User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
The X-waiver is dead
In 2016, when Erin Schanning lost her brother Ethan to an overdose, she wanted to know what could have been done to have helped him. Ethan, who had struggled with opioids since getting a prescription for the drugs after a dental procedure in middle school, had tried dozens of treatments. But at the age of 30, he was gone.
“After my brother died, I started researching and was surprised to learn that there were many evidence-based ways to treat substance use disorder that he hadn’t had access to, even though he had doggedly pursued treatment,” Ms. Schanning told me in an interview. One of those treatments, buprenorphine, is one of the most effective tools that health care providers have to treat opioid use disorder. A partial opioid agonist, it reduces cravings and prevents overdose, decreasing mortality more effectively than almost any medication for any disease. Yet most providers have never prescribed it.
That may be about to change. The special license to prescribe the medication, commonly known as the “X-waiver,” was officially eliminated as part of the passage of the Mainstreaming Addiction Treatment (MAT) Act. Immediately, following the passage of the Act, any provider with a DEA license became eligible to prescribe buprenorphine to treat opioid use disorder, and limits on the number of patients they could treat were eliminated.
Previously, buprenorphine, which has a better safety profile than almost any other prescription opioid because of its ceiling effect on respiratory depression, nonetheless required providers to obtain a special license to prescribe it, and – prior to an executive order from the Biden administration – 8 to 24 hours of training to do so. This led to a misconception that buprenorphine was dangerous, and created barriers for treatment during the worst overdose crisis in our country’s history. More than 110,00 overdose deaths occurred in 2021, representing a 468% increase in the last 2 decades.
Along with the MAT Act, the Medication Access and Training Expansion Act was passed in the same spending bill, requiring all prescribers who obtain a DEA license to do 8 hours of training on the treatment of substance use disorders. According to the Act, addiction specialty societies will have a role in creating trainings. Medical schools and residencies will also be able to fulfill this requirement with a “comprehensive” curriculum that covers all approved medications for the treatment of substance use disorders.
The DEA has not yet confirmed what training will be accepted, according to the Chief Medical Officer of the Substance Abuse and Mental Health Services Administration, Neeraj Gandotra, MD, who spoke to me in an interview. However, it is required to do so by April 5, 2023. Dr. Gandotra also emphasized that state and local laws, as well as insurance requirements, remain in place, and may place other constraints on prescribing. According to the Act, this new rule will be in effect by June 2023.
As an addiction medicine specialist and longtime buprenorphine prescriber, I am excited about these changes but wary of lingering resistance among health care providers. Will providers who have chosen not to get an X-waiver now look for another reason to not treat patients with substance use disorders?
Ms. Schanning remains hopeful. “I’m incredibly optimistic that health care providers are going to learn about buprenorphine and prescribe it to patients, and that patients are going to start asking about this medication,” she told me. “Seven in 10 providers say that they do feel an obligation to treat their patients with [opioid use disorder], but the federal government has made it very difficult to do so.”
Now with the X-waiver gone, providers and patients may be able to push for a long overdue shift in how we treat and conceptualize substance use disorders, she noted.
“Health care providers need to recognize substance use disorder as a medical condition that deserves treatment, and to speak about it like a medical condition,” Ms. Schanning said, by, for instance, moving away from using words such as “abuse” and “clean” and, instead, talking about treatable substance use disorders that can improve with evidence-based care, such as buprenorphine and methadone. “We also need to share stories of success and hope with people,” she added. “Once you’ve seen how someone can be transformed by treatment, it’s really difficult to say that substance use disorder is a character flaw, or their fault.”
A patient-centered approach
Over the past decade of practicing medicine, I have experienced this transformation personally. In residency, I believed that people had to be ready for help, to stop using, to change. I failed to recognize that many of those same people were asking me for help, and I wasn’t offering what they needed. The person who had to change was me.
As I moved toward a patient-centered approach, lowering barriers to starting and remaining in treatment, and collaborating with teams that could meet people wherever they might be, addictions became the most rewarding part of my practice.
I have never had more people thank me spontaneously and deeply for the care I provide. Plus, I have never seen a more profound change in the students I work with than when they witness someone with a substance use disorder offered treatment that works.
The X-waiver was not the only barrier to care, and the overdose crisis is not slowing down. But maybe with a new tool widely accessible, more of us will be ready to help.
Dr. Poorman is board certified in internal medicine and addiction medicine, assistant professor of medicine, University of Illinois at Chicago, and provides primary care and addiction services in Chicago. Her views do not necessarily reflect the views of her employer. She has reported no relevant disclosures, and she serves on the editorial advisory board of Internal Medicine News.
In 2016, when Erin Schanning lost her brother Ethan to an overdose, she wanted to know what could have been done to have helped him. Ethan, who had struggled with opioids since getting a prescription for the drugs after a dental procedure in middle school, had tried dozens of treatments. But at the age of 30, he was gone.
“After my brother died, I started researching and was surprised to learn that there were many evidence-based ways to treat substance use disorder that he hadn’t had access to, even though he had doggedly pursued treatment,” Ms. Schanning told me in an interview. One of those treatments, buprenorphine, is one of the most effective tools that health care providers have to treat opioid use disorder. A partial opioid agonist, it reduces cravings and prevents overdose, decreasing mortality more effectively than almost any medication for any disease. Yet most providers have never prescribed it.
That may be about to change. The special license to prescribe the medication, commonly known as the “X-waiver,” was officially eliminated as part of the passage of the Mainstreaming Addiction Treatment (MAT) Act. Immediately, following the passage of the Act, any provider with a DEA license became eligible to prescribe buprenorphine to treat opioid use disorder, and limits on the number of patients they could treat were eliminated.
Previously, buprenorphine, which has a better safety profile than almost any other prescription opioid because of its ceiling effect on respiratory depression, nonetheless required providers to obtain a special license to prescribe it, and – prior to an executive order from the Biden administration – 8 to 24 hours of training to do so. This led to a misconception that buprenorphine was dangerous, and created barriers for treatment during the worst overdose crisis in our country’s history. More than 110,00 overdose deaths occurred in 2021, representing a 468% increase in the last 2 decades.
Along with the MAT Act, the Medication Access and Training Expansion Act was passed in the same spending bill, requiring all prescribers who obtain a DEA license to do 8 hours of training on the treatment of substance use disorders. According to the Act, addiction specialty societies will have a role in creating trainings. Medical schools and residencies will also be able to fulfill this requirement with a “comprehensive” curriculum that covers all approved medications for the treatment of substance use disorders.
The DEA has not yet confirmed what training will be accepted, according to the Chief Medical Officer of the Substance Abuse and Mental Health Services Administration, Neeraj Gandotra, MD, who spoke to me in an interview. However, it is required to do so by April 5, 2023. Dr. Gandotra also emphasized that state and local laws, as well as insurance requirements, remain in place, and may place other constraints on prescribing. According to the Act, this new rule will be in effect by June 2023.
As an addiction medicine specialist and longtime buprenorphine prescriber, I am excited about these changes but wary of lingering resistance among health care providers. Will providers who have chosen not to get an X-waiver now look for another reason to not treat patients with substance use disorders?
Ms. Schanning remains hopeful. “I’m incredibly optimistic that health care providers are going to learn about buprenorphine and prescribe it to patients, and that patients are going to start asking about this medication,” she told me. “Seven in 10 providers say that they do feel an obligation to treat their patients with [opioid use disorder], but the federal government has made it very difficult to do so.”
Now with the X-waiver gone, providers and patients may be able to push for a long overdue shift in how we treat and conceptualize substance use disorders, she noted.
“Health care providers need to recognize substance use disorder as a medical condition that deserves treatment, and to speak about it like a medical condition,” Ms. Schanning said, by, for instance, moving away from using words such as “abuse” and “clean” and, instead, talking about treatable substance use disorders that can improve with evidence-based care, such as buprenorphine and methadone. “We also need to share stories of success and hope with people,” she added. “Once you’ve seen how someone can be transformed by treatment, it’s really difficult to say that substance use disorder is a character flaw, or their fault.”
A patient-centered approach
Over the past decade of practicing medicine, I have experienced this transformation personally. In residency, I believed that people had to be ready for help, to stop using, to change. I failed to recognize that many of those same people were asking me for help, and I wasn’t offering what they needed. The person who had to change was me.
As I moved toward a patient-centered approach, lowering barriers to starting and remaining in treatment, and collaborating with teams that could meet people wherever they might be, addictions became the most rewarding part of my practice.
I have never had more people thank me spontaneously and deeply for the care I provide. Plus, I have never seen a more profound change in the students I work with than when they witness someone with a substance use disorder offered treatment that works.
The X-waiver was not the only barrier to care, and the overdose crisis is not slowing down. But maybe with a new tool widely accessible, more of us will be ready to help.
Dr. Poorman is board certified in internal medicine and addiction medicine, assistant professor of medicine, University of Illinois at Chicago, and provides primary care and addiction services in Chicago. Her views do not necessarily reflect the views of her employer. She has reported no relevant disclosures, and she serves on the editorial advisory board of Internal Medicine News.
In 2016, when Erin Schanning lost her brother Ethan to an overdose, she wanted to know what could have been done to have helped him. Ethan, who had struggled with opioids since getting a prescription for the drugs after a dental procedure in middle school, had tried dozens of treatments. But at the age of 30, he was gone.
“After my brother died, I started researching and was surprised to learn that there were many evidence-based ways to treat substance use disorder that he hadn’t had access to, even though he had doggedly pursued treatment,” Ms. Schanning told me in an interview. One of those treatments, buprenorphine, is one of the most effective tools that health care providers have to treat opioid use disorder. A partial opioid agonist, it reduces cravings and prevents overdose, decreasing mortality more effectively than almost any medication for any disease. Yet most providers have never prescribed it.
That may be about to change. The special license to prescribe the medication, commonly known as the “X-waiver,” was officially eliminated as part of the passage of the Mainstreaming Addiction Treatment (MAT) Act. Immediately, following the passage of the Act, any provider with a DEA license became eligible to prescribe buprenorphine to treat opioid use disorder, and limits on the number of patients they could treat were eliminated.
Previously, buprenorphine, which has a better safety profile than almost any other prescription opioid because of its ceiling effect on respiratory depression, nonetheless required providers to obtain a special license to prescribe it, and – prior to an executive order from the Biden administration – 8 to 24 hours of training to do so. This led to a misconception that buprenorphine was dangerous, and created barriers for treatment during the worst overdose crisis in our country’s history. More than 110,00 overdose deaths occurred in 2021, representing a 468% increase in the last 2 decades.
Along with the MAT Act, the Medication Access and Training Expansion Act was passed in the same spending bill, requiring all prescribers who obtain a DEA license to do 8 hours of training on the treatment of substance use disorders. According to the Act, addiction specialty societies will have a role in creating trainings. Medical schools and residencies will also be able to fulfill this requirement with a “comprehensive” curriculum that covers all approved medications for the treatment of substance use disorders.
The DEA has not yet confirmed what training will be accepted, according to the Chief Medical Officer of the Substance Abuse and Mental Health Services Administration, Neeraj Gandotra, MD, who spoke to me in an interview. However, it is required to do so by April 5, 2023. Dr. Gandotra also emphasized that state and local laws, as well as insurance requirements, remain in place, and may place other constraints on prescribing. According to the Act, this new rule will be in effect by June 2023.
As an addiction medicine specialist and longtime buprenorphine prescriber, I am excited about these changes but wary of lingering resistance among health care providers. Will providers who have chosen not to get an X-waiver now look for another reason to not treat patients with substance use disorders?
Ms. Schanning remains hopeful. “I’m incredibly optimistic that health care providers are going to learn about buprenorphine and prescribe it to patients, and that patients are going to start asking about this medication,” she told me. “Seven in 10 providers say that they do feel an obligation to treat their patients with [opioid use disorder], but the federal government has made it very difficult to do so.”
Now with the X-waiver gone, providers and patients may be able to push for a long overdue shift in how we treat and conceptualize substance use disorders, she noted.
“Health care providers need to recognize substance use disorder as a medical condition that deserves treatment, and to speak about it like a medical condition,” Ms. Schanning said, by, for instance, moving away from using words such as “abuse” and “clean” and, instead, talking about treatable substance use disorders that can improve with evidence-based care, such as buprenorphine and methadone. “We also need to share stories of success and hope with people,” she added. “Once you’ve seen how someone can be transformed by treatment, it’s really difficult to say that substance use disorder is a character flaw, or their fault.”
A patient-centered approach
Over the past decade of practicing medicine, I have experienced this transformation personally. In residency, I believed that people had to be ready for help, to stop using, to change. I failed to recognize that many of those same people were asking me for help, and I wasn’t offering what they needed. The person who had to change was me.
As I moved toward a patient-centered approach, lowering barriers to starting and remaining in treatment, and collaborating with teams that could meet people wherever they might be, addictions became the most rewarding part of my practice.
I have never had more people thank me spontaneously and deeply for the care I provide. Plus, I have never seen a more profound change in the students I work with than when they witness someone with a substance use disorder offered treatment that works.
The X-waiver was not the only barrier to care, and the overdose crisis is not slowing down. But maybe with a new tool widely accessible, more of us will be ready to help.
Dr. Poorman is board certified in internal medicine and addiction medicine, assistant professor of medicine, University of Illinois at Chicago, and provides primary care and addiction services in Chicago. Her views do not necessarily reflect the views of her employer. She has reported no relevant disclosures, and she serves on the editorial advisory board of Internal Medicine News.
Unexpected link between light drinking and dementia risk
new research suggests.
Investigators assessed dementia risk using changes in alcohol consumption over a 2-year period in nearly 4 million people in South Korea. After about 7 years, dementia was 21% less likely in mild drinkers and 17% less likely in moderate drinkers. Heavy drinking was linked to an 8% increased risk.
Other studies of the relationship between alcohol and dementia have yielded mixed results, and this study does little to clear those murky waters. Nor do the results mean that drinking is recommended, the investigators note.
But the study does offer new information on how risk changes over time as people change their drinking habits, lead investigator Keun Hye Jeon, MD, assistant professor of family medicine at Cha Gumi Medical Center at Cha University, Gumi, South Korea, told this news organization.
“Although numerous studies have shown a relationship between alcohol consumption and dementia, there is a paucity of understanding as to how the incidence of dementia changes with changes in drinking habits,” Dr. Jeon said.
“By measuring alcohol consumption at two time points, we were able to study the relationship between reducing, ceasing, maintaining, and increasing alcohol consumption and incident dementia,” he added.
The findings were published online in JAMA Network Open.
Tracking drinking habits
Researchers analyzed data from nearly 4 million individuals aged 40 years and older in the Korean National Health Insurance Service who completed questionnaires and underwent physical exams in 2009 and 2011.
Study participants completed questionnaires on their drinking habits and were assigned to one of five groups according to change in alcohol consumption during the study period. These groups consisted of sustained nondrinkers; those who stopped drinking (quitters); those who reduced their consumption of alcohol but did not stop drinking (reducers); those who maintained the same level of consumption (sustainers); and those who increased their level of consumption (increasers).
A standard drink in the United States contains 14 g of alcohol. For this study, mild drinking was defined as less than 15 g/day, or one drink; moderate consumption as 15-29.9 g/day, or one to two drinks; and heavy drinking as 30 g/day or more, or three or more drinks.
At baseline, 54.8% of participants were nondrinkers, 26.7% were mild drinkers, 11.0% were moderate drinkers, and 7.5% were heavy drinkers.
From 2009 to 2011, 24.2% of mild drinkers, 8.4% of moderate drinkers, and 7.6% of heavy drinkers became quitters. In the same period, 13.9% of nondrinkers, 16.1% of mild drinkers, and 17.4% of moderate drinkers increased their drinking level.
After a mean follow-up of 6.3 years, 2.5% of participants were diagnosed with dementia, 2.0% with Alzheimer’s disease, and 0.3% with vascular dementia.
Unexpected finding
Compared with consistently not drinking, mild and moderate alcohol consumption was associated with a 21% (adjust hazard ratio, 0.79; 95% confidence interval, 0.77-0.81) and 17% (aHR, 0.83; 95% CI, 0.79-0.88) decreased risk for dementia, respectively.
Heavy drinking was linked to an 8% increased risk (aHR, 1.08; 95% CI, 1.03-1.12).
Similar associations were found between alcohol consumption and risk for Alzheimer’s disease and vascular dementia.
Reducing drinking habits from heavy to moderate led to a reduction in risk for dementia and Alzheimer’s, and increasing drinking levels led to an increase in risk for both conditions.
But when the researchers analyzed dementia risk for nondrinkers who began drinking at mild levels during the study period, they found something unexpected – the risk in this group decreased by 7% for dementia (aHR, 0.93; 95% CI, 0.90-0.96) and by 8% for Alzheimer’s (aHR, 0.92; 95% CI, 0.89-0.95), compared with sustained mild drinkers.
“Our study showed that initiation of mild alcohol consumption leads to a reduced risk of all-cause dementia and Alzheimer’s disease, which has never been reported in previous studies,” Dr. Jeon said.
However, Dr. Jeon was quick to point out that this doesn’t mean that people who don’t drink should start.
Previous studies have shown that heavy alcohol use can triple an individual’s dementia risk, while other studies have shown that no amount of alcohol consumption is good for the brain.
“None of the existing health guidelines recommend starting alcohol drinking,” Dr. Jeon said. “Our findings regarding an initiation of mild alcohol consumption cannot be directly translated into clinical recommendations,” but the findings do warrant additional study, he added.
Risks persist
Commenting on the findings, Percy Griffin, PhD, director of scientific engagement for the Alzheimer’s Association in Chicago, agrees.
“While this study is interesting, and this topic deserves further study, no one should drink alcohol as a method of reducing risk of Alzheimer’s disease or other dementia based on this study,” said Dr. Griffin, who was not part of the study.
The exact tipping point in alcohol consumption that can lead to problems with cognition or increased dementia risk is unknown, Dr. Griffin said. Nor do researchers understand why mild drinking may have a protective effect.
“We do know, however, that excessive alcohol consumption has negative effects on heart health and general health, which can lead to problems with brain function,” he said. “Clinicians should have discussions with their patients around their alcohol consumption patterns and the risks associated with drinking in excess, including potential damage to their cognition.”
