User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Pneumococcal serotypes likely to cause invasive disease dropped after PCV13 introduced
The number of pneumococcal serotypes with high potential for causing invasive disease decreased in France after the introduction of the 13-valent pneumococcal conjugate vaccine (PCV13), according to a study published in Vaccine by Dr. Emmanuelle Varon of the Hospital Européen Georges Pompidou in Paris and her colleagues.
Only two nonvaccine serotypes, 24F and 12F, had a high invasive disease potential in children under the age of 2 years after PCV13 was made available in 2010, researchers found. This is a decrease from five serotypes – 7F, 3, 1, 24F and 19A – previously known to have had a high invasive disease potential, when children were offered a seven-valent vaccine (PCV7).
During two study periods, 2008-2009 (before PCV13) and 2012-2013 (after PCV13), researchers looked at 355 pneumococci isolated from 1,212 healthy children aged 6-24 months and from 569 children with invasive pneumococcal disease (IPD), including 166 with meningitis, 114 with pneumonia, and 289 with other IPDs. During the second study period, the number of pneumococcal isolates was reduced for overall IPD (53%), meningitis (37%), pneumonia (66%), and other IPDs (56%). PCV7 serotypes almost disappeared between the two time frames.
Ongoing surveys are needed “to further characterize the little-known [nonvaccine serotypes], which are still poorly represented,” the authors wrote.
Read the article in Vaccine (doi: 10.1016/j.vaccine.2015.10.015).
The number of pneumococcal serotypes with high potential for causing invasive disease decreased in France after the introduction of the 13-valent pneumococcal conjugate vaccine (PCV13), according to a study published in Vaccine by Dr. Emmanuelle Varon of the Hospital Européen Georges Pompidou in Paris and her colleagues.
Only two nonvaccine serotypes, 24F and 12F, had a high invasive disease potential in children under the age of 2 years after PCV13 was made available in 2010, researchers found. This is a decrease from five serotypes – 7F, 3, 1, 24F and 19A – previously known to have had a high invasive disease potential, when children were offered a seven-valent vaccine (PCV7).
During two study periods, 2008-2009 (before PCV13) and 2012-2013 (after PCV13), researchers looked at 355 pneumococci isolated from 1,212 healthy children aged 6-24 months and from 569 children with invasive pneumococcal disease (IPD), including 166 with meningitis, 114 with pneumonia, and 289 with other IPDs. During the second study period, the number of pneumococcal isolates was reduced for overall IPD (53%), meningitis (37%), pneumonia (66%), and other IPDs (56%). PCV7 serotypes almost disappeared between the two time frames.
Ongoing surveys are needed “to further characterize the little-known [nonvaccine serotypes], which are still poorly represented,” the authors wrote.
Read the article in Vaccine (doi: 10.1016/j.vaccine.2015.10.015).
The number of pneumococcal serotypes with high potential for causing invasive disease decreased in France after the introduction of the 13-valent pneumococcal conjugate vaccine (PCV13), according to a study published in Vaccine by Dr. Emmanuelle Varon of the Hospital Européen Georges Pompidou in Paris and her colleagues.
Only two nonvaccine serotypes, 24F and 12F, had a high invasive disease potential in children under the age of 2 years after PCV13 was made available in 2010, researchers found. This is a decrease from five serotypes – 7F, 3, 1, 24F and 19A – previously known to have had a high invasive disease potential, when children were offered a seven-valent vaccine (PCV7).
During two study periods, 2008-2009 (before PCV13) and 2012-2013 (after PCV13), researchers looked at 355 pneumococci isolated from 1,212 healthy children aged 6-24 months and from 569 children with invasive pneumococcal disease (IPD), including 166 with meningitis, 114 with pneumonia, and 289 with other IPDs. During the second study period, the number of pneumococcal isolates was reduced for overall IPD (53%), meningitis (37%), pneumonia (66%), and other IPDs (56%). PCV7 serotypes almost disappeared between the two time frames.
Ongoing surveys are needed “to further characterize the little-known [nonvaccine serotypes], which are still poorly represented,” the authors wrote.
Read the article in Vaccine (doi: 10.1016/j.vaccine.2015.10.015).
FROM VACCINE
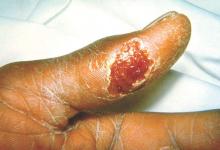
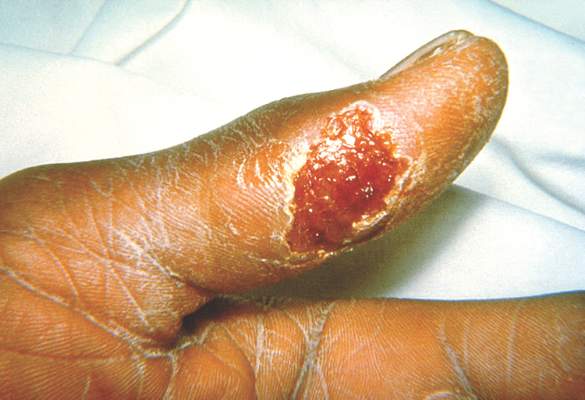
Tularemia outbreak in four U.S. states
One hundred cases of tularemia have been reported in 2015 among residents of Colorado, Nebraska, South Dakota, and Wyoming, according to a report by the U.S. Centers for Disease Control and Prevention. This represents a substantial increase in the annual mean number of cases (2 to 7) reported in each of these states during the past decade.
Although the cause for the increase in cases is unclear, Dr. Caitlin Pedati of the CDC’s Epidemic Intelligence Service and colleagues wrote in the Dec. 4 issue of Morbidity and Mortality Weekly Report that possible factors include increased rainfall promoting vegetation growth, pathogen survival, increased rodent and rabbit populations, and increased disease awareness (MMWR. 2015 Dec 4;64[47]:1317-8).
The infected patients ranged in age from 10 months to 89 years, and most commonly presented with the pneumonic form of respiratory disease, skin lesions with lymphadenopathy, and a general febrile illness without localizing signs. A total of 48 people were hospitalized and one died. Possible exposure routes included animal contact, environmental aerosolizing activities, and arthropod bites.
Clinical disease signs of tularemia could include fever and chills with muscle and joint pain, cough or difficulty breathing, swollen lymph nodes with or without skin lesions, conjunctivitis, pharyngitis, or abdominal pain with vomiting and diarrhea, the authors noted. Case fatality rates range from <2% to 24%, depending on the strain. Streptomycin is considered the drug of choice for treatment.
“Health care providers should be aware of the elevated risk for tularemia within these states and consider a diagnosis of tularemia in any person nationwide with compatible signs and symptoms,” wrote Dr. Pedati and colleagues.
Read the full article in Morbidity and Mortality Weekly Report.
One hundred cases of tularemia have been reported in 2015 among residents of Colorado, Nebraska, South Dakota, and Wyoming, according to a report by the U.S. Centers for Disease Control and Prevention. This represents a substantial increase in the annual mean number of cases (2 to 7) reported in each of these states during the past decade.
Although the cause for the increase in cases is unclear, Dr. Caitlin Pedati of the CDC’s Epidemic Intelligence Service and colleagues wrote in the Dec. 4 issue of Morbidity and Mortality Weekly Report that possible factors include increased rainfall promoting vegetation growth, pathogen survival, increased rodent and rabbit populations, and increased disease awareness (MMWR. 2015 Dec 4;64[47]:1317-8).
The infected patients ranged in age from 10 months to 89 years, and most commonly presented with the pneumonic form of respiratory disease, skin lesions with lymphadenopathy, and a general febrile illness without localizing signs. A total of 48 people were hospitalized and one died. Possible exposure routes included animal contact, environmental aerosolizing activities, and arthropod bites.
Clinical disease signs of tularemia could include fever and chills with muscle and joint pain, cough or difficulty breathing, swollen lymph nodes with or without skin lesions, conjunctivitis, pharyngitis, or abdominal pain with vomiting and diarrhea, the authors noted. Case fatality rates range from <2% to 24%, depending on the strain. Streptomycin is considered the drug of choice for treatment.
“Health care providers should be aware of the elevated risk for tularemia within these states and consider a diagnosis of tularemia in any person nationwide with compatible signs and symptoms,” wrote Dr. Pedati and colleagues.
Read the full article in Morbidity and Mortality Weekly Report.
One hundred cases of tularemia have been reported in 2015 among residents of Colorado, Nebraska, South Dakota, and Wyoming, according to a report by the U.S. Centers for Disease Control and Prevention. This represents a substantial increase in the annual mean number of cases (2 to 7) reported in each of these states during the past decade.
Although the cause for the increase in cases is unclear, Dr. Caitlin Pedati of the CDC’s Epidemic Intelligence Service and colleagues wrote in the Dec. 4 issue of Morbidity and Mortality Weekly Report that possible factors include increased rainfall promoting vegetation growth, pathogen survival, increased rodent and rabbit populations, and increased disease awareness (MMWR. 2015 Dec 4;64[47]:1317-8).
The infected patients ranged in age from 10 months to 89 years, and most commonly presented with the pneumonic form of respiratory disease, skin lesions with lymphadenopathy, and a general febrile illness without localizing signs. A total of 48 people were hospitalized and one died. Possible exposure routes included animal contact, environmental aerosolizing activities, and arthropod bites.
Clinical disease signs of tularemia could include fever and chills with muscle and joint pain, cough or difficulty breathing, swollen lymph nodes with or without skin lesions, conjunctivitis, pharyngitis, or abdominal pain with vomiting and diarrhea, the authors noted. Case fatality rates range from <2% to 24%, depending on the strain. Streptomycin is considered the drug of choice for treatment.
“Health care providers should be aware of the elevated risk for tularemia within these states and consider a diagnosis of tularemia in any person nationwide with compatible signs and symptoms,” wrote Dr. Pedati and colleagues.
Read the full article in Morbidity and Mortality Weekly Report.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
AHA: COPD Doubles Sudden Cardiac Death Risk in Hypertensives
ORLANDO – A second, confirmatory major study has shown that chronic obstructive pulmonary disease independently increases the risk of sudden cardiac death severalfold.
COPD was associated with a roughly twofold increased risk of sudden cardiac death (SCD) in hypertensive patients with COPD, compared with those without the pulmonary disease, in the Scandinavian Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial, Dr. Peter M. Okin reported at the American Heart Association scientific sessions.
Moreover, aggressive blood pressure lowering in the hypertensive COPD patients didn’t negate this risk, added Dr. Okin of Cornell University in New York.
The impetus for his secondary analysis of LIFE data was an earlier report from the landmark, population-based Rotterdam Heart Study. Among 1,615 participants with COPD, the age- and sex-adjusted risk of SCD was 1.34-fold greater than in nearly 12,000 controls. This increased SCD risk climbed to 2.12-fold during the first 2,000 days following diagnosis of COPD and reached 3.58-fold among those with frequent COPD exacerbations during this period (Eur Heart J. 2015 Jul 14;36[27]:1754-61).
Dr. Okin’s secondary analysis of LIFE data included 9,193 hypertensive subjects with ECG evidence of left ventricular hypertrophy who were randomized to lisinopril- or atenolol-based blood pressure lowering to a target of 140/90 mm Hg or less. A history of COPD was present in 385 patients (4.2%) at enrollment.
During a mean 4.8 years of prospective follow-up, 178 patients experienced SCD, a prespecified secondary endpoint in the LIFE trial. The incidence rate was 9 cases per 1,000 patient-years in those with COPD and 3.8 per 1,000 person-years in those without the pulmonary disease.
