User login
17-year-old girl • abdominal pain • lower-leg itching • dark urine and yellow eyes • Dx?
THE CASE
A 17-year-old White girl with no known past medical history presented to the emergency department (ED) with complaints of abdominal pain and pruritus. The abdominal pain had started 9 days prior and lasted for 3 days. One day after resolution, she developed bilateral lower extremity itching, which was not relieved with loratadine.
Review of systems included dark urine and yellow eyes noted for several days. The patient denied nausea, vomiting, diarrhea, constipation, fevers, chills, arthralgias, recent illness, travel, or sick contacts. Immunizations were up to date. The patient had no history of surgery or liver disease and no pertinent family history. Her current medications included ethinyl estradiol/norethindrone acetate for birth control and minocycline for acne vulgaris. She had been taking the latter medication for 2 years. No additional medications were noted, including vitamins, over-the-counter medications, or supplements. She denied smoking and alcohol or recreational drug use.
In the ED, the patient had normal vital signs. Physical exam findings included bilateral scleral icterus and scattered skin excoriations on the hands, arms, back of the neck, and feet. At the time of hospital admission, the patient’s minocycline and birth control were held under the initial presumption that one or both might be contributing to her presentation.
Pertinent laboratory findings included aspartate transaminase (AST), 828 U/L (normal range, 2-40 U/L); alanine aminotransferase (ALT), 784 U/L (normal range, 3-30 U/L); lactic acid dehydrogenase, 520 U/L (normal range, 140-280 U/L); alkaline phosphatase, 119 U/L (normal range, 44-147 U/L); total bilirubin, 1.9 µmol/L (normal range, 2-18 µmol/L); and direct bilirubin, 1.3 µmol/L (normal range, 0-4 µmol/L). Baseline liver function test results (prior to admission) were unknown. Results of a coagulation panel, complete blood count, basic metabolic panel, amylase, lipase, urine toxicology, and urinalysis all were within normal limits.
Ultrasound of the abdomen revealed a normal abdomen, liver, pancreas, gallbladder, and common bile duct. This imaging study was negative for other obstructive pathologies.
THE DIAGNOSIS
During hospital admission, a noninvasive liver work-up was pursued by Gastroenterology. A hepatitis panel, Epstein-Barr virus testing, and levels of ceruloplasmin and acetaminophen were all found to be within normal limits, excluding additional causes of liver disease. Serum antinuclear antibody (ANA) testing was significantly positive, with a titer of 1:640 (range, < 1:20) and, as noted above, liver transaminases were severely elevated, leading to a presumptive diagnosis of drug-induced liver pathology.
Continue to: During outpatient follow-up...
During outpatient follow-up with Gastroenterology 2 days after discharge, the patient’s liver transaminases and bilirubin continued to trend upward (to a maximum ALT of 871 U/L; AST, 1097 U/L; alkaline phosphatase, 122 U/L; and bilirubin, 2.9 µmol/L). Immunoglobulin G was 1342 mg/mL (normal range, 694-1618 mg/mL).
An ultrasound-guided liver biopsy was performed; it demonstrated lobular, portal, and periportal hepatitis with focal bridging necrosis, consistent with a diagnosis of autoimmune hepatitis. Mild-to-moderate focal cholestasis was demonstrated, consistent with cholestatic hepatitis.
DISCUSSION
Autoimmune hepatitis is characterized by inflammation of the liver, secondary to the presence of circulating antibodies or hypergammaglobulinemia. The pathogenesis is thought to involve a T-cell–mediated immune attack on the liver. Based on case reports,the use of minocycline is associated with risk for liver injury, although the incidence is rare.1-4 Use of this medication may be associated with autoimmune disease in patients who are predisposed to autoimmune tendencies or who have genetic predeterminants.
Diagnosis is typically made based on abnormalities in aminotransferases (AST, ALT), elevation in serum immunoglobulins, and positive auto-antibody titers including ANA, smooth muscle antibodies, and anti-liver kidney microsomal type 1 antibodies. Although clinical presentations tend to differ, the confirmatory diagnosis is typically made histologically, with the presence of lobular and perivenular necro-inflammatory changes and plasma cell infiltration.5
Other infectious and metabolic causes of hepatitis should be excluded. Many medications and herbal agents have been noted to cause autoimmune hepatitis or similar syndromes that mimic the condition.
Medication history. Review of the case patient’s medication list identified ethinyl estradiol/norethindrone acetate and minocycline as potential culprits. Ethinyl estradiol/norethindrone acetate is a low-dose combination oral contraceptive pill (OCP). Although earlier formulations of OCPs were associated with hepatobiliary complications, these adverse effects are noted to be rare in the absence of predisposing conditions.6 In some cases, OCPs have been linked to cholestasis, chronic hepatocellular carcinoma, or hepatic adenomas, but studies have shown that these medications do not affect the course of acute liver failure.7
Continue to: Minocycline...
Minocycline is a second-generation tetracycline commonly used to treat acne vulgaris. Long-term treatment with minocycline has been associated with severe adverse effects, including autoimmune and hypersensitivity reactions.8 Minocycline-associated hepatotoxicity can be due to a systemic hypersensitivity reaction, occurring within a few weeks of therapy initiation, whereas autoimmune hepatitis manifests after a year or more of exposure to the medication (as in this case). Patients may present acutely several months after starting the medication, with symptoms of jaundice, fatigue, and/or joint aches. The acute liver injury is typically self-limited and often resolves with cessation of the drug. However, patients may require corticosteroids and immunosuppressive therapy.
Which is it? Histologically, drug-induced autoimmune hepatitis is indistinguishable from idiopathic autoimmune hepatitis.3 The estimated incidence of idiopathic autoimmune liver disease ranges from 0.7 to 2 out of 100,000 population.9 A systematic review of the literature identified 65 reported cases of liver damage associated with minocycline specifically.1
In this case, given the patient’s 2-year history of minocycline use, it is possible that she developed an acute presentation of autoimmune hepatitis. With drug-induced autoimmune liver injury, complete resolution occurs after withdrawal of the offending medication, and a response to corticosteroid therapy supports the diagnosis. Recurrence of signs or symptoms following corticosteroid cessation may indicate idiopathic autoimmune hepatitis as opposed to a drug-induced form.2
Our patient was started on steroid and immunomodulator therapy, with prednisone 40 mg/d and mycophenolate 250 mg bid. At follow-up with Gastroenterology, the patient’s symptoms and liver function test results had improved significantly (AST, 27 U/L; ALT, 14 U/L; alkaline phosphatase, 51 U/L; and total bilirubin, 0.4 µmol/L). The patient was continued on a prednisone taper while simultaneously titrating mycophenolate. The ultimate plan of care included continuing mycophenolate for a total of 4 to 5 years.
THE TAKEAWAY
During evaluation of a patient with new-onset liver disease, it is important to inquire about prescription medications, drugs, vitamins, and herbal supplements as possible contributors to the disease process. This case highlights the importance of monitoring patients while on minocycline and of weighing the risks vs benefits of long-term therapy. It has been suggested that liver enzymes be tested before therapy initiation and about every 3 months during long-term antibiotic treatment.4 Careful consideration and caution should be taken prior to the initiation of medications that have been linked to rare, but important, adverse reactions.
ACKNOWLEDGEMENT
The authors would like to thank Frank Bauer, MD, and Eva Sotil, MD, for their contributions to this case presentation.
CORRESPONDENCE
Andrea Gillis, DO, Asylum Hill Family Medicine Center, 99 Woodland Street, Hartford, CT 06105; andrea.gillis@ trinityhealthofne.org
1. Lawrenson RA, Seaman HE, Sundström A, et al. Liver damage associated with minocycline use in acne: a systematic review of the published literature and pharmacovigilance data. Drug Saf. 2000;23:333-349.
2. Teitelbaum JE, Perez-Atayde AR, Cohen M, et al. Minocycline-related autoimmune hepatitis case series and literature review. Arch Pediatr Adolesc Med. 1998;152:1132-1136.
3. Goldstein NS, Bayati N, Silverman AL, et al. Minocycline as a cause of drug induced autoimmune hepatitis: report of four cases and comparison with autoimmune hepatitis. Am J Clinic Pathol. 2000;114:591-598.
4. Ramakrishna J, Johnson AR, Banner BF. Long-term minocycline use for acne in healthy adolescents can cause severe autoimmune hepatitis. J Clin Gastroenterol. 2009;43:787-790.
5. Nguyen Canh H, Harada K, Ouchi H, et al. Acute presentation of autoimmune hepatitis: a multicentre study with detailed histological evaluation in a large cohort of patients. J Clin Pathol. 2017;70:961-969.
6. Lindberg MC. Hepatobiliary complications of oral contraceptives. J Gen Intern Med. 1992; 7:199-209.
7. Kapp N, Tilley IB, Curtis KM. The effects of hormonal contraceptive use among women with viral hepatitis or cirrhosis of the liver: a systematic review. Contraception. 2009;80:381-386.
8. DeLemos AS, Foureau DM, Jacobs C, et al. Drug-induced liver injury with autoimmune features. Semin Liver Dis. 2014;34:194-204.
9. Jepsen P, Gronbaek L, Vilstrup H. Worldwide incidence of autoimmune liver disease. Dig Dis. 2015;33(suppl 2):2-12.
THE CASE
A 17-year-old White girl with no known past medical history presented to the emergency department (ED) with complaints of abdominal pain and pruritus. The abdominal pain had started 9 days prior and lasted for 3 days. One day after resolution, she developed bilateral lower extremity itching, which was not relieved with loratadine.
Review of systems included dark urine and yellow eyes noted for several days. The patient denied nausea, vomiting, diarrhea, constipation, fevers, chills, arthralgias, recent illness, travel, or sick contacts. Immunizations were up to date. The patient had no history of surgery or liver disease and no pertinent family history. Her current medications included ethinyl estradiol/norethindrone acetate for birth control and minocycline for acne vulgaris. She had been taking the latter medication for 2 years. No additional medications were noted, including vitamins, over-the-counter medications, or supplements. She denied smoking and alcohol or recreational drug use.
In the ED, the patient had normal vital signs. Physical exam findings included bilateral scleral icterus and scattered skin excoriations on the hands, arms, back of the neck, and feet. At the time of hospital admission, the patient’s minocycline and birth control were held under the initial presumption that one or both might be contributing to her presentation.
Pertinent laboratory findings included aspartate transaminase (AST), 828 U/L (normal range, 2-40 U/L); alanine aminotransferase (ALT), 784 U/L (normal range, 3-30 U/L); lactic acid dehydrogenase, 520 U/L (normal range, 140-280 U/L); alkaline phosphatase, 119 U/L (normal range, 44-147 U/L); total bilirubin, 1.9 µmol/L (normal range, 2-18 µmol/L); and direct bilirubin, 1.3 µmol/L (normal range, 0-4 µmol/L). Baseline liver function test results (prior to admission) were unknown. Results of a coagulation panel, complete blood count, basic metabolic panel, amylase, lipase, urine toxicology, and urinalysis all were within normal limits.
Ultrasound of the abdomen revealed a normal abdomen, liver, pancreas, gallbladder, and common bile duct. This imaging study was negative for other obstructive pathologies.
THE DIAGNOSIS
During hospital admission, a noninvasive liver work-up was pursued by Gastroenterology. A hepatitis panel, Epstein-Barr virus testing, and levels of ceruloplasmin and acetaminophen were all found to be within normal limits, excluding additional causes of liver disease. Serum antinuclear antibody (ANA) testing was significantly positive, with a titer of 1:640 (range, < 1:20) and, as noted above, liver transaminases were severely elevated, leading to a presumptive diagnosis of drug-induced liver pathology.
Continue to: During outpatient follow-up...
During outpatient follow-up with Gastroenterology 2 days after discharge, the patient’s liver transaminases and bilirubin continued to trend upward (to a maximum ALT of 871 U/L; AST, 1097 U/L; alkaline phosphatase, 122 U/L; and bilirubin, 2.9 µmol/L). Immunoglobulin G was 1342 mg/mL (normal range, 694-1618 mg/mL).
An ultrasound-guided liver biopsy was performed; it demonstrated lobular, portal, and periportal hepatitis with focal bridging necrosis, consistent with a diagnosis of autoimmune hepatitis. Mild-to-moderate focal cholestasis was demonstrated, consistent with cholestatic hepatitis.
DISCUSSION
Autoimmune hepatitis is characterized by inflammation of the liver, secondary to the presence of circulating antibodies or hypergammaglobulinemia. The pathogenesis is thought to involve a T-cell–mediated immune attack on the liver. Based on case reports,the use of minocycline is associated with risk for liver injury, although the incidence is rare.1-4 Use of this medication may be associated with autoimmune disease in patients who are predisposed to autoimmune tendencies or who have genetic predeterminants.
Diagnosis is typically made based on abnormalities in aminotransferases (AST, ALT), elevation in serum immunoglobulins, and positive auto-antibody titers including ANA, smooth muscle antibodies, and anti-liver kidney microsomal type 1 antibodies. Although clinical presentations tend to differ, the confirmatory diagnosis is typically made histologically, with the presence of lobular and perivenular necro-inflammatory changes and plasma cell infiltration.5
Other infectious and metabolic causes of hepatitis should be excluded. Many medications and herbal agents have been noted to cause autoimmune hepatitis or similar syndromes that mimic the condition.
Medication history. Review of the case patient’s medication list identified ethinyl estradiol/norethindrone acetate and minocycline as potential culprits. Ethinyl estradiol/norethindrone acetate is a low-dose combination oral contraceptive pill (OCP). Although earlier formulations of OCPs were associated with hepatobiliary complications, these adverse effects are noted to be rare in the absence of predisposing conditions.6 In some cases, OCPs have been linked to cholestasis, chronic hepatocellular carcinoma, or hepatic adenomas, but studies have shown that these medications do not affect the course of acute liver failure.7
Continue to: Minocycline...
Minocycline is a second-generation tetracycline commonly used to treat acne vulgaris. Long-term treatment with minocycline has been associated with severe adverse effects, including autoimmune and hypersensitivity reactions.8 Minocycline-associated hepatotoxicity can be due to a systemic hypersensitivity reaction, occurring within a few weeks of therapy initiation, whereas autoimmune hepatitis manifests after a year or more of exposure to the medication (as in this case). Patients may present acutely several months after starting the medication, with symptoms of jaundice, fatigue, and/or joint aches. The acute liver injury is typically self-limited and often resolves with cessation of the drug. However, patients may require corticosteroids and immunosuppressive therapy.
Which is it? Histologically, drug-induced autoimmune hepatitis is indistinguishable from idiopathic autoimmune hepatitis.3 The estimated incidence of idiopathic autoimmune liver disease ranges from 0.7 to 2 out of 100,000 population.9 A systematic review of the literature identified 65 reported cases of liver damage associated with minocycline specifically.1
In this case, given the patient’s 2-year history of minocycline use, it is possible that she developed an acute presentation of autoimmune hepatitis. With drug-induced autoimmune liver injury, complete resolution occurs after withdrawal of the offending medication, and a response to corticosteroid therapy supports the diagnosis. Recurrence of signs or symptoms following corticosteroid cessation may indicate idiopathic autoimmune hepatitis as opposed to a drug-induced form.2
Our patient was started on steroid and immunomodulator therapy, with prednisone 40 mg/d and mycophenolate 250 mg bid. At follow-up with Gastroenterology, the patient’s symptoms and liver function test results had improved significantly (AST, 27 U/L; ALT, 14 U/L; alkaline phosphatase, 51 U/L; and total bilirubin, 0.4 µmol/L). The patient was continued on a prednisone taper while simultaneously titrating mycophenolate. The ultimate plan of care included continuing mycophenolate for a total of 4 to 5 years.
THE TAKEAWAY
During evaluation of a patient with new-onset liver disease, it is important to inquire about prescription medications, drugs, vitamins, and herbal supplements as possible contributors to the disease process. This case highlights the importance of monitoring patients while on minocycline and of weighing the risks vs benefits of long-term therapy. It has been suggested that liver enzymes be tested before therapy initiation and about every 3 months during long-term antibiotic treatment.4 Careful consideration and caution should be taken prior to the initiation of medications that have been linked to rare, but important, adverse reactions.
ACKNOWLEDGEMENT
The authors would like to thank Frank Bauer, MD, and Eva Sotil, MD, for their contributions to this case presentation.
CORRESPONDENCE
Andrea Gillis, DO, Asylum Hill Family Medicine Center, 99 Woodland Street, Hartford, CT 06105; andrea.gillis@ trinityhealthofne.org
THE CASE
A 17-year-old White girl with no known past medical history presented to the emergency department (ED) with complaints of abdominal pain and pruritus. The abdominal pain had started 9 days prior and lasted for 3 days. One day after resolution, she developed bilateral lower extremity itching, which was not relieved with loratadine.
Review of systems included dark urine and yellow eyes noted for several days. The patient denied nausea, vomiting, diarrhea, constipation, fevers, chills, arthralgias, recent illness, travel, or sick contacts. Immunizations were up to date. The patient had no history of surgery or liver disease and no pertinent family history. Her current medications included ethinyl estradiol/norethindrone acetate for birth control and minocycline for acne vulgaris. She had been taking the latter medication for 2 years. No additional medications were noted, including vitamins, over-the-counter medications, or supplements. She denied smoking and alcohol or recreational drug use.
In the ED, the patient had normal vital signs. Physical exam findings included bilateral scleral icterus and scattered skin excoriations on the hands, arms, back of the neck, and feet. At the time of hospital admission, the patient’s minocycline and birth control were held under the initial presumption that one or both might be contributing to her presentation.
Pertinent laboratory findings included aspartate transaminase (AST), 828 U/L (normal range, 2-40 U/L); alanine aminotransferase (ALT), 784 U/L (normal range, 3-30 U/L); lactic acid dehydrogenase, 520 U/L (normal range, 140-280 U/L); alkaline phosphatase, 119 U/L (normal range, 44-147 U/L); total bilirubin, 1.9 µmol/L (normal range, 2-18 µmol/L); and direct bilirubin, 1.3 µmol/L (normal range, 0-4 µmol/L). Baseline liver function test results (prior to admission) were unknown. Results of a coagulation panel, complete blood count, basic metabolic panel, amylase, lipase, urine toxicology, and urinalysis all were within normal limits.
Ultrasound of the abdomen revealed a normal abdomen, liver, pancreas, gallbladder, and common bile duct. This imaging study was negative for other obstructive pathologies.
THE DIAGNOSIS
During hospital admission, a noninvasive liver work-up was pursued by Gastroenterology. A hepatitis panel, Epstein-Barr virus testing, and levels of ceruloplasmin and acetaminophen were all found to be within normal limits, excluding additional causes of liver disease. Serum antinuclear antibody (ANA) testing was significantly positive, with a titer of 1:640 (range, < 1:20) and, as noted above, liver transaminases were severely elevated, leading to a presumptive diagnosis of drug-induced liver pathology.
Continue to: During outpatient follow-up...
During outpatient follow-up with Gastroenterology 2 days after discharge, the patient’s liver transaminases and bilirubin continued to trend upward (to a maximum ALT of 871 U/L; AST, 1097 U/L; alkaline phosphatase, 122 U/L; and bilirubin, 2.9 µmol/L). Immunoglobulin G was 1342 mg/mL (normal range, 694-1618 mg/mL).
An ultrasound-guided liver biopsy was performed; it demonstrated lobular, portal, and periportal hepatitis with focal bridging necrosis, consistent with a diagnosis of autoimmune hepatitis. Mild-to-moderate focal cholestasis was demonstrated, consistent with cholestatic hepatitis.
DISCUSSION
Autoimmune hepatitis is characterized by inflammation of the liver, secondary to the presence of circulating antibodies or hypergammaglobulinemia. The pathogenesis is thought to involve a T-cell–mediated immune attack on the liver. Based on case reports,the use of minocycline is associated with risk for liver injury, although the incidence is rare.1-4 Use of this medication may be associated with autoimmune disease in patients who are predisposed to autoimmune tendencies or who have genetic predeterminants.
Diagnosis is typically made based on abnormalities in aminotransferases (AST, ALT), elevation in serum immunoglobulins, and positive auto-antibody titers including ANA, smooth muscle antibodies, and anti-liver kidney microsomal type 1 antibodies. Although clinical presentations tend to differ, the confirmatory diagnosis is typically made histologically, with the presence of lobular and perivenular necro-inflammatory changes and plasma cell infiltration.5
Other infectious and metabolic causes of hepatitis should be excluded. Many medications and herbal agents have been noted to cause autoimmune hepatitis or similar syndromes that mimic the condition.
Medication history. Review of the case patient’s medication list identified ethinyl estradiol/norethindrone acetate and minocycline as potential culprits. Ethinyl estradiol/norethindrone acetate is a low-dose combination oral contraceptive pill (OCP). Although earlier formulations of OCPs were associated with hepatobiliary complications, these adverse effects are noted to be rare in the absence of predisposing conditions.6 In some cases, OCPs have been linked to cholestasis, chronic hepatocellular carcinoma, or hepatic adenomas, but studies have shown that these medications do not affect the course of acute liver failure.7
Continue to: Minocycline...
Minocycline is a second-generation tetracycline commonly used to treat acne vulgaris. Long-term treatment with minocycline has been associated with severe adverse effects, including autoimmune and hypersensitivity reactions.8 Minocycline-associated hepatotoxicity can be due to a systemic hypersensitivity reaction, occurring within a few weeks of therapy initiation, whereas autoimmune hepatitis manifests after a year or more of exposure to the medication (as in this case). Patients may present acutely several months after starting the medication, with symptoms of jaundice, fatigue, and/or joint aches. The acute liver injury is typically self-limited and often resolves with cessation of the drug. However, patients may require corticosteroids and immunosuppressive therapy.
Which is it? Histologically, drug-induced autoimmune hepatitis is indistinguishable from idiopathic autoimmune hepatitis.3 The estimated incidence of idiopathic autoimmune liver disease ranges from 0.7 to 2 out of 100,000 population.9 A systematic review of the literature identified 65 reported cases of liver damage associated with minocycline specifically.1
In this case, given the patient’s 2-year history of minocycline use, it is possible that she developed an acute presentation of autoimmune hepatitis. With drug-induced autoimmune liver injury, complete resolution occurs after withdrawal of the offending medication, and a response to corticosteroid therapy supports the diagnosis. Recurrence of signs or symptoms following corticosteroid cessation may indicate idiopathic autoimmune hepatitis as opposed to a drug-induced form.2
Our patient was started on steroid and immunomodulator therapy, with prednisone 40 mg/d and mycophenolate 250 mg bid. At follow-up with Gastroenterology, the patient’s symptoms and liver function test results had improved significantly (AST, 27 U/L; ALT, 14 U/L; alkaline phosphatase, 51 U/L; and total bilirubin, 0.4 µmol/L). The patient was continued on a prednisone taper while simultaneously titrating mycophenolate. The ultimate plan of care included continuing mycophenolate for a total of 4 to 5 years.
THE TAKEAWAY
During evaluation of a patient with new-onset liver disease, it is important to inquire about prescription medications, drugs, vitamins, and herbal supplements as possible contributors to the disease process. This case highlights the importance of monitoring patients while on minocycline and of weighing the risks vs benefits of long-term therapy. It has been suggested that liver enzymes be tested before therapy initiation and about every 3 months during long-term antibiotic treatment.4 Careful consideration and caution should be taken prior to the initiation of medications that have been linked to rare, but important, adverse reactions.
ACKNOWLEDGEMENT
The authors would like to thank Frank Bauer, MD, and Eva Sotil, MD, for their contributions to this case presentation.
CORRESPONDENCE
Andrea Gillis, DO, Asylum Hill Family Medicine Center, 99 Woodland Street, Hartford, CT 06105; andrea.gillis@ trinityhealthofne.org
1. Lawrenson RA, Seaman HE, Sundström A, et al. Liver damage associated with minocycline use in acne: a systematic review of the published literature and pharmacovigilance data. Drug Saf. 2000;23:333-349.
2. Teitelbaum JE, Perez-Atayde AR, Cohen M, et al. Minocycline-related autoimmune hepatitis case series and literature review. Arch Pediatr Adolesc Med. 1998;152:1132-1136.
3. Goldstein NS, Bayati N, Silverman AL, et al. Minocycline as a cause of drug induced autoimmune hepatitis: report of four cases and comparison with autoimmune hepatitis. Am J Clinic Pathol. 2000;114:591-598.
4. Ramakrishna J, Johnson AR, Banner BF. Long-term minocycline use for acne in healthy adolescents can cause severe autoimmune hepatitis. J Clin Gastroenterol. 2009;43:787-790.
5. Nguyen Canh H, Harada K, Ouchi H, et al. Acute presentation of autoimmune hepatitis: a multicentre study with detailed histological evaluation in a large cohort of patients. J Clin Pathol. 2017;70:961-969.
6. Lindberg MC. Hepatobiliary complications of oral contraceptives. J Gen Intern Med. 1992; 7:199-209.
7. Kapp N, Tilley IB, Curtis KM. The effects of hormonal contraceptive use among women with viral hepatitis or cirrhosis of the liver: a systematic review. Contraception. 2009;80:381-386.
8. DeLemos AS, Foureau DM, Jacobs C, et al. Drug-induced liver injury with autoimmune features. Semin Liver Dis. 2014;34:194-204.
9. Jepsen P, Gronbaek L, Vilstrup H. Worldwide incidence of autoimmune liver disease. Dig Dis. 2015;33(suppl 2):2-12.
1. Lawrenson RA, Seaman HE, Sundström A, et al. Liver damage associated with minocycline use in acne: a systematic review of the published literature and pharmacovigilance data. Drug Saf. 2000;23:333-349.
2. Teitelbaum JE, Perez-Atayde AR, Cohen M, et al. Minocycline-related autoimmune hepatitis case series and literature review. Arch Pediatr Adolesc Med. 1998;152:1132-1136.
3. Goldstein NS, Bayati N, Silverman AL, et al. Minocycline as a cause of drug induced autoimmune hepatitis: report of four cases and comparison with autoimmune hepatitis. Am J Clinic Pathol. 2000;114:591-598.
4. Ramakrishna J, Johnson AR, Banner BF. Long-term minocycline use for acne in healthy adolescents can cause severe autoimmune hepatitis. J Clin Gastroenterol. 2009;43:787-790.
5. Nguyen Canh H, Harada K, Ouchi H, et al. Acute presentation of autoimmune hepatitis: a multicentre study with detailed histological evaluation in a large cohort of patients. J Clin Pathol. 2017;70:961-969.
6. Lindberg MC. Hepatobiliary complications of oral contraceptives. J Gen Intern Med. 1992; 7:199-209.
7. Kapp N, Tilley IB, Curtis KM. The effects of hormonal contraceptive use among women with viral hepatitis or cirrhosis of the liver: a systematic review. Contraception. 2009;80:381-386.
8. DeLemos AS, Foureau DM, Jacobs C, et al. Drug-induced liver injury with autoimmune features. Semin Liver Dis. 2014;34:194-204.
9. Jepsen P, Gronbaek L, Vilstrup H. Worldwide incidence of autoimmune liver disease. Dig Dis. 2015;33(suppl 2):2-12.
Herpes Zoster May Be a Marker for COVID-19 Infection During Pregnancy
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the most recently identified member of the zoonotic pathogens of coronaviruses. It caused an outbreak of pneumonia in December 2019 in Wuhan, China.1 Among all related acute respiratory syndromes (SARS-CoV, Middle East respiratory syndrome coronavirus), SARS-CoV-2 remains to be the most infectious, has the highest potential for human transmission, and can eventually result in acute respiratory distress syndrome.2,3
Only 15% of coronavirus disease 2019 (COVID-19) cases progress to pneumonia, and approximately 5% of these cases develop acute respiratory distress syndrome, septic shock, and/or multiple organ failure. The majority of cases only exhibit mild to moderate symptoms.4,5 A wide array of skin manifestations in COVID-19 infection have been reported, including maculopapular eruptions, morbilliform rashes, urticaria, chickenpoxlike lesions, livedo reticularis, COVID toes, erythema multiforme, pityriasis rosea, and several other patterns.6 We report a case of herpes zoster (HZ) complication in a COVID-19–positive woman who was 27 weeks pregnant.
