more than ample, highly consistent evidence via lab studies and clinical outcomes to support the bivalent’s benefit over the original booster.”
Among other evidence, he looked at eight studies, including four that used a live virus as part of the research. Six of the eight studies showed the bivalent booster is more effective against the BA.5 variant, compared with the original booster shots. Two others showed no real difference.
“The four live virus studies offer consistent evidence of broadened immunity for the BA.5 vaccine that is improved over the original booster shots,” Dr. Topol wrote. The evidence also found the bivalent antibody response superior against XBB, he wrote.
Dr. Topol also cited CDC data that supports the benefits of the bivalent shot on hospitalization in older adults. During November, hospitalization of adults 65 and above was 2.5 times higher for those vaccinated who did not get the booster, compared to those who got the updated bivalent booster.
Boosters do matter, Dr. Offit says. “But not for all.” In a perspective published Jan. 11 in the New England Journal of Medicine – the same issue that published the two studies finding few differences between the original and bivalent – Dr. Offit wrote that boosting is best reserved for vulnerable groups.
Chasing the variants with a bivalent vaccine, he says, “has not panned out. There remains no evidence that a bivalent vaccine is any better than what we had. Please, show me the data that one is better than the other.”
Dr. Offit believes the goal should not be to prevent all symptomatic infections in healthy, young people by boosting them “with vaccines containing mRNA from strains that might disappear a few months later.”
The CDC needs to parse the data by subgroups, Dr. Offit says. “The critical question is, ‘Who gets hospitalized and who is dying? Who are they?’ ”
That data should take into account age, ethnicity, vaccine history, and other factors, Dr. Offit says, because right now, there is no great data to say, “OK, everyone gets a boost.”
Future vaccine costs
Another debate – for not only current boosters but future ones, too – centers on cost. Without congressional action to fund more vaccines, vaccine makers have suggested their prices may reach $130 a dose, compared with the average $20-per-dose cost the federal government pays now, according to a Kaiser Family Foundation report.
The government has spent more than $30 billion on COVID-19 vaccines, including the bivalent, to provide them free of charge.
The suggested price increase infuriated many. On Jan. 10, Sen. Bernie Sanders (I-Vt.), incoming chair of the Senate Committee on Health, Education, Labor and Pensions, sent a letter to Moderna CEO Stéphane Bancel, urging him to reconsider and refrain from any price increase.
“The huge increase in price that you have proposed will have a significantly negative impact on the budgets of Medicaid, Medicare and other government programs that will continue covering the vaccine without cost-sharing for patients.”
He pointed out, too, the $19 billion in profits Moderna has made over the past 2 years.
While most people with health insurance would likely still get the vaccines and booster for free, according to the Kaiser analysis, will a higher price discourage people from keeping up with recommended vaccinations, including a possible new booster?
“I think so, yes,” Dr. Hotez says, noting that vaccine reluctance is high as it is, even with free vaccinations and easy access.
“The government is balking at paying for the boosters,” he says. “I think it’s very tone deaf from the pharmaceutical companies [to increase the price]. Given all the help they’ve gotten from the American people, I think they should not be gouging at this point.”
He noted that the federal government provided not just money to the companies for the vaccines, but a “glide path” through the FDA for the vaccine approvals.
Are new, variant-specific boosters coming?
Are Moderna, Pfizer-BioNTech, and others developing more variant-specific vaccines, boosters, or other advances?
Novavax, approved in July 2022 as a primary series and in some cases as a booster, is “also developing an Omicron-containing bivalent vaccine at the direction of public health agencies,” says spokesperson Alison Chartan.
Pfizer responded: “When and if we have something to share we will let you know.”
Moderna did not respond.
A version of this article first appeared on WebMD.com.
For some people who have received a two-dose primary series and all the recommended boosters, that could mean a sixth shot since COVID-19 vaccines became available. But is even that enough (or too much)?
At this point, no one knows for sure, but new guidance may be on the docket.
On Jan. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee is meeting. On the agenda is discussion about plans for future vaccinations for COVID-19.The committee, made up of external advisers, evaluates data on vaccines and other products for the agency.
According to the FDA announcement, after the meeting, “the FDA will consider whether to recommend adjustments to the current authorizations and approvals, and the FDA will consider the most efficient and transparent process to use for selection of strains for inclusion in the primary and booster vaccines.”
From there, the CDC will take up the issue and decide on recommendations.
The issue is important, as more than 550 Americans a day are still dying from COVID-19, as of the week ending Jan. 13, the CDC reported. That’s up from 346 a day for the week ending Dec. 28.
Yet, uptake of the newest vaccine, the bivalent booster, has been slow. As of Jan. 11, just 15.9% of the population 5 years and up has gotten it; for those most vulnerable to COVID19 – those 65 and up – the number is just 39%.
COVID vaccines, 2023 and beyond
Meanwhile, infectious disease experts have widely differing views on what the vaccination landscape of 2023 and beyond should look like. Among the areas of disagreement are how effective the bivalent vaccine is, which people most need another shot, and what type of vaccine is best.
“I think we probably will need another booster,” says Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine, and codirector of the Center for Vaccine Development at Texas Children’s Hospital in Houston. “The question is, what is it going to be? Is it going to be the same bivalent that we just got, or will it be a new bivalent or even a trivalent?”
The trivalent booster, he suggested, might include something more protective against XBB.1.5.
The bivalent booster gives “broadened immunity” that is improved from the original booster shots, says Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, WebMD’s sister site for health professionals.
In his publication Ground Truths, Dr. Topol on Jan. 11 explained how new data caused him to reverse his previously skeptical view of how the FDA authorized the bivalent vaccine in September without data on how it affected humans at the time.
