User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Should we use antibiotics to treat sore throats?
The use of antibiotics to treat a sore throat remains contentious, with guidelines from around the world providing contradictory advice. This topic generated a lively debate at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
Lauri Ivaska, MD, of the department of pediatrics and adolescent medicine at Turku (Finland) University Hospital, argued for the use of antibiotics, while Borbála Zsigmond, MD, of Heim Pál Children’s Hospital in Budapest, made the case against their use. Interestingly, this debate occurred against the background of a poll conducted before the debate, which found that only 11% of the audience voted in favor of using antibiotics to treat sore throats.
Both speakers began by exploring their approach to the treatment of a recent clinical case involving a 4-year-old girl presenting with sore throat. Dr. Ivaska stressed the difference between a sore throat, pharyngitis, and tonsillitis: the latter two refer to a physical finding, while the former is a subjective symptom.
International guidelines differ on the subject
The debate moved to discussing the international guidelines for treating pharyngitis and tonsillitis. Dr. Zsigmond believes that these are flawed and unhelpful, arguing that they differ depending on what part of the world a physician is practicing in. For example, the 2012 Infectious Diseases Society of America guidelines recommend using best clinical judgment and then backing this up by testing. If testing proves positive for group A Streptococcus pyogenes (GAS), the physician should universally treat. By comparison, the European Society of Clinical Microbiology and Infectious Diseases Sore Throat Guideline Group focuses on severity rather than the cause of the infection. If the case is deemed to be serious, antibiotics can be prescribed without a positive test.
Sore throat is frequently associated with a common cold. In a recent study, more that 80% of students with an acute viral respiratory tract infection had soreness at the beginning of their illness.
Reporting from his own research, Dr. Ivaska argued that viruses can be detected in almost two-thirds of children with pharyngitis using polymerase chain reaction analysis. He thinks antibiotics should be reserved for those 30%-40% of patients with a confirmed GAS infection. The potential role of Fusobacterium necrophorum was raised, but there is no evidence of the benefits of antibiotic treatment in such cases.
There are diagnostic aids for GAS infection
It was suggested that, instead of concentrating on sore throat, the debate should be about whether to use antibiotics to treat GAS infection. But how can the diagnosis be confirmed simply in a clinical setting? Dr. Ivaska recommended adopting diagnostic aids such as Centor, McIsaac, and FeverPAIN, which award scores for several common disease features – the higher the score, the more likely a patient is to be suffering from a GAS infection.
Dr. Zsigmond also likes scoring symptoms but believes they are often inaccurate, especially in young children. She pointed to a report that examined the use of the Centor tool among 441 children attending a pediatric ED. The authors concluded that the Centor criteria were ineffective in predicting a positive GAS culture in throat swabs taken from symptomatic patients.
When are antibiotics warranted?
It is widely accepted that antibiotics should be avoided for viral infections. Returning to the case described at the start of this debate, Dr. Zsigmond calculated that her patient with a 2-day history of sore throat, elevated temperature, pussy tonsils, and enlarged cervical lymph glands but no cough or rhinitis had a FeverPAIN score of 4-5 and a Centor score of 4, meaning that, according to the European guidelines, she should receive antibiotic treatment. However, viral swabs proved positive for adenovirus.
Dr. Ivaska responded with his recent experiences of a similar case, where a 5-year-old boy had a FeverPAIN score of 4-5 and Centor score of 3. Cultures from his throat were GAS positive, illustrating the problem of differentiating between bacterial and viral infections.
But does a GAS-positive pharyngeal culture necessarily mean that antibiotic treatment is indicated? Dr. Ivaska believes it does, citing the importance of preventing serious complications such as rheumatic fever. Dr. Zsigmind countered by pointing out the low levels of acute rheumatic fever in developed nations. In her own country, Hungary, there has not been a case in the last 30 years. Giving antibiotics for historical reasons cannot, in her view, be justified.
Dr. Ivaska responded that perhaps this is because of early treatment in children with sore throats.
Another complication of tonsillitis is quinsy. Dr. Zsigmond cited a study showing that there is no statistically significant evidence demonstrating that antibiotics prevent quinsy. She attributed this to quinsy appearing quickly, typically within 2 days. Delay in seeking help means that the window to treat is often missed. However, should symptoms present early, there is no statistical evidence that prior antibiotic use can prevent quinsy. Also, given the rarity of this condition, prevention would mean excessive use of antibiotics.
Are there other possible benefits of antibiotic treatment in patients with a sore throat? Dr. Ivaska referred to a Cochrane review that found a shortening in duration of throat soreness and fever. Furthermore, compared with placebo, antibiotics reduced the incidence of suppurative complications such as acute otitis media and sinusitis following a sore throat. Other studies have also pointed to the potential benefits of reduced transmission in families where one member with pharyngitis was GAS positive.
As the debate ended, Dr. Zsigmond reported evidence of global antibiotic overprescribing for sore throat ranging from 53% in Europe to 94% in Australia. She also highlighted risks such as altered gut flora, drug resistance, and rashes.
Robin Marlow from the University of Bristol (England), PhD, MBBS, commented that “one of the most enjoyable parts of the ESPID meeting is hearing different viewpoints rationally explained from across the world. As [antibiotic prescription for a sore throat is] a clinical conundrum that faces pediatricians every day, I thought this debate was a really great example of how, despite our different health care systems and ways of working, we are all striving together to improve children’s health using the best evidence available.”
The presenters had no financial conflicts of interest to declare.
The use of antibiotics to treat a sore throat remains contentious, with guidelines from around the world providing contradictory advice. This topic generated a lively debate at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
Lauri Ivaska, MD, of the department of pediatrics and adolescent medicine at Turku (Finland) University Hospital, argued for the use of antibiotics, while Borbála Zsigmond, MD, of Heim Pál Children’s Hospital in Budapest, made the case against their use. Interestingly, this debate occurred against the background of a poll conducted before the debate, which found that only 11% of the audience voted in favor of using antibiotics to treat sore throats.
Both speakers began by exploring their approach to the treatment of a recent clinical case involving a 4-year-old girl presenting with sore throat. Dr. Ivaska stressed the difference between a sore throat, pharyngitis, and tonsillitis: the latter two refer to a physical finding, while the former is a subjective symptom.
International guidelines differ on the subject
The debate moved to discussing the international guidelines for treating pharyngitis and tonsillitis. Dr. Zsigmond believes that these are flawed and unhelpful, arguing that they differ depending on what part of the world a physician is practicing in. For example, the 2012 Infectious Diseases Society of America guidelines recommend using best clinical judgment and then backing this up by testing. If testing proves positive for group A Streptococcus pyogenes (GAS), the physician should universally treat. By comparison, the European Society of Clinical Microbiology and Infectious Diseases Sore Throat Guideline Group focuses on severity rather than the cause of the infection. If the case is deemed to be serious, antibiotics can be prescribed without a positive test.
Sore throat is frequently associated with a common cold. In a recent study, more that 80% of students with an acute viral respiratory tract infection had soreness at the beginning of their illness.
Reporting from his own research, Dr. Ivaska argued that viruses can be detected in almost two-thirds of children with pharyngitis using polymerase chain reaction analysis. He thinks antibiotics should be reserved for those 30%-40% of patients with a confirmed GAS infection. The potential role of Fusobacterium necrophorum was raised, but there is no evidence of the benefits of antibiotic treatment in such cases.
There are diagnostic aids for GAS infection
It was suggested that, instead of concentrating on sore throat, the debate should be about whether to use antibiotics to treat GAS infection. But how can the diagnosis be confirmed simply in a clinical setting? Dr. Ivaska recommended adopting diagnostic aids such as Centor, McIsaac, and FeverPAIN, which award scores for several common disease features – the higher the score, the more likely a patient is to be suffering from a GAS infection.
Dr. Zsigmond also likes scoring symptoms but believes they are often inaccurate, especially in young children. She pointed to a report that examined the use of the Centor tool among 441 children attending a pediatric ED. The authors concluded that the Centor criteria were ineffective in predicting a positive GAS culture in throat swabs taken from symptomatic patients.
When are antibiotics warranted?
It is widely accepted that antibiotics should be avoided for viral infections. Returning to the case described at the start of this debate, Dr. Zsigmond calculated that her patient with a 2-day history of sore throat, elevated temperature, pussy tonsils, and enlarged cervical lymph glands but no cough or rhinitis had a FeverPAIN score of 4-5 and a Centor score of 4, meaning that, according to the European guidelines, she should receive antibiotic treatment. However, viral swabs proved positive for adenovirus.
Dr. Ivaska responded with his recent experiences of a similar case, where a 5-year-old boy had a FeverPAIN score of 4-5 and Centor score of 3. Cultures from his throat were GAS positive, illustrating the problem of differentiating between bacterial and viral infections.
But does a GAS-positive pharyngeal culture necessarily mean that antibiotic treatment is indicated? Dr. Ivaska believes it does, citing the importance of preventing serious complications such as rheumatic fever. Dr. Zsigmind countered by pointing out the low levels of acute rheumatic fever in developed nations. In her own country, Hungary, there has not been a case in the last 30 years. Giving antibiotics for historical reasons cannot, in her view, be justified.
Dr. Ivaska responded that perhaps this is because of early treatment in children with sore throats.
Another complication of tonsillitis is quinsy. Dr. Zsigmond cited a study showing that there is no statistically significant evidence demonstrating that antibiotics prevent quinsy. She attributed this to quinsy appearing quickly, typically within 2 days. Delay in seeking help means that the window to treat is often missed. However, should symptoms present early, there is no statistical evidence that prior antibiotic use can prevent quinsy. Also, given the rarity of this condition, prevention would mean excessive use of antibiotics.
Are there other possible benefits of antibiotic treatment in patients with a sore throat? Dr. Ivaska referred to a Cochrane review that found a shortening in duration of throat soreness and fever. Furthermore, compared with placebo, antibiotics reduced the incidence of suppurative complications such as acute otitis media and sinusitis following a sore throat. Other studies have also pointed to the potential benefits of reduced transmission in families where one member with pharyngitis was GAS positive.
As the debate ended, Dr. Zsigmond reported evidence of global antibiotic overprescribing for sore throat ranging from 53% in Europe to 94% in Australia. She also highlighted risks such as altered gut flora, drug resistance, and rashes.
Robin Marlow from the University of Bristol (England), PhD, MBBS, commented that “one of the most enjoyable parts of the ESPID meeting is hearing different viewpoints rationally explained from across the world. As [antibiotic prescription for a sore throat is] a clinical conundrum that faces pediatricians every day, I thought this debate was a really great example of how, despite our different health care systems and ways of working, we are all striving together to improve children’s health using the best evidence available.”
The presenters had no financial conflicts of interest to declare.
The use of antibiotics to treat a sore throat remains contentious, with guidelines from around the world providing contradictory advice. This topic generated a lively debate at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
Lauri Ivaska, MD, of the department of pediatrics and adolescent medicine at Turku (Finland) University Hospital, argued for the use of antibiotics, while Borbála Zsigmond, MD, of Heim Pál Children’s Hospital in Budapest, made the case against their use. Interestingly, this debate occurred against the background of a poll conducted before the debate, which found that only 11% of the audience voted in favor of using antibiotics to treat sore throats.
Both speakers began by exploring their approach to the treatment of a recent clinical case involving a 4-year-old girl presenting with sore throat. Dr. Ivaska stressed the difference between a sore throat, pharyngitis, and tonsillitis: the latter two refer to a physical finding, while the former is a subjective symptom.
International guidelines differ on the subject
The debate moved to discussing the international guidelines for treating pharyngitis and tonsillitis. Dr. Zsigmond believes that these are flawed and unhelpful, arguing that they differ depending on what part of the world a physician is practicing in. For example, the 2012 Infectious Diseases Society of America guidelines recommend using best clinical judgment and then backing this up by testing. If testing proves positive for group A Streptococcus pyogenes (GAS), the physician should universally treat. By comparison, the European Society of Clinical Microbiology and Infectious Diseases Sore Throat Guideline Group focuses on severity rather than the cause of the infection. If the case is deemed to be serious, antibiotics can be prescribed without a positive test.
Sore throat is frequently associated with a common cold. In a recent study, more that 80% of students with an acute viral respiratory tract infection had soreness at the beginning of their illness.
Reporting from his own research, Dr. Ivaska argued that viruses can be detected in almost two-thirds of children with pharyngitis using polymerase chain reaction analysis. He thinks antibiotics should be reserved for those 30%-40% of patients with a confirmed GAS infection. The potential role of Fusobacterium necrophorum was raised, but there is no evidence of the benefits of antibiotic treatment in such cases.
There are diagnostic aids for GAS infection
It was suggested that, instead of concentrating on sore throat, the debate should be about whether to use antibiotics to treat GAS infection. But how can the diagnosis be confirmed simply in a clinical setting? Dr. Ivaska recommended adopting diagnostic aids such as Centor, McIsaac, and FeverPAIN, which award scores for several common disease features – the higher the score, the more likely a patient is to be suffering from a GAS infection.
Dr. Zsigmond also likes scoring symptoms but believes they are often inaccurate, especially in young children. She pointed to a report that examined the use of the Centor tool among 441 children attending a pediatric ED. The authors concluded that the Centor criteria were ineffective in predicting a positive GAS culture in throat swabs taken from symptomatic patients.
