User login
Inflammation and immunity troubles top long-COVID suspect list
“I think that it’s a much more complex picture than just inflammation, or just autoimmunity, or just immune dysregulation. And it’s probably a combination of all three causing a cascade of effects that then manifests itself as brain fog, or shortness of breath, or chronic fatigue,” says Alexander Truong, MD, a pulmonologist and assistant professor at Emory University, Atlanta, who also runs a long-COVID clinic.
Long COVID, post–COVID-19 condition, and postacute sequelae of SARS-CoV-2 (PASC) are among the terms used by the National Institutes of Health to describe the long-term health issues faced by an estimated 10%-30% of people infected with COVID-19. Symptoms – as many as 200 – can range from inconvenient to crippling, damage multiple organ systems, come and go, and relapse. Long COVID increases the risk of worsening existing health problems and triggering new ones, including cardiovascular disease and type 2 diabetes.
So far, research suggests there is no single cause, condition, or disease that explains why some people have an extensive range of symptoms long after the early COVID-19 infection has cleared up. Many experts believe some combination of biological processes – including the virus hanging around in our bodies, inflammation, autoimmunity, tiny blood clots, immune system problems, and even the reactivation of dormant viruses such as the Epstein-Barr virus – could be the culprit, a theory also supported by a comprehensive and in-depth review of long-COVID studies published in the journal Nature Reviews Microbiology.
“It’s become clear over the last couple of years that there are different [symptoms] of long COVID … that cannot all be lumped together,” says Michael Peluso, MD, an assistant professor of medicine and an infectious diseases doctor at the University of California, San Francisco.
Inflammation and a virus that hangs around
Multiple studies have shown that the virus or pieces of it can remain in many parts of the body, including the kidneys, brain, heart, and gastrointestinal system, long after the early infection.
“One major question that I think is the area of most intense investigation now is whether there is viral persistence that is driving immune dysregulation and therefore symptoms,” says Dr. Peluso.
A small Harvard University study, for example, found evidence that reservoirs of the coronavirus could linger in patients up to a year after they’re first diagnosed.
An earlier German study found that patients with post-COVID-19 symptoms had higher levels of three cytokines – small proteins that tell the body’s immune system what to do and are involved in the growth and activity of immune system cells and blood cells. Researchers said the results supported the theory that there is persistent reprogramming of certain immune cells, and that the uncontrolled “self-fueled hyperinflammation” during the early COVID-19 infection can become continued immune cell disruption that drives long-COVID symptoms.
“Long COVID is more likely due to either an inflammatory response by the body or reservoirs of virus that the body is still trying to clear … and the symptoms we’re seeing are a side effect of that,” says Rainu Kaushal, MD, senior associate dean for clinical research at Weill Cornell Medicine in New York.
Australian researchers found that immune system recovery appeared different, compared with those who were infected with other common coronaviruses.
These findings also support concerns that some experts express over the long-term risks of COVID-19 infections in general, but especially repeat infections.
“Anything that kind of revs up inflammation in the body can boil that pot over and make the symptoms worse. That’s very easily an infection or some other insult to the body. So that’s the generalized hypothesis as to why insults to the body may worsen the symptoms,” says Dr. Truong.
An autoimmune condition?
But inflammation alone does not fully explain post–COVID-19 problems.
Dr. Truong and his team, for example, have been documenting inflammatory markers in patients at the post-COVID clinic he cofounded more than 2 years ago at Emory Executive Park in Atlanta. When the clinic was first launched, high-dose nonsteroidal anti-inflammatory drugs – including ibuprofen – and prednisone were prescribed to long-COVID patients.
“It didn’t make a difference at all for any of these folks,” he says, adding that there are signs that autoimmunity is at play. But he cautions that it is still too early to suggest treating long-COVID patients with medications used for other autoimmune conditions.
In autoimmune conditions such as rheumatoid arthritis, lupus, and type 1 diabetes, a person’s immune system can’t tell normal cells from foreign pathogens and attacks healthy cells. There is typically no single diagnostic test, and many share similar symptoms, making detection and diagnosis potentially difficult, according to Johns Hopkins Medicine.
A small study published in the journal Science Translational Medicine found that, among patients who failed to regain their sense of smell long after their initial infection, there was inflammation in the nose tissue where smell nerve cells are found, even though no detectable virus remained. Fewer olfactory sensory neurons were seen, as well – findings that researchers said resembled some kind of “autoimmune-like process.”
Meanwhile, scientists in Canada found signs of autoimmunity in blood samples taken from patients who still had fatigue and shortness of breath after their initial COVID-19 infection. Two specific proteins were present a year after infection in up to 30% of patients, many of whom still had shortness of breath and fatigue, the researchers reported in the Jan. 1 issue of the European Respiratory Journal. These patients had been healthy and had no autoimmune condition or other diseases before they were infected.
Immune system problems
A number of studies have suggested that a problematic immune response could also explain why symptoms persist for some people.
Researchers in France, for example, found that the immune response problems in those with severe COVID-19 infections caused exaggerated or uncontrolled formation of a type of bug-fighting defense mechanism called a neutrophil extracellular trap (NET), which in turn triggers harmful inflammation that can result in multiorgan damage. These traps are netlike structures made from fibers composed mostly of DNA strings that bind, or trap, pathogens.
Long COVID is not like an acute infectious disease, says Alexander Charney, MD, PhD, the lead principal investigator of the RECOVER adult cohort at Mount Sinai in New York, and an associate professor at Icahn School of Medicine at Mount Sinai. It is more similar to other complex chronic diseases that have taken decades to understand, such as heart disease, mental illness, and rheumatologic diseases, he says.
Biomarkers and blood clots
Scientists are homing in on biomarkers, or detectable and measurable traits – in this case, molecular indicators – that can make diagnosing long COVID easier and give better direction for treatment. These biomarkers are also key to helping sort out the complex biology of long COVID.
In one study, data from blood samples taken from hundreds of hospitalized COVID-19 patients suggests changes are happening at the molecular level during initial severe infections. These changes may be tied to the development of longer-term symptoms, according to the study by Dr. Charney and his team at Mount Sinai published in Nature Medicine
Blood clotting issues have also been detected in long COVID patients. At least one study found signs that long-COVID patients had higher levels of a type of auto-antibody linked to the abnormal formation of clots. Researchers suspect that tiny, persistent microclots – undetectable via regular pathology tests – may be cutting off oxygen flow to tissue by blocking capillaries – and could explain many of the post-COVID symptoms described by patients.
While enormous progress has been made toward understanding long COVID, the research is still considered early and faces many challenges, including varying criteria used to define the condition, the types and quality of data used, differences in how patients are defined and recruited, and the small size of many studies. Some research also appears to conflict with other studies. And while there are specialized tools for diagnosing some aspects of the condition, standard tests often don’t detect many of the signs seen in long-COVID patients. But given the urgency and global scale of the problem, experts say more funding and support should be prioritized.
“People are suffering now, and they want answers now. ... It’s not like with COVID, where the path towards a great and meaningful solution to this unbelievable problem was clear – we need a vaccine,” says Dr. Charney.
“It’s going to be a long haul to figure out what is going on.”
A version of this article originally appeared on WebMD.com.
“I think that it’s a much more complex picture than just inflammation, or just autoimmunity, or just immune dysregulation. And it’s probably a combination of all three causing a cascade of effects that then manifests itself as brain fog, or shortness of breath, or chronic fatigue,” says Alexander Truong, MD, a pulmonologist and assistant professor at Emory University, Atlanta, who also runs a long-COVID clinic.
Long COVID, post–COVID-19 condition, and postacute sequelae of SARS-CoV-2 (PASC) are among the terms used by the National Institutes of Health to describe the long-term health issues faced by an estimated 10%-30% of people infected with COVID-19. Symptoms – as many as 200 – can range from inconvenient to crippling, damage multiple organ systems, come and go, and relapse. Long COVID increases the risk of worsening existing health problems and triggering new ones, including cardiovascular disease and type 2 diabetes.
So far, research suggests there is no single cause, condition, or disease that explains why some people have an extensive range of symptoms long after the early COVID-19 infection has cleared up. Many experts believe some combination of biological processes – including the virus hanging around in our bodies, inflammation, autoimmunity, tiny blood clots, immune system problems, and even the reactivation of dormant viruses such as the Epstein-Barr virus – could be the culprit, a theory also supported by a comprehensive and in-depth review of long-COVID studies published in the journal Nature Reviews Microbiology.
“It’s become clear over the last couple of years that there are different [symptoms] of long COVID … that cannot all be lumped together,” says Michael Peluso, MD, an assistant professor of medicine and an infectious diseases doctor at the University of California, San Francisco.
Inflammation and a virus that hangs around
Multiple studies have shown that the virus or pieces of it can remain in many parts of the body, including the kidneys, brain, heart, and gastrointestinal system, long after the early infection.
“One major question that I think is the area of most intense investigation now is whether there is viral persistence that is driving immune dysregulation and therefore symptoms,” says Dr. Peluso.
A small Harvard University study, for example, found evidence that reservoirs of the coronavirus could linger in patients up to a year after they’re first diagnosed.
An earlier German study found that patients with post-COVID-19 symptoms had higher levels of three cytokines – small proteins that tell the body’s immune system what to do and are involved in the growth and activity of immune system cells and blood cells. Researchers said the results supported the theory that there is persistent reprogramming of certain immune cells, and that the uncontrolled “self-fueled hyperinflammation” during the early COVID-19 infection can become continued immune cell disruption that drives long-COVID symptoms.
“Long COVID is more likely due to either an inflammatory response by the body or reservoirs of virus that the body is still trying to clear … and the symptoms we’re seeing are a side effect of that,” says Rainu Kaushal, MD, senior associate dean for clinical research at Weill Cornell Medicine in New York.
Australian researchers found that immune system recovery appeared different, compared with those who were infected with other common coronaviruses.
These findings also support concerns that some experts express over the long-term risks of COVID-19 infections in general, but especially repeat infections.
“Anything that kind of revs up inflammation in the body can boil that pot over and make the symptoms worse. That’s very easily an infection or some other insult to the body. So that’s the generalized hypothesis as to why insults to the body may worsen the symptoms,” says Dr. Truong.
An autoimmune condition?
But inflammation alone does not fully explain post–COVID-19 problems.
Dr. Truong and his team, for example, have been documenting inflammatory markers in patients at the post-COVID clinic he cofounded more than 2 years ago at Emory Executive Park in Atlanta. When the clinic was first launched, high-dose nonsteroidal anti-inflammatory drugs – including ibuprofen – and prednisone were prescribed to long-COVID patients.
“It didn’t make a difference at all for any of these folks,” he says, adding that there are signs that autoimmunity is at play. But he cautions that it is still too early to suggest treating long-COVID patients with medications used for other autoimmune conditions.
In autoimmune conditions such as rheumatoid arthritis, lupus, and type 1 diabetes, a person’s immune system can’t tell normal cells from foreign pathogens and attacks healthy cells. There is typically no single diagnostic test, and many share similar symptoms, making detection and diagnosis potentially difficult, according to Johns Hopkins Medicine.
A small study published in the journal Science Translational Medicine found that, among patients who failed to regain their sense of smell long after their initial infection, there was inflammation in the nose tissue where smell nerve cells are found, even though no detectable virus remained. Fewer olfactory sensory neurons were seen, as well – findings that researchers said resembled some kind of “autoimmune-like process.”
Meanwhile, scientists in Canada found signs of autoimmunity in blood samples taken from patients who still had fatigue and shortness of breath after their initial COVID-19 infection. Two specific proteins were present a year after infection in up to 30% of patients, many of whom still had shortness of breath and fatigue, the researchers reported in the Jan. 1 issue of the European Respiratory Journal. These patients had been healthy and had no autoimmune condition or other diseases before they were infected.
Immune system problems
A number of studies have suggested that a problematic immune response could also explain why symptoms persist for some people.
Researchers in France, for example, found that the immune response problems in those with severe COVID-19 infections caused exaggerated or uncontrolled formation of a type of bug-fighting defense mechanism called a neutrophil extracellular trap (NET), which in turn triggers harmful inflammation that can result in multiorgan damage. These traps are netlike structures made from fibers composed mostly of DNA strings that bind, or trap, pathogens.
Long COVID is not like an acute infectious disease, says Alexander Charney, MD, PhD, the lead principal investigator of the RECOVER adult cohort at Mount Sinai in New York, and an associate professor at Icahn School of Medicine at Mount Sinai. It is more similar to other complex chronic diseases that have taken decades to understand, such as heart disease, mental illness, and rheumatologic diseases, he says.
Biomarkers and blood clots
Scientists are homing in on biomarkers, or detectable and measurable traits – in this case, molecular indicators – that can make diagnosing long COVID easier and give better direction for treatment. These biomarkers are also key to helping sort out the complex biology of long COVID.
In one study, data from blood samples taken from hundreds of hospitalized COVID-19 patients suggests changes are happening at the molecular level during initial severe infections. These changes may be tied to the development of longer-term symptoms, according to the study by Dr. Charney and his team at Mount Sinai published in Nature Medicine
Blood clotting issues have also been detected in long COVID patients. At least one study found signs that long-COVID patients had higher levels of a type of auto-antibody linked to the abnormal formation of clots. Researchers suspect that tiny, persistent microclots – undetectable via regular pathology tests – may be cutting off oxygen flow to tissue by blocking capillaries – and could explain many of the post-COVID symptoms described by patients.
While enormous progress has been made toward understanding long COVID, the research is still considered early and faces many challenges, including varying criteria used to define the condition, the types and quality of data used, differences in how patients are defined and recruited, and the small size of many studies. Some research also appears to conflict with other studies. And while there are specialized tools for diagnosing some aspects of the condition, standard tests often don’t detect many of the signs seen in long-COVID patients. But given the urgency and global scale of the problem, experts say more funding and support should be prioritized.
“People are suffering now, and they want answers now. ... It’s not like with COVID, where the path towards a great and meaningful solution to this unbelievable problem was clear – we need a vaccine,” says Dr. Charney.
“It’s going to be a long haul to figure out what is going on.”
A version of this article originally appeared on WebMD.com.
“I think that it’s a much more complex picture than just inflammation, or just autoimmunity, or just immune dysregulation. And it’s probably a combination of all three causing a cascade of effects that then manifests itself as brain fog, or shortness of breath, or chronic fatigue,” says Alexander Truong, MD, a pulmonologist and assistant professor at Emory University, Atlanta, who also runs a long-COVID clinic.
Long COVID, post–COVID-19 condition, and postacute sequelae of SARS-CoV-2 (PASC) are among the terms used by the National Institutes of Health to describe the long-term health issues faced by an estimated 10%-30% of people infected with COVID-19. Symptoms – as many as 200 – can range from inconvenient to crippling, damage multiple organ systems, come and go, and relapse. Long COVID increases the risk of worsening existing health problems and triggering new ones, including cardiovascular disease and type 2 diabetes.
So far, research suggests there is no single cause, condition, or disease that explains why some people have an extensive range of symptoms long after the early COVID-19 infection has cleared up. Many experts believe some combination of biological processes – including the virus hanging around in our bodies, inflammation, autoimmunity, tiny blood clots, immune system problems, and even the reactivation of dormant viruses such as the Epstein-Barr virus – could be the culprit, a theory also supported by a comprehensive and in-depth review of long-COVID studies published in the journal Nature Reviews Microbiology.
“It’s become clear over the last couple of years that there are different [symptoms] of long COVID … that cannot all be lumped together,” says Michael Peluso, MD, an assistant professor of medicine and an infectious diseases doctor at the University of California, San Francisco.
Inflammation and a virus that hangs around
Multiple studies have shown that the virus or pieces of it can remain in many parts of the body, including the kidneys, brain, heart, and gastrointestinal system, long after the early infection.
“One major question that I think is the area of most intense investigation now is whether there is viral persistence that is driving immune dysregulation and therefore symptoms,” says Dr. Peluso.
A small Harvard University study, for example, found evidence that reservoirs of the coronavirus could linger in patients up to a year after they’re first diagnosed.
An earlier German study found that patients with post-COVID-19 symptoms had higher levels of three cytokines – small proteins that tell the body’s immune system what to do and are involved in the growth and activity of immune system cells and blood cells. Researchers said the results supported the theory that there is persistent reprogramming of certain immune cells, and that the uncontrolled “self-fueled hyperinflammation” during the early COVID-19 infection can become continued immune cell disruption that drives long-COVID symptoms.
“Long COVID is more likely due to either an inflammatory response by the body or reservoirs of virus that the body is still trying to clear … and the symptoms we’re seeing are a side effect of that,” says Rainu Kaushal, MD, senior associate dean for clinical research at Weill Cornell Medicine in New York.
Australian researchers found that immune system recovery appeared different, compared with those who were infected with other common coronaviruses.
These findings also support concerns that some experts express over the long-term risks of COVID-19 infections in general, but especially repeat infections.
“Anything that kind of revs up inflammation in the body can boil that pot over and make the symptoms worse. That’s very easily an infection or some other insult to the body. So that’s the generalized hypothesis as to why insults to the body may worsen the symptoms,” says Dr. Truong.
An autoimmune condition?
But inflammation alone does not fully explain post–COVID-19 problems.
Dr. Truong and his team, for example, have been documenting inflammatory markers in patients at the post-COVID clinic he cofounded more than 2 years ago at Emory Executive Park in Atlanta. When the clinic was first launched, high-dose nonsteroidal anti-inflammatory drugs – including ibuprofen – and prednisone were prescribed to long-COVID patients.
“It didn’t make a difference at all for any of these folks,” he says, adding that there are signs that autoimmunity is at play. But he cautions that it is still too early to suggest treating long-COVID patients with medications used for other autoimmune conditions.
In autoimmune conditions such as rheumatoid arthritis, lupus, and type 1 diabetes, a person’s immune system can’t tell normal cells from foreign pathogens and attacks healthy cells. There is typically no single diagnostic test, and many share similar symptoms, making detection and diagnosis potentially difficult, according to Johns Hopkins Medicine.
A small study published in the journal Science Translational Medicine found that, among patients who failed to regain their sense of smell long after their initial infection, there was inflammation in the nose tissue where smell nerve cells are found, even though no detectable virus remained. Fewer olfactory sensory neurons were seen, as well – findings that researchers said resembled some kind of “autoimmune-like process.”
Meanwhile, scientists in Canada found signs of autoimmunity in blood samples taken from patients who still had fatigue and shortness of breath after their initial COVID-19 infection. Two specific proteins were present a year after infection in up to 30% of patients, many of whom still had shortness of breath and fatigue, the researchers reported in the Jan. 1 issue of the European Respiratory Journal. These patients had been healthy and had no autoimmune condition or other diseases before they were infected.
Immune system problems
A number of studies have suggested that a problematic immune response could also explain why symptoms persist for some people.
Researchers in France, for example, found that the immune response problems in those with severe COVID-19 infections caused exaggerated or uncontrolled formation of a type of bug-fighting defense mechanism called a neutrophil extracellular trap (NET), which in turn triggers harmful inflammation that can result in multiorgan damage. These traps are netlike structures made from fibers composed mostly of DNA strings that bind, or trap, pathogens.
Long COVID is not like an acute infectious disease, says Alexander Charney, MD, PhD, the lead principal investigator of the RECOVER adult cohort at Mount Sinai in New York, and an associate professor at Icahn School of Medicine at Mount Sinai. It is more similar to other complex chronic diseases that have taken decades to understand, such as heart disease, mental illness, and rheumatologic diseases, he says.
Biomarkers and blood clots
Scientists are homing in on biomarkers, or detectable and measurable traits – in this case, molecular indicators – that can make diagnosing long COVID easier and give better direction for treatment. These biomarkers are also key to helping sort out the complex biology of long COVID.
In one study, data from blood samples taken from hundreds of hospitalized COVID-19 patients suggests changes are happening at the molecular level during initial severe infections. These changes may be tied to the development of longer-term symptoms, according to the study by Dr. Charney and his team at Mount Sinai published in Nature Medicine
Blood clotting issues have also been detected in long COVID patients. At least one study found signs that long-COVID patients had higher levels of a type of auto-antibody linked to the abnormal formation of clots. Researchers suspect that tiny, persistent microclots – undetectable via regular pathology tests – may be cutting off oxygen flow to tissue by blocking capillaries – and could explain many of the post-COVID symptoms described by patients.
While enormous progress has been made toward understanding long COVID, the research is still considered early and faces many challenges, including varying criteria used to define the condition, the types and quality of data used, differences in how patients are defined and recruited, and the small size of many studies. Some research also appears to conflict with other studies. And while there are specialized tools for diagnosing some aspects of the condition, standard tests often don’t detect many of the signs seen in long-COVID patients. But given the urgency and global scale of the problem, experts say more funding and support should be prioritized.
“People are suffering now, and they want answers now. ... It’s not like with COVID, where the path towards a great and meaningful solution to this unbelievable problem was clear – we need a vaccine,” says Dr. Charney.
“It’s going to be a long haul to figure out what is going on.”
A version of this article originally appeared on WebMD.com.
COVID emergency orders ending: What’s next?
It’s the end of an era.
The orders spanned two presidencies. The Trump administration’s Health and Human Services Secretary Alex Azar issued a public health emergency in January 2020. Then-President Donald Trump declared the COVID-19 pandemic a national emergency 2 months later. Both emergency declarations – which remained in effect under President Joe Biden – are set to expire May 11.
Read on for an overview of how the end of the public health emergency will trigger multiple federal policy changes.
Changes that affect everyone
- There will be cost-sharing changes for COVID-19 vaccines, testing, and certain treatments. One hundred–percent coverage for COVID testing, including free at-home tests, will expire May 11.
- Telemedicine cannot be used to prescribe controlled substances after May 11, 2023.
- Enhanced federal funding will be phased down through Dec. 31, 2023. This extends the time states must receive federally matched funds for COVID-related services and products, through the Consolidated Appropriations Act of 2023. Otherwise, this would have expired June 30, 2023.
- Emergency use authorizations for COVID-19 treatments and vaccinations will not be affected and/or end on May 11.
Changes that affect people with private health insurance
- Many will likely see higher costs for COVID-19 tests, as free testing expires and cost-sharing begins in the coming months.
- COVID-19 vaccinations and boosters will continue to be covered until the federal government’s vaccination supply is depleted. If that happens, you will need an in-network provider.
- You will still have access to COVID-19 treatments – but that could change when the federal supply dwindles.
Changes that affect Medicare recipients
- Medicare telehealth flexibilities will be extended through Dec. 31, 2024, regardless of public health emergency status. This means people can access telehealth services from anywhere, not just rural areas; can use a smartphone for telehealth; and can access telehealth in their homes.
- Medicare cost-sharing for testing and treatments will expire May 11, except for oral antivirals.
Changes that affect Medicaid/CHIP recipients
- Medicaid and Children’s Health Insurance Program (CHIP) recipients will continue to receive approved vaccinations free of charge, but testing and treatment without cost-sharing will expire during the third quarter of 2024.
- The Medicaid continuous enrollment provision will be separated from the public health emergency, and continuous enrollment will end March 31, 2023.
Changes that affect uninsured people
- The uninsured will no longer have access to 100% coverage for these products and services (free COVID-19 treatments, vaccines, and testing).
Changes that affect health care providers
- There will be changes to how much providers get paid for diagnosing people with COVID-19, ending the enhanced Inpatient Prospective Payment System reimbursement rate, as of May 11, 2023.
- Health Insurance Portability and Accountability Act (HIPAA) potential penalty waivers will end. This allows providers to communicate with patients through telehealth on a smartphone, for example, without violating privacy laws and incurring penalties.
What the experts are saying
This news organization asked several health experts for their thoughts on ending the emergency health declarations for COVID, and what effects this could have. Many expressed concerns about the timing of the ending, saying that the move could limit access to COVID-related treatments. Others said the move was inevitable but raised concerns about federal guidance related to the decision.
Question: Do you agree with the timing of the end to the emergency order?
Answer: Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston: “A lead time to prepare and anticipate these consequences may ease the transition, compared to an abrupt declaration that ends the declaration.”
Answer: Georges C. Benjamin, MD, executive director of the American Public Health Association: “I think it’s time to do so. It has to be done in a great, thoughtful, and organized way because we’ve attached so many different things to this public health emergency. It’s going to take time for the system to adapt. [Centers for Disease Control and Prevention] data collection most likely will continue. People are used to reporting now. The CDC needs to give guidance to the states so that we’re clear about what we’re reporting, what we’re not. If we did that abruptly, it would just be a mess.”
Answer: Bruce Farber, MD, chief public health and epidemiology officer at Northwell Health in Manhasset, N.Y.: “I would have hoped to see it delayed.”
Answer: Steven Newmark, JD, chief legal officer and director of policy at the Global Healthy Living Foundation: “While we understand that an emergency cannot last forever, we hope that expanded services such as free vaccination, promotion of widespread vaccination, increased use of pharmacists to administer vaccines, telehealth availability and reimbursement, flexibility in work-from-home opportunities, and more continues. Access to equitable health care should never backtrack or be reduced.”
Q: What will the end of free COVID vaccinations and free testing mean?
A: Dr. Farber: “There will likely be a decrease in vaccinations and testing. The vaccination rates are very low to begin with, and this will likely lower it further.”
A: Dr. Atmar: “I think it will mean that fewer people will get tested and vaccinated,” which “could lead to increased transmission, although wastewater testing suggests that there is a lot of unrecognized infection already occurring.”
A: Dr. Benjamin: “That is a big concern. It means that for people, particularly for people who are uninsured and underinsured, we’ve got to make sure they have access to those. There’s a lot of discussion and debate about what the cost of those tests and vaccines will be, and it looks like the companies are going to impose very steep, increasing costs.”
Q: How will this affect higher-risk populations, like people with weakened immune systems?
A: Dr. Farber: “Without monoclonals [drugs to treat COVID] and free Paxlovid,” people with weakened immune systems “may be undertreated.”
A: Dr. Atmar: “The implications of ongoing widespread virus transmission are that immunocompromised individuals may be more likely to be exposed and infected and to suffer the consequences of such infection, including severe illness. However, to a certain degree, this may already be happening. We are still seeing about 500 deaths/day, primarily in persons at highest risk of severe disease.”
A: Dr. Benjamin: “People who have good insurance, can afford to get immunized, and have good relations with practitioners probably will continue to be covered. But lower-income individuals and people who really can’t afford to get tested or get immunized would likely become underimmunized and more infected.
“So even though the federal emergency declaration will go away, I’m hoping that the federal government will continue to encourage all of us to emphasize those populations at the highest risk – those with chronic disease and those who are immunocompromised.”
A: Mr. Newmark: “People who are immunocompromised by their chronic illness or the medicines they take to treat acute or chronic conditions remain at higher risk for COVID-19 and its serious complications. The administration needs to support continued development of effective treatments and updated vaccines to protect the individual and public health. We’re also concerned that increased health care services - such as vaccination or telehealth – may fall back to prepandemic levels while the burden of protection, such as masking, may fall to chronic disease patients alone, which adds to the burden of living with disease.”
Q: What effect will ending Medicaid expansion money have?
A: Dr. Benjamin: Anywhere from 16 to 20 million people are going to lose in coverage. I’m hoping that states will look at their experience over these last 2 years or so and come to the decision that there were improvements in healthier populations.
Q: Will this have any effect on how the public perceives the pandemic?
A: Dr. Farber: “It is likely to give the impression that COVID is gone, which clearly is not the case.”
A: Dr. Benjamin: “It’ll be another argument by some that the pandemic is over. People should think about this as kind of like a hurricane. A hurricane comes through and tragically tears up communities, and we have an emergency during that time. But then we have to go through a period of recovery. I’m hoping people will realize that even though the public health emergencies have gone away, that we still need to go through a period of transition ... and that means that they still need to protect themselves, get vaccinated, and wear a mask when appropriate.”
A: Dr. Atmar: “There needs to be messaging that while we are transitioning away from emergency management of COVID-19, it is still a significant public health concern.”
A version of this article originally appeared on WebMD.com.
It’s the end of an era.
The orders spanned two presidencies. The Trump administration’s Health and Human Services Secretary Alex Azar issued a public health emergency in January 2020. Then-President Donald Trump declared the COVID-19 pandemic a national emergency 2 months later. Both emergency declarations – which remained in effect under President Joe Biden – are set to expire May 11.
Read on for an overview of how the end of the public health emergency will trigger multiple federal policy changes.
Changes that affect everyone
- There will be cost-sharing changes for COVID-19 vaccines, testing, and certain treatments. One hundred–percent coverage for COVID testing, including free at-home tests, will expire May 11.
- Telemedicine cannot be used to prescribe controlled substances after May 11, 2023.
- Enhanced federal funding will be phased down through Dec. 31, 2023. This extends the time states must receive federally matched funds for COVID-related services and products, through the Consolidated Appropriations Act of 2023. Otherwise, this would have expired June 30, 2023.
- Emergency use authorizations for COVID-19 treatments and vaccinations will not be affected and/or end on May 11.
Changes that affect people with private health insurance
- Many will likely see higher costs for COVID-19 tests, as free testing expires and cost-sharing begins in the coming months.
- COVID-19 vaccinations and boosters will continue to be covered until the federal government’s vaccination supply is depleted. If that happens, you will need an in-network provider.
- You will still have access to COVID-19 treatments – but that could change when the federal supply dwindles.
Changes that affect Medicare recipients
- Medicare telehealth flexibilities will be extended through Dec. 31, 2024, regardless of public health emergency status. This means people can access telehealth services from anywhere, not just rural areas; can use a smartphone for telehealth; and can access telehealth in their homes.
- Medicare cost-sharing for testing and treatments will expire May 11, except for oral antivirals.
Changes that affect Medicaid/CHIP recipients
- Medicaid and Children’s Health Insurance Program (CHIP) recipients will continue to receive approved vaccinations free of charge, but testing and treatment without cost-sharing will expire during the third quarter of 2024.
- The Medicaid continuous enrollment provision will be separated from the public health emergency, and continuous enrollment will end March 31, 2023.
Changes that affect uninsured people
- The uninsured will no longer have access to 100% coverage for these products and services (free COVID-19 treatments, vaccines, and testing).
Changes that affect health care providers
- There will be changes to how much providers get paid for diagnosing people with COVID-19, ending the enhanced Inpatient Prospective Payment System reimbursement rate, as of May 11, 2023.
- Health Insurance Portability and Accountability Act (HIPAA) potential penalty waivers will end. This allows providers to communicate with patients through telehealth on a smartphone, for example, without violating privacy laws and incurring penalties.
What the experts are saying
This news organization asked several health experts for their thoughts on ending the emergency health declarations for COVID, and what effects this could have. Many expressed concerns about the timing of the ending, saying that the move could limit access to COVID-related treatments. Others said the move was inevitable but raised concerns about federal guidance related to the decision.
Question: Do you agree with the timing of the end to the emergency order?
Answer: Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston: “A lead time to prepare and anticipate these consequences may ease the transition, compared to an abrupt declaration that ends the declaration.”
Answer: Georges C. Benjamin, MD, executive director of the American Public Health Association: “I think it’s time to do so. It has to be done in a great, thoughtful, and organized way because we’ve attached so many different things to this public health emergency. It’s going to take time for the system to adapt. [Centers for Disease Control and Prevention] data collection most likely will continue. People are used to reporting now. The CDC needs to give guidance to the states so that we’re clear about what we’re reporting, what we’re not. If we did that abruptly, it would just be a mess.”
Answer: Bruce Farber, MD, chief public health and epidemiology officer at Northwell Health in Manhasset, N.Y.: “I would have hoped to see it delayed.”
Answer: Steven Newmark, JD, chief legal officer and director of policy at the Global Healthy Living Foundation: “While we understand that an emergency cannot last forever, we hope that expanded services such as free vaccination, promotion of widespread vaccination, increased use of pharmacists to administer vaccines, telehealth availability and reimbursement, flexibility in work-from-home opportunities, and more continues. Access to equitable health care should never backtrack or be reduced.”
Q: What will the end of free COVID vaccinations and free testing mean?
A: Dr. Farber: “There will likely be a decrease in vaccinations and testing. The vaccination rates are very low to begin with, and this will likely lower it further.”
A: Dr. Atmar: “I think it will mean that fewer people will get tested and vaccinated,” which “could lead to increased transmission, although wastewater testing suggests that there is a lot of unrecognized infection already occurring.”
A: Dr. Benjamin: “That is a big concern. It means that for people, particularly for people who are uninsured and underinsured, we’ve got to make sure they have access to those. There’s a lot of discussion and debate about what the cost of those tests and vaccines will be, and it looks like the companies are going to impose very steep, increasing costs.”
Q: How will this affect higher-risk populations, like people with weakened immune systems?
A: Dr. Farber: “Without monoclonals [drugs to treat COVID] and free Paxlovid,” people with weakened immune systems “may be undertreated.”
A: Dr. Atmar: “The implications of ongoing widespread virus transmission are that immunocompromised individuals may be more likely to be exposed and infected and to suffer the consequences of such infection, including severe illness. However, to a certain degree, this may already be happening. We are still seeing about 500 deaths/day, primarily in persons at highest risk of severe disease.”
A: Dr. Benjamin: “People who have good insurance, can afford to get immunized, and have good relations with practitioners probably will continue to be covered. But lower-income individuals and people who really can’t afford to get tested or get immunized would likely become underimmunized and more infected.
“So even though the federal emergency declaration will go away, I’m hoping that the federal government will continue to encourage all of us to emphasize those populations at the highest risk – those with chronic disease and those who are immunocompromised.”
A: Mr. Newmark: “People who are immunocompromised by their chronic illness or the medicines they take to treat acute or chronic conditions remain at higher risk for COVID-19 and its serious complications. The administration needs to support continued development of effective treatments and updated vaccines to protect the individual and public health. We’re also concerned that increased health care services - such as vaccination or telehealth – may fall back to prepandemic levels while the burden of protection, such as masking, may fall to chronic disease patients alone, which adds to the burden of living with disease.”
Q: What effect will ending Medicaid expansion money have?
A: Dr. Benjamin: Anywhere from 16 to 20 million people are going to lose in coverage. I’m hoping that states will look at their experience over these last 2 years or so and come to the decision that there were improvements in healthier populations.
Q: Will this have any effect on how the public perceives the pandemic?
A: Dr. Farber: “It is likely to give the impression that COVID is gone, which clearly is not the case.”
A: Dr. Benjamin: “It’ll be another argument by some that the pandemic is over. People should think about this as kind of like a hurricane. A hurricane comes through and tragically tears up communities, and we have an emergency during that time. But then we have to go through a period of recovery. I’m hoping people will realize that even though the public health emergencies have gone away, that we still need to go through a period of transition ... and that means that they still need to protect themselves, get vaccinated, and wear a mask when appropriate.”
A: Dr. Atmar: “There needs to be messaging that while we are transitioning away from emergency management of COVID-19, it is still a significant public health concern.”
A version of this article originally appeared on WebMD.com.
It’s the end of an era.
The orders spanned two presidencies. The Trump administration’s Health and Human Services Secretary Alex Azar issued a public health emergency in January 2020. Then-President Donald Trump declared the COVID-19 pandemic a national emergency 2 months later. Both emergency declarations – which remained in effect under President Joe Biden – are set to expire May 11.
Read on for an overview of how the end of the public health emergency will trigger multiple federal policy changes.
Changes that affect everyone
- There will be cost-sharing changes for COVID-19 vaccines, testing, and certain treatments. One hundred–percent coverage for COVID testing, including free at-home tests, will expire May 11.
- Telemedicine cannot be used to prescribe controlled substances after May 11, 2023.
- Enhanced federal funding will be phased down through Dec. 31, 2023. This extends the time states must receive federally matched funds for COVID-related services and products, through the Consolidated Appropriations Act of 2023. Otherwise, this would have expired June 30, 2023.
- Emergency use authorizations for COVID-19 treatments and vaccinations will not be affected and/or end on May 11.
Changes that affect people with private health insurance
- Many will likely see higher costs for COVID-19 tests, as free testing expires and cost-sharing begins in the coming months.
- COVID-19 vaccinations and boosters will continue to be covered until the federal government’s vaccination supply is depleted. If that happens, you will need an in-network provider.
- You will still have access to COVID-19 treatments – but that could change when the federal supply dwindles.
Changes that affect Medicare recipients
- Medicare telehealth flexibilities will be extended through Dec. 31, 2024, regardless of public health emergency status. This means people can access telehealth services from anywhere, not just rural areas; can use a smartphone for telehealth; and can access telehealth in their homes.
- Medicare cost-sharing for testing and treatments will expire May 11, except for oral antivirals.
Changes that affect Medicaid/CHIP recipients
- Medicaid and Children’s Health Insurance Program (CHIP) recipients will continue to receive approved vaccinations free of charge, but testing and treatment without cost-sharing will expire during the third quarter of 2024.
- The Medicaid continuous enrollment provision will be separated from the public health emergency, and continuous enrollment will end March 31, 2023.
Changes that affect uninsured people
- The uninsured will no longer have access to 100% coverage for these products and services (free COVID-19 treatments, vaccines, and testing).
Changes that affect health care providers
- There will be changes to how much providers get paid for diagnosing people with COVID-19, ending the enhanced Inpatient Prospective Payment System reimbursement rate, as of May 11, 2023.
- Health Insurance Portability and Accountability Act (HIPAA) potential penalty waivers will end. This allows providers to communicate with patients through telehealth on a smartphone, for example, without violating privacy laws and incurring penalties.
What the experts are saying
This news organization asked several health experts for their thoughts on ending the emergency health declarations for COVID, and what effects this could have. Many expressed concerns about the timing of the ending, saying that the move could limit access to COVID-related treatments. Others said the move was inevitable but raised concerns about federal guidance related to the decision.
Question: Do you agree with the timing of the end to the emergency order?
Answer: Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston: “A lead time to prepare and anticipate these consequences may ease the transition, compared to an abrupt declaration that ends the declaration.”
Answer: Georges C. Benjamin, MD, executive director of the American Public Health Association: “I think it’s time to do so. It has to be done in a great, thoughtful, and organized way because we’ve attached so many different things to this public health emergency. It’s going to take time for the system to adapt. [Centers for Disease Control and Prevention] data collection most likely will continue. People are used to reporting now. The CDC needs to give guidance to the states so that we’re clear about what we’re reporting, what we’re not. If we did that abruptly, it would just be a mess.”
Answer: Bruce Farber, MD, chief public health and epidemiology officer at Northwell Health in Manhasset, N.Y.: “I would have hoped to see it delayed.”
Answer: Steven Newmark, JD, chief legal officer and director of policy at the Global Healthy Living Foundation: “While we understand that an emergency cannot last forever, we hope that expanded services such as free vaccination, promotion of widespread vaccination, increased use of pharmacists to administer vaccines, telehealth availability and reimbursement, flexibility in work-from-home opportunities, and more continues. Access to equitable health care should never backtrack or be reduced.”
Q: What will the end of free COVID vaccinations and free testing mean?
A: Dr. Farber: “There will likely be a decrease in vaccinations and testing. The vaccination rates are very low to begin with, and this will likely lower it further.”
A: Dr. Atmar: “I think it will mean that fewer people will get tested and vaccinated,” which “could lead to increased transmission, although wastewater testing suggests that there is a lot of unrecognized infection already occurring.”
A: Dr. Benjamin: “That is a big concern. It means that for people, particularly for people who are uninsured and underinsured, we’ve got to make sure they have access to those. There’s a lot of discussion and debate about what the cost of those tests and vaccines will be, and it looks like the companies are going to impose very steep, increasing costs.”
Q: How will this affect higher-risk populations, like people with weakened immune systems?
A: Dr. Farber: “Without monoclonals [drugs to treat COVID] and free Paxlovid,” people with weakened immune systems “may be undertreated.”
A: Dr. Atmar: “The implications of ongoing widespread virus transmission are that immunocompromised individuals may be more likely to be exposed and infected and to suffer the consequences of such infection, including severe illness. However, to a certain degree, this may already be happening. We are still seeing about 500 deaths/day, primarily in persons at highest risk of severe disease.”
A: Dr. Benjamin: “People who have good insurance, can afford to get immunized, and have good relations with practitioners probably will continue to be covered. But lower-income individuals and people who really can’t afford to get tested or get immunized would likely become underimmunized and more infected.
“So even though the federal emergency declaration will go away, I’m hoping that the federal government will continue to encourage all of us to emphasize those populations at the highest risk – those with chronic disease and those who are immunocompromised.”
A: Mr. Newmark: “People who are immunocompromised by their chronic illness or the medicines they take to treat acute or chronic conditions remain at higher risk for COVID-19 and its serious complications. The administration needs to support continued development of effective treatments and updated vaccines to protect the individual and public health. We’re also concerned that increased health care services - such as vaccination or telehealth – may fall back to prepandemic levels while the burden of protection, such as masking, may fall to chronic disease patients alone, which adds to the burden of living with disease.”
Q: What effect will ending Medicaid expansion money have?
A: Dr. Benjamin: Anywhere from 16 to 20 million people are going to lose in coverage. I’m hoping that states will look at their experience over these last 2 years or so and come to the decision that there were improvements in healthier populations.
Q: Will this have any effect on how the public perceives the pandemic?
A: Dr. Farber: “It is likely to give the impression that COVID is gone, which clearly is not the case.”
A: Dr. Benjamin: “It’ll be another argument by some that the pandemic is over. People should think about this as kind of like a hurricane. A hurricane comes through and tragically tears up communities, and we have an emergency during that time. But then we have to go through a period of recovery. I’m hoping people will realize that even though the public health emergencies have gone away, that we still need to go through a period of transition ... and that means that they still need to protect themselves, get vaccinated, and wear a mask when appropriate.”
A: Dr. Atmar: “There needs to be messaging that while we are transitioning away from emergency management of COVID-19, it is still a significant public health concern.”
A version of this article originally appeared on WebMD.com.
Washington medical board charges doctor with spreading COVID misinformation
Doctors and professional organizations are standing guard, hoping to protect patients from any harm that results from mistruths spread by colleagues.
Case in point: Several physicians and the American Board of Pathology filed complaints with Washington and Idaho medical boards alleging that Ryan Cole, MD, a board-certified pathologist who practices in Boise, Idaho, but who also holds a license in Washington, has spread antivaccine and pro-ivermectin statements on social media. Dr. Cole is one of the founders of America’s Frontline Doctors, a right-wing political organization. Dr. Cole did not respond to a request for comment.
Gary W. Procop, MD, CEO, American Board of Pathology, told this news organization that “as physicians and board-certified pathologists, we have a public trust, and we must be accountable to patients, society, and the profession. Misinformation can cause real harm to patients, which may include death. Misinformation diverts patients away from lifesaving vaccination and other preventive measures, promotes viral transmission, and recommends ineffective therapies that may be toxic instead of evidence-based medical care.”
Cavalcade of complaints
Several doctors also chimed in with formal complaints alleging that Cole is spreading unreliable information, according to a report from KTVB News. For example, a Boise doctor wrote in his complaint that Dr. Cole is “a major purveyor of misinformation” and called it “amazing” that the physician was continuing to publicly support debunked information about COVID-19 more than a year into the pandemic. The doctor also stated, “Cole is a health menace, abusing his status as a physician to mislead the public.”
As a result of such complaints, the Washington medical board has charged Cole with COVID-19–related violations. It is unclear whether or not the Idaho medical board will sanction the doctor. At least 12 medical boards have sanctioned doctors for similar violations since the start of the pandemic.
The statement of charges from the Washington medical board contends that since March 2021, Dr. Cole has made numerous misleading statements regarding the COVID-19 pandemic, vaccines, the use of ivermectin to treat COVID-19, and the effectiveness of masks.
In addition, the statement alleges that Dr. Cole treated several COVID-19 patients via telemedicine. During these sessions, he prescribed ivermectin, an antiparasite drug that has not been found to have any effectiveness in treating, curing, or preventing COVID-19. One of the patients died after receiving this treatment, according to the complaint.
Citing a study published in the New England Journal of Medicine, Dr. Procop pointed out that use of ivermectin, which is not approved by the U.S. Food and Drug Administration to treat COVID-19, is particularly troubling.
“There is a concern whenever an ineffective treatment is prescribed when more effective and scientifically proven therapies are available. Therapeutics have potential side effects, and toxicities have been associated with the use of ivermectin,” Dr. Procop said. “The benefits of therapy should always outweigh the risks of treatment.”
If the Washington medical board finds that Dr. Cole has engaged in unprofessional conduct, possible sanctions include revocation or suspension of his license. Washington state law also provides for a range of other possible sanctions, including restriction or limitation of his practice, requiring that he complete a specific program of remedial education or treatment, monitoring of his practice, censure or reprimand, probation, a fine of up to $5,000 for each violation, or refunding fees that his practice has billed to and collected from patients. Dr. Cole had until January 30 to respond to the medical board’s statement.
“The American Board of Pathology supports the actions of the Washington State Medical Board regarding their inquiries into any physician that holds license in their state who makes false and misleading medical claims, or provides medical care beyond their scope of practice, as indicated by their training,” Dr. Procop said.
Law in limbo
While medical boards are seeking to sanction professionals who spread falsehoods, the pause button has been hit on the California law that allows regulators to punish doctors for spreading false information about COVID-19 vaccinations and treatments.
The law went into effect Jan. 1 but was temporarily halted when U.S. District Judge William B. Shubb of the Eastern District of California granted a preliminary injunction against the law on Jan. 25, according to a report in the Sacramento Bee.
Mr. Shubb said the measure’s definition of “misinformation” was “unconstitutionally vague” under the due process clause of the 14th Amendment. He also criticized the law’s definition of “misinformation” as being “grammatically incoherent.”
A version of this article first appeared on Medscape.com.
Doctors and professional organizations are standing guard, hoping to protect patients from any harm that results from mistruths spread by colleagues.
Case in point: Several physicians and the American Board of Pathology filed complaints with Washington and Idaho medical boards alleging that Ryan Cole, MD, a board-certified pathologist who practices in Boise, Idaho, but who also holds a license in Washington, has spread antivaccine and pro-ivermectin statements on social media. Dr. Cole is one of the founders of America’s Frontline Doctors, a right-wing political organization. Dr. Cole did not respond to a request for comment.
Gary W. Procop, MD, CEO, American Board of Pathology, told this news organization that “as physicians and board-certified pathologists, we have a public trust, and we must be accountable to patients, society, and the profession. Misinformation can cause real harm to patients, which may include death. Misinformation diverts patients away from lifesaving vaccination and other preventive measures, promotes viral transmission, and recommends ineffective therapies that may be toxic instead of evidence-based medical care.”
Cavalcade of complaints
Several doctors also chimed in with formal complaints alleging that Cole is spreading unreliable information, according to a report from KTVB News. For example, a Boise doctor wrote in his complaint that Dr. Cole is “a major purveyor of misinformation” and called it “amazing” that the physician was continuing to publicly support debunked information about COVID-19 more than a year into the pandemic. The doctor also stated, “Cole is a health menace, abusing his status as a physician to mislead the public.”
As a result of such complaints, the Washington medical board has charged Cole with COVID-19–related violations. It is unclear whether or not the Idaho medical board will sanction the doctor. At least 12 medical boards have sanctioned doctors for similar violations since the start of the pandemic.
The statement of charges from the Washington medical board contends that since March 2021, Dr. Cole has made numerous misleading statements regarding the COVID-19 pandemic, vaccines, the use of ivermectin to treat COVID-19, and the effectiveness of masks.
In addition, the statement alleges that Dr. Cole treated several COVID-19 patients via telemedicine. During these sessions, he prescribed ivermectin, an antiparasite drug that has not been found to have any effectiveness in treating, curing, or preventing COVID-19. One of the patients died after receiving this treatment, according to the complaint.
Citing a study published in the New England Journal of Medicine, Dr. Procop pointed out that use of ivermectin, which is not approved by the U.S. Food and Drug Administration to treat COVID-19, is particularly troubling.
“There is a concern whenever an ineffective treatment is prescribed when more effective and scientifically proven therapies are available. Therapeutics have potential side effects, and toxicities have been associated with the use of ivermectin,” Dr. Procop said. “The benefits of therapy should always outweigh the risks of treatment.”
If the Washington medical board finds that Dr. Cole has engaged in unprofessional conduct, possible sanctions include revocation or suspension of his license. Washington state law also provides for a range of other possible sanctions, including restriction or limitation of his practice, requiring that he complete a specific program of remedial education or treatment, monitoring of his practice, censure or reprimand, probation, a fine of up to $5,000 for each violation, or refunding fees that his practice has billed to and collected from patients. Dr. Cole had until January 30 to respond to the medical board’s statement.
“The American Board of Pathology supports the actions of the Washington State Medical Board regarding their inquiries into any physician that holds license in their state who makes false and misleading medical claims, or provides medical care beyond their scope of practice, as indicated by their training,” Dr. Procop said.
Law in limbo
While medical boards are seeking to sanction professionals who spread falsehoods, the pause button has been hit on the California law that allows regulators to punish doctors for spreading false information about COVID-19 vaccinations and treatments.
The law went into effect Jan. 1 but was temporarily halted when U.S. District Judge William B. Shubb of the Eastern District of California granted a preliminary injunction against the law on Jan. 25, according to a report in the Sacramento Bee.
Mr. Shubb said the measure’s definition of “misinformation” was “unconstitutionally vague” under the due process clause of the 14th Amendment. He also criticized the law’s definition of “misinformation” as being “grammatically incoherent.”
A version of this article first appeared on Medscape.com.
Doctors and professional organizations are standing guard, hoping to protect patients from any harm that results from mistruths spread by colleagues.
Case in point: Several physicians and the American Board of Pathology filed complaints with Washington and Idaho medical boards alleging that Ryan Cole, MD, a board-certified pathologist who practices in Boise, Idaho, but who also holds a license in Washington, has spread antivaccine and pro-ivermectin statements on social media. Dr. Cole is one of the founders of America’s Frontline Doctors, a right-wing political organization. Dr. Cole did not respond to a request for comment.
Gary W. Procop, MD, CEO, American Board of Pathology, told this news organization that “as physicians and board-certified pathologists, we have a public trust, and we must be accountable to patients, society, and the profession. Misinformation can cause real harm to patients, which may include death. Misinformation diverts patients away from lifesaving vaccination and other preventive measures, promotes viral transmission, and recommends ineffective therapies that may be toxic instead of evidence-based medical care.”
Cavalcade of complaints
Several doctors also chimed in with formal complaints alleging that Cole is spreading unreliable information, according to a report from KTVB News. For example, a Boise doctor wrote in his complaint that Dr. Cole is “a major purveyor of misinformation” and called it “amazing” that the physician was continuing to publicly support debunked information about COVID-19 more than a year into the pandemic. The doctor also stated, “Cole is a health menace, abusing his status as a physician to mislead the public.”
As a result of such complaints, the Washington medical board has charged Cole with COVID-19–related violations. It is unclear whether or not the Idaho medical board will sanction the doctor. At least 12 medical boards have sanctioned doctors for similar violations since the start of the pandemic.
The statement of charges from the Washington medical board contends that since March 2021, Dr. Cole has made numerous misleading statements regarding the COVID-19 pandemic, vaccines, the use of ivermectin to treat COVID-19, and the effectiveness of masks.
In addition, the statement alleges that Dr. Cole treated several COVID-19 patients via telemedicine. During these sessions, he prescribed ivermectin, an antiparasite drug that has not been found to have any effectiveness in treating, curing, or preventing COVID-19. One of the patients died after receiving this treatment, according to the complaint.
Citing a study published in the New England Journal of Medicine, Dr. Procop pointed out that use of ivermectin, which is not approved by the U.S. Food and Drug Administration to treat COVID-19, is particularly troubling.
“There is a concern whenever an ineffective treatment is prescribed when more effective and scientifically proven therapies are available. Therapeutics have potential side effects, and toxicities have been associated with the use of ivermectin,” Dr. Procop said. “The benefits of therapy should always outweigh the risks of treatment.”
If the Washington medical board finds that Dr. Cole has engaged in unprofessional conduct, possible sanctions include revocation or suspension of his license. Washington state law also provides for a range of other possible sanctions, including restriction or limitation of his practice, requiring that he complete a specific program of remedial education or treatment, monitoring of his practice, censure or reprimand, probation, a fine of up to $5,000 for each violation, or refunding fees that his practice has billed to and collected from patients. Dr. Cole had until January 30 to respond to the medical board’s statement.
“The American Board of Pathology supports the actions of the Washington State Medical Board regarding their inquiries into any physician that holds license in their state who makes false and misleading medical claims, or provides medical care beyond their scope of practice, as indicated by their training,” Dr. Procop said.
Law in limbo
While medical boards are seeking to sanction professionals who spread falsehoods, the pause button has been hit on the California law that allows regulators to punish doctors for spreading false information about COVID-19 vaccinations and treatments.
The law went into effect Jan. 1 but was temporarily halted when U.S. District Judge William B. Shubb of the Eastern District of California granted a preliminary injunction against the law on Jan. 25, according to a report in the Sacramento Bee.
Mr. Shubb said the measure’s definition of “misinformation” was “unconstitutionally vague” under the due process clause of the 14th Amendment. He also criticized the law’s definition of “misinformation” as being “grammatically incoherent.”
A version of this article first appeared on Medscape.com.
Skin of Color Society Scientific Symposium Winners: 2022
The 18th Annual Skin of Color Society Scientific Symposium was held in March 2022 in Boston, Massachusetts. With a theme of Diversity in Action: Science, Healthcare & Society, researchers gathered to present new findings, share key insights, and discuss the continuing evolution of the field. Three awards were presented from the scientific posters at the symposium.
The Best Poster Presentation Award was presented to Brandyn M. White, BS, for “A Preliminary Analysis of the DDB1 Gene: Genome-Wide Association Studies in African and Admixed African American Populations—Is Our Skin Different?” authored by Brandyn M. White, BS; Chidubem A.V. Okeke, BS; Raveena Khanna, MD; Ginette A. Okoye, MD; Michael C. Campbell, PhD; and Angel S. Byrd, MD, PhD. Their research evaluated the association of variant DNA damage binding protein 1, DDB1, with African populations and highlighted the possible phenotypic variations between African and admixed African American populations. Further, it discussed the advantages of conducting future genome-wide association studies in the Washington metropolitan area to better understand dermatological diseases that disproportionately affect skin of color patients.
The Best Oral Presentation Award was presented to Erica Ogwumike, BA, for “Matching into Dermatology Residency: The Impact of Research Fellowships” authored by Erica Ogwumike, BA; Chine Chime, MS, MPH; and Rebecca Vasquez, MD. The aim of this study was to explore what variables were important for 2 events: taking a research fellowship and matching into dermatology. The authors analyzed Electronic Residency Application Service (ERAS) applications for all medical students applying to the UT Southwestern Dermatology Residency Program in the 2014-2015 cycle. They found that 1 of 5 students participated in a research fellowship prior to applying to dermatology residency, and it was not associated with increased odds of matching. They also discovered that students more likely to take a research fellowship were Latinx, attended a medical school ranked in the Top 25, and were not Alpha Omega Alpha members. Nevertheless, total publications did increase the odds of matching; therefore, the authors concluded that when looking for a research fellowship, applicants should look for one that allows productivity so that this measure can be achieved. Further investigation is needed to substantiate these results, but this study was a starting point to examine the characteristics involved in taking a research fellowship in dermatology.
Finally, the Crowd Favorite Award was presented to Jennifer Cucalon, BS, for “Non-invasive, In-Vivo RCM Monitoring of Lentigines Treated With Cryotherapy to Establish Minimum Freeze Time in Seconds (Dose) in Skin of Color” authored by Jennifer Cucalon, BS, and Babar K. Rao, MD. This pilot study showed a minimum freezing time of 3 seconds to be effective in removing lentigines in darker skin; increasing the dose to 6 and 9 seconds had no added benefit. The authors also demonstrated reflectance confocal microscopy to be an appropriate, noninvasive, in vivo tool to visualize pigmentary changes and monitor the effectiveness of treatments for various skin conditions.
The 19th Annual Scientific Symposium will take place on March 16, 2023, in New Orleans, Louisiana. The theme will be Where Science, Innovation & Inclusion Meet. For more information, visit https://skinofcolorsociety.org/19th-annual-skin-of-color-society-scientific-symposium/.
The 18th Annual Skin of Color Society Scientific Symposium was held in March 2022 in Boston, Massachusetts. With a theme of Diversity in Action: Science, Healthcare & Society, researchers gathered to present new findings, share key insights, and discuss the continuing evolution of the field. Three awards were presented from the scientific posters at the symposium.
The Best Poster Presentation Award was presented to Brandyn M. White, BS, for “A Preliminary Analysis of the DDB1 Gene: Genome-Wide Association Studies in African and Admixed African American Populations—Is Our Skin Different?” authored by Brandyn M. White, BS; Chidubem A.V. Okeke, BS; Raveena Khanna, MD; Ginette A. Okoye, MD; Michael C. Campbell, PhD; and Angel S. Byrd, MD, PhD. Their research evaluated the association of variant DNA damage binding protein 1, DDB1, with African populations and highlighted the possible phenotypic variations between African and admixed African American populations. Further, it discussed the advantages of conducting future genome-wide association studies in the Washington metropolitan area to better understand dermatological diseases that disproportionately affect skin of color patients.
The Best Oral Presentation Award was presented to Erica Ogwumike, BA, for “Matching into Dermatology Residency: The Impact of Research Fellowships” authored by Erica Ogwumike, BA; Chine Chime, MS, MPH; and Rebecca Vasquez, MD. The aim of this study was to explore what variables were important for 2 events: taking a research fellowship and matching into dermatology. The authors analyzed Electronic Residency Application Service (ERAS) applications for all medical students applying to the UT Southwestern Dermatology Residency Program in the 2014-2015 cycle. They found that 1 of 5 students participated in a research fellowship prior to applying to dermatology residency, and it was not associated with increased odds of matching. They also discovered that students more likely to take a research fellowship were Latinx, attended a medical school ranked in the Top 25, and were not Alpha Omega Alpha members. Nevertheless, total publications did increase the odds of matching; therefore, the authors concluded that when looking for a research fellowship, applicants should look for one that allows productivity so that this measure can be achieved. Further investigation is needed to substantiate these results, but this study was a starting point to examine the characteristics involved in taking a research fellowship in dermatology.
Finally, the Crowd Favorite Award was presented to Jennifer Cucalon, BS, for “Non-invasive, In-Vivo RCM Monitoring of Lentigines Treated With Cryotherapy to Establish Minimum Freeze Time in Seconds (Dose) in Skin of Color” authored by Jennifer Cucalon, BS, and Babar K. Rao, MD. This pilot study showed a minimum freezing time of 3 seconds to be effective in removing lentigines in darker skin; increasing the dose to 6 and 9 seconds had no added benefit. The authors also demonstrated reflectance confocal microscopy to be an appropriate, noninvasive, in vivo tool to visualize pigmentary changes and monitor the effectiveness of treatments for various skin conditions.
The 19th Annual Scientific Symposium will take place on March 16, 2023, in New Orleans, Louisiana. The theme will be Where Science, Innovation & Inclusion Meet. For more information, visit https://skinofcolorsociety.org/19th-annual-skin-of-color-society-scientific-symposium/.
The 18th Annual Skin of Color Society Scientific Symposium was held in March 2022 in Boston, Massachusetts. With a theme of Diversity in Action: Science, Healthcare & Society, researchers gathered to present new findings, share key insights, and discuss the continuing evolution of the field. Three awards were presented from the scientific posters at the symposium.
The Best Poster Presentation Award was presented to Brandyn M. White, BS, for “A Preliminary Analysis of the DDB1 Gene: Genome-Wide Association Studies in African and Admixed African American Populations—Is Our Skin Different?” authored by Brandyn M. White, BS; Chidubem A.V. Okeke, BS; Raveena Khanna, MD; Ginette A. Okoye, MD; Michael C. Campbell, PhD; and Angel S. Byrd, MD, PhD. Their research evaluated the association of variant DNA damage binding protein 1, DDB1, with African populations and highlighted the possible phenotypic variations between African and admixed African American populations. Further, it discussed the advantages of conducting future genome-wide association studies in the Washington metropolitan area to better understand dermatological diseases that disproportionately affect skin of color patients.
The Best Oral Presentation Award was presented to Erica Ogwumike, BA, for “Matching into Dermatology Residency: The Impact of Research Fellowships” authored by Erica Ogwumike, BA; Chine Chime, MS, MPH; and Rebecca Vasquez, MD. The aim of this study was to explore what variables were important for 2 events: taking a research fellowship and matching into dermatology. The authors analyzed Electronic Residency Application Service (ERAS) applications for all medical students applying to the UT Southwestern Dermatology Residency Program in the 2014-2015 cycle. They found that 1 of 5 students participated in a research fellowship prior to applying to dermatology residency, and it was not associated with increased odds of matching. They also discovered that students more likely to take a research fellowship were Latinx, attended a medical school ranked in the Top 25, and were not Alpha Omega Alpha members. Nevertheless, total publications did increase the odds of matching; therefore, the authors concluded that when looking for a research fellowship, applicants should look for one that allows productivity so that this measure can be achieved. Further investigation is needed to substantiate these results, but this study was a starting point to examine the characteristics involved in taking a research fellowship in dermatology.
Finally, the Crowd Favorite Award was presented to Jennifer Cucalon, BS, for “Non-invasive, In-Vivo RCM Monitoring of Lentigines Treated With Cryotherapy to Establish Minimum Freeze Time in Seconds (Dose) in Skin of Color” authored by Jennifer Cucalon, BS, and Babar K. Rao, MD. This pilot study showed a minimum freezing time of 3 seconds to be effective in removing lentigines in darker skin; increasing the dose to 6 and 9 seconds had no added benefit. The authors also demonstrated reflectance confocal microscopy to be an appropriate, noninvasive, in vivo tool to visualize pigmentary changes and monitor the effectiveness of treatments for various skin conditions.
The 19th Annual Scientific Symposium will take place on March 16, 2023, in New Orleans, Louisiana. The theme will be Where Science, Innovation & Inclusion Meet. For more information, visit https://skinofcolorsociety.org/19th-annual-skin-of-color-society-scientific-symposium/.
Managing respiratory symptoms in the ‘tripledemic’ era
Is it COVID-19, flu, or even RSV? I recently described just such a patient, an obese woman with type 2 diabetes, presenting with fever, cough, myalgia, and fatigue. I asked readers whether they agreed with my management of this patient.
Thank you for your comments as we continue to react to high rates of URIs. Your comments highlight the importance of local resources and practice habits when managing patients with URI.
It was clear that readers value testing to distinguish between infections. However, access to testing is highly variable around the world and is likely to be routinely used only in high-income countries. The Kaiser Family Foundation performed a cost analysis of testing for SARS-CoV-2 in 2020 and found, not surprisingly, wide variability in the cost of testing. Medicare covers tests at rates of $36-$143 per test; a study of list prices for SARS-CoV-2 tests at 93 hospitals found a median cost of $148 per test. And this does not include collection or facility fees. About 20% of tests cost more than $300.
These costs are prohibitive for many health systems. However, more devices have been introduced since that analysis, and competition and evolving technology should drive down prices. Generally, multiplex polymerase chain reaction (PCR) testing for multiple pathogens is less expensive than ordering two or three separate molecular tests and is more convenient for patients and practices alike.
Other reader comments focused on the challenges of getting accurate data on viral epidemiology, and there is certainly a time lag between infection trends and public health reports. This is exacerbated by underreporting of symptoms and more testing at home using antigen tests.
But please do not give up on epidemiology! If a test such as PCR is 90% sensitive for identifying infection, the yield in terms of the number of individuals infected with a particular virus should be high, and that is true when infection is in broad circulation. If 20% of a population of 1,000 has an infection and the test sensitivity is 90%, the yield of testing is 180 true cases versus 20 false positives.
However, if just 2% of the population of 1,000 has the infection in this same scenario, then only 18 true cases are identified. The effect on public health is certainly less, and a lower prevalence rate means that confounding variables, such as how long an individual might shed viral particles and the method of sample collection, have an outsized effect on results. This reduces the validity of diagnostic tests.
Even trends on a national level can provide some insight regarding whom to test. Traditionally, our practice has been to not routinely test patients for influenza or RSV from late spring to early fall unless there was a compelling reason, such as recent travel to an area where these infections were more prevalent. The loss of temporality for these infections since 2020 has altered this approach and made us pay more attention to reports from public health organizations.
I also appreciate the discussion of how to treat Agnes’s symptoms as she waits to improve, and anyone who suffers with or treats a viral URI knows that there are few interventions effective for such symptoms as cough and congestion. A systematic review of 29 randomized controlled trials of over-the-counter medications for cough yielded mixed and largely negative results.
Antihistamines alone do not seem to work, and guaifenesin was successful in only one of three trials. Combinations of different drug classes appeared to be slightly more effective.
My personal favorite for the management of acute cough is something that kids generally love: honey. In a review of 14 studies, 9 of which were limited to pediatric patients, honey was associated with significant reductions in cough frequency, cough severity, and total symptom score. However, there was a moderate risk of bias in the included research, and evidence of honey’s benefit in placebo-controlled trials was limited. Honey used in this research came in a variety of forms, so the best dosage is uncertain.
Clearly, advancements are needed. Better symptom management in viral URI will almost certainly improve productivity across the population and will probably reduce the inappropriate use of antibiotics as well. I have said for years that the scientists who can solve the Gordian knot of pediatric mucus deserve three Nobel prizes. I look forward to that golden day.
Dr. Vega is a clinical professor of family medicine at the University of California, Irvine. He reported a conflict of interest with McNeil Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Is it COVID-19, flu, or even RSV? I recently described just such a patient, an obese woman with type 2 diabetes, presenting with fever, cough, myalgia, and fatigue. I asked readers whether they agreed with my management of this patient.
Thank you for your comments as we continue to react to high rates of URIs. Your comments highlight the importance of local resources and practice habits when managing patients with URI.
It was clear that readers value testing to distinguish between infections. However, access to testing is highly variable around the world and is likely to be routinely used only in high-income countries. The Kaiser Family Foundation performed a cost analysis of testing for SARS-CoV-2 in 2020 and found, not surprisingly, wide variability in the cost of testing. Medicare covers tests at rates of $36-$143 per test; a study of list prices for SARS-CoV-2 tests at 93 hospitals found a median cost of $148 per test. And this does not include collection or facility fees. About 20% of tests cost more than $300.
These costs are prohibitive for many health systems. However, more devices have been introduced since that analysis, and competition and evolving technology should drive down prices. Generally, multiplex polymerase chain reaction (PCR) testing for multiple pathogens is less expensive than ordering two or three separate molecular tests and is more convenient for patients and practices alike.
Other reader comments focused on the challenges of getting accurate data on viral epidemiology, and there is certainly a time lag between infection trends and public health reports. This is exacerbated by underreporting of symptoms and more testing at home using antigen tests.
But please do not give up on epidemiology! If a test such as PCR is 90% sensitive for identifying infection, the yield in terms of the number of individuals infected with a particular virus should be high, and that is true when infection is in broad circulation. If 20% of a population of 1,000 has an infection and the test sensitivity is 90%, the yield of testing is 180 true cases versus 20 false positives.
However, if just 2% of the population of 1,000 has the infection in this same scenario, then only 18 true cases are identified. The effect on public health is certainly less, and a lower prevalence rate means that confounding variables, such as how long an individual might shed viral particles and the method of sample collection, have an outsized effect on results. This reduces the validity of diagnostic tests.
Even trends on a national level can provide some insight regarding whom to test. Traditionally, our practice has been to not routinely test patients for influenza or RSV from late spring to early fall unless there was a compelling reason, such as recent travel to an area where these infections were more prevalent. The loss of temporality for these infections since 2020 has altered this approach and made us pay more attention to reports from public health organizations.
I also appreciate the discussion of how to treat Agnes’s symptoms as she waits to improve, and anyone who suffers with or treats a viral URI knows that there are few interventions effective for such symptoms as cough and congestion. A systematic review of 29 randomized controlled trials of over-the-counter medications for cough yielded mixed and largely negative results.
Antihistamines alone do not seem to work, and guaifenesin was successful in only one of three trials. Combinations of different drug classes appeared to be slightly more effective.
My personal favorite for the management of acute cough is something that kids generally love: honey. In a review of 14 studies, 9 of which were limited to pediatric patients, honey was associated with significant reductions in cough frequency, cough severity, and total symptom score. However, there was a moderate risk of bias in the included research, and evidence of honey’s benefit in placebo-controlled trials was limited. Honey used in this research came in a variety of forms, so the best dosage is uncertain.
Clearly, advancements are needed. Better symptom management in viral URI will almost certainly improve productivity across the population and will probably reduce the inappropriate use of antibiotics as well. I have said for years that the scientists who can solve the Gordian knot of pediatric mucus deserve three Nobel prizes. I look forward to that golden day.
Dr. Vega is a clinical professor of family medicine at the University of California, Irvine. He reported a conflict of interest with McNeil Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Is it COVID-19, flu, or even RSV? I recently described just such a patient, an obese woman with type 2 diabetes, presenting with fever, cough, myalgia, and fatigue. I asked readers whether they agreed with my management of this patient.
Thank you for your comments as we continue to react to high rates of URIs. Your comments highlight the importance of local resources and practice habits when managing patients with URI.
It was clear that readers value testing to distinguish between infections. However, access to testing is highly variable around the world and is likely to be routinely used only in high-income countries. The Kaiser Family Foundation performed a cost analysis of testing for SARS-CoV-2 in 2020 and found, not surprisingly, wide variability in the cost of testing. Medicare covers tests at rates of $36-$143 per test; a study of list prices for SARS-CoV-2 tests at 93 hospitals found a median cost of $148 per test. And this does not include collection or facility fees. About 20% of tests cost more than $300.
These costs are prohibitive for many health systems. However, more devices have been introduced since that analysis, and competition and evolving technology should drive down prices. Generally, multiplex polymerase chain reaction (PCR) testing for multiple pathogens is less expensive than ordering two or three separate molecular tests and is more convenient for patients and practices alike.
Other reader comments focused on the challenges of getting accurate data on viral epidemiology, and there is certainly a time lag between infection trends and public health reports. This is exacerbated by underreporting of symptoms and more testing at home using antigen tests.
But please do not give up on epidemiology! If a test such as PCR is 90% sensitive for identifying infection, the yield in terms of the number of individuals infected with a particular virus should be high, and that is true when infection is in broad circulation. If 20% of a population of 1,000 has an infection and the test sensitivity is 90%, the yield of testing is 180 true cases versus 20 false positives.
However, if just 2% of the population of 1,000 has the infection in this same scenario, then only 18 true cases are identified. The effect on public health is certainly less, and a lower prevalence rate means that confounding variables, such as how long an individual might shed viral particles and the method of sample collection, have an outsized effect on results. This reduces the validity of diagnostic tests.
Even trends on a national level can provide some insight regarding whom to test. Traditionally, our practice has been to not routinely test patients for influenza or RSV from late spring to early fall unless there was a compelling reason, such as recent travel to an area where these infections were more prevalent. The loss of temporality for these infections since 2020 has altered this approach and made us pay more attention to reports from public health organizations.
I also appreciate the discussion of how to treat Agnes’s symptoms as she waits to improve, and anyone who suffers with or treats a viral URI knows that there are few interventions effective for such symptoms as cough and congestion. A systematic review of 29 randomized controlled trials of over-the-counter medications for cough yielded mixed and largely negative results.
Antihistamines alone do not seem to work, and guaifenesin was successful in only one of three trials. Combinations of different drug classes appeared to be slightly more effective.
My personal favorite for the management of acute cough is something that kids generally love: honey. In a review of 14 studies, 9 of which were limited to pediatric patients, honey was associated with significant reductions in cough frequency, cough severity, and total symptom score. However, there was a moderate risk of bias in the included research, and evidence of honey’s benefit in placebo-controlled trials was limited. Honey used in this research came in a variety of forms, so the best dosage is uncertain.
Clearly, advancements are needed. Better symptom management in viral URI will almost certainly improve productivity across the population and will probably reduce the inappropriate use of antibiotics as well. I have said for years that the scientists who can solve the Gordian knot of pediatric mucus deserve three Nobel prizes. I look forward to that golden day.
Dr. Vega is a clinical professor of family medicine at the University of California, Irvine. He reported a conflict of interest with McNeil Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Biden to end COVID emergencies in May
Doing so will have many effects, including the end of free vaccines and health services to fight the pandemic. The public health emergency has been renewed every 90 days since it was declared by the Trump administration in January 2020.
The declaration allowed major changes throughout the health care system to deal with the pandemic, including the free distribution of vaccines, testing, and treatments. In addition, telehealth services were expanded, and Medicaid and the Children’s Health Insurance Program were extended to millions more Americans.
Biden said the COVID-19 national emergency is set to expire March 1 while the declared public health emergency would currently expire on April 11. The president said both will be extended to end May 11.
There were nearly 300,000 newly reported COVID-19 cases in the United States for the week ending Jan. 25, according to CDC data, as well as more than 3,750 deaths.
A version of this article first appeared on WebMD.com.
Doing so will have many effects, including the end of free vaccines and health services to fight the pandemic. The public health emergency has been renewed every 90 days since it was declared by the Trump administration in January 2020.
The declaration allowed major changes throughout the health care system to deal with the pandemic, including the free distribution of vaccines, testing, and treatments. In addition, telehealth services were expanded, and Medicaid and the Children’s Health Insurance Program were extended to millions more Americans.
Biden said the COVID-19 national emergency is set to expire March 1 while the declared public health emergency would currently expire on April 11. The president said both will be extended to end May 11.
There were nearly 300,000 newly reported COVID-19 cases in the United States for the week ending Jan. 25, according to CDC data, as well as more than 3,750 deaths.
A version of this article first appeared on WebMD.com.
Doing so will have many effects, including the end of free vaccines and health services to fight the pandemic. The public health emergency has been renewed every 90 days since it was declared by the Trump administration in January 2020.
The declaration allowed major changes throughout the health care system to deal with the pandemic, including the free distribution of vaccines, testing, and treatments. In addition, telehealth services were expanded, and Medicaid and the Children’s Health Insurance Program were extended to millions more Americans.
Biden said the COVID-19 national emergency is set to expire March 1 while the declared public health emergency would currently expire on April 11. The president said both will be extended to end May 11.
There were nearly 300,000 newly reported COVID-19 cases in the United States for the week ending Jan. 25, according to CDC data, as well as more than 3,750 deaths.
A version of this article first appeared on WebMD.com.
Healthy habits lower T2D microvascular risks: Cohort study
People with diabetes who adhere to a healthy diet, exercise regularly, and follow other healthy lifestyle practices have a significantly lower risk of microvascular complications from the disease, such as diabetic neuropathy, retinopathy, and nephropathy, as well as foot disorders, than counterparts with diabetes who don’t, a prospective cohort study of more than 7,000 patients with type 2 diabetes has found.
“We believe this is one of the first large-scale analyses among diabetes patients that specifically examined an overall healthy lifestyle in relation to the risk of developing microvascular complications,” senior study author Qi Sun, MD, ScD, said in an interview. “The results are not surprising that the healthy lifestyle is associated with lower risk of developing these complications and the enhanced adherence to the healthy lifestyle is associated with lower risk as well. And these findings bear lots of public health significance as they suggest the important role of living a healthy lifestyle in the prevention of diabetes complications, on top of the clinical treatment.”
Dr. Sun is an associate professor of nutrition and epidemiology at the Harvard T.H. Chan School of Public Health, Boston.
The study stated that the findings “lend support” for the American Diabetes Association guidelines for healthy lifestyle practices in people with diabetes.
The study used a cohort from two large prospective cohort studies, the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS), comprising 4,982 women and 2,095 men who were diagnosed with type 2 diabetes during follow-up. They had no cardiovascular disease or cancer at the time of their diabetes diagnosis. Both NHS and HPFS used validated questionnaires to gather information on diet, lifestyle, medical history, and newly diagnosed diseases every 2-4 years. The latter study included NHS and HPFS participants who also completed supplementary questionnaires about their diabetes.
The latest study took into account five modifiable lifestyle-related factors: diet, body weight, smoking status, alcohol, and physical activity. For diet, both large studies used the 2010 Alternate Healthy Eating Index to assess diet quality; those in the upper 40th percentile of the study population were defined as healthy diet. Healthy body weight was defined at a body mass index of 18.5-25 kg/m2.
Among the latter study cohort, 2,878 incident cases of diabetic microvascular complications were documented during follow-up. Patients who adhered to a healthy lifestyle before their diabetes diagnosis, defined as having four or more low-risk lifestyle factors, had a 27% lower relative risk of developing any microvascular complication than counterparts with no low-risk lifestyle factors (relative risk, 0.73; 95% confidence interval, 0.35-1; P = .006).
The study found similar outcomes for those who adopted a healthy lifestyle after their diabetes diagnosis, with a 32% reduction in relative risk compared with those who didn’t adopt any healthy lifestyle practices (RR, 0.68; 95% CI, 0.55-0.83; P < .001).
Dr. Sun noted what was noteworthy about his group’s cohort study. “The unique design is truly the prospective follow-up over time so that we could examine the lifestyle at diabetes diagnosis as well as changes in lifestyle before and after diabetes in relation to the future risk of developing the complications,” he said.
A randomized trial would be a more rigorous way to evaluate the impact of a healthy lifestyle, he added, “although it’s much more expensive than a cohort study like what we did with this investigation.”
As for future research, Dr. Sun said, “It will be interesting to understand mechanisms underlying these observations. It’s also critical to understand why certain diabetes patients may not benefit from a healthy lifestyle, since some of them, even when living a healthy lifestyle, still develop the complications.”
This trial shows in a new light the benefits of healthy lifestyle practices on microvascular complications of type 2 diabetes, Paul S. Jellinger, MD, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., and a professor at the University of Miami, said in a comment. “These benefits have always been surmised and demonstrated in a limited way in previous trials, but not subject to the level of analysis seen in this prospective cohort trial.”
He called the study design “excellent,” adding, “ ‘Validated’ self-reported questionnaires were used widely, although minimal detail is provided about the validation process.” One limitation, he noted, was “the homogeneity of the participants; all were health professionals.”
The study “affirms” and “quantitates” the benefits of a healthy lifestyle in type 2 diabetes. “The issue is not unawareness but rather application,” Dr. Jellinger said. “Modifying long-held lifestyle habits is a real challenge. Perhaps by ‘quantitating’ the benefit, as shown in this trial and hopefully additional studies, impetus will be provided to refocus on this approach, which is too often simply given lip service.”
The National Institutes of Health provided funding for the study. Dr. Sun has no relevant disclosures. Dr. Jellinger disclosed relationships with Amgen and Esperion.
People with diabetes who adhere to a healthy diet, exercise regularly, and follow other healthy lifestyle practices have a significantly lower risk of microvascular complications from the disease, such as diabetic neuropathy, retinopathy, and nephropathy, as well as foot disorders, than counterparts with diabetes who don’t, a prospective cohort study of more than 7,000 patients with type 2 diabetes has found.
“We believe this is one of the first large-scale analyses among diabetes patients that specifically examined an overall healthy lifestyle in relation to the risk of developing microvascular complications,” senior study author Qi Sun, MD, ScD, said in an interview. “The results are not surprising that the healthy lifestyle is associated with lower risk of developing these complications and the enhanced adherence to the healthy lifestyle is associated with lower risk as well. And these findings bear lots of public health significance as they suggest the important role of living a healthy lifestyle in the prevention of diabetes complications, on top of the clinical treatment.”
Dr. Sun is an associate professor of nutrition and epidemiology at the Harvard T.H. Chan School of Public Health, Boston.
The study stated that the findings “lend support” for the American Diabetes Association guidelines for healthy lifestyle practices in people with diabetes.
The study used a cohort from two large prospective cohort studies, the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS), comprising 4,982 women and 2,095 men who were diagnosed with type 2 diabetes during follow-up. They had no cardiovascular disease or cancer at the time of their diabetes diagnosis. Both NHS and HPFS used validated questionnaires to gather information on diet, lifestyle, medical history, and newly diagnosed diseases every 2-4 years. The latter study included NHS and HPFS participants who also completed supplementary questionnaires about their diabetes.
The latest study took into account five modifiable lifestyle-related factors: diet, body weight, smoking status, alcohol, and physical activity. For diet, both large studies used the 2010 Alternate Healthy Eating Index to assess diet quality; those in the upper 40th percentile of the study population were defined as healthy diet. Healthy body weight was defined at a body mass index of 18.5-25 kg/m2.
Among the latter study cohort, 2,878 incident cases of diabetic microvascular complications were documented during follow-up. Patients who adhered to a healthy lifestyle before their diabetes diagnosis, defined as having four or more low-risk lifestyle factors, had a 27% lower relative risk of developing any microvascular complication than counterparts with no low-risk lifestyle factors (relative risk, 0.73; 95% confidence interval, 0.35-1; P = .006).
The study found similar outcomes for those who adopted a healthy lifestyle after their diabetes diagnosis, with a 32% reduction in relative risk compared with those who didn’t adopt any healthy lifestyle practices (RR, 0.68; 95% CI, 0.55-0.83; P < .001).
Dr. Sun noted what was noteworthy about his group’s cohort study. “The unique design is truly the prospective follow-up over time so that we could examine the lifestyle at diabetes diagnosis as well as changes in lifestyle before and after diabetes in relation to the future risk of developing the complications,” he said.
A randomized trial would be a more rigorous way to evaluate the impact of a healthy lifestyle, he added, “although it’s much more expensive than a cohort study like what we did with this investigation.”
As for future research, Dr. Sun said, “It will be interesting to understand mechanisms underlying these observations. It’s also critical to understand why certain diabetes patients may not benefit from a healthy lifestyle, since some of them, even when living a healthy lifestyle, still develop the complications.”
This trial shows in a new light the benefits of healthy lifestyle practices on microvascular complications of type 2 diabetes, Paul S. Jellinger, MD, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., and a professor at the University of Miami, said in a comment. “These benefits have always been surmised and demonstrated in a limited way in previous trials, but not subject to the level of analysis seen in this prospective cohort trial.”
He called the study design “excellent,” adding, “ ‘Validated’ self-reported questionnaires were used widely, although minimal detail is provided about the validation process.” One limitation, he noted, was “the homogeneity of the participants; all were health professionals.”
The study “affirms” and “quantitates” the benefits of a healthy lifestyle in type 2 diabetes. “The issue is not unawareness but rather application,” Dr. Jellinger said. “Modifying long-held lifestyle habits is a real challenge. Perhaps by ‘quantitating’ the benefit, as shown in this trial and hopefully additional studies, impetus will be provided to refocus on this approach, which is too often simply given lip service.”
The National Institutes of Health provided funding for the study. Dr. Sun has no relevant disclosures. Dr. Jellinger disclosed relationships with Amgen and Esperion.
People with diabetes who adhere to a healthy diet, exercise regularly, and follow other healthy lifestyle practices have a significantly lower risk of microvascular complications from the disease, such as diabetic neuropathy, retinopathy, and nephropathy, as well as foot disorders, than counterparts with diabetes who don’t, a prospective cohort study of more than 7,000 patients with type 2 diabetes has found.
“We believe this is one of the first large-scale analyses among diabetes patients that specifically examined an overall healthy lifestyle in relation to the risk of developing microvascular complications,” senior study author Qi Sun, MD, ScD, said in an interview. “The results are not surprising that the healthy lifestyle is associated with lower risk of developing these complications and the enhanced adherence to the healthy lifestyle is associated with lower risk as well. And these findings bear lots of public health significance as they suggest the important role of living a healthy lifestyle in the prevention of diabetes complications, on top of the clinical treatment.”
Dr. Sun is an associate professor of nutrition and epidemiology at the Harvard T.H. Chan School of Public Health, Boston.
The study stated that the findings “lend support” for the American Diabetes Association guidelines for healthy lifestyle practices in people with diabetes.
The study used a cohort from two large prospective cohort studies, the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS), comprising 4,982 women and 2,095 men who were diagnosed with type 2 diabetes during follow-up. They had no cardiovascular disease or cancer at the time of their diabetes diagnosis. Both NHS and HPFS used validated questionnaires to gather information on diet, lifestyle, medical history, and newly diagnosed diseases every 2-4 years. The latter study included NHS and HPFS participants who also completed supplementary questionnaires about their diabetes.
The latest study took into account five modifiable lifestyle-related factors: diet, body weight, smoking status, alcohol, and physical activity. For diet, both large studies used the 2010 Alternate Healthy Eating Index to assess diet quality; those in the upper 40th percentile of the study population were defined as healthy diet. Healthy body weight was defined at a body mass index of 18.5-25 kg/m2.
Among the latter study cohort, 2,878 incident cases of diabetic microvascular complications were documented during follow-up. Patients who adhered to a healthy lifestyle before their diabetes diagnosis, defined as having four or more low-risk lifestyle factors, had a 27% lower relative risk of developing any microvascular complication than counterparts with no low-risk lifestyle factors (relative risk, 0.73; 95% confidence interval, 0.35-1; P = .006).
The study found similar outcomes for those who adopted a healthy lifestyle after their diabetes diagnosis, with a 32% reduction in relative risk compared with those who didn’t adopt any healthy lifestyle practices (RR, 0.68; 95% CI, 0.55-0.83; P < .001).
Dr. Sun noted what was noteworthy about his group’s cohort study. “The unique design is truly the prospective follow-up over time so that we could examine the lifestyle at diabetes diagnosis as well as changes in lifestyle before and after diabetes in relation to the future risk of developing the complications,” he said.
A randomized trial would be a more rigorous way to evaluate the impact of a healthy lifestyle, he added, “although it’s much more expensive than a cohort study like what we did with this investigation.”
As for future research, Dr. Sun said, “It will be interesting to understand mechanisms underlying these observations. It’s also critical to understand why certain diabetes patients may not benefit from a healthy lifestyle, since some of them, even when living a healthy lifestyle, still develop the complications.”
This trial shows in a new light the benefits of healthy lifestyle practices on microvascular complications of type 2 diabetes, Paul S. Jellinger, MD, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., and a professor at the University of Miami, said in a comment. “These benefits have always been surmised and demonstrated in a limited way in previous trials, but not subject to the level of analysis seen in this prospective cohort trial.”
He called the study design “excellent,” adding, “ ‘Validated’ self-reported questionnaires were used widely, although minimal detail is provided about the validation process.” One limitation, he noted, was “the homogeneity of the participants; all were health professionals.”
The study “affirms” and “quantitates” the benefits of a healthy lifestyle in type 2 diabetes. “The issue is not unawareness but rather application,” Dr. Jellinger said. “Modifying long-held lifestyle habits is a real challenge. Perhaps by ‘quantitating’ the benefit, as shown in this trial and hopefully additional studies, impetus will be provided to refocus on this approach, which is too often simply given lip service.”
The National Institutes of Health provided funding for the study. Dr. Sun has no relevant disclosures. Dr. Jellinger disclosed relationships with Amgen and Esperion.
FROM JAMA NETWORK OPEN
Long COVID affecting more than one-third of college students, faculty
With a median age of 23 years, the study is unique for evaluating mostly healthy, young adults and for its rare look at long COVID in a university community.
The more symptoms during a bout with COVID, the greater the risk for long COVID, the researchers found. That lines up with previous studies. Also, the more vaccinations and booster shots against SARS-CoV-2, the virus that causes COVID, the lower the long COVID risk.
Women were more likely than men to be affected. Current or prior smoking, seeking medical care for COVID, and receiving antibody treatment also were linked to higher chances for developing long COVID.
Lead author Megan Landry, DrPH, MPH, and colleagues were already assessing students, staff, and faculty at George Washington University, Washington, who tested positive for COVID. Then they started seeing symptoms that lasted 28 days or more after their 10-day isolation period.
“We were starting to recognize that individuals ... were still having symptoms longer than the typical isolation period,” said Dr. Landry. So they developed a questionnaire to figure out the how long these symptoms last and how many people are affected by them.
The list of potential symptoms was long and included trouble thinking, fatigue, loss of smell or taste, shortness of breath, and more.
The study was published online in Emerging Infectious Diseases. Results are based on records and responses from 1,388 students, faculty, and staff from July 2021 to March 2022.
People had a median of four long COVID symptoms, about 63% were women, and 56% were non-Hispanic White. About three-quarters were students and the remainder were faculty and staff.
The finding that 36% of people with a history of COVID reported long COVID symptoms did not surprise Dr. Landry.
“Based on the literature that’s currently out there, it ranges from a 10% to an 80% prevalence of long COVID,” she said. “We kind of figured that we would fall somewhere in there.”
In contrast, that figure seemed high to Eric Topol, MD.
“That’s really high,” said Dr. Topol, founder and director of the Scripps Research Translational Institute in La Jolla, Calif. He added most studies estimate that about 10% of people with a history of acute infection develop long COVID.
Even at 10%, which could be an underestimate, that’s a lot of affected people globally.
“At least 65 million individuals around the world have long COVID, based on a conservative estimated incidence of 10% of infected people and more than 651 million documented COVID-19 cases worldwide; the number is likely much higher due to many undocumented cases,” Dr. Topol and colleagues wrote in a long COVID review article published in Nature Reviews Microbiology.
About 30% of study participants were fully vaccinated with an initial vaccine series, 42% had received a booster dose, and 29% were not fully vaccinated at the time of their first positive test for COVID. Those who were not fully vaccinated were significantly more likely to report symptoms of long COVID.
“I know a lot of people wish they could put COVID on the back burner or brush it under the rug, but COVID is still a real thing. We need to continue supporting vaccines and boosters and make sure people are up to date. Not only for COVID, but for flu as well,” Dr. Topol said
Research continues
“Long COVID is still evolving and we continue to learn more about it every day,” Landry said. “It’s just so new and there are still a lot of unknowns. That’s why it’s important to get this information out.”
People with long COVID often have a hard time with occupational, educational, social, or personal activities, compared with before COVID, with effects that can last for more than 6 months, the authors noted.
“I think across the board, universities in general need to consider the possibility of folks on their campuses are having symptoms of long COVID,” Dr. Landry said.
Moving forward, Dr. Landry and colleagues would like to continue investigating long COVID. For example, in the current study, they did not ask about severity of symptoms or how the symptoms affected daily functioning.
“I would like to continue this and dive deeper into how disruptive their symptoms of long COVID are to their everyday studying, teaching, or their activities to keeping a university running,” Dr. Landry said.
A version of this article originally appeared on WebMD.com.
With a median age of 23 years, the study is unique for evaluating mostly healthy, young adults and for its rare look at long COVID in a university community.
The more symptoms during a bout with COVID, the greater the risk for long COVID, the researchers found. That lines up with previous studies. Also, the more vaccinations and booster shots against SARS-CoV-2, the virus that causes COVID, the lower the long COVID risk.
Women were more likely than men to be affected. Current or prior smoking, seeking medical care for COVID, and receiving antibody treatment also were linked to higher chances for developing long COVID.
Lead author Megan Landry, DrPH, MPH, and colleagues were already assessing students, staff, and faculty at George Washington University, Washington, who tested positive for COVID. Then they started seeing symptoms that lasted 28 days or more after their 10-day isolation period.
“We were starting to recognize that individuals ... were still having symptoms longer than the typical isolation period,” said Dr. Landry. So they developed a questionnaire to figure out the how long these symptoms last and how many people are affected by them.
The list of potential symptoms was long and included trouble thinking, fatigue, loss of smell or taste, shortness of breath, and more.
The study was published online in Emerging Infectious Diseases. Results are based on records and responses from 1,388 students, faculty, and staff from July 2021 to March 2022.
People had a median of four long COVID symptoms, about 63% were women, and 56% were non-Hispanic White. About three-quarters were students and the remainder were faculty and staff.
The finding that 36% of people with a history of COVID reported long COVID symptoms did not surprise Dr. Landry.
“Based on the literature that’s currently out there, it ranges from a 10% to an 80% prevalence of long COVID,” she said. “We kind of figured that we would fall somewhere in there.”
In contrast, that figure seemed high to Eric Topol, MD.
“That’s really high,” said Dr. Topol, founder and director of the Scripps Research Translational Institute in La Jolla, Calif. He added most studies estimate that about 10% of people with a history of acute infection develop long COVID.
Even at 10%, which could be an underestimate, that’s a lot of affected people globally.
“At least 65 million individuals around the world have long COVID, based on a conservative estimated incidence of 10% of infected people and more than 651 million documented COVID-19 cases worldwide; the number is likely much higher due to many undocumented cases,” Dr. Topol and colleagues wrote in a long COVID review article published in Nature Reviews Microbiology.
About 30% of study participants were fully vaccinated with an initial vaccine series, 42% had received a booster dose, and 29% were not fully vaccinated at the time of their first positive test for COVID. Those who were not fully vaccinated were significantly more likely to report symptoms of long COVID.
“I know a lot of people wish they could put COVID on the back burner or brush it under the rug, but COVID is still a real thing. We need to continue supporting vaccines and boosters and make sure people are up to date. Not only for COVID, but for flu as well,” Dr. Topol said
Research continues
“Long COVID is still evolving and we continue to learn more about it every day,” Landry said. “It’s just so new and there are still a lot of unknowns. That’s why it’s important to get this information out.”
People with long COVID often have a hard time with occupational, educational, social, or personal activities, compared with before COVID, with effects that can last for more than 6 months, the authors noted.
“I think across the board, universities in general need to consider the possibility of folks on their campuses are having symptoms of long COVID,” Dr. Landry said.
Moving forward, Dr. Landry and colleagues would like to continue investigating long COVID. For example, in the current study, they did not ask about severity of symptoms or how the symptoms affected daily functioning.
“I would like to continue this and dive deeper into how disruptive their symptoms of long COVID are to their everyday studying, teaching, or their activities to keeping a university running,” Dr. Landry said.
A version of this article originally appeared on WebMD.com.
With a median age of 23 years, the study is unique for evaluating mostly healthy, young adults and for its rare look at long COVID in a university community.
The more symptoms during a bout with COVID, the greater the risk for long COVID, the researchers found. That lines up with previous studies. Also, the more vaccinations and booster shots against SARS-CoV-2, the virus that causes COVID, the lower the long COVID risk.
Women were more likely than men to be affected. Current or prior smoking, seeking medical care for COVID, and receiving antibody treatment also were linked to higher chances for developing long COVID.
Lead author Megan Landry, DrPH, MPH, and colleagues were already assessing students, staff, and faculty at George Washington University, Washington, who tested positive for COVID. Then they started seeing symptoms that lasted 28 days or more after their 10-day isolation period.
“We were starting to recognize that individuals ... were still having symptoms longer than the typical isolation period,” said Dr. Landry. So they developed a questionnaire to figure out the how long these symptoms last and how many people are affected by them.
The list of potential symptoms was long and included trouble thinking, fatigue, loss of smell or taste, shortness of breath, and more.
The study was published online in Emerging Infectious Diseases. Results are based on records and responses from 1,388 students, faculty, and staff from July 2021 to March 2022.
People had a median of four long COVID symptoms, about 63% were women, and 56% were non-Hispanic White. About three-quarters were students and the remainder were faculty and staff.
The finding that 36% of people with a history of COVID reported long COVID symptoms did not surprise Dr. Landry.
“Based on the literature that’s currently out there, it ranges from a 10% to an 80% prevalence of long COVID,” she said. “We kind of figured that we would fall somewhere in there.”
In contrast, that figure seemed high to Eric Topol, MD.
“That’s really high,” said Dr. Topol, founder and director of the Scripps Research Translational Institute in La Jolla, Calif. He added most studies estimate that about 10% of people with a history of acute infection develop long COVID.
Even at 10%, which could be an underestimate, that’s a lot of affected people globally.
“At least 65 million individuals around the world have long COVID, based on a conservative estimated incidence of 10% of infected people and more than 651 million documented COVID-19 cases worldwide; the number is likely much higher due to many undocumented cases,” Dr. Topol and colleagues wrote in a long COVID review article published in Nature Reviews Microbiology.
About 30% of study participants were fully vaccinated with an initial vaccine series, 42% had received a booster dose, and 29% were not fully vaccinated at the time of their first positive test for COVID. Those who were not fully vaccinated were significantly more likely to report symptoms of long COVID.
“I know a lot of people wish they could put COVID on the back burner or brush it under the rug, but COVID is still a real thing. We need to continue supporting vaccines and boosters and make sure people are up to date. Not only for COVID, but for flu as well,” Dr. Topol said
Research continues
“Long COVID is still evolving and we continue to learn more about it every day,” Landry said. “It’s just so new and there are still a lot of unknowns. That’s why it’s important to get this information out.”
People with long COVID often have a hard time with occupational, educational, social, or personal activities, compared with before COVID, with effects that can last for more than 6 months, the authors noted.
“I think across the board, universities in general need to consider the possibility of folks on their campuses are having symptoms of long COVID,” Dr. Landry said.
Moving forward, Dr. Landry and colleagues would like to continue investigating long COVID. For example, in the current study, they did not ask about severity of symptoms or how the symptoms affected daily functioning.
“I would like to continue this and dive deeper into how disruptive their symptoms of long COVID are to their everyday studying, teaching, or their activities to keeping a university running,” Dr. Landry said.
A version of this article originally appeared on WebMD.com.
FROM EMERGING INFECTIOUS DISEASES
Don’t cross the friends line with patients
All that moving can make it hard to maintain friendships. Factor in the challenges from the pandemic, and a physician’s life can be lonely. So, when a patient invites you for coffee or a game of pickleball, do you accept? For almost one-third of the physicians who responded to the Medscape Physician Friendships: The Joys and Challenges 2022, the answer might be yes.
About 29% said they develop friendships with patients. However, a lot depends on the circumstances. As one physician in the report said: “I have been a pediatrician for 35 years, and my patients have grown up and become productive adults in our small, rural, isolated area. You can’t help but know almost everyone.”
As the daughter of a cardiologist, Nishi Mehta, MD, a radiologist and founder of the largest physician-only Facebook group in the country, grew up with that small-town-everyone-knows-the-doctor model.
“When I was a kid, I’d go to the mall, and my friends and I would play a game: How long before a patient [of my dad’s] comes up to me?” she said. At the time, Dr. Mehta was embarrassed, but now she marvels that her dad knew his patients so well that they would recognize his daughter in crowded suburban mall.
In other instances, a physician may develop a friendly relationship after a patient leaves their care. For example, Leo Nissola, MD, now a full-time researcher and immunotherapy scientist in San Francisco, has stayed in touch with some of the patients he treated while at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Nissola said it was important to stay connected with the patients he had meaningful relationships with. “It becomes challenging, though, when a former patient asks for medical advice.” At that moment, “you have to be explicitly clear that the relationship has changed.”
A hard line in the sand
The blurring of lines is one reason many doctors refuse to befriend patients, even after they are no longer treating them. The American College of Physicians Ethics Manual advises against treating anyone with whom you have a close relationship, including family and friends.
“Friendships can get in the way of patients being honest with you, which can interfere with medical care,” Dr. Mehta said. “If a patient has a concern related to something they wouldn’t want you to know as friends, it can get awkward. They may elect not to tell you.”
And on the flip side, friendship can provide a view into your private life that you may not welcome in the exam room.
“Let’s say you go out for drinks [with a patient], and you’re up late, but you have surgery the next day,” said Brandi Ring, MD, an ob.gyn. and the associate medical director at the Center for Children and Women in Houston. Now, one of your patients knows you were out until midnight when you had to be in the OR at 5:00 a.m.
Worse still, your relationship could color your decisions about a patient’s care, even unconsciously. It can be hard to maintain objectivity when you have an emotional investment in someone’s well-being.
“We don’t necessarily treat family and friends to the standards of medical care,” said Dr. Ring. “We go above and beyond. We might order more tests and more scans. We don’t always follow the guidelines, especially in critical illness.”
For all these reasons and more, the ACP advises against treating friends.
Put physician before friend
But adhering to those guidelines can lead physicians to make some painful decisions. Cutting yourself off from the possibility of friendship is never easy, and the Medscape report found that physicians tend to have fewer friends than the average American.
“Especially earlier in my practice, when I was a young parent, and I would see a lot of other young parents in the same stage in life, I’d think, ‘In other circumstances, I would be hanging out at the park with this person,’ “ said Kathleen Rowland, MD, a family medicine physician and vice chair of education in the department of family medicine at Rush University, Chicago. “But the hard part is, the doctor-patient relationship always comes first.”
To a certain extent, one’s specialty may determine the feasibility of becoming friends with a patient. While Dr. Mehta has never done so, as a radiologist, she doesn’t usually see patients repeatedly. Likewise, a young gerontologist may have little in common with his octogenarian patients. And an older pediatrician is not in the same life stage as his patients’ sleep-deprived new parents, possibly making them less attractive friends.
However, practicing family medicine is all about long-term physician-patient relationships. Getting to know patients and their families over many years can lead to a certain intimacy. Dr. Rowland said that, while a wonderful part of being a physician is getting that unique trust whereby patients tell you all sorts of things about their lives, she’s never gone down the friendship path.
“There’s the assumption I’ll take care of someone for a long period of time, and their partner and their kids, maybe another generation or two,” Dr. Rowland said. “People really do rely on that relationship to contribute to their health.”
Worse, nowadays, when people may be starved for connection, many patients want to feel emotionally close and cared for by their doctor, so it’d be easy to cross the line. While patients deserve a compassionate, caring doctor, the physician is left to walk the line between those boundaries. Dr. Rowland said, “It’s up to the clinician to say: ‘My role is as a doctor. You deserve caring friends, but I have to order your mammogram and your blood counts. My role is different.’ ”
Friendly but not friends
It can be tricky to navigate the boundary between a cordial, warm relationship with a patient and that patient inviting you to their daughter’s wedding.
“People may mistake being pleasant and friendly for being friends,” said Larry Blosser, MD, chief medical officer at Central Ohio Primary Care, Westerville. In his position, he sometimes hears from patients who have misunderstood their relationship with a doctor in the practice. When that happens, he advises the physician to consider the persona they’re presenting to the patient. If you’re overly friendly, there’s the potential for confusion, but you can’t be aloof and cold, he said.
Maintaining that awareness helps to prevent a patient’s offhand invitation to catch a movie or go on a hike. And verbalizing it to your patients can make your relationship clear from the get-go.
“I tell patients we’re a team. I’m the captain, and they’re my MVP. When the match is over, whatever the results, we’re done,” said Karenne Fru, MD, PhD, a fertility specialist at Oma Fertility Atlanta. Making deep connections is essential to her practice, so Dr. Fru structures her patient interactions carefully. “Infertility is such an isolating experience. While you’re with us, we care about what’s going on in your life, your pets, and your mom’s chemo. We need mutual trust for you to be compliant with the care.”
However, that approach won’t work when you see patients regularly, as with family practice or specialties that see the same patients repeatedly throughout the year. In those circumstances, the match is never over but one in which the onus is on the physician to establish a friendly yet professional rapport without letting your self-interest, loneliness, or lack of friends interfere.
“It’s been a very difficult couple of years for a lot of us. Depending on what kind of clinical work we do, some of us took care of healthy people that got very sick or passed away,” Dr. Rowland said. “Having the chance to reconnect with people and reestablish some of that closeness, both physical and emotional, is going to be good for us.”
Just continue conveying warm, trusting compassion for your patients without blurring the friend lines.
A version of this article first appeared on Medscape.com.
All that moving can make it hard to maintain friendships. Factor in the challenges from the pandemic, and a physician’s life can be lonely. So, when a patient invites you for coffee or a game of pickleball, do you accept? For almost one-third of the physicians who responded to the Medscape Physician Friendships: The Joys and Challenges 2022, the answer might be yes.
About 29% said they develop friendships with patients. However, a lot depends on the circumstances. As one physician in the report said: “I have been a pediatrician for 35 years, and my patients have grown up and become productive adults in our small, rural, isolated area. You can’t help but know almost everyone.”
As the daughter of a cardiologist, Nishi Mehta, MD, a radiologist and founder of the largest physician-only Facebook group in the country, grew up with that small-town-everyone-knows-the-doctor model.
“When I was a kid, I’d go to the mall, and my friends and I would play a game: How long before a patient [of my dad’s] comes up to me?” she said. At the time, Dr. Mehta was embarrassed, but now she marvels that her dad knew his patients so well that they would recognize his daughter in crowded suburban mall.
In other instances, a physician may develop a friendly relationship after a patient leaves their care. For example, Leo Nissola, MD, now a full-time researcher and immunotherapy scientist in San Francisco, has stayed in touch with some of the patients he treated while at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Nissola said it was important to stay connected with the patients he had meaningful relationships with. “It becomes challenging, though, when a former patient asks for medical advice.” At that moment, “you have to be explicitly clear that the relationship has changed.”
A hard line in the sand
The blurring of lines is one reason many doctors refuse to befriend patients, even after they are no longer treating them. The American College of Physicians Ethics Manual advises against treating anyone with whom you have a close relationship, including family and friends.
“Friendships can get in the way of patients being honest with you, which can interfere with medical care,” Dr. Mehta said. “If a patient has a concern related to something they wouldn’t want you to know as friends, it can get awkward. They may elect not to tell you.”
And on the flip side, friendship can provide a view into your private life that you may not welcome in the exam room.
“Let’s say you go out for drinks [with a patient], and you’re up late, but you have surgery the next day,” said Brandi Ring, MD, an ob.gyn. and the associate medical director at the Center for Children and Women in Houston. Now, one of your patients knows you were out until midnight when you had to be in the OR at 5:00 a.m.
Worse still, your relationship could color your decisions about a patient’s care, even unconsciously. It can be hard to maintain objectivity when you have an emotional investment in someone’s well-being.
“We don’t necessarily treat family and friends to the standards of medical care,” said Dr. Ring. “We go above and beyond. We might order more tests and more scans. We don’t always follow the guidelines, especially in critical illness.”
For all these reasons and more, the ACP advises against treating friends.
Put physician before friend
But adhering to those guidelines can lead physicians to make some painful decisions. Cutting yourself off from the possibility of friendship is never easy, and the Medscape report found that physicians tend to have fewer friends than the average American.
“Especially earlier in my practice, when I was a young parent, and I would see a lot of other young parents in the same stage in life, I’d think, ‘In other circumstances, I would be hanging out at the park with this person,’ “ said Kathleen Rowland, MD, a family medicine physician and vice chair of education in the department of family medicine at Rush University, Chicago. “But the hard part is, the doctor-patient relationship always comes first.”
To a certain extent, one’s specialty may determine the feasibility of becoming friends with a patient. While Dr. Mehta has never done so, as a radiologist, she doesn’t usually see patients repeatedly. Likewise, a young gerontologist may have little in common with his octogenarian patients. And an older pediatrician is not in the same life stage as his patients’ sleep-deprived new parents, possibly making them less attractive friends.
However, practicing family medicine is all about long-term physician-patient relationships. Getting to know patients and their families over many years can lead to a certain intimacy. Dr. Rowland said that, while a wonderful part of being a physician is getting that unique trust whereby patients tell you all sorts of things about their lives, she’s never gone down the friendship path.
“There’s the assumption I’ll take care of someone for a long period of time, and their partner and their kids, maybe another generation or two,” Dr. Rowland said. “People really do rely on that relationship to contribute to their health.”
Worse, nowadays, when people may be starved for connection, many patients want to feel emotionally close and cared for by their doctor, so it’d be easy to cross the line. While patients deserve a compassionate, caring doctor, the physician is left to walk the line between those boundaries. Dr. Rowland said, “It’s up to the clinician to say: ‘My role is as a doctor. You deserve caring friends, but I have to order your mammogram and your blood counts. My role is different.’ ”
Friendly but not friends
It can be tricky to navigate the boundary between a cordial, warm relationship with a patient and that patient inviting you to their daughter’s wedding.
“People may mistake being pleasant and friendly for being friends,” said Larry Blosser, MD, chief medical officer at Central Ohio Primary Care, Westerville. In his position, he sometimes hears from patients who have misunderstood their relationship with a doctor in the practice. When that happens, he advises the physician to consider the persona they’re presenting to the patient. If you’re overly friendly, there’s the potential for confusion, but you can’t be aloof and cold, he said.
Maintaining that awareness helps to prevent a patient’s offhand invitation to catch a movie or go on a hike. And verbalizing it to your patients can make your relationship clear from the get-go.
“I tell patients we’re a team. I’m the captain, and they’re my MVP. When the match is over, whatever the results, we’re done,” said Karenne Fru, MD, PhD, a fertility specialist at Oma Fertility Atlanta. Making deep connections is essential to her practice, so Dr. Fru structures her patient interactions carefully. “Infertility is such an isolating experience. While you’re with us, we care about what’s going on in your life, your pets, and your mom’s chemo. We need mutual trust for you to be compliant with the care.”
However, that approach won’t work when you see patients regularly, as with family practice or specialties that see the same patients repeatedly throughout the year. In those circumstances, the match is never over but one in which the onus is on the physician to establish a friendly yet professional rapport without letting your self-interest, loneliness, or lack of friends interfere.
“It’s been a very difficult couple of years for a lot of us. Depending on what kind of clinical work we do, some of us took care of healthy people that got very sick or passed away,” Dr. Rowland said. “Having the chance to reconnect with people and reestablish some of that closeness, both physical and emotional, is going to be good for us.”
Just continue conveying warm, trusting compassion for your patients without blurring the friend lines.
A version of this article first appeared on Medscape.com.
All that moving can make it hard to maintain friendships. Factor in the challenges from the pandemic, and a physician’s life can be lonely. So, when a patient invites you for coffee or a game of pickleball, do you accept? For almost one-third of the physicians who responded to the Medscape Physician Friendships: The Joys and Challenges 2022, the answer might be yes.
About 29% said they develop friendships with patients. However, a lot depends on the circumstances. As one physician in the report said: “I have been a pediatrician for 35 years, and my patients have grown up and become productive adults in our small, rural, isolated area. You can’t help but know almost everyone.”
As the daughter of a cardiologist, Nishi Mehta, MD, a radiologist and founder of the largest physician-only Facebook group in the country, grew up with that small-town-everyone-knows-the-doctor model.
“When I was a kid, I’d go to the mall, and my friends and I would play a game: How long before a patient [of my dad’s] comes up to me?” she said. At the time, Dr. Mehta was embarrassed, but now she marvels that her dad knew his patients so well that they would recognize his daughter in crowded suburban mall.
In other instances, a physician may develop a friendly relationship after a patient leaves their care. For example, Leo Nissola, MD, now a full-time researcher and immunotherapy scientist in San Francisco, has stayed in touch with some of the patients he treated while at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Nissola said it was important to stay connected with the patients he had meaningful relationships with. “It becomes challenging, though, when a former patient asks for medical advice.” At that moment, “you have to be explicitly clear that the relationship has changed.”
A hard line in the sand
The blurring of lines is one reason many doctors refuse to befriend patients, even after they are no longer treating them. The American College of Physicians Ethics Manual advises against treating anyone with whom you have a close relationship, including family and friends.
“Friendships can get in the way of patients being honest with you, which can interfere with medical care,” Dr. Mehta said. “If a patient has a concern related to something they wouldn’t want you to know as friends, it can get awkward. They may elect not to tell you.”
And on the flip side, friendship can provide a view into your private life that you may not welcome in the exam room.
“Let’s say you go out for drinks [with a patient], and you’re up late, but you have surgery the next day,” said Brandi Ring, MD, an ob.gyn. and the associate medical director at the Center for Children and Women in Houston. Now, one of your patients knows you were out until midnight when you had to be in the OR at 5:00 a.m.
Worse still, your relationship could color your decisions about a patient’s care, even unconsciously. It can be hard to maintain objectivity when you have an emotional investment in someone’s well-being.
“We don’t necessarily treat family and friends to the standards of medical care,” said Dr. Ring. “We go above and beyond. We might order more tests and more scans. We don’t always follow the guidelines, especially in critical illness.”
For all these reasons and more, the ACP advises against treating friends.
Put physician before friend
But adhering to those guidelines can lead physicians to make some painful decisions. Cutting yourself off from the possibility of friendship is never easy, and the Medscape report found that physicians tend to have fewer friends than the average American.
“Especially earlier in my practice, when I was a young parent, and I would see a lot of other young parents in the same stage in life, I’d think, ‘In other circumstances, I would be hanging out at the park with this person,’ “ said Kathleen Rowland, MD, a family medicine physician and vice chair of education in the department of family medicine at Rush University, Chicago. “But the hard part is, the doctor-patient relationship always comes first.”
To a certain extent, one’s specialty may determine the feasibility of becoming friends with a patient. While Dr. Mehta has never done so, as a radiologist, she doesn’t usually see patients repeatedly. Likewise, a young gerontologist may have little in common with his octogenarian patients. And an older pediatrician is not in the same life stage as his patients’ sleep-deprived new parents, possibly making them less attractive friends.
However, practicing family medicine is all about long-term physician-patient relationships. Getting to know patients and their families over many years can lead to a certain intimacy. Dr. Rowland said that, while a wonderful part of being a physician is getting that unique trust whereby patients tell you all sorts of things about their lives, she’s never gone down the friendship path.
“There’s the assumption I’ll take care of someone for a long period of time, and their partner and their kids, maybe another generation or two,” Dr. Rowland said. “People really do rely on that relationship to contribute to their health.”
Worse, nowadays, when people may be starved for connection, many patients want to feel emotionally close and cared for by their doctor, so it’d be easy to cross the line. While patients deserve a compassionate, caring doctor, the physician is left to walk the line between those boundaries. Dr. Rowland said, “It’s up to the clinician to say: ‘My role is as a doctor. You deserve caring friends, but I have to order your mammogram and your blood counts. My role is different.’ ”
Friendly but not friends
It can be tricky to navigate the boundary between a cordial, warm relationship with a patient and that patient inviting you to their daughter’s wedding.
“People may mistake being pleasant and friendly for being friends,” said Larry Blosser, MD, chief medical officer at Central Ohio Primary Care, Westerville. In his position, he sometimes hears from patients who have misunderstood their relationship with a doctor in the practice. When that happens, he advises the physician to consider the persona they’re presenting to the patient. If you’re overly friendly, there’s the potential for confusion, but you can’t be aloof and cold, he said.
Maintaining that awareness helps to prevent a patient’s offhand invitation to catch a movie or go on a hike. And verbalizing it to your patients can make your relationship clear from the get-go.
“I tell patients we’re a team. I’m the captain, and they’re my MVP. When the match is over, whatever the results, we’re done,” said Karenne Fru, MD, PhD, a fertility specialist at Oma Fertility Atlanta. Making deep connections is essential to her practice, so Dr. Fru structures her patient interactions carefully. “Infertility is such an isolating experience. While you’re with us, we care about what’s going on in your life, your pets, and your mom’s chemo. We need mutual trust for you to be compliant with the care.”
However, that approach won’t work when you see patients regularly, as with family practice or specialties that see the same patients repeatedly throughout the year. In those circumstances, the match is never over but one in which the onus is on the physician to establish a friendly yet professional rapport without letting your self-interest, loneliness, or lack of friends interfere.
“It’s been a very difficult couple of years for a lot of us. Depending on what kind of clinical work we do, some of us took care of healthy people that got very sick or passed away,” Dr. Rowland said. “Having the chance to reconnect with people and reestablish some of that closeness, both physical and emotional, is going to be good for us.”
Just continue conveying warm, trusting compassion for your patients without blurring the friend lines.
A version of this article first appeared on Medscape.com.
More type 2 diabetes deaths from cancer than heart disease
Cancer appears to have overtaken cardiovascular disease (CVD) as a leading cause of death in adults with type 2 diabetes, a 20-year population study in England suggests.
The researchers found that, from 1998 to 2018, in more than 130,000 adults aged 35 and older with type 2 diabetes, all-cause mortality declined for all ages, but cancer mortality increased for those aged 75 and older; people with type 2 diabetes who were smokers had higher and steadily increasing cancer mortality rates; and people with type 2 diabetes had more than twice the rate of colorectal, pancreatic, liver, and endometrial cancer mortality than age- and sex-matched individuals in the general population.
The findings suggest that “cancer prevention strategies therefore deserve at least a similar level of attention as cardiovascular disease prevention, particularly in older people and for some cancers such as liver, colorectal, and pancreatic cancer,” the researchers wrote.
Tailored cancer prevention and early-detection strategies are needed to address persistent inequalities in the older population, the most deprived, and smokers, they added.
Breast cancer rates in younger women with type 2 diabetes rising
According to the researchers, “early cancer detection through changes to existing screening [programs], or more in-depth investigations for suspected/nonspecific symptoms, may reduce the number of avoidable cancer deaths in people with type 2 diabetes.”
Moreover, breast cancer rates in younger women with type 2 diabetes are rising by 4.1% per year, they wrote, which suggests such women are high risk and should be screened at a younger age, but screening age would need to be determined in cost-effectiveness analyses.
The study by Suping Ling, PhD, and colleagues was published online in Diabetologia.
Results challenge belief that preventing CVD is priority in type 2 diabetes
“The prevention of cardiovascular disease has been, and is still considered, a priority in people with diabetes,” the researchers wrote.
“Our results challenge this view by showing that cancer may have overtaken cardiovascular disease as a leading cause of death in people with type 2 diabetes.”
“The proportion of cancer deaths out of all-cause deaths remains high (> 30%) in young ages, and it was steadily increasing in older ages,” Dr. Ling, from the department of noncommunicable disease epidemiology, London School of Hygiene & Tropical Medicine, said in a comment.
“Combined with previous studies reporting decreasing CVD mortality rates,” she said, “we concluded that cancer might have overtaken CVD as the leading cause of death in people with type 2 diabetes.”
Many evidence-based cancer-prevention strategies related to lifestyle (such as being physically active, being a healthy weight, eating a better diet, stopping smoking, as summarized by the World Cancer Research Fund), are helpful for preventing both cancer and CVD, Ling observed.
However, in the medical community, many additional efforts were made for monitoring, early detection, and innovating medications for CVD, she noted. “Therefore, we would like to propose a similar level of attention and effort for cancer in people with type 2 diabetes.”
Deaths from cancer vs. all causes in patients with diabetes
The researchers identified 137,804 patients aged 35 and older who were newly diagnosed with type 2 diabetes from 1998 to 2018 in general practices in the UK that were part of the Clinical Practice Research Datalink.
Patients were a median age of 64 years and 45% were women. Most (83%) were White, followed by South Asian (3.5%), Black (2.0%), and other (3%); 8.4% had missing information for race. Patients had a median body mass index (BMI) of 30.6 kg/m2.
Researchers divided patients into socioeconomic quintiles of most to least deprived based on income, employment, education, and other factors. During a median follow-up of 8.4 years, there were 39,212 deaths (28.5%).
Cancer mortality in subgroups of patients with type 2 diabetes
Researchers analyzed annual deaths from cancer and from all causes over 20 years in subgroups of patients with type 2 diabetes.
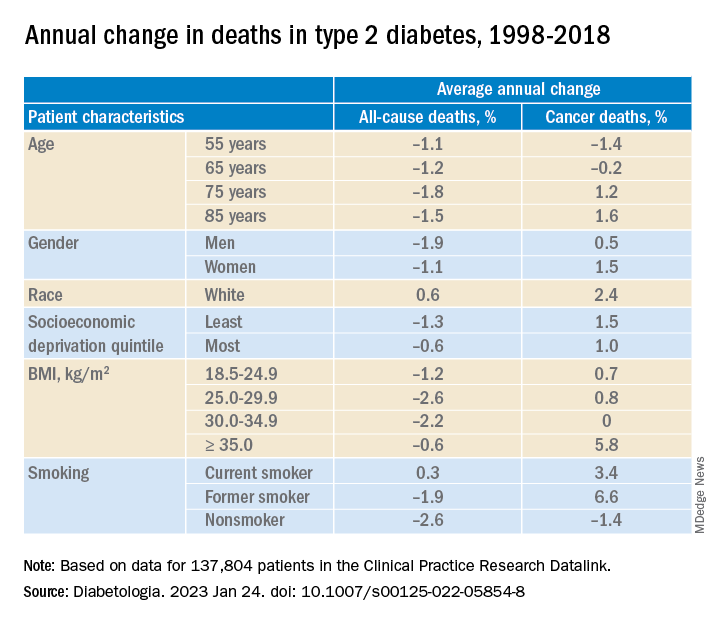
In adults with type 2 diabetes, the average percentage change in cancer mortality per year, from 1998 to 2018 decreased in people aged 55 and 65 (–1.4% and –0.2%, respectively), but increased in people aged 75 and 85 (1.2% and 1.6%, respectively); increased more in women than in men (1.5% vs 1.0%), although women had lower cancer mortality than men; and increased more in the least deprived (wealthiest) individuals than in the most deprived (1.5% vs 1.0%). Cancer mortality rates were consistently higher in the most deprived individuals, Dr. Ling noted.
Cancer mortality also increased more in people with class III obesity (BMI ≥ 35) versus normal weight (5.8% vs 0.7%) and versus other weights. In addition, there was an upward trend in cancer mortality in people who were White or former/current smokers.
Deaths from specific cancers in diabetes vs. general population
Next, researchers determined cancer mortality ratios – the cancer mortality of the patients with diabetes divided by the cancer mortality of the general population.
They determined this for all cancers, the four most common cancers in the United Kingdom (lung, colorectal, breast, and prostate), and cancers caused by type 2 diabetes (pancreatic, liver, gallbladder, and endometrial cancer), standardized by sex and age.
Mortality from all cancer was 18% higher in patients with type 2 diabetes, compared with the general population.
Overall, mortality from colorectal cancer, pancreatic cancer, and liver cancer was 2.4 times, 2.12 times, and 2.13 times higher, respectively, in patients with type 2 diabetes than in the general population.
Mortality from breast cancer was 9% higher and mortality from endometrial cancer was 2.08 times higher in women with type 2 diabetes than in women in the general population.
There was a constant upward trend for mortality rates for pancreatic, liver, and lung cancer at all ages, colorectal cancer at most ages, breast cancer at younger ages, and prostate and endometrial cancer at older ages.
The study was funded by Hope Against Cancer. Dr. Ling reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cancer appears to have overtaken cardiovascular disease (CVD) as a leading cause of death in adults with type 2 diabetes, a 20-year population study in England suggests.
The researchers found that, from 1998 to 2018, in more than 130,000 adults aged 35 and older with type 2 diabetes, all-cause mortality declined for all ages, but cancer mortality increased for those aged 75 and older; people with type 2 diabetes who were smokers had higher and steadily increasing cancer mortality rates; and people with type 2 diabetes had more than twice the rate of colorectal, pancreatic, liver, and endometrial cancer mortality than age- and sex-matched individuals in the general population.
The findings suggest that “cancer prevention strategies therefore deserve at least a similar level of attention as cardiovascular disease prevention, particularly in older people and for some cancers such as liver, colorectal, and pancreatic cancer,” the researchers wrote.
Tailored cancer prevention and early-detection strategies are needed to address persistent inequalities in the older population, the most deprived, and smokers, they added.
Breast cancer rates in younger women with type 2 diabetes rising
According to the researchers, “early cancer detection through changes to existing screening [programs], or more in-depth investigations for suspected/nonspecific symptoms, may reduce the number of avoidable cancer deaths in people with type 2 diabetes.”
Moreover, breast cancer rates in younger women with type 2 diabetes are rising by 4.1% per year, they wrote, which suggests such women are high risk and should be screened at a younger age, but screening age would need to be determined in cost-effectiveness analyses.
The study by Suping Ling, PhD, and colleagues was published online in Diabetologia.
Results challenge belief that preventing CVD is priority in type 2 diabetes
“The prevention of cardiovascular disease has been, and is still considered, a priority in people with diabetes,” the researchers wrote.
“Our results challenge this view by showing that cancer may have overtaken cardiovascular disease as a leading cause of death in people with type 2 diabetes.”
“The proportion of cancer deaths out of all-cause deaths remains high (> 30%) in young ages, and it was steadily increasing in older ages,” Dr. Ling, from the department of noncommunicable disease epidemiology, London School of Hygiene & Tropical Medicine, said in a comment.
“Combined with previous studies reporting decreasing CVD mortality rates,” she said, “we concluded that cancer might have overtaken CVD as the leading cause of death in people with type 2 diabetes.”
Many evidence-based cancer-prevention strategies related to lifestyle (such as being physically active, being a healthy weight, eating a better diet, stopping smoking, as summarized by the World Cancer Research Fund), are helpful for preventing both cancer and CVD, Ling observed.
However, in the medical community, many additional efforts were made for monitoring, early detection, and innovating medications for CVD, she noted. “Therefore, we would like to propose a similar level of attention and effort for cancer in people with type 2 diabetes.”
Deaths from cancer vs. all causes in patients with diabetes
The researchers identified 137,804 patients aged 35 and older who were newly diagnosed with type 2 diabetes from 1998 to 2018 in general practices in the UK that were part of the Clinical Practice Research Datalink.
Patients were a median age of 64 years and 45% were women. Most (83%) were White, followed by South Asian (3.5%), Black (2.0%), and other (3%); 8.4% had missing information for race. Patients had a median body mass index (BMI) of 30.6 kg/m2.
Researchers divided patients into socioeconomic quintiles of most to least deprived based on income, employment, education, and other factors. During a median follow-up of 8.4 years, there were 39,212 deaths (28.5%).
Cancer mortality in subgroups of patients with type 2 diabetes
Researchers analyzed annual deaths from cancer and from all causes over 20 years in subgroups of patients with type 2 diabetes.

In adults with type 2 diabetes, the average percentage change in cancer mortality per year, from 1998 to 2018 decreased in people aged 55 and 65 (–1.4% and –0.2%, respectively), but increased in people aged 75 and 85 (1.2% and 1.6%, respectively); increased more in women than in men (1.5% vs 1.0%), although women had lower cancer mortality than men; and increased more in the least deprived (wealthiest) individuals than in the most deprived (1.5% vs 1.0%). Cancer mortality rates were consistently higher in the most deprived individuals, Dr. Ling noted.
Cancer mortality also increased more in people with class III obesity (BMI ≥ 35) versus normal weight (5.8% vs 0.7%) and versus other weights. In addition, there was an upward trend in cancer mortality in people who were White or former/current smokers.
Deaths from specific cancers in diabetes vs. general population
Next, researchers determined cancer mortality ratios – the cancer mortality of the patients with diabetes divided by the cancer mortality of the general population.
They determined this for all cancers, the four most common cancers in the United Kingdom (lung, colorectal, breast, and prostate), and cancers caused by type 2 diabetes (pancreatic, liver, gallbladder, and endometrial cancer), standardized by sex and age.
Mortality from all cancer was 18% higher in patients with type 2 diabetes, compared with the general population.
Overall, mortality from colorectal cancer, pancreatic cancer, and liver cancer was 2.4 times, 2.12 times, and 2.13 times higher, respectively, in patients with type 2 diabetes than in the general population.
Mortality from breast cancer was 9% higher and mortality from endometrial cancer was 2.08 times higher in women with type 2 diabetes than in women in the general population.
There was a constant upward trend for mortality rates for pancreatic, liver, and lung cancer at all ages, colorectal cancer at most ages, breast cancer at younger ages, and prostate and endometrial cancer at older ages.
The study was funded by Hope Against Cancer. Dr. Ling reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cancer appears to have overtaken cardiovascular disease (CVD) as a leading cause of death in adults with type 2 diabetes, a 20-year population study in England suggests.
The researchers found that, from 1998 to 2018, in more than 130,000 adults aged 35 and older with type 2 diabetes, all-cause mortality declined for all ages, but cancer mortality increased for those aged 75 and older; people with type 2 diabetes who were smokers had higher and steadily increasing cancer mortality rates; and people with type 2 diabetes had more than twice the rate of colorectal, pancreatic, liver, and endometrial cancer mortality than age- and sex-matched individuals in the general population.
The findings suggest that “cancer prevention strategies therefore deserve at least a similar level of attention as cardiovascular disease prevention, particularly in older people and for some cancers such as liver, colorectal, and pancreatic cancer,” the researchers wrote.
Tailored cancer prevention and early-detection strategies are needed to address persistent inequalities in the older population, the most deprived, and smokers, they added.
Breast cancer rates in younger women with type 2 diabetes rising
According to the researchers, “early cancer detection through changes to existing screening [programs], or more in-depth investigations for suspected/nonspecific symptoms, may reduce the number of avoidable cancer deaths in people with type 2 diabetes.”
Moreover, breast cancer rates in younger women with type 2 diabetes are rising by 4.1% per year, they wrote, which suggests such women are high risk and should be screened at a younger age, but screening age would need to be determined in cost-effectiveness analyses.
The study by Suping Ling, PhD, and colleagues was published online in Diabetologia.
Results challenge belief that preventing CVD is priority in type 2 diabetes
“The prevention of cardiovascular disease has been, and is still considered, a priority in people with diabetes,” the researchers wrote.
“Our results challenge this view by showing that cancer may have overtaken cardiovascular disease as a leading cause of death in people with type 2 diabetes.”
“The proportion of cancer deaths out of all-cause deaths remains high (> 30%) in young ages, and it was steadily increasing in older ages,” Dr. Ling, from the department of noncommunicable disease epidemiology, London School of Hygiene & Tropical Medicine, said in a comment.
“Combined with previous studies reporting decreasing CVD mortality rates,” she said, “we concluded that cancer might have overtaken CVD as the leading cause of death in people with type 2 diabetes.”
Many evidence-based cancer-prevention strategies related to lifestyle (such as being physically active, being a healthy weight, eating a better diet, stopping smoking, as summarized by the World Cancer Research Fund), are helpful for preventing both cancer and CVD, Ling observed.
However, in the medical community, many additional efforts were made for monitoring, early detection, and innovating medications for CVD, she noted. “Therefore, we would like to propose a similar level of attention and effort for cancer in people with type 2 diabetes.”
Deaths from cancer vs. all causes in patients with diabetes
The researchers identified 137,804 patients aged 35 and older who were newly diagnosed with type 2 diabetes from 1998 to 2018 in general practices in the UK that were part of the Clinical Practice Research Datalink.
Patients were a median age of 64 years and 45% were women. Most (83%) were White, followed by South Asian (3.5%), Black (2.0%), and other (3%); 8.4% had missing information for race. Patients had a median body mass index (BMI) of 30.6 kg/m2.
Researchers divided patients into socioeconomic quintiles of most to least deprived based on income, employment, education, and other factors. During a median follow-up of 8.4 years, there were 39,212 deaths (28.5%).
Cancer mortality in subgroups of patients with type 2 diabetes
Researchers analyzed annual deaths from cancer and from all causes over 20 years in subgroups of patients with type 2 diabetes.

In adults with type 2 diabetes, the average percentage change in cancer mortality per year, from 1998 to 2018 decreased in people aged 55 and 65 (–1.4% and –0.2%, respectively), but increased in people aged 75 and 85 (1.2% and 1.6%, respectively); increased more in women than in men (1.5% vs 1.0%), although women had lower cancer mortality than men; and increased more in the least deprived (wealthiest) individuals than in the most deprived (1.5% vs 1.0%). Cancer mortality rates were consistently higher in the most deprived individuals, Dr. Ling noted.
Cancer mortality also increased more in people with class III obesity (BMI ≥ 35) versus normal weight (5.8% vs 0.7%) and versus other weights. In addition, there was an upward trend in cancer mortality in people who were White or former/current smokers.
Deaths from specific cancers in diabetes vs. general population
Next, researchers determined cancer mortality ratios – the cancer mortality of the patients with diabetes divided by the cancer mortality of the general population.
They determined this for all cancers, the four most common cancers in the United Kingdom (lung, colorectal, breast, and prostate), and cancers caused by type 2 diabetes (pancreatic, liver, gallbladder, and endometrial cancer), standardized by sex and age.
Mortality from all cancer was 18% higher in patients with type 2 diabetes, compared with the general population.
Overall, mortality from colorectal cancer, pancreatic cancer, and liver cancer was 2.4 times, 2.12 times, and 2.13 times higher, respectively, in patients with type 2 diabetes than in the general population.
Mortality from breast cancer was 9% higher and mortality from endometrial cancer was 2.08 times higher in women with type 2 diabetes than in women in the general population.
There was a constant upward trend for mortality rates for pancreatic, liver, and lung cancer at all ages, colorectal cancer at most ages, breast cancer at younger ages, and prostate and endometrial cancer at older ages.
The study was funded by Hope Against Cancer. Dr. Ling reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM DIABETOLOGIA


