User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Updates in Pediatrics
TALKING OUT CHILDHOOD OBESITY
Resnicow K, McMaster F, Bocian A, et al. Motivational interviewing and dietary counseling for obesity in primary care: an RCT. Pediatrics. 2015;135(4):649-657.
Counseling parents of overweight children using motivational interviewing from both health care providers and registered dietitians can significantly improve BMI, according to a study of 42 practices in the Pediatric Research in Office Settings Network of the American Academy of Pediatrics.
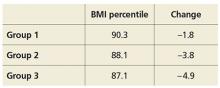
Researchers randomly assigned parents of overweight children, ages 2 through 8, to one of three groups: (1) usual care, (2) four provider-delivered motivational interviewing sessions over two years, or (3) four provider-delivered motivational interviewing sessions plus six sessions with a registered dietitian over two years. At study end, BMI percentile and change in BMI for the different groups were as follows:
COMMENTARY
The results of this study are exciting. Motivational interviewing is a technique in which the practitioner asks questions of a patient and allows the patient to discover his/her own conclusions about the topic. By so doing, the patient is more engaged in the discussion and is less resistant to input. This technique, with excellent evidence of effectiveness in the area of drug and alcohol abuse, has been shown to facilitate effective behavioral change in many areas and is recommended by the American Heart Association for behavioral change in adults.1,2 This is an exciting paper demonstrating evidence-based efficacy in addressing childhood obesity—a critical health issue—and is worth trying in the office.
1. Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55(513):305-312.
2. Spring B, Ockene JK, Gidding SS, et al; American Heart Association Behavior Change Committee of the Council on Epidemiology and Prevention, Council on Lifestyle and Cardiometabolic Health, Council for High Blood Pressure Research, and Council on Cardiovascular and Stroke Nursing. Better population health through behavior change in adults: a call to action. Circulation. 2013;128(19):2169-2176. doi: 10.1161/01.cir.0000435173.25936.e1.
Continue for long-acting reversible contraception among teens >>
LONG-ACTING REVERSIBLE CONTRACEPTION AMONG TEENS
Romero L, Pazol K, Warner L, et al. Vital signs: trends in use of long-acting reversible contraception among teens aged 15-19 years seeking contraceptive services – United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2015;64(13):363-369.
Efforts to improve teen access to long-acting reversible contraception (LARC) have increased use of these methods, according to a CDC review of services provided at Title X National Family Planning Program centers. The report found
• LARC rates among teen patients increased from 0.4% in 2005 to 7.2% in 2013.
• In 2013, 2.8% of those seeking contraception used IUDs and 4.3% used implants.
• Among Title X patients, 7.6% of 18- and 19-year-olds used LARC, compared with 6.5% of 15- to 17-year-olds.
• Rates of LARC were lowest in Mississippi (0.7%) and highest in Colorado (25.8%).
COMMENTARY
LARCs, which include IUD and implantable hormonal contraceptive devices, require no effort for adherence on the part of the user; once in place, they are effective without further action. Current CDC guidelines on contraceptive use clearly recommend LARC for teenagers based on the efficacy and safety.1 LARCs are favored for teenagers because poor compliance has yielded suboptimal effectiveness of oral contraceptives and condoms in teenagers, who often forget to take their birth control pills or don’t use condoms when they should. Many clinicians have been slow to recommend LARCs in teenagers based on safety concerns related to adverse experience with IUDs 20 to 30 years ago. According to CDC guidelines, IUDs and implantable contraceptive devices now have robust safety data, and this article shows that they are being increasingly made available to teenagers who need them.
1. Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. US Selected Practice Recommendations for Contraceptive Use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep. 2013;62(RR-05):1-60.
Continue to testing for celiac in pediatric rheumatology patients >>
TESTING FOR CELIAC IN PEDIATRIC RHEUMATOLOGY PATIENTS
Sherman Y, Karanicolas R, DiMarco B, et al. Unrecognized celiac disease in children presenting for rheumatology evaluation. Pediatrics. 2015; [Epub ahead of print].
Children presenting for rheumatology evaluation should be screened for celiac disease, according to a review of 2,125 pediatric patients who were screened for celiac as part of the standard initial serologic evaluation.
Researchers identified 36 new cases of celiac disease (2.0% prevalence rate). The most common presenting complaints among these patients were myalgia, arthralgia, and rash. Less frequent complaints included gastrointestinal complaints of abdominal pain, nausea, and diarrhea.
After initiating a gluten-free diet, all of the patients with celiac disease reported improvement or complete resolution of musculoskeletal symptoms.
COMMENTARY
Celiac disease has a prevalence of 0.5% to 1% in the US and can present with a range of symptoms that include diarrhea, abdominal discomfort, and weight loss. In addition to these typical symptoms, celiac disease can cause a wide range of systemic symptoms, including osteopenia, abnormal liver function tests, anemia, neurologic symptoms, and general malaise and fatigue.1 This study suggests that celiac testing be considered in children presenting with rheumatologic symptoms, including myalgia, arthralgia, and rash.
1. Presutti RJ, Cangemi JR, Cassidy HD, et al. Celiac disease. Am Fam Physician. 2007;76(12):1795-1802, 1809-1810.
Continue for educating parents about antibiotic use >>
EDUCATING PARENTS ABOUT ANTIBIOTIC USE
Vaz LE, Kleinman KP, Lakoma MD, et al. Prevalence of parental misconceptions about antibiotic use. Pediatrics. 2015;136:221-231.
Misperceptions about antibiotic use persist and continue to be more prevalent among parents of Medicaid-insured children, according to a study of 1,500 Massachusetts parents.
Investigators examined antibiotic-related knowledge and attitudes among both Medicaid-insured and private-insured parents and found
• Fewer Medicaid parents answered questions correctly, except for one regarding bronchitis.
• Medicaid patients were more likely to request unnecessary antibiotics.
• More parents in 2013 understood that green nasal discharge did not require antibiotics than in 2000.
• Medicaid-insured parents were younger, less likely to be white, and had less education than those commercially insured.
COMMENTARY
Decreasing the unnecessary use of antibiotics for viral infections is an important component of decreasing the development of antibiotic-resistant organisms. An important driver of clinician use of antibiotics is patients’ expectations for antibiotics. This study shows that much work remains to be done in changing patient expectations, which is not surprising to any practicing clinician. This study also suggests that the expectation for antibiotics is greater among those individuals with Medicaid insurance, which suggests that an opportunity exists for Medicaid insurance plans to do targeted patient education on this issue—which should improve patient outcomes, decrease cost from use of unneeded antibiotics, and decrease the development of antibiotic-resistant organisms.
Continue for newborn pulmonary hypertension and maternal antidepressant use >>
NEWBORN PULMONARY HYPERTENSION AND MATERNAL ANTIDEPRESSANT USE
Huybrechts KF, Bateman BT, Palmsten K, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA. 2015;313(21):2142-2151.
Taking antidepressants during late pregnancy may increase the risk for persistent pulmonary hypertension of the newborn (PPHN), according to a nested cohort study of more than 3.7 million pregnant women in the 2000-2010 Medicaid Analytic eXtract.
Investigators compared offspring of mothers who used selective serotonin reuptake inhibitors (SSRIs) or non-SSRI monotherapy in the last 90 days of pregnancy to those who did not and found 3.4% of women filled at least one prescription for antidepressants late in pregnancy, primarily SSRIs.
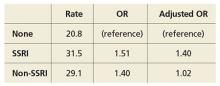
Rates and odds ratios (ORs) of PPHN stratified by use and type of antidepressant were as follows:
The study authors note the absolute risk is small, and the increased risk is more modest than previous studies found.
COMMENTARY
Depression affects more than 12% of pregnancies and has important consequences, including increased risk for suicide, preterm birth, poor fetal growth, and impaired fetal and infant development.1 PPHN is a serious condition that can require intubation and can be fatal in 10% to 20% of cases. This study adds to the conflicting data suggesting that SSRI use in pregnancy can lead to an increase in this rare condition. The decision to use an antidepressant during pregnancy is a difficult one, because depression has serious consequences but so, potentially, does treatment. The treatment of depression during pregnancy requires thoughtful, informed discussion between patient and provider.
1. Stewart DE. Clinical practice. Depression during pregnancy. N Engl J Med. 2011;365(17):1605-1611. doi:10.1056/NEJMcp1102730.
Continue for new infant vaccine treats 6 diseases >>
NEW INFANT VACCINE TREATS 6 DISEASES
Marshall GS, Adams GL, Leonardi ML, et al. Immunogenicity, safety, and tolerability of a hexavalent vaccine in infants. Pediatrics. 2015:136(2):323-332.
The safety and immunogenicity of DTaP5-IPV-Hib-HepB fully liquid investigational hexavalent vaccine are comparable with the analogous licensed component vaccines and provide a new combination vaccine option aligned with the recommended US infant immunization schedule, according to a phase III study of 1,465 participants. Overall, 981 healthy infants were vaccinated in group 1 with the hexavalent vaccine and 484 in group 2 with the analogous licensed component vaccines.
The study found
• Immune responses in group 1 to all antigens contained in the vaccine one month after dose 3 were essentially noninferior to those in group 2.
• Adverse event rates after any dose were similar in both groups.
COMMENTARY
Vaccine administration has led to many diseases, including Haemophilus influenzae type b, polio, and measles, becoming quite rare. As we have recently seen with the resurgence of measles, continued vigilance and high immunization rates are important in ensuring that these diseases remain rare. The development of a hexavalent vaccine is another step in making immunization easier for clinicians to administer and for patients to accept.
Continue for treating infants with bronchiolitis >>
TREATING INFANTS WITH BRONCHIOLITIS
Silver AH, Esteban-Cruciani N, Azzarone G, et al. 3% hypertonic saline versus normal saline in inpatient bronchiolitis: a randomized controlled trial. Pediatrics. 2015;136:1036-1043.
Infants hospitalized with bronchiolitis saw no difference in length of stay or seven-day readmission rates when treated with nebulized 3% hypertonic saline (HS) compared with nebulized normal saline (NS), according to a randomized, controlled study of 227 infants who were younger than 12 months when admitted. Patients received either 4 mL nebulized 3% HS (113 infants) or 4 mL 0.9% NS (114 infants) every four hours from enrollment until hospital discharge. Researchers found
• Median length of stay of HS and NS groups was 2.1 days vs 2.1 days, respectively.
• Seven-day readmission rates for HS and NS groups were 4.3% vs 3.1%, respectively.
• Clinical worsening events were similar between groups.
COMMENTARY
Bronchiolitis, the most common lower respiratory tract infection in infants, is usually due to a viral infection, most often respiratory syncytial virus, and can cause disease that ranges in severity from mild to life-threatening. Infants with bronchiolitis typically present with rhinitis, tachypnea, wheezing, and cough, and occasionally crackles and use of accessory muscles. While many medications are used, supportive care and monitoring are the mainstays of therapy. Maintaining pulse above 90% is important, using supplemental oxygen when needed to achieve this. Alpha-adrenergic and beta-adrenergic bronchodilators are often used; though the evidence suggests that they are not usually helpful, they can be tried and continued if they appear to help. Systemic steroids are often used, but they too lack evidence of efficacy.1 This study shows that hypertonic saline can now join the list of interventions used with evidence of a lack of efficacy.
1. Diagnosis and management of bronchiolitis. Subcommittee on diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774-1793. doi:10.1542/peds.2006-2223.
Continue for azithromycin and preschool children >>
AZITHROMYCIN AND PRESCHOOL CHILDREN: CAN ANTIBIOTICS LESSEN ONSET OF SEVERE LRTIs?
Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314(19):2034-2044.
The use of azithromycin early during an apparent respiratory tract illness (RTI) reduced the likelihood of severe lower RTI (LRTI) among young children with a history of recurrent severe LRTI, compared with placebo, according to a study of 607 children ages 12 to 71 months. Participants were randomized in a 1:1 ratio to receive either azithromycin (12 mg/kg/d for 5 d) or matching placebo at the start of an RTI. Researchers found
• A total of 937 treated RTIs were experienced by 443 children, including 92 severe LRTIs (azithromycin group, 35; placebo group, 57).
• Azithromycin significantly reduced the risk for progression to severe LRTI relative to placebo (HR, 0.64).
• Induction of azithromycin-resistant organisms and adverse events were infrequent.
COMMENTARY
Recurrent episodes of severe wheezing with RTI are an important and common occurrence, affecting up to 15% to 20% of children prior to age 6.1 LRTI was defined in this study as RTI that required the use of additional rescue medication. The current approach to RTI is to try to minimize the use of antibiotics unless an infection is clearly bacterial in origin, and to treat severe LRTI when it occurs. The results of this trial suggest that in children at high risk for severe LRTI, identified by their episodes of recurrent wheezing, early treatment of RTI with azithromycin may decrease the development of severe LRTI by more than 35%—an important result. It is important to recognize that this study does not suggest treating all RTIs with antibiotics, but rather that the use of a macrolide antibiotic may be considered, perhaps, in the select group of children similar to those studied, with a history of recurrent wheezing with previous RTIs.
1. Ly NP, Gold DR, Weiss ST, Celedón JC. Recurrent wheeze in early childhood and asthma among children at risk for atopy. Pediatrics. 2006;117(6):e1132-e1138.
TALKING OUT CHILDHOOD OBESITY
Resnicow K, McMaster F, Bocian A, et al. Motivational interviewing and dietary counseling for obesity in primary care: an RCT. Pediatrics. 2015;135(4):649-657.
Counseling parents of overweight children using motivational interviewing from both health care providers and registered dietitians can significantly improve BMI, according to a study of 42 practices in the Pediatric Research in Office Settings Network of the American Academy of Pediatrics.
Researchers randomly assigned parents of overweight children, ages 2 through 8, to one of three groups: (1) usual care, (2) four provider-delivered motivational interviewing sessions over two years, or (3) four provider-delivered motivational interviewing sessions plus six sessions with a registered dietitian over two years. At study end, BMI percentile and change in BMI for the different groups were as follows:
COMMENTARY
The results of this study are exciting. Motivational interviewing is a technique in which the practitioner asks questions of a patient and allows the patient to discover his/her own conclusions about the topic. By so doing, the patient is more engaged in the discussion and is less resistant to input. This technique, with excellent evidence of effectiveness in the area of drug and alcohol abuse, has been shown to facilitate effective behavioral change in many areas and is recommended by the American Heart Association for behavioral change in adults.1,2 This is an exciting paper demonstrating evidence-based efficacy in addressing childhood obesity—a critical health issue—and is worth trying in the office.
1. Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55(513):305-312.
2. Spring B, Ockene JK, Gidding SS, et al; American Heart Association Behavior Change Committee of the Council on Epidemiology and Prevention, Council on Lifestyle and Cardiometabolic Health, Council for High Blood Pressure Research, and Council on Cardiovascular and Stroke Nursing. Better population health through behavior change in adults: a call to action. Circulation. 2013;128(19):2169-2176. doi: 10.1161/01.cir.0000435173.25936.e1.
Continue for long-acting reversible contraception among teens >>
LONG-ACTING REVERSIBLE CONTRACEPTION AMONG TEENS
Romero L, Pazol K, Warner L, et al. Vital signs: trends in use of long-acting reversible contraception among teens aged 15-19 years seeking contraceptive services – United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2015;64(13):363-369.
Efforts to improve teen access to long-acting reversible contraception (LARC) have increased use of these methods, according to a CDC review of services provided at Title X National Family Planning Program centers. The report found
• LARC rates among teen patients increased from 0.4% in 2005 to 7.2% in 2013.