When are antibiotics warranted?
It is widely accepted that antibiotics should be avoided for viral infections. Returning to the case described at the start of this debate, Dr. Zsigmond calculated that her patient with a 2-day history of sore throat, elevated temperature, pussy tonsils, and enlarged cervical lymph glands but no cough or rhinitis had a FeverPAIN score of 4-5 and a Centor score of 4, meaning that, according to the European guidelines, she should receive antibiotic treatment. However, viral swabs proved positive for adenovirus.
Dr. Ivaska responded with his recent experiences of a similar case, where a 5-year-old boy had a FeverPAIN score of 4-5 and Centor score of 3. Cultures from his throat were GAS positive, illustrating the problem of differentiating between bacterial and viral infections.
But does a GAS-positive pharyngeal culture necessarily mean that antibiotic treatment is indicated? Dr. Ivaska believes it does, citing the importance of preventing serious complications such as rheumatic fever. Dr. Zsigmind countered by pointing out the low levels of acute rheumatic fever in developed nations. In her own country, Hungary, there has not been a case in the last 30 years. Giving antibiotics for historical reasons cannot, in her view, be justified.
Dr. Ivaska responded that perhaps this is because of early treatment in children with sore throats.
Another complication of tonsillitis is quinsy. Dr. Zsigmond cited a study showing that there is no statistically significant evidence demonstrating that antibiotics prevent quinsy. She attributed this to quinsy appearing quickly, typically within 2 days. Delay in seeking help means that the window to treat is often missed. However, should symptoms present early, there is no statistical evidence that prior antibiotic use can prevent quinsy. Also, given the rarity of this condition, prevention would mean excessive use of antibiotics.
Are there other possible benefits of antibiotic treatment in patients with a sore throat? Dr. Ivaska referred to a Cochrane review that found a shortening in duration of throat soreness and fever. Furthermore, compared with placebo, antibiotics reduced the incidence of suppurative complications such as acute otitis media and sinusitis following a sore throat. Other studies have also pointed to the potential benefits of reduced transmission in families where one member with pharyngitis was GAS positive.
As the debate ended, Dr. Zsigmond reported evidence of global antibiotic overprescribing for sore throat ranging from 53% in Europe to 94% in Australia. She also highlighted risks such as altered gut flora, drug resistance, and rashes.
Robin Marlow from the University of Bristol (England), PhD, MBBS, commented that “one of the most enjoyable parts of the ESPID meeting is hearing different viewpoints rationally explained from across the world. As [antibiotic prescription for a sore throat is] a clinical conundrum that faces pediatricians every day, I thought this debate was a really great example of how, despite our different health care systems and ways of working, we are all striving together to improve children’s health using the best evidence available.”
The presenters had no financial conflicts of interest to declare.
FROM ESPID 2020
E-cigarette use tied to increased COPD, asthma risk
Results from a large national prospective cohort study of adults demonstrated that the use of electronic cigarettes is associated with an increased risk of asthma, chronic obstructive pulmonary disease (COPD), emphysema, and chronic bronchitis – independent of cigarette smoking and other combustible tobacco product use.
“Our longitudinal results are consistent with the findings of prior population studies,” researchers led by Wubin Xie, DrPH, MPH, wrote in a study published online in JAMA Network Open. “With a more refined study design assessing multiple respiratory conditions and extensive sensitivity checks to mitigate bias from reverse causation and residual confounding by cigarette smoking and other tobacco product use, our results strengthen the evidence of the potential role of e-cigarette use in pulmonary disease pathogenesis. The findings may be used to inform counseling of patients on the potential risks of e-cigarette use.”
Dr. Xie of Boston University, and colleagues used data from the Population Assessment of Tobacco and Health (PATH) study waves 1-4 to examine the association of e-cigarette use with incident respiratory conditions, including COPD, emphysema, chronic bronchitis, and asthma. An earlier analysis of PATH data found an association between e-cigarette use with a composite respiratory disease outcome, but it did not consider the timing of respiratory events over follow-up and was underpowered to evaluate specific respiratory conditions.
The current analysis included data from 21,618 U.S. adults who were surveyed in four waves of PATH between 2013 and 2018. Of these, 49% were men, 65% were non-Hispanic White, 12% were non-Hispanic Black, 16% were Hispanic, and the remainder were non-Hispanic other. Their mean pack-years was 6.7 at baseline, 26% had self-reported hypertension, and their mean body mass index was 27.8 kg/m2. The analysis was limited to data from the wave 1 cohort of adults and the prospective follow-up at waves 2-4 from public use files. It excluded adults who reported a history of a respiratory condition such as COPD, emphysema, chronic bronchitis, or asthma at wave 1 (baseline).
Two-thirds of respondents (66%) were never e-cigarette users, 12% were former e-cigarette users, and 5% were current e-cigarette users. After the researchers adjusted for cigarette and other combustible tobacco product use, demographic characteristics, and chronic health conditions, they observed an increased risk of respiratory disease among former e-cigarette users (incidence rate ratio, 1.28) and current e-cigarette users (IRR, 1.31). Among respondents with good self-reported health, the IRR for former e-cigarette users was 1.21 and the IRR for current e-cigarette users was 1.43. As for specific respiratory diseases among current e-cigarette users, the IRR was 1.33 for chronic bronchitis, 1.69 for emphysema, 1.57 for COPD, and 1.31 for asthma.
“Our findings on clinical outcome were consistent with studies assessing in vivo biomarkers of e-cigarette exposure in animal subjects, human participants, and population studies,” the authors wrote. “Studies have documented that exclusive e-cigarette use may be associated with higher exposure to harmful and potentially harmful constituents, compared with tobacco nonuse. The potential mechanisms of the association of e-cigarette exposure with pulmonary diseases include pulmonary inflammation, increased oxidative stress, and inhibited immune response. Animal studies have generated substantial evidence on e-cigarette exposure and emphysematous lung destruction, loss of pulmonary capillaries, reduced small airway function, and airway hyperresponsiveness, suggesting the plausibility of e-cigarettes causing chronic lung diseases.”
They acknowledged certain limitations of the study, including its reliance on self-reported measures of e-cigarette and other tobacco product use and its reliance on self-reported diagnoses of respiratory diseases.
The study was supported by grants from the National Heart, Lung, and Blood Institute; the Food and Drug Administration Center for Tobacco Products; and the American Lung Association Public Policy Research Award. Dr. Xie reported having no financial disclosures. His coauthors reported having received research grants and personal fees from a variety of sources.
SOURCE: Xie W et al. JAMA Netw Open. 2020 Nov 12. doi: 10.1001/jamanetworkopen.2020.20816
Results from a large national prospective cohort study of adults demonstrated that the use of electronic cigarettes is associated with an increased risk of asthma, chronic obstructive pulmonary disease (COPD), emphysema, and chronic bronchitis – independent of cigarette smoking and other combustible tobacco product use.
“Our longitudinal results are consistent with the findings of prior population studies,” researchers led by Wubin Xie, DrPH, MPH, wrote in a study published online in JAMA Network Open. “With a more refined study design assessing multiple respiratory conditions and extensive sensitivity checks to mitigate bias from reverse causation and residual confounding by cigarette smoking and other tobacco product use, our results strengthen the evidence of the potential role of e-cigarette use in pulmonary disease pathogenesis. The findings may be used to inform counseling of patients on the potential risks of e-cigarette use.”
Dr. Xie of Boston University, and colleagues used data from the Population Assessment of Tobacco and Health (PATH) study waves 1-4 to examine the association of e-cigarette use with incident respiratory conditions, including COPD, emphysema, chronic bronchitis, and asthma. An earlier analysis of PATH data found an association between e-cigarette use with a composite respiratory disease outcome, but it did not consider the timing of respiratory events over follow-up and was underpowered to evaluate specific respiratory conditions.
The current analysis included data from 21,618 U.S. adults who were surveyed in four waves of PATH between 2013 and 2018. Of these, 49% were men, 65% were non-Hispanic White, 12% were non-Hispanic Black, 16% were Hispanic, and the remainder were non-Hispanic other. Their mean pack-years was 6.7 at baseline, 26% had self-reported hypertension, and their mean body mass index was 27.8 kg/m2. The analysis was limited to data from the wave 1 cohort of adults and the prospective follow-up at waves 2-4 from public use files. It excluded adults who reported a history of a respiratory condition such as COPD, emphysema, chronic bronchitis, or asthma at wave 1 (baseline).
Two-thirds of respondents (66%) were never e-cigarette users, 12% were former e-cigarette users, and 5% were current e-cigarette users. After the researchers adjusted for cigarette and other combustible tobacco product use, demographic characteristics, and chronic health conditions, they observed an increased risk of respiratory disease among former e-cigarette users (incidence rate ratio, 1.28) and current e-cigarette users (IRR, 1.31). Among respondents with good self-reported health, the IRR for former e-cigarette users was 1.21 and the IRR for current e-cigarette users was 1.43. As for specific respiratory diseases among current e-cigarette users, the IRR was 1.33 for chronic bronchitis, 1.69 for emphysema, 1.57 for COPD, and 1.31 for asthma.
“Our findings on clinical outcome were consistent with studies assessing in vivo biomarkers of e-cigarette exposure in animal subjects, human participants, and population studies,” the authors wrote. “Studies have documented that exclusive e-cigarette use may be associated with higher exposure to harmful and potentially harmful constituents, compared with tobacco nonuse. The potential mechanisms of the association of e-cigarette exposure with pulmonary diseases include pulmonary inflammation, increased oxidative stress, and inhibited immune response. Animal studies have generated substantial evidence on e-cigarette exposure and emphysematous lung destruction, loss of pulmonary capillaries, reduced small airway function, and airway hyperresponsiveness, suggesting the plausibility of e-cigarettes causing chronic lung diseases.”
They acknowledged certain limitations of the study, including its reliance on self-reported measures of e-cigarette and other tobacco product use and its reliance on self-reported diagnoses of respiratory diseases.
The study was supported by grants from the National Heart, Lung, and Blood Institute; the Food and Drug Administration Center for Tobacco Products; and the American Lung Association Public Policy Research Award. Dr. Xie reported having no financial disclosures. His coauthors reported having received research grants and personal fees from a variety of sources.
SOURCE: Xie W et al. JAMA Netw Open. 2020 Nov 12. doi: 10.1001/jamanetworkopen.2020.20816
Results from a large national prospective cohort study of adults demonstrated that the use of electronic cigarettes is associated with an increased risk of asthma, chronic obstructive pulmonary disease (COPD), emphysema, and chronic bronchitis – independent of cigarette smoking and other combustible tobacco product use.
“Our longitudinal results are consistent with the findings of prior population studies,” researchers led by Wubin Xie, DrPH, MPH, wrote in a study published online in JAMA Network Open. “With a more refined study design assessing multiple respiratory conditions and extensive sensitivity checks to mitigate bias from reverse causation and residual confounding by cigarette smoking and other tobacco product use, our results strengthen the evidence of the potential role of e-cigarette use in pulmonary disease pathogenesis. The findings may be used to inform counseling of patients on the potential risks of e-cigarette use.”
Dr. Xie of Boston University, and colleagues used data from the Population Assessment of Tobacco and Health (PATH) study waves 1-4 to examine the association of e-cigarette use with incident respiratory conditions, including COPD, emphysema, chronic bronchitis, and asthma. An earlier analysis of PATH data found an association between e-cigarette use with a composite respiratory disease outcome, but it did not consider the timing of respiratory events over follow-up and was underpowered to evaluate specific respiratory conditions.
The current analysis included data from 21,618 U.S. adults who were surveyed in four waves of PATH between 2013 and 2018. Of these, 49% were men, 65% were non-Hispanic White, 12% were non-Hispanic Black, 16% were Hispanic, and the remainder were non-Hispanic other. Their mean pack-years was 6.7 at baseline, 26% had self-reported hypertension, and their mean body mass index was 27.8 kg/m2. The analysis was limited to data from the wave 1 cohort of adults and the prospective follow-up at waves 2-4 from public use files. It excluded adults who reported a history of a respiratory condition such as COPD, emphysema, chronic bronchitis, or asthma at wave 1 (baseline).
Two-thirds of respondents (66%) were never e-cigarette users, 12% were former e-cigarette users, and 5% were current e-cigarette users. After the researchers adjusted for cigarette and other combustible tobacco product use, demographic characteristics, and chronic health conditions, they observed an increased risk of respiratory disease among former e-cigarette users (incidence rate ratio, 1.28) and current e-cigarette users (IRR, 1.31). Among respondents with good self-reported health, the IRR for former e-cigarette users was 1.21 and the IRR for current e-cigarette users was 1.43. As for specific respiratory diseases among current e-cigarette users, the IRR was 1.33 for chronic bronchitis, 1.69 for emphysema, 1.57 for COPD, and 1.31 for asthma.
“Our findings on clinical outcome were consistent with studies assessing in vivo biomarkers of e-cigarette exposure in animal subjects, human participants, and population studies,” the authors wrote. “Studies have documented that exclusive e-cigarette use may be associated with higher exposure to harmful and potentially harmful constituents, compared with tobacco nonuse. The potential mechanisms of the association of e-cigarette exposure with pulmonary diseases include pulmonary inflammation, increased oxidative stress, and inhibited immune response. Animal studies have generated substantial evidence on e-cigarette exposure and emphysematous lung destruction, loss of pulmonary capillaries, reduced small airway function, and airway hyperresponsiveness, suggesting the plausibility of e-cigarettes causing chronic lung diseases.”