Paul Offit, MD, director of the Vaccine Education Center and a professor of pediatrics at the Children’s Hospital of Philadelphia, is a member of the FDA advisory committee for vaccines. He still takes a dimmer view of more bivalent booster vaccines, at least as a blanket recommendation.
While he acknowledges that boosters can help some groups – such as older adults, people with multiple health conditions, and those with compromised immune systems – he opposes a recommendation that’s population-wide.
“People who fall into those three groups do benefit,” he says, “but the recommendation is everyone over 6 months get the bivalent, and what I’m asking is, ‘Where is the data that a healthy 12-year-old boy needs a booster to stay out of the hospital?’ ”
Evolving research
“We are trying to understand how to stay one step ahead rather than several steps behind [the virus],“ says Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Among the key questions: How well can a vaccine work against a single subvariant, when no one can say for sure what the next predominant subvariant will be?
Much more research has become available recently about the bivalent vaccine and its effectiveness, Dr. Osterholm says. “The bivalent vaccine is working as well as we could have expected,” he says, especially in high-risk people and in those over age 65. “The challenge we have is, what does that mean going forward?”
In his review, Dr. Topol concludes: “There is now more than ample, highly consistent evidence via lab studies and clinical outcomes to support the bivalent’s benefit over the original booster.”
Among other evidence, he looked at eight studies, including four that used a live virus as part of the research. Six of the eight studies showed the bivalent booster is more effective against the BA.5 variant, compared with the original booster shots. Two others showed no real difference.
“The four live virus studies offer consistent evidence of broadened immunity for the BA.5 vaccine that is improved over the original booster shots,” Dr. Topol wrote. The evidence also found the bivalent antibody response superior against XBB, he wrote.
Dr. Topol also cited CDC data that supports the benefits of the bivalent shot on hospitalization in older adults. During November, hospitalization of adults 65 and above was 2.5 times higher for those vaccinated who did not get the booster, compared to those who got the updated bivalent booster.
Boosters do matter, Dr. Offit says. “But not for all.” In a perspective published Jan. 11 in the New England Journal of Medicine – the same issue that published the two studies finding few differences between the original and bivalent – Dr. Offit wrote that boosting is best reserved for vulnerable groups.
Chasing the variants with a bivalent vaccine, he says, “has not panned out. There remains no evidence that a bivalent vaccine is any better than what we had. Please, show me the data that one is better than the other.”
Dr. Offit believes the goal should not be to prevent all symptomatic infections in healthy, young people by boosting them “with vaccines containing mRNA from strains that might disappear a few months later.”
The CDC needs to parse the data by subgroups, Dr. Offit says. “The critical question is, ‘Who gets hospitalized and who is dying? Who are they?’ ”
That data should take into account age, ethnicity, vaccine history, and other factors, Dr. Offit says, because right now, there is no great data to say, “OK, everyone gets a boost.”
Future vaccine costs
Another debate – for not only current boosters but future ones, too – centers on cost. Without congressional action to fund more vaccines, vaccine makers have suggested their prices may reach $130 a dose, compared with the average $20-per-dose cost the federal government pays now, according to a Kaiser Family Foundation report.
The government has spent more than $30 billion on COVID-19 vaccines, including the bivalent, to provide them free of charge.
The suggested price increase infuriated many. On Jan. 10, Sen. Bernie Sanders (I-Vt.), incoming chair of the Senate Committee on Health, Education, Labor and Pensions, sent a letter to Moderna CEO Stéphane Bancel, urging him to reconsider and refrain from any price increase.
“The huge increase in price that you have proposed will have a significantly negative impact on the budgets of Medicaid, Medicare and other government programs that will continue covering the vaccine without cost-sharing for patients.”
He pointed out, too, the $19 billion in profits Moderna has made over the past 2 years.
While most people with health insurance would likely still get the vaccines and booster for free, according to the Kaiser analysis, will a higher price discourage people from keeping up with recommended vaccinations, including a possible new booster?
“I think so, yes,” Dr. Hotez says, noting that vaccine reluctance is high as it is, even with free vaccinations and easy access.
“The government is balking at paying for the boosters,” he says. “I think it’s very tone deaf from the pharmaceutical companies [to increase the price]. Given all the help they’ve gotten from the American people, I think they should not be gouging at this point.”
He noted that the federal government provided not just money to the companies for the vaccines, but a “glide path” through the FDA for the vaccine approvals.
Are new, variant-specific boosters coming?
Are Moderna, Pfizer-BioNTech, and others developing more variant-specific vaccines, boosters, or other advances?
Novavax, approved in July 2022 as a primary series and in some cases as a booster, is “also developing an Omicron-containing bivalent vaccine at the direction of public health agencies,” says spokesperson Alison Chartan.
Pfizer responded: “When and if we have something to share we will let you know.”
Moderna did not respond.
A version of this article first appeared on WebMD.com.
For some people who have received a two-dose primary series and all the recommended boosters, that could mean a sixth shot since COVID-19 vaccines became available. But is even that enough (or too much)?
At this point, no one knows for sure, but new guidance may be on the docket.
On Jan. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee is meeting. On the agenda is discussion about plans for future vaccinations for COVID-19.The committee, made up of external advisers, evaluates data on vaccines and other products for the agency.
According to the FDA announcement, after the meeting, “the FDA will consider whether to recommend adjustments to the current authorizations and approvals, and the FDA will consider the most efficient and transparent process to use for selection of strains for inclusion in the primary and booster vaccines.”
