User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Women’s Health Initiative may account for insomnia’s upward trend
DENVER – Much of the rising prevalence of insomnia among U.S. and Canadian adults may be driven by the sharp reduction in the use of hormone replacement therapy following the 2002 report from the Women’s Health Initiative, Sheila N. Garland, PhD, said at the annual meeting of the Associated Professional Sleep Societies.
“In 2002 the Women’s Health Initiative came out with findings that hormone replacement therapy increased the risk of coronary heart disease, breast cancer, stroke, and pulmonary embolism [JAMA. 2002 Jul 17;288(3):321-33]. A lot of women on hormone replacement therapy up until 2002 stopped using it then. The increase in difficulty sleeping we found between 2002 and 2012 could perhaps represent an untreated menopausal symptom,” according to Dr. Garland, a psychologist at Memorial University of Newfoundland in St. John’s.
She presented an analysis of data from the Canadian Community Health Survey, a national once-per-decade survey, which included 34,118 adults aged 20-80 years and older in the 2002 version and 23,089 in 2012.
The key finding from the standpoint of sleep medicine, the prevalence of self-reported trouble sleeping, increased from 15.6% in 2002 to 17.1% in 2012. And this increase was concentrated in 40- to 59-year-old women, where the prevalence of insomnia or poor sleep rose from 19% to 24.3%, the highest of any age group. Rates remained flat over time in men and women aged 20-39 years and 60-80 years and older as well as in 40- to 59-year-old men.
The sleep-related question put to survey participants in face-to-face or telephone interviews was this: “How often do you have trouble going to sleep or staying asleep?” If they answered “most of the time” or “all of the time” they were classified as having poor sleep.
When Dr. Garland saw the survey results she immediately asked herself, “What is happening with Canadian women?” She came up with two theories. They are not mutually exclusive. One hinges on the dramatic drop off in the use of hormone replacement therapy in response to the Women’s Health Initiative report. The other theory is that the increasing prevalence of insomnia in middle-aged women is a manifestation of stress among the growing population of what has been called “the sandwich generation,” people who care for their aging parents while also supporting their own children. Those responsibilities most often fall upon the mother.
She noted that a similar rise in the prevalence of insomnia or trouble sleeping is occurring south of the border – in the United States – as documented in a Centers for Disease Control and Prevention analysis. The CDC investigators examined data on 30,970 adult participants in the 2002 National Health Interview Survey, 23,344 in the 2007 survey, and 34,509 in the 2012 survey. The prevalence of insomnia or trouble sleeping increased from 17.5% in 2002 to 19.2% in 2012. That absolute 1.7% increase pairs well with the 1.5% rise over the same time frame in the Canadian survey. The prevalence was higher in U.S. women than men in all three survey years (Sleep Med. 2015 Mar;16[3]:372-8).
Dr. Garland said the Canadian national survey contains data on self-perceived stress levels. She plans to analyze those results to learn if being a member of the sandwich generation is a significant contributor to poor sleep.
Regardless of the underlying mechanism, she continued, it’s clear from these large national surveys that middle-aged women are particularly vulnerable to poor sleep.
“Increased recognition is necessary. Prevention and intervention programs may be warranted, given the individual and societal consequences of poor sleep, including increased psychiatric and medical disorders, work absenteeism, and health care costs,” she said.
One audience member rose to congratulate Dr. Garland on her detective work. He commented that her notion that the rise in insomnia can be traced to fallout from the 2002 Women’s Health Initiative is consistent with his own clinical experience.
“The most common way women over age 40 present to our sleep center with insomnia is because they’re menopausal and not on hormone replacement therapy,” he said.
The Canadian Community Health Survey is sponsored by Statistics Canada. Dr. Garland reported having no financial conflicts of interest.
DENVER – Much of the rising prevalence of insomnia among U.S. and Canadian adults may be driven by the sharp reduction in the use of hormone replacement therapy following the 2002 report from the Women’s Health Initiative, Sheila N. Garland, PhD, said at the annual meeting of the Associated Professional Sleep Societies.
“In 2002 the Women’s Health Initiative came out with findings that hormone replacement therapy increased the risk of coronary heart disease, breast cancer, stroke, and pulmonary embolism [JAMA. 2002 Jul 17;288(3):321-33]. A lot of women on hormone replacement therapy up until 2002 stopped using it then. The increase in difficulty sleeping we found between 2002 and 2012 could perhaps represent an untreated menopausal symptom,” according to Dr. Garland, a psychologist at Memorial University of Newfoundland in St. John’s.
She presented an analysis of data from the Canadian Community Health Survey, a national once-per-decade survey, which included 34,118 adults aged 20-80 years and older in the 2002 version and 23,089 in 2012.
The key finding from the standpoint of sleep medicine, the prevalence of self-reported trouble sleeping, increased from 15.6% in 2002 to 17.1% in 2012. And this increase was concentrated in 40- to 59-year-old women, where the prevalence of insomnia or poor sleep rose from 19% to 24.3%, the highest of any age group. Rates remained flat over time in men and women aged 20-39 years and 60-80 years and older as well as in 40- to 59-year-old men.
The sleep-related question put to survey participants in face-to-face or telephone interviews was this: “How often do you have trouble going to sleep or staying asleep?” If they answered “most of the time” or “all of the time” they were classified as having poor sleep.
When Dr. Garland saw the survey results she immediately asked herself, “What is happening with Canadian women?” She came up with two theories. They are not mutually exclusive. One hinges on the dramatic drop off in the use of hormone replacement therapy in response to the Women’s Health Initiative report. The other theory is that the increasing prevalence of insomnia in middle-aged women is a manifestation of stress among the growing population of what has been called “the sandwich generation,” people who care for their aging parents while also supporting their own children. Those responsibilities most often fall upon the mother.
She noted that a similar rise in the prevalence of insomnia or trouble sleeping is occurring south of the border – in the United States – as documented in a Centers for Disease Control and Prevention analysis. The CDC investigators examined data on 30,970 adult participants in the 2002 National Health Interview Survey, 23,344 in the 2007 survey, and 34,509 in the 2012 survey. The prevalence of insomnia or trouble sleeping increased from 17.5% in 2002 to 19.2% in 2012. That absolute 1.7% increase pairs well with the 1.5% rise over the same time frame in the Canadian survey. The prevalence was higher in U.S. women than men in all three survey years (Sleep Med. 2015 Mar;16[3]:372-8).
Dr. Garland said the Canadian national survey contains data on self-perceived stress levels. She plans to analyze those results to learn if being a member of the sandwich generation is a significant contributor to poor sleep.
Regardless of the underlying mechanism, she continued, it’s clear from these large national surveys that middle-aged women are particularly vulnerable to poor sleep.
“Increased recognition is necessary. Prevention and intervention programs may be warranted, given the individual and societal consequences of poor sleep, including increased psychiatric and medical disorders, work absenteeism, and health care costs,” she said.
One audience member rose to congratulate Dr. Garland on her detective work. He commented that her notion that the rise in insomnia can be traced to fallout from the 2002 Women’s Health Initiative is consistent with his own clinical experience.
“The most common way women over age 40 present to our sleep center with insomnia is because they’re menopausal and not on hormone replacement therapy,” he said.
The Canadian Community Health Survey is sponsored by Statistics Canada. Dr. Garland reported having no financial conflicts of interest.
DENVER – Much of the rising prevalence of insomnia among U.S. and Canadian adults may be driven by the sharp reduction in the use of hormone replacement therapy following the 2002 report from the Women’s Health Initiative, Sheila N. Garland, PhD, said at the annual meeting of the Associated Professional Sleep Societies.
“In 2002 the Women’s Health Initiative came out with findings that hormone replacement therapy increased the risk of coronary heart disease, breast cancer, stroke, and pulmonary embolism [JAMA. 2002 Jul 17;288(3):321-33]. A lot of women on hormone replacement therapy up until 2002 stopped using it then. The increase in difficulty sleeping we found between 2002 and 2012 could perhaps represent an untreated menopausal symptom,” according to Dr. Garland, a psychologist at Memorial University of Newfoundland in St. John’s.
She presented an analysis of data from the Canadian Community Health Survey, a national once-per-decade survey, which included 34,118 adults aged 20-80 years and older in the 2002 version and 23,089 in 2012.
The key finding from the standpoint of sleep medicine, the prevalence of self-reported trouble sleeping, increased from 15.6% in 2002 to 17.1% in 2012. And this increase was concentrated in 40- to 59-year-old women, where the prevalence of insomnia or poor sleep rose from 19% to 24.3%, the highest of any age group. Rates remained flat over time in men and women aged 20-39 years and 60-80 years and older as well as in 40- to 59-year-old men.
The sleep-related question put to survey participants in face-to-face or telephone interviews was this: “How often do you have trouble going to sleep or staying asleep?” If they answered “most of the time” or “all of the time” they were classified as having poor sleep.
When Dr. Garland saw the survey results she immediately asked herself, “What is happening with Canadian women?” She came up with two theories. They are not mutually exclusive. One hinges on the dramatic drop off in the use of hormone replacement therapy in response to the Women’s Health Initiative report. The other theory is that the increasing prevalence of insomnia in middle-aged women is a manifestation of stress among the growing population of what has been called “the sandwich generation,” people who care for their aging parents while also supporting their own children. Those responsibilities most often fall upon the mother.
She noted that a similar rise in the prevalence of insomnia or trouble sleeping is occurring south of the border – in the United States – as documented in a Centers for Disease Control and Prevention analysis. The CDC investigators examined data on 30,970 adult participants in the 2002 National Health Interview Survey, 23,344 in the 2007 survey, and 34,509 in the 2012 survey. The prevalence of insomnia or trouble sleeping increased from 17.5% in 2002 to 19.2% in 2012. That absolute 1.7% increase pairs well with the 1.5% rise over the same time frame in the Canadian survey. The prevalence was higher in U.S. women than men in all three survey years (Sleep Med. 2015 Mar;16[3]:372-8).
Dr. Garland said the Canadian national survey contains data on self-perceived stress levels. She plans to analyze those results to learn if being a member of the sandwich generation is a significant contributor to poor sleep.
Regardless of the underlying mechanism, she continued, it’s clear from these large national surveys that middle-aged women are particularly vulnerable to poor sleep.
“Increased recognition is necessary. Prevention and intervention programs may be warranted, given the individual and societal consequences of poor sleep, including increased psychiatric and medical disorders, work absenteeism, and health care costs,” she said.
One audience member rose to congratulate Dr. Garland on her detective work. He commented that her notion that the rise in insomnia can be traced to fallout from the 2002 Women’s Health Initiative is consistent with his own clinical experience.
“The most common way women over age 40 present to our sleep center with insomnia is because they’re menopausal and not on hormone replacement therapy,” he said.
The Canadian Community Health Survey is sponsored by Statistics Canada. Dr. Garland reported having no financial conflicts of interest.
AT SLEEP 2016
Key clinical point: Middle-aged women are increasingly vulnerable to insomnia.
Major finding: The prevalence of insomnia or poor sleep among 40- to 59-year-old Canadian women climbed from 19% in 2002 to 24.3% in 2012.
Data source: The Canadian Community Health Survey included 34,118 adults aged 20-80 years and older in 2002 and 23,089 in 2012.
Disclosures: The survey is sponsored by Statistics Canada. The presenter reported having no financial conflicts of interest.
Primary care management of sepsis survivors does not improve mental health quality of life
Patients who have survived sepsis or septic shock do not receive any significant benefit in the quality of their mental health by receiving primary care management intervention, according to a new study published by JAMA.
“Many survivors of sepsis have multiple medical comorbidities that are typically managed in primary care [but] interventions for managing sepsis sequelae in primary care have not been developed,” states the study, which was led by Jochen Gensichen, MD, of the Institute of General Practice & Family Medicine at Jena (Germany) University Hospital.
“To our knowledge, this is the first large-scale, randomized controlled clinical trial of an intervention to improve outcomes in survivors of sepsis in primary care,” Dr. Gensichen and his coinvestigators added.
The study recruited sepsis and septic shock survivors from nine ICUs across Germany between February 2011 and December 2014, excluding any patients with cognitive impairment, defined as a Telephone Interview of Cognitive Status score no greater than 27. Ultimately, 291 patients aged 18 years or older (mean age of 61.6 years) were selected for inclusion and randomized into cohorts receiving either primary care–based intervention (n = 148) or usual care (n = 143) (JAMA. 2016;315:2703-11. doi: 10.1001/jama.2016.7207).
Those assigned to the usual care cohort received the standard care that their primary care providers would normally carry out, which included “periodic contacts, referrals to specialists, and prescription of medication and therapeutic aids at quantities comparable with those for other populations with multiple chronic conditions.” Those in the other cohort were given active monitoring of symptoms from providers who had been given evidence-based care training and clinical decision support from nurses who underwent training to become case managers. Case managers would take patients through an hour-long face-to-face training on sepsis sequelae within 2-20 days of ICU discharge, along with subsequent follow-up conversations over the phone.
“Case managers monitored patients’ symptoms using validated screening tools to assess critical illness polyneuropathy/myopathy, wasting, neurocognitive deficits, [posttraumatic stress disorder], depressive and pain symptoms, as well as patient self-management behaviors focusing on physical activity and individual self-management goals,” the authors said, noting that case managers would report their results to a consulting physician who “supervised the case managers and provided clinical decision support to the [primary care physicians].”
Baseline Mental Component Summary (MCS) scores were taken for subjects in both cohorts to determine mental health-based quality of life, which averaged 49.1 for the intervention cohort and 49.3 for the control. MCS scores at 6 months’ follow-up were 52.9 for the intervention group (95% confidence interval, 1.05-6.54) and 51.0 for the control group (95% CI, –1.22-4.51), for a mean change of 3.8 in the intervention cohort and 1.6 for the control group. The mean treatment effect was 2.15 (95% CI, –1.79-6.09; P = .28), indicating no significant difference between the two.
“There was no evidence for a differential treatment effect on the study’s primary outcome, postsepsis MCS scores,” the authors concluded. “This finding is similar to those from previous trials of care management interventions following critical illness.”
The authors added that “further research is needed to determine if modified approaches to primary care management may be more effective.”
The study was funded by the Center for Sepsis Control and Care, the German Federal Ministry of Education and Research, and the German Sepsis Society. Dr. Gensichen reported receiving personal fees from the Primary Health Care Foundation and receiving a grant from the German Federal Ministry of Education and Research.
