User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Maternal vaccination against pertussis can protect premature infants
Maternal immunization in the early third trimester (from 28 weeks’ gestation) may protect premature infants from pertussis, study results found.
This was the finding of an observational substudy of a larger multicenter, randomized, controlled vaccination trial of premature infants (the PUNS trial), which compared pertussis antibody concentrations before and after primary immunization in premature infants born to mothers who were or were not vaccinated with Repevax. Dr. Alison Kent of St George’s, University of London, and colleagues assessed the levels of the five vaccine antigens present in the maternal combination Repevax vaccine (pertussis toxoid, filamentous hemagglutinin, fimbriae types 2 and 3, diphtheria toxoid, tetanus toxoid, and inactivated poliovirus) in premature infants born to mothers who either received or did not receive Repevax from 28 weeks’ gestation. Antigen quantifications were conducted in these premature infants at approximately 2, 5, and 12 months of age.
Thirty-one (19%) of the 160 premature infants in the substudy were born to mothers who had been vaccinated. Two months after their premature birth, infants born to vaccinated mothers had significantly higher concentrations of all five measured antigens, compared with those born to unvaccinated mothers (all P values less than .001). Although fewer infants were sampled at 5 months of age, significantly higher concentrations of filamentous hemagglutinin and diphtheria toxoid were still found in those born to vaccinated mothers (both P = .003). Data collected at the 12-month assessment indicated that only tetanus antibody concentrations remained significantly higher in those born to vaccinated mothers (P = .015). A positive correlation between the number of days from maternal vaccination to delivery was found for all measured antigens, with the exception of fimbriae types 2 and 3.
“The emergency introduction of a maternal immunization program to control a national pertussis outbreak serendipitously provided an opportunity to assess antibody concentrations to maternal vaccine antigens in premature infants,” Dr. Kent and associates noted in Pediatrics (June 2016 doi: 10.1542/peds.2015-3854). This unexpected opportunity resulted in evidence supporting a protective effect against pertussis in the early lives of infants born prematurely to mothers immunized in their early third trimester.
Pfizer and the National Institute for Health Research Clinical Research Network funded the study. Professor Heath and Dr. Ladhani disclosed conducting studies on behalf of St George’s, University of London funded by vaccine manufacturers without receiving personal payments or travel support. The other authors reported no conflicts of interest.
Maternal immunization in the early third trimester (from 28 weeks’ gestation) may protect premature infants from pertussis, study results found.
This was the finding of an observational substudy of a larger multicenter, randomized, controlled vaccination trial of premature infants (the PUNS trial), which compared pertussis antibody concentrations before and after primary immunization in premature infants born to mothers who were or were not vaccinated with Repevax. Dr. Alison Kent of St George’s, University of London, and colleagues assessed the levels of the five vaccine antigens present in the maternal combination Repevax vaccine (pertussis toxoid, filamentous hemagglutinin, fimbriae types 2 and 3, diphtheria toxoid, tetanus toxoid, and inactivated poliovirus) in premature infants born to mothers who either received or did not receive Repevax from 28 weeks’ gestation. Antigen quantifications were conducted in these premature infants at approximately 2, 5, and 12 months of age.
Thirty-one (19%) of the 160 premature infants in the substudy were born to mothers who had been vaccinated. Two months after their premature birth, infants born to vaccinated mothers had significantly higher concentrations of all five measured antigens, compared with those born to unvaccinated mothers (all P values less than .001). Although fewer infants were sampled at 5 months of age, significantly higher concentrations of filamentous hemagglutinin and diphtheria toxoid were still found in those born to vaccinated mothers (both P = .003). Data collected at the 12-month assessment indicated that only tetanus antibody concentrations remained significantly higher in those born to vaccinated mothers (P = .015). A positive correlation between the number of days from maternal vaccination to delivery was found for all measured antigens, with the exception of fimbriae types 2 and 3.
“The emergency introduction of a maternal immunization program to control a national pertussis outbreak serendipitously provided an opportunity to assess antibody concentrations to maternal vaccine antigens in premature infants,” Dr. Kent and associates noted in Pediatrics (June 2016 doi: 10.1542/peds.2015-3854). This unexpected opportunity resulted in evidence supporting a protective effect against pertussis in the early lives of infants born prematurely to mothers immunized in their early third trimester.
Pfizer and the National Institute for Health Research Clinical Research Network funded the study. Professor Heath and Dr. Ladhani disclosed conducting studies on behalf of St George’s, University of London funded by vaccine manufacturers without receiving personal payments or travel support. The other authors reported no conflicts of interest.
Maternal immunization in the early third trimester (from 28 weeks’ gestation) may protect premature infants from pertussis, study results found.
This was the finding of an observational substudy of a larger multicenter, randomized, controlled vaccination trial of premature infants (the PUNS trial), which compared pertussis antibody concentrations before and after primary immunization in premature infants born to mothers who were or were not vaccinated with Repevax. Dr. Alison Kent of St George’s, University of London, and colleagues assessed the levels of the five vaccine antigens present in the maternal combination Repevax vaccine (pertussis toxoid, filamentous hemagglutinin, fimbriae types 2 and 3, diphtheria toxoid, tetanus toxoid, and inactivated poliovirus) in premature infants born to mothers who either received or did not receive Repevax from 28 weeks’ gestation. Antigen quantifications were conducted in these premature infants at approximately 2, 5, and 12 months of age.
Thirty-one (19%) of the 160 premature infants in the substudy were born to mothers who had been vaccinated. Two months after their premature birth, infants born to vaccinated mothers had significantly higher concentrations of all five measured antigens, compared with those born to unvaccinated mothers (all P values less than .001). Although fewer infants were sampled at 5 months of age, significantly higher concentrations of filamentous hemagglutinin and diphtheria toxoid were still found in those born to vaccinated mothers (both P = .003). Data collected at the 12-month assessment indicated that only tetanus antibody concentrations remained significantly higher in those born to vaccinated mothers (P = .015). A positive correlation between the number of days from maternal vaccination to delivery was found for all measured antigens, with the exception of fimbriae types 2 and 3.
“The emergency introduction of a maternal immunization program to control a national pertussis outbreak serendipitously provided an opportunity to assess antibody concentrations to maternal vaccine antigens in premature infants,” Dr. Kent and associates noted in Pediatrics (June 2016 doi: 10.1542/peds.2015-3854). This unexpected opportunity resulted in evidence supporting a protective effect against pertussis in the early lives of infants born prematurely to mothers immunized in their early third trimester.
Pfizer and the National Institute for Health Research Clinical Research Network funded the study. Professor Heath and Dr. Ladhani disclosed conducting studies on behalf of St George’s, University of London funded by vaccine manufacturers without receiving personal payments or travel support. The other authors reported no conflicts of interest.
FROM PEDIATRICS
Key clinical point: Vaccination of pregnant women early in their third trimester may protect premature infants against pertussis.
Major finding: At 2 months after birth, infants born to vaccinated mothers displayed higher antibody concentrations to vaccine antigens than did infants born to unvaccinated mothers.
Data source: Premature infants born to mothers that did or did not receive Repevax from 28 weeks of pregnancy.
Disclosures: Pfizer Ltd. and the National Institute for Health Research Clinical Research Network funded the study. Professor Heath and Dr. Ladhani disclosed conducting studies on behalf of St George’s, University of London funded by vaccine manufacturers without receiving personal payments or travel support. The other authors reported no conflicts of interest.
E-cigarettes: How “safe” are they?
› Inform patients that e-cigarette vapors contain toxic substances, including the heavy metals lead, cadmium, and nickel. A
› Educate all patients—particularly young people and those who are pregnant or lactating—about the potential health risks of e-cigarettes. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Electronic cigarettes (e-cigarettes) have become increasingly popular over the last decade. Although they are perceived by many to be safer than traditional cigarettes, many of the devices still contain nicotine, and inhaling their vapors exposes users to toxic substances, including lead, cadmium, and nickel—heavy metals that are associated with significant health problems.1 (For more on how e-cigarettes work, see “Cigarettes vs e-cigarettes: How does the experience (and cost) compare?”)
In addition, many people use e-cigarettes as a means to stop smoking, but few who do so achieve abstinence.2,3 They frequently end up utilizing both, increasing their health risks by exposing themselves to the dangers of 2 products instead of one.1
Further complicating the issue is that the manufacture and distribution of e-cigarettes has not been well regulated. Without regulation, there is no way to know with certainty how much nicotine the devices contain and what else is in them.
Things, however, are changing. The Food and Drug Administration (FDA) recently announced that e-cigarettes and other tobacco products like cigars and hookahs will now be regulated in the same way the government regulates tobacco cigarettes and smokeless tobacco.4 The rule will not take effect immediately because companies requested time to comply, but once it is enacted, packaging will be required to list what the products contain, among other changes.
Keeping up on the latest information on e-cigarettes is now—and will continue to be—important as family physicians are increasingly asked about them. What follows is a review of what we know about their potential risks.
A nicotine system developed by a pharmacist
E-cigarettes, or electronic nicotine delivery systems, were patented in 2003 by a Chinese pharmacist.5 Since their introduction to North America and Europe in 2007, the devices have become known by over 400 different brand names.6 Consumption among adults doubled by 2012, and by 2014, about 4% of US adults used e-cigarettes every day or some days.7 Many of them are dual users of tobacco and electronic cigarettes. In fact, Jenkins and colleagues reports in this issue of JFP (see "E-cigarettes: Who's using them and why?") that over half of cigarette smokers (52%) in their study use e-cigarettes, usually to either lower their cigarette consumption or aid in smoking cessation. (Throughout this article, we will use “cigarettes” and “smoking” to refer to the use of traditional tobacco cigarettes.)
In addition to concern over an increase in use among the general population, there is significant concern about the increase in e-cigarette use among US middle and high school students.1,8,9 In 2015, e-cigarettes were the most commonly used smoking product among middle and high school students, with 620,000 middle school students and nearly 2.4 million high school students using the battery-powered devices in the past 30 days.10
Many factors have contributed to the growing popularity of e-cigarettes.
- Perceived safety. With tobacco’s dangers so thoroughly documented, many advertising campaigns tout e-cigarettes as less dangerous than conventional cigarettes in terms of their ability to cause cardiac and lung diseases and low birth weights. This is largely because e-cigarettes do not produce the combustion products of tar, ash, or carbon monoxide. In addition, many consumers are mistakenly less fearful about the nicotine added to many e-cigarettes.
- Expectation that it helps smokers quit. Many smokers view e-cigarettes as an aid to smoking cessation.6 In fact, testimonials of efficacy in tobacco cessation abound in promotional materials and on the Web, and e-cigarettes are recommended by some physicians as a means to quit or lessen smoking of tobacco cigarettes.11
- Wide availability and opportunities for use. The use of electronic nicotine delivery devices is sometimes permitted in places where smoking of conventional cigarettes is banned, although rules vary widely in different parts of the country. In addition, e-cigarettes are readily available for purchase on the Internet without age verification.
- Extensive advertising. There are increasing concerns that advertising campaigns unduly target adolescents, young adults, and women.12-155 In addition to advertising, the media and social influences play significant roles in young people’s experimentation with “vaping,” the term for inhaling electronic cigarette aerosols.14,15
- Regulation, legislation remain controversial. Currently, e-cigarettes are not required to be tested before marketing,16 but that may change with the FDA’s new regulations. The British National Public Health body, Public Health England, has documented public health benefits of e-cigarettes when used as a way to quit smoking, and provides evidence that the devices are less dangerous than traditional cigarettes.17 But this issue and public policy are the subject of ongoing debate. In 2015, the United Kingdom made it illegal to sell e-cigarettes or e-liquids to people younger than 18 years of age and urged child-proof packaging.
What’s “in” an e-cigarette—and are the ingredients toxic?
Because e-cigarettes are relatively new to the global marketplace, little research exists regarding the long-term effects and safety of their use, especially among habitual users.
Vapor/refills. E-liquids may contain a variety of substances because they have been largely unregulated, but they generally include some combination of nicotine, propylene glycol, glycerin, and flavorings. In fact, up to 7000 flavors are available,6 including such kid-friendly flavors as chocolate, cherry crush, and bubble gum.
When the refills do contain nicotine, users generally derive less of the substance from the electronic devices than they do from a conventional cigarette. Researchers found that individual puffs from an e-cigarette contained 0 to 35 µg nicotine per puff.1,18 Assuming an amount at the high end of the spectrum (30 µg nicotine), it would take about 30 puffs of an e-cigarette to derive the same amount of nicotine (1 mg) typically delivered by a conventional cigarette.
The chemical make-up of the vapor and the biologic effects on animal models have been investigated using 42 different liquid refills.19,20 All contained potentially harmful compounds, but the levels were within exposure limits authorized by the FDA. These potentially dangerous chemicals include the known toxins formaldehyde, acrolein, and hydrocarbons.20
An inflammatory response to the inhalation of the vapors was demonstrated in mouse lungs; exposure to e-cigarette aerosols reduced lung glutathione—an important enzyme in maintaining oxidation-reduction balance—to a degree similar to that of cigarette smoke exposure.20 Less of the enzyme facilitates increased pulmonary inflammation.
In addition, human lung cells release pro-inflammatory cytokines when exposed to e-cigarette aerosols.20 Other health risks include:
Harm to indoor air quality/secondhand exposure. Even though e-cigarettes do not emit smoke, bystanders are exposed to the aerosol or vapor exhaled by the user, and researchers have found varying levels of such substances as formaldehyde, acetaldehyde, isoprene, acetic acid, acetone, propanol, propylene glycol, and nicotine in the air. However, it is unclear at this time whether the ultra-fine particles in the e-cigarette vapor have health effects commensurate with the emissions of conventional cigarettes.1,21,22
Cartridge refill ingestion by children. Accidental nicotine poisonings, particularly among children drawn to the colors, flavors, and scents of the e-liquids, have been problematic. In 2014, for example, over 3500 exposures occurred and more than half of those were in children younger than 6 years of age. (Exposure is defined as contact with the substance in some way including ingestion, inhalation, absorption by the skin/eyes, etc; not all exposures are poisonings or overdoses).23 Although incidence has tapered off somewhat, the American Association of Poison Control Centers reports that there were 623 exposures across all age groups between January 1, 2016 and April 30, 2016.23
Environmental impact of discarded e-cigarettes. Discarded e-cigarettes filling our landfills is a new and emerging public health concern. Their batteries, as do all batteries, pollute the land and water and have the potential to leach lead into the environment.24 Similarly, incompletely used liquid cartridges and refills may contain nicotine and heavy metals, which add to these risks.24
Explosions. Fires and explosions have been documented with e-cigarette use, mostly due to malfunctioning lithium-ion batteries.25 Thermal injuries to the face and hands can be significant.
Heavy metals. The presence of lead, cadmium, and nickel in inhaled e-cigarette vapor is another area of significant concern, particularly for younger people who might have long-term exposure.1 All 3 heavy metals are known to be toxic to humans, and safe levels of inhalation have not been established.
Inhalation and/or ingestion of lead, in particular, can cause severe neurologic damage, especially to the developing brains of children.26 Lead also results in hematologic dysfunction. Because of the risks associated with inhalation of this heavy metal, the substance was removed from gasoline years ago.
Inhaled cadmium induces kidney, liver, bone, and respiratory tract pathology27 and can cause organ failure, hypertension, anemias, fractures, osteoporosis, and/or osteomalacia.28 And inhaling nickel produces an inflammatory pulmonary reaction.29
Pregnancy/lactation. Since no clear evidence exists on the safety of e-cigarette use during pregnancy, women should avoid exposure to these vapors during the entire perinatal period. Similarly, the effects of e-cigarettes on infants who are breastfeeding are not established. Pregnant and breastfeeding women should not replace cigarettes with e-cigarettes.30,31 For pregnant women who smoke, the US Preventive Services Task Force (USPSTF) advises using only behavioral methods to stop cigarette use.32 And until more information becomes available, exposing infants and young children to e-cigarette vapor during breastfeeding is not recommended.
On the flip side, without tobacco, tar, ash, or carbon monoxide, e-cigarettes may have some advantages when compared with the use of traditional cigarettes, but that has not been substantiated.
Cigarettes vs e-cigarettes: How does the experience (and cost) compare?
If you were to ask a smoker to describe how cigarette smoking compares to using e-cigarettes, he or she would probably tell you that while the process of drawing on an e-cigarette is similar to that of a conventional cigarette, the experience in terms of reaching that state of relaxation or getting that “smoker’s high” is not.
In fact, a recent national survey of current and former smokers found that more than three-quarters of current smokers (77%) rated e-cigarettes less satisfying than conventional cigarettes and stopped using them.1 “Being less harmful” was the most highly rated reason for continuing to use the devices among people who switched from conventional to e-cigarettes.
How do they work? E-cigarettes do not burn anything and users do not light them. E-cigarettes work in much the same way as a smoke or fog machine. They use battery power (usually a rechargeable lithium battery) to heat a solution—usually containing nicotine, flavorings, and other chemicals—to the point that it turns into vapor. Much of whatever substances are in the vapor enter the bloodstream through the buccal mucosa, rather than the lungs.
Devices typically have an on/off button or switch, an atomizer containing a heating coil, a battery, and an LED light, which is designed to simulate a burning cigarette. A sensor detects when a user takes a drag and activates the atomizer and light. Some of the devices can be charged with a USB cord.
Because e-cigarettes don’t burn anything, they don’t have any smoke. They also don’t have any tar, ash, carbon monoxide, or odor (except perhaps a faint, short-lived scent matching the flavor liquid chosen). But the issues of second-hand exposure and effects on air quality are still being investigated.
With over 500 brands available, devices generally fall into one of 3 categories:2
- Cigalikes: About the same size and shape of a conventional cigarette, these cigarette look-alikes may come pre-filled with about a day’s worth of liquid and then may be discarded, or they may be non-disposable and have a replaceable cartridge.
- eGo’s: Also known as "vape pens," these devices tend to be longer and wider than cigalikes, have a more powerful battery, and usually are refillable or have a replaceable cartridge.
- Mods: Short for “modules,” these “vaporizers” tend to be the largest and most expensive type of e-cigarette. They may be refilled with e-liquid or accept replaceable cartridges and have even more powerful batteries.
What do they cost? A pack of cigarettes (containing 20 cigarettes) costs anywhere from $5 to $14, depending on where one lives.3 The price of e-cigarette devices starts at about $8 and can climb higher than $100. A 5-pack of flavor cartridges or a refill tank of e-liquid (which may last as long as about 150 cigarettes) costs about $10 to $15.4
To put this in perspective, a pack-a-day smoker in New York might spend about $5000 a year on cigarettes ($14 per pack x 365 days in a year), whereas someone who uses an e-cigarette device ($10) plus a refill tank per week ($14 x 52 weeks per year) will spend about $740 a year. (The actual cost will be higher because atomizers or devices as a whole must be replaced periodically, with some lasting only days and others lasting weeks or months, depending largely on how often one uses them. Although the cost of atomizers ranges widely, many can be found for $3-$5.)
Of course, the difference between cigarettes and e-cigarettes will be less dramatic in states where cigarettes are cheaper.
References
1. Pechacek TF, Nayak P, Gregory KR, et al. The potential that electronic delivery systems can be a disruptive technology: results from a national survey. Nicotine Tob Res. 2016. Available at: http://ntr.oxfordjournals.org/content/early/2016/05/03/ntr.ntw102.abstract. Accessed May 13, 2016.
2. Center for Environmental Health. A smoking gun: cancer-causing chemicals in e-cigarettes. Available at: http://www.ceh.org/wp-content/uploads/CEH-2015-report_A-Smoking-Gun_-Cancer-Causing-Chemicals-in-E-Cigarettes_alt.pdf. Accessed May 11, 2016.
3. Holmes H. The price of being an American. What a pack of cigarettes costs, in every state. August 28, 2015. Available at: http://www.theawl.com/2015/08/what-a-pack-of-cigarettes-costs-in-every-state. Accessed May 11, 2016.
4. Blu. How much do e-cigs cost? E-cig & vapor cigarette prices. Available at: http://www.blucigs.com/much-e-cigs-cost/. Accessed May 13, 2016.
Don’t substitute one form of nicotine for another
The USPSTF has not determined the benefit-to-harm ratio of using e-cigarettes as a smoking cessation aid, but recommends prescribing behavioral techniques and/or pharmacologic alternatives instead.32 Because the devices have been promoted as an aid to smoking cessation, intention to quit using tobacco products is a reason often stated for utilizing e-cigarettes.2,33,34 Indeed, use of e-cigarettes is much more likely among those who already utilize tobacco products.35-37
At least one study reports that e-cigarettes have efficacy similar to nicotine patches in achieving smoking abstinence among smokers who want to quit.38 Former smokers who used e-cigarettes to quit smoking reported fewer withdrawal symptoms than those who used nicotine skin patches.39 In addition, former smokers were more likely to endorse e-cigarettes than nicotine patches as a tobacco cigarette cessation aid. Significant reduction in tobacco smoke exposure has been demonstrated in dual users of tobacco and electronic cigarettes;40,41 however, both of these nicotine delivery systems sustain nicotine addiction.
Despite many ongoing studies to determine if e-cigarettes are useful as a smoking cessation aid, the results vary widely and are inconclusive at this time.42
E-cigarettes do not increase long-term tobacco abstinence
Contrary to popular belief, research shows that e-cigarette use among smokers is not associated with long-term tobacco abstinence.1 E-cigarette users, however, may make more attempts to quit smoking compared with smokers not using them.43 In addition, even though there is some evidence that e-cigarettes help smokers reduce the number of cigarettes smoked per day, simply reducing the daily number of cigarettes does not equate with safety.44 Smoking just one to 4 cigarettes per day poses 3 times the risk of myocardial infarction and lung cancer compared with not smoking.44 And since many individuals continue to use traditional and electronic cigarettes, they end up in double jeopardy of toxicity through exposure to the dangers of both.
A gateway to other substances of abuse?
There is also fear that nicotine exposure via e-cigarettes, especially in young people, serves as a “gateway” to tobacco consumption and other substance abuses, and increases the risk for nicotine addiction.34 Such nicotine-induced effects are a result of changes in brain chemistry, and have been documented in humans and animals.34
These concerns about negative health consequences, combined with the fact that e-cigarettes are undocumented as a smoking cessation aid, add urgency to the need for legislative and regulatory actions that hopefully can curb all nicotine exposures, particularly for our nation’s youth. In the meantime, it is important for physicians to advise patients—and the public—about the risks of e-cigarettes and the importance of quitting all forms of nicotine inhalation because nicotine—regardless of how it is delivered—is still an addictive drug.
CORRESPONDENCE
Steven Lippmann, MD, University of Louisville School of Medicine, 401 E. Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
1. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129:1972-1986.
2. Vickerman KA, Carpenter KM, Altman T, et al. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787-1791.
3. Grana R, Popova L, Ling P. A longitudinal analysis of electronic cigarette use and smoking cessation. JAMA Int Med. 2014;174:812-813.
4. U.S. Food and Drug Administration. Vaporizers, e-cigarettes, and other electronic nicotine delivery systems (ENDS). Available at: http://www.fda.gov/TobaccoProducts/Labeling/ProductsIngredientsComponents/ucm456610.htm. Accessed May 12, 2016.
5. Grana R, Benowitz N, Glantz SA. Background paper on E-cigarettes (electronic nicotine delivery systems). Center for Tobacco Control Research and Education, University of California, San Francisco, a WHO Collaborating Center on Tobacco Control. Prepared for World Health Organization Tobacco Free Initiative. December 2013. Available at: http://pvw.escholarship.org/uc/item/13p2b72n. Accessed March 31, 2014.
6. Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23:iii3-iii9.
7. Electronic Cigarette Use Among Adults: United States, 2014. NCHStats: A blog of the National Center for Health Statistics. Available at: http://nchstats.com/2015/10/28/electronic-cigarette-use-among-adults-united-states-2014/. Accessed April 22, 2016.
8. Centers for Disease Control and Prevention. E-cigarette use more than doubles among U.S. middle and high school students from 2011-2012. Available at: http://www.cdc.gov/media/releases/2013/p0905-ecigarette-use.html. Accessed April 22, 2016.
9. Centers for Disease Control and Prevention. Notes from the field: electronic cigarette use among middle and high school students — United States, 2011-2012. MMWR Morb Mortal Wkly Rep. 2013;62:729-730.
10. Singh T, Arrazola RA, Corey CG, et al. Tobacco use among middle and high school students—United States, 2011-2015. MMWR Morb Mortal Wkly Rpt. 2016;65:361-367.
11. Kandra KL, Ranney LM, Lee JG, et al. Physicians’ attitudes and use of e-cigarettes as cessation devices, North Carolina, 2013. PloS One. 2014;9:e103462.
12. Schraufnagel DE. Electronic cigarettes: vulnerability of youth. Pediatr Allergy Immunol Pulmonol. 2015;28:2-6.
13. White J, Li J, Newcombe R, et al. Tripling use of electronic cigarettes among New Zealand adolescents between 2012 and 2014. J Adolesc Health. 2015;56:522-528.
14. Duke JC, Lee YO, Kim AE, et al. Exposure to electronic cigarette television advertisements among youth and young adults. Pediatrics. 2014;134:29-36.
15. Huang J, Kornfield R, Szczypka G, et al. A cross-sectional examination of marketing of electronic cigarettes on Twitter. Tob Control. 2014;23:iii26-iii30.
16. Rojewski AM, Coleman N, Toll BA. Position Statement: Emerging policy issues regarding electronic nicotine delivery systems: a need for regulation. Society of Behavioral Medicine. 2016. Available at: http://www.sbm.org/UserFiles/file/e-cig-statement_v2_lores.pdf. Accessed April 22, 2016.
17. McNeill A, Brose LS, Calder R, et al. E-cigarettes: an evidence update. A report commissioned by Public Health England. 2015. Available at: https://www.gov.uk/government/publications/e-cigarettes-an-evidence-update. Accessed April 22, 2016.
18. Goniewicz ML, Kuma T, Gawron M, et al. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15:158-166.
19. Varlet V, Farsalinos K, Augsburger M, et al. Toxicity assessment of refill liquids for electronic cigarettes. Int J Environ Res Public Health. 2015;12:4796-4815.
20. Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10:e0116732.
21. Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217:628-637.
22. Schripp T, Markewitz D, Uhde E, et al. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23:25-31.
23. The American Association of Poison Control Centers. E-cigarettes and liquid nicotine. Available at: http://www.aapcc.org/alerts/e-cigarettes/. Accessed May 12, 2016.
24. Krause MJ, Townsend TG. Hazardous waste status of discarded electronic cigarettes. Waste Manag. 2015;39:57-62.
25. U.S. Fire Administration. Electronic cigarette fires and explosions. October 2014. Available at: https://www.usfa.fema.gov/downloads/pdf/publications/electronic_cigarettes.pdf. Accessed May 17, 2016.
26. Skerfving S, Löfmark L, Lundh T, et al. Late effects of low blood lead concentrations in children on school performance and cognitive functions. Neurotoxicology. 2015;49:114-120.
27. Bernhoft RA. Cadmium toxicity and treatment. Scientific World Journal. 2013;394652.
28. Agency for Toxic Substances and Disease Registry. Case studies in environmental medicine (CSEM) Cadmium Toxicity. Available at: http://www.atsdr.cdc.gov/csem/cadmium/docs/cadmium.pdf. Accessed April 22, 2016.
29. Das KK, Buchner V. Effect of nickel exposure on peripheral tissues: role of oxidative stress in toxicity and possible protection by ascorbic acid. Rev Environ Health. 2007;22:157-173.
30. England LJ, Bunnell RE, Pechacek TF, et al. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am J Prev Med. 2015;49:286-293.
31. Suter MA, Mastrobattista J, Sachs M, et al. Is there evidence for potential harm of electronic cigarette use in pregnancy? Birth defects research. Birth Defects Res A Clin Mol Teratol. 2015;103:186-195.
32. U.S. Preventive Services Task Force. Draft Recommendation Statement. Tobacco smoking cessation in adults and pregnant women: behavioral and pharmacotherapy interventions. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement147/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1. Accessed March 22, 2016.
33. Peters EN, Harrell PT, Hendricks PS, et al. Electronic cigarettes in adults in outpatient substance use treatment: awareness, perceptions, use, and reasons for use. Am J Addict. 2015;24:233-239.
34. Kandel ER, Kandel DB. A molecular basis for nicotine as a gateway drug. N Engl J Med. 2014;371:932-943.
35. King BA, Patel R, Nguyen KH, et al. Trends in awareness and use of electronic cigarettes among US Adults, 2010-2013. Nicotine Tob Res. 2015;17:219-227.
36. McMillen RC, Gottlieb MA, Shaefer RM, et al. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;1195-1202.
37. Lee S, Grana RA, Glantz SA. Electronic cigarette use among Korean adolescents: a cross-sectional study of market penetration, dual use, and relationship to quit attempts and former smoking. J Adolesc Health. 2014;54:684-690.
38. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629-1637.
39. Nelson VA, Goniewicz ML, Beard E, et al. Comparison of the characteristics of long-term users of electronic cigarettes versus nicotine replacement therapy: a cross-sectional survey of English ex-smokers and current smokers. Drug Alcohol Depend. 2015;153:300-305.
40. Caponnetto P, Campagna D, Cibella F, et al. Efficiency and safety of an electronic cigarette (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8:e66317.
41. Polosa R, Caponnetto P, Morjaria JB, et al. Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health. 2011;11:786.
42. Malas M, van der Tempel J, Schwartz R, et al. Electronic cigarettes for smoking cessation: a systematic review. Nicotine Tob Res. 2016. [Epub ahead of print].
43. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
44. Bjartveit K, Tverdal A. Health consequences of smoking 1-4 cigarettes per day. Tob Control. 2005;14:315-320.
› Inform patients that e-cigarette vapors contain toxic substances, including the heavy metals lead, cadmium, and nickel. A
› Educate all patients—particularly young people and those who are pregnant or lactating—about the potential health risks of e-cigarettes. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Electronic cigarettes (e-cigarettes) have become increasingly popular over the last decade. Although they are perceived by many to be safer than traditional cigarettes, many of the devices still contain nicotine, and inhaling their vapors exposes users to toxic substances, including lead, cadmium, and nickel—heavy metals that are associated with significant health problems.1 (For more on how e-cigarettes work, see “Cigarettes vs e-cigarettes: How does the experience (and cost) compare?”)
In addition, many people use e-cigarettes as a means to stop smoking, but few who do so achieve abstinence.2,3 They frequently end up utilizing both, increasing their health risks by exposing themselves to the dangers of 2 products instead of one.1
Further complicating the issue is that the manufacture and distribution of e-cigarettes has not been well regulated. Without regulation, there is no way to know with certainty how much nicotine the devices contain and what else is in them.
Things, however, are changing. The Food and Drug Administration (FDA) recently announced that e-cigarettes and other tobacco products like cigars and hookahs will now be regulated in the same way the government regulates tobacco cigarettes and smokeless tobacco.4 The rule will not take effect immediately because companies requested time to comply, but once it is enacted, packaging will be required to list what the products contain, among other changes.
Keeping up on the latest information on e-cigarettes is now—and will continue to be—important as family physicians are increasingly asked about them. What follows is a review of what we know about their potential risks.
A nicotine system developed by a pharmacist
E-cigarettes, or electronic nicotine delivery systems, were patented in 2003 by a Chinese pharmacist.5 Since their introduction to North America and Europe in 2007, the devices have become known by over 400 different brand names.6 Consumption among adults doubled by 2012, and by 2014, about 4% of US adults used e-cigarettes every day or some days.7 Many of them are dual users of tobacco and electronic cigarettes. In fact, Jenkins and colleagues reports in this issue of JFP (see "E-cigarettes: Who's using them and why?") that over half of cigarette smokers (52%) in their study use e-cigarettes, usually to either lower their cigarette consumption or aid in smoking cessation. (Throughout this article, we will use “cigarettes” and “smoking” to refer to the use of traditional tobacco cigarettes.)
In addition to concern over an increase in use among the general population, there is significant concern about the increase in e-cigarette use among US middle and high school students.1,8,9 In 2015, e-cigarettes were the most commonly used smoking product among middle and high school students, with 620,000 middle school students and nearly 2.4 million high school students using the battery-powered devices in the past 30 days.10
Many factors have contributed to the growing popularity of e-cigarettes.
- Perceived safety. With tobacco’s dangers so thoroughly documented, many advertising campaigns tout e-cigarettes as less dangerous than conventional cigarettes in terms of their ability to cause cardiac and lung diseases and low birth weights. This is largely because e-cigarettes do not produce the combustion products of tar, ash, or carbon monoxide. In addition, many consumers are mistakenly less fearful about the nicotine added to many e-cigarettes.
- Expectation that it helps smokers quit. Many smokers view e-cigarettes as an aid to smoking cessation.6 In fact, testimonials of efficacy in tobacco cessation abound in promotional materials and on the Web, and e-cigarettes are recommended by some physicians as a means to quit or lessen smoking of tobacco cigarettes.11
- Wide availability and opportunities for use. The use of electronic nicotine delivery devices is sometimes permitted in places where smoking of conventional cigarettes is banned, although rules vary widely in different parts of the country. In addition, e-cigarettes are readily available for purchase on the Internet without age verification.
- Extensive advertising. There are increasing concerns that advertising campaigns unduly target adolescents, young adults, and women.12-155 In addition to advertising, the media and social influences play significant roles in young people’s experimentation with “vaping,” the term for inhaling electronic cigarette aerosols.14,15
- Regulation, legislation remain controversial. Currently, e-cigarettes are not required to be tested before marketing,16 but that may change with the FDA’s new regulations. The British National Public Health body, Public Health England, has documented public health benefits of e-cigarettes when used as a way to quit smoking, and provides evidence that the devices are less dangerous than traditional cigarettes.17 But this issue and public policy are the subject of ongoing debate. In 2015, the United Kingdom made it illegal to sell e-cigarettes or e-liquids to people younger than 18 years of age and urged child-proof packaging.
What’s “in” an e-cigarette—and are the ingredients toxic?
Because e-cigarettes are relatively new to the global marketplace, little research exists regarding the long-term effects and safety of their use, especially among habitual users.
Vapor/refills. E-liquids may contain a variety of substances because they have been largely unregulated, but they generally include some combination of nicotine, propylene glycol, glycerin, and flavorings. In fact, up to 7000 flavors are available,6 including such kid-friendly flavors as chocolate, cherry crush, and bubble gum.
When the refills do contain nicotine, users generally derive less of the substance from the electronic devices than they do from a conventional cigarette. Researchers found that individual puffs from an e-cigarette contained 0 to 35 µg nicotine per puff.1,18 Assuming an amount at the high end of the spectrum (30 µg nicotine), it would take about 30 puffs of an e-cigarette to derive the same amount of nicotine (1 mg) typically delivered by a conventional cigarette.
The chemical make-up of the vapor and the biologic effects on animal models have been investigated using 42 different liquid refills.19,20 All contained potentially harmful compounds, but the levels were within exposure limits authorized by the FDA. These potentially dangerous chemicals include the known toxins formaldehyde, acrolein, and hydrocarbons.20
An inflammatory response to the inhalation of the vapors was demonstrated in mouse lungs; exposure to e-cigarette aerosols reduced lung glutathione—an important enzyme in maintaining oxidation-reduction balance—to a degree similar to that of cigarette smoke exposure.20 Less of the enzyme facilitates increased pulmonary inflammation.
In addition, human lung cells release pro-inflammatory cytokines when exposed to e-cigarette aerosols.20 Other health risks include:
Harm to indoor air quality/secondhand exposure. Even though e-cigarettes do not emit smoke, bystanders are exposed to the aerosol or vapor exhaled by the user, and researchers have found varying levels of such substances as formaldehyde, acetaldehyde, isoprene, acetic acid, acetone, propanol, propylene glycol, and nicotine in the air. However, it is unclear at this time whether the ultra-fine particles in the e-cigarette vapor have health effects commensurate with the emissions of conventional cigarettes.1,21,22
Cartridge refill ingestion by children. Accidental nicotine poisonings, particularly among children drawn to the colors, flavors, and scents of the e-liquids, have been problematic. In 2014, for example, over 3500 exposures occurred and more than half of those were in children younger than 6 years of age. (Exposure is defined as contact with the substance in some way including ingestion, inhalation, absorption by the skin/eyes, etc; not all exposures are poisonings or overdoses).23 Although incidence has tapered off somewhat, the American Association of Poison Control Centers reports that there were 623 exposures across all age groups between January 1, 2016 and April 30, 2016.23
Environmental impact of discarded e-cigarettes. Discarded e-cigarettes filling our landfills is a new and emerging public health concern. Their batteries, as do all batteries, pollute the land and water and have the potential to leach lead into the environment.24 Similarly, incompletely used liquid cartridges and refills may contain nicotine and heavy metals, which add to these risks.24
Explosions. Fires and explosions have been documented with e-cigarette use, mostly due to malfunctioning lithium-ion batteries.25 Thermal injuries to the face and hands can be significant.
Heavy metals. The presence of lead, cadmium, and nickel in inhaled e-cigarette vapor is another area of significant concern, particularly for younger people who might have long-term exposure.1 All 3 heavy metals are known to be toxic to humans, and safe levels of inhalation have not been established.
Inhalation and/or ingestion of lead, in particular, can cause severe neurologic damage, especially to the developing brains of children.26 Lead also results in hematologic dysfunction. Because of the risks associated with inhalation of this heavy metal, the substance was removed from gasoline years ago.
Inhaled cadmium induces kidney, liver, bone, and respiratory tract pathology27 and can cause organ failure, hypertension, anemias, fractures, osteoporosis, and/or osteomalacia.28 And inhaling nickel produces an inflammatory pulmonary reaction.29
Pregnancy/lactation. Since no clear evidence exists on the safety of e-cigarette use during pregnancy, women should avoid exposure to these vapors during the entire perinatal period. Similarly, the effects of e-cigarettes on infants who are breastfeeding are not established. Pregnant and breastfeeding women should not replace cigarettes with e-cigarettes.30,31 For pregnant women who smoke, the US Preventive Services Task Force (USPSTF) advises using only behavioral methods to stop cigarette use.32 And until more information becomes available, exposing infants and young children to e-cigarette vapor during breastfeeding is not recommended.
On the flip side, without tobacco, tar, ash, or carbon monoxide, e-cigarettes may have some advantages when compared with the use of traditional cigarettes, but that has not been substantiated.
Cigarettes vs e-cigarettes: How does the experience (and cost) compare?
If you were to ask a smoker to describe how cigarette smoking compares to using e-cigarettes, he or she would probably tell you that while the process of drawing on an e-cigarette is similar to that of a conventional cigarette, the experience in terms of reaching that state of relaxation or getting that “smoker’s high” is not.
In fact, a recent national survey of current and former smokers found that more than three-quarters of current smokers (77%) rated e-cigarettes less satisfying than conventional cigarettes and stopped using them.1 “Being less harmful” was the most highly rated reason for continuing to use the devices among people who switched from conventional to e-cigarettes.
How do they work? E-cigarettes do not burn anything and users do not light them. E-cigarettes work in much the same way as a smoke or fog machine. They use battery power (usually a rechargeable lithium battery) to heat a solution—usually containing nicotine, flavorings, and other chemicals—to the point that it turns into vapor. Much of whatever substances are in the vapor enter the bloodstream through the buccal mucosa, rather than the lungs.
Devices typically have an on/off button or switch, an atomizer containing a heating coil, a battery, and an LED light, which is designed to simulate a burning cigarette. A sensor detects when a user takes a drag and activates the atomizer and light. Some of the devices can be charged with a USB cord.
Because e-cigarettes don’t burn anything, they don’t have any smoke. They also don’t have any tar, ash, carbon monoxide, or odor (except perhaps a faint, short-lived scent matching the flavor liquid chosen). But the issues of second-hand exposure and effects on air quality are still being investigated.
With over 500 brands available, devices generally fall into one of 3 categories:2
- Cigalikes: About the same size and shape of a conventional cigarette, these cigarette look-alikes may come pre-filled with about a day’s worth of liquid and then may be discarded, or they may be non-disposable and have a replaceable cartridge.
- eGo’s: Also known as "vape pens," these devices tend to be longer and wider than cigalikes, have a more powerful battery, and usually are refillable or have a replaceable cartridge.
- Mods: Short for “modules,” these “vaporizers” tend to be the largest and most expensive type of e-cigarette. They may be refilled with e-liquid or accept replaceable cartridges and have even more powerful batteries.
What do they cost? A pack of cigarettes (containing 20 cigarettes) costs anywhere from $5 to $14, depending on where one lives.3 The price of e-cigarette devices starts at about $8 and can climb higher than $100. A 5-pack of flavor cartridges or a refill tank of e-liquid (which may last as long as about 150 cigarettes) costs about $10 to $15.4
To put this in perspective, a pack-a-day smoker in New York might spend about $5000 a year on cigarettes ($14 per pack x 365 days in a year), whereas someone who uses an e-cigarette device ($10) plus a refill tank per week ($14 x 52 weeks per year) will spend about $740 a year. (The actual cost will be higher because atomizers or devices as a whole must be replaced periodically, with some lasting only days and others lasting weeks or months, depending largely on how often one uses them. Although the cost of atomizers ranges widely, many can be found for $3-$5.)
Of course, the difference between cigarettes and e-cigarettes will be less dramatic in states where cigarettes are cheaper.
References
1. Pechacek TF, Nayak P, Gregory KR, et al. The potential that electronic delivery systems can be a disruptive technology: results from a national survey. Nicotine Tob Res. 2016. Available at: http://ntr.oxfordjournals.org/content/early/2016/05/03/ntr.ntw102.abstract. Accessed May 13, 2016.
2. Center for Environmental Health. A smoking gun: cancer-causing chemicals in e-cigarettes. Available at: http://www.ceh.org/wp-content/uploads/CEH-2015-report_A-Smoking-Gun_-Cancer-Causing-Chemicals-in-E-Cigarettes_alt.pdf. Accessed May 11, 2016.
3. Holmes H. The price of being an American. What a pack of cigarettes costs, in every state. August 28, 2015. Available at: http://www.theawl.com/2015/08/what-a-pack-of-cigarettes-costs-in-every-state. Accessed May 11, 2016.
4. Blu. How much do e-cigs cost? E-cig & vapor cigarette prices. Available at: http://www.blucigs.com/much-e-cigs-cost/. Accessed May 13, 2016.
Don’t substitute one form of nicotine for another
The USPSTF has not determined the benefit-to-harm ratio of using e-cigarettes as a smoking cessation aid, but recommends prescribing behavioral techniques and/or pharmacologic alternatives instead.32 Because the devices have been promoted as an aid to smoking cessation, intention to quit using tobacco products is a reason often stated for utilizing e-cigarettes.2,33,34 Indeed, use of e-cigarettes is much more likely among those who already utilize tobacco products.35-37
At least one study reports that e-cigarettes have efficacy similar to nicotine patches in achieving smoking abstinence among smokers who want to quit.38 Former smokers who used e-cigarettes to quit smoking reported fewer withdrawal symptoms than those who used nicotine skin patches.39 In addition, former smokers were more likely to endorse e-cigarettes than nicotine patches as a tobacco cigarette cessation aid. Significant reduction in tobacco smoke exposure has been demonstrated in dual users of tobacco and electronic cigarettes;40,41 however, both of these nicotine delivery systems sustain nicotine addiction.
Despite many ongoing studies to determine if e-cigarettes are useful as a smoking cessation aid, the results vary widely and are inconclusive at this time.42
E-cigarettes do not increase long-term tobacco abstinence
Contrary to popular belief, research shows that e-cigarette use among smokers is not associated with long-term tobacco abstinence.1 E-cigarette users, however, may make more attempts to quit smoking compared with smokers not using them.43 In addition, even though there is some evidence that e-cigarettes help smokers reduce the number of cigarettes smoked per day, simply reducing the daily number of cigarettes does not equate with safety.44 Smoking just one to 4 cigarettes per day poses 3 times the risk of myocardial infarction and lung cancer compared with not smoking.44 And since many individuals continue to use traditional and electronic cigarettes, they end up in double jeopardy of toxicity through exposure to the dangers of both.
A gateway to other substances of abuse?
There is also fear that nicotine exposure via e-cigarettes, especially in young people, serves as a “gateway” to tobacco consumption and other substance abuses, and increases the risk for nicotine addiction.34 Such nicotine-induced effects are a result of changes in brain chemistry, and have been documented in humans and animals.34
These concerns about negative health consequences, combined with the fact that e-cigarettes are undocumented as a smoking cessation aid, add urgency to the need for legislative and regulatory actions that hopefully can curb all nicotine exposures, particularly for our nation’s youth. In the meantime, it is important for physicians to advise patients—and the public—about the risks of e-cigarettes and the importance of quitting all forms of nicotine inhalation because nicotine—regardless of how it is delivered—is still an addictive drug.
CORRESPONDENCE
Steven Lippmann, MD, University of Louisville School of Medicine, 401 E. Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
› Inform patients that e-cigarette vapors contain toxic substances, including the heavy metals lead, cadmium, and nickel. A
› Educate all patients—particularly young people and those who are pregnant or lactating—about the potential health risks of e-cigarettes. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Electronic cigarettes (e-cigarettes) have become increasingly popular over the last decade. Although they are perceived by many to be safer than traditional cigarettes, many of the devices still contain nicotine, and inhaling their vapors exposes users to toxic substances, including lead, cadmium, and nickel—heavy metals that are associated with significant health problems.1 (For more on how e-cigarettes work, see “Cigarettes vs e-cigarettes: How does the experience (and cost) compare?”)
In addition, many people use e-cigarettes as a means to stop smoking, but few who do so achieve abstinence.2,3 They frequently end up utilizing both, increasing their health risks by exposing themselves to the dangers of 2 products instead of one.1
Further complicating the issue is that the manufacture and distribution of e-cigarettes has not been well regulated. Without regulation, there is no way to know with certainty how much nicotine the devices contain and what else is in them.
Things, however, are changing. The Food and Drug Administration (FDA) recently announced that e-cigarettes and other tobacco products like cigars and hookahs will now be regulated in the same way the government regulates tobacco cigarettes and smokeless tobacco.4 The rule will not take effect immediately because companies requested time to comply, but once it is enacted, packaging will be required to list what the products contain, among other changes.
Keeping up on the latest information on e-cigarettes is now—and will continue to be—important as family physicians are increasingly asked about them. What follows is a review of what we know about their potential risks.
A nicotine system developed by a pharmacist
E-cigarettes, or electronic nicotine delivery systems, were patented in 2003 by a Chinese pharmacist.5 Since their introduction to North America and Europe in 2007, the devices have become known by over 400 different brand names.6 Consumption among adults doubled by 2012, and by 2014, about 4% of US adults used e-cigarettes every day or some days.7 Many of them are dual users of tobacco and electronic cigarettes. In fact, Jenkins and colleagues reports in this issue of JFP (see "E-cigarettes: Who's using them and why?") that over half of cigarette smokers (52%) in their study use e-cigarettes, usually to either lower their cigarette consumption or aid in smoking cessation. (Throughout this article, we will use “cigarettes” and “smoking” to refer to the use of traditional tobacco cigarettes.)
In addition to concern over an increase in use among the general population, there is significant concern about the increase in e-cigarette use among US middle and high school students.1,8,9 In 2015, e-cigarettes were the most commonly used smoking product among middle and high school students, with 620,000 middle school students and nearly 2.4 million high school students using the battery-powered devices in the past 30 days.10
Many factors have contributed to the growing popularity of e-cigarettes.
- Perceived safety. With tobacco’s dangers so thoroughly documented, many advertising campaigns tout e-cigarettes as less dangerous than conventional cigarettes in terms of their ability to cause cardiac and lung diseases and low birth weights. This is largely because e-cigarettes do not produce the combustion products of tar, ash, or carbon monoxide. In addition, many consumers are mistakenly less fearful about the nicotine added to many e-cigarettes.
- Expectation that it helps smokers quit. Many smokers view e-cigarettes as an aid to smoking cessation.6 In fact, testimonials of efficacy in tobacco cessation abound in promotional materials and on the Web, and e-cigarettes are recommended by some physicians as a means to quit or lessen smoking of tobacco cigarettes.11
- Wide availability and opportunities for use. The use of electronic nicotine delivery devices is sometimes permitted in places where smoking of conventional cigarettes is banned, although rules vary widely in different parts of the country. In addition, e-cigarettes are readily available for purchase on the Internet without age verification.
- Extensive advertising. There are increasing concerns that advertising campaigns unduly target adolescents, young adults, and women.12-155 In addition to advertising, the media and social influences play significant roles in young people’s experimentation with “vaping,” the term for inhaling electronic cigarette aerosols.14,15
- Regulation, legislation remain controversial. Currently, e-cigarettes are not required to be tested before marketing,16 but that may change with the FDA’s new regulations. The British National Public Health body, Public Health England, has documented public health benefits of e-cigarettes when used as a way to quit smoking, and provides evidence that the devices are less dangerous than traditional cigarettes.17 But this issue and public policy are the subject of ongoing debate. In 2015, the United Kingdom made it illegal to sell e-cigarettes or e-liquids to people younger than 18 years of age and urged child-proof packaging.
What’s “in” an e-cigarette—and are the ingredients toxic?
Because e-cigarettes are relatively new to the global marketplace, little research exists regarding the long-term effects and safety of their use, especially among habitual users.
Vapor/refills. E-liquids may contain a variety of substances because they have been largely unregulated, but they generally include some combination of nicotine, propylene glycol, glycerin, and flavorings. In fact, up to 7000 flavors are available,6 including such kid-friendly flavors as chocolate, cherry crush, and bubble gum.
When the refills do contain nicotine, users generally derive less of the substance from the electronic devices than they do from a conventional cigarette. Researchers found that individual puffs from an e-cigarette contained 0 to 35 µg nicotine per puff.1,18 Assuming an amount at the high end of the spectrum (30 µg nicotine), it would take about 30 puffs of an e-cigarette to derive the same amount of nicotine (1 mg) typically delivered by a conventional cigarette.
The chemical make-up of the vapor and the biologic effects on animal models have been investigated using 42 different liquid refills.19,20 All contained potentially harmful compounds, but the levels were within exposure limits authorized by the FDA. These potentially dangerous chemicals include the known toxins formaldehyde, acrolein, and hydrocarbons.20
An inflammatory response to the inhalation of the vapors was demonstrated in mouse lungs; exposure to e-cigarette aerosols reduced lung glutathione—an important enzyme in maintaining oxidation-reduction balance—to a degree similar to that of cigarette smoke exposure.20 Less of the enzyme facilitates increased pulmonary inflammation.
In addition, human lung cells release pro-inflammatory cytokines when exposed to e-cigarette aerosols.20 Other health risks include:
Harm to indoor air quality/secondhand exposure. Even though e-cigarettes do not emit smoke, bystanders are exposed to the aerosol or vapor exhaled by the user, and researchers have found varying levels of such substances as formaldehyde, acetaldehyde, isoprene, acetic acid, acetone, propanol, propylene glycol, and nicotine in the air. However, it is unclear at this time whether the ultra-fine particles in the e-cigarette vapor have health effects commensurate with the emissions of conventional cigarettes.1,21,22
Cartridge refill ingestion by children. Accidental nicotine poisonings, particularly among children drawn to the colors, flavors, and scents of the e-liquids, have been problematic. In 2014, for example, over 3500 exposures occurred and more than half of those were in children younger than 6 years of age. (Exposure is defined as contact with the substance in some way including ingestion, inhalation, absorption by the skin/eyes, etc; not all exposures are poisonings or overdoses).23 Although incidence has tapered off somewhat, the American Association of Poison Control Centers reports that there were 623 exposures across all age groups between January 1, 2016 and April 30, 2016.23
Environmental impact of discarded e-cigarettes. Discarded e-cigarettes filling our landfills is a new and emerging public health concern. Their batteries, as do all batteries, pollute the land and water and have the potential to leach lead into the environment.24 Similarly, incompletely used liquid cartridges and refills may contain nicotine and heavy metals, which add to these risks.24
Explosions. Fires and explosions have been documented with e-cigarette use, mostly due to malfunctioning lithium-ion batteries.25 Thermal injuries to the face and hands can be significant.
Heavy metals. The presence of lead, cadmium, and nickel in inhaled e-cigarette vapor is another area of significant concern, particularly for younger people who might have long-term exposure.1 All 3 heavy metals are known to be toxic to humans, and safe levels of inhalation have not been established.
Inhalation and/or ingestion of lead, in particular, can cause severe neurologic damage, especially to the developing brains of children.26 Lead also results in hematologic dysfunction. Because of the risks associated with inhalation of this heavy metal, the substance was removed from gasoline years ago.
Inhaled cadmium induces kidney, liver, bone, and respiratory tract pathology27 and can cause organ failure, hypertension, anemias, fractures, osteoporosis, and/or osteomalacia.28 And inhaling nickel produces an inflammatory pulmonary reaction.29
Pregnancy/lactation. Since no clear evidence exists on the safety of e-cigarette use during pregnancy, women should avoid exposure to these vapors during the entire perinatal period. Similarly, the effects of e-cigarettes on infants who are breastfeeding are not established. Pregnant and breastfeeding women should not replace cigarettes with e-cigarettes.30,31 For pregnant women who smoke, the US Preventive Services Task Force (USPSTF) advises using only behavioral methods to stop cigarette use.32 And until more information becomes available, exposing infants and young children to e-cigarette vapor during breastfeeding is not recommended.
On the flip side, without tobacco, tar, ash, or carbon monoxide, e-cigarettes may have some advantages when compared with the use of traditional cigarettes, but that has not been substantiated.
Cigarettes vs e-cigarettes: How does the experience (and cost) compare?
If you were to ask a smoker to describe how cigarette smoking compares to using e-cigarettes, he or she would probably tell you that while the process of drawing on an e-cigarette is similar to that of a conventional cigarette, the experience in terms of reaching that state of relaxation or getting that “smoker’s high” is not.
In fact, a recent national survey of current and former smokers found that more than three-quarters of current smokers (77%) rated e-cigarettes less satisfying than conventional cigarettes and stopped using them.1 “Being less harmful” was the most highly rated reason for continuing to use the devices among people who switched from conventional to e-cigarettes.
How do they work? E-cigarettes do not burn anything and users do not light them. E-cigarettes work in much the same way as a smoke or fog machine. They use battery power (usually a rechargeable lithium battery) to heat a solution—usually containing nicotine, flavorings, and other chemicals—to the point that it turns into vapor. Much of whatever substances are in the vapor enter the bloodstream through the buccal mucosa, rather than the lungs.
Devices typically have an on/off button or switch, an atomizer containing a heating coil, a battery, and an LED light, which is designed to simulate a burning cigarette. A sensor detects when a user takes a drag and activates the atomizer and light. Some of the devices can be charged with a USB cord.
Because e-cigarettes don’t burn anything, they don’t have any smoke. They also don’t have any tar, ash, carbon monoxide, or odor (except perhaps a faint, short-lived scent matching the flavor liquid chosen). But the issues of second-hand exposure and effects on air quality are still being investigated.
With over 500 brands available, devices generally fall into one of 3 categories:2
- Cigalikes: About the same size and shape of a conventional cigarette, these cigarette look-alikes may come pre-filled with about a day’s worth of liquid and then may be discarded, or they may be non-disposable and have a replaceable cartridge.
- eGo’s: Also known as "vape pens," these devices tend to be longer and wider than cigalikes, have a more powerful battery, and usually are refillable or have a replaceable cartridge.
- Mods: Short for “modules,” these “vaporizers” tend to be the largest and most expensive type of e-cigarette. They may be refilled with e-liquid or accept replaceable cartridges and have even more powerful batteries.
What do they cost? A pack of cigarettes (containing 20 cigarettes) costs anywhere from $5 to $14, depending on where one lives.3 The price of e-cigarette devices starts at about $8 and can climb higher than $100. A 5-pack of flavor cartridges or a refill tank of e-liquid (which may last as long as about 150 cigarettes) costs about $10 to $15.4
To put this in perspective, a pack-a-day smoker in New York might spend about $5000 a year on cigarettes ($14 per pack x 365 days in a year), whereas someone who uses an e-cigarette device ($10) plus a refill tank per week ($14 x 52 weeks per year) will spend about $740 a year. (The actual cost will be higher because atomizers or devices as a whole must be replaced periodically, with some lasting only days and others lasting weeks or months, depending largely on how often one uses them. Although the cost of atomizers ranges widely, many can be found for $3-$5.)
Of course, the difference between cigarettes and e-cigarettes will be less dramatic in states where cigarettes are cheaper.
References
1. Pechacek TF, Nayak P, Gregory KR, et al. The potential that electronic delivery systems can be a disruptive technology: results from a national survey. Nicotine Tob Res. 2016. Available at: http://ntr.oxfordjournals.org/content/early/2016/05/03/ntr.ntw102.abstract. Accessed May 13, 2016.
2. Center for Environmental Health. A smoking gun: cancer-causing chemicals in e-cigarettes. Available at: http://www.ceh.org/wp-content/uploads/CEH-2015-report_A-Smoking-Gun_-Cancer-Causing-Chemicals-in-E-Cigarettes_alt.pdf. Accessed May 11, 2016.
3. Holmes H. The price of being an American. What a pack of cigarettes costs, in every state. August 28, 2015. Available at: http://www.theawl.com/2015/08/what-a-pack-of-cigarettes-costs-in-every-state. Accessed May 11, 2016.
4. Blu. How much do e-cigs cost? E-cig & vapor cigarette prices. Available at: http://www.blucigs.com/much-e-cigs-cost/. Accessed May 13, 2016.
Don’t substitute one form of nicotine for another
The USPSTF has not determined the benefit-to-harm ratio of using e-cigarettes as a smoking cessation aid, but recommends prescribing behavioral techniques and/or pharmacologic alternatives instead.32 Because the devices have been promoted as an aid to smoking cessation, intention to quit using tobacco products is a reason often stated for utilizing e-cigarettes.2,33,34 Indeed, use of e-cigarettes is much more likely among those who already utilize tobacco products.35-37
At least one study reports that e-cigarettes have efficacy similar to nicotine patches in achieving smoking abstinence among smokers who want to quit.38 Former smokers who used e-cigarettes to quit smoking reported fewer withdrawal symptoms than those who used nicotine skin patches.39 In addition, former smokers were more likely to endorse e-cigarettes than nicotine patches as a tobacco cigarette cessation aid. Significant reduction in tobacco smoke exposure has been demonstrated in dual users of tobacco and electronic cigarettes;40,41 however, both of these nicotine delivery systems sustain nicotine addiction.
Despite many ongoing studies to determine if e-cigarettes are useful as a smoking cessation aid, the results vary widely and are inconclusive at this time.42
E-cigarettes do not increase long-term tobacco abstinence
Contrary to popular belief, research shows that e-cigarette use among smokers is not associated with long-term tobacco abstinence.1 E-cigarette users, however, may make more attempts to quit smoking compared with smokers not using them.43 In addition, even though there is some evidence that e-cigarettes help smokers reduce the number of cigarettes smoked per day, simply reducing the daily number of cigarettes does not equate with safety.44 Smoking just one to 4 cigarettes per day poses 3 times the risk of myocardial infarction and lung cancer compared with not smoking.44 And since many individuals continue to use traditional and electronic cigarettes, they end up in double jeopardy of toxicity through exposure to the dangers of both.
A gateway to other substances of abuse?
There is also fear that nicotine exposure via e-cigarettes, especially in young people, serves as a “gateway” to tobacco consumption and other substance abuses, and increases the risk for nicotine addiction.34 Such nicotine-induced effects are a result of changes in brain chemistry, and have been documented in humans and animals.34
These concerns about negative health consequences, combined with the fact that e-cigarettes are undocumented as a smoking cessation aid, add urgency to the need for legislative and regulatory actions that hopefully can curb all nicotine exposures, particularly for our nation’s youth. In the meantime, it is important for physicians to advise patients—and the public—about the risks of e-cigarettes and the importance of quitting all forms of nicotine inhalation because nicotine—regardless of how it is delivered—is still an addictive drug.
CORRESPONDENCE
Steven Lippmann, MD, University of Louisville School of Medicine, 401 E. Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
1. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129:1972-1986.
2. Vickerman KA, Carpenter KM, Altman T, et al. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787-1791.
3. Grana R, Popova L, Ling P. A longitudinal analysis of electronic cigarette use and smoking cessation. JAMA Int Med. 2014;174:812-813.
4. U.S. Food and Drug Administration. Vaporizers, e-cigarettes, and other electronic nicotine delivery systems (ENDS). Available at: http://www.fda.gov/TobaccoProducts/Labeling/ProductsIngredientsComponents/ucm456610.htm. Accessed May 12, 2016.
5. Grana R, Benowitz N, Glantz SA. Background paper on E-cigarettes (electronic nicotine delivery systems). Center for Tobacco Control Research and Education, University of California, San Francisco, a WHO Collaborating Center on Tobacco Control. Prepared for World Health Organization Tobacco Free Initiative. December 2013. Available at: http://pvw.escholarship.org/uc/item/13p2b72n. Accessed March 31, 2014.
6. Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23:iii3-iii9.
7. Electronic Cigarette Use Among Adults: United States, 2014. NCHStats: A blog of the National Center for Health Statistics. Available at: http://nchstats.com/2015/10/28/electronic-cigarette-use-among-adults-united-states-2014/. Accessed April 22, 2016.
8. Centers for Disease Control and Prevention. E-cigarette use more than doubles among U.S. middle and high school students from 2011-2012. Available at: http://www.cdc.gov/media/releases/2013/p0905-ecigarette-use.html. Accessed April 22, 2016.
9. Centers for Disease Control and Prevention. Notes from the field: electronic cigarette use among middle and high school students — United States, 2011-2012. MMWR Morb Mortal Wkly Rep. 2013;62:729-730.
10. Singh T, Arrazola RA, Corey CG, et al. Tobacco use among middle and high school students—United States, 2011-2015. MMWR Morb Mortal Wkly Rpt. 2016;65:361-367.
11. Kandra KL, Ranney LM, Lee JG, et al. Physicians’ attitudes and use of e-cigarettes as cessation devices, North Carolina, 2013. PloS One. 2014;9:e103462.
12. Schraufnagel DE. Electronic cigarettes: vulnerability of youth. Pediatr Allergy Immunol Pulmonol. 2015;28:2-6.
13. White J, Li J, Newcombe R, et al. Tripling use of electronic cigarettes among New Zealand adolescents between 2012 and 2014. J Adolesc Health. 2015;56:522-528.
14. Duke JC, Lee YO, Kim AE, et al. Exposure to electronic cigarette television advertisements among youth and young adults. Pediatrics. 2014;134:29-36.
15. Huang J, Kornfield R, Szczypka G, et al. A cross-sectional examination of marketing of electronic cigarettes on Twitter. Tob Control. 2014;23:iii26-iii30.
16. Rojewski AM, Coleman N, Toll BA. Position Statement: Emerging policy issues regarding electronic nicotine delivery systems: a need for regulation. Society of Behavioral Medicine. 2016. Available at: http://www.sbm.org/UserFiles/file/e-cig-statement_v2_lores.pdf. Accessed April 22, 2016.
17. McNeill A, Brose LS, Calder R, et al. E-cigarettes: an evidence update. A report commissioned by Public Health England. 2015. Available at: https://www.gov.uk/government/publications/e-cigarettes-an-evidence-update. Accessed April 22, 2016.
18. Goniewicz ML, Kuma T, Gawron M, et al. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15:158-166.
19. Varlet V, Farsalinos K, Augsburger M, et al. Toxicity assessment of refill liquids for electronic cigarettes. Int J Environ Res Public Health. 2015;12:4796-4815.
20. Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10:e0116732.
21. Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217:628-637.
22. Schripp T, Markewitz D, Uhde E, et al. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23:25-31.
23. The American Association of Poison Control Centers. E-cigarettes and liquid nicotine. Available at: http://www.aapcc.org/alerts/e-cigarettes/. Accessed May 12, 2016.
24. Krause MJ, Townsend TG. Hazardous waste status of discarded electronic cigarettes. Waste Manag. 2015;39:57-62.
25. U.S. Fire Administration. Electronic cigarette fires and explosions. October 2014. Available at: https://www.usfa.fema.gov/downloads/pdf/publications/electronic_cigarettes.pdf. Accessed May 17, 2016.
26. Skerfving S, Löfmark L, Lundh T, et al. Late effects of low blood lead concentrations in children on school performance and cognitive functions. Neurotoxicology. 2015;49:114-120.
27. Bernhoft RA. Cadmium toxicity and treatment. Scientific World Journal. 2013;394652.
28. Agency for Toxic Substances and Disease Registry. Case studies in environmental medicine (CSEM) Cadmium Toxicity. Available at: http://www.atsdr.cdc.gov/csem/cadmium/docs/cadmium.pdf. Accessed April 22, 2016.
29. Das KK, Buchner V. Effect of nickel exposure on peripheral tissues: role of oxidative stress in toxicity and possible protection by ascorbic acid. Rev Environ Health. 2007;22:157-173.
30. England LJ, Bunnell RE, Pechacek TF, et al. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am J Prev Med. 2015;49:286-293.
31. Suter MA, Mastrobattista J, Sachs M, et al. Is there evidence for potential harm of electronic cigarette use in pregnancy? Birth defects research. Birth Defects Res A Clin Mol Teratol. 2015;103:186-195.
32. U.S. Preventive Services Task Force. Draft Recommendation Statement. Tobacco smoking cessation in adults and pregnant women: behavioral and pharmacotherapy interventions. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement147/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1. Accessed March 22, 2016.
33. Peters EN, Harrell PT, Hendricks PS, et al. Electronic cigarettes in adults in outpatient substance use treatment: awareness, perceptions, use, and reasons for use. Am J Addict. 2015;24:233-239.
34. Kandel ER, Kandel DB. A molecular basis for nicotine as a gateway drug. N Engl J Med. 2014;371:932-943.
35. King BA, Patel R, Nguyen KH, et al. Trends in awareness and use of electronic cigarettes among US Adults, 2010-2013. Nicotine Tob Res. 2015;17:219-227.
36. McMillen RC, Gottlieb MA, Shaefer RM, et al. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;1195-1202.
37. Lee S, Grana RA, Glantz SA. Electronic cigarette use among Korean adolescents: a cross-sectional study of market penetration, dual use, and relationship to quit attempts and former smoking. J Adolesc Health. 2014;54:684-690.
38. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629-1637.
39. Nelson VA, Goniewicz ML, Beard E, et al. Comparison of the characteristics of long-term users of electronic cigarettes versus nicotine replacement therapy: a cross-sectional survey of English ex-smokers and current smokers. Drug Alcohol Depend. 2015;153:300-305.
40. Caponnetto P, Campagna D, Cibella F, et al. Efficiency and safety of an electronic cigarette (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8:e66317.
41. Polosa R, Caponnetto P, Morjaria JB, et al. Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health. 2011;11:786.
42. Malas M, van der Tempel J, Schwartz R, et al. Electronic cigarettes for smoking cessation: a systematic review. Nicotine Tob Res. 2016. [Epub ahead of print].
43. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
44. Bjartveit K, Tverdal A. Health consequences of smoking 1-4 cigarettes per day. Tob Control. 2005;14:315-320.
1. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129:1972-1986.
2. Vickerman KA, Carpenter KM, Altman T, et al. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787-1791.
3. Grana R, Popova L, Ling P. A longitudinal analysis of electronic cigarette use and smoking cessation. JAMA Int Med. 2014;174:812-813.
4. U.S. Food and Drug Administration. Vaporizers, e-cigarettes, and other electronic nicotine delivery systems (ENDS). Available at: http://www.fda.gov/TobaccoProducts/Labeling/ProductsIngredientsComponents/ucm456610.htm. Accessed May 12, 2016.
5. Grana R, Benowitz N, Glantz SA. Background paper on E-cigarettes (electronic nicotine delivery systems). Center for Tobacco Control Research and Education, University of California, San Francisco, a WHO Collaborating Center on Tobacco Control. Prepared for World Health Organization Tobacco Free Initiative. December 2013. Available at: http://pvw.escholarship.org/uc/item/13p2b72n. Accessed March 31, 2014.
6. Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23:iii3-iii9.
7. Electronic Cigarette Use Among Adults: United States, 2014. NCHStats: A blog of the National Center for Health Statistics. Available at: http://nchstats.com/2015/10/28/electronic-cigarette-use-among-adults-united-states-2014/. Accessed April 22, 2016.
8. Centers for Disease Control and Prevention. E-cigarette use more than doubles among U.S. middle and high school students from 2011-2012. Available at: http://www.cdc.gov/media/releases/2013/p0905-ecigarette-use.html. Accessed April 22, 2016.
9. Centers for Disease Control and Prevention. Notes from the field: electronic cigarette use among middle and high school students — United States, 2011-2012. MMWR Morb Mortal Wkly Rep. 2013;62:729-730.
10. Singh T, Arrazola RA, Corey CG, et al. Tobacco use among middle and high school students—United States, 2011-2015. MMWR Morb Mortal Wkly Rpt. 2016;65:361-367.
11. Kandra KL, Ranney LM, Lee JG, et al. Physicians’ attitudes and use of e-cigarettes as cessation devices, North Carolina, 2013. PloS One. 2014;9:e103462.
12. Schraufnagel DE. Electronic cigarettes: vulnerability of youth. Pediatr Allergy Immunol Pulmonol. 2015;28:2-6.
13. White J, Li J, Newcombe R, et al. Tripling use of electronic cigarettes among New Zealand adolescents between 2012 and 2014. J Adolesc Health. 2015;56:522-528.
14. Duke JC, Lee YO, Kim AE, et al. Exposure to electronic cigarette television advertisements among youth and young adults. Pediatrics. 2014;134:29-36.
15. Huang J, Kornfield R, Szczypka G, et al. A cross-sectional examination of marketing of electronic cigarettes on Twitter. Tob Control. 2014;23:iii26-iii30.
16. Rojewski AM, Coleman N, Toll BA. Position Statement: Emerging policy issues regarding electronic nicotine delivery systems: a need for regulation. Society of Behavioral Medicine. 2016. Available at: http://www.sbm.org/UserFiles/file/e-cig-statement_v2_lores.pdf. Accessed April 22, 2016.
17. McNeill A, Brose LS, Calder R, et al. E-cigarettes: an evidence update. A report commissioned by Public Health England. 2015. Available at: https://www.gov.uk/government/publications/e-cigarettes-an-evidence-update. Accessed April 22, 2016.
18. Goniewicz ML, Kuma T, Gawron M, et al. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15:158-166.
19. Varlet V, Farsalinos K, Augsburger M, et al. Toxicity assessment of refill liquids for electronic cigarettes. Int J Environ Res Public Health. 2015;12:4796-4815.
20. Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10:e0116732.
21. Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217:628-637.
22. Schripp T, Markewitz D, Uhde E, et al. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23:25-31.
23. The American Association of Poison Control Centers. E-cigarettes and liquid nicotine. Available at: http://www.aapcc.org/alerts/e-cigarettes/. Accessed May 12, 2016.
24. Krause MJ, Townsend TG. Hazardous waste status of discarded electronic cigarettes. Waste Manag. 2015;39:57-62.
25. U.S. Fire Administration. Electronic cigarette fires and explosions. October 2014. Available at: https://www.usfa.fema.gov/downloads/pdf/publications/electronic_cigarettes.pdf. Accessed May 17, 2016.
26. Skerfving S, Löfmark L, Lundh T, et al. Late effects of low blood lead concentrations in children on school performance and cognitive functions. Neurotoxicology. 2015;49:114-120.
27. Bernhoft RA. Cadmium toxicity and treatment. Scientific World Journal. 2013;394652.
28. Agency for Toxic Substances and Disease Registry. Case studies in environmental medicine (CSEM) Cadmium Toxicity. Available at: http://www.atsdr.cdc.gov/csem/cadmium/docs/cadmium.pdf. Accessed April 22, 2016.
29. Das KK, Buchner V. Effect of nickel exposure on peripheral tissues: role of oxidative stress in toxicity and possible protection by ascorbic acid. Rev Environ Health. 2007;22:157-173.
30. England LJ, Bunnell RE, Pechacek TF, et al. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am J Prev Med. 2015;49:286-293.
31. Suter MA, Mastrobattista J, Sachs M, et al. Is there evidence for potential harm of electronic cigarette use in pregnancy? Birth defects research. Birth Defects Res A Clin Mol Teratol. 2015;103:186-195.
32. U.S. Preventive Services Task Force. Draft Recommendation Statement. Tobacco smoking cessation in adults and pregnant women: behavioral and pharmacotherapy interventions. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement147/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1. Accessed March 22, 2016.
33. Peters EN, Harrell PT, Hendricks PS, et al. Electronic cigarettes in adults in outpatient substance use treatment: awareness, perceptions, use, and reasons for use. Am J Addict. 2015;24:233-239.
34. Kandel ER, Kandel DB. A molecular basis for nicotine as a gateway drug. N Engl J Med. 2014;371:932-943.
35. King BA, Patel R, Nguyen KH, et al. Trends in awareness and use of electronic cigarettes among US Adults, 2010-2013. Nicotine Tob Res. 2015;17:219-227.
36. McMillen RC, Gottlieb MA, Shaefer RM, et al. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;1195-1202.
37. Lee S, Grana RA, Glantz SA. Electronic cigarette use among Korean adolescents: a cross-sectional study of market penetration, dual use, and relationship to quit attempts and former smoking. J Adolesc Health. 2014;54:684-690.
38. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629-1637.
39. Nelson VA, Goniewicz ML, Beard E, et al. Comparison of the characteristics of long-term users of electronic cigarettes versus nicotine replacement therapy: a cross-sectional survey of English ex-smokers and current smokers. Drug Alcohol Depend. 2015;153:300-305.
40. Caponnetto P, Campagna D, Cibella F, et al. Efficiency and safety of an electronic cigarette (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8:e66317.
41. Polosa R, Caponnetto P, Morjaria JB, et al. Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health. 2011;11:786.
42. Malas M, van der Tempel J, Schwartz R, et al. Electronic cigarettes for smoking cessation: a systematic review. Nicotine Tob Res. 2016. [Epub ahead of print].
43. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
44. Bjartveit K, Tverdal A. Health consequences of smoking 1-4 cigarettes per day. Tob Control. 2005;14:315-320.
From The Journal of Family Practice | 2016;65(6):380-385.
Time of day matters for flu vaccine administration in older adults
A simple and cost-neutral manipulation of the timing of flu vaccine administration – vaccinating older adults in the morning – may improve protection from the influenza virus, according to a study published in Vaccine.
Anna C. Phillips, PhD, of the School of Sport, Exercise, and Rehabilitation Sciences at the University of Birmingham (England), and her associates assessed the change in antibody titers to three vaccine influenza strains (A/H1N1, A/H3N2, and B) from prevaccination to one month postvaccination in a non-blinded cluster-randomized trial of 276 adults aged 65 or older receiving vaccinations in the morning or afternoon between October 28, 2011 and November 12, 2013. Because diurnal variations in immune cell responses and/or levels of hormones with immune modifying properties, such as cortisol or inflammatory cytokines, may provide an advantageous period for vaccination responses to occur, their levels were analyzed at baseline to identify relationships with antibody responses (Vaccine. 2016 May;34[24]:2679-85. doi: 10.1016/j.vaccine.2016.04.032).
The study results indicated significant effects of time of day on the A/H1N1 and B strain antibody responses, but not for the A/H3N2 strain. More specifically, morning vaccinations produced greater antibody responses for the A/H1N1 and B strains as compared with those vaccinated in the afternoon, while the A/H3N2 strain antibody responses did not differ between morning and afternoon administration. Furthermore, both men and women were equally likely to show these effects.
Given their known diurnal rhythms, expected significant differences between groups were found for cortisol, the cortisol:cortisone ratio, corticosterone, dehydroepiandrosterone (DHEA), and androstenedione. However, none of the measured steroid hormone or cytokine levels showed any relationship between the time of day and antibody responses.
Dr. Phillips and her associates said that the strength of their study was its first-of-a-kind, large-scale randomized design for the assessment of different times of vaccination, which provided evidence for the enhancement of the antibody responses to the influenza vaccine following morning administration. Limitations included the inability to reach the recruitment goal of 400 participants over three years, which may have reduced the statistical power of the study.
The study was funded by a Medical Research Council Lifelong Health and Wellbeing Collaborative Research Grant to the University of Birmingham. The authors declared no conflicts of interest.
A simple and cost-neutral manipulation of the timing of flu vaccine administration – vaccinating older adults in the morning – may improve protection from the influenza virus, according to a study published in Vaccine.
Anna C. Phillips, PhD, of the School of Sport, Exercise, and Rehabilitation Sciences at the University of Birmingham (England), and her associates assessed the change in antibody titers to three vaccine influenza strains (A/H1N1, A/H3N2, and B) from prevaccination to one month postvaccination in a non-blinded cluster-randomized trial of 276 adults aged 65 or older receiving vaccinations in the morning or afternoon between October 28, 2011 and November 12, 2013. Because diurnal variations in immune cell responses and/or levels of hormones with immune modifying properties, such as cortisol or inflammatory cytokines, may provide an advantageous period for vaccination responses to occur, their levels were analyzed at baseline to identify relationships with antibody responses (Vaccine. 2016 May;34[24]:2679-85. doi: 10.1016/j.vaccine.2016.04.032).
The study results indicated significant effects of time of day on the A/H1N1 and B strain antibody responses, but not for the A/H3N2 strain. More specifically, morning vaccinations produced greater antibody responses for the A/H1N1 and B strains as compared with those vaccinated in the afternoon, while the A/H3N2 strain antibody responses did not differ between morning and afternoon administration. Furthermore, both men and women were equally likely to show these effects.
Given their known diurnal rhythms, expected significant differences between groups were found for cortisol, the cortisol:cortisone ratio, corticosterone, dehydroepiandrosterone (DHEA), and androstenedione. However, none of the measured steroid hormone or cytokine levels showed any relationship between the time of day and antibody responses.
Dr. Phillips and her associates said that the strength of their study was its first-of-a-kind, large-scale randomized design for the assessment of different times of vaccination, which provided evidence for the enhancement of the antibody responses to the influenza vaccine following morning administration. Limitations included the inability to reach the recruitment goal of 400 participants over three years, which may have reduced the statistical power of the study.
The study was funded by a Medical Research Council Lifelong Health and Wellbeing Collaborative Research Grant to the University of Birmingham. The authors declared no conflicts of interest.
A simple and cost-neutral manipulation of the timing of flu vaccine administration – vaccinating older adults in the morning – may improve protection from the influenza virus, according to a study published in Vaccine.
Anna C. Phillips, PhD, of the School of Sport, Exercise, and Rehabilitation Sciences at the University of Birmingham (England), and her associates assessed the change in antibody titers to three vaccine influenza strains (A/H1N1, A/H3N2, and B) from prevaccination to one month postvaccination in a non-blinded cluster-randomized trial of 276 adults aged 65 or older receiving vaccinations in the morning or afternoon between October 28, 2011 and November 12, 2013. Because diurnal variations in immune cell responses and/or levels of hormones with immune modifying properties, such as cortisol or inflammatory cytokines, may provide an advantageous period for vaccination responses to occur, their levels were analyzed at baseline to identify relationships with antibody responses (Vaccine. 2016 May;34[24]:2679-85. doi: 10.1016/j.vaccine.2016.04.032).
The study results indicated significant effects of time of day on the A/H1N1 and B strain antibody responses, but not for the A/H3N2 strain. More specifically, morning vaccinations produced greater antibody responses for the A/H1N1 and B strains as compared with those vaccinated in the afternoon, while the A/H3N2 strain antibody responses did not differ between morning and afternoon administration. Furthermore, both men and women were equally likely to show these effects.
Given their known diurnal rhythms, expected significant differences between groups were found for cortisol, the cortisol:cortisone ratio, corticosterone, dehydroepiandrosterone (DHEA), and androstenedione. However, none of the measured steroid hormone or cytokine levels showed any relationship between the time of day and antibody responses.
Dr. Phillips and her associates said that the strength of their study was its first-of-a-kind, large-scale randomized design for the assessment of different times of vaccination, which provided evidence for the enhancement of the antibody responses to the influenza vaccine following morning administration. Limitations included the inability to reach the recruitment goal of 400 participants over three years, which may have reduced the statistical power of the study.
The study was funded by a Medical Research Council Lifelong Health and Wellbeing Collaborative Research Grant to the University of Birmingham. The authors declared no conflicts of interest.
Key clinical point: Vaccinating older adults in the morning may improve protection from the influenza virus.
Major finding: Antibody responses to two of three influenza strains were higher when vaccinations were administered in the morning.
Data sources: Participants aged 65 years or older, recruited from 24 Primary Care General Practices within the West Midlands (England), vaccinated in the morning or afternoon between 2011 and 2013.
Disclosures: The study was funded by a Medical Research Council Lifelong Health and Wellbeing Collaborative Research Grant to the University of Birmingham. The authors declared no conflicts of interest.
What do we really know about e-cigarettes?
It’s been about 2 years since I had my first e-cigarette discussion with a patient. He was a smoker in his 30s and, since we routinely screen for tobacco use in our practice, I asked him if he was interested in quitting. He said he was cutting down by using e-cigarettes, but had not yet stopped smoking.
According to the 2 articles on e-cigarettes in this issue—one original research study about the prevalence of e-cigarette use in rural Illinois and one review of the safety of e-cigarettes—my experience with this patient is typical of e-cigarette users. Many are “dual users” who turn to e-cigarettes to try to cut down on their tobacco use.
As these 2 articles discuss, we still have a great deal to learn about the potential harms and benefits of e-cigarettes. What chemicals are people taking into their bodies and how dangerous are they? And even if they pose health risks, do e-cigarettes have value as smoking cessation aids if they are less harmful than tobacco?
One could simply take a “just say No” approach, as does my wife who says, “Any chemical you inhale into your lungs can’t be good for you!” Or, one can assume the more moderate lesser-of-two-evils stance of the British health system, which posits that there may be some benefit to e-cigarettes if they help people cut down or stop using tobacco products.
In writing this editorial, I conducted a quick literature search that yielded only 5 legitimate randomized trials of e-cigarettes to reduce or eliminate tobacco use, and the results were underwhelming. At best, e-cigarettes appear to be as effective as other forms of nicotine replacement, such as patches, which do not have chemical additives.
Fortunately, researchers are taking e-cigarettes seriously, and research is ongoing. Using the search term “e-cigarette” yielded 2058 references, indicating a respectable amount of e-cigarette research conducted over the past 6 years. Most of the research so far has been about the chemical constituents of the vapor people inhale or about use patterns. There is still a lack of definitive research on whether e-cigarettes are an effective smoking cessation method or a “gateway” to the use of tobacco and other substances of abuse.
Or perhaps they are both.
Hopefully, in 5 years we will know a great deal more, but until we do, I am happy to see that the US Food and Drug Administration has decided to regulate e-cigarettes like tobacco.
It’s been about 2 years since I had my first e-cigarette discussion with a patient. He was a smoker in his 30s and, since we routinely screen for tobacco use in our practice, I asked him if he was interested in quitting. He said he was cutting down by using e-cigarettes, but had not yet stopped smoking.
According to the 2 articles on e-cigarettes in this issue—one original research study about the prevalence of e-cigarette use in rural Illinois and one review of the safety of e-cigarettes—my experience with this patient is typical of e-cigarette users. Many are “dual users” who turn to e-cigarettes to try to cut down on their tobacco use.
As these 2 articles discuss, we still have a great deal to learn about the potential harms and benefits of e-cigarettes. What chemicals are people taking into their bodies and how dangerous are they? And even if they pose health risks, do e-cigarettes have value as smoking cessation aids if they are less harmful than tobacco?
One could simply take a “just say No” approach, as does my wife who says, “Any chemical you inhale into your lungs can’t be good for you!” Or, one can assume the more moderate lesser-of-two-evils stance of the British health system, which posits that there may be some benefit to e-cigarettes if they help people cut down or stop using tobacco products.
In writing this editorial, I conducted a quick literature search that yielded only 5 legitimate randomized trials of e-cigarettes to reduce or eliminate tobacco use, and the results were underwhelming. At best, e-cigarettes appear to be as effective as other forms of nicotine replacement, such as patches, which do not have chemical additives.
Fortunately, researchers are taking e-cigarettes seriously, and research is ongoing. Using the search term “e-cigarette” yielded 2058 references, indicating a respectable amount of e-cigarette research conducted over the past 6 years. Most of the research so far has been about the chemical constituents of the vapor people inhale or about use patterns. There is still a lack of definitive research on whether e-cigarettes are an effective smoking cessation method or a “gateway” to the use of tobacco and other substances of abuse.
Or perhaps they are both.
Hopefully, in 5 years we will know a great deal more, but until we do, I am happy to see that the US Food and Drug Administration has decided to regulate e-cigarettes like tobacco.
It’s been about 2 years since I had my first e-cigarette discussion with a patient. He was a smoker in his 30s and, since we routinely screen for tobacco use in our practice, I asked him if he was interested in quitting. He said he was cutting down by using e-cigarettes, but had not yet stopped smoking.
According to the 2 articles on e-cigarettes in this issue—one original research study about the prevalence of e-cigarette use in rural Illinois and one review of the safety of e-cigarettes—my experience with this patient is typical of e-cigarette users. Many are “dual users” who turn to e-cigarettes to try to cut down on their tobacco use.
As these 2 articles discuss, we still have a great deal to learn about the potential harms and benefits of e-cigarettes. What chemicals are people taking into their bodies and how dangerous are they? And even if they pose health risks, do e-cigarettes have value as smoking cessation aids if they are less harmful than tobacco?
One could simply take a “just say No” approach, as does my wife who says, “Any chemical you inhale into your lungs can’t be good for you!” Or, one can assume the more moderate lesser-of-two-evils stance of the British health system, which posits that there may be some benefit to e-cigarettes if they help people cut down or stop using tobacco products.
In writing this editorial, I conducted a quick literature search that yielded only 5 legitimate randomized trials of e-cigarettes to reduce or eliminate tobacco use, and the results were underwhelming. At best, e-cigarettes appear to be as effective as other forms of nicotine replacement, such as patches, which do not have chemical additives.
Fortunately, researchers are taking e-cigarettes seriously, and research is ongoing. Using the search term “e-cigarette” yielded 2058 references, indicating a respectable amount of e-cigarette research conducted over the past 6 years. Most of the research so far has been about the chemical constituents of the vapor people inhale or about use patterns. There is still a lack of definitive research on whether e-cigarettes are an effective smoking cessation method or a “gateway” to the use of tobacco and other substances of abuse.
Or perhaps they are both.
Hopefully, in 5 years we will know a great deal more, but until we do, I am happy to see that the US Food and Drug Administration has decided to regulate e-cigarettes like tobacco.
E-cigarettes: Who’s using them and why?
ABSTRACT
Background Electronic cigarettes (e-cigarettes) are often marketed as safe and effective aids for quitting cigarette smoking, but concerns remain that use of e-cigarettes might actually reduce the number of quit attempts. To address these issues, we characterized the utilization and demographic correlates of dual use of e-cigarettes and traditional cigarettes (referred to here as simply “cigarettes”) among smokers in a rural population of Illinois.
Methods The majority of survey participants were recruited from the 2014 Illinois State Fair and from another event—the Springfield Mile (a motorcycle racing event)—in Springfield, Ill. Survey questions explored participant demographics and cigarette and e-cigarette use history.
Results Of 201 total cigarette smokers, 79 smoked only tobacco cigarettes (smokers), while 122 also used e-cigarettes (dual users). Dual users did not differ significantly from smokers in gender, age, income, or education. Compared to smokers, dual users were more likely to smoke within 30 minutes of awakening (odds ratio [OR]=3.3; 95% confidence interval [CI], 1.8-6.3), but did not smoke more cigarettes per day or perceive a greater likelihood of quit success. Non-white dual users smoked fewer cigarettes per day than smokers. In addition, 79.5% of all dual users reported that they were using e-cigarettes to quit smoking or reduce the number of cigarettes smoked, and white respondents were 6 times more likely than non-whites to use e-cigarettes for ‘trying to quit smoking’ (OR=6.0; 95% CI, 1.1-32.9). Males and respondents with lower income were less likely to say they were using e-cigarettes to reduce the number of cigarettes smoked than females or participants with higher income (OR=0.2; 95% CI, 0.1-0.8 and OR=0.1; 95% CI, 0.0-0.5, respectively).
Conclusions E-cigarettes may significantly alter the landscape of nicotine physical dependence, and local influences likely are associated with use patterns. Future research should continue to examine whether dual use of traditional and electronic cigarettes impacts smoking cessation, and clinicians should be aware that local norms may create differences from national level data.
Approximately 21% of US adults use tobacco products at least occasionally.1 Although smoking prevalence has declined in recent years (from 21% in 2005 to 18% in 2013), it remains high among certain groups (eg, males and those with a high school education or less).2 As we know, the health burden of smoking—as a cause of death from cancer, pulmonary disease, and heart disease—is substantial,3,4 and rural areas experience a significantly higher prevalence of smoking compared to urban areas.2,5,6
However, it is unknown if the context and habits surrounding tobacco use in rural and/or Midwestern areas are similar to those of urban or nationally-representative populations. For example, while many urban residents may encounter a multitude of media messages encouraging smoking cessation resulting in less community acceptance of smoking, rural residents may be exposed to substantially fewer messages (eg, no city bus signs, billboards, subway posters, etc.) and the community may be more accommodating and tolerant of smoking.
Do e-cigarettes increase cigarette smoking?
Public health professionals are concerned about the increased use of e-cigarettes, particularly among young people, and whether this use increases the likelihood that individuals will start smoking tobacco cigarettes.7(Throughout this paper, we will use “cigarettes” and “smoking” to refer to the use of traditional tobacco cigarettes.) A recent study found that adolescents who used electronic nicotine delivery systems were twice as likely as non-users to have tried cigarettes in the past year.8
An onslaught of advertising. There are also concerns that e-cigarettes may serve to ‘renormalize’ nicotine addiction, in part through large-scale advertising, which was seen by nearly 70% of the participants in the 2014 National Youth Tobacco Survey.9 Largely as a result of that advertising, e-cigarette sales exceed $1.7 billion in the United States alone.10 With 15% of all US adults having ever tried electronic nicotine delivery systems and more than half (52%) of smokers having done so, questions regarding their health impact cannot be taken lightly.11
Do e-cigarettes help people quit smoking? E-cigarettes are often marketed as a safe and effective means for quitting cigarette smoking.12-14 (See "E-cigarettes: How "safe" are they?") Nearly two-thirds of physicians report being asked about e-cigarettes by their patients and approximately one-third of physicians recommend using them as a smoking cessation aid.15
Claims regarding the usefulness of e-cigarettes in smoking cessation, however, have not been substantiated by high-quality randomized controlled trials (RCTs). In fact, no RCTs have shown them to be safer or more effective than cessation treatments currently approved by the US Food and Drug Administration.16,17
Two studies reflect the conflicting data that are currently available. One small study found intensive e-cigarette users were 6 times more likely than non-users/triers to report successful smoking cessation.18 However, researchers surveying callers of a cigarette quit line found that smokers who used e-cigarettes (dual users) were less likely to quit smoking than non-users.19
The lack of good-quality data substantiates the concern that dual use might discourage quitting by normalizing cigarette use and reducing perceptions of harm.20,21 Dual use may also hamper smoking cessation efforts by increasing nicotine physical dependence and associated withdrawal symptoms when trying to quit.22 And finally, dual use may expose users to more carcinogens and toxins than those who use only one product, and the average number of cigarettes smoked per day may be significantly higher among dual users.23
Unique demographic factors at work? Finally, the social and community context within which smoking occurs, and the prevalence of smoking-associated demographic risk factors, may vary significantly between rural and urban areas and between seemingly similar rural areas.24-27 Few studies have examined differences in e-cigarette use between rural and urban areas. Those that have are contradictory, reporting that rural residents use e-cigarettes both more and less than their urban peers,28,29 but many of these studies were conducted outside the United States, where the context and norms associated with smoking and e-cigarette use likely vary.
For these reasons, we sought to examine e-cigarette use among residents of Illinois, the nation’s fifth largest state and one with a rural population exceeding 1.5 million.30 We compared dual users of e-cigarettes and cigarettes to smokers of cigarettes only in terms of demographic characteristics, nicotine physical dependence, and smoking cessation beliefs, and explored dual smokers' reasons for using both types of cigarettes.
MATERIALS AND METHODS
A survey was fielded during August and September 2014 in Springfield, Ill. To obtain responses, a booth was set up at both the Illinois State Fair and the Springfield Mile (a motorcycle racing event), and participants were recruited via direct solicitation by project staff. This was supplemented by an email invitation to all employees of the Southern Illinois University School of Medicine. The 2 venues and the email strategy were chosen because they draw from a large area of central and southern Illinois and were convenient to the location of the study team. Individuals were eligible to participate if they were ≥18 years of age and used any tobacco product or e-cigarettes. Survey elements were derived from 2 national surveys of health and behavior—the Minnesota Adult Tobacco Survey 201031 and the Brief Smoking Consequences Questionnaire-Adult.32
Survey questions assessed cigarette use, nicotine physical dependence, social norms, perceived risks and benefits, and smoking cessation beliefs and behaviors. Questions were slightly reworded to address not only the use of traditional cigarettes, but the use of e-cigarettes, as well. Ultimately, each participant answered a similarly-worded set of questions for both regular and e-cigarettes. Dual use of cigarettes and e-cigarettes was also assessed. Participants self-reported all data and survey responses on an electronic tablet and received a $10 (cash or gift card) incentive. This project was reviewed and approved by the Springfield Committee for Research Involving Human Subjects.
Stratification of results. Race was dichotomized into white and non-white. Education was stratified into 3 categories: up to and including high school graduation, some college but not a Bachelor’s degree, and Bachelor’s degree and above. Income was divided as being ≤$20,000 or >$20,000, and age was split into 2 groups by the median value. Analyses included descriptions of participant demographics, dual use status, measures of nicotine physical dependence, quit attempts, and e-cigarette use motivations. Bivariate relationships between dual use status and demographic characteristics, nicotine physical dependence, and smoking cessation beliefs were analyzed by chi-square (categorical variables) and ANOVA (continuous/Likert variables).
Multivariable logistic regression modeling of the demographic variables and dual use status (cigarette smoker only vs dual user) was performed to predict 3 factors: number of cigarettes smoked per day (≤10 vs 11+); time to first cigarette (≤30 vs 31+ minutes from waking); and perceived likelihood of quit attempt success (very/somewhat likely vs very/somewhat unlikely). Multivariable models examining the reasons for dual use included the demographic, nicotine physical dependence, and cessation belief items described previously.
RESULTS
Of 309 total survey participants (Fair=288; Race=12; Email=9), there were 235 current cigarette smokers consisting of 79 who smoked only cigarettes (smokers); 122 who used both cigarettes and e-cigarettes (dual users); and 34 former e-cigarette users. Only smokers and dual users were included in this analysis (N=201, although for the purposes of TABLE 1, N=200 or 199 because at least one participant did not provide answers to all of the questions). Approximately 51% of the smokers were male, 78% were white, 12% were 4-year college graduates, and 57% reported incomes >$20,000. The mean age was 37.7 years (SD=14.4); 50% of respondents were <35 years of age. Dual users did not vary significantly from smokers in terms of gender, age, education, or income (all P>.05). However, a greater proportion of whites vs non-whites were dual users (54.9% vs 42.3%; P=.035).
Click here to see an enlarged version of the table.
No big quit differences. Bivariate analyses revealed that dual users were no more likely than smokers to have attempted to quit smoking within the past year (X2=2.3; P=.14), consider quitting in the next one or 6 months (X2=1.1; P=.34), or differ in perceived likelihood of cessation success (X2=0.0; P=1.00). The proportion of dual users who smoked 11+ cigarettes per day did not differ from that of cigarette smokers for the group as a whole or when the group was stratified by gender, income, education, or age. However, among non-whites, dual users smoked fewer cigarettes than cigarette smokers (TABLE 1).
Predicting physical dependence. Significant differences also were observed regarding the timing of the first cigarette of the day, with dual users approximately 3 times more likely than smokers to smoke within 30 minutes of awakening (80% vs 54.4%; OR=3.3; 95% CI, 1.8-6.3), and this difference was upheld among males, females, whites, those with an income >$20,000, those with a high school education or less and those with some college education, and age >34 years. There was no association, however, between dual use and perceived likelihood of quit success.
We then performed multivariable logistic modeling on dual users to determine which variables might predict 3 measures of physical dependence: number of cigarettes smoked per day (≤10 vs 11+), time between waking and smoking the first cigarette of the day (≤30 vs 31+ minutes), and perceived likelihood of cessation success (TABLE 2). Male gender (OR=3.4; 95% CI, 1.8-6.5) and white race (OR=4.4; 95% CI, 1.9-10.1) were significant for predicting smoking 11+ cigarettes a day, while dual use status was insignificant (P=.104). Regarding time to first cigarette, only dual use was significant (OR=3.1; 95% CI, 1.6-5.9), with dual users approximately 3 times more likely than smokers to have their first cigarette within 30 minutes of waking. No variables were significant in predicting perceived likelihood of quit success.
Reasons for dual use. We examined reasons for dual use with the question: Do you use e-cigarettes to reduce your regular tobacco use? Here, 79.5% of smokers reported using e-cigarettes to quit smoking or reduce the number of cigarettes smoked.
A multivariable polynomial logistic regression that included only dual users was performed to examine which variables might predict use for tobacco cessation (“trying to quit smoking”) vs reduction in smoking intensity (“trying to reduce the number of regular cigarettes I smoke per day”) vs no change (“use the same amount of tobacco as always”) (TABLE 2). Whites were approximately 6 times more likely than non-whites to indicate they engage in dual use to try to quit smoking (OR=6.0; 95% CI, 1.1-32.9). Males and people with lower incomes were much less likely to indicate they engaged in dual use to try to reduce the number of regular cigarettes smoked than females or those with higher incomes (OR=0.2; 95% CI, 0.1-0.8 and OR=0.1, 95% CI, 0.0-0.5, respectively). No other demographic variables or measures of nicotine physical dependence were significantly different between dual users and smokers.
Click here to see an enlarged version of the table.
DISCUSSION
E-cigarettes are used by approximately half of smokers (52%), which is much higher than that reported by Delnevo, et al, in their analysis of the National Health Interview Study.33 There, prevalence of dual use of both cigarettes and e-cigarettes ranged from 3.4% to 12.7%. This substantial difference raises important questions regarding study population characterization. Were participants in our study representative of central Illinois, state fair attendees, or the agricultural profession? Further work to identify this group with an increased propensity for dual use will assist clinicians in developing appropriate intervention strategies.
Dual use in our study did not vary by many customary demographic variables. Nor was it associated with different rates of past or future quit attempts or perceived ability to successfully quit if quitting was attempted. These factors—high rates of dual use and insignificant effect on quit attempts—may have implications for local physicians counseling patients who smoke.
In our study, the majority of smokers already use e-cigarettes, and this does not seem to increase their ability/likelihood to quit smoking. Further, dual use did not seem to be associated with overall cigarette consumption; males and white participants smoked more cigarettes than females and non-whites. But dual use was associated with a measure of increased nicotine physical dependence (earlier first cigarette of the day). As a result, physicians may want to think twice before recommending e-cigarette use as a means of smoking cessation.
In addition to the high prevalence of e-cigarette use among smokers, a number of other interesting findings surfaced that run counter to some of the current literature. First, dual users are no more likely than smokers to have tried to quit in the past or to try to quit in the future.21,22,34 It could be that for the relatively small geographical area from which our participants were recruited (central Illinois; ~77% of participants from Sangamon County alone), the local context and culture of smoking differs from that associated with participants in other studies, who were mostly recruited from national and regional online surveys. However, there is no a priori reason to suspect Sangamon County is especially different, as it is quite similar to Illinois as a whole by many measures (eg, percentage rural: 14.1% vs 11.5%; percentage black (only): 12.4% vs 14.7%; education to at least a Bachelor’s degree: 33.0% vs 31.9%; and median household income: $55,565 vs $57,166).30
While we found that dual users did have one measure of increased nicotine physical dependence, the total number of cigarettes consumed per day was not significantly different from that of smokers.23-25 This is contrary to another study of nicotine physical dependence, but, unlike that study, we did not assess length of time of concurrent use.35 There is much uncertainty surrounding the issue of nicotine physical dependence and e-cigarette use, largely because the level of nicotine delivered by various e-products varies significantly.36
Cross-sectional nature, small sample size limit utility of data
There are significant limitations to this study, including the cross-sectional nature of the data, the small sample size, the use of self-report, and the limited scope of recruitment. The relatively small sample size limits our ability to observe small differences and effect sizes. However, small differences often lack practical significance. Finally, participation was limited to those attending a state fair or a local sporting event and those employed by a local medical school. Thus, the results may not be generalizable to populations outside central Illinois. On the other hand, the very low income sample recruited from the Midwestern US, which is underrepresented in prior e-cigarette research, might represent some of the strengths of this work.
Future investigations. Future studies should more closely examine e-cigarette use prevalence on smaller geographic scales and especially in rural areas where there is a paucity of research. As the majority of our respondents came from a single county in central Illinois, one has to ask the questions, “Is this a ‘hot spot’ for e-cigarette use?" And "Do other rural areas experience similar use?” It may be important to know if national surveys are sensitive enough to observe significant local variations. Research also should examine how e-cigarette use and the influence of local culture vary across wider areas.
Several specific areas of study would help to inform policy and intervention development. For example, is tobacco cigarette quit success impacted by concurrent e-cigarette use? While our study showed no difference in past or possible future quit attempts among dual users as compared with smokers, we did not assess actual quit success, and multiple participants in our study anecdotally described using e-cigarettes to successfully quit smoking.
In the end, the rapid increase in the use of e-cigarettes has the potential to significantly alter the landscape of nicotine physical dependence, and local culture and other influences are likely associated with use patterns.
CORRESPONDENCE
Wiley D. Jenkins, PhD, MPH, Science Director, Population Health Science Program, Southern Illinois University School of Medicine, 201 E. Madison St., Springfield, IL 62794-9664; [email protected].
1. Agaku IT, King BA, Husten CG, et al; Centers for Disease Control and Prevention (CDC). Tobacco product use among adults—United States, 2012-2013. MMWR Morb Mortal Wkly Rep. 2014;63:542-547.
2. Jamal A, Agaku IT, O’Connor E, et al. Current cigarette smoking among adults—United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2014;63:1108-1112.
3. Siegel RL, Jacobs EJ, Newton CC, et al. Deaths due to cigarette smoking for 12 smoking-related cancers in the United States. JAMA Intern Med. 2015;175:1574-1576.
4. US Department of Health and Human Services. Surgeon General. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014. Available at: http://www.surgeongeneral.gov/library/reports/50-years-of-progress/index.html. Accessed January 22, 2014.
5. Gamm LD, Hutchison LL, Dabney BJ, et al, eds. (2003). Rural Healthy People 2010: A companion document to Healthy People 2010. Volume 2. College Station, TX: The Texas A&M University System Health Science Center, School of Rural Public Health, Southwest Rural Health Research Center.
6. Doescher MP, Jackson JE, Jerant A, et al. Prevalence and trends in smoking: a national rural study. J Rural Health. 2006;22:112-118.
7. Bunnell RE, Agaku IT, Arrazola RA, et al. Intentions to smoke cigarettes among never-smoking US middle and high school electronic cigarette users: National Youth Tobacco Survey, 2011-2013. Nicotine Tob Res. 2015;17:228-235.
8. Cardenas VM, Evans VL, Balamurugan A, et al. Use of electronic nicotine delivery systems and recent initiation of smoking among US youth. Int J Public Health. 2016;61:237-241.
9. Auf R, Trepka MJ, Cano MA, et al. Electronic cigarettes: the renormalisation of nicotine use. BMJ. 2016;352:i425.
10. CNBC. E-cigarette sales are smoking hot, set to hit $1.7 billion. Available at: http://www.cnbc.com/id/100991511. Accessed April 5, 2016.
11. Weaver SR, Majeed BA, Pechacek TF, et al. Use of electronic nicotine delivery systems and other tobacco products among USA adults, 2014: results from a national survey. Int J Public Health. 2016;61:177-188.
12. Richardson A, Ganz O, Vallone D. Tobacco on the web: surveillance and characterisation of online tobacco and e-cigarette advertising. Tob Control. 2015;24:341-347.
13. Paek HJ, Kim S, Hove T, et al. Reduced harm or another gateway to smoking? source, message, and information characteristics of E-cigarette videos on YouTube. J Health Commun. 2014;19:545-560.
14. Kim AE, Arnold KY, Makarenko O. E-cigarette advertising expenditures in the U.S., 2011-2012. Am J Prev Med. 2014;46:409-412.
15. Steinberg MB, Giovenco DP, Delnevo CD. Patient-physician communication regarding electronic cigarettes. Prev Med Rep. 2015;2:96-98.
16. Gualano MR, Passi S, Bert F, et al. Electronic cigarettes: assessing the efficacy and the adverse effects through a systematic review of published studies. J Public Health (Oxf). 2015:37:488-497.
17. U.S. National Institutes of Health. ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/results?term=%22electronic+cigarette%22&Search=Search. Accessed July 10, 2015.
18. Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2015;17:127-133.
19. Vickerman KA, Carpenter KM, Altman T, et al. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787-1791.
20. Center for Disease Control and Prevention. Press Release February 28,2013. Available at: http://www.cdc.gov/media/releases/2013/p0228_electronic_cigarettes.html. Accessed July 8, 2015.
21. Pisinger C. Why public health people are more worried than excited over e-cigarettes. BMC Med. 2014;12:226.
22. Post A, Gilljam H, Rosendahl I, et al. Symptoms of nicotine dependence in a cohort of Swedish youths: a comparison between smokers, smokeless tobacco users and dual tobacco users. Addiction. 2010;105:740-746.
23. Mazurek JM, Syamlal G, King BA, et al; Division of Respiratory Disease Studies, National Institute for Occupational Safety and Health, CDC. Smokeless tobacco use among working adults—United States, 2005 and 2010. MMWR Morb Mortal Wkly Rep. 2014;63:477-482.
24. Hutcheson TD, Greiner KA, Ellerbeck EF, et al. Understanding smoking cessation in rural communities. J Rural Health. 2008;24:116-124.
25. McMillen R, Breen J, Cosby AG. Rural-urban differences in the social climate surrounding environmental tobacco smoke: a report from the 2002 Social Climate Survey of Tobacco Control. J Rural Health. 2004;20:7-16.
26. Butler KM, Rayens MK, Adkins S, et al. Culturally-specific smoking cessation outreach in a rural community. Public Health Nurs. 2014;31:44-54.
27. Butler KM, Hedgecock S, Record RA, et al. An evidence-based cessation strategy using rural smokers’ experiences with tobacco. Nurs Clin North Am. 2012;47:31-43.
28. Hamilton HA, Ferrence R, Boak A, et al. Ever use of nicotine and nonnicotine electronic cigarettes among high school students in Ontario, Canada. Nicotine Tob Res. 2015;17:1212-1218.
29. Goniewicz ML, Zielinska-Danch W. Electronic cigarette use among teenagers and young adults in Poland. Pediatrics. 2012;130:e879-e885.
30. US Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Available at: http://www.census.gov/geo/reference/ua/urban-rural-2010.html. Accessed March 13, 2016.
31. Minnesota Adult Tobacco Survey. Tobacco use in Minnesota: 1999-2014. Available at: http://www.mnadulttobaccosurvey.org/. Accessed April 27, 2016.
32. Rash CJ, Copeland AL. The Brief Smoking Consequences Questionnaire-Adult (BSCQ-A): development of a short form of the SCQ-A. Nicotine Tob Res. 2008;10:1633-1643.
33. Delnevo CD, Giovenco DP, Steinberg MB, et al. Patterns of electronic cigarette use among adults in the United States. Nicotine Tob Res. 2016;18:715-719.
34. Lee YO, Hebert CJ, Nonnemaker JM, et al. Multiple tobacco product use among adults in the United States: cigarettes, cigars, electronic cigarettes, hookah, smokeless tobacco, and snus. Prev Med. 2014;62:14-19.
35. Etter JF, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015;147:68-75.
36. Cobb CO, Hendricks PS, Eissenberg T. Electronic cigarettes and nicotine dependence: evolving products, evolving problems. BMC Med. 2015;13:119.
ABSTRACT
Background Electronic cigarettes (e-cigarettes) are often marketed as safe and effective aids for quitting cigarette smoking, but concerns remain that use of e-cigarettes might actually reduce the number of quit attempts. To address these issues, we characterized the utilization and demographic correlates of dual use of e-cigarettes and traditional cigarettes (referred to here as simply “cigarettes”) among smokers in a rural population of Illinois.
Methods The majority of survey participants were recruited from the 2014 Illinois State Fair and from another event—the Springfield Mile (a motorcycle racing event)—in Springfield, Ill. Survey questions explored participant demographics and cigarette and e-cigarette use history.
Results Of 201 total cigarette smokers, 79 smoked only tobacco cigarettes (smokers), while 122 also used e-cigarettes (dual users). Dual users did not differ significantly from smokers in gender, age, income, or education. Compared to smokers, dual users were more likely to smoke within 30 minutes of awakening (odds ratio [OR]=3.3; 95% confidence interval [CI], 1.8-6.3), but did not smoke more cigarettes per day or perceive a greater likelihood of quit success. Non-white dual users smoked fewer cigarettes per day than smokers. In addition, 79.5% of all dual users reported that they were using e-cigarettes to quit smoking or reduce the number of cigarettes smoked, and white respondents were 6 times more likely than non-whites to use e-cigarettes for ‘trying to quit smoking’ (OR=6.0; 95% CI, 1.1-32.9). Males and respondents with lower income were less likely to say they were using e-cigarettes to reduce the number of cigarettes smoked than females or participants with higher income (OR=0.2; 95% CI, 0.1-0.8 and OR=0.1; 95% CI, 0.0-0.5, respectively).
Conclusions E-cigarettes may significantly alter the landscape of nicotine physical dependence, and local influences likely are associated with use patterns. Future research should continue to examine whether dual use of traditional and electronic cigarettes impacts smoking cessation, and clinicians should be aware that local norms may create differences from national level data.
Approximately 21% of US adults use tobacco products at least occasionally.1 Although smoking prevalence has declined in recent years (from 21% in 2005 to 18% in 2013), it remains high among certain groups (eg, males and those with a high school education or less).2 As we know, the health burden of smoking—as a cause of death from cancer, pulmonary disease, and heart disease—is substantial,3,4 and rural areas experience a significantly higher prevalence of smoking compared to urban areas.2,5,6
However, it is unknown if the context and habits surrounding tobacco use in rural and/or Midwestern areas are similar to those of urban or nationally-representative populations. For example, while many urban residents may encounter a multitude of media messages encouraging smoking cessation resulting in less community acceptance of smoking, rural residents may be exposed to substantially fewer messages (eg, no city bus signs, billboards, subway posters, etc.) and the community may be more accommodating and tolerant of smoking.
Do e-cigarettes increase cigarette smoking?
Public health professionals are concerned about the increased use of e-cigarettes, particularly among young people, and whether this use increases the likelihood that individuals will start smoking tobacco cigarettes.7(Throughout this paper, we will use “cigarettes” and “smoking” to refer to the use of traditional tobacco cigarettes.) A recent study found that adolescents who used electronic nicotine delivery systems were twice as likely as non-users to have tried cigarettes in the past year.8
An onslaught of advertising. There are also concerns that e-cigarettes may serve to ‘renormalize’ nicotine addiction, in part through large-scale advertising, which was seen by nearly 70% of the participants in the 2014 National Youth Tobacco Survey.9 Largely as a result of that advertising, e-cigarette sales exceed $1.7 billion in the United States alone.10 With 15% of all US adults having ever tried electronic nicotine delivery systems and more than half (52%) of smokers having done so, questions regarding their health impact cannot be taken lightly.11
Do e-cigarettes help people quit smoking? E-cigarettes are often marketed as a safe and effective means for quitting cigarette smoking.12-14 (See "E-cigarettes: How "safe" are they?") Nearly two-thirds of physicians report being asked about e-cigarettes by their patients and approximately one-third of physicians recommend using them as a smoking cessation aid.15
Claims regarding the usefulness of e-cigarettes in smoking cessation, however, have not been substantiated by high-quality randomized controlled trials (RCTs). In fact, no RCTs have shown them to be safer or more effective than cessation treatments currently approved by the US Food and Drug Administration.16,17
Two studies reflect the conflicting data that are currently available. One small study found intensive e-cigarette users were 6 times more likely than non-users/triers to report successful smoking cessation.18 However, researchers surveying callers of a cigarette quit line found that smokers who used e-cigarettes (dual users) were less likely to quit smoking than non-users.19
The lack of good-quality data substantiates the concern that dual use might discourage quitting by normalizing cigarette use and reducing perceptions of harm.20,21 Dual use may also hamper smoking cessation efforts by increasing nicotine physical dependence and associated withdrawal symptoms when trying to quit.22 And finally, dual use may expose users to more carcinogens and toxins than those who use only one product, and the average number of cigarettes smoked per day may be significantly higher among dual users.23
Unique demographic factors at work? Finally, the social and community context within which smoking occurs, and the prevalence of smoking-associated demographic risk factors, may vary significantly between rural and urban areas and between seemingly similar rural areas.24-27 Few studies have examined differences in e-cigarette use between rural and urban areas. Those that have are contradictory, reporting that rural residents use e-cigarettes both more and less than their urban peers,28,29 but many of these studies were conducted outside the United States, where the context and norms associated with smoking and e-cigarette use likely vary.
For these reasons, we sought to examine e-cigarette use among residents of Illinois, the nation’s fifth largest state and one with a rural population exceeding 1.5 million.30 We compared dual users of e-cigarettes and cigarettes to smokers of cigarettes only in terms of demographic characteristics, nicotine physical dependence, and smoking cessation beliefs, and explored dual smokers' reasons for using both types of cigarettes.
MATERIALS AND METHODS
A survey was fielded during August and September 2014 in Springfield, Ill. To obtain responses, a booth was set up at both the Illinois State Fair and the Springfield Mile (a motorcycle racing event), and participants were recruited via direct solicitation by project staff. This was supplemented by an email invitation to all employees of the Southern Illinois University School of Medicine. The 2 venues and the email strategy were chosen because they draw from a large area of central and southern Illinois and were convenient to the location of the study team. Individuals were eligible to participate if they were ≥18 years of age and used any tobacco product or e-cigarettes. Survey elements were derived from 2 national surveys of health and behavior—the Minnesota Adult Tobacco Survey 201031 and the Brief Smoking Consequences Questionnaire-Adult.32
Survey questions assessed cigarette use, nicotine physical dependence, social norms, perceived risks and benefits, and smoking cessation beliefs and behaviors. Questions were slightly reworded to address not only the use of traditional cigarettes, but the use of e-cigarettes, as well. Ultimately, each participant answered a similarly-worded set of questions for both regular and e-cigarettes. Dual use of cigarettes and e-cigarettes was also assessed. Participants self-reported all data and survey responses on an electronic tablet and received a $10 (cash or gift card) incentive. This project was reviewed and approved by the Springfield Committee for Research Involving Human Subjects.
Stratification of results. Race was dichotomized into white and non-white. Education was stratified into 3 categories: up to and including high school graduation, some college but not a Bachelor’s degree, and Bachelor’s degree and above. Income was divided as being ≤$20,000 or >$20,000, and age was split into 2 groups by the median value. Analyses included descriptions of participant demographics, dual use status, measures of nicotine physical dependence, quit attempts, and e-cigarette use motivations. Bivariate relationships between dual use status and demographic characteristics, nicotine physical dependence, and smoking cessation beliefs were analyzed by chi-square (categorical variables) and ANOVA (continuous/Likert variables).
Multivariable logistic regression modeling of the demographic variables and dual use status (cigarette smoker only vs dual user) was performed to predict 3 factors: number of cigarettes smoked per day (≤10 vs 11+); time to first cigarette (≤30 vs 31+ minutes from waking); and perceived likelihood of quit attempt success (very/somewhat likely vs very/somewhat unlikely). Multivariable models examining the reasons for dual use included the demographic, nicotine physical dependence, and cessation belief items described previously.
RESULTS
Of 309 total survey participants (Fair=288; Race=12; Email=9), there were 235 current cigarette smokers consisting of 79 who smoked only cigarettes (smokers); 122 who used both cigarettes and e-cigarettes (dual users); and 34 former e-cigarette users. Only smokers and dual users were included in this analysis (N=201, although for the purposes of TABLE 1, N=200 or 199 because at least one participant did not provide answers to all of the questions). Approximately 51% of the smokers were male, 78% were white, 12% were 4-year college graduates, and 57% reported incomes >$20,000. The mean age was 37.7 years (SD=14.4); 50% of respondents were <35 years of age. Dual users did not vary significantly from smokers in terms of gender, age, education, or income (all P>.05). However, a greater proportion of whites vs non-whites were dual users (54.9% vs 42.3%; P=.035).
Click here to see an enlarged version of the table.
No big quit differences. Bivariate analyses revealed that dual users were no more likely than smokers to have attempted to quit smoking within the past year (X2=2.3; P=.14), consider quitting in the next one or 6 months (X2=1.1; P=.34), or differ in perceived likelihood of cessation success (X2=0.0; P=1.00). The proportion of dual users who smoked 11+ cigarettes per day did not differ from that of cigarette smokers for the group as a whole or when the group was stratified by gender, income, education, or age. However, among non-whites, dual users smoked fewer cigarettes than cigarette smokers (TABLE 1).
Predicting physical dependence. Significant differences also were observed regarding the timing of the first cigarette of the day, with dual users approximately 3 times more likely than smokers to smoke within 30 minutes of awakening (80% vs 54.4%; OR=3.3; 95% CI, 1.8-6.3), and this difference was upheld among males, females, whites, those with an income >$20,000, those with a high school education or less and those with some college education, and age >34 years. There was no association, however, between dual use and perceived likelihood of quit success.
We then performed multivariable logistic modeling on dual users to determine which variables might predict 3 measures of physical dependence: number of cigarettes smoked per day (≤10 vs 11+), time between waking and smoking the first cigarette of the day (≤30 vs 31+ minutes), and perceived likelihood of cessation success (TABLE 2). Male gender (OR=3.4; 95% CI, 1.8-6.5) and white race (OR=4.4; 95% CI, 1.9-10.1) were significant for predicting smoking 11+ cigarettes a day, while dual use status was insignificant (P=.104). Regarding time to first cigarette, only dual use was significant (OR=3.1; 95% CI, 1.6-5.9), with dual users approximately 3 times more likely than smokers to have their first cigarette within 30 minutes of waking. No variables were significant in predicting perceived likelihood of quit success.
Reasons for dual use. We examined reasons for dual use with the question: Do you use e-cigarettes to reduce your regular tobacco use? Here, 79.5% of smokers reported using e-cigarettes to quit smoking or reduce the number of cigarettes smoked.
A multivariable polynomial logistic regression that included only dual users was performed to examine which variables might predict use for tobacco cessation (“trying to quit smoking”) vs reduction in smoking intensity (“trying to reduce the number of regular cigarettes I smoke per day”) vs no change (“use the same amount of tobacco as always”) (TABLE 2). Whites were approximately 6 times more likely than non-whites to indicate they engage in dual use to try to quit smoking (OR=6.0; 95% CI, 1.1-32.9). Males and people with lower incomes were much less likely to indicate they engaged in dual use to try to reduce the number of regular cigarettes smoked than females or those with higher incomes (OR=0.2; 95% CI, 0.1-0.8 and OR=0.1, 95% CI, 0.0-0.5, respectively). No other demographic variables or measures of nicotine physical dependence were significantly different between dual users and smokers.
Click here to see an enlarged version of the table.
DISCUSSION
E-cigarettes are used by approximately half of smokers (52%), which is much higher than that reported by Delnevo, et al, in their analysis of the National Health Interview Study.33 There, prevalence of dual use of both cigarettes and e-cigarettes ranged from 3.4% to 12.7%. This substantial difference raises important questions regarding study population characterization. Were participants in our study representative of central Illinois, state fair attendees, or the agricultural profession? Further work to identify this group with an increased propensity for dual use will assist clinicians in developing appropriate intervention strategies.
Dual use in our study did not vary by many customary demographic variables. Nor was it associated with different rates of past or future quit attempts or perceived ability to successfully quit if quitting was attempted. These factors—high rates of dual use and insignificant effect on quit attempts—may have implications for local physicians counseling patients who smoke.
In our study, the majority of smokers already use e-cigarettes, and this does not seem to increase their ability/likelihood to quit smoking. Further, dual use did not seem to be associated with overall cigarette consumption; males and white participants smoked more cigarettes than females and non-whites. But dual use was associated with a measure of increased nicotine physical dependence (earlier first cigarette of the day). As a result, physicians may want to think twice before recommending e-cigarette use as a means of smoking cessation.
In addition to the high prevalence of e-cigarette use among smokers, a number of other interesting findings surfaced that run counter to some of the current literature. First, dual users are no more likely than smokers to have tried to quit in the past or to try to quit in the future.21,22,34 It could be that for the relatively small geographical area from which our participants were recruited (central Illinois; ~77% of participants from Sangamon County alone), the local context and culture of smoking differs from that associated with participants in other studies, who were mostly recruited from national and regional online surveys. However, there is no a priori reason to suspect Sangamon County is especially different, as it is quite similar to Illinois as a whole by many measures (eg, percentage rural: 14.1% vs 11.5%; percentage black (only): 12.4% vs 14.7%; education to at least a Bachelor’s degree: 33.0% vs 31.9%; and median household income: $55,565 vs $57,166).30
While we found that dual users did have one measure of increased nicotine physical dependence, the total number of cigarettes consumed per day was not significantly different from that of smokers.23-25 This is contrary to another study of nicotine physical dependence, but, unlike that study, we did not assess length of time of concurrent use.35 There is much uncertainty surrounding the issue of nicotine physical dependence and e-cigarette use, largely because the level of nicotine delivered by various e-products varies significantly.36
Cross-sectional nature, small sample size limit utility of data
There are significant limitations to this study, including the cross-sectional nature of the data, the small sample size, the use of self-report, and the limited scope of recruitment. The relatively small sample size limits our ability to observe small differences and effect sizes. However, small differences often lack practical significance. Finally, participation was limited to those attending a state fair or a local sporting event and those employed by a local medical school. Thus, the results may not be generalizable to populations outside central Illinois. On the other hand, the very low income sample recruited from the Midwestern US, which is underrepresented in prior e-cigarette research, might represent some of the strengths of this work.
Future investigations. Future studies should more closely examine e-cigarette use prevalence on smaller geographic scales and especially in rural areas where there is a paucity of research. As the majority of our respondents came from a single county in central Illinois, one has to ask the questions, “Is this a ‘hot spot’ for e-cigarette use?" And "Do other rural areas experience similar use?” It may be important to know if national surveys are sensitive enough to observe significant local variations. Research also should examine how e-cigarette use and the influence of local culture vary across wider areas.
Several specific areas of study would help to inform policy and intervention development. For example, is tobacco cigarette quit success impacted by concurrent e-cigarette use? While our study showed no difference in past or possible future quit attempts among dual users as compared with smokers, we did not assess actual quit success, and multiple participants in our study anecdotally described using e-cigarettes to successfully quit smoking.
In the end, the rapid increase in the use of e-cigarettes has the potential to significantly alter the landscape of nicotine physical dependence, and local culture and other influences are likely associated with use patterns.
CORRESPONDENCE
Wiley D. Jenkins, PhD, MPH, Science Director, Population Health Science Program, Southern Illinois University School of Medicine, 201 E. Madison St., Springfield, IL 62794-9664; [email protected].
ABSTRACT
Background Electronic cigarettes (e-cigarettes) are often marketed as safe and effective aids for quitting cigarette smoking, but concerns remain that use of e-cigarettes might actually reduce the number of quit attempts. To address these issues, we characterized the utilization and demographic correlates of dual use of e-cigarettes and traditional cigarettes (referred to here as simply “cigarettes”) among smokers in a rural population of Illinois.
Methods The majority of survey participants were recruited from the 2014 Illinois State Fair and from another event—the Springfield Mile (a motorcycle racing event)—in Springfield, Ill. Survey questions explored participant demographics and cigarette and e-cigarette use history.
Results Of 201 total cigarette smokers, 79 smoked only tobacco cigarettes (smokers), while 122 also used e-cigarettes (dual users). Dual users did not differ significantly from smokers in gender, age, income, or education. Compared to smokers, dual users were more likely to smoke within 30 minutes of awakening (odds ratio [OR]=3.3; 95% confidence interval [CI], 1.8-6.3), but did not smoke more cigarettes per day or perceive a greater likelihood of quit success. Non-white dual users smoked fewer cigarettes per day than smokers. In addition, 79.5% of all dual users reported that they were using e-cigarettes to quit smoking or reduce the number of cigarettes smoked, and white respondents were 6 times more likely than non-whites to use e-cigarettes for ‘trying to quit smoking’ (OR=6.0; 95% CI, 1.1-32.9). Males and respondents with lower income were less likely to say they were using e-cigarettes to reduce the number of cigarettes smoked than females or participants with higher income (OR=0.2; 95% CI, 0.1-0.8 and OR=0.1; 95% CI, 0.0-0.5, respectively).
Conclusions E-cigarettes may significantly alter the landscape of nicotine physical dependence, and local influences likely are associated with use patterns. Future research should continue to examine whether dual use of traditional and electronic cigarettes impacts smoking cessation, and clinicians should be aware that local norms may create differences from national level data.
Approximately 21% of US adults use tobacco products at least occasionally.1 Although smoking prevalence has declined in recent years (from 21% in 2005 to 18% in 2013), it remains high among certain groups (eg, males and those with a high school education or less).2 As we know, the health burden of smoking—as a cause of death from cancer, pulmonary disease, and heart disease—is substantial,3,4 and rural areas experience a significantly higher prevalence of smoking compared to urban areas.2,5,6
However, it is unknown if the context and habits surrounding tobacco use in rural and/or Midwestern areas are similar to those of urban or nationally-representative populations. For example, while many urban residents may encounter a multitude of media messages encouraging smoking cessation resulting in less community acceptance of smoking, rural residents may be exposed to substantially fewer messages (eg, no city bus signs, billboards, subway posters, etc.) and the community may be more accommodating and tolerant of smoking.
Do e-cigarettes increase cigarette smoking?
Public health professionals are concerned about the increased use of e-cigarettes, particularly among young people, and whether this use increases the likelihood that individuals will start smoking tobacco cigarettes.7(Throughout this paper, we will use “cigarettes” and “smoking” to refer to the use of traditional tobacco cigarettes.) A recent study found that adolescents who used electronic nicotine delivery systems were twice as likely as non-users to have tried cigarettes in the past year.8
An onslaught of advertising. There are also concerns that e-cigarettes may serve to ‘renormalize’ nicotine addiction, in part through large-scale advertising, which was seen by nearly 70% of the participants in the 2014 National Youth Tobacco Survey.9 Largely as a result of that advertising, e-cigarette sales exceed $1.7 billion in the United States alone.10 With 15% of all US adults having ever tried electronic nicotine delivery systems and more than half (52%) of smokers having done so, questions regarding their health impact cannot be taken lightly.11
Do e-cigarettes help people quit smoking? E-cigarettes are often marketed as a safe and effective means for quitting cigarette smoking.12-14 (See "E-cigarettes: How "safe" are they?") Nearly two-thirds of physicians report being asked about e-cigarettes by their patients and approximately one-third of physicians recommend using them as a smoking cessation aid.15
Claims regarding the usefulness of e-cigarettes in smoking cessation, however, have not been substantiated by high-quality randomized controlled trials (RCTs). In fact, no RCTs have shown them to be safer or more effective than cessation treatments currently approved by the US Food and Drug Administration.16,17
Two studies reflect the conflicting data that are currently available. One small study found intensive e-cigarette users were 6 times more likely than non-users/triers to report successful smoking cessation.18 However, researchers surveying callers of a cigarette quit line found that smokers who used e-cigarettes (dual users) were less likely to quit smoking than non-users.19
The lack of good-quality data substantiates the concern that dual use might discourage quitting by normalizing cigarette use and reducing perceptions of harm.20,21 Dual use may also hamper smoking cessation efforts by increasing nicotine physical dependence and associated withdrawal symptoms when trying to quit.22 And finally, dual use may expose users to more carcinogens and toxins than those who use only one product, and the average number of cigarettes smoked per day may be significantly higher among dual users.23
Unique demographic factors at work? Finally, the social and community context within which smoking occurs, and the prevalence of smoking-associated demographic risk factors, may vary significantly between rural and urban areas and between seemingly similar rural areas.24-27 Few studies have examined differences in e-cigarette use between rural and urban areas. Those that have are contradictory, reporting that rural residents use e-cigarettes both more and less than their urban peers,28,29 but many of these studies were conducted outside the United States, where the context and norms associated with smoking and e-cigarette use likely vary.
For these reasons, we sought to examine e-cigarette use among residents of Illinois, the nation’s fifth largest state and one with a rural population exceeding 1.5 million.30 We compared dual users of e-cigarettes and cigarettes to smokers of cigarettes only in terms of demographic characteristics, nicotine physical dependence, and smoking cessation beliefs, and explored dual smokers' reasons for using both types of cigarettes.
MATERIALS AND METHODS
A survey was fielded during August and September 2014 in Springfield, Ill. To obtain responses, a booth was set up at both the Illinois State Fair and the Springfield Mile (a motorcycle racing event), and participants were recruited via direct solicitation by project staff. This was supplemented by an email invitation to all employees of the Southern Illinois University School of Medicine. The 2 venues and the email strategy were chosen because they draw from a large area of central and southern Illinois and were convenient to the location of the study team. Individuals were eligible to participate if they were ≥18 years of age and used any tobacco product or e-cigarettes. Survey elements were derived from 2 national surveys of health and behavior—the Minnesota Adult Tobacco Survey 201031 and the Brief Smoking Consequences Questionnaire-Adult.32
Survey questions assessed cigarette use, nicotine physical dependence, social norms, perceived risks and benefits, and smoking cessation beliefs and behaviors. Questions were slightly reworded to address not only the use of traditional cigarettes, but the use of e-cigarettes, as well. Ultimately, each participant answered a similarly-worded set of questions for both regular and e-cigarettes. Dual use of cigarettes and e-cigarettes was also assessed. Participants self-reported all data and survey responses on an electronic tablet and received a $10 (cash or gift card) incentive. This project was reviewed and approved by the Springfield Committee for Research Involving Human Subjects.
Stratification of results. Race was dichotomized into white and non-white. Education was stratified into 3 categories: up to and including high school graduation, some college but not a Bachelor’s degree, and Bachelor’s degree and above. Income was divided as being ≤$20,000 or >$20,000, and age was split into 2 groups by the median value. Analyses included descriptions of participant demographics, dual use status, measures of nicotine physical dependence, quit attempts, and e-cigarette use motivations. Bivariate relationships between dual use status and demographic characteristics, nicotine physical dependence, and smoking cessation beliefs were analyzed by chi-square (categorical variables) and ANOVA (continuous/Likert variables).
Multivariable logistic regression modeling of the demographic variables and dual use status (cigarette smoker only vs dual user) was performed to predict 3 factors: number of cigarettes smoked per day (≤10 vs 11+); time to first cigarette (≤30 vs 31+ minutes from waking); and perceived likelihood of quit attempt success (very/somewhat likely vs very/somewhat unlikely). Multivariable models examining the reasons for dual use included the demographic, nicotine physical dependence, and cessation belief items described previously.
RESULTS
Of 309 total survey participants (Fair=288; Race=12; Email=9), there were 235 current cigarette smokers consisting of 79 who smoked only cigarettes (smokers); 122 who used both cigarettes and e-cigarettes (dual users); and 34 former e-cigarette users. Only smokers and dual users were included in this analysis (N=201, although for the purposes of TABLE 1, N=200 or 199 because at least one participant did not provide answers to all of the questions). Approximately 51% of the smokers were male, 78% were white, 12% were 4-year college graduates, and 57% reported incomes >$20,000. The mean age was 37.7 years (SD=14.4); 50% of respondents were <35 years of age. Dual users did not vary significantly from smokers in terms of gender, age, education, or income (all P>.05). However, a greater proportion of whites vs non-whites were dual users (54.9% vs 42.3%; P=.035).
Click here to see an enlarged version of the table.
No big quit differences. Bivariate analyses revealed that dual users were no more likely than smokers to have attempted to quit smoking within the past year (X2=2.3; P=.14), consider quitting in the next one or 6 months (X2=1.1; P=.34), or differ in perceived likelihood of cessation success (X2=0.0; P=1.00). The proportion of dual users who smoked 11+ cigarettes per day did not differ from that of cigarette smokers for the group as a whole or when the group was stratified by gender, income, education, or age. However, among non-whites, dual users smoked fewer cigarettes than cigarette smokers (TABLE 1).
Predicting physical dependence. Significant differences also were observed regarding the timing of the first cigarette of the day, with dual users approximately 3 times more likely than smokers to smoke within 30 minutes of awakening (80% vs 54.4%; OR=3.3; 95% CI, 1.8-6.3), and this difference was upheld among males, females, whites, those with an income >$20,000, those with a high school education or less and those with some college education, and age >34 years. There was no association, however, between dual use and perceived likelihood of quit success.
We then performed multivariable logistic modeling on dual users to determine which variables might predict 3 measures of physical dependence: number of cigarettes smoked per day (≤10 vs 11+), time between waking and smoking the first cigarette of the day (≤30 vs 31+ minutes), and perceived likelihood of cessation success (TABLE 2). Male gender (OR=3.4; 95% CI, 1.8-6.5) and white race (OR=4.4; 95% CI, 1.9-10.1) were significant for predicting smoking 11+ cigarettes a day, while dual use status was insignificant (P=.104). Regarding time to first cigarette, only dual use was significant (OR=3.1; 95% CI, 1.6-5.9), with dual users approximately 3 times more likely than smokers to have their first cigarette within 30 minutes of waking. No variables were significant in predicting perceived likelihood of quit success.
Reasons for dual use. We examined reasons for dual use with the question: Do you use e-cigarettes to reduce your regular tobacco use? Here, 79.5% of smokers reported using e-cigarettes to quit smoking or reduce the number of cigarettes smoked.
A multivariable polynomial logistic regression that included only dual users was performed to examine which variables might predict use for tobacco cessation (“trying to quit smoking”) vs reduction in smoking intensity (“trying to reduce the number of regular cigarettes I smoke per day”) vs no change (“use the same amount of tobacco as always”) (TABLE 2). Whites were approximately 6 times more likely than non-whites to indicate they engage in dual use to try to quit smoking (OR=6.0; 95% CI, 1.1-32.9). Males and people with lower incomes were much less likely to indicate they engaged in dual use to try to reduce the number of regular cigarettes smoked than females or those with higher incomes (OR=0.2; 95% CI, 0.1-0.8 and OR=0.1, 95% CI, 0.0-0.5, respectively). No other demographic variables or measures of nicotine physical dependence were significantly different between dual users and smokers.
Click here to see an enlarged version of the table.
DISCUSSION
E-cigarettes are used by approximately half of smokers (52%), which is much higher than that reported by Delnevo, et al, in their analysis of the National Health Interview Study.33 There, prevalence of dual use of both cigarettes and e-cigarettes ranged from 3.4% to 12.7%. This substantial difference raises important questions regarding study population characterization. Were participants in our study representative of central Illinois, state fair attendees, or the agricultural profession? Further work to identify this group with an increased propensity for dual use will assist clinicians in developing appropriate intervention strategies.
Dual use in our study did not vary by many customary demographic variables. Nor was it associated with different rates of past or future quit attempts or perceived ability to successfully quit if quitting was attempted. These factors—high rates of dual use and insignificant effect on quit attempts—may have implications for local physicians counseling patients who smoke.
In our study, the majority of smokers already use e-cigarettes, and this does not seem to increase their ability/likelihood to quit smoking. Further, dual use did not seem to be associated with overall cigarette consumption; males and white participants smoked more cigarettes than females and non-whites. But dual use was associated with a measure of increased nicotine physical dependence (earlier first cigarette of the day). As a result, physicians may want to think twice before recommending e-cigarette use as a means of smoking cessation.
In addition to the high prevalence of e-cigarette use among smokers, a number of other interesting findings surfaced that run counter to some of the current literature. First, dual users are no more likely than smokers to have tried to quit in the past or to try to quit in the future.21,22,34 It could be that for the relatively small geographical area from which our participants were recruited (central Illinois; ~77% of participants from Sangamon County alone), the local context and culture of smoking differs from that associated with participants in other studies, who were mostly recruited from national and regional online surveys. However, there is no a priori reason to suspect Sangamon County is especially different, as it is quite similar to Illinois as a whole by many measures (eg, percentage rural: 14.1% vs 11.5%; percentage black (only): 12.4% vs 14.7%; education to at least a Bachelor’s degree: 33.0% vs 31.9%; and median household income: $55,565 vs $57,166).30
While we found that dual users did have one measure of increased nicotine physical dependence, the total number of cigarettes consumed per day was not significantly different from that of smokers.23-25 This is contrary to another study of nicotine physical dependence, but, unlike that study, we did not assess length of time of concurrent use.35 There is much uncertainty surrounding the issue of nicotine physical dependence and e-cigarette use, largely because the level of nicotine delivered by various e-products varies significantly.36
Cross-sectional nature, small sample size limit utility of data
There are significant limitations to this study, including the cross-sectional nature of the data, the small sample size, the use of self-report, and the limited scope of recruitment. The relatively small sample size limits our ability to observe small differences and effect sizes. However, small differences often lack practical significance. Finally, participation was limited to those attending a state fair or a local sporting event and those employed by a local medical school. Thus, the results may not be generalizable to populations outside central Illinois. On the other hand, the very low income sample recruited from the Midwestern US, which is underrepresented in prior e-cigarette research, might represent some of the strengths of this work.
Future investigations. Future studies should more closely examine e-cigarette use prevalence on smaller geographic scales and especially in rural areas where there is a paucity of research. As the majority of our respondents came from a single county in central Illinois, one has to ask the questions, “Is this a ‘hot spot’ for e-cigarette use?" And "Do other rural areas experience similar use?” It may be important to know if national surveys are sensitive enough to observe significant local variations. Research also should examine how e-cigarette use and the influence of local culture vary across wider areas.
Several specific areas of study would help to inform policy and intervention development. For example, is tobacco cigarette quit success impacted by concurrent e-cigarette use? While our study showed no difference in past or possible future quit attempts among dual users as compared with smokers, we did not assess actual quit success, and multiple participants in our study anecdotally described using e-cigarettes to successfully quit smoking.
In the end, the rapid increase in the use of e-cigarettes has the potential to significantly alter the landscape of nicotine physical dependence, and local culture and other influences are likely associated with use patterns.
CORRESPONDENCE
Wiley D. Jenkins, PhD, MPH, Science Director, Population Health Science Program, Southern Illinois University School of Medicine, 201 E. Madison St., Springfield, IL 62794-9664; [email protected].
1. Agaku IT, King BA, Husten CG, et al; Centers for Disease Control and Prevention (CDC). Tobacco product use among adults—United States, 2012-2013. MMWR Morb Mortal Wkly Rep. 2014;63:542-547.
2. Jamal A, Agaku IT, O’Connor E, et al. Current cigarette smoking among adults—United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2014;63:1108-1112.
3. Siegel RL, Jacobs EJ, Newton CC, et al. Deaths due to cigarette smoking for 12 smoking-related cancers in the United States. JAMA Intern Med. 2015;175:1574-1576.
4. US Department of Health and Human Services. Surgeon General. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014. Available at: http://www.surgeongeneral.gov/library/reports/50-years-of-progress/index.html. Accessed January 22, 2014.
5. Gamm LD, Hutchison LL, Dabney BJ, et al, eds. (2003). Rural Healthy People 2010: A companion document to Healthy People 2010. Volume 2. College Station, TX: The Texas A&M University System Health Science Center, School of Rural Public Health, Southwest Rural Health Research Center.
6. Doescher MP, Jackson JE, Jerant A, et al. Prevalence and trends in smoking: a national rural study. J Rural Health. 2006;22:112-118.
7. Bunnell RE, Agaku IT, Arrazola RA, et al. Intentions to smoke cigarettes among never-smoking US middle and high school electronic cigarette users: National Youth Tobacco Survey, 2011-2013. Nicotine Tob Res. 2015;17:228-235.
8. Cardenas VM, Evans VL, Balamurugan A, et al. Use of electronic nicotine delivery systems and recent initiation of smoking among US youth. Int J Public Health. 2016;61:237-241.
9. Auf R, Trepka MJ, Cano MA, et al. Electronic cigarettes: the renormalisation of nicotine use. BMJ. 2016;352:i425.
10. CNBC. E-cigarette sales are smoking hot, set to hit $1.7 billion. Available at: http://www.cnbc.com/id/100991511. Accessed April 5, 2016.
11. Weaver SR, Majeed BA, Pechacek TF, et al. Use of electronic nicotine delivery systems and other tobacco products among USA adults, 2014: results from a national survey. Int J Public Health. 2016;61:177-188.
12. Richardson A, Ganz O, Vallone D. Tobacco on the web: surveillance and characterisation of online tobacco and e-cigarette advertising. Tob Control. 2015;24:341-347.
13. Paek HJ, Kim S, Hove T, et al. Reduced harm or another gateway to smoking? source, message, and information characteristics of E-cigarette videos on YouTube. J Health Commun. 2014;19:545-560.
14. Kim AE, Arnold KY, Makarenko O. E-cigarette advertising expenditures in the U.S., 2011-2012. Am J Prev Med. 2014;46:409-412.
15. Steinberg MB, Giovenco DP, Delnevo CD. Patient-physician communication regarding electronic cigarettes. Prev Med Rep. 2015;2:96-98.
16. Gualano MR, Passi S, Bert F, et al. Electronic cigarettes: assessing the efficacy and the adverse effects through a systematic review of published studies. J Public Health (Oxf). 2015:37:488-497.
17. U.S. National Institutes of Health. ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/results?term=%22electronic+cigarette%22&Search=Search. Accessed July 10, 2015.
18. Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2015;17:127-133.
19. Vickerman KA, Carpenter KM, Altman T, et al. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787-1791.
20. Center for Disease Control and Prevention. Press Release February 28,2013. Available at: http://www.cdc.gov/media/releases/2013/p0228_electronic_cigarettes.html. Accessed July 8, 2015.
21. Pisinger C. Why public health people are more worried than excited over e-cigarettes. BMC Med. 2014;12:226.
22. Post A, Gilljam H, Rosendahl I, et al. Symptoms of nicotine dependence in a cohort of Swedish youths: a comparison between smokers, smokeless tobacco users and dual tobacco users. Addiction. 2010;105:740-746.
23. Mazurek JM, Syamlal G, King BA, et al; Division of Respiratory Disease Studies, National Institute for Occupational Safety and Health, CDC. Smokeless tobacco use among working adults—United States, 2005 and 2010. MMWR Morb Mortal Wkly Rep. 2014;63:477-482.
24. Hutcheson TD, Greiner KA, Ellerbeck EF, et al. Understanding smoking cessation in rural communities. J Rural Health. 2008;24:116-124.
25. McMillen R, Breen J, Cosby AG. Rural-urban differences in the social climate surrounding environmental tobacco smoke: a report from the 2002 Social Climate Survey of Tobacco Control. J Rural Health. 2004;20:7-16.
26. Butler KM, Rayens MK, Adkins S, et al. Culturally-specific smoking cessation outreach in a rural community. Public Health Nurs. 2014;31:44-54.
27. Butler KM, Hedgecock S, Record RA, et al. An evidence-based cessation strategy using rural smokers’ experiences with tobacco. Nurs Clin North Am. 2012;47:31-43.
28. Hamilton HA, Ferrence R, Boak A, et al. Ever use of nicotine and nonnicotine electronic cigarettes among high school students in Ontario, Canada. Nicotine Tob Res. 2015;17:1212-1218.
29. Goniewicz ML, Zielinska-Danch W. Electronic cigarette use among teenagers and young adults in Poland. Pediatrics. 2012;130:e879-e885.
30. US Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Available at: http://www.census.gov/geo/reference/ua/urban-rural-2010.html. Accessed March 13, 2016.
31. Minnesota Adult Tobacco Survey. Tobacco use in Minnesota: 1999-2014. Available at: http://www.mnadulttobaccosurvey.org/. Accessed April 27, 2016.
32. Rash CJ, Copeland AL. The Brief Smoking Consequences Questionnaire-Adult (BSCQ-A): development of a short form of the SCQ-A. Nicotine Tob Res. 2008;10:1633-1643.
33. Delnevo CD, Giovenco DP, Steinberg MB, et al. Patterns of electronic cigarette use among adults in the United States. Nicotine Tob Res. 2016;18:715-719.
34. Lee YO, Hebert CJ, Nonnemaker JM, et al. Multiple tobacco product use among adults in the United States: cigarettes, cigars, electronic cigarettes, hookah, smokeless tobacco, and snus. Prev Med. 2014;62:14-19.
35. Etter JF, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015;147:68-75.
36. Cobb CO, Hendricks PS, Eissenberg T. Electronic cigarettes and nicotine dependence: evolving products, evolving problems. BMC Med. 2015;13:119.
1. Agaku IT, King BA, Husten CG, et al; Centers for Disease Control and Prevention (CDC). Tobacco product use among adults—United States, 2012-2013. MMWR Morb Mortal Wkly Rep. 2014;63:542-547.
2. Jamal A, Agaku IT, O’Connor E, et al. Current cigarette smoking among adults—United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2014;63:1108-1112.
3. Siegel RL, Jacobs EJ, Newton CC, et al. Deaths due to cigarette smoking for 12 smoking-related cancers in the United States. JAMA Intern Med. 2015;175:1574-1576.
4. US Department of Health and Human Services. Surgeon General. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014. Available at: http://www.surgeongeneral.gov/library/reports/50-years-of-progress/index.html. Accessed January 22, 2014.
5. Gamm LD, Hutchison LL, Dabney BJ, et al, eds. (2003). Rural Healthy People 2010: A companion document to Healthy People 2010. Volume 2. College Station, TX: The Texas A&M University System Health Science Center, School of Rural Public Health, Southwest Rural Health Research Center.
6. Doescher MP, Jackson JE, Jerant A, et al. Prevalence and trends in smoking: a national rural study. J Rural Health. 2006;22:112-118.
7. Bunnell RE, Agaku IT, Arrazola RA, et al. Intentions to smoke cigarettes among never-smoking US middle and high school electronic cigarette users: National Youth Tobacco Survey, 2011-2013. Nicotine Tob Res. 2015;17:228-235.
8. Cardenas VM, Evans VL, Balamurugan A, et al. Use of electronic nicotine delivery systems and recent initiation of smoking among US youth. Int J Public Health. 2016;61:237-241.
9. Auf R, Trepka MJ, Cano MA, et al. Electronic cigarettes: the renormalisation of nicotine use. BMJ. 2016;352:i425.
10. CNBC. E-cigarette sales are smoking hot, set to hit $1.7 billion. Available at: http://www.cnbc.com/id/100991511. Accessed April 5, 2016.
11. Weaver SR, Majeed BA, Pechacek TF, et al. Use of electronic nicotine delivery systems and other tobacco products among USA adults, 2014: results from a national survey. Int J Public Health. 2016;61:177-188.
12. Richardson A, Ganz O, Vallone D. Tobacco on the web: surveillance and characterisation of online tobacco and e-cigarette advertising. Tob Control. 2015;24:341-347.
13. Paek HJ, Kim S, Hove T, et al. Reduced harm or another gateway to smoking? source, message, and information characteristics of E-cigarette videos on YouTube. J Health Commun. 2014;19:545-560.
14. Kim AE, Arnold KY, Makarenko O. E-cigarette advertising expenditures in the U.S., 2011-2012. Am J Prev Med. 2014;46:409-412.
15. Steinberg MB, Giovenco DP, Delnevo CD. Patient-physician communication regarding electronic cigarettes. Prev Med Rep. 2015;2:96-98.
16. Gualano MR, Passi S, Bert F, et al. Electronic cigarettes: assessing the efficacy and the adverse effects through a systematic review of published studies. J Public Health (Oxf). 2015:37:488-497.
17. U.S. National Institutes of Health. ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/results?term=%22electronic+cigarette%22&Search=Search. Accessed July 10, 2015.
18. Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2015;17:127-133.
19. Vickerman KA, Carpenter KM, Altman T, et al. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787-1791.
20. Center for Disease Control and Prevention. Press Release February 28,2013. Available at: http://www.cdc.gov/media/releases/2013/p0228_electronic_cigarettes.html. Accessed July 8, 2015.
21. Pisinger C. Why public health people are more worried than excited over e-cigarettes. BMC Med. 2014;12:226.
22. Post A, Gilljam H, Rosendahl I, et al. Symptoms of nicotine dependence in a cohort of Swedish youths: a comparison between smokers, smokeless tobacco users and dual tobacco users. Addiction. 2010;105:740-746.
23. Mazurek JM, Syamlal G, King BA, et al; Division of Respiratory Disease Studies, National Institute for Occupational Safety and Health, CDC. Smokeless tobacco use among working adults—United States, 2005 and 2010. MMWR Morb Mortal Wkly Rep. 2014;63:477-482.
24. Hutcheson TD, Greiner KA, Ellerbeck EF, et al. Understanding smoking cessation in rural communities. J Rural Health. 2008;24:116-124.
25. McMillen R, Breen J, Cosby AG. Rural-urban differences in the social climate surrounding environmental tobacco smoke: a report from the 2002 Social Climate Survey of Tobacco Control. J Rural Health. 2004;20:7-16.
26. Butler KM, Rayens MK, Adkins S, et al. Culturally-specific smoking cessation outreach in a rural community. Public Health Nurs. 2014;31:44-54.
27. Butler KM, Hedgecock S, Record RA, et al. An evidence-based cessation strategy using rural smokers’ experiences with tobacco. Nurs Clin North Am. 2012;47:31-43.
28. Hamilton HA, Ferrence R, Boak A, et al. Ever use of nicotine and nonnicotine electronic cigarettes among high school students in Ontario, Canada. Nicotine Tob Res. 2015;17:1212-1218.
29. Goniewicz ML, Zielinska-Danch W. Electronic cigarette use among teenagers and young adults in Poland. Pediatrics. 2012;130:e879-e885.
30. US Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Available at: http://www.census.gov/geo/reference/ua/urban-rural-2010.html. Accessed March 13, 2016.
31. Minnesota Adult Tobacco Survey. Tobacco use in Minnesota: 1999-2014. Available at: http://www.mnadulttobaccosurvey.org/. Accessed April 27, 2016.
32. Rash CJ, Copeland AL. The Brief Smoking Consequences Questionnaire-Adult (BSCQ-A): development of a short form of the SCQ-A. Nicotine Tob Res. 2008;10:1633-1643.
33. Delnevo CD, Giovenco DP, Steinberg MB, et al. Patterns of electronic cigarette use among adults in the United States. Nicotine Tob Res. 2016;18:715-719.
34. Lee YO, Hebert CJ, Nonnemaker JM, et al. Multiple tobacco product use among adults in the United States: cigarettes, cigars, electronic cigarettes, hookah, smokeless tobacco, and snus. Prev Med. 2014;62:14-19.
35. Etter JF, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015;147:68-75.
36. Cobb CO, Hendricks PS, Eissenberg T. Electronic cigarettes and nicotine dependence: evolving products, evolving problems. BMC Med. 2015;13:119.
Respiratory exacerbations rate high in patients not meeting COPD criteria
The clinical definition of chronic obstructive pulmonary disease (COPD) may need to be revised, based on results from a multicenter observational study of 2,736 individuals.
Respiratory symptoms of COPD were present in 425 of 849 current or former smokers who did not meet the standard spirometry criteria for diagnosing COPD. The 425 study participants who were symptomatic for COPD but were considered to have preserved pulmonary function had a significantly higher rate of respiratory exacerbations, compared with that of the 424 current or former smokers who were asymptomatic for COPD and were not classified as having the disease (0.27 +/- 0.67 events vs. 0.08 +/- 0.31 events; P less than .001).
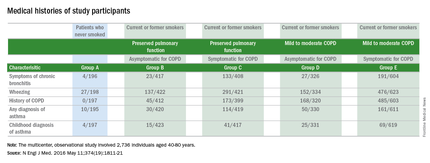
Using spirometry to define who should receive a diagnosis of COPD does not address all people with symptomatic smoking-related lung disease. This large population needs to be studied to better define appropriate treatment strategies, Dr. Prescott G. Woodruff, a professor of medicine at the University of California, San Francisco, and his associates noted (N Engl J Med. 2016 May 11;[19]374:1811-21.).
Study participants were classified as not having COPD if the ratios of their forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) was 0.70 or more after bronchodilator use and if their FVC was above the lower limit of the normal range. During a stable phase of disease, which was defined as greater than six weeks after a respiratory exacerbation, patients participated in the COPD Assessment Test (CAT), an eight-question health status instrument. Patients with a CAT score of greater than or equal to 10 were considered to be symptomatic for COPD and those with CAT scores of less than 10 were considered to be asymptomatic for COPD.
While 963 of the 1,812 study participants who were current or former smokers were classified as having Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 1 or 2 COPD, half of the current or former smokers who were not classified as having COPD were still symptomatic for the disease. Additionally, among the 199 study participants who had never smoked, 16% had COPD symptoms.
Current or former smokers classified as not having COPD, who were symptomatic for the disease, had elevations in all components of the CAT score, were younger, were more likely to be nonwhite or Hispanic, and had a higher body mass index than those with a CAT score of less than 10. These patients with preserved pulmonary function despite being symptomatic for COPD also were more likely to be current smokers, report symptoms of chronic bronchitis, report a history of wheezing and asthma, and report a previous diagnosis of COPD, compared with the asymptomatic patients with preserved pulmonary function.
From 2010 through 2015, 2,736 individuals, aged 40-80 years, were enrolled in the study. The study participants had either never smoked (defined as having smoked less than or equal to 1 pack-year of tobacco use) or were current or former smokers with a smoking history of more than 20 pack-years. Spirometry was used to determine if study participants had COPD. This was performed before and after four inhalations each of albuterol, at a dose of 90 mcg per inhalation, and ipratropium, at a dose of 18 mcg per inhalation.
Among the other data obtained by the researchers were the patients’ 6-minute walk distances. The average 6-minute walk distance was shorter for patients with symptoms of COPD, compared with those with CAT scores of less than 10.
This study shows that the CAT can identify smokers at risk for exacerbations and provides evidence that individuals with COPD symptoms may have airway disease, according to the researchers.
“Clinical trials are needed to determine whether maintenance therapy with bronchodilators or inhaled glucocorticoids will alleviate symptoms and reduce the rate of respiratory exacerbations in [current or former smokers with elevated respiratory exacerbation rates who do not meet the standard criteria for having COPD],” the researchers said.
This study was supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health and by the Foundation for the National Institutes of Health through contributions made to an external advisory board composed of members from several pharmaceutical companies. The authors’ disclosures are available at NEJM.org.
It is often symptoms that bring patients to seek hospital care. This study points out a split between smoking-related respiratory symptoms and the preserved spirometry that was found in a portion of the study group. Further research may better characterize this population of symptomatic current and former smokers. With identification of effective treatments and improved symptom management, we may be able to promote better respiratory health, thereby reducing hospital utilization.
Dr. Vera De Palo is Editor in Chief of CHEST Physician. She is the Chief Medical Officer at Signature Healthcare in Brockton, Massachusetts.
It is often symptoms that bring patients to seek hospital care. This study points out a split between smoking-related respiratory symptoms and the preserved spirometry that was found in a portion of the study group. Further research may better characterize this population of symptomatic current and former smokers. With identification of effective treatments and improved symptom management, we may be able to promote better respiratory health, thereby reducing hospital utilization.
Dr. Vera De Palo is Editor in Chief of CHEST Physician. She is the Chief Medical Officer at Signature Healthcare in Brockton, Massachusetts.
It is often symptoms that bring patients to seek hospital care. This study points out a split between smoking-related respiratory symptoms and the preserved spirometry that was found in a portion of the study group. Further research may better characterize this population of symptomatic current and former smokers. With identification of effective treatments and improved symptom management, we may be able to promote better respiratory health, thereby reducing hospital utilization.
Dr. Vera De Palo is Editor in Chief of CHEST Physician. She is the Chief Medical Officer at Signature Healthcare in Brockton, Massachusetts.
The clinical definition of chronic obstructive pulmonary disease (COPD) may need to be revised, based on results from a multicenter observational study of 2,736 individuals.
Respiratory symptoms of COPD were present in 425 of 849 current or former smokers who did not meet the standard spirometry criteria for diagnosing COPD. The 425 study participants who were symptomatic for COPD but were considered to have preserved pulmonary function had a significantly higher rate of respiratory exacerbations, compared with that of the 424 current or former smokers who were asymptomatic for COPD and were not classified as having the disease (0.27 +/- 0.67 events vs. 0.08 +/- 0.31 events; P less than .001).

Using spirometry to define who should receive a diagnosis of COPD does not address all people with symptomatic smoking-related lung disease. This large population needs to be studied to better define appropriate treatment strategies, Dr. Prescott G. Woodruff, a professor of medicine at the University of California, San Francisco, and his associates noted (N Engl J Med. 2016 May 11;[19]374:1811-21.).
Study participants were classified as not having COPD if the ratios of their forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) was 0.70 or more after bronchodilator use and if their FVC was above the lower limit of the normal range. During a stable phase of disease, which was defined as greater than six weeks after a respiratory exacerbation, patients participated in the COPD Assessment Test (CAT), an eight-question health status instrument. Patients with a CAT score of greater than or equal to 10 were considered to be symptomatic for COPD and those with CAT scores of less than 10 were considered to be asymptomatic for COPD.
While 963 of the 1,812 study participants who were current or former smokers were classified as having Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 1 or 2 COPD, half of the current or former smokers who were not classified as having COPD were still symptomatic for the disease. Additionally, among the 199 study participants who had never smoked, 16% had COPD symptoms.
Current or former smokers classified as not having COPD, who were symptomatic for the disease, had elevations in all components of the CAT score, were younger, were more likely to be nonwhite or Hispanic, and had a higher body mass index than those with a CAT score of less than 10. These patients with preserved pulmonary function despite being symptomatic for COPD also were more likely to be current smokers, report symptoms of chronic bronchitis, report a history of wheezing and asthma, and report a previous diagnosis of COPD, compared with the asymptomatic patients with preserved pulmonary function.
From 2010 through 2015, 2,736 individuals, aged 40-80 years, were enrolled in the study. The study participants had either never smoked (defined as having smoked less than or equal to 1 pack-year of tobacco use) or were current or former smokers with a smoking history of more than 20 pack-years. Spirometry was used to determine if study participants had COPD. This was performed before and after four inhalations each of albuterol, at a dose of 90 mcg per inhalation, and ipratropium, at a dose of 18 mcg per inhalation.
Among the other data obtained by the researchers were the patients’ 6-minute walk distances. The average 6-minute walk distance was shorter for patients with symptoms of COPD, compared with those with CAT scores of less than 10.
This study shows that the CAT can identify smokers at risk for exacerbations and provides evidence that individuals with COPD symptoms may have airway disease, according to the researchers.
“Clinical trials are needed to determine whether maintenance therapy with bronchodilators or inhaled glucocorticoids will alleviate symptoms and reduce the rate of respiratory exacerbations in [current or former smokers with elevated respiratory exacerbation rates who do not meet the standard criteria for having COPD],” the researchers said.
This study was supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health and by the Foundation for the National Institutes of Health through contributions made to an external advisory board composed of members from several pharmaceutical companies. The authors’ disclosures are available at NEJM.org.
The clinical definition of chronic obstructive pulmonary disease (COPD) may need to be revised, based on results from a multicenter observational study of 2,736 individuals.
Respiratory symptoms of COPD were present in 425 of 849 current or former smokers who did not meet the standard spirometry criteria for diagnosing COPD. The 425 study participants who were symptomatic for COPD but were considered to have preserved pulmonary function had a significantly higher rate of respiratory exacerbations, compared with that of the 424 current or former smokers who were asymptomatic for COPD and were not classified as having the disease (0.27 +/- 0.67 events vs. 0.08 +/- 0.31 events; P less than .001).

Using spirometry to define who should receive a diagnosis of COPD does not address all people with symptomatic smoking-related lung disease. This large population needs to be studied to better define appropriate treatment strategies, Dr. Prescott G. Woodruff, a professor of medicine at the University of California, San Francisco, and his associates noted (N Engl J Med. 2016 May 11;[19]374:1811-21.).
Study participants were classified as not having COPD if the ratios of their forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) was 0.70 or more after bronchodilator use and if their FVC was above the lower limit of the normal range. During a stable phase of disease, which was defined as greater than six weeks after a respiratory exacerbation, patients participated in the COPD Assessment Test (CAT), an eight-question health status instrument. Patients with a CAT score of greater than or equal to 10 were considered to be symptomatic for COPD and those with CAT scores of less than 10 were considered to be asymptomatic for COPD.
While 963 of the 1,812 study participants who were current or former smokers were classified as having Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 1 or 2 COPD, half of the current or former smokers who were not classified as having COPD were still symptomatic for the disease. Additionally, among the 199 study participants who had never smoked, 16% had COPD symptoms.
Current or former smokers classified as not having COPD, who were symptomatic for the disease, had elevations in all components of the CAT score, were younger, were more likely to be nonwhite or Hispanic, and had a higher body mass index than those with a CAT score of less than 10. These patients with preserved pulmonary function despite being symptomatic for COPD also were more likely to be current smokers, report symptoms of chronic bronchitis, report a history of wheezing and asthma, and report a previous diagnosis of COPD, compared with the asymptomatic patients with preserved pulmonary function.
From 2010 through 2015, 2,736 individuals, aged 40-80 years, were enrolled in the study. The study participants had either never smoked (defined as having smoked less than or equal to 1 pack-year of tobacco use) or were current or former smokers with a smoking history of more than 20 pack-years. Spirometry was used to determine if study participants had COPD. This was performed before and after four inhalations each of albuterol, at a dose of 90 mcg per inhalation, and ipratropium, at a dose of 18 mcg per inhalation.
Among the other data obtained by the researchers were the patients’ 6-minute walk distances. The average 6-minute walk distance was shorter for patients with symptoms of COPD, compared with those with CAT scores of less than 10.
This study shows that the CAT can identify smokers at risk for exacerbations and provides evidence that individuals with COPD symptoms may have airway disease, according to the researchers.
“Clinical trials are needed to determine whether maintenance therapy with bronchodilators or inhaled glucocorticoids will alleviate symptoms and reduce the rate of respiratory exacerbations in [current or former smokers with elevated respiratory exacerbation rates who do not meet the standard criteria for having COPD],” the researchers said.
This study was supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health and by the Foundation for the National Institutes of Health through contributions made to an external advisory board composed of members from several pharmaceutical companies. The authors’ disclosures are available at NEJM.org.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Respiratory symptoms of COPD were present in 425 of 849 current or former smokers who did not meet the standard spirometry criteria for diagnosing COPD.
Major finding: The 425 study participants who were symptomatic for COPD but did not meet the standard spirometry criteria for diagnosing COPD had a significantly higher rate of respiratory exacerbations, compared with that of the 424 current or former smokers who were asymptomatic for COPD and were not classified as having the disease (0.27 +/- 0.67 events vs. 0.08 +/- 0.31 events; P less than .001).
Data source: Results are from a multicenter observational study that enrolled 2,736 individuals from 2010 to 2015.
Disclosures: This study was supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health and by the Foundation for the National Institutes of Health through contributions made to an external advisory board composed of members from several pharmaceutical companies. The authors’ disclosures are available at NEJM.org.
Vaping: Are Its “Benefits” a Lot of Hot Air?
I was sitting in a restaurant bar a few days ago when a huge puff of cherry-scented smoke engulfed the area. As a former firefighter, I immediately looked around to find the source. You guessed it: a group of young adults were “vaping” nearby. This method of smoking is accomplished with an electronic “cigarette.” A sensor inside the e-cigarette detects airflow and initiates a heating element that vaporizes a liquid solution containing propylene glycol (organic compound with the chemical formula C₃H₈O₂), the flavoring of choice, and nicotine.1
I knew of this fad but didn’t give it much thought until recently, when I realized how pervasive it has become. Frankly, I have always thought, At least they are not smoking cigarettes and inhaling all that benzene, carbon dioxide, and formaldehyde.
We all know smoking cessation is valuable to the health of the population, but what do we know about the effects of vaping? For one thing, use of e-cigarettes (vapes) has increased considerably since they were first introduced (0.3% to 6.8% between 2007 and 2010).This is cause for concern, because while some research on e-cigarettes has emerged since their appearance, there are few definitive answers regarding their effect on human health.2
We also know that nicotine is addictive and toxic (in high doses), but we do not know the effects of propylene glycol, although it is generally recognized as “safe.” Symptoms that may occur as a result of vaporized propylene glycol inhalation include throat and ocular irritation, cough, mild airway obstruction, throat and vocal cord inflammation, headache, and dizziness. In spite of this, since the manufacturers of e-cigarettes have not made any therapeutic claims about their products, the FDA initially did not regulate them.
With e-cigarettes appearing in vaping shops, gas stations, and convenience stores—alongside advertising copy that claims vaping can help smokers curtail their habit by inhaling “harmless water vapor”—what should we tell our patients? These advertisements tout vaping as the “lesser of two evils” when compared to cigarettes. How can you knock that logic when we know cigarette smoking causes one in five deaths in the US each year and is a leading risk factor for COPD?3
Continue for the conundrum >>
The conundrum, as I see it, is threefold. The first step is to determine if vaping is a significant alternative to smoking cessation. The second is to determine if any components of vaping (nicotine, propylene glycol, or combustion) are safe for humans. Lastly, we must establish how to regulate e-cigarettes, given scientific uncertainty as to their therapeutic effects.4
In 2013, Palazzolo did a literature review of 66 articles related to e-cigarettes and vaping. He found that, when compared to the effects of smoking, vaping could be a substitute for smoking and a conceivable means for smoking reduction. It was unclear, however, if vaping could reduce nicotine addiction. He reported that the effects of vaping on human health are questionable, due to the extreme scarcity of empiric research.5
Although there has been a paucity of research on this topic, a study by Goniewicz and colleagues reports on the toxicants emitted by tobacco cigarettes and e-cigarettes.6 Their results indicate that e-cigarettes emit fewer toxicants than traditional tobacco cigarettes (formaldehyde, 0.20-5.61 µg and 1.6-52 µg, respectively; acetaldehyde, 0.11-1.36 µg and 52-140 µg).7 Despite this evidence, more studies need to be done on the effects of propylene glycol inhalation to determine the safety of e-cigarettes.
Another concern has been the lack of an age restriction on e-cigarettes and their growing popularity among grade and high school students.E-cigarette use doubled among US middle and high school students from 2011 to 2012, resulting in an estimated 1.78 million students who have used e-cigarettes as of 2012. There is serious concern about the possible harmful impact of nicotine on adolescent brain development,as well as the risk for nicotine addiction.8
Amid these growing questions and concerns, the FDA issued a warning that e-cigarettes may be as bad as the real thing and has recommended against their use.9 Just last month, the agency finalized a regulation on all tobacco products, including vaporizers, vape pens, hookah pens, e-cigs, and e-pipes. They will now regulate the manufacturing, import, packaging, labeling, and distribution of e-cigarettes to ensure that ingredients are assessed and determined to be safe for human use.FDA Commissioner Robert M. Califf, MD, said, “We must do our job under the Tobacco Control Act to reduce the harms caused by tobacco. That includes ensuring that consumers have the information they need to make informed decisions about tobacco use and making sure that new tobacco products for purchase come under comprehensive FDA review.”10,11
Well, is it just a bunch of smoke, or are the new regulations and health warnings about e-cigarettes long overdue? I would love to hear your experiences and additional advice for our colleagues and patients regarding the use of e-cigarettes and vaping. You can reach me at [email protected].
References
1. Jerry JM, Collins GB, Streem D. E-cigarettes: safe to recommend to patients? Cleve Clin J Med. 2015;82(8):521-526.
2. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
3. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed May 10, 2016.
4. Gostin LO, Glasner AY. E-cigarettes, vaping, and youth. JAMA. 2014;312(6):595-596.
5. Palazzolo DL. Electronic cigarettes and vaping: a new challenge in clinical medicine and public health. A literature review. Front Public Health. 2013;1(56):1-20.
6. Goniewicz ML, Hajek P, McRobbie H. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications. Addiction. 2014;109(3):500-507.
7. Arnold C. Vaping and health: what do we know about e-cigarettes? Environ Health Perspect. 2014;122(9):A244-A249. http://ehp.niehs.nih.gov/122-a244. Accessed May 10, 2016.
8. Electronic cigarette use among middle and high school students. Medscape. September 6, 2013. www.medscape.com/viewarticle/811008. Accessed May 10, 2016.
9. FDA: E-cigarettes may be as bad as real thing. NBC Nightly News. July 22, 2015. www.nbcnews.com/video/nightly-news/32091534#32091534. Accessed May 10, 2016.
10. Caudle J. Why we need new rules on e-cigs. CNN. May 6, 2016. www.cnn.com/2016/05/06/opinions/fda-electronic-cigarettes-caudle. Accessed May 10, 2016.
11. FDA. FDA takes significant steps to protect Americans from dangers of tobacco through new regulation [news release]. May 5, 2016. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm499234.htm. Accessed May 10, 2016.
I was sitting in a restaurant bar a few days ago when a huge puff of cherry-scented smoke engulfed the area. As a former firefighter, I immediately looked around to find the source. You guessed it: a group of young adults were “vaping” nearby. This method of smoking is accomplished with an electronic “cigarette.” A sensor inside the e-cigarette detects airflow and initiates a heating element that vaporizes a liquid solution containing propylene glycol (organic compound with the chemical formula C₃H₈O₂), the flavoring of choice, and nicotine.1
I knew of this fad but didn’t give it much thought until recently, when I realized how pervasive it has become. Frankly, I have always thought, At least they are not smoking cigarettes and inhaling all that benzene, carbon dioxide, and formaldehyde.
We all know smoking cessation is valuable to the health of the population, but what do we know about the effects of vaping? For one thing, use of e-cigarettes (vapes) has increased considerably since they were first introduced (0.3% to 6.8% between 2007 and 2010).This is cause for concern, because while some research on e-cigarettes has emerged since their appearance, there are few definitive answers regarding their effect on human health.2
We also know that nicotine is addictive and toxic (in high doses), but we do not know the effects of propylene glycol, although it is generally recognized as “safe.” Symptoms that may occur as a result of vaporized propylene glycol inhalation include throat and ocular irritation, cough, mild airway obstruction, throat and vocal cord inflammation, headache, and dizziness. In spite of this, since the manufacturers of e-cigarettes have not made any therapeutic claims about their products, the FDA initially did not regulate them.
With e-cigarettes appearing in vaping shops, gas stations, and convenience stores—alongside advertising copy that claims vaping can help smokers curtail their habit by inhaling “harmless water vapor”—what should we tell our patients? These advertisements tout vaping as the “lesser of two evils” when compared to cigarettes. How can you knock that logic when we know cigarette smoking causes one in five deaths in the US each year and is a leading risk factor for COPD?3
Continue for the conundrum >>
The conundrum, as I see it, is threefold. The first step is to determine if vaping is a significant alternative to smoking cessation. The second is to determine if any components of vaping (nicotine, propylene glycol, or combustion) are safe for humans. Lastly, we must establish how to regulate e-cigarettes, given scientific uncertainty as to their therapeutic effects.4
In 2013, Palazzolo did a literature review of 66 articles related to e-cigarettes and vaping. He found that, when compared to the effects of smoking, vaping could be a substitute for smoking and a conceivable means for smoking reduction. It was unclear, however, if vaping could reduce nicotine addiction. He reported that the effects of vaping on human health are questionable, due to the extreme scarcity of empiric research.5
Although there has been a paucity of research on this topic, a study by Goniewicz and colleagues reports on the toxicants emitted by tobacco cigarettes and e-cigarettes.6 Their results indicate that e-cigarettes emit fewer toxicants than traditional tobacco cigarettes (formaldehyde, 0.20-5.61 µg and 1.6-52 µg, respectively; acetaldehyde, 0.11-1.36 µg and 52-140 µg).7 Despite this evidence, more studies need to be done on the effects of propylene glycol inhalation to determine the safety of e-cigarettes.
Another concern has been the lack of an age restriction on e-cigarettes and their growing popularity among grade and high school students.E-cigarette use doubled among US middle and high school students from 2011 to 2012, resulting in an estimated 1.78 million students who have used e-cigarettes as of 2012. There is serious concern about the possible harmful impact of nicotine on adolescent brain development,as well as the risk for nicotine addiction.8
Amid these growing questions and concerns, the FDA issued a warning that e-cigarettes may be as bad as the real thing and has recommended against their use.9 Just last month, the agency finalized a regulation on all tobacco products, including vaporizers, vape pens, hookah pens, e-cigs, and e-pipes. They will now regulate the manufacturing, import, packaging, labeling, and distribution of e-cigarettes to ensure that ingredients are assessed and determined to be safe for human use.FDA Commissioner Robert M. Califf, MD, said, “We must do our job under the Tobacco Control Act to reduce the harms caused by tobacco. That includes ensuring that consumers have the information they need to make informed decisions about tobacco use and making sure that new tobacco products for purchase come under comprehensive FDA review.”10,11
Well, is it just a bunch of smoke, or are the new regulations and health warnings about e-cigarettes long overdue? I would love to hear your experiences and additional advice for our colleagues and patients regarding the use of e-cigarettes and vaping. You can reach me at [email protected].
References
1. Jerry JM, Collins GB, Streem D. E-cigarettes: safe to recommend to patients? Cleve Clin J Med. 2015;82(8):521-526.
2. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
3. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed May 10, 2016.
4. Gostin LO, Glasner AY. E-cigarettes, vaping, and youth. JAMA. 2014;312(6):595-596.
5. Palazzolo DL. Electronic cigarettes and vaping: a new challenge in clinical medicine and public health. A literature review. Front Public Health. 2013;1(56):1-20.
6. Goniewicz ML, Hajek P, McRobbie H. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications. Addiction. 2014;109(3):500-507.
7. Arnold C. Vaping and health: what do we know about e-cigarettes? Environ Health Perspect. 2014;122(9):A244-A249. http://ehp.niehs.nih.gov/122-a244. Accessed May 10, 2016.
8. Electronic cigarette use among middle and high school students. Medscape. September 6, 2013. www.medscape.com/viewarticle/811008. Accessed May 10, 2016.
9. FDA: E-cigarettes may be as bad as real thing. NBC Nightly News. July 22, 2015. www.nbcnews.com/video/nightly-news/32091534#32091534. Accessed May 10, 2016.
10. Caudle J. Why we need new rules on e-cigs. CNN. May 6, 2016. www.cnn.com/2016/05/06/opinions/fda-electronic-cigarettes-caudle. Accessed May 10, 2016.
11. FDA. FDA takes significant steps to protect Americans from dangers of tobacco through new regulation [news release]. May 5, 2016. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm499234.htm. Accessed May 10, 2016.
I was sitting in a restaurant bar a few days ago when a huge puff of cherry-scented smoke engulfed the area. As a former firefighter, I immediately looked around to find the source. You guessed it: a group of young adults were “vaping” nearby. This method of smoking is accomplished with an electronic “cigarette.” A sensor inside the e-cigarette detects airflow and initiates a heating element that vaporizes a liquid solution containing propylene glycol (organic compound with the chemical formula C₃H₈O₂), the flavoring of choice, and nicotine.1
I knew of this fad but didn’t give it much thought until recently, when I realized how pervasive it has become. Frankly, I have always thought, At least they are not smoking cigarettes and inhaling all that benzene, carbon dioxide, and formaldehyde.
We all know smoking cessation is valuable to the health of the population, but what do we know about the effects of vaping? For one thing, use of e-cigarettes (vapes) has increased considerably since they were first introduced (0.3% to 6.8% between 2007 and 2010).This is cause for concern, because while some research on e-cigarettes has emerged since their appearance, there are few definitive answers regarding their effect on human health.2
We also know that nicotine is addictive and toxic (in high doses), but we do not know the effects of propylene glycol, although it is generally recognized as “safe.” Symptoms that may occur as a result of vaporized propylene glycol inhalation include throat and ocular irritation, cough, mild airway obstruction, throat and vocal cord inflammation, headache, and dizziness. In spite of this, since the manufacturers of e-cigarettes have not made any therapeutic claims about their products, the FDA initially did not regulate them.
With e-cigarettes appearing in vaping shops, gas stations, and convenience stores—alongside advertising copy that claims vaping can help smokers curtail their habit by inhaling “harmless water vapor”—what should we tell our patients? These advertisements tout vaping as the “lesser of two evils” when compared to cigarettes. How can you knock that logic when we know cigarette smoking causes one in five deaths in the US each year and is a leading risk factor for COPD?3
Continue for the conundrum >>
The conundrum, as I see it, is threefold. The first step is to determine if vaping is a significant alternative to smoking cessation. The second is to determine if any components of vaping (nicotine, propylene glycol, or combustion) are safe for humans. Lastly, we must establish how to regulate e-cigarettes, given scientific uncertainty as to their therapeutic effects.4
In 2013, Palazzolo did a literature review of 66 articles related to e-cigarettes and vaping. He found that, when compared to the effects of smoking, vaping could be a substitute for smoking and a conceivable means for smoking reduction. It was unclear, however, if vaping could reduce nicotine addiction. He reported that the effects of vaping on human health are questionable, due to the extreme scarcity of empiric research.5
Although there has been a paucity of research on this topic, a study by Goniewicz and colleagues reports on the toxicants emitted by tobacco cigarettes and e-cigarettes.6 Their results indicate that e-cigarettes emit fewer toxicants than traditional tobacco cigarettes (formaldehyde, 0.20-5.61 µg and 1.6-52 µg, respectively; acetaldehyde, 0.11-1.36 µg and 52-140 µg).7 Despite this evidence, more studies need to be done on the effects of propylene glycol inhalation to determine the safety of e-cigarettes.
Another concern has been the lack of an age restriction on e-cigarettes and their growing popularity among grade and high school students.E-cigarette use doubled among US middle and high school students from 2011 to 2012, resulting in an estimated 1.78 million students who have used e-cigarettes as of 2012. There is serious concern about the possible harmful impact of nicotine on adolescent brain development,as well as the risk for nicotine addiction.8
Amid these growing questions and concerns, the FDA issued a warning that e-cigarettes may be as bad as the real thing and has recommended against their use.9 Just last month, the agency finalized a regulation on all tobacco products, including vaporizers, vape pens, hookah pens, e-cigs, and e-pipes. They will now regulate the manufacturing, import, packaging, labeling, and distribution of e-cigarettes to ensure that ingredients are assessed and determined to be safe for human use.FDA Commissioner Robert M. Califf, MD, said, “We must do our job under the Tobacco Control Act to reduce the harms caused by tobacco. That includes ensuring that consumers have the information they need to make informed decisions about tobacco use and making sure that new tobacco products for purchase come under comprehensive FDA review.”10,11
Well, is it just a bunch of smoke, or are the new regulations and health warnings about e-cigarettes long overdue? I would love to hear your experiences and additional advice for our colleagues and patients regarding the use of e-cigarettes and vaping. You can reach me at [email protected].
References
1. Jerry JM, Collins GB, Streem D. E-cigarettes: safe to recommend to patients? Cleve Clin J Med. 2015;82(8):521-526.
2. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
3. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed May 10, 2016.
4. Gostin LO, Glasner AY. E-cigarettes, vaping, and youth. JAMA. 2014;312(6):595-596.
5. Palazzolo DL. Electronic cigarettes and vaping: a new challenge in clinical medicine and public health. A literature review. Front Public Health. 2013;1(56):1-20.
6. Goniewicz ML, Hajek P, McRobbie H. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications. Addiction. 2014;109(3):500-507.
7. Arnold C. Vaping and health: what do we know about e-cigarettes? Environ Health Perspect. 2014;122(9):A244-A249. http://ehp.niehs.nih.gov/122-a244. Accessed May 10, 2016.
8. Electronic cigarette use among middle and high school students. Medscape. September 6, 2013. www.medscape.com/viewarticle/811008. Accessed May 10, 2016.
9. FDA: E-cigarettes may be as bad as real thing. NBC Nightly News. July 22, 2015. www.nbcnews.com/video/nightly-news/32091534#32091534. Accessed May 10, 2016.
10. Caudle J. Why we need new rules on e-cigs. CNN. May 6, 2016. www.cnn.com/2016/05/06/opinions/fda-electronic-cigarettes-caudle. Accessed May 10, 2016.
11. FDA. FDA takes significant steps to protect Americans from dangers of tobacco through new regulation [news release]. May 5, 2016. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm499234.htm. Accessed May 10, 2016.
RENEW: Endobronchial coils improve exercise tolerance in severe emphysema
SAN FRANCISCO – Compressing damaged lung tissue with endobronchial coils improves exercise tolerance in patients with severe emphysema, albeit with the tradeoff of more adverse events, concludes the phase 3 RENEW trial.
After a year of treatment, the 6-minute walk distance had improved in patients given coils, whereas it had worsened in patients managed with usual care, with a difference of nearly 15 meters between groups, investigators reported at an international conference of the American Thoracic Society and simultaneously published (JAMA. doi:10.1001/jama.2016.6261. Published online May 15, 2016).
However, the median gain with coils fell short of the study’s predefined minimal clinically important difference of 25 meters. Additionally, major complications, mainly lower respiratory tract infections, were more common with the coils, although they resolved with time.
“Participants in the RENEW trial had advanced disease. Seventy-seven percent had homogeneous emphysema. This is a group that has very few therapeutic options,” commented lead investigator Dr. Frank C. Sciurba, director of both the Emphysema Research Center and the Pulmonary Function Exercise Physiology Laboratory at the University of Pittsburgh.
“The response rates of endobronchial coils to improve quality of life and exercise tolerance in these severely symptomatic patients balanced against peri-procedural adverse events in this population provides an evidence-based choice for symptomatic patients and treating physicians when there are few other options in these patients,” he said.
RENEW (Lung Volume Reduction Coil Treatment in Patients With Emphysema) was conducted among 315 patients from the United States, Canada, the United Kingdom, Germany, the Netherlands, and France who had emphysema with severe air trapping.
“This was a very inclusive study. In contrast to the surgical and valvular studies, we randomized nearly half of those screened because we allowed patients with homogeneous disease and of course didn’t select based on fissure integrity, which is a selection criterion for other studies,” Dr. Sciurba commented.
The patients received either guideline-based usual care alone (including pulmonary rehabilitation and bronchodilators) or with the addition of bilateral, bronchoscopically placed coils (RePneu Lung Volume Reduction Coil System, currently investigational in the United States).
At 12 months, the median 6-minute walk distance had improved by 10.3 meters with coil treatment but worsened by 7.6 meters with usual care (P = .02). The proportion of patients attaining an improvement of at least 25 meters was higher in the coil group (40.0% vs. 26.9%; P = .01).
In exploratory analyses, patients having more nonpulmonary comorbidities at baseline derived lesser benefit in walk distance from coil treatment, Dr. Sciurba noted.
The coils also netted greater improvement in the median change in forced exploratory volume in 1 second (FEV1) (difference between groups, 7.0%; P < .001) and in scores on the St. George’s Respiratory Questionnaire (difference between groups, −8.9 points).
At the same time, patients in the coil group had higher rates of major complications such as pneumonia requiring hospitalization and other potentially life-threatening or fatal events (34.8% vs. 19.1%, P = .002) and of other serious adverse events such as pneumonia (20% vs. 4.5%) and pneumothorax (9.7% vs. 0.6%).
“All of these adverse events returned to baseline at 9 to 12 months,” Dr. Sciurba reported. Additionally, there was no significant difference between groups in mortality rate.
Of note, 35% of the 40 cases of coil-associated opacities initially thought to be pneumonia were in fact determined to likely be a noninfectious inflammatory reaction to the coils. “These adjudicated noninfectious coil-associated opacities were associated with a better response,” he noted.
Finally, in stratified analyses, patients with greater air trapping at baseline had better-than-average improvements in outcomes with the coils, regardless of whether they had homogeneous or heterogeneous disease. Among patients with lesser air trapping, those with homogeneous disease derived much less benefit than the average from coils, while the group with heterogeneous disease was too small to draw any conclusions.
Dr. Sciurba disclosed that he receives institutional support from PneumRx and Pulmonx. The study was sponsored by PneumRx.
Dr. Vera De Palo, FCCP, comments: When patients are functionally limited, as physicians we like to be able to offer options. The results of this trial indicate that another option may exist to improve functionality. As with all treatment decisions, matching the patient to the best therapeutic option and weighing the risks and benefits of the choice will be important.
Dr. Vera De Palo, FCCP, comments: When patients are functionally limited, as physicians we like to be able to offer options. The results of this trial indicate that another option may exist to improve functionality. As with all treatment decisions, matching the patient to the best therapeutic option and weighing the risks and benefits of the choice will be important.
Dr. Vera De Palo, FCCP, comments: When patients are functionally limited, as physicians we like to be able to offer options. The results of this trial indicate that another option may exist to improve functionality. As with all treatment decisions, matching the patient to the best therapeutic option and weighing the risks and benefits of the choice will be important.
SAN FRANCISCO – Compressing damaged lung tissue with endobronchial coils improves exercise tolerance in patients with severe emphysema, albeit with the tradeoff of more adverse events, concludes the phase 3 RENEW trial.
After a year of treatment, the 6-minute walk distance had improved in patients given coils, whereas it had worsened in patients managed with usual care, with a difference of nearly 15 meters between groups, investigators reported at an international conference of the American Thoracic Society and simultaneously published (JAMA. doi:10.1001/jama.2016.6261. Published online May 15, 2016).
However, the median gain with coils fell short of the study’s predefined minimal clinically important difference of 25 meters. Additionally, major complications, mainly lower respiratory tract infections, were more common with the coils, although they resolved with time.
“Participants in the RENEW trial had advanced disease. Seventy-seven percent had homogeneous emphysema. This is a group that has very few therapeutic options,” commented lead investigator Dr. Frank C. Sciurba, director of both the Emphysema Research Center and the Pulmonary Function Exercise Physiology Laboratory at the University of Pittsburgh.
“The response rates of endobronchial coils to improve quality of life and exercise tolerance in these severely symptomatic patients balanced against peri-procedural adverse events in this population provides an evidence-based choice for symptomatic patients and treating physicians when there are few other options in these patients,” he said.
RENEW (Lung Volume Reduction Coil Treatment in Patients With Emphysema) was conducted among 315 patients from the United States, Canada, the United Kingdom, Germany, the Netherlands, and France who had emphysema with severe air trapping.
“This was a very inclusive study. In contrast to the surgical and valvular studies, we randomized nearly half of those screened because we allowed patients with homogeneous disease and of course didn’t select based on fissure integrity, which is a selection criterion for other studies,” Dr. Sciurba commented.
The patients received either guideline-based usual care alone (including pulmonary rehabilitation and bronchodilators) or with the addition of bilateral, bronchoscopically placed coils (RePneu Lung Volume Reduction Coil System, currently investigational in the United States).
At 12 months, the median 6-minute walk distance had improved by 10.3 meters with coil treatment but worsened by 7.6 meters with usual care (P = .02). The proportion of patients attaining an improvement of at least 25 meters was higher in the coil group (40.0% vs. 26.9%; P = .01).
In exploratory analyses, patients having more nonpulmonary comorbidities at baseline derived lesser benefit in walk distance from coil treatment, Dr. Sciurba noted.
The coils also netted greater improvement in the median change in forced exploratory volume in 1 second (FEV1) (difference between groups, 7.0%; P < .001) and in scores on the St. George’s Respiratory Questionnaire (difference between groups, −8.9 points).
At the same time, patients in the coil group had higher rates of major complications such as pneumonia requiring hospitalization and other potentially life-threatening or fatal events (34.8% vs. 19.1%, P = .002) and of other serious adverse events such as pneumonia (20% vs. 4.5%) and pneumothorax (9.7% vs. 0.6%).
“All of these adverse events returned to baseline at 9 to 12 months,” Dr. Sciurba reported. Additionally, there was no significant difference between groups in mortality rate.
Of note, 35% of the 40 cases of coil-associated opacities initially thought to be pneumonia were in fact determined to likely be a noninfectious inflammatory reaction to the coils. “These adjudicated noninfectious coil-associated opacities were associated with a better response,” he noted.
Finally, in stratified analyses, patients with greater air trapping at baseline had better-than-average improvements in outcomes with the coils, regardless of whether they had homogeneous or heterogeneous disease. Among patients with lesser air trapping, those with homogeneous disease derived much less benefit than the average from coils, while the group with heterogeneous disease was too small to draw any conclusions.
Dr. Sciurba disclosed that he receives institutional support from PneumRx and Pulmonx. The study was sponsored by PneumRx.
SAN FRANCISCO – Compressing damaged lung tissue with endobronchial coils improves exercise tolerance in patients with severe emphysema, albeit with the tradeoff of more adverse events, concludes the phase 3 RENEW trial.
After a year of treatment, the 6-minute walk distance had improved in patients given coils, whereas it had worsened in patients managed with usual care, with a difference of nearly 15 meters between groups, investigators reported at an international conference of the American Thoracic Society and simultaneously published (JAMA. doi:10.1001/jama.2016.6261. Published online May 15, 2016).
However, the median gain with coils fell short of the study’s predefined minimal clinically important difference of 25 meters. Additionally, major complications, mainly lower respiratory tract infections, were more common with the coils, although they resolved with time.
“Participants in the RENEW trial had advanced disease. Seventy-seven percent had homogeneous emphysema. This is a group that has very few therapeutic options,” commented lead investigator Dr. Frank C. Sciurba, director of both the Emphysema Research Center and the Pulmonary Function Exercise Physiology Laboratory at the University of Pittsburgh.
“The response rates of endobronchial coils to improve quality of life and exercise tolerance in these severely symptomatic patients balanced against peri-procedural adverse events in this population provides an evidence-based choice for symptomatic patients and treating physicians when there are few other options in these patients,” he said.
RENEW (Lung Volume Reduction Coil Treatment in Patients With Emphysema) was conducted among 315 patients from the United States, Canada, the United Kingdom, Germany, the Netherlands, and France who had emphysema with severe air trapping.
“This was a very inclusive study. In contrast to the surgical and valvular studies, we randomized nearly half of those screened because we allowed patients with homogeneous disease and of course didn’t select based on fissure integrity, which is a selection criterion for other studies,” Dr. Sciurba commented.
The patients received either guideline-based usual care alone (including pulmonary rehabilitation and bronchodilators) or with the addition of bilateral, bronchoscopically placed coils (RePneu Lung Volume Reduction Coil System, currently investigational in the United States).
At 12 months, the median 6-minute walk distance had improved by 10.3 meters with coil treatment but worsened by 7.6 meters with usual care (P = .02). The proportion of patients attaining an improvement of at least 25 meters was higher in the coil group (40.0% vs. 26.9%; P = .01).
In exploratory analyses, patients having more nonpulmonary comorbidities at baseline derived lesser benefit in walk distance from coil treatment, Dr. Sciurba noted.
The coils also netted greater improvement in the median change in forced exploratory volume in 1 second (FEV1) (difference between groups, 7.0%; P < .001) and in scores on the St. George’s Respiratory Questionnaire (difference between groups, −8.9 points).
At the same time, patients in the coil group had higher rates of major complications such as pneumonia requiring hospitalization and other potentially life-threatening or fatal events (34.8% vs. 19.1%, P = .002) and of other serious adverse events such as pneumonia (20% vs. 4.5%) and pneumothorax (9.7% vs. 0.6%).
“All of these adverse events returned to baseline at 9 to 12 months,” Dr. Sciurba reported. Additionally, there was no significant difference between groups in mortality rate.
Of note, 35% of the 40 cases of coil-associated opacities initially thought to be pneumonia were in fact determined to likely be a noninfectious inflammatory reaction to the coils. “These adjudicated noninfectious coil-associated opacities were associated with a better response,” he noted.
Finally, in stratified analyses, patients with greater air trapping at baseline had better-than-average improvements in outcomes with the coils, regardless of whether they had homogeneous or heterogeneous disease. Among patients with lesser air trapping, those with homogeneous disease derived much less benefit than the average from coils, while the group with heterogeneous disease was too small to draw any conclusions.
Dr. Sciurba disclosed that he receives institutional support from PneumRx and Pulmonx. The study was sponsored by PneumRx.
AT ATS 2016
Key clinical point: Endobronchial coils modestly improve exercise tolerance in severe emphysema with the tradeoff of more adverse events.
Major finding: After a year, the median 6-minute walk distance had improved by 10.3 meters with coil treatment but worsened by 7.6 meters with usual care (P = .02).
Data source: A randomized phase 3 trial among 315 patients with emphysema and severe air trapping.
Disclosures: Dr. Sciurba disclosed that he receives institutional support from PneumRx and Pulmonx. The study was sponsored by PneumRx.
Lebrikizumab boosts lung function in asthma
LOS ANGELES – The investigational interleukin-13 inhibitor lebrikizumab provides a clinically meaningful improvement in measures of lung function within 1 week after the first dose in patients with moderate-to-severe uncontrolled asthma on standard-of-care therapy and a high baseline serum periostin level, Dr. Jonathan Corren reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He presented a post hoc analysis of three phase II randomized trials of lebrikizumab as add-on therapy in a total of 558 patients with uncontrolled asthma while on a moderate- or high-dose inhaled corticosteroid plus at least one other controller medication, most often a long-acting beta agonist. The post hoc analysis included 333 asthma patients who received lebrikizumab subcutaneously at 125 or 250 mg every 4 weeks for 12 weeks and 225 who got placebo. Baseline serum periostin levels were 50 ng/mL or higher in 252 participants.
One week after the first dose of lebrikizumab, the high serum periostin group demonstrated a placebo-subtracted mean 147-mL improvement from baseline in pre-bronchodilator forced expiratory volume in 1 second (FEV1). The week 1 improvement in FEV1 with lebrikizumab in the low serum periostin group was more modest: a placebo-subtracted 57 mL.
The response to lebrikizumab was maintained through 12 weeks of once-monthly therapy, with a mean placebo-subtracted week 12 improvement in FEV1 of 198 mL in the high-periostin group, compared with 74 mL in low-periostin patients. The lebrikizumab-treated group with high baseline periostin had a 16% improvement from baseline in FEV1 as compared with a 5% improvement in placebo-treated patients with high periostin.
The three trials were known by the acronyms MILLY, LUTE, and VERSE. Dr. Corren was first author of the MILLY study (N Engl J Med. 2011 Sep 22;365(12):1088-98), which was the initial report that lebrikizumab performed markedly better in patients with uncontrolled asthma and a high baseline serum periostin – a biomarker for IL-13 activity – and that periostin was a better predictor of response to lebrikizumab than either blood eosinophil count or serum IgE.
The new pooled post hoc analysis was performed to boost sample size and confirm the key MILLY findings, as well as to more closely examine the speed of improvement in airflow in response to therapy, said Dr. Corren of the University of California, Los Angeles.
Lebrikizumab is an IgG4 humanized monoclonal antibody that binds to IL-13 with high affinity. Its efficacy in the phase II trials confirms the importance of IL-13 as a mediator of disease activity in a subset of asthma patients with activation of Type 2 lymphocytes.
“We know specifically that IL-13 has some very important effects in asthma, including upregulation of adhesion molecules that allow eosinophils to stick in the lung, as well as promoting hyperplasia of smooth muscle and mucus cell hyperplasia with increased mucus secretion. Immunologically, it allows switching from IgM to IgE on the surface of B cells. So IL-13 is a cytokine that literally makes people atopic,” Dr. Corren explained in an interview.
Several ongoing phase III randomized trials of lebrikizumab in adults with uncontrolled asthma despite standard-of-care therapy are due to be completed in the first half of 2017. A phase III trial in adolescents is also underway.
Dr. Corren reported receiving research funding from Roche/Genentech, which sponsored the studies.
LOS ANGELES – The investigational interleukin-13 inhibitor lebrikizumab provides a clinically meaningful improvement in measures of lung function within 1 week after the first dose in patients with moderate-to-severe uncontrolled asthma on standard-of-care therapy and a high baseline serum periostin level, Dr. Jonathan Corren reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He presented a post hoc analysis of three phase II randomized trials of lebrikizumab as add-on therapy in a total of 558 patients with uncontrolled asthma while on a moderate- or high-dose inhaled corticosteroid plus at least one other controller medication, most often a long-acting beta agonist. The post hoc analysis included 333 asthma patients who received lebrikizumab subcutaneously at 125 or 250 mg every 4 weeks for 12 weeks and 225 who got placebo. Baseline serum periostin levels were 50 ng/mL or higher in 252 participants.
One week after the first dose of lebrikizumab, the high serum periostin group demonstrated a placebo-subtracted mean 147-mL improvement from baseline in pre-bronchodilator forced expiratory volume in 1 second (FEV1). The week 1 improvement in FEV1 with lebrikizumab in the low serum periostin group was more modest: a placebo-subtracted 57 mL.
The response to lebrikizumab was maintained through 12 weeks of once-monthly therapy, with a mean placebo-subtracted week 12 improvement in FEV1 of 198 mL in the high-periostin group, compared with 74 mL in low-periostin patients. The lebrikizumab-treated group with high baseline periostin had a 16% improvement from baseline in FEV1 as compared with a 5% improvement in placebo-treated patients with high periostin.
The three trials were known by the acronyms MILLY, LUTE, and VERSE. Dr. Corren was first author of the MILLY study (N Engl J Med. 2011 Sep 22;365(12):1088-98), which was the initial report that lebrikizumab performed markedly better in patients with uncontrolled asthma and a high baseline serum periostin – a biomarker for IL-13 activity – and that periostin was a better predictor of response to lebrikizumab than either blood eosinophil count or serum IgE.
The new pooled post hoc analysis was performed to boost sample size and confirm the key MILLY findings, as well as to more closely examine the speed of improvement in airflow in response to therapy, said Dr. Corren of the University of California, Los Angeles.
Lebrikizumab is an IgG4 humanized monoclonal antibody that binds to IL-13 with high affinity. Its efficacy in the phase II trials confirms the importance of IL-13 as a mediator of disease activity in a subset of asthma patients with activation of Type 2 lymphocytes.
“We know specifically that IL-13 has some very important effects in asthma, including upregulation of adhesion molecules that allow eosinophils to stick in the lung, as well as promoting hyperplasia of smooth muscle and mucus cell hyperplasia with increased mucus secretion. Immunologically, it allows switching from IgM to IgE on the surface of B cells. So IL-13 is a cytokine that literally makes people atopic,” Dr. Corren explained in an interview.
Several ongoing phase III randomized trials of lebrikizumab in adults with uncontrolled asthma despite standard-of-care therapy are due to be completed in the first half of 2017. A phase III trial in adolescents is also underway.
Dr. Corren reported receiving research funding from Roche/Genentech, which sponsored the studies.
LOS ANGELES – The investigational interleukin-13 inhibitor lebrikizumab provides a clinically meaningful improvement in measures of lung function within 1 week after the first dose in patients with moderate-to-severe uncontrolled asthma on standard-of-care therapy and a high baseline serum periostin level, Dr. Jonathan Corren reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He presented a post hoc analysis of three phase II randomized trials of lebrikizumab as add-on therapy in a total of 558 patients with uncontrolled asthma while on a moderate- or high-dose inhaled corticosteroid plus at least one other controller medication, most often a long-acting beta agonist. The post hoc analysis included 333 asthma patients who received lebrikizumab subcutaneously at 125 or 250 mg every 4 weeks for 12 weeks and 225 who got placebo. Baseline serum periostin levels were 50 ng/mL or higher in 252 participants.
One week after the first dose of lebrikizumab, the high serum periostin group demonstrated a placebo-subtracted mean 147-mL improvement from baseline in pre-bronchodilator forced expiratory volume in 1 second (FEV1). The week 1 improvement in FEV1 with lebrikizumab in the low serum periostin group was more modest: a placebo-subtracted 57 mL.
The response to lebrikizumab was maintained through 12 weeks of once-monthly therapy, with a mean placebo-subtracted week 12 improvement in FEV1 of 198 mL in the high-periostin group, compared with 74 mL in low-periostin patients. The lebrikizumab-treated group with high baseline periostin had a 16% improvement from baseline in FEV1 as compared with a 5% improvement in placebo-treated patients with high periostin.
The three trials were known by the acronyms MILLY, LUTE, and VERSE. Dr. Corren was first author of the MILLY study (N Engl J Med. 2011 Sep 22;365(12):1088-98), which was the initial report that lebrikizumab performed markedly better in patients with uncontrolled asthma and a high baseline serum periostin – a biomarker for IL-13 activity – and that periostin was a better predictor of response to lebrikizumab than either blood eosinophil count or serum IgE.
The new pooled post hoc analysis was performed to boost sample size and confirm the key MILLY findings, as well as to more closely examine the speed of improvement in airflow in response to therapy, said Dr. Corren of the University of California, Los Angeles.
Lebrikizumab is an IgG4 humanized monoclonal antibody that binds to IL-13 with high affinity. Its efficacy in the phase II trials confirms the importance of IL-13 as a mediator of disease activity in a subset of asthma patients with activation of Type 2 lymphocytes.
“We know specifically that IL-13 has some very important effects in asthma, including upregulation of adhesion molecules that allow eosinophils to stick in the lung, as well as promoting hyperplasia of smooth muscle and mucus cell hyperplasia with increased mucus secretion. Immunologically, it allows switching from IgM to IgE on the surface of B cells. So IL-13 is a cytokine that literally makes people atopic,” Dr. Corren explained in an interview.
Several ongoing phase III randomized trials of lebrikizumab in adults with uncontrolled asthma despite standard-of-care therapy are due to be completed in the first half of 2017. A phase III trial in adolescents is also underway.
Dr. Corren reported receiving research funding from Roche/Genentech, which sponsored the studies.
AT 2016 AAAAI ANNUAL MEETING
Key clinical point: Lebrikizumab shows promise for treatment of patients with moderate-to-severe type 2 lymphocyte-mediated asthma.
Major finding: One week after patients with uncontrolled asthma and a high baseline serum periostin level received their first dose of lebrikizumab, they demonstrated a mean 16% improvement over baseline in forced expiratory volume in 1 second.
Data source: This was a post hoc analysis of three phase II, randomized, placebo-controlled clinical trials totaling 558 patients with moderate-to-severe uncontrolled asthma while on standard of care therapy.
Disclosures: The study presenter reported receiving research funding from Roche/Genentech, which is developing lebrikizumab.
Flu vaccination cut hospitalizations in heart failure patients
FLORENCE, ITALY – Influenza vaccination of heart failure patients cut their rate of hospitalization for cardiovascular disease by nearly a third during the year following vaccination in a study of more than 59,000 British heart failure patients.
Influenza vaccination of heart failure patients also cut their rate of hospitalization for respiratory infections by a statistically significant 16% during the year following vaccination, Dr. Kazem Rahimi said at a meeting held by the Heart Failure Association of the ESC.
“In the absence of randomized trials, this [observational] study of 59,202 heart failure patients provides the most compelling evidence to date for the protective effect of influenza vaccination on hospital admissions,” said Dr. Rahimi, a cardiologist and epidemiologist who is deputy director of the George Institute for Global Health at the University of Oxford (England).
The analysis also showed that influenza vaccination of heart failure patients had no significant effect on all-cause hospitalizations.
Dr. Rahimi and his associates analyzed electronic health records from primary and secondary care settings in England during 1990-2013, from which they identified 59,202 heart failure patients with records for at least 1 year of influenza vaccination and at least 1 year without vaccination. The patients averaged 75 years old and were divided equally among women and men.
To control for potential confounding factors, they used a self-control model in which hospitalizations for each heart failure patient during the year following an influenza vaccination were compared with an adjacent year for that same patient when no vaccination occurred.
The results showed that the incidence of hospitalizations for cardiovascular diseases fell by a statistically significant 30% in the year following an influenza vaccination, compared with one or more adjacent years without vaccination. The protection again hospitalization was strongest during the second month following vaccination and then gradually waned over the ensuing year; by about 10 months following vaccination the protection effect had disappeared.
The study also looked at influenza vaccine uptake by heart failure patients through the 24-year period examined. During that time, the vaccination uptake rate rose from a low of less than 10% in 1990 to a peak rate of just over 60% in 2006, after which the rate gradually declined to a rate of just under 50% in 2013. Dr. Rahimi attributed the rise in uptake during the period from 1990 to 2006 in part to incentives that primary care physicians in England began receiving to administer influenza vaccine to their patients. “Higher uptake of annual vaccination in heart failure patients may help alleviate the burden of influenza-related hospital admissions,” he said.
Dr. Rahimi had no disclosures.
On Twitter @mitchelzoler
The main problem with observational studies is confounding, and the observational studies done until now that had looked at the protective role of influenza vaccination in heart failure patients had not been very convincing. Dr. Rahimi and his associates performed a high-quality study that adds to the evidence and underlines recommendations for influenza vaccination of heart failure patients. Their study was very large, and it used a self-control approach to adjust for potential confounding. I think they did their best to eliminate confounding.
 |
Dr. Arno W. Hoes |
The newly released, updated guidelines from the European Society of Cardiology for the diagnosis and treatment of acute and chronic heart failure recommend annual vaccination of heart failure patients against influenza and pneumococcal disease. The data reported by Dr. Rahimi also document that only about half of heart failure patients in England currently receive an annual influenza vaccine. That percentage needs to increase.
Dr. Arno W. Hoes is professor of clinical epidemiology at the University Medical Center in Utrecht, the Netherlands. He made these comments as designated discussant for the study. He had no disclosures.
The main problem with observational studies is confounding, and the observational studies done until now that had looked at the protective role of influenza vaccination in heart failure patients had not been very convincing. Dr. Rahimi and his associates performed a high-quality study that adds to the evidence and underlines recommendations for influenza vaccination of heart failure patients. Their study was very large, and it used a self-control approach to adjust for potential confounding. I think they did their best to eliminate confounding.
 |
Dr. Arno W. Hoes |
The newly released, updated guidelines from the European Society of Cardiology for the diagnosis and treatment of acute and chronic heart failure recommend annual vaccination of heart failure patients against influenza and pneumococcal disease. The data reported by Dr. Rahimi also document that only about half of heart failure patients in England currently receive an annual influenza vaccine. That percentage needs to increase.
Dr. Arno W. Hoes is professor of clinical epidemiology at the University Medical Center in Utrecht, the Netherlands. He made these comments as designated discussant for the study. He had no disclosures.
The main problem with observational studies is confounding, and the observational studies done until now that had looked at the protective role of influenza vaccination in heart failure patients had not been very convincing. Dr. Rahimi and his associates performed a high-quality study that adds to the evidence and underlines recommendations for influenza vaccination of heart failure patients. Their study was very large, and it used a self-control approach to adjust for potential confounding. I think they did their best to eliminate confounding.
 |
Dr. Arno W. Hoes |
The newly released, updated guidelines from the European Society of Cardiology for the diagnosis and treatment of acute and chronic heart failure recommend annual vaccination of heart failure patients against influenza and pneumococcal disease. The data reported by Dr. Rahimi also document that only about half of heart failure patients in England currently receive an annual influenza vaccine. That percentage needs to increase.
Dr. Arno W. Hoes is professor of clinical epidemiology at the University Medical Center in Utrecht, the Netherlands. He made these comments as designated discussant for the study. He had no disclosures.
FLORENCE, ITALY – Influenza vaccination of heart failure patients cut their rate of hospitalization for cardiovascular disease by nearly a third during the year following vaccination in a study of more than 59,000 British heart failure patients.
Influenza vaccination of heart failure patients also cut their rate of hospitalization for respiratory infections by a statistically significant 16% during the year following vaccination, Dr. Kazem Rahimi said at a meeting held by the Heart Failure Association of the ESC.
“In the absence of randomized trials, this [observational] study of 59,202 heart failure patients provides the most compelling evidence to date for the protective effect of influenza vaccination on hospital admissions,” said Dr. Rahimi, a cardiologist and epidemiologist who is deputy director of the George Institute for Global Health at the University of Oxford (England).
The analysis also showed that influenza vaccination of heart failure patients had no significant effect on all-cause hospitalizations.
Dr. Rahimi and his associates analyzed electronic health records from primary and secondary care settings in England during 1990-2013, from which they identified 59,202 heart failure patients with records for at least 1 year of influenza vaccination and at least 1 year without vaccination. The patients averaged 75 years old and were divided equally among women and men.
To control for potential confounding factors, they used a self-control model in which hospitalizations for each heart failure patient during the year following an influenza vaccination were compared with an adjacent year for that same patient when no vaccination occurred.
The results showed that the incidence of hospitalizations for cardiovascular diseases fell by a statistically significant 30% in the year following an influenza vaccination, compared with one or more adjacent years without vaccination. The protection again hospitalization was strongest during the second month following vaccination and then gradually waned over the ensuing year; by about 10 months following vaccination the protection effect had disappeared.
The study also looked at influenza vaccine uptake by heart failure patients through the 24-year period examined. During that time, the vaccination uptake rate rose from a low of less than 10% in 1990 to a peak rate of just over 60% in 2006, after which the rate gradually declined to a rate of just under 50% in 2013. Dr. Rahimi attributed the rise in uptake during the period from 1990 to 2006 in part to incentives that primary care physicians in England began receiving to administer influenza vaccine to their patients. “Higher uptake of annual vaccination in heart failure patients may help alleviate the burden of influenza-related hospital admissions,” he said.
Dr. Rahimi had no disclosures.
On Twitter @mitchelzoler
FLORENCE, ITALY – Influenza vaccination of heart failure patients cut their rate of hospitalization for cardiovascular disease by nearly a third during the year following vaccination in a study of more than 59,000 British heart failure patients.
Influenza vaccination of heart failure patients also cut their rate of hospitalization for respiratory infections by a statistically significant 16% during the year following vaccination, Dr. Kazem Rahimi said at a meeting held by the Heart Failure Association of the ESC.
“In the absence of randomized trials, this [observational] study of 59,202 heart failure patients provides the most compelling evidence to date for the protective effect of influenza vaccination on hospital admissions,” said Dr. Rahimi, a cardiologist and epidemiologist who is deputy director of the George Institute for Global Health at the University of Oxford (England).
The analysis also showed that influenza vaccination of heart failure patients had no significant effect on all-cause hospitalizations.
Dr. Rahimi and his associates analyzed electronic health records from primary and secondary care settings in England during 1990-2013, from which they identified 59,202 heart failure patients with records for at least 1 year of influenza vaccination and at least 1 year without vaccination. The patients averaged 75 years old and were divided equally among women and men.
To control for potential confounding factors, they used a self-control model in which hospitalizations for each heart failure patient during the year following an influenza vaccination were compared with an adjacent year for that same patient when no vaccination occurred.
The results showed that the incidence of hospitalizations for cardiovascular diseases fell by a statistically significant 30% in the year following an influenza vaccination, compared with one or more adjacent years without vaccination. The protection again hospitalization was strongest during the second month following vaccination and then gradually waned over the ensuing year; by about 10 months following vaccination the protection effect had disappeared.
The study also looked at influenza vaccine uptake by heart failure patients through the 24-year period examined. During that time, the vaccination uptake rate rose from a low of less than 10% in 1990 to a peak rate of just over 60% in 2006, after which the rate gradually declined to a rate of just under 50% in 2013. Dr. Rahimi attributed the rise in uptake during the period from 1990 to 2006 in part to incentives that primary care physicians in England began receiving to administer influenza vaccine to their patients. “Higher uptake of annual vaccination in heart failure patients may help alleviate the burden of influenza-related hospital admissions,” he said.
Dr. Rahimi had no disclosures.
On Twitter @mitchelzoler
AT HEART FAILURE 2016
Key clinical point: When heart failure patients received an influenza vaccination, their rate of hospitalization for cardiovascular disease and for respiratory infection fell significantly during the year following vaccination.
Major finding: Cardiovascular hospitalizations fell by 30% in the year following influenza vaccination, compared with adjacent years with no vaccination.
Data source: Review of electronic health records for 59,202 heart failure patients in England during 1990-2013.
Disclosures: Dr. Rahimi had no disclosures.