User login
52-year-old man • hematemesis • history of cirrhosis • persistent fevers • Dx?
THE CASE
A 52-year-old man presented to the emergency department after vomiting a large volume of blood and was admitted to the intensive care unit. His past medical history was remarkable for untreated chronic hepatitis C resulting from injection drug use and cirrhosis without prior history of esophageal varices.
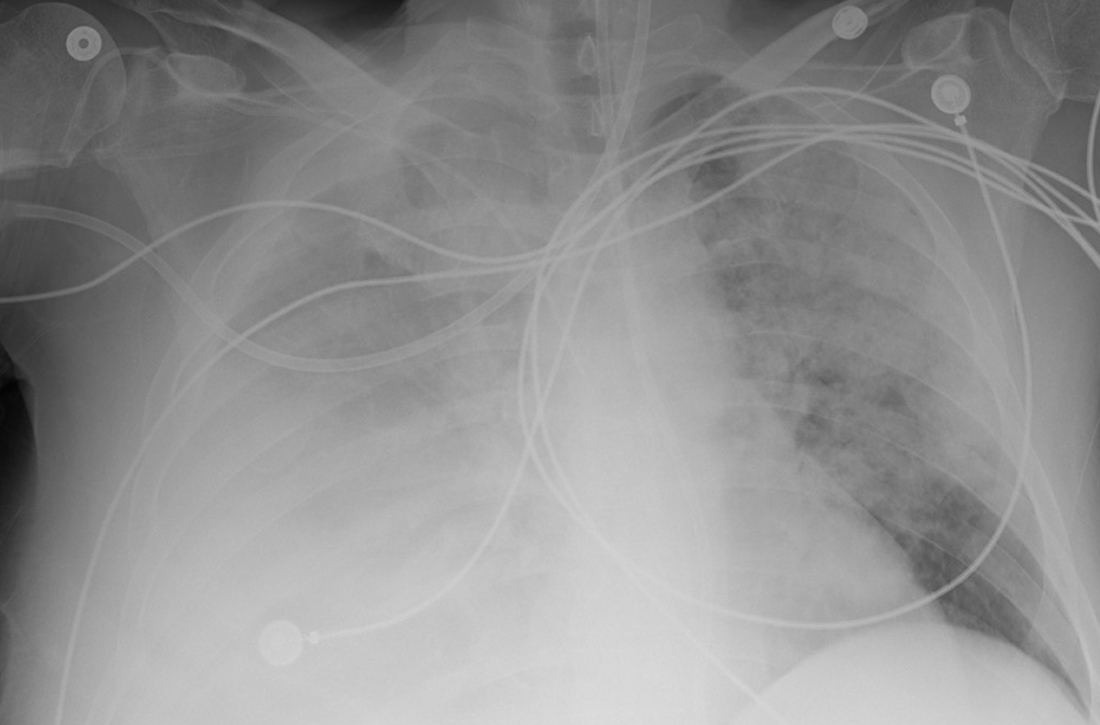
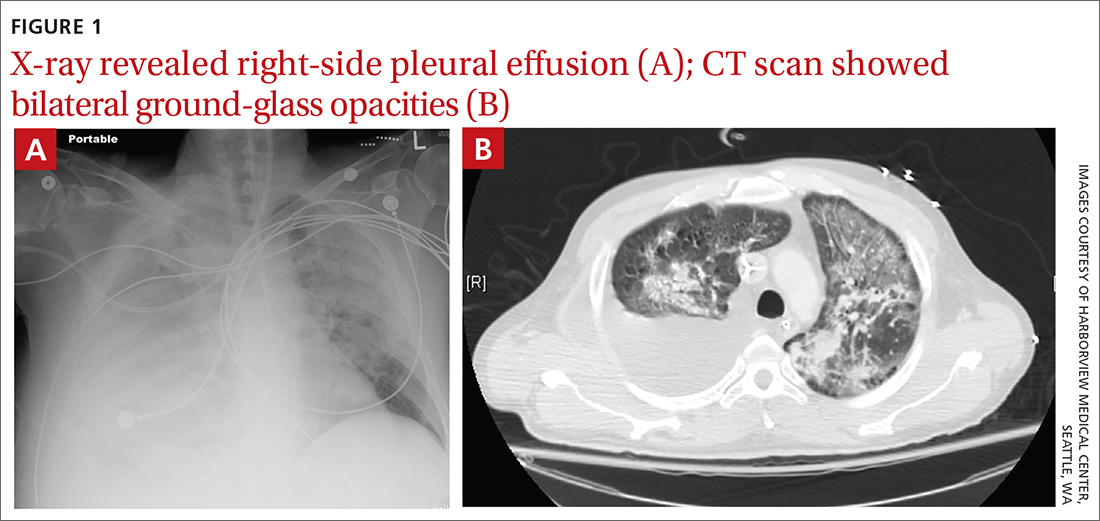
Due to ongoing hematemesis, he was intubated for airway protection and underwent esophagogastroduodenoscopy with banding of large esophageal varices on hospital day (HD) 1. He was extubated on HD 2 after clinical stability was achieved; however, he became encephalopathic over the subsequent days despite treatment with lactulose. On HD 4, the patient required re-intubation for progressive respiratory failure. Chest imaging revealed a large, simple-appearing right pleural effusion and extensive bilateral patchy ground-glass opacities (FIGURE 1).
Thoracentesis was ordered and revealed transudative pleural fluid; this finding, along with negative infectious studies, was consistent with hepatic hydrothorax. In the setting of initial decompensation, empiric treatment with vancomycin and meropenem was started for suspected hospital-acquired pneumonia.
The patient had persistent fevers that had developed during his hospital stay and pulmonary opacities, despite 72 hours of treatment with broad-spectrum antibiotics. Thus, a diagnostic bronchoscopy with bronchoalveolar lavage (BAL) was performed. BAL cell count and differential revealed 363 nucleated cells/µL, with profound eosinophilia (42% eosinophils, 44% macrophages, 14% neutrophils).
Bacterial and fungal cultures and a viral polymerase chain reaction panel were negative. HIV antibody-antigen and RNA testing were also negative. The patient had no evidence or history of underlying malignancy, autoimmune disease, or recent immunosuppressive therapy, including corticosteroids. Due to consistent imaging findings and lack of improvement with appropriate treatment for bacterial pneumonia, further work-up was pursued.
THE DIAGNOSIS
Given the consistent radiographic pattern, the differential diagnosis for this patient included pneumocystis pneumonia (PCP), a potentially life-threatening opportunistic infection. Work-up therefore included direct fluorescent antibody testing, which was positive for Pneumocystis jirovecii, a fungus that can cause PCP.
Of note, the patient’s white blood cell count was elevated on admission (11.44 × 103/µL) but low for much of his hospital stay (nadir = 1.97 × 103/µL), with associated lymphopenia (nadir = 0.22 × 103/µl). No peripheral eosinophilia was noted.
Continue to: DISCUSSION
DISCUSSION
PCP typically occurs in immunocompromised individuals and may be related to HIV infection, malignancy, or exposure to immunosuppressive therapies.1,2 While rare cases of PCP have been described in adults without predisposing factors, many of these cases occurred at the beginning of the AIDS epidemic, prior to reliable HIV testing.3-5
Uncharted territory. We were confident in our diagnosis because immunofluorescence testing has very few false-positives and a high specificity.6-8 But there were informational gaps. The eosinophilia recorded on BAL is poorly described in HIV-negative patients with PCP but well-described in HIV-positive patients, with the level of eosinophilia associated with disease severity.9,10 Eosinophils are thought to contribute to pulmonary inflammation, which may explain the severity of our patient’s course.10
A first of its kind case?
To our knowledge, this is the first report of PCP in a patient with cirrhosis from chronic hepatitis C virus infection and no other predisposing conditions or preceding immunosuppressive therapy. We suspect that his lymphopenia, which was noted during his critical illness, predisposed him to PCP.
Lymphocytes (in particular CD4+ T cells) have been shown to play an important role, along with alveolar macrophages and neutrophils, in directing the host defense against
Typical risk factors for lymphopenia had not been observed in this patient. However, cirrhosis has been associated with low CD4+ T-cell counts and disruption of cell-mediated immunity, even in HIV-seronegative patients.14,15 There are several postulated mechanisms for low CD4+ T-cell counts in cirrhosis, including splenic sequestration, impaired T-cell production (due to impaired thymopoiesis), increased T-cell consumption, and apoptosis (due to persistent immune system activation from bacterial translocation and an overall pro-inflammatory state).16,17
Continue to: Predisposing factors guide treatment
Predisposing factors guide treatment
Routine treatment for PCP in patients without HIV is a 21-day course of trimethoprim/sulfamethoxazole (Bactrim). Dosing for patients with normal renal function is 15 to 20 mg/kg orally or intravenously per day. Patients with allergy to trimethoprim/sulfamethoxazole should ideally undergo desensitization, given its effectiveness against PCP.
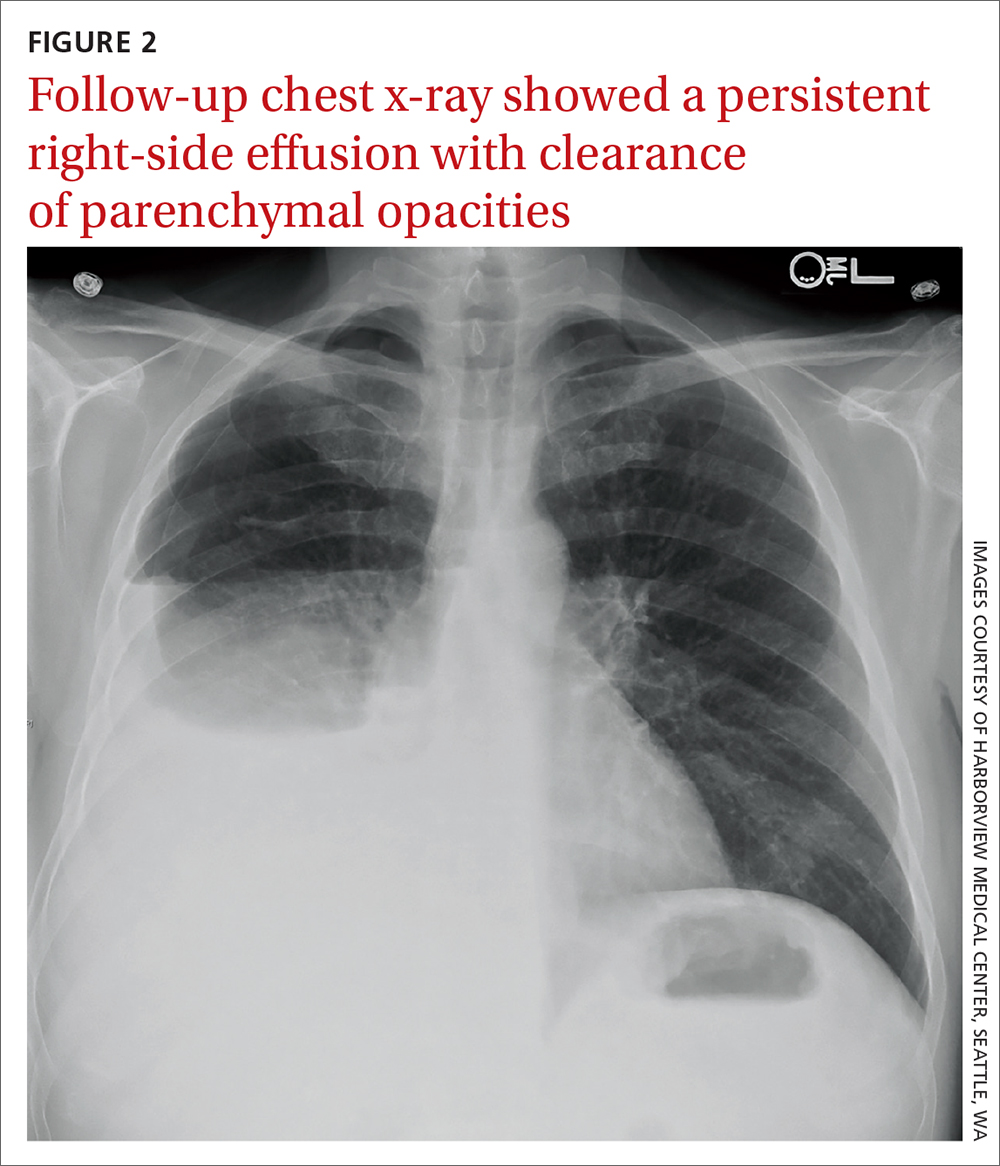
Due to a sulfonamide allergy, our patient was started on primaquine 30 mg/d, clindamycin 600 mg tid, and prednisone 40 mg bid. (The corticosteroid was added because of the severity of the disease.) Three days after starting treatment—and 10 days into his hospital stay—the patient had significant improvement in his respiratory status and was successfully extubated. He underwent trimethoprim/sulfamethoxazole desensitization and completed a 21-day course of treatment for PCP with complete resolution of respiratory symptoms. Follow-up chest radiograph 2 months later (FIGURE 2) confirmed clearance of opacities.
THE TAKEAWAY
PCP remains a rare disease in patients without the typical immunosuppressive risk factors. However, it should be considered in patients with cirrhosis who develop respiratory failure, especially those with compatible radiographic findings and negative microbiologic evaluation for other, more typical, organisms.
CORRESPONDENCE
Tyler Albert, MD, VA Puget Sound Healthcare System, 1660 South Columbian Way, S-111-Pulm, Seattle, WA 98108; [email protected]
1. Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487-2498. doi: 10.1056/NEJMra032588
2. Walzer PD, Perl DP, Krogstad DJ, et al. Pneumocystis carinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974;80:83-93. doi: 10.7326/0003-4819-80-1-83
3. Sepkowitz KA. Pneumocystis carinii pneumonia in patients without AIDS. Clin Infect Dis. 1993;17 suppl 2:S416-422. doi: 10.1093/clinids/17.supplement_2.s416
4. Al Soub H, Taha RY, El Deeb Y, et al. Pneumocystis carinii pneumonia in a patient without a predisposing illness: case report and review. Scand J Infect Dis. 2004;36:618-621. doi: 10.1080/00365540410017608
5. Jacobs JL, Libby DM, Winters RA, et al. A cluster of Pneumocystis carinii pneumonia in adults without predisposing illnesses. N Engl J Med. 1991;324:246-250. doi: 10.1056/NEJM199101243240407
6. Ng VL, Yajko DM, McPhaul LW, et al. Evaluation of an indirect fluorescent-antibody stain for detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:975-979. doi: 10.1128/jcm.28.5.975-979.1990
7. Cregan P, Yamamoto A, Lum A, et al. Comparison of four methods for rapid detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:2432-2436. doi: 10.1128/jcm.28.11.2432-2436.1990
8. Turner D, Schwarz Y, Yust I. Induced sputum for diagnosing Pneumocystis carinii pneumonia in HIV patients: new data, new issues. Eur Respir J. 2003;21:204-208. doi: 10.1183/09031936.03.00035303
9. Smith RL, el-Sadr WM, Lewis ML. Correlation of bronchoalveolar lavage cell populations with clinical severity of Pneumocystis carinii pneumonia. Chest. 1988;93:60-64. doi: 10.1378/chest.93.1.60
10. Fleury-Feith J, Van Nhieu JT, Picard C, et al. Bronchoalveolar lavage eosinophilia associated with Pneumocystis carinii pneumonitis in AIDS patients. Comparative study with non-AIDS patients. Chest. 1989;95:1198-1201. doi: 10.1378/chest.95.6.1198
11. Thomas CF Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol. 2007;5:298-308. doi: 10.1038/nrmicro1621
12. Toh BH, Roberts-Thomson IC, Mathews JD, et al. Depression of cell-mediated immunity in old age and the immunopathic diseases, lupus erythematosus, chronic hepatitis and rheumatoid arthritis. Clin Exp Immunol. 1973;14:193-202.
13. Mansharamani NG, Balachandran D, Vernovsky I, et al. Peripheral blood CD4 + T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest. 2000;118:712-720. doi: 10.1378/chest.118.3.712
14. McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431-437. doi: 10.1086/509580
15. Bienvenu AL, Traore K, Plekhanova I, et al. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int J Infect Dis. 2016;46:11-17. doi: 10.1016/j.ijid.2016.03.018
16. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. doi: 10.1016/j.jhep.2014.08.010
17. Lario M, Muñoz L, Ubeda M, et al. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J Hepatol. 2013;59:723-730. doi: 10.1016/j.jhep.2013.05.042
THE CASE
A 52-year-old man presented to the emergency department after vomiting a large volume of blood and was admitted to the intensive care unit. His past medical history was remarkable for untreated chronic hepatitis C resulting from injection drug use and cirrhosis without prior history of esophageal varices.
Due to ongoing hematemesis, he was intubated for airway protection and underwent esophagogastroduodenoscopy with banding of large esophageal varices on hospital day (HD) 1. He was extubated on HD 2 after clinical stability was achieved; however, he became encephalopathic over the subsequent days despite treatment with lactulose. On HD 4, the patient required re-intubation for progressive respiratory failure. Chest imaging revealed a large, simple-appearing right pleural effusion and extensive bilateral patchy ground-glass opacities (FIGURE 1).

Thoracentesis was ordered and revealed transudative pleural fluid; this finding, along with negative infectious studies, was consistent with hepatic hydrothorax. In the setting of initial decompensation, empiric treatment with vancomycin and meropenem was started for suspected hospital-acquired pneumonia.
The patient had persistent fevers that had developed during his hospital stay and pulmonary opacities, despite 72 hours of treatment with broad-spectrum antibiotics. Thus, a diagnostic bronchoscopy with bronchoalveolar lavage (BAL) was performed. BAL cell count and differential revealed 363 nucleated cells/µL, with profound eosinophilia (42% eosinophils, 44% macrophages, 14% neutrophils).
Bacterial and fungal cultures and a viral polymerase chain reaction panel were negative. HIV antibody-antigen and RNA testing were also negative. The patient had no evidence or history of underlying malignancy, autoimmune disease, or recent immunosuppressive therapy, including corticosteroids. Due to consistent imaging findings and lack of improvement with appropriate treatment for bacterial pneumonia, further work-up was pursued.
THE DIAGNOSIS
Given the consistent radiographic pattern, the differential diagnosis for this patient included pneumocystis pneumonia (PCP), a potentially life-threatening opportunistic infection. Work-up therefore included direct fluorescent antibody testing, which was positive for Pneumocystis jirovecii, a fungus that can cause PCP.
Of note, the patient’s white blood cell count was elevated on admission (11.44 × 103/µL) but low for much of his hospital stay (nadir = 1.97 × 103/µL), with associated lymphopenia (nadir = 0.22 × 103/µl). No peripheral eosinophilia was noted.
Continue to: DISCUSSION
DISCUSSION
PCP typically occurs in immunocompromised individuals and may be related to HIV infection, malignancy, or exposure to immunosuppressive therapies.1,2 While rare cases of PCP have been described in adults without predisposing factors, many of these cases occurred at the beginning of the AIDS epidemic, prior to reliable HIV testing.3-5
Uncharted territory. We were confident in our diagnosis because immunofluorescence testing has very few false-positives and a high specificity.6-8 But there were informational gaps. The eosinophilia recorded on BAL is poorly described in HIV-negative patients with PCP but well-described in HIV-positive patients, with the level of eosinophilia associated with disease severity.9,10 Eosinophils are thought to contribute to pulmonary inflammation, which may explain the severity of our patient’s course.10
A first of its kind case?
To our knowledge, this is the first report of PCP in a patient with cirrhosis from chronic hepatitis C virus infection and no other predisposing conditions or preceding immunosuppressive therapy. We suspect that his lymphopenia, which was noted during his critical illness, predisposed him to PCP.
Lymphocytes (in particular CD4+ T cells) have been shown to play an important role, along with alveolar macrophages and neutrophils, in directing the host defense against
Typical risk factors for lymphopenia had not been observed in this patient. However, cirrhosis has been associated with low CD4+ T-cell counts and disruption of cell-mediated immunity, even in HIV-seronegative patients.14,15 There are several postulated mechanisms for low CD4+ T-cell counts in cirrhosis, including splenic sequestration, impaired T-cell production (due to impaired thymopoiesis), increased T-cell consumption, and apoptosis (due to persistent immune system activation from bacterial translocation and an overall pro-inflammatory state).16,17
Continue to: Predisposing factors guide treatment
Predisposing factors guide treatment
Routine treatment for PCP in patients without HIV is a 21-day course of trimethoprim/sulfamethoxazole (Bactrim). Dosing for patients with normal renal function is 15 to 20 mg/kg orally or intravenously per day. Patients with allergy to trimethoprim/sulfamethoxazole should ideally undergo desensitization, given its effectiveness against PCP.
Due to a sulfonamide allergy, our patient was started on primaquine 30 mg/d, clindamycin 600 mg tid, and prednisone 40 mg bid. (The corticosteroid was added because of the severity of the disease.) Three days after starting treatment—and 10 days into his hospital stay—the patient had significant improvement in his respiratory status and was successfully extubated. He underwent trimethoprim/sulfamethoxazole desensitization and completed a 21-day course of treatment for PCP with complete resolution of respiratory symptoms. Follow-up chest radiograph 2 months later (FIGURE 2) confirmed clearance of opacities.

THE TAKEAWAY
PCP remains a rare disease in patients without the typical immunosuppressive risk factors. However, it should be considered in patients with cirrhosis who develop respiratory failure, especially those with compatible radiographic findings and negative microbiologic evaluation for other, more typical, organisms.
CORRESPONDENCE
Tyler Albert, MD, VA Puget Sound Healthcare System, 1660 South Columbian Way, S-111-Pulm, Seattle, WA 98108; [email protected]
THE CASE
A 52-year-old man presented to the emergency department after vomiting a large volume of blood and was admitted to the intensive care unit. His past medical history was remarkable for untreated chronic hepatitis C resulting from injection drug use and cirrhosis without prior history of esophageal varices.
Due to ongoing hematemesis, he was intubated for airway protection and underwent esophagogastroduodenoscopy with banding of large esophageal varices on hospital day (HD) 1. He was extubated on HD 2 after clinical stability was achieved; however, he became encephalopathic over the subsequent days despite treatment with lactulose. On HD 4, the patient required re-intubation for progressive respiratory failure. Chest imaging revealed a large, simple-appearing right pleural effusion and extensive bilateral patchy ground-glass opacities (FIGURE 1).

Thoracentesis was ordered and revealed transudative pleural fluid; this finding, along with negative infectious studies, was consistent with hepatic hydrothorax. In the setting of initial decompensation, empiric treatment with vancomycin and meropenem was started for suspected hospital-acquired pneumonia.
The patient had persistent fevers that had developed during his hospital stay and pulmonary opacities, despite 72 hours of treatment with broad-spectrum antibiotics. Thus, a diagnostic bronchoscopy with bronchoalveolar lavage (BAL) was performed. BAL cell count and differential revealed 363 nucleated cells/µL, with profound eosinophilia (42% eosinophils, 44% macrophages, 14% neutrophils).
Bacterial and fungal cultures and a viral polymerase chain reaction panel were negative. HIV antibody-antigen and RNA testing were also negative. The patient had no evidence or history of underlying malignancy, autoimmune disease, or recent immunosuppressive therapy, including corticosteroids. Due to consistent imaging findings and lack of improvement with appropriate treatment for bacterial pneumonia, further work-up was pursued.
THE DIAGNOSIS
Given the consistent radiographic pattern, the differential diagnosis for this patient included pneumocystis pneumonia (PCP), a potentially life-threatening opportunistic infection. Work-up therefore included direct fluorescent antibody testing, which was positive for Pneumocystis jirovecii, a fungus that can cause PCP.
Of note, the patient’s white blood cell count was elevated on admission (11.44 × 103/µL) but low for much of his hospital stay (nadir = 1.97 × 103/µL), with associated lymphopenia (nadir = 0.22 × 103/µl). No peripheral eosinophilia was noted.
Continue to: DISCUSSION
DISCUSSION
PCP typically occurs in immunocompromised individuals and may be related to HIV infection, malignancy, or exposure to immunosuppressive therapies.1,2 While rare cases of PCP have been described in adults without predisposing factors, many of these cases occurred at the beginning of the AIDS epidemic, prior to reliable HIV testing.3-5
Uncharted territory. We were confident in our diagnosis because immunofluorescence testing has very few false-positives and a high specificity.6-8 But there were informational gaps. The eosinophilia recorded on BAL is poorly described in HIV-negative patients with PCP but well-described in HIV-positive patients, with the level of eosinophilia associated with disease severity.9,10 Eosinophils are thought to contribute to pulmonary inflammation, which may explain the severity of our patient’s course.10
A first of its kind case?
To our knowledge, this is the first report of PCP in a patient with cirrhosis from chronic hepatitis C virus infection and no other predisposing conditions or preceding immunosuppressive therapy. We suspect that his lymphopenia, which was noted during his critical illness, predisposed him to PCP.
Lymphocytes (in particular CD4+ T cells) have been shown to play an important role, along with alveolar macrophages and neutrophils, in directing the host defense against
Typical risk factors for lymphopenia had not been observed in this patient. However, cirrhosis has been associated with low CD4+ T-cell counts and disruption of cell-mediated immunity, even in HIV-seronegative patients.14,15 There are several postulated mechanisms for low CD4+ T-cell counts in cirrhosis, including splenic sequestration, impaired T-cell production (due to impaired thymopoiesis), increased T-cell consumption, and apoptosis (due to persistent immune system activation from bacterial translocation and an overall pro-inflammatory state).16,17
Continue to: Predisposing factors guide treatment
Predisposing factors guide treatment
Routine treatment for PCP in patients without HIV is a 21-day course of trimethoprim/sulfamethoxazole (Bactrim). Dosing for patients with normal renal function is 15 to 20 mg/kg orally or intravenously per day. Patients with allergy to trimethoprim/sulfamethoxazole should ideally undergo desensitization, given its effectiveness against PCP.
Due to a sulfonamide allergy, our patient was started on primaquine 30 mg/d, clindamycin 600 mg tid, and prednisone 40 mg bid. (The corticosteroid was added because of the severity of the disease.) Three days after starting treatment—and 10 days into his hospital stay—the patient had significant improvement in his respiratory status and was successfully extubated. He underwent trimethoprim/sulfamethoxazole desensitization and completed a 21-day course of treatment for PCP with complete resolution of respiratory symptoms. Follow-up chest radiograph 2 months later (FIGURE 2) confirmed clearance of opacities.

THE TAKEAWAY
PCP remains a rare disease in patients without the typical immunosuppressive risk factors. However, it should be considered in patients with cirrhosis who develop respiratory failure, especially those with compatible radiographic findings and negative microbiologic evaluation for other, more typical, organisms.
CORRESPONDENCE
Tyler Albert, MD, VA Puget Sound Healthcare System, 1660 South Columbian Way, S-111-Pulm, Seattle, WA 98108; [email protected]
1. Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487-2498. doi: 10.1056/NEJMra032588
2. Walzer PD, Perl DP, Krogstad DJ, et al. Pneumocystis carinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974;80:83-93. doi: 10.7326/0003-4819-80-1-83
3. Sepkowitz KA. Pneumocystis carinii pneumonia in patients without AIDS. Clin Infect Dis. 1993;17 suppl 2:S416-422. doi: 10.1093/clinids/17.supplement_2.s416
4. Al Soub H, Taha RY, El Deeb Y, et al. Pneumocystis carinii pneumonia in a patient without a predisposing illness: case report and review. Scand J Infect Dis. 2004;36:618-621. doi: 10.1080/00365540410017608
5. Jacobs JL, Libby DM, Winters RA, et al. A cluster of Pneumocystis carinii pneumonia in adults without predisposing illnesses. N Engl J Med. 1991;324:246-250. doi: 10.1056/NEJM199101243240407
6. Ng VL, Yajko DM, McPhaul LW, et al. Evaluation of an indirect fluorescent-antibody stain for detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:975-979. doi: 10.1128/jcm.28.5.975-979.1990
7. Cregan P, Yamamoto A, Lum A, et al. Comparison of four methods for rapid detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:2432-2436. doi: 10.1128/jcm.28.11.2432-2436.1990
8. Turner D, Schwarz Y, Yust I. Induced sputum for diagnosing Pneumocystis carinii pneumonia in HIV patients: new data, new issues. Eur Respir J. 2003;21:204-208. doi: 10.1183/09031936.03.00035303
9. Smith RL, el-Sadr WM, Lewis ML. Correlation of bronchoalveolar lavage cell populations with clinical severity of Pneumocystis carinii pneumonia. Chest. 1988;93:60-64. doi: 10.1378/chest.93.1.60
10. Fleury-Feith J, Van Nhieu JT, Picard C, et al. Bronchoalveolar lavage eosinophilia associated with Pneumocystis carinii pneumonitis in AIDS patients. Comparative study with non-AIDS patients. Chest. 1989;95:1198-1201. doi: 10.1378/chest.95.6.1198
11. Thomas CF Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol. 2007;5:298-308. doi: 10.1038/nrmicro1621
12. Toh BH, Roberts-Thomson IC, Mathews JD, et al. Depression of cell-mediated immunity in old age and the immunopathic diseases, lupus erythematosus, chronic hepatitis and rheumatoid arthritis. Clin Exp Immunol. 1973;14:193-202.
13. Mansharamani NG, Balachandran D, Vernovsky I, et al. Peripheral blood CD4 + T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest. 2000;118:712-720. doi: 10.1378/chest.118.3.712
14. McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431-437. doi: 10.1086/509580
15. Bienvenu AL, Traore K, Plekhanova I, et al. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int J Infect Dis. 2016;46:11-17. doi: 10.1016/j.ijid.2016.03.018
16. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. doi: 10.1016/j.jhep.2014.08.010
17. Lario M, Muñoz L, Ubeda M, et al. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J Hepatol. 2013;59:723-730. doi: 10.1016/j.jhep.2013.05.042
1. Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487-2498. doi: 10.1056/NEJMra032588
2. Walzer PD, Perl DP, Krogstad DJ, et al. Pneumocystis carinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974;80:83-93. doi: 10.7326/0003-4819-80-1-83
3. Sepkowitz KA. Pneumocystis carinii pneumonia in patients without AIDS. Clin Infect Dis. 1993;17 suppl 2:S416-422. doi: 10.1093/clinids/17.supplement_2.s416
4. Al Soub H, Taha RY, El Deeb Y, et al. Pneumocystis carinii pneumonia in a patient without a predisposing illness: case report and review. Scand J Infect Dis. 2004;36:618-621. doi: 10.1080/00365540410017608
5. Jacobs JL, Libby DM, Winters RA, et al. A cluster of Pneumocystis carinii pneumonia in adults without predisposing illnesses. N Engl J Med. 1991;324:246-250. doi: 10.1056/NEJM199101243240407
6. Ng VL, Yajko DM, McPhaul LW, et al. Evaluation of an indirect fluorescent-antibody stain for detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:975-979. doi: 10.1128/jcm.28.5.975-979.1990
7. Cregan P, Yamamoto A, Lum A, et al. Comparison of four methods for rapid detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:2432-2436. doi: 10.1128/jcm.28.11.2432-2436.1990
8. Turner D, Schwarz Y, Yust I. Induced sputum for diagnosing Pneumocystis carinii pneumonia in HIV patients: new data, new issues. Eur Respir J. 2003;21:204-208. doi: 10.1183/09031936.03.00035303
9. Smith RL, el-Sadr WM, Lewis ML. Correlation of bronchoalveolar lavage cell populations with clinical severity of Pneumocystis carinii pneumonia. Chest. 1988;93:60-64. doi: 10.1378/chest.93.1.60
10. Fleury-Feith J, Van Nhieu JT, Picard C, et al. Bronchoalveolar lavage eosinophilia associated with Pneumocystis carinii pneumonitis in AIDS patients. Comparative study with non-AIDS patients. Chest. 1989;95:1198-1201. doi: 10.1378/chest.95.6.1198
11. Thomas CF Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol. 2007;5:298-308. doi: 10.1038/nrmicro1621
12. Toh BH, Roberts-Thomson IC, Mathews JD, et al. Depression of cell-mediated immunity in old age and the immunopathic diseases, lupus erythematosus, chronic hepatitis and rheumatoid arthritis. Clin Exp Immunol. 1973;14:193-202.
13. Mansharamani NG, Balachandran D, Vernovsky I, et al. Peripheral blood CD4 + T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest. 2000;118:712-720. doi: 10.1378/chest.118.3.712
14. McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431-437. doi: 10.1086/509580
15. Bienvenu AL, Traore K, Plekhanova I, et al. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int J Infect Dis. 2016;46:11-17. doi: 10.1016/j.ijid.2016.03.018
16. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. doi: 10.1016/j.jhep.2014.08.010
17. Lario M, Muñoz L, Ubeda M, et al. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J Hepatol. 2013;59:723-730. doi: 10.1016/j.jhep.2013.05.042
61-year-old woman • nausea • paresthesia • cold allodynia • Dx?
THE CASE
An active 61-year-old woman (140 lbs) in good health became ill during a sailing holiday in the Virgin Islands. During the trip, she ate various fish in local restaurants; after one lunch, she developed nausea, diarrhea, dizziness, headache, and light-headedness. In the following days, she suffered “intense itching” in the ears, dizziness, malaise, a “fluttering feeling” throughout her body, genitourinary sensitivity, and a “rhythmic buzzing sensation near the rectum.”
She said that cold objects and beverages felt uncomfortably hot (cold allodynia). She noted heightened senses of smell and taste, as well as paresthesia down her spine, and described feeling “moody.” She reduced her workload, took many days off from work, and ceased consuming meat and alcohol because these items seemed to aggravate her symptoms.
The paresthesia persisted, and she consulted her family physician one month later. Laboratory tests—including a complete blood count, hematocrit, thyroid-stimulating hormone, antinuclear antibodies, and titers for Ehrlichia chaffeensis, Lyme disease, and Anaplasma phagocytophila—all yielded normal results. Her symptoms continued for 3 more months before referral to Medical Toxicology.
THE DIAGNOSIS
The patient’s symptoms and history were consistent with ciguatera poisoning. Features supporting this diagnosis included an acute gastrointestinal illness after eating fish caught in tropical waters and subsequent persistent paresthesia, including cold allodynia.1 Laboratory testing excluded acute infection, anemia, thyroid dysfunction, vitamin B12 deficiency, lupus, rheumatoid arthritis, Lyme disease, ehrlichiosis, and anaplasmosis.
DISCUSSION
Ciguatera results from ciguatoxin, a class of heat-stable polycyclic toxins produced in warm tropical waters by microscopic dinoflagellates (most often Gambierdiscus toxicus).2,3 Small variations exist in the Caribbean, Pacific, and Indian Ocean forms. Ciguatoxin bio-accumulates in the food chain, and humans most often ingest it by eating larger fish (typically barracuda, snapper, grouper, or amberjack).4 Because ciguatoxin confers no characteristic taste or smell to the fish, people who prepare or eat contaminated seafood have no reliable means to detect and avoid it.
Ciguatoxin opens neuronal voltage-gated sodium channels and blocks delayed-rectifier potassium channels.5 These cause repetitive, spontaneous action potentials that explain the paresthesia. Sodium influx triggers an increase in intracellular calcium concentrations. Increased intracellular sodium and calcium concentrations draw water into the intracellular space and cause neuronal edema.
Death is rarely associated with ciguatera (< 0.1% in the largest observational study).1 Even without treatment (discussed shortly), symptoms of ciguatera will gradually resolve over several weeks to several months in most cases.1,4,5 However, after recovery, patients often briefly experience milder symptoms after consuming fish, alcohol, or nuts.6
Continue to: Treatment of ciguatera
Treatment of ciguatera may include intravenous (IV) mannitol infusion. Other treatments, such as amitriptyline, gabapentin, pregabalin, and tocainide, have been used, but there is limited supporting evidence and they appear variably effective.7
Mannitol reverses the effects of ciguatoxin, with suppression of spontaneous action potentials and reversal of neuronal edema.8,9 It is reasonable to offer mannitol for acute or persistent symptoms of ciguatera fish poisoning even after a delay of several weeks.
A recent systematic review found that mannitol has the largest body of evidence supporting its use, although that evidence is generally of low quality (case reports and large case series).7 While these reports10-13 describe beneficial effects of mannitol, a single randomized trial suggested that mannitol is no more effective than normal saline.14 However, this study was underpowered and had inadequate treatment concealment; twice as many saline control patients as mannitol-treated patients requested a rescue dose of mannitol.14
Mannitol may be most effective when given early in the course of ciguatera but has shown some success when given later.5,12,13 In 1 large case series, the longest interval from symptom onset to successful treatment was 70 days, although most patients with satisfactory results received mannitol in the first few days.5
Our patient was administered an IV infusion of 100 g of 20% mannitol over 1 hour. She received the infusion 140 days after the onset of her symptoms and experienced rapid symptom relief.
Continue to: At a follow-up visit...
At a follow-up visit 2 weeks later, she described increased energy and further improvement in her paresthesia. She returned to a full work schedule and resumed all of her daily activities. However, she continued to avoid alcohol and proteins, as she had experienced a mild recurrence that she temporally related to eating meat and drinking alcohol.
At the 2-month follow-up, the patient reported continued improvement in her paresthesia but continued to experience occasional gastrointestinal symptoms and fatigue associated with meat and alcohol consumption.
The Takeaway
Ciguatera fish poisoning is largely a clinical diagnosis. It is based on early gastrointestinal symptoms followed by persistent paresthesia and cold allodynia after consumption of fish caught in tropical waters. Family physicians may see ciguatera in returning travelers or people who have consumed certain fish imported from endemic areas. Untreated symptoms may last for many weeks or months. IV mannitol may relieve symptoms of ciguatera poisoning even when administered several months after symptom onset.
We are grateful to our patient, who allowed us to share her story in the hope of helping other travelers.
CORRESPONDENCE
Michael E. Mullins, MD, Division of Medical Toxicology, Department of Emergency Medicine, Washington University School of Medicine, Campus Box 8072, 660 South Euclid Avenue, Saint Louis, MO 63110; [email protected]
1. Bagnis R, Kuberski T, Laugier S. Clinical Observations of 3,009 cases of ciguatera (fish poisoning) in the South Pacific. Am J Trop Med Hyg. 1979;28:1067-1073. doi: 10.4269/ajtmh.1979.28.1067
2. Morris JG Jr, Lewin P, Smith CW, et al. Ciguatera fish poisoning: epidemiology of the disease on St. Thomas, US Virgin Islands. Am J Trop Med Hyg. 1982;31:574-578. doi: 10.4269/ajtmh.1982.31.574
3. Radke EG, Grattan LM, Cook RL, et al. Ciguatera incidence in the US Virgin Islands has not increased over a 30-year time period despite rising seawater temperatures. Am J Trop Med Hyg. 2013;88:908-913. doi: 10.4269/ajtmh.12-0676
4. Goodman DM, Rogers J, Livingston EH. Ciguatera fish poisoning.
5. Blythe DG, De Sylva DP, Fleming LE, et al. Clinical experience with IV mannitol in the treatment of ciguatera. Bull Soc Pathol Exot. 1992;85:425-426.
6. Lewis, RJ. The changing face of ciguatera. Toxicon. 2001;39:97-106. doi: 10.1016/s0041-0101(00)00161-6
7. Mullins ME, Hoffman RS. Is mannitol the treatment of choice for ciguatera fish poisoning? Clin Toxicol (Phila). 2017;55:947-955. doi: 10.1080/15563650.2017.1327664
8. Nicholson GM, Lewis, RJ. Ciguatoxins: cyclic polyether modulators of voltage-gated ion channel function. Mar Drugs. 2006;4:82-118.
9. Mattei C, Molgo J, Marquais M, et al. Hyperosmolar D-mannitol reverses the increased membrane excitability and the nodal swelling caused by Caribbean ciguatoxin-1 in single frog myelinated neurons. Brain Res. 1999;847:50-58. doi: 10.1016/s0006-8993(99)02032-6
10. Palafox NA, Jain LG, Pinano AZ, et al. Successful treatment of ciguatera fish poisoning with intravenous mannitol. JAMA. 1988;259:2740-2742.
11. Pearn JH, Lewis RJ, Ruff T, et al. Ciguatera and mannitol: experience with a new treatment regimen. Med J Aust. 1989;151:77-80. doi: 10.5694/j.1326-5377.1989.tb101165.x
12. Eastaugh JA. Delayed use of intravenous mannitol in ciguatera (fish poisoning). Ann Emerg Med. 1996;28:105-106. doi: 10.1016/s0196-0644(96)70151-8
13. Schwarz ES, Mullins ME, Brooks CB. Ciguatera poisoning successfully treated with delayed mannitol. Ann Emerg Med. 2008;52:476-477. doi: 10.1016/j.annemergmed.2008.05.015
14. Schnorf H, Taurarii M, Cundy T. Ciguatera fish poisoning: a double-blind randomized trial of mannitol therapy. Neurology. 2002;58:873-880. doi: 10.1212/wnl.58.6.873
THE CASE
An active 61-year-old woman (140 lbs) in good health became ill during a sailing holiday in the Virgin Islands. During the trip, she ate various fish in local restaurants; after one lunch, she developed nausea, diarrhea, dizziness, headache, and light-headedness. In the following days, she suffered “intense itching” in the ears, dizziness, malaise, a “fluttering feeling” throughout her body, genitourinary sensitivity, and a “rhythmic buzzing sensation near the rectum.”
She said that cold objects and beverages felt uncomfortably hot (cold allodynia). She noted heightened senses of smell and taste, as well as paresthesia down her spine, and described feeling “moody.” She reduced her workload, took many days off from work, and ceased consuming meat and alcohol because these items seemed to aggravate her symptoms.
The paresthesia persisted, and she consulted her family physician one month later. Laboratory tests—including a complete blood count, hematocrit, thyroid-stimulating hormone, antinuclear antibodies, and titers for Ehrlichia chaffeensis, Lyme disease, and Anaplasma phagocytophila—all yielded normal results. Her symptoms continued for 3 more months before referral to Medical Toxicology.
THE DIAGNOSIS
The patient’s symptoms and history were consistent with ciguatera poisoning. Features supporting this diagnosis included an acute gastrointestinal illness after eating fish caught in tropical waters and subsequent persistent paresthesia, including cold allodynia.1 Laboratory testing excluded acute infection, anemia, thyroid dysfunction, vitamin B12 deficiency, lupus, rheumatoid arthritis, Lyme disease, ehrlichiosis, and anaplasmosis.
DISCUSSION
Ciguatera results from ciguatoxin, a class of heat-stable polycyclic toxins produced in warm tropical waters by microscopic dinoflagellates (most often Gambierdiscus toxicus).2,3 Small variations exist in the Caribbean, Pacific, and Indian Ocean forms. Ciguatoxin bio-accumulates in the food chain, and humans most often ingest it by eating larger fish (typically barracuda, snapper, grouper, or amberjack).4 Because ciguatoxin confers no characteristic taste or smell to the fish, people who prepare or eat contaminated seafood have no reliable means to detect and avoid it.
Ciguatoxin opens neuronal voltage-gated sodium channels and blocks delayed-rectifier potassium channels.5 These cause repetitive, spontaneous action potentials that explain the paresthesia. Sodium influx triggers an increase in intracellular calcium concentrations. Increased intracellular sodium and calcium concentrations draw water into the intracellular space and cause neuronal edema.
Death is rarely associated with ciguatera (< 0.1% in the largest observational study).1 Even without treatment (discussed shortly), symptoms of ciguatera will gradually resolve over several weeks to several months in most cases.1,4,5 However, after recovery, patients often briefly experience milder symptoms after consuming fish, alcohol, or nuts.6
Continue to: Treatment of ciguatera
Treatment of ciguatera may include intravenous (IV) mannitol infusion. Other treatments, such as amitriptyline, gabapentin, pregabalin, and tocainide, have been used, but there is limited supporting evidence and they appear variably effective.7
Mannitol reverses the effects of ciguatoxin, with suppression of spontaneous action potentials and reversal of neuronal edema.8,9 It is reasonable to offer mannitol for acute or persistent symptoms of ciguatera fish poisoning even after a delay of several weeks.
A recent systematic review found that mannitol has the largest body of evidence supporting its use, although that evidence is generally of low quality (case reports and large case series).7 While these reports10-13 describe beneficial effects of mannitol, a single randomized trial suggested that mannitol is no more effective than normal saline.14 However, this study was underpowered and had inadequate treatment concealment; twice as many saline control patients as mannitol-treated patients requested a rescue dose of mannitol.14
Mannitol may be most effective when given early in the course of ciguatera but has shown some success when given later.5,12,13 In 1 large case series, the longest interval from symptom onset to successful treatment was 70 days, although most patients with satisfactory results received mannitol in the first few days.5
Our patient was administered an IV infusion of 100 g of 20% mannitol over 1 hour. She received the infusion 140 days after the onset of her symptoms and experienced rapid symptom relief.
Continue to: At a follow-up visit...
At a follow-up visit 2 weeks later, she described increased energy and further improvement in her paresthesia. She returned to a full work schedule and resumed all of her daily activities. However, she continued to avoid alcohol and proteins, as she had experienced a mild recurrence that she temporally related to eating meat and drinking alcohol.
At the 2-month follow-up, the patient reported continued improvement in her paresthesia but continued to experience occasional gastrointestinal symptoms and fatigue associated with meat and alcohol consumption.
The Takeaway
Ciguatera fish poisoning is largely a clinical diagnosis. It is based on early gastrointestinal symptoms followed by persistent paresthesia and cold allodynia after consumption of fish caught in tropical waters. Family physicians may see ciguatera in returning travelers or people who have consumed certain fish imported from endemic areas. Untreated symptoms may last for many weeks or months. IV mannitol may relieve symptoms of ciguatera poisoning even when administered several months after symptom onset.
We are grateful to our patient, who allowed us to share her story in the hope of helping other travelers.
CORRESPONDENCE
Michael E. Mullins, MD, Division of Medical Toxicology, Department of Emergency Medicine, Washington University School of Medicine, Campus Box 8072, 660 South Euclid Avenue, Saint Louis, MO 63110; [email protected]
THE CASE
An active 61-year-old woman (140 lbs) in good health became ill during a sailing holiday in the Virgin Islands. During the trip, she ate various fish in local restaurants; after one lunch, she developed nausea, diarrhea, dizziness, headache, and light-headedness. In the following days, she suffered “intense itching” in the ears, dizziness, malaise, a “fluttering feeling” throughout her body, genitourinary sensitivity, and a “rhythmic buzzing sensation near the rectum.”
She said that cold objects and beverages felt uncomfortably hot (cold allodynia). She noted heightened senses of smell and taste, as well as paresthesia down her spine, and described feeling “moody.” She reduced her workload, took many days off from work, and ceased consuming meat and alcohol because these items seemed to aggravate her symptoms.
The paresthesia persisted, and she consulted her family physician one month later. Laboratory tests—including a complete blood count, hematocrit, thyroid-stimulating hormone, antinuclear antibodies, and titers for Ehrlichia chaffeensis, Lyme disease, and Anaplasma phagocytophila—all yielded normal results. Her symptoms continued for 3 more months before referral to Medical Toxicology.
THE DIAGNOSIS
The patient’s symptoms and history were consistent with ciguatera poisoning. Features supporting this diagnosis included an acute gastrointestinal illness after eating fish caught in tropical waters and subsequent persistent paresthesia, including cold allodynia.1 Laboratory testing excluded acute infection, anemia, thyroid dysfunction, vitamin B12 deficiency, lupus, rheumatoid arthritis, Lyme disease, ehrlichiosis, and anaplasmosis.
DISCUSSION
Ciguatera results from ciguatoxin, a class of heat-stable polycyclic toxins produced in warm tropical waters by microscopic dinoflagellates (most often Gambierdiscus toxicus).2,3 Small variations exist in the Caribbean, Pacific, and Indian Ocean forms. Ciguatoxin bio-accumulates in the food chain, and humans most often ingest it by eating larger fish (typically barracuda, snapper, grouper, or amberjack).4 Because ciguatoxin confers no characteristic taste or smell to the fish, people who prepare or eat contaminated seafood have no reliable means to detect and avoid it.
Ciguatoxin opens neuronal voltage-gated sodium channels and blocks delayed-rectifier potassium channels.5 These cause repetitive, spontaneous action potentials that explain the paresthesia. Sodium influx triggers an increase in intracellular calcium concentrations. Increased intracellular sodium and calcium concentrations draw water into the intracellular space and cause neuronal edema.
Death is rarely associated with ciguatera (< 0.1% in the largest observational study).1 Even without treatment (discussed shortly), symptoms of ciguatera will gradually resolve over several weeks to several months in most cases.1,4,5 However, after recovery, patients often briefly experience milder symptoms after consuming fish, alcohol, or nuts.6
Continue to: Treatment of ciguatera
Treatment of ciguatera may include intravenous (IV) mannitol infusion. Other treatments, such as amitriptyline, gabapentin, pregabalin, and tocainide, have been used, but there is limited supporting evidence and they appear variably effective.7
Mannitol reverses the effects of ciguatoxin, with suppression of spontaneous action potentials and reversal of neuronal edema.8,9 It is reasonable to offer mannitol for acute or persistent symptoms of ciguatera fish poisoning even after a delay of several weeks.
A recent systematic review found that mannitol has the largest body of evidence supporting its use, although that evidence is generally of low quality (case reports and large case series).7 While these reports10-13 describe beneficial effects of mannitol, a single randomized trial suggested that mannitol is no more effective than normal saline.14 However, this study was underpowered and had inadequate treatment concealment; twice as many saline control patients as mannitol-treated patients requested a rescue dose of mannitol.14
Mannitol may be most effective when given early in the course of ciguatera but has shown some success when given later.5,12,13 In 1 large case series, the longest interval from symptom onset to successful treatment was 70 days, although most patients with satisfactory results received mannitol in the first few days.5
Our patient was administered an IV infusion of 100 g of 20% mannitol over 1 hour. She received the infusion 140 days after the onset of her symptoms and experienced rapid symptom relief.
Continue to: At a follow-up visit...
At a follow-up visit 2 weeks later, she described increased energy and further improvement in her paresthesia. She returned to a full work schedule and resumed all of her daily activities. However, she continued to avoid alcohol and proteins, as she had experienced a mild recurrence that she temporally related to eating meat and drinking alcohol.
At the 2-month follow-up, the patient reported continued improvement in her paresthesia but continued to experience occasional gastrointestinal symptoms and fatigue associated with meat and alcohol consumption.
The Takeaway
Ciguatera fish poisoning is largely a clinical diagnosis. It is based on early gastrointestinal symptoms followed by persistent paresthesia and cold allodynia after consumption of fish caught in tropical waters. Family physicians may see ciguatera in returning travelers or people who have consumed certain fish imported from endemic areas. Untreated symptoms may last for many weeks or months. IV mannitol may relieve symptoms of ciguatera poisoning even when administered several months after symptom onset.
We are grateful to our patient, who allowed us to share her story in the hope of helping other travelers.
CORRESPONDENCE
Michael E. Mullins, MD, Division of Medical Toxicology, Department of Emergency Medicine, Washington University School of Medicine, Campus Box 8072, 660 South Euclid Avenue, Saint Louis, MO 63110; [email protected]
1. Bagnis R, Kuberski T, Laugier S. Clinical Observations of 3,009 cases of ciguatera (fish poisoning) in the South Pacific. Am J Trop Med Hyg. 1979;28:1067-1073. doi: 10.4269/ajtmh.1979.28.1067
2. Morris JG Jr, Lewin P, Smith CW, et al. Ciguatera fish poisoning: epidemiology of the disease on St. Thomas, US Virgin Islands. Am J Trop Med Hyg. 1982;31:574-578. doi: 10.4269/ajtmh.1982.31.574
3. Radke EG, Grattan LM, Cook RL, et al. Ciguatera incidence in the US Virgin Islands has not increased over a 30-year time period despite rising seawater temperatures. Am J Trop Med Hyg. 2013;88:908-913. doi: 10.4269/ajtmh.12-0676
4. Goodman DM, Rogers J, Livingston EH. Ciguatera fish poisoning.
5. Blythe DG, De Sylva DP, Fleming LE, et al. Clinical experience with IV mannitol in the treatment of ciguatera. Bull Soc Pathol Exot. 1992;85:425-426.
6. Lewis, RJ. The changing face of ciguatera. Toxicon. 2001;39:97-106. doi: 10.1016/s0041-0101(00)00161-6
7. Mullins ME, Hoffman RS. Is mannitol the treatment of choice for ciguatera fish poisoning? Clin Toxicol (Phila). 2017;55:947-955. doi: 10.1080/15563650.2017.1327664
8. Nicholson GM, Lewis, RJ. Ciguatoxins: cyclic polyether modulators of voltage-gated ion channel function. Mar Drugs. 2006;4:82-118.
9. Mattei C, Molgo J, Marquais M, et al. Hyperosmolar D-mannitol reverses the increased membrane excitability and the nodal swelling caused by Caribbean ciguatoxin-1 in single frog myelinated neurons. Brain Res. 1999;847:50-58. doi: 10.1016/s0006-8993(99)02032-6
10. Palafox NA, Jain LG, Pinano AZ, et al. Successful treatment of ciguatera fish poisoning with intravenous mannitol. JAMA. 1988;259:2740-2742.
11. Pearn JH, Lewis RJ, Ruff T, et al. Ciguatera and mannitol: experience with a new treatment regimen. Med J Aust. 1989;151:77-80. doi: 10.5694/j.1326-5377.1989.tb101165.x
12. Eastaugh JA. Delayed use of intravenous mannitol in ciguatera (fish poisoning). Ann Emerg Med. 1996;28:105-106. doi: 10.1016/s0196-0644(96)70151-8
13. Schwarz ES, Mullins ME, Brooks CB. Ciguatera poisoning successfully treated with delayed mannitol. Ann Emerg Med. 2008;52:476-477. doi: 10.1016/j.annemergmed.2008.05.015
14. Schnorf H, Taurarii M, Cundy T. Ciguatera fish poisoning: a double-blind randomized trial of mannitol therapy. Neurology. 2002;58:873-880. doi: 10.1212/wnl.58.6.873
1. Bagnis R, Kuberski T, Laugier S. Clinical Observations of 3,009 cases of ciguatera (fish poisoning) in the South Pacific. Am J Trop Med Hyg. 1979;28:1067-1073. doi: 10.4269/ajtmh.1979.28.1067
2. Morris JG Jr, Lewin P, Smith CW, et al. Ciguatera fish poisoning: epidemiology of the disease on St. Thomas, US Virgin Islands. Am J Trop Med Hyg. 1982;31:574-578. doi: 10.4269/ajtmh.1982.31.574
3. Radke EG, Grattan LM, Cook RL, et al. Ciguatera incidence in the US Virgin Islands has not increased over a 30-year time period despite rising seawater temperatures. Am J Trop Med Hyg. 2013;88:908-913. doi: 10.4269/ajtmh.12-0676
4. Goodman DM, Rogers J, Livingston EH. Ciguatera fish poisoning.
5. Blythe DG, De Sylva DP, Fleming LE, et al. Clinical experience with IV mannitol in the treatment of ciguatera. Bull Soc Pathol Exot. 1992;85:425-426.
6. Lewis, RJ. The changing face of ciguatera. Toxicon. 2001;39:97-106. doi: 10.1016/s0041-0101(00)00161-6
7. Mullins ME, Hoffman RS. Is mannitol the treatment of choice for ciguatera fish poisoning? Clin Toxicol (Phila). 2017;55:947-955. doi: 10.1080/15563650.2017.1327664
8. Nicholson GM, Lewis, RJ. Ciguatoxins: cyclic polyether modulators of voltage-gated ion channel function. Mar Drugs. 2006;4:82-118.
9. Mattei C, Molgo J, Marquais M, et al. Hyperosmolar D-mannitol reverses the increased membrane excitability and the nodal swelling caused by Caribbean ciguatoxin-1 in single frog myelinated neurons. Brain Res. 1999;847:50-58. doi: 10.1016/s0006-8993(99)02032-6
10. Palafox NA, Jain LG, Pinano AZ, et al. Successful treatment of ciguatera fish poisoning with intravenous mannitol. JAMA. 1988;259:2740-2742.
11. Pearn JH, Lewis RJ, Ruff T, et al. Ciguatera and mannitol: experience with a new treatment regimen. Med J Aust. 1989;151:77-80. doi: 10.5694/j.1326-5377.1989.tb101165.x
12. Eastaugh JA. Delayed use of intravenous mannitol in ciguatera (fish poisoning). Ann Emerg Med. 1996;28:105-106. doi: 10.1016/s0196-0644(96)70151-8
13. Schwarz ES, Mullins ME, Brooks CB. Ciguatera poisoning successfully treated with delayed mannitol. Ann Emerg Med. 2008;52:476-477. doi: 10.1016/j.annemergmed.2008.05.015
14. Schnorf H, Taurarii M, Cundy T. Ciguatera fish poisoning: a double-blind randomized trial of mannitol therapy. Neurology. 2002;58:873-880. doi: 10.1212/wnl.58.6.873
Elective Total Hip Arthroplasty: Which Surgical Approach Is Optimal?
Total hip arthroplasty (THA) is one of the most successful orthopedic interventions performed today in terms of pain relief, cost effectiveness, and clinical outcomes.1 As a definitive treatment for end-stage arthritis of the hip, more than 330,000 procedures are performed in the Unites States each year. The number performed is growing by > 5% per year and is predicted to double by 2030, partly due to patients living longer, older individuals seeking a higher level of functionality than did previous generations, and better access to health care.2,3
The THA procedure also has become increasingly common in a younger population for posttraumatic fractures and conditions that lead to early-onset secondary arthritis, such as avascular necrosis, juvenile rheumatoid arthritis, hip dysplasia, Perthes disease, and femoroacetabular impingement.4 Younger patients are more likely to need a revision. According to a study by Evans and colleagues using available arthroplasty registry data, about three-quarters of hip replacements last 15 to 20 years, and 58% of hip replacements last 25 years in patients with osteoarthritis.5
For decades, the THA procedure of choice has been a standard posterior approach (PA). The PA was used because it allowed excellent intraoperative exposure and was applicable to a wide range of hip problems.6 In the past several years, modified muscle-sparing surgical approaches have been introduced. Two performed frequently are the mini PA (MPA) and the direct anterior approach (DAA).
The MPA is a modification of the PA. Surgeons perform the THA through a small incision without cutting the abductor muscles that are critical to hip stability and gait. A study published in 2010 concluded that the MPA was associated with less pain, shorter hospital length of stay (LOS) (therefore, an economic saving), and an earlier return to walking postoperatively.7
The DAA has been around since the early days of THA. Carl Hueter first described the anterior approach to the hip in 1881 (referred to as the Hueter approach). Smith-Peterson is frequently credited with popularizing the DAA technique during his career after publishing his first description of the approach in 1917.8 About 10 years ago, the DAA showed a resurgence as another muscle-sparing alternative for THAs. The DAA is considered to be a true intermuscular approach that preserves the soft tissues around the hip joint, thereby preserving the stability of the joint.9-11 The optimal surgical approach is still the subject of debate.
We present a male with right hip end-stage degenerative joint disease (DJD) and review some medical literature. Although other approaches to THA can be used (lateral, anterolateral), the discussion focuses on 2 muscle-sparing approaches performed frequently, the MPA and the DAA, and can be of value to primary care practitioners in their discussion with patients.
Case Presentation
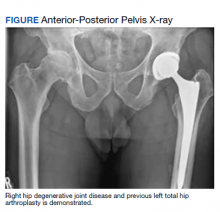
A 61-year-old male patient presented with progressive right hip pain. At age 37, he had a left THA via a PA due to hip dysplasia and a revision on the same hip at age 55 (the polyethylene liner was replaced and the cobalt chromium head was changed to ceramic), again through a PA. An orthopedic clinical evaluation and X-rays confirmed end-stage DJD of the right hip (Figure). He was informed to return to plan an elective THA when the “bad days were significantly greater than the good days” and/or when his functionality or quality of life was unacceptable. The orthopedic surgeon favored an MPA but offered a hand-off to colleagues who preferred the DAA. The patient was given information to review.
Discussion
No matter which approach is used, one study concluded that surgeons who perform > 50 hip replacements each year have better overall outcomes.12
The MPA emerged in the past decade as a muscle-sparing modification of the PA. The incision length (< 10 cm) is the simplest way of categorizing the surgery as an MPA. However, the amount of deep surgical dissection is a more important consideration for sparing muscle (for improved postoperative functionality, recovery, and joint stability) due to the gluteus maximus insertion, the quadratus femoris, and the piriformis tendons being left intact.13-16
Multiple studies have directly compared the MPA and PA, with variable results. One study concluded that the MPA was associated with lower surgical blood loss, lower pain at rest, and a faster recovery compared with that of the PA. Still, the study found no significant difference in postoperative laboratory values of possible markers of increased tissue damage and surgical invasiveness, such as creatinine phosphokinase (CPK) levels.15 Another randomized controlled trial (RCT) of 100 patients concluded that there was a trend for improved walking times and patient satisfaction at 6 weeks post-MPA vs PA.16 Other studies have found that the MPA and PA were essentially equivalent to each other regarding operative time, early postoperative outcomes, transfusion rate, hospital LOS, and postoperative complications.14 However, a recent meta-analysis found positive trends in favor of the MPA. The MPA was associated with a slight decrease in operating time, blood loss, hospital LOS, and earlier improvement in Harris hip scores. The meta-analysis found no significant decrease in the rate of dislocation or femoral fracture.13 Studies are still needed to evaluate long-term implant survival and outcomes for MPA and PA.
The DAA has received renewed attention as surgeons seek minimally invasive techniques and more rapid recoveries.6 The DAA involves a 3- to 4-inch incision on the front of the hip and enters the hip joint through the intermuscular interval between the tensor fasciae latae and gluteus medius muscles laterally and the sartorius muscle and rectus fascia medially.9 The DAA is considered a true intermuscular approach that preserves the soft tissues around the hip joint (including the posterior capsule), thereby presumably preserving the stability of the joint.9 The popularity for this approach has been attributed primarily to claims of improved recovery times, lower pain levels, improved patient satisfaction, as well as improved accuracy on both implant placement/alignment and leg length restoration.17 Orthopedic surgeons are increasingly being trained in the DAA during their residency and fellowship training.
There are many potential disadvantages to DAA. For example, DAA may present intraoperative radiation exposure for patients and surgeons during a fluoroscopy-assisted procedure. In addition, neuropraxia, particularly to the lateral femoral cutaneous nerve, can cause transient or permanent meralgia paresthetica. Wound healing may also present problems for female and obese patients, particularly those with a body mass index > 39 who are at increased risk of wound complications. DAA also increases time under anesthesia. Patients may experience proximal femoral fractures and dislocations and complex/challenging femoral exposure and bone preparation. Finally, sagittal malalignment of the stem could lead to loosening and an increased need for revision surgery.18
Another disadvantage of the DAA compared with the PA and MPA is the steep learning curve. Most studies find that the complication rate decreases only when the surgeon performs a significant number of DAA procedures. DeSteiger and colleagues noted a learning curve of 50 to 100 cases needed, and Masonis and colleagues concluded that at least 100 cases needed to be done to decrease operating and fluoroscopy times.19,20 Many orthopedic surgeons perform < 25 THA procedures a year.21
With the recent surge in popularity of the DAA, several studies have evaluated the DAA vs the MPA. A prospective RCT of 54 patients comparing the 2 approaches found that DAA patients walked without assistive devices sooner than did MPA patients: 22 days for DAA and 28 days for MPA.22 Improved cup position and a faster return of functionality were found in another study. DAA patients transitioned to a cane at 12 days vs 15.5 days for MPA patients and had a negative Trendelenburg sign at 16.7 days vs 24.8 days for MPA patients.23
Comparing DAA and MPA for inflammatory markers (serum CPK, C-reactive protein, interleukin-6, interleukin-1 β and tumor necrosis factor-α), the level of CPK postoperatively was 5.5 times higher in MPA patients, consistent with significantly more muscle damage. However, the overall physiologic burden as demonstrated by the measurement of all inflammatory markers was similar between the MPA and the DAA. This suggests that the inflammatory cascade associated with THA may be influenced more by the osteotomy and prosthesis implantation than by the surgical approach.24
Of note, some surgeons who perform the DAA recommend fewer postoperative precautions and suggest that physical therapy may not be necessary after discharge.25,26 Nevertheless, physiotherapeutic rehabilitation after all THA surgery is recommended as the standard treatment to minimize postoperative complications, such as hip dislocation, wound infection, deep venous thrombosis, and pulmonary embolism, and to maximize the patient’s functionality.27-29 RCTs are needed to look at long-term data on clinical outcomes between the MPA and DAA. Dislocation is a risk regardless of the approach used. Nevertheless, rates of dislocation, in general, are now very low, given the use of larger femoral head implants for all approaches.
Conclusions
THA is one of the most successful surgical procedures performed today. Patients desire hip pain relief and a return to function with as little interruption in their life as possible. Additionally, health care systems and insurers require THA procedures to be as efficient and cost-effective as possible. The debate regarding the most effective or preferable approach for THA continues. Although some prospective RCTs found that patients who underwent the DAA had objectively faster recovery than patients who had the MPA, it is also acknowledged that the results were dependent on surgeons who are very skilled in performing DAAs. The hope of both approaches is to get the individual moving as quickly and safely as possible to avoid a cascade of deterioration in the postoperative period. Factors other than the surgical approach, including patient selection, surgical volume and experience, careful preoperative assessments, attentive pain management, and rapid rehabilitation protocols, may be just as important as to which procedure is performed.30 The final decision should still be dependent on the patient-surgeon relationship and informed decision making.
In this case, the patient reviewed all the information he was given and independently researched the 2 procedures over many months. Ultimately, he decided to undergo a right THA via the DAA.
1. Elmallah RK, Chughtai M, Khlopas A. et al. Determining cost-effectiveness of total hip and knee arthroplasty using the Short Form-6D utility measure. J Arthroplasty. 2017;32(2):351-354. doi:10.1016/j.arth.2016.08.006
2. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630. doi:10.2106/JBJS.M.00285
3. Kurtz, S, Ong KL, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222
4. Sheahan WT, Parvataneni HK. Asymptomatic but time for a hip revision. Fed Pract. 2016;33(2):39-43.
5. Evans, JT, Evans JP, Walker RW, et al. How long does a hip replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet. 2019;393(10172):647-654. doi:10.1016/S0140-6736(18)31665-9
6. Yang X, Huang H-F, Sun L , Yang Z, Deng C-Y, Tian XB. Direct anterior approach versus posterolateral approach in total hip arthroplasty: a systematic review and meta-analysis of randomized controlled studies. Orthop Surg. 2020;12:1065-1073. doi:10.1111/os.12669
7. Varela Egocheaga JR, Suárez-Suárez MA, Fernández-Villán M, González-Sastre V, Varela-Gómez JR, Murcia-Mazón A. Minimally invasive posterior approach in total hip arthroplasty. Prospective randomized trial. An Sist Sanit Navar. 2010:33(2):133-143. doi:10.4321/s1137-66272010000300002
8. Raxhbauer F, Kain MS, Leunig M. The history of the anterior approach to the hip. Orthop Clin North Am. 2009;40(3):311-320. doi:10.1016/j.ocl.2009.02.007
9. Jia F, Guo B, Xu F, Hou Y, Tang X, Huang L. A comparison of clinical, radiographic and surgical outcomes of total hip arthroplasty between direct anterior and posterior approaches: a systematic review and meta-analysis. Hip Int. 2019;29(6):584-596. doi:10.1177/1120700018820652
10. Kennon RE Keggi JM, Wetmore RS, Zatorski LE, Huo MH, Keggi KJ. Total hip arthroplasty through a minimally invasive anterior surgical approach. J Bone Joint Surg Am. 2003;85-A(suppl 4):39-48. doi:10.2106/00004623-200300004-00005
11. Bal BS, Vallurupalli S. Minimally invasive total hip arthroplasty with the anterior approach. Indian J Orthop. 2008;42(3):301-308. doi:10.4103/0019-5413.41853
12. Katz JN, Losina E, Barrett E. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2001;83(11):1622-1629. doi:10.2106/00004623-200111000-00002
13. Berstock JR, Blom AW, Beswick AD. A systematic review and meta-analysis of the standard versus mini-incision approach to a total hip arthroplasty. J Arthroplasty. 2014;29(10):1970-1982. doi:10.1016/j.arth.2014.05.021
14. Chimento GF, Pavone V, Sharrock S, Kahn K, Cahill J, Sculco TP. Minimally invasive total hip arthroplasty: a prospective randomized study. J Arthroplasty. 2005;20(2):139-144. doi:10.1016/j.arth.2004.09.061
15. Fink B, Mittelstaedt A, Schulz MS, Sebena P, Sing J. Comparison of a minimally invasive posterior approach and the standard posterior approach for total hip arthroplasty. A prospective and comparative study. J Orthop Surg Res. 2010;5:46. doi:10.1186/1749-799X-5-46
16. Khan RJ, Maor D, Hofmann M, Haebich S. A comparison of a less invasive piriformis-sparing approach versus the standard approach to the hip: a randomized controlled trial. J Bone Joint Surg Br. 2012;94:43-50. doi:10.1302/0301-620X.94B1.27001
17. Galakatos GR. Direct anterior total hip arthroplasty. Missouri Med. 2018;115(6):537-541.
18. Flevas, DA, Tsantes AG, Mavrogenis, AE. Direct anterior approach total hip arthroplasty revisited. JBJS Rev. 2020;8(4):e0144. doi:10.2106/JBJS.RVW.19.00144
19. DeSteiger RN, Lorimer M, Solomon M. What is the learning curve for the anterior approach for total hip arthroplasty? Clin Orthop Relat Res. 2015;473(12):3860-3866. doi:10.1007/s11999-015-4565-6
20. Masonis J, Thompson C, Odum S. Safe and accurate: learning the direct anterior total hip arthroplasty. Orthopedics. 2008;31(12)(suppl 2).
21. Bal BS. Clinical faceoff: anterior total hip versus mini-posterior: Which one is better? Clin Orthop Relat Res. 2015;473(4):1192-1196. doi:10.1007/s11999-014-3684-9
22. Taunton MJ, Mason JB, Odum SM, Bryan D, Springer BD. Direct anterior total hip arthroplasty yields more rapid voluntary cessation of all walking aids: a prospective, randomized clinical trial. J Arthroplasty. 2014;29;(suppl 9):169-172. doi:10.1016/j.arth.2014.03.05
23. Nakata K, Nishikawa M, Yamamoto K, Hirota S, Yoshikawa H. A clinical comparative study of the direct anterior with mini-posterior approach: two consecutive series. J Arthroplasty. 2009;24(5):698-704. doi:10.1016/j.arth.2008.04.012
24. Bergin PF, Doppelt JD, Kephart CJ. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. Bone Joint Surg Am. 2011; 93(15):1392-1398. doi:10.2106/JBJS.J.00557
25. Carli AV, Poitras S, Clohisy JC, Beaule PE. Variation in use of postoperative precautions and equipment following total hip arthroplasty: a survey of the AAHKS and CAS membership. J Arthroplasty. 2018;33(10):3201-3205. doi:10.1016/j.arth.2018.05.043
26. Kavcˇicˇ G, Mirt PK, Tumpej J, Bedenčič. The direct anterior approach for total hip arthroplasty without specific table: surgical approach and our seven years of experience. Published June 14, 2019. Accessed March 4, 2022. https://crimsonăpublishers.com/rabs/fulltext/RABS.000520.php27. American Academy of Orthopedic Surgeons. Total hip replacement exercise guide. Published 2017. Updated February 2022. Accessed March 4, 2022. https://orthoinfo.aaos.org/en/recovery/total-hip-replacement-exercise-guide
28. Medical Advisory Secretariat. Physiotherapy rehabilitation after total knee or hip replacement: an evidence-based analysis. Ont Health Technol Assess Ser. 2005;5(8):1-91.
29. Pa˘unescu F, Didilescu A, Antonescu DM. Factors that may influence the functional outcome after primary total hip arthroplasty. Clujul Med. 2013;86(2):121-127.
30. Poehling-Monaghan KL, Kamath AF, Taunton MJ, Pagnano MW. Direct anterior versus miniposterior THA with the same advanced perioperative protocols: surprising early clinical results. Clin Orthop Relat Res. 2015;473(2):623-631. doi:10.1007/s11999-014-3827-z
Total hip arthroplasty (THA) is one of the most successful orthopedic interventions performed today in terms of pain relief, cost effectiveness, and clinical outcomes.1 As a definitive treatment for end-stage arthritis of the hip, more than 330,000 procedures are performed in the Unites States each year. The number performed is growing by > 5% per year and is predicted to double by 2030, partly due to patients living longer, older individuals seeking a higher level of functionality than did previous generations, and better access to health care.2,3
The THA procedure also has become increasingly common in a younger population for posttraumatic fractures and conditions that lead to early-onset secondary arthritis, such as avascular necrosis, juvenile rheumatoid arthritis, hip dysplasia, Perthes disease, and femoroacetabular impingement.4 Younger patients are more likely to need a revision. According to a study by Evans and colleagues using available arthroplasty registry data, about three-quarters of hip replacements last 15 to 20 years, and 58% of hip replacements last 25 years in patients with osteoarthritis.5
For decades, the THA procedure of choice has been a standard posterior approach (PA). The PA was used because it allowed excellent intraoperative exposure and was applicable to a wide range of hip problems.6 In the past several years, modified muscle-sparing surgical approaches have been introduced. Two performed frequently are the mini PA (MPA) and the direct anterior approach (DAA).
The MPA is a modification of the PA. Surgeons perform the THA through a small incision without cutting the abductor muscles that are critical to hip stability and gait. A study published in 2010 concluded that the MPA was associated with less pain, shorter hospital length of stay (LOS) (therefore, an economic saving), and an earlier return to walking postoperatively.7
The DAA has been around since the early days of THA. Carl Hueter first described the anterior approach to the hip in 1881 (referred to as the Hueter approach). Smith-Peterson is frequently credited with popularizing the DAA technique during his career after publishing his first description of the approach in 1917.8 About 10 years ago, the DAA showed a resurgence as another muscle-sparing alternative for THAs. The DAA is considered to be a true intermuscular approach that preserves the soft tissues around the hip joint, thereby preserving the stability of the joint.9-11 The optimal surgical approach is still the subject of debate.
We present a male with right hip end-stage degenerative joint disease (DJD) and review some medical literature. Although other approaches to THA can be used (lateral, anterolateral), the discussion focuses on 2 muscle-sparing approaches performed frequently, the MPA and the DAA, and can be of value to primary care practitioners in their discussion with patients.
Case Presentation
A 61-year-old male patient presented with progressive right hip pain. At age 37, he had a left THA via a PA due to hip dysplasia and a revision on the same hip at age 55 (the polyethylene liner was replaced and the cobalt chromium head was changed to ceramic), again through a PA. An orthopedic clinical evaluation and X-rays confirmed end-stage DJD of the right hip (Figure). He was informed to return to plan an elective THA when the “bad days were significantly greater than the good days” and/or when his functionality or quality of life was unacceptable. The orthopedic surgeon favored an MPA but offered a hand-off to colleagues who preferred the DAA. The patient was given information to review.
Discussion
No matter which approach is used, one study concluded that surgeons who perform > 50 hip replacements each year have better overall outcomes.12
The MPA emerged in the past decade as a muscle-sparing modification of the PA. The incision length (< 10 cm) is the simplest way of categorizing the surgery as an MPA. However, the amount of deep surgical dissection is a more important consideration for sparing muscle (for improved postoperative functionality, recovery, and joint stability) due to the gluteus maximus insertion, the quadratus femoris, and the piriformis tendons being left intact.13-16
Multiple studies have directly compared the MPA and PA, with variable results. One study concluded that the MPA was associated with lower surgical blood loss, lower pain at rest, and a faster recovery compared with that of the PA. Still, the study found no significant difference in postoperative laboratory values of possible markers of increased tissue damage and surgical invasiveness, such as creatinine phosphokinase (CPK) levels.15 Another randomized controlled trial (RCT) of 100 patients concluded that there was a trend for improved walking times and patient satisfaction at 6 weeks post-MPA vs PA.16 Other studies have found that the MPA and PA were essentially equivalent to each other regarding operative time, early postoperative outcomes, transfusion rate, hospital LOS, and postoperative complications.14 However, a recent meta-analysis found positive trends in favor of the MPA. The MPA was associated with a slight decrease in operating time, blood loss, hospital LOS, and earlier improvement in Harris hip scores. The meta-analysis found no significant decrease in the rate of dislocation or femoral fracture.13 Studies are still needed to evaluate long-term implant survival and outcomes for MPA and PA.
The DAA has received renewed attention as surgeons seek minimally invasive techniques and more rapid recoveries.6 The DAA involves a 3- to 4-inch incision on the front of the hip and enters the hip joint through the intermuscular interval between the tensor fasciae latae and gluteus medius muscles laterally and the sartorius muscle and rectus fascia medially.9 The DAA is considered a true intermuscular approach that preserves the soft tissues around the hip joint (including the posterior capsule), thereby presumably preserving the stability of the joint.9 The popularity for this approach has been attributed primarily to claims of improved recovery times, lower pain levels, improved patient satisfaction, as well as improved accuracy on both implant placement/alignment and leg length restoration.17 Orthopedic surgeons are increasingly being trained in the DAA during their residency and fellowship training.
There are many potential disadvantages to DAA. For example, DAA may present intraoperative radiation exposure for patients and surgeons during a fluoroscopy-assisted procedure. In addition, neuropraxia, particularly to the lateral femoral cutaneous nerve, can cause transient or permanent meralgia paresthetica. Wound healing may also present problems for female and obese patients, particularly those with a body mass index > 39 who are at increased risk of wound complications. DAA also increases time under anesthesia. Patients may experience proximal femoral fractures and dislocations and complex/challenging femoral exposure and bone preparation. Finally, sagittal malalignment of the stem could lead to loosening and an increased need for revision surgery.18
Another disadvantage of the DAA compared with the PA and MPA is the steep learning curve. Most studies find that the complication rate decreases only when the surgeon performs a significant number of DAA procedures. DeSteiger and colleagues noted a learning curve of 50 to 100 cases needed, and Masonis and colleagues concluded that at least 100 cases needed to be done to decrease operating and fluoroscopy times.19,20 Many orthopedic surgeons perform < 25 THA procedures a year.21
With the recent surge in popularity of the DAA, several studies have evaluated the DAA vs the MPA. A prospective RCT of 54 patients comparing the 2 approaches found that DAA patients walked without assistive devices sooner than did MPA patients: 22 days for DAA and 28 days for MPA.22 Improved cup position and a faster return of functionality were found in another study. DAA patients transitioned to a cane at 12 days vs 15.5 days for MPA patients and had a negative Trendelenburg sign at 16.7 days vs 24.8 days for MPA patients.23
Comparing DAA and MPA for inflammatory markers (serum CPK, C-reactive protein, interleukin-6, interleukin-1 β and tumor necrosis factor-α), the level of CPK postoperatively was 5.5 times higher in MPA patients, consistent with significantly more muscle damage. However, the overall physiologic burden as demonstrated by the measurement of all inflammatory markers was similar between the MPA and the DAA. This suggests that the inflammatory cascade associated with THA may be influenced more by the osteotomy and prosthesis implantation than by the surgical approach.24
Of note, some surgeons who perform the DAA recommend fewer postoperative precautions and suggest that physical therapy may not be necessary after discharge.25,26 Nevertheless, physiotherapeutic rehabilitation after all THA surgery is recommended as the standard treatment to minimize postoperative complications, such as hip dislocation, wound infection, deep venous thrombosis, and pulmonary embolism, and to maximize the patient’s functionality.27-29 RCTs are needed to look at long-term data on clinical outcomes between the MPA and DAA. Dislocation is a risk regardless of the approach used. Nevertheless, rates of dislocation, in general, are now very low, given the use of larger femoral head implants for all approaches.
Conclusions
THA is one of the most successful surgical procedures performed today. Patients desire hip pain relief and a return to function with as little interruption in their life as possible. Additionally, health care systems and insurers require THA procedures to be as efficient and cost-effective as possible. The debate regarding the most effective or preferable approach for THA continues. Although some prospective RCTs found that patients who underwent the DAA had objectively faster recovery than patients who had the MPA, it is also acknowledged that the results were dependent on surgeons who are very skilled in performing DAAs. The hope of both approaches is to get the individual moving as quickly and safely as possible to avoid a cascade of deterioration in the postoperative period. Factors other than the surgical approach, including patient selection, surgical volume and experience, careful preoperative assessments, attentive pain management, and rapid rehabilitation protocols, may be just as important as to which procedure is performed.30 The final decision should still be dependent on the patient-surgeon relationship and informed decision making.
In this case, the patient reviewed all the information he was given and independently researched the 2 procedures over many months. Ultimately, he decided to undergo a right THA via the DAA.
Total hip arthroplasty (THA) is one of the most successful orthopedic interventions performed today in terms of pain relief, cost effectiveness, and clinical outcomes.1 As a definitive treatment for end-stage arthritis of the hip, more than 330,000 procedures are performed in the Unites States each year. The number performed is growing by > 5% per year and is predicted to double by 2030, partly due to patients living longer, older individuals seeking a higher level of functionality than did previous generations, and better access to health care.2,3
The THA procedure also has become increasingly common in a younger population for posttraumatic fractures and conditions that lead to early-onset secondary arthritis, such as avascular necrosis, juvenile rheumatoid arthritis, hip dysplasia, Perthes disease, and femoroacetabular impingement.4 Younger patients are more likely to need a revision. According to a study by Evans and colleagues using available arthroplasty registry data, about three-quarters of hip replacements last 15 to 20 years, and 58% of hip replacements last 25 years in patients with osteoarthritis.5
For decades, the THA procedure of choice has been a standard posterior approach (PA). The PA was used because it allowed excellent intraoperative exposure and was applicable to a wide range of hip problems.6 In the past several years, modified muscle-sparing surgical approaches have been introduced. Two performed frequently are the mini PA (MPA) and the direct anterior approach (DAA).
The MPA is a modification of the PA. Surgeons perform the THA through a small incision without cutting the abductor muscles that are critical to hip stability and gait. A study published in 2010 concluded that the MPA was associated with less pain, shorter hospital length of stay (LOS) (therefore, an economic saving), and an earlier return to walking postoperatively.7
The DAA has been around since the early days of THA. Carl Hueter first described the anterior approach to the hip in 1881 (referred to as the Hueter approach). Smith-Peterson is frequently credited with popularizing the DAA technique during his career after publishing his first description of the approach in 1917.8 About 10 years ago, the DAA showed a resurgence as another muscle-sparing alternative for THAs. The DAA is considered to be a true intermuscular approach that preserves the soft tissues around the hip joint, thereby preserving the stability of the joint.9-11 The optimal surgical approach is still the subject of debate.
We present a male with right hip end-stage degenerative joint disease (DJD) and review some medical literature. Although other approaches to THA can be used (lateral, anterolateral), the discussion focuses on 2 muscle-sparing approaches performed frequently, the MPA and the DAA, and can be of value to primary care practitioners in their discussion with patients.
Case Presentation
A 61-year-old male patient presented with progressive right hip pain. At age 37, he had a left THA via a PA due to hip dysplasia and a revision on the same hip at age 55 (the polyethylene liner was replaced and the cobalt chromium head was changed to ceramic), again through a PA. An orthopedic clinical evaluation and X-rays confirmed end-stage DJD of the right hip (Figure). He was informed to return to plan an elective THA when the “bad days were significantly greater than the good days” and/or when his functionality or quality of life was unacceptable. The orthopedic surgeon favored an MPA but offered a hand-off to colleagues who preferred the DAA. The patient was given information to review.
Discussion
No matter which approach is used, one study concluded that surgeons who perform > 50 hip replacements each year have better overall outcomes.12
The MPA emerged in the past decade as a muscle-sparing modification of the PA. The incision length (< 10 cm) is the simplest way of categorizing the surgery as an MPA. However, the amount of deep surgical dissection is a more important consideration for sparing muscle (for improved postoperative functionality, recovery, and joint stability) due to the gluteus maximus insertion, the quadratus femoris, and the piriformis tendons being left intact.13-16
Multiple studies have directly compared the MPA and PA, with variable results. One study concluded that the MPA was associated with lower surgical blood loss, lower pain at rest, and a faster recovery compared with that of the PA. Still, the study found no significant difference in postoperative laboratory values of possible markers of increased tissue damage and surgical invasiveness, such as creatinine phosphokinase (CPK) levels.15 Another randomized controlled trial (RCT) of 100 patients concluded that there was a trend for improved walking times and patient satisfaction at 6 weeks post-MPA vs PA.16 Other studies have found that the MPA and PA were essentially equivalent to each other regarding operative time, early postoperative outcomes, transfusion rate, hospital LOS, and postoperative complications.14 However, a recent meta-analysis found positive trends in favor of the MPA. The MPA was associated with a slight decrease in operating time, blood loss, hospital LOS, and earlier improvement in Harris hip scores. The meta-analysis found no significant decrease in the rate of dislocation or femoral fracture.13 Studies are still needed to evaluate long-term implant survival and outcomes for MPA and PA.
The DAA has received renewed attention as surgeons seek minimally invasive techniques and more rapid recoveries.6 The DAA involves a 3- to 4-inch incision on the front of the hip and enters the hip joint through the intermuscular interval between the tensor fasciae latae and gluteus medius muscles laterally and the sartorius muscle and rectus fascia medially.9 The DAA is considered a true intermuscular approach that preserves the soft tissues around the hip joint (including the posterior capsule), thereby presumably preserving the stability of the joint.9 The popularity for this approach has been attributed primarily to claims of improved recovery times, lower pain levels, improved patient satisfaction, as well as improved accuracy on both implant placement/alignment and leg length restoration.17 Orthopedic surgeons are increasingly being trained in the DAA during their residency and fellowship training.
There are many potential disadvantages to DAA. For example, DAA may present intraoperative radiation exposure for patients and surgeons during a fluoroscopy-assisted procedure. In addition, neuropraxia, particularly to the lateral femoral cutaneous nerve, can cause transient or permanent meralgia paresthetica. Wound healing may also present problems for female and obese patients, particularly those with a body mass index > 39 who are at increased risk of wound complications. DAA also increases time under anesthesia. Patients may experience proximal femoral fractures and dislocations and complex/challenging femoral exposure and bone preparation. Finally, sagittal malalignment of the stem could lead to loosening and an increased need for revision surgery.18
Another disadvantage of the DAA compared with the PA and MPA is the steep learning curve. Most studies find that the complication rate decreases only when the surgeon performs a significant number of DAA procedures. DeSteiger and colleagues noted a learning curve of 50 to 100 cases needed, and Masonis and colleagues concluded that at least 100 cases needed to be done to decrease operating and fluoroscopy times.19,20 Many orthopedic surgeons perform < 25 THA procedures a year.21
With the recent surge in popularity of the DAA, several studies have evaluated the DAA vs the MPA. A prospective RCT of 54 patients comparing the 2 approaches found that DAA patients walked without assistive devices sooner than did MPA patients: 22 days for DAA and 28 days for MPA.22 Improved cup position and a faster return of functionality were found in another study. DAA patients transitioned to a cane at 12 days vs 15.5 days for MPA patients and had a negative Trendelenburg sign at 16.7 days vs 24.8 days for MPA patients.23
Comparing DAA and MPA for inflammatory markers (serum CPK, C-reactive protein, interleukin-6, interleukin-1 β and tumor necrosis factor-α), the level of CPK postoperatively was 5.5 times higher in MPA patients, consistent with significantly more muscle damage. However, the overall physiologic burden as demonstrated by the measurement of all inflammatory markers was similar between the MPA and the DAA. This suggests that the inflammatory cascade associated with THA may be influenced more by the osteotomy and prosthesis implantation than by the surgical approach.24
Of note, some surgeons who perform the DAA recommend fewer postoperative precautions and suggest that physical therapy may not be necessary after discharge.25,26 Nevertheless, physiotherapeutic rehabilitation after all THA surgery is recommended as the standard treatment to minimize postoperative complications, such as hip dislocation, wound infection, deep venous thrombosis, and pulmonary embolism, and to maximize the patient’s functionality.27-29 RCTs are needed to look at long-term data on clinical outcomes between the MPA and DAA. Dislocation is a risk regardless of the approach used. Nevertheless, rates of dislocation, in general, are now very low, given the use of larger femoral head implants for all approaches.
Conclusions
THA is one of the most successful surgical procedures performed today. Patients desire hip pain relief and a return to function with as little interruption in their life as possible. Additionally, health care systems and insurers require THA procedures to be as efficient and cost-effective as possible. The debate regarding the most effective or preferable approach for THA continues. Although some prospective RCTs found that patients who underwent the DAA had objectively faster recovery than patients who had the MPA, it is also acknowledged that the results were dependent on surgeons who are very skilled in performing DAAs. The hope of both approaches is to get the individual moving as quickly and safely as possible to avoid a cascade of deterioration in the postoperative period. Factors other than the surgical approach, including patient selection, surgical volume and experience, careful preoperative assessments, attentive pain management, and rapid rehabilitation protocols, may be just as important as to which procedure is performed.30 The final decision should still be dependent on the patient-surgeon relationship and informed decision making.
In this case, the patient reviewed all the information he was given and independently researched the 2 procedures over many months. Ultimately, he decided to undergo a right THA via the DAA.
1. Elmallah RK, Chughtai M, Khlopas A. et al. Determining cost-effectiveness of total hip and knee arthroplasty using the Short Form-6D utility measure. J Arthroplasty. 2017;32(2):351-354. doi:10.1016/j.arth.2016.08.006
2. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630. doi:10.2106/JBJS.M.00285
3. Kurtz, S, Ong KL, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222
4. Sheahan WT, Parvataneni HK. Asymptomatic but time for a hip revision. Fed Pract. 2016;33(2):39-43.
5. Evans, JT, Evans JP, Walker RW, et al. How long does a hip replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet. 2019;393(10172):647-654. doi:10.1016/S0140-6736(18)31665-9
6. Yang X, Huang H-F, Sun L , Yang Z, Deng C-Y, Tian XB. Direct anterior approach versus posterolateral approach in total hip arthroplasty: a systematic review and meta-analysis of randomized controlled studies. Orthop Surg. 2020;12:1065-1073. doi:10.1111/os.12669
7. Varela Egocheaga JR, Suárez-Suárez MA, Fernández-Villán M, González-Sastre V, Varela-Gómez JR, Murcia-Mazón A. Minimally invasive posterior approach in total hip arthroplasty. Prospective randomized trial. An Sist Sanit Navar. 2010:33(2):133-143. doi:10.4321/s1137-66272010000300002
8. Raxhbauer F, Kain MS, Leunig M. The history of the anterior approach to the hip. Orthop Clin North Am. 2009;40(3):311-320. doi:10.1016/j.ocl.2009.02.007
9. Jia F, Guo B, Xu F, Hou Y, Tang X, Huang L. A comparison of clinical, radiographic and surgical outcomes of total hip arthroplasty between direct anterior and posterior approaches: a systematic review and meta-analysis. Hip Int. 2019;29(6):584-596. doi:10.1177/1120700018820652
10. Kennon RE Keggi JM, Wetmore RS, Zatorski LE, Huo MH, Keggi KJ. Total hip arthroplasty through a minimally invasive anterior surgical approach. J Bone Joint Surg Am. 2003;85-A(suppl 4):39-48. doi:10.2106/00004623-200300004-00005
11. Bal BS, Vallurupalli S. Minimally invasive total hip arthroplasty with the anterior approach. Indian J Orthop. 2008;42(3):301-308. doi:10.4103/0019-5413.41853
12. Katz JN, Losina E, Barrett E. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2001;83(11):1622-1629. doi:10.2106/00004623-200111000-00002
13. Berstock JR, Blom AW, Beswick AD. A systematic review and meta-analysis of the standard versus mini-incision approach to a total hip arthroplasty. J Arthroplasty. 2014;29(10):1970-1982. doi:10.1016/j.arth.2014.05.021
14. Chimento GF, Pavone V, Sharrock S, Kahn K, Cahill J, Sculco TP. Minimally invasive total hip arthroplasty: a prospective randomized study. J Arthroplasty. 2005;20(2):139-144. doi:10.1016/j.arth.2004.09.061
15. Fink B, Mittelstaedt A, Schulz MS, Sebena P, Sing J. Comparison of a minimally invasive posterior approach and the standard posterior approach for total hip arthroplasty. A prospective and comparative study. J Orthop Surg Res. 2010;5:46. doi:10.1186/1749-799X-5-46
16. Khan RJ, Maor D, Hofmann M, Haebich S. A comparison of a less invasive piriformis-sparing approach versus the standard approach to the hip: a randomized controlled trial. J Bone Joint Surg Br. 2012;94:43-50. doi:10.1302/0301-620X.94B1.27001
17. Galakatos GR. Direct anterior total hip arthroplasty. Missouri Med. 2018;115(6):537-541.
18. Flevas, DA, Tsantes AG, Mavrogenis, AE. Direct anterior approach total hip arthroplasty revisited. JBJS Rev. 2020;8(4):e0144. doi:10.2106/JBJS.RVW.19.00144
19. DeSteiger RN, Lorimer M, Solomon M. What is the learning curve for the anterior approach for total hip arthroplasty? Clin Orthop Relat Res. 2015;473(12):3860-3866. doi:10.1007/s11999-015-4565-6
20. Masonis J, Thompson C, Odum S. Safe and accurate: learning the direct anterior total hip arthroplasty. Orthopedics. 2008;31(12)(suppl 2).
21. Bal BS. Clinical faceoff: anterior total hip versus mini-posterior: Which one is better? Clin Orthop Relat Res. 2015;473(4):1192-1196. doi:10.1007/s11999-014-3684-9
22. Taunton MJ, Mason JB, Odum SM, Bryan D, Springer BD. Direct anterior total hip arthroplasty yields more rapid voluntary cessation of all walking aids: a prospective, randomized clinical trial. J Arthroplasty. 2014;29;(suppl 9):169-172. doi:10.1016/j.arth.2014.03.05
23. Nakata K, Nishikawa M, Yamamoto K, Hirota S, Yoshikawa H. A clinical comparative study of the direct anterior with mini-posterior approach: two consecutive series. J Arthroplasty. 2009;24(5):698-704. doi:10.1016/j.arth.2008.04.012
24. Bergin PF, Doppelt JD, Kephart CJ. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. Bone Joint Surg Am. 2011; 93(15):1392-1398. doi:10.2106/JBJS.J.00557
25. Carli AV, Poitras S, Clohisy JC, Beaule PE. Variation in use of postoperative precautions and equipment following total hip arthroplasty: a survey of the AAHKS and CAS membership. J Arthroplasty. 2018;33(10):3201-3205. doi:10.1016/j.arth.2018.05.043
26. Kavcˇicˇ G, Mirt PK, Tumpej J, Bedenčič. The direct anterior approach for total hip arthroplasty without specific table: surgical approach and our seven years of experience. Published June 14, 2019. Accessed March 4, 2022. https://crimsonăpublishers.com/rabs/fulltext/RABS.000520.php27. American Academy of Orthopedic Surgeons. Total hip replacement exercise guide. Published 2017. Updated February 2022. Accessed March 4, 2022. https://orthoinfo.aaos.org/en/recovery/total-hip-replacement-exercise-guide
28. Medical Advisory Secretariat. Physiotherapy rehabilitation after total knee or hip replacement: an evidence-based analysis. Ont Health Technol Assess Ser. 2005;5(8):1-91.
29. Pa˘unescu F, Didilescu A, Antonescu DM. Factors that may influence the functional outcome after primary total hip arthroplasty. Clujul Med. 2013;86(2):121-127.
30. Poehling-Monaghan KL, Kamath AF, Taunton MJ, Pagnano MW. Direct anterior versus miniposterior THA with the same advanced perioperative protocols: surprising early clinical results. Clin Orthop Relat Res. 2015;473(2):623-631. doi:10.1007/s11999-014-3827-z
1. Elmallah RK, Chughtai M, Khlopas A. et al. Determining cost-effectiveness of total hip and knee arthroplasty using the Short Form-6D utility measure. J Arthroplasty. 2017;32(2):351-354. doi:10.1016/j.arth.2016.08.006
2. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630. doi:10.2106/JBJS.M.00285
3. Kurtz, S, Ong KL, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222
4. Sheahan WT, Parvataneni HK. Asymptomatic but time for a hip revision. Fed Pract. 2016;33(2):39-43.
5. Evans, JT, Evans JP, Walker RW, et al. How long does a hip replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet. 2019;393(10172):647-654. doi:10.1016/S0140-6736(18)31665-9
6. Yang X, Huang H-F, Sun L , Yang Z, Deng C-Y, Tian XB. Direct anterior approach versus posterolateral approach in total hip arthroplasty: a systematic review and meta-analysis of randomized controlled studies. Orthop Surg. 2020;12:1065-1073. doi:10.1111/os.12669
7. Varela Egocheaga JR, Suárez-Suárez MA, Fernández-Villán M, González-Sastre V, Varela-Gómez JR, Murcia-Mazón A. Minimally invasive posterior approach in total hip arthroplasty. Prospective randomized trial. An Sist Sanit Navar. 2010:33(2):133-143. doi:10.4321/s1137-66272010000300002
8. Raxhbauer F, Kain MS, Leunig M. The history of the anterior approach to the hip. Orthop Clin North Am. 2009;40(3):311-320. doi:10.1016/j.ocl.2009.02.007
9. Jia F, Guo B, Xu F, Hou Y, Tang X, Huang L. A comparison of clinical, radiographic and surgical outcomes of total hip arthroplasty between direct anterior and posterior approaches: a systematic review and meta-analysis. Hip Int. 2019;29(6):584-596. doi:10.1177/1120700018820652
10. Kennon RE Keggi JM, Wetmore RS, Zatorski LE, Huo MH, Keggi KJ. Total hip arthroplasty through a minimally invasive anterior surgical approach. J Bone Joint Surg Am. 2003;85-A(suppl 4):39-48. doi:10.2106/00004623-200300004-00005
11. Bal BS, Vallurupalli S. Minimally invasive total hip arthroplasty with the anterior approach. Indian J Orthop. 2008;42(3):301-308. doi:10.4103/0019-5413.41853
12. Katz JN, Losina E, Barrett E. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2001;83(11):1622-1629. doi:10.2106/00004623-200111000-00002
13. Berstock JR, Blom AW, Beswick AD. A systematic review and meta-analysis of the standard versus mini-incision approach to a total hip arthroplasty. J Arthroplasty. 2014;29(10):1970-1982. doi:10.1016/j.arth.2014.05.021
14. Chimento GF, Pavone V, Sharrock S, Kahn K, Cahill J, Sculco TP. Minimally invasive total hip arthroplasty: a prospective randomized study. J Arthroplasty. 2005;20(2):139-144. doi:10.1016/j.arth.2004.09.061
15. Fink B, Mittelstaedt A, Schulz MS, Sebena P, Sing J. Comparison of a minimally invasive posterior approach and the standard posterior approach for total hip arthroplasty. A prospective and comparative study. J Orthop Surg Res. 2010;5:46. doi:10.1186/1749-799X-5-46
16. Khan RJ, Maor D, Hofmann M, Haebich S. A comparison of a less invasive piriformis-sparing approach versus the standard approach to the hip: a randomized controlled trial. J Bone Joint Surg Br. 2012;94:43-50. doi:10.1302/0301-620X.94B1.27001
17. Galakatos GR. Direct anterior total hip arthroplasty. Missouri Med. 2018;115(6):537-541.
18. Flevas, DA, Tsantes AG, Mavrogenis, AE. Direct anterior approach total hip arthroplasty revisited. JBJS Rev. 2020;8(4):e0144. doi:10.2106/JBJS.RVW.19.00144
19. DeSteiger RN, Lorimer M, Solomon M. What is the learning curve for the anterior approach for total hip arthroplasty? Clin Orthop Relat Res. 2015;473(12):3860-3866. doi:10.1007/s11999-015-4565-6
20. Masonis J, Thompson C, Odum S. Safe and accurate: learning the direct anterior total hip arthroplasty. Orthopedics. 2008;31(12)(suppl 2).
21. Bal BS. Clinical faceoff: anterior total hip versus mini-posterior: Which one is better? Clin Orthop Relat Res. 2015;473(4):1192-1196. doi:10.1007/s11999-014-3684-9
22. Taunton MJ, Mason JB, Odum SM, Bryan D, Springer BD. Direct anterior total hip arthroplasty yields more rapid voluntary cessation of all walking aids: a prospective, randomized clinical trial. J Arthroplasty. 2014;29;(suppl 9):169-172. doi:10.1016/j.arth.2014.03.05
23. Nakata K, Nishikawa M, Yamamoto K, Hirota S, Yoshikawa H. A clinical comparative study of the direct anterior with mini-posterior approach: two consecutive series. J Arthroplasty. 2009;24(5):698-704. doi:10.1016/j.arth.2008.04.012
24. Bergin PF, Doppelt JD, Kephart CJ. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. Bone Joint Surg Am. 2011; 93(15):1392-1398. doi:10.2106/JBJS.J.00557
25. Carli AV, Poitras S, Clohisy JC, Beaule PE. Variation in use of postoperative precautions and equipment following total hip arthroplasty: a survey of the AAHKS and CAS membership. J Arthroplasty. 2018;33(10):3201-3205. doi:10.1016/j.arth.2018.05.043
26. Kavcˇicˇ G, Mirt PK, Tumpej J, Bedenčič. The direct anterior approach for total hip arthroplasty without specific table: surgical approach and our seven years of experience. Published June 14, 2019. Accessed March 4, 2022. https://crimsonăpublishers.com/rabs/fulltext/RABS.000520.php27. American Academy of Orthopedic Surgeons. Total hip replacement exercise guide. Published 2017. Updated February 2022. Accessed March 4, 2022. https://orthoinfo.aaos.org/en/recovery/total-hip-replacement-exercise-guide
28. Medical Advisory Secretariat. Physiotherapy rehabilitation after total knee or hip replacement: an evidence-based analysis. Ont Health Technol Assess Ser. 2005;5(8):1-91.
29. Pa˘unescu F, Didilescu A, Antonescu DM. Factors that may influence the functional outcome after primary total hip arthroplasty. Clujul Med. 2013;86(2):121-127.
30. Poehling-Monaghan KL, Kamath AF, Taunton MJ, Pagnano MW. Direct anterior versus miniposterior THA with the same advanced perioperative protocols: surprising early clinical results. Clin Orthop Relat Res. 2015;473(2):623-631. doi:10.1007/s11999-014-3827-z
Neutropenia and Leukopenia After Cross Taper From Quetiapine to Divalproex for the Treatment of Borderline Personality Disorder
Valproic acid (VPA) and its derivative, divalproex (DVP) are prescribed for a variety of indications, commonly for seizure control in patients with epilepsy, mood stabilization in patients with bipolar disorder, and migraine prophylaxis. Gastrointestinal distress and sedation are among the most reported adverse effects (AEs) with DVP therapy.1 Although serious hepatic and hematologic AEs are rare, monitoring is still recommended. DVP can cause various hematologic dyscrasias, the most common being thrombocytopenia.1,2 Neutropenia and leukopenia have been reported in isolated cases, most occurring in pediatric patients or patients with epilepsy.3-14
Several case reports of DVP-related neutropenia (absolute neutrophil count [ANC] < 1.50 103/mcL) and leukopenia (white blood cell count [WBC] < 4.0 103/mcL) were reviewed during our literature search, some caused by DVP monotherapy; others were thought to be related to concomitant use of DVP and another drug.15-25 Quetiapine was the antipsychotic most commonly implicated in causing hematologic abnormalities when combined with DVP. We report a case of neutropenia and leukopenia that presented after a cross taper from quetiapine to DVP for the treatment of borderline personality disorder (BPD).
Although no medications have been approved by the US Food and Drug Administration (FDA) for the treatment of BPD, mood stabilizers, including DVP, have literature to support their use for the treatment of affective dysregulation and impulsive behavioral dyscontrol.26-28 A therapeutic range for DVP in the treatment of BPD has not been defined; therefore, for this case report, the generally accepted range of 50 to 100 µg/mL will be considered therapeutic.1
Case Presentation
A 34-year-old male patient presented to the mental health clinic pharmacist reporting that his current psychotropic medication regimen was not effective. His medical history included posttraumatic stress disorder (PTSD), opioid use disorder, alcohol use disorder, stimulant use disorder, cannabis use, BPD, hypertension, hyperlipidemia, prediabetes, gastroesophageal reflex disease, and a pulmonary nodule. On initial presentation, the patient was prescribed buprenorphine 24 mg/naloxone 6 mg, quetiapine 400 mg, duloxetine 120 mg, and prazosin 15 mg per day. At the time of pharmacy consultation, last reported alcohol or nonprescribed opioid use was about 6 months prior, and methamphetamine use about 1 month prior, with ongoing cannabis use. The patient had a history of participating in cognitive processing therapy, dialectical behavior therapy (DBT), and residential treatment for both PTSD and substance use. Additionally, he was actively participating in contingency management for stimulant use disorder and self-management and recovery training group.
The patient reported ongoing mood lability, hypervigilance, and oversedation with current psychotropic regimen. The prescriber of his medication for opioid use disorder also reported the patient experienced labile mood, impulsive behavior, and anger outbursts. In the setting of intolerability due to oversedation with quetiapine, cardiometabolic risk, and lack of clear indication for use, the patient and health care practitioner (HCP) agreed to taper quetiapine and initiate a trial of DVP for affective dysregulation and impulsive-behavioral dyscontrol. To prevent cholinergic rebound and insomnia with abrupt discontinuation of quetiapine, DVP and quetiapine were cross tapered. The following cross taper was prescribed: quetiapine 300 mg and DVP 500 mg per day for week 1; quetiapine 200 mg and DVP 500 mg per day for week 2; quetiapine 100 mg and DVP 1000 mg per day for week 3; quetiapine 50 mg and DVP 1000 mg per day for week 4; followed by DVP 1000 mg per day and discontinuation of quetiapine.
During a 4-week follow-up appointment, the patient reported appropriate completion of cross taper but stopped taking the DVP 3 days prior to the appointment due to self-reported lack of efficacy. For this reason, serum VPA level was not obtained. After discussion with his HCP, the patient restarted DVP 1000 mg per day without retitration with plans to get laboratory tests in 1 week. The next week, laboratory tests were notable for VPA level 28.74 (reference range, 50-100) µg/mL, low WBC 3.51 (reference range, 4.00-10.00) 103/mcL, platelets 169 (reference range, 150-420) 103/mcL, and low ANC 1.00 (reference range, 1.50-7.40) 103/mcL (Table). This raised clinical concern as the patient had no history of documented neutropenia or leukopenia, with most recent complete blood count (CBC) prior to DVP initiation 3 months earlier while prescribed quetiapine.
On further review, the HCP opted to cease administration of DVP and repeat CBC with differential in 1 week. Nine days later, laboratory tests were performed and compared with those collected the week before, revealing resolution of neutropenia and leukopenia. A score of 7 on the Naranjo Adverse Drug Reaction Probability Scale (NADRPS) was determined based on previous conclusive reports on the reaction (+1), appeared after suspected drug administration (+2), improved with drug discontinuation (+1), confirmed by objective evidence (+1), and no alternative causes could be found (+2).29 With a NADRPS score of 7, an AE of probable DVP-induced neutropenia was documented and medication was not resumed.
Discussion
Our case report describes isolated neutropenia and leukopenia that developed after a cross taper from quetiapine to DVP. Hematologic abnormalities resolved after discontinuation of DVP, suggesting a likely correlation. DVP has a well-established, dose-related prevalence of thrombocytopenia occurring in up to 27% of patients.1 Fewer case reports exist on neutropenia and leukopenia. DVP-induced neutropenia is thought to be a result of direct bone marrow suppression, whereas the more commonly occurring blood dyscrasia, thrombocytopenia, is thought to be caused by an antibody-mediated destruction of platelets.6
Management of DVP-induced thrombocytopenia is often dependent on the severity of the reaction. In mild-to-moderate cases, intervention may not be necessary as thrombocytopenia has been shown to resolve without adjustment to DVP therapy.1 In more severe or symptomatic cases, dose reduction or discontinuation of the offending agent is recommended, typically resulting in resolution shortly following pharmacologic intervention.
Guidance on the management of other drug-induced hematologic abnormalities, such as neutropenia and leukopenia are not as well established. A 2019 systematic review of idiosyncratic drug-induced neutropenia suggested that continuing the offending drug with strict monitoring could be considered in cases of mild neutropenia. In cases of moderate neutropenia, the author suggests temporary cessation of the drug and reinstatement once neutrophil count normalizes and definitive cessation of the drug in severe cases.30
In our case, continuing the offending agent with close monitoring was considered, similar to the well-established management of clozapine-induced neutropenia. However, due to the concern that the ANC was bordering moderate neutropenia in the absence of a therapeutic VPA level as well as a significant reduction in platelets, although not meeting criteria for thrombocytopenia, the decision was made to err on the side of caution and discontinue the most likely offending agent.
It is important to highlight that DVP was replacing quetiapine in the form of a cross taper. Quetiapine is structurally similar to clozapine. While clozapine has strict monitoring requirements related to neutropenia, blood dyscrasias with quetiapine therapy are rare. Quetiapine-induced hematologic abnormalities may be due to direct toxicity or to an immune-mediated mechanism, leading to bone marrow suppression.20 Case reports documenting blood dyscrasias with the combination of DVP and quetiapine were identified during literature review.15-19 Despite these case reports, we believe DVP was the primary offending agent in our case as the patient’s last dose of quetiapine was 2 weeks before obtaining the abnormal CBC. There was no history of blood dyscrasias with quetiapine monotherapy; however, the effect of the combination of DVP and quetiapine is unknown as no CBC was obtained during the cross-taper period.
Although there are no FDA-approved medications for the treatment of BPD, mood stabilizers, including DVP, have some research to support their use for the treatment of affective dysregulation and impulsive-behavioral dyscontrol.26-28 In our case, DVP was selected due to the evidence for use in BPD and ability to assess adherence with therapeutic monitoring. Although polypharmacy is a concern in patients with BPD, in our case we believed that the patient’s ongoing mood lability and impulsive behaviors warranted pharmacologic intervention. Additionally, DVP provided an advantage in its ability to quickly titrate to therapeutic dose when compared with lamotrigine and a lower risk of cognitive AEs when compared with topiramate.
Conclusions
To our knowledge, this case report demonstrates the first published case of neutropenia and leukopenia related to DVP therapy for the treatment of BPD. Routine CBC monitoring is recommended with DVP therapy, and our case highlights the importance of evaluating for not only thrombocytopenia, but also other blood dyscrasias during the titration phase even in the absence of a therapeutic VPA level. Further studies are warranted to determine incidence of DVP-related neutropenia and leukopenia and to evaluate the safety of continuing DVP in cases of mild-to-moderate neutropenia with close monitoring.
1. Depakote (valproic acid). Package insert. Abbott Laboratories; June 2000.
2. Conley EL, Coley KC, Pollock BG, Dapos SV, Maxwell R, Branch RA. Prevalence and risk of thrombocytopenia with valproic acid: experience at a psychiatric teaching hospital. Pharmacotherapy. 2001;21(11):1325-1330. doi:10.1592/phco.21.17.1325.34418
3. Jaeken J, van Goethem C, Casaer P, Devlieger H, Eggermont E, Pilet M. Neutropenia during sodium valproate therapy. Arch Dis Child. 1979;54(12):986-987. doi:10.1136/adc.54.12.986
4. Barr RD, Copeland SA, Stockwell MC, Morris N, Kelton JC. Valproic acid and immune thrombocytopenia. Arch Dis Child. 1982;57(9):681-684. doi:10.1136/adc.57.9.681
5. Symon DNK, Russell G. Sodium valproate and neutropenia (letter). Arch Dis Child. 1983;58:235. doi:10.1136/adc.58.3.235
6. Watts RG, Emanuel PD, Zuckerman KS, Howard TH. Valproic acid-induced cytopenias: evidence for a dose-related suppression of hematopoiesis. J Pediatr. 1990;117(3):495-499. doi:10.1016/s0022-3476(05)81105-9
7. Blackburn SC, Oliart AD, García-Rodríguez LA, Pérez Gutthann S. Antiepileptics and blood dyscrasias: a cohort study. Pharmacotherapy. 1998;18(6):1277-1283.
8. Acharya S, Bussel JB. Hematologic toxicity of sodium valproate. J Pediatr Hematol Oncol. 2000;22(1):62-65. doi:10.1097/00043426-200001000-00012
9. Vesta KS, Medina PJ. Valproic acid-induced neutropenia. Ann Pharmacother. 2003;37(6):819-821. doi:10.1345/aph.1C381
10. Kohli U, Gulati, S. Sodium valproate induced isolated neutropenia. Indian J Pediatr. 2006;73(9):844-844. doi:10.1007/BF02790401
11. Hsu HC, Tseng HK, Wang SC, Wang YY. Valproic acid-induced agranulocytosis. Int J Gerontol. 2009;3(2):137-139. doi:10.1016/S1873-9598(09)70036-5
12. Chakraborty S, Chakraborty J, Mandal S, Ghosal MK. A rare occurrence of isolated neutropenia with valproic acid: a case report. J Indian Med Assoc. 2011;109(5):345-346.
13. Stoner SC, Deal E, Lurk JT. Delayed-onset neutropenia with divalproex sodium. Ann Pharmacother. 2008;42(10):1507-1510. doi:10.1345/aph.1L239
14. Storch DD. Severe leukopenia with valproate. J Am Acad Child Adolesc Psychiatry. 2000;39(10):1208-1209. doi:10.1097/00004583-200010000-00003
15. Rahman A, Mican LM, Fischer C, Campbell AH. Evaluating the incidence of leukopenia and neutropenia with valproate, quetiapine, or the combination in children and adolescents. Ann Pharmacother. 2009;43:822-830. doi:10.1345/aph.1L617
16. Hung WC, Hsieh MH. Neutropenia associated with the comedication of quetiapine and valproate in 2 elderly patients. J Clin Psychopharmacol. 2012;32(3):416-417. doi:10.1097/JCP.0b013e3182549d2d
17. Park HJ, Kim JY. Incidence of neutropenia with valproate and quetiapine combination treatment in subjects with acquired brain injuries. Arch Phys Med Rehabil. 2016;97(2):183-188. doi:10.1016/j.apmr.2015.09.004
18. Estabrook KR, Pheister M. A case of quetiapine XR and divalproex-associated neutropenia followed by successful use of ziprasidone. J Clin Psychopharmacol. 2012;32(3):417-418. doi:10.1097/JCP.0b013e318253a071
19. Nair P, Lippmann S. Is leukopenia associated with divalproex and/or quetiapine? Psychosomatics. 2005;46(2):188-189. doi:10.1176/appi.psy.46.2.188
20. Cowan C, Oakley C. Leukopenia and neutropenia induced by quetiapine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):292-294. doi:10.1016/j.pnpbp.2006.07.003
21. Fan KY, Chen WY, Huang MC. Quetiapine-associated leucopenia and thrombocytopenia: a case report. BMC Psychiatry. 2015;15:110. doi:10.1186/s12888-015-0495-9
22. Malik S, Lally J, Ajnakina O, et al. Sodium valproate and clozapine induced neutropenia: A case control study using register data. Schizophr Res. 2018;195:267-273. doi:10.1016/j.schres.2017.08.041
23. Pantelis C, Adesanya A. Increased risk of neutropaenia and agranulocytosis with sodium valproate used adjunctively with clozapine. Aust N Z J Psychiatry. 2001;35(4):544-545. doi:10.1046/j.1440-1614.2001.0911f.x
24. Madeb R, Hirschmann S, Kurs R, Turkie A, Modai I. Combined clozapine and valproic acid treatment-induced agranulocytosis. Eur Psychiatry. 2002;17(4):238-239. doi:10.1016/s0924-9338(02)00659-4
25. Dose M, Hellweg R, Yassouridis A, Theison M, Emrich HM. Combined treatment of schizophrenic psychoses with haloperidol and valproate. Pharmacopsychiatry. 1998;31(4):122-125. doi:10.1055/s-2007-979312
26. Ingenhoven T, Lafay P, Rinne T, Passchier J, Duivenvoorden H. Effectiveness of pharmacotherapy for severe personality disorders: meta-analyses of randomized controlled trials. J Clin Psychiatry. 2010;71:14. doi:10.4088/jcp.08r04526gre
27. Mercer D, Douglass AB, Links PS. Meta-analyses of mood stabilizers, antidepressants and antipsychotics in the treatment of borderline personality disorder: effectiveness for depression and anger symptoms. J Pers Disord. 2009;23(2):156-174. doi:10.1521/pedi.2009.23.2.156
28. Hollander E, Swann AC, Coccaro EF, Jiang P, Smith TB. Impact of trait impulsivity and state aggression on divalproex versus placebo response in borderline personality disorder. Am J Psychiatry. 2005;162(3):621-624. doi:10.1176/appi.ajp.162.3.621
29. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245. doi:10.1038/clpt.1981.154
30. Andrès E, Villalba NL, Zulfiqar AA, Serraj K, Mourot-Cottet R, Gottenberg AJ. State of art of idiosyncratic drug-induced neutropenia or agranulocytosis, with a focus on biotherapies. J Clin Med. 2019;8(9):1351. doi:10.3390/jcm8091351
Valproic acid (VPA) and its derivative, divalproex (DVP) are prescribed for a variety of indications, commonly for seizure control in patients with epilepsy, mood stabilization in patients with bipolar disorder, and migraine prophylaxis. Gastrointestinal distress and sedation are among the most reported adverse effects (AEs) with DVP therapy.1 Although serious hepatic and hematologic AEs are rare, monitoring is still recommended. DVP can cause various hematologic dyscrasias, the most common being thrombocytopenia.1,2 Neutropenia and leukopenia have been reported in isolated cases, most occurring in pediatric patients or patients with epilepsy.3-14
Several case reports of DVP-related neutropenia (absolute neutrophil count [ANC] < 1.50 103/mcL) and leukopenia (white blood cell count [WBC] < 4.0 103/mcL) were reviewed during our literature search, some caused by DVP monotherapy; others were thought to be related to concomitant use of DVP and another drug.15-25 Quetiapine was the antipsychotic most commonly implicated in causing hematologic abnormalities when combined with DVP. We report a case of neutropenia and leukopenia that presented after a cross taper from quetiapine to DVP for the treatment of borderline personality disorder (BPD).
Although no medications have been approved by the US Food and Drug Administration (FDA) for the treatment of BPD, mood stabilizers, including DVP, have literature to support their use for the treatment of affective dysregulation and impulsive behavioral dyscontrol.26-28 A therapeutic range for DVP in the treatment of BPD has not been defined; therefore, for this case report, the generally accepted range of 50 to 100 µg/mL will be considered therapeutic.1
Case Presentation
A 34-year-old male patient presented to the mental health clinic pharmacist reporting that his current psychotropic medication regimen was not effective. His medical history included posttraumatic stress disorder (PTSD), opioid use disorder, alcohol use disorder, stimulant use disorder, cannabis use, BPD, hypertension, hyperlipidemia, prediabetes, gastroesophageal reflex disease, and a pulmonary nodule. On initial presentation, the patient was prescribed buprenorphine 24 mg/naloxone 6 mg, quetiapine 400 mg, duloxetine 120 mg, and prazosin 15 mg per day. At the time of pharmacy consultation, last reported alcohol or nonprescribed opioid use was about 6 months prior, and methamphetamine use about 1 month prior, with ongoing cannabis use. The patient had a history of participating in cognitive processing therapy, dialectical behavior therapy (DBT), and residential treatment for both PTSD and substance use. Additionally, he was actively participating in contingency management for stimulant use disorder and self-management and recovery training group.
The patient reported ongoing mood lability, hypervigilance, and oversedation with current psychotropic regimen. The prescriber of his medication for opioid use disorder also reported the patient experienced labile mood, impulsive behavior, and anger outbursts. In the setting of intolerability due to oversedation with quetiapine, cardiometabolic risk, and lack of clear indication for use, the patient and health care practitioner (HCP) agreed to taper quetiapine and initiate a trial of DVP for affective dysregulation and impulsive-behavioral dyscontrol. To prevent cholinergic rebound and insomnia with abrupt discontinuation of quetiapine, DVP and quetiapine were cross tapered. The following cross taper was prescribed: quetiapine 300 mg and DVP 500 mg per day for week 1; quetiapine 200 mg and DVP 500 mg per day for week 2; quetiapine 100 mg and DVP 1000 mg per day for week 3; quetiapine 50 mg and DVP 1000 mg per day for week 4; followed by DVP 1000 mg per day and discontinuation of quetiapine.
During a 4-week follow-up appointment, the patient reported appropriate completion of cross taper but stopped taking the DVP 3 days prior to the appointment due to self-reported lack of efficacy. For this reason, serum VPA level was not obtained. After discussion with his HCP, the patient restarted DVP 1000 mg per day without retitration with plans to get laboratory tests in 1 week. The next week, laboratory tests were notable for VPA level 28.74 (reference range, 50-100) µg/mL, low WBC 3.51 (reference range, 4.00-10.00) 103/mcL, platelets 169 (reference range, 150-420) 103/mcL, and low ANC 1.00 (reference range, 1.50-7.40) 103/mcL (Table). This raised clinical concern as the patient had no history of documented neutropenia or leukopenia, with most recent complete blood count (CBC) prior to DVP initiation 3 months earlier while prescribed quetiapine.
On further review, the HCP opted to cease administration of DVP and repeat CBC with differential in 1 week. Nine days later, laboratory tests were performed and compared with those collected the week before, revealing resolution of neutropenia and leukopenia. A score of 7 on the Naranjo Adverse Drug Reaction Probability Scale (NADRPS) was determined based on previous conclusive reports on the reaction (+1), appeared after suspected drug administration (+2), improved with drug discontinuation (+1), confirmed by objective evidence (+1), and no alternative causes could be found (+2).29 With a NADRPS score of 7, an AE of probable DVP-induced neutropenia was documented and medication was not resumed.
Discussion
Our case report describes isolated neutropenia and leukopenia that developed after a cross taper from quetiapine to DVP. Hematologic abnormalities resolved after discontinuation of DVP, suggesting a likely correlation. DVP has a well-established, dose-related prevalence of thrombocytopenia occurring in up to 27% of patients.1 Fewer case reports exist on neutropenia and leukopenia. DVP-induced neutropenia is thought to be a result of direct bone marrow suppression, whereas the more commonly occurring blood dyscrasia, thrombocytopenia, is thought to be caused by an antibody-mediated destruction of platelets.6
Management of DVP-induced thrombocytopenia is often dependent on the severity of the reaction. In mild-to-moderate cases, intervention may not be necessary as thrombocytopenia has been shown to resolve without adjustment to DVP therapy.1 In more severe or symptomatic cases, dose reduction or discontinuation of the offending agent is recommended, typically resulting in resolution shortly following pharmacologic intervention.
Guidance on the management of other drug-induced hematologic abnormalities, such as neutropenia and leukopenia are not as well established. A 2019 systematic review of idiosyncratic drug-induced neutropenia suggested that continuing the offending drug with strict monitoring could be considered in cases of mild neutropenia. In cases of moderate neutropenia, the author suggests temporary cessation of the drug and reinstatement once neutrophil count normalizes and definitive cessation of the drug in severe cases.30
In our case, continuing the offending agent with close monitoring was considered, similar to the well-established management of clozapine-induced neutropenia. However, due to the concern that the ANC was bordering moderate neutropenia in the absence of a therapeutic VPA level as well as a significant reduction in platelets, although not meeting criteria for thrombocytopenia, the decision was made to err on the side of caution and discontinue the most likely offending agent.
It is important to highlight that DVP was replacing quetiapine in the form of a cross taper. Quetiapine is structurally similar to clozapine. While clozapine has strict monitoring requirements related to neutropenia, blood dyscrasias with quetiapine therapy are rare. Quetiapine-induced hematologic abnormalities may be due to direct toxicity or to an immune-mediated mechanism, leading to bone marrow suppression.20 Case reports documenting blood dyscrasias with the combination of DVP and quetiapine were identified during literature review.15-19 Despite these case reports, we believe DVP was the primary offending agent in our case as the patient’s last dose of quetiapine was 2 weeks before obtaining the abnormal CBC. There was no history of blood dyscrasias with quetiapine monotherapy; however, the effect of the combination of DVP and quetiapine is unknown as no CBC was obtained during the cross-taper period.
Although there are no FDA-approved medications for the treatment of BPD, mood stabilizers, including DVP, have some research to support their use for the treatment of affective dysregulation and impulsive-behavioral dyscontrol.26-28 In our case, DVP was selected due to the evidence for use in BPD and ability to assess adherence with therapeutic monitoring. Although polypharmacy is a concern in patients with BPD, in our case we believed that the patient’s ongoing mood lability and impulsive behaviors warranted pharmacologic intervention. Additionally, DVP provided an advantage in its ability to quickly titrate to therapeutic dose when compared with lamotrigine and a lower risk of cognitive AEs when compared with topiramate.
Conclusions
To our knowledge, this case report demonstrates the first published case of neutropenia and leukopenia related to DVP therapy for the treatment of BPD. Routine CBC monitoring is recommended with DVP therapy, and our case highlights the importance of evaluating for not only thrombocytopenia, but also other blood dyscrasias during the titration phase even in the absence of a therapeutic VPA level. Further studies are warranted to determine incidence of DVP-related neutropenia and leukopenia and to evaluate the safety of continuing DVP in cases of mild-to-moderate neutropenia with close monitoring.
Valproic acid (VPA) and its derivative, divalproex (DVP) are prescribed for a variety of indications, commonly for seizure control in patients with epilepsy, mood stabilization in patients with bipolar disorder, and migraine prophylaxis. Gastrointestinal distress and sedation are among the most reported adverse effects (AEs) with DVP therapy.1 Although serious hepatic and hematologic AEs are rare, monitoring is still recommended. DVP can cause various hematologic dyscrasias, the most common being thrombocytopenia.1,2 Neutropenia and leukopenia have been reported in isolated cases, most occurring in pediatric patients or patients with epilepsy.3-14
Several case reports of DVP-related neutropenia (absolute neutrophil count [ANC] < 1.50 103/mcL) and leukopenia (white blood cell count [WBC] < 4.0 103/mcL) were reviewed during our literature search, some caused by DVP monotherapy; others were thought to be related to concomitant use of DVP and another drug.15-25 Quetiapine was the antipsychotic most commonly implicated in causing hematologic abnormalities when combined with DVP. We report a case of neutropenia and leukopenia that presented after a cross taper from quetiapine to DVP for the treatment of borderline personality disorder (BPD).
Although no medications have been approved by the US Food and Drug Administration (FDA) for the treatment of BPD, mood stabilizers, including DVP, have literature to support their use for the treatment of affective dysregulation and impulsive behavioral dyscontrol.26-28 A therapeutic range for DVP in the treatment of BPD has not been defined; therefore, for this case report, the generally accepted range of 50 to 100 µg/mL will be considered therapeutic.1
Case Presentation
A 34-year-old male patient presented to the mental health clinic pharmacist reporting that his current psychotropic medication regimen was not effective. His medical history included posttraumatic stress disorder (PTSD), opioid use disorder, alcohol use disorder, stimulant use disorder, cannabis use, BPD, hypertension, hyperlipidemia, prediabetes, gastroesophageal reflex disease, and a pulmonary nodule. On initial presentation, the patient was prescribed buprenorphine 24 mg/naloxone 6 mg, quetiapine 400 mg, duloxetine 120 mg, and prazosin 15 mg per day. At the time of pharmacy consultation, last reported alcohol or nonprescribed opioid use was about 6 months prior, and methamphetamine use about 1 month prior, with ongoing cannabis use. The patient had a history of participating in cognitive processing therapy, dialectical behavior therapy (DBT), and residential treatment for both PTSD and substance use. Additionally, he was actively participating in contingency management for stimulant use disorder and self-management and recovery training group.
The patient reported ongoing mood lability, hypervigilance, and oversedation with current psychotropic regimen. The prescriber of his medication for opioid use disorder also reported the patient experienced labile mood, impulsive behavior, and anger outbursts. In the setting of intolerability due to oversedation with quetiapine, cardiometabolic risk, and lack of clear indication for use, the patient and health care practitioner (HCP) agreed to taper quetiapine and initiate a trial of DVP for affective dysregulation and impulsive-behavioral dyscontrol. To prevent cholinergic rebound and insomnia with abrupt discontinuation of quetiapine, DVP and quetiapine were cross tapered. The following cross taper was prescribed: quetiapine 300 mg and DVP 500 mg per day for week 1; quetiapine 200 mg and DVP 500 mg per day for week 2; quetiapine 100 mg and DVP 1000 mg per day for week 3; quetiapine 50 mg and DVP 1000 mg per day for week 4; followed by DVP 1000 mg per day and discontinuation of quetiapine.
During a 4-week follow-up appointment, the patient reported appropriate completion of cross taper but stopped taking the DVP 3 days prior to the appointment due to self-reported lack of efficacy. For this reason, serum VPA level was not obtained. After discussion with his HCP, the patient restarted DVP 1000 mg per day without retitration with plans to get laboratory tests in 1 week. The next week, laboratory tests were notable for VPA level 28.74 (reference range, 50-100) µg/mL, low WBC 3.51 (reference range, 4.00-10.00) 103/mcL, platelets 169 (reference range, 150-420) 103/mcL, and low ANC 1.00 (reference range, 1.50-7.40) 103/mcL (Table). This raised clinical concern as the patient had no history of documented neutropenia or leukopenia, with most recent complete blood count (CBC) prior to DVP initiation 3 months earlier while prescribed quetiapine.
On further review, the HCP opted to cease administration of DVP and repeat CBC with differential in 1 week. Nine days later, laboratory tests were performed and compared with those collected the week before, revealing resolution of neutropenia and leukopenia. A score of 7 on the Naranjo Adverse Drug Reaction Probability Scale (NADRPS) was determined based on previous conclusive reports on the reaction (+1), appeared after suspected drug administration (+2), improved with drug discontinuation (+1), confirmed by objective evidence (+1), and no alternative causes could be found (+2).29 With a NADRPS score of 7, an AE of probable DVP-induced neutropenia was documented and medication was not resumed.
Discussion
Our case report describes isolated neutropenia and leukopenia that developed after a cross taper from quetiapine to DVP. Hematologic abnormalities resolved after discontinuation of DVP, suggesting a likely correlation. DVP has a well-established, dose-related prevalence of thrombocytopenia occurring in up to 27% of patients.1 Fewer case reports exist on neutropenia and leukopenia. DVP-induced neutropenia is thought to be a result of direct bone marrow suppression, whereas the more commonly occurring blood dyscrasia, thrombocytopenia, is thought to be caused by an antibody-mediated destruction of platelets.6
Management of DVP-induced thrombocytopenia is often dependent on the severity of the reaction. In mild-to-moderate cases, intervention may not be necessary as thrombocytopenia has been shown to resolve without adjustment to DVP therapy.1 In more severe or symptomatic cases, dose reduction or discontinuation of the offending agent is recommended, typically resulting in resolution shortly following pharmacologic intervention.
Guidance on the management of other drug-induced hematologic abnormalities, such as neutropenia and leukopenia are not as well established. A 2019 systematic review of idiosyncratic drug-induced neutropenia suggested that continuing the offending drug with strict monitoring could be considered in cases of mild neutropenia. In cases of moderate neutropenia, the author suggests temporary cessation of the drug and reinstatement once neutrophil count normalizes and definitive cessation of the drug in severe cases.30
In our case, continuing the offending agent with close monitoring was considered, similar to the well-established management of clozapine-induced neutropenia. However, due to the concern that the ANC was bordering moderate neutropenia in the absence of a therapeutic VPA level as well as a significant reduction in platelets, although not meeting criteria for thrombocytopenia, the decision was made to err on the side of caution and discontinue the most likely offending agent.
It is important to highlight that DVP was replacing quetiapine in the form of a cross taper. Quetiapine is structurally similar to clozapine. While clozapine has strict monitoring requirements related to neutropenia, blood dyscrasias with quetiapine therapy are rare. Quetiapine-induced hematologic abnormalities may be due to direct toxicity or to an immune-mediated mechanism, leading to bone marrow suppression.20 Case reports documenting blood dyscrasias with the combination of DVP and quetiapine were identified during literature review.15-19 Despite these case reports, we believe DVP was the primary offending agent in our case as the patient’s last dose of quetiapine was 2 weeks before obtaining the abnormal CBC. There was no history of blood dyscrasias with quetiapine monotherapy; however, the effect of the combination of DVP and quetiapine is unknown as no CBC was obtained during the cross-taper period.
Although there are no FDA-approved medications for the treatment of BPD, mood stabilizers, including DVP, have some research to support their use for the treatment of affective dysregulation and impulsive-behavioral dyscontrol.26-28 In our case, DVP was selected due to the evidence for use in BPD and ability to assess adherence with therapeutic monitoring. Although polypharmacy is a concern in patients with BPD, in our case we believed that the patient’s ongoing mood lability and impulsive behaviors warranted pharmacologic intervention. Additionally, DVP provided an advantage in its ability to quickly titrate to therapeutic dose when compared with lamotrigine and a lower risk of cognitive AEs when compared with topiramate.
Conclusions
To our knowledge, this case report demonstrates the first published case of neutropenia and leukopenia related to DVP therapy for the treatment of BPD. Routine CBC monitoring is recommended with DVP therapy, and our case highlights the importance of evaluating for not only thrombocytopenia, but also other blood dyscrasias during the titration phase even in the absence of a therapeutic VPA level. Further studies are warranted to determine incidence of DVP-related neutropenia and leukopenia and to evaluate the safety of continuing DVP in cases of mild-to-moderate neutropenia with close monitoring.
1. Depakote (valproic acid). Package insert. Abbott Laboratories; June 2000.
2. Conley EL, Coley KC, Pollock BG, Dapos SV, Maxwell R, Branch RA. Prevalence and risk of thrombocytopenia with valproic acid: experience at a psychiatric teaching hospital. Pharmacotherapy. 2001;21(11):1325-1330. doi:10.1592/phco.21.17.1325.34418
3. Jaeken J, van Goethem C, Casaer P, Devlieger H, Eggermont E, Pilet M. Neutropenia during sodium valproate therapy. Arch Dis Child. 1979;54(12):986-987. doi:10.1136/adc.54.12.986
4. Barr RD, Copeland SA, Stockwell MC, Morris N, Kelton JC. Valproic acid and immune thrombocytopenia. Arch Dis Child. 1982;57(9):681-684. doi:10.1136/adc.57.9.681
5. Symon DNK, Russell G. Sodium valproate and neutropenia (letter). Arch Dis Child. 1983;58:235. doi:10.1136/adc.58.3.235
6. Watts RG, Emanuel PD, Zuckerman KS, Howard TH. Valproic acid-induced cytopenias: evidence for a dose-related suppression of hematopoiesis. J Pediatr. 1990;117(3):495-499. doi:10.1016/s0022-3476(05)81105-9
7. Blackburn SC, Oliart AD, García-Rodríguez LA, Pérez Gutthann S. Antiepileptics and blood dyscrasias: a cohort study. Pharmacotherapy. 1998;18(6):1277-1283.
8. Acharya S, Bussel JB. Hematologic toxicity of sodium valproate. J Pediatr Hematol Oncol. 2000;22(1):62-65. doi:10.1097/00043426-200001000-00012
9. Vesta KS, Medina PJ. Valproic acid-induced neutropenia. Ann Pharmacother. 2003;37(6):819-821. doi:10.1345/aph.1C381
10. Kohli U, Gulati, S. Sodium valproate induced isolated neutropenia. Indian J Pediatr. 2006;73(9):844-844. doi:10.1007/BF02790401
11. Hsu HC, Tseng HK, Wang SC, Wang YY. Valproic acid-induced agranulocytosis. Int J Gerontol. 2009;3(2):137-139. doi:10.1016/S1873-9598(09)70036-5
12. Chakraborty S, Chakraborty J, Mandal S, Ghosal MK. A rare occurrence of isolated neutropenia with valproic acid: a case report. J Indian Med Assoc. 2011;109(5):345-346.
13. Stoner SC, Deal E, Lurk JT. Delayed-onset neutropenia with divalproex sodium. Ann Pharmacother. 2008;42(10):1507-1510. doi:10.1345/aph.1L239
14. Storch DD. Severe leukopenia with valproate. J Am Acad Child Adolesc Psychiatry. 2000;39(10):1208-1209. doi:10.1097/00004583-200010000-00003
15. Rahman A, Mican LM, Fischer C, Campbell AH. Evaluating the incidence of leukopenia and neutropenia with valproate, quetiapine, or the combination in children and adolescents. Ann Pharmacother. 2009;43:822-830. doi:10.1345/aph.1L617
16. Hung WC, Hsieh MH. Neutropenia associated with the comedication of quetiapine and valproate in 2 elderly patients. J Clin Psychopharmacol. 2012;32(3):416-417. doi:10.1097/JCP.0b013e3182549d2d
17. Park HJ, Kim JY. Incidence of neutropenia with valproate and quetiapine combination treatment in subjects with acquired brain injuries. Arch Phys Med Rehabil. 2016;97(2):183-188. doi:10.1016/j.apmr.2015.09.004
18. Estabrook KR, Pheister M. A case of quetiapine XR and divalproex-associated neutropenia followed by successful use of ziprasidone. J Clin Psychopharmacol. 2012;32(3):417-418. doi:10.1097/JCP.0b013e318253a071
19. Nair P, Lippmann S. Is leukopenia associated with divalproex and/or quetiapine? Psychosomatics. 2005;46(2):188-189. doi:10.1176/appi.psy.46.2.188
20. Cowan C, Oakley C. Leukopenia and neutropenia induced by quetiapine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):292-294. doi:10.1016/j.pnpbp.2006.07.003
21. Fan KY, Chen WY, Huang MC. Quetiapine-associated leucopenia and thrombocytopenia: a case report. BMC Psychiatry. 2015;15:110. doi:10.1186/s12888-015-0495-9
22. Malik S, Lally J, Ajnakina O, et al. Sodium valproate and clozapine induced neutropenia: A case control study using register data. Schizophr Res. 2018;195:267-273. doi:10.1016/j.schres.2017.08.041
23. Pantelis C, Adesanya A. Increased risk of neutropaenia and agranulocytosis with sodium valproate used adjunctively with clozapine. Aust N Z J Psychiatry. 2001;35(4):544-545. doi:10.1046/j.1440-1614.2001.0911f.x
24. Madeb R, Hirschmann S, Kurs R, Turkie A, Modai I. Combined clozapine and valproic acid treatment-induced agranulocytosis. Eur Psychiatry. 2002;17(4):238-239. doi:10.1016/s0924-9338(02)00659-4
25. Dose M, Hellweg R, Yassouridis A, Theison M, Emrich HM. Combined treatment of schizophrenic psychoses with haloperidol and valproate. Pharmacopsychiatry. 1998;31(4):122-125. doi:10.1055/s-2007-979312
26. Ingenhoven T, Lafay P, Rinne T, Passchier J, Duivenvoorden H. Effectiveness of pharmacotherapy for severe personality disorders: meta-analyses of randomized controlled trials. J Clin Psychiatry. 2010;71:14. doi:10.4088/jcp.08r04526gre
27. Mercer D, Douglass AB, Links PS. Meta-analyses of mood stabilizers, antidepressants and antipsychotics in the treatment of borderline personality disorder: effectiveness for depression and anger symptoms. J Pers Disord. 2009;23(2):156-174. doi:10.1521/pedi.2009.23.2.156
28. Hollander E, Swann AC, Coccaro EF, Jiang P, Smith TB. Impact of trait impulsivity and state aggression on divalproex versus placebo response in borderline personality disorder. Am J Psychiatry. 2005;162(3):621-624. doi:10.1176/appi.ajp.162.3.621
29. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245. doi:10.1038/clpt.1981.154
30. Andrès E, Villalba NL, Zulfiqar AA, Serraj K, Mourot-Cottet R, Gottenberg AJ. State of art of idiosyncratic drug-induced neutropenia or agranulocytosis, with a focus on biotherapies. J Clin Med. 2019;8(9):1351. doi:10.3390/jcm8091351
1. Depakote (valproic acid). Package insert. Abbott Laboratories; June 2000.
2. Conley EL, Coley KC, Pollock BG, Dapos SV, Maxwell R, Branch RA. Prevalence and risk of thrombocytopenia with valproic acid: experience at a psychiatric teaching hospital. Pharmacotherapy. 2001;21(11):1325-1330. doi:10.1592/phco.21.17.1325.34418
3. Jaeken J, van Goethem C, Casaer P, Devlieger H, Eggermont E, Pilet M. Neutropenia during sodium valproate therapy. Arch Dis Child. 1979;54(12):986-987. doi:10.1136/adc.54.12.986
4. Barr RD, Copeland SA, Stockwell MC, Morris N, Kelton JC. Valproic acid and immune thrombocytopenia. Arch Dis Child. 1982;57(9):681-684. doi:10.1136/adc.57.9.681
5. Symon DNK, Russell G. Sodium valproate and neutropenia (letter). Arch Dis Child. 1983;58:235. doi:10.1136/adc.58.3.235
6. Watts RG, Emanuel PD, Zuckerman KS, Howard TH. Valproic acid-induced cytopenias: evidence for a dose-related suppression of hematopoiesis. J Pediatr. 1990;117(3):495-499. doi:10.1016/s0022-3476(05)81105-9
7. Blackburn SC, Oliart AD, García-Rodríguez LA, Pérez Gutthann S. Antiepileptics and blood dyscrasias: a cohort study. Pharmacotherapy. 1998;18(6):1277-1283.
8. Acharya S, Bussel JB. Hematologic toxicity of sodium valproate. J Pediatr Hematol Oncol. 2000;22(1):62-65. doi:10.1097/00043426-200001000-00012
9. Vesta KS, Medina PJ. Valproic acid-induced neutropenia. Ann Pharmacother. 2003;37(6):819-821. doi:10.1345/aph.1C381
10. Kohli U, Gulati, S. Sodium valproate induced isolated neutropenia. Indian J Pediatr. 2006;73(9):844-844. doi:10.1007/BF02790401
11. Hsu HC, Tseng HK, Wang SC, Wang YY. Valproic acid-induced agranulocytosis. Int J Gerontol. 2009;3(2):137-139. doi:10.1016/S1873-9598(09)70036-5
12. Chakraborty S, Chakraborty J, Mandal S, Ghosal MK. A rare occurrence of isolated neutropenia with valproic acid: a case report. J Indian Med Assoc. 2011;109(5):345-346.
13. Stoner SC, Deal E, Lurk JT. Delayed-onset neutropenia with divalproex sodium. Ann Pharmacother. 2008;42(10):1507-1510. doi:10.1345/aph.1L239
14. Storch DD. Severe leukopenia with valproate. J Am Acad Child Adolesc Psychiatry. 2000;39(10):1208-1209. doi:10.1097/00004583-200010000-00003
15. Rahman A, Mican LM, Fischer C, Campbell AH. Evaluating the incidence of leukopenia and neutropenia with valproate, quetiapine, or the combination in children and adolescents. Ann Pharmacother. 2009;43:822-830. doi:10.1345/aph.1L617
16. Hung WC, Hsieh MH. Neutropenia associated with the comedication of quetiapine and valproate in 2 elderly patients. J Clin Psychopharmacol. 2012;32(3):416-417. doi:10.1097/JCP.0b013e3182549d2d
17. Park HJ, Kim JY. Incidence of neutropenia with valproate and quetiapine combination treatment in subjects with acquired brain injuries. Arch Phys Med Rehabil. 2016;97(2):183-188. doi:10.1016/j.apmr.2015.09.004
18. Estabrook KR, Pheister M. A case of quetiapine XR and divalproex-associated neutropenia followed by successful use of ziprasidone. J Clin Psychopharmacol. 2012;32(3):417-418. doi:10.1097/JCP.0b013e318253a071
19. Nair P, Lippmann S. Is leukopenia associated with divalproex and/or quetiapine? Psychosomatics. 2005;46(2):188-189. doi:10.1176/appi.psy.46.2.188
20. Cowan C, Oakley C. Leukopenia and neutropenia induced by quetiapine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):292-294. doi:10.1016/j.pnpbp.2006.07.003
21. Fan KY, Chen WY, Huang MC. Quetiapine-associated leucopenia and thrombocytopenia: a case report. BMC Psychiatry. 2015;15:110. doi:10.1186/s12888-015-0495-9
22. Malik S, Lally J, Ajnakina O, et al. Sodium valproate and clozapine induced neutropenia: A case control study using register data. Schizophr Res. 2018;195:267-273. doi:10.1016/j.schres.2017.08.041
23. Pantelis C, Adesanya A. Increased risk of neutropaenia and agranulocytosis with sodium valproate used adjunctively with clozapine. Aust N Z J Psychiatry. 2001;35(4):544-545. doi:10.1046/j.1440-1614.2001.0911f.x
24. Madeb R, Hirschmann S, Kurs R, Turkie A, Modai I. Combined clozapine and valproic acid treatment-induced agranulocytosis. Eur Psychiatry. 2002;17(4):238-239. doi:10.1016/s0924-9338(02)00659-4
25. Dose M, Hellweg R, Yassouridis A, Theison M, Emrich HM. Combined treatment of schizophrenic psychoses with haloperidol and valproate. Pharmacopsychiatry. 1998;31(4):122-125. doi:10.1055/s-2007-979312
26. Ingenhoven T, Lafay P, Rinne T, Passchier J, Duivenvoorden H. Effectiveness of pharmacotherapy for severe personality disorders: meta-analyses of randomized controlled trials. J Clin Psychiatry. 2010;71:14. doi:10.4088/jcp.08r04526gre
27. Mercer D, Douglass AB, Links PS. Meta-analyses of mood stabilizers, antidepressants and antipsychotics in the treatment of borderline personality disorder: effectiveness for depression and anger symptoms. J Pers Disord. 2009;23(2):156-174. doi:10.1521/pedi.2009.23.2.156
28. Hollander E, Swann AC, Coccaro EF, Jiang P, Smith TB. Impact of trait impulsivity and state aggression on divalproex versus placebo response in borderline personality disorder. Am J Psychiatry. 2005;162(3):621-624. doi:10.1176/appi.ajp.162.3.621
29. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245. doi:10.1038/clpt.1981.154
30. Andrès E, Villalba NL, Zulfiqar AA, Serraj K, Mourot-Cottet R, Gottenberg AJ. State of art of idiosyncratic drug-induced neutropenia or agranulocytosis, with a focus on biotherapies. J Clin Med. 2019;8(9):1351. doi:10.3390/jcm8091351
The Molting Man: Anasarca-Induced Full-Body Desquamation
Edema blisters are a common but often underreported entity most commonly seen on the lower extremities in the setting of acute edema. 1 Reported risk factors and associations include chronic venous insufficiency, congestive heart failure, hereditary angioedema, and medications (eg, amlodipine). 1,2 We report a newly described variant that we have termed anasarca-induced desquamation in which a patient sloughed the entire cutaneous surface of the body after gaining almost 40 pounds over 5 days.
Case Report
A 50-year-old man without a home was found minimally responsive in a yard. His core body temperature was 25.5 °C. He was profoundly acidotic (pH, <6.733 [reference range, 7.35–7.45]; lactic acid, 20.5 mmol/L [reference range, 0.5–2.2 mmol/L]) at admission. His medical history was notable for diabetes mellitus, hypertension, alcohol abuse, and pulmonary embolism. The patient was resuscitated with rewarming and intravenous fluids in the setting of acute renal insufficiency. By day 5 of the hospital stay, he had a net positive intake of 21.8 L and an 18-kg (39.7-lb) weight gain.
Dermatology was consulted for skin sloughing. Physical examination revealed nonpainful desquamation of the vermilion lip, periorbital skin, right shoulder, and hips without notable mucosal changes. Two 4-mm punch biopsies of the shoulder revealed an intracorneal split with desquamation of the stratum corneum and a mild dermal lymphocytic infiltrate, consistent with exfoliation secondary to edema or staphylococcal scalded skin syndrome (Figure 1). No staphylococcal growth was noted on blood, urine, nasal, wound, and ocular cultures throughout the hospital stay.
As the patient’s anasarca improved with diuretics and continuous renal replacement therapy, the entire cutaneous surface—head to toe—underwent desquamation, including the palms and soles. He was managed with supportive skin care. The anasarca healed completely with residual hypopigmentation (Figures 2 and 3).
Comment
Anasarca-induced desquamation represents a more diffuse form of a known entity: edema blisters. Occurring most commonly in the setting of acute exacerbation of chronic venous insufficiency, edema blisters can mimic other vesiculobullous conditions, such as bullous pemphigoid and herpes zoster.3
Pathogenesis of Edema Blisters—Edema develops in the skin when the capillary filtration rate, determined by the hydrostatic and oncotic pressures of the capillaries and interstitium, exceeds venous and lymphatic drainage. The appearance of edema blisters in the acute setting likely is related to the speed at which edema develops in skin.1 Although edema blisters often are described as tense, there is a paucity of histologic data at the anatomical level of split in the skin.In our patient, desquamation was within the stratum corneum and likely multifactorial. His weight gain of nearly 40 lb, the result of intravenous instillation of fluids and low urine output, was undeniably a contributing factor. The anasarca was aggravated by hypoalbuminemia (2.1 g/dL) in the setting of known liver disease. Other possible contributing factors were hypotension, which required vasopressor therapy that led to hypoperfusion of the skin, and treatment of hypothermia, with resulting reactive vasodilation and capillary leak.
Management—Treatment of acute edema blisters is focused on the underlying cause of the edema. In a study of 13 patients with edema blisters, all had blisters on the legs that resolved with treatment, such as diuretics or compression therapy.1
Anasarca-induced desquamation is an inherently benign condition that mimics potentially fatal disorders, such as Stevens-Johnson syndrome, staphylococcal scalded skin syndrome, and toxic shock syndrome. Therefore, patients presenting with diffuse superficial desquamation should be assessed for the mucosal changes of Stevens-Johnson syndrome and a history of acute edema in the affected areas to avoid potentially harmful empiric treatments, such as corticosteroids and intravenous antibiotics.
Conclusion
Anasarca-induced desquamation represents a more diffuse form of edema blisters. This desquamation can mimic a potentially fatal rash, such as Stevens-Johnson syndrome and staphylococcal scalded skin syndrome.
- Bhushan M, Chalmers RJ, Cox NH. Acute oedema blisters: a report of 13 cases. Br J Dermatol. 2001;144:580-582. doi:10.1046/j.1365-2133.2001.04087.x
- Fabiani J, Bork K. Acute edema blisters on a skin swelling: an unusual manifestation of hereditary angioedema. Acta Derm Venereol. 2016;96:556-557. doi:10.2340/00015555-2252
- Chen SX, Cohen PR. Edema bullae mimicking disseminated herpes zoster. Cureus. 2017;9:E1780. doi:10.7759/cureus.1780
Edema blisters are a common but often underreported entity most commonly seen on the lower extremities in the setting of acute edema. 1 Reported risk factors and associations include chronic venous insufficiency, congestive heart failure, hereditary angioedema, and medications (eg, amlodipine). 1,2 We report a newly described variant that we have termed anasarca-induced desquamation in which a patient sloughed the entire cutaneous surface of the body after gaining almost 40 pounds over 5 days.
Case Report
A 50-year-old man without a home was found minimally responsive in a yard. His core body temperature was 25.5 °C. He was profoundly acidotic (pH, <6.733 [reference range, 7.35–7.45]; lactic acid, 20.5 mmol/L [reference range, 0.5–2.2 mmol/L]) at admission. His medical history was notable for diabetes mellitus, hypertension, alcohol abuse, and pulmonary embolism. The patient was resuscitated with rewarming and intravenous fluids in the setting of acute renal insufficiency. By day 5 of the hospital stay, he had a net positive intake of 21.8 L and an 18-kg (39.7-lb) weight gain.

Dermatology was consulted for skin sloughing. Physical examination revealed nonpainful desquamation of the vermilion lip, periorbital skin, right shoulder, and hips without notable mucosal changes. Two 4-mm punch biopsies of the shoulder revealed an intracorneal split with desquamation of the stratum corneum and a mild dermal lymphocytic infiltrate, consistent with exfoliation secondary to edema or staphylococcal scalded skin syndrome (Figure 1). No staphylococcal growth was noted on blood, urine, nasal, wound, and ocular cultures throughout the hospital stay.

As the patient’s anasarca improved with diuretics and continuous renal replacement therapy, the entire cutaneous surface—head to toe—underwent desquamation, including the palms and soles. He was managed with supportive skin care. The anasarca healed completely with residual hypopigmentation (Figures 2 and 3).

Comment
Anasarca-induced desquamation represents a more diffuse form of a known entity: edema blisters. Occurring most commonly in the setting of acute exacerbation of chronic venous insufficiency, edema blisters can mimic other vesiculobullous conditions, such as bullous pemphigoid and herpes zoster.3
Pathogenesis of Edema Blisters—Edema develops in the skin when the capillary filtration rate, determined by the hydrostatic and oncotic pressures of the capillaries and interstitium, exceeds venous and lymphatic drainage. The appearance of edema blisters in the acute setting likely is related to the speed at which edema develops in skin.1 Although edema blisters often are described as tense, there is a paucity of histologic data at the anatomical level of split in the skin.In our patient, desquamation was within the stratum corneum and likely multifactorial. His weight gain of nearly 40 lb, the result of intravenous instillation of fluids and low urine output, was undeniably a contributing factor. The anasarca was aggravated by hypoalbuminemia (2.1 g/dL) in the setting of known liver disease. Other possible contributing factors were hypotension, which required vasopressor therapy that led to hypoperfusion of the skin, and treatment of hypothermia, with resulting reactive vasodilation and capillary leak.
Management—Treatment of acute edema blisters is focused on the underlying cause of the edema. In a study of 13 patients with edema blisters, all had blisters on the legs that resolved with treatment, such as diuretics or compression therapy.1
Anasarca-induced desquamation is an inherently benign condition that mimics potentially fatal disorders, such as Stevens-Johnson syndrome, staphylococcal scalded skin syndrome, and toxic shock syndrome. Therefore, patients presenting with diffuse superficial desquamation should be assessed for the mucosal changes of Stevens-Johnson syndrome and a history of acute edema in the affected areas to avoid potentially harmful empiric treatments, such as corticosteroids and intravenous antibiotics.
Conclusion
Anasarca-induced desquamation represents a more diffuse form of edema blisters. This desquamation can mimic a potentially fatal rash, such as Stevens-Johnson syndrome and staphylococcal scalded skin syndrome.
Edema blisters are a common but often underreported entity most commonly seen on the lower extremities in the setting of acute edema. 1 Reported risk factors and associations include chronic venous insufficiency, congestive heart failure, hereditary angioedema, and medications (eg, amlodipine). 1,2 We report a newly described variant that we have termed anasarca-induced desquamation in which a patient sloughed the entire cutaneous surface of the body after gaining almost 40 pounds over 5 days.
Case Report
A 50-year-old man without a home was found minimally responsive in a yard. His core body temperature was 25.5 °C. He was profoundly acidotic (pH, <6.733 [reference range, 7.35–7.45]; lactic acid, 20.5 mmol/L [reference range, 0.5–2.2 mmol/L]) at admission. His medical history was notable for diabetes mellitus, hypertension, alcohol abuse, and pulmonary embolism. The patient was resuscitated with rewarming and intravenous fluids in the setting of acute renal insufficiency. By day 5 of the hospital stay, he had a net positive intake of 21.8 L and an 18-kg (39.7-lb) weight gain.

Dermatology was consulted for skin sloughing. Physical examination revealed nonpainful desquamation of the vermilion lip, periorbital skin, right shoulder, and hips without notable mucosal changes. Two 4-mm punch biopsies of the shoulder revealed an intracorneal split with desquamation of the stratum corneum and a mild dermal lymphocytic infiltrate, consistent with exfoliation secondary to edema or staphylococcal scalded skin syndrome (Figure 1). No staphylococcal growth was noted on blood, urine, nasal, wound, and ocular cultures throughout the hospital stay.

As the patient’s anasarca improved with diuretics and continuous renal replacement therapy, the entire cutaneous surface—head to toe—underwent desquamation, including the palms and soles. He was managed with supportive skin care. The anasarca healed completely with residual hypopigmentation (Figures 2 and 3).

Comment
Anasarca-induced desquamation represents a more diffuse form of a known entity: edema blisters. Occurring most commonly in the setting of acute exacerbation of chronic venous insufficiency, edema blisters can mimic other vesiculobullous conditions, such as bullous pemphigoid and herpes zoster.3
Pathogenesis of Edema Blisters—Edema develops in the skin when the capillary filtration rate, determined by the hydrostatic and oncotic pressures of the capillaries and interstitium, exceeds venous and lymphatic drainage. The appearance of edema blisters in the acute setting likely is related to the speed at which edema develops in skin.1 Although edema blisters often are described as tense, there is a paucity of histologic data at the anatomical level of split in the skin.In our patient, desquamation was within the stratum corneum and likely multifactorial. His weight gain of nearly 40 lb, the result of intravenous instillation of fluids and low urine output, was undeniably a contributing factor. The anasarca was aggravated by hypoalbuminemia (2.1 g/dL) in the setting of known liver disease. Other possible contributing factors were hypotension, which required vasopressor therapy that led to hypoperfusion of the skin, and treatment of hypothermia, with resulting reactive vasodilation and capillary leak.
Management—Treatment of acute edema blisters is focused on the underlying cause of the edema. In a study of 13 patients with edema blisters, all had blisters on the legs that resolved with treatment, such as diuretics or compression therapy.1
Anasarca-induced desquamation is an inherently benign condition that mimics potentially fatal disorders, such as Stevens-Johnson syndrome, staphylococcal scalded skin syndrome, and toxic shock syndrome. Therefore, patients presenting with diffuse superficial desquamation should be assessed for the mucosal changes of Stevens-Johnson syndrome and a history of acute edema in the affected areas to avoid potentially harmful empiric treatments, such as corticosteroids and intravenous antibiotics.
Conclusion
Anasarca-induced desquamation represents a more diffuse form of edema blisters. This desquamation can mimic a potentially fatal rash, such as Stevens-Johnson syndrome and staphylococcal scalded skin syndrome.
- Bhushan M, Chalmers RJ, Cox NH. Acute oedema blisters: a report of 13 cases. Br J Dermatol. 2001;144:580-582. doi:10.1046/j.1365-2133.2001.04087.x
- Fabiani J, Bork K. Acute edema blisters on a skin swelling: an unusual manifestation of hereditary angioedema. Acta Derm Venereol. 2016;96:556-557. doi:10.2340/00015555-2252
- Chen SX, Cohen PR. Edema bullae mimicking disseminated herpes zoster. Cureus. 2017;9:E1780. doi:10.7759/cureus.1780
- Bhushan M, Chalmers RJ, Cox NH. Acute oedema blisters: a report of 13 cases. Br J Dermatol. 2001;144:580-582. doi:10.1046/j.1365-2133.2001.04087.x
- Fabiani J, Bork K. Acute edema blisters on a skin swelling: an unusual manifestation of hereditary angioedema. Acta Derm Venereol. 2016;96:556-557. doi:10.2340/00015555-2252
- Chen SX, Cohen PR. Edema bullae mimicking disseminated herpes zoster. Cureus. 2017;9:E1780. doi:10.7759/cureus.1780
Practice Points
- The appearance of anasarca-induced desquamation can be similar to staphylococcal scalded skin syndrome and Stevens-Johnson syndrome.
- Histopathologic evaluation of this condition shows desquamation localized to the stratum corneum without epidermal necrosis.
- Careful evaluation, including bacterial culture, is required to rule out an infectious cause.
- Early diagnosis of anasarca-induced desquamation reduces the potential for providing harmful empiric treatment, such as systemic steroids and intravenous antibiotics, especially in patients known to have comorbidities.
Acral Papulovesicular Eruption in a Soldier Following Smallpox Vaccination
Following the attacks of September 11, 2001, heightened concerns over bioterrorism and the potential use of smallpox as a biological weapon made smallpox vaccination a critical component of military readiness. Therefore, the US Military resumed its smallpox vaccination program in 2002 using the first-generation smallpox vaccine (Dryvax, Wyeth Pharmaceuticals), a live vaccinia virus vaccine created in the late 19th century. This vaccine was developed by pooling vaccinia strains from the skin of infected cows1 and had previously been used during the worldwide vaccination campaign in the 1970s. Dryvax was associated with various cardiac and cutaneous complications, from benign hypersensitivity reactions to life-threatening eczema vaccinatum and progressive vaccinia.
Due to concerns that the remaining supply of Dryvax was insufficient to vaccinate the US population in the case of a bioterrorism attack, investigators developed the second-generation smallpox vaccine (ACAM2000, Sanofi Pasteur Biologics Co) using advances in vaccine technology.2 ACAM2000 is a plaque-purified isolate of vaccinia virus propagated in cell culture, thereby reducing contaminants and lot-to-lot variation.1 Clinical trials demonstrated comparable immunogenicity and frequency of adverse events compared with Dryvax,2 and ACAM2000 replaced Dryvax in 2008. However, these trials focused on serious adverse events, such as cardiac complications and postvaccinal encephalitis, with less specific characterization and description of cutaneous eruptions.3
Since 2008, there have been few reports of cutaneous adverse reactions following vaccination with ACAM2000. Beachkofsky et al4 described 7 cases of papulovesicular eruptions and 1 case of generalized vaccinia. Freeman and Lenz5 described 4 cases of papulovesicular eruptions, and there has been 1 case of progressive vaccinia reported in a soldier with newly diagnosed acute myelogenous leukemia.6 Kramer7 described a patient with multiple vesiculopustular lesions secondary to autoinoculation. The distinct pruritic acral papulovesicular eruptions following ACAM2000 vaccination have occurred in healthy military service members at different locations since the introduction of ACAM2000. We describe an additional case of this unique cutaneous eruption, followed by a review of previously described cutaneous adverse events associated with smallpox vaccination.
Case Report
A 21-year-old female soldier who was otherwise healthy presented to the dermatology clinic with a pruritic papular eruption involving the upper and lower extremities of 1 week’s duration. The lesions first appeared 8 days after she received the ACAM2000 vaccine. She received no other concurrent vaccines, had no history of atopic dermatitis, and had no systemic symptoms. Physical examination revealed numerous erythematous indurated papules involving the dorsolateral hands and fingers, as well as the extensor surfaces of the elbows, knees, and thighs (Figures 1 and 2). Based on the clinical presentation, the differential diagnosis included lichen planus, verruca plana, dyshidrotic eczema, and smallpox vaccine reaction. Erythema multiforme was considered; however, the absence of palmoplantar involvement and typical targetoid lesions made this diagnosis less likely.
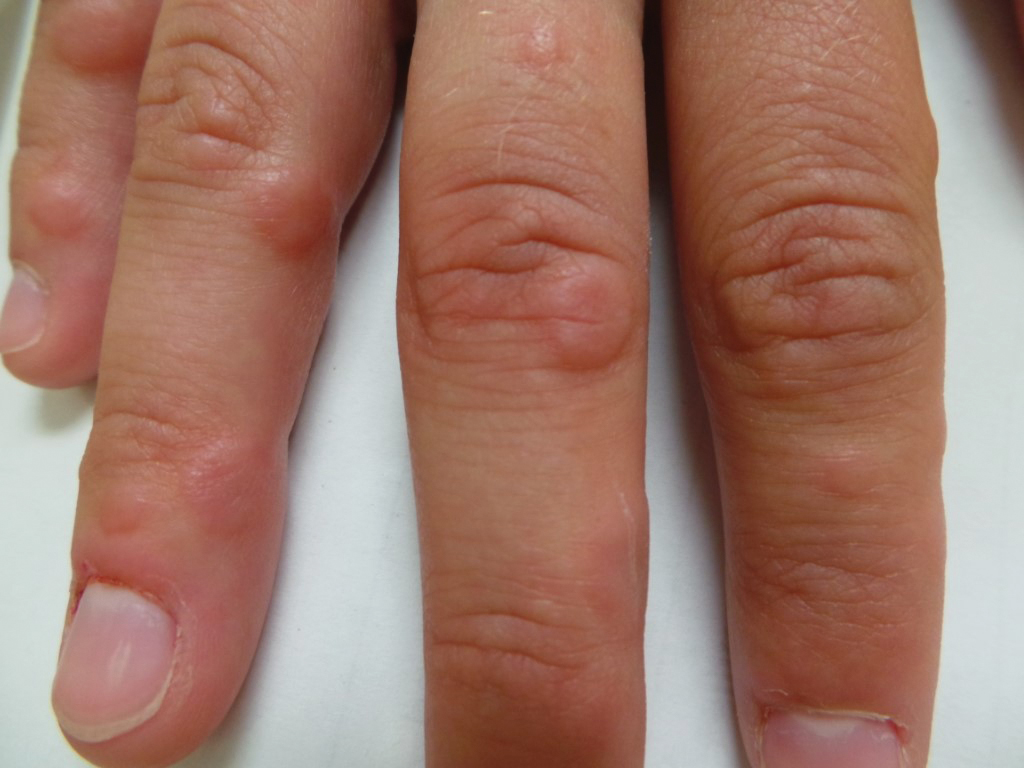
Biopsies of lesions on the arm and thigh were performed. Histologic findings revealed interface and spongiotic dermatitis with scattered necrotic keratinocytes and extravasated erythrocytes (Figure 3). There was no evidence of viral cytopathic effects. Similar clinical and histologic findings have been reported in the literature as acral papulovesicular eruptions following smallpox vaccination or papular spongiotic dermatitis of smallpox vaccination.8 The presence of eosinophils was not conspicuous in the current case and was only a notable finding in 1 of 2 cases previously described by Gaertner et al.8 This may simply be due to an idiosyncratic drug reaction. Furthermore, in the cases described by Beachkofsky et al,4 there were essentially 2 histologic groups. The first group demonstrated a dermal hypersensitivity-type reaction, and the second group demonstrated a lymphocytic capillaritis.
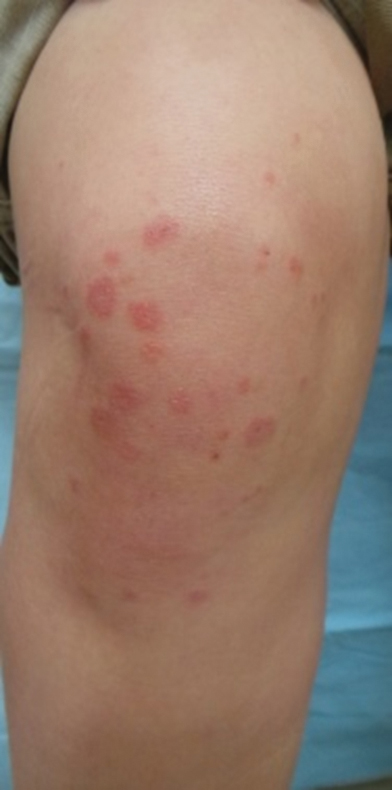
Based on these findings, the patient was diagnosed with an acral papulovesicular eruption following smallpox vaccination. Of note, the patient’s presentation was not consistent with other described smallpox vaccine reactions, which included eczema vaccinatum, autoinoculation, generalized vaccinia, and progressive vaccinia. The patient was treated supportively with triamcinolone acetonide cream 0.1%, cool compresses, and oral diphenhydramine as needed for pruritus. The lesions notably improved within the first week of treatment.
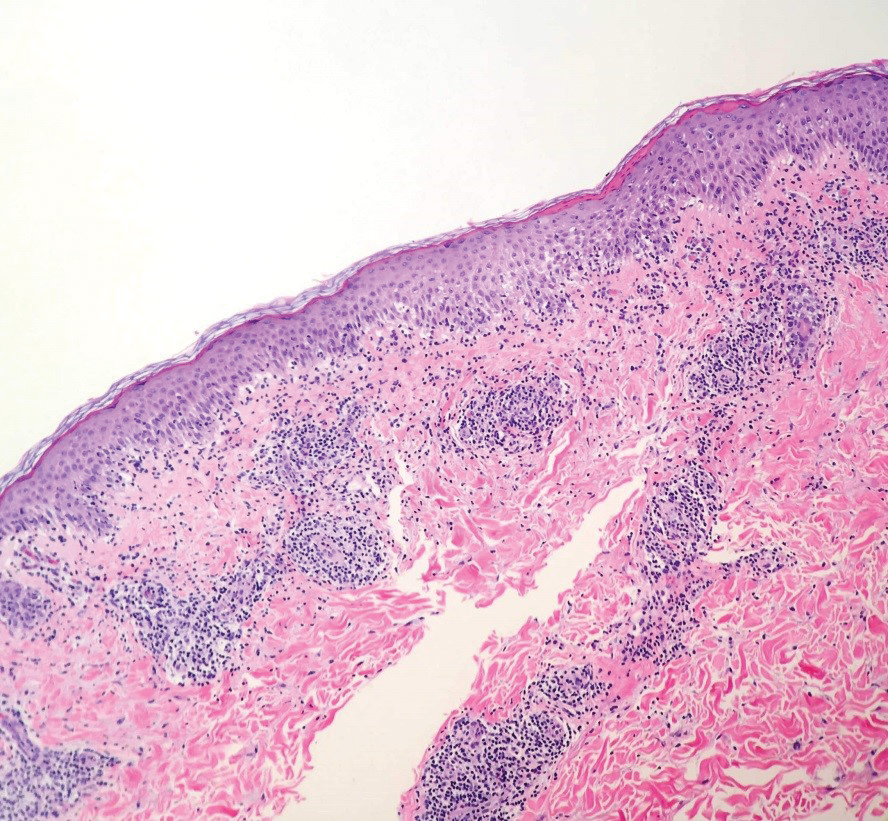
Comment
Reported cases of acral papulovesicular eruption4-6 demonstrated an onset of cutaneous symptoms an average of 14 days following vaccination (range, 8–18 days postvaccination). Lesions were benign and self-limited in all cases, with resolution within an average of 25 days (range, 7–71 days). All patients were active-duty military adults with a mean age of 24 years. Supportive treatment varied from topical steroids and oral antihistamines to tapering oral prednisone doses. Of note, all previously reported cases of this reaction occurred in patients who also had received other concurrent or near-concurrent vaccines, including anthrax, hepatitis B, influenza, and typhoid. Our patient represents a unique case of a papulovesicular eruption following smallpox vaccination with no history of concurrent vaccines.
Since the 1970s, smallpox vaccination has been associated with numerous cutaneous reactions, most of which have been reported with the first-generation Dryvax. Minor local reactions occurred in approximately 2% to 6% of vaccinees in clinical trials.9 These reactions included local edema involving the upper arm, satellite lesions within 2.5 cm of the vaccination site, local lymphadenopathy, intense inflammation or viral cellulitis surrounding the inoculation site, and viral lymphangitis tracking to axillary lymph nodes. In clinical trials, these reactions were self-limited and required only symptomatic treatment.9
Autoinoculation is another cutaneous reaction that can occur because Dryvax and ACAM2000 both contain live-attenuated replicating vaccinia virus. Accidental implantation may occur when the high titers of virus present at the vaccine site are subsequently transferred to other sites, especially abnormal mucosa or skin, resulting in an additional primary inoculation site.10
Eczema vaccinatum is a potentially life-threatening reaction that may occur in patients with disruptive skin disorders, such as atopic dermatitis. These patients are at risk for massive confluent vaccinia infection of the skin.10 In patients with atopic dermatitis, the virus rapidly disseminates due to both skin barrier dysfunction and impaired immunomodulation, resulting in large confluent skin lesions and the potential for viremia, septic shock, and death.10,11 Mortality from eczema vaccinatum may be reduced by administration of vaccinia immune globulin.10
The vaccinia virus also may spread hematogenously in healthy individuals,10 resulting in a benign reaction called generalized vaccinia. These patients develop pustules on areas of the skin other than the vaccination site. Although typically benign and self-limited, Beachkofsky et al4 described a case of generalized vaccinia in a healthy 34-year-old man resulting in a rapidly progressive vesiculopustular eruption with associated fever and pancytopenia. The patient made a complete recovery over the course of the following month.4
Alternatively, progressive vaccinia is a severe complication of smallpox vaccination seen in patients with impaired cell-mediated immunity. It also is known as vaccinia gangrenosum or vaccinia necrosum. These patients develop expanding ulcers due to exaggerated viral replication and cell-to-cell spread of the vaccinia virus.10,11 Hematogenous spread may result in viral implantation at distant sites of the body. This disease slowly progresses over weeks to months, and it often is resistant to treatment and fatal in patients with severe T-cell deficiency.10
Acral papulovesicular eruption is a distinct cutaneous adverse event following smallpox vaccination. Although further research is needed to discern the pathogenesis of this reaction, it is benign and self-limited, and patients have fully recovered with supportive care. In addition, a modified vaccinia Ankara vaccine (Bavarian Nordic) was approved by the US Food and Drug Administration in 2019.12,13 It is a nonreplicating attenuated viral vaccine that had fewer adverse events compared to ACAM2000 in clinical trials.13 To date, papulovesicular eruptions have not been reported following vaccination with the modified vaccinia Ankara vaccine; however, continued monitoring will help to further characterize any cutaneous reactions to this newer vaccine.
- Nalca A, Zumbrun EE. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des Devel Ther. 2010;4:71-79.
- Monath TP, Caldwell JR, Mundt W, et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain)—a second-generation smallpox vaccine for biological defense. Int J Infect Dis. 2004;8:S31-S44.
- Thomas TN, Reef S, Neff L, et al. A review of the smallpox vaccine adverse events active surveillance system. Clin Infect Dis. 2008;46:S212-S220.
- Beachkofsky TM, Carrizales SC, Bidinger JJ, et al. Adverse events following smallpox vaccination with ACAM2000 in a military population. Arch Dermatol. 2010;146:656-661.
- Freeman R, Lenz B. Cutaneous reactions associated with ACAM2000 smallpox vaccination in a deploying U.S. Army unit. Mil Med. 2015;180:E152-E156.
- Centers for Disease Control and Prevention. Progressive vaccinia in a military smallpox vaccinee—United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Kramer TR. Post–smallpox vaccination skin eruption in a marine. Mil Med. 2018;183:E649-E653.
- Gaertner EM, Groo S, Kim J. Papular spongiotic dermatitis of smallpox vaccination: report of 2 cases with review of the literature. Arch Pathol Lab Med. 2004;128:1173-1175.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part I. background, vaccination technique, normal vaccination and revaccination, and expected normal reactions. Clin Infect Dis. 2003;37:241-250.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Bray M. Understanding smallpox vaccination. J Infect Dis. 2011;203:1037-1039.
- Greenberg RN, Hay CM, Stapleton JT, et al. A randomized, double-blind, placebo-controlled phase II trial investigating the safety and immunogenicity of modified vaccinia ankara smallpox vaccine (MVA-BN®) in 56-80-year-old subjects. PLoS One. 2016;11:E0157335.
- Pittman PR, Hahn M, Lee HS, et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N Engl J Med. 2019;381:1897-1908.
Following the attacks of September 11, 2001, heightened concerns over bioterrorism and the potential use of smallpox as a biological weapon made smallpox vaccination a critical component of military readiness. Therefore, the US Military resumed its smallpox vaccination program in 2002 using the first-generation smallpox vaccine (Dryvax, Wyeth Pharmaceuticals), a live vaccinia virus vaccine created in the late 19th century. This vaccine was developed by pooling vaccinia strains from the skin of infected cows1 and had previously been used during the worldwide vaccination campaign in the 1970s. Dryvax was associated with various cardiac and cutaneous complications, from benign hypersensitivity reactions to life-threatening eczema vaccinatum and progressive vaccinia.
Due to concerns that the remaining supply of Dryvax was insufficient to vaccinate the US population in the case of a bioterrorism attack, investigators developed the second-generation smallpox vaccine (ACAM2000, Sanofi Pasteur Biologics Co) using advances in vaccine technology.2 ACAM2000 is a plaque-purified isolate of vaccinia virus propagated in cell culture, thereby reducing contaminants and lot-to-lot variation.1 Clinical trials demonstrated comparable immunogenicity and frequency of adverse events compared with Dryvax,2 and ACAM2000 replaced Dryvax in 2008. However, these trials focused on serious adverse events, such as cardiac complications and postvaccinal encephalitis, with less specific characterization and description of cutaneous eruptions.3
Since 2008, there have been few reports of cutaneous adverse reactions following vaccination with ACAM2000. Beachkofsky et al4 described 7 cases of papulovesicular eruptions and 1 case of generalized vaccinia. Freeman and Lenz5 described 4 cases of papulovesicular eruptions, and there has been 1 case of progressive vaccinia reported in a soldier with newly diagnosed acute myelogenous leukemia.6 Kramer7 described a patient with multiple vesiculopustular lesions secondary to autoinoculation. The distinct pruritic acral papulovesicular eruptions following ACAM2000 vaccination have occurred in healthy military service members at different locations since the introduction of ACAM2000. We describe an additional case of this unique cutaneous eruption, followed by a review of previously described cutaneous adverse events associated with smallpox vaccination.
Case Report
A 21-year-old female soldier who was otherwise healthy presented to the dermatology clinic with a pruritic papular eruption involving the upper and lower extremities of 1 week’s duration. The lesions first appeared 8 days after she received the ACAM2000 vaccine. She received no other concurrent vaccines, had no history of atopic dermatitis, and had no systemic symptoms. Physical examination revealed numerous erythematous indurated papules involving the dorsolateral hands and fingers, as well as the extensor surfaces of the elbows, knees, and thighs (Figures 1 and 2). Based on the clinical presentation, the differential diagnosis included lichen planus, verruca plana, dyshidrotic eczema, and smallpox vaccine reaction. Erythema multiforme was considered; however, the absence of palmoplantar involvement and typical targetoid lesions made this diagnosis less likely.

Biopsies of lesions on the arm and thigh were performed. Histologic findings revealed interface and spongiotic dermatitis with scattered necrotic keratinocytes and extravasated erythrocytes (Figure 3). There was no evidence of viral cytopathic effects. Similar clinical and histologic findings have been reported in the literature as acral papulovesicular eruptions following smallpox vaccination or papular spongiotic dermatitis of smallpox vaccination.8 The presence of eosinophils was not conspicuous in the current case and was only a notable finding in 1 of 2 cases previously described by Gaertner et al.8 This may simply be due to an idiosyncratic drug reaction. Furthermore, in the cases described by Beachkofsky et al,4 there were essentially 2 histologic groups. The first group demonstrated a dermal hypersensitivity-type reaction, and the second group demonstrated a lymphocytic capillaritis.

Based on these findings, the patient was diagnosed with an acral papulovesicular eruption following smallpox vaccination. Of note, the patient’s presentation was not consistent with other described smallpox vaccine reactions, which included eczema vaccinatum, autoinoculation, generalized vaccinia, and progressive vaccinia. The patient was treated supportively with triamcinolone acetonide cream 0.1%, cool compresses, and oral diphenhydramine as needed for pruritus. The lesions notably improved within the first week of treatment.

Comment
Reported cases of acral papulovesicular eruption4-6 demonstrated an onset of cutaneous symptoms an average of 14 days following vaccination (range, 8–18 days postvaccination). Lesions were benign and self-limited in all cases, with resolution within an average of 25 days (range, 7–71 days). All patients were active-duty military adults with a mean age of 24 years. Supportive treatment varied from topical steroids and oral antihistamines to tapering oral prednisone doses. Of note, all previously reported cases of this reaction occurred in patients who also had received other concurrent or near-concurrent vaccines, including anthrax, hepatitis B, influenza, and typhoid. Our patient represents a unique case of a papulovesicular eruption following smallpox vaccination with no history of concurrent vaccines.
Since the 1970s, smallpox vaccination has been associated with numerous cutaneous reactions, most of which have been reported with the first-generation Dryvax. Minor local reactions occurred in approximately 2% to 6% of vaccinees in clinical trials.9 These reactions included local edema involving the upper arm, satellite lesions within 2.5 cm of the vaccination site, local lymphadenopathy, intense inflammation or viral cellulitis surrounding the inoculation site, and viral lymphangitis tracking to axillary lymph nodes. In clinical trials, these reactions were self-limited and required only symptomatic treatment.9
Autoinoculation is another cutaneous reaction that can occur because Dryvax and ACAM2000 both contain live-attenuated replicating vaccinia virus. Accidental implantation may occur when the high titers of virus present at the vaccine site are subsequently transferred to other sites, especially abnormal mucosa or skin, resulting in an additional primary inoculation site.10
Eczema vaccinatum is a potentially life-threatening reaction that may occur in patients with disruptive skin disorders, such as atopic dermatitis. These patients are at risk for massive confluent vaccinia infection of the skin.10 In patients with atopic dermatitis, the virus rapidly disseminates due to both skin barrier dysfunction and impaired immunomodulation, resulting in large confluent skin lesions and the potential for viremia, septic shock, and death.10,11 Mortality from eczema vaccinatum may be reduced by administration of vaccinia immune globulin.10
The vaccinia virus also may spread hematogenously in healthy individuals,10 resulting in a benign reaction called generalized vaccinia. These patients develop pustules on areas of the skin other than the vaccination site. Although typically benign and self-limited, Beachkofsky et al4 described a case of generalized vaccinia in a healthy 34-year-old man resulting in a rapidly progressive vesiculopustular eruption with associated fever and pancytopenia. The patient made a complete recovery over the course of the following month.4
Alternatively, progressive vaccinia is a severe complication of smallpox vaccination seen in patients with impaired cell-mediated immunity. It also is known as vaccinia gangrenosum or vaccinia necrosum. These patients develop expanding ulcers due to exaggerated viral replication and cell-to-cell spread of the vaccinia virus.10,11 Hematogenous spread may result in viral implantation at distant sites of the body. This disease slowly progresses over weeks to months, and it often is resistant to treatment and fatal in patients with severe T-cell deficiency.10
Acral papulovesicular eruption is a distinct cutaneous adverse event following smallpox vaccination. Although further research is needed to discern the pathogenesis of this reaction, it is benign and self-limited, and patients have fully recovered with supportive care. In addition, a modified vaccinia Ankara vaccine (Bavarian Nordic) was approved by the US Food and Drug Administration in 2019.12,13 It is a nonreplicating attenuated viral vaccine that had fewer adverse events compared to ACAM2000 in clinical trials.13 To date, papulovesicular eruptions have not been reported following vaccination with the modified vaccinia Ankara vaccine; however, continued monitoring will help to further characterize any cutaneous reactions to this newer vaccine.
Following the attacks of September 11, 2001, heightened concerns over bioterrorism and the potential use of smallpox as a biological weapon made smallpox vaccination a critical component of military readiness. Therefore, the US Military resumed its smallpox vaccination program in 2002 using the first-generation smallpox vaccine (Dryvax, Wyeth Pharmaceuticals), a live vaccinia virus vaccine created in the late 19th century. This vaccine was developed by pooling vaccinia strains from the skin of infected cows1 and had previously been used during the worldwide vaccination campaign in the 1970s. Dryvax was associated with various cardiac and cutaneous complications, from benign hypersensitivity reactions to life-threatening eczema vaccinatum and progressive vaccinia.
Due to concerns that the remaining supply of Dryvax was insufficient to vaccinate the US population in the case of a bioterrorism attack, investigators developed the second-generation smallpox vaccine (ACAM2000, Sanofi Pasteur Biologics Co) using advances in vaccine technology.2 ACAM2000 is a plaque-purified isolate of vaccinia virus propagated in cell culture, thereby reducing contaminants and lot-to-lot variation.1 Clinical trials demonstrated comparable immunogenicity and frequency of adverse events compared with Dryvax,2 and ACAM2000 replaced Dryvax in 2008. However, these trials focused on serious adverse events, such as cardiac complications and postvaccinal encephalitis, with less specific characterization and description of cutaneous eruptions.3
Since 2008, there have been few reports of cutaneous adverse reactions following vaccination with ACAM2000. Beachkofsky et al4 described 7 cases of papulovesicular eruptions and 1 case of generalized vaccinia. Freeman and Lenz5 described 4 cases of papulovesicular eruptions, and there has been 1 case of progressive vaccinia reported in a soldier with newly diagnosed acute myelogenous leukemia.6 Kramer7 described a patient with multiple vesiculopustular lesions secondary to autoinoculation. The distinct pruritic acral papulovesicular eruptions following ACAM2000 vaccination have occurred in healthy military service members at different locations since the introduction of ACAM2000. We describe an additional case of this unique cutaneous eruption, followed by a review of previously described cutaneous adverse events associated with smallpox vaccination.
Case Report
A 21-year-old female soldier who was otherwise healthy presented to the dermatology clinic with a pruritic papular eruption involving the upper and lower extremities of 1 week’s duration. The lesions first appeared 8 days after she received the ACAM2000 vaccine. She received no other concurrent vaccines, had no history of atopic dermatitis, and had no systemic symptoms. Physical examination revealed numerous erythematous indurated papules involving the dorsolateral hands and fingers, as well as the extensor surfaces of the elbows, knees, and thighs (Figures 1 and 2). Based on the clinical presentation, the differential diagnosis included lichen planus, verruca plana, dyshidrotic eczema, and smallpox vaccine reaction. Erythema multiforme was considered; however, the absence of palmoplantar involvement and typical targetoid lesions made this diagnosis less likely.

Biopsies of lesions on the arm and thigh were performed. Histologic findings revealed interface and spongiotic dermatitis with scattered necrotic keratinocytes and extravasated erythrocytes (Figure 3). There was no evidence of viral cytopathic effects. Similar clinical and histologic findings have been reported in the literature as acral papulovesicular eruptions following smallpox vaccination or papular spongiotic dermatitis of smallpox vaccination.8 The presence of eosinophils was not conspicuous in the current case and was only a notable finding in 1 of 2 cases previously described by Gaertner et al.8 This may simply be due to an idiosyncratic drug reaction. Furthermore, in the cases described by Beachkofsky et al,4 there were essentially 2 histologic groups. The first group demonstrated a dermal hypersensitivity-type reaction, and the second group demonstrated a lymphocytic capillaritis.

Based on these findings, the patient was diagnosed with an acral papulovesicular eruption following smallpox vaccination. Of note, the patient’s presentation was not consistent with other described smallpox vaccine reactions, which included eczema vaccinatum, autoinoculation, generalized vaccinia, and progressive vaccinia. The patient was treated supportively with triamcinolone acetonide cream 0.1%, cool compresses, and oral diphenhydramine as needed for pruritus. The lesions notably improved within the first week of treatment.

Comment
Reported cases of acral papulovesicular eruption4-6 demonstrated an onset of cutaneous symptoms an average of 14 days following vaccination (range, 8–18 days postvaccination). Lesions were benign and self-limited in all cases, with resolution within an average of 25 days (range, 7–71 days). All patients were active-duty military adults with a mean age of 24 years. Supportive treatment varied from topical steroids and oral antihistamines to tapering oral prednisone doses. Of note, all previously reported cases of this reaction occurred in patients who also had received other concurrent or near-concurrent vaccines, including anthrax, hepatitis B, influenza, and typhoid. Our patient represents a unique case of a papulovesicular eruption following smallpox vaccination with no history of concurrent vaccines.
Since the 1970s, smallpox vaccination has been associated with numerous cutaneous reactions, most of which have been reported with the first-generation Dryvax. Minor local reactions occurred in approximately 2% to 6% of vaccinees in clinical trials.9 These reactions included local edema involving the upper arm, satellite lesions within 2.5 cm of the vaccination site, local lymphadenopathy, intense inflammation or viral cellulitis surrounding the inoculation site, and viral lymphangitis tracking to axillary lymph nodes. In clinical trials, these reactions were self-limited and required only symptomatic treatment.9
Autoinoculation is another cutaneous reaction that can occur because Dryvax and ACAM2000 both contain live-attenuated replicating vaccinia virus. Accidental implantation may occur when the high titers of virus present at the vaccine site are subsequently transferred to other sites, especially abnormal mucosa or skin, resulting in an additional primary inoculation site.10
Eczema vaccinatum is a potentially life-threatening reaction that may occur in patients with disruptive skin disorders, such as atopic dermatitis. These patients are at risk for massive confluent vaccinia infection of the skin.10 In patients with atopic dermatitis, the virus rapidly disseminates due to both skin barrier dysfunction and impaired immunomodulation, resulting in large confluent skin lesions and the potential for viremia, septic shock, and death.10,11 Mortality from eczema vaccinatum may be reduced by administration of vaccinia immune globulin.10
The vaccinia virus also may spread hematogenously in healthy individuals,10 resulting in a benign reaction called generalized vaccinia. These patients develop pustules on areas of the skin other than the vaccination site. Although typically benign and self-limited, Beachkofsky et al4 described a case of generalized vaccinia in a healthy 34-year-old man resulting in a rapidly progressive vesiculopustular eruption with associated fever and pancytopenia. The patient made a complete recovery over the course of the following month.4
Alternatively, progressive vaccinia is a severe complication of smallpox vaccination seen in patients with impaired cell-mediated immunity. It also is known as vaccinia gangrenosum or vaccinia necrosum. These patients develop expanding ulcers due to exaggerated viral replication and cell-to-cell spread of the vaccinia virus.10,11 Hematogenous spread may result in viral implantation at distant sites of the body. This disease slowly progresses over weeks to months, and it often is resistant to treatment and fatal in patients with severe T-cell deficiency.10
Acral papulovesicular eruption is a distinct cutaneous adverse event following smallpox vaccination. Although further research is needed to discern the pathogenesis of this reaction, it is benign and self-limited, and patients have fully recovered with supportive care. In addition, a modified vaccinia Ankara vaccine (Bavarian Nordic) was approved by the US Food and Drug Administration in 2019.12,13 It is a nonreplicating attenuated viral vaccine that had fewer adverse events compared to ACAM2000 in clinical trials.13 To date, papulovesicular eruptions have not been reported following vaccination with the modified vaccinia Ankara vaccine; however, continued monitoring will help to further characterize any cutaneous reactions to this newer vaccine.
- Nalca A, Zumbrun EE. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des Devel Ther. 2010;4:71-79.
- Monath TP, Caldwell JR, Mundt W, et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain)—a second-generation smallpox vaccine for biological defense. Int J Infect Dis. 2004;8:S31-S44.
- Thomas TN, Reef S, Neff L, et al. A review of the smallpox vaccine adverse events active surveillance system. Clin Infect Dis. 2008;46:S212-S220.
- Beachkofsky TM, Carrizales SC, Bidinger JJ, et al. Adverse events following smallpox vaccination with ACAM2000 in a military population. Arch Dermatol. 2010;146:656-661.
- Freeman R, Lenz B. Cutaneous reactions associated with ACAM2000 smallpox vaccination in a deploying U.S. Army unit. Mil Med. 2015;180:E152-E156.
- Centers for Disease Control and Prevention. Progressive vaccinia in a military smallpox vaccinee—United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Kramer TR. Post–smallpox vaccination skin eruption in a marine. Mil Med. 2018;183:E649-E653.
- Gaertner EM, Groo S, Kim J. Papular spongiotic dermatitis of smallpox vaccination: report of 2 cases with review of the literature. Arch Pathol Lab Med. 2004;128:1173-1175.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part I. background, vaccination technique, normal vaccination and revaccination, and expected normal reactions. Clin Infect Dis. 2003;37:241-250.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Bray M. Understanding smallpox vaccination. J Infect Dis. 2011;203:1037-1039.
- Greenberg RN, Hay CM, Stapleton JT, et al. A randomized, double-blind, placebo-controlled phase II trial investigating the safety and immunogenicity of modified vaccinia ankara smallpox vaccine (MVA-BN®) in 56-80-year-old subjects. PLoS One. 2016;11:E0157335.
- Pittman PR, Hahn M, Lee HS, et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N Engl J Med. 2019;381:1897-1908.
- Nalca A, Zumbrun EE. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des Devel Ther. 2010;4:71-79.
- Monath TP, Caldwell JR, Mundt W, et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain)—a second-generation smallpox vaccine for biological defense. Int J Infect Dis. 2004;8:S31-S44.
- Thomas TN, Reef S, Neff L, et al. A review of the smallpox vaccine adverse events active surveillance system. Clin Infect Dis. 2008;46:S212-S220.
- Beachkofsky TM, Carrizales SC, Bidinger JJ, et al. Adverse events following smallpox vaccination with ACAM2000 in a military population. Arch Dermatol. 2010;146:656-661.
- Freeman R, Lenz B. Cutaneous reactions associated with ACAM2000 smallpox vaccination in a deploying U.S. Army unit. Mil Med. 2015;180:E152-E156.
- Centers for Disease Control and Prevention. Progressive vaccinia in a military smallpox vaccinee—United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Kramer TR. Post–smallpox vaccination skin eruption in a marine. Mil Med. 2018;183:E649-E653.
- Gaertner EM, Groo S, Kim J. Papular spongiotic dermatitis of smallpox vaccination: report of 2 cases with review of the literature. Arch Pathol Lab Med. 2004;128:1173-1175.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part I. background, vaccination technique, normal vaccination and revaccination, and expected normal reactions. Clin Infect Dis. 2003;37:241-250.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Bray M. Understanding smallpox vaccination. J Infect Dis. 2011;203:1037-1039.
- Greenberg RN, Hay CM, Stapleton JT, et al. A randomized, double-blind, placebo-controlled phase II trial investigating the safety and immunogenicity of modified vaccinia ankara smallpox vaccine (MVA-BN®) in 56-80-year-old subjects. PLoS One. 2016;11:E0157335.
- Pittman PR, Hahn M, Lee HS, et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N Engl J Med. 2019;381:1897-1908.
Practice Points
- There are several potential cutaneous adverse reactions associated with smallpox vaccination, ranging from benign self-limited hypersensitivity reactions to life-threatening eczema vaccinatum and progressive vaccinia.
- Acral papulovesicular eruption is a distinct presentation that has been described in the US Military following vaccination with the second-generation live smallpox vaccine (ACAM2000).
Vesicular Eruption Secondary to Bites by Larval Amblyomma americanum
Case Report
A 58-year-old woman presented to the dermatology office with a widespread pruritic eruption of 3 days’ duration that started in the groin and spread to the rest of the body. No treatments had been attempted. She had no notable medical history, and she denied any recent illness, change in personal care products, or new medications or supplements. She reported a camping trip 2 weeks prior to presentation on the east end of Long Island, New York. She later learned that others on the same trip developed a similar, albeit less widespread, eruption.
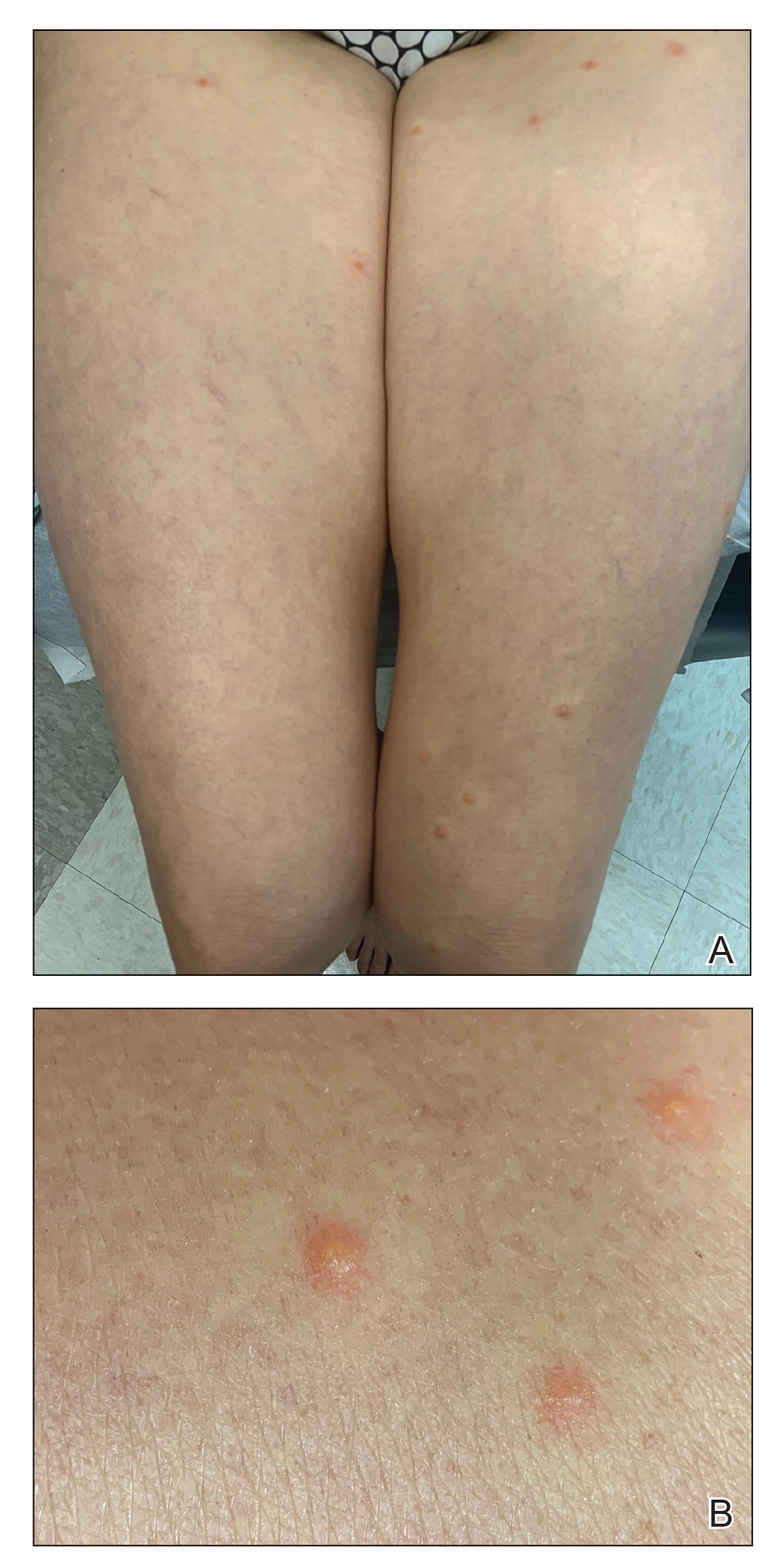
Physical examination revealed clear vesicles on the arms, legs, trunk, and pubic area (Figure 1). Dermoscopy revealed a small lone star tick larva in the center of one of the vesicles (Figure 2). The type of tick larva was identified using resources from the Centers for Disease Control and Prevention (Figure 3).1 Careful inspection revealed dark marks on various vesicles, mostly in the perineum, yielding nearly 20 larvae, which were removed with forceps. The patient was counseled to cover herself in petrolatum for 2 to 3 hours with the hope of smothering any remaining tick larvae. She was given triamcinolone cream and was encouraged to take a nonsedating antihistamine for itch. The patient was seen back in clinic 2 weeks later and the eruption had resolved.
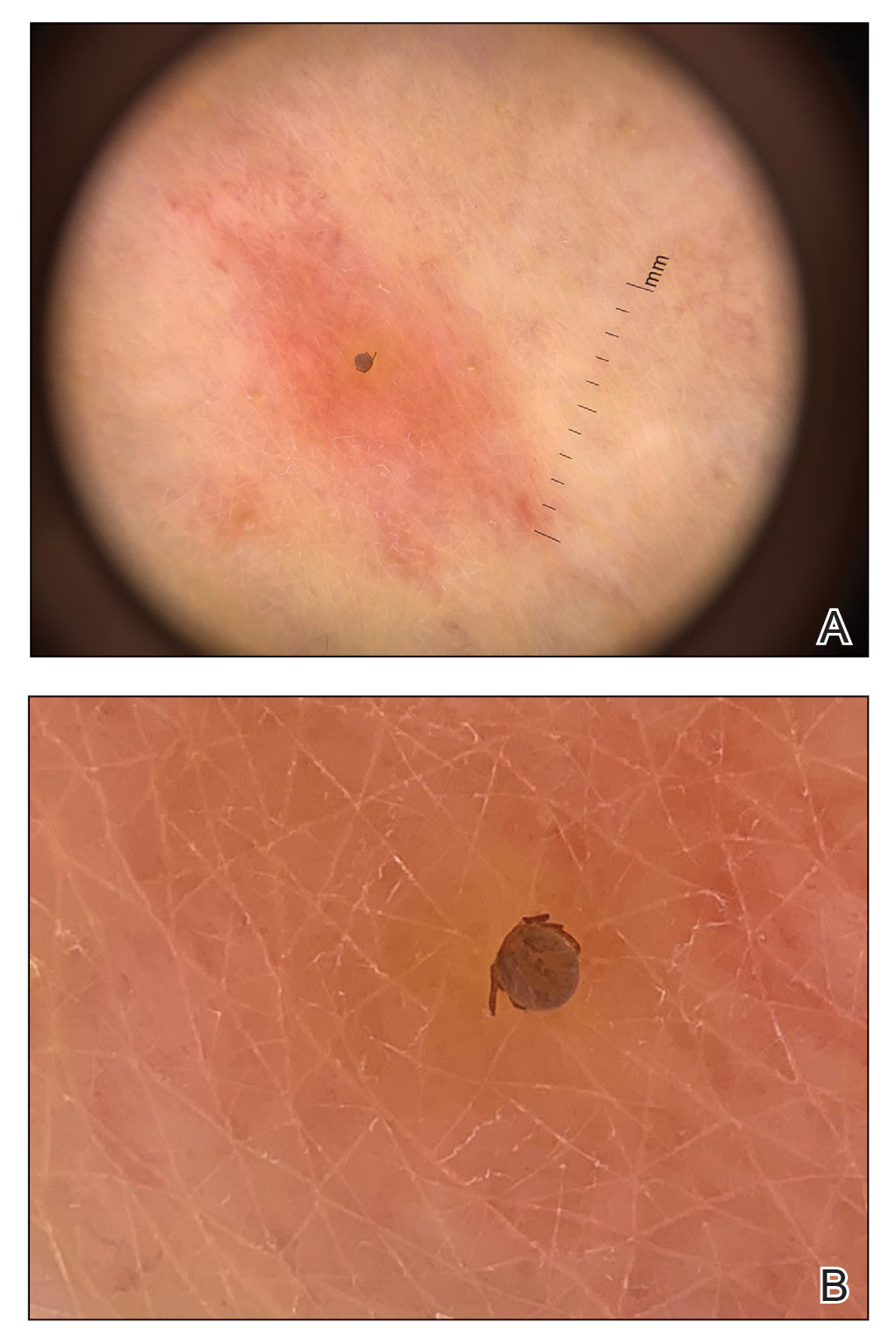
Comment
Spread of Tick-Borne Disease—Ticks and tick-borne disease are increasing major health concerns for humans, domesticated animals, and livestock. Reported cases of bacterial and protozoan tick-borne disease doubled in the United States between 2004 and 2016. Ninety percent of the nearly 60,000 cases of nationally notifiable vector-borne diseases reported in 2017 were linked to ticks.2 Geographic ranges of multiple tick species continue to expand, which is thought to be secondary to rising global temperatures, ecologic changes, reforestation, and increases in commerce and travel (Figure 4).3 Not only have warming temperatures contributed to geographic range expansion, they also may extend ticks’ active season. The lone star tick (Amblyomma americanum) is widely distributed throughout much of the eastern United States.4 The range of A americanum has expanded north in recent years from its prior core range in the southeastern United States.2 One study found that from 2006 to 2016, the vector tick species most commonly collected from humans and submitted to a tick surveillance system in New Jersey shifted from Ixodes scapularis to A americanum.5
Bites by Amblyomma Ticks—As with most hard ticks, the life cycle of A americanum lasts 2 years and includes the egg, the 6-legged larva or “seed tick,” the 8-legged immature nymph, and the 8-legged reproductively mature adult (Figure 3). Amblyomma americanum can lay several thousand eggs.2 Because our patient had numerous bites, it is plausible that she came into contact with a nest of newly hatched tick larvae. Morphogenesis from larva to nymph, then nymph to adult, requires a blood meal.6,7 The larvae emerge from eggs deposited on the ground and then crawl up low vegetation where they can easily attach to passing hosts. The tick clings to hair or clothing and waits until the host is at rest before moving to a favorable location and then bites.8 When attaching, ticks inject an anesthetic akin to lidocaine, making the bite painless. A tick may spend up to 24 hours on the host prior to biting and then feed for 2 hours to 7 days before releasing.9 For the majority of tick-borne illnesses, the tick must remain attached for 24 to 48 hours before disease is transmitted.10
All stages of
Even when the ticks do not transmit disease, tick bites can cause impressive local reactions. Uncomplicated bites can be painful and leave a puncture wound that can take 1 to 2 weeks to heal.13 Rarely, bites can cause a delayed hypersensitivity reaction including fever, pruritus, and urticaria. Granulomas can develop if a tick is improperly removed.9 Other reports describe prurigo lesions, skin hemorrhage, papular urticaria, diffuse papules, vesicles and bullae, necrotic ulcers, and patchy alopecia.14,15 A 2015 systematic controlled study of human bite reactions from A americanum demonstrated the development of itchy erythematous papules and vesicles within 48 hours of larval tick attachment to research participants. The study found tissue damage from A americanum mouthparts, and degranulating mast cells may be evident in as little as 15 minutes.16 The severity of individual skin reaction is hypothesized to depend on several variables, such as the duration of feeding, size of mouthparts, type of tick secretions, changes in secretions during feeding, and prior exposures of the host.14
Tick Removal—If patients present to clinic with ticks attached, removal can be challenging. Removal recommendations call for use of blunt forceps or tweezers. Ticks should be grasped near the skin with consistent pressure, and the tick should be pulled straight out, perpendicular to the skin. Twisting motions can cause the head to separate from the body and remain in the bite wound. Immediately following removal, the area should be cleansed with a disinfectant.10,17 After the tick is removed, some studies recommend storing the tick at −20 °C; should the patient develop disease, the tick could be sent for evaluation.6,17 If there is no clinical or serologic evidence of infection, testing for the presence of antibodies against tick-borne bacteria at presentation and at 3 and 6 weeks is not recommended due to low sensitivity, low positive predictive value, and cost. Clinicians must only observe and treat if disease occurs.17
Prevention of Tick Bites—Tick bites are best prevented by avoiding tick-infested areas; when these areas are unavoidable, tick bites may be prevented by wearing long pants with the pant legs tucked into boots. In addition, applying topical DEET (N,N-diethyl-m-toluamide) repellent to exposed skin and treating clothing with permethrin can be helpful.17 When used alone, DEET provides greater than 90% protection for up to 2.7 hours against A americanum.18 Permethrin-treated clothing alone is 79% to 100% effective at killing A americanum ticks or disabling them for several hours.19
Conclusion
Tick-borne illness is an increasingly important cause of human infectious disease. In addition to their role as a disease vector, ticks can produce primary skin disorders. This case posed a diagnostic challenge because of the unusually large number and wide distribution of bites as well as the subsequent vesicular reaction that ensued. It is important to keep tick larvae or adult tick bites in the differential when evaluating a patient to expedite tick removal and begin clinical monitoring. Recognition of A americanum larvae as a potential cause of pruritic papules may be helpful in similar cases. In addition, it is important for dermatologists to be aware of the tick species in their area.
- Centers for Disease Control and Prevention. Tick ID. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tickID.html
- Molaei G, Little EAH, Williams SC, et al. Bracing for the worst—range expansion of the lone star tick in the northeastern United States. N Engl J Med. 2019;381:2189-2192.
- Centers for Disease Control and Prevention, Division of Vector-Borne Diseases. Lone star tick (Amblyomma americanum). Accessed March 23, 2022. https://www.cdc.gov/ticks/maps/lone_star_tick.pdf
- Reynolds HH, Elston DM. What’s eating you? lone star tick (Amblyomma americanum). Cutis. 2017;99:111-114.
- Jordan RA, Egizi A. The growing importance of lone star ticks in a Lyme disease endemic county: passive tick surveillance in Monmouth County, NJ, 2006–2016. PLoS One. 2019;14:E0211778.
- Singh-Behl D, La Rosa SP, Tomecki KJ. Tick-borne infections. Dermatol Clin. 2003;21:237-244, v.
- Spach DH, Liles WC, Campbell GL, et al. Tick-borne diseases in the United States. N Engl J Med. 1993;329:936-947.
- Duckworth PF Jr, Hayden GF, Reed CN. Human infestation by Amblyomma americanum larvae (“seed ticks”). South Med J. 1985;78:751-753.
- Middleton DB. Tick-borne infections. what starts as a tiny bite may have a serious outcome. Postgrad Med. 1994;95:131-139.
- Moody EK, Barker RW, White JL, et al. Ticks and tick-borne diseases in Oklahoma. J Okla State Med Assoc. 1998;91:438-445.
- Jones BE. Human ‘seed tick’ infestation. Amblyomma americanum larvae. Arch Dermatol. 1981;117:812-814.
- Centers for Disease Control and Prevention. Tick bite prophylaxis. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tick-bite-prophylaxis.html
- Fisher EJ, Mo J, Lucky AW. Multiple pruritic papules from lone star tick larvae bites. Arch Dermatol. 2006;142:491-494.
- Krinsky WL. Dermatoses associated with the bites of mites and ticks (Arthropoda: Acari). Int J Dermatol. 1983;22:75-91.
- Yesudian P, Thambiah AS. Persistent papules after tick-bites. Dermatologica. 1973;147:214-218.
- Goddard J, Portugal JS. Cutaneous lesions due to bites by larval Amblyomma americanum ticks. JAMA Dermatol. 2015;151:1373-1375.
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928.
- Solberg VB, Klein TA, McPherson KR, et al. Field evaluation of DEET and a piperidine repellent (AI3-37220) against Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 1995;32:870-875.
- Evans SR, Korch GW Jr, Lawson MA. Comparative field evaluation of permethrin and DEET-treated military uniforms for personal protection against ticks (Acari). J Med Entomol. 1990;27:829-834.
Case Report
A 58-year-old woman presented to the dermatology office with a widespread pruritic eruption of 3 days’ duration that started in the groin and spread to the rest of the body. No treatments had been attempted. She had no notable medical history, and she denied any recent illness, change in personal care products, or new medications or supplements. She reported a camping trip 2 weeks prior to presentation on the east end of Long Island, New York. She later learned that others on the same trip developed a similar, albeit less widespread, eruption.

Physical examination revealed clear vesicles on the arms, legs, trunk, and pubic area (Figure 1). Dermoscopy revealed a small lone star tick larva in the center of one of the vesicles (Figure 2). The type of tick larva was identified using resources from the Centers for Disease Control and Prevention (Figure 3).1 Careful inspection revealed dark marks on various vesicles, mostly in the perineum, yielding nearly 20 larvae, which were removed with forceps. The patient was counseled to cover herself in petrolatum for 2 to 3 hours with the hope of smothering any remaining tick larvae. She was given triamcinolone cream and was encouraged to take a nonsedating antihistamine for itch. The patient was seen back in clinic 2 weeks later and the eruption had resolved.

Comment
Spread of Tick-Borne Disease—Ticks and tick-borne disease are increasing major health concerns for humans, domesticated animals, and livestock. Reported cases of bacterial and protozoan tick-borne disease doubled in the United States between 2004 and 2016. Ninety percent of the nearly 60,000 cases of nationally notifiable vector-borne diseases reported in 2017 were linked to ticks.2 Geographic ranges of multiple tick species continue to expand, which is thought to be secondary to rising global temperatures, ecologic changes, reforestation, and increases in commerce and travel (Figure 4).3 Not only have warming temperatures contributed to geographic range expansion, they also may extend ticks’ active season. The lone star tick (Amblyomma americanum) is widely distributed throughout much of the eastern United States.4 The range of A americanum has expanded north in recent years from its prior core range in the southeastern United States.2 One study found that from 2006 to 2016, the vector tick species most commonly collected from humans and submitted to a tick surveillance system in New Jersey shifted from Ixodes scapularis to A americanum.5

Bites by Amblyomma Ticks—As with most hard ticks, the life cycle of A americanum lasts 2 years and includes the egg, the 6-legged larva or “seed tick,” the 8-legged immature nymph, and the 8-legged reproductively mature adult (Figure 3). Amblyomma americanum can lay several thousand eggs.2 Because our patient had numerous bites, it is plausible that she came into contact with a nest of newly hatched tick larvae. Morphogenesis from larva to nymph, then nymph to adult, requires a blood meal.6,7 The larvae emerge from eggs deposited on the ground and then crawl up low vegetation where they can easily attach to passing hosts. The tick clings to hair or clothing and waits until the host is at rest before moving to a favorable location and then bites.8 When attaching, ticks inject an anesthetic akin to lidocaine, making the bite painless. A tick may spend up to 24 hours on the host prior to biting and then feed for 2 hours to 7 days before releasing.9 For the majority of tick-borne illnesses, the tick must remain attached for 24 to 48 hours before disease is transmitted.10

All stages of
Even when the ticks do not transmit disease, tick bites can cause impressive local reactions. Uncomplicated bites can be painful and leave a puncture wound that can take 1 to 2 weeks to heal.13 Rarely, bites can cause a delayed hypersensitivity reaction including fever, pruritus, and urticaria. Granulomas can develop if a tick is improperly removed.9 Other reports describe prurigo lesions, skin hemorrhage, papular urticaria, diffuse papules, vesicles and bullae, necrotic ulcers, and patchy alopecia.14,15 A 2015 systematic controlled study of human bite reactions from A americanum demonstrated the development of itchy erythematous papules and vesicles within 48 hours of larval tick attachment to research participants. The study found tissue damage from A americanum mouthparts, and degranulating mast cells may be evident in as little as 15 minutes.16 The severity of individual skin reaction is hypothesized to depend on several variables, such as the duration of feeding, size of mouthparts, type of tick secretions, changes in secretions during feeding, and prior exposures of the host.14
Tick Removal—If patients present to clinic with ticks attached, removal can be challenging. Removal recommendations call for use of blunt forceps or tweezers. Ticks should be grasped near the skin with consistent pressure, and the tick should be pulled straight out, perpendicular to the skin. Twisting motions can cause the head to separate from the body and remain in the bite wound. Immediately following removal, the area should be cleansed with a disinfectant.10,17 After the tick is removed, some studies recommend storing the tick at −20 °C; should the patient develop disease, the tick could be sent for evaluation.6,17 If there is no clinical or serologic evidence of infection, testing for the presence of antibodies against tick-borne bacteria at presentation and at 3 and 6 weeks is not recommended due to low sensitivity, low positive predictive value, and cost. Clinicians must only observe and treat if disease occurs.17
Prevention of Tick Bites—Tick bites are best prevented by avoiding tick-infested areas; when these areas are unavoidable, tick bites may be prevented by wearing long pants with the pant legs tucked into boots. In addition, applying topical DEET (N,N-diethyl-m-toluamide) repellent to exposed skin and treating clothing with permethrin can be helpful.17 When used alone, DEET provides greater than 90% protection for up to 2.7 hours against A americanum.18 Permethrin-treated clothing alone is 79% to 100% effective at killing A americanum ticks or disabling them for several hours.19
Conclusion
Tick-borne illness is an increasingly important cause of human infectious disease. In addition to their role as a disease vector, ticks can produce primary skin disorders. This case posed a diagnostic challenge because of the unusually large number and wide distribution of bites as well as the subsequent vesicular reaction that ensued. It is important to keep tick larvae or adult tick bites in the differential when evaluating a patient to expedite tick removal and begin clinical monitoring. Recognition of A americanum larvae as a potential cause of pruritic papules may be helpful in similar cases. In addition, it is important for dermatologists to be aware of the tick species in their area.
Case Report
A 58-year-old woman presented to the dermatology office with a widespread pruritic eruption of 3 days’ duration that started in the groin and spread to the rest of the body. No treatments had been attempted. She had no notable medical history, and she denied any recent illness, change in personal care products, or new medications or supplements. She reported a camping trip 2 weeks prior to presentation on the east end of Long Island, New York. She later learned that others on the same trip developed a similar, albeit less widespread, eruption.

Physical examination revealed clear vesicles on the arms, legs, trunk, and pubic area (Figure 1). Dermoscopy revealed a small lone star tick larva in the center of one of the vesicles (Figure 2). The type of tick larva was identified using resources from the Centers for Disease Control and Prevention (Figure 3).1 Careful inspection revealed dark marks on various vesicles, mostly in the perineum, yielding nearly 20 larvae, which were removed with forceps. The patient was counseled to cover herself in petrolatum for 2 to 3 hours with the hope of smothering any remaining tick larvae. She was given triamcinolone cream and was encouraged to take a nonsedating antihistamine for itch. The patient was seen back in clinic 2 weeks later and the eruption had resolved.

Comment
Spread of Tick-Borne Disease—Ticks and tick-borne disease are increasing major health concerns for humans, domesticated animals, and livestock. Reported cases of bacterial and protozoan tick-borne disease doubled in the United States between 2004 and 2016. Ninety percent of the nearly 60,000 cases of nationally notifiable vector-borne diseases reported in 2017 were linked to ticks.2 Geographic ranges of multiple tick species continue to expand, which is thought to be secondary to rising global temperatures, ecologic changes, reforestation, and increases in commerce and travel (Figure 4).3 Not only have warming temperatures contributed to geographic range expansion, they also may extend ticks’ active season. The lone star tick (Amblyomma americanum) is widely distributed throughout much of the eastern United States.4 The range of A americanum has expanded north in recent years from its prior core range in the southeastern United States.2 One study found that from 2006 to 2016, the vector tick species most commonly collected from humans and submitted to a tick surveillance system in New Jersey shifted from Ixodes scapularis to A americanum.5

Bites by Amblyomma Ticks—As with most hard ticks, the life cycle of A americanum lasts 2 years and includes the egg, the 6-legged larva or “seed tick,” the 8-legged immature nymph, and the 8-legged reproductively mature adult (Figure 3). Amblyomma americanum can lay several thousand eggs.2 Because our patient had numerous bites, it is plausible that she came into contact with a nest of newly hatched tick larvae. Morphogenesis from larva to nymph, then nymph to adult, requires a blood meal.6,7 The larvae emerge from eggs deposited on the ground and then crawl up low vegetation where they can easily attach to passing hosts. The tick clings to hair or clothing and waits until the host is at rest before moving to a favorable location and then bites.8 When attaching, ticks inject an anesthetic akin to lidocaine, making the bite painless. A tick may spend up to 24 hours on the host prior to biting and then feed for 2 hours to 7 days before releasing.9 For the majority of tick-borne illnesses, the tick must remain attached for 24 to 48 hours before disease is transmitted.10

All stages of
Even when the ticks do not transmit disease, tick bites can cause impressive local reactions. Uncomplicated bites can be painful and leave a puncture wound that can take 1 to 2 weeks to heal.13 Rarely, bites can cause a delayed hypersensitivity reaction including fever, pruritus, and urticaria. Granulomas can develop if a tick is improperly removed.9 Other reports describe prurigo lesions, skin hemorrhage, papular urticaria, diffuse papules, vesicles and bullae, necrotic ulcers, and patchy alopecia.14,15 A 2015 systematic controlled study of human bite reactions from A americanum demonstrated the development of itchy erythematous papules and vesicles within 48 hours of larval tick attachment to research participants. The study found tissue damage from A americanum mouthparts, and degranulating mast cells may be evident in as little as 15 minutes.16 The severity of individual skin reaction is hypothesized to depend on several variables, such as the duration of feeding, size of mouthparts, type of tick secretions, changes in secretions during feeding, and prior exposures of the host.14
Tick Removal—If patients present to clinic with ticks attached, removal can be challenging. Removal recommendations call for use of blunt forceps or tweezers. Ticks should be grasped near the skin with consistent pressure, and the tick should be pulled straight out, perpendicular to the skin. Twisting motions can cause the head to separate from the body and remain in the bite wound. Immediately following removal, the area should be cleansed with a disinfectant.10,17 After the tick is removed, some studies recommend storing the tick at −20 °C; should the patient develop disease, the tick could be sent for evaluation.6,17 If there is no clinical or serologic evidence of infection, testing for the presence of antibodies against tick-borne bacteria at presentation and at 3 and 6 weeks is not recommended due to low sensitivity, low positive predictive value, and cost. Clinicians must only observe and treat if disease occurs.17
Prevention of Tick Bites—Tick bites are best prevented by avoiding tick-infested areas; when these areas are unavoidable, tick bites may be prevented by wearing long pants with the pant legs tucked into boots. In addition, applying topical DEET (N,N-diethyl-m-toluamide) repellent to exposed skin and treating clothing with permethrin can be helpful.17 When used alone, DEET provides greater than 90% protection for up to 2.7 hours against A americanum.18 Permethrin-treated clothing alone is 79% to 100% effective at killing A americanum ticks or disabling them for several hours.19
Conclusion
Tick-borne illness is an increasingly important cause of human infectious disease. In addition to their role as a disease vector, ticks can produce primary skin disorders. This case posed a diagnostic challenge because of the unusually large number and wide distribution of bites as well as the subsequent vesicular reaction that ensued. It is important to keep tick larvae or adult tick bites in the differential when evaluating a patient to expedite tick removal and begin clinical monitoring. Recognition of A americanum larvae as a potential cause of pruritic papules may be helpful in similar cases. In addition, it is important for dermatologists to be aware of the tick species in their area.
- Centers for Disease Control and Prevention. Tick ID. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tickID.html
- Molaei G, Little EAH, Williams SC, et al. Bracing for the worst—range expansion of the lone star tick in the northeastern United States. N Engl J Med. 2019;381:2189-2192.
- Centers for Disease Control and Prevention, Division of Vector-Borne Diseases. Lone star tick (Amblyomma americanum). Accessed March 23, 2022. https://www.cdc.gov/ticks/maps/lone_star_tick.pdf
- Reynolds HH, Elston DM. What’s eating you? lone star tick (Amblyomma americanum). Cutis. 2017;99:111-114.
- Jordan RA, Egizi A. The growing importance of lone star ticks in a Lyme disease endemic county: passive tick surveillance in Monmouth County, NJ, 2006–2016. PLoS One. 2019;14:E0211778.
- Singh-Behl D, La Rosa SP, Tomecki KJ. Tick-borne infections. Dermatol Clin. 2003;21:237-244, v.
- Spach DH, Liles WC, Campbell GL, et al. Tick-borne diseases in the United States. N Engl J Med. 1993;329:936-947.
- Duckworth PF Jr, Hayden GF, Reed CN. Human infestation by Amblyomma americanum larvae (“seed ticks”). South Med J. 1985;78:751-753.
- Middleton DB. Tick-borne infections. what starts as a tiny bite may have a serious outcome. Postgrad Med. 1994;95:131-139.
- Moody EK, Barker RW, White JL, et al. Ticks and tick-borne diseases in Oklahoma. J Okla State Med Assoc. 1998;91:438-445.
- Jones BE. Human ‘seed tick’ infestation. Amblyomma americanum larvae. Arch Dermatol. 1981;117:812-814.
- Centers for Disease Control and Prevention. Tick bite prophylaxis. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tick-bite-prophylaxis.html
- Fisher EJ, Mo J, Lucky AW. Multiple pruritic papules from lone star tick larvae bites. Arch Dermatol. 2006;142:491-494.
- Krinsky WL. Dermatoses associated with the bites of mites and ticks (Arthropoda: Acari). Int J Dermatol. 1983;22:75-91.
- Yesudian P, Thambiah AS. Persistent papules after tick-bites. Dermatologica. 1973;147:214-218.
- Goddard J, Portugal JS. Cutaneous lesions due to bites by larval Amblyomma americanum ticks. JAMA Dermatol. 2015;151:1373-1375.
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928.
- Solberg VB, Klein TA, McPherson KR, et al. Field evaluation of DEET and a piperidine repellent (AI3-37220) against Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 1995;32:870-875.
- Evans SR, Korch GW Jr, Lawson MA. Comparative field evaluation of permethrin and DEET-treated military uniforms for personal protection against ticks (Acari). J Med Entomol. 1990;27:829-834.
- Centers for Disease Control and Prevention. Tick ID. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tickID.html
- Molaei G, Little EAH, Williams SC, et al. Bracing for the worst—range expansion of the lone star tick in the northeastern United States. N Engl J Med. 2019;381:2189-2192.
- Centers for Disease Control and Prevention, Division of Vector-Borne Diseases. Lone star tick (Amblyomma americanum). Accessed March 23, 2022. https://www.cdc.gov/ticks/maps/lone_star_tick.pdf
- Reynolds HH, Elston DM. What’s eating you? lone star tick (Amblyomma americanum). Cutis. 2017;99:111-114.
- Jordan RA, Egizi A. The growing importance of lone star ticks in a Lyme disease endemic county: passive tick surveillance in Monmouth County, NJ, 2006–2016. PLoS One. 2019;14:E0211778.
- Singh-Behl D, La Rosa SP, Tomecki KJ. Tick-borne infections. Dermatol Clin. 2003;21:237-244, v.
- Spach DH, Liles WC, Campbell GL, et al. Tick-borne diseases in the United States. N Engl J Med. 1993;329:936-947.
- Duckworth PF Jr, Hayden GF, Reed CN. Human infestation by Amblyomma americanum larvae (“seed ticks”). South Med J. 1985;78:751-753.
- Middleton DB. Tick-borne infections. what starts as a tiny bite may have a serious outcome. Postgrad Med. 1994;95:131-139.
- Moody EK, Barker RW, White JL, et al. Ticks and tick-borne diseases in Oklahoma. J Okla State Med Assoc. 1998;91:438-445.
- Jones BE. Human ‘seed tick’ infestation. Amblyomma americanum larvae. Arch Dermatol. 1981;117:812-814.
- Centers for Disease Control and Prevention. Tick bite prophylaxis. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tick-bite-prophylaxis.html
- Fisher EJ, Mo J, Lucky AW. Multiple pruritic papules from lone star tick larvae bites. Arch Dermatol. 2006;142:491-494.
- Krinsky WL. Dermatoses associated with the bites of mites and ticks (Arthropoda: Acari). Int J Dermatol. 1983;22:75-91.
- Yesudian P, Thambiah AS. Persistent papules after tick-bites. Dermatologica. 1973;147:214-218.
- Goddard J, Portugal JS. Cutaneous lesions due to bites by larval Amblyomma americanum ticks. JAMA Dermatol. 2015;151:1373-1375.
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928.
- Solberg VB, Klein TA, McPherson KR, et al. Field evaluation of DEET and a piperidine repellent (AI3-37220) against Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 1995;32:870-875.
- Evans SR, Korch GW Jr, Lawson MA. Comparative field evaluation of permethrin and DEET-treated military uniforms for personal protection against ticks (Acari). J Med Entomol. 1990;27:829-834.
Practice Points
- The range of Amblyomma americanum has expanded north in recent years from its core range in the southeastern United States. Warming temperatures also have increased the duration of the ticks’ active season.
- Amblyomma americanum can lay several thousand eggs. A person happening upon a newly hatched nest of larval ticks could sustain a widespread vesicular eruption secondary to tick bites.
- It is important to keep larval tick infestation in the differential when evaluating a patient with a new widespread vesicular eruption to expedite prompt removal of the offending ticks and to begin clinical monitoring.
Angioimmunoblastic T-cell Lymphoma Mimicking DRESS Syndrome
Angioimmunoblastic T-cell lymphoma (AITL) is a rare and aggressive lymphoma arising from follicular T-helper cells that predominantly affects older adults and carries a 5-year overall survival rate of 32%.1 Notably, as many as 50% of AITL patients present with a skin rash in addition to the more common but nonspecific acute-onset generalized lymphadenopathy, hepatosplenomegaly, and anemia.2 At presentation, most AITL patients are already at an advanced (III/IV) stage of disease.
Formerly known as angioimmunoblastic lymphadenopathy with dysproteinemia, AITL was once considered a benign entity that carried a risk for malignant transformation. As more cases have been identified and explored, this entity has been recategorized as a frank lymphoma.3 Therefore, it is critical that AITL be diagnosed and treated as early as possible.
We present the case of a 65-year-old man with clinical features that resembled drug reaction with eosinophilia and systemic symptoms (DRESS syndrome). After extensive workup, he was found to have AITL. This atypical case highlights the importance of maintaining a flexible differential diagnosis in patients with a persistent rash that does not improve with appropriate drug withdrawal and therapy.
Case Report
A 65-year-old Filipino man whose medical history was notable for hepatitis B that had been treated with entecavir for years without issue was admitted to the internal medicine service with fever of unknown origin and malaise of approximately 6 weeks’ duration. Six days prior to admission and 5 days after completing courses of the antiviral oseltamivir phosphate and amoxicillin for an upper respiratory tract infection and sinusitis, he developed worsening of an intermittently pruritic rash of approximately 1 month's duration. The dermatology department was consulted the day of hospital admission for evaluation of the rash. Chronic home medications included entecavir, lisinopril/hydrochlorothiazide, amlodipine, atorvastatin, metformin, salsalate, and over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) as needed.
Physical examination was notable for mild erythema and scale distributed across the entire face; mild facial edema; and a blanchable, nonconfluent, macular erythema distributed across the trunk and upper and proximal lower extremities (Figure). In addition, the patient displayed conjunctival injection, pitting edema of the hands, and bilateral cervical and inguinal lymphadenopathy.
Laboratory tests revealed mild leukocytosis (11.6×109/L, [reference range, 4.0–10.5×109/L]), anemia (hemoglobin, 125 g/L (reference range, 138–170 g/L); hematocrit, 36.9%, [reference range, 40.0%–50.0%)], eosinophilia (1.07×109/L [reference range, 0.00–0.70×109/L)], hyponatremia, hypokalemia, and a mildly elevated creatinine level. Computed tomography and full-body positron-emission tomography (PET) scans during admission demonstrated diffuse lymphadenopathy. A skin biopsy from the left chest and a left inguinal lymph node biopsy also were performed.
Despite the lack of a clear medication trigger within the usual timeline for severe cutaneous drug-induced hypersensitivity reactions, DRESS syndrome was high on the differential diagnosis at the time of the initial presentation given the diffuse morbilliform eruption with pruritus, facial edema, eosinophilia, and lymphadenopathy.
Home medications were discontinued except for amlodipine, atorvastatin, and entecavir. The patient was treated symptomatically with topical steroids because it was believed that, if the clinical presentation represented DRESS syndrome, it was a mild variant that could be treated topically.4 His case was considered mild because of a lack of confirmed organ dysfunction and a mild protracted course.
After discharge following a 3-day inpatient stay, the patient was followed in the clinic weekly for 3 weeks without considerable change in the skin or laboratory findings. Discontinuation of entecavir was discussed and approved by his hepatologist.
Posthospitalization analysis of the punch biopsy specimen from the chest performed during the patient’s hospital stay revealed a superficial and deep dermal lymphoid infiltrate comprising CD3-, CD5-, and programmed cell death protein 1–positive cells with cytologic atypia in a perivascular distribution. Analysis of the lymph node biopsy specimen performed during the hospitalization showed effacement of the nodal architecture, a polymorphous lymphoid cell population with irregular nuclear contour, and abundant clear cytoplasm associated with high endothelial venules (HEVs). Cells of interest were positive for CD3, CD4, CD2, CD5, and CD7, with a subset staining positive for programmed cell death protein 1, inducible costimulator, CD10, and chemokine (C-X-C motif) ligand (CXCL) 13. CD21 demonstrated an expanded follicular dendritic cell meshwork in association with HEVs. Polymerase chain reaction revealed a clonal T-cell population. These findings of the skin and lymph node biopsies were consistent with AITL. Subsequent bone marrow biopsy with flow cytometry showed a normal CD4:CD8 ratio in T cells and no increase in natural killer cells.
Cyclophosphamide–hydroxydaunorubicin–Oncovin–prednisone (CHOP) chemotherapy was initiated; the patient completed a total of 6 cycles. He has had near resolution of the skin findings and is considered in remission based on a PET scan performed approximately 7 months after the initial presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a rare peripheral T-cell lymphoma, part of a group of aggressive neoplasms that constitute approximately 15% of peripheral T-cell lymphomas and approximately 2% of non-Hodgkin lymphomas in adults worldwide.5 Cutaneous involvement occurs in approximately half of AITL cases and can be the first manifestation of disease.2 Skin findings are largely nonspecific, ranging from simple morbilliform rashes to erythroderma, at times manifesting with purpura.
Given this variability in the presentation of AITL, early diagnosis is challenging in the absence of more specific signs and symptoms.2 It can conceivably be mistaken for common entities such as viral exanthems or drug eruptions, depending on the history and context. DRESS syndrome, a T cell-mediated, delayed type-IV hypersensitivity drug reaction can present in a manner highly similar to that of AITL, with cutaneous involvement (diffuse morbilliform rash, fever, facial edema, and generalized lymphadenopathy) and variable systemic involvement. Laboratory findings of eosinophilia, atypical lymphocytes, and thrombocytopenia also might be seen in both entities.6 Furthermore, the AITL in our patient was accompanied by electrolyte disturbances that were concerning for syndrome of inappropriate antidiuretic hormone secretion, a rare complication of patients with DRESS syndrome complicated by encephalitis.7,8
Our patient met 4 RegiSCAR criteria for DRESS syndrome, warranting high clinical suspicion for an offending drug.9 DRESS syndrome can be caused by numerous medications—most commonly anticonvulsants, sulfonamides, antibiotics, allopurinol, and NSAIDs. A review of our patient’s medication list identified NSAIDs (including salsalate), entecavir, and amoxicillin, as possible culpable medications. Notably, the only new addition to the patient’s regimen was amoxicillin, which did not fit the typical 2- to 8-week timeline for a DRESS syndrome nidus.10 Our patient’s fever began well before the antibiotic was initiated, and skin findings appeared within 1 week after the course of amoxicillin was completed. Although there is documented variability in the latency of onset of DRESS syndrome following administration of a culprit medication,11 it is critical to maintain a broad differential diagnosis to allow for further diagnostic information to be obtained, especially when the medication timeline does not align with the clinical presentation.
DRESS syndrome is far more common than AITL. Similarities in their clinical presentation pose a substantial challenge and often cause a delay in the diagnosis of AITL, which is made by excisional tissue biopsy, most commonly of a lymph node, with assessment of morphology and immunophenotyping. Histologic assessment of tissue reveals a polymorphous infiltrate of variably sized atypical lymphocytes with prominent arborizing HEVs as well as expanded populations of follicular dendritic cells that can be detected by CD21 staining. Cells express CD3 and CD4, variably express BCL6 (B-cell lymphoma 6 antigen) and CD10, and also may have partial or complete loss of expression of a subset of pan T-cell antigens (CD2, CD3, CD5, and CD7).12-18
The treatment approach to AITL mirrors that of other nodal peripheral T-cell lymphomas, including chemotherapy and consideration of autologous stem-cell transplantation. Recent prospective trials of CHOP and CHOP-like chemotherapy have reported 3-year event-free survival and overall survival rates of 50% and 68%, respectively.19 Novel chemotherapeutic targets and gene-expression profiling are being investigated as potential therapeutic avenues.20
Conclusion
DRESS syndrome and AITL can have near-identical presentations. Clinicians should maintain a high index of suspicion for AITL in patients with presumed DRESS syndrome whose rash does not improve with appropriate drug withdrawal and steroid therapy or who lack a strong offending medication history. In such cases, skin and lymph node biopsies should be performed as early as possible to evaluate for AITL and so that appropriate therapy can be initiated.
- Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31:240-246. doi:10.1200/JCO.2011.37.3647
- Botros N, Cerroni L, Shawwa A, et al. Cutaneous manifestations of angioimmunoblastic T-cell lymphoma: clinical and pathological characteristics. Am J Dermatopathol. 2015;37:274-283. doi:10.1097/DAD.0000000000000144
- Sachsida-Colombo E, Barbosa Mariano LC, Bastos FQ, et al. A difficult case of angioimmunoblastic T-cell lymphoma to diagnose. Rev Bras Hematol Hemoter. 2016;38:82-85. doi:10.1016/j.bjhh.2015.11.002
- Funck-Brentano E, Duong T-A, Bouvresse S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252. doi:10.1016/j.jaad.2014.10.032
- Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood. 2017;129:1095-1102. doi:10.1182/blood-2016-09-692541
- Sato R, Itoh M, Suzuki H, et al. Pathological findings of lymphadenopathy in drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic syndrome (DRESS): similarities with angioimmunoblastic T-cell lymphoma. Eur J Dermatol. 2017;27:201-202. doi:10.1684/ejd.2016.2954
- Osizik L, Tanriover MD, Saka E. Autoimmune limbic encephalitis and syndrome of inappropriate antidiuretic hormone secretion associated with lamotrigine-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Intern Med. 2015;55:1393-1396. doi:10.2169/internalmedicine.55.6035
- Sakuma K, Kano Y, Fukuhara M, et al. Syndrome of inappropriate secretion of antidiuretic hormone associated with limbic encephalitis in a patient with drug-induced hypersensitivity syndrome. Clin Exp Dermatol. 2008;33:287-290. doi:10.1111/j.1365-2230.2007.02645.x
- Pannu AK, Saroch A. Diagnostic criteria for drug rash and eosinophilia with systemic symptoms. J Family Med Prim Care. 2017;6:693-694. doi:10.4103/2249-4863.222050
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al; RegiSCAR study group. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080. doi:10.1111/bjd.12501
- Soria A, Bernier C, Veyrac G, et al. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: a retrospective study. J Am Acad Dermatol. 2020;82:606.
- Loghavi S, Wang SA, Medeiros LJ, et al. Immunophenotypic and diagnostic characterization of angioimmunoblastic T-cell lymphoma by advanced flow cytometric technology. Leuk Lymphoma. 2016;57:2804-2812. doi:10.3109/10428194.2016.1170827
- Lee S-S, R, Odenwald T, et al. Angioimmunoblastic T cell lymphoma is derived from mature T-helper cells with varying expression and loss of detectable CD4. Int J Cancer. 2003;103:12-20. doi:10.1002/ijc.10758
- Feller AC, Griesser H, Schilling CV, et al. Clonal gene rearrangement patterns correlate with immunophenotype and clinical parameters in patients with angioimmunoblastic lymphadenopathy. Am J Pathol. 1988;133:549-556.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; 2008.
- Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633. doi:10.1182/blood.v99.2.627
- Mourad N, Mounier N, J, et al; Groupe d’Etude des Lymphomes de l’Adulte. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470. doi:10.1182/blood-2007-08-105759
- Marafioti T, Paterson JC, Ballabio E, et al. The inducible T-cell co-stimulator molecule is expressed on subsets of T cells and is a new marker of lymphomas of T follicular helper cell-derivation. Haematologica. 2010;95:432-439. doi:10.3324/haematol.2009.010991
- Schmitz N, L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients withT-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418-3425. doi:10.1182/blood-2010-02-270785
- Moskowitz AJ. Practical treatment approach for angioimmunoblastic T-cell lymphoma. J Oncol Pract. 2019;15:137-143. doi:10.1200/JOP.18.00511
Angioimmunoblastic T-cell lymphoma (AITL) is a rare and aggressive lymphoma arising from follicular T-helper cells that predominantly affects older adults and carries a 5-year overall survival rate of 32%.1 Notably, as many as 50% of AITL patients present with a skin rash in addition to the more common but nonspecific acute-onset generalized lymphadenopathy, hepatosplenomegaly, and anemia.2 At presentation, most AITL patients are already at an advanced (III/IV) stage of disease.
Formerly known as angioimmunoblastic lymphadenopathy with dysproteinemia, AITL was once considered a benign entity that carried a risk for malignant transformation. As more cases have been identified and explored, this entity has been recategorized as a frank lymphoma.3 Therefore, it is critical that AITL be diagnosed and treated as early as possible.
We present the case of a 65-year-old man with clinical features that resembled drug reaction with eosinophilia and systemic symptoms (DRESS syndrome). After extensive workup, he was found to have AITL. This atypical case highlights the importance of maintaining a flexible differential diagnosis in patients with a persistent rash that does not improve with appropriate drug withdrawal and therapy.
Case Report
A 65-year-old Filipino man whose medical history was notable for hepatitis B that had been treated with entecavir for years without issue was admitted to the internal medicine service with fever of unknown origin and malaise of approximately 6 weeks’ duration. Six days prior to admission and 5 days after completing courses of the antiviral oseltamivir phosphate and amoxicillin for an upper respiratory tract infection and sinusitis, he developed worsening of an intermittently pruritic rash of approximately 1 month's duration. The dermatology department was consulted the day of hospital admission for evaluation of the rash. Chronic home medications included entecavir, lisinopril/hydrochlorothiazide, amlodipine, atorvastatin, metformin, salsalate, and over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) as needed.
Physical examination was notable for mild erythema and scale distributed across the entire face; mild facial edema; and a blanchable, nonconfluent, macular erythema distributed across the trunk and upper and proximal lower extremities (Figure). In addition, the patient displayed conjunctival injection, pitting edema of the hands, and bilateral cervical and inguinal lymphadenopathy.

Laboratory tests revealed mild leukocytosis (11.6×109/L, [reference range, 4.0–10.5×109/L]), anemia (hemoglobin, 125 g/L (reference range, 138–170 g/L); hematocrit, 36.9%, [reference range, 40.0%–50.0%)], eosinophilia (1.07×109/L [reference range, 0.00–0.70×109/L)], hyponatremia, hypokalemia, and a mildly elevated creatinine level. Computed tomography and full-body positron-emission tomography (PET) scans during admission demonstrated diffuse lymphadenopathy. A skin biopsy from the left chest and a left inguinal lymph node biopsy also were performed.
Despite the lack of a clear medication trigger within the usual timeline for severe cutaneous drug-induced hypersensitivity reactions, DRESS syndrome was high on the differential diagnosis at the time of the initial presentation given the diffuse morbilliform eruption with pruritus, facial edema, eosinophilia, and lymphadenopathy.
Home medications were discontinued except for amlodipine, atorvastatin, and entecavir. The patient was treated symptomatically with topical steroids because it was believed that, if the clinical presentation represented DRESS syndrome, it was a mild variant that could be treated topically.4 His case was considered mild because of a lack of confirmed organ dysfunction and a mild protracted course.
After discharge following a 3-day inpatient stay, the patient was followed in the clinic weekly for 3 weeks without considerable change in the skin or laboratory findings. Discontinuation of entecavir was discussed and approved by his hepatologist.
Posthospitalization analysis of the punch biopsy specimen from the chest performed during the patient’s hospital stay revealed a superficial and deep dermal lymphoid infiltrate comprising CD3-, CD5-, and programmed cell death protein 1–positive cells with cytologic atypia in a perivascular distribution. Analysis of the lymph node biopsy specimen performed during the hospitalization showed effacement of the nodal architecture, a polymorphous lymphoid cell population with irregular nuclear contour, and abundant clear cytoplasm associated with high endothelial venules (HEVs). Cells of interest were positive for CD3, CD4, CD2, CD5, and CD7, with a subset staining positive for programmed cell death protein 1, inducible costimulator, CD10, and chemokine (C-X-C motif) ligand (CXCL) 13. CD21 demonstrated an expanded follicular dendritic cell meshwork in association with HEVs. Polymerase chain reaction revealed a clonal T-cell population. These findings of the skin and lymph node biopsies were consistent with AITL. Subsequent bone marrow biopsy with flow cytometry showed a normal CD4:CD8 ratio in T cells and no increase in natural killer cells.
Cyclophosphamide–hydroxydaunorubicin–Oncovin–prednisone (CHOP) chemotherapy was initiated; the patient completed a total of 6 cycles. He has had near resolution of the skin findings and is considered in remission based on a PET scan performed approximately 7 months after the initial presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a rare peripheral T-cell lymphoma, part of a group of aggressive neoplasms that constitute approximately 15% of peripheral T-cell lymphomas and approximately 2% of non-Hodgkin lymphomas in adults worldwide.5 Cutaneous involvement occurs in approximately half of AITL cases and can be the first manifestation of disease.2 Skin findings are largely nonspecific, ranging from simple morbilliform rashes to erythroderma, at times manifesting with purpura.
Given this variability in the presentation of AITL, early diagnosis is challenging in the absence of more specific signs and symptoms.2 It can conceivably be mistaken for common entities such as viral exanthems or drug eruptions, depending on the history and context. DRESS syndrome, a T cell-mediated, delayed type-IV hypersensitivity drug reaction can present in a manner highly similar to that of AITL, with cutaneous involvement (diffuse morbilliform rash, fever, facial edema, and generalized lymphadenopathy) and variable systemic involvement. Laboratory findings of eosinophilia, atypical lymphocytes, and thrombocytopenia also might be seen in both entities.6 Furthermore, the AITL in our patient was accompanied by electrolyte disturbances that were concerning for syndrome of inappropriate antidiuretic hormone secretion, a rare complication of patients with DRESS syndrome complicated by encephalitis.7,8
Our patient met 4 RegiSCAR criteria for DRESS syndrome, warranting high clinical suspicion for an offending drug.9 DRESS syndrome can be caused by numerous medications—most commonly anticonvulsants, sulfonamides, antibiotics, allopurinol, and NSAIDs. A review of our patient’s medication list identified NSAIDs (including salsalate), entecavir, and amoxicillin, as possible culpable medications. Notably, the only new addition to the patient’s regimen was amoxicillin, which did not fit the typical 2- to 8-week timeline for a DRESS syndrome nidus.10 Our patient’s fever began well before the antibiotic was initiated, and skin findings appeared within 1 week after the course of amoxicillin was completed. Although there is documented variability in the latency of onset of DRESS syndrome following administration of a culprit medication,11 it is critical to maintain a broad differential diagnosis to allow for further diagnostic information to be obtained, especially when the medication timeline does not align with the clinical presentation.
DRESS syndrome is far more common than AITL. Similarities in their clinical presentation pose a substantial challenge and often cause a delay in the diagnosis of AITL, which is made by excisional tissue biopsy, most commonly of a lymph node, with assessment of morphology and immunophenotyping. Histologic assessment of tissue reveals a polymorphous infiltrate of variably sized atypical lymphocytes with prominent arborizing HEVs as well as expanded populations of follicular dendritic cells that can be detected by CD21 staining. Cells express CD3 and CD4, variably express BCL6 (B-cell lymphoma 6 antigen) and CD10, and also may have partial or complete loss of expression of a subset of pan T-cell antigens (CD2, CD3, CD5, and CD7).12-18
The treatment approach to AITL mirrors that of other nodal peripheral T-cell lymphomas, including chemotherapy and consideration of autologous stem-cell transplantation. Recent prospective trials of CHOP and CHOP-like chemotherapy have reported 3-year event-free survival and overall survival rates of 50% and 68%, respectively.19 Novel chemotherapeutic targets and gene-expression profiling are being investigated as potential therapeutic avenues.20
Conclusion
DRESS syndrome and AITL can have near-identical presentations. Clinicians should maintain a high index of suspicion for AITL in patients with presumed DRESS syndrome whose rash does not improve with appropriate drug withdrawal and steroid therapy or who lack a strong offending medication history. In such cases, skin and lymph node biopsies should be performed as early as possible to evaluate for AITL and so that appropriate therapy can be initiated.
Angioimmunoblastic T-cell lymphoma (AITL) is a rare and aggressive lymphoma arising from follicular T-helper cells that predominantly affects older adults and carries a 5-year overall survival rate of 32%.1 Notably, as many as 50% of AITL patients present with a skin rash in addition to the more common but nonspecific acute-onset generalized lymphadenopathy, hepatosplenomegaly, and anemia.2 At presentation, most AITL patients are already at an advanced (III/IV) stage of disease.
Formerly known as angioimmunoblastic lymphadenopathy with dysproteinemia, AITL was once considered a benign entity that carried a risk for malignant transformation. As more cases have been identified and explored, this entity has been recategorized as a frank lymphoma.3 Therefore, it is critical that AITL be diagnosed and treated as early as possible.
We present the case of a 65-year-old man with clinical features that resembled drug reaction with eosinophilia and systemic symptoms (DRESS syndrome). After extensive workup, he was found to have AITL. This atypical case highlights the importance of maintaining a flexible differential diagnosis in patients with a persistent rash that does not improve with appropriate drug withdrawal and therapy.
Case Report
A 65-year-old Filipino man whose medical history was notable for hepatitis B that had been treated with entecavir for years without issue was admitted to the internal medicine service with fever of unknown origin and malaise of approximately 6 weeks’ duration. Six days prior to admission and 5 days after completing courses of the antiviral oseltamivir phosphate and amoxicillin for an upper respiratory tract infection and sinusitis, he developed worsening of an intermittently pruritic rash of approximately 1 month's duration. The dermatology department was consulted the day of hospital admission for evaluation of the rash. Chronic home medications included entecavir, lisinopril/hydrochlorothiazide, amlodipine, atorvastatin, metformin, salsalate, and over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) as needed.
Physical examination was notable for mild erythema and scale distributed across the entire face; mild facial edema; and a blanchable, nonconfluent, macular erythema distributed across the trunk and upper and proximal lower extremities (Figure). In addition, the patient displayed conjunctival injection, pitting edema of the hands, and bilateral cervical and inguinal lymphadenopathy.

Laboratory tests revealed mild leukocytosis (11.6×109/L, [reference range, 4.0–10.5×109/L]), anemia (hemoglobin, 125 g/L (reference range, 138–170 g/L); hematocrit, 36.9%, [reference range, 40.0%–50.0%)], eosinophilia (1.07×109/L [reference range, 0.00–0.70×109/L)], hyponatremia, hypokalemia, and a mildly elevated creatinine level. Computed tomography and full-body positron-emission tomography (PET) scans during admission demonstrated diffuse lymphadenopathy. A skin biopsy from the left chest and a left inguinal lymph node biopsy also were performed.
Despite the lack of a clear medication trigger within the usual timeline for severe cutaneous drug-induced hypersensitivity reactions, DRESS syndrome was high on the differential diagnosis at the time of the initial presentation given the diffuse morbilliform eruption with pruritus, facial edema, eosinophilia, and lymphadenopathy.
Home medications were discontinued except for amlodipine, atorvastatin, and entecavir. The patient was treated symptomatically with topical steroids because it was believed that, if the clinical presentation represented DRESS syndrome, it was a mild variant that could be treated topically.4 His case was considered mild because of a lack of confirmed organ dysfunction and a mild protracted course.
After discharge following a 3-day inpatient stay, the patient was followed in the clinic weekly for 3 weeks without considerable change in the skin or laboratory findings. Discontinuation of entecavir was discussed and approved by his hepatologist.
Posthospitalization analysis of the punch biopsy specimen from the chest performed during the patient’s hospital stay revealed a superficial and deep dermal lymphoid infiltrate comprising CD3-, CD5-, and programmed cell death protein 1–positive cells with cytologic atypia in a perivascular distribution. Analysis of the lymph node biopsy specimen performed during the hospitalization showed effacement of the nodal architecture, a polymorphous lymphoid cell population with irregular nuclear contour, and abundant clear cytoplasm associated with high endothelial venules (HEVs). Cells of interest were positive for CD3, CD4, CD2, CD5, and CD7, with a subset staining positive for programmed cell death protein 1, inducible costimulator, CD10, and chemokine (C-X-C motif) ligand (CXCL) 13. CD21 demonstrated an expanded follicular dendritic cell meshwork in association with HEVs. Polymerase chain reaction revealed a clonal T-cell population. These findings of the skin and lymph node biopsies were consistent with AITL. Subsequent bone marrow biopsy with flow cytometry showed a normal CD4:CD8 ratio in T cells and no increase in natural killer cells.
Cyclophosphamide–hydroxydaunorubicin–Oncovin–prednisone (CHOP) chemotherapy was initiated; the patient completed a total of 6 cycles. He has had near resolution of the skin findings and is considered in remission based on a PET scan performed approximately 7 months after the initial presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a rare peripheral T-cell lymphoma, part of a group of aggressive neoplasms that constitute approximately 15% of peripheral T-cell lymphomas and approximately 2% of non-Hodgkin lymphomas in adults worldwide.5 Cutaneous involvement occurs in approximately half of AITL cases and can be the first manifestation of disease.2 Skin findings are largely nonspecific, ranging from simple morbilliform rashes to erythroderma, at times manifesting with purpura.
Given this variability in the presentation of AITL, early diagnosis is challenging in the absence of more specific signs and symptoms.2 It can conceivably be mistaken for common entities such as viral exanthems or drug eruptions, depending on the history and context. DRESS syndrome, a T cell-mediated, delayed type-IV hypersensitivity drug reaction can present in a manner highly similar to that of AITL, with cutaneous involvement (diffuse morbilliform rash, fever, facial edema, and generalized lymphadenopathy) and variable systemic involvement. Laboratory findings of eosinophilia, atypical lymphocytes, and thrombocytopenia also might be seen in both entities.6 Furthermore, the AITL in our patient was accompanied by electrolyte disturbances that were concerning for syndrome of inappropriate antidiuretic hormone secretion, a rare complication of patients with DRESS syndrome complicated by encephalitis.7,8
Our patient met 4 RegiSCAR criteria for DRESS syndrome, warranting high clinical suspicion for an offending drug.9 DRESS syndrome can be caused by numerous medications—most commonly anticonvulsants, sulfonamides, antibiotics, allopurinol, and NSAIDs. A review of our patient’s medication list identified NSAIDs (including salsalate), entecavir, and amoxicillin, as possible culpable medications. Notably, the only new addition to the patient’s regimen was amoxicillin, which did not fit the typical 2- to 8-week timeline for a DRESS syndrome nidus.10 Our patient’s fever began well before the antibiotic was initiated, and skin findings appeared within 1 week after the course of amoxicillin was completed. Although there is documented variability in the latency of onset of DRESS syndrome following administration of a culprit medication,11 it is critical to maintain a broad differential diagnosis to allow for further diagnostic information to be obtained, especially when the medication timeline does not align with the clinical presentation.
DRESS syndrome is far more common than AITL. Similarities in their clinical presentation pose a substantial challenge and often cause a delay in the diagnosis of AITL, which is made by excisional tissue biopsy, most commonly of a lymph node, with assessment of morphology and immunophenotyping. Histologic assessment of tissue reveals a polymorphous infiltrate of variably sized atypical lymphocytes with prominent arborizing HEVs as well as expanded populations of follicular dendritic cells that can be detected by CD21 staining. Cells express CD3 and CD4, variably express BCL6 (B-cell lymphoma 6 antigen) and CD10, and also may have partial or complete loss of expression of a subset of pan T-cell antigens (CD2, CD3, CD5, and CD7).12-18
The treatment approach to AITL mirrors that of other nodal peripheral T-cell lymphomas, including chemotherapy and consideration of autologous stem-cell transplantation. Recent prospective trials of CHOP and CHOP-like chemotherapy have reported 3-year event-free survival and overall survival rates of 50% and 68%, respectively.19 Novel chemotherapeutic targets and gene-expression profiling are being investigated as potential therapeutic avenues.20
Conclusion
DRESS syndrome and AITL can have near-identical presentations. Clinicians should maintain a high index of suspicion for AITL in patients with presumed DRESS syndrome whose rash does not improve with appropriate drug withdrawal and steroid therapy or who lack a strong offending medication history. In such cases, skin and lymph node biopsies should be performed as early as possible to evaluate for AITL and so that appropriate therapy can be initiated.
- Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31:240-246. doi:10.1200/JCO.2011.37.3647
- Botros N, Cerroni L, Shawwa A, et al. Cutaneous manifestations of angioimmunoblastic T-cell lymphoma: clinical and pathological characteristics. Am J Dermatopathol. 2015;37:274-283. doi:10.1097/DAD.0000000000000144
- Sachsida-Colombo E, Barbosa Mariano LC, Bastos FQ, et al. A difficult case of angioimmunoblastic T-cell lymphoma to diagnose. Rev Bras Hematol Hemoter. 2016;38:82-85. doi:10.1016/j.bjhh.2015.11.002
- Funck-Brentano E, Duong T-A, Bouvresse S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252. doi:10.1016/j.jaad.2014.10.032
- Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood. 2017;129:1095-1102. doi:10.1182/blood-2016-09-692541
- Sato R, Itoh M, Suzuki H, et al. Pathological findings of lymphadenopathy in drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic syndrome (DRESS): similarities with angioimmunoblastic T-cell lymphoma. Eur J Dermatol. 2017;27:201-202. doi:10.1684/ejd.2016.2954
- Osizik L, Tanriover MD, Saka E. Autoimmune limbic encephalitis and syndrome of inappropriate antidiuretic hormone secretion associated with lamotrigine-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Intern Med. 2015;55:1393-1396. doi:10.2169/internalmedicine.55.6035
- Sakuma K, Kano Y, Fukuhara M, et al. Syndrome of inappropriate secretion of antidiuretic hormone associated with limbic encephalitis in a patient with drug-induced hypersensitivity syndrome. Clin Exp Dermatol. 2008;33:287-290. doi:10.1111/j.1365-2230.2007.02645.x
- Pannu AK, Saroch A. Diagnostic criteria for drug rash and eosinophilia with systemic symptoms. J Family Med Prim Care. 2017;6:693-694. doi:10.4103/2249-4863.222050
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al; RegiSCAR study group. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080. doi:10.1111/bjd.12501
- Soria A, Bernier C, Veyrac G, et al. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: a retrospective study. J Am Acad Dermatol. 2020;82:606.
- Loghavi S, Wang SA, Medeiros LJ, et al. Immunophenotypic and diagnostic characterization of angioimmunoblastic T-cell lymphoma by advanced flow cytometric technology. Leuk Lymphoma. 2016;57:2804-2812. doi:10.3109/10428194.2016.1170827
- Lee S-S, R, Odenwald T, et al. Angioimmunoblastic T cell lymphoma is derived from mature T-helper cells with varying expression and loss of detectable CD4. Int J Cancer. 2003;103:12-20. doi:10.1002/ijc.10758
- Feller AC, Griesser H, Schilling CV, et al. Clonal gene rearrangement patterns correlate with immunophenotype and clinical parameters in patients with angioimmunoblastic lymphadenopathy. Am J Pathol. 1988;133:549-556.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; 2008.
- Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633. doi:10.1182/blood.v99.2.627
- Mourad N, Mounier N, J, et al; Groupe d’Etude des Lymphomes de l’Adulte. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470. doi:10.1182/blood-2007-08-105759
- Marafioti T, Paterson JC, Ballabio E, et al. The inducible T-cell co-stimulator molecule is expressed on subsets of T cells and is a new marker of lymphomas of T follicular helper cell-derivation. Haematologica. 2010;95:432-439. doi:10.3324/haematol.2009.010991
- Schmitz N, L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients withT-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418-3425. doi:10.1182/blood-2010-02-270785
- Moskowitz AJ. Practical treatment approach for angioimmunoblastic T-cell lymphoma. J Oncol Pract. 2019;15:137-143. doi:10.1200/JOP.18.00511
- Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31:240-246. doi:10.1200/JCO.2011.37.3647
- Botros N, Cerroni L, Shawwa A, et al. Cutaneous manifestations of angioimmunoblastic T-cell lymphoma: clinical and pathological characteristics. Am J Dermatopathol. 2015;37:274-283. doi:10.1097/DAD.0000000000000144
- Sachsida-Colombo E, Barbosa Mariano LC, Bastos FQ, et al. A difficult case of angioimmunoblastic T-cell lymphoma to diagnose. Rev Bras Hematol Hemoter. 2016;38:82-85. doi:10.1016/j.bjhh.2015.11.002
- Funck-Brentano E, Duong T-A, Bouvresse S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252. doi:10.1016/j.jaad.2014.10.032
- Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood. 2017;129:1095-1102. doi:10.1182/blood-2016-09-692541
- Sato R, Itoh M, Suzuki H, et al. Pathological findings of lymphadenopathy in drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic syndrome (DRESS): similarities with angioimmunoblastic T-cell lymphoma. Eur J Dermatol. 2017;27:201-202. doi:10.1684/ejd.2016.2954
- Osizik L, Tanriover MD, Saka E. Autoimmune limbic encephalitis and syndrome of inappropriate antidiuretic hormone secretion associated with lamotrigine-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Intern Med. 2015;55:1393-1396. doi:10.2169/internalmedicine.55.6035
- Sakuma K, Kano Y, Fukuhara M, et al. Syndrome of inappropriate secretion of antidiuretic hormone associated with limbic encephalitis in a patient with drug-induced hypersensitivity syndrome. Clin Exp Dermatol. 2008;33:287-290. doi:10.1111/j.1365-2230.2007.02645.x
- Pannu AK, Saroch A. Diagnostic criteria for drug rash and eosinophilia with systemic symptoms. J Family Med Prim Care. 2017;6:693-694. doi:10.4103/2249-4863.222050
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al; RegiSCAR study group. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080. doi:10.1111/bjd.12501
- Soria A, Bernier C, Veyrac G, et al. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: a retrospective study. J Am Acad Dermatol. 2020;82:606.
- Loghavi S, Wang SA, Medeiros LJ, et al. Immunophenotypic and diagnostic characterization of angioimmunoblastic T-cell lymphoma by advanced flow cytometric technology. Leuk Lymphoma. 2016;57:2804-2812. doi:10.3109/10428194.2016.1170827
- Lee S-S, R, Odenwald T, et al. Angioimmunoblastic T cell lymphoma is derived from mature T-helper cells with varying expression and loss of detectable CD4. Int J Cancer. 2003;103:12-20. doi:10.1002/ijc.10758
- Feller AC, Griesser H, Schilling CV, et al. Clonal gene rearrangement patterns correlate with immunophenotype and clinical parameters in patients with angioimmunoblastic lymphadenopathy. Am J Pathol. 1988;133:549-556.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; 2008.
- Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633. doi:10.1182/blood.v99.2.627
- Mourad N, Mounier N, J, et al; Groupe d’Etude des Lymphomes de l’Adulte. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470. doi:10.1182/blood-2007-08-105759
- Marafioti T, Paterson JC, Ballabio E, et al. The inducible T-cell co-stimulator molecule is expressed on subsets of T cells and is a new marker of lymphomas of T follicular helper cell-derivation. Haematologica. 2010;95:432-439. doi:10.3324/haematol.2009.010991
- Schmitz N, L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients withT-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418-3425. doi:10.1182/blood-2010-02-270785
- Moskowitz AJ. Practical treatment approach for angioimmunoblastic T-cell lymphoma. J Oncol Pract. 2019;15:137-143. doi:10.1200/JOP.18.00511
Practice Points
- It is important to maintain a high index of suspicion for angioimmunoblastic T-cell lymphoma in older patients with a longstanding rash and no clear culprit for drug reaction with eosinophilia and systemic symptoms (DRESS syndrome).
- Consider performing a lymph node biopsy early in the course of disease in patients with presumed DRESS syndrome who do not improve with drug withdrawal and steroid therapy.