User login
LGBTQ+ teens in homophobic high schools
I am a psychiatrist now but had another life teaching English in public high school for 17 years. My teaching life, in which I was an openly gay teacher, spanned 2001-2018 and was divided between two urban California schools – in Berkeley and San Leandro. I came out by responding honestly to student questions about whether I had a girlfriend, and what I did over the weekend. At Berkeley High my openness wasn’t an issue at all. The school had a vibrant Gay Straight Alliance/GSA for years, there were many openly gay staff and many openly gay students. No students felt the need to come out to me in search of a gay mentor.
Two years later, I began teaching in San Leandro, 20 miles away, and it was a lesson in how even the San Francisco Bay Area, an LGBTQ+ bastion, could harbor homophobia. When I was hired in 2003, San Leandro High had one openly gay teacher, Q. I quickly realized how much braver his coming out was compared with mine in Berkeley.
In San Leandro, gay slurs were heard nonstop in the hallways, no students were out, and by the end of my first year Q had quit, confiding in me that he couldn’t handle the homophobic harassment from students anymore. There was no GSA. A few years ago, two lesbians had held hands during lunch and inspired the wrath of a group of parents who advocated for their expulsion. In response, a teacher tried to introduce gay sensitivity training into his class and the same group of parents tried to get him fired. He was reprimanded by the principal, he countersued in a case that went all the way to the California Supreme Court, and won. Comparing these two local high schools reinforced to me how visibility really matters in creating a childhood experience that is nurturing versus traumatizing.1
Two Chinese girls in love
N and T were two Chinese girls who grew up in San Leandro. They went to the same elementary school and had crushes on each other since then. In their junior year, they joined our first student GSA, becoming president and vice-president. They were out. And, of course, they must’ve known that their families, who would not have been supportive, would become aware. I remember sitting at an outdoor concert when I got a text from N warning me her father had found out and blamed me for having corrupted her. He planned on coming to school to demand I be fired. And such was the unrelenting pressure that N and T faced every time they went home from school and sat at their dinner tables. Eventually, they broke up. They didn’t do so tearfully, but more wearily.
This story illustrates how difficult it is for love between two LGBTQ+ teens to be nurtured. Love in youth can already be volatile because of the lack of emotional regulation and experience. The questioning of identity and the threat of family disintegration at a time when these teens do not have the economic means to protect themselves makes love dangerous. It is no wonder that gay teens are at increased risk for homelessness.2
The family incident that led to the girls’ breakup reveals how culture affects homophobic pressure. N resisted her parents’ disapproval for months, but she capitulated when her father had a heart attack and blamed it on her. “And it’s true,” N confided. “After my parents found out, they were continually stressed. I could see it affect their health. And it breaks my heart to see my dad in the hospital.”
For N, she had not capitulated from fear, but perhaps because of filial piety, or one’s obligation to protect one’s parent. It was a choice between two heartbreaks. Double minorities, like N and T, face a double threat and often can find no safe place. One of my patients who is gay and Black put it best: “It’s like being beaten up at school only to come home to another beating.” This double threat is evidenced by the higher suicide risk of ethnicities who are LGBTQ+ relative to their white counterparts.3
The confusion of a gay athlete
R was a star point guard, a senior who had secured an athletic scholarship, and was recognized as the best athlete in our county. A popular boy, he flaunted his physique and flirted with all the girls. And then when he was enrolled in my class, he began flirting with all the boys, too. There was gossip that R was bisexual. Then one day, not unexpectedly, he came out to me as gay. He admitted he only flirted with girls for his reputation.
By this time many students had come out to me but he flirted with me with his revelation. I corrected him and warned him unequivocally that it was inappropriate but I was worried because I knew he had placed his trust in me. I also knew he came from a homophobic family that was violent – his father had attacked him physically at a school game and our coaches had to pull him off.
Instinctively, I felt I had to have a witness so I confided in another teacher and documented the situation meticulously. Then, one day, just as I feared, he went too far. He stayed after class and said he wanted to show me something on his phone. And that something turned out to be a picture of himself naked. I immediately confiscated the phone and reported it to the administration. This was not how I wanted him to come out: His family notified by the police that he had sexually harassed his teacher, expulsion pending, and scholarship inevitably revoked. Fortunately, we did find a resolution that restored R’s future.
Let’s examine the circumstances that could’ve informed his transgressive behavior. If we consider sexual harassment a form of bullying, R’s history of having a father who publicly bullied him – and may have bullied others in front of him – is a known risk factor.4 It is also common knowledge that organized team sports were and still are a bastion of homophobia and that gay athletes had to accept a culture of explicit homophobia.5
So, it is not hard to understand the constant public pressures that R faced in addition to those from his family. Let’s also consider that appropriate sexual behaviors are not something we are born with, but something that is learned. Of course, inappropriate sexual behavior also happens in the heterosexual world. But heterosexual sexual behavior often has more accepted paths of trial and error. Children experiment with these behaviors and are corrected by adults and older peers as they mature.
However, for homosexual behaviors, there is not usually the fine-tuning about what is appropriate.
Summary
An educational environment where LGBTQ+ persons are highly visible and accepted is a more nurturing environment for LGBTQ teens than one that is not. Specific subcultures within the LGBTQ population involving race, culture, gender, and athletics modulate the experience of coming out and the nature of homophobic oppression.
Dr. Nguyen is a first-year psychiatry resident at the University of San Francisco School of Medicine at Fresno.
References
1. Kosciw JG et al. The effect of negative school climate on academic outcomes for LGBT youth and the role of in-school supports. J Sch Violence. 2013;12(1):45-63.
2. Center for American Progress. Gay and Transgender Youth Homelessness by the Numbers. June 21, 2010).
3. O’Donnell S et al. Increased risk of suicide attempts among Black and Latino lesbians, gay men, and bisexuals. Am J Public Health. 2011;101(6):1055-9.
4. Farrington D and Baldry A. Individual risk factors for school bullying. J Aggress Confl Peace Res. 2010 Jan;2(1):4-16.
5. Anderson E. Openly gay athletes: Contesting hegemonic masculinity in a homophobic environment Gend Soc. 2002 Dec:16(6):860-77.
I am a psychiatrist now but had another life teaching English in public high school for 17 years. My teaching life, in which I was an openly gay teacher, spanned 2001-2018 and was divided between two urban California schools – in Berkeley and San Leandro. I came out by responding honestly to student questions about whether I had a girlfriend, and what I did over the weekend. At Berkeley High my openness wasn’t an issue at all. The school had a vibrant Gay Straight Alliance/GSA for years, there were many openly gay staff and many openly gay students. No students felt the need to come out to me in search of a gay mentor.
Two years later, I began teaching in San Leandro, 20 miles away, and it was a lesson in how even the San Francisco Bay Area, an LGBTQ+ bastion, could harbor homophobia. When I was hired in 2003, San Leandro High had one openly gay teacher, Q. I quickly realized how much braver his coming out was compared with mine in Berkeley.
In San Leandro, gay slurs were heard nonstop in the hallways, no students were out, and by the end of my first year Q had quit, confiding in me that he couldn’t handle the homophobic harassment from students anymore. There was no GSA. A few years ago, two lesbians had held hands during lunch and inspired the wrath of a group of parents who advocated for their expulsion. In response, a teacher tried to introduce gay sensitivity training into his class and the same group of parents tried to get him fired. He was reprimanded by the principal, he countersued in a case that went all the way to the California Supreme Court, and won. Comparing these two local high schools reinforced to me how visibility really matters in creating a childhood experience that is nurturing versus traumatizing.1
Two Chinese girls in love
N and T were two Chinese girls who grew up in San Leandro. They went to the same elementary school and had crushes on each other since then. In their junior year, they joined our first student GSA, becoming president and vice-president. They were out. And, of course, they must’ve known that their families, who would not have been supportive, would become aware. I remember sitting at an outdoor concert when I got a text from N warning me her father had found out and blamed me for having corrupted her. He planned on coming to school to demand I be fired. And such was the unrelenting pressure that N and T faced every time they went home from school and sat at their dinner tables. Eventually, they broke up. They didn’t do so tearfully, but more wearily.
This story illustrates how difficult it is for love between two LGBTQ+ teens to be nurtured. Love in youth can already be volatile because of the lack of emotional regulation and experience. The questioning of identity and the threat of family disintegration at a time when these teens do not have the economic means to protect themselves makes love dangerous. It is no wonder that gay teens are at increased risk for homelessness.2
The family incident that led to the girls’ breakup reveals how culture affects homophobic pressure. N resisted her parents’ disapproval for months, but she capitulated when her father had a heart attack and blamed it on her. “And it’s true,” N confided. “After my parents found out, they were continually stressed. I could see it affect their health. And it breaks my heart to see my dad in the hospital.”
For N, she had not capitulated from fear, but perhaps because of filial piety, or one’s obligation to protect one’s parent. It was a choice between two heartbreaks. Double minorities, like N and T, face a double threat and often can find no safe place. One of my patients who is gay and Black put it best: “It’s like being beaten up at school only to come home to another beating.” This double threat is evidenced by the higher suicide risk of ethnicities who are LGBTQ+ relative to their white counterparts.3
The confusion of a gay athlete
R was a star point guard, a senior who had secured an athletic scholarship, and was recognized as the best athlete in our county. A popular boy, he flaunted his physique and flirted with all the girls. And then when he was enrolled in my class, he began flirting with all the boys, too. There was gossip that R was bisexual. Then one day, not unexpectedly, he came out to me as gay. He admitted he only flirted with girls for his reputation.
By this time many students had come out to me but he flirted with me with his revelation. I corrected him and warned him unequivocally that it was inappropriate but I was worried because I knew he had placed his trust in me. I also knew he came from a homophobic family that was violent – his father had attacked him physically at a school game and our coaches had to pull him off.
Instinctively, I felt I had to have a witness so I confided in another teacher and documented the situation meticulously. Then, one day, just as I feared, he went too far. He stayed after class and said he wanted to show me something on his phone. And that something turned out to be a picture of himself naked. I immediately confiscated the phone and reported it to the administration. This was not how I wanted him to come out: His family notified by the police that he had sexually harassed his teacher, expulsion pending, and scholarship inevitably revoked. Fortunately, we did find a resolution that restored R’s future.
Let’s examine the circumstances that could’ve informed his transgressive behavior. If we consider sexual harassment a form of bullying, R’s history of having a father who publicly bullied him – and may have bullied others in front of him – is a known risk factor.4 It is also common knowledge that organized team sports were and still are a bastion of homophobia and that gay athletes had to accept a culture of explicit homophobia.5
So, it is not hard to understand the constant public pressures that R faced in addition to those from his family. Let’s also consider that appropriate sexual behaviors are not something we are born with, but something that is learned. Of course, inappropriate sexual behavior also happens in the heterosexual world. But heterosexual sexual behavior often has more accepted paths of trial and error. Children experiment with these behaviors and are corrected by adults and older peers as they mature.
However, for homosexual behaviors, there is not usually the fine-tuning about what is appropriate.
Summary
An educational environment where LGBTQ+ persons are highly visible and accepted is a more nurturing environment for LGBTQ teens than one that is not. Specific subcultures within the LGBTQ population involving race, culture, gender, and athletics modulate the experience of coming out and the nature of homophobic oppression.
Dr. Nguyen is a first-year psychiatry resident at the University of San Francisco School of Medicine at Fresno.
References
1. Kosciw JG et al. The effect of negative school climate on academic outcomes for LGBT youth and the role of in-school supports. J Sch Violence. 2013;12(1):45-63.
2. Center for American Progress. Gay and Transgender Youth Homelessness by the Numbers. June 21, 2010).
3. O’Donnell S et al. Increased risk of suicide attempts among Black and Latino lesbians, gay men, and bisexuals. Am J Public Health. 2011;101(6):1055-9.
4. Farrington D and Baldry A. Individual risk factors for school bullying. J Aggress Confl Peace Res. 2010 Jan;2(1):4-16.
5. Anderson E. Openly gay athletes: Contesting hegemonic masculinity in a homophobic environment Gend Soc. 2002 Dec:16(6):860-77.
I am a psychiatrist now but had another life teaching English in public high school for 17 years. My teaching life, in which I was an openly gay teacher, spanned 2001-2018 and was divided between two urban California schools – in Berkeley and San Leandro. I came out by responding honestly to student questions about whether I had a girlfriend, and what I did over the weekend. At Berkeley High my openness wasn’t an issue at all. The school had a vibrant Gay Straight Alliance/GSA for years, there were many openly gay staff and many openly gay students. No students felt the need to come out to me in search of a gay mentor.
Two years later, I began teaching in San Leandro, 20 miles away, and it was a lesson in how even the San Francisco Bay Area, an LGBTQ+ bastion, could harbor homophobia. When I was hired in 2003, San Leandro High had one openly gay teacher, Q. I quickly realized how much braver his coming out was compared with mine in Berkeley.
In San Leandro, gay slurs were heard nonstop in the hallways, no students were out, and by the end of my first year Q had quit, confiding in me that he couldn’t handle the homophobic harassment from students anymore. There was no GSA. A few years ago, two lesbians had held hands during lunch and inspired the wrath of a group of parents who advocated for their expulsion. In response, a teacher tried to introduce gay sensitivity training into his class and the same group of parents tried to get him fired. He was reprimanded by the principal, he countersued in a case that went all the way to the California Supreme Court, and won. Comparing these two local high schools reinforced to me how visibility really matters in creating a childhood experience that is nurturing versus traumatizing.1
Two Chinese girls in love
N and T were two Chinese girls who grew up in San Leandro. They went to the same elementary school and had crushes on each other since then. In their junior year, they joined our first student GSA, becoming president and vice-president. They were out. And, of course, they must’ve known that their families, who would not have been supportive, would become aware. I remember sitting at an outdoor concert when I got a text from N warning me her father had found out and blamed me for having corrupted her. He planned on coming to school to demand I be fired. And such was the unrelenting pressure that N and T faced every time they went home from school and sat at their dinner tables. Eventually, they broke up. They didn’t do so tearfully, but more wearily.
This story illustrates how difficult it is for love between two LGBTQ+ teens to be nurtured. Love in youth can already be volatile because of the lack of emotional regulation and experience. The questioning of identity and the threat of family disintegration at a time when these teens do not have the economic means to protect themselves makes love dangerous. It is no wonder that gay teens are at increased risk for homelessness.2
The family incident that led to the girls’ breakup reveals how culture affects homophobic pressure. N resisted her parents’ disapproval for months, but she capitulated when her father had a heart attack and blamed it on her. “And it’s true,” N confided. “After my parents found out, they were continually stressed. I could see it affect their health. And it breaks my heart to see my dad in the hospital.”
For N, she had not capitulated from fear, but perhaps because of filial piety, or one’s obligation to protect one’s parent. It was a choice between two heartbreaks. Double minorities, like N and T, face a double threat and often can find no safe place. One of my patients who is gay and Black put it best: “It’s like being beaten up at school only to come home to another beating.” This double threat is evidenced by the higher suicide risk of ethnicities who are LGBTQ+ relative to their white counterparts.3
The confusion of a gay athlete
R was a star point guard, a senior who had secured an athletic scholarship, and was recognized as the best athlete in our county. A popular boy, he flaunted his physique and flirted with all the girls. And then when he was enrolled in my class, he began flirting with all the boys, too. There was gossip that R was bisexual. Then one day, not unexpectedly, he came out to me as gay. He admitted he only flirted with girls for his reputation.
By this time many students had come out to me but he flirted with me with his revelation. I corrected him and warned him unequivocally that it was inappropriate but I was worried because I knew he had placed his trust in me. I also knew he came from a homophobic family that was violent – his father had attacked him physically at a school game and our coaches had to pull him off.
Instinctively, I felt I had to have a witness so I confided in another teacher and documented the situation meticulously. Then, one day, just as I feared, he went too far. He stayed after class and said he wanted to show me something on his phone. And that something turned out to be a picture of himself naked. I immediately confiscated the phone and reported it to the administration. This was not how I wanted him to come out: His family notified by the police that he had sexually harassed his teacher, expulsion pending, and scholarship inevitably revoked. Fortunately, we did find a resolution that restored R’s future.
Let’s examine the circumstances that could’ve informed his transgressive behavior. If we consider sexual harassment a form of bullying, R’s history of having a father who publicly bullied him – and may have bullied others in front of him – is a known risk factor.4 It is also common knowledge that organized team sports were and still are a bastion of homophobia and that gay athletes had to accept a culture of explicit homophobia.5
So, it is not hard to understand the constant public pressures that R faced in addition to those from his family. Let’s also consider that appropriate sexual behaviors are not something we are born with, but something that is learned. Of course, inappropriate sexual behavior also happens in the heterosexual world. But heterosexual sexual behavior often has more accepted paths of trial and error. Children experiment with these behaviors and are corrected by adults and older peers as they mature.
However, for homosexual behaviors, there is not usually the fine-tuning about what is appropriate.
Summary
An educational environment where LGBTQ+ persons are highly visible and accepted is a more nurturing environment for LGBTQ teens than one that is not. Specific subcultures within the LGBTQ population involving race, culture, gender, and athletics modulate the experience of coming out and the nature of homophobic oppression.
Dr. Nguyen is a first-year psychiatry resident at the University of San Francisco School of Medicine at Fresno.
References
1. Kosciw JG et al. The effect of negative school climate on academic outcomes for LGBT youth and the role of in-school supports. J Sch Violence. 2013;12(1):45-63.
2. Center for American Progress. Gay and Transgender Youth Homelessness by the Numbers. June 21, 2010).
3. O’Donnell S et al. Increased risk of suicide attempts among Black and Latino lesbians, gay men, and bisexuals. Am J Public Health. 2011;101(6):1055-9.
4. Farrington D and Baldry A. Individual risk factors for school bullying. J Aggress Confl Peace Res. 2010 Jan;2(1):4-16.
5. Anderson E. Openly gay athletes: Contesting hegemonic masculinity in a homophobic environment Gend Soc. 2002 Dec:16(6):860-77.
What do I have? How to tell patients you’re not sure
Physicians often struggle with telling patients when they are unsure about a diagnosis. In the absence of clarity, doctors may fear losing a patient’s trust by appearing unsure.
Yet diagnostic uncertainty is an inevitable part of medicine.
“It’s often uncertain what is really going on. People have lots of unspecific symptoms,” said Gordon D. Schiff, MD, a patient safety researcher at Harvard Medical School and Brigham and Women’s Hospital in Boston.
By one estimate, more than one-third of patients are discharged from an emergency department without a clear diagnosis. Physicians may order more tests to try to resolve uncertainty, but this method is not foolproof and may lead to increased health care costs. Physicians can use an uncertain diagnosis as an opportunity to improve conversations with patients, Dr. Schiff said.
“How do you talk to patients about that? How do you convey that?” Dr. Schiff asked.
To begin to answer these questions, The scenarios included an enlarged lymph node in a patient in remission for lymphoma, which could suggest recurrence of the disease but not necessarily; a patient with a new-onset headache; and another patient with an unexplained fever and a respiratory tract infection.
For each vignette, the researchers also asked patient advocates – many of whom had experienced receiving an incorrect diagnosis – for their thoughts on how the conversation should go.
Almost 70 people were consulted (24 primary care physicians, 40 patients, and five experts in informatics and quality and safety). Dr. Schiff and his colleagues produced six standardized elements that should be part of a conversation whenever a diagnosis is unclear.
- The most likely diagnosis, along with any alternatives if this isn’t certain, with phrases such as, “Sometimes we don’t have the answers, but we will keep trying to figure out what is going on.”
- Next steps – lab tests, return visits, etc.
- Expected time frame for patient’s improvement and recovery.
- Full disclosure of the limitations of the physical examination or any lab tests.
- Ways to contact the physician going forward.
- Patient insights on their experience and reaction to what they just heard.
The researchers, who published their findings in JAMA Network Open, recommend that the conversation be transcribed in real time using voice recognition software and a microphone, and then printed for the patient to take home. The physician should make eye contact with the patient during the conversation, they suggested.
“Patients felt it was a conversation, that they actually understood what was said. Most patients felt like they were partners during the encounter,” said Maram Khazen, PhD, a coauthor of the paper, who studies communication dynamics. Dr. Khazen was a visiting postdoctoral fellow with Dr. Schiff during the study, and is now a lecturer at the Max Stern Yezreel Valley College in Israel.
Hardeep Singh, MD, MPH, a patient safety researcher at the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine in Houston, called the new work “a great start,” but said that the complexity of the field warrants more research into the tool. Dr. Singh was not involved in the study.
Dr. Singh pointed out that many of the patient voices came from spokespeople for advocacy groups, and that these participants are not necessarily representative of actual people with unclear diagnoses.
“The choice of words really matters,” said Dr. Singh, who led a 2018 study that showed that people reacted more negatively when physicians bluntly acknowledged uncertainty than when they walked patients through different possible diagnoses. Dr. Schiff and Dr. Khazen’s framework offers good principles for discussing uncertainty, he added, but further research is needed on the optimal language to use during conversations.
“It’s really encouraging that we’re seeing high-quality research like this, that leverages patient engagement principles,” said Dimitrios Papanagnou, MD, MPH, an emergency medicine physician and vice dean of medicine at Thomas Jefferson University in Philadelphia.
Dr. Papanagnou, who was not part of the study, called for diverse patients to be part of conversations about diagnostic uncertainty.
“Are we having patients from diverse experiences, from underrepresented groups, participate in this kind of work?” Dr. Papanagnou asked. Dr. Schiff and Dr. Khazen said they agree that the tool needs to be tested in larger samples of diverse patients.
Some common themes about how to communicate diagnostic uncertainty are emerging in multiple areas of medicine. Dr. Papanagnou helped develop an uncertainty communication checklist for discharging patients from an emergency department to home, with principles similar to those that Dr. Schiff and Dr. Khazen recommend for primary care providers.
The study was funded by Harvard Hospitals’ malpractice insurer, the Controlled Risk Insurance Company. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Physicians often struggle with telling patients when they are unsure about a diagnosis. In the absence of clarity, doctors may fear losing a patient’s trust by appearing unsure.
Yet diagnostic uncertainty is an inevitable part of medicine.
“It’s often uncertain what is really going on. People have lots of unspecific symptoms,” said Gordon D. Schiff, MD, a patient safety researcher at Harvard Medical School and Brigham and Women’s Hospital in Boston.
By one estimate, more than one-third of patients are discharged from an emergency department without a clear diagnosis. Physicians may order more tests to try to resolve uncertainty, but this method is not foolproof and may lead to increased health care costs. Physicians can use an uncertain diagnosis as an opportunity to improve conversations with patients, Dr. Schiff said.
“How do you talk to patients about that? How do you convey that?” Dr. Schiff asked.
To begin to answer these questions, The scenarios included an enlarged lymph node in a patient in remission for lymphoma, which could suggest recurrence of the disease but not necessarily; a patient with a new-onset headache; and another patient with an unexplained fever and a respiratory tract infection.
For each vignette, the researchers also asked patient advocates – many of whom had experienced receiving an incorrect diagnosis – for their thoughts on how the conversation should go.
Almost 70 people were consulted (24 primary care physicians, 40 patients, and five experts in informatics and quality and safety). Dr. Schiff and his colleagues produced six standardized elements that should be part of a conversation whenever a diagnosis is unclear.
- The most likely diagnosis, along with any alternatives if this isn’t certain, with phrases such as, “Sometimes we don’t have the answers, but we will keep trying to figure out what is going on.”
- Next steps – lab tests, return visits, etc.
- Expected time frame for patient’s improvement and recovery.
- Full disclosure of the limitations of the physical examination or any lab tests.
- Ways to contact the physician going forward.
- Patient insights on their experience and reaction to what they just heard.
The researchers, who published their findings in JAMA Network Open, recommend that the conversation be transcribed in real time using voice recognition software and a microphone, and then printed for the patient to take home. The physician should make eye contact with the patient during the conversation, they suggested.
“Patients felt it was a conversation, that they actually understood what was said. Most patients felt like they were partners during the encounter,” said Maram Khazen, PhD, a coauthor of the paper, who studies communication dynamics. Dr. Khazen was a visiting postdoctoral fellow with Dr. Schiff during the study, and is now a lecturer at the Max Stern Yezreel Valley College in Israel.
Hardeep Singh, MD, MPH, a patient safety researcher at the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine in Houston, called the new work “a great start,” but said that the complexity of the field warrants more research into the tool. Dr. Singh was not involved in the study.
Dr. Singh pointed out that many of the patient voices came from spokespeople for advocacy groups, and that these participants are not necessarily representative of actual people with unclear diagnoses.
“The choice of words really matters,” said Dr. Singh, who led a 2018 study that showed that people reacted more negatively when physicians bluntly acknowledged uncertainty than when they walked patients through different possible diagnoses. Dr. Schiff and Dr. Khazen’s framework offers good principles for discussing uncertainty, he added, but further research is needed on the optimal language to use during conversations.
“It’s really encouraging that we’re seeing high-quality research like this, that leverages patient engagement principles,” said Dimitrios Papanagnou, MD, MPH, an emergency medicine physician and vice dean of medicine at Thomas Jefferson University in Philadelphia.
Dr. Papanagnou, who was not part of the study, called for diverse patients to be part of conversations about diagnostic uncertainty.
“Are we having patients from diverse experiences, from underrepresented groups, participate in this kind of work?” Dr. Papanagnou asked. Dr. Schiff and Dr. Khazen said they agree that the tool needs to be tested in larger samples of diverse patients.
Some common themes about how to communicate diagnostic uncertainty are emerging in multiple areas of medicine. Dr. Papanagnou helped develop an uncertainty communication checklist for discharging patients from an emergency department to home, with principles similar to those that Dr. Schiff and Dr. Khazen recommend for primary care providers.
The study was funded by Harvard Hospitals’ malpractice insurer, the Controlled Risk Insurance Company. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Physicians often struggle with telling patients when they are unsure about a diagnosis. In the absence of clarity, doctors may fear losing a patient’s trust by appearing unsure.
Yet diagnostic uncertainty is an inevitable part of medicine.
“It’s often uncertain what is really going on. People have lots of unspecific symptoms,” said Gordon D. Schiff, MD, a patient safety researcher at Harvard Medical School and Brigham and Women’s Hospital in Boston.
By one estimate, more than one-third of patients are discharged from an emergency department without a clear diagnosis. Physicians may order more tests to try to resolve uncertainty, but this method is not foolproof and may lead to increased health care costs. Physicians can use an uncertain diagnosis as an opportunity to improve conversations with patients, Dr. Schiff said.
“How do you talk to patients about that? How do you convey that?” Dr. Schiff asked.
To begin to answer these questions, The scenarios included an enlarged lymph node in a patient in remission for lymphoma, which could suggest recurrence of the disease but not necessarily; a patient with a new-onset headache; and another patient with an unexplained fever and a respiratory tract infection.
For each vignette, the researchers also asked patient advocates – many of whom had experienced receiving an incorrect diagnosis – for their thoughts on how the conversation should go.
Almost 70 people were consulted (24 primary care physicians, 40 patients, and five experts in informatics and quality and safety). Dr. Schiff and his colleagues produced six standardized elements that should be part of a conversation whenever a diagnosis is unclear.
- The most likely diagnosis, along with any alternatives if this isn’t certain, with phrases such as, “Sometimes we don’t have the answers, but we will keep trying to figure out what is going on.”
- Next steps – lab tests, return visits, etc.
- Expected time frame for patient’s improvement and recovery.
- Full disclosure of the limitations of the physical examination or any lab tests.
- Ways to contact the physician going forward.
- Patient insights on their experience and reaction to what they just heard.
The researchers, who published their findings in JAMA Network Open, recommend that the conversation be transcribed in real time using voice recognition software and a microphone, and then printed for the patient to take home. The physician should make eye contact with the patient during the conversation, they suggested.
“Patients felt it was a conversation, that they actually understood what was said. Most patients felt like they were partners during the encounter,” said Maram Khazen, PhD, a coauthor of the paper, who studies communication dynamics. Dr. Khazen was a visiting postdoctoral fellow with Dr. Schiff during the study, and is now a lecturer at the Max Stern Yezreel Valley College in Israel.
Hardeep Singh, MD, MPH, a patient safety researcher at the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine in Houston, called the new work “a great start,” but said that the complexity of the field warrants more research into the tool. Dr. Singh was not involved in the study.
Dr. Singh pointed out that many of the patient voices came from spokespeople for advocacy groups, and that these participants are not necessarily representative of actual people with unclear diagnoses.
“The choice of words really matters,” said Dr. Singh, who led a 2018 study that showed that people reacted more negatively when physicians bluntly acknowledged uncertainty than when they walked patients through different possible diagnoses. Dr. Schiff and Dr. Khazen’s framework offers good principles for discussing uncertainty, he added, but further research is needed on the optimal language to use during conversations.
“It’s really encouraging that we’re seeing high-quality research like this, that leverages patient engagement principles,” said Dimitrios Papanagnou, MD, MPH, an emergency medicine physician and vice dean of medicine at Thomas Jefferson University in Philadelphia.
Dr. Papanagnou, who was not part of the study, called for diverse patients to be part of conversations about diagnostic uncertainty.
“Are we having patients from diverse experiences, from underrepresented groups, participate in this kind of work?” Dr. Papanagnou asked. Dr. Schiff and Dr. Khazen said they agree that the tool needs to be tested in larger samples of diverse patients.
Some common themes about how to communicate diagnostic uncertainty are emerging in multiple areas of medicine. Dr. Papanagnou helped develop an uncertainty communication checklist for discharging patients from an emergency department to home, with principles similar to those that Dr. Schiff and Dr. Khazen recommend for primary care providers.
The study was funded by Harvard Hospitals’ malpractice insurer, the Controlled Risk Insurance Company. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Cultivating strength: Psychological well-being after nonfatal suicide attempts
A study of three separate nationally representative samples of nearly 9,000 U.S. military veterans found psychological well-being – defined in terms of having a high sense of purpose, social connectedness, and happiness – to be significantly diminished among veteran suicide attempt survivors relative to nonattempters, even decades after their last attempt.1
Despite the trend toward diminished well-being, many veterans who survived a suicide attempt reported average to optimal levels of well-being. Specifically, 52%-60% of veterans reporting a prior suicide attempt also reported experiencing as much purpose, social connection, and happiness as veterans without a suicide attempt history. Remarkably, a small subset (2-7%) of veteran attempt survivors even reported higher levels of well-being than veterans without a suicide attempt history.
Thus,
These data are notable because, in 2021, approximately 1.4 million U.S. adults made a nonfatal suicide attempt. Historically, suicide research has understandably emphasized the study of risk factors that increase the likelihood that someone dies by suicide. Given that a prior suicide attempt is among the top risk factors for suicide, virtually all research on suicide attempt survivors has focused on their elevated risk for future suicidality. Yet, 9 out of 10 people who have made a nonfatal suicide attempt do not go on to die by suicide. It is thus critical to investigate the quality of life of the millions of suicide attempt survivors.
To date, we know little about a question keenly important to suicide attempt survivors and their loved ones: What is the possibility of rebuilding a meaningful, high-quality life after a suicide attempt?
In addition to reporting on the prevalence of high levels of psychological well-being after a nonfatal suicide attempt, it is pivotal to investigate factors that may help facilitate this outcome. To that end, we identified personal characteristics associated with high levels of well-being. Notably, it was malleable psychological strengths such as optimism and a curious mindset, more than the mere absence of symptoms, that were linked to higher levels of well-being among veteran suicide attempt survivors.
Current suicide prevention interventions and treatments, which often focus on mitigating immediate suicide risk by treating symptoms, may be overlooking the importance of cultivating and building psychological strengths that may help promote greater well-being and enriched lives. Moreover, treatments that emphasize such strengths might be particularly fruitful in mitigating suicide risk in veterans, as veterans may be more receptive to prevention and treatment initiatives that embrace the cultivation and bolstering of strengths that are inherent in military culture and values, such as resilience and perseverance in the face of life challenges.2
One notable caveat to this study is that the data were cross-sectional, meaning they were collected at a single time point. As such, the authors cannot conclude that factors such as curiosity necessarily caused higher levels of well-being in veterans, as opposed to well-being causing higher levels of curiosity.
Similarly, while one can infer that psychological well-being was near-absent at the time of a suicide attempt, well-being of attempt survivors was not assessed before their attempt. Longitudinal studies that follow attempt survivors over time are needed to understand how well-being changes over time for suicide attempt survivors and the causal chain in what predicts that change.
Nevertheless, the results of this large, multicohort study serve as an important first step toward a more comprehensive view of prognosis after a suicide attempt. Just as the process that leads to a suicide attempt is complex, so too is the process of recovery after an attempt. While this study provides sound estimates of well-being outcomes and some possible candidates that might facilitate these outcomes, a critical next step for future research is to replicate and extend these findings. To do so, it is pivotal to extend the assessment scope beyond symptom-based measures and include measures of well-being.
Additionally, the investment in resources into longer-term examinations following suicide attempts is essential to understand different pathways toward achieving greater well-being. Providing hope is vital and potentially lifesaving, as one of the most common experiences reported before a suicide attempt is an unremitting sense of hopelessness. Continued research on well-being has the potential to impart a more balanced, nuanced prognosis after a suicide attempt that challenges perceptions of an invariably bleak prospect of recovery after suicidality.
Collectively, these results highlight the importance of broadening the scope of how the mental health field views and treats psychiatric difficulties to include a greater focus on recovery-based outcomes and personal strengths that help facilitate recovery from adverse life experiences such as suicide attempts.
People desire lives that they enjoy and find meaningful, and having a history of suicide attempts does not preclude the prospect of such a life. It is time that suicide research reflects the vast landscape of potential outcomes after a suicide attempt that goes beyond the prediction of future suicide risk.
Mr. Brown is a doctoral student of clinical psychology at the University of South Florida, Tampa. Dr. Rottenberg is director of the Mood and Emotion Lab and area director of the department of clinical psychology, University of South Florida.
References
1. Brown BA et al. Psychological well-being in US veterans with non-fatal suicide attempts: A multi-cohort population-based study. J Affect Disord. 2022 Oct 1;314:34-43. doi: 10.1016/j.jad.2022.07.003.
2. Bryan CJ et al. Understanding and preventing military suicide. Arch Suicide Res. 2012;16(2):95-110. doi: 10.1080/13811118.2012.667321.
A study of three separate nationally representative samples of nearly 9,000 U.S. military veterans found psychological well-being – defined in terms of having a high sense of purpose, social connectedness, and happiness – to be significantly diminished among veteran suicide attempt survivors relative to nonattempters, even decades after their last attempt.1
Despite the trend toward diminished well-being, many veterans who survived a suicide attempt reported average to optimal levels of well-being. Specifically, 52%-60% of veterans reporting a prior suicide attempt also reported experiencing as much purpose, social connection, and happiness as veterans without a suicide attempt history. Remarkably, a small subset (2-7%) of veteran attempt survivors even reported higher levels of well-being than veterans without a suicide attempt history.
Thus,
These data are notable because, in 2021, approximately 1.4 million U.S. adults made a nonfatal suicide attempt. Historically, suicide research has understandably emphasized the study of risk factors that increase the likelihood that someone dies by suicide. Given that a prior suicide attempt is among the top risk factors for suicide, virtually all research on suicide attempt survivors has focused on their elevated risk for future suicidality. Yet, 9 out of 10 people who have made a nonfatal suicide attempt do not go on to die by suicide. It is thus critical to investigate the quality of life of the millions of suicide attempt survivors.
To date, we know little about a question keenly important to suicide attempt survivors and their loved ones: What is the possibility of rebuilding a meaningful, high-quality life after a suicide attempt?
In addition to reporting on the prevalence of high levels of psychological well-being after a nonfatal suicide attempt, it is pivotal to investigate factors that may help facilitate this outcome. To that end, we identified personal characteristics associated with high levels of well-being. Notably, it was malleable psychological strengths such as optimism and a curious mindset, more than the mere absence of symptoms, that were linked to higher levels of well-being among veteran suicide attempt survivors.
Current suicide prevention interventions and treatments, which often focus on mitigating immediate suicide risk by treating symptoms, may be overlooking the importance of cultivating and building psychological strengths that may help promote greater well-being and enriched lives. Moreover, treatments that emphasize such strengths might be particularly fruitful in mitigating suicide risk in veterans, as veterans may be more receptive to prevention and treatment initiatives that embrace the cultivation and bolstering of strengths that are inherent in military culture and values, such as resilience and perseverance in the face of life challenges.2
One notable caveat to this study is that the data were cross-sectional, meaning they were collected at a single time point. As such, the authors cannot conclude that factors such as curiosity necessarily caused higher levels of well-being in veterans, as opposed to well-being causing higher levels of curiosity.
Similarly, while one can infer that psychological well-being was near-absent at the time of a suicide attempt, well-being of attempt survivors was not assessed before their attempt. Longitudinal studies that follow attempt survivors over time are needed to understand how well-being changes over time for suicide attempt survivors and the causal chain in what predicts that change.
Nevertheless, the results of this large, multicohort study serve as an important first step toward a more comprehensive view of prognosis after a suicide attempt. Just as the process that leads to a suicide attempt is complex, so too is the process of recovery after an attempt. While this study provides sound estimates of well-being outcomes and some possible candidates that might facilitate these outcomes, a critical next step for future research is to replicate and extend these findings. To do so, it is pivotal to extend the assessment scope beyond symptom-based measures and include measures of well-being.
Additionally, the investment in resources into longer-term examinations following suicide attempts is essential to understand different pathways toward achieving greater well-being. Providing hope is vital and potentially lifesaving, as one of the most common experiences reported before a suicide attempt is an unremitting sense of hopelessness. Continued research on well-being has the potential to impart a more balanced, nuanced prognosis after a suicide attempt that challenges perceptions of an invariably bleak prospect of recovery after suicidality.
Collectively, these results highlight the importance of broadening the scope of how the mental health field views and treats psychiatric difficulties to include a greater focus on recovery-based outcomes and personal strengths that help facilitate recovery from adverse life experiences such as suicide attempts.
People desire lives that they enjoy and find meaningful, and having a history of suicide attempts does not preclude the prospect of such a life. It is time that suicide research reflects the vast landscape of potential outcomes after a suicide attempt that goes beyond the prediction of future suicide risk.
Mr. Brown is a doctoral student of clinical psychology at the University of South Florida, Tampa. Dr. Rottenberg is director of the Mood and Emotion Lab and area director of the department of clinical psychology, University of South Florida.
References
1. Brown BA et al. Psychological well-being in US veterans with non-fatal suicide attempts: A multi-cohort population-based study. J Affect Disord. 2022 Oct 1;314:34-43. doi: 10.1016/j.jad.2022.07.003.
2. Bryan CJ et al. Understanding and preventing military suicide. Arch Suicide Res. 2012;16(2):95-110. doi: 10.1080/13811118.2012.667321.
A study of three separate nationally representative samples of nearly 9,000 U.S. military veterans found psychological well-being – defined in terms of having a high sense of purpose, social connectedness, and happiness – to be significantly diminished among veteran suicide attempt survivors relative to nonattempters, even decades after their last attempt.1
Despite the trend toward diminished well-being, many veterans who survived a suicide attempt reported average to optimal levels of well-being. Specifically, 52%-60% of veterans reporting a prior suicide attempt also reported experiencing as much purpose, social connection, and happiness as veterans without a suicide attempt history. Remarkably, a small subset (2-7%) of veteran attempt survivors even reported higher levels of well-being than veterans without a suicide attempt history.
Thus,
These data are notable because, in 2021, approximately 1.4 million U.S. adults made a nonfatal suicide attempt. Historically, suicide research has understandably emphasized the study of risk factors that increase the likelihood that someone dies by suicide. Given that a prior suicide attempt is among the top risk factors for suicide, virtually all research on suicide attempt survivors has focused on their elevated risk for future suicidality. Yet, 9 out of 10 people who have made a nonfatal suicide attempt do not go on to die by suicide. It is thus critical to investigate the quality of life of the millions of suicide attempt survivors.
To date, we know little about a question keenly important to suicide attempt survivors and their loved ones: What is the possibility of rebuilding a meaningful, high-quality life after a suicide attempt?
In addition to reporting on the prevalence of high levels of psychological well-being after a nonfatal suicide attempt, it is pivotal to investigate factors that may help facilitate this outcome. To that end, we identified personal characteristics associated with high levels of well-being. Notably, it was malleable psychological strengths such as optimism and a curious mindset, more than the mere absence of symptoms, that were linked to higher levels of well-being among veteran suicide attempt survivors.
Current suicide prevention interventions and treatments, which often focus on mitigating immediate suicide risk by treating symptoms, may be overlooking the importance of cultivating and building psychological strengths that may help promote greater well-being and enriched lives. Moreover, treatments that emphasize such strengths might be particularly fruitful in mitigating suicide risk in veterans, as veterans may be more receptive to prevention and treatment initiatives that embrace the cultivation and bolstering of strengths that are inherent in military culture and values, such as resilience and perseverance in the face of life challenges.2
One notable caveat to this study is that the data were cross-sectional, meaning they were collected at a single time point. As such, the authors cannot conclude that factors such as curiosity necessarily caused higher levels of well-being in veterans, as opposed to well-being causing higher levels of curiosity.
Similarly, while one can infer that psychological well-being was near-absent at the time of a suicide attempt, well-being of attempt survivors was not assessed before their attempt. Longitudinal studies that follow attempt survivors over time are needed to understand how well-being changes over time for suicide attempt survivors and the causal chain in what predicts that change.
Nevertheless, the results of this large, multicohort study serve as an important first step toward a more comprehensive view of prognosis after a suicide attempt. Just as the process that leads to a suicide attempt is complex, so too is the process of recovery after an attempt. While this study provides sound estimates of well-being outcomes and some possible candidates that might facilitate these outcomes, a critical next step for future research is to replicate and extend these findings. To do so, it is pivotal to extend the assessment scope beyond symptom-based measures and include measures of well-being.
Additionally, the investment in resources into longer-term examinations following suicide attempts is essential to understand different pathways toward achieving greater well-being. Providing hope is vital and potentially lifesaving, as one of the most common experiences reported before a suicide attempt is an unremitting sense of hopelessness. Continued research on well-being has the potential to impart a more balanced, nuanced prognosis after a suicide attempt that challenges perceptions of an invariably bleak prospect of recovery after suicidality.
Collectively, these results highlight the importance of broadening the scope of how the mental health field views and treats psychiatric difficulties to include a greater focus on recovery-based outcomes and personal strengths that help facilitate recovery from adverse life experiences such as suicide attempts.
People desire lives that they enjoy and find meaningful, and having a history of suicide attempts does not preclude the prospect of such a life. It is time that suicide research reflects the vast landscape of potential outcomes after a suicide attempt that goes beyond the prediction of future suicide risk.
Mr. Brown is a doctoral student of clinical psychology at the University of South Florida, Tampa. Dr. Rottenberg is director of the Mood and Emotion Lab and area director of the department of clinical psychology, University of South Florida.
References
1. Brown BA et al. Psychological well-being in US veterans with non-fatal suicide attempts: A multi-cohort population-based study. J Affect Disord. 2022 Oct 1;314:34-43. doi: 10.1016/j.jad.2022.07.003.
2. Bryan CJ et al. Understanding and preventing military suicide. Arch Suicide Res. 2012;16(2):95-110. doi: 10.1080/13811118.2012.667321.
Spinosad: New kid on the block for treating scabies
HONOLULU – , Anthony J. Mancini, MD, said during a presentation at the Hawaii Dermatology Seminar provided by MedscapeLIVE!
In April 2021, spinosad topical suspension 0.9%, was approved by the Food and Drug Administration for treating scabies infestations in adult and pediatric patients 4 years of age and older – a first-in-class drug and the first new scabicide approved in 31 years. It was also approved for treating head lice in adults and children aged 6 months of age and older.
“Scabies has been described as the worst itch one can experience,” said Dr. Mancini, professor of pediatrics and dermatology at Northwestern University, Chicago. “It’s a hallmark of the disease, it can persist for weeks, it’s most intense at night, and patients report various sensations. It’s believed to be a both type I and type IV hypersensitivity reaction.”
The microscopic scabies mite burrows into the upper layer of the skin where it lives and lays its eggs. Besides intense itching, the classic presentation consists of a skin rash composed of inflammatory papules, linear burrows and crusted papules (especially on the hands, feet, and groin), and at times, larger red nodules. “Scabies nodules can persist for many months,” he said.
The Global Burden of Disease Study 2015 cited scabies as having the greatest burden of disease in tropical regions, especially among children, adolescents, and the elderly. The greatest burden of disability-adjusted life years (DALYs) occurred in East and Southeast Asia, Oceana, and tropical South America, but in North America, there was a 24% increase in the DALY rate between 1990 and 2015.
In addition, the World Health Organization designated scabies as a neglected tropical disease in 2017 and included it in its 10-year road map for neglected tropical diseases 2021-2030 with goals of promoting disease awareness and encouraging research and achieving global control.
“In our country, we typically see scabies treated successfully without complications, but there can be complications, especially in underdeveloped areas, like Staph aureus and Group A beta-hemolytic streptococcal infections,” which can be fatal, said Dr. Mancini, who is also head of pediatric dermatology at Ann & Robert H. Lurie Children’s Hospital of Chicago.
Permethrin 5% cream is typically offered as first-line topical therapy in the United States for the treatment of scabies. However, in vitro studies and small investigator-initiated in vivo studies have reported that efficacy appears to be decreasing. In one of the trials, Italian researchers enrolled 155 patients who were treated with permethrin 5% for 8 hours for 2 consecutive days and repeated the treatment 5 days later . Following the course of permethrin, only 34 responded, 96 failed treatment, and 25 were lost to follow-up.
“The study authors concluded that mite resistance to permethrin 5% seems to be increasing, following a path like other ectoparasite resistance,” said Dr. Mancini, who was not involved with the study. “We may even be seeing more ivermectin resistance in some geographic locations, as well.”
According to new scabicide efficacy criteria established by the FDA in 2016, complete cure is now defined as meeting both clinical and confirmatory criteria. A clinical cure means that all signs and symptoms of scabies have completely resolved, including burrows, inflammatory/noninflammatory lesions, and pruritus. A confirmatory cure means there is an absence of mites, eggs, scybala (feces), and burrows via microscopy or dermoscopy.
Enter spinosad, which is derived from a naturally occurring soil microorganism known as Saccharopolyspora spinosa and is composed of two active molecules: spinosyn A and spinosyn D. According to Dr. Mancini, spinosad’s mechanism of action is unique from other medications used to treat ectoparasites. It activates nicotinic and GABA-gated sodium channels, leads to sodium influx in the insect nerves, hyperexcitation, then paralysis and death. Cross-resistance to other insecticides has not been reported, he added, and there is no known evidence of resistance to its active compound.
Approval of the drug was based on data from two phase 3 randomized clinical trials involving 551 index cases and household contacts. In the intent-to-treat population, with the two trials combined, complete cure was achieved in 78.1% of the spinosad-treated group, compared with 39.6% in the vehicle group (P < .0001), clinical cure was achieved in 79.6% of the spinosad group, compared with 41.2% in the vehicle group (P < .001), and microscopic cure occurred in 85.9% of the spinosad group, compared with 52.6% in the vehicle group (P < .001).
Of the 306 participants in the study, the only adverse events reported by more than one patient each included abdominal pain, back pain, cough, headache, neck pain, and decreased weight in two patients each (0.8%), which investigators believed were not attributable to the study drug. Adverse events that investigators considered to be potentially related to the study drug included burning sensation in two participants (0.7%) and dry skin in another (0.3%). In clinical trials reported in the prescribing information, adverse events occurring in greater than 1% of subjects included application-site irritation (3% spinosad vs. 0% vehicle) and dry skin (2% spinosad vs. 0% vehicle).
“Spinosad met the FDA’s new stringent criteria, with all signs and symptoms of scabies completely resolved and confirmed via microscopy or dermoscopy,” said Dr. Mancini, who was not involved in the trials. “The patented formulation drives the active compound to the stratum corneum, where mites live and breed. It’s a single full-body application, without any resistance observed to date. This is an exciting newer option for treating our scabies patients.”
In an interview at the meeting, John S. Barbieri, MD, MBA, of the department of dermatology, Brigham and Women’s Hospital, Boston, said that, while he has no clinical experience with spinosad for scabies, he welcomes a new option for the condition. “The fact that it has a different mechanism of action than permethrin is a good thing,” he said.
Dr. Mancini disclosed that he is a consultant or an adviser for ParaPRO, the manufacturer of spinosad, and Cassiopea, Castle Creek, Novan, Novartis, and Verrica. He was not involved in clinical trials of spinosad. Dr. Barbieri disclosed that he receives consulting fees from Dexcel.
Medscape and this news organization are owned by the same parent company.
HONOLULU – , Anthony J. Mancini, MD, said during a presentation at the Hawaii Dermatology Seminar provided by MedscapeLIVE!
In April 2021, spinosad topical suspension 0.9%, was approved by the Food and Drug Administration for treating scabies infestations in adult and pediatric patients 4 years of age and older – a first-in-class drug and the first new scabicide approved in 31 years. It was also approved for treating head lice in adults and children aged 6 months of age and older.
“Scabies has been described as the worst itch one can experience,” said Dr. Mancini, professor of pediatrics and dermatology at Northwestern University, Chicago. “It’s a hallmark of the disease, it can persist for weeks, it’s most intense at night, and patients report various sensations. It’s believed to be a both type I and type IV hypersensitivity reaction.”
The microscopic scabies mite burrows into the upper layer of the skin where it lives and lays its eggs. Besides intense itching, the classic presentation consists of a skin rash composed of inflammatory papules, linear burrows and crusted papules (especially on the hands, feet, and groin), and at times, larger red nodules. “Scabies nodules can persist for many months,” he said.
The Global Burden of Disease Study 2015 cited scabies as having the greatest burden of disease in tropical regions, especially among children, adolescents, and the elderly. The greatest burden of disability-adjusted life years (DALYs) occurred in East and Southeast Asia, Oceana, and tropical South America, but in North America, there was a 24% increase in the DALY rate between 1990 and 2015.
In addition, the World Health Organization designated scabies as a neglected tropical disease in 2017 and included it in its 10-year road map for neglected tropical diseases 2021-2030 with goals of promoting disease awareness and encouraging research and achieving global control.
“In our country, we typically see scabies treated successfully without complications, but there can be complications, especially in underdeveloped areas, like Staph aureus and Group A beta-hemolytic streptococcal infections,” which can be fatal, said Dr. Mancini, who is also head of pediatric dermatology at Ann & Robert H. Lurie Children’s Hospital of Chicago.
Permethrin 5% cream is typically offered as first-line topical therapy in the United States for the treatment of scabies. However, in vitro studies and small investigator-initiated in vivo studies have reported that efficacy appears to be decreasing. In one of the trials, Italian researchers enrolled 155 patients who were treated with permethrin 5% for 8 hours for 2 consecutive days and repeated the treatment 5 days later . Following the course of permethrin, only 34 responded, 96 failed treatment, and 25 were lost to follow-up.
“The study authors concluded that mite resistance to permethrin 5% seems to be increasing, following a path like other ectoparasite resistance,” said Dr. Mancini, who was not involved with the study. “We may even be seeing more ivermectin resistance in some geographic locations, as well.”
According to new scabicide efficacy criteria established by the FDA in 2016, complete cure is now defined as meeting both clinical and confirmatory criteria. A clinical cure means that all signs and symptoms of scabies have completely resolved, including burrows, inflammatory/noninflammatory lesions, and pruritus. A confirmatory cure means there is an absence of mites, eggs, scybala (feces), and burrows via microscopy or dermoscopy.
Enter spinosad, which is derived from a naturally occurring soil microorganism known as Saccharopolyspora spinosa and is composed of two active molecules: spinosyn A and spinosyn D. According to Dr. Mancini, spinosad’s mechanism of action is unique from other medications used to treat ectoparasites. It activates nicotinic and GABA-gated sodium channels, leads to sodium influx in the insect nerves, hyperexcitation, then paralysis and death. Cross-resistance to other insecticides has not been reported, he added, and there is no known evidence of resistance to its active compound.
Approval of the drug was based on data from two phase 3 randomized clinical trials involving 551 index cases and household contacts. In the intent-to-treat population, with the two trials combined, complete cure was achieved in 78.1% of the spinosad-treated group, compared with 39.6% in the vehicle group (P < .0001), clinical cure was achieved in 79.6% of the spinosad group, compared with 41.2% in the vehicle group (P < .001), and microscopic cure occurred in 85.9% of the spinosad group, compared with 52.6% in the vehicle group (P < .001).
Of the 306 participants in the study, the only adverse events reported by more than one patient each included abdominal pain, back pain, cough, headache, neck pain, and decreased weight in two patients each (0.8%), which investigators believed were not attributable to the study drug. Adverse events that investigators considered to be potentially related to the study drug included burning sensation in two participants (0.7%) and dry skin in another (0.3%). In clinical trials reported in the prescribing information, adverse events occurring in greater than 1% of subjects included application-site irritation (3% spinosad vs. 0% vehicle) and dry skin (2% spinosad vs. 0% vehicle).
“Spinosad met the FDA’s new stringent criteria, with all signs and symptoms of scabies completely resolved and confirmed via microscopy or dermoscopy,” said Dr. Mancini, who was not involved in the trials. “The patented formulation drives the active compound to the stratum corneum, where mites live and breed. It’s a single full-body application, without any resistance observed to date. This is an exciting newer option for treating our scabies patients.”
In an interview at the meeting, John S. Barbieri, MD, MBA, of the department of dermatology, Brigham and Women’s Hospital, Boston, said that, while he has no clinical experience with spinosad for scabies, he welcomes a new option for the condition. “The fact that it has a different mechanism of action than permethrin is a good thing,” he said.
Dr. Mancini disclosed that he is a consultant or an adviser for ParaPRO, the manufacturer of spinosad, and Cassiopea, Castle Creek, Novan, Novartis, and Verrica. He was not involved in clinical trials of spinosad. Dr. Barbieri disclosed that he receives consulting fees from Dexcel.
Medscape and this news organization are owned by the same parent company.
HONOLULU – , Anthony J. Mancini, MD, said during a presentation at the Hawaii Dermatology Seminar provided by MedscapeLIVE!
In April 2021, spinosad topical suspension 0.9%, was approved by the Food and Drug Administration for treating scabies infestations in adult and pediatric patients 4 years of age and older – a first-in-class drug and the first new scabicide approved in 31 years. It was also approved for treating head lice in adults and children aged 6 months of age and older.
“Scabies has been described as the worst itch one can experience,” said Dr. Mancini, professor of pediatrics and dermatology at Northwestern University, Chicago. “It’s a hallmark of the disease, it can persist for weeks, it’s most intense at night, and patients report various sensations. It’s believed to be a both type I and type IV hypersensitivity reaction.”
The microscopic scabies mite burrows into the upper layer of the skin where it lives and lays its eggs. Besides intense itching, the classic presentation consists of a skin rash composed of inflammatory papules, linear burrows and crusted papules (especially on the hands, feet, and groin), and at times, larger red nodules. “Scabies nodules can persist for many months,” he said.
The Global Burden of Disease Study 2015 cited scabies as having the greatest burden of disease in tropical regions, especially among children, adolescents, and the elderly. The greatest burden of disability-adjusted life years (DALYs) occurred in East and Southeast Asia, Oceana, and tropical South America, but in North America, there was a 24% increase in the DALY rate between 1990 and 2015.
In addition, the World Health Organization designated scabies as a neglected tropical disease in 2017 and included it in its 10-year road map for neglected tropical diseases 2021-2030 with goals of promoting disease awareness and encouraging research and achieving global control.
“In our country, we typically see scabies treated successfully without complications, but there can be complications, especially in underdeveloped areas, like Staph aureus and Group A beta-hemolytic streptococcal infections,” which can be fatal, said Dr. Mancini, who is also head of pediatric dermatology at Ann & Robert H. Lurie Children’s Hospital of Chicago.
Permethrin 5% cream is typically offered as first-line topical therapy in the United States for the treatment of scabies. However, in vitro studies and small investigator-initiated in vivo studies have reported that efficacy appears to be decreasing. In one of the trials, Italian researchers enrolled 155 patients who were treated with permethrin 5% for 8 hours for 2 consecutive days and repeated the treatment 5 days later . Following the course of permethrin, only 34 responded, 96 failed treatment, and 25 were lost to follow-up.
“The study authors concluded that mite resistance to permethrin 5% seems to be increasing, following a path like other ectoparasite resistance,” said Dr. Mancini, who was not involved with the study. “We may even be seeing more ivermectin resistance in some geographic locations, as well.”
According to new scabicide efficacy criteria established by the FDA in 2016, complete cure is now defined as meeting both clinical and confirmatory criteria. A clinical cure means that all signs and symptoms of scabies have completely resolved, including burrows, inflammatory/noninflammatory lesions, and pruritus. A confirmatory cure means there is an absence of mites, eggs, scybala (feces), and burrows via microscopy or dermoscopy.
Enter spinosad, which is derived from a naturally occurring soil microorganism known as Saccharopolyspora spinosa and is composed of two active molecules: spinosyn A and spinosyn D. According to Dr. Mancini, spinosad’s mechanism of action is unique from other medications used to treat ectoparasites. It activates nicotinic and GABA-gated sodium channels, leads to sodium influx in the insect nerves, hyperexcitation, then paralysis and death. Cross-resistance to other insecticides has not been reported, he added, and there is no known evidence of resistance to its active compound.
Approval of the drug was based on data from two phase 3 randomized clinical trials involving 551 index cases and household contacts. In the intent-to-treat population, with the two trials combined, complete cure was achieved in 78.1% of the spinosad-treated group, compared with 39.6% in the vehicle group (P < .0001), clinical cure was achieved in 79.6% of the spinosad group, compared with 41.2% in the vehicle group (P < .001), and microscopic cure occurred in 85.9% of the spinosad group, compared with 52.6% in the vehicle group (P < .001).
Of the 306 participants in the study, the only adverse events reported by more than one patient each included abdominal pain, back pain, cough, headache, neck pain, and decreased weight in two patients each (0.8%), which investigators believed were not attributable to the study drug. Adverse events that investigators considered to be potentially related to the study drug included burning sensation in two participants (0.7%) and dry skin in another (0.3%). In clinical trials reported in the prescribing information, adverse events occurring in greater than 1% of subjects included application-site irritation (3% spinosad vs. 0% vehicle) and dry skin (2% spinosad vs. 0% vehicle).
“Spinosad met the FDA’s new stringent criteria, with all signs and symptoms of scabies completely resolved and confirmed via microscopy or dermoscopy,” said Dr. Mancini, who was not involved in the trials. “The patented formulation drives the active compound to the stratum corneum, where mites live and breed. It’s a single full-body application, without any resistance observed to date. This is an exciting newer option for treating our scabies patients.”
In an interview at the meeting, John S. Barbieri, MD, MBA, of the department of dermatology, Brigham and Women’s Hospital, Boston, said that, while he has no clinical experience with spinosad for scabies, he welcomes a new option for the condition. “The fact that it has a different mechanism of action than permethrin is a good thing,” he said.
Dr. Mancini disclosed that he is a consultant or an adviser for ParaPRO, the manufacturer of spinosad, and Cassiopea, Castle Creek, Novan, Novartis, and Verrica. He was not involved in clinical trials of spinosad. Dr. Barbieri disclosed that he receives consulting fees from Dexcel.
Medscape and this news organization are owned by the same parent company.
AT THE MEDSCAPELIVE! HAWAII DERMATOLOGY SEMINAR
Antioxidants may ease anxiety and depression
The prevalence of anxiety and depression has increased worldwide, especially in the wake of the COVID-19 pandemic. “Therefore, identifying specific interventions that improve depressive status is critical for public health policy,” wrote Huan Wang, MD, of First Hospital of Jilin University, Changchun, China, and colleagues.
Recent evidence suggests that modifiable lifestyle factors, including nutrition, may have a positive impact on symptoms of anxiety and depression, and observational studies have shown that antioxidant supplements affect depressive status, but data from randomized, controlled trials are limited by small sample sizes, they wrote.
In a study published in the Journal of Affective Disorders, the researchers conducted a meta-analysis of 52 studies with a total of 4,049 patients. Of these, 2,004 received antioxidant supplements and 2,045 received a placebo supplement or no supplements. The median treatment duration was 11 weeks; treatment durations ranged from 2 weeks to 2 years. All 52 studies addressed the effect of antioxidants on depressive status, and 21 studies also assessed anxiety status. The studies used a range of depression scales, including the Beck Depression Inventory, the Edinburgh Postnatal Depression Scale, Montgomery-Asberg Depression Rating Scale, Hamilton Depression Rating Scale, Center for Epidemiologic Studies–Depression, and Hospital Anxiety and Depression Scale.
Overall, the meta-analysis revealed a statistically significant improvement in depressive status associated with antioxidant supplement use (standardized mean difference, 0.60; P < .00001). When broken down by supplement, significant positive effects appeared for magnesium (SMD = 0.16; P = .03), zinc (SMD = 0.59; P = .01), selenium (SMD = 0.33; P = .009), CoQ10 (SMD = 0.97; P = .05), tea and coffee (SMD = 1.15; P = .001) and crocin (MD = 6.04; P < .00001).
As a secondary outcome, antioxidant supplementation had a significantly positive effect on anxiety (SMD = 0.40; P < .00001).
The mechanism of action for the effect of antioxidants remains unclear, the researchers wrote in their discussion, but, “Depriving or boosting the supply of food components with antioxidant capabilities might worsen or lessen oxidative stress,” they said.
The researchers attempted a subgroup analysis across countries, and found that, while antioxidant supplementation improved depressive status in populations from Iran, China, and Italy, “no significant improvement was found in the United States, Australia, Italy and other countries.” The reasons for this difference might be related to fewer studies from these countries, or “the improvement brought about by antioxidants might be particularly pronounced in people with significant depression and higher depression scores,” they wrote. “Studies have shown that Asian countries have fewer psychiatrists and more expensive treatments,” they added.
The findings were limited by several factors including the inability to include all types of antioxidant supplements, the range of depression rating scales, and insufficient subgroup analysis of the range of populations from the included studies, the researchers noted.
“Additional data from large clinical trials are needed to confirm the efficacy and safety of antioxidant supplements in improving depressive status,” they said. However, the results suggest that antioxidants may play a role as an adjunct treatment to conventional antidepressants, they concluded.
The study was funded by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
The prevalence of anxiety and depression has increased worldwide, especially in the wake of the COVID-19 pandemic. “Therefore, identifying specific interventions that improve depressive status is critical for public health policy,” wrote Huan Wang, MD, of First Hospital of Jilin University, Changchun, China, and colleagues.
Recent evidence suggests that modifiable lifestyle factors, including nutrition, may have a positive impact on symptoms of anxiety and depression, and observational studies have shown that antioxidant supplements affect depressive status, but data from randomized, controlled trials are limited by small sample sizes, they wrote.
In a study published in the Journal of Affective Disorders, the researchers conducted a meta-analysis of 52 studies with a total of 4,049 patients. Of these, 2,004 received antioxidant supplements and 2,045 received a placebo supplement or no supplements. The median treatment duration was 11 weeks; treatment durations ranged from 2 weeks to 2 years. All 52 studies addressed the effect of antioxidants on depressive status, and 21 studies also assessed anxiety status. The studies used a range of depression scales, including the Beck Depression Inventory, the Edinburgh Postnatal Depression Scale, Montgomery-Asberg Depression Rating Scale, Hamilton Depression Rating Scale, Center for Epidemiologic Studies–Depression, and Hospital Anxiety and Depression Scale.
Overall, the meta-analysis revealed a statistically significant improvement in depressive status associated with antioxidant supplement use (standardized mean difference, 0.60; P < .00001). When broken down by supplement, significant positive effects appeared for magnesium (SMD = 0.16; P = .03), zinc (SMD = 0.59; P = .01), selenium (SMD = 0.33; P = .009), CoQ10 (SMD = 0.97; P = .05), tea and coffee (SMD = 1.15; P = .001) and crocin (MD = 6.04; P < .00001).
As a secondary outcome, antioxidant supplementation had a significantly positive effect on anxiety (SMD = 0.40; P < .00001).
The mechanism of action for the effect of antioxidants remains unclear, the researchers wrote in their discussion, but, “Depriving or boosting the supply of food components with antioxidant capabilities might worsen or lessen oxidative stress,” they said.
The researchers attempted a subgroup analysis across countries, and found that, while antioxidant supplementation improved depressive status in populations from Iran, China, and Italy, “no significant improvement was found in the United States, Australia, Italy and other countries.” The reasons for this difference might be related to fewer studies from these countries, or “the improvement brought about by antioxidants might be particularly pronounced in people with significant depression and higher depression scores,” they wrote. “Studies have shown that Asian countries have fewer psychiatrists and more expensive treatments,” they added.
The findings were limited by several factors including the inability to include all types of antioxidant supplements, the range of depression rating scales, and insufficient subgroup analysis of the range of populations from the included studies, the researchers noted.
“Additional data from large clinical trials are needed to confirm the efficacy and safety of antioxidant supplements in improving depressive status,” they said. However, the results suggest that antioxidants may play a role as an adjunct treatment to conventional antidepressants, they concluded.
The study was funded by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
The prevalence of anxiety and depression has increased worldwide, especially in the wake of the COVID-19 pandemic. “Therefore, identifying specific interventions that improve depressive status is critical for public health policy,” wrote Huan Wang, MD, of First Hospital of Jilin University, Changchun, China, and colleagues.
Recent evidence suggests that modifiable lifestyle factors, including nutrition, may have a positive impact on symptoms of anxiety and depression, and observational studies have shown that antioxidant supplements affect depressive status, but data from randomized, controlled trials are limited by small sample sizes, they wrote.
In a study published in the Journal of Affective Disorders, the researchers conducted a meta-analysis of 52 studies with a total of 4,049 patients. Of these, 2,004 received antioxidant supplements and 2,045 received a placebo supplement or no supplements. The median treatment duration was 11 weeks; treatment durations ranged from 2 weeks to 2 years. All 52 studies addressed the effect of antioxidants on depressive status, and 21 studies also assessed anxiety status. The studies used a range of depression scales, including the Beck Depression Inventory, the Edinburgh Postnatal Depression Scale, Montgomery-Asberg Depression Rating Scale, Hamilton Depression Rating Scale, Center for Epidemiologic Studies–Depression, and Hospital Anxiety and Depression Scale.
Overall, the meta-analysis revealed a statistically significant improvement in depressive status associated with antioxidant supplement use (standardized mean difference, 0.60; P < .00001). When broken down by supplement, significant positive effects appeared for magnesium (SMD = 0.16; P = .03), zinc (SMD = 0.59; P = .01), selenium (SMD = 0.33; P = .009), CoQ10 (SMD = 0.97; P = .05), tea and coffee (SMD = 1.15; P = .001) and crocin (MD = 6.04; P < .00001).
As a secondary outcome, antioxidant supplementation had a significantly positive effect on anxiety (SMD = 0.40; P < .00001).
The mechanism of action for the effect of antioxidants remains unclear, the researchers wrote in their discussion, but, “Depriving or boosting the supply of food components with antioxidant capabilities might worsen or lessen oxidative stress,” they said.
The researchers attempted a subgroup analysis across countries, and found that, while antioxidant supplementation improved depressive status in populations from Iran, China, and Italy, “no significant improvement was found in the United States, Australia, Italy and other countries.” The reasons for this difference might be related to fewer studies from these countries, or “the improvement brought about by antioxidants might be particularly pronounced in people with significant depression and higher depression scores,” they wrote. “Studies have shown that Asian countries have fewer psychiatrists and more expensive treatments,” they added.
The findings were limited by several factors including the inability to include all types of antioxidant supplements, the range of depression rating scales, and insufficient subgroup analysis of the range of populations from the included studies, the researchers noted.
“Additional data from large clinical trials are needed to confirm the efficacy and safety of antioxidant supplements in improving depressive status,” they said. However, the results suggest that antioxidants may play a role as an adjunct treatment to conventional antidepressants, they concluded.
The study was funded by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Notes on direct admission of pediatric patients
Scenario: Yesterday you saw a 6-month-old infant with what appeared to be viral gastroenteritis and mild dehydration. When you called his parents today to check on his condition he was not improving despite your recommendations about his diet and oral rehydration. Should you have him brought to your office for a reevaluation, have his parents take him to the local emergency department for evaluation and probable hospital admission, or ask his parents to take him to the hospital telling them that you will call and arrange for a direct admission.
Obviously, I haven’t given you enough background information to allow you to give me an answer you are comfortable with. What time of day is it? Is it a holiday weekend? What’s the weather like? How far is it from the patient’s home to your office? To the emergency department? How is the local ED staffed? Are there hospitalists? What is their training?
Whether or not you choose to see the patient first in the office, is direct admission to the hospital an option that you are likely to choose? What steps do you take to see that it happens smoothly?
At least one-quarter of the unscheduled pediatric hospitalizations begin with a direct admission, meaning that the patients are not first evaluated in that hospital’s ED. In a recent policy statement, the American Academy of Pediatrics Committee on Hospital Care explored the pluses and minuses of direct admission and issued a list of seven recommendations. Among the concerns raised by the authors are “potential delays in initial evaluation and treatment, inconsistent admission processes, and difficulties in determining the appropriateness of patients for direct admission.” The committee makes it clear that they understand each community has it own strengths and challenges and the unique needs of each patient make it difficult to define a set of recommendations that fits all.
However, as I read through the committee’s seven recommendations, one leapt off the screen as a unifying concept that should apply in every situation. Recommendation No. 2 reads, “[There should be] clear systems of communication between members of the health care team and with families of children requiring admission.”
First, who is on this “health care team”? Are you a team member with the hospital folks – the ED nurses and doctors, the hospitalists, the floor nurses? Do you share an employer? Are you in the same town? Have your ever met them face to face? Do you do so regularly?
I assume you call the ED or the pediatric floor to arrange a direct admit? Maybe you don’t. I can recall working in situations where several infamous “local docs” would just send the patients in with a scribbled note (or not) and no phone call. Will you be speaking to folks who are even vaguely familiar with you or even your name? Do you get to speak with people who will be hands on with the patient?
Obviously, where I’m going with this is that, if you and the hospital staff are truly on the same health care team, communication should flow freely among the members and having some familiarity allows this to happen more smoothly. It can start on our end as the referring physician by making the call personally. Likewise, the receiving hospital must make frontline people available so you can speak with staff who will be working with the patient. Do you have enough information to tell the family what to expect?
Of course legible and complete records are a must. But nothing beats personal contact and a name. If you can tell a parent “I spoke to Martha, the nurse who will meet you on the floor,” that can be a giant first step forward in the healing process.
Most of us trained at hospitals that accepted direct admit patients and can remember the challenges. And most of us recall EDs that weren’t pediatric friendly. Whether our local situation favors direct admission or ED preadmission evaluation, it is our job to make the communication flow with the patient’s safety and the family’s comfort in mind.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Scenario: Yesterday you saw a 6-month-old infant with what appeared to be viral gastroenteritis and mild dehydration. When you called his parents today to check on his condition he was not improving despite your recommendations about his diet and oral rehydration. Should you have him brought to your office for a reevaluation, have his parents take him to the local emergency department for evaluation and probable hospital admission, or ask his parents to take him to the hospital telling them that you will call and arrange for a direct admission.
Obviously, I haven’t given you enough background information to allow you to give me an answer you are comfortable with. What time of day is it? Is it a holiday weekend? What’s the weather like? How far is it from the patient’s home to your office? To the emergency department? How is the local ED staffed? Are there hospitalists? What is their training?
Whether or not you choose to see the patient first in the office, is direct admission to the hospital an option that you are likely to choose? What steps do you take to see that it happens smoothly?
At least one-quarter of the unscheduled pediatric hospitalizations begin with a direct admission, meaning that the patients are not first evaluated in that hospital’s ED. In a recent policy statement, the American Academy of Pediatrics Committee on Hospital Care explored the pluses and minuses of direct admission and issued a list of seven recommendations. Among the concerns raised by the authors are “potential delays in initial evaluation and treatment, inconsistent admission processes, and difficulties in determining the appropriateness of patients for direct admission.” The committee makes it clear that they understand each community has it own strengths and challenges and the unique needs of each patient make it difficult to define a set of recommendations that fits all.
However, as I read through the committee’s seven recommendations, one leapt off the screen as a unifying concept that should apply in every situation. Recommendation No. 2 reads, “[There should be] clear systems of communication between members of the health care team and with families of children requiring admission.”
First, who is on this “health care team”? Are you a team member with the hospital folks – the ED nurses and doctors, the hospitalists, the floor nurses? Do you share an employer? Are you in the same town? Have your ever met them face to face? Do you do so regularly?
I assume you call the ED or the pediatric floor to arrange a direct admit? Maybe you don’t. I can recall working in situations where several infamous “local docs” would just send the patients in with a scribbled note (or not) and no phone call. Will you be speaking to folks who are even vaguely familiar with you or even your name? Do you get to speak with people who will be hands on with the patient?
Obviously, where I’m going with this is that, if you and the hospital staff are truly on the same health care team, communication should flow freely among the members and having some familiarity allows this to happen more smoothly. It can start on our end as the referring physician by making the call personally. Likewise, the receiving hospital must make frontline people available so you can speak with staff who will be working with the patient. Do you have enough information to tell the family what to expect?
Of course legible and complete records are a must. But nothing beats personal contact and a name. If you can tell a parent “I spoke to Martha, the nurse who will meet you on the floor,” that can be a giant first step forward in the healing process.
Most of us trained at hospitals that accepted direct admit patients and can remember the challenges. And most of us recall EDs that weren’t pediatric friendly. Whether our local situation favors direct admission or ED preadmission evaluation, it is our job to make the communication flow with the patient’s safety and the family’s comfort in mind.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Scenario: Yesterday you saw a 6-month-old infant with what appeared to be viral gastroenteritis and mild dehydration. When you called his parents today to check on his condition he was not improving despite your recommendations about his diet and oral rehydration. Should you have him brought to your office for a reevaluation, have his parents take him to the local emergency department for evaluation and probable hospital admission, or ask his parents to take him to the hospital telling them that you will call and arrange for a direct admission.
Obviously, I haven’t given you enough background information to allow you to give me an answer you are comfortable with. What time of day is it? Is it a holiday weekend? What’s the weather like? How far is it from the patient’s home to your office? To the emergency department? How is the local ED staffed? Are there hospitalists? What is their training?
Whether or not you choose to see the patient first in the office, is direct admission to the hospital an option that you are likely to choose? What steps do you take to see that it happens smoothly?
At least one-quarter of the unscheduled pediatric hospitalizations begin with a direct admission, meaning that the patients are not first evaluated in that hospital’s ED. In a recent policy statement, the American Academy of Pediatrics Committee on Hospital Care explored the pluses and minuses of direct admission and issued a list of seven recommendations. Among the concerns raised by the authors are “potential delays in initial evaluation and treatment, inconsistent admission processes, and difficulties in determining the appropriateness of patients for direct admission.” The committee makes it clear that they understand each community has it own strengths and challenges and the unique needs of each patient make it difficult to define a set of recommendations that fits all.
However, as I read through the committee’s seven recommendations, one leapt off the screen as a unifying concept that should apply in every situation. Recommendation No. 2 reads, “[There should be] clear systems of communication between members of the health care team and with families of children requiring admission.”
First, who is on this “health care team”? Are you a team member with the hospital folks – the ED nurses and doctors, the hospitalists, the floor nurses? Do you share an employer? Are you in the same town? Have your ever met them face to face? Do you do so regularly?
I assume you call the ED or the pediatric floor to arrange a direct admit? Maybe you don’t. I can recall working in situations where several infamous “local docs” would just send the patients in with a scribbled note (or not) and no phone call. Will you be speaking to folks who are even vaguely familiar with you or even your name? Do you get to speak with people who will be hands on with the patient?
Obviously, where I’m going with this is that, if you and the hospital staff are truly on the same health care team, communication should flow freely among the members and having some familiarity allows this to happen more smoothly. It can start on our end as the referring physician by making the call personally. Likewise, the receiving hospital must make frontline people available so you can speak with staff who will be working with the patient. Do you have enough information to tell the family what to expect?
Of course legible and complete records are a must. But nothing beats personal contact and a name. If you can tell a parent “I spoke to Martha, the nurse who will meet you on the floor,” that can be a giant first step forward in the healing process.
Most of us trained at hospitals that accepted direct admit patients and can remember the challenges. And most of us recall EDs that weren’t pediatric friendly. Whether our local situation favors direct admission or ED preadmission evaluation, it is our job to make the communication flow with the patient’s safety and the family’s comfort in mind.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Pandemic hit Black children harder, study shows
Black children had almost three times as many COVID-related deaths as White children and about twice as many hospitalizations, according to a new study.
The study said that 1,556 children have died from the start of the pandemic until Nov. 30, 2022, with 593 of those children being 4 and under. Black children died of COVID-related causes 2.7 times more often than White children and were hospitalized 2.2 times more often than White children, the study said.
Lower vaccination rates for Black people may be a factor. The study said 43.6% of White children have received two or more vaccinations, compared with 40.2% of Black children.
“First and foremost, this study repudiates the misunderstanding that COVID-19 has not been of consequence to children who have had more than 15.5 million reported cases, representing 18 percent of all cases in the United States,” Reed Tuckson, MD, a member of the Black Coalition Against COVID board of directors and former District of Columbia public health commissioner, said in a news release.
“And second, our research shows that like their adult counterparts, Black and other children of color have shouldered more of the burden of COVID-19 than the White population.”
The study was commissioned by BCAC and conducted by the Satcher Health Leadership Institute of the Morehouse School of Medicine, Atlanta. It’s based on studies conducted by other agencies over 2 years.
Black and Hispanic children also had more severe COVID cases, the study said. Among 281 pediatric patients in New York, New Jersey, and Connecticut, 23.3% of severe cases were Black and 51% of severe cases were Hispanic.
The study says 1 in 310 Black children lost a parent or caregiver to COVID between April 2020 and June 2012, compared with 1 in 738 White children.
Economic and health-related hardships were experienced by 31% of Black households, 29% of Latino households, and 16% of White households, the study said.
“Children with COVID-19 in communities of color were sicker, [were] hospitalized and died at higher rates than White children,” Sandra Harris-Hooker, the interim executive director at the Satcher Health Leadership Institute of Morehouse School, said in the release. “We can now fully understand the devastating impact the virus had on communities of color across generations.”
The study recommends several changes, such as modifying eligibility requirements for the Children’s Health Insurance Program to help more children who fall into coverage gaps and expanding the Child Tax Credit.
A version of this article first appeared on WebMD.com.
Black children had almost three times as many COVID-related deaths as White children and about twice as many hospitalizations, according to a new study.
The study said that 1,556 children have died from the start of the pandemic until Nov. 30, 2022, with 593 of those children being 4 and under. Black children died of COVID-related causes 2.7 times more often than White children and were hospitalized 2.2 times more often than White children, the study said.
Lower vaccination rates for Black people may be a factor. The study said 43.6% of White children have received two or more vaccinations, compared with 40.2% of Black children.
“First and foremost, this study repudiates the misunderstanding that COVID-19 has not been of consequence to children who have had more than 15.5 million reported cases, representing 18 percent of all cases in the United States,” Reed Tuckson, MD, a member of the Black Coalition Against COVID board of directors and former District of Columbia public health commissioner, said in a news release.
“And second, our research shows that like their adult counterparts, Black and other children of color have shouldered more of the burden of COVID-19 than the White population.”
The study was commissioned by BCAC and conducted by the Satcher Health Leadership Institute of the Morehouse School of Medicine, Atlanta. It’s based on studies conducted by other agencies over 2 years.
Black and Hispanic children also had more severe COVID cases, the study said. Among 281 pediatric patients in New York, New Jersey, and Connecticut, 23.3% of severe cases were Black and 51% of severe cases were Hispanic.
The study says 1 in 310 Black children lost a parent or caregiver to COVID between April 2020 and June 2012, compared with 1 in 738 White children.
Economic and health-related hardships were experienced by 31% of Black households, 29% of Latino households, and 16% of White households, the study said.
“Children with COVID-19 in communities of color were sicker, [were] hospitalized and died at higher rates than White children,” Sandra Harris-Hooker, the interim executive director at the Satcher Health Leadership Institute of Morehouse School, said in the release. “We can now fully understand the devastating impact the virus had on communities of color across generations.”
The study recommends several changes, such as modifying eligibility requirements for the Children’s Health Insurance Program to help more children who fall into coverage gaps and expanding the Child Tax Credit.
A version of this article first appeared on WebMD.com.
Black children had almost three times as many COVID-related deaths as White children and about twice as many hospitalizations, according to a new study.
The study said that 1,556 children have died from the start of the pandemic until Nov. 30, 2022, with 593 of those children being 4 and under. Black children died of COVID-related causes 2.7 times more often than White children and were hospitalized 2.2 times more often than White children, the study said.
Lower vaccination rates for Black people may be a factor. The study said 43.6% of White children have received two or more vaccinations, compared with 40.2% of Black children.
“First and foremost, this study repudiates the misunderstanding that COVID-19 has not been of consequence to children who have had more than 15.5 million reported cases, representing 18 percent of all cases in the United States,” Reed Tuckson, MD, a member of the Black Coalition Against COVID board of directors and former District of Columbia public health commissioner, said in a news release.
“And second, our research shows that like their adult counterparts, Black and other children of color have shouldered more of the burden of COVID-19 than the White population.”
The study was commissioned by BCAC and conducted by the Satcher Health Leadership Institute of the Morehouse School of Medicine, Atlanta. It’s based on studies conducted by other agencies over 2 years.
Black and Hispanic children also had more severe COVID cases, the study said. Among 281 pediatric patients in New York, New Jersey, and Connecticut, 23.3% of severe cases were Black and 51% of severe cases were Hispanic.
The study says 1 in 310 Black children lost a parent or caregiver to COVID between April 2020 and June 2012, compared with 1 in 738 White children.
Economic and health-related hardships were experienced by 31% of Black households, 29% of Latino households, and 16% of White households, the study said.
“Children with COVID-19 in communities of color were sicker, [were] hospitalized and died at higher rates than White children,” Sandra Harris-Hooker, the interim executive director at the Satcher Health Leadership Institute of Morehouse School, said in the release. “We can now fully understand the devastating impact the virus had on communities of color across generations.”
The study recommends several changes, such as modifying eligibility requirements for the Children’s Health Insurance Program to help more children who fall into coverage gaps and expanding the Child Tax Credit.
A version of this article first appeared on WebMD.com.
Navigating your childcare options in a post-COVID world
When we found out we were expecting our first child, we were ecstatic. Our excitement soon gave way to panic, however, as we realized that we needed a plan for childcare. As full-time physicians early in our careers, neither of us was prepared to drop to part-time or become a stay-at-home caregiver. Not knowing where to start, we turned to our friends and colleagues, and of course, the Internet, for advice on our options.
In our research, we discovered three things. First, with COVID-19, the cost of childcare has skyrocketed, and availability has decreased. Second, there are several options for childcare, each with its own benefits and drawbacks. Third, there is no one-size-fits-all solution.
Family
Using family members to provide childcare is often cost-effective and provides a familiar, supportive environment for children. Proximity does not guarantee a willingness or ability to provide long-term care, however, and it can cause strain on family relationships, lead to intrusions and boundary issues, and create feelings of obligation and guilt. It is important to have very honest, up-front discussions with family members about hopes and expectations if this is your childcare plan.
Daycare, facility-based
Daycare centers are commercial facilities that offer care to multiple children of varying ages, starting from as young as 6 weeks. They have trained professionals and provide structured activities and educational programs for children. Many daycares also provide snacks and lunch, which is included in their tuition. They are a popular choice for families seeking full-time childcare and the social and educational benefits that come with a structured setting.
Daycares also have some downsides. They usually operate during normal workday hours, from 7:00 a.m. to 6:00 p.m., which may not be convenient for physicians who work outside of these hours. Even with feasible hours, getting children dressed, ready, and dropped off each morning could add significant time and stress to your morning routine. Additionally, most daycares have policies that prohibit attendance if a child is sick or febrile, which is a common occurrence, particularly for daycare kids. In case of an illness outbreak, the daycare may even close for several days. Both scenarios require at least one parent to take a day off or have an alternative childcare plan available on short notice.
Availability of daycare can be limited, particularly since the COVID pandemic, creating waitlists that can be several months long. Early registration, even during pregnancy, is recommended to secure a spot. It can be helpful to find out if your employer has an agreement with a specific daycare that has “physician-friendly” hours and gives waitlist priority to trainees or even attending physicians. The cost of daycare for one child is typically affordable, around $12,000 per year on average, but can be as high as $25,000 in cities with high cost of living. A sibling discount may be offered, but the cost of daycare for multiple children could still exceed in-home childcare options.1
Daycare, home-based (also known as family care centers)
Family care centers offer a home-like alternative to daycares, with smaller staff-to-child ratios and often more personalized care. They are favored by families seeking a more intimate setting. They might offer more flexible scheduling and are typically less expensive than facility-based daycares, at up to 25% lower cost.1 They may lack the same structure and educational opportunities as facility-based daycares, however, and are not subject to the same health and safety regulations.
Nannies
Nannies are professional caregivers who provide in-home childcare services. Their responsibilities may include feeding, changing, dressing, bathing, and playing with children. In some cases, they may also be expected to do light housekeeping tasks like meal preparation, laundry, and cleaning. It is common for nannies in high-demand markets to refuse to perform these additional tasks, however. Nannies are preferred by families with hectic schedules due to their flexibility. They can work early, late, or even overnight shifts, and provide care in the comfort of your home, avoiding the hassle of drop-off and pick-up times. Nannies also can provide personalized care to meet each child’s specific needs, and they can care for children who are sick or febrile.
When hiring a nanny, it is important to have a written contract outlining their expected hours, wages, benefits, and duties to prevent misunderstandings in the future. Finding a trustworthy and reliable nanny can be a challenge, and families have several options for finding one. They can post jobs on free websites and browse nanny CVs or use a fee-based nanny agency. The cost of using an agency can range from a few hundred to several thousand dollars, so it is important to ask friends and colleagues for recommendations before paying for an agency’s services.
The cost of hiring a nanny is one of its main drawbacks. Nannies typically earn $15 to $30 per hour, and if they work in the family’s home, they are typically considered “household employees” by the IRS. Household employees are entitled to overtime pay for work beyond 40 hours per week, and the employer (you!) is responsible for payroll taxes, withholding, and providing an annual W-2 tax statement.2 There are affordable online nanny payroll services that handle payroll and tax-filing to simplify the process, however. The average annual cost of a full-time nanny is around $40,000 and can be as high as $75,000 in some markets.1 A nanny-share with other families can lower costs, but it may also result in less control over the caregiver and schedule.
It is important to consult a tax professional or the IRS for guidance on nanny wages, taxes, and payroll, as a nanny might rarely be considered an “independent contractor” if they meet certain criteria.
Au pair
An au pair is a live-in childcare provider who travels to a host family’s home from a foreign country on a special J-1 visa. The goal is to provide care for children and participate in cultural exchange activities. Au pairs bring many benefits, such as cost savings compared to traditional childcare options and greater flexibility and customization. They can work up to 10 hours per day and 45 hours a week, performing tasks such as light housekeeping, meal preparation, and transportation for the children. Host families must provide a safe and comfortable living environment, including a private room, meals, and some travel and education expenses.1
The process of hiring an au pair involves working with a designated agency that matches families with applicants and sponsors the J-1 visa. The entire process can take several months, and average program fees cost around $10,000 per placement. Au pairs are hired on a 12-month J-1 visa, which can be extended for up to an additional 12 months, allowing families up to 2 years with the same au pair before needing to find a new placement.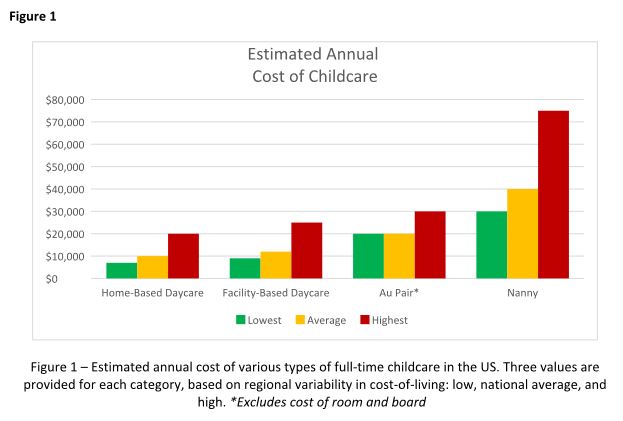
Au pairs earn a minimum weekly stipend of $195.75, set forth by the U.S. State Department.3 Currently, au pairs are not subject to local and state wage requirements, but legal proceedings in various states have recently questioned whether au pairs should be protected under local regulations. Massachusetts has been the most progressive, explicitly protecting au pairs as domestic workers under state labor laws, raising their weekly stipend to roughly $600 to comply with state minimum wage requirements.4 The federal government is expected to provide clarity on this issue, but for the time being, au pairs remain an affordable alternative to a nanny in most states.
Conclusion
Choosing childcare is a complicated process with multiple factors to consider. Figure 1 breaks down the estimated annual cost of each of the options outlined above for a single child in low, average, and high cost-of-living areas. But your decision likely hinges on much more than just cost, and may include family dynamics, scheduling needs, and personal preferences. Gather as much advice and information as possible, but remember to trust your instincts and make the decision that works best for your family. At the end of the day, what matters most is the happiness and well-being of your child.
Dr. Hathorn and Dr. Creighton are married, and both work full-time with a 1-year-old child. Dr. Hathorn is a bariatric and advanced therapeutic endoscopist at the University of North Carolina at Chapel Hill. Dr. Creighton is an anesthesiologist at UNC Chapel Hill. Neither reported any conflicts of interest.
References
1. Care.com. This is how much childcare costs in 2022. 2022 Jun 15.
2. Internal Revenue Service. Publication 926 - Household Employer’s Tax Guide 2023.
3. U.S. Department of State. Au Pair.
4. Commonwealth of Massachusetts. Domestic workers.
Disclaimer
The financial and tax information presented in this article are believed to be true and accurate at the time of writing. However, it’s important to note that tax laws and regulations are subject to change. The authors are not certified financial advisers or tax specialists. It is recommended to seek verification from a local tax expert or the Internal Revenue Service to discuss your specific situation.
When we found out we were expecting our first child, we were ecstatic. Our excitement soon gave way to panic, however, as we realized that we needed a plan for childcare. As full-time physicians early in our careers, neither of us was prepared to drop to part-time or become a stay-at-home caregiver. Not knowing where to start, we turned to our friends and colleagues, and of course, the Internet, for advice on our options.
In our research, we discovered three things. First, with COVID-19, the cost of childcare has skyrocketed, and availability has decreased. Second, there are several options for childcare, each with its own benefits and drawbacks. Third, there is no one-size-fits-all solution.
Family
Using family members to provide childcare is often cost-effective and provides a familiar, supportive environment for children. Proximity does not guarantee a willingness or ability to provide long-term care, however, and it can cause strain on family relationships, lead to intrusions and boundary issues, and create feelings of obligation and guilt. It is important to have very honest, up-front discussions with family members about hopes and expectations if this is your childcare plan.
Daycare, facility-based
Daycare centers are commercial facilities that offer care to multiple children of varying ages, starting from as young as 6 weeks. They have trained professionals and provide structured activities and educational programs for children. Many daycares also provide snacks and lunch, which is included in their tuition. They are a popular choice for families seeking full-time childcare and the social and educational benefits that come with a structured setting.
Daycares also have some downsides. They usually operate during normal workday hours, from 7:00 a.m. to 6:00 p.m., which may not be convenient for physicians who work outside of these hours. Even with feasible hours, getting children dressed, ready, and dropped off each morning could add significant time and stress to your morning routine. Additionally, most daycares have policies that prohibit attendance if a child is sick or febrile, which is a common occurrence, particularly for daycare kids. In case of an illness outbreak, the daycare may even close for several days. Both scenarios require at least one parent to take a day off or have an alternative childcare plan available on short notice.
Availability of daycare can be limited, particularly since the COVID pandemic, creating waitlists that can be several months long. Early registration, even during pregnancy, is recommended to secure a spot. It can be helpful to find out if your employer has an agreement with a specific daycare that has “physician-friendly” hours and gives waitlist priority to trainees or even attending physicians. The cost of daycare for one child is typically affordable, around $12,000 per year on average, but can be as high as $25,000 in cities with high cost of living. A sibling discount may be offered, but the cost of daycare for multiple children could still exceed in-home childcare options.1
Daycare, home-based (also known as family care centers)
Family care centers offer a home-like alternative to daycares, with smaller staff-to-child ratios and often more personalized care. They are favored by families seeking a more intimate setting. They might offer more flexible scheduling and are typically less expensive than facility-based daycares, at up to 25% lower cost.1 They may lack the same structure and educational opportunities as facility-based daycares, however, and are not subject to the same health and safety regulations.
Nannies
Nannies are professional caregivers who provide in-home childcare services. Their responsibilities may include feeding, changing, dressing, bathing, and playing with children. In some cases, they may also be expected to do light housekeeping tasks like meal preparation, laundry, and cleaning. It is common for nannies in high-demand markets to refuse to perform these additional tasks, however. Nannies are preferred by families with hectic schedules due to their flexibility. They can work early, late, or even overnight shifts, and provide care in the comfort of your home, avoiding the hassle of drop-off and pick-up times. Nannies also can provide personalized care to meet each child’s specific needs, and they can care for children who are sick or febrile.
When hiring a nanny, it is important to have a written contract outlining their expected hours, wages, benefits, and duties to prevent misunderstandings in the future. Finding a trustworthy and reliable nanny can be a challenge, and families have several options for finding one. They can post jobs on free websites and browse nanny CVs or use a fee-based nanny agency. The cost of using an agency can range from a few hundred to several thousand dollars, so it is important to ask friends and colleagues for recommendations before paying for an agency’s services.
The cost of hiring a nanny is one of its main drawbacks. Nannies typically earn $15 to $30 per hour, and if they work in the family’s home, they are typically considered “household employees” by the IRS. Household employees are entitled to overtime pay for work beyond 40 hours per week, and the employer (you!) is responsible for payroll taxes, withholding, and providing an annual W-2 tax statement.2 There are affordable online nanny payroll services that handle payroll and tax-filing to simplify the process, however. The average annual cost of a full-time nanny is around $40,000 and can be as high as $75,000 in some markets.1 A nanny-share with other families can lower costs, but it may also result in less control over the caregiver and schedule.
It is important to consult a tax professional or the IRS for guidance on nanny wages, taxes, and payroll, as a nanny might rarely be considered an “independent contractor” if they meet certain criteria.
Au pair
An au pair is a live-in childcare provider who travels to a host family’s home from a foreign country on a special J-1 visa. The goal is to provide care for children and participate in cultural exchange activities. Au pairs bring many benefits, such as cost savings compared to traditional childcare options and greater flexibility and customization. They can work up to 10 hours per day and 45 hours a week, performing tasks such as light housekeeping, meal preparation, and transportation for the children. Host families must provide a safe and comfortable living environment, including a private room, meals, and some travel and education expenses.1
The process of hiring an au pair involves working with a designated agency that matches families with applicants and sponsors the J-1 visa. The entire process can take several months, and average program fees cost around $10,000 per placement. Au pairs are hired on a 12-month J-1 visa, which can be extended for up to an additional 12 months, allowing families up to 2 years with the same au pair before needing to find a new placement.
Au pairs earn a minimum weekly stipend of $195.75, set forth by the U.S. State Department.3 Currently, au pairs are not subject to local and state wage requirements, but legal proceedings in various states have recently questioned whether au pairs should be protected under local regulations. Massachusetts has been the most progressive, explicitly protecting au pairs as domestic workers under state labor laws, raising their weekly stipend to roughly $600 to comply with state minimum wage requirements.4 The federal government is expected to provide clarity on this issue, but for the time being, au pairs remain an affordable alternative to a nanny in most states.
Conclusion
Choosing childcare is a complicated process with multiple factors to consider. Figure 1 breaks down the estimated annual cost of each of the options outlined above for a single child in low, average, and high cost-of-living areas. But your decision likely hinges on much more than just cost, and may include family dynamics, scheduling needs, and personal preferences. Gather as much advice and information as possible, but remember to trust your instincts and make the decision that works best for your family. At the end of the day, what matters most is the happiness and well-being of your child.
Dr. Hathorn and Dr. Creighton are married, and both work full-time with a 1-year-old child. Dr. Hathorn is a bariatric and advanced therapeutic endoscopist at the University of North Carolina at Chapel Hill. Dr. Creighton is an anesthesiologist at UNC Chapel Hill. Neither reported any conflicts of interest.
References
1. Care.com. This is how much childcare costs in 2022. 2022 Jun 15.
2. Internal Revenue Service. Publication 926 - Household Employer’s Tax Guide 2023.
3. U.S. Department of State. Au Pair.
4. Commonwealth of Massachusetts. Domestic workers.
Disclaimer
The financial and tax information presented in this article are believed to be true and accurate at the time of writing. However, it’s important to note that tax laws and regulations are subject to change. The authors are not certified financial advisers or tax specialists. It is recommended to seek verification from a local tax expert or the Internal Revenue Service to discuss your specific situation.
When we found out we were expecting our first child, we were ecstatic. Our excitement soon gave way to panic, however, as we realized that we needed a plan for childcare. As full-time physicians early in our careers, neither of us was prepared to drop to part-time or become a stay-at-home caregiver. Not knowing where to start, we turned to our friends and colleagues, and of course, the Internet, for advice on our options.
In our research, we discovered three things. First, with COVID-19, the cost of childcare has skyrocketed, and availability has decreased. Second, there are several options for childcare, each with its own benefits and drawbacks. Third, there is no one-size-fits-all solution.
Family
Using family members to provide childcare is often cost-effective and provides a familiar, supportive environment for children. Proximity does not guarantee a willingness or ability to provide long-term care, however, and it can cause strain on family relationships, lead to intrusions and boundary issues, and create feelings of obligation and guilt. It is important to have very honest, up-front discussions with family members about hopes and expectations if this is your childcare plan.
Daycare, facility-based
Daycare centers are commercial facilities that offer care to multiple children of varying ages, starting from as young as 6 weeks. They have trained professionals and provide structured activities and educational programs for children. Many daycares also provide snacks and lunch, which is included in their tuition. They are a popular choice for families seeking full-time childcare and the social and educational benefits that come with a structured setting.
Daycares also have some downsides. They usually operate during normal workday hours, from 7:00 a.m. to 6:00 p.m., which may not be convenient for physicians who work outside of these hours. Even with feasible hours, getting children dressed, ready, and dropped off each morning could add significant time and stress to your morning routine. Additionally, most daycares have policies that prohibit attendance if a child is sick or febrile, which is a common occurrence, particularly for daycare kids. In case of an illness outbreak, the daycare may even close for several days. Both scenarios require at least one parent to take a day off or have an alternative childcare plan available on short notice.
Availability of daycare can be limited, particularly since the COVID pandemic, creating waitlists that can be several months long. Early registration, even during pregnancy, is recommended to secure a spot. It can be helpful to find out if your employer has an agreement with a specific daycare that has “physician-friendly” hours and gives waitlist priority to trainees or even attending physicians. The cost of daycare for one child is typically affordable, around $12,000 per year on average, but can be as high as $25,000 in cities with high cost of living. A sibling discount may be offered, but the cost of daycare for multiple children could still exceed in-home childcare options.1
Daycare, home-based (also known as family care centers)
Family care centers offer a home-like alternative to daycares, with smaller staff-to-child ratios and often more personalized care. They are favored by families seeking a more intimate setting. They might offer more flexible scheduling and are typically less expensive than facility-based daycares, at up to 25% lower cost.1 They may lack the same structure and educational opportunities as facility-based daycares, however, and are not subject to the same health and safety regulations.
Nannies
Nannies are professional caregivers who provide in-home childcare services. Their responsibilities may include feeding, changing, dressing, bathing, and playing with children. In some cases, they may also be expected to do light housekeeping tasks like meal preparation, laundry, and cleaning. It is common for nannies in high-demand markets to refuse to perform these additional tasks, however. Nannies are preferred by families with hectic schedules due to their flexibility. They can work early, late, or even overnight shifts, and provide care in the comfort of your home, avoiding the hassle of drop-off and pick-up times. Nannies also can provide personalized care to meet each child’s specific needs, and they can care for children who are sick or febrile.
When hiring a nanny, it is important to have a written contract outlining their expected hours, wages, benefits, and duties to prevent misunderstandings in the future. Finding a trustworthy and reliable nanny can be a challenge, and families have several options for finding one. They can post jobs on free websites and browse nanny CVs or use a fee-based nanny agency. The cost of using an agency can range from a few hundred to several thousand dollars, so it is important to ask friends and colleagues for recommendations before paying for an agency’s services.
The cost of hiring a nanny is one of its main drawbacks. Nannies typically earn $15 to $30 per hour, and if they work in the family’s home, they are typically considered “household employees” by the IRS. Household employees are entitled to overtime pay for work beyond 40 hours per week, and the employer (you!) is responsible for payroll taxes, withholding, and providing an annual W-2 tax statement.2 There are affordable online nanny payroll services that handle payroll and tax-filing to simplify the process, however. The average annual cost of a full-time nanny is around $40,000 and can be as high as $75,000 in some markets.1 A nanny-share with other families can lower costs, but it may also result in less control over the caregiver and schedule.
It is important to consult a tax professional or the IRS for guidance on nanny wages, taxes, and payroll, as a nanny might rarely be considered an “independent contractor” if they meet certain criteria.
Au pair
An au pair is a live-in childcare provider who travels to a host family’s home from a foreign country on a special J-1 visa. The goal is to provide care for children and participate in cultural exchange activities. Au pairs bring many benefits, such as cost savings compared to traditional childcare options and greater flexibility and customization. They can work up to 10 hours per day and 45 hours a week, performing tasks such as light housekeeping, meal preparation, and transportation for the children. Host families must provide a safe and comfortable living environment, including a private room, meals, and some travel and education expenses.1
The process of hiring an au pair involves working with a designated agency that matches families with applicants and sponsors the J-1 visa. The entire process can take several months, and average program fees cost around $10,000 per placement. Au pairs are hired on a 12-month J-1 visa, which can be extended for up to an additional 12 months, allowing families up to 2 years with the same au pair before needing to find a new placement.
Au pairs earn a minimum weekly stipend of $195.75, set forth by the U.S. State Department.3 Currently, au pairs are not subject to local and state wage requirements, but legal proceedings in various states have recently questioned whether au pairs should be protected under local regulations. Massachusetts has been the most progressive, explicitly protecting au pairs as domestic workers under state labor laws, raising their weekly stipend to roughly $600 to comply with state minimum wage requirements.4 The federal government is expected to provide clarity on this issue, but for the time being, au pairs remain an affordable alternative to a nanny in most states.
Conclusion
Choosing childcare is a complicated process with multiple factors to consider. Figure 1 breaks down the estimated annual cost of each of the options outlined above for a single child in low, average, and high cost-of-living areas. But your decision likely hinges on much more than just cost, and may include family dynamics, scheduling needs, and personal preferences. Gather as much advice and information as possible, but remember to trust your instincts and make the decision that works best for your family. At the end of the day, what matters most is the happiness and well-being of your child.
Dr. Hathorn and Dr. Creighton are married, and both work full-time with a 1-year-old child. Dr. Hathorn is a bariatric and advanced therapeutic endoscopist at the University of North Carolina at Chapel Hill. Dr. Creighton is an anesthesiologist at UNC Chapel Hill. Neither reported any conflicts of interest.
References
1. Care.com. This is how much childcare costs in 2022. 2022 Jun 15.
2. Internal Revenue Service. Publication 926 - Household Employer’s Tax Guide 2023.
3. U.S. Department of State. Au Pair.
4. Commonwealth of Massachusetts. Domestic workers.
Disclaimer
The financial and tax information presented in this article are believed to be true and accurate at the time of writing. However, it’s important to note that tax laws and regulations are subject to change. The authors are not certified financial advisers or tax specialists. It is recommended to seek verification from a local tax expert or the Internal Revenue Service to discuss your specific situation.
From private practice to academic medicine: My journey and lessons learned
Loyalty.
This is a quality that I value in relationships. Loyalty was a significant factor contributing to my postfellowship commitment to private practice. In 2001, I graduated from physician assistant school and accepted a job with a private practice GI group in Omaha. I was fortunate to work with supportive gastroenterologists who encouraged me to pursue further training after I expressed an interest in medical school. My goal was to become a gastroenterologist but like every medical student, I would keep an open mind. My decision did not waver, and the support from my first mentors continued. As I graduated from fellowship in 2014, I gravitated toward the same private practice largely based on loyalty and my experience as a PA.

COURTESY AMERICAN GASTROENTEROLOGICAL ASSOCIATION
My experience in private practice was positive. My focus at that time and currently is clinical medicine with a focus on inflammatory bowel disease (IBD) patients. My colleagues were supportive, and I worked with a great team of nurses and APPs. I cared for many patients in both the inpatient and outpatient setting and had an opportunity to complete a high volume and variety of procedures. Overall, the various aspects of my job were rewarding. However, something was missing, and I made personal and professional adjustments. My schedule pulled me from valued family time with my kids (mostly) in the early mornings, therefore, I altered my work schedule. My clinical interest in IBD was diluted by the emphasis to see mostly general GI patients, as is the case for many in private practice. I missed the academic environment, especially working with medical students, residents, and fellows, so I occasionally had residents shadow me. Unfortunately, adjustments did not “fix” that missing component – to me, this was a job that did not feel like a career. I was not professionally fulfilled and on several occasions during the 6 years in private practice, I connected with mentors from my medical training to explore career options while trying to define what was missing.
During the latter part of years 5 and 6, it became apparent to me that loyalty pulled me toward working with a great group of supportive gastroenterologists, but it became increasingly more apparent that this job was not in line with my career goals. I had identified that I wanted to actively participate in medical education while practicing as a gastroenterologist in an academic setting. Additionally, time with my family was a critical part to the work-life integration.
My approach to the next step in my journey was different than my initial job. My goal was to define what was important, as in what were my absolute requirements for career satisfaction and where was I willing to be flexible. Each of us has different absolute and relative requirements based on our values, and I neglected to clearly identify these components with my first job. Admittedly, I have (at times) struggled to acknowledge my values, because I might somehow appear less committed to a career. Owning these values has provided clarity in my path from private practice to academic medicine. During the 3 years I have been in my current position, I have stepped into a leadership role in the University of Nebraska Medicine GI fellowship program while providing clinical medicine at the Fred Paustian IBD Center at Nebraska Medicine. In addition, I continue to have an active endoscopy schedule and derive great satisfaction teaching the fellows how to be effective endoscopists. Personally, the difference in compensation between academic medicine and private practice was not an important factor, although this is a factor for some people (and that’s okay).
When I graduated from fellowship, my path seemed clear, and I did not anticipate the road ahead. However, with each hurdle, I was gifted with lessons that would prove to be valuable as I moved ahead. Thank you for giving me the space to share my story.
Lessons that have helped in my journey from private practice to academics
- Mentorship: Find mentors, not just one mentor. Over the years, I have had several mentors, but what I recognize is that, early in my career, I did not have a mentor. Although a mentor cannot make decisions for you, he/she can provide guidance from a place of experience (both career and life experience).
- Define a mission statement: My mentor pushed me to first define my values and then my mission statement. This serves as the foundation that I reference when making decisions that will impact my family and my career. For example, if I am invited to participate on a committee, I look at how this will impact my family and whether it aligns with my mission. This helps to clarify what I am willing to say yes to and what to pass along to another colleague. Remember that last part ... if you are saying no to something, suggest another colleague for the opportunity.
- Advocate for yourself: Only you know what is best for you. Sometimes the path to discovering what that is can be tortuous and require guidance. Throughout my journey, I worked with colleagues who were supportive of my journey back to medical school and supportive of my job in private practice, but only I could define what a career meant to me.
- Assume positive intent: In medicine, we frequently work in a high-stakes, stressful environment. Assume positive intent in your interactions with others, especially colleagues. This will serve you well.
- Life happens: Each of us will experience an unexpected event in our personal life or career path or both. This will be okay. The path forward may look different and require a pivot. This unexpected event might be that you find your job leaves you wanting something more or something different. Your journey will be right for you.
Dr. Hutchins is an assistant professor in the division of gastroenterology and hepatology at the University of Nebraska Medical Center, Omaha. She reported no conflicts of interest.
Loyalty.
This is a quality that I value in relationships. Loyalty was a significant factor contributing to my postfellowship commitment to private practice. In 2001, I graduated from physician assistant school and accepted a job with a private practice GI group in Omaha. I was fortunate to work with supportive gastroenterologists who encouraged me to pursue further training after I expressed an interest in medical school. My goal was to become a gastroenterologist but like every medical student, I would keep an open mind. My decision did not waver, and the support from my first mentors continued. As I graduated from fellowship in 2014, I gravitated toward the same private practice largely based on loyalty and my experience as a PA.

COURTESY AMERICAN GASTROENTEROLOGICAL ASSOCIATION
My experience in private practice was positive. My focus at that time and currently is clinical medicine with a focus on inflammatory bowel disease (IBD) patients. My colleagues were supportive, and I worked with a great team of nurses and APPs. I cared for many patients in both the inpatient and outpatient setting and had an opportunity to complete a high volume and variety of procedures. Overall, the various aspects of my job were rewarding. However, something was missing, and I made personal and professional adjustments. My schedule pulled me from valued family time with my kids (mostly) in the early mornings, therefore, I altered my work schedule. My clinical interest in IBD was diluted by the emphasis to see mostly general GI patients, as is the case for many in private practice. I missed the academic environment, especially working with medical students, residents, and fellows, so I occasionally had residents shadow me. Unfortunately, adjustments did not “fix” that missing component – to me, this was a job that did not feel like a career. I was not professionally fulfilled and on several occasions during the 6 years in private practice, I connected with mentors from my medical training to explore career options while trying to define what was missing.
During the latter part of years 5 and 6, it became apparent to me that loyalty pulled me toward working with a great group of supportive gastroenterologists, but it became increasingly more apparent that this job was not in line with my career goals. I had identified that I wanted to actively participate in medical education while practicing as a gastroenterologist in an academic setting. Additionally, time with my family was a critical part to the work-life integration.
My approach to the next step in my journey was different than my initial job. My goal was to define what was important, as in what were my absolute requirements for career satisfaction and where was I willing to be flexible. Each of us has different absolute and relative requirements based on our values, and I neglected to clearly identify these components with my first job. Admittedly, I have (at times) struggled to acknowledge my values, because I might somehow appear less committed to a career. Owning these values has provided clarity in my path from private practice to academic medicine. During the 3 years I have been in my current position, I have stepped into a leadership role in the University of Nebraska Medicine GI fellowship program while providing clinical medicine at the Fred Paustian IBD Center at Nebraska Medicine. In addition, I continue to have an active endoscopy schedule and derive great satisfaction teaching the fellows how to be effective endoscopists. Personally, the difference in compensation between academic medicine and private practice was not an important factor, although this is a factor for some people (and that’s okay).
When I graduated from fellowship, my path seemed clear, and I did not anticipate the road ahead. However, with each hurdle, I was gifted with lessons that would prove to be valuable as I moved ahead. Thank you for giving me the space to share my story.
Lessons that have helped in my journey from private practice to academics
- Mentorship: Find mentors, not just one mentor. Over the years, I have had several mentors, but what I recognize is that, early in my career, I did not have a mentor. Although a mentor cannot make decisions for you, he/she can provide guidance from a place of experience (both career and life experience).
- Define a mission statement: My mentor pushed me to first define my values and then my mission statement. This serves as the foundation that I reference when making decisions that will impact my family and my career. For example, if I am invited to participate on a committee, I look at how this will impact my family and whether it aligns with my mission. This helps to clarify what I am willing to say yes to and what to pass along to another colleague. Remember that last part ... if you are saying no to something, suggest another colleague for the opportunity.
- Advocate for yourself: Only you know what is best for you. Sometimes the path to discovering what that is can be tortuous and require guidance. Throughout my journey, I worked with colleagues who were supportive of my journey back to medical school and supportive of my job in private practice, but only I could define what a career meant to me.
- Assume positive intent: In medicine, we frequently work in a high-stakes, stressful environment. Assume positive intent in your interactions with others, especially colleagues. This will serve you well.
- Life happens: Each of us will experience an unexpected event in our personal life or career path or both. This will be okay. The path forward may look different and require a pivot. This unexpected event might be that you find your job leaves you wanting something more or something different. Your journey will be right for you.
Dr. Hutchins is an assistant professor in the division of gastroenterology and hepatology at the University of Nebraska Medical Center, Omaha. She reported no conflicts of interest.
Loyalty.
This is a quality that I value in relationships. Loyalty was a significant factor contributing to my postfellowship commitment to private practice. In 2001, I graduated from physician assistant school and accepted a job with a private practice GI group in Omaha. I was fortunate to work with supportive gastroenterologists who encouraged me to pursue further training after I expressed an interest in medical school. My goal was to become a gastroenterologist but like every medical student, I would keep an open mind. My decision did not waver, and the support from my first mentors continued. As I graduated from fellowship in 2014, I gravitated toward the same private practice largely based on loyalty and my experience as a PA.

COURTESY AMERICAN GASTROENTEROLOGICAL ASSOCIATION
My experience in private practice was positive. My focus at that time and currently is clinical medicine with a focus on inflammatory bowel disease (IBD) patients. My colleagues were supportive, and I worked with a great team of nurses and APPs. I cared for many patients in both the inpatient and outpatient setting and had an opportunity to complete a high volume and variety of procedures. Overall, the various aspects of my job were rewarding. However, something was missing, and I made personal and professional adjustments. My schedule pulled me from valued family time with my kids (mostly) in the early mornings, therefore, I altered my work schedule. My clinical interest in IBD was diluted by the emphasis to see mostly general GI patients, as is the case for many in private practice. I missed the academic environment, especially working with medical students, residents, and fellows, so I occasionally had residents shadow me. Unfortunately, adjustments did not “fix” that missing component – to me, this was a job that did not feel like a career. I was not professionally fulfilled and on several occasions during the 6 years in private practice, I connected with mentors from my medical training to explore career options while trying to define what was missing.
During the latter part of years 5 and 6, it became apparent to me that loyalty pulled me toward working with a great group of supportive gastroenterologists, but it became increasingly more apparent that this job was not in line with my career goals. I had identified that I wanted to actively participate in medical education while practicing as a gastroenterologist in an academic setting. Additionally, time with my family was a critical part to the work-life integration.
My approach to the next step in my journey was different than my initial job. My goal was to define what was important, as in what were my absolute requirements for career satisfaction and where was I willing to be flexible. Each of us has different absolute and relative requirements based on our values, and I neglected to clearly identify these components with my first job. Admittedly, I have (at times) struggled to acknowledge my values, because I might somehow appear less committed to a career. Owning these values has provided clarity in my path from private practice to academic medicine. During the 3 years I have been in my current position, I have stepped into a leadership role in the University of Nebraska Medicine GI fellowship program while providing clinical medicine at the Fred Paustian IBD Center at Nebraska Medicine. In addition, I continue to have an active endoscopy schedule and derive great satisfaction teaching the fellows how to be effective endoscopists. Personally, the difference in compensation between academic medicine and private practice was not an important factor, although this is a factor for some people (and that’s okay).
When I graduated from fellowship, my path seemed clear, and I did not anticipate the road ahead. However, with each hurdle, I was gifted with lessons that would prove to be valuable as I moved ahead. Thank you for giving me the space to share my story.
Lessons that have helped in my journey from private practice to academics
- Mentorship: Find mentors, not just one mentor. Over the years, I have had several mentors, but what I recognize is that, early in my career, I did not have a mentor. Although a mentor cannot make decisions for you, he/she can provide guidance from a place of experience (both career and life experience).
- Define a mission statement: My mentor pushed me to first define my values and then my mission statement. This serves as the foundation that I reference when making decisions that will impact my family and my career. For example, if I am invited to participate on a committee, I look at how this will impact my family and whether it aligns with my mission. This helps to clarify what I am willing to say yes to and what to pass along to another colleague. Remember that last part ... if you are saying no to something, suggest another colleague for the opportunity.
- Advocate for yourself: Only you know what is best for you. Sometimes the path to discovering what that is can be tortuous and require guidance. Throughout my journey, I worked with colleagues who were supportive of my journey back to medical school and supportive of my job in private practice, but only I could define what a career meant to me.
- Assume positive intent: In medicine, we frequently work in a high-stakes, stressful environment. Assume positive intent in your interactions with others, especially colleagues. This will serve you well.
- Life happens: Each of us will experience an unexpected event in our personal life or career path or both. This will be okay. The path forward may look different and require a pivot. This unexpected event might be that you find your job leaves you wanting something more or something different. Your journey will be right for you.
Dr. Hutchins is an assistant professor in the division of gastroenterology and hepatology at the University of Nebraska Medical Center, Omaha. She reported no conflicts of interest.
Widespread Erosions in Intertriginous Areas
The Diagnosis: Darier Disease
A clinical diagnosis of Darier disease was made from the skin findings of pruritic, malodorous, keratotic papules in a seborrheic distribution and pathognomonic nail dystrophy, along with a family history that demonstrated autosomal-dominant inheritance. The ulcerations were suspected to be caused by a superimposed herpes simplex virus (HSV) infection in the form of eczema herpeticum. The clinical diagnosis was later confirmed via punch biopsy. Pathology results demonstrated focal acantholytic dyskeratosis, which was consistent with Darier disease given the focal nature and lack of acanthosis. The patient’s father and sister also were confirmed to have Darier disease by an outside dermatologist.
Darier disease is a rare keratinizing autosomaldominant genodermatosis that occurs due to a mutation in the ATP2A2 gene, which encodes a sarco/endoplasmic reticulum calcium ATPase pump that decreases cell adhesion between keratinocytes, leading to epidermal acantholysis and dyskeratosis and ultimately a disrupted skin barrier.1,2 Darier disease often presents in childhood and adolescence with papules in a seborrheic distribution on the central chest and back (Figure, A); the intertriginous folds also may be involved. Darier disease can manifest with palmoplantar pits (Figure, B), a cobblestonelike texture of the oral mucosa, acrokeratosis verruciformis of Hopf, and nail findings with alternating red and white longitudinal streaks in the nail bed resembling a candy cane along with characteristic V nicking deformities of the nails themselves (Figure, C). Chronic flares may occur throughout one’s lifetime, with patients experiencing more symptoms in the summer months due to heat, sweat, and UV light exposure, as well as infections that irritate the skin and worsen dyskeratosis. Studies have revealed an association between Darier disease and neuropsychiatric conditions, including major depressive disorder, schizophrenia, and bipolar disorder.3,4
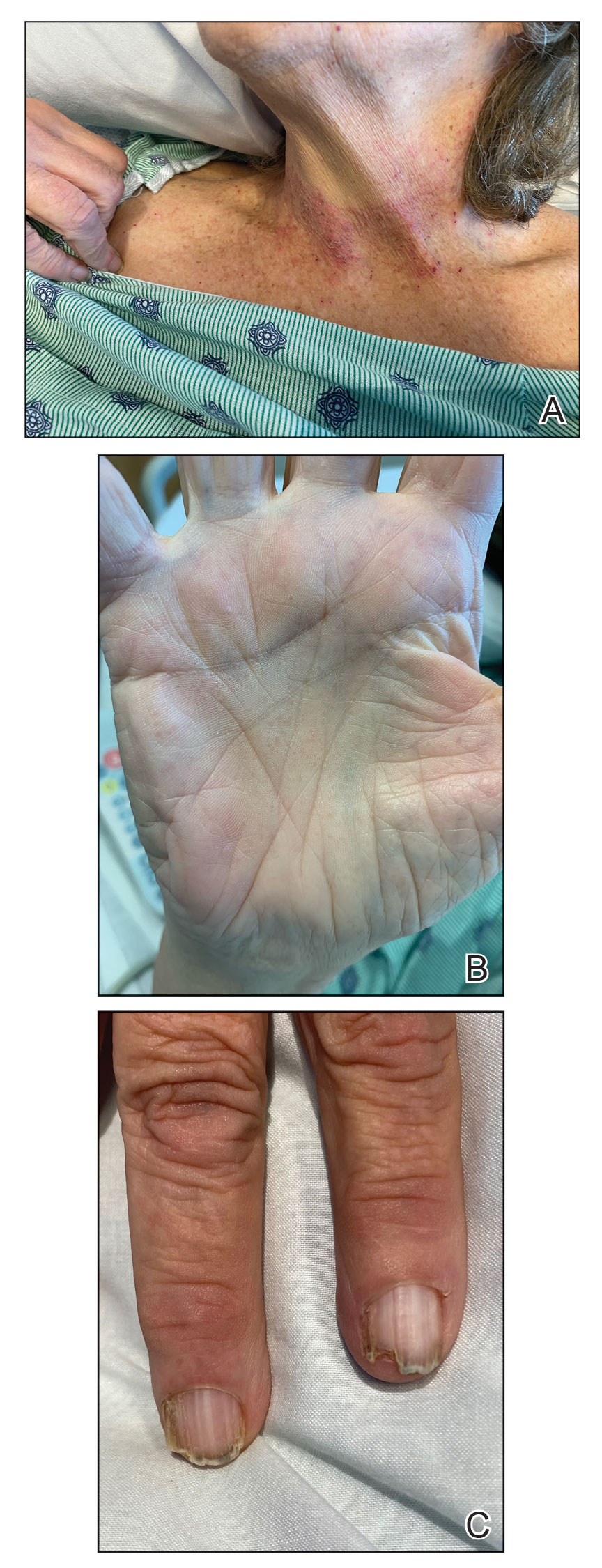
The skin barrier is compromised in patients with Darier disease, thereby making secondary infection more likely to occur. Polymerase chain reaction swabs of our patient’s purulent ulcerations were positive for HSV type 1, further strengthening a diagnosis of secondary eczema herpeticum, which occurs when patients have widespread HSV superinfecting pre-existing skin conditions such as atopic dermatitis, Darier disease, and Hailey-Hailey disease.5-7 The lesions are characterized by a monomorphic eruption of umbilicated vesicles on an erythematous base. Lesions can progress to punched-out ulcers and erosions with hemorrhagic crusts that coalesce, forming scalloped borders, similar to our patient’s presentation.8
Hailey-Hailey disease, a genodermatosis that alters calcium signaling with an autosomal-dominant inheritance pattern, was unlikely in our patient due to the presence of nail abnormalities and palmar pits that are characteristic of Darier disease. From a purely histopathologic standpoint, Grover disease was considered with skin biopsy demonstrating acantholytic dyskeratosis but was not compatible with the clinical context. Furthermore, trials of antibiotics with group A Streptococcus and Staphylococcus aureus coverage failed in our patient, and she lacked systemic symptoms that would be supportive of a cellulitis diagnosis. The punched-out lesions suggested that an isolated exacerbation of atopic dermatitis was not sufficient to explain all of the clinical findings.
Eczema herpeticum must be considered in the differential diagnosis for patients with underlying Darier disease and widespread ulcerations. Our patient had more recent punched-out ulcerations in the intertriginous regions, with other areas showing later stages of confluent ulcers with scalloped borders. Delayed diagnosis and treatment of eczema herpeticum combined with severe Darier disease can lead to increased risk for hospitalization and rarely fatality.8,9
Our patient was started on intravenous acyclovir until the lesions crusted and then was transitioned to a suppressive dose of oral valacyclovir given the widespread distribution. The Darier disease itself was managed with topical steroids and a zinc oxide barrier, serving as protectants to pathogens through microscopic breaks in the skin. Our patient also had a mild case of candidal intertrigo that was exacerbated by obesity and managed with topical ketoconazole. Gabapentin, hydromorphone, and acetaminophen were used for pain. She was discharged 10 days after admission with substantial improvement of both the HSV lesions and the irritation from her Darier disease. At follow-up visits 20 days later and again 6 months after discharge, she had been feeling well without any HSV flares.
The eczema herpeticum likely arose from our patient’s chronic skin barrier impairment attributed to Darier disease, leading to the cutaneous inoculation of HSV. Our patient and her family members had never been evaluated by a dermatologist until late in life during this hospitalization. Medication compliance with a suppressive dose of oral valacyclovir and topical steroids is vital to prevent flares of both eczema herpeticum and Darier disease, respectively. This case highlights the importance of dermatology consultation for complex cutaneous findings, as delayed diagnosis and treatment can lead to increased morbidity and mortality.
- Cooper SM, Burge SM. Darier’s disease: epidemiology, pathophysiology, and management. Am J Clin Dermatol. 2003;4:97-105. doi:10.2165/00128071-200304020-00003
- Dhitavat J, Cobbold C, Leslie N, et al. Impaired trafficking of the desmoplakins in cultured Darier’s disease keratinocytes. J Invest Dermatol. 2003;121:1349-1355. doi:10.1046/j.1523-1747.2003.12557.x
- Nakamura T, Kazuno AA, Nakajima K, et al. Loss of function mutations in ATP2A2 and psychoses: a case report and literature survey. Psychiatry Clin Neurosci. 2016;70:342-350. doi:10.1111/pcn.12395
- Gordon-Smith K, Jones LA, Burge SM, et al. The neuropsychiatric phenotype in Darier disease. Br J Dermatol. 2010;163:515-522. doi:10.1111/j.1365-2133.2010.09834.x
- Hemani SA, Edmond MB, Jaggi P, et al. Frequency and clinical features associated with eczema herpeticum in hospitalized children with presumed atopic dermatitis skin infection. Pediatr Infect Dis J. 2020;39:263-266. doi:10.1097/INF.0000000000002542
- Tayabali K, Pothiwalla H, Lowitt M. Eczema herpeticum in Darier’s disease: a topical storm. J Community Hosp Intern Med Perspect. 2019;9:347-350. doi:10.1080/20009666.2019.1650590
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314. doi:10.5021/ad.2009.21.3.311
- Nikkels AF, Beauthier F, Quatresooz P, et al. Fatal herpes simplex virus infection in Darier disease under corticotherapy. Eur J Dermatol. 2005;15:293-297.
- Vogt KA, Lohse CM, El-Azhary RA, et al. Kaposi varicelliform eruption in patients with Darier disease: a 20-year retrospective study. J Am Acad Dermatol. 2015;72:481-484. doi:10.1016/j.jaad.2014.12.001
The Diagnosis: Darier Disease
A clinical diagnosis of Darier disease was made from the skin findings of pruritic, malodorous, keratotic papules in a seborrheic distribution and pathognomonic nail dystrophy, along with a family history that demonstrated autosomal-dominant inheritance. The ulcerations were suspected to be caused by a superimposed herpes simplex virus (HSV) infection in the form of eczema herpeticum. The clinical diagnosis was later confirmed via punch biopsy. Pathology results demonstrated focal acantholytic dyskeratosis, which was consistent with Darier disease given the focal nature and lack of acanthosis. The patient’s father and sister also were confirmed to have Darier disease by an outside dermatologist.
Darier disease is a rare keratinizing autosomaldominant genodermatosis that occurs due to a mutation in the ATP2A2 gene, which encodes a sarco/endoplasmic reticulum calcium ATPase pump that decreases cell adhesion between keratinocytes, leading to epidermal acantholysis and dyskeratosis and ultimately a disrupted skin barrier.1,2 Darier disease often presents in childhood and adolescence with papules in a seborrheic distribution on the central chest and back (Figure, A); the intertriginous folds also may be involved. Darier disease can manifest with palmoplantar pits (Figure, B), a cobblestonelike texture of the oral mucosa, acrokeratosis verruciformis of Hopf, and nail findings with alternating red and white longitudinal streaks in the nail bed resembling a candy cane along with characteristic V nicking deformities of the nails themselves (Figure, C). Chronic flares may occur throughout one’s lifetime, with patients experiencing more symptoms in the summer months due to heat, sweat, and UV light exposure, as well as infections that irritate the skin and worsen dyskeratosis. Studies have revealed an association between Darier disease and neuropsychiatric conditions, including major depressive disorder, schizophrenia, and bipolar disorder.3,4

The skin barrier is compromised in patients with Darier disease, thereby making secondary infection more likely to occur. Polymerase chain reaction swabs of our patient’s purulent ulcerations were positive for HSV type 1, further strengthening a diagnosis of secondary eczema herpeticum, which occurs when patients have widespread HSV superinfecting pre-existing skin conditions such as atopic dermatitis, Darier disease, and Hailey-Hailey disease.5-7 The lesions are characterized by a monomorphic eruption of umbilicated vesicles on an erythematous base. Lesions can progress to punched-out ulcers and erosions with hemorrhagic crusts that coalesce, forming scalloped borders, similar to our patient’s presentation.8
Hailey-Hailey disease, a genodermatosis that alters calcium signaling with an autosomal-dominant inheritance pattern, was unlikely in our patient due to the presence of nail abnormalities and palmar pits that are characteristic of Darier disease. From a purely histopathologic standpoint, Grover disease was considered with skin biopsy demonstrating acantholytic dyskeratosis but was not compatible with the clinical context. Furthermore, trials of antibiotics with group A Streptococcus and Staphylococcus aureus coverage failed in our patient, and she lacked systemic symptoms that would be supportive of a cellulitis diagnosis. The punched-out lesions suggested that an isolated exacerbation of atopic dermatitis was not sufficient to explain all of the clinical findings.
Eczema herpeticum must be considered in the differential diagnosis for patients with underlying Darier disease and widespread ulcerations. Our patient had more recent punched-out ulcerations in the intertriginous regions, with other areas showing later stages of confluent ulcers with scalloped borders. Delayed diagnosis and treatment of eczema herpeticum combined with severe Darier disease can lead to increased risk for hospitalization and rarely fatality.8,9
Our patient was started on intravenous acyclovir until the lesions crusted and then was transitioned to a suppressive dose of oral valacyclovir given the widespread distribution. The Darier disease itself was managed with topical steroids and a zinc oxide barrier, serving as protectants to pathogens through microscopic breaks in the skin. Our patient also had a mild case of candidal intertrigo that was exacerbated by obesity and managed with topical ketoconazole. Gabapentin, hydromorphone, and acetaminophen were used for pain. She was discharged 10 days after admission with substantial improvement of both the HSV lesions and the irritation from her Darier disease. At follow-up visits 20 days later and again 6 months after discharge, she had been feeling well without any HSV flares.
The eczema herpeticum likely arose from our patient’s chronic skin barrier impairment attributed to Darier disease, leading to the cutaneous inoculation of HSV. Our patient and her family members had never been evaluated by a dermatologist until late in life during this hospitalization. Medication compliance with a suppressive dose of oral valacyclovir and topical steroids is vital to prevent flares of both eczema herpeticum and Darier disease, respectively. This case highlights the importance of dermatology consultation for complex cutaneous findings, as delayed diagnosis and treatment can lead to increased morbidity and mortality.
The Diagnosis: Darier Disease
A clinical diagnosis of Darier disease was made from the skin findings of pruritic, malodorous, keratotic papules in a seborrheic distribution and pathognomonic nail dystrophy, along with a family history that demonstrated autosomal-dominant inheritance. The ulcerations were suspected to be caused by a superimposed herpes simplex virus (HSV) infection in the form of eczema herpeticum. The clinical diagnosis was later confirmed via punch biopsy. Pathology results demonstrated focal acantholytic dyskeratosis, which was consistent with Darier disease given the focal nature and lack of acanthosis. The patient’s father and sister also were confirmed to have Darier disease by an outside dermatologist.
Darier disease is a rare keratinizing autosomaldominant genodermatosis that occurs due to a mutation in the ATP2A2 gene, which encodes a sarco/endoplasmic reticulum calcium ATPase pump that decreases cell adhesion between keratinocytes, leading to epidermal acantholysis and dyskeratosis and ultimately a disrupted skin barrier.1,2 Darier disease often presents in childhood and adolescence with papules in a seborrheic distribution on the central chest and back (Figure, A); the intertriginous folds also may be involved. Darier disease can manifest with palmoplantar pits (Figure, B), a cobblestonelike texture of the oral mucosa, acrokeratosis verruciformis of Hopf, and nail findings with alternating red and white longitudinal streaks in the nail bed resembling a candy cane along with characteristic V nicking deformities of the nails themselves (Figure, C). Chronic flares may occur throughout one’s lifetime, with patients experiencing more symptoms in the summer months due to heat, sweat, and UV light exposure, as well as infections that irritate the skin and worsen dyskeratosis. Studies have revealed an association between Darier disease and neuropsychiatric conditions, including major depressive disorder, schizophrenia, and bipolar disorder.3,4

The skin barrier is compromised in patients with Darier disease, thereby making secondary infection more likely to occur. Polymerase chain reaction swabs of our patient’s purulent ulcerations were positive for HSV type 1, further strengthening a diagnosis of secondary eczema herpeticum, which occurs when patients have widespread HSV superinfecting pre-existing skin conditions such as atopic dermatitis, Darier disease, and Hailey-Hailey disease.5-7 The lesions are characterized by a monomorphic eruption of umbilicated vesicles on an erythematous base. Lesions can progress to punched-out ulcers and erosions with hemorrhagic crusts that coalesce, forming scalloped borders, similar to our patient’s presentation.8
Hailey-Hailey disease, a genodermatosis that alters calcium signaling with an autosomal-dominant inheritance pattern, was unlikely in our patient due to the presence of nail abnormalities and palmar pits that are characteristic of Darier disease. From a purely histopathologic standpoint, Grover disease was considered with skin biopsy demonstrating acantholytic dyskeratosis but was not compatible with the clinical context. Furthermore, trials of antibiotics with group A Streptococcus and Staphylococcus aureus coverage failed in our patient, and she lacked systemic symptoms that would be supportive of a cellulitis diagnosis. The punched-out lesions suggested that an isolated exacerbation of atopic dermatitis was not sufficient to explain all of the clinical findings.
Eczema herpeticum must be considered in the differential diagnosis for patients with underlying Darier disease and widespread ulcerations. Our patient had more recent punched-out ulcerations in the intertriginous regions, with other areas showing later stages of confluent ulcers with scalloped borders. Delayed diagnosis and treatment of eczema herpeticum combined with severe Darier disease can lead to increased risk for hospitalization and rarely fatality.8,9
Our patient was started on intravenous acyclovir until the lesions crusted and then was transitioned to a suppressive dose of oral valacyclovir given the widespread distribution. The Darier disease itself was managed with topical steroids and a zinc oxide barrier, serving as protectants to pathogens through microscopic breaks in the skin. Our patient also had a mild case of candidal intertrigo that was exacerbated by obesity and managed with topical ketoconazole. Gabapentin, hydromorphone, and acetaminophen were used for pain. She was discharged 10 days after admission with substantial improvement of both the HSV lesions and the irritation from her Darier disease. At follow-up visits 20 days later and again 6 months after discharge, she had been feeling well without any HSV flares.
The eczema herpeticum likely arose from our patient’s chronic skin barrier impairment attributed to Darier disease, leading to the cutaneous inoculation of HSV. Our patient and her family members had never been evaluated by a dermatologist until late in life during this hospitalization. Medication compliance with a suppressive dose of oral valacyclovir and topical steroids is vital to prevent flares of both eczema herpeticum and Darier disease, respectively. This case highlights the importance of dermatology consultation for complex cutaneous findings, as delayed diagnosis and treatment can lead to increased morbidity and mortality.
- Cooper SM, Burge SM. Darier’s disease: epidemiology, pathophysiology, and management. Am J Clin Dermatol. 2003;4:97-105. doi:10.2165/00128071-200304020-00003
- Dhitavat J, Cobbold C, Leslie N, et al. Impaired trafficking of the desmoplakins in cultured Darier’s disease keratinocytes. J Invest Dermatol. 2003;121:1349-1355. doi:10.1046/j.1523-1747.2003.12557.x
- Nakamura T, Kazuno AA, Nakajima K, et al. Loss of function mutations in ATP2A2 and psychoses: a case report and literature survey. Psychiatry Clin Neurosci. 2016;70:342-350. doi:10.1111/pcn.12395
- Gordon-Smith K, Jones LA, Burge SM, et al. The neuropsychiatric phenotype in Darier disease. Br J Dermatol. 2010;163:515-522. doi:10.1111/j.1365-2133.2010.09834.x
- Hemani SA, Edmond MB, Jaggi P, et al. Frequency and clinical features associated with eczema herpeticum in hospitalized children with presumed atopic dermatitis skin infection. Pediatr Infect Dis J. 2020;39:263-266. doi:10.1097/INF.0000000000002542
- Tayabali K, Pothiwalla H, Lowitt M. Eczema herpeticum in Darier’s disease: a topical storm. J Community Hosp Intern Med Perspect. 2019;9:347-350. doi:10.1080/20009666.2019.1650590
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314. doi:10.5021/ad.2009.21.3.311
- Nikkels AF, Beauthier F, Quatresooz P, et al. Fatal herpes simplex virus infection in Darier disease under corticotherapy. Eur J Dermatol. 2005;15:293-297.
- Vogt KA, Lohse CM, El-Azhary RA, et al. Kaposi varicelliform eruption in patients with Darier disease: a 20-year retrospective study. J Am Acad Dermatol. 2015;72:481-484. doi:10.1016/j.jaad.2014.12.001
- Cooper SM, Burge SM. Darier’s disease: epidemiology, pathophysiology, and management. Am J Clin Dermatol. 2003;4:97-105. doi:10.2165/00128071-200304020-00003
- Dhitavat J, Cobbold C, Leslie N, et al. Impaired trafficking of the desmoplakins in cultured Darier’s disease keratinocytes. J Invest Dermatol. 2003;121:1349-1355. doi:10.1046/j.1523-1747.2003.12557.x
- Nakamura T, Kazuno AA, Nakajima K, et al. Loss of function mutations in ATP2A2 and psychoses: a case report and literature survey. Psychiatry Clin Neurosci. 2016;70:342-350. doi:10.1111/pcn.12395
- Gordon-Smith K, Jones LA, Burge SM, et al. The neuropsychiatric phenotype in Darier disease. Br J Dermatol. 2010;163:515-522. doi:10.1111/j.1365-2133.2010.09834.x
- Hemani SA, Edmond MB, Jaggi P, et al. Frequency and clinical features associated with eczema herpeticum in hospitalized children with presumed atopic dermatitis skin infection. Pediatr Infect Dis J. 2020;39:263-266. doi:10.1097/INF.0000000000002542
- Tayabali K, Pothiwalla H, Lowitt M. Eczema herpeticum in Darier’s disease: a topical storm. J Community Hosp Intern Med Perspect. 2019;9:347-350. doi:10.1080/20009666.2019.1650590
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314. doi:10.5021/ad.2009.21.3.311
- Nikkels AF, Beauthier F, Quatresooz P, et al. Fatal herpes simplex virus infection in Darier disease under corticotherapy. Eur J Dermatol. 2005;15:293-297.
- Vogt KA, Lohse CM, El-Azhary RA, et al. Kaposi varicelliform eruption in patients with Darier disease: a 20-year retrospective study. J Am Acad Dermatol. 2015;72:481-484. doi:10.1016/j.jaad.2014.12.001
A 72-year-old woman presented to the emergency department with painful, erythematic, pruritic, and purulent lesions in intertriginous regions including the inframammary, infra-abdominal, and inguinal folds with a burning sensation of 1 week’s duration. Her medical history was notable for obesity and major depressive disorder. She was empirically treated for cellulitis, but there was no improvement with cefazolin or clindamycin. Dermatology was consulted. Physical examination revealed gray-brown, slightly umbilicated papules in the inframammary region that were malodorous upon lifting the folds. Grouped, punched-out ulcerations with scalloped borders were superimposed onto these papules. Further examination revealed a macerated erythematous plaque in the infra-abdominal and inguinal regions with punched-out ulcers. Hemecrusted papules were observed in seborrheic areas including the anterior neck, hairline, and trunk. Few subtle keratotic pits were localized on the palms. She reported similar flares in the past but never saw a dermatologist and noted that her father and sister had similar papules in a seborrheic distribution. Nail abnormalities included red and white alternating subungual streaks with irregular texture including V nicking of the distal nails.