User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
ED visits due to anaphylaxis doubled at Canadian children’s hospital
The percentage of emergency department (ED) visits due to anaphylaxis more than doubled from 2011 to 2015 at one Canadian children’s hospital, according to a Research Letter to the Editor published in the Journal of Allergy and Clinical Immunology.
“Our results are limited to one pediatric center, but they suggest a worrisome increase in anaphylaxis rate that is consistent with the worldwide reported increase,” said Dr. Elana Hochstadter of the Hospital for Sick Children, University of Toronto, and her associates. The investigators analyzed longitudinal data in a national registry of anaphylaxis cases to track time trends for the disorder at their hospital. They identified 965 cases presenting to their ED during a 4-year period. The percentage of all ED visits accounted for by anaphylaxis rose from 0.20% to 0.41%. The overall volume of ED visits and the volume of specific ED diagnoses did not change during this interval.
As in other studies of anaphylaxis around the world, food was the most common trigger in this series, responsible for 82% of cases. Peanut was the most common food allergen, accounting for 22% of cases. Most reactions were of moderate severity, and the percentages of mild, moderate, and severe reactions remained relatively stable throughout the study period. The presence of asthma was associated with increased severity of anaphylaxis (odds ratio, 2.3), as was the presence of eczema (OR, 2.1). Only half of the patients who had an epinephrine autoinjector used it before presenting to the ED, Dr. Hochstadter and her associates said (J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.02.016). The median age of the patients was 6 years.
The reason for this rapid increase is unknown, but it parallels that reported in studies of anaphylaxis throughout North America and Europe. “An important observation in our study is that administration of epinephrine before arrival in the ED is independently associated with a decreased likelihood of requiring multiple doses of epinephrine in the ED, suggesting that prompt epinephrine administration is beneficial,” they noted.
The Allergy, Genes, and Environment Network Centres of Excellence, Health Canada, and Sanofi funded the study. Dr. Hochstadter and her associates reported having no relevant disclosures.
The percentage of emergency department (ED) visits due to anaphylaxis more than doubled from 2011 to 2015 at one Canadian children’s hospital, according to a Research Letter to the Editor published in the Journal of Allergy and Clinical Immunology.
“Our results are limited to one pediatric center, but they suggest a worrisome increase in anaphylaxis rate that is consistent with the worldwide reported increase,” said Dr. Elana Hochstadter of the Hospital for Sick Children, University of Toronto, and her associates. The investigators analyzed longitudinal data in a national registry of anaphylaxis cases to track time trends for the disorder at their hospital. They identified 965 cases presenting to their ED during a 4-year period. The percentage of all ED visits accounted for by anaphylaxis rose from 0.20% to 0.41%. The overall volume of ED visits and the volume of specific ED diagnoses did not change during this interval.
As in other studies of anaphylaxis around the world, food was the most common trigger in this series, responsible for 82% of cases. Peanut was the most common food allergen, accounting for 22% of cases. Most reactions were of moderate severity, and the percentages of mild, moderate, and severe reactions remained relatively stable throughout the study period. The presence of asthma was associated with increased severity of anaphylaxis (odds ratio, 2.3), as was the presence of eczema (OR, 2.1). Only half of the patients who had an epinephrine autoinjector used it before presenting to the ED, Dr. Hochstadter and her associates said (J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.02.016). The median age of the patients was 6 years.
The reason for this rapid increase is unknown, but it parallels that reported in studies of anaphylaxis throughout North America and Europe. “An important observation in our study is that administration of epinephrine before arrival in the ED is independently associated with a decreased likelihood of requiring multiple doses of epinephrine in the ED, suggesting that prompt epinephrine administration is beneficial,” they noted.
The Allergy, Genes, and Environment Network Centres of Excellence, Health Canada, and Sanofi funded the study. Dr. Hochstadter and her associates reported having no relevant disclosures.
The percentage of emergency department (ED) visits due to anaphylaxis more than doubled from 2011 to 2015 at one Canadian children’s hospital, according to a Research Letter to the Editor published in the Journal of Allergy and Clinical Immunology.
“Our results are limited to one pediatric center, but they suggest a worrisome increase in anaphylaxis rate that is consistent with the worldwide reported increase,” said Dr. Elana Hochstadter of the Hospital for Sick Children, University of Toronto, and her associates. The investigators analyzed longitudinal data in a national registry of anaphylaxis cases to track time trends for the disorder at their hospital. They identified 965 cases presenting to their ED during a 4-year period. The percentage of all ED visits accounted for by anaphylaxis rose from 0.20% to 0.41%. The overall volume of ED visits and the volume of specific ED diagnoses did not change during this interval.
As in other studies of anaphylaxis around the world, food was the most common trigger in this series, responsible for 82% of cases. Peanut was the most common food allergen, accounting for 22% of cases. Most reactions were of moderate severity, and the percentages of mild, moderate, and severe reactions remained relatively stable throughout the study period. The presence of asthma was associated with increased severity of anaphylaxis (odds ratio, 2.3), as was the presence of eczema (OR, 2.1). Only half of the patients who had an epinephrine autoinjector used it before presenting to the ED, Dr. Hochstadter and her associates said (J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.02.016). The median age of the patients was 6 years.
The reason for this rapid increase is unknown, but it parallels that reported in studies of anaphylaxis throughout North America and Europe. “An important observation in our study is that administration of epinephrine before arrival in the ED is independently associated with a decreased likelihood of requiring multiple doses of epinephrine in the ED, suggesting that prompt epinephrine administration is beneficial,” they noted.
The Allergy, Genes, and Environment Network Centres of Excellence, Health Canada, and Sanofi funded the study. Dr. Hochstadter and her associates reported having no relevant disclosures.
FROM JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY
Key clinical point: The percentage of ED visits due to anaphylaxis more than doubled at one Canadian children’s hospital between 2011 and 2015.
Major finding: The percentage of all ED visits accounted for by anaphylaxis rose from 0.20% to 0.41% during the 4-year study.
Data source: A single-center longitudinal analysis of 965 ED visits for anaphylaxis.
Disclosures: The Allergy, Genes, and Environment Network Centres of Excellence, Health Canada, and Sanofi funded the study. Dr. Hochstadter and her associates reported having no relevant disclosures.
Survey: Teens view e-cigarettes as less harmful than traditional tobacco products
New and emerging tobacco products such as hookah and e-cigarettes are perceived as being less harmful and more socially acceptable than traditional cigarettes, according to Maria Roditis, Ph.D., and her associates.
In a survey of 722 students aged 13-19, cigarettes were perceived as being most harmful to one’s health, to the health of friends, and to the environment. Perceived harm to one’s own health was similar for cigars and chewing tobacco, but chewing tobacco was seen as significantly less harmful to friends and to the environment. E-cigarettes were seen as least harmful overall, followed by hookah.
E-cigarettes were seen to have the least social risk, while cigarettes and cigars had the greatest social risk. Chewing tobacco had the smallest perceived social benefit, while hookah had the greatest social benefit. All tobacco products were seen as having significant long-term health risks, though the effect was smaller in older adolescents and for e-cigarettes.
“There is a clear need to expand messaging campaigns to discuss the risks related to all tobacco products and not focus solely on the risk of cigarettes alone. Although such messaging campaigns should continually be updated to reflect the current body of research, the public health community needs to actively start messaging on known risks related to all tobacco products now,” the investigators noted.
Find the full study in the Journal of Adolescent Health (doi: 10.1016/j.jadohealth.2016.01.012).
New and emerging tobacco products such as hookah and e-cigarettes are perceived as being less harmful and more socially acceptable than traditional cigarettes, according to Maria Roditis, Ph.D., and her associates.
In a survey of 722 students aged 13-19, cigarettes were perceived as being most harmful to one’s health, to the health of friends, and to the environment. Perceived harm to one’s own health was similar for cigars and chewing tobacco, but chewing tobacco was seen as significantly less harmful to friends and to the environment. E-cigarettes were seen as least harmful overall, followed by hookah.
E-cigarettes were seen to have the least social risk, while cigarettes and cigars had the greatest social risk. Chewing tobacco had the smallest perceived social benefit, while hookah had the greatest social benefit. All tobacco products were seen as having significant long-term health risks, though the effect was smaller in older adolescents and for e-cigarettes.
“There is a clear need to expand messaging campaigns to discuss the risks related to all tobacco products and not focus solely on the risk of cigarettes alone. Although such messaging campaigns should continually be updated to reflect the current body of research, the public health community needs to actively start messaging on known risks related to all tobacco products now,” the investigators noted.
Find the full study in the Journal of Adolescent Health (doi: 10.1016/j.jadohealth.2016.01.012).
New and emerging tobacco products such as hookah and e-cigarettes are perceived as being less harmful and more socially acceptable than traditional cigarettes, according to Maria Roditis, Ph.D., and her associates.
In a survey of 722 students aged 13-19, cigarettes were perceived as being most harmful to one’s health, to the health of friends, and to the environment. Perceived harm to one’s own health was similar for cigars and chewing tobacco, but chewing tobacco was seen as significantly less harmful to friends and to the environment. E-cigarettes were seen as least harmful overall, followed by hookah.
E-cigarettes were seen to have the least social risk, while cigarettes and cigars had the greatest social risk. Chewing tobacco had the smallest perceived social benefit, while hookah had the greatest social benefit. All tobacco products were seen as having significant long-term health risks, though the effect was smaller in older adolescents and for e-cigarettes.
“There is a clear need to expand messaging campaigns to discuss the risks related to all tobacco products and not focus solely on the risk of cigarettes alone. Although such messaging campaigns should continually be updated to reflect the current body of research, the public health community needs to actively start messaging on known risks related to all tobacco products now,” the investigators noted.
Find the full study in the Journal of Adolescent Health (doi: 10.1016/j.jadohealth.2016.01.012).
FROM THE JOURNAL OF ADOLESCENT HEALTH
Paperwork snarls stand between kids and at-school asthma medications
BALTIMORE – Four out of five children with asthma didn’t have access to their medication at school because the proper paperwork was missing, according to a survey of 10 inner-city Milwaukee elementary schools.
The number of students who had the required physician-signed authorization forms remained low throughout the school year, said Dr. Santiago Encalada, a pulmonary fellow at the Medical College of Wisconsin, Milwaukee.
Dr. Encalada cited administrative hurdles, lack of standardization, and challenges in school-physician-family communication as barriers to children’s access to asthma medication at school. Although school nurses in Milwaukee have standing orders for emergency albuterol administration, they otherwise need physician signatures on school-generated forms to administer both rescue and prophylactic asthma administration.
In a study whose purpose was to assess the percentage of children with asthma who had appropriate orders on file in a sample of 10 Milwaukee inner-city schools, the schools had orders on file for just 11% of students, on average, at the beginning of the 2014-2015 school year. At the second assessment in January 2015, the average number of students with orders on file at each school had risen to 22%, with schools that had performed better earlier also showing greater gains at mid-year. However, the June 2015 assessment showed that the gains did not continue, with the schools’ aggregate average of 21% of students with appropriate orders showing no improvement from mid-year.