Case Report
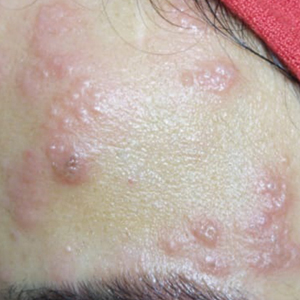
A 36-year-old woman who was 27 weeks pregnant was referred by her obstetrician to the dermatology clinic. She presented with a low-grade fever and a vesicular painful rash. Physical examination revealed painful, itchy, dysesthetic papules and vesicles on the left side of the forehead along with mild edema of the left upper eyelid but no watering of the eye or photophobia. She reported episodes of fever (temperature, 38.9°C), fatigue, and myalgia over the last week. She had bouts of dyspnea and tachycardia that she thought were related to being in the late second trimester of pregnancy. The area surrounding the vesicular eruption was tender to touch. No dry cough or any gastrointestinal or urinary tract symptoms were noted. She reported a burning sensation when splashing water on the face or when exposed to air currents. One week following the initial symptoms, she experienced a painful vesicular rash along the upper left forehead (Figure) associated with eyelid edema. Oral and ocular mucosae were free of any presentations. She had no relevant history and had not experienced any complications during pregnancy. A diagnosis of HZ was made, and she was prescribed valacyclovir 1 g 3 times daily for 7 days, acetaminophen for the fever, and calamine lotion. We recommended COVID-19 testing based on her symptoms. A chest radiograph and a positive nasopharyngeal smear were consistent with COVID-19 infection. She reported via telephone follow-up 1 week after presentation that her skin condition had improved following the treatment course and that the vesicles eventually dried, leaving a crusting appearance after 5 to 7 days. Regarding her SARS-CoV-2 condition, her oxygen saturation was 95% at presentation; she self-quarantined at home; and she was treated with oseltamivir 75 mg orally every 12 hours for 5 days, azithromycin 500 mg orally daily, acetaminophen, and vitamin C. Electronic fetal heart rate monitoring and ultrasound examinations were performed to assess the condition of the fetus and were reported normal. At the time of writing this article, she was 32 weeks pregnant and tested negative to 2 consecutive nasopharyngeal swabs for COVID-19 and was in good general condition. She continued her pregnancy according to her obstetrician’s recommendations.
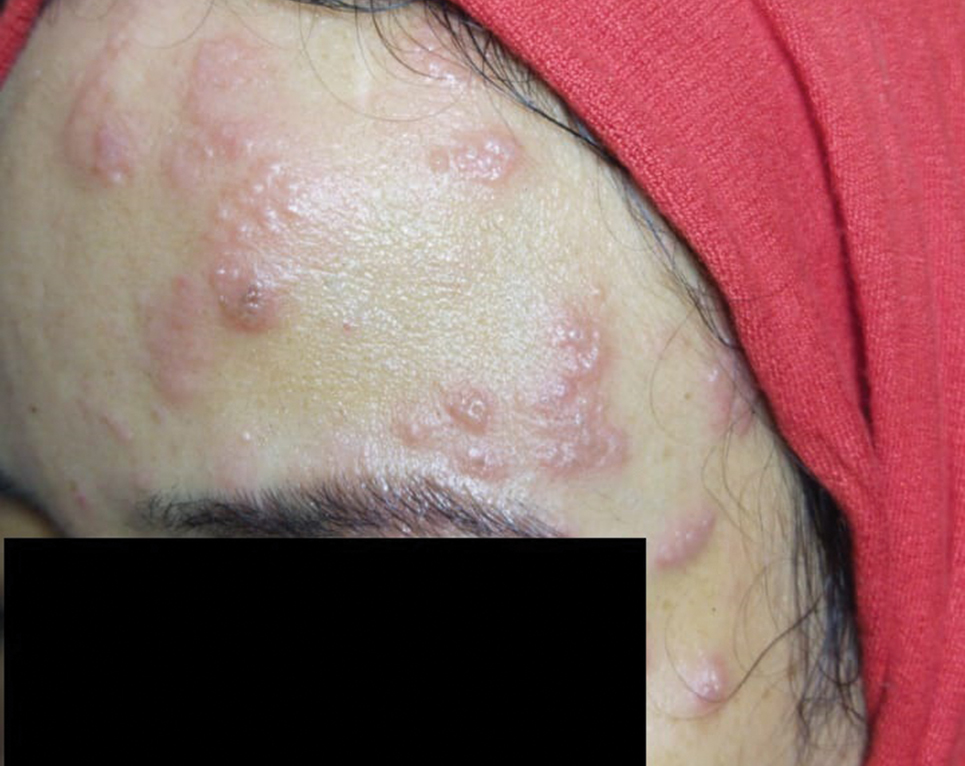
Comment
The incubation time of COVID-19 can be up to 14 days. Fever, dry cough, fatigue, and diarrhea have been speculated to be clinical symptoms; however, many cases may be asymptomatic. Aside from a medical or travel history at risk for COVID-19, diagnosis can be confirmed by detection of viral RNA by reverse transcriptase–polymerase chain reaction for nasopharyngeal swabs or bronchoalveolar fluid. Patients who are immunocompromised, older, or male or who have a history of cardiovascular conditions or debilitating chronic conditions are at an increased risk for severe disease and poor outcome compared to younger healthy individuals.7
The vesicular rash of COVID-19 has been reported to have different forms of presentation. A diffuse widespread pattern resembling hand-foot-and-mouth disease and a localized monomorphic pattern resembling chickenpox but with predilection to the trunk has been described.8
Physiologic changes in the immune and cardiopulmonary systems during pregnancy (eg, diaphragm elevation, increased oxygen consumption, edema of the respiratory tract mucosae) make pregnant women intolerant to hypoxia. The mortality rate of the 1918 influenza pandemic was 2.6% in the overall population but 37% among pregnant women.9 In 2009, pregnant women were reported to be at an increased risk for complications from the H1N1 influenza virus pandemic, with a higher estimated rate of hospital admission than the general population.10 In 2003, approximately 50% of pregnant women who received a diagnosis of SARS-CoV were admitted to the intensive care unit, approximately 33% of pregnant women with SARS-CoV required mechanical ventilation, and the mortality rate was as high as 25% for these women.11 To date, data on the effects of COVID-19 in pregnancy are limited to small case series.12-15
It was confirmed that COVID-19 infection is accompanied by a reduction in lymphocytes, monocytes, and eosinophils, along with a notable reduction of CD4/CD8 T cells, B cells, and natural killer cells. It was further revealed that nonsurvivor COVID-19 patients continued to show a decrease in lymphocyte counts along the course of their disease until death.16-18
Different mechanisms for lymphocyte depletion and deficiency were speculated among COVID-19 patients and include direct lymphocyte death through coronavirus angiotensin-converting enzyme 2–lymphocyte-expressed receptors; direct damage to lymphatic organs, such as the thymus and spleen, but this theory needs to be further investigated; direct lymphocyte apoptosis mediated by tumor necrosis factor α, IL-6, and other proinflammatory cytokines; and direct inhibition of lymphocytes by metabolic upset, such as acidosis.19,20
These causes may precipitate lymphopenia and impaired antiviral responses.21 It also has been postulated that the functional damage of CD4+ T cells may predispose patients with COVID-19 to severe disease.22 Such immune changes can render a patient more susceptible to developing shingles by reactivating varicella-zoster virus, which could be a sign of undiagnosed COVID-19 infection in younger age groups.
Two earlier reports discussed HZ among COVID-19–diagnosed patients. Shors23 presented a case of a patient who developed varicella-zoster virus reactivation of the V2 dermatome during the course of COVID-19 infection. In addition, the patient developed severe acute herpetic neuralgia despite the early initiation of antiviral therapy.23 Elsaie et al24 described 2 cases of patients during the pandemic who first presented with HZ before later being diagnosed with COVID-19 infection.
New information and cutaneous manifestations possibly related to COVID-19 are emerging every day. We report a pregnant female presenting with HZ during the course of COVID-19 infection, which suggests that the clinical presentation of HZ at the time of the current pandemic, especially if associated with other signs of COVID-19 infection, should be carefully monitored and reported for further assessment.
Acknowledgment
The authors would like to thank all the health care workers who have been fighting COVID-19 in Egypt and worldwide.
- Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199-1207.
- Zhang YZ, Holes EC. A genomic perspective on the origin and emergence of sars-cov-2. Cell. 2020;181:223-227.
- Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1‐9.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan0, China. Lancet. 2020;395:497-506.
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422.
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33:e13549.
- Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577‐582.
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol. 2020;45:872-875.
- Gottfredsson M. The Spanish flu in Iceland 1918. Lessons in medicine and history [in Icelandic]. Laeknabladid. 2008;94:737-745.
- Jamieson D, Honein M, Rasmussen S, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451-458.
- Ksiazek TG, Erdman D, Goldsmith CS. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953-1966.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809‐815.
- Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCov pneumonia. Transl Pediatr. 2020;9:51-60.
- Liu Y, Chen H, Tang K, et al. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy [published online March 4, 2020]. J Infect. doi:10.1016/j.jinf.2020.02.028.
- Zhang L, Jiang Y, Wei M, et al. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province [in Chinese]. Zhonghua Fu Chan Ke Za Zhi. 2020;55:166-171.
- Henry BM, de Oliveira MHS, Benoit S, et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021-1028.
- Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752.
- Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-884.
- Kumar A, Anil A, Sharma P, et al. Clinical features of COVID-19 and factors associated with severe clinical course: a systematic review and meta-analysis [preprint]. SSRN. doi:10.2139/ssrn.3566166.
- Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12. https://doi.org/10.1038/s41368-020-0074-x.
- Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517-1520.
- Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533-535.
- Shors AR. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection. JAAD Case Rep. 2020;6:656-657.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection [published online May 23, 2020]. Dermatol Ther. doi:10.1111/dth.13666.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the most recently identified member of the zoonotic pathogens of coronaviruses. It caused an outbreak of pneumonia in December 2019 in Wuhan, China.1 Among all related acute respiratory syndromes (SARS-CoV, Middle East respiratory syndrome coronavirus), SARS-CoV-2 remains to be the most infectious, has the highest potential for human transmission, and can eventually result in acute respiratory distress syndrome.2,3
Only 15% of coronavirus disease 2019 (COVID-19) cases progress to pneumonia, and approximately 5% of these cases develop acute respiratory distress syndrome, septic shock, and/or multiple organ failure. The majority of cases only exhibit mild to moderate symptoms.4,5 A wide array of skin manifestations in COVID-19 infection have been reported, including maculopapular eruptions, morbilliform rashes, urticaria, chickenpoxlike lesions, livedo reticularis, COVID toes, erythema multiforme, pityriasis rosea, and several other patterns.6 We report a case of herpes zoster (HZ) complication in a COVID-19–positive woman who was 27 weeks pregnant.
Case Report
A 36-year-old woman who was 27 weeks pregnant was referred by her obstetrician to the dermatology clinic. She presented with a low-grade fever and a vesicular painful rash. Physical examination revealed painful, itchy, dysesthetic papules and vesicles on the left side of the forehead along with mild edema of the left upper eyelid but no watering of the eye or photophobia. She reported episodes of fever (temperature, 38.9°C), fatigue, and myalgia over the last week. She had bouts of dyspnea and tachycardia that she thought were related to being in the late second trimester of pregnancy. The area surrounding the vesicular eruption was tender to touch. No dry cough or any gastrointestinal or urinary tract symptoms were noted. She reported a burning sensation when splashing water on the face or when exposed to air currents. One week following the initial symptoms, she experienced a painful vesicular rash along the upper left forehead (Figure) associated with eyelid edema. Oral and ocular mucosae were free of any presentations. She had no relevant history and had not experienced any complications during pregnancy. A diagnosis of HZ was made, and she was prescribed valacyclovir 1 g 3 times daily for 7 days, acetaminophen for the fever, and calamine lotion. We recommended COVID-19 testing based on her symptoms. A chest radiograph and a positive nasopharyngeal smear were consistent with COVID-19 infection. She reported via telephone follow-up 1 week after presentation that her skin condition had improved following the treatment course and that the vesicles eventually dried, leaving a crusting appearance after 5 to 7 days. Regarding her SARS-CoV-2 condition, her oxygen saturation was 95% at presentation; she self-quarantined at home; and she was treated with oseltamivir 75 mg orally every 12 hours for 5 days, azithromycin 500 mg orally daily, acetaminophen, and vitamin C. Electronic fetal heart rate monitoring and ultrasound examinations were performed to assess the condition of the fetus and were reported normal. At the time of writing this article, she was 32 weeks pregnant and tested negative to 2 consecutive nasopharyngeal swabs for COVID-19 and was in good general condition. She continued her pregnancy according to her obstetrician’s recommendations.

Comment
The incubation time of COVID-19 can be up to 14 days. Fever, dry cough, fatigue, and diarrhea have been speculated to be clinical symptoms; however, many cases may be asymptomatic. Aside from a medical or travel history at risk for COVID-19, diagnosis can be confirmed by detection of viral RNA by reverse transcriptase–polymerase chain reaction for nasopharyngeal swabs or bronchoalveolar fluid. Patients who are immunocompromised, older, or male or who have a history of cardiovascular conditions or debilitating chronic conditions are at an increased risk for severe disease and poor outcome compared to younger healthy individuals.7
The vesicular rash of COVID-19 has been reported to have different forms of presentation. A diffuse widespread pattern resembling hand-foot-and-mouth disease and a localized monomorphic pattern resembling chickenpox but with predilection to the trunk has been described.8
Physiologic changes in the immune and cardiopulmonary systems during pregnancy (eg, diaphragm elevation, increased oxygen consumption, edema of the respiratory tract mucosae) make pregnant women intolerant to hypoxia. The mortality rate of the 1918 influenza pandemic was 2.6% in the overall population but 37% among pregnant women.9 In 2009, pregnant women were reported to be at an increased risk for complications from the H1N1 influenza virus pandemic, with a higher estimated rate of hospital admission than the general population.10 In 2003, approximately 50% of pregnant women who received a diagnosis of SARS-CoV were admitted to the intensive care unit, approximately 33% of pregnant women with SARS-CoV required mechanical ventilation, and the mortality rate was as high as 25% for these women.11 To date, data on the effects of COVID-19 in pregnancy are limited to small case series.12-15
It was confirmed that COVID-19 infection is accompanied by a reduction in lymphocytes, monocytes, and eosinophils, along with a notable reduction of CD4/CD8 T cells, B cells, and natural killer cells. It was further revealed that nonsurvivor COVID-19 patients continued to show a decrease in lymphocyte counts along the course of their disease until death.16-18
Different mechanisms for lymphocyte depletion and deficiency were speculated among COVID-19 patients and include direct lymphocyte death through coronavirus angiotensin-converting enzyme 2–lymphocyte-expressed receptors; direct damage to lymphatic organs, such as the thymus and spleen, but this theory needs to be further investigated; direct lymphocyte apoptosis mediated by tumor necrosis factor α, IL-6, and other proinflammatory cytokines; and direct inhibition of lymphocytes by metabolic upset, such as acidosis.19,20
These causes may precipitate lymphopenia and impaired antiviral responses.21 It also has been postulated that the functional damage of CD4+ T cells may predispose patients with COVID-19 to severe disease.22 Such immune changes can render a patient more susceptible to developing shingles by reactivating varicella-zoster virus, which could be a sign of undiagnosed COVID-19 infection in younger age groups.
Two earlier reports discussed HZ among COVID-19–diagnosed patients. Shors23 presented a case of a patient who developed varicella-zoster virus reactivation of the V2 dermatome during the course of COVID-19 infection. In addition, the patient developed severe acute herpetic neuralgia despite the early initiation of antiviral therapy.23 Elsaie et al24 described 2 cases of patients during the pandemic who first presented with HZ before later being diagnosed with COVID-19 infection.
New information and cutaneous manifestations possibly related to COVID-19 are emerging every day. We report a pregnant female presenting with HZ during the course of COVID-19 infection, which suggests that the clinical presentation of HZ at the time of the current pandemic, especially if associated with other signs of COVID-19 infection, should be carefully monitored and reported for further assessment.
Acknowledgment
The authors would like to thank all the health care workers who have been fighting COVID-19 in Egypt and worldwide.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the most recently identified member of the zoonotic pathogens of coronaviruses. It caused an outbreak of pneumonia in December 2019 in Wuhan, China.1 Among all related acute respiratory syndromes (SARS-CoV, Middle East respiratory syndrome coronavirus), SARS-CoV-2 remains to be the most infectious, has the highest potential for human transmission, and can eventually result in acute respiratory distress syndrome.2,3
Only 15% of coronavirus disease 2019 (COVID-19) cases progress to pneumonia, and approximately 5% of these cases develop acute respiratory distress syndrome, septic shock, and/or multiple organ failure. The majority of cases only exhibit mild to moderate symptoms.4,5 A wide array of skin manifestations in COVID-19 infection have been reported, including maculopapular eruptions, morbilliform rashes, urticaria, chickenpoxlike lesions, livedo reticularis, COVID toes, erythema multiforme, pityriasis rosea, and several other patterns.6 We report a case of herpes zoster (HZ) complication in a COVID-19–positive woman who was 27 weeks pregnant.
Case Report
A 36-year-old woman who was 27 weeks pregnant was referred by her obstetrician to the dermatology clinic. She presented with a low-grade fever and a vesicular painful rash. Physical examination revealed painful, itchy, dysesthetic papules and vesicles on the left side of the forehead along with mild edema of the left upper eyelid but no watering of the eye or photophobia. She reported episodes of fever (temperature, 38.9°C), fatigue, and myalgia over the last week. She had bouts of dyspnea and tachycardia that she thought were related to being in the late second trimester of pregnancy. The area surrounding the vesicular eruption was tender to touch. No dry cough or any gastrointestinal or urinary tract symptoms were noted. She reported a burning sensation when splashing water on the face or when exposed to air currents. One week following the initial symptoms, she experienced a painful vesicular rash along the upper left forehead (Figure) associated with eyelid edema. Oral and ocular mucosae were free of any presentations. She had no relevant history and had not experienced any complications during pregnancy. A diagnosis of HZ was made, and she was prescribed valacyclovir 1 g 3 times daily for 7 days, acetaminophen for the fever, and calamine lotion. We recommended COVID-19 testing based on her symptoms. A chest radiograph and a positive nasopharyngeal smear were consistent with COVID-19 infection. She reported via telephone follow-up 1 week after presentation that her skin condition had improved following the treatment course and that the vesicles eventually dried, leaving a crusting appearance after 5 to 7 days. Regarding her SARS-CoV-2 condition, her oxygen saturation was 95% at presentation; she self-quarantined at home; and she was treated with oseltamivir 75 mg orally every 12 hours for 5 days, azithromycin 500 mg orally daily, acetaminophen, and vitamin C. Electronic fetal heart rate monitoring and ultrasound examinations were performed to assess the condition of the fetus and were reported normal. At the time of writing this article, she was 32 weeks pregnant and tested negative to 2 consecutive nasopharyngeal swabs for COVID-19 and was in good general condition. She continued her pregnancy according to her obstetrician’s recommendations.

Comment
The incubation time of COVID-19 can be up to 14 days. Fever, dry cough, fatigue, and diarrhea have been speculated to be clinical symptoms; however, many cases may be asymptomatic. Aside from a medical or travel history at risk for COVID-19, diagnosis can be confirmed by detection of viral RNA by reverse transcriptase–polymerase chain reaction for nasopharyngeal swabs or bronchoalveolar fluid. Patients who are immunocompromised, older, or male or who have a history of cardiovascular conditions or debilitating chronic conditions are at an increased risk for severe disease and poor outcome compared to younger healthy individuals.7
The vesicular rash of COVID-19 has been reported to have different forms of presentation. A diffuse widespread pattern resembling hand-foot-and-mouth disease and a localized monomorphic pattern resembling chickenpox but with predilection to the trunk has been described.8
Physiologic changes in the immune and cardiopulmonary systems during pregnancy (eg, diaphragm elevation, increased oxygen consumption, edema of the respiratory tract mucosae) make pregnant women intolerant to hypoxia. The mortality rate of the 1918 influenza pandemic was 2.6% in the overall population but 37% among pregnant women.9 In 2009, pregnant women were reported to be at an increased risk for complications from the H1N1 influenza virus pandemic, with a higher estimated rate of hospital admission than the general population.10 In 2003, approximately 50% of pregnant women who received a diagnosis of SARS-CoV were admitted to the intensive care unit, approximately 33% of pregnant women with SARS-CoV required mechanical ventilation, and the mortality rate was as high as 25% for these women.11 To date, data on the effects of COVID-19 in pregnancy are limited to small case series.12-15
It was confirmed that COVID-19 infection is accompanied by a reduction in lymphocytes, monocytes, and eosinophils, along with a notable reduction of CD4/CD8 T cells, B cells, and natural killer cells. It was further revealed that nonsurvivor COVID-19 patients continued to show a decrease in lymphocyte counts along the course of their disease until death.16-18
Different mechanisms for lymphocyte depletion and deficiency were speculated among COVID-19 patients and include direct lymphocyte death through coronavirus angiotensin-converting enzyme 2–lymphocyte-expressed receptors; direct damage to lymphatic organs, such as the thymus and spleen, but this theory needs to be further investigated; direct lymphocyte apoptosis mediated by tumor necrosis factor α, IL-6, and other proinflammatory cytokines; and direct inhibition of lymphocytes by metabolic upset, such as acidosis.19,20
These causes may precipitate lymphopenia and impaired antiviral responses.21 It also has been postulated that the functional damage of CD4+ T cells may predispose patients with COVID-19 to severe disease.22 Such immune changes can render a patient more susceptible to developing shingles by reactivating varicella-zoster virus, which could be a sign of undiagnosed COVID-19 infection in younger age groups.
Two earlier reports discussed HZ among COVID-19–diagnosed patients. Shors23 presented a case of a patient who developed varicella-zoster virus reactivation of the V2 dermatome during the course of COVID-19 infection. In addition, the patient developed severe acute herpetic neuralgia despite the early initiation of antiviral therapy.23 Elsaie et al24 described 2 cases of patients during the pandemic who first presented with HZ before later being diagnosed with COVID-19 infection.
New information and cutaneous manifestations possibly related to COVID-19 are emerging every day. We report a pregnant female presenting with HZ during the course of COVID-19 infection, which suggests that the clinical presentation of HZ at the time of the current pandemic, especially if associated with other signs of COVID-19 infection, should be carefully monitored and reported for further assessment.
Acknowledgment
The authors would like to thank all the health care workers who have been fighting COVID-19 in Egypt and worldwide.
- Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199-1207.
- Zhang YZ, Holes EC. A genomic perspective on the origin and emergence of sars-cov-2. Cell. 2020;181:223-227.
- Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1‐9.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan0, China. Lancet. 2020;395:497-506.
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422.
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33:e13549.
- Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577‐582.
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol. 2020;45:872-875.
- Gottfredsson M. The Spanish flu in Iceland 1918. Lessons in medicine and history [in Icelandic]. Laeknabladid. 2008;94:737-745.
- Jamieson D, Honein M, Rasmussen S, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451-458.
- Ksiazek TG, Erdman D, Goldsmith CS. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953-1966.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809‐815.
- Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCov pneumonia. Transl Pediatr. 2020;9:51-60.
- Liu Y, Chen H, Tang K, et al. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy [published online March 4, 2020]. J Infect. doi:10.1016/j.jinf.2020.02.028.
- Zhang L, Jiang Y, Wei M, et al. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province [in Chinese]. Zhonghua Fu Chan Ke Za Zhi. 2020;55:166-171.
- Henry BM, de Oliveira MHS, Benoit S, et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021-1028.
- Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752.
- Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-884.
- Kumar A, Anil A, Sharma P, et al. Clinical features of COVID-19 and factors associated with severe clinical course: a systematic review and meta-analysis [preprint]. SSRN. doi:10.2139/ssrn.3566166.
- Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12. https://doi.org/10.1038/s41368-020-0074-x.
- Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517-1520.
- Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533-535.
- Shors AR. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection. JAAD Case Rep. 2020;6:656-657.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection [published online May 23, 2020]. Dermatol Ther. doi:10.1111/dth.13666.
- Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199-1207.
- Zhang YZ, Holes EC. A genomic perspective on the origin and emergence of sars-cov-2. Cell. 2020;181:223-227.
- Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1‐9.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan0, China. Lancet. 2020;395:497-506.
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422.
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33:e13549.
- Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577‐582.
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol. 2020;45:872-875.
- Gottfredsson M. The Spanish flu in Iceland 1918. Lessons in medicine and history [in Icelandic]. Laeknabladid. 2008;94:737-745.
- Jamieson D, Honein M, Rasmussen S, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451-458.
- Ksiazek TG, Erdman D, Goldsmith CS. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953-1966.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809‐815.
- Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCov pneumonia. Transl Pediatr. 2020;9:51-60.
- Liu Y, Chen H, Tang K, et al. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy [published online March 4, 2020]. J Infect. doi:10.1016/j.jinf.2020.02.028.
- Zhang L, Jiang Y, Wei M, et al. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province [in Chinese]. Zhonghua Fu Chan Ke Za Zhi. 2020;55:166-171.
- Henry BM, de Oliveira MHS, Benoit S, et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021-1028.
- Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752.
- Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-884.
- Kumar A, Anil A, Sharma P, et al. Clinical features of COVID-19 and factors associated with severe clinical course: a systematic review and meta-analysis [preprint]. SSRN. doi:10.2139/ssrn.3566166.
- Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12. https://doi.org/10.1038/s41368-020-0074-x.
- Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517-1520.
- Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533-535.
- Shors AR. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection. JAAD Case Rep. 2020;6:656-657.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection [published online May 23, 2020]. Dermatol Ther. doi:10.1111/dth.13666.
Practice Points
- The vesicular rash of coronavirus disease 2019 (COVID-19) has been reported to have different forms of presentation.
- Pregnant women appear to be at increased risk for complications from COVID-19 infection.
- The clinical presentation of herpes zoster should be carefully monitored and reported for further assessment, especially if associated with other signs of COVID-19 infection.
Skin Eruption and Gastrointestinal Symptoms as Presentation of COVID-19
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) started an outbreak of respiratory illnesses in Wuhan, China. The respiratory disease was termed coronavirus disease 2019 (COVID-19) and rapidly spread worldwide, resulting in a pandemic classification on March 11, 2020. 1 Recently, several cutaneous manifestations of COVID-19 have been reported. Skin manifestations have been reported to be similar to other common viral infections. 2 However, there is a paucity of published clinical images of more atypical presentations.
Case Report
A 52-year-old black man presented via urgent store-and-forward teledermatology consultation from his primary care provider with a self-described “vesicular,” highly pruritic rash of both arms and legs of 1 week’s duration without involvement of the trunk, axillae, groin, face, genitalia, or any mucous membranes. He noted nausea, loss of appetite, and nonbloody diarrhea 4 days later. He denied fever, chills, dry cough, shortness of breath, or dyspnea. He had a history of hypertension and type 2 diabetes mellitus. There were no changes in medications; no outdoor activities, gardening, or yard work; no exposure to plants or metals; and no use of new personal care products.
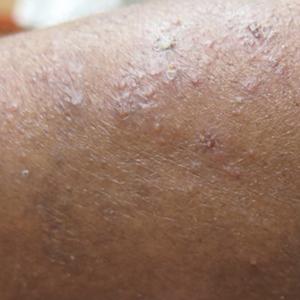
The digital images showed zones of flesh-colored to slightly erythematous, somewhat “juicy” papules with some coalescence into ill-defined plaques. There were scattered foci of scale and hemorrhagic crust that involved both palms, forearms (Figure, A), and legs (Figure, B). There were no intact vesicles, and a herald patch was not identified. Vital signs at the time of imaging were normal, with the exception of a low-grade fever (temperature, 37.3°C). Basic laboratory testing showed only mild leukocytosis with mild neutropenia and mild aspartate aminotransaminase elevation. A skin biopsy was not performed. Pulmonary imaging and workup were not performed because of the lack of respiratory symptoms.
