User login
Mohs micrographic surgery (MMS) frequently is used in surgical removal of cancerous cutaneous lesions on cosmetically sensitive areas and anatomically challenging sites, including the ears. The vascular supply of the ear is complex and includes several watershed regions that are susceptible to injury during surgical resection or operative closure.
Case Reports
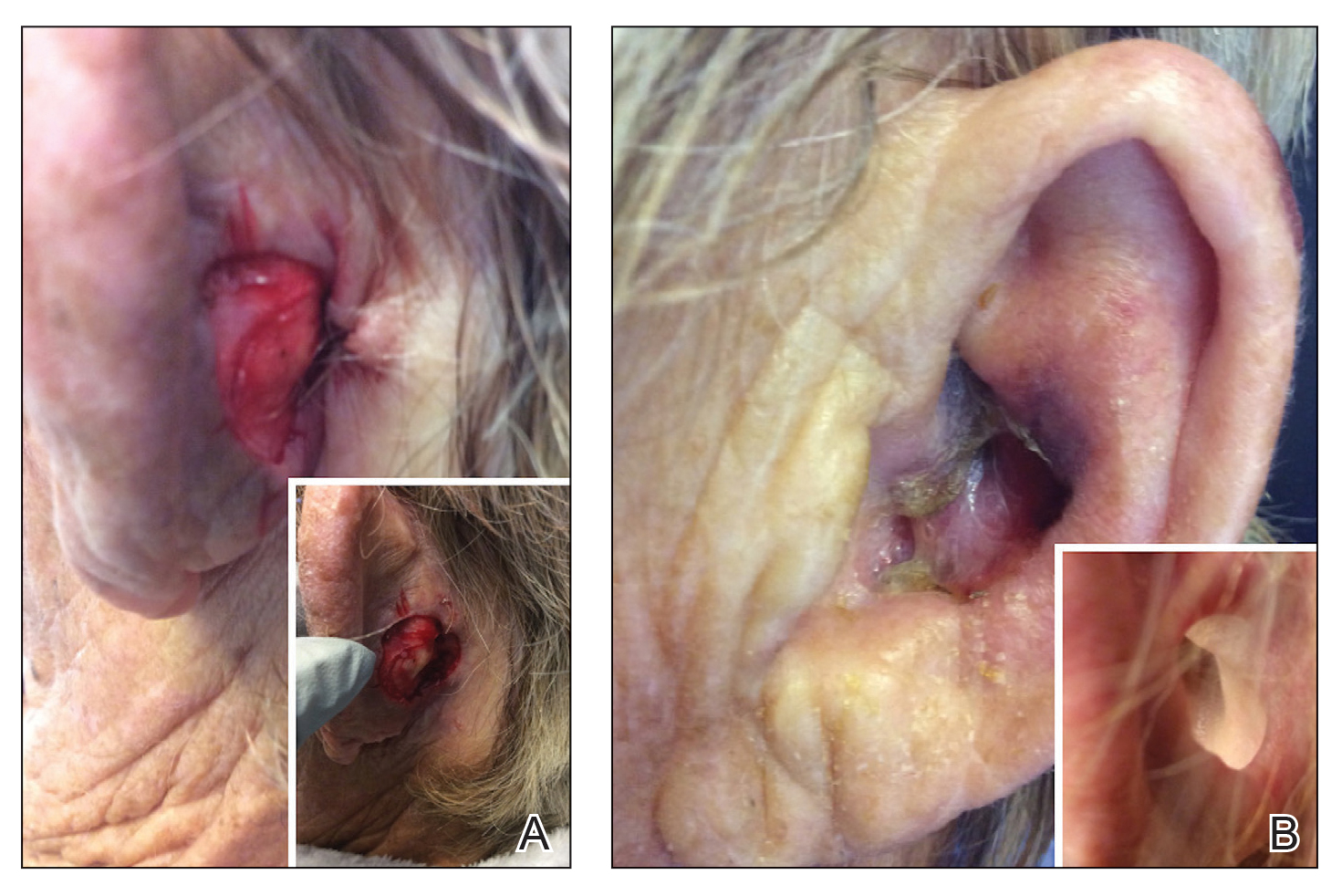
Patient 1—An 82-year-old woman with a 100-pack-year smoking history and no known history of diabetes mellitus or coronary artery disease presented with a superficial and micronodular basal cell carcinoma (BCC) of the left postauricular skin of approximately 18 months’ duration. Mohs micrographic surgery was performed for lesion removal. The BCC was noted to be deeply penetrating and by the second stage was to the depth of the deep subcutaneous tissue (Figure 1A [inset]). Frozen section histopathology revealed a micronodular and superficial BCC. A 2.1×2.0-cm postoperative defect including the posterior surface of the ear, postauricular sulcus, and postauricular scalp remained. To minimize the area left to heal via secondary intention, partial layered closure was performed by placing four 4-0 polyglactin sutures from the scalp side of the defect on the postauricular skin to the postauricular sulcus (Figure 1A).
The patient presented to the clinic on postoperative day (POD) 4, noting pain and redness since the evening of the surgery on the anterior surface of the ear, specifically the cavum concha. Physical examination revealed that the incision site appeared to be healing as expected, but the cavum concha demonstrated erosions and ecchymosis (Figure 1B). A fluid culture was collected, and the patient was started on doxycycline 100 mg twice daily for 10 days. The patient returned to the clinic at POD 10 with skin sloughing and a small border of dark purple discoloration, consistent with early necrosis.
At the 1-month postsurgery follow-up visit, the wound had persistent anterior sloughing and discoloration with adherent debris suggestive of vascular compromise. At the 5-month wound check, the left conchal bowl had a 1-cm through-and-through defect of the concha cavum (Figure 1B [inset]). The favored etiology was occlusion of the posterior auricular artery during the patient’s MMS and reconstruction. Once healed, options including reconstruction, prosthesis, and no treatment were discussed with the patient. The patient decided to pursue partial closure of the defect.
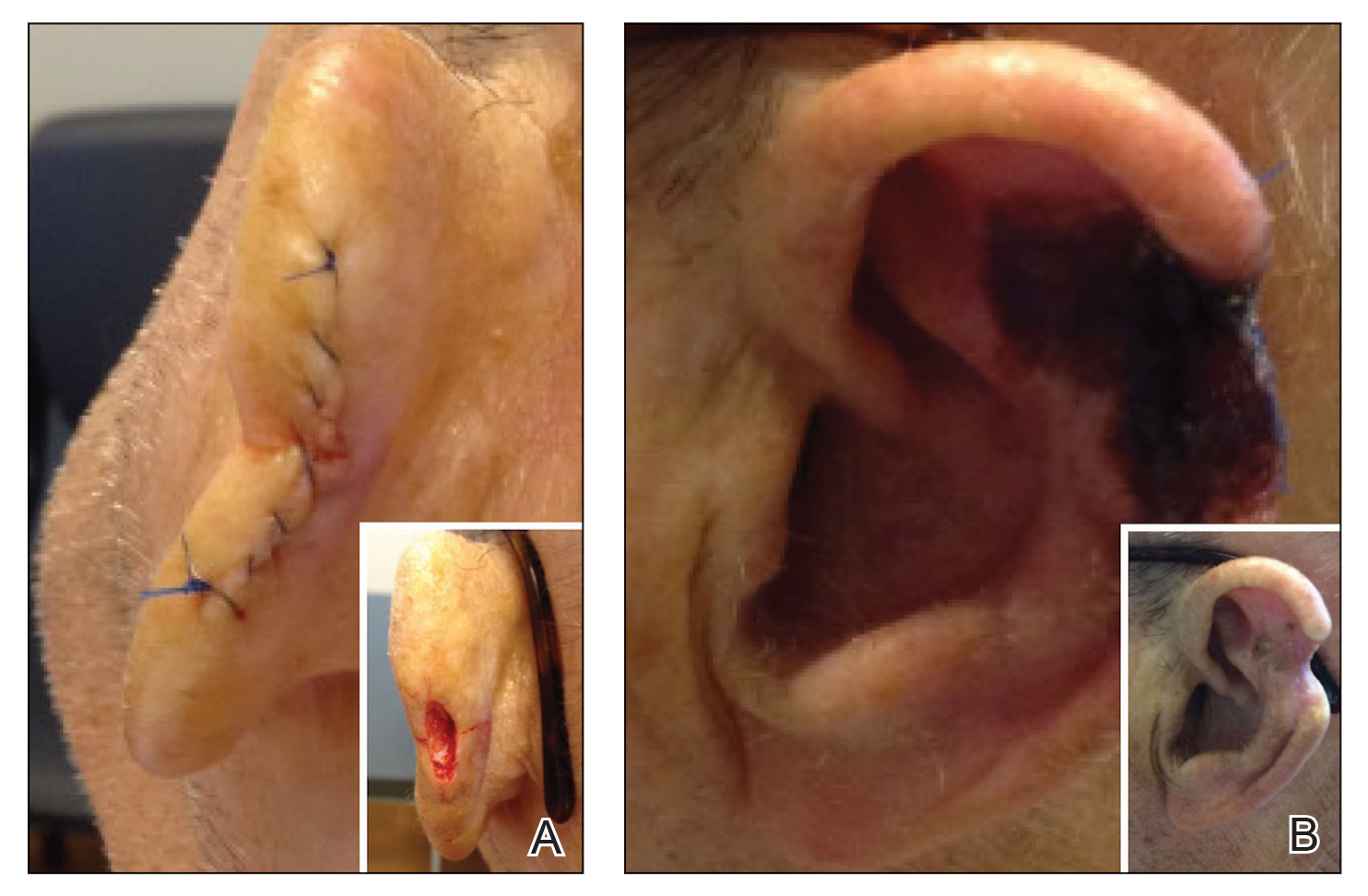
Patient 2—A 71-year-old man with coronary artery disease and no known smoking or diabetes mellitus history presented with a 0.7×0.6-cm cutaneous squamous cell carcinoma of the left helix (Figure 2A [inset]). Mohs micrographic surgery was completed, resulting in a 1.1×1.0-cm defect that extended to the perichondrium. Given the location and size, a linear closure was performed with a deep layer of 5-0 polyglactin sutures and a cutaneous layer of 6-0 polypropylene sutures. The final closure length was 2.1 cm (Figure 2A).
On POD 14, the patient presented for suture removal and reported the onset of brown discoloration of the ear on POD 3. Physical examination revealed the left ear appeared dusky around the mid helix with extension onto the antihelix (Figure 2B). Because one of the main concerns was necrosis, a thin layer of nitropaste ointment 2% was prescribed to be applied twice daily to the affected area, in addition to liberal application of petroleum jelly. On POD 21, the left mid helix demonstrated a well-defined area of necrosis on the helical rim extending to the antihelix, and conservative treatment was continued. Four weeks later, the left ear had a prominent eschar, which was debrided. On follow-up 6 weeks later, the area was well healed with an obvious notched defect of the helix and scaphoid fossa (Figure 2B [inset]). The favored etiology was occlusion of the middle helical arcade during the patient’s MMS and reconstruction. Reconstructive options were discussed with the patient; however, he declined any further reconstructive intervention.
Comment
Auricular Vasculature—In our patients, the auricular vascular supply was compromised during routine MMS followed by reconstruction, resulting in tissue necrosis. Given the relative frequency of these procedures and the risk for tissue necrosis, a review of the auricular vasculature with special attention to the conchal bowl and helical rim was warranted (Figure 3).
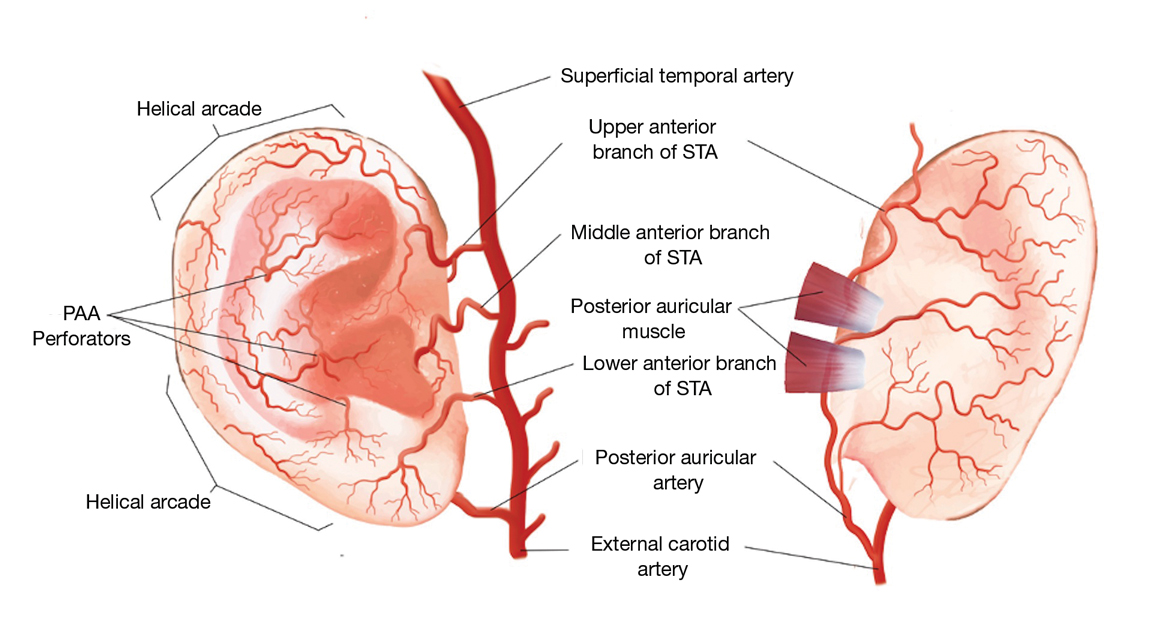
The auricle is supplied by 2 main arterial sources arising from the external carotid artery: the superficial temporal artery (STA) supplying the anterior auricle and the posterior auricular artery (PAA) supplying the posterior auricle and the concha.1 Anastomoses between these 2 blood supplies occur through perforating arteries and vascular arcades.
As the STA courses cranially, it moves from a deep position—deep to the parotidomasseteric fascia—to the superficial temporal fascia approximately 1 cm anterior and superior to the tragus. In approximately 80% of patients, 3 perpendicular branches stem from the STA—the upper, middle, and lower anterior branches—which supply the ascending helix, tragus, and lower margin of the earlobe, respectively.2 The upper anterior branch of the STA joins other branches to form 2 dominant arcades: the first with the nonperforating branches of the PAA forming the upper third of the helical arcade, and the second with the lower anterior branch of the STA forming the middle portion of the helical arcade.3,4 In 75% of patients, the middle helical arcade was identified as a single connecting artery, whereas in the remaining 25% of patients, a robust capillary network was formed.2 In patient 2, the middle helical arcade was likely disrupted during closure, resulting in the helical necrosis seen postoperatively.
The second main blood supply of the auricle is the PAA, which enters in a more superficial position after traversing superiorly from the meatal cartilage, between the mastoid process and the posterior surface of the concha. From this point, the PAA runs in the deep subcutaneous tissue in the groove formed by the conchal cartilage and the mastoid process. Near the midpoint of the postauricular groove, it passes inferior to the postauricular muscle. The PAA has multiple radial branches that anastomose with helical branches; it also sends perforating branches (there were 2–4 branches in a recent study2) through the cartilage to the anterior surface of the concha. The 2 primary perforating arteries most commonly are located at the level of the antihelix and the antitragus.5 These arteries transverse through a vascular foramen located approximately 11 mm from the tragus in the horizontal plane and supply blood to the conchal bowl.6 In patient 1, the PAA itself, or the perforating arteries that course anteriorly through the vascular foramen, was likely disrupted, resulting in the conchal defect.
Special Considerations Before Surgery—As evidenced by these cases, special attention is needed during operative planning to account for the external ear vascular arcades. Damage to the helical arcades (patient 2) or the perforating arteries within the conchal bowl (patient 1) can lead to unintended consequences such as postoperative tissue necrosis. Tissue manipulation in these areas should be approached cautiously and with the least invasive treatment and closure options available. In doing so, blood flow and tissue integrity can be maintained, resulting in improved postoperative outcomes. Further research is warranted to identify the best intervention in cases involving these watershed regions.
- Park C, Lineaweaver WC, Rumly TO, et al. Arterial supply of the anterior ear. Plast Reconstr Surg. 1992;90:38-44. doi:10.1097/00006534-199207000-00005
- Zilinsky I, Erdmann D, Weissman O, et al. Reevaluation of the arterial blood supply of the auricle. J Anat. 2017;230:315-324. doi:10.1111/joa.12550
- Erdmann D, Bruno AD, Follmar KE, et al. The helical arcade: anatomic basis for survival in near-total ear avulsion. J Craniofac Surg. 2009;20:245-248. doi:10.1097/SCS.0b013e318184343a
- Zilinsky I, Cotofana S, Hammer N, et al. The arterial blood supply of the helical rim and the earlobe-based advancement flap (ELBAF): a new strategy for reconstructions of helical rim defects. J Plast Reconstr Aesthet Surg. 2015;68:56-62. doi:10.1016/j.bjps.2014.08.062
- Henoux M, Espitalier F, Hamel A, et al. Vascular supply of the auricle: anatomical study and applications to external ear reconstruction. Dermatol Surg. 2017;43:87-97. doi:10.1097/dss.0000000000000928
- Wilson C, Iwanaga J, Simonds E, et al. The conchal vascular foramen of the posterior auricular artery: application to conchal cartilage grafting. Kurume Med J. 2018;65:7-10. doi:10.2739/kurumemedj.MS651002
Mohs micrographic surgery (MMS) frequently is used in surgical removal of cancerous cutaneous lesions on cosmetically sensitive areas and anatomically challenging sites, including the ears. The vascular supply of the ear is complex and includes several watershed regions that are susceptible to injury during surgical resection or operative closure.
Case Reports
Patient 1—An 82-year-old woman with a 100-pack-year smoking history and no known history of diabetes mellitus or coronary artery disease presented with a superficial and micronodular basal cell carcinoma (BCC) of the left postauricular skin of approximately 18 months’ duration. Mohs micrographic surgery was performed for lesion removal. The BCC was noted to be deeply penetrating and by the second stage was to the depth of the deep subcutaneous tissue (Figure 1A [inset]). Frozen section histopathology revealed a micronodular and superficial BCC. A 2.1×2.0-cm postoperative defect including the posterior surface of the ear, postauricular sulcus, and postauricular scalp remained. To minimize the area left to heal via secondary intention, partial layered closure was performed by placing four 4-0 polyglactin sutures from the scalp side of the defect on the postauricular skin to the postauricular sulcus (Figure 1A).
The patient presented to the clinic on postoperative day (POD) 4, noting pain and redness since the evening of the surgery on the anterior surface of the ear, specifically the cavum concha. Physical examination revealed that the incision site appeared to be healing as expected, but the cavum concha demonstrated erosions and ecchymosis (Figure 1B). A fluid culture was collected, and the patient was started on doxycycline 100 mg twice daily for 10 days. The patient returned to the clinic at POD 10 with skin sloughing and a small border of dark purple discoloration, consistent with early necrosis.
At the 1-month postsurgery follow-up visit, the wound had persistent anterior sloughing and discoloration with adherent debris suggestive of vascular compromise. At the 5-month wound check, the left conchal bowl had a 1-cm through-and-through defect of the concha cavum (Figure 1B [inset]). The favored etiology was occlusion of the posterior auricular artery during the patient’s MMS and reconstruction. Once healed, options including reconstruction, prosthesis, and no treatment were discussed with the patient. The patient decided to pursue partial closure of the defect.

Patient 2—A 71-year-old man with coronary artery disease and no known smoking or diabetes mellitus history presented with a 0.7×0.6-cm cutaneous squamous cell carcinoma of the left helix (Figure 2A [inset]). Mohs micrographic surgery was completed, resulting in a 1.1×1.0-cm defect that extended to the perichondrium. Given the location and size, a linear closure was performed with a deep layer of 5-0 polyglactin sutures and a cutaneous layer of 6-0 polypropylene sutures. The final closure length was 2.1 cm (Figure 2A).
On POD 14, the patient presented for suture removal and reported the onset of brown discoloration of the ear on POD 3. Physical examination revealed the left ear appeared dusky around the mid helix with extension onto the antihelix (Figure 2B). Because one of the main concerns was necrosis, a thin layer of nitropaste ointment 2% was prescribed to be applied twice daily to the affected area, in addition to liberal application of petroleum jelly. On POD 21, the left mid helix demonstrated a well-defined area of necrosis on the helical rim extending to the antihelix, and conservative treatment was continued. Four weeks later, the left ear had a prominent eschar, which was debrided. On follow-up 6 weeks later, the area was well healed with an obvious notched defect of the helix and scaphoid fossa (Figure 2B [inset]). The favored etiology was occlusion of the middle helical arcade during the patient’s MMS and reconstruction. Reconstructive options were discussed with the patient; however, he declined any further reconstructive intervention.