Patients who have survived sepsis or septic shock do not receive any significant benefit in the quality of their mental health by receiving primary care management intervention, according to a new study published by JAMA.
“Many survivors of sepsis have multiple medical comorbidities that are typically managed in primary care [but] interventions for managing sepsis sequelae in primary care have not been developed,” states the study, which was led by Jochen Gensichen, MD, of the Institute of General Practice & Family Medicine at Jena (Germany) University Hospital.
“To our knowledge, this is the first large-scale, randomized controlled clinical trial of an intervention to improve outcomes in survivors of sepsis in primary care,” Dr. Gensichen and his coinvestigators added.
The study recruited sepsis and septic shock survivors from nine ICUs across Germany between February 2011 and December 2014, excluding any patients with cognitive impairment, defined as a Telephone Interview of Cognitive Status score no greater than 27. Ultimately, 291 patients aged 18 years or older (mean age of 61.6 years) were selected for inclusion and randomized into cohorts receiving either primary care–based intervention (n = 148) or usual care (n = 143) (JAMA. 2016;315:2703-11. doi: 10.1001/jama.2016.7207).
Those assigned to the usual care cohort received the standard care that their primary care providers would normally carry out, which included “periodic contacts, referrals to specialists, and prescription of medication and therapeutic aids at quantities comparable with those for other populations with multiple chronic conditions.” Those in the other cohort were given active monitoring of symptoms from providers who had been given evidence-based care training and clinical decision support from nurses who underwent training to become case managers. Case managers would take patients through an hour-long face-to-face training on sepsis sequelae within 2-20 days of ICU discharge, along with subsequent follow-up conversations over the phone.
“Case managers monitored patients’ symptoms using validated screening tools to assess critical illness polyneuropathy/myopathy, wasting, neurocognitive deficits, [posttraumatic stress disorder], depressive and pain symptoms, as well as patient self-management behaviors focusing on physical activity and individual self-management goals,” the authors said, noting that case managers would report their results to a consulting physician who “supervised the case managers and provided clinical decision support to the [primary care physicians].”
Baseline Mental Component Summary (MCS) scores were taken for subjects in both cohorts to determine mental health-based quality of life, which averaged 49.1 for the intervention cohort and 49.3 for the control. MCS scores at 6 months’ follow-up were 52.9 for the intervention group (95% confidence interval, 1.05-6.54) and 51.0 for the control group (95% CI, –1.22-4.51), for a mean change of 3.8 in the intervention cohort and 1.6 for the control group. The mean treatment effect was 2.15 (95% CI, –1.79-6.09; P = .28), indicating no significant difference between the two.
“There was no evidence for a differential treatment effect on the study’s primary outcome, postsepsis MCS scores,” the authors concluded. “This finding is similar to those from previous trials of care management interventions following critical illness.”
The authors added that “further research is needed to determine if modified approaches to primary care management may be more effective.”
The study was funded by the Center for Sepsis Control and Care, the German Federal Ministry of Education and Research, and the German Sepsis Society. Dr. Gensichen reported receiving personal fees from the Primary Health Care Foundation and receiving a grant from the German Federal Ministry of Education and Research.
Patients who have survived sepsis or septic shock do not receive any significant benefit in the quality of their mental health by receiving primary care management intervention, according to a new study published by JAMA.
“Many survivors of sepsis have multiple medical comorbidities that are typically managed in primary care [but] interventions for managing sepsis sequelae in primary care have not been developed,” states the study, which was led by Jochen Gensichen, MD, of the Institute of General Practice & Family Medicine at Jena (Germany) University Hospital.
“To our knowledge, this is the first large-scale, randomized controlled clinical trial of an intervention to improve outcomes in survivors of sepsis in primary care,” Dr. Gensichen and his coinvestigators added.
The study recruited sepsis and septic shock survivors from nine ICUs across Germany between February 2011 and December 2014, excluding any patients with cognitive impairment, defined as a Telephone Interview of Cognitive Status score no greater than 27. Ultimately, 291 patients aged 18 years or older (mean age of 61.6 years) were selected for inclusion and randomized into cohorts receiving either primary care–based intervention (n = 148) or usual care (n = 143) (JAMA. 2016;315:2703-11. doi: 10.1001/jama.2016.7207).
Those assigned to the usual care cohort received the standard care that their primary care providers would normally carry out, which included “periodic contacts, referrals to specialists, and prescription of medication and therapeutic aids at quantities comparable with those for other populations with multiple chronic conditions.” Those in the other cohort were given active monitoring of symptoms from providers who had been given evidence-based care training and clinical decision support from nurses who underwent training to become case managers. Case managers would take patients through an hour-long face-to-face training on sepsis sequelae within 2-20 days of ICU discharge, along with subsequent follow-up conversations over the phone.
“Case managers monitored patients’ symptoms using validated screening tools to assess critical illness polyneuropathy/myopathy, wasting, neurocognitive deficits, [posttraumatic stress disorder], depressive and pain symptoms, as well as patient self-management behaviors focusing on physical activity and individual self-management goals,” the authors said, noting that case managers would report their results to a consulting physician who “supervised the case managers and provided clinical decision support to the [primary care physicians].”
Baseline Mental Component Summary (MCS) scores were taken for subjects in both cohorts to determine mental health-based quality of life, which averaged 49.1 for the intervention cohort and 49.3 for the control. MCS scores at 6 months’ follow-up were 52.9 for the intervention group (95% confidence interval, 1.05-6.54) and 51.0 for the control group (95% CI, –1.22-4.51), for a mean change of 3.8 in the intervention cohort and 1.6 for the control group. The mean treatment effect was 2.15 (95% CI, –1.79-6.09; P = .28), indicating no significant difference between the two.
“There was no evidence for a differential treatment effect on the study’s primary outcome, postsepsis MCS scores,” the authors concluded. “This finding is similar to those from previous trials of care management interventions following critical illness.”
The authors added that “further research is needed to determine if modified approaches to primary care management may be more effective.”
The study was funded by the Center for Sepsis Control and Care, the German Federal Ministry of Education and Research, and the German Sepsis Society. Dr. Gensichen reported receiving personal fees from the Primary Health Care Foundation and receiving a grant from the German Federal Ministry of Education and Research.
FROM JAMA
Key clinical point: Primary care intervention does not improve mental health–related quality of life in survivors of sepsis or septic shock.
Major finding: Mean Mental Component Summary (MCS) scores showed no significant change between the time of ICU discharge (49.1) versus at 6 months postdischarge (52.9) (95% CI, 1.05-6.54), compared with the control group: 49.3 at baseline vs. 51.0 at 6 months follow-up (95% CI, –1.22-4.51).
Data source: A multicenter, unblinded, two-group randomized clinical trial of 291 adult sepsis or septic shock survivors recruited from nine German ICUs from February 2011 through December 2014.
Disclosures: Study funded by the Center for Sepsis Control and Care, the German Federal Ministry of Education and Research, and the German Sepsis Society. Dr. Gensichen reported receiving personal fees from The Primary Health Care Foundation and receiving a grant from the German Federal Ministry of Education and Research.
ICU-based therapy fails to shorten hospital stay
Standardized rehabilitation therapy did not reduce hospital length of stay in patients with acute respiratory failure, based on data from a randomized trial of 300 adults published online in JAMA.
Hospital length of stay averaged 10 days for patients in the standardized rehabilitation therapy group (SRT) and 10 days in the control group that received usual ICU care, wrote Dr. Peter E. Morris of the division of pulmonary, critical care and sleep medicine at the University of Kentucky, Lexington, and his colleagues (JAMA. 2016 Jun;315:2694-702. doi: 10.1001/jama.2016.7201).
The patients were followed for 6 months; 84 patients in the SRT group and 81 in the usual group completed the study.
Patients in the SRT group received daily therapy including passive range of motion, physical therapy, and progressive-resistance exercises. The usual care group received weekday physical therapy as determined by the clinical team.
The researchers also assessed secondary outcomes related to physical function and quality of life, including ventilator days, Short Physical Performance Battery (SPPB) score, handgrip, Mini-Mental State Examination, and Functional Performance Inventory (FPI).
Overall, there was no difference in duration of ventilation or ICU care between the two groups, and score of handgrip strength and mental health also were similar at 6 months’ follow up. However, the SF-36 physical function scores were significantly higher in the SRT group (difference, 12.2; 95% confidence interval, 3.8-20.7; P = .001), and the FPI scores and SPPB scores were higher, compared with the usual care group at 6 months.
“These findings from the exploratory analysis may highlight the emerging role of placing long-term outcomes within critical care clinical trial design not only as a secondary outcome, but possibly as the primary outcome,” the researchers noted. “In view of the SPPB, SF-36 PFS, and FPI data at 6 months, the SRT group demonstrated a potential signal of improvement compared with the usual care group that was not evident at hospital discharge,” they wrote.
The study was supported by the National Institutes of Health, National Institute of Nursing Research, and National Heart, Lung, and Blood Institute. Lead author, Dr. Morris, had no financial conflicts to disclose.
Standardized rehabilitation therapy did not reduce hospital length of stay in patients with acute respiratory failure, based on data from a randomized trial of 300 adults published online in JAMA.
Hospital length of stay averaged 10 days for patients in the standardized rehabilitation therapy group (SRT) and 10 days in the control group that received usual ICU care, wrote Dr. Peter E. Morris of the division of pulmonary, critical care and sleep medicine at the University of Kentucky, Lexington, and his colleagues (JAMA. 2016 Jun;315:2694-702. doi: 10.1001/jama.2016.7201).
The patients were followed for 6 months; 84 patients in the SRT group and 81 in the usual group completed the study.
Patients in the SRT group received daily therapy including passive range of motion, physical therapy, and progressive-resistance exercises. The usual care group received weekday physical therapy as determined by the clinical team.
The researchers also assessed secondary outcomes related to physical function and quality of life, including ventilator days, Short Physical Performance Battery (SPPB) score, handgrip, Mini-Mental State Examination, and Functional Performance Inventory (FPI).
Overall, there was no difference in duration of ventilation or ICU care between the two groups, and score of handgrip strength and mental health also were similar at 6 months’ follow up. However, the SF-36 physical function scores were significantly higher in the SRT group (difference, 12.2; 95% confidence interval, 3.8-20.7; P = .001), and the FPI scores and SPPB scores were higher, compared with the usual care group at 6 months.
“These findings from the exploratory analysis may highlight the emerging role of placing long-term outcomes within critical care clinical trial design not only as a secondary outcome, but possibly as the primary outcome,” the researchers noted. “In view of the SPPB, SF-36 PFS, and FPI data at 6 months, the SRT group demonstrated a potential signal of improvement compared with the usual care group that was not evident at hospital discharge,” they wrote.
The study was supported by the National Institutes of Health, National Institute of Nursing Research, and National Heart, Lung, and Blood Institute. Lead author, Dr. Morris, had no financial conflicts to disclose.
Standardized rehabilitation therapy did not reduce hospital length of stay in patients with acute respiratory failure, based on data from a randomized trial of 300 adults published online in JAMA.
Hospital length of stay averaged 10 days for patients in the standardized rehabilitation therapy group (SRT) and 10 days in the control group that received usual ICU care, wrote Dr. Peter E. Morris of the division of pulmonary, critical care and sleep medicine at the University of Kentucky, Lexington, and his colleagues (JAMA. 2016 Jun;315:2694-702. doi: 10.1001/jama.2016.7201).
The patients were followed for 6 months; 84 patients in the SRT group and 81 in the usual group completed the study.
Patients in the SRT group received daily therapy including passive range of motion, physical therapy, and progressive-resistance exercises. The usual care group received weekday physical therapy as determined by the clinical team.
The researchers also assessed secondary outcomes related to physical function and quality of life, including ventilator days, Short Physical Performance Battery (SPPB) score, handgrip, Mini-Mental State Examination, and Functional Performance Inventory (FPI).
Overall, there was no difference in duration of ventilation or ICU care between the two groups, and score of handgrip strength and mental health also were similar at 6 months’ follow up. However, the SF-36 physical function scores were significantly higher in the SRT group (difference, 12.2; 95% confidence interval, 3.8-20.7; P = .001), and the FPI scores and SPPB scores were higher, compared with the usual care group at 6 months.
“These findings from the exploratory analysis may highlight the emerging role of placing long-term outcomes within critical care clinical trial design not only as a secondary outcome, but possibly as the primary outcome,” the researchers noted. “In view of the SPPB, SF-36 PFS, and FPI data at 6 months, the SRT group demonstrated a potential signal of improvement compared with the usual care group that was not evident at hospital discharge,” they wrote.
The study was supported by the National Institutes of Health, National Institute of Nursing Research, and National Heart, Lung, and Blood Institute. Lead author, Dr. Morris, had no financial conflicts to disclose.
FROM JAMA
Key clinical point: Rehabilitation therapy in the ICU did not reduce hospital stay in patients with acute respiratory failure.
Major finding: The average length of stay was 10 days in both the therapy and control groups.
Data source: A randomized, single-center study including 300 adults with acute respiratory failure.
Disclosures: The study was supported by the National Institutes of Health, National Institute of Nursing Research, and National Heart, Lung, and Blood Institute. Lead author Dr. Morris had no financial conflicts to disclose.
Upper airway stimulation for obstructive sleep apnea shows continued benefit at 42 months
DENVER – The surgically implanted Inspire system for controlled upper airway stimulation as therapy for moderate to severe obstructive sleep apnea demonstrated sustained benefit at 42 months of prospective follow-up in the STAR trial, Dr. Patrick J. Strollo Jr. reported at the annual meeting of the Associated Professional Sleep Societies.
STAR was the pivotal trial whose previously reported 12-month outcomes led to Food and Drug Administration clearance of the device. Dr. Strollo was first author of that paper (N Engl J Med. 2014 Jan 9;370:139-49). At SLEEP 2016, he presented patient- and partner-reported outcomes at 42 months. Bottom line: The device had continued safety and no loss in efficacy.
“So far it seems to be a useful option for people who frequently didn’t have an option. And the technology is improving and will only get better,” said Dr. Strollo, professor of medicine and clinical and translational science, director of the Sleep Medicine Center, and codirector of the Sleep Medicine Institute at the University of Pittsburgh.
The Inspire system consists of three parts implanted by an otolaryngologist in an outpatient procedure: a small impulse generator, a breathing sensor lead inserted in the intercostal muscle, and a stimulator lead attached to the distal branch of the 10th cranial nerve, the hypoglossal nerve controlling the tongue muscles.