In a univariate analysis, a history of COPD was associated with a 2.36-fold increased risk of SCD during follow-up. In a multivariate analysis extensively adjusted for potential confounders – treatment arm, age, race, gender, history of atrial fibrillation, baseline serum creatinine and serum glucose, stroke or TIA, as well as on-treatment blood pressure, heart rate, QRS duration, HDL cholesterol level, use of a statin or hydrochlorothiazide, and incident MI or heart failure – COPD remained associated with a 1.82-fold increased risk of SCD, the cardiologist reported.
“These results suggest the need for additional studies to assess whether there are targeted therapies that can reduce the risk of SCD in patients with COPD,” he concluded.
As previously reported, the main finding in LIFE was that losartan conferred benefits beyond blood pressure control (Lancet. 2002 Mar 23;359[9311]:995-1003).
Dr. Okin reported serving as a consultant to Novartis.
ORLANDO – A second, confirmatory major study has shown that chronic obstructive pulmonary disease independently increases the risk of sudden cardiac death severalfold.
COPD was associated with a roughly twofold increased risk of sudden cardiac death (SCD) in hypertensive patients with COPD, compared with those without the pulmonary disease, in the Scandinavian Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial, Dr. Peter M. Okin reported at the American Heart Association scientific sessions.
Moreover, aggressive blood pressure lowering in the hypertensive COPD patients didn’t negate this risk, added Dr. Okin of Cornell University in New York.
The impetus for his secondary analysis of LIFE data was an earlier report from the landmark, population-based Rotterdam Heart Study. Among 1,615 participants with COPD, the age- and sex-adjusted risk of SCD was 1.34-fold greater than in nearly 12,000 controls. This increased SCD risk climbed to 2.12-fold during the first 2,000 days following diagnosis of COPD and reached 3.58-fold among those with frequent COPD exacerbations during this period (Eur Heart J. 2015 Jul 14;36[27]:1754-61).
Dr. Okin’s secondary analysis of LIFE data included 9,193 hypertensive subjects with ECG evidence of left ventricular hypertrophy who were randomized to lisinopril- or atenolol-based blood pressure lowering to a target of 140/90 mm Hg or less. A history of COPD was present in 385 patients (4.2%) at enrollment.
During a mean 4.8 years of prospective follow-up, 178 patients experienced SCD, a prespecified secondary endpoint in the LIFE trial. The incidence rate was 9 cases per 1,000 patient-years in those with COPD and 3.8 per 1,000 person-years in those without the pulmonary disease.
In a univariate analysis, a history of COPD was associated with a 2.36-fold increased risk of SCD during follow-up. In a multivariate analysis extensively adjusted for potential confounders – treatment arm, age, race, gender, history of atrial fibrillation, baseline serum creatinine and serum glucose, stroke or TIA, as well as on-treatment blood pressure, heart rate, QRS duration, HDL cholesterol level, use of a statin or hydrochlorothiazide, and incident MI or heart failure – COPD remained associated with a 1.82-fold increased risk of SCD, the cardiologist reported.
“These results suggest the need for additional studies to assess whether there are targeted therapies that can reduce the risk of SCD in patients with COPD,” he concluded.
As previously reported, the main finding in LIFE was that losartan conferred benefits beyond blood pressure control (Lancet. 2002 Mar 23;359[9311]:995-1003).
Dr. Okin reported serving as a consultant to Novartis.
ORLANDO – A second, confirmatory major study has shown that chronic obstructive pulmonary disease independently increases the risk of sudden cardiac death severalfold.
COPD was associated with a roughly twofold increased risk of sudden cardiac death (SCD) in hypertensive patients with COPD, compared with those without the pulmonary disease, in the Scandinavian Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial, Dr. Peter M. Okin reported at the American Heart Association scientific sessions.
Moreover, aggressive blood pressure lowering in the hypertensive COPD patients didn’t negate this risk, added Dr. Okin of Cornell University in New York.
The impetus for his secondary analysis of LIFE data was an earlier report from the landmark, population-based Rotterdam Heart Study. Among 1,615 participants with COPD, the age- and sex-adjusted risk of SCD was 1.34-fold greater than in nearly 12,000 controls. This increased SCD risk climbed to 2.12-fold during the first 2,000 days following diagnosis of COPD and reached 3.58-fold among those with frequent COPD exacerbations during this period (Eur Heart J. 2015 Jul 14;36[27]:1754-61).
Dr. Okin’s secondary analysis of LIFE data included 9,193 hypertensive subjects with ECG evidence of left ventricular hypertrophy who were randomized to lisinopril- or atenolol-based blood pressure lowering to a target of 140/90 mm Hg or less. A history of COPD was present in 385 patients (4.2%) at enrollment.
During a mean 4.8 years of prospective follow-up, 178 patients experienced SCD, a prespecified secondary endpoint in the LIFE trial. The incidence rate was 9 cases per 1,000 patient-years in those with COPD and 3.8 per 1,000 person-years in those without the pulmonary disease.
In a univariate analysis, a history of COPD was associated with a 2.36-fold increased risk of SCD during follow-up. In a multivariate analysis extensively adjusted for potential confounders – treatment arm, age, race, gender, history of atrial fibrillation, baseline serum creatinine and serum glucose, stroke or TIA, as well as on-treatment blood pressure, heart rate, QRS duration, HDL cholesterol level, use of a statin or hydrochlorothiazide, and incident MI or heart failure – COPD remained associated with a 1.82-fold increased risk of SCD, the cardiologist reported.
“These results suggest the need for additional studies to assess whether there are targeted therapies that can reduce the risk of SCD in patients with COPD,” he concluded.
As previously reported, the main finding in LIFE was that losartan conferred benefits beyond blood pressure control (Lancet. 2002 Mar 23;359[9311]:995-1003).
Dr. Okin reported serving as a consultant to Novartis.
AT THE AHA SCIENTIFIC SESSIONS
AHA: COPD doubles sudden cardiac death risk in hypertensives
ORLANDO – A second, confirmatory major study has shown that chronic obstructive pulmonary disease independently increases the risk of sudden cardiac death severalfold.
COPD was associated with a roughly twofold increased risk of sudden cardiac death (SCD) in hypertensive patients with COPD, compared with those without the pulmonary disease, in the Scandinavian Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial, Dr. Peter M. Okin reported at the American Heart Association scientific sessions.
Moreover, aggressive blood pressure lowering in the hypertensive COPD patients didn’t negate this risk, added Dr. Okin of Cornell University in New York.
The impetus for his secondary analysis of LIFE data was an earlier report from the landmark, population-based Rotterdam Heart Study. Among 1,615 participants with COPD, the age- and sex-adjusted risk of SCD was 1.34-fold greater than in nearly 12,000 controls. This increased SCD risk climbed to 2.12-fold during the first 2,000 days following diagnosis of COPD and reached 3.58-fold among those with frequent COPD exacerbations during this period (Eur Heart J. 2015 Jul 14;36[27]:1754-61).
Dr. Okin’s secondary analysis of LIFE data included 9,193 hypertensive subjects with ECG evidence of left ventricular hypertrophy who were randomized to lisinopril- or atenolol-based blood pressure lowering to a target of 140/90 mm Hg or less. A history of COPD was present in 385 patients (4.2%) at enrollment.
During a mean 4.8 years of prospective follow-up, 178 patients experienced SCD, a prespecified secondary endpoint in the LIFE trial. The incidence rate was 9 cases per 1,000 patient-years in those with COPD and 3.8 per 1,000 person-years in those without the pulmonary disease.
In a univariate analysis, a history of COPD was associated with a 2.36-fold increased risk of SCD during follow-up. In a multivariate analysis extensively adjusted for potential confounders – treatment arm, age, race, gender, history of atrial fibrillation, baseline serum creatinine and serum glucose, stroke or TIA, as well as on-treatment blood pressure, heart rate, QRS duration, HDL cholesterol level, use of a statin or hydrochlorothiazide, and incident MI or heart failure – COPD remained associated with a 1.82-fold increased risk of SCD, the cardiologist reported.
“These results suggest the need for additional studies to assess whether there are targeted therapies that can reduce the risk of SCD in patients with COPD,” he concluded.
As previously reported, the main finding in LIFE was that losartan conferred benefits beyond blood pressure control (Lancet. 2002 Mar 23;359[9311]:995-1003).
Dr. Okin reported serving as a consultant to Novartis.
ORLANDO – A second, confirmatory major study has shown that chronic obstructive pulmonary disease independently increases the risk of sudden cardiac death severalfold.
COPD was associated with a roughly twofold increased risk of sudden cardiac death (SCD) in hypertensive patients with COPD, compared with those without the pulmonary disease, in the Scandinavian Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial, Dr. Peter M. Okin reported at the American Heart Association scientific sessions.
Moreover, aggressive blood pressure lowering in the hypertensive COPD patients didn’t negate this risk, added Dr. Okin of Cornell University in New York.
The impetus for his secondary analysis of LIFE data was an earlier report from the landmark, population-based Rotterdam Heart Study. Among 1,615 participants with COPD, the age- and sex-adjusted risk of SCD was 1.34-fold greater than in nearly 12,000 controls. This increased SCD risk climbed to 2.12-fold during the first 2,000 days following diagnosis of COPD and reached 3.58-fold among those with frequent COPD exacerbations during this period (Eur Heart J. 2015 Jul 14;36[27]:1754-61).
Dr. Okin’s secondary analysis of LIFE data included 9,193 hypertensive subjects with ECG evidence of left ventricular hypertrophy who were randomized to lisinopril- or atenolol-based blood pressure lowering to a target of 140/90 mm Hg or less. A history of COPD was present in 385 patients (4.2%) at enrollment.
During a mean 4.8 years of prospective follow-up, 178 patients experienced SCD, a prespecified secondary endpoint in the LIFE trial. The incidence rate was 9 cases per 1,000 patient-years in those with COPD and 3.8 per 1,000 person-years in those without the pulmonary disease.
In a univariate analysis, a history of COPD was associated with a 2.36-fold increased risk of SCD during follow-up. In a multivariate analysis extensively adjusted for potential confounders – treatment arm, age, race, gender, history of atrial fibrillation, baseline serum creatinine and serum glucose, stroke or TIA, as well as on-treatment blood pressure, heart rate, QRS duration, HDL cholesterol level, use of a statin or hydrochlorothiazide, and incident MI or heart failure – COPD remained associated with a 1.82-fold increased risk of SCD, the cardiologist reported.
“These results suggest the need for additional studies to assess whether there are targeted therapies that can reduce the risk of SCD in patients with COPD,” he concluded.
As previously reported, the main finding in LIFE was that losartan conferred benefits beyond blood pressure control (Lancet. 2002 Mar 23;359[9311]:995-1003).
Dr. Okin reported serving as a consultant to Novartis.
ORLANDO – A second, confirmatory major study has shown that chronic obstructive pulmonary disease independently increases the risk of sudden cardiac death severalfold.
COPD was associated with a roughly twofold increased risk of sudden cardiac death (SCD) in hypertensive patients with COPD, compared with those without the pulmonary disease, in the Scandinavian Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial, Dr. Peter M. Okin reported at the American Heart Association scientific sessions.
Moreover, aggressive blood pressure lowering in the hypertensive COPD patients didn’t negate this risk, added Dr. Okin of Cornell University in New York.
The impetus for his secondary analysis of LIFE data was an earlier report from the landmark, population-based Rotterdam Heart Study. Among 1,615 participants with COPD, the age- and sex-adjusted risk of SCD was 1.34-fold greater than in nearly 12,000 controls. This increased SCD risk climbed to 2.12-fold during the first 2,000 days following diagnosis of COPD and reached 3.58-fold among those with frequent COPD exacerbations during this period (Eur Heart J. 2015 Jul 14;36[27]:1754-61).