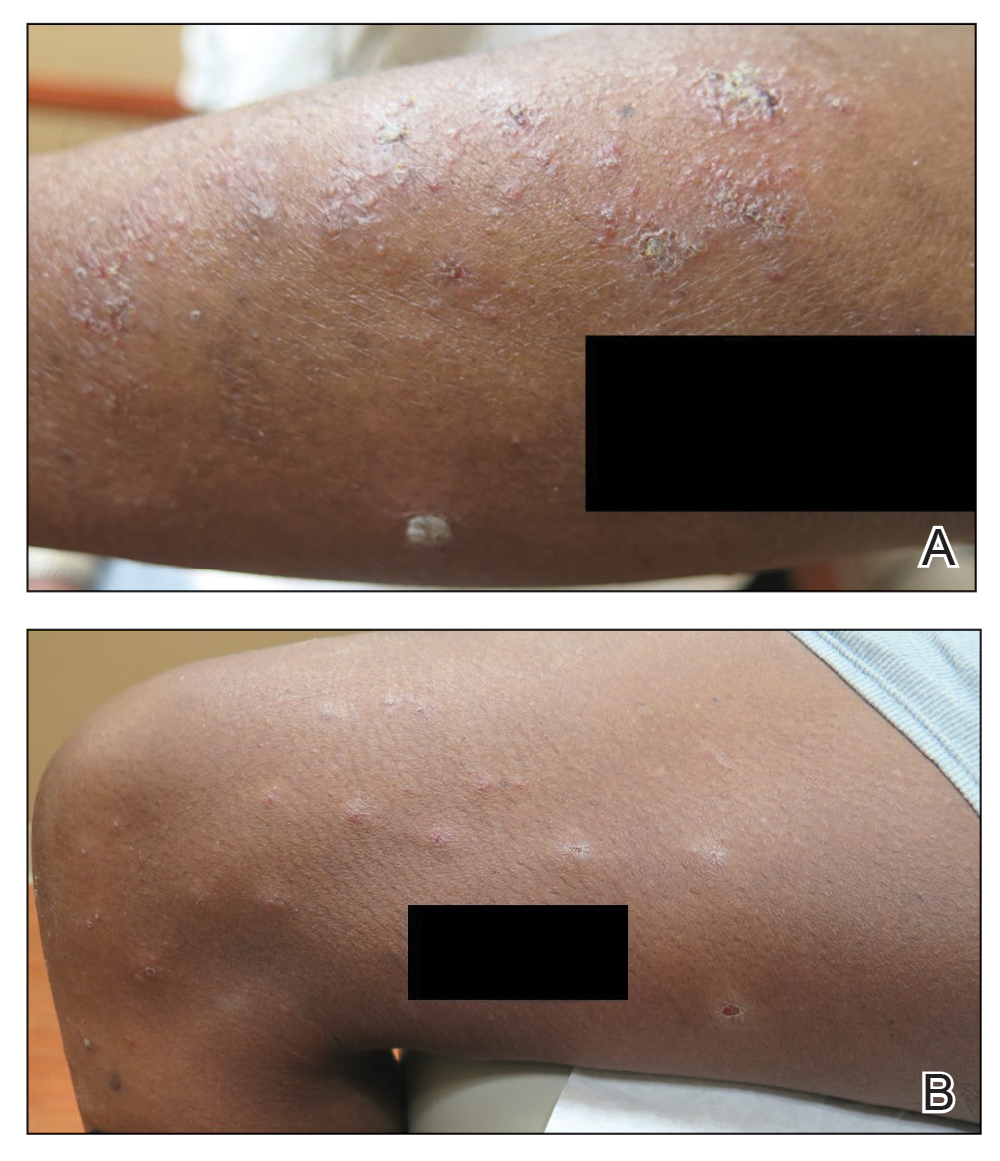
The teledermatology differential diagnosis included a drug eruption, autosensitization eruption, unusual contact dermatitis, viral exanthem, secondary syphilis, and papular pityriasis rosea with an unusual distribution. The absence of changes in the patient’s medication regimen and the lack of outdoor activity in late winter made a drug eruption and contact dermatitis less likely, respectively. A rapid plasma reagin test drawn after disappearance of the rash was negative. Although the morphology of this eruption displayed some features of papular pityriasis rosea, this diagnosis was considered to be less likely given the presence of palmar involvement and the absence of any truncal lesions. This variant of pityriasis rosea is more commonly encountered in younger, darker-skinned patients.
Given the presence of an unusual rash on the extremities followed shortly by gastrointestinal (GI) symptoms and coupled with a low-grade fever, a nasopharyngeal swab was obtained to test for COVID-19 using a reverse transcriptase–polymerase chain reaction test. The results were positive.
The patient was treated with triamcinolone 0.1% slush (triamcinolone cream 0.1% mixed 1:1 with tap water) to the affected skin of the extremities 3 times daily, and he experienced a reduction in pruritus. He developed new lesions on the face and eyelids (not imaged) 2 days after teledermatology consultation. The facial involvement was treated with hydrocortisone cream 1%. During the following week, the GI symptoms and skin eruption completely resolved. However, postinflammatory hyperpigmentation was observed in areas of the resolved papules and plaques. Over the course of this illness, the patient reported no respiratory symptoms.
Comment
Coronavirus disease 2019 is caused by SARS-CoV2, an enveloped, nonsegmented, positive-sense RNA virus of the coronavirus family. It is currently believed that SARS-CoV-2 uses the angiotensin-converting enzyme 2 receptor to gain entry into human cells, leading to infection primarily affecting the lower respiratory tract.3 Patients suspected of COVID-19 infection most often present with fever, dry cough, dyspnea, and fatigue, while GI symptoms such as nausea, vomiting, and diarrhea are uncommon.4 More recently, several reports describe a variety of skin findings associated with COVID-19. A current theory suggests that the virus does not directly target keratinocytes but triggers a systemic immune response, leading to a diversity of skin morphologies.5 The main types of described cutaneous findings include pseudochilblains, overtly vesicular, urticarial, maculopapular, and livedo/necrosis.6 Others have described petechial7 and papulosquamous eruptions.8 Most of these patients initially presented with typical COVID-19 symptoms and frequently represented more severe cases of the disease. Additionally, the vesicular and papulosquamous eruptions reportedly occurred on the trunk and not the limbs, as in our case.
This confirmed COVID-19–positive patient presented with an ill-defined vesicular and papulosquamous-type eruption on the arms and legs and later developed only mild GI symptoms. By sharing this case, we report yet another skin manifestation of COVID-19 and propose the possible expansion of testing for SARS-CoV-2 in patients presenting with rash and GI symptoms, which holds the potential to increase the identification of COVID-19 in the population, thereby increasing strict contact tracing and slowing the spread of this pandemic.
- Ng OT, Marimuthu K, Chia PY, et al. SARS-CoV-2 infection among travelers returning from Wuhan, China. N Engl J Med. 2020;382:1476-1478.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil Med Res. 2020;7:11.
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
- Gianotti R, Zerbi P, Dodiuk-Gad RP. Clinical and histopathological study of skin dermatoses in patients affected by COVID-19 infection in the Northern part of Italy. J Dermatol Sci. 2020;98:141-143.
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Diaz-Guimaraens B, Dominguez-Santas M, Suarez-Valle A, et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:820-822.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) started an outbreak of respiratory illnesses in Wuhan, China. The respiratory disease was termed coronavirus disease 2019 (COVID-19) and rapidly spread worldwide, resulting in a pandemic classification on March 11, 2020. 1 Recently, several cutaneous manifestations of COVID-19 have been reported. Skin manifestations have been reported to be similar to other common viral infections. 2 However, there is a paucity of published clinical images of more atypical presentations.
Case Report
A 52-year-old black man presented via urgent store-and-forward teledermatology consultation from his primary care provider with a self-described “vesicular,” highly pruritic rash of both arms and legs of 1 week’s duration without involvement of the trunk, axillae, groin, face, genitalia, or any mucous membranes. He noted nausea, loss of appetite, and nonbloody diarrhea 4 days later. He denied fever, chills, dry cough, shortness of breath, or dyspnea. He had a history of hypertension and type 2 diabetes mellitus. There were no changes in medications; no outdoor activities, gardening, or yard work; no exposure to plants or metals; and no use of new personal care products.
The digital images showed zones of flesh-colored to slightly erythematous, somewhat “juicy” papules with some coalescence into ill-defined plaques. There were scattered foci of scale and hemorrhagic crust that involved both palms, forearms (Figure, A), and legs (Figure, B). There were no intact vesicles, and a herald patch was not identified. Vital signs at the time of imaging were normal, with the exception of a low-grade fever (temperature, 37.3°C). Basic laboratory testing showed only mild leukocytosis with mild neutropenia and mild aspartate aminotransaminase elevation. A skin biopsy was not performed. Pulmonary imaging and workup were not performed because of the lack of respiratory symptoms.

The teledermatology differential diagnosis included a drug eruption, autosensitization eruption, unusual contact dermatitis, viral exanthem, secondary syphilis, and papular pityriasis rosea with an unusual distribution. The absence of changes in the patient’s medication regimen and the lack of outdoor activity in late winter made a drug eruption and contact dermatitis less likely, respectively. A rapid plasma reagin test drawn after disappearance of the rash was negative. Although the morphology of this eruption displayed some features of papular pityriasis rosea, this diagnosis was considered to be less likely given the presence of palmar involvement and the absence of any truncal lesions. This variant of pityriasis rosea is more commonly encountered in younger, darker-skinned patients.
Given the presence of an unusual rash on the extremities followed shortly by gastrointestinal (GI) symptoms and coupled with a low-grade fever, a nasopharyngeal swab was obtained to test for COVID-19 using a reverse transcriptase–polymerase chain reaction test. The results were positive.
The patient was treated with triamcinolone 0.1% slush (triamcinolone cream 0.1% mixed 1:1 with tap water) to the affected skin of the extremities 3 times daily, and he experienced a reduction in pruritus. He developed new lesions on the face and eyelids (not imaged) 2 days after teledermatology consultation. The facial involvement was treated with hydrocortisone cream 1%. During the following week, the GI symptoms and skin eruption completely resolved. However, postinflammatory hyperpigmentation was observed in areas of the resolved papules and plaques. Over the course of this illness, the patient reported no respiratory symptoms.
Comment
Coronavirus disease 2019 is caused by SARS-CoV2, an enveloped, nonsegmented, positive-sense RNA virus of the coronavirus family. It is currently believed that SARS-CoV-2 uses the angiotensin-converting enzyme 2 receptor to gain entry into human cells, leading to infection primarily affecting the lower respiratory tract.3 Patients suspected of COVID-19 infection most often present with fever, dry cough, dyspnea, and fatigue, while GI symptoms such as nausea, vomiting, and diarrhea are uncommon.4 More recently, several reports describe a variety of skin findings associated with COVID-19. A current theory suggests that the virus does not directly target keratinocytes but triggers a systemic immune response, leading to a diversity of skin morphologies.5 The main types of described cutaneous findings include pseudochilblains, overtly vesicular, urticarial, maculopapular, and livedo/necrosis.6 Others have described petechial7 and papulosquamous eruptions.8 Most of these patients initially presented with typical COVID-19 symptoms and frequently represented more severe cases of the disease. Additionally, the vesicular and papulosquamous eruptions reportedly occurred on the trunk and not the limbs, as in our case.
This confirmed COVID-19–positive patient presented with an ill-defined vesicular and papulosquamous-type eruption on the arms and legs and later developed only mild GI symptoms. By sharing this case, we report yet another skin manifestation of COVID-19 and propose the possible expansion of testing for SARS-CoV-2 in patients presenting with rash and GI symptoms, which holds the potential to increase the identification of COVID-19 in the population, thereby increasing strict contact tracing and slowing the spread of this pandemic.
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) started an outbreak of respiratory illnesses in Wuhan, China. The respiratory disease was termed coronavirus disease 2019 (COVID-19) and rapidly spread worldwide, resulting in a pandemic classification on March 11, 2020. 1 Recently, several cutaneous manifestations of COVID-19 have been reported. Skin manifestations have been reported to be similar to other common viral infections. 2 However, there is a paucity of published clinical images of more atypical presentations.
Case Report
A 52-year-old black man presented via urgent store-and-forward teledermatology consultation from his primary care provider with a self-described “vesicular,” highly pruritic rash of both arms and legs of 1 week’s duration without involvement of the trunk, axillae, groin, face, genitalia, or any mucous membranes. He noted nausea, loss of appetite, and nonbloody diarrhea 4 days later. He denied fever, chills, dry cough, shortness of breath, or dyspnea. He had a history of hypertension and type 2 diabetes mellitus. There were no changes in medications; no outdoor activities, gardening, or yard work; no exposure to plants or metals; and no use of new personal care products.
The digital images showed zones of flesh-colored to slightly erythematous, somewhat “juicy” papules with some coalescence into ill-defined plaques. There were scattered foci of scale and hemorrhagic crust that involved both palms, forearms (Figure, A), and legs (Figure, B). There were no intact vesicles, and a herald patch was not identified. Vital signs at the time of imaging were normal, with the exception of a low-grade fever (temperature, 37.3°C). Basic laboratory testing showed only mild leukocytosis with mild neutropenia and mild aspartate aminotransaminase elevation. A skin biopsy was not performed. Pulmonary imaging and workup were not performed because of the lack of respiratory symptoms.

The teledermatology differential diagnosis included a drug eruption, autosensitization eruption, unusual contact dermatitis, viral exanthem, secondary syphilis, and papular pityriasis rosea with an unusual distribution. The absence of changes in the patient’s medication regimen and the lack of outdoor activity in late winter made a drug eruption and contact dermatitis less likely, respectively. A rapid plasma reagin test drawn after disappearance of the rash was negative. Although the morphology of this eruption displayed some features of papular pityriasis rosea, this diagnosis was considered to be less likely given the presence of palmar involvement and the absence of any truncal lesions. This variant of pityriasis rosea is more commonly encountered in younger, darker-skinned patients.
Given the presence of an unusual rash on the extremities followed shortly by gastrointestinal (GI) symptoms and coupled with a low-grade fever, a nasopharyngeal swab was obtained to test for COVID-19 using a reverse transcriptase–polymerase chain reaction test. The results were positive.
The patient was treated with triamcinolone 0.1% slush (triamcinolone cream 0.1% mixed 1:1 with tap water) to the affected skin of the extremities 3 times daily, and he experienced a reduction in pruritus. He developed new lesions on the face and eyelids (not imaged) 2 days after teledermatology consultation. The facial involvement was treated with hydrocortisone cream 1%. During the following week, the GI symptoms and skin eruption completely resolved. However, postinflammatory hyperpigmentation was observed in areas of the resolved papules and plaques. Over the course of this illness, the patient reported no respiratory symptoms.
Comment
Coronavirus disease 2019 is caused by SARS-CoV2, an enveloped, nonsegmented, positive-sense RNA virus of the coronavirus family. It is currently believed that SARS-CoV-2 uses the angiotensin-converting enzyme 2 receptor to gain entry into human cells, leading to infection primarily affecting the lower respiratory tract.3 Patients suspected of COVID-19 infection most often present with fever, dry cough, dyspnea, and fatigue, while GI symptoms such as nausea, vomiting, and diarrhea are uncommon.4 More recently, several reports describe a variety of skin findings associated with COVID-19. A current theory suggests that the virus does not directly target keratinocytes but triggers a systemic immune response, leading to a diversity of skin morphologies.5 The main types of described cutaneous findings include pseudochilblains, overtly vesicular, urticarial, maculopapular, and livedo/necrosis.6 Others have described petechial7 and papulosquamous eruptions.8 Most of these patients initially presented with typical COVID-19 symptoms and frequently represented more severe cases of the disease. Additionally, the vesicular and papulosquamous eruptions reportedly occurred on the trunk and not the limbs, as in our case.
This confirmed COVID-19–positive patient presented with an ill-defined vesicular and papulosquamous-type eruption on the arms and legs and later developed only mild GI symptoms. By sharing this case, we report yet another skin manifestation of COVID-19 and propose the possible expansion of testing for SARS-CoV-2 in patients presenting with rash and GI symptoms, which holds the potential to increase the identification of COVID-19 in the population, thereby increasing strict contact tracing and slowing the spread of this pandemic.
- Ng OT, Marimuthu K, Chia PY, et al. SARS-CoV-2 infection among travelers returning from Wuhan, China. N Engl J Med. 2020;382:1476-1478.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil Med Res. 2020;7:11.
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
- Gianotti R, Zerbi P, Dodiuk-Gad RP. Clinical and histopathological study of skin dermatoses in patients affected by COVID-19 infection in the Northern part of Italy. J Dermatol Sci. 2020;98:141-143.
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Diaz-Guimaraens B, Dominguez-Santas M, Suarez-Valle A, et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:820-822.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
- Ng OT, Marimuthu K, Chia PY, et al. SARS-CoV-2 infection among travelers returning from Wuhan, China. N Engl J Med. 2020;382:1476-1478.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil Med Res. 2020;7:11.
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
- Gianotti R, Zerbi P, Dodiuk-Gad RP. Clinical and histopathological study of skin dermatoses in patients affected by COVID-19 infection in the Northern part of Italy. J Dermatol Sci. 2020;98:141-143.
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Diaz-Guimaraens B, Dominguez-Santas M, Suarez-Valle A, et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:820-822.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
Practice Points
- Patients with coronavirus disease 2019 (COVID-19) typically present with fever, dry cough, dyspnea, and fatigue, but cutaneous manifestations also have been reported.
- Awareness of atypical presentations of COVID-19, including uncommon cutaneous manifestations, may identify more cases and help slow the expansion of this pandemic.
Left Ventricular Compression and Hypotension Due to Acute Colonic Pseudo-Obstruction
Acute colonic pseudo-obstruction is a postsurgical dilatation of the colon that presents with abdominal distension, pain, nausea, vomiting, constipation, or diarrhea and may lead to colonic ischemia and bowel perforation.
A cute colonic pseudo-obstruction, or Ogilvie syndrome, is dilatation of the colon without mechanical obstruction. It is often seen postoperatively after cesarean section , pelvic , spinal, or other orthopedic surgery, such as knee arthroplasty. 1 One study demonstrated an incidence of acute colonic pseudo-obstruction of 1.3% following hip replacement surgery. 2
The most common symptoms are abdominal distension, pain, nausea, vomiting, constipation, or diarrhea. Bowel sounds are present in the majority of cases.3 It is important to recognize the varied presentations of ileus in the postoperative setting. The most serious complications of acute colonic pseudo-obstruction are colonic ischemia and bowel perforation.
Case Presentation
An 84-year-old man underwent a total left hip arthroplasty revision. The evening after his surgery, his blood pressure (BP) decreased from 93/54 to 71/47 mm Hg, and his heart rate was 73 beats per minute. He was awake, in no acute distress, but reported loose stools. Results of cardiac and pulmonary examinations were normal, showing a regular rate and rhythm with no murmurs and clear lungs. There was normal jugular venous pressure and chronic pitting edema of the lower extremities bilaterally.
An abdominal examination revealed positive bowel sounds, a large ventral hernia, which was easily reducible, and a distended abdomen. His BP remained unchanged after IV normal saline 4 L, and urine output was 200 cc over 4 hours, which the care team determined represented adequate resuscitation. An infection workup, including chest X-ray, urinalysis, and blood and urine cultures, was unrevealing. Hemoglobin was stable at 8.5 g/dL (normal range 14-18), and creatinine level 0.66 mg/dL (normal range 0.7-1.2) at baseline. A transthoracic echocardiogram showed impaired diastolic filling suggestive of extrinsic compression of the left ventricle by mediastinal contents (Figure 1). An abdominal X-ray revealed diffuse dilatation of large bowel loops up to 10 cm, causing elevation and rightward shift of the heart (Figure 2A). A computed tomography scan of the abdomen showed total colonic dilatation without obstruction (Figure 2B).
The patient was diagnosed with postoperative ileus and acute colonic pseudo-obstruction. Nasogastric and rectal tubes were placed for decompression, and the patient was placed on nothing by mouth status. By postoperative day 3, his hypotension had resolved and his BP had improved to 111/58 mm Hg. The patient was able to resume a regular diet.
Discussion
We present an unusual case of left ventricular compression leading to hypotension due to acute colonic pseudo-obstruction. Our patient presented with the rare complication of hypotension due to cardiac compression, which we have not previously seen reported in the literature. Analogous instance of cardiac compression may arise from hiatal hernias and diaphragmatic paralysis. 4-6
Management of acute colonic pseudo-obstruction is through nothing by mouth status and abdominal decompression. For more severe cases, neostigmine, colonoscopic decompression, and surgery can be considered.
This surgical complication was diagnosed by internal medicine hospitalist consultants on a surgical comanagement service. In the comanagement model, the surgical specialties of orthopedic surgery, neurosurgery, and podiatry at San Francisco Veterans Affairs Medical Center in California have hospitalists who work with the team as active consultants for the medical care of the patients. Hospitalists develop a unique skill set in which they can anticipate new diagnoses, prevent or identify early complications, and individualize a patient’s postoperative care.7 One study found that a surgical comanagement service was associated with a decrease in the number of patients with at least 1 surgical complication, decrease in length of stay and 30-day readmissions for a medical cause, decreased consultant use, and an average cost savings per patient of about $2,600 to $4,300.8
Conclusions
With the increasing prevalence of hospitalist comanagement services, it is important for surgeons and nonsurgeons alike to recognize acute colonic pseudo-obstruction as a possible surgical complication.
1. Bernardi M, Warrier S, Lynch C, Heriot A. Acute and chronic pseudo-obstruction: a current update. ANZ J Surg. 2015;85(10):709-714. doi:10.1111/ans.13148
2. Norwood MGA, Lykostratis H, Garcea G, Berry DP. Acute colonic pseudo-obstruction following major orthopaedic surgery. Colorectal Dis. 2005;7(5):496-499. doi:10.1111/j.1463-1318.2005.00790.x
3. Vanek VW, Al-Salti M. Acute pseudo-obstruction of the colon (Ogilvie’s syndrome). An analysis of 400 cases. Dis Colon Rectum. 1986;29(3):203-210. doi:10.1007/BF02555027
4. Devabhandari MP, Khan MA, Hooper TL. Cardiac compression following cardiac surgery due to unrecognised hiatus hernia. Eur J Cardiothoracic Surg. 2007;32(5):813-815. doi:10.1016/j.ejcts.2007.08.002
5. Asti E, Bonavina L, Lombardi M, Bandera F, Secchi F, Guazzi M. Reversibility of cardiopulmonary impairment after laparoscopic repair of large hiatal hernia. Int J Surg Case Rep. 2015;14:33-35. doi:10.1016/j.ijscr.2015.07.005
6. Tayyareci Y, Bayazit P, Taştan CP, Aksoy H. Right atrial compression due to idiopathic right diaphragm paralysis detected incidentally by transthoracic echocardiography. Turk Kardiyol Dern Ars. 2008;36(6):412-414.
7. Rohatgi N, Schulman K, Ahuja N. Comanagement by hospitalists: why it makes clinical and fiscal sense. Am J Med. 2020;133(3):257-258. doi:10.1016/j.amjmed.2019.07.053
8. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: a propensity score analysis. Ann Surg. 2016;264(2):275-282. doi:10.1097/SLA.0000000000001629
Acute colonic pseudo-obstruction is a postsurgical dilatation of the colon that presents with abdominal distension, pain, nausea, vomiting, constipation, or diarrhea and may lead to colonic ischemia and bowel perforation.
Acute colonic pseudo-obstruction is a postsurgical dilatation of the colon that presents with abdominal distension, pain, nausea, vomiting, constipation, or diarrhea and may lead to colonic ischemia and bowel perforation.
A cute colonic pseudo-obstruction, or Ogilvie syndrome, is dilatation of the colon without mechanical obstruction. It is often seen postoperatively after cesarean section , pelvic , spinal, or other orthopedic surgery, such as knee arthroplasty. 1 One study demonstrated an incidence of acute colonic pseudo-obstruction of 1.3% following hip replacement surgery. 2
The most common symptoms are abdominal distension, pain, nausea, vomiting, constipation, or diarrhea. Bowel sounds are present in the majority of cases.3 It is important to recognize the varied presentations of ileus in the postoperative setting. The most serious complications of acute colonic pseudo-obstruction are colonic ischemia and bowel perforation.
Case Presentation
An 84-year-old man underwent a total left hip arthroplasty revision. The evening after his surgery, his blood pressure (BP) decreased from 93/54 to 71/47 mm Hg, and his heart rate was 73 beats per minute. He was awake, in no acute distress, but reported loose stools. Results of cardiac and pulmonary examinations were normal, showing a regular rate and rhythm with no murmurs and clear lungs. There was normal jugular venous pressure and chronic pitting edema of the lower extremities bilaterally.
An abdominal examination revealed positive bowel sounds, a large ventral hernia, which was easily reducible, and a distended abdomen. His BP remained unchanged after IV normal saline 4 L, and urine output was 200 cc over 4 hours, which the care team determined represented adequate resuscitation. An infection workup, including chest X-ray, urinalysis, and blood and urine cultures, was unrevealing. Hemoglobin was stable at 8.5 g/dL (normal range 14-18), and creatinine level 0.66 mg/dL (normal range 0.7-1.2) at baseline. A transthoracic echocardiogram showed impaired diastolic filling suggestive of extrinsic compression of the left ventricle by mediastinal contents (Figure 1). An abdominal X-ray revealed diffuse dilatation of large bowel loops up to 10 cm, causing elevation and rightward shift of the heart (Figure 2A). A computed tomography scan of the abdomen showed total colonic dilatation without obstruction (Figure 2B).
The patient was diagnosed with postoperative ileus and acute colonic pseudo-obstruction. Nasogastric and rectal tubes were placed for decompression, and the patient was placed on nothing by mouth status. By postoperative day 3, his hypotension had resolved and his BP had improved to 111/58 mm Hg. The patient was able to resume a regular diet.
Discussion
We present an unusual case of left ventricular compression leading to hypotension due to acute colonic pseudo-obstruction. Our patient presented with the rare complication of hypotension due to cardiac compression, which we have not previously seen reported in the literature. Analogous instance of cardiac compression may arise from hiatal hernias and diaphragmatic paralysis. 4-6
Management of acute colonic pseudo-obstruction is through nothing by mouth status and abdominal decompression. For more severe cases, neostigmine, colonoscopic decompression, and surgery can be considered.
This surgical complication was diagnosed by internal medicine hospitalist consultants on a surgical comanagement service. In the comanagement model, the surgical specialties of orthopedic surgery, neurosurgery, and podiatry at San Francisco Veterans Affairs Medical Center in California have hospitalists who work with the team as active consultants for the medical care of the patients. Hospitalists develop a unique skill set in which they can anticipate new diagnoses, prevent or identify early complications, and individualize a patient’s postoperative care.7 One study found that a surgical comanagement service was associated with a decrease in the number of patients with at least 1 surgical complication, decrease in length of stay and 30-day readmissions for a medical cause, decreased consultant use, and an average cost savings per patient of about $2,600 to $4,300.8
Conclusions
With the increasing prevalence of hospitalist comanagement services, it is important for surgeons and nonsurgeons alike to recognize acute colonic pseudo-obstruction as a possible surgical complication.
A cute colonic pseudo-obstruction, or Ogilvie syndrome, is dilatation of the colon without mechanical obstruction. It is often seen postoperatively after cesarean section , pelvic , spinal, or other orthopedic surgery, such as knee arthroplasty. 1 One study demonstrated an incidence of acute colonic pseudo-obstruction of 1.3% following hip replacement surgery. 2
The most common symptoms are abdominal distension, pain, nausea, vomiting, constipation, or diarrhea. Bowel sounds are present in the majority of cases.3 It is important to recognize the varied presentations of ileus in the postoperative setting. The most serious complications of acute colonic pseudo-obstruction are colonic ischemia and bowel perforation.
Case Presentation
An 84-year-old man underwent a total left hip arthroplasty revision. The evening after his surgery, his blood pressure (BP) decreased from 93/54 to 71/47 mm Hg, and his heart rate was 73 beats per minute. He was awake, in no acute distress, but reported loose stools. Results of cardiac and pulmonary examinations were normal, showing a regular rate and rhythm with no murmurs and clear lungs. There was normal jugular venous pressure and chronic pitting edema of the lower extremities bilaterally.
An abdominal examination revealed positive bowel sounds, a large ventral hernia, which was easily reducible, and a distended abdomen. His BP remained unchanged after IV normal saline 4 L, and urine output was 200 cc over 4 hours, which the care team determined represented adequate resuscitation. An infection workup, including chest X-ray, urinalysis, and blood and urine cultures, was unrevealing. Hemoglobin was stable at 8.5 g/dL (normal range 14-18), and creatinine level 0.66 mg/dL (normal range 0.7-1.2) at baseline. A transthoracic echocardiogram showed impaired diastolic filling suggestive of extrinsic compression of the left ventricle by mediastinal contents (Figure 1). An abdominal X-ray revealed diffuse dilatation of large bowel loops up to 10 cm, causing elevation and rightward shift of the heart (Figure 2A). A computed tomography scan of the abdomen showed total colonic dilatation without obstruction (Figure 2B).
The patient was diagnosed with postoperative ileus and acute colonic pseudo-obstruction. Nasogastric and rectal tubes were placed for decompression, and the patient was placed on nothing by mouth status. By postoperative day 3, his hypotension had resolved and his BP had improved to 111/58 mm Hg. The patient was able to resume a regular diet.