The device is programmed to discharge at the end of expiration and continue through the inspiratory phase, causing the tongue to move forward and the retrolingual and retropalatal airways to open, he explained in an interview.
Upper airway stimulation is approved for commercial use in patients such as those enrolled in the STAR trial on the basis of pilot studies that identified most likely responders. The key selection criteria include moderate to severe obstructive sleep apnea as defined by an apnea-hypopnea index of 20-50, nonadherence to continuous positive airway pressure (CPAP), a body mass index of 32 kg/m2 or less, and absence of concentric collapse of the airway at the level of the palate during sedated endoscopy.
STAR included 126 participants who received the upper airway stimulation device. There have been two explants: one from septic arthritis, the other elective.
A total of 97 STAR participants had 42-month follow-up data available. Among the key findings were that:
• Mean scores on the Epworth Sleepiness Scale decreased from 11.6 at baseline to 7 at 12 months and 7.1 at 42 months.
• Scores on the Functional Outcomes of Sleep Questionnaire improved from 14.3 at baseline to 17.3 at 12 months and 17.5 at 42 months.
• The scores on both the Epworth Sleepiness Scale and Functional Outcomes of Sleep Questionnaire were abnormal at baseline and converted to normal range at both 12 and 42 months of follow-up.
• At baseline, 29% of the patients’ sleeping partners characterized the snoring as loud, 24% rated it ‘very intense,’ and 30% left the bedroom. At 32 months, 11% of partners called the snoring loud, 3% deemed it very intense, and only 4% left the room.
• At 42 months, 81% of patients reported using the device nightly. That’s consistent with the objective evidence of adherence Dr. Strollo and his coinvestigators obtained in a study of postmarketing device implants in which they found device usage averaged about 7 hours per night.
“That’s much better than we see with CPAP in patients who can tolerate that therapy,” Dr. Strollo observed.
The planned 5-year follow-up of STAR participants includes a full laboratory polysomnography study to obtain objective apnea-hypopnea index figures.
The other major development is the launch of a comprehensive registry of patients who receive a post-marketing commercial implant. Roughly 1,000 implants have been done worldwide to date, but now that the device is approved, that number will quickly grow. The registry should prove a rich source for research.
“The goal is to try to refine the selection criteria,” according to Dr. Strollo.
Given that only about 50% of patients with moderate to severe sleep apnea are able to tolerate CPAP long term, where does the Inspire system fit into today’s practice of sleep medicine?
“Upper airway stimulation is another tool, another option for patients,” he said. “In my practice, normally I’d let patients try positive pressure first. I want to make sure they’ve tried CPAP, and they’ve tried more advanced therapy like autotitrating bilevel positive airway pressure, which is more comfortable than CPAP. Bilevel positive airway pressure allows you to salvage a fair number of patients who can’t tolerate CPAP. And I also offer an oral appliance, although the robustness of an oral appliance is not great as apnea becomes more severe.”
The STAR trial is supported by Inspire Medical Systems. Dr. Strollo reported receiving a research grant from the company.
DENVER – The surgically implanted Inspire system for controlled upper airway stimulation as therapy for moderate to severe obstructive sleep apnea demonstrated sustained benefit at 42 months of prospective follow-up in the STAR trial, Dr. Patrick J. Strollo Jr. reported at the annual meeting of the Associated Professional Sleep Societies.
STAR was the pivotal trial whose previously reported 12-month outcomes led to Food and Drug Administration clearance of the device. Dr. Strollo was first author of that paper (N Engl J Med. 2014 Jan 9;370:139-49). At SLEEP 2016, he presented patient- and partner-reported outcomes at 42 months. Bottom line: The device had continued safety and no loss in efficacy.
“So far it seems to be a useful option for people who frequently didn’t have an option. And the technology is improving and will only get better,” said Dr. Strollo, professor of medicine and clinical and translational science, director of the Sleep Medicine Center, and codirector of the Sleep Medicine Institute at the University of Pittsburgh.
The Inspire system consists of three parts implanted by an otolaryngologist in an outpatient procedure: a small impulse generator, a breathing sensor lead inserted in the intercostal muscle, and a stimulator lead attached to the distal branch of the 10th cranial nerve, the hypoglossal nerve controlling the tongue muscles.
The device is programmed to discharge at the end of expiration and continue through the inspiratory phase, causing the tongue to move forward and the retrolingual and retropalatal airways to open, he explained in an interview.
Upper airway stimulation is approved for commercial use in patients such as those enrolled in the STAR trial on the basis of pilot studies that identified most likely responders. The key selection criteria include moderate to severe obstructive sleep apnea as defined by an apnea-hypopnea index of 20-50, nonadherence to continuous positive airway pressure (CPAP), a body mass index of 32 kg/m2 or less, and absence of concentric collapse of the airway at the level of the palate during sedated endoscopy.
STAR included 126 participants who received the upper airway stimulation device. There have been two explants: one from septic arthritis, the other elective.
A total of 97 STAR participants had 42-month follow-up data available. Among the key findings were that:
• Mean scores on the Epworth Sleepiness Scale decreased from 11.6 at baseline to 7 at 12 months and 7.1 at 42 months.
• Scores on the Functional Outcomes of Sleep Questionnaire improved from 14.3 at baseline to 17.3 at 12 months and 17.5 at 42 months.
• The scores on both the Epworth Sleepiness Scale and Functional Outcomes of Sleep Questionnaire were abnormal at baseline and converted to normal range at both 12 and 42 months of follow-up.
• At baseline, 29% of the patients’ sleeping partners characterized the snoring as loud, 24% rated it ‘very intense,’ and 30% left the bedroom. At 32 months, 11% of partners called the snoring loud, 3% deemed it very intense, and only 4% left the room.
• At 42 months, 81% of patients reported using the device nightly. That’s consistent with the objective evidence of adherence Dr. Strollo and his coinvestigators obtained in a study of postmarketing device implants in which they found device usage averaged about 7 hours per night.
“That’s much better than we see with CPAP in patients who can tolerate that therapy,” Dr. Strollo observed.
The planned 5-year follow-up of STAR participants includes a full laboratory polysomnography study to obtain objective apnea-hypopnea index figures.
The other major development is the launch of a comprehensive registry of patients who receive a post-marketing commercial implant. Roughly 1,000 implants have been done worldwide to date, but now that the device is approved, that number will quickly grow. The registry should prove a rich source for research.
“The goal is to try to refine the selection criteria,” according to Dr. Strollo.
Given that only about 50% of patients with moderate to severe sleep apnea are able to tolerate CPAP long term, where does the Inspire system fit into today’s practice of sleep medicine?
“Upper airway stimulation is another tool, another option for patients,” he said. “In my practice, normally I’d let patients try positive pressure first. I want to make sure they’ve tried CPAP, and they’ve tried more advanced therapy like autotitrating bilevel positive airway pressure, which is more comfortable than CPAP. Bilevel positive airway pressure allows you to salvage a fair number of patients who can’t tolerate CPAP. And I also offer an oral appliance, although the robustness of an oral appliance is not great as apnea becomes more severe.”
The STAR trial is supported by Inspire Medical Systems. Dr. Strollo reported receiving a research grant from the company.
DENVER – The surgically implanted Inspire system for controlled upper airway stimulation as therapy for moderate to severe obstructive sleep apnea demonstrated sustained benefit at 42 months of prospective follow-up in the STAR trial, Dr. Patrick J. Strollo Jr. reported at the annual meeting of the Associated Professional Sleep Societies.
STAR was the pivotal trial whose previously reported 12-month outcomes led to Food and Drug Administration clearance of the device. Dr. Strollo was first author of that paper (N Engl J Med. 2014 Jan 9;370:139-49). At SLEEP 2016, he presented patient- and partner-reported outcomes at 42 months. Bottom line: The device had continued safety and no loss in efficacy.
“So far it seems to be a useful option for people who frequently didn’t have an option. And the technology is improving and will only get better,” said Dr. Strollo, professor of medicine and clinical and translational science, director of the Sleep Medicine Center, and codirector of the Sleep Medicine Institute at the University of Pittsburgh.
The Inspire system consists of three parts implanted by an otolaryngologist in an outpatient procedure: a small impulse generator, a breathing sensor lead inserted in the intercostal muscle, and a stimulator lead attached to the distal branch of the 10th cranial nerve, the hypoglossal nerve controlling the tongue muscles.
The device is programmed to discharge at the end of expiration and continue through the inspiratory phase, causing the tongue to move forward and the retrolingual and retropalatal airways to open, he explained in an interview.
Upper airway stimulation is approved for commercial use in patients such as those enrolled in the STAR trial on the basis of pilot studies that identified most likely responders. The key selection criteria include moderate to severe obstructive sleep apnea as defined by an apnea-hypopnea index of 20-50, nonadherence to continuous positive airway pressure (CPAP), a body mass index of 32 kg/m2 or less, and absence of concentric collapse of the airway at the level of the palate during sedated endoscopy.
STAR included 126 participants who received the upper airway stimulation device. There have been two explants: one from septic arthritis, the other elective.
A total of 97 STAR participants had 42-month follow-up data available. Among the key findings were that:
• Mean scores on the Epworth Sleepiness Scale decreased from 11.6 at baseline to 7 at 12 months and 7.1 at 42 months.
• Scores on the Functional Outcomes of Sleep Questionnaire improved from 14.3 at baseline to 17.3 at 12 months and 17.5 at 42 months.
• The scores on both the Epworth Sleepiness Scale and Functional Outcomes of Sleep Questionnaire were abnormal at baseline and converted to normal range at both 12 and 42 months of follow-up.
• At baseline, 29% of the patients’ sleeping partners characterized the snoring as loud, 24% rated it ‘very intense,’ and 30% left the bedroom. At 32 months, 11% of partners called the snoring loud, 3% deemed it very intense, and only 4% left the room.
• At 42 months, 81% of patients reported using the device nightly. That’s consistent with the objective evidence of adherence Dr. Strollo and his coinvestigators obtained in a study of postmarketing device implants in which they found device usage averaged about 7 hours per night.
“That’s much better than we see with CPAP in patients who can tolerate that therapy,” Dr. Strollo observed.
The planned 5-year follow-up of STAR participants includes a full laboratory polysomnography study to obtain objective apnea-hypopnea index figures.
The other major development is the launch of a comprehensive registry of patients who receive a post-marketing commercial implant. Roughly 1,000 implants have been done worldwide to date, but now that the device is approved, that number will quickly grow. The registry should prove a rich source for research.
“The goal is to try to refine the selection criteria,” according to Dr. Strollo.
Given that only about 50% of patients with moderate to severe sleep apnea are able to tolerate CPAP long term, where does the Inspire system fit into today’s practice of sleep medicine?
“Upper airway stimulation is another tool, another option for patients,” he said. “In my practice, normally I’d let patients try positive pressure first. I want to make sure they’ve tried CPAP, and they’ve tried more advanced therapy like autotitrating bilevel positive airway pressure, which is more comfortable than CPAP. Bilevel positive airway pressure allows you to salvage a fair number of patients who can’t tolerate CPAP. And I also offer an oral appliance, although the robustness of an oral appliance is not great as apnea becomes more severe.”
The STAR trial is supported by Inspire Medical Systems. Dr. Strollo reported receiving a research grant from the company.
AT SLEEP 2016
Key clinical point: Device therapy for stimulation of the hyperglossal nerve as treatment for obstructive sleep apnea showed continued strong results at 42 months of follow-up.
Major finding: Scores on the Epworth Sleepiness Scale went from 11.6 at baseline to 7.0 at 12 months follow-up following implantation of the Inspire upper airway stimulation device and 7.1 at 42 months.
Data source: This presentation features the prospective 42-month follow-up of 97 participants in the pivotal STAR trial, whose 12-month data earned Food and Drug Administration clearance of the Inspire device.
Disclosures: The study was supported by Inspire Medical Systems. The presenter reported receiving a research grant from the company.
Infant bronchiolitis risk linked to gut flora composition
Infants with gut flora dominated by the genus Bacteroides have more than four times greater odds of developing bronchiolitis than those with microbiota dominated by Enterobacter and Veillonella combined, according to results of a recent study. Two other bacterial profiles, one dominated by Bifidobacterium and another by Escherichia, were not associated with any higher or lower risk of bronchiolitis.
“Our observations, in conjunction with earlier studies, suggest a causal pathway linking the gut microbiota in early infancy to the respiratory tract immune response against viral infection,” wrote Dr. Kohei Hasegawa of Harvard Medical School in Boston, and his associates (Pediatrics. 2016 June 27. doi: 10.1542/peds.2016-0218). “That is, the Bacteroides-dominant gut microbiota in early infancy attenuates the development of immune function in the respiratory tract and thereby leads to an increased susceptibility to bronchiolitis.”
The researchers collected stool samples from 115 healthy infants from a Massachusetts General Hospital primary care group practice, and from 40 infants who were hospitalized with bronchiolitis from November 2013 through April 2014 at one of three children’s hospitals in Wilmington, Del.; Boston; and Louisville, Ky. The groups were age matched, and the infants overall were a median 3 months old. Just over half were male, and just over half were white. Of those with bronchiolitis, 65% had respiratory syncytial virus and 23% had rhinovirus.
Further, “compared with healthy controls, infants with bronchiolitis were [significantly] more likely to have a parental history of asthma, maternal antibiotic use during pregnancy, a history of premature birth, a sibling at home, and corticosteroid use before the enrollment, but they were less likely to be breastfed,” the authors reported.
The researchers used a 16S rRNA gene-sequencing method similar to the one used by the Human Microbiome Project to identify the composition of the fecal samples’ microbiota. Four different bacterial profiles emerged. The most common was an Escherichia-dominant profile, occurring in 30% of the infants overall. Microbiota dominated by Bacteroides followed next, occurring in 28% of infants, while 22% of infants had a Enterobacter/Veillonella–dominant profile, and 21% had a Bifidobacterium-dominant profile. Those with a Bacteroides-dominant profile were older, more likely to be born vaginally, and more likely to be prenatally exposed to maternal smoking.
In infants with bronchiolitis, however, flora dominated by Bacteroides was most common, occurring in 44% of the ill infants. Enterobacter/Veillonella–dominant microbiota occurred in only 15% of the infants with bronchiolitis. Infants’ risk of bronchiolitis was not significantly different among those with Bifidobacterium-dominant or Escherichia-dominant profiles, compared with the Enterobacter/Veillonella–dominant profile.
