User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Managing hyperhidrosis, HS: Ask questions first
A wide variety of medications exists for treating hyperhidrosis, a dermatologist told colleagues, but before prescribing anything to a pediatric patient, he recommended, ask the patient a simple question: “What bothers you the most?”
The answer will provide guidance for developing a step-by-step treatment strategy and help provide the patient “a set of realistic expectations in terms of what the response will look like,” George Hightower, MD, PhD, a pediatric dermatologist at Rady Children’s Hospital and the University of California, San Diego, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
A similar question-based approach will help guide therapy for patients with hidradenitis suppurativa (HS), he said.
With regards to hyperhidrosis, Dr. Hightower said that patients most commonly complain that their underarms are too smelly, too sweaty, and red, itchy, or painful. Causes, he said, can include irritation/contact dermatitis, folliculitis, and seborrheic dermatitis, as well as hyperhidrosis or HS.
Primary focal axillary hyperhidrosis is defined as focal, visible, excessive sweating for at least 6 months without an apparent cause plus at least two of the following characteristics: Sweating is bilateral and relatively symmetric, it impairs daily activities, it starts before the age of 25 with at least one episode per week (many patients have it daily), a family history of idiopathic hyperhidrosis is present, and focal sweating does not occur during sleep.
Secondary hyperhidrosis can be linked to other conditions, such as a spinal column injury, Dr. Hightower noted.
The first step on the treatment ladder is topical 20% aluminum chloride, which is available over the counter. This should be applied nightly for 1 week then every 1-2 weeks, Dr. Hightower recommended. All of his patients with hyperhidrosis have had at least one trial of this treatment.
The next option is daily topical treatment with 2.4% glycopyrronium tosylate (Qbrexza) cloths, approved by the Food and Drug Administration in 2018 for primary axillary hyperhidrosis in patients aged 9 and older. According to the prescribing information, dry mouth was by far the most common treatment-associated adverse effect in clinical trials (24% versus almost 6% among those on vehicle). As for skin reactions, erythema occurred in about 17% of both the intervention and vehicle groups, and burning/stinging occurred in 14% of those on treatment and almost 17% of those on vehicle.
“If they’re not able to get access to the cloths due to [insurance] coverage issues, or they don’t allow them to reach the clinical endpoint desired, then I use an oral daily glycopyrrolate pill,” Dr. Hightower said.
He recommends 1 mg to 6 mg daily of the anticholinergic drug, which has been used off-label for hyperhidrosis for several years. A 2012 study of 31 children with hyperhidrosis, he noted, supported the use of the drug. The retrospective study found that 90% of the patients, at a mean daily dose of 2 mg, experienced improvements, reported as major in 71%. In addition, patients experienced improvement within hours of taking the medication, and benefits disappeared within a day of stopping the medication. In the study, patients were on the treatment for an average of 2.1 years, and 29% experienced side effects, which were dose related; the most common were dry mouth in 26% and dry eyes in 10%.
According to goodrx.com, a month’s supply of 2 mg of the drug costs as little as $13 with a discount or coupon.
The next steps in treatment are procedural interventions such as microwave-based therapies.
Dr. Hightower said that patients should be advised that treatment may take years, and to encourage them to return for follow-up. He suggested this helpful message: “We’re still trying to find the best treatment for you, and we’ll need to see you back in the office.”
Hidradenitis suppurativa
Dr. Hightower said that too often, HS goes undiagnosed for a significant period of time, preventing patients from seeing a dermatologist for treatment. Hallmarks of HS include inflammatory nodules, abscesses, and scarring, he said. “It can be disfiguring, painful, embarrassing, and associated with significantly decreased quality of life. Early recognition in terms of making and solidifying the diagnosis is important so we can prevent further worsening of the disease.”
The goal of treatment include preventing scars and unnecessary emergency department visits, and stopping flares from worsening, Dr. Hightower said. For specifics, he pointed to clinical management guidelines released by the United States and Canadian hidradenitis suppurativa foundations in 2019.
Make sure to set individualized treatment goals and understand the impact of treatment on the patient’s interactions with family, school, and peers, he said. And keep in mind that “parent-defined goals may be different from patient-defined goals.”
Dr. Hightower reported no relevant disclosures. MedscapeLive and this news organization are owned by the same parent company
A wide variety of medications exists for treating hyperhidrosis, a dermatologist told colleagues, but before prescribing anything to a pediatric patient, he recommended, ask the patient a simple question: “What bothers you the most?”
The answer will provide guidance for developing a step-by-step treatment strategy and help provide the patient “a set of realistic expectations in terms of what the response will look like,” George Hightower, MD, PhD, a pediatric dermatologist at Rady Children’s Hospital and the University of California, San Diego, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
A similar question-based approach will help guide therapy for patients with hidradenitis suppurativa (HS), he said.
With regards to hyperhidrosis, Dr. Hightower said that patients most commonly complain that their underarms are too smelly, too sweaty, and red, itchy, or painful. Causes, he said, can include irritation/contact dermatitis, folliculitis, and seborrheic dermatitis, as well as hyperhidrosis or HS.
Primary focal axillary hyperhidrosis is defined as focal, visible, excessive sweating for at least 6 months without an apparent cause plus at least two of the following characteristics: Sweating is bilateral and relatively symmetric, it impairs daily activities, it starts before the age of 25 with at least one episode per week (many patients have it daily), a family history of idiopathic hyperhidrosis is present, and focal sweating does not occur during sleep.
Secondary hyperhidrosis can be linked to other conditions, such as a spinal column injury, Dr. Hightower noted.
The first step on the treatment ladder is topical 20% aluminum chloride, which is available over the counter. This should be applied nightly for 1 week then every 1-2 weeks, Dr. Hightower recommended. All of his patients with hyperhidrosis have had at least one trial of this treatment.
The next option is daily topical treatment with 2.4% glycopyrronium tosylate (Qbrexza) cloths, approved by the Food and Drug Administration in 2018 for primary axillary hyperhidrosis in patients aged 9 and older. According to the prescribing information, dry mouth was by far the most common treatment-associated adverse effect in clinical trials (24% versus almost 6% among those on vehicle). As for skin reactions, erythema occurred in about 17% of both the intervention and vehicle groups, and burning/stinging occurred in 14% of those on treatment and almost 17% of those on vehicle.
“If they’re not able to get access to the cloths due to [insurance] coverage issues, or they don’t allow them to reach the clinical endpoint desired, then I use an oral daily glycopyrrolate pill,” Dr. Hightower said.
He recommends 1 mg to 6 mg daily of the anticholinergic drug, which has been used off-label for hyperhidrosis for several years. A 2012 study of 31 children with hyperhidrosis, he noted, supported the use of the drug. The retrospective study found that 90% of the patients, at a mean daily dose of 2 mg, experienced improvements, reported as major in 71%. In addition, patients experienced improvement within hours of taking the medication, and benefits disappeared within a day of stopping the medication. In the study, patients were on the treatment for an average of 2.1 years, and 29% experienced side effects, which were dose related; the most common were dry mouth in 26% and dry eyes in 10%.
According to goodrx.com, a month’s supply of 2 mg of the drug costs as little as $13 with a discount or coupon.
The next steps in treatment are procedural interventions such as microwave-based therapies.
Dr. Hightower said that patients should be advised that treatment may take years, and to encourage them to return for follow-up. He suggested this helpful message: “We’re still trying to find the best treatment for you, and we’ll need to see you back in the office.”
Hidradenitis suppurativa
Dr. Hightower said that too often, HS goes undiagnosed for a significant period of time, preventing patients from seeing a dermatologist for treatment. Hallmarks of HS include inflammatory nodules, abscesses, and scarring, he said. “It can be disfiguring, painful, embarrassing, and associated with significantly decreased quality of life. Early recognition in terms of making and solidifying the diagnosis is important so we can prevent further worsening of the disease.”
The goal of treatment include preventing scars and unnecessary emergency department visits, and stopping flares from worsening, Dr. Hightower said. For specifics, he pointed to clinical management guidelines released by the United States and Canadian hidradenitis suppurativa foundations in 2019.
Make sure to set individualized treatment goals and understand the impact of treatment on the patient’s interactions with family, school, and peers, he said. And keep in mind that “parent-defined goals may be different from patient-defined goals.”
Dr. Hightower reported no relevant disclosures. MedscapeLive and this news organization are owned by the same parent company
A wide variety of medications exists for treating hyperhidrosis, a dermatologist told colleagues, but before prescribing anything to a pediatric patient, he recommended, ask the patient a simple question: “What bothers you the most?”
The answer will provide guidance for developing a step-by-step treatment strategy and help provide the patient “a set of realistic expectations in terms of what the response will look like,” George Hightower, MD, PhD, a pediatric dermatologist at Rady Children’s Hospital and the University of California, San Diego, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
A similar question-based approach will help guide therapy for patients with hidradenitis suppurativa (HS), he said.
With regards to hyperhidrosis, Dr. Hightower said that patients most commonly complain that their underarms are too smelly, too sweaty, and red, itchy, or painful. Causes, he said, can include irritation/contact dermatitis, folliculitis, and seborrheic dermatitis, as well as hyperhidrosis or HS.
Primary focal axillary hyperhidrosis is defined as focal, visible, excessive sweating for at least 6 months without an apparent cause plus at least two of the following characteristics: Sweating is bilateral and relatively symmetric, it impairs daily activities, it starts before the age of 25 with at least one episode per week (many patients have it daily), a family history of idiopathic hyperhidrosis is present, and focal sweating does not occur during sleep.
Secondary hyperhidrosis can be linked to other conditions, such as a spinal column injury, Dr. Hightower noted.
The first step on the treatment ladder is topical 20% aluminum chloride, which is available over the counter. This should be applied nightly for 1 week then every 1-2 weeks, Dr. Hightower recommended. All of his patients with hyperhidrosis have had at least one trial of this treatment.
The next option is daily topical treatment with 2.4% glycopyrronium tosylate (Qbrexza) cloths, approved by the Food and Drug Administration in 2018 for primary axillary hyperhidrosis in patients aged 9 and older. According to the prescribing information, dry mouth was by far the most common treatment-associated adverse effect in clinical trials (24% versus almost 6% among those on vehicle). As for skin reactions, erythema occurred in about 17% of both the intervention and vehicle groups, and burning/stinging occurred in 14% of those on treatment and almost 17% of those on vehicle.
“If they’re not able to get access to the cloths due to [insurance] coverage issues, or they don’t allow them to reach the clinical endpoint desired, then I use an oral daily glycopyrrolate pill,” Dr. Hightower said.
He recommends 1 mg to 6 mg daily of the anticholinergic drug, which has been used off-label for hyperhidrosis for several years. A 2012 study of 31 children with hyperhidrosis, he noted, supported the use of the drug. The retrospective study found that 90% of the patients, at a mean daily dose of 2 mg, experienced improvements, reported as major in 71%. In addition, patients experienced improvement within hours of taking the medication, and benefits disappeared within a day of stopping the medication. In the study, patients were on the treatment for an average of 2.1 years, and 29% experienced side effects, which were dose related; the most common were dry mouth in 26% and dry eyes in 10%.
According to goodrx.com, a month’s supply of 2 mg of the drug costs as little as $13 with a discount or coupon.
The next steps in treatment are procedural interventions such as microwave-based therapies.
Dr. Hightower said that patients should be advised that treatment may take years, and to encourage them to return for follow-up. He suggested this helpful message: “We’re still trying to find the best treatment for you, and we’ll need to see you back in the office.”
Hidradenitis suppurativa
Dr. Hightower said that too often, HS goes undiagnosed for a significant period of time, preventing patients from seeing a dermatologist for treatment. Hallmarks of HS include inflammatory nodules, abscesses, and scarring, he said. “It can be disfiguring, painful, embarrassing, and associated with significantly decreased quality of life. Early recognition in terms of making and solidifying the diagnosis is important so we can prevent further worsening of the disease.”
The goal of treatment include preventing scars and unnecessary emergency department visits, and stopping flares from worsening, Dr. Hightower said. For specifics, he pointed to clinical management guidelines released by the United States and Canadian hidradenitis suppurativa foundations in 2019.
Make sure to set individualized treatment goals and understand the impact of treatment on the patient’s interactions with family, school, and peers, he said. And keep in mind that “parent-defined goals may be different from patient-defined goals.”
Dr. Hightower reported no relevant disclosures. MedscapeLive and this news organization are owned by the same parent company
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Myth busting: SARS-CoV-2 vaccine
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
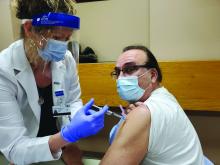
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
Bacteriotherapy passes early test in phase 1 atopic dermatitis study
that also demonstrated “encouraging clinical and mechanistic results,” Richard L. Gallo, MD, PhD, and his coinvestigators have reported in Nature Medicine.
Findings from the 1-week, 54-patient trial of a topical formulation containing Staphylococcus hominis A9 (ShA9) offer evidence that the strain directly kills S. aureus, inhibits the production of S. aureus–generated toxins, and enables expansion of a healthy bacterial community, “allowing the rest of the microbiome to start to recover to normal,” Dr. Gallo, professor and chairman of the department of dermatology at the University of California, San Diego, said in an interview.
“And perhaps most exciting,” Dr. Gallo added, is the finding that the subset of patients with AD who were most responsive to the ShA9 compound – approximately two-thirds of the participants who were randomized to receive it – showed improvement in local EASI (Eczema Area and Severity Index) and SCORAD (Scoring Atopic Dermatitis) scores used to assess inflammation. Plans are underway for a larger and longer trial, he said.
S. aureus commonly colonizes patients with AD and exacerbates disease by causing inflammation. In recent years, Dr. Gallo and other investigators have come to believe that AD is a cyclic disease in which the skin’s microbiome affects the host, and the host affects the microbiome. The goal of bacteriotherapy is to break the cycle of S. aureus colonization and improve the skin immune and barrier dysfunction characteristics of AD, Dr. Gallo said.
ShA9, a bacterium isolated from healthy human skin, was chosen as a potential topical therapy for AD based on its capacity both to selectively kill S. aureus and to inhibit toxin production by S. aureus. Dr. Gallo’s team’s preclinical work involved screening thousands of isolates of coagulase-negative staphylococci for gene products that perform these two functions by expressing both antimicrobial peptides (AMPs) and autoinducing peptides (AIPs), the latter of which inhibit the S. aureus quorum-sending system that leads to toxin production. Most patients with AD lack protective strains of coagulase-negative staphylococci, including S. hominis, prior research has found.
The double-blind phase 1 trial randomized 54 adults with moderate-severe AD affecting the ventral forearms in a 2:1 fashion to receive the proprietary lyophilized preparation of ShA9 or an ShA9-free formulation twice daily for 1 week. All participants were culture positive for S. aureus.
Clinical assessments and skin swabs were obtained before and within an hour after the first application of day 1, and swabs were collected on days 4 and 7 within 4 hours of the first application.
Blinded physician assessments and skin swabs were also obtained at 24, 48, and 96 hours after the final dose on day 7.
Based on structured daily diaries, there were no serious adverse events, and significantly fewer adverse events in those treated with ShA9, compared with the vehicle alone; 55.6% versus 83.3%, respectively, were considered to have adverse events.
The adverse event–reporting system captured the normal fluctuation of eczema and considered any report of fluctuation above baseline to be an adverse event. “Patients treated with the [placebo formulation] had the expected high frequency of itching, burning, and pain that you see with AD but it was encouraging that the frequency of reporting these events was significantly less in those treated with the active [formulation],” Dr. Gallo said in the interview.
Their report describes a decrease in S. aureus in participants treated with ShA9, and increases in ShA9 DNA. Not all S. aureus strains were directly killed by ShA9, but all strains had reduced expression of mRNA for psm-alpha, an important virulence factor. That reduced expression correlated with ShA9 AIPs and improved EASI scores, the latter of which was observed in a post-hoc analysis. “Participants with S. aureus not killed by ShA9 were still sensitive to inhibition of toxin production, a mechanistic outcome that predicted clinical improvement in mice and may require longer therapy to observe clinical improvement in humans,” the investigators wrote.
Local eczema severity was not significantly different between the bacteriotherapy and control groups. But the post-hoc analysis showed that after 7 days of treatment, and up to 4 days after treatment was discontinued, the patients with S. aureus that was sensitive to killing by ShA9 (21 out of 35 total who received the bacteriotherapy) showed improvement in EASI and SCORAD scores, compared with control patients.
Future research will assess the compound in both S. aureus culture-positive and culture-negative patients, and in patients with mild disease, Dr. Gallo said.
The trial was conducted at USCD and the National Jewish Health General Clinical Research Center in Denver, and was sponsored by the National Institute of Allergy and Infectious Diseases. The ShA9 formulation and related technology are licensed to MatriSys Bioscience, of which Dr. Gallo is the cofounder and an advisory board member. Dr. Gallo holds equity interest in the company.
that also demonstrated “encouraging clinical and mechanistic results,” Richard L. Gallo, MD, PhD, and his coinvestigators have reported in Nature Medicine.
Findings from the 1-week, 54-patient trial of a topical formulation containing Staphylococcus hominis A9 (ShA9) offer evidence that the strain directly kills S. aureus, inhibits the production of S. aureus–generated toxins, and enables expansion of a healthy bacterial community, “allowing the rest of the microbiome to start to recover to normal,” Dr. Gallo, professor and chairman of the department of dermatology at the University of California, San Diego, said in an interview.
“And perhaps most exciting,” Dr. Gallo added, is the finding that the subset of patients with AD who were most responsive to the ShA9 compound – approximately two-thirds of the participants who were randomized to receive it – showed improvement in local EASI (Eczema Area and Severity Index) and SCORAD (Scoring Atopic Dermatitis) scores used to assess inflammation. Plans are underway for a larger and longer trial, he said.
S. aureus commonly colonizes patients with AD and exacerbates disease by causing inflammation. In recent years, Dr. Gallo and other investigators have come to believe that AD is a cyclic disease in which the skin’s microbiome affects the host, and the host affects the microbiome. The goal of bacteriotherapy is to break the cycle of S. aureus colonization and improve the skin immune and barrier dysfunction characteristics of AD, Dr. Gallo said.
ShA9, a bacterium isolated from healthy human skin, was chosen as a potential topical therapy for AD based on its capacity both to selectively kill S. aureus and to inhibit toxin production by S. aureus. Dr. Gallo’s team’s preclinical work involved screening thousands of isolates of coagulase-negative staphylococci for gene products that perform these two functions by expressing both antimicrobial peptides (AMPs) and autoinducing peptides (AIPs), the latter of which inhibit the S. aureus quorum-sending system that leads to toxin production. Most patients with AD lack protective strains of coagulase-negative staphylococci, including S. hominis, prior research has found.
The double-blind phase 1 trial randomized 54 adults with moderate-severe AD affecting the ventral forearms in a 2:1 fashion to receive the proprietary lyophilized preparation of ShA9 or an ShA9-free formulation twice daily for 1 week. All participants were culture positive for S. aureus.
Clinical assessments and skin swabs were obtained before and within an hour after the first application of day 1, and swabs were collected on days 4 and 7 within 4 hours of the first application.
Blinded physician assessments and skin swabs were also obtained at 24, 48, and 96 hours after the final dose on day 7.
Based on structured daily diaries, there were no serious adverse events, and significantly fewer adverse events in those treated with ShA9, compared with the vehicle alone; 55.6% versus 83.3%, respectively, were considered to have adverse events.
The adverse event–reporting system captured the normal fluctuation of eczema and considered any report of fluctuation above baseline to be an adverse event. “Patients treated with the [placebo formulation] had the expected high frequency of itching, burning, and pain that you see with AD but it was encouraging that the frequency of reporting these events was significantly less in those treated with the active [formulation],” Dr. Gallo said in the interview.
Their report describes a decrease in S. aureus in participants treated with ShA9, and increases in ShA9 DNA. Not all S. aureus strains were directly killed by ShA9, but all strains had reduced expression of mRNA for psm-alpha, an important virulence factor. That reduced expression correlated with ShA9 AIPs and improved EASI scores, the latter of which was observed in a post-hoc analysis. “Participants with S. aureus not killed by ShA9 were still sensitive to inhibition of toxin production, a mechanistic outcome that predicted clinical improvement in mice and may require longer therapy to observe clinical improvement in humans,” the investigators wrote.
Local eczema severity was not significantly different between the bacteriotherapy and control groups. But the post-hoc analysis showed that after 7 days of treatment, and up to 4 days after treatment was discontinued, the patients with S. aureus that was sensitive to killing by ShA9 (21 out of 35 total who received the bacteriotherapy) showed improvement in EASI and SCORAD scores, compared with control patients.
Future research will assess the compound in both S. aureus culture-positive and culture-negative patients, and in patients with mild disease, Dr. Gallo said.
The trial was conducted at USCD and the National Jewish Health General Clinical Research Center in Denver, and was sponsored by the National Institute of Allergy and Infectious Diseases. The ShA9 formulation and related technology are licensed to MatriSys Bioscience, of which Dr. Gallo is the cofounder and an advisory board member. Dr. Gallo holds equity interest in the company.
that also demonstrated “encouraging clinical and mechanistic results,” Richard L. Gallo, MD, PhD, and his coinvestigators have reported in Nature Medicine.
Findings from the 1-week, 54-patient trial of a topical formulation containing Staphylococcus hominis A9 (ShA9) offer evidence that the strain directly kills S. aureus, inhibits the production of S. aureus–generated toxins, and enables expansion of a healthy bacterial community, “allowing the rest of the microbiome to start to recover to normal,” Dr. Gallo, professor and chairman of the department of dermatology at the University of California, San Diego, said in an interview.