From there, the CDC will take up the issue and decide on recommendations.
The issue is important, as more than 550 Americans a day are still dying from COVID-19, as of the week ending Jan. 13, the CDC reported. That’s up from 346 a day for the week ending Dec. 28.
Yet, uptake of the newest vaccine, the bivalent booster, has been slow. As of Jan. 11, just 15.9% of the population 5 years and up has gotten it; for those most vulnerable to COVID19 – those 65 and up – the number is just 39%.
COVID vaccines, 2023 and beyond
Meanwhile, infectious disease experts have widely differing views on what the vaccination landscape of 2023 and beyond should look like. Among the areas of disagreement are how effective the bivalent vaccine is, which people most need another shot, and what type of vaccine is best.
“I think we probably will need another booster,” says Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine, and codirector of the Center for Vaccine Development at Texas Children’s Hospital in Houston. “The question is, what is it going to be? Is it going to be the same bivalent that we just got, or will it be a new bivalent or even a trivalent?”
The trivalent booster, he suggested, might include something more protective against XBB.1.5.
The bivalent booster gives “broadened immunity” that is improved from the original booster shots, says Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, WebMD’s sister site for health professionals.
In his publication Ground Truths, Dr. Topol on Jan. 11 explained how new data caused him to reverse his previously skeptical view of how the FDA authorized the bivalent vaccine in September without data on how it affected humans at the time.
Paul Offit, MD, director of the Vaccine Education Center and a professor of pediatrics at the Children’s Hospital of Philadelphia, is a member of the FDA advisory committee for vaccines. He still takes a dimmer view of more bivalent booster vaccines, at least as a blanket recommendation.
While he acknowledges that boosters can help some groups – such as older adults, people with multiple health conditions, and those with compromised immune systems – he opposes a recommendation that’s population-wide.
“People who fall into those three groups do benefit,” he says, “but the recommendation is everyone over 6 months get the bivalent, and what I’m asking is, ‘Where is the data that a healthy 12-year-old boy needs a booster to stay out of the hospital?’ ”
Evolving research
“We are trying to understand how to stay one step ahead rather than several steps behind [the virus],“ says Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Among the key questions: How well can a vaccine work against a single subvariant, when no one can say for sure what the next predominant subvariant will be?
Much more research has become available recently about the bivalent vaccine and its effectiveness, Dr. Osterholm says. “The bivalent vaccine is working as well as we could have expected,” he says, especially in high-risk people and in those over age 65. “The challenge we have is, what does that mean going forward?”
In his review, Dr. Topol concludes: “There is now more than ample, highly consistent evidence via lab studies and clinical outcomes to support the bivalent’s benefit over the original booster.”
Among other evidence, he looked at eight studies, including four that used a live virus as part of the research. Six of the eight studies showed the bivalent booster is more effective against the BA.5 variant, compared with the original booster shots. Two others showed no real difference.
“The four live virus studies offer consistent evidence of broadened immunity for the BA.5 vaccine that is improved over the original booster shots,” Dr. Topol wrote. The evidence also found the bivalent antibody response superior against XBB, he wrote.
Dr. Topol also cited CDC data that supports the benefits of the bivalent shot on hospitalization in older adults. During November, hospitalization of adults 65 and above was 2.5 times higher for those vaccinated who did not get the booster, compared to those who got the updated bivalent booster.
Boosters do matter, Dr. Offit says. “But not for all.” In a perspective published Jan. 11 in the New England Journal of Medicine – the same issue that published the two studies finding few differences between the original and bivalent – Dr. Offit wrote that boosting is best reserved for vulnerable groups.
Chasing the variants with a bivalent vaccine, he says, “has not panned out. There remains no evidence that a bivalent vaccine is any better than what we had. Please, show me the data that one is better than the other.”
Dr. Offit believes the goal should not be to prevent all symptomatic infections in healthy, young people by boosting them “with vaccines containing mRNA from strains that might disappear a few months later.”
The CDC needs to parse the data by subgroups, Dr. Offit says. “The critical question is, ‘Who gets hospitalized and who is dying? Who are they?’ ”
That data should take into account age, ethnicity, vaccine history, and other factors, Dr. Offit says, because right now, there is no great data to say, “OK, everyone gets a boost.”
Future vaccine costs
Another debate – for not only current boosters but future ones, too – centers on cost. Without congressional action to fund more vaccines, vaccine makers have suggested their prices may reach $130 a dose, compared with the average $20-per-dose cost the federal government pays now, according to a Kaiser Family Foundation report.
The government has spent more than $30 billion on COVID-19 vaccines, including the bivalent, to provide them free of charge.
The suggested price increase infuriated many. On Jan. 10, Sen. Bernie Sanders (I-Vt.), incoming chair of the Senate Committee on Health, Education, Labor and Pensions, sent a letter to Moderna CEO Stéphane Bancel, urging him to reconsider and refrain from any price increase.
“The huge increase in price that you have proposed will have a significantly negative impact on the budgets of Medicaid, Medicare and other government programs that will continue covering the vaccine without cost-sharing for patients.”
He pointed out, too, the $19 billion in profits Moderna has made over the past 2 years.
While most people with health insurance would likely still get the vaccines and booster for free, according to the Kaiser analysis, will a higher price discourage people from keeping up with recommended vaccinations, including a possible new booster?
“I think so, yes,” Dr. Hotez says, noting that vaccine reluctance is high as it is, even with free vaccinations and easy access.
“The government is balking at paying for the boosters,” he says. “I think it’s very tone deaf from the pharmaceutical companies [to increase the price]. Given all the help they’ve gotten from the American people, I think they should not be gouging at this point.”