Dr. Okin’s secondary analysis of LIFE data included 9,193 hypertensive subjects with ECG evidence of left ventricular hypertrophy who were randomized to lisinopril- or atenolol-based blood pressure lowering to a target of 140/90 mm Hg or less. A history of COPD was present in 385 patients (4.2%) at enrollment.
During a mean 4.8 years of prospective follow-up, 178 patients experienced SCD, a prespecified secondary endpoint in the LIFE trial. The incidence rate was 9 cases per 1,000 patient-years in those with COPD and 3.8 per 1,000 person-years in those without the pulmonary disease.
In a univariate analysis, a history of COPD was associated with a 2.36-fold increased risk of SCD during follow-up. In a multivariate analysis extensively adjusted for potential confounders – treatment arm, age, race, gender, history of atrial fibrillation, baseline serum creatinine and serum glucose, stroke or TIA, as well as on-treatment blood pressure, heart rate, QRS duration, HDL cholesterol level, use of a statin or hydrochlorothiazide, and incident MI or heart failure – COPD remained associated with a 1.82-fold increased risk of SCD, the cardiologist reported.
“These results suggest the need for additional studies to assess whether there are targeted therapies that can reduce the risk of SCD in patients with COPD,” he concluded.
As previously reported, the main finding in LIFE was that losartan conferred benefits beyond blood pressure control (Lancet. 2002 Mar 23;359[9311]:995-1003).
Dr. Okin reported serving as a consultant to Novartis.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Two large studies link chronic obstructive pulmonary disease with increased risk of sudden cardiac death.
Major finding: Patients with COPD and hypertension had nearly a twofold increased risk of sudden cardiac death, compared with hypertensives without the pulmonary disease.
Data source: This was a secondary analysis comparing sudden cardiac death rates in 385 hypertensive patients with and nearly 12,000 without COPD, all participants in the LIFE trial.
Disclosures: The presenter reported serving as a consultant to Novartis.
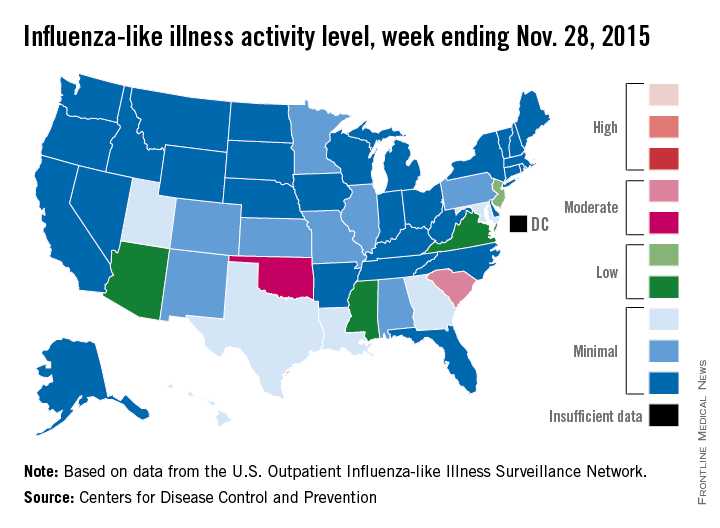
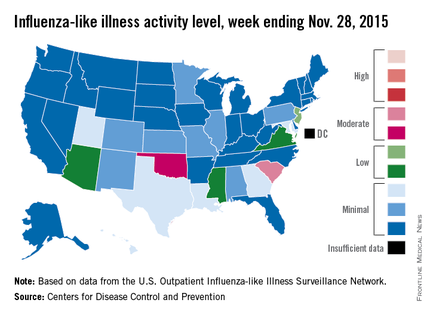
‘Moderate’ flu activity seen in two U.S. states
Two U.S. states experienced “moderate” activity of influenza-like illness for the week ending Nov. 28, 2015 – week 7 of the 2015-2016 flu season – the Centers for Disease Control and Prevention reported Dec. 4.
South Carolina had the highest (level 7) activity for the week, with Oklahoma joined by Puerto Rico at level 6. New Jersey was at the highest level (level 5) of “low” activity, while Arizona, Mississippi, and Virginia were a notch lower (level 4) but still in the “low” zone. All told, 20 states had flu activity of level 2 or higher, according to the CDC.
There were no influenza-associated pediatric deaths reported during the week, with two such deaths reported for the 2015-2016 season so far. For week 7 nationwide, 1.9% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network were the result of influenza-like illness – defined as a temperature of 100° F or greater and cough and/or sore throat – which is below the national baseline of 2.1%, the CDC said.
During week 7, 1.5% of the 11,288 specimens tested were positive for influenza, with 60% positive for influenza A and 40% positive for influenza B. For the season overall, 1.2% of the 102,675 specimens tested have been positive, with a 61/39 split for influenza A and B, the CDC noted.
Two U.S. states experienced “moderate” activity of influenza-like illness for the week ending Nov. 28, 2015 – week 7 of the 2015-2016 flu season – the Centers for Disease Control and Prevention reported Dec. 4.
South Carolina had the highest (level 7) activity for the week, with Oklahoma joined by Puerto Rico at level 6. New Jersey was at the highest level (level 5) of “low” activity, while Arizona, Mississippi, and Virginia were a notch lower (level 4) but still in the “low” zone. All told, 20 states had flu activity of level 2 or higher, according to the CDC.

There were no influenza-associated pediatric deaths reported during the week, with two such deaths reported for the 2015-2016 season so far. For week 7 nationwide, 1.9% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network were the result of influenza-like illness – defined as a temperature of 100° F or greater and cough and/or sore throat – which is below the national baseline of 2.1%, the CDC said.
During week 7, 1.5% of the 11,288 specimens tested were positive for influenza, with 60% positive for influenza A and 40% positive for influenza B. For the season overall, 1.2% of the 102,675 specimens tested have been positive, with a 61/39 split for influenza A and B, the CDC noted.
Two U.S. states experienced “moderate” activity of influenza-like illness for the week ending Nov. 28, 2015 – week 7 of the 2015-2016 flu season – the Centers for Disease Control and Prevention reported Dec. 4.
South Carolina had the highest (level 7) activity for the week, with Oklahoma joined by Puerto Rico at level 6. New Jersey was at the highest level (level 5) of “low” activity, while Arizona, Mississippi, and Virginia were a notch lower (level 4) but still in the “low” zone. All told, 20 states had flu activity of level 2 or higher, according to the CDC.

There were no influenza-associated pediatric deaths reported during the week, with two such deaths reported for the 2015-2016 season so far. For week 7 nationwide, 1.9% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network were the result of influenza-like illness – defined as a temperature of 100° F or greater and cough and/or sore throat – which is below the national baseline of 2.1%, the CDC said.
During week 7, 1.5% of the 11,288 specimens tested were positive for influenza, with 60% positive for influenza A and 40% positive for influenza B. For the season overall, 1.2% of the 102,675 specimens tested have been positive, with a 61/39 split for influenza A and B, the CDC noted.
Flu vaccines highly effective for pregnant women and their children
Administering flu vaccines to pregnant women during their second and third trimesters results in high seroprotection against all influenza strains for most women and for more than half of their newly born babies, reported Dr. M.P. Kostinov and colleagues at the I.I. Mechnikov Scientific Research Institute of Vaccines and Sera, at Ul’yanovsk State University, Moscow. The study was published in the Journal of Vaccines & Vaccination.
Researchers gave influenza vaccines (the Grippol Plus vaccine) to 27 women in their second trimesters and 21 women in their third trimesters of pregnancy during 2010-2012. Each 0.5-mL dose of the preservative-free vaccine contained antigens of the following strains: A/California/7/2009/H1N1/v-like (5 mcg), A/H3N2/(Victoria)-like (5 mcg), and B/Brisbane-like (5 mcg) flu.
Within 1 month after vaccination, the seroprotection rate against all influenza strains was above the recommended threshold level of 1:40 in more than 70% of pregnant women. A gradual decrease in the seroprotection rates against all three influenza strains was reported in the postpartum period.
In infants, protective levels of antibodies were detected within 2-3 days of delivery and ranged from 52% to 62% regardless of the trimester when the vaccination was given. Within 3 months, this seroprotection decreased, and within 6 months it disappeared. The mothers’ protective levels against vaccine strains were 46%-65% after delivery.
Read the article in the Journal of Vaccines & Vaccination (Pakhomov et al. J Vaccines Vaccin. 2015,6:5).
Administering flu vaccines to pregnant women during their second and third trimesters results in high seroprotection against all influenza strains for most women and for more than half of their newly born babies, reported Dr. M.P. Kostinov and colleagues at the I.I. Mechnikov Scientific Research Institute of Vaccines and Sera, at Ul’yanovsk State University, Moscow. The study was published in the Journal of Vaccines & Vaccination.
Researchers gave influenza vaccines (the Grippol Plus vaccine) to 27 women in their second trimesters and 21 women in their third trimesters of pregnancy during 2010-2012. Each 0.5-mL dose of the preservative-free vaccine contained antigens of the following strains: A/California/7/2009/H1N1/v-like (5 mcg), A/H3N2/(Victoria)-like (5 mcg), and B/Brisbane-like (5 mcg) flu.
Within 1 month after vaccination, the seroprotection rate against all influenza strains was above the recommended threshold level of 1:40 in more than 70% of pregnant women. A gradual decrease in the seroprotection rates against all three influenza strains was reported in the postpartum period.
In infants, protective levels of antibodies were detected within 2-3 days of delivery and ranged from 52% to 62% regardless of the trimester when the vaccination was given. Within 3 months, this seroprotection decreased, and within 6 months it disappeared. The mothers’ protective levels against vaccine strains were 46%-65% after delivery.
Read the article in the Journal of Vaccines & Vaccination (Pakhomov et al. J Vaccines Vaccin. 2015,6:5).
Administering flu vaccines to pregnant women during their second and third trimesters results in high seroprotection against all influenza strains for most women and for more than half of their newly born babies, reported Dr. M.P. Kostinov and colleagues at the I.I. Mechnikov Scientific Research Institute of Vaccines and Sera, at Ul’yanovsk State University, Moscow. The study was published in the Journal of Vaccines & Vaccination.
Researchers gave influenza vaccines (the Grippol Plus vaccine) to 27 women in their second trimesters and 21 women in their third trimesters of pregnancy during 2010-2012. Each 0.5-mL dose of the preservative-free vaccine contained antigens of the following strains: A/California/7/2009/H1N1/v-like (5 mcg), A/H3N2/(Victoria)-like (5 mcg), and B/Brisbane-like (5 mcg) flu.
Within 1 month after vaccination, the seroprotection rate against all influenza strains was above the recommended threshold level of 1:40 in more than 70% of pregnant women. A gradual decrease in the seroprotection rates against all three influenza strains was reported in the postpartum period.
In infants, protective levels of antibodies were detected within 2-3 days of delivery and ranged from 52% to 62% regardless of the trimester when the vaccination was given. Within 3 months, this seroprotection decreased, and within 6 months it disappeared. The mothers’ protective levels against vaccine strains were 46%-65% after delivery.
Read the article in the Journal of Vaccines & Vaccination (Pakhomov et al. J Vaccines Vaccin. 2015,6:5).
FROM JOURNAL OF VACCINES & VACCINATION
Study: Exposure history critical to design of universal flu vaccine
In a study with implications for the development of new influenza vaccine strategies, researchers discovered that – among patients who received the 2009 H1N1 influenza vaccine – individuals with low levels of H1N1-specific antibodies prior to vaccination produced a more broadly protective immune response against the influenza virus than patients with high levels of H1N1-specific antibodies prior to vaccination.