They acknowledged certain limitations of the study, including its reliance on self-reported measures of e-cigarette and other tobacco product use and its reliance on self-reported diagnoses of respiratory diseases.
The study was supported by grants from the National Heart, Lung, and Blood Institute; the Food and Drug Administration Center for Tobacco Products; and the American Lung Association Public Policy Research Award. Dr. Xie reported having no financial disclosures. His coauthors reported having received research grants and personal fees from a variety of sources.
SOURCE: Xie W et al. JAMA Netw Open. 2020 Nov 12. doi: 10.1001/jamanetworkopen.2020.20816
FROM JAMA NETWORK OPEN
Pediatricians want kids to be part of COVID vaccine trials
If clinical trials for COVID-19 vaccines aren’t expanded soon to include children, it’s unlikely that even kids in their teens will be vaccinated in time for the next school year.
The hurdle is that COVID vaccine makers are only in the early stages of testing their products on children. The Pfizer vaccine authorized for use by the Food and Drug Administration on Friday was greenlighted only for people aged 16 years and older. Moderna just started trials for 12- to 17-year-olds for its vaccine, likely to be authorized later this month.
It will take months to approve use of the vaccines for middle- and high school–aged kids, and months more to test them in younger children. But some pediatricians say that concerns about the safety of the front-runner vaccines make the wait worthwhile.
Although most pediatricians believe the eventual vaccination of children will be crucial to subduing the COVID virus, they’re split on how fast to move toward that, says James Campbell, MD, professor of pediatrics at the University of Maryland’s Center for Vaccine Development and Global Health in Baltimore. Dr. Campbell and colleagues said it’s a matter of urgency to get the vaccines tested in kids, while others want to hold off on those trials until millions of adults have been safely vaccinated.
Much of the debate centers on two issues: the degree of harm COVID-19 causes children and the extent to which children are spreading the virus to their friends, teachers, parents and grandparents.
COVID-19’s impact on children represents a tiny fraction of the suffering and death experienced by vulnerable adults. Yet it would qualify as a pretty serious childhood disease, having caused 154 deaths and more than 7,500 hospitalizations as of Dec. 3 among aged people 19 years and younger in the United States. Those numbers rank it as worse than a typical year of influenza, and worse than diseases like mumps or hepatitis B in children before the vaccination era.
Studies thus far show that 1%-2% of children infected with the virus end up requiring intensive care, Stanley Plotkin, MD, professor emeritus of pediatrics at the University of Pennsylvania, Philadelphia told a federal panel. That’s in line with the percentage who become gravely ill as result of infections like Haemophilus influenzae type B, for which doctors have vaccinated children since the 1980s, he pointed out.
Dr. Campbell, who with colleagues has developed a plan for how to run pediatric COVID vaccine trials, pointed out that, “in a universe where COVID mainly affected children the way it’s affecting them now, and we had potential vaccines, people would be clamoring for them.”
The evidence that teens can transmit the disease is pretty clear, and transmission has been documented in children as young as 8. Fear of spread by children has been enough to close schools, and led the American Academy of Pediatrics to demand that children be quickly included in vaccine testing.
“The longer we take to start kids in trials, the longer it will take them to get vaccinated and to break the chains of transmission,” said Yvonne Maldonado, MD, a professor of pediatrics at Stanford (Calif.) University who chairs the AAP’s infectious disease committee. “If you want kids to go back to school and not have the teachers union terrified, you have to make sure they aren’t a risk.”
Other pediatricians worry that early pediatric trials could backfire. Cody Meissner, MD, chief of pediatric infectious diseases at Tufts Medical Center and a member of the FDA’s advisory committee on vaccines, is worried that whatever causes multisystem inflammatory syndrome in children, a rare but frightening COVID-related disorder, might also be triggered, however rarely, by vaccination.
Dr. Meissner abstained from the committee’s vote Thursday that supported, by a 17-4 vote, an emergency authorization of the Pfizer vaccine for people aged 16 years and older.
“I have trouble justifying it for children so unlikely to get the disease,” he said during debate on the measure.
But panel member Ofer Levy, MD, PhD, director of the Precision Vaccines Program at Boston Children’s Hospital, said the 16-years-and-up authorization would speed the vaccine’s testing in and approval for younger children. That is vital for the world’s protection from COVID-19, he said, since in the United States and most places “most vaccines are delivered early in life.”
While vaccines given to tens of thousands of people so far appear to be safe, the lack of understanding of the inflammatory syndrome means that children in any trials should be followed closely, said Emily Erbelding, MD, MPH, director of the division of microbiology and infectious diseases at the National Institute of Allergy and Infectious Diseases.
Under a 2003 law, vaccine companies are required eventually to test all their products on children. By late November, Pfizer had vaccinated approximately 100 children aged 12-15 years, said spokesperson Jerica Pitts.
Moderna has started enrolling 3,000 children aged 12 years and over in another clinical trial, and other companies have similar plans. Assuming the trials show the vaccines are safe and provide a good immune response, future tests could include progressively younger children, moving to, say, 6- to 12-year-olds next, then 2- to 6-year-olds. Eventually, trials could include younger toddlers and infants.
Similar step-down approaches were taken to test vaccines against human papillomavirus (HPV), influenza and other diseases in the past, Dr. Erbelding noted. Such trials are easiest to conduct when researchers know that a measurable immune response, like antibody levels in the blood, translates to effective protection against disease. Armed with such knowledge, they can see whether children were protected without them having to be exposed to the virus. Federal scientists hope to get that data from the Moderna and Pfizer adult vaccine trials, she said.
Vaccine trials geared to tweens or younger children may involve testing half-doses, which, if protective, would require less vaccine and might cause fewer incidents of sore arms and fevers that afflicted many who’ve received the Pfizer and Moderna vaccines, Dr. Campbell said.
But unless additional studies begin quickly, the window for having an FDA-authorized vaccine available before the next school year “will be closed even for our oldest children,” said Evan Anderson, MD, a pediatrics professor at Emory University, Atlanta. “Our younger children are almost certainly going into next school year without a vaccine option available for them.”
In the meantime, teachers are likely to be high on the priority list for vaccination. Protecting school staff could allow more schools to reopen even if most children can’t be vaccinated, Dr. Erbelding said.
Eventually, if the SARS-CoV-2 virus remains in circulation, governments may want to mandate childhood vaccination against the virus to protect them as they grow up and protect society as a whole, Dr. Plotkin said.
In the 1960s, Dr. Plotkin invented the rubella vaccine that has been given to hundreds of millions of children since. Like COVID-19, rubella – or German measles – is not usually a serious illness for children. But congenital rubella syndrome afflicted babies in the womb with blindness, deafness, developmental delays, and autism. Immunizing toddlers, which, in turn, protects their pregnant mothers, has indirectly prevented hundreds of thousands of such cases.
“We don’t want to use children to protect everyone in the community,” said Dr. Campbell. “But when you can protect both children and their community, that’s important.”
And while a coronavirus infection may not be bad for most children, missed school, absent friends, and distanced families have caused them immense suffering, he said.
“It’s a huge burden on a child to have their entire world flipped around,” Dr. Campbell said. “If vaccinating could help to flip it back, we should begin testing to see if that’s possible.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
If clinical trials for COVID-19 vaccines aren’t expanded soon to include children, it’s unlikely that even kids in their teens will be vaccinated in time for the next school year.
The hurdle is that COVID vaccine makers are only in the early stages of testing their products on children. The Pfizer vaccine authorized for use by the Food and Drug Administration on Friday was greenlighted only for people aged 16 years and older. Moderna just started trials for 12- to 17-year-olds for its vaccine, likely to be authorized later this month.
It will take months to approve use of the vaccines for middle- and high school–aged kids, and months more to test them in younger children. But some pediatricians say that concerns about the safety of the front-runner vaccines make the wait worthwhile.
Although most pediatricians believe the eventual vaccination of children will be crucial to subduing the COVID virus, they’re split on how fast to move toward that, says James Campbell, MD, professor of pediatrics at the University of Maryland’s Center for Vaccine Development and Global Health in Baltimore. Dr. Campbell and colleagues said it’s a matter of urgency to get the vaccines tested in kids, while others want to hold off on those trials until millions of adults have been safely vaccinated.
Much of the debate centers on two issues: the degree of harm COVID-19 causes children and the extent to which children are spreading the virus to their friends, teachers, parents and grandparents.
COVID-19’s impact on children represents a tiny fraction of the suffering and death experienced by vulnerable adults. Yet it would qualify as a pretty serious childhood disease, having caused 154 deaths and more than 7,500 hospitalizations as of Dec. 3 among aged people 19 years and younger in the United States. Those numbers rank it as worse than a typical year of influenza, and worse than diseases like mumps or hepatitis B in children before the vaccination era.
Studies thus far show that 1%-2% of children infected with the virus end up requiring intensive care, Stanley Plotkin, MD, professor emeritus of pediatrics at the University of Pennsylvania, Philadelphia told a federal panel. That’s in line with the percentage who become gravely ill as result of infections like Haemophilus influenzae type B, for which doctors have vaccinated children since the 1980s, he pointed out.
Dr. Campbell, who with colleagues has developed a plan for how to run pediatric COVID vaccine trials, pointed out that, “in a universe where COVID mainly affected children the way it’s affecting them now, and we had potential vaccines, people would be clamoring for them.”
The evidence that teens can transmit the disease is pretty clear, and transmission has been documented in children as young as 8. Fear of spread by children has been enough to close schools, and led the American Academy of Pediatrics to demand that children be quickly included in vaccine testing.
“The longer we take to start kids in trials, the longer it will take them to get vaccinated and to break the chains of transmission,” said Yvonne Maldonado, MD, a professor of pediatrics at Stanford (Calif.) University who chairs the AAP’s infectious disease committee. “If you want kids to go back to school and not have the teachers union terrified, you have to make sure they aren’t a risk.”
Other pediatricians worry that early pediatric trials could backfire. Cody Meissner, MD, chief of pediatric infectious diseases at Tufts Medical Center and a member of the FDA’s advisory committee on vaccines, is worried that whatever causes multisystem inflammatory syndrome in children, a rare but frightening COVID-related disorder, might also be triggered, however rarely, by vaccination.
Dr. Meissner abstained from the committee’s vote Thursday that supported, by a 17-4 vote, an emergency authorization of the Pfizer vaccine for people aged 16 years and older.
“I have trouble justifying it for children so unlikely to get the disease,” he said during debate on the measure.
But panel member Ofer Levy, MD, PhD, director of the Precision Vaccines Program at Boston Children’s Hospital, said the 16-years-and-up authorization would speed the vaccine’s testing in and approval for younger children. That is vital for the world’s protection from COVID-19, he said, since in the United States and most places “most vaccines are delivered early in life.”
While vaccines given to tens of thousands of people so far appear to be safe, the lack of understanding of the inflammatory syndrome means that children in any trials should be followed closely, said Emily Erbelding, MD, MPH, director of the division of microbiology and infectious diseases at the National Institute of Allergy and Infectious Diseases.
Under a 2003 law, vaccine companies are required eventually to test all their products on children. By late November, Pfizer had vaccinated approximately 100 children aged 12-15 years, said spokesperson Jerica Pitts.
Moderna has started enrolling 3,000 children aged 12 years and over in another clinical trial, and other companies have similar plans. Assuming the trials show the vaccines are safe and provide a good immune response, future tests could include progressively younger children, moving to, say, 6- to 12-year-olds next, then 2- to 6-year-olds. Eventually, trials could include younger toddlers and infants.
Similar step-down approaches were taken to test vaccines against human papillomavirus (HPV), influenza and other diseases in the past, Dr. Erbelding noted. Such trials are easiest to conduct when researchers know that a measurable immune response, like antibody levels in the blood, translates to effective protection against disease. Armed with such knowledge, they can see whether children were protected without them having to be exposed to the virus. Federal scientists hope to get that data from the Moderna and Pfizer adult vaccine trials, she said.
Vaccine trials geared to tweens or younger children may involve testing half-doses, which, if protective, would require less vaccine and might cause fewer incidents of sore arms and fevers that afflicted many who’ve received the Pfizer and Moderna vaccines, Dr. Campbell said.
But unless additional studies begin quickly, the window for having an FDA-authorized vaccine available before the next school year “will be closed even for our oldest children,” said Evan Anderson, MD, a pediatrics professor at Emory University, Atlanta. “Our younger children are almost certainly going into next school year without a vaccine option available for them.”
In the meantime, teachers are likely to be high on the priority list for vaccination. Protecting school staff could allow more schools to reopen even if most children can’t be vaccinated, Dr. Erbelding said.
Eventually, if the SARS-CoV-2 virus remains in circulation, governments may want to mandate childhood vaccination against the virus to protect them as they grow up and protect society as a whole, Dr. Plotkin said.
In the 1960s, Dr. Plotkin invented the rubella vaccine that has been given to hundreds of millions of children since. Like COVID-19, rubella – or German measles – is not usually a serious illness for children. But congenital rubella syndrome afflicted babies in the womb with blindness, deafness, developmental delays, and autism. Immunizing toddlers, which, in turn, protects their pregnant mothers, has indirectly prevented hundreds of thousands of such cases.