Funding for the study was not disclosed. Dr. Jeon and Dr. Griffin report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Investigators assessed dementia risk using changes in alcohol consumption over a 2-year period in nearly 4 million people in South Korea. After about 7 years, dementia was 21% less likely in mild drinkers and 17% less likely in moderate drinkers. Heavy drinking was linked to an 8% increased risk.
Other studies of the relationship between alcohol and dementia have yielded mixed results, and this study does little to clear those murky waters. Nor do the results mean that drinking is recommended, the investigators note.
But the study does offer new information on how risk changes over time as people change their drinking habits, lead investigator Keun Hye Jeon, MD, assistant professor of family medicine at Cha Gumi Medical Center at Cha University, Gumi, South Korea, told this news organization.
“Although numerous studies have shown a relationship between alcohol consumption and dementia, there is a paucity of understanding as to how the incidence of dementia changes with changes in drinking habits,” Dr. Jeon said.
“By measuring alcohol consumption at two time points, we were able to study the relationship between reducing, ceasing, maintaining, and increasing alcohol consumption and incident dementia,” he added.
The findings were published online in JAMA Network Open.
Tracking drinking habits
Researchers analyzed data from nearly 4 million individuals aged 40 years and older in the Korean National Health Insurance Service who completed questionnaires and underwent physical exams in 2009 and 2011.
Study participants completed questionnaires on their drinking habits and were assigned to one of five groups according to change in alcohol consumption during the study period. These groups consisted of sustained nondrinkers; those who stopped drinking (quitters); those who reduced their consumption of alcohol but did not stop drinking (reducers); those who maintained the same level of consumption (sustainers); and those who increased their level of consumption (increasers).
A standard drink in the United States contains 14 g of alcohol. For this study, mild drinking was defined as less than 15 g/day, or one drink; moderate consumption as 15-29.9 g/day, or one to two drinks; and heavy drinking as 30 g/day or more, or three or more drinks.
At baseline, 54.8% of participants were nondrinkers, 26.7% were mild drinkers, 11.0% were moderate drinkers, and 7.5% were heavy drinkers.
From 2009 to 2011, 24.2% of mild drinkers, 8.4% of moderate drinkers, and 7.6% of heavy drinkers became quitters. In the same period, 13.9% of nondrinkers, 16.1% of mild drinkers, and 17.4% of moderate drinkers increased their drinking level.
After a mean follow-up of 6.3 years, 2.5% of participants were diagnosed with dementia, 2.0% with Alzheimer’s disease, and 0.3% with vascular dementia.
Unexpected finding
Compared with consistently not drinking, mild and moderate alcohol consumption was associated with a 21% (adjust hazard ratio, 0.79; 95% confidence interval, 0.77-0.81) and 17% (aHR, 0.83; 95% CI, 0.79-0.88) decreased risk for dementia, respectively.
Heavy drinking was linked to an 8% increased risk (aHR, 1.08; 95% CI, 1.03-1.12).
Similar associations were found between alcohol consumption and risk for Alzheimer’s disease and vascular dementia.
Reducing drinking habits from heavy to moderate led to a reduction in risk for dementia and Alzheimer’s, and increasing drinking levels led to an increase in risk for both conditions.
But when the researchers analyzed dementia risk for nondrinkers who began drinking at mild levels during the study period, they found something unexpected – the risk in this group decreased by 7% for dementia (aHR, 0.93; 95% CI, 0.90-0.96) and by 8% for Alzheimer’s (aHR, 0.92; 95% CI, 0.89-0.95), compared with sustained mild drinkers.
“Our study showed that initiation of mild alcohol consumption leads to a reduced risk of all-cause dementia and Alzheimer’s disease, which has never been reported in previous studies,” Dr. Jeon said.
However, Dr. Jeon was quick to point out that this doesn’t mean that people who don’t drink should start.
Previous studies have shown that heavy alcohol use can triple an individual’s dementia risk, while other studies have shown that no amount of alcohol consumption is good for the brain.
“None of the existing health guidelines recommend starting alcohol drinking,” Dr. Jeon said. “Our findings regarding an initiation of mild alcohol consumption cannot be directly translated into clinical recommendations,” but the findings do warrant additional study, he added.
Risks persist
Commenting on the findings, Percy Griffin, PhD, director of scientific engagement for the Alzheimer’s Association in Chicago, agrees.
“While this study is interesting, and this topic deserves further study, no one should drink alcohol as a method of reducing risk of Alzheimer’s disease or other dementia based on this study,” said Dr. Griffin, who was not part of the study.
The exact tipping point in alcohol consumption that can lead to problems with cognition or increased dementia risk is unknown, Dr. Griffin said. Nor do researchers understand why mild drinking may have a protective effect.
“We do know, however, that excessive alcohol consumption has negative effects on heart health and general health, which can lead to problems with brain function,” he said. “Clinicians should have discussions with their patients around their alcohol consumption patterns and the risks associated with drinking in excess, including potential damage to their cognition.”
Funding for the study was not disclosed. Dr. Jeon and Dr. Griffin report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Investigators assessed dementia risk using changes in alcohol consumption over a 2-year period in nearly 4 million people in South Korea. After about 7 years, dementia was 21% less likely in mild drinkers and 17% less likely in moderate drinkers. Heavy drinking was linked to an 8% increased risk.
Other studies of the relationship between alcohol and dementia have yielded mixed results, and this study does little to clear those murky waters. Nor do the results mean that drinking is recommended, the investigators note.
But the study does offer new information on how risk changes over time as people change their drinking habits, lead investigator Keun Hye Jeon, MD, assistant professor of family medicine at Cha Gumi Medical Center at Cha University, Gumi, South Korea, told this news organization.
“Although numerous studies have shown a relationship between alcohol consumption and dementia, there is a paucity of understanding as to how the incidence of dementia changes with changes in drinking habits,” Dr. Jeon said.
“By measuring alcohol consumption at two time points, we were able to study the relationship between reducing, ceasing, maintaining, and increasing alcohol consumption and incident dementia,” he added.
The findings were published online in JAMA Network Open.
Tracking drinking habits
Researchers analyzed data from nearly 4 million individuals aged 40 years and older in the Korean National Health Insurance Service who completed questionnaires and underwent physical exams in 2009 and 2011.
Study participants completed questionnaires on their drinking habits and were assigned to one of five groups according to change in alcohol consumption during the study period. These groups consisted of sustained nondrinkers; those who stopped drinking (quitters); those who reduced their consumption of alcohol but did not stop drinking (reducers); those who maintained the same level of consumption (sustainers); and those who increased their level of consumption (increasers).
A standard drink in the United States contains 14 g of alcohol. For this study, mild drinking was defined as less than 15 g/day, or one drink; moderate consumption as 15-29.9 g/day, or one to two drinks; and heavy drinking as 30 g/day or more, or three or more drinks.
At baseline, 54.8% of participants were nondrinkers, 26.7% were mild drinkers, 11.0% were moderate drinkers, and 7.5% were heavy drinkers.
From 2009 to 2011, 24.2% of mild drinkers, 8.4% of moderate drinkers, and 7.6% of heavy drinkers became quitters. In the same period, 13.9% of nondrinkers, 16.1% of mild drinkers, and 17.4% of moderate drinkers increased their drinking level.
After a mean follow-up of 6.3 years, 2.5% of participants were diagnosed with dementia, 2.0% with Alzheimer’s disease, and 0.3% with vascular dementia.
Unexpected finding
Compared with consistently not drinking, mild and moderate alcohol consumption was associated with a 21% (adjust hazard ratio, 0.79; 95% confidence interval, 0.77-0.81) and 17% (aHR, 0.83; 95% CI, 0.79-0.88) decreased risk for dementia, respectively.
Heavy drinking was linked to an 8% increased risk (aHR, 1.08; 95% CI, 1.03-1.12).
Similar associations were found between alcohol consumption and risk for Alzheimer’s disease and vascular dementia.
Reducing drinking habits from heavy to moderate led to a reduction in risk for dementia and Alzheimer’s, and increasing drinking levels led to an increase in risk for both conditions.
But when the researchers analyzed dementia risk for nondrinkers who began drinking at mild levels during the study period, they found something unexpected – the risk in this group decreased by 7% for dementia (aHR, 0.93; 95% CI, 0.90-0.96) and by 8% for Alzheimer’s (aHR, 0.92; 95% CI, 0.89-0.95), compared with sustained mild drinkers.
“Our study showed that initiation of mild alcohol consumption leads to a reduced risk of all-cause dementia and Alzheimer’s disease, which has never been reported in previous studies,” Dr. Jeon said.
However, Dr. Jeon was quick to point out that this doesn’t mean that people who don’t drink should start.
Previous studies have shown that heavy alcohol use can triple an individual’s dementia risk, while other studies have shown that no amount of alcohol consumption is good for the brain.
“None of the existing health guidelines recommend starting alcohol drinking,” Dr. Jeon said. “Our findings regarding an initiation of mild alcohol consumption cannot be directly translated into clinical recommendations,” but the findings do warrant additional study, he added.
Risks persist
Commenting on the findings, Percy Griffin, PhD, director of scientific engagement for the Alzheimer’s Association in Chicago, agrees.
“While this study is interesting, and this topic deserves further study, no one should drink alcohol as a method of reducing risk of Alzheimer’s disease or other dementia based on this study,” said Dr. Griffin, who was not part of the study.
The exact tipping point in alcohol consumption that can lead to problems with cognition or increased dementia risk is unknown, Dr. Griffin said. Nor do researchers understand why mild drinking may have a protective effect.
“We do know, however, that excessive alcohol consumption has negative effects on heart health and general health, which can lead to problems with brain function,” he said. “Clinicians should have discussions with their patients around their alcohol consumption patterns and the risks associated with drinking in excess, including potential damage to their cognition.”
Funding for the study was not disclosed. Dr. Jeon and Dr. Griffin report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Finding catatonia requires knowing what to look for
Catatonia is a psychomotor syndrome identified by its clinical phenotype. Unlike common psychiatric syndromes such as major depression that are characterized by self-report of symptoms, catatonia is identified chiefly by empirically evaluated signs on clinical evaluation. Its signs are recognized through observation, physical examination, or elicitation by clinical maneuvers or the presentation of stimuli. However, catatonia is often overlooked even though its clinical signs are often visibly apparent, including to the casual observer.
Why is catatonia underdiagnosed? A key modifiable factor appears to be a prevalent misunderstanding over what catatonia looks like.1 We have sought to address this in a few ways.
First identified was the need for comprehensive educational resources on how to assess for and recognize catatonia. Using the Bush-Francis Catatonia Rating Scale – the most widely used scale for catatonia in both research and clinical settings and the most cited publication in the catatonia literature – our team developed the BFCRS Training Manual and Coding Guide.2,3 This manual expands on the definitions of each BFCRS item based on how it was originally operationalized by the scale’s authors. Subsequently, we created a comprehensive set of educational resources including videos illustrating how to assess for catatonia, a video for each of the 23 items on the BFCRS, and self-assessment tools. All resources are freely available online at https://bfcrs.urmc.edu.4
Through this project it became apparent that there are many discrepancies across the field regarding the phenotype of catatonia. Specifically, a recent review inspired by this project set about to characterize the scope of distinctions across diagnostic systems and rating scales.5 For instance, each diagnostic system and rating scale includes a unique set of signs, approaches diagnostic thresholds differently, and often operationalizes clinical features in ways that lead either to criterion overlap (for example, combativeness would be scored both as combativeness and agitation on ICD-11) or contradictions with other systems or scales (for example, varied definitions of waxy flexibility). In the face of so many inconsistencies, what is a clinician to do? What follows is a discussion of how to apply the insights from this recent review in clinical and research settings.
Starting with DSM-5-TR and ICD-11 – the current editions of the two leading diagnostic systems – one might ask: How do they compare?6,7 Overall, these two systems are broadly aligned in terms of the catatonic syndrome. Both systems identify individual clinical signs (as opposed to symptom complexes). Both require three features as a diagnostic threshold. Most of the same clinical signs are included in both systems, and the definitions of individual items are largely equivalent. Additionally, both systems allow for diagnosis of catatonia in association with psychiatric and medical conditions and include a category for unspecified catatonia.
Despite these core agreements, though, there are several important distinctions. First, whereas all 12 signs included in DSM-5-TR count toward an ICD-11 catatonia diagnosis, the opposite cannot be said. ICD-11 includes several features that are not in DSM-5-TR: rigidity, verbigeration, withdrawal, staring, ambitendency, impulsivity, and combativeness. Next, autonomic abnormality, which signifies the most severe type of catatonia called malignant catatonia, is included as a potential comorbidity in ICD-11 but not mentioned in DSM-5-TR. Third, ICD-11 includes a separate diagnosis for substance-induced catatonia, whereas this condition would be diagnosed as unspecified catatonia in DSM-5-TR.
There are also elements missing from both systems. The most notable of these is that neither system specifies the period over which findings must be present for diagnosis. By clinical convention, the practical definition of 24 hours is appropriate in most instances. The clinical features identified during direct evaluation are usually sufficient for diagnosis, but additional signs observed or documented over the prior 24 hours should be incorporated as part of the clinical evaluation. Another distinction is how to handle clinical features before and after lorazepam challenge. As noted in the BFCRS Training Manual, it would be appropriate to compare “state assessments” (that is, restricted to features identified only during direct, in-person assessment) from before and after lorazepam administration to document improvement.4
Whereas DSM-5-TR and ICD-11 are broadly in agreement, comparing these systems with catatonia rating scales reveals many sources of potential confusion, but also concrete guidance on operationalizing individual items.5 How exactly should each of catatonia’s clinical signs be defined? Descriptions differ, and thresholds of duration and frequency vary considerably across scales. As a result, clinicians who use different scales and then convert these results to diagnostic criteria are liable to come to different clinical conclusions. For instance, both echophenomena and negativism must be elicited more than five times to be scored per Northoff,8 but even a single convincing instance of either would be scored on the BFCRS as “occasional.”2
Such discrepancies are important because, whereas the psychometric properties of several catatonia scales have been documented, there are no analogous studies on the DSM-5-TR and ICD-11 criteria. Therefore, it is essential for clinicians and researchers to document how diagnostic criteria have been operationalized. The most practical and evidence-based way to do this is to use a clinically validated scale and convert these to diagnostic criteria, yet in doing so a few modifications will be necessary.
Of the available clinical scales, the BFCRS is best positioned for clinical use. The BFCRS has been validated clinically and has good reliability, detailed item definitions and audiovisual examples available. In addition, it is the only scale with a published semistructured evaluation (see initial paper and Training Manual), which takes about 5 minutes.2,4 In terms of utility, all 12 signs included by DSM-5-TR are among the first 14 items on the BFCRS, which constitutes a standalone tool known as the Bush-Francis Catatonia Screening Instrument (BFCSI, see Table).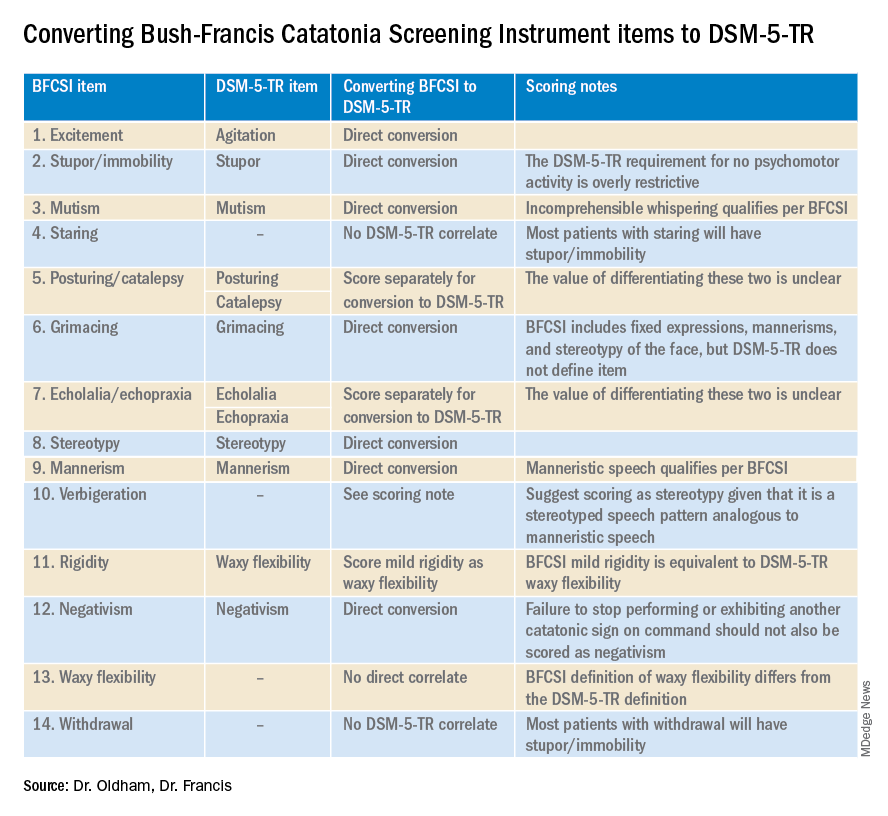
Many fundamental questions remain about catatonia,but the importance of a shared understanding of its clinical features is clear.9 Catatonia should be on the differential whenever a patient exhibits a markedly altered level of activity or grossly abnormal behavior, especially when inappropriate to context. We encourage readers to familiarize themselves with the phenotype of catatonia through online educational resources4 because the optimal care of patients with catatonia requires – at a minimum – that we know what we’re looking for.
Dr. Oldham is assistant professor of psychiatry at the University of Rochester (N.Y.) Medical Center. Dr. Francis is professor of psychiatry at Penn State University, Hershey. The authors declare no relevant conflicts of interest. Funding for the educational project hosted at https://bfcrs.urmc.edu was provided by the department of psychiatry at the University of Rochester Medical Center. Dr. Oldham is currently supported by a K23 career development award from the National Institute on Aging (AG072383). The educational resources referenced in this piece could not have been created were it not for the intellectual and thespian collaboration of Joshua R. Wortzel, MD, who is currently a fellow in child and adolescent psychiatry at Brown University, Providence, R.I. The authors are also indebted to Hochang B. Lee, MD, for his gracious support of this project.
References
1. Wortzel JR et al. J Clin Psychiatry. 2021 Aug 17;82(5):21m14025. doi: 10.4088/JCP.21m14025.
2. Bush G et al. Acta Psychiatr Scand. 1996 Feb;93(2):129-36. doi: 10.1111/j.1600-0447.1996.tb09814.x.
3. Weleff J et al. J Acad Consult Liaison Psychiatry. 2023 Jan-Feb;64(1):13-27. doi:10.1016/j.jaclp.2022.07.002.
4. Oldham MA et al. Bush-Francis Catatonia Rating Scale Assessment Resources. University of Rochester Medical Center, Department of Psychiatry. https://bfcrs.urmc.edu.
5. Oldham MA. Schizophr Res. 2022 Aug 19;S0920-9964(22)00294-8. doi: 10.1016/j.schres.2022.08.002.
6. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5-TR. Washington, D.C.: American Psychiatric Association Publishing, 2022.
7. World Health Organization. ICD-11 for Mortality and Morbidity Stastistics. 2022. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/486722075.
8. Northoff G et al. Mov Disord. May 1999;14(3):404-16. doi: 10.1002/1531-8257(199905)14:3<404::AID-MDS1004>3.0.CO;2-5.
9. Walther S et al. The Lancet Psychiatry. 2019 Jul;6(7):610-9. doi: 10.1016/S2215-0366(18)30474-7.
Catatonia is a psychomotor syndrome identified by its clinical phenotype. Unlike common psychiatric syndromes such as major depression that are characterized by self-report of symptoms, catatonia is identified chiefly by empirically evaluated signs on clinical evaluation. Its signs are recognized through observation, physical examination, or elicitation by clinical maneuvers or the presentation of stimuli. However, catatonia is often overlooked even though its clinical signs are often visibly apparent, including to the casual observer.
Why is catatonia underdiagnosed? A key modifiable factor appears to be a prevalent misunderstanding over what catatonia looks like.1 We have sought to address this in a few ways.
First identified was the need for comprehensive educational resources on how to assess for and recognize catatonia. Using the Bush-Francis Catatonia Rating Scale – the most widely used scale for catatonia in both research and clinical settings and the most cited publication in the catatonia literature – our team developed the BFCRS Training Manual and Coding Guide.2,3 This manual expands on the definitions of each BFCRS item based on how it was originally operationalized by the scale’s authors. Subsequently, we created a comprehensive set of educational resources including videos illustrating how to assess for catatonia, a video for each of the 23 items on the BFCRS, and self-assessment tools. All resources are freely available online at https://bfcrs.urmc.edu.4
Through this project it became apparent that there are many discrepancies across the field regarding the phenotype of catatonia. Specifically, a recent review inspired by this project set about to characterize the scope of distinctions across diagnostic systems and rating scales.5 For instance, each diagnostic system and rating scale includes a unique set of signs, approaches diagnostic thresholds differently, and often operationalizes clinical features in ways that lead either to criterion overlap (for example, combativeness would be scored both as combativeness and agitation on ICD-11) or contradictions with other systems or scales (for example, varied definitions of waxy flexibility). In the face of so many inconsistencies, what is a clinician to do? What follows is a discussion of how to apply the insights from this recent review in clinical and research settings.
Starting with DSM-5-TR and ICD-11 – the current editions of the two leading diagnostic systems – one might ask: How do they compare?6,7 Overall, these two systems are broadly aligned in terms of the catatonic syndrome. Both systems identify individual clinical signs (as opposed to symptom complexes). Both require three features as a diagnostic threshold. Most of the same clinical signs are included in both systems, and the definitions of individual items are largely equivalent. Additionally, both systems allow for diagnosis of catatonia in association with psychiatric and medical conditions and include a category for unspecified catatonia.
Despite these core agreements, though, there are several important distinctions. First, whereas all 12 signs included in DSM-5-TR count toward an ICD-11 catatonia diagnosis, the opposite cannot be said. ICD-11 includes several features that are not in DSM-5-TR: rigidity, verbigeration, withdrawal, staring, ambitendency, impulsivity, and combativeness. Next, autonomic abnormality, which signifies the most severe type of catatonia called malignant catatonia, is included as a potential comorbidity in ICD-11 but not mentioned in DSM-5-TR. Third, ICD-11 includes a separate diagnosis for substance-induced catatonia, whereas this condition would be diagnosed as unspecified catatonia in DSM-5-TR.
There are also elements missing from both systems. The most notable of these is that neither system specifies the period over which findings must be present for diagnosis. By clinical convention, the practical definition of 24 hours is appropriate in most instances. The clinical features identified during direct evaluation are usually sufficient for diagnosis, but additional signs observed or documented over the prior 24 hours should be incorporated as part of the clinical evaluation. Another distinction is how to handle clinical features before and after lorazepam challenge. As noted in the BFCRS Training Manual, it would be appropriate to compare “state assessments” (that is, restricted to features identified only during direct, in-person assessment) from before and after lorazepam administration to document improvement.4
Whereas DSM-5-TR and ICD-11 are broadly in agreement, comparing these systems with catatonia rating scales reveals many sources of potential confusion, but also concrete guidance on operationalizing individual items.5 How exactly should each of catatonia’s clinical signs be defined? Descriptions differ, and thresholds of duration and frequency vary considerably across scales. As a result, clinicians who use different scales and then convert these results to diagnostic criteria are liable to come to different clinical conclusions. For instance, both echophenomena and negativism must be elicited more than five times to be scored per Northoff,8 but even a single convincing instance of either would be scored on the BFCRS as “occasional.”2
Such discrepancies are important because, whereas the psychometric properties of several catatonia scales have been documented, there are no analogous studies on the DSM-5-TR and ICD-11 criteria. Therefore, it is essential for clinicians and researchers to document how diagnostic criteria have been operationalized. The most practical and evidence-based way to do this is to use a clinically validated scale and convert these to diagnostic criteria, yet in doing so a few modifications will be necessary.
Of the available clinical scales, the BFCRS is best positioned for clinical use. The BFCRS has been validated clinically and has good reliability, detailed item definitions and audiovisual examples available. In addition, it is the only scale with a published semistructured evaluation (see initial paper and Training Manual), which takes about 5 minutes.2,4 In terms of utility, all 12 signs included by DSM-5-TR are among the first 14 items on the BFCRS, which constitutes a standalone tool known as the Bush-Francis Catatonia Screening Instrument (BFCSI, see Table).
Many fundamental questions remain about catatonia,but the importance of a shared understanding of its clinical features is clear.9 Catatonia should be on the differential whenever a patient exhibits a markedly altered level of activity or grossly abnormal behavior, especially when inappropriate to context. We encourage readers to familiarize themselves with the phenotype of catatonia through online educational resources4 because the optimal care of patients with catatonia requires – at a minimum – that we know what we’re looking for.
Dr. Oldham is assistant professor of psychiatry at the University of Rochester (N.Y.) Medical Center. Dr. Francis is professor of psychiatry at Penn State University, Hershey. The authors declare no relevant conflicts of interest. Funding for the educational project hosted at https://bfcrs.urmc.edu was provided by the department of psychiatry at the University of Rochester Medical Center. Dr. Oldham is currently supported by a K23 career development award from the National Institute on Aging (AG072383). The educational resources referenced in this piece could not have been created were it not for the intellectual and thespian collaboration of Joshua R. Wortzel, MD, who is currently a fellow in child and adolescent psychiatry at Brown University, Providence, R.I. The authors are also indebted to Hochang B. Lee, MD, for his gracious support of this project.
References
1. Wortzel JR et al. J Clin Psychiatry. 2021 Aug 17;82(5):21m14025. doi: 10.4088/JCP.21m14025.
2. Bush G et al. Acta Psychiatr Scand. 1996 Feb;93(2):129-36. doi: 10.1111/j.1600-0447.1996.tb09814.x.
3. Weleff J et al. J Acad Consult Liaison Psychiatry. 2023 Jan-Feb;64(1):13-27. doi:10.1016/j.jaclp.2022.07.002.
4. Oldham MA et al. Bush-Francis Catatonia Rating Scale Assessment Resources. University of Rochester Medical Center, Department of Psychiatry. https://bfcrs.urmc.edu.
5. Oldham MA. Schizophr Res. 2022 Aug 19;S0920-9964(22)00294-8. doi: 10.1016/j.schres.2022.08.002.
6. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5-TR. Washington, D.C.: American Psychiatric Association Publishing, 2022.
7. World Health Organization. ICD-11 for Mortality and Morbidity Stastistics. 2022. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/486722075.
8. Northoff G et al. Mov Disord. May 1999;14(3):404-16. doi: 10.1002/1531-8257(199905)14:3<404::AID-MDS1004>3.0.CO;2-5.
9. Walther S et al. The Lancet Psychiatry. 2019 Jul;6(7):610-9. doi: 10.1016/S2215-0366(18)30474-7.
Catatonia is a psychomotor syndrome identified by its clinical phenotype. Unlike common psychiatric syndromes such as major depression that are characterized by self-report of symptoms, catatonia is identified chiefly by empirically evaluated signs on clinical evaluation. Its signs are recognized through observation, physical examination, or elicitation by clinical maneuvers or the presentation of stimuli. However, catatonia is often overlooked even though its clinical signs are often visibly apparent, including to the casual observer.
Why is catatonia underdiagnosed? A key modifiable factor appears to be a prevalent misunderstanding over what catatonia looks like.1 We have sought to address this in a few ways.
First identified was the need for comprehensive educational resources on how to assess for and recognize catatonia. Using the Bush-Francis Catatonia Rating Scale – the most widely used scale for catatonia in both research and clinical settings and the most cited publication in the catatonia literature – our team developed the BFCRS Training Manual and Coding Guide.2,3 This manual expands on the definitions of each BFCRS item based on how it was originally operationalized by the scale’s authors. Subsequently, we created a comprehensive set of educational resources including videos illustrating how to assess for catatonia, a video for each of the 23 items on the BFCRS, and self-assessment tools. All resources are freely available online at https://bfcrs.urmc.edu.4
Through this project it became apparent that there are many discrepancies across the field regarding the phenotype of catatonia. Specifically, a recent review inspired by this project set about to characterize the scope of distinctions across diagnostic systems and rating scales.5 For instance, each diagnostic system and rating scale includes a unique set of signs, approaches diagnostic thresholds differently, and often operationalizes clinical features in ways that lead either to criterion overlap (for example, combativeness would be scored both as combativeness and agitation on ICD-11) or contradictions with other systems or scales (for example, varied definitions of waxy flexibility). In the face of so many inconsistencies, what is a clinician to do? What follows is a discussion of how to apply the insights from this recent review in clinical and research settings.
Starting with DSM-5-TR and ICD-11 – the current editions of the two leading diagnostic systems – one might ask: How do they compare?6,7 Overall, these two systems are broadly aligned in terms of the catatonic syndrome. Both systems identify individual clinical signs (as opposed to symptom complexes). Both require three features as a diagnostic threshold. Most of the same clinical signs are included in both systems, and the definitions of individual items are largely equivalent. Additionally, both systems allow for diagnosis of catatonia in association with psychiatric and medical conditions and include a category for unspecified catatonia.
Despite these core agreements, though, there are several important distinctions. First, whereas all 12 signs included in DSM-5-TR count toward an ICD-11 catatonia diagnosis, the opposite cannot be said. ICD-11 includes several features that are not in DSM-5-TR: rigidity, verbigeration, withdrawal, staring, ambitendency, impulsivity, and combativeness. Next, autonomic abnormality, which signifies the most severe type of catatonia called malignant catatonia, is included as a potential comorbidity in ICD-11 but not mentioned in DSM-5-TR. Third, ICD-11 includes a separate diagnosis for substance-induced catatonia, whereas this condition would be diagnosed as unspecified catatonia in DSM-5-TR.
There are also elements missing from both systems. The most notable of these is that neither system specifies the period over which findings must be present for diagnosis. By clinical convention, the practical definition of 24 hours is appropriate in most instances. The clinical features identified during direct evaluation are usually sufficient for diagnosis, but additional signs observed or documented over the prior 24 hours should be incorporated as part of the clinical evaluation. Another distinction is how to handle clinical features before and after lorazepam challenge. As noted in the BFCRS Training Manual, it would be appropriate to compare “state assessments” (that is, restricted to features identified only during direct, in-person assessment) from before and after lorazepam administration to document improvement.4
Whereas DSM-5-TR and ICD-11 are broadly in agreement, comparing these systems with catatonia rating scales reveals many sources of potential confusion, but also concrete guidance on operationalizing individual items.5 How exactly should each of catatonia’s clinical signs be defined? Descriptions differ, and thresholds of duration and frequency vary considerably across scales. As a result, clinicians who use different scales and then convert these results to diagnostic criteria are liable to come to different clinical conclusions. For instance, both echophenomena and negativism must be elicited more than five times to be scored per Northoff,8 but even a single convincing instance of either would be scored on the BFCRS as “occasional.”2
Such discrepancies are important because, whereas the psychometric properties of several catatonia scales have been documented, there are no analogous studies on the DSM-5-TR and ICD-11 criteria. Therefore, it is essential for clinicians and researchers to document how diagnostic criteria have been operationalized. The most practical and evidence-based way to do this is to use a clinically validated scale and convert these to diagnostic criteria, yet in doing so a few modifications will be necessary.
Of the available clinical scales, the BFCRS is best positioned for clinical use. The BFCRS has been validated clinically and has good reliability, detailed item definitions and audiovisual examples available. In addition, it is the only scale with a published semistructured evaluation (see initial paper and Training Manual), which takes about 5 minutes.2,4 In terms of utility, all 12 signs included by DSM-5-TR are among the first 14 items on the BFCRS, which constitutes a standalone tool known as the Bush-Francis Catatonia Screening Instrument (BFCSI, see Table).
Many fundamental questions remain about catatonia,but the importance of a shared understanding of its clinical features is clear.9 Catatonia should be on the differential whenever a patient exhibits a markedly altered level of activity or grossly abnormal behavior, especially when inappropriate to context. We encourage readers to familiarize themselves with the phenotype of catatonia through online educational resources4 because the optimal care of patients with catatonia requires – at a minimum – that we know what we’re looking for.
Dr. Oldham is assistant professor of psychiatry at the University of Rochester (N.Y.) Medical Center. Dr. Francis is professor of psychiatry at Penn State University, Hershey. The authors declare no relevant conflicts of interest. Funding for the educational project hosted at https://bfcrs.urmc.edu was provided by the department of psychiatry at the University of Rochester Medical Center. Dr. Oldham is currently supported by a K23 career development award from the National Institute on Aging (AG072383). The educational resources referenced in this piece could not have been created were it not for the intellectual and thespian collaboration of Joshua R. Wortzel, MD, who is currently a fellow in child and adolescent psychiatry at Brown University, Providence, R.I. The authors are also indebted to Hochang B. Lee, MD, for his gracious support of this project.
References
1. Wortzel JR et al. J Clin Psychiatry. 2021 Aug 17;82(5):21m14025. doi: 10.4088/JCP.21m14025.
2. Bush G et al. Acta Psychiatr Scand. 1996 Feb;93(2):129-36. doi: 10.1111/j.1600-0447.1996.tb09814.x.
3. Weleff J et al. J Acad Consult Liaison Psychiatry. 2023 Jan-Feb;64(1):13-27. doi:10.1016/j.jaclp.2022.07.002.
4. Oldham MA et al. Bush-Francis Catatonia Rating Scale Assessment Resources. University of Rochester Medical Center, Department of Psychiatry. https://bfcrs.urmc.edu.
5. Oldham MA. Schizophr Res. 2022 Aug 19;S0920-9964(22)00294-8. doi: 10.1016/j.schres.2022.08.002.
6. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5-TR. Washington, D.C.: American Psychiatric Association Publishing, 2022.
7. World Health Organization. ICD-11 for Mortality and Morbidity Stastistics. 2022. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/486722075.
8. Northoff G et al. Mov Disord. May 1999;14(3):404-16. doi: 10.1002/1531-8257(199905)14:3<404::AID-MDS1004>3.0.CO;2-5.
9. Walther S et al. The Lancet Psychiatry. 2019 Jul;6(7):610-9. doi: 10.1016/S2215-0366(18)30474-7.
Nature, not nurture, the culprit in OCD
new research suggests.
This finding from a large, register-based study is particularly surprising because results from previous studies of major depression and anxiety disorder have shown a significant effect of parenting and a child’s home environment on the risk for these disorders, the investigators noted.
While the results likely won’t change patient treatment, one expert said it could alleviate concerns of some parents with OCD who fear that witnessing their obsessive behaviors might put their children at higher risk for the disorder.
“The evidence is consistent with the idea that the psychological transmission of OCD from parent to child, if it exists, is really pretty weak,” lead author Kenneth S. Kendler, MD, professor of psychiatry and director of the Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond, said in an interview.
The findings were published online in JAMA Psychiatry.
Family analysis
The study is the first to include adoptive parents in an analysis of OCD transmission, which allowed investigators to answer the nature versus nurture question that is often difficult to decipher.
Working with Swedish population registries, researchers identified more than 2.4 million offspring. Of these, 27,141 individuals (1.1%) had a lifetime diagnosis of OCD.
Families were divided into four types: intact families, with kids who lived at home with their biological parents from birth to at least age 15 years; families with kids who never lived with their biological father; families with children who did not live with their biological fathers between birth and age 15 years but who lived with a stepfather for at least 10 of those years; and families with children who were adopted before the age of 5 by people with no biological connection to the child.
After analyzing data from all parent-child relationships, researchers found that genes plus rearing (odds ratio, 3.94; 95% confidence interval, 3.58-4.33) and genes only (OR, 3.34; 95% CI, 2.27-4.93) were significantly more likely to be correlated to transmission of OCD from parent to offspring than rearing alone. Rearing only (OR, 1.4; 95% CI, 0.45-4.39) was not significantly correlated with OCD transmission
“It appears from our data that the only substantial transmission that occurs is in the genes parents transmit, not by the modeling of behavior,” Dr. Kendler said.
“There’s an idea that you can learn some things from your parents from psychopathology, but we didn’t see that kids picked that up much in the case of OCD,” he added.
However, there was one outlier: Children raised by stepparents or adoptive parents with an anxiety disorder had a greater risk of developing OCD.
Given the lack of evidence of a strong rearing effect in other analyses, Dr. Kendler noted that this rogue finding could be caused by an underpowered sample; the researchers plan to study the data further.
“Psychiatric disorders, like many other conditions, are often correlated with neighboring conditions,” he said. “Our study would suggest that some of the molecular genetic variants between OCD and generalized anxiety disorder or other anxiety disorders would be shared, but some would be unique.”
Answers an old question
In a comment, Jon Grant, JD, MD, MPH, professor of psychiatry and director of the Addictive, Compulsive, and Impulsive Disorders Research Lab at the University of Chicago, said the findings fill an important gap in what is known about OCD.
“I think the findings are really answering this old question of: ‘Is OCD due to the rearing patterns in a family versus genetics?’ This was able to get at that information showing that it’s virtually all due to genetics within families, and that’s really good to know,” said Dr. Grant, who was not a part of the study.
He was also struck by the finding of a strong genetic relationship between OCD and generalized anxiety disorder (GAD).
While identifying that OCD and GAD are genetically linked likely won’t change clinical care, “I think it at least allows clinicians to know when we see that comorbidity that it may be much more genetically linked in the case of GAD,” Dr. Grant said.
The study was funded by the Swedish Research Council, as well as Avtal om Läkarutbildning och Forskning funding from Region Skåne. Dr. Kendler and Dr. Grant reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
This finding from a large, register-based study is particularly surprising because results from previous studies of major depression and anxiety disorder have shown a significant effect of parenting and a child’s home environment on the risk for these disorders, the investigators noted.
While the results likely won’t change patient treatment, one expert said it could alleviate concerns of some parents with OCD who fear that witnessing their obsessive behaviors might put their children at higher risk for the disorder.
“The evidence is consistent with the idea that the psychological transmission of OCD from parent to child, if it exists, is really pretty weak,” lead author Kenneth S. Kendler, MD, professor of psychiatry and director of the Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond, said in an interview.
The findings were published online in JAMA Psychiatry.
Family analysis
The study is the first to include adoptive parents in an analysis of OCD transmission, which allowed investigators to answer the nature versus nurture question that is often difficult to decipher.
Working with Swedish population registries, researchers identified more than 2.4 million offspring. Of these, 27,141 individuals (1.1%) had a lifetime diagnosis of OCD.
Families were divided into four types: intact families, with kids who lived at home with their biological parents from birth to at least age 15 years; families with kids who never lived with their biological father; families with children who did not live with their biological fathers between birth and age 15 years but who lived with a stepfather for at least 10 of those years; and families with children who were adopted before the age of 5 by people with no biological connection to the child.
After analyzing data from all parent-child relationships, researchers found that genes plus rearing (odds ratio, 3.94; 95% confidence interval, 3.58-4.33) and genes only (OR, 3.34; 95% CI, 2.27-4.93) were significantly more likely to be correlated to transmission of OCD from parent to offspring than rearing alone. Rearing only (OR, 1.4; 95% CI, 0.45-4.39) was not significantly correlated with OCD transmission
“It appears from our data that the only substantial transmission that occurs is in the genes parents transmit, not by the modeling of behavior,” Dr. Kendler said.
“There’s an idea that you can learn some things from your parents from psychopathology, but we didn’t see that kids picked that up much in the case of OCD,” he added.
However, there was one outlier: Children raised by stepparents or adoptive parents with an anxiety disorder had a greater risk of developing OCD.
Given the lack of evidence of a strong rearing effect in other analyses, Dr. Kendler noted that this rogue finding could be caused by an underpowered sample; the researchers plan to study the data further.
“Psychiatric disorders, like many other conditions, are often correlated with neighboring conditions,” he said. “Our study would suggest that some of the molecular genetic variants between OCD and generalized anxiety disorder or other anxiety disorders would be shared, but some would be unique.”
Answers an old question
In a comment, Jon Grant, JD, MD, MPH, professor of psychiatry and director of the Addictive, Compulsive, and Impulsive Disorders Research Lab at the University of Chicago, said the findings fill an important gap in what is known about OCD.
“I think the findings are really answering this old question of: ‘Is OCD due to the rearing patterns in a family versus genetics?’ This was able to get at that information showing that it’s virtually all due to genetics within families, and that’s really good to know,” said Dr. Grant, who was not a part of the study.
He was also struck by the finding of a strong genetic relationship between OCD and generalized anxiety disorder (GAD).
While identifying that OCD and GAD are genetically linked likely won’t change clinical care, “I think it at least allows clinicians to know when we see that comorbidity that it may be much more genetically linked in the case of GAD,” Dr. Grant said.
The study was funded by the Swedish Research Council, as well as Avtal om Läkarutbildning och Forskning funding from Region Skåne. Dr. Kendler and Dr. Grant reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
This finding from a large, register-based study is particularly surprising because results from previous studies of major depression and anxiety disorder have shown a significant effect of parenting and a child’s home environment on the risk for these disorders, the investigators noted.
While the results likely won’t change patient treatment, one expert said it could alleviate concerns of some parents with OCD who fear that witnessing their obsessive behaviors might put their children at higher risk for the disorder.
“The evidence is consistent with the idea that the psychological transmission of OCD from parent to child, if it exists, is really pretty weak,” lead author Kenneth S. Kendler, MD, professor of psychiatry and director of the Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond, said in an interview.
The findings were published online in JAMA Psychiatry.
Family analysis
The study is the first to include adoptive parents in an analysis of OCD transmission, which allowed investigators to answer the nature versus nurture question that is often difficult to decipher.
Working with Swedish population registries, researchers identified more than 2.4 million offspring. Of these, 27,141 individuals (1.1%) had a lifetime diagnosis of OCD.
Families were divided into four types: intact families, with kids who lived at home with their biological parents from birth to at least age 15 years; families with kids who never lived with their biological father; families with children who did not live with their biological fathers between birth and age 15 years but who lived with a stepfather for at least 10 of those years; and families with children who were adopted before the age of 5 by people with no biological connection to the child.
After analyzing data from all parent-child relationships, researchers found that genes plus rearing (odds ratio, 3.94; 95% confidence interval, 3.58-4.33) and genes only (OR, 3.34; 95% CI, 2.27-4.93) were significantly more likely to be correlated to transmission of OCD from parent to offspring than rearing alone. Rearing only (OR, 1.4; 95% CI, 0.45-4.39) was not significantly correlated with OCD transmission
“It appears from our data that the only substantial transmission that occurs is in the genes parents transmit, not by the modeling of behavior,” Dr. Kendler said.
“There’s an idea that you can learn some things from your parents from psychopathology, but we didn’t see that kids picked that up much in the case of OCD,” he added.
However, there was one outlier: Children raised by stepparents or adoptive parents with an anxiety disorder had a greater risk of developing OCD.
Given the lack of evidence of a strong rearing effect in other analyses, Dr. Kendler noted that this rogue finding could be caused by an underpowered sample; the researchers plan to study the data further.
“Psychiatric disorders, like many other conditions, are often correlated with neighboring conditions,” he said. “Our study would suggest that some of the molecular genetic variants between OCD and generalized anxiety disorder or other anxiety disorders would be shared, but some would be unique.”
Answers an old question
In a comment, Jon Grant, JD, MD, MPH, professor of psychiatry and director of the Addictive, Compulsive, and Impulsive Disorders Research Lab at the University of Chicago, said the findings fill an important gap in what is known about OCD.
“I think the findings are really answering this old question of: ‘Is OCD due to the rearing patterns in a family versus genetics?’ This was able to get at that information showing that it’s virtually all due to genetics within families, and that’s really good to know,” said Dr. Grant, who was not a part of the study.
He was also struck by the finding of a strong genetic relationship between OCD and generalized anxiety disorder (GAD).
While identifying that OCD and GAD are genetically linked likely won’t change clinical care, “I think it at least allows clinicians to know when we see that comorbidity that it may be much more genetically linked in the case of GAD,” Dr. Grant said.
The study was funded by the Swedish Research Council, as well as Avtal om Läkarutbildning och Forskning funding from Region Skåne. Dr. Kendler and Dr. Grant reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Three wild technologies about to change health care
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
Can a hormone shot rescue low libido?
according to results from two small randomized controlled trials.
The data suggest that injections of kisspeptin can boost sexual desire in men and women and can increase penile rigidity in men.
Together, these two studies provide proof of concept for the development of kisspeptin-based therapeutics for men and women with distressing hypoactive sexual desire disorder (HSDD), study investigator Alexander Comninos, MD, PhD, Imperial College London, said in a news release.
One study was published online Feb. 3, 2022, in JAMA Network Open. The other was published in October 2022.
Unmet need
HSDD affects up to 10% of women and 8% of men worldwide and leads to psychological and social harm, the news release noted.
“There is a real unmet need to find new, safer, and more effective therapies for this distressing condition for both women and men seeking treatment,” Dr. Comninos said.
Kisspeptin is a naturally occurring reproductive hormone that serves as a crucial activator of the reproductive system. Emerging evidence from animal models shows that kisspeptin signaling has key roles in modulating reproductive behavior, including sexual motivation and erections.
In a double-blind, placebo-controlled, crossover study, the researchers enrolled 32 healthy heterosexual men (mean age, 37.9 years) who had HSDD.
At the first study visit, the men were given an infusion of kisspeptin-54 (1 nmol/kg per hour) or placebo (saline) over 75 minutes. The participants then crossed over to the other treatment at a second study visit at least 7 days later.
The active treatment significantly increased circulating kisspeptin levels. A steady state was reached after 30-75 minutes of infusion, the researchers reported.
Similar data in men, women
While the men viewed sexual videos, kisspeptin significantly modulated brain activity on fMRI in key structures of the sexual-processing network, compared with placebo (P = .003).
In addition, the treatment led to significant increases in penile tumescence in response to sexual stimuli (by up to 56% more than placebo; P = .02) and behavioral measures of sexual desire – most notably increased happiness about sex (P = .02).
Given the significant stimulatory effect of kisspeptin administration on penile rigidity, coupled with its demonstrated proerectile effect in rodents, future studies should examine the use of kisspeptin for patients with erectile dysfunction, the researchers wrote.
The second study included 32 women with HSDD and had the same design. Its results also showed that kisspeptin restored sexual and attraction brain processing without adverse effects.
“It is highly encouraging to see the same boosting effect in both women and men, although the precise brain pathways were slightly different, as might be expected,” coinvestigator Waljit Dhillo, PhD, Imperial College London, said in the news release.
“Collectively, the results suggest that kisspeptin may offer a safe and much-needed treatment for HSDD that affects millions of people around the world; and we look forward to taking this forward in future larger studies and in other patient groups,” Dr. Dhillo added.
The study was funded by the National Institute for Health and Care Research Imperial Biomedical Research Centre and the Medical Research Council, part of UK Research and Innovation. Dr. Comninos reported no relevant financial relationships. Dr. Dhillo reported receiving consulting fees from Myovant Sciences and KaNDy Therapeutics outside the submitted work.
A version of this article first appeared on Medscape.com.
according to results from two small randomized controlled trials.
The data suggest that injections of kisspeptin can boost sexual desire in men and women and can increase penile rigidity in men.
Together, these two studies provide proof of concept for the development of kisspeptin-based therapeutics for men and women with distressing hypoactive sexual desire disorder (HSDD), study investigator Alexander Comninos, MD, PhD, Imperial College London, said in a news release.
One study was published online Feb. 3, 2022, in JAMA Network Open. The other was published in October 2022.
Unmet need
HSDD affects up to 10% of women and 8% of men worldwide and leads to psychological and social harm, the news release noted.
“There is a real unmet need to find new, safer, and more effective therapies for this distressing condition for both women and men seeking treatment,” Dr. Comninos said.
Kisspeptin is a naturally occurring reproductive hormone that serves as a crucial activator of the reproductive system. Emerging evidence from animal models shows that kisspeptin signaling has key roles in modulating reproductive behavior, including sexual motivation and erections.
In a double-blind, placebo-controlled, crossover study, the researchers enrolled 32 healthy heterosexual men (mean age, 37.9 years) who had HSDD.
At the first study visit, the men were given an infusion of kisspeptin-54 (1 nmol/kg per hour) or placebo (saline) over 75 minutes. The participants then crossed over to the other treatment at a second study visit at least 7 days later.
The active treatment significantly increased circulating kisspeptin levels. A steady state was reached after 30-75 minutes of infusion, the researchers reported.
Similar data in men, women
While the men viewed sexual videos, kisspeptin significantly modulated brain activity on fMRI in key structures of the sexual-processing network, compared with placebo (P = .003).
In addition, the treatment led to significant increases in penile tumescence in response to sexual stimuli (by up to 56% more than placebo; P = .02) and behavioral measures of sexual desire – most notably increased happiness about sex (P = .02).
Given the significant stimulatory effect of kisspeptin administration on penile rigidity, coupled with its demonstrated proerectile effect in rodents, future studies should examine the use of kisspeptin for patients with erectile dysfunction, the researchers wrote.
The second study included 32 women with HSDD and had the same design. Its results also showed that kisspeptin restored sexual and attraction brain processing without adverse effects.
“It is highly encouraging to see the same boosting effect in both women and men, although the precise brain pathways were slightly different, as might be expected,” coinvestigator Waljit Dhillo, PhD, Imperial College London, said in the news release.
“Collectively, the results suggest that kisspeptin may offer a safe and much-needed treatment for HSDD that affects millions of people around the world; and we look forward to taking this forward in future larger studies and in other patient groups,” Dr. Dhillo added.
The study was funded by the National Institute for Health and Care Research Imperial Biomedical Research Centre and the Medical Research Council, part of UK Research and Innovation. Dr. Comninos reported no relevant financial relationships. Dr. Dhillo reported receiving consulting fees from Myovant Sciences and KaNDy Therapeutics outside the submitted work.
A version of this article first appeared on Medscape.com.
according to results from two small randomized controlled trials.
The data suggest that injections of kisspeptin can boost sexual desire in men and women and can increase penile rigidity in men.
Together, these two studies provide proof of concept for the development of kisspeptin-based therapeutics for men and women with distressing hypoactive sexual desire disorder (HSDD), study investigator Alexander Comninos, MD, PhD, Imperial College London, said in a news release.
One study was published online Feb. 3, 2022, in JAMA Network Open. The other was published in October 2022.
Unmet need
HSDD affects up to 10% of women and 8% of men worldwide and leads to psychological and social harm, the news release noted.
“There is a real unmet need to find new, safer, and more effective therapies for this distressing condition for both women and men seeking treatment,” Dr. Comninos said.
Kisspeptin is a naturally occurring reproductive hormone that serves as a crucial activator of the reproductive system. Emerging evidence from animal models shows that kisspeptin signaling has key roles in modulating reproductive behavior, including sexual motivation and erections.
In a double-blind, placebo-controlled, crossover study, the researchers enrolled 32 healthy heterosexual men (mean age, 37.9 years) who had HSDD.
At the first study visit, the men were given an infusion of kisspeptin-54 (1 nmol/kg per hour) or placebo (saline) over 75 minutes. The participants then crossed over to the other treatment at a second study visit at least 7 days later.
The active treatment significantly increased circulating kisspeptin levels. A steady state was reached after 30-75 minutes of infusion, the researchers reported.
Similar data in men, women
While the men viewed sexual videos, kisspeptin significantly modulated brain activity on fMRI in key structures of the sexual-processing network, compared with placebo (P = .003).
In addition, the treatment led to significant increases in penile tumescence in response to sexual stimuli (by up to 56% more than placebo; P = .02) and behavioral measures of sexual desire – most notably increased happiness about sex (P = .02).
Given the significant stimulatory effect of kisspeptin administration on penile rigidity, coupled with its demonstrated proerectile effect in rodents, future studies should examine the use of kisspeptin for patients with erectile dysfunction, the researchers wrote.
The second study included 32 women with HSDD and had the same design. Its results also showed that kisspeptin restored sexual and attraction brain processing without adverse effects.
“It is highly encouraging to see the same boosting effect in both women and men, although the precise brain pathways were slightly different, as might be expected,” coinvestigator Waljit Dhillo, PhD, Imperial College London, said in the news release.
“Collectively, the results suggest that kisspeptin may offer a safe and much-needed treatment for HSDD that affects millions of people around the world; and we look forward to taking this forward in future larger studies and in other patient groups,” Dr. Dhillo added.
The study was funded by the National Institute for Health and Care Research Imperial Biomedical Research Centre and the Medical Research Council, part of UK Research and Innovation. Dr. Comninos reported no relevant financial relationships. Dr. Dhillo reported receiving consulting fees from Myovant Sciences and KaNDy Therapeutics outside the submitted work.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Pound of flesh buys less prison time
Pound of flesh buys less prison time
We should all have more Shakespeare in our lives. Yeah, yeah, Shakespeare is meant to be played, not read, and it can be a struggle to herd teenagers through the Bard’s interesting and bloody tragedies, but even a perfunctory reading of “The Merchant of Venice” would hopefully have prevented the dystopian nightmare Massachusetts has presented us with today.
The United States has a massive shortage of donor organs. This is an unfortunate truth. So, to combat this issue, a pair of Massachusetts congresspeople have proposed HD 3822, which would allow prisoners to donate organs and/or bone marrow (a pound of flesh, so to speak) in exchange for up to a year in reduced prison time. Yes, that’s right. Give up pieces of yourself and the state of Massachusetts will deign to reduce your long prison sentence.
Oh, and before you dismiss this as typical Republican antics, the bill was sponsored by two Democrats, and in a statement one of them hoped to address racial disparities in organ donation, as people of color are much less likely to receive organs. Never mind that Black people are imprisoned at a much higher rate than Whites.
Yeah, this whole thing is what people in the business like to call an ethical disaster.
Fortunately, the bill will likely never be passed and it’s probably illegal anyway. A federal law from 1984 (how’s that for a coincidence) prevents people from donating organs for use in human transplantation in exchange for “valuable consideration.” In other words, you can’t sell your organs for profit, and in this case, reducing prison time would probably count as valuable consideration in the eyes of the courts.
Oh, and in case you’ve never read Merchant of Venice, Shylock, the character looking for the pound of flesh as payment for a debt? He’s the villain. In fact, it’s pretty safe to say that anyone looking to extract payment from human dismemberment is probably the bad guy of the story. Apparently that wasn’t clear.
How do you stop a fungi? With a deadly guy
Thanks to the new HBO series “The Last of Us,” there’s been a lot of talk about the upcoming fungi-pocalypse, as the show depicts the real-life “zombie fungus” Cordyceps turning humans into, you know, zombies.