Patients with Bacteroides-dominant microflora had 4.59 greater odds of severe bronchiolitis than those with Enterobacter/Veillonella–dominant microbiota (P = .008). These odds dropped only to 3.89 after adjustments for age, sex, prematurity, mode of birth, and a history of systemic antibiotic use (P = .03). Similarly, adjusting for age, sex, parental history of asthma, maternal antibiotic use during pregnancy, and systemic corticosteroid use before enrollment resulted in 4.12 greater odds of bronchiolitis in those with a Bacteroides-dominant profile (P = .02).
The research was funded by the National Institutes of Health. Dr. Hasegawa reported having no disclosures. Two authors reported owning shares at the microbiome research company Diversigen, and one had consulted on bronchiolitis for Regeneron. The other authors reported having no disclosures.
This cross-sectional, case-control study raises multiple hypotheses about the relationship between different gut microbiota compositions and the presence of bronchiolitis while also exposing limitations in the study. For instance, polysaccharide A of Bacteroides suppresses T-cell responses to inflammatory stimuli. Inappropriate suppression of “cellular learning” in infancy may alter subsequent mucosal immunity upon infection, resulting in exacerbated inflammatory responses to environmental challenges. Thus, an increased abundance of enteric Bacteroides before a viral challenge may be hypothesized to increase the likelihood of reduced viral immunity and an inappropriate response to an infection.
However, in the study by Hasegawa et al., the gut microbiota was sampled only at the time of hospitalization for infection and once in age-matched controls. Any of the observed microbiota profiles may not reflect earlier states of the microbiota and critical windows of early immune priming. Therefore, prospective longitudinal studies will be essential to determine whether the observed microbiota profiles at the time of bronchiolitis preceded symptoms, were concurrent with the disease onset, or occurred after the disease was well under way. Only through these types of studies, coupled with preclinical mechanistic models of bronchiolitis, can causality be established.
The associations identified by Hasegawa et al., if upheld by the necessary prospective and causal studies, may yield new insights into the failures of antibiotic therapy and suggest alternative approaches to therapeutically modify the microbiota and thus reduce the risk and severity of viral bronchiolitis in infants.
Respiratory tract research has entered a new era. Through a combination of clinical and preclinical models, genomics, immunology, and metabolomics, investigations into the gut-lung axis are expected to drive a paradigm shift in which pulmonary health is viewed through a wider lens of multisystem interactions that includes the microbiota, and through which new preventive strategies, diagnostics and therapeutics may be envisioned for common respiratory diseases.
These comments were condensed from an editorial by Dr. Patrick C. Seed that was published in Pediatrics (doi: 10.1542/peds.2016-1377) alongside the original research. Dr. Seed is supported by the National Institutes of Health, and he reported having no disclosures.
This cross-sectional, case-control study raises multiple hypotheses about the relationship between different gut microbiota compositions and the presence of bronchiolitis while also exposing limitations in the study. For instance, polysaccharide A of Bacteroides suppresses T-cell responses to inflammatory stimuli. Inappropriate suppression of “cellular learning” in infancy may alter subsequent mucosal immunity upon infection, resulting in exacerbated inflammatory responses to environmental challenges. Thus, an increased abundance of enteric Bacteroides before a viral challenge may be hypothesized to increase the likelihood of reduced viral immunity and an inappropriate response to an infection.
However, in the study by Hasegawa et al., the gut microbiota was sampled only at the time of hospitalization for infection and once in age-matched controls. Any of the observed microbiota profiles may not reflect earlier states of the microbiota and critical windows of early immune priming. Therefore, prospective longitudinal studies will be essential to determine whether the observed microbiota profiles at the time of bronchiolitis preceded symptoms, were concurrent with the disease onset, or occurred after the disease was well under way. Only through these types of studies, coupled with preclinical mechanistic models of bronchiolitis, can causality be established.
The associations identified by Hasegawa et al., if upheld by the necessary prospective and causal studies, may yield new insights into the failures of antibiotic therapy and suggest alternative approaches to therapeutically modify the microbiota and thus reduce the risk and severity of viral bronchiolitis in infants.
Respiratory tract research has entered a new era. Through a combination of clinical and preclinical models, genomics, immunology, and metabolomics, investigations into the gut-lung axis are expected to drive a paradigm shift in which pulmonary health is viewed through a wider lens of multisystem interactions that includes the microbiota, and through which new preventive strategies, diagnostics and therapeutics may be envisioned for common respiratory diseases.
These comments were condensed from an editorial by Dr. Patrick C. Seed that was published in Pediatrics (doi: 10.1542/peds.2016-1377) alongside the original research. Dr. Seed is supported by the National Institutes of Health, and he reported having no disclosures.
This cross-sectional, case-control study raises multiple hypotheses about the relationship between different gut microbiota compositions and the presence of bronchiolitis while also exposing limitations in the study. For instance, polysaccharide A of Bacteroides suppresses T-cell responses to inflammatory stimuli. Inappropriate suppression of “cellular learning” in infancy may alter subsequent mucosal immunity upon infection, resulting in exacerbated inflammatory responses to environmental challenges. Thus, an increased abundance of enteric Bacteroides before a viral challenge may be hypothesized to increase the likelihood of reduced viral immunity and an inappropriate response to an infection.
However, in the study by Hasegawa et al., the gut microbiota was sampled only at the time of hospitalization for infection and once in age-matched controls. Any of the observed microbiota profiles may not reflect earlier states of the microbiota and critical windows of early immune priming. Therefore, prospective longitudinal studies will be essential to determine whether the observed microbiota profiles at the time of bronchiolitis preceded symptoms, were concurrent with the disease onset, or occurred after the disease was well under way. Only through these types of studies, coupled with preclinical mechanistic models of bronchiolitis, can causality be established.
The associations identified by Hasegawa et al., if upheld by the necessary prospective and causal studies, may yield new insights into the failures of antibiotic therapy and suggest alternative approaches to therapeutically modify the microbiota and thus reduce the risk and severity of viral bronchiolitis in infants.
Respiratory tract research has entered a new era. Through a combination of clinical and preclinical models, genomics, immunology, and metabolomics, investigations into the gut-lung axis are expected to drive a paradigm shift in which pulmonary health is viewed through a wider lens of multisystem interactions that includes the microbiota, and through which new preventive strategies, diagnostics and therapeutics may be envisioned for common respiratory diseases.
These comments were condensed from an editorial by Dr. Patrick C. Seed that was published in Pediatrics (doi: 10.1542/peds.2016-1377) alongside the original research. Dr. Seed is supported by the National Institutes of Health, and he reported having no disclosures.
Infants with gut flora dominated by the genus Bacteroides have more than four times greater odds of developing bronchiolitis than those with microbiota dominated by Enterobacter and Veillonella combined, according to results of a recent study. Two other bacterial profiles, one dominated by Bifidobacterium and another by Escherichia, were not associated with any higher or lower risk of bronchiolitis.
“Our observations, in conjunction with earlier studies, suggest a causal pathway linking the gut microbiota in early infancy to the respiratory tract immune response against viral infection,” wrote Dr. Kohei Hasegawa of Harvard Medical School in Boston, and his associates (Pediatrics. 2016 June 27. doi: 10.1542/peds.2016-0218). “That is, the Bacteroides-dominant gut microbiota in early infancy attenuates the development of immune function in the respiratory tract and thereby leads to an increased susceptibility to bronchiolitis.”
The researchers collected stool samples from 115 healthy infants from a Massachusetts General Hospital primary care group practice, and from 40 infants who were hospitalized with bronchiolitis from November 2013 through April 2014 at one of three children’s hospitals in Wilmington, Del.; Boston; and Louisville, Ky. The groups were age matched, and the infants overall were a median 3 months old. Just over half were male, and just over half were white. Of those with bronchiolitis, 65% had respiratory syncytial virus and 23% had rhinovirus.
Further, “compared with healthy controls, infants with bronchiolitis were [significantly] more likely to have a parental history of asthma, maternal antibiotic use during pregnancy, a history of premature birth, a sibling at home, and corticosteroid use before the enrollment, but they were less likely to be breastfed,” the authors reported.
The researchers used a 16S rRNA gene-sequencing method similar to the one used by the Human Microbiome Project to identify the composition of the fecal samples’ microbiota. Four different bacterial profiles emerged. The most common was an Escherichia-dominant profile, occurring in 30% of the infants overall. Microbiota dominated by Bacteroides followed next, occurring in 28% of infants, while 22% of infants had a Enterobacter/Veillonella–dominant profile, and 21% had a Bifidobacterium-dominant profile. Those with a Bacteroides-dominant profile were older, more likely to be born vaginally, and more likely to be prenatally exposed to maternal smoking.
In infants with bronchiolitis, however, flora dominated by Bacteroides was most common, occurring in 44% of the ill infants. Enterobacter/Veillonella–dominant microbiota occurred in only 15% of the infants with bronchiolitis. Infants’ risk of bronchiolitis was not significantly different among those with Bifidobacterium-dominant or Escherichia-dominant profiles, compared with the Enterobacter/Veillonella–dominant profile.
Patients with Bacteroides-dominant microflora had 4.59 greater odds of severe bronchiolitis than those with Enterobacter/Veillonella–dominant microbiota (P = .008). These odds dropped only to 3.89 after adjustments for age, sex, prematurity, mode of birth, and a history of systemic antibiotic use (P = .03). Similarly, adjusting for age, sex, parental history of asthma, maternal antibiotic use during pregnancy, and systemic corticosteroid use before enrollment resulted in 4.12 greater odds of bronchiolitis in those with a Bacteroides-dominant profile (P = .02).
The research was funded by the National Institutes of Health. Dr. Hasegawa reported having no disclosures. Two authors reported owning shares at the microbiome research company Diversigen, and one had consulted on bronchiolitis for Regeneron. The other authors reported having no disclosures.
Infants with gut flora dominated by the genus Bacteroides have more than four times greater odds of developing bronchiolitis than those with microbiota dominated by Enterobacter and Veillonella combined, according to results of a recent study. Two other bacterial profiles, one dominated by Bifidobacterium and another by Escherichia, were not associated with any higher or lower risk of bronchiolitis.
“Our observations, in conjunction with earlier studies, suggest a causal pathway linking the gut microbiota in early infancy to the respiratory tract immune response against viral infection,” wrote Dr. Kohei Hasegawa of Harvard Medical School in Boston, and his associates (Pediatrics. 2016 June 27. doi: 10.1542/peds.2016-0218). “That is, the Bacteroides-dominant gut microbiota in early infancy attenuates the development of immune function in the respiratory tract and thereby leads to an increased susceptibility to bronchiolitis.”
The researchers collected stool samples from 115 healthy infants from a Massachusetts General Hospital primary care group practice, and from 40 infants who were hospitalized with bronchiolitis from November 2013 through April 2014 at one of three children’s hospitals in Wilmington, Del.; Boston; and Louisville, Ky. The groups were age matched, and the infants overall were a median 3 months old. Just over half were male, and just over half were white. Of those with bronchiolitis, 65% had respiratory syncytial virus and 23% had rhinovirus.
Further, “compared with healthy controls, infants with bronchiolitis were [significantly] more likely to have a parental history of asthma, maternal antibiotic use during pregnancy, a history of premature birth, a sibling at home, and corticosteroid use before the enrollment, but they were less likely to be breastfed,” the authors reported.
The researchers used a 16S rRNA gene-sequencing method similar to the one used by the Human Microbiome Project to identify the composition of the fecal samples’ microbiota. Four different bacterial profiles emerged. The most common was an Escherichia-dominant profile, occurring in 30% of the infants overall. Microbiota dominated by Bacteroides followed next, occurring in 28% of infants, while 22% of infants had a Enterobacter/Veillonella–dominant profile, and 21% had a Bifidobacterium-dominant profile. Those with a Bacteroides-dominant profile were older, more likely to be born vaginally, and more likely to be prenatally exposed to maternal smoking.
In infants with bronchiolitis, however, flora dominated by Bacteroides was most common, occurring in 44% of the ill infants. Enterobacter/Veillonella–dominant microbiota occurred in only 15% of the infants with bronchiolitis. Infants’ risk of bronchiolitis was not significantly different among those with Bifidobacterium-dominant or Escherichia-dominant profiles, compared with the Enterobacter/Veillonella–dominant profile.
Patients with Bacteroides-dominant microflora had 4.59 greater odds of severe bronchiolitis than those with Enterobacter/Veillonella–dominant microbiota (P = .008). These odds dropped only to 3.89 after adjustments for age, sex, prematurity, mode of birth, and a history of systemic antibiotic use (P = .03). Similarly, adjusting for age, sex, parental history of asthma, maternal antibiotic use during pregnancy, and systemic corticosteroid use before enrollment resulted in 4.12 greater odds of bronchiolitis in those with a Bacteroides-dominant profile (P = .02).
The research was funded by the National Institutes of Health. Dr. Hasegawa reported having no disclosures. Two authors reported owning shares at the microbiome research company Diversigen, and one had consulted on bronchiolitis for Regeneron. The other authors reported having no disclosures.
PEDIATRICS
Key clinical point: Infants with Bacteroides-dominant microbiota have the highest risk of bronchiolitis.
Major finding: 44% of infants with bronchiolitis had Bacteroides-dominant fecal microbiota, compared with 15% with Enterobacter/Veillonella–dominant microbiota.
Data source: The findings are based on a case-control study involving 155 infants from three children’s hospitals in Delaware, Massachusetts, and Kentucky, of whom 40 had bronchiolitis between November 2013 and April 2014.
Disclosures: The research was funded by the National Institutes of Health. Dr. Hasegawa reported having no disclosures. Two authors reported owning shares at the microbiome research company Diversigen, and one had consulted on bronchiolitis for Regeneron. The other authors reported having no disclosures.
Peds, FPs flunk screening children for obstructive sleep apnea
DENVER – A high degree of unwarranted practice variation exists among pediatricians and family physicians with regard to screening children for obstructive sleep apnea in accord with current evidence-based practice guidelines, Sarah M. Honaker, Ph.D., said at the annual meeting of the Associated Professional Sleep Societies.