“We don’t want to use children to protect everyone in the community,” said Dr. Campbell. “But when you can protect both children and their community, that’s important.”
And while a coronavirus infection may not be bad for most children, missed school, absent friends, and distanced families have caused them immense suffering, he said.
“It’s a huge burden on a child to have their entire world flipped around,” Dr. Campbell said. “If vaccinating could help to flip it back, we should begin testing to see if that’s possible.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
If clinical trials for COVID-19 vaccines aren’t expanded soon to include children, it’s unlikely that even kids in their teens will be vaccinated in time for the next school year.
The hurdle is that COVID vaccine makers are only in the early stages of testing their products on children. The Pfizer vaccine authorized for use by the Food and Drug Administration on Friday was greenlighted only for people aged 16 years and older. Moderna just started trials for 12- to 17-year-olds for its vaccine, likely to be authorized later this month.
It will take months to approve use of the vaccines for middle- and high school–aged kids, and months more to test them in younger children. But some pediatricians say that concerns about the safety of the front-runner vaccines make the wait worthwhile.
Although most pediatricians believe the eventual vaccination of children will be crucial to subduing the COVID virus, they’re split on how fast to move toward that, says James Campbell, MD, professor of pediatrics at the University of Maryland’s Center for Vaccine Development and Global Health in Baltimore. Dr. Campbell and colleagues said it’s a matter of urgency to get the vaccines tested in kids, while others want to hold off on those trials until millions of adults have been safely vaccinated.
Much of the debate centers on two issues: the degree of harm COVID-19 causes children and the extent to which children are spreading the virus to their friends, teachers, parents and grandparents.
COVID-19’s impact on children represents a tiny fraction of the suffering and death experienced by vulnerable adults. Yet it would qualify as a pretty serious childhood disease, having caused 154 deaths and more than 7,500 hospitalizations as of Dec. 3 among aged people 19 years and younger in the United States. Those numbers rank it as worse than a typical year of influenza, and worse than diseases like mumps or hepatitis B in children before the vaccination era.
Studies thus far show that 1%-2% of children infected with the virus end up requiring intensive care, Stanley Plotkin, MD, professor emeritus of pediatrics at the University of Pennsylvania, Philadelphia told a federal panel. That’s in line with the percentage who become gravely ill as result of infections like Haemophilus influenzae type B, for which doctors have vaccinated children since the 1980s, he pointed out.
Dr. Campbell, who with colleagues has developed a plan for how to run pediatric COVID vaccine trials, pointed out that, “in a universe where COVID mainly affected children the way it’s affecting them now, and we had potential vaccines, people would be clamoring for them.”
The evidence that teens can transmit the disease is pretty clear, and transmission has been documented in children as young as 8. Fear of spread by children has been enough to close schools, and led the American Academy of Pediatrics to demand that children be quickly included in vaccine testing.
“The longer we take to start kids in trials, the longer it will take them to get vaccinated and to break the chains of transmission,” said Yvonne Maldonado, MD, a professor of pediatrics at Stanford (Calif.) University who chairs the AAP’s infectious disease committee. “If you want kids to go back to school and not have the teachers union terrified, you have to make sure they aren’t a risk.”
Other pediatricians worry that early pediatric trials could backfire. Cody Meissner, MD, chief of pediatric infectious diseases at Tufts Medical Center and a member of the FDA’s advisory committee on vaccines, is worried that whatever causes multisystem inflammatory syndrome in children, a rare but frightening COVID-related disorder, might also be triggered, however rarely, by vaccination.
Dr. Meissner abstained from the committee’s vote Thursday that supported, by a 17-4 vote, an emergency authorization of the Pfizer vaccine for people aged 16 years and older.
“I have trouble justifying it for children so unlikely to get the disease,” he said during debate on the measure.
But panel member Ofer Levy, MD, PhD, director of the Precision Vaccines Program at Boston Children’s Hospital, said the 16-years-and-up authorization would speed the vaccine’s testing in and approval for younger children. That is vital for the world’s protection from COVID-19, he said, since in the United States and most places “most vaccines are delivered early in life.”
While vaccines given to tens of thousands of people so far appear to be safe, the lack of understanding of the inflammatory syndrome means that children in any trials should be followed closely, said Emily Erbelding, MD, MPH, director of the division of microbiology and infectious diseases at the National Institute of Allergy and Infectious Diseases.
Under a 2003 law, vaccine companies are required eventually to test all their products on children. By late November, Pfizer had vaccinated approximately 100 children aged 12-15 years, said spokesperson Jerica Pitts.
Moderna has started enrolling 3,000 children aged 12 years and over in another clinical trial, and other companies have similar plans. Assuming the trials show the vaccines are safe and provide a good immune response, future tests could include progressively younger children, moving to, say, 6- to 12-year-olds next, then 2- to 6-year-olds. Eventually, trials could include younger toddlers and infants.
Similar step-down approaches were taken to test vaccines against human papillomavirus (HPV), influenza and other diseases in the past, Dr. Erbelding noted. Such trials are easiest to conduct when researchers know that a measurable immune response, like antibody levels in the blood, translates to effective protection against disease. Armed with such knowledge, they can see whether children were protected without them having to be exposed to the virus. Federal scientists hope to get that data from the Moderna and Pfizer adult vaccine trials, she said.
Vaccine trials geared to tweens or younger children may involve testing half-doses, which, if protective, would require less vaccine and might cause fewer incidents of sore arms and fevers that afflicted many who’ve received the Pfizer and Moderna vaccines, Dr. Campbell said.
But unless additional studies begin quickly, the window for having an FDA-authorized vaccine available before the next school year “will be closed even for our oldest children,” said Evan Anderson, MD, a pediatrics professor at Emory University, Atlanta. “Our younger children are almost certainly going into next school year without a vaccine option available for them.”
In the meantime, teachers are likely to be high on the priority list for vaccination. Protecting school staff could allow more schools to reopen even if most children can’t be vaccinated, Dr. Erbelding said.
Eventually, if the SARS-CoV-2 virus remains in circulation, governments may want to mandate childhood vaccination against the virus to protect them as they grow up and protect society as a whole, Dr. Plotkin said.
In the 1960s, Dr. Plotkin invented the rubella vaccine that has been given to hundreds of millions of children since. Like COVID-19, rubella – or German measles – is not usually a serious illness for children. But congenital rubella syndrome afflicted babies in the womb with blindness, deafness, developmental delays, and autism. Immunizing toddlers, which, in turn, protects their pregnant mothers, has indirectly prevented hundreds of thousands of such cases.
“We don’t want to use children to protect everyone in the community,” said Dr. Campbell. “But when you can protect both children and their community, that’s important.”
And while a coronavirus infection may not be bad for most children, missed school, absent friends, and distanced families have caused them immense suffering, he said.
“It’s a huge burden on a child to have their entire world flipped around,” Dr. Campbell said. “If vaccinating could help to flip it back, we should begin testing to see if that’s possible.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Teenage bone density declines following sleeve gastrectomy
Adolescents who undergo sleeve gastrectomy have lower bone density and higher bone marrow fat at 1 year following surgery, new research shows.
“It’s almost paradoxical,” Miriam Bredella, MD, of Massachusetts General Hospital in Boston, told Medscape Medical News. “Despite marked loss of body fat, these children have more fat in their bones and decreased bone density.”
She explained that the dissected part of the stomach is filled with anabolic cells that are important for building bone mass. “When those cells are cut out, the body cannot produce the hormones for building up bone.” It’s a malabsorption problem, she added. “Cutting out parts of the stomach or gut leads to less absorption.”
It is well known that bariatric surgery in adults has long-term effects on bone, she said, but this is the first time it has been studied in children.
“Nobody thinks about bone loss in children, but it’s extremely important,” Bredella reports. “The adolescent years up to age 25 are when we accrue bone density, so if something happens during this critical time, it can lead to weak bones later in life.” In the case of these adolescents, peak bone mass is never reached.
To investigate the effects of sleeve gastrectomy on bone density and marrow adipose tissue in extremely obese teenagers, researchers at Massachusetts General Hospital and Harvard Medical School recruited 52 adolescents with a mean body mass index (BMI) of 45. They measured volumetric bone mineral density using quantitative computer tomography (QCT) of the lumbar spine.
“We used QCT instead of DEXA [dual energy x-ray absorptiometry] scan because it isn’t affected by changes in soft tissue; it’s less susceptible to extreme changes in body weight,” Bredella said. “With DEXA scan there are too many artifacts.”
Half of the group (n = 26) underwent surgery. At 1 year, those who underwent surgery lost an average of 34 kg (75 lb). Adolescents in the control group lost an average of 0.2 kg (0.5 lb) (P < .0001).
Both groups repeated the QCT scan at the 1-year follow-up. Researchers found a decrease in bone density in those who underwent sleeve gastrectomy vs. controls (P = .046).
In her presentation, Bredella showed the QCT of the L2 spine in a 17-year old female before surgery and 12 months later. Her volumetric bone mineral density decreased from 183 mg/cm3 to 146 mg/cm3.
“Sleeve gastrectomy in children is bad for bones,” Bradella said. “You have to take care of your bones. This is something people are not thinking about and it probably won’t be a problem when they’re young but will likely affect these patients with osteoporosis when they are older.”
Patients need to be aware of this, she warns, and take steps to combat the bone loss. “Drinking milk, taking vitamin D, and doing weight-bearing exercise may help increase the bone density,” she said.
The increased fat in the bone is also concerning, she said. “Increased fat in the bone is a phenomenon that we see in anorexic patients,” Bredella explained.
The body appears to store the fat in bone in case of need later on, she explained. “We know that in severe states of malnutrition the body has the ability to metabolize the fat in the bones.”
The obesity epidemic in America has given way to a 100-fold increase in sleeve gastrectomy procedures in teenagers between 2005 and 2014. “These patients need this surgery so they don›t die of cardiac arrest or diabetes,” she said. “But we need to make sure they get their bone mineral density checked frequently.”
“The results of this study are important,” Marc Michalsky, MD, Nationwide Children’s Hospital, Columbus, Ohio, told Medscape Medical News. “But they need to be put into context.”
“There is an impetus and argument to support bariatric surgery as it offers a significant reduction in BMI and an associated reversal and complete amelioration of obesity related diseases.”
What this study doesn’t address, he said, is whether this population will experience an increase in bone density-related fractures down the road.
“These results are a snapshot in time — a picture of one postoperative time point,” Michalsky pointed out. “Are we seeing a process that represents continued change in bone mineralization? It’s not unreasonable to assume that the radiological findings here may lead to real clinical impact, but we don’t know.”
Bredella and Michalsky have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Adolescents who undergo sleeve gastrectomy have lower bone density and higher bone marrow fat at 1 year following surgery, new research shows.
“It’s almost paradoxical,” Miriam Bredella, MD, of Massachusetts General Hospital in Boston, told Medscape Medical News. “Despite marked loss of body fat, these children have more fat in their bones and decreased bone density.”
She explained that the dissected part of the stomach is filled with anabolic cells that are important for building bone mass. “When those cells are cut out, the body cannot produce the hormones for building up bone.” It’s a malabsorption problem, she added. “Cutting out parts of the stomach or gut leads to less absorption.”
It is well known that bariatric surgery in adults has long-term effects on bone, she said, but this is the first time it has been studied in children.
“Nobody thinks about bone loss in children, but it’s extremely important,” Bredella reports. “The adolescent years up to age 25 are when we accrue bone density, so if something happens during this critical time, it can lead to weak bones later in life.” In the case of these adolescents, peak bone mass is never reached.
To investigate the effects of sleeve gastrectomy on bone density and marrow adipose tissue in extremely obese teenagers, researchers at Massachusetts General Hospital and Harvard Medical School recruited 52 adolescents with a mean body mass index (BMI) of 45. They measured volumetric bone mineral density using quantitative computer tomography (QCT) of the lumbar spine.
“We used QCT instead of DEXA [dual energy x-ray absorptiometry] scan because it isn’t affected by changes in soft tissue; it’s less susceptible to extreme changes in body weight,” Bredella said. “With DEXA scan there are too many artifacts.”
Half of the group (n = 26) underwent surgery. At 1 year, those who underwent surgery lost an average of 34 kg (75 lb). Adolescents in the control group lost an average of 0.2 kg (0.5 lb) (P < .0001).
Both groups repeated the QCT scan at the 1-year follow-up. Researchers found a decrease in bone density in those who underwent sleeve gastrectomy vs. controls (P = .046).
In her presentation, Bredella showed the QCT of the L2 spine in a 17-year old female before surgery and 12 months later. Her volumetric bone mineral density decreased from 183 mg/cm3 to 146 mg/cm3.
“Sleeve gastrectomy in children is bad for bones,” Bradella said. “You have to take care of your bones. This is something people are not thinking about and it probably won’t be a problem when they’re young but will likely affect these patients with osteoporosis when they are older.”
Patients need to be aware of this, she warns, and take steps to combat the bone loss. “Drinking milk, taking vitamin D, and doing weight-bearing exercise may help increase the bone density,” she said.
The increased fat in the bone is also concerning, she said. “Increased fat in the bone is a phenomenon that we see in anorexic patients,” Bredella explained.
The body appears to store the fat in bone in case of need later on, she explained. “We know that in severe states of malnutrition the body has the ability to metabolize the fat in the bones.”
