User login
MINDFULNESS-BASED COGNITIVE THERAPY AND ANTIDEPRESSANTS
Kuyken W, Hayes R, Barrett B, et al. Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): a randomised controlled trial. Lancet. 2015;386(9988):63-73. doi:10.1016/S0140-6736(14)62222-4.
Mindfulness-based cognitive therapy—a group-based psychosocial intervention designed to enhance self-management of prodromal symptoms associated with depressive relapse—with support to taper or discontinue antidepressant treatment (MBCT-TS) is neither superior nor inferior to maintenance antidepressant treatment for preventing a depressive relapse, according to the PREVENT trial.
Researchers randomly assigned 424 patients to MBCT-TS or maintenance therapy and found no difference in time to relapse or recurrence of depression between the two groups. Rates of adverse effects were similar in both groups.
The study authors note that both treatments were associated with positive outcomes regarding relapse or recurrence, residual depressive symptoms, and quality of life.
COMMENTARY
Patients with recurrent depression have a 50% to 80% lifetime rate of relapse, making a prevention strategy an important part of their care. Current recommendations suggest long-term continuation of antidepressant treatment decreases recurrence by 50% to 60%.1 However, antidepressant medication only works for as long as you take it, and many people do not want to be on antidepressants long term. A previous study compared MBCT-TS, continuation of antidepressant medication, and placebo; the respective relapse rates of 28%, 27%, and 71% indicate that both MBCT-TS and antidepressant medication substantially decrease the rate of depression relapse.2 This study provides further evidence that MBCT-TS is an excellent alternative to antidepressant medication for decreasing depression relapse.
1. Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361:653-661.
2. Segal ZV, Bieling P, Young T, et al. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Arch Gen Psych. 2010;67:1256-1264. doi:10.1001/archgenpsychiatry.2010.168.
Continue for treating preconception depression: To stop SSRIs or not >>
TREATING PRECONCEPTION DEPRESSION: TO STOP SSRIs OR NOT
Andersen JT, Andersen NL, Horwitz H, et al. Exposure to selective serotonin reuptake inhibitors in early pregnancy and the risk of miscarriage. Obstet Gynecol. 2014;124(4):655-661. doi: 10.1097/AOG.0000000000000447.
Miscarriage rates in women taking selective serotonin reuptake inhibitors (SSRIs) in early pregnancy were higher than in those not taking SSRIs but similar to those who discontinued SSRI treatment prior to pregnancy, a Danish cohort study revealed.
Out of 1.3 million pregnancies between 1997 and 2010, researchers identified 22,884 women who were exposed to an SSRI during the first 35 days of pregnancy and found miscarriage rates of 13% in those exposed to the antidepressants, compared to 11% for those not exposed. Investigators also identified 14,016 women who discontinued SSRI treatment three to 12 months prior to conception and found a miscarriage rate of 14%.
The adjusted hazard ratio for miscarriage while taking SSRIs in early pregnancy was 1.27, and for miscarriage after discontinuing SSRIs prior to pregnancy, 1.24. When the data were stratified according to specific SSRIs, rates were lowest among those taking fluoxetine during pregnancy (1.10) and highest among those taking sertraline (1.45). Miscarriage rates among women who stopped SSRIs prior to pregnancy were lowest for fluoxetine (1.2) and highest for escitalopram (1.33).
“Because the risk for miscarriage is elevated in both groups compared with an unexposed population, there is likely no benefit in discontinuing SSRI use before pregnancy to decrease one’s chances of miscarriage,” the study authors conclude.
COMMENTARY
The effects of depression on a woman’s experience during pregnancy are large, as are the effects of depression on pregnancy outcomes. Depression during pregnancy is associated with increased rates of prematurity, low birth weight, and preeclampsia.1 Depression during pregnancy is also an important risk factor for postpartum depression, which affects babies as well as mothers and is associated with maternal suicide.
At the same time, use of SSRIs in pregnancy has been inconsistently associated with miscarriage, cardiac defects, premature birth, and primary pulmonary hypertension in the newborn.2 This study is reassuring in that SSRIs are unlikely to be a significant contributor to miscarriage. But it is important to realize that this article only addresses miscarriage rates, not other potential effects of SSRIs on the fetus. The decision about the use of SSRIs in pregnancy remains a difficult one, balancing risk and benefit. When determining that balance, bear in mind that cognitive behavioral therapy (CBT) has been shown in other studies to be equally effective to medication in treating depression and may also be considered in our range of options for treatment of depression in pregnancy.3,4
The decision about whether to use or continue an SSRI and whether to use or supplement with CBT instead is an important one and always requires detailed discussion with the mother-to-be.
1. Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psych. 2013;74:e321-341.
2. Meltzer-Brody S. Treating perinatal depression: risks and stigma. Obstet Gynecol. 2014;124(4):653-654. doi: 10.1097/AOG.0000000000000498.
3. Keller MB, McCullough JP, Klein DN, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression [published correction appears in N Engl J Med. 2001;345(3):232]. N Engl J Med. 2000;342(20): 1462-1470.
4. Cuijpers P, Hollon SD, van Straten A, et al. Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open. 2013;3(4). pii: e002542. doi: 10.1136/bmjopen-2012-002542.
Continue for suicide, self-harm rates, and antidepressants >>
SUICIDE, SELF-HARM RATES, AND ANTIDEPRESSANTS
Coupland C, Hill T, Morriss R, et al. Antidepressant use and risk of suicide and attempted suicide or self harm in people aged 20 to 64: cohort study using a primary care database. BMJ. 2015;350:h517. doi: 10.1136/bmj.h517.
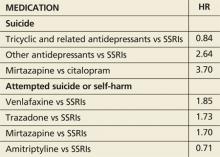
In patients with clinical depression, rates of suicide and self-harm are similar among those treated with selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants but significantly higher among those treated with other antidepressants, according to a review of 238,963 patients who were diagnosed with depression.
During an average five years’ follow-up, researchers noted 198 cases of suicide and 5,243 cases of attempted suicide or self-harm. The following hazard ratios (HR) were associated with antidepressant use:
Absolute risk for suicide over one year ranged from 0.02% for amitriptyline to 0.19% for mirtazapine.
COMMENTARY
This large study suggests suicide rates may be greater with non-SSRI antidepressants than with SSRIs. The data are far from solid, though, because of the small number of events and the potential for systematic differences in how these antidepressants are prescribed. For instance, if dual norepinephrine and serotonin agents are prescribed more often to individuals with more severe depression, then the increased suicide risk with use of combined norepinephrine/serotonin agents (eg, venlafaxine) could relate to the severity of the depression treated, not to an effect of the medication. Of importance is that the rate of suicide was increased in the first 28 days after starting an antidepressant and in the 28 days after stopping the antidepressant, times when we should have increased vigilance for suicidal ideation.
MINDFULNESS-BASED COGNITIVE THERAPY AND ANTIDEPRESSANTS
Kuyken W, Hayes R, Barrett B, et al. Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): a randomised controlled trial. Lancet. 2015;386(9988):63-73. doi:10.1016/S0140-6736(14)62222-4.
Mindfulness-based cognitive therapy—a group-based psychosocial intervention designed to enhance self-management of prodromal symptoms associated with depressive relapse—with support to taper or discontinue antidepressant treatment (MBCT-TS) is neither superior nor inferior to maintenance antidepressant treatment for preventing a depressive relapse, according to the PREVENT trial.
Researchers randomly assigned 424 patients to MBCT-TS or maintenance therapy and found no difference in time to relapse or recurrence of depression between the two groups. Rates of adverse effects were similar in both groups.
The study authors note that both treatments were associated with positive outcomes regarding relapse or recurrence, residual depressive symptoms, and quality of life.
COMMENTARY
Patients with recurrent depression have a 50% to 80% lifetime rate of relapse, making a prevention strategy an important part of their care. Current recommendations suggest long-term continuation of antidepressant treatment decreases recurrence by 50% to 60%.1 However, antidepressant medication only works for as long as you take it, and many people do not want to be on antidepressants long term. A previous study compared MBCT-TS, continuation of antidepressant medication, and placebo; the respective relapse rates of 28%, 27%, and 71% indicate that both MBCT-TS and antidepressant medication substantially decrease the rate of depression relapse.2 This study provides further evidence that MBCT-TS is an excellent alternative to antidepressant medication for decreasing depression relapse.
1. Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361:653-661.
2. Segal ZV, Bieling P, Young T, et al. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Arch Gen Psych. 2010;67:1256-1264. doi:10.1001/archgenpsychiatry.2010.168.
Continue for treating preconception depression: To stop SSRIs or not >>
TREATING PRECONCEPTION DEPRESSION: TO STOP SSRIs OR NOT
Andersen JT, Andersen NL, Horwitz H, et al. Exposure to selective serotonin reuptake inhibitors in early pregnancy and the risk of miscarriage. Obstet Gynecol. 2014;124(4):655-661. doi: 10.1097/AOG.0000000000000447.
Miscarriage rates in women taking selective serotonin reuptake inhibitors (SSRIs) in early pregnancy were higher than in those not taking SSRIs but similar to those who discontinued SSRI treatment prior to pregnancy, a Danish cohort study revealed.
Out of 1.3 million pregnancies between 1997 and 2010, researchers identified 22,884 women who were exposed to an SSRI during the first 35 days of pregnancy and found miscarriage rates of 13% in those exposed to the antidepressants, compared to 11% for those not exposed. Investigators also identified 14,016 women who discontinued SSRI treatment three to 12 months prior to conception and found a miscarriage rate of 14%.
The adjusted hazard ratio for miscarriage while taking SSRIs in early pregnancy was 1.27, and for miscarriage after discontinuing SSRIs prior to pregnancy, 1.24. When the data were stratified according to specific SSRIs, rates were lowest among those taking fluoxetine during pregnancy (1.10) and highest among those taking sertraline (1.45). Miscarriage rates among women who stopped SSRIs prior to pregnancy were lowest for fluoxetine (1.2) and highest for escitalopram (1.33).
“Because the risk for miscarriage is elevated in both groups compared with an unexposed population, there is likely no benefit in discontinuing SSRI use before pregnancy to decrease one’s chances of miscarriage,” the study authors conclude.
COMMENTARY
The effects of depression on a woman’s experience during pregnancy are large, as are the effects of depression on pregnancy outcomes. Depression during pregnancy is associated with increased rates of prematurity, low birth weight, and preeclampsia.1 Depression during pregnancy is also an important risk factor for postpartum depression, which affects babies as well as mothers and is associated with maternal suicide.
At the same time, use of SSRIs in pregnancy has been inconsistently associated with miscarriage, cardiac defects, premature birth, and primary pulmonary hypertension in the newborn.2 This study is reassuring in that SSRIs are unlikely to be a significant contributor to miscarriage. But it is important to realize that this article only addresses miscarriage rates, not other potential effects of SSRIs on the fetus. The decision about the use of SSRIs in pregnancy remains a difficult one, balancing risk and benefit. When determining that balance, bear in mind that cognitive behavioral therapy (CBT) has been shown in other studies to be equally effective to medication in treating depression and may also be considered in our range of options for treatment of depression in pregnancy.3,4
The decision about whether to use or continue an SSRI and whether to use or supplement with CBT instead is an important one and always requires detailed discussion with the mother-to-be.
1. Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psych. 2013;74:e321-341.
2. Meltzer-Brody S. Treating perinatal depression: risks and stigma. Obstet Gynecol. 2014;124(4):653-654. doi: 10.1097/AOG.0000000000000498.
3. Keller MB, McCullough JP, Klein DN, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression [published correction appears in N Engl J Med. 2001;345(3):232]. N Engl J Med. 2000;342(20): 1462-1470.
4. Cuijpers P, Hollon SD, van Straten A, et al. Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open. 2013;3(4). pii: e002542. doi: 10.1136/bmjopen-2012-002542.
Continue for suicide, self-harm rates, and antidepressants >>
SUICIDE, SELF-HARM RATES, AND ANTIDEPRESSANTS
Coupland C, Hill T, Morriss R, et al. Antidepressant use and risk of suicide and attempted suicide or self harm in people aged 20 to 64: cohort study using a primary care database. BMJ. 2015;350:h517. doi: 10.1136/bmj.h517.
In patients with clinical depression, rates of suicide and self-harm are similar among those treated with selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants but significantly higher among those treated with other antidepressants, according to a review of 238,963 patients who were diagnosed with depression.
During an average five years’ follow-up, researchers noted 198 cases of suicide and 5,243 cases of attempted suicide or self-harm. The following hazard ratios (HR) were associated with antidepressant use:
Absolute risk for suicide over one year ranged from 0.02% for amitriptyline to 0.19% for mirtazapine.
COMMENTARY
This large study suggests suicide rates may be greater with non-SSRI antidepressants than with SSRIs. The data are far from solid, though, because of the small number of events and the potential for systematic differences in how these antidepressants are prescribed. For instance, if dual norepinephrine and serotonin agents are prescribed more often to individuals with more severe depression, then the increased suicide risk with use of combined norepinephrine/serotonin agents (eg, venlafaxine) could relate to the severity of the depression treated, not to an effect of the medication. Of importance is that the rate of suicide was increased in the first 28 days after starting an antidepressant and in the 28 days after stopping the antidepressant, times when we should have increased vigilance for suicidal ideation.
MINDFULNESS-BASED COGNITIVE THERAPY AND ANTIDEPRESSANTS
Kuyken W, Hayes R, Barrett B, et al. Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): a randomised controlled trial. Lancet. 2015;386(9988):63-73. doi:10.1016/S0140-6736(14)62222-4.
Mindfulness-based cognitive therapy—a group-based psychosocial intervention designed to enhance self-management of prodromal symptoms associated with depressive relapse—with support to taper or discontinue antidepressant treatment (MBCT-TS) is neither superior nor inferior to maintenance antidepressant treatment for preventing a depressive relapse, according to the PREVENT trial.
Researchers randomly assigned 424 patients to MBCT-TS or maintenance therapy and found no difference in time to relapse or recurrence of depression between the two groups. Rates of adverse effects were similar in both groups.
The study authors note that both treatments were associated with positive outcomes regarding relapse or recurrence, residual depressive symptoms, and quality of life.
COMMENTARY
Patients with recurrent depression have a 50% to 80% lifetime rate of relapse, making a prevention strategy an important part of their care. Current recommendations suggest long-term continuation of antidepressant treatment decreases recurrence by 50% to 60%.1 However, antidepressant medication only works for as long as you take it, and many people do not want to be on antidepressants long term. A previous study compared MBCT-TS, continuation of antidepressant medication, and placebo; the respective relapse rates of 28%, 27%, and 71% indicate that both MBCT-TS and antidepressant medication substantially decrease the rate of depression relapse.2 This study provides further evidence that MBCT-TS is an excellent alternative to antidepressant medication for decreasing depression relapse.
1. Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361:653-661.
2. Segal ZV, Bieling P, Young T, et al. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Arch Gen Psych. 2010;67:1256-1264. doi:10.1001/archgenpsychiatry.2010.168.
Continue for treating preconception depression: To stop SSRIs or not >>
TREATING PRECONCEPTION DEPRESSION: TO STOP SSRIs OR NOT
Andersen JT, Andersen NL, Horwitz H, et al. Exposure to selective serotonin reuptake inhibitors in early pregnancy and the risk of miscarriage. Obstet Gynecol. 2014;124(4):655-661. doi: 10.1097/AOG.0000000000000447.
Miscarriage rates in women taking selective serotonin reuptake inhibitors (SSRIs) in early pregnancy were higher than in those not taking SSRIs but similar to those who discontinued SSRI treatment prior to pregnancy, a Danish cohort study revealed.
Out of 1.3 million pregnancies between 1997 and 2010, researchers identified 22,884 women who were exposed to an SSRI during the first 35 days of pregnancy and found miscarriage rates of 13% in those exposed to the antidepressants, compared to 11% for those not exposed. Investigators also identified 14,016 women who discontinued SSRI treatment three to 12 months prior to conception and found a miscarriage rate of 14%.
The adjusted hazard ratio for miscarriage while taking SSRIs in early pregnancy was 1.27, and for miscarriage after discontinuing SSRIs prior to pregnancy, 1.24. When the data were stratified according to specific SSRIs, rates were lowest among those taking fluoxetine during pregnancy (1.10) and highest among those taking sertraline (1.45). Miscarriage rates among women who stopped SSRIs prior to pregnancy were lowest for fluoxetine (1.2) and highest for escitalopram (1.33).
“Because the risk for miscarriage is elevated in both groups compared with an unexposed population, there is likely no benefit in discontinuing SSRI use before pregnancy to decrease one’s chances of miscarriage,” the study authors conclude.
COMMENTARY
The effects of depression on a woman’s experience during pregnancy are large, as are the effects of depression on pregnancy outcomes. Depression during pregnancy is associated with increased rates of prematurity, low birth weight, and preeclampsia.1 Depression during pregnancy is also an important risk factor for postpartum depression, which affects babies as well as mothers and is associated with maternal suicide.
At the same time, use of SSRIs in pregnancy has been inconsistently associated with miscarriage, cardiac defects, premature birth, and primary pulmonary hypertension in the newborn.2 This study is reassuring in that SSRIs are unlikely to be a significant contributor to miscarriage. But it is important to realize that this article only addresses miscarriage rates, not other potential effects of SSRIs on the fetus. The decision about the use of SSRIs in pregnancy remains a difficult one, balancing risk and benefit. When determining that balance, bear in mind that cognitive behavioral therapy (CBT) has been shown in other studies to be equally effective to medication in treating depression and may also be considered in our range of options for treatment of depression in pregnancy.3,4
The decision about whether to use or continue an SSRI and whether to use or supplement with CBT instead is an important one and always requires detailed discussion with the mother-to-be.
1. Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psych. 2013;74:e321-341.
2. Meltzer-Brody S. Treating perinatal depression: risks and stigma. Obstet Gynecol. 2014;124(4):653-654. doi: 10.1097/AOG.0000000000000498.
3. Keller MB, McCullough JP, Klein DN, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression [published correction appears in N Engl J Med. 2001;345(3):232]. N Engl J Med. 2000;342(20): 1462-1470.
4. Cuijpers P, Hollon SD, van Straten A, et al. Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open. 2013;3(4). pii: e002542. doi: 10.1136/bmjopen-2012-002542.
Continue for suicide, self-harm rates, and antidepressants >>
SUICIDE, SELF-HARM RATES, AND ANTIDEPRESSANTS
Coupland C, Hill T, Morriss R, et al. Antidepressant use and risk of suicide and attempted suicide or self harm in people aged 20 to 64: cohort study using a primary care database. BMJ. 2015;350:h517. doi: 10.1136/bmj.h517.
In patients with clinical depression, rates of suicide and self-harm are similar among those treated with selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants but significantly higher among those treated with other antidepressants, according to a review of 238,963 patients who were diagnosed with depression.
During an average five years’ follow-up, researchers noted 198 cases of suicide and 5,243 cases of attempted suicide or self-harm. The following hazard ratios (HR) were associated with antidepressant use:
Absolute risk for suicide over one year ranged from 0.02% for amitriptyline to 0.19% for mirtazapine.
COMMENTARY
This large study suggests suicide rates may be greater with non-SSRI antidepressants than with SSRIs. The data are far from solid, though, because of the small number of events and the potential for systematic differences in how these antidepressants are prescribed. For instance, if dual norepinephrine and serotonin agents are prescribed more often to individuals with more severe depression, then the increased suicide risk with use of combined norepinephrine/serotonin agents (eg, venlafaxine) could relate to the severity of the depression treated, not to an effect of the medication. Of importance is that the rate of suicide was increased in the first 28 days after starting an antidepressant and in the 28 days after stopping the antidepressant, times when we should have increased vigilance for suicidal ideation.