The number of students with asthma in schools varied from about 40 to nearly 200. Numbers varied through the school year as enrollments shifted in these high-need schools, said Dr. Encalada, who presented his findings during a poster session at the annual meeting of the Pediatric Academic Societies. In general, the schools with lower enrollments tended to do better with having orders on file, although statistical analysis was not performed for this variable.
“On average, 80% of asthmatic students in the inner city schools we studied did not have school forms or orders available for life-saving asthma rescue medications, with significant variation between schools. Our findings show that access to even basic asthma care necessities are lagging for this vulnerable population, and a significant disparity exists even within this population,” said senior author Nicholas Antos*, associate director of the Cystic Fibrosis Center at Milwaukee’s Children’s Hospital of Wisconsin.
In interviews and discussion with school nurses and physicians’ offices, Dr. Antos* and Dr. Encalada found that there were often simple but fundamental misunderstandings that impeded the proper flow of paperwork. For example, schools in Milwaukee do not have standardized forms that authorize administration of prescription medications at school, so forms may be confusing to providers and their staff. Privacy concerns sometimes impeded the ability of clinic staff to authorize treatment for students. Also, the inevitable shuffle of paperwork in school-aged families meant that the forms sometimes were simply lost on the way to school.
Understanding the barriers in the process both on the school side and in physician offices has helped Dr. Antos*, Dr. Encalada, and their colleagues to start to build a better pathway. For example, a module has been built into the EHR asthma visit template that allows easy generation of a school form and asks for patient consent for release of information to the schools.
Dr. Antos* said in an interview that the work is ongoing: “To help address these problems, we have devised interventions to improve the way school nurses can contact clinicians, and helped design innovative standardized Asthma Action Plans that can double as school orders.”
In addition to working with local providers and schools, Dr. Encalada and Dr. Antos* have reached out to pediatric societies and the American Academy of Asthma, Allergy, and Immunology (AAAAI). Emphasizing the need for “education of stakeholders of all types,” Dr. Antos* said that change “may be difficult, but we hope with the support of pediatric organizations, the AAAAI, and school administrators, we can begin to break down the barriers preventing quality and timely communication with school nurses.”
The authors had no financial disclosures. The study was funded by the Centers for Disease Control and Prevention through the Wisconsin Asthma Coalition (WAC).
On Twitter @karioakes
*In a previous version, Dr. Antos' name was misspelled.
Dr. Susan Millard, FCCP: comments: The issues identified in this article are huge and not just an occurrence in the inner cities. The critical problem is that the children are even more at risk when living in the inner cities and for sudden death due to asthma. Having one form for the whole state would help tremendously because we could print out an asthma action plan and the form for the school and then fax it directly!
Dr. Susan Millard, FCCP: comments: The issues identified in this article are huge and not just an occurrence in the inner cities. The critical problem is that the children are even more at risk when living in the inner cities and for sudden death due to asthma. Having one form for the whole state would help tremendously because we could print out an asthma action plan and the form for the school and then fax it directly!
Dr. Susan Millard, FCCP: comments: The issues identified in this article are huge and not just an occurrence in the inner cities. The critical problem is that the children are even more at risk when living in the inner cities and for sudden death due to asthma. Having one form for the whole state would help tremendously because we could print out an asthma action plan and the form for the school and then fax it directly!
BALTIMORE – Four out of five children with asthma didn’t have access to their medication at school because the proper paperwork was missing, according to a survey of 10 inner-city Milwaukee elementary schools.
The number of students who had the required physician-signed authorization forms remained low throughout the school year, said Dr. Santiago Encalada, a pulmonary fellow at the Medical College of Wisconsin, Milwaukee.
Dr. Encalada cited administrative hurdles, lack of standardization, and challenges in school-physician-family communication as barriers to children’s access to asthma medication at school. Although school nurses in Milwaukee have standing orders for emergency albuterol administration, they otherwise need physician signatures on school-generated forms to administer both rescue and prophylactic asthma administration.
In a study whose purpose was to assess the percentage of children with asthma who had appropriate orders on file in a sample of 10 Milwaukee inner-city schools, the schools had orders on file for just 11% of students, on average, at the beginning of the 2014-2015 school year. At the second assessment in January 2015, the average number of students with orders on file at each school had risen to 22%, with schools that had performed better earlier also showing greater gains at mid-year. However, the June 2015 assessment showed that the gains did not continue, with the schools’ aggregate average of 21% of students with appropriate orders showing no improvement from mid-year.
The number of students with asthma in schools varied from about 40 to nearly 200. Numbers varied through the school year as enrollments shifted in these high-need schools, said Dr. Encalada, who presented his findings during a poster session at the annual meeting of the Pediatric Academic Societies. In general, the schools with lower enrollments tended to do better with having orders on file, although statistical analysis was not performed for this variable.
“On average, 80% of asthmatic students in the inner city schools we studied did not have school forms or orders available for life-saving asthma rescue medications, with significant variation between schools. Our findings show that access to even basic asthma care necessities are lagging for this vulnerable population, and a significant disparity exists even within this population,” said senior author Nicholas Antos*, associate director of the Cystic Fibrosis Center at Milwaukee’s Children’s Hospital of Wisconsin.
In interviews and discussion with school nurses and physicians’ offices, Dr. Antos* and Dr. Encalada found that there were often simple but fundamental misunderstandings that impeded the proper flow of paperwork. For example, schools in Milwaukee do not have standardized forms that authorize administration of prescription medications at school, so forms may be confusing to providers and their staff. Privacy concerns sometimes impeded the ability of clinic staff to authorize treatment for students. Also, the inevitable shuffle of paperwork in school-aged families meant that the forms sometimes were simply lost on the way to school.
Understanding the barriers in the process both on the school side and in physician offices has helped Dr. Antos*, Dr. Encalada, and their colleagues to start to build a better pathway. For example, a module has been built into the EHR asthma visit template that allows easy generation of a school form and asks for patient consent for release of information to the schools.
Dr. Antos* said in an interview that the work is ongoing: “To help address these problems, we have devised interventions to improve the way school nurses can contact clinicians, and helped design innovative standardized Asthma Action Plans that can double as school orders.”
In addition to working with local providers and schools, Dr. Encalada and Dr. Antos* have reached out to pediatric societies and the American Academy of Asthma, Allergy, and Immunology (AAAAI). Emphasizing the need for “education of stakeholders of all types,” Dr. Antos* said that change “may be difficult, but we hope with the support of pediatric organizations, the AAAAI, and school administrators, we can begin to break down the barriers preventing quality and timely communication with school nurses.”
The authors had no financial disclosures. The study was funded by the Centers for Disease Control and Prevention through the Wisconsin Asthma Coalition (WAC).
On Twitter @karioakes
*In a previous version, Dr. Antos' name was misspelled.
BALTIMORE – Four out of five children with asthma didn’t have access to their medication at school because the proper paperwork was missing, according to a survey of 10 inner-city Milwaukee elementary schools.
The number of students who had the required physician-signed authorization forms remained low throughout the school year, said Dr. Santiago Encalada, a pulmonary fellow at the Medical College of Wisconsin, Milwaukee.
Dr. Encalada cited administrative hurdles, lack of standardization, and challenges in school-physician-family communication as barriers to children’s access to asthma medication at school. Although school nurses in Milwaukee have standing orders for emergency albuterol administration, they otherwise need physician signatures on school-generated forms to administer both rescue and prophylactic asthma administration.
In a study whose purpose was to assess the percentage of children with asthma who had appropriate orders on file in a sample of 10 Milwaukee inner-city schools, the schools had orders on file for just 11% of students, on average, at the beginning of the 2014-2015 school year. At the second assessment in January 2015, the average number of students with orders on file at each school had risen to 22%, with schools that had performed better earlier also showing greater gains at mid-year. However, the June 2015 assessment showed that the gains did not continue, with the schools’ aggregate average of 21% of students with appropriate orders showing no improvement from mid-year.
The number of students with asthma in schools varied from about 40 to nearly 200. Numbers varied through the school year as enrollments shifted in these high-need schools, said Dr. Encalada, who presented his findings during a poster session at the annual meeting of the Pediatric Academic Societies. In general, the schools with lower enrollments tended to do better with having orders on file, although statistical analysis was not performed for this variable.
“On average, 80% of asthmatic students in the inner city schools we studied did not have school forms or orders available for life-saving asthma rescue medications, with significant variation between schools. Our findings show that access to even basic asthma care necessities are lagging for this vulnerable population, and a significant disparity exists even within this population,” said senior author Nicholas Antos*, associate director of the Cystic Fibrosis Center at Milwaukee’s Children’s Hospital of Wisconsin.
In interviews and discussion with school nurses and physicians’ offices, Dr. Antos* and Dr. Encalada found that there were often simple but fundamental misunderstandings that impeded the proper flow of paperwork. For example, schools in Milwaukee do not have standardized forms that authorize administration of prescription medications at school, so forms may be confusing to providers and their staff. Privacy concerns sometimes impeded the ability of clinic staff to authorize treatment for students. Also, the inevitable shuffle of paperwork in school-aged families meant that the forms sometimes were simply lost on the way to school.
Understanding the barriers in the process both on the school side and in physician offices has helped Dr. Antos*, Dr. Encalada, and their colleagues to start to build a better pathway. For example, a module has been built into the EHR asthma visit template that allows easy generation of a school form and asks for patient consent for release of information to the schools.
Dr. Antos* said in an interview that the work is ongoing: “To help address these problems, we have devised interventions to improve the way school nurses can contact clinicians, and helped design innovative standardized Asthma Action Plans that can double as school orders.”
In addition to working with local providers and schools, Dr. Encalada and Dr. Antos* have reached out to pediatric societies and the American Academy of Asthma, Allergy, and Immunology (AAAAI). Emphasizing the need for “education of stakeholders of all types,” Dr. Antos* said that change “may be difficult, but we hope with the support of pediatric organizations, the AAAAI, and school administrators, we can begin to break down the barriers preventing quality and timely communication with school nurses.”
The authors had no financial disclosures. The study was funded by the Centers for Disease Control and Prevention through the Wisconsin Asthma Coalition (WAC).
On Twitter @karioakes
*In a previous version, Dr. Antos' name was misspelled.
AT THE PAS ANNUAL MEETING
Key clinical point: Four out of five high-risk elementary school children lacked proper orders for at-school asthma medication administration.
Major finding: The average number of elementary school children with asthma medication orders on file was 21% at year’s end.
Data source: Yearlong study of 10 inner-city Milwaukee elementary schools; enrollees with asthma ranged from about 40 to nearly 200.
Disclosures: The study was funded by the Centers for Disease Control and Prevention through the Wisconsin Asthma Coalition (WAC).
Seasonal flu holding strong in New Jersey
The 2015-2016 seasonal influenza virus has gotten hold of New Jersey and just won’t let go, according to the latest data from the Centers for Disease Control and Prevention.