The American Academy of Pediatrics and American Academy of Sleep Medicine recommend that children with frequent snoring be referred for a sleep study or to a sleep specialist or otolaryngologist because of the damaging consequences of living with untreated obstructive sleep apnea (OSA). But Dr. Honaker’s study of 8,135 children aged 1-12 years seen at five university-affiliated urban primary care pediatric clinics demonstrated that the identification rate of suspected OSA was abysmally low, and physician practice on this score varied enormously.
“There is a critical need to develop enhanced implementation strategies to support primary care providers’ adherence to evidence-based practice for pediatric OSA,” declared Dr. Honaker of Indiana University in Indianapolis.
She and her coinvestigators have developed a computer decision support system designed to do just that. Known as CHICA, for Child Health Improvement through Computer Automation, the system works like this: While in the clinic waiting room, the parent is handed a computer tablet and asked to complete 20 yes/no items designed to identify priority areas for the primary care provider to address during the current visit. The items are individually tailored based upon the child’s age, past medical history, and previous responses.
One item asks if the child snores three or more nights per week, a well-established indicator of increased risk for OSA. If the answer is yes, CHICA instantly sends a prompt to the child’s electronic medical record noting that the parent reports the child is a frequent snorer and this might indicate OSA.
The primary care provider can either ignore the prompt or respond during or after the visit in one of three ways: “I am concerned about OSA,” “I do not suspect OSA,” or “The parent in the examining room denies hearing frequent snoring.”
The mean age of the patients in Dr. Honaker’s study was 5.8 years, and 83% of the 8,135 children had Medicaid coverage. Parental cooperation with CHICA was high: 98.5% of parents addressed the snoring question. They reported that 28.5% of the children snored at least 3 nights per week, generating a total of 1,094 CHICA prompts to the primary care providers. Forty-four percent of providers didn’t respond to the prompt, which Dr. Honaker said is a typical rate for this sort of computerized assist intervention. Of those who did respond, only 15.9% indicated they suspected OSA. Sixty-three percent declared they didn’t suspect OSA, and the remainder said the parent in the examining room didn’t report frequent snoring.
Although responding providers indicated they suspected OSA in 15.9% of the children, that’s a low figure for frequent snorers. Moreover, 31% of children who got the CHICA frequent snoring prompt were overweight or obese, and 17% had attention-deficit hyperactivity disorder symptoms, both known risk factors for OSA. Some of the kids had both risk factors, but 39% had at least one in addition to their frequent snoring, Dr. Honaker noted.
The investigators carried out multivariate logistic regression analyses of child, provider, and clinic characteristics in search of predictors associated with physician concern that a child might have OSA. It turned out that none of the provider characteristics had any bearing on this endpoint. It didn’t matter if a physician’s specialty was pediatrics or family medicine. Providers had been in practice for 3-42 years, with a mean of 15.1 years, but years in practice weren’t associated with a physician’s rate of identification of possible pediatric OSA. Individual provider rates varied enormously, though. Some physicians never suspected OSA, others did so in nearly 50% of children flagged by the CHICA prompt.
“It’s not clear that type of training plays a large role in this area,” she commented.
The only relevant patient factor was age: children aged 1-2.5 years were 73% less likely to generate physician suspicion of OSA.
“Surprisingly, none of the patient health factors were predictive. So having ADHD symptoms or being overweight or obese did not make it more likely that a child would elicit concern for OSA,” Dr. Honaker observed.
However, which of the five clinics the child attended turned out to make a big difference. Rates of suspected OSA in children with a CHICA snoring prompt ranged from a low of 5% at one clinic to 27% at another.
Dr. Honaker’s recent review of the literature led her to conclude that the Indiana University experience is hardly unique. Despite documented high rates of pediatric sleep disorders in primary care settings, screening and treatment rates are low. Primary care physicians receive little training in sleep medicine (Sleep Med Rev. 2016;25:31-9).
What’s next for CHICA?
Dr. Honaker and coworkers have developed a beefed up CHICA decision support system known as the CHICA OSA Module. In addition to generating a prompt in the medical record if a parent indicates the child snores three or more nights per week, additional OSA signs and symptoms, if present, will be noted on the screen, along with a comment that American Academy of Pediatrics guidelines recommend referral when OSA is suspected. Dr. Honaker plans to conduct a controlled trial in which some clinics get the CHICA OSA Module while others use the older CHICA system.
Her study was funded by the American Sleep Medicine Foundation. She reported having no financial conflicts.
DENVER – A high degree of unwarranted practice variation exists among pediatricians and family physicians with regard to screening children for obstructive sleep apnea in accord with current evidence-based practice guidelines, Sarah M. Honaker, Ph.D., said at the annual meeting of the Associated Professional Sleep Societies.
The American Academy of Pediatrics and American Academy of Sleep Medicine recommend that children with frequent snoring be referred for a sleep study or to a sleep specialist or otolaryngologist because of the damaging consequences of living with untreated obstructive sleep apnea (OSA). But Dr. Honaker’s study of 8,135 children aged 1-12 years seen at five university-affiliated urban primary care pediatric clinics demonstrated that the identification rate of suspected OSA was abysmally low, and physician practice on this score varied enormously.
“There is a critical need to develop enhanced implementation strategies to support primary care providers’ adherence to evidence-based practice for pediatric OSA,” declared Dr. Honaker of Indiana University in Indianapolis.
She and her coinvestigators have developed a computer decision support system designed to do just that. Known as CHICA, for Child Health Improvement through Computer Automation, the system works like this: While in the clinic waiting room, the parent is handed a computer tablet and asked to complete 20 yes/no items designed to identify priority areas for the primary care provider to address during the current visit. The items are individually tailored based upon the child’s age, past medical history, and previous responses.
One item asks if the child snores three or more nights per week, a well-established indicator of increased risk for OSA. If the answer is yes, CHICA instantly sends a prompt to the child’s electronic medical record noting that the parent reports the child is a frequent snorer and this might indicate OSA.
The primary care provider can either ignore the prompt or respond during or after the visit in one of three ways: “I am concerned about OSA,” “I do not suspect OSA,” or “The parent in the examining room denies hearing frequent snoring.”
The mean age of the patients in Dr. Honaker’s study was 5.8 years, and 83% of the 8,135 children had Medicaid coverage. Parental cooperation with CHICA was high: 98.5% of parents addressed the snoring question. They reported that 28.5% of the children snored at least 3 nights per week, generating a total of 1,094 CHICA prompts to the primary care providers. Forty-four percent of providers didn’t respond to the prompt, which Dr. Honaker said is a typical rate for this sort of computerized assist intervention. Of those who did respond, only 15.9% indicated they suspected OSA. Sixty-three percent declared they didn’t suspect OSA, and the remainder said the parent in the examining room didn’t report frequent snoring.
Although responding providers indicated they suspected OSA in 15.9% of the children, that’s a low figure for frequent snorers. Moreover, 31% of children who got the CHICA frequent snoring prompt were overweight or obese, and 17% had attention-deficit hyperactivity disorder symptoms, both known risk factors for OSA. Some of the kids had both risk factors, but 39% had at least one in addition to their frequent snoring, Dr. Honaker noted.
The investigators carried out multivariate logistic regression analyses of child, provider, and clinic characteristics in search of predictors associated with physician concern that a child might have OSA. It turned out that none of the provider characteristics had any bearing on this endpoint. It didn’t matter if a physician’s specialty was pediatrics or family medicine. Providers had been in practice for 3-42 years, with a mean of 15.1 years, but years in practice weren’t associated with a physician’s rate of identification of possible pediatric OSA. Individual provider rates varied enormously, though. Some physicians never suspected OSA, others did so in nearly 50% of children flagged by the CHICA prompt.
“It’s not clear that type of training plays a large role in this area,” she commented.
The only relevant patient factor was age: children aged 1-2.5 years were 73% less likely to generate physician suspicion of OSA.
“Surprisingly, none of the patient health factors were predictive. So having ADHD symptoms or being overweight or obese did not make it more likely that a child would elicit concern for OSA,” Dr. Honaker observed.
However, which of the five clinics the child attended turned out to make a big difference. Rates of suspected OSA in children with a CHICA snoring prompt ranged from a low of 5% at one clinic to 27% at another.
Dr. Honaker’s recent review of the literature led her to conclude that the Indiana University experience is hardly unique. Despite documented high rates of pediatric sleep disorders in primary care settings, screening and treatment rates are low. Primary care physicians receive little training in sleep medicine (Sleep Med Rev. 2016;25:31-9).
What’s next for CHICA?
Dr. Honaker and coworkers have developed a beefed up CHICA decision support system known as the CHICA OSA Module. In addition to generating a prompt in the medical record if a parent indicates the child snores three or more nights per week, additional OSA signs and symptoms, if present, will be noted on the screen, along with a comment that American Academy of Pediatrics guidelines recommend referral when OSA is suspected. Dr. Honaker plans to conduct a controlled trial in which some clinics get the CHICA OSA Module while others use the older CHICA system.
Her study was funded by the American Sleep Medicine Foundation. She reported having no financial conflicts.
DENVER – A high degree of unwarranted practice variation exists among pediatricians and family physicians with regard to screening children for obstructive sleep apnea in accord with current evidence-based practice guidelines, Sarah M. Honaker, Ph.D., said at the annual meeting of the Associated Professional Sleep Societies.
The American Academy of Pediatrics and American Academy of Sleep Medicine recommend that children with frequent snoring be referred for a sleep study or to a sleep specialist or otolaryngologist because of the damaging consequences of living with untreated obstructive sleep apnea (OSA). But Dr. Honaker’s study of 8,135 children aged 1-12 years seen at five university-affiliated urban primary care pediatric clinics demonstrated that the identification rate of suspected OSA was abysmally low, and physician practice on this score varied enormously.
“There is a critical need to develop enhanced implementation strategies to support primary care providers’ adherence to evidence-based practice for pediatric OSA,” declared Dr. Honaker of Indiana University in Indianapolis.
She and her coinvestigators have developed a computer decision support system designed to do just that. Known as CHICA, for Child Health Improvement through Computer Automation, the system works like this: While in the clinic waiting room, the parent is handed a computer tablet and asked to complete 20 yes/no items designed to identify priority areas for the primary care provider to address during the current visit. The items are individually tailored based upon the child’s age, past medical history, and previous responses.
One item asks if the child snores three or more nights per week, a well-established indicator of increased risk for OSA. If the answer is yes, CHICA instantly sends a prompt to the child’s electronic medical record noting that the parent reports the child is a frequent snorer and this might indicate OSA.
The primary care provider can either ignore the prompt or respond during or after the visit in one of three ways: “I am concerned about OSA,” “I do not suspect OSA,” or “The parent in the examining room denies hearing frequent snoring.”
The mean age of the patients in Dr. Honaker’s study was 5.8 years, and 83% of the 8,135 children had Medicaid coverage. Parental cooperation with CHICA was high: 98.5% of parents addressed the snoring question. They reported that 28.5% of the children snored at least 3 nights per week, generating a total of 1,094 CHICA prompts to the primary care providers. Forty-four percent of providers didn’t respond to the prompt, which Dr. Honaker said is a typical rate for this sort of computerized assist intervention. Of those who did respond, only 15.9% indicated they suspected OSA. Sixty-three percent declared they didn’t suspect OSA, and the remainder said the parent in the examining room didn’t report frequent snoring.
Although responding providers indicated they suspected OSA in 15.9% of the children, that’s a low figure for frequent snorers. Moreover, 31% of children who got the CHICA frequent snoring prompt were overweight or obese, and 17% had attention-deficit hyperactivity disorder symptoms, both known risk factors for OSA. Some of the kids had both risk factors, but 39% had at least one in addition to their frequent snoring, Dr. Honaker noted.
The investigators carried out multivariate logistic regression analyses of child, provider, and clinic characteristics in search of predictors associated with physician concern that a child might have OSA. It turned out that none of the provider characteristics had any bearing on this endpoint. It didn’t matter if a physician’s specialty was pediatrics or family medicine. Providers had been in practice for 3-42 years, with a mean of 15.1 years, but years in practice weren’t associated with a physician’s rate of identification of possible pediatric OSA. Individual provider rates varied enormously, though. Some physicians never suspected OSA, others did so in nearly 50% of children flagged by the CHICA prompt.
“It’s not clear that type of training plays a large role in this area,” she commented.
The only relevant patient factor was age: children aged 1-2.5 years were 73% less likely to generate physician suspicion of OSA.
“Surprisingly, none of the patient health factors were predictive. So having ADHD symptoms or being overweight or obese did not make it more likely that a child would elicit concern for OSA,” Dr. Honaker observed.
However, which of the five clinics the child attended turned out to make a big difference. Rates of suspected OSA in children with a CHICA snoring prompt ranged from a low of 5% at one clinic to 27% at another.
Dr. Honaker’s recent review of the literature led her to conclude that the Indiana University experience is hardly unique. Despite documented high rates of pediatric sleep disorders in primary care settings, screening and treatment rates are low. Primary care physicians receive little training in sleep medicine (Sleep Med Rev. 2016;25:31-9).
What’s next for CHICA?
Dr. Honaker and coworkers have developed a beefed up CHICA decision support system known as the CHICA OSA Module. In addition to generating a prompt in the medical record if a parent indicates the child snores three or more nights per week, additional OSA signs and symptoms, if present, will be noted on the screen, along with a comment that American Academy of Pediatrics guidelines recommend referral when OSA is suspected. Dr. Honaker plans to conduct a controlled trial in which some clinics get the CHICA OSA Module while others use the older CHICA system.
Her study was funded by the American Sleep Medicine Foundation. She reported having no financial conflicts.
AT SLEEP 2016
Key clinical point: Huge unwarranted practice variations exist in primary care physicians’ adherence to evidence-based guidelines for identification of obstructive sleep apnea in children.
Major finding: Although 39% of a large group of children reportedly snored at least three nights per week and had at least one additional major risk factor for obstructive sleep apnea, only 15.9% of the children elicited suspicion of the disorder on the part of primary care providers despite an electronic prompt.
Data source: This was an observational study in which 8,135 children aged 1-12 years were screened for obstructive sleep apnea using a computer-assisted decision support system known as CHICA, linked to the patients’ electronic medical record.
Disclosures: The study was funded by the American Sleep Medicine Foundation. The presenter reported having no financial conflicts.
ACIP votes to scrap LAIV vaccine for 2016-2017 influenza season
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices has voted to scrap the use of the live attenuated influenza vaccine for the 2016-2017 flu season.
ACIP’s interim recommendation guidance to the CDC is that “no live attenuated influenza vaccines (LAIV) should be used in any setting,” after reviewing data showing that for three consecutive influenza seasons, LAIV’s vaccine effectiveness (VE) against any flu virus was 3% (95% confidence interval, -49%-37%) in children aged 2-17 years. Meanwhile, injectable inactivated influenza vaccine (IIV) had a VE estimate of 63% (95% CI, 52%-72%) against any flu virus in that age group. Findings were similar across age groups.
CDC epidemiologist Brendan Flannery, Ph.D., painted a damning picture of the intranasal LAIV, presenting preliminary data from the U.S. Flu Vaccine Effectiveness Network for 2015-2016 that showed quadrivalent LAIV offered children “no significant protection against influenza A(H1N1)pdm09.” He also presented 2015-2016 national cohort data from Finland showing that unadjusted vaccine effectiveness (VE) against the H1N1 strain for LAIV in 2-year-olds was 47%, compared with 78% for IIV. Dr. Flannery also cited a series of other U.S. and international studies that did not support the use of LAIV, including unpublished Department of Defense 2015-2016 influenza season data for military families indicating that VE for LAIV against the strain last season was “insignificant” in children aged 2-17 years.
Data from an industry study of the quadrivalent LAIV FluMist (MedImmune) also indicated that in this cohort, for the 2015-2016 influenza season, LAIV underperformed compared with IIV, 46% vs. 65%.
Still more bad news for LAIV came from an English study published online just as the ACIP meeting was getting underway. Those data showed that from October 2015 through May 2016, LAIV in children aged 2-17 years had a VE of 57.6% against influenza A and B.
Debate over whether to pull support for LAIV entirely or to allow the use of it in certain circumstances – such as when a person declined a flu shot – hinged on the projected upheaval pulling LAIV is likely to cause.
Concerns were raised around what would happen in the case of LAIV vaccine orders already placed, over whether pulling LAIV would mean a shortage of other vaccine alternatives, and how communicating contingencies might only confuse the public, but several committee members spoke forcefully in favor of what they said the data compelled them to do.
“The science simply shows that LAIV has not worked for the past 3 years, whereas IIV has,” American Academy of Pediatrics Infectious Disease Committee’s Red Book editor, and AAP liaison to ACIP, Dr. David Kimberlin said before the vote.
Restricting but not prohibiting LAIV also would have allowed for an easier transition back to LAIV if the vaccine were successfully reformulated, ACIP Influenza Work Group Chair Dr. Ruth Karron said while presenting potential policy recommendations.
During the debate over whether to pull or limit support for the inhaled vaccine, Dr. Karron reminded the committee that at the time the Food and Drug Administration approved LAIV, serum antibody responses to both the tri- and quadrivalent vaccine were modest, prompting the FDA to call for effectiveness studies. “So, my question to the FDA now is, ‘What are their plans?’ ”
“We’ve seen the studies, but we’ve not really reviewed all the data,” Dr. Wellington Sun, the FDA’s ACIP liaison responded. “I think we have to acknowledge that LAIV has offered advantages over IIV in the past. At this point, we’re not ready to undertake a program for changing the prescriber’s information. We want to continue to work with MedImmune to find out the root cause of this phenomenon.”
“We’ve increased our research into understanding the biology of the H1N1pdm09 LAIV strains so we can improve their effectiveness in future seasons,” Dr. Chris Ambrose, vice president of U.S. medical affairs for infectious disease at MedImmune’s parent company AstraZeneca, told the committee.
“I think this is a very sad day for the influenza vaccination program,” ACIP chair Dr. Nancy Bennett said before adjourning the meeting. “We all had great hopes. It’s not over. If you’ve seen 1 influenza year, you’ve seen 1 influenza year. We may be back here next year having a very different discussion.”
The CDC is not obligated to adopt ACIP recommendations, but typically does. The committee also accepted a resolution to update the Vaccines for Children program in accordance with the recommendation to deny support for LAIV this flu season.
Thirteen members of the committee voted in favor of the recommendation. Dr. Edward Belongia abstained citing a conflict of interest, and Ms. Cynthia Pelligrini voted no, citing “insufficient time to consider this information.” The ICICLE trial NCT01997450, was sponsored by MedImmune, a subsidiary of AstraZeneca where Dr. Ambrose is vice president of U.S. medical affairs for infectious disease.
On Twitter @whitneymcknight
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices has voted to scrap the use of the live attenuated influenza vaccine for the 2016-2017 flu season.
ACIP’s interim recommendation guidance to the CDC is that “no live attenuated influenza vaccines (LAIV) should be used in any setting,” after reviewing data showing that for three consecutive influenza seasons, LAIV’s vaccine effectiveness (VE) against any flu virus was 3% (95% confidence interval, -49%-37%) in children aged 2-17 years. Meanwhile, injectable inactivated influenza vaccine (IIV) had a VE estimate of 63% (95% CI, 52%-72%) against any flu virus in that age group. Findings were similar across age groups.
CDC epidemiologist Brendan Flannery, Ph.D., painted a damning picture of the intranasal LAIV, presenting preliminary data from the U.S. Flu Vaccine Effectiveness Network for 2015-2016 that showed quadrivalent LAIV offered children “no significant protection against influenza A(H1N1)pdm09.” He also presented 2015-2016 national cohort data from Finland showing that unadjusted vaccine effectiveness (VE) against the H1N1 strain for LAIV in 2-year-olds was 47%, compared with 78% for IIV. Dr. Flannery also cited a series of other U.S. and international studies that did not support the use of LAIV, including unpublished Department of Defense 2015-2016 influenza season data for military families indicating that VE for LAIV against the strain last season was “insignificant” in children aged 2-17 years.
Data from an industry study of the quadrivalent LAIV FluMist (MedImmune) also indicated that in this cohort, for the 2015-2016 influenza season, LAIV underperformed compared with IIV, 46% vs. 65%.
Still more bad news for LAIV came from an English study published online just as the ACIP meeting was getting underway. Those data showed that from October 2015 through May 2016, LAIV in children aged 2-17 years had a VE of 57.6% against influenza A and B.
Debate over whether to pull support for LAIV entirely or to allow the use of it in certain circumstances – such as when a person declined a flu shot – hinged on the projected upheaval pulling LAIV is likely to cause.
Concerns were raised around what would happen in the case of LAIV vaccine orders already placed, over whether pulling LAIV would mean a shortage of other vaccine alternatives, and how communicating contingencies might only confuse the public, but several committee members spoke forcefully in favor of what they said the data compelled them to do.
“The science simply shows that LAIV has not worked for the past 3 years, whereas IIV has,” American Academy of Pediatrics Infectious Disease Committee’s Red Book editor, and AAP liaison to ACIP, Dr. David Kimberlin said before the vote.
Restricting but not prohibiting LAIV also would have allowed for an easier transition back to LAIV if the vaccine were successfully reformulated, ACIP Influenza Work Group Chair Dr. Ruth Karron said while presenting potential policy recommendations.
During the debate over whether to pull or limit support for the inhaled vaccine, Dr. Karron reminded the committee that at the time the Food and Drug Administration approved LAIV, serum antibody responses to both the tri- and quadrivalent vaccine were modest, prompting the FDA to call for effectiveness studies. “So, my question to the FDA now is, ‘What are their plans?’ ”
“We’ve seen the studies, but we’ve not really reviewed all the data,” Dr. Wellington Sun, the FDA’s ACIP liaison responded. “I think we have to acknowledge that LAIV has offered advantages over IIV in the past. At this point, we’re not ready to undertake a program for changing the prescriber’s information. We want to continue to work with MedImmune to find out the root cause of this phenomenon.”
“We’ve increased our research into understanding the biology of the H1N1pdm09 LAIV strains so we can improve their effectiveness in future seasons,” Dr. Chris Ambrose, vice president of U.S. medical affairs for infectious disease at MedImmune’s parent company AstraZeneca, told the committee.
“I think this is a very sad day for the influenza vaccination program,” ACIP chair Dr. Nancy Bennett said before adjourning the meeting. “We all had great hopes. It’s not over. If you’ve seen 1 influenza year, you’ve seen 1 influenza year. We may be back here next year having a very different discussion.”
The CDC is not obligated to adopt ACIP recommendations, but typically does. The committee also accepted a resolution to update the Vaccines for Children program in accordance with the recommendation to deny support for LAIV this flu season.
Thirteen members of the committee voted in favor of the recommendation. Dr. Edward Belongia abstained citing a conflict of interest, and Ms. Cynthia Pelligrini voted no, citing “insufficient time to consider this information.” The ICICLE trial NCT01997450, was sponsored by MedImmune, a subsidiary of AstraZeneca where Dr. Ambrose is vice president of U.S. medical affairs for infectious disease.
On Twitter @whitneymcknight
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices has voted to scrap the use of the live attenuated influenza vaccine for the 2016-2017 flu season.
ACIP’s interim recommendation guidance to the CDC is that “no live attenuated influenza vaccines (LAIV) should be used in any setting,” after reviewing data showing that for three consecutive influenza seasons, LAIV’s vaccine effectiveness (VE) against any flu virus was 3% (95% confidence interval, -49%-37%) in children aged 2-17 years. Meanwhile, injectable inactivated influenza vaccine (IIV) had a VE estimate of 63% (95% CI, 52%-72%) against any flu virus in that age group. Findings were similar across age groups.
CDC epidemiologist Brendan Flannery, Ph.D., painted a damning picture of the intranasal LAIV, presenting preliminary data from the U.S. Flu Vaccine Effectiveness Network for 2015-2016 that showed quadrivalent LAIV offered children “no significant protection against influenza A(H1N1)pdm09.” He also presented 2015-2016 national cohort data from Finland showing that unadjusted vaccine effectiveness (VE) against the H1N1 strain for LAIV in 2-year-olds was 47%, compared with 78% for IIV. Dr. Flannery also cited a series of other U.S. and international studies that did not support the use of LAIV, including unpublished Department of Defense 2015-2016 influenza season data for military families indicating that VE for LAIV against the strain last season was “insignificant” in children aged 2-17 years.
Data from an industry study of the quadrivalent LAIV FluMist (MedImmune) also indicated that in this cohort, for the 2015-2016 influenza season, LAIV underperformed compared with IIV, 46% vs. 65%.
Still more bad news for LAIV came from an English study published online just as the ACIP meeting was getting underway. Those data showed that from October 2015 through May 2016, LAIV in children aged 2-17 years had a VE of 57.6% against influenza A and B.
Debate over whether to pull support for LAIV entirely or to allow the use of it in certain circumstances – such as when a person declined a flu shot – hinged on the projected upheaval pulling LAIV is likely to cause.
Concerns were raised around what would happen in the case of LAIV vaccine orders already placed, over whether pulling LAIV would mean a shortage of other vaccine alternatives, and how communicating contingencies might only confuse the public, but several committee members spoke forcefully in favor of what they said the data compelled them to do.
“The science simply shows that LAIV has not worked for the past 3 years, whereas IIV has,” American Academy of Pediatrics Infectious Disease Committee’s Red Book editor, and AAP liaison to ACIP, Dr. David Kimberlin said before the vote.
Restricting but not prohibiting LAIV also would have allowed for an easier transition back to LAIV if the vaccine were successfully reformulated, ACIP Influenza Work Group Chair Dr. Ruth Karron said while presenting potential policy recommendations.
During the debate over whether to pull or limit support for the inhaled vaccine, Dr. Karron reminded the committee that at the time the Food and Drug Administration approved LAIV, serum antibody responses to both the tri- and quadrivalent vaccine were modest, prompting the FDA to call for effectiveness studies. “So, my question to the FDA now is, ‘What are their plans?’ ”
“We’ve seen the studies, but we’ve not really reviewed all the data,” Dr. Wellington Sun, the FDA’s ACIP liaison responded. “I think we have to acknowledge that LAIV has offered advantages over IIV in the past. At this point, we’re not ready to undertake a program for changing the prescriber’s information. We want to continue to work with MedImmune to find out the root cause of this phenomenon.”
“We’ve increased our research into understanding the biology of the H1N1pdm09 LAIV strains so we can improve their effectiveness in future seasons,” Dr. Chris Ambrose, vice president of U.S. medical affairs for infectious disease at MedImmune’s parent company AstraZeneca, told the committee.
“I think this is a very sad day for the influenza vaccination program,” ACIP chair Dr. Nancy Bennett said before adjourning the meeting. “We all had great hopes. It’s not over. If you’ve seen 1 influenza year, you’ve seen 1 influenza year. We may be back here next year having a very different discussion.”
The CDC is not obligated to adopt ACIP recommendations, but typically does. The committee also accepted a resolution to update the Vaccines for Children program in accordance with the recommendation to deny support for LAIV this flu season.
Thirteen members of the committee voted in favor of the recommendation. Dr. Edward Belongia abstained citing a conflict of interest, and Ms. Cynthia Pelligrini voted no, citing “insufficient time to consider this information.” The ICICLE trial NCT01997450, was sponsored by MedImmune, a subsidiary of AstraZeneca where Dr. Ambrose is vice president of U.S. medical affairs for infectious disease.
On Twitter @whitneymcknight
FROM AN ACIP MEETING
The promise of peanut allergy prevention lies in draft guidelines
Updated guidelines from the National Institute of Allergy and Infectious Diseases for the early introduction of peanut-containing foods to children at increased risk for peanut allergies are on the horizon, pending final approval.
“Two studies recently showed that infants at high risk of developing peanut allergy [infants with egg allergy and or severe eczema] were much less likely to have peanut allergy at age 5 years if they were able to incorporate peanut regularly into the diet between 4 and 11 months of age,” said Dr. Scott H. Sicherer, the Elliot and Roslyn Jaffe Professor of Pediatrics, Allergy and Immunology, and chief of the division of allergy and immunology in the department of pediatrics at the Icahn School of Medicine at Mount Sinai, New York.
“However, adding peanut to the diet at this age requires caution because these infants may already be allergic to peanut, and so allergy testing and care in adding peanut to the diet with medical supervision is needed in this high-risk group,” noted Dr. Sicherer, a member of the expert panel that worked on the guidelines.
The draft guidelines include 43 clinical recommendations for the diagnosis and management of food allergies in children, according to the NIAID website. In particular, the draft guidelines recommend introducing peanut-containing foods to infants aged 4-6 months who are at increased risk for peanut allergy because of severe eczema and/or egg allergies, after an evaluation with skin prick testing or peanut-specific IgE testing.
“Peanut allergy is relatively common and often persistent, and so a strategy that could prevent the allergy is very important,” Dr. Sicherer said in an interview. “However, peanut can be a choking hazard as peanuts or peanut butter, and so families should talk to their pediatrician about how and when to incorporate peanut into the diet, and whether allergy testing and referral to an allergist is needed.”
Support for the guidelines comes from several large studies with promising results, notably the LEAP (Learning Early about Peanut Allergy) trial. A recent extension of that study, known as LEAP-On (Persistence of Oral Tolerance to Peanut), showed that regular consumption of peanut-containing foods from infancy to 5 years provided ongoing protection against allergies, even 6 years after peanut consumption was discontinued for a 1-year period in 550 children (N Eng J Med. 2016 Apr 14;374:1435-43).
In the original LEAP study, 640 infants aged 4-11 months with severe eczema, egg allergy, or both were randomized to dietary peanut consumption or avoidance (N Engl J Med. 2015 Feb 26;372[9]:803-13). The prevalence of peanut allergy at 5 years of age was approximately 2% in the peanut-consumption group, compared with 14% in the peanut-avoidance group.
Another significant randomized trial, the EAT study (Enquiring About Tolerance) tested not only peanut, but also the early introduction of cooked egg, cow’s milk, sesame, wheat, and fish to 1,303 infants aged 3 months and older in the general population. The study’s strict protocol made adherence difficult, but researchers found a significant 67% reduction in the prevalence of food allergies at age 3 years among the children who followed the protocol, compared with controls, with relative risk reductions of 100% and 75%, respectively, for peanut and egg allergies (N Engl J Med. 2016 May 5;374:1733-43).
The next steps for research should make early introduction of peanut-containing foods even more effective at allergy prevention, Dr. Sicherer noted.
“We need to learn more about how much peanut should be incorporated into the diet, how long the protein has to be kept in the diet to have the best preventative effect, and whether this strategy applies to other foods,” he said.
Updated guidelines from the National Institute of Allergy and Infectious Diseases for the early introduction of peanut-containing foods to children at increased risk for peanut allergies are on the horizon, pending final approval.
“Two studies recently showed that infants at high risk of developing peanut allergy [infants with egg allergy and or severe eczema] were much less likely to have peanut allergy at age 5 years if they were able to incorporate peanut regularly into the diet between 4 and 11 months of age,” said Dr. Scott H. Sicherer, the Elliot and Roslyn Jaffe Professor of Pediatrics, Allergy and Immunology, and chief of the division of allergy and immunology in the department of pediatrics at the Icahn School of Medicine at Mount Sinai, New York.
“However, adding peanut to the diet at this age requires caution because these infants may already be allergic to peanut, and so allergy testing and care in adding peanut to the diet with medical supervision is needed in this high-risk group,” noted Dr. Sicherer, a member of the expert panel that worked on the guidelines.
The draft guidelines include 43 clinical recommendations for the diagnosis and management of food allergies in children, according to the NIAID website. In particular, the draft guidelines recommend introducing peanut-containing foods to infants aged 4-6 months who are at increased risk for peanut allergy because of severe eczema and/or egg allergies, after an evaluation with skin prick testing or peanut-specific IgE testing.
“Peanut allergy is relatively common and often persistent, and so a strategy that could prevent the allergy is very important,” Dr. Sicherer said in an interview. “However, peanut can be a choking hazard as peanuts or peanut butter, and so families should talk to their pediatrician about how and when to incorporate peanut into the diet, and whether allergy testing and referral to an allergist is needed.”
Support for the guidelines comes from several large studies with promising results, notably the LEAP (Learning Early about Peanut Allergy) trial. A recent extension of that study, known as LEAP-On (Persistence of Oral Tolerance to Peanut), showed that regular consumption of peanut-containing foods from infancy to 5 years provided ongoing protection against allergies, even 6 years after peanut consumption was discontinued for a 1-year period in 550 children (N Eng J Med. 2016 Apr 14;374:1435-43).
In the original LEAP study, 640 infants aged 4-11 months with severe eczema, egg allergy, or both were randomized to dietary peanut consumption or avoidance (N Engl J Med. 2015 Feb 26;372[9]:803-13). The prevalence of peanut allergy at 5 years of age was approximately 2% in the peanut-consumption group, compared with 14% in the peanut-avoidance group.
Another significant randomized trial, the EAT study (Enquiring About Tolerance) tested not only peanut, but also the early introduction of cooked egg, cow’s milk, sesame, wheat, and fish to 1,303 infants aged 3 months and older in the general population. The study’s strict protocol made adherence difficult, but researchers found a significant 67% reduction in the prevalence of food allergies at age 3 years among the children who followed the protocol, compared with controls, with relative risk reductions of 100% and 75%, respectively, for peanut and egg allergies (N Engl J Med. 2016 May 5;374:1733-43).
The next steps for research should make early introduction of peanut-containing foods even more effective at allergy prevention, Dr. Sicherer noted.
“We need to learn more about how much peanut should be incorporated into the diet, how long the protein has to be kept in the diet to have the best preventative effect, and whether this strategy applies to other foods,” he said.
Updated guidelines from the National Institute of Allergy and Infectious Diseases for the early introduction of peanut-containing foods to children at increased risk for peanut allergies are on the horizon, pending final approval.
“Two studies recently showed that infants at high risk of developing peanut allergy [infants with egg allergy and or severe eczema] were much less likely to have peanut allergy at age 5 years if they were able to incorporate peanut regularly into the diet between 4 and 11 months of age,” said Dr. Scott H. Sicherer, the Elliot and Roslyn Jaffe Professor of Pediatrics, Allergy and Immunology, and chief of the division of allergy and immunology in the department of pediatrics at the Icahn School of Medicine at Mount Sinai, New York.
“However, adding peanut to the diet at this age requires caution because these infants may already be allergic to peanut, and so allergy testing and care in adding peanut to the diet with medical supervision is needed in this high-risk group,” noted Dr. Sicherer, a member of the expert panel that worked on the guidelines.
The draft guidelines include 43 clinical recommendations for the diagnosis and management of food allergies in children, according to the NIAID website. In particular, the draft guidelines recommend introducing peanut-containing foods to infants aged 4-6 months who are at increased risk for peanut allergy because of severe eczema and/or egg allergies, after an evaluation with skin prick testing or peanut-specific IgE testing.
“Peanut allergy is relatively common and often persistent, and so a strategy that could prevent the allergy is very important,” Dr. Sicherer said in an interview. “However, peanut can be a choking hazard as peanuts or peanut butter, and so families should talk to their pediatrician about how and when to incorporate peanut into the diet, and whether allergy testing and referral to an allergist is needed.”
Support for the guidelines comes from several large studies with promising results, notably the LEAP (Learning Early about Peanut Allergy) trial. A recent extension of that study, known as LEAP-On (Persistence of Oral Tolerance to Peanut), showed that regular consumption of peanut-containing foods from infancy to 5 years provided ongoing protection against allergies, even 6 years after peanut consumption was discontinued for a 1-year period in 550 children (N Eng J Med. 2016 Apr 14;374:1435-43).
In the original LEAP study, 640 infants aged 4-11 months with severe eczema, egg allergy, or both were randomized to dietary peanut consumption or avoidance (N Engl J Med. 2015 Feb 26;372[9]:803-13). The prevalence of peanut allergy at 5 years of age was approximately 2% in the peanut-consumption group, compared with 14% in the peanut-avoidance group.
Another significant randomized trial, the EAT study (Enquiring About Tolerance) tested not only peanut, but also the early introduction of cooked egg, cow’s milk, sesame, wheat, and fish to 1,303 infants aged 3 months and older in the general population. The study’s strict protocol made adherence difficult, but researchers found a significant 67% reduction in the prevalence of food allergies at age 3 years among the children who followed the protocol, compared with controls, with relative risk reductions of 100% and 75%, respectively, for peanut and egg allergies (N Engl J Med. 2016 May 5;374:1733-43).
The next steps for research should make early introduction of peanut-containing foods even more effective at allergy prevention, Dr. Sicherer noted.
“We need to learn more about how much peanut should be incorporated into the diet, how long the protein has to be kept in the diet to have the best preventative effect, and whether this strategy applies to other foods,” he said.
Sleep apnea in later life more than doubles subsequent Alzheimer’s risk
DENVER – Obstructive sleep apnea diagnosed later in life is associated with an increased likelihood of subsequent Alzheimer’s disease, Dr. Omonigho Bubu reported at the annual meeting of the Associated Professional Sleep Societies.
He presented a retrospective cohort study in which a dose-reponse relationship was apparent. The more severe an individual’s obstructive sleep apnea (OSA) as reflected in a higher apnea-hypopnea index on polysomnography, the greater the risk of later being diagnosed with Alzheimer’s disease, compared with matched controls during up to 13 years of follow-up.
The study also identified several possible contributing factors for the observed OSA/Alzheimer’s relationship. Those OSA patients with more severe sleep fragmentation, nocturnal hypoxia, and abnormal sleep duration were significantly more likely to subsequently develop Alzheimer’s disease than were OSA patients with less severely disrupted sleep measures, added Dr. Bubu of the University of South Florida, Tampa.
The study included 756 patients aged 65 years and older with no history of cognitive decline when diagnosed with OSA by polysomnography at Tampa General Hospital during 2001-2005. They were matched by age, race, sex, body mass index, and zip code to two control groups totaling 3,780 subjects. The controls, drawn from outpatient medical clinics at the hospital, had a variety of medical problems but no sleep disorders or cognitive impairment.
During a mean 10.5-year follow-up period, 513 subjects were diagnosed with Alzheimer’s disease, according to Medicare data. In a Cox proportional hazards analysis adjusted for age, sex, race, body mass index, and education level, OSA was independently associated with a 2.2-fold increased risk. Further adjustment for alcohol intake, smoking, use of sleep medications, and chronic medical conditions didn’t substantially change the results.
However, the investigators were not able to control for apolipoprotein E (APOE)–epsilon 4 allele status, which is a known risk factor for both OSA and Alzheimer’s disease, so it makes one wonder whether the association is “all related to APOE,” said Dr. Richard J. Caselli, professor of neurology at the Mayo Clinic, Scottsdale, Ariz., when asked to comment on the study.
Time to onset of Alzheimer’s disease was shorter in the OSA patients: The mean time to diagnosis was 60.8 months after diagnosis of OSA, compared with 73 and 78 months in members of the two control groups who developed the dementia.
When the risk of developing Alzheimer’s disease was stratified according to baseline OSA severity, a dose-response effect was seen. Mild OSA, defined as 5-14 apnea-hypopnea events per hour of sleep, was associated with a 1.67-fold greater risk than in controls. The moderate OSA group, who had 15-29 events per hour, had a 1.81-fold increased risk. Patients with severe OSA, with 30 or more events per hour, had a 2.63-fold increased risk.
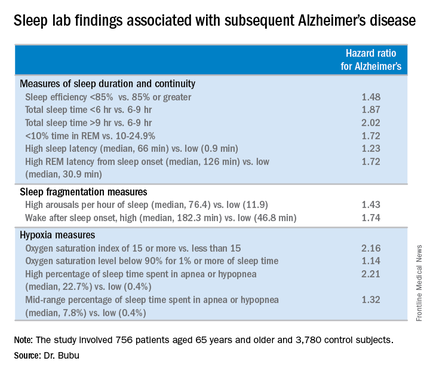
Gender, race, and education modified the relationship between OSA and Alzheimer’s disease, Dr. Bubu said. Women with OSA had a 2.28-fold greater risk of later developing the disease, compared with controls; men had a 1.42-fold increased risk. African-Americans with OSA were at 2.56-fold greater risk than were controls, while Hispanics with OSA were at 1.8-fold increased risk and non-Hispanic whites were at 1.87-fold increased risk. OSA patients with a high school education or less were at 2.73 times greater risk of Alzheimer’s disease than were controls, those with at least some college or technical school were at 1.82-fold risk, and OSA patients who’d been to graduate school had a 1.31-fold increased risk.
“Our results definitely show that OSA precedes the onset of Alzheimer’s disease. But we cannot say that’s causation. That will be left to future research examining the potential mechanisms we’ve identified,” Dr. Bubu said in an interview.
A key missing link in establishing a causal relationship is the lack of data on how many of the older patients diagnosed with OSA accepted treatment for the condition, and what their response rates were. In other words, it remains to be seen whether OSA occurring later in life is a modifiable risk factor for Alzheimer’s disease as opposed to an early expression of the dementing disease process whereby treatment of the sleep disorder doesn’t affect the progressive cognitive decline.
Both short sleep duration of less than 6 hours as well as a mean total sleep time greater than 9 hours in patients with OSA were associated with significantly increased risk of Alzheimer’s disease, compared with a sleep time of 6-9 hours. Patients with a high sleep-onset latency in the sleep lab, a high REM latency from sleep onset, a low percentage of time spent in REM, an oxygen saturation level of less than 90% for at least 1% of sleep time, and/or a high number of arousals per hour of sleep were also at increased risk of subsequent Alzheimer’s disease.
The study was supported by the Byrd Alzheimer’s Institute. Dr. Bubu reported having no financial conflicts.
DENVER – Obstructive sleep apnea diagnosed later in life is associated with an increased likelihood of subsequent Alzheimer’s disease, Dr. Omonigho Bubu reported at the annual meeting of the Associated Professional Sleep Societies.
He presented a retrospective cohort study in which a dose-reponse relationship was apparent. The more severe an individual’s obstructive sleep apnea (OSA) as reflected in a higher apnea-hypopnea index on polysomnography, the greater the risk of later being diagnosed with Alzheimer’s disease, compared with matched controls during up to 13 years of follow-up.
The study also identified several possible contributing factors for the observed OSA/Alzheimer’s relationship. Those OSA patients with more severe sleep fragmentation, nocturnal hypoxia, and abnormal sleep duration were significantly more likely to subsequently develop Alzheimer’s disease than were OSA patients with less severely disrupted sleep measures, added Dr. Bubu of the University of South Florida, Tampa.
The study included 756 patients aged 65 years and older with no history of cognitive decline when diagnosed with OSA by polysomnography at Tampa General Hospital during 2001-2005. They were matched by age, race, sex, body mass index, and zip code to two control groups totaling 3,780 subjects. The controls, drawn from outpatient medical clinics at the hospital, had a variety of medical problems but no sleep disorders or cognitive impairment.
During a mean 10.5-year follow-up period, 513 subjects were diagnosed with Alzheimer’s disease, according to Medicare data. In a Cox proportional hazards analysis adjusted for age, sex, race, body mass index, and education level, OSA was independently associated with a 2.2-fold increased risk. Further adjustment for alcohol intake, smoking, use of sleep medications, and chronic medical conditions didn’t substantially change the results.
However, the investigators were not able to control for apolipoprotein E (APOE)–epsilon 4 allele status, which is a known risk factor for both OSA and Alzheimer’s disease, so it makes one wonder whether the association is “all related to APOE,” said Dr. Richard J. Caselli, professor of neurology at the Mayo Clinic, Scottsdale, Ariz., when asked to comment on the study.
Time to onset of Alzheimer’s disease was shorter in the OSA patients: The mean time to diagnosis was 60.8 months after diagnosis of OSA, compared with 73 and 78 months in members of the two control groups who developed the dementia.
When the risk of developing Alzheimer’s disease was stratified according to baseline OSA severity, a dose-response effect was seen. Mild OSA, defined as 5-14 apnea-hypopnea events per hour of sleep, was associated with a 1.67-fold greater risk than in controls. The moderate OSA group, who had 15-29 events per hour, had a 1.81-fold increased risk. Patients with severe OSA, with 30 or more events per hour, had a 2.63-fold increased risk.

Gender, race, and education modified the relationship between OSA and Alzheimer’s disease, Dr. Bubu said. Women with OSA had a 2.28-fold greater risk of later developing the disease, compared with controls; men had a 1.42-fold increased risk. African-Americans with OSA were at 2.56-fold greater risk than were controls, while Hispanics with OSA were at 1.8-fold increased risk and non-Hispanic whites were at 1.87-fold increased risk. OSA patients with a high school education or less were at 2.73 times greater risk of Alzheimer’s disease than were controls, those with at least some college or technical school were at 1.82-fold risk, and OSA patients who’d been to graduate school had a 1.31-fold increased risk.
“Our results definitely show that OSA precedes the onset of Alzheimer’s disease. But we cannot say that’s causation. That will be left to future research examining the potential mechanisms we’ve identified,” Dr. Bubu said in an interview.
A key missing link in establishing a causal relationship is the lack of data on how many of the older patients diagnosed with OSA accepted treatment for the condition, and what their response rates were. In other words, it remains to be seen whether OSA occurring later in life is a modifiable risk factor for Alzheimer’s disease as opposed to an early expression of the dementing disease process whereby treatment of the sleep disorder doesn’t affect the progressive cognitive decline.
Both short sleep duration of less than 6 hours as well as a mean total sleep time greater than 9 hours in patients with OSA were associated with significantly increased risk of Alzheimer’s disease, compared with a sleep time of 6-9 hours. Patients with a high sleep-onset latency in the sleep lab, a high REM latency from sleep onset, a low percentage of time spent in REM, an oxygen saturation level of less than 90% for at least 1% of sleep time, and/or a high number of arousals per hour of sleep were also at increased risk of subsequent Alzheimer’s disease.
The study was supported by the Byrd Alzheimer’s Institute. Dr. Bubu reported having no financial conflicts.
DENVER – Obstructive sleep apnea diagnosed later in life is associated with an increased likelihood of subsequent Alzheimer’s disease, Dr. Omonigho Bubu reported at the annual meeting of the Associated Professional Sleep Societies.
He presented a retrospective cohort study in which a dose-reponse relationship was apparent. The more severe an individual’s obstructive sleep apnea (OSA) as reflected in a higher apnea-hypopnea index on polysomnography, the greater the risk of later being diagnosed with Alzheimer’s disease, compared with matched controls during up to 13 years of follow-up.
The study also identified several possible contributing factors for the observed OSA/Alzheimer’s relationship. Those OSA patients with more severe sleep fragmentation, nocturnal hypoxia, and abnormal sleep duration were significantly more likely to subsequently develop Alzheimer’s disease than were OSA patients with less severely disrupted sleep measures, added Dr. Bubu of the University of South Florida, Tampa.
The study included 756 patients aged 65 years and older with no history of cognitive decline when diagnosed with OSA by polysomnography at Tampa General Hospital during 2001-2005. They were matched by age, race, sex, body mass index, and zip code to two control groups totaling 3,780 subjects. The controls, drawn from outpatient medical clinics at the hospital, had a variety of medical problems but no sleep disorders or cognitive impairment.
During a mean 10.5-year follow-up period, 513 subjects were diagnosed with Alzheimer’s disease, according to Medicare data. In a Cox proportional hazards analysis adjusted for age, sex, race, body mass index, and education level, OSA was independently associated with a 2.2-fold increased risk. Further adjustment for alcohol intake, smoking, use of sleep medications, and chronic medical conditions didn’t substantially change the results.
However, the investigators were not able to control for apolipoprotein E (APOE)–epsilon 4 allele status, which is a known risk factor for both OSA and Alzheimer’s disease, so it makes one wonder whether the association is “all related to APOE,” said Dr. Richard J. Caselli, professor of neurology at the Mayo Clinic, Scottsdale, Ariz., when asked to comment on the study.
Time to onset of Alzheimer’s disease was shorter in the OSA patients: The mean time to diagnosis was 60.8 months after diagnosis of OSA, compared with 73 and 78 months in members of the two control groups who developed the dementia.
When the risk of developing Alzheimer’s disease was stratified according to baseline OSA severity, a dose-response effect was seen. Mild OSA, defined as 5-14 apnea-hypopnea events per hour of sleep, was associated with a 1.67-fold greater risk than in controls. The moderate OSA group, who had 15-29 events per hour, had a 1.81-fold increased risk. Patients with severe OSA, with 30 or more events per hour, had a 2.63-fold increased risk.

Gender, race, and education modified the relationship between OSA and Alzheimer’s disease, Dr. Bubu said. Women with OSA had a 2.28-fold greater risk of later developing the disease, compared with controls; men had a 1.42-fold increased risk. African-Americans with OSA were at 2.56-fold greater risk than were controls, while Hispanics with OSA were at 1.8-fold increased risk and non-Hispanic whites were at 1.87-fold increased risk. OSA patients with a high school education or less were at 2.73 times greater risk of Alzheimer’s disease than were controls, those with at least some college or technical school were at 1.82-fold risk, and OSA patients who’d been to graduate school had a 1.31-fold increased risk.
“Our results definitely show that OSA precedes the onset of Alzheimer’s disease. But we cannot say that’s causation. That will be left to future research examining the potential mechanisms we’ve identified,” Dr. Bubu said in an interview.
A key missing link in establishing a causal relationship is the lack of data on how many of the older patients diagnosed with OSA accepted treatment for the condition, and what their response rates were. In other words, it remains to be seen whether OSA occurring later in life is a modifiable risk factor for Alzheimer’s disease as opposed to an early expression of the dementing disease process whereby treatment of the sleep disorder doesn’t affect the progressive cognitive decline.
Both short sleep duration of less than 6 hours as well as a mean total sleep time greater than 9 hours in patients with OSA were associated with significantly increased risk of Alzheimer’s disease, compared with a sleep time of 6-9 hours. Patients with a high sleep-onset latency in the sleep lab, a high REM latency from sleep onset, a low percentage of time spent in REM, an oxygen saturation level of less than 90% for at least 1% of sleep time, and/or a high number of arousals per hour of sleep were also at increased risk of subsequent Alzheimer’s disease.
The study was supported by the Byrd Alzheimer’s Institute. Dr. Bubu reported having no financial conflicts.
AT SLEEP 2016
Key clinical point: Patients diagnosed with obstructive sleep apnea after age 65 may be at increased risk of later developing Alzheimer’s disease.
Major finding: A dose-response effect was seen: obstructive sleep apnea classified as mild was associated with a 1.67-fold increased risk of subsequent Alzheimer’s disease, moderate with a 1.81-fold risk, and severe obstructive sleep apnea carried a 2.63-fold increased risk.
Data source: This was a retrospective cohort study including 756 patients age 65 or older with no history of cognitive impairment who were diagnosed with obstructive sleep apnea during 2001-2005 and 3,780 matched controls, all followed for up to 13 years.
Disclosures: The study was supported by the Byrd Alzheimer’s Institute. The presenter reported having no financial conflicts.
Insomnia is pervasive in adult neurodevelopmental disorders
DENVER – Adults with ADHD or autism spectrum disorder experience a high burden of sleep disturbances, especially insomnia, Dr. Anastasios Galanopoulos reported at the annual meeting of the Associated Professional Sleep Societies.
This has previously been established to be the case in pediatric patients with these neurodevelopmental disorders. However, sleep pathology hasn’t previously been well studied in affected adults, according to Dr. Galanopoulos, a consulting psychiatrist at Maudsley Hospital and King’s College London.
He presented a cross-sectional study of insomnia in a clinically representative sample comprising 164 adult patients: 98 with a DSM-5 diagnosis of ADHD, 30 with autism spectrum disorder, and 34 carrying both diagnoses.
Fully 91% of participants fell into the “poor” sleep category on the Pittsburgh Sleep Quality Index. Moreover, 44% of subjects had either moderate or severe clinical insomnia as reflected in a score of 15 or more on the Insomnia Severity Index. The rate of high sleep disruption scores was similar, regardless of whether the diagnosis was ADHD, autism spectrum disorder, or both.
Anxiety but not depression ratings on the Hospital Anxiety and Depression Scale correlated with insomnia scores, regardless of neurodevelopmental diagnosis. Insomnia scores also increased in concert with higher levels of hyperactivity symptoms as scored on the Barkley Adult ADHD Rating Scale. In contrast, inattentiveness scores were unrelated to insomnia.
Hyperactivity scores on the Barkley scale were significantly higher in adults with ADHD than in those with autism spectrum disorder. On the other hand, anxiety scores were higher in adults with autism spectrum disorder.
While the burden of sleep disturbances is similarly high in adults with ADHD and autism spectrum disorder, the underlying mechanism may well be different. For this reason, Dr. Galanopoulos and coinvestigators have begun studies systematically looking at various possible interventions for sleep disorders in adults with neurodevelopmental disorders.
He reported having no financial conflicts regarding this study, which was conducted without commercial support.
DENVER – Adults with ADHD or autism spectrum disorder experience a high burden of sleep disturbances, especially insomnia, Dr. Anastasios Galanopoulos reported at the annual meeting of the Associated Professional Sleep Societies.
This has previously been established to be the case in pediatric patients with these neurodevelopmental disorders. However, sleep pathology hasn’t previously been well studied in affected adults, according to Dr. Galanopoulos, a consulting psychiatrist at Maudsley Hospital and King’s College London.
He presented a cross-sectional study of insomnia in a clinically representative sample comprising 164 adult patients: 98 with a DSM-5 diagnosis of ADHD, 30 with autism spectrum disorder, and 34 carrying both diagnoses.
Fully 91% of participants fell into the “poor” sleep category on the Pittsburgh Sleep Quality Index. Moreover, 44% of subjects had either moderate or severe clinical insomnia as reflected in a score of 15 or more on the Insomnia Severity Index. The rate of high sleep disruption scores was similar, regardless of whether the diagnosis was ADHD, autism spectrum disorder, or both.
Anxiety but not depression ratings on the Hospital Anxiety and Depression Scale correlated with insomnia scores, regardless of neurodevelopmental diagnosis. Insomnia scores also increased in concert with higher levels of hyperactivity symptoms as scored on the Barkley Adult ADHD Rating Scale. In contrast, inattentiveness scores were unrelated to insomnia.
Hyperactivity scores on the Barkley scale were significantly higher in adults with ADHD than in those with autism spectrum disorder. On the other hand, anxiety scores were higher in adults with autism spectrum disorder.
While the burden of sleep disturbances is similarly high in adults with ADHD and autism spectrum disorder, the underlying mechanism may well be different. For this reason, Dr. Galanopoulos and coinvestigators have begun studies systematically looking at various possible interventions for sleep disorders in adults with neurodevelopmental disorders.
He reported having no financial conflicts regarding this study, which was conducted without commercial support.
DENVER – Adults with ADHD or autism spectrum disorder experience a high burden of sleep disturbances, especially insomnia, Dr. Anastasios Galanopoulos reported at the annual meeting of the Associated Professional Sleep Societies.
This has previously been established to be the case in pediatric patients with these neurodevelopmental disorders. However, sleep pathology hasn’t previously been well studied in affected adults, according to Dr. Galanopoulos, a consulting psychiatrist at Maudsley Hospital and King’s College London.
He presented a cross-sectional study of insomnia in a clinically representative sample comprising 164 adult patients: 98 with a DSM-5 diagnosis of ADHD, 30 with autism spectrum disorder, and 34 carrying both diagnoses.
Fully 91% of participants fell into the “poor” sleep category on the Pittsburgh Sleep Quality Index. Moreover, 44% of subjects had either moderate or severe clinical insomnia as reflected in a score of 15 or more on the Insomnia Severity Index. The rate of high sleep disruption scores was similar, regardless of whether the diagnosis was ADHD, autism spectrum disorder, or both.
Anxiety but not depression ratings on the Hospital Anxiety and Depression Scale correlated with insomnia scores, regardless of neurodevelopmental diagnosis. Insomnia scores also increased in concert with higher levels of hyperactivity symptoms as scored on the Barkley Adult ADHD Rating Scale. In contrast, inattentiveness scores were unrelated to insomnia.
Hyperactivity scores on the Barkley scale were significantly higher in adults with ADHD than in those with autism spectrum disorder. On the other hand, anxiety scores were higher in adults with autism spectrum disorder.
While the burden of sleep disturbances is similarly high in adults with ADHD and autism spectrum disorder, the underlying mechanism may well be different. For this reason, Dr. Galanopoulos and coinvestigators have begun studies systematically looking at various possible interventions for sleep disorders in adults with neurodevelopmental disorders.
He reported having no financial conflicts regarding this study, which was conducted without commercial support.
AT SLEEP 2016
Key clinical point: Clinically significant insomnia is extremely common in adults with ADHD or autism spectrum disorder.
Major finding: 44% of adults with ADHD, autism spectrum disorder, or both diagnoses had moderate or severe insomnia on the validated Insomnia Severity Index.
Data source: This was a cross-sectional study of insomnia in 164 adults with neurodevelopmental disorders.
Disclosures: The presenter reported having no financial conflicts regarding this study, which was conducted without commercial support.