Comment
Auricular Vasculature—In our patients, the auricular vascular supply was compromised during routine MMS followed by reconstruction, resulting in tissue necrosis. Given the relative frequency of these procedures and the risk for tissue necrosis, a review of the auricular vasculature with special attention to the conchal bowl and helical rim was warranted (Figure 3).

The auricle is supplied by 2 main arterial sources arising from the external carotid artery: the superficial temporal artery (STA) supplying the anterior auricle and the posterior auricular artery (PAA) supplying the posterior auricle and the concha.1 Anastomoses between these 2 blood supplies occur through perforating arteries and vascular arcades.
As the STA courses cranially, it moves from a deep position—deep to the parotidomasseteric fascia—to the superficial temporal fascia approximately 1 cm anterior and superior to the tragus. In approximately 80% of patients, 3 perpendicular branches stem from the STA—the upper, middle, and lower anterior branches—which supply the ascending helix, tragus, and lower margin of the earlobe, respectively.2 The upper anterior branch of the STA joins other branches to form 2 dominant arcades: the first with the nonperforating branches of the PAA forming the upper third of the helical arcade, and the second with the lower anterior branch of the STA forming the middle portion of the helical arcade.3,4 In 75% of patients, the middle helical arcade was identified as a single connecting artery, whereas in the remaining 25% of patients, a robust capillary network was formed.2 In patient 2, the middle helical arcade was likely disrupted during closure, resulting in the helical necrosis seen postoperatively.
The second main blood supply of the auricle is the PAA, which enters in a more superficial position after traversing superiorly from the meatal cartilage, between the mastoid process and the posterior surface of the concha. From this point, the PAA runs in the deep subcutaneous tissue in the groove formed by the conchal cartilage and the mastoid process. Near the midpoint of the postauricular groove, it passes inferior to the postauricular muscle. The PAA has multiple radial branches that anastomose with helical branches; it also sends perforating branches (there were 2–4 branches in a recent study2) through the cartilage to the anterior surface of the concha. The 2 primary perforating arteries most commonly are located at the level of the antihelix and the antitragus.5 These arteries transverse through a vascular foramen located approximately 11 mm from the tragus in the horizontal plane and supply blood to the conchal bowl.6 In patient 1, the PAA itself, or the perforating arteries that course anteriorly through the vascular foramen, was likely disrupted, resulting in the conchal defect.
Special Considerations Before Surgery—As evidenced by these cases, special attention is needed during operative planning to account for the external ear vascular arcades. Damage to the helical arcades (patient 2) or the perforating arteries within the conchal bowl (patient 1) can lead to unintended consequences such as postoperative tissue necrosis. Tissue manipulation in these areas should be approached cautiously and with the least invasive treatment and closure options available. In doing so, blood flow and tissue integrity can be maintained, resulting in improved postoperative outcomes. Further research is warranted to identify the best intervention in cases involving these watershed regions.
Mohs micrographic surgery (MMS) frequently is used in surgical removal of cancerous cutaneous lesions on cosmetically sensitive areas and anatomically challenging sites, including the ears. The vascular supply of the ear is complex and includes several watershed regions that are susceptible to injury during surgical resection or operative closure.
Case Reports
Patient 1—An 82-year-old woman with a 100-pack-year smoking history and no known history of diabetes mellitus or coronary artery disease presented with a superficial and micronodular basal cell carcinoma (BCC) of the left postauricular skin of approximately 18 months’ duration. Mohs micrographic surgery was performed for lesion removal. The BCC was noted to be deeply penetrating and by the second stage was to the depth of the deep subcutaneous tissue (Figure 1A [inset]). Frozen section histopathology revealed a micronodular and superficial BCC. A 2.1×2.0-cm postoperative defect including the posterior surface of the ear, postauricular sulcus, and postauricular scalp remained. To minimize the area left to heal via secondary intention, partial layered closure was performed by placing four 4-0 polyglactin sutures from the scalp side of the defect on the postauricular skin to the postauricular sulcus (Figure 1A).