• In 2013, 2.8% of those seeking contraception used IUDs and 4.3% used implants.
• Among Title X patients, 7.6% of 18- and 19-year-olds used LARC, compared with 6.5% of 15- to 17-year-olds.
• Rates of LARC were lowest in Mississippi (0.7%) and highest in Colorado (25.8%).
COMMENTARY
LARCs, which include IUD and implantable hormonal contraceptive devices, require no effort for adherence on the part of the user; once in place, they are effective without further action. Current CDC guidelines on contraceptive use clearly recommend LARC for teenagers based on the efficacy and safety.1 LARCs are favored for teenagers because poor compliance has yielded suboptimal effectiveness of oral contraceptives and condoms in teenagers, who often forget to take their birth control pills or don’t use condoms when they should. Many clinicians have been slow to recommend LARCs in teenagers based on safety concerns related to adverse experience with IUDs 20 to 30 years ago. According to CDC guidelines, IUDs and implantable contraceptive devices now have robust safety data, and this article shows that they are being increasingly made available to teenagers who need them.
1. Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. US Selected Practice Recommendations for Contraceptive Use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep. 2013;62(RR-05):1-60.
Continue to testing for celiac in pediatric rheumatology patients >>
TESTING FOR CELIAC IN PEDIATRIC RHEUMATOLOGY PATIENTS
Sherman Y, Karanicolas R, DiMarco B, et al. Unrecognized celiac disease in children presenting for rheumatology evaluation. Pediatrics. 2015; [Epub ahead of print].
Children presenting for rheumatology evaluation should be screened for celiac disease, according to a review of 2,125 pediatric patients who were screened for celiac as part of the standard initial serologic evaluation.
Researchers identified 36 new cases of celiac disease (2.0% prevalence rate). The most common presenting complaints among these patients were myalgia, arthralgia, and rash. Less frequent complaints included gastrointestinal complaints of abdominal pain, nausea, and diarrhea.
After initiating a gluten-free diet, all of the patients with celiac disease reported improvement or complete resolution of musculoskeletal symptoms.
COMMENTARY
Celiac disease has a prevalence of 0.5% to 1% in the US and can present with a range of symptoms that include diarrhea, abdominal discomfort, and weight loss. In addition to these typical symptoms, celiac disease can cause a wide range of systemic symptoms, including osteopenia, abnormal liver function tests, anemia, neurologic symptoms, and general malaise and fatigue.1 This study suggests that celiac testing be considered in children presenting with rheumatologic symptoms, including myalgia, arthralgia, and rash.
1. Presutti RJ, Cangemi JR, Cassidy HD, et al. Celiac disease. Am Fam Physician. 2007;76(12):1795-1802, 1809-1810.
Continue for educating parents about antibiotic use >>
EDUCATING PARENTS ABOUT ANTIBIOTIC USE
Vaz LE, Kleinman KP, Lakoma MD, et al. Prevalence of parental misconceptions about antibiotic use. Pediatrics. 2015;136:221-231.
Misperceptions about antibiotic use persist and continue to be more prevalent among parents of Medicaid-insured children, according to a study of 1,500 Massachusetts parents.
Investigators examined antibiotic-related knowledge and attitudes among both Medicaid-insured and private-insured parents and found
• Fewer Medicaid parents answered questions correctly, except for one regarding bronchitis.
• Medicaid patients were more likely to request unnecessary antibiotics.
• More parents in 2013 understood that green nasal discharge did not require antibiotics than in 2000.
• Medicaid-insured parents were younger, less likely to be white, and had less education than those commercially insured.
COMMENTARY
Decreasing the unnecessary use of antibiotics for viral infections is an important component of decreasing the development of antibiotic-resistant organisms. An important driver of clinician use of antibiotics is patients’ expectations for antibiotics. This study shows that much work remains to be done in changing patient expectations, which is not surprising to any practicing clinician. This study also suggests that the expectation for antibiotics is greater among those individuals with Medicaid insurance, which suggests that an opportunity exists for Medicaid insurance plans to do targeted patient education on this issue—which should improve patient outcomes, decrease cost from use of unneeded antibiotics, and decrease the development of antibiotic-resistant organisms.
Continue for newborn pulmonary hypertension and maternal antidepressant use >>
NEWBORN PULMONARY HYPERTENSION AND MATERNAL ANTIDEPRESSANT USE
Huybrechts KF, Bateman BT, Palmsten K, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA. 2015;313(21):2142-2151.
Taking antidepressants during late pregnancy may increase the risk for persistent pulmonary hypertension of the newborn (PPHN), according to a nested cohort study of more than 3.7 million pregnant women in the 2000-2010 Medicaid Analytic eXtract.
Investigators compared offspring of mothers who used selective serotonin reuptake inhibitors (SSRIs) or non-SSRI monotherapy in the last 90 days of pregnancy to those who did not and found 3.4% of women filled at least one prescription for antidepressants late in pregnancy, primarily SSRIs.
Rates and odds ratios (ORs) of PPHN stratified by use and type of antidepressant were as follows:
The study authors note the absolute risk is small, and the increased risk is more modest than previous studies found.
COMMENTARY
Depression affects more than 12% of pregnancies and has important consequences, including increased risk for suicide, preterm birth, poor fetal growth, and impaired fetal and infant development.1 PPHN is a serious condition that can require intubation and can be fatal in 10% to 20% of cases. This study adds to the conflicting data suggesting that SSRI use in pregnancy can lead to an increase in this rare condition. The decision to use an antidepressant during pregnancy is a difficult one, because depression has serious consequences but so, potentially, does treatment. The treatment of depression during pregnancy requires thoughtful, informed discussion between patient and provider.
1. Stewart DE. Clinical practice. Depression during pregnancy. N Engl J Med. 2011;365(17):1605-1611. doi:10.1056/NEJMcp1102730.
Continue for new infant vaccine treats 6 diseases >>
NEW INFANT VACCINE TREATS 6 DISEASES
Marshall GS, Adams GL, Leonardi ML, et al. Immunogenicity, safety, and tolerability of a hexavalent vaccine in infants. Pediatrics. 2015:136(2):323-332.
The safety and immunogenicity of DTaP5-IPV-Hib-HepB fully liquid investigational hexavalent vaccine are comparable with the analogous licensed component vaccines and provide a new combination vaccine option aligned with the recommended US infant immunization schedule, according to a phase III study of 1,465 participants. Overall, 981 healthy infants were vaccinated in group 1 with the hexavalent vaccine and 484 in group 2 with the analogous licensed component vaccines.
The study found
• Immune responses in group 1 to all antigens contained in the vaccine one month after dose 3 were essentially noninferior to those in group 2.
• Adverse event rates after any dose were similar in both groups.
COMMENTARY
Vaccine administration has led to many diseases, including Haemophilus influenzae type b, polio, and measles, becoming quite rare. As we have recently seen with the resurgence of measles, continued vigilance and high immunization rates are important in ensuring that these diseases remain rare. The development of a hexavalent vaccine is another step in making immunization easier for clinicians to administer and for patients to accept.
Continue for treating infants with bronchiolitis >>
TREATING INFANTS WITH BRONCHIOLITIS
Silver AH, Esteban-Cruciani N, Azzarone G, et al. 3% hypertonic saline versus normal saline in inpatient bronchiolitis: a randomized controlled trial. Pediatrics. 2015;136:1036-1043.
Infants hospitalized with bronchiolitis saw no difference in length of stay or seven-day readmission rates when treated with nebulized 3% hypertonic saline (HS) compared with nebulized normal saline (NS), according to a randomized, controlled study of 227 infants who were younger than 12 months when admitted. Patients received either 4 mL nebulized 3% HS (113 infants) or 4 mL 0.9% NS (114 infants) every four hours from enrollment until hospital discharge. Researchers found
• Median length of stay of HS and NS groups was 2.1 days vs 2.1 days, respectively.
• Seven-day readmission rates for HS and NS groups were 4.3% vs 3.1%, respectively.
• Clinical worsening events were similar between groups.
COMMENTARY
Bronchiolitis, the most common lower respiratory tract infection in infants, is usually due to a viral infection, most often respiratory syncytial virus, and can cause disease that ranges in severity from mild to life-threatening. Infants with bronchiolitis typically present with rhinitis, tachypnea, wheezing, and cough, and occasionally crackles and use of accessory muscles. While many medications are used, supportive care and monitoring are the mainstays of therapy. Maintaining pulse above 90% is important, using supplemental oxygen when needed to achieve this. Alpha-adrenergic and beta-adrenergic bronchodilators are often used; though the evidence suggests that they are not usually helpful, they can be tried and continued if they appear to help. Systemic steroids are often used, but they too lack evidence of efficacy.1 This study shows that hypertonic saline can now join the list of interventions used with evidence of a lack of efficacy.
1. Diagnosis and management of bronchiolitis. Subcommittee on diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774-1793. doi:10.1542/peds.2006-2223.
Continue for azithromycin and preschool children >>
AZITHROMYCIN AND PRESCHOOL CHILDREN: CAN ANTIBIOTICS LESSEN ONSET OF SEVERE LRTIs?
Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314(19):2034-2044.
The use of azithromycin early during an apparent respiratory tract illness (RTI) reduced the likelihood of severe lower RTI (LRTI) among young children with a history of recurrent severe LRTI, compared with placebo, according to a study of 607 children ages 12 to 71 months. Participants were randomized in a 1:1 ratio to receive either azithromycin (12 mg/kg/d for 5 d) or matching placebo at the start of an RTI. Researchers found
• A total of 937 treated RTIs were experienced by 443 children, including 92 severe LRTIs (azithromycin group, 35; placebo group, 57).
• Azithromycin significantly reduced the risk for progression to severe LRTI relative to placebo (HR, 0.64).
• Induction of azithromycin-resistant organisms and adverse events were infrequent.
COMMENTARY
Recurrent episodes of severe wheezing with RTI are an important and common occurrence, affecting up to 15% to 20% of children prior to age 6.1 LRTI was defined in this study as RTI that required the use of additional rescue medication. The current approach to RTI is to try to minimize the use of antibiotics unless an infection is clearly bacterial in origin, and to treat severe LRTI when it occurs. The results of this trial suggest that in children at high risk for severe LRTI, identified by their episodes of recurrent wheezing, early treatment of RTI with azithromycin may decrease the development of severe LRTI by more than 35%—an important result. It is important to recognize that this study does not suggest treating all RTIs with antibiotics, but rather that the use of a macrolide antibiotic may be considered, perhaps, in the select group of children similar to those studied, with a history of recurrent wheezing with previous RTIs.
1. Ly NP, Gold DR, Weiss ST, Celedón JC. Recurrent wheeze in early childhood and asthma among children at risk for atopy. Pediatrics. 2006;117(6):e1132-e1138.
TALKING OUT CHILDHOOD OBESITY
Resnicow K, McMaster F, Bocian A, et al. Motivational interviewing and dietary counseling for obesity in primary care: an RCT. Pediatrics. 2015;135(4):649-657.
Counseling parents of overweight children using motivational interviewing from both health care providers and registered dietitians can significantly improve BMI, according to a study of 42 practices in the Pediatric Research in Office Settings Network of the American Academy of Pediatrics.
Researchers randomly assigned parents of overweight children, ages 2 through 8, to one of three groups: (1) usual care, (2) four provider-delivered motivational interviewing sessions over two years, or (3) four provider-delivered motivational interviewing sessions plus six sessions with a registered dietitian over two years. At study end, BMI percentile and change in BMI for the different groups were as follows:
COMMENTARY
The results of this study are exciting. Motivational interviewing is a technique in which the practitioner asks questions of a patient and allows the patient to discover his/her own conclusions about the topic. By so doing, the patient is more engaged in the discussion and is less resistant to input. This technique, with excellent evidence of effectiveness in the area of drug and alcohol abuse, has been shown to facilitate effective behavioral change in many areas and is recommended by the American Heart Association for behavioral change in adults.1,2 This is an exciting paper demonstrating evidence-based efficacy in addressing childhood obesity—a critical health issue—and is worth trying in the office.
1. Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55(513):305-312.
2. Spring B, Ockene JK, Gidding SS, et al; American Heart Association Behavior Change Committee of the Council on Epidemiology and Prevention, Council on Lifestyle and Cardiometabolic Health, Council for High Blood Pressure Research, and Council on Cardiovascular and Stroke Nursing. Better population health through behavior change in adults: a call to action. Circulation. 2013;128(19):2169-2176. doi: 10.1161/01.cir.0000435173.25936.e1.
Continue for long-acting reversible contraception among teens >>
LONG-ACTING REVERSIBLE CONTRACEPTION AMONG TEENS
Romero L, Pazol K, Warner L, et al. Vital signs: trends in use of long-acting reversible contraception among teens aged 15-19 years seeking contraceptive services – United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2015;64(13):363-369.
Efforts to improve teen access to long-acting reversible contraception (LARC) have increased use of these methods, according to a CDC review of services provided at Title X National Family Planning Program centers. The report found
• LARC rates among teen patients increased from 0.4% in 2005 to 7.2% in 2013.
• In 2013, 2.8% of those seeking contraception used IUDs and 4.3% used implants.
• Among Title X patients, 7.6% of 18- and 19-year-olds used LARC, compared with 6.5% of 15- to 17-year-olds.
• Rates of LARC were lowest in Mississippi (0.7%) and highest in Colorado (25.8%).
COMMENTARY
LARCs, which include IUD and implantable hormonal contraceptive devices, require no effort for adherence on the part of the user; once in place, they are effective without further action. Current CDC guidelines on contraceptive use clearly recommend LARC for teenagers based on the efficacy and safety.1 LARCs are favored for teenagers because poor compliance has yielded suboptimal effectiveness of oral contraceptives and condoms in teenagers, who often forget to take their birth control pills or don’t use condoms when they should. Many clinicians have been slow to recommend LARCs in teenagers based on safety concerns related to adverse experience with IUDs 20 to 30 years ago. According to CDC guidelines, IUDs and implantable contraceptive devices now have robust safety data, and this article shows that they are being increasingly made available to teenagers who need them.
1. Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. US Selected Practice Recommendations for Contraceptive Use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep. 2013;62(RR-05):1-60.
Continue to testing for celiac in pediatric rheumatology patients >>
TESTING FOR CELIAC IN PEDIATRIC RHEUMATOLOGY PATIENTS
Sherman Y, Karanicolas R, DiMarco B, et al. Unrecognized celiac disease in children presenting for rheumatology evaluation. Pediatrics. 2015; [Epub ahead of print].
Children presenting for rheumatology evaluation should be screened for celiac disease, according to a review of 2,125 pediatric patients who were screened for celiac as part of the standard initial serologic evaluation.
Researchers identified 36 new cases of celiac disease (2.0% prevalence rate). The most common presenting complaints among these patients were myalgia, arthralgia, and rash. Less frequent complaints included gastrointestinal complaints of abdominal pain, nausea, and diarrhea.
After initiating a gluten-free diet, all of the patients with celiac disease reported improvement or complete resolution of musculoskeletal symptoms.
COMMENTARY
Celiac disease has a prevalence of 0.5% to 1% in the US and can present with a range of symptoms that include diarrhea, abdominal discomfort, and weight loss. In addition to these typical symptoms, celiac disease can cause a wide range of systemic symptoms, including osteopenia, abnormal liver function tests, anemia, neurologic symptoms, and general malaise and fatigue.1 This study suggests that celiac testing be considered in children presenting with rheumatologic symptoms, including myalgia, arthralgia, and rash.