Discussion
We present an unusual case of left ventricular compression leading to hypotension due to acute colonic pseudo-obstruction. Our patient presented with the rare complication of hypotension due to cardiac compression, which we have not previously seen reported in the literature. Analogous instance of cardiac compression may arise from hiatal hernias and diaphragmatic paralysis. 4-6
Management of acute colonic pseudo-obstruction is through nothing by mouth status and abdominal decompression. For more severe cases, neostigmine, colonoscopic decompression, and surgery can be considered.
This surgical complication was diagnosed by internal medicine hospitalist consultants on a surgical comanagement service. In the comanagement model, the surgical specialties of orthopedic surgery, neurosurgery, and podiatry at San Francisco Veterans Affairs Medical Center in California have hospitalists who work with the team as active consultants for the medical care of the patients. Hospitalists develop a unique skill set in which they can anticipate new diagnoses, prevent or identify early complications, and individualize a patient’s postoperative care.7 One study found that a surgical comanagement service was associated with a decrease in the number of patients with at least 1 surgical complication, decrease in length of stay and 30-day readmissions for a medical cause, decreased consultant use, and an average cost savings per patient of about $2,600 to $4,300.8
Conclusions
With the increasing prevalence of hospitalist comanagement services, it is important for surgeons and nonsurgeons alike to recognize acute colonic pseudo-obstruction as a possible surgical complication.
1. Bernardi M, Warrier S, Lynch C, Heriot A. Acute and chronic pseudo-obstruction: a current update. ANZ J Surg. 2015;85(10):709-714. doi:10.1111/ans.13148
2. Norwood MGA, Lykostratis H, Garcea G, Berry DP. Acute colonic pseudo-obstruction following major orthopaedic surgery. Colorectal Dis. 2005;7(5):496-499. doi:10.1111/j.1463-1318.2005.00790.x
3. Vanek VW, Al-Salti M. Acute pseudo-obstruction of the colon (Ogilvie’s syndrome). An analysis of 400 cases. Dis Colon Rectum. 1986;29(3):203-210. doi:10.1007/BF02555027
4. Devabhandari MP, Khan MA, Hooper TL. Cardiac compression following cardiac surgery due to unrecognised hiatus hernia. Eur J Cardiothoracic Surg. 2007;32(5):813-815. doi:10.1016/j.ejcts.2007.08.002
5. Asti E, Bonavina L, Lombardi M, Bandera F, Secchi F, Guazzi M. Reversibility of cardiopulmonary impairment after laparoscopic repair of large hiatal hernia. Int J Surg Case Rep. 2015;14:33-35. doi:10.1016/j.ijscr.2015.07.005
6. Tayyareci Y, Bayazit P, Taştan CP, Aksoy H. Right atrial compression due to idiopathic right diaphragm paralysis detected incidentally by transthoracic echocardiography. Turk Kardiyol Dern Ars. 2008;36(6):412-414.
7. Rohatgi N, Schulman K, Ahuja N. Comanagement by hospitalists: why it makes clinical and fiscal sense. Am J Med. 2020;133(3):257-258. doi:10.1016/j.amjmed.2019.07.053
8. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: a propensity score analysis. Ann Surg. 2016;264(2):275-282. doi:10.1097/SLA.0000000000001629
1. Bernardi M, Warrier S, Lynch C, Heriot A. Acute and chronic pseudo-obstruction: a current update. ANZ J Surg. 2015;85(10):709-714. doi:10.1111/ans.13148
2. Norwood MGA, Lykostratis H, Garcea G, Berry DP. Acute colonic pseudo-obstruction following major orthopaedic surgery. Colorectal Dis. 2005;7(5):496-499. doi:10.1111/j.1463-1318.2005.00790.x
3. Vanek VW, Al-Salti M. Acute pseudo-obstruction of the colon (Ogilvie’s syndrome). An analysis of 400 cases. Dis Colon Rectum. 1986;29(3):203-210. doi:10.1007/BF02555027
4. Devabhandari MP, Khan MA, Hooper TL. Cardiac compression following cardiac surgery due to unrecognised hiatus hernia. Eur J Cardiothoracic Surg. 2007;32(5):813-815. doi:10.1016/j.ejcts.2007.08.002
5. Asti E, Bonavina L, Lombardi M, Bandera F, Secchi F, Guazzi M. Reversibility of cardiopulmonary impairment after laparoscopic repair of large hiatal hernia. Int J Surg Case Rep. 2015;14:33-35. doi:10.1016/j.ijscr.2015.07.005
6. Tayyareci Y, Bayazit P, Taştan CP, Aksoy H. Right atrial compression due to idiopathic right diaphragm paralysis detected incidentally by transthoracic echocardiography. Turk Kardiyol Dern Ars. 2008;36(6):412-414.
7. Rohatgi N, Schulman K, Ahuja N. Comanagement by hospitalists: why it makes clinical and fiscal sense. Am J Med. 2020;133(3):257-258. doi:10.1016/j.amjmed.2019.07.053
8. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: a propensity score analysis. Ann Surg. 2016;264(2):275-282. doi:10.1097/SLA.0000000000001629
Renal Replacement Therapy in a Patient Diagnosed With Pancreatitis Secondary to Severe Leptospirosis
In areas where the zoonotic disease leptospirosis is endemic, reduced morbidity and mortality is strongly linked to quick initiation of renal replacement therapy.
Leptospirosis (LS) is considered the most common and widespread zoonotic disease in the world. Numerous outbreaks have occurred in the past 10 years. Due to its technically difficult diagnosis, LS is severely underrecognized, underdiagnosed, and therefore, underreported.1,2 The Centers for Disease Control and Prevention (CDC) estimate 100 to 150 cases of LS are identified annually in the US, with about 50% of those cases occurring in Puerto Rico (PR).3 Specifically in PR, about 15 to 100 cases of suspected LS were reported annually between 2000 and 2009, with 59 cases and 1 death reported in 2010. The data are thought to be severely underreported due to a lack of widespread diagnostic testing availability in PR and no formal veterinary and environmental surveillance programs to monitor the incidence of animal cases and actual circulating serovars.4
A recent systematic review of 80 studies from 34 countries on morbidity and mortality of LS revealed that the global incidence and mortality is about 1.03 million cases and 58,900 deaths every year. Almost half of the reported deaths were adult males aged 20 to 49 years.5 Although mild cases of LS are not associated with an elevated mortality, icteric LS with renal failure (Weil disease) carries a mortality rate of 10%.6 In patients who develop hemorrhagic pneumonitis, mortality may be as high as 50 to 70%.7 Therefore, it is pivotal that clinicians recognize the disease early, that novel modalities of treatment continue to be developed, and that their impact on patient morbidity and mortality are studied and documented.
Case Presentation
A 43-year-old man with a medical history of schizophrenia presented to the emergency department at the US Department of Veterans Affairs (VA) Caribbean Healthcare System in San Juan, PR, after experiencing 1 week of intermittent fever, myalgia, and general weakness. Emergency medical services had found him disheveled and in a rodent-infested swamp area several days before admission. Initial vital signs were within normal limits.
On physical examination, the patient was afebrile, without acute distress, but he had diffuse jaundice and mild epigastric tenderness without evidence of peritoneal irritation. His complete blood count was remarkable for leukocytosis with left shifting, adequate hemoglobin levels but with 9 × 103 U/L platelets. The complete metabolic panel demonstrated an aspartate aminotransferase level of 564 U/L, alanine transaminase level of 462 U/L, total bilirubin of 12 mg/dL, which 10.2 mg/dL were direct bilirubin, and an alkaline phosphate of 345 U/L. Lipase levels were measured at 626 U/L. Marked coagulopathy also was present. The toxicology panel, including acetaminophen and salicylate acid levels, did not reveal the presence of any of the tested substances, and chest imaging did not demonstrate any infiltrates.
An abdominal ultrasound was negative for acute cholestatic pathologies, such as cholelithiasis, cholecystitis, or choledocholithiasis. Nonetheless, a noncontrast abdominopelvic computed tomography was remarkable for peripancreatic fat stranding, which raised suspicion for a diagnosis of pancreatitis.
Once the patient was transferred to the intensive care unit, he developed several episodes of hematemesis, leading to hemodynamical instability and severe respiratory distress. Due to anticipated respiratory failure and need for airway securement, endotracheal intubation was performed. Multiple packed red blood cells were transfused, and the patient was started in vasopressor support.
Diagnosis
A presumptive diagnosis of LS was made due to a considerable history of rodent exposure. The patient was started on broad-spectrum parenteral antibiotics, vancomycin 750 mg every 24 hours, metronidazole 500 mg every 8 hours, and ceftriaxone 2 g IV daily for adequate coverage against Leptospira spp. Despite 72 hours of antibiotic treatment, the patient’s clinical state deteriorated. He required high dosages of norepinephrine (1.5 mcg/kg/min) and vasopressin (0.03 U/min) to maintain adequate organ perfusion. Despite lung protective settings with low tidal volume and a high positive end-expiratory pressure, there was difficulty maintaining adequate oxygenation. Chest imaging was remarkable for bilateral infiltrates concerning for acute respiratory distress syndrome (ARDS).
The coagulopathy and cholestasis continued to worsen, and the renal failure progressed from nonoliguric to anuric. Because of this progression, the patient was started on continuous renal replacement therapy (CRRT) by hemodialysis. Within 24 hours of initiating CRRT, the patient’s clinical status improved dramatically. Vasopressor support was weaned, the coagulopathy resolved, and the cholestasis was improving. The patient’s respiratory status improved in such a manner that he was extubated by the seventh day after being placed on mechanical ventilation. The urine and blood samples sent for identification of Leptospira spp. through polymerase chain reaction (PCR) returned positive by the ninth day of admission. While on CRRT, the patient’s renal function eventually returned to baseline, and he was discharged 12 days after admission.
Discussion
The spirochetes of the genus Leptospira include both saprophytic and pathogenic species. These pathogenic Leptospira spp. have adapted to a grand variety of zoonotic hosts, the most important being rodents. They serve as vectors for the contraction of the disease by humans. Initial infection in rodents by Leptospira spp. causes a systemic illness followed by a persistent colonization of renal tubules from which they are excreted in the urine and into the environment. Humans, in turn, are an incidental host unable to induce a carrier state for the transmission of the pathogenic organism.1 The time from exposure to onset of symptoms, or incubation phase, averages 7 to 12 days but may range from 3 to 30 days.8
LS has been described as having 2 discernable but often coexisting phases. The first, an acute febrile bacteremic phase, has been noted to last about 9 days in about 85% of patients, although a minority have persistent fever from 2 weeks to > 30 days. A second phase, the immune or inflammatory phase, is characterized by a second fever spike preceded by 1 to 5 afebrile days in which there is presence of IgM antibodies and resolution of leptospiremia but positive urine cultures.9 Weil disease may present as the second phase of the disease or as a single, progressive illness from its first manifestation. It is characterized by a triad of jaundice, renal failure, and hemorrhage or coagulopathy.10 Weil disease is of great concern and importance due to its associated higher mortality than that found with the mildest form of the disease.
There are studies that advocate for RRT as an intricate part of the treatment regimen in LS to remove the inflammatory cytokines produced as a reaction to the spirochete.11 In tropical countries with a higher incidence of the disease, leptospirosis is an important cause of acute kidney injury (AKI), depending on multiple factors, including the AKI definition that is used.12 Renal invasion by Leptospira spp. produces acute tubular necrosis (ATN) and cell edema during the first week and then could progress to acute interstitial nephritis (AIN) in 2 to 3 weeks. It is believed that the mechanism for the Leptospira spp. invasion of the tubules that results in damage is associated with the antigenic components in its outer membrane; the most important outer membrane protein expressed during infection is LipL32. This protein increases the production of proinflammatory proteins, such as inducible nitric oxide synthase, monocyte chemotactic protein-1 (CCL2/MCP-1), T cells, and tumor necrosis factor.13
Although doxycycline has been recommended for the prophylaxis and treatment of mild LS, the preferred agent and the conferred benefits of antibiotic treatment overall for the severe form of the disease has been controversial. Traditionally, penicillin G sodium has been recommended as the first-line antibiotic treatment for moderate-to-severe LS.14 Nonetheless, there has been an increasing pattern of penicillin resistance among Leptospira spp. that has prompted the study and use of alternative agents.
An open-label, randomized comparison of parenteral cefotaxime, penicillin G sodium, and doxycycline for the treatment of suspected severe leptospirosis conducted by Suputtamongkol and colleagues showed no difference in mortality, defervescence, or time to resolution of abnormal laboratory findings.15 Current CDC recommendations include the use of parenteral penicillin 1.5 MU every 6 hours as the drug of choice, with ceftriaxone 1 g administered IV every 24 hours equally as effective.3
In addition to antimicrobial therapy, supportive care has shifted to include hemodialysis in those patients who develop AKI as part of the disease. Andrade and colleagues conducted a study of 33 patients with LS in Brazil that was set to compare the impact of door-to-dialysis time and dosage of hemodialysis on mortality. In patients with a quicker door-to-dialysis time and daily hemodialysis sessions, there was a 50% (16.7% vs 66.7%) absolute mortality reduction when compared with those with delayed initiation and alternate-day hemodialysis sessions.11 A follow-up prospective study compared the use of traditional sustained low-efficiency dialysis (SLED) with the use of extended SLED via hemodiafiltration in patients with LS presenting with ARDS and AKI. Although hemodiafiltration resulted in a relative decrease in serum levels of interleukin (IL)-17, IL-7, and CCL2/MCP-1, there was no significant difference in mortality.16 The most important prognostic factor in severe LS presenting with AKI and relating to RRT is a shorter door-to-dialysis time and increased dose, not the mode of dialysis clearance. Nonetheless, both RRT methods resulted in a progressive decrease in inflammatory mediators that have been associated with ATN and AIN in the context of LS.16 The authors argue that using CRRT instead of SLED via hemodiafiltration could have accentuated the effects of the reduction that inflammatory mediators may have on mortality in patients with severe LS.
Conclusions
LS continues to be of interest due to its current status as the most common zoonotic disease and its widespread prevalence throughout the globe. Novel treatment modalities for LS, specifically for Weil disease, continue to be developed with the goal of reducing the current mortality rate associated with the disease.
In endemic areas, prompt recognition is essential to initiate the recommended therapy. Parenteral antibiotics, such as penicillin G sodium and ceftriaxone, continue to be the mainstay of treatment and constitute the current CDC recommendations. Nonetheless, early initiation of CRRT has been shown to greatly reduce the mortality associated with Weil disease and, when available, should be considered in these patients.
Our patient failed to improve while receiving parenteral antibiotics alone but showed marked improvement after being placed on CRRT. Furthermore, initiation of CRRT resulted in near-complete resolution of his organ dysfunction and eventual discharge from the hospital. This case serves to further support the use of early CRRT as part of the standard of care in severe LS.
1. Ko AI, Goarant C, Picardeau M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol. 2009;7(10):736-747. doi:10.1038/nrmicro2208
2. Hartskeerl RA, Collares-Pereira M, Ellis WA. Emergence, control and re-emerging leptospirosis: dynamics of infection in the changing world. Clin Microbiol Infect. 2011;17(4):494-501. doi:10.1111/j.1469-0691.2011.03474.x
3. Centers for Disease Control and Prevention. Leptospirosis fact sheet for clinicians, CS287535B. https://www.cdc.gov/leptospirosis/pdf/fs-leptospirosis-clinicians-eng-508.pdf. Published January 30, 2018. Accessed October 9, 2020.
4. Martinez-Recio C, Rodriguez-Cintron W, Galarza-Vargas S, et al. The brief case: cases from 3 hospitals in Puerto Rico. ACP Hosp. https://acphospitalist.org/archives/2014/09/briefcase.htm. Published September 2014. Accessed October 9, 2020.
5. Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898. doi:10.1371/journal.pntd.0003898
6. Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296-326. doi:10.1128/CMR.14.2.296-326.2001
7. Vijayachari P, Sugunan AP, Shriram AN. Leptospirosis: an emerging global public health problem. J Biosci. 2008;33(4):557-569. doi:10.1007/s12038-008-0074-z
8. Haake DA, Levett PN. Leptospirosis in humans. In: Adler B, ed. Leptospira and Leptospirosis. Berlin, Heidelberg: Springer-Verlag Berlin Heidelberg; 2015:65-97. doi:10.1007/978-3-662-45059-8_5
9. Berman SJ. Sporadic anicteric leptospirosis in South Vietnam: a study in 150 patients. Ann Intern Med. 1973;79(2):167. doi:10.7326/0003-4819-79-2-167
10. Bharti AR, Nally JE, Ricaldi JN, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3(12):757-771. doi:10.1016/S1473-3099(03)00830-2
11. Andrade L, Cleto S, Seguro AC. Door-to-dialysis time and daily hemodialysis in patients with leptospirosis: impact on mortality. Clin J Am Soc Nephrol. 2007;2(4):739–744. doi: 10.2215/CJN.00680207
12. Mathew A, George J. Acute kidney injury in the tropics. Ann Saudi Med. 2011;31(5):451-456. doi:10.4103/0256-4947.84620
13. Daher EF, Silva GB Jr, Karbage NNN, et al. Predictors of oliguric acute kidney injury in leptospirosis. Nephron Clin Pract. 2009;112(1):c25-c30. doi:10.1159/000210571
14. Panaphut T, Domrongkitchaiporn S, Vibhagool A, Thinkamrop B, Susaengrat W. Ceftriaxone compared with sodium penicillin g for treatment of severe leptospirosis. Clin Infect Dis. 2003;36(12):1507-1513. doi:10.1086/375226
15. Suputtamongkol Y, Niwattayakul K, Suttinont C, et al. An open, randomized, controlled trial of penicillin, doxycycline, and cefotaxime for patients with severe leptospirosis. Clin Infect Dis. 2004;39(10):1417-1424. doi:10.1086/425001
16. Cleto SA, Rodrigues CE, Malaque CM, Sztajnbok J, Seguro AC, Andrade L. Hemodiafiltration decreases serum levels of inflammatory mediators in severe leptospirosis: a prospective study. PLoS ONE. 2016;11(8):e0160010. doi:10.1371/journal.pone.0160010
In areas where the zoonotic disease leptospirosis is endemic, reduced morbidity and mortality is strongly linked to quick initiation of renal replacement therapy.
In areas where the zoonotic disease leptospirosis is endemic, reduced morbidity and mortality is strongly linked to quick initiation of renal replacement therapy.
Leptospirosis (LS) is considered the most common and widespread zoonotic disease in the world. Numerous outbreaks have occurred in the past 10 years. Due to its technically difficult diagnosis, LS is severely underrecognized, underdiagnosed, and therefore, underreported.1,2 The Centers for Disease Control and Prevention (CDC) estimate 100 to 150 cases of LS are identified annually in the US, with about 50% of those cases occurring in Puerto Rico (PR).3 Specifically in PR, about 15 to 100 cases of suspected LS were reported annually between 2000 and 2009, with 59 cases and 1 death reported in 2010. The data are thought to be severely underreported due to a lack of widespread diagnostic testing availability in PR and no formal veterinary and environmental surveillance programs to monitor the incidence of animal cases and actual circulating serovars.4
A recent systematic review of 80 studies from 34 countries on morbidity and mortality of LS revealed that the global incidence and mortality is about 1.03 million cases and 58,900 deaths every year. Almost half of the reported deaths were adult males aged 20 to 49 years.5 Although mild cases of LS are not associated with an elevated mortality, icteric LS with renal failure (Weil disease) carries a mortality rate of 10%.6 In patients who develop hemorrhagic pneumonitis, mortality may be as high as 50 to 70%.7 Therefore, it is pivotal that clinicians recognize the disease early, that novel modalities of treatment continue to be developed, and that their impact on patient morbidity and mortality are studied and documented.
Case Presentation
A 43-year-old man with a medical history of schizophrenia presented to the emergency department at the US Department of Veterans Affairs (VA) Caribbean Healthcare System in San Juan, PR, after experiencing 1 week of intermittent fever, myalgia, and general weakness. Emergency medical services had found him disheveled and in a rodent-infested swamp area several days before admission. Initial vital signs were within normal limits.
On physical examination, the patient was afebrile, without acute distress, but he had diffuse jaundice and mild epigastric tenderness without evidence of peritoneal irritation. His complete blood count was remarkable for leukocytosis with left shifting, adequate hemoglobin levels but with 9 × 103 U/L platelets. The complete metabolic panel demonstrated an aspartate aminotransferase level of 564 U/L, alanine transaminase level of 462 U/L, total bilirubin of 12 mg/dL, which 10.2 mg/dL were direct bilirubin, and an alkaline phosphate of 345 U/L. Lipase levels were measured at 626 U/L. Marked coagulopathy also was present. The toxicology panel, including acetaminophen and salicylate acid levels, did not reveal the presence of any of the tested substances, and chest imaging did not demonstrate any infiltrates.
An abdominal ultrasound was negative for acute cholestatic pathologies, such as cholelithiasis, cholecystitis, or choledocholithiasis. Nonetheless, a noncontrast abdominopelvic computed tomography was remarkable for peripancreatic fat stranding, which raised suspicion for a diagnosis of pancreatitis.
Once the patient was transferred to the intensive care unit, he developed several episodes of hematemesis, leading to hemodynamical instability and severe respiratory distress. Due to anticipated respiratory failure and need for airway securement, endotracheal intubation was performed. Multiple packed red blood cells were transfused, and the patient was started in vasopressor support.
Diagnosis
A presumptive diagnosis of LS was made due to a considerable history of rodent exposure. The patient was started on broad-spectrum parenteral antibiotics, vancomycin 750 mg every 24 hours, metronidazole 500 mg every 8 hours, and ceftriaxone 2 g IV daily for adequate coverage against Leptospira spp. Despite 72 hours of antibiotic treatment, the patient’s clinical state deteriorated. He required high dosages of norepinephrine (1.5 mcg/kg/min) and vasopressin (0.03 U/min) to maintain adequate organ perfusion. Despite lung protective settings with low tidal volume and a high positive end-expiratory pressure, there was difficulty maintaining adequate oxygenation. Chest imaging was remarkable for bilateral infiltrates concerning for acute respiratory distress syndrome (ARDS).
The coagulopathy and cholestasis continued to worsen, and the renal failure progressed from nonoliguric to anuric. Because of this progression, the patient was started on continuous renal replacement therapy (CRRT) by hemodialysis. Within 24 hours of initiating CRRT, the patient’s clinical status improved dramatically. Vasopressor support was weaned, the coagulopathy resolved, and the cholestasis was improving. The patient’s respiratory status improved in such a manner that he was extubated by the seventh day after being placed on mechanical ventilation. The urine and blood samples sent for identification of Leptospira spp. through polymerase chain reaction (PCR) returned positive by the ninth day of admission. While on CRRT, the patient’s renal function eventually returned to baseline, and he was discharged 12 days after admission.
Discussion
The spirochetes of the genus Leptospira include both saprophytic and pathogenic species. These pathogenic Leptospira spp. have adapted to a grand variety of zoonotic hosts, the most important being rodents. They serve as vectors for the contraction of the disease by humans. Initial infection in rodents by Leptospira spp. causes a systemic illness followed by a persistent colonization of renal tubules from which they are excreted in the urine and into the environment. Humans, in turn, are an incidental host unable to induce a carrier state for the transmission of the pathogenic organism.1 The time from exposure to onset of symptoms, or incubation phase, averages 7 to 12 days but may range from 3 to 30 days.8
LS has been described as having 2 discernable but often coexisting phases. The first, an acute febrile bacteremic phase, has been noted to last about 9 days in about 85% of patients, although a minority have persistent fever from 2 weeks to > 30 days. A second phase, the immune or inflammatory phase, is characterized by a second fever spike preceded by 1 to 5 afebrile days in which there is presence of IgM antibodies and resolution of leptospiremia but positive urine cultures.9 Weil disease may present as the second phase of the disease or as a single, progressive illness from its first manifestation. It is characterized by a triad of jaundice, renal failure, and hemorrhage or coagulopathy.10 Weil disease is of great concern and importance due to its associated higher mortality than that found with the mildest form of the disease.
There are studies that advocate for RRT as an intricate part of the treatment regimen in LS to remove the inflammatory cytokines produced as a reaction to the spirochete.11 In tropical countries with a higher incidence of the disease, leptospirosis is an important cause of acute kidney injury (AKI), depending on multiple factors, including the AKI definition that is used.12 Renal invasion by Leptospira spp. produces acute tubular necrosis (ATN) and cell edema during the first week and then could progress to acute interstitial nephritis (AIN) in 2 to 3 weeks. It is believed that the mechanism for the Leptospira spp. invasion of the tubules that results in damage is associated with the antigenic components in its outer membrane; the most important outer membrane protein expressed during infection is LipL32. This protein increases the production of proinflammatory proteins, such as inducible nitric oxide synthase, monocyte chemotactic protein-1 (CCL2/MCP-1), T cells, and tumor necrosis factor.13
Although doxycycline has been recommended for the prophylaxis and treatment of mild LS, the preferred agent and the conferred benefits of antibiotic treatment overall for the severe form of the disease has been controversial. Traditionally, penicillin G sodium has been recommended as the first-line antibiotic treatment for moderate-to-severe LS.14 Nonetheless, there has been an increasing pattern of penicillin resistance among Leptospira spp. that has prompted the study and use of alternative agents.
An open-label, randomized comparison of parenteral cefotaxime, penicillin G sodium, and doxycycline for the treatment of suspected severe leptospirosis conducted by Suputtamongkol and colleagues showed no difference in mortality, defervescence, or time to resolution of abnormal laboratory findings.15 Current CDC recommendations include the use of parenteral penicillin 1.5 MU every 6 hours as the drug of choice, with ceftriaxone 1 g administered IV every 24 hours equally as effective.3
In addition to antimicrobial therapy, supportive care has shifted to include hemodialysis in those patients who develop AKI as part of the disease. Andrade and colleagues conducted a study of 33 patients with LS in Brazil that was set to compare the impact of door-to-dialysis time and dosage of hemodialysis on mortality. In patients with a quicker door-to-dialysis time and daily hemodialysis sessions, there was a 50% (16.7% vs 66.7%) absolute mortality reduction when compared with those with delayed initiation and alternate-day hemodialysis sessions.11 A follow-up prospective study compared the use of traditional sustained low-efficiency dialysis (SLED) with the use of extended SLED via hemodiafiltration in patients with LS presenting with ARDS and AKI. Although hemodiafiltration resulted in a relative decrease in serum levels of interleukin (IL)-17, IL-7, and CCL2/MCP-1, there was no significant difference in mortality.16 The most important prognostic factor in severe LS presenting with AKI and relating to RRT is a shorter door-to-dialysis time and increased dose, not the mode of dialysis clearance. Nonetheless, both RRT methods resulted in a progressive decrease in inflammatory mediators that have been associated with ATN and AIN in the context of LS.16 The authors argue that using CRRT instead of SLED via hemodiafiltration could have accentuated the effects of the reduction that inflammatory mediators may have on mortality in patients with severe LS.
Conclusions
LS continues to be of interest due to its current status as the most common zoonotic disease and its widespread prevalence throughout the globe. Novel treatment modalities for LS, specifically for Weil disease, continue to be developed with the goal of reducing the current mortality rate associated with the disease.
In endemic areas, prompt recognition is essential to initiate the recommended therapy. Parenteral antibiotics, such as penicillin G sodium and ceftriaxone, continue to be the mainstay of treatment and constitute the current CDC recommendations. Nonetheless, early initiation of CRRT has been shown to greatly reduce the mortality associated with Weil disease and, when available, should be considered in these patients.
Our patient failed to improve while receiving parenteral antibiotics alone but showed marked improvement after being placed on CRRT. Furthermore, initiation of CRRT resulted in near-complete resolution of his organ dysfunction and eventual discharge from the hospital. This case serves to further support the use of early CRRT as part of the standard of care in severe LS.
Leptospirosis (LS) is considered the most common and widespread zoonotic disease in the world. Numerous outbreaks have occurred in the past 10 years. Due to its technically difficult diagnosis, LS is severely underrecognized, underdiagnosed, and therefore, underreported.1,2 The Centers for Disease Control and Prevention (CDC) estimate 100 to 150 cases of LS are identified annually in the US, with about 50% of those cases occurring in Puerto Rico (PR).3 Specifically in PR, about 15 to 100 cases of suspected LS were reported annually between 2000 and 2009, with 59 cases and 1 death reported in 2010. The data are thought to be severely underreported due to a lack of widespread diagnostic testing availability in PR and no formal veterinary and environmental surveillance programs to monitor the incidence of animal cases and actual circulating serovars.4
A recent systematic review of 80 studies from 34 countries on morbidity and mortality of LS revealed that the global incidence and mortality is about 1.03 million cases and 58,900 deaths every year. Almost half of the reported deaths were adult males aged 20 to 49 years.5 Although mild cases of LS are not associated with an elevated mortality, icteric LS with renal failure (Weil disease) carries a mortality rate of 10%.6 In patients who develop hemorrhagic pneumonitis, mortality may be as high as 50 to 70%.7 Therefore, it is pivotal that clinicians recognize the disease early, that novel modalities of treatment continue to be developed, and that their impact on patient morbidity and mortality are studied and documented.