The patient presented to the clinic on postoperative day (POD) 4, noting pain and redness since the evening of the surgery on the anterior surface of the ear, specifically the cavum concha. Physical examination revealed that the incision site appeared to be healing as expected, but the cavum concha demonstrated erosions and ecchymosis (Figure 1B). A fluid culture was collected, and the patient was started on doxycycline 100 mg twice daily for 10 days. The patient returned to the clinic at POD 10 with skin sloughing and a small border of dark purple discoloration, consistent with early necrosis.
At the 1-month postsurgery follow-up visit, the wound had persistent anterior sloughing and discoloration with adherent debris suggestive of vascular compromise. At the 5-month wound check, the left conchal bowl had a 1-cm through-and-through defect of the concha cavum (Figure 1B [inset]). The favored etiology was occlusion of the posterior auricular artery during the patient’s MMS and reconstruction. Once healed, options including reconstruction, prosthesis, and no treatment were discussed with the patient. The patient decided to pursue partial closure of the defect.

Patient 2—A 71-year-old man with coronary artery disease and no known smoking or diabetes mellitus history presented with a 0.7×0.6-cm cutaneous squamous cell carcinoma of the left helix (Figure 2A [inset]). Mohs micrographic surgery was completed, resulting in a 1.1×1.0-cm defect that extended to the perichondrium. Given the location and size, a linear closure was performed with a deep layer of 5-0 polyglactin sutures and a cutaneous layer of 6-0 polypropylene sutures. The final closure length was 2.1 cm (Figure 2A).
On POD 14, the patient presented for suture removal and reported the onset of brown discoloration of the ear on POD 3. Physical examination revealed the left ear appeared dusky around the mid helix with extension onto the antihelix (Figure 2B). Because one of the main concerns was necrosis, a thin layer of nitropaste ointment 2% was prescribed to be applied twice daily to the affected area, in addition to liberal application of petroleum jelly. On POD 21, the left mid helix demonstrated a well-defined area of necrosis on the helical rim extending to the antihelix, and conservative treatment was continued. Four weeks later, the left ear had a prominent eschar, which was debrided. On follow-up 6 weeks later, the area was well healed with an obvious notched defect of the helix and scaphoid fossa (Figure 2B [inset]). The favored etiology was occlusion of the middle helical arcade during the patient’s MMS and reconstruction. Reconstructive options were discussed with the patient; however, he declined any further reconstructive intervention.

Comment
Auricular Vasculature—In our patients, the auricular vascular supply was compromised during routine MMS followed by reconstruction, resulting in tissue necrosis. Given the relative frequency of these procedures and the risk for tissue necrosis, a review of the auricular vasculature with special attention to the conchal bowl and helical rim was warranted (Figure 3).

The auricle is supplied by 2 main arterial sources arising from the external carotid artery: the superficial temporal artery (STA) supplying the anterior auricle and the posterior auricular artery (PAA) supplying the posterior auricle and the concha.1 Anastomoses between these 2 blood supplies occur through perforating arteries and vascular arcades.
As the STA courses cranially, it moves from a deep position—deep to the parotidomasseteric fascia—to the superficial temporal fascia approximately 1 cm anterior and superior to the tragus. In approximately 80% of patients, 3 perpendicular branches stem from the STA—the upper, middle, and lower anterior branches—which supply the ascending helix, tragus, and lower margin of the earlobe, respectively.2 The upper anterior branch of the STA joins other branches to form 2 dominant arcades: the first with the nonperforating branches of the PAA forming the upper third of the helical arcade, and the second with the lower anterior branch of the STA forming the middle portion of the helical arcade.3,4 In 75% of patients, the middle helical arcade was identified as a single connecting artery, whereas in the remaining 25% of patients, a robust capillary network was formed.2 In patient 2, the middle helical arcade was likely disrupted during closure, resulting in the helical necrosis seen postoperatively.