1. Presutti RJ, Cangemi JR, Cassidy HD, et al. Celiac disease. Am Fam Physician. 2007;76(12):1795-1802, 1809-1810.
Continue for educating parents about antibiotic use >>
EDUCATING PARENTS ABOUT ANTIBIOTIC USE
Vaz LE, Kleinman KP, Lakoma MD, et al. Prevalence of parental misconceptions about antibiotic use. Pediatrics. 2015;136:221-231.
Misperceptions about antibiotic use persist and continue to be more prevalent among parents of Medicaid-insured children, according to a study of 1,500 Massachusetts parents.
Investigators examined antibiotic-related knowledge and attitudes among both Medicaid-insured and private-insured parents and found
• Fewer Medicaid parents answered questions correctly, except for one regarding bronchitis.
• Medicaid patients were more likely to request unnecessary antibiotics.
• More parents in 2013 understood that green nasal discharge did not require antibiotics than in 2000.
• Medicaid-insured parents were younger, less likely to be white, and had less education than those commercially insured.
COMMENTARY
Decreasing the unnecessary use of antibiotics for viral infections is an important component of decreasing the development of antibiotic-resistant organisms. An important driver of clinician use of antibiotics is patients’ expectations for antibiotics. This study shows that much work remains to be done in changing patient expectations, which is not surprising to any practicing clinician. This study also suggests that the expectation for antibiotics is greater among those individuals with Medicaid insurance, which suggests that an opportunity exists for Medicaid insurance plans to do targeted patient education on this issue—which should improve patient outcomes, decrease cost from use of unneeded antibiotics, and decrease the development of antibiotic-resistant organisms.
Continue for newborn pulmonary hypertension and maternal antidepressant use >>
NEWBORN PULMONARY HYPERTENSION AND MATERNAL ANTIDEPRESSANT USE
Huybrechts KF, Bateman BT, Palmsten K, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA. 2015;313(21):2142-2151.
Taking antidepressants during late pregnancy may increase the risk for persistent pulmonary hypertension of the newborn (PPHN), according to a nested cohort study of more than 3.7 million pregnant women in the 2000-2010 Medicaid Analytic eXtract.
Investigators compared offspring of mothers who used selective serotonin reuptake inhibitors (SSRIs) or non-SSRI monotherapy in the last 90 days of pregnancy to those who did not and found 3.4% of women filled at least one prescription for antidepressants late in pregnancy, primarily SSRIs.
Rates and odds ratios (ORs) of PPHN stratified by use and type of antidepressant were as follows:
The study authors note the absolute risk is small, and the increased risk is more modest than previous studies found.
COMMENTARY
Depression affects more than 12% of pregnancies and has important consequences, including increased risk for suicide, preterm birth, poor fetal growth, and impaired fetal and infant development.1 PPHN is a serious condition that can require intubation and can be fatal in 10% to 20% of cases. This study adds to the conflicting data suggesting that SSRI use in pregnancy can lead to an increase in this rare condition. The decision to use an antidepressant during pregnancy is a difficult one, because depression has serious consequences but so, potentially, does treatment. The treatment of depression during pregnancy requires thoughtful, informed discussion between patient and provider.
1. Stewart DE. Clinical practice. Depression during pregnancy. N Engl J Med. 2011;365(17):1605-1611. doi:10.1056/NEJMcp1102730.
Continue for new infant vaccine treats 6 diseases >>
NEW INFANT VACCINE TREATS 6 DISEASES
Marshall GS, Adams GL, Leonardi ML, et al. Immunogenicity, safety, and tolerability of a hexavalent vaccine in infants. Pediatrics. 2015:136(2):323-332.
The safety and immunogenicity of DTaP5-IPV-Hib-HepB fully liquid investigational hexavalent vaccine are comparable with the analogous licensed component vaccines and provide a new combination vaccine option aligned with the recommended US infant immunization schedule, according to a phase III study of 1,465 participants. Overall, 981 healthy infants were vaccinated in group 1 with the hexavalent vaccine and 484 in group 2 with the analogous licensed component vaccines.
The study found
• Immune responses in group 1 to all antigens contained in the vaccine one month after dose 3 were essentially noninferior to those in group 2.
• Adverse event rates after any dose were similar in both groups.
COMMENTARY
Vaccine administration has led to many diseases, including Haemophilus influenzae type b, polio, and measles, becoming quite rare. As we have recently seen with the resurgence of measles, continued vigilance and high immunization rates are important in ensuring that these diseases remain rare. The development of a hexavalent vaccine is another step in making immunization easier for clinicians to administer and for patients to accept.
Continue for treating infants with bronchiolitis >>
TREATING INFANTS WITH BRONCHIOLITIS
Silver AH, Esteban-Cruciani N, Azzarone G, et al. 3% hypertonic saline versus normal saline in inpatient bronchiolitis: a randomized controlled trial. Pediatrics. 2015;136:1036-1043.
Infants hospitalized with bronchiolitis saw no difference in length of stay or seven-day readmission rates when treated with nebulized 3% hypertonic saline (HS) compared with nebulized normal saline (NS), according to a randomized, controlled study of 227 infants who were younger than 12 months when admitted. Patients received either 4 mL nebulized 3% HS (113 infants) or 4 mL 0.9% NS (114 infants) every four hours from enrollment until hospital discharge. Researchers found
• Median length of stay of HS and NS groups was 2.1 days vs 2.1 days, respectively.
• Seven-day readmission rates for HS and NS groups were 4.3% vs 3.1%, respectively.
• Clinical worsening events were similar between groups.
COMMENTARY
Bronchiolitis, the most common lower respiratory tract infection in infants, is usually due to a viral infection, most often respiratory syncytial virus, and can cause disease that ranges in severity from mild to life-threatening. Infants with bronchiolitis typically present with rhinitis, tachypnea, wheezing, and cough, and occasionally crackles and use of accessory muscles. While many medications are used, supportive care and monitoring are the mainstays of therapy. Maintaining pulse above 90% is important, using supplemental oxygen when needed to achieve this. Alpha-adrenergic and beta-adrenergic bronchodilators are often used; though the evidence suggests that they are not usually helpful, they can be tried and continued if they appear to help. Systemic steroids are often used, but they too lack evidence of efficacy.1 This study shows that hypertonic saline can now join the list of interventions used with evidence of a lack of efficacy.
1. Diagnosis and management of bronchiolitis. Subcommittee on diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774-1793. doi:10.1542/peds.2006-2223.
Continue for azithromycin and preschool children >>
AZITHROMYCIN AND PRESCHOOL CHILDREN: CAN ANTIBIOTICS LESSEN ONSET OF SEVERE LRTIs?
Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314(19):2034-2044.
The use of azithromycin early during an apparent respiratory tract illness (RTI) reduced the likelihood of severe lower RTI (LRTI) among young children with a history of recurrent severe LRTI, compared with placebo, according to a study of 607 children ages 12 to 71 months. Participants were randomized in a 1:1 ratio to receive either azithromycin (12 mg/kg/d for 5 d) or matching placebo at the start of an RTI. Researchers found
• A total of 937 treated RTIs were experienced by 443 children, including 92 severe LRTIs (azithromycin group, 35; placebo group, 57).
• Azithromycin significantly reduced the risk for progression to severe LRTI relative to placebo (HR, 0.64).
• Induction of azithromycin-resistant organisms and adverse events were infrequent.
COMMENTARY
Recurrent episodes of severe wheezing with RTI are an important and common occurrence, affecting up to 15% to 20% of children prior to age 6.1 LRTI was defined in this study as RTI that required the use of additional rescue medication. The current approach to RTI is to try to minimize the use of antibiotics unless an infection is clearly bacterial in origin, and to treat severe LRTI when it occurs. The results of this trial suggest that in children at high risk for severe LRTI, identified by their episodes of recurrent wheezing, early treatment of RTI with azithromycin may decrease the development of severe LRTI by more than 35%—an important result. It is important to recognize that this study does not suggest treating all RTIs with antibiotics, but rather that the use of a macrolide antibiotic may be considered, perhaps, in the select group of children similar to those studied, with a history of recurrent wheezing with previous RTIs.
1. Ly NP, Gold DR, Weiss ST, Celedón JC. Recurrent wheeze in early childhood and asthma among children at risk for atopy. Pediatrics. 2006;117(6):e1132-e1138.
European societies issue aspergillosis diagnosis, management guidelines
European respiratory disease and infectious disease specialists have banded together to issue new clinical guidelines on the diagnosis and management of an uncommon but serious problem: chronic pulmonary aspergillosis (CPA).
Pulmonary infections with Aspergillus species, although uncommon, are a complicating factor in several lung diseases, especially tuberculosis, and aspergillosis is a serious, often fatal opportunistic infection in transplant recipients who are on chronic immunosuppression or patients who are immunocompromised from disease or cytotoxic chemotherapy.
Approximately 240,000 people in Europe and 3 million people worldwide have chronic pulmonary aspergillosis (CPA). The Centers for Disease Control and Prevention notes that because aspergillosis is not classified as a reportable disease, data on the actual incidence of infections in the United States are hard to come by.
“You don’t see this every day, whether you’re an infectious disease specialist or pulmonologist, so you really can’t rely on your experience to guide you in managing these cases, which is why guidelines such as this can be very helpful,” commented Dr. Norman Edelman, a pulmonologist and senior consultant for scientific affairs for the American Lung Association.
The guidelines, issued by the European Society for Clinical Microbiology and Infectious Diseases in cooperation with the European Confederation of Medical Mycology and the European Respiratory Society, are an attempt to provide clinicians with the best possible evidence-based guidance on managing patients with aspergillosis, primarily those with CPA (Eur Respir J. 2015. doi: 10.1183/13993003.00583-2015).
Dr. Edelman noted that the most frequent presentation he sees – and that very infrequently – is allergic bronchopulmonary aspergillosis in patients with asthma.
The most recent U.S. guidelines, issued under the aegis of the Infectious Diseases Society of America (IDSA) in 2000 and revised in 2008 (CID 2008;46:327-360), differ from the European recommendations in their level of detail, explained Prof. David W. Denning, professor of infectious diseases in global health at the University of Manchester (England) and lead author of the European guidelines.
“The IDSA guidelines assume that you know how to make the diagnosis, but actually for chronic pulmonary aspergillosis that’s not so easy with some patients,” he said in an interview.
“The European ones go into in great detail the diagnosis, the radiology, whether this test is better than that test, how they all add up, and all that sort of stuff,” he said,
The European guidelines also make recommendations for duration of therapy and comment on the use of steroids and immunotherapy with interferon-gamma, Dr. Denning noted.
Diagnostic criteria
The European guidelines categorize Aspergillus infections according to differences in clinical management:
• Simple aspergilloma. A single pulmonary cavity containing a fungal ball, supported by serologic or microbiologic evidence of infections with Aspergillus species in patients who are not immunocompromised and are asymptomatic or have only minor symptoms and no radiographic evidence of progression for at least 3 months.
• Chronic cavitary pulmonary aspergillosis (CCPA). The presence of one or more pulmonary cavities that may contain one or more aspergillomas or irregular intraluminal material, evidence of Aspergillus species, significant pulmonary/systemic symptoms, and overt progression on radiography over 3 or more months of observations.
• Chronic fibrosing pulmonary aspergillosis (CFPA). Severe, fibrotic destruction of at least two lung lobes as a complication of CCPA, causing a major loss of lung function. The guidelines note that destruction of a single lobe is designated as CCPA of that lobe.
• Aspergillus nodules. This unusual presentation is marked by the presence of one or more nodules that may or may not cavitate. The nodules may resemble tuberculoma, carcinoma of the lung, or coccidioidomycosis; histology is required to make an accurate diagnosis.
• Subacute invasive aspergillosis (SAIA). This can occur over the course of 1-3 months in patients who are mildly immunocompromised. Radiologic features can vary, and may include cavitation, the presence of nodules, and progressive consolidation with the appearance of abscess formation. Fungal hyphae (filaments) can be seen in biopsied lung tissues, and there may be evidence of Aspergillus galactomannan antigen in respiratory fluids or blood.
Treatment
The guidelines note that most of the evidence for managing CPA are based on cohort studies and case reports rather than randomized clinical trials, and that there have been no head-to-head trials comparing oral triazole agents.
For treatment of CPA, the European guidelines recommend:
• Itraconazole 200 mg twice daily, with therapeutic drug monitoring and dose adjustment as necessary (Grade A [strong] recommendation).
• Voriconazole 150-200 mg twice daily, with monitoring and dose adjustment. The guidelines recommend lower doses for patients older than 70 years, those with low body weight, significant liver disease, and/or those of Northeast Asian descent, who may be genetically inclined to slow drug metabolism (Grade A).
• Posaconazole liquid 400 mg twice daily, or tablets 300 mg once daily (Grade B [moderate] recommendation].
In general, the recommended duration of therapy for control of infection in patients with CPA or curative intent for patients with SAIA or chronic necrotizing pulmonary aspergillosis is 6 months or more, depending on patient status and drug tolerance.
For patients with CPA with progressive disease, those whom therapy has failed, or those who are intolerant of or have disease resistant to triazoles, intravenous therapy with micafungin, 150 mg day (Grade B); amphotericin B deoxycholate, 0.7-1.0 mg/kg per day (Grade C [marginal] recommendation); liposomal amphotericin B, 3 mg/kg per day (Grade B); or caspofungin, 50-70 mg/day (Grade C) are recommended.
The guidelines also recommend surgical excision of simple aspergilloma, preferably by a video-assisted thoracic surgery technique, if technically feasible.
“In my own experience, we resort to surgery very infrequently,” Dr. Edelman said.
He noted that it would be helpful if the guidelines had also allergic bronchopulmonary aspergillosis as a separate entity.
Ideal not always achievable
Prof. Denning points out that the optimum therapies and practices described in the guidelines can’t always be implemented. Worldwide, he said, antifungal therapy is not widely available, with the exception of fluconazole, which has no activity against Aspergillus, and is inferior to itraconazole and other extended azoles for other fungal diseases such as histoplasmosis, blastomycosis, and paracoccidioidomycosis.
The price of antifungal therapies can also be a barrier to effective treatment in many parts of the world.
“If you’re having to pay for your medicines and you’re living on $5 or $10 a day in Kenya, say, you can’t afford to buy them. So even if the drugs are physically there, it may not be really affordable for a course of therapy for these patients, and there’s some advocacy to be done around that for the whole world,” he said.
The guidelines were funded primarily by grants from ESCMID and ERS with additional support from ECMM. Authors’ travel expenses were funded jointly by ESCMID and ERS. Dr. Denning has received grant support and founder shares in F2G, and has received grants from the Fungal Research Trust, Wellcome Trust, Moulton Trust, Medical Research Council, Chronic Granulomatous Disease Research Trust, National Institute of Allergy and Infectious Diseases, National Institute of Health Research and the European Union, and AstraZeneca. Dr. Edelman reported no relevant disclosures.
European respiratory disease and infectious disease specialists have banded together to issue new clinical guidelines on the diagnosis and management of an uncommon but serious problem: chronic pulmonary aspergillosis (CPA).
Pulmonary infections with Aspergillus species, although uncommon, are a complicating factor in several lung diseases, especially tuberculosis, and aspergillosis is a serious, often fatal opportunistic infection in transplant recipients who are on chronic immunosuppression or patients who are immunocompromised from disease or cytotoxic chemotherapy.
Approximately 240,000 people in Europe and 3 million people worldwide have chronic pulmonary aspergillosis (CPA). The Centers for Disease Control and Prevention notes that because aspergillosis is not classified as a reportable disease, data on the actual incidence of infections in the United States are hard to come by.