Case Presentation
A 43-year-old man with a medical history of schizophrenia presented to the emergency department at the US Department of Veterans Affairs (VA) Caribbean Healthcare System in San Juan, PR, after experiencing 1 week of intermittent fever, myalgia, and general weakness. Emergency medical services had found him disheveled and in a rodent-infested swamp area several days before admission. Initial vital signs were within normal limits.
On physical examination, the patient was afebrile, without acute distress, but he had diffuse jaundice and mild epigastric tenderness without evidence of peritoneal irritation. His complete blood count was remarkable for leukocytosis with left shifting, adequate hemoglobin levels but with 9 × 103 U/L platelets. The complete metabolic panel demonstrated an aspartate aminotransferase level of 564 U/L, alanine transaminase level of 462 U/L, total bilirubin of 12 mg/dL, which 10.2 mg/dL were direct bilirubin, and an alkaline phosphate of 345 U/L. Lipase levels were measured at 626 U/L. Marked coagulopathy also was present. The toxicology panel, including acetaminophen and salicylate acid levels, did not reveal the presence of any of the tested substances, and chest imaging did not demonstrate any infiltrates.
An abdominal ultrasound was negative for acute cholestatic pathologies, such as cholelithiasis, cholecystitis, or choledocholithiasis. Nonetheless, a noncontrast abdominopelvic computed tomography was remarkable for peripancreatic fat stranding, which raised suspicion for a diagnosis of pancreatitis.
Once the patient was transferred to the intensive care unit, he developed several episodes of hematemesis, leading to hemodynamical instability and severe respiratory distress. Due to anticipated respiratory failure and need for airway securement, endotracheal intubation was performed. Multiple packed red blood cells were transfused, and the patient was started in vasopressor support.
Diagnosis
A presumptive diagnosis of LS was made due to a considerable history of rodent exposure. The patient was started on broad-spectrum parenteral antibiotics, vancomycin 750 mg every 24 hours, metronidazole 500 mg every 8 hours, and ceftriaxone 2 g IV daily for adequate coverage against Leptospira spp. Despite 72 hours of antibiotic treatment, the patient’s clinical state deteriorated. He required high dosages of norepinephrine (1.5 mcg/kg/min) and vasopressin (0.03 U/min) to maintain adequate organ perfusion. Despite lung protective settings with low tidal volume and a high positive end-expiratory pressure, there was difficulty maintaining adequate oxygenation. Chest imaging was remarkable for bilateral infiltrates concerning for acute respiratory distress syndrome (ARDS).
The coagulopathy and cholestasis continued to worsen, and the renal failure progressed from nonoliguric to anuric. Because of this progression, the patient was started on continuous renal replacement therapy (CRRT) by hemodialysis. Within 24 hours of initiating CRRT, the patient’s clinical status improved dramatically. Vasopressor support was weaned, the coagulopathy resolved, and the cholestasis was improving. The patient’s respiratory status improved in such a manner that he was extubated by the seventh day after being placed on mechanical ventilation. The urine and blood samples sent for identification of Leptospira spp. through polymerase chain reaction (PCR) returned positive by the ninth day of admission. While on CRRT, the patient’s renal function eventually returned to baseline, and he was discharged 12 days after admission.
Discussion
The spirochetes of the genus Leptospira include both saprophytic and pathogenic species. These pathogenic Leptospira spp. have adapted to a grand variety of zoonotic hosts, the most important being rodents. They serve as vectors for the contraction of the disease by humans. Initial infection in rodents by Leptospira spp. causes a systemic illness followed by a persistent colonization of renal tubules from which they are excreted in the urine and into the environment. Humans, in turn, are an incidental host unable to induce a carrier state for the transmission of the pathogenic organism.1 The time from exposure to onset of symptoms, or incubation phase, averages 7 to 12 days but may range from 3 to 30 days.8
LS has been described as having 2 discernable but often coexisting phases. The first, an acute febrile bacteremic phase, has been noted to last about 9 days in about 85% of patients, although a minority have persistent fever from 2 weeks to > 30 days. A second phase, the immune or inflammatory phase, is characterized by a second fever spike preceded by 1 to 5 afebrile days in which there is presence of IgM antibodies and resolution of leptospiremia but positive urine cultures.9 Weil disease may present as the second phase of the disease or as a single, progressive illness from its first manifestation. It is characterized by a triad of jaundice, renal failure, and hemorrhage or coagulopathy.10 Weil disease is of great concern and importance due to its associated higher mortality than that found with the mildest form of the disease.
There are studies that advocate for RRT as an intricate part of the treatment regimen in LS to remove the inflammatory cytokines produced as a reaction to the spirochete.11 In tropical countries with a higher incidence of the disease, leptospirosis is an important cause of acute kidney injury (AKI), depending on multiple factors, including the AKI definition that is used.12 Renal invasion by Leptospira spp. produces acute tubular necrosis (ATN) and cell edema during the first week and then could progress to acute interstitial nephritis (AIN) in 2 to 3 weeks. It is believed that the mechanism for the Leptospira spp. invasion of the tubules that results in damage is associated with the antigenic components in its outer membrane; the most important outer membrane protein expressed during infection is LipL32. This protein increases the production of proinflammatory proteins, such as inducible nitric oxide synthase, monocyte chemotactic protein-1 (CCL2/MCP-1), T cells, and tumor necrosis factor.13
Although doxycycline has been recommended for the prophylaxis and treatment of mild LS, the preferred agent and the conferred benefits of antibiotic treatment overall for the severe form of the disease has been controversial. Traditionally, penicillin G sodium has been recommended as the first-line antibiotic treatment for moderate-to-severe LS.14 Nonetheless, there has been an increasing pattern of penicillin resistance among Leptospira spp. that has prompted the study and use of alternative agents.
An open-label, randomized comparison of parenteral cefotaxime, penicillin G sodium, and doxycycline for the treatment of suspected severe leptospirosis conducted by Suputtamongkol and colleagues showed no difference in mortality, defervescence, or time to resolution of abnormal laboratory findings.15 Current CDC recommendations include the use of parenteral penicillin 1.5 MU every 6 hours as the drug of choice, with ceftriaxone 1 g administered IV every 24 hours equally as effective.3
In addition to antimicrobial therapy, supportive care has shifted to include hemodialysis in those patients who develop AKI as part of the disease. Andrade and colleagues conducted a study of 33 patients with LS in Brazil that was set to compare the impact of door-to-dialysis time and dosage of hemodialysis on mortality. In patients with a quicker door-to-dialysis time and daily hemodialysis sessions, there was a 50% (16.7% vs 66.7%) absolute mortality reduction when compared with those with delayed initiation and alternate-day hemodialysis sessions.11 A follow-up prospective study compared the use of traditional sustained low-efficiency dialysis (SLED) with the use of extended SLED via hemodiafiltration in patients with LS presenting with ARDS and AKI. Although hemodiafiltration resulted in a relative decrease in serum levels of interleukin (IL)-17, IL-7, and CCL2/MCP-1, there was no significant difference in mortality.16 The most important prognostic factor in severe LS presenting with AKI and relating to RRT is a shorter door-to-dialysis time and increased dose, not the mode of dialysis clearance. Nonetheless, both RRT methods resulted in a progressive decrease in inflammatory mediators that have been associated with ATN and AIN in the context of LS.16 The authors argue that using CRRT instead of SLED via hemodiafiltration could have accentuated the effects of the reduction that inflammatory mediators may have on mortality in patients with severe LS.
Conclusions
LS continues to be of interest due to its current status as the most common zoonotic disease and its widespread prevalence throughout the globe. Novel treatment modalities for LS, specifically for Weil disease, continue to be developed with the goal of reducing the current mortality rate associated with the disease.
In endemic areas, prompt recognition is essential to initiate the recommended therapy. Parenteral antibiotics, such as penicillin G sodium and ceftriaxone, continue to be the mainstay of treatment and constitute the current CDC recommendations. Nonetheless, early initiation of CRRT has been shown to greatly reduce the mortality associated with Weil disease and, when available, should be considered in these patients.
Our patient failed to improve while receiving parenteral antibiotics alone but showed marked improvement after being placed on CRRT. Furthermore, initiation of CRRT resulted in near-complete resolution of his organ dysfunction and eventual discharge from the hospital. This case serves to further support the use of early CRRT as part of the standard of care in severe LS.
1. Ko AI, Goarant C, Picardeau M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol. 2009;7(10):736-747. doi:10.1038/nrmicro2208
2. Hartskeerl RA, Collares-Pereira M, Ellis WA. Emergence, control and re-emerging leptospirosis: dynamics of infection in the changing world. Clin Microbiol Infect. 2011;17(4):494-501. doi:10.1111/j.1469-0691.2011.03474.x
3. Centers for Disease Control and Prevention. Leptospirosis fact sheet for clinicians, CS287535B. https://www.cdc.gov/leptospirosis/pdf/fs-leptospirosis-clinicians-eng-508.pdf. Published January 30, 2018. Accessed October 9, 2020.
4. Martinez-Recio C, Rodriguez-Cintron W, Galarza-Vargas S, et al. The brief case: cases from 3 hospitals in Puerto Rico. ACP Hosp. https://acphospitalist.org/archives/2014/09/briefcase.htm. Published September 2014. Accessed October 9, 2020.
5. Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898. doi:10.1371/journal.pntd.0003898
6. Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296-326. doi:10.1128/CMR.14.2.296-326.2001
7. Vijayachari P, Sugunan AP, Shriram AN. Leptospirosis: an emerging global public health problem. J Biosci. 2008;33(4):557-569. doi:10.1007/s12038-008-0074-z
8. Haake DA, Levett PN. Leptospirosis in humans. In: Adler B, ed. Leptospira and Leptospirosis. Berlin, Heidelberg: Springer-Verlag Berlin Heidelberg; 2015:65-97. doi:10.1007/978-3-662-45059-8_5
9. Berman SJ. Sporadic anicteric leptospirosis in South Vietnam: a study in 150 patients. Ann Intern Med. 1973;79(2):167. doi:10.7326/0003-4819-79-2-167
10. Bharti AR, Nally JE, Ricaldi JN, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3(12):757-771. doi:10.1016/S1473-3099(03)00830-2
11. Andrade L, Cleto S, Seguro AC. Door-to-dialysis time and daily hemodialysis in patients with leptospirosis: impact on mortality. Clin J Am Soc Nephrol. 2007;2(4):739–744. doi: 10.2215/CJN.00680207
12. Mathew A, George J. Acute kidney injury in the tropics. Ann Saudi Med. 2011;31(5):451-456. doi:10.4103/0256-4947.84620
13. Daher EF, Silva GB Jr, Karbage NNN, et al. Predictors of oliguric acute kidney injury in leptospirosis. Nephron Clin Pract. 2009;112(1):c25-c30. doi:10.1159/000210571
14. Panaphut T, Domrongkitchaiporn S, Vibhagool A, Thinkamrop B, Susaengrat W. Ceftriaxone compared with sodium penicillin g for treatment of severe leptospirosis. Clin Infect Dis. 2003;36(12):1507-1513. doi:10.1086/375226
15. Suputtamongkol Y, Niwattayakul K, Suttinont C, et al. An open, randomized, controlled trial of penicillin, doxycycline, and cefotaxime for patients with severe leptospirosis. Clin Infect Dis. 2004;39(10):1417-1424. doi:10.1086/425001
16. Cleto SA, Rodrigues CE, Malaque CM, Sztajnbok J, Seguro AC, Andrade L. Hemodiafiltration decreases serum levels of inflammatory mediators in severe leptospirosis: a prospective study. PLoS ONE. 2016;11(8):e0160010. doi:10.1371/journal.pone.0160010
1. Ko AI, Goarant C, Picardeau M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol. 2009;7(10):736-747. doi:10.1038/nrmicro2208
2. Hartskeerl RA, Collares-Pereira M, Ellis WA. Emergence, control and re-emerging leptospirosis: dynamics of infection in the changing world. Clin Microbiol Infect. 2011;17(4):494-501. doi:10.1111/j.1469-0691.2011.03474.x
3. Centers for Disease Control and Prevention. Leptospirosis fact sheet for clinicians, CS287535B. https://www.cdc.gov/leptospirosis/pdf/fs-leptospirosis-clinicians-eng-508.pdf. Published January 30, 2018. Accessed October 9, 2020.
4. Martinez-Recio C, Rodriguez-Cintron W, Galarza-Vargas S, et al. The brief case: cases from 3 hospitals in Puerto Rico. ACP Hosp. https://acphospitalist.org/archives/2014/09/briefcase.htm. Published September 2014. Accessed October 9, 2020.
5. Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898. doi:10.1371/journal.pntd.0003898
6. Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296-326. doi:10.1128/CMR.14.2.296-326.2001
7. Vijayachari P, Sugunan AP, Shriram AN. Leptospirosis: an emerging global public health problem. J Biosci. 2008;33(4):557-569. doi:10.1007/s12038-008-0074-z
8. Haake DA, Levett PN. Leptospirosis in humans. In: Adler B, ed. Leptospira and Leptospirosis. Berlin, Heidelberg: Springer-Verlag Berlin Heidelberg; 2015:65-97. doi:10.1007/978-3-662-45059-8_5
9. Berman SJ. Sporadic anicteric leptospirosis in South Vietnam: a study in 150 patients. Ann Intern Med. 1973;79(2):167. doi:10.7326/0003-4819-79-2-167
10. Bharti AR, Nally JE, Ricaldi JN, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3(12):757-771. doi:10.1016/S1473-3099(03)00830-2
11. Andrade L, Cleto S, Seguro AC. Door-to-dialysis time and daily hemodialysis in patients with leptospirosis: impact on mortality. Clin J Am Soc Nephrol. 2007;2(4):739–744. doi: 10.2215/CJN.00680207
12. Mathew A, George J. Acute kidney injury in the tropics. Ann Saudi Med. 2011;31(5):451-456. doi:10.4103/0256-4947.84620
13. Daher EF, Silva GB Jr, Karbage NNN, et al. Predictors of oliguric acute kidney injury in leptospirosis. Nephron Clin Pract. 2009;112(1):c25-c30. doi:10.1159/000210571
14. Panaphut T, Domrongkitchaiporn S, Vibhagool A, Thinkamrop B, Susaengrat W. Ceftriaxone compared with sodium penicillin g for treatment of severe leptospirosis. Clin Infect Dis. 2003;36(12):1507-1513. doi:10.1086/375226
15. Suputtamongkol Y, Niwattayakul K, Suttinont C, et al. An open, randomized, controlled trial of penicillin, doxycycline, and cefotaxime for patients with severe leptospirosis. Clin Infect Dis. 2004;39(10):1417-1424. doi:10.1086/425001
16. Cleto SA, Rodrigues CE, Malaque CM, Sztajnbok J, Seguro AC, Andrade L. Hemodiafiltration decreases serum levels of inflammatory mediators in severe leptospirosis: a prospective study. PLoS ONE. 2016;11(8):e0160010. doi:10.1371/journal.pone.0160010
Burnt Out ? The Phenomenon of Type 2 Diabetes Mellitus in End-Stage Renal Disease
In patients with T2DM and ESRD, insulin is the antidiabetic medication of choice with a hemoglobin A1c target of 6 to 8%, using fructosamine levels or other measures for better assessment of glycemic control.
More than 34 million adults in the US have type 2 diabetes mellitus (T2DM), a chronic progressive disease identified by worsening hyperglycemia and micro- and macrovascular complications.1 Consequently, 12.2% of the US adult population is currently at risk for macrovascular diseases, such as stroke and coronary artery disease (CAD) and microvascular diseases, such as neuropathy and diabetic nephropathy.1
T2DM is the most common comorbid risk factor for chronic kidney disease (CKD) and the leading cause of end-stage renal disease (ESRD). As of 2017, about 750,000 Americans have CKD stage 5 requiring dialysis, and 50% of these patients have preexisting diabetic nephropathy.2 Rates of mortality and morbidity are observed to be higher in patients with both CKD and T2DM compared with patients with CKD without T2DM.2 Previous clinical trials, including the United Kingdom Prospective Diabetes Study of 1998, have proven that optimal glycemic control decreases the risk of complications of T2DM (ie, nephropathy) in the general population.3 Conversely, tight glycemic control that targets hemoglobin A1c (HbA1c) < 7%, in patients with T2DM with ESRD has not shown the same benefits and may lead to worse outcomes. It is postulated that this may be due to the increased incidence of hypoglycemia in this patient population.4
Dialysis has varying effects on patients both with and without T2DM. While patients with ESRD without T2DM have the potential to develop impaired glucose tolerance and T2DM, about 33% of patients with T2DM on dialysis actually have HbA1c < 6%.5 In these patients, glycemic control improves spontaneously as their disease progresses, leading to a decrease or cessation of insulin or other antidiabetic medications. This phenomenon, known as burnt-out diabetes, is characterized by (1) alterations in glucose homeostasis and normoglycemia without antidiabetic treatment; (2) HbA1c levels < 6% despite having established T2DM; (3) decline in insulin requirements or cessation of insulin altogether; and (4) spontaneous hypoglycemia.
There is a misconception that burnt-out diabetes is a favorable condition due to the alteration of the natural course of T2DM. Although this may be true, patients with this condition are prone to develop hypoglycemic episodes and may be linked to poor survival outcomes due to low HbA1c.6,7
Since Kalantar-Zadeh and colleagues presented a 2009 case study, there has been a lack of research regarding this unique condition.8 The purpose of this case study is to shed further light on burnt-out diabetes and present a patient case pertaining to the challenges of glycemic control in ESRD.
Case Presentation
Mr. A is a 49-year-old Hispanic male veteran with a history of ESRD on hemodialysis (HD) for 6 years, anemia of CKD, and T2DM for 22 years. The patient also has an extensive cardiovascular disease history, including hypertension, hyperlipidemia, and CAD status post-4-vessel coronary artery bypass graft in December 2014. The patient receives in-home HD Monday, Wednesday, and Friday and is on the wait list for kidney transplantation. The patient’s T2DM is managed by a primary care clinical pharmacy specialist (CPS) at the
Mr. A’s antidiabetic regimen is 45 units of subcutaneous insulin glargine every morning; insulin aspart sliding scale (about 15-27 units) subcutaneous 3 times daily with meals; and saxagliptin 2.5 mg by mouth once daily.
At a follow-up visit with the CPS, Mr. A stated, “I feel fine except for the occasional low blood sugar episode.” The patient’s most recent HbA1c was 6.1%, and he reported medication adherence and no signs or symptoms of hyperglycemia (ie, polydipsia, polyphagia, nocturia, visual disturbances). Mr. A reported no use of alcohol, tobacco, or illicit drugs. He walks 1 mile every other day and participates in self-monitoring blood glucose (SMBG) about 2 to 3 times daily (Table 1).
Although Mr. A’s most recent HbA1c was well controlled, his estimated fasting blood glucose at the same laboratory draw was 224 mg/dL. His SMBG readings in the past month also were elevated with higher readings in the evening. Mr. A attributed the elevated readings to dietary excursions and a high carbohydrate intake. At this visit, the CPS increased his insulin glargine dose to 50 units subcutaneous every morning and educated him on lifestyle modifications. Follow-up with the CPS was scheduled for 2 months from the day of the visit.
Analysis
Few articles on potential contributors to burnt-out diabetes have been published.6,7 These articles discuss decreased renal and hepatic clearance of insulin (which increases its half-life) hypoglycemia during HD, and low HbA1c due to preexisting anemia. Inappropriately low HbA1c levels may be secondary to, but not limited to, hemolysis, recent blood transfusion, acute blood loss, and medications, such as erythropoietin-stimulating agents (ESAs).9 The conditions that affect red blood cell turnover are common in patients with advanced CKD and may result in discrepancies in HbA1c levels.
Glycated hemoglobin is a series of minor hemoglobin components formed by the adduction of various carbohydrate molecules to hemoglobin. HbA1c is the largest fraction formed and the most consistent index of the concentration of glucose in the blood.10 Hence, HbA1c is the traditional indicator of overall glycemic control. The current HbA1c goals recommended by the American Diabetes Association are derived from landmark trials conducted with patients in the general adult diabetic non-CKD population. However, hemoglobin measurements can be confounded by conditions present in ESRD and tend to underestimate glucose measurements in patients with T2DM on HD. Despite this, HbA1c is still regarded as a reasonable measure of glycemic control even in patients with ESRD; however, alternative markers of glycemia may be preferable.11
Although HbA1c is the gold standard, there are other laboratory measures of average glycemic control available. Fructosamine is a ketoamine formed when glucose binds to serum proteins. When these proteins are exposed to high concentrations of glucose, they experience increased glycation. Fructosamine assays measure the total glycated serum proteins, of which albumin accounts for about 90%.11 Because the half-life of serum proteins is about 20 days, fructosamine levels can reflect glycemic control over a 2- to 3-week period. This is advantageous in conditions that affect the average age of red blood cells, in pregnancy where frequent monitoring and measures of short-term glucose control are especially important, and in the evaluation of a medication adjustment in the management of T2DM. However, this test is not without its limitations. It is less reliable in settings of decreased protein levels (eg, liver disease), there is a lack of availability in routine practice, and reference levels have not been established.11
Fructosamine has been shown to be strongly associated with mean blood glucose and HbA1c (Table 2). In 2010, Mittman and colleagues published a study that compared HbA1c with fructosamine and their correlation to glycemic control and morbidity, defined as rates of hospitalization and infection.12 The study included 100 patients with T2DM on HD with a mean age of 63 years, 54% were women, mean HbA1c of 7.2%, and mean dialysis duration of 3 years. Average follow-up was 3 years. At the end of follow-up, Mittman and colleagues found that HbA1c and fructosamine were highly correlated and associated with serum glucose (P < .01). However, fructosamine was found to be more highly correlated with mean glucose levels when those levels were below 150 mg/dL (P = .01). A higher fructosamine level, not HbA1c was a more significant predictor of hospitalization (P = .007) and infection (P = .001). Mittman and colleagues presented evidence for the use of fructosamine over HbA1c in patients with T2DM on HD.12
Hypoglycemic Episodes
At the 2-month follow-up visit with the CPS, Mr. A reported having 5 hypoglycemic episodes in the past 30 days. He also stated he would forget to take his insulin aspart dose before dinner about 3 to 4 times a week but would take it 30 to 60 minutes after the meal. Mr. A did not bring his glucometer or SMBG readings to the visit, but he indicated that his blood glucose levels continued to fluctuate and were elevated when consuming carbohydrates.
Laboratory tests 1 month prior to the 2-month follow-up visit showed HbA1c of 7.3%, which had increased from his previous level of 6.1%. He was counseled on the proper administration of insulin aspart and lifestyle modifications. A fructosamine level was ordered at this visit to further assess his glycemic control. A follow-up appointment and laboratory workup (fructosamine and HbA1c) were scheduled for 2 months from the visit (Table 3).
Mr. A was educated on the unreliability of his HbA1c levels secondary to his condition of ESRD on HD. He was counseled on the purpose of fructosamine and how it may be a better predictor of his glycemic control and morbidity. Mr. A continued to be followed closely by the primary care CPS for T2DM management.
Discussion
Management of T2DM in patients with ESRD presents challenges for clinicians in determining HbA1c goals and selecting appropriate medication options. The 2012 Kidney Disease Outcomes Quality Initiative (KDOQI) diabetes guideline does not recommend treatment for patients with substantially reduced kidney function to a target HbA1c < 7% due to risk of hypoglycemia.13 Although a target HbA1c > 7% is suggested for these patients, little is known about appropriate glycemic control in these patients as there is a paucity of prospective, randomized clinical trials that include patients with advanced CKD.13
Moreover, many oral antidiabetic medications and their metabolites are cleared by the kidneys and, therefore, pose with potential harm for patients with CKD. Because of this, insulin is the medication of choice for patients with ESRD.7 Although insulin requirements may diminish with worsening kidney function, insulin provides the safest method of glycemic control. Insulin dosing can be individualized according to a patient’s renal status as there is no uniformity in renal dose adjustments. There are some noninsulin antidiabetic agents that can be used in ESRD, but use of these agents requires close monitoring and evaluation of the medication’s pharmacokinetics (Table 4). Overall, medication management can be a difficult task for patients with T2DM and ESRD, but antidiabetic regimens may be reduced or discontinued altogether in burnt-out diabetes.
One of 3 patients with T2DM and ESRD on dialysis has burnt-out diabetes, defined as a phenomenon in which glucose homeostasis is altered to cause normoglycemia, spontaneous hypoglycemia, and decreased insulin requirements in established patients with T2DM.5 Although Mr. A had a normal-to-low HbA1c, he did not meet these criteria. Due to his elevated SMBG readings, he did not have normoglycemia and did require an increase in his basal insulin dose. Therefore, our patient did not have burnt-out diabetes.
Mr. A represents the relevant issue of inappropriately and unreliably low HbA1c levels due to various factors in ESRD. Our patient did not receive a blood transfusion in the past 2 years and was not on ESA therapy; nevertheless, Mr. A was a patient with ESRD on HD with a diagnosis of anemia. These diagnoses are confounders for low HbA1c values. When fructosamine levels were drawn for Mr. A on September 11, 2018 and November 6, 2018, they correlated well with his serum glucose and SMBG readings. This indicated to the CPS that the patient’s glycemic control was poor despite a promising HbA1c level.
This patient’s case and supporting evidence suggests that other measures of glycemic control (eg, fructosamine) can be used to supplement HbA1c, serum glucose, and glucometer readings to provide an accurate assessment of glycemic control in T2DM. Fructosamine also can assist HbA1c with predicting morbidity and potentially mortality, which are of great importance in this patient population.
Kalantar-Zadeh and colleagues conducted a study of 23,618 patients with T2DM on dialysis to observe mortality in association with HbA1c.5 This analysis showed that patients with HbA1c levels < 5% or > 8% had a higher risk of mortality; higher values of HbA1c (> 10%) were associated with increased death risk vs all other values. In the unadjusted analysis, HbA1c levels between 6 and 8% had the lowest death risk (hazard ratios [HR] 0.8 - 0.9, 95% CI) compared with those of higher and lower HbA1c ranges.5 In nonanemic patients, HbA1c > 6% was associated with increased death risk, whereas anemic patients did not show this trend.
Other studies made similar observations. In 2001, Morioka and colleagues published an observational study of 150 patients with DM on intermittent hemodialysis. The study analyzed survival and HbA1c levels at 1, 3, and 5 years. The study found that at 1, 3, and 5 years, patients with HbA1c < 7.5% had better survival than did patients with HbA1c > 7.5% (3.6 years vs 2.0 years, P = .008). Morioka and colleagues also found that there was a 13% increase in death per 1% increase in HbA1c.14 Oomichi and colleagues conducted an observational study of 114 patients with T2DM and ESRD on intermittent hemodialysis. Patients with fair control (HbA1c 6.5 - 8%) and good control (HbA1c < 6.5%) were compared with patients with poor control (HbA1c > 8%); it was found that the poor control group had nearly triple the mortality when compared with the good and fair control groups (HR = 2.89, P = .01).15 Park and colleagues also saw a similar observation in a study of 1,239 patients with ESRD and DM; 70% of these patients were on intermittent hemodialysis. Patients with poor control (HbA1c ≥ 8%) had worse survival outcomes than those with HbA1c < 8% (HR 2.2, P < .001).16
Our patient case forced us to ask the question, “What should our patient’s HbA1c goals be?” In the study by Oomichi and colleagues, a HbA1c level of 8% has usefulness as a “signpost for management of glycemic control.”15 All patients’ goals should be individualized based on various factors (eg, age, comorbidities), but based on the survival studies above, a HbA1c goal range of 6 to 8% may be optimal.
Conclusions
Patients with T2DM and ESRD on dialysis may have higher morbidity and mortality rates than the rates of those without T2DM. It has been shown in various studies that very low HbA1c (< 5%) and high HbA1c (> 8%) are associated with poor survival. Some patients with T2DM on dialysis may experience burnt-out diabetes in which they may have normoglycemia and a HbA1c below goal; despite these facts, this condition is not positive and can be linked to bad outcomes. In patients with T2DM and ESRD, insulin is the antidiabetic medication of choice, and we recommend a HbA1c target of 6 to 8%. In this patient population, consider using fructosamine levels or other measures of glycemic control to supplement HbA1c and glucose values to provide a better assessment of glycemic control, morbidity, and mortality. Larger clinical trials are needed to assist in answering questions regarding mortality and optimal HbA1c targets in burnt-out diabetes.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. https://www.cdc.gov/diabetes/data/statistics-report/index.html. Updated August 28, 2020. Accessed November 17, 2020.
