User login
Catheter-Directed Retrieval of an Infected Fragment in a Vietnam War Veteran
Shrapnel injuries are commonly encountered in war zones.1 Shrapnel injuries can remain asymptomatic or become systemic, with health effects of the retained foreign body ranging from local to systemic toxicities depending on the patient’s reaction to the chemical composition and corrosiveness of the fragments in vivo.2 We present a case of a reactivating shrapnel injury in the form of a retroperitoneal infection and subsequent iliopsoas abscess. A collaborative procedure was performed between surgery and interventional radiology to snare and remove the infected fragment and drain the abscess.
Case Presentation
While serving in Vietnam, a soldier sustained a fragment injury to his left lower abdomen. He underwent a laparotomy, small bowel resection, and a temporary ileostomy at the time of the injury. Nearly 50 years later, the patient presented with chronic left lower quadrant pain and a low-grade fever. He was diagnosed clinically in the emergency department (ED) with diverticulitis and treated with antibiotics. The patient initially responded to treatment but returned 6 months later with similar symptoms, low-grade fever, and mild leukocytosis. A computed tomography (CT) scan during that encounter without IV contrast revealed a few scattered colonic diverticula without definite diverticulitis as well as a metallic fragment embedded in the left iliopsoas with increased soft tissue density.
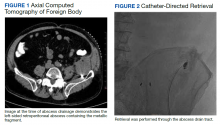
The patient was diagnosed with a pelvic/abdominal wall hematoma and was discharged with pain medication. The patient reported recurrent attacks of left lower quadrant pain, fever, and changes in bowel habits, prompting gastrointestinal consultation and a colonoscopy that was unremarkable. Ten months later, the patient again presented to the ED, with recurrent symptoms, a fever of 102 °F, and leukocytosis with a white blood cell count of 11.7 × 109/L. CT scan with IV contrast revealed a large left iliopsoas abscess associated with an approximately 1-cm metallic fragment (Figure 1). A drainage catheter was placed under CT guidance and approximately 270 mL of purulent fluid was drained. Culture of the fluid was positive for Escherichia coli (E coli). Two days after drain placement, the fragment was removed as a joint procedure with interventional radiology and surgery. Using the drainage catheter tract as a point of entry, multiple attempts were made to retrieve the fragment with Olympus EndoJaw endoscopic forceps without success.
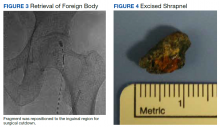
Ultimately a stiff directional sheath from a Cook Medical transjugular liver biopsy kit was used with a Merit Medical EnSnare to relocate the fragment to the left inguinal region for surgical excision (Figures 2, 3, and 4). The fragment was removed and swabbed for culture and sensitivity and a BLAKE drain was placed in the evacuated abscess cavity. The patient tolerated the procedure well and was discharged the following day. Three days later, culture and sensitivity grew E coli and Acinetobacter, thus confirming infection and a nidus for the surrounding abscess formation. On follow-up with general surgery 7 days later, the patient reported he was doing well, and the drain was removed without difficulty.
Discussion
Foreign body injuries can be benign or debilitating depending on the initial damage, anatomical location of the foreign body, composition of the foreign body, and the patient’s response to it. Retained shrapnel deep within the muscle tissue rarely causes complications. Although many times embedded objects can be asymptomatic and require no further management, migration of the foreign body or the formation of a fistula is possible, causing symptoms and requiring surgical intervention.1 One case involved the formation of a purulent fistula appearing a year after an explosive wound to the lumbosacral spine, which was treated with antimicrobials. Recurrence of the fistula several times after treatment led to surgical removal of the shrapnel along with antibiotic treatment of the osteomyelitis.3 Although uncommon, lead exposure that occurs due to retained foreign body fragments from gunshot or military-related injuries can cause systemic lead toxicity. Symptoms may range from abdominal pain, nausea, and constipation to jaundice and hepatitis.4 The severity has also been stated to correlate with the surface area of the lead exposed for dissolution.5 Migration of foreign bodies and shrapnel to other sites in the body, such as movement from soft tissues into distantly located body cavities, have been reported as well. Such a case involved the spontaneous onset of knee synovitis due to an intra-articular metallic object that was introduced via a blast injury to the upper third of the ipsilateral thigh.1
In this patient’s case, a large intramuscular abscess had formed nearly 50 years after the initial combat injury, requiring drainage of the abscess and removal of the fragment. By snaring the foreign body to a more superficial site, the surgical removal only required a minor incision, decreasing recovery time and the likelihood of postoperative complications that would have been associated with a large retroperitoneal dissection. While loop snare is often the first-line technique for the removal of intravascular foreign bodies, its use in soft tissue retained materials is scarcely reported.6 The more typical uses involve the removal of intraluminal materials, such as partially fractured venous catheters, guide wires, stents, and vena cava filters. The same report mentioned that in all 16 cases of percutaneous foreign body retrieval, no surgical intervention was required.7 In the case of most nonvascular foreign bodies, however, surgical retrieval is usually performed.8
Surgical removal of foreign bodies can be difficult in cases where a foreign body is anatomically located next to vital structures.9 An additional challenge with a sole surgical approach to foreign body retrieval is when it is small in size and lies deep within the soft tissue, as was the case for our patient. In such cases, the surgical procedure can be time consuming and lead to more trauma to the surrounding tissues.10 These factors alone necessitate consideration of postoperative morbidity and mortality.
In our patient, the retained fragment was embedded in the wall of an abscess located retroperitoneally in his iliopsoas muscle. When considering the proximity of the iliopsoas muscle to the digestive tract, urinary tract, and iliac lymph nodes, it is reasonable for infectious material to come in contact with the foreign body from these nearby structures, resulting in secondary infection.11 Surgery was previously considered the first-line treatment for retroperitoneal abscesses until the advent of imaging-guided percutaneous drainage.12
In some instances, surgical drainage may still be attempted, such as if there are different disease processes requiring open surgery or if percutaneous catheter drainage is not technically possible due to the location of the abscess, thick exudate, loculation/septations, or phlegmon. In these cases, laparoscopic drainage as opposed to open surgical drainage can provide the benefits of an open procedure (ie, total drainage and resection of infected tissue) but is less invasive, requires a smaller incision, and heals faster.13 Percutaneous drainage is the current first-line treatment due to the lack of need for general anesthesia, lower cost, and better morbidity and mortality outcomes compared to surgical methods.12 While percutaneous drainage proved to be immediately therapeutic for our patient, the risk of abscess recurrence with the retained infected fragment necessitated coordination of procedures across specialties to provide the best outcome for the patient.
Conclusions
This case demonstrates a multidisciplinary approach to transforming an otherwise large retroperitoneal dissection to a minimally invasive and technically efficient abscess drainage and foreign body retrieval.
1. Schroeder JE, Lowe J, Chaimsky G, Liebergall M, Mosheiff R. Migrating shrapnel: a rare cause of knee synovitis. Mil Med. 2010;175(11):929-930. doi:10.7205/milmed-d-09-00254
2. Centeno JA, Rogers DA, van der Voet GB, et al. Embedded fragments from U.S. military personnel—chemical analysis and potential health implications. Int J Environ Res Public Health. 2014;11(2):1261-1278. Published 2014 Jan 23. doi:10.3390/ijerph110201261
3. Carija R, Busic Z, Bradaric N, Bulovic B, Borzic Z, Pavicic-Perkovic S. Surgical removal of metallic foreign body (shrapnel) from the lumbosacral spine and the treatment of chronic osteomyelitis: a case report. West Indian Med J. 2014;63(4):373-375. doi:10.7727/wimj.2012.290
4. Grasso I, Blattner M, Short T, Downs J. Severe systemic lead toxicity resulting from extra-articular retained shrapnel presenting as jaundice and hepatitis: a case report and review of the literature. Mil Med. 2017;182(3-4):e1843-e1848. doi:10.7205/MILMED-D-16-00231
5. Dillman RO, Crumb CK, Lidsky MJ. Lead poisoning from a gunshot wound: report of a case and review of the literature. Am J Med. 1979;66(3):509-514. doi:10.1016/0002-9343(79)91083-0
6. Woodhouse JB, Uberoi R. Techniques for intravascular foreign body retrieval. Cardiovasc Intervent Radiol. 2013;36(4):888-897. doi:10.1007/s00270-012-0488-8
7. Mallmann CV, Wolf KJ, Wacker FK. Retrieval of vascular foreign bodies using a self-made wire snare. Acta Radiol. 2008;49(10):1124-1128. doi:10.1080/02841850802454741
8. Nosher JL, Siegel R. Percutaneous retrieval of nonvascular foreign bodies. Radiology. 1993;187(3):649-651. doi:10.1148/radiology.187.3.8497610
9. Fu Y, Cui LG, Romagnoli C, Li ZQ, Lei YT. Ultrasound-guided removal of retained soft tissue foreign body with late presentation. Chin Med J (Engl). 2017;130(14):1753-1754. doi:10.4103/0366-6999.209910
10. Liang HD, Li H, Feng H, Zhao ZN, Song WJ, Yuan B. Application of intraoperative navigation and positioning system in the removal of deep foreign bodies in the limbs. Chin Med J (Engl). 2019;132(11):1375-1377. doi:10.1097/CM9.0000000000000253
11. Moriarty CM, Baker RJ. A pain in the psoas. Sports Health. 2016;8(6):568-572. doi:10.1177/1941738116665112
12. Akhan O, Durmaz H, Balcı S, Birgi E, Çiftçi T, Akıncı D. Percutaneous drainage of retroperitoneal abscesses: variables for success, failure, and recurrence. Diagn Interv Radiol. 2020;26(2):124-130. doi:10.5152/dir.2019.19199
13. Hong CH, Hong YC, Bae SH, et al. Laparoscopic drainage as a minimally invasive treatment for a psoas abscess: a single center case series and literature review. Medicine (Baltimore). 2020;99(14):e19640. doi:10.1097/MD.0000000000019640
Shrapnel injuries are commonly encountered in war zones.1 Shrapnel injuries can remain asymptomatic or become systemic, with health effects of the retained foreign body ranging from local to systemic toxicities depending on the patient’s reaction to the chemical composition and corrosiveness of the fragments in vivo.2 We present a case of a reactivating shrapnel injury in the form of a retroperitoneal infection and subsequent iliopsoas abscess. A collaborative procedure was performed between surgery and interventional radiology to snare and remove the infected fragment and drain the abscess.
Case Presentation
While serving in Vietnam, a soldier sustained a fragment injury to his left lower abdomen. He underwent a laparotomy, small bowel resection, and a temporary ileostomy at the time of the injury. Nearly 50 years later, the patient presented with chronic left lower quadrant pain and a low-grade fever. He was diagnosed clinically in the emergency department (ED) with diverticulitis and treated with antibiotics. The patient initially responded to treatment but returned 6 months later with similar symptoms, low-grade fever, and mild leukocytosis. A computed tomography (CT) scan during that encounter without IV contrast revealed a few scattered colonic diverticula without definite diverticulitis as well as a metallic fragment embedded in the left iliopsoas with increased soft tissue density.
The patient was diagnosed with a pelvic/abdominal wall hematoma and was discharged with pain medication. The patient reported recurrent attacks of left lower quadrant pain, fever, and changes in bowel habits, prompting gastrointestinal consultation and a colonoscopy that was unremarkable. Ten months later, the patient again presented to the ED, with recurrent symptoms, a fever of 102 °F, and leukocytosis with a white blood cell count of 11.7 × 109/L. CT scan with IV contrast revealed a large left iliopsoas abscess associated with an approximately 1-cm metallic fragment (Figure 1). A drainage catheter was placed under CT guidance and approximately 270 mL of purulent fluid was drained. Culture of the fluid was positive for Escherichia coli (E coli). Two days after drain placement, the fragment was removed as a joint procedure with interventional radiology and surgery. Using the drainage catheter tract as a point of entry, multiple attempts were made to retrieve the fragment with Olympus EndoJaw endoscopic forceps without success.
Ultimately a stiff directional sheath from a Cook Medical transjugular liver biopsy kit was used with a Merit Medical EnSnare to relocate the fragment to the left inguinal region for surgical excision (Figures 2, 3, and 4). The fragment was removed and swabbed for culture and sensitivity and a BLAKE drain was placed in the evacuated abscess cavity. The patient tolerated the procedure well and was discharged the following day. Three days later, culture and sensitivity grew E coli and Acinetobacter, thus confirming infection and a nidus for the surrounding abscess formation. On follow-up with general surgery 7 days later, the patient reported he was doing well, and the drain was removed without difficulty.
Discussion
Foreign body injuries can be benign or debilitating depending on the initial damage, anatomical location of the foreign body, composition of the foreign body, and the patient’s response to it. Retained shrapnel deep within the muscle tissue rarely causes complications. Although many times embedded objects can be asymptomatic and require no further management, migration of the foreign body or the formation of a fistula is possible, causing symptoms and requiring surgical intervention.1 One case involved the formation of a purulent fistula appearing a year after an explosive wound to the lumbosacral spine, which was treated with antimicrobials. Recurrence of the fistula several times after treatment led to surgical removal of the shrapnel along with antibiotic treatment of the osteomyelitis.3 Although uncommon, lead exposure that occurs due to retained foreign body fragments from gunshot or military-related injuries can cause systemic lead toxicity. Symptoms may range from abdominal pain, nausea, and constipation to jaundice and hepatitis.4 The severity has also been stated to correlate with the surface area of the lead exposed for dissolution.5 Migration of foreign bodies and shrapnel to other sites in the body, such as movement from soft tissues into distantly located body cavities, have been reported as well. Such a case involved the spontaneous onset of knee synovitis due to an intra-articular metallic object that was introduced via a blast injury to the upper third of the ipsilateral thigh.1
In this patient’s case, a large intramuscular abscess had formed nearly 50 years after the initial combat injury, requiring drainage of the abscess and removal of the fragment. By snaring the foreign body to a more superficial site, the surgical removal only required a minor incision, decreasing recovery time and the likelihood of postoperative complications that would have been associated with a large retroperitoneal dissection. While loop snare is often the first-line technique for the removal of intravascular foreign bodies, its use in soft tissue retained materials is scarcely reported.6 The more typical uses involve the removal of intraluminal materials, such as partially fractured venous catheters, guide wires, stents, and vena cava filters. The same report mentioned that in all 16 cases of percutaneous foreign body retrieval, no surgical intervention was required.7 In the case of most nonvascular foreign bodies, however, surgical retrieval is usually performed.8
Surgical removal of foreign bodies can be difficult in cases where a foreign body is anatomically located next to vital structures.9 An additional challenge with a sole surgical approach to foreign body retrieval is when it is small in size and lies deep within the soft tissue, as was the case for our patient. In such cases, the surgical procedure can be time consuming and lead to more trauma to the surrounding tissues.10 These factors alone necessitate consideration of postoperative morbidity and mortality.
In our patient, the retained fragment was embedded in the wall of an abscess located retroperitoneally in his iliopsoas muscle. When considering the proximity of the iliopsoas muscle to the digestive tract, urinary tract, and iliac lymph nodes, it is reasonable for infectious material to come in contact with the foreign body from these nearby structures, resulting in secondary infection.11 Surgery was previously considered the first-line treatment for retroperitoneal abscesses until the advent of imaging-guided percutaneous drainage.12
In some instances, surgical drainage may still be attempted, such as if there are different disease processes requiring open surgery or if percutaneous catheter drainage is not technically possible due to the location of the abscess, thick exudate, loculation/septations, or phlegmon. In these cases, laparoscopic drainage as opposed to open surgical drainage can provide the benefits of an open procedure (ie, total drainage and resection of infected tissue) but is less invasive, requires a smaller incision, and heals faster.13 Percutaneous drainage is the current first-line treatment due to the lack of need for general anesthesia, lower cost, and better morbidity and mortality outcomes compared to surgical methods.12 While percutaneous drainage proved to be immediately therapeutic for our patient, the risk of abscess recurrence with the retained infected fragment necessitated coordination of procedures across specialties to provide the best outcome for the patient.
Conclusions
This case demonstrates a multidisciplinary approach to transforming an otherwise large retroperitoneal dissection to a minimally invasive and technically efficient abscess drainage and foreign body retrieval.
Shrapnel injuries are commonly encountered in war zones.1 Shrapnel injuries can remain asymptomatic or become systemic, with health effects of the retained foreign body ranging from local to systemic toxicities depending on the patient’s reaction to the chemical composition and corrosiveness of the fragments in vivo.2 We present a case of a reactivating shrapnel injury in the form of a retroperitoneal infection and subsequent iliopsoas abscess. A collaborative procedure was performed between surgery and interventional radiology to snare and remove the infected fragment and drain the abscess.
Case Presentation
While serving in Vietnam, a soldier sustained a fragment injury to his left lower abdomen. He underwent a laparotomy, small bowel resection, and a temporary ileostomy at the time of the injury. Nearly 50 years later, the patient presented with chronic left lower quadrant pain and a low-grade fever. He was diagnosed clinically in the emergency department (ED) with diverticulitis and treated with antibiotics. The patient initially responded to treatment but returned 6 months later with similar symptoms, low-grade fever, and mild leukocytosis. A computed tomography (CT) scan during that encounter without IV contrast revealed a few scattered colonic diverticula without definite diverticulitis as well as a metallic fragment embedded in the left iliopsoas with increased soft tissue density.
The patient was diagnosed with a pelvic/abdominal wall hematoma and was discharged with pain medication. The patient reported recurrent attacks of left lower quadrant pain, fever, and changes in bowel habits, prompting gastrointestinal consultation and a colonoscopy that was unremarkable. Ten months later, the patient again presented to the ED, with recurrent symptoms, a fever of 102 °F, and leukocytosis with a white blood cell count of 11.7 × 109/L. CT scan with IV contrast revealed a large left iliopsoas abscess associated with an approximately 1-cm metallic fragment (Figure 1). A drainage catheter was placed under CT guidance and approximately 270 mL of purulent fluid was drained. Culture of the fluid was positive for Escherichia coli (E coli). Two days after drain placement, the fragment was removed as a joint procedure with interventional radiology and surgery. Using the drainage catheter tract as a point of entry, multiple attempts were made to retrieve the fragment with Olympus EndoJaw endoscopic forceps without success.
Ultimately a stiff directional sheath from a Cook Medical transjugular liver biopsy kit was used with a Merit Medical EnSnare to relocate the fragment to the left inguinal region for surgical excision (Figures 2, 3, and 4). The fragment was removed and swabbed for culture and sensitivity and a BLAKE drain was placed in the evacuated abscess cavity. The patient tolerated the procedure well and was discharged the following day. Three days later, culture and sensitivity grew E coli and Acinetobacter, thus confirming infection and a nidus for the surrounding abscess formation. On follow-up with general surgery 7 days later, the patient reported he was doing well, and the drain was removed without difficulty.
Discussion
Foreign body injuries can be benign or debilitating depending on the initial damage, anatomical location of the foreign body, composition of the foreign body, and the patient’s response to it. Retained shrapnel deep within the muscle tissue rarely causes complications. Although many times embedded objects can be asymptomatic and require no further management, migration of the foreign body or the formation of a fistula is possible, causing symptoms and requiring surgical intervention.1 One case involved the formation of a purulent fistula appearing a year after an explosive wound to the lumbosacral spine, which was treated with antimicrobials. Recurrence of the fistula several times after treatment led to surgical removal of the shrapnel along with antibiotic treatment of the osteomyelitis.3 Although uncommon, lead exposure that occurs due to retained foreign body fragments from gunshot or military-related injuries can cause systemic lead toxicity. Symptoms may range from abdominal pain, nausea, and constipation to jaundice and hepatitis.4 The severity has also been stated to correlate with the surface area of the lead exposed for dissolution.5 Migration of foreign bodies and shrapnel to other sites in the body, such as movement from soft tissues into distantly located body cavities, have been reported as well. Such a case involved the spontaneous onset of knee synovitis due to an intra-articular metallic object that was introduced via a blast injury to the upper third of the ipsilateral thigh.1
In this patient’s case, a large intramuscular abscess had formed nearly 50 years after the initial combat injury, requiring drainage of the abscess and removal of the fragment. By snaring the foreign body to a more superficial site, the surgical removal only required a minor incision, decreasing recovery time and the likelihood of postoperative complications that would have been associated with a large retroperitoneal dissection. While loop snare is often the first-line technique for the removal of intravascular foreign bodies, its use in soft tissue retained materials is scarcely reported.6 The more typical uses involve the removal of intraluminal materials, such as partially fractured venous catheters, guide wires, stents, and vena cava filters. The same report mentioned that in all 16 cases of percutaneous foreign body retrieval, no surgical intervention was required.7 In the case of most nonvascular foreign bodies, however, surgical retrieval is usually performed.8
Surgical removal of foreign bodies can be difficult in cases where a foreign body is anatomically located next to vital structures.9 An additional challenge with a sole surgical approach to foreign body retrieval is when it is small in size and lies deep within the soft tissue, as was the case for our patient. In such cases, the surgical procedure can be time consuming and lead to more trauma to the surrounding tissues.10 These factors alone necessitate consideration of postoperative morbidity and mortality.
In our patient, the retained fragment was embedded in the wall of an abscess located retroperitoneally in his iliopsoas muscle. When considering the proximity of the iliopsoas muscle to the digestive tract, urinary tract, and iliac lymph nodes, it is reasonable for infectious material to come in contact with the foreign body from these nearby structures, resulting in secondary infection.11 Surgery was previously considered the first-line treatment for retroperitoneal abscesses until the advent of imaging-guided percutaneous drainage.12
In some instances, surgical drainage may still be attempted, such as if there are different disease processes requiring open surgery or if percutaneous catheter drainage is not technically possible due to the location of the abscess, thick exudate, loculation/septations, or phlegmon. In these cases, laparoscopic drainage as opposed to open surgical drainage can provide the benefits of an open procedure (ie, total drainage and resection of infected tissue) but is less invasive, requires a smaller incision, and heals faster.13 Percutaneous drainage is the current first-line treatment due to the lack of need for general anesthesia, lower cost, and better morbidity and mortality outcomes compared to surgical methods.12 While percutaneous drainage proved to be immediately therapeutic for our patient, the risk of abscess recurrence with the retained infected fragment necessitated coordination of procedures across specialties to provide the best outcome for the patient.
Conclusions
This case demonstrates a multidisciplinary approach to transforming an otherwise large retroperitoneal dissection to a minimally invasive and technically efficient abscess drainage and foreign body retrieval.
1. Schroeder JE, Lowe J, Chaimsky G, Liebergall M, Mosheiff R. Migrating shrapnel: a rare cause of knee synovitis. Mil Med. 2010;175(11):929-930. doi:10.7205/milmed-d-09-00254
2. Centeno JA, Rogers DA, van der Voet GB, et al. Embedded fragments from U.S. military personnel—chemical analysis and potential health implications. Int J Environ Res Public Health. 2014;11(2):1261-1278. Published 2014 Jan 23. doi:10.3390/ijerph110201261
3. Carija R, Busic Z, Bradaric N, Bulovic B, Borzic Z, Pavicic-Perkovic S. Surgical removal of metallic foreign body (shrapnel) from the lumbosacral spine and the treatment of chronic osteomyelitis: a case report. West Indian Med J. 2014;63(4):373-375. doi:10.7727/wimj.2012.290
4. Grasso I, Blattner M, Short T, Downs J. Severe systemic lead toxicity resulting from extra-articular retained shrapnel presenting as jaundice and hepatitis: a case report and review of the literature. Mil Med. 2017;182(3-4):e1843-e1848. doi:10.7205/MILMED-D-16-00231
5. Dillman RO, Crumb CK, Lidsky MJ. Lead poisoning from a gunshot wound: report of a case and review of the literature. Am J Med. 1979;66(3):509-514. doi:10.1016/0002-9343(79)91083-0
6. Woodhouse JB, Uberoi R. Techniques for intravascular foreign body retrieval. Cardiovasc Intervent Radiol. 2013;36(4):888-897. doi:10.1007/s00270-012-0488-8
7. Mallmann CV, Wolf KJ, Wacker FK. Retrieval of vascular foreign bodies using a self-made wire snare. Acta Radiol. 2008;49(10):1124-1128. doi:10.1080/02841850802454741
8. Nosher JL, Siegel R. Percutaneous retrieval of nonvascular foreign bodies. Radiology. 1993;187(3):649-651. doi:10.1148/radiology.187.3.8497610
9. Fu Y, Cui LG, Romagnoli C, Li ZQ, Lei YT. Ultrasound-guided removal of retained soft tissue foreign body with late presentation. Chin Med J (Engl). 2017;130(14):1753-1754. doi:10.4103/0366-6999.209910
10. Liang HD, Li H, Feng H, Zhao ZN, Song WJ, Yuan B. Application of intraoperative navigation and positioning system in the removal of deep foreign bodies in the limbs. Chin Med J (Engl). 2019;132(11):1375-1377. doi:10.1097/CM9.0000000000000253
11. Moriarty CM, Baker RJ. A pain in the psoas. Sports Health. 2016;8(6):568-572. doi:10.1177/1941738116665112
12. Akhan O, Durmaz H, Balcı S, Birgi E, Çiftçi T, Akıncı D. Percutaneous drainage of retroperitoneal abscesses: variables for success, failure, and recurrence. Diagn Interv Radiol. 2020;26(2):124-130. doi:10.5152/dir.2019.19199
13. Hong CH, Hong YC, Bae SH, et al. Laparoscopic drainage as a minimally invasive treatment for a psoas abscess: a single center case series and literature review. Medicine (Baltimore). 2020;99(14):e19640. doi:10.1097/MD.0000000000019640
1. Schroeder JE, Lowe J, Chaimsky G, Liebergall M, Mosheiff R. Migrating shrapnel: a rare cause of knee synovitis. Mil Med. 2010;175(11):929-930. doi:10.7205/milmed-d-09-00254
2. Centeno JA, Rogers DA, van der Voet GB, et al. Embedded fragments from U.S. military personnel—chemical analysis and potential health implications. Int J Environ Res Public Health. 2014;11(2):1261-1278. Published 2014 Jan 23. doi:10.3390/ijerph110201261
3. Carija R, Busic Z, Bradaric N, Bulovic B, Borzic Z, Pavicic-Perkovic S. Surgical removal of metallic foreign body (shrapnel) from the lumbosacral spine and the treatment of chronic osteomyelitis: a case report. West Indian Med J. 2014;63(4):373-375. doi:10.7727/wimj.2012.290
4. Grasso I, Blattner M, Short T, Downs J. Severe systemic lead toxicity resulting from extra-articular retained shrapnel presenting as jaundice and hepatitis: a case report and review of the literature. Mil Med. 2017;182(3-4):e1843-e1848. doi:10.7205/MILMED-D-16-00231
5. Dillman RO, Crumb CK, Lidsky MJ. Lead poisoning from a gunshot wound: report of a case and review of the literature. Am J Med. 1979;66(3):509-514. doi:10.1016/0002-9343(79)91083-0
6. Woodhouse JB, Uberoi R. Techniques for intravascular foreign body retrieval. Cardiovasc Intervent Radiol. 2013;36(4):888-897. doi:10.1007/s00270-012-0488-8
7. Mallmann CV, Wolf KJ, Wacker FK. Retrieval of vascular foreign bodies using a self-made wire snare. Acta Radiol. 2008;49(10):1124-1128. doi:10.1080/02841850802454741
8. Nosher JL, Siegel R. Percutaneous retrieval of nonvascular foreign bodies. Radiology. 1993;187(3):649-651. doi:10.1148/radiology.187.3.8497610
9. Fu Y, Cui LG, Romagnoli C, Li ZQ, Lei YT. Ultrasound-guided removal of retained soft tissue foreign body with late presentation. Chin Med J (Engl). 2017;130(14):1753-1754. doi:10.4103/0366-6999.209910
10. Liang HD, Li H, Feng H, Zhao ZN, Song WJ, Yuan B. Application of intraoperative navigation and positioning system in the removal of deep foreign bodies in the limbs. Chin Med J (Engl). 2019;132(11):1375-1377. doi:10.1097/CM9.0000000000000253
11. Moriarty CM, Baker RJ. A pain in the psoas. Sports Health. 2016;8(6):568-572. doi:10.1177/1941738116665112
12. Akhan O, Durmaz H, Balcı S, Birgi E, Çiftçi T, Akıncı D. Percutaneous drainage of retroperitoneal abscesses: variables for success, failure, and recurrence. Diagn Interv Radiol. 2020;26(2):124-130. doi:10.5152/dir.2019.19199
13. Hong CH, Hong YC, Bae SH, et al. Laparoscopic drainage as a minimally invasive treatment for a psoas abscess: a single center case series and literature review. Medicine (Baltimore). 2020;99(14):e19640. doi:10.1097/MD.0000000000019640
Successful Use of Lanadelumab in an Older Patient With Type II Hereditary Angioedema
Hereditary angioedema (HAE) is a rare genetic disorder affecting about 1 in 67,000 individuals and may lead to increased morbidity and mortality.1,2 HAE is characterized by recurring episodes of subcutaneous and/or submucosal edema without urticaria due to an excess of bradykinin.2,3 Autosomal dominant inheritance is present in 75% of patients with HAE and is classified into 2 main types.2 Type I HAE is caused by deficiency of C1 esterase inhibitor, accounting for 85% of cases.2 Type II HAE is marked by normal to elevated levels of C1 esterase inhibitor but with reduced activity.2
Cutaneous and abdominal angioedema attacks are the most common presentation.1 However, any location may be affected, including the face, oropharynx, and larynx.1 Only 0.9% of all HAE attacks cause laryngeal edema, but 50% of HAE patients have experienced a laryngeal attack, which may be lethal.1 An angioedema attack can range in severity, depending on the location and degree of edema.3 In addition, patients with HAE often are diagnosed with anxiety and depression secondary to their poor quality of life.4 Thus, long-term prophylaxis of attacks is crucial to reduce the physical and psychological implications.
Previously, HAE was treated with antifibrinolytic agents and attenuated androgens for short- and long-term prophylaxis.1 These treatment modalities are now considered second-line since the development of novel medications with improved efficacy and limited adverse effects (AEs).1 For long-term prophylaxis, subcutaneous and IV C1 esterase inhibitor has been proven effective in both types I and II HAE.1 Another option, lanadelumab, a subcutaneously delivered monoclonal antibody inhibitor of plasma kallikrein, has been proven to decrease the frequency of HAE attacks without significant AEs.5 Lanadelumab works by binding to the active site of plasma kallikrein, which reduces its activity and slows the production of bradykinin.6 This results in decreasing vascular permeability and swelling episodes in patients with HAE.7 Data, however, are limited, specifically regarding patients with type II HAE and patients aged ≥ 65 years.5 This article reports on an older male with type II HAE successfully treated with lanadelumab.
Case Presentation
An 81-year-old male patient with hypertension, hypertriglyceridemia, and aortic aneurysm had recurrent, frequent episodes of severe abdominal pain with a remote history of extremity and scrotal swelling since adolescence. He was misdiagnosed for years and was eventually determined to have HAE at age 75 years after his niece was diagnosed, prompting him to be reevaluated for his frequent bouts of abdominal pain. His laboratory findings were consistent with HAE type II with low C4 (7.8 mg/dL), normal C1 esterase inhibitor levels (24 mg/dL), and low levels of C1 esterase inhibitor activity (28% of normal).
Initially, he described having weekly attacks of abdominal pain that could last 1 to several days. At worst, these attacks would last up to a month, causing a decrease in appetite and weight loss. At age 77 years, he began an on-demand treatment, icatibant, a bradykinin receptor blocker. After initiating icatibant during an acute attack, the pain would diminish within 1 to 2 hours, and within several hours, he would be pain free. Previously, pain relief would take several days to weeks. He continued to use icatibant on-demand, typically requiring treatment every 1 to 2 months for only the more severe attacks.
After an increasing frequency of abdominal pain attacks, prophylactic medication was recommended. Therefore, subcutaneous lanadelumab 300 mg every 2 weeks was initiated for long-term prophylaxis. The patient went from requiring on-demand treatment 2 to 3 times per month to once in 6 months after starting lanadelumab. In addition, he tolerated the medication well without any AEs.
Discussion
According to the international WAO/EAACI 2021 guidelines, HAE treatment goals are “to achieve complete control of the disease and to normalize patients’ lives.”8 On-demand treatment options include C1 esterase inhibitor, icatibant, or ecallantide (a kallikrein inhibitor).8 Long-term prophylaxis in HAE should be considered, accounting for disease activity, burden, control, and patient preference. Five medications have been used for long-term prophylaxis: antifibrinolytic agents (not recommended), attenuated androgens (considered second-line), C1 esterase inhibitor, berotralstat, and lanadelumab.8
Antifibrinolytics are no longer recommended for long-term prophylactic treatment as their efficacy is poor and was not considered for our patient. Attenuated androgens, such as danazol, have a history of prophylactic use in patients with HAE due to their good efficacy but are suboptimal due to their significant AE profile and many drug-drug interactions.8 In addition, androgens have many contraindications, including hypertension and hypertriglyceridemia, which were both present in our patient. Consequently, danazol was not an advised treatment for our patient. C1 esterase inhibitor is often used to prevent HAE attacks and can be given intravenously or subcutaneously, typically administered biweekly. A potential AE of C1 esterase inhibitor is thrombosis.Therefore, C1 esterase inhibitor was not a preferred choice in our older patient with a history of hypercoagulability. Berotralstat, a plasma kallikrein inhibitor, is an oral treatment option that also has shown efficacy in long-term prophylaxis. The most common AEs of berotralstat tend to be gastrointestinal symptoms, and the medication requires dose adjustment for patients with hepatic impairment.8 Berotralstat was not considered because it was not an approved treatment option at the time of this patient’s treatment. Lanadelumab is a human monoclonal antibody against plasma kallikrein, which decreases bradykinin production in patients with HAE, thus preventing angioedema attacks.5 Data regarding the use of lanadelumab in patients with type II HAE are limited, but because HAE with normal C1 esterase inhibitor levels involves the production of bradykinin via kallikrein, lanadelumab should still be effective.1 Lanadelumab was chosen for our patient because of its minimal AEs and is not known to increase the risk of thrombosis.
Lanadelumab is a novel medication, recently approved in 2018 by the US Food and Drug Administration for the treatment of type I and type 2 HAE in patients aged ≥ 12 years.7 The phase 3 Hereditary Angioedema Long-term Prophylaxis (HELP) study concluded that treatment with subcutaneous lanadelumab for 26 weeks significantly decreased the frequency of angioedema attacks compared with placebo.5 However, 113 (90.4%) of patients in the phase III HELP study had type I HAE.5 Of the 125 patients that completed this randomized, double-blind study, only 12 had type II HAE.5 In addition, this study only included 5 patients aged ≥ 65 years.5 Also, no patients aged ≥ 65 years were part of the treatment arms that included a lanadelumab dose of 300 mg.5 In a case series of 12 patients in Canada, treatment with lanadelumab decreased angioedema attacks by 72%.9 However, the series only included 1 patient with type II HAE who was aged 36 years.9 Therefore, our case demonstrates the efficacy of lanadelumab in a patient aged ≥ 65 years with type II HAE.
Conclusions
HAE is a rare and potentially fatal disease characterized by recurrent, unpredictable attacks of edema throughout the body. The disease burden adversely affects a patient’s quality of life. Therefore, long-term prophylaxis is critical to managing patients with HAE. Lanadelumab has been proven as an effective long-term prophylactic treatment option for HAE attacks. This case supports the use of lanadelumab in patients with type II HAE and patients aged ≥ 65 years.
Acknowledgments
The patient was initially written up based on his delayed diagnosis as a case report.3 An earlier version of this article was presented by Samuel Weiss, MD, and Derek Smith, MD, as a poster at the American Academy of Allergy, Asthma, and Immunology virtual conference February 26 to March 1, 2021.
1. Busse PJ, Christiansen SC. Hereditary angioedema. N Engl J Med. 2020;382(12):1136-1148. doi:10.1056/NEJMra1808012
2. Bernstein JA. Severity of hereditary angioedema, prevalence, and diagnostic considerations. Am J Manag Care. 2018;24(14)(suppl):S292-S298.
3. Berger J, Carroll MP Jr, Champoux E, Coop CA. Extremely delayed diagnosis of type II hereditary angioedema: case report and review of the literature. Mil Med. 2018;183(11-12):e765-e767. doi:10.1093/milmed/usy031
4. Fouche AS, Saunders EF, Craig T. Depression and anxiety in patients with hereditary angioedema. Ann Allergy Asthma Immunol. 2014;112(4):371-375. doi:10.1016/j.anai.2013.05.028
5. Banerji A, Riedl MA, Bernstein JA, et al; HELP Investigators. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: a randomized clinical trial. JAMA. 2018;320(20):2108-2121. doi:10.1001/jama.2018.16773
6. Busse PJ, Farkas H, Banerji A, et al. Lanadelumab for the prophylactic treatment of hereditary angioedema with C1 inhibitor deficiency: a review of preclinical and phase I studies. BioDrugs. 2019;33(1):33-43. doi:10.1007/s40259-018-0325-y
7. Riedl MA, Maurer M, Bernstein JA, et al. Lanadelumab demonstrates rapid and sustained prevention of hereditary angioedema attacks. Allergy. 2020;75(11):2879-2887. doi:10.1111/all.14416
8. Maurer M, Magerl M, Betschel S, et al. The international WAO/EAACI guideline for the management of hereditary angioedema—the 2021 revision and update. Allergy. 2022;77(7):1961-1990. doi:10.1111/all.15214
9. Iaboni A, Kanani A, Lacuesta G, Song C, Kan M, Betschel SD. Impact of lanadelumab in hereditary angioedema: a case series of 12 patients in Canada. Allergy Asthma Clin Immunol. 2021;17(1):78. Published 2021 Jul 23. doi:10.1186/s13223-021-00579-6
Hereditary angioedema (HAE) is a rare genetic disorder affecting about 1 in 67,000 individuals and may lead to increased morbidity and mortality.1,2 HAE is characterized by recurring episodes of subcutaneous and/or submucosal edema without urticaria due to an excess of bradykinin.2,3 Autosomal dominant inheritance is present in 75% of patients with HAE and is classified into 2 main types.2 Type I HAE is caused by deficiency of C1 esterase inhibitor, accounting for 85% of cases.2 Type II HAE is marked by normal to elevated levels of C1 esterase inhibitor but with reduced activity.2
Cutaneous and abdominal angioedema attacks are the most common presentation.1 However, any location may be affected, including the face, oropharynx, and larynx.1 Only 0.9% of all HAE attacks cause laryngeal edema, but 50% of HAE patients have experienced a laryngeal attack, which may be lethal.1 An angioedema attack can range in severity, depending on the location and degree of edema.3 In addition, patients with HAE often are diagnosed with anxiety and depression secondary to their poor quality of life.4 Thus, long-term prophylaxis of attacks is crucial to reduce the physical and psychological implications.
Previously, HAE was treated with antifibrinolytic agents and attenuated androgens for short- and long-term prophylaxis.1 These treatment modalities are now considered second-line since the development of novel medications with improved efficacy and limited adverse effects (AEs).1 For long-term prophylaxis, subcutaneous and IV C1 esterase inhibitor has been proven effective in both types I and II HAE.1 Another option, lanadelumab, a subcutaneously delivered monoclonal antibody inhibitor of plasma kallikrein, has been proven to decrease the frequency of HAE attacks without significant AEs.5 Lanadelumab works by binding to the active site of plasma kallikrein, which reduces its activity and slows the production of bradykinin.6 This results in decreasing vascular permeability and swelling episodes in patients with HAE.7 Data, however, are limited, specifically regarding patients with type II HAE and patients aged ≥ 65 years.5 This article reports on an older male with type II HAE successfully treated with lanadelumab.
Case Presentation
An 81-year-old male patient with hypertension, hypertriglyceridemia, and aortic aneurysm had recurrent, frequent episodes of severe abdominal pain with a remote history of extremity and scrotal swelling since adolescence. He was misdiagnosed for years and was eventually determined to have HAE at age 75 years after his niece was diagnosed, prompting him to be reevaluated for his frequent bouts of abdominal pain. His laboratory findings were consistent with HAE type II with low C4 (7.8 mg/dL), normal C1 esterase inhibitor levels (24 mg/dL), and low levels of C1 esterase inhibitor activity (28% of normal).
Initially, he described having weekly attacks of abdominal pain that could last 1 to several days. At worst, these attacks would last up to a month, causing a decrease in appetite and weight loss. At age 77 years, he began an on-demand treatment, icatibant, a bradykinin receptor blocker. After initiating icatibant during an acute attack, the pain would diminish within 1 to 2 hours, and within several hours, he would be pain free. Previously, pain relief would take several days to weeks. He continued to use icatibant on-demand, typically requiring treatment every 1 to 2 months for only the more severe attacks.
After an increasing frequency of abdominal pain attacks, prophylactic medication was recommended. Therefore, subcutaneous lanadelumab 300 mg every 2 weeks was initiated for long-term prophylaxis. The patient went from requiring on-demand treatment 2 to 3 times per month to once in 6 months after starting lanadelumab. In addition, he tolerated the medication well without any AEs.
Discussion
According to the international WAO/EAACI 2021 guidelines, HAE treatment goals are “to achieve complete control of the disease and to normalize patients’ lives.”8 On-demand treatment options include C1 esterase inhibitor, icatibant, or ecallantide (a kallikrein inhibitor).8 Long-term prophylaxis in HAE should be considered, accounting for disease activity, burden, control, and patient preference. Five medications have been used for long-term prophylaxis: antifibrinolytic agents (not recommended), attenuated androgens (considered second-line), C1 esterase inhibitor, berotralstat, and lanadelumab.8
Antifibrinolytics are no longer recommended for long-term prophylactic treatment as their efficacy is poor and was not considered for our patient. Attenuated androgens, such as danazol, have a history of prophylactic use in patients with HAE due to their good efficacy but are suboptimal due to their significant AE profile and many drug-drug interactions.8 In addition, androgens have many contraindications, including hypertension and hypertriglyceridemia, which were both present in our patient. Consequently, danazol was not an advised treatment for our patient. C1 esterase inhibitor is often used to prevent HAE attacks and can be given intravenously or subcutaneously, typically administered biweekly. A potential AE of C1 esterase inhibitor is thrombosis.Therefore, C1 esterase inhibitor was not a preferred choice in our older patient with a history of hypercoagulability. Berotralstat, a plasma kallikrein inhibitor, is an oral treatment option that also has shown efficacy in long-term prophylaxis. The most common AEs of berotralstat tend to be gastrointestinal symptoms, and the medication requires dose adjustment for patients with hepatic impairment.8 Berotralstat was not considered because it was not an approved treatment option at the time of this patient’s treatment. Lanadelumab is a human monoclonal antibody against plasma kallikrein, which decreases bradykinin production in patients with HAE, thus preventing angioedema attacks.5 Data regarding the use of lanadelumab in patients with type II HAE are limited, but because HAE with normal C1 esterase inhibitor levels involves the production of bradykinin via kallikrein, lanadelumab should still be effective.1 Lanadelumab was chosen for our patient because of its minimal AEs and is not known to increase the risk of thrombosis.
Lanadelumab is a novel medication, recently approved in 2018 by the US Food and Drug Administration for the treatment of type I and type 2 HAE in patients aged ≥ 12 years.7 The phase 3 Hereditary Angioedema Long-term Prophylaxis (HELP) study concluded that treatment with subcutaneous lanadelumab for 26 weeks significantly decreased the frequency of angioedema attacks compared with placebo.5 However, 113 (90.4%) of patients in the phase III HELP study had type I HAE.5 Of the 125 patients that completed this randomized, double-blind study, only 12 had type II HAE.5 In addition, this study only included 5 patients aged ≥ 65 years.5 Also, no patients aged ≥ 65 years were part of the treatment arms that included a lanadelumab dose of 300 mg.5 In a case series of 12 patients in Canada, treatment with lanadelumab decreased angioedema attacks by 72%.9 However, the series only included 1 patient with type II HAE who was aged 36 years.9 Therefore, our case demonstrates the efficacy of lanadelumab in a patient aged ≥ 65 years with type II HAE.
Conclusions
HAE is a rare and potentially fatal disease characterized by recurrent, unpredictable attacks of edema throughout the body. The disease burden adversely affects a patient’s quality of life. Therefore, long-term prophylaxis is critical to managing patients with HAE. Lanadelumab has been proven as an effective long-term prophylactic treatment option for HAE attacks. This case supports the use of lanadelumab in patients with type II HAE and patients aged ≥ 65 years.
Acknowledgments
The patient was initially written up based on his delayed diagnosis as a case report.3 An earlier version of this article was presented by Samuel Weiss, MD, and Derek Smith, MD, as a poster at the American Academy of Allergy, Asthma, and Immunology virtual conference February 26 to March 1, 2021.