“You don’t see this every day, whether you’re an infectious disease specialist or pulmonologist, so you really can’t rely on your experience to guide you in managing these cases, which is why guidelines such as this can be very helpful,” commented Dr. Norman Edelman, a pulmonologist and senior consultant for scientific affairs for the American Lung Association.
The guidelines, issued by the European Society for Clinical Microbiology and Infectious Diseases in cooperation with the European Confederation of Medical Mycology and the European Respiratory Society, are an attempt to provide clinicians with the best possible evidence-based guidance on managing patients with aspergillosis, primarily those with CPA (Eur Respir J. 2015. doi: 10.1183/13993003.00583-2015).
Dr. Edelman noted that the most frequent presentation he sees – and that very infrequently – is allergic bronchopulmonary aspergillosis in patients with asthma.
The most recent U.S. guidelines, issued under the aegis of the Infectious Diseases Society of America (IDSA) in 2000 and revised in 2008 (CID 2008;46:327-360), differ from the European recommendations in their level of detail, explained Prof. David W. Denning, professor of infectious diseases in global health at the University of Manchester (England) and lead author of the European guidelines.
“The IDSA guidelines assume that you know how to make the diagnosis, but actually for chronic pulmonary aspergillosis that’s not so easy with some patients,” he said in an interview.
“The European ones go into in great detail the diagnosis, the radiology, whether this test is better than that test, how they all add up, and all that sort of stuff,” he said,
The European guidelines also make recommendations for duration of therapy and comment on the use of steroids and immunotherapy with interferon-gamma, Dr. Denning noted.
Diagnostic criteria
The European guidelines categorize Aspergillus infections according to differences in clinical management:
• Simple aspergilloma. A single pulmonary cavity containing a fungal ball, supported by serologic or microbiologic evidence of infections with Aspergillus species in patients who are not immunocompromised and are asymptomatic or have only minor symptoms and no radiographic evidence of progression for at least 3 months.
• Chronic cavitary pulmonary aspergillosis (CCPA). The presence of one or more pulmonary cavities that may contain one or more aspergillomas or irregular intraluminal material, evidence of Aspergillus species, significant pulmonary/systemic symptoms, and overt progression on radiography over 3 or more months of observations.
• Chronic fibrosing pulmonary aspergillosis (CFPA). Severe, fibrotic destruction of at least two lung lobes as a complication of CCPA, causing a major loss of lung function. The guidelines note that destruction of a single lobe is designated as CCPA of that lobe.
• Aspergillus nodules. This unusual presentation is marked by the presence of one or more nodules that may or may not cavitate. The nodules may resemble tuberculoma, carcinoma of the lung, or coccidioidomycosis; histology is required to make an accurate diagnosis.
• Subacute invasive aspergillosis (SAIA). This can occur over the course of 1-3 months in patients who are mildly immunocompromised. Radiologic features can vary, and may include cavitation, the presence of nodules, and progressive consolidation with the appearance of abscess formation. Fungal hyphae (filaments) can be seen in biopsied lung tissues, and there may be evidence of Aspergillus galactomannan antigen in respiratory fluids or blood.
Treatment
The guidelines note that most of the evidence for managing CPA are based on cohort studies and case reports rather than randomized clinical trials, and that there have been no head-to-head trials comparing oral triazole agents.
For treatment of CPA, the European guidelines recommend:
• Itraconazole 200 mg twice daily, with therapeutic drug monitoring and dose adjustment as necessary (Grade A [strong] recommendation).
• Voriconazole 150-200 mg twice daily, with monitoring and dose adjustment. The guidelines recommend lower doses for patients older than 70 years, those with low body weight, significant liver disease, and/or those of Northeast Asian descent, who may be genetically inclined to slow drug metabolism (Grade A).
• Posaconazole liquid 400 mg twice daily, or tablets 300 mg once daily (Grade B [moderate] recommendation].
In general, the recommended duration of therapy for control of infection in patients with CPA or curative intent for patients with SAIA or chronic necrotizing pulmonary aspergillosis is 6 months or more, depending on patient status and drug tolerance.
For patients with CPA with progressive disease, those whom therapy has failed, or those who are intolerant of or have disease resistant to triazoles, intravenous therapy with micafungin, 150 mg day (Grade B); amphotericin B deoxycholate, 0.7-1.0 mg/kg per day (Grade C [marginal] recommendation); liposomal amphotericin B, 3 mg/kg per day (Grade B); or caspofungin, 50-70 mg/day (Grade C) are recommended.
The guidelines also recommend surgical excision of simple aspergilloma, preferably by a video-assisted thoracic surgery technique, if technically feasible.
“In my own experience, we resort to surgery very infrequently,” Dr. Edelman said.
He noted that it would be helpful if the guidelines had also allergic bronchopulmonary aspergillosis as a separate entity.
Ideal not always achievable
Prof. Denning points out that the optimum therapies and practices described in the guidelines can’t always be implemented. Worldwide, he said, antifungal therapy is not widely available, with the exception of fluconazole, which has no activity against Aspergillus, and is inferior to itraconazole and other extended azoles for other fungal diseases such as histoplasmosis, blastomycosis, and paracoccidioidomycosis.
The price of antifungal therapies can also be a barrier to effective treatment in many parts of the world.
“If you’re having to pay for your medicines and you’re living on $5 or $10 a day in Kenya, say, you can’t afford to buy them. So even if the drugs are physically there, it may not be really affordable for a course of therapy for these patients, and there’s some advocacy to be done around that for the whole world,” he said.
The guidelines were funded primarily by grants from ESCMID and ERS with additional support from ECMM. Authors’ travel expenses were funded jointly by ESCMID and ERS. Dr. Denning has received grant support and founder shares in F2G, and has received grants from the Fungal Research Trust, Wellcome Trust, Moulton Trust, Medical Research Council, Chronic Granulomatous Disease Research Trust, National Institute of Allergy and Infectious Diseases, National Institute of Health Research and the European Union, and AstraZeneca. Dr. Edelman reported no relevant disclosures.
European respiratory disease and infectious disease specialists have banded together to issue new clinical guidelines on the diagnosis and management of an uncommon but serious problem: chronic pulmonary aspergillosis (CPA).
Pulmonary infections with Aspergillus species, although uncommon, are a complicating factor in several lung diseases, especially tuberculosis, and aspergillosis is a serious, often fatal opportunistic infection in transplant recipients who are on chronic immunosuppression or patients who are immunocompromised from disease or cytotoxic chemotherapy.
Approximately 240,000 people in Europe and 3 million people worldwide have chronic pulmonary aspergillosis (CPA). The Centers for Disease Control and Prevention notes that because aspergillosis is not classified as a reportable disease, data on the actual incidence of infections in the United States are hard to come by.
“You don’t see this every day, whether you’re an infectious disease specialist or pulmonologist, so you really can’t rely on your experience to guide you in managing these cases, which is why guidelines such as this can be very helpful,” commented Dr. Norman Edelman, a pulmonologist and senior consultant for scientific affairs for the American Lung Association.
The guidelines, issued by the European Society for Clinical Microbiology and Infectious Diseases in cooperation with the European Confederation of Medical Mycology and the European Respiratory Society, are an attempt to provide clinicians with the best possible evidence-based guidance on managing patients with aspergillosis, primarily those with CPA (Eur Respir J. 2015. doi: 10.1183/13993003.00583-2015).
Dr. Edelman noted that the most frequent presentation he sees – and that very infrequently – is allergic bronchopulmonary aspergillosis in patients with asthma.
The most recent U.S. guidelines, issued under the aegis of the Infectious Diseases Society of America (IDSA) in 2000 and revised in 2008 (CID 2008;46:327-360), differ from the European recommendations in their level of detail, explained Prof. David W. Denning, professor of infectious diseases in global health at the University of Manchester (England) and lead author of the European guidelines.
“The IDSA guidelines assume that you know how to make the diagnosis, but actually for chronic pulmonary aspergillosis that’s not so easy with some patients,” he said in an interview.
“The European ones go into in great detail the diagnosis, the radiology, whether this test is better than that test, how they all add up, and all that sort of stuff,” he said,
The European guidelines also make recommendations for duration of therapy and comment on the use of steroids and immunotherapy with interferon-gamma, Dr. Denning noted.
Diagnostic criteria
The European guidelines categorize Aspergillus infections according to differences in clinical management:
• Simple aspergilloma. A single pulmonary cavity containing a fungal ball, supported by serologic or microbiologic evidence of infections with Aspergillus species in patients who are not immunocompromised and are asymptomatic or have only minor symptoms and no radiographic evidence of progression for at least 3 months.
• Chronic cavitary pulmonary aspergillosis (CCPA). The presence of one or more pulmonary cavities that may contain one or more aspergillomas or irregular intraluminal material, evidence of Aspergillus species, significant pulmonary/systemic symptoms, and overt progression on radiography over 3 or more months of observations.
• Chronic fibrosing pulmonary aspergillosis (CFPA). Severe, fibrotic destruction of at least two lung lobes as a complication of CCPA, causing a major loss of lung function. The guidelines note that destruction of a single lobe is designated as CCPA of that lobe.
• Aspergillus nodules. This unusual presentation is marked by the presence of one or more nodules that may or may not cavitate. The nodules may resemble tuberculoma, carcinoma of the lung, or coccidioidomycosis; histology is required to make an accurate diagnosis.
• Subacute invasive aspergillosis (SAIA). This can occur over the course of 1-3 months in patients who are mildly immunocompromised. Radiologic features can vary, and may include cavitation, the presence of nodules, and progressive consolidation with the appearance of abscess formation. Fungal hyphae (filaments) can be seen in biopsied lung tissues, and there may be evidence of Aspergillus galactomannan antigen in respiratory fluids or blood.
Treatment
The guidelines note that most of the evidence for managing CPA are based on cohort studies and case reports rather than randomized clinical trials, and that there have been no head-to-head trials comparing oral triazole agents.
For treatment of CPA, the European guidelines recommend:
• Itraconazole 200 mg twice daily, with therapeutic drug monitoring and dose adjustment as necessary (Grade A [strong] recommendation).
• Voriconazole 150-200 mg twice daily, with monitoring and dose adjustment. The guidelines recommend lower doses for patients older than 70 years, those with low body weight, significant liver disease, and/or those of Northeast Asian descent, who may be genetically inclined to slow drug metabolism (Grade A).
• Posaconazole liquid 400 mg twice daily, or tablets 300 mg once daily (Grade B [moderate] recommendation].
In general, the recommended duration of therapy for control of infection in patients with CPA or curative intent for patients with SAIA or chronic necrotizing pulmonary aspergillosis is 6 months or more, depending on patient status and drug tolerance.
For patients with CPA with progressive disease, those whom therapy has failed, or those who are intolerant of or have disease resistant to triazoles, intravenous therapy with micafungin, 150 mg day (Grade B); amphotericin B deoxycholate, 0.7-1.0 mg/kg per day (Grade C [marginal] recommendation); liposomal amphotericin B, 3 mg/kg per day (Grade B); or caspofungin, 50-70 mg/day (Grade C) are recommended.
The guidelines also recommend surgical excision of simple aspergilloma, preferably by a video-assisted thoracic surgery technique, if technically feasible.
“In my own experience, we resort to surgery very infrequently,” Dr. Edelman said.
He noted that it would be helpful if the guidelines had also allergic bronchopulmonary aspergillosis as a separate entity.
Ideal not always achievable
Prof. Denning points out that the optimum therapies and practices described in the guidelines can’t always be implemented. Worldwide, he said, antifungal therapy is not widely available, with the exception of fluconazole, which has no activity against Aspergillus, and is inferior to itraconazole and other extended azoles for other fungal diseases such as histoplasmosis, blastomycosis, and paracoccidioidomycosis.
The price of antifungal therapies can also be a barrier to effective treatment in many parts of the world.
“If you’re having to pay for your medicines and you’re living on $5 or $10 a day in Kenya, say, you can’t afford to buy them. So even if the drugs are physically there, it may not be really affordable for a course of therapy for these patients, and there’s some advocacy to be done around that for the whole world,” he said.
The guidelines were funded primarily by grants from ESCMID and ERS with additional support from ECMM. Authors’ travel expenses were funded jointly by ESCMID and ERS. Dr. Denning has received grant support and founder shares in F2G, and has received grants from the Fungal Research Trust, Wellcome Trust, Moulton Trust, Medical Research Council, Chronic Granulomatous Disease Research Trust, National Institute of Allergy and Infectious Diseases, National Institute of Health Research and the European Union, and AstraZeneca. Dr. Edelman reported no relevant disclosures.
FROM JOURNAL OF DRUGS IN DERMATOLOGY
FDA approves selexipag for pulmonary arterial hypertension
Selexipag has been approved to treat pulmonary arterial hypertension, the Food and Drug Administration announced on Dec. 21 in a statement.
“The FDA supports continued efforts to provide new treatment options for rare diseases,” Dr. Ellis Unger, director of the Office of Drug Evaluation I in the FDA’s Center for Drug Evaluation and Research, said in the statement. Selexipag (Uptravi) was granted orphan drug designation, which provides incentives such as tax credits, user fee waivers, and eligibility for exclusivity to assist and encourage the development of drugs for rare diseases.
Selexipag is an oral IP prostacyclin receptor agonist that dilates blood vessels, decreasing elevated pressure in pulmonary vessels.
In a trial of 1,156 patients with PAH, selexipag use for a median duration of 1.4 years was associated with fewer hospitalizations for PAH and a lower risk of disease progression, compared with placebo.
Common side effects observed in those treated with selexipag in the trial include headache, diarrhea, jaw pain, nausea, myalgia, vomiting, pain in an extremity, and flushing.
Uptravi is marketed by San Francisco–based Actelion Pharmaceuticals US.
On Twitter @maryjodales
Selexipag has been approved to treat pulmonary arterial hypertension, the Food and Drug Administration announced on Dec. 21 in a statement.
“The FDA supports continued efforts to provide new treatment options for rare diseases,” Dr. Ellis Unger, director of the Office of Drug Evaluation I in the FDA’s Center for Drug Evaluation and Research, said in the statement. Selexipag (Uptravi) was granted orphan drug designation, which provides incentives such as tax credits, user fee waivers, and eligibility for exclusivity to assist and encourage the development of drugs for rare diseases.
Selexipag is an oral IP prostacyclin receptor agonist that dilates blood vessels, decreasing elevated pressure in pulmonary vessels.
In a trial of 1,156 patients with PAH, selexipag use for a median duration of 1.4 years was associated with fewer hospitalizations for PAH and a lower risk of disease progression, compared with placebo.
Common side effects observed in those treated with selexipag in the trial include headache, diarrhea, jaw pain, nausea, myalgia, vomiting, pain in an extremity, and flushing.
Uptravi is marketed by San Francisco–based Actelion Pharmaceuticals US.
On Twitter @maryjodales
Selexipag has been approved to treat pulmonary arterial hypertension, the Food and Drug Administration announced on Dec. 21 in a statement.
“The FDA supports continued efforts to provide new treatment options for rare diseases,” Dr. Ellis Unger, director of the Office of Drug Evaluation I in the FDA’s Center for Drug Evaluation and Research, said in the statement. Selexipag (Uptravi) was granted orphan drug designation, which provides incentives such as tax credits, user fee waivers, and eligibility for exclusivity to assist and encourage the development of drugs for rare diseases.
Selexipag is an oral IP prostacyclin receptor agonist that dilates blood vessels, decreasing elevated pressure in pulmonary vessels.
In a trial of 1,156 patients with PAH, selexipag use for a median duration of 1.4 years was associated with fewer hospitalizations for PAH and a lower risk of disease progression, compared with placebo.
Common side effects observed in those treated with selexipag in the trial include headache, diarrhea, jaw pain, nausea, myalgia, vomiting, pain in an extremity, and flushing.
Uptravi is marketed by San Francisco–based Actelion Pharmaceuticals US.
On Twitter @maryjodales
Men seen as main source of TB transmission
More than half of tuberculosis infections among men, women, and children were seen to derive from contacts with adult men, according to findings from research teams working in the United Kingdom, Zambia, and South Africa.
Understanding TB infection incidence by age and sex of source cases, and where and how often contacts occur, is critical to controlling transmission and directing prevention and treatment efforts. For this study, published online in the American Journal of Epidemiology (Am J Epidemiol. 2015 Dec 8. doi: 10.1093/aje/kwv160), researchers compared TB prevalence data in adults from a recent study in South Africa and Zambia and from an earlier, related study in children with data on daily contact patterns gathered through interviews with more than 3,500 adults 18 and older (53% female).
Close contacts were defined as face-to-face conversations, while casual contacts were defined as sharing indoor space, a potentially important source of TB transmission. In the interviews, adults reported an average of 4.9 close contacts and 10.4 casual contacts daily. They also reported the age groups and sexes with which their contacts occurred.
The authors of the study, led by Dr. Peter J. Dodd of the University of Sheffield (U.K.), said the finding on adult males as the likely drivers of TB transmission in both genders was surprising, because the study also revealed that daily close contacts occurred preferentially among age groups and among members of the same sex.
More than 63% of women’s close contacts and 61% of men’s were among members of the same sex. Adults averaged 0.8 close contacts per day with children under 12, though women had a slightly higher rate of contact than men. Among adults, estimated infections from contact with adult men was 57.3% in South Africa and 65.7% in Zambia. Estimated transmission from contact with men was 50% or higher in all age groups, in both sexes, and in both countries, except in girls 12 and younger and boys 4 and younger in South Africa.
“We noticed in the study that there was preferential mixing by gender,” Dr. Dodd said in an interview. “If there was the same amount of TB in men and women, you’d have expected women to be responsible for the majority of transmission to women and vice versa. But in fact there’s so much more TB in men, that they’re still responsible for the majority of transmission in women.”
Young children’s high proportion of TB infection attributable to contact with men was surprising, they noted, because their contact rates with women were higher. This suggests “that even in this age group, the higher prevalence in males tended to outweigh the higher contact rates between young children and women,” the researchers wrote in their analysis.
Prevalence of TB was markedly higher in men than women (0.9% vs. 0.4% in Zambia, and 3% vs. 2% in South Africa). While the reasons for more TB in men are not well understood, these likely include both biological and social factors, including time before seeking care, and access to care. If men are slower to report or get diagnosed, because of work or other considerations, they will have higher prevalence of infectious TB disease, Dr. Dodd said. Preferential mixing by gender means that men are also more likely to be exposed to infectious TB by other men.
The study also found that incidence of infection was between 1.5 and 6 times higher in adults than was measured in children. “Adults are likely to have higher rates of exposure to TB, because adults on average have more contact with other adults than they do with children – and it’s the adults who tend to have infectious TB disease,” Dr. Dodd said.
Tuberculosis infection incidence based on surveys in children might underestimate true incidence in adults in TB-endemic settings, the researchers cautioned in their analysis.
“For hyperendemic communities in South Africa, which may have an incidence of M. tuberculosis infection in children as high as 4% per year, our findings imply that incidence rates in never-infected adults may be as high as 10% per year, rates which have rarely been seen outside institutional settings.”
Care and control of tuberculosis in males, the researchers concluded, “is critical to protecting men, women, and children from tuberculosis.”
Individual researchers were funded by the Bill and Melinda Gates Foundation, the Medical Research Council (U.K.), and the Wellcome Trust. None of the researchers reported conflicts of interest.
More than half of tuberculosis infections among men, women, and children were seen to derive from contacts with adult men, according to findings from research teams working in the United Kingdom, Zambia, and South Africa.
Understanding TB infection incidence by age and sex of source cases, and where and how often contacts occur, is critical to controlling transmission and directing prevention and treatment efforts. For this study, published online in the American Journal of Epidemiology (Am J Epidemiol. 2015 Dec 8. doi: 10.1093/aje/kwv160), researchers compared TB prevalence data in adults from a recent study in South Africa and Zambia and from an earlier, related study in children with data on daily contact patterns gathered through interviews with more than 3,500 adults 18 and older (53% female).
Close contacts were defined as face-to-face conversations, while casual contacts were defined as sharing indoor space, a potentially important source of TB transmission. In the interviews, adults reported an average of 4.9 close contacts and 10.4 casual contacts daily. They also reported the age groups and sexes with which their contacts occurred.
The authors of the study, led by Dr. Peter J. Dodd of the University of Sheffield (U.K.), said the finding on adult males as the likely drivers of TB transmission in both genders was surprising, because the study also revealed that daily close contacts occurred preferentially among age groups and among members of the same sex.
More than 63% of women’s close contacts and 61% of men’s were among members of the same sex. Adults averaged 0.8 close contacts per day with children under 12, though women had a slightly higher rate of contact than men. Among adults, estimated infections from contact with adult men was 57.3% in South Africa and 65.7% in Zambia. Estimated transmission from contact with men was 50% or higher in all age groups, in both sexes, and in both countries, except in girls 12 and younger and boys 4 and younger in South Africa.
“We noticed in the study that there was preferential mixing by gender,” Dr. Dodd said in an interview. “If there was the same amount of TB in men and women, you’d have expected women to be responsible for the majority of transmission to women and vice versa. But in fact there’s so much more TB in men, that they’re still responsible for the majority of transmission in women.”
Young children’s high proportion of TB infection attributable to contact with men was surprising, they noted, because their contact rates with women were higher. This suggests “that even in this age group, the higher prevalence in males tended to outweigh the higher contact rates between young children and women,” the researchers wrote in their analysis.
Prevalence of TB was markedly higher in men than women (0.9% vs. 0.4% in Zambia, and 3% vs. 2% in South Africa). While the reasons for more TB in men are not well understood, these likely include both biological and social factors, including time before seeking care, and access to care. If men are slower to report or get diagnosed, because of work or other considerations, they will have higher prevalence of infectious TB disease, Dr. Dodd said. Preferential mixing by gender means that men are also more likely to be exposed to infectious TB by other men.
The study also found that incidence of infection was between 1.5 and 6 times higher in adults than was measured in children. “Adults are likely to have higher rates of exposure to TB, because adults on average have more contact with other adults than they do with children – and it’s the adults who tend to have infectious TB disease,” Dr. Dodd said.
Tuberculosis infection incidence based on surveys in children might underestimate true incidence in adults in TB-endemic settings, the researchers cautioned in their analysis.
“For hyperendemic communities in South Africa, which may have an incidence of M. tuberculosis infection in children as high as 4% per year, our findings imply that incidence rates in never-infected adults may be as high as 10% per year, rates which have rarely been seen outside institutional settings.”
Care and control of tuberculosis in males, the researchers concluded, “is critical to protecting men, women, and children from tuberculosis.”
Individual researchers were funded by the Bill and Melinda Gates Foundation, the Medical Research Council (U.K.), and the Wellcome Trust. None of the researchers reported conflicts of interest.
More than half of tuberculosis infections among men, women, and children were seen to derive from contacts with adult men, according to findings from research teams working in the United Kingdom, Zambia, and South Africa.
Understanding TB infection incidence by age and sex of source cases, and where and how often contacts occur, is critical to controlling transmission and directing prevention and treatment efforts. For this study, published online in the American Journal of Epidemiology (Am J Epidemiol. 2015 Dec 8. doi: 10.1093/aje/kwv160), researchers compared TB prevalence data in adults from a recent study in South Africa and Zambia and from an earlier, related study in children with data on daily contact patterns gathered through interviews with more than 3,500 adults 18 and older (53% female).
Close contacts were defined as face-to-face conversations, while casual contacts were defined as sharing indoor space, a potentially important source of TB transmission. In the interviews, adults reported an average of 4.9 close contacts and 10.4 casual contacts daily. They also reported the age groups and sexes with which their contacts occurred.
The authors of the study, led by Dr. Peter J. Dodd of the University of Sheffield (U.K.), said the finding on adult males as the likely drivers of TB transmission in both genders was surprising, because the study also revealed that daily close contacts occurred preferentially among age groups and among members of the same sex.
More than 63% of women’s close contacts and 61% of men’s were among members of the same sex. Adults averaged 0.8 close contacts per day with children under 12, though women had a slightly higher rate of contact than men. Among adults, estimated infections from contact with adult men was 57.3% in South Africa and 65.7% in Zambia. Estimated transmission from contact with men was 50% or higher in all age groups, in both sexes, and in both countries, except in girls 12 and younger and boys 4 and younger in South Africa.
“We noticed in the study that there was preferential mixing by gender,” Dr. Dodd said in an interview. “If there was the same amount of TB in men and women, you’d have expected women to be responsible for the majority of transmission to women and vice versa. But in fact there’s so much more TB in men, that they’re still responsible for the majority of transmission in women.”
Young children’s high proportion of TB infection attributable to contact with men was surprising, they noted, because their contact rates with women were higher. This suggests “that even in this age group, the higher prevalence in males tended to outweigh the higher contact rates between young children and women,” the researchers wrote in their analysis.
Prevalence of TB was markedly higher in men than women (0.9% vs. 0.4% in Zambia, and 3% vs. 2% in South Africa). While the reasons for more TB in men are not well understood, these likely include both biological and social factors, including time before seeking care, and access to care. If men are slower to report or get diagnosed, because of work or other considerations, they will have higher prevalence of infectious TB disease, Dr. Dodd said. Preferential mixing by gender means that men are also more likely to be exposed to infectious TB by other men.
The study also found that incidence of infection was between 1.5 and 6 times higher in adults than was measured in children. “Adults are likely to have higher rates of exposure to TB, because adults on average have more contact with other adults than they do with children – and it’s the adults who tend to have infectious TB disease,” Dr. Dodd said.
Tuberculosis infection incidence based on surveys in children might underestimate true incidence in adults in TB-endemic settings, the researchers cautioned in their analysis.
“For hyperendemic communities in South Africa, which may have an incidence of M. tuberculosis infection in children as high as 4% per year, our findings imply that incidence rates in never-infected adults may be as high as 10% per year, rates which have rarely been seen outside institutional settings.”
Care and control of tuberculosis in males, the researchers concluded, “is critical to protecting men, women, and children from tuberculosis.”
Individual researchers were funded by the Bill and Melinda Gates Foundation, the Medical Research Council (U.K.), and the Wellcome Trust. None of the researchers reported conflicts of interest.
FROM THE AMERICAN JOURNAL OF EPIDEMIOLOGY
Key clinical point: More than half of TB infections among men, women, and children were attributable to contact with adult men in 2 African cohorts.
Major finding: Estimated overall adult infections attributable to contact with adult men was 57.3% in South Africa and 65.7% in Zambia, and more than 50% overall for all ages including children, with estimated incidence in adults 1.5-6 times higher than that in children.
Data source: Interviews with 3,528 adults on daily close and casual contacts conducted in 24 South African and Zambian communities in 2011, compared with recent empirical incidence data by age group and sex for the same regions.
Disclosures: Individual researchers were funded by the Bill and Melinda Gates Foundation, the Medical Research Council (U.K.), and the Wellcome Trust. None reported conflicts of interest.
Minority of U.S. hospitals mandate flu vaccination
Less than 50% of U.S. hospitals require health care workers to receive annual flu shots, according to a survey study with responses from nearly 500 facilities.
The study, published online in Infection Control & Hospital Epidemiology (2015 Nov 27. doi: 10.1017/ice.2015.277), also found that only 1.3% of U.S. Veterans Affairs hospitals mandate flu shots, despite no law preventing them from doing so.
Dr. M. Todd Greene of the University of Michigan, Ann Arbor, and the Veterans Affairs/University of Michigan Patient Safety Enhancement Program, led the study, which asked hospital infection specialists to report on their institutions’ policies regarding annual vaccines, the stated reasons behind these policies, and other efforts to promote vaccination or discourage nonvaccination in 2013. Only 42.7% of respondents from 386 non-VA hospitals said their institutions required universal vaccination of personnel. However, many reported policies promoting uptake and/or mandating declination forms and face masks for personnel who opted out.
Among non-VA hospitals without mandatory vaccination, 22% said their administrations were unwilling to require it, while another 22% said vaccination was “strongly recommended” or otherwise promoted, and 21% said face masks and signed declination forms were mandatory for nonvaccinated personnel. Union concerns were cited by 8% as a reason vaccination was not required. In the VA system, meanwhile, 57% of hospitals without mandatory vaccination cited federal agency status as a reason, and more than a quarter cited union issues.
Dr. Greene and colleagues noted that while the VA does not have a national vaccination requirement, individual hospitals are free to determine their own policies. The Veterans Hospital Administration estimates vaccine uptake among workers at its hospitals to be only 55% in recent flu seasons, while a recent study by the Centers for Disease Control and Prevention found that overall about 77% of U.S. health care workers in diverse clinical settings received flu shots in 2014-2015, and that those settings with vaccination requirements saw 96% of personnel covered.
Dr. Greene and colleagues’ study was funded by the Blue Cross Blue Shield of Michigan Foundation, the Department of Veterans Affairs, and the National Center for Patient Safety. None of its authors disclosed conflicts of interest.
Less than 50% of U.S. hospitals require health care workers to receive annual flu shots, according to a survey study with responses from nearly 500 facilities.
The study, published online in Infection Control & Hospital Epidemiology (2015 Nov 27. doi: 10.1017/ice.2015.277), also found that only 1.3% of U.S. Veterans Affairs hospitals mandate flu shots, despite no law preventing them from doing so.
Dr. M. Todd Greene of the University of Michigan, Ann Arbor, and the Veterans Affairs/University of Michigan Patient Safety Enhancement Program, led the study, which asked hospital infection specialists to report on their institutions’ policies regarding annual vaccines, the stated reasons behind these policies, and other efforts to promote vaccination or discourage nonvaccination in 2013. Only 42.7% of respondents from 386 non-VA hospitals said their institutions required universal vaccination of personnel. However, many reported policies promoting uptake and/or mandating declination forms and face masks for personnel who opted out.
Among non-VA hospitals without mandatory vaccination, 22% said their administrations were unwilling to require it, while another 22% said vaccination was “strongly recommended” or otherwise promoted, and 21% said face masks and signed declination forms were mandatory for nonvaccinated personnel. Union concerns were cited by 8% as a reason vaccination was not required. In the VA system, meanwhile, 57% of hospitals without mandatory vaccination cited federal agency status as a reason, and more than a quarter cited union issues.
Dr. Greene and colleagues noted that while the VA does not have a national vaccination requirement, individual hospitals are free to determine their own policies. The Veterans Hospital Administration estimates vaccine uptake among workers at its hospitals to be only 55% in recent flu seasons, while a recent study by the Centers for Disease Control and Prevention found that overall about 77% of U.S. health care workers in diverse clinical settings received flu shots in 2014-2015, and that those settings with vaccination requirements saw 96% of personnel covered.
Dr. Greene and colleagues’ study was funded by the Blue Cross Blue Shield of Michigan Foundation, the Department of Veterans Affairs, and the National Center for Patient Safety. None of its authors disclosed conflicts of interest.
Less than 50% of U.S. hospitals require health care workers to receive annual flu shots, according to a survey study with responses from nearly 500 facilities.
