User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
USPSTF opposes screening for obstructive sleep apnea in draft recommendation
The U.S. Preventive Services Task Force has issued a draft recommendation opposing screening for obstructive sleep apnea (OSA) in adults who are asymptomatic for the breathing disorder.
The USPSTF’s opposition is based on its determination that there is insufficient evidence to assess the balance of benefits and harms of screening for OSA in asymptomatic adults in primary care settings, giving the service an “I” grade. The recommendation and a draft evidence review are available for public comment until July 11 at 8:00 p.m. EST.
The draft recommendation is the first that the USPSTF has ever made about sleep apnea, according to the draft evidence review. The recommendation “applies to asymptomatic adults (aged 18 years and older) and adults with unrecognized symptoms of OSA.” It does not apply to children, adolescents, pregnant women, persons presenting with symptoms of or concerns about OSA, those who are being referred for evaluation or treatment of suspected OSA, and those who have acute conditions that could trigger the onset of OSA.
“Reported estimates of OSA prevalence vary due to differing definitions of OSA, sampling bias, and year of study publication. A 2013 systematic review reported an estimated prevalence of 2%-14% based on four community-based studies, while two U.S.-based studies conducted in the 1990s reported an estimated prevalence of 10% for mild OSA and 3.8%-6.5% for moderate or severe OSA,” according to the recommendation.
The USPSTF was unable to find adequate evidence on the direct harms of screening for OSA or the benefits of screening for OSA in asymptomatic populations, including their magnitude.
Most primary care clinicians do not routinely screen for OSA, according to the recommendation. While the Epworth Sleepiness Scale, STOP Questionnaire, STOPBang Questionnaire, Berlin Questionnaire, and Wisconsin Sleep Questionnaire are potential screening tests for OSA, none of these questionnaires has been validated in a primary care setting.
“There is uncertainty about the clinical utility of all potential screening tools,” and the USPSTF found no studies that prospectively evaluated screening questionnaires or clinical prediction tools to report calibration or clinical utility for improving health outcomes,” the draft evidence review said.
The USPSTF also found no studies evaluating the effect of screening for OSA on health outcomes or that directly evaluated benefits or harms of screening for OSA.
The recommendation calls for further research on the health outcomes of screening for OSA in asymptomatic persons and the role of sleepiness in determining health outcomes. The following are needed:
• The identification of valid and reliable clinical prediction tools that could accurately determine which asymptomatic persons (or persons with unrecognized symptoms) would benefit from further evaluation and testing for OSA.
• Studies that evaluate the effect of OSA treatments or interventions on health outcomes that are adequately powered and have an appropriate length of follow-up.
• Studies that evaluate whether improvement in the apnea-hypopnea index leads to improvement in health outcomes.
• More data on the natural history of mild sleep apnea.
The final evidence review will be used to inform the final USPSTF recommendation statement.

|
| Dr. David Schulman, FCCP |
Dr. David A. Schulman, FCCP, comments: The draft statement from the Preventative Services Task Force recommending against screening asymptomatic patients with standardized OSA questionnaires warrants a careful read. Many sleepy patients may not complain of their fatigue unless specifically asked, choosing to attribute their symptoms to inactivity, age, weight or a lack of exercise instead of a potential underlying sleep disorder.
Assessment of patients’ sleep habits and patterns by primary care physicians remains a critical component of preventative health to improve identification of the twenty-plus million Americans with sleep disordered breathing.

|
| Dr. David Schulman, FCCP |
Dr. David A. Schulman, FCCP, comments: The draft statement from the Preventative Services Task Force recommending against screening asymptomatic patients with standardized OSA questionnaires warrants a careful read. Many sleepy patients may not complain of their fatigue unless specifically asked, choosing to attribute their symptoms to inactivity, age, weight or a lack of exercise instead of a potential underlying sleep disorder.
Assessment of patients’ sleep habits and patterns by primary care physicians remains a critical component of preventative health to improve identification of the twenty-plus million Americans with sleep disordered breathing.

|
| Dr. David Schulman, FCCP |
Dr. David A. Schulman, FCCP, comments: The draft statement from the Preventative Services Task Force recommending against screening asymptomatic patients with standardized OSA questionnaires warrants a careful read. Many sleepy patients may not complain of their fatigue unless specifically asked, choosing to attribute their symptoms to inactivity, age, weight or a lack of exercise instead of a potential underlying sleep disorder.
Assessment of patients’ sleep habits and patterns by primary care physicians remains a critical component of preventative health to improve identification of the twenty-plus million Americans with sleep disordered breathing.
The U.S. Preventive Services Task Force has issued a draft recommendation opposing screening for obstructive sleep apnea (OSA) in adults who are asymptomatic for the breathing disorder.
The USPSTF’s opposition is based on its determination that there is insufficient evidence to assess the balance of benefits and harms of screening for OSA in asymptomatic adults in primary care settings, giving the service an “I” grade. The recommendation and a draft evidence review are available for public comment until July 11 at 8:00 p.m. EST.
The draft recommendation is the first that the USPSTF has ever made about sleep apnea, according to the draft evidence review. The recommendation “applies to asymptomatic adults (aged 18 years and older) and adults with unrecognized symptoms of OSA.” It does not apply to children, adolescents, pregnant women, persons presenting with symptoms of or concerns about OSA, those who are being referred for evaluation or treatment of suspected OSA, and those who have acute conditions that could trigger the onset of OSA.
“Reported estimates of OSA prevalence vary due to differing definitions of OSA, sampling bias, and year of study publication. A 2013 systematic review reported an estimated prevalence of 2%-14% based on four community-based studies, while two U.S.-based studies conducted in the 1990s reported an estimated prevalence of 10% for mild OSA and 3.8%-6.5% for moderate or severe OSA,” according to the recommendation.
The USPSTF was unable to find adequate evidence on the direct harms of screening for OSA or the benefits of screening for OSA in asymptomatic populations, including their magnitude.
Most primary care clinicians do not routinely screen for OSA, according to the recommendation. While the Epworth Sleepiness Scale, STOP Questionnaire, STOPBang Questionnaire, Berlin Questionnaire, and Wisconsin Sleep Questionnaire are potential screening tests for OSA, none of these questionnaires has been validated in a primary care setting.
“There is uncertainty about the clinical utility of all potential screening tools,” and the USPSTF found no studies that prospectively evaluated screening questionnaires or clinical prediction tools to report calibration or clinical utility for improving health outcomes,” the draft evidence review said.
The USPSTF also found no studies evaluating the effect of screening for OSA on health outcomes or that directly evaluated benefits or harms of screening for OSA.
The recommendation calls for further research on the health outcomes of screening for OSA in asymptomatic persons and the role of sleepiness in determining health outcomes. The following are needed:
• The identification of valid and reliable clinical prediction tools that could accurately determine which asymptomatic persons (or persons with unrecognized symptoms) would benefit from further evaluation and testing for OSA.
• Studies that evaluate the effect of OSA treatments or interventions on health outcomes that are adequately powered and have an appropriate length of follow-up.
• Studies that evaluate whether improvement in the apnea-hypopnea index leads to improvement in health outcomes.
• More data on the natural history of mild sleep apnea.
The final evidence review will be used to inform the final USPSTF recommendation statement.
The U.S. Preventive Services Task Force has issued a draft recommendation opposing screening for obstructive sleep apnea (OSA) in adults who are asymptomatic for the breathing disorder.
The USPSTF’s opposition is based on its determination that there is insufficient evidence to assess the balance of benefits and harms of screening for OSA in asymptomatic adults in primary care settings, giving the service an “I” grade. The recommendation and a draft evidence review are available for public comment until July 11 at 8:00 p.m. EST.
The draft recommendation is the first that the USPSTF has ever made about sleep apnea, according to the draft evidence review. The recommendation “applies to asymptomatic adults (aged 18 years and older) and adults with unrecognized symptoms of OSA.” It does not apply to children, adolescents, pregnant women, persons presenting with symptoms of or concerns about OSA, those who are being referred for evaluation or treatment of suspected OSA, and those who have acute conditions that could trigger the onset of OSA.
“Reported estimates of OSA prevalence vary due to differing definitions of OSA, sampling bias, and year of study publication. A 2013 systematic review reported an estimated prevalence of 2%-14% based on four community-based studies, while two U.S.-based studies conducted in the 1990s reported an estimated prevalence of 10% for mild OSA and 3.8%-6.5% for moderate or severe OSA,” according to the recommendation.
The USPSTF was unable to find adequate evidence on the direct harms of screening for OSA or the benefits of screening for OSA in asymptomatic populations, including their magnitude.
Most primary care clinicians do not routinely screen for OSA, according to the recommendation. While the Epworth Sleepiness Scale, STOP Questionnaire, STOPBang Questionnaire, Berlin Questionnaire, and Wisconsin Sleep Questionnaire are potential screening tests for OSA, none of these questionnaires has been validated in a primary care setting.
“There is uncertainty about the clinical utility of all potential screening tools,” and the USPSTF found no studies that prospectively evaluated screening questionnaires or clinical prediction tools to report calibration or clinical utility for improving health outcomes,” the draft evidence review said.
The USPSTF also found no studies evaluating the effect of screening for OSA on health outcomes or that directly evaluated benefits or harms of screening for OSA.
The recommendation calls for further research on the health outcomes of screening for OSA in asymptomatic persons and the role of sleepiness in determining health outcomes. The following are needed:
• The identification of valid and reliable clinical prediction tools that could accurately determine which asymptomatic persons (or persons with unrecognized symptoms) would benefit from further evaluation and testing for OSA.
• Studies that evaluate the effect of OSA treatments or interventions on health outcomes that are adequately powered and have an appropriate length of follow-up.
• Studies that evaluate whether improvement in the apnea-hypopnea index leads to improvement in health outcomes.
• More data on the natural history of mild sleep apnea.
The final evidence review will be used to inform the final USPSTF recommendation statement.
Diffuse alveolar damage ups death risk in acute respiratory distress syndrome
Among patients with acute respiratory distress syndrome (ARDS), those who are also diagnosed with diffuse alveolar damage (DAD) via an open lung biopsy face nearly twice as high a mortality risk as did those without DAD, according to a systematic review and a meta-analysis by Dr. Pablo Cardinal-Fernandez and his colleagues.
The results of this and other studies “support the hypothesis that ARDS with DAD is a specific clinicopathological entity different from ARDS without DAD,” said Dr. Cardinal-Fernandez of the department of genetic medicine, Cornell University, New York, and his colleagues (CHEST 2016 149:1155-64.).
“Our meta-analysis underscores the need for less-invasive approaches to individualize therapy for patients with ARDS, including the development of biomarkers for predicting responses to treatments.”
The researchers analyzed studies identified using MEDLINE, EMBASE, Cochrane Register of Controlled Trials, LILACS, and citation review from Jan. 1, 1967, to Sept. 1, 2015. Eight studies involving 350 patients satisfied the researchers’ criteria for inclusion in the review. All of such studies included patients who received an open lung biopsy after being diagnosed with ARDS, histologic results indicating the presence or absence of DAD based on the open lung biopsy, and the mortalities of both a group of patients diagnosed with DAD and a group of patients not diagnosed with DAD. Studies were excluded from the review if they included fewer than five participants.
The pooled odds ratio for mortality in patients who had ARDS with DAD, compared with patients with ARDs who did not have DAD was 1.81.
At the time of ARDS diagnosis, the meta-differences for sequential organ failure assessment scores and the index of hypoxemia (PaO2/FiO2) ratio between the patients who had DAD and those who did not were 0.26 and 4.36, respectively. On the day of open lung biopsy, the meta-differences for the sequential organ failure assessment score and the PaO2/FiO2 ratio between the two patient groups were also small; the meta-difference for sequential organ failure was 0.45 and the meta-difference for the PaO2/FiO2 ratio was –8.63.
The higher mortality risk in the group of patients with DAD – despite patients in both groups having similar severities of illness – suggests that “the presence of DAD confers additional prognostic information,” according to the researchers.
“The mortality heterogeneity of this meta-analysis was low, suggesting that no other variables affect the results (that is, the observed effect depends mainly on the presence of DAD). Conditions that were identified in patients without DAD included pneumonia, pulmonary embolism, edema, or no pathologic abnormality. It appears that these subsets of patients have a different and better prognosis than did patients with ARDS and DAD, perhaps as a result of favorable responses to specific treatments, with the possible exception of lower tidal volume, which appears to be beneficial in all subgroups,” the researchers said.
No financial or nonfinancial disclosures were declared.
Among patients with acute respiratory distress syndrome (ARDS), those who are also diagnosed with diffuse alveolar damage (DAD) via an open lung biopsy face nearly twice as high a mortality risk as did those without DAD, according to a systematic review and a meta-analysis by Dr. Pablo Cardinal-Fernandez and his colleagues.
The results of this and other studies “support the hypothesis that ARDS with DAD is a specific clinicopathological entity different from ARDS without DAD,” said Dr. Cardinal-Fernandez of the department of genetic medicine, Cornell University, New York, and his colleagues (CHEST 2016 149:1155-64.).
“Our meta-analysis underscores the need for less-invasive approaches to individualize therapy for patients with ARDS, including the development of biomarkers for predicting responses to treatments.”
The researchers analyzed studies identified using MEDLINE, EMBASE, Cochrane Register of Controlled Trials, LILACS, and citation review from Jan. 1, 1967, to Sept. 1, 2015. Eight studies involving 350 patients satisfied the researchers’ criteria for inclusion in the review. All of such studies included patients who received an open lung biopsy after being diagnosed with ARDS, histologic results indicating the presence or absence of DAD based on the open lung biopsy, and the mortalities of both a group of patients diagnosed with DAD and a group of patients not diagnosed with DAD. Studies were excluded from the review if they included fewer than five participants.
The pooled odds ratio for mortality in patients who had ARDS with DAD, compared with patients with ARDs who did not have DAD was 1.81.
At the time of ARDS diagnosis, the meta-differences for sequential organ failure assessment scores and the index of hypoxemia (PaO2/FiO2) ratio between the patients who had DAD and those who did not were 0.26 and 4.36, respectively. On the day of open lung biopsy, the meta-differences for the sequential organ failure assessment score and the PaO2/FiO2 ratio between the two patient groups were also small; the meta-difference for sequential organ failure was 0.45 and the meta-difference for the PaO2/FiO2 ratio was –8.63.
The higher mortality risk in the group of patients with DAD – despite patients in both groups having similar severities of illness – suggests that “the presence of DAD confers additional prognostic information,” according to the researchers.
“The mortality heterogeneity of this meta-analysis was low, suggesting that no other variables affect the results (that is, the observed effect depends mainly on the presence of DAD). Conditions that were identified in patients without DAD included pneumonia, pulmonary embolism, edema, or no pathologic abnormality. It appears that these subsets of patients have a different and better prognosis than did patients with ARDS and DAD, perhaps as a result of favorable responses to specific treatments, with the possible exception of lower tidal volume, which appears to be beneficial in all subgroups,” the researchers said.
No financial or nonfinancial disclosures were declared.
Among patients with acute respiratory distress syndrome (ARDS), those who are also diagnosed with diffuse alveolar damage (DAD) via an open lung biopsy face nearly twice as high a mortality risk as did those without DAD, according to a systematic review and a meta-analysis by Dr. Pablo Cardinal-Fernandez and his colleagues.
The results of this and other studies “support the hypothesis that ARDS with DAD is a specific clinicopathological entity different from ARDS without DAD,” said Dr. Cardinal-Fernandez of the department of genetic medicine, Cornell University, New York, and his colleagues (CHEST 2016 149:1155-64.).
“Our meta-analysis underscores the need for less-invasive approaches to individualize therapy for patients with ARDS, including the development of biomarkers for predicting responses to treatments.”
The researchers analyzed studies identified using MEDLINE, EMBASE, Cochrane Register of Controlled Trials, LILACS, and citation review from Jan. 1, 1967, to Sept. 1, 2015. Eight studies involving 350 patients satisfied the researchers’ criteria for inclusion in the review. All of such studies included patients who received an open lung biopsy after being diagnosed with ARDS, histologic results indicating the presence or absence of DAD based on the open lung biopsy, and the mortalities of both a group of patients diagnosed with DAD and a group of patients not diagnosed with DAD. Studies were excluded from the review if they included fewer than five participants.
The pooled odds ratio for mortality in patients who had ARDS with DAD, compared with patients with ARDs who did not have DAD was 1.81.
At the time of ARDS diagnosis, the meta-differences for sequential organ failure assessment scores and the index of hypoxemia (PaO2/FiO2) ratio between the patients who had DAD and those who did not were 0.26 and 4.36, respectively. On the day of open lung biopsy, the meta-differences for the sequential organ failure assessment score and the PaO2/FiO2 ratio between the two patient groups were also small; the meta-difference for sequential organ failure was 0.45 and the meta-difference for the PaO2/FiO2 ratio was –8.63.
The higher mortality risk in the group of patients with DAD – despite patients in both groups having similar severities of illness – suggests that “the presence of DAD confers additional prognostic information,” according to the researchers.
“The mortality heterogeneity of this meta-analysis was low, suggesting that no other variables affect the results (that is, the observed effect depends mainly on the presence of DAD). Conditions that were identified in patients without DAD included pneumonia, pulmonary embolism, edema, or no pathologic abnormality. It appears that these subsets of patients have a different and better prognosis than did patients with ARDS and DAD, perhaps as a result of favorable responses to specific treatments, with the possible exception of lower tidal volume, which appears to be beneficial in all subgroups,” the researchers said.
No financial or nonfinancial disclosures were declared.
FROM CHEST
Key clinical point: Among patients with ARDS, those who are also diagnosed with DAD faced nearly twice as high a mortality risk as did those without DAD.
Major finding: The pooled odds ratio for mortality in patients who had acute respiratory distress syndrome with diffuse alveolar damage, compared with patients with ARDs who did not have DAD was 1.81.
Data source: A systematic review and meta-analysis of eight studies, including 350 patients, published between 1967 and 2015.
Disclosures: No financial or nonfinancial disclosures were declared.
SLEEP TIGHT: CPAP may be vasculoprotective in stroke/TIA
DENVER – Long-term continuous positive airway pressure (CPAP) for treatment of sleep apnea in patients with a recent mild stroke or transient ischemic attack resulted in improved cardiovascular and metabolic risk factors, better neurologic function, and a reduction in the recurrent vascular event rate, compared with usual care in the SLEEP TIGHT study.
“Up to 25% of patients will have a stroke, cardiovascular event, or death within 90 days after a minor stroke or TIA [transient ischemic attack] despite current preventive strategies. And, importantly, patients with a TIA or stroke have a high prevalence of obstructive sleep apnea – on the order of 60%-80%,” explained Dr. H. Klar Yaggi at the annual meeting of the Associated Professional Sleep Societies.
SLEEP TIGHT’s findings support the hypothesis that diagnosis and treatment of sleep apnea in patients with a recent minor stroke or TIA will address a major unmet need for better methods of reducing the high vascular risk present in this population, said Dr. Yaggi of Yale University in New Haven, Conn.
SLEEP TIGHT was a National Heart, Lung, and Blood Institute–sponsored phase II, 12-month, multicenter, single-blind, randomized, proof-of-concept study. It included 252 patients, 80% of whom had a recent minor stroke, the rest a TIA. These were patients with high levels of cardiovascular risk factors: two-thirds had hypertension, half were hyperlipidemic, 40% had diabetes, 15% had a prior MI, 10% had atrial fibrillation, and the group’s mean body mass index was 30 kg/m2. Polysomnography revealed that 76% of subjects had sleep apnea as defined by an apnea-hypopnea index of at least 5 events per hour. In fact, they averaged about 23 events per hour, putting them in the moderate-severity range. As is common among stroke/TIA patients with sleep apnea, they experienced less daytime sleepiness than is typical in a sleep clinic population, with a mean baseline Epworth Sleepiness Scale score of 7.
Participants were randomized to one of three groups: a usual care control group, a CPAP arm, or an enhanced CPAP arm. The enhanced intervention protocol was designed to boost CPAP adherence; it included targeted education, a customized cognitive intervention, and additional CPAP support beyond the standard CPAP protocols used in sleep medicine clinics. Patients with sleep apnea in the two intervention arms were then placed on CPAP.
At 1 year of follow-up, the stroke rate was 8.7 per 100 patient-years in the usual care group, compared with 5.5 per 100 person-years in the combined intervention arms. The composite cardiovascular event rate, composed of all-cause mortality, acute MI, stroke, hospitalization for unstable angina, or urgent coronary revascularization, was 13.1 per 100 person-years with usual care and 11.0 in the CPAP intervention arms. While these results are encouraging, SLEEP TIGHT wasn’t powered to show significant differences in these hard events.
Outcomes across the board didn’t differ significantly between the CPAP and enhanced CPAP groups. And since the mean number of hours of CPAP use per night was also similar in the two groups – 3.9 hours with standard CPAP and 4.3 hours with enhanced CPAP – it’s likely that the phase III trial will rely upon the much simpler standard CPAP intervention, according to Dr. Yaggi.
He deemed CPAP adherence in this stroke/TIA population to be similar to the rates typically seen in routine sleep medicine practice. Roughly 40% of the stroke/TIA patients were rated as having good adherence, 30% made some use of the therapy, and 30% had no or poor adherence.
Nonetheless, patients in the two intervention arms did significantly better than the usual care group in terms of 1-year changes in insulin resistance and glycosylated hemoglobin. They also had lower 24-hour mean systolic blood pressure and were more likely to convert to a favorable pattern of nocturnal blood pressure dipping. However, no differences between the intervention and usual care groups were seen in levels of high-sensitivity C-reactive protein and interleukin-6, the two markers of systemic inflammation analyzed. Nor did the CPAP intervention provide any benefit in terms of heart rate variability and other measures of autonomic function.
Fifty-eight percent of patients in the intervention arms ended up with a desirable National Institutes of Health Stroke Scale score of 0-1, compared with 38% of the usual care group. In addition, daytime sleepiness as reflected in Epworth Sleepiness Scale scores was reduced at last follow-up to a significantly greater extent in the CPAP groups, Dr. Yaggi noted.
Greater CPAP use was associated with a favorable trend for improvement in the modified Rankin score, a measure of functional ability: a 0.3-point reduction with no or poor CPAP use, a 0.4-point decrease with some use, and a 0.9-point reduction with good use.
The encouraging results will be helpful in designing a planned much larger, event-driven, definitive phase III trial, Dr. Yaggi said.
Dr. Yaggi reported having no financial conflicts regarding this National Heart, Lung and Blood Institute-sponsored study.
DENVER – Long-term continuous positive airway pressure (CPAP) for treatment of sleep apnea in patients with a recent mild stroke or transient ischemic attack resulted in improved cardiovascular and metabolic risk factors, better neurologic function, and a reduction in the recurrent vascular event rate, compared with usual care in the SLEEP TIGHT study.
“Up to 25% of patients will have a stroke, cardiovascular event, or death within 90 days after a minor stroke or TIA [transient ischemic attack] despite current preventive strategies. And, importantly, patients with a TIA or stroke have a high prevalence of obstructive sleep apnea – on the order of 60%-80%,” explained Dr. H. Klar Yaggi at the annual meeting of the Associated Professional Sleep Societies.
SLEEP TIGHT’s findings support the hypothesis that diagnosis and treatment of sleep apnea in patients with a recent minor stroke or TIA will address a major unmet need for better methods of reducing the high vascular risk present in this population, said Dr. Yaggi of Yale University in New Haven, Conn.
SLEEP TIGHT was a National Heart, Lung, and Blood Institute–sponsored phase II, 12-month, multicenter, single-blind, randomized, proof-of-concept study. It included 252 patients, 80% of whom had a recent minor stroke, the rest a TIA. These were patients with high levels of cardiovascular risk factors: two-thirds had hypertension, half were hyperlipidemic, 40% had diabetes, 15% had a prior MI, 10% had atrial fibrillation, and the group’s mean body mass index was 30 kg/m2. Polysomnography revealed that 76% of subjects had sleep apnea as defined by an apnea-hypopnea index of at least 5 events per hour. In fact, they averaged about 23 events per hour, putting them in the moderate-severity range. As is common among stroke/TIA patients with sleep apnea, they experienced less daytime sleepiness than is typical in a sleep clinic population, with a mean baseline Epworth Sleepiness Scale score of 7.
Participants were randomized to one of three groups: a usual care control group, a CPAP arm, or an enhanced CPAP arm. The enhanced intervention protocol was designed to boost CPAP adherence; it included targeted education, a customized cognitive intervention, and additional CPAP support beyond the standard CPAP protocols used in sleep medicine clinics. Patients with sleep apnea in the two intervention arms were then placed on CPAP.
At 1 year of follow-up, the stroke rate was 8.7 per 100 patient-years in the usual care group, compared with 5.5 per 100 person-years in the combined intervention arms. The composite cardiovascular event rate, composed of all-cause mortality, acute MI, stroke, hospitalization for unstable angina, or urgent coronary revascularization, was 13.1 per 100 person-years with usual care and 11.0 in the CPAP intervention arms. While these results are encouraging, SLEEP TIGHT wasn’t powered to show significant differences in these hard events.
Outcomes across the board didn’t differ significantly between the CPAP and enhanced CPAP groups. And since the mean number of hours of CPAP use per night was also similar in the two groups – 3.9 hours with standard CPAP and 4.3 hours with enhanced CPAP – it’s likely that the phase III trial will rely upon the much simpler standard CPAP intervention, according to Dr. Yaggi.
He deemed CPAP adherence in this stroke/TIA population to be similar to the rates typically seen in routine sleep medicine practice. Roughly 40% of the stroke/TIA patients were rated as having good adherence, 30% made some use of the therapy, and 30% had no or poor adherence.
Nonetheless, patients in the two intervention arms did significantly better than the usual care group in terms of 1-year changes in insulin resistance and glycosylated hemoglobin. They also had lower 24-hour mean systolic blood pressure and were more likely to convert to a favorable pattern of nocturnal blood pressure dipping. However, no differences between the intervention and usual care groups were seen in levels of high-sensitivity C-reactive protein and interleukin-6, the two markers of systemic inflammation analyzed. Nor did the CPAP intervention provide any benefit in terms of heart rate variability and other measures of autonomic function.
Fifty-eight percent of patients in the intervention arms ended up with a desirable National Institutes of Health Stroke Scale score of 0-1, compared with 38% of the usual care group. In addition, daytime sleepiness as reflected in Epworth Sleepiness Scale scores was reduced at last follow-up to a significantly greater extent in the CPAP groups, Dr. Yaggi noted.
Greater CPAP use was associated with a favorable trend for improvement in the modified Rankin score, a measure of functional ability: a 0.3-point reduction with no or poor CPAP use, a 0.4-point decrease with some use, and a 0.9-point reduction with good use.
The encouraging results will be helpful in designing a planned much larger, event-driven, definitive phase III trial, Dr. Yaggi said.
Dr. Yaggi reported having no financial conflicts regarding this National Heart, Lung and Blood Institute-sponsored study.
DENVER – Long-term continuous positive airway pressure (CPAP) for treatment of sleep apnea in patients with a recent mild stroke or transient ischemic attack resulted in improved cardiovascular and metabolic risk factors, better neurologic function, and a reduction in the recurrent vascular event rate, compared with usual care in the SLEEP TIGHT study.
“Up to 25% of patients will have a stroke, cardiovascular event, or death within 90 days after a minor stroke or TIA [transient ischemic attack] despite current preventive strategies. And, importantly, patients with a TIA or stroke have a high prevalence of obstructive sleep apnea – on the order of 60%-80%,” explained Dr. H. Klar Yaggi at the annual meeting of the Associated Professional Sleep Societies.
SLEEP TIGHT’s findings support the hypothesis that diagnosis and treatment of sleep apnea in patients with a recent minor stroke or TIA will address a major unmet need for better methods of reducing the high vascular risk present in this population, said Dr. Yaggi of Yale University in New Haven, Conn.
SLEEP TIGHT was a National Heart, Lung, and Blood Institute–sponsored phase II, 12-month, multicenter, single-blind, randomized, proof-of-concept study. It included 252 patients, 80% of whom had a recent minor stroke, the rest a TIA. These were patients with high levels of cardiovascular risk factors: two-thirds had hypertension, half were hyperlipidemic, 40% had diabetes, 15% had a prior MI, 10% had atrial fibrillation, and the group’s mean body mass index was 30 kg/m2. Polysomnography revealed that 76% of subjects had sleep apnea as defined by an apnea-hypopnea index of at least 5 events per hour. In fact, they averaged about 23 events per hour, putting them in the moderate-severity range. As is common among stroke/TIA patients with sleep apnea, they experienced less daytime sleepiness than is typical in a sleep clinic population, with a mean baseline Epworth Sleepiness Scale score of 7.
Participants were randomized to one of three groups: a usual care control group, a CPAP arm, or an enhanced CPAP arm. The enhanced intervention protocol was designed to boost CPAP adherence; it included targeted education, a customized cognitive intervention, and additional CPAP support beyond the standard CPAP protocols used in sleep medicine clinics. Patients with sleep apnea in the two intervention arms were then placed on CPAP.
At 1 year of follow-up, the stroke rate was 8.7 per 100 patient-years in the usual care group, compared with 5.5 per 100 person-years in the combined intervention arms. The composite cardiovascular event rate, composed of all-cause mortality, acute MI, stroke, hospitalization for unstable angina, or urgent coronary revascularization, was 13.1 per 100 person-years with usual care and 11.0 in the CPAP intervention arms. While these results are encouraging, SLEEP TIGHT wasn’t powered to show significant differences in these hard events.
Outcomes across the board didn’t differ significantly between the CPAP and enhanced CPAP groups. And since the mean number of hours of CPAP use per night was also similar in the two groups – 3.9 hours with standard CPAP and 4.3 hours with enhanced CPAP – it’s likely that the phase III trial will rely upon the much simpler standard CPAP intervention, according to Dr. Yaggi.
He deemed CPAP adherence in this stroke/TIA population to be similar to the rates typically seen in routine sleep medicine practice. Roughly 40% of the stroke/TIA patients were rated as having good adherence, 30% made some use of the therapy, and 30% had no or poor adherence.
Nonetheless, patients in the two intervention arms did significantly better than the usual care group in terms of 1-year changes in insulin resistance and glycosylated hemoglobin. They also had lower 24-hour mean systolic blood pressure and were more likely to convert to a favorable pattern of nocturnal blood pressure dipping. However, no differences between the intervention and usual care groups were seen in levels of high-sensitivity C-reactive protein and interleukin-6, the two markers of systemic inflammation analyzed. Nor did the CPAP intervention provide any benefit in terms of heart rate variability and other measures of autonomic function.
Fifty-eight percent of patients in the intervention arms ended up with a desirable National Institutes of Health Stroke Scale score of 0-1, compared with 38% of the usual care group. In addition, daytime sleepiness as reflected in Epworth Sleepiness Scale scores was reduced at last follow-up to a significantly greater extent in the CPAP groups, Dr. Yaggi noted.
Greater CPAP use was associated with a favorable trend for improvement in the modified Rankin score, a measure of functional ability: a 0.3-point reduction with no or poor CPAP use, a 0.4-point decrease with some use, and a 0.9-point reduction with good use.
The encouraging results will be helpful in designing a planned much larger, event-driven, definitive phase III trial, Dr. Yaggi said.
Dr. Yaggi reported having no financial conflicts regarding this National Heart, Lung and Blood Institute-sponsored study.
AT SLEEP 2016
Key clinical point: CPAP treatment of obstructive sleep apnea in patients with a recent TIA or mild stroke appears to reduce their risk of further vascular events.
Major finding: At 1 year of follow-up, the stroke rate in patients randomized to CPAP, including the large subgroup with poor or no adherence, was 5.5 events per 100 person-years, compared with 8.7 in usual care controls.
Data source: SLEEP TIGHT was a 12-month, multicenter, prospective, randomized, single-blind, phase II trial including 252 patients.
Disclosures: The study presenter reported having no financial conflicts regarding this National Heart, Lung, and Blood Institute–sponsored trial.
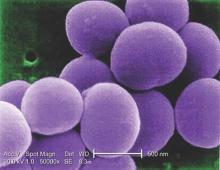
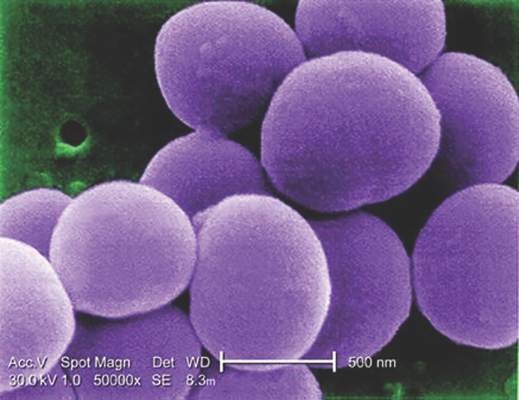
Glucocorticoids increase risk of S. aureus bacteremia
Use of systemic glucocorticoids significantly increased risk for community-acquired Staphylococcus aureus bacteremia (CA-SAB) in a dose-dependent fashion, based on data from a large Danish registry.
On average, current users of systemic glucocorticoids had an adjusted 2.5-fold increased risk of CA-SAB, compared with nonusers. The risk was most pronounced in long-term users of glucocorticoids, including patients with connective tissue disease and patients with chronic pulmonary disease. Among new users of glucocorticoids, the risk of CA-SAB was highest for patients with cancer, in the retrospective, case-control study published by Mayo Clinic Proceedings.
Dr. Jesper Smit of Aalborg (Denmark) University and his colleagues, looked at all 2,638 patients admitted with first-time CA-SAB and 26,379 matched population controls in Northern Denmark medical databases between January 1, 2000, and December 31, 2011.
New glucocorticoid users had an odds ratio for CA-SAB of 2.7, slightly higher than the OR of 2.3 for long-term users. Former glucocorticoid users had a considerably lower OR for CA-SAB of 1.3.
Risk of CA-SAB rose in a dose-dependent fashion as 90-day cumulative doses increased. For subjects taking a cumulative dose of 150 mg or less, the adjusted OR for CA-SAB was 1.3. At a cumulative dose of 500-1000 mg, OR rose to 2.4. At a cumulative dose greater than 1000 mg, OR was 6.2.
Risk did not differ based on individuals’ sex, age group, or the severity of any comorbidity.
“This is the first study to specifically investigate whether the use of glucocorticoids is associated with increased risk of CA-SAB,” the authors concluded, adding that “these results extend the current knowledge of risk factors for CA-SAB and may serve as a reminder for clinicians to carefully weigh the elevated risk against the potential beneficial effect of glucocorticoid therapy, particularly in patients with concomitant CA-SAB risk factors.”
This study was supported by grants from Heinrich Kopp, Hertha Christensen, and North Denmark Health Sciences Research foundation. The authors did not report any relevant financial disclosures.
Use of systemic glucocorticoids significantly increased risk for community-acquired Staphylococcus aureus bacteremia (CA-SAB) in a dose-dependent fashion, based on data from a large Danish registry.
On average, current users of systemic glucocorticoids had an adjusted 2.5-fold increased risk of CA-SAB, compared with nonusers. The risk was most pronounced in long-term users of glucocorticoids, including patients with connective tissue disease and patients with chronic pulmonary disease. Among new users of glucocorticoids, the risk of CA-SAB was highest for patients with cancer, in the retrospective, case-control study published by Mayo Clinic Proceedings.
Dr. Jesper Smit of Aalborg (Denmark) University and his colleagues, looked at all 2,638 patients admitted with first-time CA-SAB and 26,379 matched population controls in Northern Denmark medical databases between January 1, 2000, and December 31, 2011.
New glucocorticoid users had an odds ratio for CA-SAB of 2.7, slightly higher than the OR of 2.3 for long-term users. Former glucocorticoid users had a considerably lower OR for CA-SAB of 1.3.
Risk of CA-SAB rose in a dose-dependent fashion as 90-day cumulative doses increased. For subjects taking a cumulative dose of 150 mg or less, the adjusted OR for CA-SAB was 1.3. At a cumulative dose of 500-1000 mg, OR rose to 2.4. At a cumulative dose greater than 1000 mg, OR was 6.2.
Risk did not differ based on individuals’ sex, age group, or the severity of any comorbidity.
“This is the first study to specifically investigate whether the use of glucocorticoids is associated with increased risk of CA-SAB,” the authors concluded, adding that “these results extend the current knowledge of risk factors for CA-SAB and may serve as a reminder for clinicians to carefully weigh the elevated risk against the potential beneficial effect of glucocorticoid therapy, particularly in patients with concomitant CA-SAB risk factors.”
This study was supported by grants from Heinrich Kopp, Hertha Christensen, and North Denmark Health Sciences Research foundation. The authors did not report any relevant financial disclosures.
Use of systemic glucocorticoids significantly increased risk for community-acquired Staphylococcus aureus bacteremia (CA-SAB) in a dose-dependent fashion, based on data from a large Danish registry.
On average, current users of systemic glucocorticoids had an adjusted 2.5-fold increased risk of CA-SAB, compared with nonusers. The risk was most pronounced in long-term users of glucocorticoids, including patients with connective tissue disease and patients with chronic pulmonary disease. Among new users of glucocorticoids, the risk of CA-SAB was highest for patients with cancer, in the retrospective, case-control study published by Mayo Clinic Proceedings.
Dr. Jesper Smit of Aalborg (Denmark) University and his colleagues, looked at all 2,638 patients admitted with first-time CA-SAB and 26,379 matched population controls in Northern Denmark medical databases between January 1, 2000, and December 31, 2011.
New glucocorticoid users had an odds ratio for CA-SAB of 2.7, slightly higher than the OR of 2.3 for long-term users. Former glucocorticoid users had a considerably lower OR for CA-SAB of 1.3.
Risk of CA-SAB rose in a dose-dependent fashion as 90-day cumulative doses increased. For subjects taking a cumulative dose of 150 mg or less, the adjusted OR for CA-SAB was 1.3. At a cumulative dose of 500-1000 mg, OR rose to 2.4. At a cumulative dose greater than 1000 mg, OR was 6.2.
Risk did not differ based on individuals’ sex, age group, or the severity of any comorbidity.
“This is the first study to specifically investigate whether the use of glucocorticoids is associated with increased risk of CA-SAB,” the authors concluded, adding that “these results extend the current knowledge of risk factors for CA-SAB and may serve as a reminder for clinicians to carefully weigh the elevated risk against the potential beneficial effect of glucocorticoid therapy, particularly in patients with concomitant CA-SAB risk factors.”
This study was supported by grants from Heinrich Kopp, Hertha Christensen, and North Denmark Health Sciences Research foundation. The authors did not report any relevant financial disclosures.
FROM MAYO CLINIC PROCEEDINGS
Key clinical point: Taking glucocorticoids can significantly increase the risk of contracting community-acquired Staphylococcus aureus bacteremia (CA-SAB).
Major finding: New glucocorticoid users had an odds ratio for CA-SAB of 2.7, slightly higher than the OR of 2.3 for long-term users. Former glucocorticoid users had a considerably lower OR for CA-SAB of 1.3.
Data source: Retrospective, case-control study of all adults with first-time CA-SAB in Northern Denmark medical registries between 2000 and 2011.
Disclosures: Study supported by grants from Heinrich Kopp, Hertha Christensen, and North Denmark Health Sciences Research foundation. The authors did not report any relevant financial disclosures.
Insomnia linked to increased risk of pregnancy loss
DENVER – Women who experience difficulty staying asleep are at increased risk of having one or more pregnancies that don’t result in a live birth, a large epidemiologic study suggests.
In contrast, other expressions of insomnia – difficulty in falling asleep, early morning awakening, or nonrestorative sleep – were not significantly associated with pregnancy loss in this analysis of a nationally representative sample comprised of 5,554 women aged 18-45 years, Sara Nowakowski, Ph.D., reported at the annual meeting of the Associated Professional Sleep Societies.
The women were participants in the National Health and Nutrition Examination Survey for 2005-2008, which collected data on reproductive history as well as sleep patterns. Roughly 20% of the women self-reported experiencing insomnia. Eighty-three percent of the 18- to 45-year-old women had been pregnant at least once, and 1,870 (40%) of them had one or more prior pregnancies that didn’t result in a live birth.
In a multivariate logistic regression analysis adjusted for age, race, education level, and frequency of sleep apnea symptoms, such as snoring or snorting/gasping, frequent difficulty in maintaining sleep was independently associated with an 85% increased risk of having experienced a pregnancy that didn’t result in a live birth, according to Dr. Nowakowski, a clinical psychologist in the department of ob.gyn. at the University of Texas, Galveston.
In an interview, she was quick to note that these are correlational, hypothesis-generating data, and that an epidemiologic study such as this can’t establish causality.
Dr. Nowakowski and her coinvestigators hope to conduct a prospective randomized trial of cognitive behavioral therapy for insomnia – a well-established treatment – in a group of women with prior spontaneous abortion, miscarriage, or other infertility issues, to determine whether insomnia is a modifiable risk factor for adverse pregnancy outcomes.
One physician at the meeting remarked that if women with fertility problems and insomnia knew of Dr. Nowakowski’s work showing an association between insomnia and unsuccessful pregnancies, they would be pounding down the doors of sleep specialists. Dr. Nowakowski agreed.
“I surf the online networks set up for infertile women. They’re very distraught over their trouble conceiving. They’re doing yoga, de-stress programs, taking all sorts of supplements – including melatonin – to try to improve their chances of fertility,” she said. “If insomnia turns out to partially account for the risk of having pregnancies that don’t result in live birth, and treating the insomnia reduces that risk, there would be a huge amount of patient interest.”
The study was supported by the National Institutes of Health. Dr. Nowakowski reported having no financial conflicts.
DENVER – Women who experience difficulty staying asleep are at increased risk of having one or more pregnancies that don’t result in a live birth, a large epidemiologic study suggests.
In contrast, other expressions of insomnia – difficulty in falling asleep, early morning awakening, or nonrestorative sleep – were not significantly associated with pregnancy loss in this analysis of a nationally representative sample comprised of 5,554 women aged 18-45 years, Sara Nowakowski, Ph.D., reported at the annual meeting of the Associated Professional Sleep Societies.
The women were participants in the National Health and Nutrition Examination Survey for 2005-2008, which collected data on reproductive history as well as sleep patterns. Roughly 20% of the women self-reported experiencing insomnia. Eighty-three percent of the 18- to 45-year-old women had been pregnant at least once, and 1,870 (40%) of them had one or more prior pregnancies that didn’t result in a live birth.
In a multivariate logistic regression analysis adjusted for age, race, education level, and frequency of sleep apnea symptoms, such as snoring or snorting/gasping, frequent difficulty in maintaining sleep was independently associated with an 85% increased risk of having experienced a pregnancy that didn’t result in a live birth, according to Dr. Nowakowski, a clinical psychologist in the department of ob.gyn. at the University of Texas, Galveston.
In an interview, she was quick to note that these are correlational, hypothesis-generating data, and that an epidemiologic study such as this can’t establish causality.
Dr. Nowakowski and her coinvestigators hope to conduct a prospective randomized trial of cognitive behavioral therapy for insomnia – a well-established treatment – in a group of women with prior spontaneous abortion, miscarriage, or other infertility issues, to determine whether insomnia is a modifiable risk factor for adverse pregnancy outcomes.
One physician at the meeting remarked that if women with fertility problems and insomnia knew of Dr. Nowakowski’s work showing an association between insomnia and unsuccessful pregnancies, they would be pounding down the doors of sleep specialists. Dr. Nowakowski agreed.
“I surf the online networks set up for infertile women. They’re very distraught over their trouble conceiving. They’re doing yoga, de-stress programs, taking all sorts of supplements – including melatonin – to try to improve their chances of fertility,” she said. “If insomnia turns out to partially account for the risk of having pregnancies that don’t result in live birth, and treating the insomnia reduces that risk, there would be a huge amount of patient interest.”
The study was supported by the National Institutes of Health. Dr. Nowakowski reported having no financial conflicts.
DENVER – Women who experience difficulty staying asleep are at increased risk of having one or more pregnancies that don’t result in a live birth, a large epidemiologic study suggests.
In contrast, other expressions of insomnia – difficulty in falling asleep, early morning awakening, or nonrestorative sleep – were not significantly associated with pregnancy loss in this analysis of a nationally representative sample comprised of 5,554 women aged 18-45 years, Sara Nowakowski, Ph.D., reported at the annual meeting of the Associated Professional Sleep Societies.
The women were participants in the National Health and Nutrition Examination Survey for 2005-2008, which collected data on reproductive history as well as sleep patterns. Roughly 20% of the women self-reported experiencing insomnia. Eighty-three percent of the 18- to 45-year-old women had been pregnant at least once, and 1,870 (40%) of them had one or more prior pregnancies that didn’t result in a live birth.
In a multivariate logistic regression analysis adjusted for age, race, education level, and frequency of sleep apnea symptoms, such as snoring or snorting/gasping, frequent difficulty in maintaining sleep was independently associated with an 85% increased risk of having experienced a pregnancy that didn’t result in a live birth, according to Dr. Nowakowski, a clinical psychologist in the department of ob.gyn. at the University of Texas, Galveston.
In an interview, she was quick to note that these are correlational, hypothesis-generating data, and that an epidemiologic study such as this can’t establish causality.
Dr. Nowakowski and her coinvestigators hope to conduct a prospective randomized trial of cognitive behavioral therapy for insomnia – a well-established treatment – in a group of women with prior spontaneous abortion, miscarriage, or other infertility issues, to determine whether insomnia is a modifiable risk factor for adverse pregnancy outcomes.
One physician at the meeting remarked that if women with fertility problems and insomnia knew of Dr. Nowakowski’s work showing an association between insomnia and unsuccessful pregnancies, they would be pounding down the doors of sleep specialists. Dr. Nowakowski agreed.
“I surf the online networks set up for infertile women. They’re very distraught over their trouble conceiving. They’re doing yoga, de-stress programs, taking all sorts of supplements – including melatonin – to try to improve their chances of fertility,” she said. “If insomnia turns out to partially account for the risk of having pregnancies that don’t result in live birth, and treating the insomnia reduces that risk, there would be a huge amount of patient interest.”
The study was supported by the National Institutes of Health. Dr. Nowakowski reported having no financial conflicts.
AT SLEEP 2016
Key clinical point: Insomnia may be a factor in some cases of poor pregnancy outcomes.
Major finding: Reproductive-age women who experienced difficulty staying asleep had an 85% greater likelihood of having a pregnancy that didn’t result in a live birth.
Data source: This epidemiologic study included 5,554 women aged 18-45 who provided details of their reproductive history and sleep status in the National Health and Nutrition Examination Survey.
Disclosures: This study was supported by the National Institutes of Health. Dr. Nowakowski reported having no financial conflicts.
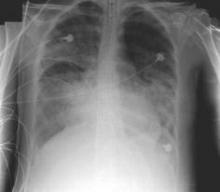
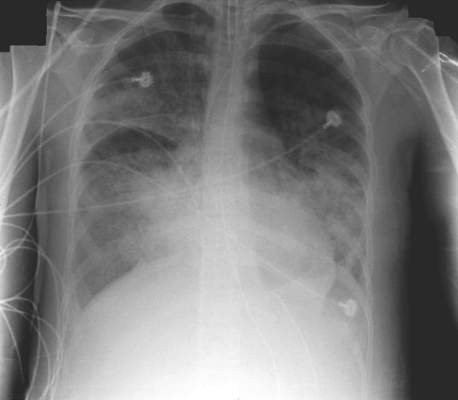
Ultrasound bests auscultation for ETT positioning
SAN DIEGO – Assessment of the trachea and pleura via point-of-care ultrasound is superior to auscultation in determining the exact location of the endotracheal tube, a randomized, single-center study found.
“It’s been reported that about 20% of the time the endotracheal tube is malpositioned,” study author Dr. Davinder S. Ramsingh said in an interview at the annual meeting of the American Society of Anesthesiologists. “Most of the time (the tube) is too deep, which can lead to severe complications.”
In a double-blinded, randomized study, Dr. Ramsingh and his associates assessed the accuracy of auscultation vs. point-of-care ultrasound in verifying the correct position of the endotracheal tube (ETT). They enrolled 42 adults who required general anesthesia with ETT and randomized them to right main bronchus, left main bronchus, or tracheal intubation, followed by fiber optically–guided visualization to place the ETT. Next, an anesthesiologist blinded to the ETT exact location used auscultation to assess the location of the ETT, while another anesthesiologist blinded to the ETT exact location used point-of-care ultrasound to assess the location of the ETT. The ultrasound exam consisted of assessing tracheal dilation via standard cuff inflation with air and evaluation of pleural lung sliding, explained Dr. Ramsingh of the department of anesthesiology and perioperative care at the University of California, Irvine.
Dr. Ramsingh reported that in differentiating tracheal versus bronchial intubations, auscultation demonstrated a sensitivity of 66% and a specificity of 59%, while ultrasound demonstrated a sensitivity of 93% and a specificity of 96%. Chi-square comparison showed a statistically significant improvement with ultrasound (P = .0005), while inter-observer agreement of the ultrasound findings was 100%.
Limitations of the study, he said, include the fact that “we don’t know the incidence of malpositioned endotracheal tubes in the operating room and that this study was evaluating patients undergoing elective surgical procedures.”
The researchers reported having no financial disclosures.
SAN DIEGO – Assessment of the trachea and pleura via point-of-care ultrasound is superior to auscultation in determining the exact location of the endotracheal tube, a randomized, single-center study found.
“It’s been reported that about 20% of the time the endotracheal tube is malpositioned,” study author Dr. Davinder S. Ramsingh said in an interview at the annual meeting of the American Society of Anesthesiologists. “Most of the time (the tube) is too deep, which can lead to severe complications.”
In a double-blinded, randomized study, Dr. Ramsingh and his associates assessed the accuracy of auscultation vs. point-of-care ultrasound in verifying the correct position of the endotracheal tube (ETT). They enrolled 42 adults who required general anesthesia with ETT and randomized them to right main bronchus, left main bronchus, or tracheal intubation, followed by fiber optically–guided visualization to place the ETT. Next, an anesthesiologist blinded to the ETT exact location used auscultation to assess the location of the ETT, while another anesthesiologist blinded to the ETT exact location used point-of-care ultrasound to assess the location of the ETT. The ultrasound exam consisted of assessing tracheal dilation via standard cuff inflation with air and evaluation of pleural lung sliding, explained Dr. Ramsingh of the department of anesthesiology and perioperative care at the University of California, Irvine.
Dr. Ramsingh reported that in differentiating tracheal versus bronchial intubations, auscultation demonstrated a sensitivity of 66% and a specificity of 59%, while ultrasound demonstrated a sensitivity of 93% and a specificity of 96%. Chi-square comparison showed a statistically significant improvement with ultrasound (P = .0005), while inter-observer agreement of the ultrasound findings was 100%.
Limitations of the study, he said, include the fact that “we don’t know the incidence of malpositioned endotracheal tubes in the operating room and that this study was evaluating patients undergoing elective surgical procedures.”
The researchers reported having no financial disclosures.
SAN DIEGO – Assessment of the trachea and pleura via point-of-care ultrasound is superior to auscultation in determining the exact location of the endotracheal tube, a randomized, single-center study found.
“It’s been reported that about 20% of the time the endotracheal tube is malpositioned,” study author Dr. Davinder S. Ramsingh said in an interview at the annual meeting of the American Society of Anesthesiologists. “Most of the time (the tube) is too deep, which can lead to severe complications.”
In a double-blinded, randomized study, Dr. Ramsingh and his associates assessed the accuracy of auscultation vs. point-of-care ultrasound in verifying the correct position of the endotracheal tube (ETT). They enrolled 42 adults who required general anesthesia with ETT and randomized them to right main bronchus, left main bronchus, or tracheal intubation, followed by fiber optically–guided visualization to place the ETT. Next, an anesthesiologist blinded to the ETT exact location used auscultation to assess the location of the ETT, while another anesthesiologist blinded to the ETT exact location used point-of-care ultrasound to assess the location of the ETT. The ultrasound exam consisted of assessing tracheal dilation via standard cuff inflation with air and evaluation of pleural lung sliding, explained Dr. Ramsingh of the department of anesthesiology and perioperative care at the University of California, Irvine.
Dr. Ramsingh reported that in differentiating tracheal versus bronchial intubations, auscultation demonstrated a sensitivity of 66% and a specificity of 59%, while ultrasound demonstrated a sensitivity of 93% and a specificity of 96%. Chi-square comparison showed a statistically significant improvement with ultrasound (P = .0005), while inter-observer agreement of the ultrasound findings was 100%.
Limitations of the study, he said, include the fact that “we don’t know the incidence of malpositioned endotracheal tubes in the operating room and that this study was evaluating patients undergoing elective surgical procedures.”
The researchers reported having no financial disclosures.
AT THE ASA ANNUAL MEETING
Key clinical point: Using point-of-care ultrasound was superior to auscultation in determining the exact location of the endotracheal tube.
Major finding: In differentiating tracheal versus bronchial intubations, auscultation demonstrated a sensitivity of 66% and a specificity of 59%, while ultrasound demonstrated a sensitivity of 93% and a specificity of 96%.
Data source: An randomized study of 42 adults who required general anesthesia with ETT.
Disclosures: The researchers reported having no financial disclosures.
Exercise improves sleep and may improve cognitive/physical function in MS
NATIONAL HARBOR, MD. – A pair of studies by the same research team has clarified how poor sleep worsens cognitive and physical function in people with multiple sclerosis (MS) and how poor sleep can be improved by exercise.
Whether the better sleep directly relates to the cognitive and physical improvements was not shown conclusively. However, a link between exercise and transient cognitive improvement has been demonstrated by others.
“Exercise may be a nonpharmacological and an inexpensive method to address sleep symptoms,” said Catherine Siengsukon, Ph.D., of the University of Kansas Medical Center, Kansas City, at the annual meeting of the Consortium of Multiple Sclerosis Centers.
About half of all people with MS experience poor sleep that results from the disease itself, medications, anxiety/depression, or other causes. The fatigue and reduced physical and psychological function diminish the quality of life and can increase the risk of mortality. “But it is unknown if poor sleep quality may impact physical function in individuals with MS,” said Dr. Siengsukon.
In healthy individuals, cognitive aspects like attention, working and long-term memory, information processing, decision making, and problem solving can all be affected by poor sleep. “But which cognitive domains are associated with poor sleep quality in people with MS is unknown,” said Dr. Siengsukon.
In the first study, 40 people (36 females) with MS (mainly relapsing-remitting MS) were analyzed through a battery of established tests of sleep quality, cognitive function, physical function, depression, anxiety, and quality of life. The subjects had a disease duration of about 12 years. All were ambulatory without the need of assistance, and none had sleep apnea.
About 68% of the subjects were considered poor sleepers with the remainder being good sleepers. They were comparable in age, sex, type of MS, disease duration, and cognitive impairment.
Compared with good sleepers, poor sleepers were significantly impaired in visuospatial memory and questionnaire-assessed physical function, were more fatigued, were more prone to be anxious and depressed, and had a worse quality of life. Independent factors of poor sleep quality included state and trait anxiety (P = .003 and .02, respectively).
“Evidence demonstrates that sleep consolidates memory. Therefore, poor sleep may selectively impair memory while not impacting other cognitive domains,” said Dr. Siengsukon.
In the second study, the influence of supervised, moderate exercise and home exercise on sleep quality was assessed in 22 other MS patients. Most had relapsing-remitting MS. The inclusion and exclusion criteria were similar to those for the first study, with additional exclusion criteria concerning cardiovascular risk of exercise.
The supervised stretching and exercise program for 12 subjects was done at a social center and utilized recumbent exercise machines, with the home-based program for 10 subjects consisting of stretching and outdoor walking. Both exercise programs were done three times weekly for 12 weeks.
Both exercise programs were beneficial in improving sleep, with the moderate-intensity program being relatively more effective than home-based exercise in two measurement scales of sleep. The greater benefit of moderate exercise might reflect the mode of exercise, with subjects feeling safer and more relaxed using a recumbent exerciser, Dr. Siengsukon said. Offering the exercise in a social setting might have been another plus.
“The results suggest that moderate-intensity exercise may improve cardiovascular fitness in people with MS. While both groups experienced moderate to large effects on sleep quality, the mechanism for improvement in sleep quality remains to be determined, as the improvement was not related to change in cardiorespiratory fitness,” said Dr. Siengsukon.
A link between treadmill exercise and transient cognitive improvement has been reported.
The studies were supported by the National Institutes of Health and the National Multiple Sclerosis Society. Dr. Siengsukon disclosed grant support from the National Multiple Sclerosis Society.
NATIONAL HARBOR, MD. – A pair of studies by the same research team has clarified how poor sleep worsens cognitive and physical function in people with multiple sclerosis (MS) and how poor sleep can be improved by exercise.
Whether the better sleep directly relates to the cognitive and physical improvements was not shown conclusively. However, a link between exercise and transient cognitive improvement has been demonstrated by others.
“Exercise may be a nonpharmacological and an inexpensive method to address sleep symptoms,” said Catherine Siengsukon, Ph.D., of the University of Kansas Medical Center, Kansas City, at the annual meeting of the Consortium of Multiple Sclerosis Centers.
About half of all people with MS experience poor sleep that results from the disease itself, medications, anxiety/depression, or other causes. The fatigue and reduced physical and psychological function diminish the quality of life and can increase the risk of mortality. “But it is unknown if poor sleep quality may impact physical function in individuals with MS,” said Dr. Siengsukon.
In healthy individuals, cognitive aspects like attention, working and long-term memory, information processing, decision making, and problem solving can all be affected by poor sleep. “But which cognitive domains are associated with poor sleep quality in people with MS is unknown,” said Dr. Siengsukon.
In the first study, 40 people (36 females) with MS (mainly relapsing-remitting MS) were analyzed through a battery of established tests of sleep quality, cognitive function, physical function, depression, anxiety, and quality of life. The subjects had a disease duration of about 12 years. All were ambulatory without the need of assistance, and none had sleep apnea.
About 68% of the subjects were considered poor sleepers with the remainder being good sleepers. They were comparable in age, sex, type of MS, disease duration, and cognitive impairment.
Compared with good sleepers, poor sleepers were significantly impaired in visuospatial memory and questionnaire-assessed physical function, were more fatigued, were more prone to be anxious and depressed, and had a worse quality of life. Independent factors of poor sleep quality included state and trait anxiety (P = .003 and .02, respectively).
“Evidence demonstrates that sleep consolidates memory. Therefore, poor sleep may selectively impair memory while not impacting other cognitive domains,” said Dr. Siengsukon.
In the second study, the influence of supervised, moderate exercise and home exercise on sleep quality was assessed in 22 other MS patients. Most had relapsing-remitting MS. The inclusion and exclusion criteria were similar to those for the first study, with additional exclusion criteria concerning cardiovascular risk of exercise.
The supervised stretching and exercise program for 12 subjects was done at a social center and utilized recumbent exercise machines, with the home-based program for 10 subjects consisting of stretching and outdoor walking. Both exercise programs were done three times weekly for 12 weeks.
Both exercise programs were beneficial in improving sleep, with the moderate-intensity program being relatively more effective than home-based exercise in two measurement scales of sleep. The greater benefit of moderate exercise might reflect the mode of exercise, with subjects feeling safer and more relaxed using a recumbent exerciser, Dr. Siengsukon said. Offering the exercise in a social setting might have been another plus.
“The results suggest that moderate-intensity exercise may improve cardiovascular fitness in people with MS. While both groups experienced moderate to large effects on sleep quality, the mechanism for improvement in sleep quality remains to be determined, as the improvement was not related to change in cardiorespiratory fitness,” said Dr. Siengsukon.
A link between treadmill exercise and transient cognitive improvement has been reported.
The studies were supported by the National Institutes of Health and the National Multiple Sclerosis Society. Dr. Siengsukon disclosed grant support from the National Multiple Sclerosis Society.
NATIONAL HARBOR, MD. – A pair of studies by the same research team has clarified how poor sleep worsens cognitive and physical function in people with multiple sclerosis (MS) and how poor sleep can be improved by exercise.
Whether the better sleep directly relates to the cognitive and physical improvements was not shown conclusively. However, a link between exercise and transient cognitive improvement has been demonstrated by others.
“Exercise may be a nonpharmacological and an inexpensive method to address sleep symptoms,” said Catherine Siengsukon, Ph.D., of the University of Kansas Medical Center, Kansas City, at the annual meeting of the Consortium of Multiple Sclerosis Centers.
About half of all people with MS experience poor sleep that results from the disease itself, medications, anxiety/depression, or other causes. The fatigue and reduced physical and psychological function diminish the quality of life and can increase the risk of mortality. “But it is unknown if poor sleep quality may impact physical function in individuals with MS,” said Dr. Siengsukon.
In healthy individuals, cognitive aspects like attention, working and long-term memory, information processing, decision making, and problem solving can all be affected by poor sleep. “But which cognitive domains are associated with poor sleep quality in people with MS is unknown,” said Dr. Siengsukon.
In the first study, 40 people (36 females) with MS (mainly relapsing-remitting MS) were analyzed through a battery of established tests of sleep quality, cognitive function, physical function, depression, anxiety, and quality of life. The subjects had a disease duration of about 12 years. All were ambulatory without the need of assistance, and none had sleep apnea.
About 68% of the subjects were considered poor sleepers with the remainder being good sleepers. They were comparable in age, sex, type of MS, disease duration, and cognitive impairment.
Compared with good sleepers, poor sleepers were significantly impaired in visuospatial memory and questionnaire-assessed physical function, were more fatigued, were more prone to be anxious and depressed, and had a worse quality of life. Independent factors of poor sleep quality included state and trait anxiety (P = .003 and .02, respectively).
“Evidence demonstrates that sleep consolidates memory. Therefore, poor sleep may selectively impair memory while not impacting other cognitive domains,” said Dr. Siengsukon.
In the second study, the influence of supervised, moderate exercise and home exercise on sleep quality was assessed in 22 other MS patients. Most had relapsing-remitting MS. The inclusion and exclusion criteria were similar to those for the first study, with additional exclusion criteria concerning cardiovascular risk of exercise.
The supervised stretching and exercise program for 12 subjects was done at a social center and utilized recumbent exercise machines, with the home-based program for 10 subjects consisting of stretching and outdoor walking. Both exercise programs were done three times weekly for 12 weeks.
Both exercise programs were beneficial in improving sleep, with the moderate-intensity program being relatively more effective than home-based exercise in two measurement scales of sleep. The greater benefit of moderate exercise might reflect the mode of exercise, with subjects feeling safer and more relaxed using a recumbent exerciser, Dr. Siengsukon said. Offering the exercise in a social setting might have been another plus.
“The results suggest that moderate-intensity exercise may improve cardiovascular fitness in people with MS. While both groups experienced moderate to large effects on sleep quality, the mechanism for improvement in sleep quality remains to be determined, as the improvement was not related to change in cardiorespiratory fitness,” said Dr. Siengsukon.
A link between treadmill exercise and transient cognitive improvement has been reported.
The studies were supported by the National Institutes of Health and the National Multiple Sclerosis Society. Dr. Siengsukon disclosed grant support from the National Multiple Sclerosis Society.
AT THE CMSC ANNUAL MEETING
Key clinical point: Moderate-intensity exercise may benefit MS patients in terms of improved sleep and perhaps improved cognitive and physical function.
Major finding: Exercise, especially a structured regimen of moderate exercise, improves sleep, and better-quality sleep improves visuospatial cognition and physical functioning in MS patients.
Data source: Two small exercise-intervention studies from the same research team.
Disclosures: Funded by the National Institutes of Health and the National Multiple Sclerosis Society. Dr. Siengsukon disclosed grant support from the National Multiple Sclerosis Society.
Vaccinations in certain combinations may slightly increase febrile seizure risk
There is an increased risk of febrile seizures when children receiving certain recommended vaccinations at the same time, but that risk is low, a study found.
Dr. Jonathan Duffy from the Immunization Safety Office of the Centers for Disease Control and Prevention and his colleagues followed up on a study that showed an increased risk of febrile seizures in children vaccinated with a trivalent inactivated influenza vaccine (IIV3) and 13-valent pneumococcal conjugate vaccine (PCV) at the same time during the 2010-2011 influenza season.
The investigators wanted to assess the effect of administering other common childhood vaccines with IIV3 on the risk for febrile seizures so they examined chart records of potential cases of febrile seizures in those aged 6-23 months from the Vaccine Safety Datalink. The data were collected between the 2006-2007 through 2010-2011 influenza seasons.
The search yielded 333 chart-confirmed cases of febrile seizures. To examine the safety of each recommended vaccination administered alone or in combination, the cases were divided into two groups, one to serve as a risk interval group (n = 103) for febrile seizures on days 0 to 1 postvaccination; and one control interval comparison group (n = 230) with febrile seizures 14-20 days postvaccination. The multivariable model used for the study indicated that IIV3, PCV, and DTaP-containing vaccines were most often associated with febrile seizures in the risk interval group, but that only PCV7 showed an independent increased risk of febrile seizures (incidence rate ratio, 1.98) after the model was adjusted to strip out concomitantly administered vaccines.
Although increased risks of febrile seizures were detected for these three combinations, the overall risk of febrile seizures was quite low, on the order of 10, 24, and 38 per 100,000 vaccinated children at 6, 12, and 15 months, respectively, for the triple concomitant administration in the risk interval.
The risk of febrile seizures also was higher after receiving three different combinations of concomitantly-administered vaccinations, IIV3 plus PCV (IRR, 3.50), IIV3 plus DTaP (IRR, 3.50), and IIV3 plus PCV plus DTaP (IRR, 5.00).
“Our results suggest that the risk of [febrile seizure] is increased after certain combinations of vaccines, but the absolute risk of [febrile seizure] after these combinations is small,” Dr. Duffy and his associates noted in Pediatrics (2016;138[1]:e20160320).
The U.S. Centers for Disease Control and Prevention funded the study. Dr. Naleway and Dr. Klein reported receiving research funding/support from multiple industry sources. The remaining authors reported no financial disclosures.
Concomitant administration of influenza, DTaP, and pneumococcal conjugate vaccine (PCV) vaccines was associated with febrile seizures at a rate of up to 30 in 100,000 children immunized. This would result in one child, at most, who would be expected to experience a febrile seizure caused by the concomitant administration of these vaccines in the first 2 years of life over a 5-10 year period in an average pediatric practice, based on a patient base including 1,000 children younger than 5 years of age, which would include 3-500 patients between 6 and 24 months of age annually.
Does this mean we should stop giving these vaccines together or stop giving them at all? We say, emphatically, no.
Febrile seizures, although frightening to parents, rarely have any long-term sequelae. The risk from these diseases far outweigh the risk from the vaccines.
This study, conducted by the Vaccine Safety Datalink, and others like it, are important as we engage in dialogue with parents about the risks and benefits of vaccines.
These comments are excerpted from a commentary by Dr. Mark H. Sawyer of the University of California, San Diego, department of pediatrics and Rady Children’s Hospital, also in San Diego. Dr. Geoff Simon of Nemours duPont Pediatrics, Wilmington, Del., and Dr. Carrie Byington of the department of pediatrics, University of Utah, Salt Lake City. Dr. Sawyer and Dr. Simon are members of and Dr. Byington is the chair of the American Academy of Pediatrics Committee on Infectious Disease. Dr. Byington has intellectual property in and receives royalties from BioFire Diagnostics; Dr. Sawyer and Dr. Simon indicated they have no financial relationships relevant to this article. Funded by the National Institutes of Health. (Pediatrics. 2016 Jun 6. doi: 10.1542/peds.2016-0976 ).
Concomitant administration of influenza, DTaP, and pneumococcal conjugate vaccine (PCV) vaccines was associated with febrile seizures at a rate of up to 30 in 100,000 children immunized. This would result in one child, at most, who would be expected to experience a febrile seizure caused by the concomitant administration of these vaccines in the first 2 years of life over a 5-10 year period in an average pediatric practice, based on a patient base including 1,000 children younger than 5 years of age, which would include 3-500 patients between 6 and 24 months of age annually.
Does this mean we should stop giving these vaccines together or stop giving them at all? We say, emphatically, no.
Febrile seizures, although frightening to parents, rarely have any long-term sequelae. The risk from these diseases far outweigh the risk from the vaccines.
This study, conducted by the Vaccine Safety Datalink, and others like it, are important as we engage in dialogue with parents about the risks and benefits of vaccines.
These comments are excerpted from a commentary by Dr. Mark H. Sawyer of the University of California, San Diego, department of pediatrics and Rady Children’s Hospital, also in San Diego. Dr. Geoff Simon of Nemours duPont Pediatrics, Wilmington, Del., and Dr. Carrie Byington of the department of pediatrics, University of Utah, Salt Lake City. Dr. Sawyer and Dr. Simon are members of and Dr. Byington is the chair of the American Academy of Pediatrics Committee on Infectious Disease. Dr. Byington has intellectual property in and receives royalties from BioFire Diagnostics; Dr. Sawyer and Dr. Simon indicated they have no financial relationships relevant to this article. Funded by the National Institutes of Health. (Pediatrics. 2016 Jun 6. doi: 10.1542/peds.2016-0976 ).
Concomitant administration of influenza, DTaP, and pneumococcal conjugate vaccine (PCV) vaccines was associated with febrile seizures at a rate of up to 30 in 100,000 children immunized. This would result in one child, at most, who would be expected to experience a febrile seizure caused by the concomitant administration of these vaccines in the first 2 years of life over a 5-10 year period in an average pediatric practice, based on a patient base including 1,000 children younger than 5 years of age, which would include 3-500 patients between 6 and 24 months of age annually.
Does this mean we should stop giving these vaccines together or stop giving them at all? We say, emphatically, no.
Febrile seizures, although frightening to parents, rarely have any long-term sequelae. The risk from these diseases far outweigh the risk from the vaccines.
This study, conducted by the Vaccine Safety Datalink, and others like it, are important as we engage in dialogue with parents about the risks and benefits of vaccines.
These comments are excerpted from a commentary by Dr. Mark H. Sawyer of the University of California, San Diego, department of pediatrics and Rady Children’s Hospital, also in San Diego. Dr. Geoff Simon of Nemours duPont Pediatrics, Wilmington, Del., and Dr. Carrie Byington of the department of pediatrics, University of Utah, Salt Lake City. Dr. Sawyer and Dr. Simon are members of and Dr. Byington is the chair of the American Academy of Pediatrics Committee on Infectious Disease. Dr. Byington has intellectual property in and receives royalties from BioFire Diagnostics; Dr. Sawyer and Dr. Simon indicated they have no financial relationships relevant to this article. Funded by the National Institutes of Health. (Pediatrics. 2016 Jun 6. doi: 10.1542/peds.2016-0976 ).
There is an increased risk of febrile seizures when children receiving certain recommended vaccinations at the same time, but that risk is low, a study found.
Dr. Jonathan Duffy from the Immunization Safety Office of the Centers for Disease Control and Prevention and his colleagues followed up on a study that showed an increased risk of febrile seizures in children vaccinated with a trivalent inactivated influenza vaccine (IIV3) and 13-valent pneumococcal conjugate vaccine (PCV) at the same time during the 2010-2011 influenza season.
The investigators wanted to assess the effect of administering other common childhood vaccines with IIV3 on the risk for febrile seizures so they examined chart records of potential cases of febrile seizures in those aged 6-23 months from the Vaccine Safety Datalink. The data were collected between the 2006-2007 through 2010-2011 influenza seasons.
The search yielded 333 chart-confirmed cases of febrile seizures. To examine the safety of each recommended vaccination administered alone or in combination, the cases were divided into two groups, one to serve as a risk interval group (n = 103) for febrile seizures on days 0 to 1 postvaccination; and one control interval comparison group (n = 230) with febrile seizures 14-20 days postvaccination. The multivariable model used for the study indicated that IIV3, PCV, and DTaP-containing vaccines were most often associated with febrile seizures in the risk interval group, but that only PCV7 showed an independent increased risk of febrile seizures (incidence rate ratio, 1.98) after the model was adjusted to strip out concomitantly administered vaccines.
Although increased risks of febrile seizures were detected for these three combinations, the overall risk of febrile seizures was quite low, on the order of 10, 24, and 38 per 100,000 vaccinated children at 6, 12, and 15 months, respectively, for the triple concomitant administration in the risk interval.
The risk of febrile seizures also was higher after receiving three different combinations of concomitantly-administered vaccinations, IIV3 plus PCV (IRR, 3.50), IIV3 plus DTaP (IRR, 3.50), and IIV3 plus PCV plus DTaP (IRR, 5.00).
“Our results suggest that the risk of [febrile seizure] is increased after certain combinations of vaccines, but the absolute risk of [febrile seizure] after these combinations is small,” Dr. Duffy and his associates noted in Pediatrics (2016;138[1]:e20160320).
The U.S. Centers for Disease Control and Prevention funded the study. Dr. Naleway and Dr. Klein reported receiving research funding/support from multiple industry sources. The remaining authors reported no financial disclosures.
There is an increased risk of febrile seizures when children receiving certain recommended vaccinations at the same time, but that risk is low, a study found.
Dr. Jonathan Duffy from the Immunization Safety Office of the Centers for Disease Control and Prevention and his colleagues followed up on a study that showed an increased risk of febrile seizures in children vaccinated with a trivalent inactivated influenza vaccine (IIV3) and 13-valent pneumococcal conjugate vaccine (PCV) at the same time during the 2010-2011 influenza season.
The investigators wanted to assess the effect of administering other common childhood vaccines with IIV3 on the risk for febrile seizures so they examined chart records of potential cases of febrile seizures in those aged 6-23 months from the Vaccine Safety Datalink. The data were collected between the 2006-2007 through 2010-2011 influenza seasons.
The search yielded 333 chart-confirmed cases of febrile seizures. To examine the safety of each recommended vaccination administered alone or in combination, the cases were divided into two groups, one to serve as a risk interval group (n = 103) for febrile seizures on days 0 to 1 postvaccination; and one control interval comparison group (n = 230) with febrile seizures 14-20 days postvaccination. The multivariable model used for the study indicated that IIV3, PCV, and DTaP-containing vaccines were most often associated with febrile seizures in the risk interval group, but that only PCV7 showed an independent increased risk of febrile seizures (incidence rate ratio, 1.98) after the model was adjusted to strip out concomitantly administered vaccines.
Although increased risks of febrile seizures were detected for these three combinations, the overall risk of febrile seizures was quite low, on the order of 10, 24, and 38 per 100,000 vaccinated children at 6, 12, and 15 months, respectively, for the triple concomitant administration in the risk interval.
The risk of febrile seizures also was higher after receiving three different combinations of concomitantly-administered vaccinations, IIV3 plus PCV (IRR, 3.50), IIV3 plus DTaP (IRR, 3.50), and IIV3 plus PCV plus DTaP (IRR, 5.00).
“Our results suggest that the risk of [febrile seizure] is increased after certain combinations of vaccines, but the absolute risk of [febrile seizure] after these combinations is small,” Dr. Duffy and his associates noted in Pediatrics (2016;138[1]:e20160320).
The U.S. Centers for Disease Control and Prevention funded the study. Dr. Naleway and Dr. Klein reported receiving research funding/support from multiple industry sources. The remaining authors reported no financial disclosures.
FROM PEDIATRICS
Key clinical point: The concomitant administration of certain childhood vaccinations may slightly increase the risk of febrile seizures.
Major finding: Although the risk of febrile seizures in the population studied was low in general, it was higher for those receiving concomitantly-administered IIV3 plus PCV, IIV3 plus DTaP, and IIV3 plus PCV plus DTaP.
Data sources: Vaccine Safety Datalink repository of vaccine safety research and surveillance.
Disclosures: The Centers for Disease Control and Prevention funded the study. Dr. Naleway and Dr. Klein reported receiving research funding/support from multiple industry sources. The remaining authors reported no financial disclosures.
Like tobacco, recent marijuana use decreases exhaled nitric oxide
Recent marijuana use may have an effect similar to recent tobacco use with regard to decreased production of exhaled nitric oxide (eNO), but a very different effect on forced vital capacity (FVC), according to the results of a study published in Chest.
Dr. Stefania I. Papatheodorou from the Cyprus International Institute for Environmental and Public Health, Limassol, Cyprus, in association with the Harvard T. H. Chan School of Public Health, and her colleagues conducted a retrospective analysis of National Health and Nutrition Examination Survey (NHANES) data collected from 10,327 people aged 18-59 years between 2007 to 2012. Respondents completed a questionnaire on illicit drug use and were given a physical examination. Outcomes of interest for this study were eNO levels and pulmonary function measurements including the forced expiratory volume in 1 second (FEV1), FVC, the FEV1/FVC ratio, and the average forced expiratory flow during the mid (25%-75%) portion of the FVC (FEF25%-75%) (Chest. 2016 Jan 16. doi: 10.1016/j.chest.2015.12.033).
The population available for analysis included 4,797 people who reported never having used marijuana, and 4,084 who reported past use. The recent users were divided into two groups; 555 and 891 respondents who reported using marijuana 5-30 days and 0-4 days before their examinations, respectively. The study results from age-adjusted analyses standardized to the 2000 U.S. Census population indicated that both past and current users had significantly lower eNO levels (in parts per billion) than those that had never used (all P less than .001). The same analysis demonstrated that FEV1, FVC, and FEV1/FVC ratios were all higher in current and past users than in never users (all P less than .001).
Using a multivariable model adjusted for age, sex, race/ethnicity, height, education level, income, marital status, asthma, tobacco use in pack-years, smoking category, and body mass index, both the 0-4 day users and the 5-30 day users showed significantly decreased eNO levels, compared with never users (-7%, 95% confidence interval -12% to -2%, P = .03; -13%, CI -18% to -8%, P less than .001, respectively). Additionally, recent users in the 0-4 day group had significantly higher FEV1 measures than never users (89 mL, CI 29-150, P = 0.005), as well as lower FEV1/FVC ratios (-.02, CI –.03 to –.01, P = .003).
Regarding the broader implications of their study findings, Dr. Papatheodorou and colleagues stated, “Given that nitric oxide plays a role in inflammatory and immune defense pathways in the respiratory system and is a mediator of vasodilation in the pulmonary and systemic vasculature, it would be useful to further explore the associations between marijuana use and vascular and pulmonary function in randomized trials.”
Dr. Mary B. Rice received funding from the Institute for Environmental Sciences. The other authors reported no conflicts of interest.
Recent marijuana use may have an effect similar to recent tobacco use with regard to decreased production of exhaled nitric oxide (eNO), but a very different effect on forced vital capacity (FVC), according to the results of a study published in Chest.
Dr. Stefania I. Papatheodorou from the Cyprus International Institute for Environmental and Public Health, Limassol, Cyprus, in association with the Harvard T. H. Chan School of Public Health, and her colleagues conducted a retrospective analysis of National Health and Nutrition Examination Survey (NHANES) data collected from 10,327 people aged 18-59 years between 2007 to 2012. Respondents completed a questionnaire on illicit drug use and were given a physical examination. Outcomes of interest for this study were eNO levels and pulmonary function measurements including the forced expiratory volume in 1 second (FEV1), FVC, the FEV1/FVC ratio, and the average forced expiratory flow during the mid (25%-75%) portion of the FVC (FEF25%-75%) (Chest. 2016 Jan 16. doi: 10.1016/j.chest.2015.12.033).
The population available for analysis included 4,797 people who reported never having used marijuana, and 4,084 who reported past use. The recent users were divided into two groups; 555 and 891 respondents who reported using marijuana 5-30 days and 0-4 days before their examinations, respectively. The study results from age-adjusted analyses standardized to the 2000 U.S. Census population indicated that both past and current users had significantly lower eNO levels (in parts per billion) than those that had never used (all P less than .001). The same analysis demonstrated that FEV1, FVC, and FEV1/FVC ratios were all higher in current and past users than in never users (all P less than .001).
Using a multivariable model adjusted for age, sex, race/ethnicity, height, education level, income, marital status, asthma, tobacco use in pack-years, smoking category, and body mass index, both the 0-4 day users and the 5-30 day users showed significantly decreased eNO levels, compared with never users (-7%, 95% confidence interval -12% to -2%, P = .03; -13%, CI -18% to -8%, P less than .001, respectively). Additionally, recent users in the 0-4 day group had significantly higher FEV1 measures than never users (89 mL, CI 29-150, P = 0.005), as well as lower FEV1/FVC ratios (-.02, CI –.03 to –.01, P = .003).
Regarding the broader implications of their study findings, Dr. Papatheodorou and colleagues stated, “Given that nitric oxide plays a role in inflammatory and immune defense pathways in the respiratory system and is a mediator of vasodilation in the pulmonary and systemic vasculature, it would be useful to further explore the associations between marijuana use and vascular and pulmonary function in randomized trials.”
Dr. Mary B. Rice received funding from the Institute for Environmental Sciences. The other authors reported no conflicts of interest.
Recent marijuana use may have an effect similar to recent tobacco use with regard to decreased production of exhaled nitric oxide (eNO), but a very different effect on forced vital capacity (FVC), according to the results of a study published in Chest.
Dr. Stefania I. Papatheodorou from the Cyprus International Institute for Environmental and Public Health, Limassol, Cyprus, in association with the Harvard T. H. Chan School of Public Health, and her colleagues conducted a retrospective analysis of National Health and Nutrition Examination Survey (NHANES) data collected from 10,327 people aged 18-59 years between 2007 to 2012. Respondents completed a questionnaire on illicit drug use and were given a physical examination. Outcomes of interest for this study were eNO levels and pulmonary function measurements including the forced expiratory volume in 1 second (FEV1), FVC, the FEV1/FVC ratio, and the average forced expiratory flow during the mid (25%-75%) portion of the FVC (FEF25%-75%) (Chest. 2016 Jan 16. doi: 10.1016/j.chest.2015.12.033).
The population available for analysis included 4,797 people who reported never having used marijuana, and 4,084 who reported past use. The recent users were divided into two groups; 555 and 891 respondents who reported using marijuana 5-30 days and 0-4 days before their examinations, respectively. The study results from age-adjusted analyses standardized to the 2000 U.S. Census population indicated that both past and current users had significantly lower eNO levels (in parts per billion) than those that had never used (all P less than .001). The same analysis demonstrated that FEV1, FVC, and FEV1/FVC ratios were all higher in current and past users than in never users (all P less than .001).
Using a multivariable model adjusted for age, sex, race/ethnicity, height, education level, income, marital status, asthma, tobacco use in pack-years, smoking category, and body mass index, both the 0-4 day users and the 5-30 day users showed significantly decreased eNO levels, compared with never users (-7%, 95% confidence interval -12% to -2%, P = .03; -13%, CI -18% to -8%, P less than .001, respectively). Additionally, recent users in the 0-4 day group had significantly higher FEV1 measures than never users (89 mL, CI 29-150, P = 0.005), as well as lower FEV1/FVC ratios (-.02, CI –.03 to –.01, P = .003).
Regarding the broader implications of their study findings, Dr. Papatheodorou and colleagues stated, “Given that nitric oxide plays a role in inflammatory and immune defense pathways in the respiratory system and is a mediator of vasodilation in the pulmonary and systemic vasculature, it would be useful to further explore the associations between marijuana use and vascular and pulmonary function in randomized trials.”
Dr. Mary B. Rice received funding from the Institute for Environmental Sciences. The other authors reported no conflicts of interest.
FROM CHEST
Key clinical point: Recent marijuana use can have acute negative effects on exhaled nitric oxide levels and aspects of pulmonary functioning.
Major finding: Current marijuana users showed significantly decreased eNO levels, compared with never users (-7% and -13% for those using within 0-4 days and within 5 to 30 days of examination, respectively).
Data sources: National Health and Nutrition Examination Survey (NHANES) data from 2007 to 2012
Disclosures: Dr. Mary B. Rice received funding from the Institute for Environmental Sciences. The other authors reported no conflicts of interest.
2015-2016 flu season slower and milder than past 3 years
The 2015-2016 flu season was less severe than the last three seasons, with a lower hospitalization rate and fewer pediatric deaths.
Cases of influenza appeared later in the season that typically seen, and activity didn’t peak until March, Stacy L. Davlin, Ph.D., wrote in the June 10 issue of the Morbidity and Mortality report (MMWR 2016; 22:567-75)
“During the most recent 18 influenza seasons, only two other seasons have peaked in March (2011-2012 and 2005-2006),” wrote Dr. Davlin, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta. This serves as a reminder that influenza can and does occur outside the traditionally expected season, and that clinicians shouldn’t discount the possibility of flu when a patient presents with typical symptoms.
“Although summer influenza activity in the United States typically is low, influenza cases and outbreaks have occurred during summer months, and clinicians should remain vigilant in considering influenza in the differential diagnosis of summer respiratory illnesses,” Dr. Davlin said.
The most common influenza virus of the last season was A(H1N1), which accounted for about half of cases in those aged 5-24 years, and about 70% of cases in those younger than 5 years and those 65 years and older.
Three novel viruses were seen as well: variants of A(H1N1), A(H1N2), and A(H3N2). The A(H1N1) variant occurred in a Minnesota resident who lived and worked in an area of swine farming, but who denied direct contact with pigs. The A(H3N2) variant occurred in a New Jersey resident who reported visiting a farm shortly before symptom onset. There was no evidence of human-to-human transmission. Both recovered fully without hospitalization. The A(H1N2) variant occurred in a Minnesota resident who was hospitalized but who recovered. This person was not interviewed so no possible source of infection was identified.
The CDC tested 2,408 viral specimens for susceptibility to antiviral medications. Among the 2,193 A(H1N1) specimens, less than 1% were resistant to oseltamivir and peramivir. All were susceptible to zanamivir. However, the testing found persistent high levels of resistant to amantadine and rimantadine in the A viruses. Amantadine is not effective on the B strains at all. Therefore, CDC does not recommend the use of amantadine as an anti-influenza medication.
Reports of influenza first exceeded the 2.1% baseline level in the week ending Dec. 26, 2015, according to the U.S. Outpatient Influenza-Like Illness Surveillance Network (ILINet). They remained elevated for the next 17 weeks, with a peak of 3.6% of all outpatient visits in the week ending March 12. From October 2015-April 2016, the overall hospitalization rate for influenza-like illness was 31 per 100,000. This was highest in those aged 65 years and older (85/100,000), and lowest in those aged 5-17 years (10/100,000). About 92% of adults hospitalized for flu-like illness had at least one underlying medical comorbidity, including obesity (42%), cardiovascular disease (40%), and metabolic disorders (38%). Almost half of children (48%) also had medical comorbidities, including asthma or other reactive airway disease (22%) and neurologic disorders (18%).
CDC’s National Center for Health Statistics Mortality Surveillance System found that the percentage of deaths attributed to pneumonia and influenza peaked at 8% during the week ending March 19. This is slightly lower than the death rate seen in the last 5 years, which ranged from 9% in 2011-2012 to 11% in 2012-2013.
Of this season’s deaths, 74 occurred in children. The mean and median ages of these patients were 7 years and 6 years, respectively; the range was 2 months-16 years. This total was lower than that recorded in any of the past three influenza seasons: 171 pediatric deaths in 2012-2013, 111 in 2013-2014, and 148 in 2014-2015.
Dr. Davlin also announced the Food and Drug Administration’s recommendations for composition of the 2016-2017 influenza vaccine.
Trivalent vaccines should contain an A/California/7/2009 (H1N1) pdm09-like virus, an A/Hong Kong/4801/2014 (H3N2)-like virus, and a B/Brisbane/60/2008-like virus (B/Victoria lineage). Quadrivalent vaccines, which have two influenza B viruses, should include the viruses recommended for the trivalent vaccines, as well as a B/Phuket/3073/2013-like virus (B/Yamagata lineage).
“The vaccine viruses recommended for inclusion in the 2016-2017 Northern Hemisphere influenza vaccines are the same vaccine viruses that were chosen for inclusion in 2016 Southern Hemisphere seasonal influenza vaccines,” Dr. Davlin noted. “These vaccine recommendations were based on a number of factors, including global influenza virologic and epidemiologic surveillance, genetic and antigenic characterization, antiviral susceptibility, and the availability of candidate vaccine viruses for production.”
As a CDC employee, Dr. Davlin had no financial disclosures.
On Twitter @Alz_Gal
The 2015-2016 flu season was less severe than the last three seasons, with a lower hospitalization rate and fewer pediatric deaths.
Cases of influenza appeared later in the season that typically seen, and activity didn’t peak until March, Stacy L. Davlin, Ph.D., wrote in the June 10 issue of the Morbidity and Mortality report (MMWR 2016; 22:567-75)
“During the most recent 18 influenza seasons, only two other seasons have peaked in March (2011-2012 and 2005-2006),” wrote Dr. Davlin, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta. This serves as a reminder that influenza can and does occur outside the traditionally expected season, and that clinicians shouldn’t discount the possibility of flu when a patient presents with typical symptoms.
“Although summer influenza activity in the United States typically is low, influenza cases and outbreaks have occurred during summer months, and clinicians should remain vigilant in considering influenza in the differential diagnosis of summer respiratory illnesses,” Dr. Davlin said.
The most common influenza virus of the last season was A(H1N1), which accounted for about half of cases in those aged 5-24 years, and about 70% of cases in those younger than 5 years and those 65 years and older.
Three novel viruses were seen as well: variants of A(H1N1), A(H1N2), and A(H3N2). The A(H1N1) variant occurred in a Minnesota resident who lived and worked in an area of swine farming, but who denied direct contact with pigs. The A(H3N2) variant occurred in a New Jersey resident who reported visiting a farm shortly before symptom onset. There was no evidence of human-to-human transmission. Both recovered fully without hospitalization. The A(H1N2) variant occurred in a Minnesota resident who was hospitalized but who recovered. This person was not interviewed so no possible source of infection was identified.
The CDC tested 2,408 viral specimens for susceptibility to antiviral medications. Among the 2,193 A(H1N1) specimens, less than 1% were resistant to oseltamivir and peramivir. All were susceptible to zanamivir. However, the testing found persistent high levels of resistant to amantadine and rimantadine in the A viruses. Amantadine is not effective on the B strains at all. Therefore, CDC does not recommend the use of amantadine as an anti-influenza medication.
Reports of influenza first exceeded the 2.1% baseline level in the week ending Dec. 26, 2015, according to the U.S. Outpatient Influenza-Like Illness Surveillance Network (ILINet). They remained elevated for the next 17 weeks, with a peak of 3.6% of all outpatient visits in the week ending March 12. From October 2015-April 2016, the overall hospitalization rate for influenza-like illness was 31 per 100,000. This was highest in those aged 65 years and older (85/100,000), and lowest in those aged 5-17 years (10/100,000). About 92% of adults hospitalized for flu-like illness had at least one underlying medical comorbidity, including obesity (42%), cardiovascular disease (40%), and metabolic disorders (38%). Almost half of children (48%) also had medical comorbidities, including asthma or other reactive airway disease (22%) and neurologic disorders (18%).
CDC’s National Center for Health Statistics Mortality Surveillance System found that the percentage of deaths attributed to pneumonia and influenza peaked at 8% during the week ending March 19. This is slightly lower than the death rate seen in the last 5 years, which ranged from 9% in 2011-2012 to 11% in 2012-2013.
Of this season’s deaths, 74 occurred in children. The mean and median ages of these patients were 7 years and 6 years, respectively; the range was 2 months-16 years. This total was lower than that recorded in any of the past three influenza seasons: 171 pediatric deaths in 2012-2013, 111 in 2013-2014, and 148 in 2014-2015.
Dr. Davlin also announced the Food and Drug Administration’s recommendations for composition of the 2016-2017 influenza vaccine.
Trivalent vaccines should contain an A/California/7/2009 (H1N1) pdm09-like virus, an A/Hong Kong/4801/2014 (H3N2)-like virus, and a B/Brisbane/60/2008-like virus (B/Victoria lineage). Quadrivalent vaccines, which have two influenza B viruses, should include the viruses recommended for the trivalent vaccines, as well as a B/Phuket/3073/2013-like virus (B/Yamagata lineage).
“The vaccine viruses recommended for inclusion in the 2016-2017 Northern Hemisphere influenza vaccines are the same vaccine viruses that were chosen for inclusion in 2016 Southern Hemisphere seasonal influenza vaccines,” Dr. Davlin noted. “These vaccine recommendations were based on a number of factors, including global influenza virologic and epidemiologic surveillance, genetic and antigenic characterization, antiviral susceptibility, and the availability of candidate vaccine viruses for production.”
As a CDC employee, Dr. Davlin had no financial disclosures.
On Twitter @Alz_Gal
The 2015-2016 flu season was less severe than the last three seasons, with a lower hospitalization rate and fewer pediatric deaths.
Cases of influenza appeared later in the season that typically seen, and activity didn’t peak until March, Stacy L. Davlin, Ph.D., wrote in the June 10 issue of the Morbidity and Mortality report (MMWR 2016; 22:567-75)
“During the most recent 18 influenza seasons, only two other seasons have peaked in March (2011-2012 and 2005-2006),” wrote Dr. Davlin, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta. This serves as a reminder that influenza can and does occur outside the traditionally expected season, and that clinicians shouldn’t discount the possibility of flu when a patient presents with typical symptoms.
“Although summer influenza activity in the United States typically is low, influenza cases and outbreaks have occurred during summer months, and clinicians should remain vigilant in considering influenza in the differential diagnosis of summer respiratory illnesses,” Dr. Davlin said.
The most common influenza virus of the last season was A(H1N1), which accounted for about half of cases in those aged 5-24 years, and about 70% of cases in those younger than 5 years and those 65 years and older.
Three novel viruses were seen as well: variants of A(H1N1), A(H1N2), and A(H3N2). The A(H1N1) variant occurred in a Minnesota resident who lived and worked in an area of swine farming, but who denied direct contact with pigs. The A(H3N2) variant occurred in a New Jersey resident who reported visiting a farm shortly before symptom onset. There was no evidence of human-to-human transmission. Both recovered fully without hospitalization. The A(H1N2) variant occurred in a Minnesota resident who was hospitalized but who recovered. This person was not interviewed so no possible source of infection was identified.
The CDC tested 2,408 viral specimens for susceptibility to antiviral medications. Among the 2,193 A(H1N1) specimens, less than 1% were resistant to oseltamivir and peramivir. All were susceptible to zanamivir. However, the testing found persistent high levels of resistant to amantadine and rimantadine in the A viruses. Amantadine is not effective on the B strains at all. Therefore, CDC does not recommend the use of amantadine as an anti-influenza medication.
Reports of influenza first exceeded the 2.1% baseline level in the week ending Dec. 26, 2015, according to the U.S. Outpatient Influenza-Like Illness Surveillance Network (ILINet). They remained elevated for the next 17 weeks, with a peak of 3.6% of all outpatient visits in the week ending March 12. From October 2015-April 2016, the overall hospitalization rate for influenza-like illness was 31 per 100,000. This was highest in those aged 65 years and older (85/100,000), and lowest in those aged 5-17 years (10/100,000). About 92% of adults hospitalized for flu-like illness had at least one underlying medical comorbidity, including obesity (42%), cardiovascular disease (40%), and metabolic disorders (38%). Almost half of children (48%) also had medical comorbidities, including asthma or other reactive airway disease (22%) and neurologic disorders (18%).
CDC’s National Center for Health Statistics Mortality Surveillance System found that the percentage of deaths attributed to pneumonia and influenza peaked at 8% during the week ending March 19. This is slightly lower than the death rate seen in the last 5 years, which ranged from 9% in 2011-2012 to 11% in 2012-2013.
Of this season’s deaths, 74 occurred in children. The mean and median ages of these patients were 7 years and 6 years, respectively; the range was 2 months-16 years. This total was lower than that recorded in any of the past three influenza seasons: 171 pediatric deaths in 2012-2013, 111 in 2013-2014, and 148 in 2014-2015.
Dr. Davlin also announced the Food and Drug Administration’s recommendations for composition of the 2016-2017 influenza vaccine.
Trivalent vaccines should contain an A/California/7/2009 (H1N1) pdm09-like virus, an A/Hong Kong/4801/2014 (H3N2)-like virus, and a B/Brisbane/60/2008-like virus (B/Victoria lineage). Quadrivalent vaccines, which have two influenza B viruses, should include the viruses recommended for the trivalent vaccines, as well as a B/Phuket/3073/2013-like virus (B/Yamagata lineage).
“The vaccine viruses recommended for inclusion in the 2016-2017 Northern Hemisphere influenza vaccines are the same vaccine viruses that were chosen for inclusion in 2016 Southern Hemisphere seasonal influenza vaccines,” Dr. Davlin noted. “These vaccine recommendations were based on a number of factors, including global influenza virologic and epidemiologic surveillance, genetic and antigenic characterization, antiviral susceptibility, and the availability of candidate vaccine viruses for production.”
As a CDC employee, Dr. Davlin had no financial disclosures.
On Twitter @Alz_Gal
FROM THE MMWR
Key clinical point: The last flu season peaked later, and killed fewer people than the last three seasons.
Major finding: The overall death rate was 31/100,000, with a peak of 8% occurring in March.
Data source: Numbers were drawn from CDC databases and other national influenza surveillance programs.
Disclosures: As a CDC employee, Dr. Davlin had no financial disclosures.