User login
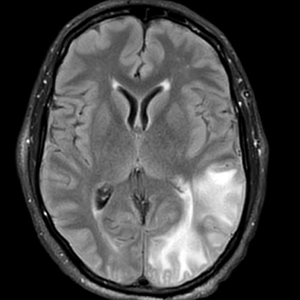
Gestational diabetes affects fetal lung development
Lung development in the fetus may be adversely affected by a mother’s gestational diabetes, based on data from in vivo, in vitro, and ex vivo studies.
Gestational diabetes mellitus (GDM) has recently been associated with fetal lung underdevelopment (FLUD) and delayed lung maturation that may lead to immediate respiratory distress in newborns and later chronic lung disease, Pengzheng Chen, PhD, of Shandong University, Jinan, China, and colleagues wrote.
Antenatal corticosteroids are considered an effective treatment for gestational fetal lung underdevelopment, but recent studies have shown adverse effects of these medications, and therefore more research is needed to identify the etiology and pathogenesis of FLUD induced by GDM, they said.
In a study published in the International Journal of Nanomedicine, the researchers collected umbilical cord blood samples from patients with GDM and matched controls at a single hospital in China.
“Using an ex vivo exosome exposure model of fetal lung explants, we observed the morphological alteration of lung explants and evaluated the expression of molecules involved in lung development,” the researchers wrote.
Fetal lung underdevelopment was more common after exposure to exosomes from the umbilical cord plasma of individuals with gestational diabetes mellitus, compared with exosomes from healthy controls.
The researchers also used mouse models to examine the effects of exosomes on fetal lung development in vivo. They found that exosomes associated with GDM impeded the growth, branching morphogenesis, and maturation of fetal lungs in mouse models. In addition, the expression of the apoptotic biomarkers known as BAX, BIM, and cleaved CASPASE-3 was up-regulated in GDMUB-exosomes and HG-exos groups, but the antiapoptotic protein BCL-2 was down-regulated; this further supported the negative impact of GDM exomes on fetal lung development, the researchers said.
The researchers then conducted miRNA sequencing, which showed that the miRNA in placenta-derived exosomes from GDM pregnancies were distinct from the miRNA in exosomes from healthy control pregnancies.
The study findings were limited by several factors including the impurity of the isolated placenta-derived exosomes from the umbilical cord blood plasma, which were not placenta specific, the researchers noted. Other limitations included the lack of data on different stages of lung development, and more research is needed to validate miRNAs and to explore the signally pathways involved in fetal lung development.
However, the study is the first known to demonstrate an adverse effect of GDM on fetal lung development via in vitro, ex vivo, and in vitro models, they said.
“These data highlight an emerging role of placenta-derived exosomes in the pathogenesis of fetal lung underdevelopment in GDM pregnancies, and provide a novel strategy for maternal-fetal communication,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Lung development in the fetus may be adversely affected by a mother’s gestational diabetes, based on data from in vivo, in vitro, and ex vivo studies.
Gestational diabetes mellitus (GDM) has recently been associated with fetal lung underdevelopment (FLUD) and delayed lung maturation that may lead to immediate respiratory distress in newborns and later chronic lung disease, Pengzheng Chen, PhD, of Shandong University, Jinan, China, and colleagues wrote.
Antenatal corticosteroids are considered an effective treatment for gestational fetal lung underdevelopment, but recent studies have shown adverse effects of these medications, and therefore more research is needed to identify the etiology and pathogenesis of FLUD induced by GDM, they said.
In a study published in the International Journal of Nanomedicine, the researchers collected umbilical cord blood samples from patients with GDM and matched controls at a single hospital in China.
“Using an ex vivo exosome exposure model of fetal lung explants, we observed the morphological alteration of lung explants and evaluated the expression of molecules involved in lung development,” the researchers wrote.
Fetal lung underdevelopment was more common after exposure to exosomes from the umbilical cord plasma of individuals with gestational diabetes mellitus, compared with exosomes from healthy controls.
The researchers also used mouse models to examine the effects of exosomes on fetal lung development in vivo. They found that exosomes associated with GDM impeded the growth, branching morphogenesis, and maturation of fetal lungs in mouse models. In addition, the expression of the apoptotic biomarkers known as BAX, BIM, and cleaved CASPASE-3 was up-regulated in GDMUB-exosomes and HG-exos groups, but the antiapoptotic protein BCL-2 was down-regulated; this further supported the negative impact of GDM exomes on fetal lung development, the researchers said.
The researchers then conducted miRNA sequencing, which showed that the miRNA in placenta-derived exosomes from GDM pregnancies were distinct from the miRNA in exosomes from healthy control pregnancies.
The study findings were limited by several factors including the impurity of the isolated placenta-derived exosomes from the umbilical cord blood plasma, which were not placenta specific, the researchers noted. Other limitations included the lack of data on different stages of lung development, and more research is needed to validate miRNAs and to explore the signally pathways involved in fetal lung development.
However, the study is the first known to demonstrate an adverse effect of GDM on fetal lung development via in vitro, ex vivo, and in vitro models, they said.
“These data highlight an emerging role of placenta-derived exosomes in the pathogenesis of fetal lung underdevelopment in GDM pregnancies, and provide a novel strategy for maternal-fetal communication,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Lung development in the fetus may be adversely affected by a mother’s gestational diabetes, based on data from in vivo, in vitro, and ex vivo studies.
Gestational diabetes mellitus (GDM) has recently been associated with fetal lung underdevelopment (FLUD) and delayed lung maturation that may lead to immediate respiratory distress in newborns and later chronic lung disease, Pengzheng Chen, PhD, of Shandong University, Jinan, China, and colleagues wrote.
Antenatal corticosteroids are considered an effective treatment for gestational fetal lung underdevelopment, but recent studies have shown adverse effects of these medications, and therefore more research is needed to identify the etiology and pathogenesis of FLUD induced by GDM, they said.
In a study published in the International Journal of Nanomedicine, the researchers collected umbilical cord blood samples from patients with GDM and matched controls at a single hospital in China.
“Using an ex vivo exosome exposure model of fetal lung explants, we observed the morphological alteration of lung explants and evaluated the expression of molecules involved in lung development,” the researchers wrote.
Fetal lung underdevelopment was more common after exposure to exosomes from the umbilical cord plasma of individuals with gestational diabetes mellitus, compared with exosomes from healthy controls.
The researchers also used mouse models to examine the effects of exosomes on fetal lung development in vivo. They found that exosomes associated with GDM impeded the growth, branching morphogenesis, and maturation of fetal lungs in mouse models. In addition, the expression of the apoptotic biomarkers known as BAX, BIM, and cleaved CASPASE-3 was up-regulated in GDMUB-exosomes and HG-exos groups, but the antiapoptotic protein BCL-2 was down-regulated; this further supported the negative impact of GDM exomes on fetal lung development, the researchers said.
The researchers then conducted miRNA sequencing, which showed that the miRNA in placenta-derived exosomes from GDM pregnancies were distinct from the miRNA in exosomes from healthy control pregnancies.
The study findings were limited by several factors including the impurity of the isolated placenta-derived exosomes from the umbilical cord blood plasma, which were not placenta specific, the researchers noted. Other limitations included the lack of data on different stages of lung development, and more research is needed to validate miRNAs and to explore the signally pathways involved in fetal lung development.
However, the study is the first known to demonstrate an adverse effect of GDM on fetal lung development via in vitro, ex vivo, and in vitro models, they said.
“These data highlight an emerging role of placenta-derived exosomes in the pathogenesis of fetal lung underdevelopment in GDM pregnancies, and provide a novel strategy for maternal-fetal communication,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM THE INTERNATIONAL JOURNAL OF NANOMEDICINE
Urine test predicts future bladder cancer 12 years before symptoms
an international team of researchers claims.
The test, if validated in further studies, has the potential to serve as a cancer screening tool for individuals at elevated risk for bladder cancer due to genetics, smoking, or from environmental exposures to known carcinogens, and it could help to reduce the frequency of unnecessary cystoscopies, say urologists who were not involved in the research.
The test involved was performed using a next-generation sequencing assay (UroAmp, Convergent Genomics, based in San Francisco) that identifies mutations in 60 genes associated with bladder cancer. New research reported at the annual congress of the European Association of Urology described the screening model that focused on 10 key genes covered in the assay.
In training and validation cohorts, the urinary comprehensive genomic profiling test accurately predicted future bladder cancer in 66% of patient urine samples, including some that had been collected more than a decade prior to being tested, reported Florence Le Calvez-Kelm, PhD, MSc, from the International Agency for Research on Cancer, Lyon, France.
“Our results provide first evidence from a population-based cohort study of preclinical urothelial cancer detection with urinary comprehensive genomic profiling,” she told the meeting.
The results were consistent both in individuals with known risk factors for bladder cancer who were undergoing cystoscopy and in those with no evidence of disease, she said.
“Research of this nature is very encouraging, as it shows that our ability to identify molecular alterations in liquid biopsies such as urine that might indicate cancer is constantly improving,” commented Joost Boormans, MD, PhD, a urologist at the Erasmus University Medical Center, Rotterdam, Netherlands, and a member of the EAU Scientific Congress Office.
“While we do need to develop more accurate diagnostics, it’s unlikely that we’ll have a mass screening program for bladder cancer in the near future,” he continued. “Where a urine test for genetic mutations could show its value is in reducing cystoscopies and scans in bladder cancer patients who are being monitored for recurrence, as well as those referred for blood in their urine. A simple urine test would be far easier for patients to undergo than invasive procedures or scans, as well as being less costly for health services.”
Dr. Le Calvez-Kelm and colleagues had previously shown that promoter mutations in the gene encoding for the enzyme telomerase reverse transcriptase (TERT) identified in urine were “promising noninvasive biomarkers” for early detection of bladder cancer.
They found that TERT mutations in urine could predict which patients were likely to develop urothelial cancer with 48% sensitivity and 100% specificity.
In the study presented at EAU23, they hypothesized that uCGP of DNA in urine could offer enhanced sensitivity for early detection of urothelial cancer.
They first used the 60-gene assay to create a training set using urine samples from 46 patients with de novo urothelial cancer, 40 with recurrent cancer, and 140 healthy controls.
They then tested the model in two validation cohorts. The first validation cohort consisted of samples from 22 patients with de novo cancer, 48 with recurrent urothelial cancer, and 96 controls from a case-control study conducted at Massachusetts General Hospital, Boston, and Ohio State University, Columbus.
The second validation cohort included 29 patients from the prospective Golestan Cohort Study who subsequently developed urothelial cancer, with 98 controls.
In all, 10 genes were identified as optimal for inclusion in a screening model, which was trained to an overall sensitivity of 88% and a 97% sensitivity for high-grade tumors, with a specificity of 94%.
In the MGH/OSU validation cohort the sensitivity of the models was 71%, and the specificity was 94%. In the Golestan cohort, the sensitivity was 66%, with a specificity of 94%. This compared favorably with the performance of the TERT-only screening model, which, as noted before, had a sensitivity of 48%, albeit with 100% specificity.
“Interestingly, when we broke down the analysis according to the lag time between urine collection and diagnosis, sensitivity increased as the time to diagnosis decreased, so the closer we got to the diagnosis, the higher was the sensitivity,” Dr. Le Calvez-Kelm said.
When the analysis was limited to urothelial cancers diagnosed within 7 years of sample collection, the sensitivity for detecting preclinical cancer improved to 86%, compared with 57% for a test of TERT promoter mutations alone.
Among the patients in the Golestan cohort, uCGP-predicted positive results were associated with a more than eightfold higher risk for worse cancer-free survival, compared with uCGP-predicted negatives (hazard ratio 8.5, P < .0001).
“Of course, further studies are needed to validate this finding and to assess the clinical utility in other longitudinal cohorts,” Dr. Le Calvez-Kelm concluded.
A version of this article first appeared on Medscape.com.
an international team of researchers claims.
The test, if validated in further studies, has the potential to serve as a cancer screening tool for individuals at elevated risk for bladder cancer due to genetics, smoking, or from environmental exposures to known carcinogens, and it could help to reduce the frequency of unnecessary cystoscopies, say urologists who were not involved in the research.
The test involved was performed using a next-generation sequencing assay (UroAmp, Convergent Genomics, based in San Francisco) that identifies mutations in 60 genes associated with bladder cancer. New research reported at the annual congress of the European Association of Urology described the screening model that focused on 10 key genes covered in the assay.
In training and validation cohorts, the urinary comprehensive genomic profiling test accurately predicted future bladder cancer in 66% of patient urine samples, including some that had been collected more than a decade prior to being tested, reported Florence Le Calvez-Kelm, PhD, MSc, from the International Agency for Research on Cancer, Lyon, France.
“Our results provide first evidence from a population-based cohort study of preclinical urothelial cancer detection with urinary comprehensive genomic profiling,” she told the meeting.
The results were consistent both in individuals with known risk factors for bladder cancer who were undergoing cystoscopy and in those with no evidence of disease, she said.
“Research of this nature is very encouraging, as it shows that our ability to identify molecular alterations in liquid biopsies such as urine that might indicate cancer is constantly improving,” commented Joost Boormans, MD, PhD, a urologist at the Erasmus University Medical Center, Rotterdam, Netherlands, and a member of the EAU Scientific Congress Office.
“While we do need to develop more accurate diagnostics, it’s unlikely that we’ll have a mass screening program for bladder cancer in the near future,” he continued. “Where a urine test for genetic mutations could show its value is in reducing cystoscopies and scans in bladder cancer patients who are being monitored for recurrence, as well as those referred for blood in their urine. A simple urine test would be far easier for patients to undergo than invasive procedures or scans, as well as being less costly for health services.”
Dr. Le Calvez-Kelm and colleagues had previously shown that promoter mutations in the gene encoding for the enzyme telomerase reverse transcriptase (TERT) identified in urine were “promising noninvasive biomarkers” for early detection of bladder cancer.
They found that TERT mutations in urine could predict which patients were likely to develop urothelial cancer with 48% sensitivity and 100% specificity.
In the study presented at EAU23, they hypothesized that uCGP of DNA in urine could offer enhanced sensitivity for early detection of urothelial cancer.
They first used the 60-gene assay to create a training set using urine samples from 46 patients with de novo urothelial cancer, 40 with recurrent cancer, and 140 healthy controls.
They then tested the model in two validation cohorts. The first validation cohort consisted of samples from 22 patients with de novo cancer, 48 with recurrent urothelial cancer, and 96 controls from a case-control study conducted at Massachusetts General Hospital, Boston, and Ohio State University, Columbus.
The second validation cohort included 29 patients from the prospective Golestan Cohort Study who subsequently developed urothelial cancer, with 98 controls.
In all, 10 genes were identified as optimal for inclusion in a screening model, which was trained to an overall sensitivity of 88% and a 97% sensitivity for high-grade tumors, with a specificity of 94%.
In the MGH/OSU validation cohort the sensitivity of the models was 71%, and the specificity was 94%. In the Golestan cohort, the sensitivity was 66%, with a specificity of 94%. This compared favorably with the performance of the TERT-only screening model, which, as noted before, had a sensitivity of 48%, albeit with 100% specificity.
“Interestingly, when we broke down the analysis according to the lag time between urine collection and diagnosis, sensitivity increased as the time to diagnosis decreased, so the closer we got to the diagnosis, the higher was the sensitivity,” Dr. Le Calvez-Kelm said.
When the analysis was limited to urothelial cancers diagnosed within 7 years of sample collection, the sensitivity for detecting preclinical cancer improved to 86%, compared with 57% for a test of TERT promoter mutations alone.
Among the patients in the Golestan cohort, uCGP-predicted positive results were associated with a more than eightfold higher risk for worse cancer-free survival, compared with uCGP-predicted negatives (hazard ratio 8.5, P < .0001).
“Of course, further studies are needed to validate this finding and to assess the clinical utility in other longitudinal cohorts,” Dr. Le Calvez-Kelm concluded.
A version of this article first appeared on Medscape.com.
an international team of researchers claims.
The test, if validated in further studies, has the potential to serve as a cancer screening tool for individuals at elevated risk for bladder cancer due to genetics, smoking, or from environmental exposures to known carcinogens, and it could help to reduce the frequency of unnecessary cystoscopies, say urologists who were not involved in the research.
The test involved was performed using a next-generation sequencing assay (UroAmp, Convergent Genomics, based in San Francisco) that identifies mutations in 60 genes associated with bladder cancer. New research reported at the annual congress of the European Association of Urology described the screening model that focused on 10 key genes covered in the assay.
In training and validation cohorts, the urinary comprehensive genomic profiling test accurately predicted future bladder cancer in 66% of patient urine samples, including some that had been collected more than a decade prior to being tested, reported Florence Le Calvez-Kelm, PhD, MSc, from the International Agency for Research on Cancer, Lyon, France.
“Our results provide first evidence from a population-based cohort study of preclinical urothelial cancer detection with urinary comprehensive genomic profiling,” she told the meeting.
The results were consistent both in individuals with known risk factors for bladder cancer who were undergoing cystoscopy and in those with no evidence of disease, she said.
“Research of this nature is very encouraging, as it shows that our ability to identify molecular alterations in liquid biopsies such as urine that might indicate cancer is constantly improving,” commented Joost Boormans, MD, PhD, a urologist at the Erasmus University Medical Center, Rotterdam, Netherlands, and a member of the EAU Scientific Congress Office.
“While we do need to develop more accurate diagnostics, it’s unlikely that we’ll have a mass screening program for bladder cancer in the near future,” he continued. “Where a urine test for genetic mutations could show its value is in reducing cystoscopies and scans in bladder cancer patients who are being monitored for recurrence, as well as those referred for blood in their urine. A simple urine test would be far easier for patients to undergo than invasive procedures or scans, as well as being less costly for health services.”
Dr. Le Calvez-Kelm and colleagues had previously shown that promoter mutations in the gene encoding for the enzyme telomerase reverse transcriptase (TERT) identified in urine were “promising noninvasive biomarkers” for early detection of bladder cancer.
They found that TERT mutations in urine could predict which patients were likely to develop urothelial cancer with 48% sensitivity and 100% specificity.
In the study presented at EAU23, they hypothesized that uCGP of DNA in urine could offer enhanced sensitivity for early detection of urothelial cancer.
They first used the 60-gene assay to create a training set using urine samples from 46 patients with de novo urothelial cancer, 40 with recurrent cancer, and 140 healthy controls.
They then tested the model in two validation cohorts. The first validation cohort consisted of samples from 22 patients with de novo cancer, 48 with recurrent urothelial cancer, and 96 controls from a case-control study conducted at Massachusetts General Hospital, Boston, and Ohio State University, Columbus.
The second validation cohort included 29 patients from the prospective Golestan Cohort Study who subsequently developed urothelial cancer, with 98 controls.
In all, 10 genes were identified as optimal for inclusion in a screening model, which was trained to an overall sensitivity of 88% and a 97% sensitivity for high-grade tumors, with a specificity of 94%.
In the MGH/OSU validation cohort the sensitivity of the models was 71%, and the specificity was 94%. In the Golestan cohort, the sensitivity was 66%, with a specificity of 94%. This compared favorably with the performance of the TERT-only screening model, which, as noted before, had a sensitivity of 48%, albeit with 100% specificity.
“Interestingly, when we broke down the analysis according to the lag time between urine collection and diagnosis, sensitivity increased as the time to diagnosis decreased, so the closer we got to the diagnosis, the higher was the sensitivity,” Dr. Le Calvez-Kelm said.
When the analysis was limited to urothelial cancers diagnosed within 7 years of sample collection, the sensitivity for detecting preclinical cancer improved to 86%, compared with 57% for a test of TERT promoter mutations alone.
Among the patients in the Golestan cohort, uCGP-predicted positive results were associated with a more than eightfold higher risk for worse cancer-free survival, compared with uCGP-predicted negatives (hazard ratio 8.5, P < .0001).
“Of course, further studies are needed to validate this finding and to assess the clinical utility in other longitudinal cohorts,” Dr. Le Calvez-Kelm concluded.
A version of this article first appeared on Medscape.com.