The second main blood supply of the auricle is the PAA, which enters in a more superficial position after traversing superiorly from the meatal cartilage, between the mastoid process and the posterior surface of the concha. From this point, the PAA runs in the deep subcutaneous tissue in the groove formed by the conchal cartilage and the mastoid process. Near the midpoint of the postauricular groove, it passes inferior to the postauricular muscle. The PAA has multiple radial branches that anastomose with helical branches; it also sends perforating branches (there were 2–4 branches in a recent study2) through the cartilage to the anterior surface of the concha. The 2 primary perforating arteries most commonly are located at the level of the antihelix and the antitragus.5 These arteries transverse through a vascular foramen located approximately 11 mm from the tragus in the horizontal plane and supply blood to the conchal bowl.6 In patient 1, the PAA itself, or the perforating arteries that course anteriorly through the vascular foramen, was likely disrupted, resulting in the conchal defect.
Special Considerations Before Surgery—As evidenced by these cases, special attention is needed during operative planning to account for the external ear vascular arcades. Damage to the helical arcades (patient 2) or the perforating arteries within the conchal bowl (patient 1) can lead to unintended consequences such as postoperative tissue necrosis. Tissue manipulation in these areas should be approached cautiously and with the least invasive treatment and closure options available. In doing so, blood flow and tissue integrity can be maintained, resulting in improved postoperative outcomes. Further research is warranted to identify the best intervention in cases involving these watershed regions.
- Park C, Lineaweaver WC, Rumly TO, et al. Arterial supply of the anterior ear. Plast Reconstr Surg. 1992;90:38-44. doi:10.1097/00006534-199207000-00005
- Zilinsky I, Erdmann D, Weissman O, et al. Reevaluation of the arterial blood supply of the auricle. J Anat. 2017;230:315-324. doi:10.1111/joa.12550
- Erdmann D, Bruno AD, Follmar KE, et al. The helical arcade: anatomic basis for survival in near-total ear avulsion. J Craniofac Surg. 2009;20:245-248. doi:10.1097/SCS.0b013e318184343a
- Zilinsky I, Cotofana S, Hammer N, et al. The arterial blood supply of the helical rim and the earlobe-based advancement flap (ELBAF): a new strategy for reconstructions of helical rim defects. J Plast Reconstr Aesthet Surg. 2015;68:56-62. doi:10.1016/j.bjps.2014.08.062
- Henoux M, Espitalier F, Hamel A, et al. Vascular supply of the auricle: anatomical study and applications to external ear reconstruction. Dermatol Surg. 2017;43:87-97. doi:10.1097/dss.0000000000000928
- Wilson C, Iwanaga J, Simonds E, et al. The conchal vascular foramen of the posterior auricular artery: application to conchal cartilage grafting. Kurume Med J. 2018;65:7-10. doi:10.2739/kurumemedj.MS651002
- Park C, Lineaweaver WC, Rumly TO, et al. Arterial supply of the anterior ear. Plast Reconstr Surg. 1992;90:38-44. doi:10.1097/00006534-199207000-00005
- Zilinsky I, Erdmann D, Weissman O, et al. Reevaluation of the arterial blood supply of the auricle. J Anat. 2017;230:315-324. doi:10.1111/joa.12550
- Erdmann D, Bruno AD, Follmar KE, et al. The helical arcade: anatomic basis for survival in near-total ear avulsion. J Craniofac Surg. 2009;20:245-248. doi:10.1097/SCS.0b013e318184343a
- Zilinsky I, Cotofana S, Hammer N, et al. The arterial blood supply of the helical rim and the earlobe-based advancement flap (ELBAF): a new strategy for reconstructions of helical rim defects. J Plast Reconstr Aesthet Surg. 2015;68:56-62. doi:10.1016/j.bjps.2014.08.062
- Henoux M, Espitalier F, Hamel A, et al. Vascular supply of the auricle: anatomical study and applications to external ear reconstruction. Dermatol Surg. 2017;43:87-97. doi:10.1097/dss.0000000000000928
- Wilson C, Iwanaga J, Simonds E, et al. The conchal vascular foramen of the posterior auricular artery: application to conchal cartilage grafting. Kurume Med J. 2018;65:7-10. doi:10.2739/kurumemedj.MS651002
Practice Points
- The auricular vasculature supply is complex and forms several anastomoses and arcades, making it susceptible to vascular compromise.
- Damage to the auricular helical arcades or perforating branches can result in postoperative tissue necrosis.
- Clinicians should pay special attention during operative planning for Mohs micrographic surgery to account for the external ear vascular arcades and, when possible, should choose the least invasive treatment and closure options available.