The study, published online in Infection Control & Hospital Epidemiology (2015 Nov 27. doi: 10.1017/ice.2015.277), also found that only 1.3% of U.S. Veterans Affairs hospitals mandate flu shots, despite no law preventing them from doing so.
Dr. M. Todd Greene of the University of Michigan, Ann Arbor, and the Veterans Affairs/University of Michigan Patient Safety Enhancement Program, led the study, which asked hospital infection specialists to report on their institutions’ policies regarding annual vaccines, the stated reasons behind these policies, and other efforts to promote vaccination or discourage nonvaccination in 2013. Only 42.7% of respondents from 386 non-VA hospitals said their institutions required universal vaccination of personnel. However, many reported policies promoting uptake and/or mandating declination forms and face masks for personnel who opted out.
Among non-VA hospitals without mandatory vaccination, 22% said their administrations were unwilling to require it, while another 22% said vaccination was “strongly recommended” or otherwise promoted, and 21% said face masks and signed declination forms were mandatory for nonvaccinated personnel. Union concerns were cited by 8% as a reason vaccination was not required. In the VA system, meanwhile, 57% of hospitals without mandatory vaccination cited federal agency status as a reason, and more than a quarter cited union issues.
Dr. Greene and colleagues noted that while the VA does not have a national vaccination requirement, individual hospitals are free to determine their own policies. The Veterans Hospital Administration estimates vaccine uptake among workers at its hospitals to be only 55% in recent flu seasons, while a recent study by the Centers for Disease Control and Prevention found that overall about 77% of U.S. health care workers in diverse clinical settings received flu shots in 2014-2015, and that those settings with vaccination requirements saw 96% of personnel covered.
Dr. Greene and colleagues’ study was funded by the Blue Cross Blue Shield of Michigan Foundation, the Department of Veterans Affairs, and the National Center for Patient Safety. None of its authors disclosed conflicts of interest.
FROM INFECTION CONTROL & HOSPITAL EPIDEMIOLOGY
Key clinical point: Despite evidence that mandatory influenza vaccination policies result in high coverage, fewer than half of U.S. hospitals, and very few VA hospitals, require workers to be vaccinated.
Major finding: 42.7% of non-VA hospitals and only 1.3% of VA hospitals required all health care workers to receive flu shots in 2013.
Data source: A survey of infection control specialists at 571 non-VA hospitals (71% responding, n = 403) and 126 VA hospitals (63% responding, n = 80) in 2013.
Disclosures: Blue Cross Blue Shield of Michigan Foundation, the Department of Veterans Affairs, and the National Center for Patient Safety funded the study, and authors reported no conflicts of interest.
Early infection-related hospitalization portends poor breast cancer prognosis
SAN ANTONIO – Hospitalization for an infection within the first year following diagnosis of primary nonmetastatic breast cancer is a red flag for increased risk of subsequent development of distant metastases or breast cancer–related death, Judith S. Brand, Ph.D., reported at the San Antonio Breast Cancer Symposium.
This increased risk for future adverse breast cancer outcomes was statistically significant and clinically meaningful for women hospitalized for respiratory tract or skin infections or sepsis. Those are the patients for whom particularly close monitoring for recurrence of breast cancer is warranted in the next 5 years, said Dr. Brand of the Karolinska Institute, Stockholm.
In contrast, hospitalization for a gastrointestinal or urinary tract infection didn’t achieve significance as an independent predictor of increased risk of adverse breast cancer outcomes.
Dr. Brand presented a prospective population-based study of 8,338 women diagnosed with stage I-III breast cancer in the Stockholm area during 2001-2008. During a median 4.9 years of follow-up after diagnosis, 720 women had an infection-related hospitalization, with the great majority of these events occurring during the first year.
The incidence of hospitalization for sepsis among breast cancer patients in their first year post diagnosis was 14-fold greater than in the general Swedish female population matched for age and year. Respiratory infections resulting in hospitalization were fourfold more frequent than in the general population, skin infections were eightfold more common, and GI infections were twice as common.
Infection-related hospitalizations had a strong and independent association with breast cancer mortality during follow-up, as seen in a multivariate analysis adjusted for age at diagnosis, comorbid conditions, infectious disease history, type of breast cancer therapy, and tumor characteristics including size, grade, hormone receptor status, and lymph node involvement.
Moreover, the risk of developing distant metastases was 50%-78% greater in breast cancer patients hospitalized for respiratory infection, sepsis, or skin infection, compared with breast cancer patients who didn’t have an infection-related hospitalization.
“We think the sepsis results are the most interesting findings,” Dr. Brand said in an interview. “Sepsis could be an expression of an immunosuppressed state. And sepsis itself can induce immunosuppression for a long time, which could trigger tumor growth. Animal studies have shown that in postseptic mice, tumor grows faster.”
Infection-related hospitalizations didn’t increase the future risk of locoregional recurrences.
Independent predictors of infection-related hospitalization included older age, comorbidities, markers indicative of greater tumor aggressiveness, and treatment with chemotherapy or axillary radiotherapy.
“This shows that the risk of infection-related hospitalizations is not only due to immunosuppression caused by chemotherapy, but that the characteristics of the tumor itself play a role, as do patient characteristics. This is the first epidemiologic study to show with very extensive data that all three elements contribute to the risk,” Dr. Brand said.
SAN ANTONIO – Hospitalization for an infection within the first year following diagnosis of primary nonmetastatic breast cancer is a red flag for increased risk of subsequent development of distant metastases or breast cancer–related death, Judith S. Brand, Ph.D., reported at the San Antonio Breast Cancer Symposium.
This increased risk for future adverse breast cancer outcomes was statistically significant and clinically meaningful for women hospitalized for respiratory tract or skin infections or sepsis. Those are the patients for whom particularly close monitoring for recurrence of breast cancer is warranted in the next 5 years, said Dr. Brand of the Karolinska Institute, Stockholm.
In contrast, hospitalization for a gastrointestinal or urinary tract infection didn’t achieve significance as an independent predictor of increased risk of adverse breast cancer outcomes.
Dr. Brand presented a prospective population-based study of 8,338 women diagnosed with stage I-III breast cancer in the Stockholm area during 2001-2008. During a median 4.9 years of follow-up after diagnosis, 720 women had an infection-related hospitalization, with the great majority of these events occurring during the first year.
The incidence of hospitalization for sepsis among breast cancer patients in their first year post diagnosis was 14-fold greater than in the general Swedish female population matched for age and year. Respiratory infections resulting in hospitalization were fourfold more frequent than in the general population, skin infections were eightfold more common, and GI infections were twice as common.
Infection-related hospitalizations had a strong and independent association with breast cancer mortality during follow-up, as seen in a multivariate analysis adjusted for age at diagnosis, comorbid conditions, infectious disease history, type of breast cancer therapy, and tumor characteristics including size, grade, hormone receptor status, and lymph node involvement.
Moreover, the risk of developing distant metastases was 50%-78% greater in breast cancer patients hospitalized for respiratory infection, sepsis, or skin infection, compared with breast cancer patients who didn’t have an infection-related hospitalization.
“We think the sepsis results are the most interesting findings,” Dr. Brand said in an interview. “Sepsis could be an expression of an immunosuppressed state. And sepsis itself can induce immunosuppression for a long time, which could trigger tumor growth. Animal studies have shown that in postseptic mice, tumor grows faster.”
Infection-related hospitalizations didn’t increase the future risk of locoregional recurrences.
Independent predictors of infection-related hospitalization included older age, comorbidities, markers indicative of greater tumor aggressiveness, and treatment with chemotherapy or axillary radiotherapy.
“This shows that the risk of infection-related hospitalizations is not only due to immunosuppression caused by chemotherapy, but that the characteristics of the tumor itself play a role, as do patient characteristics. This is the first epidemiologic study to show with very extensive data that all three elements contribute to the risk,” Dr. Brand said.
SAN ANTONIO – Hospitalization for an infection within the first year following diagnosis of primary nonmetastatic breast cancer is a red flag for increased risk of subsequent development of distant metastases or breast cancer–related death, Judith S. Brand, Ph.D., reported at the San Antonio Breast Cancer Symposium.
This increased risk for future adverse breast cancer outcomes was statistically significant and clinically meaningful for women hospitalized for respiratory tract or skin infections or sepsis. Those are the patients for whom particularly close monitoring for recurrence of breast cancer is warranted in the next 5 years, said Dr. Brand of the Karolinska Institute, Stockholm.
In contrast, hospitalization for a gastrointestinal or urinary tract infection didn’t achieve significance as an independent predictor of increased risk of adverse breast cancer outcomes.
Dr. Brand presented a prospective population-based study of 8,338 women diagnosed with stage I-III breast cancer in the Stockholm area during 2001-2008. During a median 4.9 years of follow-up after diagnosis, 720 women had an infection-related hospitalization, with the great majority of these events occurring during the first year.
The incidence of hospitalization for sepsis among breast cancer patients in their first year post diagnosis was 14-fold greater than in the general Swedish female population matched for age and year. Respiratory infections resulting in hospitalization were fourfold more frequent than in the general population, skin infections were eightfold more common, and GI infections were twice as common.
Infection-related hospitalizations had a strong and independent association with breast cancer mortality during follow-up, as seen in a multivariate analysis adjusted for age at diagnosis, comorbid conditions, infectious disease history, type of breast cancer therapy, and tumor characteristics including size, grade, hormone receptor status, and lymph node involvement.
Moreover, the risk of developing distant metastases was 50%-78% greater in breast cancer patients hospitalized for respiratory infection, sepsis, or skin infection, compared with breast cancer patients who didn’t have an infection-related hospitalization.
“We think the sepsis results are the most interesting findings,” Dr. Brand said in an interview. “Sepsis could be an expression of an immunosuppressed state. And sepsis itself can induce immunosuppression for a long time, which could trigger tumor growth. Animal studies have shown that in postseptic mice, tumor grows faster.”
Infection-related hospitalizations didn’t increase the future risk of locoregional recurrences.
Independent predictors of infection-related hospitalization included older age, comorbidities, markers indicative of greater tumor aggressiveness, and treatment with chemotherapy or axillary radiotherapy.
“This shows that the risk of infection-related hospitalizations is not only due to immunosuppression caused by chemotherapy, but that the characteristics of the tumor itself play a role, as do patient characteristics. This is the first epidemiologic study to show with very extensive data that all three elements contribute to the risk,” Dr. Brand said.
AT SABCS 2015
Key clinical point: Particularly close monitoring for adverse breast cancer outcomes is warranted for women with an infection-related hospitalization during the first year after breast cancer diagnosis.
Major finding: Women hospitalized for sepsis or a respiratory or skin infection during the first year after being diagnosed with stage I-III breast cancer were up to 4.8 times more likely to subsequently die of breast cancer than those without an infection-related hospitalization.
Data source: This was a prospective observational study of 8,338 Stockholm-area breast cancer patients followed for a median of 4.9 years post diagnosis.
Disclosures: The study presenter reported having no financial conflicts regarding this university-funded study.
Measures that don’t account for DNR status could unfairly penalize hospitals
Mortality-based quality measures that do not account for do-not-resuscitate orders paint a skewed picture of hospital performance, said authors of a multicenter retrospective cohort study.
“Our findings suggest that current methods of comparing hospitals, which do not account for patient DNR status, penalize potentially high-quality hospitals [that admit] a larger proportion of patients who had chosen to forgo resuscitation,” Dr. Allan J. Walkey of Boston University, and his associates wrote online Dec. 14 in JAMA Internal Medicine. Accounting for DNR status when evaluating mortality outcomes “may substantially affect publicly reportable hospital rankings and hospital reimbursements,” the researchers added.
Early DNR orders typically reflect patient-specific variables, such as baseline comorbidities and attitudes about end-of-life care, the researchers noted. “Despite the potential for hospital variation in DNR orders to influence patients’ end-of-life experiences and outcomes, DNR status is generally unreported by hospitals and unaccounted for in hospital outcome measures,” they added. Their population-based cohort study assessed DNR status and mortality for more than 90,000 pneumonia cases at 303 hospitals in California during 2011 (JAMA Intern Med. 2015 Dec. 14. doi: 10.1001/jamainternmed.2015.6324).
The lower and upper quartiles for DNR rates were about 9% and 22%, said the researchers. Without accounting for these differences, hospitals in the highest quartile had significantly greater patient mortality than did those in the lowest quartile (adjusted odds ratio, 1.17; 95% confidence interval, 1.04-1.32), corresponding to worse mortality rankings. But this trend actually reversed after the investigators accounted for DNR rates (adjusted OR, 0.79; 95% CI, 0.70-0.89), as did the link between hospital mortality rankings and DNR rates.
Only about half of hospitals that were low-performing outliers without accounting for DNR status remained outliers after adjustment, the researchers noted. And although DNR rates did not significantly correlate with composite quality measures of pneumonia care, they were positively linked to patient satisfaction scores (P less than .001). Based on the findings, “stakeholders should seek to improve methods to standardize and report DNR status in hospital discharge records,” they concluded.
The study was funded by the National Institutes of Health, the National Heart, Lung, and Blood Institute of the NIH, the Agency for Healthcare Research and Quality, the Edith Nourse Rogers Memorial Veterans Affairs Hospital, and Boston University. The researchers had no disclosures.
Mortality-based quality measures that do not account for do-not-resuscitate orders paint a skewed picture of hospital performance, said authors of a multicenter retrospective cohort study.
“Our findings suggest that current methods of comparing hospitals, which do not account for patient DNR status, penalize potentially high-quality hospitals [that admit] a larger proportion of patients who had chosen to forgo resuscitation,” Dr. Allan J. Walkey of Boston University, and his associates wrote online Dec. 14 in JAMA Internal Medicine. Accounting for DNR status when evaluating mortality outcomes “may substantially affect publicly reportable hospital rankings and hospital reimbursements,” the researchers added.
Early DNR orders typically reflect patient-specific variables, such as baseline comorbidities and attitudes about end-of-life care, the researchers noted. “Despite the potential for hospital variation in DNR orders to influence patients’ end-of-life experiences and outcomes, DNR status is generally unreported by hospitals and unaccounted for in hospital outcome measures,” they added. Their population-based cohort study assessed DNR status and mortality for more than 90,000 pneumonia cases at 303 hospitals in California during 2011 (JAMA Intern Med. 2015 Dec. 14. doi: 10.1001/jamainternmed.2015.6324).
The lower and upper quartiles for DNR rates were about 9% and 22%, said the researchers. Without accounting for these differences, hospitals in the highest quartile had significantly greater patient mortality than did those in the lowest quartile (adjusted odds ratio, 1.17; 95% confidence interval, 1.04-1.32), corresponding to worse mortality rankings. But this trend actually reversed after the investigators accounted for DNR rates (adjusted OR, 0.79; 95% CI, 0.70-0.89), as did the link between hospital mortality rankings and DNR rates.
Only about half of hospitals that were low-performing outliers without accounting for DNR status remained outliers after adjustment, the researchers noted. And although DNR rates did not significantly correlate with composite quality measures of pneumonia care, they were positively linked to patient satisfaction scores (P less than .001). Based on the findings, “stakeholders should seek to improve methods to standardize and report DNR status in hospital discharge records,” they concluded.
The study was funded by the National Institutes of Health, the National Heart, Lung, and Blood Institute of the NIH, the Agency for Healthcare Research and Quality, the Edith Nourse Rogers Memorial Veterans Affairs Hospital, and Boston University. The researchers had no disclosures.
Mortality-based quality measures that do not account for do-not-resuscitate orders paint a skewed picture of hospital performance, said authors of a multicenter retrospective cohort study.
“Our findings suggest that current methods of comparing hospitals, which do not account for patient DNR status, penalize potentially high-quality hospitals [that admit] a larger proportion of patients who had chosen to forgo resuscitation,” Dr. Allan J. Walkey of Boston University, and his associates wrote online Dec. 14 in JAMA Internal Medicine. Accounting for DNR status when evaluating mortality outcomes “may substantially affect publicly reportable hospital rankings and hospital reimbursements,” the researchers added.
Early DNR orders typically reflect patient-specific variables, such as baseline comorbidities and attitudes about end-of-life care, the researchers noted. “Despite the potential for hospital variation in DNR orders to influence patients’ end-of-life experiences and outcomes, DNR status is generally unreported by hospitals and unaccounted for in hospital outcome measures,” they added. Their population-based cohort study assessed DNR status and mortality for more than 90,000 pneumonia cases at 303 hospitals in California during 2011 (JAMA Intern Med. 2015 Dec. 14. doi: 10.1001/jamainternmed.2015.6324).
The lower and upper quartiles for DNR rates were about 9% and 22%, said the researchers. Without accounting for these differences, hospitals in the highest quartile had significantly greater patient mortality than did those in the lowest quartile (adjusted odds ratio, 1.17; 95% confidence interval, 1.04-1.32), corresponding to worse mortality rankings. But this trend actually reversed after the investigators accounted for DNR rates (adjusted OR, 0.79; 95% CI, 0.70-0.89), as did the link between hospital mortality rankings and DNR rates.
Only about half of hospitals that were low-performing outliers without accounting for DNR status remained outliers after adjustment, the researchers noted. And although DNR rates did not significantly correlate with composite quality measures of pneumonia care, they were positively linked to patient satisfaction scores (P less than .001). Based on the findings, “stakeholders should seek to improve methods to standardize and report DNR status in hospital discharge records,” they concluded.
The study was funded by the National Institutes of Health, the National Heart, Lung, and Blood Institute of the NIH, the Agency for Healthcare Research and Quality, the Edith Nourse Rogers Memorial Veterans Affairs Hospital, and Boston University. The researchers had no disclosures.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Quality measures that fail to account for DNR orders could lead to unfair hospital penalties.
Major finding: Without accounting for DNR status, hospitals whose rates were in the highest quartile had significantly greater mortality rates (adjusted odds ratio, 1.17) than did those in the lowest quartile, corresponding with worse mortality rankings.
Data source: A retrospective, population-based cohort study of 90,644 cases of pneumonia at 303 hospitals in California.
Disclosures: The study was funded by the National Institutes of Health, the National Heart, Lung, and Blood Institute of the NIH, the Agency for Healthcare Research and Quality, the Edith Nourse Rogers Memorial Veterans Affairs Hospital, and Boston University. The researchers had no disclosures.
Yoga Performs Like Pulmonary Rehab for COPD Patients
MONTREAL – A structured regimen of regular yoga exercises was as effective as a standard pulmonary rehabilitation program in patients with chronic obstructive pulmonary disease for improving lung function, exercise tolerance, dyspnea severity, and quality of life in a single-center, randomized comparison of the two strategies with 60 patients.
In addition, chronic obstructive pulmonary disease (COPD) patients had a higher level of acceptance of yoga and were more comfortable doing it, compared with standard pulmonary rehabilitation, and it is a cost-effective approach given the minimal equipment required, Dr. Randeep Guleria said at the annual meeting of the American College of Chest Physicians.
“Patients with difficulty walking, osteoarthritis, knee problems, or unable to do exercises like cycling or treadmill found yoga to be much more acceptable,” said Dr. Guleria in an interview. Acceptance of yoga was also higher than standard rehabilitation among patients with more severe COPD, said Dr. Guleria, professor and head of the department of pulmonary medicine and sleep disorders at the All India Institute of Medical Sciences in New Delhi.
“I think that yoga could be a very valuable adjunct” to pulmonary rehabilitation in COPD patients, commented Dr. Roger S. Goldstein, director of the divisional program in respiratory rehabilitation at the University of Toronto. Dr. Goldstein speculated that even better than comparing yoga against conventional pulmonary rehabilitation would be a study that compared a combined yoga plus rehabilitation program against standard rehabilitation alone. Yoga is “a tremendous opportunity,” he said.
The 12-week study enrolled 60 patients who averaged 56 years old who had been diagnosed with COPD for an average of 8 years. Just under a third of the patients had moderate COPD, 42% had severe COPD, and 28% had very severe COPD.
Dr. Guleria and his associates randomized 30 patients into a yoga program that included 4 weeks of biweekly 1-hour sessions that instructed patients in a series of specially designed yoga exercises. That was followed by 8 weeks during which patients were mostly left to perform their learned exercises on their own, but with a supervised session once every 2 weeks. The other 30 patients participated in a standard pulmonary rehabilitation program for 12 weeks.
The researchers measured several parameters at baseline and after 12 weeks, including two measures of dyspnea severity, 6-minute walk distance, a quality of life assessment, and two serum markers of inflammation, C-reactive protein and interleukin 6.
Both interventions resulted in modest but statistically significant improvements, such as increases in 6-minute walk distance and a reduced modified Borg scale assessment. The Borg scale score fell from an average of 1.5 at baseline to 1.0 after 12 weeks in the yoga patients, and from an average 3.0 at baseline to 0.5 after 12 weeks in the rehabilitation patients.
A score that measured total quality of life improved by an average of 32% in the yoga patients and by an average of 21% in the rehabilitation patients, changes that approached statistical significance in both subgroups.
Comparing the assessment measures after 12 weeks in both arms of the study showed no statistically significant between-group differences, Dr. Guleria reported.
The researchers commissioned a specially designed yoga program from a professional yoga instructor who had been briefed about COPD. The yoga exercises included physical postures, breathing technique, and meditation and relaxation. The pulmonary rehabilitation program included patient education, upper and lower limb exercises, and breathing exercises.
Dr. Guleria said he believed the opportunity exists to further modify the yoga program to better optimize its potential to benefit COPD patients.
Dr. Guleria had no relevant disclosures. Dr. Goldstein had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter@mitchelzoler
MONTREAL – A structured regimen of regular yoga exercises was as effective as a standard pulmonary rehabilitation program in patients with chronic obstructive pulmonary disease for improving lung function, exercise tolerance, dyspnea severity, and quality of life in a single-center, randomized comparison of the two strategies with 60 patients.
In addition, chronic obstructive pulmonary disease (COPD) patients had a higher level of acceptance of yoga and were more comfortable doing it, compared with standard pulmonary rehabilitation, and it is a cost-effective approach given the minimal equipment required, Dr. Randeep Guleria said at the annual meeting of the American College of Chest Physicians.
“Patients with difficulty walking, osteoarthritis, knee problems, or unable to do exercises like cycling or treadmill found yoga to be much more acceptable,” said Dr. Guleria in an interview. Acceptance of yoga was also higher than standard rehabilitation among patients with more severe COPD, said Dr. Guleria, professor and head of the department of pulmonary medicine and sleep disorders at the All India Institute of Medical Sciences in New Delhi.
“I think that yoga could be a very valuable adjunct” to pulmonary rehabilitation in COPD patients, commented Dr. Roger S. Goldstein, director of the divisional program in respiratory rehabilitation at the University of Toronto. Dr. Goldstein speculated that even better than comparing yoga against conventional pulmonary rehabilitation would be a study that compared a combined yoga plus rehabilitation program against standard rehabilitation alone. Yoga is “a tremendous opportunity,” he said.
The 12-week study enrolled 60 patients who averaged 56 years old who had been diagnosed with COPD for an average of 8 years. Just under a third of the patients had moderate COPD, 42% had severe COPD, and 28% had very severe COPD.
Dr. Guleria and his associates randomized 30 patients into a yoga program that included 4 weeks of biweekly 1-hour sessions that instructed patients in a series of specially designed yoga exercises. That was followed by 8 weeks during which patients were mostly left to perform their learned exercises on their own, but with a supervised session once every 2 weeks. The other 30 patients participated in a standard pulmonary rehabilitation program for 12 weeks.
The researchers measured several parameters at baseline and after 12 weeks, including two measures of dyspnea severity, 6-minute walk distance, a quality of life assessment, and two serum markers of inflammation, C-reactive protein and interleukin 6.
Both interventions resulted in modest but statistically significant improvements, such as increases in 6-minute walk distance and a reduced modified Borg scale assessment. The Borg scale score fell from an average of 1.5 at baseline to 1.0 after 12 weeks in the yoga patients, and from an average 3.0 at baseline to 0.5 after 12 weeks in the rehabilitation patients.
A score that measured total quality of life improved by an average of 32% in the yoga patients and by an average of 21% in the rehabilitation patients, changes that approached statistical significance in both subgroups.
Comparing the assessment measures after 12 weeks in both arms of the study showed no statistically significant between-group differences, Dr. Guleria reported.
The researchers commissioned a specially designed yoga program from a professional yoga instructor who had been briefed about COPD. The yoga exercises included physical postures, breathing technique, and meditation and relaxation. The pulmonary rehabilitation program included patient education, upper and lower limb exercises, and breathing exercises.
Dr. Guleria said he believed the opportunity exists to further modify the yoga program to better optimize its potential to benefit COPD patients.
Dr. Guleria had no relevant disclosures. Dr. Goldstein had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter@mitchelzoler
MONTREAL – A structured regimen of regular yoga exercises was as effective as a standard pulmonary rehabilitation program in patients with chronic obstructive pulmonary disease for improving lung function, exercise tolerance, dyspnea severity, and quality of life in a single-center, randomized comparison of the two strategies with 60 patients.
In addition, chronic obstructive pulmonary disease (COPD) patients had a higher level of acceptance of yoga and were more comfortable doing it, compared with standard pulmonary rehabilitation, and it is a cost-effective approach given the minimal equipment required, Dr. Randeep Guleria said at the annual meeting of the American College of Chest Physicians.
“Patients with difficulty walking, osteoarthritis, knee problems, or unable to do exercises like cycling or treadmill found yoga to be much more acceptable,” said Dr. Guleria in an interview. Acceptance of yoga was also higher than standard rehabilitation among patients with more severe COPD, said Dr. Guleria, professor and head of the department of pulmonary medicine and sleep disorders at the All India Institute of Medical Sciences in New Delhi.
“I think that yoga could be a very valuable adjunct” to pulmonary rehabilitation in COPD patients, commented Dr. Roger S. Goldstein, director of the divisional program in respiratory rehabilitation at the University of Toronto. Dr. Goldstein speculated that even better than comparing yoga against conventional pulmonary rehabilitation would be a study that compared a combined yoga plus rehabilitation program against standard rehabilitation alone. Yoga is “a tremendous opportunity,” he said.
The 12-week study enrolled 60 patients who averaged 56 years old who had been diagnosed with COPD for an average of 8 years. Just under a third of the patients had moderate COPD, 42% had severe COPD, and 28% had very severe COPD.
Dr. Guleria and his associates randomized 30 patients into a yoga program that included 4 weeks of biweekly 1-hour sessions that instructed patients in a series of specially designed yoga exercises. That was followed by 8 weeks during which patients were mostly left to perform their learned exercises on their own, but with a supervised session once every 2 weeks. The other 30 patients participated in a standard pulmonary rehabilitation program for 12 weeks.
The researchers measured several parameters at baseline and after 12 weeks, including two measures of dyspnea severity, 6-minute walk distance, a quality of life assessment, and two serum markers of inflammation, C-reactive protein and interleukin 6.
Both interventions resulted in modest but statistically significant improvements, such as increases in 6-minute walk distance and a reduced modified Borg scale assessment. The Borg scale score fell from an average of 1.5 at baseline to 1.0 after 12 weeks in the yoga patients, and from an average 3.0 at baseline to 0.5 after 12 weeks in the rehabilitation patients.
A score that measured total quality of life improved by an average of 32% in the yoga patients and by an average of 21% in the rehabilitation patients, changes that approached statistical significance in both subgroups.
Comparing the assessment measures after 12 weeks in both arms of the study showed no statistically significant between-group differences, Dr. Guleria reported.
The researchers commissioned a specially designed yoga program from a professional yoga instructor who had been briefed about COPD. The yoga exercises included physical postures, breathing technique, and meditation and relaxation. The pulmonary rehabilitation program included patient education, upper and lower limb exercises, and breathing exercises.
Dr. Guleria said he believed the opportunity exists to further modify the yoga program to better optimize its potential to benefit COPD patients.
Dr. Guleria had no relevant disclosures. Dr. Goldstein had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter@mitchelzoler
AT CHEST 2015
Self-reported poor functional status predicts perioperative morbidity
SAN DIEGO – Among patients with pulmonary hypertension presenting for elective surgery, self-reported poor functional status is associated with multiple comorbidities and is independently predictive of longer hospital length of stay, results from an ongoing single-center study suggest.
“Patients with pulmonary hypertension (PHTN) presenting for elective surgery are at significantly higher risk for adverse perioperative outcomes, including increased hospital length of stay, right ventricular failure, cardiac arrhythmia, persistent postoperative hypoxemia, coronary ischemia and death,” researchers led by Dr. Aalap C. Shah wrote in an abstract presented at the at the annual meeting of the American Society of Anesthesiologists. “The diagnosis of PHTN is based on costly echocardiographic examination and right heart catheterization and should be reserved for high-risk patients. No studies have assessed the role of self-reported functional classification on PHTN severity stratification, and few studies have achieved a sufficiently large patient sample size.”
In an effort to evaluate the predictive value of self-reported exercise tolerance on echocardiogram findings, outcomes, and length of stay (LOS) after noncardiac, nonobstetric surgery, the researchers queried the University of Washington database for all PHTN seen in preoperative anesthesia clinic for noncardiac, nonobstetric procedures from April 2007 through September 2013. Inclusion criteria required an echocardiogram less than 1 year prior to the procedure and available patient-reported functional status, which was defined as less than four metabolic equivalents (METS) in exercise testing or four METS or greater. Dr. Shah, formerly a resident in the University of Washington’s department of anesthesiology and pain medicine, and his associates used univariate analyses to compare functional status with echocardiographic findings, complication rates, and length of stay (LOS). At the meeting he presented results from 294 patients evaluated to date: 143 with normal functional status and 151 with poor functional status. Their mean age was 62 years, and 51% of patients were female.
Compared with their counterparts with normal functional status, patients with poor functional status trended toward a higher complication rate at hospital discharge (14.6% vs. 7%, respectively; P = .041) and had a higher cumulative rate of complications (33 vs. 15; P = .035). However, no association between functional status and complications was observed 30 days postoperatively.
Patients with poor functional status had a significantly longer average LOS, compared with patients with normal functional status (7.21 vs. 4.73 days; P = .047). Open surgical approach was also an independent predictor of increased LOS (odds ratio 2.39; P = .005). No significant independent predictors of complications were observed at discharge or 30 days postoperatively.
“Going forward, the goal is to use these data to create a risk stratification algorithm to figure out: Does a patient with good functional status and pulmonary hypertension undergoing toe surgery, for example, really need an echocardiogram before getting surgery?” said Dr. Shah said, who is now an anesthesiology fellow at Boston Children’s Hospital. “Hopefully we can show that using these risk stratification algorithms can decrease the costs and decrease the time to actually getting surgery.”
The researchers reported having no financial disclosures.
SAN DIEGO – Among patients with pulmonary hypertension presenting for elective surgery, self-reported poor functional status is associated with multiple comorbidities and is independently predictive of longer hospital length of stay, results from an ongoing single-center study suggest.
“Patients with pulmonary hypertension (PHTN) presenting for elective surgery are at significantly higher risk for adverse perioperative outcomes, including increased hospital length of stay, right ventricular failure, cardiac arrhythmia, persistent postoperative hypoxemia, coronary ischemia and death,” researchers led by Dr. Aalap C. Shah wrote in an abstract presented at the at the annual meeting of the American Society of Anesthesiologists. “The diagnosis of PHTN is based on costly echocardiographic examination and right heart catheterization and should be reserved for high-risk patients. No studies have assessed the role of self-reported functional classification on PHTN severity stratification, and few studies have achieved a sufficiently large patient sample size.”
In an effort to evaluate the predictive value of self-reported exercise tolerance on echocardiogram findings, outcomes, and length of stay (LOS) after noncardiac, nonobstetric surgery, the researchers queried the University of Washington database for all PHTN seen in preoperative anesthesia clinic for noncardiac, nonobstetric procedures from April 2007 through September 2013. Inclusion criteria required an echocardiogram less than 1 year prior to the procedure and available patient-reported functional status, which was defined as less than four metabolic equivalents (METS) in exercise testing or four METS or greater. Dr. Shah, formerly a resident in the University of Washington’s department of anesthesiology and pain medicine, and his associates used univariate analyses to compare functional status with echocardiographic findings, complication rates, and length of stay (LOS). At the meeting he presented results from 294 patients evaluated to date: 143 with normal functional status and 151 with poor functional status. Their mean age was 62 years, and 51% of patients were female.
Compared with their counterparts with normal functional status, patients with poor functional status trended toward a higher complication rate at hospital discharge (14.6% vs. 7%, respectively; P = .041) and had a higher cumulative rate of complications (33 vs. 15; P = .035). However, no association between functional status and complications was observed 30 days postoperatively.
Patients with poor functional status had a significantly longer average LOS, compared with patients with normal functional status (7.21 vs. 4.73 days; P = .047). Open surgical approach was also an independent predictor of increased LOS (odds ratio 2.39; P = .005). No significant independent predictors of complications were observed at discharge or 30 days postoperatively.
“Going forward, the goal is to use these data to create a risk stratification algorithm to figure out: Does a patient with good functional status and pulmonary hypertension undergoing toe surgery, for example, really need an echocardiogram before getting surgery?” said Dr. Shah said, who is now an anesthesiology fellow at Boston Children’s Hospital. “Hopefully we can show that using these risk stratification algorithms can decrease the costs and decrease the time to actually getting surgery.”
The researchers reported having no financial disclosures.
SAN DIEGO – Among patients with pulmonary hypertension presenting for elective surgery, self-reported poor functional status is associated with multiple comorbidities and is independently predictive of longer hospital length of stay, results from an ongoing single-center study suggest.
“Patients with pulmonary hypertension (PHTN) presenting for elective surgery are at significantly higher risk for adverse perioperative outcomes, including increased hospital length of stay, right ventricular failure, cardiac arrhythmia, persistent postoperative hypoxemia, coronary ischemia and death,” researchers led by Dr. Aalap C. Shah wrote in an abstract presented at the at the annual meeting of the American Society of Anesthesiologists. “The diagnosis of PHTN is based on costly echocardiographic examination and right heart catheterization and should be reserved for high-risk patients. No studies have assessed the role of self-reported functional classification on PHTN severity stratification, and few studies have achieved a sufficiently large patient sample size.”
In an effort to evaluate the predictive value of self-reported exercise tolerance on echocardiogram findings, outcomes, and length of stay (LOS) after noncardiac, nonobstetric surgery, the researchers queried the University of Washington database for all PHTN seen in preoperative anesthesia clinic for noncardiac, nonobstetric procedures from April 2007 through September 2013. Inclusion criteria required an echocardiogram less than 1 year prior to the procedure and available patient-reported functional status, which was defined as less than four metabolic equivalents (METS) in exercise testing or four METS or greater. Dr. Shah, formerly a resident in the University of Washington’s department of anesthesiology and pain medicine, and his associates used univariate analyses to compare functional status with echocardiographic findings, complication rates, and length of stay (LOS). At the meeting he presented results from 294 patients evaluated to date: 143 with normal functional status and 151 with poor functional status. Their mean age was 62 years, and 51% of patients were female.
Compared with their counterparts with normal functional status, patients with poor functional status trended toward a higher complication rate at hospital discharge (14.6% vs. 7%, respectively; P = .041) and had a higher cumulative rate of complications (33 vs. 15; P = .035). However, no association between functional status and complications was observed 30 days postoperatively.
Patients with poor functional status had a significantly longer average LOS, compared with patients with normal functional status (7.21 vs. 4.73 days; P = .047). Open surgical approach was also an independent predictor of increased LOS (odds ratio 2.39; P = .005). No significant independent predictors of complications were observed at discharge or 30 days postoperatively.
“Going forward, the goal is to use these data to create a risk stratification algorithm to figure out: Does a patient with good functional status and pulmonary hypertension undergoing toe surgery, for example, really need an echocardiogram before getting surgery?” said Dr. Shah said, who is now an anesthesiology fellow at Boston Children’s Hospital. “Hopefully we can show that using these risk stratification algorithms can decrease the costs and decrease the time to actually getting surgery.”
The researchers reported having no financial disclosures.
AT THE ASA ANNUAL MEETING
Key clinical point:Poor self-reported exercise tolerance by patients with pulmonary hypertension is associated with multiple comorbidities and increased hospital length of stay.
Major finding: Compared with their counterparts with normal functional status, patients with poor functional status trended toward a higher complication rate at hospital discharge (14.6% vs. 7%, respectively; P = .041) and had a higher cumulative rate of complications (33 vs. 15; P = .035).
Data source: A study 294 PHTN patients seen in preoperative anesthesia clinic at the University of Washington for non-cardiac, nonobstetric procedures from April 2007 through September 2013.
Disclosures: The researchers reported having no financial disclosures.
Reslizumab: FDA panel recommends approval for adults, but not children
SILVER SPRING, MD. Members of the FDA Pulmonary-Allergy Drugs Advisory Committee voted 11-3 on Dec. 9 to recommend the approval of reslizumab, a humanized monoclonal antibody, for the treatment of asthma and elevated blood eosinophils in patients aged 18 years and older but did not recommend approval for children aged 12-17 years.
The advisers were tasked to consider a dosage of 3 mg/kg of reslizumab, administered intravenously once every 4 weeks for the management of severe asthma. The lack of a recommendation for the pediatric population is because of what panel members considered to be insufficient data culled from a small sample population (19 patients), along with results that did not provide enough evidence that the treatment was of significant benefit to adolescents.
There were “limited data” presented to support use of reslizumab in pediatrics, and “all the evidence was going in the wrong direction,” according to panel member Erica H. Brittain, Ph.D., of the National Institute of Allergy and Infectious Diseases, Bethesda, Md.
Panelist Dr. Thomas A.E. Platts-Mills, professor of medicine at the University of Virginia, Charlottesville, added that a larger study is needed in order to more accurately assess the drug’s efficacy, and should also include patients younger than the age of 12 years.
Both of the questions posited to the panel by the FDA regarding reslizumab approval for pediatric patients, “Do the efficacy data provide substantial evidence of a clinically meaningful benefit?” and “Do the available efficacy and safety data support approval of reslizumab?” received unanimous “No” votes from the 14-member voting panel.
The advisory panel members considered data from five separate trials evaluating the safety and efficacy of reslizumab to be marketed as Cinqair by Teva Pharmaceuticals. Those trials included two 16-week lung-function studies examining forced expiratory volume in 1 second (FEV1), two year-long asthma exacerbation studies, and an open-label safety extension study.
Advisers generally agreed that reslizumab demonstrated substantial improvement in FEV1 and asthma exacerbation in the adult population. Specifically, in the two exacerbation studies, clinical asthma exacerbations did not occur at all over the 12-month study period in 61% and 73% of subjects on reslizumab, vs. 44% and 52% of subjects in the control cohort, respectively, with FEV1 dropping.
However, panelists voiced their concern about the risk of muscle toxicity and, especially, anaphylaxis. In a presentation regarding the treatment’s immunogenicity issues, João A. Pedras-Vasconcelos, Ph.D., of the FDA Office of Pharmaceutical Quality, cautioned that enough work was not done by the sponsors to “thoroughly investigate [the] root causes of anaphylaxis.”
Ultimately, the advisory committee largely agreed that the unmet need for reslizumab outweighed the potential risks associated with it.
In casting his “yes” vote regarding the adequacy of reslizumab’s safety profile, panel chair Dr. Dennis R. Ownby, professor of pediatrics at Georgia Regents University in Augusta, admitted that he was “reluctant” to endorse reslizumab, but said that he believes “this is a drug that clinicians will use very cautiously, [so] I’m placing faith on our practicing physicians” to prescribe the drug responsibly.
Advisers voted 11-3 to recommend approval of reslizumab as a safe and effective treatment of severe asthma and elevated blood eosinophils. Approval of reslizumab would make it the third monoclonal antibody to be FDA approved to treat asthma.
The FDA is not required to follow the advice of its advisory panels, but often does. No members of the panel reported any relevant financial conflicts of interest.
SILVER SPRING, MD. Members of the FDA Pulmonary-Allergy Drugs Advisory Committee voted 11-3 on Dec. 9 to recommend the approval of reslizumab, a humanized monoclonal antibody, for the treatment of asthma and elevated blood eosinophils in patients aged 18 years and older but did not recommend approval for children aged 12-17 years.
The advisers were tasked to consider a dosage of 3 mg/kg of reslizumab, administered intravenously once every 4 weeks for the management of severe asthma. The lack of a recommendation for the pediatric population is because of what panel members considered to be insufficient data culled from a small sample population (19 patients), along with results that did not provide enough evidence that the treatment was of significant benefit to adolescents.
There were “limited data” presented to support use of reslizumab in pediatrics, and “all the evidence was going in the wrong direction,” according to panel member Erica H. Brittain, Ph.D., of the National Institute of Allergy and Infectious Diseases, Bethesda, Md.
Panelist Dr. Thomas A.E. Platts-Mills, professor of medicine at the University of Virginia, Charlottesville, added that a larger study is needed in order to more accurately assess the drug’s efficacy, and should also include patients younger than the age of 12 years.
Both of the questions posited to the panel by the FDA regarding reslizumab approval for pediatric patients, “Do the efficacy data provide substantial evidence of a clinically meaningful benefit?” and “Do the available efficacy and safety data support approval of reslizumab?” received unanimous “No” votes from the 14-member voting panel.
The advisory panel members considered data from five separate trials evaluating the safety and efficacy of reslizumab to be marketed as Cinqair by Teva Pharmaceuticals. Those trials included two 16-week lung-function studies examining forced expiratory volume in 1 second (FEV1), two year-long asthma exacerbation studies, and an open-label safety extension study.
Advisers generally agreed that reslizumab demonstrated substantial improvement in FEV1 and asthma exacerbation in the adult population. Specifically, in the two exacerbation studies, clinical asthma exacerbations did not occur at all over the 12-month study period in 61% and 73% of subjects on reslizumab, vs. 44% and 52% of subjects in the control cohort, respectively, with FEV1 dropping.
However, panelists voiced their concern about the risk of muscle toxicity and, especially, anaphylaxis. In a presentation regarding the treatment’s immunogenicity issues, João A. Pedras-Vasconcelos, Ph.D., of the FDA Office of Pharmaceutical Quality, cautioned that enough work was not done by the sponsors to “thoroughly investigate [the] root causes of anaphylaxis.”
Ultimately, the advisory committee largely agreed that the unmet need for reslizumab outweighed the potential risks associated with it.
In casting his “yes” vote regarding the adequacy of reslizumab’s safety profile, panel chair Dr. Dennis R. Ownby, professor of pediatrics at Georgia Regents University in Augusta, admitted that he was “reluctant” to endorse reslizumab, but said that he believes “this is a drug that clinicians will use very cautiously, [so] I’m placing faith on our practicing physicians” to prescribe the drug responsibly.
Advisers voted 11-3 to recommend approval of reslizumab as a safe and effective treatment of severe asthma and elevated blood eosinophils. Approval of reslizumab would make it the third monoclonal antibody to be FDA approved to treat asthma.
The FDA is not required to follow the advice of its advisory panels, but often does. No members of the panel reported any relevant financial conflicts of interest.
SILVER SPRING, MD. Members of the FDA Pulmonary-Allergy Drugs Advisory Committee voted 11-3 on Dec. 9 to recommend the approval of reslizumab, a humanized monoclonal antibody, for the treatment of asthma and elevated blood eosinophils in patients aged 18 years and older but did not recommend approval for children aged 12-17 years.
The advisers were tasked to consider a dosage of 3 mg/kg of reslizumab, administered intravenously once every 4 weeks for the management of severe asthma. The lack of a recommendation for the pediatric population is because of what panel members considered to be insufficient data culled from a small sample population (19 patients), along with results that did not provide enough evidence that the treatment was of significant benefit to adolescents.
There were “limited data” presented to support use of reslizumab in pediatrics, and “all the evidence was going in the wrong direction,” according to panel member Erica H. Brittain, Ph.D., of the National Institute of Allergy and Infectious Diseases, Bethesda, Md.
Panelist Dr. Thomas A.E. Platts-Mills, professor of medicine at the University of Virginia, Charlottesville, added that a larger study is needed in order to more accurately assess the drug’s efficacy, and should also include patients younger than the age of 12 years.
Both of the questions posited to the panel by the FDA regarding reslizumab approval for pediatric patients, “Do the efficacy data provide substantial evidence of a clinically meaningful benefit?” and “Do the available efficacy and safety data support approval of reslizumab?” received unanimous “No” votes from the 14-member voting panel.
The advisory panel members considered data from five separate trials evaluating the safety and efficacy of reslizumab to be marketed as Cinqair by Teva Pharmaceuticals. Those trials included two 16-week lung-function studies examining forced expiratory volume in 1 second (FEV1), two year-long asthma exacerbation studies, and an open-label safety extension study.
Advisers generally agreed that reslizumab demonstrated substantial improvement in FEV1 and asthma exacerbation in the adult population. Specifically, in the two exacerbation studies, clinical asthma exacerbations did not occur at all over the 12-month study period in 61% and 73% of subjects on reslizumab, vs. 44% and 52% of subjects in the control cohort, respectively, with FEV1 dropping.
However, panelists voiced their concern about the risk of muscle toxicity and, especially, anaphylaxis. In a presentation regarding the treatment’s immunogenicity issues, João A. Pedras-Vasconcelos, Ph.D., of the FDA Office of Pharmaceutical Quality, cautioned that enough work was not done by the sponsors to “thoroughly investigate [the] root causes of anaphylaxis.”
Ultimately, the advisory committee largely agreed that the unmet need for reslizumab outweighed the potential risks associated with it.
In casting his “yes” vote regarding the adequacy of reslizumab’s safety profile, panel chair Dr. Dennis R. Ownby, professor of pediatrics at Georgia Regents University in Augusta, admitted that he was “reluctant” to endorse reslizumab, but said that he believes “this is a drug that clinicians will use very cautiously, [so] I’m placing faith on our practicing physicians” to prescribe the drug responsibly.
Advisers voted 11-3 to recommend approval of reslizumab as a safe and effective treatment of severe asthma and elevated blood eosinophils. Approval of reslizumab would make it the third monoclonal antibody to be FDA approved to treat asthma.
The FDA is not required to follow the advice of its advisory panels, but often does. No members of the panel reported any relevant financial conflicts of interest.
AT AN FDA ADVISORY COMMITTEE MEETING