“And perhaps most exciting,” Dr. Gallo added, is the finding that the subset of patients with AD who were most responsive to the ShA9 compound – approximately two-thirds of the participants who were randomized to receive it – showed improvement in local EASI (Eczema Area and Severity Index) and SCORAD (Scoring Atopic Dermatitis) scores used to assess inflammation. Plans are underway for a larger and longer trial, he said.
S. aureus commonly colonizes patients with AD and exacerbates disease by causing inflammation. In recent years, Dr. Gallo and other investigators have come to believe that AD is a cyclic disease in which the skin’s microbiome affects the host, and the host affects the microbiome. The goal of bacteriotherapy is to break the cycle of S. aureus colonization and improve the skin immune and barrier dysfunction characteristics of AD, Dr. Gallo said.
ShA9, a bacterium isolated from healthy human skin, was chosen as a potential topical therapy for AD based on its capacity both to selectively kill S. aureus and to inhibit toxin production by S. aureus. Dr. Gallo’s team’s preclinical work involved screening thousands of isolates of coagulase-negative staphylococci for gene products that perform these two functions by expressing both antimicrobial peptides (AMPs) and autoinducing peptides (AIPs), the latter of which inhibit the S. aureus quorum-sending system that leads to toxin production. Most patients with AD lack protective strains of coagulase-negative staphylococci, including S. hominis, prior research has found.
The double-blind phase 1 trial randomized 54 adults with moderate-severe AD affecting the ventral forearms in a 2:1 fashion to receive the proprietary lyophilized preparation of ShA9 or an ShA9-free formulation twice daily for 1 week. All participants were culture positive for S. aureus.
Clinical assessments and skin swabs were obtained before and within an hour after the first application of day 1, and swabs were collected on days 4 and 7 within 4 hours of the first application.
Blinded physician assessments and skin swabs were also obtained at 24, 48, and 96 hours after the final dose on day 7.
Based on structured daily diaries, there were no serious adverse events, and significantly fewer adverse events in those treated with ShA9, compared with the vehicle alone; 55.6% versus 83.3%, respectively, were considered to have adverse events.
The adverse event–reporting system captured the normal fluctuation of eczema and considered any report of fluctuation above baseline to be an adverse event. “Patients treated with the [placebo formulation] had the expected high frequency of itching, burning, and pain that you see with AD but it was encouraging that the frequency of reporting these events was significantly less in those treated with the active [formulation],” Dr. Gallo said in the interview.
Their report describes a decrease in S. aureus in participants treated with ShA9, and increases in ShA9 DNA. Not all S. aureus strains were directly killed by ShA9, but all strains had reduced expression of mRNA for psm-alpha, an important virulence factor. That reduced expression correlated with ShA9 AIPs and improved EASI scores, the latter of which was observed in a post-hoc analysis. “Participants with S. aureus not killed by ShA9 were still sensitive to inhibition of toxin production, a mechanistic outcome that predicted clinical improvement in mice and may require longer therapy to observe clinical improvement in humans,” the investigators wrote.
Local eczema severity was not significantly different between the bacteriotherapy and control groups. But the post-hoc analysis showed that after 7 days of treatment, and up to 4 days after treatment was discontinued, the patients with S. aureus that was sensitive to killing by ShA9 (21 out of 35 total who received the bacteriotherapy) showed improvement in EASI and SCORAD scores, compared with control patients.
Future research will assess the compound in both S. aureus culture-positive and culture-negative patients, and in patients with mild disease, Dr. Gallo said.
The trial was conducted at USCD and the National Jewish Health General Clinical Research Center in Denver, and was sponsored by the National Institute of Allergy and Infectious Diseases. The ShA9 formulation and related technology are licensed to MatriSys Bioscience, of which Dr. Gallo is the cofounder and an advisory board member. Dr. Gallo holds equity interest in the company.
FROM NATURE MEDICINE
CDC data strengthen link between obesity and severe COVID
Officials have previously linked being overweight or obese to a greater risk for more severe COVID-19. A report today from the U.S. Centers for Disease Control and Prevention adds numbers and some nuance to the association.
Data from nearly 150,000 U.S. adults hospitalized with COVID-19 nationwide indicate that risk for more severe disease outcomes increases along with body mass index (BMI). The risk of COVID-19–related hospitalization and death associated with obesity was particularly high among people younger than 65.
“As clinicians develop care plans for COVID-19 patients, they should consider the risk for severe outcomes in patients with higher BMIs, especially for those with severe obesity,” the researchers note. They add that their findings suggest “progressively intensive management of COVID-19 might be needed for patients with more severe obesity.”
People with COVID-19 close to the border between a healthy and overweight BMI – from 23.7 kg/m2 to 25.9 kg/m2 – had the lowest risks for adverse outcomes.
The study was published online today in Morbidity and Mortality Weekly Report.
Greater need for critical care
The risk of ICU admission was particularly associated with severe obesity. For example, those with a BMI in the 40-44.9 kg/m2 category had a 6% increased risk, which jumped to 16% higher among those with a BMI of 45 or greater.
Compared to people with a healthy BMI, the need for invasive mechanical ventilation was 12% more likely among overweight adults with a BMI of 25-29.2. The risked jumped to 108% greater among the most obese people, those with a BMI of 45 or greater, lead CDC researcher Lyudmyla Kompaniyets, PhD, and colleagues reported.
Moreover, the risks for hospitalization and death increased in a dose-response relationship with obesity.
For example, risks of being hospitalized were 7% greater for adults with a BMI between 30 and 34.9 and climbed to 33% greater for those with a BMI of 45. Risks were calculated as adjusted relative risks compared with people with a healthy BMI between 18.5 and 24.9.
Interestingly, being underweight was associated with elevated risk for COVID-19 hospitalization as well. For example, people with a BMI of less than 18.5 had a 20% greater chance of admission vs. people in the healthy BMI range. Unknown underlying medical conditions or issues related to nutrition or immune function could be contributing factors, the researchers note.
Elevated risk of dying
The risk of death in adults with obesity ranged from 8% higher in the 30-34.9 range up to 61% greater for those with a BMI of 45.
Chronic inflammation or impaired lung function from excess weight are possible reasons that higher BMI imparts greater risk, the researchers note.
The CDC researchers evaluated 148,494 adults from 238 hospitals participating in PHD-SR database. Because the study was limited to people hospitalized with COVID-19, the findings may not apply to all adults with COVID-19.
Another potential limitation is that investigators were unable to calculate BMI for all patients in the database because about 28% of participating hospitals did not report height and weight.
The study authors had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
Officials have previously linked being overweight or obese to a greater risk for more severe COVID-19. A report today from the U.S. Centers for Disease Control and Prevention adds numbers and some nuance to the association.
Data from nearly 150,000 U.S. adults hospitalized with COVID-19 nationwide indicate that risk for more severe disease outcomes increases along with body mass index (BMI). The risk of COVID-19–related hospitalization and death associated with obesity was particularly high among people younger than 65.
“As clinicians develop care plans for COVID-19 patients, they should consider the risk for severe outcomes in patients with higher BMIs, especially for those with severe obesity,” the researchers note. They add that their findings suggest “progressively intensive management of COVID-19 might be needed for patients with more severe obesity.”
People with COVID-19 close to the border between a healthy and overweight BMI – from 23.7 kg/m2 to 25.9 kg/m2 – had the lowest risks for adverse outcomes.
The study was published online today in Morbidity and Mortality Weekly Report.
Greater need for critical care
The risk of ICU admission was particularly associated with severe obesity. For example, those with a BMI in the 40-44.9 kg/m2 category had a 6% increased risk, which jumped to 16% higher among those with a BMI of 45 or greater.
Compared to people with a healthy BMI, the need for invasive mechanical ventilation was 12% more likely among overweight adults with a BMI of 25-29.2. The risked jumped to 108% greater among the most obese people, those with a BMI of 45 or greater, lead CDC researcher Lyudmyla Kompaniyets, PhD, and colleagues reported.
Moreover, the risks for hospitalization and death increased in a dose-response relationship with obesity.
For example, risks of being hospitalized were 7% greater for adults with a BMI between 30 and 34.9 and climbed to 33% greater for those with a BMI of 45. Risks were calculated as adjusted relative risks compared with people with a healthy BMI between 18.5 and 24.9.
Interestingly, being underweight was associated with elevated risk for COVID-19 hospitalization as well. For example, people with a BMI of less than 18.5 had a 20% greater chance of admission vs. people in the healthy BMI range. Unknown underlying medical conditions or issues related to nutrition or immune function could be contributing factors, the researchers note.
Elevated risk of dying
The risk of death in adults with obesity ranged from 8% higher in the 30-34.9 range up to 61% greater for those with a BMI of 45.
Chronic inflammation or impaired lung function from excess weight are possible reasons that higher BMI imparts greater risk, the researchers note.
The CDC researchers evaluated 148,494 adults from 238 hospitals participating in PHD-SR database. Because the study was limited to people hospitalized with COVID-19, the findings may not apply to all adults with COVID-19.
Another potential limitation is that investigators were unable to calculate BMI for all patients in the database because about 28% of participating hospitals did not report height and weight.
The study authors had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
Officials have previously linked being overweight or obese to a greater risk for more severe COVID-19. A report today from the U.S. Centers for Disease Control and Prevention adds numbers and some nuance to the association.
Data from nearly 150,000 U.S. adults hospitalized with COVID-19 nationwide indicate that risk for more severe disease outcomes increases along with body mass index (BMI). The risk of COVID-19–related hospitalization and death associated with obesity was particularly high among people younger than 65.
“As clinicians develop care plans for COVID-19 patients, they should consider the risk for severe outcomes in patients with higher BMIs, especially for those with severe obesity,” the researchers note. They add that their findings suggest “progressively intensive management of COVID-19 might be needed for patients with more severe obesity.”
People with COVID-19 close to the border between a healthy and overweight BMI – from 23.7 kg/m2 to 25.9 kg/m2 – had the lowest risks for adverse outcomes.
The study was published online today in Morbidity and Mortality Weekly Report.
Greater need for critical care
The risk of ICU admission was particularly associated with severe obesity. For example, those with a BMI in the 40-44.9 kg/m2 category had a 6% increased risk, which jumped to 16% higher among those with a BMI of 45 or greater.
Compared to people with a healthy BMI, the need for invasive mechanical ventilation was 12% more likely among overweight adults with a BMI of 25-29.2. The risked jumped to 108% greater among the most obese people, those with a BMI of 45 or greater, lead CDC researcher Lyudmyla Kompaniyets, PhD, and colleagues reported.
Moreover, the risks for hospitalization and death increased in a dose-response relationship with obesity.
For example, risks of being hospitalized were 7% greater for adults with a BMI between 30 and 34.9 and climbed to 33% greater for those with a BMI of 45. Risks were calculated as adjusted relative risks compared with people with a healthy BMI between 18.5 and 24.9.
Interestingly, being underweight was associated with elevated risk for COVID-19 hospitalization as well. For example, people with a BMI of less than 18.5 had a 20% greater chance of admission vs. people in the healthy BMI range. Unknown underlying medical conditions or issues related to nutrition or immune function could be contributing factors, the researchers note.