The obesity epidemic in America has given way to a 100-fold increase in sleeve gastrectomy procedures in teenagers between 2005 and 2014. “These patients need this surgery so they don›t die of cardiac arrest or diabetes,” she said. “But we need to make sure they get their bone mineral density checked frequently.”
“The results of this study are important,” Marc Michalsky, MD, Nationwide Children’s Hospital, Columbus, Ohio, told Medscape Medical News. “But they need to be put into context.”
“There is an impetus and argument to support bariatric surgery as it offers a significant reduction in BMI and an associated reversal and complete amelioration of obesity related diseases.”
What this study doesn’t address, he said, is whether this population will experience an increase in bone density-related fractures down the road.
“These results are a snapshot in time — a picture of one postoperative time point,” Michalsky pointed out. “Are we seeing a process that represents continued change in bone mineralization? It’s not unreasonable to assume that the radiological findings here may lead to real clinical impact, but we don’t know.”
Bredella and Michalsky have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Adolescents who undergo sleeve gastrectomy have lower bone density and higher bone marrow fat at 1 year following surgery, new research shows.
“It’s almost paradoxical,” Miriam Bredella, MD, of Massachusetts General Hospital in Boston, told Medscape Medical News. “Despite marked loss of body fat, these children have more fat in their bones and decreased bone density.”
She explained that the dissected part of the stomach is filled with anabolic cells that are important for building bone mass. “When those cells are cut out, the body cannot produce the hormones for building up bone.” It’s a malabsorption problem, she added. “Cutting out parts of the stomach or gut leads to less absorption.”
It is well known that bariatric surgery in adults has long-term effects on bone, she said, but this is the first time it has been studied in children.
“Nobody thinks about bone loss in children, but it’s extremely important,” Bredella reports. “The adolescent years up to age 25 are when we accrue bone density, so if something happens during this critical time, it can lead to weak bones later in life.” In the case of these adolescents, peak bone mass is never reached.
To investigate the effects of sleeve gastrectomy on bone density and marrow adipose tissue in extremely obese teenagers, researchers at Massachusetts General Hospital and Harvard Medical School recruited 52 adolescents with a mean body mass index (BMI) of 45. They measured volumetric bone mineral density using quantitative computer tomography (QCT) of the lumbar spine.
“We used QCT instead of DEXA [dual energy x-ray absorptiometry] scan because it isn’t affected by changes in soft tissue; it’s less susceptible to extreme changes in body weight,” Bredella said. “With DEXA scan there are too many artifacts.”
Half of the group (n = 26) underwent surgery. At 1 year, those who underwent surgery lost an average of 34 kg (75 lb). Adolescents in the control group lost an average of 0.2 kg (0.5 lb) (P < .0001).
Both groups repeated the QCT scan at the 1-year follow-up. Researchers found a decrease in bone density in those who underwent sleeve gastrectomy vs. controls (P = .046).
In her presentation, Bredella showed the QCT of the L2 spine in a 17-year old female before surgery and 12 months later. Her volumetric bone mineral density decreased from 183 mg/cm3 to 146 mg/cm3.
“Sleeve gastrectomy in children is bad for bones,” Bradella said. “You have to take care of your bones. This is something people are not thinking about and it probably won’t be a problem when they’re young but will likely affect these patients with osteoporosis when they are older.”
Patients need to be aware of this, she warns, and take steps to combat the bone loss. “Drinking milk, taking vitamin D, and doing weight-bearing exercise may help increase the bone density,” she said.
The increased fat in the bone is also concerning, she said. “Increased fat in the bone is a phenomenon that we see in anorexic patients,” Bredella explained.
The body appears to store the fat in bone in case of need later on, she explained. “We know that in severe states of malnutrition the body has the ability to metabolize the fat in the bones.”
The obesity epidemic in America has given way to a 100-fold increase in sleeve gastrectomy procedures in teenagers between 2005 and 2014. “These patients need this surgery so they don›t die of cardiac arrest or diabetes,” she said. “But we need to make sure they get their bone mineral density checked frequently.”
“The results of this study are important,” Marc Michalsky, MD, Nationwide Children’s Hospital, Columbus, Ohio, told Medscape Medical News. “But they need to be put into context.”
“There is an impetus and argument to support bariatric surgery as it offers a significant reduction in BMI and an associated reversal and complete amelioration of obesity related diseases.”
What this study doesn’t address, he said, is whether this population will experience an increase in bone density-related fractures down the road.
“These results are a snapshot in time — a picture of one postoperative time point,” Michalsky pointed out. “Are we seeing a process that represents continued change in bone mineralization? It’s not unreasonable to assume that the radiological findings here may lead to real clinical impact, but we don’t know.”
Bredella and Michalsky have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Vaccine-preventable infection risk high for pediatric hematopoietic cell transplantation recipients
Vaccine-preventable infections (VPIs) in pediatric hematopoietic cell transplantation (HCT) recipients cause significant morbidity, health care burden, and mortality.
Dana Danino, MD, and colleagues presented their evaluation of the prevalence and epidemiology of pediatric VPI-associated hospitalizations occurring within 5 years post HCT at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
“Pediatric HCT recipients are at increased risk of VPIs, and HCT recipients have poor outcomes from VPIs, compared with the general population,” explained Dr. Danino, of the department of pediatrics, and divisions of infectious diseases and host defense at the Ohio State University, Columbus. “However, the contemporary prevalence, risk factors, morbidity and mortality resulting from VPIs in children post HCT are not well known.”
Their epidemiological study, using the Pediatric Health Information System (PHIS) database, identified all children under 18 years that underwent allogeneic or autologous HCT in an 8-year period. A total of 9,591 unique HCT recipients were identified.
The researchers demonstrated that 7.1% of this cohort were hospitalized for a VPI in the first 5 years post HCT. Dr. Danino explained that 67% of VPI hospitalizations occurred during the first year, at a median of 222 days, and 22% of VPIs occurred during the initial HCT admission.
As to the type of infection, Dr. Danino and colleagues found that, the prevalence of VPI hospitalizations were highest for influenza, followed by varicella and invasive pneumococcal infections. They identified no hospitalizations due to measles or rubella during the study period.
The study findings revealed that the influenza infections occurred a median 231 days post HCT; varicella infections occurred a median 190 days; and invasive pneumococcal infections occurred a median 311 days post HCT.
“When we did a multivariate analysis by time post HCT, we found that age at transplantation, primary immune deficiency as an indication for transplantation, and graft versus host disease were independent predictors of VPIs during the initial HCT admission,” said Dr. Danino.
Children with a VPI who spent longer in hospital were more likely to be admitted to an ICU and have higher mortality, compared with children without a VPI diagnosis.
“VPIs led to longer duration of hospitalization, higher rates of ICU admission, and higher mortality, compared to HCT recipients without VPIs,” Dr. Danino explained. It was not possible in this retrospective study to determine whether increased mortality was VPI related.
These results underline the seriousness of infections in vulnerable children after HCT. Dr. Danino concluded by saying that “efforts to optimize vaccination strategies early post HCT are warranted to decrease VPIs.”
Dr. Danino had nothing to disclose.
Vaccine-preventable infections (VPIs) in pediatric hematopoietic cell transplantation (HCT) recipients cause significant morbidity, health care burden, and mortality.
Dana Danino, MD, and colleagues presented their evaluation of the prevalence and epidemiology of pediatric VPI-associated hospitalizations occurring within 5 years post HCT at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
“Pediatric HCT recipients are at increased risk of VPIs, and HCT recipients have poor outcomes from VPIs, compared with the general population,” explained Dr. Danino, of the department of pediatrics, and divisions of infectious diseases and host defense at the Ohio State University, Columbus. “However, the contemporary prevalence, risk factors, morbidity and mortality resulting from VPIs in children post HCT are not well known.”
Their epidemiological study, using the Pediatric Health Information System (PHIS) database, identified all children under 18 years that underwent allogeneic or autologous HCT in an 8-year period. A total of 9,591 unique HCT recipients were identified.
The researchers demonstrated that 7.1% of this cohort were hospitalized for a VPI in the first 5 years post HCT. Dr. Danino explained that 67% of VPI hospitalizations occurred during the first year, at a median of 222 days, and 22% of VPIs occurred during the initial HCT admission.
As to the type of infection, Dr. Danino and colleagues found that, the prevalence of VPI hospitalizations were highest for influenza, followed by varicella and invasive pneumococcal infections. They identified no hospitalizations due to measles or rubella during the study period.
The study findings revealed that the influenza infections occurred a median 231 days post HCT; varicella infections occurred a median 190 days; and invasive pneumococcal infections occurred a median 311 days post HCT.
“When we did a multivariate analysis by time post HCT, we found that age at transplantation, primary immune deficiency as an indication for transplantation, and graft versus host disease were independent predictors of VPIs during the initial HCT admission,” said Dr. Danino.
Children with a VPI who spent longer in hospital were more likely to be admitted to an ICU and have higher mortality, compared with children without a VPI diagnosis.
“VPIs led to longer duration of hospitalization, higher rates of ICU admission, and higher mortality, compared to HCT recipients without VPIs,” Dr. Danino explained. It was not possible in this retrospective study to determine whether increased mortality was VPI related.
These results underline the seriousness of infections in vulnerable children after HCT. Dr. Danino concluded by saying that “efforts to optimize vaccination strategies early post HCT are warranted to decrease VPIs.”
Dr. Danino had nothing to disclose.
Vaccine-preventable infections (VPIs) in pediatric hematopoietic cell transplantation (HCT) recipients cause significant morbidity, health care burden, and mortality.
Dana Danino, MD, and colleagues presented their evaluation of the prevalence and epidemiology of pediatric VPI-associated hospitalizations occurring within 5 years post HCT at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
“Pediatric HCT recipients are at increased risk of VPIs, and HCT recipients have poor outcomes from VPIs, compared with the general population,” explained Dr. Danino, of the department of pediatrics, and divisions of infectious diseases and host defense at the Ohio State University, Columbus. “However, the contemporary prevalence, risk factors, morbidity and mortality resulting from VPIs in children post HCT are not well known.”
Their epidemiological study, using the Pediatric Health Information System (PHIS) database, identified all children under 18 years that underwent allogeneic or autologous HCT in an 8-year period. A total of 9,591 unique HCT recipients were identified.
The researchers demonstrated that 7.1% of this cohort were hospitalized for a VPI in the first 5 years post HCT. Dr. Danino explained that 67% of VPI hospitalizations occurred during the first year, at a median of 222 days, and 22% of VPIs occurred during the initial HCT admission.
As to the type of infection, Dr. Danino and colleagues found that, the prevalence of VPI hospitalizations were highest for influenza, followed by varicella and invasive pneumococcal infections. They identified no hospitalizations due to measles or rubella during the study period.
The study findings revealed that the influenza infections occurred a median 231 days post HCT; varicella infections occurred a median 190 days; and invasive pneumococcal infections occurred a median 311 days post HCT.
“When we did a multivariate analysis by time post HCT, we found that age at transplantation, primary immune deficiency as an indication for transplantation, and graft versus host disease were independent predictors of VPIs during the initial HCT admission,” said Dr. Danino.
Children with a VPI who spent longer in hospital were more likely to be admitted to an ICU and have higher mortality, compared with children without a VPI diagnosis.
“VPIs led to longer duration of hospitalization, higher rates of ICU admission, and higher mortality, compared to HCT recipients without VPIs,” Dr. Danino explained. It was not possible in this retrospective study to determine whether increased mortality was VPI related.
These results underline the seriousness of infections in vulnerable children after HCT. Dr. Danino concluded by saying that “efforts to optimize vaccination strategies early post HCT are warranted to decrease VPIs.”
Dr. Danino had nothing to disclose.
FROM ESPID 2020
COVID-19 vaccines: Safe for immunocompromised patients?
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
Parents favored virtual learning over in-person school attendance
Parents of school-aged children were generally more comfortable with full-time virtual learning in schools in the fall of 2020, compared with full-capacity in-person attendance, according to a survey conducted in July.
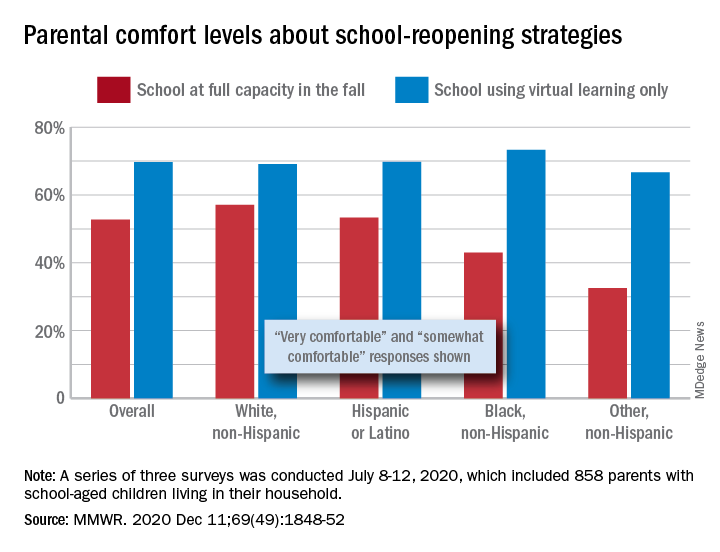
Those of racial/ethnic minorities, however, “were less likely to feel that schools should reopen for all students and were more concerned about” several aspects of in-person instruction than were White parents, Leah K. Gilbert, MD, and associates at the Centers for Disease Control and Prevention’s COVID-19 Response Team said in the Morbidity and Mortality Weekly Report.
A slim majority, just under 53% of the 858 parents surveyed, said that they were very or somewhat comfortable with their children returning to schools that were reopening at full capacity, while almost 70% said they were very/somewhat comfortable with schools going exclusively with virtual learning, the investigators reported.
The question about full-capacity attendance in particular showed considerable variation by race and ethnicity, with 57% of White parents saying they were very/somewhat comfortable, versus 53% of Hispanic or Latino parents, 43% of Black parents, and 32.5% of parents of other races/ethnicities (American Indian/Alaska Native, Asian, or multiracial).
Comfort levels were closer regarding virtual learning: Parents of other races/ethnicities were lowest at 67% and Black parents were highest at 73%. When asked about schools reopening at 50% capacity and 50% virtual learning, Black parents were again lowest at 58% with strong or moderate comfort and White parents were highest at 68%, Dr. Gilbert and associates said.
“Although the majority of parent respondents had concerns about both school reopening for in-person instruction and virtual learning, the perceived risk for SARS-CoV-2 infection and poor health outcomes might account for the differences in parental attitudes and concerns by race and ethnicity,” they wrote.
SOURCE: Gilbert LK et al. MMWR. 2020 Dec 11;69(49):1848-52.
Parents of school-aged children were generally more comfortable with full-time virtual learning in schools in the fall of 2020, compared with full-capacity in-person attendance, according to a survey conducted in July.

Those of racial/ethnic minorities, however, “were less likely to feel that schools should reopen for all students and were more concerned about” several aspects of in-person instruction than were White parents, Leah K. Gilbert, MD, and associates at the Centers for Disease Control and Prevention’s COVID-19 Response Team said in the Morbidity and Mortality Weekly Report.
A slim majority, just under 53% of the 858 parents surveyed, said that they were very or somewhat comfortable with their children returning to schools that were reopening at full capacity, while almost 70% said they were very/somewhat comfortable with schools going exclusively with virtual learning, the investigators reported.
The question about full-capacity attendance in particular showed considerable variation by race and ethnicity, with 57% of White parents saying they were very/somewhat comfortable, versus 53% of Hispanic or Latino parents, 43% of Black parents, and 32.5% of parents of other races/ethnicities (American Indian/Alaska Native, Asian, or multiracial).
Comfort levels were closer regarding virtual learning: Parents of other races/ethnicities were lowest at 67% and Black parents were highest at 73%. When asked about schools reopening at 50% capacity and 50% virtual learning, Black parents were again lowest at 58% with strong or moderate comfort and White parents were highest at 68%, Dr. Gilbert and associates said.
“Although the majority of parent respondents had concerns about both school reopening for in-person instruction and virtual learning, the perceived risk for SARS-CoV-2 infection and poor health outcomes might account for the differences in parental attitudes and concerns by race and ethnicity,” they wrote.
SOURCE: Gilbert LK et al. MMWR. 2020 Dec 11;69(49):1848-52.
Parents of school-aged children were generally more comfortable with full-time virtual learning in schools in the fall of 2020, compared with full-capacity in-person attendance, according to a survey conducted in July.

Those of racial/ethnic minorities, however, “were less likely to feel that schools should reopen for all students and were more concerned about” several aspects of in-person instruction than were White parents, Leah K. Gilbert, MD, and associates at the Centers for Disease Control and Prevention’s COVID-19 Response Team said in the Morbidity and Mortality Weekly Report.
A slim majority, just under 53% of the 858 parents surveyed, said that they were very or somewhat comfortable with their children returning to schools that were reopening at full capacity, while almost 70% said they were very/somewhat comfortable with schools going exclusively with virtual learning, the investigators reported.
The question about full-capacity attendance in particular showed considerable variation by race and ethnicity, with 57% of White parents saying they were very/somewhat comfortable, versus 53% of Hispanic or Latino parents, 43% of Black parents, and 32.5% of parents of other races/ethnicities (American Indian/Alaska Native, Asian, or multiracial).
Comfort levels were closer regarding virtual learning: Parents of other races/ethnicities were lowest at 67% and Black parents were highest at 73%. When asked about schools reopening at 50% capacity and 50% virtual learning, Black parents were again lowest at 58% with strong or moderate comfort and White parents were highest at 68%, Dr. Gilbert and associates said.
“Although the majority of parent respondents had concerns about both school reopening for in-person instruction and virtual learning, the perceived risk for SARS-CoV-2 infection and poor health outcomes might account for the differences in parental attitudes and concerns by race and ethnicity,” they wrote.
SOURCE: Gilbert LK et al. MMWR. 2020 Dec 11;69(49):1848-52.
FROM MMWR
Preadolescent acne: Management from birth requires increasing vigilance
No treatment may be necessary for acne in the first few months of life, but the condition can leave scars in children as young as ages 3-6 months, said Andrea L. Zaenglein, MD, professor of dermatology and pediatric dermatology, Penn State University, Hershey, Penn., said in a presentation at MedscapeLive’s virtual Women’s & Pediatric Dermatology Seminar.
Neonatal acne occurs in more than 20% of newborns aged 2 weeks to 3 months. “Typically we don’t need to treat it. But if you do, you could use a topical antifungal like clotrimazole cream twice a day,” but in most babies, “this will just improve over time and resolve without any scarring or sequelae,” she said.
Infantile acne begins about 3-6 months of age typically, or a little bit older, and lasts up to 2 years of age, Dr. Zaenglein said. “You will see comedones in infantile acne, so this is actually a true form of acne. It’s due to increased adrenal production of androgens.”
She added: “The scarring can be permanent. It’s important that you recognize infantile acne and treat it, even though it seems pretty mild.”
For infantile acne, she recommends performing a full-skin exam to rule out hyperandrogenic disorders such as Cushing syndrome, congenital adrenal hyperplasia, premature adrenarche, a gonadal/adrenal tumor and precocious puberty.
Treatments are similar to those in teenagers, she said, “but make sure you use baby-friendly formulations,” with lower concentrations of active ingredients – and avoid tetracyclines and benzoyl peroxide (BPO) washes. BPO can be used in leave-on formulations/creams at lower strengths (2.5%-5%).
One possible combination option is tretinoin 0.025% cream or adapalene 0.1% gel plus BPO 2.5% cream or clindamycin/BPO gel. Another combination is adapalene/BPO 2.5% gel.
Erythromycin can be appropriate at 30-50 mg/kg per day divided in two or three doses a day, but beware of possible gastrointestinal upset. Azithromycin at 5 mg/kg per day is another option.
“Rarely do we have to go to isotretinoin,” Dr. Zaenglein said. “I think in all my years, I’ve only treated one baby with isotretinoin for infantile acne. But severe forms can occur.”
Midchildhood and preadolescent acne conditions occur in children starting at ages 1 up to 10 years, Dr. Zaenglein said. In this population, she also recommends ruling out hyperandrogenism by looking for secondary sexual characteristics with full-body skin exams. “The workup can be broad and includes looking at adrenal androgens and total and free testosterone, as well as looking at growth charts and bone age. Typically, you’ll refer these kids to pediatric endocrinology.”
Keep in mind, she said, that early adrenarche starts at ages 6-7 years in girls and 7-8 years in boys. “That’s when we expect to start seeing that very early acne. You can see it even earlier in patients with elevated BMI, and it’s more common in Hispanic and Black children as well.”
She added that it’s important to remember that early adrenarche is a risk factor for polycystic ovarian syndrome (PCOS). “So ask patients about their family history and look for other signs of PCOS as they move further into adolescence.”
Milder cases of acne in this age group can be treated with “salicylic acid wipes and things that are kind of a rite of passage. But if they have any more severe acne, you’re going to want to treat it more or less like you do adolescent acne.”
MedscapeLive and this news organization are owned by the same parent company. Dr. Zaenglein disclosed receiving consulting fees from Cassiopea, Dermata, and Regeneron and fees for contracted research support from Incyte.
No treatment may be necessary for acne in the first few months of life, but the condition can leave scars in children as young as ages 3-6 months, said Andrea L. Zaenglein, MD, professor of dermatology and pediatric dermatology, Penn State University, Hershey, Penn., said in a presentation at MedscapeLive’s virtual Women’s & Pediatric Dermatology Seminar.
Neonatal acne occurs in more than 20% of newborns aged 2 weeks to 3 months. “Typically we don’t need to treat it. But if you do, you could use a topical antifungal like clotrimazole cream twice a day,” but in most babies, “this will just improve over time and resolve without any scarring or sequelae,” she said.
Infantile acne begins about 3-6 months of age typically, or a little bit older, and lasts up to 2 years of age, Dr. Zaenglein said. “You will see comedones in infantile acne, so this is actually a true form of acne. It’s due to increased adrenal production of androgens.”
She added: “The scarring can be permanent. It’s important that you recognize infantile acne and treat it, even though it seems pretty mild.”
For infantile acne, she recommends performing a full-skin exam to rule out hyperandrogenic disorders such as Cushing syndrome, congenital adrenal hyperplasia, premature adrenarche, a gonadal/adrenal tumor and precocious puberty.
Treatments are similar to those in teenagers, she said, “but make sure you use baby-friendly formulations,” with lower concentrations of active ingredients – and avoid tetracyclines and benzoyl peroxide (BPO) washes. BPO can be used in leave-on formulations/creams at lower strengths (2.5%-5%).
One possible combination option is tretinoin 0.025% cream or adapalene 0.1% gel plus BPO 2.5% cream or clindamycin/BPO gel. Another combination is adapalene/BPO 2.5% gel.
Erythromycin can be appropriate at 30-50 mg/kg per day divided in two or three doses a day, but beware of possible gastrointestinal upset. Azithromycin at 5 mg/kg per day is another option.
“Rarely do we have to go to isotretinoin,” Dr. Zaenglein said. “I think in all my years, I’ve only treated one baby with isotretinoin for infantile acne. But severe forms can occur.”
Midchildhood and preadolescent acne conditions occur in children starting at ages 1 up to 10 years, Dr. Zaenglein said. In this population, she also recommends ruling out hyperandrogenism by looking for secondary sexual characteristics with full-body skin exams. “The workup can be broad and includes looking at adrenal androgens and total and free testosterone, as well as looking at growth charts and bone age. Typically, you’ll refer these kids to pediatric endocrinology.”
Keep in mind, she said, that early adrenarche starts at ages 6-7 years in girls and 7-8 years in boys. “That’s when we expect to start seeing that very early acne. You can see it even earlier in patients with elevated BMI, and it’s more common in Hispanic and Black children as well.”
She added that it’s important to remember that early adrenarche is a risk factor for polycystic ovarian syndrome (PCOS). “So ask patients about their family history and look for other signs of PCOS as they move further into adolescence.”
Milder cases of acne in this age group can be treated with “salicylic acid wipes and things that are kind of a rite of passage. But if they have any more severe acne, you’re going to want to treat it more or less like you do adolescent acne.”
MedscapeLive and this news organization are owned by the same parent company. Dr. Zaenglein disclosed receiving consulting fees from Cassiopea, Dermata, and Regeneron and fees for contracted research support from Incyte.
No treatment may be necessary for acne in the first few months of life, but the condition can leave scars in children as young as ages 3-6 months, said Andrea L. Zaenglein, MD, professor of dermatology and pediatric dermatology, Penn State University, Hershey, Penn., said in a presentation at MedscapeLive’s virtual Women’s & Pediatric Dermatology Seminar.
Neonatal acne occurs in more than 20% of newborns aged 2 weeks to 3 months. “Typically we don’t need to treat it. But if you do, you could use a topical antifungal like clotrimazole cream twice a day,” but in most babies, “this will just improve over time and resolve without any scarring or sequelae,” she said.
Infantile acne begins about 3-6 months of age typically, or a little bit older, and lasts up to 2 years of age, Dr. Zaenglein said. “You will see comedones in infantile acne, so this is actually a true form of acne. It’s due to increased adrenal production of androgens.”
She added: “The scarring can be permanent. It’s important that you recognize infantile acne and treat it, even though it seems pretty mild.”
For infantile acne, she recommends performing a full-skin exam to rule out hyperandrogenic disorders such as Cushing syndrome, congenital adrenal hyperplasia, premature adrenarche, a gonadal/adrenal tumor and precocious puberty.
Treatments are similar to those in teenagers, she said, “but make sure you use baby-friendly formulations,” with lower concentrations of active ingredients – and avoid tetracyclines and benzoyl peroxide (BPO) washes. BPO can be used in leave-on formulations/creams at lower strengths (2.5%-5%).
One possible combination option is tretinoin 0.025% cream or adapalene 0.1% gel plus BPO 2.5% cream or clindamycin/BPO gel. Another combination is adapalene/BPO 2.5% gel.
Erythromycin can be appropriate at 30-50 mg/kg per day divided in two or three doses a day, but beware of possible gastrointestinal upset. Azithromycin at 5 mg/kg per day is another option.
“Rarely do we have to go to isotretinoin,” Dr. Zaenglein said. “I think in all my years, I’ve only treated one baby with isotretinoin for infantile acne. But severe forms can occur.”
Midchildhood and preadolescent acne conditions occur in children starting at ages 1 up to 10 years, Dr. Zaenglein said. In this population, she also recommends ruling out hyperandrogenism by looking for secondary sexual characteristics with full-body skin exams. “The workup can be broad and includes looking at adrenal androgens and total and free testosterone, as well as looking at growth charts and bone age. Typically, you’ll refer these kids to pediatric endocrinology.”
Keep in mind, she said, that early adrenarche starts at ages 6-7 years in girls and 7-8 years in boys. “That’s when we expect to start seeing that very early acne. You can see it even earlier in patients with elevated BMI, and it’s more common in Hispanic and Black children as well.”
She added that it’s important to remember that early adrenarche is a risk factor for polycystic ovarian syndrome (PCOS). “So ask patients about their family history and look for other signs of PCOS as they move further into adolescence.”
Milder cases of acne in this age group can be treated with “salicylic acid wipes and things that are kind of a rite of passage. But if they have any more severe acne, you’re going to want to treat it more or less like you do adolescent acne.”
MedscapeLive and this news organization are owned by the same parent company. Dr. Zaenglein disclosed receiving consulting fees from Cassiopea, Dermata, and Regeneron and fees for contracted research support from Incyte.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
FDA clears first OTC rapid at-home COVID diagnostic test
The Food and Drug Administration has issued an emergency-use authorization (EUA) for the first COVID-19 diagnostic test that can be completed at home without a prescription.
Authorization of the Ellume COVID-19 Home Test is “a major milestone in diagnostic testing for COVID-19,” FDA Commissioner Stephen M. Hahn, MD, said in a news release.
“By authorizing a test for over-the-counter use, the FDA allows it to be sold in places like drug stores, where a patient can buy it, swab their nose, run the test, and find out their results in as little as 20 minutes,” said Dr. Hahn.
The Ellume COVID-19 Home Test is a rapid antigen test that detects fragments of the SARS-CoV-2 virus from a nasal swab sample taken from anyone aged 2 years and older, including those not showing any symptoms.
In testing, the Ellume COVID-19 Home Test correctly identified 96% of positive samples and 100% of negative samples in individuals with symptoms.
In people without symptoms, the test correctly identified 91% of positive samples and 96% of negative samples, the FDA said.
The test includes a sterile nasal swab, a dropper, processing fluid, and a Bluetooth-connected analyzer for use with an app on the user’s smartphone. The sample is analyzed and results are automatically transmitted to the user’s smartphone.
“The Ellume COVID-19 home test’s core technology combines ultra-sensitive optics, electronics, and proprietary software to leverage best-in-class digital immunoassay technology with next-generation multi-quantum dot fluorescence technology,” the company said in a news release.
The mobile app requires individuals to input their ZIP code and date of birth, with optional fields including name and email address. The app automatically reports the results as appropriate to public health authorities to monitor disease prevalence.
Ellume expects to produce more than 3 million tests in January 2021. The company said the test will cost around $30.
FDA authorization of this first fully at-home nonprescription COVID-19 diagnostic test follows last month’s EUA for the first prescription COVID-19 test for home use, as reported this news organization.
Since the start of the pandemic, the FDA has authorized more than 225 diagnostic tests for COVID-19, including more than 25 tests that allow for home collection of samples, which are then sent to a lab for testing.
“As we continue to authorize additional tests for home use, we are helping expand Americans’ access to testing, reducing the burden on laboratories and test supplies, and giving Americans more testing options from the comfort and safety of their own homes,” Dr. Hahn said.
“This test, like other antigen tests, is less sensitive and less specific than typical molecular tests run in a lab,” said Jeffrey Shuren, MD, JD, director of FDA’s Center for Devices and Radiological Health, in the release. “However, the fact that it can be used completely at home and return results quickly means that it can play an important role in response to the pandemic.”
As with other antigen tests, a small percentage of positive and negative results from the Ellume test may be false. In patients without symptoms, positive results should be treated as presumptively positive until confirmed by another test as soon as possible, the FDA advised.
This is especially true if there are fewer infections in a particular community, as false-positive results can be more common when antigen tests are used in populations where there is a low prevalence of COVID-19, the agency said.
Because all tests can give false-negative and false-positive results, individuals with positive results should self-isolate and seek additional care from their health care provider.
Individuals who test negative and have symptoms of COVID-19 should follow up with their health care provider, as negative results don’t preclude an individual from SARS-CoV-2 infection.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has issued an emergency-use authorization (EUA) for the first COVID-19 diagnostic test that can be completed at home without a prescription.
Authorization of the Ellume COVID-19 Home Test is “a major milestone in diagnostic testing for COVID-19,” FDA Commissioner Stephen M. Hahn, MD, said in a news release.
“By authorizing a test for over-the-counter use, the FDA allows it to be sold in places like drug stores, where a patient can buy it, swab their nose, run the test, and find out their results in as little as 20 minutes,” said Dr. Hahn.
The Ellume COVID-19 Home Test is a rapid antigen test that detects fragments of the SARS-CoV-2 virus from a nasal swab sample taken from anyone aged 2 years and older, including those not showing any symptoms.
In testing, the Ellume COVID-19 Home Test correctly identified 96% of positive samples and 100% of negative samples in individuals with symptoms.
In people without symptoms, the test correctly identified 91% of positive samples and 96% of negative samples, the FDA said.
The test includes a sterile nasal swab, a dropper, processing fluid, and a Bluetooth-connected analyzer for use with an app on the user’s smartphone. The sample is analyzed and results are automatically transmitted to the user’s smartphone.
“The Ellume COVID-19 home test’s core technology combines ultra-sensitive optics, electronics, and proprietary software to leverage best-in-class digital immunoassay technology with next-generation multi-quantum dot fluorescence technology,” the company said in a news release.
The mobile app requires individuals to input their ZIP code and date of birth, with optional fields including name and email address. The app automatically reports the results as appropriate to public health authorities to monitor disease prevalence.
Ellume expects to produce more than 3 million tests in January 2021. The company said the test will cost around $30.
FDA authorization of this first fully at-home nonprescription COVID-19 diagnostic test follows last month’s EUA for the first prescription COVID-19 test for home use, as reported this news organization.
Since the start of the pandemic, the FDA has authorized more than 225 diagnostic tests for COVID-19, including more than 25 tests that allow for home collection of samples, which are then sent to a lab for testing.
“As we continue to authorize additional tests for home use, we are helping expand Americans’ access to testing, reducing the burden on laboratories and test supplies, and giving Americans more testing options from the comfort and safety of their own homes,” Dr. Hahn said.
“This test, like other antigen tests, is less sensitive and less specific than typical molecular tests run in a lab,” said Jeffrey Shuren, MD, JD, director of FDA’s Center for Devices and Radiological Health, in the release. “However, the fact that it can be used completely at home and return results quickly means that it can play an important role in response to the pandemic.”
As with other antigen tests, a small percentage of positive and negative results from the Ellume test may be false. In patients without symptoms, positive results should be treated as presumptively positive until confirmed by another test as soon as possible, the FDA advised.
This is especially true if there are fewer infections in a particular community, as false-positive results can be more common when antigen tests are used in populations where there is a low prevalence of COVID-19, the agency said.
Because all tests can give false-negative and false-positive results, individuals with positive results should self-isolate and seek additional care from their health care provider.
Individuals who test negative and have symptoms of COVID-19 should follow up with their health care provider, as negative results don’t preclude an individual from SARS-CoV-2 infection.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has issued an emergency-use authorization (EUA) for the first COVID-19 diagnostic test that can be completed at home without a prescription.
Authorization of the Ellume COVID-19 Home Test is “a major milestone in diagnostic testing for COVID-19,” FDA Commissioner Stephen M. Hahn, MD, said in a news release.
“By authorizing a test for over-the-counter use, the FDA allows it to be sold in places like drug stores, where a patient can buy it, swab their nose, run the test, and find out their results in as little as 20 minutes,” said Dr. Hahn.
The Ellume COVID-19 Home Test is a rapid antigen test that detects fragments of the SARS-CoV-2 virus from a nasal swab sample taken from anyone aged 2 years and older, including those not showing any symptoms.
In testing, the Ellume COVID-19 Home Test correctly identified 96% of positive samples and 100% of negative samples in individuals with symptoms.
In people without symptoms, the test correctly identified 91% of positive samples and 96% of negative samples, the FDA said.
The test includes a sterile nasal swab, a dropper, processing fluid, and a Bluetooth-connected analyzer for use with an app on the user’s smartphone. The sample is analyzed and results are automatically transmitted to the user’s smartphone.
“The Ellume COVID-19 home test’s core technology combines ultra-sensitive optics, electronics, and proprietary software to leverage best-in-class digital immunoassay technology with next-generation multi-quantum dot fluorescence technology,” the company said in a news release.
The mobile app requires individuals to input their ZIP code and date of birth, with optional fields including name and email address. The app automatically reports the results as appropriate to public health authorities to monitor disease prevalence.
Ellume expects to produce more than 3 million tests in January 2021. The company said the test will cost around $30.
FDA authorization of this first fully at-home nonprescription COVID-19 diagnostic test follows last month’s EUA for the first prescription COVID-19 test for home use, as reported this news organization.
Since the start of the pandemic, the FDA has authorized more than 225 diagnostic tests for COVID-19, including more than 25 tests that allow for home collection of samples, which are then sent to a lab for testing.
“As we continue to authorize additional tests for home use, we are helping expand Americans’ access to testing, reducing the burden on laboratories and test supplies, and giving Americans more testing options from the comfort and safety of their own homes,” Dr. Hahn said.
“This test, like other antigen tests, is less sensitive and less specific than typical molecular tests run in a lab,” said Jeffrey Shuren, MD, JD, director of FDA’s Center for Devices and Radiological Health, in the release. “However, the fact that it can be used completely at home and return results quickly means that it can play an important role in response to the pandemic.”
As with other antigen tests, a small percentage of positive and negative results from the Ellume test may be false. In patients without symptoms, positive results should be treated as presumptively positive until confirmed by another test as soon as possible, the FDA advised.
This is especially true if there are fewer infections in a particular community, as false-positive results can be more common when antigen tests are used in populations where there is a low prevalence of COVID-19, the agency said.
Because all tests can give false-negative and false-positive results, individuals with positive results should self-isolate and seek additional care from their health care provider.
Individuals who test negative and have symptoms of COVID-19 should follow up with their health care provider, as negative results don’t preclude an individual from SARS-CoV-2 infection.
A version of this article first appeared on Medscape.com.
Endocrine societies push back on discriminatory transgender health policies
Science should be the cornerstone for health policy, and decisions on medical care of transgender and gender-diverse (TGD) individuals should be between a patient and their doctor.
That’s according to a joint policy statement from the Endocrine Society and Pediatric Endocrine Society published in the Journal of Clinical Endocrinology & Metabolism expressing concern about recent proposed legislation that would limit access to medical care for TGD individuals.
“The main emphasis is that we are simply medical people trying to be conservative and science driven in the care of our patients,” Joshua D. Safer, MD, coauthor and executive director of the Center for Transgender Medicine and Surgery at Mount Sinai Health System, and professor of medicine at Icahn School of Medicine at Mount Sinai, New York, said in an interview. “Why the health care for a particular group of people should be considered political is a mystery to me.”
TGD individuals have seen a recent uptick in efforts to limit or restrict their access to medical care at the federal and state levels. In June 2020, the Department of Health & Human Services finalized a revision to Section 1557 of the Affordable Care Act, rolling back a 2016 rule that determined the phrase “on the basis of sex” included nondiscrimination based on a person’s sex and gender identity. The Endocrine Society opposed this rule revision, arguing that it would allow “providers to deny care to TGD persons as well as discourage patients from seeking routine and gender-affirming care or reporting discrimination.”
Over a dozen U.S. states have introduced proposed legislation concerning medical care of TGD individuals that contain erroneous and misleading information. Proposed laws in Alabama, Missouri, and Texas, for example, would prohibit any use of medical treatments for minors for the purpose of gender-affirming medical care, including “gonadotropin-releasing hormone agonist therapy for pubertal suppression and gender-affirming hormonal therapy,” the authors of the joint statement wrote. In some cases, medical professionals who provide medical care for TGD patients could face criminal charges.
Outside the United States, three High Court judges in the United Kingdom recently ruled that minors aged under 16 years could not legally consent to pubertal suppression. “The recent U.K. court decision could be very disruptive because it would raise a barrier to transgender children receiving puberty blockers at exactly the ages that puberty blockers would be typically used,” Dr. Safer said.
Misleading characterizations of gender-affirming medical care for TGD individuals have also been spread to the general public. A recent Republican primary ballot proposition in Texas asked whether the state should ban “chemical castration, puberty blockers, cross-sex hormones, and genital mutilation surgery on all minor children for transition purposes,” falsely asserting that “Texas children as young as 3 are being transitioned from their biological sex to the opposite sex,” referencing a high-profile custody battle of a transgender child in Texas.
There are several tiers of misinformation that exist within these statements, Dr. Safer noted. “Some statements have suggested that gender-affirming treatment for young children can include hormone therapy or even surgery. Of course, there are no medical treatments for transgender and gender-diverse children prior to puberty.”
For adolescents aged under 18 years, Endocrine Society guidelines released in 2017 state that pubertal suppression is fully reversible and “offered to adolescents who meet diagnostic and treatment criteria, and are requesting care, for gender dysphoria/gender incongruence after they exhibit physical changes of puberty,” Dr. Safer and coauthors wrote in the joint policy statement. Other, more permanent – but still partially reversible – treatments such as hormone therapy are available as options for adolescents with confirmed and persistent gender dysphoria/gender incongruence, after meeting with a team of medical and mental health professionals and giving informed consent, according to the guidelines.
Dr. Safer expressed surprise at the opposition to puberty blockers in proposed state legislation. “Puberty blockers are the conservative option so that we can avoid permanent changes while thoughtful decisions are being made by our adolescent patients with their families and health care providers,” he said.
The perception that puberty blockers will lead to hormone therapy is another misunderstanding and source of misinformation, Dr. Safer explained.
“The fear is that these data suggest that puberty blockers are a ‘gateway drug’ of some sort. But that is false. The reason that most adolescents who take puberty blockers go on to hormone therapy is because most of the adolescents who are identified in our conservative systems are actually transgender and interested in more gender-affirming care as they age,” he said.
Effects of discrimination on TGD persons
Many of these proposed state bills have not advanced through state legislatures, but a few – such as the proposed laws in Alabama, Missouri, and Texas – are still currently under consideration.
“In the United States, most recent efforts to single out transgender and gender-diverse people for discrimination in health care have failed. However, the demonization of trans people and attempts to disrupt care have been the source of much stress among our patients,” Dr. Safer said.
Restricting access to health care has “multiple implications” for TGD patients. “In the era when we did not provide care to transgender youth, we had a situation where approximately 40% of transgender people had considered suicide in their lives,” Dr. Safer said. In contrast, having access to these treatments has been shown to improve mental health outcomes in these patients, according to an Endocrine Society position statement.
The purpose of earlier interventions such as puberty blockers is to allow an adolescent to “explore options and live in the experienced gender before making a decision to proceed with gender-affirming hormone therapy,” the authors of the Endocrine Society and Pediatric Endocrine Society joint statement said.
Blocking access to puberty blockers, on the other hand, forces transgender youth to experience a puberty that doesn’t match their gender identity, Dr. Safer noted. “The puberty will include permanent changes which will then have to be reversed with surgery. Why would we intentionally allow that to happen?”
Dr. Safer reported that his spouse is an employee of Parexel. Dr. Tangpricha is the current president of the World Association for Transgender Health and a board member of the American Association of Clinical Endocrinology. The other authors reported no relevant conflicts of interests.
SOURCE: Safer JD et al. J Clin Endocrinol Metab. 2020. doi: 10.1210/clinem/dgaa816.
Science should be the cornerstone for health policy, and decisions on medical care of transgender and gender-diverse (TGD) individuals should be between a patient and their doctor.
That’s according to a joint policy statement from the Endocrine Society and Pediatric Endocrine Society published in the Journal of Clinical Endocrinology & Metabolism expressing concern about recent proposed legislation that would limit access to medical care for TGD individuals.
“The main emphasis is that we are simply medical people trying to be conservative and science driven in the care of our patients,” Joshua D. Safer, MD, coauthor and executive director of the Center for Transgender Medicine and Surgery at Mount Sinai Health System, and professor of medicine at Icahn School of Medicine at Mount Sinai, New York, said in an interview. “Why the health care for a particular group of people should be considered political is a mystery to me.”
TGD individuals have seen a recent uptick in efforts to limit or restrict their access to medical care at the federal and state levels. In June 2020, the Department of Health & Human Services finalized a revision to Section 1557 of the Affordable Care Act, rolling back a 2016 rule that determined the phrase “on the basis of sex” included nondiscrimination based on a person’s sex and gender identity. The Endocrine Society opposed this rule revision, arguing that it would allow “providers to deny care to TGD persons as well as discourage patients from seeking routine and gender-affirming care or reporting discrimination.”
Over a dozen U.S. states have introduced proposed legislation concerning medical care of TGD individuals that contain erroneous and misleading information. Proposed laws in Alabama, Missouri, and Texas, for example, would prohibit any use of medical treatments for minors for the purpose of gender-affirming medical care, including “gonadotropin-releasing hormone agonist therapy for pubertal suppression and gender-affirming hormonal therapy,” the authors of the joint statement wrote. In some cases, medical professionals who provide medical care for TGD patients could face criminal charges.
Outside the United States, three High Court judges in the United Kingdom recently ruled that minors aged under 16 years could not legally consent to pubertal suppression. “The recent U.K. court decision could be very disruptive because it would raise a barrier to transgender children receiving puberty blockers at exactly the ages that puberty blockers would be typically used,” Dr. Safer said.
Misleading characterizations of gender-affirming medical care for TGD individuals have also been spread to the general public. A recent Republican primary ballot proposition in Texas asked whether the state should ban “chemical castration, puberty blockers, cross-sex hormones, and genital mutilation surgery on all minor children for transition purposes,” falsely asserting that “Texas children as young as 3 are being transitioned from their biological sex to the opposite sex,” referencing a high-profile custody battle of a transgender child in Texas.
There are several tiers of misinformation that exist within these statements, Dr. Safer noted. “Some statements have suggested that gender-affirming treatment for young children can include hormone therapy or even surgery. Of course, there are no medical treatments for transgender and gender-diverse children prior to puberty.”
For adolescents aged under 18 years, Endocrine Society guidelines released in 2017 state that pubertal suppression is fully reversible and “offered to adolescents who meet diagnostic and treatment criteria, and are requesting care, for gender dysphoria/gender incongruence after they exhibit physical changes of puberty,” Dr. Safer and coauthors wrote in the joint policy statement. Other, more permanent – but still partially reversible – treatments such as hormone therapy are available as options for adolescents with confirmed and persistent gender dysphoria/gender incongruence, after meeting with a team of medical and mental health professionals and giving informed consent, according to the guidelines.
Dr. Safer expressed surprise at the opposition to puberty blockers in proposed state legislation. “Puberty blockers are the conservative option so that we can avoid permanent changes while thoughtful decisions are being made by our adolescent patients with their families and health care providers,” he said.
The perception that puberty blockers will lead to hormone therapy is another misunderstanding and source of misinformation, Dr. Safer explained.
“The fear is that these data suggest that puberty blockers are a ‘gateway drug’ of some sort. But that is false. The reason that most adolescents who take puberty blockers go on to hormone therapy is because most of the adolescents who are identified in our conservative systems are actually transgender and interested in more gender-affirming care as they age,” he said.
Effects of discrimination on TGD persons
Many of these proposed state bills have not advanced through state legislatures, but a few – such as the proposed laws in Alabama, Missouri, and Texas – are still currently under consideration.
“In the United States, most recent efforts to single out transgender and gender-diverse people for discrimination in health care have failed. However, the demonization of trans people and attempts to disrupt care have been the source of much stress among our patients,” Dr. Safer said.
Restricting access to health care has “multiple implications” for TGD patients. “In the era when we did not provide care to transgender youth, we had a situation where approximately 40% of transgender people had considered suicide in their lives,” Dr. Safer said. In contrast, having access to these treatments has been shown to improve mental health outcomes in these patients, according to an Endocrine Society position statement.
The purpose of earlier interventions such as puberty blockers is to allow an adolescent to “explore options and live in the experienced gender before making a decision to proceed with gender-affirming hormone therapy,” the authors of the Endocrine Society and Pediatric Endocrine Society joint statement said.
Blocking access to puberty blockers, on the other hand, forces transgender youth to experience a puberty that doesn’t match their gender identity, Dr. Safer noted. “The puberty will include permanent changes which will then have to be reversed with surgery. Why would we intentionally allow that to happen?”
Dr. Safer reported that his spouse is an employee of Parexel. Dr. Tangpricha is the current president of the World Association for Transgender Health and a board member of the American Association of Clinical Endocrinology. The other authors reported no relevant conflicts of interests.
SOURCE: Safer JD et al. J Clin Endocrinol Metab. 2020. doi: 10.1210/clinem/dgaa816.
Science should be the cornerstone for health policy, and decisions on medical care of transgender and gender-diverse (TGD) individuals should be between a patient and their doctor.
That’s according to a joint policy statement from the Endocrine Society and Pediatric Endocrine Society published in the Journal of Clinical Endocrinology & Metabolism expressing concern about recent proposed legislation that would limit access to medical care for TGD individuals.
“The main emphasis is that we are simply medical people trying to be conservative and science driven in the care of our patients,” Joshua D. Safer, MD, coauthor and executive director of the Center for Transgender Medicine and Surgery at Mount Sinai Health System, and professor of medicine at Icahn School of Medicine at Mount Sinai, New York, said in an interview. “Why the health care for a particular group of people should be considered political is a mystery to me.”
TGD individuals have seen a recent uptick in efforts to limit or restrict their access to medical care at the federal and state levels. In June 2020, the Department of Health & Human Services finalized a revision to Section 1557 of the Affordable Care Act, rolling back a 2016 rule that determined the phrase “on the basis of sex” included nondiscrimination based on a person’s sex and gender identity. The Endocrine Society opposed this rule revision, arguing that it would allow “providers to deny care to TGD persons as well as discourage patients from seeking routine and gender-affirming care or reporting discrimination.”
Over a dozen U.S. states have introduced proposed legislation concerning medical care of TGD individuals that contain erroneous and misleading information. Proposed laws in Alabama, Missouri, and Texas, for example, would prohibit any use of medical treatments for minors for the purpose of gender-affirming medical care, including “gonadotropin-releasing hormone agonist therapy for pubertal suppression and gender-affirming hormonal therapy,” the authors of the joint statement wrote. In some cases, medical professionals who provide medical care for TGD patients could face criminal charges.
Outside the United States, three High Court judges in the United Kingdom recently ruled that minors aged under 16 years could not legally consent to pubertal suppression. “The recent U.K. court decision could be very disruptive because it would raise a barrier to transgender children receiving puberty blockers at exactly the ages that puberty blockers would be typically used,” Dr. Safer said.
Misleading characterizations of gender-affirming medical care for TGD individuals have also been spread to the general public. A recent Republican primary ballot proposition in Texas asked whether the state should ban “chemical castration, puberty blockers, cross-sex hormones, and genital mutilation surgery on all minor children for transition purposes,” falsely asserting that “Texas children as young as 3 are being transitioned from their biological sex to the opposite sex,” referencing a high-profile custody battle of a transgender child in Texas.
There are several tiers of misinformation that exist within these statements, Dr. Safer noted. “Some statements have suggested that gender-affirming treatment for young children can include hormone therapy or even surgery. Of course, there are no medical treatments for transgender and gender-diverse children prior to puberty.”
For adolescents aged under 18 years, Endocrine Society guidelines released in 2017 state that pubertal suppression is fully reversible and “offered to adolescents who meet diagnostic and treatment criteria, and are requesting care, for gender dysphoria/gender incongruence after they exhibit physical changes of puberty,” Dr. Safer and coauthors wrote in the joint policy statement. Other, more permanent – but still partially reversible – treatments such as hormone therapy are available as options for adolescents with confirmed and persistent gender dysphoria/gender incongruence, after meeting with a team of medical and mental health professionals and giving informed consent, according to the guidelines.
Dr. Safer expressed surprise at the opposition to puberty blockers in proposed state legislation. “Puberty blockers are the conservative option so that we can avoid permanent changes while thoughtful decisions are being made by our adolescent patients with their families and health care providers,” he said.
The perception that puberty blockers will lead to hormone therapy is another misunderstanding and source of misinformation, Dr. Safer explained.
“The fear is that these data suggest that puberty blockers are a ‘gateway drug’ of some sort. But that is false. The reason that most adolescents who take puberty blockers go on to hormone therapy is because most of the adolescents who are identified in our conservative systems are actually transgender and interested in more gender-affirming care as they age,” he said.
Effects of discrimination on TGD persons
Many of these proposed state bills have not advanced through state legislatures, but a few – such as the proposed laws in Alabama, Missouri, and Texas – are still currently under consideration.
“In the United States, most recent efforts to single out transgender and gender-diverse people for discrimination in health care have failed. However, the demonization of trans people and attempts to disrupt care have been the source of much stress among our patients,” Dr. Safer said.
Restricting access to health care has “multiple implications” for TGD patients. “In the era when we did not provide care to transgender youth, we had a situation where approximately 40% of transgender people had considered suicide in their lives,” Dr. Safer said. In contrast, having access to these treatments has been shown to improve mental health outcomes in these patients, according to an Endocrine Society position statement.
The purpose of earlier interventions such as puberty blockers is to allow an adolescent to “explore options and live in the experienced gender before making a decision to proceed with gender-affirming hormone therapy,” the authors of the Endocrine Society and Pediatric Endocrine Society joint statement said.
Blocking access to puberty blockers, on the other hand, forces transgender youth to experience a puberty that doesn’t match their gender identity, Dr. Safer noted. “The puberty will include permanent changes which will then have to be reversed with surgery. Why would we intentionally allow that to happen?”
Dr. Safer reported that his spouse is an employee of Parexel. Dr. Tangpricha is the current president of the World Association for Transgender Health and a board member of the American Association of Clinical Endocrinology. The other authors reported no relevant conflicts of interests.
SOURCE: Safer JD et al. J Clin Endocrinol Metab. 2020. doi: 10.1210/clinem/dgaa816.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM