User login
News and Views that Matter to Pediatricians
The leading independent newspaper covering news and commentary in pediatrics.
Does obesity blunt effects of vitamin D supplementation?
compared with normal-weight individuals in a new analysis of a randomized trial.
“There seems to be something different happening with vitamin D metabolism at higher body weights, and this study may help explain diminished outcomes of supplementation for individuals with an elevated body mass index (BMI),” said first author Deirdre K. Tobias, ScD, an associate epidemiologist at Brigham and Women’s Hospital’s division of preventive medicine in Boston. She made the comments in a press statement issued with the study, published online in JAMA Network Open.
The findings are from a post hoc analysis of the large-scale Vitamin D and Omega-3 Trial (VITAL), which overall, showed no benefits among those randomized to 5 years of vitamin D supplementation (2,000 IU/day) versus placebo in terms of the primary endpoints of cancer or major cardiovascular disease outcomes.
However, prespecified secondary analyses according to body weight showed that those of normal weight (body mass index < 25.0 kg/m2) did have significant benefits from supplementation versus placebo in terms of cancer incidence (24% lower), cancer mortality (42% lower), and autoimmune disease (22% lower), while no corresponding benefits were observed among those who were overweight or had obesity.
The new analysis adds important context to the trial’s overall findings, noted Katherine N. Bachmann, MD, in an accompanying editorial.
“Thanks to its very large sample size and detailed biomarker analyses, the current study is able to provide novel evidence that responses to vitamin D supplementation may be attenuated in individuals with overweight and obesity, and that this may contribute to the differential outcomes by BMI noted in the original VITAL,” she wrote.
“Further studies are warranted to determine the optimal dose or circulating vitamin D level for individuals with obesity for nonskeletal health-related outcomes,” added Dr. Bachmann, division of diabetes, endocrinology, and metabolism at Vanderbilt University Medical Center, Nashville, Tenn.
New analysis examined vitamin D and biomarkers at baseline and 2 years
To take a closer look at the specific changes in vitamin D serum and biomarker levels between the different body-weight groups, Dr. Tobias and colleagues evaluated data from 16,515 participants in the trial (of the 25,000 originally included in VITAL) and looked at changes in key vitamin D serum levels and biomarkers at baseline and follow-up.
Consistent with common observations of lower vitamin D levels with obesity, participants in the higher BMI categories had incrementally lower mean levels of serum total 25-hydroxyvitamin D (25-OHD) prior to randomization, with levels ranging from 32.3 ng/mL for normal weight individuals to 28.0 ng/mL for those with obesity class II (P < .001 for a linear trend).
Baseline levels of other vitamin D biomarkers were also lower with higher BMI, including total 25-OHD 3, free vitamin D (FVD), and bioavailable vitamin D (BioD).
Among 2,742 participants with repeated blood collections at year 2, significant mean increases were observed overall at the end of the study period in serum 25-OHD levels (11.9 ng/mL) among those randomized to vitamin D supplementation, compared with little change in the placebo group (–0.7 ng/mL).
There were also significant increases, overall, in mean total 25-OHD, 25-OHD3, FVD, and BioD levels at 2 years among those receiving supplementation, with little or no change in the placebo group.
When stratified by BMI level, however, the magnitude of increase was lower among those with higher baseline BMI (all treatment effect interactions P < .001). For instance, the mean increases in total 25-OHD level at 2 years for supplementation versus placebo were 13.5 ng/mL for those with a BMI less than 25.0 versus only 10.0 ng/mL for those with a BMI of at least 35.0.
Importantly, even after controlling for baseline vitamin D status of sufficiency or insufficiency, BMI was still significantly associated with changes seen with supplementation.
“It was surprising that, even in the context of low vitamin D levels, those with higher BMI still had a blunted response to supplementation, suggesting the interaction between supplementation and BMI with health outcomes is not simply due to higher prevalence of deficiency,” Dr. Tobias said in an interview. “It really does seem that, even with insufficient or low levels at baseline, those with higher BMI are not able to catch up to sufficient levels as well as those with normal BMI.”
Mechanisms?
Among leading theories as to why higher BMI would be associated with lower serum vitamin D levels and a lower response to supplementation is that because vitamin D is a fat-soluble vitamin, the increased adiposity and fat storage capacity with higher BMI results in greater removal of the vitamin from circulation.
“Our results are largely consistent with this hypothesis,” the authors noted.
They added that weight-loss studies, including those involving bariatric surgery, have further shown greater increases in serum 25-OHD or circulating vitamin D levels after weight loss compared with baseline.
Other theories suggest that obesity-induced hepatic dysfunction can contribute to impaired vitamin D metabolism.
Without a clear understanding of the exact mechanisms, the potential for addressing the lower vitamin D levels with, for instance, higher doses of supplementation among those with obesity, also remains unclear, Dr. Tobias noted.
“I think once there’s more clarity on what the mechanism is, then it would make sense to consider what doses could be necessary to achieve the internal levels desired,” she said.
The VITAL study received funding from a grant from the National Center for Complementary and Integrative Health and other sources.
A version of this article first appeared on Medscape.com.
compared with normal-weight individuals in a new analysis of a randomized trial.
“There seems to be something different happening with vitamin D metabolism at higher body weights, and this study may help explain diminished outcomes of supplementation for individuals with an elevated body mass index (BMI),” said first author Deirdre K. Tobias, ScD, an associate epidemiologist at Brigham and Women’s Hospital’s division of preventive medicine in Boston. She made the comments in a press statement issued with the study, published online in JAMA Network Open.
The findings are from a post hoc analysis of the large-scale Vitamin D and Omega-3 Trial (VITAL), which overall, showed no benefits among those randomized to 5 years of vitamin D supplementation (2,000 IU/day) versus placebo in terms of the primary endpoints of cancer or major cardiovascular disease outcomes.
However, prespecified secondary analyses according to body weight showed that those of normal weight (body mass index < 25.0 kg/m2) did have significant benefits from supplementation versus placebo in terms of cancer incidence (24% lower), cancer mortality (42% lower), and autoimmune disease (22% lower), while no corresponding benefits were observed among those who were overweight or had obesity.
The new analysis adds important context to the trial’s overall findings, noted Katherine N. Bachmann, MD, in an accompanying editorial.
“Thanks to its very large sample size and detailed biomarker analyses, the current study is able to provide novel evidence that responses to vitamin D supplementation may be attenuated in individuals with overweight and obesity, and that this may contribute to the differential outcomes by BMI noted in the original VITAL,” she wrote.
“Further studies are warranted to determine the optimal dose or circulating vitamin D level for individuals with obesity for nonskeletal health-related outcomes,” added Dr. Bachmann, division of diabetes, endocrinology, and metabolism at Vanderbilt University Medical Center, Nashville, Tenn.
New analysis examined vitamin D and biomarkers at baseline and 2 years
To take a closer look at the specific changes in vitamin D serum and biomarker levels between the different body-weight groups, Dr. Tobias and colleagues evaluated data from 16,515 participants in the trial (of the 25,000 originally included in VITAL) and looked at changes in key vitamin D serum levels and biomarkers at baseline and follow-up.
Consistent with common observations of lower vitamin D levels with obesity, participants in the higher BMI categories had incrementally lower mean levels of serum total 25-hydroxyvitamin D (25-OHD) prior to randomization, with levels ranging from 32.3 ng/mL for normal weight individuals to 28.0 ng/mL for those with obesity class II (P < .001 for a linear trend).
Baseline levels of other vitamin D biomarkers were also lower with higher BMI, including total 25-OHD 3, free vitamin D (FVD), and bioavailable vitamin D (BioD).
Among 2,742 participants with repeated blood collections at year 2, significant mean increases were observed overall at the end of the study period in serum 25-OHD levels (11.9 ng/mL) among those randomized to vitamin D supplementation, compared with little change in the placebo group (–0.7 ng/mL).
There were also significant increases, overall, in mean total 25-OHD, 25-OHD3, FVD, and BioD levels at 2 years among those receiving supplementation, with little or no change in the placebo group.
When stratified by BMI level, however, the magnitude of increase was lower among those with higher baseline BMI (all treatment effect interactions P < .001). For instance, the mean increases in total 25-OHD level at 2 years for supplementation versus placebo were 13.5 ng/mL for those with a BMI less than 25.0 versus only 10.0 ng/mL for those with a BMI of at least 35.0.
Importantly, even after controlling for baseline vitamin D status of sufficiency or insufficiency, BMI was still significantly associated with changes seen with supplementation.
“It was surprising that, even in the context of low vitamin D levels, those with higher BMI still had a blunted response to supplementation, suggesting the interaction between supplementation and BMI with health outcomes is not simply due to higher prevalence of deficiency,” Dr. Tobias said in an interview. “It really does seem that, even with insufficient or low levels at baseline, those with higher BMI are not able to catch up to sufficient levels as well as those with normal BMI.”
Mechanisms?
Among leading theories as to why higher BMI would be associated with lower serum vitamin D levels and a lower response to supplementation is that because vitamin D is a fat-soluble vitamin, the increased adiposity and fat storage capacity with higher BMI results in greater removal of the vitamin from circulation.
“Our results are largely consistent with this hypothesis,” the authors noted.
They added that weight-loss studies, including those involving bariatric surgery, have further shown greater increases in serum 25-OHD or circulating vitamin D levels after weight loss compared with baseline.
Other theories suggest that obesity-induced hepatic dysfunction can contribute to impaired vitamin D metabolism.
Without a clear understanding of the exact mechanisms, the potential for addressing the lower vitamin D levels with, for instance, higher doses of supplementation among those with obesity, also remains unclear, Dr. Tobias noted.
“I think once there’s more clarity on what the mechanism is, then it would make sense to consider what doses could be necessary to achieve the internal levels desired,” she said.
The VITAL study received funding from a grant from the National Center for Complementary and Integrative Health and other sources.
A version of this article first appeared on Medscape.com.
compared with normal-weight individuals in a new analysis of a randomized trial.
“There seems to be something different happening with vitamin D metabolism at higher body weights, and this study may help explain diminished outcomes of supplementation for individuals with an elevated body mass index (BMI),” said first author Deirdre K. Tobias, ScD, an associate epidemiologist at Brigham and Women’s Hospital’s division of preventive medicine in Boston. She made the comments in a press statement issued with the study, published online in JAMA Network Open.
The findings are from a post hoc analysis of the large-scale Vitamin D and Omega-3 Trial (VITAL), which overall, showed no benefits among those randomized to 5 years of vitamin D supplementation (2,000 IU/day) versus placebo in terms of the primary endpoints of cancer or major cardiovascular disease outcomes.
However, prespecified secondary analyses according to body weight showed that those of normal weight (body mass index < 25.0 kg/m2) did have significant benefits from supplementation versus placebo in terms of cancer incidence (24% lower), cancer mortality (42% lower), and autoimmune disease (22% lower), while no corresponding benefits were observed among those who were overweight or had obesity.
The new analysis adds important context to the trial’s overall findings, noted Katherine N. Bachmann, MD, in an accompanying editorial.
“Thanks to its very large sample size and detailed biomarker analyses, the current study is able to provide novel evidence that responses to vitamin D supplementation may be attenuated in individuals with overweight and obesity, and that this may contribute to the differential outcomes by BMI noted in the original VITAL,” she wrote.
“Further studies are warranted to determine the optimal dose or circulating vitamin D level for individuals with obesity for nonskeletal health-related outcomes,” added Dr. Bachmann, division of diabetes, endocrinology, and metabolism at Vanderbilt University Medical Center, Nashville, Tenn.
New analysis examined vitamin D and biomarkers at baseline and 2 years
To take a closer look at the specific changes in vitamin D serum and biomarker levels between the different body-weight groups, Dr. Tobias and colleagues evaluated data from 16,515 participants in the trial (of the 25,000 originally included in VITAL) and looked at changes in key vitamin D serum levels and biomarkers at baseline and follow-up.
Consistent with common observations of lower vitamin D levels with obesity, participants in the higher BMI categories had incrementally lower mean levels of serum total 25-hydroxyvitamin D (25-OHD) prior to randomization, with levels ranging from 32.3 ng/mL for normal weight individuals to 28.0 ng/mL for those with obesity class II (P < .001 for a linear trend).
Baseline levels of other vitamin D biomarkers were also lower with higher BMI, including total 25-OHD 3, free vitamin D (FVD), and bioavailable vitamin D (BioD).
Among 2,742 participants with repeated blood collections at year 2, significant mean increases were observed overall at the end of the study period in serum 25-OHD levels (11.9 ng/mL) among those randomized to vitamin D supplementation, compared with little change in the placebo group (–0.7 ng/mL).
There were also significant increases, overall, in mean total 25-OHD, 25-OHD3, FVD, and BioD levels at 2 years among those receiving supplementation, with little or no change in the placebo group.
When stratified by BMI level, however, the magnitude of increase was lower among those with higher baseline BMI (all treatment effect interactions P < .001). For instance, the mean increases in total 25-OHD level at 2 years for supplementation versus placebo were 13.5 ng/mL for those with a BMI less than 25.0 versus only 10.0 ng/mL for those with a BMI of at least 35.0.
Importantly, even after controlling for baseline vitamin D status of sufficiency or insufficiency, BMI was still significantly associated with changes seen with supplementation.
“It was surprising that, even in the context of low vitamin D levels, those with higher BMI still had a blunted response to supplementation, suggesting the interaction between supplementation and BMI with health outcomes is not simply due to higher prevalence of deficiency,” Dr. Tobias said in an interview. “It really does seem that, even with insufficient or low levels at baseline, those with higher BMI are not able to catch up to sufficient levels as well as those with normal BMI.”
Mechanisms?
Among leading theories as to why higher BMI would be associated with lower serum vitamin D levels and a lower response to supplementation is that because vitamin D is a fat-soluble vitamin, the increased adiposity and fat storage capacity with higher BMI results in greater removal of the vitamin from circulation.
“Our results are largely consistent with this hypothesis,” the authors noted.
They added that weight-loss studies, including those involving bariatric surgery, have further shown greater increases in serum 25-OHD or circulating vitamin D levels after weight loss compared with baseline.
Other theories suggest that obesity-induced hepatic dysfunction can contribute to impaired vitamin D metabolism.
Without a clear understanding of the exact mechanisms, the potential for addressing the lower vitamin D levels with, for instance, higher doses of supplementation among those with obesity, also remains unclear, Dr. Tobias noted.
“I think once there’s more clarity on what the mechanism is, then it would make sense to consider what doses could be necessary to achieve the internal levels desired,” she said.
The VITAL study received funding from a grant from the National Center for Complementary and Integrative Health and other sources.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
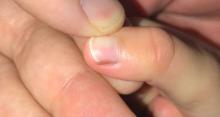
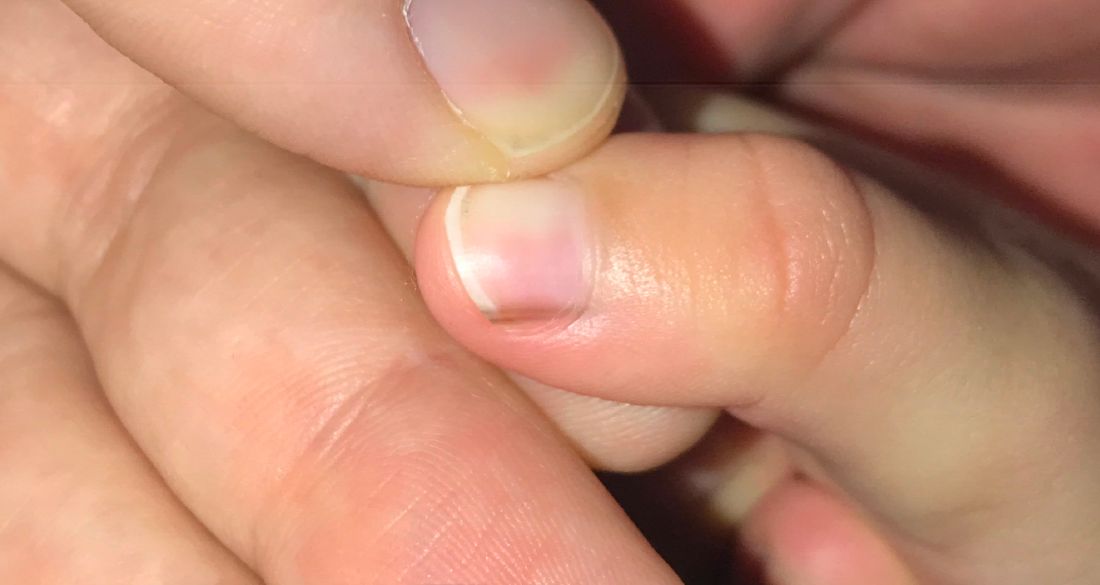
A toddler presents with a dark line on a fingernail
Given the over 1-year history of an unchanging longitudinal band of pigment without extension to the proximal or lateral nailfolds or any other nail findings, the most likely diagnosis is benign longitudinal melanonychia.
Longitudinal melanonychia, also known as melanonychia striata, describes a brown to black streak of pigment extending from the nail matrix to the free edge of the nail.1,2
This disorder can occur secondary to a wide variety of benign and pathologic causes including lentigines, nevi, melanoma, chronic trauma, inflammatory skin diseases, systemic diseases, iatrogenic causes, and genetic syndromes.3 In melanocytic causes of longitudinal melanonychia, either melanocytic activation or hyperplasia drive pigmentary development leading to the brown to black band seen in the nail.4 Benign causes of longitudinal melanonychia include benign melanocyte activation, lentigo, and benign nevus.1
What’s the differential diagnosis?