He noted that the federal government provided not just money to the companies for the vaccines, but a “glide path” through the FDA for the vaccine approvals.
Are new, variant-specific boosters coming?
Are Moderna, Pfizer-BioNTech, and others developing more variant-specific vaccines, boosters, or other advances?
Novavax, approved in July 2022 as a primary series and in some cases as a booster, is “also developing an Omicron-containing bivalent vaccine at the direction of public health agencies,” says spokesperson Alison Chartan.
Pfizer responded: “When and if we have something to share we will let you know.”
Moderna did not respond.
A version of this article first appeared on WebMD.com.
Nitrite food additives may increase risk of type 2 diabetes
Consuming a large amount of nitrites from food additives versus none was associated with a greater risk of developing type 2 diabetes in the NutriNet-Santé study in France, researchers report.
However, a few experts who were not involved with this research question the strength of the findings because of study limitations.
The study involved more than 100,000 adults with a mean age of 43, and 79% were women.
Individuals with the highest intakes of nitrites from food additives (top third) had a 53% higher risk of developing type 2 diabetes during a median follow-up of 7 years compared with those with the lowest intake of this food additive after controlling for intake of sugars, red and processed meats, heme iron, salt, and saturated fatty acids. Consumption of nitrates from food additives was not associated with risk of type 2 diabetes.
“Our findings suggest a direct association between additives-originated nitrites and [type 2 diabetes] risk and corroborate previously suggested associations between total dietary nitrites and [type 2 diabetes],” the researchers report in an article published online in PLoS Medicine.
However, “as this is the first large-scale study finding these associations, these results need to be replicated in other large-scale cohorts,” senior author Mathilde Touvier, PhD, head of the Nutritional Epidemiology Research Team (EREN-CRESS), INSERM, INRAE, Sorbonne Paris Nord University, and lead author Bernard Srour, PhD, PharmD, a scientist at the same institution, said in a joint email to this news organization.
Short-term intervention studies to determine insulin resistance could also be tested, they add.
In the meantime, “this study adds further evidence to the existing strong link between nitrites and colorectal cancer risk, and supports the importance of further regulation of nitrites as food additives and nitrogen fertilizers,” they say.
According to Dr. Touvier and Dr. Srour, the takeaway message for clinicians is the finding that nitrites from food additives are associated with type 2 diabetes, “support existing guidelines recommending [limiting] the consumption of processed meats to prevent chronic diseases. However, the consumption of vegetables should be encouraged as they contain several beneficial compounds and contribute to chronic disease prevention.”
Some experts are skeptical
But three experts who were not involved with the research were skeptical about the conclusions, in comments made to the U.K. Science Media Centre.
“The fundamental weakness of this study is how the food additive intake was assessed,” said Tom Sanders, DSc, PhD, professor emeritus of nutrition and dietetics, King’s College London. “Estimates of intake were based on recalls of dietary intake on two separate occasions at the beginning of the study with no further estimates in the follow-up period of over 7 years,” he noted.
Other limitations include the relatively young age of the cohort and relatively low incidence of new cases of type 2 diabetes (about 1% of the study population over 7 years).
Moreover, the level of nitrite food additive ingestion is much lower than the acceptable daily intake. The findings would need to be replicated with appropriate adjustment for differences in body weight.
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading, England, said that “the study does not support the claim in the press release and paper that food additives are responsible for the increased risk.”
He pointed out that “nitrite from additives contributes only about 4%-6% of total nitrite intake in the population, and it is not clear why this should have a stronger impact on risk than nitrite from other sources,” such as nitrate found in food and water.
Duane Mellor, PhD, registered dietitian and senior lecturer, Aston University, Birmingham, England, said: “It could be questioned how accurate estimating intakes of individual additives like sodium nitrite, which was less than 1 mg per day from a record of just 2 days food intake per year, as it assumes people ate the same the other 363 days of the year.”
Moreover, “it is perhaps worth noting that the use of nitrites as an additive is often as sodium nitrite, which is used to cure meats like bacon, which if someone is seeking to reduce their risk of type 2 diabetes would be something people would be encouraged to eat less of [anyway].”
“The best way to reduce your risk of developing type 2 diabetes,” he said, “is to be physically active, maintain a healthy weight for you, and eat a varied diet based on vegetables, pulses, nuts, seeds, and fruit along with wholegrain and moderate intakes of dairy foods and meat (especially processed meats).”
Study details
Nitrites and nitrates are used as food additives to prevent bacterial growth, mainly in processed meats, and they are also found in foods (mainly green leafy vegetables) and water (nitrates from the use of nitrogen fertilizer can enter the water supply).
The researchers analyzed data from 104,168 participants in NutriNet-Santé who had no diabetes at baseline and who completed 24-hour dietary intake records. They investigated the association between exposure to nitrites and nitrates (in food and water or in additives) and incident type 2 diabetes.
Most nitrites came from food (95.3%), and less often from food additives (4.7%) and water (< 0.01%). The nitrites in foods were mainly from vegetables (60%) and seasonings (23%).
Most nitrates also came from food (93%), followed by water (6.9%) and food additives (0.1%). The nitrates in foods were mainly from vegetables (41%), processed meat (19%), and meat (17%).
During a median follow-up of 7.3 years, there were 969 incident cases of type 2 diabetes.
Compared with individuals in the lowest third of nitrites from food and water, those in the highest tertile had a 27% higher risk of incident type 2 diabetes, after adjusting for multiple variables (hazard ratio, 1.27; P = .009).
