User login
The controversy over long-acting beta agonists: Examining the evidence
Bariatric surgery: Part of the answer to the obesity epidemic
Risks and benefits of bariatric surgery: Current evidence
Sunless Tanning: A Review
Protect yourself against patient assault
Wayne Fenton, MD, an associate director of the National Institute of Mental Health (NIMH), was murdered September 3—allegedly by a patient—in his Bethesda, MD, office. The case has led other mental health professionals to wonder how susceptible they are to assault and whether they are doing all they can to protect themselves.
To explore these safety issues, Current Psychiatry Deputy Editor Lois E. Krahn, MD, talked with John Battaglia, MD, medical director of the Program of Assertive Community Treatment (PACT) in Madison, WI.
Dr. Battaglia’s work takes him into the community to treat patients with severe chronic mental illnesses. The Madison PACT program uses an intensive, team-based approach for patients who have been inadequately treated in usual mental health services. Patients with complicated psychiatric, social, and legal problems are seen in their homes, at work, or on the streets in an assertive and comprehensive style of case management.
Dr. Krahn: Dr. Fenton’s death was a tremendous loss to the psychiatric community.
Dr. Battaglia: We were all shaken; my first reaction was horror and sadness.
Dr. Krahn: Dr. Fenton was a very experienced psychiatrist (Box 1). His murder makes us think about our own vulnerability and wonder if such an assault could happen to us.
Dr. Battaglia: Yes, it’s very common for psychiatrists or mental health providers to be assaulted (Box 2).
Dr. Fenton devoted his life to schizophrenia, through his compassion for those afflicted and his research that aided untold numbers of the mentally ill and their caregivers.
So it was especially sad that Dr. Fenton died while reaching out to a patient in need. On September 3, the NIMH associate director answered an urgent call to help a distressed, psychotic young man. A short time later, Dr. Fenton was found beaten to death at his Bethesda, MD, office.
Dr. Fenton was just 53 when he died, but his accomplishments were great. He joined NIMH in 1999, helping the organization find new treatments to enable schizophrenia patients to function in society. In this role, he galvanized colleagues nationwide to tackle the complex issue of difficult-to-treat schizophrenia. Before joining NIMH, Dr. Fenton was director and CEO of the Chestnut Lodge Hospital in Rockville, MD, where he did pivotal long-term studies of therapies for schizophrenia. From 2000 to 2005, he was deputy editor-in-chief of the journal Schizophrenia Bulletin. He served on numerous boards and in advocacy roles and won numerous awards.
In addition to these responsibilities, Dr. Fenton made time for his patients. And he gave his life, as he had lived it, trying to help. His obituary in the Washington Post included this quotation from Dr. Fenton, whom the newspaper interviewed in 2002:
All one has to do is walk through a downtown area to appreciate that the availability of adequate treatment for patients with schizophrenia and other mental illnesses is a serious problem for the country. We wouldn’t let our 80-year-old mother with Alzheimer’s live on a grate. Why is it all right for a 30-year-old daughter with schizophrenia?
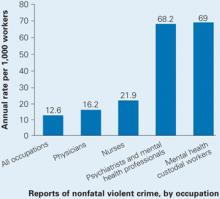
In one study, more than 50% of psychiatrists and 75% of mental health nurses reported experiencing an act or threat of violence within the past year.1
Dr. Krahn: Have you been assaulted by a patient?
Dr. Battaglia: Yes I have, and I think we need to define assault. A 15-year analysis of assaults on staff in a Massachusetts mental health system divided the acts into four types: physical, sexual, nonverbal threats/intimidation, and verbal assault.2 And you might think physical assault would be worse than verbal assaults. But a threat from a patient—especially one aimed toward your family—can leave you feeling vulnerable, stressed, and hypervigilant. Every sound at night makes you wonder if that person is coming after your family.
Dr. Krahn: What kinds of patients are associated with violence and assault?
Dr. Battaglia: The DSM-IV-TR diagnosis that comes up most often is schizophrenia, but it’s debatable whether diagnosis alone increases the risk of violence.
A study in Sweden published this year found a definite correlation between severe mental illness and violent crime. The authors concluded that about 5% of violent crimes in that country were committed by persons with severe mental illness.3
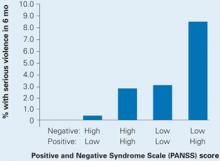
Also this year, a study of data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) found an increased risk of violence in schizophrenia patients with positive psychotic symptoms but a decreased risk in those with predominantly negative symptoms such as social withdrawal. Those with a combination of above-median positive and below-median negative symptoms were at highest risk for serious violence (Box 3).
Among a sample of 1,410 chronic schizophrenia patients enrolled in the NIMH-sponsored CATIE, 19% were involved in either minor or serious violent behavior in the past 6 months and 3.6% in serious violent behavior.4
Nobody argues that someone with schizophrenia is clearly at higher risk of becoming violent when in a high arousal state with positive symptoms or unpleasant delusions or hallucinations. A person with schizophrenia who is in an agitated, aroused psychotic state with active paranoid delusions and hallucinations is clearly at higher risk for committing violence.5,6 The patient who has been charged in the beating death of Dr. Fenton was a 19-year-old man with severe psychosis.
Dr. Krahn: Are there other disorders, such as bipolar mania, that are high risk for patient violence?
Dr. Battaglia: Acute manic states are higher risk.7 But, again, the diagnosis of bipolar disorder in and of itself does not show an increased incidence of violence. Personality disorders can be higher risk, as can nonspecific neurologic abnormalities, such as abnormal EEGs or neurologic “soft signs” by exam or testing.
Dr. Krahn: What about substance abuse?
Dr. Battaglia: The risk of violence is higher in patients who are under the influence of certain stimulants such as cocaine and methamphetamines, as opposed to marijuana or sedatives.8
Dr. Krahn: How can we predict whether a patient is at high risk for assault?
Dr. Battaglia: The best predictor is a history of violence, especially when the act was unprovoked or resulted in injury.9 A small number of patients is responsible for the majority of aggression. One study showed that recidivists committed 53% of all violent acts in a health care setting.10
Dr. Krahn: What if the patient’s history is unknown?
Dr. Battaglia: Most assaults in health care occur in high arousal states. Planned, methodical assaults are significantly less frequent. So, in the case of patients making threats against staff—let’s say you terminated your relationship with a patient and obtained a restraining order—very commonly that patient’s passion toward the clinic will wane over time.
Dr. Krahn: But not every arousal state results in assault.
Dr. Battaglia: Right. I have a colleague who says, “Risk factors make you worry more, and nothing makes you worry less.” That’s the attitude to have. Nothing should make you lower your antenna.
Source: U.S. Department of Justice, National Crime Victimization Survey, 1993 to 1999
Dr. Krahn: Is the risk higher with a new patient, or does it go down as you establish a relationship?
Dr. Battaglia: Clearly, untreated patients in high arousal states are a much greater risk. Does risk go down with somebody you’ve known for a while? I don’t know. My own experiences with assault have sometimes occurred with people I’ve grown to trust and when I let my guard down.
Dr. Krahn: So we might relax once we know the patient, but then we might be more vulnerable. Any clues that should put us on high alert?
Dr. Battaglia: The first clue—and this is going to sound obvious—is our internal, visceral, emotional sense of impending danger. In my experience, psychiatrists have a very good sense of that, but we override or don’t pay attention to it. Part of that inattention is an occupational hazard; we have to turn off our sense of danger again and again so that we can stay in situations that would repulse most people.
For instance, medical students with no psychiatric experience might sit in an interview with an agitated patient and feel an intense need to flee. Their antennae are telling them the situation looks dangerous. Seasoned psychiatrists, however, will calm themselves and stay through the interview. We are so used to being healers and helpers that we often turn off or dampen our sense of danger.
Dr. Krahn: Can you elaborate?
Dr. Battaglia: A nurse and I were with a patient who was highly agitated. He was labile; he was angry; he was spitting as he was speaking. In any other context, people would be keeping their distance because the signals were so powerful. Instead, the nurse leaned in, held his hand, and started telling him, “Come on now (Bob), you need to settle down. This is scaring us.”
That’s what I call the “leaning-in response.” We do that day in and day out. We turn off our danger signals in order to be therapeutic, and that makes us vulnerable.
Dr. Krahn: So, how do we keep our signals tuned?
Dr. Battaglia: When our senses are telling us we’re scared or we’re noticing a feeling of wanting to flee, we have to shift away from the goal of being therapeutic and focus on the goal of harm reduction. In assault cases, two clinician errors I see are:
- people had a sense that something was dangerous, but they ignored or dampened it
- people were passive when tension was mounting and didn’t abort an assault situation.
Anger is easy to recognize. Raised voice, inappropriate staring, clenched fists, agitation, and verbal threats are common before a violent episode. This seems self-evident, yet it’s surprising—even when these signs are obvious—that clinicians often took no de-escalation measures to ward off violence. A verbal threat is a red flag to prepare for violence.
Dr. Krahn: So, your senses are tingling. What do you do?
Dr. Battaglia: If the patient is threatening you and is in a negative affective arousal state that does not allow verbal redirection, you need to get away. Before you make your move, however, announce your behavior so that the patient will not interpret it as an attack (“Bob, I am standing up now because I need to leave the room”).
Schizophrenia symptoms associated with violent behavior
Schizophrenia patients with combined low negative and high positive PANSS scores were at highest risk to cause bodily injury or harm someone with a weapon in the past 6 months.
Dr. Krahn: Can that be a difficult call?
Dr. Battaglia: I think you learn when to shift gears. You undergo a number of incidents where you question yourself, and you go to an experienced colleague and say, “I was in a session with this patient. Here’s what I did. Do you think I was exposing myself unnecessarily?” Go over the incident in detail with someone who is supportive and understanding but also has a critical eye.
Dr. Krahn: Any suggestions as to how the room or other staff can be positioned to keep the risk as low as possible? Do you recommend alarms inside offices?
Dr. Battaglia: I think it’s smart to have an alarm system. And you need to think about the physical layout of the room ahead of time. You and the patient may need to have equal access to the door. If the patient is high-risk, you might want to arrange seating at a 90-degree angle rather than face-to-face to limit sustained confrontational eye contact. You might want to place your chair greater than an arm swing or leg kick away. You need to decide whether it’s safe to be alone, and whether to have the door open or to have security posted.
Dr. Krahn: What kind of training should staff be given?
Dr. Battaglia: Every office should have policies and protocols for handling behavioral emergencies. Who calls 911? What are each person’s responsibilities? Also, staff should be confident but not confrontational. That, in itself, may dissuade a patient from acting out.
Everyone should be taught de-escalation techniques. Body language can send threatening signals or they can signal a person that you’re not a threat and you’re going to work with them.
Dr. Krahn: Can you give an example where training might have helped?
Dr. Battaglia: I recently reviewed an incident where a nurse and a psychologist had a delusional, paranoid patient in their office and he wanted to leave. He was relapsed and clearly agitated; he was psychotic; he needed to be hospitalized. He wanted to escape, and they barred the door because they wanted to get him in the hospital.
The patient punched the nurse. If you bar someone’s escape, you’re very likely to get hurt. Let the patient go and call the police, who are trained to bring people in.
Dr. Krahn: What about building security? I know of a situation where a patient was found waiting for a psychiatrist in the parking garage. If there are threats, should an escort system be in place?
Dr. Battaglia: Security needs to work with the staff to come up with a plan.
Dr. Krahn: If someone in your office is assaulted, how do you handle the aftermath?
Dr. Battaglia: The person who is assaulted needs to get help. Crisis debriefing has been debated in trauma treatment, but there’s no debate about the benefit of “psychological first aid.” It provides an opportunity for the person to talk in confidence with another professional about what’s happened and how it may be affecting him or her.
Dr. Krahn: Can you continue to treat someone who has assaulted you?
Dr. Battaglia: That decision has to be made on a case-by-case basis. The main question is whether you feel safe enough to be therapeutic with the person in the future. Outside of a controlled setting, I don’t think you can effectively treat a patient you fear.
Dr. Krahn: Dr. Fenton’s death brings home that we need to be vigilant each day. We meet new patients every week, and any of them may have the disorders and risk factors that can lead to violence.
Dr. Battaglia: That’s true, yet being in a constant state of fear can impair mental health professionals’ ability to do our work. It’s a dynamic balance—we attempt a measured calmness in our work yet pay attention to external and visceral cues of impending danger.
Dr. Krahn: I think some psychiatrists feel patient violence occurs only in correctional settings or emergency rooms—not in their world. But Dr. Fenton’s death shows that it can happen anywhere. You just don’t know.
Related resources
- Joint Commission on Accreditation of HealthCare Organizations (JCAHO). Rules on application of seclusion and restraint. www.jointcommission.org.
Acknowledgment
This article was edited by Lynn Waltz, a medical writer and editor in Norfolk, VA, from the transcript of the September 29, 2006 interview of Dr. Battaglia by Dr. Krahn.
1. Nolan P, Dallender J, Soares J, et al. Violence in mental health care: the experiences of mental health nurses and psychiatrists. J Adv Nurs 1999;30:934-41.
2. Flannery RB, Jr, Juliano J, Cronin S, Walker AP. Characteristics of assaultive psychiatric patients: fifteen-year analysis of the Assaulted Staff Action Program (ASAP). Psychiatr Q 2006;77(3):239-49.
3. Fazel S, Grann M. The population impact of severe mental illness on violent crime. Am J Psychiatry 2006;163(8):1397-403.
4. Swanson JW, Swartz MS, Van Dorn RA, et al. A national study of violent behavior in persons with schizophrenia. Arch Gen Psychiatry 2006;63(5):490-9.
5. Cheung P, Schweitzer I, Crowley K, et al. Violence in schizophrenia: role of hallucinations and delusions. Schizophr Res 1997;26:181-90.
6. Binder R, McNiel D. Effects of diagnosis and context on dangerousness. Am J Psychiatry 1988;145:728-32.
7. Hyman S. The violent patient. In: Hyman S (ed). Manual of psychiatric emergencies. Boston: Little, Brown and Co, 1988;23-31.
8. Swartz M, Swanson J, Hiday V, et al. Violence and severe mental illness: the effects of substance abuse and nonadherence to medication. Am J Psychiatry 1998;155:226-31.
9. Convit A, Isay D, Otis D, et al. Characteristics of repeatedly assaultive psychiatric inpatients. Hosp Community Psychiatry 1990;41:1112-5.
10. Taylor P. Motives for offending among violent and psychotic men. Br J Psychiatry 1985;147:491-8.
Wayne Fenton, MD, an associate director of the National Institute of Mental Health (NIMH), was murdered September 3—allegedly by a patient—in his Bethesda, MD, office. The case has led other mental health professionals to wonder how susceptible they are to assault and whether they are doing all they can to protect themselves.
To explore these safety issues, Current Psychiatry Deputy Editor Lois E. Krahn, MD, talked with John Battaglia, MD, medical director of the Program of Assertive Community Treatment (PACT) in Madison, WI.
Dr. Battaglia’s work takes him into the community to treat patients with severe chronic mental illnesses. The Madison PACT program uses an intensive, team-based approach for patients who have been inadequately treated in usual mental health services. Patients with complicated psychiatric, social, and legal problems are seen in their homes, at work, or on the streets in an assertive and comprehensive style of case management.
Dr. Krahn: Dr. Fenton’s death was a tremendous loss to the psychiatric community.
Dr. Battaglia: We were all shaken; my first reaction was horror and sadness.
Dr. Krahn: Dr. Fenton was a very experienced psychiatrist (Box 1). His murder makes us think about our own vulnerability and wonder if such an assault could happen to us.
Dr. Battaglia: Yes, it’s very common for psychiatrists or mental health providers to be assaulted (Box 2).
Dr. Fenton devoted his life to schizophrenia, through his compassion for those afflicted and his research that aided untold numbers of the mentally ill and their caregivers.
So it was especially sad that Dr. Fenton died while reaching out to a patient in need. On September 3, the NIMH associate director answered an urgent call to help a distressed, psychotic young man. A short time later, Dr. Fenton was found beaten to death at his Bethesda, MD, office.
Dr. Fenton was just 53 when he died, but his accomplishments were great. He joined NIMH in 1999, helping the organization find new treatments to enable schizophrenia patients to function in society. In this role, he galvanized colleagues nationwide to tackle the complex issue of difficult-to-treat schizophrenia. Before joining NIMH, Dr. Fenton was director and CEO of the Chestnut Lodge Hospital in Rockville, MD, where he did pivotal long-term studies of therapies for schizophrenia. From 2000 to 2005, he was deputy editor-in-chief of the journal Schizophrenia Bulletin. He served on numerous boards and in advocacy roles and won numerous awards.
In addition to these responsibilities, Dr. Fenton made time for his patients. And he gave his life, as he had lived it, trying to help. His obituary in the Washington Post included this quotation from Dr. Fenton, whom the newspaper interviewed in 2002:
All one has to do is walk through a downtown area to appreciate that the availability of adequate treatment for patients with schizophrenia and other mental illnesses is a serious problem for the country. We wouldn’t let our 80-year-old mother with Alzheimer’s live on a grate. Why is it all right for a 30-year-old daughter with schizophrenia?
In one study, more than 50% of psychiatrists and 75% of mental health nurses reported experiencing an act or threat of violence within the past year.1
Dr. Krahn: Have you been assaulted by a patient?
Dr. Battaglia: Yes I have, and I think we need to define assault. A 15-year analysis of assaults on staff in a Massachusetts mental health system divided the acts into four types: physical, sexual, nonverbal threats/intimidation, and verbal assault.2 And you might think physical assault would be worse than verbal assaults. But a threat from a patient—especially one aimed toward your family—can leave you feeling vulnerable, stressed, and hypervigilant. Every sound at night makes you wonder if that person is coming after your family.
Dr. Krahn: What kinds of patients are associated with violence and assault?
Dr. Battaglia: The DSM-IV-TR diagnosis that comes up most often is schizophrenia, but it’s debatable whether diagnosis alone increases the risk of violence.
A study in Sweden published this year found a definite correlation between severe mental illness and violent crime. The authors concluded that about 5% of violent crimes in that country were committed by persons with severe mental illness.3
Also this year, a study of data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) found an increased risk of violence in schizophrenia patients with positive psychotic symptoms but a decreased risk in those with predominantly negative symptoms such as social withdrawal. Those with a combination of above-median positive and below-median negative symptoms were at highest risk for serious violence (Box 3).
Among a sample of 1,410 chronic schizophrenia patients enrolled in the NIMH-sponsored CATIE, 19% were involved in either minor or serious violent behavior in the past 6 months and 3.6% in serious violent behavior.4
Nobody argues that someone with schizophrenia is clearly at higher risk of becoming violent when in a high arousal state with positive symptoms or unpleasant delusions or hallucinations. A person with schizophrenia who is in an agitated, aroused psychotic state with active paranoid delusions and hallucinations is clearly at higher risk for committing violence.5,6 The patient who has been charged in the beating death of Dr. Fenton was a 19-year-old man with severe psychosis.
Dr. Krahn: Are there other disorders, such as bipolar mania, that are high risk for patient violence?
Dr. Battaglia: Acute manic states are higher risk.7 But, again, the diagnosis of bipolar disorder in and of itself does not show an increased incidence of violence. Personality disorders can be higher risk, as can nonspecific neurologic abnormalities, such as abnormal EEGs or neurologic “soft signs” by exam or testing.
Dr. Krahn: What about substance abuse?
Dr. Battaglia: The risk of violence is higher in patients who are under the influence of certain stimulants such as cocaine and methamphetamines, as opposed to marijuana or sedatives.8
Dr. Krahn: How can we predict whether a patient is at high risk for assault?
Dr. Battaglia: The best predictor is a history of violence, especially when the act was unprovoked or resulted in injury.9 A small number of patients is responsible for the majority of aggression. One study showed that recidivists committed 53% of all violent acts in a health care setting.10
Dr. Krahn: What if the patient’s history is unknown?
Dr. Battaglia: Most assaults in health care occur in high arousal states. Planned, methodical assaults are significantly less frequent. So, in the case of patients making threats against staff—let’s say you terminated your relationship with a patient and obtained a restraining order—very commonly that patient’s passion toward the clinic will wane over time.
Dr. Krahn: But not every arousal state results in assault.
Dr. Battaglia: Right. I have a colleague who says, “Risk factors make you worry more, and nothing makes you worry less.” That’s the attitude to have. Nothing should make you lower your antenna.
Source: U.S. Department of Justice, National Crime Victimization Survey, 1993 to 1999
Dr. Krahn: Is the risk higher with a new patient, or does it go down as you establish a relationship?
Dr. Battaglia: Clearly, untreated patients in high arousal states are a much greater risk. Does risk go down with somebody you’ve known for a while? I don’t know. My own experiences with assault have sometimes occurred with people I’ve grown to trust and when I let my guard down.
Dr. Krahn: So we might relax once we know the patient, but then we might be more vulnerable. Any clues that should put us on high alert?
Dr. Battaglia: The first clue—and this is going to sound obvious—is our internal, visceral, emotional sense of impending danger. In my experience, psychiatrists have a very good sense of that, but we override or don’t pay attention to it. Part of that inattention is an occupational hazard; we have to turn off our sense of danger again and again so that we can stay in situations that would repulse most people.
For instance, medical students with no psychiatric experience might sit in an interview with an agitated patient and feel an intense need to flee. Their antennae are telling them the situation looks dangerous. Seasoned psychiatrists, however, will calm themselves and stay through the interview. We are so used to being healers and helpers that we often turn off or dampen our sense of danger.
Dr. Krahn: Can you elaborate?
Dr. Battaglia: A nurse and I were with a patient who was highly agitated. He was labile; he was angry; he was spitting as he was speaking. In any other context, people would be keeping their distance because the signals were so powerful. Instead, the nurse leaned in, held his hand, and started telling him, “Come on now (Bob), you need to settle down. This is scaring us.”
That’s what I call the “leaning-in response.” We do that day in and day out. We turn off our danger signals in order to be therapeutic, and that makes us vulnerable.
Dr. Krahn: So, how do we keep our signals tuned?
Dr. Battaglia: When our senses are telling us we’re scared or we’re noticing a feeling of wanting to flee, we have to shift away from the goal of being therapeutic and focus on the goal of harm reduction. In assault cases, two clinician errors I see are:
- people had a sense that something was dangerous, but they ignored or dampened it
- people were passive when tension was mounting and didn’t abort an assault situation.
Anger is easy to recognize. Raised voice, inappropriate staring, clenched fists, agitation, and verbal threats are common before a violent episode. This seems self-evident, yet it’s surprising—even when these signs are obvious—that clinicians often took no de-escalation measures to ward off violence. A verbal threat is a red flag to prepare for violence.
Dr. Krahn: So, your senses are tingling. What do you do?
Dr. Battaglia: If the patient is threatening you and is in a negative affective arousal state that does not allow verbal redirection, you need to get away. Before you make your move, however, announce your behavior so that the patient will not interpret it as an attack (“Bob, I am standing up now because I need to leave the room”).
Schizophrenia symptoms associated with violent behavior
Schizophrenia patients with combined low negative and high positive PANSS scores were at highest risk to cause bodily injury or harm someone with a weapon in the past 6 months.
Dr. Krahn: Can that be a difficult call?
Dr. Battaglia: I think you learn when to shift gears. You undergo a number of incidents where you question yourself, and you go to an experienced colleague and say, “I was in a session with this patient. Here’s what I did. Do you think I was exposing myself unnecessarily?” Go over the incident in detail with someone who is supportive and understanding but also has a critical eye.
Dr. Krahn: Any suggestions as to how the room or other staff can be positioned to keep the risk as low as possible? Do you recommend alarms inside offices?
Dr. Battaglia: I think it’s smart to have an alarm system. And you need to think about the physical layout of the room ahead of time. You and the patient may need to have equal access to the door. If the patient is high-risk, you might want to arrange seating at a 90-degree angle rather than face-to-face to limit sustained confrontational eye contact. You might want to place your chair greater than an arm swing or leg kick away. You need to decide whether it’s safe to be alone, and whether to have the door open or to have security posted.
Dr. Krahn: What kind of training should staff be given?
Dr. Battaglia: Every office should have policies and protocols for handling behavioral emergencies. Who calls 911? What are each person’s responsibilities? Also, staff should be confident but not confrontational. That, in itself, may dissuade a patient from acting out.
Everyone should be taught de-escalation techniques. Body language can send threatening signals or they can signal a person that you’re not a threat and you’re going to work with them.
Dr. Krahn: Can you give an example where training might have helped?
Dr. Battaglia: I recently reviewed an incident where a nurse and a psychologist had a delusional, paranoid patient in their office and he wanted to leave. He was relapsed and clearly agitated; he was psychotic; he needed to be hospitalized. He wanted to escape, and they barred the door because they wanted to get him in the hospital.
The patient punched the nurse. If you bar someone’s escape, you’re very likely to get hurt. Let the patient go and call the police, who are trained to bring people in.
Dr. Krahn: What about building security? I know of a situation where a patient was found waiting for a psychiatrist in the parking garage. If there are threats, should an escort system be in place?
Dr. Battaglia: Security needs to work with the staff to come up with a plan.
Dr. Krahn: If someone in your office is assaulted, how do you handle the aftermath?
Dr. Battaglia: The person who is assaulted needs to get help. Crisis debriefing has been debated in trauma treatment, but there’s no debate about the benefit of “psychological first aid.” It provides an opportunity for the person to talk in confidence with another professional about what’s happened and how it may be affecting him or her.
Dr. Krahn: Can you continue to treat someone who has assaulted you?
Dr. Battaglia: That decision has to be made on a case-by-case basis. The main question is whether you feel safe enough to be therapeutic with the person in the future. Outside of a controlled setting, I don’t think you can effectively treat a patient you fear.
Dr. Krahn: Dr. Fenton’s death brings home that we need to be vigilant each day. We meet new patients every week, and any of them may have the disorders and risk factors that can lead to violence.
Dr. Battaglia: That’s true, yet being in a constant state of fear can impair mental health professionals’ ability to do our work. It’s a dynamic balance—we attempt a measured calmness in our work yet pay attention to external and visceral cues of impending danger.
Dr. Krahn: I think some psychiatrists feel patient violence occurs only in correctional settings or emergency rooms—not in their world. But Dr. Fenton’s death shows that it can happen anywhere. You just don’t know.
Related resources
- Joint Commission on Accreditation of HealthCare Organizations (JCAHO). Rules on application of seclusion and restraint. www.jointcommission.org.
Acknowledgment
This article was edited by Lynn Waltz, a medical writer and editor in Norfolk, VA, from the transcript of the September 29, 2006 interview of Dr. Battaglia by Dr. Krahn.
Wayne Fenton, MD, an associate director of the National Institute of Mental Health (NIMH), was murdered September 3—allegedly by a patient—in his Bethesda, MD, office. The case has led other mental health professionals to wonder how susceptible they are to assault and whether they are doing all they can to protect themselves.
To explore these safety issues, Current Psychiatry Deputy Editor Lois E. Krahn, MD, talked with John Battaglia, MD, medical director of the Program of Assertive Community Treatment (PACT) in Madison, WI.
Dr. Battaglia’s work takes him into the community to treat patients with severe chronic mental illnesses. The Madison PACT program uses an intensive, team-based approach for patients who have been inadequately treated in usual mental health services. Patients with complicated psychiatric, social, and legal problems are seen in their homes, at work, or on the streets in an assertive and comprehensive style of case management.
Dr. Krahn: Dr. Fenton’s death was a tremendous loss to the psychiatric community.
Dr. Battaglia: We were all shaken; my first reaction was horror and sadness.
Dr. Krahn: Dr. Fenton was a very experienced psychiatrist (Box 1). His murder makes us think about our own vulnerability and wonder if such an assault could happen to us.
Dr. Battaglia: Yes, it’s very common for psychiatrists or mental health providers to be assaulted (Box 2).
Dr. Fenton devoted his life to schizophrenia, through his compassion for those afflicted and his research that aided untold numbers of the mentally ill and their caregivers.
So it was especially sad that Dr. Fenton died while reaching out to a patient in need. On September 3, the NIMH associate director answered an urgent call to help a distressed, psychotic young man. A short time later, Dr. Fenton was found beaten to death at his Bethesda, MD, office.
Dr. Fenton was just 53 when he died, but his accomplishments were great. He joined NIMH in 1999, helping the organization find new treatments to enable schizophrenia patients to function in society. In this role, he galvanized colleagues nationwide to tackle the complex issue of difficult-to-treat schizophrenia. Before joining NIMH, Dr. Fenton was director and CEO of the Chestnut Lodge Hospital in Rockville, MD, where he did pivotal long-term studies of therapies for schizophrenia. From 2000 to 2005, he was deputy editor-in-chief of the journal Schizophrenia Bulletin. He served on numerous boards and in advocacy roles and won numerous awards.
In addition to these responsibilities, Dr. Fenton made time for his patients. And he gave his life, as he had lived it, trying to help. His obituary in the Washington Post included this quotation from Dr. Fenton, whom the newspaper interviewed in 2002:
All one has to do is walk through a downtown area to appreciate that the availability of adequate treatment for patients with schizophrenia and other mental illnesses is a serious problem for the country. We wouldn’t let our 80-year-old mother with Alzheimer’s live on a grate. Why is it all right for a 30-year-old daughter with schizophrenia?
In one study, more than 50% of psychiatrists and 75% of mental health nurses reported experiencing an act or threat of violence within the past year.1
Dr. Krahn: Have you been assaulted by a patient?
Dr. Battaglia: Yes I have, and I think we need to define assault. A 15-year analysis of assaults on staff in a Massachusetts mental health system divided the acts into four types: physical, sexual, nonverbal threats/intimidation, and verbal assault.2 And you might think physical assault would be worse than verbal assaults. But a threat from a patient—especially one aimed toward your family—can leave you feeling vulnerable, stressed, and hypervigilant. Every sound at night makes you wonder if that person is coming after your family.
Dr. Krahn: What kinds of patients are associated with violence and assault?
Dr. Battaglia: The DSM-IV-TR diagnosis that comes up most often is schizophrenia, but it’s debatable whether diagnosis alone increases the risk of violence.
A study in Sweden published this year found a definite correlation between severe mental illness and violent crime. The authors concluded that about 5% of violent crimes in that country were committed by persons with severe mental illness.3
Also this year, a study of data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) found an increased risk of violence in schizophrenia patients with positive psychotic symptoms but a decreased risk in those with predominantly negative symptoms such as social withdrawal. Those with a combination of above-median positive and below-median negative symptoms were at highest risk for serious violence (Box 3).
Among a sample of 1,410 chronic schizophrenia patients enrolled in the NIMH-sponsored CATIE, 19% were involved in either minor or serious violent behavior in the past 6 months and 3.6% in serious violent behavior.4
Nobody argues that someone with schizophrenia is clearly at higher risk of becoming violent when in a high arousal state with positive symptoms or unpleasant delusions or hallucinations. A person with schizophrenia who is in an agitated, aroused psychotic state with active paranoid delusions and hallucinations is clearly at higher risk for committing violence.5,6 The patient who has been charged in the beating death of Dr. Fenton was a 19-year-old man with severe psychosis.
Dr. Krahn: Are there other disorders, such as bipolar mania, that are high risk for patient violence?
Dr. Battaglia: Acute manic states are higher risk.7 But, again, the diagnosis of bipolar disorder in and of itself does not show an increased incidence of violence. Personality disorders can be higher risk, as can nonspecific neurologic abnormalities, such as abnormal EEGs or neurologic “soft signs” by exam or testing.
Dr. Krahn: What about substance abuse?
Dr. Battaglia: The risk of violence is higher in patients who are under the influence of certain stimulants such as cocaine and methamphetamines, as opposed to marijuana or sedatives.8
Dr. Krahn: How can we predict whether a patient is at high risk for assault?
Dr. Battaglia: The best predictor is a history of violence, especially when the act was unprovoked or resulted in injury.9 A small number of patients is responsible for the majority of aggression. One study showed that recidivists committed 53% of all violent acts in a health care setting.10
Dr. Krahn: What if the patient’s history is unknown?
Dr. Battaglia: Most assaults in health care occur in high arousal states. Planned, methodical assaults are significantly less frequent. So, in the case of patients making threats against staff—let’s say you terminated your relationship with a patient and obtained a restraining order—very commonly that patient’s passion toward the clinic will wane over time.
Dr. Krahn: But not every arousal state results in assault.
Dr. Battaglia: Right. I have a colleague who says, “Risk factors make you worry more, and nothing makes you worry less.” That’s the attitude to have. Nothing should make you lower your antenna.
Source: U.S. Department of Justice, National Crime Victimization Survey, 1993 to 1999
Dr. Krahn: Is the risk higher with a new patient, or does it go down as you establish a relationship?
Dr. Battaglia: Clearly, untreated patients in high arousal states are a much greater risk. Does risk go down with somebody you’ve known for a while? I don’t know. My own experiences with assault have sometimes occurred with people I’ve grown to trust and when I let my guard down.
Dr. Krahn: So we might relax once we know the patient, but then we might be more vulnerable. Any clues that should put us on high alert?
Dr. Battaglia: The first clue—and this is going to sound obvious—is our internal, visceral, emotional sense of impending danger. In my experience, psychiatrists have a very good sense of that, but we override or don’t pay attention to it. Part of that inattention is an occupational hazard; we have to turn off our sense of danger again and again so that we can stay in situations that would repulse most people.
For instance, medical students with no psychiatric experience might sit in an interview with an agitated patient and feel an intense need to flee. Their antennae are telling them the situation looks dangerous. Seasoned psychiatrists, however, will calm themselves and stay through the interview. We are so used to being healers and helpers that we often turn off or dampen our sense of danger.
Dr. Krahn: Can you elaborate?
Dr. Battaglia: A nurse and I were with a patient who was highly agitated. He was labile; he was angry; he was spitting as he was speaking. In any other context, people would be keeping their distance because the signals were so powerful. Instead, the nurse leaned in, held his hand, and started telling him, “Come on now (Bob), you need to settle down. This is scaring us.”
That’s what I call the “leaning-in response.” We do that day in and day out. We turn off our danger signals in order to be therapeutic, and that makes us vulnerable.
Dr. Krahn: So, how do we keep our signals tuned?
Dr. Battaglia: When our senses are telling us we’re scared or we’re noticing a feeling of wanting to flee, we have to shift away from the goal of being therapeutic and focus on the goal of harm reduction. In assault cases, two clinician errors I see are:
- people had a sense that something was dangerous, but they ignored or dampened it
- people were passive when tension was mounting and didn’t abort an assault situation.
Anger is easy to recognize. Raised voice, inappropriate staring, clenched fists, agitation, and verbal threats are common before a violent episode. This seems self-evident, yet it’s surprising—even when these signs are obvious—that clinicians often took no de-escalation measures to ward off violence. A verbal threat is a red flag to prepare for violence.
Dr. Krahn: So, your senses are tingling. What do you do?
Dr. Battaglia: If the patient is threatening you and is in a negative affective arousal state that does not allow verbal redirection, you need to get away. Before you make your move, however, announce your behavior so that the patient will not interpret it as an attack (“Bob, I am standing up now because I need to leave the room”).
Schizophrenia symptoms associated with violent behavior
Schizophrenia patients with combined low negative and high positive PANSS scores were at highest risk to cause bodily injury or harm someone with a weapon in the past 6 months.
Dr. Krahn: Can that be a difficult call?
Dr. Battaglia: I think you learn when to shift gears. You undergo a number of incidents where you question yourself, and you go to an experienced colleague and say, “I was in a session with this patient. Here’s what I did. Do you think I was exposing myself unnecessarily?” Go over the incident in detail with someone who is supportive and understanding but also has a critical eye.
Dr. Krahn: Any suggestions as to how the room or other staff can be positioned to keep the risk as low as possible? Do you recommend alarms inside offices?
Dr. Battaglia: I think it’s smart to have an alarm system. And you need to think about the physical layout of the room ahead of time. You and the patient may need to have equal access to the door. If the patient is high-risk, you might want to arrange seating at a 90-degree angle rather than face-to-face to limit sustained confrontational eye contact. You might want to place your chair greater than an arm swing or leg kick away. You need to decide whether it’s safe to be alone, and whether to have the door open or to have security posted.
Dr. Krahn: What kind of training should staff be given?
Dr. Battaglia: Every office should have policies and protocols for handling behavioral emergencies. Who calls 911? What are each person’s responsibilities? Also, staff should be confident but not confrontational. That, in itself, may dissuade a patient from acting out.
Everyone should be taught de-escalation techniques. Body language can send threatening signals or they can signal a person that you’re not a threat and you’re going to work with them.
Dr. Krahn: Can you give an example where training might have helped?
Dr. Battaglia: I recently reviewed an incident where a nurse and a psychologist had a delusional, paranoid patient in their office and he wanted to leave. He was relapsed and clearly agitated; he was psychotic; he needed to be hospitalized. He wanted to escape, and they barred the door because they wanted to get him in the hospital.
The patient punched the nurse. If you bar someone’s escape, you’re very likely to get hurt. Let the patient go and call the police, who are trained to bring people in.
Dr. Krahn: What about building security? I know of a situation where a patient was found waiting for a psychiatrist in the parking garage. If there are threats, should an escort system be in place?
Dr. Battaglia: Security needs to work with the staff to come up with a plan.
Dr. Krahn: If someone in your office is assaulted, how do you handle the aftermath?
Dr. Battaglia: The person who is assaulted needs to get help. Crisis debriefing has been debated in trauma treatment, but there’s no debate about the benefit of “psychological first aid.” It provides an opportunity for the person to talk in confidence with another professional about what’s happened and how it may be affecting him or her.
Dr. Krahn: Can you continue to treat someone who has assaulted you?
Dr. Battaglia: That decision has to be made on a case-by-case basis. The main question is whether you feel safe enough to be therapeutic with the person in the future. Outside of a controlled setting, I don’t think you can effectively treat a patient you fear.
Dr. Krahn: Dr. Fenton’s death brings home that we need to be vigilant each day. We meet new patients every week, and any of them may have the disorders and risk factors that can lead to violence.
Dr. Battaglia: That’s true, yet being in a constant state of fear can impair mental health professionals’ ability to do our work. It’s a dynamic balance—we attempt a measured calmness in our work yet pay attention to external and visceral cues of impending danger.
Dr. Krahn: I think some psychiatrists feel patient violence occurs only in correctional settings or emergency rooms—not in their world. But Dr. Fenton’s death shows that it can happen anywhere. You just don’t know.
Related resources
- Joint Commission on Accreditation of HealthCare Organizations (JCAHO). Rules on application of seclusion and restraint. www.jointcommission.org.
Acknowledgment
This article was edited by Lynn Waltz, a medical writer and editor in Norfolk, VA, from the transcript of the September 29, 2006 interview of Dr. Battaglia by Dr. Krahn.
1. Nolan P, Dallender J, Soares J, et al. Violence in mental health care: the experiences of mental health nurses and psychiatrists. J Adv Nurs 1999;30:934-41.
2. Flannery RB, Jr, Juliano J, Cronin S, Walker AP. Characteristics of assaultive psychiatric patients: fifteen-year analysis of the Assaulted Staff Action Program (ASAP). Psychiatr Q 2006;77(3):239-49.
3. Fazel S, Grann M. The population impact of severe mental illness on violent crime. Am J Psychiatry 2006;163(8):1397-403.
4. Swanson JW, Swartz MS, Van Dorn RA, et al. A national study of violent behavior in persons with schizophrenia. Arch Gen Psychiatry 2006;63(5):490-9.
5. Cheung P, Schweitzer I, Crowley K, et al. Violence in schizophrenia: role of hallucinations and delusions. Schizophr Res 1997;26:181-90.
6. Binder R, McNiel D. Effects of diagnosis and context on dangerousness. Am J Psychiatry 1988;145:728-32.
7. Hyman S. The violent patient. In: Hyman S (ed). Manual of psychiatric emergencies. Boston: Little, Brown and Co, 1988;23-31.
8. Swartz M, Swanson J, Hiday V, et al. Violence and severe mental illness: the effects of substance abuse and nonadherence to medication. Am J Psychiatry 1998;155:226-31.
9. Convit A, Isay D, Otis D, et al. Characteristics of repeatedly assaultive psychiatric inpatients. Hosp Community Psychiatry 1990;41:1112-5.
10. Taylor P. Motives for offending among violent and psychotic men. Br J Psychiatry 1985;147:491-8.
1. Nolan P, Dallender J, Soares J, et al. Violence in mental health care: the experiences of mental health nurses and psychiatrists. J Adv Nurs 1999;30:934-41.
2. Flannery RB, Jr, Juliano J, Cronin S, Walker AP. Characteristics of assaultive psychiatric patients: fifteen-year analysis of the Assaulted Staff Action Program (ASAP). Psychiatr Q 2006;77(3):239-49.
3. Fazel S, Grann M. The population impact of severe mental illness on violent crime. Am J Psychiatry 2006;163(8):1397-403.
4. Swanson JW, Swartz MS, Van Dorn RA, et al. A national study of violent behavior in persons with schizophrenia. Arch Gen Psychiatry 2006;63(5):490-9.
5. Cheung P, Schweitzer I, Crowley K, et al. Violence in schizophrenia: role of hallucinations and delusions. Schizophr Res 1997;26:181-90.
6. Binder R, McNiel D. Effects of diagnosis and context on dangerousness. Am J Psychiatry 1988;145:728-32.
7. Hyman S. The violent patient. In: Hyman S (ed). Manual of psychiatric emergencies. Boston: Little, Brown and Co, 1988;23-31.
8. Swartz M, Swanson J, Hiday V, et al. Violence and severe mental illness: the effects of substance abuse and nonadherence to medication. Am J Psychiatry 1998;155:226-31.
9. Convit A, Isay D, Otis D, et al. Characteristics of repeatedly assaultive psychiatric inpatients. Hosp Community Psychiatry 1990;41:1112-5.
10. Taylor P. Motives for offending among violent and psychotic men. Br J Psychiatry 1985;147:491-8.
2 procedures in 10 days will trigger bundling
If the physician is taking the patient to surgery to do only the keloid excision, you have several codes to select from, depending on the type of closure. The excision of the keloid scar would be reported using 11400–11406 (Excision, benign lesion including margins, except skin tag [unless listed elsewhere], trunk, arms or legs), where the code selected depends on the documented size of the scar removed.
If it is simple closure, no additional code is reported, but if the closure is either intermediate or complex, you will add a code from the repair section (12031–12037 or 13100–13102). But again the size in centimeters must be documented in order to use these codes.
Also remember that if the surgeon performs the cesarean within 10 days of the keloid excision, he/she will be in the global period for these codes and might have to use a modifier -79 (Unrelated procedure or service by the same physician during the postoperative period) on the global OB code you report. If the keloid is excised at the time of the cesarean, it will be included by most payers as part of establishing the operative site and incision closure.
If the physician is taking the patient to surgery to do only the keloid excision, you have several codes to select from, depending on the type of closure. The excision of the keloid scar would be reported using 11400–11406 (Excision, benign lesion including margins, except skin tag [unless listed elsewhere], trunk, arms or legs), where the code selected depends on the documented size of the scar removed.
If it is simple closure, no additional code is reported, but if the closure is either intermediate or complex, you will add a code from the repair section (12031–12037 or 13100–13102). But again the size in centimeters must be documented in order to use these codes.
Also remember that if the surgeon performs the cesarean within 10 days of the keloid excision, he/she will be in the global period for these codes and might have to use a modifier -79 (Unrelated procedure or service by the same physician during the postoperative period) on the global OB code you report. If the keloid is excised at the time of the cesarean, it will be included by most payers as part of establishing the operative site and incision closure.
If the physician is taking the patient to surgery to do only the keloid excision, you have several codes to select from, depending on the type of closure. The excision of the keloid scar would be reported using 11400–11406 (Excision, benign lesion including margins, except skin tag [unless listed elsewhere], trunk, arms or legs), where the code selected depends on the documented size of the scar removed.
If it is simple closure, no additional code is reported, but if the closure is either intermediate or complex, you will add a code from the repair section (12031–12037 or 13100–13102). But again the size in centimeters must be documented in order to use these codes.
Also remember that if the surgeon performs the cesarean within 10 days of the keloid excision, he/she will be in the global period for these codes and might have to use a modifier -79 (Unrelated procedure or service by the same physician during the postoperative period) on the global OB code you report. If the keloid is excised at the time of the cesarean, it will be included by most payers as part of establishing the operative site and incision closure.
Modifier needed to bill for anesthesia
If you did perform the version procedure as well as providing the anesthesia to the patient, you would need to indicate this by adding a modifier -47 (Anesthesia by surgeon) to code 59412 (External cephalic version, with or without tocolysis). You would then report a 2nd code for the type of regional anesthesia you administered. For instance, if you used epidural anesthesia, you would report 59412-47, 62311 (Injection, single [not via indwelling catheter], not including neurolytic substances, with or without contrast [for either localization or epidurography], of diagnostic or therapeutic substance[s] [including anesthetic, antispasmodic, opioid, steroid, other solution], epidural or subarachnoid; lumbar, sacral [caudal]).
If you were only providing the anesthesia, then code 01958 is correct, but now the payer is indicating a mismatch between the CPT code and the diagnosis code.
You have indicated that you used code 652.2 (Breech presentation without mention of version). But as you are billing for anesthesia for a version, this code would no longer be correct. In this case, the more correct code would be 652.13 (Breech or other malpresentation successfully converted to cephalic presentation; antepartum condition or complication) if the version was successful or 652.03 (Unstable lie; antepartum condition or complication) if it was not.
If you did perform the version procedure as well as providing the anesthesia to the patient, you would need to indicate this by adding a modifier -47 (Anesthesia by surgeon) to code 59412 (External cephalic version, with or without tocolysis). You would then report a 2nd code for the type of regional anesthesia you administered. For instance, if you used epidural anesthesia, you would report 59412-47, 62311 (Injection, single [not via indwelling catheter], not including neurolytic substances, with or without contrast [for either localization or epidurography], of diagnostic or therapeutic substance[s] [including anesthetic, antispasmodic, opioid, steroid, other solution], epidural or subarachnoid; lumbar, sacral [caudal]).
If you were only providing the anesthesia, then code 01958 is correct, but now the payer is indicating a mismatch between the CPT code and the diagnosis code.
You have indicated that you used code 652.2 (Breech presentation without mention of version). But as you are billing for anesthesia for a version, this code would no longer be correct. In this case, the more correct code would be 652.13 (Breech or other malpresentation successfully converted to cephalic presentation; antepartum condition or complication) if the version was successful or 652.03 (Unstable lie; antepartum condition or complication) if it was not.
If you did perform the version procedure as well as providing the anesthesia to the patient, you would need to indicate this by adding a modifier -47 (Anesthesia by surgeon) to code 59412 (External cephalic version, with or without tocolysis). You would then report a 2nd code for the type of regional anesthesia you administered. For instance, if you used epidural anesthesia, you would report 59412-47, 62311 (Injection, single [not via indwelling catheter], not including neurolytic substances, with or without contrast [for either localization or epidurography], of diagnostic or therapeutic substance[s] [including anesthetic, antispasmodic, opioid, steroid, other solution], epidural or subarachnoid; lumbar, sacral [caudal]).
If you were only providing the anesthesia, then code 01958 is correct, but now the payer is indicating a mismatch between the CPT code and the diagnosis code.
You have indicated that you used code 652.2 (Breech presentation without mention of version). But as you are billing for anesthesia for a version, this code would no longer be correct. In this case, the more correct code would be 652.13 (Breech or other malpresentation successfully converted to cephalic presentation; antepartum condition or complication) if the version was successful or 652.03 (Unstable lie; antepartum condition or complication) if it was not.
Few payers deny unlisted procedures
2 options
This leaves you with 2 coding options. Because the cervix is part of the uterus, the code 58578 (Unlisted laparoscopy procedure, uterus) would be appropriate. If you choose this option, you would report 58661, 58578-51. Alternatively, you could add a modifier -22 (Unusual procedural services) to code 58661. Whichever option you choose, you will need to send documentation with the claim to explain the unlisted procedure or the additional work.
I prefer the first option because it will give you the opportunity to set your fee to account for the actual work performed.
Most payers will not deny unlisted procedures so long as they are not considered investigational or experimental, a concept that should not apply to this surgery.
2 options
This leaves you with 2 coding options. Because the cervix is part of the uterus, the code 58578 (Unlisted laparoscopy procedure, uterus) would be appropriate. If you choose this option, you would report 58661, 58578-51. Alternatively, you could add a modifier -22 (Unusual procedural services) to code 58661. Whichever option you choose, you will need to send documentation with the claim to explain the unlisted procedure or the additional work.
I prefer the first option because it will give you the opportunity to set your fee to account for the actual work performed.
Most payers will not deny unlisted procedures so long as they are not considered investigational or experimental, a concept that should not apply to this surgery.
2 options
This leaves you with 2 coding options. Because the cervix is part of the uterus, the code 58578 (Unlisted laparoscopy procedure, uterus) would be appropriate. If you choose this option, you would report 58661, 58578-51. Alternatively, you could add a modifier -22 (Unusual procedural services) to code 58661. Whichever option you choose, you will need to send documentation with the claim to explain the unlisted procedure or the additional work.
I prefer the first option because it will give you the opportunity to set your fee to account for the actual work performed.
Most payers will not deny unlisted procedures so long as they are not considered investigational or experimental, a concept that should not apply to this surgery.
Use of “complication” triggers Medicare denial
I assume that you appropriately used the ICD-9-CM code 998.2 (Accidental puncture or laceration during a procedure) when billing for the suture of the bladder (51860, Cystorrhaphy, suture of bladder wound, injury or rupture; simple or 51865,.......; complicated).
Although neither of these codes is bundled with the sling procedure (57288, Sling operation for stress incontinence [eg, fascia or synthetic]), the general rules for NCCI state: “When a complication described by codes defining complications arises during an operative session, a separate service for treating the complication is not to be reported.” The use of the complication diagnosis would trigger the denial.
In addition, you apparently billed code 52000 (Cystourethroscopy [separate procedure]), and this code is bundled into code 57288 with a “0” indicator, which means that the edit cannot be bypassed using any modifier.
The good news
These rules would only apply to Medicare or to payers who use Medicare rules. Although you may find that 52000 may be a common bundle by many payers, you will not usually find commercial insurance denying the repair of the complication during surgery.
I assume that you appropriately used the ICD-9-CM code 998.2 (Accidental puncture or laceration during a procedure) when billing for the suture of the bladder (51860, Cystorrhaphy, suture of bladder wound, injury or rupture; simple or 51865,.......; complicated).
Although neither of these codes is bundled with the sling procedure (57288, Sling operation for stress incontinence [eg, fascia or synthetic]), the general rules for NCCI state: “When a complication described by codes defining complications arises during an operative session, a separate service for treating the complication is not to be reported.” The use of the complication diagnosis would trigger the denial.
In addition, you apparently billed code 52000 (Cystourethroscopy [separate procedure]), and this code is bundled into code 57288 with a “0” indicator, which means that the edit cannot be bypassed using any modifier.
The good news
These rules would only apply to Medicare or to payers who use Medicare rules. Although you may find that 52000 may be a common bundle by many payers, you will not usually find commercial insurance denying the repair of the complication during surgery.
I assume that you appropriately used the ICD-9-CM code 998.2 (Accidental puncture or laceration during a procedure) when billing for the suture of the bladder (51860, Cystorrhaphy, suture of bladder wound, injury or rupture; simple or 51865,.......; complicated).
Although neither of these codes is bundled with the sling procedure (57288, Sling operation for stress incontinence [eg, fascia or synthetic]), the general rules for NCCI state: “When a complication described by codes defining complications arises during an operative session, a separate service for treating the complication is not to be reported.” The use of the complication diagnosis would trigger the denial.
In addition, you apparently billed code 52000 (Cystourethroscopy [separate procedure]), and this code is bundled into code 57288 with a “0” indicator, which means that the edit cannot be bypassed using any modifier.
The good news
These rules would only apply to Medicare or to payers who use Medicare rules. Although you may find that 52000 may be a common bundle by many payers, you will not usually find commercial insurance denying the repair of the complication during surgery.
Establishing a Rapid Response Team
Medical emergency teams (METs) were introduced more than a decade ago in Australia and the United Kingdom to rapidly identify and manage seriously ill patients at risk of cardiopulmonary arrest and other high‐risk conditions.1 METs, known in the United States as rapid response teams (RRTs), have been slow to be adopted thus far but are quickly gaining ground. Despite numerous studies indicating long‐term patient outcomes are poor following cardiac resuscitation in the hospital, the benefits of early intervention have sometimes been overlooked.25 Several observational studies and a retrospective analysis that included the Medical Emergency Response Improvement Team (MERIT) in Pittsburgh showed that introduction of a MET apparently has the potential to decrease the incidence of unanticipated intensive care unit (ICU) admissions and in‐hospital morbidity and mortality from unexpected cardiopulmonary arrest.69 Furthermore, the use of a MET as a quality improvement tool to detect medical errors and effect systemwide interventions is promising.10 Most recently, the Institute for Healthcare Improvement (IHI) and the American Hospital Association challenged health care organizations to redesign patient safety systems to prevent avoidable deaths in its 100K Lives Campaign. One of the 6 proposed core interventions was the deployment of rapid response teams at the first sign of patient decline.11
Despite these reports of success, a recent large cluster‐randomized controlled trial did not yield the same positive results. In this well‐designed study of 23 Australian hospitals, the Medical Early Response, Intervention and Therapy (MERIT) study investigators found the incidence of cardiac arrest, unplanned ICU admissions, and unexpected death essentially unchanged despite large increases in how often the emergency team was called.12 One possible explanation why these findings conflicted with previous favorable results is that the ultimate impact of a MET may depend on the effectiveness of implementation strategies. To derive the benefits of a MET/RRT, hospitals must increasingly focus on identifying barriers to implementation and address practical issues that may undermine their long‐term effectiveness.
In this article we describe in detail the process of establishing an RRT at our urban, academic hospital and the modifications that became necessary as we rolled out the intervention and encountered obstacles. This analysis was undertaken as a quality improvement (QI) activity. To our knowledge, this is one of the few recent published descriptions of the experiences of implementing an RRT in the United States since earlier work in Pittsburgh.9, 13
METHODS
Temple University Hospital is a tertiary care academic hospital in urban Philadelphia, Pennsylvania. Our RRT was first implemented July 1, 2004, and in the first 12 months of initiation, it was activated 307 times. The RRT at Temple University Hospital was designed to be accessible 24 hours a day, 7 days a week. The daytime team (8 am‐5 pm) is composed of an attending physician (a hospitalist trained as a general internist), a senior internal medicine resident, a critical care nurse, a nurse manager, a pharmacist, and a respiratory therapist. In addition, both a transporter and a member of the admissions office respond to all rapid response team calls but do not get clinically involved in patient care. For nighttime (5 pm‐8 am) and weekend coverage the hospitalist is replaced by an on‐site pulmonary critical care physician, but the remainder of the team is unchanged. All RRT members carry beepers synchronized to provide the location of an RRT activation. In addition, all RRT calls are simultaneously announced on the overhead paging system. No changes were made to the existing cardiac arrest team (code team) at the hospital, which remained a 24‐hour response team for patients found to be in true cardiopulmonary arrest and was comprised of on‐call internal medicine house staff (but no hospitalist attending physician), a respiratory therapist, a pharmacist, a critical care nurse, a nurse manager, and, most notably, an anesthesiologist for emergent intubation and airway management.
The RRT was intended for use within the physical confines of Temple University Hospital and its immediately adjacent grounds. Within the hospital the main locations defined were: inpatient areas, including patient rooms and hallways of the medical‐surgical units of the inpatient tower, as well as the burn, coronary, medical, neurological, neurosurgical, and surgical intensive care units; off‐unit/procedural areas, including diagnostic/emnterventional radiology, the gastroenterology endoscopy suite, the pulmonary procedure suite and pulmonary function lab, the cardiac catheterization/ECHO/stress Lab, the inpatient dialysis unit, and the physical therapy gym, all areas where inpatients are routinely transported during their hospital admission for workup/treatment and where outpatients go for scheduled procedures and therapies; and outpatient/common areas, including all the general medical and subspecialty outpatient clinics in 2 separate outpatient towers (Outpatient Building and Parkinson Pavilion) with direct access from the main hospital building, the outpatient pharmacy, the elevators, the hallways in the outpatient sections of the hospital, all lobbies, and the immediately adjacent outside grounds.
Prior to the launch date of the RRT, clinical criteria were established to help guide staff about when an RRT might be called (Fig. 1). These were based in part on early literature on the clinical markers that most often precede clinical deterioration.14, 15 In addition, 2 much broader categories for RRT activation were added (Inability to reach the patient's primary team of treating physicians for any of the above and Any potentially serious medical errors or adverse events) in order to minimize the need for a very specific physiologic definition to be met in order to activate the team. Physicians, nurses, and other staff with significant daily contact with inpatients and outpatients were in‐serviced about the purpose of the RRT and how to activate the system via the hospital paging operator. Laminated cards with RRT criteria were distributed to all hospital personnel, and educational posters were displayed prominently throughout the hospital.

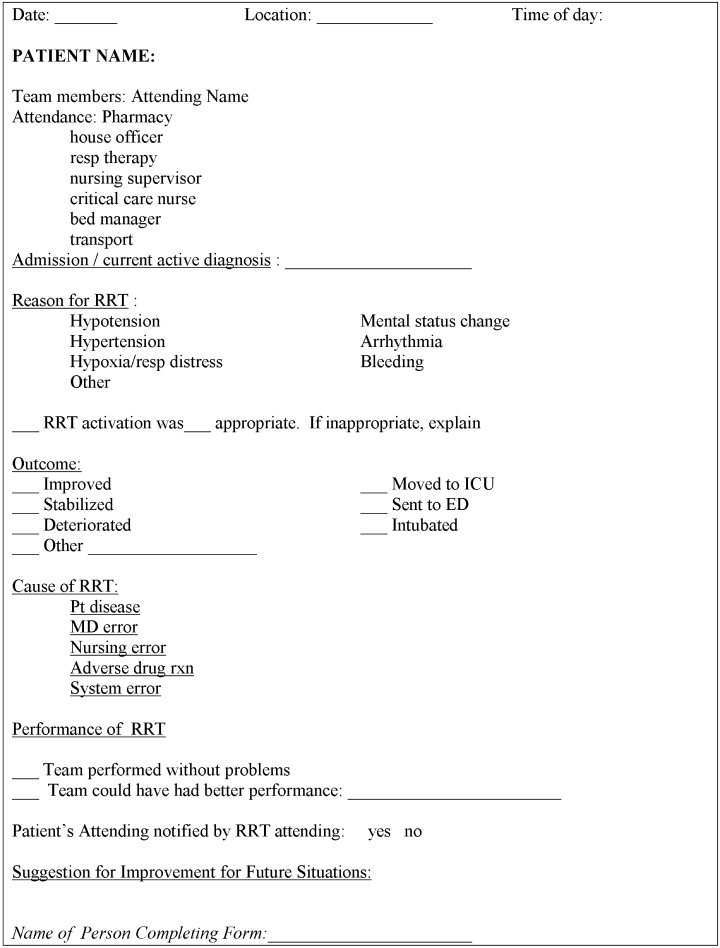
Each RRT event was to be assessed by team members using a standardized evaluation form (Fig. 2), with primary responsibility going to the physician team leader. In the initial phases of implementation, these forms were kept in the offices of the Section of Hospital Medicine for the use of hospitalist attending physician team leaders. Later on in the year they were kept in the pharmacist's RRT medication bag. These forms were collected at the completion of each RRT event or faxed to a central location and then entered into a database maintained by the hospital's Department of Patient Safety Operations. Weekly debriefing meetings to review all RRT events from the preceding week were attended by representatives from patient safety, respiratory, nursing, hospital medicine, and the pharmacy. Attempts were made to identify the issues that led to selected RRT activations, to obtain patient follow‐up from the clinical event, and to evaluate the performance of the team. Throughout these weekly meetings, QI strategies for improving the effectiveness of the RRT were identified and implemented.
The core outcome measures that were used to assess RRT performance were: appropriateness of the RRT activation, percentage of patients who were stabilized, percentage of patients who were transferred to a higher level of care, and overall team performance.
In the weekly meeting of the RRT evaluation committee, at which each RRT was reviewed by the clinical team, each scenario and details of the event were reviewed to determine whether the RRT activation was appropriate, whether the intervention was successful, and whether there were any issues with the team performance. After a thorough discussion of each case and review of additional data from the chart if necessary, the RRT evaluation committee reached a consensus about each of these measures.
We also tracked the number of code team activations from the year preceding establishment of the RRT (2003‐2004) through the year during which the RRT was established (2004‐2005). Because all calls for both the RRT and the code team go first to the hospital operator, we reviewed the hospital paging operators' logs for the entire 12‐month period to track the rate of code team events to RRT events on a monthly basis.
RESULTS
In a 12‐month period, the RRT was activated 307 times, as recorded in the hospital operator logs. In the year preceding inception of the RRT, there were 272 code team activations. In the first 12 months concurrent with RRT implementation, the code team was activated 258 times. Overall, at their discretion the team leaders converted 13% of the 307 RRT activations to traditional code team activations.
There were 11 RRT activations in July, the first month of implementation, and 14 activations in the second month. At that point, the internal hospital newsletter released a feature on the new RRT, and our patient safety officer/director of patient safety operations made a concerted effort to educate hospital administration and the Graduate Medical Education Committee (GMEC); as a result, utilization picked up. From September onward through the remainder of the academic year, an average of 28 RRT activations occurred each month (range 20‐37), whereas an average of 22 codes took place each month (range 12‐27). The numbers of RRT versus code team activations are plotted in Figure 3. A trend line for the number of code team activations per month in 2003, the year prior to implementation of the RRT, was added for comparison; it conveys the slight overall decrease in the number of codes as the RRT took effect (average of 23 codes per month, range 15‐31).
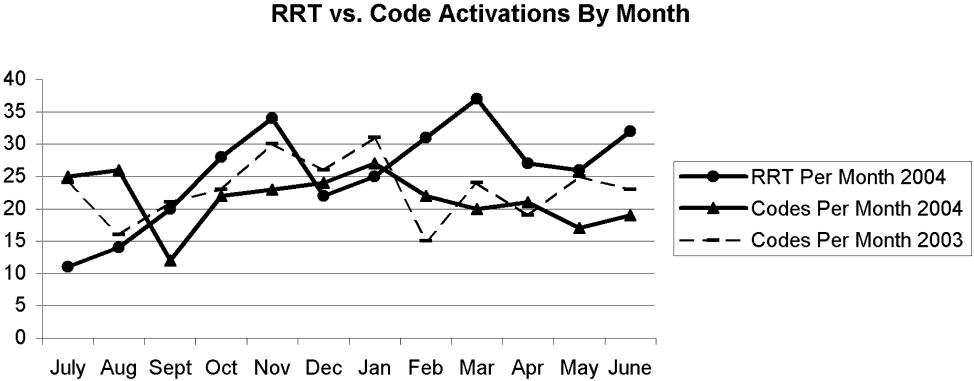
Physician evaluation forms were returned for 170 of the 307 RRT events (55%). The main inpatient tower was the site of 42% of these RRT activations, followed by the outpatient/common areas, where 19% of the activations occurred, and off‐unit/procedural areas, the site of 18%. Table 2 provides information on specific location, reason for call, and disposition of a sample of the RRT activations in the non‐inpatient areas. Time of day was noted in 76.8% of events. Of these, 82.9% occurred during the traditional day shift (7 am‐7 pm) and 17.1% on night shift (7 pm‐7 am). Most RRT activations occurred between 8 am and 4 pm. Daytime events heavily outnumbered nighttime events regardless of location.
Physician team leaders largely believed a specific underlying clinical diagnosis was responsible for 59% of the RRT activations, followed by adverse drug reactions (3.5%), physician error (1.8%), and nursing error (0.6%). When an underlying clinical diagnosis or organ system was suspected, it was most frequently pulmonary (32%), followed by neurological (14%) and cardiac (11%). It was believed that 32% of events were for other reason not listed. Table 1 provides the breakdown of other underlying diagnoses in RRT events.
| Pulmonary | 32% |
| Hypoxia/Respiratory Distress (32%) | |
| Neurological | 14% |
| Change of mental status (7%) | |
| Syncope (7%) | |
| Cardiac | 11% |
| Hypotension (8%) | |
| Arrhythmia (2%) | |
| Hypertension (1%) | |
| Hematologic | 2% |
| Bleeding (2%) | |
| Endocrine | 1% |
| Hypoglycemia (1%) | |
| Other reason not listed | 32% |
| No reason given | 9% |
| Location | Reason for RRT call | Disposition | |
|---|---|---|---|
| Outpatient clinical | Outpatient orthopedics | Dysrhythmia | ED |
| Outpatient medicine clinic | Hypoxia/respiratory Distress | Stabilized | |
| Outpatient urology | Vomiting | ED | |
| Outpatient Parkinson | Asthma | ED | |
| Outpatient Parkinson | Seizure | ED | |
| Common area/nonclinical | Preadmissions testing | Changed mental status | Unknown |
| Admissions | Changed mental status | Stabilized | |
| Hypoxia/respiratory distress | Stabilized | ||
| Syncope/bradycardia | ED | ||
| Security | Syncope | Improved | |
| Lobby | Hypoxia/respiratory distress | Unknown | |
| Changed mental status | ED | ||
| Hypoxia/respiratory distress | Improved | ||
| Procedures/Off‐unit clinical | Stress test lab | Hypoxia/respiratory distress | Improved |
| Cardiac catheterization lab | Chest pain | ED | |
| Diagnostic imaging | Changed mental status | Improved | |
| Mucus plug in tracheostomy | Improved | ||
| Seizure | ICU | ||
| Syncope | ED | ||
| Hypoxia/respiratory distress | Unknown | ||
| Hypoglycemia | ED | ||
| Dialysis | Bleeding | Stabilized | |
| Gastroenterology procedures | Hypoxia/respiratory distress | ICU | |
| Hypoxia/respiratory distress | Stabilized | ||
| Hypoxia/respiratory distress | ICU | ||
| Interventional radiology | Hypotension/dehydration | Unknown | |
| Hypoxia/respiratory distress | ICU | ||
| Changed mental status | Stabilized | ||
| Hypoxia/Respiratory distress | ICU | ||
| Hypoxia/Respiratory distress | ICU | ||
| Changed mental status | ED | ||
| Hypoxia/Respiratory distress | ICU | ||
| MRI | Hypoxia/Respiratory distress | ED | |
| Hypoxia/respiratory distress | ED | ||
| Hypoxia/respiratory distress | ED | ||
| Changed mental status | ED | ||
| Occupational therapy | Hypotension | ED | |
| Physical therapy | Hypotension | Stabilized | |
| Physical medicine/rehab | Hypoxia/respiratory distress | Unknown | |
| Short procedure unit | Syncope | Stabilized | |
| Hypotension | ICU |
In the judgment of evaluators, the system was utilized appropriately in 98% of the evaluated events. Eighty‐five percent of RRT activations were believed to have prevented further clinical deterioration, though it was also thought that 3% of patients deteriorated despite the efforts of the team. Disposition of the patient following an RRT event was noted 87% of the time, and it was believed that 88% of the patients were stabilized. Of the formally evaluated RRT events, team members were largely satisfied with the response and the functioning of the team, stating for 68% of the events that the team performed without a problem.
Problems Identified and Addressed During Implementation
Though it was encouraging that those surveyed believed the team performed without a problem in 68% of the activations, another way to look at it is that team performance was inadequate in 32% of the cases. Any issues cited on the evaluation sheets, ranging from delays in arrival of team members to missing/delayed arrival of equipment, were seen as opportunities for improvement. For example, very early on in the implementation process, team leaders specifically noted repeatedly encountering a diagnosis of suspected hypoglycemia in patients with a known history of diabetes found with altered mental status. Early clinical assessments by the RRT were severely limited and judged problematic without a simple way to objectively rule out this possibility and/or to attempt immediate treatment, especially because this frequently occurred in non‐inpatient settings. Team members suggested and quickly obtained approval to carry both glucometers and glucose tablets and Glucagon in the pharmacist's fanny pack. In another case, our respiratory therapists arrived promptly to the scene of an RRT call for shortness of breath but were hampered by lack of readily available oxygen tanks. This was promptly remedied, at the recommendation of the committee, by placing additional oxygen tanks near all hospital security stations. Placement of code (crash) carts has also been modified to increase accessibility, especially in nonclinical areas, where delays were perceived to have contributed to poor outcomes. In the future, alphanumeric pagers will be used to allow for more specific and efficient deployment of the team.
Other changes that have been made include the addition to respiratory/pharmacy fanny packs of other key medications such as lorazepam for seizures, equipment such as peripheral catheters for intravenous access, and syringes/needles. It is hoped that in the near future, a state‐of‐the‐art point‐of‐care blood‐testing device, I‐stat, capable of quickly analyzing a blood sample for basic stat lab tests will be added to the pack to expedite triage.16 Perhaps most important, the committee reached a consensus that to improve and encourage real‐time evaluations, it might be best to have the RRT evaluation forms and other paperwork at the point of care to increase yield. The pharmacist now carries blank forms in the fanny pack for convenience. Early on in our RRT implementation process, all these items were noted to be lacking at various times and were requested by team leaders, nurses, and pharmacists in order to be better prepared for various clinical scenarios. In addition, ongoing analysis of the most common RRT diagnoses in the database guided our final decisions in order to keep the size of the fanny pack down to a minimum while providing crucial equipment.
DISCUSSION
We have found the RRT to be an effective but challenging‐to‐implement QI intervention to increase patient safety at our academic institution. The Australian MERIT investigators recently suggested that despite growing evidence of the benefits of MET/RRT systems, long‐term success may depend most on effective implementation strategies.12 We experienced firsthand these challenges in the first year of our new RRT system.
Large system changes in a hospital are especially fraught with danger because of the unique aspects of health care delivery systems. As Reid commented in an editorial about the emerging use of the MET system in the United Kingdom, Despite potential advantages to patients, ensuring appropriate utilization was difficult because of cultural barriers. Traditional hierarchical behaviors that dictate how doctors and nurses react and work got in the way of people calling these life saving teams.17
Our weekly multidisciplinary RRT debriefings were the most crucial component of our implementation strategy. Many latent systems issues were uncovered, as well as more subtle problems such as lack of coordination of care, communication errors, gaps in patient handoffs or sign‐out. Previous studies by the Pittsburgh MERIT team have validated such retrospective categorization of errors uncovered by MET responses.10
However, neither that group nor the Australian MERIT study investigators specifically addressed the importance of the feedback process in RRT implementation. A strength of our system is that modifications to the RRT are made prospectively and in real time based on feedback from active RRT members during debriefing. In fact, the success of our RRT underscores the importance of open communication among hospitalists, house staff, nurses, pharmacists, and ancillary staff in multidisciplinary patient safety and QI endeavors. Everything from the responsibilities of team members to equipment evolved over the 12‐month period in order to improve the function and effectiveness of the team and was almost entirely based on feedback from the RRT doctors and nurses on the front lines. Suggestions from the evaluation forms were given serious consideration at every RRT evaluation committee debriefing. By optimizing the efficient operation of the RRT, we hope to continue to improve outcomes.
We believe a key to the success of our debriefing process was the constant attendance of our patient safety officer/chief medical officer and director of patient safety operations, who both encouraged active participation. Early on in the process, comments were made principally by physician and critical care nurse RRT members, and the dynamic was a bit one‐sided. However, we quickly saw a noticeable and sustained increase in participation by pharmacists and respiratory therapists, and by year's end, they had offered some of the most valuable practical suggestions, which resulted in a more efficient response. As the year went on and real changes were made quickly, all groups were much more vocal and willing to bounce ideas around the room, and the team dynamic and spirit of the group effort improved substantially.
Previous studies have focused on the impact of METs/RRTs on the rate of inpatient cardiac arrests. However, we found that nearly as many RRT events occurred off the inpatient units, for instance, when admitted patients were transported to other areas such as radiology, procedural suites, physical therapy, or dialysis and when scheduled outpatients arrived for their appointments. In addition, a large number of RRT calls came from outpatient departments and common areas of the hospital such as lobbies, hallways, and waiting rooms, mostly involving outpatients and visitors, but not infrequently hospital employees were involved as well. This unexpected and, to our knowledge, previously unreported finding is mirrored in the distribution of RRT activations throughout the course of the day. Most events occurred during the traditional day shift of 7 am‐7 pm, and were heavily clustered between 8 am and 4 pm. In most American hospitals, these are the hours during which outpatients and visitors make up a significant proportion of the hospital population and during which most elective procedures on inpatients occur. Prior to the introduction of our RRT, no specific system was in place for emergent triage, assessment, and expedited treatment of off‐unit patients, outpatients, and visitors. Most often, the code team was mobilized, sometimes taking them to remote locations and making them unavailable for true inpatient cardiopulmonary arrests. Our RRT seems to have the potential to fill a much‐needed gap in patient safety, offering off‐unit patients, outpatients, and visitors a safety net while in our hospital. No prior descriptions of RRT or MET implementation have touched on this area. It would be interesting to see if other hospitals with RRTs have had a similar experience in order to determine whether having an RRT dedicated specifically to the outpatient and common areas of the hospital might provide even more targeted efforts and efficient response times. Thus, the benefits of our RRT seemed to extend beyond a simple reduction in the number of in‐hospital cardiopulmonary arrests and into an unanticipated patient safety black hole.
Implementation of the RRT specifically in academic medical centers has been limited to date. In our opinion, the academic environment is an ideal area for RRTs (because the most critically ill patients often are cared for on teaching services by junior house officers), but it is also a challenging arena in which to make change (because of the complex hierarchy of teaching hospitals). We chose to have an attending physician lead our RRT efforts for the most part. However, residents always participated, and not infrequently led, as key team members. As a commentator on the Australian RRT system pointed out, it is important that junior medical staff [feel empowered] to call for immediate assistance when they are concerned about their patient, but may not have the experience, knowledge, confidence or skills necessary to manage them appropriately.18 We believe that the RRT serves as a valuable educational forum for resident education. Academic centers that develop RRTs must work to integrate the teams into an educational context while simultaneously providing patients with the most experienced and knowledgeable clinical team to address their needs at a time when appropriate clinical decision making is critical. Therefore, the residents who participate in our RRT are formally evaluated by the hospitalists using a standard program evaluation form that encompasses the Accreditation Council for Graduate Medical Education (ACGME) core competencies.19
Through the first year of our RRT system and beyond, activation of the code team and RRT shifted as more RRT activations were recorded and fewer codes were called. Concerted educational efforts and reinforcement of the criteria for calling the RRT had a definite sustained impact of helping staff to become comfortable with using the system. At our institution, it has been difficult to definitively conclude whether RRT calls prevented codes or merely substituted for them at times, especially because 13% of all RRT activations were subsequently converted to code team calls. The Australian MERIT study investigators, despite an excellent study design of a large multicenter trial, also were unable to demonstrate a true decrease in the cardiac arrest rate.12 Much more significant to us, especially in the first year of implementation, was learning that the vast majority of physician RRT leaders perceived activation of the team to occur appropriately and to play a role in preventing clinical deterioration of patients. None of the other RRT or MET implementation studies that we reviewed commented specifically on these areas. It will be interesting to continue to follow these trends, as we expect the use of RRTs to become even more defined. Over time, we will no doubt be better able to determine whether RRTs have a true, sustained impact on preventing patient deterioration and inpatient cardiopulmonary arrests while maintaining a high rate of physician satisfaction that the team is being activated for legitimate reasons.
Our descriptive study had some limitations. The number of RRT evaluations received, while adequate for preliminary analysis, may not accurately represent the 307 activations of the system that occurred in the first 12 months. We suspect that this underreporting, especially in the first half of the year, was in large part a result of relying on team leaders to voluntarily return data forms at the conclusion of each RRT event. RRT evaluations in the second half of the year were more actively distributed at the point of care to the team leader directly by the pharmacist and were more diligently followed up on. Forms are now readily available in the team pharmacist's fanny pack, which was done because of quality improvement feedback from physicians at a debriefing meeting. Since those interventions, there has been a dramatic improvement in the capture of event data and the timely submission of forms. We expect and have demanded close to a 100% return of the forms in the second year of our RRT system, which will vastly improve our analysis. We were also surprised that despite the comprehensiveness of our RRT activation criteria, 32% of physicians were unable to find a match with a clinical indication on the list, indicating unanticipated reasons for calling an RRT. We will continually strive to improve the specificity of future data for planning purposes and training initiatives. However, in some way this confirms our belief that RRTs occur for such a wide variety of reasons that they cannot always be limited to the major clinical categories. On a similar note, we regret not adding a specific category under Outcomes on the evaluation form to include the possibility that RRT members might have offered palliative care or changes in code/do not resuscitate (DNR) status to patients or families. Given that our hospital has both a code team and an RRT begs the question of whether mortality rates might be affected if patients who prior to the RRT might have had a full resuscitation effort were made DNR. In the future, this would be an interesting issue to consider in analysis. Carefully categorizing RRT events is critical to continued success. Further work involving formal team skills training for RRT members, including use of the medical school's clinical simulators for mock RRT scenarios, is planned. These sessions are planned to review performance and clinical decision making for the most common scenarios that we have found to be involved in RRT activations. The 307 activations of the RRT in our first year have clearly set us on the path toward defining predictive rules and directed skills training for earlier identification of patient problems. Further outcome analyses of these efforts will be crucial.
CONCLUSIONS
An RRT was successfully introduced into an academic medical center. The team was heavily utilized in the first 12 months after the program was initiated, especially for off‐unit inpatients and those in outpatient/common areas, perhaps filling a gap in hospital patient safety. The keys to the early success of implementation of our RRT were multidisciplinary input and improvements made in real time. The long‐term effects of the RRT on the culture of patient safety in our institution and throughout the United States remain to be seen but are promising.
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,, et al.Quality of cardiopulmonary resuscitation during in‐hospital cardiac arrest.JAMA.2005;293:363–365.
- ,,.In‐hospital cardiopulmonary resuscitation.Medicine.1995;74:163–175.
- ,,, et al.In‐hospital cardiac arrest: survival depends mainly on the effectiveness of the emergency response.Resuscitation.2004;62:291–297.
- ,,.Factors influencing survival after in‐hospital cardiopulmonary resuscitation.Resuscitation.2005;66:317–321.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.Br Med J.2002;324:1–5.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–204.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,,,,.Use of medical emergency team (MET) responses to detect medical errors.Qual Saf Health Care.2004;13:255–259.
- Institute for Healthcare Improvement. 100K Lives Campaign [IHI website]. Available at: http://www.ihi.org/IHI/Programs/campaign. Accessed November 10,2005.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,, et al.Improving the utilization of medical crisis teams (condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,.Developing strategies to prevent in‐hospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22:244–247.
- ,,,,.Clinical Antecedents to In‐Hospital Cardiopulmonary Arrest.Chest.1990;98:1388–1392.
- Abbot Point of Care: Abbot Laboratories Online. Available at: http://www.istat.com/website/www/products/analyzers.htm. Accessed November 10,2005.
- .Developing and implementing organisational practice that delivers better, safer care.Qual Saf Health Care.2004;13:247.
- ,.The medical emergency team: does it really make a difference?Intern Med J.2003;33:511–514.
- Accreditation Council for Graduate Medical Education (ACGME). Program requirements for residency education in internal medicine. Effective July 2003; revised July 1, 2004. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/140pr703_u704.pdf. Accessed February 17,2006.
Medical emergency teams (METs) were introduced more than a decade ago in Australia and the United Kingdom to rapidly identify and manage seriously ill patients at risk of cardiopulmonary arrest and other high‐risk conditions.1 METs, known in the United States as rapid response teams (RRTs), have been slow to be adopted thus far but are quickly gaining ground. Despite numerous studies indicating long‐term patient outcomes are poor following cardiac resuscitation in the hospital, the benefits of early intervention have sometimes been overlooked.25 Several observational studies and a retrospective analysis that included the Medical Emergency Response Improvement Team (MERIT) in Pittsburgh showed that introduction of a MET apparently has the potential to decrease the incidence of unanticipated intensive care unit (ICU) admissions and in‐hospital morbidity and mortality from unexpected cardiopulmonary arrest.69 Furthermore, the use of a MET as a quality improvement tool to detect medical errors and effect systemwide interventions is promising.10 Most recently, the Institute for Healthcare Improvement (IHI) and the American Hospital Association challenged health care organizations to redesign patient safety systems to prevent avoidable deaths in its 100K Lives Campaign. One of the 6 proposed core interventions was the deployment of rapid response teams at the first sign of patient decline.11
Despite these reports of success, a recent large cluster‐randomized controlled trial did not yield the same positive results. In this well‐designed study of 23 Australian hospitals, the Medical Early Response, Intervention and Therapy (MERIT) study investigators found the incidence of cardiac arrest, unplanned ICU admissions, and unexpected death essentially unchanged despite large increases in how often the emergency team was called.12 One possible explanation why these findings conflicted with previous favorable results is that the ultimate impact of a MET may depend on the effectiveness of implementation strategies. To derive the benefits of a MET/RRT, hospitals must increasingly focus on identifying barriers to implementation and address practical issues that may undermine their long‐term effectiveness.
In this article we describe in detail the process of establishing an RRT at our urban, academic hospital and the modifications that became necessary as we rolled out the intervention and encountered obstacles. This analysis was undertaken as a quality improvement (QI) activity. To our knowledge, this is one of the few recent published descriptions of the experiences of implementing an RRT in the United States since earlier work in Pittsburgh.9, 13
METHODS
Temple University Hospital is a tertiary care academic hospital in urban Philadelphia, Pennsylvania. Our RRT was first implemented July 1, 2004, and in the first 12 months of initiation, it was activated 307 times. The RRT at Temple University Hospital was designed to be accessible 24 hours a day, 7 days a week. The daytime team (8 am‐5 pm) is composed of an attending physician (a hospitalist trained as a general internist), a senior internal medicine resident, a critical care nurse, a nurse manager, a pharmacist, and a respiratory therapist. In addition, both a transporter and a member of the admissions office respond to all rapid response team calls but do not get clinically involved in patient care. For nighttime (5 pm‐8 am) and weekend coverage the hospitalist is replaced by an on‐site pulmonary critical care physician, but the remainder of the team is unchanged. All RRT members carry beepers synchronized to provide the location of an RRT activation. In addition, all RRT calls are simultaneously announced on the overhead paging system. No changes were made to the existing cardiac arrest team (code team) at the hospital, which remained a 24‐hour response team for patients found to be in true cardiopulmonary arrest and was comprised of on‐call internal medicine house staff (but no hospitalist attending physician), a respiratory therapist, a pharmacist, a critical care nurse, a nurse manager, and, most notably, an anesthesiologist for emergent intubation and airway management.
The RRT was intended for use within the physical confines of Temple University Hospital and its immediately adjacent grounds. Within the hospital the main locations defined were: inpatient areas, including patient rooms and hallways of the medical‐surgical units of the inpatient tower, as well as the burn, coronary, medical, neurological, neurosurgical, and surgical intensive care units; off‐unit/procedural areas, including diagnostic/emnterventional radiology, the gastroenterology endoscopy suite, the pulmonary procedure suite and pulmonary function lab, the cardiac catheterization/ECHO/stress Lab, the inpatient dialysis unit, and the physical therapy gym, all areas where inpatients are routinely transported during their hospital admission for workup/treatment and where outpatients go for scheduled procedures and therapies; and outpatient/common areas, including all the general medical and subspecialty outpatient clinics in 2 separate outpatient towers (Outpatient Building and Parkinson Pavilion) with direct access from the main hospital building, the outpatient pharmacy, the elevators, the hallways in the outpatient sections of the hospital, all lobbies, and the immediately adjacent outside grounds.
Prior to the launch date of the RRT, clinical criteria were established to help guide staff about when an RRT might be called (Fig. 1). These were based in part on early literature on the clinical markers that most often precede clinical deterioration.14, 15 In addition, 2 much broader categories for RRT activation were added (Inability to reach the patient's primary team of treating physicians for any of the above and Any potentially serious medical errors or adverse events) in order to minimize the need for a very specific physiologic definition to be met in order to activate the team. Physicians, nurses, and other staff with significant daily contact with inpatients and outpatients were in‐serviced about the purpose of the RRT and how to activate the system via the hospital paging operator. Laminated cards with RRT criteria were distributed to all hospital personnel, and educational posters were displayed prominently throughout the hospital.

Each RRT event was to be assessed by team members using a standardized evaluation form (Fig. 2), with primary responsibility going to the physician team leader. In the initial phases of implementation, these forms were kept in the offices of the Section of Hospital Medicine for the use of hospitalist attending physician team leaders. Later on in the year they were kept in the pharmacist's RRT medication bag. These forms were collected at the completion of each RRT event or faxed to a central location and then entered into a database maintained by the hospital's Department of Patient Safety Operations. Weekly debriefing meetings to review all RRT events from the preceding week were attended by representatives from patient safety, respiratory, nursing, hospital medicine, and the pharmacy. Attempts were made to identify the issues that led to selected RRT activations, to obtain patient follow‐up from the clinical event, and to evaluate the performance of the team. Throughout these weekly meetings, QI strategies for improving the effectiveness of the RRT were identified and implemented.

The core outcome measures that were used to assess RRT performance were: appropriateness of the RRT activation, percentage of patients who were stabilized, percentage of patients who were transferred to a higher level of care, and overall team performance.
In the weekly meeting of the RRT evaluation committee, at which each RRT was reviewed by the clinical team, each scenario and details of the event were reviewed to determine whether the RRT activation was appropriate, whether the intervention was successful, and whether there were any issues with the team performance. After a thorough discussion of each case and review of additional data from the chart if necessary, the RRT evaluation committee reached a consensus about each of these measures.
We also tracked the number of code team activations from the year preceding establishment of the RRT (2003‐2004) through the year during which the RRT was established (2004‐2005). Because all calls for both the RRT and the code team go first to the hospital operator, we reviewed the hospital paging operators' logs for the entire 12‐month period to track the rate of code team events to RRT events on a monthly basis.
RESULTS
In a 12‐month period, the RRT was activated 307 times, as recorded in the hospital operator logs. In the year preceding inception of the RRT, there were 272 code team activations. In the first 12 months concurrent with RRT implementation, the code team was activated 258 times. Overall, at their discretion the team leaders converted 13% of the 307 RRT activations to traditional code team activations.
There were 11 RRT activations in July, the first month of implementation, and 14 activations in the second month. At that point, the internal hospital newsletter released a feature on the new RRT, and our patient safety officer/director of patient safety operations made a concerted effort to educate hospital administration and the Graduate Medical Education Committee (GMEC); as a result, utilization picked up. From September onward through the remainder of the academic year, an average of 28 RRT activations occurred each month (range 20‐37), whereas an average of 22 codes took place each month (range 12‐27). The numbers of RRT versus code team activations are plotted in Figure 3. A trend line for the number of code team activations per month in 2003, the year prior to implementation of the RRT, was added for comparison; it conveys the slight overall decrease in the number of codes as the RRT took effect (average of 23 codes per month, range 15‐31).

Physician evaluation forms were returned for 170 of the 307 RRT events (55%). The main inpatient tower was the site of 42% of these RRT activations, followed by the outpatient/common areas, where 19% of the activations occurred, and off‐unit/procedural areas, the site of 18%. Table 2 provides information on specific location, reason for call, and disposition of a sample of the RRT activations in the non‐inpatient areas. Time of day was noted in 76.8% of events. Of these, 82.9% occurred during the traditional day shift (7 am‐7 pm) and 17.1% on night shift (7 pm‐7 am). Most RRT activations occurred between 8 am and 4 pm. Daytime events heavily outnumbered nighttime events regardless of location.
Physician team leaders largely believed a specific underlying clinical diagnosis was responsible for 59% of the RRT activations, followed by adverse drug reactions (3.5%), physician error (1.8%), and nursing error (0.6%). When an underlying clinical diagnosis or organ system was suspected, it was most frequently pulmonary (32%), followed by neurological (14%) and cardiac (11%). It was believed that 32% of events were for other reason not listed. Table 1 provides the breakdown of other underlying diagnoses in RRT events.
| Pulmonary | 32% |
| Hypoxia/Respiratory Distress (32%) | |
| Neurological | 14% |
| Change of mental status (7%) | |
| Syncope (7%) | |
| Cardiac | 11% |
| Hypotension (8%) | |
| Arrhythmia (2%) | |
| Hypertension (1%) | |
| Hematologic | 2% |
| Bleeding (2%) | |
| Endocrine | 1% |
| Hypoglycemia (1%) | |
| Other reason not listed | 32% |
| No reason given | 9% |
| Location | Reason for RRT call | Disposition | |
|---|---|---|---|
| Outpatient clinical | Outpatient orthopedics | Dysrhythmia | ED |
| Outpatient medicine clinic | Hypoxia/respiratory Distress | Stabilized | |
| Outpatient urology | Vomiting | ED | |
| Outpatient Parkinson | Asthma | ED | |
| Outpatient Parkinson | Seizure | ED | |
| Common area/nonclinical | Preadmissions testing | Changed mental status | Unknown |
| Admissions | Changed mental status | Stabilized | |
| Hypoxia/respiratory distress | Stabilized | ||
| Syncope/bradycardia | ED | ||
| Security | Syncope | Improved | |
| Lobby | Hypoxia/respiratory distress | Unknown | |
| Changed mental status | ED | ||
| Hypoxia/respiratory distress | Improved | ||
| Procedures/Off‐unit clinical | Stress test lab | Hypoxia/respiratory distress | Improved |
| Cardiac catheterization lab | Chest pain | ED | |
| Diagnostic imaging | Changed mental status | Improved | |
| Mucus plug in tracheostomy | Improved | ||
| Seizure | ICU | ||
| Syncope | ED | ||
| Hypoxia/respiratory distress | Unknown | ||
| Hypoglycemia | ED | ||
| Dialysis | Bleeding | Stabilized | |
| Gastroenterology procedures | Hypoxia/respiratory distress | ICU | |
| Hypoxia/respiratory distress | Stabilized | ||
| Hypoxia/respiratory distress | ICU | ||
| Interventional radiology | Hypotension/dehydration | Unknown | |
| Hypoxia/respiratory distress | ICU | ||
| Changed mental status | Stabilized | ||
| Hypoxia/Respiratory distress | ICU | ||
| Hypoxia/Respiratory distress | ICU | ||
| Changed mental status | ED | ||
| Hypoxia/Respiratory distress | ICU | ||
| MRI | Hypoxia/Respiratory distress | ED | |
| Hypoxia/respiratory distress | ED | ||
| Hypoxia/respiratory distress | ED | ||
| Changed mental status | ED | ||
| Occupational therapy | Hypotension | ED | |
| Physical therapy | Hypotension | Stabilized | |
| Physical medicine/rehab | Hypoxia/respiratory distress | Unknown | |
| Short procedure unit | Syncope | Stabilized | |
| Hypotension | ICU |
In the judgment of evaluators, the system was utilized appropriately in 98% of the evaluated events. Eighty‐five percent of RRT activations were believed to have prevented further clinical deterioration, though it was also thought that 3% of patients deteriorated despite the efforts of the team. Disposition of the patient following an RRT event was noted 87% of the time, and it was believed that 88% of the patients were stabilized. Of the formally evaluated RRT events, team members were largely satisfied with the response and the functioning of the team, stating for 68% of the events that the team performed without a problem.
Problems Identified and Addressed During Implementation
Though it was encouraging that those surveyed believed the team performed without a problem in 68% of the activations, another way to look at it is that team performance was inadequate in 32% of the cases. Any issues cited on the evaluation sheets, ranging from delays in arrival of team members to missing/delayed arrival of equipment, were seen as opportunities for improvement. For example, very early on in the implementation process, team leaders specifically noted repeatedly encountering a diagnosis of suspected hypoglycemia in patients with a known history of diabetes found with altered mental status. Early clinical assessments by the RRT were severely limited and judged problematic without a simple way to objectively rule out this possibility and/or to attempt immediate treatment, especially because this frequently occurred in non‐inpatient settings. Team members suggested and quickly obtained approval to carry both glucometers and glucose tablets and Glucagon in the pharmacist's fanny pack. In another case, our respiratory therapists arrived promptly to the scene of an RRT call for shortness of breath but were hampered by lack of readily available oxygen tanks. This was promptly remedied, at the recommendation of the committee, by placing additional oxygen tanks near all hospital security stations. Placement of code (crash) carts has also been modified to increase accessibility, especially in nonclinical areas, where delays were perceived to have contributed to poor outcomes. In the future, alphanumeric pagers will be used to allow for more specific and efficient deployment of the team.
Other changes that have been made include the addition to respiratory/pharmacy fanny packs of other key medications such as lorazepam for seizures, equipment such as peripheral catheters for intravenous access, and syringes/needles. It is hoped that in the near future, a state‐of‐the‐art point‐of‐care blood‐testing device, I‐stat, capable of quickly analyzing a blood sample for basic stat lab tests will be added to the pack to expedite triage.16 Perhaps most important, the committee reached a consensus that to improve and encourage real‐time evaluations, it might be best to have the RRT evaluation forms and other paperwork at the point of care to increase yield. The pharmacist now carries blank forms in the fanny pack for convenience. Early on in our RRT implementation process, all these items were noted to be lacking at various times and were requested by team leaders, nurses, and pharmacists in order to be better prepared for various clinical scenarios. In addition, ongoing analysis of the most common RRT diagnoses in the database guided our final decisions in order to keep the size of the fanny pack down to a minimum while providing crucial equipment.
DISCUSSION
We have found the RRT to be an effective but challenging‐to‐implement QI intervention to increase patient safety at our academic institution. The Australian MERIT investigators recently suggested that despite growing evidence of the benefits of MET/RRT systems, long‐term success may depend most on effective implementation strategies.12 We experienced firsthand these challenges in the first year of our new RRT system.
Large system changes in a hospital are especially fraught with danger because of the unique aspects of health care delivery systems. As Reid commented in an editorial about the emerging use of the MET system in the United Kingdom, Despite potential advantages to patients, ensuring appropriate utilization was difficult because of cultural barriers. Traditional hierarchical behaviors that dictate how doctors and nurses react and work got in the way of people calling these life saving teams.17
Our weekly multidisciplinary RRT debriefings were the most crucial component of our implementation strategy. Many latent systems issues were uncovered, as well as more subtle problems such as lack of coordination of care, communication errors, gaps in patient handoffs or sign‐out. Previous studies by the Pittsburgh MERIT team have validated such retrospective categorization of errors uncovered by MET responses.10
However, neither that group nor the Australian MERIT study investigators specifically addressed the importance of the feedback process in RRT implementation. A strength of our system is that modifications to the RRT are made prospectively and in real time based on feedback from active RRT members during debriefing. In fact, the success of our RRT underscores the importance of open communication among hospitalists, house staff, nurses, pharmacists, and ancillary staff in multidisciplinary patient safety and QI endeavors. Everything from the responsibilities of team members to equipment evolved over the 12‐month period in order to improve the function and effectiveness of the team and was almost entirely based on feedback from the RRT doctors and nurses on the front lines. Suggestions from the evaluation forms were given serious consideration at every RRT evaluation committee debriefing. By optimizing the efficient operation of the RRT, we hope to continue to improve outcomes.
We believe a key to the success of our debriefing process was the constant attendance of our patient safety officer/chief medical officer and director of patient safety operations, who both encouraged active participation. Early on in the process, comments were made principally by physician and critical care nurse RRT members, and the dynamic was a bit one‐sided. However, we quickly saw a noticeable and sustained increase in participation by pharmacists and respiratory therapists, and by year's end, they had offered some of the most valuable practical suggestions, which resulted in a more efficient response. As the year went on and real changes were made quickly, all groups were much more vocal and willing to bounce ideas around the room, and the team dynamic and spirit of the group effort improved substantially.
Previous studies have focused on the impact of METs/RRTs on the rate of inpatient cardiac arrests. However, we found that nearly as many RRT events occurred off the inpatient units, for instance, when admitted patients were transported to other areas such as radiology, procedural suites, physical therapy, or dialysis and when scheduled outpatients arrived for their appointments. In addition, a large number of RRT calls came from outpatient departments and common areas of the hospital such as lobbies, hallways, and waiting rooms, mostly involving outpatients and visitors, but not infrequently hospital employees were involved as well. This unexpected and, to our knowledge, previously unreported finding is mirrored in the distribution of RRT activations throughout the course of the day. Most events occurred during the traditional day shift of 7 am‐7 pm, and were heavily clustered between 8 am and 4 pm. In most American hospitals, these are the hours during which outpatients and visitors make up a significant proportion of the hospital population and during which most elective procedures on inpatients occur. Prior to the introduction of our RRT, no specific system was in place for emergent triage, assessment, and expedited treatment of off‐unit patients, outpatients, and visitors. Most often, the code team was mobilized, sometimes taking them to remote locations and making them unavailable for true inpatient cardiopulmonary arrests. Our RRT seems to have the potential to fill a much‐needed gap in patient safety, offering off‐unit patients, outpatients, and visitors a safety net while in our hospital. No prior descriptions of RRT or MET implementation have touched on this area. It would be interesting to see if other hospitals with RRTs have had a similar experience in order to determine whether having an RRT dedicated specifically to the outpatient and common areas of the hospital might provide even more targeted efforts and efficient response times. Thus, the benefits of our RRT seemed to extend beyond a simple reduction in the number of in‐hospital cardiopulmonary arrests and into an unanticipated patient safety black hole.
Implementation of the RRT specifically in academic medical centers has been limited to date. In our opinion, the academic environment is an ideal area for RRTs (because the most critically ill patients often are cared for on teaching services by junior house officers), but it is also a challenging arena in which to make change (because of the complex hierarchy of teaching hospitals). We chose to have an attending physician lead our RRT efforts for the most part. However, residents always participated, and not infrequently led, as key team members. As a commentator on the Australian RRT system pointed out, it is important that junior medical staff [feel empowered] to call for immediate assistance when they are concerned about their patient, but may not have the experience, knowledge, confidence or skills necessary to manage them appropriately.18 We believe that the RRT serves as a valuable educational forum for resident education. Academic centers that develop RRTs must work to integrate the teams into an educational context while simultaneously providing patients with the most experienced and knowledgeable clinical team to address their needs at a time when appropriate clinical decision making is critical. Therefore, the residents who participate in our RRT are formally evaluated by the hospitalists using a standard program evaluation form that encompasses the Accreditation Council for Graduate Medical Education (ACGME) core competencies.19
Through the first year of our RRT system and beyond, activation of the code team and RRT shifted as more RRT activations were recorded and fewer codes were called. Concerted educational efforts and reinforcement of the criteria for calling the RRT had a definite sustained impact of helping staff to become comfortable with using the system. At our institution, it has been difficult to definitively conclude whether RRT calls prevented codes or merely substituted for them at times, especially because 13% of all RRT activations were subsequently converted to code team calls. The Australian MERIT study investigators, despite an excellent study design of a large multicenter trial, also were unable to demonstrate a true decrease in the cardiac arrest rate.12 Much more significant to us, especially in the first year of implementation, was learning that the vast majority of physician RRT leaders perceived activation of the team to occur appropriately and to play a role in preventing clinical deterioration of patients. None of the other RRT or MET implementation studies that we reviewed commented specifically on these areas. It will be interesting to continue to follow these trends, as we expect the use of RRTs to become even more defined. Over time, we will no doubt be better able to determine whether RRTs have a true, sustained impact on preventing patient deterioration and inpatient cardiopulmonary arrests while maintaining a high rate of physician satisfaction that the team is being activated for legitimate reasons.
Our descriptive study had some limitations. The number of RRT evaluations received, while adequate for preliminary analysis, may not accurately represent the 307 activations of the system that occurred in the first 12 months. We suspect that this underreporting, especially in the first half of the year, was in large part a result of relying on team leaders to voluntarily return data forms at the conclusion of each RRT event. RRT evaluations in the second half of the year were more actively distributed at the point of care to the team leader directly by the pharmacist and were more diligently followed up on. Forms are now readily available in the team pharmacist's fanny pack, which was done because of quality improvement feedback from physicians at a debriefing meeting. Since those interventions, there has been a dramatic improvement in the capture of event data and the timely submission of forms. We expect and have demanded close to a 100% return of the forms in the second year of our RRT system, which will vastly improve our analysis. We were also surprised that despite the comprehensiveness of our RRT activation criteria, 32% of physicians were unable to find a match with a clinical indication on the list, indicating unanticipated reasons for calling an RRT. We will continually strive to improve the specificity of future data for planning purposes and training initiatives. However, in some way this confirms our belief that RRTs occur for such a wide variety of reasons that they cannot always be limited to the major clinical categories. On a similar note, we regret not adding a specific category under Outcomes on the evaluation form to include the possibility that RRT members might have offered palliative care or changes in code/do not resuscitate (DNR) status to patients or families. Given that our hospital has both a code team and an RRT begs the question of whether mortality rates might be affected if patients who prior to the RRT might have had a full resuscitation effort were made DNR. In the future, this would be an interesting issue to consider in analysis. Carefully categorizing RRT events is critical to continued success. Further work involving formal team skills training for RRT members, including use of the medical school's clinical simulators for mock RRT scenarios, is planned. These sessions are planned to review performance and clinical decision making for the most common scenarios that we have found to be involved in RRT activations. The 307 activations of the RRT in our first year have clearly set us on the path toward defining predictive rules and directed skills training for earlier identification of patient problems. Further outcome analyses of these efforts will be crucial.
CONCLUSIONS
An RRT was successfully introduced into an academic medical center. The team was heavily utilized in the first 12 months after the program was initiated, especially for off‐unit inpatients and those in outpatient/common areas, perhaps filling a gap in hospital patient safety. The keys to the early success of implementation of our RRT were multidisciplinary input and improvements made in real time. The long‐term effects of the RRT on the culture of patient safety in our institution and throughout the United States remain to be seen but are promising.
Medical emergency teams (METs) were introduced more than a decade ago in Australia and the United Kingdom to rapidly identify and manage seriously ill patients at risk of cardiopulmonary arrest and other high‐risk conditions.1 METs, known in the United States as rapid response teams (RRTs), have been slow to be adopted thus far but are quickly gaining ground. Despite numerous studies indicating long‐term patient outcomes are poor following cardiac resuscitation in the hospital, the benefits of early intervention have sometimes been overlooked.25 Several observational studies and a retrospective analysis that included the Medical Emergency Response Improvement Team (MERIT) in Pittsburgh showed that introduction of a MET apparently has the potential to decrease the incidence of unanticipated intensive care unit (ICU) admissions and in‐hospital morbidity and mortality from unexpected cardiopulmonary arrest.69 Furthermore, the use of a MET as a quality improvement tool to detect medical errors and effect systemwide interventions is promising.10 Most recently, the Institute for Healthcare Improvement (IHI) and the American Hospital Association challenged health care organizations to redesign patient safety systems to prevent avoidable deaths in its 100K Lives Campaign. One of the 6 proposed core interventions was the deployment of rapid response teams at the first sign of patient decline.11
Despite these reports of success, a recent large cluster‐randomized controlled trial did not yield the same positive results. In this well‐designed study of 23 Australian hospitals, the Medical Early Response, Intervention and Therapy (MERIT) study investigators found the incidence of cardiac arrest, unplanned ICU admissions, and unexpected death essentially unchanged despite large increases in how often the emergency team was called.12 One possible explanation why these findings conflicted with previous favorable results is that the ultimate impact of a MET may depend on the effectiveness of implementation strategies. To derive the benefits of a MET/RRT, hospitals must increasingly focus on identifying barriers to implementation and address practical issues that may undermine their long‐term effectiveness.
In this article we describe in detail the process of establishing an RRT at our urban, academic hospital and the modifications that became necessary as we rolled out the intervention and encountered obstacles. This analysis was undertaken as a quality improvement (QI) activity. To our knowledge, this is one of the few recent published descriptions of the experiences of implementing an RRT in the United States since earlier work in Pittsburgh.9, 13
METHODS
Temple University Hospital is a tertiary care academic hospital in urban Philadelphia, Pennsylvania. Our RRT was first implemented July 1, 2004, and in the first 12 months of initiation, it was activated 307 times. The RRT at Temple University Hospital was designed to be accessible 24 hours a day, 7 days a week. The daytime team (8 am‐5 pm) is composed of an attending physician (a hospitalist trained as a general internist), a senior internal medicine resident, a critical care nurse, a nurse manager, a pharmacist, and a respiratory therapist. In addition, both a transporter and a member of the admissions office respond to all rapid response team calls but do not get clinically involved in patient care. For nighttime (5 pm‐8 am) and weekend coverage the hospitalist is replaced by an on‐site pulmonary critical care physician, but the remainder of the team is unchanged. All RRT members carry beepers synchronized to provide the location of an RRT activation. In addition, all RRT calls are simultaneously announced on the overhead paging system. No changes were made to the existing cardiac arrest team (code team) at the hospital, which remained a 24‐hour response team for patients found to be in true cardiopulmonary arrest and was comprised of on‐call internal medicine house staff (but no hospitalist attending physician), a respiratory therapist, a pharmacist, a critical care nurse, a nurse manager, and, most notably, an anesthesiologist for emergent intubation and airway management.
The RRT was intended for use within the physical confines of Temple University Hospital and its immediately adjacent grounds. Within the hospital the main locations defined were: inpatient areas, including patient rooms and hallways of the medical‐surgical units of the inpatient tower, as well as the burn, coronary, medical, neurological, neurosurgical, and surgical intensive care units; off‐unit/procedural areas, including diagnostic/emnterventional radiology, the gastroenterology endoscopy suite, the pulmonary procedure suite and pulmonary function lab, the cardiac catheterization/ECHO/stress Lab, the inpatient dialysis unit, and the physical therapy gym, all areas where inpatients are routinely transported during their hospital admission for workup/treatment and where outpatients go for scheduled procedures and therapies; and outpatient/common areas, including all the general medical and subspecialty outpatient clinics in 2 separate outpatient towers (Outpatient Building and Parkinson Pavilion) with direct access from the main hospital building, the outpatient pharmacy, the elevators, the hallways in the outpatient sections of the hospital, all lobbies, and the immediately adjacent outside grounds.
Prior to the launch date of the RRT, clinical criteria were established to help guide staff about when an RRT might be called (Fig. 1). These were based in part on early literature on the clinical markers that most often precede clinical deterioration.14, 15 In addition, 2 much broader categories for RRT activation were added (Inability to reach the patient's primary team of treating physicians for any of the above and Any potentially serious medical errors or adverse events) in order to minimize the need for a very specific physiologic definition to be met in order to activate the team. Physicians, nurses, and other staff with significant daily contact with inpatients and outpatients were in‐serviced about the purpose of the RRT and how to activate the system via the hospital paging operator. Laminated cards with RRT criteria were distributed to all hospital personnel, and educational posters were displayed prominently throughout the hospital.

Each RRT event was to be assessed by team members using a standardized evaluation form (Fig. 2), with primary responsibility going to the physician team leader. In the initial phases of implementation, these forms were kept in the offices of the Section of Hospital Medicine for the use of hospitalist attending physician team leaders. Later on in the year they were kept in the pharmacist's RRT medication bag. These forms were collected at the completion of each RRT event or faxed to a central location and then entered into a database maintained by the hospital's Department of Patient Safety Operations. Weekly debriefing meetings to review all RRT events from the preceding week were attended by representatives from patient safety, respiratory, nursing, hospital medicine, and the pharmacy. Attempts were made to identify the issues that led to selected RRT activations, to obtain patient follow‐up from the clinical event, and to evaluate the performance of the team. Throughout these weekly meetings, QI strategies for improving the effectiveness of the RRT were identified and implemented.

The core outcome measures that were used to assess RRT performance were: appropriateness of the RRT activation, percentage of patients who were stabilized, percentage of patients who were transferred to a higher level of care, and overall team performance.
In the weekly meeting of the RRT evaluation committee, at which each RRT was reviewed by the clinical team, each scenario and details of the event were reviewed to determine whether the RRT activation was appropriate, whether the intervention was successful, and whether there were any issues with the team performance. After a thorough discussion of each case and review of additional data from the chart if necessary, the RRT evaluation committee reached a consensus about each of these measures.
We also tracked the number of code team activations from the year preceding establishment of the RRT (2003‐2004) through the year during which the RRT was established (2004‐2005). Because all calls for both the RRT and the code team go first to the hospital operator, we reviewed the hospital paging operators' logs for the entire 12‐month period to track the rate of code team events to RRT events on a monthly basis.
RESULTS
In a 12‐month period, the RRT was activated 307 times, as recorded in the hospital operator logs. In the year preceding inception of the RRT, there were 272 code team activations. In the first 12 months concurrent with RRT implementation, the code team was activated 258 times. Overall, at their discretion the team leaders converted 13% of the 307 RRT activations to traditional code team activations.
There were 11 RRT activations in July, the first month of implementation, and 14 activations in the second month. At that point, the internal hospital newsletter released a feature on the new RRT, and our patient safety officer/director of patient safety operations made a concerted effort to educate hospital administration and the Graduate Medical Education Committee (GMEC); as a result, utilization picked up. From September onward through the remainder of the academic year, an average of 28 RRT activations occurred each month (range 20‐37), whereas an average of 22 codes took place each month (range 12‐27). The numbers of RRT versus code team activations are plotted in Figure 3. A trend line for the number of code team activations per month in 2003, the year prior to implementation of the RRT, was added for comparison; it conveys the slight overall decrease in the number of codes as the RRT took effect (average of 23 codes per month, range 15‐31).

Physician evaluation forms were returned for 170 of the 307 RRT events (55%). The main inpatient tower was the site of 42% of these RRT activations, followed by the outpatient/common areas, where 19% of the activations occurred, and off‐unit/procedural areas, the site of 18%. Table 2 provides information on specific location, reason for call, and disposition of a sample of the RRT activations in the non‐inpatient areas. Time of day was noted in 76.8% of events. Of these, 82.9% occurred during the traditional day shift (7 am‐7 pm) and 17.1% on night shift (7 pm‐7 am). Most RRT activations occurred between 8 am and 4 pm. Daytime events heavily outnumbered nighttime events regardless of location.
Physician team leaders largely believed a specific underlying clinical diagnosis was responsible for 59% of the RRT activations, followed by adverse drug reactions (3.5%), physician error (1.8%), and nursing error (0.6%). When an underlying clinical diagnosis or organ system was suspected, it was most frequently pulmonary (32%), followed by neurological (14%) and cardiac (11%). It was believed that 32% of events were for other reason not listed. Table 1 provides the breakdown of other underlying diagnoses in RRT events.
| Pulmonary | 32% |
| Hypoxia/Respiratory Distress (32%) | |
| Neurological | 14% |
| Change of mental status (7%) | |
| Syncope (7%) | |
| Cardiac | 11% |
| Hypotension (8%) | |
| Arrhythmia (2%) | |
| Hypertension (1%) | |
| Hematologic | 2% |
| Bleeding (2%) | |
| Endocrine | 1% |
| Hypoglycemia (1%) | |
| Other reason not listed | 32% |
| No reason given | 9% |
| Location | Reason for RRT call | Disposition | |
|---|---|---|---|
| Outpatient clinical | Outpatient orthopedics | Dysrhythmia | ED |
| Outpatient medicine clinic | Hypoxia/respiratory Distress | Stabilized | |
| Outpatient urology | Vomiting | ED | |
| Outpatient Parkinson | Asthma | ED | |
| Outpatient Parkinson | Seizure | ED | |
| Common area/nonclinical | Preadmissions testing | Changed mental status | Unknown |
| Admissions | Changed mental status | Stabilized | |
| Hypoxia/respiratory distress | Stabilized | ||
| Syncope/bradycardia | ED | ||
| Security | Syncope | Improved | |
| Lobby | Hypoxia/respiratory distress | Unknown | |
| Changed mental status | ED | ||
| Hypoxia/respiratory distress | Improved | ||
| Procedures/Off‐unit clinical | Stress test lab | Hypoxia/respiratory distress | Improved |
| Cardiac catheterization lab | Chest pain | ED | |
| Diagnostic imaging | Changed mental status | Improved | |
| Mucus plug in tracheostomy | Improved | ||
| Seizure | ICU | ||
| Syncope | ED | ||
| Hypoxia/respiratory distress | Unknown | ||
| Hypoglycemia | ED | ||
| Dialysis | Bleeding | Stabilized | |
| Gastroenterology procedures | Hypoxia/respiratory distress | ICU | |
| Hypoxia/respiratory distress | Stabilized | ||
| Hypoxia/respiratory distress | ICU | ||
| Interventional radiology | Hypotension/dehydration | Unknown | |
| Hypoxia/respiratory distress | ICU | ||
| Changed mental status | Stabilized | ||
| Hypoxia/Respiratory distress | ICU | ||
| Hypoxia/Respiratory distress | ICU | ||
| Changed mental status | ED | ||
| Hypoxia/Respiratory distress | ICU | ||
| MRI | Hypoxia/Respiratory distress | ED | |
| Hypoxia/respiratory distress | ED | ||
| Hypoxia/respiratory distress | ED | ||
| Changed mental status | ED | ||
| Occupational therapy | Hypotension | ED | |
| Physical therapy | Hypotension | Stabilized | |
| Physical medicine/rehab | Hypoxia/respiratory distress | Unknown | |
| Short procedure unit | Syncope | Stabilized | |
| Hypotension | ICU |
In the judgment of evaluators, the system was utilized appropriately in 98% of the evaluated events. Eighty‐five percent of RRT activations were believed to have prevented further clinical deterioration, though it was also thought that 3% of patients deteriorated despite the efforts of the team. Disposition of the patient following an RRT event was noted 87% of the time, and it was believed that 88% of the patients were stabilized. Of the formally evaluated RRT events, team members were largely satisfied with the response and the functioning of the team, stating for 68% of the events that the team performed without a problem.
Problems Identified and Addressed During Implementation
Though it was encouraging that those surveyed believed the team performed without a problem in 68% of the activations, another way to look at it is that team performance was inadequate in 32% of the cases. Any issues cited on the evaluation sheets, ranging from delays in arrival of team members to missing/delayed arrival of equipment, were seen as opportunities for improvement. For example, very early on in the implementation process, team leaders specifically noted repeatedly encountering a diagnosis of suspected hypoglycemia in patients with a known history of diabetes found with altered mental status. Early clinical assessments by the RRT were severely limited and judged problematic without a simple way to objectively rule out this possibility and/or to attempt immediate treatment, especially because this frequently occurred in non‐inpatient settings. Team members suggested and quickly obtained approval to carry both glucometers and glucose tablets and Glucagon in the pharmacist's fanny pack. In another case, our respiratory therapists arrived promptly to the scene of an RRT call for shortness of breath but were hampered by lack of readily available oxygen tanks. This was promptly remedied, at the recommendation of the committee, by placing additional oxygen tanks near all hospital security stations. Placement of code (crash) carts has also been modified to increase accessibility, especially in nonclinical areas, where delays were perceived to have contributed to poor outcomes. In the future, alphanumeric pagers will be used to allow for more specific and efficient deployment of the team.
Other changes that have been made include the addition to respiratory/pharmacy fanny packs of other key medications such as lorazepam for seizures, equipment such as peripheral catheters for intravenous access, and syringes/needles. It is hoped that in the near future, a state‐of‐the‐art point‐of‐care blood‐testing device, I‐stat, capable of quickly analyzing a blood sample for basic stat lab tests will be added to the pack to expedite triage.16 Perhaps most important, the committee reached a consensus that to improve and encourage real‐time evaluations, it might be best to have the RRT evaluation forms and other paperwork at the point of care to increase yield. The pharmacist now carries blank forms in the fanny pack for convenience. Early on in our RRT implementation process, all these items were noted to be lacking at various times and were requested by team leaders, nurses, and pharmacists in order to be better prepared for various clinical scenarios. In addition, ongoing analysis of the most common RRT diagnoses in the database guided our final decisions in order to keep the size of the fanny pack down to a minimum while providing crucial equipment.
DISCUSSION
We have found the RRT to be an effective but challenging‐to‐implement QI intervention to increase patient safety at our academic institution. The Australian MERIT investigators recently suggested that despite growing evidence of the benefits of MET/RRT systems, long‐term success may depend most on effective implementation strategies.12 We experienced firsthand these challenges in the first year of our new RRT system.
Large system changes in a hospital are especially fraught with danger because of the unique aspects of health care delivery systems. As Reid commented in an editorial about the emerging use of the MET system in the United Kingdom, Despite potential advantages to patients, ensuring appropriate utilization was difficult because of cultural barriers. Traditional hierarchical behaviors that dictate how doctors and nurses react and work got in the way of people calling these life saving teams.17
Our weekly multidisciplinary RRT debriefings were the most crucial component of our implementation strategy. Many latent systems issues were uncovered, as well as more subtle problems such as lack of coordination of care, communication errors, gaps in patient handoffs or sign‐out. Previous studies by the Pittsburgh MERIT team have validated such retrospective categorization of errors uncovered by MET responses.10
However, neither that group nor the Australian MERIT study investigators specifically addressed the importance of the feedback process in RRT implementation. A strength of our system is that modifications to the RRT are made prospectively and in real time based on feedback from active RRT members during debriefing. In fact, the success of our RRT underscores the importance of open communication among hospitalists, house staff, nurses, pharmacists, and ancillary staff in multidisciplinary patient safety and QI endeavors. Everything from the responsibilities of team members to equipment evolved over the 12‐month period in order to improve the function and effectiveness of the team and was almost entirely based on feedback from the RRT doctors and nurses on the front lines. Suggestions from the evaluation forms were given serious consideration at every RRT evaluation committee debriefing. By optimizing the efficient operation of the RRT, we hope to continue to improve outcomes.
We believe a key to the success of our debriefing process was the constant attendance of our patient safety officer/chief medical officer and director of patient safety operations, who both encouraged active participation. Early on in the process, comments were made principally by physician and critical care nurse RRT members, and the dynamic was a bit one‐sided. However, we quickly saw a noticeable and sustained increase in participation by pharmacists and respiratory therapists, and by year's end, they had offered some of the most valuable practical suggestions, which resulted in a more efficient response. As the year went on and real changes were made quickly, all groups were much more vocal and willing to bounce ideas around the room, and the team dynamic and spirit of the group effort improved substantially.
Previous studies have focused on the impact of METs/RRTs on the rate of inpatient cardiac arrests. However, we found that nearly as many RRT events occurred off the inpatient units, for instance, when admitted patients were transported to other areas such as radiology, procedural suites, physical therapy, or dialysis and when scheduled outpatients arrived for their appointments. In addition, a large number of RRT calls came from outpatient departments and common areas of the hospital such as lobbies, hallways, and waiting rooms, mostly involving outpatients and visitors, but not infrequently hospital employees were involved as well. This unexpected and, to our knowledge, previously unreported finding is mirrored in the distribution of RRT activations throughout the course of the day. Most events occurred during the traditional day shift of 7 am‐7 pm, and were heavily clustered between 8 am and 4 pm. In most American hospitals, these are the hours during which outpatients and visitors make up a significant proportion of the hospital population and during which most elective procedures on inpatients occur. Prior to the introduction of our RRT, no specific system was in place for emergent triage, assessment, and expedited treatment of off‐unit patients, outpatients, and visitors. Most often, the code team was mobilized, sometimes taking them to remote locations and making them unavailable for true inpatient cardiopulmonary arrests. Our RRT seems to have the potential to fill a much‐needed gap in patient safety, offering off‐unit patients, outpatients, and visitors a safety net while in our hospital. No prior descriptions of RRT or MET implementation have touched on this area. It would be interesting to see if other hospitals with RRTs have had a similar experience in order to determine whether having an RRT dedicated specifically to the outpatient and common areas of the hospital might provide even more targeted efforts and efficient response times. Thus, the benefits of our RRT seemed to extend beyond a simple reduction in the number of in‐hospital cardiopulmonary arrests and into an unanticipated patient safety black hole.
Implementation of the RRT specifically in academic medical centers has been limited to date. In our opinion, the academic environment is an ideal area for RRTs (because the most critically ill patients often are cared for on teaching services by junior house officers), but it is also a challenging arena in which to make change (because of the complex hierarchy of teaching hospitals). We chose to have an attending physician lead our RRT efforts for the most part. However, residents always participated, and not infrequently led, as key team members. As a commentator on the Australian RRT system pointed out, it is important that junior medical staff [feel empowered] to call for immediate assistance when they are concerned about their patient, but may not have the experience, knowledge, confidence or skills necessary to manage them appropriately.18 We believe that the RRT serves as a valuable educational forum for resident education. Academic centers that develop RRTs must work to integrate the teams into an educational context while simultaneously providing patients with the most experienced and knowledgeable clinical team to address their needs at a time when appropriate clinical decision making is critical. Therefore, the residents who participate in our RRT are formally evaluated by the hospitalists using a standard program evaluation form that encompasses the Accreditation Council for Graduate Medical Education (ACGME) core competencies.19
Through the first year of our RRT system and beyond, activation of the code team and RRT shifted as more RRT activations were recorded and fewer codes were called. Concerted educational efforts and reinforcement of the criteria for calling the RRT had a definite sustained impact of helping staff to become comfortable with using the system. At our institution, it has been difficult to definitively conclude whether RRT calls prevented codes or merely substituted for them at times, especially because 13% of all RRT activations were subsequently converted to code team calls. The Australian MERIT study investigators, despite an excellent study design of a large multicenter trial, also were unable to demonstrate a true decrease in the cardiac arrest rate.12 Much more significant to us, especially in the first year of implementation, was learning that the vast majority of physician RRT leaders perceived activation of the team to occur appropriately and to play a role in preventing clinical deterioration of patients. None of the other RRT or MET implementation studies that we reviewed commented specifically on these areas. It will be interesting to continue to follow these trends, as we expect the use of RRTs to become even more defined. Over time, we will no doubt be better able to determine whether RRTs have a true, sustained impact on preventing patient deterioration and inpatient cardiopulmonary arrests while maintaining a high rate of physician satisfaction that the team is being activated for legitimate reasons.
Our descriptive study had some limitations. The number of RRT evaluations received, while adequate for preliminary analysis, may not accurately represent the 307 activations of the system that occurred in the first 12 months. We suspect that this underreporting, especially in the first half of the year, was in large part a result of relying on team leaders to voluntarily return data forms at the conclusion of each RRT event. RRT evaluations in the second half of the year were more actively distributed at the point of care to the team leader directly by the pharmacist and were more diligently followed up on. Forms are now readily available in the team pharmacist's fanny pack, which was done because of quality improvement feedback from physicians at a debriefing meeting. Since those interventions, there has been a dramatic improvement in the capture of event data and the timely submission of forms. We expect and have demanded close to a 100% return of the forms in the second year of our RRT system, which will vastly improve our analysis. We were also surprised that despite the comprehensiveness of our RRT activation criteria, 32% of physicians were unable to find a match with a clinical indication on the list, indicating unanticipated reasons for calling an RRT. We will continually strive to improve the specificity of future data for planning purposes and training initiatives. However, in some way this confirms our belief that RRTs occur for such a wide variety of reasons that they cannot always be limited to the major clinical categories. On a similar note, we regret not adding a specific category under Outcomes on the evaluation form to include the possibility that RRT members might have offered palliative care or changes in code/do not resuscitate (DNR) status to patients or families. Given that our hospital has both a code team and an RRT begs the question of whether mortality rates might be affected if patients who prior to the RRT might have had a full resuscitation effort were made DNR. In the future, this would be an interesting issue to consider in analysis. Carefully categorizing RRT events is critical to continued success. Further work involving formal team skills training for RRT members, including use of the medical school's clinical simulators for mock RRT scenarios, is planned. These sessions are planned to review performance and clinical decision making for the most common scenarios that we have found to be involved in RRT activations. The 307 activations of the RRT in our first year have clearly set us on the path toward defining predictive rules and directed skills training for earlier identification of patient problems. Further outcome analyses of these efforts will be crucial.
CONCLUSIONS
An RRT was successfully introduced into an academic medical center. The team was heavily utilized in the first 12 months after the program was initiated, especially for off‐unit inpatients and those in outpatient/common areas, perhaps filling a gap in hospital patient safety. The keys to the early success of implementation of our RRT were multidisciplinary input and improvements made in real time. The long‐term effects of the RRT on the culture of patient safety in our institution and throughout the United States remain to be seen but are promising.
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,, et al.Quality of cardiopulmonary resuscitation during in‐hospital cardiac arrest.JAMA.2005;293:363–365.
- ,,.In‐hospital cardiopulmonary resuscitation.Medicine.1995;74:163–175.
- ,,, et al.In‐hospital cardiac arrest: survival depends mainly on the effectiveness of the emergency response.Resuscitation.2004;62:291–297.
- ,,.Factors influencing survival after in‐hospital cardiopulmonary resuscitation.Resuscitation.2005;66:317–321.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.Br Med J.2002;324:1–5.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–204.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,,,,.Use of medical emergency team (MET) responses to detect medical errors.Qual Saf Health Care.2004;13:255–259.
- Institute for Healthcare Improvement. 100K Lives Campaign [IHI website]. Available at: http://www.ihi.org/IHI/Programs/campaign. Accessed November 10,2005.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,, et al.Improving the utilization of medical crisis teams (condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,.Developing strategies to prevent in‐hospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22:244–247.
- ,,,,.Clinical Antecedents to In‐Hospital Cardiopulmonary Arrest.Chest.1990;98:1388–1392.
- Abbot Point of Care: Abbot Laboratories Online. Available at: http://www.istat.com/website/www/products/analyzers.htm. Accessed November 10,2005.
- .Developing and implementing organisational practice that delivers better, safer care.Qual Saf Health Care.2004;13:247.
- ,.The medical emergency team: does it really make a difference?Intern Med J.2003;33:511–514.
- Accreditation Council for Graduate Medical Education (ACGME). Program requirements for residency education in internal medicine. Effective July 2003; revised July 1, 2004. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/140pr703_u704.pdf. Accessed February 17,2006.
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,, et al.Quality of cardiopulmonary resuscitation during in‐hospital cardiac arrest.JAMA.2005;293:363–365.
- ,,.In‐hospital cardiopulmonary resuscitation.Medicine.1995;74:163–175.
- ,,, et al.In‐hospital cardiac arrest: survival depends mainly on the effectiveness of the emergency response.Resuscitation.2004;62:291–297.
- ,,.Factors influencing survival after in‐hospital cardiopulmonary resuscitation.Resuscitation.2005;66:317–321.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.Br Med J.2002;324:1–5.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–204.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,,,,.Use of medical emergency team (MET) responses to detect medical errors.Qual Saf Health Care.2004;13:255–259.
- Institute for Healthcare Improvement. 100K Lives Campaign [IHI website]. Available at: http://www.ihi.org/IHI/Programs/campaign. Accessed November 10,2005.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,, et al.Improving the utilization of medical crisis teams (condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,.Developing strategies to prevent in‐hospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22:244–247.
- ,,,,.Clinical Antecedents to In‐Hospital Cardiopulmonary Arrest.Chest.1990;98:1388–1392.
- Abbot Point of Care: Abbot Laboratories Online. Available at: http://www.istat.com/website/www/products/analyzers.htm. Accessed November 10,2005.
- .Developing and implementing organisational practice that delivers better, safer care.Qual Saf Health Care.2004;13:247.
- ,.The medical emergency team: does it really make a difference?Intern Med J.2003;33:511–514.
- Accreditation Council for Graduate Medical Education (ACGME). Program requirements for residency education in internal medicine. Effective July 2003; revised July 1, 2004. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/140pr703_u704.pdf. Accessed February 17,2006.