Hereditary angioedema (HAE) is a rare genetic disorder affecting about 1 in 67,000 individuals and may lead to increased morbidity and mortality.1,2 HAE is characterized by recurring episodes of subcutaneous and/or submucosal edema without urticaria due to an excess of bradykinin.2,3 Autosomal dominant inheritance is present in 75% of patients with HAE and is classified into 2 main types.2 Type I HAE is caused by deficiency of C1 esterase inhibitor, accounting for 85% of cases.2 Type II HAE is marked by normal to elevated levels of C1 esterase inhibitor but with reduced activity.2
Cutaneous and abdominal angioedema attacks are the most common presentation.1 However, any location may be affected, including the face, oropharynx, and larynx.1 Only 0.9% of all HAE attacks cause laryngeal edema, but 50% of HAE patients have experienced a laryngeal attack, which may be lethal.1 An angioedema attack can range in severity, depending on the location and degree of edema.3 In addition, patients with HAE often are diagnosed with anxiety and depression secondary to their poor quality of life.4 Thus, long-term prophylaxis of attacks is crucial to reduce the physical and psychological implications.
Previously, HAE was treated with antifibrinolytic agents and attenuated androgens for short- and long-term prophylaxis.1 These treatment modalities are now considered second-line since the development of novel medications with improved efficacy and limited adverse effects (AEs).1 For long-term prophylaxis, subcutaneous and IV C1 esterase inhibitor has been proven effective in both types I and II HAE.1 Another option, lanadelumab, a subcutaneously delivered monoclonal antibody inhibitor of plasma kallikrein, has been proven to decrease the frequency of HAE attacks without significant AEs.5 Lanadelumab works by binding to the active site of plasma kallikrein, which reduces its activity and slows the production of bradykinin.6 This results in decreasing vascular permeability and swelling episodes in patients with HAE.7 Data, however, are limited, specifically regarding patients with type II HAE and patients aged ≥ 65 years.5 This article reports on an older male with type II HAE successfully treated with lanadelumab.
Case Presentation
An 81-year-old male patient with hypertension, hypertriglyceridemia, and aortic aneurysm had recurrent, frequent episodes of severe abdominal pain with a remote history of extremity and scrotal swelling since adolescence. He was misdiagnosed for years and was eventually determined to have HAE at age 75 years after his niece was diagnosed, prompting him to be reevaluated for his frequent bouts of abdominal pain. His laboratory findings were consistent with HAE type II with low C4 (7.8 mg/dL), normal C1 esterase inhibitor levels (24 mg/dL), and low levels of C1 esterase inhibitor activity (28% of normal).
Initially, he described having weekly attacks of abdominal pain that could last 1 to several days. At worst, these attacks would last up to a month, causing a decrease in appetite and weight loss. At age 77 years, he began an on-demand treatment, icatibant, a bradykinin receptor blocker. After initiating icatibant during an acute attack, the pain would diminish within 1 to 2 hours, and within several hours, he would be pain free. Previously, pain relief would take several days to weeks. He continued to use icatibant on-demand, typically requiring treatment every 1 to 2 months for only the more severe attacks.
After an increasing frequency of abdominal pain attacks, prophylactic medication was recommended. Therefore, subcutaneous lanadelumab 300 mg every 2 weeks was initiated for long-term prophylaxis. The patient went from requiring on-demand treatment 2 to 3 times per month to once in 6 months after starting lanadelumab. In addition, he tolerated the medication well without any AEs.
Discussion
According to the international WAO/EAACI 2021 guidelines, HAE treatment goals are “to achieve complete control of the disease and to normalize patients’ lives.”8 On-demand treatment options include C1 esterase inhibitor, icatibant, or ecallantide (a kallikrein inhibitor).8 Long-term prophylaxis in HAE should be considered, accounting for disease activity, burden, control, and patient preference. Five medications have been used for long-term prophylaxis: antifibrinolytic agents (not recommended), attenuated androgens (considered second-line), C1 esterase inhibitor, berotralstat, and lanadelumab.8
Antifibrinolytics are no longer recommended for long-term prophylactic treatment as their efficacy is poor and was not considered for our patient. Attenuated androgens, such as danazol, have a history of prophylactic use in patients with HAE due to their good efficacy but are suboptimal due to their significant AE profile and many drug-drug interactions.8 In addition, androgens have many contraindications, including hypertension and hypertriglyceridemia, which were both present in our patient. Consequently, danazol was not an advised treatment for our patient. C1 esterase inhibitor is often used to prevent HAE attacks and can be given intravenously or subcutaneously, typically administered biweekly. A potential AE of C1 esterase inhibitor is thrombosis.Therefore, C1 esterase inhibitor was not a preferred choice in our older patient with a history of hypercoagulability. Berotralstat, a plasma kallikrein inhibitor, is an oral treatment option that also has shown efficacy in long-term prophylaxis. The most common AEs of berotralstat tend to be gastrointestinal symptoms, and the medication requires dose adjustment for patients with hepatic impairment.8 Berotralstat was not considered because it was not an approved treatment option at the time of this patient’s treatment. Lanadelumab is a human monoclonal antibody against plasma kallikrein, which decreases bradykinin production in patients with HAE, thus preventing angioedema attacks.5 Data regarding the use of lanadelumab in patients with type II HAE are limited, but because HAE with normal C1 esterase inhibitor levels involves the production of bradykinin via kallikrein, lanadelumab should still be effective.1 Lanadelumab was chosen for our patient because of its minimal AEs and is not known to increase the risk of thrombosis.
Lanadelumab is a novel medication, recently approved in 2018 by the US Food and Drug Administration for the treatment of type I and type 2 HAE in patients aged ≥ 12 years.7 The phase 3 Hereditary Angioedema Long-term Prophylaxis (HELP) study concluded that treatment with subcutaneous lanadelumab for 26 weeks significantly decreased the frequency of angioedema attacks compared with placebo.5 However, 113 (90.4%) of patients in the phase III HELP study had type I HAE.5 Of the 125 patients that completed this randomized, double-blind study, only 12 had type II HAE.5 In addition, this study only included 5 patients aged ≥ 65 years.5 Also, no patients aged ≥ 65 years were part of the treatment arms that included a lanadelumab dose of 300 mg.5 In a case series of 12 patients in Canada, treatment with lanadelumab decreased angioedema attacks by 72%.9 However, the series only included 1 patient with type II HAE who was aged 36 years.9 Therefore, our case demonstrates the efficacy of lanadelumab in a patient aged ≥ 65 years with type II HAE.
Conclusions
HAE is a rare and potentially fatal disease characterized by recurrent, unpredictable attacks of edema throughout the body. The disease burden adversely affects a patient’s quality of life. Therefore, long-term prophylaxis is critical to managing patients with HAE. Lanadelumab has been proven as an effective long-term prophylactic treatment option for HAE attacks. This case supports the use of lanadelumab in patients with type II HAE and patients aged ≥ 65 years.
Acknowledgments
The patient was initially written up based on his delayed diagnosis as a case report.3 An earlier version of this article was presented by Samuel Weiss, MD, and Derek Smith, MD, as a poster at the American Academy of Allergy, Asthma, and Immunology virtual conference February 26 to March 1, 2021.
1. Busse PJ, Christiansen SC. Hereditary angioedema. N Engl J Med. 2020;382(12):1136-1148. doi:10.1056/NEJMra1808012
2. Bernstein JA. Severity of hereditary angioedema, prevalence, and diagnostic considerations. Am J Manag Care. 2018;24(14)(suppl):S292-S298.
3. Berger J, Carroll MP Jr, Champoux E, Coop CA. Extremely delayed diagnosis of type II hereditary angioedema: case report and review of the literature. Mil Med. 2018;183(11-12):e765-e767. doi:10.1093/milmed/usy031
4. Fouche AS, Saunders EF, Craig T. Depression and anxiety in patients with hereditary angioedema. Ann Allergy Asthma Immunol. 2014;112(4):371-375. doi:10.1016/j.anai.2013.05.028
5. Banerji A, Riedl MA, Bernstein JA, et al; HELP Investigators. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: a randomized clinical trial. JAMA. 2018;320(20):2108-2121. doi:10.1001/jama.2018.16773
6. Busse PJ, Farkas H, Banerji A, et al. Lanadelumab for the prophylactic treatment of hereditary angioedema with C1 inhibitor deficiency: a review of preclinical and phase I studies. BioDrugs. 2019;33(1):33-43. doi:10.1007/s40259-018-0325-y
7. Riedl MA, Maurer M, Bernstein JA, et al. Lanadelumab demonstrates rapid and sustained prevention of hereditary angioedema attacks. Allergy. 2020;75(11):2879-2887. doi:10.1111/all.14416
8. Maurer M, Magerl M, Betschel S, et al. The international WAO/EAACI guideline for the management of hereditary angioedema—the 2021 revision and update. Allergy. 2022;77(7):1961-1990. doi:10.1111/all.15214
9. Iaboni A, Kanani A, Lacuesta G, Song C, Kan M, Betschel SD. Impact of lanadelumab in hereditary angioedema: a case series of 12 patients in Canada. Allergy Asthma Clin Immunol. 2021;17(1):78. Published 2021 Jul 23. doi:10.1186/s13223-021-00579-6
1. Busse PJ, Christiansen SC. Hereditary angioedema. N Engl J Med. 2020;382(12):1136-1148. doi:10.1056/NEJMra1808012
2. Bernstein JA. Severity of hereditary angioedema, prevalence, and diagnostic considerations. Am J Manag Care. 2018;24(14)(suppl):S292-S298.
3. Berger J, Carroll MP Jr, Champoux E, Coop CA. Extremely delayed diagnosis of type II hereditary angioedema: case report and review of the literature. Mil Med. 2018;183(11-12):e765-e767. doi:10.1093/milmed/usy031
4. Fouche AS, Saunders EF, Craig T. Depression and anxiety in patients with hereditary angioedema. Ann Allergy Asthma Immunol. 2014;112(4):371-375. doi:10.1016/j.anai.2013.05.028
5. Banerji A, Riedl MA, Bernstein JA, et al; HELP Investigators. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: a randomized clinical trial. JAMA. 2018;320(20):2108-2121. doi:10.1001/jama.2018.16773
6. Busse PJ, Farkas H, Banerji A, et al. Lanadelumab for the prophylactic treatment of hereditary angioedema with C1 inhibitor deficiency: a review of preclinical and phase I studies. BioDrugs. 2019;33(1):33-43. doi:10.1007/s40259-018-0325-y
7. Riedl MA, Maurer M, Bernstein JA, et al. Lanadelumab demonstrates rapid and sustained prevention of hereditary angioedema attacks. Allergy. 2020;75(11):2879-2887. doi:10.1111/all.14416
8. Maurer M, Magerl M, Betschel S, et al. The international WAO/EAACI guideline for the management of hereditary angioedema—the 2021 revision and update. Allergy. 2022;77(7):1961-1990. doi:10.1111/all.15214
9. Iaboni A, Kanani A, Lacuesta G, Song C, Kan M, Betschel SD. Impact of lanadelumab in hereditary angioedema: a case series of 12 patients in Canada. Allergy Asthma Clin Immunol. 2021;17(1):78. Published 2021 Jul 23. doi:10.1186/s13223-021-00579-6
56-year-old man • increased heart rate • weakness • intense sweating • horseradish consumption • Dx?
THE CASE
A 56-year-old physician (CUL) visited a local seafood restaurant, after having fasted since the prior evening. He had a history of hypertension that was well controlled with lisinopril/hydrochlorothiazide.
The physician and his party were seated outside, where the temperature was in the mid-70s. The group ordered oysters on the half shell accompanied by mignonette sauce, cocktail sauce, and horseradish. The physician ate an olive-size amount of horseradish with an oyster. He immediately complained of a sharp burning sensation in his stomach and remarked that the horseradish was significantly stronger than what he was accustomed to. Within 30 seconds, he noted an increased heart rate, weakness, and intense sweating. There was no increase in nasal secretions. Observers noted that he was very pale.
About 5 minutes after eating the horseradish, the physician leaned his head back and briefly lost consciousness. His wife, while supporting his head and checking his pulse, instructed other diners to call for emergency services, at which point the physician regained consciousness and the dispatcher was told that an ambulance was no longer necessary. Within a matter of minutes, all symptoms had abated, except for some mild weakness.
THE DIAGNOSIS
Ten minutes after the event, the physician identified his symptoms as a horseradish-induced vasovagal syncope (VVS), based on a case report published in JAMA in 1988, which his wife found after he asked her to do an Internet search of his symptoms.1
THE DISCUSSION
Horseradish’s active component is isothiocyanate. Horseradish-induced syncope is also called Seder syncope after the Jewish Passover holiday dinner at which observant Jews are required to eat “bitter herbs.”1,2 This type of syncope is thought to occur when horseradish vapors directly irritate the gastric or respiratory tract mucosa.
VVS commonly manifests for the first time at around age 13 years; however, the timing of that first occurrence can vary significantly among individuals (as in this case)
The loss of consciousness may be caused by an emotional trigger (eg, sight of blood, cast removal,8 blood or platelet donations9,10), a painful event (eg, an injection11), an orthostatic trigger12 (eg, prolonged standing), or visceral reflexes such as swallowing.13 In approximately 30% of cases, loss of consciousness is associated with memory loss.14 Loss of consciousness with VVS may be associated with injury in 33% of cases.15
Continue to: The recovery with awareness
The recovery with awareness of time, place, and person may be a feature of VVS, which would differentiate it from seizures and brainstem vascular events. Autonomic prodromal symptoms—including abdominal discomfort, pallor, sweating, and nausea—may precede the loss of consciousness.8
An evolutionary response?
VVS may have developed as a trait through evolution, although modern medicine treats it as a disease. Many potential explanations for VVS as a body defense mechanism have been proposed. Examples include fainting at the sight of blood, which developed during the Old Stone Age—a period with extreme human-to-human violence—or acting like a “possum playing dead” as a tactic designed to confuse an attacker.16
Another theory involves clot production and suggests that VVS-induced hypotension is a defense against bleeding by improving clot formation.17
A psychological defense theory maintains that the fainting and memory loss are designed to prevent a painful or overwhelming experience from being remembered. None of these theories, however, explain orthostatic VVS.18
The brain defense theory could explain all forms of VVS. It postulates that hypotension causes decreased cerebral perfusion, which leads to syncope resulting in the body returning to a more orthostatic position with increased cerebral profusion.19
Continue to: The patient
The patient in this case was able to leave the restaurant on his own volition 30 minutes after the event and resume normal activities. Ten days later, an electrocardiogram was performed, with negative results. In this case, the use of a potassium-wasting diuretic exacerbated the risk of a fluid-deprived state, hypokalemia, and hypotension, possibly contributing to the syncope. The patient has since “gotten back on the horseradish” without ill effect.
THE TAKEAWAY
Consumers and health care providers should be aware of the risks associated with consumption of fresh horseradish and should allow it to rest prior to ingestion to allow some evaporation of its active ingredient. An old case report saved the patient from an unnecessary (and costly) emergency department visit.
ACKNOWLEDGEMENTS
The authors would like to thank Terry J. Hannan, MBBS, FRACP, FACHI, FACMI for his critical review of the manuscript.
CORRESPONDENCE
Christoph U. Lehmann, MD, Clinical Informatics Center, 5323 Harry Hines Boulevard, Dallas, TX 75390; [email protected]
1. Rubin HR, Wu AW. The bitter herbs of Seder: more on horseradish horrors. JAMA. 1988;259:1943. doi: 10.1001/jama.259.13.1943b
2. Seder syncope. The Free Dictionary. Accessed July 20, 2022. https://medical-dictionary.thefreedictionary.com/Horseradish+Syncope
3. Sheldon RS, Sheldon AG, Connolly SJ, et al. Age of first faint in patients with vasovagal syncope. J Cardiovasc Electrophysiol. 2006;17:49-54. doi: 10.1111/j.1540-8167.2005.00267.x
4. Wallin BG, Sundlöf G. Sympathetic outflow to muscles during vasovagal syncope. J Auton Nerv Syst. 1982;6:287-291. doi: 10.1016/0165-1838(82)90001-7
5. Jardine DL, Melton IC, Crozier IG, et al. Decrease in cardiac output and muscle sympathetic activity during vasovagal syncope. Am J Physiol Heart Circ Physiol. 2002;282:H1804-H1809. doi: 10.1152/ajpheart.00640.2001
6. Waxman MB, Asta JA, Cameron DA. Localization of the reflex pathway responsible for the vasodepressor reaction induced by inferior vena caval occlusion and isoproterenol. Can J Physiol Pharmacol. 1992;70:882-889. doi: 10.1139/y92-118
7. Alboni P, Alboni M. Typical vasovagal syncope as a “defense mechanism” for the heart by contrasting sympathetic overactivity. Clin Auton Res. 2017;27:253-261. doi: 10.1007/s10286-017-0446-2
8. Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30:2631-2671. doi: 10.1093/eurheartj/ehp298
9. Davies J, MacDonald L, Sivakumar B, et al. Prospective analysis of syncope/pre-syncope in a tertiary paediatric orthopaedic fracture outpatient clinic. ANZ J Surg. 2021;91:668-672. doi: 10.1111/ans.16664
10. Almutairi H, Salam M, Batarfi K, et al. Incidence and severity of adverse events among platelet donors: a three-year retrospective study. Medicine (Baltimore). 2020;99:e23648. doi: 10.1097/MD.0000000000023648
11. Coakley A, Bailey A, Tao J, et al. Video education to improve clinical skills in the prevention of and response to vasovagal syncopal episodes. Int J Womens Dermatol. 2020;6:186-190. doi: 10.1016/j.ijwd.2020.02.002
12. Thijs RD, Brignole M, Falup-Pecurariu C, et al. Recommendations for tilt table testing and other provocative cardiovascular autonomic tests in conditions that may cause transient loss of consciousness: consensus statement of the European Federation of Autonomic Societies (EFAS) endorsed by the American Autonomic Society (AAS) and the European Academy of Neurology (EAN). Auton Neurosci. 2021;233:102792. doi: 10.1016/j.autneu.2021.102792
13. Nakagawa S, Hisanaga S, Kondoh H, et al. A case of swallow syncope induced by vagotonic visceral reflex resulting in atrioventricular node suppression. J Electrocardiol. 1987;20:65-69. doi: 10.1016/0022-0736(87)90010-0
14. O’Dwyer C, Bennett K, Langan Y, et al. Amnesia for loss of consciousness is common in vasovagal syncope. Europace. 2011;13:1040-1045. doi: 10.1093/europace/eur069
15. Jorge JG, Raj SR, Teixeira PS, et al. Likelihood of injury due to vasovagal syncope: a systematic review and meta-analysis. Europace. 2021;23:1092-1099. doi: 10.1093/europace/euab041
16. Bracha HS, Bracha AS, Williams AE, et al. The human fear-circuitry and fear-induced fainting in healthy individuals—the paleolithic-threat hypothesis. Clin Auton Res. 2005;15:238-241. doi: 10.1007/s10286-005-0245-z
17. Diehl RR. Vasovagal syncope and Darwinian fitness. Clin Auton Res. 2005;15:126-129. doi: 10.1007/s10286-005-0244-0
18. Engel CL, Romano J. Studies of syncope; biologic interpretation of vasodepressor syncope. Psychosom Med. 1947;9:288-294. doi: 10.1097/00006842-194709000-00002
19. Blanc JJ, Benditt DG. Vasovagal syncope: hypothesis focusing on its being a clinical feature unique to humans. J Cardiovasc Electrophysiol. 2016;27:623-629. doi: 10.1111/jce.12945
THE CASE
A 56-year-old physician (CUL) visited a local seafood restaurant, after having fasted since the prior evening. He had a history of hypertension that was well controlled with lisinopril/hydrochlorothiazide.
The physician and his party were seated outside, where the temperature was in the mid-70s. The group ordered oysters on the half shell accompanied by mignonette sauce, cocktail sauce, and horseradish. The physician ate an olive-size amount of horseradish with an oyster. He immediately complained of a sharp burning sensation in his stomach and remarked that the horseradish was significantly stronger than what he was accustomed to. Within 30 seconds, he noted an increased heart rate, weakness, and intense sweating. There was no increase in nasal secretions. Observers noted that he was very pale.
About 5 minutes after eating the horseradish, the physician leaned his head back and briefly lost consciousness. His wife, while supporting his head and checking his pulse, instructed other diners to call for emergency services, at which point the physician regained consciousness and the dispatcher was told that an ambulance was no longer necessary. Within a matter of minutes, all symptoms had abated, except for some mild weakness.
THE DIAGNOSIS
Ten minutes after the event, the physician identified his symptoms as a horseradish-induced vasovagal syncope (VVS), based on a case report published in JAMA in 1988, which his wife found after he asked her to do an Internet search of his symptoms.1
THE DISCUSSION
Horseradish’s active component is isothiocyanate. Horseradish-induced syncope is also called Seder syncope after the Jewish Passover holiday dinner at which observant Jews are required to eat “bitter herbs.”1,2 This type of syncope is thought to occur when horseradish vapors directly irritate the gastric or respiratory tract mucosa.
VVS commonly manifests for the first time at around age 13 years; however, the timing of that first occurrence can vary significantly among individuals (as in this case)
The loss of consciousness may be caused by an emotional trigger (eg, sight of blood, cast removal,8 blood or platelet donations9,10), a painful event (eg, an injection11), an orthostatic trigger12 (eg, prolonged standing), or visceral reflexes such as swallowing.13 In approximately 30% of cases, loss of consciousness is associated with memory loss.14 Loss of consciousness with VVS may be associated with injury in 33% of cases.15
Continue to: The recovery with awareness
The recovery with awareness of time, place, and person may be a feature of VVS, which would differentiate it from seizures and brainstem vascular events. Autonomic prodromal symptoms—including abdominal discomfort, pallor, sweating, and nausea—may precede the loss of consciousness.8
An evolutionary response?
VVS may have developed as a trait through evolution, although modern medicine treats it as a disease. Many potential explanations for VVS as a body defense mechanism have been proposed. Examples include fainting at the sight of blood, which developed during the Old Stone Age—a period with extreme human-to-human violence—or acting like a “possum playing dead” as a tactic designed to confuse an attacker.16
Another theory involves clot production and suggests that VVS-induced hypotension is a defense against bleeding by improving clot formation.17
A psychological defense theory maintains that the fainting and memory loss are designed to prevent a painful or overwhelming experience from being remembered. None of these theories, however, explain orthostatic VVS.18
The brain defense theory could explain all forms of VVS. It postulates that hypotension causes decreased cerebral perfusion, which leads to syncope resulting in the body returning to a more orthostatic position with increased cerebral profusion.19
Continue to: The patient
The patient in this case was able to leave the restaurant on his own volition 30 minutes after the event and resume normal activities. Ten days later, an electrocardiogram was performed, with negative results. In this case, the use of a potassium-wasting diuretic exacerbated the risk of a fluid-deprived state, hypokalemia, and hypotension, possibly contributing to the syncope. The patient has since “gotten back on the horseradish” without ill effect.
THE TAKEAWAY
Consumers and health care providers should be aware of the risks associated with consumption of fresh horseradish and should allow it to rest prior to ingestion to allow some evaporation of its active ingredient. An old case report saved the patient from an unnecessary (and costly) emergency department visit.
ACKNOWLEDGEMENTS
The authors would like to thank Terry J. Hannan, MBBS, FRACP, FACHI, FACMI for his critical review of the manuscript.
CORRESPONDENCE
Christoph U. Lehmann, MD, Clinical Informatics Center, 5323 Harry Hines Boulevard, Dallas, TX 75390; [email protected]
THE CASE
A 56-year-old physician (CUL) visited a local seafood restaurant, after having fasted since the prior evening. He had a history of hypertension that was well controlled with lisinopril/hydrochlorothiazide.
The physician and his party were seated outside, where the temperature was in the mid-70s. The group ordered oysters on the half shell accompanied by mignonette sauce, cocktail sauce, and horseradish. The physician ate an olive-size amount of horseradish with an oyster. He immediately complained of a sharp burning sensation in his stomach and remarked that the horseradish was significantly stronger than what he was accustomed to. Within 30 seconds, he noted an increased heart rate, weakness, and intense sweating. There was no increase in nasal secretions. Observers noted that he was very pale.
About 5 minutes after eating the horseradish, the physician leaned his head back and briefly lost consciousness. His wife, while supporting his head and checking his pulse, instructed other diners to call for emergency services, at which point the physician regained consciousness and the dispatcher was told that an ambulance was no longer necessary. Within a matter of minutes, all symptoms had abated, except for some mild weakness.
THE DIAGNOSIS
Ten minutes after the event, the physician identified his symptoms as a horseradish-induced vasovagal syncope (VVS), based on a case report published in JAMA in 1988, which his wife found after he asked her to do an Internet search of his symptoms.1
THE DISCUSSION
Horseradish’s active component is isothiocyanate. Horseradish-induced syncope is also called Seder syncope after the Jewish Passover holiday dinner at which observant Jews are required to eat “bitter herbs.”1,2 This type of syncope is thought to occur when horseradish vapors directly irritate the gastric or respiratory tract mucosa.
VVS commonly manifests for the first time at around age 13 years; however, the timing of that first occurrence can vary significantly among individuals (as in this case)
The loss of consciousness may be caused by an emotional trigger (eg, sight of blood, cast removal,8 blood or platelet donations9,10), a painful event (eg, an injection11), an orthostatic trigger12 (eg, prolonged standing), or visceral reflexes such as swallowing.13 In approximately 30% of cases, loss of consciousness is associated with memory loss.14 Loss of consciousness with VVS may be associated with injury in 33% of cases.15
Continue to: The recovery with awareness
The recovery with awareness of time, place, and person may be a feature of VVS, which would differentiate it from seizures and brainstem vascular events. Autonomic prodromal symptoms—including abdominal discomfort, pallor, sweating, and nausea—may precede the loss of consciousness.8
An evolutionary response?
VVS may have developed as a trait through evolution, although modern medicine treats it as a disease. Many potential explanations for VVS as a body defense mechanism have been proposed. Examples include fainting at the sight of blood, which developed during the Old Stone Age—a period with extreme human-to-human violence—or acting like a “possum playing dead” as a tactic designed to confuse an attacker.16
Another theory involves clot production and suggests that VVS-induced hypotension is a defense against bleeding by improving clot formation.17
A psychological defense theory maintains that the fainting and memory loss are designed to prevent a painful or overwhelming experience from being remembered. None of these theories, however, explain orthostatic VVS.18
The brain defense theory could explain all forms of VVS. It postulates that hypotension causes decreased cerebral perfusion, which leads to syncope resulting in the body returning to a more orthostatic position with increased cerebral profusion.19
Continue to: The patient
The patient in this case was able to leave the restaurant on his own volition 30 minutes after the event and resume normal activities. Ten days later, an electrocardiogram was performed, with negative results. In this case, the use of a potassium-wasting diuretic exacerbated the risk of a fluid-deprived state, hypokalemia, and hypotension, possibly contributing to the syncope. The patient has since “gotten back on the horseradish” without ill effect.
THE TAKEAWAY
Consumers and health care providers should be aware of the risks associated with consumption of fresh horseradish and should allow it to rest prior to ingestion to allow some evaporation of its active ingredient. An old case report saved the patient from an unnecessary (and costly) emergency department visit.
ACKNOWLEDGEMENTS
The authors would like to thank Terry J. Hannan, MBBS, FRACP, FACHI, FACMI for his critical review of the manuscript.
CORRESPONDENCE
Christoph U. Lehmann, MD, Clinical Informatics Center, 5323 Harry Hines Boulevard, Dallas, TX 75390; [email protected]
1. Rubin HR, Wu AW. The bitter herbs of Seder: more on horseradish horrors. JAMA. 1988;259:1943. doi: 10.1001/jama.259.13.1943b
2. Seder syncope. The Free Dictionary. Accessed July 20, 2022. https://medical-dictionary.thefreedictionary.com/Horseradish+Syncope
3. Sheldon RS, Sheldon AG, Connolly SJ, et al. Age of first faint in patients with vasovagal syncope. J Cardiovasc Electrophysiol. 2006;17:49-54. doi: 10.1111/j.1540-8167.2005.00267.x
4. Wallin BG, Sundlöf G. Sympathetic outflow to muscles during vasovagal syncope. J Auton Nerv Syst. 1982;6:287-291. doi: 10.1016/0165-1838(82)90001-7
5. Jardine DL, Melton IC, Crozier IG, et al. Decrease in cardiac output and muscle sympathetic activity during vasovagal syncope. Am J Physiol Heart Circ Physiol. 2002;282:H1804-H1809. doi: 10.1152/ajpheart.00640.2001
6. Waxman MB, Asta JA, Cameron DA. Localization of the reflex pathway responsible for the vasodepressor reaction induced by inferior vena caval occlusion and isoproterenol. Can J Physiol Pharmacol. 1992;70:882-889. doi: 10.1139/y92-118
7. Alboni P, Alboni M. Typical vasovagal syncope as a “defense mechanism” for the heart by contrasting sympathetic overactivity. Clin Auton Res. 2017;27:253-261. doi: 10.1007/s10286-017-0446-2
8. Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30:2631-2671. doi: 10.1093/eurheartj/ehp298
9. Davies J, MacDonald L, Sivakumar B, et al. Prospective analysis of syncope/pre-syncope in a tertiary paediatric orthopaedic fracture outpatient clinic. ANZ J Surg. 2021;91:668-672. doi: 10.1111/ans.16664
10. Almutairi H, Salam M, Batarfi K, et al. Incidence and severity of adverse events among platelet donors: a three-year retrospective study. Medicine (Baltimore). 2020;99:e23648. doi: 10.1097/MD.0000000000023648
11. Coakley A, Bailey A, Tao J, et al. Video education to improve clinical skills in the prevention of and response to vasovagal syncopal episodes. Int J Womens Dermatol. 2020;6:186-190. doi: 10.1016/j.ijwd.2020.02.002
12. Thijs RD, Brignole M, Falup-Pecurariu C, et al. Recommendations for tilt table testing and other provocative cardiovascular autonomic tests in conditions that may cause transient loss of consciousness: consensus statement of the European Federation of Autonomic Societies (EFAS) endorsed by the American Autonomic Society (AAS) and the European Academy of Neurology (EAN). Auton Neurosci. 2021;233:102792. doi: 10.1016/j.autneu.2021.102792
13. Nakagawa S, Hisanaga S, Kondoh H, et al. A case of swallow syncope induced by vagotonic visceral reflex resulting in atrioventricular node suppression. J Electrocardiol. 1987;20:65-69. doi: 10.1016/0022-0736(87)90010-0
14. O’Dwyer C, Bennett K, Langan Y, et al. Amnesia for loss of consciousness is common in vasovagal syncope. Europace. 2011;13:1040-1045. doi: 10.1093/europace/eur069
15. Jorge JG, Raj SR, Teixeira PS, et al. Likelihood of injury due to vasovagal syncope: a systematic review and meta-analysis. Europace. 2021;23:1092-1099. doi: 10.1093/europace/euab041
16. Bracha HS, Bracha AS, Williams AE, et al. The human fear-circuitry and fear-induced fainting in healthy individuals—the paleolithic-threat hypothesis. Clin Auton Res. 2005;15:238-241. doi: 10.1007/s10286-005-0245-z
17. Diehl RR. Vasovagal syncope and Darwinian fitness. Clin Auton Res. 2005;15:126-129. doi: 10.1007/s10286-005-0244-0
18. Engel CL, Romano J. Studies of syncope; biologic interpretation of vasodepressor syncope. Psychosom Med. 1947;9:288-294. doi: 10.1097/00006842-194709000-00002
19. Blanc JJ, Benditt DG. Vasovagal syncope: hypothesis focusing on its being a clinical feature unique to humans. J Cardiovasc Electrophysiol. 2016;27:623-629. doi: 10.1111/jce.12945
1. Rubin HR, Wu AW. The bitter herbs of Seder: more on horseradish horrors. JAMA. 1988;259:1943. doi: 10.1001/jama.259.13.1943b
2. Seder syncope. The Free Dictionary. Accessed July 20, 2022. https://medical-dictionary.thefreedictionary.com/Horseradish+Syncope
3. Sheldon RS, Sheldon AG, Connolly SJ, et al. Age of first faint in patients with vasovagal syncope. J Cardiovasc Electrophysiol. 2006;17:49-54. doi: 10.1111/j.1540-8167.2005.00267.x
4. Wallin BG, Sundlöf G. Sympathetic outflow to muscles during vasovagal syncope. J Auton Nerv Syst. 1982;6:287-291. doi: 10.1016/0165-1838(82)90001-7
5. Jardine DL, Melton IC, Crozier IG, et al. Decrease in cardiac output and muscle sympathetic activity during vasovagal syncope. Am J Physiol Heart Circ Physiol. 2002;282:H1804-H1809. doi: 10.1152/ajpheart.00640.2001
6. Waxman MB, Asta JA, Cameron DA. Localization of the reflex pathway responsible for the vasodepressor reaction induced by inferior vena caval occlusion and isoproterenol. Can J Physiol Pharmacol. 1992;70:882-889. doi: 10.1139/y92-118
7. Alboni P, Alboni M. Typical vasovagal syncope as a “defense mechanism” for the heart by contrasting sympathetic overactivity. Clin Auton Res. 2017;27:253-261. doi: 10.1007/s10286-017-0446-2
8. Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30:2631-2671. doi: 10.1093/eurheartj/ehp298
9. Davies J, MacDonald L, Sivakumar B, et al. Prospective analysis of syncope/pre-syncope in a tertiary paediatric orthopaedic fracture outpatient clinic. ANZ J Surg. 2021;91:668-672. doi: 10.1111/ans.16664
10. Almutairi H, Salam M, Batarfi K, et al. Incidence and severity of adverse events among platelet donors: a three-year retrospective study. Medicine (Baltimore). 2020;99:e23648. doi: 10.1097/MD.0000000000023648
11. Coakley A, Bailey A, Tao J, et al. Video education to improve clinical skills in the prevention of and response to vasovagal syncopal episodes. Int J Womens Dermatol. 2020;6:186-190. doi: 10.1016/j.ijwd.2020.02.002
12. Thijs RD, Brignole M, Falup-Pecurariu C, et al. Recommendations for tilt table testing and other provocative cardiovascular autonomic tests in conditions that may cause transient loss of consciousness: consensus statement of the European Federation of Autonomic Societies (EFAS) endorsed by the American Autonomic Society (AAS) and the European Academy of Neurology (EAN). Auton Neurosci. 2021;233:102792. doi: 10.1016/j.autneu.2021.102792
13. Nakagawa S, Hisanaga S, Kondoh H, et al. A case of swallow syncope induced by vagotonic visceral reflex resulting in atrioventricular node suppression. J Electrocardiol. 1987;20:65-69. doi: 10.1016/0022-0736(87)90010-0
14. O’Dwyer C, Bennett K, Langan Y, et al. Amnesia for loss of consciousness is common in vasovagal syncope. Europace. 2011;13:1040-1045. doi: 10.1093/europace/eur069
15. Jorge JG, Raj SR, Teixeira PS, et al. Likelihood of injury due to vasovagal syncope: a systematic review and meta-analysis. Europace. 2021;23:1092-1099. doi: 10.1093/europace/euab041
16. Bracha HS, Bracha AS, Williams AE, et al. The human fear-circuitry and fear-induced fainting in healthy individuals—the paleolithic-threat hypothesis. Clin Auton Res. 2005;15:238-241. doi: 10.1007/s10286-005-0245-z
17. Diehl RR. Vasovagal syncope and Darwinian fitness. Clin Auton Res. 2005;15:126-129. doi: 10.1007/s10286-005-0244-0
18. Engel CL, Romano J. Studies of syncope; biologic interpretation of vasodepressor syncope. Psychosom Med. 1947;9:288-294. doi: 10.1097/00006842-194709000-00002
19. Blanc JJ, Benditt DG. Vasovagal syncope: hypothesis focusing on its being a clinical feature unique to humans. J Cardiovasc Electrophysiol. 2016;27:623-629. doi: 10.1111/jce.12945
Angiolymphoid Hyperplasia with Eosinophilia in a Patient With Coccidioidomycosis
Angiolymphoid hyperplasia with eosinophilia (ALHE) is a rare nodular unencapsulated mass that is characterized by benign anomalous vascular hyperplasia of epithelioidlike endothelial cells attached to dilated blood vessels. The mass is surrounded by lymphocytes and eosinophils that can present clinically as papules, plaques, or nodules.1 The etiology of ALHE is unknown; it is hypothesized that it is a vascular neoplasm or a lymphoproliferative disorder.
Coccidioidomycosis (CM) is a prevalent deep fungal infection endemic to the southwestern United States caused by Coccidioides immitis and Coccidioides posadasii. Infection can occur from direct inoculation through abrasions or direct trauma but usually occurs through the inhalation of spores and can result in a reactive rash (eg, Sweet syndrome, erythema nodosum, interstitial granulomatous dermatitis).2 Coccidioidomycosis also can result in respiratory pneumonia and dissemination from pulmonary infection of the skin. As such, it is important to distinguish CM and its immunologically mediated eruptions for accurate diagnosis and treatment.
We report a novel case of ALHE as a reactive dermatologic presentation in a patient with CM.
Case Report
A 72-year-old woman presented to the dermatology clinic with itchy papules and plaques on the arms and legs of 17 years’ duration. Her medical history included coronary artery disease and hypercholesterolemia as well as a remote history of cutaneous marginal zone B-cell lymphoma of the nose, which was confirmed by histology and treated more than 10 years prior and has remained in remission for 6 years. Her current medications included aspirin, atorvastatin, lisinopril, and metoprolol succinate.
Our patient first presented to our dermatology clinic for itchy nodules and papules on the legs and arms. The patient previously had been seen by another dermatologist 2 months prior for the same condition. At that time, biopsies of the lesions were reported as prurigo nodules. Physical examination at the current presentation revealed round, pink to flesh-colored, raised papules and plaques scattered on the arms and legs (Figure 1). The differential diagnosis included lymphomatoid papulosis, cutaneous B-cell lymphoma, pseudolymphoma, cutaneous CM, and papular mucinosis.
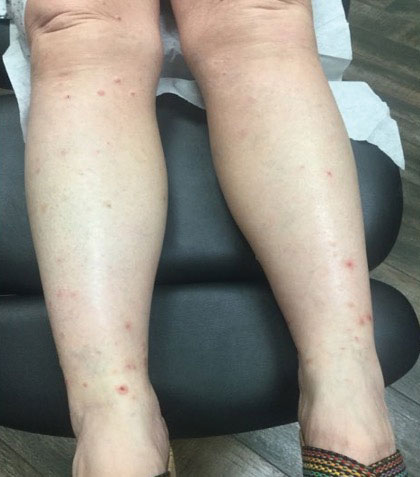
Four-mm punch biopsies of the right proximal pretibial region and left knee region were taken and sent for histologic analysis, direct immunofluorescence testing, and tissue culture. Testing for atypical mycobacteria and deep fungal infection was negative; bacterial cultures and sensitivity testing were negative. Direct immunofluorescence testing was negative. Microscopic examination of material from the right proximal pretibial region showed widely dilated, variously shaped, large blood vessels in a multinodular pattern; the vessels also were surrounded by an inflammatory cell infiltrate containing eosinophils. Histologic findings were consistent with ALHE.
Subsequent biopsies were completed 2 weeks and 1 month from the initial presentation. Both histology reports—from 2 different histopathology laboratories—were consistent with ALHE (Figure 2). Additional work-up during the patient’s initial visit to our clinic for the rash included CM serologic testing, which demonstrated IgM and IgG antibodies. Subsequently, chest radiography revealed a 2.2×2.3-cm mass in the right lower lobe of the lung. Follow-up computed tomography 1 month later confirmed the nodule in the same area to be 2.3×2.1×1.8 cm.
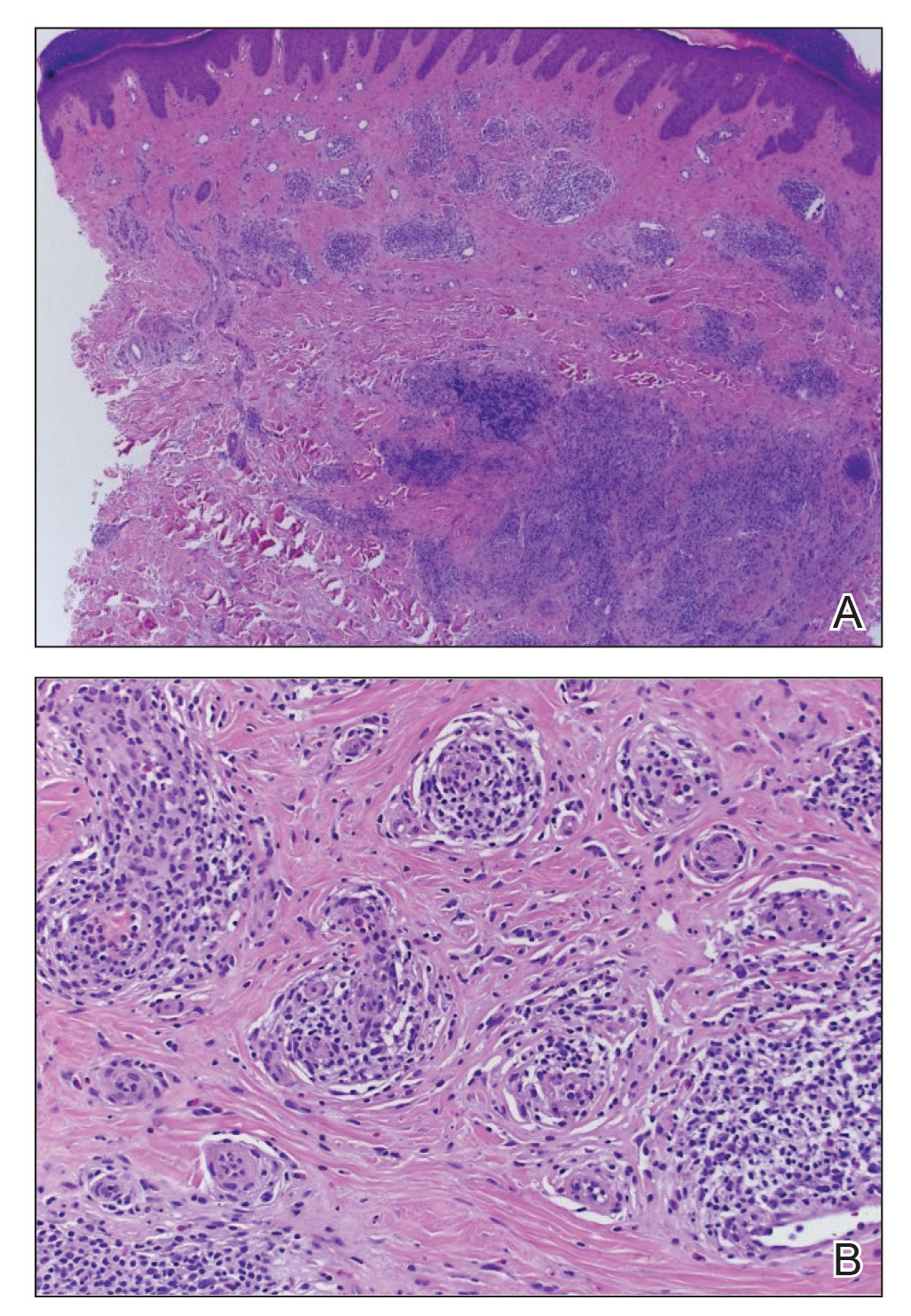
The patient was referred to pulmonology and was treated for pulmonary CM with oral fluconazole 200 mg twice daily for 4 months. Initial treatment also included clobetasol cream 0.05% applied twice daily, which did not produce marked improvement in pruritus. Narrowband UVB phototherapy was attempted, but the patient could not complete the course because of travel time to the office; however, the patient’s ALHE improved considerably with the fluconazole treatment for pulmonary CM.
Oral doxycycline 100 mg twice daily was added to the fluconazole 2 months after her initial visit to our office, which kept the ALHE at bay and helped with the pruritus (Figure 3). Pulmonology and primary care comanaged the pulmonary CM with oral fluconazole 200 mg twice daily. Repeat serologic testing for CM was negative for IgG and IgM after 14 months since the initial visit to the office.
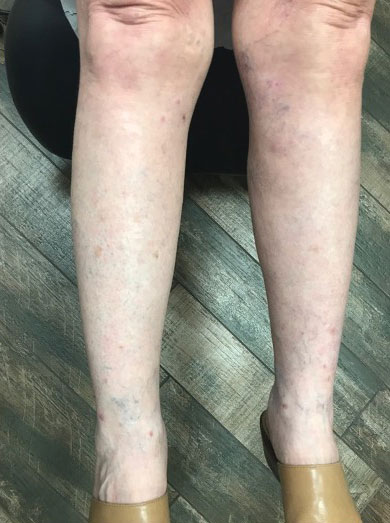
Comment
Pulmonary CM infection has varying dermatologic manifestations. A PubMed search of articles indexed for MEDLINE using the terms ALHE and coccidioidomycosis yielded no case reports; in fact, there have been few reported cases of ALHE at all. Notable conditions associated with ALHE include membranous nephropathy and arteriovenous malformations treated with corticosteroids and surgery, respectively.3,4 Our case is a rare presentation of CM infection manifesting with ALHE. Following treatment and remission for our patient’s CM infection, the ALHE lesion decreased in size.
Standard treatment of uncomplicated CM involves azole antifungals, typically oral fluconazole or itraconazole 400 to 600 mg/d. In more severe cases (eg, immunocompromised patients) amphotericin B can be used.5 Our patient was treated with oral fluconazole 200 mg twice daily for 4 months.
In the literature, treatment via surgical excision, steroid injection, pulsed-dye laser therapy, and radiotherapy also has been described.6-8 Antibiotics including clindamycin, doxycycline, and amoxicillin-clavulanate also have been shown to be effective.9
In our patient, ALHE improved when oral doxycycline 100 mg twice daily was added to the oral fluconazole. In fact, after 4 months of treatment, the CM infection and ALHE lesions both improved to a point at which the lesions were not visible. When those lesions recurred 15 months later, they responded with another course of doxycycline and fluconazole.
Upon recurrence, the patient was asked to have her care transferred to her pulmonologist, who then managed the fluconazole regimen. During the pulmonologist’s workup, no peripheral eosinophilia was found. This is important because eosinophils can be a marker for CM infection; in this case, however, the ALHE lesion was a reactive process to the infection. Classically known to play a reactive role in fungal infection, these white blood cells demonstrate reactivity to the environmental fungus Alternaria alternata by contact-dependent killing, utilizing β2 integrins and CD11b to recognize and adhere to β-glucan. Eosinophils react through contact-dependent killing, releasing cytotoxic granule proteins and proinflammatory mediators, and have been documented to occur in CM and Paracoccidioides brasiliensis infection, in which they deposit major basic protein on the organism.10 Most pertinent to our case with ALHE and CM is the ability of eosinophils to communicate with other immune cells. Eosinophils play a role in the active inflammation of CM through cytokine signaling, which may propagate formation of ALHE.
The function of eosinophils in ALHE is poorly understood; it is unclear whether they act as a primary driver of pathogenesis or are simply indicators of secondary infiltration or infection. Our review of the current literature suggests that eosinophils are unnecessary for progression of ALHE but might be involved at its onset. As reported, even monoclonal antibody therapy (eg, mepolizumab and benralizumab) that effectively depletes eosinophil levels by negating IL-5 signaling do not slow progression of ALHE.11 Symptomatic changes are modest at best (ie, simply softening the ALHE nodules).
Our patient had no peripheral eosinophilia, suggesting that the onset of ALHE might not be caused by eosinophilia but a different inflammatory process—in this patient, by CM. Because peripheral eosinophilia was not seen in our patient, the presence of eosinophils in the ALHE lesion likely is unnecessary for its onset or progression but is a secondary process that exacerbates the lesion. The pathogenesis is unknown but could be directed toward lymphocytes and plasma cells, with eosinophils as part of the dynamic process.11
Conclusion
Because reports of an association between CM and ALHE are limited, our case is distinguished by a unique clinical presentation of ALHE. When a patient is given a diagnosis of ALHE, it therefore is important to consider exposure to CM as a cause, especially in patients who reside in or travel to a region where CM is endemic.
- Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol. 1969;81:1-14. doi:10.1111/j.1365-2133.1969.tb15914.x
- DiCaudo D. Coccidioidomycosis. Semin Cutan Med Surg. 2014;33:140-145. doi:10.12788/j.sder.0111
- Onishi Y, Ohara K. Angiolymphoid hyperplasia with eosinophilia associated with arteriovenous malformation: a clinicopathological correlation with angiography and serial estimation of serum levels of renin, eosinophil cationic protein and interleukin 5. Br J Dermatol. 1999;140:1153-1156. doi:10.1046/j.1365-2133.1999.02880.x
- Matsumoto A, Matsui I, Namba T, et al. VEGF-A links angiolymphoid hyperplasia with eosinophilia (ALHE) to THSD7A membranous nephropathy: a report of 2 cases. Am J Kidney Dis. 2019;73:880-885. doi:10.1053/j.ajkd.2018.10.009
- Bercovitch RS, Catanzaro A, Schwartz BS, et al. Coccidioidomycosis during pregnancy: a review and recommendations for management. Clin Infect Dis. 2011;53:363-368. doi:10.1093/cid/cir410
- Youssef A, Hasan AR, Youssef Y, et al. Angiolymphoid hyperplasia with eosinophilia: a case report. J Med Case Rep. 2018;12:89. doi:10.1186/s13256-018-1599-x
- Abrahamson TG, Davis DA. Angiolymphoid hyperplasia witheosinophilia responsive to pulsed dye laser. J Am Acad Dermatol. 2003;49(2 suppl case reports):S195-S196. doi:10.1067/mjd.2003.314
- Lembo S, Balato A, Cirillo T, et al. A long-term follow-up of angiolymphoid hyperplasia with eosinophilia treated by corticosteroids: when a traditional therapy is still up-to-date. Case Rep Dermatol. 2011;3:64-67. doi:10.1159/000323182
- Cleveland E. Atypical presentation of angiolymphomatous hyperplasia with eosinophilia. J Am Acad Dermatol. 2018;79(3 suppl 1):AB53. doi:10.1016/j.jaad.2018.05.249
- Ravin KA, Loy M. The eosinophil in infection. Clin Rev Allergy Immunol. 2015;50:214-227. doi:10.1007/s12016-015-8525-4
- Grünewald M, Stölzl D, Wehkamp U, et al. Role of eosinophils in angiolymphoid hyperplasia with eosinophilia. JAMA Dermatol. 2021;157:1241-1243. doi:10.1001/jamadermatol.2021.2732
Angiolymphoid hyperplasia with eosinophilia (ALHE) is a rare nodular unencapsulated mass that is characterized by benign anomalous vascular hyperplasia of epithelioidlike endothelial cells attached to dilated blood vessels. The mass is surrounded by lymphocytes and eosinophils that can present clinically as papules, plaques, or nodules.1 The etiology of ALHE is unknown; it is hypothesized that it is a vascular neoplasm or a lymphoproliferative disorder.
Coccidioidomycosis (CM) is a prevalent deep fungal infection endemic to the southwestern United States caused by Coccidioides immitis and Coccidioides posadasii. Infection can occur from direct inoculation through abrasions or direct trauma but usually occurs through the inhalation of spores and can result in a reactive rash (eg, Sweet syndrome, erythema nodosum, interstitial granulomatous dermatitis).2 Coccidioidomycosis also can result in respiratory pneumonia and dissemination from pulmonary infection of the skin. As such, it is important to distinguish CM and its immunologically mediated eruptions for accurate diagnosis and treatment.
We report a novel case of ALHE as a reactive dermatologic presentation in a patient with CM.
Case Report
A 72-year-old woman presented to the dermatology clinic with itchy papules and plaques on the arms and legs of 17 years’ duration. Her medical history included coronary artery disease and hypercholesterolemia as well as a remote history of cutaneous marginal zone B-cell lymphoma of the nose, which was confirmed by histology and treated more than 10 years prior and has remained in remission for 6 years. Her current medications included aspirin, atorvastatin, lisinopril, and metoprolol succinate.
Our patient first presented to our dermatology clinic for itchy nodules and papules on the legs and arms. The patient previously had been seen by another dermatologist 2 months prior for the same condition. At that time, biopsies of the lesions were reported as prurigo nodules. Physical examination at the current presentation revealed round, pink to flesh-colored, raised papules and plaques scattered on the arms and legs (Figure 1). The differential diagnosis included lymphomatoid papulosis, cutaneous B-cell lymphoma, pseudolymphoma, cutaneous CM, and papular mucinosis.