A research team led by Patrick C. Wilson, Ph.D., of the Knapp Center for Lupus and Immunology Research at the University of Chicago, studied the B cell response in patients who received the pandemic 2009 H1N1 vaccine 2 years in a row and had varied histories of influenza exposure. All patients were 18 years or older, healthy, and had not received the yearly influenza vaccine prior to participating in the study. The researchers compared the patients’ “vaccine-induced plasmablast response upon first vaccination with the pandemic H1N1 strain in 2009-2010” with the patients’ plasmablast response upon revaccination with this same strain in 2010-2011 or 2011-2012. Each of the 21 study participants provided the researchers with at least four H1N1-specific plasmablasts.
The researchers “analyzed the immunoglobulin regions, strain specificity, and functional properties of the antibodies produced by this plasmablast population at the single-cell level across multiple years,” which allowed them to directly evaluate the effect of immune memory on the specificity of the current response to the virus.
Among the study’s findings was that “only individuals with low preexisting serological levels of pandemic H1N1 specific antibodies generated a broadly neutralizing plasmablast response directed toward the [hemagglutinin] stalk,” which is part of the hemagglutinin protein located on the surface of the influenza virus.
“[W]e demonstrate that the immune subdominance of the [hemagglutinin] stalk is a function of both the poor accessibility to the broadly protective epitopes and the inherent polyreactivity of the antibodies that can bind. We conclude that immunological memory profoundly shapes the viral epitopes targeted upon exposure with divergent influenza strains and determines the likelihood of generating a broadly protective response,” said Dr. Wilson and his coauthors. The authors reported no conflicts of interest.
Read the full study in Science Translational Medicine (doi: 10.1126/scitranslmed.aad0522).
In a study with implications for the development of new influenza vaccine strategies, researchers discovered that – among patients who received the 2009 H1N1 influenza vaccine – individuals with low levels of H1N1-specific antibodies prior to vaccination produced a more broadly protective immune response against the influenza virus than patients with high levels of H1N1-specific antibodies prior to vaccination.
A research team led by Patrick C. Wilson, Ph.D., of the Knapp Center for Lupus and Immunology Research at the University of Chicago, studied the B cell response in patients who received the pandemic 2009 H1N1 vaccine 2 years in a row and had varied histories of influenza exposure. All patients were 18 years or older, healthy, and had not received the yearly influenza vaccine prior to participating in the study. The researchers compared the patients’ “vaccine-induced plasmablast response upon first vaccination with the pandemic H1N1 strain in 2009-2010” with the patients’ plasmablast response upon revaccination with this same strain in 2010-2011 or 2011-2012. Each of the 21 study participants provided the researchers with at least four H1N1-specific plasmablasts.
The researchers “analyzed the immunoglobulin regions, strain specificity, and functional properties of the antibodies produced by this plasmablast population at the single-cell level across multiple years,” which allowed them to directly evaluate the effect of immune memory on the specificity of the current response to the virus.
Among the study’s findings was that “only individuals with low preexisting serological levels of pandemic H1N1 specific antibodies generated a broadly neutralizing plasmablast response directed toward the [hemagglutinin] stalk,” which is part of the hemagglutinin protein located on the surface of the influenza virus.
“[W]e demonstrate that the immune subdominance of the [hemagglutinin] stalk is a function of both the poor accessibility to the broadly protective epitopes and the inherent polyreactivity of the antibodies that can bind. We conclude that immunological memory profoundly shapes the viral epitopes targeted upon exposure with divergent influenza strains and determines the likelihood of generating a broadly protective response,” said Dr. Wilson and his coauthors. The authors reported no conflicts of interest.
Read the full study in Science Translational Medicine (doi: 10.1126/scitranslmed.aad0522).
In a study with implications for the development of new influenza vaccine strategies, researchers discovered that – among patients who received the 2009 H1N1 influenza vaccine – individuals with low levels of H1N1-specific antibodies prior to vaccination produced a more broadly protective immune response against the influenza virus than patients with high levels of H1N1-specific antibodies prior to vaccination.
A research team led by Patrick C. Wilson, Ph.D., of the Knapp Center for Lupus and Immunology Research at the University of Chicago, studied the B cell response in patients who received the pandemic 2009 H1N1 vaccine 2 years in a row and had varied histories of influenza exposure. All patients were 18 years or older, healthy, and had not received the yearly influenza vaccine prior to participating in the study. The researchers compared the patients’ “vaccine-induced plasmablast response upon first vaccination with the pandemic H1N1 strain in 2009-2010” with the patients’ plasmablast response upon revaccination with this same strain in 2010-2011 or 2011-2012. Each of the 21 study participants provided the researchers with at least four H1N1-specific plasmablasts.
The researchers “analyzed the immunoglobulin regions, strain specificity, and functional properties of the antibodies produced by this plasmablast population at the single-cell level across multiple years,” which allowed them to directly evaluate the effect of immune memory on the specificity of the current response to the virus.
Among the study’s findings was that “only individuals with low preexisting serological levels of pandemic H1N1 specific antibodies generated a broadly neutralizing plasmablast response directed toward the [hemagglutinin] stalk,” which is part of the hemagglutinin protein located on the surface of the influenza virus.
“[W]e demonstrate that the immune subdominance of the [hemagglutinin] stalk is a function of both the poor accessibility to the broadly protective epitopes and the inherent polyreactivity of the antibodies that can bind. We conclude that immunological memory profoundly shapes the viral epitopes targeted upon exposure with divergent influenza strains and determines the likelihood of generating a broadly protective response,” said Dr. Wilson and his coauthors. The authors reported no conflicts of interest.
Read the full study in Science Translational Medicine (doi: 10.1126/scitranslmed.aad0522).
FROM SCIENCE TRANSLATIONAL MEDICINE
CPAP, oral devices reduced blood pressure in sleep apnea
Continuous positive airway pressure (CPAP) and mandibular advancement devices (MADs) both achieved similar reductions in blood pressure in individuals with obstructive sleep apnea, compared with inactive controls.
In a systematic review and meta-analysis of 51 studies involving 4,888 patients, researchers found that CPAP use was associated with a significant mean systolic blood pressure reduction of 2.5 mm Hg and mean diastolic reduction of 2 mm Hg, compared with inactive controls. Each 1-hour increase in mean CPAP use was associated with a significant additional 1.5 mm Hg systolic and 0.9 mm Hg diastolic blood pressure reduction.
Similarly, MADs were associated with a significant 2.1 mm Hg reduction in systolic pressure and 1.9 mm Hg reduction in diastolic pressure, compared with inactive controls.
“This is partly in contrast to a previous meta-analysis, which did not find a beneficial association with MADs, perhaps due to including only two [randomized controlled trials] and thus having inadequate power to detect a difference,” wrote Daniel J. Bratton, Ph.D., of the department of pulmonology, University Hospital, Zurich, and coauthors (JAMA. 2015 Dec 1;314:2280-93).
Overall, the authors found no significant differences between CPAP and MADs in the associated changes in systolic or diastolic blood pressure, although they noted that CPAP showed the strongest association with systolic blood pressure reductions.
The Swiss National Science Foundation and the University of Zurich supported the study. The authors declared no conflicts of interest.
Continuous positive airway pressure (CPAP) and mandibular advancement devices (MADs) both achieved similar reductions in blood pressure in individuals with obstructive sleep apnea, compared with inactive controls.
In a systematic review and meta-analysis of 51 studies involving 4,888 patients, researchers found that CPAP use was associated with a significant mean systolic blood pressure reduction of 2.5 mm Hg and mean diastolic reduction of 2 mm Hg, compared with inactive controls. Each 1-hour increase in mean CPAP use was associated with a significant additional 1.5 mm Hg systolic and 0.9 mm Hg diastolic blood pressure reduction.
Similarly, MADs were associated with a significant 2.1 mm Hg reduction in systolic pressure and 1.9 mm Hg reduction in diastolic pressure, compared with inactive controls.
“This is partly in contrast to a previous meta-analysis, which did not find a beneficial association with MADs, perhaps due to including only two [randomized controlled trials] and thus having inadequate power to detect a difference,” wrote Daniel J. Bratton, Ph.D., of the department of pulmonology, University Hospital, Zurich, and coauthors (JAMA. 2015 Dec 1;314:2280-93).
Overall, the authors found no significant differences between CPAP and MADs in the associated changes in systolic or diastolic blood pressure, although they noted that CPAP showed the strongest association with systolic blood pressure reductions.
The Swiss National Science Foundation and the University of Zurich supported the study. The authors declared no conflicts of interest.
Continuous positive airway pressure (CPAP) and mandibular advancement devices (MADs) both achieved similar reductions in blood pressure in individuals with obstructive sleep apnea, compared with inactive controls.
In a systematic review and meta-analysis of 51 studies involving 4,888 patients, researchers found that CPAP use was associated with a significant mean systolic blood pressure reduction of 2.5 mm Hg and mean diastolic reduction of 2 mm Hg, compared with inactive controls. Each 1-hour increase in mean CPAP use was associated with a significant additional 1.5 mm Hg systolic and 0.9 mm Hg diastolic blood pressure reduction.
Similarly, MADs were associated with a significant 2.1 mm Hg reduction in systolic pressure and 1.9 mm Hg reduction in diastolic pressure, compared with inactive controls.
“This is partly in contrast to a previous meta-analysis, which did not find a beneficial association with MADs, perhaps due to including only two [randomized controlled trials] and thus having inadequate power to detect a difference,” wrote Daniel J. Bratton, Ph.D., of the department of pulmonology, University Hospital, Zurich, and coauthors (JAMA. 2015 Dec 1;314:2280-93).
Overall, the authors found no significant differences between CPAP and MADs in the associated changes in systolic or diastolic blood pressure, although they noted that CPAP showed the strongest association with systolic blood pressure reductions.
The Swiss National Science Foundation and the University of Zurich supported the study. The authors declared no conflicts of interest.
FROM JAMA
Key clinical point: Continuous positive airway pressure and mandibular advancement devices both achieve similar reductions in blood pressure in individuals with obstructive sleep apnea.
Major finding: CPAP use was associated with a mean systolic blood pressure reduction of 2.5 mm Hg, and MADs were associated with a 2.1 mm Hg reduction, compared with inactive controls.
Data source: A systematic review and meta-analysis of 51 studies involving 4,888 patients.
Disclosures: The Swiss National Science Foundation and the University of Zurich supported the study. The authors declared no conflicts of interest.
Home apnea monitors—when to discontinue use
› Tell parents that home apnea monitoring has not been shown to prevent sudden unexpected death in infants. C
› Consider discontinuing home apnea monitoring for infants at risk for recurrent apnea at approximately 43 weeks postmenstrual age or after the cessation of extreme episodes. B
› Educate parents about steps they can take to reduce their child’s risk of sudden infant death syndrome, such as putting him to sleep on his back, breastfeeding him, and refraining from sleeping in the same bed with him. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year, more than one in every 100 infants are born at less than 32 weeks postmenstrual age.1 In industrialized countries, many of these infants are discharged from the neonatal intensive care unit (NICU) with home apnea monitors,1 which alert caregivers to episodes of apnea and bradycardia, while also capturing and storing data surrounding significant events for later analysis.2
Evidence supporting the use of home apnea monitoring is sparse, and recommendations highlight the need to use this technology sparingly and to discontinue use once it is no longer necessary (TABLE).3 Counseling parents is critical. It’s important to explain that home apnea monitoring can be used to help reduce the likelihood that a child will die in his or her sleep, but it affords users no “guarantees.” In addition, home apnea monitoring can adversely affect parents. Parents who use home apnea monitoring for their infants have been shown to have higher stress scores, greater levels of fatigue, and poorer health than parents of infants without home apnea monitors.4-8
As a family physician, you are likely to encounter home apnea monitoring in one of 3 scenarios: at the first visit after discharge by a premature infant who experienced apnea while hospitalized, at a follow-up visit after discharge from the hospital by an infant who experienced an apparent life-threatening event (ALTE), and at a follow-up visit by an infant whose sibling had died from sudden infant death syndrome (SIDS). This article presents case studies that illustrate each of these scenarios, and explains what to tell parents who ask about how long they should continue home apnea monitoring.