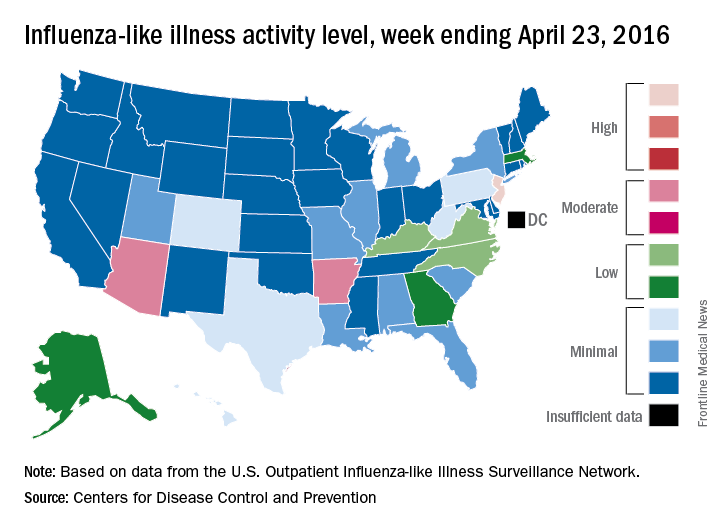
For the week ending April 23, 2016, influenza-like illness (ILI) activity in the United States remained at level 10 on the CDC’s 1-10 scale for the 11th consecutive week, even as the country’s overall proportion of outpatient visits for ILI dropped to 2.0%, which is below the national baseline of 2.1%, the CDC reported.
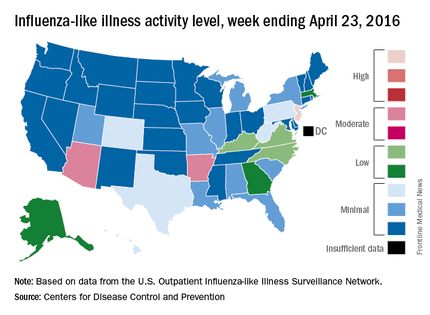
Two other states – Arizona and Arkansas – joined New Jersey in bucking the trend of decreasing ILI activity, as both moved up to level 7 and the high end of the “moderate” range. Arizona had been at level 5 the week before, while Arkansas was at level 4. No other state was above level 5 for the most recent week, and 27 states were at level 1, data from the CDC’s Influenza-like Illness Surveillance Network (ILINet) show.
Four flu-related pediatric deaths were reported during the week ending April 23, only one of which occurred that week. The total number of pediatric deaths rose to 60 for the 2015-2016 season, with 27 states and Puerto Rico reporting deaths so far, the CDC noted.
The CDC also reported a cumulative influenza-associated hospitalization rate for the season of 29.8 such hospitalizations per 100,000 population. This data was based on 8,239 laboratory-confirmed influenza-associated hospitalizations reported between October 1, 2015 and April 23, 2016. The highest rate of hospitalization was among adults aged 65 years or older (79.6 per 100,000 population), followed by adults aged 50-64 (43.1 per 100,000 population) and children aged 0-4 years (40.5 per 100,000 population). Among all hospitalizations, 6,254 (75.9%) were associated with influenza A, 1,905 (23.1%) with influenza B, 41 (0.5%) with influenza A and B co-infection, and 39 (0.5%) had no virus type information.
The 2015-2016 seasonal influenza virus has gotten hold of New Jersey and just won’t let go, according to the latest data from the Centers for Disease Control and Prevention.
For the week ending April 23, 2016, influenza-like illness (ILI) activity in the United States remained at level 10 on the CDC’s 1-10 scale for the 11th consecutive week, even as the country’s overall proportion of outpatient visits for ILI dropped to 2.0%, which is below the national baseline of 2.1%, the CDC reported.

Two other states – Arizona and Arkansas – joined New Jersey in bucking the trend of decreasing ILI activity, as both moved up to level 7 and the high end of the “moderate” range. Arizona had been at level 5 the week before, while Arkansas was at level 4. No other state was above level 5 for the most recent week, and 27 states were at level 1, data from the CDC’s Influenza-like Illness Surveillance Network (ILINet) show.
Four flu-related pediatric deaths were reported during the week ending April 23, only one of which occurred that week. The total number of pediatric deaths rose to 60 for the 2015-2016 season, with 27 states and Puerto Rico reporting deaths so far, the CDC noted.
The CDC also reported a cumulative influenza-associated hospitalization rate for the season of 29.8 such hospitalizations per 100,000 population. This data was based on 8,239 laboratory-confirmed influenza-associated hospitalizations reported between October 1, 2015 and April 23, 2016. The highest rate of hospitalization was among adults aged 65 years or older (79.6 per 100,000 population), followed by adults aged 50-64 (43.1 per 100,000 population) and children aged 0-4 years (40.5 per 100,000 population). Among all hospitalizations, 6,254 (75.9%) were associated with influenza A, 1,905 (23.1%) with influenza B, 41 (0.5%) with influenza A and B co-infection, and 39 (0.5%) had no virus type information.
The 2015-2016 seasonal influenza virus has gotten hold of New Jersey and just won’t let go, according to the latest data from the Centers for Disease Control and Prevention.
For the week ending April 23, 2016, influenza-like illness (ILI) activity in the United States remained at level 10 on the CDC’s 1-10 scale for the 11th consecutive week, even as the country’s overall proportion of outpatient visits for ILI dropped to 2.0%, which is below the national baseline of 2.1%, the CDC reported.

Two other states – Arizona and Arkansas – joined New Jersey in bucking the trend of decreasing ILI activity, as both moved up to level 7 and the high end of the “moderate” range. Arizona had been at level 5 the week before, while Arkansas was at level 4. No other state was above level 5 for the most recent week, and 27 states were at level 1, data from the CDC’s Influenza-like Illness Surveillance Network (ILINet) show.
Four flu-related pediatric deaths were reported during the week ending April 23, only one of which occurred that week. The total number of pediatric deaths rose to 60 for the 2015-2016 season, with 27 states and Puerto Rico reporting deaths so far, the CDC noted.
The CDC also reported a cumulative influenza-associated hospitalization rate for the season of 29.8 such hospitalizations per 100,000 population. This data was based on 8,239 laboratory-confirmed influenza-associated hospitalizations reported between October 1, 2015 and April 23, 2016. The highest rate of hospitalization was among adults aged 65 years or older (79.6 per 100,000 population), followed by adults aged 50-64 (43.1 per 100,000 population) and children aged 0-4 years (40.5 per 100,000 population). Among all hospitalizations, 6,254 (75.9%) were associated with influenza A, 1,905 (23.1%) with influenza B, 41 (0.5%) with influenza A and B co-infection, and 39 (0.5%) had no virus type information.
Vedolizumab use linked to high rate of postoperative complications in IBD patients
LOS ANGELES – Overall, 44% of inflammatory bowel disease (IBD) patients on vedolizumab had some form of infectious complication following intra-abdominal or anorectal surgery, results from a small single-center study suggest.
According to lead study author Dr. Samuel Eisenstein, there are currently no published surgical outcomes of patients receiving vedolizumab, an integrin receptor antagonist which was approved in May 2014 for the treatment of adults with moderate to severe ulcerative colitis as well as those with moderate to severe Crohn’s disease. “We’re not trying to alienate people who are proponents of the medication,” Dr. Eisenstein said in an interview in advance of the annual meeting of the American Society of Colon and Rectal Surgeons. “It’s an effective medication for treating Crohn’s and ulcerative colitis. We need to have a high index of suspicion that patients may have complications after these surgeries and to treat them with caution until we have better data.”
Dr. Eisenstein and his associates in the section of colon and rectal surgery at Moores Cancer Center, University of California, San Diego, Health System, retrospectively analyzed the medical records of 26 patients with IBD who underwent intra-abdominal or anorectal surgery at the center following treatment with vedolizumab. The patients underwent a total of 36 operations: 27 that were intra-abdominal and 9 that were anorectal. Their mean age was 31 years and 46% were female.
Dr. Eisenstein reported that 17 of the 26 patients (65%) had a Clavien-Dindo grade II or greater complication following 19 operations. In all, 26 complications occurred following these 19 operations, and 53% were infectious in nature. The overall rate of infectious complications following any operation was 44%. In addition, the rate of anastomotic leak was 15%, and two patients died from culture-negative sepsis following abdominal surgery, for an overall mortality rate of 7.7%.
The researchers also observed that there were 23 visits to the emergency room following surgery and 10 hospital readmissions. The only preoperative characteristics that differed significantly between patients who had complications and those who did not were level of hemoglobin (10.6 g/dL vs. 11.9 g/dL, respectively; P = .02) and platelet count (349 vs. 287 K/mm3; P = .025). No differences in the rate of complications were observed based on the number of biologic medications each patient failed prior to the initiation of vedolizumab (P = .718). Compared with patients who had no postoperative complications, those who did were more likely to have undergone intra-abdominal surgery (17 vs. 10 patients; P = .034), require postoperative transfusion (4 vs. none; P = .045), visit the emergency department (10 vs. none; P less than .001), or require hospital readmission (10 vs. none; P less than .001).
Dr. Eisenstein acknowledged certain limitations of the study including its small sample size, single-center, retrospective design, and the potential for selection bias. “The patients who were getting vedolizumab are the patients who failed all of the anti-TNFs, so we’re really selecting patients with the worst, most medically refractory disease,” he noted. “Because of that we can’t say for sure [if the complications] are due to their severity of disease or due to the medication itself.”
The data are “preliminary and retrospectively analyzed, but there is some concern that patients on these types of medications may have an increased risk of postoperative complications,” he concluded. “What we really need are bigger studies. To that end, we are actually starting an IBD collaborative based on some of the findings we have here, because we really want to analyze these data over a much larger population of patients.”
The researchers reported having no financial disclosures.
LOS ANGELES – Overall, 44% of inflammatory bowel disease (IBD) patients on vedolizumab had some form of infectious complication following intra-abdominal or anorectal surgery, results from a small single-center study suggest.
According to lead study author Dr. Samuel Eisenstein, there are currently no published surgical outcomes of patients receiving vedolizumab, an integrin receptor antagonist which was approved in May 2014 for the treatment of adults with moderate to severe ulcerative colitis as well as those with moderate to severe Crohn’s disease. “We’re not trying to alienate people who are proponents of the medication,” Dr. Eisenstein said in an interview in advance of the annual meeting of the American Society of Colon and Rectal Surgeons. “It’s an effective medication for treating Crohn’s and ulcerative colitis. We need to have a high index of suspicion that patients may have complications after these surgeries and to treat them with caution until we have better data.”
Dr. Eisenstein and his associates in the section of colon and rectal surgery at Moores Cancer Center, University of California, San Diego, Health System, retrospectively analyzed the medical records of 26 patients with IBD who underwent intra-abdominal or anorectal surgery at the center following treatment with vedolizumab. The patients underwent a total of 36 operations: 27 that were intra-abdominal and 9 that were anorectal. Their mean age was 31 years and 46% were female.
Dr. Eisenstein reported that 17 of the 26 patients (65%) had a Clavien-Dindo grade II or greater complication following 19 operations. In all, 26 complications occurred following these 19 operations, and 53% were infectious in nature. The overall rate of infectious complications following any operation was 44%. In addition, the rate of anastomotic leak was 15%, and two patients died from culture-negative sepsis following abdominal surgery, for an overall mortality rate of 7.7%.
The researchers also observed that there were 23 visits to the emergency room following surgery and 10 hospital readmissions. The only preoperative characteristics that differed significantly between patients who had complications and those who did not were level of hemoglobin (10.6 g/dL vs. 11.9 g/dL, respectively; P = .02) and platelet count (349 vs. 287 K/mm3; P = .025). No differences in the rate of complications were observed based on the number of biologic medications each patient failed prior to the initiation of vedolizumab (P = .718). Compared with patients who had no postoperative complications, those who did were more likely to have undergone intra-abdominal surgery (17 vs. 10 patients; P = .034), require postoperative transfusion (4 vs. none; P = .045), visit the emergency department (10 vs. none; P less than .001), or require hospital readmission (10 vs. none; P less than .001).
Dr. Eisenstein acknowledged certain limitations of the study including its small sample size, single-center, retrospective design, and the potential for selection bias. “The patients who were getting vedolizumab are the patients who failed all of the anti-TNFs, so we’re really selecting patients with the worst, most medically refractory disease,” he noted. “Because of that we can’t say for sure [if the complications] are due to their severity of disease or due to the medication itself.”
The data are “preliminary and retrospectively analyzed, but there is some concern that patients on these types of medications may have an increased risk of postoperative complications,” he concluded. “What we really need are bigger studies. To that end, we are actually starting an IBD collaborative based on some of the findings we have here, because we really want to analyze these data over a much larger population of patients.”
The researchers reported having no financial disclosures.
LOS ANGELES – Overall, 44% of inflammatory bowel disease (IBD) patients on vedolizumab had some form of infectious complication following intra-abdominal or anorectal surgery, results from a small single-center study suggest.
According to lead study author Dr. Samuel Eisenstein, there are currently no published surgical outcomes of patients receiving vedolizumab, an integrin receptor antagonist which was approved in May 2014 for the treatment of adults with moderate to severe ulcerative colitis as well as those with moderate to severe Crohn’s disease. “We’re not trying to alienate people who are proponents of the medication,” Dr. Eisenstein said in an interview in advance of the annual meeting of the American Society of Colon and Rectal Surgeons. “It’s an effective medication for treating Crohn’s and ulcerative colitis. We need to have a high index of suspicion that patients may have complications after these surgeries and to treat them with caution until we have better data.”
Dr. Eisenstein and his associates in the section of colon and rectal surgery at Moores Cancer Center, University of California, San Diego, Health System, retrospectively analyzed the medical records of 26 patients with IBD who underwent intra-abdominal or anorectal surgery at the center following treatment with vedolizumab. The patients underwent a total of 36 operations: 27 that were intra-abdominal and 9 that were anorectal. Their mean age was 31 years and 46% were female.
Dr. Eisenstein reported that 17 of the 26 patients (65%) had a Clavien-Dindo grade II or greater complication following 19 operations. In all, 26 complications occurred following these 19 operations, and 53% were infectious in nature. The overall rate of infectious complications following any operation was 44%. In addition, the rate of anastomotic leak was 15%, and two patients died from culture-negative sepsis following abdominal surgery, for an overall mortality rate of 7.7%.
The researchers also observed that there were 23 visits to the emergency room following surgery and 10 hospital readmissions. The only preoperative characteristics that differed significantly between patients who had complications and those who did not were level of hemoglobin (10.6 g/dL vs. 11.9 g/dL, respectively; P = .02) and platelet count (349 vs. 287 K/mm3; P = .025). No differences in the rate of complications were observed based on the number of biologic medications each patient failed prior to the initiation of vedolizumab (P = .718). Compared with patients who had no postoperative complications, those who did were more likely to have undergone intra-abdominal surgery (17 vs. 10 patients; P = .034), require postoperative transfusion (4 vs. none; P = .045), visit the emergency department (10 vs. none; P less than .001), or require hospital readmission (10 vs. none; P less than .001).
Dr. Eisenstein acknowledged certain limitations of the study including its small sample size, single-center, retrospective design, and the potential for selection bias. “The patients who were getting vedolizumab are the patients who failed all of the anti-TNFs, so we’re really selecting patients with the worst, most medically refractory disease,” he noted. “Because of that we can’t say for sure [if the complications] are due to their severity of disease or due to the medication itself.”
The data are “preliminary and retrospectively analyzed, but there is some concern that patients on these types of medications may have an increased risk of postoperative complications,” he concluded. “What we really need are bigger studies. To that end, we are actually starting an IBD collaborative based on some of the findings we have here, because we really want to analyze these data over a much larger population of patients.”
The researchers reported having no financial disclosures.
AT THE ASCRS ANNUAL MEETING
Key clinical point:Patients on vedolizumab have a high rate of postoperative complications.
Major finding: The overall rate of infectious complications following intra-abdominal or anorectal surgery was 44%.
Data source: A retrospective study of 26 patients with IBD who underwent intra-abdominal or anorectal surgery following treatment with vedolizumab.
Disclosures: Dr. Eisenstein reported having no financial disclosures.
Food allergy development linked to S. aureus colonization in children with AD
Staphylococcus aureus colonization is associated with development of food allergy in children with atopic dermatitis (AD), according to a letter to the editor from Dr. Andrea L. Jones and her associates.
In a study of 718 patients with AD, median food allergen–specific IgE levels to peanut were highest in patients with methicillin-resistant Staphylococcus aureus (MRSA) at 77.7 kilounits of allergen per liter. Patients with methicillin-sensitive S. aureus (MSSA) had median food allergen–specific IgE (sIgE) levels to peanut of 38.9 kUA/L, and patients without S. aureus had median sIgE levels to peanut of 4.3 kUA/L, below the 95% positive predictive value of oral food challenge reaction in patients of 14 kUA/L.
Total IgE levels were highest in AD patients with MRSA at 4,498 kU/L, but were also elevated in patients with MSSA at 2,709 kU/L, compared with 217 kU/L for patients without S. aureus colonization.
“Studies are needed to assess the association between S. aureus skin colonization and food allergy in patients with AD. Confirmation of our current observations opens up the possibility that therapy directed at eradicating S. aureus colonization will be important in the prevention of food allergen sensitization and possibly food allergy in patients with AD,” the investigators concluded.
Find the full letter in the Journal of Allergy and Clinical Immunology (2016 Apr. doi: 10.1016/j.jaci.2016.01.010).
Staphylococcus aureus colonization is associated with development of food allergy in children with atopic dermatitis (AD), according to a letter to the editor from Dr. Andrea L. Jones and her associates.
In a study of 718 patients with AD, median food allergen–specific IgE levels to peanut were highest in patients with methicillin-resistant Staphylococcus aureus (MRSA) at 77.7 kilounits of allergen per liter. Patients with methicillin-sensitive S. aureus (MSSA) had median food allergen–specific IgE (sIgE) levels to peanut of 38.9 kUA/L, and patients without S. aureus had median sIgE levels to peanut of 4.3 kUA/L, below the 95% positive predictive value of oral food challenge reaction in patients of 14 kUA/L.
Total IgE levels were highest in AD patients with MRSA at 4,498 kU/L, but were also elevated in patients with MSSA at 2,709 kU/L, compared with 217 kU/L for patients without S. aureus colonization.
“Studies are needed to assess the association between S. aureus skin colonization and food allergy in patients with AD. Confirmation of our current observations opens up the possibility that therapy directed at eradicating S. aureus colonization will be important in the prevention of food allergen sensitization and possibly food allergy in patients with AD,” the investigators concluded.
Find the full letter in the Journal of Allergy and Clinical Immunology (2016 Apr. doi: 10.1016/j.jaci.2016.01.010).
Staphylococcus aureus colonization is associated with development of food allergy in children with atopic dermatitis (AD), according to a letter to the editor from Dr. Andrea L. Jones and her associates.
In a study of 718 patients with AD, median food allergen–specific IgE levels to peanut were highest in patients with methicillin-resistant Staphylococcus aureus (MRSA) at 77.7 kilounits of allergen per liter. Patients with methicillin-sensitive S. aureus (MSSA) had median food allergen–specific IgE (sIgE) levels to peanut of 38.9 kUA/L, and patients without S. aureus had median sIgE levels to peanut of 4.3 kUA/L, below the 95% positive predictive value of oral food challenge reaction in patients of 14 kUA/L.
Total IgE levels were highest in AD patients with MRSA at 4,498 kU/L, but were also elevated in patients with MSSA at 2,709 kU/L, compared with 217 kU/L for patients without S. aureus colonization.
“Studies are needed to assess the association between S. aureus skin colonization and food allergy in patients with AD. Confirmation of our current observations opens up the possibility that therapy directed at eradicating S. aureus colonization will be important in the prevention of food allergen sensitization and possibly food allergy in patients with AD,” the investigators concluded.
Find the full letter in the Journal of Allergy and Clinical Immunology (2016 Apr. doi: 10.1016/j.jaci.2016.01.010).
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY
Study reaffirms that maternal flu immunization reduces infants’ risk of flu
Infants born to mothers who received flu immunization during pregnancy were 70% less likely to contract lab-confirmed influenza and 81% less likely to be hospitalized for flu before age 6 months, compared with infants of unimmunized mothers, a study reaffirmed.
Yet just one in ten pregnant women received the vaccine, a proportion that has steadily risen since the 2009-2010 H1N1 flu season.
“The results of this large retrospective study support the conclusions of prospective studies regarding the protective benefit of maternal influenza immunization during pregnancy,” reported Dr. Julie H. Shakib of the University of Utah, Salt Lake City, and her associates (Pediatrics. 2016 May 3. doi: 10.1542/peds.2015-2360). “Interventions that target both healthy pregnant women and those with chronic conditions are needed to increase vaccine uptake,” they wrote.
The researchers analyzed self-reported seasonal influenza immunization uptake in the 245,386 women who gave birth between December 2005 and March 2014 in the Intermountain Healthcare facilities in Utah and Idaho. Although 10% of the women overall received flu vaccinations, just 2.2% of the women who delivered before the 2009-2010 H1N1 pandemic had received them. That number rose after the pandemic to 21% (P < .001). More than half (52%) of the women giving birth during the 2013-2014 flu season reported getting the seasonal flu vaccine.
Among the women’s 249,387 infants, 866 had at least one influenza-like illness (ILI), including 32 born to vaccinated women and 834 born to unvaccinated women. The infants born to women receiving the flu vaccine during pregnancy were 64% less likely to develop an ILI, with illnesses in 1.34 per 1,000 born to vaccinated women and 3.7 per 1,000 born to unvaccinated women (relative risk, 0.36).
The rates of laboratory-confirmed influenza in the 658 children who contracted it were 0.84 per 1,000 children born to vaccinated mothers and 2.83 per 1,000 children born to unvaccinated mothers, translating to a 70% lower risk of flu in those born to vaccinated mothers (RR, 0.30). Similarly, infants born to vaccinated mothers were 81% less likely to be hospitalized for lab-confirmed influenza (RR, 0.19, P = .005). Just 3 of 151 hospitalized infants had been born to mothers who received the flu vaccine, for a rate of 0.13 per 1,000 for children of vaccinated mothers and 0.66 per 1,000 for children of unvaccinated mothers.
Pregnant women with public insurance or no insurance were less likely to report getting the seasonal vaccine than were privately insured women, but those with chronic conditions were more likely to be vaccinated. Uptake also was lower among women with incomes below the federal poverty level and among women living in either rural or frontier areas or in the Urban South or Southwest Intermountain regions.
“The Intermountain Urban South region includes Utah County, 1 of 30 U.S. counties with the largest estimated numbers of unvaccinated children from 1995-2001 CDC National Immunization Surveys (NIS) data,” the authors wrote. “It is possible that factors leading to parental vaccine hesitancy in children may similarly affect pregnant women considering maternal immunization during pregnancy.”
Because of widespread testing for respiratory viruses at Intermountain facilities, the researchers also could determine that flu vaccine receipt among the mothers did not affect incidence of RSV.
“Our study strengthens the evidence that maternal immunization provides passive protection against influenza to infants during the vulnerable period before they are old enough to receive active immunization,” Dr. Shakib and her associates wrote.
The research was funded by the National Institutes of Health, the University of Utah Children’s Health Research Center and the Pediatric Clinical and Translational Scholar Program, the H.A. and Edna Benning Presidential Endowment, and the University of Utah Center for Clinical and Translational Science through the National Center for Research Resources and the National Center for Advancing Translational Sciences. The authors reported no disclosures.
Infants born to mothers who received flu immunization during pregnancy were 70% less likely to contract lab-confirmed influenza and 81% less likely to be hospitalized for flu before age 6 months, compared with infants of unimmunized mothers, a study reaffirmed.
Yet just one in ten pregnant women received the vaccine, a proportion that has steadily risen since the 2009-2010 H1N1 flu season.
“The results of this large retrospective study support the conclusions of prospective studies regarding the protective benefit of maternal influenza immunization during pregnancy,” reported Dr. Julie H. Shakib of the University of Utah, Salt Lake City, and her associates (Pediatrics. 2016 May 3. doi: 10.1542/peds.2015-2360). “Interventions that target both healthy pregnant women and those with chronic conditions are needed to increase vaccine uptake,” they wrote.
The researchers analyzed self-reported seasonal influenza immunization uptake in the 245,386 women who gave birth between December 2005 and March 2014 in the Intermountain Healthcare facilities in Utah and Idaho. Although 10% of the women overall received flu vaccinations, just 2.2% of the women who delivered before the 2009-2010 H1N1 pandemic had received them. That number rose after the pandemic to 21% (P < .001). More than half (52%) of the women giving birth during the 2013-2014 flu season reported getting the seasonal flu vaccine.
Among the women’s 249,387 infants, 866 had at least one influenza-like illness (ILI), including 32 born to vaccinated women and 834 born to unvaccinated women. The infants born to women receiving the flu vaccine during pregnancy were 64% less likely to develop an ILI, with illnesses in 1.34 per 1,000 born to vaccinated women and 3.7 per 1,000 born to unvaccinated women (relative risk, 0.36).
The rates of laboratory-confirmed influenza in the 658 children who contracted it were 0.84 per 1,000 children born to vaccinated mothers and 2.83 per 1,000 children born to unvaccinated mothers, translating to a 70% lower risk of flu in those born to vaccinated mothers (RR, 0.30). Similarly, infants born to vaccinated mothers were 81% less likely to be hospitalized for lab-confirmed influenza (RR, 0.19, P = .005). Just 3 of 151 hospitalized infants had been born to mothers who received the flu vaccine, for a rate of 0.13 per 1,000 for children of vaccinated mothers and 0.66 per 1,000 for children of unvaccinated mothers.
Pregnant women with public insurance or no insurance were less likely to report getting the seasonal vaccine than were privately insured women, but those with chronic conditions were more likely to be vaccinated. Uptake also was lower among women with incomes below the federal poverty level and among women living in either rural or frontier areas or in the Urban South or Southwest Intermountain regions.
“The Intermountain Urban South region includes Utah County, 1 of 30 U.S. counties with the largest estimated numbers of unvaccinated children from 1995-2001 CDC National Immunization Surveys (NIS) data,” the authors wrote. “It is possible that factors leading to parental vaccine hesitancy in children may similarly affect pregnant women considering maternal immunization during pregnancy.”
Because of widespread testing for respiratory viruses at Intermountain facilities, the researchers also could determine that flu vaccine receipt among the mothers did not affect incidence of RSV.
“Our study strengthens the evidence that maternal immunization provides passive protection against influenza to infants during the vulnerable period before they are old enough to receive active immunization,” Dr. Shakib and her associates wrote.
The research was funded by the National Institutes of Health, the University of Utah Children’s Health Research Center and the Pediatric Clinical and Translational Scholar Program, the H.A. and Edna Benning Presidential Endowment, and the University of Utah Center for Clinical and Translational Science through the National Center for Research Resources and the National Center for Advancing Translational Sciences. The authors reported no disclosures.
Infants born to mothers who received flu immunization during pregnancy were 70% less likely to contract lab-confirmed influenza and 81% less likely to be hospitalized for flu before age 6 months, compared with infants of unimmunized mothers, a study reaffirmed.
Yet just one in ten pregnant women received the vaccine, a proportion that has steadily risen since the 2009-2010 H1N1 flu season.
“The results of this large retrospective study support the conclusions of prospective studies regarding the protective benefit of maternal influenza immunization during pregnancy,” reported Dr. Julie H. Shakib of the University of Utah, Salt Lake City, and her associates (Pediatrics. 2016 May 3. doi: 10.1542/peds.2015-2360). “Interventions that target both healthy pregnant women and those with chronic conditions are needed to increase vaccine uptake,” they wrote.
The researchers analyzed self-reported seasonal influenza immunization uptake in the 245,386 women who gave birth between December 2005 and March 2014 in the Intermountain Healthcare facilities in Utah and Idaho. Although 10% of the women overall received flu vaccinations, just 2.2% of the women who delivered before the 2009-2010 H1N1 pandemic had received them. That number rose after the pandemic to 21% (P < .001). More than half (52%) of the women giving birth during the 2013-2014 flu season reported getting the seasonal flu vaccine.
Among the women’s 249,387 infants, 866 had at least one influenza-like illness (ILI), including 32 born to vaccinated women and 834 born to unvaccinated women. The infants born to women receiving the flu vaccine during pregnancy were 64% less likely to develop an ILI, with illnesses in 1.34 per 1,000 born to vaccinated women and 3.7 per 1,000 born to unvaccinated women (relative risk, 0.36).
The rates of laboratory-confirmed influenza in the 658 children who contracted it were 0.84 per 1,000 children born to vaccinated mothers and 2.83 per 1,000 children born to unvaccinated mothers, translating to a 70% lower risk of flu in those born to vaccinated mothers (RR, 0.30). Similarly, infants born to vaccinated mothers were 81% less likely to be hospitalized for lab-confirmed influenza (RR, 0.19, P = .005). Just 3 of 151 hospitalized infants had been born to mothers who received the flu vaccine, for a rate of 0.13 per 1,000 for children of vaccinated mothers and 0.66 per 1,000 for children of unvaccinated mothers.
Pregnant women with public insurance or no insurance were less likely to report getting the seasonal vaccine than were privately insured women, but those with chronic conditions were more likely to be vaccinated. Uptake also was lower among women with incomes below the federal poverty level and among women living in either rural or frontier areas or in the Urban South or Southwest Intermountain regions.
“The Intermountain Urban South region includes Utah County, 1 of 30 U.S. counties with the largest estimated numbers of unvaccinated children from 1995-2001 CDC National Immunization Surveys (NIS) data,” the authors wrote. “It is possible that factors leading to parental vaccine hesitancy in children may similarly affect pregnant women considering maternal immunization during pregnancy.”
Because of widespread testing for respiratory viruses at Intermountain facilities, the researchers also could determine that flu vaccine receipt among the mothers did not affect incidence of RSV.
“Our study strengthens the evidence that maternal immunization provides passive protection against influenza to infants during the vulnerable period before they are old enough to receive active immunization,” Dr. Shakib and her associates wrote.
The research was funded by the National Institutes of Health, the University of Utah Children’s Health Research Center and the Pediatric Clinical and Translational Scholar Program, the H.A. and Edna Benning Presidential Endowment, and the University of Utah Center for Clinical and Translational Science through the National Center for Research Resources and the National Center for Advancing Translational Sciences. The authors reported no disclosures.
FROM PEDIATRICS
Key clinical point: Young infants born to mothers receiving the flu vaccine were less likely to develop the flu and serious complications.
Major finding: Infants under 6 months old were 64% less likely to develop influenza-like illness, 70% less likely to develop lab-confirmed flu, and 81% less likely to be hospitalized for flu if born to vaccinated mothers.
Data source: The findings are based on a retrospective cohort study of 245,386 women who gave birth to 249,387 infants between December 2005 and March 2014 in the Intermountain Healthcare facilities in Utah and Idaho.
Disclosures: The research was funded by the National Institutes of Health, the University of Utah Children’s Health Research Center and the Pediatric Clinical and Translational Scholar Program, the H.A. and Edna Benning Presidential Endowment, and the University of Utah Center for Clinical and Translational Science through the National Center for Research Resources and the National Center for Advancing Translational Sciences. The authors reported no disclosures.
Vitamin D supplementation cuts dust mite atopy
BALTIMORE – Three months of daily, oral treatment with a relatively high but safe dosage of a vitamin D supplement to pregnant mothers during late gestation followed by continued oral supplementation to their neonates during the first 6 months of life led to a significant reduction in the prevalence of dust-mite skin reactivity in those children once they reached 18 months old in a randomized, controlled trial with 259 mothers and infants.
And in a preliminary assessment that tallied the number of children who required primary care office visits for asthma through age 18 months, children who had received the highest vitamin D supplementation also showed a statistically significant reduction of these visits, compared with the placebo control children, Dr. Cameron C. Grant reported at the annual meeting of the Pediatric Academic Societies.
This suggestion that the vitamin D intervention could cut asthma development is not completely certain because in 18-month-old children, diagnosis of asthma is “very insecure,” noted Dr. Grant, a pediatrician at the University of Auckland, New Zealand and at Starship Children’s Hospital, also in Auckland. In addition, a limitation of the observed effect on dust mite atopy on skin-test challenge was that this follow-up occurred in only 186 (72%) of the 259 infants who participated in the study.
The study’s premise was that vitamin D is an immune system modulator, and that New Zealand provides an excellent setting to test the hypothesis that normalized vitamin D levels can help prevent development of atopy and asthma because many of the country’s residents are vitamin D deficient due to their diet and sun avoidance to prevent skin cancers. Results from prior studies had shown that 57% of New Zealand neonates have inadequate levels of vitamin D at birth, defined as a serum level of 25-hydroxyvitamin D of less than 20 ng/ml (less than 50 nmol/L), Dr. Grant noted.
“I think this intervention will only work in populations that are vitamin D deficient,” Dr. Grant said in an interview. In his study, the average serum level of 25-hydroxyvitamin D among control neonates was 38 nmol/L (about 15 ng/mL). In contrast, neonates born to mothers who had received a daily, higher-dose vitamin D supplement during the third trimester had serum measures that were roughly twice that level.
The study enrolled 260 pregnant women from the Auckland area with a single pregnancy at 26-30 weeks’ gestation; average gestational age at baseline was 27 weeks. Dr. Grant and his associates randomized the mothers to receive 1,000 IU oral vitamin D daily, 2,000 oral vitamin D daily, or placebo. The women delivered 259 infants. Infants born to women on the lower dosage supplement then received 400 IU vitamin daily for 6 months, those born to mothers on the higher level supplement received 800 IU vitamin D daily for 6 months, and those born to mothers in the placebo group received placebo supplements daily for 6 months.
Both supplement regimens led to statistically significant increases in serum levels of 25-hydroxyvitamin D in maternal serum at 36 weeks’ gestation, in cord blood at delivery, in the neonates’ serum at ages 2 months and 4 months, and in infant serum in the higher dosage group at 6 months of age, compared with similar measures taken at all these time points in the placebo group.
In addition, the neonates in the higher dosage group had significantly higher serum levels at 2, 4, and 6 months, compared with the lower dosage group. When measured a final time at 18-month follow-up, a year after the end of vitamin D supplementation, average serum levels of 25-hydroxyvitamin D in an three subgroups of children were virtually identical and similar to maternal serum levels at baseline. Dr. Grant and his associates had previously reported these findings and also had documented the safety of both the low and high levels of vitamin D supplements for both mothers and their children (Pediatrics. 2014 Jan;133[1]:e143-53).
The new findings reported by Dr. Grant focused on clinical outcomes at 18 months. He and his colleagues ran skin-prick testing on 186 of the 259 (72%) children in the study (the remaining children weren’t available for this follow-up assessment). They tested three aeroallergens: cat, pollen, and house dust mite. They saw no significant differences in the prevalence of positive skin-prick reactions among the three study groups to cat and pollen, but prevalence levels of positive reactions to dust mite were 9% in the controls, 3% of children in the low-dosage group, and none in the high dosage group. The difference between the controls and high dosage groups was statistically significant; the difference between the controls and the low dosage group was not significant, Dr. Grant said. Additional testing of specific IgE responses to four different dust mite antigens showed statistically significant reductions in responses to each of the four antigens among the high dosage children, compared with the controls and with the low dosage children.
The researchers also tallied the number of acute, primary care office visits during the first 18 months of life among the children in each of the three subgroups for a variety of respiratory diagnoses. The three groups showed no significant differences in total number of office visits for most of these diagnoses, including colds, otitis media, croup, and bronchitis. However, about 12% of children in the control group had been seen in a primary care office for a diagnosis of asthma, compared with none of the children in the low dosage group and about 4% in the high-dosage group. The differences between the two intervention groups and the control group were statistically significant. Dr. Grant cautioned that this finding is very preliminary and that any conclusions about the impact of vitamin D supplements on asthma incidence must await studies with larger numbers of children who are followed to an older age.
Dr. Grant had no disclosures.
On Twitter @mitchelzoler
BALTIMORE – Three months of daily, oral treatment with a relatively high but safe dosage of a vitamin D supplement to pregnant mothers during late gestation followed by continued oral supplementation to their neonates during the first 6 months of life led to a significant reduction in the prevalence of dust-mite skin reactivity in those children once they reached 18 months old in a randomized, controlled trial with 259 mothers and infants.
And in a preliminary assessment that tallied the number of children who required primary care office visits for asthma through age 18 months, children who had received the highest vitamin D supplementation also showed a statistically significant reduction of these visits, compared with the placebo control children, Dr. Cameron C. Grant reported at the annual meeting of the Pediatric Academic Societies.
This suggestion that the vitamin D intervention could cut asthma development is not completely certain because in 18-month-old children, diagnosis of asthma is “very insecure,” noted Dr. Grant, a pediatrician at the University of Auckland, New Zealand and at Starship Children’s Hospital, also in Auckland. In addition, a limitation of the observed effect on dust mite atopy on skin-test challenge was that this follow-up occurred in only 186 (72%) of the 259 infants who participated in the study.
The study’s premise was that vitamin D is an immune system modulator, and that New Zealand provides an excellent setting to test the hypothesis that normalized vitamin D levels can help prevent development of atopy and asthma because many of the country’s residents are vitamin D deficient due to their diet and sun avoidance to prevent skin cancers. Results from prior studies had shown that 57% of New Zealand neonates have inadequate levels of vitamin D at birth, defined as a serum level of 25-hydroxyvitamin D of less than 20 ng/ml (less than 50 nmol/L), Dr. Grant noted.
“I think this intervention will only work in populations that are vitamin D deficient,” Dr. Grant said in an interview. In his study, the average serum level of 25-hydroxyvitamin D among control neonates was 38 nmol/L (about 15 ng/mL). In contrast, neonates born to mothers who had received a daily, higher-dose vitamin D supplement during the third trimester had serum measures that were roughly twice that level.
The study enrolled 260 pregnant women from the Auckland area with a single pregnancy at 26-30 weeks’ gestation; average gestational age at baseline was 27 weeks. Dr. Grant and his associates randomized the mothers to receive 1,000 IU oral vitamin D daily, 2,000 oral vitamin D daily, or placebo. The women delivered 259 infants. Infants born to women on the lower dosage supplement then received 400 IU vitamin daily for 6 months, those born to mothers on the higher level supplement received 800 IU vitamin D daily for 6 months, and those born to mothers in the placebo group received placebo supplements daily for 6 months.
Both supplement regimens led to statistically significant increases in serum levels of 25-hydroxyvitamin D in maternal serum at 36 weeks’ gestation, in cord blood at delivery, in the neonates’ serum at ages 2 months and 4 months, and in infant serum in the higher dosage group at 6 months of age, compared with similar measures taken at all these time points in the placebo group.
In addition, the neonates in the higher dosage group had significantly higher serum levels at 2, 4, and 6 months, compared with the lower dosage group. When measured a final time at 18-month follow-up, a year after the end of vitamin D supplementation, average serum levels of 25-hydroxyvitamin D in an three subgroups of children were virtually identical and similar to maternal serum levels at baseline. Dr. Grant and his associates had previously reported these findings and also had documented the safety of both the low and high levels of vitamin D supplements for both mothers and their children (Pediatrics. 2014 Jan;133[1]:e143-53).
The new findings reported by Dr. Grant focused on clinical outcomes at 18 months. He and his colleagues ran skin-prick testing on 186 of the 259 (72%) children in the study (the remaining children weren’t available for this follow-up assessment). They tested three aeroallergens: cat, pollen, and house dust mite. They saw no significant differences in the prevalence of positive skin-prick reactions among the three study groups to cat and pollen, but prevalence levels of positive reactions to dust mite were 9% in the controls, 3% of children in the low-dosage group, and none in the high dosage group. The difference between the controls and high dosage groups was statistically significant; the difference between the controls and the low dosage group was not significant, Dr. Grant said. Additional testing of specific IgE responses to four different dust mite antigens showed statistically significant reductions in responses to each of the four antigens among the high dosage children, compared with the controls and with the low dosage children.
The researchers also tallied the number of acute, primary care office visits during the first 18 months of life among the children in each of the three subgroups for a variety of respiratory diagnoses. The three groups showed no significant differences in total number of office visits for most of these diagnoses, including colds, otitis media, croup, and bronchitis. However, about 12% of children in the control group had been seen in a primary care office for a diagnosis of asthma, compared with none of the children in the low dosage group and about 4% in the high-dosage group. The differences between the two intervention groups and the control group were statistically significant. Dr. Grant cautioned that this finding is very preliminary and that any conclusions about the impact of vitamin D supplements on asthma incidence must await studies with larger numbers of children who are followed to an older age.
Dr. Grant had no disclosures.
On Twitter @mitchelzoler
BALTIMORE – Three months of daily, oral treatment with a relatively high but safe dosage of a vitamin D supplement to pregnant mothers during late gestation followed by continued oral supplementation to their neonates during the first 6 months of life led to a significant reduction in the prevalence of dust-mite skin reactivity in those children once they reached 18 months old in a randomized, controlled trial with 259 mothers and infants.
And in a preliminary assessment that tallied the number of children who required primary care office visits for asthma through age 18 months, children who had received the highest vitamin D supplementation also showed a statistically significant reduction of these visits, compared with the placebo control children, Dr. Cameron C. Grant reported at the annual meeting of the Pediatric Academic Societies.
This suggestion that the vitamin D intervention could cut asthma development is not completely certain because in 18-month-old children, diagnosis of asthma is “very insecure,” noted Dr. Grant, a pediatrician at the University of Auckland, New Zealand and at Starship Children’s Hospital, also in Auckland. In addition, a limitation of the observed effect on dust mite atopy on skin-test challenge was that this follow-up occurred in only 186 (72%) of the 259 infants who participated in the study.
The study’s premise was that vitamin D is an immune system modulator, and that New Zealand provides an excellent setting to test the hypothesis that normalized vitamin D levels can help prevent development of atopy and asthma because many of the country’s residents are vitamin D deficient due to their diet and sun avoidance to prevent skin cancers. Results from prior studies had shown that 57% of New Zealand neonates have inadequate levels of vitamin D at birth, defined as a serum level of 25-hydroxyvitamin D of less than 20 ng/ml (less than 50 nmol/L), Dr. Grant noted.
“I think this intervention will only work in populations that are vitamin D deficient,” Dr. Grant said in an interview. In his study, the average serum level of 25-hydroxyvitamin D among control neonates was 38 nmol/L (about 15 ng/mL). In contrast, neonates born to mothers who had received a daily, higher-dose vitamin D supplement during the third trimester had serum measures that were roughly twice that level.
The study enrolled 260 pregnant women from the Auckland area with a single pregnancy at 26-30 weeks’ gestation; average gestational age at baseline was 27 weeks. Dr. Grant and his associates randomized the mothers to receive 1,000 IU oral vitamin D daily, 2,000 oral vitamin D daily, or placebo. The women delivered 259 infants. Infants born to women on the lower dosage supplement then received 400 IU vitamin daily for 6 months, those born to mothers on the higher level supplement received 800 IU vitamin D daily for 6 months, and those born to mothers in the placebo group received placebo supplements daily for 6 months.
Both supplement regimens led to statistically significant increases in serum levels of 25-hydroxyvitamin D in maternal serum at 36 weeks’ gestation, in cord blood at delivery, in the neonates’ serum at ages 2 months and 4 months, and in infant serum in the higher dosage group at 6 months of age, compared with similar measures taken at all these time points in the placebo group.
In addition, the neonates in the higher dosage group had significantly higher serum levels at 2, 4, and 6 months, compared with the lower dosage group. When measured a final time at 18-month follow-up, a year after the end of vitamin D supplementation, average serum levels of 25-hydroxyvitamin D in an three subgroups of children were virtually identical and similar to maternal serum levels at baseline. Dr. Grant and his associates had previously reported these findings and also had documented the safety of both the low and high levels of vitamin D supplements for both mothers and their children (Pediatrics. 2014 Jan;133[1]:e143-53).