The risk of type 2 diabetes associated with the highest intake of nitrites from additives was as previously described, 53% higher, than that for those with the lowest intake.
There was no evidence of an association between nitrates and risk of type 2 diabetes.
The researchers acknowledge that study limitations include potential errors in assessment of nitrate and nitrate exposure, potential selection bias (participants in the web-based study may have had healthier behaviors than the general population), and potential unaccounted confounders (because it was an observational study).
A version of this article first appeared on Medscape.com.
Consuming a large amount of nitrites from food additives versus none was associated with a greater risk of developing type 2 diabetes in the NutriNet-Santé study in France, researchers report.
However, a few experts who were not involved with this research question the strength of the findings because of study limitations.
The study involved more than 100,000 adults with a mean age of 43, and 79% were women.
Individuals with the highest intakes of nitrites from food additives (top third) had a 53% higher risk of developing type 2 diabetes during a median follow-up of 7 years compared with those with the lowest intake of this food additive after controlling for intake of sugars, red and processed meats, heme iron, salt, and saturated fatty acids. Consumption of nitrates from food additives was not associated with risk of type 2 diabetes.
“Our findings suggest a direct association between additives-originated nitrites and [type 2 diabetes] risk and corroborate previously suggested associations between total dietary nitrites and [type 2 diabetes],” the researchers report in an article published online in PLoS Medicine.
However, “as this is the first large-scale study finding these associations, these results need to be replicated in other large-scale cohorts,” senior author Mathilde Touvier, PhD, head of the Nutritional Epidemiology Research Team (EREN-CRESS), INSERM, INRAE, Sorbonne Paris Nord University, and lead author Bernard Srour, PhD, PharmD, a scientist at the same institution, said in a joint email to this news organization.
Short-term intervention studies to determine insulin resistance could also be tested, they add.
In the meantime, “this study adds further evidence to the existing strong link between nitrites and colorectal cancer risk, and supports the importance of further regulation of nitrites as food additives and nitrogen fertilizers,” they say.
According to Dr. Touvier and Dr. Srour, the takeaway message for clinicians is the finding that nitrites from food additives are associated with type 2 diabetes, “support existing guidelines recommending [limiting] the consumption of processed meats to prevent chronic diseases. However, the consumption of vegetables should be encouraged as they contain several beneficial compounds and contribute to chronic disease prevention.”
Some experts are skeptical
But three experts who were not involved with the research were skeptical about the conclusions, in comments made to the U.K. Science Media Centre.
“The fundamental weakness of this study is how the food additive intake was assessed,” said Tom Sanders, DSc, PhD, professor emeritus of nutrition and dietetics, King’s College London. “Estimates of intake were based on recalls of dietary intake on two separate occasions at the beginning of the study with no further estimates in the follow-up period of over 7 years,” he noted.
Other limitations include the relatively young age of the cohort and relatively low incidence of new cases of type 2 diabetes (about 1% of the study population over 7 years).
Moreover, the level of nitrite food additive ingestion is much lower than the acceptable daily intake. The findings would need to be replicated with appropriate adjustment for differences in body weight.
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading, England, said that “the study does not support the claim in the press release and paper that food additives are responsible for the increased risk.”
He pointed out that “nitrite from additives contributes only about 4%-6% of total nitrite intake in the population, and it is not clear why this should have a stronger impact on risk than nitrite from other sources,” such as nitrate found in food and water.
Duane Mellor, PhD, registered dietitian and senior lecturer, Aston University, Birmingham, England, said: “It could be questioned how accurate estimating intakes of individual additives like sodium nitrite, which was less than 1 mg per day from a record of just 2 days food intake per year, as it assumes people ate the same the other 363 days of the year.”
Moreover, “it is perhaps worth noting that the use of nitrites as an additive is often as sodium nitrite, which is used to cure meats like bacon, which if someone is seeking to reduce their risk of type 2 diabetes would be something people would be encouraged to eat less of [anyway].”
“The best way to reduce your risk of developing type 2 diabetes,” he said, “is to be physically active, maintain a healthy weight for you, and eat a varied diet based on vegetables, pulses, nuts, seeds, and fruit along with wholegrain and moderate intakes of dairy foods and meat (especially processed meats).”
Study details
Nitrites and nitrates are used as food additives to prevent bacterial growth, mainly in processed meats, and they are also found in foods (mainly green leafy vegetables) and water (nitrates from the use of nitrogen fertilizer can enter the water supply).
The researchers analyzed data from 104,168 participants in NutriNet-Santé who had no diabetes at baseline and who completed 24-hour dietary intake records. They investigated the association between exposure to nitrites and nitrates (in food and water or in additives) and incident type 2 diabetes.
Most nitrites came from food (95.3%), and less often from food additives (4.7%) and water (< 0.01%). The nitrites in foods were mainly from vegetables (60%) and seasonings (23%).
Most nitrates also came from food (93%), followed by water (6.9%) and food additives (0.1%). The nitrates in foods were mainly from vegetables (41%), processed meat (19%), and meat (17%).
During a median follow-up of 7.3 years, there were 969 incident cases of type 2 diabetes.
Compared with individuals in the lowest third of nitrites from food and water, those in the highest tertile had a 27% higher risk of incident type 2 diabetes, after adjusting for multiple variables (hazard ratio, 1.27; P = .009).
The risk of type 2 diabetes associated with the highest intake of nitrites from additives was as previously described, 53% higher, than that for those with the lowest intake.
There was no evidence of an association between nitrates and risk of type 2 diabetes.
The researchers acknowledge that study limitations include potential errors in assessment of nitrate and nitrate exposure, potential selection bias (participants in the web-based study may have had healthier behaviors than the general population), and potential unaccounted confounders (because it was an observational study).