No need to worry, ladies and gentleman, because science has discovered a way to turn back the fungal horde. A heroic, and environmentally friendly, alternative to chemical pesticides “in the fight against resistant fungi [that] are now resistant to antimycotics – partly because they are used in large quantities in agricultural fields,” investigators at the Leibniz Institute for Natural Product Research and Infection Biology in Jena, Germany, said in a written statement.
We are, of course, talking about Keanu Reeves. Wait a second. He’s not even in “The Last of Us.” Sorry folks, we are being told that it really is Keanu Reeves. Our champion in the inevitable fungal pandemic is movie star Keanu Reeves. Sort of. It’s actually keanumycin, a substance produced by bacteria of the genus Pseudomonas.
Really? Keanumycin? “The lipopeptides kill so efficiently that we named them after Keanu Reeves because he, too, is extremely deadly in his roles,” lead author Sebastian Götze, PhD, explained.
Dr. Götze and his associates had been working with pseudomonads for quite a while before they were able to isolate the toxins responsible for their ability to kill amoebae, which resemble fungi in some characteristics. When then finally tried the keanumycin against gray mold rot on hydrangea leaves, the intensely contemplative star of “The Matrix” and “John Wick” – sorry, wrong Keanu – the bacterial derivative significantly inhibited growth of the fungus, they said.
Additional testing has shown that keanumycin is not highly toxic to human cells and is effective against fungi such as Candida albicans in very low concentrations, which makes it a good candidate for future pharmaceutical development.
To that news there can be only one response from the substance’s namesake.
High fat, bye parasites
Fat. Fat. Fat. Seems like everyone is trying to avoid it these days, but fat may be good thing when it comes to weaseling out a parasite.
The parasite in this case is the whipworm, aka Trichuris trichiura. You can find this guy in the intestines of millions of people, where it causes long-lasting infections. Yikes … Researchers have found that the plan of attack to get rid of this invasive species is to boost the immune system, but instead of vitamin C and zinc it’s fat they’re pumping in. Yes, fat.
The developing countries with poor sewage that are at the highest risk for contracting parasites such as this also are among those where people ingest cheaper diets that are generally higher in fat. The investigators were interested to see how a high-fat diet would affect immune responses to the whipworms.
And, as with almost everything else, the researchers turned to mice, which were introduced to a closely related species, Trichuris muris.
A high-fat diet, rather than obesity itself, increases a molecule on T-helper cells called ST2, and this allows an increased T-helper 2 response, effectively giving eviction notices to the parasites in the intestinal lining.
To say the least, the researchers were surprised since “high-fat diets are mostly associated with increased pathology during disease,” said senior author Richard Grencis, PhD, of the University of Manchester (England), who noted that ST2 is not normally triggered with a standard diet in mice but the high-fat diet gave it a boost and an “alternate pathway” out.
Now before you start ordering extra-large fries at the drive-through to keep the whipworms away, the researchers added that they “have previously published that weight loss can aid the expulsion of a different gut parasite worm.” Figures.
Once again, though, signs are pointing to the gut for improved health.
Pound of flesh buys less prison time
We should all have more Shakespeare in our lives. Yeah, yeah, Shakespeare is meant to be played, not read, and it can be a struggle to herd teenagers through the Bard’s interesting and bloody tragedies, but even a perfunctory reading of “The Merchant of Venice” would hopefully have prevented the dystopian nightmare Massachusetts has presented us with today.
The United States has a massive shortage of donor organs. This is an unfortunate truth. So, to combat this issue, a pair of Massachusetts congresspeople have proposed HD 3822, which would allow prisoners to donate organs and/or bone marrow (a pound of flesh, so to speak) in exchange for up to a year in reduced prison time. Yes, that’s right. Give up pieces of yourself and the state of Massachusetts will deign to reduce your long prison sentence.
Oh, and before you dismiss this as typical Republican antics, the bill was sponsored by two Democrats, and in a statement one of them hoped to address racial disparities in organ donation, as people of color are much less likely to receive organs. Never mind that Black people are imprisoned at a much higher rate than Whites.
Yeah, this whole thing is what people in the business like to call an ethical disaster.
Fortunately, the bill will likely never be passed and it’s probably illegal anyway. A federal law from 1984 (how’s that for a coincidence) prevents people from donating organs for use in human transplantation in exchange for “valuable consideration.” In other words, you can’t sell your organs for profit, and in this case, reducing prison time would probably count as valuable consideration in the eyes of the courts.
Oh, and in case you’ve never read Merchant of Venice, Shylock, the character looking for the pound of flesh as payment for a debt? He’s the villain. In fact, it’s pretty safe to say that anyone looking to extract payment from human dismemberment is probably the bad guy of the story. Apparently that wasn’t clear.
How do you stop a fungi? With a deadly guy
Thanks to the new HBO series “The Last of Us,” there’s been a lot of talk about the upcoming fungi-pocalypse, as the show depicts the real-life “zombie fungus” Cordyceps turning humans into, you know, zombies.
No need to worry, ladies and gentleman, because science has discovered a way to turn back the fungal horde. A heroic, and environmentally friendly, alternative to chemical pesticides “in the fight against resistant fungi [that] are now resistant to antimycotics – partly because they are used in large quantities in agricultural fields,” investigators at the Leibniz Institute for Natural Product Research and Infection Biology in Jena, Germany, said in a written statement.
We are, of course, talking about Keanu Reeves. Wait a second. He’s not even in “The Last of Us.” Sorry folks, we are being told that it really is Keanu Reeves. Our champion in the inevitable fungal pandemic is movie star Keanu Reeves. Sort of. It’s actually keanumycin, a substance produced by bacteria of the genus Pseudomonas.
Really? Keanumycin? “The lipopeptides kill so efficiently that we named them after Keanu Reeves because he, too, is extremely deadly in his roles,” lead author Sebastian Götze, PhD, explained.
Dr. Götze and his associates had been working with pseudomonads for quite a while before they were able to isolate the toxins responsible for their ability to kill amoebae, which resemble fungi in some characteristics. When then finally tried the keanumycin against gray mold rot on hydrangea leaves, the intensely contemplative star of “The Matrix” and “John Wick” – sorry, wrong Keanu – the bacterial derivative significantly inhibited growth of the fungus, they said.
Additional testing has shown that keanumycin is not highly toxic to human cells and is effective against fungi such as Candida albicans in very low concentrations, which makes it a good candidate for future pharmaceutical development.
To that news there can be only one response from the substance’s namesake.
High fat, bye parasites
Fat. Fat. Fat. Seems like everyone is trying to avoid it these days, but fat may be good thing when it comes to weaseling out a parasite.
The parasite in this case is the whipworm, aka Trichuris trichiura. You can find this guy in the intestines of millions of people, where it causes long-lasting infections. Yikes … Researchers have found that the plan of attack to get rid of this invasive species is to boost the immune system, but instead of vitamin C and zinc it’s fat they’re pumping in. Yes, fat.
The developing countries with poor sewage that are at the highest risk for contracting parasites such as this also are among those where people ingest cheaper diets that are generally higher in fat. The investigators were interested to see how a high-fat diet would affect immune responses to the whipworms.
And, as with almost everything else, the researchers turned to mice, which were introduced to a closely related species, Trichuris muris.
A high-fat diet, rather than obesity itself, increases a molecule on T-helper cells called ST2, and this allows an increased T-helper 2 response, effectively giving eviction notices to the parasites in the intestinal lining.
To say the least, the researchers were surprised since “high-fat diets are mostly associated with increased pathology during disease,” said senior author Richard Grencis, PhD, of the University of Manchester (England), who noted that ST2 is not normally triggered with a standard diet in mice but the high-fat diet gave it a boost and an “alternate pathway” out.
Now before you start ordering extra-large fries at the drive-through to keep the whipworms away, the researchers added that they “have previously published that weight loss can aid the expulsion of a different gut parasite worm.” Figures.
Once again, though, signs are pointing to the gut for improved health.
Pound of flesh buys less prison time
We should all have more Shakespeare in our lives. Yeah, yeah, Shakespeare is meant to be played, not read, and it can be a struggle to herd teenagers through the Bard’s interesting and bloody tragedies, but even a perfunctory reading of “The Merchant of Venice” would hopefully have prevented the dystopian nightmare Massachusetts has presented us with today.
The United States has a massive shortage of donor organs. This is an unfortunate truth. So, to combat this issue, a pair of Massachusetts congresspeople have proposed HD 3822, which would allow prisoners to donate organs and/or bone marrow (a pound of flesh, so to speak) in exchange for up to a year in reduced prison time. Yes, that’s right. Give up pieces of yourself and the state of Massachusetts will deign to reduce your long prison sentence.
Oh, and before you dismiss this as typical Republican antics, the bill was sponsored by two Democrats, and in a statement one of them hoped to address racial disparities in organ donation, as people of color are much less likely to receive organs. Never mind that Black people are imprisoned at a much higher rate than Whites.
Yeah, this whole thing is what people in the business like to call an ethical disaster.
Fortunately, the bill will likely never be passed and it’s probably illegal anyway. A federal law from 1984 (how’s that for a coincidence) prevents people from donating organs for use in human transplantation in exchange for “valuable consideration.” In other words, you can’t sell your organs for profit, and in this case, reducing prison time would probably count as valuable consideration in the eyes of the courts.
Oh, and in case you’ve never read Merchant of Venice, Shylock, the character looking for the pound of flesh as payment for a debt? He’s the villain. In fact, it’s pretty safe to say that anyone looking to extract payment from human dismemberment is probably the bad guy of the story. Apparently that wasn’t clear.
How do you stop a fungi? With a deadly guy
Thanks to the new HBO series “The Last of Us,” there’s been a lot of talk about the upcoming fungi-pocalypse, as the show depicts the real-life “zombie fungus” Cordyceps turning humans into, you know, zombies.
No need to worry, ladies and gentleman, because science has discovered a way to turn back the fungal horde. A heroic, and environmentally friendly, alternative to chemical pesticides “in the fight against resistant fungi [that] are now resistant to antimycotics – partly because they are used in large quantities in agricultural fields,” investigators at the Leibniz Institute for Natural Product Research and Infection Biology in Jena, Germany, said in a written statement.
We are, of course, talking about Keanu Reeves. Wait a second. He’s not even in “The Last of Us.” Sorry folks, we are being told that it really is Keanu Reeves. Our champion in the inevitable fungal pandemic is movie star Keanu Reeves. Sort of. It’s actually keanumycin, a substance produced by bacteria of the genus Pseudomonas.
Really? Keanumycin? “The lipopeptides kill so efficiently that we named them after Keanu Reeves because he, too, is extremely deadly in his roles,” lead author Sebastian Götze, PhD, explained.
Dr. Götze and his associates had been working with pseudomonads for quite a while before they were able to isolate the toxins responsible for their ability to kill amoebae, which resemble fungi in some characteristics. When then finally tried the keanumycin against gray mold rot on hydrangea leaves, the intensely contemplative star of “The Matrix” and “John Wick” – sorry, wrong Keanu – the bacterial derivative significantly inhibited growth of the fungus, they said.
Additional testing has shown that keanumycin is not highly toxic to human cells and is effective against fungi such as Candida albicans in very low concentrations, which makes it a good candidate for future pharmaceutical development.
To that news there can be only one response from the substance’s namesake.
High fat, bye parasites
Fat. Fat. Fat. Seems like everyone is trying to avoid it these days, but fat may be good thing when it comes to weaseling out a parasite.
The parasite in this case is the whipworm, aka Trichuris trichiura. You can find this guy in the intestines of millions of people, where it causes long-lasting infections. Yikes … Researchers have found that the plan of attack to get rid of this invasive species is to boost the immune system, but instead of vitamin C and zinc it’s fat they’re pumping in. Yes, fat.
The developing countries with poor sewage that are at the highest risk for contracting parasites such as this also are among those where people ingest cheaper diets that are generally higher in fat. The investigators were interested to see how a high-fat diet would affect immune responses to the whipworms.
And, as with almost everything else, the researchers turned to mice, which were introduced to a closely related species, Trichuris muris.
A high-fat diet, rather than obesity itself, increases a molecule on T-helper cells called ST2, and this allows an increased T-helper 2 response, effectively giving eviction notices to the parasites in the intestinal lining.
To say the least, the researchers were surprised since “high-fat diets are mostly associated with increased pathology during disease,” said senior author Richard Grencis, PhD, of the University of Manchester (England), who noted that ST2 is not normally triggered with a standard diet in mice but the high-fat diet gave it a boost and an “alternate pathway” out.
Now before you start ordering extra-large fries at the drive-through to keep the whipworms away, the researchers added that they “have previously published that weight loss can aid the expulsion of a different gut parasite worm.” Figures.
Once again, though, signs are pointing to the gut for improved health.
No spike in overdose deaths from relaxed buprenorphine regulations
Researchers say the data add weight to the argument for permanently adopting the pandemic-era prescribing regulations for buprenorphine, a treatment for opioid use disorder.
“We saw no evidence that increased availability of buprenorphine through the loosening of rules around prescribing and dispensing of buprenorphine during the pandemic increased overdose deaths,” investigator Wilson Compton, MD, deputy director of the National Institute on Drug Abuse, told this news organization.
“This is reassuring that, even when we opened up the doors to easier access to buprenorphine, we didn’t see that most serious consequence,” Dr. Compton said.
The findings were published online in JAMA Network Open .
Cause and effect
Federal agencies relaxed prescribing regulations for buprenorphine in March 2020 to make it easier for clinicians to prescribe the drug via telemedicine and for patients to take the medication at home.
The number of buprenorphine prescriptions has increased since that change, with more than 1 million people receiving the medication in 2021 from retail pharmacies in the United States.
However, questions remained about whether increased access would lead to an increase in buprenorphine-involved overdose.
Researchers with NIDA and the Centers for Disease Control and Prevention analyzed data from the State Unintentional Drug Overdose Reporting System, a CDC database that combines medical examiner and coroner reports and postmortem toxicology testing.
The study included information about overdose deaths from July 2019 to June 2021 in 46 states and the District of Columbia.
Between July 2019 and June 2021, there were 1,955 buprenorphine-involved overdose deaths, which accounted for 2.2% of all drug overdose deaths and 2.6% of opioid-involved overdose deaths.
However, researchers went beyond overall numbers and evaluated details from coroner’s and medical examiner reports, something they had not done before.
“For the first time we looked at the characteristics of decedents from buprenorphine because this has not been studied in this type of detail with a near-national sample,” Dr. Compton said.
“That allowed us to look at patterns of use of other substances as well as the circumstances that are recorded at the death scene that are in the data set,” he added.
Important insights
Reports from nearly all buprenorphine-involved deaths included the presence of at least one other drug, compared with opioid overdose deaths that typically involved only one drug.
“This is consistent with the pharmacology of buprenorphine being a partial agonist, so it may not be as fatal all by itself as some of the other opioids,” Dr. Compton said.
Deaths involving buprenorphine were less likely to include illicitly manufactured fentanyls, and other prescription medications were more often found on the scene, such as antidepressants.
Compared with opioid decedents, buprenorphine decedents were more likely to be women, age 35-44, White, and receiving treatment for mental health conditions, including for substance use disorder (SUD).
These kinds of characteristics provide important insights about potential ways to improve safety and clinical outcomes, Dr. Compton noted.
“When we see things like a little higher rate of SUD treatment and this evidence of other prescription drugs on the scene, and some higher rates of antidepressants in these decedents than I might have expected, I’m very curious about their use of other medical services outside of substance use treatment, because that might be a place where some interventions could be implemented,” he said.
A similar study showed pandemic-era policy changes that allowed methadone to be taken at home was followed by a decrease in methadone-related overdose deaths.
The new findings are consistent with those results, Dr. Compton said.
‘Chipping away’ at stigma
Commenting on the study, O. Trent Hall, DO, assistant professor of addiction medicine, Department of Psychiatry and Behavioral Health, Ohio State University Wexner Medical Center, Columbus, said that, although he welcomed the findings, they aren’t unexpected.
“Buprenorphine is well established as a safe and effective medication for opioid use disorder and as a physician who routinely cares for patients in the hospital after opioid overdose, I am not at all surprised by these results,” said Dr. Hall, who was not involved with the research.
“When my patients leave the hospital with a buprenorphine prescription, they are much less likely to return with another overdose or serious opioid-related medical problem,” he added.
U.S. drug overdose deaths topped 100,000 for the first time in 2021, and most were opioid-related. Although the latest data from the CDC shows drug overdose deaths have been declining slowly since early 2022, the numbers remain high.
Buprenorphine is one of only two drugs known to reduce the risk of opioid overdose. While prescriptions have increased since 2020, the medication remains underutilized, despite its known effectiveness in treating opioid use disorder.
Dr. Hall noted that research such as the new study could help increase buprenorphine’s use.
“Studies like this one chip away at the stigma that has been misapplied to buprenorphine,” he said. “I hope this article will encourage more providers to offer buprenorphine to patients with opioid use disorder.”
The study was funded internally by NIDA and the CDC. Dr. Compton reported owning stock in General Electric, 3M, and Pfizer outside the submitted work. Dr. Hall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers say the data add weight to the argument for permanently adopting the pandemic-era prescribing regulations for buprenorphine, a treatment for opioid use disorder.
“We saw no evidence that increased availability of buprenorphine through the loosening of rules around prescribing and dispensing of buprenorphine during the pandemic increased overdose deaths,” investigator Wilson Compton, MD, deputy director of the National Institute on Drug Abuse, told this news organization.
“This is reassuring that, even when we opened up the doors to easier access to buprenorphine, we didn’t see that most serious consequence,” Dr. Compton said.
The findings were published online in JAMA Network Open .
Cause and effect
Federal agencies relaxed prescribing regulations for buprenorphine in March 2020 to make it easier for clinicians to prescribe the drug via telemedicine and for patients to take the medication at home.
The number of buprenorphine prescriptions has increased since that change, with more than 1 million people receiving the medication in 2021 from retail pharmacies in the United States.
However, questions remained about whether increased access would lead to an increase in buprenorphine-involved overdose.
Researchers with NIDA and the Centers for Disease Control and Prevention analyzed data from the State Unintentional Drug Overdose Reporting System, a CDC database that combines medical examiner and coroner reports and postmortem toxicology testing.
The study included information about overdose deaths from July 2019 to June 2021 in 46 states and the District of Columbia.
Between July 2019 and June 2021, there were 1,955 buprenorphine-involved overdose deaths, which accounted for 2.2% of all drug overdose deaths and 2.6% of opioid-involved overdose deaths.