The new findings reported by Dr. Grant focused on clinical outcomes at 18 months. He and his colleagues ran skin-prick testing on 186 of the 259 (72%) children in the study (the remaining children weren’t available for this follow-up assessment). They tested three aeroallergens: cat, pollen, and house dust mite. They saw no significant differences in the prevalence of positive skin-prick reactions among the three study groups to cat and pollen, but prevalence levels of positive reactions to dust mite were 9% in the controls, 3% of children in the low-dosage group, and none in the high dosage group. The difference between the controls and high dosage groups was statistically significant; the difference between the controls and the low dosage group was not significant, Dr. Grant said. Additional testing of specific IgE responses to four different dust mite antigens showed statistically significant reductions in responses to each of the four antigens among the high dosage children, compared with the controls and with the low dosage children.
The researchers also tallied the number of acute, primary care office visits during the first 18 months of life among the children in each of the three subgroups for a variety of respiratory diagnoses. The three groups showed no significant differences in total number of office visits for most of these diagnoses, including colds, otitis media, croup, and bronchitis. However, about 12% of children in the control group had been seen in a primary care office for a diagnosis of asthma, compared with none of the children in the low dosage group and about 4% in the high-dosage group. The differences between the two intervention groups and the control group were statistically significant. Dr. Grant cautioned that this finding is very preliminary and that any conclusions about the impact of vitamin D supplements on asthma incidence must await studies with larger numbers of children who are followed to an older age.
Dr. Grant had no disclosures.
On Twitter @mitchelzoler
AT THE PAS ANNUAL MEETING
Key clinical point: Maternal treatment to achieve adequate vitamin D levels during late gestation followed by neonatal vitamin D supplementation significantly cut dust mite atopy at 18 months of age, along with a suggestion of reduced asthma incidence.
Major finding: Dust mite reactivity at 18 months occurred in no children treated with higher vitamin D supplementation and in 9% of controls.
Data source: A randomized, controlled, single-center study with 260 pregnant women who delivered 259 infants.
Disclosures: Dr. Grant had no disclosures.
Tiotropium inhalation spray effective for asthma regardless of allergic status
LOS ANGELES – Once-daily tiotropium bromide inhalation spray as long-term, add-on maintenance therapy in patients with poorly controlled symptomatic asthma is similarly effective in both allergic and nonallergic asthma, according to Dr. Donald P. Tashkin.
In a series of analyses presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, he and his coinvestigators showed that tiotropium bromide inhalation spray (Spiriva Respimat) given as add-on maintenance therapy resulted in significantly improved lung function, enhanced asthma symptom control, and fewer asthma exacerbations. The important new finding in these post hoc analyses was that the medication was similarly effective across the full range of baseline serum IgE and blood eosinophil levels, said Dr. Tashkin, director of the pulmonary function laboratories at the University of California, Los Angeles.
Last fall, the Food and Drug Administration approved an expanded indication for tiotropium bromide inhalation spray as long-term, add-on maintenance therapy of asthma in patients aged 12 and up who remain symptomatic despite taking other maintenance therapies. The once-daily medication had already been approved in the fall of 2014 for maintenance therapy of chronic obstructive pulmonary disease. Spiriva Respimat delivers a once-daily 2.5-mcg dose of tiotropium bromide via two 1.25-mcg puffs in a slow-moving, propellant-free mist designed to get the drug into the distal lungs independent of a patient’s skill in using a conventional metered-dose inhaler.
Dr. Tashkin and his coinvestigators presented a series of post hoc analyses combining data on 3,012 participants in the two prospective PrimoTinA-asthma and two MezzoTinA-asthma clinical trials. These phase III, double-blind, placebo-controlled trials defined participants’ allergic phenotype on the basis of the conventional cut points of a serum IgE level above or below 430 mcg/mL or a blood eosinophil count above or below 600 cells/mcL.
“The question is, are these appropriate cut points? Can we be sure that somebody below those cut points doesn’t have atopy? To answer that question, we looked at the whole spectrum of eosinophils in the blood from 5/mcL to 2,000/mcL and serum IgE levels from 2 mcg/mL to 2,000 mcg/mL. We found that the efficacy was similar across the entire spectrum of these measures of allergy,” he said in an interview.
Thus, these new findings support the use of this novel maintenance therapy without any need for lab tests to determine whether an individual patient’s asthma is T helper 2–cell dominant or not, Dr. Tashkin added.
The PrimoTinA-asthma trials were 48-week studies of add-on Spiriva Respimat or placebo conducted in patients with symptomatic asthma despite treatment with an inhaled corticosteroid plus a long-acting beta-agonist. The MezzoTinA-asthma studies were 24 weeks long and focused on patients who remained symptomatic despite at least moderate-dose inhaled corticosteroid therapy. Key endpoints included improvement in asthma symptom control as measured by the seven-question Asthma Control Questionnaire, improved peak and trough forced expiratory volume in 1 second, and time to a first severe asthma exacerbation.
Dr. Tashkin reported serving as a paid speaker on behalf of Boehringer Ingelheim, which markets Spiriva Respimat and sponsored the studies.
LOS ANGELES – Once-daily tiotropium bromide inhalation spray as long-term, add-on maintenance therapy in patients with poorly controlled symptomatic asthma is similarly effective in both allergic and nonallergic asthma, according to Dr. Donald P. Tashkin.
In a series of analyses presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, he and his coinvestigators showed that tiotropium bromide inhalation spray (Spiriva Respimat) given as add-on maintenance therapy resulted in significantly improved lung function, enhanced asthma symptom control, and fewer asthma exacerbations. The important new finding in these post hoc analyses was that the medication was similarly effective across the full range of baseline serum IgE and blood eosinophil levels, said Dr. Tashkin, director of the pulmonary function laboratories at the University of California, Los Angeles.
Last fall, the Food and Drug Administration approved an expanded indication for tiotropium bromide inhalation spray as long-term, add-on maintenance therapy of asthma in patients aged 12 and up who remain symptomatic despite taking other maintenance therapies. The once-daily medication had already been approved in the fall of 2014 for maintenance therapy of chronic obstructive pulmonary disease. Spiriva Respimat delivers a once-daily 2.5-mcg dose of tiotropium bromide via two 1.25-mcg puffs in a slow-moving, propellant-free mist designed to get the drug into the distal lungs independent of a patient’s skill in using a conventional metered-dose inhaler.
Dr. Tashkin and his coinvestigators presented a series of post hoc analyses combining data on 3,012 participants in the two prospective PrimoTinA-asthma and two MezzoTinA-asthma clinical trials. These phase III, double-blind, placebo-controlled trials defined participants’ allergic phenotype on the basis of the conventional cut points of a serum IgE level above or below 430 mcg/mL or a blood eosinophil count above or below 600 cells/mcL.
“The question is, are these appropriate cut points? Can we be sure that somebody below those cut points doesn’t have atopy? To answer that question, we looked at the whole spectrum of eosinophils in the blood from 5/mcL to 2,000/mcL and serum IgE levels from 2 mcg/mL to 2,000 mcg/mL. We found that the efficacy was similar across the entire spectrum of these measures of allergy,” he said in an interview.
Thus, these new findings support the use of this novel maintenance therapy without any need for lab tests to determine whether an individual patient’s asthma is T helper 2–cell dominant or not, Dr. Tashkin added.
The PrimoTinA-asthma trials were 48-week studies of add-on Spiriva Respimat or placebo conducted in patients with symptomatic asthma despite treatment with an inhaled corticosteroid plus a long-acting beta-agonist. The MezzoTinA-asthma studies were 24 weeks long and focused on patients who remained symptomatic despite at least moderate-dose inhaled corticosteroid therapy. Key endpoints included improvement in asthma symptom control as measured by the seven-question Asthma Control Questionnaire, improved peak and trough forced expiratory volume in 1 second, and time to a first severe asthma exacerbation.
Dr. Tashkin reported serving as a paid speaker on behalf of Boehringer Ingelheim, which markets Spiriva Respimat and sponsored the studies.
LOS ANGELES – Once-daily tiotropium bromide inhalation spray as long-term, add-on maintenance therapy in patients with poorly controlled symptomatic asthma is similarly effective in both allergic and nonallergic asthma, according to Dr. Donald P. Tashkin.
In a series of analyses presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, he and his coinvestigators showed that tiotropium bromide inhalation spray (Spiriva Respimat) given as add-on maintenance therapy resulted in significantly improved lung function, enhanced asthma symptom control, and fewer asthma exacerbations. The important new finding in these post hoc analyses was that the medication was similarly effective across the full range of baseline serum IgE and blood eosinophil levels, said Dr. Tashkin, director of the pulmonary function laboratories at the University of California, Los Angeles.
Last fall, the Food and Drug Administration approved an expanded indication for tiotropium bromide inhalation spray as long-term, add-on maintenance therapy of asthma in patients aged 12 and up who remain symptomatic despite taking other maintenance therapies. The once-daily medication had already been approved in the fall of 2014 for maintenance therapy of chronic obstructive pulmonary disease. Spiriva Respimat delivers a once-daily 2.5-mcg dose of tiotropium bromide via two 1.25-mcg puffs in a slow-moving, propellant-free mist designed to get the drug into the distal lungs independent of a patient’s skill in using a conventional metered-dose inhaler.
Dr. Tashkin and his coinvestigators presented a series of post hoc analyses combining data on 3,012 participants in the two prospective PrimoTinA-asthma and two MezzoTinA-asthma clinical trials. These phase III, double-blind, placebo-controlled trials defined participants’ allergic phenotype on the basis of the conventional cut points of a serum IgE level above or below 430 mcg/mL or a blood eosinophil count above or below 600 cells/mcL.
“The question is, are these appropriate cut points? Can we be sure that somebody below those cut points doesn’t have atopy? To answer that question, we looked at the whole spectrum of eosinophils in the blood from 5/mcL to 2,000/mcL and serum IgE levels from 2 mcg/mL to 2,000 mcg/mL. We found that the efficacy was similar across the entire spectrum of these measures of allergy,” he said in an interview.
Thus, these new findings support the use of this novel maintenance therapy without any need for lab tests to determine whether an individual patient’s asthma is T helper 2–cell dominant or not, Dr. Tashkin added.
The PrimoTinA-asthma trials were 48-week studies of add-on Spiriva Respimat or placebo conducted in patients with symptomatic asthma despite treatment with an inhaled corticosteroid plus a long-acting beta-agonist. The MezzoTinA-asthma studies were 24 weeks long and focused on patients who remained symptomatic despite at least moderate-dose inhaled corticosteroid therapy. Key endpoints included improvement in asthma symptom control as measured by the seven-question Asthma Control Questionnaire, improved peak and trough forced expiratory volume in 1 second, and time to a first severe asthma exacerbation.
Dr. Tashkin reported serving as a paid speaker on behalf of Boehringer Ingelheim, which markets Spiriva Respimat and sponsored the studies.
EXPERT ANALYSIS FROM THE 2016 AAAAI ANNUAL MEETING
Hospital intervention slashes heparin-induced thrombocytopenia
A simple, hospital-wide “avoid heparin” intervention dramatically cut the rate of suspected heparin-induced thrombocytopenia by 42%, that of positive ELISA screens for HIT by 63%, that of adjudicated HIT by 79%, and that of HIT with thrombosis by 91%, while also reducing the costs of HIT-related care by 83% at one large university hospital.
The medical literature has focused on early recognition and treatment of heparin-induced thrombocytopenia, “but its prevention has been largely overlooked,” noted Dr. Kelly E. McGowan of Sunnybrook Health Sciences Centre and the University of Toronto and her associates.
Sunnybrook introduced an “avoid heparin” program in 2006 in which most intravenous and subcutaneous unfractionated heparin was replaced with low-molecular-weight heparin (LMWH) in prophylactic or therapeutic doses; heparinized saline in arterial and central venous lines was replaced with saline flushes; order sets were modified to exclude unfractionated heparin options; and unfractionated heparin stores were removed from most nursing units.
Unfractionated heparin remained available for use in hemodialysis, cardiovascular surgery, and certain cases of acute coronary syndrome. Most hospital clinicians were unaware that LMWH was being substituted for unfractionated heparin, and none were aware that the effects of this change were being studied.
The investigators assessed all 1,118 cases of suspected heparin-induced thrombocytopenia that occurred during a 10-year period before and after this intervention was implemented. The use of LMWH rose fourfold after the program was initiated, but the annual rate of HIT associated with LMWH remained constant at 0.9 cases per 10,000 admissions over the course of the study.
The annual incidence of suspected HIT decreased from 85.5 per 10,000 admissions per year before the intervention to 49.0 afterward (relative risk reduction, 41.7%). The rate of positive ELISA screens for the disorder dropped from 16.5 to 6.1 per 10,000 (RRR, 62.9%), the rate of adjudicated HIT decreased from 10.7 to 2.2 per 10,000, and the rate of HIT with thrombosis declined from 4.6 to 0.4 per 10,000.
The program’s greatest impact was on cardiac surgery, but the burden of HIT also markedly decreased in other surgical and medical patients. HIT decreased by 77% in cardiovascular surgeries, 77% in other surgeries, 75% in cardiology patients, and 62% in medical patients, Dr. McGowan and her associates said (Blood 2016 Apr 21;127[16]:1954-9).
Patients with HIT during the preintervention years more often developed thrombosis (43%), usually venous thromboembolism, compared with those who had HIT in the postintervention years (19%), and median length of stay declined accordingly. The average estimated costs of HIT care per year dropped by about $267,000 dollars per year, from $322,000 before the program was implemented to $55,000 afterward.
The investigators added that this is the first study ever to show the success of an HIT prevention strategy. Their findings indicate that a hospital-wide “avoid heparin” program can substantially reduce morbidity, mortality, and costs associated with HIT. “The heparin avoidance strategy that we used was not complex or costly and would be feasible in other centers,” they noted.
This study is the first to show the substantial impact of large-scale removal of heparin from clinical practice in the real world. But implementing such a program at a system-wide level wouldn’t be simple.
The cost of a unit of low-molecular-weight heparin is six- to eightfold higher than that of unfractionated heparin. Hospitals may need to be convinced that unfractionated heparin is not the bargain it appears to be once the costs of heparin-induced thrombocytopenia are factored in.
In addition, unfractionated heparin remains the best option for patients undergoing cardiovascular surgery, those with renal failure, and those at high risk for bleeding that requires a rapid reversal agent.
Lori-Ann Linkins, M.D., of McMaster University, Hamilton (Ont.), made these remarks in a commentary accompanying Dr. McGowan’s report (Blood 2016 Apr 21;127[16]:1945-6). She reported receiving lecture honoraria from Pfizer and research funding from Bayer.
This study is the first to show the substantial impact of large-scale removal of heparin from clinical practice in the real world. But implementing such a program at a system-wide level wouldn’t be simple.
The cost of a unit of low-molecular-weight heparin is six- to eightfold higher than that of unfractionated heparin. Hospitals may need to be convinced that unfractionated heparin is not the bargain it appears to be once the costs of heparin-induced thrombocytopenia are factored in.
In addition, unfractionated heparin remains the best option for patients undergoing cardiovascular surgery, those with renal failure, and those at high risk for bleeding that requires a rapid reversal agent.
Lori-Ann Linkins, M.D., of McMaster University, Hamilton (Ont.), made these remarks in a commentary accompanying Dr. McGowan’s report (Blood 2016 Apr 21;127[16]:1945-6). She reported receiving lecture honoraria from Pfizer and research funding from Bayer.
This study is the first to show the substantial impact of large-scale removal of heparin from clinical practice in the real world. But implementing such a program at a system-wide level wouldn’t be simple.
The cost of a unit of low-molecular-weight heparin is six- to eightfold higher than that of unfractionated heparin. Hospitals may need to be convinced that unfractionated heparin is not the bargain it appears to be once the costs of heparin-induced thrombocytopenia are factored in.
In addition, unfractionated heparin remains the best option for patients undergoing cardiovascular surgery, those with renal failure, and those at high risk for bleeding that requires a rapid reversal agent.
Lori-Ann Linkins, M.D., of McMaster University, Hamilton (Ont.), made these remarks in a commentary accompanying Dr. McGowan’s report (Blood 2016 Apr 21;127[16]:1945-6). She reported receiving lecture honoraria from Pfizer and research funding from Bayer.
A simple, hospital-wide “avoid heparin” intervention dramatically cut the rate of suspected heparin-induced thrombocytopenia by 42%, that of positive ELISA screens for HIT by 63%, that of adjudicated HIT by 79%, and that of HIT with thrombosis by 91%, while also reducing the costs of HIT-related care by 83% at one large university hospital.
The medical literature has focused on early recognition and treatment of heparin-induced thrombocytopenia, “but its prevention has been largely overlooked,” noted Dr. Kelly E. McGowan of Sunnybrook Health Sciences Centre and the University of Toronto and her associates.
Sunnybrook introduced an “avoid heparin” program in 2006 in which most intravenous and subcutaneous unfractionated heparin was replaced with low-molecular-weight heparin (LMWH) in prophylactic or therapeutic doses; heparinized saline in arterial and central venous lines was replaced with saline flushes; order sets were modified to exclude unfractionated heparin options; and unfractionated heparin stores were removed from most nursing units.
Unfractionated heparin remained available for use in hemodialysis, cardiovascular surgery, and certain cases of acute coronary syndrome. Most hospital clinicians were unaware that LMWH was being substituted for unfractionated heparin, and none were aware that the effects of this change were being studied.
The investigators assessed all 1,118 cases of suspected heparin-induced thrombocytopenia that occurred during a 10-year period before and after this intervention was implemented. The use of LMWH rose fourfold after the program was initiated, but the annual rate of HIT associated with LMWH remained constant at 0.9 cases per 10,000 admissions over the course of the study.
The annual incidence of suspected HIT decreased from 85.5 per 10,000 admissions per year before the intervention to 49.0 afterward (relative risk reduction, 41.7%). The rate of positive ELISA screens for the disorder dropped from 16.5 to 6.1 per 10,000 (RRR, 62.9%), the rate of adjudicated HIT decreased from 10.7 to 2.2 per 10,000, and the rate of HIT with thrombosis declined from 4.6 to 0.4 per 10,000.
The program’s greatest impact was on cardiac surgery, but the burden of HIT also markedly decreased in other surgical and medical patients. HIT decreased by 77% in cardiovascular surgeries, 77% in other surgeries, 75% in cardiology patients, and 62% in medical patients, Dr. McGowan and her associates said (Blood 2016 Apr 21;127[16]:1954-9).
Patients with HIT during the preintervention years more often developed thrombosis (43%), usually venous thromboembolism, compared with those who had HIT in the postintervention years (19%), and median length of stay declined accordingly. The average estimated costs of HIT care per year dropped by about $267,000 dollars per year, from $322,000 before the program was implemented to $55,000 afterward.
The investigators added that this is the first study ever to show the success of an HIT prevention strategy. Their findings indicate that a hospital-wide “avoid heparin” program can substantially reduce morbidity, mortality, and costs associated with HIT. “The heparin avoidance strategy that we used was not complex or costly and would be feasible in other centers,” they noted.
A simple, hospital-wide “avoid heparin” intervention dramatically cut the rate of suspected heparin-induced thrombocytopenia by 42%, that of positive ELISA screens for HIT by 63%, that of adjudicated HIT by 79%, and that of HIT with thrombosis by 91%, while also reducing the costs of HIT-related care by 83% at one large university hospital.
The medical literature has focused on early recognition and treatment of heparin-induced thrombocytopenia, “but its prevention has been largely overlooked,” noted Dr. Kelly E. McGowan of Sunnybrook Health Sciences Centre and the University of Toronto and her associates.
Sunnybrook introduced an “avoid heparin” program in 2006 in which most intravenous and subcutaneous unfractionated heparin was replaced with low-molecular-weight heparin (LMWH) in prophylactic or therapeutic doses; heparinized saline in arterial and central venous lines was replaced with saline flushes; order sets were modified to exclude unfractionated heparin options; and unfractionated heparin stores were removed from most nursing units.
Unfractionated heparin remained available for use in hemodialysis, cardiovascular surgery, and certain cases of acute coronary syndrome. Most hospital clinicians were unaware that LMWH was being substituted for unfractionated heparin, and none were aware that the effects of this change were being studied.
The investigators assessed all 1,118 cases of suspected heparin-induced thrombocytopenia that occurred during a 10-year period before and after this intervention was implemented. The use of LMWH rose fourfold after the program was initiated, but the annual rate of HIT associated with LMWH remained constant at 0.9 cases per 10,000 admissions over the course of the study.
The annual incidence of suspected HIT decreased from 85.5 per 10,000 admissions per year before the intervention to 49.0 afterward (relative risk reduction, 41.7%). The rate of positive ELISA screens for the disorder dropped from 16.5 to 6.1 per 10,000 (RRR, 62.9%), the rate of adjudicated HIT decreased from 10.7 to 2.2 per 10,000, and the rate of HIT with thrombosis declined from 4.6 to 0.4 per 10,000.
The program’s greatest impact was on cardiac surgery, but the burden of HIT also markedly decreased in other surgical and medical patients. HIT decreased by 77% in cardiovascular surgeries, 77% in other surgeries, 75% in cardiology patients, and 62% in medical patients, Dr. McGowan and her associates said (Blood 2016 Apr 21;127[16]:1954-9).
Patients with HIT during the preintervention years more often developed thrombosis (43%), usually venous thromboembolism, compared with those who had HIT in the postintervention years (19%), and median length of stay declined accordingly. The average estimated costs of HIT care per year dropped by about $267,000 dollars per year, from $322,000 before the program was implemented to $55,000 afterward.
The investigators added that this is the first study ever to show the success of an HIT prevention strategy. Their findings indicate that a hospital-wide “avoid heparin” program can substantially reduce morbidity, mortality, and costs associated with HIT. “The heparin avoidance strategy that we used was not complex or costly and would be feasible in other centers,” they noted.
FROM BLOOD
Key clinical point: A simple, hospital-wide “avoid heparin” intervention dramatically cut the burden of heparin-induced thrombocytopenia at one hospital.
Major finding: The annual incidence of suspected HIT decreased from 85.5 per 10,000 admissions per year before the intervention to 49.0 afterward (relative risk reduction, 41.7%).
Data source: A retrospective comparison of 1,118 heparin-induced disorders at a large university hospital before and after the implementation of a preventive intervention.
Disclosures: No sponsors/supporters were identified for this study. Dr. McGowan reported having no relevant financial disclosures; her associates reported ties to numerous industry sources.