A version of this article first appeared on Medscape.com.
Consuming a large amount of nitrites from food additives versus none was associated with a greater risk of developing type 2 diabetes in the NutriNet-Santé study in France, researchers report.
However, a few experts who were not involved with this research question the strength of the findings because of study limitations.
The study involved more than 100,000 adults with a mean age of 43, and 79% were women.
Individuals with the highest intakes of nitrites from food additives (top third) had a 53% higher risk of developing type 2 diabetes during a median follow-up of 7 years compared with those with the lowest intake of this food additive after controlling for intake of sugars, red and processed meats, heme iron, salt, and saturated fatty acids. Consumption of nitrates from food additives was not associated with risk of type 2 diabetes.
“Our findings suggest a direct association between additives-originated nitrites and [type 2 diabetes] risk and corroborate previously suggested associations between total dietary nitrites and [type 2 diabetes],” the researchers report in an article published online in PLoS Medicine.
However, “as this is the first large-scale study finding these associations, these results need to be replicated in other large-scale cohorts,” senior author Mathilde Touvier, PhD, head of the Nutritional Epidemiology Research Team (EREN-CRESS), INSERM, INRAE, Sorbonne Paris Nord University, and lead author Bernard Srour, PhD, PharmD, a scientist at the same institution, said in a joint email to this news organization.
Short-term intervention studies to determine insulin resistance could also be tested, they add.
In the meantime, “this study adds further evidence to the existing strong link between nitrites and colorectal cancer risk, and supports the importance of further regulation of nitrites as food additives and nitrogen fertilizers,” they say.
According to Dr. Touvier and Dr. Srour, the takeaway message for clinicians is the finding that nitrites from food additives are associated with type 2 diabetes, “support existing guidelines recommending [limiting] the consumption of processed meats to prevent chronic diseases. However, the consumption of vegetables should be encouraged as they contain several beneficial compounds and contribute to chronic disease prevention.”
Some experts are skeptical
But three experts who were not involved with the research were skeptical about the conclusions, in comments made to the U.K. Science Media Centre.
“The fundamental weakness of this study is how the food additive intake was assessed,” said Tom Sanders, DSc, PhD, professor emeritus of nutrition and dietetics, King’s College London. “Estimates of intake were based on recalls of dietary intake on two separate occasions at the beginning of the study with no further estimates in the follow-up period of over 7 years,” he noted.
Other limitations include the relatively young age of the cohort and relatively low incidence of new cases of type 2 diabetes (about 1% of the study population over 7 years).
Moreover, the level of nitrite food additive ingestion is much lower than the acceptable daily intake. The findings would need to be replicated with appropriate adjustment for differences in body weight.
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading, England, said that “the study does not support the claim in the press release and paper that food additives are responsible for the increased risk.”
He pointed out that “nitrite from additives contributes only about 4%-6% of total nitrite intake in the population, and it is not clear why this should have a stronger impact on risk than nitrite from other sources,” such as nitrate found in food and water.
Duane Mellor, PhD, registered dietitian and senior lecturer, Aston University, Birmingham, England, said: “It could be questioned how accurate estimating intakes of individual additives like sodium nitrite, which was less than 1 mg per day from a record of just 2 days food intake per year, as it assumes people ate the same the other 363 days of the year.”
Moreover, “it is perhaps worth noting that the use of nitrites as an additive is often as sodium nitrite, which is used to cure meats like bacon, which if someone is seeking to reduce their risk of type 2 diabetes would be something people would be encouraged to eat less of [anyway].”
“The best way to reduce your risk of developing type 2 diabetes,” he said, “is to be physically active, maintain a healthy weight for you, and eat a varied diet based on vegetables, pulses, nuts, seeds, and fruit along with wholegrain and moderate intakes of dairy foods and meat (especially processed meats).”
Study details
Nitrites and nitrates are used as food additives to prevent bacterial growth, mainly in processed meats, and they are also found in foods (mainly green leafy vegetables) and water (nitrates from the use of nitrogen fertilizer can enter the water supply).
The researchers analyzed data from 104,168 participants in NutriNet-Santé who had no diabetes at baseline and who completed 24-hour dietary intake records. They investigated the association between exposure to nitrites and nitrates (in food and water or in additives) and incident type 2 diabetes.
Most nitrites came from food (95.3%), and less often from food additives (4.7%) and water (< 0.01%). The nitrites in foods were mainly from vegetables (60%) and seasonings (23%).
Most nitrates also came from food (93%), followed by water (6.9%) and food additives (0.1%). The nitrates in foods were mainly from vegetables (41%), processed meat (19%), and meat (17%).
During a median follow-up of 7.3 years, there were 969 incident cases of type 2 diabetes.
Compared with individuals in the lowest third of nitrites from food and water, those in the highest tertile had a 27% higher risk of incident type 2 diabetes, after adjusting for multiple variables (hazard ratio, 1.27; P = .009).
The risk of type 2 diabetes associated with the highest intake of nitrites from additives was as previously described, 53% higher, than that for those with the lowest intake.
There was no evidence of an association between nitrates and risk of type 2 diabetes.
The researchers acknowledge that study limitations include potential errors in assessment of nitrate and nitrate exposure, potential selection bias (participants in the web-based study may have had healthier behaviors than the general population), and potential unaccounted confounders (because it was an observational study).
A version of this article first appeared on Medscape.com.
FROM PLOS MEDICINE
Diet packed with fast food found hard on the liver
The study finds that getting one-fifth or more of total daily calories from fast food can increase the risk of nonalcoholic fatty liver disease, which can lead to cirrhosis and its complications, including liver failure and liver cancer.
Although the magnitude of association was modest among the general population, “striking” elevations in steatosis were evident among persons with obesity and diabetes who consumed fast food, in comparison with their counterparts who did not have obesity and diabetes, the researchers reported.
“My hope is that this study encourages people to seek out more nutritious, healthy food options and provides information that clinicians can use to counsel their patients, particularly those with underlying metabolic risk factors, of the importance of avoiding foods that are high in fat, carbohydrates, and processed sugars,” lead investigator Ani Kardashian, MD, hepatologist with the University of Southern California, Los Angeles, said in an interview.
“At a policy level, public health efforts are needed to improve access to affordable, healthy, and nutritious food options across the U.S. This is especially important as more people have turned to fast foods during the pandemic and as the price of food as risen dramatically over the past year due to food inflation,” Dr. Kardashian added.
The study was published online in Clinical Gastroenterology and Hepatology.
More fast food, greater steatosis
The findings are based on data from 3,954 adults who participated in the National Health and Nutrition Examination Survey (NHANES) of 2017-2018 and who underwent vibration-controlled transient elastography. Of these participants, data regarding 1- or 2-day dietary recall were available.
Steatosis, the primary outcome, was measured via controlled attenuation parameter (CAP). Two validated cutoffs were utilized (CAP ≥ 263 dB/m and CAP ≥ 285 dB/m).
Of those surveyed, 52% consumed any fast food, and 29% derived 20% or more of their daily calories from fast food.
Fast-food intake of 20% or more of daily calories was significantly associated with greater steatosis after multivariable adjustment, both as a continuous measure (4.6 dB/m higher CAP score) and with respect to the CAP ≥ 263 dB/m cutoff (odds ratio [OR], 1.45).
“The negative effects are particularly severe in people who already have diabetes and obesity,” Dr. Kardashian told this news organization.
For example, with diabetes and fast-food intake of 20% or more of daily calories, the ORs of meeting the CAP ≥ 263 dB/m cutoff and the CAP ≥ 285 dB/m cutoff were 2.3 and 2.48, respectively.
The researchers said their findings are particularly “alarming,” given the overall increase in fast-food consumption over the past 50 years in the United States, regardless of socioeconomic status.
Diet coaching
The finding that fast food has more deleterious impact on those with obesity and diabetes “emphasizes that it is not just one insult but multiple factors that contribute to overall health,” said Nancy Reau, MD, section chief of hepatology at Rush University Medical Center in Chicago.
“This is actually great news, because diet is modifiable, vs. your genetics, which you currently can’t change. This doesn’t mean if you’re lean you can eat whatever you want, but if you are overweight, being careful with your diet does have impact, even if it doesn’t lead to substantial weight changes,” said Dr. Reau, who is not affiliated with the study.
For people who have limited options and need to eat fast food, “there are healthy choices at most restaurants; you just need to be smart about reading labels, watching calories, and ordering the healthier options,” Dr. Reau said in an interview.
Fast food and fatty liver go “hand in hand,” Lisa Ganjhu, DO, gastroenterologist and hepatologist at NYU Langone Health in New York, told this news organization.
“I counsel and coach my patients on healthy diet and exercise, and I’ve been pretty successful,” said Dr. Ganjhu, who was not involved with the study.
“If my patient is eating at McDonald’s a lot, I basically walk through the menu with them and help them find something healthy. When patients see the benefits of cutting out fat and reducing carbohydrates, they are more apt to continue,” Dr. Ganjhu said.
The study was funded by the University of Southern California. Dr. Kardashian, Dr. Reau, and Dr. Ganjhu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The study finds that getting one-fifth or more of total daily calories from fast food can increase the risk of nonalcoholic fatty liver disease, which can lead to cirrhosis and its complications, including liver failure and liver cancer.
Although the magnitude of association was modest among the general population, “striking” elevations in steatosis were evident among persons with obesity and diabetes who consumed fast food, in comparison with their counterparts who did not have obesity and diabetes, the researchers reported.
“My hope is that this study encourages people to seek out more nutritious, healthy food options and provides information that clinicians can use to counsel their patients, particularly those with underlying metabolic risk factors, of the importance of avoiding foods that are high in fat, carbohydrates, and processed sugars,” lead investigator Ani Kardashian, MD, hepatologist with the University of Southern California, Los Angeles, said in an interview.
“At a policy level, public health efforts are needed to improve access to affordable, healthy, and nutritious food options across the U.S. This is especially important as more people have turned to fast foods during the pandemic and as the price of food as risen dramatically over the past year due to food inflation,” Dr. Kardashian added.
The study was published online in Clinical Gastroenterology and Hepatology.
More fast food, greater steatosis
The findings are based on data from 3,954 adults who participated in the National Health and Nutrition Examination Survey (NHANES) of 2017-2018 and who underwent vibration-controlled transient elastography. Of these participants, data regarding 1- or 2-day dietary recall were available.
Steatosis, the primary outcome, was measured via controlled attenuation parameter (CAP). Two validated cutoffs were utilized (CAP ≥ 263 dB/m and CAP ≥ 285 dB/m).
Of those surveyed, 52% consumed any fast food, and 29% derived 20% or more of their daily calories from fast food.
Fast-food intake of 20% or more of daily calories was significantly associated with greater steatosis after multivariable adjustment, both as a continuous measure (4.6 dB/m higher CAP score) and with respect to the CAP ≥ 263 dB/m cutoff (odds ratio [OR], 1.45).
“The negative effects are particularly severe in people who already have diabetes and obesity,” Dr. Kardashian told this news organization.
For example, with diabetes and fast-food intake of 20% or more of daily calories, the ORs of meeting the CAP ≥ 263 dB/m cutoff and the CAP ≥ 285 dB/m cutoff were 2.3 and 2.48, respectively.
The researchers said their findings are particularly “alarming,” given the overall increase in fast-food consumption over the past 50 years in the United States, regardless of socioeconomic status.
Diet coaching
The finding that fast food has more deleterious impact on those with obesity and diabetes “emphasizes that it is not just one insult but multiple factors that contribute to overall health,” said Nancy Reau, MD, section chief of hepatology at Rush University Medical Center in Chicago.
“This is actually great news, because diet is modifiable, vs. your genetics, which you currently can’t change. This doesn’t mean if you’re lean you can eat whatever you want, but if you are overweight, being careful with your diet does have impact, even if it doesn’t lead to substantial weight changes,” said Dr. Reau, who is not affiliated with the study.
For people who have limited options and need to eat fast food, “there are healthy choices at most restaurants; you just need to be smart about reading labels, watching calories, and ordering the healthier options,” Dr. Reau said in an interview.
Fast food and fatty liver go “hand in hand,” Lisa Ganjhu, DO, gastroenterologist and hepatologist at NYU Langone Health in New York, told this news organization.
“I counsel and coach my patients on healthy diet and exercise, and I’ve been pretty successful,” said Dr. Ganjhu, who was not involved with the study.
“If my patient is eating at McDonald’s a lot, I basically walk through the menu with them and help them find something healthy. When patients see the benefits of cutting out fat and reducing carbohydrates, they are more apt to continue,” Dr. Ganjhu said.
The study was funded by the University of Southern California. Dr. Kardashian, Dr. Reau, and Dr. Ganjhu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The study finds that getting one-fifth or more of total daily calories from fast food can increase the risk of nonalcoholic fatty liver disease, which can lead to cirrhosis and its complications, including liver failure and liver cancer.
Although the magnitude of association was modest among the general population, “striking” elevations in steatosis were evident among persons with obesity and diabetes who consumed fast food, in comparison with their counterparts who did not have obesity and diabetes, the researchers reported.
“My hope is that this study encourages people to seek out more nutritious, healthy food options and provides information that clinicians can use to counsel their patients, particularly those with underlying metabolic risk factors, of the importance of avoiding foods that are high in fat, carbohydrates, and processed sugars,” lead investigator Ani Kardashian, MD, hepatologist with the University of Southern California, Los Angeles, said in an interview.
“At a policy level, public health efforts are needed to improve access to affordable, healthy, and nutritious food options across the U.S. This is especially important as more people have turned to fast foods during the pandemic and as the price of food as risen dramatically over the past year due to food inflation,” Dr. Kardashian added.
The study was published online in Clinical Gastroenterology and Hepatology.
More fast food, greater steatosis
The findings are based on data from 3,954 adults who participated in the National Health and Nutrition Examination Survey (NHANES) of 2017-2018 and who underwent vibration-controlled transient elastography. Of these participants, data regarding 1- or 2-day dietary recall were available.
Steatosis, the primary outcome, was measured via controlled attenuation parameter (CAP). Two validated cutoffs were utilized (CAP ≥ 263 dB/m and CAP ≥ 285 dB/m).
Of those surveyed, 52% consumed any fast food, and 29% derived 20% or more of their daily calories from fast food.
Fast-food intake of 20% or more of daily calories was significantly associated with greater steatosis after multivariable adjustment, both as a continuous measure (4.6 dB/m higher CAP score) and with respect to the CAP ≥ 263 dB/m cutoff (odds ratio [OR], 1.45).
“The negative effects are particularly severe in people who already have diabetes and obesity,” Dr. Kardashian told this news organization.
For example, with diabetes and fast-food intake of 20% or more of daily calories, the ORs of meeting the CAP ≥ 263 dB/m cutoff and the CAP ≥ 285 dB/m cutoff were 2.3 and 2.48, respectively.
The researchers said their findings are particularly “alarming,” given the overall increase in fast-food consumption over the past 50 years in the United States, regardless of socioeconomic status.
Diet coaching
The finding that fast food has more deleterious impact on those with obesity and diabetes “emphasizes that it is not just one insult but multiple factors that contribute to overall health,” said Nancy Reau, MD, section chief of hepatology at Rush University Medical Center in Chicago.
“This is actually great news, because diet is modifiable, vs. your genetics, which you currently can’t change. This doesn’t mean if you’re lean you can eat whatever you want, but if you are overweight, being careful with your diet does have impact, even if it doesn’t lead to substantial weight changes,” said Dr. Reau, who is not affiliated with the study.
For people who have limited options and need to eat fast food, “there are healthy choices at most restaurants; you just need to be smart about reading labels, watching calories, and ordering the healthier options,” Dr. Reau said in an interview.
Fast food and fatty liver go “hand in hand,” Lisa Ganjhu, DO, gastroenterologist and hepatologist at NYU Langone Health in New York, told this news organization.
“I counsel and coach my patients on healthy diet and exercise, and I’ve been pretty successful,” said Dr. Ganjhu, who was not involved with the study.
“If my patient is eating at McDonald’s a lot, I basically walk through the menu with them and help them find something healthy. When patients see the benefits of cutting out fat and reducing carbohydrates, they are more apt to continue,” Dr. Ganjhu said.
The study was funded by the University of Southern California. Dr. Kardashian, Dr. Reau, and Dr. Ganjhu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY