User login
Leukocytoclastic Vasculitis Masquerading as Chronic ITP
Idiopathic thrombocytopenic purpura (ITP) is an immune-mediated acquired condition affecting both adults and children.1 Acute ITP is the most common form, which happens in the presence of a precipitant, leading to a drop in platelet counts. However, chronic ITP can occur when all the causes that might precipitate thrombocytopenia have been ruled out, and it is persistent for ≥ 12 months.2 Its presence can mask other diseases that exhibit somewhat similar signs and symptoms. We present a case of a patient presenting with chronic ITP with diffuse rash and was later diagnosed with idiopathic leukocytoclastic vasculitis (LCV).
Case Presentation
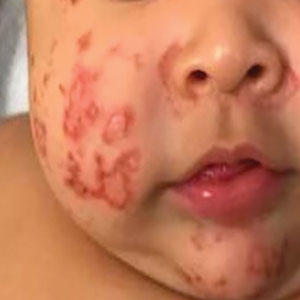
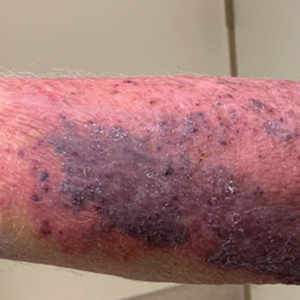
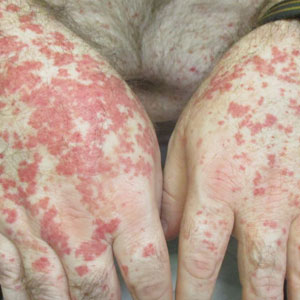
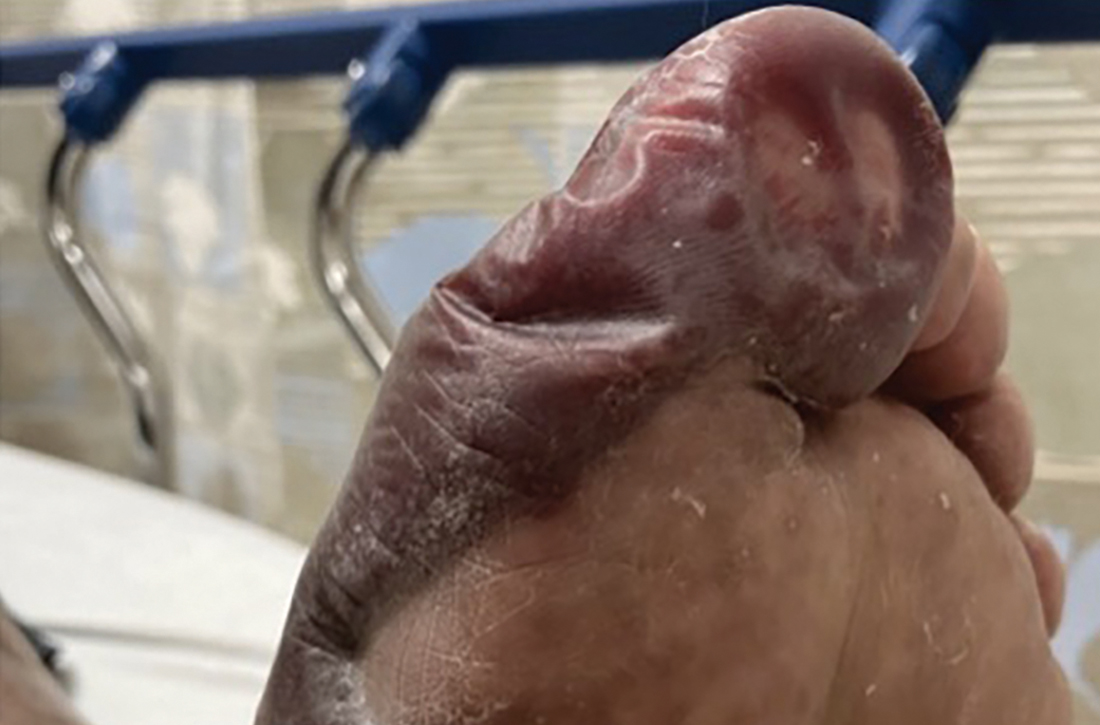
A 79-year-old presented to the hospital with 2-day history of a rash. The rash was purpureal and petechial and located on the trunk and bilateral upper and lower extremities. The rash was associated with itchiness and pain in the wrists, ankles, and small joints of the hands. The patient reported no changes in medication or diet,
The patient mentioned that at the time of diagnosis the platelet count was about 90,000 but had been fluctuating between 50 and 60,000 recently. The patient also reported no history of gum bleeding, nosebleeds, hemoptysis, hematemesis, or any miscarriages. She also had difficulty voiding for 2 to 3 days but no dysuria, frequency, urgency, or incontinence.
Laboratory results were significant for 57,000/µL platelet count (normal range, 150,000-450,000), elevated d-dimer (6.07), < 6 mg/dL C4 (normal range, 88-201). Hemoglobin level, coagulation panel, hemolytic panel, and fibrinogen level results were unremarkable. The hepatitis panel, Lyme disease, and HIV test were negative. The peripheral blood smear showed moderate thrombocytopenia, mild monocytosis, and borderline normochromic normocytic anemia without schistocytes. The autoimmune panel to evaluate thrombocytopenia showed platelet antibody against glycoprotein (GP) IIb/IIIa, GP Ib/Ix, GP Ia/IIa, suggestive toward a diagnosis of chronic idiopathic ITP. However, the skin biopsy of the rash was indicative of LCV.
An autoimmune panel for vasculitis, including antinuclear antibody and antidouble-stranded DNA, was negative. While in the hospital, the patient completed the course of ciprofloxacin for the UTI, the rash started to fade without any intervention, and the platelet count improved to 69,000/µL. The patient was discharged after 3 days with the recommendation to follow up with her hematologist.
Discussion
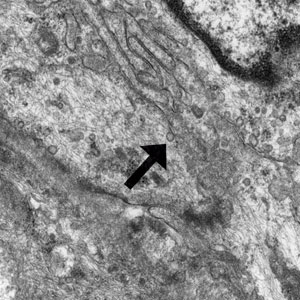
LCV is a small vessel vasculitis of the dermal capillaries and venules. Histologically, LCV is characterized by fibrinoid necrosis of the vessel wall with frequent neutrophils, nuclear dust, and extravasated erythrocytes.3
Although a thorough evaluation is recommended to determine etiology, about 50% of cases are idiopathic. The most common precipitants are acute infection or a new medication. Postinfectious LCV is most commonly seen after streptococcal upper respiratory tract infection. Among other infectious triggers, Mycobacterium, Staphylococcus aureus, chlamydia, Neisseria, HIV, hepatitis B, hepatitis C, and syphilis are noteworthy. Foods, autoimmune disease, collagen vascular disease, and malignancy are also associated with LCV.4
In our patient we could not find any specific identifiable triggers. However, the presence of a UTI as a precipitating factor cannot be ruled out.5 Moreover, the patient received ciprofloxacin and there have been several case reports of LCV associated with use of a fluroquinolone.6 Nevertheless, in the presence of chronic ITP, which also is an auto-immune condition, an idiopathic cause seemed a reasonable explanation for the patient’s etiopathogenesis.
The cutaneous manifestations of LCV may appear about 1 to 3 weeks after the triggering event if present. The major clinical findings include palpable purpura and/or petechiae that are nonblanching. These findings can easily be confused with other diagnoses especially in the presence of a similar preexisting diagnosis. For example, our patient already had chronic ITP, and in such circumstances, a diagnosis of superimposed LCV can be easily missed without a thorough investigation. Extracutaneous manifestations with LCV are less common. Systemic symptoms may include low-grade fevers, malaise, weight loss, myalgia, and arthralgia. These findings have been noted in about 30% of affected patients, with arthralgia the most common manifestation.7 Our patient also presented with pain involving multiple joints.
The mainstay of diagnosis for LCV is a skin biopsy with direct immunofluorescence. However, a workup for an underlying condition should be considered based on clinical suspicion. If a secondary cause is found, management should target treating the underlying cause, including withdrawal of the offending drug, treatment or control of the underlying infection, malignancy, or connective tissue disease. Most cases of idiopathic cutaneous LCV resolve with supportive measures, including leg elevation, rest, compression stockings, and antihistamines. In resistant cases, a 4- to 6-week tapering dose of corticosteroids and immunosuppressive steroid-sparing agents may be needed.8
Conclusions
Although most cases of LCV are mild and resolve without intervention, many cases go undiagnosed due to a delay in performing a biopsy. However, we should always look for the root cause of a patient’s condition to rule out underlying contributing conditions. Differentiating LCV from any other preexisting condition presenting similarly is important.
1. Gaurav K, Keith RM. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013;27(3): 495-520. doi:10.1016/j.hoc.2013.03.001
2. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393.
3. James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. Saunders/Elsevier; 2011.
4. Einhorn J, Levis JT. Dermatologic diagnosis: leukocytoclastic vasculitis. Perm J. 2015;19(3):77-78. doi:10.7812/TPP/15-001
5. The role of infectious agents in the pathogenesis of vasculitis. Nicolò P, Carlo S. Best Pract Res Clin Rheumatol. 2008;22(5):897-911. doi:10.7812/TPP/15-001
6. Maunz G, Conzett T, Zimmerli W. Cutaneous vasculitis associated with fluoroquinolones. Infection. 2009;37(5):466-468. doi:10.1007/s15010-009-8437-4
7. Baigrie D, Goyal A, Crane J.C. Leukocytoclastic vasculitis. StatPearls [internet]. Updated May 8, 2022. Accessed October 10, 2022. https://www.ncbi.nlm.nih.gov/books/NBK482159
8. Micheletti RG, Pagnoux C. Management of cutaneous vasculitis. Presse Med. 2020; 49(3):104033. doi:10.1016/j.lpm.2020.104033
Idiopathic thrombocytopenic purpura (ITP) is an immune-mediated acquired condition affecting both adults and children.1 Acute ITP is the most common form, which happens in the presence of a precipitant, leading to a drop in platelet counts. However, chronic ITP can occur when all the causes that might precipitate thrombocytopenia have been ruled out, and it is persistent for ≥ 12 months.2 Its presence can mask other diseases that exhibit somewhat similar signs and symptoms. We present a case of a patient presenting with chronic ITP with diffuse rash and was later diagnosed with idiopathic leukocytoclastic vasculitis (LCV).
Case Presentation
A 79-year-old presented to the hospital with 2-day history of a rash. The rash was purpureal and petechial and located on the trunk and bilateral upper and lower extremities. The rash was associated with itchiness and pain in the wrists, ankles, and small joints of the hands. The patient reported no changes in medication or diet,
The patient mentioned that at the time of diagnosis the platelet count was about 90,000 but had been fluctuating between 50 and 60,000 recently. The patient also reported no history of gum bleeding, nosebleeds, hemoptysis, hematemesis, or any miscarriages. She also had difficulty voiding for 2 to 3 days but no dysuria, frequency, urgency, or incontinence.
Laboratory results were significant for 57,000/µL platelet count (normal range, 150,000-450,000), elevated d-dimer (6.07), < 6 mg/dL C4 (normal range, 88-201). Hemoglobin level, coagulation panel, hemolytic panel, and fibrinogen level results were unremarkable. The hepatitis panel, Lyme disease, and HIV test were negative. The peripheral blood smear showed moderate thrombocytopenia, mild monocytosis, and borderline normochromic normocytic anemia without schistocytes. The autoimmune panel to evaluate thrombocytopenia showed platelet antibody against glycoprotein (GP) IIb/IIIa, GP Ib/Ix, GP Ia/IIa, suggestive toward a diagnosis of chronic idiopathic ITP. However, the skin biopsy of the rash was indicative of LCV.
An autoimmune panel for vasculitis, including antinuclear antibody and antidouble-stranded DNA, was negative. While in the hospital, the patient completed the course of ciprofloxacin for the UTI, the rash started to fade without any intervention, and the platelet count improved to 69,000/µL. The patient was discharged after 3 days with the recommendation to follow up with her hematologist.
Discussion
LCV is a small vessel vasculitis of the dermal capillaries and venules. Histologically, LCV is characterized by fibrinoid necrosis of the vessel wall with frequent neutrophils, nuclear dust, and extravasated erythrocytes.3
Although a thorough evaluation is recommended to determine etiology, about 50% of cases are idiopathic. The most common precipitants are acute infection or a new medication. Postinfectious LCV is most commonly seen after streptococcal upper respiratory tract infection. Among other infectious triggers, Mycobacterium, Staphylococcus aureus, chlamydia, Neisseria, HIV, hepatitis B, hepatitis C, and syphilis are noteworthy. Foods, autoimmune disease, collagen vascular disease, and malignancy are also associated with LCV.4
In our patient we could not find any specific identifiable triggers. However, the presence of a UTI as a precipitating factor cannot be ruled out.5 Moreover, the patient received ciprofloxacin and there have been several case reports of LCV associated with use of a fluroquinolone.6 Nevertheless, in the presence of chronic ITP, which also is an auto-immune condition, an idiopathic cause seemed a reasonable explanation for the patient’s etiopathogenesis.
The cutaneous manifestations of LCV may appear about 1 to 3 weeks after the triggering event if present. The major clinical findings include palpable purpura and/or petechiae that are nonblanching. These findings can easily be confused with other diagnoses especially in the presence of a similar preexisting diagnosis. For example, our patient already had chronic ITP, and in such circumstances, a diagnosis of superimposed LCV can be easily missed without a thorough investigation. Extracutaneous manifestations with LCV are less common. Systemic symptoms may include low-grade fevers, malaise, weight loss, myalgia, and arthralgia. These findings have been noted in about 30% of affected patients, with arthralgia the most common manifestation.7 Our patient also presented with pain involving multiple joints.
The mainstay of diagnosis for LCV is a skin biopsy with direct immunofluorescence. However, a workup for an underlying condition should be considered based on clinical suspicion. If a secondary cause is found, management should target treating the underlying cause, including withdrawal of the offending drug, treatment or control of the underlying infection, malignancy, or connective tissue disease. Most cases of idiopathic cutaneous LCV resolve with supportive measures, including leg elevation, rest, compression stockings, and antihistamines. In resistant cases, a 4- to 6-week tapering dose of corticosteroids and immunosuppressive steroid-sparing agents may be needed.8
Conclusions
Although most cases of LCV are mild and resolve without intervention, many cases go undiagnosed due to a delay in performing a biopsy. However, we should always look for the root cause of a patient’s condition to rule out underlying contributing conditions. Differentiating LCV from any other preexisting condition presenting similarly is important.
Idiopathic thrombocytopenic purpura (ITP) is an immune-mediated acquired condition affecting both adults and children.1 Acute ITP is the most common form, which happens in the presence of a precipitant, leading to a drop in platelet counts. However, chronic ITP can occur when all the causes that might precipitate thrombocytopenia have been ruled out, and it is persistent for ≥ 12 months.2 Its presence can mask other diseases that exhibit somewhat similar signs and symptoms. We present a case of a patient presenting with chronic ITP with diffuse rash and was later diagnosed with idiopathic leukocytoclastic vasculitis (LCV).
Case Presentation
A 79-year-old presented to the hospital with 2-day history of a rash. The rash was purpureal and petechial and located on the trunk and bilateral upper and lower extremities. The rash was associated with itchiness and pain in the wrists, ankles, and small joints of the hands. The patient reported no changes in medication or diet,
The patient mentioned that at the time of diagnosis the platelet count was about 90,000 but had been fluctuating between 50 and 60,000 recently. The patient also reported no history of gum bleeding, nosebleeds, hemoptysis, hematemesis, or any miscarriages. She also had difficulty voiding for 2 to 3 days but no dysuria, frequency, urgency, or incontinence.
Laboratory results were significant for 57,000/µL platelet count (normal range, 150,000-450,000), elevated d-dimer (6.07), < 6 mg/dL C4 (normal range, 88-201). Hemoglobin level, coagulation panel, hemolytic panel, and fibrinogen level results were unremarkable. The hepatitis panel, Lyme disease, and HIV test were negative. The peripheral blood smear showed moderate thrombocytopenia, mild monocytosis, and borderline normochromic normocytic anemia without schistocytes. The autoimmune panel to evaluate thrombocytopenia showed platelet antibody against glycoprotein (GP) IIb/IIIa, GP Ib/Ix, GP Ia/IIa, suggestive toward a diagnosis of chronic idiopathic ITP. However, the skin biopsy of the rash was indicative of LCV.
An autoimmune panel for vasculitis, including antinuclear antibody and antidouble-stranded DNA, was negative. While in the hospital, the patient completed the course of ciprofloxacin for the UTI, the rash started to fade without any intervention, and the platelet count improved to 69,000/µL. The patient was discharged after 3 days with the recommendation to follow up with her hematologist.
Discussion
LCV is a small vessel vasculitis of the dermal capillaries and venules. Histologically, LCV is characterized by fibrinoid necrosis of the vessel wall with frequent neutrophils, nuclear dust, and extravasated erythrocytes.3
Although a thorough evaluation is recommended to determine etiology, about 50% of cases are idiopathic. The most common precipitants are acute infection or a new medication. Postinfectious LCV is most commonly seen after streptococcal upper respiratory tract infection. Among other infectious triggers, Mycobacterium, Staphylococcus aureus, chlamydia, Neisseria, HIV, hepatitis B, hepatitis C, and syphilis are noteworthy. Foods, autoimmune disease, collagen vascular disease, and malignancy are also associated with LCV.4
In our patient we could not find any specific identifiable triggers. However, the presence of a UTI as a precipitating factor cannot be ruled out.5 Moreover, the patient received ciprofloxacin and there have been several case reports of LCV associated with use of a fluroquinolone.6 Nevertheless, in the presence of chronic ITP, which also is an auto-immune condition, an idiopathic cause seemed a reasonable explanation for the patient’s etiopathogenesis.
The cutaneous manifestations of LCV may appear about 1 to 3 weeks after the triggering event if present. The major clinical findings include palpable purpura and/or petechiae that are nonblanching. These findings can easily be confused with other diagnoses especially in the presence of a similar preexisting diagnosis. For example, our patient already had chronic ITP, and in such circumstances, a diagnosis of superimposed LCV can be easily missed without a thorough investigation. Extracutaneous manifestations with LCV are less common. Systemic symptoms may include low-grade fevers, malaise, weight loss, myalgia, and arthralgia. These findings have been noted in about 30% of affected patients, with arthralgia the most common manifestation.7 Our patient also presented with pain involving multiple joints.
The mainstay of diagnosis for LCV is a skin biopsy with direct immunofluorescence. However, a workup for an underlying condition should be considered based on clinical suspicion. If a secondary cause is found, management should target treating the underlying cause, including withdrawal of the offending drug, treatment or control of the underlying infection, malignancy, or connective tissue disease. Most cases of idiopathic cutaneous LCV resolve with supportive measures, including leg elevation, rest, compression stockings, and antihistamines. In resistant cases, a 4- to 6-week tapering dose of corticosteroids and immunosuppressive steroid-sparing agents may be needed.8
Conclusions
Although most cases of LCV are mild and resolve without intervention, many cases go undiagnosed due to a delay in performing a biopsy. However, we should always look for the root cause of a patient’s condition to rule out underlying contributing conditions. Differentiating LCV from any other preexisting condition presenting similarly is important.
1. Gaurav K, Keith RM. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013;27(3): 495-520. doi:10.1016/j.hoc.2013.03.001
2. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393.
3. James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. Saunders/Elsevier; 2011.
4. Einhorn J, Levis JT. Dermatologic diagnosis: leukocytoclastic vasculitis. Perm J. 2015;19(3):77-78. doi:10.7812/TPP/15-001
5. The role of infectious agents in the pathogenesis of vasculitis. Nicolò P, Carlo S. Best Pract Res Clin Rheumatol. 2008;22(5):897-911. doi:10.7812/TPP/15-001
6. Maunz G, Conzett T, Zimmerli W. Cutaneous vasculitis associated with fluoroquinolones. Infection. 2009;37(5):466-468. doi:10.1007/s15010-009-8437-4
7. Baigrie D, Goyal A, Crane J.C. Leukocytoclastic vasculitis. StatPearls [internet]. Updated May 8, 2022. Accessed October 10, 2022. https://www.ncbi.nlm.nih.gov/books/NBK482159
8. Micheletti RG, Pagnoux C. Management of cutaneous vasculitis. Presse Med. 2020; 49(3):104033. doi:10.1016/j.lpm.2020.104033
1. Gaurav K, Keith RM. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013;27(3): 495-520. doi:10.1016/j.hoc.2013.03.001
2. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393.
3. James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. Saunders/Elsevier; 2011.
4. Einhorn J, Levis JT. Dermatologic diagnosis: leukocytoclastic vasculitis. Perm J. 2015;19(3):77-78. doi:10.7812/TPP/15-001
5. The role of infectious agents in the pathogenesis of vasculitis. Nicolò P, Carlo S. Best Pract Res Clin Rheumatol. 2008;22(5):897-911. doi:10.7812/TPP/15-001
6. Maunz G, Conzett T, Zimmerli W. Cutaneous vasculitis associated with fluoroquinolones. Infection. 2009;37(5):466-468. doi:10.1007/s15010-009-8437-4
7. Baigrie D, Goyal A, Crane J.C. Leukocytoclastic vasculitis. StatPearls [internet]. Updated May 8, 2022. Accessed October 10, 2022. https://www.ncbi.nlm.nih.gov/books/NBK482159
8. Micheletti RG, Pagnoux C. Management of cutaneous vasculitis. Presse Med. 2020; 49(3):104033. doi:10.1016/j.lpm.2020.104033
Challenges and Considerations in Treating Negative and Cognitive Symptoms of Schizophrenia Spectrum Disorders
Schizophrenia spectrum disorders (SSDs) represent some of the most debilitating mental health disorders.1 While these disorders have myriad presentations, the prototypical patient with SSD is often thought to possess positive symptoms. More recently, clinicians and researchers are raising awareness of another presentation of SSD: predominantly negative and cognitive symptoms. This symptom profile is not a novel phenomenon; for many years this presentation was recognized as a “deficit” presentation, referring to negative symptoms as the prominent feature.2,3 However, it presents unique diagnostic and treatment considerations that are often underappreciated in clinical settings.
Negative symptoms (blunted/flat affect, avolition, alogia, anhedonia, asociality) have long been identified as key features of SSD and are widely recognized as predictive of poor prognostic outcomes for patients with SSDs.1 In many patients, negative symptoms may precede the development of positive symptoms and emerge as a more robust predictor of functional outcomes than positive symptoms.1 Negative symptoms also appear to be inextricably linked to cognitive symptoms. Specifically, patients with primary negative symptoms seem to perform poorly on measures of global cognitive functioning.1 Similar to negative symptoms, cognitive symptoms of SSDs are a primary source of functional impairment and persistent disability.1 Despite this, little attention is given in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) to the neurocognitive and social cognitive deficits seen in patients with SSDs. Previous research highlights broad deficits in a range of neurocognitive abilities, including attention, working memory, processing speed, executive functioning, learning and memory, and receptive and expressive language.4 Similarly, patients also display deficits in domains of social cognition, such as emotion processing, identifying and utilizing social cues, evaluating attributions of others, and perspective-taking.5
A predominantly negative and cognitive symptom presentation can present diagnostic and treatment challenges. We present a case of a patient with such a presentation and the unique considerations given to diagnostic clarification and her treatment.
Case Presentation
A 33-year-old female veteran presented to the emergency department (ED) at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, in 2020. She was brought to the ED by local police following an attempted assault of her neighbor. Per collateral information from the police, the veteran stated she “had the urge to hurt someone” but was unable to provide any other information about this event. The veteran demonstrated diminished speech output, providing 2- to 3-word responses before refusing to speak entirely. She also presented with markedly blunted affect and tangential speech. She was not oriented to situation, stating confusion as to how she was brought to the hospital, and appeared to be responding to internal stimuli. She was subsequently admitted to the inpatient mental health unit due to unspecified psychosis.
The veteran presented as an unreliable historian, and much of her medical history was obtained via a review of US Department of Defense (DoD) records and collateral interview with her parents. Before her hospitalization, the veteran had been diagnosed with major depressive disorder (MDD) and adjustment disorder while serving in the Navy. Her psychiatric history before her military career was otherwise unremarkable. At that time, she began a trial of sertraline 50 mg and completed 10 sessions of psychotherapy. After approximately 1 year, she elected to stop taking sertraline due to improved mental health. However, shortly after this she began experiencing significant depressive symptoms and was ultimately released early from the Navy due to her mental health concerns.
The veteran’s parents provided interim history between her discharge and establishing care at MEDVAMC as the veteran was reluctant to discuss this period of her life. According to her parents the veteran had prior diagnoses of borderline personality disorder and MDD and had difficulty adhering to her current medications (bupropion and duloxetine) for about 1 month before her hospitalization. During the previous month, her parents observed her staying in her room around the clock and “[going] mute.”
The veteran remained hospitalized for about 1 month, during which she was diagnosed with schizoaffective disorder and stabilized on injections of long-acting olanzapine 210 mg (administered every 2 weeks). She was referred for outpatient psychotherapy in a specialty clinic for veterans with SSDs. However, she did not attend her initial intake assessment.
About 2 weeks after discharge from the hospital, the veteran presented for her injection appointment. At this time, she was noted to be disorganized in her thinking and behavior, displaying thought blocking and catatonic behavior. Her parents also described concerning behavior since her discharge. They stated she went to a hotel after her discharge and spent all her available money. She then returned to her parents’ home, where she did not sleep or bathe for several days. She was observed wandering around the house aimlessly and in a confused manner and had become verbally aggressive and threatening toward her parents. The veteran was again psychiatrically admitted due to psychosis and concerns for her safety. She was discharged about 2 weeks later and continued olanzapine injections. She was also referred for outpatient psychotherapy; although she did not initially engage in psychotherapy, she was referred again about 5 months after discharge and began psychotherapy at that time.
The veteran began a course of weekly outpatient psychotherapy employing cognitive behavior therapy for psychosis (CBTp).6 During this time, she described her primary concerns as anxiety and feeling disconnected from others. She reported a history of depression but not of schizoaffective disorder. When asked about this, the veteran stated that she did not feel this diagnosis was accurate and instead believed she had severe depression. When asked why she was prescribed olanzapine, the veteran stated that this medication was for depression. As with her inpatient stays, the veteran demonstrated several negative symptoms during her course of psychotherapy. She presented with noticeably blunted affect, evidenced by lack of facial expression and monotonic speech. She also routinely displayed alogia (ie, lack of speech), often stating that she “did not feel like talking much.” She described difficulty finding motivation to initiate tasks (avolition) as well as a tendency toward social isolation (asociality).
The veteran also described concerns related to neurocognitive and social cognitive symptoms. She reported difficulties in processing speed, cognitive set-shifting (mentally switching between tasks), and inhibition, describing how these concerns interfered with her occupational functioning. She noted difficulty maintaining the expected pace of work at her previous positions, stating that she felt it took her longer to complete tasks compared with others. In addition, she displayed some difficulties with attention and memory. On more than one occasion, she seemed to have forgotten the previous day’s conversations with clinicians. Regarding social cognitive symptoms, she noted difficulties in emotion processing, indicating that it was difficult for her to identify and manage her emotions. This was especially prominent during times of depressed mood.
She also displayed a hostile attribution bias, or tendency to overattribute hostile intent to others’ ambiguous actions. For example, she described an instance where a family member sat too close to her on the couch, stating that she felt this behavior indicated the family member did not care about her. Relatedly, the veteran demonstrated difficulty with perspective taking, which became evident during cognitive restructuring regarding interpretations of her family’s behavior. Finally, the veteran displayed some deficits in social perception, or the ability to identify social context and rules based on nonverbal communication, verbal cues, and vocal intonation. She stated that she often felt conversing with others was difficult for her and indicated that she was “not good at conversations.” This may have in part been due to deficits in social perception.
During the first 2 months of psychotherapy, the veteran regularly attended sessions (conducted over telephone due to the COVID-19 pandemic) and was adherent to twice-weekly olanzapine injections. Despite this, she began experiencing an increase in depressive symptoms accompanied by a noticeable worsening of her blunted affect, alogia, and avolition. After about 2 months of psychotherapy, she described active suicidal ideation and requested to be voluntarily hospitalized. During this hospitalization, the veteran was consulted about the use of clozapine in treatment-refractory conditions and began a trial of clozapine 400 mg. She demonstrated marked improvement in her depressed mood after taking the medication and was discharged about 2 weeks after admission. The veteran completed 10 sessions of CBTp before electing to terminate due to an upcoming move. She was adherent to weekly blood draws per the requirements of clozapine and described intentions to engage in mental health care after her move. The patient’s mother contacted the clinic to inform the treatment team that the patient and her family had moved to a different city and the patient had started receiving care at the VAMC in that city.
Discussion
As the veteran’s case highlights, a predominantly negative and cognitive symptom presentation may present diagnostic challenges. Since this presentation may not be viewed as representative of SSDs, patients with this presentation may be misdiagnosed. This was evident in the current case, not only in the veteran’s prodromal phase of illness while in the Navy, but also in her reported previous diagnoses of borderline personality disorder and MDD. More than one clinician at the MEDVAMC provisionally considered a diagnosis of MDD before collecting collateral information from the veteran’s family regarding her clear psychotic symptoms. Unfortunately, such misdiagnoses may have prevented early intervention of the veteran’s schizoaffective disorder, which is found to be instrumental in reducing impairment and disability among patients with SSDs.7,8
These misdiagnoses are understandable given the considerable symptom overlap between SSDs and other mental health disorders. For instance, anhedonia and avolition are 2 key symptoms seen in depressive episodes. Both anhedonia and lack of positive emotion are often seen in posttraumatic stress disorder. Additionally, anxiety disorders may induce a lack of positive emotion, loss of interest in previously enjoyed activities, and lack of motivation secondary to primary symptoms of anxiety. Furthermore, schizoaffective disorder requires the presence of a major mood episode. In the absence of apparent positive symptoms (as is the case for patients with a predominantly negative symptom presentation), schizoaffective disorder may be easily misdiagnosed as a mood disorder.
Patients with predominantly negative or cognitive symptoms may also be less accepting of a diagnosis of SSD. A wealth of research points to the clear stigma of SSDs, with many suggesting that these disorders are among the most stigmatized mental health disorders.9 Therefore, patients with predominantly negative and cognitive symptoms may be more likely to attribute their symptoms to another, less stigmatized mental health disorder. This was seen in the current case, as the veteran repeatedly denied a diagnosis of schizoaffective disorder and instead claimed to have severe depression. This reluctance to accept a diagnosis of an SSD, coupled with the diagnostic ambiguity of negative symptoms, is likely to make it challenging for clinicians to accurately identify patients with a predominantly negative and cognitive symptom presentation of SSDs.
Clinicians working within a team-based setting may be less likely to misdiagnose patients as they can consult others. Diagnostic clarity in the current case was undoubtedly facilitated by the multidisciplinary team involved in the veteran’s care; clinicians involved in her care were able to consult with one another to determine that her symptoms were indicative of an SSD rather than a mood disorder. Mental health professionals in private practice are unlikely to have access to such multidisciplinary specialty services and may be particularly vulnerable to misdiagnoses.
Treatment Considerations
This case also highlights several psychotherapy and psychopharmacology treatment considerations for patients with a predominantly negative and cognitive symptom presentation. The veteran was initially difficult to engage in psychotherapy. Although patients with SSDs often have difficulty engaging in treatment, patients with a predominant negative and cognitive symptom profile may experience more difficulty doing so.10 Previous research suggests that both negative symptoms and cognitive symptoms are inversely related to treatment engagement.11,12
By their very nature, negative symptoms may make it difficult to fully engage in psychotherapy. First, avolition and amotivation likely make it difficult for patients to attend psychotherapy appointments. Furthermore, negative symptoms may make it difficult to emotionally engage with the content of psychotherapy, thus limiting the potential benefits. Cognitive symptoms may also make it more difficult for patients to fully reap the benefits of psychotherapy. Deficits in attention, memory, and abstract reasoning seen in other mental health and medical conditions are associated with poorer treatment outcomes in psychotherapy.13,14 Thus, it may be especially difficult to engage patients with primarily negative and cognitive symptoms of SSDs in psychotherapy. However, given the link between these symptoms and functional impairment, it is even more important to evaluate and address such barriers to treatment.
This case highlights the utility of clozapine in the treatment of SSDs. Many commonly prescribed antipsychotic medications have questionable efficacy in treating negative symptoms, and none of the currently available antipsychotics are approved for this indication.15 In our case, the veteran saw a limited reduction of her negative or cognitive symptoms from her use of olanzapine. However, case reports, naturalistic follow-up, and open-label studies suggest that clozapine may be efficacious in targeting negative symptoms of SSDs.16-19 Previous research also suggests clozapine is more effective than other antipsychotic medications, including olanzapine, quetiapine, and risperidone, in decreasing overall SSD symptoms.20,21 Additionally, there is initial evidence of the efficacy of clozapine in treating cognitive symptoms, suggesting that some areas of cognition may improve in response to this medication.22-24 On the other hand, a recent case study suggests high doses of clozapine may be associated with cognitive impairment, although cognitive impairment was still greater without medication than at this higher dose.25 Thus, further research is needed to refine our understanding of the impact of clozapine on cognitive symptoms in SSDs.
Despite the promising research behind clozapine, it remains widely underprescribed, likely due to concerns regarding the potential adverse effects.26,27 Clozapine has been associated with many adverse effects, the most concerning being neutropenia, which can lead to serious infection and death. Thus, one concern among clinicians may be the potential lethality of clozapine. However, a wealth of research indicates clozapine can be safely administered under medical supervision.26,28 In fact, clozapine has been linked to lower all-cause mortality rates and lower mortality rates by suicide compared with other antipsychotic medications.29-31 It may therefore be argued that clozapine lowers the overall risk of mortality. Prescribers may also be weary of adherence to regular blood tests that patients must undergo to monitor their risk for neutropenia. This is the most frequently cited anticipated barrier to beginning a trial of clozapine.27 These concerns may not be unfounded; indeed, if avolition and amotivation make it difficult to attend psychotherapy sessions, these factors may logically make it difficult to attend blood draw appointments. In response to such barriers, several solutions have been suggested regarding potential blood draw nonadherence, including the use of in-home treatment teams and point-of-care monitoring.32,33
Conclusions
Predominant negative and cognitive symptom presentations of SSDs require unique considerations to accurately identify and provide optimal treatment for patients with such presentations. As our case highlights, patients with such presentations may often be misdiagnosed, as negative and cognitive symptoms may be attributed to other disorders. Additionally, patients with this presentation may experience difficulty engaging in psychotherapy and may not see the same benefits from common antipsychotic medications as patients with predominantly positive symptoms. Clozapine emerges as a promising treatment for addressing negative and cognitive symptoms, although it remains widely underutilized. In cases where clinicians encounter patients with predominantly negative and cognitive symptoms, we strongly recommend consultation and referral to psychiatric care for medication management.
The current case highlights the need for individually tailored treatment plans for individuals seeking mental health care. Clinicians of patients with any mental disorder, but especially those with SSDs of predominantly negative and cognitive symptoms, should carefully formulate a treatment plan based on relevant case history, presentation, and current empirical literature. A singular, one-size-fits-all approach should not be universally implemented for such patients. Our case demonstrates how careful multidisciplinary evaluations, review of medical records, collateral information from patients’ family members, and other diagnostic and treatment considerations in patients with predominant negative and cognitive symptoms of SSDs can refine and enhance the clinical care offered to such patients.
Acknowledgments
A.K. is supported by the US Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Central Texas Veterans Affairs Health Care System, and the VISN 17 Center of Excellence for Research on Returning War Veterans.
1. Kantrowitz JT. Managing negative symptoms of schizophrenia: how far have we come? CNS Drugs. 2017;31(5):373-388. doi:10.1007/s40263-017-0428-x
2. Fenton WS, McGlashan TH. Antecedents, symptom progression, and long-term outcome of the deficit syndrome in schizophrenia. Am J Psychiatry. 1994;151(3):351-356. doi:10.1176/ajp.151.3.351
3. Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT. A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58(2):165. doi:10.1001/archpsyc.58.2.165
4. Kalkstein S, Hurford I, Gur RC. Neurocognition in schizophrenia. Curr Top Behav Neurosci. 2010;4:373-390. doi:10.1007/7854_2010_42
5. Green MF, Horan WP. Social cognition in schizophrenia. Curr Dir Psychol Sci. 2010;19(4):243-248. doi:10.1177/0963721410377600
6. Kingdon DG, Turkington D. Cognitive Therapy of Schizophrenia. Guilford Press; 2008.
7. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555. doi:10.1001/jamapsychiatry.2018.0623
8. McGorry PD. Early intervention in psychosis: obvious, effective, overdue. J Nerv Ment Dis. 2015;203(5):310-318. doi:10.1097/NMD.0000000000000284
9. Crisp AH, Gelder MG, Rix S, Meltzer HI, Rowlands OJ. Stigmatisation of people with mental illnesses. Br J Psychiatry. 2000;177(1):4-7. doi:10.1192/bjp.177.1.4
10. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15(1):13-20. doi:10.1002/wps.20306
11. Kukla M, Davis LW, Lysaker PH. Cognitive behavioral therapy and work outcomes: correlates of treatment engagement and full and partial success in schizophrenia. Behav Cogn Psychother. 2014;42(5):577-592. doi:10.1017/S1352465813000428
12. Johansen R, Hestad K, Iversen VC, et al. Cognitive and clinical factors are associated with service engagement in early-phase schizophrenia spectrum disorders. J Nerv Ment Dis. 2011;199(3):176-182. doi:10.1097/NMD.0b013e31820bc2f9
13. Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81(3):313-322. doi:10.1016/j.drugalcdep.2005.08.003
14. Aarsland D, Taylor JP, Weintraub D. Psychiatric issues in cognitive impairment. Mov Disord. 2014;29(5):651-662. doi:10.1002/mds.25873
15. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. doi:10.1016/S0140-6736(13)60733-3
16. Khan AH, Zaidi S. Clozapine: Improvement of Negative Symptoms of Schizophrenia. Cureus. 2017;9(12):e1973. Published 2017 Dec 20. doi:10.7759/cureus.1973
17. Brar JS, Chengappa KN, Parepally H, et al. The effects of clozapine on negative symptoms in patients with schizophrenia with minimal positive symptoms. Ann Clin Psychiatry. 1997;9(4):227-234. doi:10.1023/a:1022352326334
18. Llorca PM, Lancon C, Farisse J, Scotto JC. Clozapine and negative symptoms. An open study. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24(3):373-384. doi:10.1016/s0278-5846(99)00105-0
19. Siskind D, McCartney L, Goldschlager R, Kisely S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385-392. doi:10.1192/bjp.bp.115.177261
20. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610. doi:10.1176/appi.ajp.163.4.600
21. Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Comparative Effectiveness of Clozapine and Standard Antipsychotic Treatment in Adults With Schizophrenia. Am J Psychiatry. 2016;173(2):166-173. doi:10.1176/appi.ajp.2015.15030332
22. Lee MA, Thompson PA, Meltzer HY. Effects of clozapine in cognitive function in schizophrenia. J Clin Psychiatry. 1994;55(suppl B):82-87.
23. Sharma T, Hughes C, Soni W, Kumari V. Cognitive effects of olanzapine and clozapine treatment in chronic schizophrenia. Psychopharmacology (Berl). 2003;169(3-4):398-403. doi:10.1007/s00213-003-1506-y
24. Spagna A, Dong Y, Mackie MA, et al. Clozapine improves the orienting of attention in schizophrenia. Schizophr Res. 2015;168(1-2):285-291. doi:10.1016/j.schres.2015.08.009
25. Savulich G, Mezquida G, Atkinson S, Bernardo M, Fernandez-Egea E. A case study of clozapine and cognition: friend or foe? J Clin Psychopharmacol. 2018;38(2):152-153. doi:10.1097/JCP.0000000000000847
26. Bogers JPAM, Schulte PFJ, Van Dijk D, Bakker B, Cohen D. Clozapine underutilization in the treatment of schizophrenia: how can clozapine prescription rates be improved? J Clin Psychopharmacol. 2016;36(2):109-111. doi:10.1097/JCP.0000000000000478
27. Kelly DL, Freudenreich O, Sayer MA, Love RC. Addressing Barriers to Clozapine Underutilization: A National Effort. Psychiatr Serv. 2018;69(2):224-227. doi:10.1176/appi.ps.201700162
28. Honigfeld G, Arellano F, Sethi J, Bianchini A, Schein J. Reducing clozapine-related morbidity and mortality: 5 years of experience with the Clozaril National Registry. J Clin Psychiatry. 1998;59(suppl 3):3-7.
29. Cho J, Hayes RD, Jewell A, et al. Clozapine and all-cause mortality in treatment-resistant schizophrenia: a historical cohort study. Acta Psychiatr Scand. 2019;139(3):237-247. doi:10.1111/acps.12989
30. Kane JM. Clozapine Reduces All-Cause Mortality. Am J Psychiatry. 2017;174(10):920-921. doi:10.1176/appi.ajp.2017.17070770
31. Taipale H, Lähteenvuo M, Tanskanen A, Mittendorfer-Rutz E, Tiihonen J. Comparative Effectiveness of Antipsychotics for Risk of Attempted or Completed Suicide Among Persons With Schizophrenia. Schizophr Bull. 2021;47(1):23-30. doi:10.1093/schbul/sbaa111
32. Love RC, Kelly DL, Freudenreich O, Sayer MA. Clozapine underutilization: addressing the barriers. National Association of State Mental Health Program Directors; 2016. Accessed October 6, 2022. https://www.nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
33. Kelly DL, Ben-Yoav H, Payne GF, et al. Blood draw barriers for treatment with clozapine and development of a point-of-care monitoring device. Clin Schizophr Relat Psychoses. 2018;12(1):23-30. doi:10.3371/CSRP.KEBE.070415
Schizophrenia spectrum disorders (SSDs) represent some of the most debilitating mental health disorders.1 While these disorders have myriad presentations, the prototypical patient with SSD is often thought to possess positive symptoms. More recently, clinicians and researchers are raising awareness of another presentation of SSD: predominantly negative and cognitive symptoms. This symptom profile is not a novel phenomenon; for many years this presentation was recognized as a “deficit” presentation, referring to negative symptoms as the prominent feature.2,3 However, it presents unique diagnostic and treatment considerations that are often underappreciated in clinical settings.
Negative symptoms (blunted/flat affect, avolition, alogia, anhedonia, asociality) have long been identified as key features of SSD and are widely recognized as predictive of poor prognostic outcomes for patients with SSDs.1 In many patients, negative symptoms may precede the development of positive symptoms and emerge as a more robust predictor of functional outcomes than positive symptoms.1 Negative symptoms also appear to be inextricably linked to cognitive symptoms. Specifically, patients with primary negative symptoms seem to perform poorly on measures of global cognitive functioning.1 Similar to negative symptoms, cognitive symptoms of SSDs are a primary source of functional impairment and persistent disability.1 Despite this, little attention is given in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) to the neurocognitive and social cognitive deficits seen in patients with SSDs. Previous research highlights broad deficits in a range of neurocognitive abilities, including attention, working memory, processing speed, executive functioning, learning and memory, and receptive and expressive language.4 Similarly, patients also display deficits in domains of social cognition, such as emotion processing, identifying and utilizing social cues, evaluating attributions of others, and perspective-taking.5
A predominantly negative and cognitive symptom presentation can present diagnostic and treatment challenges. We present a case of a patient with such a presentation and the unique considerations given to diagnostic clarification and her treatment.
Case Presentation
A 33-year-old female veteran presented to the emergency department (ED) at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, in 2020. She was brought to the ED by local police following an attempted assault of her neighbor. Per collateral information from the police, the veteran stated she “had the urge to hurt someone” but was unable to provide any other information about this event. The veteran demonstrated diminished speech output, providing 2- to 3-word responses before refusing to speak entirely. She also presented with markedly blunted affect and tangential speech. She was not oriented to situation, stating confusion as to how she was brought to the hospital, and appeared to be responding to internal stimuli. She was subsequently admitted to the inpatient mental health unit due to unspecified psychosis.
The veteran presented as an unreliable historian, and much of her medical history was obtained via a review of US Department of Defense (DoD) records and collateral interview with her parents. Before her hospitalization, the veteran had been diagnosed with major depressive disorder (MDD) and adjustment disorder while serving in the Navy. Her psychiatric history before her military career was otherwise unremarkable. At that time, she began a trial of sertraline 50 mg and completed 10 sessions of psychotherapy. After approximately 1 year, she elected to stop taking sertraline due to improved mental health. However, shortly after this she began experiencing significant depressive symptoms and was ultimately released early from the Navy due to her mental health concerns.
The veteran’s parents provided interim history between her discharge and establishing care at MEDVAMC as the veteran was reluctant to discuss this period of her life. According to her parents the veteran had prior diagnoses of borderline personality disorder and MDD and had difficulty adhering to her current medications (bupropion and duloxetine) for about 1 month before her hospitalization. During the previous month, her parents observed her staying in her room around the clock and “[going] mute.”
The veteran remained hospitalized for about 1 month, during which she was diagnosed with schizoaffective disorder and stabilized on injections of long-acting olanzapine 210 mg (administered every 2 weeks). She was referred for outpatient psychotherapy in a specialty clinic for veterans with SSDs. However, she did not attend her initial intake assessment.
About 2 weeks after discharge from the hospital, the veteran presented for her injection appointment. At this time, she was noted to be disorganized in her thinking and behavior, displaying thought blocking and catatonic behavior. Her parents also described concerning behavior since her discharge. They stated she went to a hotel after her discharge and spent all her available money. She then returned to her parents’ home, where she did not sleep or bathe for several days. She was observed wandering around the house aimlessly and in a confused manner and had become verbally aggressive and threatening toward her parents. The veteran was again psychiatrically admitted due to psychosis and concerns for her safety. She was discharged about 2 weeks later and continued olanzapine injections. She was also referred for outpatient psychotherapy; although she did not initially engage in psychotherapy, she was referred again about 5 months after discharge and began psychotherapy at that time.
The veteran began a course of weekly outpatient psychotherapy employing cognitive behavior therapy for psychosis (CBTp).6 During this time, she described her primary concerns as anxiety and feeling disconnected from others. She reported a history of depression but not of schizoaffective disorder. When asked about this, the veteran stated that she did not feel this diagnosis was accurate and instead believed she had severe depression. When asked why she was prescribed olanzapine, the veteran stated that this medication was for depression. As with her inpatient stays, the veteran demonstrated several negative symptoms during her course of psychotherapy. She presented with noticeably blunted affect, evidenced by lack of facial expression and monotonic speech. She also routinely displayed alogia (ie, lack of speech), often stating that she “did not feel like talking much.” She described difficulty finding motivation to initiate tasks (avolition) as well as a tendency toward social isolation (asociality).
The veteran also described concerns related to neurocognitive and social cognitive symptoms. She reported difficulties in processing speed, cognitive set-shifting (mentally switching between tasks), and inhibition, describing how these concerns interfered with her occupational functioning. She noted difficulty maintaining the expected pace of work at her previous positions, stating that she felt it took her longer to complete tasks compared with others. In addition, she displayed some difficulties with attention and memory. On more than one occasion, she seemed to have forgotten the previous day’s conversations with clinicians. Regarding social cognitive symptoms, she noted difficulties in emotion processing, indicating that it was difficult for her to identify and manage her emotions. This was especially prominent during times of depressed mood.
She also displayed a hostile attribution bias, or tendency to overattribute hostile intent to others’ ambiguous actions. For example, she described an instance where a family member sat too close to her on the couch, stating that she felt this behavior indicated the family member did not care about her. Relatedly, the veteran demonstrated difficulty with perspective taking, which became evident during cognitive restructuring regarding interpretations of her family’s behavior. Finally, the veteran displayed some deficits in social perception, or the ability to identify social context and rules based on nonverbal communication, verbal cues, and vocal intonation. She stated that she often felt conversing with others was difficult for her and indicated that she was “not good at conversations.” This may have in part been due to deficits in social perception.
During the first 2 months of psychotherapy, the veteran regularly attended sessions (conducted over telephone due to the COVID-19 pandemic) and was adherent to twice-weekly olanzapine injections. Despite this, she began experiencing an increase in depressive symptoms accompanied by a noticeable worsening of her blunted affect, alogia, and avolition. After about 2 months of psychotherapy, she described active suicidal ideation and requested to be voluntarily hospitalized. During this hospitalization, the veteran was consulted about the use of clozapine in treatment-refractory conditions and began a trial of clozapine 400 mg. She demonstrated marked improvement in her depressed mood after taking the medication and was discharged about 2 weeks after admission. The veteran completed 10 sessions of CBTp before electing to terminate due to an upcoming move. She was adherent to weekly blood draws per the requirements of clozapine and described intentions to engage in mental health care after her move. The patient’s mother contacted the clinic to inform the treatment team that the patient and her family had moved to a different city and the patient had started receiving care at the VAMC in that city.
Discussion
As the veteran’s case highlights, a predominantly negative and cognitive symptom presentation may present diagnostic challenges. Since this presentation may not be viewed as representative of SSDs, patients with this presentation may be misdiagnosed. This was evident in the current case, not only in the veteran’s prodromal phase of illness while in the Navy, but also in her reported previous diagnoses of borderline personality disorder and MDD. More than one clinician at the MEDVAMC provisionally considered a diagnosis of MDD before collecting collateral information from the veteran’s family regarding her clear psychotic symptoms. Unfortunately, such misdiagnoses may have prevented early intervention of the veteran’s schizoaffective disorder, which is found to be instrumental in reducing impairment and disability among patients with SSDs.7,8
These misdiagnoses are understandable given the considerable symptom overlap between SSDs and other mental health disorders. For instance, anhedonia and avolition are 2 key symptoms seen in depressive episodes. Both anhedonia and lack of positive emotion are often seen in posttraumatic stress disorder. Additionally, anxiety disorders may induce a lack of positive emotion, loss of interest in previously enjoyed activities, and lack of motivation secondary to primary symptoms of anxiety. Furthermore, schizoaffective disorder requires the presence of a major mood episode. In the absence of apparent positive symptoms (as is the case for patients with a predominantly negative symptom presentation), schizoaffective disorder may be easily misdiagnosed as a mood disorder.
Patients with predominantly negative or cognitive symptoms may also be less accepting of a diagnosis of SSD. A wealth of research points to the clear stigma of SSDs, with many suggesting that these disorders are among the most stigmatized mental health disorders.9 Therefore, patients with predominantly negative and cognitive symptoms may be more likely to attribute their symptoms to another, less stigmatized mental health disorder. This was seen in the current case, as the veteran repeatedly denied a diagnosis of schizoaffective disorder and instead claimed to have severe depression. This reluctance to accept a diagnosis of an SSD, coupled with the diagnostic ambiguity of negative symptoms, is likely to make it challenging for clinicians to accurately identify patients with a predominantly negative and cognitive symptom presentation of SSDs.
Clinicians working within a team-based setting may be less likely to misdiagnose patients as they can consult others. Diagnostic clarity in the current case was undoubtedly facilitated by the multidisciplinary team involved in the veteran’s care; clinicians involved in her care were able to consult with one another to determine that her symptoms were indicative of an SSD rather than a mood disorder. Mental health professionals in private practice are unlikely to have access to such multidisciplinary specialty services and may be particularly vulnerable to misdiagnoses.
Treatment Considerations
This case also highlights several psychotherapy and psychopharmacology treatment considerations for patients with a predominantly negative and cognitive symptom presentation. The veteran was initially difficult to engage in psychotherapy. Although patients with SSDs often have difficulty engaging in treatment, patients with a predominant negative and cognitive symptom profile may experience more difficulty doing so.10 Previous research suggests that both negative symptoms and cognitive symptoms are inversely related to treatment engagement.11,12
By their very nature, negative symptoms may make it difficult to fully engage in psychotherapy. First, avolition and amotivation likely make it difficult for patients to attend psychotherapy appointments. Furthermore, negative symptoms may make it difficult to emotionally engage with the content of psychotherapy, thus limiting the potential benefits. Cognitive symptoms may also make it more difficult for patients to fully reap the benefits of psychotherapy. Deficits in attention, memory, and abstract reasoning seen in other mental health and medical conditions are associated with poorer treatment outcomes in psychotherapy.13,14 Thus, it may be especially difficult to engage patients with primarily negative and cognitive symptoms of SSDs in psychotherapy. However, given the link between these symptoms and functional impairment, it is even more important to evaluate and address such barriers to treatment.
This case highlights the utility of clozapine in the treatment of SSDs. Many commonly prescribed antipsychotic medications have questionable efficacy in treating negative symptoms, and none of the currently available antipsychotics are approved for this indication.15 In our case, the veteran saw a limited reduction of her negative or cognitive symptoms from her use of olanzapine. However, case reports, naturalistic follow-up, and open-label studies suggest that clozapine may be efficacious in targeting negative symptoms of SSDs.16-19 Previous research also suggests clozapine is more effective than other antipsychotic medications, including olanzapine, quetiapine, and risperidone, in decreasing overall SSD symptoms.20,21 Additionally, there is initial evidence of the efficacy of clozapine in treating cognitive symptoms, suggesting that some areas of cognition may improve in response to this medication.22-24 On the other hand, a recent case study suggests high doses of clozapine may be associated with cognitive impairment, although cognitive impairment was still greater without medication than at this higher dose.25 Thus, further research is needed to refine our understanding of the impact of clozapine on cognitive symptoms in SSDs.
Despite the promising research behind clozapine, it remains widely underprescribed, likely due to concerns regarding the potential adverse effects.26,27 Clozapine has been associated with many adverse effects, the most concerning being neutropenia, which can lead to serious infection and death. Thus, one concern among clinicians may be the potential lethality of clozapine. However, a wealth of research indicates clozapine can be safely administered under medical supervision.26,28 In fact, clozapine has been linked to lower all-cause mortality rates and lower mortality rates by suicide compared with other antipsychotic medications.29-31 It may therefore be argued that clozapine lowers the overall risk of mortality. Prescribers may also be weary of adherence to regular blood tests that patients must undergo to monitor their risk for neutropenia. This is the most frequently cited anticipated barrier to beginning a trial of clozapine.27 These concerns may not be unfounded; indeed, if avolition and amotivation make it difficult to attend psychotherapy sessions, these factors may logically make it difficult to attend blood draw appointments. In response to such barriers, several solutions have been suggested regarding potential blood draw nonadherence, including the use of in-home treatment teams and point-of-care monitoring.32,33
Conclusions
Predominant negative and cognitive symptom presentations of SSDs require unique considerations to accurately identify and provide optimal treatment for patients with such presentations. As our case highlights, patients with such presentations may often be misdiagnosed, as negative and cognitive symptoms may be attributed to other disorders. Additionally, patients with this presentation may experience difficulty engaging in psychotherapy and may not see the same benefits from common antipsychotic medications as patients with predominantly positive symptoms. Clozapine emerges as a promising treatment for addressing negative and cognitive symptoms, although it remains widely underutilized. In cases where clinicians encounter patients with predominantly negative and cognitive symptoms, we strongly recommend consultation and referral to psychiatric care for medication management.
The current case highlights the need for individually tailored treatment plans for individuals seeking mental health care. Clinicians of patients with any mental disorder, but especially those with SSDs of predominantly negative and cognitive symptoms, should carefully formulate a treatment plan based on relevant case history, presentation, and current empirical literature. A singular, one-size-fits-all approach should not be universally implemented for such patients. Our case demonstrates how careful multidisciplinary evaluations, review of medical records, collateral information from patients’ family members, and other diagnostic and treatment considerations in patients with predominant negative and cognitive symptoms of SSDs can refine and enhance the clinical care offered to such patients.
Acknowledgments
A.K. is supported by the US Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Central Texas Veterans Affairs Health Care System, and the VISN 17 Center of Excellence for Research on Returning War Veterans.
Schizophrenia spectrum disorders (SSDs) represent some of the most debilitating mental health disorders.1 While these disorders have myriad presentations, the prototypical patient with SSD is often thought to possess positive symptoms. More recently, clinicians and researchers are raising awareness of another presentation of SSD: predominantly negative and cognitive symptoms. This symptom profile is not a novel phenomenon; for many years this presentation was recognized as a “deficit” presentation, referring to negative symptoms as the prominent feature.2,3 However, it presents unique diagnostic and treatment considerations that are often underappreciated in clinical settings.
Negative symptoms (blunted/flat affect, avolition, alogia, anhedonia, asociality) have long been identified as key features of SSD and are widely recognized as predictive of poor prognostic outcomes for patients with SSDs.1 In many patients, negative symptoms may precede the development of positive symptoms and emerge as a more robust predictor of functional outcomes than positive symptoms.1 Negative symptoms also appear to be inextricably linked to cognitive symptoms. Specifically, patients with primary negative symptoms seem to perform poorly on measures of global cognitive functioning.1 Similar to negative symptoms, cognitive symptoms of SSDs are a primary source of functional impairment and persistent disability.1 Despite this, little attention is given in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) to the neurocognitive and social cognitive deficits seen in patients with SSDs. Previous research highlights broad deficits in a range of neurocognitive abilities, including attention, working memory, processing speed, executive functioning, learning and memory, and receptive and expressive language.4 Similarly, patients also display deficits in domains of social cognition, such as emotion processing, identifying and utilizing social cues, evaluating attributions of others, and perspective-taking.5
A predominantly negative and cognitive symptom presentation can present diagnostic and treatment challenges. We present a case of a patient with such a presentation and the unique considerations given to diagnostic clarification and her treatment.
Case Presentation
A 33-year-old female veteran presented to the emergency department (ED) at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, in 2020. She was brought to the ED by local police following an attempted assault of her neighbor. Per collateral information from the police, the veteran stated she “had the urge to hurt someone” but was unable to provide any other information about this event. The veteran demonstrated diminished speech output, providing 2- to 3-word responses before refusing to speak entirely. She also presented with markedly blunted affect and tangential speech. She was not oriented to situation, stating confusion as to how she was brought to the hospital, and appeared to be responding to internal stimuli. She was subsequently admitted to the inpatient mental health unit due to unspecified psychosis.
The veteran presented as an unreliable historian, and much of her medical history was obtained via a review of US Department of Defense (DoD) records and collateral interview with her parents. Before her hospitalization, the veteran had been diagnosed with major depressive disorder (MDD) and adjustment disorder while serving in the Navy. Her psychiatric history before her military career was otherwise unremarkable. At that time, she began a trial of sertraline 50 mg and completed 10 sessions of psychotherapy. After approximately 1 year, she elected to stop taking sertraline due to improved mental health. However, shortly after this she began experiencing significant depressive symptoms and was ultimately released early from the Navy due to her mental health concerns.
The veteran’s parents provided interim history between her discharge and establishing care at MEDVAMC as the veteran was reluctant to discuss this period of her life. According to her parents the veteran had prior diagnoses of borderline personality disorder and MDD and had difficulty adhering to her current medications (bupropion and duloxetine) for about 1 month before her hospitalization. During the previous month, her parents observed her staying in her room around the clock and “[going] mute.”
The veteran remained hospitalized for about 1 month, during which she was diagnosed with schizoaffective disorder and stabilized on injections of long-acting olanzapine 210 mg (administered every 2 weeks). She was referred for outpatient psychotherapy in a specialty clinic for veterans with SSDs. However, she did not attend her initial intake assessment.
About 2 weeks after discharge from the hospital, the veteran presented for her injection appointment. At this time, she was noted to be disorganized in her thinking and behavior, displaying thought blocking and catatonic behavior. Her parents also described concerning behavior since her discharge. They stated she went to a hotel after her discharge and spent all her available money. She then returned to her parents’ home, where she did not sleep or bathe for several days. She was observed wandering around the house aimlessly and in a confused manner and had become verbally aggressive and threatening toward her parents. The veteran was again psychiatrically admitted due to psychosis and concerns for her safety. She was discharged about 2 weeks later and continued olanzapine injections. She was also referred for outpatient psychotherapy; although she did not initially engage in psychotherapy, she was referred again about 5 months after discharge and began psychotherapy at that time.
The veteran began a course of weekly outpatient psychotherapy employing cognitive behavior therapy for psychosis (CBTp).6 During this time, she described her primary concerns as anxiety and feeling disconnected from others. She reported a history of depression but not of schizoaffective disorder. When asked about this, the veteran stated that she did not feel this diagnosis was accurate and instead believed she had severe depression. When asked why she was prescribed olanzapine, the veteran stated that this medication was for depression. As with her inpatient stays, the veteran demonstrated several negative symptoms during her course of psychotherapy. She presented with noticeably blunted affect, evidenced by lack of facial expression and monotonic speech. She also routinely displayed alogia (ie, lack of speech), often stating that she “did not feel like talking much.” She described difficulty finding motivation to initiate tasks (avolition) as well as a tendency toward social isolation (asociality).
The veteran also described concerns related to neurocognitive and social cognitive symptoms. She reported difficulties in processing speed, cognitive set-shifting (mentally switching between tasks), and inhibition, describing how these concerns interfered with her occupational functioning. She noted difficulty maintaining the expected pace of work at her previous positions, stating that she felt it took her longer to complete tasks compared with others. In addition, she displayed some difficulties with attention and memory. On more than one occasion, she seemed to have forgotten the previous day’s conversations with clinicians. Regarding social cognitive symptoms, she noted difficulties in emotion processing, indicating that it was difficult for her to identify and manage her emotions. This was especially prominent during times of depressed mood.
She also displayed a hostile attribution bias, or tendency to overattribute hostile intent to others’ ambiguous actions. For example, she described an instance where a family member sat too close to her on the couch, stating that she felt this behavior indicated the family member did not care about her. Relatedly, the veteran demonstrated difficulty with perspective taking, which became evident during cognitive restructuring regarding interpretations of her family’s behavior. Finally, the veteran displayed some deficits in social perception, or the ability to identify social context and rules based on nonverbal communication, verbal cues, and vocal intonation. She stated that she often felt conversing with others was difficult for her and indicated that she was “not good at conversations.” This may have in part been due to deficits in social perception.
During the first 2 months of psychotherapy, the veteran regularly attended sessions (conducted over telephone due to the COVID-19 pandemic) and was adherent to twice-weekly olanzapine injections. Despite this, she began experiencing an increase in depressive symptoms accompanied by a noticeable worsening of her blunted affect, alogia, and avolition. After about 2 months of psychotherapy, she described active suicidal ideation and requested to be voluntarily hospitalized. During this hospitalization, the veteran was consulted about the use of clozapine in treatment-refractory conditions and began a trial of clozapine 400 mg. She demonstrated marked improvement in her depressed mood after taking the medication and was discharged about 2 weeks after admission. The veteran completed 10 sessions of CBTp before electing to terminate due to an upcoming move. She was adherent to weekly blood draws per the requirements of clozapine and described intentions to engage in mental health care after her move. The patient’s mother contacted the clinic to inform the treatment team that the patient and her family had moved to a different city and the patient had started receiving care at the VAMC in that city.
Discussion
As the veteran’s case highlights, a predominantly negative and cognitive symptom presentation may present diagnostic challenges. Since this presentation may not be viewed as representative of SSDs, patients with this presentation may be misdiagnosed. This was evident in the current case, not only in the veteran’s prodromal phase of illness while in the Navy, but also in her reported previous diagnoses of borderline personality disorder and MDD. More than one clinician at the MEDVAMC provisionally considered a diagnosis of MDD before collecting collateral information from the veteran’s family regarding her clear psychotic symptoms. Unfortunately, such misdiagnoses may have prevented early intervention of the veteran’s schizoaffective disorder, which is found to be instrumental in reducing impairment and disability among patients with SSDs.7,8
These misdiagnoses are understandable given the considerable symptom overlap between SSDs and other mental health disorders. For instance, anhedonia and avolition are 2 key symptoms seen in depressive episodes. Both anhedonia and lack of positive emotion are often seen in posttraumatic stress disorder. Additionally, anxiety disorders may induce a lack of positive emotion, loss of interest in previously enjoyed activities, and lack of motivation secondary to primary symptoms of anxiety. Furthermore, schizoaffective disorder requires the presence of a major mood episode. In the absence of apparent positive symptoms (as is the case for patients with a predominantly negative symptom presentation), schizoaffective disorder may be easily misdiagnosed as a mood disorder.
Patients with predominantly negative or cognitive symptoms may also be less accepting of a diagnosis of SSD. A wealth of research points to the clear stigma of SSDs, with many suggesting that these disorders are among the most stigmatized mental health disorders.9 Therefore, patients with predominantly negative and cognitive symptoms may be more likely to attribute their symptoms to another, less stigmatized mental health disorder. This was seen in the current case, as the veteran repeatedly denied a diagnosis of schizoaffective disorder and instead claimed to have severe depression. This reluctance to accept a diagnosis of an SSD, coupled with the diagnostic ambiguity of negative symptoms, is likely to make it challenging for clinicians to accurately identify patients with a predominantly negative and cognitive symptom presentation of SSDs.
Clinicians working within a team-based setting may be less likely to misdiagnose patients as they can consult others. Diagnostic clarity in the current case was undoubtedly facilitated by the multidisciplinary team involved in the veteran’s care; clinicians involved in her care were able to consult with one another to determine that her symptoms were indicative of an SSD rather than a mood disorder. Mental health professionals in private practice are unlikely to have access to such multidisciplinary specialty services and may be particularly vulnerable to misdiagnoses.
Treatment Considerations
This case also highlights several psychotherapy and psychopharmacology treatment considerations for patients with a predominantly negative and cognitive symptom presentation. The veteran was initially difficult to engage in psychotherapy. Although patients with SSDs often have difficulty engaging in treatment, patients with a predominant negative and cognitive symptom profile may experience more difficulty doing so.10 Previous research suggests that both negative symptoms and cognitive symptoms are inversely related to treatment engagement.11,12
By their very nature, negative symptoms may make it difficult to fully engage in psychotherapy. First, avolition and amotivation likely make it difficult for patients to attend psychotherapy appointments. Furthermore, negative symptoms may make it difficult to emotionally engage with the content of psychotherapy, thus limiting the potential benefits. Cognitive symptoms may also make it more difficult for patients to fully reap the benefits of psychotherapy. Deficits in attention, memory, and abstract reasoning seen in other mental health and medical conditions are associated with poorer treatment outcomes in psychotherapy.13,14 Thus, it may be especially difficult to engage patients with primarily negative and cognitive symptoms of SSDs in psychotherapy. However, given the link between these symptoms and functional impairment, it is even more important to evaluate and address such barriers to treatment.
This case highlights the utility of clozapine in the treatment of SSDs. Many commonly prescribed antipsychotic medications have questionable efficacy in treating negative symptoms, and none of the currently available antipsychotics are approved for this indication.15 In our case, the veteran saw a limited reduction of her negative or cognitive symptoms from her use of olanzapine. However, case reports, naturalistic follow-up, and open-label studies suggest that clozapine may be efficacious in targeting negative symptoms of SSDs.16-19 Previous research also suggests clozapine is more effective than other antipsychotic medications, including olanzapine, quetiapine, and risperidone, in decreasing overall SSD symptoms.20,21 Additionally, there is initial evidence of the efficacy of clozapine in treating cognitive symptoms, suggesting that some areas of cognition may improve in response to this medication.22-24 On the other hand, a recent case study suggests high doses of clozapine may be associated with cognitive impairment, although cognitive impairment was still greater without medication than at this higher dose.25 Thus, further research is needed to refine our understanding of the impact of clozapine on cognitive symptoms in SSDs.
Despite the promising research behind clozapine, it remains widely underprescribed, likely due to concerns regarding the potential adverse effects.26,27 Clozapine has been associated with many adverse effects, the most concerning being neutropenia, which can lead to serious infection and death. Thus, one concern among clinicians may be the potential lethality of clozapine. However, a wealth of research indicates clozapine can be safely administered under medical supervision.26,28 In fact, clozapine has been linked to lower all-cause mortality rates and lower mortality rates by suicide compared with other antipsychotic medications.29-31 It may therefore be argued that clozapine lowers the overall risk of mortality. Prescribers may also be weary of adherence to regular blood tests that patients must undergo to monitor their risk for neutropenia. This is the most frequently cited anticipated barrier to beginning a trial of clozapine.27 These concerns may not be unfounded; indeed, if avolition and amotivation make it difficult to attend psychotherapy sessions, these factors may logically make it difficult to attend blood draw appointments. In response to such barriers, several solutions have been suggested regarding potential blood draw nonadherence, including the use of in-home treatment teams and point-of-care monitoring.32,33
Conclusions
Predominant negative and cognitive symptom presentations of SSDs require unique considerations to accurately identify and provide optimal treatment for patients with such presentations. As our case highlights, patients with such presentations may often be misdiagnosed, as negative and cognitive symptoms may be attributed to other disorders. Additionally, patients with this presentation may experience difficulty engaging in psychotherapy and may not see the same benefits from common antipsychotic medications as patients with predominantly positive symptoms. Clozapine emerges as a promising treatment for addressing negative and cognitive symptoms, although it remains widely underutilized. In cases where clinicians encounter patients with predominantly negative and cognitive symptoms, we strongly recommend consultation and referral to psychiatric care for medication management.
The current case highlights the need for individually tailored treatment plans for individuals seeking mental health care. Clinicians of patients with any mental disorder, but especially those with SSDs of predominantly negative and cognitive symptoms, should carefully formulate a treatment plan based on relevant case history, presentation, and current empirical literature. A singular, one-size-fits-all approach should not be universally implemented for such patients. Our case demonstrates how careful multidisciplinary evaluations, review of medical records, collateral information from patients’ family members, and other diagnostic and treatment considerations in patients with predominant negative and cognitive symptoms of SSDs can refine and enhance the clinical care offered to such patients.
Acknowledgments
A.K. is supported by the US Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Central Texas Veterans Affairs Health Care System, and the VISN 17 Center of Excellence for Research on Returning War Veterans.
1. Kantrowitz JT. Managing negative symptoms of schizophrenia: how far have we come? CNS Drugs. 2017;31(5):373-388. doi:10.1007/s40263-017-0428-x
2. Fenton WS, McGlashan TH. Antecedents, symptom progression, and long-term outcome of the deficit syndrome in schizophrenia. Am J Psychiatry. 1994;151(3):351-356. doi:10.1176/ajp.151.3.351
3. Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT. A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58(2):165. doi:10.1001/archpsyc.58.2.165
4. Kalkstein S, Hurford I, Gur RC. Neurocognition in schizophrenia. Curr Top Behav Neurosci. 2010;4:373-390. doi:10.1007/7854_2010_42
5. Green MF, Horan WP. Social cognition in schizophrenia. Curr Dir Psychol Sci. 2010;19(4):243-248. doi:10.1177/0963721410377600
6. Kingdon DG, Turkington D. Cognitive Therapy of Schizophrenia. Guilford Press; 2008.
7. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555. doi:10.1001/jamapsychiatry.2018.0623
8. McGorry PD. Early intervention in psychosis: obvious, effective, overdue. J Nerv Ment Dis. 2015;203(5):310-318. doi:10.1097/NMD.0000000000000284
9. Crisp AH, Gelder MG, Rix S, Meltzer HI, Rowlands OJ. Stigmatisation of people with mental illnesses. Br J Psychiatry. 2000;177(1):4-7. doi:10.1192/bjp.177.1.4
10. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15(1):13-20. doi:10.1002/wps.20306
11. Kukla M, Davis LW, Lysaker PH. Cognitive behavioral therapy and work outcomes: correlates of treatment engagement and full and partial success in schizophrenia. Behav Cogn Psychother. 2014;42(5):577-592. doi:10.1017/S1352465813000428
12. Johansen R, Hestad K, Iversen VC, et al. Cognitive and clinical factors are associated with service engagement in early-phase schizophrenia spectrum disorders. J Nerv Ment Dis. 2011;199(3):176-182. doi:10.1097/NMD.0b013e31820bc2f9
13. Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81(3):313-322. doi:10.1016/j.drugalcdep.2005.08.003
14. Aarsland D, Taylor JP, Weintraub D. Psychiatric issues in cognitive impairment. Mov Disord. 2014;29(5):651-662. doi:10.1002/mds.25873
15. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. doi:10.1016/S0140-6736(13)60733-3
16. Khan AH, Zaidi S. Clozapine: Improvement of Negative Symptoms of Schizophrenia. Cureus. 2017;9(12):e1973. Published 2017 Dec 20. doi:10.7759/cureus.1973
17. Brar JS, Chengappa KN, Parepally H, et al. The effects of clozapine on negative symptoms in patients with schizophrenia with minimal positive symptoms. Ann Clin Psychiatry. 1997;9(4):227-234. doi:10.1023/a:1022352326334
18. Llorca PM, Lancon C, Farisse J, Scotto JC. Clozapine and negative symptoms. An open study. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24(3):373-384. doi:10.1016/s0278-5846(99)00105-0
19. Siskind D, McCartney L, Goldschlager R, Kisely S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385-392. doi:10.1192/bjp.bp.115.177261
20. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610. doi:10.1176/appi.ajp.163.4.600
21. Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Comparative Effectiveness of Clozapine and Standard Antipsychotic Treatment in Adults With Schizophrenia. Am J Psychiatry. 2016;173(2):166-173. doi:10.1176/appi.ajp.2015.15030332
22. Lee MA, Thompson PA, Meltzer HY. Effects of clozapine in cognitive function in schizophrenia. J Clin Psychiatry. 1994;55(suppl B):82-87.
23. Sharma T, Hughes C, Soni W, Kumari V. Cognitive effects of olanzapine and clozapine treatment in chronic schizophrenia. Psychopharmacology (Berl). 2003;169(3-4):398-403. doi:10.1007/s00213-003-1506-y
24. Spagna A, Dong Y, Mackie MA, et al. Clozapine improves the orienting of attention in schizophrenia. Schizophr Res. 2015;168(1-2):285-291. doi:10.1016/j.schres.2015.08.009
25. Savulich G, Mezquida G, Atkinson S, Bernardo M, Fernandez-Egea E. A case study of clozapine and cognition: friend or foe? J Clin Psychopharmacol. 2018;38(2):152-153. doi:10.1097/JCP.0000000000000847
26. Bogers JPAM, Schulte PFJ, Van Dijk D, Bakker B, Cohen D. Clozapine underutilization in the treatment of schizophrenia: how can clozapine prescription rates be improved? J Clin Psychopharmacol. 2016;36(2):109-111. doi:10.1097/JCP.0000000000000478
27. Kelly DL, Freudenreich O, Sayer MA, Love RC. Addressing Barriers to Clozapine Underutilization: A National Effort. Psychiatr Serv. 2018;69(2):224-227. doi:10.1176/appi.ps.201700162
28. Honigfeld G, Arellano F, Sethi J, Bianchini A, Schein J. Reducing clozapine-related morbidity and mortality: 5 years of experience with the Clozaril National Registry. J Clin Psychiatry. 1998;59(suppl 3):3-7.
29. Cho J, Hayes RD, Jewell A, et al. Clozapine and all-cause mortality in treatment-resistant schizophrenia: a historical cohort study. Acta Psychiatr Scand. 2019;139(3):237-247. doi:10.1111/acps.12989
30. Kane JM. Clozapine Reduces All-Cause Mortality. Am J Psychiatry. 2017;174(10):920-921. doi:10.1176/appi.ajp.2017.17070770
31. Taipale H, Lähteenvuo M, Tanskanen A, Mittendorfer-Rutz E, Tiihonen J. Comparative Effectiveness of Antipsychotics for Risk of Attempted or Completed Suicide Among Persons With Schizophrenia. Schizophr Bull. 2021;47(1):23-30. doi:10.1093/schbul/sbaa111
32. Love RC, Kelly DL, Freudenreich O, Sayer MA. Clozapine underutilization: addressing the barriers. National Association of State Mental Health Program Directors; 2016. Accessed October 6, 2022. https://www.nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
33. Kelly DL, Ben-Yoav H, Payne GF, et al. Blood draw barriers for treatment with clozapine and development of a point-of-care monitoring device. Clin Schizophr Relat Psychoses. 2018;12(1):23-30. doi:10.3371/CSRP.KEBE.070415
1. Kantrowitz JT. Managing negative symptoms of schizophrenia: how far have we come? CNS Drugs. 2017;31(5):373-388. doi:10.1007/s40263-017-0428-x
2. Fenton WS, McGlashan TH. Antecedents, symptom progression, and long-term outcome of the deficit syndrome in schizophrenia. Am J Psychiatry. 1994;151(3):351-356. doi:10.1176/ajp.151.3.351
3. Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT. A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58(2):165. doi:10.1001/archpsyc.58.2.165
4. Kalkstein S, Hurford I, Gur RC. Neurocognition in schizophrenia. Curr Top Behav Neurosci. 2010;4:373-390. doi:10.1007/7854_2010_42
5. Green MF, Horan WP. Social cognition in schizophrenia. Curr Dir Psychol Sci. 2010;19(4):243-248. doi:10.1177/0963721410377600
6. Kingdon DG, Turkington D. Cognitive Therapy of Schizophrenia. Guilford Press; 2008.
7. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555. doi:10.1001/jamapsychiatry.2018.0623
8. McGorry PD. Early intervention in psychosis: obvious, effective, overdue. J Nerv Ment Dis. 2015;203(5):310-318. doi:10.1097/NMD.0000000000000284
9. Crisp AH, Gelder MG, Rix S, Meltzer HI, Rowlands OJ. Stigmatisation of people with mental illnesses. Br J Psychiatry. 2000;177(1):4-7. doi:10.1192/bjp.177.1.4
10. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15(1):13-20. doi:10.1002/wps.20306
11. Kukla M, Davis LW, Lysaker PH. Cognitive behavioral therapy and work outcomes: correlates of treatment engagement and full and partial success in schizophrenia. Behav Cogn Psychother. 2014;42(5):577-592. doi:10.1017/S1352465813000428
12. Johansen R, Hestad K, Iversen VC, et al. Cognitive and clinical factors are associated with service engagement in early-phase schizophrenia spectrum disorders. J Nerv Ment Dis. 2011;199(3):176-182. doi:10.1097/NMD.0b013e31820bc2f9
13. Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81(3):313-322. doi:10.1016/j.drugalcdep.2005.08.003
14. Aarsland D, Taylor JP, Weintraub D. Psychiatric issues in cognitive impairment. Mov Disord. 2014;29(5):651-662. doi:10.1002/mds.25873
15. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. doi:10.1016/S0140-6736(13)60733-3
16. Khan AH, Zaidi S. Clozapine: Improvement of Negative Symptoms of Schizophrenia. Cureus. 2017;9(12):e1973. Published 2017 Dec 20. doi:10.7759/cureus.1973
17. Brar JS, Chengappa KN, Parepally H, et al. The effects of clozapine on negative symptoms in patients with schizophrenia with minimal positive symptoms. Ann Clin Psychiatry. 1997;9(4):227-234. doi:10.1023/a:1022352326334
18. Llorca PM, Lancon C, Farisse J, Scotto JC. Clozapine and negative symptoms. An open study. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24(3):373-384. doi:10.1016/s0278-5846(99)00105-0
19. Siskind D, McCartney L, Goldschlager R, Kisely S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385-392. doi:10.1192/bjp.bp.115.177261
20. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610. doi:10.1176/appi.ajp.163.4.600
21. Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Comparative Effectiveness of Clozapine and Standard Antipsychotic Treatment in Adults With Schizophrenia. Am J Psychiatry. 2016;173(2):166-173. doi:10.1176/appi.ajp.2015.15030332
22. Lee MA, Thompson PA, Meltzer HY. Effects of clozapine in cognitive function in schizophrenia. J Clin Psychiatry. 1994;55(suppl B):82-87.
23. Sharma T, Hughes C, Soni W, Kumari V. Cognitive effects of olanzapine and clozapine treatment in chronic schizophrenia. Psychopharmacology (Berl). 2003;169(3-4):398-403. doi:10.1007/s00213-003-1506-y
24. Spagna A, Dong Y, Mackie MA, et al. Clozapine improves the orienting of attention in schizophrenia. Schizophr Res. 2015;168(1-2):285-291. doi:10.1016/j.schres.2015.08.009
25. Savulich G, Mezquida G, Atkinson S, Bernardo M, Fernandez-Egea E. A case study of clozapine and cognition: friend or foe? J Clin Psychopharmacol. 2018;38(2):152-153. doi:10.1097/JCP.0000000000000847
26. Bogers JPAM, Schulte PFJ, Van Dijk D, Bakker B, Cohen D. Clozapine underutilization in the treatment of schizophrenia: how can clozapine prescription rates be improved? J Clin Psychopharmacol. 2016;36(2):109-111. doi:10.1097/JCP.0000000000000478
27. Kelly DL, Freudenreich O, Sayer MA, Love RC. Addressing Barriers to Clozapine Underutilization: A National Effort. Psychiatr Serv. 2018;69(2):224-227. doi:10.1176/appi.ps.201700162
28. Honigfeld G, Arellano F, Sethi J, Bianchini A, Schein J. Reducing clozapine-related morbidity and mortality: 5 years of experience with the Clozaril National Registry. J Clin Psychiatry. 1998;59(suppl 3):3-7.
29. Cho J, Hayes RD, Jewell A, et al. Clozapine and all-cause mortality in treatment-resistant schizophrenia: a historical cohort study. Acta Psychiatr Scand. 2019;139(3):237-247. doi:10.1111/acps.12989
30. Kane JM. Clozapine Reduces All-Cause Mortality. Am J Psychiatry. 2017;174(10):920-921. doi:10.1176/appi.ajp.2017.17070770
31. Taipale H, Lähteenvuo M, Tanskanen A, Mittendorfer-Rutz E, Tiihonen J. Comparative Effectiveness of Antipsychotics for Risk of Attempted or Completed Suicide Among Persons With Schizophrenia. Schizophr Bull. 2021;47(1):23-30. doi:10.1093/schbul/sbaa111
32. Love RC, Kelly DL, Freudenreich O, Sayer MA. Clozapine underutilization: addressing the barriers. National Association of State Mental Health Program Directors; 2016. Accessed October 6, 2022. https://www.nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
33. Kelly DL, Ben-Yoav H, Payne GF, et al. Blood draw barriers for treatment with clozapine and development of a point-of-care monitoring device. Clin Schizophr Relat Psychoses. 2018;12(1):23-30. doi:10.3371/CSRP.KEBE.070415
Cutaneous and Subcutaneous Perineuriomas in 2 Pediatric Patients
Perineuriomas are benign, slow-growing tumors derived from perineurial cells,1 which form the structurally supportive perineurium that surrounds individual nerve fascicles.2,3 Perineuriomas are classified into 2 main forms: intraneural or extraneural.4 Intraneural perineuriomas are found within the border of the peripheral nerve,5 while extraneural perineuriomas usually are found in soft tissue and skin. Extraneural perineuriomas can be further classified into variants based on their histologic appearance, including reticular, sclerosing, and plexiform subtypes. Extraneural perineuriomas usually present on the extremities or trunk of young to middle-aged adults as a well-circumscribed, painless, subcutaneous masses.1 These tumors are especially unusual in children.4 We present 2 extraneural perineurioma cases in children, and we review the pertinent diagnostic features of perineurioma as well as the presentation in the pediatric population.
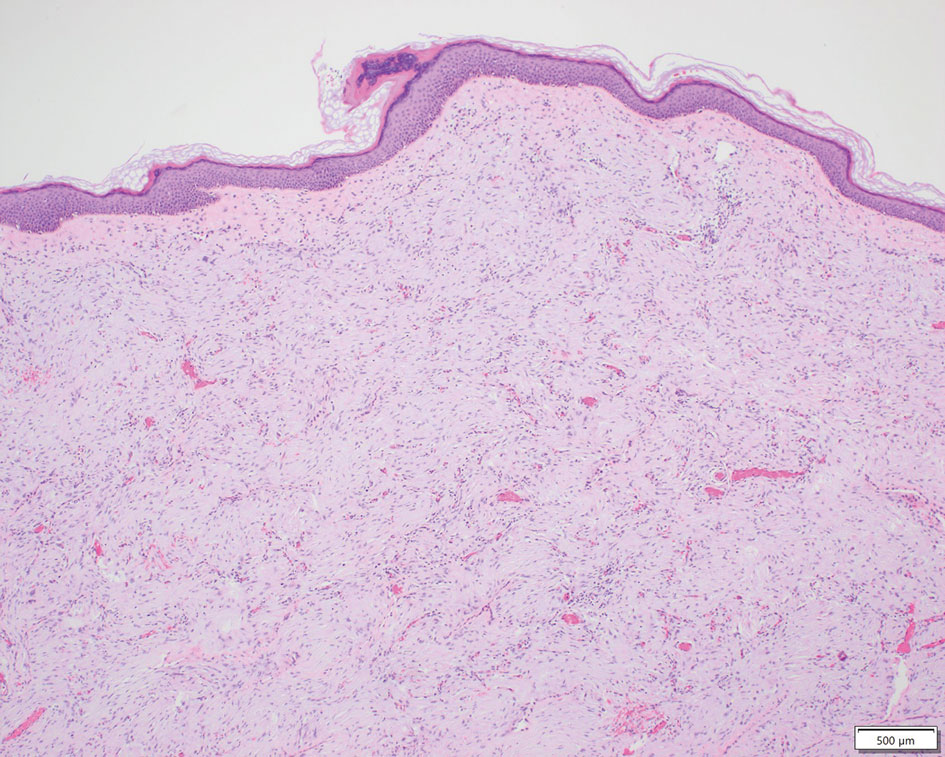
Case Reports
Patient 1—A 10-year-old boy with a history of cerebral palsy and related comorbidities presented to the clinic for evaluation of a lesion on the thigh with no associated pain, irritation, erythema, or drainage. Physical examination revealed a soft, pedunculated, mobile nodule on the right medial thigh. An elliptical excision was performed. Gross examination demonstrated a 2.0×2.0×1.8-cm polypoid nodule. Histologic examination showed a dermal-based proliferation of bland spindle cells (Figure 1). The cytomorphology was characterized by elongated tapering nuclei and many areas with delicate bipolar cytoplasmic processes. The constituent cells were arranged in a whorled pattern in a variably myxoid to collagenous stroma. The tumor cells were multifocally positive for CD34; focally positive for smooth muscle actin (SMA); and negative for S-100, epithelial membrane antigen (EMA), GLUT1, claudin-1, STAT6, and desmin. Rb protein was intact. The CD34 immunostain highlighted the cytoplasmic processes. Electron microscopy was performed because the immunohistochemical results were nonspecific despite the favorable histologic features for perineurioma and showed pinocytic vesicles with delicate cytoplasmic processes, characteristic of perineurioma (Figure 2). Follow-up visits were related to the management of multiple comorbidities; no known recurrence of the lesion was documented.
Patient 2—A 15-year-old adolescent boy with no notable medical history presented to the pediatric clinic for a bump on the right upper arm of 4 to 5 months’ duration. He did not recall an injury to the area and denied change in size, redness, bruising, or pain of the lesion. Ultrasonography demonstrated a 2.6×2.3×1.3-cm hypoechoic and slightly heterogeneous, well-circumscribed, subcutaneous mass with internal vascularity. The patient was then referred to a pediatric surgeon. The clinical differential included a lipoma, lymphadenopathy, or sebaceous cyst. An excision was performed. Gross inspection demonstrated a 7-g, 2.8×2.6×1.8-cm, homogeneous, tan-pink, rubbery nodule with minimal surrounding soft tissue. Histologic examination showed a bland proliferation of spindle cells with storiform and whorled patterns (Figure 3). No notable nuclear atypia or necrosis was identified. The tumor cells were focally positive for EMA (Figure 4), claudin-1, and CD34 and negative for S-100, SOX10, GLUT1, desmin, STAT6, pankeratin AE1/AE3, and SMA. The diagnosis of perineurioma was rendered. No recurrence of the lesion was appreciated clinically on a 6-month follow-up examination.
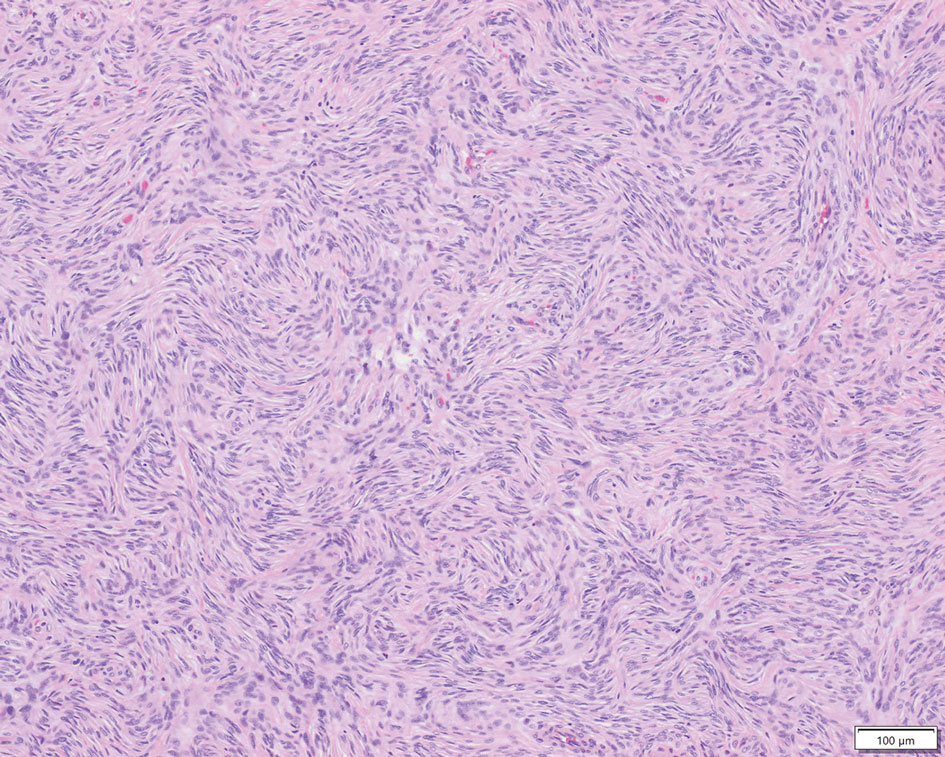
Comment
Characteristics of Perineuriomas—On gross evaluation, perineuriomas are firm, gray-white, and well circumscribed but not encapsulated. Histologically, perineuriomas can have a storiform, whorled, or lamellar pattern of spindle cells. Perivascular whorls can be a histologic clue. The spindle cells are bland appearing and typically are elongated and slender but can appear slightly ovoid and plump. The background stroma can be myxoid, collagenous, or mixed. There usually is no atypia, and mitotic figures are rare.2,3,6,7 Intraneural perineuriomas vary architecturally in that they display a unique onion bulb–like appearance in which whorls of cytoplasmic material of variable sizes surround central axons.3
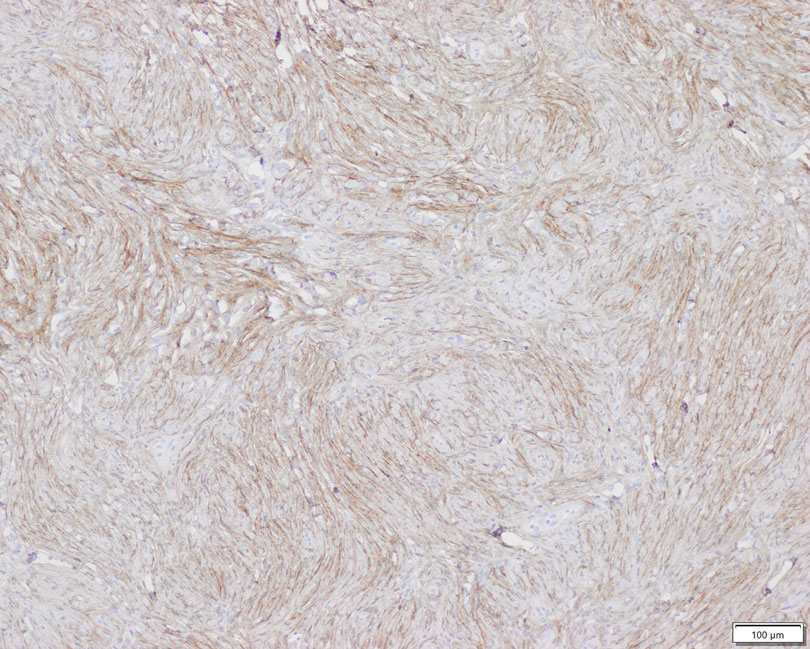
Diagnosis—The diagnosis of perineuriomas usually requires characteristic immunohistochemical and sometimes ultrastructural features. Perineuriomas are positive for EMA and GLUT1 and variable for CD34.6 Approximately 20% to 91% will be positive for claudin-1, a tight junction protein associated with perineuriomas.8 Of note, EMA and GLUT1 usually are positive in both neoplastic and nonneoplastic perineurial cells.9,10 Occasionally, these tumors can be focally positive for SMA and negative for S-100 and glial fibrillary acidic protein. The bipolar, thin, delicate, cytoplasmic processes with long-tapering nuclei may be easier to appreciate on electron microscopy than on conventional light microscopy. In addition, the cells contain pinocytotic vesicles and a discontinuous external lamina, which may be helpful for diagnosis.10
Genetics—Genetic alterations in perineurioma continue to be elucidated. Although many soft tissue perineuriomas possess deletion of chromosome 22q material, this is not a consistent finding and is not pathognomonic. Notably, the NF2 tumor suppressor gene is found on chromosome 22.11 For the sclerosing variant of perineurioma, rearrangements or deletions of chromosome 10q have been described. A study of 14 soft tissue/extraneural perineuriomas using whole-exome sequencing and single nucleotide polymorphism array showed 6 cases of recurrent chromosome 22q deletions containing the NF2 locus and 4 cases with a previously unreported finding of chromosome 17q deletions containing the NF1 locus that were mutually exclusive events in all but 1 case.12 Although perineuriomas can harbor NF1 or NF2 mutations, perineuriomas are not considered to be associated with neurofibromatosis type 1 or 2 (NF1 or NF2, respectively). Patients with NF1 or NF2 and perineurioma are exceedingly rare. One pediatric patient with both soft tissue perineurioma and NF1 has been reported in the literature.13
Differential Diagnosis—Perineuriomas should be distinguished from other benign neural neoplasms of the skin and soft tissue. Commonly considered in the differential diagnosis is schwannoma and neurofibroma. Schwannomas are encapsulated epineurial nerve sheath tumors comprised of a neoplastic proliferation of Schwann cells. Schwannomas morphologically differ from perineuriomas because of the presence of the hypercellular Antoni A with Verocay bodies and the hypocellular myxoid Antoni B patterns of spindle cells with elongated wavy nuclei and tapered ends. Other features include hyalinized vessels, hemosiderin deposition, cystic degeneration, and/or degenerative atypia.3,14 Importantly, the constituent cells of schwannomas are positive for S-100 and SOX10 and negative for EMA.3 Neurofibromas consist of fascicles and whorls of Schwann cells in a background myxoid stroma with scattered mast cells, lymphocytes, fibroblasts, and perineurial cells. Similar to schwannomas, neurofibromas also are positive for S-100 and negative for EMA.3,14 Neurofibromas can have either a somatic or germline mutation of the biallelic NF1 gene on chromosome 17q11.2 with subsequent loss of protein neurofibromin activity.15 Less common but still a consideration are the hybrid peripheral nerve sheath tumors that may present with a biphasic or intermingled morphology. Combinations include neurofibroma-schwannoma, schwannoma-perineurioma, and neurofibroma-perineurioma. The hybrid schwannoma-perineurioma has a mixture of thin and plump spindle cells with tapered nuclei as well as patchy S-100 positivity corresponding to schwannian areas. Similarly, S-100 will highlight the wavy Schwann cells in neurofibroma-perineurioma as well as CD34-highlighting fibroblasts.7,15 In both aforementioned hybrid tumors, EMA will be positive in the perineurial areas. Another potential diagnostic consideration that can occur in both pediatric and adult populations is dermatofibrosarcoma protuberans (DFSP), which is comprised of a dermal proliferation of monomorphic fusiform spindle cells. Although both perineuriomas and DFSP can have a storiform architecture, DFSP is more asymmetric and infiltrative. Dermatofibrosarcoma protuberans is recognized in areas of individual adipocyte trapping, referred to as honeycombing. Dermatofibrosarcoma protuberans typically does not express EMA, though the sclerosing variant of DFSP has been reported to sometimes demonstrate focal EMA reactivity.11,14,16 For morphologically challenging cases, cytogenetic studies will show t(17;22) translocation fusing the COL1A1 and PDGFRB genes.16 Finally, for subcutaneous or deep-seated tumors, one also may consider other mesenchymal neoplasms, including solitary fibrous tumor, low-grade fibromyxoid sarcoma, or low-grade malignant peripheral nerve sheath tumor (MPNST).11
Management—Perineuriomas are considered benign. The presence of mitotic figures, pleomorphism, and degenerative nuclear atypia akin to ancient change, as seen in ancient schwannoma, does not affect their benign clinical behavior. Treatment of a perineurioma typically is surgical excision with conservative margins and minimal chance of recurrence.1,11 So-called malignant perineuriomas are better classified as MPNSTs with perineural differentiation or perineurial MPNST. They also are positive for EMA and may be distinguished from perineurioma by the presence of major atypia and an infiltrative growth pattern.17,18
Considerations in the Pediatric Population—Few pediatric soft tissue perineuriomas have been reported. A clinicopathologic analysis by Hornick and Fletcher1 of patients with soft tissue perineurioma showed that only 6 of 81 patients were younger than 20 years. The youngest reported case of perineurioma occurred as an extraneural perineurioma on the scalp in an infant.19 Only 1 soft tissue perineural MPNST has been reported in the pediatric population, arising on the face of an 11-year-old boy. In a case series of 11 pediatric perineuriomas, including extraneural and intraneural, there was no evidence of recurrence or metastasis at follow-up.4
Conclusion
Perineuriomas are rare benign peripheral nerve sheath tumors with unique histologic and immunohistochemical features. Soft tissue perineuriomas in the pediatric population are an important diagnostic consideration, especially for the pediatrician or dermatologist when encountering a well-circumscribed nodular soft tissue lesion of the extremity or when encountering a neural-appearing tumor in the subcutaneous tissue.
Acknowledgment—We would like to thank Christopher Fletcher, MD (Boston, Massachusetts), for his expertise in outside consultation for patient 1.
- Hornick J, Fletcher C. Soft tissue perineurioma. Am J Surg Pathol. 2005;29:845-858.
- Tsang WY, Chan JK, Chow LT, et al. Perineurioma: an uncommon soft tissue neoplasm distinct from localized hypertrophic neuropathy and neurofibroma. Am J Surg Pathol. 1992;16:756-763.
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Balarezo FS, Muller RC, Weiss RG, et al. Soft tissue perineuriomas in children: report of three cases and review of the literature. Pediatr Dev Pathol. 2003;6:137-141. Published correction appears in Pediatr Dev Pathol. 2003;6:following 364.
- Macarenco R, Ellinger F, Oliveira A. Perineurioma: a distinctive and underrecognized peripheral nerve sheath neoplasm. Arch Pathol Lab Med. 2007;131:625-636.
- Agaimy A, Buslei R, Coras R, et al. Comparative study of soft tissue perineurioma and meningioma using a five-marker immunohistochemical panel. Histopathology. 2014;65:60-70.
- Greenson JK, Hornick JL, Longacre TA, et al. Sternberg’s Diagnostic Surgical Pathology. Wolters Kluwer; 2015.
- Folpe A, Billings S, McKenney J, et al. Expression of claudin-1, a recently described tight junction-associated protein, distinguishes soft tissue perineurioma from potential mimics. Am J Surg Pathol. 2002;26:1620-1626.
- Hirose T, Tani T, Shimada T, et al. Immunohistochemical demonstration of EMA/Glut1-positive perineurial cells and CD34-positive fibroblastic cells in peripheral nerve sheath tumors. Mod Pathol. 2003;16:293-298.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. Perineurioma. WHO Classification of Tumours of Soft Tissue and Bone. IARC Press; 2013:176-178.
- Hornick JL. Practical Soft Tissue Pathology: A Diagnostic Approach. Elsevier Saunders; 2013.
- Carter JM, Wu Y, Blessing MM, et al. Recurrent genomic alterations in soft tissue perineuriomas. Am J Surg Pathol. 2018;42:1708-1714.
- Al-Adnani M. Soft tissue perineurioma in a child with neurofibromatosis type 1: a case report and review of the literature. Pediatr Dev Pathol. 2017;20:444-448.
- Reddy VB, David O, Spitz DJ, et al. Gattuso’s Differential Diagnosis in Surgical Pathology. Elsevier Saunders; 2022.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Abdaljaleel MY, North JP. Sclerosing dermatofibrosarcoma protuberans shows significant overlap with sclerotic fibroma in both routine and immunohistochemical analysis: a potential diagnostic pitfall. Am J Dermatopathol. 2017;39:83-88.
- Rosenberg AS, Langee CL, Stevens GL, et al. Malignant peripheral nerve sheath tumor with perineurial differentiation: “malignant perineurioma.” J Cutan Pathol. 2002;29:362-367.
- Mitchell A, Scheithauer BW, Doyon J, et al. Malignant perineurioma (malignant peripheral nerve sheath tumor with perineural differentiation). Clin Neuropathol. 2012;31:424-429.
- Duhan A, Rana P, Beniwal K, et al. Perineurioma of scalp in an infant: a case report with short review of literature. Asian J Neurosurg. 2016;11:81-83.
Perineuriomas are benign, slow-growing tumors derived from perineurial cells,1 which form the structurally supportive perineurium that surrounds individual nerve fascicles.2,3 Perineuriomas are classified into 2 main forms: intraneural or extraneural.4 Intraneural perineuriomas are found within the border of the peripheral nerve,5 while extraneural perineuriomas usually are found in soft tissue and skin. Extraneural perineuriomas can be further classified into variants based on their histologic appearance, including reticular, sclerosing, and plexiform subtypes. Extraneural perineuriomas usually present on the extremities or trunk of young to middle-aged adults as a well-circumscribed, painless, subcutaneous masses.1 These tumors are especially unusual in children.4 We present 2 extraneural perineurioma cases in children, and we review the pertinent diagnostic features of perineurioma as well as the presentation in the pediatric population.

Case Reports
Patient 1—A 10-year-old boy with a history of cerebral palsy and related comorbidities presented to the clinic for evaluation of a lesion on the thigh with no associated pain, irritation, erythema, or drainage. Physical examination revealed a soft, pedunculated, mobile nodule on the right medial thigh. An elliptical excision was performed. Gross examination demonstrated a 2.0×2.0×1.8-cm polypoid nodule. Histologic examination showed a dermal-based proliferation of bland spindle cells (Figure 1). The cytomorphology was characterized by elongated tapering nuclei and many areas with delicate bipolar cytoplasmic processes. The constituent cells were arranged in a whorled pattern in a variably myxoid to collagenous stroma. The tumor cells were multifocally positive for CD34; focally positive for smooth muscle actin (SMA); and negative for S-100, epithelial membrane antigen (EMA), GLUT1, claudin-1, STAT6, and desmin. Rb protein was intact. The CD34 immunostain highlighted the cytoplasmic processes. Electron microscopy was performed because the immunohistochemical results were nonspecific despite the favorable histologic features for perineurioma and showed pinocytic vesicles with delicate cytoplasmic processes, characteristic of perineurioma (Figure 2). Follow-up visits were related to the management of multiple comorbidities; no known recurrence of the lesion was documented.

Patient 2—A 15-year-old adolescent boy with no notable medical history presented to the pediatric clinic for a bump on the right upper arm of 4 to 5 months’ duration. He did not recall an injury to the area and denied change in size, redness, bruising, or pain of the lesion. Ultrasonography demonstrated a 2.6×2.3×1.3-cm hypoechoic and slightly heterogeneous, well-circumscribed, subcutaneous mass with internal vascularity. The patient was then referred to a pediatric surgeon. The clinical differential included a lipoma, lymphadenopathy, or sebaceous cyst. An excision was performed. Gross inspection demonstrated a 7-g, 2.8×2.6×1.8-cm, homogeneous, tan-pink, rubbery nodule with minimal surrounding soft tissue. Histologic examination showed a bland proliferation of spindle cells with storiform and whorled patterns (Figure 3). No notable nuclear atypia or necrosis was identified. The tumor cells were focally positive for EMA (Figure 4), claudin-1, and CD34 and negative for S-100, SOX10, GLUT1, desmin, STAT6, pankeratin AE1/AE3, and SMA. The diagnosis of perineurioma was rendered. No recurrence of the lesion was appreciated clinically on a 6-month follow-up examination.

Comment
Characteristics of Perineuriomas—On gross evaluation, perineuriomas are firm, gray-white, and well circumscribed but not encapsulated. Histologically, perineuriomas can have a storiform, whorled, or lamellar pattern of spindle cells. Perivascular whorls can be a histologic clue. The spindle cells are bland appearing and typically are elongated and slender but can appear slightly ovoid and plump. The background stroma can be myxoid, collagenous, or mixed. There usually is no atypia, and mitotic figures are rare.2,3,6,7 Intraneural perineuriomas vary architecturally in that they display a unique onion bulb–like appearance in which whorls of cytoplasmic material of variable sizes surround central axons.3

Diagnosis—The diagnosis of perineuriomas usually requires characteristic immunohistochemical and sometimes ultrastructural features. Perineuriomas are positive for EMA and GLUT1 and variable for CD34.6 Approximately 20% to 91% will be positive for claudin-1, a tight junction protein associated with perineuriomas.8 Of note, EMA and GLUT1 usually are positive in both neoplastic and nonneoplastic perineurial cells.9,10 Occasionally, these tumors can be focally positive for SMA and negative for S-100 and glial fibrillary acidic protein. The bipolar, thin, delicate, cytoplasmic processes with long-tapering nuclei may be easier to appreciate on electron microscopy than on conventional light microscopy. In addition, the cells contain pinocytotic vesicles and a discontinuous external lamina, which may be helpful for diagnosis.10
Genetics—Genetic alterations in perineurioma continue to be elucidated. Although many soft tissue perineuriomas possess deletion of chromosome 22q material, this is not a consistent finding and is not pathognomonic. Notably, the NF2 tumor suppressor gene is found on chromosome 22.11 For the sclerosing variant of perineurioma, rearrangements or deletions of chromosome 10q have been described. A study of 14 soft tissue/extraneural perineuriomas using whole-exome sequencing and single nucleotide polymorphism array showed 6 cases of recurrent chromosome 22q deletions containing the NF2 locus and 4 cases with a previously unreported finding of chromosome 17q deletions containing the NF1 locus that were mutually exclusive events in all but 1 case.12 Although perineuriomas can harbor NF1 or NF2 mutations, perineuriomas are not considered to be associated with neurofibromatosis type 1 or 2 (NF1 or NF2, respectively). Patients with NF1 or NF2 and perineurioma are exceedingly rare. One pediatric patient with both soft tissue perineurioma and NF1 has been reported in the literature.13
Differential Diagnosis—Perineuriomas should be distinguished from other benign neural neoplasms of the skin and soft tissue. Commonly considered in the differential diagnosis is schwannoma and neurofibroma. Schwannomas are encapsulated epineurial nerve sheath tumors comprised of a neoplastic proliferation of Schwann cells. Schwannomas morphologically differ from perineuriomas because of the presence of the hypercellular Antoni A with Verocay bodies and the hypocellular myxoid Antoni B patterns of spindle cells with elongated wavy nuclei and tapered ends. Other features include hyalinized vessels, hemosiderin deposition, cystic degeneration, and/or degenerative atypia.3,14 Importantly, the constituent cells of schwannomas are positive for S-100 and SOX10 and negative for EMA.3 Neurofibromas consist of fascicles and whorls of Schwann cells in a background myxoid stroma with scattered mast cells, lymphocytes, fibroblasts, and perineurial cells. Similar to schwannomas, neurofibromas also are positive for S-100 and negative for EMA.3,14 Neurofibromas can have either a somatic or germline mutation of the biallelic NF1 gene on chromosome 17q11.2 with subsequent loss of protein neurofibromin activity.15 Less common but still a consideration are the hybrid peripheral nerve sheath tumors that may present with a biphasic or intermingled morphology. Combinations include neurofibroma-schwannoma, schwannoma-perineurioma, and neurofibroma-perineurioma. The hybrid schwannoma-perineurioma has a mixture of thin and plump spindle cells with tapered nuclei as well as patchy S-100 positivity corresponding to schwannian areas. Similarly, S-100 will highlight the wavy Schwann cells in neurofibroma-perineurioma as well as CD34-highlighting fibroblasts.7,15 In both aforementioned hybrid tumors, EMA will be positive in the perineurial areas. Another potential diagnostic consideration that can occur in both pediatric and adult populations is dermatofibrosarcoma protuberans (DFSP), which is comprised of a dermal proliferation of monomorphic fusiform spindle cells. Although both perineuriomas and DFSP can have a storiform architecture, DFSP is more asymmetric and infiltrative. Dermatofibrosarcoma protuberans is recognized in areas of individual adipocyte trapping, referred to as honeycombing. Dermatofibrosarcoma protuberans typically does not express EMA, though the sclerosing variant of DFSP has been reported to sometimes demonstrate focal EMA reactivity.11,14,16 For morphologically challenging cases, cytogenetic studies will show t(17;22) translocation fusing the COL1A1 and PDGFRB genes.16 Finally, for subcutaneous or deep-seated tumors, one also may consider other mesenchymal neoplasms, including solitary fibrous tumor, low-grade fibromyxoid sarcoma, or low-grade malignant peripheral nerve sheath tumor (MPNST).11
Management—Perineuriomas are considered benign. The presence of mitotic figures, pleomorphism, and degenerative nuclear atypia akin to ancient change, as seen in ancient schwannoma, does not affect their benign clinical behavior. Treatment of a perineurioma typically is surgical excision with conservative margins and minimal chance of recurrence.1,11 So-called malignant perineuriomas are better classified as MPNSTs with perineural differentiation or perineurial MPNST. They also are positive for EMA and may be distinguished from perineurioma by the presence of major atypia and an infiltrative growth pattern.17,18
Considerations in the Pediatric Population—Few pediatric soft tissue perineuriomas have been reported. A clinicopathologic analysis by Hornick and Fletcher1 of patients with soft tissue perineurioma showed that only 6 of 81 patients were younger than 20 years. The youngest reported case of perineurioma occurred as an extraneural perineurioma on the scalp in an infant.19 Only 1 soft tissue perineural MPNST has been reported in the pediatric population, arising on the face of an 11-year-old boy. In a case series of 11 pediatric perineuriomas, including extraneural and intraneural, there was no evidence of recurrence or metastasis at follow-up.4
Conclusion
Perineuriomas are rare benign peripheral nerve sheath tumors with unique histologic and immunohistochemical features. Soft tissue perineuriomas in the pediatric population are an important diagnostic consideration, especially for the pediatrician or dermatologist when encountering a well-circumscribed nodular soft tissue lesion of the extremity or when encountering a neural-appearing tumor in the subcutaneous tissue.
Acknowledgment—We would like to thank Christopher Fletcher, MD (Boston, Massachusetts), for his expertise in outside consultation for patient 1.
Perineuriomas are benign, slow-growing tumors derived from perineurial cells,1 which form the structurally supportive perineurium that surrounds individual nerve fascicles.2,3 Perineuriomas are classified into 2 main forms: intraneural or extraneural.4 Intraneural perineuriomas are found within the border of the peripheral nerve,5 while extraneural perineuriomas usually are found in soft tissue and skin. Extraneural perineuriomas can be further classified into variants based on their histologic appearance, including reticular, sclerosing, and plexiform subtypes. Extraneural perineuriomas usually present on the extremities or trunk of young to middle-aged adults as a well-circumscribed, painless, subcutaneous masses.1 These tumors are especially unusual in children.4 We present 2 extraneural perineurioma cases in children, and we review the pertinent diagnostic features of perineurioma as well as the presentation in the pediatric population.

Case Reports
Patient 1—A 10-year-old boy with a history of cerebral palsy and related comorbidities presented to the clinic for evaluation of a lesion on the thigh with no associated pain, irritation, erythema, or drainage. Physical examination revealed a soft, pedunculated, mobile nodule on the right medial thigh. An elliptical excision was performed. Gross examination demonstrated a 2.0×2.0×1.8-cm polypoid nodule. Histologic examination showed a dermal-based proliferation of bland spindle cells (Figure 1). The cytomorphology was characterized by elongated tapering nuclei and many areas with delicate bipolar cytoplasmic processes. The constituent cells were arranged in a whorled pattern in a variably myxoid to collagenous stroma. The tumor cells were multifocally positive for CD34; focally positive for smooth muscle actin (SMA); and negative for S-100, epithelial membrane antigen (EMA), GLUT1, claudin-1, STAT6, and desmin. Rb protein was intact. The CD34 immunostain highlighted the cytoplasmic processes. Electron microscopy was performed because the immunohistochemical results were nonspecific despite the favorable histologic features for perineurioma and showed pinocytic vesicles with delicate cytoplasmic processes, characteristic of perineurioma (Figure 2). Follow-up visits were related to the management of multiple comorbidities; no known recurrence of the lesion was documented.

Patient 2—A 15-year-old adolescent boy with no notable medical history presented to the pediatric clinic for a bump on the right upper arm of 4 to 5 months’ duration. He did not recall an injury to the area and denied change in size, redness, bruising, or pain of the lesion. Ultrasonography demonstrated a 2.6×2.3×1.3-cm hypoechoic and slightly heterogeneous, well-circumscribed, subcutaneous mass with internal vascularity. The patient was then referred to a pediatric surgeon. The clinical differential included a lipoma, lymphadenopathy, or sebaceous cyst. An excision was performed. Gross inspection demonstrated a 7-g, 2.8×2.6×1.8-cm, homogeneous, tan-pink, rubbery nodule with minimal surrounding soft tissue. Histologic examination showed a bland proliferation of spindle cells with storiform and whorled patterns (Figure 3). No notable nuclear atypia or necrosis was identified. The tumor cells were focally positive for EMA (Figure 4), claudin-1, and CD34 and negative for S-100, SOX10, GLUT1, desmin, STAT6, pankeratin AE1/AE3, and SMA. The diagnosis of perineurioma was rendered. No recurrence of the lesion was appreciated clinically on a 6-month follow-up examination.

Comment
Characteristics of Perineuriomas—On gross evaluation, perineuriomas are firm, gray-white, and well circumscribed but not encapsulated. Histologically, perineuriomas can have a storiform, whorled, or lamellar pattern of spindle cells. Perivascular whorls can be a histologic clue. The spindle cells are bland appearing and typically are elongated and slender but can appear slightly ovoid and plump. The background stroma can be myxoid, collagenous, or mixed. There usually is no atypia, and mitotic figures are rare.2,3,6,7 Intraneural perineuriomas vary architecturally in that they display a unique onion bulb–like appearance in which whorls of cytoplasmic material of variable sizes surround central axons.3

Diagnosis—The diagnosis of perineuriomas usually requires characteristic immunohistochemical and sometimes ultrastructural features. Perineuriomas are positive for EMA and GLUT1 and variable for CD34.6 Approximately 20% to 91% will be positive for claudin-1, a tight junction protein associated with perineuriomas.8 Of note, EMA and GLUT1 usually are positive in both neoplastic and nonneoplastic perineurial cells.9,10 Occasionally, these tumors can be focally positive for SMA and negative for S-100 and glial fibrillary acidic protein. The bipolar, thin, delicate, cytoplasmic processes with long-tapering nuclei may be easier to appreciate on electron microscopy than on conventional light microscopy. In addition, the cells contain pinocytotic vesicles and a discontinuous external lamina, which may be helpful for diagnosis.10
Genetics—Genetic alterations in perineurioma continue to be elucidated. Although many soft tissue perineuriomas possess deletion of chromosome 22q material, this is not a consistent finding and is not pathognomonic. Notably, the NF2 tumor suppressor gene is found on chromosome 22.11 For the sclerosing variant of perineurioma, rearrangements or deletions of chromosome 10q have been described. A study of 14 soft tissue/extraneural perineuriomas using whole-exome sequencing and single nucleotide polymorphism array showed 6 cases of recurrent chromosome 22q deletions containing the NF2 locus and 4 cases with a previously unreported finding of chromosome 17q deletions containing the NF1 locus that were mutually exclusive events in all but 1 case.12 Although perineuriomas can harbor NF1 or NF2 mutations, perineuriomas are not considered to be associated with neurofibromatosis type 1 or 2 (NF1 or NF2, respectively). Patients with NF1 or NF2 and perineurioma are exceedingly rare. One pediatric patient with both soft tissue perineurioma and NF1 has been reported in the literature.13
Differential Diagnosis—Perineuriomas should be distinguished from other benign neural neoplasms of the skin and soft tissue. Commonly considered in the differential diagnosis is schwannoma and neurofibroma. Schwannomas are encapsulated epineurial nerve sheath tumors comprised of a neoplastic proliferation of Schwann cells. Schwannomas morphologically differ from perineuriomas because of the presence of the hypercellular Antoni A with Verocay bodies and the hypocellular myxoid Antoni B patterns of spindle cells with elongated wavy nuclei and tapered ends. Other features include hyalinized vessels, hemosiderin deposition, cystic degeneration, and/or degenerative atypia.3,14 Importantly, the constituent cells of schwannomas are positive for S-100 and SOX10 and negative for EMA.3 Neurofibromas consist of fascicles and whorls of Schwann cells in a background myxoid stroma with scattered mast cells, lymphocytes, fibroblasts, and perineurial cells. Similar to schwannomas, neurofibromas also are positive for S-100 and negative for EMA.3,14 Neurofibromas can have either a somatic or germline mutation of the biallelic NF1 gene on chromosome 17q11.2 with subsequent loss of protein neurofibromin activity.15 Less common but still a consideration are the hybrid peripheral nerve sheath tumors that may present with a biphasic or intermingled morphology. Combinations include neurofibroma-schwannoma, schwannoma-perineurioma, and neurofibroma-perineurioma. The hybrid schwannoma-perineurioma has a mixture of thin and plump spindle cells with tapered nuclei as well as patchy S-100 positivity corresponding to schwannian areas. Similarly, S-100 will highlight the wavy Schwann cells in neurofibroma-perineurioma as well as CD34-highlighting fibroblasts.7,15 In both aforementioned hybrid tumors, EMA will be positive in the perineurial areas. Another potential diagnostic consideration that can occur in both pediatric and adult populations is dermatofibrosarcoma protuberans (DFSP), which is comprised of a dermal proliferation of monomorphic fusiform spindle cells. Although both perineuriomas and DFSP can have a storiform architecture, DFSP is more asymmetric and infiltrative. Dermatofibrosarcoma protuberans is recognized in areas of individual adipocyte trapping, referred to as honeycombing. Dermatofibrosarcoma protuberans typically does not express EMA, though the sclerosing variant of DFSP has been reported to sometimes demonstrate focal EMA reactivity.11,14,16 For morphologically challenging cases, cytogenetic studies will show t(17;22) translocation fusing the COL1A1 and PDGFRB genes.16 Finally, for subcutaneous or deep-seated tumors, one also may consider other mesenchymal neoplasms, including solitary fibrous tumor, low-grade fibromyxoid sarcoma, or low-grade malignant peripheral nerve sheath tumor (MPNST).11
Management—Perineuriomas are considered benign. The presence of mitotic figures, pleomorphism, and degenerative nuclear atypia akin to ancient change, as seen in ancient schwannoma, does not affect their benign clinical behavior. Treatment of a perineurioma typically is surgical excision with conservative margins and minimal chance of recurrence.1,11 So-called malignant perineuriomas are better classified as MPNSTs with perineural differentiation or perineurial MPNST. They also are positive for EMA and may be distinguished from perineurioma by the presence of major atypia and an infiltrative growth pattern.17,18
Considerations in the Pediatric Population—Few pediatric soft tissue perineuriomas have been reported. A clinicopathologic analysis by Hornick and Fletcher1 of patients with soft tissue perineurioma showed that only 6 of 81 patients were younger than 20 years. The youngest reported case of perineurioma occurred as an extraneural perineurioma on the scalp in an infant.19 Only 1 soft tissue perineural MPNST has been reported in the pediatric population, arising on the face of an 11-year-old boy. In a case series of 11 pediatric perineuriomas, including extraneural and intraneural, there was no evidence of recurrence or metastasis at follow-up.4
Conclusion
Perineuriomas are rare benign peripheral nerve sheath tumors with unique histologic and immunohistochemical features. Soft tissue perineuriomas in the pediatric population are an important diagnostic consideration, especially for the pediatrician or dermatologist when encountering a well-circumscribed nodular soft tissue lesion of the extremity or when encountering a neural-appearing tumor in the subcutaneous tissue.
Acknowledgment—We would like to thank Christopher Fletcher, MD (Boston, Massachusetts), for his expertise in outside consultation for patient 1.
- Hornick J, Fletcher C. Soft tissue perineurioma. Am J Surg Pathol. 2005;29:845-858.
- Tsang WY, Chan JK, Chow LT, et al. Perineurioma: an uncommon soft tissue neoplasm distinct from localized hypertrophic neuropathy and neurofibroma. Am J Surg Pathol. 1992;16:756-763.
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Balarezo FS, Muller RC, Weiss RG, et al. Soft tissue perineuriomas in children: report of three cases and review of the literature. Pediatr Dev Pathol. 2003;6:137-141. Published correction appears in Pediatr Dev Pathol. 2003;6:following 364.
- Macarenco R, Ellinger F, Oliveira A. Perineurioma: a distinctive and underrecognized peripheral nerve sheath neoplasm. Arch Pathol Lab Med. 2007;131:625-636.
- Agaimy A, Buslei R, Coras R, et al. Comparative study of soft tissue perineurioma and meningioma using a five-marker immunohistochemical panel. Histopathology. 2014;65:60-70.
- Greenson JK, Hornick JL, Longacre TA, et al. Sternberg’s Diagnostic Surgical Pathology. Wolters Kluwer; 2015.
- Folpe A, Billings S, McKenney J, et al. Expression of claudin-1, a recently described tight junction-associated protein, distinguishes soft tissue perineurioma from potential mimics. Am J Surg Pathol. 2002;26:1620-1626.
- Hirose T, Tani T, Shimada T, et al. Immunohistochemical demonstration of EMA/Glut1-positive perineurial cells and CD34-positive fibroblastic cells in peripheral nerve sheath tumors. Mod Pathol. 2003;16:293-298.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. Perineurioma. WHO Classification of Tumours of Soft Tissue and Bone. IARC Press; 2013:176-178.
- Hornick JL. Practical Soft Tissue Pathology: A Diagnostic Approach. Elsevier Saunders; 2013.
- Carter JM, Wu Y, Blessing MM, et al. Recurrent genomic alterations in soft tissue perineuriomas. Am J Surg Pathol. 2018;42:1708-1714.
- Al-Adnani M. Soft tissue perineurioma in a child with neurofibromatosis type 1: a case report and review of the literature. Pediatr Dev Pathol. 2017;20:444-448.
- Reddy VB, David O, Spitz DJ, et al. Gattuso’s Differential Diagnosis in Surgical Pathology. Elsevier Saunders; 2022.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Abdaljaleel MY, North JP. Sclerosing dermatofibrosarcoma protuberans shows significant overlap with sclerotic fibroma in both routine and immunohistochemical analysis: a potential diagnostic pitfall. Am J Dermatopathol. 2017;39:83-88.
- Rosenberg AS, Langee CL, Stevens GL, et al. Malignant peripheral nerve sheath tumor with perineurial differentiation: “malignant perineurioma.” J Cutan Pathol. 2002;29:362-367.
- Mitchell A, Scheithauer BW, Doyon J, et al. Malignant perineurioma (malignant peripheral nerve sheath tumor with perineural differentiation). Clin Neuropathol. 2012;31:424-429.
- Duhan A, Rana P, Beniwal K, et al. Perineurioma of scalp in an infant: a case report with short review of literature. Asian J Neurosurg. 2016;11:81-83.
- Hornick J, Fletcher C. Soft tissue perineurioma. Am J Surg Pathol. 2005;29:845-858.
- Tsang WY, Chan JK, Chow LT, et al. Perineurioma: an uncommon soft tissue neoplasm distinct from localized hypertrophic neuropathy and neurofibroma. Am J Surg Pathol. 1992;16:756-763.
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Balarezo FS, Muller RC, Weiss RG, et al. Soft tissue perineuriomas in children: report of three cases and review of the literature. Pediatr Dev Pathol. 2003;6:137-141. Published correction appears in Pediatr Dev Pathol. 2003;6:following 364.
- Macarenco R, Ellinger F, Oliveira A. Perineurioma: a distinctive and underrecognized peripheral nerve sheath neoplasm. Arch Pathol Lab Med. 2007;131:625-636.
- Agaimy A, Buslei R, Coras R, et al. Comparative study of soft tissue perineurioma and meningioma using a five-marker immunohistochemical panel. Histopathology. 2014;65:60-70.
- Greenson JK, Hornick JL, Longacre TA, et al. Sternberg’s Diagnostic Surgical Pathology. Wolters Kluwer; 2015.
- Folpe A, Billings S, McKenney J, et al. Expression of claudin-1, a recently described tight junction-associated protein, distinguishes soft tissue perineurioma from potential mimics. Am J Surg Pathol. 2002;26:1620-1626.
- Hirose T, Tani T, Shimada T, et al. Immunohistochemical demonstration of EMA/Glut1-positive perineurial cells and CD34-positive fibroblastic cells in peripheral nerve sheath tumors. Mod Pathol. 2003;16:293-298.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. Perineurioma. WHO Classification of Tumours of Soft Tissue and Bone. IARC Press; 2013:176-178.
- Hornick JL. Practical Soft Tissue Pathology: A Diagnostic Approach. Elsevier Saunders; 2013.
- Carter JM, Wu Y, Blessing MM, et al. Recurrent genomic alterations in soft tissue perineuriomas. Am J Surg Pathol. 2018;42:1708-1714.
- Al-Adnani M. Soft tissue perineurioma in a child with neurofibromatosis type 1: a case report and review of the literature. Pediatr Dev Pathol. 2017;20:444-448.
- Reddy VB, David O, Spitz DJ, et al. Gattuso’s Differential Diagnosis in Surgical Pathology. Elsevier Saunders; 2022.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Abdaljaleel MY, North JP. Sclerosing dermatofibrosarcoma protuberans shows significant overlap with sclerotic fibroma in both routine and immunohistochemical analysis: a potential diagnostic pitfall. Am J Dermatopathol. 2017;39:83-88.
- Rosenberg AS, Langee CL, Stevens GL, et al. Malignant peripheral nerve sheath tumor with perineurial differentiation: “malignant perineurioma.” J Cutan Pathol. 2002;29:362-367.
- Mitchell A, Scheithauer BW, Doyon J, et al. Malignant perineurioma (malignant peripheral nerve sheath tumor with perineural differentiation). Clin Neuropathol. 2012;31:424-429.
- Duhan A, Rana P, Beniwal K, et al. Perineurioma of scalp in an infant: a case report with short review of literature. Asian J Neurosurg. 2016;11:81-83.
Practice Points
- Perineuriomas are rare benign peripheral nerve sheath tumors that most commonly occur in young to middle-aged adults but rarely can present in children.
- Immunohistochemically, perineuriomas show positive staining with epithelial membrane antigen, GLUT1, claudin-1, and frequently with CD34; they are negative for S-100 and glial fibrillary acidic protein.
- Perineuriomas should be considered in the differential diagnosis in children who present with a well-circumscribed nodular lesion in the subcutaneous tissue.
Acquired Acrodermatitis Enteropathica in an Infant
Acrodermatitis enteropathica (AE) is a rare disorder of zinc metabolism that typically presents in infancy.1 Although it is clinically characterized by acral and periorificial dermatitis, alopecia, and diarrhea, only 20% of cases present with this triad.2 Zinc deficiency in AE can either be acquired or inborn (congenital). Acquired forms can occur from dietary inadequacy or malabsorption, whereas genetic causes are related to an autosomal-recessive disorder affecting zinc transporters.1 We report a case of a 3-month-old female infant with acquired AE who was successfully treated with zinc supplementation over the course of 3 weeks.
Case Report
A 3-month-old female infant presented to the emergency department with a rash of 2 weeks’ duration. She was born full term with no birth complications. The patient’s mother reported that the rash started on the cheeks, then enlarged and spread to the neck, back, and perineum. The patient also had been having diarrhea during this time. She previously had received mupirocin and cephalexin with no response to treatment. Maternal history was negative for lupus, and the mother’s diet consisted of a variety of foods but not many vegetables. The patient was exclusively breastfed, and there was no pertinent history of similar rashes occurring in other family members.
Physical examination revealed the patient had annular and polycyclic, hyperkeratotic, crusted papules and plaques on the cheeks, neck, back, and axillae, as well as the perineum/groin and perianal regions (Figure 1). The differential diagnosis at the time included neonatal lupus, zinc deficiency, and syphilis. Relevant laboratory testing and a shave biopsy of the left axilla were obtained.
Pertinent laboratory findings included a low zinc level (23 μg/dL [reference range, 26–141 μg/dL]), low alkaline phosphatase level (74 U/L [reference range, 94–486 U/L]), and thrombocytosis (826×109/L [reference range, 150–400×109/L). Results for antinuclear antibody and anti–Sjögren syndrome–related antigen A and B antibody testing were negative. A rapid plasma reagin test was nonreactive. Histologic examination revealed psoriasiform hyperplasia with overlying confluent parakeratosis, focal spongiosis, multiple dyskeratotic keratinocytes, and mitotic figures (Figure 2). Ballooning was evident in focal cells in the subcorneal region in addition to an accompanying lymphocytic infiltrate and occasional neutrophils.
The patient was given a 10-mg/mL suspension of elemental zinc and was advised to take 1 mL (10 mg) by mouth twice daily with food. This dosage equated to 3 mg/kg/d. On follow-up 3 weeks later, the skin began to clear (Figure 3). Follow-up laboratory testing showed an increase in zinc (114 μg/dL) and alkaline phosphatase levels (313 U/L). The patient was able to discontinue the zinc supplementation, and follow-up during the next year revealed no recurrence.
Comment
Etiology of AE—Acrodermatitis enteropathica was first identified in 1942 as an acral rash associated with diarrhea3; in 1973, Barnes and Moynahan4 discovered zinc deficiency as a causal agent for these findings. The causes of AE are further subclassified as either an acquired or inborn etiology. Congenital causes commonly are seen in infants within the first few months of life, whereas acquired forms are seen at any age. Acquired forms in infants can occur from failure of the mother to secrete zinc in breast milk, low maternal serum zinc levels, or other reasons causing low nutritional intake. A single mutation in the SLC30A2 gene has been found to markedly reduce zinc concentrations in breast milk, thus causing zinc deficiency in breastfed infants.5 Other acquired forms can be caused by malabsorption, sometimes after surgery such as intestinal bypass or from intravenous nutrition without sufficient zinc.1 The congenital form of AE is an autosomal-recessive disorder occurring from mutations in the SLC39A4 gene located on band 8q24.3. Affected individuals have a decreased ability to absorb zinc in the small intestine because of defects in zinc transporters ZIP and ZnT.6 Based on our patient’s laboratory findings and history, it is believed that the zinc deficiency was acquired, as the condition normalized with repletion and has not required any supplementation in the year of follow-up. In addition, the absence of a pertinent family history supported an acquired diagnosis, which has various etiologies, whereas the congenital form primarily is a genetic disease.
Management—Treatment of AE includes supplementation with oral elemental zinc; however, there are scant evidence-based recommendations on the exact dose of zinc to be given. Generally, the recommended amount is 3 mg/kg/d.8 For individuals with the congenital form of AE, lifelong zinc supplementation is additionally recommended.9 It is important to recognize this presentation because the patient can develop worsening irritability, severe diarrhea, nail dystrophy, hair loss, immune dysfunction, and numerous ophthalmic disorders if left untreated. Acute zinc toxicity due to excess administration is rare, with symptoms of nausea and vomiting occurring with dosages of 50 to 100 mg/d. Additionally, dosages of up to 70 mg twice weekly have been provided without any toxic effect.10 In our case, 3 mg/kg/d of oral zinc supplementation proved to be effective in resolving the patient’s symptoms of acquired zinc deficiency.
Differential Diagnosis—It is important to note that deficiencies of other nutrients may present as an AE-like eruption called acrodermatitis dysmetabolica (AD). Both diseases may present with the triad of dermatitis, alopecia, and diarrhea; however, AD is associated with inborn errors of metabolism. There have been cases that describe AD in patients with a zinc deficiency in conjunction with a deficiency of branched-chain amino acids.11,12 It is important to consider AD in the differential diagnosis of an AE eruption, especially in the context of a metabolic disorder, as it may affect the treatment plan. One case described the dermatitis of AD as not responding to zinc supplementation alone, while another described improvement after increasing an isoleucine supplementation dose.11,12
Other considerations in the differential diagnoses include AE-like conditions such as biotinidase deficiency, multiple carboxylase deficiency, and essential fatty acid deficiency. An AE-like condition may present with the triad of dermatitis, alopecia, and diarrhea. However, unlike in true AE, zinc and alkaline phosphatase levels tend to be normal in these conditions. Other features seen in AE-like conditions depend on the underlying cause but often include failure to thrive, neurologic defects, ophthalmic abnormalities, and metabolic abnormalities.13
- Acrodermatitis enteropathica. National Organization for Rare Disorders. Accessed October 16, 2022. https://rarediseases.org/rare-diseases/acrodermatitis-enteropathica/
- Perafán-Riveros C, França LFS, Alves ACF, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- Danbolt N. Acrodermatitis enteropathica. Br J Dermatol. 1979;100:37-40.
- Barnes PM, Moynahan EJ. Zinc deficiency in acrodermatitis enteropathica: multiple dietary intolerance treated with synthetic diet. Proc R Soc Med. 1973;66:327-329.
- Lee S, Zhou Y, Gill DL, et al. A genetic variant in SLC30A2 causes breast dysfunction during lactation by inducing ER stress, oxidative stress and epithelial barrier defects. Sci Rep. 2018;8:3542.
- Kaur S, Sangwan A, Sahu P, et al. Clinical variants of acrodermatitis enteropathica and its co-relation with genetics. Indian J Paediatr Dermatol. 2016;17:35-37.
- Dela Rosa KM, James WD. Acrodermatitis enteropathica workup. Medscape. Updated June 4, 2021. Accessed October 16, 2022. https://emedicine.medscape.com/article/1102575-workup#showall
- Ngan V, Gangakhedkar A, Oakley A. Acrodermatitis enteropathica. DermNet. Accessed October 16, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica/
- Ranugha P, Sethi P, Veeranna S. Acrodermatitis enteropathica: the need for sustained high dose zinc supplementation. Dermatol Online J. 2018;24:13030/qt1w9002sr.
- Larson CP, Roy SK, Khan AI, et al. Zinc treatment to under-five children: applications to improve child survival and reduce burden of disease. J Health Popul Nutr. 2008;26:356-365.
- Samady JA, Schwartz RA, Shih LY, et al. Acrodermatitis enteropathica-like eruption in an infant with nonketotic hyperglycinemia. J Dermatol. 2000;27:604-608.
- Flores K, Chikowski R, Morrell DS. Acrodermatitis dysmetabolica in an infant with maple syrup urine disease. Clin Exp Dermatol. 2016;41:651-654.
- Jones L, Oakley A. Acrodermatitis enteropathica-like conditions. DermNet. Accessed August 30, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica-like-conditions
Acrodermatitis enteropathica (AE) is a rare disorder of zinc metabolism that typically presents in infancy.1 Although it is clinically characterized by acral and periorificial dermatitis, alopecia, and diarrhea, only 20% of cases present with this triad.2 Zinc deficiency in AE can either be acquired or inborn (congenital). Acquired forms can occur from dietary inadequacy or malabsorption, whereas genetic causes are related to an autosomal-recessive disorder affecting zinc transporters.1 We report a case of a 3-month-old female infant with acquired AE who was successfully treated with zinc supplementation over the course of 3 weeks.
Case Report
A 3-month-old female infant presented to the emergency department with a rash of 2 weeks’ duration. She was born full term with no birth complications. The patient’s mother reported that the rash started on the cheeks, then enlarged and spread to the neck, back, and perineum. The patient also had been having diarrhea during this time. She previously had received mupirocin and cephalexin with no response to treatment. Maternal history was negative for lupus, and the mother’s diet consisted of a variety of foods but not many vegetables. The patient was exclusively breastfed, and there was no pertinent history of similar rashes occurring in other family members.
Physical examination revealed the patient had annular and polycyclic, hyperkeratotic, crusted papules and plaques on the cheeks, neck, back, and axillae, as well as the perineum/groin and perianal regions (Figure 1). The differential diagnosis at the time included neonatal lupus, zinc deficiency, and syphilis. Relevant laboratory testing and a shave biopsy of the left axilla were obtained.

Pertinent laboratory findings included a low zinc level (23 μg/dL [reference range, 26–141 μg/dL]), low alkaline phosphatase level (74 U/L [reference range, 94–486 U/L]), and thrombocytosis (826×109/L [reference range, 150–400×109/L). Results for antinuclear antibody and anti–Sjögren syndrome–related antigen A and B antibody testing were negative. A rapid plasma reagin test was nonreactive. Histologic examination revealed psoriasiform hyperplasia with overlying confluent parakeratosis, focal spongiosis, multiple dyskeratotic keratinocytes, and mitotic figures (Figure 2). Ballooning was evident in focal cells in the subcorneal region in addition to an accompanying lymphocytic infiltrate and occasional neutrophils.

The patient was given a 10-mg/mL suspension of elemental zinc and was advised to take 1 mL (10 mg) by mouth twice daily with food. This dosage equated to 3 mg/kg/d. On follow-up 3 weeks later, the skin began to clear (Figure 3). Follow-up laboratory testing showed an increase in zinc (114 μg/dL) and alkaline phosphatase levels (313 U/L). The patient was able to discontinue the zinc supplementation, and follow-up during the next year revealed no recurrence.

Comment
Etiology of AE—Acrodermatitis enteropathica was first identified in 1942 as an acral rash associated with diarrhea3; in 1973, Barnes and Moynahan4 discovered zinc deficiency as a causal agent for these findings. The causes of AE are further subclassified as either an acquired or inborn etiology. Congenital causes commonly are seen in infants within the first few months of life, whereas acquired forms are seen at any age. Acquired forms in infants can occur from failure of the mother to secrete zinc in breast milk, low maternal serum zinc levels, or other reasons causing low nutritional intake. A single mutation in the SLC30A2 gene has been found to markedly reduce zinc concentrations in breast milk, thus causing zinc deficiency in breastfed infants.5 Other acquired forms can be caused by malabsorption, sometimes after surgery such as intestinal bypass or from intravenous nutrition without sufficient zinc.1 The congenital form of AE is an autosomal-recessive disorder occurring from mutations in the SLC39A4 gene located on band 8q24.3. Affected individuals have a decreased ability to absorb zinc in the small intestine because of defects in zinc transporters ZIP and ZnT.6 Based on our patient’s laboratory findings and history, it is believed that the zinc deficiency was acquired, as the condition normalized with repletion and has not required any supplementation in the year of follow-up. In addition, the absence of a pertinent family history supported an acquired diagnosis, which has various etiologies, whereas the congenital form primarily is a genetic disease.
Management—Treatment of AE includes supplementation with oral elemental zinc; however, there are scant evidence-based recommendations on the exact dose of zinc to be given. Generally, the recommended amount is 3 mg/kg/d.8 For individuals with the congenital form of AE, lifelong zinc supplementation is additionally recommended.9 It is important to recognize this presentation because the patient can develop worsening irritability, severe diarrhea, nail dystrophy, hair loss, immune dysfunction, and numerous ophthalmic disorders if left untreated. Acute zinc toxicity due to excess administration is rare, with symptoms of nausea and vomiting occurring with dosages of 50 to 100 mg/d. Additionally, dosages of up to 70 mg twice weekly have been provided without any toxic effect.10 In our case, 3 mg/kg/d of oral zinc supplementation proved to be effective in resolving the patient’s symptoms of acquired zinc deficiency.
Differential Diagnosis—It is important to note that deficiencies of other nutrients may present as an AE-like eruption called acrodermatitis dysmetabolica (AD). Both diseases may present with the triad of dermatitis, alopecia, and diarrhea; however, AD is associated with inborn errors of metabolism. There have been cases that describe AD in patients with a zinc deficiency in conjunction with a deficiency of branched-chain amino acids.11,12 It is important to consider AD in the differential diagnosis of an AE eruption, especially in the context of a metabolic disorder, as it may affect the treatment plan. One case described the dermatitis of AD as not responding to zinc supplementation alone, while another described improvement after increasing an isoleucine supplementation dose.11,12
Other considerations in the differential diagnoses include AE-like conditions such as biotinidase deficiency, multiple carboxylase deficiency, and essential fatty acid deficiency. An AE-like condition may present with the triad of dermatitis, alopecia, and diarrhea. However, unlike in true AE, zinc and alkaline phosphatase levels tend to be normal in these conditions. Other features seen in AE-like conditions depend on the underlying cause but often include failure to thrive, neurologic defects, ophthalmic abnormalities, and metabolic abnormalities.13
Acrodermatitis enteropathica (AE) is a rare disorder of zinc metabolism that typically presents in infancy.1 Although it is clinically characterized by acral and periorificial dermatitis, alopecia, and diarrhea, only 20% of cases present with this triad.2 Zinc deficiency in AE can either be acquired or inborn (congenital). Acquired forms can occur from dietary inadequacy or malabsorption, whereas genetic causes are related to an autosomal-recessive disorder affecting zinc transporters.1 We report a case of a 3-month-old female infant with acquired AE who was successfully treated with zinc supplementation over the course of 3 weeks.
Case Report
A 3-month-old female infant presented to the emergency department with a rash of 2 weeks’ duration. She was born full term with no birth complications. The patient’s mother reported that the rash started on the cheeks, then enlarged and spread to the neck, back, and perineum. The patient also had been having diarrhea during this time. She previously had received mupirocin and cephalexin with no response to treatment. Maternal history was negative for lupus, and the mother’s diet consisted of a variety of foods but not many vegetables. The patient was exclusively breastfed, and there was no pertinent history of similar rashes occurring in other family members.
Physical examination revealed the patient had annular and polycyclic, hyperkeratotic, crusted papules and plaques on the cheeks, neck, back, and axillae, as well as the perineum/groin and perianal regions (Figure 1). The differential diagnosis at the time included neonatal lupus, zinc deficiency, and syphilis. Relevant laboratory testing and a shave biopsy of the left axilla were obtained.

Pertinent laboratory findings included a low zinc level (23 μg/dL [reference range, 26–141 μg/dL]), low alkaline phosphatase level (74 U/L [reference range, 94–486 U/L]), and thrombocytosis (826×109/L [reference range, 150–400×109/L). Results for antinuclear antibody and anti–Sjögren syndrome–related antigen A and B antibody testing were negative. A rapid plasma reagin test was nonreactive. Histologic examination revealed psoriasiform hyperplasia with overlying confluent parakeratosis, focal spongiosis, multiple dyskeratotic keratinocytes, and mitotic figures (Figure 2). Ballooning was evident in focal cells in the subcorneal region in addition to an accompanying lymphocytic infiltrate and occasional neutrophils.

The patient was given a 10-mg/mL suspension of elemental zinc and was advised to take 1 mL (10 mg) by mouth twice daily with food. This dosage equated to 3 mg/kg/d. On follow-up 3 weeks later, the skin began to clear (Figure 3). Follow-up laboratory testing showed an increase in zinc (114 μg/dL) and alkaline phosphatase levels (313 U/L). The patient was able to discontinue the zinc supplementation, and follow-up during the next year revealed no recurrence.

Comment
Etiology of AE—Acrodermatitis enteropathica was first identified in 1942 as an acral rash associated with diarrhea3; in 1973, Barnes and Moynahan4 discovered zinc deficiency as a causal agent for these findings. The causes of AE are further subclassified as either an acquired or inborn etiology. Congenital causes commonly are seen in infants within the first few months of life, whereas acquired forms are seen at any age. Acquired forms in infants can occur from failure of the mother to secrete zinc in breast milk, low maternal serum zinc levels, or other reasons causing low nutritional intake. A single mutation in the SLC30A2 gene has been found to markedly reduce zinc concentrations in breast milk, thus causing zinc deficiency in breastfed infants.5 Other acquired forms can be caused by malabsorption, sometimes after surgery such as intestinal bypass or from intravenous nutrition without sufficient zinc.1 The congenital form of AE is an autosomal-recessive disorder occurring from mutations in the SLC39A4 gene located on band 8q24.3. Affected individuals have a decreased ability to absorb zinc in the small intestine because of defects in zinc transporters ZIP and ZnT.6 Based on our patient’s laboratory findings and history, it is believed that the zinc deficiency was acquired, as the condition normalized with repletion and has not required any supplementation in the year of follow-up. In addition, the absence of a pertinent family history supported an acquired diagnosis, which has various etiologies, whereas the congenital form primarily is a genetic disease.
Management—Treatment of AE includes supplementation with oral elemental zinc; however, there are scant evidence-based recommendations on the exact dose of zinc to be given. Generally, the recommended amount is 3 mg/kg/d.8 For individuals with the congenital form of AE, lifelong zinc supplementation is additionally recommended.9 It is important to recognize this presentation because the patient can develop worsening irritability, severe diarrhea, nail dystrophy, hair loss, immune dysfunction, and numerous ophthalmic disorders if left untreated. Acute zinc toxicity due to excess administration is rare, with symptoms of nausea and vomiting occurring with dosages of 50 to 100 mg/d. Additionally, dosages of up to 70 mg twice weekly have been provided without any toxic effect.10 In our case, 3 mg/kg/d of oral zinc supplementation proved to be effective in resolving the patient’s symptoms of acquired zinc deficiency.
Differential Diagnosis—It is important to note that deficiencies of other nutrients may present as an AE-like eruption called acrodermatitis dysmetabolica (AD). Both diseases may present with the triad of dermatitis, alopecia, and diarrhea; however, AD is associated with inborn errors of metabolism. There have been cases that describe AD in patients with a zinc deficiency in conjunction with a deficiency of branched-chain amino acids.11,12 It is important to consider AD in the differential diagnosis of an AE eruption, especially in the context of a metabolic disorder, as it may affect the treatment plan. One case described the dermatitis of AD as not responding to zinc supplementation alone, while another described improvement after increasing an isoleucine supplementation dose.11,12
Other considerations in the differential diagnoses include AE-like conditions such as biotinidase deficiency, multiple carboxylase deficiency, and essential fatty acid deficiency. An AE-like condition may present with the triad of dermatitis, alopecia, and diarrhea. However, unlike in true AE, zinc and alkaline phosphatase levels tend to be normal in these conditions. Other features seen in AE-like conditions depend on the underlying cause but often include failure to thrive, neurologic defects, ophthalmic abnormalities, and metabolic abnormalities.13
- Acrodermatitis enteropathica. National Organization for Rare Disorders. Accessed October 16, 2022. https://rarediseases.org/rare-diseases/acrodermatitis-enteropathica/
- Perafán-Riveros C, França LFS, Alves ACF, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- Danbolt N. Acrodermatitis enteropathica. Br J Dermatol. 1979;100:37-40.
- Barnes PM, Moynahan EJ. Zinc deficiency in acrodermatitis enteropathica: multiple dietary intolerance treated with synthetic diet. Proc R Soc Med. 1973;66:327-329.
- Lee S, Zhou Y, Gill DL, et al. A genetic variant in SLC30A2 causes breast dysfunction during lactation by inducing ER stress, oxidative stress and epithelial barrier defects. Sci Rep. 2018;8:3542.
- Kaur S, Sangwan A, Sahu P, et al. Clinical variants of acrodermatitis enteropathica and its co-relation with genetics. Indian J Paediatr Dermatol. 2016;17:35-37.
- Dela Rosa KM, James WD. Acrodermatitis enteropathica workup. Medscape. Updated June 4, 2021. Accessed October 16, 2022. https://emedicine.medscape.com/article/1102575-workup#showall
- Ngan V, Gangakhedkar A, Oakley A. Acrodermatitis enteropathica. DermNet. Accessed October 16, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica/
- Ranugha P, Sethi P, Veeranna S. Acrodermatitis enteropathica: the need for sustained high dose zinc supplementation. Dermatol Online J. 2018;24:13030/qt1w9002sr.
- Larson CP, Roy SK, Khan AI, et al. Zinc treatment to under-five children: applications to improve child survival and reduce burden of disease. J Health Popul Nutr. 2008;26:356-365.
- Samady JA, Schwartz RA, Shih LY, et al. Acrodermatitis enteropathica-like eruption in an infant with nonketotic hyperglycinemia. J Dermatol. 2000;27:604-608.
- Flores K, Chikowski R, Morrell DS. Acrodermatitis dysmetabolica in an infant with maple syrup urine disease. Clin Exp Dermatol. 2016;41:651-654.
- Jones L, Oakley A. Acrodermatitis enteropathica-like conditions. DermNet. Accessed August 30, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica-like-conditions
- Acrodermatitis enteropathica. National Organization for Rare Disorders. Accessed October 16, 2022. https://rarediseases.org/rare-diseases/acrodermatitis-enteropathica/
- Perafán-Riveros C, França LFS, Alves ACF, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- Danbolt N. Acrodermatitis enteropathica. Br J Dermatol. 1979;100:37-40.
- Barnes PM, Moynahan EJ. Zinc deficiency in acrodermatitis enteropathica: multiple dietary intolerance treated with synthetic diet. Proc R Soc Med. 1973;66:327-329.
- Lee S, Zhou Y, Gill DL, et al. A genetic variant in SLC30A2 causes breast dysfunction during lactation by inducing ER stress, oxidative stress and epithelial barrier defects. Sci Rep. 2018;8:3542.
- Kaur S, Sangwan A, Sahu P, et al. Clinical variants of acrodermatitis enteropathica and its co-relation with genetics. Indian J Paediatr Dermatol. 2016;17:35-37.
- Dela Rosa KM, James WD. Acrodermatitis enteropathica workup. Medscape. Updated June 4, 2021. Accessed October 16, 2022. https://emedicine.medscape.com/article/1102575-workup#showall
- Ngan V, Gangakhedkar A, Oakley A. Acrodermatitis enteropathica. DermNet. Accessed October 16, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica/
- Ranugha P, Sethi P, Veeranna S. Acrodermatitis enteropathica: the need for sustained high dose zinc supplementation. Dermatol Online J. 2018;24:13030/qt1w9002sr.
- Larson CP, Roy SK, Khan AI, et al. Zinc treatment to under-five children: applications to improve child survival and reduce burden of disease. J Health Popul Nutr. 2008;26:356-365.
- Samady JA, Schwartz RA, Shih LY, et al. Acrodermatitis enteropathica-like eruption in an infant with nonketotic hyperglycinemia. J Dermatol. 2000;27:604-608.
- Flores K, Chikowski R, Morrell DS. Acrodermatitis dysmetabolica in an infant with maple syrup urine disease. Clin Exp Dermatol. 2016;41:651-654.
- Jones L, Oakley A. Acrodermatitis enteropathica-like conditions. DermNet. Accessed August 30, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica-like-conditions
Practice Points
- Although clinically characterized by the triad of acral and periorificial dermatitis, alopecia, and diarrhea, most cases of acrodermatitis enteropathica (AE) present with only partial features of this syndrome.
- Low levels of zinc-dependent enzymes such as alkaline phosphatase may support the diagnosis of AE.
Mycetomalike Skin Infection Due to Gordonia bronchialis in an Immunocompetent Patient
Mycetoma is a chronic subcutaneous infection due to fungal (eumycetoma) or aerobic actinomycetes (actinomycetoma) organisms. Clinical lesions develop from a granulomatous infiltrate organizing around the infectious organism. Patients can present with extensive subcutaneous nodularity and draining sinuses that can lead to deformation of the affected extremity. These infections are rare in developed countries, and the prevalence and incidence remain unknown. It has been reported that actinomycetes represent 60% of mycetoma cases worldwide, with the majority of cases in Central America from Nocardia (86%) and Actinomadura madurae (10%). 1Gordonia species are aerobic, partially acid-fast, gram-positive actinobacteria that may comprise a notable minority of actinomycete isolates. 2 The species Gordonia bronchialis is of particular interest as a human pathogen because of increasing reports of nosocomial infections. 3,4 We describe a case of a mycetomalike infection due to G bronchialis in an immunocompetent patient with complete resolution after 3 months of antibiotics.
Case Report
An 86-year-old man presented to the emergency department with a pruritic rash on the right forearm. He had a history of chronic kidney disease, hypertension, and inverse psoriasis complicated by steroid atrophy. He reported trauma to the right antecubital fossa approximately 1 to 2 months prior from a car door; he received wound care over several weeks at an outside hospital. The initial wound healed completely, but he subsequently noticed erythema spreading down the forearm. At the current presentation, he was empirically treated with mid-potency topical steroids and cefuroxime for 7 days. Initial laboratory results were notable for a white blood cell count of 5.7×103 cells/μL (reference range,3.7–8.4×103 cells/μL) and a creatinine level of 1.5 mg/dL (reference range, 0.57–1.25 mg/dL). The patient returned to the emergency department 2 weeks later with spreading of the initial rash and worsening pruritus. Dermatologic evaluation revealed the patient was afebrile and had violaceous papules and nodules that coalesced into plaques on the right arm, with the largest measuring approximately 15 cm. Areas of superficial erosion and crusting were noted (Figure 1A). The patient denied constitutional symptoms and had no axillary or cervical lymphadenopathy. The differential initially included an atypical infection vs a neoplasm. Two 5-mm punch biopsies were performed, which demonstrated a suppurative granulomatous infiltrate in the dermis with extension into the subcutis (Figure 2A). Focal vacuolations within the dermis demonstrated aggregates of gram-positive pseudofilamentous organisms (Figures 2B and 2C). Aerobic tissue cultures grew G bronchialis that was susceptible to all antibiotics tested and Staphylococcus epidermidis. Fungal and mycobacterial cultures were negative. The patient was placed on amoxicillin 875 mg–clavulanate 125 mg twice daily for 3 weeks. However, he demonstrated progression of the rash, with increased induration and confluence of plaques on the forearm (Figure 1B). A repeat excisional biopsy was performed, and a tissue sample was sent for 16S ribosomal RNA sequencing identification. However, neither conventional cultures nor sequencing demonstrated evidence of G bronchialis or any other pathogen. Additionally, bacterial, fungal, and mycobacterial blood cultures were negative. Amoxicillin-clavulanate was stopped, and he was placed on trimethoprim-sulfamethoxazole for 2 weeks, then changed to linezolid (600 mg twice daily) due to continued lack of improvement of the rash. After 2 weeks of linezolid, the rash was slightly improved, but the patient had notable side effects (eg, nausea, mucositis). Therefore, he was switched back to trimethoprim-sulfamethoxazole for another 6 weeks. Antibiotic therapy was discontinued after there was notable regression of indurated plaques (Figure 1C); he received more than 3 months of antibiotics in all. At 1 month after completion of antibiotic therapy, the patient had no evidence of recurrence.
Comment
Microbiology of Gordonia Species—Gordonia bronchialis originally was isolated in 1971 by Tsukamura et al5 from the sputum of patients with cavitary tuberculosis and bronchiectasis in Japan. Other Gordonia species (formerly Rhodococcus or Gordona) later were identified in soil, seawater, sediment, and wastewater. Gordonia bronchialis is a gram-positive aerobic actinomycete short rod that organizes in cordlike compact groups. It is weakly acid fast, nonmotile, and nonsporulating. Colonies exhibit pinkish-brown pigmentation. Our understanding of the clinical significance of this organism continues to evolve, and it is not always clearly pathogenic. Because Gordonia isolates may be dismissed as commensals or misidentified as Nocardia or Rhodococcus by routine biochemical tests, it is possible that infections may go undetected. Speciation requires gene sequencing; as our utilization of molecular methods has increased, the identification of clinically relevant aerobic actinomycetes, including Gordonia, has improved,6 and the following species have been recognized as pathogens: Gordonia araii, G bronchialis, Gordonia effusa, Gordonia otitidis, Gordonia polyisoprenivorans, Gordonia rubirpertincta, Gordonia sputi, and Gordonia terrae.7
Cases Reported in the Literature—A PubMed search of articles indexed for MEDLINE using the term Gordonia bronchialis yielded 35 previously reported human cases of G bronchialis infection, most often associated with medical devices or procedures.8-31 Eighteen of these cases were sternal surgical site infections in patients with a history of cardiac surgery,3,4,12-16,30 including 2 outbreaks following coronary artery bypass grafting that were thought to be related to intraoperative transmission from a nurse.3,4 Of the remaining cases, 12 were linked to a procedure or an indwelling catheter: 4 cases of peritonitis in the setting of continuous ambulatory peritoneal dialysis17,18,26,27; 3 cases of skin and soft tissue infection (1 at the site of a prior needle injection,10 1 after acupuncture,11 and 1 after breast reduction surgery29); 1 case of ventriculitis in a premature neonate with an underlying intraventricular shunt19; 2 cases of pacemaker-induced endocarditis20,28; 1 case of tibial osteomyelitis related to a bioresorbable polymer screw21; and 1 case of chronic endophthalmitis with underlying intraocular lens implants.22 The Table lists all cases of G bronchialis skin or surgical site infections encountered in our literature search as well as the treatment provided in each case.
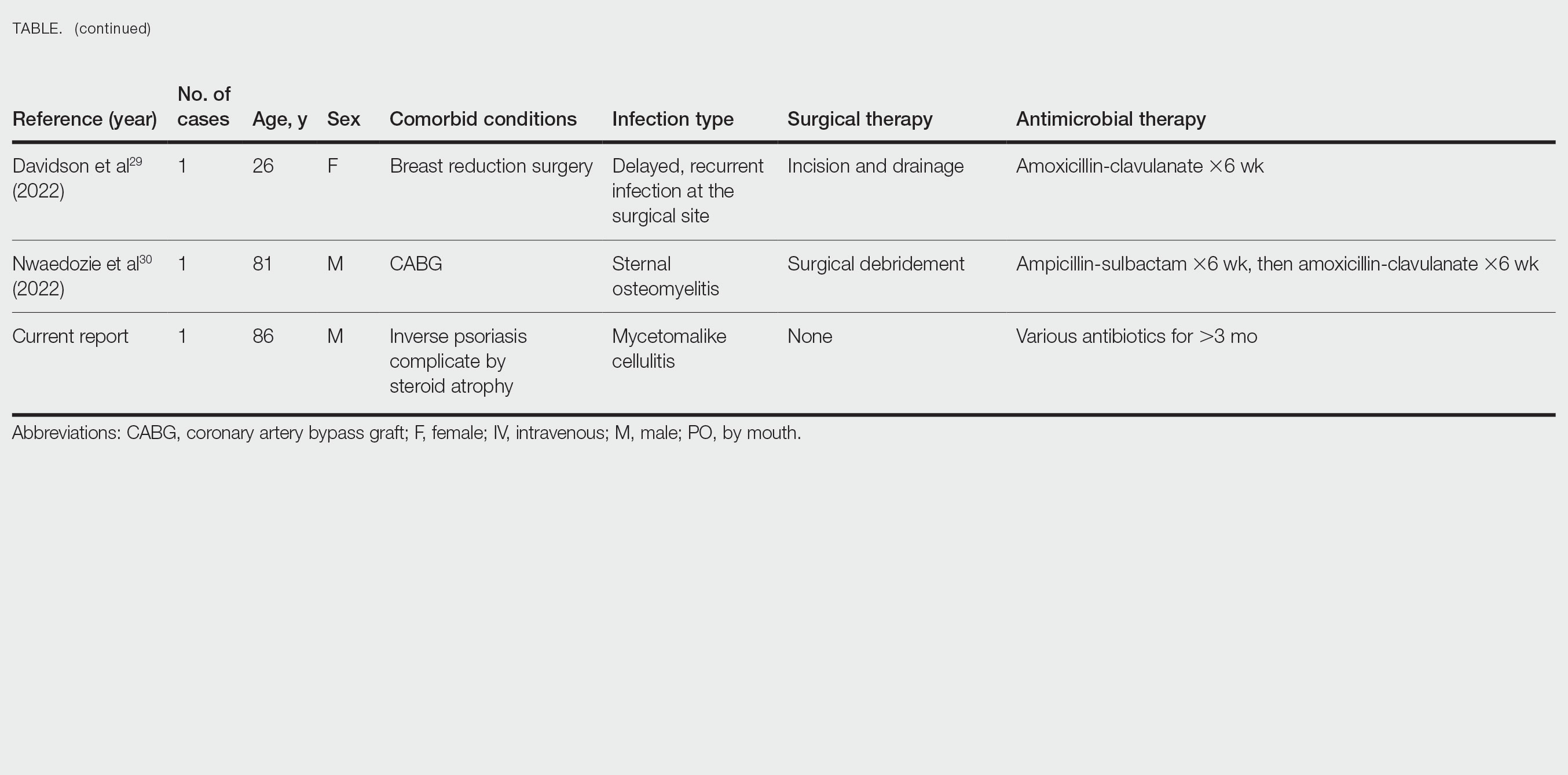
Only 4 of these 35 cases of G bronchialis infections were skin and soft tissue infections. All 4 occurred in immunocompetent hosts, and 3 were associated with needle punctures or surgery. The fourth case involved a recurrent breast abscess that occurred in a patient without known risk factors or recent procedures.23 Other Gordonia species have been associated with cutaneous infections, including Gordonia amicalis, G terrae, and recently Gordonia westfalica, with the latter 2 demonstrating actinomycetoma formation.32-34 Our case is remarkable in that it represents actinomycetoma due to G bronchialis. Of note, our patient was immunocompetent and did not have any radiation or chronic lymphedema involving the affected extremity. However, his history of steroid-induced skin atrophy may have predisposed him to this rare infection.
Clinical Presentation—Classic mycetoma demonstrate organismal granules within the dermis, surrounded by a neutrophilic infiltrate, which is in turn surrounded by histiocytes and multinucleated giant cells. Periodic acid–Schiff and silver stains can identify fungal organisms, while Gram stain helps to elucidate bacterial etiologies.1 In our patient, a biopsy revealed several dermal aggregates of pseudofilamentous gram-positive organisms surrounded by a neutrophilic and histiocytic infiltrate.8 Because this case presented over weeks to months rather than months to years, it progressed more rapidly than a classic mycetoma. However, the dermatologic and histologic features were consistent with mycetoma.
Management—General treatment of actinomycetoma requires identification of the causative organism and prolonged administration of antibiotics, typically in combination.35-37 Most G bronchialis infections associated with surgical intervention or implants in the literature required surgical debridement and removal of contaminated material for clinical cure, with the exception of 3 cases of sternal wound infection and 1 case of peritonitis that recovered with antimicrobial therapy alone.3,17 Combination therapy often was used, but monotherapy, particularly with a fluoroquinolone, has been reported. Susceptibility data are limited, but in general, Gordonia species appear susceptible to imipenem, ciprofloxacin, amikacin, gentamicin, and linezolid, with variable susceptibility to vancomycin (89% of isolates), third-generation cephalosporins (80%–90% of isolates), tetracyclines (≤85% of isolates), penicillin (≤70% of isolates), and trimethoprim-sulfamethoxazole (≤65% of isolates).7,10,19,38-40 Although there are no standardized recommendations for the treatment of these infections, the most commonly used drugs to treat Gordonia are carbapenems and fluoroquinolones, with or without an aminoglycoside, followed by third-generation cephalosporins and vancomycin, depending on susceptibilities. Additional antibiotics (alone or in combination) that have previously been used with favorable outcomes include amoxicillin or amoxicillin-clavulanate, piperacillin-tazobactam, rifampicin, trimethoprim-sulfamethoxazole, minocycline, doxycycline, and daptomycin.
Our patient received amoxicillin-clavulanate, trimethoprim-sulfamethoxazole, and linezolid. We considered combination therapy but decided against it due to concern for toxicity, given his age and poor renal function. The antibiotic that was most important to his recovery was unclear; the patient insisted that his body, not antibiotics, deserved most of the credit for healing his arm. Although cultures and polymerase chain reaction assays were negative after 3 weeks of amoxicillin-clavulanate, the patient did not show clinical improvement—reasons could be because the antibiotic reduced but did not eliminate the bacterial burden, sampling error of the biopsy, or it takes much longer for the body to heal than it takes to kill the bacteria. Most likely a combination of factors was at play.
Conclusion
Gordonia bronchialis is an emerging cause of human infections typically occurring after trauma, inoculation, or surgery. Most infections are localized; however, the present case highlights the ability of this species to form a massive cutaneous infection. Treatment should be tailored to susceptibility, with close follow-up to ensure improvement and resolution. For clinicians encountering a similar case, we encourage biopsy prior to empiric antibiotics, as antibiotic therapy can decrease the yield of subsequent testing. Treatment should be guided by the clinical course and may need to last weeks to months. Combination therapy for Gordonia infections should be considered in severe cases, in cases presenting as actinomycetoma, in those not responding to therapy, or when the susceptibility profile is unknown or unreliable.
Acknowledgments—The authors thank this veteran for allowing us to participate in his care and to learn from his experience. He gave his consent for us to share his story and the photographs of the arm.
- Arenas R, Fernandez Martinez RF, Torres-Guerrero E, et al. Actinomycetoma: an update on diagnosis and treatment. Cutis. 2017;99:E11-E15.
- Poonwan N, Mekha N, Yazawa K, et al. Characterization of clinical isolates of pathogenic Nocardia strains and related actinomycetes in Thailand from 1996 to 2003. Mycopathologia. 2005;159:361-368.
- Richet HM, Craven PC, Brown JM, et al. A cluster of Rhodococcus (Gordona) bronchialis sternal-wound infections after coronary-artery bypass surgery. N Engl J Med. 1991;324:104-109.
- Wright SN, Gerry JS, Busowski MT, et al. Gordonia bronchialis sternal wound infection in 3 patients following open heart surgery: intraoperative transmission from a healthcare worker. Infect Control Hosp Epidemiol. 2012;33:1238-1241.
- Tsukamura M. Proposal of a new genus, Gordona, for slightly acid-fast organisms occurring in sputa of patients with pulmonary disease and in soil. J Gen Microbiol. 1971;68:15-26.
- Wang T, Kong F, Chen S, et al. Improved identification of Gordonia, Rhodococcus and Tsukamurella species by 5′-end 16s rRNA gene sequencing. Pathology. 2011;43:58-63.
- Aoyama K, Kang Y, Yazawa K, et al. Characterization of clinical isolates of Gordonia species in Japanese clinical samples during 1998-2008. Mycopathologia. 2009;168:175-183.
- Ivanova N, Sikorski J, Jando M, et al. Complete genome sequence of Gordonia bronchialis type strain (3410 T). Stand Genomic Sci. 2010;2:19-28.
- Johnson JA, Onderdonk AB, Cosimi LA, et al. Gordonia bronchialis bacteremia and pleural infection: case report and review of the literature. J Clin Microbiol. 2011;49:1662-1666.
- Bartolomé-Álvarez J, Sáez-Nieto JA, Escudero-Jiménez A, et al. Cutaneous abscess due to Gordonia bronchialis: case report and literature review. Rev Esp Quimioter. 2016;29:170-173.
- Choi ME, Jung CJ, Won CH, et al. Case report of cutaneous nodule caused by Gordonia bronchialis in an immunocompetent patient after receiving acupuncture. J Dermatol. 2019;46:343-346.
- Nguyen DB, Gupta N, Abou-Daoud A, et al. A polymicrobial outbreak of surgical site infections following cardiac surgery at a community hospital in Florida, 2011-2012. Am J Infect Control. 2014;42:432-435.
- Chang JH, Ji M, Hong HL, et al. Sternal osteomyelitis caused byGordonia bronchialis after open-heart surgery. Infect Chemother. 2014;46:110-114.
- Rodriguez-Lozano J, Pérez-Llantada E, Agüero J, et al. Sternal wound infection caused by Gordonia bronchialis: identification by MALDI-TOF MS. JMM Case Rep. 2016;3:e005067.
- Akrami K, Coletta J, Mehta S, et al. Gordonia sternal wound infection treated with ceftaroline: case report and literature review. JMM Case Rep. 2017;4:e005113.
- Ambesh P, Kapoor A, Kazmi D, et al. Sternal osteomyelitis by Gordonia bronchialis in an immunocompetent patient after open heart surgery. Ann Card Anaesth. 2019;22:221-224.
- Ma TKW, Chow KM, Kwan BCH, et al. Peritoneal-dialysis related peritonitis caused by Gordonia species: report of four cases and literature review. Nephrology. 2014;19:379-383.
- Lam JYW, Wu AKL, Leung WS, et al. Gordonia species as emerging causes of continuous-ambulatory-peritoneal-dialysis-related peritonitis identified by 16S rRNA and secA1 gene sequencing and matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). J Clin Microbiol. 2015;53:671-676.
- Blaschke AJ, Bender J, Byington CL, et al. Gordonia species: emerging pathogens in pediatric patients that are identified by 16S ribosomal RNA gene sequencing. Clin Infect Dis. 2007;45:483-486.
- Titécat M, Loïez C, Courcol RJ, et al. Difficulty with Gordonia bronchialis identification by Microflex mass spectrometer in a pacemaker‐induced endocarditis. JMM Case Rep. 2014;1:E003681.
- Siddiqui N, Toumeh A, Georgescu C. Tibial osteomyelitis caused by Gordonia bronchialis in an immunocompetent patient. J Clin Microbiol. 2012;50:3119-3121.
- Choi R, Strnad L, Flaxel CJ, et al. Gordonia bronchialis–associated endophthalmitis. Emerg Infect Dis. 2019;25:1017-1019.
- Werno AM, Anderson TP, Chambers ST, et al. Recurrent breast abscess caused by Gordonia bronchialis in an immunocompetent patient. J Clin Microbiol. 2005;43:3009-3010.
- Sng LH, Koh TH, Toney SR, et al. Bacteremia caused by Gordonia bronchialis in a patient with sequestrated lung. J Clin Microbiol. 2004;42:2870-2871.
- Ramanan P, Deziel PJ, Wengenack NL. Gordonia bacteremia. J Clin Microbiol. 2013;51:3443-3447.
- Sukackiene D, Rimsevicius L, Kiveryte S, et al. A case of successfully treated relapsing peritoneal dialysis-associated peritonitis caused by Gordonia bronchialis in a farmer. Nephrol Ther. 2018;14:109-111.
- Bruno V, Tjon J, Lin S, et al. Peritoneal dialysis-related peritonitis caused by Gordonia bronchialis: first pediatric report. Pediatr Nephrol. 2022;37:217-220. doi: 10.1007/s00467-021-05313-3
- Mormeneo Bayo S, Palacián Ruíz MP, Asin Samper U, et al. Pacemaker-induced endocarditis by Gordonia bronchialis. Enferm Infecc Microbiol Clin (Engl Ed). 2022;40:255-257.
- Davidson AL, Driscoll CR, Luther VP, et al. Recurrent skin and soft tissue infection following breast reduction surgery caused by Gordonia bronchialis: a case report. Plast Reconstr Surg Glob Open. 2022;10:E4395.
- Nwaedozie S, Mojarrab JN, Gopinath P, et al. Sternal osteomyelitis caused by Gordonia bronchialis in an immunocompetent patient following coronary artery bypass surgery. IDCases. 2022;29:E01548.
- Nakahama H, Hanada S, Takada K, et al. Obstructive pneumonia caused by Gordonia bronchialis with a bronchial foreign body. Int J Infect Dis. 2022;124:157-158. doi:10.1016/j.ijid.2022.09.028
- Lai CC, Hsieh JH, Tsai HY, et al. Cutaneous infection caused by Gordonia amicalis after a traumatic injury. J Clin Microbiol. 2012;50:1821-1822.
- Bakker XR, Spauwen PHM, Dolmans WMV. Mycetoma of the hand caused by Gordona terrae: a case report. J Hand Surg Am. 2004;29:188-190.
- Gueneau R, Blanchet D, Rodriguez-Nava V, et al. Actinomycetoma caused by Gordonia westfalica: first reported case of human infection. New Microbes New Infect. 2020;34:100658.
- Auwaerter PG, ed. The Johns Hopkins POC-IT ABX Guide. Johns Hopkins Medicine; 2021.
- Welsh O, Sauceda E, Gonzalez J, et al. Amikacin alone andin combination with trimethoprim-sulfamethoxazole in the treatment of actinomycotic mycetoma. J Am Acad Dermatol. 1987;17:443-448.
- Zijlstra EE, van de Sande WWJ, Welsh O, et al. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis. 2016;16:100-112.
- Pham AS, Dé I, Rolston KV, et al. Catheter-related bacteremia caused by the nocardioform actinomycete Gordonia terrae. Clin Infect Dis. 2003;36:524-527.
- Renvoise A, Harle JR, Raoult D, et al. Gordonia sputi bacteremia. Emerg Infect Dis. 2009;15:1535-1537.
- Moser BD, Pellegrini GJ, Lasker BA, et al. Pattern of antimicrobial susceptibility obtained from blood isolates of a rare but emerging human pathogen, Gordonia polyisoprenivorans. Antimicrob Agents Chemother. 2012;56:4991-4993.
Mycetoma is a chronic subcutaneous infection due to fungal (eumycetoma) or aerobic actinomycetes (actinomycetoma) organisms. Clinical lesions develop from a granulomatous infiltrate organizing around the infectious organism. Patients can present with extensive subcutaneous nodularity and draining sinuses that can lead to deformation of the affected extremity. These infections are rare in developed countries, and the prevalence and incidence remain unknown. It has been reported that actinomycetes represent 60% of mycetoma cases worldwide, with the majority of cases in Central America from Nocardia (86%) and Actinomadura madurae (10%). 1Gordonia species are aerobic, partially acid-fast, gram-positive actinobacteria that may comprise a notable minority of actinomycete isolates. 2 The species Gordonia bronchialis is of particular interest as a human pathogen because of increasing reports of nosocomial infections. 3,4 We describe a case of a mycetomalike infection due to G bronchialis in an immunocompetent patient with complete resolution after 3 months of antibiotics.

Case Report
An 86-year-old man presented to the emergency department with a pruritic rash on the right forearm. He had a history of chronic kidney disease, hypertension, and inverse psoriasis complicated by steroid atrophy. He reported trauma to the right antecubital fossa approximately 1 to 2 months prior from a car door; he received wound care over several weeks at an outside hospital. The initial wound healed completely, but he subsequently noticed erythema spreading down the forearm. At the current presentation, he was empirically treated with mid-potency topical steroids and cefuroxime for 7 days. Initial laboratory results were notable for a white blood cell count of 5.7×103 cells/μL (reference range,3.7–8.4×103 cells/μL) and a creatinine level of 1.5 mg/dL (reference range, 0.57–1.25 mg/dL). The patient returned to the emergency department 2 weeks later with spreading of the initial rash and worsening pruritus. Dermatologic evaluation revealed the patient was afebrile and had violaceous papules and nodules that coalesced into plaques on the right arm, with the largest measuring approximately 15 cm. Areas of superficial erosion and crusting were noted (Figure 1A). The patient denied constitutional symptoms and had no axillary or cervical lymphadenopathy. The differential initially included an atypical infection vs a neoplasm. Two 5-mm punch biopsies were performed, which demonstrated a suppurative granulomatous infiltrate in the dermis with extension into the subcutis (Figure 2A). Focal vacuolations within the dermis demonstrated aggregates of gram-positive pseudofilamentous organisms (Figures 2B and 2C). Aerobic tissue cultures grew G bronchialis that was susceptible to all antibiotics tested and Staphylococcus epidermidis. Fungal and mycobacterial cultures were negative. The patient was placed on amoxicillin 875 mg–clavulanate 125 mg twice daily for 3 weeks. However, he demonstrated progression of the rash, with increased induration and confluence of plaques on the forearm (Figure 1B). A repeat excisional biopsy was performed, and a tissue sample was sent for 16S ribosomal RNA sequencing identification. However, neither conventional cultures nor sequencing demonstrated evidence of G bronchialis or any other pathogen. Additionally, bacterial, fungal, and mycobacterial blood cultures were negative. Amoxicillin-clavulanate was stopped, and he was placed on trimethoprim-sulfamethoxazole for 2 weeks, then changed to linezolid (600 mg twice daily) due to continued lack of improvement of the rash. After 2 weeks of linezolid, the rash was slightly improved, but the patient had notable side effects (eg, nausea, mucositis). Therefore, he was switched back to trimethoprim-sulfamethoxazole for another 6 weeks. Antibiotic therapy was discontinued after there was notable regression of indurated plaques (Figure 1C); he received more than 3 months of antibiotics in all. At 1 month after completion of antibiotic therapy, the patient had no evidence of recurrence.

Comment
Microbiology of Gordonia Species—Gordonia bronchialis originally was isolated in 1971 by Tsukamura et al5 from the sputum of patients with cavitary tuberculosis and bronchiectasis in Japan. Other Gordonia species (formerly Rhodococcus or Gordona) later were identified in soil, seawater, sediment, and wastewater. Gordonia bronchialis is a gram-positive aerobic actinomycete short rod that organizes in cordlike compact groups. It is weakly acid fast, nonmotile, and nonsporulating. Colonies exhibit pinkish-brown pigmentation. Our understanding of the clinical significance of this organism continues to evolve, and it is not always clearly pathogenic. Because Gordonia isolates may be dismissed as commensals or misidentified as Nocardia or Rhodococcus by routine biochemical tests, it is possible that infections may go undetected. Speciation requires gene sequencing; as our utilization of molecular methods has increased, the identification of clinically relevant aerobic actinomycetes, including Gordonia, has improved,6 and the following species have been recognized as pathogens: Gordonia araii, G bronchialis, Gordonia effusa, Gordonia otitidis, Gordonia polyisoprenivorans, Gordonia rubirpertincta, Gordonia sputi, and Gordonia terrae.7
Cases Reported in the Literature—A PubMed search of articles indexed for MEDLINE using the term Gordonia bronchialis yielded 35 previously reported human cases of G bronchialis infection, most often associated with medical devices or procedures.8-31 Eighteen of these cases were sternal surgical site infections in patients with a history of cardiac surgery,3,4,12-16,30 including 2 outbreaks following coronary artery bypass grafting that were thought to be related to intraoperative transmission from a nurse.3,4 Of the remaining cases, 12 were linked to a procedure or an indwelling catheter: 4 cases of peritonitis in the setting of continuous ambulatory peritoneal dialysis17,18,26,27; 3 cases of skin and soft tissue infection (1 at the site of a prior needle injection,10 1 after acupuncture,11 and 1 after breast reduction surgery29); 1 case of ventriculitis in a premature neonate with an underlying intraventricular shunt19; 2 cases of pacemaker-induced endocarditis20,28; 1 case of tibial osteomyelitis related to a bioresorbable polymer screw21; and 1 case of chronic endophthalmitis with underlying intraocular lens implants.22 The Table lists all cases of G bronchialis skin or surgical site infections encountered in our literature search as well as the treatment provided in each case.


Only 4 of these 35 cases of G bronchialis infections were skin and soft tissue infections. All 4 occurred in immunocompetent hosts, and 3 were associated with needle punctures or surgery. The fourth case involved a recurrent breast abscess that occurred in a patient without known risk factors or recent procedures.23 Other Gordonia species have been associated with cutaneous infections, including Gordonia amicalis, G terrae, and recently Gordonia westfalica, with the latter 2 demonstrating actinomycetoma formation.32-34 Our case is remarkable in that it represents actinomycetoma due to G bronchialis. Of note, our patient was immunocompetent and did not have any radiation or chronic lymphedema involving the affected extremity. However, his history of steroid-induced skin atrophy may have predisposed him to this rare infection.
Clinical Presentation—Classic mycetoma demonstrate organismal granules within the dermis, surrounded by a neutrophilic infiltrate, which is in turn surrounded by histiocytes and multinucleated giant cells. Periodic acid–Schiff and silver stains can identify fungal organisms, while Gram stain helps to elucidate bacterial etiologies.1 In our patient, a biopsy revealed several dermal aggregates of pseudofilamentous gram-positive organisms surrounded by a neutrophilic and histiocytic infiltrate.8 Because this case presented over weeks to months rather than months to years, it progressed more rapidly than a classic mycetoma. However, the dermatologic and histologic features were consistent with mycetoma.
Management—General treatment of actinomycetoma requires identification of the causative organism and prolonged administration of antibiotics, typically in combination.35-37 Most G bronchialis infections associated with surgical intervention or implants in the literature required surgical debridement and removal of contaminated material for clinical cure, with the exception of 3 cases of sternal wound infection and 1 case of peritonitis that recovered with antimicrobial therapy alone.3,17 Combination therapy often was used, but monotherapy, particularly with a fluoroquinolone, has been reported. Susceptibility data are limited, but in general, Gordonia species appear susceptible to imipenem, ciprofloxacin, amikacin, gentamicin, and linezolid, with variable susceptibility to vancomycin (89% of isolates), third-generation cephalosporins (80%–90% of isolates), tetracyclines (≤85% of isolates), penicillin (≤70% of isolates), and trimethoprim-sulfamethoxazole (≤65% of isolates).7,10,19,38-40 Although there are no standardized recommendations for the treatment of these infections, the most commonly used drugs to treat Gordonia are carbapenems and fluoroquinolones, with or without an aminoglycoside, followed by third-generation cephalosporins and vancomycin, depending on susceptibilities. Additional antibiotics (alone or in combination) that have previously been used with favorable outcomes include amoxicillin or amoxicillin-clavulanate, piperacillin-tazobactam, rifampicin, trimethoprim-sulfamethoxazole, minocycline, doxycycline, and daptomycin.
Our patient received amoxicillin-clavulanate, trimethoprim-sulfamethoxazole, and linezolid. We considered combination therapy but decided against it due to concern for toxicity, given his age and poor renal function. The antibiotic that was most important to his recovery was unclear; the patient insisted that his body, not antibiotics, deserved most of the credit for healing his arm. Although cultures and polymerase chain reaction assays were negative after 3 weeks of amoxicillin-clavulanate, the patient did not show clinical improvement—reasons could be because the antibiotic reduced but did not eliminate the bacterial burden, sampling error of the biopsy, or it takes much longer for the body to heal than it takes to kill the bacteria. Most likely a combination of factors was at play.
Conclusion
Gordonia bronchialis is an emerging cause of human infections typically occurring after trauma, inoculation, or surgery. Most infections are localized; however, the present case highlights the ability of this species to form a massive cutaneous infection. Treatment should be tailored to susceptibility, with close follow-up to ensure improvement and resolution. For clinicians encountering a similar case, we encourage biopsy prior to empiric antibiotics, as antibiotic therapy can decrease the yield of subsequent testing. Treatment should be guided by the clinical course and may need to last weeks to months. Combination therapy for Gordonia infections should be considered in severe cases, in cases presenting as actinomycetoma, in those not responding to therapy, or when the susceptibility profile is unknown or unreliable.
Acknowledgments—The authors thank this veteran for allowing us to participate in his care and to learn from his experience. He gave his consent for us to share his story and the photographs of the arm.
Mycetoma is a chronic subcutaneous infection due to fungal (eumycetoma) or aerobic actinomycetes (actinomycetoma) organisms. Clinical lesions develop from a granulomatous infiltrate organizing around the infectious organism. Patients can present with extensive subcutaneous nodularity and draining sinuses that can lead to deformation of the affected extremity. These infections are rare in developed countries, and the prevalence and incidence remain unknown. It has been reported that actinomycetes represent 60% of mycetoma cases worldwide, with the majority of cases in Central America from Nocardia (86%) and Actinomadura madurae (10%). 1Gordonia species are aerobic, partially acid-fast, gram-positive actinobacteria that may comprise a notable minority of actinomycete isolates. 2 The species Gordonia bronchialis is of particular interest as a human pathogen because of increasing reports of nosocomial infections. 3,4 We describe a case of a mycetomalike infection due to G bronchialis in an immunocompetent patient with complete resolution after 3 months of antibiotics.

Case Report
An 86-year-old man presented to the emergency department with a pruritic rash on the right forearm. He had a history of chronic kidney disease, hypertension, and inverse psoriasis complicated by steroid atrophy. He reported trauma to the right antecubital fossa approximately 1 to 2 months prior from a car door; he received wound care over several weeks at an outside hospital. The initial wound healed completely, but he subsequently noticed erythema spreading down the forearm. At the current presentation, he was empirically treated with mid-potency topical steroids and cefuroxime for 7 days. Initial laboratory results were notable for a white blood cell count of 5.7×103 cells/μL (reference range,3.7–8.4×103 cells/μL) and a creatinine level of 1.5 mg/dL (reference range, 0.57–1.25 mg/dL). The patient returned to the emergency department 2 weeks later with spreading of the initial rash and worsening pruritus. Dermatologic evaluation revealed the patient was afebrile and had violaceous papules and nodules that coalesced into plaques on the right arm, with the largest measuring approximately 15 cm. Areas of superficial erosion and crusting were noted (Figure 1A). The patient denied constitutional symptoms and had no axillary or cervical lymphadenopathy. The differential initially included an atypical infection vs a neoplasm. Two 5-mm punch biopsies were performed, which demonstrated a suppurative granulomatous infiltrate in the dermis with extension into the subcutis (Figure 2A). Focal vacuolations within the dermis demonstrated aggregates of gram-positive pseudofilamentous organisms (Figures 2B and 2C). Aerobic tissue cultures grew G bronchialis that was susceptible to all antibiotics tested and Staphylococcus epidermidis. Fungal and mycobacterial cultures were negative. The patient was placed on amoxicillin 875 mg–clavulanate 125 mg twice daily for 3 weeks. However, he demonstrated progression of the rash, with increased induration and confluence of plaques on the forearm (Figure 1B). A repeat excisional biopsy was performed, and a tissue sample was sent for 16S ribosomal RNA sequencing identification. However, neither conventional cultures nor sequencing demonstrated evidence of G bronchialis or any other pathogen. Additionally, bacterial, fungal, and mycobacterial blood cultures were negative. Amoxicillin-clavulanate was stopped, and he was placed on trimethoprim-sulfamethoxazole for 2 weeks, then changed to linezolid (600 mg twice daily) due to continued lack of improvement of the rash. After 2 weeks of linezolid, the rash was slightly improved, but the patient had notable side effects (eg, nausea, mucositis). Therefore, he was switched back to trimethoprim-sulfamethoxazole for another 6 weeks. Antibiotic therapy was discontinued after there was notable regression of indurated plaques (Figure 1C); he received more than 3 months of antibiotics in all. At 1 month after completion of antibiotic therapy, the patient had no evidence of recurrence.

Comment
Microbiology of Gordonia Species—Gordonia bronchialis originally was isolated in 1971 by Tsukamura et al5 from the sputum of patients with cavitary tuberculosis and bronchiectasis in Japan. Other Gordonia species (formerly Rhodococcus or Gordona) later were identified in soil, seawater, sediment, and wastewater. Gordonia bronchialis is a gram-positive aerobic actinomycete short rod that organizes in cordlike compact groups. It is weakly acid fast, nonmotile, and nonsporulating. Colonies exhibit pinkish-brown pigmentation. Our understanding of the clinical significance of this organism continues to evolve, and it is not always clearly pathogenic. Because Gordonia isolates may be dismissed as commensals or misidentified as Nocardia or Rhodococcus by routine biochemical tests, it is possible that infections may go undetected. Speciation requires gene sequencing; as our utilization of molecular methods has increased, the identification of clinically relevant aerobic actinomycetes, including Gordonia, has improved,6 and the following species have been recognized as pathogens: Gordonia araii, G bronchialis, Gordonia effusa, Gordonia otitidis, Gordonia polyisoprenivorans, Gordonia rubirpertincta, Gordonia sputi, and Gordonia terrae.7
Cases Reported in the Literature—A PubMed search of articles indexed for MEDLINE using the term Gordonia bronchialis yielded 35 previously reported human cases of G bronchialis infection, most often associated with medical devices or procedures.8-31 Eighteen of these cases were sternal surgical site infections in patients with a history of cardiac surgery,3,4,12-16,30 including 2 outbreaks following coronary artery bypass grafting that were thought to be related to intraoperative transmission from a nurse.3,4 Of the remaining cases, 12 were linked to a procedure or an indwelling catheter: 4 cases of peritonitis in the setting of continuous ambulatory peritoneal dialysis17,18,26,27; 3 cases of skin and soft tissue infection (1 at the site of a prior needle injection,10 1 after acupuncture,11 and 1 after breast reduction surgery29); 1 case of ventriculitis in a premature neonate with an underlying intraventricular shunt19; 2 cases of pacemaker-induced endocarditis20,28; 1 case of tibial osteomyelitis related to a bioresorbable polymer screw21; and 1 case of chronic endophthalmitis with underlying intraocular lens implants.22 The Table lists all cases of G bronchialis skin or surgical site infections encountered in our literature search as well as the treatment provided in each case.


Only 4 of these 35 cases of G bronchialis infections were skin and soft tissue infections. All 4 occurred in immunocompetent hosts, and 3 were associated with needle punctures or surgery. The fourth case involved a recurrent breast abscess that occurred in a patient without known risk factors or recent procedures.23 Other Gordonia species have been associated with cutaneous infections, including Gordonia amicalis, G terrae, and recently Gordonia westfalica, with the latter 2 demonstrating actinomycetoma formation.32-34 Our case is remarkable in that it represents actinomycetoma due to G bronchialis. Of note, our patient was immunocompetent and did not have any radiation or chronic lymphedema involving the affected extremity. However, his history of steroid-induced skin atrophy may have predisposed him to this rare infection.
Clinical Presentation—Classic mycetoma demonstrate organismal granules within the dermis, surrounded by a neutrophilic infiltrate, which is in turn surrounded by histiocytes and multinucleated giant cells. Periodic acid–Schiff and silver stains can identify fungal organisms, while Gram stain helps to elucidate bacterial etiologies.1 In our patient, a biopsy revealed several dermal aggregates of pseudofilamentous gram-positive organisms surrounded by a neutrophilic and histiocytic infiltrate.8 Because this case presented over weeks to months rather than months to years, it progressed more rapidly than a classic mycetoma. However, the dermatologic and histologic features were consistent with mycetoma.
Management—General treatment of actinomycetoma requires identification of the causative organism and prolonged administration of antibiotics, typically in combination.35-37 Most G bronchialis infections associated with surgical intervention or implants in the literature required surgical debridement and removal of contaminated material for clinical cure, with the exception of 3 cases of sternal wound infection and 1 case of peritonitis that recovered with antimicrobial therapy alone.3,17 Combination therapy often was used, but monotherapy, particularly with a fluoroquinolone, has been reported. Susceptibility data are limited, but in general, Gordonia species appear susceptible to imipenem, ciprofloxacin, amikacin, gentamicin, and linezolid, with variable susceptibility to vancomycin (89% of isolates), third-generation cephalosporins (80%–90% of isolates), tetracyclines (≤85% of isolates), penicillin (≤70% of isolates), and trimethoprim-sulfamethoxazole (≤65% of isolates).7,10,19,38-40 Although there are no standardized recommendations for the treatment of these infections, the most commonly used drugs to treat Gordonia are carbapenems and fluoroquinolones, with or without an aminoglycoside, followed by third-generation cephalosporins and vancomycin, depending on susceptibilities. Additional antibiotics (alone or in combination) that have previously been used with favorable outcomes include amoxicillin or amoxicillin-clavulanate, piperacillin-tazobactam, rifampicin, trimethoprim-sulfamethoxazole, minocycline, doxycycline, and daptomycin.
Our patient received amoxicillin-clavulanate, trimethoprim-sulfamethoxazole, and linezolid. We considered combination therapy but decided against it due to concern for toxicity, given his age and poor renal function. The antibiotic that was most important to his recovery was unclear; the patient insisted that his body, not antibiotics, deserved most of the credit for healing his arm. Although cultures and polymerase chain reaction assays were negative after 3 weeks of amoxicillin-clavulanate, the patient did not show clinical improvement—reasons could be because the antibiotic reduced but did not eliminate the bacterial burden, sampling error of the biopsy, or it takes much longer for the body to heal than it takes to kill the bacteria. Most likely a combination of factors was at play.
Conclusion
Gordonia bronchialis is an emerging cause of human infections typically occurring after trauma, inoculation, or surgery. Most infections are localized; however, the present case highlights the ability of this species to form a massive cutaneous infection. Treatment should be tailored to susceptibility, with close follow-up to ensure improvement and resolution. For clinicians encountering a similar case, we encourage biopsy prior to empiric antibiotics, as antibiotic therapy can decrease the yield of subsequent testing. Treatment should be guided by the clinical course and may need to last weeks to months. Combination therapy for Gordonia infections should be considered in severe cases, in cases presenting as actinomycetoma, in those not responding to therapy, or when the susceptibility profile is unknown or unreliable.
Acknowledgments—The authors thank this veteran for allowing us to participate in his care and to learn from his experience. He gave his consent for us to share his story and the photographs of the arm.
- Arenas R, Fernandez Martinez RF, Torres-Guerrero E, et al. Actinomycetoma: an update on diagnosis and treatment. Cutis. 2017;99:E11-E15.
- Poonwan N, Mekha N, Yazawa K, et al. Characterization of clinical isolates of pathogenic Nocardia strains and related actinomycetes in Thailand from 1996 to 2003. Mycopathologia. 2005;159:361-368.
- Richet HM, Craven PC, Brown JM, et al. A cluster of Rhodococcus (Gordona) bronchialis sternal-wound infections after coronary-artery bypass surgery. N Engl J Med. 1991;324:104-109.
- Wright SN, Gerry JS, Busowski MT, et al. Gordonia bronchialis sternal wound infection in 3 patients following open heart surgery: intraoperative transmission from a healthcare worker. Infect Control Hosp Epidemiol. 2012;33:1238-1241.
- Tsukamura M. Proposal of a new genus, Gordona, for slightly acid-fast organisms occurring in sputa of patients with pulmonary disease and in soil. J Gen Microbiol. 1971;68:15-26.
- Wang T, Kong F, Chen S, et al. Improved identification of Gordonia, Rhodococcus and Tsukamurella species by 5′-end 16s rRNA gene sequencing. Pathology. 2011;43:58-63.
- Aoyama K, Kang Y, Yazawa K, et al. Characterization of clinical isolates of Gordonia species in Japanese clinical samples during 1998-2008. Mycopathologia. 2009;168:175-183.
- Ivanova N, Sikorski J, Jando M, et al. Complete genome sequence of Gordonia bronchialis type strain (3410 T). Stand Genomic Sci. 2010;2:19-28.
- Johnson JA, Onderdonk AB, Cosimi LA, et al. Gordonia bronchialis bacteremia and pleural infection: case report and review of the literature. J Clin Microbiol. 2011;49:1662-1666.
- Bartolomé-Álvarez J, Sáez-Nieto JA, Escudero-Jiménez A, et al. Cutaneous abscess due to Gordonia bronchialis: case report and literature review. Rev Esp Quimioter. 2016;29:170-173.
- Choi ME, Jung CJ, Won CH, et al. Case report of cutaneous nodule caused by Gordonia bronchialis in an immunocompetent patient after receiving acupuncture. J Dermatol. 2019;46:343-346.
- Nguyen DB, Gupta N, Abou-Daoud A, et al. A polymicrobial outbreak of surgical site infections following cardiac surgery at a community hospital in Florida, 2011-2012. Am J Infect Control. 2014;42:432-435.
- Chang JH, Ji M, Hong HL, et al. Sternal osteomyelitis caused byGordonia bronchialis after open-heart surgery. Infect Chemother. 2014;46:110-114.
- Rodriguez-Lozano J, Pérez-Llantada E, Agüero J, et al. Sternal wound infection caused by Gordonia bronchialis: identification by MALDI-TOF MS. JMM Case Rep. 2016;3:e005067.
- Akrami K, Coletta J, Mehta S, et al. Gordonia sternal wound infection treated with ceftaroline: case report and literature review. JMM Case Rep. 2017;4:e005113.
- Ambesh P, Kapoor A, Kazmi D, et al. Sternal osteomyelitis by Gordonia bronchialis in an immunocompetent patient after open heart surgery. Ann Card Anaesth. 2019;22:221-224.
- Ma TKW, Chow KM, Kwan BCH, et al. Peritoneal-dialysis related peritonitis caused by Gordonia species: report of four cases and literature review. Nephrology. 2014;19:379-383.
- Lam JYW, Wu AKL, Leung WS, et al. Gordonia species as emerging causes of continuous-ambulatory-peritoneal-dialysis-related peritonitis identified by 16S rRNA and secA1 gene sequencing and matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). J Clin Microbiol. 2015;53:671-676.
- Blaschke AJ, Bender J, Byington CL, et al. Gordonia species: emerging pathogens in pediatric patients that are identified by 16S ribosomal RNA gene sequencing. Clin Infect Dis. 2007;45:483-486.
- Titécat M, Loïez C, Courcol RJ, et al. Difficulty with Gordonia bronchialis identification by Microflex mass spectrometer in a pacemaker‐induced endocarditis. JMM Case Rep. 2014;1:E003681.
- Siddiqui N, Toumeh A, Georgescu C. Tibial osteomyelitis caused by Gordonia bronchialis in an immunocompetent patient. J Clin Microbiol. 2012;50:3119-3121.
- Choi R, Strnad L, Flaxel CJ, et al. Gordonia bronchialis–associated endophthalmitis. Emerg Infect Dis. 2019;25:1017-1019.
- Werno AM, Anderson TP, Chambers ST, et al. Recurrent breast abscess caused by Gordonia bronchialis in an immunocompetent patient. J Clin Microbiol. 2005;43:3009-3010.
- Sng LH, Koh TH, Toney SR, et al. Bacteremia caused by Gordonia bronchialis in a patient with sequestrated lung. J Clin Microbiol. 2004;42:2870-2871.
- Ramanan P, Deziel PJ, Wengenack NL. Gordonia bacteremia. J Clin Microbiol. 2013;51:3443-3447.
- Sukackiene D, Rimsevicius L, Kiveryte S, et al. A case of successfully treated relapsing peritoneal dialysis-associated peritonitis caused by Gordonia bronchialis in a farmer. Nephrol Ther. 2018;14:109-111.
- Bruno V, Tjon J, Lin S, et al. Peritoneal dialysis-related peritonitis caused by Gordonia bronchialis: first pediatric report. Pediatr Nephrol. 2022;37:217-220. doi: 10.1007/s00467-021-05313-3
- Mormeneo Bayo S, Palacián Ruíz MP, Asin Samper U, et al. Pacemaker-induced endocarditis by Gordonia bronchialis. Enferm Infecc Microbiol Clin (Engl Ed). 2022;40:255-257.
- Davidson AL, Driscoll CR, Luther VP, et al. Recurrent skin and soft tissue infection following breast reduction surgery caused by Gordonia bronchialis: a case report. Plast Reconstr Surg Glob Open. 2022;10:E4395.
- Nwaedozie S, Mojarrab JN, Gopinath P, et al. Sternal osteomyelitis caused by Gordonia bronchialis in an immunocompetent patient following coronary artery bypass surgery. IDCases. 2022;29:E01548.
- Nakahama H, Hanada S, Takada K, et al. Obstructive pneumonia caused by Gordonia bronchialis with a bronchial foreign body. Int J Infect Dis. 2022;124:157-158. doi:10.1016/j.ijid.2022.09.028
- Lai CC, Hsieh JH, Tsai HY, et al. Cutaneous infection caused by Gordonia amicalis after a traumatic injury. J Clin Microbiol. 2012;50:1821-1822.
- Bakker XR, Spauwen PHM, Dolmans WMV. Mycetoma of the hand caused by Gordona terrae: a case report. J Hand Surg Am. 2004;29:188-190.
- Gueneau R, Blanchet D, Rodriguez-Nava V, et al. Actinomycetoma caused by Gordonia westfalica: first reported case of human infection. New Microbes New Infect. 2020;34:100658.
- Auwaerter PG, ed. The Johns Hopkins POC-IT ABX Guide. Johns Hopkins Medicine; 2021.
- Welsh O, Sauceda E, Gonzalez J, et al. Amikacin alone andin combination with trimethoprim-sulfamethoxazole in the treatment of actinomycotic mycetoma. J Am Acad Dermatol. 1987;17:443-448.
- Zijlstra EE, van de Sande WWJ, Welsh O, et al. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis. 2016;16:100-112.
- Pham AS, Dé I, Rolston KV, et al. Catheter-related bacteremia caused by the nocardioform actinomycete Gordonia terrae. Clin Infect Dis. 2003;36:524-527.
- Renvoise A, Harle JR, Raoult D, et al. Gordonia sputi bacteremia. Emerg Infect Dis. 2009;15:1535-1537.
- Moser BD, Pellegrini GJ, Lasker BA, et al. Pattern of antimicrobial susceptibility obtained from blood isolates of a rare but emerging human pathogen, Gordonia polyisoprenivorans. Antimicrob Agents Chemother. 2012;56:4991-4993.
- Arenas R, Fernandez Martinez RF, Torres-Guerrero E, et al. Actinomycetoma: an update on diagnosis and treatment. Cutis. 2017;99:E11-E15.
- Poonwan N, Mekha N, Yazawa K, et al. Characterization of clinical isolates of pathogenic Nocardia strains and related actinomycetes in Thailand from 1996 to 2003. Mycopathologia. 2005;159:361-368.
- Richet HM, Craven PC, Brown JM, et al. A cluster of Rhodococcus (Gordona) bronchialis sternal-wound infections after coronary-artery bypass surgery. N Engl J Med. 1991;324:104-109.
- Wright SN, Gerry JS, Busowski MT, et al. Gordonia bronchialis sternal wound infection in 3 patients following open heart surgery: intraoperative transmission from a healthcare worker. Infect Control Hosp Epidemiol. 2012;33:1238-1241.
- Tsukamura M. Proposal of a new genus, Gordona, for slightly acid-fast organisms occurring in sputa of patients with pulmonary disease and in soil. J Gen Microbiol. 1971;68:15-26.
- Wang T, Kong F, Chen S, et al. Improved identification of Gordonia, Rhodococcus and Tsukamurella species by 5′-end 16s rRNA gene sequencing. Pathology. 2011;43:58-63.
- Aoyama K, Kang Y, Yazawa K, et al. Characterization of clinical isolates of Gordonia species in Japanese clinical samples during 1998-2008. Mycopathologia. 2009;168:175-183.
- Ivanova N, Sikorski J, Jando M, et al. Complete genome sequence of Gordonia bronchialis type strain (3410 T). Stand Genomic Sci. 2010;2:19-28.
- Johnson JA, Onderdonk AB, Cosimi LA, et al. Gordonia bronchialis bacteremia and pleural infection: case report and review of the literature. J Clin Microbiol. 2011;49:1662-1666.
- Bartolomé-Álvarez J, Sáez-Nieto JA, Escudero-Jiménez A, et al. Cutaneous abscess due to Gordonia bronchialis: case report and literature review. Rev Esp Quimioter. 2016;29:170-173.
- Choi ME, Jung CJ, Won CH, et al. Case report of cutaneous nodule caused by Gordonia bronchialis in an immunocompetent patient after receiving acupuncture. J Dermatol. 2019;46:343-346.
- Nguyen DB, Gupta N, Abou-Daoud A, et al. A polymicrobial outbreak of surgical site infections following cardiac surgery at a community hospital in Florida, 2011-2012. Am J Infect Control. 2014;42:432-435.
- Chang JH, Ji M, Hong HL, et al. Sternal osteomyelitis caused byGordonia bronchialis after open-heart surgery. Infect Chemother. 2014;46:110-114.
- Rodriguez-Lozano J, Pérez-Llantada E, Agüero J, et al. Sternal wound infection caused by Gordonia bronchialis: identification by MALDI-TOF MS. JMM Case Rep. 2016;3:e005067.
- Akrami K, Coletta J, Mehta S, et al. Gordonia sternal wound infection treated with ceftaroline: case report and literature review. JMM Case Rep. 2017;4:e005113.
- Ambesh P, Kapoor A, Kazmi D, et al. Sternal osteomyelitis by Gordonia bronchialis in an immunocompetent patient after open heart surgery. Ann Card Anaesth. 2019;22:221-224.
- Ma TKW, Chow KM, Kwan BCH, et al. Peritoneal-dialysis related peritonitis caused by Gordonia species: report of four cases and literature review. Nephrology. 2014;19:379-383.
- Lam JYW, Wu AKL, Leung WS, et al. Gordonia species as emerging causes of continuous-ambulatory-peritoneal-dialysis-related peritonitis identified by 16S rRNA and secA1 gene sequencing and matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). J Clin Microbiol. 2015;53:671-676.
- Blaschke AJ, Bender J, Byington CL, et al. Gordonia species: emerging pathogens in pediatric patients that are identified by 16S ribosomal RNA gene sequencing. Clin Infect Dis. 2007;45:483-486.
- Titécat M, Loïez C, Courcol RJ, et al. Difficulty with Gordonia bronchialis identification by Microflex mass spectrometer in a pacemaker‐induced endocarditis. JMM Case Rep. 2014;1:E003681.
- Siddiqui N, Toumeh A, Georgescu C. Tibial osteomyelitis caused by Gordonia bronchialis in an immunocompetent patient. J Clin Microbiol. 2012;50:3119-3121.
- Choi R, Strnad L, Flaxel CJ, et al. Gordonia bronchialis–associated endophthalmitis. Emerg Infect Dis. 2019;25:1017-1019.
- Werno AM, Anderson TP, Chambers ST, et al. Recurrent breast abscess caused by Gordonia bronchialis in an immunocompetent patient. J Clin Microbiol. 2005;43:3009-3010.
- Sng LH, Koh TH, Toney SR, et al. Bacteremia caused by Gordonia bronchialis in a patient with sequestrated lung. J Clin Microbiol. 2004;42:2870-2871.
- Ramanan P, Deziel PJ, Wengenack NL. Gordonia bacteremia. J Clin Microbiol. 2013;51:3443-3447.
- Sukackiene D, Rimsevicius L, Kiveryte S, et al. A case of successfully treated relapsing peritoneal dialysis-associated peritonitis caused by Gordonia bronchialis in a farmer. Nephrol Ther. 2018;14:109-111.
- Bruno V, Tjon J, Lin S, et al. Peritoneal dialysis-related peritonitis caused by Gordonia bronchialis: first pediatric report. Pediatr Nephrol. 2022;37:217-220. doi: 10.1007/s00467-021-05313-3
- Mormeneo Bayo S, Palacián Ruíz MP, Asin Samper U, et al. Pacemaker-induced endocarditis by Gordonia bronchialis. Enferm Infecc Microbiol Clin (Engl Ed). 2022;40:255-257.
- Davidson AL, Driscoll CR, Luther VP, et al. Recurrent skin and soft tissue infection following breast reduction surgery caused by Gordonia bronchialis: a case report. Plast Reconstr Surg Glob Open. 2022;10:E4395.
- Nwaedozie S, Mojarrab JN, Gopinath P, et al. Sternal osteomyelitis caused by Gordonia bronchialis in an immunocompetent patient following coronary artery bypass surgery. IDCases. 2022;29:E01548.
- Nakahama H, Hanada S, Takada K, et al. Obstructive pneumonia caused by Gordonia bronchialis with a bronchial foreign body. Int J Infect Dis. 2022;124:157-158. doi:10.1016/j.ijid.2022.09.028
- Lai CC, Hsieh JH, Tsai HY, et al. Cutaneous infection caused by Gordonia amicalis after a traumatic injury. J Clin Microbiol. 2012;50:1821-1822.
- Bakker XR, Spauwen PHM, Dolmans WMV. Mycetoma of the hand caused by Gordona terrae: a case report. J Hand Surg Am. 2004;29:188-190.
- Gueneau R, Blanchet D, Rodriguez-Nava V, et al. Actinomycetoma caused by Gordonia westfalica: first reported case of human infection. New Microbes New Infect. 2020;34:100658.
- Auwaerter PG, ed. The Johns Hopkins POC-IT ABX Guide. Johns Hopkins Medicine; 2021.
- Welsh O, Sauceda E, Gonzalez J, et al. Amikacin alone andin combination with trimethoprim-sulfamethoxazole in the treatment of actinomycotic mycetoma. J Am Acad Dermatol. 1987;17:443-448.
- Zijlstra EE, van de Sande WWJ, Welsh O, et al. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis. 2016;16:100-112.
- Pham AS, Dé I, Rolston KV, et al. Catheter-related bacteremia caused by the nocardioform actinomycete Gordonia terrae. Clin Infect Dis. 2003;36:524-527.
- Renvoise A, Harle JR, Raoult D, et al. Gordonia sputi bacteremia. Emerg Infect Dis. 2009;15:1535-1537.
- Moser BD, Pellegrini GJ, Lasker BA, et al. Pattern of antimicrobial susceptibility obtained from blood isolates of a rare but emerging human pathogen, Gordonia polyisoprenivorans. Antimicrob Agents Chemother. 2012;56:4991-4993.
Practice Points
- Gordonia bronchialis is an emerging cause of human skin and soft tissue infection, typically occurring after trauma, inoculation, or surgery.
- Gordonia species can cause a mycetomalike skin infection.
- Increasing use of molecular methods to identify bacteria has improved identification of clinically relevant actinomycetes, such as Helvetica Neue LT StdGordonia, and increases the likelihood that clinicians will see these organisms on culture results.
Iron Screening in Alopecia Areata Patients May Catch Hereditary Hemochromatosis Early
The role of micronutrients in the hair follicle cycle is not fully understood; thus deficiency and/or excess of certain micronutrients may be a modifiable risk factor associated with the development and/or treatment of some types of hair loss and therefore may be included in the workup during an alopecia consultation.
Hereditary hemochromatosis (HHC) is the most common genetic disorder identified in White individuals, with a worldwide prevalence of 1 in 220 to 1 in 250 individuals for a homozygous mutation. It most commonly affects individuals of Northern European descent.1 Men usually present in the fourth to sixth decades of life, while women usually develop symptoms after menopause, as pregnancy and menstruation delay the onset of the disease.2 Early symptoms of HHC include fatigue, joint pain, abdominal pain, and weight loss. Men are more likely to develop complications; in fact, 1 in 10 men with HHC will develop severe liver disease.3 As the disease progresses, affected individuals can present with cardiomyopathy (restrictive and dilated), cirrhosis, hypogonadism (usually hypogonadotrophic), arthropathy, diabetes mellitus, hepatomegaly, hepatic cirrhosis, and primary liver cancer (eg, hepatocellular carcinoma, cholangiocarcinoma).2 Approximately 90% of patients with HHC present with hyperpigmentation at the time of diagnosis.4 Thinning or loss of hair is another finding in HHC, primarily reported in the axillae and pubic regions, and is ascribed to hepatotesticular insufficiency.5
Alopecia areata (AA) is the most common cause of autoimmune, inflammation-induced hair loss, with a calculated lifetime risk of 2%.6 This disease manifests as loss of hair in well-circumscribed patches of skin, most commonly on the scalp; AA also may affect other hair-bearing sites on the body. It is associated with an increased risk for other autoimmune disorders, such as psoriasis, thyroid disease, rheumatoid arthritis, systemic lupus erythematosus, and vitiligo.7
Alopecia areata is induced by an inflammatory infiltrate of CD4+ and CD8+ T lymphocytes around hair follicles in the anagen stage, the active growth phase.8 Although the diagnosis is clinical, some clinicians order laboratory thyroid studies to investigate conditions that may be associated with AA. Common treatments include topical, intralesional, and/or systemic corticosteroids; contact immunotherapy; topical and more recently oral minoxidil; phototherapy; and topical and systemic JAK inhibitors, including tofacitinib.4,9
We reviewed the medical records of 533 patients who were seen in The University of Texas Southwestern (Dallas, Texas) dermatology clinic from January 2015 through January 2020 and were diagnosed with AA. We examined their demographic data and medical history. We sought to determine any relationship between various types of alopecia and certain micronutrient levels through laboratory test results. Ferritin and iron saturation studies were evaluated. We report 4 cases of HHC concurrent with AA, of which 2 HHC diagnoses were uncovered through iron studies as part of the alopecia evaluation.
Case Reports
Patient 1—A 55-year-old White woman presented to the clinic for an alopecia consultation. She had a medical history of hypothyroidism and AA that was treated unsuccessfully with triamcinolone acetonide steroid injections; topical minoxidil; topical steroids; and systemic steroids, specifically oral prednisone. Following evaluation, she successfully transitioned to treatment with oral tofacitinib and continued to do well on tofacitinib.
The patient’s alopecia workup revealed a ferritin level of 245 ng/mL (reference range, 13–150 ng/mL) and iron saturation of 60% (reference range, 20%–50%). She was referred to the hematology department for further evaluation and was diagnosed with HHC. Genetic testing revealed a heterozygous H63D mutation; therapeutic phlebotomy was recommended. Her sister also was recently diagnosed with HHC.
Patient 2—A 55-year-old White man was referred for evaluation and treatment of alopecia universalis. He had a medical history of skin cancer and vitiligo. He attempted contact immunotherapy with diphenylcyclopropenone scalp treatment but stopped due to intolerable inflammation. Intervention with a topical steroid and topical minoxidil was unsuccessful, but use of triamcinolone acetonide steroid injection on the scalp and topical bimatoprost 0.03% on the eyebrows produced satisfactory results.
The patient’s alopecia workup revealed a ferritin level of 422 ng/mL (reference range, 30–400 ng/mL), which prompted a hematology consultation for further evaluation. Notably, the patient ate red meat several times a week, used iron skillets, and denied receiving blood transfusions. His social habits included 3 alcoholic beverages a night, 5 days a week. Ultrasonography of the liver was recommended to assess potential damage from iron overload and alcohol consumption; the results suggested chronic liver disease, not definitive for cirrhosis, and no evidence of hepatocellular carcinoma. Genetic analysis later revealed the heterozygous H63D variant; therapeutic phlebotomy was recommended.
Patient 3—A 22-year-old White man presented with AA involving his facial beard. He had a medical history of vitiligo and psoriasis and a family history of AA as well as other autoimmune diseases including Hashimoto thyroiditis, psoriasis, eczema, and autoimmune hepatitis. Diphenylcyclopropenone treatment was not successful.
Laboratory studies revealed mildly elevated transaminase and ferritin levels. The patient also presented to the gastroenterologist for evaluation of abdominal pain. Subsequent hematology evaluation confirmed the presence of compound heterozygous C282Y and H63D mutations in the HFE gene, and the patient’s mother was later determined to be homozygous for the C282Y mutation with no elevated ferritin level. The patient’s ferritin level at diagnosis was approximately 500 ng/mL (reference range, 22–322 ng/mL); he required a modest number of therapeutic phlebotomies to normalize his ferritin level.
Patient 4—A 62-year-old White woman presented for evaluation and treatment of patchy hair loss on the scalp of 7 months’ duration. She was subsequently diagnosed with AA. After unsuccessful treatment with a triamcinolone acetonide steroid injection, topical immunotherapy with diphenylcyclopropenone was recommended. The patient achieved full hair regrowth after 35 treatments administered at 3-week intervals.
The patient had a medical history of HHC, including homozygosity for the C282Y mutation, and a family history of HHC in 1 sister. Treatment was therapeutic phlebotomy.
Comment
HHC in the Setting of AA—We presented 4 White patients with both HHC and AA. A PubMed search of articles indexed for MEDLINE using the terms HHC and AA yielded only 1 other reported case of newly identified HHC in a 56-year-old man who presented with pigmented purpuric dermatitis and AA that affected the beard.10 Because HHC is the most common genetic disorder identified in White individuals and has a varied clinical presentation, the documentation of AA may be an important cutaneous clue to help clinicians diagnose HHC early.
Iron Overload in Patients With HHC—The genetic association between HHC and AA, if any, is unknown. What is known is that iron overload can catalyze reactive oxygen species, which can overwhelm cellular antioxidant capacities at particular levels and cause injury to its constituents.11 Data show that the levels of oxidative stress are elevated in the scalp of patients with AA compared to controls and increased 2-fold during the early phase of disease vs late-phase disease.12 Thus, it is possible that increased iron levels in HHC may contribute to AA in genetically susceptible individuals by direct toxicity that ultimately results in the AA hair disorder that is CD8+ T-cell mediated.
Data show that 78% (31/40) of men and 36% (14/39) of women identified with homozygous C282Y mutations determined from family genetic analyses exhibited iron overload.13 In general, a normal life expectancy is possible for patients promptly treated with appropriate therapeutic phlebotomies.14 Thus, early diagnosis and appropriate therapy can prevent consequences of iron overload, which include cirrhosis, diabetes mellitus, and cardiomyopathy.13Iron Screening in the Alopecia Workup—Our cases illustrate how iron screening tests as part of the alopecia workup identified a cohort of White patients with iron overload and subsequently led to an early diagnosis of HHC. The calculated 2% lifetime risk for developing AA highlights the importance of evaluating iron status as part of the AA workup, particularly for White men, and the potential health benefit from early diagnosis of HHC. Limitations of this case series included its retrospective nature and small patient number.
- Bacon BR, Adams PC, Kowdley KV, et al. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:328-343.
- Barton JC, Edwards CQ. HFE hemochromatosis. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews® [Internet]. University of Washington, Seattle; 1993-2020.
- Centers for Disease Control and Prevention. Hereditary hemochromatosis. Accessed September 13, 2022. https://www.cdc.gov/genomics/disease/hemochromatosis.htm
- Ibrahim O, Bayart CB, Hogan S, et al. Treatment of alopecia areata with tofacitinib. JAMA Dermatol. 2017;153:600-602.
- Tweed MJ, Roland JM. Haemochromatosis as an endocrine cause of subfertility. BMJ. 1998;316:915-916. doi:10.1136/bmj.316.7135.915
- Gilhar A, Etzioni A, Paus R. Alopecia areata. N Engl J Med. 2012;366:1515-1525.
- Barahmani N, Schabath MB, Duvic M, et al. History of atopy or autoimmunity increases risk of alopecia areata. J Am Acad Dermatol. 2009;61:581-591.
- McElwee KJ, Freyschmidt-Paul P, Hoffmann R, et al. Transfer of CD8(+) cells induces localized hair loss whereas CD4(+)/CD25(−) cells promote systemic alopecia areata and CD4(+)/CD25(+) cells blockade disease onset in the C3H/HeJ mouse model. J Invest Dermatol. 2005;124:947-957.
- MacDonald Hull SP, Wood ML, Hutchinson PE, et al. Guidelines for the management of alopecia areata. Br J Dermatol. 2003;149:692-699.
- Sredoja Tišma V, Bulimbašic´ S, Jaganjac M, et al. Progressive pigmented purpuric dermatitis and alopecia areata as unusual skin manifestations in recognizing hereditary hemochromatosis. Acta Dermatovenerol Croat. 2012;20:181-186.
- Cabantchik ZI. Labile iron in cells and body fluids: physiology, pathology, and pharmacology. Front Pharmacol. 2014;5:45.
- Akar A, Arca E, Erbil H, et al. Antioxidant enzymes and lipid peroxidation in the scalp of patients with alopecia areata. J Dermatol Sci. 2002;29:85-90.
- Ryan E, Byrnes V, Coughlan B, et al. Underdiagnosis of hereditary haemochromatosis: lack of presentation or penetration? Gut. 2002;51:108-112.
- Niederau C, Strohmeyer G. Strategies for early diagnosis of haemochromatosis. Eur J Gastroenterol Hepatol. 2002;14:217-221.
The role of micronutrients in the hair follicle cycle is not fully understood; thus deficiency and/or excess of certain micronutrients may be a modifiable risk factor associated with the development and/or treatment of some types of hair loss and therefore may be included in the workup during an alopecia consultation.
Hereditary hemochromatosis (HHC) is the most common genetic disorder identified in White individuals, with a worldwide prevalence of 1 in 220 to 1 in 250 individuals for a homozygous mutation. It most commonly affects individuals of Northern European descent.1 Men usually present in the fourth to sixth decades of life, while women usually develop symptoms after menopause, as pregnancy and menstruation delay the onset of the disease.2 Early symptoms of HHC include fatigue, joint pain, abdominal pain, and weight loss. Men are more likely to develop complications; in fact, 1 in 10 men with HHC will develop severe liver disease.3 As the disease progresses, affected individuals can present with cardiomyopathy (restrictive and dilated), cirrhosis, hypogonadism (usually hypogonadotrophic), arthropathy, diabetes mellitus, hepatomegaly, hepatic cirrhosis, and primary liver cancer (eg, hepatocellular carcinoma, cholangiocarcinoma).2 Approximately 90% of patients with HHC present with hyperpigmentation at the time of diagnosis.4 Thinning or loss of hair is another finding in HHC, primarily reported in the axillae and pubic regions, and is ascribed to hepatotesticular insufficiency.5
Alopecia areata (AA) is the most common cause of autoimmune, inflammation-induced hair loss, with a calculated lifetime risk of 2%.6 This disease manifests as loss of hair in well-circumscribed patches of skin, most commonly on the scalp; AA also may affect other hair-bearing sites on the body. It is associated with an increased risk for other autoimmune disorders, such as psoriasis, thyroid disease, rheumatoid arthritis, systemic lupus erythematosus, and vitiligo.7
Alopecia areata is induced by an inflammatory infiltrate of CD4+ and CD8+ T lymphocytes around hair follicles in the anagen stage, the active growth phase.8 Although the diagnosis is clinical, some clinicians order laboratory thyroid studies to investigate conditions that may be associated with AA. Common treatments include topical, intralesional, and/or systemic corticosteroids; contact immunotherapy; topical and more recently oral minoxidil; phototherapy; and topical and systemic JAK inhibitors, including tofacitinib.4,9
We reviewed the medical records of 533 patients who were seen in The University of Texas Southwestern (Dallas, Texas) dermatology clinic from January 2015 through January 2020 and were diagnosed with AA. We examined their demographic data and medical history. We sought to determine any relationship between various types of alopecia and certain micronutrient levels through laboratory test results. Ferritin and iron saturation studies were evaluated. We report 4 cases of HHC concurrent with AA, of which 2 HHC diagnoses were uncovered through iron studies as part of the alopecia evaluation.
Case Reports
Patient 1—A 55-year-old White woman presented to the clinic for an alopecia consultation. She had a medical history of hypothyroidism and AA that was treated unsuccessfully with triamcinolone acetonide steroid injections; topical minoxidil; topical steroids; and systemic steroids, specifically oral prednisone. Following evaluation, she successfully transitioned to treatment with oral tofacitinib and continued to do well on tofacitinib.
The patient’s alopecia workup revealed a ferritin level of 245 ng/mL (reference range, 13–150 ng/mL) and iron saturation of 60% (reference range, 20%–50%). She was referred to the hematology department for further evaluation and was diagnosed with HHC. Genetic testing revealed a heterozygous H63D mutation; therapeutic phlebotomy was recommended. Her sister also was recently diagnosed with HHC.
Patient 2—A 55-year-old White man was referred for evaluation and treatment of alopecia universalis. He had a medical history of skin cancer and vitiligo. He attempted contact immunotherapy with diphenylcyclopropenone scalp treatment but stopped due to intolerable inflammation. Intervention with a topical steroid and topical minoxidil was unsuccessful, but use of triamcinolone acetonide steroid injection on the scalp and topical bimatoprost 0.03% on the eyebrows produced satisfactory results.
The patient’s alopecia workup revealed a ferritin level of 422 ng/mL (reference range, 30–400 ng/mL), which prompted a hematology consultation for further evaluation. Notably, the patient ate red meat several times a week, used iron skillets, and denied receiving blood transfusions. His social habits included 3 alcoholic beverages a night, 5 days a week. Ultrasonography of the liver was recommended to assess potential damage from iron overload and alcohol consumption; the results suggested chronic liver disease, not definitive for cirrhosis, and no evidence of hepatocellular carcinoma. Genetic analysis later revealed the heterozygous H63D variant; therapeutic phlebotomy was recommended.
Patient 3—A 22-year-old White man presented with AA involving his facial beard. He had a medical history of vitiligo and psoriasis and a family history of AA as well as other autoimmune diseases including Hashimoto thyroiditis, psoriasis, eczema, and autoimmune hepatitis. Diphenylcyclopropenone treatment was not successful.
Laboratory studies revealed mildly elevated transaminase and ferritin levels. The patient also presented to the gastroenterologist for evaluation of abdominal pain. Subsequent hematology evaluation confirmed the presence of compound heterozygous C282Y and H63D mutations in the HFE gene, and the patient’s mother was later determined to be homozygous for the C282Y mutation with no elevated ferritin level. The patient’s ferritin level at diagnosis was approximately 500 ng/mL (reference range, 22–322 ng/mL); he required a modest number of therapeutic phlebotomies to normalize his ferritin level.
Patient 4—A 62-year-old White woman presented for evaluation and treatment of patchy hair loss on the scalp of 7 months’ duration. She was subsequently diagnosed with AA. After unsuccessful treatment with a triamcinolone acetonide steroid injection, topical immunotherapy with diphenylcyclopropenone was recommended. The patient achieved full hair regrowth after 35 treatments administered at 3-week intervals.
The patient had a medical history of HHC, including homozygosity for the C282Y mutation, and a family history of HHC in 1 sister. Treatment was therapeutic phlebotomy.
Comment
HHC in the Setting of AA—We presented 4 White patients with both HHC and AA. A PubMed search of articles indexed for MEDLINE using the terms HHC and AA yielded only 1 other reported case of newly identified HHC in a 56-year-old man who presented with pigmented purpuric dermatitis and AA that affected the beard.10 Because HHC is the most common genetic disorder identified in White individuals and has a varied clinical presentation, the documentation of AA may be an important cutaneous clue to help clinicians diagnose HHC early.
Iron Overload in Patients With HHC—The genetic association between HHC and AA, if any, is unknown. What is known is that iron overload can catalyze reactive oxygen species, which can overwhelm cellular antioxidant capacities at particular levels and cause injury to its constituents.11 Data show that the levels of oxidative stress are elevated in the scalp of patients with AA compared to controls and increased 2-fold during the early phase of disease vs late-phase disease.12 Thus, it is possible that increased iron levels in HHC may contribute to AA in genetically susceptible individuals by direct toxicity that ultimately results in the AA hair disorder that is CD8+ T-cell mediated.
Data show that 78% (31/40) of men and 36% (14/39) of women identified with homozygous C282Y mutations determined from family genetic analyses exhibited iron overload.13 In general, a normal life expectancy is possible for patients promptly treated with appropriate therapeutic phlebotomies.14 Thus, early diagnosis and appropriate therapy can prevent consequences of iron overload, which include cirrhosis, diabetes mellitus, and cardiomyopathy.13Iron Screening in the Alopecia Workup—Our cases illustrate how iron screening tests as part of the alopecia workup identified a cohort of White patients with iron overload and subsequently led to an early diagnosis of HHC. The calculated 2% lifetime risk for developing AA highlights the importance of evaluating iron status as part of the AA workup, particularly for White men, and the potential health benefit from early diagnosis of HHC. Limitations of this case series included its retrospective nature and small patient number.
The role of micronutrients in the hair follicle cycle is not fully understood; thus deficiency and/or excess of certain micronutrients may be a modifiable risk factor associated with the development and/or treatment of some types of hair loss and therefore may be included in the workup during an alopecia consultation.
Hereditary hemochromatosis (HHC) is the most common genetic disorder identified in White individuals, with a worldwide prevalence of 1 in 220 to 1 in 250 individuals for a homozygous mutation. It most commonly affects individuals of Northern European descent.1 Men usually present in the fourth to sixth decades of life, while women usually develop symptoms after menopause, as pregnancy and menstruation delay the onset of the disease.2 Early symptoms of HHC include fatigue, joint pain, abdominal pain, and weight loss. Men are more likely to develop complications; in fact, 1 in 10 men with HHC will develop severe liver disease.3 As the disease progresses, affected individuals can present with cardiomyopathy (restrictive and dilated), cirrhosis, hypogonadism (usually hypogonadotrophic), arthropathy, diabetes mellitus, hepatomegaly, hepatic cirrhosis, and primary liver cancer (eg, hepatocellular carcinoma, cholangiocarcinoma).2 Approximately 90% of patients with HHC present with hyperpigmentation at the time of diagnosis.4 Thinning or loss of hair is another finding in HHC, primarily reported in the axillae and pubic regions, and is ascribed to hepatotesticular insufficiency.5
Alopecia areata (AA) is the most common cause of autoimmune, inflammation-induced hair loss, with a calculated lifetime risk of 2%.6 This disease manifests as loss of hair in well-circumscribed patches of skin, most commonly on the scalp; AA also may affect other hair-bearing sites on the body. It is associated with an increased risk for other autoimmune disorders, such as psoriasis, thyroid disease, rheumatoid arthritis, systemic lupus erythematosus, and vitiligo.7
Alopecia areata is induced by an inflammatory infiltrate of CD4+ and CD8+ T lymphocytes around hair follicles in the anagen stage, the active growth phase.8 Although the diagnosis is clinical, some clinicians order laboratory thyroid studies to investigate conditions that may be associated with AA. Common treatments include topical, intralesional, and/or systemic corticosteroids; contact immunotherapy; topical and more recently oral minoxidil; phototherapy; and topical and systemic JAK inhibitors, including tofacitinib.4,9
We reviewed the medical records of 533 patients who were seen in The University of Texas Southwestern (Dallas, Texas) dermatology clinic from January 2015 through January 2020 and were diagnosed with AA. We examined their demographic data and medical history. We sought to determine any relationship between various types of alopecia and certain micronutrient levels through laboratory test results. Ferritin and iron saturation studies were evaluated. We report 4 cases of HHC concurrent with AA, of which 2 HHC diagnoses were uncovered through iron studies as part of the alopecia evaluation.
Case Reports
Patient 1—A 55-year-old White woman presented to the clinic for an alopecia consultation. She had a medical history of hypothyroidism and AA that was treated unsuccessfully with triamcinolone acetonide steroid injections; topical minoxidil; topical steroids; and systemic steroids, specifically oral prednisone. Following evaluation, she successfully transitioned to treatment with oral tofacitinib and continued to do well on tofacitinib.
The patient’s alopecia workup revealed a ferritin level of 245 ng/mL (reference range, 13–150 ng/mL) and iron saturation of 60% (reference range, 20%–50%). She was referred to the hematology department for further evaluation and was diagnosed with HHC. Genetic testing revealed a heterozygous H63D mutation; therapeutic phlebotomy was recommended. Her sister also was recently diagnosed with HHC.
Patient 2—A 55-year-old White man was referred for evaluation and treatment of alopecia universalis. He had a medical history of skin cancer and vitiligo. He attempted contact immunotherapy with diphenylcyclopropenone scalp treatment but stopped due to intolerable inflammation. Intervention with a topical steroid and topical minoxidil was unsuccessful, but use of triamcinolone acetonide steroid injection on the scalp and topical bimatoprost 0.03% on the eyebrows produced satisfactory results.
The patient’s alopecia workup revealed a ferritin level of 422 ng/mL (reference range, 30–400 ng/mL), which prompted a hematology consultation for further evaluation. Notably, the patient ate red meat several times a week, used iron skillets, and denied receiving blood transfusions. His social habits included 3 alcoholic beverages a night, 5 days a week. Ultrasonography of the liver was recommended to assess potential damage from iron overload and alcohol consumption; the results suggested chronic liver disease, not definitive for cirrhosis, and no evidence of hepatocellular carcinoma. Genetic analysis later revealed the heterozygous H63D variant; therapeutic phlebotomy was recommended.
Patient 3—A 22-year-old White man presented with AA involving his facial beard. He had a medical history of vitiligo and psoriasis and a family history of AA as well as other autoimmune diseases including Hashimoto thyroiditis, psoriasis, eczema, and autoimmune hepatitis. Diphenylcyclopropenone treatment was not successful.
Laboratory studies revealed mildly elevated transaminase and ferritin levels. The patient also presented to the gastroenterologist for evaluation of abdominal pain. Subsequent hematology evaluation confirmed the presence of compound heterozygous C282Y and H63D mutations in the HFE gene, and the patient’s mother was later determined to be homozygous for the C282Y mutation with no elevated ferritin level. The patient’s ferritin level at diagnosis was approximately 500 ng/mL (reference range, 22–322 ng/mL); he required a modest number of therapeutic phlebotomies to normalize his ferritin level.
Patient 4—A 62-year-old White woman presented for evaluation and treatment of patchy hair loss on the scalp of 7 months’ duration. She was subsequently diagnosed with AA. After unsuccessful treatment with a triamcinolone acetonide steroid injection, topical immunotherapy with diphenylcyclopropenone was recommended. The patient achieved full hair regrowth after 35 treatments administered at 3-week intervals.
The patient had a medical history of HHC, including homozygosity for the C282Y mutation, and a family history of HHC in 1 sister. Treatment was therapeutic phlebotomy.
Comment
HHC in the Setting of AA—We presented 4 White patients with both HHC and AA. A PubMed search of articles indexed for MEDLINE using the terms HHC and AA yielded only 1 other reported case of newly identified HHC in a 56-year-old man who presented with pigmented purpuric dermatitis and AA that affected the beard.10 Because HHC is the most common genetic disorder identified in White individuals and has a varied clinical presentation, the documentation of AA may be an important cutaneous clue to help clinicians diagnose HHC early.
Iron Overload in Patients With HHC—The genetic association between HHC and AA, if any, is unknown. What is known is that iron overload can catalyze reactive oxygen species, which can overwhelm cellular antioxidant capacities at particular levels and cause injury to its constituents.11 Data show that the levels of oxidative stress are elevated in the scalp of patients with AA compared to controls and increased 2-fold during the early phase of disease vs late-phase disease.12 Thus, it is possible that increased iron levels in HHC may contribute to AA in genetically susceptible individuals by direct toxicity that ultimately results in the AA hair disorder that is CD8+ T-cell mediated.
Data show that 78% (31/40) of men and 36% (14/39) of women identified with homozygous C282Y mutations determined from family genetic analyses exhibited iron overload.13 In general, a normal life expectancy is possible for patients promptly treated with appropriate therapeutic phlebotomies.14 Thus, early diagnosis and appropriate therapy can prevent consequences of iron overload, which include cirrhosis, diabetes mellitus, and cardiomyopathy.13Iron Screening in the Alopecia Workup—Our cases illustrate how iron screening tests as part of the alopecia workup identified a cohort of White patients with iron overload and subsequently led to an early diagnosis of HHC. The calculated 2% lifetime risk for developing AA highlights the importance of evaluating iron status as part of the AA workup, particularly for White men, and the potential health benefit from early diagnosis of HHC. Limitations of this case series included its retrospective nature and small patient number.
- Bacon BR, Adams PC, Kowdley KV, et al. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:328-343.
- Barton JC, Edwards CQ. HFE hemochromatosis. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews® [Internet]. University of Washington, Seattle; 1993-2020.
- Centers for Disease Control and Prevention. Hereditary hemochromatosis. Accessed September 13, 2022. https://www.cdc.gov/genomics/disease/hemochromatosis.htm
- Ibrahim O, Bayart CB, Hogan S, et al. Treatment of alopecia areata with tofacitinib. JAMA Dermatol. 2017;153:600-602.
- Tweed MJ, Roland JM. Haemochromatosis as an endocrine cause of subfertility. BMJ. 1998;316:915-916. doi:10.1136/bmj.316.7135.915
- Gilhar A, Etzioni A, Paus R. Alopecia areata. N Engl J Med. 2012;366:1515-1525.
- Barahmani N, Schabath MB, Duvic M, et al. History of atopy or autoimmunity increases risk of alopecia areata. J Am Acad Dermatol. 2009;61:581-591.
- McElwee KJ, Freyschmidt-Paul P, Hoffmann R, et al. Transfer of CD8(+) cells induces localized hair loss whereas CD4(+)/CD25(−) cells promote systemic alopecia areata and CD4(+)/CD25(+) cells blockade disease onset in the C3H/HeJ mouse model. J Invest Dermatol. 2005;124:947-957.
- MacDonald Hull SP, Wood ML, Hutchinson PE, et al. Guidelines for the management of alopecia areata. Br J Dermatol. 2003;149:692-699.
- Sredoja Tišma V, Bulimbašic´ S, Jaganjac M, et al. Progressive pigmented purpuric dermatitis and alopecia areata as unusual skin manifestations in recognizing hereditary hemochromatosis. Acta Dermatovenerol Croat. 2012;20:181-186.
- Cabantchik ZI. Labile iron in cells and body fluids: physiology, pathology, and pharmacology. Front Pharmacol. 2014;5:45.
- Akar A, Arca E, Erbil H, et al. Antioxidant enzymes and lipid peroxidation in the scalp of patients with alopecia areata. J Dermatol Sci. 2002;29:85-90.
- Ryan E, Byrnes V, Coughlan B, et al. Underdiagnosis of hereditary haemochromatosis: lack of presentation or penetration? Gut. 2002;51:108-112.
- Niederau C, Strohmeyer G. Strategies for early diagnosis of haemochromatosis. Eur J Gastroenterol Hepatol. 2002;14:217-221.
- Bacon BR, Adams PC, Kowdley KV, et al. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:328-343.
- Barton JC, Edwards CQ. HFE hemochromatosis. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews® [Internet]. University of Washington, Seattle; 1993-2020.
- Centers for Disease Control and Prevention. Hereditary hemochromatosis. Accessed September 13, 2022. https://www.cdc.gov/genomics/disease/hemochromatosis.htm
- Ibrahim O, Bayart CB, Hogan S, et al. Treatment of alopecia areata with tofacitinib. JAMA Dermatol. 2017;153:600-602.
- Tweed MJ, Roland JM. Haemochromatosis as an endocrine cause of subfertility. BMJ. 1998;316:915-916. doi:10.1136/bmj.316.7135.915
- Gilhar A, Etzioni A, Paus R. Alopecia areata. N Engl J Med. 2012;366:1515-1525.
- Barahmani N, Schabath MB, Duvic M, et al. History of atopy or autoimmunity increases risk of alopecia areata. J Am Acad Dermatol. 2009;61:581-591.
- McElwee KJ, Freyschmidt-Paul P, Hoffmann R, et al. Transfer of CD8(+) cells induces localized hair loss whereas CD4(+)/CD25(−) cells promote systemic alopecia areata and CD4(+)/CD25(+) cells blockade disease onset in the C3H/HeJ mouse model. J Invest Dermatol. 2005;124:947-957.
- MacDonald Hull SP, Wood ML, Hutchinson PE, et al. Guidelines for the management of alopecia areata. Br J Dermatol. 2003;149:692-699.
- Sredoja Tišma V, Bulimbašic´ S, Jaganjac M, et al. Progressive pigmented purpuric dermatitis and alopecia areata as unusual skin manifestations in recognizing hereditary hemochromatosis. Acta Dermatovenerol Croat. 2012;20:181-186.
- Cabantchik ZI. Labile iron in cells and body fluids: physiology, pathology, and pharmacology. Front Pharmacol. 2014;5:45.
- Akar A, Arca E, Erbil H, et al. Antioxidant enzymes and lipid peroxidation in the scalp of patients with alopecia areata. J Dermatol Sci. 2002;29:85-90.
- Ryan E, Byrnes V, Coughlan B, et al. Underdiagnosis of hereditary haemochromatosis: lack of presentation or penetration? Gut. 2002;51:108-112.
- Niederau C, Strohmeyer G. Strategies for early diagnosis of haemochromatosis. Eur J Gastroenterol Hepatol. 2002;14:217-221.
Practice Points
- Hereditary hemochromatosis (HHC) is a disorder of iron overload that presents with clinical phenotypic heterogeneity. Complications can be mitigated with early intervention.
- Alopecia areata (AA) may be a rare early cutaneous manifestation of HHC in individuals with a predisposition for autoimmunity; therefore, it is important to evaluate iron status as part of the AA workup.
IgA Vasculitis in the Setting of Biologic Therapy for Psoriasis and Recurrent Cutaneous Methicillin-Resistant Staphylococcus aureus Colonization
Case Report
A 47-year-old man presented with a sudden-onset rash consisting of red bumps on the abdomen and legs that had been ongoing for several days. He had known psoriasis and psoriatic arthritis that had been well controlled with adalimumab for the last 18 months. He reported concurrent onset of nausea but denied fevers, chills, night sweats, unintentional weight loss, abdominal pain, and pruritus. He endorsed prior cutaneous infections of methicillin-resistant Staphylococcus aureus (MRSA). His medical history also included diabetes mellitus, hypertension, and obesity. His other medications included oral losartan-hydrochlorothiazide, amlodipine, naproxen, and atorvastatin.
Physical examination revealed numerous thin purpuric papules—some with adherent scale—distributed on the lower legs, extensor forearms, and abdomen. Abdominal lesions were confined to weight-related striae (Figure 1). The palms, soles, oral mucosa, and face were spared. Three punch biopsies were performed, including 1 for direct immunofluorescence (DIF), and the patient was instructed to apply clobetasol to the affected areas twice daily until further notice.
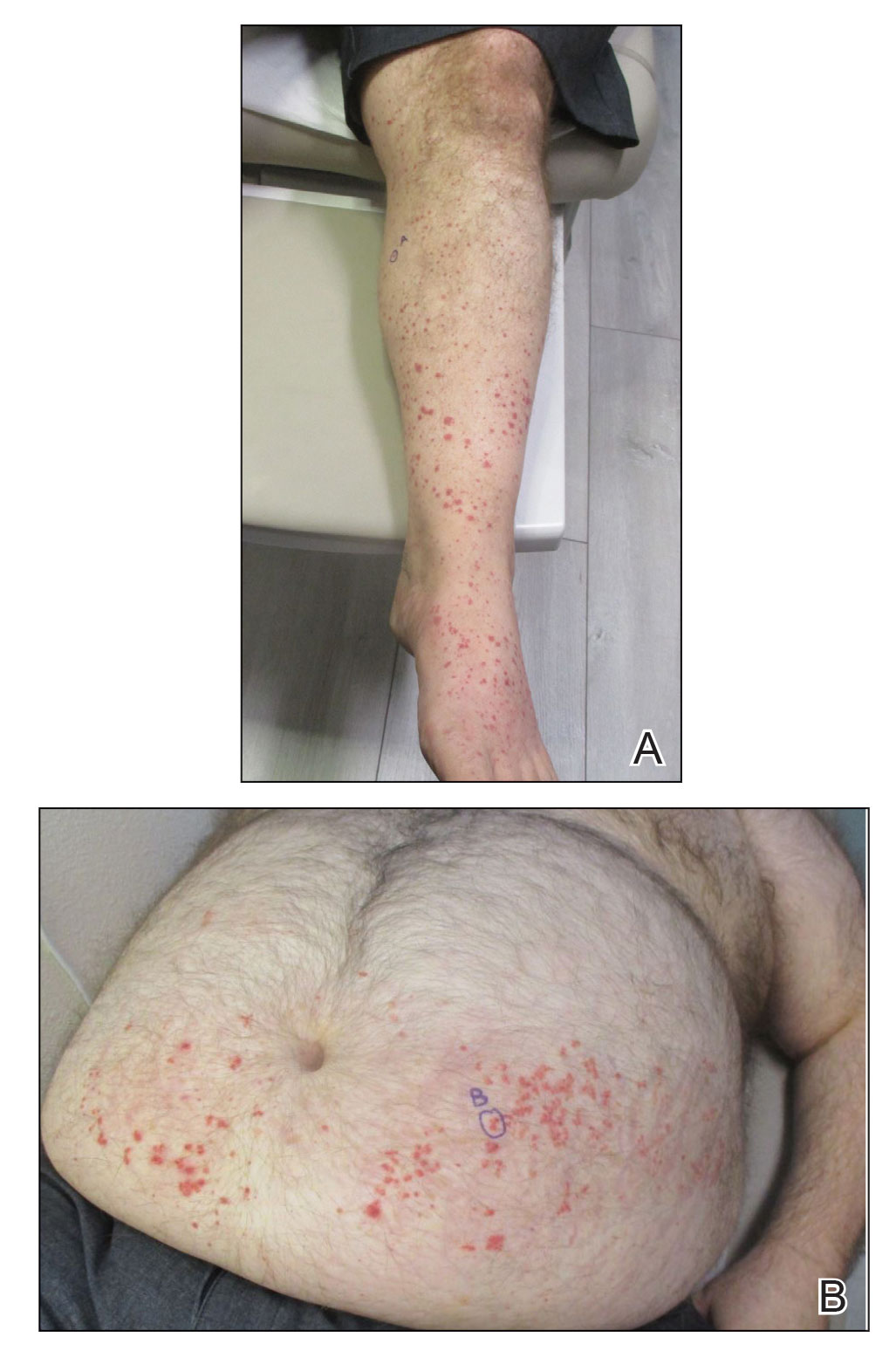
Pathology showed perivascular extravasation of erythrocytes, neutrophils, eosinophils, and leukocytoclasis surrounding blood vessels associated with fibrin (Figure 2). Direct immunofluorescence showed granular deposition of IgA, complement component 3, and fibrinogen in a superficial dermal vascular pattern (Figure 3). These results were consistent with IgA small-vessel vasculitis. One specimen was consistent with the patient’s known psoriasis.
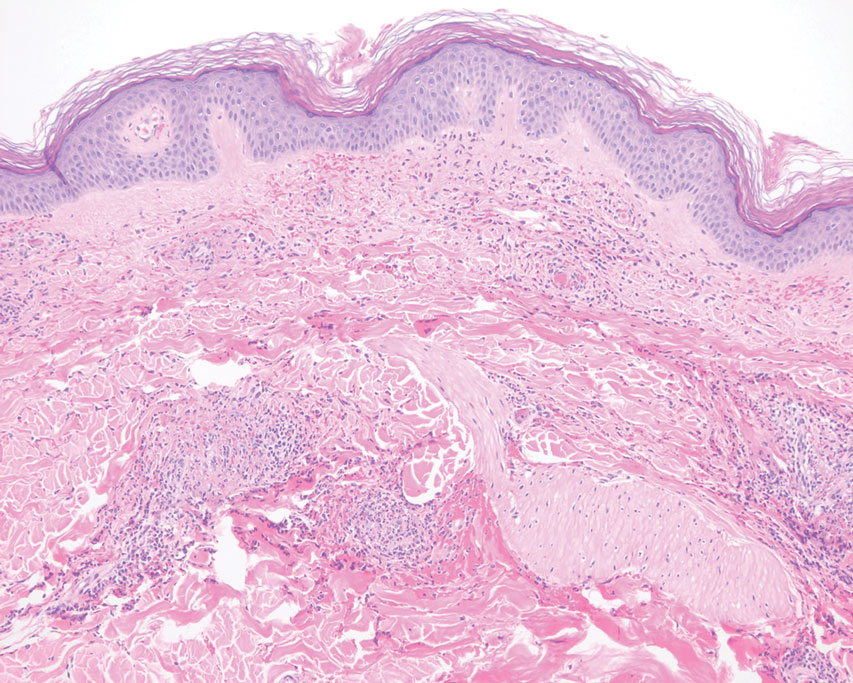
Urinalysis revealed moderate hemoglobinuria, and urine microscopy showed 174 red blood cells per high-power field. Creatinine was high at 1.87 mg/dL (reference range, <1.34 mg/dL; patient’s baseline, 0.81 mg/dL) and glomerular filtration rate was low (42 mL/min, patient’s baseline, >60 mL/min [reference range, 90–120 mL/min]). Erythrocyte sedimentation rate (21 mm/h [reference range, 0–22 mm/h]) and C-reactive protein were elevated (2.2 mg/dL [reference range, 0.3–1.0 mg/dL]). Given his history of cutaneous MRSA infections, a bacterial culture swab was collected from the skin surface to check for colonization, which showed moderate growth of MRSA. Naproxen was discontinued over concern of worsening the patient’s renal status. The patient was instructed to rest at home with his legs elevated, wear compression socks when ambulatory, use chlorhexidine antiseptic daily as a body wash when showering, and apply mupirocin three times daily to the biopsy sites. He was referred to urology for his microhematuria, where cystoscopy revealed no abnormalities.A month passed with no improvement of the patient’s cutaneous vasculitis, and his psoriatic arthritis worsened without his usual use of naproxen. He developed abdominal pain and loss of appetite. A prednisone taper was ordered starting at 40 mg/d (28.8 mg/kg), which provided relief of the skin and joint symptoms only until the course was completed 12 days later.
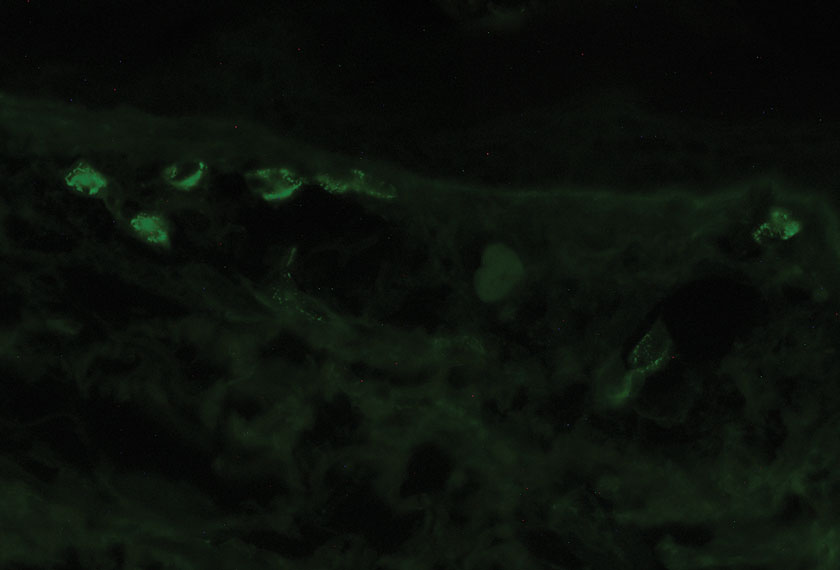
Five weeks after the initial presentation, the patient returned with a more severe eruption consisting of innumerable purpuric papules that coalesced in plaques on the abdomen, arms, and legs. He also had erythematous facial pustules and mild palmar petechiae (Figure 4). Three biopsies were performed, including 1 for DIF and 1 from a pustule on the forehead. Histology and DIF were again consistent with IgA small-vessel vasculitis. The forehead biopsy was compatible with steroid acne (attributed to recent prednisone use) and psoriasis.
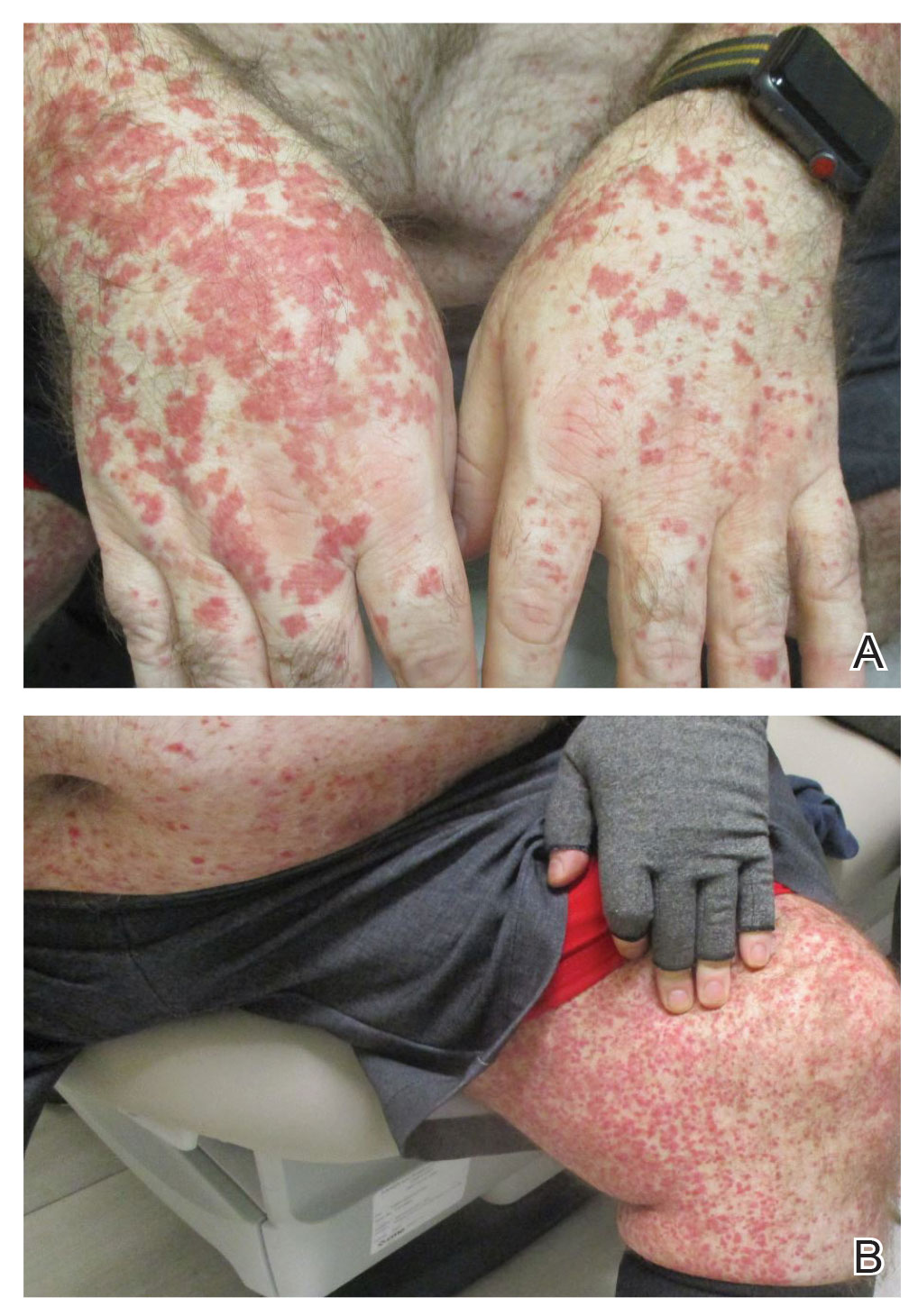
Rheumatology was consulted, and adalimumab was discontinued 6 weeks after the initial presentation out of concern for drug-induced cutaneous vasculitis. Vasculitis work-up was unremarkable, including antineutrophil cytoplasmic antibodies, rheumatoid factor, cyclic citrullinated peptide, and serum protein electrophoresis. Oral dapsone was started at 100 mg/d, with the tentative plan of starting secukinumab if cutaneous symptoms improved. For 3 weeks, the patient’s cutaneous symptoms steadily improved.
Nine weeks after initial presentation to dermatology (3 weeks after discontinuing adalimumab) the patient self-administered his first dose of secukinumab at home. Several hours later, he reported sudden reappearance of vasculitis. He denied diarrhea, abdominal pain, bowel movement urgency, fevers, fatigue, and unintentional weight loss. Antistreptolysin O and hepatitis A antibodies were negative. He was instructed to hold secukinumab indefinitely.
Four weeks after his only secukinumab injection, the patient reported another episode of acute worsening cutaneous symptoms. A 4-week prednisone taper starting at 40 mg/d was ordered. Computed tomography of the chest, abdomen, and pelvis to rule out internal malignancy was unremarkable. Around this time, the patient reported major emotional distress related to an unexpected death in his family, which added to a gradual increase in his stress level related to the COVID-19 pandemic.
Three weeks later, dapsone was increased to 100 mg twice daily on account of the patient’s adiposity and lack of cutaneous improvement on the lower dose. Subsequently, the vasculitis rapidly improved for 2 weeks. The patient then reported symptoms of headache, dizziness, and chills. He was tested for COVID-19 and was negative. Six weeks after increasing the dapsone dose (5 months after initial presentation), the skin was normalizing, showing only faintly hyperpigmented macules confined to areas of resolved vasculitis (forearms, abdomen, legs).
The patient had been on dapsone 100 mg twice daily for 3 months when he was started on ustekinumab (90 mg at weeks 0 and 4, with planned doses every 12 weeks) for psoriatic arthritis in hopes of withdrawing dapsone. His cutaneous symptoms have remained well controlled on this regimen for 18 months. Lowering of dapsone below 100 mg daily has resulted in recurrent mild vasculitis symptoms; he now maintains the once-daily dosing without negative side effects.
Comment
IgA vasculitis is a form of cutaneous small-vessel leukocytoclastic vasculitis (LCV) characterized by episodes of palpable purpura on the extensor surfaces of the arms and legs that may be associated with arthritis, abdominal pain, and/or hematuria. Although vasculitis is a known potential adverse effect of anti–tumor necrosis factor (TNF) α therapy, cases of adalimumab-induced IgA vasculitis are uncommon. As use of more targeted therapies for psoriasis and psoriatic arthritis, such as the IL-17 inhibitor secukinumab, increases so do reports of associated adverse events. Of 6 previously reported cases of secukinumab-associated vasculitis, at least 4 were IgA vasculitis (Table).1-6 Another case described one patient with rheumatoid arthritis undergoing secukinumab treatment who experienced necrotizing glomerulonephritis; however, the authors concluded secukinumab likely was not causative in that case, as serologies and urinalyses suggested gradual onset of the process prior to initiating the medication.7
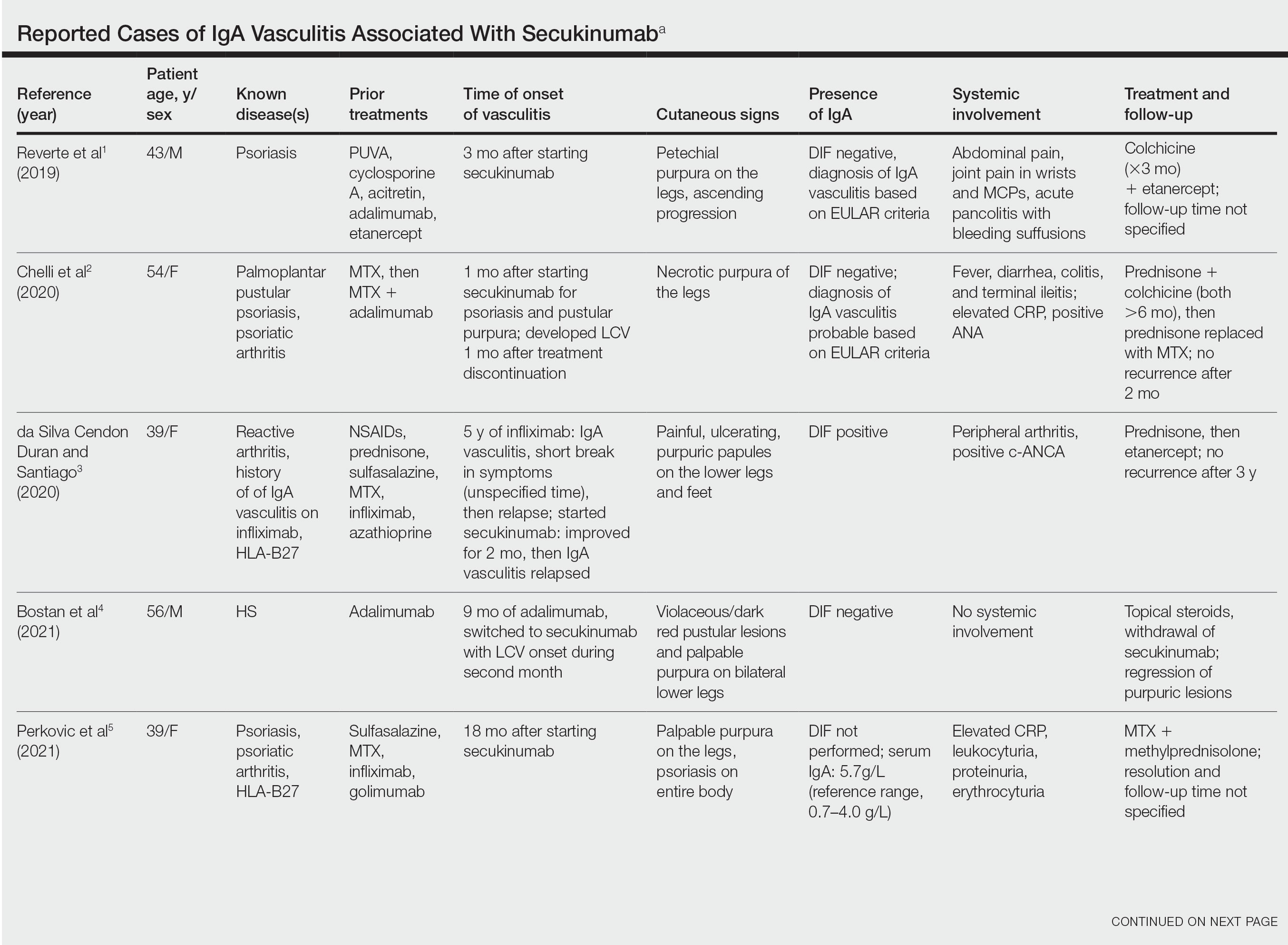
The exact pathogenesis of IgA vasculitis is unclear, but a prevailing theory involves the dysregulation of IgA synthesis and metabolism. Other than increased serum levels of transforming growth factor β, which is a major stimulating factor for IgA production, it also has been hypothesized that the presence of aberrantly hypoglycosylated IgA exposes an autoepitope for recognition by other pathogenic IgG and IgA, leading to the formation of large immune complexes that can readily deposit in postcapillary venules. The deposition of IgA immune complexes in postcapillary venules and the subsequent activation of the complement system causes direct damage to the endothelial cells of vessel walls. This complement activation is evidenced by vascular complement component 3 deposition on DIF (a nonspecific feature of LCV). Chemotaxis of neutrophils ensues, followed by their firm adherence and transendothelial migration (mediated by monocyte chemoattractant protein 1 [MCP-1]). Neutrophil degranulation releases reactive oxygen species and cytokines, which in turn recruit additional leukocytes to the area of inflammation, subsequently undergoing degeneration (leukocytoclasis). Microvascular permeability also is enhanced by MCP-1, allowing exudation of serum, erythrocytes, and fibrin. In the setting of elevated circulating TNF and IL-1, endothelium is stimulated to activate the intrinsic and extrinsic coagulation pathways. This decreases endothelial fibrinolytic activity, leading to thrombosis. The high venous pressure and low fibrinolytic activity in the lower legs explains why vasculitic lesions often are confined to or begin in this distribution.1,8-10
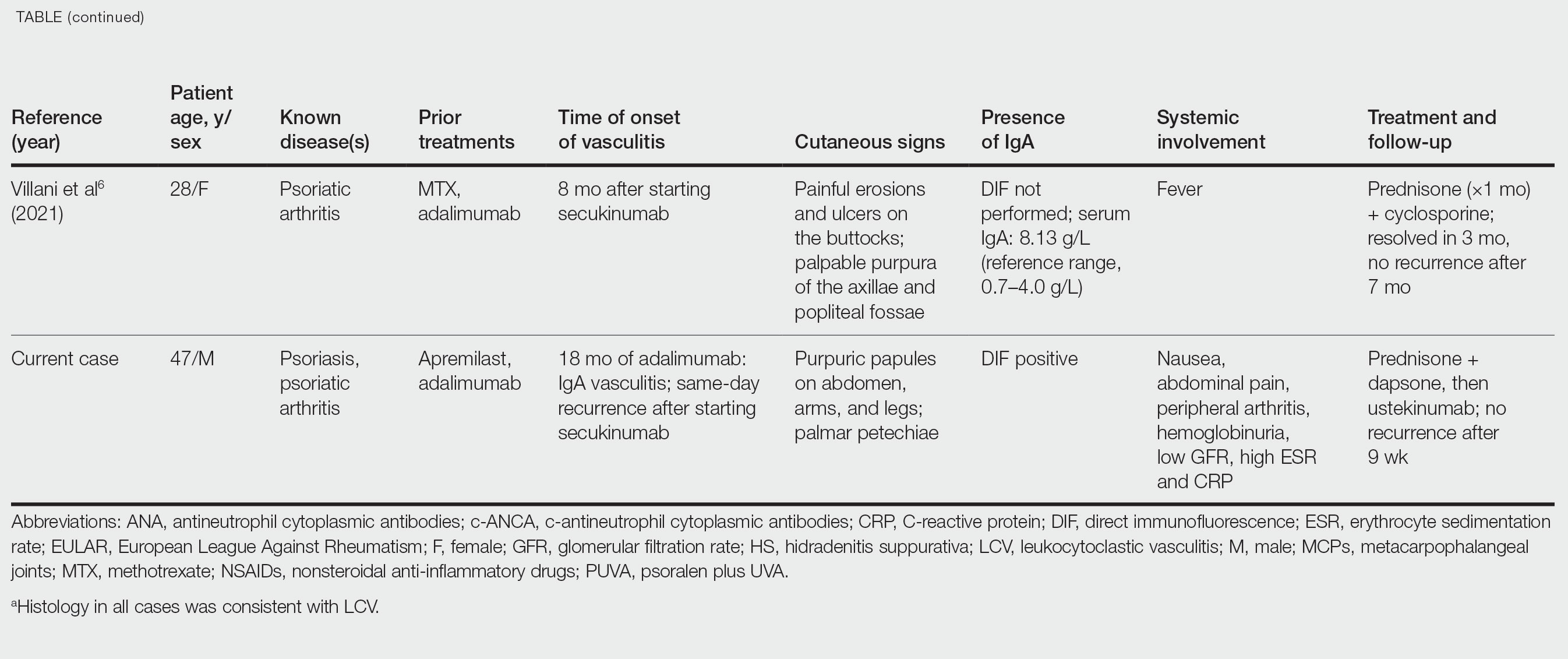
There also are noteworthy roles for cytokines in LCV. Circulating transforming growth factor β and IL-6—which are necessary for development of T helper 17 (TH17) cells and production of IL-17—are higher in patients with LCV compared to controls. Peripheral blood monocytes in patients with LCV demonstrate higher production of IL-17. Once TH17 cells develop, their survival and phenotype are maintained by IL-23 (considered the master regulator of TH17 differentiation). IL-17 is a potent chemoattractant of IL-8 (CXCL8) and MCP-1, both of which promote neutrophil-mediated perivascular inflammation. The IL-23 and IL-17 pathways implicated in the pathogenesis of psoriasis also cause neutrophil activation and upregulate transcription of proinflammatory cytokines (IL-1, IL-6, IL-8, and TNF-α), which overlap with those implicated in LCV. Autoimmune disease generally entails some positive feedback loop of progressively severe self-recognition and tissue destruction by the immune system. These shared cytokinetic processes may explain how the internal environment of psoriasis could perpetuate IgA vasculitis.1,2,8,10-12
The mechanisms underlying vasculitis associated with adalimumab are unclear, but hypotheses involve direct toxicity on vessels, capillary deposition of anti-TNF/TNF immune complexes, or an inflammatory process resulting in autoantibodies. Similar hypotheses are posited for secukinumab-associated vasculitis, including deposition of secukinumab–IL-17 complexes. Anti–TNF-α medications may increase TH17 cell numbers, leading to increased production of IL-22 and a resultant immunologic microenvironment conducive to vasculitis. All 6 published cases of secukinumab-associated vasculitis that we found had received prior treatment with a TNF-α blocker, but only 1 had occurrence of vasculitis during that treatment.1-6,10
In the 6 cases we reviewed, the time from starting secukinumab to onset of vasculitis ranged from 1 to 18 months. Our patient’s same-day re-emergence of vasculitis after his first secukinumab dose was so acute that we were skeptical of secukinumab as a potential trigger; this may simply have been coincident to the natural waxing and waning of the vasculitis (although onset of IgA vasculitis within 1 day of starting anti–TNF-α therapy has been reported).1-6,13
Specific associations of IgA vasculitis are many and can include bacterial organisms such as Helicobacter pylori, streptococci, and staphylococci. Although internal mucous membrane infections are considered more linked because of the surveillance role of IgA predominantly in mucosal tissues, it is possible that our patient with cutaneous MRSA harbored the same within the nasal mucosa. Our patient also received multiple vaccinations outside our department throughout his clinical course (2 hepatitis B and 1 pneumococcal conjugate), which are known potential triggers for vasculitis. Psychological stress is a known trigger for psoriasis, and given the cytokinetic relationship of psoriasis to vasculitis described previously, it may have indirectly contributed to vasculitis in our case. The anxiety associated with being immunosuppressed during the COVID-19 pandemic and bereavement of losing a family member may have contributed to the refractory nature of our patient’s condition. Renal involvement is relatively common in adults with IgA vasculitis and so should be ruled out, as should occult internal malignancy.8,10,14
It is unclear which of the above factors was causative in our case, but a multifactorial process is likely. Treatment of monoclonal antibody–associated vasculitis entails investigating for triggers and systemic involvement, removing the most likely culprit, quelling the vasculitis acutely, avoiding known potential exacerbators, and introducing an alternative long-term immunomodulant. In all 6 reported similar cases, discontinuation of secukinumab and initiation of prednisone or colchicine led to resolution.1-6 Dapsone also is acceptable for acute control of IgA vasculitis, although this medication is highly lipid soluble and penetrates well into various tissues.15 Thus, lower doses may prove ineffective for obese patients, as was demonstrated in our case. Given the known potential of vaccinations, infections, and other factors (eg, alcohol, penicillin) to trigger IgA vasculitis, these should be avoided.10
Blockade of IL-23 with ustekinumab has been suggested by other authors encountering secukinumab-associated vasculitis, as IL-23 is the main driver and sustainer of TH17 cell differentiation.8 Although 6 previously reported cases of secukinumab-associated vasculitis achieved resolution without long-term recurrence, none did so using an IL-23 inhibitor (nor had any of the described patients received IL-23 inhibitors previously).1-6 Given the established safety of IL-23 inhibitors and that they theoretically are well suited for this unique circumstance (by ceasing the main causative cytokine cascades “upstream”) and were efficacious in quickly resolving our patient’s vasculitis, we suggest that ustekinumab may represent
- Reverte M, Etienne M, Fouchard M, et al. Occurrence of Henoch-Schönlein purpura in a patient treated with secukinumab. J Eur Acad Dermatol Venereol. 2019;33:E455-E457.
- Chelli C, Loget J, Vanhaecke C, et al. Cutaneous vasculitis with gut involvement during secukinumab treatment for psoriatic arthritis. Acta Derm Venereol. 2020;100:adv00077.
- da Silva Cendon Duran C, Santiago MB. Cutaneous vasculitis during secukinumab treatment. Eur J Case Rep Intern Med. 2020;7:001815.
- Bostan E, Gulseren D, Yalici-Armagan B, et al. Vasculitis during certolizumab pegol and secukinumab treatment: report of two cases. Dermatol Ther. 2021;34:E15007.
- Perkovic D, Simac P, Katic J. IgA vasculitis during secukinumab therapy. Clin Rheumatol. 2021;40:2071-2073.
- Villani A, DE Fata Salvatores G, Nappa P, et al. Cutaneous leucocytoclastic vasculitis during secukinumab treatment. Ital J Dermatol Venerol. 2021;156(suppl 1 to no. 6):9-10.
- Góis M, Messias A, Carvalho D, et al. MPO-ANCA-associated necrotizing glomerulonephritis in rheumatoid arthritis; a case report and review of literature. J Nephropathol. 2017;6:58-62.
- Jen HY, Chuang YH, Lin SC, et al. Increased serum interleukin-17 and peripheral Th17 cells in children with acute Henoch-Schönlein purpura. Pediatr Allergy Immunol. 2011;22:862-868.
- Hetland LE, Susrud KS, Lindahl KH, et al. Henoch-Schönlein purpura: a literature review. Acta Derm Venereol 2017;97:1160-1166.
- Weedon D. The vasculopathic reaction pattern. In: Houston M, Davie B, eds. Weedon’s Skin Pathology. 3rd ed. Elsevier Limited; 2010:207-211.
- Puig L. Paradoxical reactions: anti-TNFα ants, ustekinumab, secukinumab, ixekizumab, and others. Curr Probl Dermatol. 2018;53:49-63.
- Nestle F, Kaplan D, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Pinheiro RR, Lencastre A. Henoch-Schönlein purpura during anti-TNFα therapy: a fortuitous event or an indication to stop therapy? Eur J Dermatol. 2017;27:304-305.
- Hello CL, Cohen P, Bousser MG, et al. Suspected hepatitis B vaccination related vasculitis. J Rheumatol. 1999;26:191-194.
- Wolverton SE. Dapsone. In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier, Inc; 2021:222-231.
Case Report
A 47-year-old man presented with a sudden-onset rash consisting of red bumps on the abdomen and legs that had been ongoing for several days. He had known psoriasis and psoriatic arthritis that had been well controlled with adalimumab for the last 18 months. He reported concurrent onset of nausea but denied fevers, chills, night sweats, unintentional weight loss, abdominal pain, and pruritus. He endorsed prior cutaneous infections of methicillin-resistant Staphylococcus aureus (MRSA). His medical history also included diabetes mellitus, hypertension, and obesity. His other medications included oral losartan-hydrochlorothiazide, amlodipine, naproxen, and atorvastatin.
Physical examination revealed numerous thin purpuric papules—some with adherent scale—distributed on the lower legs, extensor forearms, and abdomen. Abdominal lesions were confined to weight-related striae (Figure 1). The palms, soles, oral mucosa, and face were spared. Three punch biopsies were performed, including 1 for direct immunofluorescence (DIF), and the patient was instructed to apply clobetasol to the affected areas twice daily until further notice.

Pathology showed perivascular extravasation of erythrocytes, neutrophils, eosinophils, and leukocytoclasis surrounding blood vessels associated with fibrin (Figure 2). Direct immunofluorescence showed granular deposition of IgA, complement component 3, and fibrinogen in a superficial dermal vascular pattern (Figure 3). These results were consistent with IgA small-vessel vasculitis. One specimen was consistent with the patient’s known psoriasis.

Urinalysis revealed moderate hemoglobinuria, and urine microscopy showed 174 red blood cells per high-power field. Creatinine was high at 1.87 mg/dL (reference range, <1.34 mg/dL; patient’s baseline, 0.81 mg/dL) and glomerular filtration rate was low (42 mL/min, patient’s baseline, >60 mL/min [reference range, 90–120 mL/min]). Erythrocyte sedimentation rate (21 mm/h [reference range, 0–22 mm/h]) and C-reactive protein were elevated (2.2 mg/dL [reference range, 0.3–1.0 mg/dL]). Given his history of cutaneous MRSA infections, a bacterial culture swab was collected from the skin surface to check for colonization, which showed moderate growth of MRSA. Naproxen was discontinued over concern of worsening the patient’s renal status. The patient was instructed to rest at home with his legs elevated, wear compression socks when ambulatory, use chlorhexidine antiseptic daily as a body wash when showering, and apply mupirocin three times daily to the biopsy sites. He was referred to urology for his microhematuria, where cystoscopy revealed no abnormalities.A month passed with no improvement of the patient’s cutaneous vasculitis, and his psoriatic arthritis worsened without his usual use of naproxen. He developed abdominal pain and loss of appetite. A prednisone taper was ordered starting at 40 mg/d (28.8 mg/kg), which provided relief of the skin and joint symptoms only until the course was completed 12 days later.

Five weeks after the initial presentation, the patient returned with a more severe eruption consisting of innumerable purpuric papules that coalesced in plaques on the abdomen, arms, and legs. He also had erythematous facial pustules and mild palmar petechiae (Figure 4). Three biopsies were performed, including 1 for DIF and 1 from a pustule on the forehead. Histology and DIF were again consistent with IgA small-vessel vasculitis. The forehead biopsy was compatible with steroid acne (attributed to recent prednisone use) and psoriasis.

Rheumatology was consulted, and adalimumab was discontinued 6 weeks after the initial presentation out of concern for drug-induced cutaneous vasculitis. Vasculitis work-up was unremarkable, including antineutrophil cytoplasmic antibodies, rheumatoid factor, cyclic citrullinated peptide, and serum protein electrophoresis. Oral dapsone was started at 100 mg/d, with the tentative plan of starting secukinumab if cutaneous symptoms improved. For 3 weeks, the patient’s cutaneous symptoms steadily improved.
Nine weeks after initial presentation to dermatology (3 weeks after discontinuing adalimumab) the patient self-administered his first dose of secukinumab at home. Several hours later, he reported sudden reappearance of vasculitis. He denied diarrhea, abdominal pain, bowel movement urgency, fevers, fatigue, and unintentional weight loss. Antistreptolysin O and hepatitis A antibodies were negative. He was instructed to hold secukinumab indefinitely.
Four weeks after his only secukinumab injection, the patient reported another episode of acute worsening cutaneous symptoms. A 4-week prednisone taper starting at 40 mg/d was ordered. Computed tomography of the chest, abdomen, and pelvis to rule out internal malignancy was unremarkable. Around this time, the patient reported major emotional distress related to an unexpected death in his family, which added to a gradual increase in his stress level related to the COVID-19 pandemic.
Three weeks later, dapsone was increased to 100 mg twice daily on account of the patient’s adiposity and lack of cutaneous improvement on the lower dose. Subsequently, the vasculitis rapidly improved for 2 weeks. The patient then reported symptoms of headache, dizziness, and chills. He was tested for COVID-19 and was negative. Six weeks after increasing the dapsone dose (5 months after initial presentation), the skin was normalizing, showing only faintly hyperpigmented macules confined to areas of resolved vasculitis (forearms, abdomen, legs).
The patient had been on dapsone 100 mg twice daily for 3 months when he was started on ustekinumab (90 mg at weeks 0 and 4, with planned doses every 12 weeks) for psoriatic arthritis in hopes of withdrawing dapsone. His cutaneous symptoms have remained well controlled on this regimen for 18 months. Lowering of dapsone below 100 mg daily has resulted in recurrent mild vasculitis symptoms; he now maintains the once-daily dosing without negative side effects.
Comment
IgA vasculitis is a form of cutaneous small-vessel leukocytoclastic vasculitis (LCV) characterized by episodes of palpable purpura on the extensor surfaces of the arms and legs that may be associated with arthritis, abdominal pain, and/or hematuria. Although vasculitis is a known potential adverse effect of anti–tumor necrosis factor (TNF) α therapy, cases of adalimumab-induced IgA vasculitis are uncommon. As use of more targeted therapies for psoriasis and psoriatic arthritis, such as the IL-17 inhibitor secukinumab, increases so do reports of associated adverse events. Of 6 previously reported cases of secukinumab-associated vasculitis, at least 4 were IgA vasculitis (Table).1-6 Another case described one patient with rheumatoid arthritis undergoing secukinumab treatment who experienced necrotizing glomerulonephritis; however, the authors concluded secukinumab likely was not causative in that case, as serologies and urinalyses suggested gradual onset of the process prior to initiating the medication.7

The exact pathogenesis of IgA vasculitis is unclear, but a prevailing theory involves the dysregulation of IgA synthesis and metabolism. Other than increased serum levels of transforming growth factor β, which is a major stimulating factor for IgA production, it also has been hypothesized that the presence of aberrantly hypoglycosylated IgA exposes an autoepitope for recognition by other pathogenic IgG and IgA, leading to the formation of large immune complexes that can readily deposit in postcapillary venules. The deposition of IgA immune complexes in postcapillary venules and the subsequent activation of the complement system causes direct damage to the endothelial cells of vessel walls. This complement activation is evidenced by vascular complement component 3 deposition on DIF (a nonspecific feature of LCV). Chemotaxis of neutrophils ensues, followed by their firm adherence and transendothelial migration (mediated by monocyte chemoattractant protein 1 [MCP-1]). Neutrophil degranulation releases reactive oxygen species and cytokines, which in turn recruit additional leukocytes to the area of inflammation, subsequently undergoing degeneration (leukocytoclasis). Microvascular permeability also is enhanced by MCP-1, allowing exudation of serum, erythrocytes, and fibrin. In the setting of elevated circulating TNF and IL-1, endothelium is stimulated to activate the intrinsic and extrinsic coagulation pathways. This decreases endothelial fibrinolytic activity, leading to thrombosis. The high venous pressure and low fibrinolytic activity in the lower legs explains why vasculitic lesions often are confined to or begin in this distribution.1,8-10

There also are noteworthy roles for cytokines in LCV. Circulating transforming growth factor β and IL-6—which are necessary for development of T helper 17 (TH17) cells and production of IL-17—are higher in patients with LCV compared to controls. Peripheral blood monocytes in patients with LCV demonstrate higher production of IL-17. Once TH17 cells develop, their survival and phenotype are maintained by IL-23 (considered the master regulator of TH17 differentiation). IL-17 is a potent chemoattractant of IL-8 (CXCL8) and MCP-1, both of which promote neutrophil-mediated perivascular inflammation. The IL-23 and IL-17 pathways implicated in the pathogenesis of psoriasis also cause neutrophil activation and upregulate transcription of proinflammatory cytokines (IL-1, IL-6, IL-8, and TNF-α), which overlap with those implicated in LCV. Autoimmune disease generally entails some positive feedback loop of progressively severe self-recognition and tissue destruction by the immune system. These shared cytokinetic processes may explain how the internal environment of psoriasis could perpetuate IgA vasculitis.1,2,8,10-12
The mechanisms underlying vasculitis associated with adalimumab are unclear, but hypotheses involve direct toxicity on vessels, capillary deposition of anti-TNF/TNF immune complexes, or an inflammatory process resulting in autoantibodies. Similar hypotheses are posited for secukinumab-associated vasculitis, including deposition of secukinumab–IL-17 complexes. Anti–TNF-α medications may increase TH17 cell numbers, leading to increased production of IL-22 and a resultant immunologic microenvironment conducive to vasculitis. All 6 published cases of secukinumab-associated vasculitis that we found had received prior treatment with a TNF-α blocker, but only 1 had occurrence of vasculitis during that treatment.1-6,10
In the 6 cases we reviewed, the time from starting secukinumab to onset of vasculitis ranged from 1 to 18 months. Our patient’s same-day re-emergence of vasculitis after his first secukinumab dose was so acute that we were skeptical of secukinumab as a potential trigger; this may simply have been coincident to the natural waxing and waning of the vasculitis (although onset of IgA vasculitis within 1 day of starting anti–TNF-α therapy has been reported).1-6,13
Specific associations of IgA vasculitis are many and can include bacterial organisms such as Helicobacter pylori, streptococci, and staphylococci. Although internal mucous membrane infections are considered more linked because of the surveillance role of IgA predominantly in mucosal tissues, it is possible that our patient with cutaneous MRSA harbored the same within the nasal mucosa. Our patient also received multiple vaccinations outside our department throughout his clinical course (2 hepatitis B and 1 pneumococcal conjugate), which are known potential triggers for vasculitis. Psychological stress is a known trigger for psoriasis, and given the cytokinetic relationship of psoriasis to vasculitis described previously, it may have indirectly contributed to vasculitis in our case. The anxiety associated with being immunosuppressed during the COVID-19 pandemic and bereavement of losing a family member may have contributed to the refractory nature of our patient’s condition. Renal involvement is relatively common in adults with IgA vasculitis and so should be ruled out, as should occult internal malignancy.8,10,14
It is unclear which of the above factors was causative in our case, but a multifactorial process is likely. Treatment of monoclonal antibody–associated vasculitis entails investigating for triggers and systemic involvement, removing the most likely culprit, quelling the vasculitis acutely, avoiding known potential exacerbators, and introducing an alternative long-term immunomodulant. In all 6 reported similar cases, discontinuation of secukinumab and initiation of prednisone or colchicine led to resolution.1-6 Dapsone also is acceptable for acute control of IgA vasculitis, although this medication is highly lipid soluble and penetrates well into various tissues.15 Thus, lower doses may prove ineffective for obese patients, as was demonstrated in our case. Given the known potential of vaccinations, infections, and other factors (eg, alcohol, penicillin) to trigger IgA vasculitis, these should be avoided.10
Blockade of IL-23 with ustekinumab has been suggested by other authors encountering secukinumab-associated vasculitis, as IL-23 is the main driver and sustainer of TH17 cell differentiation.8 Although 6 previously reported cases of secukinumab-associated vasculitis achieved resolution without long-term recurrence, none did so using an IL-23 inhibitor (nor had any of the described patients received IL-23 inhibitors previously).1-6 Given the established safety of IL-23 inhibitors and that they theoretically are well suited for this unique circumstance (by ceasing the main causative cytokine cascades “upstream”) and were efficacious in quickly resolving our patient’s vasculitis, we suggest that ustekinumab may represent
Case Report
A 47-year-old man presented with a sudden-onset rash consisting of red bumps on the abdomen and legs that had been ongoing for several days. He had known psoriasis and psoriatic arthritis that had been well controlled with adalimumab for the last 18 months. He reported concurrent onset of nausea but denied fevers, chills, night sweats, unintentional weight loss, abdominal pain, and pruritus. He endorsed prior cutaneous infections of methicillin-resistant Staphylococcus aureus (MRSA). His medical history also included diabetes mellitus, hypertension, and obesity. His other medications included oral losartan-hydrochlorothiazide, amlodipine, naproxen, and atorvastatin.
Physical examination revealed numerous thin purpuric papules—some with adherent scale—distributed on the lower legs, extensor forearms, and abdomen. Abdominal lesions were confined to weight-related striae (Figure 1). The palms, soles, oral mucosa, and face were spared. Three punch biopsies were performed, including 1 for direct immunofluorescence (DIF), and the patient was instructed to apply clobetasol to the affected areas twice daily until further notice.

Pathology showed perivascular extravasation of erythrocytes, neutrophils, eosinophils, and leukocytoclasis surrounding blood vessels associated with fibrin (Figure 2). Direct immunofluorescence showed granular deposition of IgA, complement component 3, and fibrinogen in a superficial dermal vascular pattern (Figure 3). These results were consistent with IgA small-vessel vasculitis. One specimen was consistent with the patient’s known psoriasis.

Urinalysis revealed moderate hemoglobinuria, and urine microscopy showed 174 red blood cells per high-power field. Creatinine was high at 1.87 mg/dL (reference range, <1.34 mg/dL; patient’s baseline, 0.81 mg/dL) and glomerular filtration rate was low (42 mL/min, patient’s baseline, >60 mL/min [reference range, 90–120 mL/min]). Erythrocyte sedimentation rate (21 mm/h [reference range, 0–22 mm/h]) and C-reactive protein were elevated (2.2 mg/dL [reference range, 0.3–1.0 mg/dL]). Given his history of cutaneous MRSA infections, a bacterial culture swab was collected from the skin surface to check for colonization, which showed moderate growth of MRSA. Naproxen was discontinued over concern of worsening the patient’s renal status. The patient was instructed to rest at home with his legs elevated, wear compression socks when ambulatory, use chlorhexidine antiseptic daily as a body wash when showering, and apply mupirocin three times daily to the biopsy sites. He was referred to urology for his microhematuria, where cystoscopy revealed no abnormalities.A month passed with no improvement of the patient’s cutaneous vasculitis, and his psoriatic arthritis worsened without his usual use of naproxen. He developed abdominal pain and loss of appetite. A prednisone taper was ordered starting at 40 mg/d (28.8 mg/kg), which provided relief of the skin and joint symptoms only until the course was completed 12 days later.

Five weeks after the initial presentation, the patient returned with a more severe eruption consisting of innumerable purpuric papules that coalesced in plaques on the abdomen, arms, and legs. He also had erythematous facial pustules and mild palmar petechiae (Figure 4). Three biopsies were performed, including 1 for DIF and 1 from a pustule on the forehead. Histology and DIF were again consistent with IgA small-vessel vasculitis. The forehead biopsy was compatible with steroid acne (attributed to recent prednisone use) and psoriasis.

Rheumatology was consulted, and adalimumab was discontinued 6 weeks after the initial presentation out of concern for drug-induced cutaneous vasculitis. Vasculitis work-up was unremarkable, including antineutrophil cytoplasmic antibodies, rheumatoid factor, cyclic citrullinated peptide, and serum protein electrophoresis. Oral dapsone was started at 100 mg/d, with the tentative plan of starting secukinumab if cutaneous symptoms improved. For 3 weeks, the patient’s cutaneous symptoms steadily improved.
Nine weeks after initial presentation to dermatology (3 weeks after discontinuing adalimumab) the patient self-administered his first dose of secukinumab at home. Several hours later, he reported sudden reappearance of vasculitis. He denied diarrhea, abdominal pain, bowel movement urgency, fevers, fatigue, and unintentional weight loss. Antistreptolysin O and hepatitis A antibodies were negative. He was instructed to hold secukinumab indefinitely.
Four weeks after his only secukinumab injection, the patient reported another episode of acute worsening cutaneous symptoms. A 4-week prednisone taper starting at 40 mg/d was ordered. Computed tomography of the chest, abdomen, and pelvis to rule out internal malignancy was unremarkable. Around this time, the patient reported major emotional distress related to an unexpected death in his family, which added to a gradual increase in his stress level related to the COVID-19 pandemic.
Three weeks later, dapsone was increased to 100 mg twice daily on account of the patient’s adiposity and lack of cutaneous improvement on the lower dose. Subsequently, the vasculitis rapidly improved for 2 weeks. The patient then reported symptoms of headache, dizziness, and chills. He was tested for COVID-19 and was negative. Six weeks after increasing the dapsone dose (5 months after initial presentation), the skin was normalizing, showing only faintly hyperpigmented macules confined to areas of resolved vasculitis (forearms, abdomen, legs).
The patient had been on dapsone 100 mg twice daily for 3 months when he was started on ustekinumab (90 mg at weeks 0 and 4, with planned doses every 12 weeks) for psoriatic arthritis in hopes of withdrawing dapsone. His cutaneous symptoms have remained well controlled on this regimen for 18 months. Lowering of dapsone below 100 mg daily has resulted in recurrent mild vasculitis symptoms; he now maintains the once-daily dosing without negative side effects.
Comment
IgA vasculitis is a form of cutaneous small-vessel leukocytoclastic vasculitis (LCV) characterized by episodes of palpable purpura on the extensor surfaces of the arms and legs that may be associated with arthritis, abdominal pain, and/or hematuria. Although vasculitis is a known potential adverse effect of anti–tumor necrosis factor (TNF) α therapy, cases of adalimumab-induced IgA vasculitis are uncommon. As use of more targeted therapies for psoriasis and psoriatic arthritis, such as the IL-17 inhibitor secukinumab, increases so do reports of associated adverse events. Of 6 previously reported cases of secukinumab-associated vasculitis, at least 4 were IgA vasculitis (Table).1-6 Another case described one patient with rheumatoid arthritis undergoing secukinumab treatment who experienced necrotizing glomerulonephritis; however, the authors concluded secukinumab likely was not causative in that case, as serologies and urinalyses suggested gradual onset of the process prior to initiating the medication.7

The exact pathogenesis of IgA vasculitis is unclear, but a prevailing theory involves the dysregulation of IgA synthesis and metabolism. Other than increased serum levels of transforming growth factor β, which is a major stimulating factor for IgA production, it also has been hypothesized that the presence of aberrantly hypoglycosylated IgA exposes an autoepitope for recognition by other pathogenic IgG and IgA, leading to the formation of large immune complexes that can readily deposit in postcapillary venules. The deposition of IgA immune complexes in postcapillary venules and the subsequent activation of the complement system causes direct damage to the endothelial cells of vessel walls. This complement activation is evidenced by vascular complement component 3 deposition on DIF (a nonspecific feature of LCV). Chemotaxis of neutrophils ensues, followed by their firm adherence and transendothelial migration (mediated by monocyte chemoattractant protein 1 [MCP-1]). Neutrophil degranulation releases reactive oxygen species and cytokines, which in turn recruit additional leukocytes to the area of inflammation, subsequently undergoing degeneration (leukocytoclasis). Microvascular permeability also is enhanced by MCP-1, allowing exudation of serum, erythrocytes, and fibrin. In the setting of elevated circulating TNF and IL-1, endothelium is stimulated to activate the intrinsic and extrinsic coagulation pathways. This decreases endothelial fibrinolytic activity, leading to thrombosis. The high venous pressure and low fibrinolytic activity in the lower legs explains why vasculitic lesions often are confined to or begin in this distribution.1,8-10

There also are noteworthy roles for cytokines in LCV. Circulating transforming growth factor β and IL-6—which are necessary for development of T helper 17 (TH17) cells and production of IL-17—are higher in patients with LCV compared to controls. Peripheral blood monocytes in patients with LCV demonstrate higher production of IL-17. Once TH17 cells develop, their survival and phenotype are maintained by IL-23 (considered the master regulator of TH17 differentiation). IL-17 is a potent chemoattractant of IL-8 (CXCL8) and MCP-1, both of which promote neutrophil-mediated perivascular inflammation. The IL-23 and IL-17 pathways implicated in the pathogenesis of psoriasis also cause neutrophil activation and upregulate transcription of proinflammatory cytokines (IL-1, IL-6, IL-8, and TNF-α), which overlap with those implicated in LCV. Autoimmune disease generally entails some positive feedback loop of progressively severe self-recognition and tissue destruction by the immune system. These shared cytokinetic processes may explain how the internal environment of psoriasis could perpetuate IgA vasculitis.1,2,8,10-12
The mechanisms underlying vasculitis associated with adalimumab are unclear, but hypotheses involve direct toxicity on vessels, capillary deposition of anti-TNF/TNF immune complexes, or an inflammatory process resulting in autoantibodies. Similar hypotheses are posited for secukinumab-associated vasculitis, including deposition of secukinumab–IL-17 complexes. Anti–TNF-α medications may increase TH17 cell numbers, leading to increased production of IL-22 and a resultant immunologic microenvironment conducive to vasculitis. All 6 published cases of secukinumab-associated vasculitis that we found had received prior treatment with a TNF-α blocker, but only 1 had occurrence of vasculitis during that treatment.1-6,10
In the 6 cases we reviewed, the time from starting secukinumab to onset of vasculitis ranged from 1 to 18 months. Our patient’s same-day re-emergence of vasculitis after his first secukinumab dose was so acute that we were skeptical of secukinumab as a potential trigger; this may simply have been coincident to the natural waxing and waning of the vasculitis (although onset of IgA vasculitis within 1 day of starting anti–TNF-α therapy has been reported).1-6,13
Specific associations of IgA vasculitis are many and can include bacterial organisms such as Helicobacter pylori, streptococci, and staphylococci. Although internal mucous membrane infections are considered more linked because of the surveillance role of IgA predominantly in mucosal tissues, it is possible that our patient with cutaneous MRSA harbored the same within the nasal mucosa. Our patient also received multiple vaccinations outside our department throughout his clinical course (2 hepatitis B and 1 pneumococcal conjugate), which are known potential triggers for vasculitis. Psychological stress is a known trigger for psoriasis, and given the cytokinetic relationship of psoriasis to vasculitis described previously, it may have indirectly contributed to vasculitis in our case. The anxiety associated with being immunosuppressed during the COVID-19 pandemic and bereavement of losing a family member may have contributed to the refractory nature of our patient’s condition. Renal involvement is relatively common in adults with IgA vasculitis and so should be ruled out, as should occult internal malignancy.8,10,14
It is unclear which of the above factors was causative in our case, but a multifactorial process is likely. Treatment of monoclonal antibody–associated vasculitis entails investigating for triggers and systemic involvement, removing the most likely culprit, quelling the vasculitis acutely, avoiding known potential exacerbators, and introducing an alternative long-term immunomodulant. In all 6 reported similar cases, discontinuation of secukinumab and initiation of prednisone or colchicine led to resolution.1-6 Dapsone also is acceptable for acute control of IgA vasculitis, although this medication is highly lipid soluble and penetrates well into various tissues.15 Thus, lower doses may prove ineffective for obese patients, as was demonstrated in our case. Given the known potential of vaccinations, infections, and other factors (eg, alcohol, penicillin) to trigger IgA vasculitis, these should be avoided.10
Blockade of IL-23 with ustekinumab has been suggested by other authors encountering secukinumab-associated vasculitis, as IL-23 is the main driver and sustainer of TH17 cell differentiation.8 Although 6 previously reported cases of secukinumab-associated vasculitis achieved resolution without long-term recurrence, none did so using an IL-23 inhibitor (nor had any of the described patients received IL-23 inhibitors previously).1-6 Given the established safety of IL-23 inhibitors and that they theoretically are well suited for this unique circumstance (by ceasing the main causative cytokine cascades “upstream”) and were efficacious in quickly resolving our patient’s vasculitis, we suggest that ustekinumab may represent
- Reverte M, Etienne M, Fouchard M, et al. Occurrence of Henoch-Schönlein purpura in a patient treated with secukinumab. J Eur Acad Dermatol Venereol. 2019;33:E455-E457.
- Chelli C, Loget J, Vanhaecke C, et al. Cutaneous vasculitis with gut involvement during secukinumab treatment for psoriatic arthritis. Acta Derm Venereol. 2020;100:adv00077.
- da Silva Cendon Duran C, Santiago MB. Cutaneous vasculitis during secukinumab treatment. Eur J Case Rep Intern Med. 2020;7:001815.
- Bostan E, Gulseren D, Yalici-Armagan B, et al. Vasculitis during certolizumab pegol and secukinumab treatment: report of two cases. Dermatol Ther. 2021;34:E15007.
- Perkovic D, Simac P, Katic J. IgA vasculitis during secukinumab therapy. Clin Rheumatol. 2021;40:2071-2073.
- Villani A, DE Fata Salvatores G, Nappa P, et al. Cutaneous leucocytoclastic vasculitis during secukinumab treatment. Ital J Dermatol Venerol. 2021;156(suppl 1 to no. 6):9-10.
- Góis M, Messias A, Carvalho D, et al. MPO-ANCA-associated necrotizing glomerulonephritis in rheumatoid arthritis; a case report and review of literature. J Nephropathol. 2017;6:58-62.
- Jen HY, Chuang YH, Lin SC, et al. Increased serum interleukin-17 and peripheral Th17 cells in children with acute Henoch-Schönlein purpura. Pediatr Allergy Immunol. 2011;22:862-868.
- Hetland LE, Susrud KS, Lindahl KH, et al. Henoch-Schönlein purpura: a literature review. Acta Derm Venereol 2017;97:1160-1166.
- Weedon D. The vasculopathic reaction pattern. In: Houston M, Davie B, eds. Weedon’s Skin Pathology. 3rd ed. Elsevier Limited; 2010:207-211.
- Puig L. Paradoxical reactions: anti-TNFα ants, ustekinumab, secukinumab, ixekizumab, and others. Curr Probl Dermatol. 2018;53:49-63.
- Nestle F, Kaplan D, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Pinheiro RR, Lencastre A. Henoch-Schönlein purpura during anti-TNFα therapy: a fortuitous event or an indication to stop therapy? Eur J Dermatol. 2017;27:304-305.
- Hello CL, Cohen P, Bousser MG, et al. Suspected hepatitis B vaccination related vasculitis. J Rheumatol. 1999;26:191-194.
- Wolverton SE. Dapsone. In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier, Inc; 2021:222-231.
- Reverte M, Etienne M, Fouchard M, et al. Occurrence of Henoch-Schönlein purpura in a patient treated with secukinumab. J Eur Acad Dermatol Venereol. 2019;33:E455-E457.
- Chelli C, Loget J, Vanhaecke C, et al. Cutaneous vasculitis with gut involvement during secukinumab treatment for psoriatic arthritis. Acta Derm Venereol. 2020;100:adv00077.
- da Silva Cendon Duran C, Santiago MB. Cutaneous vasculitis during secukinumab treatment. Eur J Case Rep Intern Med. 2020;7:001815.
- Bostan E, Gulseren D, Yalici-Armagan B, et al. Vasculitis during certolizumab pegol and secukinumab treatment: report of two cases. Dermatol Ther. 2021;34:E15007.
- Perkovic D, Simac P, Katic J. IgA vasculitis during secukinumab therapy. Clin Rheumatol. 2021;40:2071-2073.
- Villani A, DE Fata Salvatores G, Nappa P, et al. Cutaneous leucocytoclastic vasculitis during secukinumab treatment. Ital J Dermatol Venerol. 2021;156(suppl 1 to no. 6):9-10.
- Góis M, Messias A, Carvalho D, et al. MPO-ANCA-associated necrotizing glomerulonephritis in rheumatoid arthritis; a case report and review of literature. J Nephropathol. 2017;6:58-62.
- Jen HY, Chuang YH, Lin SC, et al. Increased serum interleukin-17 and peripheral Th17 cells in children with acute Henoch-Schönlein purpura. Pediatr Allergy Immunol. 2011;22:862-868.
- Hetland LE, Susrud KS, Lindahl KH, et al. Henoch-Schönlein purpura: a literature review. Acta Derm Venereol 2017;97:1160-1166.
- Weedon D. The vasculopathic reaction pattern. In: Houston M, Davie B, eds. Weedon’s Skin Pathology. 3rd ed. Elsevier Limited; 2010:207-211.
- Puig L. Paradoxical reactions: anti-TNFα ants, ustekinumab, secukinumab, ixekizumab, and others. Curr Probl Dermatol. 2018;53:49-63.
- Nestle F, Kaplan D, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Pinheiro RR, Lencastre A. Henoch-Schönlein purpura during anti-TNFα therapy: a fortuitous event or an indication to stop therapy? Eur J Dermatol. 2017;27:304-305.
- Hello CL, Cohen P, Bousser MG, et al. Suspected hepatitis B vaccination related vasculitis. J Rheumatol. 1999;26:191-194.
- Wolverton SE. Dapsone. In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier, Inc; 2021:222-231.
Practice Points
- Biologic medications including adalimumab and more rarely secukinumab may be associated with leukocytoclastic vasculitis; a smaller subset of patients may experience IgA vasculitis.
- The IL-23 blocker ustekinumab may represent an ideal therapeutic agent when secukinumabassociated vasculitis is suspected. Because IL-23 is the main driver and sustainer of TH17 cell differentiation, it may cease the main causative cytokine cascades “upstream.”
A Patient With Recurrent Immune Stromal Keratitis and Adherence Challenges
Herpes simplex keratitis (HSK) is a common yet potentially blinding condition caused by a primary or reactivated herpetic infection of the cornea.1 The Herpetic Eye Disease Study established the standard of care in HSK management.2 Treatments range from oral antivirals and artificial tears to topical antibiotics, amniotic membranes, and corneal transplantation.3 Patients with immune stromal keratitis (ISK) may experience low-grade chronic keratitis for years.4 ISK is classified by a cellular and neovascularization infiltration of the cornea.5 We present a case of a patient with recurrent ISK and review its presentation, diagnosis, and management.
Case Presentation
A 52-year-old man presented to the eye clinic with a watery and itchy right eye with mildly blurred vision. His ocular history was unremarkable. His medical history was notable for hepatitis C, hypertension, alcohol and drug dependence, homelessness, and a COVID-19–induced coma. His medications included trazodone, nifedipine, clonidine HCl, and buprenorphine/naloxone.
On clinical examination, the patient’s best-corrected visual acuity was 20/40 in the right eye and 20/20 in the left. Corneal sensitivity was absent in the right eye and intact in the left. Anterior segment findings in the right eye included 360-degree superficial corneal neovascularization, deep neovascularization temporally, scattered patches of corneal haze, epithelial irregularity, and 2+ diffuse bulbar conjunctival injection (Figure 1). The anterior segment of the left eye and the posterior segments of both eyes were unremarkable. The differential diagnosis included HSK, syphilis, Cogan syndrome, varicella-zoster virus keratitis, Epstein-Barr virus keratitis, and Lyme disease. With consultation from a corneal specialist, the patient was given the presumptive diagnosis of ISK in the right eye based on unilateral corneal presentation and lack of corneal sensitivity. He was treated with
The patient returned a week later having only used the prednisolone drops for 2 days before discontinuing. Examination showed no change in his corneal appearance from the previous week. The patient was counseled on the importance of adherence to the regimen of topical prednisolone and oral valacyclovir.
The patient followed up 2 weeks later. He reported good adherence to the ISK medication regimen. His symptoms had resolved, and his visual acuity returned to 20/20 in the right eye. Slit-lamp examination showed improvement in injection, and the superficial corneal neovascularization had cleared. A trace ghost vessel was seen temporally at a site of deep neovascularization (Figure 2). He was instructed to continue valacyclovir once daily and prednisolone drops once daily in the right eye and to follow up in 1 month.
At the 1-month follow-up, the patient’s signs and symptoms had reverted to his original presentation. The patient reported poor adherence to the medication regimen, having missed multiple doses of prednisolone drops as well as valacyclovir. The patient was counseled again on the ISK regimen, and the prednisolone drops and 1-g oral valacyclovir were refilled. A follow-up visit was scheduled for 2 weeks. Additional follow-up revealed a resolved corneal appearance and bimonthly follow-ups were scheduled thereafter.
Discussion
HSK is the most common infectious cause of unilateral blindness and vision impairment in the world.2 This case highlights the diagnosis and management of a patient with ISK, a type of HSK characterized by decreased corneal sensitivity and unilateral stromal opacification or neovascularization.6
ISK is caused by the herpes simplex virus (HSV), a double-stranded enveloped DNA virus that occurs worldwide with little variation, replicates in many types of cells, has rapid growth, and is cytolytic, causing necrosis of nearby cells. Transmission is via direct contact and there is a lifelong latency period in the trigeminal ganglia. Both primary and reactivation infections of HSK can affect a broad array of ocular structures, from the lids to the retina. Infectious epithelial keratitis, also known as dendritic keratitis, is the reactivation of the live virus and is the most common presentation of HSK. ISK is responsible for 20% to 48% of recurrent HSV disease and is the leading cause of vision loss. ISK is the result of an immune-mediated inflammatory response due to a retained viral antigen within the stromal tissue.7 Inflammation in the corneal stroma leads to corneal haze and eventually focal or diffuse scarring, reducing the visual potential.7 This presentation may occur days to years after the initial epithelial episode and may persist for years. Although this patient did not present with infectious epithelial keratitis, it is possible he had a previous episode not mentioned as a history was difficult to obtain, and it can be subtle or innocuous, like pink eye.
Symptoms of ISK include unilateral redness, photophobia, tearing, eye pain, and blurred vision, as described by this patient. On examination, initial manifestations of ISK include corneal haze, edema, scarring, and neovascularization.7 Again, this patient presented with edema and neovascularization. These signs may improve with prompt diagnosis and treatment. More frequent reactivated disease leads to a higher propensity of corneal scarring and irregular astigmatism, reducing the visual outcome.
The standard of care established by the Herpetic Eye Disease Study recommends that a patient with presumed ISK should be started on oral antiviral therapy and, in the absence of epithelial disease, topical steroids. Oral antivirals, such as acyclovir and valacyclovir, have good ocular penetration, a good safety profile, a low susceptibility of resistance, and are well tolerated with long-term treatment.2,8 There were no known interactions between any of the patient’s medications and valacyclovir. Oral antivirals should be used in the initial presentation and for maintenance therapy to help reduce the chance of recurrent disease. Initial treatment for ISK is 1-g valacyclovir 3 times daily. When the eye becomes quiet, that dosage can be tapered to 1 g twice daily, to 1 g once daily, and eventually to a maintenance dose of 500 mg daily. Topical steroids block the inflammatory cascade, therefore reducing the corneal inflammation and potential scarring, further reducing the risk of visual impairment.9 Initial treatment is 1 drop 3 times daily, then can be tapered at the same schedule as the oral acyclovir to help simplify adherence for the patient. After 1 drop once daily, steroids may be discontinued while the oral antiviral maintenance dosage continues. Follow-ups should be performed on a monthly to bimonthly basis to evaluate intraocular pressure, ensuring there is no steroid response.
As seen in this patient, adherence with a treatment regimen and awareness of factors, such as a complex psychosocial history that may impact this adherence, are of utmost importance.7
Conclusions
ISK presents unilaterally with decreased or absent corneal sensitivity and nonspecific symptoms. It should be at the top of the list in the differential diagnosis in any patient with unilateral corneal edema, opacification, or neovascularization, and the patient should be started on oral antiviral therapy.
1. Sibley D, Larkin DFP. Update on Herpes simplex keratitis management. Eye (Lond). 2020;34(12):2219-2226. doi:10.1038/s41433-020-01153-x
2. Chodosh J, Ung L. Adoption of innovation in herpes simplex virus keratitis. Cornea. 2020;39(1)(suppl 1):S7-S18. doi:10.1097/ICO.0000000000002425
3. Pérez-Bartolomé F, Botín DM, de Dompablo P, de Arriba P, Arnalich Montiel F, Muñoz Negrete FJ. Post-herpes neurotrophic keratopathy: pathogenesis, clinical signs and current therapies. Arch Soc Esp Oftalmol. 2019;94(4):171-183. doi:10.1016/j.oftal.2019.01.002
4. Holland EJ, Schwartz GS. Classification of herpes simplex virus keratitis. Cornea. 1999;18(2):144-154.
5. Gauthier AS, Noureddine S, Delbosc B. Interstitial keratitis diagnosis and treatment. J Fr Ophtalmol. 2019;42(6):e229-e237. doi:10.1016/j.jfo.2019.04.001
6. Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012;5(57):448-462. doi:10.1016/jsurvophthal.2012.01.005
7. Wang L, Wang R, Xu C, Zhou H. Pathogenesis of herpes stromal keratitis: immune inflammatory response mediated by inflammatory regulators. Front Immunol. 2020;11:766. Published 2020 May 13. doi:10.3389/fimmu.2020.00766
8. Tyring SK, Baker D, Snowden W. Valacyclovir for herpes simplex virus infection: long-term safety and sustained efficacy after 20 years’ experience with acyclovir. J Infect Dis. 2002;186(suppl 1):S40-S46. doi:10.1086/342966
9. Dawson CR. The herpetic eye disease study. Arch Ophthalmol. 1990;108(2):191-192. doi:10.1001/archopht.1990.01070040043027
Herpes simplex keratitis (HSK) is a common yet potentially blinding condition caused by a primary or reactivated herpetic infection of the cornea.1 The Herpetic Eye Disease Study established the standard of care in HSK management.2 Treatments range from oral antivirals and artificial tears to topical antibiotics, amniotic membranes, and corneal transplantation.3 Patients with immune stromal keratitis (ISK) may experience low-grade chronic keratitis for years.4 ISK is classified by a cellular and neovascularization infiltration of the cornea.5 We present a case of a patient with recurrent ISK and review its presentation, diagnosis, and management.
Case Presentation
A 52-year-old man presented to the eye clinic with a watery and itchy right eye with mildly blurred vision. His ocular history was unremarkable. His medical history was notable for hepatitis C, hypertension, alcohol and drug dependence, homelessness, and a COVID-19–induced coma. His medications included trazodone, nifedipine, clonidine HCl, and buprenorphine/naloxone.
On clinical examination, the patient’s best-corrected visual acuity was 20/40 in the right eye and 20/20 in the left. Corneal sensitivity was absent in the right eye and intact in the left. Anterior segment findings in the right eye included 360-degree superficial corneal neovascularization, deep neovascularization temporally, scattered patches of corneal haze, epithelial irregularity, and 2+ diffuse bulbar conjunctival injection (Figure 1). The anterior segment of the left eye and the posterior segments of both eyes were unremarkable. The differential diagnosis included HSK, syphilis, Cogan syndrome, varicella-zoster virus keratitis, Epstein-Barr virus keratitis, and Lyme disease. With consultation from a corneal specialist, the patient was given the presumptive diagnosis of ISK in the right eye based on unilateral corneal presentation and lack of corneal sensitivity. He was treated with
The patient returned a week later having only used the prednisolone drops for 2 days before discontinuing. Examination showed no change in his corneal appearance from the previous week. The patient was counseled on the importance of adherence to the regimen of topical prednisolone and oral valacyclovir.
The patient followed up 2 weeks later. He reported good adherence to the ISK medication regimen. His symptoms had resolved, and his visual acuity returned to 20/20 in the right eye. Slit-lamp examination showed improvement in injection, and the superficial corneal neovascularization had cleared. A trace ghost vessel was seen temporally at a site of deep neovascularization (Figure 2). He was instructed to continue valacyclovir once daily and prednisolone drops once daily in the right eye and to follow up in 1 month.
At the 1-month follow-up, the patient’s signs and symptoms had reverted to his original presentation. The patient reported poor adherence to the medication regimen, having missed multiple doses of prednisolone drops as well as valacyclovir. The patient was counseled again on the ISK regimen, and the prednisolone drops and 1-g oral valacyclovir were refilled. A follow-up visit was scheduled for 2 weeks. Additional follow-up revealed a resolved corneal appearance and bimonthly follow-ups were scheduled thereafter.
Discussion
HSK is the most common infectious cause of unilateral blindness and vision impairment in the world.2 This case highlights the diagnosis and management of a patient with ISK, a type of HSK characterized by decreased corneal sensitivity and unilateral stromal opacification or neovascularization.6
ISK is caused by the herpes simplex virus (HSV), a double-stranded enveloped DNA virus that occurs worldwide with little variation, replicates in many types of cells, has rapid growth, and is cytolytic, causing necrosis of nearby cells. Transmission is via direct contact and there is a lifelong latency period in the trigeminal ganglia. Both primary and reactivation infections of HSK can affect a broad array of ocular structures, from the lids to the retina. Infectious epithelial keratitis, also known as dendritic keratitis, is the reactivation of the live virus and is the most common presentation of HSK. ISK is responsible for 20% to 48% of recurrent HSV disease and is the leading cause of vision loss. ISK is the result of an immune-mediated inflammatory response due to a retained viral antigen within the stromal tissue.7 Inflammation in the corneal stroma leads to corneal haze and eventually focal or diffuse scarring, reducing the visual potential.7 This presentation may occur days to years after the initial epithelial episode and may persist for years. Although this patient did not present with infectious epithelial keratitis, it is possible he had a previous episode not mentioned as a history was difficult to obtain, and it can be subtle or innocuous, like pink eye.
Symptoms of ISK include unilateral redness, photophobia, tearing, eye pain, and blurred vision, as described by this patient. On examination, initial manifestations of ISK include corneal haze, edema, scarring, and neovascularization.7 Again, this patient presented with edema and neovascularization. These signs may improve with prompt diagnosis and treatment. More frequent reactivated disease leads to a higher propensity of corneal scarring and irregular astigmatism, reducing the visual outcome.
The standard of care established by the Herpetic Eye Disease Study recommends that a patient with presumed ISK should be started on oral antiviral therapy and, in the absence of epithelial disease, topical steroids. Oral antivirals, such as acyclovir and valacyclovir, have good ocular penetration, a good safety profile, a low susceptibility of resistance, and are well tolerated with long-term treatment.2,8 There were no known interactions between any of the patient’s medications and valacyclovir. Oral antivirals should be used in the initial presentation and for maintenance therapy to help reduce the chance of recurrent disease. Initial treatment for ISK is 1-g valacyclovir 3 times daily. When the eye becomes quiet, that dosage can be tapered to 1 g twice daily, to 1 g once daily, and eventually to a maintenance dose of 500 mg daily. Topical steroids block the inflammatory cascade, therefore reducing the corneal inflammation and potential scarring, further reducing the risk of visual impairment.9 Initial treatment is 1 drop 3 times daily, then can be tapered at the same schedule as the oral acyclovir to help simplify adherence for the patient. After 1 drop once daily, steroids may be discontinued while the oral antiviral maintenance dosage continues. Follow-ups should be performed on a monthly to bimonthly basis to evaluate intraocular pressure, ensuring there is no steroid response.
As seen in this patient, adherence with a treatment regimen and awareness of factors, such as a complex psychosocial history that may impact this adherence, are of utmost importance.7
Conclusions
ISK presents unilaterally with decreased or absent corneal sensitivity and nonspecific symptoms. It should be at the top of the list in the differential diagnosis in any patient with unilateral corneal edema, opacification, or neovascularization, and the patient should be started on oral antiviral therapy.
Herpes simplex keratitis (HSK) is a common yet potentially blinding condition caused by a primary or reactivated herpetic infection of the cornea.1 The Herpetic Eye Disease Study established the standard of care in HSK management.2 Treatments range from oral antivirals and artificial tears to topical antibiotics, amniotic membranes, and corneal transplantation.3 Patients with immune stromal keratitis (ISK) may experience low-grade chronic keratitis for years.4 ISK is classified by a cellular and neovascularization infiltration of the cornea.5 We present a case of a patient with recurrent ISK and review its presentation, diagnosis, and management.
Case Presentation
A 52-year-old man presented to the eye clinic with a watery and itchy right eye with mildly blurred vision. His ocular history was unremarkable. His medical history was notable for hepatitis C, hypertension, alcohol and drug dependence, homelessness, and a COVID-19–induced coma. His medications included trazodone, nifedipine, clonidine HCl, and buprenorphine/naloxone.
On clinical examination, the patient’s best-corrected visual acuity was 20/40 in the right eye and 20/20 in the left. Corneal sensitivity was absent in the right eye and intact in the left. Anterior segment findings in the right eye included 360-degree superficial corneal neovascularization, deep neovascularization temporally, scattered patches of corneal haze, epithelial irregularity, and 2+ diffuse bulbar conjunctival injection (Figure 1). The anterior segment of the left eye and the posterior segments of both eyes were unremarkable. The differential diagnosis included HSK, syphilis, Cogan syndrome, varicella-zoster virus keratitis, Epstein-Barr virus keratitis, and Lyme disease. With consultation from a corneal specialist, the patient was given the presumptive diagnosis of ISK in the right eye based on unilateral corneal presentation and lack of corneal sensitivity. He was treated with
The patient returned a week later having only used the prednisolone drops for 2 days before discontinuing. Examination showed no change in his corneal appearance from the previous week. The patient was counseled on the importance of adherence to the regimen of topical prednisolone and oral valacyclovir.
The patient followed up 2 weeks later. He reported good adherence to the ISK medication regimen. His symptoms had resolved, and his visual acuity returned to 20/20 in the right eye. Slit-lamp examination showed improvement in injection, and the superficial corneal neovascularization had cleared. A trace ghost vessel was seen temporally at a site of deep neovascularization (Figure 2). He was instructed to continue valacyclovir once daily and prednisolone drops once daily in the right eye and to follow up in 1 month.
At the 1-month follow-up, the patient’s signs and symptoms had reverted to his original presentation. The patient reported poor adherence to the medication regimen, having missed multiple doses of prednisolone drops as well as valacyclovir. The patient was counseled again on the ISK regimen, and the prednisolone drops and 1-g oral valacyclovir were refilled. A follow-up visit was scheduled for 2 weeks. Additional follow-up revealed a resolved corneal appearance and bimonthly follow-ups were scheduled thereafter.
Discussion
HSK is the most common infectious cause of unilateral blindness and vision impairment in the world.2 This case highlights the diagnosis and management of a patient with ISK, a type of HSK characterized by decreased corneal sensitivity and unilateral stromal opacification or neovascularization.6
ISK is caused by the herpes simplex virus (HSV), a double-stranded enveloped DNA virus that occurs worldwide with little variation, replicates in many types of cells, has rapid growth, and is cytolytic, causing necrosis of nearby cells. Transmission is via direct contact and there is a lifelong latency period in the trigeminal ganglia. Both primary and reactivation infections of HSK can affect a broad array of ocular structures, from the lids to the retina. Infectious epithelial keratitis, also known as dendritic keratitis, is the reactivation of the live virus and is the most common presentation of HSK. ISK is responsible for 20% to 48% of recurrent HSV disease and is the leading cause of vision loss. ISK is the result of an immune-mediated inflammatory response due to a retained viral antigen within the stromal tissue.7 Inflammation in the corneal stroma leads to corneal haze and eventually focal or diffuse scarring, reducing the visual potential.7 This presentation may occur days to years after the initial epithelial episode and may persist for years. Although this patient did not present with infectious epithelial keratitis, it is possible he had a previous episode not mentioned as a history was difficult to obtain, and it can be subtle or innocuous, like pink eye.
Symptoms of ISK include unilateral redness, photophobia, tearing, eye pain, and blurred vision, as described by this patient. On examination, initial manifestations of ISK include corneal haze, edema, scarring, and neovascularization.7 Again, this patient presented with edema and neovascularization. These signs may improve with prompt diagnosis and treatment. More frequent reactivated disease leads to a higher propensity of corneal scarring and irregular astigmatism, reducing the visual outcome.
The standard of care established by the Herpetic Eye Disease Study recommends that a patient with presumed ISK should be started on oral antiviral therapy and, in the absence of epithelial disease, topical steroids. Oral antivirals, such as acyclovir and valacyclovir, have good ocular penetration, a good safety profile, a low susceptibility of resistance, and are well tolerated with long-term treatment.2,8 There were no known interactions between any of the patient’s medications and valacyclovir. Oral antivirals should be used in the initial presentation and for maintenance therapy to help reduce the chance of recurrent disease. Initial treatment for ISK is 1-g valacyclovir 3 times daily. When the eye becomes quiet, that dosage can be tapered to 1 g twice daily, to 1 g once daily, and eventually to a maintenance dose of 500 mg daily. Topical steroids block the inflammatory cascade, therefore reducing the corneal inflammation and potential scarring, further reducing the risk of visual impairment.9 Initial treatment is 1 drop 3 times daily, then can be tapered at the same schedule as the oral acyclovir to help simplify adherence for the patient. After 1 drop once daily, steroids may be discontinued while the oral antiviral maintenance dosage continues. Follow-ups should be performed on a monthly to bimonthly basis to evaluate intraocular pressure, ensuring there is no steroid response.
As seen in this patient, adherence with a treatment regimen and awareness of factors, such as a complex psychosocial history that may impact this adherence, are of utmost importance.7
Conclusions
ISK presents unilaterally with decreased or absent corneal sensitivity and nonspecific symptoms. It should be at the top of the list in the differential diagnosis in any patient with unilateral corneal edema, opacification, or neovascularization, and the patient should be started on oral antiviral therapy.
1. Sibley D, Larkin DFP. Update on Herpes simplex keratitis management. Eye (Lond). 2020;34(12):2219-2226. doi:10.1038/s41433-020-01153-x
2. Chodosh J, Ung L. Adoption of innovation in herpes simplex virus keratitis. Cornea. 2020;39(1)(suppl 1):S7-S18. doi:10.1097/ICO.0000000000002425
3. Pérez-Bartolomé F, Botín DM, de Dompablo P, de Arriba P, Arnalich Montiel F, Muñoz Negrete FJ. Post-herpes neurotrophic keratopathy: pathogenesis, clinical signs and current therapies. Arch Soc Esp Oftalmol. 2019;94(4):171-183. doi:10.1016/j.oftal.2019.01.002
4. Holland EJ, Schwartz GS. Classification of herpes simplex virus keratitis. Cornea. 1999;18(2):144-154.
5. Gauthier AS, Noureddine S, Delbosc B. Interstitial keratitis diagnosis and treatment. J Fr Ophtalmol. 2019;42(6):e229-e237. doi:10.1016/j.jfo.2019.04.001
6. Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012;5(57):448-462. doi:10.1016/jsurvophthal.2012.01.005
7. Wang L, Wang R, Xu C, Zhou H. Pathogenesis of herpes stromal keratitis: immune inflammatory response mediated by inflammatory regulators. Front Immunol. 2020;11:766. Published 2020 May 13. doi:10.3389/fimmu.2020.00766
8. Tyring SK, Baker D, Snowden W. Valacyclovir for herpes simplex virus infection: long-term safety and sustained efficacy after 20 years’ experience with acyclovir. J Infect Dis. 2002;186(suppl 1):S40-S46. doi:10.1086/342966
9. Dawson CR. The herpetic eye disease study. Arch Ophthalmol. 1990;108(2):191-192. doi:10.1001/archopht.1990.01070040043027
1. Sibley D, Larkin DFP. Update on Herpes simplex keratitis management. Eye (Lond). 2020;34(12):2219-2226. doi:10.1038/s41433-020-01153-x
2. Chodosh J, Ung L. Adoption of innovation in herpes simplex virus keratitis. Cornea. 2020;39(1)(suppl 1):S7-S18. doi:10.1097/ICO.0000000000002425
3. Pérez-Bartolomé F, Botín DM, de Dompablo P, de Arriba P, Arnalich Montiel F, Muñoz Negrete FJ. Post-herpes neurotrophic keratopathy: pathogenesis, clinical signs and current therapies. Arch Soc Esp Oftalmol. 2019;94(4):171-183. doi:10.1016/j.oftal.2019.01.002
4. Holland EJ, Schwartz GS. Classification of herpes simplex virus keratitis. Cornea. 1999;18(2):144-154.
5. Gauthier AS, Noureddine S, Delbosc B. Interstitial keratitis diagnosis and treatment. J Fr Ophtalmol. 2019;42(6):e229-e237. doi:10.1016/j.jfo.2019.04.001
6. Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012;5(57):448-462. doi:10.1016/jsurvophthal.2012.01.005
7. Wang L, Wang R, Xu C, Zhou H. Pathogenesis of herpes stromal keratitis: immune inflammatory response mediated by inflammatory regulators. Front Immunol. 2020;11:766. Published 2020 May 13. doi:10.3389/fimmu.2020.00766
8. Tyring SK, Baker D, Snowden W. Valacyclovir for herpes simplex virus infection: long-term safety and sustained efficacy after 20 years’ experience with acyclovir. J Infect Dis. 2002;186(suppl 1):S40-S46. doi:10.1086/342966
9. Dawson CR. The herpetic eye disease study. Arch Ophthalmol. 1990;108(2):191-192. doi:10.1001/archopht.1990.01070040043027
57-year-old man • type 2 diabetes • neuropathy • bilateral foot blisters • Dx?
THE CASE
A 57-year-old man with type 2 diabetes, hyperlipidemia, and obesity presented to the emergency department (ED) for bilateral foot blisters, both of which appeared 1 day prior to evaluation. The patient’s history also included right-side Charcot foot diagnosed 4 years earlier and right foot osteomyelitis diagnosed 2 years prior. He had ongoing neuropathy in both feet but denied any significant pain.
The patient wore orthotics daily and he’d had new orthotics made 6 months prior; however, a recent COVID-19 diagnosis and prolonged hospital stay resulted in a 30-pound weight loss and decreased swelling in his ankles. He acquired new shoes 2 weeks prior to ED presentation.
Physical examination revealed large blisters along the medial aspect of the patient’s feet, with both hemorrhagic and serous fluid-filled bullae. The lesions were flaccid but intact, without drainage or surrounding erythema, warmth, or tenderness. The blister on the left foot measured 8 x 5 cm and extended from the great toe to mid-arch (FIGURE), while the one on the right foot measured 8 x 3 cm and extended from the great toe to the base of the proximal arch. Sensation was decreased in the bilateral first and second digits but unchanged from prior documented exams. Bilateral dorsalis pedis pulses were normal.
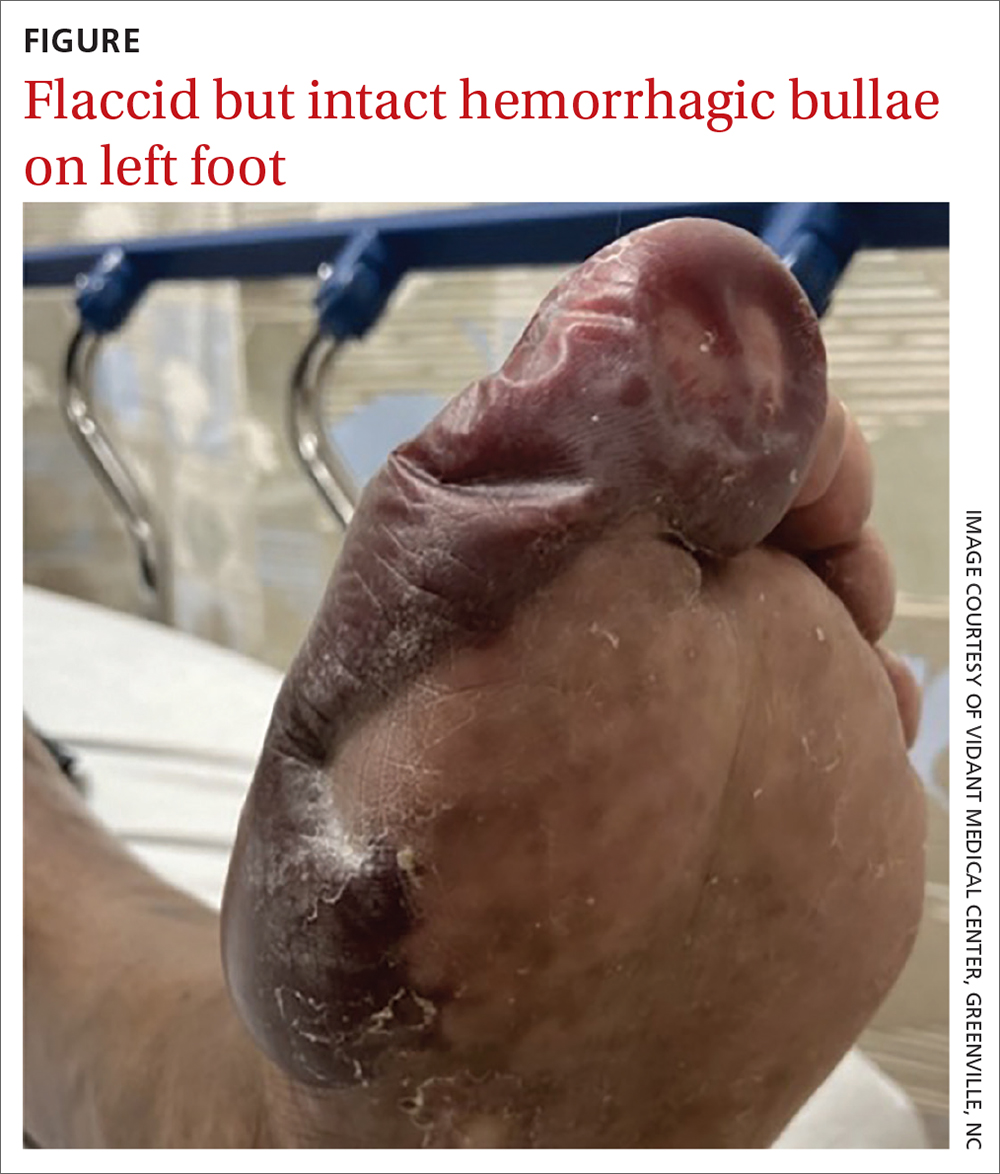
Work-up included imaging and lab work. The patient’s complete blood count was normal, as were his erythrocyte sedimentation rate and C-reactive protein level. Radiographs of the right foot were normal, but those of the left foot were concerning, although inconclusive, for osteomyelitis. Further evaluation with magnetic resonance imaging of his left foot revealed a deformity of the first digit with some subchondral signal change that was thought to be posttraumatic or degenerative, but unlikely osteomyelitis.
THE DIAGNOSIS
Podiatry was consulted for blister management. Based on atraumatic history, rapid appearance, location of blisters, unremarkable lab work and imaging, and concurrent diabetes, the patient received a diagnosis of bilateral bullous diabeticorum (BD).
DISCUSSION
Roughly one-third of patients with diabetes will experience some cutaneous adverse effect because of the disease.1 Common iterations include acanthosis nigricans, rash, or even infection.2 BD is a rare bullous skin lesion that occurs in patients with diabetes; it has a reported annual incidence of 0.16% and may be underdiagnosed.1
Cases of BD have been described both in patients with longstanding diabetes and in those newly diagnosed, although the former group is more often affected.1 BD is reported more frequently in males than females, at a ratio of 2:1.1,3 Patients ages 17 to 80 years (average age, 55 years) have received a diagnosis of BD.1 Most affected patients will have a concomitant peripheral neuropathy and sometimes nephropathy or retinopathy.1
Continue to: The etiology of BD...
The etiology of BD is unclear but appears to be multifactorial. Hypotheses suggest that there’s a link to neuropathy/nephropathy, excessive exposure to ultraviolet light, or a vascular cause secondary to hyaline deposition in the capillary walls.4,5
What you’ll see at presentation
The typical manifestation of BD is the rapid appearance of tense blisters, which may occur overnight or even within hours.1 They are usually painless; common locations include the feet, distal legs, hands, and forearms.1,5 The bullae can be serous or hemorrhagic.1
Most notable in the patient’s history will be a lack of trauma or injury to the area.1 Although A1C values do not correlate with blister formation, patients with hypoglycemic episodes and highly varying blood glucose values seem to have higher rates of occurrence.1
Other sources of blistering must be ruled out
The diagnosis of BD is clinical and based on history, exam, and exclusion of other bullous diagnoses.6 A key clue in the history is the spontaneous and rapid onset without associated trauma in a patient with diabetes.6 Direct immunofluorescence, although nonspecific, can be helpful to rule out other disorders (such as porphyria cutanea tarda and bullous pemphigoid) if the history and exam are inconclusive. Direct and indirect immunofluorescence is typically negative in BD.4,6
The differential diagnosis includes other conditions that involve bullae—such as frictional bullae, bullous pemphigoid, and bullous systemic lupus erythematosus—as well as porphyria, erythema multiforme, insect bites, or even fixed drug eruption.2,7
Continue to: Porphyria
Porphyria tends to develop on the hands, whereas BD most commonly occurs on the feet.5
Erythema multiforme typically includes inflammatory skin changes.5
Trauma or fixed drug eruption as a cause of blistering lesions would be revealed during history taking.
Considerations for treatment and follow-up
Without treatment, blisters often self-resolve in 2 to 6 weeks, but there is high likelihood of recurrence.6,8 There is no consensus on treatment, although a typical course of action is to deroof the blister and examine the area to rule out infection.6 The wound is then covered with wet-to-dry gauze that is changed regularly. If there is suspicion for or signs of underlying infection, such as an ulcer or skin necrosis, antibiotics should be included in the treatment plan.7
Additional considerations. Patients will often need therapeutic footwear if the blisters are located on the feet. Given the higher prevalence of microvascular complications in patients with diabetes who develop BD, routine ophthalmologic examination and renal function testing to monitor for microalbuminuria are recommended.5
Our patient underwent bedside incision and drainage and was discharged home with appropriate wound care and follow-up.
THE TAKEAWAY
BD cases may be underdiagnosed in clinical practice, perhaps due to patients not seeking help for a seemingly nonthreatening condition or lack of clinician recognition that bullae are related to a patient’s diabetes status. Prompt recognition and proper wound care are important to prevent poor outcomes, such as ulceration or necrosis.
CORRESPONDENCE
Kathleen S. Kinderwater, MD, 101 Heart Drive, Greenville, NC 27834; [email protected]
1. Larsen K, Jensen T, Karlsmark T, et al. Incidence of bullosis diabeticorum—a controversial cause of chronic foot ulceration. Int Wound J. 2008;5:591-596. doi: 10.1111/j.1742-481X.2008.00476.x
2. Lipsky BA, Baker PD, Ahroni JH. Diabetic bullae: 12 cases of a purportedly rare cutaneous disorder. Int J Dermatol. 2000;39:196-200. doi: 10.1046/j.1365-4362.2000.00947.x
3. Gupta V, Gulati N, Bahl J, et al. Bullosis diabeticorum: rare presentation in a common disease. Case Rep Endocrinol. 2014;2014:862912.
4. Sonani H, Abdul Salim S, Garla VV, et al. Bullosis diabeticorum: a rare presentation with immunoglobulin G (IgG) deposition related vasculopathy. Case report and focused review. Am J Case Rep. 2018;19:52-56. doi: 10.12659/ajcr.905452
5. Chouk C, Litaiem N. Bullosis diabeticorum. StatPearls [Internet]. Updated June 5, 2021. Accessed July 14, 2022. www.ncbi.nlm.nih.gov/books/NBK539872/
6. Chatterjee D, Radotra A, Radotra BD, et al. Bullous diabeticorum: a rare blistering manifestation of diabetes. Indian Dermatol Online J. 2017;8:274-275. doi: 10.4103/idoj.IDOJ_340_16
7. Kansal NK, Anuragi RP. Bullous lesions in diabetes mellitus: bullous diabeticorum (diabetic bulla). BMJ Case Rep. 2020;13:e238617. doi: 10.1136/bcr-2020-238617
8. Bello F, Samaila OM, Lawal Y, et al. 2 cases of bullosis diabeticorum following long-distance journeys by road: a report of 2 cases. Case Rep Endocrinol. 2012;2012:367218. doi: 10.1155/2012/367218
THE CASE
A 57-year-old man with type 2 diabetes, hyperlipidemia, and obesity presented to the emergency department (ED) for bilateral foot blisters, both of which appeared 1 day prior to evaluation. The patient’s history also included right-side Charcot foot diagnosed 4 years earlier and right foot osteomyelitis diagnosed 2 years prior. He had ongoing neuropathy in both feet but denied any significant pain.
The patient wore orthotics daily and he’d had new orthotics made 6 months prior; however, a recent COVID-19 diagnosis and prolonged hospital stay resulted in a 30-pound weight loss and decreased swelling in his ankles. He acquired new shoes 2 weeks prior to ED presentation.
Physical examination revealed large blisters along the medial aspect of the patient’s feet, with both hemorrhagic and serous fluid-filled bullae. The lesions were flaccid but intact, without drainage or surrounding erythema, warmth, or tenderness. The blister on the left foot measured 8 x 5 cm and extended from the great toe to mid-arch (FIGURE), while the one on the right foot measured 8 x 3 cm and extended from the great toe to the base of the proximal arch. Sensation was decreased in the bilateral first and second digits but unchanged from prior documented exams. Bilateral dorsalis pedis pulses were normal.

Work-up included imaging and lab work. The patient’s complete blood count was normal, as were his erythrocyte sedimentation rate and C-reactive protein level. Radiographs of the right foot were normal, but those of the left foot were concerning, although inconclusive, for osteomyelitis. Further evaluation with magnetic resonance imaging of his left foot revealed a deformity of the first digit with some subchondral signal change that was thought to be posttraumatic or degenerative, but unlikely osteomyelitis.
THE DIAGNOSIS
Podiatry was consulted for blister management. Based on atraumatic history, rapid appearance, location of blisters, unremarkable lab work and imaging, and concurrent diabetes, the patient received a diagnosis of bilateral bullous diabeticorum (BD).
DISCUSSION
Roughly one-third of patients with diabetes will experience some cutaneous adverse effect because of the disease.1 Common iterations include acanthosis nigricans, rash, or even infection.2 BD is a rare bullous skin lesion that occurs in patients with diabetes; it has a reported annual incidence of 0.16% and may be underdiagnosed.1
Cases of BD have been described both in patients with longstanding diabetes and in those newly diagnosed, although the former group is more often affected.1 BD is reported more frequently in males than females, at a ratio of 2:1.1,3 Patients ages 17 to 80 years (average age, 55 years) have received a diagnosis of BD.1 Most affected patients will have a concomitant peripheral neuropathy and sometimes nephropathy or retinopathy.1
Continue to: The etiology of BD...
The etiology of BD is unclear but appears to be multifactorial. Hypotheses suggest that there’s a link to neuropathy/nephropathy, excessive exposure to ultraviolet light, or a vascular cause secondary to hyaline deposition in the capillary walls.4,5
What you’ll see at presentation
The typical manifestation of BD is the rapid appearance of tense blisters, which may occur overnight or even within hours.1 They are usually painless; common locations include the feet, distal legs, hands, and forearms.1,5 The bullae can be serous or hemorrhagic.1
Most notable in the patient’s history will be a lack of trauma or injury to the area.1 Although A1C values do not correlate with blister formation, patients with hypoglycemic episodes and highly varying blood glucose values seem to have higher rates of occurrence.1
Other sources of blistering must be ruled out
The diagnosis of BD is clinical and based on history, exam, and exclusion of other bullous diagnoses.6 A key clue in the history is the spontaneous and rapid onset without associated trauma in a patient with diabetes.6 Direct immunofluorescence, although nonspecific, can be helpful to rule out other disorders (such as porphyria cutanea tarda and bullous pemphigoid) if the history and exam are inconclusive. Direct and indirect immunofluorescence is typically negative in BD.4,6
The differential diagnosis includes other conditions that involve bullae—such as frictional bullae, bullous pemphigoid, and bullous systemic lupus erythematosus—as well as porphyria, erythema multiforme, insect bites, or even fixed drug eruption.2,7
Continue to: Porphyria
Porphyria tends to develop on the hands, whereas BD most commonly occurs on the feet.5
Erythema multiforme typically includes inflammatory skin changes.5
Trauma or fixed drug eruption as a cause of blistering lesions would be revealed during history taking.
Considerations for treatment and follow-up
Without treatment, blisters often self-resolve in 2 to 6 weeks, but there is high likelihood of recurrence.6,8 There is no consensus on treatment, although a typical course of action is to deroof the blister and examine the area to rule out infection.6 The wound is then covered with wet-to-dry gauze that is changed regularly. If there is suspicion for or signs of underlying infection, such as an ulcer or skin necrosis, antibiotics should be included in the treatment plan.7
Additional considerations. Patients will often need therapeutic footwear if the blisters are located on the feet. Given the higher prevalence of microvascular complications in patients with diabetes who develop BD, routine ophthalmologic examination and renal function testing to monitor for microalbuminuria are recommended.5
Our patient underwent bedside incision and drainage and was discharged home with appropriate wound care and follow-up.
THE TAKEAWAY
BD cases may be underdiagnosed in clinical practice, perhaps due to patients not seeking help for a seemingly nonthreatening condition or lack of clinician recognition that bullae are related to a patient’s diabetes status. Prompt recognition and proper wound care are important to prevent poor outcomes, such as ulceration or necrosis.
CORRESPONDENCE
Kathleen S. Kinderwater, MD, 101 Heart Drive, Greenville, NC 27834; [email protected]
THE CASE
A 57-year-old man with type 2 diabetes, hyperlipidemia, and obesity presented to the emergency department (ED) for bilateral foot blisters, both of which appeared 1 day prior to evaluation. The patient’s history also included right-side Charcot foot diagnosed 4 years earlier and right foot osteomyelitis diagnosed 2 years prior. He had ongoing neuropathy in both feet but denied any significant pain.
The patient wore orthotics daily and he’d had new orthotics made 6 months prior; however, a recent COVID-19 diagnosis and prolonged hospital stay resulted in a 30-pound weight loss and decreased swelling in his ankles. He acquired new shoes 2 weeks prior to ED presentation.
Physical examination revealed large blisters along the medial aspect of the patient’s feet, with both hemorrhagic and serous fluid-filled bullae. The lesions were flaccid but intact, without drainage or surrounding erythema, warmth, or tenderness. The blister on the left foot measured 8 x 5 cm and extended from the great toe to mid-arch (FIGURE), while the one on the right foot measured 8 x 3 cm and extended from the great toe to the base of the proximal arch. Sensation was decreased in the bilateral first and second digits but unchanged from prior documented exams. Bilateral dorsalis pedis pulses were normal.

Work-up included imaging and lab work. The patient’s complete blood count was normal, as were his erythrocyte sedimentation rate and C-reactive protein level. Radiographs of the right foot were normal, but those of the left foot were concerning, although inconclusive, for osteomyelitis. Further evaluation with magnetic resonance imaging of his left foot revealed a deformity of the first digit with some subchondral signal change that was thought to be posttraumatic or degenerative, but unlikely osteomyelitis.
THE DIAGNOSIS
Podiatry was consulted for blister management. Based on atraumatic history, rapid appearance, location of blisters, unremarkable lab work and imaging, and concurrent diabetes, the patient received a diagnosis of bilateral bullous diabeticorum (BD).
DISCUSSION
Roughly one-third of patients with diabetes will experience some cutaneous adverse effect because of the disease.1 Common iterations include acanthosis nigricans, rash, or even infection.2 BD is a rare bullous skin lesion that occurs in patients with diabetes; it has a reported annual incidence of 0.16% and may be underdiagnosed.1
Cases of BD have been described both in patients with longstanding diabetes and in those newly diagnosed, although the former group is more often affected.1 BD is reported more frequently in males than females, at a ratio of 2:1.1,3 Patients ages 17 to 80 years (average age, 55 years) have received a diagnosis of BD.1 Most affected patients will have a concomitant peripheral neuropathy and sometimes nephropathy or retinopathy.1
Continue to: The etiology of BD...
The etiology of BD is unclear but appears to be multifactorial. Hypotheses suggest that there’s a link to neuropathy/nephropathy, excessive exposure to ultraviolet light, or a vascular cause secondary to hyaline deposition in the capillary walls.4,5
What you’ll see at presentation
The typical manifestation of BD is the rapid appearance of tense blisters, which may occur overnight or even within hours.1 They are usually painless; common locations include the feet, distal legs, hands, and forearms.1,5 The bullae can be serous or hemorrhagic.1
Most notable in the patient’s history will be a lack of trauma or injury to the area.1 Although A1C values do not correlate with blister formation, patients with hypoglycemic episodes and highly varying blood glucose values seem to have higher rates of occurrence.1
Other sources of blistering must be ruled out
The diagnosis of BD is clinical and based on history, exam, and exclusion of other bullous diagnoses.6 A key clue in the history is the spontaneous and rapid onset without associated trauma in a patient with diabetes.6 Direct immunofluorescence, although nonspecific, can be helpful to rule out other disorders (such as porphyria cutanea tarda and bullous pemphigoid) if the history and exam are inconclusive. Direct and indirect immunofluorescence is typically negative in BD.4,6
The differential diagnosis includes other conditions that involve bullae—such as frictional bullae, bullous pemphigoid, and bullous systemic lupus erythematosus—as well as porphyria, erythema multiforme, insect bites, or even fixed drug eruption.2,7
Continue to: Porphyria
Porphyria tends to develop on the hands, whereas BD most commonly occurs on the feet.5
Erythema multiforme typically includes inflammatory skin changes.5
Trauma or fixed drug eruption as a cause of blistering lesions would be revealed during history taking.
Considerations for treatment and follow-up
Without treatment, blisters often self-resolve in 2 to 6 weeks, but there is high likelihood of recurrence.6,8 There is no consensus on treatment, although a typical course of action is to deroof the blister and examine the area to rule out infection.6 The wound is then covered with wet-to-dry gauze that is changed regularly. If there is suspicion for or signs of underlying infection, such as an ulcer or skin necrosis, antibiotics should be included in the treatment plan.7
Additional considerations. Patients will often need therapeutic footwear if the blisters are located on the feet. Given the higher prevalence of microvascular complications in patients with diabetes who develop BD, routine ophthalmologic examination and renal function testing to monitor for microalbuminuria are recommended.5
Our patient underwent bedside incision and drainage and was discharged home with appropriate wound care and follow-up.
THE TAKEAWAY
BD cases may be underdiagnosed in clinical practice, perhaps due to patients not seeking help for a seemingly nonthreatening condition or lack of clinician recognition that bullae are related to a patient’s diabetes status. Prompt recognition and proper wound care are important to prevent poor outcomes, such as ulceration or necrosis.
CORRESPONDENCE
Kathleen S. Kinderwater, MD, 101 Heart Drive, Greenville, NC 27834; [email protected]
1. Larsen K, Jensen T, Karlsmark T, et al. Incidence of bullosis diabeticorum—a controversial cause of chronic foot ulceration. Int Wound J. 2008;5:591-596. doi: 10.1111/j.1742-481X.2008.00476.x
2. Lipsky BA, Baker PD, Ahroni JH. Diabetic bullae: 12 cases of a purportedly rare cutaneous disorder. Int J Dermatol. 2000;39:196-200. doi: 10.1046/j.1365-4362.2000.00947.x
3. Gupta V, Gulati N, Bahl J, et al. Bullosis diabeticorum: rare presentation in a common disease. Case Rep Endocrinol. 2014;2014:862912.
4. Sonani H, Abdul Salim S, Garla VV, et al. Bullosis diabeticorum: a rare presentation with immunoglobulin G (IgG) deposition related vasculopathy. Case report and focused review. Am J Case Rep. 2018;19:52-56. doi: 10.12659/ajcr.905452
5. Chouk C, Litaiem N. Bullosis diabeticorum. StatPearls [Internet]. Updated June 5, 2021. Accessed July 14, 2022. www.ncbi.nlm.nih.gov/books/NBK539872/
6. Chatterjee D, Radotra A, Radotra BD, et al. Bullous diabeticorum: a rare blistering manifestation of diabetes. Indian Dermatol Online J. 2017;8:274-275. doi: 10.4103/idoj.IDOJ_340_16
7. Kansal NK, Anuragi RP. Bullous lesions in diabetes mellitus: bullous diabeticorum (diabetic bulla). BMJ Case Rep. 2020;13:e238617. doi: 10.1136/bcr-2020-238617
8. Bello F, Samaila OM, Lawal Y, et al. 2 cases of bullosis diabeticorum following long-distance journeys by road: a report of 2 cases. Case Rep Endocrinol. 2012;2012:367218. doi: 10.1155/2012/367218
1. Larsen K, Jensen T, Karlsmark T, et al. Incidence of bullosis diabeticorum—a controversial cause of chronic foot ulceration. Int Wound J. 2008;5:591-596. doi: 10.1111/j.1742-481X.2008.00476.x
2. Lipsky BA, Baker PD, Ahroni JH. Diabetic bullae: 12 cases of a purportedly rare cutaneous disorder. Int J Dermatol. 2000;39:196-200. doi: 10.1046/j.1365-4362.2000.00947.x
3. Gupta V, Gulati N, Bahl J, et al. Bullosis diabeticorum: rare presentation in a common disease. Case Rep Endocrinol. 2014;2014:862912.
4. Sonani H, Abdul Salim S, Garla VV, et al. Bullosis diabeticorum: a rare presentation with immunoglobulin G (IgG) deposition related vasculopathy. Case report and focused review. Am J Case Rep. 2018;19:52-56. doi: 10.12659/ajcr.905452
5. Chouk C, Litaiem N. Bullosis diabeticorum. StatPearls [Internet]. Updated June 5, 2021. Accessed July 14, 2022. www.ncbi.nlm.nih.gov/books/NBK539872/
6. Chatterjee D, Radotra A, Radotra BD, et al. Bullous diabeticorum: a rare blistering manifestation of diabetes. Indian Dermatol Online J. 2017;8:274-275. doi: 10.4103/idoj.IDOJ_340_16
7. Kansal NK, Anuragi RP. Bullous lesions in diabetes mellitus: bullous diabeticorum (diabetic bulla). BMJ Case Rep. 2020;13:e238617. doi: 10.1136/bcr-2020-238617
8. Bello F, Samaila OM, Lawal Y, et al. 2 cases of bullosis diabeticorum following long-distance journeys by road: a report of 2 cases. Case Rep Endocrinol. 2012;2012:367218. doi: 10.1155/2012/367218