CASE 1 › Apnea of prematurity
Jacob is a newborn who is brought in to your clinic by his parents for an initial visit. The infant was born prematurely at 32 weeks and required a prolonged NICU stay. His mother says that Jacob spent 4 weeks there and was discharged home with home apnea monitoring. On exam, the infant has a monitor attached via a chest band. Jacob appears healthy and his exam is normal. The mother asks you how long her son should use the home monitor.
Pathologic apnea is a respiratory pause that lasts at least 20 seconds or is associated with cyanosis; abrupt, marked pallor or hypotonia; or bradycardia.2 Apnea of prematurity is present in almost all infants born at <29 weeks postmenstrual age or who weigh <1000 g.9 It is found in 54% of infants born at 30 to 31 weeks, 15% born at 32 to 33 weeks, and 7% of infants born at 34 to 35 weeks.10
Apnea of prematurity is primarily due to an immature respiratory control system, which results in impaired breathing regulation, immature respiratory responses to hypercapnia and hypoxia, and an exaggerated inhibitory response to stimulation of airway receptors.11-13 Although apnea of prematurity usually resolves by 36 to 40 weeks postmenstrual age, it often persists beyond 38 to 40 weeks in infants born before 28 weeks.10 In these infants, by 43 to 44 weeks postmenstrual age, the frequency of apneic episodes decreases to that of full-term infants.14
The differences in long-term outcomes of infants with apnea of prematurity vs infants without it are subtle, if present at all.14,15 There does seem to be a correlation between the number of days with apnea and poor outcomes. Neurodevelopmental impairment and death are more likely in neonates who experience a greater number of days with apnea episodes.16,17 However, apnea of prematurity is not associated with an increased risk of SIDS.18
According to the American Academy of Pediatrics (AAP), home apnea monitoring may be warranted for premature infants who are at high risk of recurrent episodes of apnea, bradycardia, and hypoxemia after hospital discharge.3 While there is general consensus that all infants born prior to 29 weeks meet this criterion, the use of home apnea monitors in older preterm infants varies significantly, and the decision to initiate monitoring in these patients is made by the discharging physician.3 Once initiated, the AAP recommends that the use of home apnea monitoring in this population be discontinued after approximately 43 weeks postmenstrual age or after the cessation of extreme episodes, whichever comes last.3
In Jacob’s case, the monitoring should be discontinued at approximately week 12 of life, or about age 3 months.
CASE 2 › Apparent life-threatening event
Sarah is brought to your office after being hospitalized for an ALTE. Her mother reports that she had witnessed her 13-day-old daughter not breathing for “about a minute.” Upon realizing what was happening, she “blew into the baby’s face,” whereupon Sarah awakened. The mother then called 911 and they went by ambulance to the emergency room. The newborn was admitted for observation overnight and received a thorough evaluation. She was discharged with a home apnea monitor.
You review the work-up and find nothing worrisome. Sarah is in a car seat attached to the apnea monitor with a chest strap. An examination of the child is normal. The mother asks you when they should stop using the home monitor.
An ALTE is “an event that is frightening to the observer and ... is characterized by some combination of apnea (central or occasionally obstructive), color change (usually cyanotic or pallid but occasionally erythematous or plethoric), marked change in muscle tone (usually marked limpness), choking, or gagging.”2 ALTE is a descriptive term, and not a definitive diagnosis.
The true incidence of ALTE is unknown, but is reported to be 0.5% to 6%; most events occur in children younger than age 1.19,20 The risk for ALTE is increased for premature infants, particularly those with respiratory syncytial virus or who had undergone general anesthesia; infants who feed rapidly, cough frequently, or choke during feeding; and male infants.19,21
The most common causes of ALTE (in descending order) are gastroesophageal reflux, seizure disorder, and lower respiratory tract infection.22 The etiology is unknown for about half of patients with ALTE.23
Tell parents that if their infant experiences an ALTE, they should seek medical attention without delay. The fear is that failing to respond to this concern will ultimately result in a sudden unexpected infant death, specifically as a result of SIDS.24
SIDS is very rare, occurring in only 40 per 100,000 births. One analysis found that children who die from SIDS and those who experience ALTE have very similar histories and clinical factors.25 Approximately 7% of infants who die from SIDS have had an ALTE.2 Overall, the long-term prognosis for infants who have had an ALTE is very good, although it depends on seriousness of the underlying etiology.8,26-28
Guidance on the effective use of home apnea monitors in infants who experience an ALTE is sparse. Despite this, the National Institutes of Health (NIH) Consensus Statement on Infantile Apnea and Home Monitoring2 and the American Academy of Pediatrics policy statement on apnea, sudden infant death syndrome, and home monitoring3 recommend the use of home apnea monitoring for certain infants who’ve had an ALTE. The NIH Consensus Statement specifies home monitoring for infants with one or more severe episodes of ALTEs that require mouth-to-mouth resuscitation or vigorous stimulation.2 There are no specific guidelines regarding the duration of monitoring.2,3
In Sarah’s case, home monitoring should be discontinued as soon as the mother is comfortable with the decision.
CASE 3 › Sudden infant death syndrome
The parents of a 2-month-old boy, Stephen, come to your office to establish care. They recently relocated and their previous care provider had prescribed a home apnea monitor because a child they’d had 3 years ago had died of SIDS. Stephen is in a car seat attached to the apnea monitor with a chest strap. Your examination of him is normal. Stephen’s parents would like to stop using the home monitor, and ask you if it’s safe to do so.
SIDS is the death of an infant or young child that is unexplained by history and in which postmortem examination fails to find an adequate explanation of cause of death.2 Since the introduction of the Back to Sleep campaign in the early 1990s, the incidence of SIDS has decreased by more than 50%.8 In 2013, approximately 1500 infant deaths were attributed to SIDS.24 Three-quarters of deaths due to SIDS occur between 2 to 4 months of age, and 95% of deaths occur before 9 months of age.29 Risk factors for SIDS include sleep environment (prone and side sleeping, bed sharing, soft bedding), prenatal and postnatal maternal tobacco use, exposure to tobacco smoke, maternal mental illness or substance abuse, male sex, poverty, prematurity, low birth weight (less than 2500 g), and no or poor prenatal care.30
The etiology of SIDS is unclear.31 The leading hypothesis is the “triple-risk model,” which proposes that death from SIDS is due to 3 overlapping factors: a vulnerable infant, a critical developmental period in homeostatic control, and an exogenous stressor.32
Although the NIH Consensus Statement suggests home apnea monitoring is indicated for infants who are siblings of 2 or more SIDS victims,2 more recent policy statements from the AAP recommend against using home apnea monitors to reduce the incidence of SIDS due to a lack of evidence.3,8
With this in mind, Stephen’s monitor should be discontinued and his parents should be educated on proven methods of preventing SIDS, including placing him on his back to sleep, breastfeeding him, letting him use a pacifier during sleep, and not sleeping in the same bed with him or overdressing him when putting him to sleep.3,8
CORRESPONDENCE
Allen Perkins, MD, MPH, Department of Family Medicine, University of South Alabama, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604; [email protected].
1. Centers for Disease Control and Prevention. Births and natality. Centers for Disease Control and Prevention/National Center for Health Statistics Web site. Available at: http://www.cdc.gov/nchs/fastats/births.htm. Accessed August 18, 2015.
2. National Institutes of Health Consensus Development Program. Infantile apnea and home monitoring. National Institutes of Health Consensus Development Statement. 1986. National Institutes of Health Consensus Development Program Web site. Available at: https://consensus.nih.gov/1986/1986InfantApneaMonitoring058html.htm. Accessed November 2, 2015.
3. Committee on Fetus and Newborn, American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics. 2003;111:914-917.
4. Kahn A, Sottiaux M, Appelboom-Fondu J, et al. Long-term development of children monitored as infants for an apparent lifethreatening event during sleep: a 10-year follow-up study. Pediatrics. 1989;83:668-673.
5. Vohr BR, Chen A, Garcia Coll C, et al. Mothers of preterm and full-term infants on home apnea monitors. Am J Dis Child. 1988;142:229-231.
6. Williams PD, Press A, Williams AR, et al. Fatigue in mothers of infants discharged to the home on apnea monitors. Appl Nurs Res. 1999;12:69-77.
7. Ahmann E, Wulff L, Meny RG. Home apnea monitoring and disruption in family life: a multidimensional controlled study. Am J Public Health. 1992;82:719-722.
8. Task Force on Sudden Infant Death Syndrome, Moon R. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:1030-1039.
9. Eichenwald EC, Aina A, Stark AR. Apnea frequently persists beyond term gestation in infants delivered at 24 to 28 weeks. Pediatrics. 1997;100:354-359.
10. Martin RJ, Abu-Shaweesh JM, Baird TM. Apnoea of prematurity. Paediatr Respir Rev. 2004;5:S377-S382.
11. Miller MJ, Martin RJ. Pathophysiology of apnea of prematurity. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004: 905-918.
12. Miller JM, Haxhiu MA, Martin RJ. Chemical control of breathing from fetal through newborn life. In: Matthew OP, ed. Respiratory Control and Disorders in the Newborn. New York, NY: Marcel Dekker; 2003:83-113.
13. Rigatto H. Control of breathing in fetal life and onset and control of breathing in the neonate. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004:890-899.
14. Koons AH, Mojica N, Jadeja N, et al. Neurodevelopmental outcome of infants with apnea of infancy. Am J Perinatol. 1993;10:208-211.
15. Perlman JM. Neurobehavioral deficits in premature graduates of intensive care—potential medical and neonatal environmental risk factors. Pediatrics. 2001;108:1339-1348.
16. Janvier A, Khairy M, Kokkotis A, et al. Apnea is associated with neurodevelopmental impairment in very low birth weight infants. J Perinatol. 2004;24:763-768.
17. Pillekamp F, Hermann C, Keller T, et al. Factors influencing apnea and bradycardia of prematurity—implications for neurodevelopment. Neonatology. 2007;91:155-161.
18. Hoffman HJ, Damus K, Hillman L, et al. Risk factors for SIDS. Results for the National Institutes of Child Health and Human Development SIDS Cooperative Epidemiological Study. Ann N Y Acad Sci. 1988;533:13-30.
19. Brooks JG. Apparent life-threatening events and apnea of infancy. Clin Perinatol. 1992;19:809-838.
20. Semmekrot BA, van Sleuwan BE, Engelberts AC, et al. Surveillance study of apparent life-threatening events (ALTE) in the Netherlands. Eur J Pediatr. 2010;169:229-236.
21. Carroll JL. Apparent Life Threatening Event (ALTE) assessment. Pediatr Pulmonol Suppl. 2004;26:108-109.
22. McGovern MC, Smith MB. Causes of apparent life threatening events in infants: a systematic review. Arch Dis Child. 2004;89:1043-1048.
23. Hall KL, Zalman B. Evaluation and management of apparent life-threatening events in children. Am Fam Physician. 2005;71:2301-2308.
24. Centers for Disease Control and Prevention. Sudden unexpected infant death and sudden infant death syndrome. Updated May 11, 2015. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/sids/data.htm. Accessed June 30, 2015.
25. Kahn A, Blum D, Hennart P, et al. A critical comparison of the history of sudden-death infants and infants hospitalized for nearmiss for SIDS. Eur J Pediatr. 1984;143:103-107.