2. Saran R, Robinson B, et al. US renal data system 2019 annual data report: epidemiology of klidney disease in the United States. Am J Kidney Dis. 2020 Jan;75(1 suppl 1):A6-A7. doi:10.1053/j.ajkd.2019.09.003. Epub 2019 Nov 5.
3. UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854-865.
4. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559. doi:10.1056/NEJMoa0802743
5. Kalantar-Zadeh K, Kopple JD, Regidor DL, et al. A1c and survival in maintenance hemodialysis patients. Diabetes Care. 2007;30(5):1049-10.55. doi:10.2337/dc06-2127
6. Park J, Lertdumrongluk P, Molnar MZ, Kovesdy CP, Kalantar-Zadeh K. Glycemic control in diabetic dialysis patients and the burnt-out diabetes phenomenon. Curr Diab Rep. 2012;12(4):432-439. doi:10.1007/s11892-012-0286-3
7. Rhee CM, Leung AM, Kovesdy CP, Lynch KE, Brent GA, Kalantar-Zadeh K. Updates on the management of diabetes in dialysis patients. Semin Dial. 2014;27(2):135-145. doi:10.1111/sdi.12198
8. Kalantar-Zadeh K, Derose SF, Nicholas S, Benner D, Sharma K, Kovesdy CP. Burnt-out diabetes: impact of chronic kidney disease progression on the natural course of diabetes mellitus. J Ren Nutr. 2009;19(1):33-37. doi:10.1053/j.jrn.2008.11.012
9. Unnikrishnan R, Anjana RM, Mohan V. Drugs affecting HbA1c levels. Indian J Endocrinol Metab. 2012;16(4):528-531. doi:10.4103/2230-8210.98004
10. Makris K, Spanou L. Is there a relationship between mean blood glucose and glycated hemoglobin? J Diabetes Sci Technol. 2011;5(6):1572-1583. doi:10.1177/193229681100500634
11. Wright LAC, Hirsch IB. The challenge of the use of glycemic biomarkers in diabetes: reflecting on hemoglobin A1c, 1,5-anhydroglucitol, and the glycated proteins fructosamine and glycated albumin. Diabetes Spectr. 2012;25(3):141-148. doi:10.2337/diaspect.25.3.141
12. Mittman N, Desiraju B, Fazil I, et al. Serum fructosamine versus glycosylated hemoglobin as an index of glycemic control, hospitalization, and infection in diabetic hemodialysis patients. Kidney Int. 2010;78 (suppl 117):S41-S45. doi:10.1038/ki.2010.193
13. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850-886. doi:10.1053/j.ajkd.2012.07.005
14. Morioka T, Emoto M, Tabata T, et al. Glycemic control is a predictor of survival for diabetic patients on hemodialysis. Diabetes Care. 2001;24(5):909-913. doi.10.2337/diacare.24.5.909

15. Oomichi T, Emoto M, Tabata T, et al. Impact of glycemic control on survival of diabetic patients on chronic regular hemodialysis: a 7-year observational study. Diabetes Care. 2006;29(7):1496-1500. doi:10.2337/dc05-1887
16. Park JI, Bae E, Kim YL, et al. Glycemic control and mortality in diabetic patients undergoing dialysis focusing on the effects of age and dialysis type: a prospective cohort study in Korea. PLoS ONE. 2015;10(8):e0136085. doi:10.1371/journal.pone.0136085
17. Glucotrol tablets [
18. Amaryl [package insert]. Bridgewater, NJ: Sanofi-Aventis; December 2018.
19. Glucophage [package insert]. Princeton, NJ: Bristol-Myers Squibb; May 2018.
20. Actos [package insert]. Deerfield, IL: Takeda Pharmaceuticals America Inc; December 2017.
21. Precose [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals; March 2015.
22. Nesina [package insert]. Deerfield, IL: Takeda Pharmaceuticals America Inc; June 2019.
23. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; June 2019.
24. Jardiance [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc; October 2018.
In patients with T2DM and ESRD, insulin is the antidiabetic medication of choice with a hemoglobin A1c target of 6 to 8%, using fructosamine levels or other measures for better assessment of glycemic control.
In patients with T2DM and ESRD, insulin is the antidiabetic medication of choice with a hemoglobin A1c target of 6 to 8%, using fructosamine levels or other measures for better assessment of glycemic control.
More than 34 million adults in the US have type 2 diabetes mellitus (T2DM), a chronic progressive disease identified by worsening hyperglycemia and micro- and macrovascular complications.1 Consequently, 12.2% of the US adult population is currently at risk for macrovascular diseases, such as stroke and coronary artery disease (CAD) and microvascular diseases, such as neuropathy and diabetic nephropathy.1
T2DM is the most common comorbid risk factor for chronic kidney disease (CKD) and the leading cause of end-stage renal disease (ESRD). As of 2017, about 750,000 Americans have CKD stage 5 requiring dialysis, and 50% of these patients have preexisting diabetic nephropathy.2 Rates of mortality and morbidity are observed to be higher in patients with both CKD and T2DM compared with patients with CKD without T2DM.2 Previous clinical trials, including the United Kingdom Prospective Diabetes Study of 1998, have proven that optimal glycemic control decreases the risk of complications of T2DM (ie, nephropathy) in the general population.3 Conversely, tight glycemic control that targets hemoglobin A1c (HbA1c) < 7%, in patients with T2DM with ESRD has not shown the same benefits and may lead to worse outcomes. It is postulated that this may be due to the increased incidence of hypoglycemia in this patient population.4
Dialysis has varying effects on patients both with and without T2DM. While patients with ESRD without T2DM have the potential to develop impaired glucose tolerance and T2DM, about 33% of patients with T2DM on dialysis actually have HbA1c < 6%.5 In these patients, glycemic control improves spontaneously as their disease progresses, leading to a decrease or cessation of insulin or other antidiabetic medications. This phenomenon, known as burnt-out diabetes, is characterized by (1) alterations in glucose homeostasis and normoglycemia without antidiabetic treatment; (2) HbA1c levels < 6% despite having established T2DM; (3) decline in insulin requirements or cessation of insulin altogether; and (4) spontaneous hypoglycemia.
There is a misconception that burnt-out diabetes is a favorable condition due to the alteration of the natural course of T2DM. Although this may be true, patients with this condition are prone to develop hypoglycemic episodes and may be linked to poor survival outcomes due to low HbA1c.6,7
Since Kalantar-Zadeh and colleagues presented a 2009 case study, there has been a lack of research regarding this unique condition.8 The purpose of this case study is to shed further light on burnt-out diabetes and present a patient case pertaining to the challenges of glycemic control in ESRD.
Case Presentation
Mr. A is a 49-year-old Hispanic male veteran with a history of ESRD on hemodialysis (HD) for 6 years, anemia of CKD, and T2DM for 22 years. The patient also has an extensive cardiovascular disease history, including hypertension, hyperlipidemia, and CAD status post-4-vessel coronary artery bypass graft in December 2014. The patient receives in-home HD Monday, Wednesday, and Friday and is on the wait list for kidney transplantation. The patient’s T2DM is managed by a primary care clinical pharmacy specialist (CPS) at the
Mr. A’s antidiabetic regimen is 45 units of subcutaneous insulin glargine every morning; insulin aspart sliding scale (about 15-27 units) subcutaneous 3 times daily with meals; and saxagliptin 2.5 mg by mouth once daily.
At a follow-up visit with the CPS, Mr. A stated, “I feel fine except for the occasional low blood sugar episode.” The patient’s most recent HbA1c was 6.1%, and he reported medication adherence and no signs or symptoms of hyperglycemia (ie, polydipsia, polyphagia, nocturia, visual disturbances). Mr. A reported no use of alcohol, tobacco, or illicit drugs. He walks 1 mile every other day and participates in self-monitoring blood glucose (SMBG) about 2 to 3 times daily (Table 1).
Although Mr. A’s most recent HbA1c was well controlled, his estimated fasting blood glucose at the same laboratory draw was 224 mg/dL. His SMBG readings in the past month also were elevated with higher readings in the evening. Mr. A attributed the elevated readings to dietary excursions and a high carbohydrate intake. At this visit, the CPS increased his insulin glargine dose to 50 units subcutaneous every morning and educated him on lifestyle modifications. Follow-up with the CPS was scheduled for 2 months from the day of the visit.
Analysis
Few articles on potential contributors to burnt-out diabetes have been published.6,7 These articles discuss decreased renal and hepatic clearance of insulin (which increases its half-life) hypoglycemia during HD, and low HbA1c due to preexisting anemia. Inappropriately low HbA1c levels may be secondary to, but not limited to, hemolysis, recent blood transfusion, acute blood loss, and medications, such as erythropoietin-stimulating agents (ESAs).9 The conditions that affect red blood cell turnover are common in patients with advanced CKD and may result in discrepancies in HbA1c levels.
Glycated hemoglobin is a series of minor hemoglobin components formed by the adduction of various carbohydrate molecules to hemoglobin. HbA1c is the largest fraction formed and the most consistent index of the concentration of glucose in the blood.10 Hence, HbA1c is the traditional indicator of overall glycemic control. The current HbA1c goals recommended by the American Diabetes Association are derived from landmark trials conducted with patients in the general adult diabetic non-CKD population. However, hemoglobin measurements can be confounded by conditions present in ESRD and tend to underestimate glucose measurements in patients with T2DM on HD. Despite this, HbA1c is still regarded as a reasonable measure of glycemic control even in patients with ESRD; however, alternative markers of glycemia may be preferable.11
Although HbA1c is the gold standard, there are other laboratory measures of average glycemic control available. Fructosamine is a ketoamine formed when glucose binds to serum proteins. When these proteins are exposed to high concentrations of glucose, they experience increased glycation. Fructosamine assays measure the total glycated serum proteins, of which albumin accounts for about 90%.11 Because the half-life of serum proteins is about 20 days, fructosamine levels can reflect glycemic control over a 2- to 3-week period. This is advantageous in conditions that affect the average age of red blood cells, in pregnancy where frequent monitoring and measures of short-term glucose control are especially important, and in the evaluation of a medication adjustment in the management of T2DM. However, this test is not without its limitations. It is less reliable in settings of decreased protein levels (eg, liver disease), there is a lack of availability in routine practice, and reference levels have not been established.11
Fructosamine has been shown to be strongly associated with mean blood glucose and HbA1c (Table 2). In 2010, Mittman and colleagues published a study that compared HbA1c with fructosamine and their correlation to glycemic control and morbidity, defined as rates of hospitalization and infection.12 The study included 100 patients with T2DM on HD with a mean age of 63 years, 54% were women, mean HbA1c of 7.2%, and mean dialysis duration of 3 years. Average follow-up was 3 years. At the end of follow-up, Mittman and colleagues found that HbA1c and fructosamine were highly correlated and associated with serum glucose (P < .01). However, fructosamine was found to be more highly correlated with mean glucose levels when those levels were below 150 mg/dL (P = .01). A higher fructosamine level, not HbA1c was a more significant predictor of hospitalization (P = .007) and infection (P = .001). Mittman and colleagues presented evidence for the use of fructosamine over HbA1c in patients with T2DM on HD.12
Hypoglycemic Episodes
At the 2-month follow-up visit with the CPS, Mr. A reported having 5 hypoglycemic episodes in the past 30 days. He also stated he would forget to take his insulin aspart dose before dinner about 3 to 4 times a week but would take it 30 to 60 minutes after the meal. Mr. A did not bring his glucometer or SMBG readings to the visit, but he indicated that his blood glucose levels continued to fluctuate and were elevated when consuming carbohydrates.
Laboratory tests 1 month prior to the 2-month follow-up visit showed HbA1c of 7.3%, which had increased from his previous level of 6.1%. He was counseled on the proper administration of insulin aspart and lifestyle modifications. A fructosamine level was ordered at this visit to further assess his glycemic control. A follow-up appointment and laboratory workup (fructosamine and HbA1c) were scheduled for 2 months from the visit (Table 3).
Mr. A was educated on the unreliability of his HbA1c levels secondary to his condition of ESRD on HD. He was counseled on the purpose of fructosamine and how it may be a better predictor of his glycemic control and morbidity. Mr. A continued to be followed closely by the primary care CPS for T2DM management.
Discussion
Management of T2DM in patients with ESRD presents challenges for clinicians in determining HbA1c goals and selecting appropriate medication options. The 2012 Kidney Disease Outcomes Quality Initiative (KDOQI) diabetes guideline does not recommend treatment for patients with substantially reduced kidney function to a target HbA1c < 7% due to risk of hypoglycemia.13 Although a target HbA1c > 7% is suggested for these patients, little is known about appropriate glycemic control in these patients as there is a paucity of prospective, randomized clinical trials that include patients with advanced CKD.13
Moreover, many oral antidiabetic medications and their metabolites are cleared by the kidneys and, therefore, pose with potential harm for patients with CKD. Because of this, insulin is the medication of choice for patients with ESRD.7 Although insulin requirements may diminish with worsening kidney function, insulin provides the safest method of glycemic control. Insulin dosing can be individualized according to a patient’s renal status as there is no uniformity in renal dose adjustments. There are some noninsulin antidiabetic agents that can be used in ESRD, but use of these agents requires close monitoring and evaluation of the medication’s pharmacokinetics (Table 4). Overall, medication management can be a difficult task for patients with T2DM and ESRD, but antidiabetic regimens may be reduced or discontinued altogether in burnt-out diabetes.
One of 3 patients with T2DM and ESRD on dialysis has burnt-out diabetes, defined as a phenomenon in which glucose homeostasis is altered to cause normoglycemia, spontaneous hypoglycemia, and decreased insulin requirements in established patients with T2DM.5 Although Mr. A had a normal-to-low HbA1c, he did not meet these criteria. Due to his elevated SMBG readings, he did not have normoglycemia and did require an increase in his basal insulin dose. Therefore, our patient did not have burnt-out diabetes.
Mr. A represents the relevant issue of inappropriately and unreliably low HbA1c levels due to various factors in ESRD. Our patient did not receive a blood transfusion in the past 2 years and was not on ESA therapy; nevertheless, Mr. A was a patient with ESRD on HD with a diagnosis of anemia. These diagnoses are confounders for low HbA1c values. When fructosamine levels were drawn for Mr. A on September 11, 2018 and November 6, 2018, they correlated well with his serum glucose and SMBG readings. This indicated to the CPS that the patient’s glycemic control was poor despite a promising HbA1c level.
This patient’s case and supporting evidence suggests that other measures of glycemic control (eg, fructosamine) can be used to supplement HbA1c, serum glucose, and glucometer readings to provide an accurate assessment of glycemic control in T2DM. Fructosamine also can assist HbA1c with predicting morbidity and potentially mortality, which are of great importance in this patient population.
Kalantar-Zadeh and colleagues conducted a study of 23,618 patients with T2DM on dialysis to observe mortality in association with HbA1c.5 This analysis showed that patients with HbA1c levels < 5% or > 8% had a higher risk of mortality; higher values of HbA1c (> 10%) were associated with increased death risk vs all other values. In the unadjusted analysis, HbA1c levels between 6 and 8% had the lowest death risk (hazard ratios [HR] 0.8 - 0.9, 95% CI) compared with those of higher and lower HbA1c ranges.5 In nonanemic patients, HbA1c > 6% was associated with increased death risk, whereas anemic patients did not show this trend.
Other studies made similar observations. In 2001, Morioka and colleagues published an observational study of 150 patients with DM on intermittent hemodialysis. The study analyzed survival and HbA1c levels at 1, 3, and 5 years. The study found that at 1, 3, and 5 years, patients with HbA1c < 7.5% had better survival than did patients with HbA1c > 7.5% (3.6 years vs 2.0 years, P = .008). Morioka and colleagues also found that there was a 13% increase in death per 1% increase in HbA1c.14 Oomichi and colleagues conducted an observational study of 114 patients with T2DM and ESRD on intermittent hemodialysis. Patients with fair control (HbA1c 6.5 - 8%) and good control (HbA1c < 6.5%) were compared with patients with poor control (HbA1c > 8%); it was found that the poor control group had nearly triple the mortality when compared with the good and fair control groups (HR = 2.89, P = .01).15 Park and colleagues also saw a similar observation in a study of 1,239 patients with ESRD and DM; 70% of these patients were on intermittent hemodialysis. Patients with poor control (HbA1c ≥ 8%) had worse survival outcomes than those with HbA1c < 8% (HR 2.2, P < .001).16
Our patient case forced us to ask the question, “What should our patient’s HbA1c goals be?” In the study by Oomichi and colleagues, a HbA1c level of 8% has usefulness as a “signpost for management of glycemic control.”15 All patients’ goals should be individualized based on various factors (eg, age, comorbidities), but based on the survival studies above, a HbA1c goal range of 6 to 8% may be optimal.
Conclusions
Patients with T2DM and ESRD on dialysis may have higher morbidity and mortality rates than the rates of those without T2DM. It has been shown in various studies that very low HbA1c (< 5%) and high HbA1c (> 8%) are associated with poor survival. Some patients with T2DM on dialysis may experience burnt-out diabetes in which they may have normoglycemia and a HbA1c below goal; despite these facts, this condition is not positive and can be linked to bad outcomes. In patients with T2DM and ESRD, insulin is the antidiabetic medication of choice, and we recommend a HbA1c target of 6 to 8%. In this patient population, consider using fructosamine levels or other measures of glycemic control to supplement HbA1c and glucose values to provide a better assessment of glycemic control, morbidity, and mortality. Larger clinical trials are needed to assist in answering questions regarding mortality and optimal HbA1c targets in burnt-out diabetes.
More than 34 million adults in the US have type 2 diabetes mellitus (T2DM), a chronic progressive disease identified by worsening hyperglycemia and micro- and macrovascular complications.1 Consequently, 12.2% of the US adult population is currently at risk for macrovascular diseases, such as stroke and coronary artery disease (CAD) and microvascular diseases, such as neuropathy and diabetic nephropathy.1
T2DM is the most common comorbid risk factor for chronic kidney disease (CKD) and the leading cause of end-stage renal disease (ESRD). As of 2017, about 750,000 Americans have CKD stage 5 requiring dialysis, and 50% of these patients have preexisting diabetic nephropathy.2 Rates of mortality and morbidity are observed to be higher in patients with both CKD and T2DM compared with patients with CKD without T2DM.2 Previous clinical trials, including the United Kingdom Prospective Diabetes Study of 1998, have proven that optimal glycemic control decreases the risk of complications of T2DM (ie, nephropathy) in the general population.3 Conversely, tight glycemic control that targets hemoglobin A1c (HbA1c) < 7%, in patients with T2DM with ESRD has not shown the same benefits and may lead to worse outcomes. It is postulated that this may be due to the increased incidence of hypoglycemia in this patient population.4
Dialysis has varying effects on patients both with and without T2DM. While patients with ESRD without T2DM have the potential to develop impaired glucose tolerance and T2DM, about 33% of patients with T2DM on dialysis actually have HbA1c < 6%.5 In these patients, glycemic control improves spontaneously as their disease progresses, leading to a decrease or cessation of insulin or other antidiabetic medications. This phenomenon, known as burnt-out diabetes, is characterized by (1) alterations in glucose homeostasis and normoglycemia without antidiabetic treatment; (2) HbA1c levels < 6% despite having established T2DM; (3) decline in insulin requirements or cessation of insulin altogether; and (4) spontaneous hypoglycemia.
There is a misconception that burnt-out diabetes is a favorable condition due to the alteration of the natural course of T2DM. Although this may be true, patients with this condition are prone to develop hypoglycemic episodes and may be linked to poor survival outcomes due to low HbA1c.6,7
Since Kalantar-Zadeh and colleagues presented a 2009 case study, there has been a lack of research regarding this unique condition.8 The purpose of this case study is to shed further light on burnt-out diabetes and present a patient case pertaining to the challenges of glycemic control in ESRD.
Case Presentation
Mr. A is a 49-year-old Hispanic male veteran with a history of ESRD on hemodialysis (HD) for 6 years, anemia of CKD, and T2DM for 22 years. The patient also has an extensive cardiovascular disease history, including hypertension, hyperlipidemia, and CAD status post-4-vessel coronary artery bypass graft in December 2014. The patient receives in-home HD Monday, Wednesday, and Friday and is on the wait list for kidney transplantation. The patient’s T2DM is managed by a primary care clinical pharmacy specialist (CPS) at the
Mr. A’s antidiabetic regimen is 45 units of subcutaneous insulin glargine every morning; insulin aspart sliding scale (about 15-27 units) subcutaneous 3 times daily with meals; and saxagliptin 2.5 mg by mouth once daily.
At a follow-up visit with the CPS, Mr. A stated, “I feel fine except for the occasional low blood sugar episode.” The patient’s most recent HbA1c was 6.1%, and he reported medication adherence and no signs or symptoms of hyperglycemia (ie, polydipsia, polyphagia, nocturia, visual disturbances). Mr. A reported no use of alcohol, tobacco, or illicit drugs. He walks 1 mile every other day and participates in self-monitoring blood glucose (SMBG) about 2 to 3 times daily (Table 1).
Although Mr. A’s most recent HbA1c was well controlled, his estimated fasting blood glucose at the same laboratory draw was 224 mg/dL. His SMBG readings in the past month also were elevated with higher readings in the evening. Mr. A attributed the elevated readings to dietary excursions and a high carbohydrate intake. At this visit, the CPS increased his insulin glargine dose to 50 units subcutaneous every morning and educated him on lifestyle modifications. Follow-up with the CPS was scheduled for 2 months from the day of the visit.
Analysis
Few articles on potential contributors to burnt-out diabetes have been published.6,7 These articles discuss decreased renal and hepatic clearance of insulin (which increases its half-life) hypoglycemia during HD, and low HbA1c due to preexisting anemia. Inappropriately low HbA1c levels may be secondary to, but not limited to, hemolysis, recent blood transfusion, acute blood loss, and medications, such as erythropoietin-stimulating agents (ESAs).9 The conditions that affect red blood cell turnover are common in patients with advanced CKD and may result in discrepancies in HbA1c levels.
Glycated hemoglobin is a series of minor hemoglobin components formed by the adduction of various carbohydrate molecules to hemoglobin. HbA1c is the largest fraction formed and the most consistent index of the concentration of glucose in the blood.10 Hence, HbA1c is the traditional indicator of overall glycemic control. The current HbA1c goals recommended by the American Diabetes Association are derived from landmark trials conducted with patients in the general adult diabetic non-CKD population. However, hemoglobin measurements can be confounded by conditions present in ESRD and tend to underestimate glucose measurements in patients with T2DM on HD. Despite this, HbA1c is still regarded as a reasonable measure of glycemic control even in patients with ESRD; however, alternative markers of glycemia may be preferable.11
Although HbA1c is the gold standard, there are other laboratory measures of average glycemic control available. Fructosamine is a ketoamine formed when glucose binds to serum proteins. When these proteins are exposed to high concentrations of glucose, they experience increased glycation. Fructosamine assays measure the total glycated serum proteins, of which albumin accounts for about 90%.11 Because the half-life of serum proteins is about 20 days, fructosamine levels can reflect glycemic control over a 2- to 3-week period. This is advantageous in conditions that affect the average age of red blood cells, in pregnancy where frequent monitoring and measures of short-term glucose control are especially important, and in the evaluation of a medication adjustment in the management of T2DM. However, this test is not without its limitations. It is less reliable in settings of decreased protein levels (eg, liver disease), there is a lack of availability in routine practice, and reference levels have not been established.11
Fructosamine has been shown to be strongly associated with mean blood glucose and HbA1c (Table 2). In 2010, Mittman and colleagues published a study that compared HbA1c with fructosamine and their correlation to glycemic control and morbidity, defined as rates of hospitalization and infection.12 The study included 100 patients with T2DM on HD with a mean age of 63 years, 54% were women, mean HbA1c of 7.2%, and mean dialysis duration of 3 years. Average follow-up was 3 years. At the end of follow-up, Mittman and colleagues found that HbA1c and fructosamine were highly correlated and associated with serum glucose (P < .01). However, fructosamine was found to be more highly correlated with mean glucose levels when those levels were below 150 mg/dL (P = .01). A higher fructosamine level, not HbA1c was a more significant predictor of hospitalization (P = .007) and infection (P = .001). Mittman and colleagues presented evidence for the use of fructosamine over HbA1c in patients with T2DM on HD.12
Hypoglycemic Episodes
At the 2-month follow-up visit with the CPS, Mr. A reported having 5 hypoglycemic episodes in the past 30 days. He also stated he would forget to take his insulin aspart dose before dinner about 3 to 4 times a week but would take it 30 to 60 minutes after the meal. Mr. A did not bring his glucometer or SMBG readings to the visit, but he indicated that his blood glucose levels continued to fluctuate and were elevated when consuming carbohydrates.
Laboratory tests 1 month prior to the 2-month follow-up visit showed HbA1c of 7.3%, which had increased from his previous level of 6.1%. He was counseled on the proper administration of insulin aspart and lifestyle modifications. A fructosamine level was ordered at this visit to further assess his glycemic control. A follow-up appointment and laboratory workup (fructosamine and HbA1c) were scheduled for 2 months from the visit (Table 3).
Mr. A was educated on the unreliability of his HbA1c levels secondary to his condition of ESRD on HD. He was counseled on the purpose of fructosamine and how it may be a better predictor of his glycemic control and morbidity. Mr. A continued to be followed closely by the primary care CPS for T2DM management.
Discussion
Management of T2DM in patients with ESRD presents challenges for clinicians in determining HbA1c goals and selecting appropriate medication options. The 2012 Kidney Disease Outcomes Quality Initiative (KDOQI) diabetes guideline does not recommend treatment for patients with substantially reduced kidney function to a target HbA1c < 7% due to risk of hypoglycemia.13 Although a target HbA1c > 7% is suggested for these patients, little is known about appropriate glycemic control in these patients as there is a paucity of prospective, randomized clinical trials that include patients with advanced CKD.13
Moreover, many oral antidiabetic medications and their metabolites are cleared by the kidneys and, therefore, pose with potential harm for patients with CKD. Because of this, insulin is the medication of choice for patients with ESRD.7 Although insulin requirements may diminish with worsening kidney function, insulin provides the safest method of glycemic control. Insulin dosing can be individualized according to a patient’s renal status as there is no uniformity in renal dose adjustments. There are some noninsulin antidiabetic agents that can be used in ESRD, but use of these agents requires close monitoring and evaluation of the medication’s pharmacokinetics (Table 4). Overall, medication management can be a difficult task for patients with T2DM and ESRD, but antidiabetic regimens may be reduced or discontinued altogether in burnt-out diabetes.
One of 3 patients with T2DM and ESRD on dialysis has burnt-out diabetes, defined as a phenomenon in which glucose homeostasis is altered to cause normoglycemia, spontaneous hypoglycemia, and decreased insulin requirements in established patients with T2DM.5 Although Mr. A had a normal-to-low HbA1c, he did not meet these criteria. Due to his elevated SMBG readings, he did not have normoglycemia and did require an increase in his basal insulin dose. Therefore, our patient did not have burnt-out diabetes.
Mr. A represents the relevant issue of inappropriately and unreliably low HbA1c levels due to various factors in ESRD. Our patient did not receive a blood transfusion in the past 2 years and was not on ESA therapy; nevertheless, Mr. A was a patient with ESRD on HD with a diagnosis of anemia. These diagnoses are confounders for low HbA1c values. When fructosamine levels were drawn for Mr. A on September 11, 2018 and November 6, 2018, they correlated well with his serum glucose and SMBG readings. This indicated to the CPS that the patient’s glycemic control was poor despite a promising HbA1c level.
This patient’s case and supporting evidence suggests that other measures of glycemic control (eg, fructosamine) can be used to supplement HbA1c, serum glucose, and glucometer readings to provide an accurate assessment of glycemic control in T2DM. Fructosamine also can assist HbA1c with predicting morbidity and potentially mortality, which are of great importance in this patient population.
Kalantar-Zadeh and colleagues conducted a study of 23,618 patients with T2DM on dialysis to observe mortality in association with HbA1c.5 This analysis showed that patients with HbA1c levels < 5% or > 8% had a higher risk of mortality; higher values of HbA1c (> 10%) were associated with increased death risk vs all other values. In the unadjusted analysis, HbA1c levels between 6 and 8% had the lowest death risk (hazard ratios [HR] 0.8 - 0.9, 95% CI) compared with those of higher and lower HbA1c ranges.5 In nonanemic patients, HbA1c > 6% was associated with increased death risk, whereas anemic patients did not show this trend.
