User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Beware a pair of dermatologic emergencies in children
in a presentation at MedscapeLive’s virtual Women’s & Pediatric Dermatology Seminar.
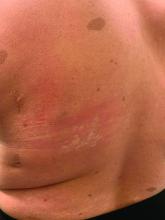
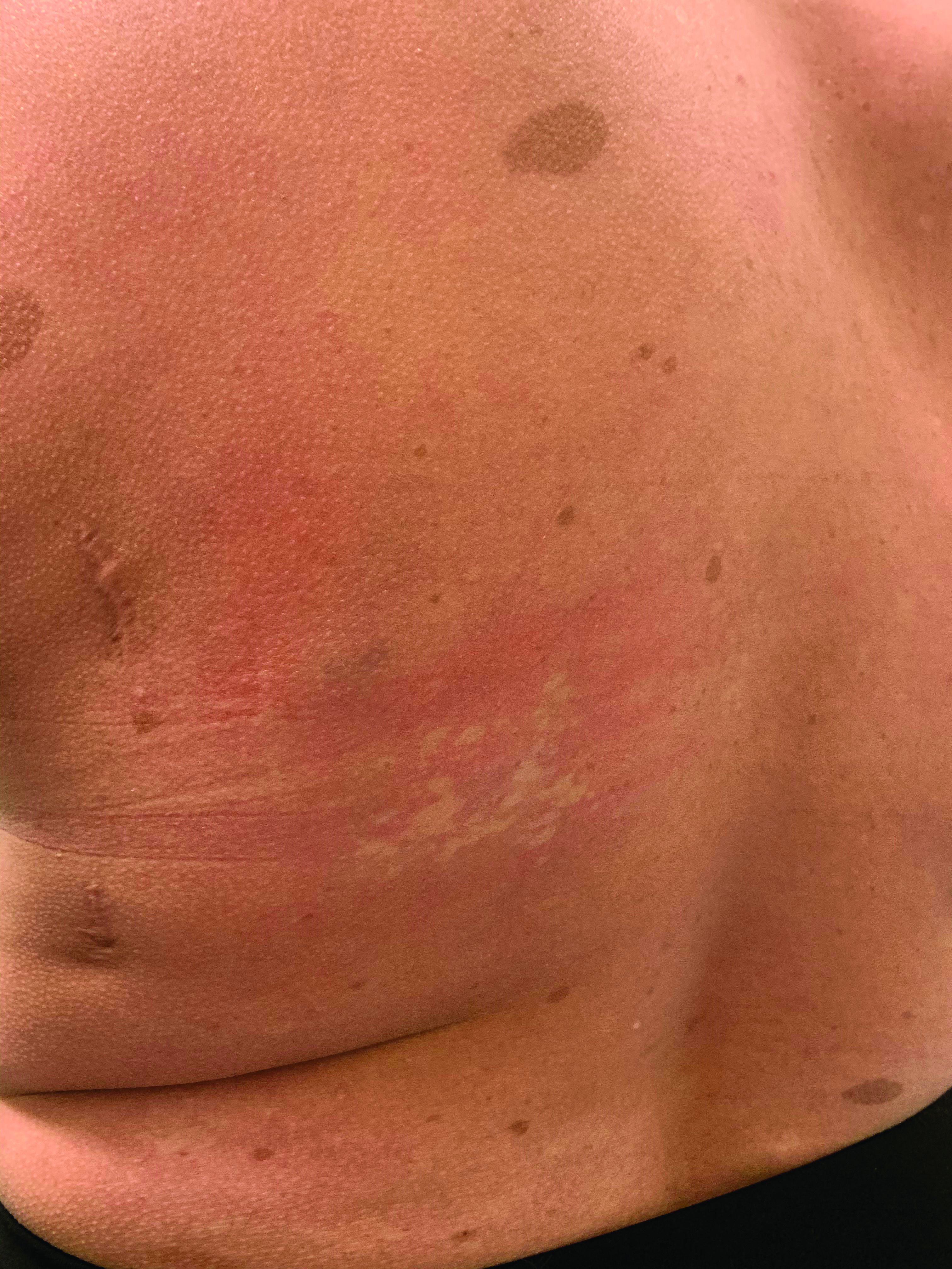
Eczema herpeticum is a condition in which a herpes simplex virus (HSV-1 or HSV-2) is superimposed over preexisting eczema. “The infection may be primary and sustained from a close contact or result in some of our older patients from reactivation and spread through autoinoculation,” said Dr. Hightower, of Rady Children’s Hospital and the University of California, both in San Diego.
Signs, he said, include acute worsening of atopic dermatitis with new-onset vesicles, pustules, and “punched-out” hemorrhagic crusted erosions. “Presentation ranges from mild to transient to life threatening.”
Potential complications include meningitis, encephalitis, hepatitis, and chronic conjunctivitis. “That’s why immediate ophthalmological evaluation is needed when there’s involvement on the face near the eye,” he said.
As for management and care, “where I have concern for HSV patients, I get HSV [polymerase chain reaction] as well as a bacterial culture,” he said. But even before the results are available, empiric treatment with acyclovir can be appropriate. “It’s got to be systemic for these kids with severe involvement,” he said, and they should also be started on medication for staphylococci and streptococci.
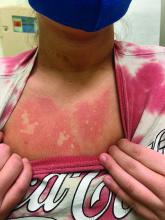
During his presentation, Dr. Hightower also highlighted staphylococcal scalded skin syndrome. Patients with the disease commonly have concurrent skin pain (which can appear to be fussiness), fever, irritability, malaise, and poor feeding. Examination may reveal widespread erythema with accentuation at folds/peeling at hands and large sheets of superficial peeling scale with diffuse erythema.
Widespread skin involvement “results not from the presence of staph throughout the skin, but the exotoxin that it produces that becomes systemic,” he said. “Clinical diagnosis is supported by presence of S. aureus on bacterial culture, but the presence of staph is not necessary to make the diagnosis. When in doubt, histopathology is helpful. But again, it’s not necessary to make the diagnosis.”
Cases can be managed with a first- or second-generation cephalosporin, he said. Alternative therapies include antistaphylococcus penicillinase-resistant penicillins (oxacillin or nafcillin) or vancomycin.
While Dr. Hightower doesn’t use clindamycin in these patients, he said it’s an option that some dermatologists consider because of its antistaphylococcus activity. “Historically, people thought it may decrease exotoxin production. The big concern if you are going to use clindamycin is that there are high rates of community resistance,” he said. “So you want to be careful that you know your resistance patterns wherever you are. Follow up on culture to make sure that you have adequate coverage for the bug that the kiddo in front of you has.”
Dr. Hightower reported no relevant disclosures. MedscapeLive and this news organization are owned by the same parent company.
in a presentation at MedscapeLive’s virtual Women’s & Pediatric Dermatology Seminar.
Eczema herpeticum is a condition in which a herpes simplex virus (HSV-1 or HSV-2) is superimposed over preexisting eczema. “The infection may be primary and sustained from a close contact or result in some of our older patients from reactivation and spread through autoinoculation,” said Dr. Hightower, of Rady Children’s Hospital and the University of California, both in San Diego.
Signs, he said, include acute worsening of atopic dermatitis with new-onset vesicles, pustules, and “punched-out” hemorrhagic crusted erosions. “Presentation ranges from mild to transient to life threatening.”
Potential complications include meningitis, encephalitis, hepatitis, and chronic conjunctivitis. “That’s why immediate ophthalmological evaluation is needed when there’s involvement on the face near the eye,” he said.
As for management and care, “where I have concern for HSV patients, I get HSV [polymerase chain reaction] as well as a bacterial culture,” he said. But even before the results are available, empiric treatment with acyclovir can be appropriate. “It’s got to be systemic for these kids with severe involvement,” he said, and they should also be started on medication for staphylococci and streptococci.
During his presentation, Dr. Hightower also highlighted staphylococcal scalded skin syndrome. Patients with the disease commonly have concurrent skin pain (which can appear to be fussiness), fever, irritability, malaise, and poor feeding. Examination may reveal widespread erythema with accentuation at folds/peeling at hands and large sheets of superficial peeling scale with diffuse erythema.
Widespread skin involvement “results not from the presence of staph throughout the skin, but the exotoxin that it produces that becomes systemic,” he said. “Clinical diagnosis is supported by presence of S. aureus on bacterial culture, but the presence of staph is not necessary to make the diagnosis. When in doubt, histopathology is helpful. But again, it’s not necessary to make the diagnosis.”
Cases can be managed with a first- or second-generation cephalosporin, he said. Alternative therapies include antistaphylococcus penicillinase-resistant penicillins (oxacillin or nafcillin) or vancomycin.
While Dr. Hightower doesn’t use clindamycin in these patients, he said it’s an option that some dermatologists consider because of its antistaphylococcus activity. “Historically, people thought it may decrease exotoxin production. The big concern if you are going to use clindamycin is that there are high rates of community resistance,” he said. “So you want to be careful that you know your resistance patterns wherever you are. Follow up on culture to make sure that you have adequate coverage for the bug that the kiddo in front of you has.”
Dr. Hightower reported no relevant disclosures. MedscapeLive and this news organization are owned by the same parent company.
in a presentation at MedscapeLive’s virtual Women’s & Pediatric Dermatology Seminar.
Eczema herpeticum is a condition in which a herpes simplex virus (HSV-1 or HSV-2) is superimposed over preexisting eczema. “The infection may be primary and sustained from a close contact or result in some of our older patients from reactivation and spread through autoinoculation,” said Dr. Hightower, of Rady Children’s Hospital and the University of California, both in San Diego.
Signs, he said, include acute worsening of atopic dermatitis with new-onset vesicles, pustules, and “punched-out” hemorrhagic crusted erosions. “Presentation ranges from mild to transient to life threatening.”
Potential complications include meningitis, encephalitis, hepatitis, and chronic conjunctivitis. “That’s why immediate ophthalmological evaluation is needed when there’s involvement on the face near the eye,” he said.
As for management and care, “where I have concern for HSV patients, I get HSV [polymerase chain reaction] as well as a bacterial culture,” he said. But even before the results are available, empiric treatment with acyclovir can be appropriate. “It’s got to be systemic for these kids with severe involvement,” he said, and they should also be started on medication for staphylococci and streptococci.
During his presentation, Dr. Hightower also highlighted staphylococcal scalded skin syndrome. Patients with the disease commonly have concurrent skin pain (which can appear to be fussiness), fever, irritability, malaise, and poor feeding. Examination may reveal widespread erythema with accentuation at folds/peeling at hands and large sheets of superficial peeling scale with diffuse erythema.
Widespread skin involvement “results not from the presence of staph throughout the skin, but the exotoxin that it produces that becomes systemic,” he said. “Clinical diagnosis is supported by presence of S. aureus on bacterial culture, but the presence of staph is not necessary to make the diagnosis. When in doubt, histopathology is helpful. But again, it’s not necessary to make the diagnosis.”
Cases can be managed with a first- or second-generation cephalosporin, he said. Alternative therapies include antistaphylococcus penicillinase-resistant penicillins (oxacillin or nafcillin) or vancomycin.
While Dr. Hightower doesn’t use clindamycin in these patients, he said it’s an option that some dermatologists consider because of its antistaphylococcus activity. “Historically, people thought it may decrease exotoxin production. The big concern if you are going to use clindamycin is that there are high rates of community resistance,” he said. “So you want to be careful that you know your resistance patterns wherever you are. Follow up on culture to make sure that you have adequate coverage for the bug that the kiddo in front of you has.”
Dr. Hightower reported no relevant disclosures. MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Six big changes coming for office-visit coding
Betsy Nicoletti, MS, a nationally recognized coding expert, will take your coding questions via email and provide guidance on how to code properly to maximize reimbursement. Have a question about coding? Send it to [email protected].
thanks to the American Medical Association.
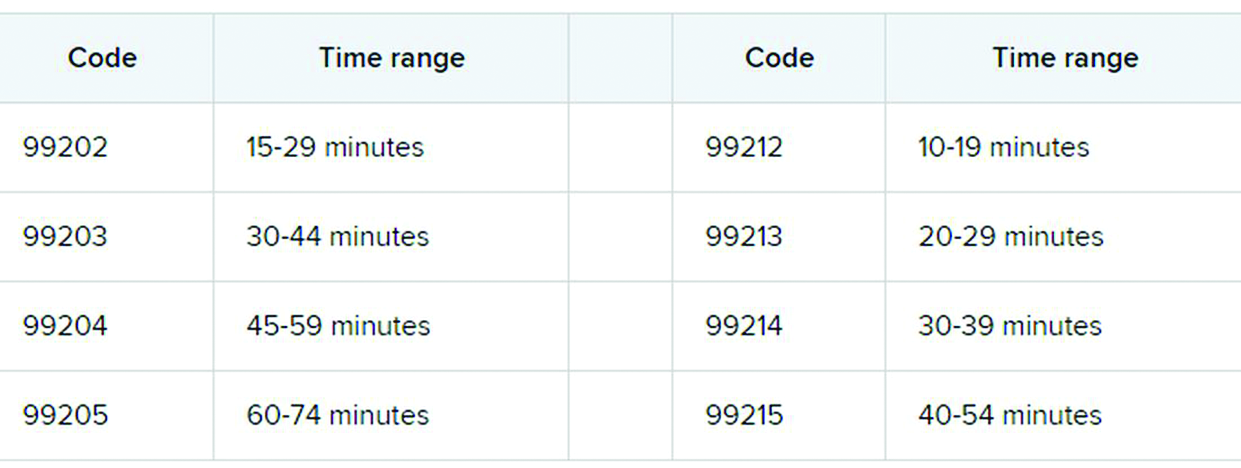
The first major changes to the definitions for E/M services will be in effect as of Jan. 1, 2021, with all payers expected to adopt these new guidelines. In particular, the AMA has revised the definitions for E/M codes 99202-99215 in the Current Procedural Terminology (CPT) 2021 codebook. The existing guidelines were developed in 1995 and 1997 and remain in effect for all other E/M services determined by history, exam, and medical decision-making (MDM).
What do the new changes mean to you? In 2021, for new and established office and other outpatient services reported with codes 99202-99215, a clinician may select the code on the basis of time or MDM.
There are three elements in MDM, and two of three are required. These elements are the number and complexity of problems addressed, amount and/or complexity of data to be reviewed and analyzed, and risk of complications and/or morbidity or mortality of patient management.
Make sure you familiarize yourself with these six big changes. It may take a bit of time to integrate these new processes into your daily routine, but wrapping your head around them as soon as possible can help boost your bottom line:
1. History and exam don’t count toward level of service
Physicians, advanced practice registered nurses, and physician assistants won’t use history or exam to select what level of code to bill for office visits 99202-99215, as they did in the past. They need only document a medically appropriate history and exam. The history may be obtained by staff members and reviewed by the billing practitioner.
While specific history and exam requirements disappear for office visit codes, they remain for all other types of visits, selected on the basis of history, exam, and MDM, such as hospital services, nursing facility services, and home and domiciliary care. So, say goodbye to “all other systems reviewed and negative” in office notes, but keep it handy for those other E/M codes.
2. All time spent caring for the patient on a particular day counts
This includes all time spent on the day of service, including preparing to see the patient, seeing the patient, phone calls or other work done after the visit (if not billed with a care management or other CPT code), and documenting in the medical record. The AMA developed new guidelines for using time for office and other outpatient services. For codes 99202-99215, count all of the face-to-face and non–face-to-face time spent by the billing clinician on the day of the visit. Counseling does not need to be more than 50% of the total time.
Do not include any staff time or time spent on any days before or after the visit. This allows clinicians to capture the work when a significant amount of it takes place before or after the visit with the patient, and to bill for it on the day of the visit.
According to the 2021 CPT codebook, physician or other qualified health care professional time includes the following activities:
- Preparing to see the patient (e.g., review of tests).