Four-mm punch biopsies of the right proximal pretibial region and left knee region were taken and sent for histologic analysis, direct immunofluorescence testing, and tissue culture. Testing for atypical mycobacteria and deep fungal infection was negative; bacterial cultures and sensitivity testing were negative. Direct immunofluorescence testing was negative. Microscopic examination of material from the right proximal pretibial region showed widely dilated, variously shaped, large blood vessels in a multinodular pattern; the vessels also were surrounded by an inflammatory cell infiltrate containing eosinophils. Histologic findings were consistent with ALHE.
Subsequent biopsies were completed 2 weeks and 1 month from the initial presentation. Both histology reports—from 2 different histopathology laboratories—were consistent with ALHE (Figure 2). Additional work-up during the patient’s initial visit to our clinic for the rash included CM serologic testing, which demonstrated IgM and IgG antibodies. Subsequently, chest radiography revealed a 2.2×2.3-cm mass in the right lower lobe of the lung. Follow-up computed tomography 1 month later confirmed the nodule in the same area to be 2.3×2.1×1.8 cm.

The patient was referred to pulmonology and was treated for pulmonary CM with oral fluconazole 200 mg twice daily for 4 months. Initial treatment also included clobetasol cream 0.05% applied twice daily, which did not produce marked improvement in pruritus. Narrowband UVB phototherapy was attempted, but the patient could not complete the course because of travel time to the office; however, the patient’s ALHE improved considerably with the fluconazole treatment for pulmonary CM.
Oral doxycycline 100 mg twice daily was added to the fluconazole 2 months after her initial visit to our office, which kept the ALHE at bay and helped with the pruritus (Figure 3). Pulmonology and primary care comanaged the pulmonary CM with oral fluconazole 200 mg twice daily. Repeat serologic testing for CM was negative for IgG and IgM after 14 months since the initial visit to the office.

Comment
Pulmonary CM infection has varying dermatologic manifestations. A PubMed search of articles indexed for MEDLINE using the terms ALHE and coccidioidomycosis yielded no case reports; in fact, there have been few reported cases of ALHE at all. Notable conditions associated with ALHE include membranous nephropathy and arteriovenous malformations treated with corticosteroids and surgery, respectively.3,4 Our case is a rare presentation of CM infection manifesting with ALHE. Following treatment and remission for our patient’s CM infection, the ALHE lesion decreased in size.
Standard treatment of uncomplicated CM involves azole antifungals, typically oral fluconazole or itraconazole 400 to 600 mg/d. In more severe cases (eg, immunocompromised patients) amphotericin B can be used.5 Our patient was treated with oral fluconazole 200 mg twice daily for 4 months.
In the literature, treatment via surgical excision, steroid injection, pulsed-dye laser therapy, and radiotherapy also has been described.6-8 Antibiotics including clindamycin, doxycycline, and amoxicillin-clavulanate also have been shown to be effective.9
In our patient, ALHE improved when oral doxycycline 100 mg twice daily was added to the oral fluconazole. In fact, after 4 months of treatment, the CM infection and ALHE lesions both improved to a point at which the lesions were not visible. When those lesions recurred 15 months later, they responded with another course of doxycycline and fluconazole.
Upon recurrence, the patient was asked to have her care transferred to her pulmonologist, who then managed the fluconazole regimen. During the pulmonologist’s workup, no peripheral eosinophilia was found. This is important because eosinophils can be a marker for CM infection; in this case, however, the ALHE lesion was a reactive process to the infection. Classically known to play a reactive role in fungal infection, these white blood cells demonstrate reactivity to the environmental fungus Alternaria alternata by contact-dependent killing, utilizing β2 integrins and CD11b to recognize and adhere to β-glucan. Eosinophils react through contact-dependent killing, releasing cytotoxic granule proteins and proinflammatory mediators, and have been documented to occur in CM and Paracoccidioides brasiliensis infection, in which they deposit major basic protein on the organism.10 Most pertinent to our case with ALHE and CM is the ability of eosinophils to communicate with other immune cells. Eosinophils play a role in the active inflammation of CM through cytokine signaling, which may propagate formation of ALHE.
The function of eosinophils in ALHE is poorly understood; it is unclear whether they act as a primary driver of pathogenesis or are simply indicators of secondary infiltration or infection. Our review of the current literature suggests that eosinophils are unnecessary for progression of ALHE but might be involved at its onset. As reported, even monoclonal antibody therapy (eg, mepolizumab and benralizumab) that effectively depletes eosinophil levels by negating IL-5 signaling do not slow progression of ALHE.11 Symptomatic changes are modest at best (ie, simply softening the ALHE nodules).
Our patient had no peripheral eosinophilia, suggesting that the onset of ALHE might not be caused by eosinophilia but a different inflammatory process—in this patient, by CM. Because peripheral eosinophilia was not seen in our patient, the presence of eosinophils in the ALHE lesion likely is unnecessary for its onset or progression but is a secondary process that exacerbates the lesion. The pathogenesis is unknown but could be directed toward lymphocytes and plasma cells, with eosinophils as part of the dynamic process.11
Conclusion
Because reports of an association between CM and ALHE are limited, our case is distinguished by a unique clinical presentation of ALHE. When a patient is given a diagnosis of ALHE, it therefore is important to consider exposure to CM as a cause, especially in patients who reside in or travel to a region where CM is endemic.
Angiolymphoid hyperplasia with eosinophilia (ALHE) is a rare nodular unencapsulated mass that is characterized by benign anomalous vascular hyperplasia of epithelioidlike endothelial cells attached to dilated blood vessels. The mass is surrounded by lymphocytes and eosinophils that can present clinically as papules, plaques, or nodules.1 The etiology of ALHE is unknown; it is hypothesized that it is a vascular neoplasm or a lymphoproliferative disorder.
Coccidioidomycosis (CM) is a prevalent deep fungal infection endemic to the southwestern United States caused by Coccidioides immitis and Coccidioides posadasii. Infection can occur from direct inoculation through abrasions or direct trauma but usually occurs through the inhalation of spores and can result in a reactive rash (eg, Sweet syndrome, erythema nodosum, interstitial granulomatous dermatitis).2 Coccidioidomycosis also can result in respiratory pneumonia and dissemination from pulmonary infection of the skin. As such, it is important to distinguish CM and its immunologically mediated eruptions for accurate diagnosis and treatment.
We report a novel case of ALHE as a reactive dermatologic presentation in a patient with CM.
Case Report
A 72-year-old woman presented to the dermatology clinic with itchy papules and plaques on the arms and legs of 17 years’ duration. Her medical history included coronary artery disease and hypercholesterolemia as well as a remote history of cutaneous marginal zone B-cell lymphoma of the nose, which was confirmed by histology and treated more than 10 years prior and has remained in remission for 6 years. Her current medications included aspirin, atorvastatin, lisinopril, and metoprolol succinate.
Our patient first presented to our dermatology clinic for itchy nodules and papules on the legs and arms. The patient previously had been seen by another dermatologist 2 months prior for the same condition. At that time, biopsies of the lesions were reported as prurigo nodules. Physical examination at the current presentation revealed round, pink to flesh-colored, raised papules and plaques scattered on the arms and legs (Figure 1). The differential diagnosis included lymphomatoid papulosis, cutaneous B-cell lymphoma, pseudolymphoma, cutaneous CM, and papular mucinosis.

Four-mm punch biopsies of the right proximal pretibial region and left knee region were taken and sent for histologic analysis, direct immunofluorescence testing, and tissue culture. Testing for atypical mycobacteria and deep fungal infection was negative; bacterial cultures and sensitivity testing were negative. Direct immunofluorescence testing was negative. Microscopic examination of material from the right proximal pretibial region showed widely dilated, variously shaped, large blood vessels in a multinodular pattern; the vessels also were surrounded by an inflammatory cell infiltrate containing eosinophils. Histologic findings were consistent with ALHE.
Subsequent biopsies were completed 2 weeks and 1 month from the initial presentation. Both histology reports—from 2 different histopathology laboratories—were consistent with ALHE (Figure 2). Additional work-up during the patient’s initial visit to our clinic for the rash included CM serologic testing, which demonstrated IgM and IgG antibodies. Subsequently, chest radiography revealed a 2.2×2.3-cm mass in the right lower lobe of the lung. Follow-up computed tomography 1 month later confirmed the nodule in the same area to be 2.3×2.1×1.8 cm.

The patient was referred to pulmonology and was treated for pulmonary CM with oral fluconazole 200 mg twice daily for 4 months. Initial treatment also included clobetasol cream 0.05% applied twice daily, which did not produce marked improvement in pruritus. Narrowband UVB phototherapy was attempted, but the patient could not complete the course because of travel time to the office; however, the patient’s ALHE improved considerably with the fluconazole treatment for pulmonary CM.
Oral doxycycline 100 mg twice daily was added to the fluconazole 2 months after her initial visit to our office, which kept the ALHE at bay and helped with the pruritus (Figure 3). Pulmonology and primary care comanaged the pulmonary CM with oral fluconazole 200 mg twice daily. Repeat serologic testing for CM was negative for IgG and IgM after 14 months since the initial visit to the office.

Comment
Pulmonary CM infection has varying dermatologic manifestations. A PubMed search of articles indexed for MEDLINE using the terms ALHE and coccidioidomycosis yielded no case reports; in fact, there have been few reported cases of ALHE at all. Notable conditions associated with ALHE include membranous nephropathy and arteriovenous malformations treated with corticosteroids and surgery, respectively.3,4 Our case is a rare presentation of CM infection manifesting with ALHE. Following treatment and remission for our patient’s CM infection, the ALHE lesion decreased in size.
Standard treatment of uncomplicated CM involves azole antifungals, typically oral fluconazole or itraconazole 400 to 600 mg/d. In more severe cases (eg, immunocompromised patients) amphotericin B can be used.5 Our patient was treated with oral fluconazole 200 mg twice daily for 4 months.
In the literature, treatment via surgical excision, steroid injection, pulsed-dye laser therapy, and radiotherapy also has been described.6-8 Antibiotics including clindamycin, doxycycline, and amoxicillin-clavulanate also have been shown to be effective.9
In our patient, ALHE improved when oral doxycycline 100 mg twice daily was added to the oral fluconazole. In fact, after 4 months of treatment, the CM infection and ALHE lesions both improved to a point at which the lesions were not visible. When those lesions recurred 15 months later, they responded with another course of doxycycline and fluconazole.
Upon recurrence, the patient was asked to have her care transferred to her pulmonologist, who then managed the fluconazole regimen. During the pulmonologist’s workup, no peripheral eosinophilia was found. This is important because eosinophils can be a marker for CM infection; in this case, however, the ALHE lesion was a reactive process to the infection. Classically known to play a reactive role in fungal infection, these white blood cells demonstrate reactivity to the environmental fungus Alternaria alternata by contact-dependent killing, utilizing β2 integrins and CD11b to recognize and adhere to β-glucan. Eosinophils react through contact-dependent killing, releasing cytotoxic granule proteins and proinflammatory mediators, and have been documented to occur in CM and Paracoccidioides brasiliensis infection, in which they deposit major basic protein on the organism.10 Most pertinent to our case with ALHE and CM is the ability of eosinophils to communicate with other immune cells. Eosinophils play a role in the active inflammation of CM through cytokine signaling, which may propagate formation of ALHE.
The function of eosinophils in ALHE is poorly understood; it is unclear whether they act as a primary driver of pathogenesis or are simply indicators of secondary infiltration or infection. Our review of the current literature suggests that eosinophils are unnecessary for progression of ALHE but might be involved at its onset. As reported, even monoclonal antibody therapy (eg, mepolizumab and benralizumab) that effectively depletes eosinophil levels by negating IL-5 signaling do not slow progression of ALHE.11 Symptomatic changes are modest at best (ie, simply softening the ALHE nodules).
Our patient had no peripheral eosinophilia, suggesting that the onset of ALHE might not be caused by eosinophilia but a different inflammatory process—in this patient, by CM. Because peripheral eosinophilia was not seen in our patient, the presence of eosinophils in the ALHE lesion likely is unnecessary for its onset or progression but is a secondary process that exacerbates the lesion. The pathogenesis is unknown but could be directed toward lymphocytes and plasma cells, with eosinophils as part of the dynamic process.11
Conclusion
Because reports of an association between CM and ALHE are limited, our case is distinguished by a unique clinical presentation of ALHE. When a patient is given a diagnosis of ALHE, it therefore is important to consider exposure to CM as a cause, especially in patients who reside in or travel to a region where CM is endemic.
- Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol. 1969;81:1-14. doi:10.1111/j.1365-2133.1969.tb15914.x
- DiCaudo D. Coccidioidomycosis. Semin Cutan Med Surg. 2014;33:140-145. doi:10.12788/j.sder.0111
- Onishi Y, Ohara K. Angiolymphoid hyperplasia with eosinophilia associated with arteriovenous malformation: a clinicopathological correlation with angiography and serial estimation of serum levels of renin, eosinophil cationic protein and interleukin 5. Br J Dermatol. 1999;140:1153-1156. doi:10.1046/j.1365-2133.1999.02880.x
- Matsumoto A, Matsui I, Namba T, et al. VEGF-A links angiolymphoid hyperplasia with eosinophilia (ALHE) to THSD7A membranous nephropathy: a report of 2 cases. Am J Kidney Dis. 2019;73:880-885. doi:10.1053/j.ajkd.2018.10.009
- Bercovitch RS, Catanzaro A, Schwartz BS, et al. Coccidioidomycosis during pregnancy: a review and recommendations for management. Clin Infect Dis. 2011;53:363-368. doi:10.1093/cid/cir410
- Youssef A, Hasan AR, Youssef Y, et al. Angiolymphoid hyperplasia with eosinophilia: a case report. J Med Case Rep. 2018;12:89. doi:10.1186/s13256-018-1599-x
- Abrahamson TG, Davis DA. Angiolymphoid hyperplasia witheosinophilia responsive to pulsed dye laser. J Am Acad Dermatol. 2003;49(2 suppl case reports):S195-S196. doi:10.1067/mjd.2003.314
- Lembo S, Balato A, Cirillo T, et al. A long-term follow-up of angiolymphoid hyperplasia with eosinophilia treated by corticosteroids: when a traditional therapy is still up-to-date. Case Rep Dermatol. 2011;3:64-67. doi:10.1159/000323182
- Cleveland E. Atypical presentation of angiolymphomatous hyperplasia with eosinophilia. J Am Acad Dermatol. 2018;79(3 suppl 1):AB53. doi:10.1016/j.jaad.2018.05.249
- Ravin KA, Loy M. The eosinophil in infection. Clin Rev Allergy Immunol. 2015;50:214-227. doi:10.1007/s12016-015-8525-4
- Grünewald M, Stölzl D, Wehkamp U, et al. Role of eosinophils in angiolymphoid hyperplasia with eosinophilia. JAMA Dermatol. 2021;157:1241-1243. doi:10.1001/jamadermatol.2021.2732
- Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol. 1969;81:1-14. doi:10.1111/j.1365-2133.1969.tb15914.x
- DiCaudo D. Coccidioidomycosis. Semin Cutan Med Surg. 2014;33:140-145. doi:10.12788/j.sder.0111
- Onishi Y, Ohara K. Angiolymphoid hyperplasia with eosinophilia associated with arteriovenous malformation: a clinicopathological correlation with angiography and serial estimation of serum levels of renin, eosinophil cationic protein and interleukin 5. Br J Dermatol. 1999;140:1153-1156. doi:10.1046/j.1365-2133.1999.02880.x
- Matsumoto A, Matsui I, Namba T, et al. VEGF-A links angiolymphoid hyperplasia with eosinophilia (ALHE) to THSD7A membranous nephropathy: a report of 2 cases. Am J Kidney Dis. 2019;73:880-885. doi:10.1053/j.ajkd.2018.10.009
- Bercovitch RS, Catanzaro A, Schwartz BS, et al. Coccidioidomycosis during pregnancy: a review and recommendations for management. Clin Infect Dis. 2011;53:363-368. doi:10.1093/cid/cir410
- Youssef A, Hasan AR, Youssef Y, et al. Angiolymphoid hyperplasia with eosinophilia: a case report. J Med Case Rep. 2018;12:89. doi:10.1186/s13256-018-1599-x
- Abrahamson TG, Davis DA. Angiolymphoid hyperplasia witheosinophilia responsive to pulsed dye laser. J Am Acad Dermatol. 2003;49(2 suppl case reports):S195-S196. doi:10.1067/mjd.2003.314
- Lembo S, Balato A, Cirillo T, et al. A long-term follow-up of angiolymphoid hyperplasia with eosinophilia treated by corticosteroids: when a traditional therapy is still up-to-date. Case Rep Dermatol. 2011;3:64-67. doi:10.1159/000323182
- Cleveland E. Atypical presentation of angiolymphomatous hyperplasia with eosinophilia. J Am Acad Dermatol. 2018;79(3 suppl 1):AB53. doi:10.1016/j.jaad.2018.05.249
- Ravin KA, Loy M. The eosinophil in infection. Clin Rev Allergy Immunol. 2015;50:214-227. doi:10.1007/s12016-015-8525-4
- Grünewald M, Stölzl D, Wehkamp U, et al. Role of eosinophils in angiolymphoid hyperplasia with eosinophilia. JAMA Dermatol. 2021;157:1241-1243. doi:10.1001/jamadermatol.2021.2732
Practice Points
- Angiolymphoid hyperplasia with eosinophilia (ALHE) is a rare entity of unknown etiology.
- There is an association between ALHE and coccidioidomycosis (CM). Patients who present with ALHE and reside in a CM-endemic region should be examined for CM.
Prolonged Drug-Induced Hypersensitivity Syndrome/DRESS With Alopecia Areata and Autoimmune Thyroiditis
Drug-induced hypersensitivity syndrome (DIHS), also called drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, is a potentially fatal drug-induced hypersensitivity reaction that is characterized by a cutaneous eruption, multiorgan involvement, viral reactivation, and hematologic abnormalities. As the nomenclature of this disease advances, consensus groups have adopted DIHS/DRESS to underscore that both names refer to the same clinical phenomenon.1 Autoimmune sequelae have been reported after DIHS/DRESS that include vitiligo, thyroid disease, and type 1 diabetes mellitus (T1DM). We present a case of lamotrigine-associated DIHS/DRESS complicated by an unusually prolonged course requiring oral corticosteroids and narrow-band ultraviolet B (UVB) treatment and with development of extensive alopecia areata and autoimmune thyroiditis.
Case Presentation
A 35-year-old female Filipino patient was prescribed lamotrigine 25 mg daily for bipolar II disorder and titrated to 100 mg twice daily after 1 month. One week after the increase, the patient developed a diffuse morbilliform rash covering their entire body along with facial swelling and generalized pruritus. Lamotrigine was discontinued after lamotrigine allergy was diagnosed. The patient improved following a 9-day oral prednisone taper and was placed on oxcarbazepine 300 mg twice daily to manage their bipolar disorder. One day after completing the taper, the patient presented again with worsening rash, swelling, and cervical lymphadenopathy. Oxcarbazepine was discontinued, and oral prednisone 60 mg was reinstituted for an additional 11 days.
Dermatology evaluated the patient 10 days after completion of the second oral steroid taper (1 month after cessation of lamotrigine). The patient had erythroderma along with malaise, fevers, chills, and fatigue and a diffuse burning sensation (Figure 1). The patient was hypotensive and tachycardic with significant eosinophilia (42%; reference range, 0%-8%), transaminitis, and renal insufficiency. The patient was diagnosed with DIHS/DRESS based on their clinical presentation and calculated RegiSCAR score of 7 (score > 5 corresponds with definite DIHS/DRESS and points were given for fever, enlarged lymph nodes, eosinophilia ≥ 20%, skin rash extending > 50% of their body, edema and scaling, and 2 organs involved).2 A punch biopsy was confirmatory (Figure 2A).3 The patient was started on prednisone 80 mg once daily along with topical fluocinonide 0.05% ointment. However, the patient’s clinical status deteriorated, requiring hospital admission for heart failure evaluation. The echocardiogram revealed hyperdynamic circulation but was otherwise unremarkable.
The patient was maintained on prednisone 70 to 80 mg daily for 2 months before improvement of the rash and pruritus. The prednisone was slowly tapered over a 6-week period and then discontinued. Shortly after discontinuation, the patient redeveloped erythroderma. Skin biopsy and complete blood count (17.3% eosinophilia) confirmed the suspected DIHS/DRESS relapse (Figure 2B). In addition, the patient reported upper respiratory tract symptoms and concurrently tested positive for human herpesvirus 6 (HHV-6). The patient was restarted on prednisone and low-dose narrow-band UVB (nbUVB) therapy was added. Over the following 2 months, they responded well to low-dose nbUVB therapy. By the end of nbUVB treatment, about 5 months after initial presentation, the patient’s erythroderma improved, eosinophilia resolved, and they were able to tolerate prednisone taper. Ten months after cessation of lamotrigine, prednisone was finally discontinued. Two weeks later, the patient was screened for adrenal insufficiency (AI) given the prolonged steroid course. Their serum morning cortisol level was within normal limits.
Four months after DIHS/DRESS resolution and cessation of steroids, the patient noted significant patches of smooth alopecia on their posterior scalp and was diagnosed with alopecia areata. Treatment with intralesional triamcinolone over 2 months resulted in regrowth of hair (Figure 3). A month later, the patient reported increasing fatigue and anorexia. The patient was evaluated once more for AI, this time with low morning cortisol and low adrenocorticotrophic hormone (ACTH) levels—consistent with AI secondary to prolonged glucocorticoid therapy. The patient also was concomitantly evaluated for hypothyroidism with significantly elevated thyroperoxidase antibodies—confirming the diagnosis of Hashimoto thyroiditis.
Discussion
DIHS/DRESS syndrome is a rare, but potentially life-threatening hypersensitivity to a medication, often beginning 2 to 6 weeks after exposure to the causative agent. The incidence of DIHS/DRESS in the general population is about 2 per 100,000.3 Our patient presented with DIHS/DRESS 33 days after starting lamotrigine, which corresponds with the published mean onset of anticonvulsant-induced DIHS/DRESS (29.7-33.3 days).4 Recent evidence shows that time from drug exposure to DIHS/DRESS symptoms may vary by drug class, with antibiotics implicated as precipitating DIHS/DRESS in < 15 days.3 The diagnosis of DIHS/DRESS may be complicated for many reasons. The accompanying rash may be morbilliform, erythroderma, or exfoliative dermatitis with multiple anatomic regions affected.5 Systemic involvement with various internal organs occurs in > 90% of cases, with the liver and kidney involved most frequently.5 Overall mortality rate may be as high as 10% most commonly due to acute liver failure.5 Biopsy may be helpful in the diagnosis but is not always specific.5 Diagnostic criteria include RegiSCAR and J-SCAR scores; our patient met criteria for both (Table).5
The pathogenesis of DIHS/DRESS remains unclear. Proposed mechanisms include genetic predisposition with human leukocyte antigen (HLA) haplotypes, autoimmune with a delayed cell-mediated immune response associated with herpesviruses, and abnormal enzymatic pathways that metabolize medications.2 Although no HLA has been identified between lamotrigine and DIHS, HLA-A*02:07 and HLA-B*15:02 have been associated with lamotrigine-induced cutaneous drug reactions in patients of Thai ancestry.6 Immunosuppression also is a risk factor, especially when accompanied by a primary or reactivated HHV-6 infection, as seen in our patient.2 Additionally, HHV-6 infection may be a common link between DIHS/DRESS and autoimmune thyroiditis but is believed to involve elevated levels of interferon-γ-induced protein-10 (IP-10) that may lead to excessive recruitment of cytotoxic T cells into target tissues.7 Elevated levels of IP-10 are seen in many autoimmune conditions, such as autoimmune thyroiditis, Sjögren syndrome, and Graves disease.8
DIHS/DRESS syndrome has been associated with development of autoimmune diseases as long-term sequelae. The most commonly affected organs are the thyroid and pancreas; approximately 4.8% of patients develop autoimmune thyroiditis and 3.5% develop fulminant T1DM.9 The time from onset of DIHS/DRESS to development of autoimmune thyroiditis can range from 2 months to 2 years, whereas the range from DIHS/DRESS onset to fulminant T1DM is about 40 days.9 Alopecia had been reported in 1, occurring 4 months after DIHS/DRESS onset. Our patient’s alopecia areata and Hashimoto thyroiditis occurred 14 and 15 months after DIHS/DRESS presentation, respectively.
Treatment
For management, early recognition and discontinuation of the offending agent is paramount. Systemic corticosteroids are the accepted treatment standard. Symptoms of DIHS/DRESS usually resolve between 3 and 18 weeks, with the mean resolution time at 7 weeks.10 Our patient developed a prolonged course with persistent eosinophilia for 20 weeks and cutaneous symptoms for 32 weeks—requiring 40 weeks of oral prednisone. The most significant clinical improvement occurred during the 8-week period low-dose nbUVB was used (Figure 4). There also are reports outlining the successful use of intravenous immunoglobulin, cyclosporine, cyclophosphamide, rituximab, or plasma exchange in cases refractory to oral corticosteroids.11
A recent retrospective case control study showed that treatment of DIHS/DRESS with cyclosporine in patients who had a contraindication to steroids resulted in faster resolution of symptoms, shorter treatment durations, and shorter hospitalizations than did those treated with corticosteroids.12 However, the data are limited by a significantly smaller number of patients treated with cyclosporine than steroids and the cyclosporine treatment group having milder cases of DIHS/DRESS.12
The risk of AI is increased for patients who have taken > 20 mg of prednisone daily ≥ 3 weeks, an evening dose ≥ 5 mg for a few weeks, or have a Cushingoid appearance.13 Patients may not regain full adrenal function for 12 to 18 months.14 Our patient had a normal basal serum cortisol level 2 weeks after prednisone cessation and then presented 5 months later with AI. While the reason for this period of normality is unclear, it may partly be due to the variable length of hypothalamic-pituitary-adrenal axis recovery time. Thus, ACTH stimulation tests in addition to serum cortisol may be done in patients with suspected AI for higher diagnostic certainty.10
Conclusions
DIHS/DRESS is a severe cutaneous adverse reaction that may require a prolonged treatment course until symptom resolution (40 weeks of oral prednisone in our patient). Oral corticosteroids are the mainstay of treatment, but long-term use is associated with significant adverse effects, such as AI in our patient. Alternative therapies, such as cyclosporine, look promising, but further studies are needed to determine safety profile and efficacy.12 Additionally, patients with DIHS/DRESS should be educated and followed for potential autoimmune sequelae; in our patient alopecia areata and autoimmune thyroiditis were late sequelae, occurring 14 and 15 months, respectively, after onset of DIHS/DRESS.
1. RegiSCAR. Accessed June 3, 2022. http://www.regiscar.org
2. Shiohara T, Mizukawa Y. Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): an update in 2019. Allergol Int. 2019;68(3):301-308. doi:10.1016/j.alit.2019.03.006
3. Wolfson AR, Zhou L, Li Y, Phadke NA, Chow OA, Blumenthal KG. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome identified in the electronic health record allergy module. J Allergy Clin Immunol Pract. 2019;7(2):633-640. doi:10.1016/j.jaip.2018.08.013
4. Sasidharanpillai S, Govindan A, Riyaz N, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a histopathology based analysis. Indian J Dermatol Venereol Leprol. 2016;82(1):28. doi:10.4103/0378-6323.168934
5. Kardaun SH, Sekula P, Valeyrie‐Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169(5):1071-1080. doi:10.1111/bjd.12501
6. Koomdee N, Pratoomwun J, Jantararoungtong T, et al. Association of HLA-A and HLA-B alleles with lamotrigine-induced cutaneous adverse drug reactions in the Thai population. Front Pharmacol. 2017;8. doi:10.3389/fphar.2017.00879
7. Yang C-W, Cho Y-T, Hsieh Y-C, Hsu S-H, Chen K-L, Chu C-Y. The interferon-γ-induced protein 10/CXCR3 axis is associated with human herpesvirus-6 reactivation and the development of sequelae in drug reaction with eosinophilia and systemic symptoms. Br J Dermatol. 2020;183(5):909-919. doi:10.1111/bjd.18942
8. Ruffilli I, Ferrari SM, Colaci M, Ferri C, Fallahi P, Antonelli A. IP-10 in autoimmune thyroiditis. Horm Metab Res. 2014;46(9):597-602. doi:10.1055/s-0034-1382053
9. Kano Y, Tohyama M, Aihara M, et al. Sequelae in 145 patients with drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J Dermatol. 2015;42(3):276-282. doi:10.1111/1346-8138.12770
10. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124(7):588-597. doi:10.1016/j.amjmed.2011.01.017
11. Bommersbach TJ, Lapid MI, Leung JG, Cunningham JL, Rummans TA, Kung S. Management of psychotropic drug-induced dress syndrome: a systematic review. Mayo Clin Proc. 2016;91(6):787-801. doi:10.1016/j.mayocp.2016.03.006
12. Nguyen E, Yanes D, Imadojemu S, Kroshinsky D. Evaluation of cyclosporine for the treatment of DRESS syndrome. JAMA Dermatol. 2020;156(6):704-706. doi:10.1001/jamadermatol.2020.0048
13. Joseph RM, Hunter AL, Ray DW, Dixon WG. Systemic glucocorticoid therapy and adrenal insufficiency in adults: a systematic review. Semin Arthritis Rheum. 2016;46(1):133-141. doi:10.1016/j.semarthrit.2016.03.001
14. Jamilloux Y, Liozon E, Pugnet G, et al. Recovery of adrenal function after long-term glucocorticoid therapy for giant cell arteritis: a cohort study. PLoS ONE. 2013;8(7):e68713. doi:10.1371/journal.pone.0068713
Drug-induced hypersensitivity syndrome (DIHS), also called drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, is a potentially fatal drug-induced hypersensitivity reaction that is characterized by a cutaneous eruption, multiorgan involvement, viral reactivation, and hematologic abnormalities. As the nomenclature of this disease advances, consensus groups have adopted DIHS/DRESS to underscore that both names refer to the same clinical phenomenon.1 Autoimmune sequelae have been reported after DIHS/DRESS that include vitiligo, thyroid disease, and type 1 diabetes mellitus (T1DM). We present a case of lamotrigine-associated DIHS/DRESS complicated by an unusually prolonged course requiring oral corticosteroids and narrow-band ultraviolet B (UVB) treatment and with development of extensive alopecia areata and autoimmune thyroiditis.
Case Presentation
A 35-year-old female Filipino patient was prescribed lamotrigine 25 mg daily for bipolar II disorder and titrated to 100 mg twice daily after 1 month. One week after the increase, the patient developed a diffuse morbilliform rash covering their entire body along with facial swelling and generalized pruritus. Lamotrigine was discontinued after lamotrigine allergy was diagnosed. The patient improved following a 9-day oral prednisone taper and was placed on oxcarbazepine 300 mg twice daily to manage their bipolar disorder. One day after completing the taper, the patient presented again with worsening rash, swelling, and cervical lymphadenopathy. Oxcarbazepine was discontinued, and oral prednisone 60 mg was reinstituted for an additional 11 days.
Dermatology evaluated the patient 10 days after completion of the second oral steroid taper (1 month after cessation of lamotrigine). The patient had erythroderma along with malaise, fevers, chills, and fatigue and a diffuse burning sensation (Figure 1). The patient was hypotensive and tachycardic with significant eosinophilia (42%; reference range, 0%-8%), transaminitis, and renal insufficiency. The patient was diagnosed with DIHS/DRESS based on their clinical presentation and calculated RegiSCAR score of 7 (score > 5 corresponds with definite DIHS/DRESS and points were given for fever, enlarged lymph nodes, eosinophilia ≥ 20%, skin rash extending > 50% of their body, edema and scaling, and 2 organs involved).2 A punch biopsy was confirmatory (Figure 2A).3 The patient was started on prednisone 80 mg once daily along with topical fluocinonide 0.05% ointment. However, the patient’s clinical status deteriorated, requiring hospital admission for heart failure evaluation. The echocardiogram revealed hyperdynamic circulation but was otherwise unremarkable.
The patient was maintained on prednisone 70 to 80 mg daily for 2 months before improvement of the rash and pruritus. The prednisone was slowly tapered over a 6-week period and then discontinued. Shortly after discontinuation, the patient redeveloped erythroderma. Skin biopsy and complete blood count (17.3% eosinophilia) confirmed the suspected DIHS/DRESS relapse (Figure 2B). In addition, the patient reported upper respiratory tract symptoms and concurrently tested positive for human herpesvirus 6 (HHV-6). The patient was restarted on prednisone and low-dose narrow-band UVB (nbUVB) therapy was added. Over the following 2 months, they responded well to low-dose nbUVB therapy. By the end of nbUVB treatment, about 5 months after initial presentation, the patient’s erythroderma improved, eosinophilia resolved, and they were able to tolerate prednisone taper. Ten months after cessation of lamotrigine, prednisone was finally discontinued. Two weeks later, the patient was screened for adrenal insufficiency (AI) given the prolonged steroid course. Their serum morning cortisol level was within normal limits.
Four months after DIHS/DRESS resolution and cessation of steroids, the patient noted significant patches of smooth alopecia on their posterior scalp and was diagnosed with alopecia areata. Treatment with intralesional triamcinolone over 2 months resulted in regrowth of hair (Figure 3). A month later, the patient reported increasing fatigue and anorexia. The patient was evaluated once more for AI, this time with low morning cortisol and low adrenocorticotrophic hormone (ACTH) levels—consistent with AI secondary to prolonged glucocorticoid therapy. The patient also was concomitantly evaluated for hypothyroidism with significantly elevated thyroperoxidase antibodies—confirming the diagnosis of Hashimoto thyroiditis.
Discussion
DIHS/DRESS syndrome is a rare, but potentially life-threatening hypersensitivity to a medication, often beginning 2 to 6 weeks after exposure to the causative agent. The incidence of DIHS/DRESS in the general population is about 2 per 100,000.3 Our patient presented with DIHS/DRESS 33 days after starting lamotrigine, which corresponds with the published mean onset of anticonvulsant-induced DIHS/DRESS (29.7-33.3 days).4 Recent evidence shows that time from drug exposure to DIHS/DRESS symptoms may vary by drug class, with antibiotics implicated as precipitating DIHS/DRESS in < 15 days.3 The diagnosis of DIHS/DRESS may be complicated for many reasons. The accompanying rash may be morbilliform, erythroderma, or exfoliative dermatitis with multiple anatomic regions affected.5 Systemic involvement with various internal organs occurs in > 90% of cases, with the liver and kidney involved most frequently.5 Overall mortality rate may be as high as 10% most commonly due to acute liver failure.5 Biopsy may be helpful in the diagnosis but is not always specific.5 Diagnostic criteria include RegiSCAR and J-SCAR scores; our patient met criteria for both (Table).5
The pathogenesis of DIHS/DRESS remains unclear. Proposed mechanisms include genetic predisposition with human leukocyte antigen (HLA) haplotypes, autoimmune with a delayed cell-mediated immune response associated with herpesviruses, and abnormal enzymatic pathways that metabolize medications.2 Although no HLA has been identified between lamotrigine and DIHS, HLA-A*02:07 and HLA-B*15:02 have been associated with lamotrigine-induced cutaneous drug reactions in patients of Thai ancestry.6 Immunosuppression also is a risk factor, especially when accompanied by a primary or reactivated HHV-6 infection, as seen in our patient.2 Additionally, HHV-6 infection may be a common link between DIHS/DRESS and autoimmune thyroiditis but is believed to involve elevated levels of interferon-γ-induced protein-10 (IP-10) that may lead to excessive recruitment of cytotoxic T cells into target tissues.7 Elevated levels of IP-10 are seen in many autoimmune conditions, such as autoimmune thyroiditis, Sjögren syndrome, and Graves disease.8
DIHS/DRESS syndrome has been associated with development of autoimmune diseases as long-term sequelae. The most commonly affected organs are the thyroid and pancreas; approximately 4.8% of patients develop autoimmune thyroiditis and 3.5% develop fulminant T1DM.9 The time from onset of DIHS/DRESS to development of autoimmune thyroiditis can range from 2 months to 2 years, whereas the range from DIHS/DRESS onset to fulminant T1DM is about 40 days.9 Alopecia had been reported in 1, occurring 4 months after DIHS/DRESS onset. Our patient’s alopecia areata and Hashimoto thyroiditis occurred 14 and 15 months after DIHS/DRESS presentation, respectively.
Treatment
For management, early recognition and discontinuation of the offending agent is paramount. Systemic corticosteroids are the accepted treatment standard. Symptoms of DIHS/DRESS usually resolve between 3 and 18 weeks, with the mean resolution time at 7 weeks.10 Our patient developed a prolonged course with persistent eosinophilia for 20 weeks and cutaneous symptoms for 32 weeks—requiring 40 weeks of oral prednisone. The most significant clinical improvement occurred during the 8-week period low-dose nbUVB was used (Figure 4). There also are reports outlining the successful use of intravenous immunoglobulin, cyclosporine, cyclophosphamide, rituximab, or plasma exchange in cases refractory to oral corticosteroids.11
A recent retrospective case control study showed that treatment of DIHS/DRESS with cyclosporine in patients who had a contraindication to steroids resulted in faster resolution of symptoms, shorter treatment durations, and shorter hospitalizations than did those treated with corticosteroids.12 However, the data are limited by a significantly smaller number of patients treated with cyclosporine than steroids and the cyclosporine treatment group having milder cases of DIHS/DRESS.12
The risk of AI is increased for patients who have taken > 20 mg of prednisone daily ≥ 3 weeks, an evening dose ≥ 5 mg for a few weeks, or have a Cushingoid appearance.13 Patients may not regain full adrenal function for 12 to 18 months.14 Our patient had a normal basal serum cortisol level 2 weeks after prednisone cessation and then presented 5 months later with AI. While the reason for this period of normality is unclear, it may partly be due to the variable length of hypothalamic-pituitary-adrenal axis recovery time. Thus, ACTH stimulation tests in addition to serum cortisol may be done in patients with suspected AI for higher diagnostic certainty.10
Conclusions
DIHS/DRESS is a severe cutaneous adverse reaction that may require a prolonged treatment course until symptom resolution (40 weeks of oral prednisone in our patient). Oral corticosteroids are the mainstay of treatment, but long-term use is associated with significant adverse effects, such as AI in our patient. Alternative therapies, such as cyclosporine, look promising, but further studies are needed to determine safety profile and efficacy.12 Additionally, patients with DIHS/DRESS should be educated and followed for potential autoimmune sequelae; in our patient alopecia areata and autoimmune thyroiditis were late sequelae, occurring 14 and 15 months, respectively, after onset of DIHS/DRESS.
Drug-induced hypersensitivity syndrome (DIHS), also called drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, is a potentially fatal drug-induced hypersensitivity reaction that is characterized by a cutaneous eruption, multiorgan involvement, viral reactivation, and hematologic abnormalities. As the nomenclature of this disease advances, consensus groups have adopted DIHS/DRESS to underscore that both names refer to the same clinical phenomenon.1 Autoimmune sequelae have been reported after DIHS/DRESS that include vitiligo, thyroid disease, and type 1 diabetes mellitus (T1DM). We present a case of lamotrigine-associated DIHS/DRESS complicated by an unusually prolonged course requiring oral corticosteroids and narrow-band ultraviolet B (UVB) treatment and with development of extensive alopecia areata and autoimmune thyroiditis.
Case Presentation
A 35-year-old female Filipino patient was prescribed lamotrigine 25 mg daily for bipolar II disorder and titrated to 100 mg twice daily after 1 month. One week after the increase, the patient developed a diffuse morbilliform rash covering their entire body along with facial swelling and generalized pruritus. Lamotrigine was discontinued after lamotrigine allergy was diagnosed. The patient improved following a 9-day oral prednisone taper and was placed on oxcarbazepine 300 mg twice daily to manage their bipolar disorder. One day after completing the taper, the patient presented again with worsening rash, swelling, and cervical lymphadenopathy. Oxcarbazepine was discontinued, and oral prednisone 60 mg was reinstituted for an additional 11 days.
Dermatology evaluated the patient 10 days after completion of the second oral steroid taper (1 month after cessation of lamotrigine). The patient had erythroderma along with malaise, fevers, chills, and fatigue and a diffuse burning sensation (Figure 1). The patient was hypotensive and tachycardic with significant eosinophilia (42%; reference range, 0%-8%), transaminitis, and renal insufficiency. The patient was diagnosed with DIHS/DRESS based on their clinical presentation and calculated RegiSCAR score of 7 (score > 5 corresponds with definite DIHS/DRESS and points were given for fever, enlarged lymph nodes, eosinophilia ≥ 20%, skin rash extending > 50% of their body, edema and scaling, and 2 organs involved).2 A punch biopsy was confirmatory (Figure 2A).3 The patient was started on prednisone 80 mg once daily along with topical fluocinonide 0.05% ointment. However, the patient’s clinical status deteriorated, requiring hospital admission for heart failure evaluation. The echocardiogram revealed hyperdynamic circulation but was otherwise unremarkable.
The patient was maintained on prednisone 70 to 80 mg daily for 2 months before improvement of the rash and pruritus. The prednisone was slowly tapered over a 6-week period and then discontinued. Shortly after discontinuation, the patient redeveloped erythroderma. Skin biopsy and complete blood count (17.3% eosinophilia) confirmed the suspected DIHS/DRESS relapse (Figure 2B). In addition, the patient reported upper respiratory tract symptoms and concurrently tested positive for human herpesvirus 6 (HHV-6). The patient was restarted on prednisone and low-dose narrow-band UVB (nbUVB) therapy was added. Over the following 2 months, they responded well to low-dose nbUVB therapy. By the end of nbUVB treatment, about 5 months after initial presentation, the patient’s erythroderma improved, eosinophilia resolved, and they were able to tolerate prednisone taper. Ten months after cessation of lamotrigine, prednisone was finally discontinued. Two weeks later, the patient was screened for adrenal insufficiency (AI) given the prolonged steroid course. Their serum morning cortisol level was within normal limits.
Four months after DIHS/DRESS resolution and cessation of steroids, the patient noted significant patches of smooth alopecia on their posterior scalp and was diagnosed with alopecia areata. Treatment with intralesional triamcinolone over 2 months resulted in regrowth of hair (Figure 3). A month later, the patient reported increasing fatigue and anorexia. The patient was evaluated once more for AI, this time with low morning cortisol and low adrenocorticotrophic hormone (ACTH) levels—consistent with AI secondary to prolonged glucocorticoid therapy. The patient also was concomitantly evaluated for hypothyroidism with significantly elevated thyroperoxidase antibodies—confirming the diagnosis of Hashimoto thyroiditis.
Discussion
DIHS/DRESS syndrome is a rare, but potentially life-threatening hypersensitivity to a medication, often beginning 2 to 6 weeks after exposure to the causative agent. The incidence of DIHS/DRESS in the general population is about 2 per 100,000.3 Our patient presented with DIHS/DRESS 33 days after starting lamotrigine, which corresponds with the published mean onset of anticonvulsant-induced DIHS/DRESS (29.7-33.3 days).4 Recent evidence shows that time from drug exposure to DIHS/DRESS symptoms may vary by drug class, with antibiotics implicated as precipitating DIHS/DRESS in < 15 days.3 The diagnosis of DIHS/DRESS may be complicated for many reasons. The accompanying rash may be morbilliform, erythroderma, or exfoliative dermatitis with multiple anatomic regions affected.5 Systemic involvement with various internal organs occurs in > 90% of cases, with the liver and kidney involved most frequently.5 Overall mortality rate may be as high as 10% most commonly due to acute liver failure.5 Biopsy may be helpful in the diagnosis but is not always specific.5 Diagnostic criteria include RegiSCAR and J-SCAR scores; our patient met criteria for both (Table).5
The pathogenesis of DIHS/DRESS remains unclear. Proposed mechanisms include genetic predisposition with human leukocyte antigen (HLA) haplotypes, autoimmune with a delayed cell-mediated immune response associated with herpesviruses, and abnormal enzymatic pathways that metabolize medications.2 Although no HLA has been identified between lamotrigine and DIHS, HLA-A*02:07 and HLA-B*15:02 have been associated with lamotrigine-induced cutaneous drug reactions in patients of Thai ancestry.6 Immunosuppression also is a risk factor, especially when accompanied by a primary or reactivated HHV-6 infection, as seen in our patient.2 Additionally, HHV-6 infection may be a common link between DIHS/DRESS and autoimmune thyroiditis but is believed to involve elevated levels of interferon-γ-induced protein-10 (IP-10) that may lead to excessive recruitment of cytotoxic T cells into target tissues.7 Elevated levels of IP-10 are seen in many autoimmune conditions, such as autoimmune thyroiditis, Sjögren syndrome, and Graves disease.8
DIHS/DRESS syndrome has been associated with development of autoimmune diseases as long-term sequelae. The most commonly affected organs are the thyroid and pancreas; approximately 4.8% of patients develop autoimmune thyroiditis and 3.5% develop fulminant T1DM.9 The time from onset of DIHS/DRESS to development of autoimmune thyroiditis can range from 2 months to 2 years, whereas the range from DIHS/DRESS onset to fulminant T1DM is about 40 days.9 Alopecia had been reported in 1, occurring 4 months after DIHS/DRESS onset. Our patient’s alopecia areata and Hashimoto thyroiditis occurred 14 and 15 months after DIHS/DRESS presentation, respectively.
Treatment
For management, early recognition and discontinuation of the offending agent is paramount. Systemic corticosteroids are the accepted treatment standard. Symptoms of DIHS/DRESS usually resolve between 3 and 18 weeks, with the mean resolution time at 7 weeks.10 Our patient developed a prolonged course with persistent eosinophilia for 20 weeks and cutaneous symptoms for 32 weeks—requiring 40 weeks of oral prednisone. The most significant clinical improvement occurred during the 8-week period low-dose nbUVB was used (Figure 4). There also are reports outlining the successful use of intravenous immunoglobulin, cyclosporine, cyclophosphamide, rituximab, or plasma exchange in cases refractory to oral corticosteroids.11
A recent retrospective case control study showed that treatment of DIHS/DRESS with cyclosporine in patients who had a contraindication to steroids resulted in faster resolution of symptoms, shorter treatment durations, and shorter hospitalizations than did those treated with corticosteroids.12 However, the data are limited by a significantly smaller number of patients treated with cyclosporine than steroids and the cyclosporine treatment group having milder cases of DIHS/DRESS.12
The risk of AI is increased for patients who have taken > 20 mg of prednisone daily ≥ 3 weeks, an evening dose ≥ 5 mg for a few weeks, or have a Cushingoid appearance.13 Patients may not regain full adrenal function for 12 to 18 months.14 Our patient had a normal basal serum cortisol level 2 weeks after prednisone cessation and then presented 5 months later with AI. While the reason for this period of normality is unclear, it may partly be due to the variable length of hypothalamic-pituitary-adrenal axis recovery time. Thus, ACTH stimulation tests in addition to serum cortisol may be done in patients with suspected AI for higher diagnostic certainty.10
Conclusions
DIHS/DRESS is a severe cutaneous adverse reaction that may require a prolonged treatment course until symptom resolution (40 weeks of oral prednisone in our patient). Oral corticosteroids are the mainstay of treatment, but long-term use is associated with significant adverse effects, such as AI in our patient. Alternative therapies, such as cyclosporine, look promising, but further studies are needed to determine safety profile and efficacy.12 Additionally, patients with DIHS/DRESS should be educated and followed for potential autoimmune sequelae; in our patient alopecia areata and autoimmune thyroiditis were late sequelae, occurring 14 and 15 months, respectively, after onset of DIHS/DRESS.
1. RegiSCAR. Accessed June 3, 2022. http://www.regiscar.org
2. Shiohara T, Mizukawa Y. Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): an update in 2019. Allergol Int. 2019;68(3):301-308. doi:10.1016/j.alit.2019.03.006
3. Wolfson AR, Zhou L, Li Y, Phadke NA, Chow OA, Blumenthal KG. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome identified in the electronic health record allergy module. J Allergy Clin Immunol Pract. 2019;7(2):633-640. doi:10.1016/j.jaip.2018.08.013
4. Sasidharanpillai S, Govindan A, Riyaz N, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a histopathology based analysis. Indian J Dermatol Venereol Leprol. 2016;82(1):28. doi:10.4103/0378-6323.168934
5. Kardaun SH, Sekula P, Valeyrie‐Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169(5):1071-1080. doi:10.1111/bjd.12501
6. Koomdee N, Pratoomwun J, Jantararoungtong T, et al. Association of HLA-A and HLA-B alleles with lamotrigine-induced cutaneous adverse drug reactions in the Thai population. Front Pharmacol. 2017;8. doi:10.3389/fphar.2017.00879
7. Yang C-W, Cho Y-T, Hsieh Y-C, Hsu S-H, Chen K-L, Chu C-Y. The interferon-γ-induced protein 10/CXCR3 axis is associated with human herpesvirus-6 reactivation and the development of sequelae in drug reaction with eosinophilia and systemic symptoms. Br J Dermatol. 2020;183(5):909-919. doi:10.1111/bjd.18942
8. Ruffilli I, Ferrari SM, Colaci M, Ferri C, Fallahi P, Antonelli A. IP-10 in autoimmune thyroiditis. Horm Metab Res. 2014;46(9):597-602. doi:10.1055/s-0034-1382053
9. Kano Y, Tohyama M, Aihara M, et al. Sequelae in 145 patients with drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J Dermatol. 2015;42(3):276-282. doi:10.1111/1346-8138.12770
10. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124(7):588-597. doi:10.1016/j.amjmed.2011.01.017
11. Bommersbach TJ, Lapid MI, Leung JG, Cunningham JL, Rummans TA, Kung S. Management of psychotropic drug-induced dress syndrome: a systematic review. Mayo Clin Proc. 2016;91(6):787-801. doi:10.1016/j.mayocp.2016.03.006
12. Nguyen E, Yanes D, Imadojemu S, Kroshinsky D. Evaluation of cyclosporine for the treatment of DRESS syndrome. JAMA Dermatol. 2020;156(6):704-706. doi:10.1001/jamadermatol.2020.0048
13. Joseph RM, Hunter AL, Ray DW, Dixon WG. Systemic glucocorticoid therapy and adrenal insufficiency in adults: a systematic review. Semin Arthritis Rheum. 2016;46(1):133-141. doi:10.1016/j.semarthrit.2016.03.001
14. Jamilloux Y, Liozon E, Pugnet G, et al. Recovery of adrenal function after long-term glucocorticoid therapy for giant cell arteritis: a cohort study. PLoS ONE. 2013;8(7):e68713. doi:10.1371/journal.pone.0068713
1. RegiSCAR. Accessed June 3, 2022. http://www.regiscar.org
2. Shiohara T, Mizukawa Y. Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): an update in 2019. Allergol Int. 2019;68(3):301-308. doi:10.1016/j.alit.2019.03.006
3. Wolfson AR, Zhou L, Li Y, Phadke NA, Chow OA, Blumenthal KG. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome identified in the electronic health record allergy module. J Allergy Clin Immunol Pract. 2019;7(2):633-640. doi:10.1016/j.jaip.2018.08.013
4. Sasidharanpillai S, Govindan A, Riyaz N, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a histopathology based analysis. Indian J Dermatol Venereol Leprol. 2016;82(1):28. doi:10.4103/0378-6323.168934
5. Kardaun SH, Sekula P, Valeyrie‐Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169(5):1071-1080. doi:10.1111/bjd.12501
6. Koomdee N, Pratoomwun J, Jantararoungtong T, et al. Association of HLA-A and HLA-B alleles with lamotrigine-induced cutaneous adverse drug reactions in the Thai population. Front Pharmacol. 2017;8. doi:10.3389/fphar.2017.00879
7. Yang C-W, Cho Y-T, Hsieh Y-C, Hsu S-H, Chen K-L, Chu C-Y. The interferon-γ-induced protein 10/CXCR3 axis is associated with human herpesvirus-6 reactivation and the development of sequelae in drug reaction with eosinophilia and systemic symptoms. Br J Dermatol. 2020;183(5):909-919. doi:10.1111/bjd.18942
8. Ruffilli I, Ferrari SM, Colaci M, Ferri C, Fallahi P, Antonelli A. IP-10 in autoimmune thyroiditis. Horm Metab Res. 2014;46(9):597-602. doi:10.1055/s-0034-1382053
9. Kano Y, Tohyama M, Aihara M, et al. Sequelae in 145 patients with drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J Dermatol. 2015;42(3):276-282. doi:10.1111/1346-8138.12770
10. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124(7):588-597. doi:10.1016/j.amjmed.2011.01.017
11. Bommersbach TJ, Lapid MI, Leung JG, Cunningham JL, Rummans TA, Kung S. Management of psychotropic drug-induced dress syndrome: a systematic review. Mayo Clin Proc. 2016;91(6):787-801. doi:10.1016/j.mayocp.2016.03.006
12. Nguyen E, Yanes D, Imadojemu S, Kroshinsky D. Evaluation of cyclosporine for the treatment of DRESS syndrome. JAMA Dermatol. 2020;156(6):704-706. doi:10.1001/jamadermatol.2020.0048
13. Joseph RM, Hunter AL, Ray DW, Dixon WG. Systemic glucocorticoid therapy and adrenal insufficiency in adults: a systematic review. Semin Arthritis Rheum. 2016;46(1):133-141. doi:10.1016/j.semarthrit.2016.03.001
14. Jamilloux Y, Liozon E, Pugnet G, et al. Recovery of adrenal function after long-term glucocorticoid therapy for giant cell arteritis: a cohort study. PLoS ONE. 2013;8(7):e68713. doi:10.1371/journal.pone.0068713
Unique Treatment for Alopecia Areata Combining Epinephrine With an Intralesional Steroid
Alopecia areata (AA) is an autoimmune disorder characterized by transient hair loss with preservation of the hair follicle (HF). The lifetime incidence risk of AA is approximately 2%,1 with a mean age of onset of 25 to 36 years and with no clinically relevant significant differences between sex or ethnicity.2 Most commonly, it presents as round, well-demarcated patches of alopecia on the scalp and spontaneously resolves in nearly 30% of patients. However, severe disease is associated with younger age of presentation and can progress to a total loss of scalp or body hair—referred to as alopecia totalis and alopecia universalis, respectively—thus severely impacting quality of life.3,4
First-line treatment options for AA include potent topical steroids5,6 and intralesional (IL) steroids, most commonly IL triamcinolone acetonide (ILTA). Intralesional steroids have been found to be more effective than topicals in stimulating hair growth at the injection site.7,8 A recent systemic therapy—the Janus kinase inhibitor baricitinib—was approved by the US Food and Drug Administration for AA.9 Other systemic therapies such as oral corticosteroids have been studied in small trials with promising results.10 However, the risks of systemic therapies may outweigh the benefits.9,10
Another less common topical therapy is contact immunotherapy, which involves topical application of an unlicensed non–pharmaceutical-grade agent to areas affected with AA. It is reported to have a wide range of response rates (29%–87%).11
We report 2 cases of extensive AA that were treated with a novel combination regimen— 2.5 mg/mL of ILTA diluted with lidocaine 1% and epinephrine 1:100,000 in place of normal saline (NS)— which is a modification to an already widely used first-line treatment.
Case Reports
Patient 1—An 11-year-old girl presented with nonscarring alopecia of the vertex and occipital scalp. Three years prior she was treated with topical and IL corticosteroids by a different provider. Physical examination revealed almost complete alopecia involving the bottom two-thirds of the occipital scalp as well as the medial eyebrows (Figures 1A and 1B). Over the span of 1 year she was treated with betamethasone dipropionate cream 0.05% and several rounds of ILTA 2.5 mg/mL buffered with NS, with minimal improvement. A year after the initial presentation, the decision was made to initiate monthly injections of ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000. Some hair regrowth of the occipital scalp was noted by 3 months, with near-complete regrowth of the scalp hair and eyebrows by 7 months and 5 months, respectively (Figures 1C and 1D). During this period, the patient continued to develop new areas of alopecia of the scalp and eyebrows, which also were injected with this combination. In total, the patient received 8 rounds of IL injections 4 to 6 weeks apart in the scalp and 6 rounds in the eyebrows. The treated areas showed resolution over a follow-up period of 14 months, though there was recurrence at the right medial eyebrow at 5 months. No localized skin atrophy or other adverse effects were noted.
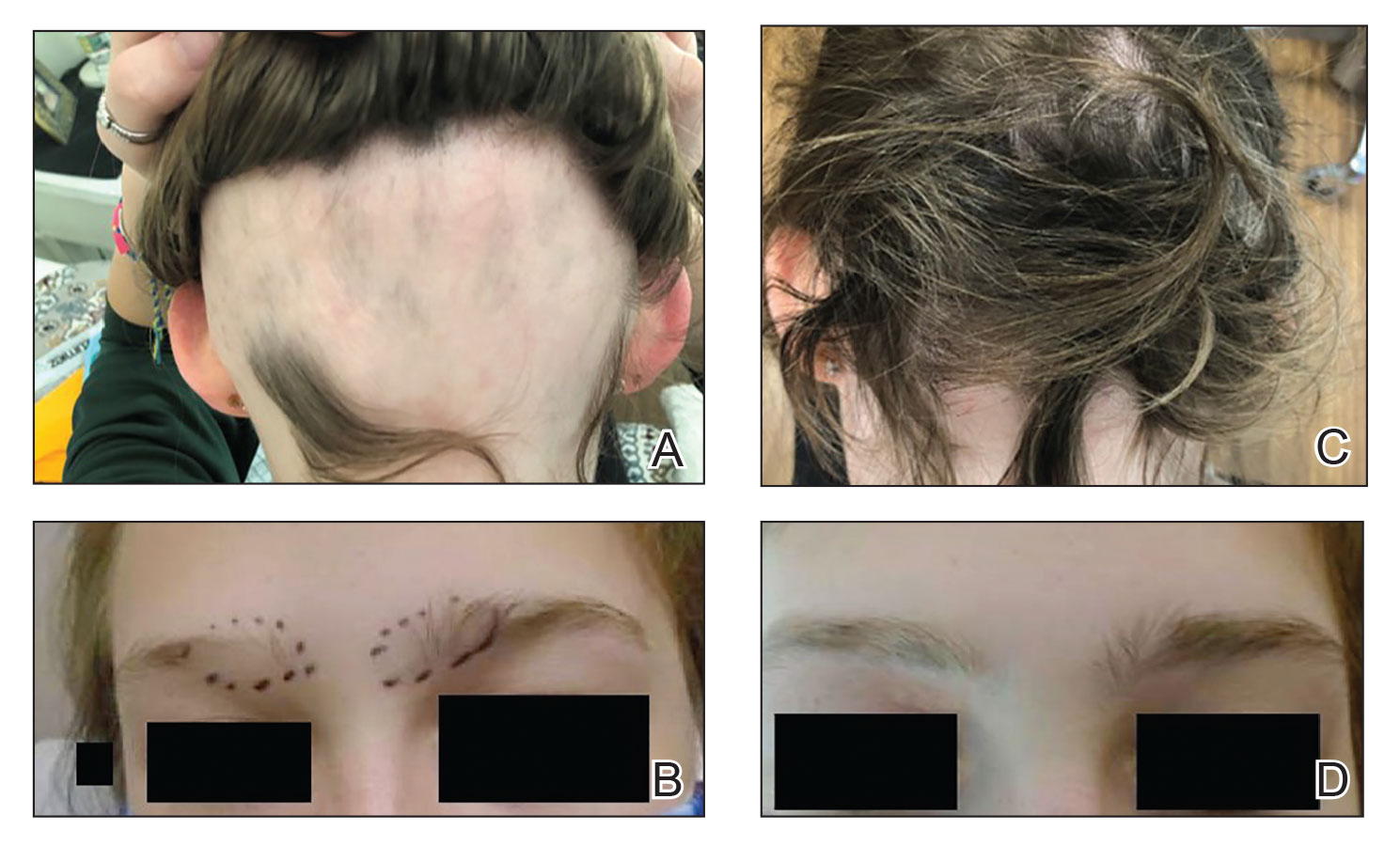
Patient 2—A 34-year-old woman who was otherwise healthy presented with previously untreated AA involving the scalp of 2 months’ duration. Physical examination revealed the following areas of nonscarring alopecia: a 10×10-cm area of the right occipital scalp with some regrowth; a 10×14-cm area of the left parieto-occipital scalp; and a 1-cm area posterior to the vertex (Figure 2A). Given the extensive involvement, the decision was made to initiate ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000 once monthly. Appreciable hair regrowth was noted within 1 month, mostly on the parietal scalp. Substantial improvement was noted after 3 months in all affected areas of the hair-bearing scalp, with near-complete regrowth on the left occipital scalp and greater than 50% regrowth on the right occipital scalp (Figure 2B). No adverse effects were noted. She currently has no alopecia.
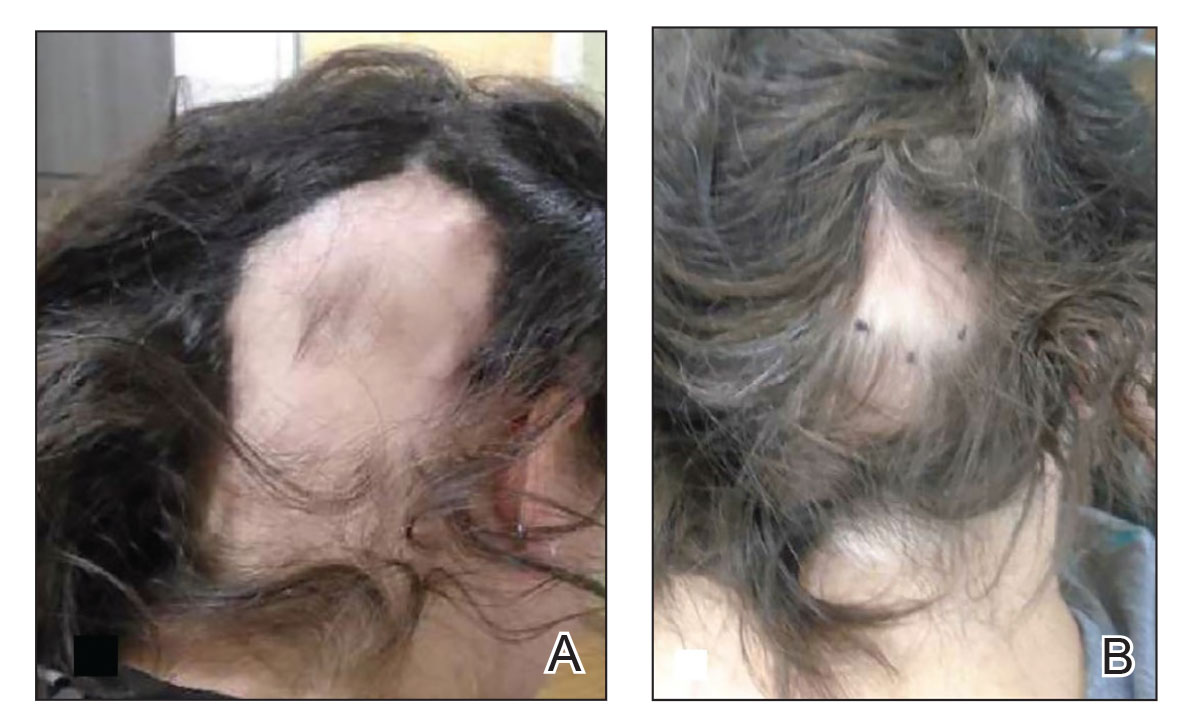
Comment
Alopecia Pathogenesis—The most widely adopted theory of AA etiology implicates an aberrant immune response. The HF, which is a dynamic “mini-organ” with its own immune and hormonal microenvironment, is considered an “immune-privileged site”—meaning it is less exposed to immune responses than most other body areas. It is hypothesized that AA results from a breakdown in this immune privilege, with the subsequent attack on the peribulbar part of the follicle by CD8+ T lymphocytes. This lymphocytic infiltrate induces apoptosis in the HF keratinocytes, resulting in inhibition of hair shaft production.12 Other theories suggest a link to the sympathetic-adrenal-medullary system and hypothalamic-pituitary-adrenal axis.13
Therapies for Alopecia—Topical and IL corticosteroids are the first-line therapies for localized AA in patients with less than 50% scalp involvement. Triamcinolone acetonide generally is the IL steroid of choice because it is widely available and less atrophogenic than other steroids. Unlike topicals, ILTA bypasses the epidermis when injected, achieving direct access to the HF.14
High-quality controlled studies regarding the use of ILTA in AA are scarce. A meta-analysis concluded that 5 mg/mL and 10 mg/mL of ILTA diluted in NS were equally effective (80.9% [P<.05] vs 76.4% [P<.005], respectively). Concentrations of less than 5 mg/mL of ILTA resulted in lower rates of hair regrowth (62.3%; P=.04).15 The role of diluents other than NS has not been studied.
Benefits of Epinephrine in ILTA Therapy—The role of epinephrine 1:100,000 is to decrease the rate of clearance of triamcinolone acetonide from the HF, allowing for a better therapeutic effect. Laser Doppler blood flowmeter studies have shown that epinephrine 1:100,000 injected in the scalp causes vasoconstriction, thereby decreasing the blood flow rate of clearance of other substances in the same solution.16 Also, a more gradual systemic absorption is achieved, decreasing systemic side effects such as osteoporosis.17
Another potential benefit of epinephrine has been suggested in animal studies that demonstrate the important role of the sympathetic nervous system in HF growth. In a mouse study, chemical sympathectomy led to diminished norepinephrine levels in the skin, accompanied by a decreased keratinocyte proliferation and hair growth. Conversely, norepinephrine was found to promote HF growth in an organotypic skin culture model.18 Topically applied isoproterenol, a panadrenergic receptor agonist, accelerated HF growth in an organotypic skin culture. It also has been shown that external light and temperature changes stimulate hair growth via the sympathetic nervous system, promoting anagen HF growth in cultured skin explants, further linking HF activity with sympathetic nerve activity.19
In our experience, cases of AA that at first failed ILTA 5 mg/mL in NS have been successfully treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000. One such case was alopecia totalis, though we do not have high-quality photographs to present for this report. The 2 cases presented here are the ones with the best photographs to demonstrate our outcomes. Both were treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000 administered using a 0.5-in long 30-gauge needle, with 0.05 to 0.1 mL per injection approximately 0.51-cm apart. The treatment intervals were 4 weeks, with a maximal dose of 20 mg per session. In addition to the 2 cases reported here, the Table includes 2 other patients in our practice who were successfully treated with this novel regimen.
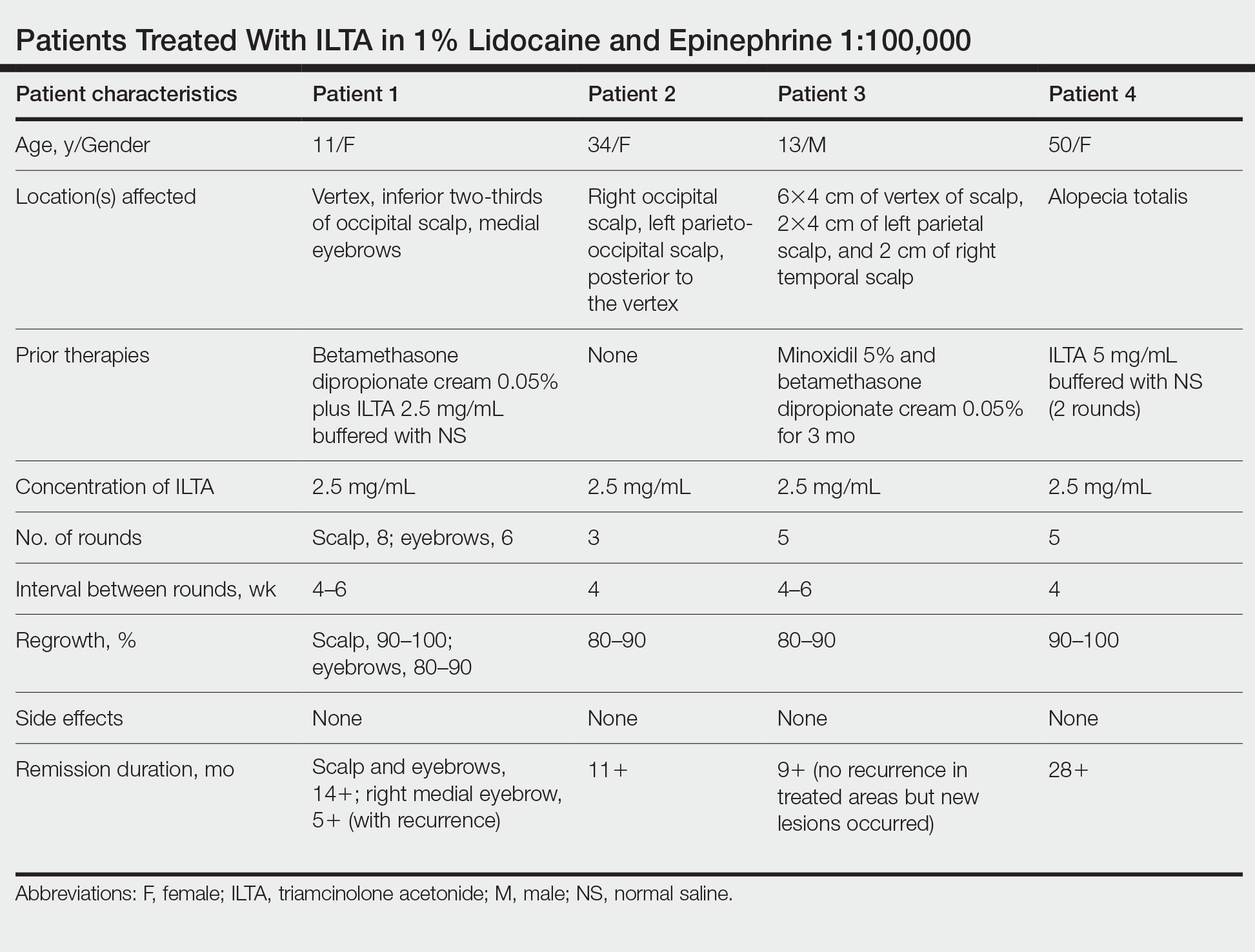
Prior to adopting this combination regimen, our standard therapy for AA was 5 mg/mL ILTA buffered with NS. Instead of NS, we now use the widely available 1% lidocaine with epinephrine 1:100,000 and dilute the ILTA to 2.5 mg/mL. We postulate that epinephrine 1:100,000 enhances therapeutic efficacy via local vasoconstriction, thus keeping the ILTA in situ longer than NS. This effect allows for a lower concentration of ILTA (2.5 mg/mL) to be effective. Furthermore, epinephrine 1:100,000 may have an independent effect, as suggested in mouse studies.18
Our first case demonstrated the ophiasis subtype of AA (symmetric bandlike hair loss), which has a poorer prognosis and is less responsive to therapy.20 In this patient, prior treatment with topical corticosteroids and ILTA in NS failed to induce a response. After a series of injections with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000, she entered remission. Our second case is one of alopecia subtotalis, which responded quickly, and the patient entered remission after just 3 months of treatment. These 2 cases are illustrative of the results that we regularly get and have come to expect with this treatment.
Conclusion
Our novel modified regimen of 2.5 mg/mL ILTA diluted with 1% lidocaine and epinephrine 1:100,000 has yielded a series of excellent outcomes in many of our most challenging AA cases without any untoward effects. Two cases are presented here. Higher-powered studies are needed to validate this new yet simple approach. A split-scalp or split-lesion study comparing ILTA with and without epinephrine 1:100,000 would be warranted for further investigation.
- Mirzoyev SA, Schrum AG, Davis MDP, et al. Lifetime incidence risk of alopecia areata estimated at 2.1 percent by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Villasante Fricke AC, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;8:397-403.
- Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55:438-441.
- Walker SA, Rothman S. A statistical study and consideration of endocrine influences. J Invest Dermatol. 1950;14:403-413.
- Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276-1277.
- Tosti A, Iorizzo M, Botta GL, et al. Efficacy and safety of a new clobetasol propionate 0.05% foam in alopecia areata: a randomized, double-blind placebo-controlled trial. J Eur Acad Dermatol Venereol. 2006;20:1243-1247.
- Kubeyinje EP. Intralesional triamcinolone acetonide in alopecia areata amongst 62 Saudi Arabs. East Afr Med J. 1994;71:674-675.
- Porter D, Burton JL. A comparison of intra-lesional triamcinolonehexacetonide and triamcinolone acetonide in alopecia areata. Br J Dermatol. 1971;85:272-273.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Lai VWY, Chen G, Gin D, et al. Systemic treatments for alopeciaareata: a systematic review. Australas J Dermatol. 2019;60:E1-E13. doi:10.1111/ajd.12913
- Rokhsar CK, Shupack JL, Vafai JJ, et al. Efficacy of topical sensitizers in the treatment of alopecia areata. J Am Acad Dermatol. 1998;39:751-761.
- Dainichi T, Kabashima K. Alopecia areata: what’s new in epidemiology, pathogenesis, diagnosis, and therapeutic options? J Dermatol Sci. 2017;86:3-12.
- Ito T. Recent advances in the pathogenesis of autoimmune hair loss disease alopecia areata. Clin Dev Immunol. 2013;2013:348546.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Consensus on the treatment of alopecia areata—Brazilian Society of Dermatology. An Bras Dermatol. 2020;95(suppl 1):39-52.
- Yee BE, Tong Y, Goldenberg A, et al. Efficacy of different concentrations of intralesional triamcinolone acetonide for alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1018-1021.
- Na YC, Park R, Jeong HS, et al. Epinephrine vasoconstriction effect time in the scalp differs according to injection site and concentration. Dermatol Surg. 2016;42:1054-1060.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Kong Y, Liu Y, Pan L, et al. Norepinephrine regulates keratinocyte proliferation to promote the growth of hair follicles. Cells Tissues Organs. 2015-2016;201:423-435.
- Fan SM, Chang YT, Chen CL, et al. External light activates hair follicle stem cells through eyes via an ipRGC-SCN-sympathetic neural pathway. Proc Natl Acad Sci U S A. 2018;115:E6880-E6889. Erratum appears in Proc Natl Acad Sci U S A. 2018;115:E12121.
- Spano F, Donovan JC. Alopecia areata: part 1: pathogenesis, diagnosis, and prognosis. Can Fam Physician. 2015;61:751-755.
Alopecia areata (AA) is an autoimmune disorder characterized by transient hair loss with preservation of the hair follicle (HF). The lifetime incidence risk of AA is approximately 2%,1 with a mean age of onset of 25 to 36 years and with no clinically relevant significant differences between sex or ethnicity.2 Most commonly, it presents as round, well-demarcated patches of alopecia on the scalp and spontaneously resolves in nearly 30% of patients. However, severe disease is associated with younger age of presentation and can progress to a total loss of scalp or body hair—referred to as alopecia totalis and alopecia universalis, respectively—thus severely impacting quality of life.3,4
First-line treatment options for AA include potent topical steroids5,6 and intralesional (IL) steroids, most commonly IL triamcinolone acetonide (ILTA). Intralesional steroids have been found to be more effective than topicals in stimulating hair growth at the injection site.7,8 A recent systemic therapy—the Janus kinase inhibitor baricitinib—was approved by the US Food and Drug Administration for AA.9 Other systemic therapies such as oral corticosteroids have been studied in small trials with promising results.10 However, the risks of systemic therapies may outweigh the benefits.9,10
Another less common topical therapy is contact immunotherapy, which involves topical application of an unlicensed non–pharmaceutical-grade agent to areas affected with AA. It is reported to have a wide range of response rates (29%–87%).11
We report 2 cases of extensive AA that were treated with a novel combination regimen— 2.5 mg/mL of ILTA diluted with lidocaine 1% and epinephrine 1:100,000 in place of normal saline (NS)— which is a modification to an already widely used first-line treatment.
Case Reports
Patient 1—An 11-year-old girl presented with nonscarring alopecia of the vertex and occipital scalp. Three years prior she was treated with topical and IL corticosteroids by a different provider. Physical examination revealed almost complete alopecia involving the bottom two-thirds of the occipital scalp as well as the medial eyebrows (Figures 1A and 1B). Over the span of 1 year she was treated with betamethasone dipropionate cream 0.05% and several rounds of ILTA 2.5 mg/mL buffered with NS, with minimal improvement. A year after the initial presentation, the decision was made to initiate monthly injections of ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000. Some hair regrowth of the occipital scalp was noted by 3 months, with near-complete regrowth of the scalp hair and eyebrows by 7 months and 5 months, respectively (Figures 1C and 1D). During this period, the patient continued to develop new areas of alopecia of the scalp and eyebrows, which also were injected with this combination. In total, the patient received 8 rounds of IL injections 4 to 6 weeks apart in the scalp and 6 rounds in the eyebrows. The treated areas showed resolution over a follow-up period of 14 months, though there was recurrence at the right medial eyebrow at 5 months. No localized skin atrophy or other adverse effects were noted.