FROM EAU23
CDC recommends screening all adults for hepatitis B
This is the first update to HBV screening guidelines since 2008, the agency said.
“Risk-based testing alone has not identified most persons living with chronic HBV infection and is considered inefficient for providers to implement,” the authors wrote in the new guidance, published in the CDC’s Morbidity and Mortality Weekly Report. “Universal screening of adults for HBV infection is cost-effective, compared with risk-based screening and averts liver disease and death. Although a curative treatment is not yet available, early diagnosis and treatment of chronic HBV infections reduces the risk for cirrhosis, liver cancer, and death.”
Howard Lee, MD, an assistant professor in the section of gastroenterology and hepatology at Baylor College of Medicine in Houston, agreed that risk-based screening has not been effective. A universal screening approach “is the way to go,” he said. With this new screening approach, patients can get tested without having to admit that they may be at risk for a chronic disease like HIV and HBV, which can be stigmatizing, said Dr. Lee, who was not involved with making these recommendations.
An estimated 580,000 to 2.4 million individuals are living with HBV infection in the United States, and two-thirds may be unaware they are infected, according to the CDC. The virus spreads through contact with blood, semen, and other body fluids of an infected person.
The guidance now recommends using the triple panel (HBsAg, anti-HBs, total anti-HBc) for initial screening.
“It can help identify persons who have an active HBV infection and could be linked to care; have resolved infection and might be susceptible to reactivation (for example, immunosuppressed persons); are susceptible and need vaccination; or are vaccinated,” the authors wrote.
Patients with previous HBV infection can have the infection reactivated with immunosuppressive treatments, Dr. Lee said, which is why detecting prior infection via the triple panel screening is important.
Women who are pregnant should be screened, ideally, in the first trimester of each pregnancy, regardless of vaccination status or testing history. If they have already received timely triple panel screening for hepatitis B and have no new HBV exposures, pregnant women only need HBsAg screening, the guidelines state.
The guidelines also specify that higher risk groups, specifically those incarcerated or formerly incarcerated, adults with current or past hepatitis C virus infection, and those with current or past sexually transmitted infections and multiple sex partners.
People who are susceptible for infection, refuse vaccination and are at higher risk for HBV should be screened periodically, but how often they should be screened should be based on shared decision-making between the provider and patient as well as individual risk and immune status.
Additional research into the optimal frequency of periodic testing is necessary, the authors say.
“Along with vaccination strategies, universal screening of adults and appropriate testing of persons at increased risk for HBV infection will improve health outcomes, reduce the prevalence of HBV infection in the United States, and advance viral hepatitis elimination goals,” the authors wrote.
The new recommendations now contrast with the 2020 screening guidelines issued by the U.S. Preventive Services Task Force (USPSTF) that recommend risk-based screening for hepatitis B.
“When that recommendation was published, the Task Force was aligned with several other organizations, including the CDC, in supporting screening for hepatitis B in high-risk populations — and importantly, we’re all still aligned in making sure that people get the care that they need,” said Michael Barry, MD, chair of the USPSTF, in an emailed statement. “The evidence on clinical preventive services is always changing, and the Task Force aims to keep all recommendations current, updating each recommendation approximately every 5 years.”
“In the meantime, we always encourage clinicians to use their judgment as they provide care for their patients — including those who may benefit from screening for hepatitis B — and to decide together with each patient which preventive services can best help them live a long and healthy life,” Dr. Barry said.
The American Association for the Study of Liver Diseases is currently updating their HBV screening recommendations, Dr. Lee said, and he expects other professional societies to follow the CDC recommendations.
“It’s not uncommon that we see the CDC or societies making recommendations and the USPSTF following along, so hopefully that’s the case for hepatitis B as well,” he said.
The authors reported no potential conflicts of interest.
A version of this article originally appeared on Medscape.com.
This is the first update to HBV screening guidelines since 2008, the agency said.
“Risk-based testing alone has not identified most persons living with chronic HBV infection and is considered inefficient for providers to implement,” the authors wrote in the new guidance, published in the CDC’s Morbidity and Mortality Weekly Report. “Universal screening of adults for HBV infection is cost-effective, compared with risk-based screening and averts liver disease and death. Although a curative treatment is not yet available, early diagnosis and treatment of chronic HBV infections reduces the risk for cirrhosis, liver cancer, and death.”
Howard Lee, MD, an assistant professor in the section of gastroenterology and hepatology at Baylor College of Medicine in Houston, agreed that risk-based screening has not been effective. A universal screening approach “is the way to go,” he said. With this new screening approach, patients can get tested without having to admit that they may be at risk for a chronic disease like HIV and HBV, which can be stigmatizing, said Dr. Lee, who was not involved with making these recommendations.
An estimated 580,000 to 2.4 million individuals are living with HBV infection in the United States, and two-thirds may be unaware they are infected, according to the CDC. The virus spreads through contact with blood, semen, and other body fluids of an infected person.
The guidance now recommends using the triple panel (HBsAg, anti-HBs, total anti-HBc) for initial screening.
“It can help identify persons who have an active HBV infection and could be linked to care; have resolved infection and might be susceptible to reactivation (for example, immunosuppressed persons); are susceptible and need vaccination; or are vaccinated,” the authors wrote.
Patients with previous HBV infection can have the infection reactivated with immunosuppressive treatments, Dr. Lee said, which is why detecting prior infection via the triple panel screening is important.
Women who are pregnant should be screened, ideally, in the first trimester of each pregnancy, regardless of vaccination status or testing history. If they have already received timely triple panel screening for hepatitis B and have no new HBV exposures, pregnant women only need HBsAg screening, the guidelines state.
The guidelines also specify that higher risk groups, specifically those incarcerated or formerly incarcerated, adults with current or past hepatitis C virus infection, and those with current or past sexually transmitted infections and multiple sex partners.
People who are susceptible for infection, refuse vaccination and are at higher risk for HBV should be screened periodically, but how often they should be screened should be based on shared decision-making between the provider and patient as well as individual risk and immune status.
Additional research into the optimal frequency of periodic testing is necessary, the authors say.
“Along with vaccination strategies, universal screening of adults and appropriate testing of persons at increased risk for HBV infection will improve health outcomes, reduce the prevalence of HBV infection in the United States, and advance viral hepatitis elimination goals,” the authors wrote.
The new recommendations now contrast with the 2020 screening guidelines issued by the U.S. Preventive Services Task Force (USPSTF) that recommend risk-based screening for hepatitis B.
“When that recommendation was published, the Task Force was aligned with several other organizations, including the CDC, in supporting screening for hepatitis B in high-risk populations — and importantly, we’re all still aligned in making sure that people get the care that they need,” said Michael Barry, MD, chair of the USPSTF, in an emailed statement. “The evidence on clinical preventive services is always changing, and the Task Force aims to keep all recommendations current, updating each recommendation approximately every 5 years.”
“In the meantime, we always encourage clinicians to use their judgment as they provide care for their patients — including those who may benefit from screening for hepatitis B — and to decide together with each patient which preventive services can best help them live a long and healthy life,” Dr. Barry said.
The American Association for the Study of Liver Diseases is currently updating their HBV screening recommendations, Dr. Lee said, and he expects other professional societies to follow the CDC recommendations.
“It’s not uncommon that we see the CDC or societies making recommendations and the USPSTF following along, so hopefully that’s the case for hepatitis B as well,” he said.
The authors reported no potential conflicts of interest.
A version of this article originally appeared on Medscape.com.
This is the first update to HBV screening guidelines since 2008, the agency said.
“Risk-based testing alone has not identified most persons living with chronic HBV infection and is considered inefficient for providers to implement,” the authors wrote in the new guidance, published in the CDC’s Morbidity and Mortality Weekly Report. “Universal screening of adults for HBV infection is cost-effective, compared with risk-based screening and averts liver disease and death. Although a curative treatment is not yet available, early diagnosis and treatment of chronic HBV infections reduces the risk for cirrhosis, liver cancer, and death.”
Howard Lee, MD, an assistant professor in the section of gastroenterology and hepatology at Baylor College of Medicine in Houston, agreed that risk-based screening has not been effective. A universal screening approach “is the way to go,” he said. With this new screening approach, patients can get tested without having to admit that they may be at risk for a chronic disease like HIV and HBV, which can be stigmatizing, said Dr. Lee, who was not involved with making these recommendations.
An estimated 580,000 to 2.4 million individuals are living with HBV infection in the United States, and two-thirds may be unaware they are infected, according to the CDC. The virus spreads through contact with blood, semen, and other body fluids of an infected person.
The guidance now recommends using the triple panel (HBsAg, anti-HBs, total anti-HBc) for initial screening.
“It can help identify persons who have an active HBV infection and could be linked to care; have resolved infection and might be susceptible to reactivation (for example, immunosuppressed persons); are susceptible and need vaccination; or are vaccinated,” the authors wrote.
Patients with previous HBV infection can have the infection reactivated with immunosuppressive treatments, Dr. Lee said, which is why detecting prior infection via the triple panel screening is important.
Women who are pregnant should be screened, ideally, in the first trimester of each pregnancy, regardless of vaccination status or testing history. If they have already received timely triple panel screening for hepatitis B and have no new HBV exposures, pregnant women only need HBsAg screening, the guidelines state.
The guidelines also specify that higher risk groups, specifically those incarcerated or formerly incarcerated, adults with current or past hepatitis C virus infection, and those with current or past sexually transmitted infections and multiple sex partners.
People who are susceptible for infection, refuse vaccination and are at higher risk for HBV should be screened periodically, but how often they should be screened should be based on shared decision-making between the provider and patient as well as individual risk and immune status.
Additional research into the optimal frequency of periodic testing is necessary, the authors say.
“Along with vaccination strategies, universal screening of adults and appropriate testing of persons at increased risk for HBV infection will improve health outcomes, reduce the prevalence of HBV infection in the United States, and advance viral hepatitis elimination goals,” the authors wrote.
The new recommendations now contrast with the 2020 screening guidelines issued by the U.S. Preventive Services Task Force (USPSTF) that recommend risk-based screening for hepatitis B.
“When that recommendation was published, the Task Force was aligned with several other organizations, including the CDC, in supporting screening for hepatitis B in high-risk populations — and importantly, we’re all still aligned in making sure that people get the care that they need,” said Michael Barry, MD, chair of the USPSTF, in an emailed statement. “The evidence on clinical preventive services is always changing, and the Task Force aims to keep all recommendations current, updating each recommendation approximately every 5 years.”
“In the meantime, we always encourage clinicians to use their judgment as they provide care for their patients — including those who may benefit from screening for hepatitis B — and to decide together with each patient which preventive services can best help them live a long and healthy life,” Dr. Barry said.
The American Association for the Study of Liver Diseases is currently updating their HBV screening recommendations, Dr. Lee said, and he expects other professional societies to follow the CDC recommendations.
“It’s not uncommon that we see the CDC or societies making recommendations and the USPSTF following along, so hopefully that’s the case for hepatitis B as well,” he said.
The authors reported no potential conflicts of interest.
A version of this article originally appeared on Medscape.com.
Are early childhood viral infections linked with asthma?
MARSEILLE, France – It is well known that viral infections, especially respiratory syncytial virus (RSV) and rhinovirus (RV), exacerbate symptoms of asthma. But could they also play a part in triggering the onset of asthma?
The link between RSV and RV infections in early childhood and the development of asthma symptoms is well established, said Camille Taillé, MD, PhD, of the department of respiratory medicine and the rare diseases center of excellence at Bichat Hospital, Paris. But getting asthma is probably not just a matter of having a viral infection at a young age or of having a severe form of it. Gene polymorphisms, immune system disorders, and preexisting atopy are also associated with the risk of asthma. This was the focus of the 27th French-language respiratory medicine conference, held in Marseille, France.
RV and RSV
Persons with asthma are vulnerable to certain viral respiratory infections, in particular the flu and RV, which can exacerbate asthma symptoms. Inhaled corticosteroids have an overall protective effect against viral-induced exacerbations. For worsening asthma symptoms during an epidemic or pandemic, there is no contraindication to inhaled or oral corticosteroids.
Young children from the time of birth to 4 years of age are particularly susceptible to viral respiratory infections. According to data from France’s clinical surveillance network, Sentinelles, from the period covering winter 2021-2022, the rate of incidence per 100,000 inhabitants was systematically greater for the 0 to 4-year age range than for older age ranges.
Of the most common viruses that infect young children, RV, the virus that causes the common cold, is a nonenveloped RNA virus from the enterovirus family. There are 160 types, which are classified into three strains (A, B, and C). Of those strains, A and C confer the most severe infections. The virus is highly variable, which makes developing a vaccine challenging. The virus circulates year round, usually peaking in the fall and at the end of spring. RSV is an RNA virus that is classed as a respiratory virus. It comprises two serotypes: type A and B. Almost all children will have been infected with RSV by the time they are 2 years old. Epidemics occur each year during winter or in early spring in temperate climates. Vaccines are currently being developed and will soon be marketed. A monoclonal antibody (palivizumab), which targets fusion proteins of the virus, is available as prophylactic treatment for at-risk children.
RSV infection
During an RSV infection, the severe inflammation of the bronchial and alveolar wall causes acute respiratory distress. “But not all infants will develop severe forms of bronchiolitis,” said Dr. Taillé. “The risk factors for the severe form of the illness are well known: being under 6 months of age, prematurity, comorbidities (neurovascular, cardiovascular, respiratory, etc.), history of a stay in a neonatal intensive care unit at birth, living in low socioeconomic status towns, and exposure to smoking.”
Asthma development
The issue of whether or not viral diseases cause asthma has been the subject of intense debate. The studies are starting to stack up, however. They seem to show that RSV or RV infections are associated with the risk of subsequent asthma development. “For example, in a study published in 2022,” said Dr. Taillé, “in children admitted with an RSV infection, 60% of those who had been admitted to neonatal intensive care presented with symptoms of asthma between 3 and 6 years of age, compared with 18% of those who had had a milder case of RSV (admitted to nonintensive care settings). A serious RSV infection is a risk factor for later development of asthma.”
However, the link between RSV and later onset of asthma is also seen in milder cases of the infection. The American COAST study was designed to examine the effect of childhood respiratory infections on the risk of developing asthma. Researchers followed 259 newborns prospectively for 1, 3, and 6 years. To qualify, at least one parent was required to have respiratory allergies (defined as one or more positive aeroallergen skin tests) or a history of physician-diagnosed asthma. Regular samples taken during infectious episodes identified a virus in 90% of cases.
“We now know that RSV is not the only pathogen responsible for bronchiolitis. RV is often found, now that it can routinely be detected by PCR tests,” said Dr. Taillé. In the COAST study, the onset of wheezing during an RSV or RV infection in children aged 0-3 years was associated with an increased risk of asthma at 6 years of age. Globally, 28% of children infected by either virus were deemed to have asthma at 6 years of age. “There is clearly a link between having had a respiratory virus like RV or RSV and getting asthma symptoms at 6 years of age,” said Dr. Taillé. “What’s more, the effect of RV is not changed in this study by allergic sensitization.”
Many articles have been published on this topic. The results of cohort studies, from Japan to Finland and the United States, Italy, and Australia, are consistent with each other. Persons who have contracted RV or RSV are more likely to suffer from recurrent wheezing or asthma, especially if the infection is contracted in infancy or if it is severe. “Some studies even suggest that viral-induced asthma is more severe,” said Dr. Taillé. “For example, a Scottish study ... showed that children with a previous history of RSV infection had more hospital admissions and required more medication than asthmatics with no history of an RSV infection, suggesting the link between a previous history of RSV infection and the development of a more severe form of asthma.”
Reaching adulthood
Few longitudinal cohorts explore this issue in adulthood. A relatively old study reported an increased rate of asthma among adults who had required hospital admission for bronchiolitis in early childhood, as well as the effect on respiratory function. A 2023 study of the effects of respiratory illnesses in childhood reported similar findings. The authors evaluated lung structure and function via CT scans of 39 patients aged 26 years and concluded that participants who had been infected with RSV in childhood presented with increased air trapping, which is suggestive of airway abnormalities, possibly linked to a direct effect of viruses on lung development.
Mechanisms of action
“The real question is understanding if it’s the virus itself that causes asthma, or if the virus is simply uncovering underlying asthma in predisposed children,” said Dr. Taillé. From 30% to 40% of children who have had RSV will go on to develop wheezing or asthma in childhood. This observation suggests that there are factors favoring the development of asthma after infection with RSV. It has been shown that there is a genetic predisposition for RV. The roles of cigarette smoke, air pollution, environmental exposures to allergens, rapid urbanization, low vitamin D levels, low maternal omega-3 long-chain polyunsaturated fatty acid levels, maternal stress, and depression have also been highlighted.
It would seem that RSV and RV are a bit different. RV is thought to be associated with the development of asthma and wheezing, especially in people with a preexisting atopy or a reduced interferon immune response, while RSV, which occurs at a younger age and among the most vulnerable populations, seems to act independently of a person’s predisposition to allergies. RV stands out from other viral factors, owing to its tendency to create a Th2-biased inflammatory environment and its association with specific risk genes in people predisposed to asthma development (CDHR3).
Dr. Taillé has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MARSEILLE, France – It is well known that viral infections, especially respiratory syncytial virus (RSV) and rhinovirus (RV), exacerbate symptoms of asthma. But could they also play a part in triggering the onset of asthma?
The link between RSV and RV infections in early childhood and the development of asthma symptoms is well established, said Camille Taillé, MD, PhD, of the department of respiratory medicine and the rare diseases center of excellence at Bichat Hospital, Paris. But getting asthma is probably not just a matter of having a viral infection at a young age or of having a severe form of it. Gene polymorphisms, immune system disorders, and preexisting atopy are also associated with the risk of asthma. This was the focus of the 27th French-language respiratory medicine conference, held in Marseille, France.
RV and RSV
Persons with asthma are vulnerable to certain viral respiratory infections, in particular the flu and RV, which can exacerbate asthma symptoms. Inhaled corticosteroids have an overall protective effect against viral-induced exacerbations. For worsening asthma symptoms during an epidemic or pandemic, there is no contraindication to inhaled or oral corticosteroids.
Young children from the time of birth to 4 years of age are particularly susceptible to viral respiratory infections. According to data from France’s clinical surveillance network, Sentinelles, from the period covering winter 2021-2022, the rate of incidence per 100,000 inhabitants was systematically greater for the 0 to 4-year age range than for older age ranges.
Of the most common viruses that infect young children, RV, the virus that causes the common cold, is a nonenveloped RNA virus from the enterovirus family. There are 160 types, which are classified into three strains (A, B, and C). Of those strains, A and C confer the most severe infections. The virus is highly variable, which makes developing a vaccine challenging. The virus circulates year round, usually peaking in the fall and at the end of spring. RSV is an RNA virus that is classed as a respiratory virus. It comprises two serotypes: type A and B. Almost all children will have been infected with RSV by the time they are 2 years old. Epidemics occur each year during winter or in early spring in temperate climates. Vaccines are currently being developed and will soon be marketed. A monoclonal antibody (palivizumab), which targets fusion proteins of the virus, is available as prophylactic treatment for at-risk children.
RSV infection
During an RSV infection, the severe inflammation of the bronchial and alveolar wall causes acute respiratory distress. “But not all infants will develop severe forms of bronchiolitis,” said Dr. Taillé. “The risk factors for the severe form of the illness are well known: being under 6 months of age, prematurity, comorbidities (neurovascular, cardiovascular, respiratory, etc.), history of a stay in a neonatal intensive care unit at birth, living in low socioeconomic status towns, and exposure to smoking.”
Asthma development
The issue of whether or not viral diseases cause asthma has been the subject of intense debate. The studies are starting to stack up, however. They seem to show that RSV or RV infections are associated with the risk of subsequent asthma development. “For example, in a study published in 2022,” said Dr. Taillé, “in children admitted with an RSV infection, 60% of those who had been admitted to neonatal intensive care presented with symptoms of asthma between 3 and 6 years of age, compared with 18% of those who had had a milder case of RSV (admitted to nonintensive care settings). A serious RSV infection is a risk factor for later development of asthma.”
However, the link between RSV and later onset of asthma is also seen in milder cases of the infection. The American COAST study was designed to examine the effect of childhood respiratory infections on the risk of developing asthma. Researchers followed 259 newborns prospectively for 1, 3, and 6 years. To qualify, at least one parent was required to have respiratory allergies (defined as one or more positive aeroallergen skin tests) or a history of physician-diagnosed asthma. Regular samples taken during infectious episodes identified a virus in 90% of cases.
“We now know that RSV is not the only pathogen responsible for bronchiolitis. RV is often found, now that it can routinely be detected by PCR tests,” said Dr. Taillé. In the COAST study, the onset of wheezing during an RSV or RV infection in children aged 0-3 years was associated with an increased risk of asthma at 6 years of age. Globally, 28% of children infected by either virus were deemed to have asthma at 6 years of age. “There is clearly a link between having had a respiratory virus like RV or RSV and getting asthma symptoms at 6 years of age,” said Dr. Taillé. “What’s more, the effect of RV is not changed in this study by allergic sensitization.”
Many articles have been published on this topic. The results of cohort studies, from Japan to Finland and the United States, Italy, and Australia, are consistent with each other. Persons who have contracted RV or RSV are more likely to suffer from recurrent wheezing or asthma, especially if the infection is contracted in infancy or if it is severe. “Some studies even suggest that viral-induced asthma is more severe,” said Dr. Taillé. “For example, a Scottish study ... showed that children with a previous history of RSV infection had more hospital admissions and required more medication than asthmatics with no history of an RSV infection, suggesting the link between a previous history of RSV infection and the development of a more severe form of asthma.”
Reaching adulthood
Few longitudinal cohorts explore this issue in adulthood. A relatively old study reported an increased rate of asthma among adults who had required hospital admission for bronchiolitis in early childhood, as well as the effect on respiratory function. A 2023 study of the effects of respiratory illnesses in childhood reported similar findings. The authors evaluated lung structure and function via CT scans of 39 patients aged 26 years and concluded that participants who had been infected with RSV in childhood presented with increased air trapping, which is suggestive of airway abnormalities, possibly linked to a direct effect of viruses on lung development.
Mechanisms of action
“The real question is understanding if it’s the virus itself that causes asthma, or if the virus is simply uncovering underlying asthma in predisposed children,” said Dr. Taillé. From 30% to 40% of children who have had RSV will go on to develop wheezing or asthma in childhood. This observation suggests that there are factors favoring the development of asthma after infection with RSV. It has been shown that there is a genetic predisposition for RV. The roles of cigarette smoke, air pollution, environmental exposures to allergens, rapid urbanization, low vitamin D levels, low maternal omega-3 long-chain polyunsaturated fatty acid levels, maternal stress, and depression have also been highlighted.
It would seem that RSV and RV are a bit different. RV is thought to be associated with the development of asthma and wheezing, especially in people with a preexisting atopy or a reduced interferon immune response, while RSV, which occurs at a younger age and among the most vulnerable populations, seems to act independently of a person’s predisposition to allergies. RV stands out from other viral factors, owing to its tendency to create a Th2-biased inflammatory environment and its association with specific risk genes in people predisposed to asthma development (CDHR3).
Dr. Taillé has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MARSEILLE, France – It is well known that viral infections, especially respiratory syncytial virus (RSV) and rhinovirus (RV), exacerbate symptoms of asthma. But could they also play a part in triggering the onset of asthma?
The link between RSV and RV infections in early childhood and the development of asthma symptoms is well established, said Camille Taillé, MD, PhD, of the department of respiratory medicine and the rare diseases center of excellence at Bichat Hospital, Paris. But getting asthma is probably not just a matter of having a viral infection at a young age or of having a severe form of it. Gene polymorphisms, immune system disorders, and preexisting atopy are also associated with the risk of asthma. This was the focus of the 27th French-language respiratory medicine conference, held in Marseille, France.
RV and RSV
Persons with asthma are vulnerable to certain viral respiratory infections, in particular the flu and RV, which can exacerbate asthma symptoms. Inhaled corticosteroids have an overall protective effect against viral-induced exacerbations. For worsening asthma symptoms during an epidemic or pandemic, there is no contraindication to inhaled or oral corticosteroids.
Young children from the time of birth to 4 years of age are particularly susceptible to viral respiratory infections. According to data from France’s clinical surveillance network, Sentinelles, from the period covering winter 2021-2022, the rate of incidence per 100,000 inhabitants was systematically greater for the 0 to 4-year age range than for older age ranges.
Of the most common viruses that infect young children, RV, the virus that causes the common cold, is a nonenveloped RNA virus from the enterovirus family. There are 160 types, which are classified into three strains (A, B, and C). Of those strains, A and C confer the most severe infections. The virus is highly variable, which makes developing a vaccine challenging. The virus circulates year round, usually peaking in the fall and at the end of spring. RSV is an RNA virus that is classed as a respiratory virus. It comprises two serotypes: type A and B. Almost all children will have been infected with RSV by the time they are 2 years old. Epidemics occur each year during winter or in early spring in temperate climates. Vaccines are currently being developed and will soon be marketed. A monoclonal antibody (palivizumab), which targets fusion proteins of the virus, is available as prophylactic treatment for at-risk children.
RSV infection
During an RSV infection, the severe inflammation of the bronchial and alveolar wall causes acute respiratory distress. “But not all infants will develop severe forms of bronchiolitis,” said Dr. Taillé. “The risk factors for the severe form of the illness are well known: being under 6 months of age, prematurity, comorbidities (neurovascular, cardiovascular, respiratory, etc.), history of a stay in a neonatal intensive care unit at birth, living in low socioeconomic status towns, and exposure to smoking.”
Asthma development
The issue of whether or not viral diseases cause asthma has been the subject of intense debate. The studies are starting to stack up, however. They seem to show that RSV or RV infections are associated with the risk of subsequent asthma development. “For example, in a study published in 2022,” said Dr. Taillé, “in children admitted with an RSV infection, 60% of those who had been admitted to neonatal intensive care presented with symptoms of asthma between 3 and 6 years of age, compared with 18% of those who had had a milder case of RSV (admitted to nonintensive care settings). A serious RSV infection is a risk factor for later development of asthma.”
However, the link between RSV and later onset of asthma is also seen in milder cases of the infection. The American COAST study was designed to examine the effect of childhood respiratory infections on the risk of developing asthma. Researchers followed 259 newborns prospectively for 1, 3, and 6 years. To qualify, at least one parent was required to have respiratory allergies (defined as one or more positive aeroallergen skin tests) or a history of physician-diagnosed asthma. Regular samples taken during infectious episodes identified a virus in 90% of cases.
“We now know that RSV is not the only pathogen responsible for bronchiolitis. RV is often found, now that it can routinely be detected by PCR tests,” said Dr. Taillé. In the COAST study, the onset of wheezing during an RSV or RV infection in children aged 0-3 years was associated with an increased risk of asthma at 6 years of age. Globally, 28% of children infected by either virus were deemed to have asthma at 6 years of age. “There is clearly a link between having had a respiratory virus like RV or RSV and getting asthma symptoms at 6 years of age,” said Dr. Taillé. “What’s more, the effect of RV is not changed in this study by allergic sensitization.”
Many articles have been published on this topic. The results of cohort studies, from Japan to Finland and the United States, Italy, and Australia, are consistent with each other. Persons who have contracted RV or RSV are more likely to suffer from recurrent wheezing or asthma, especially if the infection is contracted in infancy or if it is severe. “Some studies even suggest that viral-induced asthma is more severe,” said Dr. Taillé. “For example, a Scottish study ... showed that children with a previous history of RSV infection had more hospital admissions and required more medication than asthmatics with no history of an RSV infection, suggesting the link between a previous history of RSV infection and the development of a more severe form of asthma.”
Reaching adulthood
Few longitudinal cohorts explore this issue in adulthood. A relatively old study reported an increased rate of asthma among adults who had required hospital admission for bronchiolitis in early childhood, as well as the effect on respiratory function. A 2023 study of the effects of respiratory illnesses in childhood reported similar findings. The authors evaluated lung structure and function via CT scans of 39 patients aged 26 years and concluded that participants who had been infected with RSV in childhood presented with increased air trapping, which is suggestive of airway abnormalities, possibly linked to a direct effect of viruses on lung development.
Mechanisms of action
“The real question is understanding if it’s the virus itself that causes asthma, or if the virus is simply uncovering underlying asthma in predisposed children,” said Dr. Taillé. From 30% to 40% of children who have had RSV will go on to develop wheezing or asthma in childhood. This observation suggests that there are factors favoring the development of asthma after infection with RSV. It has been shown that there is a genetic predisposition for RV. The roles of cigarette smoke, air pollution, environmental exposures to allergens, rapid urbanization, low vitamin D levels, low maternal omega-3 long-chain polyunsaturated fatty acid levels, maternal stress, and depression have also been highlighted.
It would seem that RSV and RV are a bit different. RV is thought to be associated with the development of asthma and wheezing, especially in people with a preexisting atopy or a reduced interferon immune response, while RSV, which occurs at a younger age and among the most vulnerable populations, seems to act independently of a person’s predisposition to allergies. RV stands out from other viral factors, owing to its tendency to create a Th2-biased inflammatory environment and its association with specific risk genes in people predisposed to asthma development (CDHR3).
Dr. Taillé has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA warns about anaphylaxis after false-negative allergen tests
The Food and Drug Administration has issued a warning about the potential for patients to experience anaphylactic reactions after a negative skin test with any allergenic extract used to diagnose food allergies.
The FDA is requiring that an anaphylaxis warning after false-negative food allergen skin test results be added to the labels of these products in light of reports to the FDA’s Adverse Event Reporting System (FAERS), according to a March 3 statement.
The action follows the recognition of an increase in adverse event reports of false-negative test results with specific lots of “ALK-Abello’s Allergenic Extract-Peanut (Arachis hypogaea) – For Diagnostic Use Only.” Some of these reports “were associated with life-threatening anaphylaxis from subsequent exposure to peanut,” according to the statement. “FDA determined that the risk of anaphylaxis following false-negative food allergen skin test results is applicable to all allergenic extracts for the diagnosis of food allergies,” the statement notes.
To date, four lots of allergenic extracts have been voluntarily withdrawn from the market by the manufacturer, in November and December 2022, and should not be used.
Although some allergenic extracts are standardized, those used in the diagnosis of food allergy currently licensed by the FDA for use in the United States are nonstandardized, so potency may vary by lot.
The FDA advises health care professionals to consider confirming a negative skin test with serologic testing for peanut-specific IgE or conducting a medically supervised oral food challenge in patients, “based on the patient’s clinical history and the index of suspicion.”
The FDA also urges patients to discuss negative food allergen skin test results with their health care providers to determine the possible need for additional testing and to review the symptoms of a severe allergic reaction.
Any adverse events or side effects associated with allergenic products should be reported to the FDA via the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has issued a warning about the potential for patients to experience anaphylactic reactions after a negative skin test with any allergenic extract used to diagnose food allergies.
The FDA is requiring that an anaphylaxis warning after false-negative food allergen skin test results be added to the labels of these products in light of reports to the FDA’s Adverse Event Reporting System (FAERS), according to a March 3 statement.
The action follows the recognition of an increase in adverse event reports of false-negative test results with specific lots of “ALK-Abello’s Allergenic Extract-Peanut (Arachis hypogaea) – For Diagnostic Use Only.” Some of these reports “were associated with life-threatening anaphylaxis from subsequent exposure to peanut,” according to the statement. “FDA determined that the risk of anaphylaxis following false-negative food allergen skin test results is applicable to all allergenic extracts for the diagnosis of food allergies,” the statement notes.
To date, four lots of allergenic extracts have been voluntarily withdrawn from the market by the manufacturer, in November and December 2022, and should not be used.
Although some allergenic extracts are standardized, those used in the diagnosis of food allergy currently licensed by the FDA for use in the United States are nonstandardized, so potency may vary by lot.
The FDA advises health care professionals to consider confirming a negative skin test with serologic testing for peanut-specific IgE or conducting a medically supervised oral food challenge in patients, “based on the patient’s clinical history and the index of suspicion.”
The FDA also urges patients to discuss negative food allergen skin test results with their health care providers to determine the possible need for additional testing and to review the symptoms of a severe allergic reaction.
Any adverse events or side effects associated with allergenic products should be reported to the FDA via the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has issued a warning about the potential for patients to experience anaphylactic reactions after a negative skin test with any allergenic extract used to diagnose food allergies.
The FDA is requiring that an anaphylaxis warning after false-negative food allergen skin test results be added to the labels of these products in light of reports to the FDA’s Adverse Event Reporting System (FAERS), according to a March 3 statement.
The action follows the recognition of an increase in adverse event reports of false-negative test results with specific lots of “ALK-Abello’s Allergenic Extract-Peanut (Arachis hypogaea) – For Diagnostic Use Only.” Some of these reports “were associated with life-threatening anaphylaxis from subsequent exposure to peanut,” according to the statement. “FDA determined that the risk of anaphylaxis following false-negative food allergen skin test results is applicable to all allergenic extracts for the diagnosis of food allergies,” the statement notes.
To date, four lots of allergenic extracts have been voluntarily withdrawn from the market by the manufacturer, in November and December 2022, and should not be used.
Although some allergenic extracts are standardized, those used in the diagnosis of food allergy currently licensed by the FDA for use in the United States are nonstandardized, so potency may vary by lot.
The FDA advises health care professionals to consider confirming a negative skin test with serologic testing for peanut-specific IgE or conducting a medically supervised oral food challenge in patients, “based on the patient’s clinical history and the index of suspicion.”
The FDA also urges patients to discuss negative food allergen skin test results with their health care providers to determine the possible need for additional testing and to review the symptoms of a severe allergic reaction.
Any adverse events or side effects associated with allergenic products should be reported to the FDA via the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
A version of this article first appeared on Medscape.com.
Opioid overdose is an important cause of postpartum death
(OUD), according to research published in Obstetrics and Gynecology.
Opioid overdose deaths account for up to 10% of pregnancy-associated deaths in the United States, and 75% of the deliveries of women with OUD are covered by Medicaid, according to lead author Elizabeth Suarez, PhD, MPH, with the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital and Harvard Medical School in Boston, and colleagues.
Nearly 5 million deliveries studied
Researchers studied claims data from Medicaid and the National Death Index database in the United States from 2006 to 2013 for 4,972,061 deliveries. They also identified a subgroup of women with a documented history of OUD in the 3 months before delivery.
They found the incidence of postpartum opioid overdose deaths was 5.4 per 100,000 deliveries (95% confidence interval, 4.5-6.4) among all in the study and 118 per 100,000 (95% CI, 84-163) among individuals with OUD.
Incidence of all-cause postpartum death was six times higher in women with OUD than in all the women studied. Common causes of death of those with OUD were other drug- and alcohol-related deaths (47/100,000); suicide (26/100,000); and other injuries, including accidents and falls (33/100,000).
Risk factors strongly linked with postpartum opioid overdose death included mental health and other substance use disorders.
Medication significantly lowers death risk
The authors also documented the benefit of buprenorphine or methadone for OUD.
For women with OUD who used medication to treat OUD post partum, odds of opioid overdose death were 60% lower (odds ratio, 0.4; 95% CI 0.1-0.9).
As important as use of medication, Marcela Smid, MD, MS, writes in an accompanying editorial, is noting that 80% of the women in this study who died of opioid overdoses had contact with a health care provider before death.
“Both of these results indicate that we have the means and opportunity to prevent these deaths,” writes Dr. Smid, with the division of maternal fetal medicine, University of Utah Health in Salt Lake City.
Dismal numbers on ob.gyns. trained to prescribe medications
She points out some barriers, however. Most clinicians, she notes, lack time and training to prescribe buprenorphine, and in 2019, fewer than 2% of ob.gyns. who accept Medicaid were able to prescribe it.
Her charge to ob.gyns.: “We need to help identify individuals who are at high risk of OUD or opioid overdose by screening.” A validated screening tool should be used at prenatal and postpartum appointments.
On a bigger scale, she urges Medicaid to be expanded for a full year post partum through the American Rescue Act’s State Plan Amendment, something only 28 states and Washington, D.C., have done so far.
Dr. Smid points out some good news, however: President Joe Biden signed the Consolidated Appropriations Act 2023, which eliminated the “X” waiver.
Now all clinicians who have a Drug Enforcement Administration registration that includes Schedule III authority can prescribe buprenorphine for OUD if applicable state law allows it.
But that calls for medical schools and residency programs to prioritize addiction medicine as a core competency, Dr. Smid says.
Getting naloxone to patients, families
One of the potential interventions the study authors suggest is providing naloxone prescriptions and training to pregnant and postpartum women who have a substance use history and to their partners and significant others.
However, Mishka Terplan, MD, MPH, told this publication, “It’s one thing to write a prescription; it’s another thing for the person to actually get the medication.” He is medical director of the Friends Research Institute in Baltimore, an ob.gyn. who specializes in addiction medicine.
“What can we do?” We can think about how to get naloxone into people’s hands at discharge from the hospital after they give birth, instead of prescribing. That would mean that health systems need to prioritize this, he said. “We give people discharge medications all the time.”
Still, naloxone can’t be seen as the answer, he said.
He compares it to defibrillators in public places, which are for rescues, not reversing a population problem.
“Some people think that naloxone reversals are doing something about OUD. It’s doing about as much about OUD as defibrillators do for cardiovascular disease,” he said.
The best help, he says, will be continuation of treatment.
“Addiction is a chronic condition,” he says, “but often we only provide episodic care. We see that particularly in pregnancy. Once the pregnancy is finished, there’s not categorical continuation of insurance.”
Even if you do have insurance, it’s hard to find a clinic that’s family friendly, he notes. “You might not feel comfortable taking your newborn and standing in line in the morning to get your daily methodone dose. We have to make those environments more welcoming.”
Problem probably understated
He also says that though the study was well done given the data available, he’s frustrated that researchers still have to depend on billing data and can’t capture factors such as child care availability, living wages, and continuation of health insurance. Additionally, not everyone is coded correctly for OUD.
“It’s all Medicaid, so it’s only people who continued with care,” he pointed out. That means these numbers may actually underrepresent the problem.
Still, he says it’s important to realize the magnitude of deaths this study does highlight in this population.
In people with OUD in the postpartum period, the deaths are more than 1 in 1,000.
“That should be alarming,” Dr. Terplan said. “That’s a very big number from a public health perspective.”
Coauthor Kathryn J. Gray received payment from Aetion Inc., Roche, and BillionToOne. Funds were paid to the University of Utah for Dr. Smid from Alydia Inc. for being the site principal investigator for a study of the JADA device, and from Gilead for Dr. Smid’s study of hepatitis C in pregnancy; she was also a consultant for Organon and Rhia Ventures. Dr. Terplan reports no relevant financial relationships.
(OUD), according to research published in Obstetrics and Gynecology.
Opioid overdose deaths account for up to 10% of pregnancy-associated deaths in the United States, and 75% of the deliveries of women with OUD are covered by Medicaid, according to lead author Elizabeth Suarez, PhD, MPH, with the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital and Harvard Medical School in Boston, and colleagues.
Nearly 5 million deliveries studied
Researchers studied claims data from Medicaid and the National Death Index database in the United States from 2006 to 2013 for 4,972,061 deliveries. They also identified a subgroup of women with a documented history of OUD in the 3 months before delivery.
They found the incidence of postpartum opioid overdose deaths was 5.4 per 100,000 deliveries (95% confidence interval, 4.5-6.4) among all in the study and 118 per 100,000 (95% CI, 84-163) among individuals with OUD.
Incidence of all-cause postpartum death was six times higher in women with OUD than in all the women studied. Common causes of death of those with OUD were other drug- and alcohol-related deaths (47/100,000); suicide (26/100,000); and other injuries, including accidents and falls (33/100,000).
Risk factors strongly linked with postpartum opioid overdose death included mental health and other substance use disorders.
Medication significantly lowers death risk
The authors also documented the benefit of buprenorphine or methadone for OUD.
For women with OUD who used medication to treat OUD post partum, odds of opioid overdose death were 60% lower (odds ratio, 0.4; 95% CI 0.1-0.9).
As important as use of medication, Marcela Smid, MD, MS, writes in an accompanying editorial, is noting that 80% of the women in this study who died of opioid overdoses had contact with a health care provider before death.
“Both of these results indicate that we have the means and opportunity to prevent these deaths,” writes Dr. Smid, with the division of maternal fetal medicine, University of Utah Health in Salt Lake City.
Dismal numbers on ob.gyns. trained to prescribe medications
She points out some barriers, however. Most clinicians, she notes, lack time and training to prescribe buprenorphine, and in 2019, fewer than 2% of ob.gyns. who accept Medicaid were able to prescribe it.
Her charge to ob.gyns.: “We need to help identify individuals who are at high risk of OUD or opioid overdose by screening.” A validated screening tool should be used at prenatal and postpartum appointments.
On a bigger scale, she urges Medicaid to be expanded for a full year post partum through the American Rescue Act’s State Plan Amendment, something only 28 states and Washington, D.C., have done so far.
Dr. Smid points out some good news, however: President Joe Biden signed the Consolidated Appropriations Act 2023, which eliminated the “X” waiver.
Now all clinicians who have a Drug Enforcement Administration registration that includes Schedule III authority can prescribe buprenorphine for OUD if applicable state law allows it.
But that calls for medical schools and residency programs to prioritize addiction medicine as a core competency, Dr. Smid says.
Getting naloxone to patients, families
One of the potential interventions the study authors suggest is providing naloxone prescriptions and training to pregnant and postpartum women who have a substance use history and to their partners and significant others.
However, Mishka Terplan, MD, MPH, told this publication, “It’s one thing to write a prescription; it’s another thing for the person to actually get the medication.” He is medical director of the Friends Research Institute in Baltimore, an ob.gyn. who specializes in addiction medicine.
“What can we do?” We can think about how to get naloxone into people’s hands at discharge from the hospital after they give birth, instead of prescribing. That would mean that health systems need to prioritize this, he said. “We give people discharge medications all the time.”
Still, naloxone can’t be seen as the answer, he said.
He compares it to defibrillators in public places, which are for rescues, not reversing a population problem.
“Some people think that naloxone reversals are doing something about OUD. It’s doing about as much about OUD as defibrillators do for cardiovascular disease,” he said.
The best help, he says, will be continuation of treatment.
“Addiction is a chronic condition,” he says, “but often we only provide episodic care. We see that particularly in pregnancy. Once the pregnancy is finished, there’s not categorical continuation of insurance.”
Even if you do have insurance, it’s hard to find a clinic that’s family friendly, he notes. “You might not feel comfortable taking your newborn and standing in line in the morning to get your daily methodone dose. We have to make those environments more welcoming.”
Problem probably understated
He also says that though the study was well done given the data available, he’s frustrated that researchers still have to depend on billing data and can’t capture factors such as child care availability, living wages, and continuation of health insurance. Additionally, not everyone is coded correctly for OUD.
“It’s all Medicaid, so it’s only people who continued with care,” he pointed out. That means these numbers may actually underrepresent the problem.
Still, he says it’s important to realize the magnitude of deaths this study does highlight in this population.
In people with OUD in the postpartum period, the deaths are more than 1 in 1,000.
“That should be alarming,” Dr. Terplan said. “That’s a very big number from a public health perspective.”
Coauthor Kathryn J. Gray received payment from Aetion Inc., Roche, and BillionToOne. Funds were paid to the University of Utah for Dr. Smid from Alydia Inc. for being the site principal investigator for a study of the JADA device, and from Gilead for Dr. Smid’s study of hepatitis C in pregnancy; she was also a consultant for Organon and Rhia Ventures. Dr. Terplan reports no relevant financial relationships.
(OUD), according to research published in Obstetrics and Gynecology.
Opioid overdose deaths account for up to 10% of pregnancy-associated deaths in the United States, and 75% of the deliveries of women with OUD are covered by Medicaid, according to lead author Elizabeth Suarez, PhD, MPH, with the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital and Harvard Medical School in Boston, and colleagues.
Nearly 5 million deliveries studied
Researchers studied claims data from Medicaid and the National Death Index database in the United States from 2006 to 2013 for 4,972,061 deliveries. They also identified a subgroup of women with a documented history of OUD in the 3 months before delivery.
They found the incidence of postpartum opioid overdose deaths was 5.4 per 100,000 deliveries (95% confidence interval, 4.5-6.4) among all in the study and 118 per 100,000 (95% CI, 84-163) among individuals with OUD.
Incidence of all-cause postpartum death was six times higher in women with OUD than in all the women studied. Common causes of death of those with OUD were other drug- and alcohol-related deaths (47/100,000); suicide (26/100,000); and other injuries, including accidents and falls (33/100,000).
Risk factors strongly linked with postpartum opioid overdose death included mental health and other substance use disorders.
Medication significantly lowers death risk
The authors also documented the benefit of buprenorphine or methadone for OUD.
For women with OUD who used medication to treat OUD post partum, odds of opioid overdose death were 60% lower (odds ratio, 0.4; 95% CI 0.1-0.9).
As important as use of medication, Marcela Smid, MD, MS, writes in an accompanying editorial, is noting that 80% of the women in this study who died of opioid overdoses had contact with a health care provider before death.
“Both of these results indicate that we have the means and opportunity to prevent these deaths,” writes Dr. Smid, with the division of maternal fetal medicine, University of Utah Health in Salt Lake City.
Dismal numbers on ob.gyns. trained to prescribe medications
She points out some barriers, however. Most clinicians, she notes, lack time and training to prescribe buprenorphine, and in 2019, fewer than 2% of ob.gyns. who accept Medicaid were able to prescribe it.
Her charge to ob.gyns.: “We need to help identify individuals who are at high risk of OUD or opioid overdose by screening.” A validated screening tool should be used at prenatal and postpartum appointments.
On a bigger scale, she urges Medicaid to be expanded for a full year post partum through the American Rescue Act’s State Plan Amendment, something only 28 states and Washington, D.C., have done so far.
Dr. Smid points out some good news, however: President Joe Biden signed the Consolidated Appropriations Act 2023, which eliminated the “X” waiver.
Now all clinicians who have a Drug Enforcement Administration registration that includes Schedule III authority can prescribe buprenorphine for OUD if applicable state law allows it.
But that calls for medical schools and residency programs to prioritize addiction medicine as a core competency, Dr. Smid says.
Getting naloxone to patients, families
One of the potential interventions the study authors suggest is providing naloxone prescriptions and training to pregnant and postpartum women who have a substance use history and to their partners and significant others.
However, Mishka Terplan, MD, MPH, told this publication, “It’s one thing to write a prescription; it’s another thing for the person to actually get the medication.” He is medical director of the Friends Research Institute in Baltimore, an ob.gyn. who specializes in addiction medicine.
“What can we do?” We can think about how to get naloxone into people’s hands at discharge from the hospital after they give birth, instead of prescribing. That would mean that health systems need to prioritize this, he said. “We give people discharge medications all the time.”
Still, naloxone can’t be seen as the answer, he said.
He compares it to defibrillators in public places, which are for rescues, not reversing a population problem.
“Some people think that naloxone reversals are doing something about OUD. It’s doing about as much about OUD as defibrillators do for cardiovascular disease,” he said.
The best help, he says, will be continuation of treatment.
“Addiction is a chronic condition,” he says, “but often we only provide episodic care. We see that particularly in pregnancy. Once the pregnancy is finished, there’s not categorical continuation of insurance.”
Even if you do have insurance, it’s hard to find a clinic that’s family friendly, he notes. “You might not feel comfortable taking your newborn and standing in line in the morning to get your daily methodone dose. We have to make those environments more welcoming.”
Problem probably understated
He also says that though the study was well done given the data available, he’s frustrated that researchers still have to depend on billing data and can’t capture factors such as child care availability, living wages, and continuation of health insurance. Additionally, not everyone is coded correctly for OUD.
“It’s all Medicaid, so it’s only people who continued with care,” he pointed out. That means these numbers may actually underrepresent the problem.
Still, he says it’s important to realize the magnitude of deaths this study does highlight in this population.
In people with OUD in the postpartum period, the deaths are more than 1 in 1,000.
“That should be alarming,” Dr. Terplan said. “That’s a very big number from a public health perspective.”
Coauthor Kathryn J. Gray received payment from Aetion Inc., Roche, and BillionToOne. Funds were paid to the University of Utah for Dr. Smid from Alydia Inc. for being the site principal investigator for a study of the JADA device, and from Gilead for Dr. Smid’s study of hepatitis C in pregnancy; she was also a consultant for Organon and Rhia Ventures. Dr. Terplan reports no relevant financial relationships.
FROM OBSTETRICS AND GYNECOLOGY
Experts share early details prescribing avacopan for ANCA-associated vasculitis
When the Food and Drug Administration approved avacopan (Tavneos) as an adjunctive treatment for severe, active antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitis (AAV) in October 2021, the oral complement C5a receptor inhibitor was hailed by its developer, ChemoCentryx, as a “new hope” for patients with the disease.
But avacopan’s novelty as a new drug for the rare diseases granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA), coupled with its approval as an adjunctive to standard therapy, including glucocorticoids, rather than strictly as a glucocorticoid-sparing agent as it was tested, has so far led to little reported real-world experience with the drug.
In the phase 3 ADVOCATE trial, the pivotal trial that served as the basis for avacopan’s approval, 331 patients with active newly diagnosed or relapsing GPA or MPA received either avacopan or an oral prednisone taper over 20 weeks on a background of cyclophosphamide followed by azathioprine or rituximab. The results of the trial showed avacopan was noninferior to the group that received prednisone taper for remission at 26 weeks and superior to prednisone taper for sustained remission at 52 weeks, but the FDA was concerned that its complex design made it difficult to define the clinically meaningful benefit of avacopan and its role in the management of AAV.
The FDA noted that, in the avacopan arm of the trial, 86% of patients received glucocorticoids outside of the study protocol. Despite this, avacopan reduced the cumulative glucocorticoid dose over the trial’s 52 weeks by nearly two-thirds, compared with the prednisone group (1,349 mg vs. 3,655 mg).
The data also indicate a higher sustained remission rate at 52 weeks in patients who received induction with rituximab, compared with cyclophosphamide. But trial did not include a maintenance therapy dose of rituximab and is thereby not a good comparison against the standard of care, the FDA said. (ADVOCATE began enrolling patients prior to the FDA's 2018 approval of an expanded indication for patients with GPA or MPA who have achieved disease control after induction treatment.)
At the FDA’s Arthritis Advisory Committee meeting in May 2021, committee members were split on whether to recommend avacopan for approval. The committee voted 9-9 on whether the ADVOCATE trial showed efficacy supporting approval of avacopan, 10-8 in favor of whether the drug’s safety profile supported approval, and 10-8 in favor of the overall benefit-risk profile of avacopan for approval. But rather than give an indication to avacopan to reduce the use of glucocorticoids in adults with GPA or MPA, the agency approved avacopan as an adjunctive treatment for severe, active disease, noting in particular that avacopan “does not eliminate glucocorticoid use.”
The European Union’s marketing authorization for avacopan states its indication for use in combination with a rituximab or cyclophosphamide regimen for the treatment of adult patients with severe, active GPA or MPA and does not mention a role for reducing glucocorticoids. Avacopan will appear in forthcoming guidelines on management of AAV released by the European Alliance of Associations for Rheumatology.
In North America, the Canadian Vasculitis Research Network recently released an addendum to their guidelines on AAV specifically for avacopan, which includes recommendations to consider adding oral avacopan (30 mg twice daily) for induction of remission in patients with new or relapsing GPA or MPA who are also receiving cyclophosphamide or rituximab. The guidelines also recommend clinicians consider a glucocorticoid tapering schedule that aims for discontinuation at 4 weeks, and continuing avacopan for at least 1 year after induction therapy. The American College of Rheumatology guideline for AAV management, updated in 2021, acknowledges avacopan but did not consider its inclusion prior to FDA approval.
There have been few real-world studies of how patients with AAV are responding to avacopan, but recent studies from researchers in the Netherlands and in France have evaluated prednisone tapering and clinical outcomes.
Anisha B. Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago, said those real-world studies “seemed to re-enforce the findings from the ADVOCATE study demonstrating the efficacy of avacopan in severe disease with steroid-sparing effects.”
However, Carol Langford, MD, MHS, director of the Center for Vasculitis Care and Research at the Cleveland Clinic, emphasized caution is needed when drawing conclusions about avacopan use outside formal studies.
“We are all interested in what other settings this might be used. I think those are things that really require formal investigation to really try and understand better as far as through a study process,” she said.
Prescribing experience with avacopan
A spokesperson from Amgen, which recently acquired ChemoCentryx, said in an interview that over 800 physicians in the United States have prescribed avacopan to patients with new or relapsing ANCA-associated vasculitis as induction or maintenance treatment, and physicians have reported outcomes consistent with the ADVOCATE trial.
Many rheumatologists are likely familiar with avacopan but are not used to prescribing it, said Lindsay S. Lally, MD, a rheumatologist with Hospital for Special Surgery in New York.
“Rituximab was approved for GPA and MPA a decade ago at this point. It was a drug that we as rheumatologists were used to using. We used it for other indications. Avacopan is a totally new drug, a new mechanism of action, so there’s not a lot of extractable data that we have in terms of comfort with the drug, and so I think that’s one of the biggest hurdles,” she said.
Mehrnaz Hojjati, MD, a rheumatologist with Loma Linda (Calif.) University Health, said that, when the FDA approved avacopan, it was an “exciting time” in her practice. “I have used avacopan now in a handful of my patients with severe ANCA-associated vasculitis, and the results are similar to what [was] reported in the ADVOCATE trial.”
Amgen offers help for clinicians in obtaining avacopan for patients, financial assistance for patients, and support in navigating insurance, which several rheumatologists noted was important for patients. Dr. Langford said the process of working with the manufacturer to get avacopan while insurance information is being processed has been “fairly smooth.”
“Certainly, the ability to get a very rapid 30-day supply with the goal of trying to initiate this as early as possible in the disease process has been helpful,” she said.
In Dr. Dua’s experience, while there were “some glitches or difficulty for providers early on” in how to access and prescribe avacopan, since then “it has been much easier to obtain the medication with the first month being provided to patients free while the authorization process is managed.”
Prescribing avacopan from inpatient pharmacies has been more challenging, she said. “The inpatient side is trickier because each hospital system has their own pharmacy system and regulations that have to be navigated. For outpatients, all the provider needs to do is fill out the start form available on their website, have the patients sign it, and then have it sent in.”
Concerns about affordability, insurance approval
Another consideration is cost, with avacopan having an estimated price of $150,000-$200,000 per patient per year.
Dr. Hojjati noted that, while it is easy to prescribe, avacopan is hard to get approved through insurance. “We face the same challenge every time a new medication comes to the market on how to convince the payers to pay for it given higher prices,” she said.
Rheumatologist Michael Putman, MD, MSCI, assistant professor of medicine at the Medical College of Wisconsin, Milwaukee, also acknowledged some difficulties in prescribing the medication. “The insurance companies have no interest in spending $150,000 on a drug that they know nothing about, and patients are a little hesitant to take it because it’s just so new,” he said.
While Dr. Lally said avacopan has not been difficult to get for patients with commercial insurance, reimbursement through Medicare has been problematic. “In many of the Medicare patients it has not really been a feasible option for them to be on the drug for the year of therapy.”
Patient response
Dr. Dua said almost all her patients with new or relapsing AAV who require induction are being prescribed avacopan, and that the medication is well tolerated. “The remission and ability to wean prednisone has really paralleled the findings from the clinical trial.”
In her practice, Dr. Hojjati starts patients on avacopan immediately after discharge from the hospital after a major vasculitis flare requiring high-dose glucocorticoids. “Avacopan does not eliminate/replace GC [glucocorticoid] use but has a notable GC-sparing effect and assists in rapid tapering of the GC while treating our severe ANCA-associated vasculitis patients,” she said.
Dr. Lally said her patients are tolerating avacopan well and hasn’t seen any of the safety signals seen in the trial, including liver function abnormalities. She has treated about 20-25 patients with avacopan.
Dr. Putman noted that he has treated about five patients with avacopan but hasn’t seen dramatic efficacy or side effects in his practice, compared with standard therapy.
Unanswered questions about avacopan
A key unanswered question with avacopan is the timeline for tapering glucocorticoids once patients start treatment. “I would like to see much more data on how prednisone is being tapered in clinical practice as well as outcomes in patients who are treated with the standard of care second dose of rituximab at 6 months,” Dr. Dua said.
Dr. Lally noted she has tried to expedite the steroid taper in her patients. “That’s really where I feel this drug is going to have most relevance, is getting it started early in active disease and getting patients off of the reliance on high doses of oral steroids. I have been able to see that in practice, and I do think ultimately that’s going to lead to better outcomes and quality of life for these patients.”
Of the rheumatologists Dr. Lally has spoken to about avacopan, there is “some confusion about what type of patients are appropriate, [and] how sick or not sick the patient needs to be.”
Dr. Putman noted he is unsure which of his patients should be receiving avacopan. “I don’t totally have a sense for where avacopan stands and how often we should be using it” outside of patients with severe disease. He added that the drug is still trying to find a niche because most patients with AAV who take rituximab and steroids get better without additional treatments.
“I think we do a pretty good job treating these diseases even in the preavacopan era. But it’s really a matter of how to really optimize these outcomes, reduce damage, reduce steroid-related and treatment-related toxicity for our patients,” Dr. Lally said.
Dr. Dua reported being a consultant and serving on advisory boards for ChemoCentryx; she was also a site principal investigator for the ADVOCATE trial. Dr. Hojjati reported being on the speaker’s bureau for Amgen. Dr. Langford reported being an investigator in the ADVOCATE trial, and her institution received funding to conduct the trial. Dr. Lally reported being a consultant for Amgen on avacopan. Dr. Putman reported no relevant financial disclosures.
*This story was updated 3/15/2023.
When the Food and Drug Administration approved avacopan (Tavneos) as an adjunctive treatment for severe, active antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitis (AAV) in October 2021, the oral complement C5a receptor inhibitor was hailed by its developer, ChemoCentryx, as a “new hope” for patients with the disease.
But avacopan’s novelty as a new drug for the rare diseases granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA), coupled with its approval as an adjunctive to standard therapy, including glucocorticoids, rather than strictly as a glucocorticoid-sparing agent as it was tested, has so far led to little reported real-world experience with the drug.
In the phase 3 ADVOCATE trial, the pivotal trial that served as the basis for avacopan’s approval, 331 patients with active newly diagnosed or relapsing GPA or MPA received either avacopan or an oral prednisone taper over 20 weeks on a background of cyclophosphamide followed by azathioprine or rituximab. The results of the trial showed avacopan was noninferior to the group that received prednisone taper for remission at 26 weeks and superior to prednisone taper for sustained remission at 52 weeks, but the FDA was concerned that its complex design made it difficult to define the clinically meaningful benefit of avacopan and its role in the management of AAV.
The FDA noted that, in the avacopan arm of the trial, 86% of patients received glucocorticoids outside of the study protocol. Despite this, avacopan reduced the cumulative glucocorticoid dose over the trial’s 52 weeks by nearly two-thirds, compared with the prednisone group (1,349 mg vs. 3,655 mg).
The data also indicate a higher sustained remission rate at 52 weeks in patients who received induction with rituximab, compared with cyclophosphamide. But trial did not include a maintenance therapy dose of rituximab and is thereby not a good comparison against the standard of care, the FDA said. (ADVOCATE began enrolling patients prior to the FDA's 2018 approval of an expanded indication for patients with GPA or MPA who have achieved disease control after induction treatment.)
At the FDA’s Arthritis Advisory Committee meeting in May 2021, committee members were split on whether to recommend avacopan for approval. The committee voted 9-9 on whether the ADVOCATE trial showed efficacy supporting approval of avacopan, 10-8 in favor of whether the drug’s safety profile supported approval, and 10-8 in favor of the overall benefit-risk profile of avacopan for approval. But rather than give an indication to avacopan to reduce the use of glucocorticoids in adults with GPA or MPA, the agency approved avacopan as an adjunctive treatment for severe, active disease, noting in particular that avacopan “does not eliminate glucocorticoid use.”
The European Union’s marketing authorization for avacopan states its indication for use in combination with a rituximab or cyclophosphamide regimen for the treatment of adult patients with severe, active GPA or MPA and does not mention a role for reducing glucocorticoids. Avacopan will appear in forthcoming guidelines on management of AAV released by the European Alliance of Associations for Rheumatology.
In North America, the Canadian Vasculitis Research Network recently released an addendum to their guidelines on AAV specifically for avacopan, which includes recommendations to consider adding oral avacopan (30 mg twice daily) for induction of remission in patients with new or relapsing GPA or MPA who are also receiving cyclophosphamide or rituximab. The guidelines also recommend clinicians consider a glucocorticoid tapering schedule that aims for discontinuation at 4 weeks, and continuing avacopan for at least 1 year after induction therapy. The American College of Rheumatology guideline for AAV management, updated in 2021, acknowledges avacopan but did not consider its inclusion prior to FDA approval.
There have been few real-world studies of how patients with AAV are responding to avacopan, but recent studies from researchers in the Netherlands and in France have evaluated prednisone tapering and clinical outcomes.
Anisha B. Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago, said those real-world studies “seemed to re-enforce the findings from the ADVOCATE study demonstrating the efficacy of avacopan in severe disease with steroid-sparing effects.”
However, Carol Langford, MD, MHS, director of the Center for Vasculitis Care and Research at the Cleveland Clinic, emphasized caution is needed when drawing conclusions about avacopan use outside formal studies.
“We are all interested in what other settings this might be used. I think those are things that really require formal investigation to really try and understand better as far as through a study process,” she said.
Prescribing experience with avacopan
A spokesperson from Amgen, which recently acquired ChemoCentryx, said in an interview that over 800 physicians in the United States have prescribed avacopan to patients with new or relapsing ANCA-associated vasculitis as induction or maintenance treatment, and physicians have reported outcomes consistent with the ADVOCATE trial.
Many rheumatologists are likely familiar with avacopan but are not used to prescribing it, said Lindsay S. Lally, MD, a rheumatologist with Hospital for Special Surgery in New York.
“Rituximab was approved for GPA and MPA a decade ago at this point. It was a drug that we as rheumatologists were used to using. We used it for other indications. Avacopan is a totally new drug, a new mechanism of action, so there’s not a lot of extractable data that we have in terms of comfort with the drug, and so I think that’s one of the biggest hurdles,” she said.
Mehrnaz Hojjati, MD, a rheumatologist with Loma Linda (Calif.) University Health, said that, when the FDA approved avacopan, it was an “exciting time” in her practice. “I have used avacopan now in a handful of my patients with severe ANCA-associated vasculitis, and the results are similar to what [was] reported in the ADVOCATE trial.”
Amgen offers help for clinicians in obtaining avacopan for patients, financial assistance for patients, and support in navigating insurance, which several rheumatologists noted was important for patients. Dr. Langford said the process of working with the manufacturer to get avacopan while insurance information is being processed has been “fairly smooth.”
“Certainly, the ability to get a very rapid 30-day supply with the goal of trying to initiate this as early as possible in the disease process has been helpful,” she said.
In Dr. Dua’s experience, while there were “some glitches or difficulty for providers early on” in how to access and prescribe avacopan, since then “it has been much easier to obtain the medication with the first month being provided to patients free while the authorization process is managed.”
Prescribing avacopan from inpatient pharmacies has been more challenging, she said. “The inpatient side is trickier because each hospital system has their own pharmacy system and regulations that have to be navigated. For outpatients, all the provider needs to do is fill out the start form available on their website, have the patients sign it, and then have it sent in.”
Concerns about affordability, insurance approval
Another consideration is cost, with avacopan having an estimated price of $150,000-$200,000 per patient per year.
Dr. Hojjati noted that, while it is easy to prescribe, avacopan is hard to get approved through insurance. “We face the same challenge every time a new medication comes to the market on how to convince the payers to pay for it given higher prices,” she said.
Rheumatologist Michael Putman, MD, MSCI, assistant professor of medicine at the Medical College of Wisconsin, Milwaukee, also acknowledged some difficulties in prescribing the medication. “The insurance companies have no interest in spending $150,000 on a drug that they know nothing about, and patients are a little hesitant to take it because it’s just so new,” he said.
While Dr. Lally said avacopan has not been difficult to get for patients with commercial insurance, reimbursement through Medicare has been problematic. “In many of the Medicare patients it has not really been a feasible option for them to be on the drug for the year of therapy.”
Patient response
Dr. Dua said almost all her patients with new or relapsing AAV who require induction are being prescribed avacopan, and that the medication is well tolerated. “The remission and ability to wean prednisone has really paralleled the findings from the clinical trial.”
In her practice, Dr. Hojjati starts patients on avacopan immediately after discharge from the hospital after a major vasculitis flare requiring high-dose glucocorticoids. “Avacopan does not eliminate/replace GC [glucocorticoid] use but has a notable GC-sparing effect and assists in rapid tapering of the GC while treating our severe ANCA-associated vasculitis patients,” she said.
Dr. Lally said her patients are tolerating avacopan well and hasn’t seen any of the safety signals seen in the trial, including liver function abnormalities. She has treated about 20-25 patients with avacopan.
Dr. Putman noted that he has treated about five patients with avacopan but hasn’t seen dramatic efficacy or side effects in his practice, compared with standard therapy.
Unanswered questions about avacopan
A key unanswered question with avacopan is the timeline for tapering glucocorticoids once patients start treatment. “I would like to see much more data on how prednisone is being tapered in clinical practice as well as outcomes in patients who are treated with the standard of care second dose of rituximab at 6 months,” Dr. Dua said.
Dr. Lally noted she has tried to expedite the steroid taper in her patients. “That’s really where I feel this drug is going to have most relevance, is getting it started early in active disease and getting patients off of the reliance on high doses of oral steroids. I have been able to see that in practice, and I do think ultimately that’s going to lead to better outcomes and quality of life for these patients.”
Of the rheumatologists Dr. Lally has spoken to about avacopan, there is “some confusion about what type of patients are appropriate, [and] how sick or not sick the patient needs to be.”
Dr. Putman noted he is unsure which of his patients should be receiving avacopan. “I don’t totally have a sense for where avacopan stands and how often we should be using it” outside of patients with severe disease. He added that the drug is still trying to find a niche because most patients with AAV who take rituximab and steroids get better without additional treatments.
“I think we do a pretty good job treating these diseases even in the preavacopan era. But it’s really a matter of how to really optimize these outcomes, reduce damage, reduce steroid-related and treatment-related toxicity for our patients,” Dr. Lally said.
Dr. Dua reported being a consultant and serving on advisory boards for ChemoCentryx; she was also a site principal investigator for the ADVOCATE trial. Dr. Hojjati reported being on the speaker’s bureau for Amgen. Dr. Langford reported being an investigator in the ADVOCATE trial, and her institution received funding to conduct the trial. Dr. Lally reported being a consultant for Amgen on avacopan. Dr. Putman reported no relevant financial disclosures.
*This story was updated 3/15/2023.
When the Food and Drug Administration approved avacopan (Tavneos) as an adjunctive treatment for severe, active antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitis (AAV) in October 2021, the oral complement C5a receptor inhibitor was hailed by its developer, ChemoCentryx, as a “new hope” for patients with the disease.
But avacopan’s novelty as a new drug for the rare diseases granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA), coupled with its approval as an adjunctive to standard therapy, including glucocorticoids, rather than strictly as a glucocorticoid-sparing agent as it was tested, has so far led to little reported real-world experience with the drug.
In the phase 3 ADVOCATE trial, the pivotal trial that served as the basis for avacopan’s approval, 331 patients with active newly diagnosed or relapsing GPA or MPA received either avacopan or an oral prednisone taper over 20 weeks on a background of cyclophosphamide followed by azathioprine or rituximab. The results of the trial showed avacopan was noninferior to the group that received prednisone taper for remission at 26 weeks and superior to prednisone taper for sustained remission at 52 weeks, but the FDA was concerned that its complex design made it difficult to define the clinically meaningful benefit of avacopan and its role in the management of AAV.
The FDA noted that, in the avacopan arm of the trial, 86% of patients received glucocorticoids outside of the study protocol. Despite this, avacopan reduced the cumulative glucocorticoid dose over the trial’s 52 weeks by nearly two-thirds, compared with the prednisone group (1,349 mg vs. 3,655 mg).
The data also indicate a higher sustained remission rate at 52 weeks in patients who received induction with rituximab, compared with cyclophosphamide. But trial did not include a maintenance therapy dose of rituximab and is thereby not a good comparison against the standard of care, the FDA said. (ADVOCATE began enrolling patients prior to the FDA's 2018 approval of an expanded indication for patients with GPA or MPA who have achieved disease control after induction treatment.)
At the FDA’s Arthritis Advisory Committee meeting in May 2021, committee members were split on whether to recommend avacopan for approval. The committee voted 9-9 on whether the ADVOCATE trial showed efficacy supporting approval of avacopan, 10-8 in favor of whether the drug’s safety profile supported approval, and 10-8 in favor of the overall benefit-risk profile of avacopan for approval. But rather than give an indication to avacopan to reduce the use of glucocorticoids in adults with GPA or MPA, the agency approved avacopan as an adjunctive treatment for severe, active disease, noting in particular that avacopan “does not eliminate glucocorticoid use.”
The European Union’s marketing authorization for avacopan states its indication for use in combination with a rituximab or cyclophosphamide regimen for the treatment of adult patients with severe, active GPA or MPA and does not mention a role for reducing glucocorticoids. Avacopan will appear in forthcoming guidelines on management of AAV released by the European Alliance of Associations for Rheumatology.
In North America, the Canadian Vasculitis Research Network recently released an addendum to their guidelines on AAV specifically for avacopan, which includes recommendations to consider adding oral avacopan (30 mg twice daily) for induction of remission in patients with new or relapsing GPA or MPA who are also receiving cyclophosphamide or rituximab. The guidelines also recommend clinicians consider a glucocorticoid tapering schedule that aims for discontinuation at 4 weeks, and continuing avacopan for at least 1 year after induction therapy. The American College of Rheumatology guideline for AAV management, updated in 2021, acknowledges avacopan but did not consider its inclusion prior to FDA approval.
There have been few real-world studies of how patients with AAV are responding to avacopan, but recent studies from researchers in the Netherlands and in France have evaluated prednisone tapering and clinical outcomes.
Anisha B. Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago, said those real-world studies “seemed to re-enforce the findings from the ADVOCATE study demonstrating the efficacy of avacopan in severe disease with steroid-sparing effects.”
However, Carol Langford, MD, MHS, director of the Center for Vasculitis Care and Research at the Cleveland Clinic, emphasized caution is needed when drawing conclusions about avacopan use outside formal studies.
“We are all interested in what other settings this might be used. I think those are things that really require formal investigation to really try and understand better as far as through a study process,” she said.
Prescribing experience with avacopan
A spokesperson from Amgen, which recently acquired ChemoCentryx, said in an interview that over 800 physicians in the United States have prescribed avacopan to patients with new or relapsing ANCA-associated vasculitis as induction or maintenance treatment, and physicians have reported outcomes consistent with the ADVOCATE trial.
Many rheumatologists are likely familiar with avacopan but are not used to prescribing it, said Lindsay S. Lally, MD, a rheumatologist with Hospital for Special Surgery in New York.
“Rituximab was approved for GPA and MPA a decade ago at this point. It was a drug that we as rheumatologists were used to using. We used it for other indications. Avacopan is a totally new drug, a new mechanism of action, so there’s not a lot of extractable data that we have in terms of comfort with the drug, and so I think that’s one of the biggest hurdles,” she said.
Mehrnaz Hojjati, MD, a rheumatologist with Loma Linda (Calif.) University Health, said that, when the FDA approved avacopan, it was an “exciting time” in her practice. “I have used avacopan now in a handful of my patients with severe ANCA-associated vasculitis, and the results are similar to what [was] reported in the ADVOCATE trial.”
Amgen offers help for clinicians in obtaining avacopan for patients, financial assistance for patients, and support in navigating insurance, which several rheumatologists noted was important for patients. Dr. Langford said the process of working with the manufacturer to get avacopan while insurance information is being processed has been “fairly smooth.”
“Certainly, the ability to get a very rapid 30-day supply with the goal of trying to initiate this as early as possible in the disease process has been helpful,” she said.
In Dr. Dua’s experience, while there were “some glitches or difficulty for providers early on” in how to access and prescribe avacopan, since then “it has been much easier to obtain the medication with the first month being provided to patients free while the authorization process is managed.”
Prescribing avacopan from inpatient pharmacies has been more challenging, she said. “The inpatient side is trickier because each hospital system has their own pharmacy system and regulations that have to be navigated. For outpatients, all the provider needs to do is fill out the start form available on their website, have the patients sign it, and then have it sent in.”
Concerns about affordability, insurance approval
Another consideration is cost, with avacopan having an estimated price of $150,000-$200,000 per patient per year.
Dr. Hojjati noted that, while it is easy to prescribe, avacopan is hard to get approved through insurance. “We face the same challenge every time a new medication comes to the market on how to convince the payers to pay for it given higher prices,” she said.
Rheumatologist Michael Putman, MD, MSCI, assistant professor of medicine at the Medical College of Wisconsin, Milwaukee, also acknowledged some difficulties in prescribing the medication. “The insurance companies have no interest in spending $150,000 on a drug that they know nothing about, and patients are a little hesitant to take it because it’s just so new,” he said.
While Dr. Lally said avacopan has not been difficult to get for patients with commercial insurance, reimbursement through Medicare has been problematic. “In many of the Medicare patients it has not really been a feasible option for them to be on the drug for the year of therapy.”
Patient response
Dr. Dua said almost all her patients with new or relapsing AAV who require induction are being prescribed avacopan, and that the medication is well tolerated. “The remission and ability to wean prednisone has really paralleled the findings from the clinical trial.”
In her practice, Dr. Hojjati starts patients on avacopan immediately after discharge from the hospital after a major vasculitis flare requiring high-dose glucocorticoids. “Avacopan does not eliminate/replace GC [glucocorticoid] use but has a notable GC-sparing effect and assists in rapid tapering of the GC while treating our severe ANCA-associated vasculitis patients,” she said.
Dr. Lally said her patients are tolerating avacopan well and hasn’t seen any of the safety signals seen in the trial, including liver function abnormalities. She has treated about 20-25 patients with avacopan.
Dr. Putman noted that he has treated about five patients with avacopan but hasn’t seen dramatic efficacy or side effects in his practice, compared with standard therapy.
Unanswered questions about avacopan
A key unanswered question with avacopan is the timeline for tapering glucocorticoids once patients start treatment. “I would like to see much more data on how prednisone is being tapered in clinical practice as well as outcomes in patients who are treated with the standard of care second dose of rituximab at 6 months,” Dr. Dua said.
Dr. Lally noted she has tried to expedite the steroid taper in her patients. “That’s really where I feel this drug is going to have most relevance, is getting it started early in active disease and getting patients off of the reliance on high doses of oral steroids. I have been able to see that in practice, and I do think ultimately that’s going to lead to better outcomes and quality of life for these patients.”
Of the rheumatologists Dr. Lally has spoken to about avacopan, there is “some confusion about what type of patients are appropriate, [and] how sick or not sick the patient needs to be.”
Dr. Putman noted he is unsure which of his patients should be receiving avacopan. “I don’t totally have a sense for where avacopan stands and how often we should be using it” outside of patients with severe disease. He added that the drug is still trying to find a niche because most patients with AAV who take rituximab and steroids get better without additional treatments.
“I think we do a pretty good job treating these diseases even in the preavacopan era. But it’s really a matter of how to really optimize these outcomes, reduce damage, reduce steroid-related and treatment-related toxicity for our patients,” Dr. Lally said.
Dr. Dua reported being a consultant and serving on advisory boards for ChemoCentryx; she was also a site principal investigator for the ADVOCATE trial. Dr. Hojjati reported being on the speaker’s bureau for Amgen. Dr. Langford reported being an investigator in the ADVOCATE trial, and her institution received funding to conduct the trial. Dr. Lally reported being a consultant for Amgen on avacopan. Dr. Putman reported no relevant financial disclosures.
*This story was updated 3/15/2023.
Cyclosporine-Induced Posterior Reversible Encephalopathy Syndrome: An Adverse Effect in a Patient With Atopic Dermatitis
To the Editor:
Cyclosporine is an immunomodulatory medication that impacts T-lymphocyte function through calcineurin inhibition and suppression of IL-2 expression. Oral cyclosporine at low doses (1–3 mg/kg/d) is one of the more common systemic treatment options for moderate to severe atopic dermatitis. At these doses it has been shown to have therapeutic benefit in several skin conditions, including chronic spontaneous urticaria,1 psoriasis,2 and atopic dermatitis.3 When used at higher doses for conditions such as glomerulonephritis or transplantation, adverse effects may be notable, and close monitoring of drug metabolism as well as end-organ function is required. In contrast, severe adverse effects are uncommon with the lower doses of cyclosporine used for cutaneous conditions, and monitoring serum drug levels is not routinely practiced.4
A 58-year-old man was referred to clinic with severe atopic dermatitis refractory to maximal topical therapy prescribed by an outside physician. He was started on cyclosporine as an anticipated bridge to dupilumab biologic therapy. He had no history of hypertension, renal disease, or hepatic insufficiency prior to starting therapy. He demonstrated notable clinical improvement at a cyclosporine dosage of 300 mg/d (equating to 3.7 mg/kg/d). Three months after initiation of therapy, the patient presented to a local emergency department with new-onset seizurelike activity, confusion, and agitation. He was normotensive with clinical concern for status epilepticus. An initial laboratory assessment included a complete blood cell count, serum electrolyte panel, and urine toxicology screen, which were unremarkable. Computed tomography of the head showed confluent white-matter hypodensities in the left parietal-temporal-occipital lobes. Magnetic resonance imaging (MRI) of the brain showed innumerable peripherally distributed foci of microhemorrhage and vasogenic edema within the left parietal-temporal-occipital lobes (Figure).
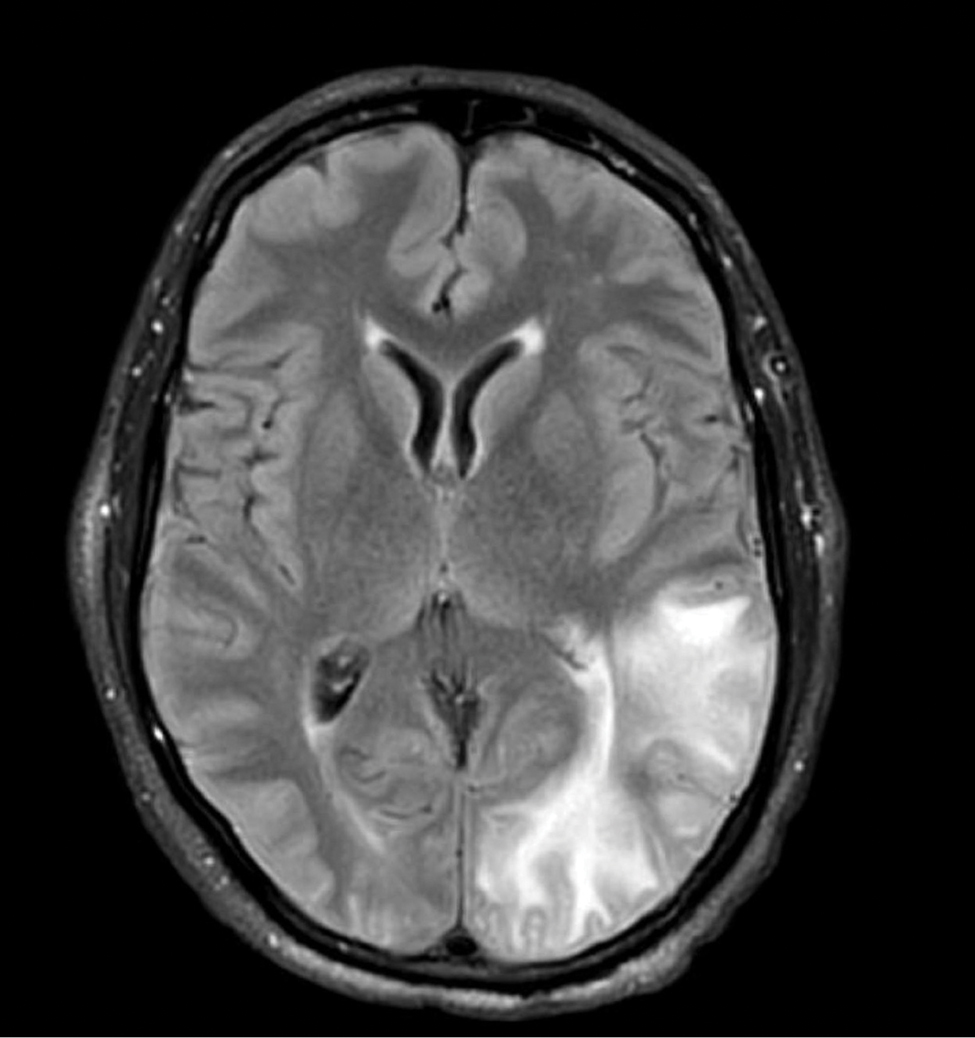
He was intubated and sedated with admission to the medical intensive care unit, where a random cyclosporine level drawn approximately 9 hours after the prior dose was noted to be 263 ng/mL. Although target therapeutic levels for cyclosporine vary based on indication, toxic supratherapeutic levels generally are considered to be greater than 400 ng/mL.5 He had no evidence of acute kidney injury, uremia, or hypertension throughout hospitalization. An electroencephalogram showed left parieto-occipital periodic epileptiform discharges with generalized slowing. Cyclosporine was discontinued, and he was started on levetiracetam. His clinical and neuroimaging findings improved over the course of the 1-week hospitalization without any further intervention. Four weeks after hospitalization, he had full neurologic, electroencephalogram, and imaging recovery. Based on the presenting symptoms, transient neuroimaging findings, and full recovery with discontinuation of cyclosporine, the patient was diagnosed with cyclosporine-induced posterior reversible encephalopathy syndrome (PRES).
The diagnosis of PRES requires evidence of acute neurologic symptoms and radiographic findings of cortical/subcortical white-matter changes on computed tomography or MRI consistent with edema. The pathophysiology is not fully understood but appears to be related to vasogenic edema, primarily impacting the posterior aspect of the brain. There have been many reported offending agents, and symptoms typically resolve following cessation of these medications. Cases of cyclosporine-induced PRES have been reported, but most occurred at higher doses within weeks of medication initiation. Two cases of cyclosporine-induced PRES treated with cutaneous dosing have been reported; neither patient was taking it for atopic dermatitis.6
Cyclosporine-induced PRES remains a pathophysiologic conundrum. However, there is evidence to support direct endothelial damage causing cellular apoptosis in the brain of mouse models that is medication specific and not necessarily related to the dosages used.7 Our case highlights a rare but important adverse event associated with even low-dose cyclosporine use that should be considered in patients currently taking cyclosporine who present with acute neurologic changes.
- Kulthanan K, Chaweekulrat P, Komoltri C, et al. Cyclosporine for chronic spontaneous urticaria: a meta-analysis and systematic review. J Allergy Clin Immunol Pract. 2018;6:586-599. doi:10.1016/j.jaip.2017.07.017
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323:1945-1960. doi:10.1001/jama.2020.4006
- Seger EW, Wechter T, Strowd L, et al. Relative efficacy of systemic treatments for atopic dermatitis [published online October 6, 2018]. J Am Acad Dermatol. 2019;80:411-416.e4. doi:10.1016/j.jaad.2018.09.053
- Blake SC, Murrell DF. Monitoring trough levels in cyclosporine for atopic dermatitis: a systematic review. Pediatr Dermatol. 2019;36:843-853. doi:10.1111/pde.13999
- Tapia C, Nessel TA, Zito PM. Cyclosporine. StatPearls Publishing: 2022. https://www.ncbi.nlm.nih.gov/books/NBK482450/
- Cosottini M, Lazzarotti G, Ceravolo R, et al. Cyclosporine‐related posterior reversible encephalopathy syndrome (PRES) in non‐transplant patient: a case report and literature review. Eur J Neurol. 2003;10:461-462. doi:10.1046/j.1468-1331.2003.00608_1.x
- Kochi S, Takanaga H, Matsuo H, et al. Induction of apoptosis in mouse brain capillary endothelial cells by cyclosporin A and tacrolimus. Life Sci. 2000;66:2255-2260. doi:10.1016/s0024-3205(00)00554-3
To the Editor:
Cyclosporine is an immunomodulatory medication that impacts T-lymphocyte function through calcineurin inhibition and suppression of IL-2 expression. Oral cyclosporine at low doses (1–3 mg/kg/d) is one of the more common systemic treatment options for moderate to severe atopic dermatitis. At these doses it has been shown to have therapeutic benefit in several skin conditions, including chronic spontaneous urticaria,1 psoriasis,2 and atopic dermatitis.3 When used at higher doses for conditions such as glomerulonephritis or transplantation, adverse effects may be notable, and close monitoring of drug metabolism as well as end-organ function is required. In contrast, severe adverse effects are uncommon with the lower doses of cyclosporine used for cutaneous conditions, and monitoring serum drug levels is not routinely practiced.4
A 58-year-old man was referred to clinic with severe atopic dermatitis refractory to maximal topical therapy prescribed by an outside physician. He was started on cyclosporine as an anticipated bridge to dupilumab biologic therapy. He had no history of hypertension, renal disease, or hepatic insufficiency prior to starting therapy. He demonstrated notable clinical improvement at a cyclosporine dosage of 300 mg/d (equating to 3.7 mg/kg/d). Three months after initiation of therapy, the patient presented to a local emergency department with new-onset seizurelike activity, confusion, and agitation. He was normotensive with clinical concern for status epilepticus. An initial laboratory assessment included a complete blood cell count, serum electrolyte panel, and urine toxicology screen, which were unremarkable. Computed tomography of the head showed confluent white-matter hypodensities in the left parietal-temporal-occipital lobes. Magnetic resonance imaging (MRI) of the brain showed innumerable peripherally distributed foci of microhemorrhage and vasogenic edema within the left parietal-temporal-occipital lobes (Figure).

He was intubated and sedated with admission to the medical intensive care unit, where a random cyclosporine level drawn approximately 9 hours after the prior dose was noted to be 263 ng/mL. Although target therapeutic levels for cyclosporine vary based on indication, toxic supratherapeutic levels generally are considered to be greater than 400 ng/mL.5 He had no evidence of acute kidney injury, uremia, or hypertension throughout hospitalization. An electroencephalogram showed left parieto-occipital periodic epileptiform discharges with generalized slowing. Cyclosporine was discontinued, and he was started on levetiracetam. His clinical and neuroimaging findings improved over the course of the 1-week hospitalization without any further intervention. Four weeks after hospitalization, he had full neurologic, electroencephalogram, and imaging recovery. Based on the presenting symptoms, transient neuroimaging findings, and full recovery with discontinuation of cyclosporine, the patient was diagnosed with cyclosporine-induced posterior reversible encephalopathy syndrome (PRES).
The diagnosis of PRES requires evidence of acute neurologic symptoms and radiographic findings of cortical/subcortical white-matter changes on computed tomography or MRI consistent with edema. The pathophysiology is not fully understood but appears to be related to vasogenic edema, primarily impacting the posterior aspect of the brain. There have been many reported offending agents, and symptoms typically resolve following cessation of these medications. Cases of cyclosporine-induced PRES have been reported, but most occurred at higher doses within weeks of medication initiation. Two cases of cyclosporine-induced PRES treated with cutaneous dosing have been reported; neither patient was taking it for atopic dermatitis.6
Cyclosporine-induced PRES remains a pathophysiologic conundrum. However, there is evidence to support direct endothelial damage causing cellular apoptosis in the brain of mouse models that is medication specific and not necessarily related to the dosages used.7 Our case highlights a rare but important adverse event associated with even low-dose cyclosporine use that should be considered in patients currently taking cyclosporine who present with acute neurologic changes.
To the Editor:
Cyclosporine is an immunomodulatory medication that impacts T-lymphocyte function through calcineurin inhibition and suppression of IL-2 expression. Oral cyclosporine at low doses (1–3 mg/kg/d) is one of the more common systemic treatment options for moderate to severe atopic dermatitis. At these doses it has been shown to have therapeutic benefit in several skin conditions, including chronic spontaneous urticaria,1 psoriasis,2 and atopic dermatitis.3 When used at higher doses for conditions such as glomerulonephritis or transplantation, adverse effects may be notable, and close monitoring of drug metabolism as well as end-organ function is required. In contrast, severe adverse effects are uncommon with the lower doses of cyclosporine used for cutaneous conditions, and monitoring serum drug levels is not routinely practiced.4
A 58-year-old man was referred to clinic with severe atopic dermatitis refractory to maximal topical therapy prescribed by an outside physician. He was started on cyclosporine as an anticipated bridge to dupilumab biologic therapy. He had no history of hypertension, renal disease, or hepatic insufficiency prior to starting therapy. He demonstrated notable clinical improvement at a cyclosporine dosage of 300 mg/d (equating to 3.7 mg/kg/d). Three months after initiation of therapy, the patient presented to a local emergency department with new-onset seizurelike activity, confusion, and agitation. He was normotensive with clinical concern for status epilepticus. An initial laboratory assessment included a complete blood cell count, serum electrolyte panel, and urine toxicology screen, which were unremarkable. Computed tomography of the head showed confluent white-matter hypodensities in the left parietal-temporal-occipital lobes. Magnetic resonance imaging (MRI) of the brain showed innumerable peripherally distributed foci of microhemorrhage and vasogenic edema within the left parietal-temporal-occipital lobes (Figure).

He was intubated and sedated with admission to the medical intensive care unit, where a random cyclosporine level drawn approximately 9 hours after the prior dose was noted to be 263 ng/mL. Although target therapeutic levels for cyclosporine vary based on indication, toxic supratherapeutic levels generally are considered to be greater than 400 ng/mL.5 He had no evidence of acute kidney injury, uremia, or hypertension throughout hospitalization. An electroencephalogram showed left parieto-occipital periodic epileptiform discharges with generalized slowing. Cyclosporine was discontinued, and he was started on levetiracetam. His clinical and neuroimaging findings improved over the course of the 1-week hospitalization without any further intervention. Four weeks after hospitalization, he had full neurologic, electroencephalogram, and imaging recovery. Based on the presenting symptoms, transient neuroimaging findings, and full recovery with discontinuation of cyclosporine, the patient was diagnosed with cyclosporine-induced posterior reversible encephalopathy syndrome (PRES).
The diagnosis of PRES requires evidence of acute neurologic symptoms and radiographic findings of cortical/subcortical white-matter changes on computed tomography or MRI consistent with edema. The pathophysiology is not fully understood but appears to be related to vasogenic edema, primarily impacting the posterior aspect of the brain. There have been many reported offending agents, and symptoms typically resolve following cessation of these medications. Cases of cyclosporine-induced PRES have been reported, but most occurred at higher doses within weeks of medication initiation. Two cases of cyclosporine-induced PRES treated with cutaneous dosing have been reported; neither patient was taking it for atopic dermatitis.6
Cyclosporine-induced PRES remains a pathophysiologic conundrum. However, there is evidence to support direct endothelial damage causing cellular apoptosis in the brain of mouse models that is medication specific and not necessarily related to the dosages used.7 Our case highlights a rare but important adverse event associated with even low-dose cyclosporine use that should be considered in patients currently taking cyclosporine who present with acute neurologic changes.
- Kulthanan K, Chaweekulrat P, Komoltri C, et al. Cyclosporine for chronic spontaneous urticaria: a meta-analysis and systematic review. J Allergy Clin Immunol Pract. 2018;6:586-599. doi:10.1016/j.jaip.2017.07.017
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323:1945-1960. doi:10.1001/jama.2020.4006
- Seger EW, Wechter T, Strowd L, et al. Relative efficacy of systemic treatments for atopic dermatitis [published online October 6, 2018]. J Am Acad Dermatol. 2019;80:411-416.e4. doi:10.1016/j.jaad.2018.09.053
- Blake SC, Murrell DF. Monitoring trough levels in cyclosporine for atopic dermatitis: a systematic review. Pediatr Dermatol. 2019;36:843-853. doi:10.1111/pde.13999
- Tapia C, Nessel TA, Zito PM. Cyclosporine. StatPearls Publishing: 2022. https://www.ncbi.nlm.nih.gov/books/NBK482450/
- Cosottini M, Lazzarotti G, Ceravolo R, et al. Cyclosporine‐related posterior reversible encephalopathy syndrome (PRES) in non‐transplant patient: a case report and literature review. Eur J Neurol. 2003;10:461-462. doi:10.1046/j.1468-1331.2003.00608_1.x
- Kochi S, Takanaga H, Matsuo H, et al. Induction of apoptosis in mouse brain capillary endothelial cells by cyclosporin A and tacrolimus. Life Sci. 2000;66:2255-2260. doi:10.1016/s0024-3205(00)00554-3
- Kulthanan K, Chaweekulrat P, Komoltri C, et al. Cyclosporine for chronic spontaneous urticaria: a meta-analysis and systematic review. J Allergy Clin Immunol Pract. 2018;6:586-599. doi:10.1016/j.jaip.2017.07.017
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323:1945-1960. doi:10.1001/jama.2020.4006
- Seger EW, Wechter T, Strowd L, et al. Relative efficacy of systemic treatments for atopic dermatitis [published online October 6, 2018]. J Am Acad Dermatol. 2019;80:411-416.e4. doi:10.1016/j.jaad.2018.09.053
- Blake SC, Murrell DF. Monitoring trough levels in cyclosporine for atopic dermatitis: a systematic review. Pediatr Dermatol. 2019;36:843-853. doi:10.1111/pde.13999
- Tapia C, Nessel TA, Zito PM. Cyclosporine. StatPearls Publishing: 2022. https://www.ncbi.nlm.nih.gov/books/NBK482450/
- Cosottini M, Lazzarotti G, Ceravolo R, et al. Cyclosporine‐related posterior reversible encephalopathy syndrome (PRES) in non‐transplant patient: a case report and literature review. Eur J Neurol. 2003;10:461-462. doi:10.1046/j.1468-1331.2003.00608_1.x
- Kochi S, Takanaga H, Matsuo H, et al. Induction of apoptosis in mouse brain capillary endothelial cells by cyclosporin A and tacrolimus. Life Sci. 2000;66:2255-2260. doi:10.1016/s0024-3205(00)00554-3
Practice Points
- Cyclosporine is an immunomodulatory therapeutic utilized for several indications in dermatology practice, most commonly in low doses.
- Posterior reversible encephalopathy syndrome (PRES) is a known but rare adverse effect of cyclosporine presenting with acute encephalopathic changes and radiographic findings on central imaging.
- Knowledge of this association is critical, as symptoms are reversible with prompt recognition, appropriate inpatient supportive care, and discontinuation of offending medications.
Kaposi Varicelliform Eruption of Mpox in a Peeling Sunburn
To the Editor:
The recent global mpox (monkeypox) outbreak that started in May 2022 has distinctive risk factors, clinical features, and patient attributes that can portend dissemination of infection. We report a case of Kaposi varicelliform eruption (KVE) over a peeling sunburn after mpox infection. Dermatologists should recognize cutaneous risk factors for dissemination of mpox.
A 35-year-old man who was otherwise healthy presented with a papulopustular eruption that began on the shoulders in an area that had been sunburned 24 to 48 hours earlier. He experienced fever (temperature, 38.6 °C)[101.5 °F]), chills, malaise, and the appearance of a painful penile ulcer. He reported a recent male sexual partner a week prior to the eruption during travel to eastern Asia and a subsequent male partner in the United States 5 days prior to eruption. Physical examination revealed a peeling sunburn with sharp clothing demarcation. Locations with the most notable desquamation—the superior shoulders, dorsal arms, upper chest, and ventral thighs—positively correlated with the highest density of scattered, discrete, erythematous-based pustules and pink papules, some with crusted umbilication (Figures 1 and 2). Lesions spared sun-protected locations except a punctate painful ulcer on the buccal mucosa and a tender well-demarcated ulcer with elevated borders on the ventral penile shaft. HIV antigen/antibody testing was negative; syphilis antibody testing was positive due to a prior infection 1 year earlier with titers down to 1:1. A penile ulcer swab did not detect herpes simplex virus types 1/2 DNA. Pharyngeal, penile, and rectal swabs were negative for chlamydia or gonorrhea DNA. A polymerase chain reaction assay of a pustule was positive for orthopoxvirus, and the Centers for Disease Control and Prevention confirmed Monkeypox virus. On day 12, a penile ulcer biopsy was nonspecific with dense mixed inflammation; immunohistochemical stains for Treponema pallidum and herpes simplex virus types 1/2 were negative. Consideration was given to starting antiviral treatment with tecovirimat, which is approved by the US Food and Drug Administration for smallpox caused by variola virus, through the Centers for Disease Control and Prevention expanded access protocol, but the patient’s symptoms and lesions cleared quickly without intervention. The patient’s recent sexual contact in the United States later tested positive for mpox. Given that the density of our patient’s mpox lesions positively correlated with areas of peeling sunburn with rapid spread during the period of desquamation, he was diagnosed with KVE due to mpox in the setting of a peeling sunburn.
The recent mpox outbreak began in May 2022, and within 3 months there were more than 31,000 confirmed mpox cases worldwide, with more than 11,000 of those cases within the United States across 49 states and Puerto Rico.1 Gay, bisexual, and other men who have sex with men have constituted the majority of cases. Although prior outbreaks have exhibited cases of classic mpox lesions, the current cases are clinically distinctive from classic mpox due to prevalent orogenital involvement and generalized symptoms that often are mild, nonexistent, or can occur after the cutaneous lesions.2
Although most current cases of mpox have been mildly symptomatic, several patients have been ill enough to require hospital admission, including patients with severe anogenital ulcerative lesions or bacterial superinfection.3 Antiviral treatment with tecovirimat may be warranted for patients with severe disease or those at risk of becoming severe due to immunosuppression, pregnancy/breastfeeding, complications (as determined by the provider), younger age (ie, pediatric patients), or skin barrier disruption. Dermatologists play a particularly important role in identifying cutaneous risk factors that may indicate progression of infection (eg, atopic dermatitis, severe acne, intertrigo, Darier disease). Kaposi varicelliform eruption is the phenomenon where a more typically localized vesicular infection is disseminated to areas with a defective skin barrier.2 Eczema herpeticum refers to the most common type of KVE due to herpes simplex virus, but other known etiologies of KVE include coxsackievirus A16, vaccinia virus, varicella-zoster virus, and smallpox.2 Although classic mpox previously had only the theoretical potential to lead to a secondary KVE, we expect the literature to evolve as cases spread, with one recent report of eczema monkeypoxicum in the setting of atopic dermatitis.4
At the time of publication, mpox cases have notably dropped globally due to public health interventions; however, mpox infections are ongoing in areas previously identified as nonendemic. Given the distinctive risk factors and clinical presentations of this most recent outbreak, clinicians will need to be adept at identifying not only infection but also risk for dissemination, including skin barrier disruption.
- Centers for Disease Control and Prevention. Mpox: 2022 US map & case count. Updated February 15, 2023. Accessed February 23, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/us-map.html
- Karray M, Kwan E, Souissi A. Kaposi varicelliform eruption. StatPearls. Updated September 12, 2022. Accessed February 24, 2023. https://www.ncbi.nlm.nih.gov/books/NBK482432
- Girometti N, Byrne R, Bracchi M, et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Lancet Infect Dis. 2022;S1473-3099(22)00411-X. doi:10.1016/S1473-3099(22)00411-X
- Xia J, Huang CL, Chu P, et al. Eczema monkeypoxicum: report of monkeypox transmission in patients with atopic dermatitis. JAAD Case Reports. 2022;29:95-99.
To the Editor:
The recent global mpox (monkeypox) outbreak that started in May 2022 has distinctive risk factors, clinical features, and patient attributes that can portend dissemination of infection. We report a case of Kaposi varicelliform eruption (KVE) over a peeling sunburn after mpox infection. Dermatologists should recognize cutaneous risk factors for dissemination of mpox.
A 35-year-old man who was otherwise healthy presented with a papulopustular eruption that began on the shoulders in an area that had been sunburned 24 to 48 hours earlier. He experienced fever (temperature, 38.6 °C)[101.5 °F]), chills, malaise, and the appearance of a painful penile ulcer. He reported a recent male sexual partner a week prior to the eruption during travel to eastern Asia and a subsequent male partner in the United States 5 days prior to eruption. Physical examination revealed a peeling sunburn with sharp clothing demarcation. Locations with the most notable desquamation—the superior shoulders, dorsal arms, upper chest, and ventral thighs—positively correlated with the highest density of scattered, discrete, erythematous-based pustules and pink papules, some with crusted umbilication (Figures 1 and 2). Lesions spared sun-protected locations except a punctate painful ulcer on the buccal mucosa and a tender well-demarcated ulcer with elevated borders on the ventral penile shaft. HIV antigen/antibody testing was negative; syphilis antibody testing was positive due to a prior infection 1 year earlier with titers down to 1:1. A penile ulcer swab did not detect herpes simplex virus types 1/2 DNA. Pharyngeal, penile, and rectal swabs were negative for chlamydia or gonorrhea DNA. A polymerase chain reaction assay of a pustule was positive for orthopoxvirus, and the Centers for Disease Control and Prevention confirmed Monkeypox virus. On day 12, a penile ulcer biopsy was nonspecific with dense mixed inflammation; immunohistochemical stains for Treponema pallidum and herpes simplex virus types 1/2 were negative. Consideration was given to starting antiviral treatment with tecovirimat, which is approved by the US Food and Drug Administration for smallpox caused by variola virus, through the Centers for Disease Control and Prevention expanded access protocol, but the patient’s symptoms and lesions cleared quickly without intervention. The patient’s recent sexual contact in the United States later tested positive for mpox. Given that the density of our patient’s mpox lesions positively correlated with areas of peeling sunburn with rapid spread during the period of desquamation, he was diagnosed with KVE due to mpox in the setting of a peeling sunburn.

The recent mpox outbreak began in May 2022, and within 3 months there were more than 31,000 confirmed mpox cases worldwide, with more than 11,000 of those cases within the United States across 49 states and Puerto Rico.1 Gay, bisexual, and other men who have sex with men have constituted the majority of cases. Although prior outbreaks have exhibited cases of classic mpox lesions, the current cases are clinically distinctive from classic mpox due to prevalent orogenital involvement and generalized symptoms that often are mild, nonexistent, or can occur after the cutaneous lesions.2

Although most current cases of mpox have been mildly symptomatic, several patients have been ill enough to require hospital admission, including patients with severe anogenital ulcerative lesions or bacterial superinfection.3 Antiviral treatment with tecovirimat may be warranted for patients with severe disease or those at risk of becoming severe due to immunosuppression, pregnancy/breastfeeding, complications (as determined by the provider), younger age (ie, pediatric patients), or skin barrier disruption. Dermatologists play a particularly important role in identifying cutaneous risk factors that may indicate progression of infection (eg, atopic dermatitis, severe acne, intertrigo, Darier disease). Kaposi varicelliform eruption is the phenomenon where a more typically localized vesicular infection is disseminated to areas with a defective skin barrier.2 Eczema herpeticum refers to the most common type of KVE due to herpes simplex virus, but other known etiologies of KVE include coxsackievirus A16, vaccinia virus, varicella-zoster virus, and smallpox.2 Although classic mpox previously had only the theoretical potential to lead to a secondary KVE, we expect the literature to evolve as cases spread, with one recent report of eczema monkeypoxicum in the setting of atopic dermatitis.4
At the time of publication, mpox cases have notably dropped globally due to public health interventions; however, mpox infections are ongoing in areas previously identified as nonendemic. Given the distinctive risk factors and clinical presentations of this most recent outbreak, clinicians will need to be adept at identifying not only infection but also risk for dissemination, including skin barrier disruption.
To the Editor:
The recent global mpox (monkeypox) outbreak that started in May 2022 has distinctive risk factors, clinical features, and patient attributes that can portend dissemination of infection. We report a case of Kaposi varicelliform eruption (KVE) over a peeling sunburn after mpox infection. Dermatologists should recognize cutaneous risk factors for dissemination of mpox.
A 35-year-old man who was otherwise healthy presented with a papulopustular eruption that began on the shoulders in an area that had been sunburned 24 to 48 hours earlier. He experienced fever (temperature, 38.6 °C)[101.5 °F]), chills, malaise, and the appearance of a painful penile ulcer. He reported a recent male sexual partner a week prior to the eruption during travel to eastern Asia and a subsequent male partner in the United States 5 days prior to eruption. Physical examination revealed a peeling sunburn with sharp clothing demarcation. Locations with the most notable desquamation—the superior shoulders, dorsal arms, upper chest, and ventral thighs—positively correlated with the highest density of scattered, discrete, erythematous-based pustules and pink papules, some with crusted umbilication (Figures 1 and 2). Lesions spared sun-protected locations except a punctate painful ulcer on the buccal mucosa and a tender well-demarcated ulcer with elevated borders on the ventral penile shaft. HIV antigen/antibody testing was negative; syphilis antibody testing was positive due to a prior infection 1 year earlier with titers down to 1:1. A penile ulcer swab did not detect herpes simplex virus types 1/2 DNA. Pharyngeal, penile, and rectal swabs were negative for chlamydia or gonorrhea DNA. A polymerase chain reaction assay of a pustule was positive for orthopoxvirus, and the Centers for Disease Control and Prevention confirmed Monkeypox virus. On day 12, a penile ulcer biopsy was nonspecific with dense mixed inflammation; immunohistochemical stains for Treponema pallidum and herpes simplex virus types 1/2 were negative. Consideration was given to starting antiviral treatment with tecovirimat, which is approved by the US Food and Drug Administration for smallpox caused by variola virus, through the Centers for Disease Control and Prevention expanded access protocol, but the patient’s symptoms and lesions cleared quickly without intervention. The patient’s recent sexual contact in the United States later tested positive for mpox. Given that the density of our patient’s mpox lesions positively correlated with areas of peeling sunburn with rapid spread during the period of desquamation, he was diagnosed with KVE due to mpox in the setting of a peeling sunburn.

The recent mpox outbreak began in May 2022, and within 3 months there were more than 31,000 confirmed mpox cases worldwide, with more than 11,000 of those cases within the United States across 49 states and Puerto Rico.1 Gay, bisexual, and other men who have sex with men have constituted the majority of cases. Although prior outbreaks have exhibited cases of classic mpox lesions, the current cases are clinically distinctive from classic mpox due to prevalent orogenital involvement and generalized symptoms that often are mild, nonexistent, or can occur after the cutaneous lesions.2

Although most current cases of mpox have been mildly symptomatic, several patients have been ill enough to require hospital admission, including patients with severe anogenital ulcerative lesions or bacterial superinfection.3 Antiviral treatment with tecovirimat may be warranted for patients with severe disease or those at risk of becoming severe due to immunosuppression, pregnancy/breastfeeding, complications (as determined by the provider), younger age (ie, pediatric patients), or skin barrier disruption. Dermatologists play a particularly important role in identifying cutaneous risk factors that may indicate progression of infection (eg, atopic dermatitis, severe acne, intertrigo, Darier disease). Kaposi varicelliform eruption is the phenomenon where a more typically localized vesicular infection is disseminated to areas with a defective skin barrier.2 Eczema herpeticum refers to the most common type of KVE due to herpes simplex virus, but other known etiologies of KVE include coxsackievirus A16, vaccinia virus, varicella-zoster virus, and smallpox.2 Although classic mpox previously had only the theoretical potential to lead to a secondary KVE, we expect the literature to evolve as cases spread, with one recent report of eczema monkeypoxicum in the setting of atopic dermatitis.4
At the time of publication, mpox cases have notably dropped globally due to public health interventions; however, mpox infections are ongoing in areas previously identified as nonendemic. Given the distinctive risk factors and clinical presentations of this most recent outbreak, clinicians will need to be adept at identifying not only infection but also risk for dissemination, including skin barrier disruption.
- Centers for Disease Control and Prevention. Mpox: 2022 US map & case count. Updated February 15, 2023. Accessed February 23, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/us-map.html
- Karray M, Kwan E, Souissi A. Kaposi varicelliform eruption. StatPearls. Updated September 12, 2022. Accessed February 24, 2023. https://www.ncbi.nlm.nih.gov/books/NBK482432
- Girometti N, Byrne R, Bracchi M, et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Lancet Infect Dis. 2022;S1473-3099(22)00411-X. doi:10.1016/S1473-3099(22)00411-X
- Xia J, Huang CL, Chu P, et al. Eczema monkeypoxicum: report of monkeypox transmission in patients with atopic dermatitis. JAAD Case Reports. 2022;29:95-99.
- Centers for Disease Control and Prevention. Mpox: 2022 US map & case count. Updated February 15, 2023. Accessed February 23, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/us-map.html
- Karray M, Kwan E, Souissi A. Kaposi varicelliform eruption. StatPearls. Updated September 12, 2022. Accessed February 24, 2023. https://www.ncbi.nlm.nih.gov/books/NBK482432
- Girometti N, Byrne R, Bracchi M, et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Lancet Infect Dis. 2022;S1473-3099(22)00411-X. doi:10.1016/S1473-3099(22)00411-X
- Xia J, Huang CL, Chu P, et al. Eczema monkeypoxicum: report of monkeypox transmission in patients with atopic dermatitis. JAAD Case Reports. 2022;29:95-99.
Practice Points
- Desquamation can be associated with dissemination and higher severity course in the setting of mpox (monkeypox) viral infection.
- Antiviral treatment with tecovirimat is warranted in those with severe mpox infection or those at risk of severe infection including skin barrier disruption.
- Kaposi varicelliform–like eruptions can happen in the setting of barrier disruption from peeling sunburns, atopic dermatitis, severe acne, and other dermatologic conditions.
No Wrong Floor on the Elevator: A Vision for the VA as an Age-Friendly Health System
One morning I stepped into the elevator in the lobby of the US Department of Veterans Affairs (VA) medical center where I work, holding a cup of coffee, joining another staffer, a middle-aged man, wearing a veteran’s pin on his employee badge. An older veteran slowly approached the elevator doors, shuffling with each step, and since he was at the front of the elevator, he cheerfully bellowed “Which floor?” as he offered to push the button for us.
“What’s on 12?” he asked in a jovial voice. I smiled. “Aging research,” referring to the Geriatrics Research Education and Clinical Center where I work.1
“I definitely need that—I forgot where I’m going!” he joked, his fingers hovering over the elevator buttons.
As we reached his floor, the doors opened, he waved with a smile and unsteadily made his way out of the elevator and down the hall to his appointment. As the elevator doors closed behind him, the other staffer turned to me and said with a shrug, “That’ll be me one day,” as he got off at the next floor.
When I got off the elevator and walked toward my office, I reflected on the care that I as a geriatrician and we at the VA hope to provide to aging veterans, now and in the future: Age-Friendly care. Age-Friendly means the compassionate care that we want for those who have served our country, for our loved ones, and for ourselves as we age. Age-Friendly means person-centered, evidence-based care that as we grow older will help us to address challenges that may come with older age, such as falls, cognitive impairment, and polypharmacy. Too often the health care system remains focused on the chief concern or on a clinician’s specialty and may not focus on those important areas where we can potentially intervene to support aging veterans.
The VA has set a goal to become the largest Age-Friendly Health System (AFHS) in the country.2 Led by the Institute for Healthcare Improvement and funded by the John A. Hartford Foundation, the Age-Friendly Health Systems Initiative aims to help clinicians and care settings “follow an essential set of evidence-based practices; cause no harm; and align with what matters to the older adult and their family caregivers.”3 An AFHS cares for older adults with attention to the 4Ms—What Matters, Mobility, Mentation, and Medications.4 Specifically, in an AFHS, older adults are asked what matters to them so we can align their health care with their goals; clinicians evaluate veterans for safe mobility and fall risk reduction, cognitive impairment and mood disorders, and identify and avoid high-risk medications.5 In an AFHS, the 4Ms are practiced as a set, reliably, across settings, so that there should be no wrong door or wrong floor for an older veteran to receive Age-Friendly care within the VA health care system.6
I thought of the veteran with the sense of humor getting off the elevator and wondered whether the clinician seeing him would have training in some of the many VA resources available for delivering Age-Friendly care (Table).
Too often our health care system and health professions education have left clinicians unprepared to care for older adults using an Age-Friendly framework; rather, we have been trained in problem-based or disease-based care that can miss the forest for the trees in an older adult living with multiple chronic conditions and/or frailty. We may focus on providing evidence-based care for individual medical conditions while neglecting the often practical interventions that can help an older person age in place by focusing on what matters, supporting safe mobility, addressing cognition and mood, and optimizing medications.18
The vision of the VA as the largest AFHS in America is urgently needed; nearly half of the veteran population is aged 65 ≥ years, compared with 16% of the general population.19 Building on the VA’s legacy of creativity and innovation in geriatrics, and the VA’s goal of being a high reliability organization, becoming an AFHS will ensure that for that older veteran stepping off that elevator there is no wrong floor, and no wrong door to receive the Age-Friendly care he deserves and that we all hope for as we age.1,5,19,20
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Veterans Affairs Boston Healthcare System and the New England Geriatric Research Education and Clinical Center.
1. Supiano MA, Alessi C, Chernoff R, Goldberg A, Morley JE, Schmader KE, Shay K; GRECC Directors Association. Department of Veterans Affairs Geriatric Research, Education and Clinical Centers: translating aging research into clinical geriatrics. J Am Geriatr Soc. 2012;60(7):1347-1356. doi:10.1111/j.1532-5415.2012.04004.x
2. US Department of Veterans Affairs. VA geriatrics and extended care: the Age-Friendly Health Systems Initiative. Updated July 29, 2022. Accessed February 8, 2023. https://www.va.gov/geriatrics/pages/VA_Age_Friendly_Health_Systems_Initiative.asp
3. What is an age-friendly health system? Accessed November 15, 2022. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/default.aspx
4. Mate KS, Berman A, Laderman M, Kabcenell A, Fulmer T. Creating age-friendly health systems - a vision for better care of older adults. Healthc (Amst). 2018;6(1):4-6. doi:10.1016/j.hjdsi.2017.05.005
5. Church K, Munro S, Shaughnessy M, Clancy C. Age-friendly health systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. Published online December 7, 2022. doi:10.1111/1475-6773.14110
6. Emery-Tiburcio EE, Berg-Weger M, Husser EK, et al. The geriatrics education and care revolution: diverse implementation of age-friendly health systems. J Am Geriatr Soc. Published online October 8, 2021. doi:10.1111/jgs.17497
7. James K, Schwartz AW, Orkaby AR. Mobility assessment in older adults. N Engl J Med. 2021;385(8):e22. doi:10.1056/NEJMvcm2009406
8. Harris R, Bean J. The Llive Long Walk Strong clinical rehabilitation program. Arch Phys Med Rehabil. 2019;100(12):e205. doi:10.1016/j.arrct.2022.100205
9. Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit program. J Am Geriatr Soc. 2018;66(5):1009-1016. doi:10.1111/jgs.15276
10. McCarten JR, Anderson P, Kuskowski MA, McPherson SE, Borson S. Screening for cognitive impairment in an elderly veteran population: acceptability and results using different versions of the Mini-Cog. J Am Geriatr Soc. 2011;59(2):309-313. doi:10.1111/j.1532-5415.2010.03249.x
11. American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
12. Linsky A, Gellad WF, Linder JA, Friedberg MW. Advancing the science of deprescribing: a novel comprehensive conceptual framework. J Am Geriatr Soc. 2019;67(10):2018-2022. doi:10.1111/jgs.16136
13. Battar S, Watson Dickerson KR, Sedgwick C, Cmelik T. Understanding principles of high reliability organizations through the eyes of VIONE: a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
14. Tinetti ME, Naik AD, Dindo L, et al. Association of patient priorities-aligned decision-making with patient outcomes and ambulatory health care burden among older adults with multiple chronic conditions: a nonrandomized clinical trial. JAMA Intern Med. 2019;179(12):1688. doi:10.1001/jamainternmed.2019.4235
15. Levy C, Ersek M, Scott W, et al. Life-sustaining treatment decisions initiative: early implementation results of a national Veterans Affairs program to honor veterans’ care preferences. J Gen Intern Med. 2020;35(6):1803-1812. doi:10.1007/s11606-020-05697-2
16. Nathan S, Fiore LL, Saunders S, et al. My life, my story: teaching patient centered care competencies for older adults through life story work. Gerontol Geriatr Educ. 2022;43(2):225-238. doi:10.1080/02701960.2019.1665038
17. Reddy KP, Schult TM, Whitehead AM, Bokhour BG. Veterans Health Administration’s whole health system of care: supporting the health, well-being, and resiliency of employees. Glob Adv Health Med. 2021;10:21649561211022696. doi:10.1177/21649561211022698
18. Aronson L. Necessary steps: how health care fails older patients, and how it can be done better. Health Aff (Millwood). 2015;34(3):528-532. doi:10.1377/hlthaff.2014.1238
19. Farrell TW, Volden TA, Butler JM, et al. Age-friendly care in the Veterans Health Administration: past, present, and future. J Am Geriatr Soc. 2023;71(1):18-25. doi:10.1111/jgs.18070
20. Burke RE, Brown RT, Kinosian B. Selecting implementation strategies to drive age-friendly health system adoption. J Am Geriatr Soc. 2022;70(1):313-318. doi:10.1111/jgs.17489
21. Centers for Disease Control and Prevention. STEADI- older adult fall prevention. July 26,2021. Updated July 26, 2021. Accessed February 6, 2023. https://www.cdc.gov/steadi/index.html
22. Exercise and physical activity. National Institute on Aging. Accessed February 6, 2023. https://www.nia.nih.gov/health/topics/exercise-and-physical-activity
23. Hastings SN, Sloane R, Morey MC, Pavon JM, Hoenig H. Assisted early mobility for hospitalized older veterans: preliminary data from the STRIDE program. J Am Geriatr Soc. 2014;62(11):2180-2184.
24. Ashcroft T, Middleton A, Driver JA, Ruopp M, Harris R, Bean JF. An innovative rehabilitation program for the Veterans Affairs post-acute skilled nursing setting: preliminary results. J Am Geriatr Soc. 2023;10.1111/jgs.18214. doi:10.1111/jgs.18214
25. AGS CoCare. Accessed February 6, 2023. https://www.americangeriatrics.org/programs/ags-cocarer
26. Jedele JM, Curyto K, Ludwin BM, Karel MJ. Addressing behavioral symptoms of dementia through STAR-VA implementation: do outcomes vary by behavior type? Am J Alzheimers Dis Other Demen. 2020;35:1533317520911577.
27. Phung E, Triantafylidis L, Zhang H, Yeh IM. New Media, Part 5: Online Deprescribing Tools. J Palliat Med. 2018;21(2):269-270.
28. Freytag J, Dindo L, Catic A, et al. Feasibility of clinicians aligning health care with patient priorities in geriatrics ambulatory care. J Am Geriatr Soc. 2020;68(9):2112-2116.
29. The Conversation Project. Accessed February 22, 2023. https://theconversationproject.org
30. Daubman BR, Bernacki R, Stoltenberg M, Wilson E, Jacobsen J. Best practices for teaching clinicians to use a serious illness conversation guide. Palliat Med Rep. 2020;1(1):135-142. Published 2020 Jul 28. doi:10.1089/pmr.2020.0066
31. Freytag J, Street RL Jr, Barnes DE, et al. Empowering older adults to discuss advance care planning during clinical visits: The PREPARE Randomized Trial. J Am Geriatr Soc. 2020;68(6):1210-1217. doi:10.1111/jgs.16405
One morning I stepped into the elevator in the lobby of the US Department of Veterans Affairs (VA) medical center where I work, holding a cup of coffee, joining another staffer, a middle-aged man, wearing a veteran’s pin on his employee badge. An older veteran slowly approached the elevator doors, shuffling with each step, and since he was at the front of the elevator, he cheerfully bellowed “Which floor?” as he offered to push the button for us.
“What’s on 12?” he asked in a jovial voice. I smiled. “Aging research,” referring to the Geriatrics Research Education and Clinical Center where I work.1
“I definitely need that—I forgot where I’m going!” he joked, his fingers hovering over the elevator buttons.
As we reached his floor, the doors opened, he waved with a smile and unsteadily made his way out of the elevator and down the hall to his appointment. As the elevator doors closed behind him, the other staffer turned to me and said with a shrug, “That’ll be me one day,” as he got off at the next floor.
When I got off the elevator and walked toward my office, I reflected on the care that I as a geriatrician and we at the VA hope to provide to aging veterans, now and in the future: Age-Friendly care. Age-Friendly means the compassionate care that we want for those who have served our country, for our loved ones, and for ourselves as we age. Age-Friendly means person-centered, evidence-based care that as we grow older will help us to address challenges that may come with older age, such as falls, cognitive impairment, and polypharmacy. Too often the health care system remains focused on the chief concern or on a clinician’s specialty and may not focus on those important areas where we can potentially intervene to support aging veterans.
The VA has set a goal to become the largest Age-Friendly Health System (AFHS) in the country.2 Led by the Institute for Healthcare Improvement and funded by the John A. Hartford Foundation, the Age-Friendly Health Systems Initiative aims to help clinicians and care settings “follow an essential set of evidence-based practices; cause no harm; and align with what matters to the older adult and their family caregivers.”3 An AFHS cares for older adults with attention to the 4Ms—What Matters, Mobility, Mentation, and Medications.4 Specifically, in an AFHS, older adults are asked what matters to them so we can align their health care with their goals; clinicians evaluate veterans for safe mobility and fall risk reduction, cognitive impairment and mood disorders, and identify and avoid high-risk medications.5 In an AFHS, the 4Ms are practiced as a set, reliably, across settings, so that there should be no wrong door or wrong floor for an older veteran to receive Age-Friendly care within the VA health care system.6
I thought of the veteran with the sense of humor getting off the elevator and wondered whether the clinician seeing him would have training in some of the many VA resources available for delivering Age-Friendly care (Table).
Too often our health care system and health professions education have left clinicians unprepared to care for older adults using an Age-Friendly framework; rather, we have been trained in problem-based or disease-based care that can miss the forest for the trees in an older adult living with multiple chronic conditions and/or frailty. We may focus on providing evidence-based care for individual medical conditions while neglecting the often practical interventions that can help an older person age in place by focusing on what matters, supporting safe mobility, addressing cognition and mood, and optimizing medications.18
The vision of the VA as the largest AFHS in America is urgently needed; nearly half of the veteran population is aged 65 ≥ years, compared with 16% of the general population.19 Building on the VA’s legacy of creativity and innovation in geriatrics, and the VA’s goal of being a high reliability organization, becoming an AFHS will ensure that for that older veteran stepping off that elevator there is no wrong floor, and no wrong door to receive the Age-Friendly care he deserves and that we all hope for as we age.1,5,19,20
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Veterans Affairs Boston Healthcare System and the New England Geriatric Research Education and Clinical Center.
One morning I stepped into the elevator in the lobby of the US Department of Veterans Affairs (VA) medical center where I work, holding a cup of coffee, joining another staffer, a middle-aged man, wearing a veteran’s pin on his employee badge. An older veteran slowly approached the elevator doors, shuffling with each step, and since he was at the front of the elevator, he cheerfully bellowed “Which floor?” as he offered to push the button for us.
“What’s on 12?” he asked in a jovial voice. I smiled. “Aging research,” referring to the Geriatrics Research Education and Clinical Center where I work.1
“I definitely need that—I forgot where I’m going!” he joked, his fingers hovering over the elevator buttons.
As we reached his floor, the doors opened, he waved with a smile and unsteadily made his way out of the elevator and down the hall to his appointment. As the elevator doors closed behind him, the other staffer turned to me and said with a shrug, “That’ll be me one day,” as he got off at the next floor.
When I got off the elevator and walked toward my office, I reflected on the care that I as a geriatrician and we at the VA hope to provide to aging veterans, now and in the future: Age-Friendly care. Age-Friendly means the compassionate care that we want for those who have served our country, for our loved ones, and for ourselves as we age. Age-Friendly means person-centered, evidence-based care that as we grow older will help us to address challenges that may come with older age, such as falls, cognitive impairment, and polypharmacy. Too often the health care system remains focused on the chief concern or on a clinician’s specialty and may not focus on those important areas where we can potentially intervene to support aging veterans.
The VA has set a goal to become the largest Age-Friendly Health System (AFHS) in the country.2 Led by the Institute for Healthcare Improvement and funded by the John A. Hartford Foundation, the Age-Friendly Health Systems Initiative aims to help clinicians and care settings “follow an essential set of evidence-based practices; cause no harm; and align with what matters to the older adult and their family caregivers.”3 An AFHS cares for older adults with attention to the 4Ms—What Matters, Mobility, Mentation, and Medications.4 Specifically, in an AFHS, older adults are asked what matters to them so we can align their health care with their goals; clinicians evaluate veterans for safe mobility and fall risk reduction, cognitive impairment and mood disorders, and identify and avoid high-risk medications.5 In an AFHS, the 4Ms are practiced as a set, reliably, across settings, so that there should be no wrong door or wrong floor for an older veteran to receive Age-Friendly care within the VA health care system.6
I thought of the veteran with the sense of humor getting off the elevator and wondered whether the clinician seeing him would have training in some of the many VA resources available for delivering Age-Friendly care (Table).
Too often our health care system and health professions education have left clinicians unprepared to care for older adults using an Age-Friendly framework; rather, we have been trained in problem-based or disease-based care that can miss the forest for the trees in an older adult living with multiple chronic conditions and/or frailty. We may focus on providing evidence-based care for individual medical conditions while neglecting the often practical interventions that can help an older person age in place by focusing on what matters, supporting safe mobility, addressing cognition and mood, and optimizing medications.18
The vision of the VA as the largest AFHS in America is urgently needed; nearly half of the veteran population is aged 65 ≥ years, compared with 16% of the general population.19 Building on the VA’s legacy of creativity and innovation in geriatrics, and the VA’s goal of being a high reliability organization, becoming an AFHS will ensure that for that older veteran stepping off that elevator there is no wrong floor, and no wrong door to receive the Age-Friendly care he deserves and that we all hope for as we age.1,5,19,20
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Veterans Affairs Boston Healthcare System and the New England Geriatric Research Education and Clinical Center.
1. Supiano MA, Alessi C, Chernoff R, Goldberg A, Morley JE, Schmader KE, Shay K; GRECC Directors Association. Department of Veterans Affairs Geriatric Research, Education and Clinical Centers: translating aging research into clinical geriatrics. J Am Geriatr Soc. 2012;60(7):1347-1356. doi:10.1111/j.1532-5415.2012.04004.x
2. US Department of Veterans Affairs. VA geriatrics and extended care: the Age-Friendly Health Systems Initiative. Updated July 29, 2022. Accessed February 8, 2023. https://www.va.gov/geriatrics/pages/VA_Age_Friendly_Health_Systems_Initiative.asp
3. What is an age-friendly health system? Accessed November 15, 2022. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/default.aspx
4. Mate KS, Berman A, Laderman M, Kabcenell A, Fulmer T. Creating age-friendly health systems - a vision for better care of older adults. Healthc (Amst). 2018;6(1):4-6. doi:10.1016/j.hjdsi.2017.05.005
5. Church K, Munro S, Shaughnessy M, Clancy C. Age-friendly health systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. Published online December 7, 2022. doi:10.1111/1475-6773.14110
6. Emery-Tiburcio EE, Berg-Weger M, Husser EK, et al. The geriatrics education and care revolution: diverse implementation of age-friendly health systems. J Am Geriatr Soc. Published online October 8, 2021. doi:10.1111/jgs.17497
7. James K, Schwartz AW, Orkaby AR. Mobility assessment in older adults. N Engl J Med. 2021;385(8):e22. doi:10.1056/NEJMvcm2009406
8. Harris R, Bean J. The Llive Long Walk Strong clinical rehabilitation program. Arch Phys Med Rehabil. 2019;100(12):e205. doi:10.1016/j.arrct.2022.100205
9. Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit program. J Am Geriatr Soc. 2018;66(5):1009-1016. doi:10.1111/jgs.15276
10. McCarten JR, Anderson P, Kuskowski MA, McPherson SE, Borson S. Screening for cognitive impairment in an elderly veteran population: acceptability and results using different versions of the Mini-Cog. J Am Geriatr Soc. 2011;59(2):309-313. doi:10.1111/j.1532-5415.2010.03249.x
11. American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
12. Linsky A, Gellad WF, Linder JA, Friedberg MW. Advancing the science of deprescribing: a novel comprehensive conceptual framework. J Am Geriatr Soc. 2019;67(10):2018-2022. doi:10.1111/jgs.16136
13. Battar S, Watson Dickerson KR, Sedgwick C, Cmelik T. Understanding principles of high reliability organizations through the eyes of VIONE: a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
14. Tinetti ME, Naik AD, Dindo L, et al. Association of patient priorities-aligned decision-making with patient outcomes and ambulatory health care burden among older adults with multiple chronic conditions: a nonrandomized clinical trial. JAMA Intern Med. 2019;179(12):1688. doi:10.1001/jamainternmed.2019.4235
15. Levy C, Ersek M, Scott W, et al. Life-sustaining treatment decisions initiative: early implementation results of a national Veterans Affairs program to honor veterans’ care preferences. J Gen Intern Med. 2020;35(6):1803-1812. doi:10.1007/s11606-020-05697-2
16. Nathan S, Fiore LL, Saunders S, et al. My life, my story: teaching patient centered care competencies for older adults through life story work. Gerontol Geriatr Educ. 2022;43(2):225-238. doi:10.1080/02701960.2019.1665038
17. Reddy KP, Schult TM, Whitehead AM, Bokhour BG. Veterans Health Administration’s whole health system of care: supporting the health, well-being, and resiliency of employees. Glob Adv Health Med. 2021;10:21649561211022696. doi:10.1177/21649561211022698
18. Aronson L. Necessary steps: how health care fails older patients, and how it can be done better. Health Aff (Millwood). 2015;34(3):528-532. doi:10.1377/hlthaff.2014.1238
19. Farrell TW, Volden TA, Butler JM, et al. Age-friendly care in the Veterans Health Administration: past, present, and future. J Am Geriatr Soc. 2023;71(1):18-25. doi:10.1111/jgs.18070
20. Burke RE, Brown RT, Kinosian B. Selecting implementation strategies to drive age-friendly health system adoption. J Am Geriatr Soc. 2022;70(1):313-318. doi:10.1111/jgs.17489
21. Centers for Disease Control and Prevention. STEADI- older adult fall prevention. July 26,2021. Updated July 26, 2021. Accessed February 6, 2023. https://www.cdc.gov/steadi/index.html
22. Exercise and physical activity. National Institute on Aging. Accessed February 6, 2023. https://www.nia.nih.gov/health/topics/exercise-and-physical-activity
23. Hastings SN, Sloane R, Morey MC, Pavon JM, Hoenig H. Assisted early mobility for hospitalized older veterans: preliminary data from the STRIDE program. J Am Geriatr Soc. 2014;62(11):2180-2184.
24. Ashcroft T, Middleton A, Driver JA, Ruopp M, Harris R, Bean JF. An innovative rehabilitation program for the Veterans Affairs post-acute skilled nursing setting: preliminary results. J Am Geriatr Soc. 2023;10.1111/jgs.18214. doi:10.1111/jgs.18214
25. AGS CoCare. Accessed February 6, 2023. https://www.americangeriatrics.org/programs/ags-cocarer
26. Jedele JM, Curyto K, Ludwin BM, Karel MJ. Addressing behavioral symptoms of dementia through STAR-VA implementation: do outcomes vary by behavior type? Am J Alzheimers Dis Other Demen. 2020;35:1533317520911577.
27. Phung E, Triantafylidis L, Zhang H, Yeh IM. New Media, Part 5: Online Deprescribing Tools. J Palliat Med. 2018;21(2):269-270.
28. Freytag J, Dindo L, Catic A, et al. Feasibility of clinicians aligning health care with patient priorities in geriatrics ambulatory care. J Am Geriatr Soc. 2020;68(9):2112-2116.
29. The Conversation Project. Accessed February 22, 2023. https://theconversationproject.org
30. Daubman BR, Bernacki R, Stoltenberg M, Wilson E, Jacobsen J. Best practices for teaching clinicians to use a serious illness conversation guide. Palliat Med Rep. 2020;1(1):135-142. Published 2020 Jul 28. doi:10.1089/pmr.2020.0066
31. Freytag J, Street RL Jr, Barnes DE, et al. Empowering older adults to discuss advance care planning during clinical visits: The PREPARE Randomized Trial. J Am Geriatr Soc. 2020;68(6):1210-1217. doi:10.1111/jgs.16405
1. Supiano MA, Alessi C, Chernoff R, Goldberg A, Morley JE, Schmader KE, Shay K; GRECC Directors Association. Department of Veterans Affairs Geriatric Research, Education and Clinical Centers: translating aging research into clinical geriatrics. J Am Geriatr Soc. 2012;60(7):1347-1356. doi:10.1111/j.1532-5415.2012.04004.x
2. US Department of Veterans Affairs. VA geriatrics and extended care: the Age-Friendly Health Systems Initiative. Updated July 29, 2022. Accessed February 8, 2023. https://www.va.gov/geriatrics/pages/VA_Age_Friendly_Health_Systems_Initiative.asp
3. What is an age-friendly health system? Accessed November 15, 2022. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/default.aspx
4. Mate KS, Berman A, Laderman M, Kabcenell A, Fulmer T. Creating age-friendly health systems - a vision for better care of older adults. Healthc (Amst). 2018;6(1):4-6. doi:10.1016/j.hjdsi.2017.05.005
5. Church K, Munro S, Shaughnessy M, Clancy C. Age-friendly health systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. Published online December 7, 2022. doi:10.1111/1475-6773.14110
6. Emery-Tiburcio EE, Berg-Weger M, Husser EK, et al. The geriatrics education and care revolution: diverse implementation of age-friendly health systems. J Am Geriatr Soc. Published online October 8, 2021. doi:10.1111/jgs.17497
7. James K, Schwartz AW, Orkaby AR. Mobility assessment in older adults. N Engl J Med. 2021;385(8):e22. doi:10.1056/NEJMvcm2009406
8. Harris R, Bean J. The Llive Long Walk Strong clinical rehabilitation program. Arch Phys Med Rehabil. 2019;100(12):e205. doi:10.1016/j.arrct.2022.100205
9. Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit program. J Am Geriatr Soc. 2018;66(5):1009-1016. doi:10.1111/jgs.15276
10. McCarten JR, Anderson P, Kuskowski MA, McPherson SE, Borson S. Screening for cognitive impairment in an elderly veteran population: acceptability and results using different versions of the Mini-Cog. J Am Geriatr Soc. 2011;59(2):309-313. doi:10.1111/j.1532-5415.2010.03249.x
11. American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
12. Linsky A, Gellad WF, Linder JA, Friedberg MW. Advancing the science of deprescribing: a novel comprehensive conceptual framework. J Am Geriatr Soc. 2019;67(10):2018-2022. doi:10.1111/jgs.16136
13. Battar S, Watson Dickerson KR, Sedgwick C, Cmelik T. Understanding principles of high reliability organizations through the eyes of VIONE: a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
14. Tinetti ME, Naik AD, Dindo L, et al. Association of patient priorities-aligned decision-making with patient outcomes and ambulatory health care burden among older adults with multiple chronic conditions: a nonrandomized clinical trial. JAMA Intern Med. 2019;179(12):1688. doi:10.1001/jamainternmed.2019.4235
15. Levy C, Ersek M, Scott W, et al. Life-sustaining treatment decisions initiative: early implementation results of a national Veterans Affairs program to honor veterans’ care preferences. J Gen Intern Med. 2020;35(6):1803-1812. doi:10.1007/s11606-020-05697-2
16. Nathan S, Fiore LL, Saunders S, et al. My life, my story: teaching patient centered care competencies for older adults through life story work. Gerontol Geriatr Educ. 2022;43(2):225-238. doi:10.1080/02701960.2019.1665038
17. Reddy KP, Schult TM, Whitehead AM, Bokhour BG. Veterans Health Administration’s whole health system of care: supporting the health, well-being, and resiliency of employees. Glob Adv Health Med. 2021;10:21649561211022696. doi:10.1177/21649561211022698
18. Aronson L. Necessary steps: how health care fails older patients, and how it can be done better. Health Aff (Millwood). 2015;34(3):528-532. doi:10.1377/hlthaff.2014.1238
19. Farrell TW, Volden TA, Butler JM, et al. Age-friendly care in the Veterans Health Administration: past, present, and future. J Am Geriatr Soc. 2023;71(1):18-25. doi:10.1111/jgs.18070
20. Burke RE, Brown RT, Kinosian B. Selecting implementation strategies to drive age-friendly health system adoption. J Am Geriatr Soc. 2022;70(1):313-318. doi:10.1111/jgs.17489
21. Centers for Disease Control and Prevention. STEADI- older adult fall prevention. July 26,2021. Updated July 26, 2021. Accessed February 6, 2023. https://www.cdc.gov/steadi/index.html
22. Exercise and physical activity. National Institute on Aging. Accessed February 6, 2023. https://www.nia.nih.gov/health/topics/exercise-and-physical-activity
23. Hastings SN, Sloane R, Morey MC, Pavon JM, Hoenig H. Assisted early mobility for hospitalized older veterans: preliminary data from the STRIDE program. J Am Geriatr Soc. 2014;62(11):2180-2184.
24. Ashcroft T, Middleton A, Driver JA, Ruopp M, Harris R, Bean JF. An innovative rehabilitation program for the Veterans Affairs post-acute skilled nursing setting: preliminary results. J Am Geriatr Soc. 2023;10.1111/jgs.18214. doi:10.1111/jgs.18214
25. AGS CoCare. Accessed February 6, 2023. https://www.americangeriatrics.org/programs/ags-cocarer
26. Jedele JM, Curyto K, Ludwin BM, Karel MJ. Addressing behavioral symptoms of dementia through STAR-VA implementation: do outcomes vary by behavior type? Am J Alzheimers Dis Other Demen. 2020;35:1533317520911577.
27. Phung E, Triantafylidis L, Zhang H, Yeh IM. New Media, Part 5: Online Deprescribing Tools. J Palliat Med. 2018;21(2):269-270.
28. Freytag J, Dindo L, Catic A, et al. Feasibility of clinicians aligning health care with patient priorities in geriatrics ambulatory care. J Am Geriatr Soc. 2020;68(9):2112-2116.
29. The Conversation Project. Accessed February 22, 2023. https://theconversationproject.org
30. Daubman BR, Bernacki R, Stoltenberg M, Wilson E, Jacobsen J. Best practices for teaching clinicians to use a serious illness conversation guide. Palliat Med Rep. 2020;1(1):135-142. Published 2020 Jul 28. doi:10.1089/pmr.2020.0066
31. Freytag J, Street RL Jr, Barnes DE, et al. Empowering older adults to discuss advance care planning during clinical visits: The PREPARE Randomized Trial. J Am Geriatr Soc. 2020;68(6):1210-1217. doi:10.1111/jgs.16405