The differential diagnosis for longitudinal melanonychia can include a wide variety of local and systemic causes. For our discussion, we will limit our differential to other locally involved disorders of the nail including subungual melanoma, subungual hematoma, onychomycosis, and glomus tumor.
Subungual melanoma is a rare subtype of acral lentiginous melanoma that most often presents as longitudinal melanonychia. Subungual melanoma is more common in those aged 50-70 years, individuals with personal or family history of melanoma or dysplastic nevus syndrome, and persons with African American, Native American, and Asian descent. Longitudinal melanonychia features that can be concerning for subungual melanoma include the presence of multiple colors, width greater than or equal to 3 mm, blurry borders, rapid increase in size, and extension to the proximal or lateral nailfolds (Hutchinson’s sign). Biopsy is required to make the diagnosis of subungual melanoma but is not necessary for melanonychia without atypical features.
Treatment of subungual melanoma depends on disease stage and can range from wide local excision of the nail apparatus to amputation of the affected digit and management with a medical oncologist. Given the absence of concerning neoplastic findings or personal or family history of melanoma, subungual melanoma is unlikely in this patient.
Subungual hematoma is an accumulation of blood underneath the nail plate that is typically the result of acute or chronic trauma to the distal phalanx. It can present as purple, red, pink, brown, or black discoloration under the nail plate and is most commonly found on the first toe. With acute trauma, pain is usually present upon initial injury. Subungual hematomas typically resolve on their own with normal nail growth. The absence of a history of trauma or pain, and the linear appearance of the lesion in our patient are inconsistent with a subungual hematoma.
Onychomycosis is a fungal infection of the nail caused by dermatophytes, nondermatophytes, or yeasts. It may present with longitudinal melanonychia; however, it more often presents with other nail abnormalities such as nail thickening, yellow discoloration, onycholysis, splitting, subungual hyperkeratosis, and nail plate destruction, which are not present in this patient. Furthermore, onychomycosis is more common in adults than children. Diagnosis is usually made with potassium hydroxide (KOH) preparations, histopathologic examination of nail clippings with a periodic acid-Schiff stain, fungal culture, or PCR.
Glomus tumor is a rare, benign neoplasm originating from cells of the glomus body. It is often found in the subungual region, in addition to other areas rich in glomus bodies such as the fingertips, palms, wrists, and forearms. Subungual glomus tumors present as a red, purple, or blueish lesions under the nail plate. Distal notching or an overlying longitudinal fissure may be present. Subungual glomus tumors are typically associated with pinpoint tenderness, paroxysmal pain, and cold sensitivity, features that are not present in our patient. The history and examination of our patient are much more consistent with benign longitudinal melanonychia.
It appears that melanoma associated with longitudinal melanonychia is very rare in children. According to one review published in 2020, only 12 cases of pediatric subungual melanoma have been reported.5 Recent series have observed longitudinal melanonychia in large sets of children, with findings that demonstrate that the vast majority of longitudinal melanonychia either stops progressing or regresses. These investigations therefore recommend serial observation of longitudinal melanonychia except in rare circumstances.6,7
Given the lack of troubling findings or concerning history, our patient was managed with observation. On follow-up 6 months later, he was found to have no change in his nail pigmentation.
Dr. Haft is an inflammatory skin disease fellow in the division of pediatric and adolescent dermatology; Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Mannava KA et al. Hand Surg. 2013;18(1):133-9.
2. Leung AKC et al. Int J Dermatol. 2019;58(11):1239-45.
3. Andre J and Lateur N. Dermatol Clin. 2006;24(3):329-39.
4. Lee DK and Lipner SR. Ann Med. 2022;54(1):694-712.
5. Smith RJ and Rubin AI. Curr Opin Pediatr. 2020;32(4):506-15. .
6. Matsui Y et al. J Am Acad Dermatol. 2022;86(4):946-8.
7. Lee JS et al. J Am Acad Dermatol. 2022;87(2):366-72.
Given the over 1-year history of an unchanging longitudinal band of pigment without extension to the proximal or lateral nailfolds or any other nail findings, the most likely diagnosis is benign longitudinal melanonychia.
Longitudinal melanonychia, also known as melanonychia striata, describes a brown to black streak of pigment extending from the nail matrix to the free edge of the nail.1,2
This disorder can occur secondary to a wide variety of benign and pathologic causes including lentigines, nevi, melanoma, chronic trauma, inflammatory skin diseases, systemic diseases, iatrogenic causes, and genetic syndromes.3 In melanocytic causes of longitudinal melanonychia, either melanocytic activation or hyperplasia drive pigmentary development leading to the brown to black band seen in the nail.4 Benign causes of longitudinal melanonychia include benign melanocyte activation, lentigo, and benign nevus.1
What’s the differential diagnosis?
The differential diagnosis for longitudinal melanonychia can include a wide variety of local and systemic causes. For our discussion, we will limit our differential to other locally involved disorders of the nail including subungual melanoma, subungual hematoma, onychomycosis, and glomus tumor.
Subungual melanoma is a rare subtype of acral lentiginous melanoma that most often presents as longitudinal melanonychia. Subungual melanoma is more common in those aged 50-70 years, individuals with personal or family history of melanoma or dysplastic nevus syndrome, and persons with African American, Native American, and Asian descent. Longitudinal melanonychia features that can be concerning for subungual melanoma include the presence of multiple colors, width greater than or equal to 3 mm, blurry borders, rapid increase in size, and extension to the proximal or lateral nailfolds (Hutchinson’s sign). Biopsy is required to make the diagnosis of subungual melanoma but is not necessary for melanonychia without atypical features.
Treatment of subungual melanoma depends on disease stage and can range from wide local excision of the nail apparatus to amputation of the affected digit and management with a medical oncologist. Given the absence of concerning neoplastic findings or personal or family history of melanoma, subungual melanoma is unlikely in this patient.
Subungual hematoma is an accumulation of blood underneath the nail plate that is typically the result of acute or chronic trauma to the distal phalanx. It can present as purple, red, pink, brown, or black discoloration under the nail plate and is most commonly found on the first toe. With acute trauma, pain is usually present upon initial injury. Subungual hematomas typically resolve on their own with normal nail growth. The absence of a history of trauma or pain, and the linear appearance of the lesion in our patient are inconsistent with a subungual hematoma.
Onychomycosis is a fungal infection of the nail caused by dermatophytes, nondermatophytes, or yeasts. It may present with longitudinal melanonychia; however, it more often presents with other nail abnormalities such as nail thickening, yellow discoloration, onycholysis, splitting, subungual hyperkeratosis, and nail plate destruction, which are not present in this patient. Furthermore, onychomycosis is more common in adults than children. Diagnosis is usually made with potassium hydroxide (KOH) preparations, histopathologic examination of nail clippings with a periodic acid-Schiff stain, fungal culture, or PCR.
Glomus tumor is a rare, benign neoplasm originating from cells of the glomus body. It is often found in the subungual region, in addition to other areas rich in glomus bodies such as the fingertips, palms, wrists, and forearms. Subungual glomus tumors present as a red, purple, or blueish lesions under the nail plate. Distal notching or an overlying longitudinal fissure may be present. Subungual glomus tumors are typically associated with pinpoint tenderness, paroxysmal pain, and cold sensitivity, features that are not present in our patient. The history and examination of our patient are much more consistent with benign longitudinal melanonychia.
It appears that melanoma associated with longitudinal melanonychia is very rare in children. According to one review published in 2020, only 12 cases of pediatric subungual melanoma have been reported.5 Recent series have observed longitudinal melanonychia in large sets of children, with findings that demonstrate that the vast majority of longitudinal melanonychia either stops progressing or regresses. These investigations therefore recommend serial observation of longitudinal melanonychia except in rare circumstances.6,7
Given the lack of troubling findings or concerning history, our patient was managed with observation. On follow-up 6 months later, he was found to have no change in his nail pigmentation.
Dr. Haft is an inflammatory skin disease fellow in the division of pediatric and adolescent dermatology; Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Mannava KA et al. Hand Surg. 2013;18(1):133-9.
2. Leung AKC et al. Int J Dermatol. 2019;58(11):1239-45.
3. Andre J and Lateur N. Dermatol Clin. 2006;24(3):329-39.
4. Lee DK and Lipner SR. Ann Med. 2022;54(1):694-712.
5. Smith RJ and Rubin AI. Curr Opin Pediatr. 2020;32(4):506-15. .
6. Matsui Y et al. J Am Acad Dermatol. 2022;86(4):946-8.
7. Lee JS et al. J Am Acad Dermatol. 2022;87(2):366-72.
Given the over 1-year history of an unchanging longitudinal band of pigment without extension to the proximal or lateral nailfolds or any other nail findings, the most likely diagnosis is benign longitudinal melanonychia.
Longitudinal melanonychia, also known as melanonychia striata, describes a brown to black streak of pigment extending from the nail matrix to the free edge of the nail.1,2
This disorder can occur secondary to a wide variety of benign and pathologic causes including lentigines, nevi, melanoma, chronic trauma, inflammatory skin diseases, systemic diseases, iatrogenic causes, and genetic syndromes.3 In melanocytic causes of longitudinal melanonychia, either melanocytic activation or hyperplasia drive pigmentary development leading to the brown to black band seen in the nail.4 Benign causes of longitudinal melanonychia include benign melanocyte activation, lentigo, and benign nevus.1
What’s the differential diagnosis?
The differential diagnosis for longitudinal melanonychia can include a wide variety of local and systemic causes. For our discussion, we will limit our differential to other locally involved disorders of the nail including subungual melanoma, subungual hematoma, onychomycosis, and glomus tumor.
Subungual melanoma is a rare subtype of acral lentiginous melanoma that most often presents as longitudinal melanonychia. Subungual melanoma is more common in those aged 50-70 years, individuals with personal or family history of melanoma or dysplastic nevus syndrome, and persons with African American, Native American, and Asian descent. Longitudinal melanonychia features that can be concerning for subungual melanoma include the presence of multiple colors, width greater than or equal to 3 mm, blurry borders, rapid increase in size, and extension to the proximal or lateral nailfolds (Hutchinson’s sign). Biopsy is required to make the diagnosis of subungual melanoma but is not necessary for melanonychia without atypical features.
Treatment of subungual melanoma depends on disease stage and can range from wide local excision of the nail apparatus to amputation of the affected digit and management with a medical oncologist. Given the absence of concerning neoplastic findings or personal or family history of melanoma, subungual melanoma is unlikely in this patient.
Subungual hematoma is an accumulation of blood underneath the nail plate that is typically the result of acute or chronic trauma to the distal phalanx. It can present as purple, red, pink, brown, or black discoloration under the nail plate and is most commonly found on the first toe. With acute trauma, pain is usually present upon initial injury. Subungual hematomas typically resolve on their own with normal nail growth. The absence of a history of trauma or pain, and the linear appearance of the lesion in our patient are inconsistent with a subungual hematoma.
Onychomycosis is a fungal infection of the nail caused by dermatophytes, nondermatophytes, or yeasts. It may present with longitudinal melanonychia; however, it more often presents with other nail abnormalities such as nail thickening, yellow discoloration, onycholysis, splitting, subungual hyperkeratosis, and nail plate destruction, which are not present in this patient. Furthermore, onychomycosis is more common in adults than children. Diagnosis is usually made with potassium hydroxide (KOH) preparations, histopathologic examination of nail clippings with a periodic acid-Schiff stain, fungal culture, or PCR.
Glomus tumor is a rare, benign neoplasm originating from cells of the glomus body. It is often found in the subungual region, in addition to other areas rich in glomus bodies such as the fingertips, palms, wrists, and forearms. Subungual glomus tumors present as a red, purple, or blueish lesions under the nail plate. Distal notching or an overlying longitudinal fissure may be present. Subungual glomus tumors are typically associated with pinpoint tenderness, paroxysmal pain, and cold sensitivity, features that are not present in our patient. The history and examination of our patient are much more consistent with benign longitudinal melanonychia.
It appears that melanoma associated with longitudinal melanonychia is very rare in children. According to one review published in 2020, only 12 cases of pediatric subungual melanoma have been reported.5 Recent series have observed longitudinal melanonychia in large sets of children, with findings that demonstrate that the vast majority of longitudinal melanonychia either stops progressing or regresses. These investigations therefore recommend serial observation of longitudinal melanonychia except in rare circumstances.6,7
Given the lack of troubling findings or concerning history, our patient was managed with observation. On follow-up 6 months later, he was found to have no change in his nail pigmentation.
Dr. Haft is an inflammatory skin disease fellow in the division of pediatric and adolescent dermatology; Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Mannava KA et al. Hand Surg. 2013;18(1):133-9.
2. Leung AKC et al. Int J Dermatol. 2019;58(11):1239-45.
3. Andre J and Lateur N. Dermatol Clin. 2006;24(3):329-39.
4. Lee DK and Lipner SR. Ann Med. 2022;54(1):694-712.
5. Smith RJ and Rubin AI. Curr Opin Pediatr. 2020;32(4):506-15. .
6. Matsui Y et al. J Am Acad Dermatol. 2022;86(4):946-8.
7. Lee JS et al. J Am Acad Dermatol. 2022;87(2):366-72.
Examination findings reveal a 2-mm brown longitudinal band on the radial aspect of the right thumbnail that does not extend into the proximal or lateral nailfolds. The rest of the skin and nail exam is unremarkable.
Updated celiac disease guideline addresses common clinical questions
The American College of Gastroenterology issued updated guidelines for celiac disease diagnosis, management, and screening that incorporates research conducted since the last update in 2013.
The guidelines offer evidence-based recommendations for common clinical questions on topics that include nonbiopsy diagnosis, gluten-free oats, probiotic use, and gluten-detection devices. They also point to areas for ongoing research.
“The main message of the guideline is all about quality of care,” Alberto Rubio-Tapia, MD, a gastroenterologist at the Cleveland Clinic, said in an interview.
“A precise celiac disease diagnosis is just the beginning of the role of the gastroenterologist,” he said. “But most importantly, we need to take care of our patients’ needs with good goal-directed follow-up using a multidisciplinary approach, with experienced dietitians playing an important role.”
The update was published in the American Journal of Gastroenterology.
Diagnosis recommendations
The ACG assembled a team of celiac disease experts and expert guideline methodologists to develop an update with high-quality evidence, Dr. Rubio-Tapia said. The authors made recommendations and suggestions for future research regarding eight questions concerning diagnosis, disease management, and screening.
For diagnosis, the guidelines recommend esophagogastroduodenoscopy (EGD) with multiple duodenal biopsies – one or two from the bulb and four from the distal duodenum – for confirmation in children and adults with suspicion of celiac disease. EGD and duodenal biopsies can also be useful for the differential diagnosis of other malabsorptive disorders or enteropathies, the authors wrote.
For children, a nonbiopsy option may be considered to be reliable for diagnosis. This option includes a combination of high-level tissue transglutaminase (TTG) IgA – at greater than 10 times the upper limit of normal – and a positive endomysial antibody finding in a second blood sample. The same criteria may be considered after the fact for symptomatic adults who are unwilling or unable to undergo upper GI endoscopy.
For children younger than 2 years, the TTG-IgA is the preferred test for those who are not IgA deficient. For children with IgA deficiency, testing should be performed using IgG-based antibodies.
Disease management guidance
After diagnosis, intestinal healing should be the endpoint for a gluten-free diet, the guidelines recommended. Clinicians and patients should discuss individualized goals of the gluten-free diet beyond clinical and serologic remission.
The standard of care for assessing patients’ diet adherence is an interview with a dietician who has expertise in gluten-free diets, the recommendations stated. Subsequent visits should be encouraged as needed to reinforce adherence.
During disease management, upper endoscopy with intestinal biopsies can be helpful for monitoring cases in which there is a lack of clinical response or in which symptoms relapse despite a gluten-free diet, the authors noted.
In addition, after a shared decision-making conversation between the patient and provider, a follow-up biopsy could be considered for assessment of mucosal healing in adults who don’t have symptoms 2 years after starting a gluten-free diet, they wrote.
“Although most patients do well on a gluten-free diet, it’s a heavy burden of care and an important issue that impacts patients,” Joseph Murray, MD, a gastroenterologist at the Mayo Clinic in Rochester, Minn., said in an interview.
Dr. Murray, who wasn’t involved with this guideline update, contributed to the 2013 guidelines and the 2019 American Gastroenterological Association practice update on diagnosing and monitoring celiac disease. He agreed with many of the recommendations in this update.
“The goal of achieving healing is a good goal to reach. We do that routinely in my practice,” he said. “The older the patient, perhaps the more important it is to discuss, including the risk for complications. There’s a nuance involved with shared decision-making.”
Nutrition advice
The guidelines recommended against routine use of gluten-detection devices for food or biospecimens for patients with celiac disease. Although multiple devices have become commercially available in recent years, they are not regulated by the Food and Drug Administration and have sensitivity problems that can lead to false positive and false negative results, the authors noted. There’s also a lack of evidence that the devices enhance diet adherence or quality of life.
The evidence is insufficient to recommend for or against the use of probiotics for the treatment of celiac disease, the recommendations stated. Although dysbiosis is a feature of celiac disease, its role in disease pathogenesis and symptomatology is uncertain, the authors wrote.
Probiotics may help with functional disorders, such as irritable bowel syndrome, but because probiotics are marketed as supplements and regulations are lax, some products may contain detectable gluten despite being labeled gluten free, they added.
On the other hand, the authors recommended gluten-free oats as part of a gluten-free diet. Oat consumption appears to be safe for most patients with celiac disease, but it may be immunogenic in a subset of patients, depending on the products or quantity consumed. Given the small risk for an immune reaction to the oat protein avenin, monitoring for oat tolerance through symptoms and serology should be conducted, although the intervals for monitoring remain unknown.
Vaccination and screening
The guidelines also support vaccination against pneumococcal disease, since adults with celiac disease are at significantly increased risk of infection and complications. Vaccination is widely recommended for people aged 65 and older, for smokers aged 19-64, and for adults with underlying conditions that place them at higher risk, the authors noted.
Overall, the guidelines recommended case findings to increase detection of celiac disease in clinical practice but recommend against mass screening in the community. Patients with symptoms for whom there is lab evidence of malabsorption should be tested, as well as those for whom celiac disease could be a treatable cause of symptoms, the authors wrote. Those with a first-degree family member who has a confirmed diagnosis should also be tested if they have possible symptoms, and asymptomatic relatives should consider testing as well.
The updated guidelines include changes that are important for patients and patient care, and they emphasize the need for continued research on key questions, Isabel Hujoel, MD, a gastroenterologist at the University of Washington Medical Center, Seattle, told this news organization.
“In particular, the discussion on the lack of evidence behind gluten-detection devices and probiotic use in celiac disease addresses conversations that come up frequently in clinic,” said Dr. Hujoel, who wasn’t involved with the update. “The guidelines also include a new addition below each recommendation where future research questions are raised. Many of these questions address gaps in our understanding on celiac disease, such as the possibility of a nonbiopsy diagnosis in adults, which will potentially dramatically impact patient care if addressed.”
The update received no funding. The authors, Dr. Murray, and Dr. Hujoel have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The American College of Gastroenterology issued updated guidelines for celiac disease diagnosis, management, and screening that incorporates research conducted since the last update in 2013.
The guidelines offer evidence-based recommendations for common clinical questions on topics that include nonbiopsy diagnosis, gluten-free oats, probiotic use, and gluten-detection devices. They also point to areas for ongoing research.
“The main message of the guideline is all about quality of care,” Alberto Rubio-Tapia, MD, a gastroenterologist at the Cleveland Clinic, said in an interview.
“A precise celiac disease diagnosis is just the beginning of the role of the gastroenterologist,” he said. “But most importantly, we need to take care of our patients’ needs with good goal-directed follow-up using a multidisciplinary approach, with experienced dietitians playing an important role.”
The update was published in the American Journal of Gastroenterology.
Diagnosis recommendations
The ACG assembled a team of celiac disease experts and expert guideline methodologists to develop an update with high-quality evidence, Dr. Rubio-Tapia said. The authors made recommendations and suggestions for future research regarding eight questions concerning diagnosis, disease management, and screening.
For diagnosis, the guidelines recommend esophagogastroduodenoscopy (EGD) with multiple duodenal biopsies – one or two from the bulb and four from the distal duodenum – for confirmation in children and adults with suspicion of celiac disease. EGD and duodenal biopsies can also be useful for the differential diagnosis of other malabsorptive disorders or enteropathies, the authors wrote.
For children, a nonbiopsy option may be considered to be reliable for diagnosis. This option includes a combination of high-level tissue transglutaminase (TTG) IgA – at greater than 10 times the upper limit of normal – and a positive endomysial antibody finding in a second blood sample. The same criteria may be considered after the fact for symptomatic adults who are unwilling or unable to undergo upper GI endoscopy.
For children younger than 2 years, the TTG-IgA is the preferred test for those who are not IgA deficient. For children with IgA deficiency, testing should be performed using IgG-based antibodies.
Disease management guidance
After diagnosis, intestinal healing should be the endpoint for a gluten-free diet, the guidelines recommended. Clinicians and patients should discuss individualized goals of the gluten-free diet beyond clinical and serologic remission.
The standard of care for assessing patients’ diet adherence is an interview with a dietician who has expertise in gluten-free diets, the recommendations stated. Subsequent visits should be encouraged as needed to reinforce adherence.
During disease management, upper endoscopy with intestinal biopsies can be helpful for monitoring cases in which there is a lack of clinical response or in which symptoms relapse despite a gluten-free diet, the authors noted.
In addition, after a shared decision-making conversation between the patient and provider, a follow-up biopsy could be considered for assessment of mucosal healing in adults who don’t have symptoms 2 years after starting a gluten-free diet, they wrote.
“Although most patients do well on a gluten-free diet, it’s a heavy burden of care and an important issue that impacts patients,” Joseph Murray, MD, a gastroenterologist at the Mayo Clinic in Rochester, Minn., said in an interview.
Dr. Murray, who wasn’t involved with this guideline update, contributed to the 2013 guidelines and the 2019 American Gastroenterological Association practice update on diagnosing and monitoring celiac disease. He agreed with many of the recommendations in this update.
“The goal of achieving healing is a good goal to reach. We do that routinely in my practice,” he said. “The older the patient, perhaps the more important it is to discuss, including the risk for complications. There’s a nuance involved with shared decision-making.”
Nutrition advice
The guidelines recommended against routine use of gluten-detection devices for food or biospecimens for patients with celiac disease. Although multiple devices have become commercially available in recent years, they are not regulated by the Food and Drug Administration and have sensitivity problems that can lead to false positive and false negative results, the authors noted. There’s also a lack of evidence that the devices enhance diet adherence or quality of life.
The evidence is insufficient to recommend for or against the use of probiotics for the treatment of celiac disease, the recommendations stated. Although dysbiosis is a feature of celiac disease, its role in disease pathogenesis and symptomatology is uncertain, the authors wrote.
Probiotics may help with functional disorders, such as irritable bowel syndrome, but because probiotics are marketed as supplements and regulations are lax, some products may contain detectable gluten despite being labeled gluten free, they added.
On the other hand, the authors recommended gluten-free oats as part of a gluten-free diet. Oat consumption appears to be safe for most patients with celiac disease, but it may be immunogenic in a subset of patients, depending on the products or quantity consumed. Given the small risk for an immune reaction to the oat protein avenin, monitoring for oat tolerance through symptoms and serology should be conducted, although the intervals for monitoring remain unknown.
Vaccination and screening
The guidelines also support vaccination against pneumococcal disease, since adults with celiac disease are at significantly increased risk of infection and complications. Vaccination is widely recommended for people aged 65 and older, for smokers aged 19-64, and for adults with underlying conditions that place them at higher risk, the authors noted.
Overall, the guidelines recommended case findings to increase detection of celiac disease in clinical practice but recommend against mass screening in the community. Patients with symptoms for whom there is lab evidence of malabsorption should be tested, as well as those for whom celiac disease could be a treatable cause of symptoms, the authors wrote. Those with a first-degree family member who has a confirmed diagnosis should also be tested if they have possible symptoms, and asymptomatic relatives should consider testing as well.
The updated guidelines include changes that are important for patients and patient care, and they emphasize the need for continued research on key questions, Isabel Hujoel, MD, a gastroenterologist at the University of Washington Medical Center, Seattle, told this news organization.
“In particular, the discussion on the lack of evidence behind gluten-detection devices and probiotic use in celiac disease addresses conversations that come up frequently in clinic,” said Dr. Hujoel, who wasn’t involved with the update. “The guidelines also include a new addition below each recommendation where future research questions are raised. Many of these questions address gaps in our understanding on celiac disease, such as the possibility of a nonbiopsy diagnosis in adults, which will potentially dramatically impact patient care if addressed.”
The update received no funding. The authors, Dr. Murray, and Dr. Hujoel have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The American College of Gastroenterology issued updated guidelines for celiac disease diagnosis, management, and screening that incorporates research conducted since the last update in 2013.
The guidelines offer evidence-based recommendations for common clinical questions on topics that include nonbiopsy diagnosis, gluten-free oats, probiotic use, and gluten-detection devices. They also point to areas for ongoing research.
“The main message of the guideline is all about quality of care,” Alberto Rubio-Tapia, MD, a gastroenterologist at the Cleveland Clinic, said in an interview.
“A precise celiac disease diagnosis is just the beginning of the role of the gastroenterologist,” he said. “But most importantly, we need to take care of our patients’ needs with good goal-directed follow-up using a multidisciplinary approach, with experienced dietitians playing an important role.”
The update was published in the American Journal of Gastroenterology.
Diagnosis recommendations
The ACG assembled a team of celiac disease experts and expert guideline methodologists to develop an update with high-quality evidence, Dr. Rubio-Tapia said. The authors made recommendations and suggestions for future research regarding eight questions concerning diagnosis, disease management, and screening.
For diagnosis, the guidelines recommend esophagogastroduodenoscopy (EGD) with multiple duodenal biopsies – one or two from the bulb and four from the distal duodenum – for confirmation in children and adults with suspicion of celiac disease. EGD and duodenal biopsies can also be useful for the differential diagnosis of other malabsorptive disorders or enteropathies, the authors wrote.
For children, a nonbiopsy option may be considered to be reliable for diagnosis. This option includes a combination of high-level tissue transglutaminase (TTG) IgA – at greater than 10 times the upper limit of normal – and a positive endomysial antibody finding in a second blood sample. The same criteria may be considered after the fact for symptomatic adults who are unwilling or unable to undergo upper GI endoscopy.
For children younger than 2 years, the TTG-IgA is the preferred test for those who are not IgA deficient. For children with IgA deficiency, testing should be performed using IgG-based antibodies.
Disease management guidance
After diagnosis, intestinal healing should be the endpoint for a gluten-free diet, the guidelines recommended. Clinicians and patients should discuss individualized goals of the gluten-free diet beyond clinical and serologic remission.
The standard of care for assessing patients’ diet adherence is an interview with a dietician who has expertise in gluten-free diets, the recommendations stated. Subsequent visits should be encouraged as needed to reinforce adherence.
During disease management, upper endoscopy with intestinal biopsies can be helpful for monitoring cases in which there is a lack of clinical response or in which symptoms relapse despite a gluten-free diet, the authors noted.
In addition, after a shared decision-making conversation between the patient and provider, a follow-up biopsy could be considered for assessment of mucosal healing in adults who don’t have symptoms 2 years after starting a gluten-free diet, they wrote.
“Although most patients do well on a gluten-free diet, it’s a heavy burden of care and an important issue that impacts patients,” Joseph Murray, MD, a gastroenterologist at the Mayo Clinic in Rochester, Minn., said in an interview.
Dr. Murray, who wasn’t involved with this guideline update, contributed to the 2013 guidelines and the 2019 American Gastroenterological Association practice update on diagnosing and monitoring celiac disease. He agreed with many of the recommendations in this update.
“The goal of achieving healing is a good goal to reach. We do that routinely in my practice,” he said. “The older the patient, perhaps the more important it is to discuss, including the risk for complications. There’s a nuance involved with shared decision-making.”
Nutrition advice
The guidelines recommended against routine use of gluten-detection devices for food or biospecimens for patients with celiac disease. Although multiple devices have become commercially available in recent years, they are not regulated by the Food and Drug Administration and have sensitivity problems that can lead to false positive and false negative results, the authors noted. There’s also a lack of evidence that the devices enhance diet adherence or quality of life.
The evidence is insufficient to recommend for or against the use of probiotics for the treatment of celiac disease, the recommendations stated. Although dysbiosis is a feature of celiac disease, its role in disease pathogenesis and symptomatology is uncertain, the authors wrote.
Probiotics may help with functional disorders, such as irritable bowel syndrome, but because probiotics are marketed as supplements and regulations are lax, some products may contain detectable gluten despite being labeled gluten free, they added.
On the other hand, the authors recommended gluten-free oats as part of a gluten-free diet. Oat consumption appears to be safe for most patients with celiac disease, but it may be immunogenic in a subset of patients, depending on the products or quantity consumed. Given the small risk for an immune reaction to the oat protein avenin, monitoring for oat tolerance through symptoms and serology should be conducted, although the intervals for monitoring remain unknown.
Vaccination and screening
The guidelines also support vaccination against pneumococcal disease, since adults with celiac disease are at significantly increased risk of infection and complications. Vaccination is widely recommended for people aged 65 and older, for smokers aged 19-64, and for adults with underlying conditions that place them at higher risk, the authors noted.
Overall, the guidelines recommended case findings to increase detection of celiac disease in clinical practice but recommend against mass screening in the community. Patients with symptoms for whom there is lab evidence of malabsorption should be tested, as well as those for whom celiac disease could be a treatable cause of symptoms, the authors wrote. Those with a first-degree family member who has a confirmed diagnosis should also be tested if they have possible symptoms, and asymptomatic relatives should consider testing as well.
The updated guidelines include changes that are important for patients and patient care, and they emphasize the need for continued research on key questions, Isabel Hujoel, MD, a gastroenterologist at the University of Washington Medical Center, Seattle, told this news organization.
“In particular, the discussion on the lack of evidence behind gluten-detection devices and probiotic use in celiac disease addresses conversations that come up frequently in clinic,” said Dr. Hujoel, who wasn’t involved with the update. “The guidelines also include a new addition below each recommendation where future research questions are raised. Many of these questions address gaps in our understanding on celiac disease, such as the possibility of a nonbiopsy diagnosis in adults, which will potentially dramatically impact patient care if addressed.”
The update received no funding. The authors, Dr. Murray, and Dr. Hujoel have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
The Respect for Marriage Act: How this law supports the health and well-being of LGBTQ+ youth
Childhood and adolescence are periods of life with rapid growth and development in which the psychosocial factors of one’s environment can have a profound effect on health. There is increasing evidence that adverse childhood experiences (ACEs) can have significant negative effects on long-term health with effects persisting into subsequent generations.1 Youth themselves, however, often do not have the voice, ability, or political power to advocate for safe and more supportive environments that are essential to their well-being. Thus, advocacy has been central to the profession of pediatrics since its inception, where providers can partner with their patients, families, and communities to push for changes in the environments in which youth live and grow.2
LGBTQ+ youth are known to be at increased risk for ACEs because of the stress that comes from being part of a minority group and the discrimination they experience by their families, communities, and society at large. These factors within their environments have been shown to be associated with increased rates of anxiety, depression, substance use, sexually transmitted infections, and homelessness.3 As with other health outcomes that have been linked to the social determinants of health, these disparities are not inevitable and could be greatly improved upon through advocacy and changes in the environments of LGBTQ+ youth.
Marriage equality (the recognition that same-sex couples have the same legal right to marry as opposite-sex couples) has been shown to be not only a political issue, but one that affects health. The debates surrounding marriage equality have contributed to minority stress by questioning the validity of same-sex relationships and assigning them less value relative to opposite-sex relationships.4 In 1996, the U.S. Congress passed the Defense of Marriage Act (DOMA), which federally defined marriage as being legally recognized only between opposite-sex couples.
Individual states then continued the marriage equality debate by passing individual state laws either allowing or prohibiting same-sex marriage. During this time, it was shown that, in states where same-sex marriage was legally prohibited, LGBTQ+ adults reported significantly higher rates of generalized anxiety disorder, alcohol use disorder, any mood disorder, and psychiatric comorbidity when compared with states without a legal ban on same-sex marriage.5
Using data from the Youth Risk Behavior Surveillance System, it was shown that state policies recognizing same-sex marriage were associated with a 7% relative reduction in suicide attempts reported by adolescent sexual minority students compared with before these policies.6 It was also shown that children with same-sex parents were overall less likely to have private health insurance, but this disparity was improved in states that legally recognized same-sex marriage and allowed second-parent adoptions.7
In 2013, the U.S. Supreme Court ruled that DOMA was unconstitutional, requiring the federal government to legally recognize same-sex marriages for the purposes of federal benefits. In 2015, the U.S. Supreme Court further ruled that same-sex couples are guaranteed the fundamental right to marry, requiring that all states issue marriage licenses to same-sex couples. These rulings were associated with a decrease in reported levels of stigma over time and increased reported levels of family support, particularly for those in same-sex relationships.8
The Respect for Marriage Act (RFMA) was passed by the U.S. Congress and signed into law by President Biden on Dec. 13, 2022. This law officially repeals DOMA and requires all states and the federal government to recognize same-sex marriages performed in any U.S. state or territory.9
If the U.S. Supreme Court were to overturn the 2015 marriage equality decision, individual state laws ensuring or banning same-sex marriage would again be in effect. However, the RFMA ensures that all states continue to recognize same-sex marriages performed in any U.S. state or territory (even if that state itself bans same-sex marriage). While we do not yet have any studies or data regarding the effect of the RFMA on public health, we can expect positive effects by drawing on the previous evidence on the effect of marriage equality and its effect on the health and well-being of LGBTQ+ individuals. By establishing marriage equality in the United States, our government institutions are affirming the relationships and identities of those in same-sex relationships, with the potential effect of helping to destigmatize the LGBTQ+ community.
Since 2002, the American Academy of Pediatrics has recommended that pediatricians “support the right of every child and family to the financial, psychological, and legal security that results from having legally recognized parents who are committed to each other and to the welfare of their children,” acknowledging that “legislative initiatives assuring legal status equivalent to marriage for gay and lesbian partners … can also attend to providing security and permanence for the children of those partnerships.”10 While changes in legal marriage equality are likely to have a positive effect on those within the LGBTQ+ community, it should also be understood that this will not solve all of the psychosocial effects and resultant health disparities that these children face.
A recent scoping review highlights that, as the result of marriage equality progress, sexual minority adults have reported increased social acceptance and reduced stigma across individual, community, and societal levels, but that sexual minority stigma continues to persist across all levels.11
As pediatricians, we can continue to support LGBTQ+ patients and parents by providing care in a safe and affirming environment in which families understand and embrace the healthy development of gender identity and sexuality in an open and destigmatized manner. Delivering care using this approach in and of itself can be seen as advocacy to promote health and well-being within minoritized populations. Pediatricians are also encouraged to become engaged in local and national advocacy initiatives to have a broader effect in the fight for health equity in minority populations, including LGBTQ+ families and youth.
Pediatricians should work with their patients, families, and communities to advocate for structural change needed to address the social determinants of health for optimal growth and development.
Dr. Warus is an adolescent medicine physician who specializes in care for transgender and gender-nonconforming youth, and LGBTQ health for youth at Children’s Hospital of Los Angeles. He is an assistant professor of pediatrics at University of Southern California, Los Angeles.
Resources
Bright Futures – Promoting Healthy Development of Sexuality and Gender Identity (Implementation Tip Sheet): https://downloads.aap.org/AAP/PDF/BF_HealthySexualityGenderIdentity_Tipsheet.pdf
Bright Futures – Implementing Social Determinants of Health Into Health Supervision Visits (Implementation Tip Sheet): https://downloads.aap.org/AAP/PDF/Bright%20Futures/BF_IntegrateSDoH_Tipsheet.pdf?_ga=2.214227031.1330574154.1673910248-58875083.1673910248
American Academy of Pediatrics – Advocacy Website: https://www.aap.org/en/advocacy/
References
1. Hughes K et al. Lancet Public Health. 2017;2(8):e356-66.
2. Camero K and Javier JR. Pediatr Clin N Am. 2023;70:43-51.
3. Lund EM and Burgess CM. Prim Care Clin Office Pract. 2021;48:179-89.
4. Buffie WC. Am J Public Health. 2011;101(6):986-90.
5. Hatzenbuehler ML et al. Am J Public Health. 2010;100:452-9.
6. Raifman J et al. JAMA Pediatr. 2017;171(4):350-6.
7. Gonzales G and Blewett LA. Pediatrics. 2013;132(4):703-11.
8. Ogolsky BG et al. J Fam Psychol. 2019;33(4):422-32.
9. Library of Congress. H.R.8404 – 117th Congress (2021-2022): Respect for Marriage Act. 2022 Dec 13. www.congress.gov/bill/117th-congress/house-bill/8404/text.
10. Perrin EC and Committee on Psychosocial Aspects of Child and Family Health. Pediatrics. 2002;109(2):341-4.
11. Drabble LA et al. PLoS ONE. 2021;16(5):e0249125.
Childhood and adolescence are periods of life with rapid growth and development in which the psychosocial factors of one’s environment can have a profound effect on health. There is increasing evidence that adverse childhood experiences (ACEs) can have significant negative effects on long-term health with effects persisting into subsequent generations.1 Youth themselves, however, often do not have the voice, ability, or political power to advocate for safe and more supportive environments that are essential to their well-being. Thus, advocacy has been central to the profession of pediatrics since its inception, where providers can partner with their patients, families, and communities to push for changes in the environments in which youth live and grow.2
LGBTQ+ youth are known to be at increased risk for ACEs because of the stress that comes from being part of a minority group and the discrimination they experience by their families, communities, and society at large. These factors within their environments have been shown to be associated with increased rates of anxiety, depression, substance use, sexually transmitted infections, and homelessness.3 As with other health outcomes that have been linked to the social determinants of health, these disparities are not inevitable and could be greatly improved upon through advocacy and changes in the environments of LGBTQ+ youth.
Marriage equality (the recognition that same-sex couples have the same legal right to marry as opposite-sex couples) has been shown to be not only a political issue, but one that affects health. The debates surrounding marriage equality have contributed to minority stress by questioning the validity of same-sex relationships and assigning them less value relative to opposite-sex relationships.4 In 1996, the U.S. Congress passed the Defense of Marriage Act (DOMA), which federally defined marriage as being legally recognized only between opposite-sex couples.
Individual states then continued the marriage equality debate by passing individual state laws either allowing or prohibiting same-sex marriage. During this time, it was shown that, in states where same-sex marriage was legally prohibited, LGBTQ+ adults reported significantly higher rates of generalized anxiety disorder, alcohol use disorder, any mood disorder, and psychiatric comorbidity when compared with states without a legal ban on same-sex marriage.5
Using data from the Youth Risk Behavior Surveillance System, it was shown that state policies recognizing same-sex marriage were associated with a 7% relative reduction in suicide attempts reported by adolescent sexual minority students compared with before these policies.6 It was also shown that children with same-sex parents were overall less likely to have private health insurance, but this disparity was improved in states that legally recognized same-sex marriage and allowed second-parent adoptions.7
In 2013, the U.S. Supreme Court ruled that DOMA was unconstitutional, requiring the federal government to legally recognize same-sex marriages for the purposes of federal benefits. In 2015, the U.S. Supreme Court further ruled that same-sex couples are guaranteed the fundamental right to marry, requiring that all states issue marriage licenses to same-sex couples. These rulings were associated with a decrease in reported levels of stigma over time and increased reported levels of family support, particularly for those in same-sex relationships.8
The Respect for Marriage Act (RFMA) was passed by the U.S. Congress and signed into law by President Biden on Dec. 13, 2022. This law officially repeals DOMA and requires all states and the federal government to recognize same-sex marriages performed in any U.S. state or territory.9
If the U.S. Supreme Court were to overturn the 2015 marriage equality decision, individual state laws ensuring or banning same-sex marriage would again be in effect. However, the RFMA ensures that all states continue to recognize same-sex marriages performed in any U.S. state or territory (even if that state itself bans same-sex marriage). While we do not yet have any studies or data regarding the effect of the RFMA on public health, we can expect positive effects by drawing on the previous evidence on the effect of marriage equality and its effect on the health and well-being of LGBTQ+ individuals. By establishing marriage equality in the United States, our government institutions are affirming the relationships and identities of those in same-sex relationships, with the potential effect of helping to destigmatize the LGBTQ+ community.
Since 2002, the American Academy of Pediatrics has recommended that pediatricians “support the right of every child and family to the financial, psychological, and legal security that results from having legally recognized parents who are committed to each other and to the welfare of their children,” acknowledging that “legislative initiatives assuring legal status equivalent to marriage for gay and lesbian partners … can also attend to providing security and permanence for the children of those partnerships.”10 While changes in legal marriage equality are likely to have a positive effect on those within the LGBTQ+ community, it should also be understood that this will not solve all of the psychosocial effects and resultant health disparities that these children face.
A recent scoping review highlights that, as the result of marriage equality progress, sexual minority adults have reported increased social acceptance and reduced stigma across individual, community, and societal levels, but that sexual minority stigma continues to persist across all levels.11
As pediatricians, we can continue to support LGBTQ+ patients and parents by providing care in a safe and affirming environment in which families understand and embrace the healthy development of gender identity and sexuality in an open and destigmatized manner. Delivering care using this approach in and of itself can be seen as advocacy to promote health and well-being within minoritized populations. Pediatricians are also encouraged to become engaged in local and national advocacy initiatives to have a broader effect in the fight for health equity in minority populations, including LGBTQ+ families and youth.
Pediatricians should work with their patients, families, and communities to advocate for structural change needed to address the social determinants of health for optimal growth and development.
Dr. Warus is an adolescent medicine physician who specializes in care for transgender and gender-nonconforming youth, and LGBTQ health for youth at Children’s Hospital of Los Angeles. He is an assistant professor of pediatrics at University of Southern California, Los Angeles.
Resources
Bright Futures – Promoting Healthy Development of Sexuality and Gender Identity (Implementation Tip Sheet): https://downloads.aap.org/AAP/PDF/BF_HealthySexualityGenderIdentity_Tipsheet.pdf
Bright Futures – Implementing Social Determinants of Health Into Health Supervision Visits (Implementation Tip Sheet): https://downloads.aap.org/AAP/PDF/Bright%20Futures/BF_IntegrateSDoH_Tipsheet.pdf?_ga=2.214227031.1330574154.1673910248-58875083.1673910248
American Academy of Pediatrics – Advocacy Website: https://www.aap.org/en/advocacy/
References
1. Hughes K et al. Lancet Public Health. 2017;2(8):e356-66.
2. Camero K and Javier JR. Pediatr Clin N Am. 2023;70:43-51.
3. Lund EM and Burgess CM. Prim Care Clin Office Pract. 2021;48:179-89.
4. Buffie WC. Am J Public Health. 2011;101(6):986-90.
5. Hatzenbuehler ML et al. Am J Public Health. 2010;100:452-9.
6. Raifman J et al. JAMA Pediatr. 2017;171(4):350-6.
7. Gonzales G and Blewett LA. Pediatrics. 2013;132(4):703-11.
8. Ogolsky BG et al. J Fam Psychol. 2019;33(4):422-32.
9. Library of Congress. H.R.8404 – 117th Congress (2021-2022): Respect for Marriage Act. 2022 Dec 13. www.congress.gov/bill/117th-congress/house-bill/8404/text.
10. Perrin EC and Committee on Psychosocial Aspects of Child and Family Health. Pediatrics. 2002;109(2):341-4.
11. Drabble LA et al. PLoS ONE. 2021;16(5):e0249125.
Childhood and adolescence are periods of life with rapid growth and development in which the psychosocial factors of one’s environment can have a profound effect on health. There is increasing evidence that adverse childhood experiences (ACEs) can have significant negative effects on long-term health with effects persisting into subsequent generations.1 Youth themselves, however, often do not have the voice, ability, or political power to advocate for safe and more supportive environments that are essential to their well-being. Thus, advocacy has been central to the profession of pediatrics since its inception, where providers can partner with their patients, families, and communities to push for changes in the environments in which youth live and grow.2
LGBTQ+ youth are known to be at increased risk for ACEs because of the stress that comes from being part of a minority group and the discrimination they experience by their families, communities, and society at large. These factors within their environments have been shown to be associated with increased rates of anxiety, depression, substance use, sexually transmitted infections, and homelessness.3 As with other health outcomes that have been linked to the social determinants of health, these disparities are not inevitable and could be greatly improved upon through advocacy and changes in the environments of LGBTQ+ youth.
Marriage equality (the recognition that same-sex couples have the same legal right to marry as opposite-sex couples) has been shown to be not only a political issue, but one that affects health. The debates surrounding marriage equality have contributed to minority stress by questioning the validity of same-sex relationships and assigning them less value relative to opposite-sex relationships.4 In 1996, the U.S. Congress passed the Defense of Marriage Act (DOMA), which federally defined marriage as being legally recognized only between opposite-sex couples.
Individual states then continued the marriage equality debate by passing individual state laws either allowing or prohibiting same-sex marriage. During this time, it was shown that, in states where same-sex marriage was legally prohibited, LGBTQ+ adults reported significantly higher rates of generalized anxiety disorder, alcohol use disorder, any mood disorder, and psychiatric comorbidity when compared with states without a legal ban on same-sex marriage.5
Using data from the Youth Risk Behavior Surveillance System, it was shown that state policies recognizing same-sex marriage were associated with a 7% relative reduction in suicide attempts reported by adolescent sexual minority students compared with before these policies.6 It was also shown that children with same-sex parents were overall less likely to have private health insurance, but this disparity was improved in states that legally recognized same-sex marriage and allowed second-parent adoptions.7
In 2013, the U.S. Supreme Court ruled that DOMA was unconstitutional, requiring the federal government to legally recognize same-sex marriages for the purposes of federal benefits. In 2015, the U.S. Supreme Court further ruled that same-sex couples are guaranteed the fundamental right to marry, requiring that all states issue marriage licenses to same-sex couples. These rulings were associated with a decrease in reported levels of stigma over time and increased reported levels of family support, particularly for those in same-sex relationships.8
The Respect for Marriage Act (RFMA) was passed by the U.S. Congress and signed into law by President Biden on Dec. 13, 2022. This law officially repeals DOMA and requires all states and the federal government to recognize same-sex marriages performed in any U.S. state or territory.9
If the U.S. Supreme Court were to overturn the 2015 marriage equality decision, individual state laws ensuring or banning same-sex marriage would again be in effect. However, the RFMA ensures that all states continue to recognize same-sex marriages performed in any U.S. state or territory (even if that state itself bans same-sex marriage). While we do not yet have any studies or data regarding the effect of the RFMA on public health, we can expect positive effects by drawing on the previous evidence on the effect of marriage equality and its effect on the health and well-being of LGBTQ+ individuals. By establishing marriage equality in the United States, our government institutions are affirming the relationships and identities of those in same-sex relationships, with the potential effect of helping to destigmatize the LGBTQ+ community.
Since 2002, the American Academy of Pediatrics has recommended that pediatricians “support the right of every child and family to the financial, psychological, and legal security that results from having legally recognized parents who are committed to each other and to the welfare of their children,” acknowledging that “legislative initiatives assuring legal status equivalent to marriage for gay and lesbian partners … can also attend to providing security and permanence for the children of those partnerships.”10 While changes in legal marriage equality are likely to have a positive effect on those within the LGBTQ+ community, it should also be understood that this will not solve all of the psychosocial effects and resultant health disparities that these children face.
A recent scoping review highlights that, as the result of marriage equality progress, sexual minority adults have reported increased social acceptance and reduced stigma across individual, community, and societal levels, but that sexual minority stigma continues to persist across all levels.11
As pediatricians, we can continue to support LGBTQ+ patients and parents by providing care in a safe and affirming environment in which families understand and embrace the healthy development of gender identity and sexuality in an open and destigmatized manner. Delivering care using this approach in and of itself can be seen as advocacy to promote health and well-being within minoritized populations. Pediatricians are also encouraged to become engaged in local and national advocacy initiatives to have a broader effect in the fight for health equity in minority populations, including LGBTQ+ families and youth.
Pediatricians should work with their patients, families, and communities to advocate for structural change needed to address the social determinants of health for optimal growth and development.
Dr. Warus is an adolescent medicine physician who specializes in care for transgender and gender-nonconforming youth, and LGBTQ health for youth at Children’s Hospital of Los Angeles. He is an assistant professor of pediatrics at University of Southern California, Los Angeles.
Resources
Bright Futures – Promoting Healthy Development of Sexuality and Gender Identity (Implementation Tip Sheet): https://downloads.aap.org/AAP/PDF/BF_HealthySexualityGenderIdentity_Tipsheet.pdf
Bright Futures – Implementing Social Determinants of Health Into Health Supervision Visits (Implementation Tip Sheet): https://downloads.aap.org/AAP/PDF/Bright%20Futures/BF_IntegrateSDoH_Tipsheet.pdf?_ga=2.214227031.1330574154.1673910248-58875083.1673910248
American Academy of Pediatrics – Advocacy Website: https://www.aap.org/en/advocacy/
References
1. Hughes K et al. Lancet Public Health. 2017;2(8):e356-66.
2. Camero K and Javier JR. Pediatr Clin N Am. 2023;70:43-51.
3. Lund EM and Burgess CM. Prim Care Clin Office Pract. 2021;48:179-89.
4. Buffie WC. Am J Public Health. 2011;101(6):986-90.
5. Hatzenbuehler ML et al. Am J Public Health. 2010;100:452-9.
6. Raifman J et al. JAMA Pediatr. 2017;171(4):350-6.
7. Gonzales G and Blewett LA. Pediatrics. 2013;132(4):703-11.
8. Ogolsky BG et al. J Fam Psychol. 2019;33(4):422-32.
9. Library of Congress. H.R.8404 – 117th Congress (2021-2022): Respect for Marriage Act. 2022 Dec 13. www.congress.gov/bill/117th-congress/house-bill/8404/text.
10. Perrin EC and Committee on Psychosocial Aspects of Child and Family Health. Pediatrics. 2002;109(2):341-4.
11. Drabble LA et al. PLoS ONE. 2021;16(5):e0249125.
CDC frets over further dip in kindergarten vaccination rates
The percentage of kindergarteners in the United States who have received routine vaccines to protect against illnesses such as measles, whooping cough, and polio has declined for 2 straight years, a new study has found.
Drops in vaccine coverage leave communities more susceptible to outbreaks of vaccine-preventable diseases, such as those that occurred in 2022, public health officials said.
Coverage for four vaccines – against measles, mumps, and rubella (MMR); diphtheria, tetanus, and acellular pertussis (DTaP); poliovirus; and varicella – among kindergarten students was about 95% in 2019-2020.
The rate fell to 94% the following year.
For the 2021-2022 school year, coverage dropped another point, to 93%, according to the report, published online in Morbidity and Mortality Weekly Report.
The rate of vaccination overall remains high, but about 250,000 kindergarten students may not be protected against measles, the researchers estimate. Measles, which is highly infectious, can lead to serious illness and even death in children who have not been vaccinated against the virus.
“In 2022, two communities in the United States responded to outbreaks of measles where children have been hospitalized,” Georgina Peacock, MD, MPH, director of the immunization services division of the Centers for Disease Control and Prevention, said in a media briefing about the report. “One community reported a case of paralytic polio in an unvaccinated person. These outbreaks were preventable. The best way to prevent these diseases and their devastating impact on children is through vaccination.”
Exemptions steady
For the new study, Ranee Seither, MPH, with the CDC’s National Center for Immunization and Respiratory Diseases and her colleagues analyzed data reported by states to estimate nationwide coverage for the four routine vaccines.
The number of students with exemptions remained low, at 2.6%, but another 3.9% who were without exemptions were not up to date with the MMR vaccine, the investigators report.
In a separate study, researchers found that vaccination coverage for 2-year-olds has increased. Approximately 70% of children were up to date with a seven-vaccine series by age 24 months. The coverage rate was higher for children born during 2018-2019 than for those born during 2016-2017.
Although the COVID-19 pandemic was not associated with decreased vaccination rates in this younger age group overall, coverage fell by 4-5 percentage points for children living below the poverty level or in rural areas, according to the study.
In addition, uninsured children were eight times more likely than those with private insurance to not be vaccinated by their second birthday, the researchers found.
Strategies to increase vaccination coverage include enforcing school vaccination requirements and holding vaccination clinics at schools, the CDC said.
“Providers should review children’s histories and recommend needed vaccinations during every clinical encounter and address parental hesitancy to help reduce disparities and ensure that all children are protected from vaccine-preventable diseases,” the agency said.
To that end, the agency launched an initiative this week called Let’s RISE (Routine Immunizations on Schedule for Everyone) to provide clinicians with resources to help patients get on track with their immunizations.
Hundreds of thousands unprotected
MMR vaccination coverage for kindergartners is the lowest it has been in over a decade, Dr. Peacock noted. Decreased coverage for kindergarten students might be tied to pandemic-related disruptions in health care systems and schools, she said. School administrators and parents may have been less focused on routine vaccination paperwork amid the return to in-person learning, for instance.
Hesitancy about COVID vaccines could be affecting routine vaccinations. “That’s something that we are watching very closely,” Dr. Peacock said.
The 2-point decrease in vaccination coverage “translates to hundreds of thousands of children starting school without being fully protected” against preventable diseases that can spread easily in classrooms, Sean O’Leary, MD, chair of the American Academy of Pediatrics’ Committee on Infectious Diseases, said.
Despite the drop in coverage, Dr. O’Leary said he saw some encouraging signs in the data: Nonmedical exemptions for kindergarten students have not increased. And the vast majority of parents are still having their children vaccinated. At the same time, the reports highlight a need to address child poverty and improve vaccine access in rural areas, he said.
A version of this article first appeared on Medscape.com.
The percentage of kindergarteners in the United States who have received routine vaccines to protect against illnesses such as measles, whooping cough, and polio has declined for 2 straight years, a new study has found.
Drops in vaccine coverage leave communities more susceptible to outbreaks of vaccine-preventable diseases, such as those that occurred in 2022, public health officials said.
Coverage for four vaccines – against measles, mumps, and rubella (MMR); diphtheria, tetanus, and acellular pertussis (DTaP); poliovirus; and varicella – among kindergarten students was about 95% in 2019-2020.
The rate fell to 94% the following year.
For the 2021-2022 school year, coverage dropped another point, to 93%, according to the report, published online in Morbidity and Mortality Weekly Report.
The rate of vaccination overall remains high, but about 250,000 kindergarten students may not be protected against measles, the researchers estimate. Measles, which is highly infectious, can lead to serious illness and even death in children who have not been vaccinated against the virus.
“In 2022, two communities in the United States responded to outbreaks of measles where children have been hospitalized,” Georgina Peacock, MD, MPH, director of the immunization services division of the Centers for Disease Control and Prevention, said in a media briefing about the report. “One community reported a case of paralytic polio in an unvaccinated person. These outbreaks were preventable. The best way to prevent these diseases and their devastating impact on children is through vaccination.”
Exemptions steady
For the new study, Ranee Seither, MPH, with the CDC’s National Center for Immunization and Respiratory Diseases and her colleagues analyzed data reported by states to estimate nationwide coverage for the four routine vaccines.
The number of students with exemptions remained low, at 2.6%, but another 3.9% who were without exemptions were not up to date with the MMR vaccine, the investigators report.
In a separate study, researchers found that vaccination coverage for 2-year-olds has increased. Approximately 70% of children were up to date with a seven-vaccine series by age 24 months. The coverage rate was higher for children born during 2018-2019 than for those born during 2016-2017.
Although the COVID-19 pandemic was not associated with decreased vaccination rates in this younger age group overall, coverage fell by 4-5 percentage points for children living below the poverty level or in rural areas, according to the study.
In addition, uninsured children were eight times more likely than those with private insurance to not be vaccinated by their second birthday, the researchers found.
Strategies to increase vaccination coverage include enforcing school vaccination requirements and holding vaccination clinics at schools, the CDC said.
“Providers should review children’s histories and recommend needed vaccinations during every clinical encounter and address parental hesitancy to help reduce disparities and ensure that all children are protected from vaccine-preventable diseases,” the agency said.
To that end, the agency launched an initiative this week called Let’s RISE (Routine Immunizations on Schedule for Everyone) to provide clinicians with resources to help patients get on track with their immunizations.
Hundreds of thousands unprotected
MMR vaccination coverage for kindergartners is the lowest it has been in over a decade, Dr. Peacock noted. Decreased coverage for kindergarten students might be tied to pandemic-related disruptions in health care systems and schools, she said. School administrators and parents may have been less focused on routine vaccination paperwork amid the return to in-person learning, for instance.
Hesitancy about COVID vaccines could be affecting routine vaccinations. “That’s something that we are watching very closely,” Dr. Peacock said.
The 2-point decrease in vaccination coverage “translates to hundreds of thousands of children starting school without being fully protected” against preventable diseases that can spread easily in classrooms, Sean O’Leary, MD, chair of the American Academy of Pediatrics’ Committee on Infectious Diseases, said.
Despite the drop in coverage, Dr. O’Leary said he saw some encouraging signs in the data: Nonmedical exemptions for kindergarten students have not increased. And the vast majority of parents are still having their children vaccinated. At the same time, the reports highlight a need to address child poverty and improve vaccine access in rural areas, he said.
A version of this article first appeared on Medscape.com.
The percentage of kindergarteners in the United States who have received routine vaccines to protect against illnesses such as measles, whooping cough, and polio has declined for 2 straight years, a new study has found.
Drops in vaccine coverage leave communities more susceptible to outbreaks of vaccine-preventable diseases, such as those that occurred in 2022, public health officials said.
Coverage for four vaccines – against measles, mumps, and rubella (MMR); diphtheria, tetanus, and acellular pertussis (DTaP); poliovirus; and varicella – among kindergarten students was about 95% in 2019-2020.
The rate fell to 94% the following year.
For the 2021-2022 school year, coverage dropped another point, to 93%, according to the report, published online in Morbidity and Mortality Weekly Report.
The rate of vaccination overall remains high, but about 250,000 kindergarten students may not be protected against measles, the researchers estimate. Measles, which is highly infectious, can lead to serious illness and even death in children who have not been vaccinated against the virus.
“In 2022, two communities in the United States responded to outbreaks of measles where children have been hospitalized,” Georgina Peacock, MD, MPH, director of the immunization services division of the Centers for Disease Control and Prevention, said in a media briefing about the report. “One community reported a case of paralytic polio in an unvaccinated person. These outbreaks were preventable. The best way to prevent these diseases and their devastating impact on children is through vaccination.”
Exemptions steady
For the new study, Ranee Seither, MPH, with the CDC’s National Center for Immunization and Respiratory Diseases and her colleagues analyzed data reported by states to estimate nationwide coverage for the four routine vaccines.
The number of students with exemptions remained low, at 2.6%, but another 3.9% who were without exemptions were not up to date with the MMR vaccine, the investigators report.
In a separate study, researchers found that vaccination coverage for 2-year-olds has increased. Approximately 70% of children were up to date with a seven-vaccine series by age 24 months. The coverage rate was higher for children born during 2018-2019 than for those born during 2016-2017.
Although the COVID-19 pandemic was not associated with decreased vaccination rates in this younger age group overall, coverage fell by 4-5 percentage points for children living below the poverty level or in rural areas, according to the study.
In addition, uninsured children were eight times more likely than those with private insurance to not be vaccinated by their second birthday, the researchers found.
Strategies to increase vaccination coverage include enforcing school vaccination requirements and holding vaccination clinics at schools, the CDC said.
“Providers should review children’s histories and recommend needed vaccinations during every clinical encounter and address parental hesitancy to help reduce disparities and ensure that all children are protected from vaccine-preventable diseases,” the agency said.
To that end, the agency launched an initiative this week called Let’s RISE (Routine Immunizations on Schedule for Everyone) to provide clinicians with resources to help patients get on track with their immunizations.
Hundreds of thousands unprotected
MMR vaccination coverage for kindergartners is the lowest it has been in over a decade, Dr. Peacock noted. Decreased coverage for kindergarten students might be tied to pandemic-related disruptions in health care systems and schools, she said. School administrators and parents may have been less focused on routine vaccination paperwork amid the return to in-person learning, for instance.
Hesitancy about COVID vaccines could be affecting routine vaccinations. “That’s something that we are watching very closely,” Dr. Peacock said.
The 2-point decrease in vaccination coverage “translates to hundreds of thousands of children starting school without being fully protected” against preventable diseases that can spread easily in classrooms, Sean O’Leary, MD, chair of the American Academy of Pediatrics’ Committee on Infectious Diseases, said.
Despite the drop in coverage, Dr. O’Leary said he saw some encouraging signs in the data: Nonmedical exemptions for kindergarten students have not increased. And the vast majority of parents are still having their children vaccinated. At the same time, the reports highlight a need to address child poverty and improve vaccine access in rural areas, he said.
A version of this article first appeared on Medscape.com.
FROM THE MMWR
AD outcomes improved with lebrikizumab and topical steroids
, according to results of the 16-week phase 3 ADhere trial.
“Lebrikizumab, a monoclonal antibody inhibiting interleukin-13, combined with TCS was associated with reduced overall disease severity of moderate to severe AD in adolescents and adults, and had a safety profile consistent with previous lebrikizumab AD studies,” noted lead author Eric L. Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, and coauthors in their article on the study, which was published in JAMA Dermatology.
The double-blind trial, conducted at 54 sites across Germany, Poland, Canada, and the United States, included 211 patients, mean age 37.2 years, of whom 48.8% were female and roughly 22% were adolescents. Almost 15% were Asian, and about 13% were Black.
At baseline, participants had a score of 16 or higher on the Eczema Area and Severity Index (EASI), a score of 3 or higher on the Investigator’s Global Assessment (IGA) scale, AD covering a body surface area of 10% or greater, and a history of inadequate response to treatment with topical medications.
After a minimum 1-week washout period from topical and systemic therapy, participants were randomized in a 2:1 ratio to receive lebrikizumab plus TCS (n = 145) or placebo plus TCS (n = 66) for 16 weeks.
Lebrikizumab or placebo was administered by subcutaneous injection every 2 weeks; the loading and week-2 doses of lebrikizumab were 500 mg, followed by 250 mg thereafter. All patients were instructed to use low- to mid-potency TCS at their own discretion. Study sites provided a mid-potency TCS (triamcinolone acetonide 0.1% cream) and a low-potency TCS (hydrocortisone 1% cream), with topical calcineurin inhibitors permitted for sensitive skin areas.
Primary outcomes at 16 weeks included a 2-point or more reduction in IGA score from baseline and EASI-75 response. Patients in the lebrikizumab arm had superior responses on both of these outcomes, with statistical significance achieved as early as week 8 and week 4, respectively, and maintained through week 16. Specifically, 41.2% of those treated with lebrikizumab had an IGA reduction of 2 points or more, compared with 22.1% of those receiving placebo plus TCS (P = .01), and the proportion of patients achieving EASI-75 responses was 69.5% vs. 42.2%, respectively (P < .001).
Patients treated with lebrikizumab also showed statistically significant improvements, compared with TCS alone in all key secondary endpoints, “including skin clearance, improvement in itch, itch interference on sleep, and enhanced QoL [quality of life],” noted the authors. “This study captured the clinical benefit of lebrikizumab through the combined end point of physician-assessed clinical sign of skin clearance (EASI-75) and patient-reported outcome of improvement in itch (Pruritus NRS).”
The percentage of patients who achieved the combined endpoint was more than double for the lebrikizumab plus TCS group vs. the group on TCS alone, indicating that patients treated with lebrikizumab plus TCS “were more likely to experience improvement in skin symptoms and itch,” the investigators added.
The authors noted that most treatment-emergent adverse events “were nonserious, mild, or moderate in severity, and did not lead to study discontinuation.” These included conjunctivitis (4.8%), headache (4.8%), hypertension (2.8%), injection-site reactions (2.8%), and herpes infection (3.4%) – all of which occurred in 1.5% or less of patients in the placebo group.
“The higher incidence of conjunctivitis has also been reported in other biologics inhibiting IL [interleukin]–13 and/or IL-4 signaling, as well as lebrikizumab monotherapy studies,” they noted. The 4.8% rate of conjunctivitis reported in the combination study, they added, is “compared with 7.5% frequency in 16-week data from the lebrikizumab monotherapy studies. Although the mechanism remains unclear, it has been reported that conjunctival goblet cell scarcity due to IL-13 and IL-4 inhibition, and subsequent effects on the homeostasis of the conjunctival mucosal surface, results in ocular AEs [adverse events].”
“This truly is a time of great hope and promise for our patients with AD,” commented Zelma Chiesa Fuxench, MD, who was not involved in the study. “The advent of newer, targeted therapeutic agents for AD continues to revolutionize the treatment experience for our patients, offering the possibility of greater AD disease control with a favorable risk profile and less need for blood work monitoring compared to traditional systemic agents.”
On the basis of the study results, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said in an interview that “lebrikizumab represents an additional option in the treatment armamentarium for providers who care for patients with AD.” She added that, “while head-to-head trials comparing lebrikizumab to dupilumab, the first FDA-approved biologic for AD, would be beneficial, to the best of my knowledge this data is currently lacking. However, based on the results of this study, we would expect lebrikizumab to work at least similarly to dupilumab, based on the reported improvements in IGA and EASI score.”
Additionally, lebrikizumab showed a favorable safety profile, “with most treatment-emergent adverse effects reported as nonserious and not leading to drug discontinuation,” she said. “Of interest to clinicians may be the reported rates of conjunctivitis in this study. Rates of conjunctivitis for lebrikizumab appear to be lower than those reported in the LIBERTY AD CHRONOS study for dupilumab – a finding that merits further scrutiny in my opinion, as this one of the most frequent treatment-emergent adverse events that I encounter in my clinical practice.”
The study was funded by Dermira, a subsidiary of Eli Lilly. Dr. Simpson reported personal fees and grants from multiple sources, including Dermira and Eli Lilly, the companies developing lebrikizumab. Several authors were employees of Eli Lilly. Dr. Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
A version of this article first appeared on Medscape.com.
, according to results of the 16-week phase 3 ADhere trial.
“Lebrikizumab, a monoclonal antibody inhibiting interleukin-13, combined with TCS was associated with reduced overall disease severity of moderate to severe AD in adolescents and adults, and had a safety profile consistent with previous lebrikizumab AD studies,” noted lead author Eric L. Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, and coauthors in their article on the study, which was published in JAMA Dermatology.
The double-blind trial, conducted at 54 sites across Germany, Poland, Canada, and the United States, included 211 patients, mean age 37.2 years, of whom 48.8% were female and roughly 22% were adolescents. Almost 15% were Asian, and about 13% were Black.
At baseline, participants had a score of 16 or higher on the Eczema Area and Severity Index (EASI), a score of 3 or higher on the Investigator’s Global Assessment (IGA) scale, AD covering a body surface area of 10% or greater, and a history of inadequate response to treatment with topical medications.
After a minimum 1-week washout period from topical and systemic therapy, participants were randomized in a 2:1 ratio to receive lebrikizumab plus TCS (n = 145) or placebo plus TCS (n = 66) for 16 weeks.
Lebrikizumab or placebo was administered by subcutaneous injection every 2 weeks; the loading and week-2 doses of lebrikizumab were 500 mg, followed by 250 mg thereafter. All patients were instructed to use low- to mid-potency TCS at their own discretion. Study sites provided a mid-potency TCS (triamcinolone acetonide 0.1% cream) and a low-potency TCS (hydrocortisone 1% cream), with topical calcineurin inhibitors permitted for sensitive skin areas.
Primary outcomes at 16 weeks included a 2-point or more reduction in IGA score from baseline and EASI-75 response. Patients in the lebrikizumab arm had superior responses on both of these outcomes, with statistical significance achieved as early as week 8 and week 4, respectively, and maintained through week 16. Specifically, 41.2% of those treated with lebrikizumab had an IGA reduction of 2 points or more, compared with 22.1% of those receiving placebo plus TCS (P = .01), and the proportion of patients achieving EASI-75 responses was 69.5% vs. 42.2%, respectively (P < .001).
Patients treated with lebrikizumab also showed statistically significant improvements, compared with TCS alone in all key secondary endpoints, “including skin clearance, improvement in itch, itch interference on sleep, and enhanced QoL [quality of life],” noted the authors. “This study captured the clinical benefit of lebrikizumab through the combined end point of physician-assessed clinical sign of skin clearance (EASI-75) and patient-reported outcome of improvement in itch (Pruritus NRS).”
The percentage of patients who achieved the combined endpoint was more than double for the lebrikizumab plus TCS group vs. the group on TCS alone, indicating that patients treated with lebrikizumab plus TCS “were more likely to experience improvement in skin symptoms and itch,” the investigators added.
The authors noted that most treatment-emergent adverse events “were nonserious, mild, or moderate in severity, and did not lead to study discontinuation.” These included conjunctivitis (4.8%), headache (4.8%), hypertension (2.8%), injection-site reactions (2.8%), and herpes infection (3.4%) – all of which occurred in 1.5% or less of patients in the placebo group.
“The higher incidence of conjunctivitis has also been reported in other biologics inhibiting IL [interleukin]–13 and/or IL-4 signaling, as well as lebrikizumab monotherapy studies,” they noted. The 4.8% rate of conjunctivitis reported in the combination study, they added, is “compared with 7.5% frequency in 16-week data from the lebrikizumab monotherapy studies. Although the mechanism remains unclear, it has been reported that conjunctival goblet cell scarcity due to IL-13 and IL-4 inhibition, and subsequent effects on the homeostasis of the conjunctival mucosal surface, results in ocular AEs [adverse events].”
“This truly is a time of great hope and promise for our patients with AD,” commented Zelma Chiesa Fuxench, MD, who was not involved in the study. “The advent of newer, targeted therapeutic agents for AD continues to revolutionize the treatment experience for our patients, offering the possibility of greater AD disease control with a favorable risk profile and less need for blood work monitoring compared to traditional systemic agents.”
On the basis of the study results, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said in an interview that “lebrikizumab represents an additional option in the treatment armamentarium for providers who care for patients with AD.” She added that, “while head-to-head trials comparing lebrikizumab to dupilumab, the first FDA-approved biologic for AD, would be beneficial, to the best of my knowledge this data is currently lacking. However, based on the results of this study, we would expect lebrikizumab to work at least similarly to dupilumab, based on the reported improvements in IGA and EASI score.”
Additionally, lebrikizumab showed a favorable safety profile, “with most treatment-emergent adverse effects reported as nonserious and not leading to drug discontinuation,” she said. “Of interest to clinicians may be the reported rates of conjunctivitis in this study. Rates of conjunctivitis for lebrikizumab appear to be lower than those reported in the LIBERTY AD CHRONOS study for dupilumab – a finding that merits further scrutiny in my opinion, as this one of the most frequent treatment-emergent adverse events that I encounter in my clinical practice.”
The study was funded by Dermira, a subsidiary of Eli Lilly. Dr. Simpson reported personal fees and grants from multiple sources, including Dermira and Eli Lilly, the companies developing lebrikizumab. Several authors were employees of Eli Lilly. Dr. Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
A version of this article first appeared on Medscape.com.
, according to results of the 16-week phase 3 ADhere trial.
“Lebrikizumab, a monoclonal antibody inhibiting interleukin-13, combined with TCS was associated with reduced overall disease severity of moderate to severe AD in adolescents and adults, and had a safety profile consistent with previous lebrikizumab AD studies,” noted lead author Eric L. Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, and coauthors in their article on the study, which was published in JAMA Dermatology.
The double-blind trial, conducted at 54 sites across Germany, Poland, Canada, and the United States, included 211 patients, mean age 37.2 years, of whom 48.8% were female and roughly 22% were adolescents. Almost 15% were Asian, and about 13% were Black.
At baseline, participants had a score of 16 or higher on the Eczema Area and Severity Index (EASI), a score of 3 or higher on the Investigator’s Global Assessment (IGA) scale, AD covering a body surface area of 10% or greater, and a history of inadequate response to treatment with topical medications.
After a minimum 1-week washout period from topical and systemic therapy, participants were randomized in a 2:1 ratio to receive lebrikizumab plus TCS (n = 145) or placebo plus TCS (n = 66) for 16 weeks.
Lebrikizumab or placebo was administered by subcutaneous injection every 2 weeks; the loading and week-2 doses of lebrikizumab were 500 mg, followed by 250 mg thereafter. All patients were instructed to use low- to mid-potency TCS at their own discretion. Study sites provided a mid-potency TCS (triamcinolone acetonide 0.1% cream) and a low-potency TCS (hydrocortisone 1% cream), with topical calcineurin inhibitors permitted for sensitive skin areas.
Primary outcomes at 16 weeks included a 2-point or more reduction in IGA score from baseline and EASI-75 response. Patients in the lebrikizumab arm had superior responses on both of these outcomes, with statistical significance achieved as early as week 8 and week 4, respectively, and maintained through week 16. Specifically, 41.2% of those treated with lebrikizumab had an IGA reduction of 2 points or more, compared with 22.1% of those receiving placebo plus TCS (P = .01), and the proportion of patients achieving EASI-75 responses was 69.5% vs. 42.2%, respectively (P < .001).
Patients treated with lebrikizumab also showed statistically significant improvements, compared with TCS alone in all key secondary endpoints, “including skin clearance, improvement in itch, itch interference on sleep, and enhanced QoL [quality of life],” noted the authors. “This study captured the clinical benefit of lebrikizumab through the combined end point of physician-assessed clinical sign of skin clearance (EASI-75) and patient-reported outcome of improvement in itch (Pruritus NRS).”
The percentage of patients who achieved the combined endpoint was more than double for the lebrikizumab plus TCS group vs. the group on TCS alone, indicating that patients treated with lebrikizumab plus TCS “were more likely to experience improvement in skin symptoms and itch,” the investigators added.
The authors noted that most treatment-emergent adverse events “were nonserious, mild, or moderate in severity, and did not lead to study discontinuation.” These included conjunctivitis (4.8%), headache (4.8%), hypertension (2.8%), injection-site reactions (2.8%), and herpes infection (3.4%) – all of which occurred in 1.5% or less of patients in the placebo group.
“The higher incidence of conjunctivitis has also been reported in other biologics inhibiting IL [interleukin]–13 and/or IL-4 signaling, as well as lebrikizumab monotherapy studies,” they noted. The 4.8% rate of conjunctivitis reported in the combination study, they added, is “compared with 7.5% frequency in 16-week data from the lebrikizumab monotherapy studies. Although the mechanism remains unclear, it has been reported that conjunctival goblet cell scarcity due to IL-13 and IL-4 inhibition, and subsequent effects on the homeostasis of the conjunctival mucosal surface, results in ocular AEs [adverse events].”
“This truly is a time of great hope and promise for our patients with AD,” commented Zelma Chiesa Fuxench, MD, who was not involved in the study. “The advent of newer, targeted therapeutic agents for AD continues to revolutionize the treatment experience for our patients, offering the possibility of greater AD disease control with a favorable risk profile and less need for blood work monitoring compared to traditional systemic agents.”
On the basis of the study results, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said in an interview that “lebrikizumab represents an additional option in the treatment armamentarium for providers who care for patients with AD.” She added that, “while head-to-head trials comparing lebrikizumab to dupilumab, the first FDA-approved biologic for AD, would be beneficial, to the best of my knowledge this data is currently lacking. However, based on the results of this study, we would expect lebrikizumab to work at least similarly to dupilumab, based on the reported improvements in IGA and EASI score.”
Additionally, lebrikizumab showed a favorable safety profile, “with most treatment-emergent adverse effects reported as nonserious and not leading to drug discontinuation,” she said. “Of interest to clinicians may be the reported rates of conjunctivitis in this study. Rates of conjunctivitis for lebrikizumab appear to be lower than those reported in the LIBERTY AD CHRONOS study for dupilumab – a finding that merits further scrutiny in my opinion, as this one of the most frequent treatment-emergent adverse events that I encounter in my clinical practice.”
The study was funded by Dermira, a subsidiary of Eli Lilly. Dr. Simpson reported personal fees and grants from multiple sources, including Dermira and Eli Lilly, the companies developing lebrikizumab. Several authors were employees of Eli Lilly. Dr. Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Children and COVID: ED visits and hospitalizations start to fall again
Emergency department visits and hospitalizations for COVID-19 in children appear to be following the declining trend set by weekly cases since early December, based on data from the Centers for Disease Control and Prevention.
. New cases took a different path that had the weekly total falling through November before taking a big jump during the week of Nov. 27 to Dec. 3 – the count doubled from 30,000 the previous week to 63,000 – and then decreased again, the CDC reported.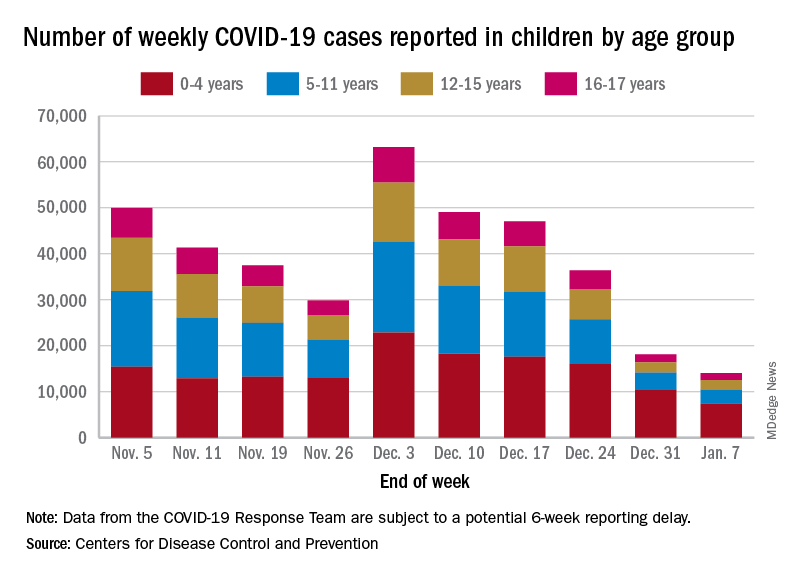
The proportion of ED visits with COVID, which was down to 1.0% of all ED visits (7-day average) for children aged 0-4 years on Nov. 4, was up to 3.2% on Jan. 3 but slipped to 2.5% as of Jan. 10. The patterns for older children are similar, with some differences in timing and lower peaks (1.7% for 12- to 15-year-olds and 1.9% for those aged 16-17), according to the CDC’s COVID Data Tracker.
The trend for new hospital admissions of children with confirmed COVID showed a similar rise through December, and the latest data for the very beginning of January suggest an even faster drop, although there is more of a reporting lag with hospitalization data, compared with ED visits, the CDC noted.
The most current data (Dec. 30 to Jan. 5) available from the American Academy of Pediatrics and the Children’s Hospital Association show less volatility in the number of weekly cases through November and December, with the peak being about 48,000 in mid-December. The AAP/CHA totals for the last 2 weeks, however, were both higher than the CDC’s corresponding counts, which are more preliminary and subject to revision.
The CDC puts the total number of COVID cases in children at 16.7 million – about 17.2% of all cases – as of Jan. 11, with 1,981 deaths reported so far. The AAP and CHA are not tracking deaths, but their case total as of Jan. 5 was 15.2 million, which represents 18.1% of cases in all ages. The AAP/CHA report is based on data reported publicly by an ever-decreasing number of states and territories.
Emergency department visits and hospitalizations for COVID-19 in children appear to be following the declining trend set by weekly cases since early December, based on data from the Centers for Disease Control and Prevention.
. New cases took a different path that had the weekly total falling through November before taking a big jump during the week of Nov. 27 to Dec. 3 – the count doubled from 30,000 the previous week to 63,000 – and then decreased again, the CDC reported.
The proportion of ED visits with COVID, which was down to 1.0% of all ED visits (7-day average) for children aged 0-4 years on Nov. 4, was up to 3.2% on Jan. 3 but slipped to 2.5% as of Jan. 10. The patterns for older children are similar, with some differences in timing and lower peaks (1.7% for 12- to 15-year-olds and 1.9% for those aged 16-17), according to the CDC’s COVID Data Tracker.
The trend for new hospital admissions of children with confirmed COVID showed a similar rise through December, and the latest data for the very beginning of January suggest an even faster drop, although there is more of a reporting lag with hospitalization data, compared with ED visits, the CDC noted.
The most current data (Dec. 30 to Jan. 5) available from the American Academy of Pediatrics and the Children’s Hospital Association show less volatility in the number of weekly cases through November and December, with the peak being about 48,000 in mid-December. The AAP/CHA totals for the last 2 weeks, however, were both higher than the CDC’s corresponding counts, which are more preliminary and subject to revision.
The CDC puts the total number of COVID cases in children at 16.7 million – about 17.2% of all cases – as of Jan. 11, with 1,981 deaths reported so far. The AAP and CHA are not tracking deaths, but their case total as of Jan. 5 was 15.2 million, which represents 18.1% of cases in all ages. The AAP/CHA report is based on data reported publicly by an ever-decreasing number of states and territories.
Emergency department visits and hospitalizations for COVID-19 in children appear to be following the declining trend set by weekly cases since early December, based on data from the Centers for Disease Control and Prevention.
. New cases took a different path that had the weekly total falling through November before taking a big jump during the week of Nov. 27 to Dec. 3 – the count doubled from 30,000 the previous week to 63,000 – and then decreased again, the CDC reported.
The proportion of ED visits with COVID, which was down to 1.0% of all ED visits (7-day average) for children aged 0-4 years on Nov. 4, was up to 3.2% on Jan. 3 but slipped to 2.5% as of Jan. 10. The patterns for older children are similar, with some differences in timing and lower peaks (1.7% for 12- to 15-year-olds and 1.9% for those aged 16-17), according to the CDC’s COVID Data Tracker.
The trend for new hospital admissions of children with confirmed COVID showed a similar rise through December, and the latest data for the very beginning of January suggest an even faster drop, although there is more of a reporting lag with hospitalization data, compared with ED visits, the CDC noted.
The most current data (Dec. 30 to Jan. 5) available from the American Academy of Pediatrics and the Children’s Hospital Association show less volatility in the number of weekly cases through November and December, with the peak being about 48,000 in mid-December. The AAP/CHA totals for the last 2 weeks, however, were both higher than the CDC’s corresponding counts, which are more preliminary and subject to revision.
The CDC puts the total number of COVID cases in children at 16.7 million – about 17.2% of all cases – as of Jan. 11, with 1,981 deaths reported so far. The AAP and CHA are not tracking deaths, but their case total as of Jan. 5 was 15.2 million, which represents 18.1% of cases in all ages. The AAP/CHA report is based on data reported publicly by an ever-decreasing number of states and territories.
Ecopipam reduces Tourette’s tics without common side effects in phase 2 trial
Ecopipam, in development for Tourette syndrome in children and adolescents, has shown in a randomized, controlled trial that, compared with placebo, it reduced tics and reduced the risk for some of the common side effects of other treatments, including weight gain.
Findings of the multicenter, double-blind, trial funded by the drug maker, Emalex Biosciences, were published online in Pediatrics. The trial was conducted at 68 sites in the United States, Canada, Germany, France, and Poland between May 2019 and September 2021.
Donald L. Gilbert, MD, MS, with the division of neurology at Cincinnati Children’s Hospital, and colleagues noted that all Food and Drug Administration–approved medications for Tourette syndrome are antipsychotics. The medications carry a risk of weight gain, electrocardiogram abnormalities, metabolic changes, and drug-induced movement disorders.
First-in-class medication ecopipam, targets the D1 dopamine receptor, while currently approved medications block the D2 receptor. It “may be a safe and effective treatment of Tourette syndrome with advantages over other currently approved therapeutic agents,” the authors wrote.
The study included 153 individuals at least 6 years old up to age 18 with a baseline Yale Global Tic Severity Score Total Tic Score of at least 20.
They were randomly assigned 1:1 to ecopipam or placebo.
Significant reduction in tic severity
Researchers saw a 30% reduction in the tic severity score from baseline to week 12 for the ecopipam group compared with the placebo group.
The data showed a least-squares mean difference of 3.44 (95% confidence interval [CI], 6.09-0.79, P = .01). Researchers also saw improvement in Clinical Global Impression of Tourette Syndrome Severity in the ecopipam group (P = .03).
Sara Pawlowski, MD, division chief for primary care mental health integration at University of Vermont Health Network and assistant professor of psychiatry, University of Vermont, Burlington, said in an interview that several things should be considered with this research.
One is that, though the results show a reduction in tics, the study lasted only 12 weeks and “tics can last a lifetime,” she noted.
“They also can ebb and flow with major life events, stressors, and various other variables. So, I wonder how the effects of improvement can be teased out from the natural ebb and flow of the condition in a 3-month window, which is a snapshot into the course of a known relapsing, remitting, lifetime, and chronically variable condition,” she said.
Headaches, insomnia among side effects
Weight gain was larger in the placebo group than in the ecopipam group: 17.1% in the ecopipam group and 20.3% of those who got a placebo had a weight gain of more than 7% over the study period.
The most common side effects of the study drug were headache (15.8%), insomnia (14.5%), fatigue (7.9%), and somnolence (7.9%).
A limitation of the study was lack of racial and ethnic diversity, as 93.5% of those in the placebo group and 86.8% in the ecopipam group were White.
Guidelines in North America and Europe agree that behavioral treatments should be the first-line therapy.
Dr. Pawlowski said that although effective medications are needed, she urges focusing on better access to nonmedication treatments “that work for children and adolescents” as children who start taking the medications early may take them for the rest of their lives.
Also, while the research didn’t find weight gain in the ecopipam group, the side effects they did find in the group, including headache and insomnia, “do impact a child’s life,” she noted.
“We also can’t be reassured that over the course of chronic treatment there wouldn’t be movement disorders or metabolic disorders that emerge. Those are side effects or disorders that can emerge surreptitiously over time, and more time than 12 weeks,” she said.
The study was funded by Emalex Biosciences. Dr. Gilbert has received consulting fees from Biogen and PTC therapeutics. Study coauthors disclosed ties with Emalex, Alkermes, and Paragon Biosciences. Dr. Pawlowski reports no relevant financial relationships.
Ecopipam, in development for Tourette syndrome in children and adolescents, has shown in a randomized, controlled trial that, compared with placebo, it reduced tics and reduced the risk for some of the common side effects of other treatments, including weight gain.
Findings of the multicenter, double-blind, trial funded by the drug maker, Emalex Biosciences, were published online in Pediatrics. The trial was conducted at 68 sites in the United States, Canada, Germany, France, and Poland between May 2019 and September 2021.
Donald L. Gilbert, MD, MS, with the division of neurology at Cincinnati Children’s Hospital, and colleagues noted that all Food and Drug Administration–approved medications for Tourette syndrome are antipsychotics. The medications carry a risk of weight gain, electrocardiogram abnormalities, metabolic changes, and drug-induced movement disorders.
First-in-class medication ecopipam, targets the D1 dopamine receptor, while currently approved medications block the D2 receptor. It “may be a safe and effective treatment of Tourette syndrome with advantages over other currently approved therapeutic agents,” the authors wrote.
The study included 153 individuals at least 6 years old up to age 18 with a baseline Yale Global Tic Severity Score Total Tic Score of at least 20.
They were randomly assigned 1:1 to ecopipam or placebo.
Significant reduction in tic severity
Researchers saw a 30% reduction in the tic severity score from baseline to week 12 for the ecopipam group compared with the placebo group.
The data showed a least-squares mean difference of 3.44 (95% confidence interval [CI], 6.09-0.79, P = .01). Researchers also saw improvement in Clinical Global Impression of Tourette Syndrome Severity in the ecopipam group (P = .03).
Sara Pawlowski, MD, division chief for primary care mental health integration at University of Vermont Health Network and assistant professor of psychiatry, University of Vermont, Burlington, said in an interview that several things should be considered with this research.
One is that, though the results show a reduction in tics, the study lasted only 12 weeks and “tics can last a lifetime,” she noted.
“They also can ebb and flow with major life events, stressors, and various other variables. So, I wonder how the effects of improvement can be teased out from the natural ebb and flow of the condition in a 3-month window, which is a snapshot into the course of a known relapsing, remitting, lifetime, and chronically variable condition,” she said.
Headaches, insomnia among side effects
Weight gain was larger in the placebo group than in the ecopipam group: 17.1% in the ecopipam group and 20.3% of those who got a placebo had a weight gain of more than 7% over the study period.
The most common side effects of the study drug were headache (15.8%), insomnia (14.5%), fatigue (7.9%), and somnolence (7.9%).
A limitation of the study was lack of racial and ethnic diversity, as 93.5% of those in the placebo group and 86.8% in the ecopipam group were White.
Guidelines in North America and Europe agree that behavioral treatments should be the first-line therapy.
Dr. Pawlowski said that although effective medications are needed, she urges focusing on better access to nonmedication treatments “that work for children and adolescents” as children who start taking the medications early may take them for the rest of their lives.
Also, while the research didn’t find weight gain in the ecopipam group, the side effects they did find in the group, including headache and insomnia, “do impact a child’s life,” she noted.
“We also can’t be reassured that over the course of chronic treatment there wouldn’t be movement disorders or metabolic disorders that emerge. Those are side effects or disorders that can emerge surreptitiously over time, and more time than 12 weeks,” she said.
The study was funded by Emalex Biosciences. Dr. Gilbert has received consulting fees from Biogen and PTC therapeutics. Study coauthors disclosed ties with Emalex, Alkermes, and Paragon Biosciences. Dr. Pawlowski reports no relevant financial relationships.
Ecopipam, in development for Tourette syndrome in children and adolescents, has shown in a randomized, controlled trial that, compared with placebo, it reduced tics and reduced the risk for some of the common side effects of other treatments, including weight gain.
Findings of the multicenter, double-blind, trial funded by the drug maker, Emalex Biosciences, were published online in Pediatrics. The trial was conducted at 68 sites in the United States, Canada, Germany, France, and Poland between May 2019 and September 2021.
Donald L. Gilbert, MD, MS, with the division of neurology at Cincinnati Children’s Hospital, and colleagues noted that all Food and Drug Administration–approved medications for Tourette syndrome are antipsychotics. The medications carry a risk of weight gain, electrocardiogram abnormalities, metabolic changes, and drug-induced movement disorders.
First-in-class medication ecopipam, targets the D1 dopamine receptor, while currently approved medications block the D2 receptor. It “may be a safe and effective treatment of Tourette syndrome with advantages over other currently approved therapeutic agents,” the authors wrote.
The study included 153 individuals at least 6 years old up to age 18 with a baseline Yale Global Tic Severity Score Total Tic Score of at least 20.
They were randomly assigned 1:1 to ecopipam or placebo.
Significant reduction in tic severity
Researchers saw a 30% reduction in the tic severity score from baseline to week 12 for the ecopipam group compared with the placebo group.
The data showed a least-squares mean difference of 3.44 (95% confidence interval [CI], 6.09-0.79, P = .01). Researchers also saw improvement in Clinical Global Impression of Tourette Syndrome Severity in the ecopipam group (P = .03).
Sara Pawlowski, MD, division chief for primary care mental health integration at University of Vermont Health Network and assistant professor of psychiatry, University of Vermont, Burlington, said in an interview that several things should be considered with this research.
One is that, though the results show a reduction in tics, the study lasted only 12 weeks and “tics can last a lifetime,” she noted.
“They also can ebb and flow with major life events, stressors, and various other variables. So, I wonder how the effects of improvement can be teased out from the natural ebb and flow of the condition in a 3-month window, which is a snapshot into the course of a known relapsing, remitting, lifetime, and chronically variable condition,” she said.
Headaches, insomnia among side effects
Weight gain was larger in the placebo group than in the ecopipam group: 17.1% in the ecopipam group and 20.3% of those who got a placebo had a weight gain of more than 7% over the study period.
The most common side effects of the study drug were headache (15.8%), insomnia (14.5%), fatigue (7.9%), and somnolence (7.9%).
A limitation of the study was lack of racial and ethnic diversity, as 93.5% of those in the placebo group and 86.8% in the ecopipam group were White.
Guidelines in North America and Europe agree that behavioral treatments should be the first-line therapy.
Dr. Pawlowski said that although effective medications are needed, she urges focusing on better access to nonmedication treatments “that work for children and adolescents” as children who start taking the medications early may take them for the rest of their lives.
Also, while the research didn’t find weight gain in the ecopipam group, the side effects they did find in the group, including headache and insomnia, “do impact a child’s life,” she noted.
“We also can’t be reassured that over the course of chronic treatment there wouldn’t be movement disorders or metabolic disorders that emerge. Those are side effects or disorders that can emerge surreptitiously over time, and more time than 12 weeks,” she said.
The study was funded by Emalex Biosciences. Dr. Gilbert has received consulting fees from Biogen and PTC therapeutics. Study coauthors disclosed ties with Emalex, Alkermes, and Paragon Biosciences. Dr. Pawlowski reports no relevant financial relationships.
FROM PEDIATRICS
Add this to the list of long COVID symptoms: Stigma
Most people with long COVID find they’re facing stigma due to their condition, according to a new report from researchers in the United Kingdom. In short: Relatives and friends may not believe they’re truly sick.
The U.K. team found that more than three-quarters of people studied had experienced stigma often or always.
In fact, 95% of people with long COVID faced at least one type of stigma at least sometimes, according to the study, published in November in the journal PLOS One.
Those conclusions had surprised the study’s lead researcher, Marija Pantelic, PhD, a public health lecturer at Brighton and Sussex Medical School, England.
“After years of working on HIV-related stigma, I was shocked to see how many people were turning a blind eye to and dismissing the difficulties experienced by people with long COVID,” Dr. Pantelic says. “It has also been clear to me from the start that this stigma is detrimental not just for people’s dignity, but also public health.”
Even some doctors argue that the growing attention paid to long COVID is excessive.
“It’s often normal to experience mild fatigue or weaknesses for weeks after being sick and inactive and not eating well. Calling these cases long COVID is the medicalization of modern life,” Marty Makary, MD, a surgeon and public policy researcher at Johns Hopkins University, Baltimore, wrote in a commentary in the Wall Street Journal.
Other doctors strongly disagree, including Alba Azola, MD, codirector of the Johns Hopkins Post-Acute COVID-19 Team and an expert in the stigma surrounding long COVID.
“Putting that spin on things, it’s just hurting people,” she says.
One example is people who cannot return to work.
“A lot of their family members tell me that they’re being lazy,” Dr. Azola says. “That’s part of the public stigma, that these are people just trying to get out of work.”
Some experts say the U.K. study represents a landmark.
“When you have data like this on long COVID stigma, it becomes more difficult to deny its existence or address it,” says Naomi Torres-Mackie, PhD, a clinical psychologist at Lenox Hill Hospital in New York. She also is head of research at the New York–based Mental Health Coalition, a group of experts working to end the stigma surrounding mental health.
She recalls her first patient with long COVID.
“She experienced the discomfort and pain itself, and then she had this crushing feeling that it wasn’t valid, or real. She felt very alone in it,” Dr. Torres-Mackie says.
Another one of her patients is working at her job from home but facing doubt about her condition from her employers.
“Every month, her medical doctor has to produce a letter confirming her medical condition,” Dr. Torres-Mackie says.
Taking part in the British stigma survey were 1,166 people, including 966 residents of the United Kingdom, with the average age of 48. Nearly 85% were female, and more than three-quarters were educated at the university level or higher.
Half of them said they had a clinical diagnosis of long COVID.
More than 60% of them said that at least some of the time, they were cautious about who they talked to about their condition. And fully 34% of those who did disclose their diagnosis said that they regretted having done so.
That’s a difficult experience for those with long COVID, says Leonard Jason, PhD, a professor of psychology at DePaul University in Chicago.
“It’s like they’re traumatized by the initial experience of being sick, and retraumatized by the response of others to them,” he says.
Unexplained illnesses are not well-regarded by the general public, Dr. Jason says.
He gave the example of multiple sclerosis. Before the 1980s, those with MS were considered to have a psychological illness, he says. “Then, in the 1980s, there were biomarkers that said, ‘Here’s the evidence.’ ”
The British study described three types of stigma stemming from the long COVID diagnosis of those questioned:
- Enacted stigma: People were directly treated unfairly because of their condition.
- Internalized stigma: People felt embarrassed by that condition.
- Anticipated stigma: People expected they would be treated poorly because of their diagnosis.
Dr. Azola calls the medical community a major problem when it comes to dealing with long COVID.
“What I see with my patients is medical trauma,” she says. They may have symptoms that send them to the emergency room, and then the tests come back negative. “Instead of tracking the patients’ symptoms, patients get told, ‘Everything looks good, you can go home, this is a panic attack,’ ” she says.
Some people go online to search for treatments, sometimes launching GoFundMe campaigns to raise money for unreliable treatments.
Long COVID patients may have gone through 5 to 10 doctors before they arrive for treatment with the Johns Hopkins Post-Acute COVID-19 Team. The clinic began in April 2020 remotely and in August of that year in person.
Today, the clinic staff spends an hour with a first-time long COVID patient, hearing their stories and helping relieve anxiety, Dr. Azola says.
The phenomenon of long COVID is similar to what patients have had with chronic fatigue syndrome, lupus, or fibromyalgia, where people have symptoms that are hard to explain, says Jennifer Chevinsky, MD, deputy public health officer for Riverside County, Calif.
“Stigma within medicine or health care is nothing new,” she says.
In Chicago, Dr. Jason notes that the federal government’s decision to invest hundreds of millions of dollars in long COVID research “shows the government is helping destigmatize it.”
Dr. Pantelic says she and her colleagues are continuing their research.
“We are interested in understanding the impacts of this stigma, and how to mitigate any adverse outcomes for patients and services,” she says.
A version of this article first appeared on WebMD.com.
Most people with long COVID find they’re facing stigma due to their condition, according to a new report from researchers in the United Kingdom. In short: Relatives and friends may not believe they’re truly sick.
The U.K. team found that more than three-quarters of people studied had experienced stigma often or always.
In fact, 95% of people with long COVID faced at least one type of stigma at least sometimes, according to the study, published in November in the journal PLOS One.
Those conclusions had surprised the study’s lead researcher, Marija Pantelic, PhD, a public health lecturer at Brighton and Sussex Medical School, England.
“After years of working on HIV-related stigma, I was shocked to see how many people were turning a blind eye to and dismissing the difficulties experienced by people with long COVID,” Dr. Pantelic says. “It has also been clear to me from the start that this stigma is detrimental not just for people’s dignity, but also public health.”
Even some doctors argue that the growing attention paid to long COVID is excessive.
“It’s often normal to experience mild fatigue or weaknesses for weeks after being sick and inactive and not eating well. Calling these cases long COVID is the medicalization of modern life,” Marty Makary, MD, a surgeon and public policy researcher at Johns Hopkins University, Baltimore, wrote in a commentary in the Wall Street Journal.
Other doctors strongly disagree, including Alba Azola, MD, codirector of the Johns Hopkins Post-Acute COVID-19 Team and an expert in the stigma surrounding long COVID.
“Putting that spin on things, it’s just hurting people,” she says.
One example is people who cannot return to work.
“A lot of their family members tell me that they’re being lazy,” Dr. Azola says. “That’s part of the public stigma, that these are people just trying to get out of work.”
Some experts say the U.K. study represents a landmark.
“When you have data like this on long COVID stigma, it becomes more difficult to deny its existence or address it,” says Naomi Torres-Mackie, PhD, a clinical psychologist at Lenox Hill Hospital in New York. She also is head of research at the New York–based Mental Health Coalition, a group of experts working to end the stigma surrounding mental health.
She recalls her first patient with long COVID.
“She experienced the discomfort and pain itself, and then she had this crushing feeling that it wasn’t valid, or real. She felt very alone in it,” Dr. Torres-Mackie says.
Another one of her patients is working at her job from home but facing doubt about her condition from her employers.
“Every month, her medical doctor has to produce a letter confirming her medical condition,” Dr. Torres-Mackie says.
Taking part in the British stigma survey were 1,166 people, including 966 residents of the United Kingdom, with the average age of 48. Nearly 85% were female, and more than three-quarters were educated at the university level or higher.
Half of them said they had a clinical diagnosis of long COVID.
More than 60% of them said that at least some of the time, they were cautious about who they talked to about their condition. And fully 34% of those who did disclose their diagnosis said that they regretted having done so.
That’s a difficult experience for those with long COVID, says Leonard Jason, PhD, a professor of psychology at DePaul University in Chicago.
“It’s like they’re traumatized by the initial experience of being sick, and retraumatized by the response of others to them,” he says.
Unexplained illnesses are not well-regarded by the general public, Dr. Jason says.
He gave the example of multiple sclerosis. Before the 1980s, those with MS were considered to have a psychological illness, he says. “Then, in the 1980s, there were biomarkers that said, ‘Here’s the evidence.’ ”
The British study described three types of stigma stemming from the long COVID diagnosis of those questioned:
- Enacted stigma: People were directly treated unfairly because of their condition.
- Internalized stigma: People felt embarrassed by that condition.
- Anticipated stigma: People expected they would be treated poorly because of their diagnosis.
Dr. Azola calls the medical community a major problem when it comes to dealing with long COVID.
“What I see with my patients is medical trauma,” she says. They may have symptoms that send them to the emergency room, and then the tests come back negative. “Instead of tracking the patients’ symptoms, patients get told, ‘Everything looks good, you can go home, this is a panic attack,’ ” she says.
Some people go online to search for treatments, sometimes launching GoFundMe campaigns to raise money for unreliable treatments.
Long COVID patients may have gone through 5 to 10 doctors before they arrive for treatment with the Johns Hopkins Post-Acute COVID-19 Team. The clinic began in April 2020 remotely and in August of that year in person.
Today, the clinic staff spends an hour with a first-time long COVID patient, hearing their stories and helping relieve anxiety, Dr. Azola says.
The phenomenon of long COVID is similar to what patients have had with chronic fatigue syndrome, lupus, or fibromyalgia, where people have symptoms that are hard to explain, says Jennifer Chevinsky, MD, deputy public health officer for Riverside County, Calif.
“Stigma within medicine or health care is nothing new,” she says.
In Chicago, Dr. Jason notes that the federal government’s decision to invest hundreds of millions of dollars in long COVID research “shows the government is helping destigmatize it.”
Dr. Pantelic says she and her colleagues are continuing their research.
“We are interested in understanding the impacts of this stigma, and how to mitigate any adverse outcomes for patients and services,” she says.
A version of this article first appeared on WebMD.com.
Most people with long COVID find they’re facing stigma due to their condition, according to a new report from researchers in the United Kingdom. In short: Relatives and friends may not believe they’re truly sick.
The U.K. team found that more than three-quarters of people studied had experienced stigma often or always.
In fact, 95% of people with long COVID faced at least one type of stigma at least sometimes, according to the study, published in November in the journal PLOS One.
Those conclusions had surprised the study’s lead researcher, Marija Pantelic, PhD, a public health lecturer at Brighton and Sussex Medical School, England.
“After years of working on HIV-related stigma, I was shocked to see how many people were turning a blind eye to and dismissing the difficulties experienced by people with long COVID,” Dr. Pantelic says. “It has also been clear to me from the start that this stigma is detrimental not just for people’s dignity, but also public health.”
Even some doctors argue that the growing attention paid to long COVID is excessive.
“It’s often normal to experience mild fatigue or weaknesses for weeks after being sick and inactive and not eating well. Calling these cases long COVID is the medicalization of modern life,” Marty Makary, MD, a surgeon and public policy researcher at Johns Hopkins University, Baltimore, wrote in a commentary in the Wall Street Journal.
Other doctors strongly disagree, including Alba Azola, MD, codirector of the Johns Hopkins Post-Acute COVID-19 Team and an expert in the stigma surrounding long COVID.
“Putting that spin on things, it’s just hurting people,” she says.
One example is people who cannot return to work.
“A lot of their family members tell me that they’re being lazy,” Dr. Azola says. “That’s part of the public stigma, that these are people just trying to get out of work.”
Some experts say the U.K. study represents a landmark.
“When you have data like this on long COVID stigma, it becomes more difficult to deny its existence or address it,” says Naomi Torres-Mackie, PhD, a clinical psychologist at Lenox Hill Hospital in New York. She also is head of research at the New York–based Mental Health Coalition, a group of experts working to end the stigma surrounding mental health.
She recalls her first patient with long COVID.
“She experienced the discomfort and pain itself, and then she had this crushing feeling that it wasn’t valid, or real. She felt very alone in it,” Dr. Torres-Mackie says.
Another one of her patients is working at her job from home but facing doubt about her condition from her employers.
“Every month, her medical doctor has to produce a letter confirming her medical condition,” Dr. Torres-Mackie says.
Taking part in the British stigma survey were 1,166 people, including 966 residents of the United Kingdom, with the average age of 48. Nearly 85% were female, and more than three-quarters were educated at the university level or higher.
Half of them said they had a clinical diagnosis of long COVID.
More than 60% of them said that at least some of the time, they were cautious about who they talked to about their condition. And fully 34% of those who did disclose their diagnosis said that they regretted having done so.
That’s a difficult experience for those with long COVID, says Leonard Jason, PhD, a professor of psychology at DePaul University in Chicago.
“It’s like they’re traumatized by the initial experience of being sick, and retraumatized by the response of others to them,” he says.
Unexplained illnesses are not well-regarded by the general public, Dr. Jason says.
He gave the example of multiple sclerosis. Before the 1980s, those with MS were considered to have a psychological illness, he says. “Then, in the 1980s, there were biomarkers that said, ‘Here’s the evidence.’ ”
The British study described three types of stigma stemming from the long COVID diagnosis of those questioned:
- Enacted stigma: People were directly treated unfairly because of their condition.
- Internalized stigma: People felt embarrassed by that condition.
- Anticipated stigma: People expected they would be treated poorly because of their diagnosis.
Dr. Azola calls the medical community a major problem when it comes to dealing with long COVID.
“What I see with my patients is medical trauma,” she says. They may have symptoms that send them to the emergency room, and then the tests come back negative. “Instead of tracking the patients’ symptoms, patients get told, ‘Everything looks good, you can go home, this is a panic attack,’ ” she says.
Some people go online to search for treatments, sometimes launching GoFundMe campaigns to raise money for unreliable treatments.
Long COVID patients may have gone through 5 to 10 doctors before they arrive for treatment with the Johns Hopkins Post-Acute COVID-19 Team. The clinic began in April 2020 remotely and in August of that year in person.
Today, the clinic staff spends an hour with a first-time long COVID patient, hearing their stories and helping relieve anxiety, Dr. Azola says.
The phenomenon of long COVID is similar to what patients have had with chronic fatigue syndrome, lupus, or fibromyalgia, where people have symptoms that are hard to explain, says Jennifer Chevinsky, MD, deputy public health officer for Riverside County, Calif.
“Stigma within medicine or health care is nothing new,” she says.
In Chicago, Dr. Jason notes that the federal government’s decision to invest hundreds of millions of dollars in long COVID research “shows the government is helping destigmatize it.”
Dr. Pantelic says she and her colleagues are continuing their research.
“We are interested in understanding the impacts of this stigma, and how to mitigate any adverse outcomes for patients and services,” she says.
A version of this article first appeared on WebMD.com.
PLOS ONE
Can siRNA improve compliance in patients with hypertension?
Many approaches have been explored in recent years to make life easier for patients living with chronic conditions that require them to take daily medication: subcutaneous implantable devices, nanogels, and, more specifically in the case of hypertension, renal denervation or small interfering RNA (siRNA) with a long half-life.
It’s siRNA that Michel Azizi, MD, PhD, head of the blood pressure clinic at Georges Pompidou European Hospital (HEGP) in Paris, discussed at the International Meeting of the French Society of Hypertension.
These small molecules have already shown their worth in treating rare diseases such as transthyretin amyloidosis. More recently, treating hypercholesterolemia with the PCSK9 inhibitor inclisiran has proven effective. “One subcutaneous injection of inclisiran reduces LDL cholesterol by 50% for a period of 210 days,” said Dr. Azizi.
The benefit of a new therapeutic siRNA – zilebesiran, administered subcutaneously – in treating hypertension is currently the subject of a phase II clinical trial.
This is a double-stranded RNA. One of the strands is linked to a sugar, N-acetylgalactosamine (GalNAc), which protects these highly fragile siRNA and binds with a very strong affinity in the liver. The second strand binds to a specific area of the RNA to prevent synthesis of the precursor peptide of angiotensin, angiotensinogen. The resulting effect is suppression of the production of angiotensin I and II, which leads to a long-lasting lowering of blood pressure.
Lasting efficacy
Phase I studies with zilebesiran have demonstrated a long-term effect, with a reduction of greater than 90% in circulating angiotensinogen over 6 months after a single subcutaneous dose (800 mg). The peak in reduction of circulating angiotensinogen occurs after approximately 3 weeks.
“It’s extremely powerful,” said Dr. Azizi.
Lasting reductions in blood pressure have also been observed, with 24-hour ambulatory blood pressure monitoring showing a reduction in systolic BP of greater than 15 mm Hg 8 weeks after administration of a single dose of zilebesiran (800 mg).
Zilebesiran was also well tolerated, with only mild to moderate reactions at the site of the injection (n = 5/56) and no serious treatment-related adverse events, hypotension, or significant changes in kidney or liver function.
“In terms of benefits, the effect is ongoing. Zilebesiran leads to reduced medication use and causes less variability in blood pressure response. Nevertheless, interfering RNA acts slowly, meaning that zilebesiran would not be suitable for people presenting with a hypertensive crisis. The fact that it blocks the renin-angiotensin system [RAS] for a very long period of time also poses the question of how to reverse its hypotensive effects,” said Dr. Azizi.
Unanswered questions
The lasting RAS antagonist and blood pressure–lowering effects pose a potential safety problem in circumstances involving patients in a state of hypovolemia and hypotension who require rapid blood pressure–raising interventions to prevent morbidity and mortality.
In recent studies, Estrellita Uijl et al. have thus examined strategies to counteract the blood pressure–lowering effect of siRNA in spontaneously hypertensive rats.
Fludrocortisone and a high-salt diet were both successful in gradually increasing blood pressure, which returned to its baseline levels on days 5 and 7, respectively. Yet this rate of response would be wholly inadequate in an urgent clinical situation.
However, midodrine could not reduce blood pressure to normal levels, whether administered subcutaneously or orally.
A rapid and short-lasting increase in blood pressure was observed with bolus doses of vasopressors, but clinically, these would need to be administered intravenously to achieve a lasting effect. Such administration would require hospitalization, close monitoring, and the use of human resources and additional health care provisions.
Encouragingly, the laboratory that created this molecule, Alnylam Pharmaceuticals, has come up with an antidote: Reversir. It is a GalNAc-conjugated, single-stranded, high-affinity oligonucleotide complementary to the zilebesiran strand that achieves effective reversal of siRNA activity in 24 hours.
In the future, after the phase 2 trials have been completed, whether or not zilebesiran reduces the incidence of cardiovascular events and mortality remains to be seen. But as for Dr. Azizi, the director of HEGP’s blood pressure clinic in Paris, he has no doubt that “this approach is about to shake up how we treat patients in the cardiovascular field.”
On the horizon
Zilebesiran is being studied in phase 2 trials in patients with mild to moderate hypertension not taking antihypertensive drugs (KARDIA-1: 375 patients; double-blind, placebo-controlled, five-arm trial; zilebesiran at 150, 300, and 600 mg twice per year and 300 mg once every 3 months) and in patients whose blood pressure is not controlled (KARDIA-2: 800 patients; initial open-label start-up period of 4 weeks with indapamide/amlodipine/olmesartan, followed by a double-blind, placebo-controlled study over 6 months, then an open-label extension study for up to 12 additional months; zilebesiran at 600 mg on the first day of the initial double-blind period, then every 6 months during the open-label extension period).
This article was translated from the Medscape French edition and a version appeared on Medscape.com.
Many approaches have been explored in recent years to make life easier for patients living with chronic conditions that require them to take daily medication: subcutaneous implantable devices, nanogels, and, more specifically in the case of hypertension, renal denervation or small interfering RNA (siRNA) with a long half-life.
It’s siRNA that Michel Azizi, MD, PhD, head of the blood pressure clinic at Georges Pompidou European Hospital (HEGP) in Paris, discussed at the International Meeting of the French Society of Hypertension.
These small molecules have already shown their worth in treating rare diseases such as transthyretin amyloidosis. More recently, treating hypercholesterolemia with the PCSK9 inhibitor inclisiran has proven effective. “One subcutaneous injection of inclisiran reduces LDL cholesterol by 50% for a period of 210 days,” said Dr. Azizi.
The benefit of a new therapeutic siRNA – zilebesiran, administered subcutaneously – in treating hypertension is currently the subject of a phase II clinical trial.
This is a double-stranded RNA. One of the strands is linked to a sugar, N-acetylgalactosamine (GalNAc), which protects these highly fragile siRNA and binds with a very strong affinity in the liver. The second strand binds to a specific area of the RNA to prevent synthesis of the precursor peptide of angiotensin, angiotensinogen. The resulting effect is suppression of the production of angiotensin I and II, which leads to a long-lasting lowering of blood pressure.
Lasting efficacy
Phase I studies with zilebesiran have demonstrated a long-term effect, with a reduction of greater than 90% in circulating angiotensinogen over 6 months after a single subcutaneous dose (800 mg). The peak in reduction of circulating angiotensinogen occurs after approximately 3 weeks.
“It’s extremely powerful,” said Dr. Azizi.
Lasting reductions in blood pressure have also been observed, with 24-hour ambulatory blood pressure monitoring showing a reduction in systolic BP of greater than 15 mm Hg 8 weeks after administration of a single dose of zilebesiran (800 mg).
Zilebesiran was also well tolerated, with only mild to moderate reactions at the site of the injection (n = 5/56) and no serious treatment-related adverse events, hypotension, or significant changes in kidney or liver function.
“In terms of benefits, the effect is ongoing. Zilebesiran leads to reduced medication use and causes less variability in blood pressure response. Nevertheless, interfering RNA acts slowly, meaning that zilebesiran would not be suitable for people presenting with a hypertensive crisis. The fact that it blocks the renin-angiotensin system [RAS] for a very long period of time also poses the question of how to reverse its hypotensive effects,” said Dr. Azizi.
Unanswered questions
The lasting RAS antagonist and blood pressure–lowering effects pose a potential safety problem in circumstances involving patients in a state of hypovolemia and hypotension who require rapid blood pressure–raising interventions to prevent morbidity and mortality.
In recent studies, Estrellita Uijl et al. have thus examined strategies to counteract the blood pressure–lowering effect of siRNA in spontaneously hypertensive rats.
Fludrocortisone and a high-salt diet were both successful in gradually increasing blood pressure, which returned to its baseline levels on days 5 and 7, respectively. Yet this rate of response would be wholly inadequate in an urgent clinical situation.
However, midodrine could not reduce blood pressure to normal levels, whether administered subcutaneously or orally.
A rapid and short-lasting increase in blood pressure was observed with bolus doses of vasopressors, but clinically, these would need to be administered intravenously to achieve a lasting effect. Such administration would require hospitalization, close monitoring, and the use of human resources and additional health care provisions.
Encouragingly, the laboratory that created this molecule, Alnylam Pharmaceuticals, has come up with an antidote: Reversir. It is a GalNAc-conjugated, single-stranded, high-affinity oligonucleotide complementary to the zilebesiran strand that achieves effective reversal of siRNA activity in 24 hours.
In the future, after the phase 2 trials have been completed, whether or not zilebesiran reduces the incidence of cardiovascular events and mortality remains to be seen. But as for Dr. Azizi, the director of HEGP’s blood pressure clinic in Paris, he has no doubt that “this approach is about to shake up how we treat patients in the cardiovascular field.”
On the horizon
Zilebesiran is being studied in phase 2 trials in patients with mild to moderate hypertension not taking antihypertensive drugs (KARDIA-1: 375 patients; double-blind, placebo-controlled, five-arm trial; zilebesiran at 150, 300, and 600 mg twice per year and 300 mg once every 3 months) and in patients whose blood pressure is not controlled (KARDIA-2: 800 patients; initial open-label start-up period of 4 weeks with indapamide/amlodipine/olmesartan, followed by a double-blind, placebo-controlled study over 6 months, then an open-label extension study for up to 12 additional months; zilebesiran at 600 mg on the first day of the initial double-blind period, then every 6 months during the open-label extension period).
This article was translated from the Medscape French edition and a version appeared on Medscape.com.
Many approaches have been explored in recent years to make life easier for patients living with chronic conditions that require them to take daily medication: subcutaneous implantable devices, nanogels, and, more specifically in the case of hypertension, renal denervation or small interfering RNA (siRNA) with a long half-life.
It’s siRNA that Michel Azizi, MD, PhD, head of the blood pressure clinic at Georges Pompidou European Hospital (HEGP) in Paris, discussed at the International Meeting of the French Society of Hypertension.
These small molecules have already shown their worth in treating rare diseases such as transthyretin amyloidosis. More recently, treating hypercholesterolemia with the PCSK9 inhibitor inclisiran has proven effective. “One subcutaneous injection of inclisiran reduces LDL cholesterol by 50% for a period of 210 days,” said Dr. Azizi.
The benefit of a new therapeutic siRNA – zilebesiran, administered subcutaneously – in treating hypertension is currently the subject of a phase II clinical trial.
This is a double-stranded RNA. One of the strands is linked to a sugar, N-acetylgalactosamine (GalNAc), which protects these highly fragile siRNA and binds with a very strong affinity in the liver. The second strand binds to a specific area of the RNA to prevent synthesis of the precursor peptide of angiotensin, angiotensinogen. The resulting effect is suppression of the production of angiotensin I and II, which leads to a long-lasting lowering of blood pressure.
Lasting efficacy
Phase I studies with zilebesiran have demonstrated a long-term effect, with a reduction of greater than 90% in circulating angiotensinogen over 6 months after a single subcutaneous dose (800 mg). The peak in reduction of circulating angiotensinogen occurs after approximately 3 weeks.
“It’s extremely powerful,” said Dr. Azizi.
Lasting reductions in blood pressure have also been observed, with 24-hour ambulatory blood pressure monitoring showing a reduction in systolic BP of greater than 15 mm Hg 8 weeks after administration of a single dose of zilebesiran (800 mg).
Zilebesiran was also well tolerated, with only mild to moderate reactions at the site of the injection (n = 5/56) and no serious treatment-related adverse events, hypotension, or significant changes in kidney or liver function.
“In terms of benefits, the effect is ongoing. Zilebesiran leads to reduced medication use and causes less variability in blood pressure response. Nevertheless, interfering RNA acts slowly, meaning that zilebesiran would not be suitable for people presenting with a hypertensive crisis. The fact that it blocks the renin-angiotensin system [RAS] for a very long period of time also poses the question of how to reverse its hypotensive effects,” said Dr. Azizi.
Unanswered questions
The lasting RAS antagonist and blood pressure–lowering effects pose a potential safety problem in circumstances involving patients in a state of hypovolemia and hypotension who require rapid blood pressure–raising interventions to prevent morbidity and mortality.
In recent studies, Estrellita Uijl et al. have thus examined strategies to counteract the blood pressure–lowering effect of siRNA in spontaneously hypertensive rats.
Fludrocortisone and a high-salt diet were both successful in gradually increasing blood pressure, which returned to its baseline levels on days 5 and 7, respectively. Yet this rate of response would be wholly inadequate in an urgent clinical situation.
However, midodrine could not reduce blood pressure to normal levels, whether administered subcutaneously or orally.
A rapid and short-lasting increase in blood pressure was observed with bolus doses of vasopressors, but clinically, these would need to be administered intravenously to achieve a lasting effect. Such administration would require hospitalization, close monitoring, and the use of human resources and additional health care provisions.
Encouragingly, the laboratory that created this molecule, Alnylam Pharmaceuticals, has come up with an antidote: Reversir. It is a GalNAc-conjugated, single-stranded, high-affinity oligonucleotide complementary to the zilebesiran strand that achieves effective reversal of siRNA activity in 24 hours.
In the future, after the phase 2 trials have been completed, whether or not zilebesiran reduces the incidence of cardiovascular events and mortality remains to be seen. But as for Dr. Azizi, the director of HEGP’s blood pressure clinic in Paris, he has no doubt that “this approach is about to shake up how we treat patients in the cardiovascular field.”
On the horizon
Zilebesiran is being studied in phase 2 trials in patients with mild to moderate hypertension not taking antihypertensive drugs (KARDIA-1: 375 patients; double-blind, placebo-controlled, five-arm trial; zilebesiran at 150, 300, and 600 mg twice per year and 300 mg once every 3 months) and in patients whose blood pressure is not controlled (KARDIA-2: 800 patients; initial open-label start-up period of 4 weeks with indapamide/amlodipine/olmesartan, followed by a double-blind, placebo-controlled study over 6 months, then an open-label extension study for up to 12 additional months; zilebesiran at 600 mg on the first day of the initial double-blind period, then every 6 months during the open-label extension period).
This article was translated from the Medscape French edition and a version appeared on Medscape.com.
AT INTERNATIONAL MEETING OF THE FRENCH SOCIETY OF HYPERTENSION