- Obtaining and/or reviewing separately obtained history.
- Performing a medically appropriate examination and/or evaluation.
- Counseling and educating the patient/family/caregiver.
- Ordering medications, tests, or procedures.
- Referring and communicating with other health care professionals (when not separately reported).
- Documenting clinical information in the electronic or other health record.
- Independently interpreting results (not separately reported) and communicating results to the patient/family/caregiver.
- Care coordination (not separately reported).
3. Soon to be gone: ‘new to the examiner’ and ‘workup planned’
The current guidelines don’t differentiate between a new problem to the clinician or an established problem to the clinician. So it doesn’t matter whether you’re hearing about a particular problem for the first time or the fifth time. The new office and outpatient services guidelines define problems only as they relate to the patient. For example, when selecting a level of service, a chronic problem with a mild exacerbation is the same level whether it’s the primary care physician seeing the patient for the 10th time to help manage her diabetes or the endocrinologist seeing the patient for the first time.
In the current guidelines (1995 and 1997), additional weight is given in selecting the level of MDM for a problem that’s new to the examiner with a workup planned, yet when the diagnostic test couldn’t be completed at the visit. This concept is gone from element of number and complexity of new problems. Ordering diagnostic tests is part of the second element, the amount and/or complexity of data to be reviewed.
4. Different guidelines if you need a history from a parent or other source
The new guidelines recognize the additional work required by the clinician when the patient is unable to give a history or when the practitioner doesn’t find the history to be reliable.
For example, in the case of a baby or child who is unable to give a history, the parent counts as an “independent historian,” according to the new guidelines. Likewise, for a patient with dementia, the caregiver counts as a historian. Note, however, that the criteria is not met simply because the patient is accompanied by another person. The additional weight in selecting the level of service is based on the patient being unable to give a reliable history.
Bottom line: In cases where patients are unable to communicate clearly, physicians or other providers should document the necessity of getting a complete history and who provided it.
5. A new spin on social determinants of health (SDoH)
In the risk of morbidity and/or mortality element, conditions described as “social determinants of health” are considered moderate complexity. SDoH are social and environmental factors that affect a patient’s health and medical outcomes. These include homelessness, inability to afford medications, food insecurity, and occupational exposure to risk factors. These circumstances are reported with codes in categories Z55-Z65.
In the past, physicians often documented this information in their office notes but rarely added a diagnosis code that described the patient’s situation. The ICD-10-CM code set includes codes that describe these factors. Using them allows the practice to track patients who have increased needs, and it communicates to payers the complexity of caring for these patients.
6. Risks related to surgery are defined
The current guidelines assign different levels of risk to minor and major surgery. They also include differentiation for “minor surgery with no identified risk factors,” “minor surgery with identified risk factors,” “elective major surgery with no identified risk factors,” and “elective major surgery with identified risk factors.” The old guidelines didn’t state whether the risk factors pertained to the patient – such as smoking, heart disease, or high body mass index – or to the procedure itself.
The new guidelines specifically say that it’s both. In the risk column, “decision regarding minor surgery with identified patient or procedure risk factors” and “decision regarding elective major surgery without patient or procedure risk factors” are both considered moderate. “Decision regarding elective major surgery with identified patient or procedure risk factors” and “decision regarding emergency major surgery” are in the high complexity column for risk.
Keep in mind that two of three elements are required: the number and complexity of problems, amount of data, and morbidity/mortality risk. Risk of morbidity/mortality alone doesn’t count as the basis for selecting the code. Of course, when surgeons see this, they ask, “What major procedures don’t have identified risk factors?”
Note, too, that these new CPT guidelines do not define the terms “minor” and “major” surgery. For payment reasons related to the postop period, the Centers for Medicare & Medicaid Services defines minor surgery as a procedure with 0-10 global days and a major surgery as a procedure with 90 global days. However, there are many procedures with 0 global days (endoscopy, cardiac catheterization) that are not minor procedures. Hopefully, the AMA will clarify this in 2021.
What’s the take-away for clinicians?
There are sure to be shifts in coding patterns based on these new guidelines. Some specialties will find that not being able to select a service based on history and exam alone will lower the level of service for which they can bill. Some practices, on the other hand, will be able to code for more high-level visits, without the need for a complete review of systems or a comprehensive exam.
The biggest challenge will be for practices that provide services both in the hospital and in the office, because they’ll have to use both sets of guidelines, depending on which type of service they’re performing.
For more details on what’s coming your way beginning on New Year’s Day, you may want to read the 16-page AMA document .
A version of this article first appeared on Medscape.com.
Betsy Nicoletti, MS, a nationally recognized coding expert, will take your coding questions via email and provide guidance on how to code properly to maximize reimbursement. Have a question about coding? Send it to [email protected].
thanks to the American Medical Association.
The first major changes to the definitions for E/M services will be in effect as of Jan. 1, 2021, with all payers expected to adopt these new guidelines. In particular, the AMA has revised the definitions for E/M codes 99202-99215 in the Current Procedural Terminology (CPT) 2021 codebook. The existing guidelines were developed in 1995 and 1997 and remain in effect for all other E/M services determined by history, exam, and medical decision-making (MDM).
What do the new changes mean to you? In 2021, for new and established office and other outpatient services reported with codes 99202-99215, a clinician may select the code on the basis of time or MDM.
There are three elements in MDM, and two of three are required. These elements are the number and complexity of problems addressed, amount and/or complexity of data to be reviewed and analyzed, and risk of complications and/or morbidity or mortality of patient management.
Make sure you familiarize yourself with these six big changes. It may take a bit of time to integrate these new processes into your daily routine, but wrapping your head around them as soon as possible can help boost your bottom line:
1. History and exam don’t count toward level of service
Physicians, advanced practice registered nurses, and physician assistants won’t use history or exam to select what level of code to bill for office visits 99202-99215, as they did in the past. They need only document a medically appropriate history and exam. The history may be obtained by staff members and reviewed by the billing practitioner.
While specific history and exam requirements disappear for office visit codes, they remain for all other types of visits, selected on the basis of history, exam, and MDM, such as hospital services, nursing facility services, and home and domiciliary care. So, say goodbye to “all other systems reviewed and negative” in office notes, but keep it handy for those other E/M codes.
2. All time spent caring for the patient on a particular day counts
This includes all time spent on the day of service, including preparing to see the patient, seeing the patient, phone calls or other work done after the visit (if not billed with a care management or other CPT code), and documenting in the medical record. The AMA developed new guidelines for using time for office and other outpatient services. For codes 99202-99215, count all of the face-to-face and non–face-to-face time spent by the billing clinician on the day of the visit. Counseling does not need to be more than 50% of the total time.
Do not include any staff time or time spent on any days before or after the visit. This allows clinicians to capture the work when a significant amount of it takes place before or after the visit with the patient, and to bill for it on the day of the visit.
According to the 2021 CPT codebook, physician or other qualified health care professional time includes the following activities:
- Preparing to see the patient (e.g., review of tests).
- Obtaining and/or reviewing separately obtained history.
- Performing a medically appropriate examination and/or evaluation.
- Counseling and educating the patient/family/caregiver.
- Ordering medications, tests, or procedures.
- Referring and communicating with other health care professionals (when not separately reported).
- Documenting clinical information in the electronic or other health record.
- Independently interpreting results (not separately reported) and communicating results to the patient/family/caregiver.
- Care coordination (not separately reported).

3. Soon to be gone: ‘new to the examiner’ and ‘workup planned’
The current guidelines don’t differentiate between a new problem to the clinician or an established problem to the clinician. So it doesn’t matter whether you’re hearing about a particular problem for the first time or the fifth time. The new office and outpatient services guidelines define problems only as they relate to the patient. For example, when selecting a level of service, a chronic problem with a mild exacerbation is the same level whether it’s the primary care physician seeing the patient for the 10th time to help manage her diabetes or the endocrinologist seeing the patient for the first time.
In the current guidelines (1995 and 1997), additional weight is given in selecting the level of MDM for a problem that’s new to the examiner with a workup planned, yet when the diagnostic test couldn’t be completed at the visit. This concept is gone from element of number and complexity of new problems. Ordering diagnostic tests is part of the second element, the amount and/or complexity of data to be reviewed.
4. Different guidelines if you need a history from a parent or other source
The new guidelines recognize the additional work required by the clinician when the patient is unable to give a history or when the practitioner doesn’t find the history to be reliable.
For example, in the case of a baby or child who is unable to give a history, the parent counts as an “independent historian,” according to the new guidelines. Likewise, for a patient with dementia, the caregiver counts as a historian. Note, however, that the criteria is not met simply because the patient is accompanied by another person. The additional weight in selecting the level of service is based on the patient being unable to give a reliable history.
Bottom line: In cases where patients are unable to communicate clearly, physicians or other providers should document the necessity of getting a complete history and who provided it.
5. A new spin on social determinants of health (SDoH)
In the risk of morbidity and/or mortality element, conditions described as “social determinants of health” are considered moderate complexity. SDoH are social and environmental factors that affect a patient’s health and medical outcomes. These include homelessness, inability to afford medications, food insecurity, and occupational exposure to risk factors. These circumstances are reported with codes in categories Z55-Z65.
In the past, physicians often documented this information in their office notes but rarely added a diagnosis code that described the patient’s situation. The ICD-10-CM code set includes codes that describe these factors. Using them allows the practice to track patients who have increased needs, and it communicates to payers the complexity of caring for these patients.
6. Risks related to surgery are defined
The current guidelines assign different levels of risk to minor and major surgery. They also include differentiation for “minor surgery with no identified risk factors,” “minor surgery with identified risk factors,” “elective major surgery with no identified risk factors,” and “elective major surgery with identified risk factors.” The old guidelines didn’t state whether the risk factors pertained to the patient – such as smoking, heart disease, or high body mass index – or to the procedure itself.
The new guidelines specifically say that it’s both. In the risk column, “decision regarding minor surgery with identified patient or procedure risk factors” and “decision regarding elective major surgery without patient or procedure risk factors” are both considered moderate. “Decision regarding elective major surgery with identified patient or procedure risk factors” and “decision regarding emergency major surgery” are in the high complexity column for risk.
Keep in mind that two of three elements are required: the number and complexity of problems, amount of data, and morbidity/mortality risk. Risk of morbidity/mortality alone doesn’t count as the basis for selecting the code. Of course, when surgeons see this, they ask, “What major procedures don’t have identified risk factors?”
Note, too, that these new CPT guidelines do not define the terms “minor” and “major” surgery. For payment reasons related to the postop period, the Centers for Medicare & Medicaid Services defines minor surgery as a procedure with 0-10 global days and a major surgery as a procedure with 90 global days. However, there are many procedures with 0 global days (endoscopy, cardiac catheterization) that are not minor procedures. Hopefully, the AMA will clarify this in 2021.
What’s the take-away for clinicians?
There are sure to be shifts in coding patterns based on these new guidelines. Some specialties will find that not being able to select a service based on history and exam alone will lower the level of service for which they can bill. Some practices, on the other hand, will be able to code for more high-level visits, without the need for a complete review of systems or a comprehensive exam.
The biggest challenge will be for practices that provide services both in the hospital and in the office, because they’ll have to use both sets of guidelines, depending on which type of service they’re performing.
For more details on what’s coming your way beginning on New Year’s Day, you may want to read the 16-page AMA document .
A version of this article first appeared on Medscape.com.
Betsy Nicoletti, MS, a nationally recognized coding expert, will take your coding questions via email and provide guidance on how to code properly to maximize reimbursement. Have a question about coding? Send it to [email protected].
thanks to the American Medical Association.
The first major changes to the definitions for E/M services will be in effect as of Jan. 1, 2021, with all payers expected to adopt these new guidelines. In particular, the AMA has revised the definitions for E/M codes 99202-99215 in the Current Procedural Terminology (CPT) 2021 codebook. The existing guidelines were developed in 1995 and 1997 and remain in effect for all other E/M services determined by history, exam, and medical decision-making (MDM).
What do the new changes mean to you? In 2021, for new and established office and other outpatient services reported with codes 99202-99215, a clinician may select the code on the basis of time or MDM.
There are three elements in MDM, and two of three are required. These elements are the number and complexity of problems addressed, amount and/or complexity of data to be reviewed and analyzed, and risk of complications and/or morbidity or mortality of patient management.
Make sure you familiarize yourself with these six big changes. It may take a bit of time to integrate these new processes into your daily routine, but wrapping your head around them as soon as possible can help boost your bottom line:
1. History and exam don’t count toward level of service
Physicians, advanced practice registered nurses, and physician assistants won’t use history or exam to select what level of code to bill for office visits 99202-99215, as they did in the past. They need only document a medically appropriate history and exam. The history may be obtained by staff members and reviewed by the billing practitioner.
While specific history and exam requirements disappear for office visit codes, they remain for all other types of visits, selected on the basis of history, exam, and MDM, such as hospital services, nursing facility services, and home and domiciliary care. So, say goodbye to “all other systems reviewed and negative” in office notes, but keep it handy for those other E/M codes.
2. All time spent caring for the patient on a particular day counts
This includes all time spent on the day of service, including preparing to see the patient, seeing the patient, phone calls or other work done after the visit (if not billed with a care management or other CPT code), and documenting in the medical record. The AMA developed new guidelines for using time for office and other outpatient services. For codes 99202-99215, count all of the face-to-face and non–face-to-face time spent by the billing clinician on the day of the visit. Counseling does not need to be more than 50% of the total time.
Do not include any staff time or time spent on any days before or after the visit. This allows clinicians to capture the work when a significant amount of it takes place before or after the visit with the patient, and to bill for it on the day of the visit.
According to the 2021 CPT codebook, physician or other qualified health care professional time includes the following activities:
- Preparing to see the patient (e.g., review of tests).
- Obtaining and/or reviewing separately obtained history.
- Performing a medically appropriate examination and/or evaluation.
- Counseling and educating the patient/family/caregiver.
- Ordering medications, tests, or procedures.
- Referring and communicating with other health care professionals (when not separately reported).
- Documenting clinical information in the electronic or other health record.
- Independently interpreting results (not separately reported) and communicating results to the patient/family/caregiver.
- Care coordination (not separately reported).

3. Soon to be gone: ‘new to the examiner’ and ‘workup planned’
The current guidelines don’t differentiate between a new problem to the clinician or an established problem to the clinician. So it doesn’t matter whether you’re hearing about a particular problem for the first time or the fifth time. The new office and outpatient services guidelines define problems only as they relate to the patient. For example, when selecting a level of service, a chronic problem with a mild exacerbation is the same level whether it’s the primary care physician seeing the patient for the 10th time to help manage her diabetes or the endocrinologist seeing the patient for the first time.
In the current guidelines (1995 and 1997), additional weight is given in selecting the level of MDM for a problem that’s new to the examiner with a workup planned, yet when the diagnostic test couldn’t be completed at the visit. This concept is gone from element of number and complexity of new problems. Ordering diagnostic tests is part of the second element, the amount and/or complexity of data to be reviewed.
4. Different guidelines if you need a history from a parent or other source
The new guidelines recognize the additional work required by the clinician when the patient is unable to give a history or when the practitioner doesn’t find the history to be reliable.
For example, in the case of a baby or child who is unable to give a history, the parent counts as an “independent historian,” according to the new guidelines. Likewise, for a patient with dementia, the caregiver counts as a historian. Note, however, that the criteria is not met simply because the patient is accompanied by another person. The additional weight in selecting the level of service is based on the patient being unable to give a reliable history.
Bottom line: In cases where patients are unable to communicate clearly, physicians or other providers should document the necessity of getting a complete history and who provided it.
5. A new spin on social determinants of health (SDoH)
In the risk of morbidity and/or mortality element, conditions described as “social determinants of health” are considered moderate complexity. SDoH are social and environmental factors that affect a patient’s health and medical outcomes. These include homelessness, inability to afford medications, food insecurity, and occupational exposure to risk factors. These circumstances are reported with codes in categories Z55-Z65.
In the past, physicians often documented this information in their office notes but rarely added a diagnosis code that described the patient’s situation. The ICD-10-CM code set includes codes that describe these factors. Using them allows the practice to track patients who have increased needs, and it communicates to payers the complexity of caring for these patients.
6. Risks related to surgery are defined
The current guidelines assign different levels of risk to minor and major surgery. They also include differentiation for “minor surgery with no identified risk factors,” “minor surgery with identified risk factors,” “elective major surgery with no identified risk factors,” and “elective major surgery with identified risk factors.” The old guidelines didn’t state whether the risk factors pertained to the patient – such as smoking, heart disease, or high body mass index – or to the procedure itself.
The new guidelines specifically say that it’s both. In the risk column, “decision regarding minor surgery with identified patient or procedure risk factors” and “decision regarding elective major surgery without patient or procedure risk factors” are both considered moderate. “Decision regarding elective major surgery with identified patient or procedure risk factors” and “decision regarding emergency major surgery” are in the high complexity column for risk.
Keep in mind that two of three elements are required: the number and complexity of problems, amount of data, and morbidity/mortality risk. Risk of morbidity/mortality alone doesn’t count as the basis for selecting the code. Of course, when surgeons see this, they ask, “What major procedures don’t have identified risk factors?”
Note, too, that these new CPT guidelines do not define the terms “minor” and “major” surgery. For payment reasons related to the postop period, the Centers for Medicare & Medicaid Services defines minor surgery as a procedure with 0-10 global days and a major surgery as a procedure with 90 global days. However, there are many procedures with 0 global days (endoscopy, cardiac catheterization) that are not minor procedures. Hopefully, the AMA will clarify this in 2021.
What’s the take-away for clinicians?
There are sure to be shifts in coding patterns based on these new guidelines. Some specialties will find that not being able to select a service based on history and exam alone will lower the level of service for which they can bill. Some practices, on the other hand, will be able to code for more high-level visits, without the need for a complete review of systems or a comprehensive exam.
The biggest challenge will be for practices that provide services both in the hospital and in the office, because they’ll have to use both sets of guidelines, depending on which type of service they’re performing.
For more details on what’s coming your way beginning on New Year’s Day, you may want to read the 16-page AMA document .
A version of this article first appeared on Medscape.com.
Endocrine-disrupting plastics pose growing health threat
Many types of plastics pose an unrecognized threat to human health by leaching endocrine-disrupting chemicals, and a new report from the Endocrine Society and the International Pollutants Elimination Network presents their dangers and risks.
Written in a consumer-friendly form designed to guide public interest groups and policy makers, the report also can be used by clinicians to inform discussions with patients about the potential dangers of plastics and how they can reduce their exposure to endocrine-disrupting chemicals.
The report, Plastics, EDCs, & Health, defines endocrine-disrupting chemicals (EDCs) as “an exogenous chemical, or mixture of chemicals, that interferes with any aspect of hormone action.” Hormones in the body must be released at specific times, and therefore interference with their normal activity can have profound effects on health in areas including growth and reproductive development, according to the report.
The available data show “more and more information about the different chemicals and the different effects they are having,” said lead author, Jodi Flaws, PhD, of the University of Illinois at Urbana-Champaign, in a virtual press conference accompanying the release of the report.
Although numerous EDCs have been identified, a recent study suggested that many potentially dangerous chemical additives remain unknown because they are identified as confidential or simply not well described, the report authors said. In addition, creation of more plastic products will likely lead to increased exposure to EDCs and make health problems worse, said report coauthor Pauliina Damdimopoulou, PhD, of the Karolinska Institutet in Stockholm.
Lesser-known EDCs populate consumer products
Most consumers are aware of bisphenol A and phthalates as known EDCs, said Dr. Flaws, but the report identifies other lesser-known EDCs including per- and polyfluoroalkyl substances (PFAS), dioxins, flame retardants, and UV stabilizers.
For example, PFAS have been used for decades in a range of consumer products including stain resistant clothes, fast food wrappers, carpet and furniture treatments, cookware, and firefighting foams, according to the report. Consequently, PFAS have become common in many water sources including surface water, drinking water, and ground water because of how they are disposed. “Consumption of fish and other aquatic creatures caught in waterways contaminated with PFAS also poses heightened risks due to bioaccumulation of persistent chemicals in these animals,” the report authors noted. Human exposures to PFAS have been documented in urine, serum, plasma, placenta, umbilical cord, breast milk, and fetal tissues, they added.
Brominated flame retardants are another lesser-known EDC highlighted in the report. These chemical additives are used in plastics such as electronics cases to reduce the spread of fire, as well as in furniture foam and other building materials, the authors wrote. UV stabilizers, which also have been linked to health problems, often are used in manufacturing cars and other machinery.
Microplastics create large risk
Microplastics, defined as plastic particles less than 5 mm in diameter, are another source of exposure to EDCs that is not well publicized, according to the report. Plastic waste disposal often leads to the release of microplastics, which can infiltrate soil and water. Plastic waste is often dumped or burned; outdoor burning of plastic causes emission of dioxins into the air and ground.
“Not only do microplastics contain endogenous chemical additives, which are not bound to the microplastic and can leach out of the microplastic and expose the population, they can also bind and accumulate toxic chemicals from the surrounding environment such as sea water and sediment,” the report authors said.
Recycling is not an easy answer, either. Often more chemicals are created and released during the process of using plastics to make other plastics, according to the report.
Overall, more awareness of the potential for increased exposure to EDCs and support of strategies to seek out alternatives to hazardous chemicals is needed at the global level, the authors wrote. For example, the European Union has proposed a chemicals strategy that includes improved classification of EDCs and banning identified EDCs in consumer products.
New data support ongoing dangers
“It was important to produce the report at this time because several new studies came out on the effects of EDCs from plastics on human health,” Dr. Flaws said in an interview. “Further, there was not previously a single source that brought together all the information in a manner that was targeted towards the public, policy makers, and others,” she said.
Dr. Flaws said that what has surprised her most in the recent research is the fact that plastics contain such a range of chemicals and EDCs.
“A good take-home message [from the report] is that plastics can contain endocrine-disrupting chemicals that can interfere with normal hormones and lead to adverse health outcomes,” she said. “I suggest limiting the use of plastics as much as possible. I know this is very hard to do, so if someone needs to use plastic, they should not heat food or drink in plastic containers,” she emphasized. Individuals also can limit reuse of plastics over and over,” she said. “Heating and repeated use/washing often causes plastics to leach EDCs into food and drink that we then get into our bodies.”
Additional research is needed to understand the mechanisms by which EDCs from plastics cause damage, Dr. Flaws emphasized. “Given that it is not possible to eliminate plastics at this time, if we understood mechanisms of action, we could develop ways to prevent toxicity or treat EDC-induced adverse health outcomes,” she said. “We also need research designed to develop plastics or ‘green materials’ that do not contain endocrine disruptors and do not cause health problems or damage the environment,” she noted.
The report was produced as a joint effort of the Endocrine Society and International Pollutants Elimination Network. The report authors had no financial conflicts to disclose.
Many types of plastics pose an unrecognized threat to human health by leaching endocrine-disrupting chemicals, and a new report from the Endocrine Society and the International Pollutants Elimination Network presents their dangers and risks.
Written in a consumer-friendly form designed to guide public interest groups and policy makers, the report also can be used by clinicians to inform discussions with patients about the potential dangers of plastics and how they can reduce their exposure to endocrine-disrupting chemicals.
The report, Plastics, EDCs, & Health, defines endocrine-disrupting chemicals (EDCs) as “an exogenous chemical, or mixture of chemicals, that interferes with any aspect of hormone action.” Hormones in the body must be released at specific times, and therefore interference with their normal activity can have profound effects on health in areas including growth and reproductive development, according to the report.
The available data show “more and more information about the different chemicals and the different effects they are having,” said lead author, Jodi Flaws, PhD, of the University of Illinois at Urbana-Champaign, in a virtual press conference accompanying the release of the report.
Although numerous EDCs have been identified, a recent study suggested that many potentially dangerous chemical additives remain unknown because they are identified as confidential or simply not well described, the report authors said. In addition, creation of more plastic products will likely lead to increased exposure to EDCs and make health problems worse, said report coauthor Pauliina Damdimopoulou, PhD, of the Karolinska Institutet in Stockholm.
Lesser-known EDCs populate consumer products
Most consumers are aware of bisphenol A and phthalates as known EDCs, said Dr. Flaws, but the report identifies other lesser-known EDCs including per- and polyfluoroalkyl substances (PFAS), dioxins, flame retardants, and UV stabilizers.
For example, PFAS have been used for decades in a range of consumer products including stain resistant clothes, fast food wrappers, carpet and furniture treatments, cookware, and firefighting foams, according to the report. Consequently, PFAS have become common in many water sources including surface water, drinking water, and ground water because of how they are disposed. “Consumption of fish and other aquatic creatures caught in waterways contaminated with PFAS also poses heightened risks due to bioaccumulation of persistent chemicals in these animals,” the report authors noted. Human exposures to PFAS have been documented in urine, serum, plasma, placenta, umbilical cord, breast milk, and fetal tissues, they added.
Brominated flame retardants are another lesser-known EDC highlighted in the report. These chemical additives are used in plastics such as electronics cases to reduce the spread of fire, as well as in furniture foam and other building materials, the authors wrote. UV stabilizers, which also have been linked to health problems, often are used in manufacturing cars and other machinery.
Microplastics create large risk
Microplastics, defined as plastic particles less than 5 mm in diameter, are another source of exposure to EDCs that is not well publicized, according to the report. Plastic waste disposal often leads to the release of microplastics, which can infiltrate soil and water. Plastic waste is often dumped or burned; outdoor burning of plastic causes emission of dioxins into the air and ground.
“Not only do microplastics contain endogenous chemical additives, which are not bound to the microplastic and can leach out of the microplastic and expose the population, they can also bind and accumulate toxic chemicals from the surrounding environment such as sea water and sediment,” the report authors said.
Recycling is not an easy answer, either. Often more chemicals are created and released during the process of using plastics to make other plastics, according to the report.
Overall, more awareness of the potential for increased exposure to EDCs and support of strategies to seek out alternatives to hazardous chemicals is needed at the global level, the authors wrote. For example, the European Union has proposed a chemicals strategy that includes improved classification of EDCs and banning identified EDCs in consumer products.
New data support ongoing dangers
“It was important to produce the report at this time because several new studies came out on the effects of EDCs from plastics on human health,” Dr. Flaws said in an interview. “Further, there was not previously a single source that brought together all the information in a manner that was targeted towards the public, policy makers, and others,” she said.
Dr. Flaws said that what has surprised her most in the recent research is the fact that plastics contain such a range of chemicals and EDCs.
“A good take-home message [from the report] is that plastics can contain endocrine-disrupting chemicals that can interfere with normal hormones and lead to adverse health outcomes,” she said. “I suggest limiting the use of plastics as much as possible. I know this is very hard to do, so if someone needs to use plastic, they should not heat food or drink in plastic containers,” she emphasized. Individuals also can limit reuse of plastics over and over,” she said. “Heating and repeated use/washing often causes plastics to leach EDCs into food and drink that we then get into our bodies.”
Additional research is needed to understand the mechanisms by which EDCs from plastics cause damage, Dr. Flaws emphasized. “Given that it is not possible to eliminate plastics at this time, if we understood mechanisms of action, we could develop ways to prevent toxicity or treat EDC-induced adverse health outcomes,” she said. “We also need research designed to develop plastics or ‘green materials’ that do not contain endocrine disruptors and do not cause health problems or damage the environment,” she noted.
The report was produced as a joint effort of the Endocrine Society and International Pollutants Elimination Network. The report authors had no financial conflicts to disclose.
Many types of plastics pose an unrecognized threat to human health by leaching endocrine-disrupting chemicals, and a new report from the Endocrine Society and the International Pollutants Elimination Network presents their dangers and risks.
Written in a consumer-friendly form designed to guide public interest groups and policy makers, the report also can be used by clinicians to inform discussions with patients about the potential dangers of plastics and how they can reduce their exposure to endocrine-disrupting chemicals.
The report, Plastics, EDCs, & Health, defines endocrine-disrupting chemicals (EDCs) as “an exogenous chemical, or mixture of chemicals, that interferes with any aspect of hormone action.” Hormones in the body must be released at specific times, and therefore interference with their normal activity can have profound effects on health in areas including growth and reproductive development, according to the report.
The available data show “more and more information about the different chemicals and the different effects they are having,” said lead author, Jodi Flaws, PhD, of the University of Illinois at Urbana-Champaign, in a virtual press conference accompanying the release of the report.
Although numerous EDCs have been identified, a recent study suggested that many potentially dangerous chemical additives remain unknown because they are identified as confidential or simply not well described, the report authors said. In addition, creation of more plastic products will likely lead to increased exposure to EDCs and make health problems worse, said report coauthor Pauliina Damdimopoulou, PhD, of the Karolinska Institutet in Stockholm.
Lesser-known EDCs populate consumer products
Most consumers are aware of bisphenol A and phthalates as known EDCs, said Dr. Flaws, but the report identifies other lesser-known EDCs including per- and polyfluoroalkyl substances (PFAS), dioxins, flame retardants, and UV stabilizers.
For example, PFAS have been used for decades in a range of consumer products including stain resistant clothes, fast food wrappers, carpet and furniture treatments, cookware, and firefighting foams, according to the report. Consequently, PFAS have become common in many water sources including surface water, drinking water, and ground water because of how they are disposed. “Consumption of fish and other aquatic creatures caught in waterways contaminated with PFAS also poses heightened risks due to bioaccumulation of persistent chemicals in these animals,” the report authors noted. Human exposures to PFAS have been documented in urine, serum, plasma, placenta, umbilical cord, breast milk, and fetal tissues, they added.
Brominated flame retardants are another lesser-known EDC highlighted in the report. These chemical additives are used in plastics such as electronics cases to reduce the spread of fire, as well as in furniture foam and other building materials, the authors wrote. UV stabilizers, which also have been linked to health problems, often are used in manufacturing cars and other machinery.
Microplastics create large risk
Microplastics, defined as plastic particles less than 5 mm in diameter, are another source of exposure to EDCs that is not well publicized, according to the report. Plastic waste disposal often leads to the release of microplastics, which can infiltrate soil and water. Plastic waste is often dumped or burned; outdoor burning of plastic causes emission of dioxins into the air and ground.
“Not only do microplastics contain endogenous chemical additives, which are not bound to the microplastic and can leach out of the microplastic and expose the population, they can also bind and accumulate toxic chemicals from the surrounding environment such as sea water and sediment,” the report authors said.
Recycling is not an easy answer, either. Often more chemicals are created and released during the process of using plastics to make other plastics, according to the report.
Overall, more awareness of the potential for increased exposure to EDCs and support of strategies to seek out alternatives to hazardous chemicals is needed at the global level, the authors wrote. For example, the European Union has proposed a chemicals strategy that includes improved classification of EDCs and banning identified EDCs in consumer products.
New data support ongoing dangers
“It was important to produce the report at this time because several new studies came out on the effects of EDCs from plastics on human health,” Dr. Flaws said in an interview. “Further, there was not previously a single source that brought together all the information in a manner that was targeted towards the public, policy makers, and others,” she said.
Dr. Flaws said that what has surprised her most in the recent research is the fact that plastics contain such a range of chemicals and EDCs.
“A good take-home message [from the report] is that plastics can contain endocrine-disrupting chemicals that can interfere with normal hormones and lead to adverse health outcomes,” she said. “I suggest limiting the use of plastics as much as possible. I know this is very hard to do, so if someone needs to use plastic, they should not heat food or drink in plastic containers,” she emphasized. Individuals also can limit reuse of plastics over and over,” she said. “Heating and repeated use/washing often causes plastics to leach EDCs into food and drink that we then get into our bodies.”
Additional research is needed to understand the mechanisms by which EDCs from plastics cause damage, Dr. Flaws emphasized. “Given that it is not possible to eliminate plastics at this time, if we understood mechanisms of action, we could develop ways to prevent toxicity or treat EDC-induced adverse health outcomes,” she said. “We also need research designed to develop plastics or ‘green materials’ that do not contain endocrine disruptors and do not cause health problems or damage the environment,” she noted.
The report was produced as a joint effort of the Endocrine Society and International Pollutants Elimination Network. The report authors had no financial conflicts to disclose.
Etonogestrel implants may be bent, fractured by trauma or during sports
In 2017, Global Pediatric Health published a case report series associated with the use of long-acting reversible contraceptives, specifically the etonogestrel implant.
In November 2020, the makers of the etonogestrel implant (Merck) recommended a change in practice with the release of a notice to health care providers certified in the training of this product. This mass marketing blast included an updated warning and cautions for prescribers as well as patient information on the potential risks of migration, fracture, and bent devices attributable to trauma or sports. “Broken or Bent Implant (Section 5.16). The addition of the following underlined language: “There have been reports of broken or bent implants, which may be related to external forces (e.g., manipulation of the implant or contact sports) while in the patient’s arm. There have also been reports of migration of a broken implant fragment within the arm.”
Clearly the etonogestrel subdermal hormonal implant is an effective form of contraception and particularly beneficial in nonadherent sexually active teens who struggle to remember oral contraceptives. But it is important to be aware of this alert. Little is known about the type of trauma or rate of external force required to cause migration, fracture, or bend implants. This update requires adequate counseling of potential risks and complications of the etonogestrel implant, including the risk of migration, fracture, or bent devices specifically in the event of contact sports and trauma.
Ms. Thew is medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee. She is a member of the Pediatric News editorial advisory board. She had no relevant financial disclosures. Email Ms. Thew at [email protected].
In 2017, Global Pediatric Health published a case report series associated with the use of long-acting reversible contraceptives, specifically the etonogestrel implant.
In November 2020, the makers of the etonogestrel implant (Merck) recommended a change in practice with the release of a notice to health care providers certified in the training of this product. This mass marketing blast included an updated warning and cautions for prescribers as well as patient information on the potential risks of migration, fracture, and bent devices attributable to trauma or sports. “Broken or Bent Implant (Section 5.16). The addition of the following underlined language: “There have been reports of broken or bent implants, which may be related to external forces (e.g., manipulation of the implant or contact sports) while in the patient’s arm. There have also been reports of migration of a broken implant fragment within the arm.”
Clearly the etonogestrel subdermal hormonal implant is an effective form of contraception and particularly beneficial in nonadherent sexually active teens who struggle to remember oral contraceptives. But it is important to be aware of this alert. Little is known about the type of trauma or rate of external force required to cause migration, fracture, or bend implants. This update requires adequate counseling of potential risks and complications of the etonogestrel implant, including the risk of migration, fracture, or bent devices specifically in the event of contact sports and trauma.
Ms. Thew is medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee. She is a member of the Pediatric News editorial advisory board. She had no relevant financial disclosures. Email Ms. Thew at [email protected].
In 2017, Global Pediatric Health published a case report series associated with the use of long-acting reversible contraceptives, specifically the etonogestrel implant.
In November 2020, the makers of the etonogestrel implant (Merck) recommended a change in practice with the release of a notice to health care providers certified in the training of this product. This mass marketing blast included an updated warning and cautions for prescribers as well as patient information on the potential risks of migration, fracture, and bent devices attributable to trauma or sports. “Broken or Bent Implant (Section 5.16). The addition of the following underlined language: “There have been reports of broken or bent implants, which may be related to external forces (e.g., manipulation of the implant or contact sports) while in the patient’s arm. There have also been reports of migration of a broken implant fragment within the arm.”
Clearly the etonogestrel subdermal hormonal implant is an effective form of contraception and particularly beneficial in nonadherent sexually active teens who struggle to remember oral contraceptives. But it is important to be aware of this alert. Little is known about the type of trauma or rate of external force required to cause migration, fracture, or bend implants. This update requires adequate counseling of potential risks and complications of the etonogestrel implant, including the risk of migration, fracture, or bent devices specifically in the event of contact sports and trauma.
Ms. Thew is medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee. She is a member of the Pediatric News editorial advisory board. She had no relevant financial disclosures. Email Ms. Thew at [email protected].
To vape or not to vape: Is that really a question?
All pediatricians are relieved that the rates of children smoking cigarettes has dropped steadily since 2011. This decline seems to be associated with education on the dangers of cigarettes and fewer parents smoking. Perhaps less modeling of cigarette use in movies (although it increased again from 2010 to 2019) and lawsuits against advertisements targeting children also has helped.
“Whew,” we may have said, “we can relax our efforts to convince children to avoid smoking.” But, as is commonly true in medicine, the next threat was right around the corner – in this case vaping or e-cigarettes, also called vapes, e-hookahs, vape pens, tank systems, mods, and electronic nicotine delivery systems. And the size of the problem is huge – over 20% of high school students report using e-cigarettes – and immediate, as vaping can kill in the short term as well as causing long-term harm.
“E-cigarette, or vaping, product use–associated Lung Injury” – EVALI for short – has killed 68 vapers and hospitalized thousands. EVALI is thought to be caused by a vitamin E acetate additive used when vaping marijuana, particularly from informal sources like friends, family, or in-person or online dealers.
Vaping increases the risk of severe COVID-19 disease
While EVALI deaths dropped in months after being explained, the COVID-19 epidemic is now a much greater threat to vapers. Vaping increases risk of severe COVID-19 disease because of its immediate paralysis of lung cilia. Sharing vape devices and touching one’s lips while using also increase the risk of virus transmission. Vaping and smoking increase the number of ACE2 receptors to which the SARS-CoV-2 virus attaches causing the characteristic cell damage, and suppresses macrophages and neutrophils, resulting in more smokers testing positive, being twice as likely to develop a severe illness and get hospitalized because of pneumonia from COVID-19, and being less likely to recover. Unfortunately, addressing this new threat to the immediate and long-term health of our patients appears to be more complicated than for addressing smoking tobacco. First of all, vaping is much more difficult to detect than smelly cigarettes sending smoke signals from behind the garage or in the school bathrooms. Many, if not most, adults do not recognize the vaping devices when they see them, as many are tiny and some look like computer thumb drives. The aerosol emitted when in use, while containing dangerous toxins, has less odor than tobacco smoke. Vaping equipment and ads have been designed to attract youth, including linking them to sports and music events. Vaping has been advertised as a way to wean off nicotine addiction, a claim that has some scientific evidence in adults, but at a lower dose of nicotine. Warning children about the dangers of marijuana vaping has been made less credible by the rapid expansion of legalization of marijuana around the United States, eliciting “I told you it was fine” reactions from youth. And the person vaping does not know what or how much of the psychoactive components are being delivered into their bodies. One Juul pod, for example, has the equivalent in nicotine of an entire pack of 20 cigarettes. They are highly addictive, especially to the developing brain, such that youth who vape are more likely to become addicted and to smoke cigarettes in the future.
Help from federal regulation has been weak
While all 50 states ban sales to youth, adults can still buy. Food and Drug Administration limitations on kid-friendly ads, and use of sweet, fruity, and mint flavorings that are most preferred by children, apply only to new producers. The FDA does not yet regulate content of vaping solutions.
So we pediatricians are on the front line for this new threat to prevent vaping or convince youth to cut down or quit. The first step in addressing vaping is being knowledgeable about its many known and emerging health risks. It may seem obvious that the dangers of vaping microscopic particles depends on the contents. Water vapor alone is not dangerous; in fact, we prescribe it in nebulizers. Unfortunately, the contents of different vaping products vary and are not well defined in different vape products. The process of using an electric current to vaporize a substance can make it more toxic than the precursor, and teens have little idea about the substances they are inhaling. The psychoactive components vary from nicotine to tetrahydrocannabinol in varying amounts. These have the well known effects of stimulation or a high, but also the potential adverse effects of poor concentration, agitation, and even psychosis. Most e-cigarettes contain nicotine, which is highly addictive and can harm adolescent brain development, which continues into the early- to mid-20s. About two-thirds of Juul users aged 15-24 years did not know that it always contains nicotine, as do 99% of all vape solutions (Centers for Disease Control and Prevention, 2020). Earlier use of nicotine is more highly associated with later addiction to tobacco products that cause lung damage, acid reflux, insulin resistance, harm to the testes, harm to fetuses, cancer, and heart disease.
E-cigarette aerosols also contain dozens of other harmful substances besides nicotine ranging from acetone, propylene glycol, and metals to formaldehyde and ethyl benzene. These same chemicals are part of familiar toxic substances such as antifreeze, paint thinner, and pesticides. These cause ear, eye and throat irritation, and impairments in the cardiovascular system reducing athletic ability – at the least. Some flavorings in vape fluids also are toxic. Even the residual left on furniture and floors is harmful to those coming in contact, including pets.
How to encourage teens not to vaping
Trying to scare youth about health hazards is not generally effective in stopping risk behaviors since adolescence is a time of perceived singularity (it does not apply to me) and even a sense of immortality. Teens also see peers who vape as being unaffected and decide on using based on this small personal sample instead of valid statistics.
But teens do pay some attention to peer models or influencers saying why they do not use. One source of such testimony you can refer to is videos of inspiring athletes, musicians, and other “cool” young adults found on the naturalhigh.org website. You may know other examples of community teens desisting you can reference.
Parent rules, and less so advice, against smoking have been shown to be effective in deterring youth cigarette smoking. Because parents are less aware of vaping and its dangers, another step we can take is educating parents in our practices about vaping, its variable forms, its effects, and dangers, supplying authoritative materials, and advising them to talk with their children. Other steps the American Academy of Pediatrics recommends regarding smoking is for parents to be a role model of not using or try to quit, designate the house and car as smoking free, avoid children viewing smoking in media, tell their children about the side effects, and encourage their children who use to quit. Parents also can encourage schools to teach and have rules about smoking and vaping (e.g., med.stanford.edu/tobaccopreventiontoolkit.html).
Another approach we have been using is to not only screen for all substance use, but also to gather information about the teen’s strengths, activities, and life goals both to enhance rapport and to reference during motivational interviewing as reasons to avoid, reduce, or quit vaping. Motivational interviewing has been shown to help patients make healthier lifestyle choices by nonjudgmentally exploring their pros and cons in a conversation that takes into account readiness to change. This fits well with the stage of developing autonomy when teens want above all to make their own decisions. The cons of using can be discussed as including the effects and side effects of vaping interfering with their favored activities and moving towards their identified goals. Praising abstinence and asking them to show you how they could decline offers to vape are valuable reinforcement you can provide.
Finally, we all know that teens hate being manipulated. Vaping education we provide can make it clear that youth are being tricked by companies – most being large cigarette producers who know the dangers of vaping – into getting addicted so these companies can get rich on their money.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication is as a paid expert to MDedge News. Email her at [email protected].
All pediatricians are relieved that the rates of children smoking cigarettes has dropped steadily since 2011. This decline seems to be associated with education on the dangers of cigarettes and fewer parents smoking. Perhaps less modeling of cigarette use in movies (although it increased again from 2010 to 2019) and lawsuits against advertisements targeting children also has helped.
“Whew,” we may have said, “we can relax our efforts to convince children to avoid smoking.” But, as is commonly true in medicine, the next threat was right around the corner – in this case vaping or e-cigarettes, also called vapes, e-hookahs, vape pens, tank systems, mods, and electronic nicotine delivery systems. And the size of the problem is huge – over 20% of high school students report using e-cigarettes – and immediate, as vaping can kill in the short term as well as causing long-term harm.
“E-cigarette, or vaping, product use–associated Lung Injury” – EVALI for short – has killed 68 vapers and hospitalized thousands. EVALI is thought to be caused by a vitamin E acetate additive used when vaping marijuana, particularly from informal sources like friends, family, or in-person or online dealers.
Vaping increases the risk of severe COVID-19 disease
While EVALI deaths dropped in months after being explained, the COVID-19 epidemic is now a much greater threat to vapers. Vaping increases risk of severe COVID-19 disease because of its immediate paralysis of lung cilia. Sharing vape devices and touching one’s lips while using also increase the risk of virus transmission. Vaping and smoking increase the number of ACE2 receptors to which the SARS-CoV-2 virus attaches causing the characteristic cell damage, and suppresses macrophages and neutrophils, resulting in more smokers testing positive, being twice as likely to develop a severe illness and get hospitalized because of pneumonia from COVID-19, and being less likely to recover. Unfortunately, addressing this new threat to the immediate and long-term health of our patients appears to be more complicated than for addressing smoking tobacco. First of all, vaping is much more difficult to detect than smelly cigarettes sending smoke signals from behind the garage or in the school bathrooms. Many, if not most, adults do not recognize the vaping devices when they see them, as many are tiny and some look like computer thumb drives. The aerosol emitted when in use, while containing dangerous toxins, has less odor than tobacco smoke. Vaping equipment and ads have been designed to attract youth, including linking them to sports and music events. Vaping has been advertised as a way to wean off nicotine addiction, a claim that has some scientific evidence in adults, but at a lower dose of nicotine. Warning children about the dangers of marijuana vaping has been made less credible by the rapid expansion of legalization of marijuana around the United States, eliciting “I told you it was fine” reactions from youth. And the person vaping does not know what or how much of the psychoactive components are being delivered into their bodies. One Juul pod, for example, has the equivalent in nicotine of an entire pack of 20 cigarettes. They are highly addictive, especially to the developing brain, such that youth who vape are more likely to become addicted and to smoke cigarettes in the future.
Help from federal regulation has been weak
While all 50 states ban sales to youth, adults can still buy. Food and Drug Administration limitations on kid-friendly ads, and use of sweet, fruity, and mint flavorings that are most preferred by children, apply only to new producers. The FDA does not yet regulate content of vaping solutions.
So we pediatricians are on the front line for this new threat to prevent vaping or convince youth to cut down or quit. The first step in addressing vaping is being knowledgeable about its many known and emerging health risks. It may seem obvious that the dangers of vaping microscopic particles depends on the contents. Water vapor alone is not dangerous; in fact, we prescribe it in nebulizers. Unfortunately, the contents of different vaping products vary and are not well defined in different vape products. The process of using an electric current to vaporize a substance can make it more toxic than the precursor, and teens have little idea about the substances they are inhaling. The psychoactive components vary from nicotine to tetrahydrocannabinol in varying amounts. These have the well known effects of stimulation or a high, but also the potential adverse effects of poor concentration, agitation, and even psychosis. Most e-cigarettes contain nicotine, which is highly addictive and can harm adolescent brain development, which continues into the early- to mid-20s. About two-thirds of Juul users aged 15-24 years did not know that it always contains nicotine, as do 99% of all vape solutions (Centers for Disease Control and Prevention, 2020). Earlier use of nicotine is more highly associated with later addiction to tobacco products that cause lung damage, acid reflux, insulin resistance, harm to the testes, harm to fetuses, cancer, and heart disease.
E-cigarette aerosols also contain dozens of other harmful substances besides nicotine ranging from acetone, propylene glycol, and metals to formaldehyde and ethyl benzene. These same chemicals are part of familiar toxic substances such as antifreeze, paint thinner, and pesticides. These cause ear, eye and throat irritation, and impairments in the cardiovascular system reducing athletic ability – at the least. Some flavorings in vape fluids also are toxic. Even the residual left on furniture and floors is harmful to those coming in contact, including pets.
How to encourage teens not to vaping
Trying to scare youth about health hazards is not generally effective in stopping risk behaviors since adolescence is a time of perceived singularity (it does not apply to me) and even a sense of immortality. Teens also see peers who vape as being unaffected and decide on using based on this small personal sample instead of valid statistics.
But teens do pay some attention to peer models or influencers saying why they do not use. One source of such testimony you can refer to is videos of inspiring athletes, musicians, and other “cool” young adults found on the naturalhigh.org website. You may know other examples of community teens desisting you can reference.
Parent rules, and less so advice, against smoking have been shown to be effective in deterring youth cigarette smoking. Because parents are less aware of vaping and its dangers, another step we can take is educating parents in our practices about vaping, its variable forms, its effects, and dangers, supplying authoritative materials, and advising them to talk with their children. Other steps the American Academy of Pediatrics recommends regarding smoking is for parents to be a role model of not using or try to quit, designate the house and car as smoking free, avoid children viewing smoking in media, tell their children about the side effects, and encourage their children who use to quit. Parents also can encourage schools to teach and have rules about smoking and vaping (e.g., med.stanford.edu/tobaccopreventiontoolkit.html).
Another approach we have been using is to not only screen for all substance use, but also to gather information about the teen’s strengths, activities, and life goals both to enhance rapport and to reference during motivational interviewing as reasons to avoid, reduce, or quit vaping. Motivational interviewing has been shown to help patients make healthier lifestyle choices by nonjudgmentally exploring their pros and cons in a conversation that takes into account readiness to change. This fits well with the stage of developing autonomy when teens want above all to make their own decisions. The cons of using can be discussed as including the effects and side effects of vaping interfering with their favored activities and moving towards their identified goals. Praising abstinence and asking them to show you how they could decline offers to vape are valuable reinforcement you can provide.
Finally, we all know that teens hate being manipulated. Vaping education we provide can make it clear that youth are being tricked by companies – most being large cigarette producers who know the dangers of vaping – into getting addicted so these companies can get rich on their money.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication is as a paid expert to MDedge News. Email her at [email protected].
All pediatricians are relieved that the rates of children smoking cigarettes has dropped steadily since 2011. This decline seems to be associated with education on the dangers of cigarettes and fewer parents smoking. Perhaps less modeling of cigarette use in movies (although it increased again from 2010 to 2019) and lawsuits against advertisements targeting children also has helped.
“Whew,” we may have said, “we can relax our efforts to convince children to avoid smoking.” But, as is commonly true in medicine, the next threat was right around the corner – in this case vaping or e-cigarettes, also called vapes, e-hookahs, vape pens, tank systems, mods, and electronic nicotine delivery systems. And the size of the problem is huge – over 20% of high school students report using e-cigarettes – and immediate, as vaping can kill in the short term as well as causing long-term harm.
“E-cigarette, or vaping, product use–associated Lung Injury” – EVALI for short – has killed 68 vapers and hospitalized thousands. EVALI is thought to be caused by a vitamin E acetate additive used when vaping marijuana, particularly from informal sources like friends, family, or in-person or online dealers.
Vaping increases the risk of severe COVID-19 disease
While EVALI deaths dropped in months after being explained, the COVID-19 epidemic is now a much greater threat to vapers. Vaping increases risk of severe COVID-19 disease because of its immediate paralysis of lung cilia. Sharing vape devices and touching one’s lips while using also increase the risk of virus transmission. Vaping and smoking increase the number of ACE2 receptors to which the SARS-CoV-2 virus attaches causing the characteristic cell damage, and suppresses macrophages and neutrophils, resulting in more smokers testing positive, being twice as likely to develop a severe illness and get hospitalized because of pneumonia from COVID-19, and being less likely to recover. Unfortunately, addressing this new threat to the immediate and long-term health of our patients appears to be more complicated than for addressing smoking tobacco. First of all, vaping is much more difficult to detect than smelly cigarettes sending smoke signals from behind the garage or in the school bathrooms. Many, if not most, adults do not recognize the vaping devices when they see them, as many are tiny and some look like computer thumb drives. The aerosol emitted when in use, while containing dangerous toxins, has less odor than tobacco smoke. Vaping equipment and ads have been designed to attract youth, including linking them to sports and music events. Vaping has been advertised as a way to wean off nicotine addiction, a claim that has some scientific evidence in adults, but at a lower dose of nicotine. Warning children about the dangers of marijuana vaping has been made less credible by the rapid expansion of legalization of marijuana around the United States, eliciting “I told you it was fine” reactions from youth. And the person vaping does not know what or how much of the psychoactive components are being delivered into their bodies. One Juul pod, for example, has the equivalent in nicotine of an entire pack of 20 cigarettes. They are highly addictive, especially to the developing brain, such that youth who vape are more likely to become addicted and to smoke cigarettes in the future.
Help from federal regulation has been weak
While all 50 states ban sales to youth, adults can still buy. Food and Drug Administration limitations on kid-friendly ads, and use of sweet, fruity, and mint flavorings that are most preferred by children, apply only to new producers. The FDA does not yet regulate content of vaping solutions.
So we pediatricians are on the front line for this new threat to prevent vaping or convince youth to cut down or quit. The first step in addressing vaping is being knowledgeable about its many known and emerging health risks. It may seem obvious that the dangers of vaping microscopic particles depends on the contents. Water vapor alone is not dangerous; in fact, we prescribe it in nebulizers. Unfortunately, the contents of different vaping products vary and are not well defined in different vape products. The process of using an electric current to vaporize a substance can make it more toxic than the precursor, and teens have little idea about the substances they are inhaling. The psychoactive components vary from nicotine to tetrahydrocannabinol in varying amounts. These have the well known effects of stimulation or a high, but also the potential adverse effects of poor concentration, agitation, and even psychosis. Most e-cigarettes contain nicotine, which is highly addictive and can harm adolescent brain development, which continues into the early- to mid-20s. About two-thirds of Juul users aged 15-24 years did not know that it always contains nicotine, as do 99% of all vape solutions (Centers for Disease Control and Prevention, 2020). Earlier use of nicotine is more highly associated with later addiction to tobacco products that cause lung damage, acid reflux, insulin resistance, harm to the testes, harm to fetuses, cancer, and heart disease.
E-cigarette aerosols also contain dozens of other harmful substances besides nicotine ranging from acetone, propylene glycol, and metals to formaldehyde and ethyl benzene. These same chemicals are part of familiar toxic substances such as antifreeze, paint thinner, and pesticides. These cause ear, eye and throat irritation, and impairments in the cardiovascular system reducing athletic ability – at the least. Some flavorings in vape fluids also are toxic. Even the residual left on furniture and floors is harmful to those coming in contact, including pets.
How to encourage teens not to vaping
Trying to scare youth about health hazards is not generally effective in stopping risk behaviors since adolescence is a time of perceived singularity (it does not apply to me) and even a sense of immortality. Teens also see peers who vape as being unaffected and decide on using based on this small personal sample instead of valid statistics.
But teens do pay some attention to peer models or influencers saying why they do not use. One source of such testimony you can refer to is videos of inspiring athletes, musicians, and other “cool” young adults found on the naturalhigh.org website. You may know other examples of community teens desisting you can reference.
Parent rules, and less so advice, against smoking have been shown to be effective in deterring youth cigarette smoking. Because parents are less aware of vaping and its dangers, another step we can take is educating parents in our practices about vaping, its variable forms, its effects, and dangers, supplying authoritative materials, and advising them to talk with their children. Other steps the American Academy of Pediatrics recommends regarding smoking is for parents to be a role model of not using or try to quit, designate the house and car as smoking free, avoid children viewing smoking in media, tell their children about the side effects, and encourage their children who use to quit. Parents also can encourage schools to teach and have rules about smoking and vaping (e.g., med.stanford.edu/tobaccopreventiontoolkit.html).
Another approach we have been using is to not only screen for all substance use, but also to gather information about the teen’s strengths, activities, and life goals both to enhance rapport and to reference during motivational interviewing as reasons to avoid, reduce, or quit vaping. Motivational interviewing has been shown to help patients make healthier lifestyle choices by nonjudgmentally exploring their pros and cons in a conversation that takes into account readiness to change. This fits well with the stage of developing autonomy when teens want above all to make their own decisions. The cons of using can be discussed as including the effects and side effects of vaping interfering with their favored activities and moving towards their identified goals. Praising abstinence and asking them to show you how they could decline offers to vape are valuable reinforcement you can provide.
Finally, we all know that teens hate being manipulated. Vaping education we provide can make it clear that youth are being tricked by companies – most being large cigarette producers who know the dangers of vaping – into getting addicted so these companies can get rich on their money.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication is as a paid expert to MDedge News. Email her at [email protected].
Asthma guidelines update FeNO, intermittent ICS use
The updated guidelines address six priority topics, including refined recommendations for the use of fractional exhaled nitric oxide (FeNO) testing, intermittent inhaled corticosteroids (ICS), long-acting muscarinic antagonists (LAMA), and bronchial thermoplasty, but notably exclude any recommendations for the use of fast-emerging biological therapy.
“Biological therapy is the major step forward,” said William W. Busse, MD, professor of allergy and immunology at the University of Wisconsin–Madison, and lead author of the previous guidelines (Bethesda, Md.: NHLBI, 2007). “It wasn’t within the scope of work, so it’s not a criticism, but it is an important shortcoming,” he said. The omission identifies the need for the next update. “This is an area that has to be dealt with,” Dr. Busse stated in an interview.
Including biologic agents would have delayed the release of the recommendations for another year or 2, wrote the expert panel working group of the NHLBI, “and this was felt to be unacceptable.” The working group, overseen by the National Asthma Education and Prevention Program Coordinating Committee, also acknowledged the update is “not a complete revision” of the 2007 guidelines.
The update provides an evidenced-based review of six key topics in asthma care, as Mary Cataletto, MD, FCCP, professor of pediatrics at New York University Long Island, Mineola, pointed out: use of FeNO, indoor allergen mitigation, use of intermittent ICS and LAMA for asthma, role of subcutaneous and sublingual immunotherapy in the treatment of allergic asthma, and the use of bronchial thermoplasty.
“It has been 13 years since the last update and substantial progress has been made since then in understanding how to best treat children and adults with asthma,” said working group member Michael Schatz, MD, MS, FCCP, an allergy specialist at Kaiser Permanente Medical Center in San Diego.
According to Dr. Schatz, the most important updated recommendations are:
- Conditional recommendation for the use of ICS in children aged infant to 4 years with recurrent wheezing with respiratory infections.
- Use of combination ICS-formoterol for maintenance and to relieve flares in patients with moderate to severe asthma.
- Addition of the LAMA inhaled bronchodilator as add-on therapy for severe asthma not controlled by long-acting beta-agonist (LABA)/ICS combination medications.
Another important update, Dr. Cataletto said, is “shared decision-making among members of asthma teams in order to improve asthma care across all age groups.”
In all, the update includes 19 recommendations in the six subject areas. Each recommendation is notated with two values: its strength, either strong or conditional, and the certainty of evidence behind it, either low, moderate, or high. For example, the recommendation for ICS in young children that Dr. Schatz referred to has a conditional strength of recommendation with moderate certainty of evidence.
Using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) methodology to determine strength of recommendation is a notable innovation of the latest guidelines, Dr. Busse noted.
Recommendations (strength of recommendation/certainty of evidence) include:
- Use of FeNO in children and adults when the asthma diagnosis is uncertain (conditional/moderate) or in those with allergic asthma and an uncertain course of management (conditional/low).
- Avoid standalone FeNO to evaluate asthma control or the likelihood or severity of future exacerbations, or for in infants to 4-year-olds with recurrent wheezing (strong/low for both).
- Avoid allergen mitigation in routine asthma management for patients who don’t have sensitivity to specific indoor allergens (conditional/low).
- Multicomponent allergen-specific mitigation when specific allergen sensitivity has been identified and pest management alone for symptoms related to specific pest exposure (conditional/low for both).
- Impermeable bedding covers should be part of a multicomponent mitigation strategy, not as a standalone tool, for patients with asthma and dust mite sensitivity (conditional/moderate).
- Daily ICS at onset of a respiratory tract infection along with as-needed short-acting beta-agonists in children aged 4 years and younger with recurrent wheezing but no wheezing between infections rather than as-needed standalone SABA (conditional/high).
- For adults and children aged 12 years and older with mild persistent asthma, either daily low-dose ICS with as-needed SABA or as-needed ICS and SABA concomitantly (conditional/moderate).
- Avoid short-course increased ICS dosing for patients aged 4 years and older with good adherence to daily ICS therapy (conditional/low).
- For patients aged 4 years and older with moderate to severe persistent asthma, a preference for combined ICS-formoterol inhaler over higher dose ICS daily and intermittent SABA or daily ICS-LABA with intermittent SABA (strong/high [aged 12 years and older]; moderate [aged 4-11 years]).
- A preference for combined ICS-formoterol for both daily and relief therapy for patients 12 years and older with severe persistent asthma over higher-dose ICS-LABA daily and intermittent SABA (conditional/high).
- A preference for adding LABA rather than LAMA to ICS in patients aged 12 years and older with uncontrolled persistent asthma (conditional/moderate).
- If LABA isn’t used, add LAMA to ICS in patients aged 12 years and older with uncontrolled persistent asthma rather than continuing the same dose of ICS alone (conditional/moderate).
- In those same patients already on combined ICS-LABA therapy, add LAMA rather than continuing the same dose of ICS-LABA (conditional/moderate).
- Use subcutaneous immunotherapy as a potential adjunct to standard drug therapy in patients aged 5 years and older with mild to moderate allergic asthma when their asthma is controlled on immunotherapy (conditional/moderate).
- Avoid sublingual immunotherapy in patients with persistent allergic asthma (conditional/moderate).
- Avoid bronchial thermoplasty in those 18 years and older with persistent asthma, but consider it in patients who can accept the short-term worsening symptoms or unknown long-term side effects in exchange for the potential benefits (conditional/moderate).
One of the key elements of the guidelines is the use of the SMART (single maintenance and reliever therapy) approach to evaluate the comparative effectiveness of intermittent ICS with formoterol, Dr. Busse noted. “I think that’s a very significant advance. The literature is replete with evidence to support this. Secondly, it really makes life convenient for patients; you have one inhaler.”
The recommendation on SABA use is also significant, Dr. Busse said. “Data have emerged to suggest that if you’re having a need for one of these rescue medications, it’s due to an increase in inflammation in the lower airway, and you want to give an ICS which will act on the inflammation along with the bronchodilator. That’s a new concept, and it’s a very significant step forward.”
Dr. Schatz disclosed financial relationships with Merck, Teva, and ALK-Abello, but was recused from the writing, discussion, and voting related to the immunotherapy recommendation. Dr. Cataletto and Dr. Busse have no relevant relationships to disclose.
SOURCE: Schatz M et al. J Allergy Clin Immunol. 2020;146:1217-70.
The updated guidelines address six priority topics, including refined recommendations for the use of fractional exhaled nitric oxide (FeNO) testing, intermittent inhaled corticosteroids (ICS), long-acting muscarinic antagonists (LAMA), and bronchial thermoplasty, but notably exclude any recommendations for the use of fast-emerging biological therapy.
“Biological therapy is the major step forward,” said William W. Busse, MD, professor of allergy and immunology at the University of Wisconsin–Madison, and lead author of the previous guidelines (Bethesda, Md.: NHLBI, 2007). “It wasn’t within the scope of work, so it’s not a criticism, but it is an important shortcoming,” he said. The omission identifies the need for the next update. “This is an area that has to be dealt with,” Dr. Busse stated in an interview.
Including biologic agents would have delayed the release of the recommendations for another year or 2, wrote the expert panel working group of the NHLBI, “and this was felt to be unacceptable.” The working group, overseen by the National Asthma Education and Prevention Program Coordinating Committee, also acknowledged the update is “not a complete revision” of the 2007 guidelines.