Patient 2—A 34-year-old woman who was otherwise healthy presented with previously untreated AA involving the scalp of 2 months’ duration. Physical examination revealed the following areas of nonscarring alopecia: a 10×10-cm area of the right occipital scalp with some regrowth; a 10×14-cm area of the left parieto-occipital scalp; and a 1-cm area posterior to the vertex (Figure 2A). Given the extensive involvement, the decision was made to initiate ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000 once monthly. Appreciable hair regrowth was noted within 1 month, mostly on the parietal scalp. Substantial improvement was noted after 3 months in all affected areas of the hair-bearing scalp, with near-complete regrowth on the left occipital scalp and greater than 50% regrowth on the right occipital scalp (Figure 2B). No adverse effects were noted. She currently has no alopecia.

Comment
Alopecia Pathogenesis—The most widely adopted theory of AA etiology implicates an aberrant immune response. The HF, which is a dynamic “mini-organ” with its own immune and hormonal microenvironment, is considered an “immune-privileged site”—meaning it is less exposed to immune responses than most other body areas. It is hypothesized that AA results from a breakdown in this immune privilege, with the subsequent attack on the peribulbar part of the follicle by CD8+ T lymphocytes. This lymphocytic infiltrate induces apoptosis in the HF keratinocytes, resulting in inhibition of hair shaft production.12 Other theories suggest a link to the sympathetic-adrenal-medullary system and hypothalamic-pituitary-adrenal axis.13
Therapies for Alopecia—Topical and IL corticosteroids are the first-line therapies for localized AA in patients with less than 50% scalp involvement. Triamcinolone acetonide generally is the IL steroid of choice because it is widely available and less atrophogenic than other steroids. Unlike topicals, ILTA bypasses the epidermis when injected, achieving direct access to the HF.14
High-quality controlled studies regarding the use of ILTA in AA are scarce. A meta-analysis concluded that 5 mg/mL and 10 mg/mL of ILTA diluted in NS were equally effective (80.9% [P<.05] vs 76.4% [P<.005], respectively). Concentrations of less than 5 mg/mL of ILTA resulted in lower rates of hair regrowth (62.3%; P=.04).15 The role of diluents other than NS has not been studied.
Benefits of Epinephrine in ILTA Therapy—The role of epinephrine 1:100,000 is to decrease the rate of clearance of triamcinolone acetonide from the HF, allowing for a better therapeutic effect. Laser Doppler blood flowmeter studies have shown that epinephrine 1:100,000 injected in the scalp causes vasoconstriction, thereby decreasing the blood flow rate of clearance of other substances in the same solution.16 Also, a more gradual systemic absorption is achieved, decreasing systemic side effects such as osteoporosis.17
Another potential benefit of epinephrine has been suggested in animal studies that demonstrate the important role of the sympathetic nervous system in HF growth. In a mouse study, chemical sympathectomy led to diminished norepinephrine levels in the skin, accompanied by a decreased keratinocyte proliferation and hair growth. Conversely, norepinephrine was found to promote HF growth in an organotypic skin culture model.18 Topically applied isoproterenol, a panadrenergic receptor agonist, accelerated HF growth in an organotypic skin culture. It also has been shown that external light and temperature changes stimulate hair growth via the sympathetic nervous system, promoting anagen HF growth in cultured skin explants, further linking HF activity with sympathetic nerve activity.19
In our experience, cases of AA that at first failed ILTA 5 mg/mL in NS have been successfully treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000. One such case was alopecia totalis, though we do not have high-quality photographs to present for this report. The 2 cases presented here are the ones with the best photographs to demonstrate our outcomes. Both were treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000 administered using a 0.5-in long 30-gauge needle, with 0.05 to 0.1 mL per injection approximately 0.51-cm apart. The treatment intervals were 4 weeks, with a maximal dose of 20 mg per session. In addition to the 2 cases reported here, the Table includes 2 other patients in our practice who were successfully treated with this novel regimen.

Prior to adopting this combination regimen, our standard therapy for AA was 5 mg/mL ILTA buffered with NS. Instead of NS, we now use the widely available 1% lidocaine with epinephrine 1:100,000 and dilute the ILTA to 2.5 mg/mL. We postulate that epinephrine 1:100,000 enhances therapeutic efficacy via local vasoconstriction, thus keeping the ILTA in situ longer than NS. This effect allows for a lower concentration of ILTA (2.5 mg/mL) to be effective. Furthermore, epinephrine 1:100,000 may have an independent effect, as suggested in mouse studies.18
Our first case demonstrated the ophiasis subtype of AA (symmetric bandlike hair loss), which has a poorer prognosis and is less responsive to therapy.20 In this patient, prior treatment with topical corticosteroids and ILTA in NS failed to induce a response. After a series of injections with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000, she entered remission. Our second case is one of alopecia subtotalis, which responded quickly, and the patient entered remission after just 3 months of treatment. These 2 cases are illustrative of the results that we regularly get and have come to expect with this treatment.
Conclusion
Our novel modified regimen of 2.5 mg/mL ILTA diluted with 1% lidocaine and epinephrine 1:100,000 has yielded a series of excellent outcomes in many of our most challenging AA cases without any untoward effects. Two cases are presented here. Higher-powered studies are needed to validate this new yet simple approach. A split-scalp or split-lesion study comparing ILTA with and without epinephrine 1:100,000 would be warranted for further investigation.
Alopecia areata (AA) is an autoimmune disorder characterized by transient hair loss with preservation of the hair follicle (HF). The lifetime incidence risk of AA is approximately 2%,1 with a mean age of onset of 25 to 36 years and with no clinically relevant significant differences between sex or ethnicity.2 Most commonly, it presents as round, well-demarcated patches of alopecia on the scalp and spontaneously resolves in nearly 30% of patients. However, severe disease is associated with younger age of presentation and can progress to a total loss of scalp or body hair—referred to as alopecia totalis and alopecia universalis, respectively—thus severely impacting quality of life.3,4
First-line treatment options for AA include potent topical steroids5,6 and intralesional (IL) steroids, most commonly IL triamcinolone acetonide (ILTA). Intralesional steroids have been found to be more effective than topicals in stimulating hair growth at the injection site.7,8 A recent systemic therapy—the Janus kinase inhibitor baricitinib—was approved by the US Food and Drug Administration for AA.9 Other systemic therapies such as oral corticosteroids have been studied in small trials with promising results.10 However, the risks of systemic therapies may outweigh the benefits.9,10
Another less common topical therapy is contact immunotherapy, which involves topical application of an unlicensed non–pharmaceutical-grade agent to areas affected with AA. It is reported to have a wide range of response rates (29%–87%).11
We report 2 cases of extensive AA that were treated with a novel combination regimen— 2.5 mg/mL of ILTA diluted with lidocaine 1% and epinephrine 1:100,000 in place of normal saline (NS)— which is a modification to an already widely used first-line treatment.
Case Reports
Patient 1—An 11-year-old girl presented with nonscarring alopecia of the vertex and occipital scalp. Three years prior she was treated with topical and IL corticosteroids by a different provider. Physical examination revealed almost complete alopecia involving the bottom two-thirds of the occipital scalp as well as the medial eyebrows (Figures 1A and 1B). Over the span of 1 year she was treated with betamethasone dipropionate cream 0.05% and several rounds of ILTA 2.5 mg/mL buffered with NS, with minimal improvement. A year after the initial presentation, the decision was made to initiate monthly injections of ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000. Some hair regrowth of the occipital scalp was noted by 3 months, with near-complete regrowth of the scalp hair and eyebrows by 7 months and 5 months, respectively (Figures 1C and 1D). During this period, the patient continued to develop new areas of alopecia of the scalp and eyebrows, which also were injected with this combination. In total, the patient received 8 rounds of IL injections 4 to 6 weeks apart in the scalp and 6 rounds in the eyebrows. The treated areas showed resolution over a follow-up period of 14 months, though there was recurrence at the right medial eyebrow at 5 months. No localized skin atrophy or other adverse effects were noted.

Patient 2—A 34-year-old woman who was otherwise healthy presented with previously untreated AA involving the scalp of 2 months’ duration. Physical examination revealed the following areas of nonscarring alopecia: a 10×10-cm area of the right occipital scalp with some regrowth; a 10×14-cm area of the left parieto-occipital scalp; and a 1-cm area posterior to the vertex (Figure 2A). Given the extensive involvement, the decision was made to initiate ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000 once monthly. Appreciable hair regrowth was noted within 1 month, mostly on the parietal scalp. Substantial improvement was noted after 3 months in all affected areas of the hair-bearing scalp, with near-complete regrowth on the left occipital scalp and greater than 50% regrowth on the right occipital scalp (Figure 2B). No adverse effects were noted. She currently has no alopecia.

Comment
Alopecia Pathogenesis—The most widely adopted theory of AA etiology implicates an aberrant immune response. The HF, which is a dynamic “mini-organ” with its own immune and hormonal microenvironment, is considered an “immune-privileged site”—meaning it is less exposed to immune responses than most other body areas. It is hypothesized that AA results from a breakdown in this immune privilege, with the subsequent attack on the peribulbar part of the follicle by CD8+ T lymphocytes. This lymphocytic infiltrate induces apoptosis in the HF keratinocytes, resulting in inhibition of hair shaft production.12 Other theories suggest a link to the sympathetic-adrenal-medullary system and hypothalamic-pituitary-adrenal axis.13
Therapies for Alopecia—Topical and IL corticosteroids are the first-line therapies for localized AA in patients with less than 50% scalp involvement. Triamcinolone acetonide generally is the IL steroid of choice because it is widely available and less atrophogenic than other steroids. Unlike topicals, ILTA bypasses the epidermis when injected, achieving direct access to the HF.14
High-quality controlled studies regarding the use of ILTA in AA are scarce. A meta-analysis concluded that 5 mg/mL and 10 mg/mL of ILTA diluted in NS were equally effective (80.9% [P<.05] vs 76.4% [P<.005], respectively). Concentrations of less than 5 mg/mL of ILTA resulted in lower rates of hair regrowth (62.3%; P=.04).15 The role of diluents other than NS has not been studied.
Benefits of Epinephrine in ILTA Therapy—The role of epinephrine 1:100,000 is to decrease the rate of clearance of triamcinolone acetonide from the HF, allowing for a better therapeutic effect. Laser Doppler blood flowmeter studies have shown that epinephrine 1:100,000 injected in the scalp causes vasoconstriction, thereby decreasing the blood flow rate of clearance of other substances in the same solution.16 Also, a more gradual systemic absorption is achieved, decreasing systemic side effects such as osteoporosis.17
Another potential benefit of epinephrine has been suggested in animal studies that demonstrate the important role of the sympathetic nervous system in HF growth. In a mouse study, chemical sympathectomy led to diminished norepinephrine levels in the skin, accompanied by a decreased keratinocyte proliferation and hair growth. Conversely, norepinephrine was found to promote HF growth in an organotypic skin culture model.18 Topically applied isoproterenol, a panadrenergic receptor agonist, accelerated HF growth in an organotypic skin culture. It also has been shown that external light and temperature changes stimulate hair growth via the sympathetic nervous system, promoting anagen HF growth in cultured skin explants, further linking HF activity with sympathetic nerve activity.19
In our experience, cases of AA that at first failed ILTA 5 mg/mL in NS have been successfully treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000. One such case was alopecia totalis, though we do not have high-quality photographs to present for this report. The 2 cases presented here are the ones with the best photographs to demonstrate our outcomes. Both were treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000 administered using a 0.5-in long 30-gauge needle, with 0.05 to 0.1 mL per injection approximately 0.51-cm apart. The treatment intervals were 4 weeks, with a maximal dose of 20 mg per session. In addition to the 2 cases reported here, the Table includes 2 other patients in our practice who were successfully treated with this novel regimen.

Prior to adopting this combination regimen, our standard therapy for AA was 5 mg/mL ILTA buffered with NS. Instead of NS, we now use the widely available 1% lidocaine with epinephrine 1:100,000 and dilute the ILTA to 2.5 mg/mL. We postulate that epinephrine 1:100,000 enhances therapeutic efficacy via local vasoconstriction, thus keeping the ILTA in situ longer than NS. This effect allows for a lower concentration of ILTA (2.5 mg/mL) to be effective. Furthermore, epinephrine 1:100,000 may have an independent effect, as suggested in mouse studies.18
Our first case demonstrated the ophiasis subtype of AA (symmetric bandlike hair loss), which has a poorer prognosis and is less responsive to therapy.20 In this patient, prior treatment with topical corticosteroids and ILTA in NS failed to induce a response. After a series of injections with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000, she entered remission. Our second case is one of alopecia subtotalis, which responded quickly, and the patient entered remission after just 3 months of treatment. These 2 cases are illustrative of the results that we regularly get and have come to expect with this treatment.
Conclusion
Our novel modified regimen of 2.5 mg/mL ILTA diluted with 1% lidocaine and epinephrine 1:100,000 has yielded a series of excellent outcomes in many of our most challenging AA cases without any untoward effects. Two cases are presented here. Higher-powered studies are needed to validate this new yet simple approach. A split-scalp or split-lesion study comparing ILTA with and without epinephrine 1:100,000 would be warranted for further investigation.
- Mirzoyev SA, Schrum AG, Davis MDP, et al. Lifetime incidence risk of alopecia areata estimated at 2.1 percent by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Villasante Fricke AC, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;8:397-403.
- Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55:438-441.
- Walker SA, Rothman S. A statistical study and consideration of endocrine influences. J Invest Dermatol. 1950;14:403-413.
- Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276-1277.
- Tosti A, Iorizzo M, Botta GL, et al. Efficacy and safety of a new clobetasol propionate 0.05% foam in alopecia areata: a randomized, double-blind placebo-controlled trial. J Eur Acad Dermatol Venereol. 2006;20:1243-1247.
- Kubeyinje EP. Intralesional triamcinolone acetonide in alopecia areata amongst 62 Saudi Arabs. East Afr Med J. 1994;71:674-675.
- Porter D, Burton JL. A comparison of intra-lesional triamcinolonehexacetonide and triamcinolone acetonide in alopecia areata. Br J Dermatol. 1971;85:272-273.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Lai VWY, Chen G, Gin D, et al. Systemic treatments for alopeciaareata: a systematic review. Australas J Dermatol. 2019;60:E1-E13. doi:10.1111/ajd.12913
- Rokhsar CK, Shupack JL, Vafai JJ, et al. Efficacy of topical sensitizers in the treatment of alopecia areata. J Am Acad Dermatol. 1998;39:751-761.
- Dainichi T, Kabashima K. Alopecia areata: what’s new in epidemiology, pathogenesis, diagnosis, and therapeutic options? J Dermatol Sci. 2017;86:3-12.
- Ito T. Recent advances in the pathogenesis of autoimmune hair loss disease alopecia areata. Clin Dev Immunol. 2013;2013:348546.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Consensus on the treatment of alopecia areata—Brazilian Society of Dermatology. An Bras Dermatol. 2020;95(suppl 1):39-52.
- Yee BE, Tong Y, Goldenberg A, et al. Efficacy of different concentrations of intralesional triamcinolone acetonide for alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1018-1021.
- Na YC, Park R, Jeong HS, et al. Epinephrine vasoconstriction effect time in the scalp differs according to injection site and concentration. Dermatol Surg. 2016;42:1054-1060.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Kong Y, Liu Y, Pan L, et al. Norepinephrine regulates keratinocyte proliferation to promote the growth of hair follicles. Cells Tissues Organs. 2015-2016;201:423-435.
- Fan SM, Chang YT, Chen CL, et al. External light activates hair follicle stem cells through eyes via an ipRGC-SCN-sympathetic neural pathway. Proc Natl Acad Sci U S A. 2018;115:E6880-E6889. Erratum appears in Proc Natl Acad Sci U S A. 2018;115:E12121.
- Spano F, Donovan JC. Alopecia areata: part 1: pathogenesis, diagnosis, and prognosis. Can Fam Physician. 2015;61:751-755.
- Mirzoyev SA, Schrum AG, Davis MDP, et al. Lifetime incidence risk of alopecia areata estimated at 2.1 percent by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Villasante Fricke AC, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;8:397-403.
- Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55:438-441.
- Walker SA, Rothman S. A statistical study and consideration of endocrine influences. J Invest Dermatol. 1950;14:403-413.
- Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276-1277.
- Tosti A, Iorizzo M, Botta GL, et al. Efficacy and safety of a new clobetasol propionate 0.05% foam in alopecia areata: a randomized, double-blind placebo-controlled trial. J Eur Acad Dermatol Venereol. 2006;20:1243-1247.
- Kubeyinje EP. Intralesional triamcinolone acetonide in alopecia areata amongst 62 Saudi Arabs. East Afr Med J. 1994;71:674-675.
- Porter D, Burton JL. A comparison of intra-lesional triamcinolonehexacetonide and triamcinolone acetonide in alopecia areata. Br J Dermatol. 1971;85:272-273.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Lai VWY, Chen G, Gin D, et al. Systemic treatments for alopeciaareata: a systematic review. Australas J Dermatol. 2019;60:E1-E13. doi:10.1111/ajd.12913
- Rokhsar CK, Shupack JL, Vafai JJ, et al. Efficacy of topical sensitizers in the treatment of alopecia areata. J Am Acad Dermatol. 1998;39:751-761.
- Dainichi T, Kabashima K. Alopecia areata: what’s new in epidemiology, pathogenesis, diagnosis, and therapeutic options? J Dermatol Sci. 2017;86:3-12.
- Ito T. Recent advances in the pathogenesis of autoimmune hair loss disease alopecia areata. Clin Dev Immunol. 2013;2013:348546.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Consensus on the treatment of alopecia areata—Brazilian Society of Dermatology. An Bras Dermatol. 2020;95(suppl 1):39-52.
- Yee BE, Tong Y, Goldenberg A, et al. Efficacy of different concentrations of intralesional triamcinolone acetonide for alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1018-1021.
- Na YC, Park R, Jeong HS, et al. Epinephrine vasoconstriction effect time in the scalp differs according to injection site and concentration. Dermatol Surg. 2016;42:1054-1060.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Kong Y, Liu Y, Pan L, et al. Norepinephrine regulates keratinocyte proliferation to promote the growth of hair follicles. Cells Tissues Organs. 2015-2016;201:423-435.
- Fan SM, Chang YT, Chen CL, et al. External light activates hair follicle stem cells through eyes via an ipRGC-SCN-sympathetic neural pathway. Proc Natl Acad Sci U S A. 2018;115:E6880-E6889. Erratum appears in Proc Natl Acad Sci U S A. 2018;115:E12121.
- Spano F, Donovan JC. Alopecia areata: part 1: pathogenesis, diagnosis, and prognosis. Can Fam Physician. 2015;61:751-755.
Practice Points
- Patients with alopecia areata that is refractory to first-line treatments may benefit from intralesional triamcinolone acetonide (ILTA) diluted to 2.5 mg/mL in 1% lidocaine and epinephrine 1:100,000 in place of normal saline.
- Local vasoconstriction due to epinephrine may potentiate ILTA effects and play an independent role.
27-year-old man • muscle weakness • fatigue • electrolyte abnormalities • Dx?
THE CASE
A 27-year-old man with no past medical history presented to his primary care physician (PCP) for a routine physical. He reported experiencing muscle weakness and fatigue for the previous 1 to 2 months. Two blood pressure measurements were recorded: 138/80 mm Hg and 142/95 mm Hg. The patient was given a diagnosis of hypertension and started on triamterene/hydrochlorothiazide. Labwork was ordered, including a complete metabolic panel, lipid panel, urinalysis, thyroid-stimulating hormone (TSH) plus thyroxine (T4), HIV antibodies, and a complete blood count.
The samples were drawn 1 week later, and the results were notable for low-normal TSH with a T4 of 0.8 ng/dL (normal range, 0.9-2.3 ng/dL); sodium, 151 mmol/L (normal range, 136-145 mmol/L); potassium, 3.4 mmol/L (normal range, 3.6-5.2 mmol/L); and white blood cell count, 13.8 x 103/mcL. The electrolyte abnormalities were attributed to the triamterene/hydrochlorothiazide, which was stopped. One week later, repeat labs showed a persistent potassium level of 3.0 mmol/L; sodium, 141 mmol/L; and glucose, 310 mg/dL. Follow-up A1C was measured at 7.4%.
At the next appointment (2 weeks after initial evaluation), the patient received a diagnosis of type 2 diabetes in addition to new-onset essential hypertension. He expressed surprise at his diagnoses, as he said he primarily ate a balanced diet with plenty of vegetables and lots of healthy home-cooked meals. His body mass index (BMI) was in normal range, and he said he exercised regularly.
The patient was started on metformin 500 mg/d and referred to Endocrinology. After seeing the endocrinologist, who agreed with metformin for initial management, the patient contacted his PCP with concerns about worsening “muscle wasting.” Based on these ongoing symptoms, the patient was advised to go to the emergency department (ED).
In the ED, the patient reported muscle aches and weakness, weight gain, dyspnea on exertion, and polyuria. He also said that his face had widened with his weight gain, and his weakness was greatest in his thighs compared to his distal lower extremities. Labs drawn in the ED indicated hyperglycemia (glucose, 334 mg/dL) and severe hypokalemia (potassium, 2.2 mmol/L).
THE DIAGNOSIS
The patient was admitted in the afternoon for further evaluation, and a random serum cortisol measurement was ordered. The results showed an elevated cortisol level (55.2 mcg/dL; normal range, 3-20 mcg/dL). This was followed by a profoundly positive low-dose dexamethasone suppression test with a morning cortisol level of 75.9 mcg/dL (normal range, < 1.8 mcg/dL). With these findings, the diagnosis of Cushing syndrome was made and the focus of the evaluation shifted to localization.
An adrenocorticotropic hormone (ACTH) measurement was ordered, as well as magnetic resonance imaging (MRI) of the pituitary gland and of the abdomen to assess the adrenal glands. Both MRIs were negative, prompting a high-dose 8-mg dexamethasone suppression test to be performed. The patient’s morning cortisol level remained elevated (69.9 mcg/dL), confirming the diagnosis of Cushing syndrome.
Continue to: Based on the results...
Based on the results of the dexamethasone suppression test, a pituitary adenoma was unlikely (as they are often suppressed to < 5 mcg/dL with this test). The patient’s morning ACTH results came back as elevated (356.6 pg/mL; normal range, 10-60 pg/mL), suggesting inappropriate ACTH secretion, which most often has an ectopic source. However, a nuclear medicine octreotide scan and multiple computed tomography scans failed to locate such a source.
The patient eventually underwent bilateral petrosal venous sinus sampling to definitively rule out a pituitary source. Lastly, he underwent nuclear medicine positron emission tomography, which identified a nodular opacity in the anterior left lung apex, demonstrating moderate radiotracer activity (FIGURE 1).
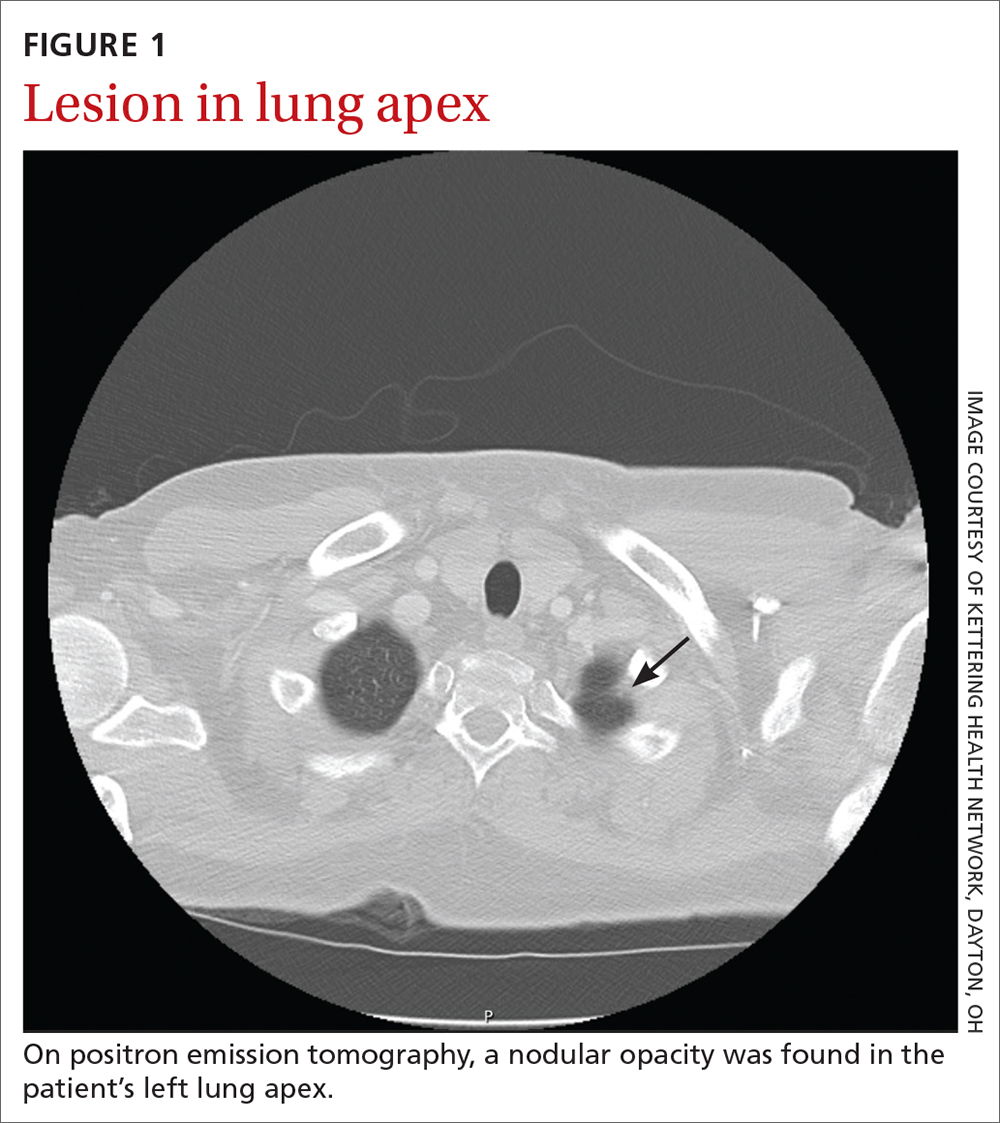
THE DISCUSSION
Cushing syndrome is rarely encountered—it is estimated to affect 2% of patients with uncontrolled diabetes1 and 1% of those with uncontrolled hypertension2—and requires a high level of clinical suspicion. This case highlights the importance of considering secondary causes of diabetes in patients who present atypically. This patient presented with symptoms consistent with Cushing syndrome that went unrecognized initially; these included high blood pressure, rounded face, weak muscles, hypokalemia, and intermittent hypernatremia in addition to new-onset hyperglycemia.2-5 Despite the atypical findings, evaluation for diabetes and potential secondary causes was neglected until an ED evaluation 1 month after initial presentation. The work-up for possible Cushing syndrome was completed in the hospital but could easily have been conducted in the outpatient setting.
Making the diagnosis. When Cushing syndrome is suspected, consider consultation with Endocrinology. It is important to exclude exogenous glucocorticoid exposure through a thorough review of the patient’s medications.2 The Endocrine Society2 recommends that one of the following tests be performed:
- 24-hour urine free cortisol (≥ 2 tests)
- Overnight 1-mg dexamethasone suppression test
- Late-night salivary cortisol test.
Results within normal range make Cushing syndrome an unlikely diagnosis; however, for patients with suggestive clinical features, further work-up may be warranted.
Continue to: Any abnormal result...
Any abnormal result is an indication to exclude a physiologic cause of hypercortisolism by repeating at least 1 of the previous studies. As with the initial testing, normal results may rule out Cushing syndrome, while abnormal results would be confirmatory. (Conflicting results require additional evaluation.)
Morbidity and mortality. Finding the etiology of Cushing syndrome can present a challenge but is also rewarding due to the reversible nature of most of the abnormalities. That said, Cushing syndrome can have a significant impact on morbidity and mortality.
Morbidity. The case patient developed compression fractures throughout his thoracic and lumbar spine, with a loss of 4 inches in height, attributed to the delay in curative treatment (FIGURE 2); these were identified about 2 months after his initial presentation to a health care facility. In addition to bone mineral density, cognitive function and quality of life can be impacted by untreated hypercortisolism and Cushing syndrome.2
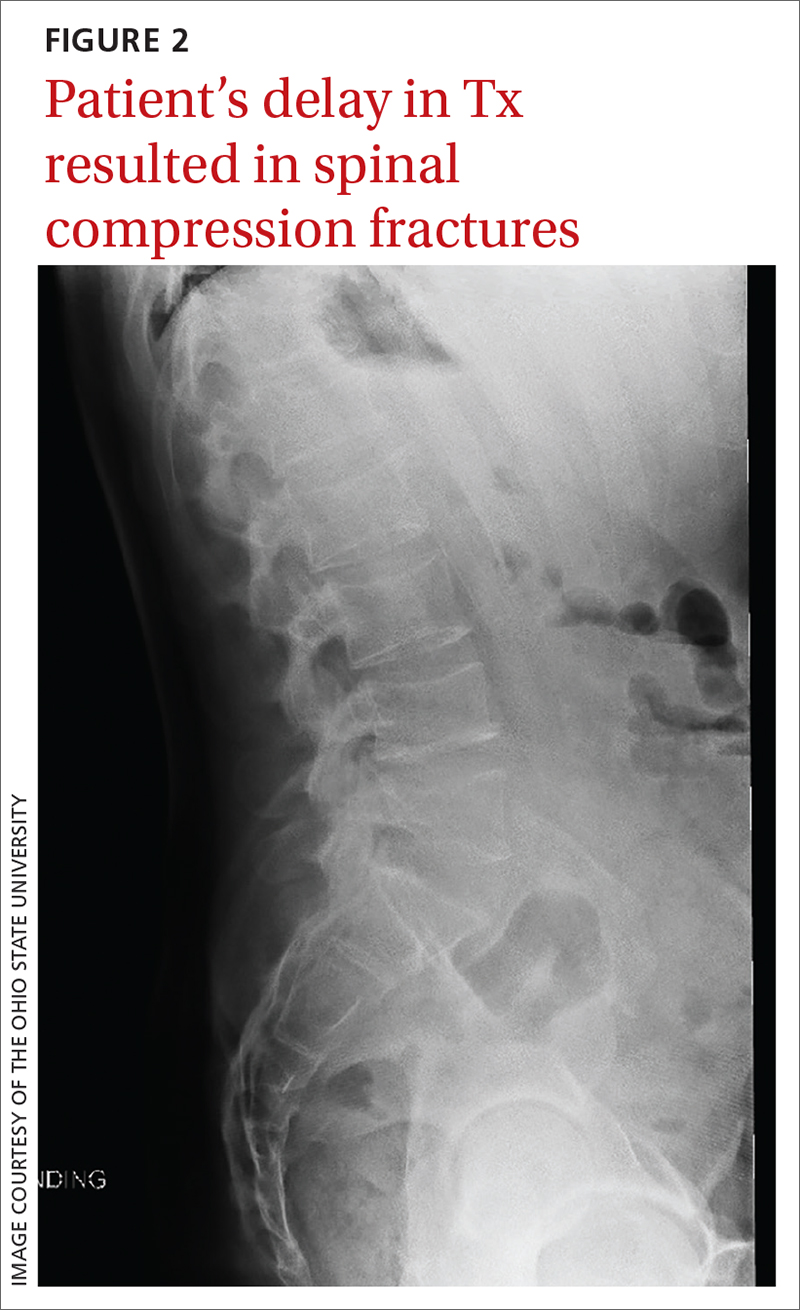
Mortality. In the earliest studies6,7 (from the 1930s-1950s), the average survival rate was about 4.6 years and the 5-year survival was just 50%—and yet, outcomes data from modern treatment modalities are scant. While there is limited data on outcomes in untreated disease, the Endocrine Society states that treatment of moderate-to-severe cases “clearly reduces mortality and morbidity” while early identification and treatment of mild cases “would reduce the risk of residual morbidity.”2
Our patient underwent video-assisted thoracoscopic surgery, during which a nodule in the anterior lingula was removed. In addition, lymph node dissection was performed. Two lymph nodes were positive for atypical well-differentiated carcinoid tumor. After surgical removal, the patient’s cortisol levels normalized and his diabetes resolved.
THE TAKEAWAY
In primary care, the frequency at which we evaluate and diagnose type 2 diabetes without secondary cause can lead to cognitive biases, such as anchoring bias, that impact patient care. In this case, the atypical secondary nature of the diabetes was missed at 3 outpatient appointments prior to presentation at the hospital ED. In an active patient who has a normal BMI and a healthy diet—but systemic symptoms—it is critical to consider secondary causes of diabetes, such as Cushing syndrome.
CORRESPONDENCE
Anna Murley Squibb, MD, 2145 North Fairfield Road, Suite 100, Beavercreek, OH 45385; [email protected]
1. Bulow B, Jansson S, Juhlin C, et al. Adrenal incidentaloma—follow-up results from a Swedish prospective study. Eur J Endocrinol. 2006;154:419-423. doi: 10.1530/eje.1.02110
2. Nieman LK, Biller BMK, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:1526-1540. doi: 10.1210/jc.2008-0125
3. Juszczak A, Morris DG, Grossman AB, et al. Chapter 13: Cushing’s syndrome. In: Jameson JL, De Groot LJ. Endocrinology: Adult and Pediatric. 7th ed. Elsevier Saunders; 2016:227-255.e11. https://doi.org/10.1016/B978-0-323-18907-1.00013-5
4. Lacroix A, Feelders RA, Stratakis CA, et al. Cushing’s syndrome. Lancet. 2015;386:913-927. doi: 10.1016/S0140-6736(14)61375-1
5. Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88:5593-5602. doi: 10.1210/jc.2003-030871
6. Cushing H. The basophil adenomas of the pituitary body and their clinical manifestations. Bull Johns Hopkins Hosp. 1932;50:137-195. doi: 10.1002/j.1550-8528.1994.tb00097.x
7. Plotz CM, Knowlton AI, Ragan C. The natural history of Cushing’s syndrome. Am J Med. 1952;13:597-614. doi: 10.1016/0002-9343(52)90027-2
THE CASE
A 27-year-old man with no past medical history presented to his primary care physician (PCP) for a routine physical. He reported experiencing muscle weakness and fatigue for the previous 1 to 2 months. Two blood pressure measurements were recorded: 138/80 mm Hg and 142/95 mm Hg. The patient was given a diagnosis of hypertension and started on triamterene/hydrochlorothiazide. Labwork was ordered, including a complete metabolic panel, lipid panel, urinalysis, thyroid-stimulating hormone (TSH) plus thyroxine (T4), HIV antibodies, and a complete blood count.
The samples were drawn 1 week later, and the results were notable for low-normal TSH with a T4 of 0.8 ng/dL (normal range, 0.9-2.3 ng/dL); sodium, 151 mmol/L (normal range, 136-145 mmol/L); potassium, 3.4 mmol/L (normal range, 3.6-5.2 mmol/L); and white blood cell count, 13.8 x 103/mcL. The electrolyte abnormalities were attributed to the triamterene/hydrochlorothiazide, which was stopped. One week later, repeat labs showed a persistent potassium level of 3.0 mmol/L; sodium, 141 mmol/L; and glucose, 310 mg/dL. Follow-up A1C was measured at 7.4%.
At the next appointment (2 weeks after initial evaluation), the patient received a diagnosis of type 2 diabetes in addition to new-onset essential hypertension. He expressed surprise at his diagnoses, as he said he primarily ate a balanced diet with plenty of vegetables and lots of healthy home-cooked meals. His body mass index (BMI) was in normal range, and he said he exercised regularly.
The patient was started on metformin 500 mg/d and referred to Endocrinology. After seeing the endocrinologist, who agreed with metformin for initial management, the patient contacted his PCP with concerns about worsening “muscle wasting.” Based on these ongoing symptoms, the patient was advised to go to the emergency department (ED).
In the ED, the patient reported muscle aches and weakness, weight gain, dyspnea on exertion, and polyuria. He also said that his face had widened with his weight gain, and his weakness was greatest in his thighs compared to his distal lower extremities. Labs drawn in the ED indicated hyperglycemia (glucose, 334 mg/dL) and severe hypokalemia (potassium, 2.2 mmol/L).
THE DIAGNOSIS
The patient was admitted in the afternoon for further evaluation, and a random serum cortisol measurement was ordered. The results showed an elevated cortisol level (55.2 mcg/dL; normal range, 3-20 mcg/dL). This was followed by a profoundly positive low-dose dexamethasone suppression test with a morning cortisol level of 75.9 mcg/dL (normal range, < 1.8 mcg/dL). With these findings, the diagnosis of Cushing syndrome was made and the focus of the evaluation shifted to localization.
An adrenocorticotropic hormone (ACTH) measurement was ordered, as well as magnetic resonance imaging (MRI) of the pituitary gland and of the abdomen to assess the adrenal glands. Both MRIs were negative, prompting a high-dose 8-mg dexamethasone suppression test to be performed. The patient’s morning cortisol level remained elevated (69.9 mcg/dL), confirming the diagnosis of Cushing syndrome.
Continue to: Based on the results...
Based on the results of the dexamethasone suppression test, a pituitary adenoma was unlikely (as they are often suppressed to < 5 mcg/dL with this test). The patient’s morning ACTH results came back as elevated (356.6 pg/mL; normal range, 10-60 pg/mL), suggesting inappropriate ACTH secretion, which most often has an ectopic source. However, a nuclear medicine octreotide scan and multiple computed tomography scans failed to locate such a source.
The patient eventually underwent bilateral petrosal venous sinus sampling to definitively rule out a pituitary source. Lastly, he underwent nuclear medicine positron emission tomography, which identified a nodular opacity in the anterior left lung apex, demonstrating moderate radiotracer activity (FIGURE 1).

THE DISCUSSION
Cushing syndrome is rarely encountered—it is estimated to affect 2% of patients with uncontrolled diabetes1 and 1% of those with uncontrolled hypertension2—and requires a high level of clinical suspicion. This case highlights the importance of considering secondary causes of diabetes in patients who present atypically. This patient presented with symptoms consistent with Cushing syndrome that went unrecognized initially; these included high blood pressure, rounded face, weak muscles, hypokalemia, and intermittent hypernatremia in addition to new-onset hyperglycemia.2-5 Despite the atypical findings, evaluation for diabetes and potential secondary causes was neglected until an ED evaluation 1 month after initial presentation. The work-up for possible Cushing syndrome was completed in the hospital but could easily have been conducted in the outpatient setting.
Making the diagnosis. When Cushing syndrome is suspected, consider consultation with Endocrinology. It is important to exclude exogenous glucocorticoid exposure through a thorough review of the patient’s medications.2 The Endocrine Society2 recommends that one of the following tests be performed:
- 24-hour urine free cortisol (≥ 2 tests)
- Overnight 1-mg dexamethasone suppression test
- Late-night salivary cortisol test.
Results within normal range make Cushing syndrome an unlikely diagnosis; however, for patients with suggestive clinical features, further work-up may be warranted.
Continue to: Any abnormal result...
Any abnormal result is an indication to exclude a physiologic cause of hypercortisolism by repeating at least 1 of the previous studies. As with the initial testing, normal results may rule out Cushing syndrome, while abnormal results would be confirmatory. (Conflicting results require additional evaluation.)
Morbidity and mortality. Finding the etiology of Cushing syndrome can present a challenge but is also rewarding due to the reversible nature of most of the abnormalities. That said, Cushing syndrome can have a significant impact on morbidity and mortality.
Morbidity. The case patient developed compression fractures throughout his thoracic and lumbar spine, with a loss of 4 inches in height, attributed to the delay in curative treatment (FIGURE 2); these were identified about 2 months after his initial presentation to a health care facility. In addition to bone mineral density, cognitive function and quality of life can be impacted by untreated hypercortisolism and Cushing syndrome.2

Mortality. In the earliest studies6,7 (from the 1930s-1950s), the average survival rate was about 4.6 years and the 5-year survival was just 50%—and yet, outcomes data from modern treatment modalities are scant. While there is limited data on outcomes in untreated disease, the Endocrine Society states that treatment of moderate-to-severe cases “clearly reduces mortality and morbidity” while early identification and treatment of mild cases “would reduce the risk of residual morbidity.”2
Our patient underwent video-assisted thoracoscopic surgery, during which a nodule in the anterior lingula was removed. In addition, lymph node dissection was performed. Two lymph nodes were positive for atypical well-differentiated carcinoid tumor. After surgical removal, the patient’s cortisol levels normalized and his diabetes resolved.
THE TAKEAWAY
In primary care, the frequency at which we evaluate and diagnose type 2 diabetes without secondary cause can lead to cognitive biases, such as anchoring bias, that impact patient care. In this case, the atypical secondary nature of the diabetes was missed at 3 outpatient appointments prior to presentation at the hospital ED. In an active patient who has a normal BMI and a healthy diet—but systemic symptoms—it is critical to consider secondary causes of diabetes, such as Cushing syndrome.
CORRESPONDENCE
Anna Murley Squibb, MD, 2145 North Fairfield Road, Suite 100, Beavercreek, OH 45385; [email protected]
THE CASE
A 27-year-old man with no past medical history presented to his primary care physician (PCP) for a routine physical. He reported experiencing muscle weakness and fatigue for the previous 1 to 2 months. Two blood pressure measurements were recorded: 138/80 mm Hg and 142/95 mm Hg. The patient was given a diagnosis of hypertension and started on triamterene/hydrochlorothiazide. Labwork was ordered, including a complete metabolic panel, lipid panel, urinalysis, thyroid-stimulating hormone (TSH) plus thyroxine (T4), HIV antibodies, and a complete blood count.
The samples were drawn 1 week later, and the results were notable for low-normal TSH with a T4 of 0.8 ng/dL (normal range, 0.9-2.3 ng/dL); sodium, 151 mmol/L (normal range, 136-145 mmol/L); potassium, 3.4 mmol/L (normal range, 3.6-5.2 mmol/L); and white blood cell count, 13.8 x 103/mcL. The electrolyte abnormalities were attributed to the triamterene/hydrochlorothiazide, which was stopped. One week later, repeat labs showed a persistent potassium level of 3.0 mmol/L; sodium, 141 mmol/L; and glucose, 310 mg/dL. Follow-up A1C was measured at 7.4%.
At the next appointment (2 weeks after initial evaluation), the patient received a diagnosis of type 2 diabetes in addition to new-onset essential hypertension. He expressed surprise at his diagnoses, as he said he primarily ate a balanced diet with plenty of vegetables and lots of healthy home-cooked meals. His body mass index (BMI) was in normal range, and he said he exercised regularly.
The patient was started on metformin 500 mg/d and referred to Endocrinology. After seeing the endocrinologist, who agreed with metformin for initial management, the patient contacted his PCP with concerns about worsening “muscle wasting.” Based on these ongoing symptoms, the patient was advised to go to the emergency department (ED).
In the ED, the patient reported muscle aches and weakness, weight gain, dyspnea on exertion, and polyuria. He also said that his face had widened with his weight gain, and his weakness was greatest in his thighs compared to his distal lower extremities. Labs drawn in the ED indicated hyperglycemia (glucose, 334 mg/dL) and severe hypokalemia (potassium, 2.2 mmol/L).
THE DIAGNOSIS
The patient was admitted in the afternoon for further evaluation, and a random serum cortisol measurement was ordered. The results showed an elevated cortisol level (55.2 mcg/dL; normal range, 3-20 mcg/dL). This was followed by a profoundly positive low-dose dexamethasone suppression test with a morning cortisol level of 75.9 mcg/dL (normal range, < 1.8 mcg/dL). With these findings, the diagnosis of Cushing syndrome was made and the focus of the evaluation shifted to localization.
An adrenocorticotropic hormone (ACTH) measurement was ordered, as well as magnetic resonance imaging (MRI) of the pituitary gland and of the abdomen to assess the adrenal glands. Both MRIs were negative, prompting a high-dose 8-mg dexamethasone suppression test to be performed. The patient’s morning cortisol level remained elevated (69.9 mcg/dL), confirming the diagnosis of Cushing syndrome.
Continue to: Based on the results...
Based on the results of the dexamethasone suppression test, a pituitary adenoma was unlikely (as they are often suppressed to < 5 mcg/dL with this test). The patient’s morning ACTH results came back as elevated (356.6 pg/mL; normal range, 10-60 pg/mL), suggesting inappropriate ACTH secretion, which most often has an ectopic source. However, a nuclear medicine octreotide scan and multiple computed tomography scans failed to locate such a source.
The patient eventually underwent bilateral petrosal venous sinus sampling to definitively rule out a pituitary source. Lastly, he underwent nuclear medicine positron emission tomography, which identified a nodular opacity in the anterior left lung apex, demonstrating moderate radiotracer activity (FIGURE 1).

THE DISCUSSION
Cushing syndrome is rarely encountered—it is estimated to affect 2% of patients with uncontrolled diabetes1 and 1% of those with uncontrolled hypertension2—and requires a high level of clinical suspicion. This case highlights the importance of considering secondary causes of diabetes in patients who present atypically. This patient presented with symptoms consistent with Cushing syndrome that went unrecognized initially; these included high blood pressure, rounded face, weak muscles, hypokalemia, and intermittent hypernatremia in addition to new-onset hyperglycemia.2-5 Despite the atypical findings, evaluation for diabetes and potential secondary causes was neglected until an ED evaluation 1 month after initial presentation. The work-up for possible Cushing syndrome was completed in the hospital but could easily have been conducted in the outpatient setting.
Making the diagnosis. When Cushing syndrome is suspected, consider consultation with Endocrinology. It is important to exclude exogenous glucocorticoid exposure through a thorough review of the patient’s medications.2 The Endocrine Society2 recommends that one of the following tests be performed:
- 24-hour urine free cortisol (≥ 2 tests)
- Overnight 1-mg dexamethasone suppression test
- Late-night salivary cortisol test.
Results within normal range make Cushing syndrome an unlikely diagnosis; however, for patients with suggestive clinical features, further work-up may be warranted.
Continue to: Any abnormal result...
Any abnormal result is an indication to exclude a physiologic cause of hypercortisolism by repeating at least 1 of the previous studies. As with the initial testing, normal results may rule out Cushing syndrome, while abnormal results would be confirmatory. (Conflicting results require additional evaluation.)
Morbidity and mortality. Finding the etiology of Cushing syndrome can present a challenge but is also rewarding due to the reversible nature of most of the abnormalities. That said, Cushing syndrome can have a significant impact on morbidity and mortality.
Morbidity. The case patient developed compression fractures throughout his thoracic and lumbar spine, with a loss of 4 inches in height, attributed to the delay in curative treatment (FIGURE 2); these were identified about 2 months after his initial presentation to a health care facility. In addition to bone mineral density, cognitive function and quality of life can be impacted by untreated hypercortisolism and Cushing syndrome.2

Mortality. In the earliest studies6,7 (from the 1930s-1950s), the average survival rate was about 4.6 years and the 5-year survival was just 50%—and yet, outcomes data from modern treatment modalities are scant. While there is limited data on outcomes in untreated disease, the Endocrine Society states that treatment of moderate-to-severe cases “clearly reduces mortality and morbidity” while early identification and treatment of mild cases “would reduce the risk of residual morbidity.”2
Our patient underwent video-assisted thoracoscopic surgery, during which a nodule in the anterior lingula was removed. In addition, lymph node dissection was performed. Two lymph nodes were positive for atypical well-differentiated carcinoid tumor. After surgical removal, the patient’s cortisol levels normalized and his diabetes resolved.
THE TAKEAWAY
In primary care, the frequency at which we evaluate and diagnose type 2 diabetes without secondary cause can lead to cognitive biases, such as anchoring bias, that impact patient care. In this case, the atypical secondary nature of the diabetes was missed at 3 outpatient appointments prior to presentation at the hospital ED. In an active patient who has a normal BMI and a healthy diet—but systemic symptoms—it is critical to consider secondary causes of diabetes, such as Cushing syndrome.
CORRESPONDENCE
Anna Murley Squibb, MD, 2145 North Fairfield Road, Suite 100, Beavercreek, OH 45385; [email protected]
1. Bulow B, Jansson S, Juhlin C, et al. Adrenal incidentaloma—follow-up results from a Swedish prospective study. Eur J Endocrinol. 2006;154:419-423. doi: 10.1530/eje.1.02110
2. Nieman LK, Biller BMK, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:1526-1540. doi: 10.1210/jc.2008-0125
3. Juszczak A, Morris DG, Grossman AB, et al. Chapter 13: Cushing’s syndrome. In: Jameson JL, De Groot LJ. Endocrinology: Adult and Pediatric. 7th ed. Elsevier Saunders; 2016:227-255.e11. https://doi.org/10.1016/B978-0-323-18907-1.00013-5
4. Lacroix A, Feelders RA, Stratakis CA, et al. Cushing’s syndrome. Lancet. 2015;386:913-927. doi: 10.1016/S0140-6736(14)61375-1
5. Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88:5593-5602. doi: 10.1210/jc.2003-030871
6. Cushing H. The basophil adenomas of the pituitary body and their clinical manifestations. Bull Johns Hopkins Hosp. 1932;50:137-195. doi: 10.1002/j.1550-8528.1994.tb00097.x
7. Plotz CM, Knowlton AI, Ragan C. The natural history of Cushing’s syndrome. Am J Med. 1952;13:597-614. doi: 10.1016/0002-9343(52)90027-2
1. Bulow B, Jansson S, Juhlin C, et al. Adrenal incidentaloma—follow-up results from a Swedish prospective study. Eur J Endocrinol. 2006;154:419-423. doi: 10.1530/eje.1.02110
2. Nieman LK, Biller BMK, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:1526-1540. doi: 10.1210/jc.2008-0125
3. Juszczak A, Morris DG, Grossman AB, et al. Chapter 13: Cushing’s syndrome. In: Jameson JL, De Groot LJ. Endocrinology: Adult and Pediatric. 7th ed. Elsevier Saunders; 2016:227-255.e11. https://doi.org/10.1016/B978-0-323-18907-1.00013-5
4. Lacroix A, Feelders RA, Stratakis CA, et al. Cushing’s syndrome. Lancet. 2015;386:913-927. doi: 10.1016/S0140-6736(14)61375-1
5. Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88:5593-5602. doi: 10.1210/jc.2003-030871
6. Cushing H. The basophil adenomas of the pituitary body and their clinical manifestations. Bull Johns Hopkins Hosp. 1932;50:137-195. doi: 10.1002/j.1550-8528.1994.tb00097.x
7. Plotz CM, Knowlton AI, Ragan C. The natural history of Cushing’s syndrome. Am J Med. 1952;13:597-614. doi: 10.1016/0002-9343(52)90027-2
52-year-old man • erectile dysfunction • insomnia • migraine headaches • disclosure of infidelity
THE CASE
A 52-year-old man requested medicine to help him with erectile dysfunction. After obtaining a medical history and performing a physical exam, the family physician (FP) asked for more details about the patient’s situation. He reported that his wife, who had recently seen the same FP for counseling related to her frustrations with her husband, was uninterested in sex. He then added that he was having an affair with a 32-year-old female co-worker and wanted to improve his sexual function.
He admitted to feeling guilty about this situation and was conflicted about whether to end the affair. He also stated that since the affair, his insomnia had worsened, he was drinking more alcohol, and he was having migraine headaches. As the FP for both patients, and with the knowledge that the wife was worried about possible infidelity, the physician felt some level of conflict about the situation. The following is a discussion of the issues that this patient encounter raised.
DISCUSSION
Issues related to infidelity are common to both men and women. They are also common in same-sex relationships; in general, however, lesbian couples have fewer outside partners, whereas gay men are more likely to seek variety by having multiple partners.1
It is widely understood that successfully committed couples spend quality time together, emphasize each other’s strengths, show respect, accept influence, and nurture their friendship. However, many couples experience infidelity at some time in the course of their marriage. It is difficult to put an exact estimate on rates of infidelity due to problems with research methodology, inaccurate reporting, and a lack of agreement on a definition for infidelity.2 General categories of infidelity include emotional only, sexual only, and combined sexual and emotional infidelity.3,4 In terms of sexual infidelity, one study found that 25% of married men and 15% of married women admitted to having had extramarital sex at least once during their relationship.5 However, other studies suggest that women are closing the “sexual infidelity” gap and engaging in sexual affairs at a similar rate to men.6 There are websites that, in fact, have made it easier for married individuals to engage in affairs.
Reasons for infidelity. Men and women often have different motives in engaging in infidelity. In general, men’s motivations are more often related to sexual dissatisfaction and women’s to emotional dissatisfaction.7,8 However, infidelity may not always be the result of marital unhappiness.
Some studies suggest that the presence of opportunity may override the positive aspects of a relationship.9 Opportunity is heightened in the work environment, as reflected by the finding that 50% of infidelity occurs in the office.10 Research suggests that all relationships may be vulnerable to infidelity if the right opportunities present themselves.11
In general, health care providers are encouraged to use caution in generalizing about infidelity, as the subject is extremely complex, nuanced, and difficult to measure with exactitude.12
Continue to: The impact of infidelity
The impact of infidelity on couples varies due to factors such as the pre-morbid health of the marriage,13 the depth of involvement with the affair partner,14 and pre-existing attitudes about infidelity.13
Infidelity is a common cause of divorce in America. However, in a sample, Schneider et al15 found that despite initial threats to leave the marriage after infidelity, less than one-quarter of partners divorced. Other studies have found that disclosure of the infidelity and a commitment to work on the marriage may be an essential component of healing.16
Emotionally focused couples therapy, with its emphasis on attachment and bonding, may hold promise for helping couples successfully work through the trauma brought on by extramarital relationships.17 Psychologist and infidelity researcher Shirley Glass found that of the two-thirds of couples who chose to stay together after an affair, 80% of them reported a better marriage after treatment.11
Initial steps to take, and questions to ask
Both male and female patients need to feel comfortable surfacing sexual concerns with their clinicians. In this case, the concerns of the husband are interwoven with broader marital issues, which are the source of emotional and psychosomatic distress. His decision regarding his affair carried with it potentially life-altering consequences for his wife, 3 children, and affair partner and her family. It also raised ethical issues for the FP, who was providing care to both the husband and the wife. Appropriate care requires that a physician in this situation
- demonstrate a nonjudgmental approach
- clarify personal ethics in response to patient behaviors
- maintain confidentiality
- apply an ethical framework to resolve value dilemmas
- avoid actions that would be harmful to patients.
Interviewing can help to elicit information that may be clarifying not only to the physician but also to the patient. When interviewing a patient such as the one in this case, it would be wise to ask:
- How long has the affair been going on?
- Why is the patient engaging in the affair?
- Is abuse (emotional or physical) a factor in the marriage?
- Does the patient still have feelings for their spouse? Does the patient want to work on the marriage?
- Has the patient talked to a friend or therapist about the situation?
- Would the patient be willing to talk to a therapist?
Continue to: Ethical and legal considerations
Ethical and legal considerations
Some therapists espouse the view that being “neutral” in the presence of an affair is as much a value judgment as taking one side or the other. In the presence of emotional or physical abuse, it might indeed be best to support a marital separation. However, in other situations when there are young children involved and the patient is undecided about what to do, the FP can discuss the pros and cons of working on a marriage that suffers from more treatable types of disrepair (ie, stress, disconnection, repetitive arguments).
Provision of care. If the patient is unwilling to end the affair, the physician needs to decide whether they feel ethically at ease with prescribing sexually enhancing performance medication, given that the patient’s wife is also a patient. A physician in this situation might feel that they are betraying the wife by providing such medications to the husband. In such cases, it might be appropriate to refer the husband to a colleague.
In all cases of infidelity, however, it is wise to discuss safe-sex practices in order to limit the risk of transmitting a sexually transmitted infection (STI) to the spouse (or affair partner) and offer testing for STIs.
Confidentiality. Despite feelings the physician might have about betraying the wife’s trust by providing the performance-enhancing medicine to the husband, there is very little justification for revealing the affair to the wife. In general, confidentiality can only be broken if there is a high level of imminent danger associated with nondisclosure. The physician needs to realize the serious legal implications of breaking confidentiality in this situation, as such disclosure may prompt the initiation of divorce proceedings.
Real-world recommendations
Check your own biases. Infidelity can trigger a whole host of emotional reactions in physicians based on their own personal and professional history. It is important to be aware of such emotions and if sufficiently triggered, discuss the case with a colleague.
Continue to: Encourage bibliotherapy and marriage therapy
Encourage bibliotherapy and marriage therapy. The conversation might go something like this:
“I would recommend you do some reading about infidelity. If you are interested in working on your marriage, you might want to consider a couples counselor who can help you. Research shows that while such counseling can help couples work through infidelity, disclosure needs to occur as part of that process. Research also indicates that about two-thirds of marriages stay together after the revelation of an affair and that such couples can experience healing if they commit to a therapeutic process. If you are unsure how you want to proceed, it might be helpful for you to explore your situation with an individual therapist. What would you like to do next?”
There are also written resources that the patient might find helpful; see “3 bibliotherapy resources for infidelity” for recommendations.
SIDEBAR
3 bibliotherapy resources for infidelity
Not ‘Just Friends’: Protect Your Relationship from Infidelity and Heal the Trauma of Betrayal (Shirley Glass)
After the Affair: Healing the Pain and Rebuilding Trust When a Partner Has Betrayed You (Janice Abrams-Spring)
How Can I Forgive You: The Courage to Forgive, the Freedom Not To (Janice Abrams-Spring)
Referral to an individual or marriage counselor is warranted if the patient wants to work through the issues alone or with their partner. Disclosure of infidelity may not always be necessary for successful reconciliation if the affair has ended. A marriage therapist to whom you refer needs to be competent in working with infidelity.
Our patient. At the completion of the initial consultation—and after a discussion focused on the issues described, including encouragement to seek counseling—the FP acceded to the patient’s request for sexual performance-enhancing medication.
Continue to: The patient returned a few months...
The patient returned a few months later. His wife had found texts between him and his affair partner and told the patient that they had to enter into couples therapy or she was going to file for divorce. The patient told his physician that he had ended the extramarital relationship and was working on his marriage with a qualified marriage therapist; however, he felt lingering feelings of loss, discomfort in the workplace, and confusion about his choices. The physician was supportive and encouraged him to share these feelings, if possible, with an individual therapist or to find a friend who could listen while being supportive of his marriage. The physician also offered his services as a sounding board.
A year later, the patient had found another job and was still working on his marriage.
THE TAKEAWAY
This case underscores the importance of some basic health care tenets. It reminds us that maintaining patient confidentiality is paramount, and that nonjudgmental interviewing can help us to help our patients navigate challenging situations. The particulars of this case also highlight the importance of referring patients out for individual or marriage counseling and making a referral to a colleague when a situation makes us feel as if we are betraying a patient’s trust.
CORRESPONDENCE
David C. Slawson, MD, 2001 Vail Avenue, Suite 400B, Mercy Medical Plaza, Charlotte, NC 28207; [email protected]
1. Blumstein P, Schwartz P. American Couples: Money, Work, Sex. William Morrow; 1983.
2. Blow A, Hartnett K. Infidelity in committed relationships I: a methodological review. J Marital Fam Ther. 2005;31:183-216. doi: 10.1111/j.1752-0606.2005.tb01555.x
3. Glass S, Wright TL. Sex differences in type of extramarital involvement and marital dissatisfaction. Sex Roles. 1985;12:1101-1120.
4. Thompson AP. Emotional and sexual components of extramarital relations. J Marriage Fam. 1984;46:35-42. doi: 10.2307/351861
5. Laumann EO, Gagnon JH, Michael RT, et al. The Social Organization of Sexuality: Sexual Practices in the United States. University of Chicago Press; 1994.
6. Oliver MB, Hyde JS. Gender differences in sexuality: a meta-analysis. Psychol Bull. 1993;114:29-51. doi: 10.1037/0033-2909.114.1.29
7. Glass SP, Wright TL. Justifications for extramarital relationships: the association between attitudes, behaviors, and gender. J Sex Res. 1992;29:361-387. doi: 10.1080/00224499209551654
8. Spanier GB, Margolis RL. Marital separation and extramarital sexual behavior. J Sex Res. 1983;19:23-48.
9. Atkins DC, Baucom DH, Jacobson NS. Understanding infidelity: correlates in a national random sample. J Fam Psychol. 2001;15:735-749. doi: 10.1037//0893-3200.15.4.735
10. Treas J, Giesen D. Sexual infidelity among married and cohabitating Americans. J Marriage Fam. 2000;62:48-60. doi: 10.1111/j.1741-3737.2000.00048.x
11. Glass SP. Not ‘Just Friends’: Protect Your Relationship From Infidelity and Heal the Trauma of Betrayal. Free Press; 2002.
12. Blow A, Hartnet K. Infidelity in committed relationships II: a substantive review. J Marital Fam Ther. 2005;31:2. doi: 10.1111/j.1752-0606.2005.tb01556.x
13. Buunk B. Conditions that promote breakups as a consequence of extradyadic involvements. J Soc Clin Psychol. 1987;5:271-284. doi: 10.1521/jscp.1987.5.3.271
14. Charn IW, Parnass S. The impact of extramarital relationships on the continuation of marriages. J Sex Marital Therapy. 1995;21:100-115. doi: 10.1080/00926239508404389
15. Schneider JP, Irons RR, Corley MD. Disclosure of extramarital sexual activities by sexually exploitative professionals and other persons with addictive or compulsive sexual disorders. J Sex Edu Therapy. 1999;24:277-287. doi: 10.1080/01614576.1999.11074316
16. Atkins DC, Eldridge KA, Baucom DH, et al. Infidelity and behavioral couple therapy: optimism in the face of betrayal. J Consult Clin Psychol. 2005;73:144-150. doi: 10.1037/0022-006X.73.1.144
17. Johnson SM, Makinen J, Millikin J. Attachment injuries in couple relationships: a new perspective on impasses in emotionally focused marital therapy. J Marital Fam Therapy. 2001;27:145-155. doi: 10.1111/j.1752-0606.2001.tb01152.x
THE CASE
A 52-year-old man requested medicine to help him with erectile dysfunction. After obtaining a medical history and performing a physical exam, the family physician (FP) asked for more details about the patient’s situation. He reported that his wife, who had recently seen the same FP for counseling related to her frustrations with her husband, was uninterested in sex. He then added that he was having an affair with a 32-year-old female co-worker and wanted to improve his sexual function.
He admitted to feeling guilty about this situation and was conflicted about whether to end the affair. He also stated that since the affair, his insomnia had worsened, he was drinking more alcohol, and he was having migraine headaches. As the FP for both patients, and with the knowledge that the wife was worried about possible infidelity, the physician felt some level of conflict about the situation. The following is a discussion of the issues that this patient encounter raised.
DISCUSSION
Issues related to infidelity are common to both men and women. They are also common in same-sex relationships; in general, however, lesbian couples have fewer outside partners, whereas gay men are more likely to seek variety by having multiple partners.1
It is widely understood that successfully committed couples spend quality time together, emphasize each other’s strengths, show respect, accept influence, and nurture their friendship. However, many couples experience infidelity at some time in the course of their marriage. It is difficult to put an exact estimate on rates of infidelity due to problems with research methodology, inaccurate reporting, and a lack of agreement on a definition for infidelity.2 General categories of infidelity include emotional only, sexual only, and combined sexual and emotional infidelity.3,4 In terms of sexual infidelity, one study found that 25% of married men and 15% of married women admitted to having had extramarital sex at least once during their relationship.5 However, other studies suggest that women are closing the “sexual infidelity” gap and engaging in sexual affairs at a similar rate to men.6 There are websites that, in fact, have made it easier for married individuals to engage in affairs.
Reasons for infidelity. Men and women often have different motives in engaging in infidelity. In general, men’s motivations are more often related to sexual dissatisfaction and women’s to emotional dissatisfaction.7,8 However, infidelity may not always be the result of marital unhappiness.
Some studies suggest that the presence of opportunity may override the positive aspects of a relationship.9 Opportunity is heightened in the work environment, as reflected by the finding that 50% of infidelity occurs in the office.10 Research suggests that all relationships may be vulnerable to infidelity if the right opportunities present themselves.11
In general, health care providers are encouraged to use caution in generalizing about infidelity, as the subject is extremely complex, nuanced, and difficult to measure with exactitude.12
Continue to: The impact of infidelity
The impact of infidelity on couples varies due to factors such as the pre-morbid health of the marriage,13 the depth of involvement with the affair partner,14 and pre-existing attitudes about infidelity.13
Infidelity is a common cause of divorce in America. However, in a sample, Schneider et al15 found that despite initial threats to leave the marriage after infidelity, less than one-quarter of partners divorced. Other studies have found that disclosure of the infidelity and a commitment to work on the marriage may be an essential component of healing.16
Emotionally focused couples therapy, with its emphasis on attachment and bonding, may hold promise for helping couples successfully work through the trauma brought on by extramarital relationships.17 Psychologist and infidelity researcher Shirley Glass found that of the two-thirds of couples who chose to stay together after an affair, 80% of them reported a better marriage after treatment.11
Initial steps to take, and questions to ask
Both male and female patients need to feel comfortable surfacing sexual concerns with their clinicians. In this case, the concerns of the husband are interwoven with broader marital issues, which are the source of emotional and psychosomatic distress. His decision regarding his affair carried with it potentially life-altering consequences for his wife, 3 children, and affair partner and her family. It also raised ethical issues for the FP, who was providing care to both the husband and the wife. Appropriate care requires that a physician in this situation
- demonstrate a nonjudgmental approach
- clarify personal ethics in response to patient behaviors
- maintain confidentiality
- apply an ethical framework to resolve value dilemmas
- avoid actions that would be harmful to patients.
Interviewing can help to elicit information that may be clarifying not only to the physician but also to the patient. When interviewing a patient such as the one in this case, it would be wise to ask:
- How long has the affair been going on?
- Why is the patient engaging in the affair?
- Is abuse (emotional or physical) a factor in the marriage?
- Does the patient still have feelings for their spouse? Does the patient want to work on the marriage?
- Has the patient talked to a friend or therapist about the situation?
- Would the patient be willing to talk to a therapist?
Continue to: Ethical and legal considerations
Ethical and legal considerations
Some therapists espouse the view that being “neutral” in the presence of an affair is as much a value judgment as taking one side or the other. In the presence of emotional or physical abuse, it might indeed be best to support a marital separation. However, in other situations when there are young children involved and the patient is undecided about what to do, the FP can discuss the pros and cons of working on a marriage that suffers from more treatable types of disrepair (ie, stress, disconnection, repetitive arguments).
Provision of care. If the patient is unwilling to end the affair, the physician needs to decide whether they feel ethically at ease with prescribing sexually enhancing performance medication, given that the patient’s wife is also a patient. A physician in this situation might feel that they are betraying the wife by providing such medications to the husband. In such cases, it might be appropriate to refer the husband to a colleague.
In all cases of infidelity, however, it is wise to discuss safe-sex practices in order to limit the risk of transmitting a sexually transmitted infection (STI) to the spouse (or affair partner) and offer testing for STIs.
Confidentiality. Despite feelings the physician might have about betraying the wife’s trust by providing the performance-enhancing medicine to the husband, there is very little justification for revealing the affair to the wife. In general, confidentiality can only be broken if there is a high level of imminent danger associated with nondisclosure. The physician needs to realize the serious legal implications of breaking confidentiality in this situation, as such disclosure may prompt the initiation of divorce proceedings.
Real-world recommendations
Check your own biases. Infidelity can trigger a whole host of emotional reactions in physicians based on their own personal and professional history. It is important to be aware of such emotions and if sufficiently triggered, discuss the case with a colleague.
Continue to: Encourage bibliotherapy and marriage therapy
Encourage bibliotherapy and marriage therapy. The conversation might go something like this:
“I would recommend you do some reading about infidelity. If you are interested in working on your marriage, you might want to consider a couples counselor who can help you. Research shows that while such counseling can help couples work through infidelity, disclosure needs to occur as part of that process. Research also indicates that about two-thirds of marriages stay together after the revelation of an affair and that such couples can experience healing if they commit to a therapeutic process. If you are unsure how you want to proceed, it might be helpful for you to explore your situation with an individual therapist. What would you like to do next?”
There are also written resources that the patient might find helpful; see “3 bibliotherapy resources for infidelity” for recommendations.
SIDEBAR
3 bibliotherapy resources for infidelity
Not ‘Just Friends’: Protect Your Relationship from Infidelity and Heal the Trauma of Betrayal (Shirley Glass)
After the Affair: Healing the Pain and Rebuilding Trust When a Partner Has Betrayed You (Janice Abrams-Spring)
How Can I Forgive You: The Courage to Forgive, the Freedom Not To (Janice Abrams-Spring)
Referral to an individual or marriage counselor is warranted if the patient wants to work through the issues alone or with their partner. Disclosure of infidelity may not always be necessary for successful reconciliation if the affair has ended. A marriage therapist to whom you refer needs to be competent in working with infidelity.
Our patient. At the completion of the initial consultation—and after a discussion focused on the issues described, including encouragement to seek counseling—the FP acceded to the patient’s request for sexual performance-enhancing medication.
Continue to: The patient returned a few months...
The patient returned a few months later. His wife had found texts between him and his affair partner and told the patient that they had to enter into couples therapy or she was going to file for divorce. The patient told his physician that he had ended the extramarital relationship and was working on his marriage with a qualified marriage therapist; however, he felt lingering feelings of loss, discomfort in the workplace, and confusion about his choices. The physician was supportive and encouraged him to share these feelings, if possible, with an individual therapist or to find a friend who could listen while being supportive of his marriage. The physician also offered his services as a sounding board.
A year later, the patient had found another job and was still working on his marriage.
THE TAKEAWAY
This case underscores the importance of some basic health care tenets. It reminds us that maintaining patient confidentiality is paramount, and that nonjudgmental interviewing can help us to help our patients navigate challenging situations. The particulars of this case also highlight the importance of referring patients out for individual or marriage counseling and making a referral to a colleague when a situation makes us feel as if we are betraying a patient’s trust.
CORRESPONDENCE
David C. Slawson, MD, 2001 Vail Avenue, Suite 400B, Mercy Medical Plaza, Charlotte, NC 28207; [email protected]
THE CASE
A 52-year-old man requested medicine to help him with erectile dysfunction. After obtaining a medical history and performing a physical exam, the family physician (FP) asked for more details about the patient’s situation. He reported that his wife, who had recently seen the same FP for counseling related to her frustrations with her husband, was uninterested in sex. He then added that he was having an affair with a 32-year-old female co-worker and wanted to improve his sexual function.
He admitted to feeling guilty about this situation and was conflicted about whether to end the affair. He also stated that since the affair, his insomnia had worsened, he was drinking more alcohol, and he was having migraine headaches. As the FP for both patients, and with the knowledge that the wife was worried about possible infidelity, the physician felt some level of conflict about the situation. The following is a discussion of the issues that this patient encounter raised.
DISCUSSION
Issues related to infidelity are common to both men and women. They are also common in same-sex relationships; in general, however, lesbian couples have fewer outside partners, whereas gay men are more likely to seek variety by having multiple partners.1
It is widely understood that successfully committed couples spend quality time together, emphasize each other’s strengths, show respect, accept influence, and nurture their friendship. However, many couples experience infidelity at some time in the course of their marriage. It is difficult to put an exact estimate on rates of infidelity due to problems with research methodology, inaccurate reporting, and a lack of agreement on a definition for infidelity.2 General categories of infidelity include emotional only, sexual only, and combined sexual and emotional infidelity.3,4 In terms of sexual infidelity, one study found that 25% of married men and 15% of married women admitted to having had extramarital sex at least once during their relationship.5 However, other studies suggest that women are closing the “sexual infidelity” gap and engaging in sexual affairs at a similar rate to men.6 There are websites that, in fact, have made it easier for married individuals to engage in affairs.
Reasons for infidelity. Men and women often have different motives in engaging in infidelity. In general, men’s motivations are more often related to sexual dissatisfaction and women’s to emotional dissatisfaction.7,8 However, infidelity may not always be the result of marital unhappiness.
Some studies suggest that the presence of opportunity may override the positive aspects of a relationship.9 Opportunity is heightened in the work environment, as reflected by the finding that 50% of infidelity occurs in the office.10 Research suggests that all relationships may be vulnerable to infidelity if the right opportunities present themselves.11
In general, health care providers are encouraged to use caution in generalizing about infidelity, as the subject is extremely complex, nuanced, and difficult to measure with exactitude.12
Continue to: The impact of infidelity
The impact of infidelity on couples varies due to factors such as the pre-morbid health of the marriage,13 the depth of involvement with the affair partner,14 and pre-existing attitudes about infidelity.13
Infidelity is a common cause of divorce in America. However, in a sample, Schneider et al15 found that despite initial threats to leave the marriage after infidelity, less than one-quarter of partners divorced. Other studies have found that disclosure of the infidelity and a commitment to work on the marriage may be an essential component of healing.16
Emotionally focused couples therapy, with its emphasis on attachment and bonding, may hold promise for helping couples successfully work through the trauma brought on by extramarital relationships.17 Psychologist and infidelity researcher Shirley Glass found that of the two-thirds of couples who chose to stay together after an affair, 80% of them reported a better marriage after treatment.11
Initial steps to take, and questions to ask
Both male and female patients need to feel comfortable surfacing sexual concerns with their clinicians. In this case, the concerns of the husband are interwoven with broader marital issues, which are the source of emotional and psychosomatic distress. His decision regarding his affair carried with it potentially life-altering consequences for his wife, 3 children, and affair partner and her family. It also raised ethical issues for the FP, who was providing care to both the husband and the wife. Appropriate care requires that a physician in this situation
- demonstrate a nonjudgmental approach
- clarify personal ethics in response to patient behaviors
- maintain confidentiality
- apply an ethical framework to resolve value dilemmas
- avoid actions that would be harmful to patients.
Interviewing can help to elicit information that may be clarifying not only to the physician but also to the patient. When interviewing a patient such as the one in this case, it would be wise to ask:
- How long has the affair been going on?
- Why is the patient engaging in the affair?
- Is abuse (emotional or physical) a factor in the marriage?
- Does the patient still have feelings for their spouse? Does the patient want to work on the marriage?
- Has the patient talked to a friend or therapist about the situation?
- Would the patient be willing to talk to a therapist?
Continue to: Ethical and legal considerations
Ethical and legal considerations
Some therapists espouse the view that being “neutral” in the presence of an affair is as much a value judgment as taking one side or the other. In the presence of emotional or physical abuse, it might indeed be best to support a marital separation. However, in other situations when there are young children involved and the patient is undecided about what to do, the FP can discuss the pros and cons of working on a marriage that suffers from more treatable types of disrepair (ie, stress, disconnection, repetitive arguments).
Provision of care. If the patient is unwilling to end the affair, the physician needs to decide whether they feel ethically at ease with prescribing sexually enhancing performance medication, given that the patient’s wife is also a patient. A physician in this situation might feel that they are betraying the wife by providing such medications to the husband. In such cases, it might be appropriate to refer the husband to a colleague.
In all cases of infidelity, however, it is wise to discuss safe-sex practices in order to limit the risk of transmitting a sexually transmitted infection (STI) to the spouse (or affair partner) and offer testing for STIs.
Confidentiality. Despite feelings the physician might have about betraying the wife’s trust by providing the performance-enhancing medicine to the husband, there is very little justification for revealing the affair to the wife. In general, confidentiality can only be broken if there is a high level of imminent danger associated with nondisclosure. The physician needs to realize the serious legal implications of breaking confidentiality in this situation, as such disclosure may prompt the initiation of divorce proceedings.
Real-world recommendations
Check your own biases. Infidelity can trigger a whole host of emotional reactions in physicians based on their own personal and professional history. It is important to be aware of such emotions and if sufficiently triggered, discuss the case with a colleague.
Continue to: Encourage bibliotherapy and marriage therapy
Encourage bibliotherapy and marriage therapy. The conversation might go something like this:
“I would recommend you do some reading about infidelity. If you are interested in working on your marriage, you might want to consider a couples counselor who can help you. Research shows that while such counseling can help couples work through infidelity, disclosure needs to occur as part of that process. Research also indicates that about two-thirds of marriages stay together after the revelation of an affair and that such couples can experience healing if they commit to a therapeutic process. If you are unsure how you want to proceed, it might be helpful for you to explore your situation with an individual therapist. What would you like to do next?”
There are also written resources that the patient might find helpful; see “3 bibliotherapy resources for infidelity” for recommendations.
SIDEBAR
3 bibliotherapy resources for infidelity
Not ‘Just Friends’: Protect Your Relationship from Infidelity and Heal the Trauma of Betrayal (Shirley Glass)
After the Affair: Healing the Pain and Rebuilding Trust When a Partner Has Betrayed You (Janice Abrams-Spring)
How Can I Forgive You: The Courage to Forgive, the Freedom Not To (Janice Abrams-Spring)
Referral to an individual or marriage counselor is warranted if the patient wants to work through the issues alone or with their partner. Disclosure of infidelity may not always be necessary for successful reconciliation if the affair has ended. A marriage therapist to whom you refer needs to be competent in working with infidelity.
Our patient. At the completion of the initial consultation—and after a discussion focused on the issues described, including encouragement to seek counseling—the FP acceded to the patient’s request for sexual performance-enhancing medication.
Continue to: The patient returned a few months...
The patient returned a few months later. His wife had found texts between him and his affair partner and told the patient that they had to enter into couples therapy or she was going to file for divorce. The patient told his physician that he had ended the extramarital relationship and was working on his marriage with a qualified marriage therapist; however, he felt lingering feelings of loss, discomfort in the workplace, and confusion about his choices. The physician was supportive and encouraged him to share these feelings, if possible, with an individual therapist or to find a friend who could listen while being supportive of his marriage. The physician also offered his services as a sounding board.
A year later, the patient had found another job and was still working on his marriage.
THE TAKEAWAY
This case underscores the importance of some basic health care tenets. It reminds us that maintaining patient confidentiality is paramount, and that nonjudgmental interviewing can help us to help our patients navigate challenging situations. The particulars of this case also highlight the importance of referring patients out for individual or marriage counseling and making a referral to a colleague when a situation makes us feel as if we are betraying a patient’s trust.
CORRESPONDENCE
David C. Slawson, MD, 2001 Vail Avenue, Suite 400B, Mercy Medical Plaza, Charlotte, NC 28207; [email protected]
1. Blumstein P, Schwartz P. American Couples: Money, Work, Sex. William Morrow; 1983.
2. Blow A, Hartnett K. Infidelity in committed relationships I: a methodological review. J Marital Fam Ther. 2005;31:183-216. doi: 10.1111/j.1752-0606.2005.tb01555.x
3. Glass S, Wright TL. Sex differences in type of extramarital involvement and marital dissatisfaction. Sex Roles. 1985;12:1101-1120.
4. Thompson AP. Emotional and sexual components of extramarital relations. J Marriage Fam. 1984;46:35-42. doi: 10.2307/351861
5. Laumann EO, Gagnon JH, Michael RT, et al. The Social Organization of Sexuality: Sexual Practices in the United States. University of Chicago Press; 1994.
6. Oliver MB, Hyde JS. Gender differences in sexuality: a meta-analysis. Psychol Bull. 1993;114:29-51. doi: 10.1037/0033-2909.114.1.29
7. Glass SP, Wright TL. Justifications for extramarital relationships: the association between attitudes, behaviors, and gender. J Sex Res. 1992;29:361-387. doi: 10.1080/00224499209551654
8. Spanier GB, Margolis RL. Marital separation and extramarital sexual behavior. J Sex Res. 1983;19:23-48.
9. Atkins DC, Baucom DH, Jacobson NS. Understanding infidelity: correlates in a national random sample. J Fam Psychol. 2001;15:735-749. doi: 10.1037//0893-3200.15.4.735
10. Treas J, Giesen D. Sexual infidelity among married and cohabitating Americans. J Marriage Fam. 2000;62:48-60. doi: 10.1111/j.1741-3737.2000.00048.x
11. Glass SP. Not ‘Just Friends’: Protect Your Relationship From Infidelity and Heal the Trauma of Betrayal. Free Press; 2002.
12. Blow A, Hartnet K. Infidelity in committed relationships II: a substantive review. J Marital Fam Ther. 2005;31:2. doi: 10.1111/j.1752-0606.2005.tb01556.x
13. Buunk B. Conditions that promote breakups as a consequence of extradyadic involvements. J Soc Clin Psychol. 1987;5:271-284. doi: 10.1521/jscp.1987.5.3.271
14. Charn IW, Parnass S. The impact of extramarital relationships on the continuation of marriages. J Sex Marital Therapy. 1995;21:100-115. doi: 10.1080/00926239508404389
15. Schneider JP, Irons RR, Corley MD. Disclosure of extramarital sexual activities by sexually exploitative professionals and other persons with addictive or compulsive sexual disorders. J Sex Edu Therapy. 1999;24:277-287. doi: 10.1080/01614576.1999.11074316
16. Atkins DC, Eldridge KA, Baucom DH, et al. Infidelity and behavioral couple therapy: optimism in the face of betrayal. J Consult Clin Psychol. 2005;73:144-150. doi: 10.1037/0022-006X.73.1.144
17. Johnson SM, Makinen J, Millikin J. Attachment injuries in couple relationships: a new perspective on impasses in emotionally focused marital therapy. J Marital Fam Therapy. 2001;27:145-155. doi: 10.1111/j.1752-0606.2001.tb01152.x
1. Blumstein P, Schwartz P. American Couples: Money, Work, Sex. William Morrow; 1983.
2. Blow A, Hartnett K. Infidelity in committed relationships I: a methodological review. J Marital Fam Ther. 2005;31:183-216. doi: 10.1111/j.1752-0606.2005.tb01555.x
3. Glass S, Wright TL. Sex differences in type of extramarital involvement and marital dissatisfaction. Sex Roles. 1985;12:1101-1120.
4. Thompson AP. Emotional and sexual components of extramarital relations. J Marriage Fam. 1984;46:35-42. doi: 10.2307/351861
5. Laumann EO, Gagnon JH, Michael RT, et al. The Social Organization of Sexuality: Sexual Practices in the United States. University of Chicago Press; 1994.
6. Oliver MB, Hyde JS. Gender differences in sexuality: a meta-analysis. Psychol Bull. 1993;114:29-51. doi: 10.1037/0033-2909.114.1.29
7. Glass SP, Wright TL. Justifications for extramarital relationships: the association between attitudes, behaviors, and gender. J Sex Res. 1992;29:361-387. doi: 10.1080/00224499209551654
8. Spanier GB, Margolis RL. Marital separation and extramarital sexual behavior. J Sex Res. 1983;19:23-48.
9. Atkins DC, Baucom DH, Jacobson NS. Understanding infidelity: correlates in a national random sample. J Fam Psychol. 2001;15:735-749. doi: 10.1037//0893-3200.15.4.735
10. Treas J, Giesen D. Sexual infidelity among married and cohabitating Americans. J Marriage Fam. 2000;62:48-60. doi: 10.1111/j.1741-3737.2000.00048.x
11. Glass SP. Not ‘Just Friends’: Protect Your Relationship From Infidelity and Heal the Trauma of Betrayal. Free Press; 2002.
12. Blow A, Hartnet K. Infidelity in committed relationships II: a substantive review. J Marital Fam Ther. 2005;31:2. doi: 10.1111/j.1752-0606.2005.tb01556.x
13. Buunk B. Conditions that promote breakups as a consequence of extradyadic involvements. J Soc Clin Psychol. 1987;5:271-284. doi: 10.1521/jscp.1987.5.3.271
14. Charn IW, Parnass S. The impact of extramarital relationships on the continuation of marriages. J Sex Marital Therapy. 1995;21:100-115. doi: 10.1080/00926239508404389
15. Schneider JP, Irons RR, Corley MD. Disclosure of extramarital sexual activities by sexually exploitative professionals and other persons with addictive or compulsive sexual disorders. J Sex Edu Therapy. 1999;24:277-287. doi: 10.1080/01614576.1999.11074316
16. Atkins DC, Eldridge KA, Baucom DH, et al. Infidelity and behavioral couple therapy: optimism in the face of betrayal. J Consult Clin Psychol. 2005;73:144-150. doi: 10.1037/0022-006X.73.1.144
17. Johnson SM, Makinen J, Millikin J. Attachment injuries in couple relationships: a new perspective on impasses in emotionally focused marital therapy. J Marital Fam Therapy. 2001;27:145-155. doi: 10.1111/j.1752-0606.2001.tb01152.x
SARS-CoV-2: A Novel Precipitant of Ischemic Priapism
Priapism is a disorder that occurs when the penis maintains a prolonged erection in the absence of appropriate stimulation. The disorder is typically divided into subgroups based on arterial flow: low flow (ischemic) and high flow (nonischemic). Ischemic priapism is the most common form and results from venous congestion due to obstructed outflow and inability of cavernous smooth muscle to contract, resulting in compartment syndrome, tissue hypoxia, hypercapnia, and acidosis.1 Conditions that result in hypercoagulable states and hyperviscosity are associated with ischemic priapism. COVID-19 is well known to cause an acute respiratory illness and systemic inflammatory response and has been increasingly associated with coagulopathy. Studies have shown that 20% to 55% of patients admitted to the hospital for COVID-19 show objective laboratory evidence of a hypercoagulable state.2
To date, there are 6 reported cases of priapism occurring in the setting of COVID-19 with all cases demonstrating the ischemic subtype. The onset of priapism from the beginning of infectious symptoms ranged from 2 days to more than a month. Five of the cases occurred in patients with critical COVID-19 and 1 in the setting of mild disease.3-8 Two critically ill patients did not receive treatment for their ischemic priapism as they were transitioned to expectant management and/or comfort measures.Most were treated with cavernosal blood aspiration and intracavernosal injections of phenylephrine or ethylephrine. Some patients were managed with prophylactic doses of anticoagulation after the identification of priapism; others were transitioned to therapeutic doses. Two patients were followed postdischarge; one patient reported normal nighttime erections with sexual desire 2 weeks postdischarge, and another patient, who underwent a bilateral T-shunt procedure after unsuccessful phenylephrine injections, reported complete erectile dysfunction at 3 months postdischarge.4,7 There was a potentially confounding variable in 2 cases in which propofol infusions were used for sedation management in the setting of mechanical ventilation.6,8 Propofol has been linked to priapism through its blockade of sympathetic activation resulting in persistent relaxation of cavernosal smooth muscle.9 We present a unique case of COVID-19–associated ischemic priapism as our patient had moderate rather than critical COVID-19.
Case Presentation
A 67-year-old male patient presented to the emergency department for a painful erection of 34-hour duration. The patient had been exposed to COVID-19 roughly 2 months prior. Since the exposure, he had experienced headache, nonproductive cough, sore throat, and decreased appetite with weight loss. His medical history included hypertension, thoracic aortic aneurysm, B-cell type chronic lymphocytic leukemia (CLL), and obstructive sleep apnea. Daily outpatient medications included atenolol 100 mg, hydrochlorothiazide 25 mg, and omeprazole 20 mg. The patient stopped tobacco use about 30 years previously. He reported no alcohol consumption or illicit drug use and had no previous episodes of prolonged erection.
The patient was afebrile, hemodynamically stable, and had an oxygen saturation of 92% on room air. Physical examination revealed clear breath sounds and an erect circumcised penis without any lesions, discoloration, or skin necrosis. Laboratory data were remarkable for the following values: 125,660 cells/μL white blood cells (WBCs), 13.82 × 103/ μL neutrophils, 110.58 × 103/μL lymphocytes, 1.26 × 103/μL monocytes, no blasts, 9.4 gm/dL hemoglobin, 100.3 fl mean corpuscular volume, 417,000 cells/μL platelets, 23,671 ng/mL D-dimer, 29.6 seconds activated partial thromboplastin time (aPTT), 16.3 seconds prothrombin time, 743 mg/dL fibrinogen, 474 U/L lactate dehydrogenase, and 202.1 mg/dL haptoglobin. A nasopharyngeal reverse transcription polymerase chain reaction test resulted positive for the SARS-CoV-2 virus, and subsequent chest X-ray revealed bilateral, hazy opacities predominantly in a peripheral distribution. Computed tomography (CT) angiogram of the chest did not reveal pulmonary emboli, pneumothorax, effusions, or lobar consolidation. However, it displayed bilateral ground-glass opacities with interstitial consolidation worst in the upper lobes. Corporal aspiration and blood gas analysis revealed a pH of 7.05, P
Differential Diagnosis
The first consideration in the differential diagnosis of priapism is to differentiate between ischemic and nonischemic. Based on the abnormal blood gas results above, this case clearly falls within the ischemic spectrum. Ischemic priapism secondary to CLL-induced hyperleukocytosis was considered. It has been noted that up to 20% of priapism cases in adults are related to hematologic disorders.10 While it is not uncommon to see hyperleukocytosis (total WBC count > 100 × 109/L) in CLL, leukostasis is rare with most reports demonstrating WBC counts > 1000 × 109/L.11 Hematology, vascular surgery, and urology services were consulted and agreed that ischemic priapism was due to microthrombi or pelvic vein thrombosis secondary to COVID-19–associated coagulopathy (CAC) was the most likely etiology.
Treatment
After corporal aspiration, intracorporal phenylephrine was administered. Diluted phenylephrine (100 ug/mL) was injected every 5 to 10 minutes while intermittently aspirating and irrigating multiple sites along the lateral length of the penile shaft. This initial procedure reduced the erection from 100% to 30% rigidity, with repeat blood gas analysis revealing minimal improvement. CT of the abdomen and pelvis with IV contrast revealed no evidence of pelvic thrombi. A second round of phenylephrine injections were administered, resulting in detumescence. The patient was treated with 2 to 3 L/min of oxygen supplementation via nasal cannula, a 5-day course of remdesivir and low-intensity heparin drip. Following the initial low-intensity heparin drip, the patient transitioned to therapeutic enoxaparin and subsequently was discharged on apixaban for a 3-month course. Since discharge, the patient followed up with hematology. He tolerated and completed the anticoagulation regimen without any recurrences of priapism or residual deficits.
Discussion
Recent studies have overwhelmingly analyzed the incidence and presentation of thrombotic complications in critically ill patients with COVID-19. CAC has been postulated to result from endotheliopathy along with immune cell activation and propagation of coagulation. While COVID-19 has been noted to create lung injury through binding angiotensin-converting enzyme 2 receptors expressed on alveolar pneumocytes, it increasingly has been found to affect endothelial cells throughout the body. Recent postmortem analyses have demonstrated direct viral infection of endothelial cells with consequent diffuse endothelial inflammation, as evidenced by viral inclusions, sequestered immune cells, and endothelial apoptosis.12,13 Manifestations of this endotheliopathy have been delineated through various studies.
An early retrospective study in Wuhan, China, illustrated that 36% of the first 99 patients hospitalized with COVID-19 demonstrated an elevated D-dimer, 6% an elevated aPTT, and 5% an elevated prothrombin time.14 Another retrospective study conducted in Wuhan found a 25% incidence of venous thromboembolic complications in critically ill patients with severe COVID-19.15 In the Netherlands, a study reported the incidence of arterial and venous thrombotic complications to be 31% in 184 critically ill patients with COVID-19, with 81% of these cases involving pulmonary emboli.16
To our knowledge, our patient is the seventh reported case of ischemic priapism occurring in the setting of a COVID-19 infection, and the first to have occurred in its moderate form. Ischemic priapism is often a consequence of penile venous outflow obstruction and resultant stasis of hypoxic blood.7 The prothrombotic state induced by CAC has been proposed to cause the obstruction of small emissary veins in the subtunical space and in turn lead to venous stasis, which propagates the formation of ischemic priapism.8 Furthermore, 4 of the previously reported cases shared laboratory data on their patients, and all demonstrated elevated D-dimer and fibrinogen levels, which strengthens this hypothesis.3,5,7,8 CLL presents a potential confounding variable in this case; however, as we have reviewed earlier, the risk of leukostasis at WBC counts < 1000 × 109/L is very low.11 It is also probable that the patient had some level of immune dysregulation secondary to CLL, leading to his prolonged course and slow clearance of the virus.
Conclusions
Although only a handful of CAC cases leading to ischemic priapism have been reported, the true incidence may be much higher. While our case highlights the importance of considering COVID-19 infection in the differential diagnosis of ischemic priapism, more research is needed to understand incidence and definitively establish a causative relationship.
1. Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1(1):116-120. doi:10.1111/j.1743-6109.2004.10117.x
2. Lee SG, Fralick M, Sholzberg M. Coagulopathy associated with COVID-19. CMAJ. 2020;192(21):E583. doi:10.1503/cmaj.200685
3. Lam G, McCarthy R, Haider R. A peculiar case of priapism: the hypercoagulable state in patients with severe COVID-19 infection. Eur J Case Rep Intern Med. 2020;7(8):001779. doi:10.12890/2020_001779
4. Addar A, Al Fraidi O, Nazer A, Althonayan N, Ghazwani Y. Priapism for 10 days in a patient with SARS-CoV-2 pneumonia: a case report. J Surg Case Rep. 2021;2021(4):rjab020. doi:10.1093/jscr/rjab020
5. Lamamri M, Chebbi A, Mamane J, et al. Priapism in a patient with coronavirus disease 2019 (COVID-19). Am J Emerg Med. 2021;39:251.e5-251.e7. doi:10.1016/j.ajem.2020.06.027
6. Silverman ML, VanDerVeer SJ, Donnelly TJ. Priapism in COVID-19: a thromboembolic complication. Am J Emerg Med. 2021;45:686.e5-686.e6. doi:10.1016/j.ajem.2020.12.072
7. Giuliano AFM, Vulpi M, Passerini F, et al. SARS-CoV-2 infection as a determining factor to the precipitation of ischemic priapism in a young patient with asymptomatic COVID-19. Case Rep Urol. 2021;2021:9936891. doi:10.1155/2021/9936891
8. Carreno BD, Perez CP, Vasquez D, Oyola JA, Suarez O, Bedoya C. Veno-occlusive priapism in COVID-19 disease. Urol Int. 2021;105(9-10):916-919. doi:10.1159/000514421
9. Senthilkumaran S, Shah S, Ganapathysubramanian, Balamurgan N, Thirumalaikolundusubramanian P. Propofol and priapism. Indian J Pharmacol. 2010;42(4):238-239. doi:10.4103/0253-7613.68430
10. Qu M, Lu X, Wang L, Liu Z, Sun Y, Gao X. Priapism secondary to chronic myeloid leukemia treated by a surgical cavernosa-corpus spongiosum shunt: case report. Asian J Urol. 2019;6(4):373-376. doi:10.1016/j.ajur.2018.12.004
11. Singh N, Singh Lubana S, Dabrowski L, Sidhu G. Leukostasis in chronic lymphocytic leukemia. Am J Case Rep. 2020;21:e924798. doi:10.12659/AJCR.924798
12. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417-1418. doi:10.1016/S0140-6736(20)30937-5
13. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033-2040. doi:10.1182/blood.2020006000
14. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi:10.1016/S0140-6736(20)30211-7
15. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421-1424. doi:10.1111/jth.14830
16. Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
Priapism is a disorder that occurs when the penis maintains a prolonged erection in the absence of appropriate stimulation. The disorder is typically divided into subgroups based on arterial flow: low flow (ischemic) and high flow (nonischemic). Ischemic priapism is the most common form and results from venous congestion due to obstructed outflow and inability of cavernous smooth muscle to contract, resulting in compartment syndrome, tissue hypoxia, hypercapnia, and acidosis.1 Conditions that result in hypercoagulable states and hyperviscosity are associated with ischemic priapism. COVID-19 is well known to cause an acute respiratory illness and systemic inflammatory response and has been increasingly associated with coagulopathy. Studies have shown that 20% to 55% of patients admitted to the hospital for COVID-19 show objective laboratory evidence of a hypercoagulable state.2
To date, there are 6 reported cases of priapism occurring in the setting of COVID-19 with all cases demonstrating the ischemic subtype. The onset of priapism from the beginning of infectious symptoms ranged from 2 days to more than a month. Five of the cases occurred in patients with critical COVID-19 and 1 in the setting of mild disease.3-8 Two critically ill patients did not receive treatment for their ischemic priapism as they were transitioned to expectant management and/or comfort measures.Most were treated with cavernosal blood aspiration and intracavernosal injections of phenylephrine or ethylephrine. Some patients were managed with prophylactic doses of anticoagulation after the identification of priapism; others were transitioned to therapeutic doses. Two patients were followed postdischarge; one patient reported normal nighttime erections with sexual desire 2 weeks postdischarge, and another patient, who underwent a bilateral T-shunt procedure after unsuccessful phenylephrine injections, reported complete erectile dysfunction at 3 months postdischarge.4,7 There was a potentially confounding variable in 2 cases in which propofol infusions were used for sedation management in the setting of mechanical ventilation.6,8 Propofol has been linked to priapism through its blockade of sympathetic activation resulting in persistent relaxation of cavernosal smooth muscle.9 We present a unique case of COVID-19–associated ischemic priapism as our patient had moderate rather than critical COVID-19.
Case Presentation
A 67-year-old male patient presented to the emergency department for a painful erection of 34-hour duration. The patient had been exposed to COVID-19 roughly 2 months prior. Since the exposure, he had experienced headache, nonproductive cough, sore throat, and decreased appetite with weight loss. His medical history included hypertension, thoracic aortic aneurysm, B-cell type chronic lymphocytic leukemia (CLL), and obstructive sleep apnea. Daily outpatient medications included atenolol 100 mg, hydrochlorothiazide 25 mg, and omeprazole 20 mg. The patient stopped tobacco use about 30 years previously. He reported no alcohol consumption or illicit drug use and had no previous episodes of prolonged erection.
The patient was afebrile, hemodynamically stable, and had an oxygen saturation of 92% on room air. Physical examination revealed clear breath sounds and an erect circumcised penis without any lesions, discoloration, or skin necrosis. Laboratory data were remarkable for the following values: 125,660 cells/μL white blood cells (WBCs), 13.82 × 103/ μL neutrophils, 110.58 × 103/μL lymphocytes, 1.26 × 103/μL monocytes, no blasts, 9.4 gm/dL hemoglobin, 100.3 fl mean corpuscular volume, 417,000 cells/μL platelets, 23,671 ng/mL D-dimer, 29.6 seconds activated partial thromboplastin time (aPTT), 16.3 seconds prothrombin time, 743 mg/dL fibrinogen, 474 U/L lactate dehydrogenase, and 202.1 mg/dL haptoglobin. A nasopharyngeal reverse transcription polymerase chain reaction test resulted positive for the SARS-CoV-2 virus, and subsequent chest X-ray revealed bilateral, hazy opacities predominantly in a peripheral distribution. Computed tomography (CT) angiogram of the chest did not reveal pulmonary emboli, pneumothorax, effusions, or lobar consolidation. However, it displayed bilateral ground-glass opacities with interstitial consolidation worst in the upper lobes. Corporal aspiration and blood gas analysis revealed a pH of 7.05, P
Differential Diagnosis
The first consideration in the differential diagnosis of priapism is to differentiate between ischemic and nonischemic. Based on the abnormal blood gas results above, this case clearly falls within the ischemic spectrum. Ischemic priapism secondary to CLL-induced hyperleukocytosis was considered. It has been noted that up to 20% of priapism cases in adults are related to hematologic disorders.10 While it is not uncommon to see hyperleukocytosis (total WBC count > 100 × 109/L) in CLL, leukostasis is rare with most reports demonstrating WBC counts > 1000 × 109/L.11 Hematology, vascular surgery, and urology services were consulted and agreed that ischemic priapism was due to microthrombi or pelvic vein thrombosis secondary to COVID-19–associated coagulopathy (CAC) was the most likely etiology.
Treatment
After corporal aspiration, intracorporal phenylephrine was administered. Diluted phenylephrine (100 ug/mL) was injected every 5 to 10 minutes while intermittently aspirating and irrigating multiple sites along the lateral length of the penile shaft. This initial procedure reduced the erection from 100% to 30% rigidity, with repeat blood gas analysis revealing minimal improvement. CT of the abdomen and pelvis with IV contrast revealed no evidence of pelvic thrombi. A second round of phenylephrine injections were administered, resulting in detumescence. The patient was treated with 2 to 3 L/min of oxygen supplementation via nasal cannula, a 5-day course of remdesivir and low-intensity heparin drip. Following the initial low-intensity heparin drip, the patient transitioned to therapeutic enoxaparin and subsequently was discharged on apixaban for a 3-month course. Since discharge, the patient followed up with hematology. He tolerated and completed the anticoagulation regimen without any recurrences of priapism or residual deficits.
Discussion
Recent studies have overwhelmingly analyzed the incidence and presentation of thrombotic complications in critically ill patients with COVID-19. CAC has been postulated to result from endotheliopathy along with immune cell activation and propagation of coagulation. While COVID-19 has been noted to create lung injury through binding angiotensin-converting enzyme 2 receptors expressed on alveolar pneumocytes, it increasingly has been found to affect endothelial cells throughout the body. Recent postmortem analyses have demonstrated direct viral infection of endothelial cells with consequent diffuse endothelial inflammation, as evidenced by viral inclusions, sequestered immune cells, and endothelial apoptosis.12,13 Manifestations of this endotheliopathy have been delineated through various studies.
An early retrospective study in Wuhan, China, illustrated that 36% of the first 99 patients hospitalized with COVID-19 demonstrated an elevated D-dimer, 6% an elevated aPTT, and 5% an elevated prothrombin time.14 Another retrospective study conducted in Wuhan found a 25% incidence of venous thromboembolic complications in critically ill patients with severe COVID-19.15 In the Netherlands, a study reported the incidence of arterial and venous thrombotic complications to be 31% in 184 critically ill patients with COVID-19, with 81% of these cases involving pulmonary emboli.16
To our knowledge, our patient is the seventh reported case of ischemic priapism occurring in the setting of a COVID-19 infection, and the first to have occurred in its moderate form. Ischemic priapism is often a consequence of penile venous outflow obstruction and resultant stasis of hypoxic blood.7 The prothrombotic state induced by CAC has been proposed to cause the obstruction of small emissary veins in the subtunical space and in turn lead to venous stasis, which propagates the formation of ischemic priapism.8 Furthermore, 4 of the previously reported cases shared laboratory data on their patients, and all demonstrated elevated D-dimer and fibrinogen levels, which strengthens this hypothesis.3,5,7,8 CLL presents a potential confounding variable in this case; however, as we have reviewed earlier, the risk of leukostasis at WBC counts < 1000 × 109/L is very low.11 It is also probable that the patient had some level of immune dysregulation secondary to CLL, leading to his prolonged course and slow clearance of the virus.
Conclusions
Although only a handful of CAC cases leading to ischemic priapism have been reported, the true incidence may be much higher. While our case highlights the importance of considering COVID-19 infection in the differential diagnosis of ischemic priapism, more research is needed to understand incidence and definitively establish a causative relationship.
Priapism is a disorder that occurs when the penis maintains a prolonged erection in the absence of appropriate stimulation. The disorder is typically divided into subgroups based on arterial flow: low flow (ischemic) and high flow (nonischemic). Ischemic priapism is the most common form and results from venous congestion due to obstructed outflow and inability of cavernous smooth muscle to contract, resulting in compartment syndrome, tissue hypoxia, hypercapnia, and acidosis.1 Conditions that result in hypercoagulable states and hyperviscosity are associated with ischemic priapism. COVID-19 is well known to cause an acute respiratory illness and systemic inflammatory response and has been increasingly associated with coagulopathy. Studies have shown that 20% to 55% of patients admitted to the hospital for COVID-19 show objective laboratory evidence of a hypercoagulable state.2
To date, there are 6 reported cases of priapism occurring in the setting of COVID-19 with all cases demonstrating the ischemic subtype. The onset of priapism from the beginning of infectious symptoms ranged from 2 days to more than a month. Five of the cases occurred in patients with critical COVID-19 and 1 in the setting of mild disease.3-8 Two critically ill patients did not receive treatment for their ischemic priapism as they were transitioned to expectant management and/or comfort measures.Most were treated with cavernosal blood aspiration and intracavernosal injections of phenylephrine or ethylephrine. Some patients were managed with prophylactic doses of anticoagulation after the identification of priapism; others were transitioned to therapeutic doses. Two patients were followed postdischarge; one patient reported normal nighttime erections with sexual desire 2 weeks postdischarge, and another patient, who underwent a bilateral T-shunt procedure after unsuccessful phenylephrine injections, reported complete erectile dysfunction at 3 months postdischarge.4,7 There was a potentially confounding variable in 2 cases in which propofol infusions were used for sedation management in the setting of mechanical ventilation.6,8 Propofol has been linked to priapism through its blockade of sympathetic activation resulting in persistent relaxation of cavernosal smooth muscle.9 We present a unique case of COVID-19–associated ischemic priapism as our patient had moderate rather than critical COVID-19.
Case Presentation
A 67-year-old male patient presented to the emergency department for a painful erection of 34-hour duration. The patient had been exposed to COVID-19 roughly 2 months prior. Since the exposure, he had experienced headache, nonproductive cough, sore throat, and decreased appetite with weight loss. His medical history included hypertension, thoracic aortic aneurysm, B-cell type chronic lymphocytic leukemia (CLL), and obstructive sleep apnea. Daily outpatient medications included atenolol 100 mg, hydrochlorothiazide 25 mg, and omeprazole 20 mg. The patient stopped tobacco use about 30 years previously. He reported no alcohol consumption or illicit drug use and had no previous episodes of prolonged erection.
The patient was afebrile, hemodynamically stable, and had an oxygen saturation of 92% on room air. Physical examination revealed clear breath sounds and an erect circumcised penis without any lesions, discoloration, or skin necrosis. Laboratory data were remarkable for the following values: 125,660 cells/μL white blood cells (WBCs), 13.82 × 103/ μL neutrophils, 110.58 × 103/μL lymphocytes, 1.26 × 103/μL monocytes, no blasts, 9.4 gm/dL hemoglobin, 100.3 fl mean corpuscular volume, 417,000 cells/μL platelets, 23,671 ng/mL D-dimer, 29.6 seconds activated partial thromboplastin time (aPTT), 16.3 seconds prothrombin time, 743 mg/dL fibrinogen, 474 U/L lactate dehydrogenase, and 202.1 mg/dL haptoglobin. A nasopharyngeal reverse transcription polymerase chain reaction test resulted positive for the SARS-CoV-2 virus, and subsequent chest X-ray revealed bilateral, hazy opacities predominantly in a peripheral distribution. Computed tomography (CT) angiogram of the chest did not reveal pulmonary emboli, pneumothorax, effusions, or lobar consolidation. However, it displayed bilateral ground-glass opacities with interstitial consolidation worst in the upper lobes. Corporal aspiration and blood gas analysis revealed a pH of 7.05, P
Differential Diagnosis
The first consideration in the differential diagnosis of priapism is to differentiate between ischemic and nonischemic. Based on the abnormal blood gas results above, this case clearly falls within the ischemic spectrum. Ischemic priapism secondary to CLL-induced hyperleukocytosis was considered. It has been noted that up to 20% of priapism cases in adults are related to hematologic disorders.10 While it is not uncommon to see hyperleukocytosis (total WBC count > 100 × 109/L) in CLL, leukostasis is rare with most reports demonstrating WBC counts > 1000 × 109/L.11 Hematology, vascular surgery, and urology services were consulted and agreed that ischemic priapism was due to microthrombi or pelvic vein thrombosis secondary to COVID-19–associated coagulopathy (CAC) was the most likely etiology.
Treatment
After corporal aspiration, intracorporal phenylephrine was administered. Diluted phenylephrine (100 ug/mL) was injected every 5 to 10 minutes while intermittently aspirating and irrigating multiple sites along the lateral length of the penile shaft. This initial procedure reduced the erection from 100% to 30% rigidity, with repeat blood gas analysis revealing minimal improvement. CT of the abdomen and pelvis with IV contrast revealed no evidence of pelvic thrombi. A second round of phenylephrine injections were administered, resulting in detumescence. The patient was treated with 2 to 3 L/min of oxygen supplementation via nasal cannula, a 5-day course of remdesivir and low-intensity heparin drip. Following the initial low-intensity heparin drip, the patient transitioned to therapeutic enoxaparin and subsequently was discharged on apixaban for a 3-month course. Since discharge, the patient followed up with hematology. He tolerated and completed the anticoagulation regimen without any recurrences of priapism or residual deficits.
Discussion
Recent studies have overwhelmingly analyzed the incidence and presentation of thrombotic complications in critically ill patients with COVID-19. CAC has been postulated to result from endotheliopathy along with immune cell activation and propagation of coagulation. While COVID-19 has been noted to create lung injury through binding angiotensin-converting enzyme 2 receptors expressed on alveolar pneumocytes, it increasingly has been found to affect endothelial cells throughout the body. Recent postmortem analyses have demonstrated direct viral infection of endothelial cells with consequent diffuse endothelial inflammation, as evidenced by viral inclusions, sequestered immune cells, and endothelial apoptosis.12,13 Manifestations of this endotheliopathy have been delineated through various studies.
An early retrospective study in Wuhan, China, illustrated that 36% of the first 99 patients hospitalized with COVID-19 demonstrated an elevated D-dimer, 6% an elevated aPTT, and 5% an elevated prothrombin time.14 Another retrospective study conducted in Wuhan found a 25% incidence of venous thromboembolic complications in critically ill patients with severe COVID-19.15 In the Netherlands, a study reported the incidence of arterial and venous thrombotic complications to be 31% in 184 critically ill patients with COVID-19, with 81% of these cases involving pulmonary emboli.16
To our knowledge, our patient is the seventh reported case of ischemic priapism occurring in the setting of a COVID-19 infection, and the first to have occurred in its moderate form. Ischemic priapism is often a consequence of penile venous outflow obstruction and resultant stasis of hypoxic blood.7 The prothrombotic state induced by CAC has been proposed to cause the obstruction of small emissary veins in the subtunical space and in turn lead to venous stasis, which propagates the formation of ischemic priapism.8 Furthermore, 4 of the previously reported cases shared laboratory data on their patients, and all demonstrated elevated D-dimer and fibrinogen levels, which strengthens this hypothesis.3,5,7,8 CLL presents a potential confounding variable in this case; however, as we have reviewed earlier, the risk of leukostasis at WBC counts < 1000 × 109/L is very low.11 It is also probable that the patient had some level of immune dysregulation secondary to CLL, leading to his prolonged course and slow clearance of the virus.
Conclusions
Although only a handful of CAC cases leading to ischemic priapism have been reported, the true incidence may be much higher. While our case highlights the importance of considering COVID-19 infection in the differential diagnosis of ischemic priapism, more research is needed to understand incidence and definitively establish a causative relationship.
1. Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1(1):116-120. doi:10.1111/j.1743-6109.2004.10117.x
2. Lee SG, Fralick M, Sholzberg M. Coagulopathy associated with COVID-19. CMAJ. 2020;192(21):E583. doi:10.1503/cmaj.200685
3. Lam G, McCarthy R, Haider R. A peculiar case of priapism: the hypercoagulable state in patients with severe COVID-19 infection. Eur J Case Rep Intern Med. 2020;7(8):001779. doi:10.12890/2020_001779
4. Addar A, Al Fraidi O, Nazer A, Althonayan N, Ghazwani Y. Priapism for 10 days in a patient with SARS-CoV-2 pneumonia: a case report. J Surg Case Rep. 2021;2021(4):rjab020. doi:10.1093/jscr/rjab020
5. Lamamri M, Chebbi A, Mamane J, et al. Priapism in a patient with coronavirus disease 2019 (COVID-19). Am J Emerg Med. 2021;39:251.e5-251.e7. doi:10.1016/j.ajem.2020.06.027
6. Silverman ML, VanDerVeer SJ, Donnelly TJ. Priapism in COVID-19: a thromboembolic complication. Am J Emerg Med. 2021;45:686.e5-686.e6. doi:10.1016/j.ajem.2020.12.072
7. Giuliano AFM, Vulpi M, Passerini F, et al. SARS-CoV-2 infection as a determining factor to the precipitation of ischemic priapism in a young patient with asymptomatic COVID-19. Case Rep Urol. 2021;2021:9936891. doi:10.1155/2021/9936891
8. Carreno BD, Perez CP, Vasquez D, Oyola JA, Suarez O, Bedoya C. Veno-occlusive priapism in COVID-19 disease. Urol Int. 2021;105(9-10):916-919. doi:10.1159/000514421
9. Senthilkumaran S, Shah S, Ganapathysubramanian, Balamurgan N, Thirumalaikolundusubramanian P. Propofol and priapism. Indian J Pharmacol. 2010;42(4):238-239. doi:10.4103/0253-7613.68430
10. Qu M, Lu X, Wang L, Liu Z, Sun Y, Gao X. Priapism secondary to chronic myeloid leukemia treated by a surgical cavernosa-corpus spongiosum shunt: case report. Asian J Urol. 2019;6(4):373-376. doi:10.1016/j.ajur.2018.12.004
11. Singh N, Singh Lubana S, Dabrowski L, Sidhu G. Leukostasis in chronic lymphocytic leukemia. Am J Case Rep. 2020;21:e924798. doi:10.12659/AJCR.924798
12. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417-1418. doi:10.1016/S0140-6736(20)30937-5
13. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033-2040. doi:10.1182/blood.2020006000
14. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi:10.1016/S0140-6736(20)30211-7
15. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421-1424. doi:10.1111/jth.14830
16. Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
1. Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1(1):116-120. doi:10.1111/j.1743-6109.2004.10117.x
2. Lee SG, Fralick M, Sholzberg M. Coagulopathy associated with COVID-19. CMAJ. 2020;192(21):E583. doi:10.1503/cmaj.200685
3. Lam G, McCarthy R, Haider R. A peculiar case of priapism: the hypercoagulable state in patients with severe COVID-19 infection. Eur J Case Rep Intern Med. 2020;7(8):001779. doi:10.12890/2020_001779
4. Addar A, Al Fraidi O, Nazer A, Althonayan N, Ghazwani Y. Priapism for 10 days in a patient with SARS-CoV-2 pneumonia: a case report. J Surg Case Rep. 2021;2021(4):rjab020. doi:10.1093/jscr/rjab020
5. Lamamri M, Chebbi A, Mamane J, et al. Priapism in a patient with coronavirus disease 2019 (COVID-19). Am J Emerg Med. 2021;39:251.e5-251.e7. doi:10.1016/j.ajem.2020.06.027
6. Silverman ML, VanDerVeer SJ, Donnelly TJ. Priapism in COVID-19: a thromboembolic complication. Am J Emerg Med. 2021;45:686.e5-686.e6. doi:10.1016/j.ajem.2020.12.072
7. Giuliano AFM, Vulpi M, Passerini F, et al. SARS-CoV-2 infection as a determining factor to the precipitation of ischemic priapism in a young patient with asymptomatic COVID-19. Case Rep Urol. 2021;2021:9936891. doi:10.1155/2021/9936891
8. Carreno BD, Perez CP, Vasquez D, Oyola JA, Suarez O, Bedoya C. Veno-occlusive priapism in COVID-19 disease. Urol Int. 2021;105(9-10):916-919. doi:10.1159/000514421
9. Senthilkumaran S, Shah S, Ganapathysubramanian, Balamurgan N, Thirumalaikolundusubramanian P. Propofol and priapism. Indian J Pharmacol. 2010;42(4):238-239. doi:10.4103/0253-7613.68430
10. Qu M, Lu X, Wang L, Liu Z, Sun Y, Gao X. Priapism secondary to chronic myeloid leukemia treated by a surgical cavernosa-corpus spongiosum shunt: case report. Asian J Urol. 2019;6(4):373-376. doi:10.1016/j.ajur.2018.12.004
11. Singh N, Singh Lubana S, Dabrowski L, Sidhu G. Leukostasis in chronic lymphocytic leukemia. Am J Case Rep. 2020;21:e924798. doi:10.12659/AJCR.924798
12. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417-1418. doi:10.1016/S0140-6736(20)30937-5
13. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033-2040. doi:10.1182/blood.2020006000
14. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi:10.1016/S0140-6736(20)30211-7
15. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421-1424. doi:10.1111/jth.14830
16. Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013