User login
News and Views that Matter to Pediatricians
The leading independent newspaper covering news and commentary in pediatrics.
TB treatment can be shortened for most children: study
The World Health Organization is expected to recommend truncating treatment of children with mild tuberculosis by 2 months – from 6 months to 4 – after a randomized trial found similar outcomes with the shorter regimen.
An international team of investigators found the abbreviated course of antibiotics was no less effective or safe than conventional treatment and saved an average of $17.34 per child – money that could be used to mitigate the toll of TB, which is estimated to sicken 1.1 million children worldwide each year.
The findings come as deaths from TB are rising as a result of the COVID-19 pandemic, which has hindered efforts to find and treat patients. In 2020, according to the WHO, an estimated 1.5 million people died from TB, the first year-over-year increase in such deaths since 2005.
Nearly a quarter of children with TB die, primarily because they go undiagnosed, according to the researchers, who published the study in the New England Journal of Medicine. Shorter treatment “translates into very large cost savings that could be used to improve screening and diagnosis to address the current case detection gap,” first author Anna Turkova, MD, of University College London, told this news organization.
The standard TB regimen is based on trials in adults with severe respiratory disease. However, about two-thirds of children have nonsevere infections.
For the study, Dr. Turkova and colleagues assigned 1,204 children with TB in four countries – Uganda, Zambia, South Africa, and India – to either a 4- or 6-month regimen with first-line medications rifampin, isoniazid, pyrazinamide, and ethambutol. Participants were aged 2 months to 15 years and had symptomatic nonsevere lung or lymph node infections with a negative test on a sputum smear microscopy. Eleven percent also had HIV.
After 18 months, 16 participants in the group that received the shortened treatment and 18 in the standard treatment group had experienced an unfavorable outcome – defined as treatment failure, recurrence of TB, loss to follow-up, or death (adjusted difference, -0.4 percentage points; 95% confidence interval, -2.2 to 1.5).
Similar numbers – 47 in the 4-month group and 48 in the 6-month group – experienced severe or life-threatening adverse events, most commonly chest infections, such as pneumonia, and liver problems, during treatment or up to 30 days after the last dose.
New guidelines coming soon
The WHO plans to issue new guidelines and a handbook for TB management in children and adolescents on March 24, World Tuberculosis Day, a spokesman for the agency told Medscape.
Anna Mandalakas, MD, PhD, director of the Global Tuberculosis Program at Baylor College of Medicine, department of pediatrics, Houston, said the shorter regimen should enable more children to successfully complete TB treatment.
“It can be challenging to convince young children to take medications on a regular basis for 6 months,” Dr. Mandalakas, a member of a WHO guidelines development group that reviewed the study, told this news organization. “Despite best intentions, parents often become fatigued and give up the medicine battle.”
Leo Martinez, PhD, an epidemiologist at Boston University School of Public Health who studies pediatric TB, noted that study’s cost-effectiveness analysis applies only to health care costs. Families often suffer financially through lost wages, transportation to health care facilities, and lost employment, fueling a cycle of poverty and disease in low-income countries, he said.
A WHO statement noted that long treatment regimens can add toxicity and risk of drug interactions for children with HIV.
Separate efforts have been underway to hasten TB treatment in different groups of patients. A study published in NEJM showed that 4 months of the potent antibiotic rifapentine, along with another antibiotic, moxifloxacin, was non-inferior to the standard 6-month regimen in patients aged 12 and older. According to the editorial accompanying that study, the research illustrated the potential for shorter treatment courses that would be cheaper and less cumbersome, although that particular combination poses hurdles such as adherence issues and potential bacterial resistance.
Experts agreed that improved diagnostic procedures are critical to significantly reducing TB pediatric deaths – an issue that Dr. Turkova said will be addressed in WHO’s forthcoming handbook.
Because no gold-standard test exists for TB, and symptoms often overlap with other infections, widespread screening of children in households where adults have been diagnosed with TB has been found to improve detection of the disease. “Training of health care workers, easy-to-implement diagnostic algorithms, and widely accessible training materials on chest radiography in childhood TB should also improve case finding and treatment initiation,” she said.
The trial was supported by U.K. government and charitable research funders. Dr. Turkova and Dr. Martinez reported no financial disclosures. Dr. Mandalakas reported honoraria from WHO to support the preparation of diagnostics and treatment chapters in the operational handbook, for providing lectures for Medscape, and for serving on a data safety monitoring board for Janssen Pharmaceuticals.
A version of this article first appeared on Medscape.com.
The World Health Organization is expected to recommend truncating treatment of children with mild tuberculosis by 2 months – from 6 months to 4 – after a randomized trial found similar outcomes with the shorter regimen.
An international team of investigators found the abbreviated course of antibiotics was no less effective or safe than conventional treatment and saved an average of $17.34 per child – money that could be used to mitigate the toll of TB, which is estimated to sicken 1.1 million children worldwide each year.
The findings come as deaths from TB are rising as a result of the COVID-19 pandemic, which has hindered efforts to find and treat patients. In 2020, according to the WHO, an estimated 1.5 million people died from TB, the first year-over-year increase in such deaths since 2005.
Nearly a quarter of children with TB die, primarily because they go undiagnosed, according to the researchers, who published the study in the New England Journal of Medicine. Shorter treatment “translates into very large cost savings that could be used to improve screening and diagnosis to address the current case detection gap,” first author Anna Turkova, MD, of University College London, told this news organization.
The standard TB regimen is based on trials in adults with severe respiratory disease. However, about two-thirds of children have nonsevere infections.
For the study, Dr. Turkova and colleagues assigned 1,204 children with TB in four countries – Uganda, Zambia, South Africa, and India – to either a 4- or 6-month regimen with first-line medications rifampin, isoniazid, pyrazinamide, and ethambutol. Participants were aged 2 months to 15 years and had symptomatic nonsevere lung or lymph node infections with a negative test on a sputum smear microscopy. Eleven percent also had HIV.
After 18 months, 16 participants in the group that received the shortened treatment and 18 in the standard treatment group had experienced an unfavorable outcome – defined as treatment failure, recurrence of TB, loss to follow-up, or death (adjusted difference, -0.4 percentage points; 95% confidence interval, -2.2 to 1.5).
Similar numbers – 47 in the 4-month group and 48 in the 6-month group – experienced severe or life-threatening adverse events, most commonly chest infections, such as pneumonia, and liver problems, during treatment or up to 30 days after the last dose.
New guidelines coming soon
The WHO plans to issue new guidelines and a handbook for TB management in children and adolescents on March 24, World Tuberculosis Day, a spokesman for the agency told Medscape.
Anna Mandalakas, MD, PhD, director of the Global Tuberculosis Program at Baylor College of Medicine, department of pediatrics, Houston, said the shorter regimen should enable more children to successfully complete TB treatment.
“It can be challenging to convince young children to take medications on a regular basis for 6 months,” Dr. Mandalakas, a member of a WHO guidelines development group that reviewed the study, told this news organization. “Despite best intentions, parents often become fatigued and give up the medicine battle.”
Leo Martinez, PhD, an epidemiologist at Boston University School of Public Health who studies pediatric TB, noted that study’s cost-effectiveness analysis applies only to health care costs. Families often suffer financially through lost wages, transportation to health care facilities, and lost employment, fueling a cycle of poverty and disease in low-income countries, he said.
A WHO statement noted that long treatment regimens can add toxicity and risk of drug interactions for children with HIV.
Separate efforts have been underway to hasten TB treatment in different groups of patients. A study published in NEJM showed that 4 months of the potent antibiotic rifapentine, along with another antibiotic, moxifloxacin, was non-inferior to the standard 6-month regimen in patients aged 12 and older. According to the editorial accompanying that study, the research illustrated the potential for shorter treatment courses that would be cheaper and less cumbersome, although that particular combination poses hurdles such as adherence issues and potential bacterial resistance.
Experts agreed that improved diagnostic procedures are critical to significantly reducing TB pediatric deaths – an issue that Dr. Turkova said will be addressed in WHO’s forthcoming handbook.
Because no gold-standard test exists for TB, and symptoms often overlap with other infections, widespread screening of children in households where adults have been diagnosed with TB has been found to improve detection of the disease. “Training of health care workers, easy-to-implement diagnostic algorithms, and widely accessible training materials on chest radiography in childhood TB should also improve case finding and treatment initiation,” she said.
The trial was supported by U.K. government and charitable research funders. Dr. Turkova and Dr. Martinez reported no financial disclosures. Dr. Mandalakas reported honoraria from WHO to support the preparation of diagnostics and treatment chapters in the operational handbook, for providing lectures for Medscape, and for serving on a data safety monitoring board for Janssen Pharmaceuticals.
A version of this article first appeared on Medscape.com.
The World Health Organization is expected to recommend truncating treatment of children with mild tuberculosis by 2 months – from 6 months to 4 – after a randomized trial found similar outcomes with the shorter regimen.
An international team of investigators found the abbreviated course of antibiotics was no less effective or safe than conventional treatment and saved an average of $17.34 per child – money that could be used to mitigate the toll of TB, which is estimated to sicken 1.1 million children worldwide each year.
The findings come as deaths from TB are rising as a result of the COVID-19 pandemic, which has hindered efforts to find and treat patients. In 2020, according to the WHO, an estimated 1.5 million people died from TB, the first year-over-year increase in such deaths since 2005.
Nearly a quarter of children with TB die, primarily because they go undiagnosed, according to the researchers, who published the study in the New England Journal of Medicine. Shorter treatment “translates into very large cost savings that could be used to improve screening and diagnosis to address the current case detection gap,” first author Anna Turkova, MD, of University College London, told this news organization.
The standard TB regimen is based on trials in adults with severe respiratory disease. However, about two-thirds of children have nonsevere infections.
For the study, Dr. Turkova and colleagues assigned 1,204 children with TB in four countries – Uganda, Zambia, South Africa, and India – to either a 4- or 6-month regimen with first-line medications rifampin, isoniazid, pyrazinamide, and ethambutol. Participants were aged 2 months to 15 years and had symptomatic nonsevere lung or lymph node infections with a negative test on a sputum smear microscopy. Eleven percent also had HIV.
After 18 months, 16 participants in the group that received the shortened treatment and 18 in the standard treatment group had experienced an unfavorable outcome – defined as treatment failure, recurrence of TB, loss to follow-up, or death (adjusted difference, -0.4 percentage points; 95% confidence interval, -2.2 to 1.5).
Similar numbers – 47 in the 4-month group and 48 in the 6-month group – experienced severe or life-threatening adverse events, most commonly chest infections, such as pneumonia, and liver problems, during treatment or up to 30 days after the last dose.
New guidelines coming soon
The WHO plans to issue new guidelines and a handbook for TB management in children and adolescents on March 24, World Tuberculosis Day, a spokesman for the agency told Medscape.
Anna Mandalakas, MD, PhD, director of the Global Tuberculosis Program at Baylor College of Medicine, department of pediatrics, Houston, said the shorter regimen should enable more children to successfully complete TB treatment.
“It can be challenging to convince young children to take medications on a regular basis for 6 months,” Dr. Mandalakas, a member of a WHO guidelines development group that reviewed the study, told this news organization. “Despite best intentions, parents often become fatigued and give up the medicine battle.”
Leo Martinez, PhD, an epidemiologist at Boston University School of Public Health who studies pediatric TB, noted that study’s cost-effectiveness analysis applies only to health care costs. Families often suffer financially through lost wages, transportation to health care facilities, and lost employment, fueling a cycle of poverty and disease in low-income countries, he said.
A WHO statement noted that long treatment regimens can add toxicity and risk of drug interactions for children with HIV.
Separate efforts have been underway to hasten TB treatment in different groups of patients. A study published in NEJM showed that 4 months of the potent antibiotic rifapentine, along with another antibiotic, moxifloxacin, was non-inferior to the standard 6-month regimen in patients aged 12 and older. According to the editorial accompanying that study, the research illustrated the potential for shorter treatment courses that would be cheaper and less cumbersome, although that particular combination poses hurdles such as adherence issues and potential bacterial resistance.
Experts agreed that improved diagnostic procedures are critical to significantly reducing TB pediatric deaths – an issue that Dr. Turkova said will be addressed in WHO’s forthcoming handbook.
Because no gold-standard test exists for TB, and symptoms often overlap with other infections, widespread screening of children in households where adults have been diagnosed with TB has been found to improve detection of the disease. “Training of health care workers, easy-to-implement diagnostic algorithms, and widely accessible training materials on chest radiography in childhood TB should also improve case finding and treatment initiation,” she said.
The trial was supported by U.K. government and charitable research funders. Dr. Turkova and Dr. Martinez reported no financial disclosures. Dr. Mandalakas reported honoraria from WHO to support the preparation of diagnostics and treatment chapters in the operational handbook, for providing lectures for Medscape, and for serving on a data safety monitoring board for Janssen Pharmaceuticals.
A version of this article first appeared on Medscape.com.
‘Bigorexia’: Why teenage boys are obsessed with bulking up
Why are teenage boys obsessed with bulking up?
While the effects of Instagram on girls’ body image has long been documented – an article in The Wall Street Journal that was published this fall reported that Facebook knew Instagram was toxic for teen girls – teenage boys are under just as much pressure.
For adolescent boys, the goal is often to get superhero-size buff – and this is leading to anxiety, stress, excessive selfies, and, often, obsessive staring in the mirror to assess their “pec” progress.
So-called “bigorexia” – or extreme gym time, excessive focus on protein diets, and intense muscle-building goals – has hit new and concerning levels, according to a recent New York Times report.
Whether it’s the pandemic or TikTok that’s to blame, teen boys are pushing hard to achieve six-pack abs, with one-third of them in the U.S. trying to bulk up, according to a study published in the Journal of Adolescent Health. What’s more, 22% reported they’re engaging in muscle-enhancing behavior, including excess exercise, taking supplements or steroids, or eating more to bulk up, according to a study published in the International Journal of Eating Disorders.
“The pandemic and social media have been a perfect storm for eating disorders and body image issues for all teens, but this has been under-recognized in boys,” says Jason Nagata, MD, a pediatrician who specializes in adolescent medicine at the University of California, San Francisco. “Both are directly connected to an increase in muscle dysmorphia.”
While “bigorexia” is a newer term coined by mental health professionals, the concept of muscular dysmorphia isn’t, says Jennifer Bahrman, PhD, a licensed psychologist with McGovern Medical School at UTHealth Houston. This may be why about a third of boys ages 11-18 reported that they aren’t enamored with their bodies, according to a small survey published in 2019 in the Californian Journal of Health Promotion.
“When we think of dysmorphia, we think of girls having it, since we see it more in females,” says Dr. Bahrman, who works extensively with adolescents and athletes. “The interesting thing about muscular dysmorphia is that it’s the only body dysmorphic disorder that’s almost exclusively present in males.”
Social media’s role
Unlike other things in boys’ lives, like movies, TV, or even the uber-buff GI Joe doll, social media has created opportunities for young men to put their bodies on display – and become an influencer or get followers because of it.
“An everyday teen can become a celebrity,” Dr. Nagata says. “Then, thanks to social media algorithms, if a teenage boy likes or interacts with a post that features a muscular guy or is all about fitness, they’ll start getting all sorts of related content. They’ll get bombarded with tons of ads for protein shakes, for example, as well as bodybuilding equipment, and that will further distort reality.”
Before-and-after photos are also known to be quite misleading.
“Some of the most popular Instagram posts among teens feature people who have experienced a massive body transformation,” Dr. Nagata says. “It’s usually someone who lost a lot of weight or someone who was scrawny and then got muscular. The most drastic changes tend to get the most likes and are perpetuated the most and shared the most often with friends.”
But as many are aware, photos posted to social media are selected to tell the best story – with the best filters, lighting, and angles possible, however exaggerated.
“A guy will post his worst picture out of a thousand for his before shot and then post the best photo out of a thousand,” Dr. Nagata says. “This, in itself, can really confuse a teenager, because the story of this person’s changed body looks so realistic.”
Worse, these images tend to be damaging to your teenager’s self-esteem.
“When you see images of people you’re aspiring to look like, it can be very upsetting,” Dr. Bahrman says. “After all, it’s easy to think, ‘I’m doing all of these pushups, and I don’t look like this.’ From there, it’s easy to begin internalizing that something is wrong with you.”
Red flags to watch out for
If you’ve noticed that your son is obsessed with his appearance, weight, food, or exercise, take note. Also, notice if he’s asking you to buy protein powder or is spending more time at the gym than with his friends.
“Pay attention if he is withdrawing from friends and family because of his concerns about his appearance,” Dr. Nagata says. “For example, we often hear that a teenager will no longer eat family meals or at a restaurant because the protein content isn’t high enough or the food is too fatty.”
If you’re concerned, always make sure to discuss this with your son’s pediatrician.
“Ultimately, you want to make sure you share your concerns before your teen son becomes even more body-image obsessed,” Dr. Nagata says.
A version of this article first appeared on WebMD.com.
Why are teenage boys obsessed with bulking up?
While the effects of Instagram on girls’ body image has long been documented – an article in The Wall Street Journal that was published this fall reported that Facebook knew Instagram was toxic for teen girls – teenage boys are under just as much pressure.
For adolescent boys, the goal is often to get superhero-size buff – and this is leading to anxiety, stress, excessive selfies, and, often, obsessive staring in the mirror to assess their “pec” progress.
So-called “bigorexia” – or extreme gym time, excessive focus on protein diets, and intense muscle-building goals – has hit new and concerning levels, according to a recent New York Times report.
Whether it’s the pandemic or TikTok that’s to blame, teen boys are pushing hard to achieve six-pack abs, with one-third of them in the U.S. trying to bulk up, according to a study published in the Journal of Adolescent Health. What’s more, 22% reported they’re engaging in muscle-enhancing behavior, including excess exercise, taking supplements or steroids, or eating more to bulk up, according to a study published in the International Journal of Eating Disorders.
“The pandemic and social media have been a perfect storm for eating disorders and body image issues for all teens, but this has been under-recognized in boys,” says Jason Nagata, MD, a pediatrician who specializes in adolescent medicine at the University of California, San Francisco. “Both are directly connected to an increase in muscle dysmorphia.”
While “bigorexia” is a newer term coined by mental health professionals, the concept of muscular dysmorphia isn’t, says Jennifer Bahrman, PhD, a licensed psychologist with McGovern Medical School at UTHealth Houston. This may be why about a third of boys ages 11-18 reported that they aren’t enamored with their bodies, according to a small survey published in 2019 in the Californian Journal of Health Promotion.
“When we think of dysmorphia, we think of girls having it, since we see it more in females,” says Dr. Bahrman, who works extensively with adolescents and athletes. “The interesting thing about muscular dysmorphia is that it’s the only body dysmorphic disorder that’s almost exclusively present in males.”
Social media’s role
Unlike other things in boys’ lives, like movies, TV, or even the uber-buff GI Joe doll, social media has created opportunities for young men to put their bodies on display – and become an influencer or get followers because of it.
“An everyday teen can become a celebrity,” Dr. Nagata says. “Then, thanks to social media algorithms, if a teenage boy likes or interacts with a post that features a muscular guy or is all about fitness, they’ll start getting all sorts of related content. They’ll get bombarded with tons of ads for protein shakes, for example, as well as bodybuilding equipment, and that will further distort reality.”
Before-and-after photos are also known to be quite misleading.
“Some of the most popular Instagram posts among teens feature people who have experienced a massive body transformation,” Dr. Nagata says. “It’s usually someone who lost a lot of weight or someone who was scrawny and then got muscular. The most drastic changes tend to get the most likes and are perpetuated the most and shared the most often with friends.”
But as many are aware, photos posted to social media are selected to tell the best story – with the best filters, lighting, and angles possible, however exaggerated.
“A guy will post his worst picture out of a thousand for his before shot and then post the best photo out of a thousand,” Dr. Nagata says. “This, in itself, can really confuse a teenager, because the story of this person’s changed body looks so realistic.”
Worse, these images tend to be damaging to your teenager’s self-esteem.
“When you see images of people you’re aspiring to look like, it can be very upsetting,” Dr. Bahrman says. “After all, it’s easy to think, ‘I’m doing all of these pushups, and I don’t look like this.’ From there, it’s easy to begin internalizing that something is wrong with you.”
Red flags to watch out for
If you’ve noticed that your son is obsessed with his appearance, weight, food, or exercise, take note. Also, notice if he’s asking you to buy protein powder or is spending more time at the gym than with his friends.
“Pay attention if he is withdrawing from friends and family because of his concerns about his appearance,” Dr. Nagata says. “For example, we often hear that a teenager will no longer eat family meals or at a restaurant because the protein content isn’t high enough or the food is too fatty.”
If you’re concerned, always make sure to discuss this with your son’s pediatrician.
“Ultimately, you want to make sure you share your concerns before your teen son becomes even more body-image obsessed,” Dr. Nagata says.
A version of this article first appeared on WebMD.com.
Why are teenage boys obsessed with bulking up?
While the effects of Instagram on girls’ body image has long been documented – an article in The Wall Street Journal that was published this fall reported that Facebook knew Instagram was toxic for teen girls – teenage boys are under just as much pressure.
For adolescent boys, the goal is often to get superhero-size buff – and this is leading to anxiety, stress, excessive selfies, and, often, obsessive staring in the mirror to assess their “pec” progress.
So-called “bigorexia” – or extreme gym time, excessive focus on protein diets, and intense muscle-building goals – has hit new and concerning levels, according to a recent New York Times report.
Whether it’s the pandemic or TikTok that’s to blame, teen boys are pushing hard to achieve six-pack abs, with one-third of them in the U.S. trying to bulk up, according to a study published in the Journal of Adolescent Health. What’s more, 22% reported they’re engaging in muscle-enhancing behavior, including excess exercise, taking supplements or steroids, or eating more to bulk up, according to a study published in the International Journal of Eating Disorders.
“The pandemic and social media have been a perfect storm for eating disorders and body image issues for all teens, but this has been under-recognized in boys,” says Jason Nagata, MD, a pediatrician who specializes in adolescent medicine at the University of California, San Francisco. “Both are directly connected to an increase in muscle dysmorphia.”
While “bigorexia” is a newer term coined by mental health professionals, the concept of muscular dysmorphia isn’t, says Jennifer Bahrman, PhD, a licensed psychologist with McGovern Medical School at UTHealth Houston. This may be why about a third of boys ages 11-18 reported that they aren’t enamored with their bodies, according to a small survey published in 2019 in the Californian Journal of Health Promotion.
“When we think of dysmorphia, we think of girls having it, since we see it more in females,” says Dr. Bahrman, who works extensively with adolescents and athletes. “The interesting thing about muscular dysmorphia is that it’s the only body dysmorphic disorder that’s almost exclusively present in males.”
Social media’s role
Unlike other things in boys’ lives, like movies, TV, or even the uber-buff GI Joe doll, social media has created opportunities for young men to put their bodies on display – and become an influencer or get followers because of it.
“An everyday teen can become a celebrity,” Dr. Nagata says. “Then, thanks to social media algorithms, if a teenage boy likes or interacts with a post that features a muscular guy or is all about fitness, they’ll start getting all sorts of related content. They’ll get bombarded with tons of ads for protein shakes, for example, as well as bodybuilding equipment, and that will further distort reality.”
Before-and-after photos are also known to be quite misleading.
“Some of the most popular Instagram posts among teens feature people who have experienced a massive body transformation,” Dr. Nagata says. “It’s usually someone who lost a lot of weight or someone who was scrawny and then got muscular. The most drastic changes tend to get the most likes and are perpetuated the most and shared the most often with friends.”
But as many are aware, photos posted to social media are selected to tell the best story – with the best filters, lighting, and angles possible, however exaggerated.
“A guy will post his worst picture out of a thousand for his before shot and then post the best photo out of a thousand,” Dr. Nagata says. “This, in itself, can really confuse a teenager, because the story of this person’s changed body looks so realistic.”
Worse, these images tend to be damaging to your teenager’s self-esteem.
“When you see images of people you’re aspiring to look like, it can be very upsetting,” Dr. Bahrman says. “After all, it’s easy to think, ‘I’m doing all of these pushups, and I don’t look like this.’ From there, it’s easy to begin internalizing that something is wrong with you.”
Red flags to watch out for
If you’ve noticed that your son is obsessed with his appearance, weight, food, or exercise, take note. Also, notice if he’s asking you to buy protein powder or is spending more time at the gym than with his friends.
“Pay attention if he is withdrawing from friends and family because of his concerns about his appearance,” Dr. Nagata says. “For example, we often hear that a teenager will no longer eat family meals or at a restaurant because the protein content isn’t high enough or the food is too fatty.”
If you’re concerned, always make sure to discuss this with your son’s pediatrician.
“Ultimately, you want to make sure you share your concerns before your teen son becomes even more body-image obsessed,” Dr. Nagata says.
A version of this article first appeared on WebMD.com.
Food insecurity linked to metabolic syndrome in Hispanic/Latino youth
Severe food insecurity was associated with metabolic syndrome and unfavorable cardiometabolic markers in Hispanic/Latino youth, researchers report.
The findings, published March 16 in Pediatrics, highlight the need to investigate interventions that address food insecurity among Hispanic/Latino youth, a segment of the U.S. population at high risk of cardiometabolic complications.
“Among Hispanic/Latino youth, no study, to our knowledge has evaluated food insecurity’s role in metabolic syndrome and metabolic syndrome–relevant cardiometabolic markers in this population,” lead author Luis E. Maldonado, PhD, of the University of North Carolina at Chapel Hill, and colleagues explained.
The researchers conducted a cross-sectional study to evaluate the associations between lower household and child food security and metabolic syndrome, as well as clinically measured cardiometabolic markers, including fasting plasma glucose, waist circumference, triglycerides, systolic and diastolic blood pressure, and high-density lipoprotein cholesterol (HDL-C).
Household food security (high, marginal, low, very low) and child food security (high, marginal, low/very low) measures were evaluated separately, and were adjusted for participant age, sex, site, parental education, and poverty-income ratio.
Data were obtained from the Hispanic Community Children’s Health Study/Study of Latino Youth, a study of offspring of adults enrolled in the Hispanic Community Health Survey/Study of Latinos.
Results
The study cohort included 1,325 Hispanic/Latino youth aged 8-16 years. For both household food security and child food security, youth in the lowest food security category had significantly lower HDL-C compared with youth with high food security (household food security, –3.17; 95% confidence interval, –5.65 to –0.70; child food security, –1.81; 95% CI, –3.54 to –0.09).
In addition, low/very low compared with high child food security was associated with higher triglycerides (beta, 8.68; 95% CI, 1.75-15.61), higher fasting plasma glucose (beta, 1.37; 95% CI, 0.08-2.65), and metabolic syndrome composite variable expected log counts (beta, 2.12; 95% CI, 0.02-0.45).
Furthermore, the researchers found statistically significant interactions between each of the two food security measures and receipt of any food assistance in the previous year in models of triglycerides (P for interactions: household food security, .03 and child food security, .005) and HDL-C (P for interactions: household food security, .01 and child food security, .04).
After evaluating the effect of parental place of birth, they found a statistically significant association for triglycerides only (P for interactions: household food security, .05 and child food security, .008).
“Our study is among the first to document adverse associations between household and child food security measures with a metabolic syndrome score variable and several metabolic syndrome–relevant cardiometabolic markers among US Hispanic/Latino youth,” the researchers wrote.
The researchers acknowledged that the cross-sectional nature of the study was a key limitation; thus, causality could not be inferred.
“In the future, we plan to conduct more qualitative work to better understand how Hispanic/Latino families respond to food insecurity, which may identify the factors that shape their response,” study author Sandra S. Albrecht, PhD, of Columbia University, New York, NY, said in an interview.
Recommendations for pediatricians
Food insecurity researcher Yankun Wang, PhD candidate at Indiana University, Bloomington, commented: “I would recommend pediatricians pay more attention to children from low-income households since they are more likely to have mental and physical health issues due to food insecurity.
“It can be very helpful if pediatricians could help families obtain SNAP benefits, enroll youth in the school breakfast and lunch programs, and promote nutrition education in schools,” Mr. Wang added.
This study was supported by grant funding from the National Heart, Lung, and Blood Institute. The authors reported no relevant disclosures.
Severe food insecurity was associated with metabolic syndrome and unfavorable cardiometabolic markers in Hispanic/Latino youth, researchers report.
The findings, published March 16 in Pediatrics, highlight the need to investigate interventions that address food insecurity among Hispanic/Latino youth, a segment of the U.S. population at high risk of cardiometabolic complications.
“Among Hispanic/Latino youth, no study, to our knowledge has evaluated food insecurity’s role in metabolic syndrome and metabolic syndrome–relevant cardiometabolic markers in this population,” lead author Luis E. Maldonado, PhD, of the University of North Carolina at Chapel Hill, and colleagues explained.
The researchers conducted a cross-sectional study to evaluate the associations between lower household and child food security and metabolic syndrome, as well as clinically measured cardiometabolic markers, including fasting plasma glucose, waist circumference, triglycerides, systolic and diastolic blood pressure, and high-density lipoprotein cholesterol (HDL-C).
Household food security (high, marginal, low, very low) and child food security (high, marginal, low/very low) measures were evaluated separately, and were adjusted for participant age, sex, site, parental education, and poverty-income ratio.
Data were obtained from the Hispanic Community Children’s Health Study/Study of Latino Youth, a study of offspring of adults enrolled in the Hispanic Community Health Survey/Study of Latinos.
Results
The study cohort included 1,325 Hispanic/Latino youth aged 8-16 years. For both household food security and child food security, youth in the lowest food security category had significantly lower HDL-C compared with youth with high food security (household food security, –3.17; 95% confidence interval, –5.65 to –0.70; child food security, –1.81; 95% CI, –3.54 to –0.09).
In addition, low/very low compared with high child food security was associated with higher triglycerides (beta, 8.68; 95% CI, 1.75-15.61), higher fasting plasma glucose (beta, 1.37; 95% CI, 0.08-2.65), and metabolic syndrome composite variable expected log counts (beta, 2.12; 95% CI, 0.02-0.45).
Furthermore, the researchers found statistically significant interactions between each of the two food security measures and receipt of any food assistance in the previous year in models of triglycerides (P for interactions: household food security, .03 and child food security, .005) and HDL-C (P for interactions: household food security, .01 and child food security, .04).
After evaluating the effect of parental place of birth, they found a statistically significant association for triglycerides only (P for interactions: household food security, .05 and child food security, .008).
“Our study is among the first to document adverse associations between household and child food security measures with a metabolic syndrome score variable and several metabolic syndrome–relevant cardiometabolic markers among US Hispanic/Latino youth,” the researchers wrote.
The researchers acknowledged that the cross-sectional nature of the study was a key limitation; thus, causality could not be inferred.
“In the future, we plan to conduct more qualitative work to better understand how Hispanic/Latino families respond to food insecurity, which may identify the factors that shape their response,” study author Sandra S. Albrecht, PhD, of Columbia University, New York, NY, said in an interview.
Recommendations for pediatricians
Food insecurity researcher Yankun Wang, PhD candidate at Indiana University, Bloomington, commented: “I would recommend pediatricians pay more attention to children from low-income households since they are more likely to have mental and physical health issues due to food insecurity.
“It can be very helpful if pediatricians could help families obtain SNAP benefits, enroll youth in the school breakfast and lunch programs, and promote nutrition education in schools,” Mr. Wang added.
This study was supported by grant funding from the National Heart, Lung, and Blood Institute. The authors reported no relevant disclosures.
Severe food insecurity was associated with metabolic syndrome and unfavorable cardiometabolic markers in Hispanic/Latino youth, researchers report.
The findings, published March 16 in Pediatrics, highlight the need to investigate interventions that address food insecurity among Hispanic/Latino youth, a segment of the U.S. population at high risk of cardiometabolic complications.
“Among Hispanic/Latino youth, no study, to our knowledge has evaluated food insecurity’s role in metabolic syndrome and metabolic syndrome–relevant cardiometabolic markers in this population,” lead author Luis E. Maldonado, PhD, of the University of North Carolina at Chapel Hill, and colleagues explained.
The researchers conducted a cross-sectional study to evaluate the associations between lower household and child food security and metabolic syndrome, as well as clinically measured cardiometabolic markers, including fasting plasma glucose, waist circumference, triglycerides, systolic and diastolic blood pressure, and high-density lipoprotein cholesterol (HDL-C).
Household food security (high, marginal, low, very low) and child food security (high, marginal, low/very low) measures were evaluated separately, and were adjusted for participant age, sex, site, parental education, and poverty-income ratio.
Data were obtained from the Hispanic Community Children’s Health Study/Study of Latino Youth, a study of offspring of adults enrolled in the Hispanic Community Health Survey/Study of Latinos.
Results
The study cohort included 1,325 Hispanic/Latino youth aged 8-16 years. For both household food security and child food security, youth in the lowest food security category had significantly lower HDL-C compared with youth with high food security (household food security, –3.17; 95% confidence interval, –5.65 to –0.70; child food security, –1.81; 95% CI, –3.54 to –0.09).
In addition, low/very low compared with high child food security was associated with higher triglycerides (beta, 8.68; 95% CI, 1.75-15.61), higher fasting plasma glucose (beta, 1.37; 95% CI, 0.08-2.65), and metabolic syndrome composite variable expected log counts (beta, 2.12; 95% CI, 0.02-0.45).
Furthermore, the researchers found statistically significant interactions between each of the two food security measures and receipt of any food assistance in the previous year in models of triglycerides (P for interactions: household food security, .03 and child food security, .005) and HDL-C (P for interactions: household food security, .01 and child food security, .04).
After evaluating the effect of parental place of birth, they found a statistically significant association for triglycerides only (P for interactions: household food security, .05 and child food security, .008).
“Our study is among the first to document adverse associations between household and child food security measures with a metabolic syndrome score variable and several metabolic syndrome–relevant cardiometabolic markers among US Hispanic/Latino youth,” the researchers wrote.
The researchers acknowledged that the cross-sectional nature of the study was a key limitation; thus, causality could not be inferred.
“In the future, we plan to conduct more qualitative work to better understand how Hispanic/Latino families respond to food insecurity, which may identify the factors that shape their response,” study author Sandra S. Albrecht, PhD, of Columbia University, New York, NY, said in an interview.
Recommendations for pediatricians
Food insecurity researcher Yankun Wang, PhD candidate at Indiana University, Bloomington, commented: “I would recommend pediatricians pay more attention to children from low-income households since they are more likely to have mental and physical health issues due to food insecurity.
“It can be very helpful if pediatricians could help families obtain SNAP benefits, enroll youth in the school breakfast and lunch programs, and promote nutrition education in schools,” Mr. Wang added.
This study was supported by grant funding from the National Heart, Lung, and Blood Institute. The authors reported no relevant disclosures.
FROM PEDIATRICS
New guidance on palliative care for neurologic disorders
Palliative care includes much more than hospice services, lead author of the new position statement Lynne P. Taylor, MD, University of Washington, Seattle, and a fellow of the AAN, said in a press release.
“Neurologists provide palliative care to people living with life-altering neurologic illnesses not just at the end of life but throughout the course of a disease, improving their lives with symptom control,” Dr. Taylor added.
The position paper, developed by a joint committee of the AAN, American Neurological Association, and Child Neurology Society, was published online March 8 in Neurology.
Guidance across the lifespan
The new paper, an update of previous position statements, includes palliative care guidance for different neurologic disorders across the lifespan. For example, neuropalliative care for neonates deserves “extra consideration,” because one-third of pediatric deaths occur during the neonatal period, most often in the neonatal intensive care unit, and after withdrawal of life-sustaining interventions, the authors note.
For older children, neuropalliative care consultation benefits families trying to maximize the quality of the remainder of their child’s life. Decisionmaking must consider the child’s cognitive abilities, the diagnosis, the perceived level of suffering, parental values, and the family’s understanding of the prognosis, the authors note.
They note that discussions about prognosis are often difficult but critical. Previous research “supports that patients desire prognostic information even when prognosis is uncertain and appreciate when their physicians disclose the presence of that uncertainty,” the authors note.
Also important is engaging in shared decisionmaking with patients and families. “This approach requires the physician to elicit a patient’s goals, make recommendations based on whether medical treatments are likely to achieve those goals, and work with patients and families to finalize a treatment plan,” according to the new guidance.
Ethical considerations
When treatments are physiologically futile, clinicians need to explain why interventions that may cause harm and have no benefit are not offered.
The authors cite cardiopulmonary resuscitation in the setting of cardiac arrest from irreversible herniation as an example of futility in the context of neurologic disease.
When life-prolonging care is no longer an option, clinicians have an obligation to shift the focus of care to preserving quality of life and comfort as much as possible, they add.
Hospices, which provide comfort-focused medical care as well as psychosocial and spiritual support, are reserved for patients believed to be in the last 6 months of their life if their disease follows the expected course.
The investigators also broached ethical considerations for individual neurologic conditions. Concerns for disorders of consciousness include misdiagnosis or inaccurate prognostication, and serial examinations are needed to re-evaluate levels of cognition, psychological state, decisionmaking capacity, and disease trajectory.
In patients with locked-in syndrome, a state of irreversible paralysis, often with respiratory and vocal paralysis, consciousness may range from a chronic minimally conscious state to intact cognition.
Without careful examination, patients with preserved consciousness may be mistaken as having a disorder of consciousness and risk their decisional capacity being ignored, the researchers note.
These patients may need assistance from speech pathologists to identify techniques to enhance communication, such as careful “yes/no” questioning, communication boards, or advanced eye-gaze technology, they add.
Stroke, dementia, Parkinson’s guidance
For stroke, the guidance suggests neurologists encourage patients with retained decisionmaking capacity to complete advance care planning given the risk of recurrent stroke and loss of capacity in the future.
For dementia, a proper and timely diagnosis can help patients and their families prepare for the consequences of cognitive dysfunction and loss of autonomy while respecting their identified values, the authors write.
They note that for Parkinson’s disease, which is marked by slow functional and cognitive decline, neurologists must aim to anticipate and treat symptoms, address psychosocial and spiritual distress and caregiver burden, and engage patients and families in advance care planning before onset of cognitive impairment.
For patients with amyotrophic lateral sclerosis (ALS) and related disorders, clinicians should aim to document goals and treatment preferences prior to extreme weakness and aphonia.
It is also important to anticipate patient preferences for future disability-specific decisions, such as those related to feeding tubes and mechanical ventilation, and to identify the patient’s minimal acceptable outcome from these life-sustaining interventions.
On the topic of withdrawal of treatment, the paper notes that competent patients have the right to refuse life-prolonging therapies, including artificial nutrition, hydration, mechanical ventilation, and antibiotics. If physicians have a moral objection to removing life-support systems, they are obligated to transfer the care of the patient to another physician, the authors add.
Once a decision is made to forgo life-sustaining treatment, physicians should minimize subsequent suffering. The investigators note most symptoms at the end of life can be managed without sedation.
In broaching the “gap” in neurology training programs, the statement referred to a survey of 49 neurology residency programs. Results showed that 42% of respondents reported being dissatisfied with their palliative care education.
Well-timed update
Kate T. Brizzi, MD, a Boston neurologist with experience in hospice and palliative care, said the updated position statement is “well-timed” as neuropalliative care has evolved dramatically over the last decade.
“In the last several years, I’ve witnessed a significant increase in trainee interest in the field, and there is growing recognition of how a palliative care approach can improve patient care and hopefully outcomes,” said Dr. Brizzi.
She praised the authors for doing “an excellent job” in highlighting the ethical challenges facing the neurology provider, particularly as it relates to prognostication in an uncertain setting.
Dr. Brizzi noted communication tools that help facilitate discussions around shared decisionmaking “have enhanced our ability to meet the palliative care needs of our patients and can be incorporated by any provider.”
However, she added that the paper only briefly comments on the role of the neurologist in “lawful physician-hastened death.”
“I anticipate that this will be an area of further discussion in the neurology and palliative care community in the future, as requests for hastened death are frequently encountered from patients with serious neurologic illness,” she said.
Dr. Brizzi also noted the importance of understanding the reasons behind the request – and addressing patient worries related to end-of-life care, which can frequently help alleviate distress.
There was no targeted funding for this paper. Coauthor Salvador Cruz-Flores, MD, department of neurology, Texas Tech University Center, El Paso, reported participation on member adjudication committees for clinical trials for Novo Nordisk, Sunovion, and Galapagos. The remaining authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Palliative care includes much more than hospice services, lead author of the new position statement Lynne P. Taylor, MD, University of Washington, Seattle, and a fellow of the AAN, said in a press release.
“Neurologists provide palliative care to people living with life-altering neurologic illnesses not just at the end of life but throughout the course of a disease, improving their lives with symptom control,” Dr. Taylor added.
The position paper, developed by a joint committee of the AAN, American Neurological Association, and Child Neurology Society, was published online March 8 in Neurology.
Guidance across the lifespan
The new paper, an update of previous position statements, includes palliative care guidance for different neurologic disorders across the lifespan. For example, neuropalliative care for neonates deserves “extra consideration,” because one-third of pediatric deaths occur during the neonatal period, most often in the neonatal intensive care unit, and after withdrawal of life-sustaining interventions, the authors note.
For older children, neuropalliative care consultation benefits families trying to maximize the quality of the remainder of their child’s life. Decisionmaking must consider the child’s cognitive abilities, the diagnosis, the perceived level of suffering, parental values, and the family’s understanding of the prognosis, the authors note.
They note that discussions about prognosis are often difficult but critical. Previous research “supports that patients desire prognostic information even when prognosis is uncertain and appreciate when their physicians disclose the presence of that uncertainty,” the authors note.
Also important is engaging in shared decisionmaking with patients and families. “This approach requires the physician to elicit a patient’s goals, make recommendations based on whether medical treatments are likely to achieve those goals, and work with patients and families to finalize a treatment plan,” according to the new guidance.
Ethical considerations
When treatments are physiologically futile, clinicians need to explain why interventions that may cause harm and have no benefit are not offered.
The authors cite cardiopulmonary resuscitation in the setting of cardiac arrest from irreversible herniation as an example of futility in the context of neurologic disease.
When life-prolonging care is no longer an option, clinicians have an obligation to shift the focus of care to preserving quality of life and comfort as much as possible, they add.
Hospices, which provide comfort-focused medical care as well as psychosocial and spiritual support, are reserved for patients believed to be in the last 6 months of their life if their disease follows the expected course.
The investigators also broached ethical considerations for individual neurologic conditions. Concerns for disorders of consciousness include misdiagnosis or inaccurate prognostication, and serial examinations are needed to re-evaluate levels of cognition, psychological state, decisionmaking capacity, and disease trajectory.
In patients with locked-in syndrome, a state of irreversible paralysis, often with respiratory and vocal paralysis, consciousness may range from a chronic minimally conscious state to intact cognition.
Without careful examination, patients with preserved consciousness may be mistaken as having a disorder of consciousness and risk their decisional capacity being ignored, the researchers note.
These patients may need assistance from speech pathologists to identify techniques to enhance communication, such as careful “yes/no” questioning, communication boards, or advanced eye-gaze technology, they add.
Stroke, dementia, Parkinson’s guidance
For stroke, the guidance suggests neurologists encourage patients with retained decisionmaking capacity to complete advance care planning given the risk of recurrent stroke and loss of capacity in the future.
For dementia, a proper and timely diagnosis can help patients and their families prepare for the consequences of cognitive dysfunction and loss of autonomy while respecting their identified values, the authors write.
They note that for Parkinson’s disease, which is marked by slow functional and cognitive decline, neurologists must aim to anticipate and treat symptoms, address psychosocial and spiritual distress and caregiver burden, and engage patients and families in advance care planning before onset of cognitive impairment.
For patients with amyotrophic lateral sclerosis (ALS) and related disorders, clinicians should aim to document goals and treatment preferences prior to extreme weakness and aphonia.
It is also important to anticipate patient preferences for future disability-specific decisions, such as those related to feeding tubes and mechanical ventilation, and to identify the patient’s minimal acceptable outcome from these life-sustaining interventions.
On the topic of withdrawal of treatment, the paper notes that competent patients have the right to refuse life-prolonging therapies, including artificial nutrition, hydration, mechanical ventilation, and antibiotics. If physicians have a moral objection to removing life-support systems, they are obligated to transfer the care of the patient to another physician, the authors add.
Once a decision is made to forgo life-sustaining treatment, physicians should minimize subsequent suffering. The investigators note most symptoms at the end of life can be managed without sedation.
In broaching the “gap” in neurology training programs, the statement referred to a survey of 49 neurology residency programs. Results showed that 42% of respondents reported being dissatisfied with their palliative care education.
Well-timed update
Kate T. Brizzi, MD, a Boston neurologist with experience in hospice and palliative care, said the updated position statement is “well-timed” as neuropalliative care has evolved dramatically over the last decade.
“In the last several years, I’ve witnessed a significant increase in trainee interest in the field, and there is growing recognition of how a palliative care approach can improve patient care and hopefully outcomes,” said Dr. Brizzi.
She praised the authors for doing “an excellent job” in highlighting the ethical challenges facing the neurology provider, particularly as it relates to prognostication in an uncertain setting.
Dr. Brizzi noted communication tools that help facilitate discussions around shared decisionmaking “have enhanced our ability to meet the palliative care needs of our patients and can be incorporated by any provider.”
However, she added that the paper only briefly comments on the role of the neurologist in “lawful physician-hastened death.”
“I anticipate that this will be an area of further discussion in the neurology and palliative care community in the future, as requests for hastened death are frequently encountered from patients with serious neurologic illness,” she said.
Dr. Brizzi also noted the importance of understanding the reasons behind the request – and addressing patient worries related to end-of-life care, which can frequently help alleviate distress.
There was no targeted funding for this paper. Coauthor Salvador Cruz-Flores, MD, department of neurology, Texas Tech University Center, El Paso, reported participation on member adjudication committees for clinical trials for Novo Nordisk, Sunovion, and Galapagos. The remaining authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Palliative care includes much more than hospice services, lead author of the new position statement Lynne P. Taylor, MD, University of Washington, Seattle, and a fellow of the AAN, said in a press release.
“Neurologists provide palliative care to people living with life-altering neurologic illnesses not just at the end of life but throughout the course of a disease, improving their lives with symptom control,” Dr. Taylor added.
The position paper, developed by a joint committee of the AAN, American Neurological Association, and Child Neurology Society, was published online March 8 in Neurology.
Guidance across the lifespan
The new paper, an update of previous position statements, includes palliative care guidance for different neurologic disorders across the lifespan. For example, neuropalliative care for neonates deserves “extra consideration,” because one-third of pediatric deaths occur during the neonatal period, most often in the neonatal intensive care unit, and after withdrawal of life-sustaining interventions, the authors note.
For older children, neuropalliative care consultation benefits families trying to maximize the quality of the remainder of their child’s life. Decisionmaking must consider the child’s cognitive abilities, the diagnosis, the perceived level of suffering, parental values, and the family’s understanding of the prognosis, the authors note.
They note that discussions about prognosis are often difficult but critical. Previous research “supports that patients desire prognostic information even when prognosis is uncertain and appreciate when their physicians disclose the presence of that uncertainty,” the authors note.
Also important is engaging in shared decisionmaking with patients and families. “This approach requires the physician to elicit a patient’s goals, make recommendations based on whether medical treatments are likely to achieve those goals, and work with patients and families to finalize a treatment plan,” according to the new guidance.
Ethical considerations
When treatments are physiologically futile, clinicians need to explain why interventions that may cause harm and have no benefit are not offered.
The authors cite cardiopulmonary resuscitation in the setting of cardiac arrest from irreversible herniation as an example of futility in the context of neurologic disease.
When life-prolonging care is no longer an option, clinicians have an obligation to shift the focus of care to preserving quality of life and comfort as much as possible, they add.
Hospices, which provide comfort-focused medical care as well as psychosocial and spiritual support, are reserved for patients believed to be in the last 6 months of their life if their disease follows the expected course.
The investigators also broached ethical considerations for individual neurologic conditions. Concerns for disorders of consciousness include misdiagnosis or inaccurate prognostication, and serial examinations are needed to re-evaluate levels of cognition, psychological state, decisionmaking capacity, and disease trajectory.
In patients with locked-in syndrome, a state of irreversible paralysis, often with respiratory and vocal paralysis, consciousness may range from a chronic minimally conscious state to intact cognition.
Without careful examination, patients with preserved consciousness may be mistaken as having a disorder of consciousness and risk their decisional capacity being ignored, the researchers note.
These patients may need assistance from speech pathologists to identify techniques to enhance communication, such as careful “yes/no” questioning, communication boards, or advanced eye-gaze technology, they add.
Stroke, dementia, Parkinson’s guidance
For stroke, the guidance suggests neurologists encourage patients with retained decisionmaking capacity to complete advance care planning given the risk of recurrent stroke and loss of capacity in the future.
For dementia, a proper and timely diagnosis can help patients and their families prepare for the consequences of cognitive dysfunction and loss of autonomy while respecting their identified values, the authors write.
They note that for Parkinson’s disease, which is marked by slow functional and cognitive decline, neurologists must aim to anticipate and treat symptoms, address psychosocial and spiritual distress and caregiver burden, and engage patients and families in advance care planning before onset of cognitive impairment.
For patients with amyotrophic lateral sclerosis (ALS) and related disorders, clinicians should aim to document goals and treatment preferences prior to extreme weakness and aphonia.
It is also important to anticipate patient preferences for future disability-specific decisions, such as those related to feeding tubes and mechanical ventilation, and to identify the patient’s minimal acceptable outcome from these life-sustaining interventions.
On the topic of withdrawal of treatment, the paper notes that competent patients have the right to refuse life-prolonging therapies, including artificial nutrition, hydration, mechanical ventilation, and antibiotics. If physicians have a moral objection to removing life-support systems, they are obligated to transfer the care of the patient to another physician, the authors add.
Once a decision is made to forgo life-sustaining treatment, physicians should minimize subsequent suffering. The investigators note most symptoms at the end of life can be managed without sedation.
In broaching the “gap” in neurology training programs, the statement referred to a survey of 49 neurology residency programs. Results showed that 42% of respondents reported being dissatisfied with their palliative care education.
Well-timed update
Kate T. Brizzi, MD, a Boston neurologist with experience in hospice and palliative care, said the updated position statement is “well-timed” as neuropalliative care has evolved dramatically over the last decade.
“In the last several years, I’ve witnessed a significant increase in trainee interest in the field, and there is growing recognition of how a palliative care approach can improve patient care and hopefully outcomes,” said Dr. Brizzi.
She praised the authors for doing “an excellent job” in highlighting the ethical challenges facing the neurology provider, particularly as it relates to prognostication in an uncertain setting.
Dr. Brizzi noted communication tools that help facilitate discussions around shared decisionmaking “have enhanced our ability to meet the palliative care needs of our patients and can be incorporated by any provider.”
However, she added that the paper only briefly comments on the role of the neurologist in “lawful physician-hastened death.”
“I anticipate that this will be an area of further discussion in the neurology and palliative care community in the future, as requests for hastened death are frequently encountered from patients with serious neurologic illness,” she said.
Dr. Brizzi also noted the importance of understanding the reasons behind the request – and addressing patient worries related to end-of-life care, which can frequently help alleviate distress.
There was no targeted funding for this paper. Coauthor Salvador Cruz-Flores, MD, department of neurology, Texas Tech University Center, El Paso, reported participation on member adjudication committees for clinical trials for Novo Nordisk, Sunovion, and Galapagos. The remaining authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From Neurology
Surveillance program highly predictive for early autism
A population-based developmental surveillance program showed high diagnostic accuracy in identifying autism in a community-based sample of infants, toddlers, and preschoolers, according to new data published online in JAMA Network Open.
Researchers, led by Josephine Barbaro, PhD, of Olga Tennison Autism Research Centre at La Trobe University, Bundoora, Australia, said their findings indicate the benefit of using early autism developmental surveillance from infancy to the preschool period rather than one-time screening.
For the study, maternal and child health nurses in Melbourne were trained to use the Social Attention and Communication Surveillance–Revised (SACS-R) and SACS-Preschool (SACS-PR) tools during well-child checkups at 11-30 months of age and at follow-up (42 months of age). Dr. Barbaro helped develop the SACS tools.
Children identified as being at high likelihood for autism (1-2 years of age: n = 327; 42 months of age: n = 168) and at low likelihood for autism plus concerns (42 months of age: n = 28) were referred by their nurse for diagnostic assessment by the researchers.
Diagnostic accuracy of the SACS-R and SACS-PR was determined by comparing likelihood for autism with children’s diagnostic outcome using clinical judgment based on standard autism assessments.
Researchers included 13,511 children ages 11 months to 42 months. Results indicated the SACS-R with SACS-PR (SACS-R+PR) had very high diagnostic accuracy for early autism detection.
According to the paper, SACS-R showed 83% positive predictive value (95% confidence interval, 0.77-0.87) and 99% estimated negative predictive value (95% CI, 0.01-0.02). Specificity (99.6%; 95% CI, 0.99-1.00) was high, with modest sensitivity (62%; 95% CI, 0.57-0.66). When the SACS-PR 42-month assessment was added, estimated sensitivity grew to 96% (95% CI, 0.94-0.98).
“Its greater accuracy, compared with psychometrics of commonly used autism screening tools when used in community-based samples, suggests that the SACS-R+PR can be used universally for the early identification of autism,” the authors wrote.
According to La Trobe University, the tool is used in 10 other countries around the world – among them China, Singapore, Poland, Japan, New Zealand, Nepal, and Bangladesh.
Early identification is crucial for children on the autism spectrum and their families because it facilitates early diagnosis and can help families get access to supports and services.
About 2% of the world’s population is on the autism spectrum. Some studies report prevalence of 4% or higher, the authors noted.
The authors called attention to a systematic review of universal autism screening in primary care, including the Infant-Toddler Checklist and the Modified Checklist for Autism in Toddlers and various versions. The authors of the review noted that few studies had enough participants to establish population sensitivity, specificity, and positive predictive value. Also, psychometric properties reported were modest and/or wide ranging, putting into question the diagnostic accuracy of the tools.
Dr. Barbaro and colleagues highlighted an advantage the current study offers. “A critical difference in this study was the use of a community-based sample rather than a clinical or high-likelihood sibling sample, which may not be representative of the general population of children on the autism spectrum because child outcomes, cognition, and autism prevalence vary by ascertainment strategy and multiplex or simplex status.”
The authors explained that, in the United States, The U.S. Preventive Services Task Force has said there is not enough evidence to recommend universal autism screening and instead recommends routine general developmental surveillance. The American Academy of Pediatrics recommends developmental surveillance between 9 and 30 months and autism-specific screening at 18 and 24 months because of the benefits of early supports and services.
Karen Pierce, PhD, codirector of the Autism Center of Excellence at University of California, San Diego, said in an interview that she was pleased to see that the researchers were able to identify a high percentage of children on the autism spectrum.
She said, however, that the system proposed in this paper involves a substantial amount of time for training the nurses.
The authors acknowledged that, saying, “there may be instances in which this could be impractical.”
Dr. Pierce said that, in the United States, parent questionnaires are combined with clinical judgment to decide which kids are at risk.
“It doesn’t take very much time to fill out these questionnaires,” she said. “That’s the sticking point. I’m not saying necessarily that it shouldn’t be adopted. It would be very hard, I think, to incorporate into current pediatric practice.”
She said a benefit of the SACS program is more hands-on observation of the child, beyond the parent report, which sometimes can reflect more emotionally how the parent is feeling about the child.
She pointed out it was impressive that the Australian team found virtually no false positives.
The researchers also identified an additional 168 children using the preschool version at 42 months who had actually passed at the earlier checkpoint, using the regular SACS-R.
“This underscores a supercritical point,” Dr. Pierce said. “Just because your child may have gotten screened at 12, 18, 24 months and they pass and everything’s looking great, it doesn’t necessarily mean at some point early in development around age 3 that there [wouldn’t] be some clearer signs of autism.”
She said in her own study, published in JAMA Pediatrics, 24% of their sample tested fine at first but were later identified as having autism.
“It underscores the need for repeat screening,” Dr. Pierce said. “That was a striking finding in this study.”
She also pointed out that the authors talk about the “false dichotomy” between screening and surveillance. “They are saying it doesn’t have to be that way. It can be a combined effort. We can have parents filling out screening tools and we can have more observational sessions with kids during checkups. It doesn’t have to be this rigid line between screening and surveillance. I would completely agree with that.”
Dr. Barbaro reported receiving grants from the Sir Robert Menzies Foundation and the Cooperative Research Centre for Living with Autism (Autism CRC) during the study. Funds are partially distributed to Dr. Barbaro for the background intellectual property. One coauthor reported grants from the Menzies Foundation and Autism CRC during the study. Another coauthor reported receiving salary from Autism CRC during the study. No other disclosures were reported. This work was supported by an Allied Health Sciences start-up grant from the Menzies Foundation and the Cooperative Research Centre for Living with Autism, established and supported under the Australian Government’s Cooperative Research Centres Program. Dr. Pierce reports no relevant financial relationships.
A population-based developmental surveillance program showed high diagnostic accuracy in identifying autism in a community-based sample of infants, toddlers, and preschoolers, according to new data published online in JAMA Network Open.
Researchers, led by Josephine Barbaro, PhD, of Olga Tennison Autism Research Centre at La Trobe University, Bundoora, Australia, said their findings indicate the benefit of using early autism developmental surveillance from infancy to the preschool period rather than one-time screening.
For the study, maternal and child health nurses in Melbourne were trained to use the Social Attention and Communication Surveillance–Revised (SACS-R) and SACS-Preschool (SACS-PR) tools during well-child checkups at 11-30 months of age and at follow-up (42 months of age). Dr. Barbaro helped develop the SACS tools.
Children identified as being at high likelihood for autism (1-2 years of age: n = 327; 42 months of age: n = 168) and at low likelihood for autism plus concerns (42 months of age: n = 28) were referred by their nurse for diagnostic assessment by the researchers.
Diagnostic accuracy of the SACS-R and SACS-PR was determined by comparing likelihood for autism with children’s diagnostic outcome using clinical judgment based on standard autism assessments.
Researchers included 13,511 children ages 11 months to 42 months. Results indicated the SACS-R with SACS-PR (SACS-R+PR) had very high diagnostic accuracy for early autism detection.
According to the paper, SACS-R showed 83% positive predictive value (95% confidence interval, 0.77-0.87) and 99% estimated negative predictive value (95% CI, 0.01-0.02). Specificity (99.6%; 95% CI, 0.99-1.00) was high, with modest sensitivity (62%; 95% CI, 0.57-0.66). When the SACS-PR 42-month assessment was added, estimated sensitivity grew to 96% (95% CI, 0.94-0.98).
“Its greater accuracy, compared with psychometrics of commonly used autism screening tools when used in community-based samples, suggests that the SACS-R+PR can be used universally for the early identification of autism,” the authors wrote.
According to La Trobe University, the tool is used in 10 other countries around the world – among them China, Singapore, Poland, Japan, New Zealand, Nepal, and Bangladesh.
Early identification is crucial for children on the autism spectrum and their families because it facilitates early diagnosis and can help families get access to supports and services.
About 2% of the world’s population is on the autism spectrum. Some studies report prevalence of 4% or higher, the authors noted.
The authors called attention to a systematic review of universal autism screening in primary care, including the Infant-Toddler Checklist and the Modified Checklist for Autism in Toddlers and various versions. The authors of the review noted that few studies had enough participants to establish population sensitivity, specificity, and positive predictive value. Also, psychometric properties reported were modest and/or wide ranging, putting into question the diagnostic accuracy of the tools.
Dr. Barbaro and colleagues highlighted an advantage the current study offers. “A critical difference in this study was the use of a community-based sample rather than a clinical or high-likelihood sibling sample, which may not be representative of the general population of children on the autism spectrum because child outcomes, cognition, and autism prevalence vary by ascertainment strategy and multiplex or simplex status.”
The authors explained that, in the United States, The U.S. Preventive Services Task Force has said there is not enough evidence to recommend universal autism screening and instead recommends routine general developmental surveillance. The American Academy of Pediatrics recommends developmental surveillance between 9 and 30 months and autism-specific screening at 18 and 24 months because of the benefits of early supports and services.
Karen Pierce, PhD, codirector of the Autism Center of Excellence at University of California, San Diego, said in an interview that she was pleased to see that the researchers were able to identify a high percentage of children on the autism spectrum.
She said, however, that the system proposed in this paper involves a substantial amount of time for training the nurses.
The authors acknowledged that, saying, “there may be instances in which this could be impractical.”
Dr. Pierce said that, in the United States, parent questionnaires are combined with clinical judgment to decide which kids are at risk.
“It doesn’t take very much time to fill out these questionnaires,” she said. “That’s the sticking point. I’m not saying necessarily that it shouldn’t be adopted. It would be very hard, I think, to incorporate into current pediatric practice.”
She said a benefit of the SACS program is more hands-on observation of the child, beyond the parent report, which sometimes can reflect more emotionally how the parent is feeling about the child.
She pointed out it was impressive that the Australian team found virtually no false positives.
The researchers also identified an additional 168 children using the preschool version at 42 months who had actually passed at the earlier checkpoint, using the regular SACS-R.
“This underscores a supercritical point,” Dr. Pierce said. “Just because your child may have gotten screened at 12, 18, 24 months and they pass and everything’s looking great, it doesn’t necessarily mean at some point early in development around age 3 that there [wouldn’t] be some clearer signs of autism.”
She said in her own study, published in JAMA Pediatrics, 24% of their sample tested fine at first but were later identified as having autism.
“It underscores the need for repeat screening,” Dr. Pierce said. “That was a striking finding in this study.”
She also pointed out that the authors talk about the “false dichotomy” between screening and surveillance. “They are saying it doesn’t have to be that way. It can be a combined effort. We can have parents filling out screening tools and we can have more observational sessions with kids during checkups. It doesn’t have to be this rigid line between screening and surveillance. I would completely agree with that.”
Dr. Barbaro reported receiving grants from the Sir Robert Menzies Foundation and the Cooperative Research Centre for Living with Autism (Autism CRC) during the study. Funds are partially distributed to Dr. Barbaro for the background intellectual property. One coauthor reported grants from the Menzies Foundation and Autism CRC during the study. Another coauthor reported receiving salary from Autism CRC during the study. No other disclosures were reported. This work was supported by an Allied Health Sciences start-up grant from the Menzies Foundation and the Cooperative Research Centre for Living with Autism, established and supported under the Australian Government’s Cooperative Research Centres Program. Dr. Pierce reports no relevant financial relationships.
A population-based developmental surveillance program showed high diagnostic accuracy in identifying autism in a community-based sample of infants, toddlers, and preschoolers, according to new data published online in JAMA Network Open.
Researchers, led by Josephine Barbaro, PhD, of Olga Tennison Autism Research Centre at La Trobe University, Bundoora, Australia, said their findings indicate the benefit of using early autism developmental surveillance from infancy to the preschool period rather than one-time screening.
For the study, maternal and child health nurses in Melbourne were trained to use the Social Attention and Communication Surveillance–Revised (SACS-R) and SACS-Preschool (SACS-PR) tools during well-child checkups at 11-30 months of age and at follow-up (42 months of age). Dr. Barbaro helped develop the SACS tools.
Children identified as being at high likelihood for autism (1-2 years of age: n = 327; 42 months of age: n = 168) and at low likelihood for autism plus concerns (42 months of age: n = 28) were referred by their nurse for diagnostic assessment by the researchers.
Diagnostic accuracy of the SACS-R and SACS-PR was determined by comparing likelihood for autism with children’s diagnostic outcome using clinical judgment based on standard autism assessments.
Researchers included 13,511 children ages 11 months to 42 months. Results indicated the SACS-R with SACS-PR (SACS-R+PR) had very high diagnostic accuracy for early autism detection.
According to the paper, SACS-R showed 83% positive predictive value (95% confidence interval, 0.77-0.87) and 99% estimated negative predictive value (95% CI, 0.01-0.02). Specificity (99.6%; 95% CI, 0.99-1.00) was high, with modest sensitivity (62%; 95% CI, 0.57-0.66). When the SACS-PR 42-month assessment was added, estimated sensitivity grew to 96% (95% CI, 0.94-0.98).
“Its greater accuracy, compared with psychometrics of commonly used autism screening tools when used in community-based samples, suggests that the SACS-R+PR can be used universally for the early identification of autism,” the authors wrote.
According to La Trobe University, the tool is used in 10 other countries around the world – among them China, Singapore, Poland, Japan, New Zealand, Nepal, and Bangladesh.
Early identification is crucial for children on the autism spectrum and their families because it facilitates early diagnosis and can help families get access to supports and services.
About 2% of the world’s population is on the autism spectrum. Some studies report prevalence of 4% or higher, the authors noted.
The authors called attention to a systematic review of universal autism screening in primary care, including the Infant-Toddler Checklist and the Modified Checklist for Autism in Toddlers and various versions. The authors of the review noted that few studies had enough participants to establish population sensitivity, specificity, and positive predictive value. Also, psychometric properties reported were modest and/or wide ranging, putting into question the diagnostic accuracy of the tools.
Dr. Barbaro and colleagues highlighted an advantage the current study offers. “A critical difference in this study was the use of a community-based sample rather than a clinical or high-likelihood sibling sample, which may not be representative of the general population of children on the autism spectrum because child outcomes, cognition, and autism prevalence vary by ascertainment strategy and multiplex or simplex status.”
The authors explained that, in the United States, The U.S. Preventive Services Task Force has said there is not enough evidence to recommend universal autism screening and instead recommends routine general developmental surveillance. The American Academy of Pediatrics recommends developmental surveillance between 9 and 30 months and autism-specific screening at 18 and 24 months because of the benefits of early supports and services.
Karen Pierce, PhD, codirector of the Autism Center of Excellence at University of California, San Diego, said in an interview that she was pleased to see that the researchers were able to identify a high percentage of children on the autism spectrum.
She said, however, that the system proposed in this paper involves a substantial amount of time for training the nurses.
The authors acknowledged that, saying, “there may be instances in which this could be impractical.”
Dr. Pierce said that, in the United States, parent questionnaires are combined with clinical judgment to decide which kids are at risk.
“It doesn’t take very much time to fill out these questionnaires,” she said. “That’s the sticking point. I’m not saying necessarily that it shouldn’t be adopted. It would be very hard, I think, to incorporate into current pediatric practice.”
She said a benefit of the SACS program is more hands-on observation of the child, beyond the parent report, which sometimes can reflect more emotionally how the parent is feeling about the child.
She pointed out it was impressive that the Australian team found virtually no false positives.
The researchers also identified an additional 168 children using the preschool version at 42 months who had actually passed at the earlier checkpoint, using the regular SACS-R.
“This underscores a supercritical point,” Dr. Pierce said. “Just because your child may have gotten screened at 12, 18, 24 months and they pass and everything’s looking great, it doesn’t necessarily mean at some point early in development around age 3 that there [wouldn’t] be some clearer signs of autism.”
She said in her own study, published in JAMA Pediatrics, 24% of their sample tested fine at first but were later identified as having autism.
“It underscores the need for repeat screening,” Dr. Pierce said. “That was a striking finding in this study.”
She also pointed out that the authors talk about the “false dichotomy” between screening and surveillance. “They are saying it doesn’t have to be that way. It can be a combined effort. We can have parents filling out screening tools and we can have more observational sessions with kids during checkups. It doesn’t have to be this rigid line between screening and surveillance. I would completely agree with that.”
Dr. Barbaro reported receiving grants from the Sir Robert Menzies Foundation and the Cooperative Research Centre for Living with Autism (Autism CRC) during the study. Funds are partially distributed to Dr. Barbaro for the background intellectual property. One coauthor reported grants from the Menzies Foundation and Autism CRC during the study. Another coauthor reported receiving salary from Autism CRC during the study. No other disclosures were reported. This work was supported by an Allied Health Sciences start-up grant from the Menzies Foundation and the Cooperative Research Centre for Living with Autism, established and supported under the Australian Government’s Cooperative Research Centres Program. Dr. Pierce reports no relevant financial relationships.
FROM JAMA NETWORK OPEN
Selling your practice
My previous column on practice valuation prompted a number of questions on the mechanics of selling a private practice. As usual, I cannot hope to cover this complex topic comprehensively in only 750 words, but here are the basics.
A generation ago, the sale of a medical practice was much like the sale of any other business: A retiring physician would sell his or her practice to a young doctor and the practice would continue on as before. Occasionally, that still happens, but changes in the business of medicine – most significantly the growth of managed care – have had a big impact on the way medical practices are bought and sold.
For one thing, there are far fewer solo practitioners these days, and polls indicate that most young physicians intend to continue that trend. .
For another, because the rules governing such sales have become so numbingly complex, the services of expert (and expensive) third parties are essential.
While these issues may complicate matters, there is still a market for the sale of medical practices. However, you must do everything possible to ensure you identify the best possible buyer and structure the best deal.
The first hurdle is the accurate valuation of your practice, which was covered in some detail in my last column. Briefly, for the protection of both parties, it is important that the appraisal be done by an experienced and neutral financial consultant, that all techniques used in the valuation be divulged and explained, and that documentation be supplied to support the conclusions reached.
Keep in mind that the valuation will not necessarily equal the purchase price; other factors may need to be considered before a final price can be agreed upon. Keep in mind, too, that there may be legal constraints on the purchase price. For example, if the buyer is a nonprofit corporation such as a hospital or HMO, by law it cannot pay in excess of fair market value for the practice – which may rule out any valuation of “good will.” In some states, the purchase of private practices by hospitals is prohibited altogether – so you might need to consider a long-term lease rather than a sale.
Once a value has been agreed upon, you must consider how the transaction will be structured. The most popular structures include purchase of assets, purchase of corporate stock, and merger.
Many buyers prefer to purchase assets, because it allows them to pick and choose only those items that have value to them. This can leave you with a bunch of “odd lot” assets to dispose of. But depending on the circumstances, an asset sale may still be to your advantage.
Sellers typically prefer to sell stock, because it allows them to sell their entire practice, which is often worth more than the sum of its parts, and often provides tax advantages.
The third option, merger, continues to grow in popularity and is a column subject in itself, and I will address it separately next month.
Tax issues must always be considered. Most private practices are corporations, and the sale of corporate stock will result in a long-term capital gain that will be taxed – currently at 15%-20%. As the saying goes, it’s not what you earn, it’s what you keep. So it may benefit you to accept a slightly lower price if the sale can be structured to provide significantly lower tax treatment. However, any gain that does not qualify as a long-term capital gain will be taxed as regular income – currently in the 32%-37% percent range – plus a Social Security tax of about 15%.
Payment in installments is a popular way to defer taxes, since they are incurred on each installment as it is paid; but such payments may be mistaken by the IRS for payments for referrals, which is illegal. And there is always the problem of making certain all payments are eventually made.
You may wish to continue working at the practice as an employee for an agreed-upon period of time, and this is often to the buyer’s advantage as well. Transitioning to new ownership in stages often maximizes the value of the business by improving patient retention, and allows patients to become accustomed to the transition. However, care must be taken, with the aid of good legal advice, to structure such an arrangement in a way that minimizes concerns of fraud and abuse.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
My previous column on practice valuation prompted a number of questions on the mechanics of selling a private practice. As usual, I cannot hope to cover this complex topic comprehensively in only 750 words, but here are the basics.
A generation ago, the sale of a medical practice was much like the sale of any other business: A retiring physician would sell his or her practice to a young doctor and the practice would continue on as before. Occasionally, that still happens, but changes in the business of medicine – most significantly the growth of managed care – have had a big impact on the way medical practices are bought and sold.
For one thing, there are far fewer solo practitioners these days, and polls indicate that most young physicians intend to continue that trend. .
For another, because the rules governing such sales have become so numbingly complex, the services of expert (and expensive) third parties are essential.
While these issues may complicate matters, there is still a market for the sale of medical practices. However, you must do everything possible to ensure you identify the best possible buyer and structure the best deal.
The first hurdle is the accurate valuation of your practice, which was covered in some detail in my last column. Briefly, for the protection of both parties, it is important that the appraisal be done by an experienced and neutral financial consultant, that all techniques used in the valuation be divulged and explained, and that documentation be supplied to support the conclusions reached.
Keep in mind that the valuation will not necessarily equal the purchase price; other factors may need to be considered before a final price can be agreed upon. Keep in mind, too, that there may be legal constraints on the purchase price. For example, if the buyer is a nonprofit corporation such as a hospital or HMO, by law it cannot pay in excess of fair market value for the practice – which may rule out any valuation of “good will.” In some states, the purchase of private practices by hospitals is prohibited altogether – so you might need to consider a long-term lease rather than a sale.
Once a value has been agreed upon, you must consider how the transaction will be structured. The most popular structures include purchase of assets, purchase of corporate stock, and merger.
Many buyers prefer to purchase assets, because it allows them to pick and choose only those items that have value to them. This can leave you with a bunch of “odd lot” assets to dispose of. But depending on the circumstances, an asset sale may still be to your advantage.
Sellers typically prefer to sell stock, because it allows them to sell their entire practice, which is often worth more than the sum of its parts, and often provides tax advantages.
The third option, merger, continues to grow in popularity and is a column subject in itself, and I will address it separately next month.
Tax issues must always be considered. Most private practices are corporations, and the sale of corporate stock will result in a long-term capital gain that will be taxed – currently at 15%-20%. As the saying goes, it’s not what you earn, it’s what you keep. So it may benefit you to accept a slightly lower price if the sale can be structured to provide significantly lower tax treatment. However, any gain that does not qualify as a long-term capital gain will be taxed as regular income – currently in the 32%-37% percent range – plus a Social Security tax of about 15%.
Payment in installments is a popular way to defer taxes, since they are incurred on each installment as it is paid; but such payments may be mistaken by the IRS for payments for referrals, which is illegal. And there is always the problem of making certain all payments are eventually made.
You may wish to continue working at the practice as an employee for an agreed-upon period of time, and this is often to the buyer’s advantage as well. Transitioning to new ownership in stages often maximizes the value of the business by improving patient retention, and allows patients to become accustomed to the transition. However, care must be taken, with the aid of good legal advice, to structure such an arrangement in a way that minimizes concerns of fraud and abuse.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
My previous column on practice valuation prompted a number of questions on the mechanics of selling a private practice. As usual, I cannot hope to cover this complex topic comprehensively in only 750 words, but here are the basics.
A generation ago, the sale of a medical practice was much like the sale of any other business: A retiring physician would sell his or her practice to a young doctor and the practice would continue on as before. Occasionally, that still happens, but changes in the business of medicine – most significantly the growth of managed care – have had a big impact on the way medical practices are bought and sold.
For one thing, there are far fewer solo practitioners these days, and polls indicate that most young physicians intend to continue that trend. .
For another, because the rules governing such sales have become so numbingly complex, the services of expert (and expensive) third parties are essential.
While these issues may complicate matters, there is still a market for the sale of medical practices. However, you must do everything possible to ensure you identify the best possible buyer and structure the best deal.
The first hurdle is the accurate valuation of your practice, which was covered in some detail in my last column. Briefly, for the protection of both parties, it is important that the appraisal be done by an experienced and neutral financial consultant, that all techniques used in the valuation be divulged and explained, and that documentation be supplied to support the conclusions reached.
Keep in mind that the valuation will not necessarily equal the purchase price; other factors may need to be considered before a final price can be agreed upon. Keep in mind, too, that there may be legal constraints on the purchase price. For example, if the buyer is a nonprofit corporation such as a hospital or HMO, by law it cannot pay in excess of fair market value for the practice – which may rule out any valuation of “good will.” In some states, the purchase of private practices by hospitals is prohibited altogether – so you might need to consider a long-term lease rather than a sale.
Once a value has been agreed upon, you must consider how the transaction will be structured. The most popular structures include purchase of assets, purchase of corporate stock, and merger.
Many buyers prefer to purchase assets, because it allows them to pick and choose only those items that have value to them. This can leave you with a bunch of “odd lot” assets to dispose of. But depending on the circumstances, an asset sale may still be to your advantage.
Sellers typically prefer to sell stock, because it allows them to sell their entire practice, which is often worth more than the sum of its parts, and often provides tax advantages.
The third option, merger, continues to grow in popularity and is a column subject in itself, and I will address it separately next month.
Tax issues must always be considered. Most private practices are corporations, and the sale of corporate stock will result in a long-term capital gain that will be taxed – currently at 15%-20%. As the saying goes, it’s not what you earn, it’s what you keep. So it may benefit you to accept a slightly lower price if the sale can be structured to provide significantly lower tax treatment. However, any gain that does not qualify as a long-term capital gain will be taxed as regular income – currently in the 32%-37% percent range – plus a Social Security tax of about 15%.
Payment in installments is a popular way to defer taxes, since they are incurred on each installment as it is paid; but such payments may be mistaken by the IRS for payments for referrals, which is illegal. And there is always the problem of making certain all payments are eventually made.
You may wish to continue working at the practice as an employee for an agreed-upon period of time, and this is often to the buyer’s advantage as well. Transitioning to new ownership in stages often maximizes the value of the business by improving patient retention, and allows patients to become accustomed to the transition. However, care must be taken, with the aid of good legal advice, to structure such an arrangement in a way that minimizes concerns of fraud and abuse.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Children and COVID: Decline in new cases reaches 7th week
New cases of COVID-19 in U.S. children have fallen to their lowest level since the beginning of the Delta surge in July of 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
. Over those 7 weeks, new cases dropped over 96% from the 1.15 million reported for Jan. 14-20, based on data collected by the AAP and CHA from state and territorial health departments.
The last time that the weekly count was below 42,000 was July 16-22, 2021, when almost 39,000 cases were reported in the midst of the Delta upsurge. That was shortly after cases had reached their lowest point, 8,447, since the early stages of the pandemic in 2020, the AAP/CHA data show.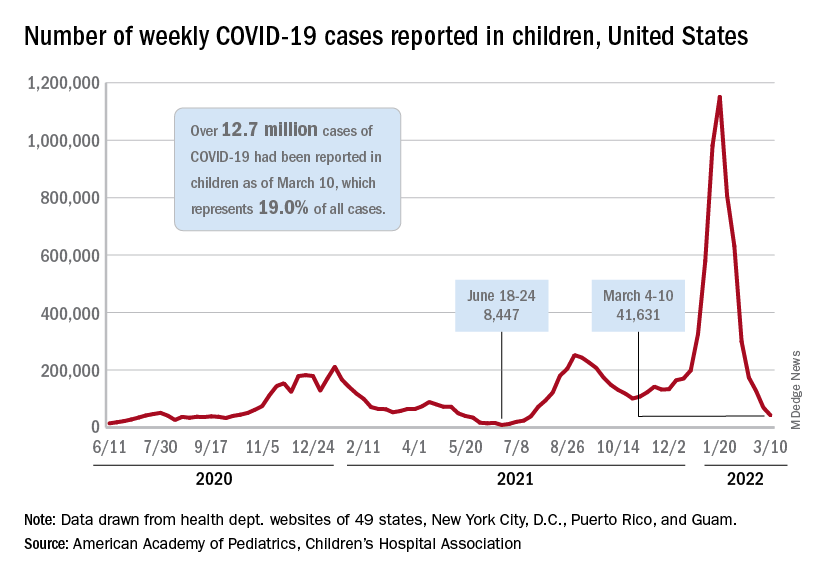
The cumulative number of pediatric cases is now up to 12.7 million, while the overall proportion of cases occurring in children held steady at 19.0% for the 4th week in a row, the AAP and CHA said in their weekly COVID-19 report. The Centers for Disease Control and Prevention, using an age range of 0-18 versus the states’ variety of ages, puts total cases at 11.7 million and deaths at 1,656 as of March 14.
Data from the CDC’s COVID-19–Associated Hospitalization Surveillance Network show that hospitalizations with laboratory-confirmed infection were down by 50% in children aged 0-4 years, by 63% among 5- to 11-year-olds, and by 58% in those aged 12-17 years for the week of Feb. 27 to March 5, compared with the week before.
The pace of vaccination continues to follow a similar trend, as the declines seen through February have continued into March. Cumulatively, 33.7% of children aged 5-11 have received at least one dose, and 26.8% are fully vaccinated, with corresponding numbers of 68.0% and 58.0% for children aged 12-17, the CDC reported on its COVID Data Tracker.
State-level data show that children aged 5-11 in Vermont, with a rate of 65%, are the most likely to have received at least one dose of COVID vaccine, while just 15% of 5- to 11-year-olds in Alabama, Louisiana, and Mississippi have gotten their first dose. Among children aged 12-17, that rate ranges from 40% in Wyoming to 94% in Hawaii, Massachusetts, and Rhode Island, the AAP said in a separate report based on CDC data.
In a recent report involving 1,364 children aged 5-15 years, two doses of the COVID-19 vaccine reduced the risk of infection from the Omicron variant by 31% in children aged 5-11 years and by 59% among children aged 12-15 years, said Ashley L. Fowlkes, ScD, of the CDC’s COVID-19 Emergency Response Team, and associates (MMWR 2022 Mar 11;71).
New cases of COVID-19 in U.S. children have fallen to their lowest level since the beginning of the Delta surge in July of 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
. Over those 7 weeks, new cases dropped over 96% from the 1.15 million reported for Jan. 14-20, based on data collected by the AAP and CHA from state and territorial health departments.
The last time that the weekly count was below 42,000 was July 16-22, 2021, when almost 39,000 cases were reported in the midst of the Delta upsurge. That was shortly after cases had reached their lowest point, 8,447, since the early stages of the pandemic in 2020, the AAP/CHA data show.
The cumulative number of pediatric cases is now up to 12.7 million, while the overall proportion of cases occurring in children held steady at 19.0% for the 4th week in a row, the AAP and CHA said in their weekly COVID-19 report. The Centers for Disease Control and Prevention, using an age range of 0-18 versus the states’ variety of ages, puts total cases at 11.7 million and deaths at 1,656 as of March 14.
Data from the CDC’s COVID-19–Associated Hospitalization Surveillance Network show that hospitalizations with laboratory-confirmed infection were down by 50% in children aged 0-4 years, by 63% among 5- to 11-year-olds, and by 58% in those aged 12-17 years for the week of Feb. 27 to March 5, compared with the week before.
The pace of vaccination continues to follow a similar trend, as the declines seen through February have continued into March. Cumulatively, 33.7% of children aged 5-11 have received at least one dose, and 26.8% are fully vaccinated, with corresponding numbers of 68.0% and 58.0% for children aged 12-17, the CDC reported on its COVID Data Tracker.
State-level data show that children aged 5-11 in Vermont, with a rate of 65%, are the most likely to have received at least one dose of COVID vaccine, while just 15% of 5- to 11-year-olds in Alabama, Louisiana, and Mississippi have gotten their first dose. Among children aged 12-17, that rate ranges from 40% in Wyoming to 94% in Hawaii, Massachusetts, and Rhode Island, the AAP said in a separate report based on CDC data.
In a recent report involving 1,364 children aged 5-15 years, two doses of the COVID-19 vaccine reduced the risk of infection from the Omicron variant by 31% in children aged 5-11 years and by 59% among children aged 12-15 years, said Ashley L. Fowlkes, ScD, of the CDC’s COVID-19 Emergency Response Team, and associates (MMWR 2022 Mar 11;71).
New cases of COVID-19 in U.S. children have fallen to their lowest level since the beginning of the Delta surge in July of 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
. Over those 7 weeks, new cases dropped over 96% from the 1.15 million reported for Jan. 14-20, based on data collected by the AAP and CHA from state and territorial health departments.
The last time that the weekly count was below 42,000 was July 16-22, 2021, when almost 39,000 cases were reported in the midst of the Delta upsurge. That was shortly after cases had reached their lowest point, 8,447, since the early stages of the pandemic in 2020, the AAP/CHA data show.
The cumulative number of pediatric cases is now up to 12.7 million, while the overall proportion of cases occurring in children held steady at 19.0% for the 4th week in a row, the AAP and CHA said in their weekly COVID-19 report. The Centers for Disease Control and Prevention, using an age range of 0-18 versus the states’ variety of ages, puts total cases at 11.7 million and deaths at 1,656 as of March 14.
Data from the CDC’s COVID-19–Associated Hospitalization Surveillance Network show that hospitalizations with laboratory-confirmed infection were down by 50% in children aged 0-4 years, by 63% among 5- to 11-year-olds, and by 58% in those aged 12-17 years for the week of Feb. 27 to March 5, compared with the week before.
The pace of vaccination continues to follow a similar trend, as the declines seen through February have continued into March. Cumulatively, 33.7% of children aged 5-11 have received at least one dose, and 26.8% are fully vaccinated, with corresponding numbers of 68.0% and 58.0% for children aged 12-17, the CDC reported on its COVID Data Tracker.
State-level data show that children aged 5-11 in Vermont, with a rate of 65%, are the most likely to have received at least one dose of COVID vaccine, while just 15% of 5- to 11-year-olds in Alabama, Louisiana, and Mississippi have gotten their first dose. Among children aged 12-17, that rate ranges from 40% in Wyoming to 94% in Hawaii, Massachusetts, and Rhode Island, the AAP said in a separate report based on CDC data.
In a recent report involving 1,364 children aged 5-15 years, two doses of the COVID-19 vaccine reduced the risk of infection from the Omicron variant by 31% in children aged 5-11 years and by 59% among children aged 12-15 years, said Ashley L. Fowlkes, ScD, of the CDC’s COVID-19 Emergency Response Team, and associates (MMWR 2022 Mar 11;71).
New JIA guidelines emphasize earlier DMARD use
Treatment of systemic juvenile idiopathic arthritis (sJIA) should emphasize early use of conventional synthetic and biologic disease-modifying antirheumatic drugs (DMARDs), compared with the previous reliance on NSAIDs and glucocorticoids, according to new guidelines from the American College of Rheumatology. The recently published 2021 guidelines focus on therapeutic approaches for oligoarthritis, temporomandibular joint (TMJ) arthritis, and sJIA.
“Systemic JIA should be treated early with biologics to rapidly bring disease under control and to avoid long-term use of glucocorticoids,” Karen Onel, MD, chief of the division of pediatric rheumatology at Weill Cornell Medicine, New York, and lead author of the guidelines, told this news organization. “Unfortunately, biologics can and are frequently denied for first-line use. For this reason, the guidelines are critically important as they demonstrate that first-line use of biologics are standard of care for the treatment of sJIA.”
The new publication is the second part of the ACR’s process to update JIA guidelines that began in 2017 and complements the release in 2019 of guidelines on the management of nonsystemic polyarthritis, sacroiliitis, and enthesitis, as well as a separate guidance on JIA-associated uveitis. The new guidelines include a second publication focused on nonpharmacologic therapies, medication monitoring, immunizations, and imaging. Both sets of guidelines grew out of a 15-member panel that included young adults with JIA and caregivers of children with JIA, and which required at least 70% agreement on recommendations.
“Though the scope of the two guidelines differed, one thing they had in common is the recognition of the importance of shared decision-making with the patient/caregiver,” Dr. Onel said. “Not every decision will be appropriate for every patient, which is why it was so instrumental to receive input from both patients and caregivers when creating these recommendations.”
Oligoarticular and TMJ arthritis
Oligoarticular and TMJ arthritis have similar recommendations, beginning with NSAIDs conditionally recommended and intra-articular glucocorticoids (IAGCs) strongly recommended as part of initial therapy. For oligoarticular arthritis, the guidelines specifically include a strong recommendation of triamcinolone hexacetonide as the preferred agent; no preferred agent is recommended for TMJ arthritis.
“The one thing that the panel was unanimous about was the use of triamcinolone hexacetonide for intra-articular steroid injections in oligoarticular kids,” Susan Shenoi, MBBS, MS, an associate professor and clinical director of pediatric rheumatology at Seattle Children’s Hospital and Research Center, said in an interview. “Triamcinolone hexacetonide has not been available recently, and through advocacy efforts, there is now a pathway to get that medication,” added Dr. Shenoi, a coauthor on the guidelines.
Dr. Onel said that “triamcinolone hexacetonide has been shown to be superior to alternative injectable glucocorticoids in achieving and maintaining remission in children with JIA,” but its unavailability meant physicians had to consider less effective, more potent, or more costly alternatives.” To address the shortage, “the FDA allowed the importation of one particular formulation of triamcinolone hexacetonide [Hexatrione 2%] specifically for joint injections in patients with JIA.”
The guidelines conditionally recommend against oral glucocorticoids for initial therapy for both oligoarticular and TMJ arthritis. In fact, throughout the guidelines it’s clear that the authors emphasize using steroids as little as possible, Dr. Shenoi said.
“Steroids are great anti-inflammatories, but in kids we worry about the long-term effects on growth and metabolism, and now we have many more DMARDs available,” Dr. Shenoi said.
The guidelines strongly recommend conventional synthetic DMARDs for patients with either of these diseases who cannot tolerate or do not respond to NSAIDs or IAGCs, with methotrexate conditionally recommended over leflunomide (Arava) for TMJ and over leflunomide, sulfasalazine (Azulfidine, Sulfazine), and hydroxychloroquine, respectively, for oligoarticular arthritis.
“NSAIDs remain widely used despite evidence supporting early use of DMARDs,” Dr. Onel said. “NSAIDs are readily available and familiar; however, they will not prevent disease progression. These guidelines should encourage short courses of NSAIDs only.”
If patients do not respond to or cannot tolerate NSAIDs, IAGCs, and at least one conventional DMARD, the guidelines strongly recommend a biologic DMARD for oligoarticular arthritis and conditionally recommend one for TMJ arthritis, without any preferences to the specific agent.
The guidelines also advise using validated disease activity measures to guide treatment decisions.
“The most important thing when you’re looking at these patients is to determine, do they have active disease or not?” Dr. Shenoi said. “If they have active disease, then you really want to step up therapy.” Using the relatively new concept of treat-to-target, Dr. Shenoi added that a crucial part of shared decision-making with the family is identifying the most appropriate target for that family “and then really trying hard to achieve that target.”
The guidelines also list risk factors for poor outcome that can be used to guide treatment decisions.
“Specific involvement of key joints, such as TMJ, wrist, sacroiliac, hip, and ankle, and other features were considered reasonable justification for early escalation of therapy,” Dr. Onel said. Other features included presence of erosive disease or enthesitis, delay in diagnosis, elevated levels of inflammation markers, and symmetric disease. “Moving quickly may be needed for a patient who is rapidly worsening, while moving slower may be appropriate for somebody who has improved substantially, but not fully.”
Systemic JIA with and without macrophage activation syndrome
For systemic JIA without macrophage activation syndrome (MAS), the guidelines similarly advise against oral glucocorticoids as initial monotherapy while conditionally recommending NSAIDs for initial monotherapy. Where the guidelines differ most from those for oligoarticular and TMJ arthritis is in progression of DMARD use, with a strong recommendation against conventional synthetic DMARDs as an initial monotherapy and interleukin-1 and IL-6 inhibitors conditionally recommended for initial monotherapy.
For patients who don’t adequately respond to NSAIDs or glucocorticoids, IL-1 and IL-6 inhibitors are strongly recommended over a single or combination of conventional DMARDs. Residual arthritis or an incomplete response to IL-1 or IL-6 inhibitors should lead next to biologic or conventional DMARDs instead of long-term glucocorticoids.
For patients with MAS, the guidelines conditionally recommend IL-1 and IL-6 inhibitors over calcineurin inhibitor monotherapy to reach inactive disease and MAS resolution, with glucocorticoids conditionally recommended in initial treatment. Again, however, for patients with incomplete responses to IL-1 or IL-6 inhibitors or with residual arthritis, the guidelines advise biologic or conventional DMARDs over long-term glucocorticoids.
In patients with sJIA with or without a history of MAS who have inactive disease, practitioners should taper and discontinue glucocorticoids (a strong recommendation). A conditional recommendation for tapering and discontinuing biologic DMARDs follows attainment of inactive disease.
Beyond pharmacology
Although many of the nonpharmacologic recommendations did not have strong evidence based on assessment with Grading of Recommendations Assessment, Development, and Evaluation methodology, consensus was more often the case than not, Dr. Onel said, such as with vaccination.
“There was strong support for the use of immunizations in children with JIA and specific guidance for children with JIA receiving immunosuppression, not on immunosuppression, and children who are underimmunized or unimmunized,” she said. “Although the supportive evidence was very low as per GRADE, panel members were strongly in favor [of immunizations], given risk of infection for immunosuppressed children as well as the preponderance of evidence in similar disease states, such as IBD [inflammatory bowel disease].”
An area with less consensus was whether to check antibody titers for vaccine-preventable childhood infections before beginning immunosuppressive medication, but more panelists opposed the practice than supported it, Dr. Onel said.
“Some panelists felt that the information might be useful for risk management in case of an outbreak or exposure,” she said. “Most believed that screening a fully immunized child was of low benefit and might delay treatment and incur unnecessary cost.”
The process of developing the documents also reveals where the biggest gaps are in research.
“One of the things that we should strive for in the future is really to do more systematic studies so we have better quality of evidence going forward,” Dr. Shenoi said. Overall, however, the guidelines also reveal the progress made in treatment of JIA.
“We now know some of the key cytokines that are involved in the disease pathogenesis, and we have effective therapies for some of these pathways,” Dr. Shenoi said. “We used to use a lot more toxic medication for systemic JIA, and in past decades, these patients used to be on steroids forever. Now we have targeted therapies, and we have some patients who don’t ever need steroids because people are moving toward targeted therapies and having good results. That’s a huge step forward in the field.”
The research was funded by the ACR. Dr. Shenoi has been a consultant for Pfizer. Dr. Onel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment of systemic juvenile idiopathic arthritis (sJIA) should emphasize early use of conventional synthetic and biologic disease-modifying antirheumatic drugs (DMARDs), compared with the previous reliance on NSAIDs and glucocorticoids, according to new guidelines from the American College of Rheumatology. The recently published 2021 guidelines focus on therapeutic approaches for oligoarthritis, temporomandibular joint (TMJ) arthritis, and sJIA.
“Systemic JIA should be treated early with biologics to rapidly bring disease under control and to avoid long-term use of glucocorticoids,” Karen Onel, MD, chief of the division of pediatric rheumatology at Weill Cornell Medicine, New York, and lead author of the guidelines, told this news organization. “Unfortunately, biologics can and are frequently denied for first-line use. For this reason, the guidelines are critically important as they demonstrate that first-line use of biologics are standard of care for the treatment of sJIA.”
The new publication is the second part of the ACR’s process to update JIA guidelines that began in 2017 and complements the release in 2019 of guidelines on the management of nonsystemic polyarthritis, sacroiliitis, and enthesitis, as well as a separate guidance on JIA-associated uveitis. The new guidelines include a second publication focused on nonpharmacologic therapies, medication monitoring, immunizations, and imaging. Both sets of guidelines grew out of a 15-member panel that included young adults with JIA and caregivers of children with JIA, and which required at least 70% agreement on recommendations.
“Though the scope of the two guidelines differed, one thing they had in common is the recognition of the importance of shared decision-making with the patient/caregiver,” Dr. Onel said. “Not every decision will be appropriate for every patient, which is why it was so instrumental to receive input from both patients and caregivers when creating these recommendations.”
Oligoarticular and TMJ arthritis
Oligoarticular and TMJ arthritis have similar recommendations, beginning with NSAIDs conditionally recommended and intra-articular glucocorticoids (IAGCs) strongly recommended as part of initial therapy. For oligoarticular arthritis, the guidelines specifically include a strong recommendation of triamcinolone hexacetonide as the preferred agent; no preferred agent is recommended for TMJ arthritis.
“The one thing that the panel was unanimous about was the use of triamcinolone hexacetonide for intra-articular steroid injections in oligoarticular kids,” Susan Shenoi, MBBS, MS, an associate professor and clinical director of pediatric rheumatology at Seattle Children’s Hospital and Research Center, said in an interview. “Triamcinolone hexacetonide has not been available recently, and through advocacy efforts, there is now a pathway to get that medication,” added Dr. Shenoi, a coauthor on the guidelines.
Dr. Onel said that “triamcinolone hexacetonide has been shown to be superior to alternative injectable glucocorticoids in achieving and maintaining remission in children with JIA,” but its unavailability meant physicians had to consider less effective, more potent, or more costly alternatives.” To address the shortage, “the FDA allowed the importation of one particular formulation of triamcinolone hexacetonide [Hexatrione 2%] specifically for joint injections in patients with JIA.”
The guidelines conditionally recommend against oral glucocorticoids for initial therapy for both oligoarticular and TMJ arthritis. In fact, throughout the guidelines it’s clear that the authors emphasize using steroids as little as possible, Dr. Shenoi said.
“Steroids are great anti-inflammatories, but in kids we worry about the long-term effects on growth and metabolism, and now we have many more DMARDs available,” Dr. Shenoi said.
The guidelines strongly recommend conventional synthetic DMARDs for patients with either of these diseases who cannot tolerate or do not respond to NSAIDs or IAGCs, with methotrexate conditionally recommended over leflunomide (Arava) for TMJ and over leflunomide, sulfasalazine (Azulfidine, Sulfazine), and hydroxychloroquine, respectively, for oligoarticular arthritis.
“NSAIDs remain widely used despite evidence supporting early use of DMARDs,” Dr. Onel said. “NSAIDs are readily available and familiar; however, they will not prevent disease progression. These guidelines should encourage short courses of NSAIDs only.”
If patients do not respond to or cannot tolerate NSAIDs, IAGCs, and at least one conventional DMARD, the guidelines strongly recommend a biologic DMARD for oligoarticular arthritis and conditionally recommend one for TMJ arthritis, without any preferences to the specific agent.
The guidelines also advise using validated disease activity measures to guide treatment decisions.
“The most important thing when you’re looking at these patients is to determine, do they have active disease or not?” Dr. Shenoi said. “If they have active disease, then you really want to step up therapy.” Using the relatively new concept of treat-to-target, Dr. Shenoi added that a crucial part of shared decision-making with the family is identifying the most appropriate target for that family “and then really trying hard to achieve that target.”
The guidelines also list risk factors for poor outcome that can be used to guide treatment decisions.
“Specific involvement of key joints, such as TMJ, wrist, sacroiliac, hip, and ankle, and other features were considered reasonable justification for early escalation of therapy,” Dr. Onel said. Other features included presence of erosive disease or enthesitis, delay in diagnosis, elevated levels of inflammation markers, and symmetric disease. “Moving quickly may be needed for a patient who is rapidly worsening, while moving slower may be appropriate for somebody who has improved substantially, but not fully.”
Systemic JIA with and without macrophage activation syndrome
For systemic JIA without macrophage activation syndrome (MAS), the guidelines similarly advise against oral glucocorticoids as initial monotherapy while conditionally recommending NSAIDs for initial monotherapy. Where the guidelines differ most from those for oligoarticular and TMJ arthritis is in progression of DMARD use, with a strong recommendation against conventional synthetic DMARDs as an initial monotherapy and interleukin-1 and IL-6 inhibitors conditionally recommended for initial monotherapy.
For patients who don’t adequately respond to NSAIDs or glucocorticoids, IL-1 and IL-6 inhibitors are strongly recommended over a single or combination of conventional DMARDs. Residual arthritis or an incomplete response to IL-1 or IL-6 inhibitors should lead next to biologic or conventional DMARDs instead of long-term glucocorticoids.
For patients with MAS, the guidelines conditionally recommend IL-1 and IL-6 inhibitors over calcineurin inhibitor monotherapy to reach inactive disease and MAS resolution, with glucocorticoids conditionally recommended in initial treatment. Again, however, for patients with incomplete responses to IL-1 or IL-6 inhibitors or with residual arthritis, the guidelines advise biologic or conventional DMARDs over long-term glucocorticoids.
In patients with sJIA with or without a history of MAS who have inactive disease, practitioners should taper and discontinue glucocorticoids (a strong recommendation). A conditional recommendation for tapering and discontinuing biologic DMARDs follows attainment of inactive disease.
Beyond pharmacology
Although many of the nonpharmacologic recommendations did not have strong evidence based on assessment with Grading of Recommendations Assessment, Development, and Evaluation methodology, consensus was more often the case than not, Dr. Onel said, such as with vaccination.
“There was strong support for the use of immunizations in children with JIA and specific guidance for children with JIA receiving immunosuppression, not on immunosuppression, and children who are underimmunized or unimmunized,” she said. “Although the supportive evidence was very low as per GRADE, panel members were strongly in favor [of immunizations], given risk of infection for immunosuppressed children as well as the preponderance of evidence in similar disease states, such as IBD [inflammatory bowel disease].”
An area with less consensus was whether to check antibody titers for vaccine-preventable childhood infections before beginning immunosuppressive medication, but more panelists opposed the practice than supported it, Dr. Onel said.
“Some panelists felt that the information might be useful for risk management in case of an outbreak or exposure,” she said. “Most believed that screening a fully immunized child was of low benefit and might delay treatment and incur unnecessary cost.”
The process of developing the documents also reveals where the biggest gaps are in research.
“One of the things that we should strive for in the future is really to do more systematic studies so we have better quality of evidence going forward,” Dr. Shenoi said. Overall, however, the guidelines also reveal the progress made in treatment of JIA.
“We now know some of the key cytokines that are involved in the disease pathogenesis, and we have effective therapies for some of these pathways,” Dr. Shenoi said. “We used to use a lot more toxic medication for systemic JIA, and in past decades, these patients used to be on steroids forever. Now we have targeted therapies, and we have some patients who don’t ever need steroids because people are moving toward targeted therapies and having good results. That’s a huge step forward in the field.”
The research was funded by the ACR. Dr. Shenoi has been a consultant for Pfizer. Dr. Onel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment of systemic juvenile idiopathic arthritis (sJIA) should emphasize early use of conventional synthetic and biologic disease-modifying antirheumatic drugs (DMARDs), compared with the previous reliance on NSAIDs and glucocorticoids, according to new guidelines from the American College of Rheumatology. The recently published 2021 guidelines focus on therapeutic approaches for oligoarthritis, temporomandibular joint (TMJ) arthritis, and sJIA.
“Systemic JIA should be treated early with biologics to rapidly bring disease under control and to avoid long-term use of glucocorticoids,” Karen Onel, MD, chief of the division of pediatric rheumatology at Weill Cornell Medicine, New York, and lead author of the guidelines, told this news organization. “Unfortunately, biologics can and are frequently denied for first-line use. For this reason, the guidelines are critically important as they demonstrate that first-line use of biologics are standard of care for the treatment of sJIA.”
The new publication is the second part of the ACR’s process to update JIA guidelines that began in 2017 and complements the release in 2019 of guidelines on the management of nonsystemic polyarthritis, sacroiliitis, and enthesitis, as well as a separate guidance on JIA-associated uveitis. The new guidelines include a second publication focused on nonpharmacologic therapies, medication monitoring, immunizations, and imaging. Both sets of guidelines grew out of a 15-member panel that included young adults with JIA and caregivers of children with JIA, and which required at least 70% agreement on recommendations.
“Though the scope of the two guidelines differed, one thing they had in common is the recognition of the importance of shared decision-making with the patient/caregiver,” Dr. Onel said. “Not every decision will be appropriate for every patient, which is why it was so instrumental to receive input from both patients and caregivers when creating these recommendations.”
Oligoarticular and TMJ arthritis
Oligoarticular and TMJ arthritis have similar recommendations, beginning with NSAIDs conditionally recommended and intra-articular glucocorticoids (IAGCs) strongly recommended as part of initial therapy. For oligoarticular arthritis, the guidelines specifically include a strong recommendation of triamcinolone hexacetonide as the preferred agent; no preferred agent is recommended for TMJ arthritis.
“The one thing that the panel was unanimous about was the use of triamcinolone hexacetonide for intra-articular steroid injections in oligoarticular kids,” Susan Shenoi, MBBS, MS, an associate professor and clinical director of pediatric rheumatology at Seattle Children’s Hospital and Research Center, said in an interview. “Triamcinolone hexacetonide has not been available recently, and through advocacy efforts, there is now a pathway to get that medication,” added Dr. Shenoi, a coauthor on the guidelines.
Dr. Onel said that “triamcinolone hexacetonide has been shown to be superior to alternative injectable glucocorticoids in achieving and maintaining remission in children with JIA,” but its unavailability meant physicians had to consider less effective, more potent, or more costly alternatives.” To address the shortage, “the FDA allowed the importation of one particular formulation of triamcinolone hexacetonide [Hexatrione 2%] specifically for joint injections in patients with JIA.”
The guidelines conditionally recommend against oral glucocorticoids for initial therapy for both oligoarticular and TMJ arthritis. In fact, throughout the guidelines it’s clear that the authors emphasize using steroids as little as possible, Dr. Shenoi said.
“Steroids are great anti-inflammatories, but in kids we worry about the long-term effects on growth and metabolism, and now we have many more DMARDs available,” Dr. Shenoi said.
The guidelines strongly recommend conventional synthetic DMARDs for patients with either of these diseases who cannot tolerate or do not respond to NSAIDs or IAGCs, with methotrexate conditionally recommended over leflunomide (Arava) for TMJ and over leflunomide, sulfasalazine (Azulfidine, Sulfazine), and hydroxychloroquine, respectively, for oligoarticular arthritis.
“NSAIDs remain widely used despite evidence supporting early use of DMARDs,” Dr. Onel said. “NSAIDs are readily available and familiar; however, they will not prevent disease progression. These guidelines should encourage short courses of NSAIDs only.”
If patients do not respond to or cannot tolerate NSAIDs, IAGCs, and at least one conventional DMARD, the guidelines strongly recommend a biologic DMARD for oligoarticular arthritis and conditionally recommend one for TMJ arthritis, without any preferences to the specific agent.
The guidelines also advise using validated disease activity measures to guide treatment decisions.
“The most important thing when you’re looking at these patients is to determine, do they have active disease or not?” Dr. Shenoi said. “If they have active disease, then you really want to step up therapy.” Using the relatively new concept of treat-to-target, Dr. Shenoi added that a crucial part of shared decision-making with the family is identifying the most appropriate target for that family “and then really trying hard to achieve that target.”
The guidelines also list risk factors for poor outcome that can be used to guide treatment decisions.
“Specific involvement of key joints, such as TMJ, wrist, sacroiliac, hip, and ankle, and other features were considered reasonable justification for early escalation of therapy,” Dr. Onel said. Other features included presence of erosive disease or enthesitis, delay in diagnosis, elevated levels of inflammation markers, and symmetric disease. “Moving quickly may be needed for a patient who is rapidly worsening, while moving slower may be appropriate for somebody who has improved substantially, but not fully.”
Systemic JIA with and without macrophage activation syndrome
For systemic JIA without macrophage activation syndrome (MAS), the guidelines similarly advise against oral glucocorticoids as initial monotherapy while conditionally recommending NSAIDs for initial monotherapy. Where the guidelines differ most from those for oligoarticular and TMJ arthritis is in progression of DMARD use, with a strong recommendation against conventional synthetic DMARDs as an initial monotherapy and interleukin-1 and IL-6 inhibitors conditionally recommended for initial monotherapy.
For patients who don’t adequately respond to NSAIDs or glucocorticoids, IL-1 and IL-6 inhibitors are strongly recommended over a single or combination of conventional DMARDs. Residual arthritis or an incomplete response to IL-1 or IL-6 inhibitors should lead next to biologic or conventional DMARDs instead of long-term glucocorticoids.
For patients with MAS, the guidelines conditionally recommend IL-1 and IL-6 inhibitors over calcineurin inhibitor monotherapy to reach inactive disease and MAS resolution, with glucocorticoids conditionally recommended in initial treatment. Again, however, for patients with incomplete responses to IL-1 or IL-6 inhibitors or with residual arthritis, the guidelines advise biologic or conventional DMARDs over long-term glucocorticoids.
In patients with sJIA with or without a history of MAS who have inactive disease, practitioners should taper and discontinue glucocorticoids (a strong recommendation). A conditional recommendation for tapering and discontinuing biologic DMARDs follows attainment of inactive disease.
Beyond pharmacology
Although many of the nonpharmacologic recommendations did not have strong evidence based on assessment with Grading of Recommendations Assessment, Development, and Evaluation methodology, consensus was more often the case than not, Dr. Onel said, such as with vaccination.
“There was strong support for the use of immunizations in children with JIA and specific guidance for children with JIA receiving immunosuppression, not on immunosuppression, and children who are underimmunized or unimmunized,” she said. “Although the supportive evidence was very low as per GRADE, panel members were strongly in favor [of immunizations], given risk of infection for immunosuppressed children as well as the preponderance of evidence in similar disease states, such as IBD [inflammatory bowel disease].”
An area with less consensus was whether to check antibody titers for vaccine-preventable childhood infections before beginning immunosuppressive medication, but more panelists opposed the practice than supported it, Dr. Onel said.
“Some panelists felt that the information might be useful for risk management in case of an outbreak or exposure,” she said. “Most believed that screening a fully immunized child was of low benefit and might delay treatment and incur unnecessary cost.”
The process of developing the documents also reveals where the biggest gaps are in research.
“One of the things that we should strive for in the future is really to do more systematic studies so we have better quality of evidence going forward,” Dr. Shenoi said. Overall, however, the guidelines also reveal the progress made in treatment of JIA.
“We now know some of the key cytokines that are involved in the disease pathogenesis, and we have effective therapies for some of these pathways,” Dr. Shenoi said. “We used to use a lot more toxic medication for systemic JIA, and in past decades, these patients used to be on steroids forever. Now we have targeted therapies, and we have some patients who don’t ever need steroids because people are moving toward targeted therapies and having good results. That’s a huge step forward in the field.”
The research was funded by the ACR. Dr. Shenoi has been a consultant for Pfizer. Dr. Onel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS & RHEUMATOLOGY
Rare pediatric cancers persist 63 years after nuclear accident
Chernobyl. Fukushima. Three Mile Island.
The world knows these names all too well because of accidents there: complete or partial meltdowns of nuclear reactors that released massive amounts of cancer-causing radiation into the air, soil, and water.
The Santa Susana Field Lab is far less well-known, but no less infamous for what took place at this former rocket engine and nuclear energy test site just 28 miles northwest of downtown Los Angeles.
In July 1959, an accident involving one of 10 experimental nuclear reactors at the SSFL site released a cloud of harmful radiation and toxic chemicals over the surrounding area, including Simi Valley, San Gabriel Valley, Chatsworth, and Canoga Park. The small reactor had no containment vessel.
This accident resulted in a release of radioactive iodine estimated to be as much as 250 times that of the partial meltdown that would occur 2 decades later at Three Mile Island, a much larger commercial reactor that had a containment vessel.
Decades-long cover-up
In 1959, the public knew nothing about what happened at the site.
According to John Pace, then an employee at SSFL, the accident was covered up. Mr. Pace recounted the cover-up in the documentary “In the Dark of the Valley,” which first aired in November 2021 on MSNBC.
In fact, the accident at SSFL remained under wraps for 2 decades, according to Daniel Hirsch, former director of the Program on Environmental and Nuclear Policy at the University of California, Santa Cruz, and now president of Committee to Bridge the Gap, a nuclear policy nongovernmental organization.
“Students working with me while I was teaching at UCLA in 1979 uncovered these Atomic Energy Commission reports from Atomics International,” he said in an interview. “We had to order the documents from the annex to the UCLA Engineering Library. They were stored offsite, and it took a few days, and when we got them, we opened them up, and there were these fold-out photographs of the fuel [rods]. As we folded out the photographs further, we saw one photo with an arrow labeled ‘longitudinal cracks,’ and then other arrows showing other kinds of cracks, and then another arrow labeled ‘melted blob.’ ”
Mr. Hirsch and his students found that other accidents had occurred at SSFL, including a fuel fabrication system that leached plutonium, fires in a “hot” lab where irradiated nuclear fuel from around the United States was handled, and open-air burn pits where radioactive and toxic chemical wastes were illegally torched.
According to the Committee to Bridge the Gap, when the 2,800-acre SSFL site was being developed under the name Rocketdyne by aircraft maker North American Aviation, the area was sparsely populated, with nearly as many grazing animals as people in its hills and valleys.
North American Aviation later became part of Rockwell International, which in turn sold its aerospace and defense business units to the Boeing Company in 1996. Boeing, now in charge of the site and the cleanup efforts, is doing everything in its power to shirk or diminish its responsibility, Mr. Hirsch and other critics say.
Parents against SSFL
Today, more than 150,000 people live within 5 miles of SSFL, and more than half a million live within 10 miles.
Melissa Bumstead is one of those residents. She and her family live 3.7 miles from the Santa Susana site. When her toddler Grace was diagnosed with a rare form of leukemia in 2014, doctors told Ms. Bumstead there were no known links between her daughter’s cancer and environmental contamination.
But during Grace’s treatment at Children’s Hospital Los Angeles, her mother began meeting other parents who lived near her and had children facing equally rare cancers.
Lauren Hammersley, whose daughter Hazel was diagnosed with a rare brain tumor called neuroblastoma at age 2, lived about 10 miles from Ms. Bumstead on the other side of a mountain and just over 4 miles from SSFL.
On her street alone, Ms. Bumstead discovered three cases of pediatric cancer, including two children in adjacent homes who had the same rare brain tumor as Hazel Hammersley.
As Ms. Bumstead told Los Angeles National Public Radio station KCRW in 2021, “I started to panic because I knew that childhood cancer is extremely rare. There’s only 15,000 new cases every year out of 72 million children in America. So, the chance of knowing your neighbors, especially at an internationally renowned hospital like Children’s Hospital Los Angeles – we knew something wasn’t right.”
After a relapse of her tumor, Hazel died in 2018, a few months after her seventh birthday.
Cancer clusters
Hoping to understand why their kids were getting so sick, Ms. Bumstead and the other parents formed a Facebook group. They plotted their homes on Google Maps and found that they all lived within roughly 10 miles of one another. It would take another year for them to realize that the SSFL site was at the center of the circle.
Once they realized that being close to SSFL could be their common thread, Ms. Bumstead and parents in her group began to gradually piece together the story, linking unusual or unexplained illnesses in their families to potential radiation or toxic chemical exposures from the lab.
“What really convinced me that this was absolutely a problem was when I learned about the epidemiological study by Dr. Hal Morgenstern that found that residents living within 2 miles of the Santa Susana Field Lab actually had a 60% higher cancer incidence rate and that over 1,500 workers have been diagnosed with cancer just from the Santa Susana Field Lab,” she told KCRW.
In 2015, Ms. Bumstead and other parents formed Parents Against Santa Susana Field Lab to hold SSFL site owner Boeing accountable for radiologic and toxic contamination and to ensure that Boeing cleans the site and surrounding areas. The group “seeks to reduce, to the greatest extent possible, the number of local families who have to hear the words, ‘Your child has cancer.’ ”
No longer quite so rare
Dr. Morgenstern, now retired from the University of Michigan, declined to be interviewed for this article. But as he and colleagues reported to the Centers for Disease Control and Prevention’s Agency for Toxic Substances and Disease Registry in 2007, there were strong signs of a link between contamination of the site and cancer.
The researchers compared cancer rates of adults living within 2 miles and 2-5 miles from SSFL with those of adults living more than 5 miles away, in Ventura and Los Angeles counties. They found that from 1988 through 1995, residents living within 2 miles of SSFL had a 60% higher rate of cancers than the control group. These included cancers of the thyroid, oral and nasal cavities, pharynx, larynx, esophagus, and bladder, as well as blood cancers such as leukemia, lymphoma, and multiple myeloma.
In separate studies, the investigators found higher rates of certain cancers among workers at SSFL who were exposed to radiation and to hydrazine, a chemical in rocket fuel.
In an interview, Dr. Saro Armenian, a pediatric hematologist-oncologist who was not involved in the studies, said the 60% increase in cancer incidence, which translated into a 1.6-fold increase in risk, merits more investigation.
“In epidemiologic studies, a 1.6-fold risk is actually a pretty strong signal because typically, most signals that you get are somewhere around 1.1- to 1.2-fold increased risk,” noted Dr. Armenian, a specialist in pediatric cancer survivorship and outcomes at City of Hope National Medical Center in Duarte, Calif.
However, Dr. Thomas Mack, former director of the Los Angeles County Cancer Surveillance Program, contends that there is insufficient evidence to support a direct link between the 1959 reactor accident and recent incident cancers. Dr. Mack is currently a professor of preventive medicine and pathology at the University of Southern California in Los Angeles.
“I have evaluated concerns about local excesses of cancer at least 100 times, usually from county residents, but for a while I represented the CDC and the California cancer registry,” Dr. Mack said, in response to an emailed request for comment.
“So far I have seen no evidence of carcinogenic radionucleotides or chemical carcinogens from Santa Susana found in any meaningful amount in any nearby community, but if someone has such evidence that would constitute evidence, that needs a response,” Dr. Mack added.
Boeing and California
Boeing has said problems at SSFL were not responsible for the high cancer rates among children in the community.
In April 2007, in a statement opposing a bill before the California State Legislature that would compel Boeing to pay for SSFL site cleanup, the company said that “in contrast to the accusations made against The Boeing Company that falsely claim increased cancer rates in the communities surrounding SSFL, a recent study conducted by the University of Michigan School of Public Health just concluded the opposite.”
Yet as Dr. Morgenstern wrote in 2007 to California state Sen. Joe Simitian, then chair of the Committee on Environmental Quality: “For the period 1996 through 2002, we found that the incidence rate of thyroid cancer was more than 60% greater among residents living within 2 miles of SSFL than for residents living more than 5 miles from SSFL. The magnitude and consistency of the thyroid finding for both periods is especially provocative because of evidence from other studies linking thyroid cancer with environmental exposures originating at SSFL and found in the surrounding communities.”
Boeing chose to ignore the results and instead focused on the methods used in the study, where the authors acknowledged that they measured distance from the site rather than environmental exposures and thus could not conclusively link excess cancer rates to exposures arising from SSFL.
But Dr. Morgenstern emphasized the conclusion of the report: “Despite the methodologic limitations of this study, the findings suggest there may be elevated incidence rates of certain cancers near SSFL that have been linked in previous studies with hazardous substances used at Rocketdyne, some of which have been observed or projected to exist offsite.”
Failure to come clean
In 2008, a law that set standards for cleanup of the site was passed. But the law was overturned in 2014 after a legal challenge by Boeing.
That left in place a 2007 order of consent between Boeing, NASA, the U.S. Department of Energy, and the California Department of Toxic Substances Control (DTSC) that required cleanup of SSFL to a much less stringent standard.
As of last year, Boeing and DTSC had begun confidential, nonbinding agreements regarding the 2007 order of consent, according to Parents Against SSFL.
Among the contaminants lingering at the site are radioactive particles, chemical compounds, heavy metals, and polluted water.
“In fact, over 300 contaminants of concern have been found at the site, and they are refusing to clean it,” Mr. Hirsch said. “This company releases large amounts of carcinogens, and perhaps significant numbers of people get sick with cancer, and the company doesn’t go to prison. They get more federal contracts.”
A version of this article first appeared on WebMD.com.
April 20, 2022 – Editor’s note: This article has been updated to include an interview with Dr. Thomas Mack, former director of the Los Angeles County Cancer Surveillance Program, who contends that there is insufficient evidence to support a direct link between the 1959 reactor accident and recent incident cancers.
Chernobyl. Fukushima. Three Mile Island.
The world knows these names all too well because of accidents there: complete or partial meltdowns of nuclear reactors that released massive amounts of cancer-causing radiation into the air, soil, and water.
The Santa Susana Field Lab is far less well-known, but no less infamous for what took place at this former rocket engine and nuclear energy test site just 28 miles northwest of downtown Los Angeles.
In July 1959, an accident involving one of 10 experimental nuclear reactors at the SSFL site released a cloud of harmful radiation and toxic chemicals over the surrounding area, including Simi Valley, San Gabriel Valley, Chatsworth, and Canoga Park. The small reactor had no containment vessel.
This accident resulted in a release of radioactive iodine estimated to be as much as 250 times that of the partial meltdown that would occur 2 decades later at Three Mile Island, a much larger commercial reactor that had a containment vessel.
Decades-long cover-up
In 1959, the public knew nothing about what happened at the site.
According to John Pace, then an employee at SSFL, the accident was covered up. Mr. Pace recounted the cover-up in the documentary “In the Dark of the Valley,” which first aired in November 2021 on MSNBC.
In fact, the accident at SSFL remained under wraps for 2 decades, according to Daniel Hirsch, former director of the Program on Environmental and Nuclear Policy at the University of California, Santa Cruz, and now president of Committee to Bridge the Gap, a nuclear policy nongovernmental organization.
“Students working with me while I was teaching at UCLA in 1979 uncovered these Atomic Energy Commission reports from Atomics International,” he said in an interview. “We had to order the documents from the annex to the UCLA Engineering Library. They were stored offsite, and it took a few days, and when we got them, we opened them up, and there were these fold-out photographs of the fuel [rods]. As we folded out the photographs further, we saw one photo with an arrow labeled ‘longitudinal cracks,’ and then other arrows showing other kinds of cracks, and then another arrow labeled ‘melted blob.’ ”
Mr. Hirsch and his students found that other accidents had occurred at SSFL, including a fuel fabrication system that leached plutonium, fires in a “hot” lab where irradiated nuclear fuel from around the United States was handled, and open-air burn pits where radioactive and toxic chemical wastes were illegally torched.
According to the Committee to Bridge the Gap, when the 2,800-acre SSFL site was being developed under the name Rocketdyne by aircraft maker North American Aviation, the area was sparsely populated, with nearly as many grazing animals as people in its hills and valleys.
North American Aviation later became part of Rockwell International, which in turn sold its aerospace and defense business units to the Boeing Company in 1996. Boeing, now in charge of the site and the cleanup efforts, is doing everything in its power to shirk or diminish its responsibility, Mr. Hirsch and other critics say.
Parents against SSFL
Today, more than 150,000 people live within 5 miles of SSFL, and more than half a million live within 10 miles.
Melissa Bumstead is one of those residents. She and her family live 3.7 miles from the Santa Susana site. When her toddler Grace was diagnosed with a rare form of leukemia in 2014, doctors told Ms. Bumstead there were no known links between her daughter’s cancer and environmental contamination.
But during Grace’s treatment at Children’s Hospital Los Angeles, her mother began meeting other parents who lived near her and had children facing equally rare cancers.
Lauren Hammersley, whose daughter Hazel was diagnosed with a rare brain tumor called neuroblastoma at age 2, lived about 10 miles from Ms. Bumstead on the other side of a mountain and just over 4 miles from SSFL.
On her street alone, Ms. Bumstead discovered three cases of pediatric cancer, including two children in adjacent homes who had the same rare brain tumor as Hazel Hammersley.
As Ms. Bumstead told Los Angeles National Public Radio station KCRW in 2021, “I started to panic because I knew that childhood cancer is extremely rare. There’s only 15,000 new cases every year out of 72 million children in America. So, the chance of knowing your neighbors, especially at an internationally renowned hospital like Children’s Hospital Los Angeles – we knew something wasn’t right.”
After a relapse of her tumor, Hazel died in 2018, a few months after her seventh birthday.
Cancer clusters
Hoping to understand why their kids were getting so sick, Ms. Bumstead and the other parents formed a Facebook group. They plotted their homes on Google Maps and found that they all lived within roughly 10 miles of one another. It would take another year for them to realize that the SSFL site was at the center of the circle.
Once they realized that being close to SSFL could be their common thread, Ms. Bumstead and parents in her group began to gradually piece together the story, linking unusual or unexplained illnesses in their families to potential radiation or toxic chemical exposures from the lab.
“What really convinced me that this was absolutely a problem was when I learned about the epidemiological study by Dr. Hal Morgenstern that found that residents living within 2 miles of the Santa Susana Field Lab actually had a 60% higher cancer incidence rate and that over 1,500 workers have been diagnosed with cancer just from the Santa Susana Field Lab,” she told KCRW.
In 2015, Ms. Bumstead and other parents formed Parents Against Santa Susana Field Lab to hold SSFL site owner Boeing accountable for radiologic and toxic contamination and to ensure that Boeing cleans the site and surrounding areas. The group “seeks to reduce, to the greatest extent possible, the number of local families who have to hear the words, ‘Your child has cancer.’ ”
No longer quite so rare
Dr. Morgenstern, now retired from the University of Michigan, declined to be interviewed for this article. But as he and colleagues reported to the Centers for Disease Control and Prevention’s Agency for Toxic Substances and Disease Registry in 2007, there were strong signs of a link between contamination of the site and cancer.
The researchers compared cancer rates of adults living within 2 miles and 2-5 miles from SSFL with those of adults living more than 5 miles away, in Ventura and Los Angeles counties. They found that from 1988 through 1995, residents living within 2 miles of SSFL had a 60% higher rate of cancers than the control group. These included cancers of the thyroid, oral and nasal cavities, pharynx, larynx, esophagus, and bladder, as well as blood cancers such as leukemia, lymphoma, and multiple myeloma.
In separate studies, the investigators found higher rates of certain cancers among workers at SSFL who were exposed to radiation and to hydrazine, a chemical in rocket fuel.
In an interview, Dr. Saro Armenian, a pediatric hematologist-oncologist who was not involved in the studies, said the 60% increase in cancer incidence, which translated into a 1.6-fold increase in risk, merits more investigation.
“In epidemiologic studies, a 1.6-fold risk is actually a pretty strong signal because typically, most signals that you get are somewhere around 1.1- to 1.2-fold increased risk,” noted Dr. Armenian, a specialist in pediatric cancer survivorship and outcomes at City of Hope National Medical Center in Duarte, Calif.
However, Dr. Thomas Mack, former director of the Los Angeles County Cancer Surveillance Program, contends that there is insufficient evidence to support a direct link between the 1959 reactor accident and recent incident cancers. Dr. Mack is currently a professor of preventive medicine and pathology at the University of Southern California in Los Angeles.
“I have evaluated concerns about local excesses of cancer at least 100 times, usually from county residents, but for a while I represented the CDC and the California cancer registry,” Dr. Mack said, in response to an emailed request for comment.
“So far I have seen no evidence of carcinogenic radionucleotides or chemical carcinogens from Santa Susana found in any meaningful amount in any nearby community, but if someone has such evidence that would constitute evidence, that needs a response,” Dr. Mack added.
Boeing and California
Boeing has said problems at SSFL were not responsible for the high cancer rates among children in the community.
In April 2007, in a statement opposing a bill before the California State Legislature that would compel Boeing to pay for SSFL site cleanup, the company said that “in contrast to the accusations made against The Boeing Company that falsely claim increased cancer rates in the communities surrounding SSFL, a recent study conducted by the University of Michigan School of Public Health just concluded the opposite.”
Yet as Dr. Morgenstern wrote in 2007 to California state Sen. Joe Simitian, then chair of the Committee on Environmental Quality: “For the period 1996 through 2002, we found that the incidence rate of thyroid cancer was more than 60% greater among residents living within 2 miles of SSFL than for residents living more than 5 miles from SSFL. The magnitude and consistency of the thyroid finding for both periods is especially provocative because of evidence from other studies linking thyroid cancer with environmental exposures originating at SSFL and found in the surrounding communities.”
Boeing chose to ignore the results and instead focused on the methods used in the study, where the authors acknowledged that they measured distance from the site rather than environmental exposures and thus could not conclusively link excess cancer rates to exposures arising from SSFL.
But Dr. Morgenstern emphasized the conclusion of the report: “Despite the methodologic limitations of this study, the findings suggest there may be elevated incidence rates of certain cancers near SSFL that have been linked in previous studies with hazardous substances used at Rocketdyne, some of which have been observed or projected to exist offsite.”
Failure to come clean
In 2008, a law that set standards for cleanup of the site was passed. But the law was overturned in 2014 after a legal challenge by Boeing.
That left in place a 2007 order of consent between Boeing, NASA, the U.S. Department of Energy, and the California Department of Toxic Substances Control (DTSC) that required cleanup of SSFL to a much less stringent standard.
As of last year, Boeing and DTSC had begun confidential, nonbinding agreements regarding the 2007 order of consent, according to Parents Against SSFL.
Among the contaminants lingering at the site are radioactive particles, chemical compounds, heavy metals, and polluted water.
“In fact, over 300 contaminants of concern have been found at the site, and they are refusing to clean it,” Mr. Hirsch said. “This company releases large amounts of carcinogens, and perhaps significant numbers of people get sick with cancer, and the company doesn’t go to prison. They get more federal contracts.”
A version of this article first appeared on WebMD.com.
April 20, 2022 – Editor’s note: This article has been updated to include an interview with Dr. Thomas Mack, former director of the Los Angeles County Cancer Surveillance Program, who contends that there is insufficient evidence to support a direct link between the 1959 reactor accident and recent incident cancers.
Chernobyl. Fukushima. Three Mile Island.
The world knows these names all too well because of accidents there: complete or partial meltdowns of nuclear reactors that released massive amounts of cancer-causing radiation into the air, soil, and water.
The Santa Susana Field Lab is far less well-known, but no less infamous for what took place at this former rocket engine and nuclear energy test site just 28 miles northwest of downtown Los Angeles.
In July 1959, an accident involving one of 10 experimental nuclear reactors at the SSFL site released a cloud of harmful radiation and toxic chemicals over the surrounding area, including Simi Valley, San Gabriel Valley, Chatsworth, and Canoga Park. The small reactor had no containment vessel.
This accident resulted in a release of radioactive iodine estimated to be as much as 250 times that of the partial meltdown that would occur 2 decades later at Three Mile Island, a much larger commercial reactor that had a containment vessel.
Decades-long cover-up
In 1959, the public knew nothing about what happened at the site.
According to John Pace, then an employee at SSFL, the accident was covered up. Mr. Pace recounted the cover-up in the documentary “In the Dark of the Valley,” which first aired in November 2021 on MSNBC.
In fact, the accident at SSFL remained under wraps for 2 decades, according to Daniel Hirsch, former director of the Program on Environmental and Nuclear Policy at the University of California, Santa Cruz, and now president of Committee to Bridge the Gap, a nuclear policy nongovernmental organization.
“Students working with me while I was teaching at UCLA in 1979 uncovered these Atomic Energy Commission reports from Atomics International,” he said in an interview. “We had to order the documents from the annex to the UCLA Engineering Library. They were stored offsite, and it took a few days, and when we got them, we opened them up, and there were these fold-out photographs of the fuel [rods]. As we folded out the photographs further, we saw one photo with an arrow labeled ‘longitudinal cracks,’ and then other arrows showing other kinds of cracks, and then another arrow labeled ‘melted blob.’ ”
Mr. Hirsch and his students found that other accidents had occurred at SSFL, including a fuel fabrication system that leached plutonium, fires in a “hot” lab where irradiated nuclear fuel from around the United States was handled, and open-air burn pits where radioactive and toxic chemical wastes were illegally torched.
According to the Committee to Bridge the Gap, when the 2,800-acre SSFL site was being developed under the name Rocketdyne by aircraft maker North American Aviation, the area was sparsely populated, with nearly as many grazing animals as people in its hills and valleys.
North American Aviation later became part of Rockwell International, which in turn sold its aerospace and defense business units to the Boeing Company in 1996. Boeing, now in charge of the site and the cleanup efforts, is doing everything in its power to shirk or diminish its responsibility, Mr. Hirsch and other critics say.
Parents against SSFL
Today, more than 150,000 people live within 5 miles of SSFL, and more than half a million live within 10 miles.
Melissa Bumstead is one of those residents. She and her family live 3.7 miles from the Santa Susana site. When her toddler Grace was diagnosed with a rare form of leukemia in 2014, doctors told Ms. Bumstead there were no known links between her daughter’s cancer and environmental contamination.
But during Grace’s treatment at Children’s Hospital Los Angeles, her mother began meeting other parents who lived near her and had children facing equally rare cancers.
Lauren Hammersley, whose daughter Hazel was diagnosed with a rare brain tumor called neuroblastoma at age 2, lived about 10 miles from Ms. Bumstead on the other side of a mountain and just over 4 miles from SSFL.
On her street alone, Ms. Bumstead discovered three cases of pediatric cancer, including two children in adjacent homes who had the same rare brain tumor as Hazel Hammersley.
As Ms. Bumstead told Los Angeles National Public Radio station KCRW in 2021, “I started to panic because I knew that childhood cancer is extremely rare. There’s only 15,000 new cases every year out of 72 million children in America. So, the chance of knowing your neighbors, especially at an internationally renowned hospital like Children’s Hospital Los Angeles – we knew something wasn’t right.”
After a relapse of her tumor, Hazel died in 2018, a few months after her seventh birthday.
Cancer clusters
Hoping to understand why their kids were getting so sick, Ms. Bumstead and the other parents formed a Facebook group. They plotted their homes on Google Maps and found that they all lived within roughly 10 miles of one another. It would take another year for them to realize that the SSFL site was at the center of the circle.
Once they realized that being close to SSFL could be their common thread, Ms. Bumstead and parents in her group began to gradually piece together the story, linking unusual or unexplained illnesses in their families to potential radiation or toxic chemical exposures from the lab.
“What really convinced me that this was absolutely a problem was when I learned about the epidemiological study by Dr. Hal Morgenstern that found that residents living within 2 miles of the Santa Susana Field Lab actually had a 60% higher cancer incidence rate and that over 1,500 workers have been diagnosed with cancer just from the Santa Susana Field Lab,” she told KCRW.
In 2015, Ms. Bumstead and other parents formed Parents Against Santa Susana Field Lab to hold SSFL site owner Boeing accountable for radiologic and toxic contamination and to ensure that Boeing cleans the site and surrounding areas. The group “seeks to reduce, to the greatest extent possible, the number of local families who have to hear the words, ‘Your child has cancer.’ ”
No longer quite so rare
Dr. Morgenstern, now retired from the University of Michigan, declined to be interviewed for this article. But as he and colleagues reported to the Centers for Disease Control and Prevention’s Agency for Toxic Substances and Disease Registry in 2007, there were strong signs of a link between contamination of the site and cancer.
The researchers compared cancer rates of adults living within 2 miles and 2-5 miles from SSFL with those of adults living more than 5 miles away, in Ventura and Los Angeles counties. They found that from 1988 through 1995, residents living within 2 miles of SSFL had a 60% higher rate of cancers than the control group. These included cancers of the thyroid, oral and nasal cavities, pharynx, larynx, esophagus, and bladder, as well as blood cancers such as leukemia, lymphoma, and multiple myeloma.
In separate studies, the investigators found higher rates of certain cancers among workers at SSFL who were exposed to radiation and to hydrazine, a chemical in rocket fuel.
In an interview, Dr. Saro Armenian, a pediatric hematologist-oncologist who was not involved in the studies, said the 60% increase in cancer incidence, which translated into a 1.6-fold increase in risk, merits more investigation.
“In epidemiologic studies, a 1.6-fold risk is actually a pretty strong signal because typically, most signals that you get are somewhere around 1.1- to 1.2-fold increased risk,” noted Dr. Armenian, a specialist in pediatric cancer survivorship and outcomes at City of Hope National Medical Center in Duarte, Calif.
However, Dr. Thomas Mack, former director of the Los Angeles County Cancer Surveillance Program, contends that there is insufficient evidence to support a direct link between the 1959 reactor accident and recent incident cancers. Dr. Mack is currently a professor of preventive medicine and pathology at the University of Southern California in Los Angeles.
“I have evaluated concerns about local excesses of cancer at least 100 times, usually from county residents, but for a while I represented the CDC and the California cancer registry,” Dr. Mack said, in response to an emailed request for comment.
“So far I have seen no evidence of carcinogenic radionucleotides or chemical carcinogens from Santa Susana found in any meaningful amount in any nearby community, but if someone has such evidence that would constitute evidence, that needs a response,” Dr. Mack added.
Boeing and California
Boeing has said problems at SSFL were not responsible for the high cancer rates among children in the community.
In April 2007, in a statement opposing a bill before the California State Legislature that would compel Boeing to pay for SSFL site cleanup, the company said that “in contrast to the accusations made against The Boeing Company that falsely claim increased cancer rates in the communities surrounding SSFL, a recent study conducted by the University of Michigan School of Public Health just concluded the opposite.”
Yet as Dr. Morgenstern wrote in 2007 to California state Sen. Joe Simitian, then chair of the Committee on Environmental Quality: “For the period 1996 through 2002, we found that the incidence rate of thyroid cancer was more than 60% greater among residents living within 2 miles of SSFL than for residents living more than 5 miles from SSFL. The magnitude and consistency of the thyroid finding for both periods is especially provocative because of evidence from other studies linking thyroid cancer with environmental exposures originating at SSFL and found in the surrounding communities.”
Boeing chose to ignore the results and instead focused on the methods used in the study, where the authors acknowledged that they measured distance from the site rather than environmental exposures and thus could not conclusively link excess cancer rates to exposures arising from SSFL.
But Dr. Morgenstern emphasized the conclusion of the report: “Despite the methodologic limitations of this study, the findings suggest there may be elevated incidence rates of certain cancers near SSFL that have been linked in previous studies with hazardous substances used at Rocketdyne, some of which have been observed or projected to exist offsite.”
Failure to come clean
In 2008, a law that set standards for cleanup of the site was passed. But the law was overturned in 2014 after a legal challenge by Boeing.
That left in place a 2007 order of consent between Boeing, NASA, the U.S. Department of Energy, and the California Department of Toxic Substances Control (DTSC) that required cleanup of SSFL to a much less stringent standard.
As of last year, Boeing and DTSC had begun confidential, nonbinding agreements regarding the 2007 order of consent, according to Parents Against SSFL.
Among the contaminants lingering at the site are radioactive particles, chemical compounds, heavy metals, and polluted water.
“In fact, over 300 contaminants of concern have been found at the site, and they are refusing to clean it,” Mr. Hirsch said. “This company releases large amounts of carcinogens, and perhaps significant numbers of people get sick with cancer, and the company doesn’t go to prison. They get more federal contracts.”
A version of this article first appeared on WebMD.com.
April 20, 2022 – Editor’s note: This article has been updated to include an interview with Dr. Thomas Mack, former director of the Los Angeles County Cancer Surveillance Program, who contends that there is insufficient evidence to support a direct link between the 1959 reactor accident and recent incident cancers.
‘Overwhelming’ need to study COVID vaccine–associated tinnitus
It’s now known that tinnitus may be an unexpected side effect of SARS-CoV-2 vaccination, and there is an urgent need to understand the precise mechanisms and best treatment for vaccine-associated tinnitus, researchers say.
As of mid-September 2021, 12,247 cases of tinnitus, or ringing in the ears, following COVID-19 vaccination had been reported to the Vaccine Adverse Event Reporting System of the U.S. Centers for Disease Control and Prevention.
“Despite several cases of tinnitus being reported following SARS-CoV-2 vaccination, the precise pathophysiology is still not clear,” write Syed Hassan Ahmed, 3rd-year MBBS student, Dow University of Health Sciences, Karachi, Pakistan, and coauthors.
The researchers review what is known and unknown about SARS-CoV-2 vaccine-associated tinnitus in an article published online Feb. 11 in Annals of Medicine and Surgery.
Molecular mimicry?
The researchers say cross-reactivity between anti-spike SARS-CoV-2 antibodies and otologic antigens is one possibility, based on the mechanisms behind other COVID-19 vaccine–induced disorders and the phenomenon of molecular mimicry.
“The heptapeptide resemblance between coronavirus spike glycoprotein and numerous human proteins further supports molecular mimicry as a potential mechanism behind such vaccine-induced disorders,” they write.
Anti-spike antibodies may react with antigens anywhere along the auditory pathway and fuel an inflammatory reaction, they point out.
“Therefore, understanding the phenomenon of cross-reactivity and molecular mimicry may be helpful in postulating potential treatment behind not only tinnitus but also the rare events of vaccination associated hearing loss and other otologic manifestations,” the authors say.
Genetic predispositions and associated conditions may also play a significant role in determining whether an individual develops vaccine-induced tinnitus.
Stress and anxiety following COVID vaccination may also play a role, inasmuch as anxiety-related adverse events following vaccination have been reported. Vaccine-related anxiety as a potential cause of tinnitus developing after vaccination needs to be explored, they write.
Jury out on best management
How best to manage COVID vaccine-associated tinnitus also remains unclear, but it starts with a well-established diagnosis, the authors say.
A well-focused and detailed history and examination are essential, with particular emphasis placed on preexisting health conditions, specifically, autoimmune diseases, such as Hashimoto thyroiditis; otologic conditions, such as sensorineural hearing loss; glaucoma; and psychological well-being. According to the review, patients often present with a history of one or more of these disorders.
“However, any such association has not yet been established and requires further investigation to be concluded as potential risk factors for vaccine-induced tinnitus,” they caution.
Routine cranial nerve examination, otoscopy, Weber test, and Rinne test, which are used for tinnitus diagnosis in general, may be helpful for confirmation of vaccine-associated tinnitus.
Owing to the significant association between tinnitus and hearing impairment, audiology should also performed, the authors say.
Although treatments for non–vaccine-induced tinnitus vary significantly, corticosteroids are the top treatment choice for SARS-CoV-2 vaccine-induced tinnitus reported in the literature.
Trials of other drug and nondrug interventions that may uniquely help with vaccine-associated tinnitus are urgently needed, the authors say.
Summing up, the reviewers say, “Although the incidence of COVID-19 vaccine-associated tinnitus is rare, there is an overwhelming need to discern the precise pathophysiology and clinical management as a better understanding of adverse events may help in encountering vaccine hesitancy and hence fostering the COVID-19 global vaccination program.
“Despite the incidence of adverse events, the benefits of the SARS-CoV-2 vaccine in reducing hospitalization and deaths continue to outweigh the rare ramifications,” they conclude.
The research had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It’s now known that tinnitus may be an unexpected side effect of SARS-CoV-2 vaccination, and there is an urgent need to understand the precise mechanisms and best treatment for vaccine-associated tinnitus, researchers say.
As of mid-September 2021, 12,247 cases of tinnitus, or ringing in the ears, following COVID-19 vaccination had been reported to the Vaccine Adverse Event Reporting System of the U.S. Centers for Disease Control and Prevention.
“Despite several cases of tinnitus being reported following SARS-CoV-2 vaccination, the precise pathophysiology is still not clear,” write Syed Hassan Ahmed, 3rd-year MBBS student, Dow University of Health Sciences, Karachi, Pakistan, and coauthors.
The researchers review what is known and unknown about SARS-CoV-2 vaccine-associated tinnitus in an article published online Feb. 11 in Annals of Medicine and Surgery.
Molecular mimicry?
The researchers say cross-reactivity between anti-spike SARS-CoV-2 antibodies and otologic antigens is one possibility, based on the mechanisms behind other COVID-19 vaccine–induced disorders and the phenomenon of molecular mimicry.
“The heptapeptide resemblance between coronavirus spike glycoprotein and numerous human proteins further supports molecular mimicry as a potential mechanism behind such vaccine-induced disorders,” they write.
Anti-spike antibodies may react with antigens anywhere along the auditory pathway and fuel an inflammatory reaction, they point out.
“Therefore, understanding the phenomenon of cross-reactivity and molecular mimicry may be helpful in postulating potential treatment behind not only tinnitus but also the rare events of vaccination associated hearing loss and other otologic manifestations,” the authors say.
Genetic predispositions and associated conditions may also play a significant role in determining whether an individual develops vaccine-induced tinnitus.
Stress and anxiety following COVID vaccination may also play a role, inasmuch as anxiety-related adverse events following vaccination have been reported. Vaccine-related anxiety as a potential cause of tinnitus developing after vaccination needs to be explored, they write.
Jury out on best management
How best to manage COVID vaccine-associated tinnitus also remains unclear, but it starts with a well-established diagnosis, the authors say.
A well-focused and detailed history and examination are essential, with particular emphasis placed on preexisting health conditions, specifically, autoimmune diseases, such as Hashimoto thyroiditis; otologic conditions, such as sensorineural hearing loss; glaucoma; and psychological well-being. According to the review, patients often present with a history of one or more of these disorders.
“However, any such association has not yet been established and requires further investigation to be concluded as potential risk factors for vaccine-induced tinnitus,” they caution.
Routine cranial nerve examination, otoscopy, Weber test, and Rinne test, which are used for tinnitus diagnosis in general, may be helpful for confirmation of vaccine-associated tinnitus.
Owing to the significant association between tinnitus and hearing impairment, audiology should also performed, the authors say.
Although treatments for non–vaccine-induced tinnitus vary significantly, corticosteroids are the top treatment choice for SARS-CoV-2 vaccine-induced tinnitus reported in the literature.
Trials of other drug and nondrug interventions that may uniquely help with vaccine-associated tinnitus are urgently needed, the authors say.
Summing up, the reviewers say, “Although the incidence of COVID-19 vaccine-associated tinnitus is rare, there is an overwhelming need to discern the precise pathophysiology and clinical management as a better understanding of adverse events may help in encountering vaccine hesitancy and hence fostering the COVID-19 global vaccination program.
“Despite the incidence of adverse events, the benefits of the SARS-CoV-2 vaccine in reducing hospitalization and deaths continue to outweigh the rare ramifications,” they conclude.
The research had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It’s now known that tinnitus may be an unexpected side effect of SARS-CoV-2 vaccination, and there is an urgent need to understand the precise mechanisms and best treatment for vaccine-associated tinnitus, researchers say.
As of mid-September 2021, 12,247 cases of tinnitus, or ringing in the ears, following COVID-19 vaccination had been reported to the Vaccine Adverse Event Reporting System of the U.S. Centers for Disease Control and Prevention.
“Despite several cases of tinnitus being reported following SARS-CoV-2 vaccination, the precise pathophysiology is still not clear,” write Syed Hassan Ahmed, 3rd-year MBBS student, Dow University of Health Sciences, Karachi, Pakistan, and coauthors.
The researchers review what is known and unknown about SARS-CoV-2 vaccine-associated tinnitus in an article published online Feb. 11 in Annals of Medicine and Surgery.
Molecular mimicry?
The researchers say cross-reactivity between anti-spike SARS-CoV-2 antibodies and otologic antigens is one possibility, based on the mechanisms behind other COVID-19 vaccine–induced disorders and the phenomenon of molecular mimicry.
“The heptapeptide resemblance between coronavirus spike glycoprotein and numerous human proteins further supports molecular mimicry as a potential mechanism behind such vaccine-induced disorders,” they write.
Anti-spike antibodies may react with antigens anywhere along the auditory pathway and fuel an inflammatory reaction, they point out.
“Therefore, understanding the phenomenon of cross-reactivity and molecular mimicry may be helpful in postulating potential treatment behind not only tinnitus but also the rare events of vaccination associated hearing loss and other otologic manifestations,” the authors say.
Genetic predispositions and associated conditions may also play a significant role in determining whether an individual develops vaccine-induced tinnitus.
Stress and anxiety following COVID vaccination may also play a role, inasmuch as anxiety-related adverse events following vaccination have been reported. Vaccine-related anxiety as a potential cause of tinnitus developing after vaccination needs to be explored, they write.
Jury out on best management
How best to manage COVID vaccine-associated tinnitus also remains unclear, but it starts with a well-established diagnosis, the authors say.
A well-focused and detailed history and examination are essential, with particular emphasis placed on preexisting health conditions, specifically, autoimmune diseases, such as Hashimoto thyroiditis; otologic conditions, such as sensorineural hearing loss; glaucoma; and psychological well-being. According to the review, patients often present with a history of one or more of these disorders.
“However, any such association has not yet been established and requires further investigation to be concluded as potential risk factors for vaccine-induced tinnitus,” they caution.
Routine cranial nerve examination, otoscopy, Weber test, and Rinne test, which are used for tinnitus diagnosis in general, may be helpful for confirmation of vaccine-associated tinnitus.
Owing to the significant association between tinnitus and hearing impairment, audiology should also performed, the authors say.
Although treatments for non–vaccine-induced tinnitus vary significantly, corticosteroids are the top treatment choice for SARS-CoV-2 vaccine-induced tinnitus reported in the literature.
Trials of other drug and nondrug interventions that may uniquely help with vaccine-associated tinnitus are urgently needed, the authors say.
Summing up, the reviewers say, “Although the incidence of COVID-19 vaccine-associated tinnitus is rare, there is an overwhelming need to discern the precise pathophysiology and clinical management as a better understanding of adverse events may help in encountering vaccine hesitancy and hence fostering the COVID-19 global vaccination program.
“Despite the incidence of adverse events, the benefits of the SARS-CoV-2 vaccine in reducing hospitalization and deaths continue to outweigh the rare ramifications,” they conclude.
The research had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF MEDICINE AND SURGERY