The update provides an evidenced-based review of six key topics in asthma care, as Mary Cataletto, MD, FCCP, professor of pediatrics at New York University Long Island, Mineola, pointed out: use of FeNO, indoor allergen mitigation, use of intermittent ICS and LAMA for asthma, role of subcutaneous and sublingual immunotherapy in the treatment of allergic asthma, and the use of bronchial thermoplasty.
“It has been 13 years since the last update and substantial progress has been made since then in understanding how to best treat children and adults with asthma,” said working group member Michael Schatz, MD, MS, FCCP, an allergy specialist at Kaiser Permanente Medical Center in San Diego.
According to Dr. Schatz, the most important updated recommendations are:
- Conditional recommendation for the use of ICS in children aged infant to 4 years with recurrent wheezing with respiratory infections.
- Use of combination ICS-formoterol for maintenance and to relieve flares in patients with moderate to severe asthma.
- Addition of the LAMA inhaled bronchodilator as add-on therapy for severe asthma not controlled by long-acting beta-agonist (LABA)/ICS combination medications.
Another important update, Dr. Cataletto said, is “shared decision-making among members of asthma teams in order to improve asthma care across all age groups.”
In all, the update includes 19 recommendations in the six subject areas. Each recommendation is notated with two values: its strength, either strong or conditional, and the certainty of evidence behind it, either low, moderate, or high. For example, the recommendation for ICS in young children that Dr. Schatz referred to has a conditional strength of recommendation with moderate certainty of evidence.
Using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) methodology to determine strength of recommendation is a notable innovation of the latest guidelines, Dr. Busse noted.
Recommendations (strength of recommendation/certainty of evidence) include:
- Use of FeNO in children and adults when the asthma diagnosis is uncertain (conditional/moderate) or in those with allergic asthma and an uncertain course of management (conditional/low).
- Avoid standalone FeNO to evaluate asthma control or the likelihood or severity of future exacerbations, or for in infants to 4-year-olds with recurrent wheezing (strong/low for both).
- Avoid allergen mitigation in routine asthma management for patients who don’t have sensitivity to specific indoor allergens (conditional/low).
- Multicomponent allergen-specific mitigation when specific allergen sensitivity has been identified and pest management alone for symptoms related to specific pest exposure (conditional/low for both).
- Impermeable bedding covers should be part of a multicomponent mitigation strategy, not as a standalone tool, for patients with asthma and dust mite sensitivity (conditional/moderate).
- Daily ICS at onset of a respiratory tract infection along with as-needed short-acting beta-agonists in children aged 4 years and younger with recurrent wheezing but no wheezing between infections rather than as-needed standalone SABA (conditional/high).
- For adults and children aged 12 years and older with mild persistent asthma, either daily low-dose ICS with as-needed SABA or as-needed ICS and SABA concomitantly (conditional/moderate).
- Avoid short-course increased ICS dosing for patients aged 4 years and older with good adherence to daily ICS therapy (conditional/low).
- For patients aged 4 years and older with moderate to severe persistent asthma, a preference for combined ICS-formoterol inhaler over higher dose ICS daily and intermittent SABA or daily ICS-LABA with intermittent SABA (strong/high [aged 12 years and older]; moderate [aged 4-11 years]).
- A preference for combined ICS-formoterol for both daily and relief therapy for patients 12 years and older with severe persistent asthma over higher-dose ICS-LABA daily and intermittent SABA (conditional/high).
- A preference for adding LABA rather than LAMA to ICS in patients aged 12 years and older with uncontrolled persistent asthma (conditional/moderate).
- If LABA isn’t used, add LAMA to ICS in patients aged 12 years and older with uncontrolled persistent asthma rather than continuing the same dose of ICS alone (conditional/moderate).
- In those same patients already on combined ICS-LABA therapy, add LAMA rather than continuing the same dose of ICS-LABA (conditional/moderate).
- Use subcutaneous immunotherapy as a potential adjunct to standard drug therapy in patients aged 5 years and older with mild to moderate allergic asthma when their asthma is controlled on immunotherapy (conditional/moderate).
- Avoid sublingual immunotherapy in patients with persistent allergic asthma (conditional/moderate).
- Avoid bronchial thermoplasty in those 18 years and older with persistent asthma, but consider it in patients who can accept the short-term worsening symptoms or unknown long-term side effects in exchange for the potential benefits (conditional/moderate).
One of the key elements of the guidelines is the use of the SMART (single maintenance and reliever therapy) approach to evaluate the comparative effectiveness of intermittent ICS with formoterol, Dr. Busse noted. “I think that’s a very significant advance. The literature is replete with evidence to support this. Secondly, it really makes life convenient for patients; you have one inhaler.”
The recommendation on SABA use is also significant, Dr. Busse said. “Data have emerged to suggest that if you’re having a need for one of these rescue medications, it’s due to an increase in inflammation in the lower airway, and you want to give an ICS which will act on the inflammation along with the bronchodilator. That’s a new concept, and it’s a very significant step forward.”
Dr. Schatz disclosed financial relationships with Merck, Teva, and ALK-Abello, but was recused from the writing, discussion, and voting related to the immunotherapy recommendation. Dr. Cataletto and Dr. Busse have no relevant relationships to disclose.
SOURCE: Schatz M et al. J Allergy Clin Immunol. 2020;146:1217-70.
The updated guidelines address six priority topics, including refined recommendations for the use of fractional exhaled nitric oxide (FeNO) testing, intermittent inhaled corticosteroids (ICS), long-acting muscarinic antagonists (LAMA), and bronchial thermoplasty, but notably exclude any recommendations for the use of fast-emerging biological therapy.
“Biological therapy is the major step forward,” said William W. Busse, MD, professor of allergy and immunology at the University of Wisconsin–Madison, and lead author of the previous guidelines (Bethesda, Md.: NHLBI, 2007). “It wasn’t within the scope of work, so it’s not a criticism, but it is an important shortcoming,” he said. The omission identifies the need for the next update. “This is an area that has to be dealt with,” Dr. Busse stated in an interview.
Including biologic agents would have delayed the release of the recommendations for another year or 2, wrote the expert panel working group of the NHLBI, “and this was felt to be unacceptable.” The working group, overseen by the National Asthma Education and Prevention Program Coordinating Committee, also acknowledged the update is “not a complete revision” of the 2007 guidelines.
The update provides an evidenced-based review of six key topics in asthma care, as Mary Cataletto, MD, FCCP, professor of pediatrics at New York University Long Island, Mineola, pointed out: use of FeNO, indoor allergen mitigation, use of intermittent ICS and LAMA for asthma, role of subcutaneous and sublingual immunotherapy in the treatment of allergic asthma, and the use of bronchial thermoplasty.
“It has been 13 years since the last update and substantial progress has been made since then in understanding how to best treat children and adults with asthma,” said working group member Michael Schatz, MD, MS, FCCP, an allergy specialist at Kaiser Permanente Medical Center in San Diego.
According to Dr. Schatz, the most important updated recommendations are:
- Conditional recommendation for the use of ICS in children aged infant to 4 years with recurrent wheezing with respiratory infections.
- Use of combination ICS-formoterol for maintenance and to relieve flares in patients with moderate to severe asthma.
- Addition of the LAMA inhaled bronchodilator as add-on therapy for severe asthma not controlled by long-acting beta-agonist (LABA)/ICS combination medications.
Another important update, Dr. Cataletto said, is “shared decision-making among members of asthma teams in order to improve asthma care across all age groups.”
In all, the update includes 19 recommendations in the six subject areas. Each recommendation is notated with two values: its strength, either strong or conditional, and the certainty of evidence behind it, either low, moderate, or high. For example, the recommendation for ICS in young children that Dr. Schatz referred to has a conditional strength of recommendation with moderate certainty of evidence.
Using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) methodology to determine strength of recommendation is a notable innovation of the latest guidelines, Dr. Busse noted.
Recommendations (strength of recommendation/certainty of evidence) include:
- Use of FeNO in children and adults when the asthma diagnosis is uncertain (conditional/moderate) or in those with allergic asthma and an uncertain course of management (conditional/low).
- Avoid standalone FeNO to evaluate asthma control or the likelihood or severity of future exacerbations, or for in infants to 4-year-olds with recurrent wheezing (strong/low for both).
- Avoid allergen mitigation in routine asthma management for patients who don’t have sensitivity to specific indoor allergens (conditional/low).
- Multicomponent allergen-specific mitigation when specific allergen sensitivity has been identified and pest management alone for symptoms related to specific pest exposure (conditional/low for both).
- Impermeable bedding covers should be part of a multicomponent mitigation strategy, not as a standalone tool, for patients with asthma and dust mite sensitivity (conditional/moderate).
- Daily ICS at onset of a respiratory tract infection along with as-needed short-acting beta-agonists in children aged 4 years and younger with recurrent wheezing but no wheezing between infections rather than as-needed standalone SABA (conditional/high).
- For adults and children aged 12 years and older with mild persistent asthma, either daily low-dose ICS with as-needed SABA or as-needed ICS and SABA concomitantly (conditional/moderate).
- Avoid short-course increased ICS dosing for patients aged 4 years and older with good adherence to daily ICS therapy (conditional/low).
- For patients aged 4 years and older with moderate to severe persistent asthma, a preference for combined ICS-formoterol inhaler over higher dose ICS daily and intermittent SABA or daily ICS-LABA with intermittent SABA (strong/high [aged 12 years and older]; moderate [aged 4-11 years]).
- A preference for combined ICS-formoterol for both daily and relief therapy for patients 12 years and older with severe persistent asthma over higher-dose ICS-LABA daily and intermittent SABA (conditional/high).
- A preference for adding LABA rather than LAMA to ICS in patients aged 12 years and older with uncontrolled persistent asthma (conditional/moderate).
- If LABA isn’t used, add LAMA to ICS in patients aged 12 years and older with uncontrolled persistent asthma rather than continuing the same dose of ICS alone (conditional/moderate).
- In those same patients already on combined ICS-LABA therapy, add LAMA rather than continuing the same dose of ICS-LABA (conditional/moderate).
- Use subcutaneous immunotherapy as a potential adjunct to standard drug therapy in patients aged 5 years and older with mild to moderate allergic asthma when their asthma is controlled on immunotherapy (conditional/moderate).
- Avoid sublingual immunotherapy in patients with persistent allergic asthma (conditional/moderate).
- Avoid bronchial thermoplasty in those 18 years and older with persistent asthma, but consider it in patients who can accept the short-term worsening symptoms or unknown long-term side effects in exchange for the potential benefits (conditional/moderate).
One of the key elements of the guidelines is the use of the SMART (single maintenance and reliever therapy) approach to evaluate the comparative effectiveness of intermittent ICS with formoterol, Dr. Busse noted. “I think that’s a very significant advance. The literature is replete with evidence to support this. Secondly, it really makes life convenient for patients; you have one inhaler.”
The recommendation on SABA use is also significant, Dr. Busse said. “Data have emerged to suggest that if you’re having a need for one of these rescue medications, it’s due to an increase in inflammation in the lower airway, and you want to give an ICS which will act on the inflammation along with the bronchodilator. That’s a new concept, and it’s a very significant step forward.”
Dr. Schatz disclosed financial relationships with Merck, Teva, and ALK-Abello, but was recused from the writing, discussion, and voting related to the immunotherapy recommendation. Dr. Cataletto and Dr. Busse have no relevant relationships to disclose.
SOURCE: Schatz M et al. J Allergy Clin Immunol. 2020;146:1217-70.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY
COVID-related harm to HCWs must be tracked more rigorously: NAS panel
A panel of scientific experts is urging the nation to do more to track morbidity and mortality among health care workers (HCWs), given the large and disproportionate number who have been infected with or died from SARS-CoV-2.
The National Academies of Sciences, Engineering, and Medicine’s Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats issued a 10-page “rapid expert consultation” on what is known about deaths and mental health problems among HCWs associated with the COVID-19 pandemic and how to protect workers.
“The absence of a uniform national framework and inconsistent requirements across states for collecting, recording, and reporting HCW mortality and morbidity data associated with COVID-19 impairs anyone’s ability to make comparisons, do combined analyses, or draw conclusions about the scale of the problem,” says the panel in the report.
Mental health, in particular, needs to be examined, it says. Although the data are still limited, the prevalence of burnout and suicide “points to a serious concern,” according to the report.
“As with mortality due to COVID-19, there are currently no national systems nor reporting standards for morbidity measures related to the pandemic, such as mental health status, provider well-being, and other psychological effects on HCWs,” the report says.
A more robust national system that collected data on circumstances and interventions that may raise or lower risk, as well as on where the infection occurred, “would support the adoption of effective mitigation strategies,” says the report. It would also facilitate epidemiologic studies on risk factors, such as face-to-face contact with COVID-19 patients and the availability and use of personal protective equipment (PPE). Studies could also examine the impact of institutional requirements for masking.
Studies have consistently shown that universal mask wearing and access to appropriate PPE support the physical safety and mental health of HCWs, says the report.
Track scale of crisis
The committee cited many gaps in the current system. The Occupational Safety and Health Administration, for instance, doesn’t count deaths from occupationally acquired infection. Many states don’t report COVID-19 deaths by profession. The Centers for Disease Control and Prevention (CDC) relies on case report forms from local health departments for all COVID-19 cases, which typically are lacking in specifics, such as occupation or job setting, says the committee’s report.
As of Nov. 3, the CDC had reported 786 deaths among HCWs that were attributable to COVID-19 – a far lower number than other sources have reported.
The committee notes that much could be done immediately. A National Academy of Medicine panel on clinician well-being and resilience in August recommended that the CDC establish a national epidemiologic tracking program to measure HCWs’ well-being, assess the acute and long-term effects of COVID-19 on those workers, and report on the outcomes of interventions.
Such a program “is needed to comprehensively acknowledge the scale of the COVID-19 crisis and protect the health care workforce that is already stretched to the breaking point in many locations,” the committee says in its report.
A version of this article originally appeared on Medscape.com.
A panel of scientific experts is urging the nation to do more to track morbidity and mortality among health care workers (HCWs), given the large and disproportionate number who have been infected with or died from SARS-CoV-2.
The National Academies of Sciences, Engineering, and Medicine’s Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats issued a 10-page “rapid expert consultation” on what is known about deaths and mental health problems among HCWs associated with the COVID-19 pandemic and how to protect workers.
“The absence of a uniform national framework and inconsistent requirements across states for collecting, recording, and reporting HCW mortality and morbidity data associated with COVID-19 impairs anyone’s ability to make comparisons, do combined analyses, or draw conclusions about the scale of the problem,” says the panel in the report.
Mental health, in particular, needs to be examined, it says. Although the data are still limited, the prevalence of burnout and suicide “points to a serious concern,” according to the report.
“As with mortality due to COVID-19, there are currently no national systems nor reporting standards for morbidity measures related to the pandemic, such as mental health status, provider well-being, and other psychological effects on HCWs,” the report says.
A more robust national system that collected data on circumstances and interventions that may raise or lower risk, as well as on where the infection occurred, “would support the adoption of effective mitigation strategies,” says the report. It would also facilitate epidemiologic studies on risk factors, such as face-to-face contact with COVID-19 patients and the availability and use of personal protective equipment (PPE). Studies could also examine the impact of institutional requirements for masking.
Studies have consistently shown that universal mask wearing and access to appropriate PPE support the physical safety and mental health of HCWs, says the report.
Track scale of crisis
The committee cited many gaps in the current system. The Occupational Safety and Health Administration, for instance, doesn’t count deaths from occupationally acquired infection. Many states don’t report COVID-19 deaths by profession. The Centers for Disease Control and Prevention (CDC) relies on case report forms from local health departments for all COVID-19 cases, which typically are lacking in specifics, such as occupation or job setting, says the committee’s report.
As of Nov. 3, the CDC had reported 786 deaths among HCWs that were attributable to COVID-19 – a far lower number than other sources have reported.
The committee notes that much could be done immediately. A National Academy of Medicine panel on clinician well-being and resilience in August recommended that the CDC establish a national epidemiologic tracking program to measure HCWs’ well-being, assess the acute and long-term effects of COVID-19 on those workers, and report on the outcomes of interventions.
Such a program “is needed to comprehensively acknowledge the scale of the COVID-19 crisis and protect the health care workforce that is already stretched to the breaking point in many locations,” the committee says in its report.
A version of this article originally appeared on Medscape.com.
A panel of scientific experts is urging the nation to do more to track morbidity and mortality among health care workers (HCWs), given the large and disproportionate number who have been infected with or died from SARS-CoV-2.
The National Academies of Sciences, Engineering, and Medicine’s Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats issued a 10-page “rapid expert consultation” on what is known about deaths and mental health problems among HCWs associated with the COVID-19 pandemic and how to protect workers.
“The absence of a uniform national framework and inconsistent requirements across states for collecting, recording, and reporting HCW mortality and morbidity data associated with COVID-19 impairs anyone’s ability to make comparisons, do combined analyses, or draw conclusions about the scale of the problem,” says the panel in the report.
Mental health, in particular, needs to be examined, it says. Although the data are still limited, the prevalence of burnout and suicide “points to a serious concern,” according to the report.
“As with mortality due to COVID-19, there are currently no national systems nor reporting standards for morbidity measures related to the pandemic, such as mental health status, provider well-being, and other psychological effects on HCWs,” the report says.
A more robust national system that collected data on circumstances and interventions that may raise or lower risk, as well as on where the infection occurred, “would support the adoption of effective mitigation strategies,” says the report. It would also facilitate epidemiologic studies on risk factors, such as face-to-face contact with COVID-19 patients and the availability and use of personal protective equipment (PPE). Studies could also examine the impact of institutional requirements for masking.
Studies have consistently shown that universal mask wearing and access to appropriate PPE support the physical safety and mental health of HCWs, says the report.
Track scale of crisis
The committee cited many gaps in the current system. The Occupational Safety and Health Administration, for instance, doesn’t count deaths from occupationally acquired infection. Many states don’t report COVID-19 deaths by profession. The Centers for Disease Control and Prevention (CDC) relies on case report forms from local health departments for all COVID-19 cases, which typically are lacking in specifics, such as occupation or job setting, says the committee’s report.
As of Nov. 3, the CDC had reported 786 deaths among HCWs that were attributable to COVID-19 – a far lower number than other sources have reported.
The committee notes that much could be done immediately. A National Academy of Medicine panel on clinician well-being and resilience in August recommended that the CDC establish a national epidemiologic tracking program to measure HCWs’ well-being, assess the acute and long-term effects of COVID-19 on those workers, and report on the outcomes of interventions.
Such a program “is needed to comprehensively acknowledge the scale of the COVID-19 crisis and protect the health care workforce that is already stretched to the breaking point in many locations,” the committee says in its report.
A version of this article originally appeared on Medscape.com.
Coronavirus has infected over 2% of U.S. children
After last week’s ever-so-slightly positive news, the COVID-19 numbers in children have gone back to their old ways.
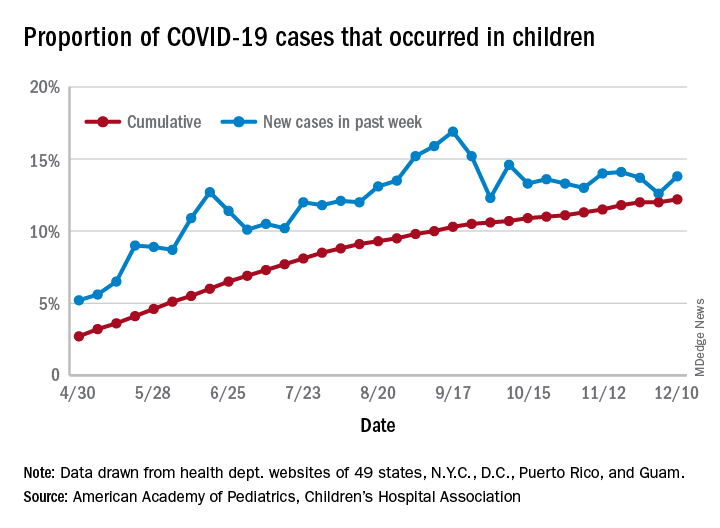
For the week ending Dec. 10, there were 178,823 new COVID-19 cases reported in U.S. children, the highest weekly total yet during the pandemic. The number of new cases had dropped the week before after setting a new high of almost 154,000 during the last full week of November, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
A new weekly high has been seen in 9 of the last 10 weeks, during which time the weekly total of child cases has gone from just over 40,000 (week ending Oct. 8) to almost 179,000, the two organizations said.
and that 2.1% of all children (2,179 per 100,000) in the United States have been infected with the coronavirus, the AAP and CHA said in their weekly report, which includes health department data from 49 states (New York does not report age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative proportion of 12.2% has been exceeded in 27 states, as well as Puerto Rico and Guam, with the highest coming in Wyoming (21.3%), South Carolina (18.1%), and Tennessee (18.1%) and the lowest in Florida (6.7%, but the state uses an age range of 0-14 years) and New Jersey (7.6%), the AAP/CHA data show.
In a separate statement, AAP president Sally Goza, MD, welcomed the approval of the Pfizer-BioNTech COVID-19 vaccine but noted that the “virus is at unprecedented levels in nearly every community in the U.S., and in many areas, our health care system is terribly overburdened. The vaccine will not solve this overnight. I urge everyone to continue to practice social distancing, and wear masks or cloth face coverings, and get a flu shot, so we can protect the people we care about.”
Dr. Goza continued: “We applaud Pfizer-BioNTech for including children ages 12 through 17 in their clinical trials and we look forward to learning more about the data from children aged 12-15. We also want to acknowledge the discussion during the committee meeting on including 16- to 17-year-olds in the EUA [emergency-use authorization]. We believe that discussion underscores the need to keep expanding these trials to the pediatric population so we can collect robust data on this age group.”
[email protected]
After last week’s ever-so-slightly positive news, the COVID-19 numbers in children have gone back to their old ways.

For the week ending Dec. 10, there were 178,823 new COVID-19 cases reported in U.S. children, the highest weekly total yet during the pandemic. The number of new cases had dropped the week before after setting a new high of almost 154,000 during the last full week of November, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
A new weekly high has been seen in 9 of the last 10 weeks, during which time the weekly total of child cases has gone from just over 40,000 (week ending Oct. 8) to almost 179,000, the two organizations said.
and that 2.1% of all children (2,179 per 100,000) in the United States have been infected with the coronavirus, the AAP and CHA said in their weekly report, which includes health department data from 49 states (New York does not report age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative proportion of 12.2% has been exceeded in 27 states, as well as Puerto Rico and Guam, with the highest coming in Wyoming (21.3%), South Carolina (18.1%), and Tennessee (18.1%) and the lowest in Florida (6.7%, but the state uses an age range of 0-14 years) and New Jersey (7.6%), the AAP/CHA data show.
In a separate statement, AAP president Sally Goza, MD, welcomed the approval of the Pfizer-BioNTech COVID-19 vaccine but noted that the “virus is at unprecedented levels in nearly every community in the U.S., and in many areas, our health care system is terribly overburdened. The vaccine will not solve this overnight. I urge everyone to continue to practice social distancing, and wear masks or cloth face coverings, and get a flu shot, so we can protect the people we care about.”
Dr. Goza continued: “We applaud Pfizer-BioNTech for including children ages 12 through 17 in their clinical trials and we look forward to learning more about the data from children aged 12-15. We also want to acknowledge the discussion during the committee meeting on including 16- to 17-year-olds in the EUA [emergency-use authorization]. We believe that discussion underscores the need to keep expanding these trials to the pediatric population so we can collect robust data on this age group.”
[email protected]
After last week’s ever-so-slightly positive news, the COVID-19 numbers in children have gone back to their old ways.

For the week ending Dec. 10, there were 178,823 new COVID-19 cases reported in U.S. children, the highest weekly total yet during the pandemic. The number of new cases had dropped the week before after setting a new high of almost 154,000 during the last full week of November, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
A new weekly high has been seen in 9 of the last 10 weeks, during which time the weekly total of child cases has gone from just over 40,000 (week ending Oct. 8) to almost 179,000, the two organizations said.
and that 2.1% of all children (2,179 per 100,000) in the United States have been infected with the coronavirus, the AAP and CHA said in their weekly report, which includes health department data from 49 states (New York does not report age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative proportion of 12.2% has been exceeded in 27 states, as well as Puerto Rico and Guam, with the highest coming in Wyoming (21.3%), South Carolina (18.1%), and Tennessee (18.1%) and the lowest in Florida (6.7%, but the state uses an age range of 0-14 years) and New Jersey (7.6%), the AAP/CHA data show.
In a separate statement, AAP president Sally Goza, MD, welcomed the approval of the Pfizer-BioNTech COVID-19 vaccine but noted that the “virus is at unprecedented levels in nearly every community in the U.S., and in many areas, our health care system is terribly overburdened. The vaccine will not solve this overnight. I urge everyone to continue to practice social distancing, and wear masks or cloth face coverings, and get a flu shot, so we can protect the people we care about.”
Dr. Goza continued: “We applaud Pfizer-BioNTech for including children ages 12 through 17 in their clinical trials and we look forward to learning more about the data from children aged 12-15. We also want to acknowledge the discussion during the committee meeting on including 16- to 17-year-olds in the EUA [emergency-use authorization]. We believe that discussion underscores the need to keep expanding these trials to the pediatric population so we can collect robust data on this age group.”
[email protected]
Proposed HIPAA overhaul to ease access to patient health info
The Department of Health & Human Services is proposing an overhaul of HIPAA that will make it easier to access patients’ personal health information, including the health records of patients with mental illness. The proposal would also do away with the requirement that all patients sign a notice of privacy practices.
The changes are contained in a 357-page proposed rule, which was unveiled by federal officials Dec. 10. Roger Severino, director of HHS’ Office for Civil Rights, said in a briefing that the sweeping proposal would empower patients, reduce the administrative burden for health care providers, and pave the way to better-coordinated care.
HHS estimated that the rule could save $3.2 billion over 5 years, but it’s not clear how much of that would accrue to clinical practices.
The most obvious cost-saving aspect for medical and dental practices is the proposal that practitioners would no longer have to provide and collect signed notifications of privacy practices.
“This has been a tremendous waste of time and effort and has caused massive confusion,” said Mr. Severino. He said some patients thought they were waiving privacy rights and that, in some cases, physicians refused to administer care unless patients signed the notices. “That was never the intent.”
Requiring that patients sign the form and that practices keep copies for 6 years is an “unnecessary burden,” said Mr. Severino. “We’ve lost whole forests from this regulation.”
Under the new proposal, health care providers would merely have to let patients know where to find their privacy policies.
Sharing mental health info
The rule would also ease the standard for sharing information about a patient who is in a mental health crisis, such as an exacerbation of a serious mental illness or a crisis related to a substance use disorder, including an overdose.
Currently, clinicians can choose to disclose protected health information – to a family member, a caregiver, a law enforcement official, a doctor, or an insurer – if they believe that doing so is advisable in their “professional judgment.” The rule proposes to ease that to a “good faith” belief that a disclosure would be in the best interest of the patient. In both instances, the patient can still object and block the disclosure.
As an example, HHS said that, in the case of a young adult who had experienced an overdose of opioids, a licensed health care professional could make the determination to “disclose relevant information to a parent who is involved in the patient’s treatment and who the young adult would expect, based on their relationship, to participate in or be involved with the patient’s recovery from the overdose.”
HHS is also proposing to let clinicians disclose information in cases in which an individual might be a threat to himself or others, provided the harm is “serious and reasonably foreseeable.”
Currently, information can only be disclosed if it appears there is a “serious and imminent” threat to health or safety. If an individual experienced suicidal ideation, for instance, a health care professional could notify family that the individual is at risk.
Fast, no-cost access
The rule also aims to make it easier for patients to get access to their own health care information quickly – within 15 days of a request – instead of the 30 days currently allowed, and sometimes at no cost.
The 30-day time frame is “a relic of a pre-Internet age that should be dispensed with,” said Mr. Severino.
Patients can also request that a treating physician get his or her records from a clinician who had previously treated the individual. The request would be fulfilled within 15 days, although extensions might be possible.
“That takes away the burden of coordination from the patient and puts it on those parties that are responsible for the actual provision of care and that are better positioned to do that coordination,” Mr. Severino said.
Health care professionals will also have to share with patients a fee schedule for records requests. However, if records are shared through a patient portal with view, download, and transmit capabilities, the provider can’t charge the patient for the time it took to upload the information into the system.
“We do not believe a patient’s personal medical record should be profit centers for providers,” Mr. Severino said.
Patients will be allowed to take photos with a smartphone of personal health information – such as an x-ray or sonogram – while receiving care.
The rule is open for public comment until mid-February. After that, it will become final in 180 days. The agency said it would not begin enforcement until 240 days after the final rule was published.
A version of this article originally appeared on Medscape.com.
The Department of Health & Human Services is proposing an overhaul of HIPAA that will make it easier to access patients’ personal health information, including the health records of patients with mental illness. The proposal would also do away with the requirement that all patients sign a notice of privacy practices.
The changes are contained in a 357-page proposed rule, which was unveiled by federal officials Dec. 10. Roger Severino, director of HHS’ Office for Civil Rights, said in a briefing that the sweeping proposal would empower patients, reduce the administrative burden for health care providers, and pave the way to better-coordinated care.
HHS estimated that the rule could save $3.2 billion over 5 years, but it’s not clear how much of that would accrue to clinical practices.
The most obvious cost-saving aspect for medical and dental practices is the proposal that practitioners would no longer have to provide and collect signed notifications of privacy practices.
“This has been a tremendous waste of time and effort and has caused massive confusion,” said Mr. Severino. He said some patients thought they were waiving privacy rights and that, in some cases, physicians refused to administer care unless patients signed the notices. “That was never the intent.”
Requiring that patients sign the form and that practices keep copies for 6 years is an “unnecessary burden,” said Mr. Severino. “We’ve lost whole forests from this regulation.”
Under the new proposal, health care providers would merely have to let patients know where to find their privacy policies.
Sharing mental health info
The rule would also ease the standard for sharing information about a patient who is in a mental health crisis, such as an exacerbation of a serious mental illness or a crisis related to a substance use disorder, including an overdose.
Currently, clinicians can choose to disclose protected health information – to a family member, a caregiver, a law enforcement official, a doctor, or an insurer – if they believe that doing so is advisable in their “professional judgment.” The rule proposes to ease that to a “good faith” belief that a disclosure would be in the best interest of the patient. In both instances, the patient can still object and block the disclosure.
As an example, HHS said that, in the case of a young adult who had experienced an overdose of opioids, a licensed health care professional could make the determination to “disclose relevant information to a parent who is involved in the patient’s treatment and who the young adult would expect, based on their relationship, to participate in or be involved with the patient’s recovery from the overdose.”
HHS is also proposing to let clinicians disclose information in cases in which an individual might be a threat to himself or others, provided the harm is “serious and reasonably foreseeable.”
Currently, information can only be disclosed if it appears there is a “serious and imminent” threat to health or safety. If an individual experienced suicidal ideation, for instance, a health care professional could notify family that the individual is at risk.
Fast, no-cost access
The rule also aims to make it easier for patients to get access to their own health care information quickly – within 15 days of a request – instead of the 30 days currently allowed, and sometimes at no cost.
The 30-day time frame is “a relic of a pre-Internet age that should be dispensed with,” said Mr. Severino.
Patients can also request that a treating physician get his or her records from a clinician who had previously treated the individual. The request would be fulfilled within 15 days, although extensions might be possible.
“That takes away the burden of coordination from the patient and puts it on those parties that are responsible for the actual provision of care and that are better positioned to do that coordination,” Mr. Severino said.
Health care professionals will also have to share with patients a fee schedule for records requests. However, if records are shared through a patient portal with view, download, and transmit capabilities, the provider can’t charge the patient for the time it took to upload the information into the system.
“We do not believe a patient’s personal medical record should be profit centers for providers,” Mr. Severino said.
Patients will be allowed to take photos with a smartphone of personal health information – such as an x-ray or sonogram – while receiving care.
The rule is open for public comment until mid-February. After that, it will become final in 180 days. The agency said it would not begin enforcement until 240 days after the final rule was published.
A version of this article originally appeared on Medscape.com.
The Department of Health & Human Services is proposing an overhaul of HIPAA that will make it easier to access patients’ personal health information, including the health records of patients with mental illness. The proposal would also do away with the requirement that all patients sign a notice of privacy practices.
The changes are contained in a 357-page proposed rule, which was unveiled by federal officials Dec. 10. Roger Severino, director of HHS’ Office for Civil Rights, said in a briefing that the sweeping proposal would empower patients, reduce the administrative burden for health care providers, and pave the way to better-coordinated care.
HHS estimated that the rule could save $3.2 billion over 5 years, but it’s not clear how much of that would accrue to clinical practices.
The most obvious cost-saving aspect for medical and dental practices is the proposal that practitioners would no longer have to provide and collect signed notifications of privacy practices.
“This has been a tremendous waste of time and effort and has caused massive confusion,” said Mr. Severino. He said some patients thought they were waiving privacy rights and that, in some cases, physicians refused to administer care unless patients signed the notices. “That was never the intent.”
Requiring that patients sign the form and that practices keep copies for 6 years is an “unnecessary burden,” said Mr. Severino. “We’ve lost whole forests from this regulation.”
Under the new proposal, health care providers would merely have to let patients know where to find their privacy policies.
Sharing mental health info
The rule would also ease the standard for sharing information about a patient who is in a mental health crisis, such as an exacerbation of a serious mental illness or a crisis related to a substance use disorder, including an overdose.
Currently, clinicians can choose to disclose protected health information – to a family member, a caregiver, a law enforcement official, a doctor, or an insurer – if they believe that doing so is advisable in their “professional judgment.” The rule proposes to ease that to a “good faith” belief that a disclosure would be in the best interest of the patient. In both instances, the patient can still object and block the disclosure.
As an example, HHS said that, in the case of a young adult who had experienced an overdose of opioids, a licensed health care professional could make the determination to “disclose relevant information to a parent who is involved in the patient’s treatment and who the young adult would expect, based on their relationship, to participate in or be involved with the patient’s recovery from the overdose.”
HHS is also proposing to let clinicians disclose information in cases in which an individual might be a threat to himself or others, provided the harm is “serious and reasonably foreseeable.”
Currently, information can only be disclosed if it appears there is a “serious and imminent” threat to health or safety. If an individual experienced suicidal ideation, for instance, a health care professional could notify family that the individual is at risk.
Fast, no-cost access
The rule also aims to make it easier for patients to get access to their own health care information quickly – within 15 days of a request – instead of the 30 days currently allowed, and sometimes at no cost.
The 30-day time frame is “a relic of a pre-Internet age that should be dispensed with,” said Mr. Severino.
Patients can also request that a treating physician get his or her records from a clinician who had previously treated the individual. The request would be fulfilled within 15 days, although extensions might be possible.
“That takes away the burden of coordination from the patient and puts it on those parties that are responsible for the actual provision of care and that are better positioned to do that coordination,” Mr. Severino said.
Health care professionals will also have to share with patients a fee schedule for records requests. However, if records are shared through a patient portal with view, download, and transmit capabilities, the provider can’t charge the patient for the time it took to upload the information into the system.
“We do not believe a patient’s personal medical record should be profit centers for providers,” Mr. Severino said.
Patients will be allowed to take photos with a smartphone of personal health information – such as an x-ray or sonogram – while receiving care.
The rule is open for public comment until mid-February. After that, it will become final in 180 days. The agency said it would not begin enforcement until 240 days after the final rule was published.
A version of this article originally appeared on Medscape.com.
A girl presents with blotchy, slightly itchy spots on her chest, back
On close evaluation of the picture on her chest, she has pale macules and patches surrounded by erythematous ill-defined patches consistent with nevus anemicus. The findings of the picture raise the suspicion for neurofibromatosis, and it was recommended for her to be evaluated in person.
She comes several days later to the clinic. The caretaker, who is her aunt, reports she does not know much of the girl’s medical history as she recently moved from South America to live with her. The girl is a very nice and pleasant 8-year-old. She reports noticing the spots on her chest for about a year and that they seem to get a little itchier and more noticeable when she is hot or when she is running. She also reports increasing headaches for several months. She is being home schooled, and according to her aunt she is at par with her cousins who are about the same age. There is no history of seizures. She has had back surgery in the past. There is no history of hypertension. There is no family history of any genetic disorder or similar lesions.
On physical exam, her vital signs are normal, but her head circumference is over the 90th percentile. She is pleasant and interactive. On skin examination, she has slightly noticeable pale macules and patches on the chest and back that become more apparent after rubbing her skin. She has multiple light brown macules and oval patches on the chest, back, and neck. She has no axillary or inguinal freckling. She has scars on the back from her prior surgery.
As she was having worsening headaches, an MRI of the brain was ordered, which showed a left optic glioma. She was then referred to ophthalmology, neurology, and genetics.
Neurofibromatosis type 1 (NF1) is a common genetic autosomal dominant disorder cause by mutations on the NF1 gene on chromosome 17, which encodes for the protein neurofibromin. This protein works in the Ras-mitogen–activated protein kinase pathway as a negative regulator. Based on the National Institute of Health criteria, children need two or more of the following to be diagnosed with NF1: more than six café au lait macules larger than 5 mm in prepubescent children and 2.5 cm after puberty; axillary or inguinal freckling; two or more Lisch nodules; optic gliomas; two or more neurofibromas or one plexiform neurofibroma; or a first degree relative with a diagnosis of NF1. With these criteria, about 70% of the children can be diagnosed before the age of 1 year.1
Nevus anemicus is an uncommon birthmark, sometimes overlooked, that is characterized by pale, hypopigmented, well-defined macules and patches that do not turn red after trauma or changes in temperature. Nevus anemicus is usually localized on the torso but can be seen on the face, neck, and extremities. These lesions are present in 1%-2% of the general population. They are thought to occur because of increased sensitivity of the affected blood vessels to catecholamines, which causes permanent vasoconstriction, which leads to hypopigmentation on the area.2 These lesions are usually present at birth and have been described in patients with tuberous sclerosis, neurofibromatosis, and phakomatosis pigmentovascularis.
Recent studies of patients with neurofibromatosis and other RASopathies have noticed that nevus anemicus is present in about 8.8%-51% of the patients studied with a diagnosis NF1, compared with only 2% of the controls.3,4 The studies failed to report any cases of nevus anemicus in patients with other RASopathies associated with café au lait macules. Bulteel and colleagues recently reported two cases of non-NF1 RASopathies also associated with nevus anemicus in a patient with Legius syndrome and a patient with Noonan syndrome with multiple lentigines.5 The nevus anemicus was reported to occur most commonly on the anterior chest and be multiple, as seen in our patient.
The authors of the published studies advocate for the introduction of nevus anemicus as part of the diagnostic criteria for NF1, especially because it can be an early finding seen in babies, which can aid in early diagnosis of NF1.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She has no relevant financial disclosures. Email Dr. Matiz at [email protected].
References
1. Pediatrics. 2000 Mar. doi: 10.1542/peds.105.3.608.
2. Nevus Anemicus. StatPearls [Internet] (Treasure Island, Fla.: StatPearls Publishing; 2020 Jan).
3. J Am Acad Dermatol. 2013 Nov. doi: 10.1016/j.jaad.2013.06.039.
4. Pediatr Dermatol. 2015 May-Jun. doi: 10.1111/pde.12525.
5. JAAD Case Rep. 2018 Apr 5. doi: 10.1016/j.jdcr.2017.09.037.
On close evaluation of the picture on her chest, she has pale macules and patches surrounded by erythematous ill-defined patches consistent with nevus anemicus. The findings of the picture raise the suspicion for neurofibromatosis, and it was recommended for her to be evaluated in person.
She comes several days later to the clinic. The caretaker, who is her aunt, reports she does not know much of the girl’s medical history as she recently moved from South America to live with her. The girl is a very nice and pleasant 8-year-old. She reports noticing the spots on her chest for about a year and that they seem to get a little itchier and more noticeable when she is hot or when she is running. She also reports increasing headaches for several months. She is being home schooled, and according to her aunt she is at par with her cousins who are about the same age. There is no history of seizures. She has had back surgery in the past. There is no history of hypertension. There is no family history of any genetic disorder or similar lesions.
On physical exam, her vital signs are normal, but her head circumference is over the 90th percentile. She is pleasant and interactive. On skin examination, she has slightly noticeable pale macules and patches on the chest and back that become more apparent after rubbing her skin. She has multiple light brown macules and oval patches on the chest, back, and neck. She has no axillary or inguinal freckling. She has scars on the back from her prior surgery.
As she was having worsening headaches, an MRI of the brain was ordered, which showed a left optic glioma. She was then referred to ophthalmology, neurology, and genetics.
Neurofibromatosis type 1 (NF1) is a common genetic autosomal dominant disorder cause by mutations on the NF1 gene on chromosome 17, which encodes for the protein neurofibromin. This protein works in the Ras-mitogen–activated protein kinase pathway as a negative regulator. Based on the National Institute of Health criteria, children need two or more of the following to be diagnosed with NF1: more than six café au lait macules larger than 5 mm in prepubescent children and 2.5 cm after puberty; axillary or inguinal freckling; two or more Lisch nodules; optic gliomas; two or more neurofibromas or one plexiform neurofibroma; or a first degree relative with a diagnosis of NF1. With these criteria, about 70% of the children can be diagnosed before the age of 1 year.1
Nevus anemicus is an uncommon birthmark, sometimes overlooked, that is characterized by pale, hypopigmented, well-defined macules and patches that do not turn red after trauma or changes in temperature. Nevus anemicus is usually localized on the torso but can be seen on the face, neck, and extremities. These lesions are present in 1%-2% of the general population. They are thought to occur because of increased sensitivity of the affected blood vessels to catecholamines, which causes permanent vasoconstriction, which leads to hypopigmentation on the area.2 These lesions are usually present at birth and have been described in patients with tuberous sclerosis, neurofibromatosis, and phakomatosis pigmentovascularis.
Recent studies of patients with neurofibromatosis and other RASopathies have noticed that nevus anemicus is present in about 8.8%-51% of the patients studied with a diagnosis NF1, compared with only 2% of the controls.3,4 The studies failed to report any cases of nevus anemicus in patients with other RASopathies associated with café au lait macules. Bulteel and colleagues recently reported two cases of non-NF1 RASopathies also associated with nevus anemicus in a patient with Legius syndrome and a patient with Noonan syndrome with multiple lentigines.5 The nevus anemicus was reported to occur most commonly on the anterior chest and be multiple, as seen in our patient.
The authors of the published studies advocate for the introduction of nevus anemicus as part of the diagnostic criteria for NF1, especially because it can be an early finding seen in babies, which can aid in early diagnosis of NF1.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She has no relevant financial disclosures. Email Dr. Matiz at [email protected].
References
1. Pediatrics. 2000 Mar. doi: 10.1542/peds.105.3.608.
2. Nevus Anemicus. StatPearls [Internet] (Treasure Island, Fla.: StatPearls Publishing; 2020 Jan).
3. J Am Acad Dermatol. 2013 Nov. doi: 10.1016/j.jaad.2013.06.039.
4. Pediatr Dermatol. 2015 May-Jun. doi: 10.1111/pde.12525.
5. JAAD Case Rep. 2018 Apr 5. doi: 10.1016/j.jdcr.2017.09.037.
On close evaluation of the picture on her chest, she has pale macules and patches surrounded by erythematous ill-defined patches consistent with nevus anemicus. The findings of the picture raise the suspicion for neurofibromatosis, and it was recommended for her to be evaluated in person.
She comes several days later to the clinic. The caretaker, who is her aunt, reports she does not know much of the girl’s medical history as she recently moved from South America to live with her. The girl is a very nice and pleasant 8-year-old. She reports noticing the spots on her chest for about a year and that they seem to get a little itchier and more noticeable when she is hot or when she is running. She also reports increasing headaches for several months. She is being home schooled, and according to her aunt she is at par with her cousins who are about the same age. There is no history of seizures. She has had back surgery in the past. There is no history of hypertension. There is no family history of any genetic disorder or similar lesions.
On physical exam, her vital signs are normal, but her head circumference is over the 90th percentile. She is pleasant and interactive. On skin examination, she has slightly noticeable pale macules and patches on the chest and back that become more apparent after rubbing her skin. She has multiple light brown macules and oval patches on the chest, back, and neck. She has no axillary or inguinal freckling. She has scars on the back from her prior surgery.
As she was having worsening headaches, an MRI of the brain was ordered, which showed a left optic glioma. She was then referred to ophthalmology, neurology, and genetics.
Neurofibromatosis type 1 (NF1) is a common genetic autosomal dominant disorder cause by mutations on the NF1 gene on chromosome 17, which encodes for the protein neurofibromin. This protein works in the Ras-mitogen–activated protein kinase pathway as a negative regulator. Based on the National Institute of Health criteria, children need two or more of the following to be diagnosed with NF1: more than six café au lait macules larger than 5 mm in prepubescent children and 2.5 cm after puberty; axillary or inguinal freckling; two or more Lisch nodules; optic gliomas; two or more neurofibromas or one plexiform neurofibroma; or a first degree relative with a diagnosis of NF1. With these criteria, about 70% of the children can be diagnosed before the age of 1 year.1
Nevus anemicus is an uncommon birthmark, sometimes overlooked, that is characterized by pale, hypopigmented, well-defined macules and patches that do not turn red after trauma or changes in temperature. Nevus anemicus is usually localized on the torso but can be seen on the face, neck, and extremities. These lesions are present in 1%-2% of the general population. They are thought to occur because of increased sensitivity of the affected blood vessels to catecholamines, which causes permanent vasoconstriction, which leads to hypopigmentation on the area.2 These lesions are usually present at birth and have been described in patients with tuberous sclerosis, neurofibromatosis, and phakomatosis pigmentovascularis.
Recent studies of patients with neurofibromatosis and other RASopathies have noticed that nevus anemicus is present in about 8.8%-51% of the patients studied with a diagnosis NF1, compared with only 2% of the controls.3,4 The studies failed to report any cases of nevus anemicus in patients with other RASopathies associated with café au lait macules. Bulteel and colleagues recently reported two cases of non-NF1 RASopathies also associated with nevus anemicus in a patient with Legius syndrome and a patient with Noonan syndrome with multiple lentigines.5 The nevus anemicus was reported to occur most commonly on the anterior chest and be multiple, as seen in our patient.
The authors of the published studies advocate for the introduction of nevus anemicus as part of the diagnostic criteria for NF1, especially because it can be an early finding seen in babies, which can aid in early diagnosis of NF1.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She has no relevant financial disclosures. Email Dr. Matiz at [email protected].
References
1. Pediatrics. 2000 Mar. doi: 10.1542/peds.105.3.608.
2. Nevus Anemicus. StatPearls [Internet] (Treasure Island, Fla.: StatPearls Publishing; 2020 Jan).
3. J Am Acad Dermatol. 2013 Nov. doi: 10.1016/j.jaad.2013.06.039.
4. Pediatr Dermatol. 2015 May-Jun. doi: 10.1111/pde.12525.
5. JAAD Case Rep. 2018 Apr 5. doi: 10.1016/j.jdcr.2017.09.037.