Other studies made similar observations. In 2001, Morioka and colleagues published an observational study of 150 patients with DM on intermittent hemodialysis. The study analyzed survival and HbA1c levels at 1, 3, and 5 years. The study found that at 1, 3, and 5 years, patients with HbA1c < 7.5% had better survival than did patients with HbA1c > 7.5% (3.6 years vs 2.0 years, P = .008). Morioka and colleagues also found that there was a 13% increase in death per 1% increase in HbA1c.14 Oomichi and colleagues conducted an observational study of 114 patients with T2DM and ESRD on intermittent hemodialysis. Patients with fair control (HbA1c 6.5 - 8%) and good control (HbA1c < 6.5%) were compared with patients with poor control (HbA1c > 8%); it was found that the poor control group had nearly triple the mortality when compared with the good and fair control groups (HR = 2.89, P = .01).15 Park and colleagues also saw a similar observation in a study of 1,239 patients with ESRD and DM; 70% of these patients were on intermittent hemodialysis. Patients with poor control (HbA1c ≥ 8%) had worse survival outcomes than those with HbA1c < 8% (HR 2.2, P < .001).16
Our patient case forced us to ask the question, “What should our patient’s HbA1c goals be?” In the study by Oomichi and colleagues, a HbA1c level of 8% has usefulness as a “signpost for management of glycemic control.”15 All patients’ goals should be individualized based on various factors (eg, age, comorbidities), but based on the survival studies above, a HbA1c goal range of 6 to 8% may be optimal.
Conclusions
Patients with T2DM and ESRD on dialysis may have higher morbidity and mortality rates than the rates of those without T2DM. It has been shown in various studies that very low HbA1c (< 5%) and high HbA1c (> 8%) are associated with poor survival. Some patients with T2DM on dialysis may experience burnt-out diabetes in which they may have normoglycemia and a HbA1c below goal; despite these facts, this condition is not positive and can be linked to bad outcomes. In patients with T2DM and ESRD, insulin is the antidiabetic medication of choice, and we recommend a HbA1c target of 6 to 8%. In this patient population, consider using fructosamine levels or other measures of glycemic control to supplement HbA1c and glucose values to provide a better assessment of glycemic control, morbidity, and mortality. Larger clinical trials are needed to assist in answering questions regarding mortality and optimal HbA1c targets in burnt-out diabetes.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. https://www.cdc.gov/diabetes/data/statistics-report/index.html. Updated August 28, 2020. Accessed November 17, 2020.
2. Saran R, Robinson B, et al. US renal data system 2019 annual data report: epidemiology of klidney disease in the United States. Am J Kidney Dis. 2020 Jan;75(1 suppl 1):A6-A7. doi:10.1053/j.ajkd.2019.09.003. Epub 2019 Nov 5.
3. UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854-865.
4. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559. doi:10.1056/NEJMoa0802743
5. Kalantar-Zadeh K, Kopple JD, Regidor DL, et al. A1c and survival in maintenance hemodialysis patients. Diabetes Care. 2007;30(5):1049-10.55. doi:10.2337/dc06-2127
6. Park J, Lertdumrongluk P, Molnar MZ, Kovesdy CP, Kalantar-Zadeh K. Glycemic control in diabetic dialysis patients and the burnt-out diabetes phenomenon. Curr Diab Rep. 2012;12(4):432-439. doi:10.1007/s11892-012-0286-3
7. Rhee CM, Leung AM, Kovesdy CP, Lynch KE, Brent GA, Kalantar-Zadeh K. Updates on the management of diabetes in dialysis patients. Semin Dial. 2014;27(2):135-145. doi:10.1111/sdi.12198
8. Kalantar-Zadeh K, Derose SF, Nicholas S, Benner D, Sharma K, Kovesdy CP. Burnt-out diabetes: impact of chronic kidney disease progression on the natural course of diabetes mellitus. J Ren Nutr. 2009;19(1):33-37. doi:10.1053/j.jrn.2008.11.012
9. Unnikrishnan R, Anjana RM, Mohan V. Drugs affecting HbA1c levels. Indian J Endocrinol Metab. 2012;16(4):528-531. doi:10.4103/2230-8210.98004
10. Makris K, Spanou L. Is there a relationship between mean blood glucose and glycated hemoglobin? J Diabetes Sci Technol. 2011;5(6):1572-1583. doi:10.1177/193229681100500634
11. Wright LAC, Hirsch IB. The challenge of the use of glycemic biomarkers in diabetes: reflecting on hemoglobin A1c, 1,5-anhydroglucitol, and the glycated proteins fructosamine and glycated albumin. Diabetes Spectr. 2012;25(3):141-148. doi:10.2337/diaspect.25.3.141
12. Mittman N, Desiraju B, Fazil I, et al. Serum fructosamine versus glycosylated hemoglobin as an index of glycemic control, hospitalization, and infection in diabetic hemodialysis patients. Kidney Int. 2010;78 (suppl 117):S41-S45. doi:10.1038/ki.2010.193
13. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850-886. doi:10.1053/j.ajkd.2012.07.005
14. Morioka T, Emoto M, Tabata T, et al. Glycemic control is a predictor of survival for diabetic patients on hemodialysis. Diabetes Care. 2001;24(5):909-913. doi.10.2337/diacare.24.5.909

15. Oomichi T, Emoto M, Tabata T, et al. Impact of glycemic control on survival of diabetic patients on chronic regular hemodialysis: a 7-year observational study. Diabetes Care. 2006;29(7):1496-1500. doi:10.2337/dc05-1887
16. Park JI, Bae E, Kim YL, et al. Glycemic control and mortality in diabetic patients undergoing dialysis focusing on the effects of age and dialysis type: a prospective cohort study in Korea. PLoS ONE. 2015;10(8):e0136085. doi:10.1371/journal.pone.0136085
17. Glucotrol tablets [
18. Amaryl [package insert]. Bridgewater, NJ: Sanofi-Aventis; December 2018.
19. Glucophage [package insert]. Princeton, NJ: Bristol-Myers Squibb; May 2018.
20. Actos [package insert]. Deerfield, IL: Takeda Pharmaceuticals America Inc; December 2017.
21. Precose [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals; March 2015.
22. Nesina [package insert]. Deerfield, IL: Takeda Pharmaceuticals America Inc; June 2019.
23. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; June 2019.
24. Jardiance [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc; October 2018.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. https://www.cdc.gov/diabetes/data/statistics-report/index.html. Updated August 28, 2020. Accessed November 17, 2020.
2. Saran R, Robinson B, et al. US renal data system 2019 annual data report: epidemiology of klidney disease in the United States. Am J Kidney Dis. 2020 Jan;75(1 suppl 1):A6-A7. doi:10.1053/j.ajkd.2019.09.003. Epub 2019 Nov 5.
3. UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854-865.
4. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559. doi:10.1056/NEJMoa0802743
5. Kalantar-Zadeh K, Kopple JD, Regidor DL, et al. A1c and survival in maintenance hemodialysis patients. Diabetes Care. 2007;30(5):1049-10.55. doi:10.2337/dc06-2127
6. Park J, Lertdumrongluk P, Molnar MZ, Kovesdy CP, Kalantar-Zadeh K. Glycemic control in diabetic dialysis patients and the burnt-out diabetes phenomenon. Curr Diab Rep. 2012;12(4):432-439. doi:10.1007/s11892-012-0286-3
7. Rhee CM, Leung AM, Kovesdy CP, Lynch KE, Brent GA, Kalantar-Zadeh K. Updates on the management of diabetes in dialysis patients. Semin Dial. 2014;27(2):135-145. doi:10.1111/sdi.12198
8. Kalantar-Zadeh K, Derose SF, Nicholas S, Benner D, Sharma K, Kovesdy CP. Burnt-out diabetes: impact of chronic kidney disease progression on the natural course of diabetes mellitus. J Ren Nutr. 2009;19(1):33-37. doi:10.1053/j.jrn.2008.11.012
9. Unnikrishnan R, Anjana RM, Mohan V. Drugs affecting HbA1c levels. Indian J Endocrinol Metab. 2012;16(4):528-531. doi:10.4103/2230-8210.98004
10. Makris K, Spanou L. Is there a relationship between mean blood glucose and glycated hemoglobin? J Diabetes Sci Technol. 2011;5(6):1572-1583. doi:10.1177/193229681100500634
11. Wright LAC, Hirsch IB. The challenge of the use of glycemic biomarkers in diabetes: reflecting on hemoglobin A1c, 1,5-anhydroglucitol, and the glycated proteins fructosamine and glycated albumin. Diabetes Spectr. 2012;25(3):141-148. doi:10.2337/diaspect.25.3.141
12. Mittman N, Desiraju B, Fazil I, et al. Serum fructosamine versus glycosylated hemoglobin as an index of glycemic control, hospitalization, and infection in diabetic hemodialysis patients. Kidney Int. 2010;78 (suppl 117):S41-S45. doi:10.1038/ki.2010.193
13. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850-886. doi:10.1053/j.ajkd.2012.07.005
14. Morioka T, Emoto M, Tabata T, et al. Glycemic control is a predictor of survival for diabetic patients on hemodialysis. Diabetes Care. 2001;24(5):909-913. doi.10.2337/diacare.24.5.909

15. Oomichi T, Emoto M, Tabata T, et al. Impact of glycemic control on survival of diabetic patients on chronic regular hemodialysis: a 7-year observational study. Diabetes Care. 2006;29(7):1496-1500. doi:10.2337/dc05-1887
16. Park JI, Bae E, Kim YL, et al. Glycemic control and mortality in diabetic patients undergoing dialysis focusing on the effects of age and dialysis type: a prospective cohort study in Korea. PLoS ONE. 2015;10(8):e0136085. doi:10.1371/journal.pone.0136085
17. Glucotrol tablets [
18. Amaryl [package insert]. Bridgewater, NJ: Sanofi-Aventis; December 2018.
19. Glucophage [package insert]. Princeton, NJ: Bristol-Myers Squibb; May 2018.
20. Actos [package insert]. Deerfield, IL: Takeda Pharmaceuticals America Inc; December 2017.
21. Precose [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals; March 2015.
22. Nesina [package insert]. Deerfield, IL: Takeda Pharmaceuticals America Inc; June 2019.
23. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; June 2019.
24. Jardiance [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc; October 2018.
90-year-old man • dyspnea • lower extremity edema • limitations in daily activities • Dx?
THE CASE
An obese 90-year-old White man presented for a 1-month follow-up with his family physician after being hospitalized for an acute exacerbation of heart failure (HF). In addition to New York Heart Association (NYHA) Class III heart failure with reduced ejection fraction (HFrEF), he had a history of tobacco abuse, hyperlipidemia, atrial fibrillation, coronary artery disease, stage 3 chronic kidney disease, and benign prostatic hyperplasia. The patient’s family accompanied him during the visit to discuss hospice care.
The patient complained of persistent shortness of breath that limited his activities of daily living (ADLs) and lower extremity and scrotal edema. He denied chest pain, orthopnea, paroxysmal nocturnal dyspnea, ascites, nocturia, and nocturnal cough.
The patient had undergone a coronary artery bypass graft 23 years earlier. His HF was being managed with metoprolol tartrate 25 mg bid, spironolactone 25 mg/d, and furosemide 80 mg/d.
Examination revealed bilateral 3+ pitting edema in the lower extremities midway up the shin, crackles to the inferior scapula bilaterally, and a 3/6 systolic murmur with regular rate and rhythm. The remainder of the physical exam was normal. The patient’s vitals were within normal limits, with an oxygen saturation of 90%.
The patient’s most recent chest x-ray demonstrated mild cardiomegaly. An echocardiogram showed an ejection fraction of 44% with severe bi-atrial enlargement, moderate-to-severe mitral regurgitation, and mild-to-moderate aortic insufficiency. His brain natriuretic peptide (BNP) was 915 pg/mL (normal range for patients ages 75-99 years, < 450 pg/mL).
THE DIAGNOSIS
The differential diagnosis for the patient’s shortness of breath included chronic obstructive pulmonary disease secondary to his smoking history, pulmonary embolus, respiratory infection, anemia, and medication-related adverse effects. The patient’s history of renal disease merited consideration of a nephrotic syndrome causing low albumin, which could explain his edema. Another possible cause of the edema was venous insufficiency. However, given the patient’s extensive cardiac history, the most likely explanation for his shortness of breath and edema was congestive HF that was unresponsive to the current diuretic regimen.
Several changes to the patient’s medications were made. Lisinopril 2.5 mg/d was started due to the mortality benefit of angiotensin-converting enzyme inhibitors in the treatment of HFrEF.1 Metoprolol tartrate 25 mg/d was transitioned to metoprolol succinate 50 mg/d, as only the longer-acting succinate version has shown mortality benefit in HFrEF.1 (Other beta-blockers with mortality benefit include carvedilol and bisoprolol.1) The furosemide 80 mg/d was replaced with torsemide 100 mg/d to provide an enhanced diuretic effect for symptomatic relief. The spironolactone dose was not increased due to concerns about the patient’s renal function. Of note, spironolactone was included in the patient’s regimen based on his NYHA classification, as well as the potential mortality benefits and improvement in edema seen in HFrEF patients.1 Spironolactone can be used with loop and/or thiazide diuretics in the treatment of HF.
Continue to: Within 5 days...
Within 5 days, the patient had lost 6 lb and his oxygen saturation had improved from 90% to 95%. He reported improvements in his breathing and was able to move around more easily.
DISCUSSION
There are several possible explanations for torsemide’s superior diuretic effect in this patient. Unlike furosemide, torsemide absorption is not influenced by intestinal edema, which is commonly seen in patients with HF. It has a longer half-life and improved bioavailability that is not altered by food intake. Torsemide also inhibits the actions of aldosterone through its interaction with the renin-angiotensin-aldosterone system and aldosterone receptor, leading to further diuresis and reduced cardiac remodeling.2
What the evidence shows. The TORIC trial was an open-label, nonrandomized, post-marketing surveillance study of 1377 patients with NYHA Class II–III HF who received diuretic therapy with torsemide 10 mg/d, furosemide 40 mg/d, or another diuretic for 12 months.3 Significantly lower total mortality and cardiac mortality was found in the torsemide group; in addition, a significantly greater proportion of patients in the torsemide group showed improvement in NYHA classification.3 Murray et al reported a reduction in hospitalization rates with torsemide therapy vs furosemide therapy in a randomized trial of 234 HF patients (32% vs 17%, P = 0.01).4 The ASCEND-HF trial, a large international acute HF trial comparing torsemide with furosemide, demonstrated a nonsignificant reduction in 30-day and 180-day events (all-cause mortality or HF hospitalization) in those receiving torsemide, after risk adjustment.5 Torsemide has also been shown to improve quality of life compared to furosemide.6
Preliminary results from the TORNADO trial,7 a multicenter randomized controlled trial, demonstrated superior symptom improvement in HF patients taking torsemide compared to those taking furosemide.8 The preliminary endpoint—a composite of improvement in NYHA class, improvement in distance of at least 50 m during a 6-minute walk test, and a decrease in fluid retention of at least 0.5 ohms at 3-month follow-up—was achieved by 94% and 58% of patients on torsemide and furosemide, respectively (P = 0.03).8 A total of 7 patients (3 in the torsemide and 4 in the furosemide group) were hospitalized for worsening HF during the follow-up period.8
A 2020 meta-analysis of more than 19,000 patients compared furosemide to torsemide and found a number needed to treat (NNT) of 23 to prevent a hospitalization due to HF; an NNT of 5 for improvement in NYHA functional status; and an NNT of 40 for reduction in cardiac mortality.9
Continue to: Our patient
Our patient reported feeling “great” at the 6-week follow-up appointment, with significant improvement in breathing and ability to perform his ADLs. His NYHA classification improved to Class II. He had lost 26 pounds (back to his weight 9 months prior), and his oxygen saturation was 97%.
On exam, the bilateral peripheral edema in his lower extremities had improved from 3+ to 1+, with the edema extending just distal to the mid-shin. Only mild crackles were present at the lung bases. The remainder of his physical examination was unchanged. His vital signs were within normal limits with no signs of hypotension. A basic metabolic panel was obtained to confirm his electrolytes were still within normal limits. His BNP had decreased to 230 pg/mL.
The patient declined the referral for hospice evaluation due to the significant improvement in his symptoms.
THE TAKEAWAY
A significant clinical improvement and improved quality of life were achieved with the transition from furosemide to torsemide. It is apparent that the patient’s furosemide had an inferior diuretic effect compared to torsemide, whether that be secondary to his dose or due to the unpredictable nature of furosemide’s bioavailability, especially in the setting of intestinal edema.
A growing body of literature9-11 suggests torsemide’s superiority over furosemide with no signs of increased adverse effects. Although additional prospective, head-to-head trials are needed, at this point in time it is appropriate to consider the use of torsemide in a patient with HF who does not seem to be fully responding to furosemide.
CORRESPONDENCE
Ryan Paulus, DO, 590 Manning Drive, Chapel Hill, NC 27599; [email protected]
1. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147-239.
2. Buggey J, Mentz RJ, Pitt B, et al. A reappraisal of loop diuretic choice in heart failure patients. Am Heart J. 2015;169:323-333.
3. Cosín J, Díez J; TORIC investigators. Torasemide in chronic heart failure: results of the TORIC study. Eur J Heart Fail. 2002;4:507-513.
4. Murray MD, Deer MM, Ferguson JA, et al. Open-label randomized trial of torsemide compared with furosemide therapy for patients with heart failure. Am J Med. 2001;111:513-520.
5. Mentz RJ, Hasselblad V, DeVore AD, et al. Torsemide versus furosemide in patients with acute heart failure (from the ASCEND-HF Trial). Am J Cardiol. 2016;117:404-411.
6. Müller K, Gamba G, Jaquet F, et al. Torasemide vs furosemide in primary care patients with chronic heart failure NYHA II to IV—efficacy and quality of life. Eur J Heart Fail. 2003;5:793-801.
7. Balsam P, Ozierański K, Tymińska A, et al. The impact of torasemide on haemodynamic and neurohormonal stress, and cardiac remodelling in heart failure—TORNADO: a study protocol for a randomized controlled trial. Trials. 2017;18:36.
8. Balsam P, Ozierański K, Marchel M, et al. Comparative effectiveness of torasemide versus furosemide in symptomatic therapy in heart failure patients: preliminary results from the randomized TORNADO trial. Cardiol J. 2019;26:661-668.
9. Abraham B, Megaly M, Sous M, et al. Meta-analysis comparing torsemide versus furosemide in patients with heart failure. Am J Cardiol. 2020;125:92-99.
10. Balsam P, Ozierański K, Kapłon-Cieślicka A, et al. Comparative analysis of long-term outcomes of torasemide and furosemide in heart failure patients in heart failure registries of the European Society of Cardiology. Cardiovasc Drugs Ther. 2019;33:77-86.
11. Täger T, Fröhlich H, Grundtvig M, et al. Comparative effectiveness of loop diuretics on mortality in the treatment of patients with chronic heart failure—a multicenter propensity score matched analysis. Int J Cardiol. 2019;289:83-90.
THE CASE
An obese 90-year-old White man presented for a 1-month follow-up with his family physician after being hospitalized for an acute exacerbation of heart failure (HF). In addition to New York Heart Association (NYHA) Class III heart failure with reduced ejection fraction (HFrEF), he had a history of tobacco abuse, hyperlipidemia, atrial fibrillation, coronary artery disease, stage 3 chronic kidney disease, and benign prostatic hyperplasia. The patient’s family accompanied him during the visit to discuss hospice care.
The patient complained of persistent shortness of breath that limited his activities of daily living (ADLs) and lower extremity and scrotal edema. He denied chest pain, orthopnea, paroxysmal nocturnal dyspnea, ascites, nocturia, and nocturnal cough.
The patient had undergone a coronary artery bypass graft 23 years earlier. His HF was being managed with metoprolol tartrate 25 mg bid, spironolactone 25 mg/d, and furosemide 80 mg/d.
Examination revealed bilateral 3+ pitting edema in the lower extremities midway up the shin, crackles to the inferior scapula bilaterally, and a 3/6 systolic murmur with regular rate and rhythm. The remainder of the physical exam was normal. The patient’s vitals were within normal limits, with an oxygen saturation of 90%.
The patient’s most recent chest x-ray demonstrated mild cardiomegaly. An echocardiogram showed an ejection fraction of 44% with severe bi-atrial enlargement, moderate-to-severe mitral regurgitation, and mild-to-moderate aortic insufficiency. His brain natriuretic peptide (BNP) was 915 pg/mL (normal range for patients ages 75-99 years, < 450 pg/mL).
THE DIAGNOSIS
The differential diagnosis for the patient’s shortness of breath included chronic obstructive pulmonary disease secondary to his smoking history, pulmonary embolus, respiratory infection, anemia, and medication-related adverse effects. The patient’s history of renal disease merited consideration of a nephrotic syndrome causing low albumin, which could explain his edema. Another possible cause of the edema was venous insufficiency. However, given the patient’s extensive cardiac history, the most likely explanation for his shortness of breath and edema was congestive HF that was unresponsive to the current diuretic regimen.
Several changes to the patient’s medications were made. Lisinopril 2.5 mg/d was started due to the mortality benefit of angiotensin-converting enzyme inhibitors in the treatment of HFrEF.1 Metoprolol tartrate 25 mg/d was transitioned to metoprolol succinate 50 mg/d, as only the longer-acting succinate version has shown mortality benefit in HFrEF.1 (Other beta-blockers with mortality benefit include carvedilol and bisoprolol.1) The furosemide 80 mg/d was replaced with torsemide 100 mg/d to provide an enhanced diuretic effect for symptomatic relief. The spironolactone dose was not increased due to concerns about the patient’s renal function. Of note, spironolactone was included in the patient’s regimen based on his NYHA classification, as well as the potential mortality benefits and improvement in edema seen in HFrEF patients.1 Spironolactone can be used with loop and/or thiazide diuretics in the treatment of HF.
Continue to: Within 5 days...
Within 5 days, the patient had lost 6 lb and his oxygen saturation had improved from 90% to 95%. He reported improvements in his breathing and was able to move around more easily.
DISCUSSION
There are several possible explanations for torsemide’s superior diuretic effect in this patient. Unlike furosemide, torsemide absorption is not influenced by intestinal edema, which is commonly seen in patients with HF. It has a longer half-life and improved bioavailability that is not altered by food intake. Torsemide also inhibits the actions of aldosterone through its interaction with the renin-angiotensin-aldosterone system and aldosterone receptor, leading to further diuresis and reduced cardiac remodeling.2
What the evidence shows. The TORIC trial was an open-label, nonrandomized, post-marketing surveillance study of 1377 patients with NYHA Class II–III HF who received diuretic therapy with torsemide 10 mg/d, furosemide 40 mg/d, or another diuretic for 12 months.3 Significantly lower total mortality and cardiac mortality was found in the torsemide group; in addition, a significantly greater proportion of patients in the torsemide group showed improvement in NYHA classification.3 Murray et al reported a reduction in hospitalization rates with torsemide therapy vs furosemide therapy in a randomized trial of 234 HF patients (32% vs 17%, P = 0.01).4 The ASCEND-HF trial, a large international acute HF trial comparing torsemide with furosemide, demonstrated a nonsignificant reduction in 30-day and 180-day events (all-cause mortality or HF hospitalization) in those receiving torsemide, after risk adjustment.5 Torsemide has also been shown to improve quality of life compared to furosemide.6
Preliminary results from the TORNADO trial,7 a multicenter randomized controlled trial, demonstrated superior symptom improvement in HF patients taking torsemide compared to those taking furosemide.8 The preliminary endpoint—a composite of improvement in NYHA class, improvement in distance of at least 50 m during a 6-minute walk test, and a decrease in fluid retention of at least 0.5 ohms at 3-month follow-up—was achieved by 94% and 58% of patients on torsemide and furosemide, respectively (P = 0.03).8 A total of 7 patients (3 in the torsemide and 4 in the furosemide group) were hospitalized for worsening HF during the follow-up period.8
A 2020 meta-analysis of more than 19,000 patients compared furosemide to torsemide and found a number needed to treat (NNT) of 23 to prevent a hospitalization due to HF; an NNT of 5 for improvement in NYHA functional status; and an NNT of 40 for reduction in cardiac mortality.9
Continue to: Our patient
Our patient reported feeling “great” at the 6-week follow-up appointment, with significant improvement in breathing and ability to perform his ADLs. His NYHA classification improved to Class II. He had lost 26 pounds (back to his weight 9 months prior), and his oxygen saturation was 97%.
On exam, the bilateral peripheral edema in his lower extremities had improved from 3+ to 1+, with the edema extending just distal to the mid-shin. Only mild crackles were present at the lung bases. The remainder of his physical examination was unchanged. His vital signs were within normal limits with no signs of hypotension. A basic metabolic panel was obtained to confirm his electrolytes were still within normal limits. His BNP had decreased to 230 pg/mL.
The patient declined the referral for hospice evaluation due to the significant improvement in his symptoms.
THE TAKEAWAY
A significant clinical improvement and improved quality of life were achieved with the transition from furosemide to torsemide. It is apparent that the patient’s furosemide had an inferior diuretic effect compared to torsemide, whether that be secondary to his dose or due to the unpredictable nature of furosemide’s bioavailability, especially in the setting of intestinal edema.
A growing body of literature9-11 suggests torsemide’s superiority over furosemide with no signs of increased adverse effects. Although additional prospective, head-to-head trials are needed, at this point in time it is appropriate to consider the use of torsemide in a patient with HF who does not seem to be fully responding to furosemide.
CORRESPONDENCE
Ryan Paulus, DO, 590 Manning Drive, Chapel Hill, NC 27599; [email protected]
THE CASE
An obese 90-year-old White man presented for a 1-month follow-up with his family physician after being hospitalized for an acute exacerbation of heart failure (HF). In addition to New York Heart Association (NYHA) Class III heart failure with reduced ejection fraction (HFrEF), he had a history of tobacco abuse, hyperlipidemia, atrial fibrillation, coronary artery disease, stage 3 chronic kidney disease, and benign prostatic hyperplasia. The patient’s family accompanied him during the visit to discuss hospice care.
The patient complained of persistent shortness of breath that limited his activities of daily living (ADLs) and lower extremity and scrotal edema. He denied chest pain, orthopnea, paroxysmal nocturnal dyspnea, ascites, nocturia, and nocturnal cough.
The patient had undergone a coronary artery bypass graft 23 years earlier. His HF was being managed with metoprolol tartrate 25 mg bid, spironolactone 25 mg/d, and furosemide 80 mg/d.
Examination revealed bilateral 3+ pitting edema in the lower extremities midway up the shin, crackles to the inferior scapula bilaterally, and a 3/6 systolic murmur with regular rate and rhythm. The remainder of the physical exam was normal. The patient’s vitals were within normal limits, with an oxygen saturation of 90%.
The patient’s most recent chest x-ray demonstrated mild cardiomegaly. An echocardiogram showed an ejection fraction of 44% with severe bi-atrial enlargement, moderate-to-severe mitral regurgitation, and mild-to-moderate aortic insufficiency. His brain natriuretic peptide (BNP) was 915 pg/mL (normal range for patients ages 75-99 years, < 450 pg/mL).
THE DIAGNOSIS
The differential diagnosis for the patient’s shortness of breath included chronic obstructive pulmonary disease secondary to his smoking history, pulmonary embolus, respiratory infection, anemia, and medication-related adverse effects. The patient’s history of renal disease merited consideration of a nephrotic syndrome causing low albumin, which could explain his edema. Another possible cause of the edema was venous insufficiency. However, given the patient’s extensive cardiac history, the most likely explanation for his shortness of breath and edema was congestive HF that was unresponsive to the current diuretic regimen.
Several changes to the patient’s medications were made. Lisinopril 2.5 mg/d was started due to the mortality benefit of angiotensin-converting enzyme inhibitors in the treatment of HFrEF.1 Metoprolol tartrate 25 mg/d was transitioned to metoprolol succinate 50 mg/d, as only the longer-acting succinate version has shown mortality benefit in HFrEF.1 (Other beta-blockers with mortality benefit include carvedilol and bisoprolol.1) The furosemide 80 mg/d was replaced with torsemide 100 mg/d to provide an enhanced diuretic effect for symptomatic relief. The spironolactone dose was not increased due to concerns about the patient’s renal function. Of note, spironolactone was included in the patient’s regimen based on his NYHA classification, as well as the potential mortality benefits and improvement in edema seen in HFrEF patients.1 Spironolactone can be used with loop and/or thiazide diuretics in the treatment of HF.
Continue to: Within 5 days...
Within 5 days, the patient had lost 6 lb and his oxygen saturation had improved from 90% to 95%. He reported improvements in his breathing and was able to move around more easily.
DISCUSSION
There are several possible explanations for torsemide’s superior diuretic effect in this patient. Unlike furosemide, torsemide absorption is not influenced by intestinal edema, which is commonly seen in patients with HF. It has a longer half-life and improved bioavailability that is not altered by food intake. Torsemide also inhibits the actions of aldosterone through its interaction with the renin-angiotensin-aldosterone system and aldosterone receptor, leading to further diuresis and reduced cardiac remodeling.2
What the evidence shows. The TORIC trial was an open-label, nonrandomized, post-marketing surveillance study of 1377 patients with NYHA Class II–III HF who received diuretic therapy with torsemide 10 mg/d, furosemide 40 mg/d, or another diuretic for 12 months.3 Significantly lower total mortality and cardiac mortality was found in the torsemide group; in addition, a significantly greater proportion of patients in the torsemide group showed improvement in NYHA classification.3 Murray et al reported a reduction in hospitalization rates with torsemide therapy vs furosemide therapy in a randomized trial of 234 HF patients (32% vs 17%, P = 0.01).4 The ASCEND-HF trial, a large international acute HF trial comparing torsemide with furosemide, demonstrated a nonsignificant reduction in 30-day and 180-day events (all-cause mortality or HF hospitalization) in those receiving torsemide, after risk adjustment.5 Torsemide has also been shown to improve quality of life compared to furosemide.6
Preliminary results from the TORNADO trial,7 a multicenter randomized controlled trial, demonstrated superior symptom improvement in HF patients taking torsemide compared to those taking furosemide.8 The preliminary endpoint—a composite of improvement in NYHA class, improvement in distance of at least 50 m during a 6-minute walk test, and a decrease in fluid retention of at least 0.5 ohms at 3-month follow-up—was achieved by 94% and 58% of patients on torsemide and furosemide, respectively (P = 0.03).8 A total of 7 patients (3 in the torsemide and 4 in the furosemide group) were hospitalized for worsening HF during the follow-up period.8
A 2020 meta-analysis of more than 19,000 patients compared furosemide to torsemide and found a number needed to treat (NNT) of 23 to prevent a hospitalization due to HF; an NNT of 5 for improvement in NYHA functional status; and an NNT of 40 for reduction in cardiac mortality.9
Continue to: Our patient
Our patient reported feeling “great” at the 6-week follow-up appointment, with significant improvement in breathing and ability to perform his ADLs. His NYHA classification improved to Class II. He had lost 26 pounds (back to his weight 9 months prior), and his oxygen saturation was 97%.
On exam, the bilateral peripheral edema in his lower extremities had improved from 3+ to 1+, with the edema extending just distal to the mid-shin. Only mild crackles were present at the lung bases. The remainder of his physical examination was unchanged. His vital signs were within normal limits with no signs of hypotension. A basic metabolic panel was obtained to confirm his electrolytes were still within normal limits. His BNP had decreased to 230 pg/mL.
The patient declined the referral for hospice evaluation due to the significant improvement in his symptoms.
THE TAKEAWAY
A significant clinical improvement and improved quality of life were achieved with the transition from furosemide to torsemide. It is apparent that the patient’s furosemide had an inferior diuretic effect compared to torsemide, whether that be secondary to his dose or due to the unpredictable nature of furosemide’s bioavailability, especially in the setting of intestinal edema.
A growing body of literature9-11 suggests torsemide’s superiority over furosemide with no signs of increased adverse effects. Although additional prospective, head-to-head trials are needed, at this point in time it is appropriate to consider the use of torsemide in a patient with HF who does not seem to be fully responding to furosemide.
CORRESPONDENCE
Ryan Paulus, DO, 590 Manning Drive, Chapel Hill, NC 27599; [email protected]
1. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147-239.
2. Buggey J, Mentz RJ, Pitt B, et al. A reappraisal of loop diuretic choice in heart failure patients. Am Heart J. 2015;169:323-333.
3. Cosín J, Díez J; TORIC investigators. Torasemide in chronic heart failure: results of the TORIC study. Eur J Heart Fail. 2002;4:507-513.
4. Murray MD, Deer MM, Ferguson JA, et al. Open-label randomized trial of torsemide compared with furosemide therapy for patients with heart failure. Am J Med. 2001;111:513-520.
5. Mentz RJ, Hasselblad V, DeVore AD, et al. Torsemide versus furosemide in patients with acute heart failure (from the ASCEND-HF Trial). Am J Cardiol. 2016;117:404-411.
6. Müller K, Gamba G, Jaquet F, et al. Torasemide vs furosemide in primary care patients with chronic heart failure NYHA II to IV—efficacy and quality of life. Eur J Heart Fail. 2003;5:793-801.
7. Balsam P, Ozierański K, Tymińska A, et al. The impact of torasemide on haemodynamic and neurohormonal stress, and cardiac remodelling in heart failure—TORNADO: a study protocol for a randomized controlled trial. Trials. 2017;18:36.
8. Balsam P, Ozierański K, Marchel M, et al. Comparative effectiveness of torasemide versus furosemide in symptomatic therapy in heart failure patients: preliminary results from the randomized TORNADO trial. Cardiol J. 2019;26:661-668.
9. Abraham B, Megaly M, Sous M, et al. Meta-analysis comparing torsemide versus furosemide in patients with heart failure. Am J Cardiol. 2020;125:92-99.
10. Balsam P, Ozierański K, Kapłon-Cieślicka A, et al. Comparative analysis of long-term outcomes of torasemide and furosemide in heart failure patients in heart failure registries of the European Society of Cardiology. Cardiovasc Drugs Ther. 2019;33:77-86.
11. Täger T, Fröhlich H, Grundtvig M, et al. Comparative effectiveness of loop diuretics on mortality in the treatment of patients with chronic heart failure—a multicenter propensity score matched analysis. Int J Cardiol. 2019;289:83-90.
1. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147-239.
2. Buggey J, Mentz RJ, Pitt B, et al. A reappraisal of loop diuretic choice in heart failure patients. Am Heart J. 2015;169:323-333.
3. Cosín J, Díez J; TORIC investigators. Torasemide in chronic heart failure: results of the TORIC study. Eur J Heart Fail. 2002;4:507-513.
4. Murray MD, Deer MM, Ferguson JA, et al. Open-label randomized trial of torsemide compared with furosemide therapy for patients with heart failure. Am J Med. 2001;111:513-520.
5. Mentz RJ, Hasselblad V, DeVore AD, et al. Torsemide versus furosemide in patients with acute heart failure (from the ASCEND-HF Trial). Am J Cardiol. 2016;117:404-411.
6. Müller K, Gamba G, Jaquet F, et al. Torasemide vs furosemide in primary care patients with chronic heart failure NYHA II to IV—efficacy and quality of life. Eur J Heart Fail. 2003;5:793-801.
7. Balsam P, Ozierański K, Tymińska A, et al. The impact of torasemide on haemodynamic and neurohormonal stress, and cardiac remodelling in heart failure—TORNADO: a study protocol for a randomized controlled trial. Trials. 2017;18:36.
8. Balsam P, Ozierański K, Marchel M, et al. Comparative effectiveness of torasemide versus furosemide in symptomatic therapy in heart failure patients: preliminary results from the randomized TORNADO trial. Cardiol J. 2019;26:661-668.
9. Abraham B, Megaly M, Sous M, et al. Meta-analysis comparing torsemide versus furosemide in patients with heart failure. Am J Cardiol. 2020;125:92-99.
10. Balsam P, Ozierański K, Kapłon-Cieślicka A, et al. Comparative analysis of long-term outcomes of torasemide and furosemide in heart failure patients in heart failure registries of the European Society of Cardiology. Cardiovasc Drugs Ther. 2019;33:77-86.
11. Täger T, Fröhlich H, Grundtvig M, et al. Comparative effectiveness of loop diuretics on mortality in the treatment of patients with chronic heart failure—a multicenter propensity score matched analysis. Int J Cardiol. 2019;289:83-90.
67-year-old man • upper extremity pain & edema • recent diagnosis of heart failure • Dx?
THE CASE
A 67-year-old man with a history of gout, tobacco use, hypertension, hyperlipidemia, prediabetes, and newly diagnosed heart failure with reduced ejection fraction presented with a new concern for sudden-onset, atraumatic right upper extremity pain and swelling. The patient had awakened with these symptoms and on the following day went to the emergency department (ED) for evaluation. Review of the ED documentation highlighted that the patient was afebrile and was found to have a slight leukocytosis (11.7 x 103/µL) and an elevated C-reactive protein level (4 mg/dL; normal range, 0.3 to 1 mg/dL). A right upper extremity x-ray was unremarkable. The patient was treated with cephalexin and colchicine for cellulitis and possible acute gout.
Three days after the ED visit, the patient presented to his primary care clinic, reporting adherence to the prescribed therapies (cephalexin and colchicine) but no improvement in symptoms. He was again afebrile, and his blood pressure was controlled to goal (118/80 mm Hg). On exam, he had significant nonpitting, unilateral edema extending from the elbow through the fingers without erythema, warmth, or rash (FIGURE). A right upper extremity ultrasound was obtained; results were negative for deep vein thrombosis.
Medication reconciliation completed during the clinic visit revealed that the patient had started and continued to take newly prescribed medications for the treatment of heart failure, including metoprolol succinate, lisinopril, and furosemide. The patient confirmed that these were started 7 days prior to symptom onset.
THE DIAGNOSIS
Given the clinical resemblance to angioedema and the recent initiation of lisinopril, the patient was asked to hold this medication. He was also advised to discontinue the cephalexin and colchicine, given low suspicion for cellulitis and gout. Six days later, he returned to clinic and reported significantly improved pain and swelling.
DISCUSSION
Angioedema is a common condition in the United States, affecting approximately 15% of the general population.1 When associated with hypotension, respiratory compromise, and other end-organ dysfunction, it is treated as anaphylaxis. Angioedema without anaphylaxis can be categorized as either histaminergic or nonhistaminergic; the former is more common.2
Certain patient and disease characteristics are more prevalent in select subsets of angioedema, although there are no features that automatically identify an etiology. Here are some factors to consider:
Recent exposures. Within the histaminergic category, allergic angioedema has the longest list of potential causes, including medications (notably, antibiotics, nonsteroidal anti-inflammatory drugs, opiates, and perioperative medications), foods, latex, and insect stings and/or bites.2 Nonhistaminergic subtypes, which include hereditary and acquired angioedema, are caused by deficiencies or mutations in complement or coagulation pathways, which can be more challenging to diagnose.
Continue to: Acquired angioedema may also...
Acquired angioedema may also be associated with the use of angiotensin-converting enzyme (ACE) inhibitors. Risk factors for ACE inhibitor–induced angioedema include history of smoking, increasing age, and female gender.3 African-American race has been correlated with increased incidence of angioedema, with rates 4 to 5 times that of Whites,1 but race is now identified as a social and not a biological construct and should not be relied on to make medical decisions about prescribing.
The rate of occurrence for ACE inhibitor–induced angioedema is highest within the first 30 days of medication use2; however, it can occur anytime. The absolute risk has been estimated as 0.3% per year.4
Patient age. Histaminergic angioedema can occur at any age. The hereditary subtype of nonhistaminergic angioedema is more common in younger individuals, typically occurring in infancy to the second decade of life, and tends to run in families, while the acquired subtype often manifests in adults older than 40.2
Physical exam findings. The typical manifestation of nonhistaminergic angioedema is firm, nonpitting, nonpruritic swelling resulting from fluid shifts to the reticular dermis and subcutaneous or submucosal tissue. In comparison, histaminergic reactions commonly involve deeper dermal tissue.
Commonly affected anatomic sites also vary by angioedema type but do not directly distinguish a cause. Allergic and ACE inhibitor–induced subtypes more commonly involve the lips, tongue, larynx, and face, whereas hereditary and other acquired etiologies are more likely to affect the periphery, abdomen, face, larynx, and genitourinary systems.2 So the way that this patient presented was a bit unusual.
Continue to: Symptom history
Symptom history. Allergic angioedema often has a rapid onset and resolution, whereas hereditary and acquired subtypes appear more gradually.2 While the presence of urticaria distinguishes a histaminergic reaction, both histaminergic and nonhistaminergic angioedema may manifest without this symptom.
In our patient, the timeline of gradual symptom manifestation and the physical exam findings, as well as the patient’s age, tobacco history, and recent initiation of an ACE inhibitor, made acquired angioedema a more likely etiology.
Treatment for ACE inhibitor–induced angioedema, in addition to airway support, entails drug discontinuation. This typically leads to symptom resolution within 24 to 48 hours.2 Treatment with corticosteroids, antihistamines, and epinephrine is usually ineffective. Switching to an alternative ACE inhibitor is not recommended, as other members of the class carry the same risk. Instead, angiotensin receptor blockers (ARBs) are an appropriate substitute, as the incidence of cross-reactivity in ACE inhibitor–intolerant patients is estimated to be 10% or less,5 and the risk for recurrence has been shown to be no different than with placebo.3,4
Our patient was transitioned to losartan 25 mg/d without recurrence of his symptoms and with continued blood pressure control (125/60 mm Hg).
THE TAKEAWAY
Angioedema is a common condition. While many medications are associated with histaminergic angioedema, ACE inhibitors are a common cause of the acquired subtype of nonhistaminergic angioedema. Commonly affected sites include the lips, tongue, and face; however, this diagnosis is not dependent on location and may manifest at other sites, as seen in this case. Treatment involves medication discontinuation. When switching the patient’s medication, other members of the ACE inhibitor class should be avoided. ARBs are an appropriate alternative without increased risk for recurrence.
CORRESPONDENCE
Katherine Montag Schafer, University of Minnesota— Department of Family Medicine and Community Health, 1414 Maryland Avenue E, St Paul, MN 55106; [email protected]
1. Temiño VM, Peebles RS Jr. The spectrum and treatment of angioedema. Am J Med. 2008;121:282-286.
2. Moellman JJ, Bernstein JA, Lindsell CA, et al; American College of Allergy, Asthma & Immunology (ACAAI), Society for Academic Emergency Medicine (SAEM). A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014;21:469-484.
3. Zuraw BL, Bernstein JA, Lang DM, et al; American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology. A focused parameter update: hereditary angioedema, acquired C1 inhibitor deficiency, and angiotensin-converting enzyme inhibitor-associated angioedema. J Allergy Clin Immunol. 2013;131:1491-1493.
4. Makani H, Messerli FH, Romero J, et al. Meta-analysis of randomized trials of angioedema as an adverse event of renin-angiotensin system inhibitors. Am J Cardiol. 2012;110:383-391.
5. Beavers CJ, Dunn SP, Macaulay TE. The role of angiotensin receptor blockers in patients with angiotensin-converting enzyme inhibitor-induced angioedema. Ann Pharmacother. 2011;45:520-524.
THE CASE
A 67-year-old man with a history of gout, tobacco use, hypertension, hyperlipidemia, prediabetes, and newly diagnosed heart failure with reduced ejection fraction presented with a new concern for sudden-onset, atraumatic right upper extremity pain and swelling. The patient had awakened with these symptoms and on the following day went to the emergency department (ED) for evaluation. Review of the ED documentation highlighted that the patient was afebrile and was found to have a slight leukocytosis (11.7 x 103/µL) and an elevated C-reactive protein level (4 mg/dL; normal range, 0.3 to 1 mg/dL). A right upper extremity x-ray was unremarkable. The patient was treated with cephalexin and colchicine for cellulitis and possible acute gout.
Three days after the ED visit, the patient presented to his primary care clinic, reporting adherence to the prescribed therapies (cephalexin and colchicine) but no improvement in symptoms. He was again afebrile, and his blood pressure was controlled to goal (118/80 mm Hg). On exam, he had significant nonpitting, unilateral edema extending from the elbow through the fingers without erythema, warmth, or rash (FIGURE). A right upper extremity ultrasound was obtained; results were negative for deep vein thrombosis.

Medication reconciliation completed during the clinic visit revealed that the patient had started and continued to take newly prescribed medications for the treatment of heart failure, including metoprolol succinate, lisinopril, and furosemide. The patient confirmed that these were started 7 days prior to symptom onset.
THE DIAGNOSIS
Given the clinical resemblance to angioedema and the recent initiation of lisinopril, the patient was asked to hold this medication. He was also advised to discontinue the cephalexin and colchicine, given low suspicion for cellulitis and gout. Six days later, he returned to clinic and reported significantly improved pain and swelling.
DISCUSSION
Angioedema is a common condition in the United States, affecting approximately 15% of the general population.1 When associated with hypotension, respiratory compromise, and other end-organ dysfunction, it is treated as anaphylaxis. Angioedema without anaphylaxis can be categorized as either histaminergic or nonhistaminergic; the former is more common.2
Certain patient and disease characteristics are more prevalent in select subsets of angioedema, although there are no features that automatically identify an etiology. Here are some factors to consider:
Recent exposures. Within the histaminergic category, allergic angioedema has the longest list of potential causes, including medications (notably, antibiotics, nonsteroidal anti-inflammatory drugs, opiates, and perioperative medications), foods, latex, and insect stings and/or bites.2 Nonhistaminergic subtypes, which include hereditary and acquired angioedema, are caused by deficiencies or mutations in complement or coagulation pathways, which can be more challenging to diagnose.
Continue to: Acquired angioedema may also...
Acquired angioedema may also be associated with the use of angiotensin-converting enzyme (ACE) inhibitors. Risk factors for ACE inhibitor–induced angioedema include history of smoking, increasing age, and female gender.3 African-American race has been correlated with increased incidence of angioedema, with rates 4 to 5 times that of Whites,1 but race is now identified as a social and not a biological construct and should not be relied on to make medical decisions about prescribing.
The rate of occurrence for ACE inhibitor–induced angioedema is highest within the first 30 days of medication use2; however, it can occur anytime. The absolute risk has been estimated as 0.3% per year.4
Patient age. Histaminergic angioedema can occur at any age. The hereditary subtype of nonhistaminergic angioedema is more common in younger individuals, typically occurring in infancy to the second decade of life, and tends to run in families, while the acquired subtype often manifests in adults older than 40.2
Physical exam findings. The typical manifestation of nonhistaminergic angioedema is firm, nonpitting, nonpruritic swelling resulting from fluid shifts to the reticular dermis and subcutaneous or submucosal tissue. In comparison, histaminergic reactions commonly involve deeper dermal tissue.
Commonly affected anatomic sites also vary by angioedema type but do not directly distinguish a cause. Allergic and ACE inhibitor–induced subtypes more commonly involve the lips, tongue, larynx, and face, whereas hereditary and other acquired etiologies are more likely to affect the periphery, abdomen, face, larynx, and genitourinary systems.2 So the way that this patient presented was a bit unusual.
Continue to: Symptom history
Symptom history. Allergic angioedema often has a rapid onset and resolution, whereas hereditary and acquired subtypes appear more gradually.2 While the presence of urticaria distinguishes a histaminergic reaction, both histaminergic and nonhistaminergic angioedema may manifest without this symptom.
In our patient, the timeline of gradual symptom manifestation and the physical exam findings, as well as the patient’s age, tobacco history, and recent initiation of an ACE inhibitor, made acquired angioedema a more likely etiology.
Treatment for ACE inhibitor–induced angioedema, in addition to airway support, entails drug discontinuation. This typically leads to symptom resolution within 24 to 48 hours.2 Treatment with corticosteroids, antihistamines, and epinephrine is usually ineffective. Switching to an alternative ACE inhibitor is not recommended, as other members of the class carry the same risk. Instead, angiotensin receptor blockers (ARBs) are an appropriate substitute, as the incidence of cross-reactivity in ACE inhibitor–intolerant patients is estimated to be 10% or less,5 and the risk for recurrence has been shown to be no different than with placebo.3,4
Our patient was transitioned to losartan 25 mg/d without recurrence of his symptoms and with continued blood pressure control (125/60 mm Hg).
THE TAKEAWAY
Angioedema is a common condition. While many medications are associated with histaminergic angioedema, ACE inhibitors are a common cause of the acquired subtype of nonhistaminergic angioedema. Commonly affected sites include the lips, tongue, and face; however, this diagnosis is not dependent on location and may manifest at other sites, as seen in this case. Treatment involves medication discontinuation. When switching the patient’s medication, other members of the ACE inhibitor class should be avoided. ARBs are an appropriate alternative without increased risk for recurrence.
CORRESPONDENCE
Katherine Montag Schafer, University of Minnesota— Department of Family Medicine and Community Health, 1414 Maryland Avenue E, St Paul, MN 55106; [email protected]
THE CASE
A 67-year-old man with a history of gout, tobacco use, hypertension, hyperlipidemia, prediabetes, and newly diagnosed heart failure with reduced ejection fraction presented with a new concern for sudden-onset, atraumatic right upper extremity pain and swelling. The patient had awakened with these symptoms and on the following day went to the emergency department (ED) for evaluation. Review of the ED documentation highlighted that the patient was afebrile and was found to have a slight leukocytosis (11.7 x 103/µL) and an elevated C-reactive protein level (4 mg/dL; normal range, 0.3 to 1 mg/dL). A right upper extremity x-ray was unremarkable. The patient was treated with cephalexin and colchicine for cellulitis and possible acute gout.
Three days after the ED visit, the patient presented to his primary care clinic, reporting adherence to the prescribed therapies (cephalexin and colchicine) but no improvement in symptoms. He was again afebrile, and his blood pressure was controlled to goal (118/80 mm Hg). On exam, he had significant nonpitting, unilateral edema extending from the elbow through the fingers without erythema, warmth, or rash (FIGURE). A right upper extremity ultrasound was obtained; results were negative for deep vein thrombosis.

Medication reconciliation completed during the clinic visit revealed that the patient had started and continued to take newly prescribed medications for the treatment of heart failure, including metoprolol succinate, lisinopril, and furosemide. The patient confirmed that these were started 7 days prior to symptom onset.
THE DIAGNOSIS
Given the clinical resemblance to angioedema and the recent initiation of lisinopril, the patient was asked to hold this medication. He was also advised to discontinue the cephalexin and colchicine, given low suspicion for cellulitis and gout. Six days later, he returned to clinic and reported significantly improved pain and swelling.
DISCUSSION
Angioedema is a common condition in the United States, affecting approximately 15% of the general population.1 When associated with hypotension, respiratory compromise, and other end-organ dysfunction, it is treated as anaphylaxis. Angioedema without anaphylaxis can be categorized as either histaminergic or nonhistaminergic; the former is more common.2
Certain patient and disease characteristics are more prevalent in select subsets of angioedema, although there are no features that automatically identify an etiology. Here are some factors to consider:
Recent exposures. Within the histaminergic category, allergic angioedema has the longest list of potential causes, including medications (notably, antibiotics, nonsteroidal anti-inflammatory drugs, opiates, and perioperative medications), foods, latex, and insect stings and/or bites.2 Nonhistaminergic subtypes, which include hereditary and acquired angioedema, are caused by deficiencies or mutations in complement or coagulation pathways, which can be more challenging to diagnose.
Continue to: Acquired angioedema may also...
Acquired angioedema may also be associated with the use of angiotensin-converting enzyme (ACE) inhibitors. Risk factors for ACE inhibitor–induced angioedema include history of smoking, increasing age, and female gender.3 African-American race has been correlated with increased incidence of angioedema, with rates 4 to 5 times that of Whites,1 but race is now identified as a social and not a biological construct and should not be relied on to make medical decisions about prescribing.
The rate of occurrence for ACE inhibitor–induced angioedema is highest within the first 30 days of medication use2; however, it can occur anytime. The absolute risk has been estimated as 0.3% per year.4
Patient age. Histaminergic angioedema can occur at any age. The hereditary subtype of nonhistaminergic angioedema is more common in younger individuals, typically occurring in infancy to the second decade of life, and tends to run in families, while the acquired subtype often manifests in adults older than 40.2
Physical exam findings. The typical manifestation of nonhistaminergic angioedema is firm, nonpitting, nonpruritic swelling resulting from fluid shifts to the reticular dermis and subcutaneous or submucosal tissue. In comparison, histaminergic reactions commonly involve deeper dermal tissue.
Commonly affected anatomic sites also vary by angioedema type but do not directly distinguish a cause. Allergic and ACE inhibitor–induced subtypes more commonly involve the lips, tongue, larynx, and face, whereas hereditary and other acquired etiologies are more likely to affect the periphery, abdomen, face, larynx, and genitourinary systems.2 So the way that this patient presented was a bit unusual.
Continue to: Symptom history
Symptom history. Allergic angioedema often has a rapid onset and resolution, whereas hereditary and acquired subtypes appear more gradually.2 While the presence of urticaria distinguishes a histaminergic reaction, both histaminergic and nonhistaminergic angioedema may manifest without this symptom.
In our patient, the timeline of gradual symptom manifestation and the physical exam findings, as well as the patient’s age, tobacco history, and recent initiation of an ACE inhibitor, made acquired angioedema a more likely etiology.
Treatment for ACE inhibitor–induced angioedema, in addition to airway support, entails drug discontinuation. This typically leads to symptom resolution within 24 to 48 hours.2 Treatment with corticosteroids, antihistamines, and epinephrine is usually ineffective. Switching to an alternative ACE inhibitor is not recommended, as other members of the class carry the same risk. Instead, angiotensin receptor blockers (ARBs) are an appropriate substitute, as the incidence of cross-reactivity in ACE inhibitor–intolerant patients is estimated to be 10% or less,5 and the risk for recurrence has been shown to be no different than with placebo.3,4
Our patient was transitioned to losartan 25 mg/d without recurrence of his symptoms and with continued blood pressure control (125/60 mm Hg).
THE TAKEAWAY
Angioedema is a common condition. While many medications are associated with histaminergic angioedema, ACE inhibitors are a common cause of the acquired subtype of nonhistaminergic angioedema. Commonly affected sites include the lips, tongue, and face; however, this diagnosis is not dependent on location and may manifest at other sites, as seen in this case. Treatment involves medication discontinuation. When switching the patient’s medication, other members of the ACE inhibitor class should be avoided. ARBs are an appropriate alternative without increased risk for recurrence.
CORRESPONDENCE
Katherine Montag Schafer, University of Minnesota— Department of Family Medicine and Community Health, 1414 Maryland Avenue E, St Paul, MN 55106; [email protected]
1. Temiño VM, Peebles RS Jr. The spectrum and treatment of angioedema. Am J Med. 2008;121:282-286.
2. Moellman JJ, Bernstein JA, Lindsell CA, et al; American College of Allergy, Asthma & Immunology (ACAAI), Society for Academic Emergency Medicine (SAEM). A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014;21:469-484.
3. Zuraw BL, Bernstein JA, Lang DM, et al; American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology. A focused parameter update: hereditary angioedema, acquired C1 inhibitor deficiency, and angiotensin-converting enzyme inhibitor-associated angioedema. J Allergy Clin Immunol. 2013;131:1491-1493.
4. Makani H, Messerli FH, Romero J, et al. Meta-analysis of randomized trials of angioedema as an adverse event of renin-angiotensin system inhibitors. Am J Cardiol. 2012;110:383-391.
5. Beavers CJ, Dunn SP, Macaulay TE. The role of angiotensin receptor blockers in patients with angiotensin-converting enzyme inhibitor-induced angioedema. Ann Pharmacother. 2011;45:520-524.
1. Temiño VM, Peebles RS Jr. The spectrum and treatment of angioedema. Am J Med. 2008;121:282-286.
2. Moellman JJ, Bernstein JA, Lindsell CA, et al; American College of Allergy, Asthma & Immunology (ACAAI), Society for Academic Emergency Medicine (SAEM). A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014;21:469-484.
3. Zuraw BL, Bernstein JA, Lang DM, et al; American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology. A focused parameter update: hereditary angioedema, acquired C1 inhibitor deficiency, and angiotensin-converting enzyme inhibitor-associated angioedema. J Allergy Clin Immunol. 2013;131:1491-1493.
4. Makani H, Messerli FH, Romero J, et al. Meta-analysis of randomized trials of angioedema as an adverse event of renin-angiotensin system inhibitors. Am J Cardiol. 2012;110:383-391.
5. Beavers CJ, Dunn SP, Macaulay TE. The role of angiotensin receptor blockers in patients with angiotensin-converting enzyme inhibitor-induced angioedema. Ann Pharmacother. 2011;45:520-524.