26. Baroni MA. Apparent life-threatening events during infancy: a follow-up study of subsequent growth and development. J Dev Behav Pediatr. 1991;12:154-161.
27. Tirosh E, Kessel A, Jaffe M, et al. Outcome of idiopathic apparent life-threatening events: infant and mother perspectives. Pediatr Pulmonol. 1999;28:47-52.
28. Koons AH. Neurodevelopmental outcome in infants with apnea. N J Med. 1992;89:688-690.
29. Poets CF. Apnea of prematurity, sudden infant death syndrome, and apparent life-threatening events. In: Taussig LM, ed. Pediatric Respiratory Medicine. Philadelphia, PA: Mosby; 2008:413-434.
30. Adams SM, Ward CE, Garcia KL. Sudden infant death syndrome. Am Fam Physician. 2015;91:778-783.
31. Goldwater PN. A perspective on SIDS pathogenesis. The hypothesis: plausibility and evidence. BMC Med. 2011;9:64.
32. Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65:194-197.
› Tell parents that home apnea monitoring has not been shown to prevent sudden unexpected death in infants. C
› Consider discontinuing home apnea monitoring for infants at risk for recurrent apnea at approximately 43 weeks postmenstrual age or after the cessation of extreme episodes. B
› Educate parents about steps they can take to reduce their child’s risk of sudden infant death syndrome, such as putting him to sleep on his back, breastfeeding him, and refraining from sleeping in the same bed with him. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year, more than one in every 100 infants are born at less than 32 weeks postmenstrual age.1 In industrialized countries, many of these infants are discharged from the neonatal intensive care unit (NICU) with home apnea monitors,1 which alert caregivers to episodes of apnea and bradycardia, while also capturing and storing data surrounding significant events for later analysis.2
Evidence supporting the use of home apnea monitoring is sparse, and recommendations highlight the need to use this technology sparingly and to discontinue use once it is no longer necessary (TABLE).3 Counseling parents is critical. It’s important to explain that home apnea monitoring can be used to help reduce the likelihood that a child will die in his or her sleep, but it affords users no “guarantees.” In addition, home apnea monitoring can adversely affect parents. Parents who use home apnea monitoring for their infants have been shown to have higher stress scores, greater levels of fatigue, and poorer health than parents of infants without home apnea monitors.4-8
As a family physician, you are likely to encounter home apnea monitoring in one of 3 scenarios: at the first visit after discharge by a premature infant who experienced apnea while hospitalized, at a follow-up visit after discharge from the hospital by an infant who experienced an apparent life-threatening event (ALTE), and at a follow-up visit by an infant whose sibling had died from sudden infant death syndrome (SIDS). This article presents case studies that illustrate each of these scenarios, and explains what to tell parents who ask about how long they should continue home apnea monitoring.
CASE 1 › Apnea of prematurity
Jacob is a newborn who is brought in to your clinic by his parents for an initial visit. The infant was born prematurely at 32 weeks and required a prolonged NICU stay. His mother says that Jacob spent 4 weeks there and was discharged home with home apnea monitoring. On exam, the infant has a monitor attached via a chest band. Jacob appears healthy and his exam is normal. The mother asks you how long her son should use the home monitor.
Pathologic apnea is a respiratory pause that lasts at least 20 seconds or is associated with cyanosis; abrupt, marked pallor or hypotonia; or bradycardia.2 Apnea of prematurity is present in almost all infants born at <29 weeks postmenstrual age or who weigh <1000 g.9 It is found in 54% of infants born at 30 to 31 weeks, 15% born at 32 to 33 weeks, and 7% of infants born at 34 to 35 weeks.10
Apnea of prematurity is primarily due to an immature respiratory control system, which results in impaired breathing regulation, immature respiratory responses to hypercapnia and hypoxia, and an exaggerated inhibitory response to stimulation of airway receptors.11-13 Although apnea of prematurity usually resolves by 36 to 40 weeks postmenstrual age, it often persists beyond 38 to 40 weeks in infants born before 28 weeks.10 In these infants, by 43 to 44 weeks postmenstrual age, the frequency of apneic episodes decreases to that of full-term infants.14
The differences in long-term outcomes of infants with apnea of prematurity vs infants without it are subtle, if present at all.14,15 There does seem to be a correlation between the number of days with apnea and poor outcomes. Neurodevelopmental impairment and death are more likely in neonates who experience a greater number of days with apnea episodes.16,17 However, apnea of prematurity is not associated with an increased risk of SIDS.18
According to the American Academy of Pediatrics (AAP), home apnea monitoring may be warranted for premature infants who are at high risk of recurrent episodes of apnea, bradycardia, and hypoxemia after hospital discharge.3 While there is general consensus that all infants born prior to 29 weeks meet this criterion, the use of home apnea monitors in older preterm infants varies significantly, and the decision to initiate monitoring in these patients is made by the discharging physician.3 Once initiated, the AAP recommends that the use of home apnea monitoring in this population be discontinued after approximately 43 weeks postmenstrual age or after the cessation of extreme episodes, whichever comes last.3
In Jacob’s case, the monitoring should be discontinued at approximately week 12 of life, or about age 3 months.
CASE 2 › Apparent life-threatening event
Sarah is brought to your office after being hospitalized for an ALTE. Her mother reports that she had witnessed her 13-day-old daughter not breathing for “about a minute.” Upon realizing what was happening, she “blew into the baby’s face,” whereupon Sarah awakened. The mother then called 911 and they went by ambulance to the emergency room. The newborn was admitted for observation overnight and received a thorough evaluation. She was discharged with a home apnea monitor.
You review the work-up and find nothing worrisome. Sarah is in a car seat attached to the apnea monitor with a chest strap. An examination of the child is normal. The mother asks you when they should stop using the home monitor.
An ALTE is “an event that is frightening to the observer and ... is characterized by some combination of apnea (central or occasionally obstructive), color change (usually cyanotic or pallid but occasionally erythematous or plethoric), marked change in muscle tone (usually marked limpness), choking, or gagging.”2 ALTE is a descriptive term, and not a definitive diagnosis.
The true incidence of ALTE is unknown, but is reported to be 0.5% to 6%; most events occur in children younger than age 1.19,20 The risk for ALTE is increased for premature infants, particularly those with respiratory syncytial virus or who had undergone general anesthesia; infants who feed rapidly, cough frequently, or choke during feeding; and male infants.19,21
The most common causes of ALTE (in descending order) are gastroesophageal reflux, seizure disorder, and lower respiratory tract infection.22 The etiology is unknown for about half of patients with ALTE.23
Tell parents that if their infant experiences an ALTE, they should seek medical attention without delay. The fear is that failing to respond to this concern will ultimately result in a sudden unexpected infant death, specifically as a result of SIDS.24
SIDS is very rare, occurring in only 40 per 100,000 births. One analysis found that children who die from SIDS and those who experience ALTE have very similar histories and clinical factors.25 Approximately 7% of infants who die from SIDS have had an ALTE.2 Overall, the long-term prognosis for infants who have had an ALTE is very good, although it depends on seriousness of the underlying etiology.8,26-28
Guidance on the effective use of home apnea monitors in infants who experience an ALTE is sparse. Despite this, the National Institutes of Health (NIH) Consensus Statement on Infantile Apnea and Home Monitoring2 and the American Academy of Pediatrics policy statement on apnea, sudden infant death syndrome, and home monitoring3 recommend the use of home apnea monitoring for certain infants who’ve had an ALTE. The NIH Consensus Statement specifies home monitoring for infants with one or more severe episodes of ALTEs that require mouth-to-mouth resuscitation or vigorous stimulation.2 There are no specific guidelines regarding the duration of monitoring.2,3
In Sarah’s case, home monitoring should be discontinued as soon as the mother is comfortable with the decision.
CASE 3 › Sudden infant death syndrome
The parents of a 2-month-old boy, Stephen, come to your office to establish care. They recently relocated and their previous care provider had prescribed a home apnea monitor because a child they’d had 3 years ago had died of SIDS. Stephen is in a car seat attached to the apnea monitor with a chest strap. Your examination of him is normal. Stephen’s parents would like to stop using the home monitor, and ask you if it’s safe to do so.
SIDS is the death of an infant or young child that is unexplained by history and in which postmortem examination fails to find an adequate explanation of cause of death.2 Since the introduction of the Back to Sleep campaign in the early 1990s, the incidence of SIDS has decreased by more than 50%.8 In 2013, approximately 1500 infant deaths were attributed to SIDS.24 Three-quarters of deaths due to SIDS occur between 2 to 4 months of age, and 95% of deaths occur before 9 months of age.29 Risk factors for SIDS include sleep environment (prone and side sleeping, bed sharing, soft bedding), prenatal and postnatal maternal tobacco use, exposure to tobacco smoke, maternal mental illness or substance abuse, male sex, poverty, prematurity, low birth weight (less than 2500 g), and no or poor prenatal care.30
The etiology of SIDS is unclear.31 The leading hypothesis is the “triple-risk model,” which proposes that death from SIDS is due to 3 overlapping factors: a vulnerable infant, a critical developmental period in homeostatic control, and an exogenous stressor.32
Although the NIH Consensus Statement suggests home apnea monitoring is indicated for infants who are siblings of 2 or more SIDS victims,2 more recent policy statements from the AAP recommend against using home apnea monitors to reduce the incidence of SIDS due to a lack of evidence.3,8
With this in mind, Stephen’s monitor should be discontinued and his parents should be educated on proven methods of preventing SIDS, including placing him on his back to sleep, breastfeeding him, letting him use a pacifier during sleep, and not sleeping in the same bed with him or overdressing him when putting him to sleep.3,8
CORRESPONDENCE
Allen Perkins, MD, MPH, Department of Family Medicine, University of South Alabama, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604; [email protected].
› Tell parents that home apnea monitoring has not been shown to prevent sudden unexpected death in infants. C
› Consider discontinuing home apnea monitoring for infants at risk for recurrent apnea at approximately 43 weeks postmenstrual age or after the cessation of extreme episodes. B
› Educate parents about steps they can take to reduce their child’s risk of sudden infant death syndrome, such as putting him to sleep on his back, breastfeeding him, and refraining from sleeping in the same bed with him. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year, more than one in every 100 infants are born at less than 32 weeks postmenstrual age.1 In industrialized countries, many of these infants are discharged from the neonatal intensive care unit (NICU) with home apnea monitors,1 which alert caregivers to episodes of apnea and bradycardia, while also capturing and storing data surrounding significant events for later analysis.2
Evidence supporting the use of home apnea monitoring is sparse, and recommendations highlight the need to use this technology sparingly and to discontinue use once it is no longer necessary (TABLE).3 Counseling parents is critical. It’s important to explain that home apnea monitoring can be used to help reduce the likelihood that a child will die in his or her sleep, but it affords users no “guarantees.” In addition, home apnea monitoring can adversely affect parents. Parents who use home apnea monitoring for their infants have been shown to have higher stress scores, greater levels of fatigue, and poorer health than parents of infants without home apnea monitors.4-8
As a family physician, you are likely to encounter home apnea monitoring in one of 3 scenarios: at the first visit after discharge by a premature infant who experienced apnea while hospitalized, at a follow-up visit after discharge from the hospital by an infant who experienced an apparent life-threatening event (ALTE), and at a follow-up visit by an infant whose sibling had died from sudden infant death syndrome (SIDS). This article presents case studies that illustrate each of these scenarios, and explains what to tell parents who ask about how long they should continue home apnea monitoring.
CASE 1 › Apnea of prematurity
Jacob is a newborn who is brought in to your clinic by his parents for an initial visit. The infant was born prematurely at 32 weeks and required a prolonged NICU stay. His mother says that Jacob spent 4 weeks there and was discharged home with home apnea monitoring. On exam, the infant has a monitor attached via a chest band. Jacob appears healthy and his exam is normal. The mother asks you how long her son should use the home monitor.
Pathologic apnea is a respiratory pause that lasts at least 20 seconds or is associated with cyanosis; abrupt, marked pallor or hypotonia; or bradycardia.2 Apnea of prematurity is present in almost all infants born at <29 weeks postmenstrual age or who weigh <1000 g.9 It is found in 54% of infants born at 30 to 31 weeks, 15% born at 32 to 33 weeks, and 7% of infants born at 34 to 35 weeks.10
Apnea of prematurity is primarily due to an immature respiratory control system, which results in impaired breathing regulation, immature respiratory responses to hypercapnia and hypoxia, and an exaggerated inhibitory response to stimulation of airway receptors.11-13 Although apnea of prematurity usually resolves by 36 to 40 weeks postmenstrual age, it often persists beyond 38 to 40 weeks in infants born before 28 weeks.10 In these infants, by 43 to 44 weeks postmenstrual age, the frequency of apneic episodes decreases to that of full-term infants.14
The differences in long-term outcomes of infants with apnea of prematurity vs infants without it are subtle, if present at all.14,15 There does seem to be a correlation between the number of days with apnea and poor outcomes. Neurodevelopmental impairment and death are more likely in neonates who experience a greater number of days with apnea episodes.16,17 However, apnea of prematurity is not associated with an increased risk of SIDS.18
According to the American Academy of Pediatrics (AAP), home apnea monitoring may be warranted for premature infants who are at high risk of recurrent episodes of apnea, bradycardia, and hypoxemia after hospital discharge.3 While there is general consensus that all infants born prior to 29 weeks meet this criterion, the use of home apnea monitors in older preterm infants varies significantly, and the decision to initiate monitoring in these patients is made by the discharging physician.3 Once initiated, the AAP recommends that the use of home apnea monitoring in this population be discontinued after approximately 43 weeks postmenstrual age or after the cessation of extreme episodes, whichever comes last.3
In Jacob’s case, the monitoring should be discontinued at approximately week 12 of life, or about age 3 months.
CASE 2 › Apparent life-threatening event
Sarah is brought to your office after being hospitalized for an ALTE. Her mother reports that she had witnessed her 13-day-old daughter not breathing for “about a minute.” Upon realizing what was happening, she “blew into the baby’s face,” whereupon Sarah awakened. The mother then called 911 and they went by ambulance to the emergency room. The newborn was admitted for observation overnight and received a thorough evaluation. She was discharged with a home apnea monitor.
You review the work-up and find nothing worrisome. Sarah is in a car seat attached to the apnea monitor with a chest strap. An examination of the child is normal. The mother asks you when they should stop using the home monitor.
An ALTE is “an event that is frightening to the observer and ... is characterized by some combination of apnea (central or occasionally obstructive), color change (usually cyanotic or pallid but occasionally erythematous or plethoric), marked change in muscle tone (usually marked limpness), choking, or gagging.”2 ALTE is a descriptive term, and not a definitive diagnosis.
The true incidence of ALTE is unknown, but is reported to be 0.5% to 6%; most events occur in children younger than age 1.19,20 The risk for ALTE is increased for premature infants, particularly those with respiratory syncytial virus or who had undergone general anesthesia; infants who feed rapidly, cough frequently, or choke during feeding; and male infants.19,21
The most common causes of ALTE (in descending order) are gastroesophageal reflux, seizure disorder, and lower respiratory tract infection.22 The etiology is unknown for about half of patients with ALTE.23
Tell parents that if their infant experiences an ALTE, they should seek medical attention without delay. The fear is that failing to respond to this concern will ultimately result in a sudden unexpected infant death, specifically as a result of SIDS.24
SIDS is very rare, occurring in only 40 per 100,000 births. One analysis found that children who die from SIDS and those who experience ALTE have very similar histories and clinical factors.25 Approximately 7% of infants who die from SIDS have had an ALTE.2 Overall, the long-term prognosis for infants who have had an ALTE is very good, although it depends on seriousness of the underlying etiology.8,26-28
Guidance on the effective use of home apnea monitors in infants who experience an ALTE is sparse. Despite this, the National Institutes of Health (NIH) Consensus Statement on Infantile Apnea and Home Monitoring2 and the American Academy of Pediatrics policy statement on apnea, sudden infant death syndrome, and home monitoring3 recommend the use of home apnea monitoring for certain infants who’ve had an ALTE. The NIH Consensus Statement specifies home monitoring for infants with one or more severe episodes of ALTEs that require mouth-to-mouth resuscitation or vigorous stimulation.2 There are no specific guidelines regarding the duration of monitoring.2,3
In Sarah’s case, home monitoring should be discontinued as soon as the mother is comfortable with the decision.
CASE 3 › Sudden infant death syndrome
The parents of a 2-month-old boy, Stephen, come to your office to establish care. They recently relocated and their previous care provider had prescribed a home apnea monitor because a child they’d had 3 years ago had died of SIDS. Stephen is in a car seat attached to the apnea monitor with a chest strap. Your examination of him is normal. Stephen’s parents would like to stop using the home monitor, and ask you if it’s safe to do so.
SIDS is the death of an infant or young child that is unexplained by history and in which postmortem examination fails to find an adequate explanation of cause of death.2 Since the introduction of the Back to Sleep campaign in the early 1990s, the incidence of SIDS has decreased by more than 50%.8 In 2013, approximately 1500 infant deaths were attributed to SIDS.24 Three-quarters of deaths due to SIDS occur between 2 to 4 months of age, and 95% of deaths occur before 9 months of age.29 Risk factors for SIDS include sleep environment (prone and side sleeping, bed sharing, soft bedding), prenatal and postnatal maternal tobacco use, exposure to tobacco smoke, maternal mental illness or substance abuse, male sex, poverty, prematurity, low birth weight (less than 2500 g), and no or poor prenatal care.30
The etiology of SIDS is unclear.31 The leading hypothesis is the “triple-risk model,” which proposes that death from SIDS is due to 3 overlapping factors: a vulnerable infant, a critical developmental period in homeostatic control, and an exogenous stressor.32
Although the NIH Consensus Statement suggests home apnea monitoring is indicated for infants who are siblings of 2 or more SIDS victims,2 more recent policy statements from the AAP recommend against using home apnea monitors to reduce the incidence of SIDS due to a lack of evidence.3,8
With this in mind, Stephen’s monitor should be discontinued and his parents should be educated on proven methods of preventing SIDS, including placing him on his back to sleep, breastfeeding him, letting him use a pacifier during sleep, and not sleeping in the same bed with him or overdressing him when putting him to sleep.3,8
CORRESPONDENCE
Allen Perkins, MD, MPH, Department of Family Medicine, University of South Alabama, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604; [email protected].
1. Centers for Disease Control and Prevention. Births and natality. Centers for Disease Control and Prevention/National Center for Health Statistics Web site. Available at: http://www.cdc.gov/nchs/fastats/births.htm. Accessed August 18, 2015.
2. National Institutes of Health Consensus Development Program. Infantile apnea and home monitoring. National Institutes of Health Consensus Development Statement. 1986. National Institutes of Health Consensus Development Program Web site. Available at: https://consensus.nih.gov/1986/1986InfantApneaMonitoring058html.htm. Accessed November 2, 2015.
3. Committee on Fetus and Newborn, American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics. 2003;111:914-917.
4. Kahn A, Sottiaux M, Appelboom-Fondu J, et al. Long-term development of children monitored as infants for an apparent lifethreatening event during sleep: a 10-year follow-up study. Pediatrics. 1989;83:668-673.
5. Vohr BR, Chen A, Garcia Coll C, et al. Mothers of preterm and full-term infants on home apnea monitors. Am J Dis Child. 1988;142:229-231.
6. Williams PD, Press A, Williams AR, et al. Fatigue in mothers of infants discharged to the home on apnea monitors. Appl Nurs Res. 1999;12:69-77.
7. Ahmann E, Wulff L, Meny RG. Home apnea monitoring and disruption in family life: a multidimensional controlled study. Am J Public Health. 1992;82:719-722.
8. Task Force on Sudden Infant Death Syndrome, Moon R. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:1030-1039.
9. Eichenwald EC, Aina A, Stark AR. Apnea frequently persists beyond term gestation in infants delivered at 24 to 28 weeks. Pediatrics. 1997;100:354-359.
10. Martin RJ, Abu-Shaweesh JM, Baird TM. Apnoea of prematurity. Paediatr Respir Rev. 2004;5:S377-S382.
11. Miller MJ, Martin RJ. Pathophysiology of apnea of prematurity. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004: 905-918.
12. Miller JM, Haxhiu MA, Martin RJ. Chemical control of breathing from fetal through newborn life. In: Matthew OP, ed. Respiratory Control and Disorders in the Newborn. New York, NY: Marcel Dekker; 2003:83-113.
13. Rigatto H. Control of breathing in fetal life and onset and control of breathing in the neonate. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004:890-899.
14. Koons AH, Mojica N, Jadeja N, et al. Neurodevelopmental outcome of infants with apnea of infancy. Am J Perinatol. 1993;10:208-211.
15. Perlman JM. Neurobehavioral deficits in premature graduates of intensive care—potential medical and neonatal environmental risk factors. Pediatrics. 2001;108:1339-1348.
16. Janvier A, Khairy M, Kokkotis A, et al. Apnea is associated with neurodevelopmental impairment in very low birth weight infants. J Perinatol. 2004;24:763-768.
17. Pillekamp F, Hermann C, Keller T, et al. Factors influencing apnea and bradycardia of prematurity—implications for neurodevelopment. Neonatology. 2007;91:155-161.
18. Hoffman HJ, Damus K, Hillman L, et al. Risk factors for SIDS. Results for the National Institutes of Child Health and Human Development SIDS Cooperative Epidemiological Study. Ann N Y Acad Sci. 1988;533:13-30.
19. Brooks JG. Apparent life-threatening events and apnea of infancy. Clin Perinatol. 1992;19:809-838.
20. Semmekrot BA, van Sleuwan BE, Engelberts AC, et al. Surveillance study of apparent life-threatening events (ALTE) in the Netherlands. Eur J Pediatr. 2010;169:229-236.
21. Carroll JL. Apparent Life Threatening Event (ALTE) assessment. Pediatr Pulmonol Suppl. 2004;26:108-109.
22. McGovern MC, Smith MB. Causes of apparent life threatening events in infants: a systematic review. Arch Dis Child. 2004;89:1043-1048.
23. Hall KL, Zalman B. Evaluation and management of apparent life-threatening events in children. Am Fam Physician. 2005;71:2301-2308.
24. Centers for Disease Control and Prevention. Sudden unexpected infant death and sudden infant death syndrome. Updated May 11, 2015. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/sids/data.htm. Accessed June 30, 2015.
25. Kahn A, Blum D, Hennart P, et al. A critical comparison of the history of sudden-death infants and infants hospitalized for nearmiss for SIDS. Eur J Pediatr. 1984;143:103-107.
26. Baroni MA. Apparent life-threatening events during infancy: a follow-up study of subsequent growth and development. J Dev Behav Pediatr. 1991;12:154-161.
27. Tirosh E, Kessel A, Jaffe M, et al. Outcome of idiopathic apparent life-threatening events: infant and mother perspectives. Pediatr Pulmonol. 1999;28:47-52.
28. Koons AH. Neurodevelopmental outcome in infants with apnea. N J Med. 1992;89:688-690.
29. Poets CF. Apnea of prematurity, sudden infant death syndrome, and apparent life-threatening events. In: Taussig LM, ed. Pediatric Respiratory Medicine. Philadelphia, PA: Mosby; 2008:413-434.
30. Adams SM, Ward CE, Garcia KL. Sudden infant death syndrome. Am Fam Physician. 2015;91:778-783.
31. Goldwater PN. A perspective on SIDS pathogenesis. The hypothesis: plausibility and evidence. BMC Med. 2011;9:64.
32. Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65:194-197.
1. Centers for Disease Control and Prevention. Births and natality. Centers for Disease Control and Prevention/National Center for Health Statistics Web site. Available at: http://www.cdc.gov/nchs/fastats/births.htm. Accessed August 18, 2015.
2. National Institutes of Health Consensus Development Program. Infantile apnea and home monitoring. National Institutes of Health Consensus Development Statement. 1986. National Institutes of Health Consensus Development Program Web site. Available at: https://consensus.nih.gov/1986/1986InfantApneaMonitoring058html.htm. Accessed November 2, 2015.
3. Committee on Fetus and Newborn, American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics. 2003;111:914-917.
4. Kahn A, Sottiaux M, Appelboom-Fondu J, et al. Long-term development of children monitored as infants for an apparent lifethreatening event during sleep: a 10-year follow-up study. Pediatrics. 1989;83:668-673.
5. Vohr BR, Chen A, Garcia Coll C, et al. Mothers of preterm and full-term infants on home apnea monitors. Am J Dis Child. 1988;142:229-231.
6. Williams PD, Press A, Williams AR, et al. Fatigue in mothers of infants discharged to the home on apnea monitors. Appl Nurs Res. 1999;12:69-77.
7. Ahmann E, Wulff L, Meny RG. Home apnea monitoring and disruption in family life: a multidimensional controlled study. Am J Public Health. 1992;82:719-722.
8. Task Force on Sudden Infant Death Syndrome, Moon R. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:1030-1039.
9. Eichenwald EC, Aina A, Stark AR. Apnea frequently persists beyond term gestation in infants delivered at 24 to 28 weeks. Pediatrics. 1997;100:354-359.
10. Martin RJ, Abu-Shaweesh JM, Baird TM. Apnoea of prematurity. Paediatr Respir Rev. 2004;5:S377-S382.
11. Miller MJ, Martin RJ. Pathophysiology of apnea of prematurity. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004: 905-918.
12. Miller JM, Haxhiu MA, Martin RJ. Chemical control of breathing from fetal through newborn life. In: Matthew OP, ed. Respiratory Control and Disorders in the Newborn. New York, NY: Marcel Dekker; 2003:83-113.
13. Rigatto H. Control of breathing in fetal life and onset and control of breathing in the neonate. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004:890-899.
14. Koons AH, Mojica N, Jadeja N, et al. Neurodevelopmental outcome of infants with apnea of infancy. Am J Perinatol. 1993;10:208-211.
15. Perlman JM. Neurobehavioral deficits in premature graduates of intensive care—potential medical and neonatal environmental risk factors. Pediatrics. 2001;108:1339-1348.
16. Janvier A, Khairy M, Kokkotis A, et al. Apnea is associated with neurodevelopmental impairment in very low birth weight infants. J Perinatol. 2004;24:763-768.
17. Pillekamp F, Hermann C, Keller T, et al. Factors influencing apnea and bradycardia of prematurity—implications for neurodevelopment. Neonatology. 2007;91:155-161.
18. Hoffman HJ, Damus K, Hillman L, et al. Risk factors for SIDS. Results for the National Institutes of Child Health and Human Development SIDS Cooperative Epidemiological Study. Ann N Y Acad Sci. 1988;533:13-30.
19. Brooks JG. Apparent life-threatening events and apnea of infancy. Clin Perinatol. 1992;19:809-838.
20. Semmekrot BA, van Sleuwan BE, Engelberts AC, et al. Surveillance study of apparent life-threatening events (ALTE) in the Netherlands. Eur J Pediatr. 2010;169:229-236.
21. Carroll JL. Apparent Life Threatening Event (ALTE) assessment. Pediatr Pulmonol Suppl. 2004;26:108-109.
22. McGovern MC, Smith MB. Causes of apparent life threatening events in infants: a systematic review. Arch Dis Child. 2004;89:1043-1048.
23. Hall KL, Zalman B. Evaluation and management of apparent life-threatening events in children. Am Fam Physician. 2005;71:2301-2308.
24. Centers for Disease Control and Prevention. Sudden unexpected infant death and sudden infant death syndrome. Updated May 11, 2015. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/sids/data.htm. Accessed June 30, 2015.
25. Kahn A, Blum D, Hennart P, et al. A critical comparison of the history of sudden-death infants and infants hospitalized for nearmiss for SIDS. Eur J Pediatr. 1984;143:103-107.
26. Baroni MA. Apparent life-threatening events during infancy: a follow-up study of subsequent growth and development. J Dev Behav Pediatr. 1991;12:154-161.
27. Tirosh E, Kessel A, Jaffe M, et al. Outcome of idiopathic apparent life-threatening events: infant and mother perspectives. Pediatr Pulmonol. 1999;28:47-52.
28. Koons AH. Neurodevelopmental outcome in infants with apnea. N J Med. 1992;89:688-690.
29. Poets CF. Apnea of prematurity, sudden infant death syndrome, and apparent life-threatening events. In: Taussig LM, ed. Pediatric Respiratory Medicine. Philadelphia, PA: Mosby; 2008:413-434.
30. Adams SM, Ward CE, Garcia KL. Sudden infant death syndrome. Am Fam Physician. 2015;91:778-783.
31. Goldwater PN. A perspective on SIDS pathogenesis. The hypothesis: plausibility and evidence. BMC Med. 2011;9:64.
32. Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65:194-197.
AHA: Spirometry Identifies Mortality Risk in Asymptomatic Adults
ORLANDO – Unselected people from the general population without clinically apparent lung disease but with low lung function had significantly increased mortality during follow-up that was independent of cardiac function, in results from more than 13,000 middle-aged Germans.
“Subtle, subclinical pulmonary impairment is a risk indicator for increased mortality independent of cardiac performance,” Dr. Christina Baum said at the American Heart Association scientific sessions.
The researchers used spirometry to measure each subject’s forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC). The results showed that “spirometry is a good screening tool that is not very expensive,” making spirometry an effective risk assessment tool for use in the general adult population, said Dr. Baum of the department of general and interventional cardiology at the University Heart Center in Hamburg, Germany.
She and her associates used data collected in the Gutenberg Health Study, which enrolled more than 15,000 German women and men aged 35-74 years during 2007-2012. The investigators excluded people with a history of pulmonary disease, resulting in a study cohort of 13,191, who averaged 55 years old, with 51% men.
At enrollment into the study, all people underwent screening spirometry and echocardiography. Their average baseline FEV1 was 2.9 L and their average FVC was 3.7 L, and 4% had heart failure based on assessments of left ventricular size and function by echocardiography. The first 5,000 enrollees also had measurements taken of their serum levels of N-terminal probrain natriuretic peptide and cardiac troponin I through use of a high-sensitivity assay. The researchers used data from patients followed for a median of 5.5 years.
During follow-up, people in the lowest tertile for FEV1 and those in the lowest tertile for FVC had higher rates of all-cause mortality, compared with those in the highest tertile for each of these two parameters.
In a multivariate analysis that adjusted for age, sex, body mass index, smoking status, hypertension, dyslipidemia, heart failure status, serum levels of N-terminal probrain natriuretic peptide and cardiac troponin I, and other parameters, people with lower FEV1 and FVC readings had significantly worse survival, Dr. Baum said. Every 1–standard deviation increase in FEV1 was linked with a statistically significant, 38% reduced mortality rate; furthermore, a similar significant inverse association existed between FVC and mortality, she reported.
ORLANDO – Unselected people from the general population without clinically apparent lung disease but with low lung function had significantly increased mortality during follow-up that was independent of cardiac function, in results from more than 13,000 middle-aged Germans.
“Subtle, subclinical pulmonary impairment is a risk indicator for increased mortality independent of cardiac performance,” Dr. Christina Baum said at the American Heart Association scientific sessions.
The researchers used spirometry to measure each subject’s forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC). The results showed that “spirometry is a good screening tool that is not very expensive,” making spirometry an effective risk assessment tool for use in the general adult population, said Dr. Baum of the department of general and interventional cardiology at the University Heart Center in Hamburg, Germany.
She and her associates used data collected in the Gutenberg Health Study, which enrolled more than 15,000 German women and men aged 35-74 years during 2007-2012. The investigators excluded people with a history of pulmonary disease, resulting in a study cohort of 13,191, who averaged 55 years old, with 51% men.
At enrollment into the study, all people underwent screening spirometry and echocardiography. Their average baseline FEV1 was 2.9 L and their average FVC was 3.7 L, and 4% had heart failure based on assessments of left ventricular size and function by echocardiography. The first 5,000 enrollees also had measurements taken of their serum levels of N-terminal probrain natriuretic peptide and cardiac troponin I through use of a high-sensitivity assay. The researchers used data from patients followed for a median of 5.5 years.
During follow-up, people in the lowest tertile for FEV1 and those in the lowest tertile for FVC had higher rates of all-cause mortality, compared with those in the highest tertile for each of these two parameters.
In a multivariate analysis that adjusted for age, sex, body mass index, smoking status, hypertension, dyslipidemia, heart failure status, serum levels of N-terminal probrain natriuretic peptide and cardiac troponin I, and other parameters, people with lower FEV1 and FVC readings had significantly worse survival, Dr. Baum said. Every 1–standard deviation increase in FEV1 was linked with a statistically significant, 38% reduced mortality rate; furthermore, a similar significant inverse association existed between FVC and mortality, she reported.
ORLANDO – Unselected people from the general population without clinically apparent lung disease but with low lung function had significantly increased mortality during follow-up that was independent of cardiac function, in results from more than 13,000 middle-aged Germans.
“Subtle, subclinical pulmonary impairment is a risk indicator for increased mortality independent of cardiac performance,” Dr. Christina Baum said at the American Heart Association scientific sessions.
The researchers used spirometry to measure each subject’s forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC). The results showed that “spirometry is a good screening tool that is not very expensive,” making spirometry an effective risk assessment tool for use in the general adult population, said Dr. Baum of the department of general and interventional cardiology at the University Heart Center in Hamburg, Germany.
She and her associates used data collected in the Gutenberg Health Study, which enrolled more than 15,000 German women and men aged 35-74 years during 2007-2012. The investigators excluded people with a history of pulmonary disease, resulting in a study cohort of 13,191, who averaged 55 years old, with 51% men.
At enrollment into the study, all people underwent screening spirometry and echocardiography. Their average baseline FEV1 was 2.9 L and their average FVC was 3.7 L, and 4% had heart failure based on assessments of left ventricular size and function by echocardiography. The first 5,000 enrollees also had measurements taken of their serum levels of N-terminal probrain natriuretic peptide and cardiac troponin I through use of a high-sensitivity assay. The researchers used data from patients followed for a median of 5.5 years.
During follow-up, people in the lowest tertile for FEV1 and those in the lowest tertile for FVC had higher rates of all-cause mortality, compared with those in the highest tertile for each of these two parameters.
In a multivariate analysis that adjusted for age, sex, body mass index, smoking status, hypertension, dyslipidemia, heart failure status, serum levels of N-terminal probrain natriuretic peptide and cardiac troponin I, and other parameters, people with lower FEV1 and FVC readings had significantly worse survival, Dr. Baum said. Every 1–standard deviation increase in FEV1 was linked with a statistically significant, 38% reduced mortality rate; furthermore, a similar significant inverse association existed between FVC and mortality, she reported.
AT THE AHA SCIENTIFIC SESSIONS