Elevated risk of dying
The risk of death in adults with obesity ranged from 8% higher in the 30-34.9 range up to 61% greater for those with a BMI of 45.
Chronic inflammation or impaired lung function from excess weight are possible reasons that higher BMI imparts greater risk, the researchers note.
The CDC researchers evaluated 148,494 adults from 238 hospitals participating in PHD-SR database. Because the study was limited to people hospitalized with COVID-19, the findings may not apply to all adults with COVID-19.
Another potential limitation is that investigators were unable to calculate BMI for all patients in the database because about 28% of participating hospitals did not report height and weight.
The study authors had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
FDA authorizes first molecular at-home, OTC COVID-19 test
The U.S. Food and Drug Administration has granted emergency use authorization (EUA) for the Cue COVID-19 Test for Home and Over The Counter Use (Cue OTC Test, Cue Health).
The Cue OTC Test is the first molecular diagnostic test available to consumers without a prescription.
The test detects genetic material from SARS-CoV-2 present in the nostrils and delivers results in about 20 minutes to the user’s mobile smart device via the Cue Health app.
In testing, the Cue OTC Test correctly identified 96% of positive nasal swab samples from individuals known to have symptoms and correctly identified 100% of positive samples from individuals without symptoms.
The test is intended for use in people aged 2 years and older with and without symptoms.
“With this authorization, consumers can purchase and self-administer one of the easiest, fastest, and most accurate tests without a prescription,” Clint Sever, cofounder and chief product officer of Cue Health, said in a news release.
“This FDA authorization will help us improve patient outcomes with a solution that provides the accuracy of central lab tests, with the speed and accessibility required to address emergent global health issues,” he said.
Cue Health expects to produce more than 100,000 single-use test kits per day by this summer. Dena Cook, the company’s chief communications officer, told this news organization that the company hasn’t announced pricing information yet, but the price will be “comparable” to other price points and other products on the market.
“The FDA continues to prioritize the availability of more at-home testing options in response to the pandemic,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement.
“Cue COVID-19 Test for Home and Over-the-Counter Use provides access to accurate and reliable testing at home, without a prescription. The FDA will continue to work collaboratively with test developers to advance effective testing options for doctors, clinicians, and the public,” he said.
In June, the FDA granted an EUA to Cue Health’s COVID-19 test for use in clinical and point-of-care settings.
The test is currently being used in hospitals, physicians’ offices, and dental clinics, as well as schools, essential businesses, nursing homes, and other congregate-care facilities. The test is also being distributed through a program led by the U.S. Department of Defense and the U.S. Department of Health & Human Services across several states.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has granted emergency use authorization (EUA) for the Cue COVID-19 Test for Home and Over The Counter Use (Cue OTC Test, Cue Health).
The Cue OTC Test is the first molecular diagnostic test available to consumers without a prescription.
The test detects genetic material from SARS-CoV-2 present in the nostrils and delivers results in about 20 minutes to the user’s mobile smart device via the Cue Health app.
In testing, the Cue OTC Test correctly identified 96% of positive nasal swab samples from individuals known to have symptoms and correctly identified 100% of positive samples from individuals without symptoms.
The test is intended for use in people aged 2 years and older with and without symptoms.
“With this authorization, consumers can purchase and self-administer one of the easiest, fastest, and most accurate tests without a prescription,” Clint Sever, cofounder and chief product officer of Cue Health, said in a news release.
“This FDA authorization will help us improve patient outcomes with a solution that provides the accuracy of central lab tests, with the speed and accessibility required to address emergent global health issues,” he said.
Cue Health expects to produce more than 100,000 single-use test kits per day by this summer. Dena Cook, the company’s chief communications officer, told this news organization that the company hasn’t announced pricing information yet, but the price will be “comparable” to other price points and other products on the market.
“The FDA continues to prioritize the availability of more at-home testing options in response to the pandemic,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement.
“Cue COVID-19 Test for Home and Over-the-Counter Use provides access to accurate and reliable testing at home, without a prescription. The FDA will continue to work collaboratively with test developers to advance effective testing options for doctors, clinicians, and the public,” he said.
In June, the FDA granted an EUA to Cue Health’s COVID-19 test for use in clinical and point-of-care settings.
The test is currently being used in hospitals, physicians’ offices, and dental clinics, as well as schools, essential businesses, nursing homes, and other congregate-care facilities. The test is also being distributed through a program led by the U.S. Department of Defense and the U.S. Department of Health & Human Services across several states.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has granted emergency use authorization (EUA) for the Cue COVID-19 Test for Home and Over The Counter Use (Cue OTC Test, Cue Health).
The Cue OTC Test is the first molecular diagnostic test available to consumers without a prescription.
The test detects genetic material from SARS-CoV-2 present in the nostrils and delivers results in about 20 minutes to the user’s mobile smart device via the Cue Health app.
In testing, the Cue OTC Test correctly identified 96% of positive nasal swab samples from individuals known to have symptoms and correctly identified 100% of positive samples from individuals without symptoms.
The test is intended for use in people aged 2 years and older with and without symptoms.
“With this authorization, consumers can purchase and self-administer one of the easiest, fastest, and most accurate tests without a prescription,” Clint Sever, cofounder and chief product officer of Cue Health, said in a news release.
“This FDA authorization will help us improve patient outcomes with a solution that provides the accuracy of central lab tests, with the speed and accessibility required to address emergent global health issues,” he said.
Cue Health expects to produce more than 100,000 single-use test kits per day by this summer. Dena Cook, the company’s chief communications officer, told this news organization that the company hasn’t announced pricing information yet, but the price will be “comparable” to other price points and other products on the market.
“The FDA continues to prioritize the availability of more at-home testing options in response to the pandemic,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement.
“Cue COVID-19 Test for Home and Over-the-Counter Use provides access to accurate and reliable testing at home, without a prescription. The FDA will continue to work collaboratively with test developers to advance effective testing options for doctors, clinicians, and the public,” he said.
In June, the FDA granted an EUA to Cue Health’s COVID-19 test for use in clinical and point-of-care settings.
The test is currently being used in hospitals, physicians’ offices, and dental clinics, as well as schools, essential businesses, nursing homes, and other congregate-care facilities. The test is also being distributed through a program led by the U.S. Department of Defense and the U.S. Department of Health & Human Services across several states.
A version of this article first appeared on Medscape.com.
Missed visits during pandemic cause ‘detrimental ripple effects’
according to a new report from the Urban Institute.
Among the adults who postponed or missed care, 32.6% said the gap worsened one or more health conditions or limited their ability to work or perform daily activities. The findings highlight “the detrimental ripple effects of delaying or forgoing care on overall health, functioning, and well-being,” researchers write.
The survey, conducted among 4,007 U.S. adults aged 18-64 in September 2020, found that adults with one or more chronic conditions were more likely than adults without chronic conditions to have delayed or missed care (40.7% vs. 26.4%). Adults with a mental health condition were particularly likely to have delayed or gone without care, write Dulce Gonzalez, MPP, a research associate in the Health Policy Center at the Urban Institute, and colleagues.
Doctors are already seeing the consequences of the missed visits, says Jacqueline W. Fincher, MD, president of the American College of Physicians.
Two of her patients with chronic conditions missed appointments last year. By the time they resumed care in 2021, their previsit lab tests showed significant kidney deterioration.
“Lo and behold, their kidneys were in failure. … One was in the hospital for 3 days and the other one was in for 5 days,” said Dr. Fincher, who practices general internal medicine in Georgia.
Dr. Fincher’s office has been proactive about calling patients with chronic diseases who missed follow-up visits or laboratory testing or who may have run out of medication, she said.
In her experience, delays mainly have been because of patients postponing visits. “We have stayed open the whole time now,” Dr. Fincher said. Her office offers telemedicine visits and in-person visits with safety precautions.
Still, some patients have decided to postpone care during the pandemic instead of asking their primary care doctor what they should do.
“We do know that chronic problems left without appropriate follow-up can create worse problems for them in terms of stroke, heart attack, and end organ damage,” Dr. Fincher said.
Lost lives
Future studies may help researchers understand the effects of delayed and missed care during the pandemic, said Russell S. Phillips, MD, director of the Center for Primary Care at Harvard Medical School, Boston.
“Although it is still early, and more data on patient outcomes will need to be collected, I anticipate that the ... delays in diagnosis, in cancer screening, and in management of chronic illness will result in lost lives and will emphasize the important role that primary care plays in saving lives,” Dr. Phillips said.
During the first several months of the pandemic, there were fewer diagnoses of hypertension, diabetes, and depression, Dr. Phillips said.
“In addition, and most importantly, the mortality rate for non-COVID conditions increased, suggesting that patients were not seeking care for symptoms of stroke or heart attack, which can be fatal if untreated,” he said. “We have also seen substantial decreases in cancer screening tests such as colonoscopy, and modeling studies suggest this will cost more lives based on delayed diagnoses of cancer.”
Vaccinating patients against COVID-19 may help primary care practices and patients get back on track, Dr. Phillips suggested.
In the meantime, some patients remain reluctant to come in. “Volumes are still lower than prepandemic, so it is challenging to overcome what is likely to be pent-up demand,” he told this news organization in an email. “Additionally, the continued burden of evaluating, testing, and monitoring patients with COVID or COVID-like symptoms makes it difficult to focus on chronic illness.”
Care most often skipped
The Urban Institute survey asked respondents about delays in prescription drugs, general doctor and specialist visits, going to a hospital, preventive health screenings or medical tests, treatment or follow-up care, dental care, mental health care or counseling, treatment or counseling for alcohol or drug use, and other types of medical care.
Dental care was the most common type of care that adults delayed or did not receive because of the pandemic (25.3%), followed by general doctor or specialist visits (20.6%) and preventive health screenings or medical tests (15.5%).
Black adults were more likely than White or Hispanic/Latinx adults to have delayed or forgone care (39.7% vs. 34.3% and 35.5%), the researchers found. Compared with adults with higher incomes, adults with lower incomes were more likely to have missed multiple types of care (26.6% vs. 20.3%).
The report by the Urban Institute researchers was supported by the Robert Wood Johnson Foundation. Dr. Phillips is an adviser to two telemedicine companies, Bicycle Health and Grow Health. Dr. Fincher has disclosed no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
according to a new report from the Urban Institute.
Among the adults who postponed or missed care, 32.6% said the gap worsened one or more health conditions or limited their ability to work or perform daily activities. The findings highlight “the detrimental ripple effects of delaying or forgoing care on overall health, functioning, and well-being,” researchers write.
The survey, conducted among 4,007 U.S. adults aged 18-64 in September 2020, found that adults with one or more chronic conditions were more likely than adults without chronic conditions to have delayed or missed care (40.7% vs. 26.4%). Adults with a mental health condition were particularly likely to have delayed or gone without care, write Dulce Gonzalez, MPP, a research associate in the Health Policy Center at the Urban Institute, and colleagues.
Doctors are already seeing the consequences of the missed visits, says Jacqueline W. Fincher, MD, president of the American College of Physicians.
Two of her patients with chronic conditions missed appointments last year. By the time they resumed care in 2021, their previsit lab tests showed significant kidney deterioration.
“Lo and behold, their kidneys were in failure. … One was in the hospital for 3 days and the other one was in for 5 days,” said Dr. Fincher, who practices general internal medicine in Georgia.
Dr. Fincher’s office has been proactive about calling patients with chronic diseases who missed follow-up visits or laboratory testing or who may have run out of medication, she said.
In her experience, delays mainly have been because of patients postponing visits. “We have stayed open the whole time now,” Dr. Fincher said. Her office offers telemedicine visits and in-person visits with safety precautions.
Still, some patients have decided to postpone care during the pandemic instead of asking their primary care doctor what they should do.
“We do know that chronic problems left without appropriate follow-up can create worse problems for them in terms of stroke, heart attack, and end organ damage,” Dr. Fincher said.
Lost lives
Future studies may help researchers understand the effects of delayed and missed care during the pandemic, said Russell S. Phillips, MD, director of the Center for Primary Care at Harvard Medical School, Boston.
“Although it is still early, and more data on patient outcomes will need to be collected, I anticipate that the ... delays in diagnosis, in cancer screening, and in management of chronic illness will result in lost lives and will emphasize the important role that primary care plays in saving lives,” Dr. Phillips said.
During the first several months of the pandemic, there were fewer diagnoses of hypertension, diabetes, and depression, Dr. Phillips said.
“In addition, and most importantly, the mortality rate for non-COVID conditions increased, suggesting that patients were not seeking care for symptoms of stroke or heart attack, which can be fatal if untreated,” he said. “We have also seen substantial decreases in cancer screening tests such as colonoscopy, and modeling studies suggest this will cost more lives based on delayed diagnoses of cancer.”
Vaccinating patients against COVID-19 may help primary care practices and patients get back on track, Dr. Phillips suggested.
In the meantime, some patients remain reluctant to come in. “Volumes are still lower than prepandemic, so it is challenging to overcome what is likely to be pent-up demand,” he told this news organization in an email. “Additionally, the continued burden of evaluating, testing, and monitoring patients with COVID or COVID-like symptoms makes it difficult to focus on chronic illness.”
Care most often skipped
The Urban Institute survey asked respondents about delays in prescription drugs, general doctor and specialist visits, going to a hospital, preventive health screenings or medical tests, treatment or follow-up care, dental care, mental health care or counseling, treatment or counseling for alcohol or drug use, and other types of medical care.
Dental care was the most common type of care that adults delayed or did not receive because of the pandemic (25.3%), followed by general doctor or specialist visits (20.6%) and preventive health screenings or medical tests (15.5%).
Black adults were more likely than White or Hispanic/Latinx adults to have delayed or forgone care (39.7% vs. 34.3% and 35.5%), the researchers found. Compared with adults with higher incomes, adults with lower incomes were more likely to have missed multiple types of care (26.6% vs. 20.3%).
The report by the Urban Institute researchers was supported by the Robert Wood Johnson Foundation. Dr. Phillips is an adviser to two telemedicine companies, Bicycle Health and Grow Health. Dr. Fincher has disclosed no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
according to a new report from the Urban Institute.
Among the adults who postponed or missed care, 32.6% said the gap worsened one or more health conditions or limited their ability to work or perform daily activities. The findings highlight “the detrimental ripple effects of delaying or forgoing care on overall health, functioning, and well-being,” researchers write.
The survey, conducted among 4,007 U.S. adults aged 18-64 in September 2020, found that adults with one or more chronic conditions were more likely than adults without chronic conditions to have delayed or missed care (40.7% vs. 26.4%). Adults with a mental health condition were particularly likely to have delayed or gone without care, write Dulce Gonzalez, MPP, a research associate in the Health Policy Center at the Urban Institute, and colleagues.
Doctors are already seeing the consequences of the missed visits, says Jacqueline W. Fincher, MD, president of the American College of Physicians.
Two of her patients with chronic conditions missed appointments last year. By the time they resumed care in 2021, their previsit lab tests showed significant kidney deterioration.
“Lo and behold, their kidneys were in failure. … One was in the hospital for 3 days and the other one was in for 5 days,” said Dr. Fincher, who practices general internal medicine in Georgia.
Dr. Fincher’s office has been proactive about calling patients with chronic diseases who missed follow-up visits or laboratory testing or who may have run out of medication, she said.
In her experience, delays mainly have been because of patients postponing visits. “We have stayed open the whole time now,” Dr. Fincher said. Her office offers telemedicine visits and in-person visits with safety precautions.
Still, some patients have decided to postpone care during the pandemic instead of asking their primary care doctor what they should do.
“We do know that chronic problems left without appropriate follow-up can create worse problems for them in terms of stroke, heart attack, and end organ damage,” Dr. Fincher said.
Lost lives
Future studies may help researchers understand the effects of delayed and missed care during the pandemic, said Russell S. Phillips, MD, director of the Center for Primary Care at Harvard Medical School, Boston.
“Although it is still early, and more data on patient outcomes will need to be collected, I anticipate that the ... delays in diagnosis, in cancer screening, and in management of chronic illness will result in lost lives and will emphasize the important role that primary care plays in saving lives,” Dr. Phillips said.
During the first several months of the pandemic, there were fewer diagnoses of hypertension, diabetes, and depression, Dr. Phillips said.
“In addition, and most importantly, the mortality rate for non-COVID conditions increased, suggesting that patients were not seeking care for symptoms of stroke or heart attack, which can be fatal if untreated,” he said. “We have also seen substantial decreases in cancer screening tests such as colonoscopy, and modeling studies suggest this will cost more lives based on delayed diagnoses of cancer.”
Vaccinating patients against COVID-19 may help primary care practices and patients get back on track, Dr. Phillips suggested.
In the meantime, some patients remain reluctant to come in. “Volumes are still lower than prepandemic, so it is challenging to overcome what is likely to be pent-up demand,” he told this news organization in an email. “Additionally, the continued burden of evaluating, testing, and monitoring patients with COVID or COVID-like symptoms makes it difficult to focus on chronic illness.”
Care most often skipped
The Urban Institute survey asked respondents about delays in prescription drugs, general doctor and specialist visits, going to a hospital, preventive health screenings or medical tests, treatment or follow-up care, dental care, mental health care or counseling, treatment or counseling for alcohol or drug use, and other types of medical care.
Dental care was the most common type of care that adults delayed or did not receive because of the pandemic (25.3%), followed by general doctor or specialist visits (20.6%) and preventive health screenings or medical tests (15.5%).
Black adults were more likely than White or Hispanic/Latinx adults to have delayed or forgone care (39.7% vs. 34.3% and 35.5%), the researchers found. Compared with adults with higher incomes, adults with lower incomes were more likely to have missed multiple types of care (26.6% vs. 20.3%).
The report by the Urban Institute researchers was supported by the Robert Wood Johnson Foundation. Dr. Phillips is an adviser to two telemedicine companies, Bicycle Health and Grow Health. Dr. Fincher has disclosed no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FDA okays novel dual-action stimulant med for ADHD
The Food and Drug Administration has approved a new, once-daily oral stimulant medication for treatment of ADHD in people aged 6 years and older.
Azstarys (KemPharm) combines extended-release serdexmethylphenidate (SDX), KemPharm’s prodrug of dexmethylphenidate (d-MPH), coformulated with immediate-release d-MPH.
Following absorption in the gastrointestinal tract, SDX is converted to d-MPH, which is gradually released throughout the day, providing symptom control both rapidly with the d-MPH and for an extended duration with SDX.
The dual action of Azstarys addresses an unmet need for a medication that has early onset of action and long duration of therapy, with steady ADHD symptom control in one capsule, Corium, the company that will lead U.S. commercialization of the drug, stated in a news release.
“The data documenting the efficacy and safety of this new dual-action medicine, the first ever to use the novel prodrug serdexmethylphenidate together with dexmethylphenidate, is welcome news for clinicians and families to consider when choosing an appropriate ADHD therapy for children,” Ann Childress, MD, president of the Center for Psychiatry and Behavioral Medicine in Las Vegas, who led the phase 3 trial of the drug, said in the release.
The study included 150 children aged 6-12 years with ADHD. Compared with placebo, treatment with Azstarys led to significant improvement in ADHD symptoms, as measured by the primary endpoint, the change from baseline in Swanson, Kotkin, Agler, M-Flynn, and Pelham Rating Scale–Combined scores averaged over 13 hours.
Adverse events seen more often with Azstarys than placebo were headache (5.4% vs. 1.3%), upper abdominal pain (4.1% vs. 1.3%), insomnia (2.7% vs. 1.3%) and pharyngitis (2.7% vs. 0%). No serious adverse events were reported.
The FDA has recommended a schedule II controlled substance classification for Azstarys and the Drug Enforcement Administration will decide on scheduling within 90 days.
Pending the DEA’s action, the launch of Azstarys is anticipated this summer. Azstarys will be available in three once-daily dosage strengths of SDX/d-MPH: 26.1/5.2 mg, 39.2/7.8 mg, and 52.3/10.4 mg.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a new, once-daily oral stimulant medication for treatment of ADHD in people aged 6 years and older.
Azstarys (KemPharm) combines extended-release serdexmethylphenidate (SDX), KemPharm’s prodrug of dexmethylphenidate (d-MPH), coformulated with immediate-release d-MPH.
Following absorption in the gastrointestinal tract, SDX is converted to d-MPH, which is gradually released throughout the day, providing symptom control both rapidly with the d-MPH and for an extended duration with SDX.
The dual action of Azstarys addresses an unmet need for a medication that has early onset of action and long duration of therapy, with steady ADHD symptom control in one capsule, Corium, the company that will lead U.S. commercialization of the drug, stated in a news release.
“The data documenting the efficacy and safety of this new dual-action medicine, the first ever to use the novel prodrug serdexmethylphenidate together with dexmethylphenidate, is welcome news for clinicians and families to consider when choosing an appropriate ADHD therapy for children,” Ann Childress, MD, president of the Center for Psychiatry and Behavioral Medicine in Las Vegas, who led the phase 3 trial of the drug, said in the release.
The study included 150 children aged 6-12 years with ADHD. Compared with placebo, treatment with Azstarys led to significant improvement in ADHD symptoms, as measured by the primary endpoint, the change from baseline in Swanson, Kotkin, Agler, M-Flynn, and Pelham Rating Scale–Combined scores averaged over 13 hours.
Adverse events seen more often with Azstarys than placebo were headache (5.4% vs. 1.3%), upper abdominal pain (4.1% vs. 1.3%), insomnia (2.7% vs. 1.3%) and pharyngitis (2.7% vs. 0%). No serious adverse events were reported.
The FDA has recommended a schedule II controlled substance classification for Azstarys and the Drug Enforcement Administration will decide on scheduling within 90 days.
Pending the DEA’s action, the launch of Azstarys is anticipated this summer. Azstarys will be available in three once-daily dosage strengths of SDX/d-MPH: 26.1/5.2 mg, 39.2/7.8 mg, and 52.3/10.4 mg.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a new, once-daily oral stimulant medication for treatment of ADHD in people aged 6 years and older.
Azstarys (KemPharm) combines extended-release serdexmethylphenidate (SDX), KemPharm’s prodrug of dexmethylphenidate (d-MPH), coformulated with immediate-release d-MPH.
Following absorption in the gastrointestinal tract, SDX is converted to d-MPH, which is gradually released throughout the day, providing symptom control both rapidly with the d-MPH and for an extended duration with SDX.
The dual action of Azstarys addresses an unmet need for a medication that has early onset of action and long duration of therapy, with steady ADHD symptom control in one capsule, Corium, the company that will lead U.S. commercialization of the drug, stated in a news release.
“The data documenting the efficacy and safety of this new dual-action medicine, the first ever to use the novel prodrug serdexmethylphenidate together with dexmethylphenidate, is welcome news for clinicians and families to consider when choosing an appropriate ADHD therapy for children,” Ann Childress, MD, president of the Center for Psychiatry and Behavioral Medicine in Las Vegas, who led the phase 3 trial of the drug, said in the release.
The study included 150 children aged 6-12 years with ADHD. Compared with placebo, treatment with Azstarys led to significant improvement in ADHD symptoms, as measured by the primary endpoint, the change from baseline in Swanson, Kotkin, Agler, M-Flynn, and Pelham Rating Scale–Combined scores averaged over 13 hours.
Adverse events seen more often with Azstarys than placebo were headache (5.4% vs. 1.3%), upper abdominal pain (4.1% vs. 1.3%), insomnia (2.7% vs. 1.3%) and pharyngitis (2.7% vs. 0%). No serious adverse events were reported.
The FDA has recommended a schedule II controlled substance classification for Azstarys and the Drug Enforcement Administration will decide on scheduling within 90 days.
Pending the DEA’s action, the launch of Azstarys is anticipated this summer. Azstarys will be available in three once-daily dosage strengths of SDX/d-MPH: 26.1/5.2 mg, 39.2/7.8 mg, and 52.3/10.4 mg.
A version of this article first appeared on Medscape.com.
Decline in weekly child COVID-19 cases has almost stopped
A third COVID-19 vaccine is now in circulation and states are starting to drop mask mandates, but the latest decline in weekly child cases barely registers as a decline, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
That’s only 702 cases – a drop of just 1.1% – the smallest by far since weekly cases peaked in mid-January, the AAP and CHA said in their weekly COVID-19 report. Since that peak, the last 7 weeks of declines have looked like this: 21.7%, 15.3%, 16.2%, 15.7%, 28.7%, 9.0%, and 1.1%.
Meanwhile, children’s share of the COVID-19 burden increased to its highest point ever: 18.0% of all new cases occurred in children during the week ending March 4, climbing from 15.7% the week before and eclipsing the previous high of 16.9%. Cumulatively, the 3.23 million cases in children represent 13.2% of all COVID-19 cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
At the state level, the new leader in cumulative share of cases is Vermont at 19.4%, which just edged past Wyoming’s 19.3% as of the week ending March 4. The other states above 18% are Alaska (19.2%) and South Carolina (18.2%). The lowest rates can be found in Florida (8.1%), New Jersey (10.2%), Iowa (10.4%), and Utah (10.5%), the AAP and CHA said.
The overall rate of COVID-19 cases nationwide was 4,294 cases per 100,000 children as of March 4, up from 4,209 per 100,000 the week before. That measure had doubled between Dec. 3 (1,941 per 100,000) and Feb. 4 (3,899) but has only risen about 10% in the last month, the AAP/CHA data show.
Perhaps the most surprising news of the week involves the number of COVID-19 deaths in children, which went from 256 the previous week to 253 after Ohio made a downward revision of its mortality data. So far, children represent just 0.06% of all coronavirus-related deaths, a figure that has held steady since last summer in the 43 states (along with New York City and Guam) that are reporting mortality data by age, the AAP and CHA said.
A third COVID-19 vaccine is now in circulation and states are starting to drop mask mandates, but the latest decline in weekly child cases barely registers as a decline, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
That’s only 702 cases – a drop of just 1.1% – the smallest by far since weekly cases peaked in mid-January, the AAP and CHA said in their weekly COVID-19 report. Since that peak, the last 7 weeks of declines have looked like this: 21.7%, 15.3%, 16.2%, 15.7%, 28.7%, 9.0%, and 1.1%.
Meanwhile, children’s share of the COVID-19 burden increased to its highest point ever: 18.0% of all new cases occurred in children during the week ending March 4, climbing from 15.7% the week before and eclipsing the previous high of 16.9%. Cumulatively, the 3.23 million cases in children represent 13.2% of all COVID-19 cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
At the state level, the new leader in cumulative share of cases is Vermont at 19.4%, which just edged past Wyoming’s 19.3% as of the week ending March 4. The other states above 18% are Alaska (19.2%) and South Carolina (18.2%). The lowest rates can be found in Florida (8.1%), New Jersey (10.2%), Iowa (10.4%), and Utah (10.5%), the AAP and CHA said.
The overall rate of COVID-19 cases nationwide was 4,294 cases per 100,000 children as of March 4, up from 4,209 per 100,000 the week before. That measure had doubled between Dec. 3 (1,941 per 100,000) and Feb. 4 (3,899) but has only risen about 10% in the last month, the AAP/CHA data show.
Perhaps the most surprising news of the week involves the number of COVID-19 deaths in children, which went from 256 the previous week to 253 after Ohio made a downward revision of its mortality data. So far, children represent just 0.06% of all coronavirus-related deaths, a figure that has held steady since last summer in the 43 states (along with New York City and Guam) that are reporting mortality data by age, the AAP and CHA said.
A third COVID-19 vaccine is now in circulation and states are starting to drop mask mandates, but the latest decline in weekly child cases barely registers as a decline, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
That’s only 702 cases – a drop of just 1.1% – the smallest by far since weekly cases peaked in mid-January, the AAP and CHA said in their weekly COVID-19 report. Since that peak, the last 7 weeks of declines have looked like this: 21.7%, 15.3%, 16.2%, 15.7%, 28.7%, 9.0%, and 1.1%.
Meanwhile, children’s share of the COVID-19 burden increased to its highest point ever: 18.0% of all new cases occurred in children during the week ending March 4, climbing from 15.7% the week before and eclipsing the previous high of 16.9%. Cumulatively, the 3.23 million cases in children represent 13.2% of all COVID-19 cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
At the state level, the new leader in cumulative share of cases is Vermont at 19.4%, which just edged past Wyoming’s 19.3% as of the week ending March 4. The other states above 18% are Alaska (19.2%) and South Carolina (18.2%). The lowest rates can be found in Florida (8.1%), New Jersey (10.2%), Iowa (10.4%), and Utah (10.5%), the AAP and CHA said.
The overall rate of COVID-19 cases nationwide was 4,294 cases per 100,000 children as of March 4, up from 4,209 per 100,000 the week before. That measure had doubled between Dec. 3 (1,941 per 100,000) and Feb. 4 (3,899) but has only risen about 10% in the last month, the AAP/CHA data show.
Perhaps the most surprising news of the week involves the number of COVID-19 deaths in children, which went from 256 the previous week to 253 after Ohio made a downward revision of its mortality data. So far, children represent just 0.06% of all coronavirus-related deaths, a figure that has held steady since last summer in the 43 states (along with New York City and Guam) that are reporting mortality data by age, the AAP and CHA said.
Call to action on obesity amid COVID-19 pandemic
Hundreds of thousands of deaths worldwide from COVID-19 could have been avoided if obesity rates were lower, a new report says.
An analysis by the World Obesity Federation found that of the 2.5 million COVID-19 deaths reported by the end of February 2021, almost 90% (2.2 million) were in countries where more than half the population is classified as overweight.
The report, released to coincide with World Obesity Day, calls for obesity to be recognized as a disease in its own right around the world, and for people with obesity to be included in priority lists for COVID-19 testing and vaccination.
“Overweight is a highly significant predictor of developing complications from COVID-19, including the need for hospitalization, for intensive care and for mechanical ventilation,” the WOF notes in the report.
It adds that in countries where less than half the adult population is classified as overweight (body mass index > 25 mg/kg2), for example, Vietnam, the likelihood of death from COVID-19 is a small fraction – around one-tenth – of the level seen in countries where more than half the population is classified as overweight.
And while it acknowledges that figures for COVID-19 deaths are affected by the age structure of national populations and a country’s relative wealth and reporting capacity, “our findings appear to be independent of these contributory factors. Furthermore, other studies have found that overweight remains a highly significant predictor of the need for COVID-19 health care after accounting for these other influences.”
As an example, based on the U.K. experience, where an estimated 36% of COVID-19 hospitalizations have been attributed to lack of physical activity and excess body weight, it can be suggested that up to a third of the costs – between $6 trillion and $7 trillion over the longer period – might be attributable to these predisposing risks.
The report said the prevalence of obesity in the United Kingdom is expected to rise from 27.8% in 2016 to more than 35% by 2025.
Rachel Batterham, lead adviser on obesity at the Royal College of Physicians, commented: “The link between high levels of obesity and deaths from COVID-19 in the U.K. is indisputable, as is the urgent need to address the factors that lead so many people to be living with obesity.
“With 30% of COVID-19 hospitalizations in the U.K. directly attributed to overweight and obesity, and three-quarters of all critically ill patients having overweight or obesity, the human and financial costs are high.”
Window of opportunity to prioritize obesity as a disease
WOF says that evolving evidence on the close association between COVID-19 and underlying obesity “provides a new urgency … for political and collective action.”
“Obesity is a disease that does not receive prioritization commensurate with its prevalence and impact, which is rising fastest in emerging economies. It is a gateway to many other noncommunicable diseases and mental-health illness and is now a major factor in COVID-19 complications and mortality.”
The WOF also shows that COVID-19 is not a special case, noting that several other respiratory viruses lead to more severe consequences in people living with excess bodyweight, giving good reasons to expect the next pandemic to have similar effects. “For these reasons we need to recognize overweight as a major risk factor for infectious diseases including respiratory viruses.”
“To prevent pandemic health crises in future requires action now: we call on all readers to support the World Obesity Federation’s call for stronger, more resilient economies that prioritize investment in people’s health.”
There is, it stresses, “a window of opportunity to advocate for, fund and implement these actions in all countries to ensure better, more resilient and sustainable health for all, “now and in our postCOVID-19 future.”
It proposes a ROOTS approach:
- Recognize that obesity is a disease in its own right.
- Obesity monitoring and surveillance must be enhanced.
- Obesity prevention strategies must be developed.
- Treatment of obesity.
- Systems-based approaches should be applied.
A version of this article first appeared on Medscape.com.
Hundreds of thousands of deaths worldwide from COVID-19 could have been avoided if obesity rates were lower, a new report says.
An analysis by the World Obesity Federation found that of the 2.5 million COVID-19 deaths reported by the end of February 2021, almost 90% (2.2 million) were in countries where more than half the population is classified as overweight.
The report, released to coincide with World Obesity Day, calls for obesity to be recognized as a disease in its own right around the world, and for people with obesity to be included in priority lists for COVID-19 testing and vaccination.
“Overweight is a highly significant predictor of developing complications from COVID-19, including the need for hospitalization, for intensive care and for mechanical ventilation,” the WOF notes in the report.
It adds that in countries where less than half the adult population is classified as overweight (body mass index > 25 mg/kg2), for example, Vietnam, the likelihood of death from COVID-19 is a small fraction – around one-tenth – of the level seen in countries where more than half the population is classified as overweight.
And while it acknowledges that figures for COVID-19 deaths are affected by the age structure of national populations and a country’s relative wealth and reporting capacity, “our findings appear to be independent of these contributory factors. Furthermore, other studies have found that overweight remains a highly significant predictor of the need for COVID-19 health care after accounting for these other influences.”
As an example, based on the U.K. experience, where an estimated 36% of COVID-19 hospitalizations have been attributed to lack of physical activity and excess body weight, it can be suggested that up to a third of the costs – between $6 trillion and $7 trillion over the longer period – might be attributable to these predisposing risks.
The report said the prevalence of obesity in the United Kingdom is expected to rise from 27.8% in 2016 to more than 35% by 2025.
Rachel Batterham, lead adviser on obesity at the Royal College of Physicians, commented: “The link between high levels of obesity and deaths from COVID-19 in the U.K. is indisputable, as is the urgent need to address the factors that lead so many people to be living with obesity.
“With 30% of COVID-19 hospitalizations in the U.K. directly attributed to overweight and obesity, and three-quarters of all critically ill patients having overweight or obesity, the human and financial costs are high.”
Window of opportunity to prioritize obesity as a disease
WOF says that evolving evidence on the close association between COVID-19 and underlying obesity “provides a new urgency … for political and collective action.”
“Obesity is a disease that does not receive prioritization commensurate with its prevalence and impact, which is rising fastest in emerging economies. It is a gateway to many other noncommunicable diseases and mental-health illness and is now a major factor in COVID-19 complications and mortality.”
The WOF also shows that COVID-19 is not a special case, noting that several other respiratory viruses lead to more severe consequences in people living with excess bodyweight, giving good reasons to expect the next pandemic to have similar effects. “For these reasons we need to recognize overweight as a major risk factor for infectious diseases including respiratory viruses.”
“To prevent pandemic health crises in future requires action now: we call on all readers to support the World Obesity Federation’s call for stronger, more resilient economies that prioritize investment in people’s health.”
There is, it stresses, “a window of opportunity to advocate for, fund and implement these actions in all countries to ensure better, more resilient and sustainable health for all, “now and in our postCOVID-19 future.”
It proposes a ROOTS approach:
- Recognize that obesity is a disease in its own right.
- Obesity monitoring and surveillance must be enhanced.
- Obesity prevention strategies must be developed.
- Treatment of obesity.
- Systems-based approaches should be applied.
A version of this article first appeared on Medscape.com.
Hundreds of thousands of deaths worldwide from COVID-19 could have been avoided if obesity rates were lower, a new report says.
An analysis by the World Obesity Federation found that of the 2.5 million COVID-19 deaths reported by the end of February 2021, almost 90% (2.2 million) were in countries where more than half the population is classified as overweight.
The report, released to coincide with World Obesity Day, calls for obesity to be recognized as a disease in its own right around the world, and for people with obesity to be included in priority lists for COVID-19 testing and vaccination.
“Overweight is a highly significant predictor of developing complications from COVID-19, including the need for hospitalization, for intensive care and for mechanical ventilation,” the WOF notes in the report.
It adds that in countries where less than half the adult population is classified as overweight (body mass index > 25 mg/kg2), for example, Vietnam, the likelihood of death from COVID-19 is a small fraction – around one-tenth – of the level seen in countries where more than half the population is classified as overweight.
And while it acknowledges that figures for COVID-19 deaths are affected by the age structure of national populations and a country’s relative wealth and reporting capacity, “our findings appear to be independent of these contributory factors. Furthermore, other studies have found that overweight remains a highly significant predictor of the need for COVID-19 health care after accounting for these other influences.”
As an example, based on the U.K. experience, where an estimated 36% of COVID-19 hospitalizations have been attributed to lack of physical activity and excess body weight, it can be suggested that up to a third of the costs – between $6 trillion and $7 trillion over the longer period – might be attributable to these predisposing risks.
The report said the prevalence of obesity in the United Kingdom is expected to rise from 27.8% in 2016 to more than 35% by 2025.
Rachel Batterham, lead adviser on obesity at the Royal College of Physicians, commented: “The link between high levels of obesity and deaths from COVID-19 in the U.K. is indisputable, as is the urgent need to address the factors that lead so many people to be living with obesity.
“With 30% of COVID-19 hospitalizations in the U.K. directly attributed to overweight and obesity, and three-quarters of all critically ill patients having overweight or obesity, the human and financial costs are high.”
Window of opportunity to prioritize obesity as a disease
WOF says that evolving evidence on the close association between COVID-19 and underlying obesity “provides a new urgency … for political and collective action.”
“Obesity is a disease that does not receive prioritization commensurate with its prevalence and impact, which is rising fastest in emerging economies. It is a gateway to many other noncommunicable diseases and mental-health illness and is now a major factor in COVID-19 complications and mortality.”
The WOF also shows that COVID-19 is not a special case, noting that several other respiratory viruses lead to more severe consequences in people living with excess bodyweight, giving good reasons to expect the next pandemic to have similar effects. “For these reasons we need to recognize overweight as a major risk factor for infectious diseases including respiratory viruses.”
“To prevent pandemic health crises in future requires action now: we call on all readers to support the World Obesity Federation’s call for stronger, more resilient economies that prioritize investment in people’s health.”
There is, it stresses, “a window of opportunity to advocate for, fund and implement these actions in all countries to ensure better, more resilient and sustainable health for all, “now and in our postCOVID-19 future.”
It proposes a ROOTS approach:
- Recognize that obesity is a disease in its own right.
- Obesity monitoring and surveillance must be enhanced.
- Obesity prevention strategies must be developed.
- Treatment of obesity.
- Systems-based approaches should be applied.
A version of this article first appeared on Medscape.com.
Risdiplam study shows promise for spinal muscular atrophy
, according to results of part 1 of the FIREFISH study.
A boost in SMN expression has been linked to improvements in survival and motor function, which was also observed in exploratory efficacy outcomes in the 2-part, phase 2-3, open-label study.
“No surviving infant was receiving permanent ventilation at month 12, and 7 of the 21 infants were able to sit without support, which is not expected in patients with type 1 spinal muscular atrophy, according to historical experience,” reported the FIREFISH Working Group led by Giovanni Baranello, MD, PhD, from the Dubowitz Neuromuscular Centre, National Institute for Health Research Great Ormond Street Hospital Biomedical Research Centre, Great Ormond Street Institute of Child Health University College London, and Great Ormond Street Hospital Trust, London.
However, “it cannot be stated with confidence that there was clinical benefit of the agent because the exploratory clinical endpoints were analyzed post hoc and can only be qualitatively compared with historical cohorts,” they added.
The findings were published online Feb. 24 in the New England Journal of Medicine.
A phase 2-3 open-label study
The study enrolled 21 infants with type 1 SMA, between the ages of 1 and 7 months. The majority (n = 17) were treated for 1 year with high-dose risdiplam, reaching 0.2 mg/kg of body weight per day by the twelfth month. Four infants in a low-dose cohort were treated with 0.08 mg/kg by the twelfth month. The medication was administered once daily orally in infants who were able to swallow, or by feeding tube for those who could not.
The primary outcomes of this first part of the study were safety, pharmacokinetics, pharmacodynamics (including the blood SMN protein concentration), and selection of the risdiplam dose for part 2 of the study. Exploratory outcomes included event-free survival, defined as being alive without tracheostomy or the use of permanent ventilation for 16 or more hours per day, and the ability to sit without support for at least 5 seconds.
In terms of safety, the study recorded 24 serious adverse events. “The most common serious adverse events were infections of the respiratory tract, and four infants died of respiratory complications; these findings are consistent with the neuromuscular respiratory failure that characterizes spinal muscular atrophy,” the authors reported. “The risdiplam-associated retinal toxic effects that had been previously observed in monkeys were not observed in the current study,” they added.
Regarding SMN protein levels, a median level of 2.1 times the baseline level was observed within 4 weeks after the initiation of treatment in the high-dose cohort, they reported. By 12 months, these median values had increased to 3.0 times and 1.9 times the baseline values in the low-dose and high-dose cohorts, respectively.
Looking at exploratory efficacy outcomes, 90% of infants survived without ventilatory support, and seven infants in the high-dose cohort were able to sit without support for at least 5 seconds. The higher dose of risdiplam (0.2 mg/kg per day) was selected for part 2 of the study.
The first oral treatment option
Risdiplam is the third SMA treatment approved by the Food and Drug Administration, “and has the potential to expand access to treatment for people with SMA,” commented Mary Schroth, MD, chief medical officer of Cure SMA, who was not involved in the research. She added that the exploratory outcomes of the FIREFISH study represent “a significant milestone for symptomatic infants with SMA type 1.”
While the other two approved SMA therapies – nusinersen and onasemnogene abeparvovec – have led to improvements in survival and motor function, they are administered either intrathecally or intravenously respectively, while risdiplam is an oral therapy.
Dr. Schroth says there are currently no studies comparing the different SMA treatments. “Cure SMA is actively collecting real-world experience with risdiplam and other SMA treatments through multiple pathways,” she said. “Every individual and family, in collaboration with their health care provider, should discuss SMA treatments and make the decision that is best for them.”
Writing in Neuroscience Insights, a few months after risdiplam’s FDA approval last summer, Ravindra N. Singh MD, from the department of biomedical sciences, Iowa State University, Ames, wrote that, as an orally deliverable small molecule, risdiplam “is a major advancement for the treatment of SMA.”
Now, the FIREFISH study is “welcome news,” he said in an interview. “The results look promising so far,” he added. “I am cautiously optimistic that risdiplam would prove to be a viable alternative to the currently available invasive approaches. However, long-term studies (with appropriate age and sex-matched cohorts) would be needed to fully rule out the potential side effects of the repeated administrations.”
The therapy “is particularly great news for a group of SMA patients that might have tolerability and/or immune response concerns when it comes to nusinersen and gene therapy,” he noted in his article, adding that the ability to store and ship the drug at ambient temperatures, as well as its comparatively low cost are added benefits.
The study was supported by F. Hoffmann–La Roche. Dr. Baranello disclosed that he serves as a consultant for AveXis, F. Hoffmann-La Roche, and Sarepta Therapeutics, as well as PTC Therapeutics, from whom he also receives speaker honoraria. Dr. Schroth disclosed no personal conflicts and is an employee of Cure SMA. Cure SMA works to develop strategic relationships with corporate partners with the goal of working together to lead the way to a world without SMA. In advancement of that mission, Cure SMA has received funding from multiple corporate sources including Aetna, Biogen, Blue Cross Blue Shield, Genentech, Kaiser Permanente, Novartis Gene Therapies, Scholar Rock, and United HealthCare. Cure SMA has no financial stake in any treatment and does not advocate for one treatment over another. Dr. Singh disclosed that Spinraza (Nusinersen), the first FDA-approved SMA drug, is based on the target (US patent # 7,838,657) that was discovered in his former laboratory at UMASS Medical School, Worcester, Mass.
, according to results of part 1 of the FIREFISH study.
A boost in SMN expression has been linked to improvements in survival and motor function, which was also observed in exploratory efficacy outcomes in the 2-part, phase 2-3, open-label study.
“No surviving infant was receiving permanent ventilation at month 12, and 7 of the 21 infants were able to sit without support, which is not expected in patients with type 1 spinal muscular atrophy, according to historical experience,” reported the FIREFISH Working Group led by Giovanni Baranello, MD, PhD, from the Dubowitz Neuromuscular Centre, National Institute for Health Research Great Ormond Street Hospital Biomedical Research Centre, Great Ormond Street Institute of Child Health University College London, and Great Ormond Street Hospital Trust, London.
However, “it cannot be stated with confidence that there was clinical benefit of the agent because the exploratory clinical endpoints were analyzed post hoc and can only be qualitatively compared with historical cohorts,” they added.
The findings were published online Feb. 24 in the New England Journal of Medicine.
A phase 2-3 open-label study
The study enrolled 21 infants with type 1 SMA, between the ages of 1 and 7 months. The majority (n = 17) were treated for 1 year with high-dose risdiplam, reaching 0.2 mg/kg of body weight per day by the twelfth month. Four infants in a low-dose cohort were treated with 0.08 mg/kg by the twelfth month. The medication was administered once daily orally in infants who were able to swallow, or by feeding tube for those who could not.
The primary outcomes of this first part of the study were safety, pharmacokinetics, pharmacodynamics (including the blood SMN protein concentration), and selection of the risdiplam dose for part 2 of the study. Exploratory outcomes included event-free survival, defined as being alive without tracheostomy or the use of permanent ventilation for 16 or more hours per day, and the ability to sit without support for at least 5 seconds.
In terms of safety, the study recorded 24 serious adverse events. “The most common serious adverse events were infections of the respiratory tract, and four infants died of respiratory complications; these findings are consistent with the neuromuscular respiratory failure that characterizes spinal muscular atrophy,” the authors reported. “The risdiplam-associated retinal toxic effects that had been previously observed in monkeys were not observed in the current study,” they added.
Regarding SMN protein levels, a median level of 2.1 times the baseline level was observed within 4 weeks after the initiation of treatment in the high-dose cohort, they reported. By 12 months, these median values had increased to 3.0 times and 1.9 times the baseline values in the low-dose and high-dose cohorts, respectively.
Looking at exploratory efficacy outcomes, 90% of infants survived without ventilatory support, and seven infants in the high-dose cohort were able to sit without support for at least 5 seconds. The higher dose of risdiplam (0.2 mg/kg per day) was selected for part 2 of the study.
The first oral treatment option
Risdiplam is the third SMA treatment approved by the Food and Drug Administration, “and has the potential to expand access to treatment for people with SMA,” commented Mary Schroth, MD, chief medical officer of Cure SMA, who was not involved in the research. She added that the exploratory outcomes of the FIREFISH study represent “a significant milestone for symptomatic infants with SMA type 1.”
While the other two approved SMA therapies – nusinersen and onasemnogene abeparvovec – have led to improvements in survival and motor function, they are administered either intrathecally or intravenously respectively, while risdiplam is an oral therapy.
Dr. Schroth says there are currently no studies comparing the different SMA treatments. “Cure SMA is actively collecting real-world experience with risdiplam and other SMA treatments through multiple pathways,” she said. “Every individual and family, in collaboration with their health care provider, should discuss SMA treatments and make the decision that is best for them.”
Writing in Neuroscience Insights, a few months after risdiplam’s FDA approval last summer, Ravindra N. Singh MD, from the department of biomedical sciences, Iowa State University, Ames, wrote that, as an orally deliverable small molecule, risdiplam “is a major advancement for the treatment of SMA.”
Now, the FIREFISH study is “welcome news,” he said in an interview. “The results look promising so far,” he added. “I am cautiously optimistic that risdiplam would prove to be a viable alternative to the currently available invasive approaches. However, long-term studies (with appropriate age and sex-matched cohorts) would be needed to fully rule out the potential side effects of the repeated administrations.”
The therapy “is particularly great news for a group of SMA patients that might have tolerability and/or immune response concerns when it comes to nusinersen and gene therapy,” he noted in his article, adding that the ability to store and ship the drug at ambient temperatures, as well as its comparatively low cost are added benefits.
The study was supported by F. Hoffmann–La Roche. Dr. Baranello disclosed that he serves as a consultant for AveXis, F. Hoffmann-La Roche, and Sarepta Therapeutics, as well as PTC Therapeutics, from whom he also receives speaker honoraria. Dr. Schroth disclosed no personal conflicts and is an employee of Cure SMA. Cure SMA works to develop strategic relationships with corporate partners with the goal of working together to lead the way to a world without SMA. In advancement of that mission, Cure SMA has received funding from multiple corporate sources including Aetna, Biogen, Blue Cross Blue Shield, Genentech, Kaiser Permanente, Novartis Gene Therapies, Scholar Rock, and United HealthCare. Cure SMA has no financial stake in any treatment and does not advocate for one treatment over another. Dr. Singh disclosed that Spinraza (Nusinersen), the first FDA-approved SMA drug, is based on the target (US patent # 7,838,657) that was discovered in his former laboratory at UMASS Medical School, Worcester, Mass.
, according to results of part 1 of the FIREFISH study.
A boost in SMN expression has been linked to improvements in survival and motor function, which was also observed in exploratory efficacy outcomes in the 2-part, phase 2-3, open-label study.
“No surviving infant was receiving permanent ventilation at month 12, and 7 of the 21 infants were able to sit without support, which is not expected in patients with type 1 spinal muscular atrophy, according to historical experience,” reported the FIREFISH Working Group led by Giovanni Baranello, MD, PhD, from the Dubowitz Neuromuscular Centre, National Institute for Health Research Great Ormond Street Hospital Biomedical Research Centre, Great Ormond Street Institute of Child Health University College London, and Great Ormond Street Hospital Trust, London.
However, “it cannot be stated with confidence that there was clinical benefit of the agent because the exploratory clinical endpoints were analyzed post hoc and can only be qualitatively compared with historical cohorts,” they added.
The findings were published online Feb. 24 in the New England Journal of Medicine.
A phase 2-3 open-label study
The study enrolled 21 infants with type 1 SMA, between the ages of 1 and 7 months. The majority (n = 17) were treated for 1 year with high-dose risdiplam, reaching 0.2 mg/kg of body weight per day by the twelfth month. Four infants in a low-dose cohort were treated with 0.08 mg/kg by the twelfth month. The medication was administered once daily orally in infants who were able to swallow, or by feeding tube for those who could not.
The primary outcomes of this first part of the study were safety, pharmacokinetics, pharmacodynamics (including the blood SMN protein concentration), and selection of the risdiplam dose for part 2 of the study. Exploratory outcomes included event-free survival, defined as being alive without tracheostomy or the use of permanent ventilation for 16 or more hours per day, and the ability to sit without support for at least 5 seconds.
In terms of safety, the study recorded 24 serious adverse events. “The most common serious adverse events were infections of the respiratory tract, and four infants died of respiratory complications; these findings are consistent with the neuromuscular respiratory failure that characterizes spinal muscular atrophy,” the authors reported. “The risdiplam-associated retinal toxic effects that had been previously observed in monkeys were not observed in the current study,” they added.
Regarding SMN protein levels, a median level of 2.1 times the baseline level was observed within 4 weeks after the initiation of treatment in the high-dose cohort, they reported. By 12 months, these median values had increased to 3.0 times and 1.9 times the baseline values in the low-dose and high-dose cohorts, respectively.
Looking at exploratory efficacy outcomes, 90% of infants survived without ventilatory support, and seven infants in the high-dose cohort were able to sit without support for at least 5 seconds. The higher dose of risdiplam (0.2 mg/kg per day) was selected for part 2 of the study.
The first oral treatment option
Risdiplam is the third SMA treatment approved by the Food and Drug Administration, “and has the potential to expand access to treatment for people with SMA,” commented Mary Schroth, MD, chief medical officer of Cure SMA, who was not involved in the research. She added that the exploratory outcomes of the FIREFISH study represent “a significant milestone for symptomatic infants with SMA type 1.”
While the other two approved SMA therapies – nusinersen and onasemnogene abeparvovec – have led to improvements in survival and motor function, they are administered either intrathecally or intravenously respectively, while risdiplam is an oral therapy.
Dr. Schroth says there are currently no studies comparing the different SMA treatments. “Cure SMA is actively collecting real-world experience with risdiplam and other SMA treatments through multiple pathways,” she said. “Every individual and family, in collaboration with their health care provider, should discuss SMA treatments and make the decision that is best for them.”
Writing in Neuroscience Insights, a few months after risdiplam’s FDA approval last summer, Ravindra N. Singh MD, from the department of biomedical sciences, Iowa State University, Ames, wrote that, as an orally deliverable small molecule, risdiplam “is a major advancement for the treatment of SMA.”
Now, the FIREFISH study is “welcome news,” he said in an interview. “The results look promising so far,” he added. “I am cautiously optimistic that risdiplam would prove to be a viable alternative to the currently available invasive approaches. However, long-term studies (with appropriate age and sex-matched cohorts) would be needed to fully rule out the potential side effects of the repeated administrations.”
The therapy “is particularly great news for a group of SMA patients that might have tolerability and/or immune response concerns when it comes to nusinersen and gene therapy,” he noted in his article, adding that the ability to store and ship the drug at ambient temperatures, as well as its comparatively low cost are added benefits.
The study was supported by F. Hoffmann–La Roche. Dr. Baranello disclosed that he serves as a consultant for AveXis, F. Hoffmann-La Roche, and Sarepta Therapeutics, as well as PTC Therapeutics, from whom he also receives speaker honoraria. Dr. Schroth disclosed no personal conflicts and is an employee of Cure SMA. Cure SMA works to develop strategic relationships with corporate partners with the goal of working together to lead the way to a world without SMA. In advancement of that mission, Cure SMA has received funding from multiple corporate sources including Aetna, Biogen, Blue Cross Blue Shield, Genentech, Kaiser Permanente, Novartis Gene Therapies, Scholar Rock, and United HealthCare. Cure SMA has no financial stake in any treatment and does not advocate for one treatment over another. Dr. Singh disclosed that Spinraza (Nusinersen), the first FDA-approved SMA drug, is based on the target (US patent # 7,838,657) that was discovered in his former laboratory at UMASS Medical School, Worcester, Mass.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE