User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
How much would you bet on a diagnosis?
“You have psoriasis,” I say all the time. I mean it when I say it, of course. But I don’t always to the same degree. Sometimes I’m trying to say, “You probably have psoriasis.” Other times I mean, “You most definitely have psoriasis.” I rarely use those terms though.
One 36-year-old man with a flaky scalp and scaly elbows wasn’t satisfied with my assessment. His dad has psoriasis. So does his older brother. He was in to see me to find out if he had psoriasis too. “Probably” was what I gave him. He pushed back, “What percent chance?” That’s a good question — must be an engineer. I’m unsure.
With the exception of the poker players, our species is notoriously bad at probabilities. We’re wired to notice the significance of events, but terrible at understanding their likelihood. This is salient in lottery ticket holders and some NFL offensive coordinators who persist despite very long odds of things working out. It’s also reflected in the language we use. Rarely do we say, there’s a sixty percent chance something will happen. Rather, we say, “it’s likely.” There are two problems here. One, we often misjudge the actual probability of something occurring and two, the terms we use are subjective and differences in interpretation can lead to misunderstandings.
Let’s take a look. A 55-year-old man with a chronic eczematous rash on his trunk and extremities is getting worse despite dupilumab. He recently had night sweats. Do you think he has atopic dermatitis or cutaneous T-cell lymphoma? If you had to place a $100 bet, would you change your answer? Immanuel Kant thinks you would. In his “Critique of Pure Reason,” the German philosopher proposes that betting helps clarify the mind, an antidote to brashness. The example Kant uses is of a physician who observes a patient and concludes he has phthisis (tuberculosis), but we really don’t know if the physician is confident. Kant proposes that if he had to bet on his conclusion, then we’d have insight into just how convinced he is of phthisis. So, what’s your bet?
If you’re a bad poker player, then you might bet he has cutaneous T-cell lymphoma. However, not having any additional information, the smart call is atopic dermatitis, which has a base rate 1000-fold higher than CTCL. It is therefore more probable to be eczema even in a case that worsens despite dupilumab or with recent night sweats, both of which could be a result of common variables such as weather and COVID. Failure to account for the base rate is a mistake we physicians sometimes make. Economists rarely do. Try to think like one before answering a likelihood question.
If you think about it, “probably” means something different even to me, depending on the situation. I might say I’ll probably go to Montana this summer and I’ll probably retire at 65. The actual likelihoods might be 95% and 70%. That’s a big difference. What about between probably and likely? Or possibly and maybe? Do they mean the same to you as to the person you’re speaking with? For much of the work we do, precise likelihoods aren’t critical. Yet, it can be important in decision making and in discussing probabilities, such as the risk of hepatitis on terbinafine or of melanoma recurrence after Mohs.
I told my patient “I say about a 70% chance you have psoriasis. I could do a biopsy today to confirm.” He thought for a second and asked, “What is the chance it’s psoriasis if the biopsy shows it?” “Eighty six percent,” I replied.
Seemed like a good bet to me.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
“You have psoriasis,” I say all the time. I mean it when I say it, of course. But I don’t always to the same degree. Sometimes I’m trying to say, “You probably have psoriasis.” Other times I mean, “You most definitely have psoriasis.” I rarely use those terms though.
One 36-year-old man with a flaky scalp and scaly elbows wasn’t satisfied with my assessment. His dad has psoriasis. So does his older brother. He was in to see me to find out if he had psoriasis too. “Probably” was what I gave him. He pushed back, “What percent chance?” That’s a good question — must be an engineer. I’m unsure.
With the exception of the poker players, our species is notoriously bad at probabilities. We’re wired to notice the significance of events, but terrible at understanding their likelihood. This is salient in lottery ticket holders and some NFL offensive coordinators who persist despite very long odds of things working out. It’s also reflected in the language we use. Rarely do we say, there’s a sixty percent chance something will happen. Rather, we say, “it’s likely.” There are two problems here. One, we often misjudge the actual probability of something occurring and two, the terms we use are subjective and differences in interpretation can lead to misunderstandings.
Let’s take a look. A 55-year-old man with a chronic eczematous rash on his trunk and extremities is getting worse despite dupilumab. He recently had night sweats. Do you think he has atopic dermatitis or cutaneous T-cell lymphoma? If you had to place a $100 bet, would you change your answer? Immanuel Kant thinks you would. In his “Critique of Pure Reason,” the German philosopher proposes that betting helps clarify the mind, an antidote to brashness. The example Kant uses is of a physician who observes a patient and concludes he has phthisis (tuberculosis), but we really don’t know if the physician is confident. Kant proposes that if he had to bet on his conclusion, then we’d have insight into just how convinced he is of phthisis. So, what’s your bet?
If you’re a bad poker player, then you might bet he has cutaneous T-cell lymphoma. However, not having any additional information, the smart call is atopic dermatitis, which has a base rate 1000-fold higher than CTCL. It is therefore more probable to be eczema even in a case that worsens despite dupilumab or with recent night sweats, both of which could be a result of common variables such as weather and COVID. Failure to account for the base rate is a mistake we physicians sometimes make. Economists rarely do. Try to think like one before answering a likelihood question.
If you think about it, “probably” means something different even to me, depending on the situation. I might say I’ll probably go to Montana this summer and I’ll probably retire at 65. The actual likelihoods might be 95% and 70%. That’s a big difference. What about between probably and likely? Or possibly and maybe? Do they mean the same to you as to the person you’re speaking with? For much of the work we do, precise likelihoods aren’t critical. Yet, it can be important in decision making and in discussing probabilities, such as the risk of hepatitis on terbinafine or of melanoma recurrence after Mohs.
I told my patient “I say about a 70% chance you have psoriasis. I could do a biopsy today to confirm.” He thought for a second and asked, “What is the chance it’s psoriasis if the biopsy shows it?” “Eighty six percent,” I replied.
Seemed like a good bet to me.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
“You have psoriasis,” I say all the time. I mean it when I say it, of course. But I don’t always to the same degree. Sometimes I’m trying to say, “You probably have psoriasis.” Other times I mean, “You most definitely have psoriasis.” I rarely use those terms though.
One 36-year-old man with a flaky scalp and scaly elbows wasn’t satisfied with my assessment. His dad has psoriasis. So does his older brother. He was in to see me to find out if he had psoriasis too. “Probably” was what I gave him. He pushed back, “What percent chance?” That’s a good question — must be an engineer. I’m unsure.
With the exception of the poker players, our species is notoriously bad at probabilities. We’re wired to notice the significance of events, but terrible at understanding their likelihood. This is salient in lottery ticket holders and some NFL offensive coordinators who persist despite very long odds of things working out. It’s also reflected in the language we use. Rarely do we say, there’s a sixty percent chance something will happen. Rather, we say, “it’s likely.” There are two problems here. One, we often misjudge the actual probability of something occurring and two, the terms we use are subjective and differences in interpretation can lead to misunderstandings.
Let’s take a look. A 55-year-old man with a chronic eczematous rash on his trunk and extremities is getting worse despite dupilumab. He recently had night sweats. Do you think he has atopic dermatitis or cutaneous T-cell lymphoma? If you had to place a $100 bet, would you change your answer? Immanuel Kant thinks you would. In his “Critique of Pure Reason,” the German philosopher proposes that betting helps clarify the mind, an antidote to brashness. The example Kant uses is of a physician who observes a patient and concludes he has phthisis (tuberculosis), but we really don’t know if the physician is confident. Kant proposes that if he had to bet on his conclusion, then we’d have insight into just how convinced he is of phthisis. So, what’s your bet?
If you’re a bad poker player, then you might bet he has cutaneous T-cell lymphoma. However, not having any additional information, the smart call is atopic dermatitis, which has a base rate 1000-fold higher than CTCL. It is therefore more probable to be eczema even in a case that worsens despite dupilumab or with recent night sweats, both of which could be a result of common variables such as weather and COVID. Failure to account for the base rate is a mistake we physicians sometimes make. Economists rarely do. Try to think like one before answering a likelihood question.
If you think about it, “probably” means something different even to me, depending on the situation. I might say I’ll probably go to Montana this summer and I’ll probably retire at 65. The actual likelihoods might be 95% and 70%. That’s a big difference. What about between probably and likely? Or possibly and maybe? Do they mean the same to you as to the person you’re speaking with? For much of the work we do, precise likelihoods aren’t critical. Yet, it can be important in decision making and in discussing probabilities, such as the risk of hepatitis on terbinafine or of melanoma recurrence after Mohs.
I told my patient “I say about a 70% chance you have psoriasis. I could do a biopsy today to confirm.” He thought for a second and asked, “What is the chance it’s psoriasis if the biopsy shows it?” “Eighty six percent,” I replied.
Seemed like a good bet to me.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
Radiation Oncologists Fight for Payment Reform Amid Cuts
The American Society for Radiation Oncology (ASTRO) recently announced its partnership with three other groups — the American College of Radiation Oncology, the American College of Radiology, and the American Society of Clinical Oncology — to change how the specialty is paid for services.
Over the past decade, radiation oncologists have seen a 23% drop in Medicare reimbursement for radiation therapy services, with more cuts to come, according to a press release from ASTRO.
Traditionally, Medicare has reimbursed on the basis of the fraction of radiation delivered. But with moves toward hypofractionated regimens, deescalated therapy, and other changes in the field, reimbursement has continued to dwindle.
The cuts have led to practice consolidation and closures that threaten patient access especially in rural and underserved areas, a spokesperson for the group told this news organization.
To reverse this trend, ASTRO recently proposed the Radiation Oncology Case Rate program, a legislative initiative to base reimbursements on patient volumes instead of fractions delivered.
ASTRO is currently drafting a congressional bill to change the current payment structure, which “has become untenable,” the spokesperson said.
A version of this article appeared on Medscape.com.
The American Society for Radiation Oncology (ASTRO) recently announced its partnership with three other groups — the American College of Radiation Oncology, the American College of Radiology, and the American Society of Clinical Oncology — to change how the specialty is paid for services.
Over the past decade, radiation oncologists have seen a 23% drop in Medicare reimbursement for radiation therapy services, with more cuts to come, according to a press release from ASTRO.
Traditionally, Medicare has reimbursed on the basis of the fraction of radiation delivered. But with moves toward hypofractionated regimens, deescalated therapy, and other changes in the field, reimbursement has continued to dwindle.
The cuts have led to practice consolidation and closures that threaten patient access especially in rural and underserved areas, a spokesperson for the group told this news organization.
To reverse this trend, ASTRO recently proposed the Radiation Oncology Case Rate program, a legislative initiative to base reimbursements on patient volumes instead of fractions delivered.
ASTRO is currently drafting a congressional bill to change the current payment structure, which “has become untenable,” the spokesperson said.
A version of this article appeared on Medscape.com.
The American Society for Radiation Oncology (ASTRO) recently announced its partnership with three other groups — the American College of Radiation Oncology, the American College of Radiology, and the American Society of Clinical Oncology — to change how the specialty is paid for services.
Over the past decade, radiation oncologists have seen a 23% drop in Medicare reimbursement for radiation therapy services, with more cuts to come, according to a press release from ASTRO.
Traditionally, Medicare has reimbursed on the basis of the fraction of radiation delivered. But with moves toward hypofractionated regimens, deescalated therapy, and other changes in the field, reimbursement has continued to dwindle.
The cuts have led to practice consolidation and closures that threaten patient access especially in rural and underserved areas, a spokesperson for the group told this news organization.
To reverse this trend, ASTRO recently proposed the Radiation Oncology Case Rate program, a legislative initiative to base reimbursements on patient volumes instead of fractions delivered.
ASTRO is currently drafting a congressional bill to change the current payment structure, which “has become untenable,” the spokesperson said.
A version of this article appeared on Medscape.com.
Oncologists Sound the Alarm About Rise of White Bagging
For years, oncologist John DiPersio, MD, PhD, had faced frustrating encounters with insurers that only cover medications through a process called white bagging.
Instead of the traditional buy-and-bill pathway where oncologists purchase specialty drugs, such as infusion medications, directly from the distributor or manufacturer, white bagging requires physicians to receive these drugs from a specialty pharmacy.
On its face, the differences may seem minor. However, as Dr. DiPersio knows well, the consequences for oncologists and patients are not.
That is why Dr. DiPersio’s cancer center does not allow white bagging.
And when insurers refuse to reconsider the white bagging policy, his cancer team is left with few options.
“Sometimes, we have to redirect patients to other places,” said Dr. DiPersio, a bone marrow transplant specialist at Siteman Cancer Center, Washington University, St. Louis.
In emergency instances where patients cannot wait, Dr. DiPersio’s team will administer their own stock of a drug. In such cases, “we accept the fact that by not allowing white bagging, there may be nonpayment. We take the hit as far as cost.”
Increasingly, white bagging mandates are becoming harder for practices to avoid.
In a 2021 survey, 87% of Association of Community Cancer Centers members said white bagging has become an insurer mandate for some of their patients.
A 2023 analysis from Adam J. Fein, PhD, of Drug Channels Institute, Philadelphia, found that white bagging accounted for 17% of infused oncology product sourcing from clinics and 38% from hospital outpatient departments, up from 15% to 28% in 2019. Another practice called brown bagging, where specialty pharmacies send drugs directly to patients, creates many of the same issues but is much less prevalent than white bagging.
This change reflects “the broader battle over oncology margins” and insurers’ “attempts to shift costs to providers, patients, and manufacturers,” Dr. Fein wrote in his 2023 report.
White Bagging: Who Benefits?
At its core, white bagging changes how drugs are covered and reimbursed. Under buy and bill, drugs fall under a patient’s medical benefit. Oncologists purchase drugs directly from the manufacturer or distributor and receive reimbursement from the insurance company for both the cost of the drug as well as for administering it to patients.
Under white bagging, drugs fall under a patient’s pharmacy benefit. In these instances, a specialty pharmacy prepares the infusion ahead of time and ships it directly to the physician’s office or clinic. Because oncologists do not purchase the drug directly, they cannot bill insurers for it; instead, the pharmacy receives reimbursement for the drug and the provider is reimbursed for administering it.
Insurance companies argue that white bagging reduces patients’ out-of-pocket costs “by preventing hospitals and physicians from charging exorbitant fees to buy and store specialty medicines themselves,” according to advocacy group America’s Health Insurance Plans (AHIP).
Data from AHIP suggested that hospitals mark up the price of cancer drugs considerably, charging about twice as much as a specialty pharmacy, and that physician’s offices also charge about 23% more. However, these figures highlight how much insurers are billed, not necessarily how much patients ultimately pay.
Other evidence shows that white bagging raises costs for patients while reducing reimbursement for oncologists and saving insurance companies money.
A recent analysis in JAMA Network Open, which looked at 50 cancer drugs associated with the highest total spending from the 2020 Medicare Part B, found that mean insurance payments to providers were more than $2000 lower for drugs distributed under bagging than traditional buy and bill: $7405 vs $9547 per patient per month. Investigators found the same pattern in median insurance payments: $5746 vs $6681. Patients also paid more out-of-pocket each month with bagging vs buy and bill: $315 vs $145.
For patients with private insurance, “out-of-pocket costs were higher under bagging practice than the traditional buy-and-bill practice,” said lead author Ya-Chen Tina Shih, PhD, a professor in the department of radiation oncology at UCLA Health, Los Angeles.
White bagging is entirely for the profit of health insurers, specialty pharmacies, and pharmacy benefit managers, the middlemen who negotiate drug prices on behalf of payers.
Many people may not realize the underlying money-making strategies behind white bagging, explained Ted Okon, executive director for Community Oncology Alliance, which opposes the practice. Often, an insurer, pharmacy benefit manager, and mail order pharmacy involved in the process are all affiliated with the same corporation. In such cases, an insurer has a financial motive to control the source of medications and steer business to its affiliated pharmacies, Mr. Okon said.
When a single corporation owns numerous parts of the drug supply chain, insurers end up having “sway over what drug to use and then how the patient is going to get it,” Mr. Okon said. If the specialty pharmacy is a 340B contract pharmacy, it likely also receives a sizable discount on the drug and can make more money through white bagging.
Dangerous to Patients?
On the safety front, proponents of white bagging say the process is safe and efficient.
Specialty pharmacies are used only for prescription drugs that can be safely delivered, said AHIP spokesman David Allen.
In addition to having the same supply chain safety requirements as any other dispensing pharmacy, “specialty pharmacies also must meet additional safety requirements for specialty drugs” to ensure “the safe storage, handling, and dispensing of the drugs,” Mr. Allen explained.
However, oncologists argue that white bagging can be dangerous.
With white bagging, specialty pharmacies send a specified dose to practices, which does not allow practices to source and mix the drug themselves or make essential last-minute dose-related changes — something that happens every day in the clinic, said Debra Patt, MD, PhD, MBA, executive vice president for policy and strategy for Texas Oncology, Dallas.
White bagging also increases the risk for drug contamination, results in drug waste if the medication can’t be used, and can create delays in care.
Essentially, white bagging takes control away from oncologists and makes patient care more unpredictable and complex, explained Dr. Patt, president of the Texas Society of Clinical Oncology, Rockville, Maryland.
Dr. Patt, who does not allow white bagging in her practice, recalled a recent patient with metastatic breast cancer who came to the clinic for trastuzumab deruxtecan. The patient had been experiencing acute abdominal pain. After an exam and CT, Dr. Patt found the breast cancer had grown and moved into the patient’s liver.
“I had to discontinue that plan and change to a different chemotherapy,” she said. “If we had white bagged, that would have been a waste of several thousand dollars. Also, the patient would have to wait for the new medication to be white bagged, a delay that would be at least a week and the patient would have to come back at another time.”
When asked about the safety concerns associated with white bagging, Lemrey “Al” Carter, MS, PharmD, RPh, executive director of the National Association of Boards of Pharmacy (NABP), said the NABP “acknowledges that all these issues exist.
“It is unfortunate if patient care or costs are negatively impacted,” Dr. Carter said, adding that “boards of pharmacy can investigate if they are made aware of safety concerns at the pharmacy level. If a violation of the pharmacy laws or rules is found, boards can take action.”
More Legislation to Prevent Bagging
As white bagging mandates from insurance companies ramp up, more practices and states are banning it.
In the Association of Community Cancer Centers’ 2021 survey, 59% of members said their cancer program or practice does not allow white bagging.
At least 15 states have introduced legislation that restricts and/or prohibits white and brown bagging practices, according to a 2023 report by the Institute for Clinical and Economic Review. Some of the proposed laws would restrict mandates by stipulating that physicians are reimbursed at the contracted amount for clinician-administered drugs, whether obtained from a pharmacy or the manufacturer.
Louisiana, Vermont, and Minnesota were the first to enact anti–white bagging laws. Louisiana’s law, for example, enacted in 2021, bans white bagging and requires insurers to reimburse providers for physician-administered drugs if obtained from out-of-network pharmacies.
When the legislation passed, white bagging was just starting to enter the healthcare market in Louisiana, and the state wanted to act proactively, said Kathy W. Oubre, MS, CEO of the Pontchartrain Cancer Center, Covington, Louisiana, and president of the Coalition of Hematology and Oncology Practices, Mountain View, California.
“We recognized the growing concern around it,” Ms. Oubre said. The state legislature at the time included physicians and pharmacists who “really understood from a practice and patient perspective, the harm that policy could do.”
Ms. Oubre would like to see more legislation in other states and believes Louisiana’s law is a good model.
At the federal level, the American Hospital Association and American Society of Health-System Pharmacists have also urged the US Food and Drug Administration to take appropriate enforcement action to protect patients from white bagging.
Legislation that bars white bagging mandates is the most reasonable way to support timely and appropriate access to cancer care, Dr. Patt said. In the absence of such legislation, she said oncologists can only opt out of insurance contracts that may require the practice.
“That is a difficult position to put oncologists in,” she said.
A version of this article appeared on Medscape.com.
For years, oncologist John DiPersio, MD, PhD, had faced frustrating encounters with insurers that only cover medications through a process called white bagging.
Instead of the traditional buy-and-bill pathway where oncologists purchase specialty drugs, such as infusion medications, directly from the distributor or manufacturer, white bagging requires physicians to receive these drugs from a specialty pharmacy.
On its face, the differences may seem minor. However, as Dr. DiPersio knows well, the consequences for oncologists and patients are not.
That is why Dr. DiPersio’s cancer center does not allow white bagging.
And when insurers refuse to reconsider the white bagging policy, his cancer team is left with few options.
“Sometimes, we have to redirect patients to other places,” said Dr. DiPersio, a bone marrow transplant specialist at Siteman Cancer Center, Washington University, St. Louis.
In emergency instances where patients cannot wait, Dr. DiPersio’s team will administer their own stock of a drug. In such cases, “we accept the fact that by not allowing white bagging, there may be nonpayment. We take the hit as far as cost.”
Increasingly, white bagging mandates are becoming harder for practices to avoid.
In a 2021 survey, 87% of Association of Community Cancer Centers members said white bagging has become an insurer mandate for some of their patients.
A 2023 analysis from Adam J. Fein, PhD, of Drug Channels Institute, Philadelphia, found that white bagging accounted for 17% of infused oncology product sourcing from clinics and 38% from hospital outpatient departments, up from 15% to 28% in 2019. Another practice called brown bagging, where specialty pharmacies send drugs directly to patients, creates many of the same issues but is much less prevalent than white bagging.
This change reflects “the broader battle over oncology margins” and insurers’ “attempts to shift costs to providers, patients, and manufacturers,” Dr. Fein wrote in his 2023 report.
White Bagging: Who Benefits?
At its core, white bagging changes how drugs are covered and reimbursed. Under buy and bill, drugs fall under a patient’s medical benefit. Oncologists purchase drugs directly from the manufacturer or distributor and receive reimbursement from the insurance company for both the cost of the drug as well as for administering it to patients.
Under white bagging, drugs fall under a patient’s pharmacy benefit. In these instances, a specialty pharmacy prepares the infusion ahead of time and ships it directly to the physician’s office or clinic. Because oncologists do not purchase the drug directly, they cannot bill insurers for it; instead, the pharmacy receives reimbursement for the drug and the provider is reimbursed for administering it.
Insurance companies argue that white bagging reduces patients’ out-of-pocket costs “by preventing hospitals and physicians from charging exorbitant fees to buy and store specialty medicines themselves,” according to advocacy group America’s Health Insurance Plans (AHIP).
Data from AHIP suggested that hospitals mark up the price of cancer drugs considerably, charging about twice as much as a specialty pharmacy, and that physician’s offices also charge about 23% more. However, these figures highlight how much insurers are billed, not necessarily how much patients ultimately pay.
Other evidence shows that white bagging raises costs for patients while reducing reimbursement for oncologists and saving insurance companies money.
A recent analysis in JAMA Network Open, which looked at 50 cancer drugs associated with the highest total spending from the 2020 Medicare Part B, found that mean insurance payments to providers were more than $2000 lower for drugs distributed under bagging than traditional buy and bill: $7405 vs $9547 per patient per month. Investigators found the same pattern in median insurance payments: $5746 vs $6681. Patients also paid more out-of-pocket each month with bagging vs buy and bill: $315 vs $145.
For patients with private insurance, “out-of-pocket costs were higher under bagging practice than the traditional buy-and-bill practice,” said lead author Ya-Chen Tina Shih, PhD, a professor in the department of radiation oncology at UCLA Health, Los Angeles.
White bagging is entirely for the profit of health insurers, specialty pharmacies, and pharmacy benefit managers, the middlemen who negotiate drug prices on behalf of payers.
Many people may not realize the underlying money-making strategies behind white bagging, explained Ted Okon, executive director for Community Oncology Alliance, which opposes the practice. Often, an insurer, pharmacy benefit manager, and mail order pharmacy involved in the process are all affiliated with the same corporation. In such cases, an insurer has a financial motive to control the source of medications and steer business to its affiliated pharmacies, Mr. Okon said.
When a single corporation owns numerous parts of the drug supply chain, insurers end up having “sway over what drug to use and then how the patient is going to get it,” Mr. Okon said. If the specialty pharmacy is a 340B contract pharmacy, it likely also receives a sizable discount on the drug and can make more money through white bagging.
Dangerous to Patients?
On the safety front, proponents of white bagging say the process is safe and efficient.
Specialty pharmacies are used only for prescription drugs that can be safely delivered, said AHIP spokesman David Allen.
In addition to having the same supply chain safety requirements as any other dispensing pharmacy, “specialty pharmacies also must meet additional safety requirements for specialty drugs” to ensure “the safe storage, handling, and dispensing of the drugs,” Mr. Allen explained.
However, oncologists argue that white bagging can be dangerous.
With white bagging, specialty pharmacies send a specified dose to practices, which does not allow practices to source and mix the drug themselves or make essential last-minute dose-related changes — something that happens every day in the clinic, said Debra Patt, MD, PhD, MBA, executive vice president for policy and strategy for Texas Oncology, Dallas.
White bagging also increases the risk for drug contamination, results in drug waste if the medication can’t be used, and can create delays in care.
Essentially, white bagging takes control away from oncologists and makes patient care more unpredictable and complex, explained Dr. Patt, president of the Texas Society of Clinical Oncology, Rockville, Maryland.
Dr. Patt, who does not allow white bagging in her practice, recalled a recent patient with metastatic breast cancer who came to the clinic for trastuzumab deruxtecan. The patient had been experiencing acute abdominal pain. After an exam and CT, Dr. Patt found the breast cancer had grown and moved into the patient’s liver.
“I had to discontinue that plan and change to a different chemotherapy,” she said. “If we had white bagged, that would have been a waste of several thousand dollars. Also, the patient would have to wait for the new medication to be white bagged, a delay that would be at least a week and the patient would have to come back at another time.”
When asked about the safety concerns associated with white bagging, Lemrey “Al” Carter, MS, PharmD, RPh, executive director of the National Association of Boards of Pharmacy (NABP), said the NABP “acknowledges that all these issues exist.
“It is unfortunate if patient care or costs are negatively impacted,” Dr. Carter said, adding that “boards of pharmacy can investigate if they are made aware of safety concerns at the pharmacy level. If a violation of the pharmacy laws or rules is found, boards can take action.”
More Legislation to Prevent Bagging
As white bagging mandates from insurance companies ramp up, more practices and states are banning it.
In the Association of Community Cancer Centers’ 2021 survey, 59% of members said their cancer program or practice does not allow white bagging.
At least 15 states have introduced legislation that restricts and/or prohibits white and brown bagging practices, according to a 2023 report by the Institute for Clinical and Economic Review. Some of the proposed laws would restrict mandates by stipulating that physicians are reimbursed at the contracted amount for clinician-administered drugs, whether obtained from a pharmacy or the manufacturer.
Louisiana, Vermont, and Minnesota were the first to enact anti–white bagging laws. Louisiana’s law, for example, enacted in 2021, bans white bagging and requires insurers to reimburse providers for physician-administered drugs if obtained from out-of-network pharmacies.
When the legislation passed, white bagging was just starting to enter the healthcare market in Louisiana, and the state wanted to act proactively, said Kathy W. Oubre, MS, CEO of the Pontchartrain Cancer Center, Covington, Louisiana, and president of the Coalition of Hematology and Oncology Practices, Mountain View, California.
“We recognized the growing concern around it,” Ms. Oubre said. The state legislature at the time included physicians and pharmacists who “really understood from a practice and patient perspective, the harm that policy could do.”
Ms. Oubre would like to see more legislation in other states and believes Louisiana’s law is a good model.
At the federal level, the American Hospital Association and American Society of Health-System Pharmacists have also urged the US Food and Drug Administration to take appropriate enforcement action to protect patients from white bagging.
Legislation that bars white bagging mandates is the most reasonable way to support timely and appropriate access to cancer care, Dr. Patt said. In the absence of such legislation, she said oncologists can only opt out of insurance contracts that may require the practice.
“That is a difficult position to put oncologists in,” she said.
A version of this article appeared on Medscape.com.
For years, oncologist John DiPersio, MD, PhD, had faced frustrating encounters with insurers that only cover medications through a process called white bagging.
Instead of the traditional buy-and-bill pathway where oncologists purchase specialty drugs, such as infusion medications, directly from the distributor or manufacturer, white bagging requires physicians to receive these drugs from a specialty pharmacy.
On its face, the differences may seem minor. However, as Dr. DiPersio knows well, the consequences for oncologists and patients are not.
That is why Dr. DiPersio’s cancer center does not allow white bagging.
And when insurers refuse to reconsider the white bagging policy, his cancer team is left with few options.
“Sometimes, we have to redirect patients to other places,” said Dr. DiPersio, a bone marrow transplant specialist at Siteman Cancer Center, Washington University, St. Louis.
In emergency instances where patients cannot wait, Dr. DiPersio’s team will administer their own stock of a drug. In such cases, “we accept the fact that by not allowing white bagging, there may be nonpayment. We take the hit as far as cost.”
Increasingly, white bagging mandates are becoming harder for practices to avoid.
In a 2021 survey, 87% of Association of Community Cancer Centers members said white bagging has become an insurer mandate for some of their patients.
A 2023 analysis from Adam J. Fein, PhD, of Drug Channels Institute, Philadelphia, found that white bagging accounted for 17% of infused oncology product sourcing from clinics and 38% from hospital outpatient departments, up from 15% to 28% in 2019. Another practice called brown bagging, where specialty pharmacies send drugs directly to patients, creates many of the same issues but is much less prevalent than white bagging.
This change reflects “the broader battle over oncology margins” and insurers’ “attempts to shift costs to providers, patients, and manufacturers,” Dr. Fein wrote in his 2023 report.
White Bagging: Who Benefits?
At its core, white bagging changes how drugs are covered and reimbursed. Under buy and bill, drugs fall under a patient’s medical benefit. Oncologists purchase drugs directly from the manufacturer or distributor and receive reimbursement from the insurance company for both the cost of the drug as well as for administering it to patients.
Under white bagging, drugs fall under a patient’s pharmacy benefit. In these instances, a specialty pharmacy prepares the infusion ahead of time and ships it directly to the physician’s office or clinic. Because oncologists do not purchase the drug directly, they cannot bill insurers for it; instead, the pharmacy receives reimbursement for the drug and the provider is reimbursed for administering it.
Insurance companies argue that white bagging reduces patients’ out-of-pocket costs “by preventing hospitals and physicians from charging exorbitant fees to buy and store specialty medicines themselves,” according to advocacy group America’s Health Insurance Plans (AHIP).
Data from AHIP suggested that hospitals mark up the price of cancer drugs considerably, charging about twice as much as a specialty pharmacy, and that physician’s offices also charge about 23% more. However, these figures highlight how much insurers are billed, not necessarily how much patients ultimately pay.
Other evidence shows that white bagging raises costs for patients while reducing reimbursement for oncologists and saving insurance companies money.
A recent analysis in JAMA Network Open, which looked at 50 cancer drugs associated with the highest total spending from the 2020 Medicare Part B, found that mean insurance payments to providers were more than $2000 lower for drugs distributed under bagging than traditional buy and bill: $7405 vs $9547 per patient per month. Investigators found the same pattern in median insurance payments: $5746 vs $6681. Patients also paid more out-of-pocket each month with bagging vs buy and bill: $315 vs $145.
For patients with private insurance, “out-of-pocket costs were higher under bagging practice than the traditional buy-and-bill practice,” said lead author Ya-Chen Tina Shih, PhD, a professor in the department of radiation oncology at UCLA Health, Los Angeles.
White bagging is entirely for the profit of health insurers, specialty pharmacies, and pharmacy benefit managers, the middlemen who negotiate drug prices on behalf of payers.
Many people may not realize the underlying money-making strategies behind white bagging, explained Ted Okon, executive director for Community Oncology Alliance, which opposes the practice. Often, an insurer, pharmacy benefit manager, and mail order pharmacy involved in the process are all affiliated with the same corporation. In such cases, an insurer has a financial motive to control the source of medications and steer business to its affiliated pharmacies, Mr. Okon said.
When a single corporation owns numerous parts of the drug supply chain, insurers end up having “sway over what drug to use and then how the patient is going to get it,” Mr. Okon said. If the specialty pharmacy is a 340B contract pharmacy, it likely also receives a sizable discount on the drug and can make more money through white bagging.
Dangerous to Patients?
On the safety front, proponents of white bagging say the process is safe and efficient.
Specialty pharmacies are used only for prescription drugs that can be safely delivered, said AHIP spokesman David Allen.
In addition to having the same supply chain safety requirements as any other dispensing pharmacy, “specialty pharmacies also must meet additional safety requirements for specialty drugs” to ensure “the safe storage, handling, and dispensing of the drugs,” Mr. Allen explained.
However, oncologists argue that white bagging can be dangerous.
With white bagging, specialty pharmacies send a specified dose to practices, which does not allow practices to source and mix the drug themselves or make essential last-minute dose-related changes — something that happens every day in the clinic, said Debra Patt, MD, PhD, MBA, executive vice president for policy and strategy for Texas Oncology, Dallas.
White bagging also increases the risk for drug contamination, results in drug waste if the medication can’t be used, and can create delays in care.
Essentially, white bagging takes control away from oncologists and makes patient care more unpredictable and complex, explained Dr. Patt, president of the Texas Society of Clinical Oncology, Rockville, Maryland.
Dr. Patt, who does not allow white bagging in her practice, recalled a recent patient with metastatic breast cancer who came to the clinic for trastuzumab deruxtecan. The patient had been experiencing acute abdominal pain. After an exam and CT, Dr. Patt found the breast cancer had grown and moved into the patient’s liver.
“I had to discontinue that plan and change to a different chemotherapy,” she said. “If we had white bagged, that would have been a waste of several thousand dollars. Also, the patient would have to wait for the new medication to be white bagged, a delay that would be at least a week and the patient would have to come back at another time.”
When asked about the safety concerns associated with white bagging, Lemrey “Al” Carter, MS, PharmD, RPh, executive director of the National Association of Boards of Pharmacy (NABP), said the NABP “acknowledges that all these issues exist.
“It is unfortunate if patient care or costs are negatively impacted,” Dr. Carter said, adding that “boards of pharmacy can investigate if they are made aware of safety concerns at the pharmacy level. If a violation of the pharmacy laws or rules is found, boards can take action.”
More Legislation to Prevent Bagging
As white bagging mandates from insurance companies ramp up, more practices and states are banning it.
In the Association of Community Cancer Centers’ 2021 survey, 59% of members said their cancer program or practice does not allow white bagging.
At least 15 states have introduced legislation that restricts and/or prohibits white and brown bagging practices, according to a 2023 report by the Institute for Clinical and Economic Review. Some of the proposed laws would restrict mandates by stipulating that physicians are reimbursed at the contracted amount for clinician-administered drugs, whether obtained from a pharmacy or the manufacturer.
Louisiana, Vermont, and Minnesota were the first to enact anti–white bagging laws. Louisiana’s law, for example, enacted in 2021, bans white bagging and requires insurers to reimburse providers for physician-administered drugs if obtained from out-of-network pharmacies.
When the legislation passed, white bagging was just starting to enter the healthcare market in Louisiana, and the state wanted to act proactively, said Kathy W. Oubre, MS, CEO of the Pontchartrain Cancer Center, Covington, Louisiana, and president of the Coalition of Hematology and Oncology Practices, Mountain View, California.
“We recognized the growing concern around it,” Ms. Oubre said. The state legislature at the time included physicians and pharmacists who “really understood from a practice and patient perspective, the harm that policy could do.”
Ms. Oubre would like to see more legislation in other states and believes Louisiana’s law is a good model.
At the federal level, the American Hospital Association and American Society of Health-System Pharmacists have also urged the US Food and Drug Administration to take appropriate enforcement action to protect patients from white bagging.
Legislation that bars white bagging mandates is the most reasonable way to support timely and appropriate access to cancer care, Dr. Patt said. In the absence of such legislation, she said oncologists can only opt out of insurance contracts that may require the practice.
“That is a difficult position to put oncologists in,” she said.
A version of this article appeared on Medscape.com.
Adequate Midlife Protein, Especially From Plants, Tied to Healthy Aging
Intake of protein, especially from plants, in middle age is associated with higher odds of healthy aging and positive mental and physical health status in older women, a recent analysis of the Nurses’ Health Study (NHS) data suggests.
The study is said to be the first to examine the long-term impact of midlife protein consumption on later health status.
Writing in the American Journal of Clinical Nutrition, a team led by Andres V. Ardisson Korat, DSc, a nutritional epidemiologist at the USDA Human Nutrition Research Center on Aging at Tufts University in Boston, Massachusetts, found the following midlife protein–related odds ratios (ORs) for later healthy aging measured at ages 70-93.
For each 3% energy increment from various protein sources:
- 1.05 (95% confidence interval, 1.01-1.10) for total protein
- 1.07 (1.02-1.11) for animal protein
- 1.14 (1.06-1.23) for dairy protein
- 1.38 (1.24-1.54) for plant protein
In substitution analyses, significant positive associations were observed for the isocaloric replacement of animal or dairy protein, carbohydrate, or fat with plant protein — with increased ORs for healthy aging of 1.22-1.58 for each 3% of energy replacement.
On the measure of physical function, for example, replacing calories from all macronutrient variables with equivalent calories from plant protein was associated with 20%-60% higher odds of having no physical function limitations. Plant protein was also associated with higher odds for good mental status.
“Other studies have looked at protein intake in older adults, but we felt midlife was a more relevant etiological window,” Dr. Ardisson Korat said in an interview. “Our findings generally align, however, with those of protein intake in older populations, which have shown that protein can reduce the risk of frailty.”
He added that the benefits of protein, especially from plant sources, would likely apply to men as well and increasing plant protein intake is not difficult. “If you want a snack during the day, eat a handful of nuts instead of potato chips,” he advised. And eating several meals a week featuring beans, peas, lentils, tofu, whole grains, or seeds is an easy way to boost dietary plant protein, which comes with health-promoting soluble and insoluble fiber as well as antioxidant and anti-inflammatory polyphenols and other phytochemicals.
Conversely, plant but not animal protein consumption in older adulthood was linked to a lower risk of frailty in a previous NHS trial.
Higher plant protein intake was associated with a better probability of achieving healthy aging defined by changes in functional impairments, self-reported health/vitality, mental health, and use of health services in the Spanish Seniors-Estudio Sobre Nutricion y Riesgo Cardiovascular.
In contrast, animal protein intake in middle adulthood has been linked to an increased risk of premature death from chronic diseases driven by cardiovascular disease mortality.
The present findings are consistent with those observed for protein intakes in older adulthood, Dr. Ardisson Korat said.
“This study underscores the health advantages for midlife adults consuming adequate dietary protein — particularly plant protein — as one component of pursuing a healthy lifestyle,” said Douglas R. Dirschl, MD, chair of orthopedic surgery at Baylor College of Medicine in Houston, Texas. Most Americans consume adequate amounts of protein, but according to Dr. Dirschl, who treats many older patients for osteoporotic fractures and other musculoskeletal conditions, many US diets are subpar in this nutrient.
While protein is essential for bone and muscle formation and maintenance, “a surprising number of Americans are protein deficient, even those who seem hale and are overweight,” he said.
Dietary Recommendations for Midlife Patients
Physicians should therefore advise midlife patients to meet or perhaps modestly exceed the recommended dietary allowance (RDA) for protein of 0.8 g/kg per day and to make plant protein a substantial component of daily dietary protein intake, Dr. Dirschl said.
Luke D. Kim, MD, MEd, a geriatrician at the Cleveland Clinic in Cleveland, Ohio, noted that patients with lower socioeconomic status or with difficulty in day-to-day functioning are likely to have suboptimal protein intake. Such patients may need encouragement to eat more protein. “But we should keep in mind that showing a higher associated odds ratio of better health with increased protein take does not mean causality,” he said.
According to Rachel L. Amdur, MD, an internist at Northwestern Medicine in Chicago, Illinois, the long-term follow-up data from the NHS are uniquely helpful. “Middle-aged persons may think they no longer need much dietary protein and need to be reminded. Sometimes eating carbohydrates is just easier,” she said in an interview. Physicians need to asses and counsel patients on nutrition at all stages of life. “As I tell my patients, it’s best to think of your future self now.”
In agreement is Louis J. Morledge, MD, an internist at Northwell Health in New York City. “I firmly counsel my patients about adequate and often increased protein intake in middle life. But this is always within a larger framework of overall nutritional health.” He added that middle-aged persons often find themselves “stuck in food ruts,” and one of his clinical focuses is to advise patients about the importance of healthier food choices so they can better adjust to mental, emotional, physical, and skeletal changes as they age.
Study Details
The NHS analysis drew on prospective data from 48,762 nurses under age 60 in 1984. Total protein, animal protein, dairy protein, and plant protein were derived from validated food-frequency questionnaires.
Adjusting for lifestyle, demographics, and health status, the investigators identified 3721 (7.6% of cohort) eligible participants. The mean age of participants at baseline was 48.6 years; 38.6% had body mass indexes (BMI; in kg/m2) greater than 25; 22.9% were current smokers; and 88.2% were married.
Healthy aging was defined as freedom from 11 major chronic diseases, good mental health, and no impairments in cognitive or physical function, as assessed in the 2014 or 2016 NHS participant questionnaires. Diseases/treatments included cancer, type 2 diabetes, myocardial infarction, coronary artery bypass graft or coronary angioplasty, congestive heart failure, stroke, kidney failure, chronic obstructive pulmonary disease, Parkinson disease, multiple sclerosis, and amyotrophic lateral sclerosis.
Mean total protein consumption as a percentage of energy was 18.3% (standard deviation 3%), slightly higher than the average 16% in the US diet. Of this, 13.3% derived from animals, 3.6% from dairy products, and 4.9% from plants.
Total protein intake was positively associated with higher education levels, being physically active, higher BMI, and a baseline history of hypertension and hypercholesterolemia. Conversely, total protein intake was inversely associated with intakes of total carbohydrates, nuts, alcohol, and sugar-sweetened beverages.
The associations between protein intake and healthy aging are complex and not fully understood, the authors stated.
Effects of Protein Intake
In studies of older adult populations lower protein intake has been associated with lean mass loss. Animal protein supplementation studies in older adults have shown lean mass gains potentially related to amino acid composition.
In terms of mechanisms, evidence suggests that protein-related activation of the rapamycin complex 1 pathway may play a role, the authors suggested. The activity of this signaling pathway decreases with age.
Rapamycin, a compound used to prevent organ transplant rejection, has been associated with delayed aging. In the body, dietary protein and exercise activate this pathway, thereby stimulating muscle protein synthesis and possibly improving physical function.
As for the differential associations of plant and animal protein on the chronic disease domain of the healthy aging phenotype, Dr. Ardisson Korat and coauthors said plant protein has been associated with favorable levels of important risk factors for cardiometabolic diseases, such as reduced LDL cholesterol, lower blood pressure, and insulin sensitivity, as well as decreased levels of proinflammatory markers.
Conversely, total and animal protein intakes have been positively associated with concentrations of insulin-like growth factor 1, which is implicated in the growth of malignant cells in breast and prostate tissue.
This study is the first step in evaluating the long-term health effect of protein intake in midlife, the relevant development window for most chronic conditions, the NHS study authors said. More research is needed, however, to corroborate the study findings in other populations and identify underlying mechanisms.
This study was supported by the USDA Agricultural Research Service and the National Institutes of Health. The authors reported no conflicts of interest. The commentators disclosed no relevant competing interests.
Intake of protein, especially from plants, in middle age is associated with higher odds of healthy aging and positive mental and physical health status in older women, a recent analysis of the Nurses’ Health Study (NHS) data suggests.
The study is said to be the first to examine the long-term impact of midlife protein consumption on later health status.
Writing in the American Journal of Clinical Nutrition, a team led by Andres V. Ardisson Korat, DSc, a nutritional epidemiologist at the USDA Human Nutrition Research Center on Aging at Tufts University in Boston, Massachusetts, found the following midlife protein–related odds ratios (ORs) for later healthy aging measured at ages 70-93.
For each 3% energy increment from various protein sources:
- 1.05 (95% confidence interval, 1.01-1.10) for total protein
- 1.07 (1.02-1.11) for animal protein
- 1.14 (1.06-1.23) for dairy protein
- 1.38 (1.24-1.54) for plant protein
In substitution analyses, significant positive associations were observed for the isocaloric replacement of animal or dairy protein, carbohydrate, or fat with plant protein — with increased ORs for healthy aging of 1.22-1.58 for each 3% of energy replacement.
On the measure of physical function, for example, replacing calories from all macronutrient variables with equivalent calories from plant protein was associated with 20%-60% higher odds of having no physical function limitations. Plant protein was also associated with higher odds for good mental status.
“Other studies have looked at protein intake in older adults, but we felt midlife was a more relevant etiological window,” Dr. Ardisson Korat said in an interview. “Our findings generally align, however, with those of protein intake in older populations, which have shown that protein can reduce the risk of frailty.”
He added that the benefits of protein, especially from plant sources, would likely apply to men as well and increasing plant protein intake is not difficult. “If you want a snack during the day, eat a handful of nuts instead of potato chips,” he advised. And eating several meals a week featuring beans, peas, lentils, tofu, whole grains, or seeds is an easy way to boost dietary plant protein, which comes with health-promoting soluble and insoluble fiber as well as antioxidant and anti-inflammatory polyphenols and other phytochemicals.
Conversely, plant but not animal protein consumption in older adulthood was linked to a lower risk of frailty in a previous NHS trial.
Higher plant protein intake was associated with a better probability of achieving healthy aging defined by changes in functional impairments, self-reported health/vitality, mental health, and use of health services in the Spanish Seniors-Estudio Sobre Nutricion y Riesgo Cardiovascular.
In contrast, animal protein intake in middle adulthood has been linked to an increased risk of premature death from chronic diseases driven by cardiovascular disease mortality.
The present findings are consistent with those observed for protein intakes in older adulthood, Dr. Ardisson Korat said.
“This study underscores the health advantages for midlife adults consuming adequate dietary protein — particularly plant protein — as one component of pursuing a healthy lifestyle,” said Douglas R. Dirschl, MD, chair of orthopedic surgery at Baylor College of Medicine in Houston, Texas. Most Americans consume adequate amounts of protein, but according to Dr. Dirschl, who treats many older patients for osteoporotic fractures and other musculoskeletal conditions, many US diets are subpar in this nutrient.
While protein is essential for bone and muscle formation and maintenance, “a surprising number of Americans are protein deficient, even those who seem hale and are overweight,” he said.
Dietary Recommendations for Midlife Patients
Physicians should therefore advise midlife patients to meet or perhaps modestly exceed the recommended dietary allowance (RDA) for protein of 0.8 g/kg per day and to make plant protein a substantial component of daily dietary protein intake, Dr. Dirschl said.
Luke D. Kim, MD, MEd, a geriatrician at the Cleveland Clinic in Cleveland, Ohio, noted that patients with lower socioeconomic status or with difficulty in day-to-day functioning are likely to have suboptimal protein intake. Such patients may need encouragement to eat more protein. “But we should keep in mind that showing a higher associated odds ratio of better health with increased protein take does not mean causality,” he said.
According to Rachel L. Amdur, MD, an internist at Northwestern Medicine in Chicago, Illinois, the long-term follow-up data from the NHS are uniquely helpful. “Middle-aged persons may think they no longer need much dietary protein and need to be reminded. Sometimes eating carbohydrates is just easier,” she said in an interview. Physicians need to asses and counsel patients on nutrition at all stages of life. “As I tell my patients, it’s best to think of your future self now.”
In agreement is Louis J. Morledge, MD, an internist at Northwell Health in New York City. “I firmly counsel my patients about adequate and often increased protein intake in middle life. But this is always within a larger framework of overall nutritional health.” He added that middle-aged persons often find themselves “stuck in food ruts,” and one of his clinical focuses is to advise patients about the importance of healthier food choices so they can better adjust to mental, emotional, physical, and skeletal changes as they age.
Study Details
The NHS analysis drew on prospective data from 48,762 nurses under age 60 in 1984. Total protein, animal protein, dairy protein, and plant protein were derived from validated food-frequency questionnaires.
Adjusting for lifestyle, demographics, and health status, the investigators identified 3721 (7.6% of cohort) eligible participants. The mean age of participants at baseline was 48.6 years; 38.6% had body mass indexes (BMI; in kg/m2) greater than 25; 22.9% were current smokers; and 88.2% were married.
Healthy aging was defined as freedom from 11 major chronic diseases, good mental health, and no impairments in cognitive or physical function, as assessed in the 2014 or 2016 NHS participant questionnaires. Diseases/treatments included cancer, type 2 diabetes, myocardial infarction, coronary artery bypass graft or coronary angioplasty, congestive heart failure, stroke, kidney failure, chronic obstructive pulmonary disease, Parkinson disease, multiple sclerosis, and amyotrophic lateral sclerosis.
Mean total protein consumption as a percentage of energy was 18.3% (standard deviation 3%), slightly higher than the average 16% in the US diet. Of this, 13.3% derived from animals, 3.6% from dairy products, and 4.9% from plants.
Total protein intake was positively associated with higher education levels, being physically active, higher BMI, and a baseline history of hypertension and hypercholesterolemia. Conversely, total protein intake was inversely associated with intakes of total carbohydrates, nuts, alcohol, and sugar-sweetened beverages.
The associations between protein intake and healthy aging are complex and not fully understood, the authors stated.
Effects of Protein Intake
In studies of older adult populations lower protein intake has been associated with lean mass loss. Animal protein supplementation studies in older adults have shown lean mass gains potentially related to amino acid composition.
In terms of mechanisms, evidence suggests that protein-related activation of the rapamycin complex 1 pathway may play a role, the authors suggested. The activity of this signaling pathway decreases with age.
Rapamycin, a compound used to prevent organ transplant rejection, has been associated with delayed aging. In the body, dietary protein and exercise activate this pathway, thereby stimulating muscle protein synthesis and possibly improving physical function.
As for the differential associations of plant and animal protein on the chronic disease domain of the healthy aging phenotype, Dr. Ardisson Korat and coauthors said plant protein has been associated with favorable levels of important risk factors for cardiometabolic diseases, such as reduced LDL cholesterol, lower blood pressure, and insulin sensitivity, as well as decreased levels of proinflammatory markers.
Conversely, total and animal protein intakes have been positively associated with concentrations of insulin-like growth factor 1, which is implicated in the growth of malignant cells in breast and prostate tissue.
This study is the first step in evaluating the long-term health effect of protein intake in midlife, the relevant development window for most chronic conditions, the NHS study authors said. More research is needed, however, to corroborate the study findings in other populations and identify underlying mechanisms.
This study was supported by the USDA Agricultural Research Service and the National Institutes of Health. The authors reported no conflicts of interest. The commentators disclosed no relevant competing interests.
Intake of protein, especially from plants, in middle age is associated with higher odds of healthy aging and positive mental and physical health status in older women, a recent analysis of the Nurses’ Health Study (NHS) data suggests.
The study is said to be the first to examine the long-term impact of midlife protein consumption on later health status.
Writing in the American Journal of Clinical Nutrition, a team led by Andres V. Ardisson Korat, DSc, a nutritional epidemiologist at the USDA Human Nutrition Research Center on Aging at Tufts University in Boston, Massachusetts, found the following midlife protein–related odds ratios (ORs) for later healthy aging measured at ages 70-93.
For each 3% energy increment from various protein sources:
- 1.05 (95% confidence interval, 1.01-1.10) for total protein
- 1.07 (1.02-1.11) for animal protein
- 1.14 (1.06-1.23) for dairy protein
- 1.38 (1.24-1.54) for plant protein
In substitution analyses, significant positive associations were observed for the isocaloric replacement of animal or dairy protein, carbohydrate, or fat with plant protein — with increased ORs for healthy aging of 1.22-1.58 for each 3% of energy replacement.
On the measure of physical function, for example, replacing calories from all macronutrient variables with equivalent calories from plant protein was associated with 20%-60% higher odds of having no physical function limitations. Plant protein was also associated with higher odds for good mental status.
“Other studies have looked at protein intake in older adults, but we felt midlife was a more relevant etiological window,” Dr. Ardisson Korat said in an interview. “Our findings generally align, however, with those of protein intake in older populations, which have shown that protein can reduce the risk of frailty.”
He added that the benefits of protein, especially from plant sources, would likely apply to men as well and increasing plant protein intake is not difficult. “If you want a snack during the day, eat a handful of nuts instead of potato chips,” he advised. And eating several meals a week featuring beans, peas, lentils, tofu, whole grains, or seeds is an easy way to boost dietary plant protein, which comes with health-promoting soluble and insoluble fiber as well as antioxidant and anti-inflammatory polyphenols and other phytochemicals.
Conversely, plant but not animal protein consumption in older adulthood was linked to a lower risk of frailty in a previous NHS trial.
Higher plant protein intake was associated with a better probability of achieving healthy aging defined by changes in functional impairments, self-reported health/vitality, mental health, and use of health services in the Spanish Seniors-Estudio Sobre Nutricion y Riesgo Cardiovascular.
In contrast, animal protein intake in middle adulthood has been linked to an increased risk of premature death from chronic diseases driven by cardiovascular disease mortality.
The present findings are consistent with those observed for protein intakes in older adulthood, Dr. Ardisson Korat said.
“This study underscores the health advantages for midlife adults consuming adequate dietary protein — particularly plant protein — as one component of pursuing a healthy lifestyle,” said Douglas R. Dirschl, MD, chair of orthopedic surgery at Baylor College of Medicine in Houston, Texas. Most Americans consume adequate amounts of protein, but according to Dr. Dirschl, who treats many older patients for osteoporotic fractures and other musculoskeletal conditions, many US diets are subpar in this nutrient.
While protein is essential for bone and muscle formation and maintenance, “a surprising number of Americans are protein deficient, even those who seem hale and are overweight,” he said.
Dietary Recommendations for Midlife Patients
Physicians should therefore advise midlife patients to meet or perhaps modestly exceed the recommended dietary allowance (RDA) for protein of 0.8 g/kg per day and to make plant protein a substantial component of daily dietary protein intake, Dr. Dirschl said.
Luke D. Kim, MD, MEd, a geriatrician at the Cleveland Clinic in Cleveland, Ohio, noted that patients with lower socioeconomic status or with difficulty in day-to-day functioning are likely to have suboptimal protein intake. Such patients may need encouragement to eat more protein. “But we should keep in mind that showing a higher associated odds ratio of better health with increased protein take does not mean causality,” he said.
According to Rachel L. Amdur, MD, an internist at Northwestern Medicine in Chicago, Illinois, the long-term follow-up data from the NHS are uniquely helpful. “Middle-aged persons may think they no longer need much dietary protein and need to be reminded. Sometimes eating carbohydrates is just easier,” she said in an interview. Physicians need to asses and counsel patients on nutrition at all stages of life. “As I tell my patients, it’s best to think of your future self now.”
In agreement is Louis J. Morledge, MD, an internist at Northwell Health in New York City. “I firmly counsel my patients about adequate and often increased protein intake in middle life. But this is always within a larger framework of overall nutritional health.” He added that middle-aged persons often find themselves “stuck in food ruts,” and one of his clinical focuses is to advise patients about the importance of healthier food choices so they can better adjust to mental, emotional, physical, and skeletal changes as they age.
Study Details
The NHS analysis drew on prospective data from 48,762 nurses under age 60 in 1984. Total protein, animal protein, dairy protein, and plant protein were derived from validated food-frequency questionnaires.
Adjusting for lifestyle, demographics, and health status, the investigators identified 3721 (7.6% of cohort) eligible participants. The mean age of participants at baseline was 48.6 years; 38.6% had body mass indexes (BMI; in kg/m2) greater than 25; 22.9% were current smokers; and 88.2% were married.
Healthy aging was defined as freedom from 11 major chronic diseases, good mental health, and no impairments in cognitive or physical function, as assessed in the 2014 or 2016 NHS participant questionnaires. Diseases/treatments included cancer, type 2 diabetes, myocardial infarction, coronary artery bypass graft or coronary angioplasty, congestive heart failure, stroke, kidney failure, chronic obstructive pulmonary disease, Parkinson disease, multiple sclerosis, and amyotrophic lateral sclerosis.
Mean total protein consumption as a percentage of energy was 18.3% (standard deviation 3%), slightly higher than the average 16% in the US diet. Of this, 13.3% derived from animals, 3.6% from dairy products, and 4.9% from plants.
Total protein intake was positively associated with higher education levels, being physically active, higher BMI, and a baseline history of hypertension and hypercholesterolemia. Conversely, total protein intake was inversely associated with intakes of total carbohydrates, nuts, alcohol, and sugar-sweetened beverages.
The associations between protein intake and healthy aging are complex and not fully understood, the authors stated.
Effects of Protein Intake
In studies of older adult populations lower protein intake has been associated with lean mass loss. Animal protein supplementation studies in older adults have shown lean mass gains potentially related to amino acid composition.
In terms of mechanisms, evidence suggests that protein-related activation of the rapamycin complex 1 pathway may play a role, the authors suggested. The activity of this signaling pathway decreases with age.
Rapamycin, a compound used to prevent organ transplant rejection, has been associated with delayed aging. In the body, dietary protein and exercise activate this pathway, thereby stimulating muscle protein synthesis and possibly improving physical function.
As for the differential associations of plant and animal protein on the chronic disease domain of the healthy aging phenotype, Dr. Ardisson Korat and coauthors said plant protein has been associated with favorable levels of important risk factors for cardiometabolic diseases, such as reduced LDL cholesterol, lower blood pressure, and insulin sensitivity, as well as decreased levels of proinflammatory markers.
Conversely, total and animal protein intakes have been positively associated with concentrations of insulin-like growth factor 1, which is implicated in the growth of malignant cells in breast and prostate tissue.
This study is the first step in evaluating the long-term health effect of protein intake in midlife, the relevant development window for most chronic conditions, the NHS study authors said. More research is needed, however, to corroborate the study findings in other populations and identify underlying mechanisms.
This study was supported by the USDA Agricultural Research Service and the National Institutes of Health. The authors reported no conflicts of interest. The commentators disclosed no relevant competing interests.
New Federal Rule for Prior Authorizations a ‘Major Win’ for Patients, Doctors
Physicians groups on January 17 hailed a new federal rule requiring health insurers to streamline and disclose more information about their prior authorization processes, saying it will improve patient care and reduce doctors’ administrative burden.
Health insurers participating in federal programs, including Medicare Advantage and Medicaid, must now respond to expedited prior authorization requests within 72 hours and other requests within 7 days under the long-awaited final rule, released on January 17 by the Centers for Medicare & Medicaid Services (CMS).
Insurers also must include their reasons for denying a prior authorization request and will be required to publicly release data on denial and approval rates for medical treatment. They’ll also need to give patients more information about their decisions to deny care. Insurers must comply with some of the rule’s provisions by January 2026 and others by January 2027.
The final rule “is an important step forward” toward the Medical Group Management Association’s goal of reducing the overall volume of prior authorization requests, said Anders Gilberg, the group’s senior vice president for government affairs, in a statement.
“Only then will medical groups find meaningful reprieve from these onerous, ill-intentioned administrative requirements that dangerously impede patient care,” Mr. Gilberg said.
Health insurers have long lobbied against increased regulation of prior authorization, arguing that it’s needed to rein in healthcare costs and prevent unnecessary treatment.
“We appreciate CMS’s announcement of enforcement discretion that will permit plans to use one standard, rather than mixing and matching, to reduce costs and speed implementation,” said America’s Health Insurance Plans, an insurers’ lobbying group, in an unsigned statement. “However, we must remember that the CMS rule is only half the picture; the Office of the Coordinator for Health Information Technology (ONC) should swiftly require vendors to build electronic prior authorization capabilities into the electronic health record so that providers can do their part, or plans will build a bridge to nowhere.”
The rule comes as health insurers have increasingly been criticized for onerous and time-consuming prior authorization procedures that physicians say unfairly delay or deny the medical treatment that their patients need. With federal legislation to rein in prior authorization overuse at a standstill, 30 states have introduced their own bills to address the problem. Regulators and lawsuits also have called attention to insurers’ increasing use of artificial intelligence and algorithms to deny claims without human review.
“Family physicians know firsthand how prior authorizations divert valuable time and resources away from direct patient care. We also know that these types of administrative requirements are driving physicians away from the workforce and worsening physician shortages,” said Steven P. Furr, MD, president of the American Academy of Family Physicians, in a statement praising the new rule.
Jesse M. Ehrenfeld, MD, MPH, president of the American Medical Association, called the final rule “ a major win” for patients and physicians, adding that its requirements for health insurers to integrate their prior authorization procedures into physicians’ electronic health records systems will also help make “the current time-consuming, manual workflow” more efficient.
A version of this article first appeared on Medscape.com.
Physicians groups on January 17 hailed a new federal rule requiring health insurers to streamline and disclose more information about their prior authorization processes, saying it will improve patient care and reduce doctors’ administrative burden.
Health insurers participating in federal programs, including Medicare Advantage and Medicaid, must now respond to expedited prior authorization requests within 72 hours and other requests within 7 days under the long-awaited final rule, released on January 17 by the Centers for Medicare & Medicaid Services (CMS).
Insurers also must include their reasons for denying a prior authorization request and will be required to publicly release data on denial and approval rates for medical treatment. They’ll also need to give patients more information about their decisions to deny care. Insurers must comply with some of the rule’s provisions by January 2026 and others by January 2027.
The final rule “is an important step forward” toward the Medical Group Management Association’s goal of reducing the overall volume of prior authorization requests, said Anders Gilberg, the group’s senior vice president for government affairs, in a statement.
“Only then will medical groups find meaningful reprieve from these onerous, ill-intentioned administrative requirements that dangerously impede patient care,” Mr. Gilberg said.
Health insurers have long lobbied against increased regulation of prior authorization, arguing that it’s needed to rein in healthcare costs and prevent unnecessary treatment.
“We appreciate CMS’s announcement of enforcement discretion that will permit plans to use one standard, rather than mixing and matching, to reduce costs and speed implementation,” said America’s Health Insurance Plans, an insurers’ lobbying group, in an unsigned statement. “However, we must remember that the CMS rule is only half the picture; the Office of the Coordinator for Health Information Technology (ONC) should swiftly require vendors to build electronic prior authorization capabilities into the electronic health record so that providers can do their part, or plans will build a bridge to nowhere.”
The rule comes as health insurers have increasingly been criticized for onerous and time-consuming prior authorization procedures that physicians say unfairly delay or deny the medical treatment that their patients need. With federal legislation to rein in prior authorization overuse at a standstill, 30 states have introduced their own bills to address the problem. Regulators and lawsuits also have called attention to insurers’ increasing use of artificial intelligence and algorithms to deny claims without human review.
“Family physicians know firsthand how prior authorizations divert valuable time and resources away from direct patient care. We also know that these types of administrative requirements are driving physicians away from the workforce and worsening physician shortages,” said Steven P. Furr, MD, president of the American Academy of Family Physicians, in a statement praising the new rule.
Jesse M. Ehrenfeld, MD, MPH, president of the American Medical Association, called the final rule “ a major win” for patients and physicians, adding that its requirements for health insurers to integrate their prior authorization procedures into physicians’ electronic health records systems will also help make “the current time-consuming, manual workflow” more efficient.
A version of this article first appeared on Medscape.com.
Physicians groups on January 17 hailed a new federal rule requiring health insurers to streamline and disclose more information about their prior authorization processes, saying it will improve patient care and reduce doctors’ administrative burden.
Health insurers participating in federal programs, including Medicare Advantage and Medicaid, must now respond to expedited prior authorization requests within 72 hours and other requests within 7 days under the long-awaited final rule, released on January 17 by the Centers for Medicare & Medicaid Services (CMS).
Insurers also must include their reasons for denying a prior authorization request and will be required to publicly release data on denial and approval rates for medical treatment. They’ll also need to give patients more information about their decisions to deny care. Insurers must comply with some of the rule’s provisions by January 2026 and others by January 2027.
The final rule “is an important step forward” toward the Medical Group Management Association’s goal of reducing the overall volume of prior authorization requests, said Anders Gilberg, the group’s senior vice president for government affairs, in a statement.
“Only then will medical groups find meaningful reprieve from these onerous, ill-intentioned administrative requirements that dangerously impede patient care,” Mr. Gilberg said.
Health insurers have long lobbied against increased regulation of prior authorization, arguing that it’s needed to rein in healthcare costs and prevent unnecessary treatment.
“We appreciate CMS’s announcement of enforcement discretion that will permit plans to use one standard, rather than mixing and matching, to reduce costs and speed implementation,” said America’s Health Insurance Plans, an insurers’ lobbying group, in an unsigned statement. “However, we must remember that the CMS rule is only half the picture; the Office of the Coordinator for Health Information Technology (ONC) should swiftly require vendors to build electronic prior authorization capabilities into the electronic health record so that providers can do their part, or plans will build a bridge to nowhere.”
The rule comes as health insurers have increasingly been criticized for onerous and time-consuming prior authorization procedures that physicians say unfairly delay or deny the medical treatment that their patients need. With federal legislation to rein in prior authorization overuse at a standstill, 30 states have introduced their own bills to address the problem. Regulators and lawsuits also have called attention to insurers’ increasing use of artificial intelligence and algorithms to deny claims without human review.
“Family physicians know firsthand how prior authorizations divert valuable time and resources away from direct patient care. We also know that these types of administrative requirements are driving physicians away from the workforce and worsening physician shortages,” said Steven P. Furr, MD, president of the American Academy of Family Physicians, in a statement praising the new rule.
Jesse M. Ehrenfeld, MD, MPH, president of the American Medical Association, called the final rule “ a major win” for patients and physicians, adding that its requirements for health insurers to integrate their prior authorization procedures into physicians’ electronic health records systems will also help make “the current time-consuming, manual workflow” more efficient.
A version of this article first appeared on Medscape.com.
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).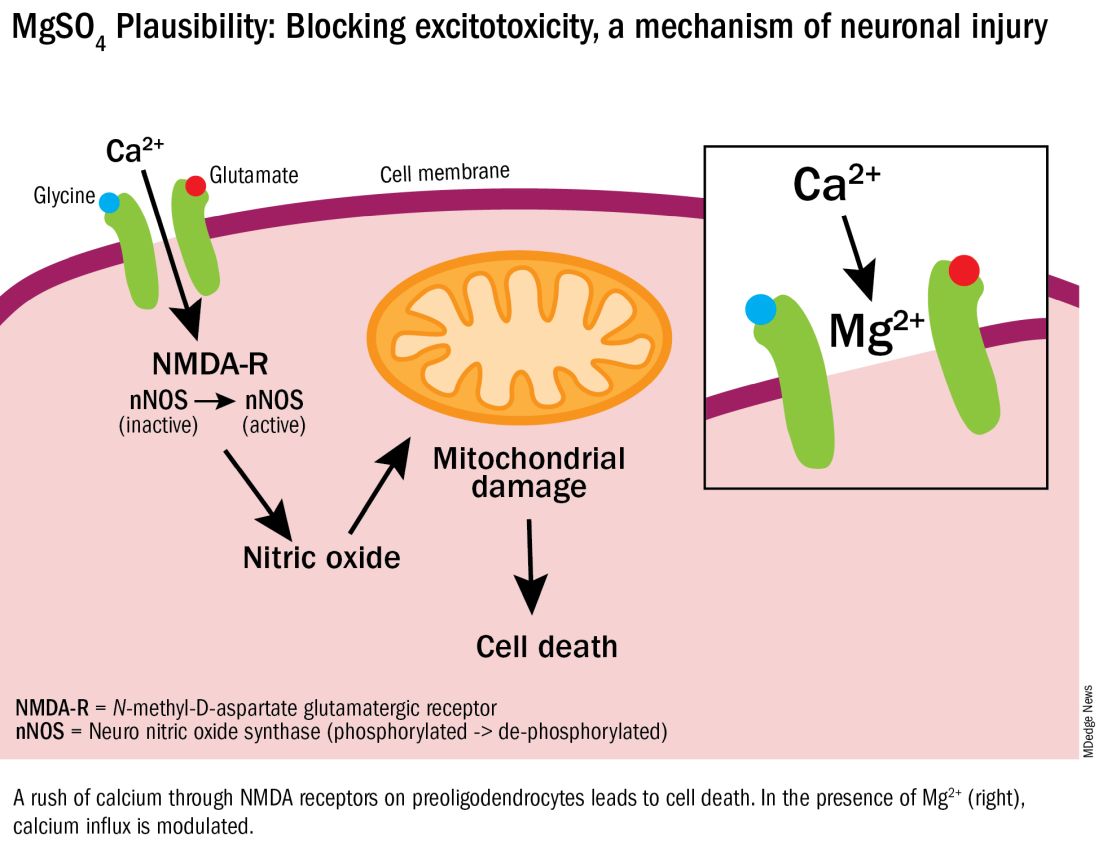
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.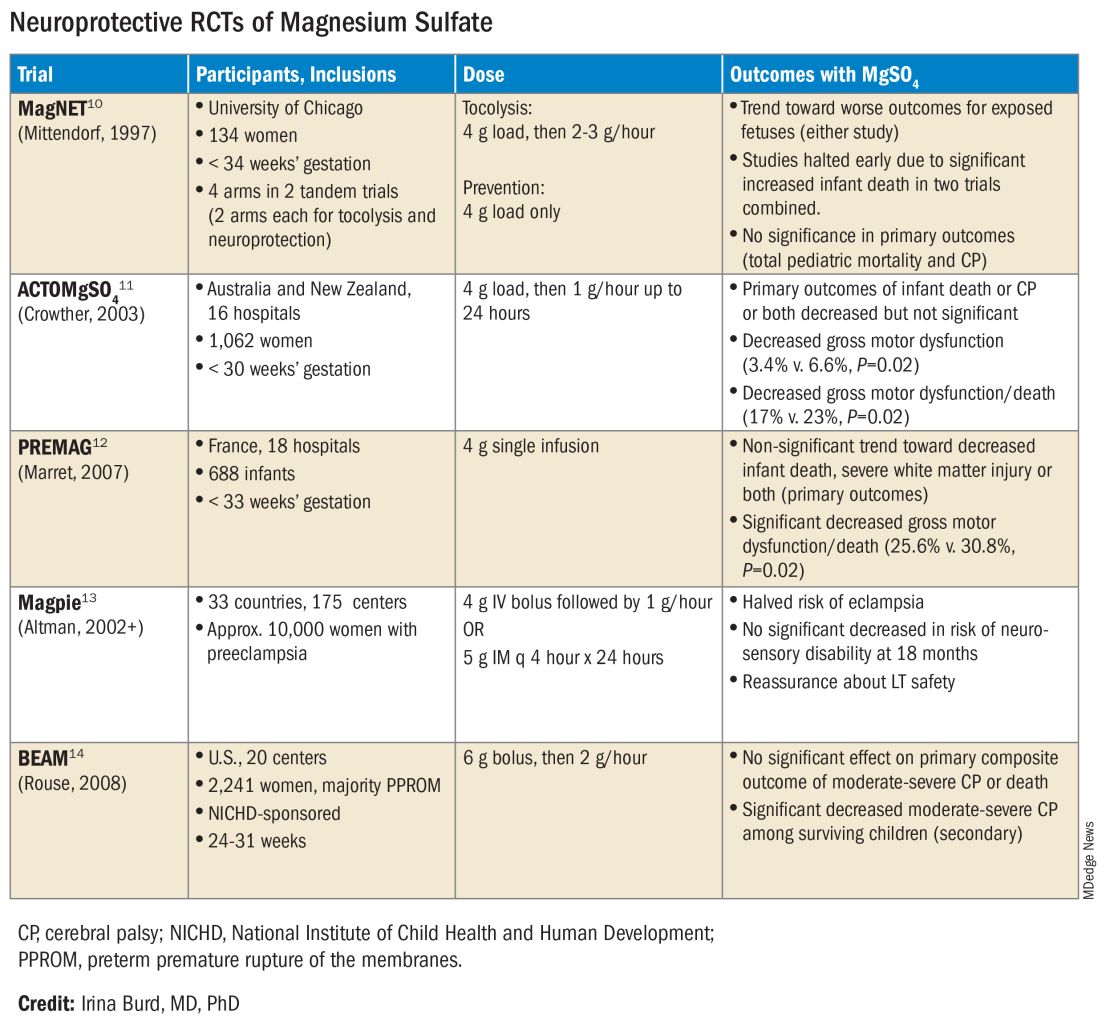
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
Gestational Diabetes May Double Chronic Kidney Disease Risk
TOPLINE:
Previous gestational diabetes mellitus (GDM) nearly doubles future chronic kidney disease (CKD) risk, irrespective of subsequent diabetes and hypertension, a study showed.
METHODOLOGY:
- A nationwide, cohort study was based on data from the Danish Medical Birth Register and included 697,622 women who gave birth between 1997 and 2018.
- Of all study participants, 3.4% reported GDM in at least one pregnancy, and 12.8% of women with GDM received insulin, a proxy for a more severe metabolic dysfunction.
- The women were followed up for a median of 11.9 years.
- Researchers studied CKD and acute kidney disease as the outcomes of interest, the mediating effects of subsequent diabetes and hypertension on future CKD, and how GDM severity affected later risk for kidney disease.
TAKEAWAY:
- Women with GDM showed significantly higher CKD risk than those without GDM (adjusted hazard ratio [aHR], 1.92; 95% CI, 1.67-2.21).
- Women who received insulin during pregnancy due to severe metabolic dysfunction but did not develop subsequent diabetes had a proportionally higher risk for CKD (aHR, 2.35; 95% CI, 1.39-3.97).
- Women with GDM who went on to develop diabetes or hypertension faced even higher risks for CKD, suggesting that preventing diabetes and hypertension after GDM may reduce the development of CKD.
- GDM did not affect the risk for acute kidney disease (aHR, 1.08; 95% CI, 0.90-1.29).
IN PRACTICE:
“Women with severe metabolic dysfunction during pregnancy constitute a high-risk group regarding future CKD,” the authors wrote. “The significantly elevated CKD risk was observed from 2 years after pregnancy and beyond.”
SOURCE:
The study, with first author Maria Hornstrup Christensen, of Odense University Hospital, Odense, Denmark, was published online on December 15 in Diabetes Care.
LIMITATIONS:
GDM may be underdiagnosed, and undiagnosed diabetes may be misclassified as GDM. The proxies of GDM and insulin treatment may not have captured the increasing severity of metabolic dysfunction. The prevalence of insulin treatment was lower than expected, perhaps due to the practice of providing a patient’s first insulin pen without a prescription and perhaps not recording it in a patient’s health record.
DISCLOSURES:
This work received financial support from the University of Southern Denmark, the Region of Southern Denmark, and the Danish Diabetes Academy, which is funded by the Novo Nordisk Foundation. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Previous gestational diabetes mellitus (GDM) nearly doubles future chronic kidney disease (CKD) risk, irrespective of subsequent diabetes and hypertension, a study showed.
METHODOLOGY:
- A nationwide, cohort study was based on data from the Danish Medical Birth Register and included 697,622 women who gave birth between 1997 and 2018.
- Of all study participants, 3.4% reported GDM in at least one pregnancy, and 12.8% of women with GDM received insulin, a proxy for a more severe metabolic dysfunction.
- The women were followed up for a median of 11.9 years.
- Researchers studied CKD and acute kidney disease as the outcomes of interest, the mediating effects of subsequent diabetes and hypertension on future CKD, and how GDM severity affected later risk for kidney disease.
TAKEAWAY:
- Women with GDM showed significantly higher CKD risk than those without GDM (adjusted hazard ratio [aHR], 1.92; 95% CI, 1.67-2.21).
- Women who received insulin during pregnancy due to severe metabolic dysfunction but did not develop subsequent diabetes had a proportionally higher risk for CKD (aHR, 2.35; 95% CI, 1.39-3.97).
- Women with GDM who went on to develop diabetes or hypertension faced even higher risks for CKD, suggesting that preventing diabetes and hypertension after GDM may reduce the development of CKD.
- GDM did not affect the risk for acute kidney disease (aHR, 1.08; 95% CI, 0.90-1.29).
IN PRACTICE:
“Women with severe metabolic dysfunction during pregnancy constitute a high-risk group regarding future CKD,” the authors wrote. “The significantly elevated CKD risk was observed from 2 years after pregnancy and beyond.”
SOURCE:
The study, with first author Maria Hornstrup Christensen, of Odense University Hospital, Odense, Denmark, was published online on December 15 in Diabetes Care.
LIMITATIONS:
GDM may be underdiagnosed, and undiagnosed diabetes may be misclassified as GDM. The proxies of GDM and insulin treatment may not have captured the increasing severity of metabolic dysfunction. The prevalence of insulin treatment was lower than expected, perhaps due to the practice of providing a patient’s first insulin pen without a prescription and perhaps not recording it in a patient’s health record.
DISCLOSURES:
This work received financial support from the University of Southern Denmark, the Region of Southern Denmark, and the Danish Diabetes Academy, which is funded by the Novo Nordisk Foundation. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Previous gestational diabetes mellitus (GDM) nearly doubles future chronic kidney disease (CKD) risk, irrespective of subsequent diabetes and hypertension, a study showed.
METHODOLOGY:
- A nationwide, cohort study was based on data from the Danish Medical Birth Register and included 697,622 women who gave birth between 1997 and 2018.
- Of all study participants, 3.4% reported GDM in at least one pregnancy, and 12.8% of women with GDM received insulin, a proxy for a more severe metabolic dysfunction.
- The women were followed up for a median of 11.9 years.
- Researchers studied CKD and acute kidney disease as the outcomes of interest, the mediating effects of subsequent diabetes and hypertension on future CKD, and how GDM severity affected later risk for kidney disease.
TAKEAWAY:
- Women with GDM showed significantly higher CKD risk than those without GDM (adjusted hazard ratio [aHR], 1.92; 95% CI, 1.67-2.21).
- Women who received insulin during pregnancy due to severe metabolic dysfunction but did not develop subsequent diabetes had a proportionally higher risk for CKD (aHR, 2.35; 95% CI, 1.39-3.97).
- Women with GDM who went on to develop diabetes or hypertension faced even higher risks for CKD, suggesting that preventing diabetes and hypertension after GDM may reduce the development of CKD.
- GDM did not affect the risk for acute kidney disease (aHR, 1.08; 95% CI, 0.90-1.29).
IN PRACTICE:
“Women with severe metabolic dysfunction during pregnancy constitute a high-risk group regarding future CKD,” the authors wrote. “The significantly elevated CKD risk was observed from 2 years after pregnancy and beyond.”
SOURCE:
The study, with first author Maria Hornstrup Christensen, of Odense University Hospital, Odense, Denmark, was published online on December 15 in Diabetes Care.
LIMITATIONS:
GDM may be underdiagnosed, and undiagnosed diabetes may be misclassified as GDM. The proxies of GDM and insulin treatment may not have captured the increasing severity of metabolic dysfunction. The prevalence of insulin treatment was lower than expected, perhaps due to the practice of providing a patient’s first insulin pen without a prescription and perhaps not recording it in a patient’s health record.
DISCLOSURES:
This work received financial support from the University of Southern Denmark, the Region of Southern Denmark, and the Danish Diabetes Academy, which is funded by the Novo Nordisk Foundation. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Autoimmune Diseases and Perinatal Depression May Share Two-Way Link
Women with autoimmune disease are more likely to have perinatal depression (PND), according to findings from a new study that also suggested the reverse relationship is true: Women with a history of PND have a higher risk of developing autoimmune disease.
The research, published online on January 9, 2024, in Molecular Psychiatry, was led by Emma Bränn, PhD, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden.
The researchers used data from the Swedish Medical Birth Register and identified all women who had given birth in Sweden between 2001 and 2013. Out of the group of approximately 815,000 women and 1.3 million pregnancies, just more than 55,000 women had been diagnosed with depression during their pregnancy or within a year after delivery.
The researchers then compared the incidence of 41 autoimmune diseases in women who had and did not have PND. They controlled for factors including genetic makeup and childhood environment.
Results indicated that women with autoimmune disease were 30% more likely to have PND (odds ratio, 1.30; 95% CI, 1.25-1.35). Conversely, women with PND were 30% more likely than women with no PND to develop an autoimmune disease (hazard ratio, 1.30; 95% CI, 1.25-1.36).
A sibling comparison helped confirm the results by controlling for some shared genetic and early life environmental factors related to the household in which sisters grew up.
Potential Shared Biological Mechanisms
The association was independent of psychiatric comorbidities, suggesting there may be shared biological mechanisms.
Dr. Bränn told this news organization that the research team wanted to do the study because previous research has shown involvement of the immune system in depression, with similarities in both the symptoms of immune system–activated diseases and depression and the molecular pathways activated by the immune system.
“Adding on top of the tremendous changes in the immune system that we see in the body of the woman during the perinatal period, we hypothesized that autoimmune diseases could be associated to perinatal depression,” she said. “This had also been shown in some previous literature but not to the extent as what we have investigated in this paper.”
She said their results help make a case for counseling women at several points in healthcare interactions — before and after conception and childbirth — and in rheumatology visits to inform women with autoimmune diseases who are contemplating motherhood of the association with developing PND. The results may also demonstrate a need for monitoring women in these groups for depression or autoimmune disease.
Fred Miller, MD, PhD, retired Scientist Emeritus of the Environmental Autoimmunity Group at the National Institute of Environmental Health Sciences, who was not part of the study, said the results seem plausible as they build on early work that demonstrated selected associations between autoimmune conditions and mental illness.
“These associations may be the result of shared genetic and environmental risk factors, including stress, hormonal changes, medications, and the proinflammatory states that can lead to both,” he said.
The novelty, he said, is in the relatively strong associations of PND with autoimmune disease overall and with specific autoimmune diseases.
Strong Link Found With Multiple Sclerosis (MS)
According to the paper, a significant positive bidirectional link was found for autoimmune thyroid disease, psoriasis, MS, ulcerative colitis, and celiac disease.
Researchers found a particularly strong association — double the risk in both directions — between PND and MS.
Dr. Miller said though it is unclear from this study why the association of PND with MS was stronger than with other autoimmune diseases, people with MS are known to be at a high risk for depression in general. That may come from greater shared genetic and environmental risk factors, he added.
Additionally, MS is one of the more common autoimmune diseases, he noted, so the population is larger for study.
He said he was surprised the researchers didn’t investigate medication use because medications used in depression have immunologic effects and medications used in autoimmune diseases could have effects on mental conditions.
The study has implications for clinicians in a wide variety of specialties, Dr. Miller noted.
“It suggests that caregivers be more alert to the signs of developing autoimmune disease in women with perinatal depression and to the signs of developing perinatal depression in those with autoimmune disease,” Dr. Miller said, “so that appropriate screening, diagnostics, and interventions may be undertaken.”
The researchers say they will continue to examine the long-term effects of depression during pregnancy and in the year after childbirth.
“Depression during this sensitive period can have serious consequences for both the mother and the baby,” Dr. Bränn said. “We hope that our results will help decision-makers to steer funding toward maternal healthcare so that more women can get help and support in time.”
The study was financed by Karolinska Institute, Forte (the Swedish Research Council for Health, Working Life and Welfare), the Swedish Research Council, and the Icelandic Research Fund.
The researchers and Dr. Miller reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Women with autoimmune disease are more likely to have perinatal depression (PND), according to findings from a new study that also suggested the reverse relationship is true: Women with a history of PND have a higher risk of developing autoimmune disease.
The research, published online on January 9, 2024, in Molecular Psychiatry, was led by Emma Bränn, PhD, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden.
The researchers used data from the Swedish Medical Birth Register and identified all women who had given birth in Sweden between 2001 and 2013. Out of the group of approximately 815,000 women and 1.3 million pregnancies, just more than 55,000 women had been diagnosed with depression during their pregnancy or within a year after delivery.
The researchers then compared the incidence of 41 autoimmune diseases in women who had and did not have PND. They controlled for factors including genetic makeup and childhood environment.
Results indicated that women with autoimmune disease were 30% more likely to have PND (odds ratio, 1.30; 95% CI, 1.25-1.35). Conversely, women with PND were 30% more likely than women with no PND to develop an autoimmune disease (hazard ratio, 1.30; 95% CI, 1.25-1.36).
A sibling comparison helped confirm the results by controlling for some shared genetic and early life environmental factors related to the household in which sisters grew up.
Potential Shared Biological Mechanisms
The association was independent of psychiatric comorbidities, suggesting there may be shared biological mechanisms.
Dr. Bränn told this news organization that the research team wanted to do the study because previous research has shown involvement of the immune system in depression, with similarities in both the symptoms of immune system–activated diseases and depression and the molecular pathways activated by the immune system.
“Adding on top of the tremendous changes in the immune system that we see in the body of the woman during the perinatal period, we hypothesized that autoimmune diseases could be associated to perinatal depression,” she said. “This had also been shown in some previous literature but not to the extent as what we have investigated in this paper.”
She said their results help make a case for counseling women at several points in healthcare interactions — before and after conception and childbirth — and in rheumatology visits to inform women with autoimmune diseases who are contemplating motherhood of the association with developing PND. The results may also demonstrate a need for monitoring women in these groups for depression or autoimmune disease.
Fred Miller, MD, PhD, retired Scientist Emeritus of the Environmental Autoimmunity Group at the National Institute of Environmental Health Sciences, who was not part of the study, said the results seem plausible as they build on early work that demonstrated selected associations between autoimmune conditions and mental illness.
“These associations may be the result of shared genetic and environmental risk factors, including stress, hormonal changes, medications, and the proinflammatory states that can lead to both,” he said.
The novelty, he said, is in the relatively strong associations of PND with autoimmune disease overall and with specific autoimmune diseases.
Strong Link Found With Multiple Sclerosis (MS)
According to the paper, a significant positive bidirectional link was found for autoimmune thyroid disease, psoriasis, MS, ulcerative colitis, and celiac disease.
Researchers found a particularly strong association — double the risk in both directions — between PND and MS.
Dr. Miller said though it is unclear from this study why the association of PND with MS was stronger than with other autoimmune diseases, people with MS are known to be at a high risk for depression in general. That may come from greater shared genetic and environmental risk factors, he added.
Additionally, MS is one of the more common autoimmune diseases, he noted, so the population is larger for study.
He said he was surprised the researchers didn’t investigate medication use because medications used in depression have immunologic effects and medications used in autoimmune diseases could have effects on mental conditions.
The study has implications for clinicians in a wide variety of specialties, Dr. Miller noted.
“It suggests that caregivers be more alert to the signs of developing autoimmune disease in women with perinatal depression and to the signs of developing perinatal depression in those with autoimmune disease,” Dr. Miller said, “so that appropriate screening, diagnostics, and interventions may be undertaken.”
The researchers say they will continue to examine the long-term effects of depression during pregnancy and in the year after childbirth.
“Depression during this sensitive period can have serious consequences for both the mother and the baby,” Dr. Bränn said. “We hope that our results will help decision-makers to steer funding toward maternal healthcare so that more women can get help and support in time.”
The study was financed by Karolinska Institute, Forte (the Swedish Research Council for Health, Working Life and Welfare), the Swedish Research Council, and the Icelandic Research Fund.
The researchers and Dr. Miller reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Women with autoimmune disease are more likely to have perinatal depression (PND), according to findings from a new study that also suggested the reverse relationship is true: Women with a history of PND have a higher risk of developing autoimmune disease.
The research, published online on January 9, 2024, in Molecular Psychiatry, was led by Emma Bränn, PhD, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden.
The researchers used data from the Swedish Medical Birth Register and identified all women who had given birth in Sweden between 2001 and 2013. Out of the group of approximately 815,000 women and 1.3 million pregnancies, just more than 55,000 women had been diagnosed with depression during their pregnancy or within a year after delivery.
The researchers then compared the incidence of 41 autoimmune diseases in women who had and did not have PND. They controlled for factors including genetic makeup and childhood environment.
Results indicated that women with autoimmune disease were 30% more likely to have PND (odds ratio, 1.30; 95% CI, 1.25-1.35). Conversely, women with PND were 30% more likely than women with no PND to develop an autoimmune disease (hazard ratio, 1.30; 95% CI, 1.25-1.36).
A sibling comparison helped confirm the results by controlling for some shared genetic and early life environmental factors related to the household in which sisters grew up.
Potential Shared Biological Mechanisms
The association was independent of psychiatric comorbidities, suggesting there may be shared biological mechanisms.
Dr. Bränn told this news organization that the research team wanted to do the study because previous research has shown involvement of the immune system in depression, with similarities in both the symptoms of immune system–activated diseases and depression and the molecular pathways activated by the immune system.
“Adding on top of the tremendous changes in the immune system that we see in the body of the woman during the perinatal period, we hypothesized that autoimmune diseases could be associated to perinatal depression,” she said. “This had also been shown in some previous literature but not to the extent as what we have investigated in this paper.”
She said their results help make a case for counseling women at several points in healthcare interactions — before and after conception and childbirth — and in rheumatology visits to inform women with autoimmune diseases who are contemplating motherhood of the association with developing PND. The results may also demonstrate a need for monitoring women in these groups for depression or autoimmune disease.
Fred Miller, MD, PhD, retired Scientist Emeritus of the Environmental Autoimmunity Group at the National Institute of Environmental Health Sciences, who was not part of the study, said the results seem plausible as they build on early work that demonstrated selected associations between autoimmune conditions and mental illness.
“These associations may be the result of shared genetic and environmental risk factors, including stress, hormonal changes, medications, and the proinflammatory states that can lead to both,” he said.
The novelty, he said, is in the relatively strong associations of PND with autoimmune disease overall and with specific autoimmune diseases.
Strong Link Found With Multiple Sclerosis (MS)
According to the paper, a significant positive bidirectional link was found for autoimmune thyroid disease, psoriasis, MS, ulcerative colitis, and celiac disease.
Researchers found a particularly strong association — double the risk in both directions — between PND and MS.
Dr. Miller said though it is unclear from this study why the association of PND with MS was stronger than with other autoimmune diseases, people with MS are known to be at a high risk for depression in general. That may come from greater shared genetic and environmental risk factors, he added.
Additionally, MS is one of the more common autoimmune diseases, he noted, so the population is larger for study.
He said he was surprised the researchers didn’t investigate medication use because medications used in depression have immunologic effects and medications used in autoimmune diseases could have effects on mental conditions.
The study has implications for clinicians in a wide variety of specialties, Dr. Miller noted.
“It suggests that caregivers be more alert to the signs of developing autoimmune disease in women with perinatal depression and to the signs of developing perinatal depression in those with autoimmune disease,” Dr. Miller said, “so that appropriate screening, diagnostics, and interventions may be undertaken.”
The researchers say they will continue to examine the long-term effects of depression during pregnancy and in the year after childbirth.
“Depression during this sensitive period can have serious consequences for both the mother and the baby,” Dr. Bränn said. “We hope that our results will help decision-makers to steer funding toward maternal healthcare so that more women can get help and support in time.”
The study was financed by Karolinska Institute, Forte (the Swedish Research Council for Health, Working Life and Welfare), the Swedish Research Council, and the Icelandic Research Fund.
The researchers and Dr. Miller reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM MOLECULAR PSYCHIATRY
SUDs rates highest in head, neck, and gastric cancer survivors
.
The association between cancer and substance use is well known, but data on the prevalence of different substance use disorders (SUDs) in different types of cancer are limited, Katie F. Jones, PhD, of the VA Boston Healthcare System, and colleagues, wrote in their paper.
“Substance use and use disorders are on the rise in general and among older adults, who represent the majority of people diagnosed with cancer, and SUDs have significant potential to complicate cancer care and negatively impact cancer outcomes,” corresponding author Devon K. Check, PhD, of Duke University, Durham, N.C., said in an interview. “We thought it was important to understand whether SUDs are more common with certain types of cancer. We can use that information to guide resources toward populations where interventions to integrate SUD treatment and cancer treatment are most needed,” he said. “In addition, because different SUDs (opioid use disorder, alcohol use disorder) might complicate cancer treatment in different ways and necessitate different types of interventions, we thought it was important to understand the distribution of specific disorders,” he explained.
In the cross-sectional study published in JAMA Oncology, the researchers reviewed data from 6,101 adult cancer survivors who participated in the National Survey of Drug Use and Health (NSDUH) between 2015 and 2020.
The study population included survivors of solid tumor cancers. SUD was defined as meeting at least one of four criteria for substance abuse or at least 3 of 6 criteria for dependence based on the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria.
Overall, 3.83% of the participants met criteria for SUD. Survivors of head and neck cancers and survivors of gastric and esophageal cancers had the highest rates of SUDs (approximately 9%), followed by cervical cancer and melanoma survivors (approximately 6%).
Alcohol use disorder was the most common SUD both overall (2.8%) and among survivors of head and neck cancers, cervical cancers, and melanoma.
Cannabis use disorder was the most prevalent SUD among esophageal and gastric cancer survivors (approximately 9%).
The prevalence of SUDs overall and within the past year (active) was approximately 4%, but the prevalence of active SUDs was significantly higher for those with head and neck cancers and cervical cancer (18.73% and 15.70%, respectively). However, the distribution of specific SUDs was different in the newly diagnosed patients. Sedative use disorder took the top spot as the most common SUD for head and neck cancer survivors (9.81%), while alcohol use disorder was the most common SUD among cervical cancer survivors (10.49%).
Limitations and Implications
The findings were limited by several factors, including the nature of the study population and the data source, said Dr. Check.
“The average prevalence of SUD (or the prevalence across cancer types) was lower than we might have expected,” but the results make sense given the mainly older and female study population, he said. SUDs are less common among older adults compared with younger adults and among women compared with men, and the study’s data source (NSDUH) has been shown in other research to underestimate the prevalence of opioid use disorder, he added.
“Otherwise, the study findings were generally consistent with what we would expect,” Dr. Check said in an interview. “For example, alcohol use disorder is the most common SUD in the general U.S. population, and that was true for our study population of cancer survivors as well. In addition, SUD prevalence was higher in cancers such as cervical cancer and head and neck cancers that are causally linked to alcohol and/or tobacco use,” he said.
Integrated care is needed
“Among people diagnosed with certain types of cancers, including cervical and head and neck cancers, the estimated prevalence of SUD is similar to those [with] medical comorbidities such as diabetes and cardiopulmonary conditions,” said Dr. Check. “Within the field, there is an increasing emphasis on ensuring that people diagnosed with cancer have access to integrated care for their comorbid medical conditions. Similar efforts for people who concurrently manage cancer and SUD are largely absent but critically needed; these efforts should prioritize cancer populations where SUD prevalence is high,” he said.
Looking ahead, “We need to understand more about the specific challenges that arise at the intersection of cancer and SUD so we can design interventions and programs to better support both patients who concurrently manage cancer and SUD and the clinicians who care for them,” Dr. Check added.
Recognize risk factors
“It is very important to study overall substance use disorders in patients with cancer, because understanding the risks of developing these issues after treatment helps us develop approaches to best support these patients following their cancer therapies,” Henry S. Park, MD, a radiation oncologist at Yale University, New Haven, Connecticut, said in an interview.
The current study findings “are generally consistent with my experience and intuition, but it is still helpful to see the actual data,” said Dr. Park, who was not involved in the study. “This may be partially because of the baseline elevated risk of preexisting SUDs for certain patients from the higher-prevalence disease sites. However, it may also be related to the intense side effects that survivors of some types of cancers, such as head and neck cancer, gastroesophageal cancer, and cervical cancer, may experience soon after treatment, and even chronically long after treatment,” he said.
Individualize risk assessment
“Ultimately, clinicians should be aware that not all patients with cancer are the same, and that the majority do not necessarily develop SUDs,” Dr. Park said in an interview. “We should be careful to treat symptoms appropriately, and not withhold therapies purely because of an elevated risk of developing SUDs. However, there are some patients who are at higher risk of SUDs who will need extra support and care from physicians, advanced practice providers, nutritionists, social workers, psychologists, dietitians, and survivorship clinics, both in the short-term and long-term,” he emphasized.
As for additional research, “more work needs to be done on which particular patients within each disease subset are most likely to develop SUDs,” said Dr. Park. “Most importantly, once we identify our high-risk group as reliably as possible, we will have to study interventions that rely on supporting and partnering with patients to decrease the risk of developing SUDs as much as possible, while adequately treating residual symptoms and quality-of-life effects following cancer treatment,” he said.
The study received no outside funding. Dr. Check disclosed grants from Duke University during the study period and grants from the National Institutes of Health and AstraZeneca unrelated to the current study. Dr. Park had no financial conflicts to disclose.
.
The association between cancer and substance use is well known, but data on the prevalence of different substance use disorders (SUDs) in different types of cancer are limited, Katie F. Jones, PhD, of the VA Boston Healthcare System, and colleagues, wrote in their paper.
“Substance use and use disorders are on the rise in general and among older adults, who represent the majority of people diagnosed with cancer, and SUDs have significant potential to complicate cancer care and negatively impact cancer outcomes,” corresponding author Devon K. Check, PhD, of Duke University, Durham, N.C., said in an interview. “We thought it was important to understand whether SUDs are more common with certain types of cancer. We can use that information to guide resources toward populations where interventions to integrate SUD treatment and cancer treatment are most needed,” he said. “In addition, because different SUDs (opioid use disorder, alcohol use disorder) might complicate cancer treatment in different ways and necessitate different types of interventions, we thought it was important to understand the distribution of specific disorders,” he explained.
In the cross-sectional study published in JAMA Oncology, the researchers reviewed data from 6,101 adult cancer survivors who participated in the National Survey of Drug Use and Health (NSDUH) between 2015 and 2020.
The study population included survivors of solid tumor cancers. SUD was defined as meeting at least one of four criteria for substance abuse or at least 3 of 6 criteria for dependence based on the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria.
Overall, 3.83% of the participants met criteria for SUD. Survivors of head and neck cancers and survivors of gastric and esophageal cancers had the highest rates of SUDs (approximately 9%), followed by cervical cancer and melanoma survivors (approximately 6%).
Alcohol use disorder was the most common SUD both overall (2.8%) and among survivors of head and neck cancers, cervical cancers, and melanoma.
Cannabis use disorder was the most prevalent SUD among esophageal and gastric cancer survivors (approximately 9%).
The prevalence of SUDs overall and within the past year (active) was approximately 4%, but the prevalence of active SUDs was significantly higher for those with head and neck cancers and cervical cancer (18.73% and 15.70%, respectively). However, the distribution of specific SUDs was different in the newly diagnosed patients. Sedative use disorder took the top spot as the most common SUD for head and neck cancer survivors (9.81%), while alcohol use disorder was the most common SUD among cervical cancer survivors (10.49%).
Limitations and Implications
The findings were limited by several factors, including the nature of the study population and the data source, said Dr. Check.
“The average prevalence of SUD (or the prevalence across cancer types) was lower than we might have expected,” but the results make sense given the mainly older and female study population, he said. SUDs are less common among older adults compared with younger adults and among women compared with men, and the study’s data source (NSDUH) has been shown in other research to underestimate the prevalence of opioid use disorder, he added.
“Otherwise, the study findings were generally consistent with what we would expect,” Dr. Check said in an interview. “For example, alcohol use disorder is the most common SUD in the general U.S. population, and that was true for our study population of cancer survivors as well. In addition, SUD prevalence was higher in cancers such as cervical cancer and head and neck cancers that are causally linked to alcohol and/or tobacco use,” he said.
Integrated care is needed
“Among people diagnosed with certain types of cancers, including cervical and head and neck cancers, the estimated prevalence of SUD is similar to those [with] medical comorbidities such as diabetes and cardiopulmonary conditions,” said Dr. Check. “Within the field, there is an increasing emphasis on ensuring that people diagnosed with cancer have access to integrated care for their comorbid medical conditions. Similar efforts for people who concurrently manage cancer and SUD are largely absent but critically needed; these efforts should prioritize cancer populations where SUD prevalence is high,” he said.
Looking ahead, “We need to understand more about the specific challenges that arise at the intersection of cancer and SUD so we can design interventions and programs to better support both patients who concurrently manage cancer and SUD and the clinicians who care for them,” Dr. Check added.
Recognize risk factors
“It is very important to study overall substance use disorders in patients with cancer, because understanding the risks of developing these issues after treatment helps us develop approaches to best support these patients following their cancer therapies,” Henry S. Park, MD, a radiation oncologist at Yale University, New Haven, Connecticut, said in an interview.
The current study findings “are generally consistent with my experience and intuition, but it is still helpful to see the actual data,” said Dr. Park, who was not involved in the study. “This may be partially because of the baseline elevated risk of preexisting SUDs for certain patients from the higher-prevalence disease sites. However, it may also be related to the intense side effects that survivors of some types of cancers, such as head and neck cancer, gastroesophageal cancer, and cervical cancer, may experience soon after treatment, and even chronically long after treatment,” he said.
Individualize risk assessment
“Ultimately, clinicians should be aware that not all patients with cancer are the same, and that the majority do not necessarily develop SUDs,” Dr. Park said in an interview. “We should be careful to treat symptoms appropriately, and not withhold therapies purely because of an elevated risk of developing SUDs. However, there are some patients who are at higher risk of SUDs who will need extra support and care from physicians, advanced practice providers, nutritionists, social workers, psychologists, dietitians, and survivorship clinics, both in the short-term and long-term,” he emphasized.
As for additional research, “more work needs to be done on which particular patients within each disease subset are most likely to develop SUDs,” said Dr. Park. “Most importantly, once we identify our high-risk group as reliably as possible, we will have to study interventions that rely on supporting and partnering with patients to decrease the risk of developing SUDs as much as possible, while adequately treating residual symptoms and quality-of-life effects following cancer treatment,” he said.
The study received no outside funding. Dr. Check disclosed grants from Duke University during the study period and grants from the National Institutes of Health and AstraZeneca unrelated to the current study. Dr. Park had no financial conflicts to disclose.
.
The association between cancer and substance use is well known, but data on the prevalence of different substance use disorders (SUDs) in different types of cancer are limited, Katie F. Jones, PhD, of the VA Boston Healthcare System, and colleagues, wrote in their paper.
“Substance use and use disorders are on the rise in general and among older adults, who represent the majority of people diagnosed with cancer, and SUDs have significant potential to complicate cancer care and negatively impact cancer outcomes,” corresponding author Devon K. Check, PhD, of Duke University, Durham, N.C., said in an interview. “We thought it was important to understand whether SUDs are more common with certain types of cancer. We can use that information to guide resources toward populations where interventions to integrate SUD treatment and cancer treatment are most needed,” he said. “In addition, because different SUDs (opioid use disorder, alcohol use disorder) might complicate cancer treatment in different ways and necessitate different types of interventions, we thought it was important to understand the distribution of specific disorders,” he explained.
In the cross-sectional study published in JAMA Oncology, the researchers reviewed data from 6,101 adult cancer survivors who participated in the National Survey of Drug Use and Health (NSDUH) between 2015 and 2020.
The study population included survivors of solid tumor cancers. SUD was defined as meeting at least one of four criteria for substance abuse or at least 3 of 6 criteria for dependence based on the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria.
Overall, 3.83% of the participants met criteria for SUD. Survivors of head and neck cancers and survivors of gastric and esophageal cancers had the highest rates of SUDs (approximately 9%), followed by cervical cancer and melanoma survivors (approximately 6%).
Alcohol use disorder was the most common SUD both overall (2.8%) and among survivors of head and neck cancers, cervical cancers, and melanoma.
Cannabis use disorder was the most prevalent SUD among esophageal and gastric cancer survivors (approximately 9%).
The prevalence of SUDs overall and within the past year (active) was approximately 4%, but the prevalence of active SUDs was significantly higher for those with head and neck cancers and cervical cancer (18.73% and 15.70%, respectively). However, the distribution of specific SUDs was different in the newly diagnosed patients. Sedative use disorder took the top spot as the most common SUD for head and neck cancer survivors (9.81%), while alcohol use disorder was the most common SUD among cervical cancer survivors (10.49%).
Limitations and Implications
The findings were limited by several factors, including the nature of the study population and the data source, said Dr. Check.
“The average prevalence of SUD (or the prevalence across cancer types) was lower than we might have expected,” but the results make sense given the mainly older and female study population, he said. SUDs are less common among older adults compared with younger adults and among women compared with men, and the study’s data source (NSDUH) has been shown in other research to underestimate the prevalence of opioid use disorder, he added.
“Otherwise, the study findings were generally consistent with what we would expect,” Dr. Check said in an interview. “For example, alcohol use disorder is the most common SUD in the general U.S. population, and that was true for our study population of cancer survivors as well. In addition, SUD prevalence was higher in cancers such as cervical cancer and head and neck cancers that are causally linked to alcohol and/or tobacco use,” he said.
Integrated care is needed
“Among people diagnosed with certain types of cancers, including cervical and head and neck cancers, the estimated prevalence of SUD is similar to those [with] medical comorbidities such as diabetes and cardiopulmonary conditions,” said Dr. Check. “Within the field, there is an increasing emphasis on ensuring that people diagnosed with cancer have access to integrated care for their comorbid medical conditions. Similar efforts for people who concurrently manage cancer and SUD are largely absent but critically needed; these efforts should prioritize cancer populations where SUD prevalence is high,” he said.
Looking ahead, “We need to understand more about the specific challenges that arise at the intersection of cancer and SUD so we can design interventions and programs to better support both patients who concurrently manage cancer and SUD and the clinicians who care for them,” Dr. Check added.
Recognize risk factors
“It is very important to study overall substance use disorders in patients with cancer, because understanding the risks of developing these issues after treatment helps us develop approaches to best support these patients following their cancer therapies,” Henry S. Park, MD, a radiation oncologist at Yale University, New Haven, Connecticut, said in an interview.
The current study findings “are generally consistent with my experience and intuition, but it is still helpful to see the actual data,” said Dr. Park, who was not involved in the study. “This may be partially because of the baseline elevated risk of preexisting SUDs for certain patients from the higher-prevalence disease sites. However, it may also be related to the intense side effects that survivors of some types of cancers, such as head and neck cancer, gastroesophageal cancer, and cervical cancer, may experience soon after treatment, and even chronically long after treatment,” he said.
Individualize risk assessment
“Ultimately, clinicians should be aware that not all patients with cancer are the same, and that the majority do not necessarily develop SUDs,” Dr. Park said in an interview. “We should be careful to treat symptoms appropriately, and not withhold therapies purely because of an elevated risk of developing SUDs. However, there are some patients who are at higher risk of SUDs who will need extra support and care from physicians, advanced practice providers, nutritionists, social workers, psychologists, dietitians, and survivorship clinics, both in the short-term and long-term,” he emphasized.
As for additional research, “more work needs to be done on which particular patients within each disease subset are most likely to develop SUDs,” said Dr. Park. “Most importantly, once we identify our high-risk group as reliably as possible, we will have to study interventions that rely on supporting and partnering with patients to decrease the risk of developing SUDs as much as possible, while adequately treating residual symptoms and quality-of-life effects following cancer treatment,” he said.
The study received no outside funding. Dr. Check disclosed grants from Duke University during the study period and grants from the National Institutes of Health and AstraZeneca unrelated to the current study. Dr. Park had no financial conflicts to disclose.
FROM JAMA ONCOLOGY
A 27-year-old Haitian woman presented with a painful umbilical mass which had been growing in size for 5 months
Endometriosis is defined as the presence of endometrial tissue outside of the uterine cavity, commonly occurring in women of reproductive age. The condition usually affects the adnexa (ovaries, Fallopian tubes, and associated ligaments and connective tissue) but can also be seen in extrapelvic structures.
Cutaneous endometriosis is an uncommon subtype that accounts for 1% of endometriosis cases and occurs when endometrial tissue is found on the surface of the skin. It is divided into primary and secondary cutaneous endometriosis. The that may lead to seeding of endometrial tissue on the skin. In the case of our patient, it appears that her laparoscopic procedure 2 years ago was the cause of endometrial seeding in the umbilicus.
Clinically, the condition may present with a palpable mass, cyclic pain, and bloody discharge from the affected area. Due to the rarity of cutaneous endometriosis, it may be hard to distinguish from other diagnoses such as keloids, dermatofibromas, hernias, or cutaneous metastasis of cancers (Sister Mary Joseph nodules).
The definitive diagnosis can be made by biopsy and histopathological assessment showing a mixture of endometrial glands and stromal tissue. Imaging studies such as computed tomography (CT) scan and magnetic resonance imaging (MRI) are helpful in excluding more common diagnoses such as hernia or cutaneous metastasis. In this patient, the mass was surgically excised. Histopathological assessment established the diagnosis of cutaneous endometriosis.
Treatment options include surgical excision and medical therapy. Medical therapy entails the use of hormonal agents such as gonadotropin-releasing hormone agonists, danazol (a pituitary gonadotropin inhibitor), and oral contraceptives, which reduce the cyclical proliferation of endothelial tissue. These agents can be used preoperatively to reduce the size of the cutaneous mass before surgical excision, or as an alternative treatment for patients who wish to avoid surgery. The rate of recurrence is observed to be higher with medical therapy rather than surgical treatment.
The case and photo were submitted by Mina Ahmed, MBBS, Brooke Resh Sateesh MD, and Nathan Uebelhoer MD, of San Diego Family Dermatology, San Diego, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Gonzalez RH et al. Am J Case Rep. 2021;22:e932493-1–e932493-4.
2. Raffi L et al. Int J Womens Dermatol. 2019 Dec;5(5):384-386.
3. Sharma A, Apostol R. Cutaneous endometriosis. Treasure Island, Fla: Statpearls Publishing, 2023.
Endometriosis is defined as the presence of endometrial tissue outside of the uterine cavity, commonly occurring in women of reproductive age. The condition usually affects the adnexa (ovaries, Fallopian tubes, and associated ligaments and connective tissue) but can also be seen in extrapelvic structures.
Cutaneous endometriosis is an uncommon subtype that accounts for 1% of endometriosis cases and occurs when endometrial tissue is found on the surface of the skin. It is divided into primary and secondary cutaneous endometriosis. The that may lead to seeding of endometrial tissue on the skin. In the case of our patient, it appears that her laparoscopic procedure 2 years ago was the cause of endometrial seeding in the umbilicus.
Clinically, the condition may present with a palpable mass, cyclic pain, and bloody discharge from the affected area. Due to the rarity of cutaneous endometriosis, it may be hard to distinguish from other diagnoses such as keloids, dermatofibromas, hernias, or cutaneous metastasis of cancers (Sister Mary Joseph nodules).
The definitive diagnosis can be made by biopsy and histopathological assessment showing a mixture of endometrial glands and stromal tissue. Imaging studies such as computed tomography (CT) scan and magnetic resonance imaging (MRI) are helpful in excluding more common diagnoses such as hernia or cutaneous metastasis. In this patient, the mass was surgically excised. Histopathological assessment established the diagnosis of cutaneous endometriosis.
Treatment options include surgical excision and medical therapy. Medical therapy entails the use of hormonal agents such as gonadotropin-releasing hormone agonists, danazol (a pituitary gonadotropin inhibitor), and oral contraceptives, which reduce the cyclical proliferation of endothelial tissue. These agents can be used preoperatively to reduce the size of the cutaneous mass before surgical excision, or as an alternative treatment for patients who wish to avoid surgery. The rate of recurrence is observed to be higher with medical therapy rather than surgical treatment.
The case and photo were submitted by Mina Ahmed, MBBS, Brooke Resh Sateesh MD, and Nathan Uebelhoer MD, of San Diego Family Dermatology, San Diego, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Gonzalez RH et al. Am J Case Rep. 2021;22:e932493-1–e932493-4.
2. Raffi L et al. Int J Womens Dermatol. 2019 Dec;5(5):384-386.
3. Sharma A, Apostol R. Cutaneous endometriosis. Treasure Island, Fla: Statpearls Publishing, 2023.
Endometriosis is defined as the presence of endometrial tissue outside of the uterine cavity, commonly occurring in women of reproductive age. The condition usually affects the adnexa (ovaries, Fallopian tubes, and associated ligaments and connective tissue) but can also be seen in extrapelvic structures.
Cutaneous endometriosis is an uncommon subtype that accounts for 1% of endometriosis cases and occurs when endometrial tissue is found on the surface of the skin. It is divided into primary and secondary cutaneous endometriosis. The that may lead to seeding of endometrial tissue on the skin. In the case of our patient, it appears that her laparoscopic procedure 2 years ago was the cause of endometrial seeding in the umbilicus.
Clinically, the condition may present with a palpable mass, cyclic pain, and bloody discharge from the affected area. Due to the rarity of cutaneous endometriosis, it may be hard to distinguish from other diagnoses such as keloids, dermatofibromas, hernias, or cutaneous metastasis of cancers (Sister Mary Joseph nodules).
The definitive diagnosis can be made by biopsy and histopathological assessment showing a mixture of endometrial glands and stromal tissue. Imaging studies such as computed tomography (CT) scan and magnetic resonance imaging (MRI) are helpful in excluding more common diagnoses such as hernia or cutaneous metastasis. In this patient, the mass was surgically excised. Histopathological assessment established the diagnosis of cutaneous endometriosis.
Treatment options include surgical excision and medical therapy. Medical therapy entails the use of hormonal agents such as gonadotropin-releasing hormone agonists, danazol (a pituitary gonadotropin inhibitor), and oral contraceptives, which reduce the cyclical proliferation of endothelial tissue. These agents can be used preoperatively to reduce the size of the cutaneous mass before surgical excision, or as an alternative treatment for patients who wish to avoid surgery. The rate of recurrence is observed to be higher with medical therapy rather than surgical treatment.
The case and photo were submitted by Mina Ahmed, MBBS, Brooke Resh Sateesh MD, and Nathan Uebelhoer MD, of San Diego Family Dermatology, San Diego, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Gonzalez RH et al. Am J Case Rep. 2021;22:e932493-1–e932493-4.
2. Raffi L et al. Int J Womens Dermatol. 2019 Dec;5(5):384-386.
3. Sharma A, Apostol R. Cutaneous endometriosis. Treasure Island, Fla: Statpearls Publishing, 2023.