However, researchers went beyond overall numbers and evaluated details from coroner’s and medical examiner reports, something they had not done before.
“For the first time we looked at the characteristics of decedents from buprenorphine because this has not been studied in this type of detail with a near-national sample,” Dr. Compton said.
“That allowed us to look at patterns of use of other substances as well as the circumstances that are recorded at the death scene that are in the data set,” he added.
Important insights
Reports from nearly all buprenorphine-involved deaths included the presence of at least one other drug, compared with opioid overdose deaths that typically involved only one drug.
“This is consistent with the pharmacology of buprenorphine being a partial agonist, so it may not be as fatal all by itself as some of the other opioids,” Dr. Compton said.
Deaths involving buprenorphine were less likely to include illicitly manufactured fentanyls, and other prescription medications were more often found on the scene, such as antidepressants.
Compared with opioid decedents, buprenorphine decedents were more likely to be women, age 35-44, White, and receiving treatment for mental health conditions, including for substance use disorder (SUD).
These kinds of characteristics provide important insights about potential ways to improve safety and clinical outcomes, Dr. Compton noted.
“When we see things like a little higher rate of SUD treatment and this evidence of other prescription drugs on the scene, and some higher rates of antidepressants in these decedents than I might have expected, I’m very curious about their use of other medical services outside of substance use treatment, because that might be a place where some interventions could be implemented,” he said.
A similar study showed pandemic-era policy changes that allowed methadone to be taken at home was followed by a decrease in methadone-related overdose deaths.
The new findings are consistent with those results, Dr. Compton said.
‘Chipping away’ at stigma
Commenting on the study, O. Trent Hall, DO, assistant professor of addiction medicine, Department of Psychiatry and Behavioral Health, Ohio State University Wexner Medical Center, Columbus, said that, although he welcomed the findings, they aren’t unexpected.
“Buprenorphine is well established as a safe and effective medication for opioid use disorder and as a physician who routinely cares for patients in the hospital after opioid overdose, I am not at all surprised by these results,” said Dr. Hall, who was not involved with the research.
“When my patients leave the hospital with a buprenorphine prescription, they are much less likely to return with another overdose or serious opioid-related medical problem,” he added.
U.S. drug overdose deaths topped 100,000 for the first time in 2021, and most were opioid-related. Although the latest data from the CDC shows drug overdose deaths have been declining slowly since early 2022, the numbers remain high.
Buprenorphine is one of only two drugs known to reduce the risk of opioid overdose. While prescriptions have increased since 2020, the medication remains underutilized, despite its known effectiveness in treating opioid use disorder.
Dr. Hall noted that research such as the new study could help increase buprenorphine’s use.
“Studies like this one chip away at the stigma that has been misapplied to buprenorphine,” he said. “I hope this article will encourage more providers to offer buprenorphine to patients with opioid use disorder.”
The study was funded internally by NIDA and the CDC. Dr. Compton reported owning stock in General Electric, 3M, and Pfizer outside the submitted work. Dr. Hall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers say the data add weight to the argument for permanently adopting the pandemic-era prescribing regulations for buprenorphine, a treatment for opioid use disorder.
“We saw no evidence that increased availability of buprenorphine through the loosening of rules around prescribing and dispensing of buprenorphine during the pandemic increased overdose deaths,” investigator Wilson Compton, MD, deputy director of the National Institute on Drug Abuse, told this news organization.
“This is reassuring that, even when we opened up the doors to easier access to buprenorphine, we didn’t see that most serious consequence,” Dr. Compton said.
The findings were published online in JAMA Network Open .
Cause and effect
Federal agencies relaxed prescribing regulations for buprenorphine in March 2020 to make it easier for clinicians to prescribe the drug via telemedicine and for patients to take the medication at home.
The number of buprenorphine prescriptions has increased since that change, with more than 1 million people receiving the medication in 2021 from retail pharmacies in the United States.
However, questions remained about whether increased access would lead to an increase in buprenorphine-involved overdose.
Researchers with NIDA and the Centers for Disease Control and Prevention analyzed data from the State Unintentional Drug Overdose Reporting System, a CDC database that combines medical examiner and coroner reports and postmortem toxicology testing.
The study included information about overdose deaths from July 2019 to June 2021 in 46 states and the District of Columbia.
Between July 2019 and June 2021, there were 1,955 buprenorphine-involved overdose deaths, which accounted for 2.2% of all drug overdose deaths and 2.6% of opioid-involved overdose deaths.
However, researchers went beyond overall numbers and evaluated details from coroner’s and medical examiner reports, something they had not done before.
“For the first time we looked at the characteristics of decedents from buprenorphine because this has not been studied in this type of detail with a near-national sample,” Dr. Compton said.
“That allowed us to look at patterns of use of other substances as well as the circumstances that are recorded at the death scene that are in the data set,” he added.
Important insights
Reports from nearly all buprenorphine-involved deaths included the presence of at least one other drug, compared with opioid overdose deaths that typically involved only one drug.
“This is consistent with the pharmacology of buprenorphine being a partial agonist, so it may not be as fatal all by itself as some of the other opioids,” Dr. Compton said.
Deaths involving buprenorphine were less likely to include illicitly manufactured fentanyls, and other prescription medications were more often found on the scene, such as antidepressants.
Compared with opioid decedents, buprenorphine decedents were more likely to be women, age 35-44, White, and receiving treatment for mental health conditions, including for substance use disorder (SUD).
These kinds of characteristics provide important insights about potential ways to improve safety and clinical outcomes, Dr. Compton noted.
“When we see things like a little higher rate of SUD treatment and this evidence of other prescription drugs on the scene, and some higher rates of antidepressants in these decedents than I might have expected, I’m very curious about their use of other medical services outside of substance use treatment, because that might be a place where some interventions could be implemented,” he said.
A similar study showed pandemic-era policy changes that allowed methadone to be taken at home was followed by a decrease in methadone-related overdose deaths.
The new findings are consistent with those results, Dr. Compton said.
‘Chipping away’ at stigma
Commenting on the study, O. Trent Hall, DO, assistant professor of addiction medicine, Department of Psychiatry and Behavioral Health, Ohio State University Wexner Medical Center, Columbus, said that, although he welcomed the findings, they aren’t unexpected.
“Buprenorphine is well established as a safe and effective medication for opioid use disorder and as a physician who routinely cares for patients in the hospital after opioid overdose, I am not at all surprised by these results,” said Dr. Hall, who was not involved with the research.
“When my patients leave the hospital with a buprenorphine prescription, they are much less likely to return with another overdose or serious opioid-related medical problem,” he added.
U.S. drug overdose deaths topped 100,000 for the first time in 2021, and most were opioid-related. Although the latest data from the CDC shows drug overdose deaths have been declining slowly since early 2022, the numbers remain high.
Buprenorphine is one of only two drugs known to reduce the risk of opioid overdose. While prescriptions have increased since 2020, the medication remains underutilized, despite its known effectiveness in treating opioid use disorder.
Dr. Hall noted that research such as the new study could help increase buprenorphine’s use.
“Studies like this one chip away at the stigma that has been misapplied to buprenorphine,” he said. “I hope this article will encourage more providers to offer buprenorphine to patients with opioid use disorder.”
The study was funded internally by NIDA and the CDC. Dr. Compton reported owning stock in General Electric, 3M, and Pfizer outside the submitted work. Dr. Hall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
What is the psychological cost of performing CPR?
One year ago, as the sun was setting on a late fall day, Andrés Snitcofsky, a 40-year-old designer from Buenos Aires, Argentina, heard harrowing cries for help. It was the niece and the wife of one of his neighbors: a man in his 60s who the women had found “passed out” in the bedroom. “I did CPR for 5 minutes straight until a friend of the victim came in and asked me to stop, telling me that the man had probably been dead for 2 or 3 hours already. But I had no idea because I’d never seen a dead body before,” Mr. Snitcofsky told this news organization. A few minutes later, the ambulance arrived. The doctor confirmed that there was nothing more that could be done.
Mr. Snitcofsky went home. Nobody had asked for his name or address or phone number. … And it wasn’t because they already knew who he was. In fact, there wasn’t any sort of relationship there. Mr. Snitcofsky had only known his neighbors by sight. His actions that day, however, “did not come without a cost. It took me weeks – months, actually – to put myself together again,” he said. The things he saw, the things he heard, everything about that night played over and over in his head. “I had trouble sleeping. I would play out different scenarios in my head. I questioned myself. I second-guessed myself, criticized myself. It’s like some taboo subject. There’s no one to share the experience with, no one who gets it. But with time, I was able to process the event.
“For 2 months, I talked to my psychologist about it all,” he continued. “That really helped me a lot. In addition to therapy, I reached out to a couple I know – they’re both physicians – and to a firefighter who teaches CPR. Their insight and guidance allowed me to get to a point where I was able to understand that what I did was a good thing and that what I did was all that could have been done. But anyone who finds themselves in the position of having to do CPR – they’re going to be affected in many, many ways. It goes beyond the euphoria of seeing a person come back to life. Of that, I’m quite certain.”
We’ve all seen campaigns encouraging people to learn CPR and to be prepared if the need arises. But in training the public (and even health care professionals), not much, if anything, is said about the “collateral damage”: the psychological and emotional consequences of carrying out the procedure. These especially come into play when you don’t know whether the person survived, when your efforts weren’t able to reverse the sudden cardiac arrest, or when the person you gave CPR to was a loved one – a case that may entail immediate therapeutic interventions to minimize or prevent the risk of suffering long-lasting trauma.
In May 2020, popular American activist and educator Kristin Flanary saw someone suffering cardiac arrest. She stepped in and started doing CPR. And she continued doing CPR … for 10 long minutes. The person she was trying to save was her 34-year-old husband, ophthalmologist and comedian Will Flanary. On Twitter, where she’s known as Lady Glaucomflecken, Ms. Flanary recently shared the following message, putting the topic of CPR and automated external defibrillator training front and center.
“Yes, everyone should learn #CPRandAED. But if we are going to ask people to perform such a brutal task, it’s imperative that we also provide them with the info and resources they need to process it mentally and emotionally. It’s traumatic and life changing. It’s irresponsible and unethical to ask people to help in such a brutal and traumatic way and then neglect to help them in return.” In less than a month, the tweet has racked up over 200,000 views.
Doing one’s duty
There are many people who work to promote CPR and strengthen the other links in the chain of survival for out-of-hospital sudden cardiac arrest, such as prompt access to and delivery of early defibrillation. According to them, any negative psychological impact of intervening is temporary and, when compared with the satisfaction of having done one’s duty, quite insignificant – even if the efforts to save a person’s life are not successful.
“In 99.9% of cases, people who have performed CPR feel a sense of satisfaction, even happiness, knowing that they’ve helped. The individuals I’ve spoken with, I’ve never heard any of them say that they felt worse after the event or that they needed to see a psychologist,” said Mario Fitz Maurice, MD, director of the Arrhythmia Council of the Argentine Society of Cardiology and head of Electrophysiology at Rivadavia Hospital in Buenos Aires. He went on to tell this news organization, “Of course, some degree of fear, sadness, or melancholy can remain afterward. But it seems to me, and there are reports saying as much, that, in the end, what stands out in the person’s mind is the fact that they tried to save a life. And for them, there’s joy in knowing this.”
Dr. Fitz Maurice, who is also the director of the National Arrhythmia Institute in Buenos Aires, pointed out that the kind of person who takes CPR classes “has a profile that’s going to allow them to be psychologically involved; they’re the caring person, the one who’s ready and willing to help people.” And he added that, at his hospital, if they can identify the individuals or first responders who have done CPR on a patient, the protocol is to always contact them to offer psychological care and assistance. “But in 99% of cases, they don’t even understand why we’re calling them, they’re extremely happy to have taken part.”
Some studies, though, paint a much different picture, one that shows that providing CPR can be emotionally challenging and have consequences in terms of one’s family and work life. A qualitative study published in 2016 looked into the experiences of 20 lay rescuers in Norway – five were health educated – who had provided CPR to 18 out-of-hospital cardiac arrest (OHCA) victims, 66% of whom survived. The time from experiencing the OHCA incident to participating in the interview ranged from 6 days to 13 years (median 5.5 years). Several participants reported the OHCA incident as a “shocking and terrifying” experience. Tiredness, exhaustion, confusion, and feeling alone about the OHCA experience were individual reactions that could vary in time from days to months. Anxiety and insomnia were also experienced following the incident.
Some lay rescuers described the influence on work and family life, and a few of them described deep sorrow, even several years after the incident. Overall, they reported repetitive self-criticism regarding whether they could have carried out anything else to achieve a better outcome for the cardiac arrest victim. All of them wanted to be informed about the outcome. And four of the lay rescuers needed professional counseling to process the OHCA experience.
In 2020, another qualitative study was conducted, this time in Taiwan. There were nine participants, none of whom were health professionals. Each had provided initial CPR and defibrillation with AED in public locations. Event-to-interview duration was within 1 year and 1-2 years. The major findings from the study were the following:
- The lay rescuers possessed helping traits and high motivation.
- The lay rescuers reported certain aspects of rescue reality that differed much from prior training and expectations, including difficulty in the depth of chest compression, and uncertainties in real emergency situations.
- The lay rescuers gained positive personal fulfillment in sharing their experience and receiving positive feedback from others, and were willing to help next time, although they experienced a short-term negative psychological impact from the event. “Measures should be taken to increase [a] layperson’s confidence and situation awareness, to reduce training-reality discrepancy, and to build up a support system to avoid negative psychological effects.” This was the conclusion of the study team, which was led by Matthew Huei-Ming Ma, MD, PhD. A professor in the department of emergency medicine at National Taiwan University in Taipei, he is also on the board of directors of the Resuscitation Council of Asia.
Potential trauma
In recalling his experience, Mr. Snitcofsky said, “The hardest part of it all was the moment that I stopped giving CPR, that moment of letting go. This became the image that kept coming back to me, the traumatic moment I hadn’t thought about.”
Psychiatrist Daniel Mosca, MD, is the founder and former president of the Argentine Society of Trauma Psychology. He is also the coordinator of the human factors team at the City of Buenos Aires Emergency Medical Care System. “Any event has the potential to be traumatic, all the more so when it’s an event where you come face to face with death and uncertainty. But how a rescuer reacts will depend on their psychological makeup.” Of the individuals who were held for months or years in the jungle as hostages of the Revolutionary Armed Forces of Colombia, “only” half developed symptoms of posttraumatic stress disorder.
Dr. Mosca believes that a comment by Frank Ochberg, MD, speaks to this finding. “In many cases, peritraumatic symptoms are a normal person’s normal response to an abnormal situation.” For a lot of people who have found themselves having to perform CPR, the symptoms associated with the initial acute stress reaction will resolve on their own in 30-90 days. “But if this doesn’t happen, and those symptoms persist, psychotherapeutic or pharmacological intervention will be necessary,” he noted.
“In CPR classes, it would be good for the instructors to talk about the warning signs that people should look out for in themselves and their fellow rescuers. So, for example, insomnia, anxiety, a heightened state of alertness, feeling disconnected from reality,” Dr. Mosca told this news organization.
“Another thing that can help rescuers is letting them know what happened to the person they gave CPR to. This way, they can get closure,” suggested Manlio Márquez Murillo, MD, a cardiologist and electrophysiologist in Mexico. He is also the coordinator of the Alliance Against Sudden Cardiac Death at the Interamerican Society of Cardiology.
“Medical and nursing societies would have to develop a brief protocol or performance standard. The goal would be to ensure that rescuers are asked for their contact information and that someone gets in touch to debrief them and to offer them care. Next would come the treatment part, to resolve any remaining aftereffects,” said in an interview.
For example, a three-stage Lay Responder Support Model (LRSM) was developed and implemented as part of a lay responder support program established in 2014 by the Peel Regional Council in Ontario. The LRSM identifies and engages individuals who witnessed or participated directly or indirectly in an OHCA, inviting them to participate in a debriefing session facilitated by a trained practitioner. Held 24-48 hours post event, the debriefing allows lay responders to contextualize their reaction to the event. The conversation also serves as an opportunity for them to fully articulate their concerns, questions, and thoughts. The facilitator can communicate stress reduction techniques and address psychological first aid needs as they emerge. Approximately 1 week post event, a secondary follow-up occurs. If the lay responder communicates a continuing struggle with symptoms impacting and interfering with everyday life, the facilitator offers a coordinated or facilitated referral for mental health support.
In an article published in the Journal of Cardiac Failure. Ms. Flanary speaks about the three kinds of language that anyone who was either forced to or inspired to perform CPR can use to help process their trauma: words that explain what happened, words that name (eg, “forgotten patients”), and words that validate the experience and allow the person to articulate their feelings. The tools and technologies that organizations and health care professionals provide can help the healing process. Empathy and compassion, too, have a place.
But there are virtually no standardized and proactive initiatives of this kind in much of the world, including Latin America. So, most people who just happened to be in the right place at the right time find that they have to navigate the “after” part all on their own.
Other obstacles
Dr. Márquez Murillo finds it unfortunate that countries in the region have yet to enact “Good Samaritan” laws. If individuals render aid to someone suffering cardiac arrest, then these laws would ensure that they will not be held liable in any way. This is the case in Argentina and Uruguay. So, the fear of things turning into a legal matter may be holding people back from taking action; that fear could also create additional stress for those who end up stepping in to help.
Even with the legal safeguards, exceptional circumstances may arise where rescuers find themselves facing unexpected emotional challenges. In Argentina, Virginia Pérez Antonelli, the 17-year-old who tried in vain to save the life of Fernando Báez Sosa, had to testify at the trial of the eight defendants accused of brutally beating him in January 2020. The press, the public – the attention of an entire country – was focused on her. She had to respond to the defense attorneys who were able to ask whether she was sure that she performed the CPR maneuvers correctly. And a few weeks ago, a medical examiner hired by the defense suggested that “the CPR may have made the situation worse” for the victim. An indignant Dr. Fitz Maurice responded on Twitter: “CPR SAVES LIVES!! Let’s not let a CHEAP AND BASELESS argument destroy all the work that’s been done…!”
Of course, there are consequences that are beyond our control and others that can, in fact, be anticipated and planned for. Dr. Fitz Maurice brought up a preventive approach: Make CPR second nature, teach it in schools, help people overcome their fears. “Cardiac deaths are 200 times more frequent than deaths resulting from fires – and we practice fire drills a lot more than we practice CPR,” he told this news organization. In a society where there is widespread training on the procedure, where people regularly practice the technique, those who have had the experience of giving someone CPR will feel less alone, will be better understood by others.
“On the other hand, beyond the initial impact and the lack of a formal support system, the medium- and long-term outcome for those who acted is also psychologically and emotionally favorable,” said Jorge Bombau, MD, an obstetrician/gynecologist in Buenos Aires. After Dr. Bombau’s 14-year-old son Beltrán suddenly died during a school sports tournament, Dr. Bombau became a prominent advocate spreading the word about CPR.
“I don’t know anyone who regrets doing CPR,” he told this news organization. “There may be a brief period when the person feels distressed or depressed, when they have trouble sleeping. But it’s been proven that doing a good deed improves one’s mood. And what better deed is there than trying to save someone’s life? Whether their efforts were successful or in vain, that person has, at the end of the day, done something meaningful and worthwhile.”
Mr. Snitcofsky shares this sentiment. For several months now, he’s been feeling he’s “in a good place.” And he’s been actively promoting CPR on social media. As he recently posted on Twitter, “I’m here to retweet everything that has to do with getting us all to become familiar with how to do CPR and working up the courage to do it. The training takes no more than a few hours.
“I want to know that, if I ever have an out-of-hospital sudden cardiac arrest, there will be neighbors, friends, or family members around who know how to do CPR. Every person who knows how to do CPR can persuade others, and those of us who’ve had to do CPR in real life are even better candidates for persuading others. And if one day a person ends up needing CPR, I want to step in again and make up for lost time. Here’s hoping it’ll do the job,” he concluded.
It’s the same for Matías Alonso, a journalist in Buenos Aires. On New Year’s Eve 15 years ago, he was at a family dinner when, a few minutes before midnight, he found himself giving CPR to his stepmother’s father. “Unfortunately, he passed away, but I continued doing CPR on him until the ambulance arrived. For some time, I felt a little guilty for not taking charge of the situation from the beginning, and because I had this idea in my head that more people pulled through and recovered. But afterwards, they really thanked me a lot. And that helped me realize that I’d done something. I didn’t stand still when faced with the inevitability of death. I understood that it was good to have tried,” Mr. Alonso told this news organization. “And next time … hopefully there won’t be a next time … but I’m more prepared, and I now know how I can do better.”
Mr. Alonso, Mr. Snitcofsky, Dr. Fitz Maurice, Dr. Mosca, Dr. Bombau, and Dr. Márquez Murillo disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from Medscape Spanish.
One year ago, as the sun was setting on a late fall day, Andrés Snitcofsky, a 40-year-old designer from Buenos Aires, Argentina, heard harrowing cries for help. It was the niece and the wife of one of his neighbors: a man in his 60s who the women had found “passed out” in the bedroom. “I did CPR for 5 minutes straight until a friend of the victim came in and asked me to stop, telling me that the man had probably been dead for 2 or 3 hours already. But I had no idea because I’d never seen a dead body before,” Mr. Snitcofsky told this news organization. A few minutes later, the ambulance arrived. The doctor confirmed that there was nothing more that could be done.
Mr. Snitcofsky went home. Nobody had asked for his name or address or phone number. … And it wasn’t because they already knew who he was. In fact, there wasn’t any sort of relationship there. Mr. Snitcofsky had only known his neighbors by sight. His actions that day, however, “did not come without a cost. It took me weeks – months, actually – to put myself together again,” he said. The things he saw, the things he heard, everything about that night played over and over in his head. “I had trouble sleeping. I would play out different scenarios in my head. I questioned myself. I second-guessed myself, criticized myself. It’s like some taboo subject. There’s no one to share the experience with, no one who gets it. But with time, I was able to process the event.
“For 2 months, I talked to my psychologist about it all,” he continued. “That really helped me a lot. In addition to therapy, I reached out to a couple I know – they’re both physicians – and to a firefighter who teaches CPR. Their insight and guidance allowed me to get to a point where I was able to understand that what I did was a good thing and that what I did was all that could have been done. But anyone who finds themselves in the position of having to do CPR – they’re going to be affected in many, many ways. It goes beyond the euphoria of seeing a person come back to life. Of that, I’m quite certain.”
We’ve all seen campaigns encouraging people to learn CPR and to be prepared if the need arises. But in training the public (and even health care professionals), not much, if anything, is said about the “collateral damage”: the psychological and emotional consequences of carrying out the procedure. These especially come into play when you don’t know whether the person survived, when your efforts weren’t able to reverse the sudden cardiac arrest, or when the person you gave CPR to was a loved one – a case that may entail immediate therapeutic interventions to minimize or prevent the risk of suffering long-lasting trauma.
In May 2020, popular American activist and educator Kristin Flanary saw someone suffering cardiac arrest. She stepped in and started doing CPR. And she continued doing CPR … for 10 long minutes. The person she was trying to save was her 34-year-old husband, ophthalmologist and comedian Will Flanary. On Twitter, where she’s known as Lady Glaucomflecken, Ms. Flanary recently shared the following message, putting the topic of CPR and automated external defibrillator training front and center.
“Yes, everyone should learn #CPRandAED. But if we are going to ask people to perform such a brutal task, it’s imperative that we also provide them with the info and resources they need to process it mentally and emotionally. It’s traumatic and life changing. It’s irresponsible and unethical to ask people to help in such a brutal and traumatic way and then neglect to help them in return.” In less than a month, the tweet has racked up over 200,000 views.
Doing one’s duty
There are many people who work to promote CPR and strengthen the other links in the chain of survival for out-of-hospital sudden cardiac arrest, such as prompt access to and delivery of early defibrillation. According to them, any negative psychological impact of intervening is temporary and, when compared with the satisfaction of having done one’s duty, quite insignificant – even if the efforts to save a person’s life are not successful.
“In 99.9% of cases, people who have performed CPR feel a sense of satisfaction, even happiness, knowing that they’ve helped. The individuals I’ve spoken with, I’ve never heard any of them say that they felt worse after the event or that they needed to see a psychologist,” said Mario Fitz Maurice, MD, director of the Arrhythmia Council of the Argentine Society of Cardiology and head of Electrophysiology at Rivadavia Hospital in Buenos Aires. He went on to tell this news organization, “Of course, some degree of fear, sadness, or melancholy can remain afterward. But it seems to me, and there are reports saying as much, that, in the end, what stands out in the person’s mind is the fact that they tried to save a life. And for them, there’s joy in knowing this.”
Dr. Fitz Maurice, who is also the director of the National Arrhythmia Institute in Buenos Aires, pointed out that the kind of person who takes CPR classes “has a profile that’s going to allow them to be psychologically involved; they’re the caring person, the one who’s ready and willing to help people.” And he added that, at his hospital, if they can identify the individuals or first responders who have done CPR on a patient, the protocol is to always contact them to offer psychological care and assistance. “But in 99% of cases, they don’t even understand why we’re calling them, they’re extremely happy to have taken part.”
Some studies, though, paint a much different picture, one that shows that providing CPR can be emotionally challenging and have consequences in terms of one’s family and work life. A qualitative study published in 2016 looked into the experiences of 20 lay rescuers in Norway – five were health educated – who had provided CPR to 18 out-of-hospital cardiac arrest (OHCA) victims, 66% of whom survived. The time from experiencing the OHCA incident to participating in the interview ranged from 6 days to 13 years (median 5.5 years). Several participants reported the OHCA incident as a “shocking and terrifying” experience. Tiredness, exhaustion, confusion, and feeling alone about the OHCA experience were individual reactions that could vary in time from days to months. Anxiety and insomnia were also experienced following the incident.
Some lay rescuers described the influence on work and family life, and a few of them described deep sorrow, even several years after the incident. Overall, they reported repetitive self-criticism regarding whether they could have carried out anything else to achieve a better outcome for the cardiac arrest victim. All of them wanted to be informed about the outcome. And four of the lay rescuers needed professional counseling to process the OHCA experience.
In 2020, another qualitative study was conducted, this time in Taiwan. There were nine participants, none of whom were health professionals. Each had provided initial CPR and defibrillation with AED in public locations. Event-to-interview duration was within 1 year and 1-2 years. The major findings from the study were the following:
- The lay rescuers possessed helping traits and high motivation.
- The lay rescuers reported certain aspects of rescue reality that differed much from prior training and expectations, including difficulty in the depth of chest compression, and uncertainties in real emergency situations.
- The lay rescuers gained positive personal fulfillment in sharing their experience and receiving positive feedback from others, and were willing to help next time, although they experienced a short-term negative psychological impact from the event. “Measures should be taken to increase [a] layperson’s confidence and situation awareness, to reduce training-reality discrepancy, and to build up a support system to avoid negative psychological effects.” This was the conclusion of the study team, which was led by Matthew Huei-Ming Ma, MD, PhD. A professor in the department of emergency medicine at National Taiwan University in Taipei, he is also on the board of directors of the Resuscitation Council of Asia.
Potential trauma
In recalling his experience, Mr. Snitcofsky said, “The hardest part of it all was the moment that I stopped giving CPR, that moment of letting go. This became the image that kept coming back to me, the traumatic moment I hadn’t thought about.”
Psychiatrist Daniel Mosca, MD, is the founder and former president of the Argentine Society of Trauma Psychology. He is also the coordinator of the human factors team at the City of Buenos Aires Emergency Medical Care System. “Any event has the potential to be traumatic, all the more so when it’s an event where you come face to face with death and uncertainty. But how a rescuer reacts will depend on their psychological makeup.” Of the individuals who were held for months or years in the jungle as hostages of the Revolutionary Armed Forces of Colombia, “only” half developed symptoms of posttraumatic stress disorder.
Dr. Mosca believes that a comment by Frank Ochberg, MD, speaks to this finding. “In many cases, peritraumatic symptoms are a normal person’s normal response to an abnormal situation.” For a lot of people who have found themselves having to perform CPR, the symptoms associated with the initial acute stress reaction will resolve on their own in 30-90 days. “But if this doesn’t happen, and those symptoms persist, psychotherapeutic or pharmacological intervention will be necessary,” he noted.
“In CPR classes, it would be good for the instructors to talk about the warning signs that people should look out for in themselves and their fellow rescuers. So, for example, insomnia, anxiety, a heightened state of alertness, feeling disconnected from reality,” Dr. Mosca told this news organization.
“Another thing that can help rescuers is letting them know what happened to the person they gave CPR to. This way, they can get closure,” suggested Manlio Márquez Murillo, MD, a cardiologist and electrophysiologist in Mexico. He is also the coordinator of the Alliance Against Sudden Cardiac Death at the Interamerican Society of Cardiology.
“Medical and nursing societies would have to develop a brief protocol or performance standard. The goal would be to ensure that rescuers are asked for their contact information and that someone gets in touch to debrief them and to offer them care. Next would come the treatment part, to resolve any remaining aftereffects,” said in an interview.
For example, a three-stage Lay Responder Support Model (LRSM) was developed and implemented as part of a lay responder support program established in 2014 by the Peel Regional Council in Ontario. The LRSM identifies and engages individuals who witnessed or participated directly or indirectly in an OHCA, inviting them to participate in a debriefing session facilitated by a trained practitioner. Held 24-48 hours post event, the debriefing allows lay responders to contextualize their reaction to the event. The conversation also serves as an opportunity for them to fully articulate their concerns, questions, and thoughts. The facilitator can communicate stress reduction techniques and address psychological first aid needs as they emerge. Approximately 1 week post event, a secondary follow-up occurs. If the lay responder communicates a continuing struggle with symptoms impacting and interfering with everyday life, the facilitator offers a coordinated or facilitated referral for mental health support.
In an article published in the Journal of Cardiac Failure. Ms. Flanary speaks about the three kinds of language that anyone who was either forced to or inspired to perform CPR can use to help process their trauma: words that explain what happened, words that name (eg, “forgotten patients”), and words that validate the experience and allow the person to articulate their feelings. The tools and technologies that organizations and health care professionals provide can help the healing process. Empathy and compassion, too, have a place.
But there are virtually no standardized and proactive initiatives of this kind in much of the world, including Latin America. So, most people who just happened to be in the right place at the right time find that they have to navigate the “after” part all on their own.
Other obstacles
Dr. Márquez Murillo finds it unfortunate that countries in the region have yet to enact “Good Samaritan” laws. If individuals render aid to someone suffering cardiac arrest, then these laws would ensure that they will not be held liable in any way. This is the case in Argentina and Uruguay. So, the fear of things turning into a legal matter may be holding people back from taking action; that fear could also create additional stress for those who end up stepping in to help.
Even with the legal safeguards, exceptional circumstances may arise where rescuers find themselves facing unexpected emotional challenges. In Argentina, Virginia Pérez Antonelli, the 17-year-old who tried in vain to save the life of Fernando Báez Sosa, had to testify at the trial of the eight defendants accused of brutally beating him in January 2020. The press, the public – the attention of an entire country – was focused on her. She had to respond to the defense attorneys who were able to ask whether she was sure that she performed the CPR maneuvers correctly. And a few weeks ago, a medical examiner hired by the defense suggested that “the CPR may have made the situation worse” for the victim. An indignant Dr. Fitz Maurice responded on Twitter: “CPR SAVES LIVES!! Let’s not let a CHEAP AND BASELESS argument destroy all the work that’s been done…!”
Of course, there are consequences that are beyond our control and others that can, in fact, be anticipated and planned for. Dr. Fitz Maurice brought up a preventive approach: Make CPR second nature, teach it in schools, help people overcome their fears. “Cardiac deaths are 200 times more frequent than deaths resulting from fires – and we practice fire drills a lot more than we practice CPR,” he told this news organization. In a society where there is widespread training on the procedure, where people regularly practice the technique, those who have had the experience of giving someone CPR will feel less alone, will be better understood by others.
“On the other hand, beyond the initial impact and the lack of a formal support system, the medium- and long-term outcome for those who acted is also psychologically and emotionally favorable,” said Jorge Bombau, MD, an obstetrician/gynecologist in Buenos Aires. After Dr. Bombau’s 14-year-old son Beltrán suddenly died during a school sports tournament, Dr. Bombau became a prominent advocate spreading the word about CPR.
“I don’t know anyone who regrets doing CPR,” he told this news organization. “There may be a brief period when the person feels distressed or depressed, when they have trouble sleeping. But it’s been proven that doing a good deed improves one’s mood. And what better deed is there than trying to save someone’s life? Whether their efforts were successful or in vain, that person has, at the end of the day, done something meaningful and worthwhile.”
Mr. Snitcofsky shares this sentiment. For several months now, he’s been feeling he’s “in a good place.” And he’s been actively promoting CPR on social media. As he recently posted on Twitter, “I’m here to retweet everything that has to do with getting us all to become familiar with how to do CPR and working up the courage to do it. The training takes no more than a few hours.
“I want to know that, if I ever have an out-of-hospital sudden cardiac arrest, there will be neighbors, friends, or family members around who know how to do CPR. Every person who knows how to do CPR can persuade others, and those of us who’ve had to do CPR in real life are even better candidates for persuading others. And if one day a person ends up needing CPR, I want to step in again and make up for lost time. Here’s hoping it’ll do the job,” he concluded.
It’s the same for Matías Alonso, a journalist in Buenos Aires. On New Year’s Eve 15 years ago, he was at a family dinner when, a few minutes before midnight, he found himself giving CPR to his stepmother’s father. “Unfortunately, he passed away, but I continued doing CPR on him until the ambulance arrived. For some time, I felt a little guilty for not taking charge of the situation from the beginning, and because I had this idea in my head that more people pulled through and recovered. But afterwards, they really thanked me a lot. And that helped me realize that I’d done something. I didn’t stand still when faced with the inevitability of death. I understood that it was good to have tried,” Mr. Alonso told this news organization. “And next time … hopefully there won’t be a next time … but I’m more prepared, and I now know how I can do better.”
Mr. Alonso, Mr. Snitcofsky, Dr. Fitz Maurice, Dr. Mosca, Dr. Bombau, and Dr. Márquez Murillo disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from Medscape Spanish.
One year ago, as the sun was setting on a late fall day, Andrés Snitcofsky, a 40-year-old designer from Buenos Aires, Argentina, heard harrowing cries for help. It was the niece and the wife of one of his neighbors: a man in his 60s who the women had found “passed out” in the bedroom. “I did CPR for 5 minutes straight until a friend of the victim came in and asked me to stop, telling me that the man had probably been dead for 2 or 3 hours already. But I had no idea because I’d never seen a dead body before,” Mr. Snitcofsky told this news organization. A few minutes later, the ambulance arrived. The doctor confirmed that there was nothing more that could be done.
Mr. Snitcofsky went home. Nobody had asked for his name or address or phone number. … And it wasn’t because they already knew who he was. In fact, there wasn’t any sort of relationship there. Mr. Snitcofsky had only known his neighbors by sight. His actions that day, however, “did not come without a cost. It took me weeks – months, actually – to put myself together again,” he said. The things he saw, the things he heard, everything about that night played over and over in his head. “I had trouble sleeping. I would play out different scenarios in my head. I questioned myself. I second-guessed myself, criticized myself. It’s like some taboo subject. There’s no one to share the experience with, no one who gets it. But with time, I was able to process the event.
“For 2 months, I talked to my psychologist about it all,” he continued. “That really helped me a lot. In addition to therapy, I reached out to a couple I know – they’re both physicians – and to a firefighter who teaches CPR. Their insight and guidance allowed me to get to a point where I was able to understand that what I did was a good thing and that what I did was all that could have been done. But anyone who finds themselves in the position of having to do CPR – they’re going to be affected in many, many ways. It goes beyond the euphoria of seeing a person come back to life. Of that, I’m quite certain.”
We’ve all seen campaigns encouraging people to learn CPR and to be prepared if the need arises. But in training the public (and even health care professionals), not much, if anything, is said about the “collateral damage”: the psychological and emotional consequences of carrying out the procedure. These especially come into play when you don’t know whether the person survived, when your efforts weren’t able to reverse the sudden cardiac arrest, or when the person you gave CPR to was a loved one – a case that may entail immediate therapeutic interventions to minimize or prevent the risk of suffering long-lasting trauma.
In May 2020, popular American activist and educator Kristin Flanary saw someone suffering cardiac arrest. She stepped in and started doing CPR. And she continued doing CPR … for 10 long minutes. The person she was trying to save was her 34-year-old husband, ophthalmologist and comedian Will Flanary. On Twitter, where she’s known as Lady Glaucomflecken, Ms. Flanary recently shared the following message, putting the topic of CPR and automated external defibrillator training front and center.
“Yes, everyone should learn #CPRandAED. But if we are going to ask people to perform such a brutal task, it’s imperative that we also provide them with the info and resources they need to process it mentally and emotionally. It’s traumatic and life changing. It’s irresponsible and unethical to ask people to help in such a brutal and traumatic way and then neglect to help them in return.” In less than a month, the tweet has racked up over 200,000 views.
Doing one’s duty
There are many people who work to promote CPR and strengthen the other links in the chain of survival for out-of-hospital sudden cardiac arrest, such as prompt access to and delivery of early defibrillation. According to them, any negative psychological impact of intervening is temporary and, when compared with the satisfaction of having done one’s duty, quite insignificant – even if the efforts to save a person’s life are not successful.
“In 99.9% of cases, people who have performed CPR feel a sense of satisfaction, even happiness, knowing that they’ve helped. The individuals I’ve spoken with, I’ve never heard any of them say that they felt worse after the event or that they needed to see a psychologist,” said Mario Fitz Maurice, MD, director of the Arrhythmia Council of the Argentine Society of Cardiology and head of Electrophysiology at Rivadavia Hospital in Buenos Aires. He went on to tell this news organization, “Of course, some degree of fear, sadness, or melancholy can remain afterward. But it seems to me, and there are reports saying as much, that, in the end, what stands out in the person’s mind is the fact that they tried to save a life. And for them, there’s joy in knowing this.”
Dr. Fitz Maurice, who is also the director of the National Arrhythmia Institute in Buenos Aires, pointed out that the kind of person who takes CPR classes “has a profile that’s going to allow them to be psychologically involved; they’re the caring person, the one who’s ready and willing to help people.” And he added that, at his hospital, if they can identify the individuals or first responders who have done CPR on a patient, the protocol is to always contact them to offer psychological care and assistance. “But in 99% of cases, they don’t even understand why we’re calling them, they’re extremely happy to have taken part.”
Some studies, though, paint a much different picture, one that shows that providing CPR can be emotionally challenging and have consequences in terms of one’s family and work life. A qualitative study published in 2016 looked into the experiences of 20 lay rescuers in Norway – five were health educated – who had provided CPR to 18 out-of-hospital cardiac arrest (OHCA) victims, 66% of whom survived. The time from experiencing the OHCA incident to participating in the interview ranged from 6 days to 13 years (median 5.5 years). Several participants reported the OHCA incident as a “shocking and terrifying” experience. Tiredness, exhaustion, confusion, and feeling alone about the OHCA experience were individual reactions that could vary in time from days to months. Anxiety and insomnia were also experienced following the incident.
Some lay rescuers described the influence on work and family life, and a few of them described deep sorrow, even several years after the incident. Overall, they reported repetitive self-criticism regarding whether they could have carried out anything else to achieve a better outcome for the cardiac arrest victim. All of them wanted to be informed about the outcome. And four of the lay rescuers needed professional counseling to process the OHCA experience.
In 2020, another qualitative study was conducted, this time in Taiwan. There were nine participants, none of whom were health professionals. Each had provided initial CPR and defibrillation with AED in public locations. Event-to-interview duration was within 1 year and 1-2 years. The major findings from the study were the following:
- The lay rescuers possessed helping traits and high motivation.
- The lay rescuers reported certain aspects of rescue reality that differed much from prior training and expectations, including difficulty in the depth of chest compression, and uncertainties in real emergency situations.
- The lay rescuers gained positive personal fulfillment in sharing their experience and receiving positive feedback from others, and were willing to help next time, although they experienced a short-term negative psychological impact from the event. “Measures should be taken to increase [a] layperson’s confidence and situation awareness, to reduce training-reality discrepancy, and to build up a support system to avoid negative psychological effects.” This was the conclusion of the study team, which was led by Matthew Huei-Ming Ma, MD, PhD. A professor in the department of emergency medicine at National Taiwan University in Taipei, he is also on the board of directors of the Resuscitation Council of Asia.
Potential trauma
In recalling his experience, Mr. Snitcofsky said, “The hardest part of it all was the moment that I stopped giving CPR, that moment of letting go. This became the image that kept coming back to me, the traumatic moment I hadn’t thought about.”
Psychiatrist Daniel Mosca, MD, is the founder and former president of the Argentine Society of Trauma Psychology. He is also the coordinator of the human factors team at the City of Buenos Aires Emergency Medical Care System. “Any event has the potential to be traumatic, all the more so when it’s an event where you come face to face with death and uncertainty. But how a rescuer reacts will depend on their psychological makeup.” Of the individuals who were held for months or years in the jungle as hostages of the Revolutionary Armed Forces of Colombia, “only” half developed symptoms of posttraumatic stress disorder.
Dr. Mosca believes that a comment by Frank Ochberg, MD, speaks to this finding. “In many cases, peritraumatic symptoms are a normal person’s normal response to an abnormal situation.” For a lot of people who have found themselves having to perform CPR, the symptoms associated with the initial acute stress reaction will resolve on their own in 30-90 days. “But if this doesn’t happen, and those symptoms persist, psychotherapeutic or pharmacological intervention will be necessary,” he noted.
“In CPR classes, it would be good for the instructors to talk about the warning signs that people should look out for in themselves and their fellow rescuers. So, for example, insomnia, anxiety, a heightened state of alertness, feeling disconnected from reality,” Dr. Mosca told this news organization.
“Another thing that can help rescuers is letting them know what happened to the person they gave CPR to. This way, they can get closure,” suggested Manlio Márquez Murillo, MD, a cardiologist and electrophysiologist in Mexico. He is also the coordinator of the Alliance Against Sudden Cardiac Death at the Interamerican Society of Cardiology.
“Medical and nursing societies would have to develop a brief protocol or performance standard. The goal would be to ensure that rescuers are asked for their contact information and that someone gets in touch to debrief them and to offer them care. Next would come the treatment part, to resolve any remaining aftereffects,” said in an interview.
For example, a three-stage Lay Responder Support Model (LRSM) was developed and implemented as part of a lay responder support program established in 2014 by the Peel Regional Council in Ontario. The LRSM identifies and engages individuals who witnessed or participated directly or indirectly in an OHCA, inviting them to participate in a debriefing session facilitated by a trained practitioner. Held 24-48 hours post event, the debriefing allows lay responders to contextualize their reaction to the event. The conversation also serves as an opportunity for them to fully articulate their concerns, questions, and thoughts. The facilitator can communicate stress reduction techniques and address psychological first aid needs as they emerge. Approximately 1 week post event, a secondary follow-up occurs. If the lay responder communicates a continuing struggle with symptoms impacting and interfering with everyday life, the facilitator offers a coordinated or facilitated referral for mental health support.
In an article published in the Journal of Cardiac Failure. Ms. Flanary speaks about the three kinds of language that anyone who was either forced to or inspired to perform CPR can use to help process their trauma: words that explain what happened, words that name (eg, “forgotten patients”), and words that validate the experience and allow the person to articulate their feelings. The tools and technologies that organizations and health care professionals provide can help the healing process. Empathy and compassion, too, have a place.
But there are virtually no standardized and proactive initiatives of this kind in much of the world, including Latin America. So, most people who just happened to be in the right place at the right time find that they have to navigate the “after” part all on their own.
Other obstacles
Dr. Márquez Murillo finds it unfortunate that countries in the region have yet to enact “Good Samaritan” laws. If individuals render aid to someone suffering cardiac arrest, then these laws would ensure that they will not be held liable in any way. This is the case in Argentina and Uruguay. So, the fear of things turning into a legal matter may be holding people back from taking action; that fear could also create additional stress for those who end up stepping in to help.
Even with the legal safeguards, exceptional circumstances may arise where rescuers find themselves facing unexpected emotional challenges. In Argentina, Virginia Pérez Antonelli, the 17-year-old who tried in vain to save the life of Fernando Báez Sosa, had to testify at the trial of the eight defendants accused of brutally beating him in January 2020. The press, the public – the attention of an entire country – was focused on her. She had to respond to the defense attorneys who were able to ask whether she was sure that she performed the CPR maneuvers correctly. And a few weeks ago, a medical examiner hired by the defense suggested that “the CPR may have made the situation worse” for the victim. An indignant Dr. Fitz Maurice responded on Twitter: “CPR SAVES LIVES!! Let’s not let a CHEAP AND BASELESS argument destroy all the work that’s been done…!”
Of course, there are consequences that are beyond our control and others that can, in fact, be anticipated and planned for. Dr. Fitz Maurice brought up a preventive approach: Make CPR second nature, teach it in schools, help people overcome their fears. “Cardiac deaths are 200 times more frequent than deaths resulting from fires – and we practice fire drills a lot more than we practice CPR,” he told this news organization. In a society where there is widespread training on the procedure, where people regularly practice the technique, those who have had the experience of giving someone CPR will feel less alone, will be better understood by others.
“On the other hand, beyond the initial impact and the lack of a formal support system, the medium- and long-term outcome for those who acted is also psychologically and emotionally favorable,” said Jorge Bombau, MD, an obstetrician/gynecologist in Buenos Aires. After Dr. Bombau’s 14-year-old son Beltrán suddenly died during a school sports tournament, Dr. Bombau became a prominent advocate spreading the word about CPR.
“I don’t know anyone who regrets doing CPR,” he told this news organization. “There may be a brief period when the person feels distressed or depressed, when they have trouble sleeping. But it’s been proven that doing a good deed improves one’s mood. And what better deed is there than trying to save someone’s life? Whether their efforts were successful or in vain, that person has, at the end of the day, done something meaningful and worthwhile.”
Mr. Snitcofsky shares this sentiment. For several months now, he’s been feeling he’s “in a good place.” And he’s been actively promoting CPR on social media. As he recently posted on Twitter, “I’m here to retweet everything that has to do with getting us all to become familiar with how to do CPR and working up the courage to do it. The training takes no more than a few hours.
“I want to know that, if I ever have an out-of-hospital sudden cardiac arrest, there will be neighbors, friends, or family members around who know how to do CPR. Every person who knows how to do CPR can persuade others, and those of us who’ve had to do CPR in real life are even better candidates for persuading others. And if one day a person ends up needing CPR, I want to step in again and make up for lost time. Here’s hoping it’ll do the job,” he concluded.
It’s the same for Matías Alonso, a journalist in Buenos Aires. On New Year’s Eve 15 years ago, he was at a family dinner when, a few minutes before midnight, he found himself giving CPR to his stepmother’s father. “Unfortunately, he passed away, but I continued doing CPR on him until the ambulance arrived. For some time, I felt a little guilty for not taking charge of the situation from the beginning, and because I had this idea in my head that more people pulled through and recovered. But afterwards, they really thanked me a lot. And that helped me realize that I’d done something. I didn’t stand still when faced with the inevitability of death. I understood that it was good to have tried,” Mr. Alonso told this news organization. “And next time … hopefully there won’t be a next time … but I’m more prepared, and I now know how I can do better.”
Mr. Alonso, Mr. Snitcofsky, Dr. Fitz Maurice, Dr. Mosca, Dr. Bombau, and Dr. Márquez Murillo disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from Medscape Spanish.
We don’t lose our keys (or other things) as much as we think
Can’t find your keys? Misplaced your glasses? No clue where you parked your car?
We all lose things from time to time. And we’ve all heard the standard-issue advice: Picture when you had the object last. Despite this common experience,
“It is well known that we have massive recognition memory for objects,” says study coauthor Jeremy Wolfe, PhD, a professor of ophthalmology and radiology at Harvard Medical School, Boston. In other words, we’re good at recognizing objects we’ve seen before. “For example, after viewing 100 objects for 2-3 seconds each, observers can discriminate those 100 old images from 100 new ones with well over 80% accuracy.”
But remembering what your keys look like won’t necessarily help you find them. “We often want to know when and where we saw [an object],” Dr. Wolfe says. “So our goal was to measure these spatial and temporal memories.”
In a series of experiments, reported in Current Biology, Wolfe and colleagues asked people in the study to remember objects placed on a grid. They viewed 300 objects (pictures of things like a vase, a wedding dress, camo pants, a wet suit) and were asked to recall each one and where it had been located on the grid.
About a third of the people remembered 100 or more locations, by choosing either the correct square on the grid or one directly next to it. Another third remembered between 50 and 100, and the rest remembered less than 50.
Results would likely be even better in the real world “because no one gives up and decides ‘I can’t remember where anything is. I will just guess in this silly experiment,’ ” Dr. Wolfe says.
Later, they were shown items one at a time and asked to click on a time line to indicate when they had seen them. Between 60% and 80% of the time, they identified when they had seen an object within 10% of the correct time. That’s a lot better than the 40% they would have achieved by guessing.
The findings build on previous research and expand our understanding of memory, Dr. Wolfe says. “We knew that people could remember where some things were located. However, no one had tried to quantify that memory,” he says.
But wait: If we’re so good at remembering the where and when, why do we struggle to locate lost objects so much? Chances are, we don’t. We just feel that way because we tend to focus on the fails and overlook the many wins.
“This [study] is showing us something about how we come to know where hundreds of things are in our world,” Dr. Wolfe says. “We tend to notice when this fails – ‘where are my keys?’ – but on a normal day, you are successfully tapping a massive memory on a regular basis.”
Next, the researchers plan to investigate whether spatial and temporal memories are correlated – if you’re good at one, are you good at the other? So far, “that correlation looks rather weak,” Dr. Wolfe says.
A version of this article first appeared on WebMD.com.
Can’t find your keys? Misplaced your glasses? No clue where you parked your car?
We all lose things from time to time. And we’ve all heard the standard-issue advice: Picture when you had the object last. Despite this common experience,
“It is well known that we have massive recognition memory for objects,” says study coauthor Jeremy Wolfe, PhD, a professor of ophthalmology and radiology at Harvard Medical School, Boston. In other words, we’re good at recognizing objects we’ve seen before. “For example, after viewing 100 objects for 2-3 seconds each, observers can discriminate those 100 old images from 100 new ones with well over 80% accuracy.”
But remembering what your keys look like won’t necessarily help you find them. “We often want to know when and where we saw [an object],” Dr. Wolfe says. “So our goal was to measure these spatial and temporal memories.”
In a series of experiments, reported in Current Biology, Wolfe and colleagues asked people in the study to remember objects placed on a grid. They viewed 300 objects (pictures of things like a vase, a wedding dress, camo pants, a wet suit) and were asked to recall each one and where it had been located on the grid.
About a third of the people remembered 100 or more locations, by choosing either the correct square on the grid or one directly next to it. Another third remembered between 50 and 100, and the rest remembered less than 50.
Results would likely be even better in the real world “because no one gives up and decides ‘I can’t remember where anything is. I will just guess in this silly experiment,’ ” Dr. Wolfe says.
Later, they were shown items one at a time and asked to click on a time line to indicate when they had seen them. Between 60% and 80% of the time, they identified when they had seen an object within 10% of the correct time. That’s a lot better than the 40% they would have achieved by guessing.
The findings build on previous research and expand our understanding of memory, Dr. Wolfe says. “We knew that people could remember where some things were located. However, no one had tried to quantify that memory,” he says.
But wait: If we’re so good at remembering the where and when, why do we struggle to locate lost objects so much? Chances are, we don’t. We just feel that way because we tend to focus on the fails and overlook the many wins.
“This [study] is showing us something about how we come to know where hundreds of things are in our world,” Dr. Wolfe says. “We tend to notice when this fails – ‘where are my keys?’ – but on a normal day, you are successfully tapping a massive memory on a regular basis.”
Next, the researchers plan to investigate whether spatial and temporal memories are correlated – if you’re good at one, are you good at the other? So far, “that correlation looks rather weak,” Dr. Wolfe says.
A version of this article first appeared on WebMD.com.
Can’t find your keys? Misplaced your glasses? No clue where you parked your car?
We all lose things from time to time. And we’ve all heard the standard-issue advice: Picture when you had the object last. Despite this common experience,
“It is well known that we have massive recognition memory for objects,” says study coauthor Jeremy Wolfe, PhD, a professor of ophthalmology and radiology at Harvard Medical School, Boston. In other words, we’re good at recognizing objects we’ve seen before. “For example, after viewing 100 objects for 2-3 seconds each, observers can discriminate those 100 old images from 100 new ones with well over 80% accuracy.”
But remembering what your keys look like won’t necessarily help you find them. “We often want to know when and where we saw [an object],” Dr. Wolfe says. “So our goal was to measure these spatial and temporal memories.”
In a series of experiments, reported in Current Biology, Wolfe and colleagues asked people in the study to remember objects placed on a grid. They viewed 300 objects (pictures of things like a vase, a wedding dress, camo pants, a wet suit) and were asked to recall each one and where it had been located on the grid.
About a third of the people remembered 100 or more locations, by choosing either the correct square on the grid or one directly next to it. Another third remembered between 50 and 100, and the rest remembered less than 50.
Results would likely be even better in the real world “because no one gives up and decides ‘I can’t remember where anything is. I will just guess in this silly experiment,’ ” Dr. Wolfe says.
Later, they were shown items one at a time and asked to click on a time line to indicate when they had seen them. Between 60% and 80% of the time, they identified when they had seen an object within 10% of the correct time. That’s a lot better than the 40% they would have achieved by guessing.
The findings build on previous research and expand our understanding of memory, Dr. Wolfe says. “We knew that people could remember where some things were located. However, no one had tried to quantify that memory,” he says.
But wait: If we’re so good at remembering the where and when, why do we struggle to locate lost objects so much? Chances are, we don’t. We just feel that way because we tend to focus on the fails and overlook the many wins.
“This [study] is showing us something about how we come to know where hundreds of things are in our world,” Dr. Wolfe says. “We tend to notice when this fails – ‘where are my keys?’ – but on a normal day, you are successfully tapping a massive memory on a regular basis.”
Next, the researchers plan to investigate whether spatial and temporal memories are correlated – if you’re good at one, are you good at the other? So far, “that correlation looks rather weak,” Dr. Wolfe says.
A version of this article first appeared on WebMD.com.
FROM CURRENT BIOLOGY








