User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Boring is good. Boring is right. Boring is … interesting
Can you keep it down? I’m trying to be boring
He chides his friends for not looking both ways before crossing the road. He is never questioned by the police because they fall asleep listening to him talk. He has won the office’s coveted perfect attendance award 10 years running. Look out, Dos Equis guy, you’ve got some new competition. That’s right, it’s the most boring man in the world.
For this boring study (sorry, study on boredom) conducted by English researchers and published in Personality and Social Psychology Bulletin, people were surveyed on various jobs and hobbies, ranking them by how exciting or boring they are, as well as how competent someone with those jobs/hobbies would be, their willingness to avoid someone with those jobs/hobbies, and how much they’d need to be paid to spend time with someone who had an undesirable job/hobby.
According to the British public, the most boring person in the world is a religious data analyst who likes to sleep and lives in a small town. In fact, spending time with this person is almost a full-time job on its own: To make it worth their while, survey subjects wanted 35 pounds a day. The boring person also was viewed as less competent, as is anyone with a boring job.
Now, there probably aren’t a lot of religious data analysts out there, but don’t worry, there are plenty of other boring jobs – accounting, tax/insurance, cleaning, and banking rounded out the top five (apparently people don’t like finances) – and hobbies – watching TV, observing animals, and mathematics filled out the top five. In case you’re curious, performing artists, scientists, journalists, health professionals, and teachers were viewed as having exciting jobs; exciting hobbies included gaming, reading, domestic tasks (really?), gardening, and writing.
Lead researcher Wijnand Van Tilburg, PhD, made an excellent point about people with boring jobs: They “have power in society – perhaps we should try not to upset them and stereotype them as boring!”
We think they should lean into it and make The Most Boring Man in the World ads: “When I drive a car off the lot, its value increases because I used the correct lending association. Batman trusts me with his Batmobile insurance. I can make those Cuban cigars tax exempt. Stay financially solvent, my friends.”
Fungi, but make it fashion
Fashion is an expensive and costly industry to sustain. Cotton production takes a toll on the environment, leather production comes with environmental and ethical/moral conundrums, and thanks to fast fashion, about 85% of textiles are being thrown away in the United States.
Researchers at the University of Borås in Sweden, however, have found a newish solution to create leather, cotton, and other textiles. And as with so many of the finer things, it starts with unsold bread from the grocery store.
Akram Zamani, PhD, and her team take that bread and turn it into breadcrumbs, then combine it with water and Rhizopus delemar, a fungus typically found in decaying food. After a couple of days of feasting on the bread, the fungus produces natural fibers made of chitin and chitosan that accumulate in the cell walls. After proteins, lipids, and other byproducts are removed, the team is left with a jelly-like substance made of those fibrous cell walls that can be spun into a fabric.
The researchers started small with very thin nonpliable sheets, but with a little layering by using tree tannins for softness and alkali for strength, their fungal leather is more like real leather than competing fungal leathers. Not to mention its being able to be produced in a fraction of the time.
This new fungal leather is fast to produce, it’s biodegradable, and it uses only natural ingredients to treat the materials. It’s the ultimate environmental fashion statement.
Who’s afraid of cancer? Not C. elegans
And now, we bring you part 2 of our ongoing series: Creatures that can diagnose cancer. Last week, we discovered that ants are well on their way to replacing dogs in our medical labs and in our hearts. This week, we present the even-more-lovable nematode.
The soil-dwelling nematode Caenorhabditis elegans, which is less than 1 mm long, is known to be “attracted or repelled by certain odors, so we came up with an idea that the roundworm could be used to detect lung cancer,” Shin Sik Choi, PhD, of Myongji University in South Korea, who is the project’s principal investigator, said in a statement on Eurekalert.
Dr. Choi’s team created a “worm-on-a-chip” that allowed the nematodes to choose between a drop of culture media from lung cancer cells and media from normal lung fibroblasts. An hour after being placed in the chip’s central chamber, more nematodes had crawled toward the lung cancer media than the normal-cell sample.
The investigators estimate that the device is about 70% effective at detecting cancer cells, but “they hope to increase both the accuracy and sensitivity of the method by using worms that were previously exposed to cancer cell media and therefore have a ‘memory’ of cancer-specific odor molecules,” according to the statement from the American Chemical Society.
Since C. elegans is easy to grow in a lab and, apparently, easy to train, the researchers hope that the worm-on-a-chip can become a quick, easy, economical, and noninvasive cancer screen.
So watch out cancer, because we never bet against the creepy crawlies.
Mosquitoes have us figured out
We are nearing mosquito season; quite possibly the most annoying and itchy time of the year. We stock up on bottles of bug spray, but somehow we still get bite after bite. It appears that mosquitoes are basically able to ignore our bug sprays, which explains why we’re still covered in bites after the Fourth of July fireworks. It turns out mosquitoes are more complex than we thought for such tiny creatures.
There’s plenty of research on the best ways to keep mosquitoes away, because not only are they incredibly annoying, but they also carry potentially harmful diseases. In a recent experiment, researchers used mosquitoes that were genetically modified to have an excessive amount of an odor receptor called AgOR2, which responds to the smell of humans.
“AgOR2 overexpression threw a wrench in the whole system by inactivating olfactory receptors in these mosquitoes,” Christopher Potter, PhD, associate professor of neuroscience at Johns Hopkins University, said in a written statement.
After testing how these genetically modified mosquitoes reacted to some of the common smells of bug spray such as lemongrass, they discovered that it’s easy for the mosquitoes to ignore the smell. We wish it were that easy for us to ignore that chemically fruity smell.
Researchers continue to work hard to figure out how to repel mosquitoes and we’re rooting for them as summer approaches, despite the mosquito’s status as a creepy crawly.
Can you keep it down? I’m trying to be boring
He chides his friends for not looking both ways before crossing the road. He is never questioned by the police because they fall asleep listening to him talk. He has won the office’s coveted perfect attendance award 10 years running. Look out, Dos Equis guy, you’ve got some new competition. That’s right, it’s the most boring man in the world.
For this boring study (sorry, study on boredom) conducted by English researchers and published in Personality and Social Psychology Bulletin, people were surveyed on various jobs and hobbies, ranking them by how exciting or boring they are, as well as how competent someone with those jobs/hobbies would be, their willingness to avoid someone with those jobs/hobbies, and how much they’d need to be paid to spend time with someone who had an undesirable job/hobby.
According to the British public, the most boring person in the world is a religious data analyst who likes to sleep and lives in a small town. In fact, spending time with this person is almost a full-time job on its own: To make it worth their while, survey subjects wanted 35 pounds a day. The boring person also was viewed as less competent, as is anyone with a boring job.
Now, there probably aren’t a lot of religious data analysts out there, but don’t worry, there are plenty of other boring jobs – accounting, tax/insurance, cleaning, and banking rounded out the top five (apparently people don’t like finances) – and hobbies – watching TV, observing animals, and mathematics filled out the top five. In case you’re curious, performing artists, scientists, journalists, health professionals, and teachers were viewed as having exciting jobs; exciting hobbies included gaming, reading, domestic tasks (really?), gardening, and writing.
Lead researcher Wijnand Van Tilburg, PhD, made an excellent point about people with boring jobs: They “have power in society – perhaps we should try not to upset them and stereotype them as boring!”
We think they should lean into it and make The Most Boring Man in the World ads: “When I drive a car off the lot, its value increases because I used the correct lending association. Batman trusts me with his Batmobile insurance. I can make those Cuban cigars tax exempt. Stay financially solvent, my friends.”
Fungi, but make it fashion
Fashion is an expensive and costly industry to sustain. Cotton production takes a toll on the environment, leather production comes with environmental and ethical/moral conundrums, and thanks to fast fashion, about 85% of textiles are being thrown away in the United States.
Researchers at the University of Borås in Sweden, however, have found a newish solution to create leather, cotton, and other textiles. And as with so many of the finer things, it starts with unsold bread from the grocery store.
Akram Zamani, PhD, and her team take that bread and turn it into breadcrumbs, then combine it with water and Rhizopus delemar, a fungus typically found in decaying food. After a couple of days of feasting on the bread, the fungus produces natural fibers made of chitin and chitosan that accumulate in the cell walls. After proteins, lipids, and other byproducts are removed, the team is left with a jelly-like substance made of those fibrous cell walls that can be spun into a fabric.
The researchers started small with very thin nonpliable sheets, but with a little layering by using tree tannins for softness and alkali for strength, their fungal leather is more like real leather than competing fungal leathers. Not to mention its being able to be produced in a fraction of the time.
This new fungal leather is fast to produce, it’s biodegradable, and it uses only natural ingredients to treat the materials. It’s the ultimate environmental fashion statement.
Who’s afraid of cancer? Not C. elegans
And now, we bring you part 2 of our ongoing series: Creatures that can diagnose cancer. Last week, we discovered that ants are well on their way to replacing dogs in our medical labs and in our hearts. This week, we present the even-more-lovable nematode.
The soil-dwelling nematode Caenorhabditis elegans, which is less than 1 mm long, is known to be “attracted or repelled by certain odors, so we came up with an idea that the roundworm could be used to detect lung cancer,” Shin Sik Choi, PhD, of Myongji University in South Korea, who is the project’s principal investigator, said in a statement on Eurekalert.
Dr. Choi’s team created a “worm-on-a-chip” that allowed the nematodes to choose between a drop of culture media from lung cancer cells and media from normal lung fibroblasts. An hour after being placed in the chip’s central chamber, more nematodes had crawled toward the lung cancer media than the normal-cell sample.
The investigators estimate that the device is about 70% effective at detecting cancer cells, but “they hope to increase both the accuracy and sensitivity of the method by using worms that were previously exposed to cancer cell media and therefore have a ‘memory’ of cancer-specific odor molecules,” according to the statement from the American Chemical Society.
Since C. elegans is easy to grow in a lab and, apparently, easy to train, the researchers hope that the worm-on-a-chip can become a quick, easy, economical, and noninvasive cancer screen.
So watch out cancer, because we never bet against the creepy crawlies.
Mosquitoes have us figured out
We are nearing mosquito season; quite possibly the most annoying and itchy time of the year. We stock up on bottles of bug spray, but somehow we still get bite after bite. It appears that mosquitoes are basically able to ignore our bug sprays, which explains why we’re still covered in bites after the Fourth of July fireworks. It turns out mosquitoes are more complex than we thought for such tiny creatures.
There’s plenty of research on the best ways to keep mosquitoes away, because not only are they incredibly annoying, but they also carry potentially harmful diseases. In a recent experiment, researchers used mosquitoes that were genetically modified to have an excessive amount of an odor receptor called AgOR2, which responds to the smell of humans.
“AgOR2 overexpression threw a wrench in the whole system by inactivating olfactory receptors in these mosquitoes,” Christopher Potter, PhD, associate professor of neuroscience at Johns Hopkins University, said in a written statement.
After testing how these genetically modified mosquitoes reacted to some of the common smells of bug spray such as lemongrass, they discovered that it’s easy for the mosquitoes to ignore the smell. We wish it were that easy for us to ignore that chemically fruity smell.
Researchers continue to work hard to figure out how to repel mosquitoes and we’re rooting for them as summer approaches, despite the mosquito’s status as a creepy crawly.
Can you keep it down? I’m trying to be boring
He chides his friends for not looking both ways before crossing the road. He is never questioned by the police because they fall asleep listening to him talk. He has won the office’s coveted perfect attendance award 10 years running. Look out, Dos Equis guy, you’ve got some new competition. That’s right, it’s the most boring man in the world.
For this boring study (sorry, study on boredom) conducted by English researchers and published in Personality and Social Psychology Bulletin, people were surveyed on various jobs and hobbies, ranking them by how exciting or boring they are, as well as how competent someone with those jobs/hobbies would be, their willingness to avoid someone with those jobs/hobbies, and how much they’d need to be paid to spend time with someone who had an undesirable job/hobby.
According to the British public, the most boring person in the world is a religious data analyst who likes to sleep and lives in a small town. In fact, spending time with this person is almost a full-time job on its own: To make it worth their while, survey subjects wanted 35 pounds a day. The boring person also was viewed as less competent, as is anyone with a boring job.
Now, there probably aren’t a lot of religious data analysts out there, but don’t worry, there are plenty of other boring jobs – accounting, tax/insurance, cleaning, and banking rounded out the top five (apparently people don’t like finances) – and hobbies – watching TV, observing animals, and mathematics filled out the top five. In case you’re curious, performing artists, scientists, journalists, health professionals, and teachers were viewed as having exciting jobs; exciting hobbies included gaming, reading, domestic tasks (really?), gardening, and writing.
Lead researcher Wijnand Van Tilburg, PhD, made an excellent point about people with boring jobs: They “have power in society – perhaps we should try not to upset them and stereotype them as boring!”
We think they should lean into it and make The Most Boring Man in the World ads: “When I drive a car off the lot, its value increases because I used the correct lending association. Batman trusts me with his Batmobile insurance. I can make those Cuban cigars tax exempt. Stay financially solvent, my friends.”
Fungi, but make it fashion
Fashion is an expensive and costly industry to sustain. Cotton production takes a toll on the environment, leather production comes with environmental and ethical/moral conundrums, and thanks to fast fashion, about 85% of textiles are being thrown away in the United States.
Researchers at the University of Borås in Sweden, however, have found a newish solution to create leather, cotton, and other textiles. And as with so many of the finer things, it starts with unsold bread from the grocery store.
Akram Zamani, PhD, and her team take that bread and turn it into breadcrumbs, then combine it with water and Rhizopus delemar, a fungus typically found in decaying food. After a couple of days of feasting on the bread, the fungus produces natural fibers made of chitin and chitosan that accumulate in the cell walls. After proteins, lipids, and other byproducts are removed, the team is left with a jelly-like substance made of those fibrous cell walls that can be spun into a fabric.
The researchers started small with very thin nonpliable sheets, but with a little layering by using tree tannins for softness and alkali for strength, their fungal leather is more like real leather than competing fungal leathers. Not to mention its being able to be produced in a fraction of the time.
This new fungal leather is fast to produce, it’s biodegradable, and it uses only natural ingredients to treat the materials. It’s the ultimate environmental fashion statement.
Who’s afraid of cancer? Not C. elegans
And now, we bring you part 2 of our ongoing series: Creatures that can diagnose cancer. Last week, we discovered that ants are well on their way to replacing dogs in our medical labs and in our hearts. This week, we present the even-more-lovable nematode.
The soil-dwelling nematode Caenorhabditis elegans, which is less than 1 mm long, is known to be “attracted or repelled by certain odors, so we came up with an idea that the roundworm could be used to detect lung cancer,” Shin Sik Choi, PhD, of Myongji University in South Korea, who is the project’s principal investigator, said in a statement on Eurekalert.
Dr. Choi’s team created a “worm-on-a-chip” that allowed the nematodes to choose between a drop of culture media from lung cancer cells and media from normal lung fibroblasts. An hour after being placed in the chip’s central chamber, more nematodes had crawled toward the lung cancer media than the normal-cell sample.
The investigators estimate that the device is about 70% effective at detecting cancer cells, but “they hope to increase both the accuracy and sensitivity of the method by using worms that were previously exposed to cancer cell media and therefore have a ‘memory’ of cancer-specific odor molecules,” according to the statement from the American Chemical Society.
Since C. elegans is easy to grow in a lab and, apparently, easy to train, the researchers hope that the worm-on-a-chip can become a quick, easy, economical, and noninvasive cancer screen.
So watch out cancer, because we never bet against the creepy crawlies.
Mosquitoes have us figured out
We are nearing mosquito season; quite possibly the most annoying and itchy time of the year. We stock up on bottles of bug spray, but somehow we still get bite after bite. It appears that mosquitoes are basically able to ignore our bug sprays, which explains why we’re still covered in bites after the Fourth of July fireworks. It turns out mosquitoes are more complex than we thought for such tiny creatures.
There’s plenty of research on the best ways to keep mosquitoes away, because not only are they incredibly annoying, but they also carry potentially harmful diseases. In a recent experiment, researchers used mosquitoes that were genetically modified to have an excessive amount of an odor receptor called AgOR2, which responds to the smell of humans.
“AgOR2 overexpression threw a wrench in the whole system by inactivating olfactory receptors in these mosquitoes,” Christopher Potter, PhD, associate professor of neuroscience at Johns Hopkins University, said in a written statement.
After testing how these genetically modified mosquitoes reacted to some of the common smells of bug spray such as lemongrass, they discovered that it’s easy for the mosquitoes to ignore the smell. We wish it were that easy for us to ignore that chemically fruity smell.
Researchers continue to work hard to figure out how to repel mosquitoes and we’re rooting for them as summer approaches, despite the mosquito’s status as a creepy crawly.
Neurodevelopmental disorders prevalent with extremely preterm birth
A large registry-based cohort study in Sweden has revealed that 75% of children born before 24 weeks of gestation had neurodevelopmental disorders, including intellectual disabilities and autism, and required habilitative services.
In addition, somatic disorders such as asthma and failure to thrive/short stature were diagnosed in 88% of the cohort. The findings, published in Acta Paediatrica, emphasize the need for further study of this population, especially as survival rates continue to increase.
“The primary aim of this large, retrospective, national study was to report clinical diagnoses registered after children born before 24 weeks were discharged from neonatal care,” explained lead author Eva Morsing, MD, PhD, of Lund (Sweden) University, and colleagues.
Data on diagnoses of neurodevelopmental disorders and selected somatic diagnoses were obtained from national Swedish registries. Study participants’ individual medical files were also examined by the researchers.
Results
The study cohort comprised 383 infants born at a median of 23.3 weeks of gestation (range, 21.9-23.9 weeks). The median birthweight of participants was 565 grams (range, 340-874 grams), with a median birthweight standard deviation (SD) of −0.40 (range, −3.63–3.17).
The majority (75%) of infants had a neurodevelopmental disorder, including speech disorders (52%), intellectual disabilities (40%), attention-deficit/hyperactivity disorder (30%), autism spectrum disorder (24%), visual impairment (22%), cerebral palsy (17%), epilepsy (10%), and hearing impairment (5%).
With respect to gender, a greater number of boys than girls born at 23 weeks had intellectual disabilities (45% vs. 27%; P < .01) and visual impairment (25% vs. 14%; P < .01). Moreover, 55% of the participants were referred for habilitative services.
With respect to somatic diagnoses, failure to thrive/short stature was diagnosed in 39% of the cohort, and it occurred more often in those born at 21 and 22 weeks than in those born at 23 weeks (49% vs. 36%; P < .05).
In addition, asthma and childhood bronchopulmonary dysplasia, pulmonary hypertension, and vocal cord paresis were diagnosed in 63%, 12%, and 13% of participants, respectively.
“Several studies have reported higher rates of preterm morbidities, and poor neurodevelopmental outcomes after extremely preterm birth in boys rather than girls,” study author Ann Hellström, MD, PhD, of the University of Gothenburg, Sweden, said in an interview.
“While the reasons for this were not studied in the present paper, reports in the literature suggest that boys have a higher average growth rate than girls and appear to be more sensitive to suboptimal neonatal nutrition than girls,” Dr. Hellström explained.
“We also know that sex steroids differ in relation to intrauterine life depending on the sex after preterm birth,” Dr. Hellström added.
In an accompanying editorial, Neil Marlow, MD, of University College London, wrote, “One headline from this study [that is interesting] is the high prevalence of autistic spectrum disorders recorded.
“This is a particular finding in extremely preterm cohorts from Sweden, who record more diagnoses than in other longitudinal studies,” Dr. Marlow added. “It certainly warrants further investigation and understanding.”
The researchers acknowledged that a key limitation of the study was the broad age range at the most recent follow-up visit, which ranged from 2 to 13 years, explaining that some diagnoses may occur later in childhood.
“Neonatal clinical practice needs to adopt a long-term perspective and clinicians treating children and adults should be aware of the complicated health problems of children born before 24 weeks,” they concluded.
This study was supported by the Swedish Medical Research Council, the Gothenburg Medical Society, and by grant funding from the Swedish government. The authors reported no relevant disclosures.
A large registry-based cohort study in Sweden has revealed that 75% of children born before 24 weeks of gestation had neurodevelopmental disorders, including intellectual disabilities and autism, and required habilitative services.
In addition, somatic disorders such as asthma and failure to thrive/short stature were diagnosed in 88% of the cohort. The findings, published in Acta Paediatrica, emphasize the need for further study of this population, especially as survival rates continue to increase.
“The primary aim of this large, retrospective, national study was to report clinical diagnoses registered after children born before 24 weeks were discharged from neonatal care,” explained lead author Eva Morsing, MD, PhD, of Lund (Sweden) University, and colleagues.
Data on diagnoses of neurodevelopmental disorders and selected somatic diagnoses were obtained from national Swedish registries. Study participants’ individual medical files were also examined by the researchers.
Results
The study cohort comprised 383 infants born at a median of 23.3 weeks of gestation (range, 21.9-23.9 weeks). The median birthweight of participants was 565 grams (range, 340-874 grams), with a median birthweight standard deviation (SD) of −0.40 (range, −3.63–3.17).
The majority (75%) of infants had a neurodevelopmental disorder, including speech disorders (52%), intellectual disabilities (40%), attention-deficit/hyperactivity disorder (30%), autism spectrum disorder (24%), visual impairment (22%), cerebral palsy (17%), epilepsy (10%), and hearing impairment (5%).
With respect to gender, a greater number of boys than girls born at 23 weeks had intellectual disabilities (45% vs. 27%; P < .01) and visual impairment (25% vs. 14%; P < .01). Moreover, 55% of the participants were referred for habilitative services.
With respect to somatic diagnoses, failure to thrive/short stature was diagnosed in 39% of the cohort, and it occurred more often in those born at 21 and 22 weeks than in those born at 23 weeks (49% vs. 36%; P < .05).
In addition, asthma and childhood bronchopulmonary dysplasia, pulmonary hypertension, and vocal cord paresis were diagnosed in 63%, 12%, and 13% of participants, respectively.
“Several studies have reported higher rates of preterm morbidities, and poor neurodevelopmental outcomes after extremely preterm birth in boys rather than girls,” study author Ann Hellström, MD, PhD, of the University of Gothenburg, Sweden, said in an interview.
“While the reasons for this were not studied in the present paper, reports in the literature suggest that boys have a higher average growth rate than girls and appear to be more sensitive to suboptimal neonatal nutrition than girls,” Dr. Hellström explained.
“We also know that sex steroids differ in relation to intrauterine life depending on the sex after preterm birth,” Dr. Hellström added.
In an accompanying editorial, Neil Marlow, MD, of University College London, wrote, “One headline from this study [that is interesting] is the high prevalence of autistic spectrum disorders recorded.
“This is a particular finding in extremely preterm cohorts from Sweden, who record more diagnoses than in other longitudinal studies,” Dr. Marlow added. “It certainly warrants further investigation and understanding.”
The researchers acknowledged that a key limitation of the study was the broad age range at the most recent follow-up visit, which ranged from 2 to 13 years, explaining that some diagnoses may occur later in childhood.
“Neonatal clinical practice needs to adopt a long-term perspective and clinicians treating children and adults should be aware of the complicated health problems of children born before 24 weeks,” they concluded.
This study was supported by the Swedish Medical Research Council, the Gothenburg Medical Society, and by grant funding from the Swedish government. The authors reported no relevant disclosures.
A large registry-based cohort study in Sweden has revealed that 75% of children born before 24 weeks of gestation had neurodevelopmental disorders, including intellectual disabilities and autism, and required habilitative services.
In addition, somatic disorders such as asthma and failure to thrive/short stature were diagnosed in 88% of the cohort. The findings, published in Acta Paediatrica, emphasize the need for further study of this population, especially as survival rates continue to increase.
“The primary aim of this large, retrospective, national study was to report clinical diagnoses registered after children born before 24 weeks were discharged from neonatal care,” explained lead author Eva Morsing, MD, PhD, of Lund (Sweden) University, and colleagues.
Data on diagnoses of neurodevelopmental disorders and selected somatic diagnoses were obtained from national Swedish registries. Study participants’ individual medical files were also examined by the researchers.
Results
The study cohort comprised 383 infants born at a median of 23.3 weeks of gestation (range, 21.9-23.9 weeks). The median birthweight of participants was 565 grams (range, 340-874 grams), with a median birthweight standard deviation (SD) of −0.40 (range, −3.63–3.17).
The majority (75%) of infants had a neurodevelopmental disorder, including speech disorders (52%), intellectual disabilities (40%), attention-deficit/hyperactivity disorder (30%), autism spectrum disorder (24%), visual impairment (22%), cerebral palsy (17%), epilepsy (10%), and hearing impairment (5%).
With respect to gender, a greater number of boys than girls born at 23 weeks had intellectual disabilities (45% vs. 27%; P < .01) and visual impairment (25% vs. 14%; P < .01). Moreover, 55% of the participants were referred for habilitative services.
With respect to somatic diagnoses, failure to thrive/short stature was diagnosed in 39% of the cohort, and it occurred more often in those born at 21 and 22 weeks than in those born at 23 weeks (49% vs. 36%; P < .05).
In addition, asthma and childhood bronchopulmonary dysplasia, pulmonary hypertension, and vocal cord paresis were diagnosed in 63%, 12%, and 13% of participants, respectively.
“Several studies have reported higher rates of preterm morbidities, and poor neurodevelopmental outcomes after extremely preterm birth in boys rather than girls,” study author Ann Hellström, MD, PhD, of the University of Gothenburg, Sweden, said in an interview.
“While the reasons for this were not studied in the present paper, reports in the literature suggest that boys have a higher average growth rate than girls and appear to be more sensitive to suboptimal neonatal nutrition than girls,” Dr. Hellström explained.
“We also know that sex steroids differ in relation to intrauterine life depending on the sex after preterm birth,” Dr. Hellström added.
In an accompanying editorial, Neil Marlow, MD, of University College London, wrote, “One headline from this study [that is interesting] is the high prevalence of autistic spectrum disorders recorded.
“This is a particular finding in extremely preterm cohorts from Sweden, who record more diagnoses than in other longitudinal studies,” Dr. Marlow added. “It certainly warrants further investigation and understanding.”
The researchers acknowledged that a key limitation of the study was the broad age range at the most recent follow-up visit, which ranged from 2 to 13 years, explaining that some diagnoses may occur later in childhood.
“Neonatal clinical practice needs to adopt a long-term perspective and clinicians treating children and adults should be aware of the complicated health problems of children born before 24 weeks,” they concluded.
This study was supported by the Swedish Medical Research Council, the Gothenburg Medical Society, and by grant funding from the Swedish government. The authors reported no relevant disclosures.
FROM ACTA PAEDIATRICA
Natural, vaccine-induced, and hybrid immunity to COVID-19
Seroprevalence surveys suggest that, from the beginning of the pandemic to 2022, more than a third of the global population had been infected with SARS-CoV-2. As large numbers of people continue to be infected, the efficacy and duration of natural immunity, in terms of protection against SARS-CoV-2 reinfections and severe disease, are of crucial significance. The virus’s epidemiologic trajectory will be influenced by the trends in vaccine-induced and hybrid immunity.
Omicron’s immune evasion
Cases of SARS-CoV-2 reinfection are increasing around the world. According to data from the U.K. Health Security Agency, 650,000 people in England have been infected twice, and most of them were reinfected in the past 2 months. Before mid-November 2021, reinfections accounted for about 1% of reported cases, but the rate has now increased to around 10%. The reinfection risk was 16 times higher between mid-December 2021 and early January 2022. Experts believe that this spike in reinfections is related to the spread of Omicron, which overtook Delta as the dominant variant. Nonetheless, other aspects should also be considered.
Omicron’s greater propensity to spread is not unrelated to its ability to evade the body’s immune defenses. This aspect was raised in a letter recently published in the New England Journal of Medicine. The authors reported that the effectiveness of previous infection in preventing reinfection against the Alpha, Beta, and Delta variants was around 90%, but it was only 56% against Omicron.
Natural immunity
Natural immunity showed roughly similar effectiveness regarding protection against reinfection across different SARS-CoV-2 variants, with the exception of the Omicron variant. The risk of hospitalization and death was also reduced in SARS-CoV-2 reinfections versus primary infections. Observational studies indicate that natural immunity may offer equal or greater protection against SARS-CoV-2 infections, compared with immunization with two doses of an mRNA vaccine, but the data are not fully consistent.
Natural immunity seems to be relatively long-lasting. Data from Denmark and Austria show no evidence that protection against reinfections wanes after 6 months. Some investigations indicate that protection against reinfection is lowest 4-5 months after initial infection and increases thereafter, a finding that might hypothetically be explained by persistent viral shedding; that is, misclassification of prolonged SARS-CoV-2 infections as reinfections. While no comparison was made against information pertaining to unvaccinated, not previously-infected individuals, preliminary data from Israel suggest that protection from reinfection can decrease from 6 to more than 12 months after the first SARS-CoV-2 infection. Taken together, epidemiologic studies indicate that protection against reinfections by natural immunity lasts over 1 year with only moderate, if any, decline over this period. Among older individuals, immunocompromised patients, and those with certain comorbidities or exposure risk (for example, health care workers), rates of reinfection may be higher. It is plausible that reinfection risk may be a function of exposure risk.
There is accumulating evidence that reinfections may be significantly less severe than primary infections with SARS-CoV-2. Reduced clinical severity of SARS-CoV-2 reinfections naturally also makes sense from a biologic point of view, inasmuch as a previously primed immune system should be better prepared for a rechallenge with this virus.
Vaccine-induced immunity
The short-term (<4 months) efficacy of mRNA vaccines against SARS-CoV-2 is high and varies from 94.1% (Moderna) to 95% (BioNTech/Pfizer). This has been confirmed by randomized controlled trials and was subsequently confirmed in effectiveness studies in real-world settings. Waning efficacy was observed with respect to protection against SARS-CoV-2 infections (for example, only approximately 20% after about half a year in Qatar), whereas protection against severe disease was either sustained or showed only a moderate decline.
In individuals who received two doses of the BioNTech/Pfizer vaccine at least 5 months earlier, an additional vaccine dose, a so-called booster, significantly lowered mortality and severe illness. These findings suggest that the booster restored and probably exceeded the initial short-term efficacy of the initial vaccination.
Data are still emerging regarding the efficacy of boosters against the Omicron variants. Preliminary data suggest a far lower ability to restore protection from infection and vaccination. However, fatalities and hospitalizations remain low.
Natural immunity vs. vaccine-induced immunity
Comparisons of natural immunity with vaccine-induced immunity are complicated by a series of biases and by combinations of biases – for example, the biases of comparisons between infected and uninfected, plus the biases of comparisons between vaccinated and nonvaccinated, with strong potential selection biases and confounding. Of particular note, the proportion of people previously infected and/or vaccinated may influence estimates of effectiveness. Regarding this point, one study compared unvaccinated patients with a prior SARS-CoV-2 infection and vaccinated individuals followed up from a week after the second vaccine dose onward versus a group of unvaccinated, not previously infected individuals. The findings showed that, compared with unvaccinated, not previously infected individuals, the natural immunity group and the vaccinated group had similar protection of 94.8% and 92.8% against infection, of 94.1% and 94.2% against hospitalization, and of 96.4% and 94.4% against severe illness, respectively.
Hybrid immunity
The combination of a previous SARS-CoV-2 infection and a respective vaccination is called hybrid immunity. This combination seems to confer the greatest protection against SARS-CoV-2 infections, but several knowledge gaps remain regarding this issue.
Data from Israel showed that, when the time since the last immunity-conferring event (either primary infection or vaccination) was the same, the rates of SARS-CoV-2 infections were similar in the following groups: individuals who had a previous infection and no vaccination, individuals who had an infection and were then vaccinated with a single dose after at least 3 months, and individuals who were vaccinated (two doses) and then infected. Severe disease was relatively rare overall.
Data on the efficacy of hybrid immunity point in the direction of hybrid immunity being superior, as compared with either vaccine-induced (without a booster) immunity or natural immunity alone. Timing and mode of vaccination of previously infected individuals to achieve optimal hybrid immunity are central questions that remain to be addressed in future studies.
Given that vaccination rates are continuously increasing and that, by the beginning of 2022, perhaps half or more of the global population had already been infected with SARS-CoV-2, with the vast majority of this group not being officially detected, it would appear logical that future infection waves, even with highly transmissible variants of SARS-CoV-2, may be limited with respect to their maximum potential health burden. The advent of Omicron suggests that massive surges can occur even in populations with extremely high rates of previous vaccination and variable rates of prior infections. However, even then, the accompanying burden of hospitalizations and deaths is far less than what was seen in 2020 and 2021. One may argue that the pandemic has already transitioned to the endemic phase and that Omicron is an endemic wave occurring in the setting of already widespread population immunity.
A version of this article first appeared on Medscape.com.
Seroprevalence surveys suggest that, from the beginning of the pandemic to 2022, more than a third of the global population had been infected with SARS-CoV-2. As large numbers of people continue to be infected, the efficacy and duration of natural immunity, in terms of protection against SARS-CoV-2 reinfections and severe disease, are of crucial significance. The virus’s epidemiologic trajectory will be influenced by the trends in vaccine-induced and hybrid immunity.
Omicron’s immune evasion
Cases of SARS-CoV-2 reinfection are increasing around the world. According to data from the U.K. Health Security Agency, 650,000 people in England have been infected twice, and most of them were reinfected in the past 2 months. Before mid-November 2021, reinfections accounted for about 1% of reported cases, but the rate has now increased to around 10%. The reinfection risk was 16 times higher between mid-December 2021 and early January 2022. Experts believe that this spike in reinfections is related to the spread of Omicron, which overtook Delta as the dominant variant. Nonetheless, other aspects should also be considered.
Omicron’s greater propensity to spread is not unrelated to its ability to evade the body’s immune defenses. This aspect was raised in a letter recently published in the New England Journal of Medicine. The authors reported that the effectiveness of previous infection in preventing reinfection against the Alpha, Beta, and Delta variants was around 90%, but it was only 56% against Omicron.
Natural immunity
Natural immunity showed roughly similar effectiveness regarding protection against reinfection across different SARS-CoV-2 variants, with the exception of the Omicron variant. The risk of hospitalization and death was also reduced in SARS-CoV-2 reinfections versus primary infections. Observational studies indicate that natural immunity may offer equal or greater protection against SARS-CoV-2 infections, compared with immunization with two doses of an mRNA vaccine, but the data are not fully consistent.
Natural immunity seems to be relatively long-lasting. Data from Denmark and Austria show no evidence that protection against reinfections wanes after 6 months. Some investigations indicate that protection against reinfection is lowest 4-5 months after initial infection and increases thereafter, a finding that might hypothetically be explained by persistent viral shedding; that is, misclassification of prolonged SARS-CoV-2 infections as reinfections. While no comparison was made against information pertaining to unvaccinated, not previously-infected individuals, preliminary data from Israel suggest that protection from reinfection can decrease from 6 to more than 12 months after the first SARS-CoV-2 infection. Taken together, epidemiologic studies indicate that protection against reinfections by natural immunity lasts over 1 year with only moderate, if any, decline over this period. Among older individuals, immunocompromised patients, and those with certain comorbidities or exposure risk (for example, health care workers), rates of reinfection may be higher. It is plausible that reinfection risk may be a function of exposure risk.
There is accumulating evidence that reinfections may be significantly less severe than primary infections with SARS-CoV-2. Reduced clinical severity of SARS-CoV-2 reinfections naturally also makes sense from a biologic point of view, inasmuch as a previously primed immune system should be better prepared for a rechallenge with this virus.
Vaccine-induced immunity
The short-term (<4 months) efficacy of mRNA vaccines against SARS-CoV-2 is high and varies from 94.1% (Moderna) to 95% (BioNTech/Pfizer). This has been confirmed by randomized controlled trials and was subsequently confirmed in effectiveness studies in real-world settings. Waning efficacy was observed with respect to protection against SARS-CoV-2 infections (for example, only approximately 20% after about half a year in Qatar), whereas protection against severe disease was either sustained or showed only a moderate decline.
In individuals who received two doses of the BioNTech/Pfizer vaccine at least 5 months earlier, an additional vaccine dose, a so-called booster, significantly lowered mortality and severe illness. These findings suggest that the booster restored and probably exceeded the initial short-term efficacy of the initial vaccination.
Data are still emerging regarding the efficacy of boosters against the Omicron variants. Preliminary data suggest a far lower ability to restore protection from infection and vaccination. However, fatalities and hospitalizations remain low.
Natural immunity vs. vaccine-induced immunity
Comparisons of natural immunity with vaccine-induced immunity are complicated by a series of biases and by combinations of biases – for example, the biases of comparisons between infected and uninfected, plus the biases of comparisons between vaccinated and nonvaccinated, with strong potential selection biases and confounding. Of particular note, the proportion of people previously infected and/or vaccinated may influence estimates of effectiveness. Regarding this point, one study compared unvaccinated patients with a prior SARS-CoV-2 infection and vaccinated individuals followed up from a week after the second vaccine dose onward versus a group of unvaccinated, not previously infected individuals. The findings showed that, compared with unvaccinated, not previously infected individuals, the natural immunity group and the vaccinated group had similar protection of 94.8% and 92.8% against infection, of 94.1% and 94.2% against hospitalization, and of 96.4% and 94.4% against severe illness, respectively.
Hybrid immunity
The combination of a previous SARS-CoV-2 infection and a respective vaccination is called hybrid immunity. This combination seems to confer the greatest protection against SARS-CoV-2 infections, but several knowledge gaps remain regarding this issue.
Data from Israel showed that, when the time since the last immunity-conferring event (either primary infection or vaccination) was the same, the rates of SARS-CoV-2 infections were similar in the following groups: individuals who had a previous infection and no vaccination, individuals who had an infection and were then vaccinated with a single dose after at least 3 months, and individuals who were vaccinated (two doses) and then infected. Severe disease was relatively rare overall.
Data on the efficacy of hybrid immunity point in the direction of hybrid immunity being superior, as compared with either vaccine-induced (without a booster) immunity or natural immunity alone. Timing and mode of vaccination of previously infected individuals to achieve optimal hybrid immunity are central questions that remain to be addressed in future studies.
Given that vaccination rates are continuously increasing and that, by the beginning of 2022, perhaps half or more of the global population had already been infected with SARS-CoV-2, with the vast majority of this group not being officially detected, it would appear logical that future infection waves, even with highly transmissible variants of SARS-CoV-2, may be limited with respect to their maximum potential health burden. The advent of Omicron suggests that massive surges can occur even in populations with extremely high rates of previous vaccination and variable rates of prior infections. However, even then, the accompanying burden of hospitalizations and deaths is far less than what was seen in 2020 and 2021. One may argue that the pandemic has already transitioned to the endemic phase and that Omicron is an endemic wave occurring in the setting of already widespread population immunity.
A version of this article first appeared on Medscape.com.
Seroprevalence surveys suggest that, from the beginning of the pandemic to 2022, more than a third of the global population had been infected with SARS-CoV-2. As large numbers of people continue to be infected, the efficacy and duration of natural immunity, in terms of protection against SARS-CoV-2 reinfections and severe disease, are of crucial significance. The virus’s epidemiologic trajectory will be influenced by the trends in vaccine-induced and hybrid immunity.
Omicron’s immune evasion
Cases of SARS-CoV-2 reinfection are increasing around the world. According to data from the U.K. Health Security Agency, 650,000 people in England have been infected twice, and most of them were reinfected in the past 2 months. Before mid-November 2021, reinfections accounted for about 1% of reported cases, but the rate has now increased to around 10%. The reinfection risk was 16 times higher between mid-December 2021 and early January 2022. Experts believe that this spike in reinfections is related to the spread of Omicron, which overtook Delta as the dominant variant. Nonetheless, other aspects should also be considered.
Omicron’s greater propensity to spread is not unrelated to its ability to evade the body’s immune defenses. This aspect was raised in a letter recently published in the New England Journal of Medicine. The authors reported that the effectiveness of previous infection in preventing reinfection against the Alpha, Beta, and Delta variants was around 90%, but it was only 56% against Omicron.
Natural immunity
Natural immunity showed roughly similar effectiveness regarding protection against reinfection across different SARS-CoV-2 variants, with the exception of the Omicron variant. The risk of hospitalization and death was also reduced in SARS-CoV-2 reinfections versus primary infections. Observational studies indicate that natural immunity may offer equal or greater protection against SARS-CoV-2 infections, compared with immunization with two doses of an mRNA vaccine, but the data are not fully consistent.
Natural immunity seems to be relatively long-lasting. Data from Denmark and Austria show no evidence that protection against reinfections wanes after 6 months. Some investigations indicate that protection against reinfection is lowest 4-5 months after initial infection and increases thereafter, a finding that might hypothetically be explained by persistent viral shedding; that is, misclassification of prolonged SARS-CoV-2 infections as reinfections. While no comparison was made against information pertaining to unvaccinated, not previously-infected individuals, preliminary data from Israel suggest that protection from reinfection can decrease from 6 to more than 12 months after the first SARS-CoV-2 infection. Taken together, epidemiologic studies indicate that protection against reinfections by natural immunity lasts over 1 year with only moderate, if any, decline over this period. Among older individuals, immunocompromised patients, and those with certain comorbidities or exposure risk (for example, health care workers), rates of reinfection may be higher. It is plausible that reinfection risk may be a function of exposure risk.
There is accumulating evidence that reinfections may be significantly less severe than primary infections with SARS-CoV-2. Reduced clinical severity of SARS-CoV-2 reinfections naturally also makes sense from a biologic point of view, inasmuch as a previously primed immune system should be better prepared for a rechallenge with this virus.
Vaccine-induced immunity
The short-term (<4 months) efficacy of mRNA vaccines against SARS-CoV-2 is high and varies from 94.1% (Moderna) to 95% (BioNTech/Pfizer). This has been confirmed by randomized controlled trials and was subsequently confirmed in effectiveness studies in real-world settings. Waning efficacy was observed with respect to protection against SARS-CoV-2 infections (for example, only approximately 20% after about half a year in Qatar), whereas protection against severe disease was either sustained or showed only a moderate decline.
In individuals who received two doses of the BioNTech/Pfizer vaccine at least 5 months earlier, an additional vaccine dose, a so-called booster, significantly lowered mortality and severe illness. These findings suggest that the booster restored and probably exceeded the initial short-term efficacy of the initial vaccination.
Data are still emerging regarding the efficacy of boosters against the Omicron variants. Preliminary data suggest a far lower ability to restore protection from infection and vaccination. However, fatalities and hospitalizations remain low.
Natural immunity vs. vaccine-induced immunity
Comparisons of natural immunity with vaccine-induced immunity are complicated by a series of biases and by combinations of biases – for example, the biases of comparisons between infected and uninfected, plus the biases of comparisons between vaccinated and nonvaccinated, with strong potential selection biases and confounding. Of particular note, the proportion of people previously infected and/or vaccinated may influence estimates of effectiveness. Regarding this point, one study compared unvaccinated patients with a prior SARS-CoV-2 infection and vaccinated individuals followed up from a week after the second vaccine dose onward versus a group of unvaccinated, not previously infected individuals. The findings showed that, compared with unvaccinated, not previously infected individuals, the natural immunity group and the vaccinated group had similar protection of 94.8% and 92.8% against infection, of 94.1% and 94.2% against hospitalization, and of 96.4% and 94.4% against severe illness, respectively.
Hybrid immunity
The combination of a previous SARS-CoV-2 infection and a respective vaccination is called hybrid immunity. This combination seems to confer the greatest protection against SARS-CoV-2 infections, but several knowledge gaps remain regarding this issue.
Data from Israel showed that, when the time since the last immunity-conferring event (either primary infection or vaccination) was the same, the rates of SARS-CoV-2 infections were similar in the following groups: individuals who had a previous infection and no vaccination, individuals who had an infection and were then vaccinated with a single dose after at least 3 months, and individuals who were vaccinated (two doses) and then infected. Severe disease was relatively rare overall.
Data on the efficacy of hybrid immunity point in the direction of hybrid immunity being superior, as compared with either vaccine-induced (without a booster) immunity or natural immunity alone. Timing and mode of vaccination of previously infected individuals to achieve optimal hybrid immunity are central questions that remain to be addressed in future studies.
Given that vaccination rates are continuously increasing and that, by the beginning of 2022, perhaps half or more of the global population had already been infected with SARS-CoV-2, with the vast majority of this group not being officially detected, it would appear logical that future infection waves, even with highly transmissible variants of SARS-CoV-2, may be limited with respect to their maximum potential health burden. The advent of Omicron suggests that massive surges can occur even in populations with extremely high rates of previous vaccination and variable rates of prior infections. However, even then, the accompanying burden of hospitalizations and deaths is far less than what was seen in 2020 and 2021. One may argue that the pandemic has already transitioned to the endemic phase and that Omicron is an endemic wave occurring in the setting of already widespread population immunity.
A version of this article first appeared on Medscape.com.
Moderna reports positive COVID-19 vaccine response in kids down to 6 months
Moderna on March 23 released interim results indicating that its mRNA-1273 COVID vaccine produced “robust” neutralizing antibody titers in children aged 6 months to 6 years – levels similar to those seen in adults.
Vaccine efficacy against infection was 43.7% in children aged 6 months to 2 years and 37.5% among children aged 2-6 years, the new data from its phase 2/3 KidCOVE study show.
The company explained the lower efficacy numbers by noting that its study involving these younger children was conducted during the Omicron wave. The same decrease in efficacy against infection was reported in adults during the Omicron surge.
A majority of COVID-19 cases were mild in the approximately 6,900 children aged 6 months to 6 years in the study. No severe COVID-19 cases, hospitalizations, or deaths were reported.
The primary series of two 25-mcg doses of the vaccine given 28 days apart was generally well tolerated. Most adverse events were mild to moderate. For example, temperature greater than 38° C (>100.4° F) was reported for 17.0% of the 6-month-old to 2-year-old group and for 14.6% of the 2- to 6-year-old group. A few children, 0.2% of each group, experienced a temperature greater than 40° C (>104° F).
Moderna plans to include these response, efficacy, and safety data in an application to the Food and Drug Administration for emergency use authorization (EUA) of the vaccine in these younger children in the coming weeks.
“We now have clinical data on the performance of our vaccine from infants 6 months of age through older adults,” Moderna CEO Stephane Bancel said in a news release. He described the interim results as “good news for parents of children under 6 years of age.”
In other news
Moderna also announced that it began the FDA EUA submission process for a 50-μg two-dose primary series for children aged 6-12 years.
The company is also updating its EUA submission for a 100-mcg two-dose primary series for children and adolescents aged 12-18 years.
Similar to its booster research in adults, Moderna plans to evaluate the potential of a booster dose for all pediatric populations, including those aged 6 months to 6 years, 6-12 years, and adolescents. The company is evaluating both a booster dose of mRNA-1273 and its bivalent booster candidate (mRNA1273.214), which includes an Omicron variant booster and mRNA-1273.
A version of this article first appeared on Medscape.com.
Moderna on March 23 released interim results indicating that its mRNA-1273 COVID vaccine produced “robust” neutralizing antibody titers in children aged 6 months to 6 years – levels similar to those seen in adults.
Vaccine efficacy against infection was 43.7% in children aged 6 months to 2 years and 37.5% among children aged 2-6 years, the new data from its phase 2/3 KidCOVE study show.
The company explained the lower efficacy numbers by noting that its study involving these younger children was conducted during the Omicron wave. The same decrease in efficacy against infection was reported in adults during the Omicron surge.
A majority of COVID-19 cases were mild in the approximately 6,900 children aged 6 months to 6 years in the study. No severe COVID-19 cases, hospitalizations, or deaths were reported.
The primary series of two 25-mcg doses of the vaccine given 28 days apart was generally well tolerated. Most adverse events were mild to moderate. For example, temperature greater than 38° C (>100.4° F) was reported for 17.0% of the 6-month-old to 2-year-old group and for 14.6% of the 2- to 6-year-old group. A few children, 0.2% of each group, experienced a temperature greater than 40° C (>104° F).
Moderna plans to include these response, efficacy, and safety data in an application to the Food and Drug Administration for emergency use authorization (EUA) of the vaccine in these younger children in the coming weeks.
“We now have clinical data on the performance of our vaccine from infants 6 months of age through older adults,” Moderna CEO Stephane Bancel said in a news release. He described the interim results as “good news for parents of children under 6 years of age.”
In other news
Moderna also announced that it began the FDA EUA submission process for a 50-μg two-dose primary series for children aged 6-12 years.
The company is also updating its EUA submission for a 100-mcg two-dose primary series for children and adolescents aged 12-18 years.
Similar to its booster research in adults, Moderna plans to evaluate the potential of a booster dose for all pediatric populations, including those aged 6 months to 6 years, 6-12 years, and adolescents. The company is evaluating both a booster dose of mRNA-1273 and its bivalent booster candidate (mRNA1273.214), which includes an Omicron variant booster and mRNA-1273.
A version of this article first appeared on Medscape.com.
Moderna on March 23 released interim results indicating that its mRNA-1273 COVID vaccine produced “robust” neutralizing antibody titers in children aged 6 months to 6 years – levels similar to those seen in adults.
Vaccine efficacy against infection was 43.7% in children aged 6 months to 2 years and 37.5% among children aged 2-6 years, the new data from its phase 2/3 KidCOVE study show.
The company explained the lower efficacy numbers by noting that its study involving these younger children was conducted during the Omicron wave. The same decrease in efficacy against infection was reported in adults during the Omicron surge.
A majority of COVID-19 cases were mild in the approximately 6,900 children aged 6 months to 6 years in the study. No severe COVID-19 cases, hospitalizations, or deaths were reported.
The primary series of two 25-mcg doses of the vaccine given 28 days apart was generally well tolerated. Most adverse events were mild to moderate. For example, temperature greater than 38° C (>100.4° F) was reported for 17.0% of the 6-month-old to 2-year-old group and for 14.6% of the 2- to 6-year-old group. A few children, 0.2% of each group, experienced a temperature greater than 40° C (>104° F).
Moderna plans to include these response, efficacy, and safety data in an application to the Food and Drug Administration for emergency use authorization (EUA) of the vaccine in these younger children in the coming weeks.
“We now have clinical data on the performance of our vaccine from infants 6 months of age through older adults,” Moderna CEO Stephane Bancel said in a news release. He described the interim results as “good news for parents of children under 6 years of age.”
In other news
Moderna also announced that it began the FDA EUA submission process for a 50-μg two-dose primary series for children aged 6-12 years.
The company is also updating its EUA submission for a 100-mcg two-dose primary series for children and adolescents aged 12-18 years.
Similar to its booster research in adults, Moderna plans to evaluate the potential of a booster dose for all pediatric populations, including those aged 6 months to 6 years, 6-12 years, and adolescents. The company is evaluating both a booster dose of mRNA-1273 and its bivalent booster candidate (mRNA1273.214), which includes an Omicron variant booster and mRNA-1273.
A version of this article first appeared on Medscape.com.
‘Profound implications’: COVID ups diabetes risk 40% a year later
COVID-19 infection appears to significantly raise the risk for diabetes by about 40% at 1 year, indicate new data from a very large Veterans Administration population.
“If patients have a prior history of COVID-19, that’s a risk factor for diabetes and they should certainly be screened for diabetes,” study coauthor Ziyad Al-Aly, MD, a nephrologist and chief of research and development at VA St. Louis Health Care, told this news organization.
“It’s still premature to make guidelines. I think we have to process the data landscape to understand what this all really means, but it’s really, really clear that all these roads are pointing in one direction, that COVID-19 increases the risk of diabetes up to a year later. The risk is small but not negligible,” he said.
The database includes over 8 million people and 180,000 with a prior COVID-19 diagnosis. Significantly increased diabetes risks compared to those not infected ranging from 31% to more than double were found in an analysis of subgroups based on diabetes risk score, body mass index, age, race, prediabetes status, and deprivation level, even after adjustment for confounding factors.
There was a gradient of diabetes risk by COVID-19 severity – i.e., whether patients had not been hospitalized, had been hospitalized, or stayed in intensive care – but a significant excess diabetes burden was seen even among those with “mild” COVID-19. The diabetes risk was also elevated compared to both contemporary and historical controls.
The study was published March 21 in The Lancet Diabetes & Endocrinology, by Yan Xie, MPH, also of VA St Louis Health Care, along with Dr. Al-Aly.
The data align with those from another study just published from a nationwide German primary care database. That study was smaller and of shorter duration than the new VA study but consistent, said Dr. Al-Aly, a clinical epidemiologist at Washington University, St. Louis.
Millions more with new diabetes as late manifestation of COVID-19
“Millions of people in the U.S. have had COVID-19, so this is going to translate to literally millions more people with new-onset diabetes. Better to identify them early so they can be adequately treated,” Dr. Al-Aly said in an interview.
“The long-term implications of SARS-CoV-2 infection increasing diabetes risk are profound,” Venkat Narayan, MD, and Lisa R. Staimez, PhD, both of the Rollins School of Public Health and Emory Global Diabetes Research Center at Emory University, Atlanta, said in an accompanying editorial.
“With large and growing numbers of people worldwide infected with SARS-CoV-2 (434,154,739 cumulative cases by Feb. 28, 2022), any COVID-19-related increases in diabetes incidence could lead to unprecedented cases of diabetes worldwide – wreaking havoc on already over-stretched and under-resourced clinical and public health systems globally, with devastating tolls in terms of deaths and suffering,” they added.
Medscape Medical News contributor Eric Topol MD, of Scripps Research Institute, La Jolla, Calif., agrees. He said these new data “are most profound. The researchers found a 40% increase in diabetes that wasn’t present at 1 month after COVID-19 but at 1 year, it was. Some kind of late manifestation is happening here.”
Dr. Al-Aly told this news organization that the mechanisms for the association are unknown and likely to be heterogeneous. Among the people who already had risk factors for type 2 diabetes, such as obesity or metabolic syndrome, SARS-CoV-2 could simply accelerate that process and “put them over the edge” to overt diabetes.
However, for those without diabetes risk factors, “COVID-19 with all the inflammation it provokes in the body could be leading to de novo disease.” (Diabetes status was ascertained by ICD-10 codes and only about 0.70% of the total were recorded as type 1 diabetes. But, since autoantibody testing wasn’t routinely conducted, it’s unknown how many of the cases may have been type 1 misclassified as type 2, Dr. Al-Aly acknowledged.)
Diabetes risk significantly increased after COVID-19 in all analyses
The analysis included 181,280 patients in the U.S. Department of Veterans Affairs health care database with a COVID-19 diagnosis who survived for at least 30 days afterward during March 2020 through Sept. 30, 2021, with 4,118,441 contemporary controls without COVID-19 seen during 2019, and a historical control group of 4,286,911 people seen at the VA in 2017. Average follow-up was about a year.
Compared with the contemporary controls, the COVID-19 group had an excess diabetes burden of 13.46 per 1,000 person-years with a hazard ratio of 1.40. They had an increased 12.35 per 1,000 person-year risk for incident use of glucose-lowering medications, with a hazard ratio of 1.85. Similar results were seen with the historical controls.
Subgroup analyses showed an increased risk for diabetes following COVID-19 infection by age (≤ 65 years and > 65 years), race (White and Black), sex (male and female), BMI categories (> 18.5 to ≤ 25 kg/m², > 25 to ≤ 30 kg/m², and > 30 kg/m²), and area deprivation index quartiles. The increased risk was also seen across diabetes risk score quartiles.
Notably, COVID-19 significantly elevated the diabetes risk by 59% even for the subgroup with BMI between 18 and 25 kg/m², and by 38% among those with the lowest diabetes risk score quartile.
The COVID-19 population included 162,096 who were not hospitalized, 15,078 hospitalized, and 4,106 admitted to intensive care. Here, the hazard ratios for diabetes compared to the contemporary controls were 1.25, 2.73, and 3.76, respectively, all significant.
Dr. Al-Aly said that his group is now further analyzing the VA data for other outcomes including cardiovascular disease and kidney disease, as well as the now well-documented long COVID symptoms including fatigue, pain, and neurocognitive dysfunction.
They’re also investigating the impact of the COVID-19 vaccine to see whether the risks are mitigated in the case of breakthrough infections: “We’re doing a broad systematic assessment. The next paper will be more comprehensive.”
Dr. Narayan and Dr. Staimez wrote: “The potential connection between COVID-19 and diabetes highlights that infectious diseases (eg, SARS-CoV-2) and chronic diseases (eg, diabetes) cannot be viewed in siloes. When we emerge out of the pandemic, the much-neglected non-communicable diseases, such as type 2 diabetes, will continue their relentless trajectory, possibly in an accelerated manner, as the leading burdens of global health.”
Dr. Al-Aly declared support from the U.S. Department of Veterans Affairs for the submitted work. He has received consultation fees from Gilead Sciences and funding (unrelated to this work) from Tonix Pharmaceuticals. He is a member of the board of directors for Veterans Research and Education Foundation of Saint Louis, associate editor for the Journal of the American Society of Nephrology, and a member of multiple editorial boards. Dr. Narayan and Dr. Staimez have received support from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
COVID-19 infection appears to significantly raise the risk for diabetes by about 40% at 1 year, indicate new data from a very large Veterans Administration population.
“If patients have a prior history of COVID-19, that’s a risk factor for diabetes and they should certainly be screened for diabetes,” study coauthor Ziyad Al-Aly, MD, a nephrologist and chief of research and development at VA St. Louis Health Care, told this news organization.
“It’s still premature to make guidelines. I think we have to process the data landscape to understand what this all really means, but it’s really, really clear that all these roads are pointing in one direction, that COVID-19 increases the risk of diabetes up to a year later. The risk is small but not negligible,” he said.
The database includes over 8 million people and 180,000 with a prior COVID-19 diagnosis. Significantly increased diabetes risks compared to those not infected ranging from 31% to more than double were found in an analysis of subgroups based on diabetes risk score, body mass index, age, race, prediabetes status, and deprivation level, even after adjustment for confounding factors.
There was a gradient of diabetes risk by COVID-19 severity – i.e., whether patients had not been hospitalized, had been hospitalized, or stayed in intensive care – but a significant excess diabetes burden was seen even among those with “mild” COVID-19. The diabetes risk was also elevated compared to both contemporary and historical controls.
The study was published March 21 in The Lancet Diabetes & Endocrinology, by Yan Xie, MPH, also of VA St Louis Health Care, along with Dr. Al-Aly.
The data align with those from another study just published from a nationwide German primary care database. That study was smaller and of shorter duration than the new VA study but consistent, said Dr. Al-Aly, a clinical epidemiologist at Washington University, St. Louis.
Millions more with new diabetes as late manifestation of COVID-19
“Millions of people in the U.S. have had COVID-19, so this is going to translate to literally millions more people with new-onset diabetes. Better to identify them early so they can be adequately treated,” Dr. Al-Aly said in an interview.
“The long-term implications of SARS-CoV-2 infection increasing diabetes risk are profound,” Venkat Narayan, MD, and Lisa R. Staimez, PhD, both of the Rollins School of Public Health and Emory Global Diabetes Research Center at Emory University, Atlanta, said in an accompanying editorial.
“With large and growing numbers of people worldwide infected with SARS-CoV-2 (434,154,739 cumulative cases by Feb. 28, 2022), any COVID-19-related increases in diabetes incidence could lead to unprecedented cases of diabetes worldwide – wreaking havoc on already over-stretched and under-resourced clinical and public health systems globally, with devastating tolls in terms of deaths and suffering,” they added.
Medscape Medical News contributor Eric Topol MD, of Scripps Research Institute, La Jolla, Calif., agrees. He said these new data “are most profound. The researchers found a 40% increase in diabetes that wasn’t present at 1 month after COVID-19 but at 1 year, it was. Some kind of late manifestation is happening here.”
Dr. Al-Aly told this news organization that the mechanisms for the association are unknown and likely to be heterogeneous. Among the people who already had risk factors for type 2 diabetes, such as obesity or metabolic syndrome, SARS-CoV-2 could simply accelerate that process and “put them over the edge” to overt diabetes.
However, for those without diabetes risk factors, “COVID-19 with all the inflammation it provokes in the body could be leading to de novo disease.” (Diabetes status was ascertained by ICD-10 codes and only about 0.70% of the total were recorded as type 1 diabetes. But, since autoantibody testing wasn’t routinely conducted, it’s unknown how many of the cases may have been type 1 misclassified as type 2, Dr. Al-Aly acknowledged.)
Diabetes risk significantly increased after COVID-19 in all analyses
The analysis included 181,280 patients in the U.S. Department of Veterans Affairs health care database with a COVID-19 diagnosis who survived for at least 30 days afterward during March 2020 through Sept. 30, 2021, with 4,118,441 contemporary controls without COVID-19 seen during 2019, and a historical control group of 4,286,911 people seen at the VA in 2017. Average follow-up was about a year.
Compared with the contemporary controls, the COVID-19 group had an excess diabetes burden of 13.46 per 1,000 person-years with a hazard ratio of 1.40. They had an increased 12.35 per 1,000 person-year risk for incident use of glucose-lowering medications, with a hazard ratio of 1.85. Similar results were seen with the historical controls.
Subgroup analyses showed an increased risk for diabetes following COVID-19 infection by age (≤ 65 years and > 65 years), race (White and Black), sex (male and female), BMI categories (> 18.5 to ≤ 25 kg/m², > 25 to ≤ 30 kg/m², and > 30 kg/m²), and area deprivation index quartiles. The increased risk was also seen across diabetes risk score quartiles.
Notably, COVID-19 significantly elevated the diabetes risk by 59% even for the subgroup with BMI between 18 and 25 kg/m², and by 38% among those with the lowest diabetes risk score quartile.
The COVID-19 population included 162,096 who were not hospitalized, 15,078 hospitalized, and 4,106 admitted to intensive care. Here, the hazard ratios for diabetes compared to the contemporary controls were 1.25, 2.73, and 3.76, respectively, all significant.
Dr. Al-Aly said that his group is now further analyzing the VA data for other outcomes including cardiovascular disease and kidney disease, as well as the now well-documented long COVID symptoms including fatigue, pain, and neurocognitive dysfunction.
They’re also investigating the impact of the COVID-19 vaccine to see whether the risks are mitigated in the case of breakthrough infections: “We’re doing a broad systematic assessment. The next paper will be more comprehensive.”
Dr. Narayan and Dr. Staimez wrote: “The potential connection between COVID-19 and diabetes highlights that infectious diseases (eg, SARS-CoV-2) and chronic diseases (eg, diabetes) cannot be viewed in siloes. When we emerge out of the pandemic, the much-neglected non-communicable diseases, such as type 2 diabetes, will continue their relentless trajectory, possibly in an accelerated manner, as the leading burdens of global health.”
Dr. Al-Aly declared support from the U.S. Department of Veterans Affairs for the submitted work. He has received consultation fees from Gilead Sciences and funding (unrelated to this work) from Tonix Pharmaceuticals. He is a member of the board of directors for Veterans Research and Education Foundation of Saint Louis, associate editor for the Journal of the American Society of Nephrology, and a member of multiple editorial boards. Dr. Narayan and Dr. Staimez have received support from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
COVID-19 infection appears to significantly raise the risk for diabetes by about 40% at 1 year, indicate new data from a very large Veterans Administration population.
“If patients have a prior history of COVID-19, that’s a risk factor for diabetes and they should certainly be screened for diabetes,” study coauthor Ziyad Al-Aly, MD, a nephrologist and chief of research and development at VA St. Louis Health Care, told this news organization.
“It’s still premature to make guidelines. I think we have to process the data landscape to understand what this all really means, but it’s really, really clear that all these roads are pointing in one direction, that COVID-19 increases the risk of diabetes up to a year later. The risk is small but not negligible,” he said.
The database includes over 8 million people and 180,000 with a prior COVID-19 diagnosis. Significantly increased diabetes risks compared to those not infected ranging from 31% to more than double were found in an analysis of subgroups based on diabetes risk score, body mass index, age, race, prediabetes status, and deprivation level, even after adjustment for confounding factors.
There was a gradient of diabetes risk by COVID-19 severity – i.e., whether patients had not been hospitalized, had been hospitalized, or stayed in intensive care – but a significant excess diabetes burden was seen even among those with “mild” COVID-19. The diabetes risk was also elevated compared to both contemporary and historical controls.
The study was published March 21 in The Lancet Diabetes & Endocrinology, by Yan Xie, MPH, also of VA St Louis Health Care, along with Dr. Al-Aly.
The data align with those from another study just published from a nationwide German primary care database. That study was smaller and of shorter duration than the new VA study but consistent, said Dr. Al-Aly, a clinical epidemiologist at Washington University, St. Louis.
Millions more with new diabetes as late manifestation of COVID-19
“Millions of people in the U.S. have had COVID-19, so this is going to translate to literally millions more people with new-onset diabetes. Better to identify them early so they can be adequately treated,” Dr. Al-Aly said in an interview.
“The long-term implications of SARS-CoV-2 infection increasing diabetes risk are profound,” Venkat Narayan, MD, and Lisa R. Staimez, PhD, both of the Rollins School of Public Health and Emory Global Diabetes Research Center at Emory University, Atlanta, said in an accompanying editorial.
“With large and growing numbers of people worldwide infected with SARS-CoV-2 (434,154,739 cumulative cases by Feb. 28, 2022), any COVID-19-related increases in diabetes incidence could lead to unprecedented cases of diabetes worldwide – wreaking havoc on already over-stretched and under-resourced clinical and public health systems globally, with devastating tolls in terms of deaths and suffering,” they added.
Medscape Medical News contributor Eric Topol MD, of Scripps Research Institute, La Jolla, Calif., agrees. He said these new data “are most profound. The researchers found a 40% increase in diabetes that wasn’t present at 1 month after COVID-19 but at 1 year, it was. Some kind of late manifestation is happening here.”
Dr. Al-Aly told this news organization that the mechanisms for the association are unknown and likely to be heterogeneous. Among the people who already had risk factors for type 2 diabetes, such as obesity or metabolic syndrome, SARS-CoV-2 could simply accelerate that process and “put them over the edge” to overt diabetes.
However, for those without diabetes risk factors, “COVID-19 with all the inflammation it provokes in the body could be leading to de novo disease.” (Diabetes status was ascertained by ICD-10 codes and only about 0.70% of the total were recorded as type 1 diabetes. But, since autoantibody testing wasn’t routinely conducted, it’s unknown how many of the cases may have been type 1 misclassified as type 2, Dr. Al-Aly acknowledged.)
Diabetes risk significantly increased after COVID-19 in all analyses
The analysis included 181,280 patients in the U.S. Department of Veterans Affairs health care database with a COVID-19 diagnosis who survived for at least 30 days afterward during March 2020 through Sept. 30, 2021, with 4,118,441 contemporary controls without COVID-19 seen during 2019, and a historical control group of 4,286,911 people seen at the VA in 2017. Average follow-up was about a year.
Compared with the contemporary controls, the COVID-19 group had an excess diabetes burden of 13.46 per 1,000 person-years with a hazard ratio of 1.40. They had an increased 12.35 per 1,000 person-year risk for incident use of glucose-lowering medications, with a hazard ratio of 1.85. Similar results were seen with the historical controls.
Subgroup analyses showed an increased risk for diabetes following COVID-19 infection by age (≤ 65 years and > 65 years), race (White and Black), sex (male and female), BMI categories (> 18.5 to ≤ 25 kg/m², > 25 to ≤ 30 kg/m², and > 30 kg/m²), and area deprivation index quartiles. The increased risk was also seen across diabetes risk score quartiles.
Notably, COVID-19 significantly elevated the diabetes risk by 59% even for the subgroup with BMI between 18 and 25 kg/m², and by 38% among those with the lowest diabetes risk score quartile.
The COVID-19 population included 162,096 who were not hospitalized, 15,078 hospitalized, and 4,106 admitted to intensive care. Here, the hazard ratios for diabetes compared to the contemporary controls were 1.25, 2.73, and 3.76, respectively, all significant.
Dr. Al-Aly said that his group is now further analyzing the VA data for other outcomes including cardiovascular disease and kidney disease, as well as the now well-documented long COVID symptoms including fatigue, pain, and neurocognitive dysfunction.
They’re also investigating the impact of the COVID-19 vaccine to see whether the risks are mitigated in the case of breakthrough infections: “We’re doing a broad systematic assessment. The next paper will be more comprehensive.”
Dr. Narayan and Dr. Staimez wrote: “The potential connection between COVID-19 and diabetes highlights that infectious diseases (eg, SARS-CoV-2) and chronic diseases (eg, diabetes) cannot be viewed in siloes. When we emerge out of the pandemic, the much-neglected non-communicable diseases, such as type 2 diabetes, will continue their relentless trajectory, possibly in an accelerated manner, as the leading burdens of global health.”
Dr. Al-Aly declared support from the U.S. Department of Veterans Affairs for the submitted work. He has received consultation fees from Gilead Sciences and funding (unrelated to this work) from Tonix Pharmaceuticals. He is a member of the board of directors for Veterans Research and Education Foundation of Saint Louis, associate editor for the Journal of the American Society of Nephrology, and a member of multiple editorial boards. Dr. Narayan and Dr. Staimez have received support from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM THE LANCET DIABETES & ENDOCRINOLOGY
Jury is out on universal screening for eating disorders
Eating disorders (binge eating disorder, bulimia nervosa, and anorexia nervosa) can cause “serious harms to physical and psychosocial health and take a tremendous toll on individuals and families,” task force member Lori Pbert, PhD, told this news organization.
“Screening for eating disorders has the potential to improve health by leading to early detection and effective treatment,” said Dr. Pbert, with the department of population and quantitative health sciences, University of Massachusetts, Worcester.
However, a “deep dive” into the available literature failed to turn up adequate evidence to recommend for or against routine screening for eating disorders for children and adolescents aged 10 years and older and for adults who have no signs or symptoms of an eating disorder or concerns about their eating and who have not previously been diagnosed with an eating disorder, Dr. Pbert said.
The task force, therefore, issued an “I” statement (insufficient evidence), meaning it cannot at this time recommend for or against screening for eating disorders.
An “I” statement is “fundamentally a call for more research,” Dr. Pbert noted.
Adolescents and adults who have signs and symptoms of an eating disorder – which include rapid weight loss; weight gain or pronounced deviation from growth trajectory; pubertal delay; bradycardia; oligomenorrhea; and amenorrhea – are not included in this recommendation.
The USPSTF recommendation statement and accompanying evidence report were published online March 15 in JAMA.
Clinical judgment key
In the absence of evidence, clinicians should use their judgment when determining whether or not to screen an individual patient for an eating disorder, Dr. Pbert advised.
One thing to consider is whether the patient is in a group at higher risk for eating disorders, such as athletes, females, young adults aged 18-29, and transgender individuals.
Another is whether the patient reports engaging in unhealthy weight control behaviors, such as fasting or skipping meals, Dr. Pbert said.
Importantly, any patient who has signs or symptoms of an eating disorder or is expressing concerns about their eating should be assessed and referred for appropriate care, Dr. Pbert said.
“The good news is that eating disorders can be treated,” she said.
Several organizations currently recommend screening in the context of monitoring changes in weight and other vital signs or signs and symptoms to determine whether a patient might have an eating disorder.
Dr. Pbert said it’s important to recognize that the USPSTF statement “doesn’t really conflict” with the recommendations of other organizations. “We all agree that patients who present with signs or symptoms of an eating disorder should be assessed further.”
Evidence gaps
The authors of an invited commentary in JAMA) say the task force has identified several “notable deficiencies” in the available data on screening for eating disorders.
“Directing attention to rigorous research to close this evidence gap will be important to find optimal approaches to identify patients with these complex disorders and improve their health outcomes,” write Evelyn Attia, MD, with Weill Cornell Medicine in New York, and Angela Guarda, MD, with Johns Hopkins University, Baltimore.
This “I” statement, they say, “highlights the need to prioritize research aimed at closing the evidence gap identified by USPSTF in a timely manner and underscores the need for new studies that address screening for eating disorders, treatment trials that enroll screen-detected populations from primary care settings, and screening in specific populations.
“Research on screening in primary care also should be paired with development and assessment of early brief intervention strategies for those individuals who screen positive, especially adolescents,” Dr. Attia and Dr. Guarda say.
Members of the USPSTF have disclosed no relevant financial relationships. Dr. Attia has received research support from the National Institute of Mental Health and the Hilda & Preston David Foundation; royalties from UpToDate; and has served as a clinical advisor to Equip Health. Dr. Guarda has received support from the Stephen and Jean Robinson Fund and research funding from the Klarman Family Foundation.
A version of this article first appeared on Medscape.com.
Eating disorders (binge eating disorder, bulimia nervosa, and anorexia nervosa) can cause “serious harms to physical and psychosocial health and take a tremendous toll on individuals and families,” task force member Lori Pbert, PhD, told this news organization.
“Screening for eating disorders has the potential to improve health by leading to early detection and effective treatment,” said Dr. Pbert, with the department of population and quantitative health sciences, University of Massachusetts, Worcester.
However, a “deep dive” into the available literature failed to turn up adequate evidence to recommend for or against routine screening for eating disorders for children and adolescents aged 10 years and older and for adults who have no signs or symptoms of an eating disorder or concerns about their eating and who have not previously been diagnosed with an eating disorder, Dr. Pbert said.
The task force, therefore, issued an “I” statement (insufficient evidence), meaning it cannot at this time recommend for or against screening for eating disorders.
An “I” statement is “fundamentally a call for more research,” Dr. Pbert noted.
Adolescents and adults who have signs and symptoms of an eating disorder – which include rapid weight loss; weight gain or pronounced deviation from growth trajectory; pubertal delay; bradycardia; oligomenorrhea; and amenorrhea – are not included in this recommendation.
The USPSTF recommendation statement and accompanying evidence report were published online March 15 in JAMA.
Clinical judgment key
In the absence of evidence, clinicians should use their judgment when determining whether or not to screen an individual patient for an eating disorder, Dr. Pbert advised.
One thing to consider is whether the patient is in a group at higher risk for eating disorders, such as athletes, females, young adults aged 18-29, and transgender individuals.
Another is whether the patient reports engaging in unhealthy weight control behaviors, such as fasting or skipping meals, Dr. Pbert said.
Importantly, any patient who has signs or symptoms of an eating disorder or is expressing concerns about their eating should be assessed and referred for appropriate care, Dr. Pbert said.
“The good news is that eating disorders can be treated,” she said.
Several organizations currently recommend screening in the context of monitoring changes in weight and other vital signs or signs and symptoms to determine whether a patient might have an eating disorder.
Dr. Pbert said it’s important to recognize that the USPSTF statement “doesn’t really conflict” with the recommendations of other organizations. “We all agree that patients who present with signs or symptoms of an eating disorder should be assessed further.”
Evidence gaps
The authors of an invited commentary in JAMA) say the task force has identified several “notable deficiencies” in the available data on screening for eating disorders.
“Directing attention to rigorous research to close this evidence gap will be important to find optimal approaches to identify patients with these complex disorders and improve their health outcomes,” write Evelyn Attia, MD, with Weill Cornell Medicine in New York, and Angela Guarda, MD, with Johns Hopkins University, Baltimore.
This “I” statement, they say, “highlights the need to prioritize research aimed at closing the evidence gap identified by USPSTF in a timely manner and underscores the need for new studies that address screening for eating disorders, treatment trials that enroll screen-detected populations from primary care settings, and screening in specific populations.
“Research on screening in primary care also should be paired with development and assessment of early brief intervention strategies for those individuals who screen positive, especially adolescents,” Dr. Attia and Dr. Guarda say.
Members of the USPSTF have disclosed no relevant financial relationships. Dr. Attia has received research support from the National Institute of Mental Health and the Hilda & Preston David Foundation; royalties from UpToDate; and has served as a clinical advisor to Equip Health. Dr. Guarda has received support from the Stephen and Jean Robinson Fund and research funding from the Klarman Family Foundation.
A version of this article first appeared on Medscape.com.
Eating disorders (binge eating disorder, bulimia nervosa, and anorexia nervosa) can cause “serious harms to physical and psychosocial health and take a tremendous toll on individuals and families,” task force member Lori Pbert, PhD, told this news organization.
“Screening for eating disorders has the potential to improve health by leading to early detection and effective treatment,” said Dr. Pbert, with the department of population and quantitative health sciences, University of Massachusetts, Worcester.
However, a “deep dive” into the available literature failed to turn up adequate evidence to recommend for or against routine screening for eating disorders for children and adolescents aged 10 years and older and for adults who have no signs or symptoms of an eating disorder or concerns about their eating and who have not previously been diagnosed with an eating disorder, Dr. Pbert said.
The task force, therefore, issued an “I” statement (insufficient evidence), meaning it cannot at this time recommend for or against screening for eating disorders.
An “I” statement is “fundamentally a call for more research,” Dr. Pbert noted.
Adolescents and adults who have signs and symptoms of an eating disorder – which include rapid weight loss; weight gain or pronounced deviation from growth trajectory; pubertal delay; bradycardia; oligomenorrhea; and amenorrhea – are not included in this recommendation.
The USPSTF recommendation statement and accompanying evidence report were published online March 15 in JAMA.
Clinical judgment key
In the absence of evidence, clinicians should use their judgment when determining whether or not to screen an individual patient for an eating disorder, Dr. Pbert advised.
One thing to consider is whether the patient is in a group at higher risk for eating disorders, such as athletes, females, young adults aged 18-29, and transgender individuals.
Another is whether the patient reports engaging in unhealthy weight control behaviors, such as fasting or skipping meals, Dr. Pbert said.
Importantly, any patient who has signs or symptoms of an eating disorder or is expressing concerns about their eating should be assessed and referred for appropriate care, Dr. Pbert said.
“The good news is that eating disorders can be treated,” she said.
Several organizations currently recommend screening in the context of monitoring changes in weight and other vital signs or signs and symptoms to determine whether a patient might have an eating disorder.
Dr. Pbert said it’s important to recognize that the USPSTF statement “doesn’t really conflict” with the recommendations of other organizations. “We all agree that patients who present with signs or symptoms of an eating disorder should be assessed further.”
Evidence gaps
The authors of an invited commentary in JAMA) say the task force has identified several “notable deficiencies” in the available data on screening for eating disorders.
“Directing attention to rigorous research to close this evidence gap will be important to find optimal approaches to identify patients with these complex disorders and improve their health outcomes,” write Evelyn Attia, MD, with Weill Cornell Medicine in New York, and Angela Guarda, MD, with Johns Hopkins University, Baltimore.
This “I” statement, they say, “highlights the need to prioritize research aimed at closing the evidence gap identified by USPSTF in a timely manner and underscores the need for new studies that address screening for eating disorders, treatment trials that enroll screen-detected populations from primary care settings, and screening in specific populations.
“Research on screening in primary care also should be paired with development and assessment of early brief intervention strategies for those individuals who screen positive, especially adolescents,” Dr. Attia and Dr. Guarda say.
Members of the USPSTF have disclosed no relevant financial relationships. Dr. Attia has received research support from the National Institute of Mental Health and the Hilda & Preston David Foundation; royalties from UpToDate; and has served as a clinical advisor to Equip Health. Dr. Guarda has received support from the Stephen and Jean Robinson Fund and research funding from the Klarman Family Foundation.
A version of this article first appeared on Medscape.com.
Children and COVID: CDC gives perspective on hospitalizations
New COVID-19 cases in children fell by 23% as the latest weekly count dropped to its lowest level since July of 2021, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
, when the early stages of the Delta surge led to 23,551 cases, the AAP and CHA said in their weekly COVID report.
The two organizations put the total number of cases at nearly 12.8 million from the start of the pandemic to March 17, with children representing 19.0% of cases among all ages. The Centers for Disease Control and Prevention puts the cumulative number of COVID-19 cases at almost 12.0 million as of March 21, or 17.5% of the nationwide total.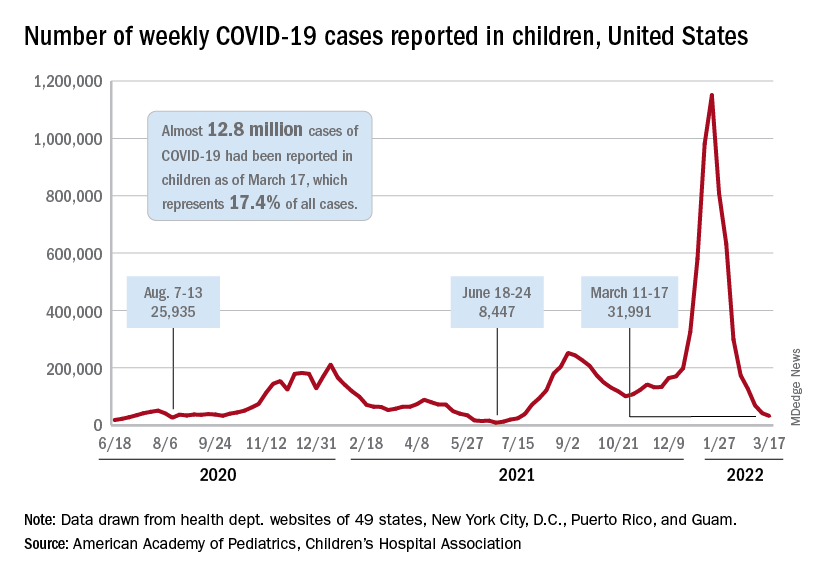
COVID-related hospitalizations also continue to fall, and two new studies from the CDC put children’s experiences during the Omicron surge and the larger pandemic into perspective.
One study showed that hospitalization rates for children aged 4 years and younger during the Omicron surge were five times higher than at the peak of the Delta surge, with the highest rates occurring in infants under 6 months of age. That report was based on the CDC’s COVID-19–Associated Hospitalization Surveillance Network (COVID-NET), which covers 99 counties across 14 states (MMWR. 2022 March 18;71[11]:429-36).
The second study compared child hospitalizations during 1 year of the COVID pandemic (Oct. 1, 2020, to Sept. 30, 2021) with three influenza seasons (2017-2018 through 2019-2020). The pre-Omicron hospitalization rate for those under age 18 years, 48.2 per 100,000 children, was higher than any of the three flu seasons: 33.5 per 100,000 in 2017-2018, 33.8 in 2018-2019, and 41.7 for 2019-2020, the investigators said in a medRxiv preprint.
Most of the increased COVID burden fell on adolescents aged 12-17, they said. The COVID hospitalization rate for that age group was 59.9 per 100,000, versus 12.2-14.1 for influenza, while children aged 5-11 had a COVID-related rate of 25.0 and flu-related rates of 24.3-31.7, and those aged 0-4 had rates of 66.8 for COVID and 70.9-91.5 for the flu, Miranda J. Delahoy of the CDC’s COVID-19 Response Team and associates reported.
New COVID-19 cases in children fell by 23% as the latest weekly count dropped to its lowest level since July of 2021, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
, when the early stages of the Delta surge led to 23,551 cases, the AAP and CHA said in their weekly COVID report.
The two organizations put the total number of cases at nearly 12.8 million from the start of the pandemic to March 17, with children representing 19.0% of cases among all ages. The Centers for Disease Control and Prevention puts the cumulative number of COVID-19 cases at almost 12.0 million as of March 21, or 17.5% of the nationwide total.
COVID-related hospitalizations also continue to fall, and two new studies from the CDC put children’s experiences during the Omicron surge and the larger pandemic into perspective.
One study showed that hospitalization rates for children aged 4 years and younger during the Omicron surge were five times higher than at the peak of the Delta surge, with the highest rates occurring in infants under 6 months of age. That report was based on the CDC’s COVID-19–Associated Hospitalization Surveillance Network (COVID-NET), which covers 99 counties across 14 states (MMWR. 2022 March 18;71[11]:429-36).
The second study compared child hospitalizations during 1 year of the COVID pandemic (Oct. 1, 2020, to Sept. 30, 2021) with three influenza seasons (2017-2018 through 2019-2020). The pre-Omicron hospitalization rate for those under age 18 years, 48.2 per 100,000 children, was higher than any of the three flu seasons: 33.5 per 100,000 in 2017-2018, 33.8 in 2018-2019, and 41.7 for 2019-2020, the investigators said in a medRxiv preprint.
Most of the increased COVID burden fell on adolescents aged 12-17, they said. The COVID hospitalization rate for that age group was 59.9 per 100,000, versus 12.2-14.1 for influenza, while children aged 5-11 had a COVID-related rate of 25.0 and flu-related rates of 24.3-31.7, and those aged 0-4 had rates of 66.8 for COVID and 70.9-91.5 for the flu, Miranda J. Delahoy of the CDC’s COVID-19 Response Team and associates reported.
New COVID-19 cases in children fell by 23% as the latest weekly count dropped to its lowest level since July of 2021, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
, when the early stages of the Delta surge led to 23,551 cases, the AAP and CHA said in their weekly COVID report.
The two organizations put the total number of cases at nearly 12.8 million from the start of the pandemic to March 17, with children representing 19.0% of cases among all ages. The Centers for Disease Control and Prevention puts the cumulative number of COVID-19 cases at almost 12.0 million as of March 21, or 17.5% of the nationwide total.
COVID-related hospitalizations also continue to fall, and two new studies from the CDC put children’s experiences during the Omicron surge and the larger pandemic into perspective.
One study showed that hospitalization rates for children aged 4 years and younger during the Omicron surge were five times higher than at the peak of the Delta surge, with the highest rates occurring in infants under 6 months of age. That report was based on the CDC’s COVID-19–Associated Hospitalization Surveillance Network (COVID-NET), which covers 99 counties across 14 states (MMWR. 2022 March 18;71[11]:429-36).
The second study compared child hospitalizations during 1 year of the COVID pandemic (Oct. 1, 2020, to Sept. 30, 2021) with three influenza seasons (2017-2018 through 2019-2020). The pre-Omicron hospitalization rate for those under age 18 years, 48.2 per 100,000 children, was higher than any of the three flu seasons: 33.5 per 100,000 in 2017-2018, 33.8 in 2018-2019, and 41.7 for 2019-2020, the investigators said in a medRxiv preprint.
Most of the increased COVID burden fell on adolescents aged 12-17, they said. The COVID hospitalization rate for that age group was 59.9 per 100,000, versus 12.2-14.1 for influenza, while children aged 5-11 had a COVID-related rate of 25.0 and flu-related rates of 24.3-31.7, and those aged 0-4 had rates of 66.8 for COVID and 70.9-91.5 for the flu, Miranda J. Delahoy of the CDC’s COVID-19 Response Team and associates reported.
Racial disparities seen in pediatric postoperative mortality rates
Among Black and White children, higher socioeconomic status (SES) was associated with lower pediatric postoperative mortality, according to a cohort study published in JAMA Network Open. However, this association was not equitable when comparing Black and White children.
The results showed that postoperative mortality rates were significantly higher in Black children in the highest income category, compared with White children in the same category.
“[We] assessed whether increasing family SES is associated with lower pediatric postoperative mortality and, if so, whether this association is equitable among Black and White children,” Brittany L. Willer, MD, of Nationwide Children’s Hospital in Columbus, Ohio, and colleagues wrote.
The researchers retrospectively analyzed data from 51 pediatric tertiary care hospitals apart of the Children’s Hospital Association Pediatric Health Information System. The cohort included children younger than 18 years who underwent inpatient surgical procedures between January 2004 and December 2020.
The exposures of interest were race and parental income quartile; the primary endpoint was risk-adjusted in-hospital mortality rates by race and parental income quartile.
Results
The study cohort included 1,378,111 participants, including 248,464 (18.0%) Black and 1,129,647 (82.0%) White children, respectively.
The overall mortality rate was 1.2%, and rates decreased as income quartile increased (1.4% in quartile 1 [lowest income]; 1.3% in quartile 2; 1.0% in quartile 3; and 0.9% in quartile 4 [highest income]; P < .001).
Among participants in the three lowest income quartiles, Black children had 33% greater odds of postoperative death versus White children (adjusted odds ratio, 1.33; 95% confidence interval, 1.27-1.39; P < .001). This difference persisted in children in the highest income quartile (aOR, 1.39; 95% CI, 1.25-1.54; P < .001).
In addition, postoperative mortality rates in Black children in the highest income quartile (1.30%; 95% CI, 1.19%-1.42%) were similar to those of White children in the lowest income quartile (1.20%; 95% CI, 1.16%-1.25%).
“These findings suggest that increasing family SES did not provide equitable advantage to Black, compared with White children, and interventions that target socioeconomic inequities alone may not fully address persistent racial disparities in pediatric postoperative mortality,” wrote Dr. Willer and colleagues. “A multifaceted approach that includes dismantling of socioeconomic barriers, equitable availability of comprehensive pediatric surgical care, and personalized care for children of all races is needed.”
The researchers acknowledged that a potential limitation of the study was the use of zip code–level median household income as a proxy for family SES.
A perspective
In an interview, Timothy Joos, MD, a Seattle internist and pediatrician in private practice, said “there is a fair dose of racism and classism inside all of us – recognizing and coming to terms with it are steps toward improving equity issues.
“As providers, we have to remind ourselves to give our most prompt and thorough care to the patients with the most acute and severe illnesses,” Dr. Joos said. “As organizations, we have to pursue feedback from all our clients, but with special outreach to those that are used to not having their voices heard.”
No funding sources were reported. The authors reported no relevant disclosures. Dr. Joos is a member of the Pediatric News editorial advisory board but had no other disclosures.
Among Black and White children, higher socioeconomic status (SES) was associated with lower pediatric postoperative mortality, according to a cohort study published in JAMA Network Open. However, this association was not equitable when comparing Black and White children.
The results showed that postoperative mortality rates were significantly higher in Black children in the highest income category, compared with White children in the same category.
“[We] assessed whether increasing family SES is associated with lower pediatric postoperative mortality and, if so, whether this association is equitable among Black and White children,” Brittany L. Willer, MD, of Nationwide Children’s Hospital in Columbus, Ohio, and colleagues wrote.
The researchers retrospectively analyzed data from 51 pediatric tertiary care hospitals apart of the Children’s Hospital Association Pediatric Health Information System. The cohort included children younger than 18 years who underwent inpatient surgical procedures between January 2004 and December 2020.
The exposures of interest were race and parental income quartile; the primary endpoint was risk-adjusted in-hospital mortality rates by race and parental income quartile.
Results
The study cohort included 1,378,111 participants, including 248,464 (18.0%) Black and 1,129,647 (82.0%) White children, respectively.
The overall mortality rate was 1.2%, and rates decreased as income quartile increased (1.4% in quartile 1 [lowest income]; 1.3% in quartile 2; 1.0% in quartile 3; and 0.9% in quartile 4 [highest income]; P < .001).
Among participants in the three lowest income quartiles, Black children had 33% greater odds of postoperative death versus White children (adjusted odds ratio, 1.33; 95% confidence interval, 1.27-1.39; P < .001). This difference persisted in children in the highest income quartile (aOR, 1.39; 95% CI, 1.25-1.54; P < .001).
In addition, postoperative mortality rates in Black children in the highest income quartile (1.30%; 95% CI, 1.19%-1.42%) were similar to those of White children in the lowest income quartile (1.20%; 95% CI, 1.16%-1.25%).
“These findings suggest that increasing family SES did not provide equitable advantage to Black, compared with White children, and interventions that target socioeconomic inequities alone may not fully address persistent racial disparities in pediatric postoperative mortality,” wrote Dr. Willer and colleagues. “A multifaceted approach that includes dismantling of socioeconomic barriers, equitable availability of comprehensive pediatric surgical care, and personalized care for children of all races is needed.”
The researchers acknowledged that a potential limitation of the study was the use of zip code–level median household income as a proxy for family SES.
A perspective
In an interview, Timothy Joos, MD, a Seattle internist and pediatrician in private practice, said “there is a fair dose of racism and classism inside all of us – recognizing and coming to terms with it are steps toward improving equity issues.
“As providers, we have to remind ourselves to give our most prompt and thorough care to the patients with the most acute and severe illnesses,” Dr. Joos said. “As organizations, we have to pursue feedback from all our clients, but with special outreach to those that are used to not having their voices heard.”
No funding sources were reported. The authors reported no relevant disclosures. Dr. Joos is a member of the Pediatric News editorial advisory board but had no other disclosures.
Among Black and White children, higher socioeconomic status (SES) was associated with lower pediatric postoperative mortality, according to a cohort study published in JAMA Network Open. However, this association was not equitable when comparing Black and White children.
The results showed that postoperative mortality rates were significantly higher in Black children in the highest income category, compared with White children in the same category.
“[We] assessed whether increasing family SES is associated with lower pediatric postoperative mortality and, if so, whether this association is equitable among Black and White children,” Brittany L. Willer, MD, of Nationwide Children’s Hospital in Columbus, Ohio, and colleagues wrote.
The researchers retrospectively analyzed data from 51 pediatric tertiary care hospitals apart of the Children’s Hospital Association Pediatric Health Information System. The cohort included children younger than 18 years who underwent inpatient surgical procedures between January 2004 and December 2020.
The exposures of interest were race and parental income quartile; the primary endpoint was risk-adjusted in-hospital mortality rates by race and parental income quartile.
Results
The study cohort included 1,378,111 participants, including 248,464 (18.0%) Black and 1,129,647 (82.0%) White children, respectively.
The overall mortality rate was 1.2%, and rates decreased as income quartile increased (1.4% in quartile 1 [lowest income]; 1.3% in quartile 2; 1.0% in quartile 3; and 0.9% in quartile 4 [highest income]; P < .001).
Among participants in the three lowest income quartiles, Black children had 33% greater odds of postoperative death versus White children (adjusted odds ratio, 1.33; 95% confidence interval, 1.27-1.39; P < .001). This difference persisted in children in the highest income quartile (aOR, 1.39; 95% CI, 1.25-1.54; P < .001).
In addition, postoperative mortality rates in Black children in the highest income quartile (1.30%; 95% CI, 1.19%-1.42%) were similar to those of White children in the lowest income quartile (1.20%; 95% CI, 1.16%-1.25%).
“These findings suggest that increasing family SES did not provide equitable advantage to Black, compared with White children, and interventions that target socioeconomic inequities alone may not fully address persistent racial disparities in pediatric postoperative mortality,” wrote Dr. Willer and colleagues. “A multifaceted approach that includes dismantling of socioeconomic barriers, equitable availability of comprehensive pediatric surgical care, and personalized care for children of all races is needed.”
The researchers acknowledged that a potential limitation of the study was the use of zip code–level median household income as a proxy for family SES.
A perspective
In an interview, Timothy Joos, MD, a Seattle internist and pediatrician in private practice, said “there is a fair dose of racism and classism inside all of us – recognizing and coming to terms with it are steps toward improving equity issues.
“As providers, we have to remind ourselves to give our most prompt and thorough care to the patients with the most acute and severe illnesses,” Dr. Joos said. “As organizations, we have to pursue feedback from all our clients, but with special outreach to those that are used to not having their voices heard.”
No funding sources were reported. The authors reported no relevant disclosures. Dr. Joos is a member of the Pediatric News editorial advisory board but had no other disclosures.
FROM JAMA NETWORK OPEN
New guidance on cannabis use for treatment-resistant epilepsy
A recent review article draws from existing clinical trials and clinical experience in New South Wales, Australia, to fill this gap with interim guidance for both pediatric and adult patients. The article was published in the British Journal of Clinical Pharmacology.
The only current U.S. guidelines are from the American Academy of Neurology’s position statement on the use of medical cannabis for neurologic disorders and the American Epilepsy Society’s position statement on cannabis as a treatment for epileptic seizures. The AAN statement “highlights the current evidence, which currently only supports [Food and Drug Administration]–approved CBD [cannabidiol] (Epidiolex) for specific epilepsy syndromes,” said Daniel Freedman, DO, an assistant professor of neurology at the University of Texas at Austin and coauthor of the AAN’s position statement.
“Rescheduling marijuana will enable researchers to study CBD, THC [tetrahydrocannabinol], and other cannabinoids in high-quality studies so that we can better understand what works and for which conditions,” said Dr. Freedman, who was not involved in the Australian guidance document. He noted that little consensus exists because little evidence exists outside the handful of trials for Epidiolex.
“There are some patients with epilepsy that can benefit from high-quality, pharmaceutical-grade CBD products,” Dr. Freedman said. “These patients need to be carefully identified by a neurologist or epileptologist and prescribed a legal, safe, quality-controlled, and FDA-regulated product.”
Appropriate patient populations
Drug-resistant epilepsy, defined as failure of two appropriate antiseizure medications, affects an estimated one third of people with epilepsy, the new guideline notes. Though many over-the-counter products are available at dispensaries in the 33 U.S. states that allow use of cannabis for medical purposes, Epidiolex (cannabidiol) is the only FDA-approved drug for epilepsy that contains a substance derived from cannabis and the only one for which evidence from randomized, controlled trials exists.
Dr. Freedman notes that hemp-derived CBD oils are classified differently in the United States than marijuana-derived CBD oil, including Epidiolex, and are loosely regulated supplements or food additives commonly seen, for example at gas station.
“The point I drive home to patients is that you wouldn’t get your antibiotics from a gas station, so please don’t get your seizure medication from there,” Dr. Freedman said. “Studies have been done on ‘over-the-counter’ CBD oils and shown that they have variable quality, sometimes no detectable CBD, and sometimes other chemicals added like THC.”
Studies of Epidiolex showed that cannabidiol more effectively reduced seizure frequency than placebo for pediatric patients with Dravet syndrome (42% reduction) and for pediatric and adult patients with Lennox-Gastaut syndrome (39% reduction) or tuberous sclerosis complex (49% reduction). Efficacy was similar across dosing from 10-50 mg/kg per day, but higher doses involved higher rates of serious adverse events.
No reliable evidence in humans exists for THC or other cannabinoids in treating epilepsy.
The Australian guidance recommends limiting cannabis treatment to patients with severe drug-resistant epilepsy; a diagnosis of Dravet syndrome, Lennox-Gastaut syndrome, or tuberous sclerosis complex; and previous treatment with four approved antiseizure medications and/or the ketogenic diet, epilepsy surgery, or neurostimulator. The authors provide specific criteria for each of these conditions and then address exceptional cases that may be considered outside that criteria, such as patients under 2 years old, severe epilepsy with extended or repeated hospitalization or ICU admission, or a dangerous seizure type. The review also includes a detailed list of exclusion criteria for CBD medicine use.
The authors advised a thorough consent process before prescribing any cannabinoids, including therapeutic goals and stopping criteria; the lack of evidence available on dosing, efficacy, and side effects; and the potential for dependence or withdrawal. Consent discussions should also note whether the products are unregistered and not covered by external payers (anything other than Epidiolex currently), any activity restrictions, and any implications for occupational drug screening.
Considerations for unapproved cannabinoids
The authors note several factors to consider if prescribing or recommending a nonapproved, nonregulated cannabis medicine, including the ”differences between registered plant-derived cannabis medicines, synthetic cannabis medicines, and unregistered hemp-derived products.” Epidiolex is plant derived while other cannabis-derived medications (Marinol, Syndos, and Cesamet) that have been approved for nonepilepsy conditions, such as nausea associated with chemotherapy, are synthetic.
The guidance document notes several reasons to use a regulated medication instead of an unregulated product:
- Manufacturing processes can differ for unregulated products, including inconsistency in batches and unknown shelf life.
- Quality control processes, including risk of impurities, are much better with regulated products, which also have a system in place for safety recalls.
- More scientific evidence is available for regulated products.
- Safety surveillance reporting is more robust and standardized for regulated products whereas adverse event reporting is less reliable for unregulated products.
- Nonregulated products are rarely covered by insurance or other reimbursement.
Legal considerations will also vary by jurisdiction. ”Right now in the U.S. we have a confused legality where state level programs are still technically illegal at the federal level and I imagine there are some quality differences amongst dispensaries and states,” Dr. Freedman said. “Whenever there is disagreement between state and federal laws, this creates tension for our patients.” He noted, for example, that a patient using a CBD product that contains THC may, even if legal in their state, be confiscated by the Transportation Security Administration at an airport since it is not FDA approved and is not legal, according to the Drug Enforcement Agency.
The authors noted that inadequate data on long-term CBD use and data on neurodevelopmental effects of THC in children, teens, and young adults means THC products should be contraindicated for these age groups. (Epidiolex has less than 2% THC.) Drug interactions should also be considered, particularly for clobazam, CYP3A4 inhibitors or inducers (including St. John’s wort), digoxin, or a mechanistic target of rapamycin inhibitor.
Dr. Freedman said that most neurologists are comfortable prescribing Epidiolex since it has FDA approval while prescribing unapproved products varies more in the field. “Now that many states have compassionate use programs for medical marijuana, some neurologists do this as well,” Dr. Freedman said. Patients often ask about unregulated CBD or CBD+THC products because they’re seen as “natural and therefore better than manufactured pharmaceuticals.”
“I think this is the naturalistic fallacy at work and try to educate my patients on that since our only high-level data to show marijuana products work for epilepsy comes from a pharmaceutical company,” Dr. Freedman said. “My reasons for hesitating on compassionate use are that there is often THC, with variable amounts of concentration, and we know that THC can harm the developing pediatric brain.”
Dosing and adverse effects
Pediatric and adult dosing differences need to be considered, and “patient response (efficacy and toxicity) to these medications varies widely,” the authors noted. They advised getting serum transaminases (ALT and AST) and total bilirubin levels before beginning treatment. All patients should begin Epidiolex at a low dose, such as 2-5 mg/kg per day of CBD in two divided doses, the authors advise, and titrate slowly while monitoring for side effects (no more than 5 mg/kg per day per week). The current dosing range for CBD is 5-20 mg/kg per day in two divided doses, with higher rates involving more risk of adverse events.
“Note that some cannabinoids auto-inhibit their own metabolism and some have active metabolites with longer half-lives,” the authors wrote. “Therefore, dose or frequency may need to be reduced over time, unless tolerance occurs.” These doses, specific to Epidiolex, “cannot necessarily be applied to other oral CBD formulations or other types of epilepsy.” This guidance also does not apply to inhaled or transdermal routes of administration.
The most common adverse events were sleepiness – which occurred in up to 60% of trial participants – as well as diarrhea, decreases in appetite and weight, and drug interactions. Risk of hepatotoxicity means there’s a need to monitor liver function and adjust dosing for patients with moderate or severe hepatic impairment. “Other short-term side effects reported only with THC-containing cannabinoid compounds include increased risk of cardiac and cerebrovascular events, anxiety and psychosis risk, dependency, and withdrawal,” the authors wrote.
Though no withdrawal syndrome has been linked to stopping CBD, the authors suggested decreasing the dose by 10% every 2 days if stopping is not urgent.
“The key points to this issue are that CBD and all marijuana products need to be safe and regulated,” Dr. Freedman said. “Any claims about them need to be backed by high-quality evidence looking at that specific product for that specific condition.”
Dr. Freedman noted the need for children to receive treatment from clinicians with expertise in their specific condition since many other evidence-based treatments exist even for patients with epilepsy syndromes that are difficult to treat, such as other medications, surgery, and specialized diets.
“We need to fix the inconsistent regulation between over-the-counter CBD products, state dispensaries, and federal laws,” Dr. Freedman added. “Any medicine being used to treat children should be held to the same FDA standard of safety and efficacy.”
Dr. Freedman and the authors had no conflicts of interest. No external funding was noted.
A recent review article draws from existing clinical trials and clinical experience in New South Wales, Australia, to fill this gap with interim guidance for both pediatric and adult patients. The article was published in the British Journal of Clinical Pharmacology.
The only current U.S. guidelines are from the American Academy of Neurology’s position statement on the use of medical cannabis for neurologic disorders and the American Epilepsy Society’s position statement on cannabis as a treatment for epileptic seizures. The AAN statement “highlights the current evidence, which currently only supports [Food and Drug Administration]–approved CBD [cannabidiol] (Epidiolex) for specific epilepsy syndromes,” said Daniel Freedman, DO, an assistant professor of neurology at the University of Texas at Austin and coauthor of the AAN’s position statement.
“Rescheduling marijuana will enable researchers to study CBD, THC [tetrahydrocannabinol], and other cannabinoids in high-quality studies so that we can better understand what works and for which conditions,” said Dr. Freedman, who was not involved in the Australian guidance document. He noted that little consensus exists because little evidence exists outside the handful of trials for Epidiolex.
“There are some patients with epilepsy that can benefit from high-quality, pharmaceutical-grade CBD products,” Dr. Freedman said. “These patients need to be carefully identified by a neurologist or epileptologist and prescribed a legal, safe, quality-controlled, and FDA-regulated product.”
Appropriate patient populations
Drug-resistant epilepsy, defined as failure of two appropriate antiseizure medications, affects an estimated one third of people with epilepsy, the new guideline notes. Though many over-the-counter products are available at dispensaries in the 33 U.S. states that allow use of cannabis for medical purposes, Epidiolex (cannabidiol) is the only FDA-approved drug for epilepsy that contains a substance derived from cannabis and the only one for which evidence from randomized, controlled trials exists.
Dr. Freedman notes that hemp-derived CBD oils are classified differently in the United States than marijuana-derived CBD oil, including Epidiolex, and are loosely regulated supplements or food additives commonly seen, for example at gas station.
“The point I drive home to patients is that you wouldn’t get your antibiotics from a gas station, so please don’t get your seizure medication from there,” Dr. Freedman said. “Studies have been done on ‘over-the-counter’ CBD oils and shown that they have variable quality, sometimes no detectable CBD, and sometimes other chemicals added like THC.”
Studies of Epidiolex showed that cannabidiol more effectively reduced seizure frequency than placebo for pediatric patients with Dravet syndrome (42% reduction) and for pediatric and adult patients with Lennox-Gastaut syndrome (39% reduction) or tuberous sclerosis complex (49% reduction). Efficacy was similar across dosing from 10-50 mg/kg per day, but higher doses involved higher rates of serious adverse events.
No reliable evidence in humans exists for THC or other cannabinoids in treating epilepsy.
The Australian guidance recommends limiting cannabis treatment to patients with severe drug-resistant epilepsy; a diagnosis of Dravet syndrome, Lennox-Gastaut syndrome, or tuberous sclerosis complex; and previous treatment with four approved antiseizure medications and/or the ketogenic diet, epilepsy surgery, or neurostimulator. The authors provide specific criteria for each of these conditions and then address exceptional cases that may be considered outside that criteria, such as patients under 2 years old, severe epilepsy with extended or repeated hospitalization or ICU admission, or a dangerous seizure type. The review also includes a detailed list of exclusion criteria for CBD medicine use.
The authors advised a thorough consent process before prescribing any cannabinoids, including therapeutic goals and stopping criteria; the lack of evidence available on dosing, efficacy, and side effects; and the potential for dependence or withdrawal. Consent discussions should also note whether the products are unregistered and not covered by external payers (anything other than Epidiolex currently), any activity restrictions, and any implications for occupational drug screening.
Considerations for unapproved cannabinoids
The authors note several factors to consider if prescribing or recommending a nonapproved, nonregulated cannabis medicine, including the ”differences between registered plant-derived cannabis medicines, synthetic cannabis medicines, and unregistered hemp-derived products.” Epidiolex is plant derived while other cannabis-derived medications (Marinol, Syndos, and Cesamet) that have been approved for nonepilepsy conditions, such as nausea associated with chemotherapy, are synthetic.
The guidance document notes several reasons to use a regulated medication instead of an unregulated product:
- Manufacturing processes can differ for unregulated products, including inconsistency in batches and unknown shelf life.
- Quality control processes, including risk of impurities, are much better with regulated products, which also have a system in place for safety recalls.
- More scientific evidence is available for regulated products.
- Safety surveillance reporting is more robust and standardized for regulated products whereas adverse event reporting is less reliable for unregulated products.
- Nonregulated products are rarely covered by insurance or other reimbursement.
Legal considerations will also vary by jurisdiction. ”Right now in the U.S. we have a confused legality where state level programs are still technically illegal at the federal level and I imagine there are some quality differences amongst dispensaries and states,” Dr. Freedman said. “Whenever there is disagreement between state and federal laws, this creates tension for our patients.” He noted, for example, that a patient using a CBD product that contains THC may, even if legal in their state, be confiscated by the Transportation Security Administration at an airport since it is not FDA approved and is not legal, according to the Drug Enforcement Agency.
The authors noted that inadequate data on long-term CBD use and data on neurodevelopmental effects of THC in children, teens, and young adults means THC products should be contraindicated for these age groups. (Epidiolex has less than 2% THC.) Drug interactions should also be considered, particularly for clobazam, CYP3A4 inhibitors or inducers (including St. John’s wort), digoxin, or a mechanistic target of rapamycin inhibitor.
Dr. Freedman said that most neurologists are comfortable prescribing Epidiolex since it has FDA approval while prescribing unapproved products varies more in the field. “Now that many states have compassionate use programs for medical marijuana, some neurologists do this as well,” Dr. Freedman said. Patients often ask about unregulated CBD or CBD+THC products because they’re seen as “natural and therefore better than manufactured pharmaceuticals.”
“I think this is the naturalistic fallacy at work and try to educate my patients on that since our only high-level data to show marijuana products work for epilepsy comes from a pharmaceutical company,” Dr. Freedman said. “My reasons for hesitating on compassionate use are that there is often THC, with variable amounts of concentration, and we know that THC can harm the developing pediatric brain.”
Dosing and adverse effects
Pediatric and adult dosing differences need to be considered, and “patient response (efficacy and toxicity) to these medications varies widely,” the authors noted. They advised getting serum transaminases (ALT and AST) and total bilirubin levels before beginning treatment. All patients should begin Epidiolex at a low dose, such as 2-5 mg/kg per day of CBD in two divided doses, the authors advise, and titrate slowly while monitoring for side effects (no more than 5 mg/kg per day per week). The current dosing range for CBD is 5-20 mg/kg per day in two divided doses, with higher rates involving more risk of adverse events.
“Note that some cannabinoids auto-inhibit their own metabolism and some have active metabolites with longer half-lives,” the authors wrote. “Therefore, dose or frequency may need to be reduced over time, unless tolerance occurs.” These doses, specific to Epidiolex, “cannot necessarily be applied to other oral CBD formulations or other types of epilepsy.” This guidance also does not apply to inhaled or transdermal routes of administration.
The most common adverse events were sleepiness – which occurred in up to 60% of trial participants – as well as diarrhea, decreases in appetite and weight, and drug interactions. Risk of hepatotoxicity means there’s a need to monitor liver function and adjust dosing for patients with moderate or severe hepatic impairment. “Other short-term side effects reported only with THC-containing cannabinoid compounds include increased risk of cardiac and cerebrovascular events, anxiety and psychosis risk, dependency, and withdrawal,” the authors wrote.
Though no withdrawal syndrome has been linked to stopping CBD, the authors suggested decreasing the dose by 10% every 2 days if stopping is not urgent.
“The key points to this issue are that CBD and all marijuana products need to be safe and regulated,” Dr. Freedman said. “Any claims about them need to be backed by high-quality evidence looking at that specific product for that specific condition.”
Dr. Freedman noted the need for children to receive treatment from clinicians with expertise in their specific condition since many other evidence-based treatments exist even for patients with epilepsy syndromes that are difficult to treat, such as other medications, surgery, and specialized diets.
“We need to fix the inconsistent regulation between over-the-counter CBD products, state dispensaries, and federal laws,” Dr. Freedman added. “Any medicine being used to treat children should be held to the same FDA standard of safety and efficacy.”
Dr. Freedman and the authors had no conflicts of interest. No external funding was noted.
A recent review article draws from existing clinical trials and clinical experience in New South Wales, Australia, to fill this gap with interim guidance for both pediatric and adult patients. The article was published in the British Journal of Clinical Pharmacology.
The only current U.S. guidelines are from the American Academy of Neurology’s position statement on the use of medical cannabis for neurologic disorders and the American Epilepsy Society’s position statement on cannabis as a treatment for epileptic seizures. The AAN statement “highlights the current evidence, which currently only supports [Food and Drug Administration]–approved CBD [cannabidiol] (Epidiolex) for specific epilepsy syndromes,” said Daniel Freedman, DO, an assistant professor of neurology at the University of Texas at Austin and coauthor of the AAN’s position statement.
“Rescheduling marijuana will enable researchers to study CBD, THC [tetrahydrocannabinol], and other cannabinoids in high-quality studies so that we can better understand what works and for which conditions,” said Dr. Freedman, who was not involved in the Australian guidance document. He noted that little consensus exists because little evidence exists outside the handful of trials for Epidiolex.
“There are some patients with epilepsy that can benefit from high-quality, pharmaceutical-grade CBD products,” Dr. Freedman said. “These patients need to be carefully identified by a neurologist or epileptologist and prescribed a legal, safe, quality-controlled, and FDA-regulated product.”
Appropriate patient populations
Drug-resistant epilepsy, defined as failure of two appropriate antiseizure medications, affects an estimated one third of people with epilepsy, the new guideline notes. Though many over-the-counter products are available at dispensaries in the 33 U.S. states that allow use of cannabis for medical purposes, Epidiolex (cannabidiol) is the only FDA-approved drug for epilepsy that contains a substance derived from cannabis and the only one for which evidence from randomized, controlled trials exists.
Dr. Freedman notes that hemp-derived CBD oils are classified differently in the United States than marijuana-derived CBD oil, including Epidiolex, and are loosely regulated supplements or food additives commonly seen, for example at gas station.
“The point I drive home to patients is that you wouldn’t get your antibiotics from a gas station, so please don’t get your seizure medication from there,” Dr. Freedman said. “Studies have been done on ‘over-the-counter’ CBD oils and shown that they have variable quality, sometimes no detectable CBD, and sometimes other chemicals added like THC.”
Studies of Epidiolex showed that cannabidiol more effectively reduced seizure frequency than placebo for pediatric patients with Dravet syndrome (42% reduction) and for pediatric and adult patients with Lennox-Gastaut syndrome (39% reduction) or tuberous sclerosis complex (49% reduction). Efficacy was similar across dosing from 10-50 mg/kg per day, but higher doses involved higher rates of serious adverse events.
No reliable evidence in humans exists for THC or other cannabinoids in treating epilepsy.
The Australian guidance recommends limiting cannabis treatment to patients with severe drug-resistant epilepsy; a diagnosis of Dravet syndrome, Lennox-Gastaut syndrome, or tuberous sclerosis complex; and previous treatment with four approved antiseizure medications and/or the ketogenic diet, epilepsy surgery, or neurostimulator. The authors provide specific criteria for each of these conditions and then address exceptional cases that may be considered outside that criteria, such as patients under 2 years old, severe epilepsy with extended or repeated hospitalization or ICU admission, or a dangerous seizure type. The review also includes a detailed list of exclusion criteria for CBD medicine use.
The authors advised a thorough consent process before prescribing any cannabinoids, including therapeutic goals and stopping criteria; the lack of evidence available on dosing, efficacy, and side effects; and the potential for dependence or withdrawal. Consent discussions should also note whether the products are unregistered and not covered by external payers (anything other than Epidiolex currently), any activity restrictions, and any implications for occupational drug screening.
Considerations for unapproved cannabinoids
The authors note several factors to consider if prescribing or recommending a nonapproved, nonregulated cannabis medicine, including the ”differences between registered plant-derived cannabis medicines, synthetic cannabis medicines, and unregistered hemp-derived products.” Epidiolex is plant derived while other cannabis-derived medications (Marinol, Syndos, and Cesamet) that have been approved for nonepilepsy conditions, such as nausea associated with chemotherapy, are synthetic.
The guidance document notes several reasons to use a regulated medication instead of an unregulated product:
- Manufacturing processes can differ for unregulated products, including inconsistency in batches and unknown shelf life.
- Quality control processes, including risk of impurities, are much better with regulated products, which also have a system in place for safety recalls.
- More scientific evidence is available for regulated products.
- Safety surveillance reporting is more robust and standardized for regulated products whereas adverse event reporting is less reliable for unregulated products.
- Nonregulated products are rarely covered by insurance or other reimbursement.
Legal considerations will also vary by jurisdiction. ”Right now in the U.S. we have a confused legality where state level programs are still technically illegal at the federal level and I imagine there are some quality differences amongst dispensaries and states,” Dr. Freedman said. “Whenever there is disagreement between state and federal laws, this creates tension for our patients.” He noted, for example, that a patient using a CBD product that contains THC may, even if legal in their state, be confiscated by the Transportation Security Administration at an airport since it is not FDA approved and is not legal, according to the Drug Enforcement Agency.
The authors noted that inadequate data on long-term CBD use and data on neurodevelopmental effects of THC in children, teens, and young adults means THC products should be contraindicated for these age groups. (Epidiolex has less than 2% THC.) Drug interactions should also be considered, particularly for clobazam, CYP3A4 inhibitors or inducers (including St. John’s wort), digoxin, or a mechanistic target of rapamycin inhibitor.
Dr. Freedman said that most neurologists are comfortable prescribing Epidiolex since it has FDA approval while prescribing unapproved products varies more in the field. “Now that many states have compassionate use programs for medical marijuana, some neurologists do this as well,” Dr. Freedman said. Patients often ask about unregulated CBD or CBD+THC products because they’re seen as “natural and therefore better than manufactured pharmaceuticals.”
“I think this is the naturalistic fallacy at work and try to educate my patients on that since our only high-level data to show marijuana products work for epilepsy comes from a pharmaceutical company,” Dr. Freedman said. “My reasons for hesitating on compassionate use are that there is often THC, with variable amounts of concentration, and we know that THC can harm the developing pediatric brain.”
Dosing and adverse effects
Pediatric and adult dosing differences need to be considered, and “patient response (efficacy and toxicity) to these medications varies widely,” the authors noted. They advised getting serum transaminases (ALT and AST) and total bilirubin levels before beginning treatment. All patients should begin Epidiolex at a low dose, such as 2-5 mg/kg per day of CBD in two divided doses, the authors advise, and titrate slowly while monitoring for side effects (no more than 5 mg/kg per day per week). The current dosing range for CBD is 5-20 mg/kg per day in two divided doses, with higher rates involving more risk of adverse events.
“Note that some cannabinoids auto-inhibit their own metabolism and some have active metabolites with longer half-lives,” the authors wrote. “Therefore, dose or frequency may need to be reduced over time, unless tolerance occurs.” These doses, specific to Epidiolex, “cannot necessarily be applied to other oral CBD formulations or other types of epilepsy.” This guidance also does not apply to inhaled or transdermal routes of administration.
The most common adverse events were sleepiness – which occurred in up to 60% of trial participants – as well as diarrhea, decreases in appetite and weight, and drug interactions. Risk of hepatotoxicity means there’s a need to monitor liver function and adjust dosing for patients with moderate or severe hepatic impairment. “Other short-term side effects reported only with THC-containing cannabinoid compounds include increased risk of cardiac and cerebrovascular events, anxiety and psychosis risk, dependency, and withdrawal,” the authors wrote.
Though no withdrawal syndrome has been linked to stopping CBD, the authors suggested decreasing the dose by 10% every 2 days if stopping is not urgent.
“The key points to this issue are that CBD and all marijuana products need to be safe and regulated,” Dr. Freedman said. “Any claims about them need to be backed by high-quality evidence looking at that specific product for that specific condition.”
Dr. Freedman noted the need for children to receive treatment from clinicians with expertise in their specific condition since many other evidence-based treatments exist even for patients with epilepsy syndromes that are difficult to treat, such as other medications, surgery, and specialized diets.
“We need to fix the inconsistent regulation between over-the-counter CBD products, state dispensaries, and federal laws,” Dr. Freedman added. “Any medicine being used to treat children should be held to the same FDA standard of safety and efficacy.”
Dr. Freedman and the authors had no conflicts of interest. No external funding was noted.
FROM THE BRITISH JOURNAL OF CLINICAL PHARMACOLOGY
Mild COVID-19 infection linked to later type 2 diabetes
People who recover from a mild case of COVID-19 appear to have an increased risk for subsequent new-onset type 2 diabetes but not other types of diabetes, new data suggest.
“If confirmed, the results of the present study indicate that diabetes screening in individuals who have recovered from even mild COVID-19 should be recommended,” say Wolfgang Rathmann, MD, of the Leibniz Center for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany, and colleagues.
The findings, from a nationwide primary care database in Germany, were recently published in Diabetologia.
These primary care data align with those from other studies of more seriously ill patients with COVID-19 that found increased rates of type 2 diabetes diagnoses in the subsequent months following illness, they point out.
“COVID-19 infection may lead to diabetes by upregulation of the immune system after remission, which may induce pancreatic beta-cell dysfunction and insulin resistance, or patients may have been at risk for developing diabetes due to having obesity or prediabetes, and the stress COVID-19 put on their bodies sped it up,” said Dr. Rathmann in a press release.
However, because the patients with COVID-19 in the study were only followed for about 3 months, “further follow-up is needed to understand whether type 2 diabetes after mild COVID-19 is just temporary and can be reversed after they have fully recovered or whether it leads to a chronic condition,” he noted.
Increase in type 2 diabetes 3 months after mild COVID-19
The retrospective cohort analysis was performed using data from the Disease Analyzer, a representative panel of 1,171 physician practices in Germany, from March 2020 to January 2021, with follow-up through July 2021.
Individuals with a history of COVID-19 or diabetes and those taking corticosteroids within 30 days after the index dates were excluded.
A total of 35,865 patients with confirmed SARS-CoV-2 infection were propensity score-matched on a one-to-one basis for sex, age, health insurance, and comorbidities with those who had acute respiratory tract infections (controls) but were COVID-19 negative. Median follow-up was 119 days for the COVID-19 group and 161 days for controls.
There was a 28% increased risk of type 2 diabetes for those who had COVID-19 versus controls (15.8 per 1,000 person-years vs. 12.3 per 1,000 person-years, respectively, which was significantly different, and an incidence rate ratio of 1.28).
The incidence of other types of diabetes or unspecified diabetes for the COVID-19 and control groups did not differ significantly (4.3 per 1,000 person-years vs. 3.7 per 1,000 person-years; IRR, 1.17).
Similar findings were seen in sensitivity analyses by glucose-lowering medication prescriptions and by ICD-10 codes.
Although type 2 diabetes is not likely to be a problem for the vast majority of people who have mild COVID-19, the authors recommend that anyone who has recovered from COVID-19 be aware of the warning signs and symptoms such as fatigue, frequent urination, and increased thirst, and seek treatment right away.
CoviDiab registry tracking type 1 and type 2 diabetes
Over the course of the pandemic, there have been conflicting data on whether COVID-19 induces or reveals a propensity for type 1 and type 2 diabetes.
The CoviDiab global registry is tracking this and will include diabetes type for adults and children.
The aim is to have “as many as possible cases of new-onset diabetes for which we can have also a minimum set of clinical data including type of diabetes and A1c,” coprincipal investigator Francesco Rubino, MD, of King’s College London, previously told this news organization.
“By looking at this information we can infer whether a role of COVID-19 in triggering diabetes is clinically plausible – or not – and what type of diabetes is most frequently associated with COVID-19.”
Rubino said that the CoviDiab team is approaching the data with the assumption that, at least in adults diagnosed with type 2 diabetes, the explanation might be that the person already had undiagnosed diabetes or the hyperglycemia may be stress-induced and temporary.
The German Diabetes Center is funded by the German Federal Ministry of Health and the Ministry of Culture and Science of the State of North Rhine-Westphalia. Dr. Rathmann has reported receiving consulting fees for attending educational sessions or advisory boards for AstraZeneca, Boehringer Ingelheim, and Novo Nordisk and institutional research grants from Novo Nordisk outside of the topic of the current work.
A version of this article first appeared on Medscape.com.
People who recover from a mild case of COVID-19 appear to have an increased risk for subsequent new-onset type 2 diabetes but not other types of diabetes, new data suggest.
“If confirmed, the results of the present study indicate that diabetes screening in individuals who have recovered from even mild COVID-19 should be recommended,” say Wolfgang Rathmann, MD, of the Leibniz Center for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany, and colleagues.
The findings, from a nationwide primary care database in Germany, were recently published in Diabetologia.
These primary care data align with those from other studies of more seriously ill patients with COVID-19 that found increased rates of type 2 diabetes diagnoses in the subsequent months following illness, they point out.
“COVID-19 infection may lead to diabetes by upregulation of the immune system after remission, which may induce pancreatic beta-cell dysfunction and insulin resistance, or patients may have been at risk for developing diabetes due to having obesity or prediabetes, and the stress COVID-19 put on their bodies sped it up,” said Dr. Rathmann in a press release.
However, because the patients with COVID-19 in the study were only followed for about 3 months, “further follow-up is needed to understand whether type 2 diabetes after mild COVID-19 is just temporary and can be reversed after they have fully recovered or whether it leads to a chronic condition,” he noted.
Increase in type 2 diabetes 3 months after mild COVID-19
The retrospective cohort analysis was performed using data from the Disease Analyzer, a representative panel of 1,171 physician practices in Germany, from March 2020 to January 2021, with follow-up through July 2021.
Individuals with a history of COVID-19 or diabetes and those taking corticosteroids within 30 days after the index dates were excluded.
A total of 35,865 patients with confirmed SARS-CoV-2 infection were propensity score-matched on a one-to-one basis for sex, age, health insurance, and comorbidities with those who had acute respiratory tract infections (controls) but were COVID-19 negative. Median follow-up was 119 days for the COVID-19 group and 161 days for controls.
There was a 28% increased risk of type 2 diabetes for those who had COVID-19 versus controls (15.8 per 1,000 person-years vs. 12.3 per 1,000 person-years, respectively, which was significantly different, and an incidence rate ratio of 1.28).
The incidence of other types of diabetes or unspecified diabetes for the COVID-19 and control groups did not differ significantly (4.3 per 1,000 person-years vs. 3.7 per 1,000 person-years; IRR, 1.17).
Similar findings were seen in sensitivity analyses by glucose-lowering medication prescriptions and by ICD-10 codes.
Although type 2 diabetes is not likely to be a problem for the vast majority of people who have mild COVID-19, the authors recommend that anyone who has recovered from COVID-19 be aware of the warning signs and symptoms such as fatigue, frequent urination, and increased thirst, and seek treatment right away.
CoviDiab registry tracking type 1 and type 2 diabetes
Over the course of the pandemic, there have been conflicting data on whether COVID-19 induces or reveals a propensity for type 1 and type 2 diabetes.
The CoviDiab global registry is tracking this and will include diabetes type for adults and children.
The aim is to have “as many as possible cases of new-onset diabetes for which we can have also a minimum set of clinical data including type of diabetes and A1c,” coprincipal investigator Francesco Rubino, MD, of King’s College London, previously told this news organization.
“By looking at this information we can infer whether a role of COVID-19 in triggering diabetes is clinically plausible – or not – and what type of diabetes is most frequently associated with COVID-19.”
Rubino said that the CoviDiab team is approaching the data with the assumption that, at least in adults diagnosed with type 2 diabetes, the explanation might be that the person already had undiagnosed diabetes or the hyperglycemia may be stress-induced and temporary.
The German Diabetes Center is funded by the German Federal Ministry of Health and the Ministry of Culture and Science of the State of North Rhine-Westphalia. Dr. Rathmann has reported receiving consulting fees for attending educational sessions or advisory boards for AstraZeneca, Boehringer Ingelheim, and Novo Nordisk and institutional research grants from Novo Nordisk outside of the topic of the current work.
A version of this article first appeared on Medscape.com.
People who recover from a mild case of COVID-19 appear to have an increased risk for subsequent new-onset type 2 diabetes but not other types of diabetes, new data suggest.
“If confirmed, the results of the present study indicate that diabetes screening in individuals who have recovered from even mild COVID-19 should be recommended,” say Wolfgang Rathmann, MD, of the Leibniz Center for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany, and colleagues.
The findings, from a nationwide primary care database in Germany, were recently published in Diabetologia.
These primary care data align with those from other studies of more seriously ill patients with COVID-19 that found increased rates of type 2 diabetes diagnoses in the subsequent months following illness, they point out.
“COVID-19 infection may lead to diabetes by upregulation of the immune system after remission, which may induce pancreatic beta-cell dysfunction and insulin resistance, or patients may have been at risk for developing diabetes due to having obesity or prediabetes, and the stress COVID-19 put on their bodies sped it up,” said Dr. Rathmann in a press release.
However, because the patients with COVID-19 in the study were only followed for about 3 months, “further follow-up is needed to understand whether type 2 diabetes after mild COVID-19 is just temporary and can be reversed after they have fully recovered or whether it leads to a chronic condition,” he noted.
Increase in type 2 diabetes 3 months after mild COVID-19
The retrospective cohort analysis was performed using data from the Disease Analyzer, a representative panel of 1,171 physician practices in Germany, from March 2020 to January 2021, with follow-up through July 2021.
Individuals with a history of COVID-19 or diabetes and those taking corticosteroids within 30 days after the index dates were excluded.
A total of 35,865 patients with confirmed SARS-CoV-2 infection were propensity score-matched on a one-to-one basis for sex, age, health insurance, and comorbidities with those who had acute respiratory tract infections (controls) but were COVID-19 negative. Median follow-up was 119 days for the COVID-19 group and 161 days for controls.
There was a 28% increased risk of type 2 diabetes for those who had COVID-19 versus controls (15.8 per 1,000 person-years vs. 12.3 per 1,000 person-years, respectively, which was significantly different, and an incidence rate ratio of 1.28).
The incidence of other types of diabetes or unspecified diabetes for the COVID-19 and control groups did not differ significantly (4.3 per 1,000 person-years vs. 3.7 per 1,000 person-years; IRR, 1.17).
Similar findings were seen in sensitivity analyses by glucose-lowering medication prescriptions and by ICD-10 codes.
Although type 2 diabetes is not likely to be a problem for the vast majority of people who have mild COVID-19, the authors recommend that anyone who has recovered from COVID-19 be aware of the warning signs and symptoms such as fatigue, frequent urination, and increased thirst, and seek treatment right away.
CoviDiab registry tracking type 1 and type 2 diabetes
Over the course of the pandemic, there have been conflicting data on whether COVID-19 induces or reveals a propensity for type 1 and type 2 diabetes.
The CoviDiab global registry is tracking this and will include diabetes type for adults and children.
The aim is to have “as many as possible cases of new-onset diabetes for which we can have also a minimum set of clinical data including type of diabetes and A1c,” coprincipal investigator Francesco Rubino, MD, of King’s College London, previously told this news organization.
“By looking at this information we can infer whether a role of COVID-19 in triggering diabetes is clinically plausible – or not – and what type of diabetes is most frequently associated with COVID-19.”
Rubino said that the CoviDiab team is approaching the data with the assumption that, at least in adults diagnosed with type 2 diabetes, the explanation might be that the person already had undiagnosed diabetes or the hyperglycemia may be stress-induced and temporary.
The German Diabetes Center is funded by the German Federal Ministry of Health and the Ministry of Culture and Science of the State of North Rhine-Westphalia. Dr. Rathmann has reported receiving consulting fees for attending educational sessions or advisory boards for AstraZeneca, Boehringer Ingelheim, and Novo Nordisk and institutional research grants from Novo Nordisk outside of the topic of the current work.
A version of this article first appeared on Medscape.com.
FROM DIABETOLOGIA






