User login
Anaphylactic Reaction to Bacitracin Ointment
Impact of Order of Application of Moisturizers on Percutaneous Absorption Kinetics: Evaluation of Sequential Application of Moisturizer Lotions and Azelaic Acid Gel 15% Using a Human Skin Model
CDC recommendations expand vaccine indications
Highlights of the 2008 recommendations of the CDC’s Advisory Committee on Immunization Practices (ACIP), detailed in the child and adult immunization schedules in the MMWR in January,1,2 include:
- an expansion of the age groups for whom an annual influenza vaccine is recommended;
- expanded indications for the pneumococcal polysaccharide vaccine;
- 2 new combination vaccines for children; and
- a second rotavirus vaccine, with revised recommendations to accommodate both vaccine products.
School-age children should get flu vaccine
Children and adolescents ages 5 through 18 years are now among those who should receive an annual flu vaccine. Previously, routine vaccination was recommended only for adults and children ages 6 months through 59 months.3
Because of the timing of vaccine purchase, ACIP recognizes that routine vaccination of 5- to 18-year-olds may not be possible in some settings until next year. Family physicians who are unable to fully incorporate this new recommendation in the 2008-2009 flu season should immunize children and adolescents who are at high risk for complications of the flu. Included in that group are 5- to 18-year-olds who are on long-term aspirin therapy; have a chronic pulmonary disease, including asthma, or a cardiovascular, renal, hepatic, hematologic, or metabolic disorder; are immunosuppressed; or have a neurological or musculoskeletal disorder that alters respiratory function or the clearance of respiratory secretions. Children and adolescents who live with others at elevated risk—kids younger than 5 years, adults older than 50 years, or individuals with medical conditions that place them at high risk for severe influenza complications—should also be vaccinated.
Pneumococcal vaccine: New indications, clarifications
Two new groups have been added to the list of people for whom the 23-valent pneumococcal polysaccharide vaccine (PPV23) is recommended: asthma patients and smokers. Smoking poses as great a risk for pneumococcal pneumonia as diabetes and other chronic illnesses that had already been noted as indications for the vaccine. The number needed to vaccinate to prevent 1 case of pneumonia in smokers is 10,000 for those between the ages of 18 to 44 years, and 4000 for those ages 45 to 64 years.
A second dose. Also in 2008, ACIP clarified its dosing recommendations for PPV23: A second dose, given 5 years after the first, is recommended for those with immune suppression, sickle cell disease, or asplenia. Individuals who are 65 years of age or older should receive a second dose if they were vaccinated 5 or more years ago and were younger than 65 at the time of primary vaccination.
Not for all Native Americans. The recommendation for the use of PPV23 among the Native American population has changed, too.
Research showing high rates of invasive pneumococcal disease in Native American communities has been performed in only a few locations and cannot be generalized to all Native Americans. Therefore, ACIP has gone from recommending routine use of the vaccine among all Native Americans to a recommendation based on the same risks and age recommendations as the general population and, in communities with high rates of disease, on public health recommendations based on the incidence and epidemiology of disease.
Combination products may mean fewer injections
Two new combination vaccine products—Pentacel4 and Kinrix5—were approved last year. Both can reduce the number of injections required to complete the child immunization recommendations.
Pentacel combines 5 vaccines—diphtheria, tetanus, and pertussis (DTaP), inactivated poliovirus (IPV), and Haemophilus influenzae type b (Hib)—and is licensed for children 6 weeks through 4 years of age. Pentacel has a 4-dose schedule, with vaccine administration at 2, 4, 6, and 15 to 18 months of age. Technically, this 4-dose schedule would fulfill requirements for 4 doses of IPV. However, this could conflict with a state school immunization schedule that requires the last dose of IPV vaccine to be administered when the child is between the ages of 4 and 6 years.6
TABLE
Rotavirus vaccines: An administration guide
| ROTATEQ | ROTARIX | |
|---|---|---|
| No. of doses | 3 | 2 |
| Recommended dosing schedule | 2, 4, and 6 mo of age | 2 and 4 mo of age |
| First dose | 6–14 wk 6 d of age | |
| Dosing interval | ≥4 wk | |
| Final dose | ≤8 mo of age | |
| Source: Centers for Disease Control and Prevention. 2009.1 | ||
Kinrix contains DTaP and IPV. The vaccine is indicated for use as the fifth dose of DTaP and the fourth dose of IPV in children 4 through 6 years of age, following a primary series using Infanrix (DTaP) and Pediarix (DTaP, hepatitis B, and IPV).
Rotavirus vaccines: Now there are 2
There are now 2 licensed rotavirus vaccines: RotaTeq was approved in 2006,7 and Rotarix in 2008.8 ACIP does not express a preference for either product, but has revised its recommendations for rotavirus vaccination to accommodate the new release. Both RotaTeq and Rotarix are live oral vaccines, but they differ in composition and schedule of administration. Rotarix should not be given to infants who are allergic to latex, as its oral applicator contains latex rubber.
Dosing requirements. RotaTeq is administered in a 3-dose series at ages 2, 4, and 6 months; Rotarix is given in a 2-dose series at 2 and 4 months of age (TABLE). The first dose of either vaccine should be administered to children between the ages of 6 weeks and 14 weeks, 6 days. (Previously, 12 weeks was the maximum age for the first dose of rotavirus vaccine.) Neither vaccine series should be initiated in infants who are 15 weeks of age or older. The minimum interval between doses is 4 weeks, and the final dose should be administered by the age of 8 months.
It is best to complete the vaccine series with the same product. If the vaccine used initially is not available, the series can be completed with the other product, but the different number of doses required must be considered. If any dose in the series was RotaTeq or you are unable to determine which rotavirus vaccine was administered previously, a total of 3 doses should be given.
HPV and meningococcal vaccine clarification
Human papilloma virus vaccine. The HPV vaccine is recommended for all females ages 11 through 26 years, but ACIP has indicated that girls as young as 9 years may be vaccinated.1
Three doses are required, with the second and third doses administered 2 and 6 months after the first. Because some providers had been administering the third dose at month 4, ACIP issued a clarification in 2008, noting that there should be a minimum of 24 weeks between the first and third dose.
MCV and MPSV. Meningococcal conjugate vaccine (MCV) is preferred over meningococcal polysaccharide vaccine (MPSV) for those 55 years of age or younger, although MPSV is an acceptable alternative. ACIP clarified recommendations for revaccination, as follows:
Individuals ages 11 to 55 years who were vaccinated with MPSV should consider revaccination with MCV after 5 years, if the risk of meningococcal meningitis persists. Children ages 2 to 10 years should be revaccinated with MCV 3 years after receiving MPSV.
1. Centers for Disease Control and Prevention (CDC). Recommended immunization schedules for persons aged 0 through 18 years—United States, 2009. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5751a5.htm. Accessed January 20, 2009.
2. CDC. Recommended adult immunization schedule—United States, 2009. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5753a6.htm. Accessed January 20, 2009.
3. CDC. Recommended immunization schedules for persons aged 0-18 years—United States, 2008. http://cdc.gov/mmwr/preview/mmwrhtml/mm5701a8.htm. Accessed January 19, 2009.
4. US Food and Drug Administration (FDA) Product approval information [memorandum]. Pentacel: recommendations regarding request for partial waiver of pediatric studies. April 25, 2008. http://www.fda.gov/CBER/products/pentacel/pentacel042508mem.htm. Accessed January 27, 2009.
5. FDA Product approval information [approval letter]. Kinrix. June 24, 2008. http://www.fda.gov/cber/approvltr/kinrix062408L.htm. Accessed January 27, 2009.
6. Immunization Action Coalition State information. State mandates on immunization and vaccine-preventable diseases. Polio: 2005-2006 requirements for kindergarten. http://www.immunize.org/laws/polio_kinder.pdf. Accessed February 3, 2009.
7. FDA. FDA approves new vaccine to prevent rotavirus gastroenteritis in infants. February 3, 2006. http://www.fda.gov/bbs/topics/news/2006/NEW01307.html. Accessed January 19, 2009.
8. FDA. FDA approves new vaccine to prevent gastroenteritis caused by rotavirus. April 3, 2008. http://www.fda.gov/bbs/topics/NEWS/2008/NEW01814.html. Accessed January 28, 2009.
Highlights of the 2008 recommendations of the CDC’s Advisory Committee on Immunization Practices (ACIP), detailed in the child and adult immunization schedules in the MMWR in January,1,2 include:
- an expansion of the age groups for whom an annual influenza vaccine is recommended;
- expanded indications for the pneumococcal polysaccharide vaccine;
- 2 new combination vaccines for children; and
- a second rotavirus vaccine, with revised recommendations to accommodate both vaccine products.
School-age children should get flu vaccine
Children and adolescents ages 5 through 18 years are now among those who should receive an annual flu vaccine. Previously, routine vaccination was recommended only for adults and children ages 6 months through 59 months.3
Because of the timing of vaccine purchase, ACIP recognizes that routine vaccination of 5- to 18-year-olds may not be possible in some settings until next year. Family physicians who are unable to fully incorporate this new recommendation in the 2008-2009 flu season should immunize children and adolescents who are at high risk for complications of the flu. Included in that group are 5- to 18-year-olds who are on long-term aspirin therapy; have a chronic pulmonary disease, including asthma, or a cardiovascular, renal, hepatic, hematologic, or metabolic disorder; are immunosuppressed; or have a neurological or musculoskeletal disorder that alters respiratory function or the clearance of respiratory secretions. Children and adolescents who live with others at elevated risk—kids younger than 5 years, adults older than 50 years, or individuals with medical conditions that place them at high risk for severe influenza complications—should also be vaccinated.
Pneumococcal vaccine: New indications, clarifications
Two new groups have been added to the list of people for whom the 23-valent pneumococcal polysaccharide vaccine (PPV23) is recommended: asthma patients and smokers. Smoking poses as great a risk for pneumococcal pneumonia as diabetes and other chronic illnesses that had already been noted as indications for the vaccine. The number needed to vaccinate to prevent 1 case of pneumonia in smokers is 10,000 for those between the ages of 18 to 44 years, and 4000 for those ages 45 to 64 years.
A second dose. Also in 2008, ACIP clarified its dosing recommendations for PPV23: A second dose, given 5 years after the first, is recommended for those with immune suppression, sickle cell disease, or asplenia. Individuals who are 65 years of age or older should receive a second dose if they were vaccinated 5 or more years ago and were younger than 65 at the time of primary vaccination.
Not for all Native Americans. The recommendation for the use of PPV23 among the Native American population has changed, too.
Research showing high rates of invasive pneumococcal disease in Native American communities has been performed in only a few locations and cannot be generalized to all Native Americans. Therefore, ACIP has gone from recommending routine use of the vaccine among all Native Americans to a recommendation based on the same risks and age recommendations as the general population and, in communities with high rates of disease, on public health recommendations based on the incidence and epidemiology of disease.
Combination products may mean fewer injections
Two new combination vaccine products—Pentacel4 and Kinrix5—were approved last year. Both can reduce the number of injections required to complete the child immunization recommendations.
Pentacel combines 5 vaccines—diphtheria, tetanus, and pertussis (DTaP), inactivated poliovirus (IPV), and Haemophilus influenzae type b (Hib)—and is licensed for children 6 weeks through 4 years of age. Pentacel has a 4-dose schedule, with vaccine administration at 2, 4, 6, and 15 to 18 months of age. Technically, this 4-dose schedule would fulfill requirements for 4 doses of IPV. However, this could conflict with a state school immunization schedule that requires the last dose of IPV vaccine to be administered when the child is between the ages of 4 and 6 years.6
TABLE
Rotavirus vaccines: An administration guide
| ROTATEQ | ROTARIX | |
|---|---|---|
| No. of doses | 3 | 2 |
| Recommended dosing schedule | 2, 4, and 6 mo of age | 2 and 4 mo of age |
| First dose | 6–14 wk 6 d of age | |
| Dosing interval | ≥4 wk | |
| Final dose | ≤8 mo of age | |
| Source: Centers for Disease Control and Prevention. 2009.1 | ||
Kinrix contains DTaP and IPV. The vaccine is indicated for use as the fifth dose of DTaP and the fourth dose of IPV in children 4 through 6 years of age, following a primary series using Infanrix (DTaP) and Pediarix (DTaP, hepatitis B, and IPV).
Rotavirus vaccines: Now there are 2
There are now 2 licensed rotavirus vaccines: RotaTeq was approved in 2006,7 and Rotarix in 2008.8 ACIP does not express a preference for either product, but has revised its recommendations for rotavirus vaccination to accommodate the new release. Both RotaTeq and Rotarix are live oral vaccines, but they differ in composition and schedule of administration. Rotarix should not be given to infants who are allergic to latex, as its oral applicator contains latex rubber.
Dosing requirements. RotaTeq is administered in a 3-dose series at ages 2, 4, and 6 months; Rotarix is given in a 2-dose series at 2 and 4 months of age (TABLE). The first dose of either vaccine should be administered to children between the ages of 6 weeks and 14 weeks, 6 days. (Previously, 12 weeks was the maximum age for the first dose of rotavirus vaccine.) Neither vaccine series should be initiated in infants who are 15 weeks of age or older. The minimum interval between doses is 4 weeks, and the final dose should be administered by the age of 8 months.
It is best to complete the vaccine series with the same product. If the vaccine used initially is not available, the series can be completed with the other product, but the different number of doses required must be considered. If any dose in the series was RotaTeq or you are unable to determine which rotavirus vaccine was administered previously, a total of 3 doses should be given.
HPV and meningococcal vaccine clarification
Human papilloma virus vaccine. The HPV vaccine is recommended for all females ages 11 through 26 years, but ACIP has indicated that girls as young as 9 years may be vaccinated.1
Three doses are required, with the second and third doses administered 2 and 6 months after the first. Because some providers had been administering the third dose at month 4, ACIP issued a clarification in 2008, noting that there should be a minimum of 24 weeks between the first and third dose.
MCV and MPSV. Meningococcal conjugate vaccine (MCV) is preferred over meningococcal polysaccharide vaccine (MPSV) for those 55 years of age or younger, although MPSV is an acceptable alternative. ACIP clarified recommendations for revaccination, as follows:
Individuals ages 11 to 55 years who were vaccinated with MPSV should consider revaccination with MCV after 5 years, if the risk of meningococcal meningitis persists. Children ages 2 to 10 years should be revaccinated with MCV 3 years after receiving MPSV.
Highlights of the 2008 recommendations of the CDC’s Advisory Committee on Immunization Practices (ACIP), detailed in the child and adult immunization schedules in the MMWR in January,1,2 include:
- an expansion of the age groups for whom an annual influenza vaccine is recommended;
- expanded indications for the pneumococcal polysaccharide vaccine;
- 2 new combination vaccines for children; and
- a second rotavirus vaccine, with revised recommendations to accommodate both vaccine products.
School-age children should get flu vaccine
Children and adolescents ages 5 through 18 years are now among those who should receive an annual flu vaccine. Previously, routine vaccination was recommended only for adults and children ages 6 months through 59 months.3
Because of the timing of vaccine purchase, ACIP recognizes that routine vaccination of 5- to 18-year-olds may not be possible in some settings until next year. Family physicians who are unable to fully incorporate this new recommendation in the 2008-2009 flu season should immunize children and adolescents who are at high risk for complications of the flu. Included in that group are 5- to 18-year-olds who are on long-term aspirin therapy; have a chronic pulmonary disease, including asthma, or a cardiovascular, renal, hepatic, hematologic, or metabolic disorder; are immunosuppressed; or have a neurological or musculoskeletal disorder that alters respiratory function or the clearance of respiratory secretions. Children and adolescents who live with others at elevated risk—kids younger than 5 years, adults older than 50 years, or individuals with medical conditions that place them at high risk for severe influenza complications—should also be vaccinated.
Pneumococcal vaccine: New indications, clarifications
Two new groups have been added to the list of people for whom the 23-valent pneumococcal polysaccharide vaccine (PPV23) is recommended: asthma patients and smokers. Smoking poses as great a risk for pneumococcal pneumonia as diabetes and other chronic illnesses that had already been noted as indications for the vaccine. The number needed to vaccinate to prevent 1 case of pneumonia in smokers is 10,000 for those between the ages of 18 to 44 years, and 4000 for those ages 45 to 64 years.
A second dose. Also in 2008, ACIP clarified its dosing recommendations for PPV23: A second dose, given 5 years after the first, is recommended for those with immune suppression, sickle cell disease, or asplenia. Individuals who are 65 years of age or older should receive a second dose if they were vaccinated 5 or more years ago and were younger than 65 at the time of primary vaccination.
Not for all Native Americans. The recommendation for the use of PPV23 among the Native American population has changed, too.
Research showing high rates of invasive pneumococcal disease in Native American communities has been performed in only a few locations and cannot be generalized to all Native Americans. Therefore, ACIP has gone from recommending routine use of the vaccine among all Native Americans to a recommendation based on the same risks and age recommendations as the general population and, in communities with high rates of disease, on public health recommendations based on the incidence and epidemiology of disease.
Combination products may mean fewer injections
Two new combination vaccine products—Pentacel4 and Kinrix5—were approved last year. Both can reduce the number of injections required to complete the child immunization recommendations.
Pentacel combines 5 vaccines—diphtheria, tetanus, and pertussis (DTaP), inactivated poliovirus (IPV), and Haemophilus influenzae type b (Hib)—and is licensed for children 6 weeks through 4 years of age. Pentacel has a 4-dose schedule, with vaccine administration at 2, 4, 6, and 15 to 18 months of age. Technically, this 4-dose schedule would fulfill requirements for 4 doses of IPV. However, this could conflict with a state school immunization schedule that requires the last dose of IPV vaccine to be administered when the child is between the ages of 4 and 6 years.6
TABLE
Rotavirus vaccines: An administration guide
| ROTATEQ | ROTARIX | |
|---|---|---|
| No. of doses | 3 | 2 |
| Recommended dosing schedule | 2, 4, and 6 mo of age | 2 and 4 mo of age |
| First dose | 6–14 wk 6 d of age | |
| Dosing interval | ≥4 wk | |
| Final dose | ≤8 mo of age | |
| Source: Centers for Disease Control and Prevention. 2009.1 | ||
Kinrix contains DTaP and IPV. The vaccine is indicated for use as the fifth dose of DTaP and the fourth dose of IPV in children 4 through 6 years of age, following a primary series using Infanrix (DTaP) and Pediarix (DTaP, hepatitis B, and IPV).
Rotavirus vaccines: Now there are 2
There are now 2 licensed rotavirus vaccines: RotaTeq was approved in 2006,7 and Rotarix in 2008.8 ACIP does not express a preference for either product, but has revised its recommendations for rotavirus vaccination to accommodate the new release. Both RotaTeq and Rotarix are live oral vaccines, but they differ in composition and schedule of administration. Rotarix should not be given to infants who are allergic to latex, as its oral applicator contains latex rubber.
Dosing requirements. RotaTeq is administered in a 3-dose series at ages 2, 4, and 6 months; Rotarix is given in a 2-dose series at 2 and 4 months of age (TABLE). The first dose of either vaccine should be administered to children between the ages of 6 weeks and 14 weeks, 6 days. (Previously, 12 weeks was the maximum age for the first dose of rotavirus vaccine.) Neither vaccine series should be initiated in infants who are 15 weeks of age or older. The minimum interval between doses is 4 weeks, and the final dose should be administered by the age of 8 months.
It is best to complete the vaccine series with the same product. If the vaccine used initially is not available, the series can be completed with the other product, but the different number of doses required must be considered. If any dose in the series was RotaTeq or you are unable to determine which rotavirus vaccine was administered previously, a total of 3 doses should be given.
HPV and meningococcal vaccine clarification
Human papilloma virus vaccine. The HPV vaccine is recommended for all females ages 11 through 26 years, but ACIP has indicated that girls as young as 9 years may be vaccinated.1
Three doses are required, with the second and third doses administered 2 and 6 months after the first. Because some providers had been administering the third dose at month 4, ACIP issued a clarification in 2008, noting that there should be a minimum of 24 weeks between the first and third dose.
MCV and MPSV. Meningococcal conjugate vaccine (MCV) is preferred over meningococcal polysaccharide vaccine (MPSV) for those 55 years of age or younger, although MPSV is an acceptable alternative. ACIP clarified recommendations for revaccination, as follows:
Individuals ages 11 to 55 years who were vaccinated with MPSV should consider revaccination with MCV after 5 years, if the risk of meningococcal meningitis persists. Children ages 2 to 10 years should be revaccinated with MCV 3 years after receiving MPSV.
1. Centers for Disease Control and Prevention (CDC). Recommended immunization schedules for persons aged 0 through 18 years—United States, 2009. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5751a5.htm. Accessed January 20, 2009.
2. CDC. Recommended adult immunization schedule—United States, 2009. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5753a6.htm. Accessed January 20, 2009.
3. CDC. Recommended immunization schedules for persons aged 0-18 years—United States, 2008. http://cdc.gov/mmwr/preview/mmwrhtml/mm5701a8.htm. Accessed January 19, 2009.
4. US Food and Drug Administration (FDA) Product approval information [memorandum]. Pentacel: recommendations regarding request for partial waiver of pediatric studies. April 25, 2008. http://www.fda.gov/CBER/products/pentacel/pentacel042508mem.htm. Accessed January 27, 2009.
5. FDA Product approval information [approval letter]. Kinrix. June 24, 2008. http://www.fda.gov/cber/approvltr/kinrix062408L.htm. Accessed January 27, 2009.
6. Immunization Action Coalition State information. State mandates on immunization and vaccine-preventable diseases. Polio: 2005-2006 requirements for kindergarten. http://www.immunize.org/laws/polio_kinder.pdf. Accessed February 3, 2009.
7. FDA. FDA approves new vaccine to prevent rotavirus gastroenteritis in infants. February 3, 2006. http://www.fda.gov/bbs/topics/news/2006/NEW01307.html. Accessed January 19, 2009.
8. FDA. FDA approves new vaccine to prevent gastroenteritis caused by rotavirus. April 3, 2008. http://www.fda.gov/bbs/topics/NEWS/2008/NEW01814.html. Accessed January 28, 2009.
1. Centers for Disease Control and Prevention (CDC). Recommended immunization schedules for persons aged 0 through 18 years—United States, 2009. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5751a5.htm. Accessed January 20, 2009.
2. CDC. Recommended adult immunization schedule—United States, 2009. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5753a6.htm. Accessed January 20, 2009.
3. CDC. Recommended immunization schedules for persons aged 0-18 years—United States, 2008. http://cdc.gov/mmwr/preview/mmwrhtml/mm5701a8.htm. Accessed January 19, 2009.
4. US Food and Drug Administration (FDA) Product approval information [memorandum]. Pentacel: recommendations regarding request for partial waiver of pediatric studies. April 25, 2008. http://www.fda.gov/CBER/products/pentacel/pentacel042508mem.htm. Accessed January 27, 2009.
5. FDA Product approval information [approval letter]. Kinrix. June 24, 2008. http://www.fda.gov/cber/approvltr/kinrix062408L.htm. Accessed January 27, 2009.
6. Immunization Action Coalition State information. State mandates on immunization and vaccine-preventable diseases. Polio: 2005-2006 requirements for kindergarten. http://www.immunize.org/laws/polio_kinder.pdf. Accessed February 3, 2009.
7. FDA. FDA approves new vaccine to prevent rotavirus gastroenteritis in infants. February 3, 2006. http://www.fda.gov/bbs/topics/news/2006/NEW01307.html. Accessed January 19, 2009.
8. FDA. FDA approves new vaccine to prevent gastroenteritis caused by rotavirus. April 3, 2008. http://www.fda.gov/bbs/topics/NEWS/2008/NEW01814.html. Accessed January 28, 2009.
Singulair-induced anaphylaxis?
When L.O., an African American boy, was 13 months old, he was taken to the emergency room by his mother for an episode of diffuse expiratory wheezing. The family had a history of asthma. L.O.’s wheezing was effectively treated with albuterol, which was prescribed for use at home. At 17 months, L.O. was diagnosed with eczema and allergy to eggs.
When the boy was 3 years old, his mother brought him to St. Dominic’s Health Clinic in Jamaica, NY, for a well-child visit. She reported that L.O. had experienced only 2 asthma attacks in the past year. We diagnosed mild intermittent asthma and advised the mother to continue using albuterol as needed. The patient returned to the clinic at age 4, with redness and swelling of both eyes typical of allergic conjunctivitis. Four months later L.O. returned with rhinorrhea, which, in conjunction with asthma, eczema, and allergic conjunctivitis, led us to diagnose atopic syndrome. This time, we prescribed 4 mg Singulair (montelukast sodium), to be taken once daily.
Immediately after taking a single Singulair tablet in the afternoon, L.O. developed pruritus. That evening he awoke from his sleep screaming; he had prominent lip, facial, and pedal edema. He also had trouble breathing and had red, blotchy hives over his entire back. His mother was unable to administer epinephrine (EpiPen), which had been prescribed for L.O.’s egg allergy. She called 911 and L.O. was taken to an emergency room. He had tachycardia and a low-grade fever. Epinephrine and diphenhydramine (Benadryl) began to lower his temperature and gradually lessened his edema and urticaria. Upon L.O.’s discharge, his mother was cautioned not to give him any more Singulair.
How common is L.O.’s experience? In a review of the literature, we found just 4 mentions of an anaphylactic response to Singulair treatment. We describe these reports here and discuss the implications.
A drug with few reported side effects
Singulair is a leukotriene receptor antagonist commonly prescribed for the prevention and treatment of asthma and for the treatment of allergic rhinitis. It is an orally active compound that binds with high affinity to the CysLT type-1 receptor, a leukotriene receptor found in a variety of human airway cells, including smooth muscle cells, macrophages, and eosinophils.1 At this receptor, Singulair inhibits the physiologic action of LTD4, a leukotriene released by various inflammatory cells that normally initiates the symptoms of asthma.
Singulair has been shown to dramatically increase forced expiratory volume, decrease usage of inhaled beta-agonists, and improve other asthma-related outcomes in both adults and children. In clinical studies, Singulair has proven safe, with few reported side effects. Some benign adverse events have been associated with this drug when compared with placebo, but causality between these events and Singulair is uncertain. Anaphylaxis was not reported in any of the premarketing clinical studies of Singulair.
4 other accounts of anaphylaxis
Singulair’s package insert mentions anaphylaxis as an adverse reaction reported after the US Food and Drug Administration approved the drug in 1998.1 Merck & Co., producer and distributor of Singulair, did not provide any specific information on reports of anaphylaxis for this review.
The Drug Safety Research Unit, an independent body associated with the University of Portsmouth in England, mentioned just one instance of anaphylaxis in a study of adverse reactions to montelukast among a cohort of more than 15,000 patients.2
A presentation given at a Healthcare Information Management Systems Society conference also briefly mentioned the case of an 8-year-old boy who experienced an anaphylactic reaction to Singulair.3
The only published description of a possible case of anaphylaxis in response to Singulair appeared in a report published by Lareb, the Dutch national pharmacovigilance system.4 A 4-year-old boy suffered facial edema, rash, coughing, and fatigue 2 days after starting montelukast 5 mg daily for asthma. The patient’s age and symptoms were strikingly similar to those of L.O.
Anaphylaxis: Always a possibility
Clearly, anaphylaxis as an adverse reaction to Singulair is rare, with only a handful of cases being reported worldwide. Nevertheless, anaphylaxis is life threatening, and we should be alert to its possibility when prescribing Singulair, especially for patients with a history of atopy.
Correspondence
Adriel Gerard, State University of New York at Buffalo School of Medicine, 99 Gold Street, Apt 1L, Brooklyn, NY 11201; [email protected]
1. Singulair (montelukast sodium) [prescribing information]. Whitehouse Station, NJ: Merck & Co., Inc; 2008.
2. Biswas P, Wilton L, Pearce G, et al. Pharmacosurveillance and safety of the leukotriene receptor antagonist (LTRA), montelukast. Clin Exp All Rev. 2001;3:300-304.
3. Millikan E. XML drug information modeling: linking evidence-based medicine with the bedside. In Proceedings of Health Information Management Systems Society. February 13-17, 2005. Available at: www.himss.org/content/files/2005proceedings/sessions/edu031.pdf. Accessed February 2, 2009.
4. An overview of reports on montelukast. Available at: www.lareb.nl/documents/kwb_2002_3_monte.pdf. Accessed February 2, 2009.
When L.O., an African American boy, was 13 months old, he was taken to the emergency room by his mother for an episode of diffuse expiratory wheezing. The family had a history of asthma. L.O.’s wheezing was effectively treated with albuterol, which was prescribed for use at home. At 17 months, L.O. was diagnosed with eczema and allergy to eggs.
When the boy was 3 years old, his mother brought him to St. Dominic’s Health Clinic in Jamaica, NY, for a well-child visit. She reported that L.O. had experienced only 2 asthma attacks in the past year. We diagnosed mild intermittent asthma and advised the mother to continue using albuterol as needed. The patient returned to the clinic at age 4, with redness and swelling of both eyes typical of allergic conjunctivitis. Four months later L.O. returned with rhinorrhea, which, in conjunction with asthma, eczema, and allergic conjunctivitis, led us to diagnose atopic syndrome. This time, we prescribed 4 mg Singulair (montelukast sodium), to be taken once daily.
Immediately after taking a single Singulair tablet in the afternoon, L.O. developed pruritus. That evening he awoke from his sleep screaming; he had prominent lip, facial, and pedal edema. He also had trouble breathing and had red, blotchy hives over his entire back. His mother was unable to administer epinephrine (EpiPen), which had been prescribed for L.O.’s egg allergy. She called 911 and L.O. was taken to an emergency room. He had tachycardia and a low-grade fever. Epinephrine and diphenhydramine (Benadryl) began to lower his temperature and gradually lessened his edema and urticaria. Upon L.O.’s discharge, his mother was cautioned not to give him any more Singulair.
How common is L.O.’s experience? In a review of the literature, we found just 4 mentions of an anaphylactic response to Singulair treatment. We describe these reports here and discuss the implications.
A drug with few reported side effects
Singulair is a leukotriene receptor antagonist commonly prescribed for the prevention and treatment of asthma and for the treatment of allergic rhinitis. It is an orally active compound that binds with high affinity to the CysLT type-1 receptor, a leukotriene receptor found in a variety of human airway cells, including smooth muscle cells, macrophages, and eosinophils.1 At this receptor, Singulair inhibits the physiologic action of LTD4, a leukotriene released by various inflammatory cells that normally initiates the symptoms of asthma.
Singulair has been shown to dramatically increase forced expiratory volume, decrease usage of inhaled beta-agonists, and improve other asthma-related outcomes in both adults and children. In clinical studies, Singulair has proven safe, with few reported side effects. Some benign adverse events have been associated with this drug when compared with placebo, but causality between these events and Singulair is uncertain. Anaphylaxis was not reported in any of the premarketing clinical studies of Singulair.
4 other accounts of anaphylaxis
Singulair’s package insert mentions anaphylaxis as an adverse reaction reported after the US Food and Drug Administration approved the drug in 1998.1 Merck & Co., producer and distributor of Singulair, did not provide any specific information on reports of anaphylaxis for this review.
The Drug Safety Research Unit, an independent body associated with the University of Portsmouth in England, mentioned just one instance of anaphylaxis in a study of adverse reactions to montelukast among a cohort of more than 15,000 patients.2
A presentation given at a Healthcare Information Management Systems Society conference also briefly mentioned the case of an 8-year-old boy who experienced an anaphylactic reaction to Singulair.3
The only published description of a possible case of anaphylaxis in response to Singulair appeared in a report published by Lareb, the Dutch national pharmacovigilance system.4 A 4-year-old boy suffered facial edema, rash, coughing, and fatigue 2 days after starting montelukast 5 mg daily for asthma. The patient’s age and symptoms were strikingly similar to those of L.O.
Anaphylaxis: Always a possibility
Clearly, anaphylaxis as an adverse reaction to Singulair is rare, with only a handful of cases being reported worldwide. Nevertheless, anaphylaxis is life threatening, and we should be alert to its possibility when prescribing Singulair, especially for patients with a history of atopy.
Correspondence
Adriel Gerard, State University of New York at Buffalo School of Medicine, 99 Gold Street, Apt 1L, Brooklyn, NY 11201; [email protected]
When L.O., an African American boy, was 13 months old, he was taken to the emergency room by his mother for an episode of diffuse expiratory wheezing. The family had a history of asthma. L.O.’s wheezing was effectively treated with albuterol, which was prescribed for use at home. At 17 months, L.O. was diagnosed with eczema and allergy to eggs.
When the boy was 3 years old, his mother brought him to St. Dominic’s Health Clinic in Jamaica, NY, for a well-child visit. She reported that L.O. had experienced only 2 asthma attacks in the past year. We diagnosed mild intermittent asthma and advised the mother to continue using albuterol as needed. The patient returned to the clinic at age 4, with redness and swelling of both eyes typical of allergic conjunctivitis. Four months later L.O. returned with rhinorrhea, which, in conjunction with asthma, eczema, and allergic conjunctivitis, led us to diagnose atopic syndrome. This time, we prescribed 4 mg Singulair (montelukast sodium), to be taken once daily.
Immediately after taking a single Singulair tablet in the afternoon, L.O. developed pruritus. That evening he awoke from his sleep screaming; he had prominent lip, facial, and pedal edema. He also had trouble breathing and had red, blotchy hives over his entire back. His mother was unable to administer epinephrine (EpiPen), which had been prescribed for L.O.’s egg allergy. She called 911 and L.O. was taken to an emergency room. He had tachycardia and a low-grade fever. Epinephrine and diphenhydramine (Benadryl) began to lower his temperature and gradually lessened his edema and urticaria. Upon L.O.’s discharge, his mother was cautioned not to give him any more Singulair.
How common is L.O.’s experience? In a review of the literature, we found just 4 mentions of an anaphylactic response to Singulair treatment. We describe these reports here and discuss the implications.
A drug with few reported side effects
Singulair is a leukotriene receptor antagonist commonly prescribed for the prevention and treatment of asthma and for the treatment of allergic rhinitis. It is an orally active compound that binds with high affinity to the CysLT type-1 receptor, a leukotriene receptor found in a variety of human airway cells, including smooth muscle cells, macrophages, and eosinophils.1 At this receptor, Singulair inhibits the physiologic action of LTD4, a leukotriene released by various inflammatory cells that normally initiates the symptoms of asthma.
Singulair has been shown to dramatically increase forced expiratory volume, decrease usage of inhaled beta-agonists, and improve other asthma-related outcomes in both adults and children. In clinical studies, Singulair has proven safe, with few reported side effects. Some benign adverse events have been associated with this drug when compared with placebo, but causality between these events and Singulair is uncertain. Anaphylaxis was not reported in any of the premarketing clinical studies of Singulair.
4 other accounts of anaphylaxis
Singulair’s package insert mentions anaphylaxis as an adverse reaction reported after the US Food and Drug Administration approved the drug in 1998.1 Merck & Co., producer and distributor of Singulair, did not provide any specific information on reports of anaphylaxis for this review.
The Drug Safety Research Unit, an independent body associated with the University of Portsmouth in England, mentioned just one instance of anaphylaxis in a study of adverse reactions to montelukast among a cohort of more than 15,000 patients.2
A presentation given at a Healthcare Information Management Systems Society conference also briefly mentioned the case of an 8-year-old boy who experienced an anaphylactic reaction to Singulair.3
The only published description of a possible case of anaphylaxis in response to Singulair appeared in a report published by Lareb, the Dutch national pharmacovigilance system.4 A 4-year-old boy suffered facial edema, rash, coughing, and fatigue 2 days after starting montelukast 5 mg daily for asthma. The patient’s age and symptoms were strikingly similar to those of L.O.
Anaphylaxis: Always a possibility
Clearly, anaphylaxis as an adverse reaction to Singulair is rare, with only a handful of cases being reported worldwide. Nevertheless, anaphylaxis is life threatening, and we should be alert to its possibility when prescribing Singulair, especially for patients with a history of atopy.
Correspondence
Adriel Gerard, State University of New York at Buffalo School of Medicine, 99 Gold Street, Apt 1L, Brooklyn, NY 11201; [email protected]
1. Singulair (montelukast sodium) [prescribing information]. Whitehouse Station, NJ: Merck & Co., Inc; 2008.
2. Biswas P, Wilton L, Pearce G, et al. Pharmacosurveillance and safety of the leukotriene receptor antagonist (LTRA), montelukast. Clin Exp All Rev. 2001;3:300-304.
3. Millikan E. XML drug information modeling: linking evidence-based medicine with the bedside. In Proceedings of Health Information Management Systems Society. February 13-17, 2005. Available at: www.himss.org/content/files/2005proceedings/sessions/edu031.pdf. Accessed February 2, 2009.
4. An overview of reports on montelukast. Available at: www.lareb.nl/documents/kwb_2002_3_monte.pdf. Accessed February 2, 2009.
1. Singulair (montelukast sodium) [prescribing information]. Whitehouse Station, NJ: Merck & Co., Inc; 2008.
2. Biswas P, Wilton L, Pearce G, et al. Pharmacosurveillance and safety of the leukotriene receptor antagonist (LTRA), montelukast. Clin Exp All Rev. 2001;3:300-304.
3. Millikan E. XML drug information modeling: linking evidence-based medicine with the bedside. In Proceedings of Health Information Management Systems Society. February 13-17, 2005. Available at: www.himss.org/content/files/2005proceedings/sessions/edu031.pdf. Accessed February 2, 2009.
4. An overview of reports on montelukast. Available at: www.lareb.nl/documents/kwb_2002_3_monte.pdf. Accessed February 2, 2009.
Postmenopausal dyspareunia— a problem for the 21st century
The author reports that he serves on the speaker’s bureau for Novogyne, TherRx, Warner-Chilcott, and Solvay, and on the advisory board for Upsher-Smith, Novogyne, QuatRx, and Wyeth.
CASE: History of dyspareunia
At her latest visit, a 56-year-old woman who is 7 years postmenopausal relates that she has been experiencing worsening pain with intercourse to the point that she now has very little sex drive at all. This problem began approximately 1 year after she discontinued hormone therapy in the wake of reports that it causes cancer and heart attack. She has been offered both local vaginal and systemic hormone therapy, but is too frightened to use any hormones at all. Sexual lubricants no longer seem to work.
How do you counsel her about these symptoms? And what therapy do you offer?
Physicians and other health-care practitioners are seeing a large and growing number of genitourinary and sexual-related complaints among menopausal women—so much so that it has reached epidemic proportions. Yet dyspareunia is underreported and undertreated, and quality of life suffers for these women.
In this article, I focus on two interrelated causes of this epidemic:
- vaginal dryness and vulvovaginal atrophy (VVA) and the impact of these conditions on women’s sexual function and psychosocial well-being
- barriers to optimal treatment.
I also explore how ObGyns’ role in this area of care is evolving—as a way to understand how you can better serve this expanding segment of our patient population.
Dyspareunia can have many causes, including endometriosis, interstitial cystitis, surgical scarring, injury that occurs during childbirth, and psychosocial origin (such as a history of sexual abuse). Our focus here is on dyspareunia due to VVA.
during sex. What should you do?
- Sexual pain as a category of female sexual dysfunction is relevant at any age; for postmenopausal women dealing with vaginal dryness as a result of estrogen deficiency, it may well be the dominant issue. When determining the cause of a sexual problem in a postmenopausal woman, put dyspareunia caused by vaginal dryness (as well as its psychosocial consequences) at the top of the list of possibilities.
- Bring up the topic of vaginal dryness and sexual pain with postmenopausal patients as part of the routine yearly exam, and explain the therapeutic capabilities of all available options.
- Estrogen therapy, either local or systemic, remains the standard when lubricants are inadequate. Make every effort to counsel the patient about the real risk:benefit ratio of estrogen use.
- If the patient is reluctant to use estrogen therapy, discuss with her the option of short-term local estrogen use, with the understanding that more acceptable options may become available in the near future. This may facilitate acceptance of short-term hormonal treatment and allow the patient to maintain her vaginal health and much of her vaginal sexual function.
- Keep abreast of both present and future options for therapy.
Just how sizable is the postmenopausal population?
About 32% of the female population is older than 50 years.1 That means that around 48 million women are currently menopausal, or will become so over the next few years.
Because average life expectancy approaches 80 years in the United States and other countries of the industrialized world,2 many women will live approximately 40 years beyond menopause or their final menstrual period. Their quality of life during the second half of their life is dependent on both physical and psychosocial health.
Postmenopausal dyspareunia isn’t new
Sexual issues arising from physical causes—dyspareunia among them—have long accounted for a large share of medical concerns reported by postmenopausal women. In a 1985 survey, for example, dyspareunia accounted for 42.5% of their complaints.3
But epidemiologic studies to determine the prevalence of female sexual dysfunction in postmenopausal women are difficult to carry out. Why? Because researchers would need to 1) address changes over time and 2) distinguish problems of sexual function from those brought on by aging.4
The techniques and methodology for researching female sexual dysfunction continue to evolve, creating new definitions of the stages of menopause and new diagnostic approaches to female sexual dysfunction.
However, based on available studies, Dennerstein and Hayes concluded that:
- postmenopausal women report a high rate of sexual dysfunction (higher than men)
- psychosocial factors can ameliorate a decline in sexual function
- “vaginal dryness and dyspareunia seem to be driven primarily by declining estradiol.”4
The WHI and its domino effect
Millions of postmenopausal women stopped taking estrogen-based therapy in the wake of widespread media coverage after 2002 publication of data from the estrogen–progestin arm of the Women’s Health Initiative (WHI), which purported to show, among other things, an increased risk of breast cancer.5
For decades, many postmenopausal women achieved medical management of VVA through long-term use of systemic hormone replacement therapy (HRT), which they used primarily to control other chronic symptoms of menopause, such as hot flashes.
After the WHI data were published (and misrepresented), reduced usage of estrogen-based HRT “unmasked” vaginal symptoms, including sexual pain, due to the effects of estrogen deficiency on the vaginal epithelium and vaginal blood flow. Since then, we have been forced to examine anew the natural history of menopause.
Within days or weeks of discontinuing HRT, women may reexperience the acute vasomotor symptoms that accompany estrogen withdrawal—most commonly hot flashes, night sweats, sleeplessness, palpitations, and headaches. Over time—anywhere from 6 months to several years—the body adjusts to the loss or withdrawal of estrogen, and these vasomotor symptoms eventually diminish or resolve. Not so for the longer-term physical effects of chronic low serum levels of estrogen, which worsen over time.
Approximately 6 months after discontinuing estrogen therapy, postmenopausal women may begin to experience vaginal dryness and VVA. As the years pass, other side effects of estrogen deficiency arise: bone loss, joint pain, mood alteration (including depression), change in skin tone, hair loss, and cardiac and central nervous system changes. These side effects do not resolve spontaneously; in fact, they grow worse as a woman ages. They may have deleterious psychosocial as well as physical impacts on her life—especially on the quality of her intimate relationship.
Clarify the report (adjust appropriately for same-sex partner)
- Where does it hurt? Describe the pain.
- When does it hurt? Does the pain occur 1) with penile contact at the opening of the vagina, 2) once the penis is partially in, 3) with full entry, 4) after some thrusting, 5) after deep thrusting, 6) with the partner’s ejaculation, 7) after withdrawal, or 8) with subsequent micturition?
- Does your body tense when your partner is attempting, or you are attempting, to insert his penis? What are your thoughts and feelings at this time?
- How long does the pain last?
- Does touching cause pain? Does it hurt when you ride a bicycle or wear tight clothes? Does penetration by tampons or fingers hurt?
Assess the pelvic floor
- Do you recognize the feeling of pelvic floor muscle tension during sexual contact?
- Do you recognize the feeling of pelvic floor muscle tension in other (nonsexual) situations?
Evaluate arousal
- Do you feel subjectively excited when you attempt intercourse?
- Does your vagina become sufficiently moist? Do you recognize the feeling of drying up?
Determine the consequences of the complaint
- What do you do when you experience pain during sexual contact? Do you continue? Or do you stop whatever is causing the pain?
- Do you continue to include intercourse or attempts at intercourse in your lovemaking, or do you use other methods of achieving sexual fulfillment? If you use other ways to make love, do you and your partner clearly understand that intercourse will not be attempted?
- What other effect does the pain have on your sexual relationship?
Explore biomedical antecedents
- When and how did the pain start?
- What tests have you undergone?
- What treatment have you received?
Source: Adapted from Basson R, et al.12
Is 60 the new 40?
Many women and men in the large cohort known as the Baby Boomer generation continue to be sexually active into their 60s, 70s, and 80s, as demonstrated by a 2007 study of sexuality and health in older adults.6 In the 57- to 64-year-old age group, 61.6% of women and 83.7% of men were sexually active (defined as sexual activity with a partner within the past 12 months). In the 65- to 74-year-old group, 39.5% of women and 67% of men were sexually active; and in the 75- to 85-year-old group, 16.7% of women and 38.5% of men were sexually active (TABLE).
These findings indicate that fewer women than men remain sexually active during their later years. One reason may be the epidemic of sexual-related symptoms among postmenopausal women. In the same survey, 34.3% of women 57 to 64 years old reported avoiding sex because of:
- pain during intercourse (17.8%)
- difficulty with lubrication (35.9%).
Across all groups, the most prevalent sexual problem was low desire (43%).6 Around 40% of postmenopausal women reported no sexual activity in the past 12 months, as well as lack of interest in sex. This number may include women who have ceased to have sex because of vaginal dryness and dyspareunia, thereby reducing the percentage reporting these symptoms (TABLE).
TABLE
Older adults are having sex—and experiencing sexual problems
| Activity or problem by gender | Number of respondents | Report, by age group (95% confidence interval*) | ||
|---|---|---|---|---|
| 57–64 yr (%) | 65–74 yr (%) | 75–85 yr (%) | ||
| Sexually active in previous 12 months† | ||||
| Men | 1,385 | 83.7 (77.6–89.8) | 67.0 (62.1–72.0) | 38.5 (33.6–43.5) |
| Women | 1,501 | 61.6 (56.7–66.4) | 39.5 (34.6–44.4) | 16.7 (12.5–21.0) |
| Difficulty with lubrication | ||||
| Women | 495 | 35.9 (29.6–42.2) | 43.2 (34.8–51.5) | 43.6 (27.0–60.2) |
| Pain during intercourse | ||||
| Men | 878 | 3.0 (1.1–4.8) | 3.2 (1.2–5.3) | 1.0 (0–2.5) |
| Women | 506 | 17.8 (13.3–22.2) | 18.6 (10.8–26.3) | 11.8 (4.3–19.4) |
| Avoidance of sex due to sexual problems** | ||||
| Men | 533 | 22.1 (17.3–26.9) | 30.1 (23.2–37.0) | 25.7 (14.9–36.4) |
| Women | 357 | 34.3 (25.0–43.7) | 30.5 (21.5–39.4) | 22.7 (9.4–35.9) |
| Source: Adapted from Lindau ST, et al.6 | ||||
| Adjusted odds ratios are based on a logistic regression including the age group and self-rated health status as covariates, estimated separately for men and women. The confidence interval is based on the inversion of the Wald tests constructed with the use of design-based standard errors. | ||||
| † These data exclude 107 respondents who reported at least one sexual problem. | ||||
| ** This question was asked only of respondents who reported at least one sexual problem. | ||||
Assessing menopause-related sexual function is a challenge
Although the transition phases of menopause have been well studied and reported for decades, few of these studies have included questions about the impact of menopause on sexual function.7 When longitudinal studies that included the classification of female sexual dysfunction began to appear, they provided evidence of the important role that VVA and psychosocial factors play in female sexual dysfunction.8
In the fourth year of the Melbourne Women’s Midlife Health Project longitudinal study, six variables related to sexual function were identified. Three were determinate of sexual function:
- feelings for the partner
- problems related to the partner
- vaginal dryness/dyspareunia.
The other three variables—sexual responsiveness, frequency of sexual activity, and libido—were dependent or outcome variables.
By the sixth year of this study, two variables had increased in significance: vaginal dryness/dyspareunia and partner problems.7
Sexual pain and relationship problems can create a vicious cycle
The interrelationship of vaginal dryness, sexual pain, flagging desire, and psychosocial parameters can produce a vicious cycle. A woman experiencing or anticipating pain may have diminished sexual desire or avoid sex altogether. During intercourse, the brain’s awareness of vaginal pain may trigger a physiologic response that can cause the muscles of the vagina to tighten and lubrication to decrease. The result? Greater vaginal pain.
This vicious cycle can contribute to relationship issues with the sexual partner and harm a woman’s psychosocial well-being. Resentment, anger, and misunderstanding may arise when a couple is dealing with problems of sexual function, and these stressors can damage many aspects of the relationship, further exacerbating sexual difficulties.
An additional and very important dimension of these issues is their potential impact on the family unit.
VVA can diminish overall well-being
In a 2007 survey reported at the North American Menopause Society (NAMS), one third to one half of 506 respondents said that VVA had a bad effect on their sexual interest, mood, self-esteem, and the intimate relationship (FIGURE 1).9 Reports from in-depth interviews were consistent with survey results and offered further insight into a woman’s emotional response to the condition of vaginal dryness and its impact on her life. Women found the condition “embarrassing,” something they had to endure but didn’t talk about, and felt that it had a major impact on their self-esteem and intimate relationship.
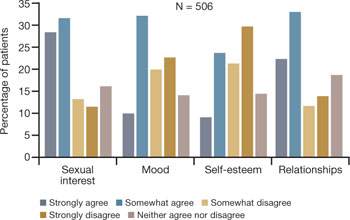
FIGURE 1 Dyspareunia affects more than interest in sex—relationships, mood, and self-esteem suffer
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
Clinicians often don’t ask about VVA, and patients are reluctant to talk
Among women of all ages, dyspareunia is underreported and undertreated. In the survey reported at NAMS, 40% of respondents said that their physician had never asked them about the problem of VVA (FIGURE 2).9
Women themselves may be reluctant to discuss the problem with physicians, nurse practitioners, or other health-care providers out of embarrassment or the assumption that there is nothing to be done about the problem. Nevertheless, more than 40% of respondents said they would be highly likely to seek treatment for VVA if they had a concern about urogenital complications of the condition (FIGURE 3).9
Another barrier may be the sense that asking the health-care provider about sex may embarrass him or her. As a result, sufferers do not anticipate help from their physician and other members of the health-care profession and fail to seek treatment or counseling for this chronic medical condition.10,11
In a 1999 telephone survey of 500 adults 25 years of age or older, 71% said they thought that their doctor would dismiss concerns about sexual problems, but 85% said they would talk to their physician anyway if they had a problem, even though they might not get treatment.11 In that survey, 91% of married men and 84% of married women rated a satisfying sex life as important to quality of life.11
Another important and often overlooked limitation on this type of discussion is the time constraints that busy clinicians face, especially with the low reimbursement offered by managed care. Sexual problems can hardly be adequately discussed in 7 to 10 minutes.
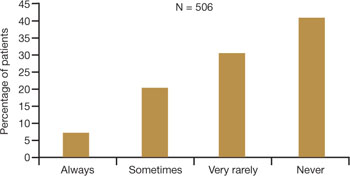
FIGURE 2 Do physicians ask about dyspareunia? Most women surveyed said “rarely” or “never”
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
FIGURE 3 Are these women likely to seek treatment?
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
Women have performance anxiety, too
It is well known that men with even a mild degree of erectile dysfunction can suffer from performance anxiety, but the fact that women can also suffer from this phenomenon is not given as much attention. Such anxiety can be a factor in relationship difficulties. With both partners perhaps feeling anxious about sexual performance, a couple may avoid even simple acts of affection, such as holding hands, to avoid raising the other’s expectations.
Exacerbating the situation is the fact that many men use widely prescribed phosphodiesterase type 5 (PDE5) inhibitors, whereas women are contending with barriers to continued sexual activity as they age. It does not take a psychologist to understand that this imbalance often adds to emotional strain and tension between partners.
Popular media address the issue
Look beyond what our postmenopausal patients tell us directly—to the popular media and online forums—to appreciate the scope of sexual pain as a major issue among postmenopausal women. Evidence of psychosocial effects is found on numerous Web sites—some from organizations, others designed by women seeking help from each other.
Red Hot Mamas
This organization aims to empower women through menopause education. Highlighted in the Winter 2007/2008 Red Hot Mamas Report is a survey done in conjunction with Harris Interactive exploring the impact of menopausal symptoms on a woman’s sex life, which found that 47% of women who have VVA have avoided or stopped sex completely because it was uncomfortable, compared with 23% of normal women.
Power Surge
This Web site offers a list of strategies for dealing with sexual pain, including an overview of hormone-based prescription and nonprescription products, along with a variety of over-the-counter, natural, holistic, and herbal therapies for treating dyspareunia.
What is the physician’s role?
Given the epidemic of sexual pain, it is crucial that physicians and others who care for postmenopausal women increase their awareness of this issue and pay special attention to its psychosocial parameters.
Ask patients about sexual function in general and dyspareunia in particular as part of the routine annual visit. A simple opening “Yes/No” question, such as “Are you sexually active?” can lead to further questions appropriate to the patient. For example, if the answer is “No,” the follow-up question might be, “Does that bother you or your partner?” Further discussion may uncover whether the lack of sexual activity is a cause of distress and identify which variables are involved.
If, instead, the answer is “Yes,” follow-up questions can identify the presence of common postmenopausal physical issues, such as vaginal dryness and difficulty with lubrication. The visit then can turn to strategies to ameliorate those conditions.
When a patient reports dyspareunia, further diagnostic information such as precise location, degree of arousal, and reaction to pain can help determine the appropriate course of treatment. For an approach to this aspect of ascertaining patient history, see the list of sample questions above.12
During the physical, pay particular attention to any physical abnormalities or organic causes of sexual pain. Questions designed to characterize the location and nature of the pain can pinpoint the cause. Sexual pain arising from VVA is likely to 1) be localized at the introitus and 2) occur with penile entry.
Since the mid-1990s, the availability of validated scales to measure female sexual function has increased rapidly and enabled researchers to better identify, quantify, and evaluate treatments for female sexual dysfunction.7 Over time, we have moved away from the somewhat mechanical sequence inherent in the linear progression of desire leading to genital stimulation followed by arousal and orgasm toward an appreciation of the multiple physical, emotional, and subjective factors that are at play in women’s sexual function.
By 1998, a classification scheme was developed to further the means to study and discuss disorders of desire, arousal, orgasm, and sexual pain.8 Further contextual definitions of sexual dysfunction are under consideration.13
Basson proposed one new model of female sexual function (see the diagram), and observed that
"…women identify many reasons they are sexual over and beyond inherent sexual drive or “hunger.” Women tell of wanting to increase emotional closeness, commitment, sharing, tenderness, and tolerance, and to show the partner that he or she has been missed (emotionally or physically). Such intimacy-based reasons motivate the woman to find a way to become sexually aroused. This arousal is not spontaneous but triggered by deliberately sought sexual stimuli."13
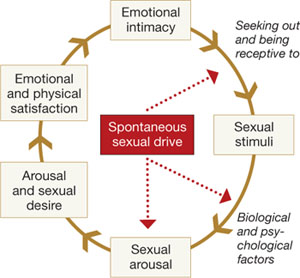
Intimacy-based model of female sexual response cycle
In this flow of physical and emotional variables involved in female sexual function, categories interact. For example, low desire can be and is frequently secondary to the anticipation of pain during sexual intercourse. Arousal can be hampered by lack of vaginal lubrication—perhaps inhibited by the anticipation of pain. Secondary orgasmic disorders can result from low desire, difficulty of arousal, and sexual pain.14 Sexual pain can affect sexual function at any point on this continuum.
Treatments in the pipeline
For decades, hormone-based treatments have been the predominant therapeutic option for vaginal dryness. Often they are a secondary benefit of hormone therapy for vasomotor symptoms and osteoporosis. Estrogen can be delivered in the form of oral tablet, transdermal patch, gel, spray, or vaginal ring for systemic use, or as vaginal cream, ring, or tablet for local use.
However, despite data to the contrary and our reassurances to the patient about overall safety, a large number of women, and many primary care providers, are no longer inclined to use short- or long-term HRT in any presentation.
Other women may have risk factors that contraindicate exogenous hormones.
Nonhormonal options for vaginal dryness and dyspareunia are limited, and there are no approved systemic or oral nonestrogen options. Over-the-counter topical lubricants can ease some of the symptoms of VVA temporarily and allow successful vaginal penetration in many cases. Some may cause vaginal warming and pleasant sensations, but overall they treat the symptom rather than the source of pain. Moreover, many patients consider local lubricants messy and inconvenient and claim they “ruin the mood.”
The use of vaginal dilators along with estrogen or lubricant therapy is an often-forgotten adjunct to therapy for dyspareunia caused by VVA (FIGURE 4).
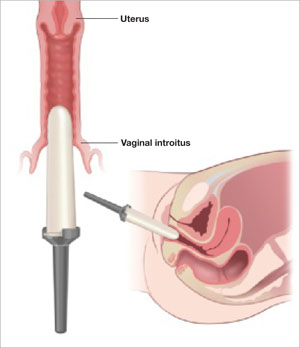
FIGURE 4 Mechanical dilation of the vagina is a useful adjunct
Mechanical dilation is often needed to restore penetration capability in the vagina, even after hormonal treatment. The focus should be on the vaginal introitus, with the top 25% to 35% of the dilator inserted into the opening once a day for 15 minutes, increasing the dilator diameter over time.
New SERMs are in development
Preclinical and clinical research into the diverse class of selective estrogen receptor modulators (SERMs) to treat estrogen-mediated disease produced tamoxifen for breast cancer prevention and raloxifene for both vertebral osteoporosis and breast cancer prevention. Each SERM seems to have unique tissue selectivity. The antiestrogenic activity of tamoxifen and raloxifene extends to the vagina and can exacerbate vaginal dryness.
A new generation of orally active SERMs is under investigation specifically for the treatment of chronic vaginal symptoms. These new agents target the nonvaginal treatment of VVA and associated symptoms. The first oral SERM for long-term treatment of these symptoms, ospemifene (Ophena), may become available in the near future. It is a novel SERM that has both anti-estrogenic and estrogenic actions, depending on the tissue. It was shown to significantly improve both vaginal dryness and dyspareunia in a large placebo-controlled trial.15
1. US Census Bureau. 2006 American community survey. S0101. Age and sex. Available at: http://fact-finder.census.gov/servlet/DatasetMainPageServlet?_program=ACS&_submenuId=&_lang=en&_ts.
2. National Center for Health Statistics. Health, United States, 2007, with Chartbook on Trends in the Health of Americans. Hyattsville, Md: NCHS; 2007. Available at: http://www.cdc.gov/nchs/fastats/lifexpec.htm. Accessed February 2, 2009.
3. Sarrel PM, Whitehead MI. Sex and menopause: defining the issues. Maturitas. 1985;7:217-224.
4. Dennerstein L, Hayes RD. Confronting the challenges: epidemiological study of female sexual dysfunction and the menopause. J Sex Med. 2005;2(suppl 3):118-132.
5. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
6. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762-774.
7. Dennerstein L, Alexander JL, Kotz K. The menopause and sexual functioning: a review of the population-based studies. Annu Rev Sex Res. 2003;14:64-82.
8. Basson R, Berman J, Burnett A, et al. Report of the international consensus development conference on female sexual dysfunction: definitions and classifications. J Urol. 2000;163:888-993.
9. Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
10. Heim LJ. Evaluation and differential diagnosis of dyspareunia. Am Fam Physician. 2001;63:1535-1552.
11. Marwick C. Survey says patients expect little physician help on sex. JAMA. 1999;281:2173-2174.
12. Basson R, Althof S, Davis S, et al. Summary of the recommendations on sexual dysfunctions in women. J Sex Med. 2004;1:24-34.
13. Basson R. Female sexual response: the role of drugs in the management of sexual dysfunction. Obstet Gynecol. 2001;98:350-353.
14. Walsh KE, Berman JR. Sexual dysfunction in the older woman: an overview of the current understanding and management. Drugs Aging. 2004;21:655-675.
15. Bachmann GA, Komi J, Hanley R. A new SERM, Ophena (ospemifene), effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Presented at the Endocrine Society annual meeting, San Francisco, Calif, June 2008.
The author reports that he serves on the speaker’s bureau for Novogyne, TherRx, Warner-Chilcott, and Solvay, and on the advisory board for Upsher-Smith, Novogyne, QuatRx, and Wyeth.
CASE: History of dyspareunia
At her latest visit, a 56-year-old woman who is 7 years postmenopausal relates that she has been experiencing worsening pain with intercourse to the point that she now has very little sex drive at all. This problem began approximately 1 year after she discontinued hormone therapy in the wake of reports that it causes cancer and heart attack. She has been offered both local vaginal and systemic hormone therapy, but is too frightened to use any hormones at all. Sexual lubricants no longer seem to work.
How do you counsel her about these symptoms? And what therapy do you offer?
Physicians and other health-care practitioners are seeing a large and growing number of genitourinary and sexual-related complaints among menopausal women—so much so that it has reached epidemic proportions. Yet dyspareunia is underreported and undertreated, and quality of life suffers for these women.
In this article, I focus on two interrelated causes of this epidemic:
- vaginal dryness and vulvovaginal atrophy (VVA) and the impact of these conditions on women’s sexual function and psychosocial well-being
- barriers to optimal treatment.
I also explore how ObGyns’ role in this area of care is evolving—as a way to understand how you can better serve this expanding segment of our patient population.
Dyspareunia can have many causes, including endometriosis, interstitial cystitis, surgical scarring, injury that occurs during childbirth, and psychosocial origin (such as a history of sexual abuse). Our focus here is on dyspareunia due to VVA.
during sex. What should you do?
- Sexual pain as a category of female sexual dysfunction is relevant at any age; for postmenopausal women dealing with vaginal dryness as a result of estrogen deficiency, it may well be the dominant issue. When determining the cause of a sexual problem in a postmenopausal woman, put dyspareunia caused by vaginal dryness (as well as its psychosocial consequences) at the top of the list of possibilities.
- Bring up the topic of vaginal dryness and sexual pain with postmenopausal patients as part of the routine yearly exam, and explain the therapeutic capabilities of all available options.
- Estrogen therapy, either local or systemic, remains the standard when lubricants are inadequate. Make every effort to counsel the patient about the real risk:benefit ratio of estrogen use.
- If the patient is reluctant to use estrogen therapy, discuss with her the option of short-term local estrogen use, with the understanding that more acceptable options may become available in the near future. This may facilitate acceptance of short-term hormonal treatment and allow the patient to maintain her vaginal health and much of her vaginal sexual function.
- Keep abreast of both present and future options for therapy.
Just how sizable is the postmenopausal population?
About 32% of the female population is older than 50 years.1 That means that around 48 million women are currently menopausal, or will become so over the next few years.
Because average life expectancy approaches 80 years in the United States and other countries of the industrialized world,2 many women will live approximately 40 years beyond menopause or their final menstrual period. Their quality of life during the second half of their life is dependent on both physical and psychosocial health.
Postmenopausal dyspareunia isn’t new
Sexual issues arising from physical causes—dyspareunia among them—have long accounted for a large share of medical concerns reported by postmenopausal women. In a 1985 survey, for example, dyspareunia accounted for 42.5% of their complaints.3
But epidemiologic studies to determine the prevalence of female sexual dysfunction in postmenopausal women are difficult to carry out. Why? Because researchers would need to 1) address changes over time and 2) distinguish problems of sexual function from those brought on by aging.4
The techniques and methodology for researching female sexual dysfunction continue to evolve, creating new definitions of the stages of menopause and new diagnostic approaches to female sexual dysfunction.
However, based on available studies, Dennerstein and Hayes concluded that:
- postmenopausal women report a high rate of sexual dysfunction (higher than men)
- psychosocial factors can ameliorate a decline in sexual function
- “vaginal dryness and dyspareunia seem to be driven primarily by declining estradiol.”4
The WHI and its domino effect
Millions of postmenopausal women stopped taking estrogen-based therapy in the wake of widespread media coverage after 2002 publication of data from the estrogen–progestin arm of the Women’s Health Initiative (WHI), which purported to show, among other things, an increased risk of breast cancer.5
For decades, many postmenopausal women achieved medical management of VVA through long-term use of systemic hormone replacement therapy (HRT), which they used primarily to control other chronic symptoms of menopause, such as hot flashes.
After the WHI data were published (and misrepresented), reduced usage of estrogen-based HRT “unmasked” vaginal symptoms, including sexual pain, due to the effects of estrogen deficiency on the vaginal epithelium and vaginal blood flow. Since then, we have been forced to examine anew the natural history of menopause.
Within days or weeks of discontinuing HRT, women may reexperience the acute vasomotor symptoms that accompany estrogen withdrawal—most commonly hot flashes, night sweats, sleeplessness, palpitations, and headaches. Over time—anywhere from 6 months to several years—the body adjusts to the loss or withdrawal of estrogen, and these vasomotor symptoms eventually diminish or resolve. Not so for the longer-term physical effects of chronic low serum levels of estrogen, which worsen over time.
Approximately 6 months after discontinuing estrogen therapy, postmenopausal women may begin to experience vaginal dryness and VVA. As the years pass, other side effects of estrogen deficiency arise: bone loss, joint pain, mood alteration (including depression), change in skin tone, hair loss, and cardiac and central nervous system changes. These side effects do not resolve spontaneously; in fact, they grow worse as a woman ages. They may have deleterious psychosocial as well as physical impacts on her life—especially on the quality of her intimate relationship.
Clarify the report (adjust appropriately for same-sex partner)
- Where does it hurt? Describe the pain.
- When does it hurt? Does the pain occur 1) with penile contact at the opening of the vagina, 2) once the penis is partially in, 3) with full entry, 4) after some thrusting, 5) after deep thrusting, 6) with the partner’s ejaculation, 7) after withdrawal, or 8) with subsequent micturition?
- Does your body tense when your partner is attempting, or you are attempting, to insert his penis? What are your thoughts and feelings at this time?
- How long does the pain last?
- Does touching cause pain? Does it hurt when you ride a bicycle or wear tight clothes? Does penetration by tampons or fingers hurt?
Assess the pelvic floor
- Do you recognize the feeling of pelvic floor muscle tension during sexual contact?
- Do you recognize the feeling of pelvic floor muscle tension in other (nonsexual) situations?
Evaluate arousal
- Do you feel subjectively excited when you attempt intercourse?
- Does your vagina become sufficiently moist? Do you recognize the feeling of drying up?
Determine the consequences of the complaint
- What do you do when you experience pain during sexual contact? Do you continue? Or do you stop whatever is causing the pain?
- Do you continue to include intercourse or attempts at intercourse in your lovemaking, or do you use other methods of achieving sexual fulfillment? If you use other ways to make love, do you and your partner clearly understand that intercourse will not be attempted?
- What other effect does the pain have on your sexual relationship?
Explore biomedical antecedents
- When and how did the pain start?
- What tests have you undergone?
- What treatment have you received?
Source: Adapted from Basson R, et al.12
Is 60 the new 40?
Many women and men in the large cohort known as the Baby Boomer generation continue to be sexually active into their 60s, 70s, and 80s, as demonstrated by a 2007 study of sexuality and health in older adults.6 In the 57- to 64-year-old age group, 61.6% of women and 83.7% of men were sexually active (defined as sexual activity with a partner within the past 12 months). In the 65- to 74-year-old group, 39.5% of women and 67% of men were sexually active; and in the 75- to 85-year-old group, 16.7% of women and 38.5% of men were sexually active (TABLE).
These findings indicate that fewer women than men remain sexually active during their later years. One reason may be the epidemic of sexual-related symptoms among postmenopausal women. In the same survey, 34.3% of women 57 to 64 years old reported avoiding sex because of:
- pain during intercourse (17.8%)
- difficulty with lubrication (35.9%).
Across all groups, the most prevalent sexual problem was low desire (43%).6 Around 40% of postmenopausal women reported no sexual activity in the past 12 months, as well as lack of interest in sex. This number may include women who have ceased to have sex because of vaginal dryness and dyspareunia, thereby reducing the percentage reporting these symptoms (TABLE).
TABLE
Older adults are having sex—and experiencing sexual problems
| Activity or problem by gender | Number of respondents | Report, by age group (95% confidence interval*) | ||
|---|---|---|---|---|
| 57–64 yr (%) | 65–74 yr (%) | 75–85 yr (%) | ||
| Sexually active in previous 12 months† | ||||
| Men | 1,385 | 83.7 (77.6–89.8) | 67.0 (62.1–72.0) | 38.5 (33.6–43.5) |
| Women | 1,501 | 61.6 (56.7–66.4) | 39.5 (34.6–44.4) | 16.7 (12.5–21.0) |
| Difficulty with lubrication | ||||
| Women | 495 | 35.9 (29.6–42.2) | 43.2 (34.8–51.5) | 43.6 (27.0–60.2) |
| Pain during intercourse | ||||
| Men | 878 | 3.0 (1.1–4.8) | 3.2 (1.2–5.3) | 1.0 (0–2.5) |
| Women | 506 | 17.8 (13.3–22.2) | 18.6 (10.8–26.3) | 11.8 (4.3–19.4) |
| Avoidance of sex due to sexual problems** | ||||
| Men | 533 | 22.1 (17.3–26.9) | 30.1 (23.2–37.0) | 25.7 (14.9–36.4) |
| Women | 357 | 34.3 (25.0–43.7) | 30.5 (21.5–39.4) | 22.7 (9.4–35.9) |
| Source: Adapted from Lindau ST, et al.6 | ||||
| Adjusted odds ratios are based on a logistic regression including the age group and self-rated health status as covariates, estimated separately for men and women. The confidence interval is based on the inversion of the Wald tests constructed with the use of design-based standard errors. | ||||
| † These data exclude 107 respondents who reported at least one sexual problem. | ||||
| ** This question was asked only of respondents who reported at least one sexual problem. | ||||
Assessing menopause-related sexual function is a challenge
Although the transition phases of menopause have been well studied and reported for decades, few of these studies have included questions about the impact of menopause on sexual function.7 When longitudinal studies that included the classification of female sexual dysfunction began to appear, they provided evidence of the important role that VVA and psychosocial factors play in female sexual dysfunction.8
In the fourth year of the Melbourne Women’s Midlife Health Project longitudinal study, six variables related to sexual function were identified. Three were determinate of sexual function:
- feelings for the partner
- problems related to the partner
- vaginal dryness/dyspareunia.
The other three variables—sexual responsiveness, frequency of sexual activity, and libido—were dependent or outcome variables.
By the sixth year of this study, two variables had increased in significance: vaginal dryness/dyspareunia and partner problems.7
Sexual pain and relationship problems can create a vicious cycle
The interrelationship of vaginal dryness, sexual pain, flagging desire, and psychosocial parameters can produce a vicious cycle. A woman experiencing or anticipating pain may have diminished sexual desire or avoid sex altogether. During intercourse, the brain’s awareness of vaginal pain may trigger a physiologic response that can cause the muscles of the vagina to tighten and lubrication to decrease. The result? Greater vaginal pain.
This vicious cycle can contribute to relationship issues with the sexual partner and harm a woman’s psychosocial well-being. Resentment, anger, and misunderstanding may arise when a couple is dealing with problems of sexual function, and these stressors can damage many aspects of the relationship, further exacerbating sexual difficulties.
An additional and very important dimension of these issues is their potential impact on the family unit.
VVA can diminish overall well-being
In a 2007 survey reported at the North American Menopause Society (NAMS), one third to one half of 506 respondents said that VVA had a bad effect on their sexual interest, mood, self-esteem, and the intimate relationship (FIGURE 1).9 Reports from in-depth interviews were consistent with survey results and offered further insight into a woman’s emotional response to the condition of vaginal dryness and its impact on her life. Women found the condition “embarrassing,” something they had to endure but didn’t talk about, and felt that it had a major impact on their self-esteem and intimate relationship.

FIGURE 1 Dyspareunia affects more than interest in sex—relationships, mood, and self-esteem suffer
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
Clinicians often don’t ask about VVA, and patients are reluctant to talk
Among women of all ages, dyspareunia is underreported and undertreated. In the survey reported at NAMS, 40% of respondents said that their physician had never asked them about the problem of VVA (FIGURE 2).9
Women themselves may be reluctant to discuss the problem with physicians, nurse practitioners, or other health-care providers out of embarrassment or the assumption that there is nothing to be done about the problem. Nevertheless, more than 40% of respondents said they would be highly likely to seek treatment for VVA if they had a concern about urogenital complications of the condition (FIGURE 3).9
Another barrier may be the sense that asking the health-care provider about sex may embarrass him or her. As a result, sufferers do not anticipate help from their physician and other members of the health-care profession and fail to seek treatment or counseling for this chronic medical condition.10,11
In a 1999 telephone survey of 500 adults 25 years of age or older, 71% said they thought that their doctor would dismiss concerns about sexual problems, but 85% said they would talk to their physician anyway if they had a problem, even though they might not get treatment.11 In that survey, 91% of married men and 84% of married women rated a satisfying sex life as important to quality of life.11
Another important and often overlooked limitation on this type of discussion is the time constraints that busy clinicians face, especially with the low reimbursement offered by managed care. Sexual problems can hardly be adequately discussed in 7 to 10 minutes.

FIGURE 2 Do physicians ask about dyspareunia? Most women surveyed said “rarely” or “never”
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
FIGURE 3 Are these women likely to seek treatment?
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
Women have performance anxiety, too
It is well known that men with even a mild degree of erectile dysfunction can suffer from performance anxiety, but the fact that women can also suffer from this phenomenon is not given as much attention. Such anxiety can be a factor in relationship difficulties. With both partners perhaps feeling anxious about sexual performance, a couple may avoid even simple acts of affection, such as holding hands, to avoid raising the other’s expectations.
Exacerbating the situation is the fact that many men use widely prescribed phosphodiesterase type 5 (PDE5) inhibitors, whereas women are contending with barriers to continued sexual activity as they age. It does not take a psychologist to understand that this imbalance often adds to emotional strain and tension between partners.
Popular media address the issue
Look beyond what our postmenopausal patients tell us directly—to the popular media and online forums—to appreciate the scope of sexual pain as a major issue among postmenopausal women. Evidence of psychosocial effects is found on numerous Web sites—some from organizations, others designed by women seeking help from each other.
Red Hot Mamas
This organization aims to empower women through menopause education. Highlighted in the Winter 2007/2008 Red Hot Mamas Report is a survey done in conjunction with Harris Interactive exploring the impact of menopausal symptoms on a woman’s sex life, which found that 47% of women who have VVA have avoided or stopped sex completely because it was uncomfortable, compared with 23% of normal women.
Power Surge
This Web site offers a list of strategies for dealing with sexual pain, including an overview of hormone-based prescription and nonprescription products, along with a variety of over-the-counter, natural, holistic, and herbal therapies for treating dyspareunia.
What is the physician’s role?
Given the epidemic of sexual pain, it is crucial that physicians and others who care for postmenopausal women increase their awareness of this issue and pay special attention to its psychosocial parameters.
Ask patients about sexual function in general and dyspareunia in particular as part of the routine annual visit. A simple opening “Yes/No” question, such as “Are you sexually active?” can lead to further questions appropriate to the patient. For example, if the answer is “No,” the follow-up question might be, “Does that bother you or your partner?” Further discussion may uncover whether the lack of sexual activity is a cause of distress and identify which variables are involved.
If, instead, the answer is “Yes,” follow-up questions can identify the presence of common postmenopausal physical issues, such as vaginal dryness and difficulty with lubrication. The visit then can turn to strategies to ameliorate those conditions.
When a patient reports dyspareunia, further diagnostic information such as precise location, degree of arousal, and reaction to pain can help determine the appropriate course of treatment. For an approach to this aspect of ascertaining patient history, see the list of sample questions above.12
During the physical, pay particular attention to any physical abnormalities or organic causes of sexual pain. Questions designed to characterize the location and nature of the pain can pinpoint the cause. Sexual pain arising from VVA is likely to 1) be localized at the introitus and 2) occur with penile entry.
Since the mid-1990s, the availability of validated scales to measure female sexual function has increased rapidly and enabled researchers to better identify, quantify, and evaluate treatments for female sexual dysfunction.7 Over time, we have moved away from the somewhat mechanical sequence inherent in the linear progression of desire leading to genital stimulation followed by arousal and orgasm toward an appreciation of the multiple physical, emotional, and subjective factors that are at play in women’s sexual function.
By 1998, a classification scheme was developed to further the means to study and discuss disorders of desire, arousal, orgasm, and sexual pain.8 Further contextual definitions of sexual dysfunction are under consideration.13
Basson proposed one new model of female sexual function (see the diagram), and observed that
"…women identify many reasons they are sexual over and beyond inherent sexual drive or “hunger.” Women tell of wanting to increase emotional closeness, commitment, sharing, tenderness, and tolerance, and to show the partner that he or she has been missed (emotionally or physically). Such intimacy-based reasons motivate the woman to find a way to become sexually aroused. This arousal is not spontaneous but triggered by deliberately sought sexual stimuli."13

Intimacy-based model of female sexual response cycle
In this flow of physical and emotional variables involved in female sexual function, categories interact. For example, low desire can be and is frequently secondary to the anticipation of pain during sexual intercourse. Arousal can be hampered by lack of vaginal lubrication—perhaps inhibited by the anticipation of pain. Secondary orgasmic disorders can result from low desire, difficulty of arousal, and sexual pain.14 Sexual pain can affect sexual function at any point on this continuum.
Treatments in the pipeline
For decades, hormone-based treatments have been the predominant therapeutic option for vaginal dryness. Often they are a secondary benefit of hormone therapy for vasomotor symptoms and osteoporosis. Estrogen can be delivered in the form of oral tablet, transdermal patch, gel, spray, or vaginal ring for systemic use, or as vaginal cream, ring, or tablet for local use.
However, despite data to the contrary and our reassurances to the patient about overall safety, a large number of women, and many primary care providers, are no longer inclined to use short- or long-term HRT in any presentation.
Other women may have risk factors that contraindicate exogenous hormones.
Nonhormonal options for vaginal dryness and dyspareunia are limited, and there are no approved systemic or oral nonestrogen options. Over-the-counter topical lubricants can ease some of the symptoms of VVA temporarily and allow successful vaginal penetration in many cases. Some may cause vaginal warming and pleasant sensations, but overall they treat the symptom rather than the source of pain. Moreover, many patients consider local lubricants messy and inconvenient and claim they “ruin the mood.”
The use of vaginal dilators along with estrogen or lubricant therapy is an often-forgotten adjunct to therapy for dyspareunia caused by VVA (FIGURE 4).

FIGURE 4 Mechanical dilation of the vagina is a useful adjunct
Mechanical dilation is often needed to restore penetration capability in the vagina, even after hormonal treatment. The focus should be on the vaginal introitus, with the top 25% to 35% of the dilator inserted into the opening once a day for 15 minutes, increasing the dilator diameter over time.
New SERMs are in development
Preclinical and clinical research into the diverse class of selective estrogen receptor modulators (SERMs) to treat estrogen-mediated disease produced tamoxifen for breast cancer prevention and raloxifene for both vertebral osteoporosis and breast cancer prevention. Each SERM seems to have unique tissue selectivity. The antiestrogenic activity of tamoxifen and raloxifene extends to the vagina and can exacerbate vaginal dryness.
A new generation of orally active SERMs is under investigation specifically for the treatment of chronic vaginal symptoms. These new agents target the nonvaginal treatment of VVA and associated symptoms. The first oral SERM for long-term treatment of these symptoms, ospemifene (Ophena), may become available in the near future. It is a novel SERM that has both anti-estrogenic and estrogenic actions, depending on the tissue. It was shown to significantly improve both vaginal dryness and dyspareunia in a large placebo-controlled trial.15
The author reports that he serves on the speaker’s bureau for Novogyne, TherRx, Warner-Chilcott, and Solvay, and on the advisory board for Upsher-Smith, Novogyne, QuatRx, and Wyeth.
CASE: History of dyspareunia
At her latest visit, a 56-year-old woman who is 7 years postmenopausal relates that she has been experiencing worsening pain with intercourse to the point that she now has very little sex drive at all. This problem began approximately 1 year after she discontinued hormone therapy in the wake of reports that it causes cancer and heart attack. She has been offered both local vaginal and systemic hormone therapy, but is too frightened to use any hormones at all. Sexual lubricants no longer seem to work.
How do you counsel her about these symptoms? And what therapy do you offer?
Physicians and other health-care practitioners are seeing a large and growing number of genitourinary and sexual-related complaints among menopausal women—so much so that it has reached epidemic proportions. Yet dyspareunia is underreported and undertreated, and quality of life suffers for these women.
In this article, I focus on two interrelated causes of this epidemic:
- vaginal dryness and vulvovaginal atrophy (VVA) and the impact of these conditions on women’s sexual function and psychosocial well-being
- barriers to optimal treatment.
I also explore how ObGyns’ role in this area of care is evolving—as a way to understand how you can better serve this expanding segment of our patient population.
Dyspareunia can have many causes, including endometriosis, interstitial cystitis, surgical scarring, injury that occurs during childbirth, and psychosocial origin (such as a history of sexual abuse). Our focus here is on dyspareunia due to VVA.
during sex. What should you do?
- Sexual pain as a category of female sexual dysfunction is relevant at any age; for postmenopausal women dealing with vaginal dryness as a result of estrogen deficiency, it may well be the dominant issue. When determining the cause of a sexual problem in a postmenopausal woman, put dyspareunia caused by vaginal dryness (as well as its psychosocial consequences) at the top of the list of possibilities.
- Bring up the topic of vaginal dryness and sexual pain with postmenopausal patients as part of the routine yearly exam, and explain the therapeutic capabilities of all available options.
- Estrogen therapy, either local or systemic, remains the standard when lubricants are inadequate. Make every effort to counsel the patient about the real risk:benefit ratio of estrogen use.
- If the patient is reluctant to use estrogen therapy, discuss with her the option of short-term local estrogen use, with the understanding that more acceptable options may become available in the near future. This may facilitate acceptance of short-term hormonal treatment and allow the patient to maintain her vaginal health and much of her vaginal sexual function.
- Keep abreast of both present and future options for therapy.
Just how sizable is the postmenopausal population?
About 32% of the female population is older than 50 years.1 That means that around 48 million women are currently menopausal, or will become so over the next few years.
Because average life expectancy approaches 80 years in the United States and other countries of the industrialized world,2 many women will live approximately 40 years beyond menopause or their final menstrual period. Their quality of life during the second half of their life is dependent on both physical and psychosocial health.
Postmenopausal dyspareunia isn’t new
Sexual issues arising from physical causes—dyspareunia among them—have long accounted for a large share of medical concerns reported by postmenopausal women. In a 1985 survey, for example, dyspareunia accounted for 42.5% of their complaints.3
But epidemiologic studies to determine the prevalence of female sexual dysfunction in postmenopausal women are difficult to carry out. Why? Because researchers would need to 1) address changes over time and 2) distinguish problems of sexual function from those brought on by aging.4
The techniques and methodology for researching female sexual dysfunction continue to evolve, creating new definitions of the stages of menopause and new diagnostic approaches to female sexual dysfunction.
However, based on available studies, Dennerstein and Hayes concluded that:
- postmenopausal women report a high rate of sexual dysfunction (higher than men)
- psychosocial factors can ameliorate a decline in sexual function
- “vaginal dryness and dyspareunia seem to be driven primarily by declining estradiol.”4
The WHI and its domino effect
Millions of postmenopausal women stopped taking estrogen-based therapy in the wake of widespread media coverage after 2002 publication of data from the estrogen–progestin arm of the Women’s Health Initiative (WHI), which purported to show, among other things, an increased risk of breast cancer.5
For decades, many postmenopausal women achieved medical management of VVA through long-term use of systemic hormone replacement therapy (HRT), which they used primarily to control other chronic symptoms of menopause, such as hot flashes.
After the WHI data were published (and misrepresented), reduced usage of estrogen-based HRT “unmasked” vaginal symptoms, including sexual pain, due to the effects of estrogen deficiency on the vaginal epithelium and vaginal blood flow. Since then, we have been forced to examine anew the natural history of menopause.
Within days or weeks of discontinuing HRT, women may reexperience the acute vasomotor symptoms that accompany estrogen withdrawal—most commonly hot flashes, night sweats, sleeplessness, palpitations, and headaches. Over time—anywhere from 6 months to several years—the body adjusts to the loss or withdrawal of estrogen, and these vasomotor symptoms eventually diminish or resolve. Not so for the longer-term physical effects of chronic low serum levels of estrogen, which worsen over time.
Approximately 6 months after discontinuing estrogen therapy, postmenopausal women may begin to experience vaginal dryness and VVA. As the years pass, other side effects of estrogen deficiency arise: bone loss, joint pain, mood alteration (including depression), change in skin tone, hair loss, and cardiac and central nervous system changes. These side effects do not resolve spontaneously; in fact, they grow worse as a woman ages. They may have deleterious psychosocial as well as physical impacts on her life—especially on the quality of her intimate relationship.
Clarify the report (adjust appropriately for same-sex partner)
- Where does it hurt? Describe the pain.
- When does it hurt? Does the pain occur 1) with penile contact at the opening of the vagina, 2) once the penis is partially in, 3) with full entry, 4) after some thrusting, 5) after deep thrusting, 6) with the partner’s ejaculation, 7) after withdrawal, or 8) with subsequent micturition?
- Does your body tense when your partner is attempting, or you are attempting, to insert his penis? What are your thoughts and feelings at this time?
- How long does the pain last?
- Does touching cause pain? Does it hurt when you ride a bicycle or wear tight clothes? Does penetration by tampons or fingers hurt?
Assess the pelvic floor
- Do you recognize the feeling of pelvic floor muscle tension during sexual contact?
- Do you recognize the feeling of pelvic floor muscle tension in other (nonsexual) situations?
Evaluate arousal
- Do you feel subjectively excited when you attempt intercourse?
- Does your vagina become sufficiently moist? Do you recognize the feeling of drying up?
Determine the consequences of the complaint
- What do you do when you experience pain during sexual contact? Do you continue? Or do you stop whatever is causing the pain?
- Do you continue to include intercourse or attempts at intercourse in your lovemaking, or do you use other methods of achieving sexual fulfillment? If you use other ways to make love, do you and your partner clearly understand that intercourse will not be attempted?
- What other effect does the pain have on your sexual relationship?
Explore biomedical antecedents
- When and how did the pain start?
- What tests have you undergone?
- What treatment have you received?
Source: Adapted from Basson R, et al.12
Is 60 the new 40?
Many women and men in the large cohort known as the Baby Boomer generation continue to be sexually active into their 60s, 70s, and 80s, as demonstrated by a 2007 study of sexuality and health in older adults.6 In the 57- to 64-year-old age group, 61.6% of women and 83.7% of men were sexually active (defined as sexual activity with a partner within the past 12 months). In the 65- to 74-year-old group, 39.5% of women and 67% of men were sexually active; and in the 75- to 85-year-old group, 16.7% of women and 38.5% of men were sexually active (TABLE).
These findings indicate that fewer women than men remain sexually active during their later years. One reason may be the epidemic of sexual-related symptoms among postmenopausal women. In the same survey, 34.3% of women 57 to 64 years old reported avoiding sex because of:
- pain during intercourse (17.8%)
- difficulty with lubrication (35.9%).
Across all groups, the most prevalent sexual problem was low desire (43%).6 Around 40% of postmenopausal women reported no sexual activity in the past 12 months, as well as lack of interest in sex. This number may include women who have ceased to have sex because of vaginal dryness and dyspareunia, thereby reducing the percentage reporting these symptoms (TABLE).
TABLE
Older adults are having sex—and experiencing sexual problems
| Activity or problem by gender | Number of respondents | Report, by age group (95% confidence interval*) | ||
|---|---|---|---|---|
| 57–64 yr (%) | 65–74 yr (%) | 75–85 yr (%) | ||
| Sexually active in previous 12 months† | ||||
| Men | 1,385 | 83.7 (77.6–89.8) | 67.0 (62.1–72.0) | 38.5 (33.6–43.5) |
| Women | 1,501 | 61.6 (56.7–66.4) | 39.5 (34.6–44.4) | 16.7 (12.5–21.0) |
| Difficulty with lubrication | ||||
| Women | 495 | 35.9 (29.6–42.2) | 43.2 (34.8–51.5) | 43.6 (27.0–60.2) |
| Pain during intercourse | ||||
| Men | 878 | 3.0 (1.1–4.8) | 3.2 (1.2–5.3) | 1.0 (0–2.5) |
| Women | 506 | 17.8 (13.3–22.2) | 18.6 (10.8–26.3) | 11.8 (4.3–19.4) |
| Avoidance of sex due to sexual problems** | ||||
| Men | 533 | 22.1 (17.3–26.9) | 30.1 (23.2–37.0) | 25.7 (14.9–36.4) |
| Women | 357 | 34.3 (25.0–43.7) | 30.5 (21.5–39.4) | 22.7 (9.4–35.9) |
| Source: Adapted from Lindau ST, et al.6 | ||||
| Adjusted odds ratios are based on a logistic regression including the age group and self-rated health status as covariates, estimated separately for men and women. The confidence interval is based on the inversion of the Wald tests constructed with the use of design-based standard errors. | ||||
| † These data exclude 107 respondents who reported at least one sexual problem. | ||||
| ** This question was asked only of respondents who reported at least one sexual problem. | ||||
Assessing menopause-related sexual function is a challenge
Although the transition phases of menopause have been well studied and reported for decades, few of these studies have included questions about the impact of menopause on sexual function.7 When longitudinal studies that included the classification of female sexual dysfunction began to appear, they provided evidence of the important role that VVA and psychosocial factors play in female sexual dysfunction.8
In the fourth year of the Melbourne Women’s Midlife Health Project longitudinal study, six variables related to sexual function were identified. Three were determinate of sexual function:
- feelings for the partner
- problems related to the partner
- vaginal dryness/dyspareunia.
The other three variables—sexual responsiveness, frequency of sexual activity, and libido—were dependent or outcome variables.
By the sixth year of this study, two variables had increased in significance: vaginal dryness/dyspareunia and partner problems.7
Sexual pain and relationship problems can create a vicious cycle
The interrelationship of vaginal dryness, sexual pain, flagging desire, and psychosocial parameters can produce a vicious cycle. A woman experiencing or anticipating pain may have diminished sexual desire or avoid sex altogether. During intercourse, the brain’s awareness of vaginal pain may trigger a physiologic response that can cause the muscles of the vagina to tighten and lubrication to decrease. The result? Greater vaginal pain.
This vicious cycle can contribute to relationship issues with the sexual partner and harm a woman’s psychosocial well-being. Resentment, anger, and misunderstanding may arise when a couple is dealing with problems of sexual function, and these stressors can damage many aspects of the relationship, further exacerbating sexual difficulties.
An additional and very important dimension of these issues is their potential impact on the family unit.
VVA can diminish overall well-being
In a 2007 survey reported at the North American Menopause Society (NAMS), one third to one half of 506 respondents said that VVA had a bad effect on their sexual interest, mood, self-esteem, and the intimate relationship (FIGURE 1).9 Reports from in-depth interviews were consistent with survey results and offered further insight into a woman’s emotional response to the condition of vaginal dryness and its impact on her life. Women found the condition “embarrassing,” something they had to endure but didn’t talk about, and felt that it had a major impact on their self-esteem and intimate relationship.

FIGURE 1 Dyspareunia affects more than interest in sex—relationships, mood, and self-esteem suffer
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
Clinicians often don’t ask about VVA, and patients are reluctant to talk
Among women of all ages, dyspareunia is underreported and undertreated. In the survey reported at NAMS, 40% of respondents said that their physician had never asked them about the problem of VVA (FIGURE 2).9
Women themselves may be reluctant to discuss the problem with physicians, nurse practitioners, or other health-care providers out of embarrassment or the assumption that there is nothing to be done about the problem. Nevertheless, more than 40% of respondents said they would be highly likely to seek treatment for VVA if they had a concern about urogenital complications of the condition (FIGURE 3).9
Another barrier may be the sense that asking the health-care provider about sex may embarrass him or her. As a result, sufferers do not anticipate help from their physician and other members of the health-care profession and fail to seek treatment or counseling for this chronic medical condition.10,11
In a 1999 telephone survey of 500 adults 25 years of age or older, 71% said they thought that their doctor would dismiss concerns about sexual problems, but 85% said they would talk to their physician anyway if they had a problem, even though they might not get treatment.11 In that survey, 91% of married men and 84% of married women rated a satisfying sex life as important to quality of life.11
Another important and often overlooked limitation on this type of discussion is the time constraints that busy clinicians face, especially with the low reimbursement offered by managed care. Sexual problems can hardly be adequately discussed in 7 to 10 minutes.

FIGURE 2 Do physicians ask about dyspareunia? Most women surveyed said “rarely” or “never”
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
FIGURE 3 Are these women likely to seek treatment?
Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
Women have performance anxiety, too
It is well known that men with even a mild degree of erectile dysfunction can suffer from performance anxiety, but the fact that women can also suffer from this phenomenon is not given as much attention. Such anxiety can be a factor in relationship difficulties. With both partners perhaps feeling anxious about sexual performance, a couple may avoid even simple acts of affection, such as holding hands, to avoid raising the other’s expectations.
Exacerbating the situation is the fact that many men use widely prescribed phosphodiesterase type 5 (PDE5) inhibitors, whereas women are contending with barriers to continued sexual activity as they age. It does not take a psychologist to understand that this imbalance often adds to emotional strain and tension between partners.
Popular media address the issue
Look beyond what our postmenopausal patients tell us directly—to the popular media and online forums—to appreciate the scope of sexual pain as a major issue among postmenopausal women. Evidence of psychosocial effects is found on numerous Web sites—some from organizations, others designed by women seeking help from each other.
Red Hot Mamas
This organization aims to empower women through menopause education. Highlighted in the Winter 2007/2008 Red Hot Mamas Report is a survey done in conjunction with Harris Interactive exploring the impact of menopausal symptoms on a woman’s sex life, which found that 47% of women who have VVA have avoided or stopped sex completely because it was uncomfortable, compared with 23% of normal women.
Power Surge
This Web site offers a list of strategies for dealing with sexual pain, including an overview of hormone-based prescription and nonprescription products, along with a variety of over-the-counter, natural, holistic, and herbal therapies for treating dyspareunia.
What is the physician’s role?
Given the epidemic of sexual pain, it is crucial that physicians and others who care for postmenopausal women increase their awareness of this issue and pay special attention to its psychosocial parameters.
Ask patients about sexual function in general and dyspareunia in particular as part of the routine annual visit. A simple opening “Yes/No” question, such as “Are you sexually active?” can lead to further questions appropriate to the patient. For example, if the answer is “No,” the follow-up question might be, “Does that bother you or your partner?” Further discussion may uncover whether the lack of sexual activity is a cause of distress and identify which variables are involved.
If, instead, the answer is “Yes,” follow-up questions can identify the presence of common postmenopausal physical issues, such as vaginal dryness and difficulty with lubrication. The visit then can turn to strategies to ameliorate those conditions.
When a patient reports dyspareunia, further diagnostic information such as precise location, degree of arousal, and reaction to pain can help determine the appropriate course of treatment. For an approach to this aspect of ascertaining patient history, see the list of sample questions above.12
During the physical, pay particular attention to any physical abnormalities or organic causes of sexual pain. Questions designed to characterize the location and nature of the pain can pinpoint the cause. Sexual pain arising from VVA is likely to 1) be localized at the introitus and 2) occur with penile entry.
Since the mid-1990s, the availability of validated scales to measure female sexual function has increased rapidly and enabled researchers to better identify, quantify, and evaluate treatments for female sexual dysfunction.7 Over time, we have moved away from the somewhat mechanical sequence inherent in the linear progression of desire leading to genital stimulation followed by arousal and orgasm toward an appreciation of the multiple physical, emotional, and subjective factors that are at play in women’s sexual function.
By 1998, a classification scheme was developed to further the means to study and discuss disorders of desire, arousal, orgasm, and sexual pain.8 Further contextual definitions of sexual dysfunction are under consideration.13
Basson proposed one new model of female sexual function (see the diagram), and observed that
"…women identify many reasons they are sexual over and beyond inherent sexual drive or “hunger.” Women tell of wanting to increase emotional closeness, commitment, sharing, tenderness, and tolerance, and to show the partner that he or she has been missed (emotionally or physically). Such intimacy-based reasons motivate the woman to find a way to become sexually aroused. This arousal is not spontaneous but triggered by deliberately sought sexual stimuli."13

Intimacy-based model of female sexual response cycle
In this flow of physical and emotional variables involved in female sexual function, categories interact. For example, low desire can be and is frequently secondary to the anticipation of pain during sexual intercourse. Arousal can be hampered by lack of vaginal lubrication—perhaps inhibited by the anticipation of pain. Secondary orgasmic disorders can result from low desire, difficulty of arousal, and sexual pain.14 Sexual pain can affect sexual function at any point on this continuum.
Treatments in the pipeline
For decades, hormone-based treatments have been the predominant therapeutic option for vaginal dryness. Often they are a secondary benefit of hormone therapy for vasomotor symptoms and osteoporosis. Estrogen can be delivered in the form of oral tablet, transdermal patch, gel, spray, or vaginal ring for systemic use, or as vaginal cream, ring, or tablet for local use.
However, despite data to the contrary and our reassurances to the patient about overall safety, a large number of women, and many primary care providers, are no longer inclined to use short- or long-term HRT in any presentation.
Other women may have risk factors that contraindicate exogenous hormones.
Nonhormonal options for vaginal dryness and dyspareunia are limited, and there are no approved systemic or oral nonestrogen options. Over-the-counter topical lubricants can ease some of the symptoms of VVA temporarily and allow successful vaginal penetration in many cases. Some may cause vaginal warming and pleasant sensations, but overall they treat the symptom rather than the source of pain. Moreover, many patients consider local lubricants messy and inconvenient and claim they “ruin the mood.”
The use of vaginal dilators along with estrogen or lubricant therapy is an often-forgotten adjunct to therapy for dyspareunia caused by VVA (FIGURE 4).

FIGURE 4 Mechanical dilation of the vagina is a useful adjunct
Mechanical dilation is often needed to restore penetration capability in the vagina, even after hormonal treatment. The focus should be on the vaginal introitus, with the top 25% to 35% of the dilator inserted into the opening once a day for 15 minutes, increasing the dilator diameter over time.
New SERMs are in development
Preclinical and clinical research into the diverse class of selective estrogen receptor modulators (SERMs) to treat estrogen-mediated disease produced tamoxifen for breast cancer prevention and raloxifene for both vertebral osteoporosis and breast cancer prevention. Each SERM seems to have unique tissue selectivity. The antiestrogenic activity of tamoxifen and raloxifene extends to the vagina and can exacerbate vaginal dryness.
A new generation of orally active SERMs is under investigation specifically for the treatment of chronic vaginal symptoms. These new agents target the nonvaginal treatment of VVA and associated symptoms. The first oral SERM for long-term treatment of these symptoms, ospemifene (Ophena), may become available in the near future. It is a novel SERM that has both anti-estrogenic and estrogenic actions, depending on the tissue. It was shown to significantly improve both vaginal dryness and dyspareunia in a large placebo-controlled trial.15
1. US Census Bureau. 2006 American community survey. S0101. Age and sex. Available at: http://fact-finder.census.gov/servlet/DatasetMainPageServlet?_program=ACS&_submenuId=&_lang=en&_ts.
2. National Center for Health Statistics. Health, United States, 2007, with Chartbook on Trends in the Health of Americans. Hyattsville, Md: NCHS; 2007. Available at: http://www.cdc.gov/nchs/fastats/lifexpec.htm. Accessed February 2, 2009.
3. Sarrel PM, Whitehead MI. Sex and menopause: defining the issues. Maturitas. 1985;7:217-224.
4. Dennerstein L, Hayes RD. Confronting the challenges: epidemiological study of female sexual dysfunction and the menopause. J Sex Med. 2005;2(suppl 3):118-132.
5. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
6. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762-774.
7. Dennerstein L, Alexander JL, Kotz K. The menopause and sexual functioning: a review of the population-based studies. Annu Rev Sex Res. 2003;14:64-82.
8. Basson R, Berman J, Burnett A, et al. Report of the international consensus development conference on female sexual dysfunction: definitions and classifications. J Urol. 2000;163:888-993.
9. Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
10. Heim LJ. Evaluation and differential diagnosis of dyspareunia. Am Fam Physician. 2001;63:1535-1552.
11. Marwick C. Survey says patients expect little physician help on sex. JAMA. 1999;281:2173-2174.
12. Basson R, Althof S, Davis S, et al. Summary of the recommendations on sexual dysfunctions in women. J Sex Med. 2004;1:24-34.
13. Basson R. Female sexual response: the role of drugs in the management of sexual dysfunction. Obstet Gynecol. 2001;98:350-353.
14. Walsh KE, Berman JR. Sexual dysfunction in the older woman: an overview of the current understanding and management. Drugs Aging. 2004;21:655-675.
15. Bachmann GA, Komi J, Hanley R. A new SERM, Ophena (ospemifene), effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Presented at the Endocrine Society annual meeting, San Francisco, Calif, June 2008.
1. US Census Bureau. 2006 American community survey. S0101. Age and sex. Available at: http://fact-finder.census.gov/servlet/DatasetMainPageServlet?_program=ACS&_submenuId=&_lang=en&_ts.
2. National Center for Health Statistics. Health, United States, 2007, with Chartbook on Trends in the Health of Americans. Hyattsville, Md: NCHS; 2007. Available at: http://www.cdc.gov/nchs/fastats/lifexpec.htm. Accessed February 2, 2009.
3. Sarrel PM, Whitehead MI. Sex and menopause: defining the issues. Maturitas. 1985;7:217-224.
4. Dennerstein L, Hayes RD. Confronting the challenges: epidemiological study of female sexual dysfunction and the menopause. J Sex Med. 2005;2(suppl 3):118-132.
5. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
6. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762-774.
7. Dennerstein L, Alexander JL, Kotz K. The menopause and sexual functioning: a review of the population-based studies. Annu Rev Sex Res. 2003;14:64-82.
8. Basson R, Berman J, Burnett A, et al. Report of the international consensus development conference on female sexual dysfunction: definitions and classifications. J Urol. 2000;163:888-993.
9. Simon JA, Komi J. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at North American Menopause Association Annual Meeting; October 3-6, 2007; Dallas, Texas.
10. Heim LJ. Evaluation and differential diagnosis of dyspareunia. Am Fam Physician. 2001;63:1535-1552.
11. Marwick C. Survey says patients expect little physician help on sex. JAMA. 1999;281:2173-2174.
12. Basson R, Althof S, Davis S, et al. Summary of the recommendations on sexual dysfunctions in women. J Sex Med. 2004;1:24-34.
13. Basson R. Female sexual response: the role of drugs in the management of sexual dysfunction. Obstet Gynecol. 2001;98:350-353.
14. Walsh KE, Berman JR. Sexual dysfunction in the older woman: an overview of the current understanding and management. Drugs Aging. 2004;21:655-675.
15. Bachmann GA, Komi J, Hanley R. A new SERM, Ophena (ospemifene), effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Presented at the Endocrine Society annual meeting, San Francisco, Calif, June 2008.
Expert tips for adnexal surgery through the laparoscope
The authors report no financial relationships relevant to this article.
CASE 1: Cystic mass in patient’s only remaining ovary
Mrs. R is a 29-year-old G1P1 who underwent a right oophorectomy, with a midline incision, for a dermoid cyst at the time of cesarean delivery. She now has a left ovarian cyst. Preoperative ultrasonography (US) reveals that it measures 3.5×4.2×3.7 cm and has both solid components and a multiloculated appearance, consistent with a dermoid cyst.
How common is this scenario?
Studies predict that one of every three women will undergo surgical management of an adnexal mass at some point in her life.1 This troubling statistic prompts several critical questions:
- How do we handle the workup for these women so that only appropriate patients undergo surgery?
- How often will a mass be malignant?
- How can we safely remove an adnexal mass to maximize patient safety, reduce overall recovery time, and prevent less favorable outcomes in women who are eventually found to have a malignancy?
A thorough workup and, sometimes, conservative management can prevent unnecessary surgery that may lead to early menopause or surgical complications. And maximizing the use of minimally invasive techniques in women who do require surgery can shorten hospital stay and recovery time. At the time of surgery, careful abdominal entry and meticulous surgical dissection and mass removal can limit the potential risks of laparoscopic excision in women who have an ultimate diagnosis of cancer.
In this article, we review the workup for women who have an adnexal mass, describe patient-selection criteria for laparoscopic surgery, including the risks and benefits of this approach ( TABLE 1 ), and present several techniques to safely manage a mass with potentially malignant histology via laparoscopy.
TABLE 1
There are benefits and risks to managing an adnexal mass laparoscopically
| Benefit | Risk |
|---|---|
| Shorter recovery Fewer adhesions Decreased overall cost Magnification Decreased pain and narcotic use Fewer wound complications | Expensive equipment* Loss of tactile sensation Concern for malignancy Risk of tumor dissemination/spillage/chemical peritonitis Trocar-site metastasis |
| *Though greater expense is not a risk per se, it does enter into decision making. | |
Begin with the physical
When a woman is known to have a pelvic mass, the aim of the office exam is to 1) identify characteristics that suggest malignancy and 2) rule out nongynecologic causes of the mass. Physical findings that are worrisome for a malignant process include:
- fixed or nodular pelvic mass
- bilateral masses
- nodular abdominal mass
- ascites
- pleural effusion on auscultation or percussion of the lung.
Although these findings can be present under benign conditions, they increase the risk that a malignancy will be detected at surgery.
Other causes of a pelvic mass should also be considered, including infection (pelvic abscess) and tumors of the colon, particularly when the pelvic mass occurs on the left side.
Some symptoms, though vague, are worth noting
Although ovarian cancer was once thought to be a silent disease, recent research has shown that bloating, pelvic or abdominal pain, early satiety, and urinary frequency and urgency are more common among women with ovarian cancer than among healthy controls and patients in high-risk screening clinics.2-4 Although these symptoms are generally nonspecific, they merit attention if they occur more than 12 times a month and have been present for less than 1 year. When they meet these criteria, the symptoms have a sensitivity for diagnosing early- and late-stage ovarian cancer of 56.7% and 79.5%, respectively.4
Sensitivity for the diagnosis of early-stage ovarian cancer may be as high as 80% when the symptom index score is combined with an elevated level of the tumor marker CA 125.3
Transvaginal US is crucial
Transvaginal US is now standard practice to obtain high-resolution images of an adnexal mass. Grayscale US has traditionally been used alone for evaluation.
Specificity is typically lower in women who are premenopausal because many benign lesions, such as endometrioma, have a similar sonographic appearance to cancer.
A number of US scoring algorithms have now been proposed to aid in the triage of women who have an adnexal mass. Sensitivity of these algorithms ranges from 65% to 100%; specificity, from 77% to 95%.5
CA 125 is the standard tumor marker
For the past two decades, CA 125 has been the standard serum marker in the screening of high-risk women for ovarian cancer and the triage of women who have an adnexal mass.
This blood test has been studied widely since its introduction in 1983. It typically has sensitivity of 75% to 85% and specificity of 85% to 95% in identifying women who have ovarian cancer. However, it is elevated in only 50% to 60% of women who have stage I ovarian cancer. Its lack of specificity and poor positive predictive value have kept researchers busy trying to identify other serum markers, for both ovarian cancer and identification of high-risk pelvic masses.
Our recommended workup and management of adnexal masses In postmenopausal women who had a pelvic mass, one study found that a CA 125 level above 65 IU/mL had sensitivity of 71% and specificity of 92.5% in the identification of ovarian cancer.6 Another group found that CA 125 levels above 65 IU/mL were more than 95% sensitive in the diagnosis of ovarian cancer in postmenopausal women.7
Several studies have combined CA 125 with other markers or with US to screen high-risk women or triage those who have an adnexal mass. These studies have shown modest improvements in sensitivity but usually lower specificity than with CA 125 testing alone.
Markers that may be used for suspected sex cord stromas and germ-cell tumors are:
- lactate dehydrogenase (LDH) for dysgerminomas
- alpha-fetoprotein (AFP) and human chorionic gonadotropin (hCG) for yolk sac tumors
- testosterone for Sertoli-Leydig cell tumors
- inhibin A and B for granulosa cell tumors.
An algorithm for working up and managing adnexal masses appears above.
How to gain abdominal access
In the opening case, the patient clearly has a benign mass. The treatment? Safe entry into the peritoneal cavity to remove the cyst and as little normal tissue as possible. This is critical in this patient because she has only one ovary.
Peritoneal access for abdominal and pelvic laparoscopy has been studied widely. Options include:
- direct insertion using a Veress needle
- open laparoscopy
- direct trocar insertion.
The technique usually depends on the preference of the surgeon. The primary goal of abdominal entry is to minimize the risk of injury, particularly unrecognized injury.
Data on complication rates show no definite benefit for open versus closed techniques in the prevention of injury to underlying viscera. However, evidence does suggest that the open technique may lower the risk of major vascular injury.8
We employ direct trocar insertion using radially expanding or optical trocars.
The Veress needle option
When the Veress needle is used to gain intraperitoneal access, data indicate that initial intraperitoneal pressure below 10 mm Hg is a reliable marker for peritoneal entry, even in obese patients.9 Insufflation pressure as high as 25 to 30 mm Hg prior to placement of the initial trocar is safe from a cardiopulmonary standpoint and may allow easier entry with a nonbladed trocar.10
Tests to confirm intraperitoneal placement of the Veress needle, such as the hanging-drop test or saline flush, do not appear to offer any additional useful information.11
Open laparoscopy is suitable when adhesions are unlikely
Open laparoscopy is typically performed by making a minilaparotomy incision at the umbilicus and then dissecting and entering the peritoneal cavity. A blunt-tip trocar is inserted.
The disadvantage of this approach is that there may be extensive adhesions under the umbilicus, and it is difficult to dissect such adhesions sufficiently to introduce a cannula and laparoscope. Adhesions left behind often obscure the field of view after introduction of the trocar.
Our preference? Left upper-quadrant insertion
In Case 1, the previous midline incision mandates an alternative approach. When abdominal entry at the umbilicus is unsuccessful or potentially difficult because of an earlier midline incision, umbilical hernia repair, or history of multiple lower abdominal or pelvic surgeries, a left upper-quadrant insertion is useful. It is, in fact, our preferred technique, and involves a small incision at the midclavicular line 5 cm below the left costal margin, at a site called Palmer’s point.
The direction of insertion usually ranges from 45° to 90°, depending on the patient’s body weight. If the trocar is placed properly, the closest organs are the stomach and the left lobe of the liver (4 to 6 cm).12 Given the stomach’s close proximity, it should be decompressed with an orogastric tube prior to trocar insertion.
Several studies have demonstrated the safety and efficacy of this entry technique.12-14 It can be helpful in cases that involve difficult access. We usually use a 5-mm primary trocar site for a 5-mm laparoscope. Modern optics allow for a pristine view with these small scopes, eliminating the need to close fascia and perhaps causing less pain at the incision.
Accessory trocar sites facilitate complex technique
We usually use three accessory sites. Two of them are lower-quadrant ports that are placed 2 cm medial and 2 cm cephalad to the anterior superior iliac spine. This area generally lies well away from the inferior epigastric vessels and remains above the area of the ilioinguinal and iliohypogastric nerves, making it a safer point of insertion.15 One trocar is 5 mm in size and the other is 10 mm. The larger one is used to extract the specimen.
We place an additional 5-mm port lateral to the rectus muscle at the level of the umbilicus. This allows the principal surgeon to use two instruments (a toothed forceps and scissors) comfortably while the assistant holds the laparoscope and assists with a grasper.
Does the type of trocar matter?
No randomized studies have directly compared all types of trocars. Options include:
- a pyramidal tip (as in reusable trocars) or shielded tip
- radial expansion
- visible entry
- blunt (Hasson-type) trocar.
Safety data on direct comparison of trocars are limited, but it appears that a radially expanding trocar may offer less port-site pain and potentially less bleeding than a traditional cutting trocar.16 Moreover, the rate of hernia at the port site appears to be relatively low with a radially expanding trocar, even when fascia is left unclosed at a 10-mm site.17
None of these trocars appears to be clearly superior at avoiding visceral or vascular injury.
Technique of laparoscopic cyst removal
A video clip of the surgery is linked to this article in the Video Library at www.obgmanagement.com. In this case, a trocar was inserted in the left upper quadrant, and a laparoscopic cystectomy was initiated using the trocars already specified.
The peritoneal cavity and adnexa were inspected, followed by pelvic washings, as detailed in TABLE 2 . Next, the ovarian cortex was incised ( FIGURE 1A ) with scissors using bipolar or unipolar energy, typically at a low power setting, such as 12 to 15 watts.
It was relatively easy to develop a tissue plane between the cortex and underlying dermoid cyst using simple or aqua-dissection ( FIGURE 1B and C ). The cyst was enucleated without rupture and inserted into a specimen bag through a 10-mm port ( FIGURE 1D ). (The specimen bags generally are available in 10-mm and 15-mm sizes.) Once the trocar was removed, the entire specimen was brought out through the incision ( FIGURE 1E ).
Cystic masses can generally be carefully aspirated using a laparoscopic needle or angiocath, or they can be incised and drained using a standard suction device. Manufacturers of most specimen bags do not recommend the morcellation of tissue within the bag because of the potential for rupture of the bag with tumor spillage or injury to underlying structures.
Occasionally, the mass remains too large to remove after drainage of the cyst fluid. Remedies include enlarging the port site with a scalpel or using a gallbladder speculum to increase the diameter of the port site ( FIGURE 1E ). If possible, the incision should be large enough to deliver the entire bag intact. Use of excessive force will rupture the bag and may cause the specimen to be lost or malignant cells to be inadvertently spilled (in the case of a cancer diagnosis).
TABLE 2
Take these 10 steps to safe laparoscopic surgery
| Examine the anesthetized patient |
| Enter the abdomen |
| Inspect the mass and peritoneal surfaces, including the diaphragm. Biopsy sites suspicious for metastasis and obtain frozen section |
| Perform pelvic and abdominal washings |
Closely inspect adnexa. If findings are not suggestive of malignancy, proceed with laparoscopy. If findings indicate obvious malignancy
|
Perform cystectomy or oophorectomy
|
| Inspect for hemostasis |
| Place cyst/ovary in endopouch |
| Open bag at abdominal wall and remove for frozen section |
| Reinspect and close |
Avoid:
|
FIGURE 1 Laparoscopic cyst removal
Begin by incising the cortex using scissors with or without an energy source.
Dissect the cyst free from the cortex using sharp dissection.
Remove the cyst from the ovary.
Place the cyst in a specimen bag, and …
… bring it to the surface for extraction. The abdominal incision may have to be enlarged to accommodate the specimen.
CASE 1: OUTCOME
The patient’s cyst is removed intact and she is discharged home. Rupture of a dermoid cyst is not associated with any problems as long as copious irrigation is used to aspirate the cyst content.18 Other cysts, such as endometriomas, may not be as easily dissected, and rupture is inevitable.
CASE 2: Symptoms suggestive of cancer
Mrs. B is a 47-year-old woman who reports abdominal bloating for the past 3 weeks. She also complains of early satiety and occasional constipation. She has no history of cancer, but her sister was given a diagnosis of breast cancer at 41 years of age, and her maternal aunt had breast cancer at 55 years.
Mrs. B is moderately obese, with a nontender abdomen and no palpable mass. Her pelvic exam also is negative for a mass or nodularity, but the extent of the exam is limited by body habitus. Her physician orders a transvaginal US, which reveals a 6-cm complex mass with thin septation and a 1-cm solid nodule, with no definite blood flow. The patient’s CA 125 level is 80 IU/mL, which we consider to be within the low-risk range for a premenopausal woman.
The patient is counseled about the need to have the mass removed and is scheduled for laparoscopic right salpingooophorectomy. Given the family history of breast cancer, the physician also requests consultation with a gynecologic oncologist, who agrees to assist with surgery and perform a laparotomy and staging in the event that a malignancy is diagnosed.
Is the mass likely to be malignant?
Given the patient’s family history of breast cancer, the recent onset of symptoms associated with ovarian cancer,2 and the characteristics of the mass (complex, with a nodule), malignancy is possible. This patient has an intermediate risk of cancer and requires additional counseling and planning.
However, most women who undergo laparoscopy for removal of an adnexal mass have benign pathologic findings.
What is the real risk of ovarian cancer?
The lifetime risk of developing ovarian cancer in the general population remains stable at approximately 1 in 70 women, with a mean age at diagnosis of 63 years.19 Ninety percent of ovarian cancer cases are sporadic, and less than 10% can be linked to genetic syndromes.
Women who have mutations in the BRCA1 gene carry a lifetime risk of ovarian cancer of up to 50%, and women who have mutations in BRCA2 have a lifetime risk of up to 25%.20,21 Women who have mutations associated with Lynch II syndrome or Hereditary Nonpolyposis Colorectal Cancer syndrome may have a lifetime risk of ovarian cancer of 12%.22,23
Some women who have a strong family history of breast and ovarian cancer do not carry a known mutation, but are likely to be at increased risk.
Additional risk factors known to be associated with ovarian cancer are nulliparity and infertility. However, the single most important risk factor for epithelial ovarian cancer is age.
Risk-reducing strategies include:
In the case of PBSO, it is imperative to ensure that all ovarian surface epithelium is removed. This means excising the infundibulopelvic ligament at least 1.5 cm above the proximal end of the ovary and excising any adjacent tissue to which the ovary is adherent (including pelvic sidewall peritoneum). Both requirements are easily achieved using the techniques outlined here.
Who should perform surgery?
The American College of Obstetricians and Gynecologists (ACOG) and the Society of Gynecologic Oncologists (SGO) have published guidelines for referral to a gynecologic oncologist ( TABLE 3 ). When Im and colleagues reviewed the records of more than 1,000 women who underwent surgery for a pelvic mass at six institutions over 12 months, they found that 70% of premenopausal women and 94% of postmenopausal women who were diagnosed with ovarian cancer were properly referred to a qualified subspecialist.27 “Over”-referral occurred in 30% to 40% of women who had a benign mass, but may be preferable given the importance of proper staging and debulking to survival.
ACOG and the SGO recommend referral for women who have:
- elevated tumor markers
- ascites
- a fixed or nodular mass
- a strong family history of breast or ovarian cancer.
Consider preoperative referral of all high-risk and, probably, intermediate-risk women, depending on the availability of qualified specialists for complete surgical staging.
In addition, women need to be counseled thoroughly about the possibility that a malignancy will be diagnosed by frozen section, necessitating additional surgical procedures.
TABLE 3
Your patient has a newly diagnosed pelvic mass. Should you refer her?
| Is she premenopausal? Then refer her when… | Is she postmenopausal? Then refer her when… |
|---|---|
| CA125 >200 IU/mL Ascites is present Evidence of abdominal or distant metastasis on exam or imaging Family history of breast or ovarian cancer in a first-degree relative | CA125 >35 IU/mL Ascites is present Nodular or fixed pelvic mass Evidence of abdominal or distant metastasis on exam or imaging Family history of breast or ovarian cancer in a first-degree relative |
Technique of laparoscopic adnexectomy
In Case 2, an umbilical site was chosen for the primary cannula. In general, the direction of insertion depends on the patient’s body habitus. Heavier patients have a thicker abdominal wall and an umbilicus below the level of the aortic bifurcation. In these women, the angle of insertion should be adjusted from the usual 45° (for patients of normal weight) to an angle nearer to 90°. Lateral ports are typical, as in Case 1.
Treat every mass like cancer
Laparoscopic excision of an adnexal mass can be safe and effective, with better outcomes and recovery than with laparotomy, provided the surgeon adheres to basic principles ( TABLE 2 ). This means treating every mass as though it is potentially malignant, and thoroughly inspecting the abdominal cavity before and after excision of the mass.
Know the retroperitoneal space
As with Case 1, successful laparoscopic excision of an adnexal mass begins with inspection of the peritoneal cavity, abdominopelvic washings, and identification of both the infundibulopelvic ligament and ureter. Knowledge of the retroperitoneal space can be of great value in difficult cases that involve significant pelvic adhesions or sidewall fibrosis. We generally use a retroperitoneal approach for laparoscopic adnexectomy ( FIGURE 2A ).
In our typical approach, we incise the peritoneum lateral to the uteroovarian ligament and continue the incision up the pelvic sidewall lateral to the infundibulopelvic ligament and up along the paracolic gutter, if needed ( FIGURE 2A ). We then mobilize the medial leaf of the broad ligament from the sidewall using blunt dissection between the external iliac vessels laterally and the ureter medially ( FIGURE 2B ).
Once we have identified the ureter, we use scissors to create a window in the medial leaf of the broad ligament just beneath the gonadal vessels. We then use an energy source to occlude and transect the pedicle. Using this technique, we secure the infundibulopelvic ligaments and safely mobilize the ureter before initiating more aggressive sidewall dissection distally for adhesions or fibrosis.
Once the ureter is mobilized, we excise the involved peritoneum along with the mass. We then seal and transect the uteroovarian ligament.
FIGURE 2 The retroperitoneal approach
Grasp and incise the peritoneum just lateral to the adnexal mass and enter the retroperitoneal space, where loose areolar tissue is visible, with the ureter seen on the medial leaf of the broad ligament.
After dissecting this tissue, identify the ureter and internal iliac vessels.
Remove the mass in a bag
Once the mass is excised, we place it in a laparoscopic bag, as described for Case 1. Solid adnexal masses are problematic because they are not amenable to drainage, and morcellation is usually discouraged. Laparoscopic excision can still be carried out, and a minilaparotomy or posterior colpotomy can be used to extract the mass in a bag. Patients managed in this way still have a complication rate similar to or lower than that of patients undergoing laparotomy for oophorectomy; they also recover faster.
Risks in cases of malignancy
The risk of tumor spillage in laparoscopic surgery can be lowered using laparoscopically guided minilaparotomy techniques. Preoperative rupture appears to be more predictive of outcome.28
The concern about metastatic implants in a laparoscopic port site in patients who have gynecologic cancer is real, with an incidence ranging from 0.97% to 1.1%. The phenomenon usually affects women who had ovarian cancer and is most common after laparoscopy with findings of ascites, carcinomatosis, or persistent disease (in the case of second-look laparoscopy performed after completion of primary therapy for ovarian cancer).
These data suggest that port-site implantation is not a concern that should deter clinicians from laparoscopic evaluation of a suspected ovarian neoplasm unless a patient presents with ascites or carcinomatosis suggestive of advanced disease.29
Data are limited regarding delays in definitive therapy. One study suggests that definitive staging on the day of rupture does not influence overall outcome, but delay by more than 2 weeks may lead to poorer prognosis.30
CASE 2: OUTCOME
Laparoscopy is performed. On initial inspection, the peritoneal cavity is unremarkable. The right ovary is multicystic with normal surface anatomy. The mass is somewhat adherent to the pelvic sidewall. Adnexectomy is performed, and frozen section reveals the mass to be a serous cystadenoma.
1. Curtin JP. Management of the adnexal mass. Gynecol Oncol. 1994;55:S42-S46.
2. Goff BA, Mandel LS, Melancon CH, Muntz HG. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291:2705-2712.
3. Andersen MR, Goff BA, Lowe KA, et al. Combining a symptoms index with CA 125 to improve detection of ovarian cancer. Cancer. 2008;113:484-489.
4. Goff BA, Mandel LS, Drescher CW, et al. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer. 2007;109:221-227.
5. Alcázar JL, Mercé LT, Laparte C, et al. A new scoring system to differentiate benign from malignant adnexal masses. Am J Obstet Gynecol. 2003;188:685-692.
6. Maggino T, Gadducci A, D’Addario V, et al. Prospective multicenter study on CA 125 in postmenopausal pelvic masses. Gynecol Oncol. 1994;54:117-123.
7. Malkasian GD, Jr, Knapp RC, Lavin PT, et al. Preoperative evaluation of serum CA 125 levels in premenopausal and postmenopausal patients with pelvic masses: discrimination of benign from malignant disease. Am J Obstet Gynecol. 1988;159:341-346.
8. Larobina M, Nottle P. Complete evidence regarding major vascular injuries during laparoscopic access. Surg Laparosc Endosc Percutan Tech. 2005;15:119-123.
9. Vilos GA, Vilos AG. Safe laparoscopic entry guided by Veress needle CO2 insufflation pressure. J Am Assoc Gynecol Laparosc. 2003;10:415-420.
10. Vilos GA, Vilos AG, Abu-Rafea B, Hollett-Caines J, Nikkhah-Abyaneh Z, Edris F. Three simple steps during closed laparoscopic entry may minimize major injuries. Surg Endosc. 2008 July 15. [Epub ahead of print]
11. Vilos GA, Ternamian A, Dempster J, Laberge PY. The Society of Obstetricians and Gynaecologists of Canada. Laparoscopic entry: a review of techniques, technologies, and complications. J Obstet Gynaecol Can. 2007;29:433-465.
12. Tulikangas PK, Nicklas A, Falcone T, Price LL. Anatomy of the left upper quadrant for cannula insertion. J Am Assoc Gynecol Laparosc. 2000;7:211-214.
13. Stepp KJ, Tulikangas PK, Goldberg JM, Attaran M, Falcone T. Laparoscopy for adnexal masses in the second trimester of pregnancy. J Am Assoc Gynecol Laparosc. 2003;10:55-59.
14. Tulikangas PK, Robinson DS, Falcone T. Left upper quadrant cannula insertion. Fertil Steril. 2003;79:411-412.
15. Whiteside JL, Barber MD, Walters MD, Falcone T. Anatomy of ilioinguinal and iliohypogastric nerves in relation to trocar placement and low transverse incisions. Am J Obstet Gynecol. 2003;189:1574-1578.
16. Yim SF, Yuen PM. Randomized double-masked comparison of radially expanding access device and conventional cutting tip trocar in laparoscopy. Obstet Gynecol. 2001;97:435-438.
17. Johnson WH, Fecher AM, McMahon RL, et al. VersaStep trocar hernia rate in unclosed fascial defects in bariatric patients. Surg Endosc. 2006;20:1584-1586.
18. Lin P, Falcone T, Tulandi T. Excision of ovarian dermoid cyst by laparoscopy and by laparotomy. Am J Obstet Gynecol. 1995;173:769-771.
19. Jemal A, Murray T, Samuels A, et al. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5-26.
20. Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingooophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609-1615.
21. King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643-646.
22. Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214-218.
23. Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105-110.
24. Dowdy SC, Stefanek M, Hartmann LC. Surgical risk reduction: prophylactic salpingooophorectomy and prophylactic mastectomy. Am J Obstet Gynecol. 2004;191:1113-1123.
25. Narod SA, Risch H, Moslehi R, et al. Oral contraceptives and the risk of hereditary ovarian cancer. Hereditary Ovarian Cancer Clinical Study Group. N Engl J Med. 1998;339:424-428.
26. Whittemore AS, Balise RR, Pharoah PD, et al. Oral contraceptive use and ovarian cancer risk among carriers of BRCA1 or BRCA2 mutations. Br J Cancer. 2004;91:1911-1915.
27. Im SS, Gordon AN, Buttin BM, et al. Validation of referral guidelines for women with pelvic masses. Obstet Gynecol. 2005;105:35-41.
28. Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357:176-182.
29. Ramirez PT, Wolf JK, Levenback C. Laparoscopic port-site metastases: etiology and prevention. Gynecol Oncol. 2003;91:179-189.
30. Lehner R, Wenzl R, Heinzl H, Husslein P, Sevelda P. Influence of delayed staging laparotomy after laparoscopic removal of ovarian masses later found malignant. Obstet Gynecol. 1998;92:967-971.
The authors report no financial relationships relevant to this article.
CASE 1: Cystic mass in patient’s only remaining ovary
Mrs. R is a 29-year-old G1P1 who underwent a right oophorectomy, with a midline incision, for a dermoid cyst at the time of cesarean delivery. She now has a left ovarian cyst. Preoperative ultrasonography (US) reveals that it measures 3.5×4.2×3.7 cm and has both solid components and a multiloculated appearance, consistent with a dermoid cyst.
How common is this scenario?
Studies predict that one of every three women will undergo surgical management of an adnexal mass at some point in her life.1 This troubling statistic prompts several critical questions:
- How do we handle the workup for these women so that only appropriate patients undergo surgery?
- How often will a mass be malignant?
- How can we safely remove an adnexal mass to maximize patient safety, reduce overall recovery time, and prevent less favorable outcomes in women who are eventually found to have a malignancy?
A thorough workup and, sometimes, conservative management can prevent unnecessary surgery that may lead to early menopause or surgical complications. And maximizing the use of minimally invasive techniques in women who do require surgery can shorten hospital stay and recovery time. At the time of surgery, careful abdominal entry and meticulous surgical dissection and mass removal can limit the potential risks of laparoscopic excision in women who have an ultimate diagnosis of cancer.
In this article, we review the workup for women who have an adnexal mass, describe patient-selection criteria for laparoscopic surgery, including the risks and benefits of this approach ( TABLE 1 ), and present several techniques to safely manage a mass with potentially malignant histology via laparoscopy.
TABLE 1
There are benefits and risks to managing an adnexal mass laparoscopically
| Benefit | Risk |
|---|---|
| Shorter recovery Fewer adhesions Decreased overall cost Magnification Decreased pain and narcotic use Fewer wound complications | Expensive equipment* Loss of tactile sensation Concern for malignancy Risk of tumor dissemination/spillage/chemical peritonitis Trocar-site metastasis |
| *Though greater expense is not a risk per se, it does enter into decision making. | |
Begin with the physical
When a woman is known to have a pelvic mass, the aim of the office exam is to 1) identify characteristics that suggest malignancy and 2) rule out nongynecologic causes of the mass. Physical findings that are worrisome for a malignant process include:
- fixed or nodular pelvic mass
- bilateral masses
- nodular abdominal mass
- ascites
- pleural effusion on auscultation or percussion of the lung.
Although these findings can be present under benign conditions, they increase the risk that a malignancy will be detected at surgery.
Other causes of a pelvic mass should also be considered, including infection (pelvic abscess) and tumors of the colon, particularly when the pelvic mass occurs on the left side.
Some symptoms, though vague, are worth noting
Although ovarian cancer was once thought to be a silent disease, recent research has shown that bloating, pelvic or abdominal pain, early satiety, and urinary frequency and urgency are more common among women with ovarian cancer than among healthy controls and patients in high-risk screening clinics.2-4 Although these symptoms are generally nonspecific, they merit attention if they occur more than 12 times a month and have been present for less than 1 year. When they meet these criteria, the symptoms have a sensitivity for diagnosing early- and late-stage ovarian cancer of 56.7% and 79.5%, respectively.4
Sensitivity for the diagnosis of early-stage ovarian cancer may be as high as 80% when the symptom index score is combined with an elevated level of the tumor marker CA 125.3
Transvaginal US is crucial
Transvaginal US is now standard practice to obtain high-resolution images of an adnexal mass. Grayscale US has traditionally been used alone for evaluation.
Specificity is typically lower in women who are premenopausal because many benign lesions, such as endometrioma, have a similar sonographic appearance to cancer.
A number of US scoring algorithms have now been proposed to aid in the triage of women who have an adnexal mass. Sensitivity of these algorithms ranges from 65% to 100%; specificity, from 77% to 95%.5
CA 125 is the standard tumor marker
For the past two decades, CA 125 has been the standard serum marker in the screening of high-risk women for ovarian cancer and the triage of women who have an adnexal mass.
This blood test has been studied widely since its introduction in 1983. It typically has sensitivity of 75% to 85% and specificity of 85% to 95% in identifying women who have ovarian cancer. However, it is elevated in only 50% to 60% of women who have stage I ovarian cancer. Its lack of specificity and poor positive predictive value have kept researchers busy trying to identify other serum markers, for both ovarian cancer and identification of high-risk pelvic masses.
Our recommended workup and management of adnexal masses In postmenopausal women who had a pelvic mass, one study found that a CA 125 level above 65 IU/mL had sensitivity of 71% and specificity of 92.5% in the identification of ovarian cancer.6 Another group found that CA 125 levels above 65 IU/mL were more than 95% sensitive in the diagnosis of ovarian cancer in postmenopausal women.7
Several studies have combined CA 125 with other markers or with US to screen high-risk women or triage those who have an adnexal mass. These studies have shown modest improvements in sensitivity but usually lower specificity than with CA 125 testing alone.
Markers that may be used for suspected sex cord stromas and germ-cell tumors are:
- lactate dehydrogenase (LDH) for dysgerminomas
- alpha-fetoprotein (AFP) and human chorionic gonadotropin (hCG) for yolk sac tumors
- testosterone for Sertoli-Leydig cell tumors
- inhibin A and B for granulosa cell tumors.
An algorithm for working up and managing adnexal masses appears above.
How to gain abdominal access
In the opening case, the patient clearly has a benign mass. The treatment? Safe entry into the peritoneal cavity to remove the cyst and as little normal tissue as possible. This is critical in this patient because she has only one ovary.
Peritoneal access for abdominal and pelvic laparoscopy has been studied widely. Options include:
- direct insertion using a Veress needle
- open laparoscopy
- direct trocar insertion.
The technique usually depends on the preference of the surgeon. The primary goal of abdominal entry is to minimize the risk of injury, particularly unrecognized injury.
Data on complication rates show no definite benefit for open versus closed techniques in the prevention of injury to underlying viscera. However, evidence does suggest that the open technique may lower the risk of major vascular injury.8
We employ direct trocar insertion using radially expanding or optical trocars.
The Veress needle option
When the Veress needle is used to gain intraperitoneal access, data indicate that initial intraperitoneal pressure below 10 mm Hg is a reliable marker for peritoneal entry, even in obese patients.9 Insufflation pressure as high as 25 to 30 mm Hg prior to placement of the initial trocar is safe from a cardiopulmonary standpoint and may allow easier entry with a nonbladed trocar.10
Tests to confirm intraperitoneal placement of the Veress needle, such as the hanging-drop test or saline flush, do not appear to offer any additional useful information.11
Open laparoscopy is suitable when adhesions are unlikely
Open laparoscopy is typically performed by making a minilaparotomy incision at the umbilicus and then dissecting and entering the peritoneal cavity. A blunt-tip trocar is inserted.
The disadvantage of this approach is that there may be extensive adhesions under the umbilicus, and it is difficult to dissect such adhesions sufficiently to introduce a cannula and laparoscope. Adhesions left behind often obscure the field of view after introduction of the trocar.
Our preference? Left upper-quadrant insertion
In Case 1, the previous midline incision mandates an alternative approach. When abdominal entry at the umbilicus is unsuccessful or potentially difficult because of an earlier midline incision, umbilical hernia repair, or history of multiple lower abdominal or pelvic surgeries, a left upper-quadrant insertion is useful. It is, in fact, our preferred technique, and involves a small incision at the midclavicular line 5 cm below the left costal margin, at a site called Palmer’s point.
The direction of insertion usually ranges from 45° to 90°, depending on the patient’s body weight. If the trocar is placed properly, the closest organs are the stomach and the left lobe of the liver (4 to 6 cm).12 Given the stomach’s close proximity, it should be decompressed with an orogastric tube prior to trocar insertion.
Several studies have demonstrated the safety and efficacy of this entry technique.12-14 It can be helpful in cases that involve difficult access. We usually use a 5-mm primary trocar site for a 5-mm laparoscope. Modern optics allow for a pristine view with these small scopes, eliminating the need to close fascia and perhaps causing less pain at the incision.
Accessory trocar sites facilitate complex technique
We usually use three accessory sites. Two of them are lower-quadrant ports that are placed 2 cm medial and 2 cm cephalad to the anterior superior iliac spine. This area generally lies well away from the inferior epigastric vessels and remains above the area of the ilioinguinal and iliohypogastric nerves, making it a safer point of insertion.15 One trocar is 5 mm in size and the other is 10 mm. The larger one is used to extract the specimen.
We place an additional 5-mm port lateral to the rectus muscle at the level of the umbilicus. This allows the principal surgeon to use two instruments (a toothed forceps and scissors) comfortably while the assistant holds the laparoscope and assists with a grasper.
Does the type of trocar matter?
No randomized studies have directly compared all types of trocars. Options include:
- a pyramidal tip (as in reusable trocars) or shielded tip
- radial expansion
- visible entry
- blunt (Hasson-type) trocar.
Safety data on direct comparison of trocars are limited, but it appears that a radially expanding trocar may offer less port-site pain and potentially less bleeding than a traditional cutting trocar.16 Moreover, the rate of hernia at the port site appears to be relatively low with a radially expanding trocar, even when fascia is left unclosed at a 10-mm site.17
None of these trocars appears to be clearly superior at avoiding visceral or vascular injury.
Technique of laparoscopic cyst removal
A video clip of the surgery is linked to this article in the Video Library at www.obgmanagement.com. In this case, a trocar was inserted in the left upper quadrant, and a laparoscopic cystectomy was initiated using the trocars already specified.
The peritoneal cavity and adnexa were inspected, followed by pelvic washings, as detailed in TABLE 2 . Next, the ovarian cortex was incised ( FIGURE 1A ) with scissors using bipolar or unipolar energy, typically at a low power setting, such as 12 to 15 watts.
It was relatively easy to develop a tissue plane between the cortex and underlying dermoid cyst using simple or aqua-dissection ( FIGURE 1B and C ). The cyst was enucleated without rupture and inserted into a specimen bag through a 10-mm port ( FIGURE 1D ). (The specimen bags generally are available in 10-mm and 15-mm sizes.) Once the trocar was removed, the entire specimen was brought out through the incision ( FIGURE 1E ).
Cystic masses can generally be carefully aspirated using a laparoscopic needle or angiocath, or they can be incised and drained using a standard suction device. Manufacturers of most specimen bags do not recommend the morcellation of tissue within the bag because of the potential for rupture of the bag with tumor spillage or injury to underlying structures.
Occasionally, the mass remains too large to remove after drainage of the cyst fluid. Remedies include enlarging the port site with a scalpel or using a gallbladder speculum to increase the diameter of the port site ( FIGURE 1E ). If possible, the incision should be large enough to deliver the entire bag intact. Use of excessive force will rupture the bag and may cause the specimen to be lost or malignant cells to be inadvertently spilled (in the case of a cancer diagnosis).
TABLE 2
Take these 10 steps to safe laparoscopic surgery
| Examine the anesthetized patient |
| Enter the abdomen |
| Inspect the mass and peritoneal surfaces, including the diaphragm. Biopsy sites suspicious for metastasis and obtain frozen section |
| Perform pelvic and abdominal washings |
Closely inspect adnexa. If findings are not suggestive of malignancy, proceed with laparoscopy. If findings indicate obvious malignancy
|
Perform cystectomy or oophorectomy
|
| Inspect for hemostasis |
| Place cyst/ovary in endopouch |
| Open bag at abdominal wall and remove for frozen section |
| Reinspect and close |
Avoid:
|
FIGURE 1 Laparoscopic cyst removal
Begin by incising the cortex using scissors with or without an energy source.
Dissect the cyst free from the cortex using sharp dissection.
Remove the cyst from the ovary.
Place the cyst in a specimen bag, and …
… bring it to the surface for extraction. The abdominal incision may have to be enlarged to accommodate the specimen.
CASE 1: OUTCOME
The patient’s cyst is removed intact and she is discharged home. Rupture of a dermoid cyst is not associated with any problems as long as copious irrigation is used to aspirate the cyst content.18 Other cysts, such as endometriomas, may not be as easily dissected, and rupture is inevitable.
CASE 2: Symptoms suggestive of cancer
Mrs. B is a 47-year-old woman who reports abdominal bloating for the past 3 weeks. She also complains of early satiety and occasional constipation. She has no history of cancer, but her sister was given a diagnosis of breast cancer at 41 years of age, and her maternal aunt had breast cancer at 55 years.
Mrs. B is moderately obese, with a nontender abdomen and no palpable mass. Her pelvic exam also is negative for a mass or nodularity, but the extent of the exam is limited by body habitus. Her physician orders a transvaginal US, which reveals a 6-cm complex mass with thin septation and a 1-cm solid nodule, with no definite blood flow. The patient’s CA 125 level is 80 IU/mL, which we consider to be within the low-risk range for a premenopausal woman.
The patient is counseled about the need to have the mass removed and is scheduled for laparoscopic right salpingooophorectomy. Given the family history of breast cancer, the physician also requests consultation with a gynecologic oncologist, who agrees to assist with surgery and perform a laparotomy and staging in the event that a malignancy is diagnosed.
Is the mass likely to be malignant?
Given the patient’s family history of breast cancer, the recent onset of symptoms associated with ovarian cancer,2 and the characteristics of the mass (complex, with a nodule), malignancy is possible. This patient has an intermediate risk of cancer and requires additional counseling and planning.
However, most women who undergo laparoscopy for removal of an adnexal mass have benign pathologic findings.
What is the real risk of ovarian cancer?
The lifetime risk of developing ovarian cancer in the general population remains stable at approximately 1 in 70 women, with a mean age at diagnosis of 63 years.19 Ninety percent of ovarian cancer cases are sporadic, and less than 10% can be linked to genetic syndromes.
Women who have mutations in the BRCA1 gene carry a lifetime risk of ovarian cancer of up to 50%, and women who have mutations in BRCA2 have a lifetime risk of up to 25%.20,21 Women who have mutations associated with Lynch II syndrome or Hereditary Nonpolyposis Colorectal Cancer syndrome may have a lifetime risk of ovarian cancer of 12%.22,23
Some women who have a strong family history of breast and ovarian cancer do not carry a known mutation, but are likely to be at increased risk.
Additional risk factors known to be associated with ovarian cancer are nulliparity and infertility. However, the single most important risk factor for epithelial ovarian cancer is age.
Risk-reducing strategies include:
In the case of PBSO, it is imperative to ensure that all ovarian surface epithelium is removed. This means excising the infundibulopelvic ligament at least 1.5 cm above the proximal end of the ovary and excising any adjacent tissue to which the ovary is adherent (including pelvic sidewall peritoneum). Both requirements are easily achieved using the techniques outlined here.
Who should perform surgery?
The American College of Obstetricians and Gynecologists (ACOG) and the Society of Gynecologic Oncologists (SGO) have published guidelines for referral to a gynecologic oncologist ( TABLE 3 ). When Im and colleagues reviewed the records of more than 1,000 women who underwent surgery for a pelvic mass at six institutions over 12 months, they found that 70% of premenopausal women and 94% of postmenopausal women who were diagnosed with ovarian cancer were properly referred to a qualified subspecialist.27 “Over”-referral occurred in 30% to 40% of women who had a benign mass, but may be preferable given the importance of proper staging and debulking to survival.
ACOG and the SGO recommend referral for women who have:
- elevated tumor markers
- ascites
- a fixed or nodular mass
- a strong family history of breast or ovarian cancer.
Consider preoperative referral of all high-risk and, probably, intermediate-risk women, depending on the availability of qualified specialists for complete surgical staging.
In addition, women need to be counseled thoroughly about the possibility that a malignancy will be diagnosed by frozen section, necessitating additional surgical procedures.
TABLE 3
Your patient has a newly diagnosed pelvic mass. Should you refer her?
| Is she premenopausal? Then refer her when… | Is she postmenopausal? Then refer her when… |
|---|---|
| CA125 >200 IU/mL Ascites is present Evidence of abdominal or distant metastasis on exam or imaging Family history of breast or ovarian cancer in a first-degree relative | CA125 >35 IU/mL Ascites is present Nodular or fixed pelvic mass Evidence of abdominal or distant metastasis on exam or imaging Family history of breast or ovarian cancer in a first-degree relative |
Technique of laparoscopic adnexectomy
In Case 2, an umbilical site was chosen for the primary cannula. In general, the direction of insertion depends on the patient’s body habitus. Heavier patients have a thicker abdominal wall and an umbilicus below the level of the aortic bifurcation. In these women, the angle of insertion should be adjusted from the usual 45° (for patients of normal weight) to an angle nearer to 90°. Lateral ports are typical, as in Case 1.
Treat every mass like cancer
Laparoscopic excision of an adnexal mass can be safe and effective, with better outcomes and recovery than with laparotomy, provided the surgeon adheres to basic principles ( TABLE 2 ). This means treating every mass as though it is potentially malignant, and thoroughly inspecting the abdominal cavity before and after excision of the mass.
Know the retroperitoneal space
As with Case 1, successful laparoscopic excision of an adnexal mass begins with inspection of the peritoneal cavity, abdominopelvic washings, and identification of both the infundibulopelvic ligament and ureter. Knowledge of the retroperitoneal space can be of great value in difficult cases that involve significant pelvic adhesions or sidewall fibrosis. We generally use a retroperitoneal approach for laparoscopic adnexectomy ( FIGURE 2A ).
In our typical approach, we incise the peritoneum lateral to the uteroovarian ligament and continue the incision up the pelvic sidewall lateral to the infundibulopelvic ligament and up along the paracolic gutter, if needed ( FIGURE 2A ). We then mobilize the medial leaf of the broad ligament from the sidewall using blunt dissection between the external iliac vessels laterally and the ureter medially ( FIGURE 2B ).
Once we have identified the ureter, we use scissors to create a window in the medial leaf of the broad ligament just beneath the gonadal vessels. We then use an energy source to occlude and transect the pedicle. Using this technique, we secure the infundibulopelvic ligaments and safely mobilize the ureter before initiating more aggressive sidewall dissection distally for adhesions or fibrosis.
Once the ureter is mobilized, we excise the involved peritoneum along with the mass. We then seal and transect the uteroovarian ligament.
FIGURE 2 The retroperitoneal approach
Grasp and incise the peritoneum just lateral to the adnexal mass and enter the retroperitoneal space, where loose areolar tissue is visible, with the ureter seen on the medial leaf of the broad ligament.
After dissecting this tissue, identify the ureter and internal iliac vessels.
Remove the mass in a bag
Once the mass is excised, we place it in a laparoscopic bag, as described for Case 1. Solid adnexal masses are problematic because they are not amenable to drainage, and morcellation is usually discouraged. Laparoscopic excision can still be carried out, and a minilaparotomy or posterior colpotomy can be used to extract the mass in a bag. Patients managed in this way still have a complication rate similar to or lower than that of patients undergoing laparotomy for oophorectomy; they also recover faster.
Risks in cases of malignancy
The risk of tumor spillage in laparoscopic surgery can be lowered using laparoscopically guided minilaparotomy techniques. Preoperative rupture appears to be more predictive of outcome.28
The concern about metastatic implants in a laparoscopic port site in patients who have gynecologic cancer is real, with an incidence ranging from 0.97% to 1.1%. The phenomenon usually affects women who had ovarian cancer and is most common after laparoscopy with findings of ascites, carcinomatosis, or persistent disease (in the case of second-look laparoscopy performed after completion of primary therapy for ovarian cancer).
These data suggest that port-site implantation is not a concern that should deter clinicians from laparoscopic evaluation of a suspected ovarian neoplasm unless a patient presents with ascites or carcinomatosis suggestive of advanced disease.29
Data are limited regarding delays in definitive therapy. One study suggests that definitive staging on the day of rupture does not influence overall outcome, but delay by more than 2 weeks may lead to poorer prognosis.30
CASE 2: OUTCOME
Laparoscopy is performed. On initial inspection, the peritoneal cavity is unremarkable. The right ovary is multicystic with normal surface anatomy. The mass is somewhat adherent to the pelvic sidewall. Adnexectomy is performed, and frozen section reveals the mass to be a serous cystadenoma.
The authors report no financial relationships relevant to this article.
CASE 1: Cystic mass in patient’s only remaining ovary
Mrs. R is a 29-year-old G1P1 who underwent a right oophorectomy, with a midline incision, for a dermoid cyst at the time of cesarean delivery. She now has a left ovarian cyst. Preoperative ultrasonography (US) reveals that it measures 3.5×4.2×3.7 cm and has both solid components and a multiloculated appearance, consistent with a dermoid cyst.
How common is this scenario?
Studies predict that one of every three women will undergo surgical management of an adnexal mass at some point in her life.1 This troubling statistic prompts several critical questions:
- How do we handle the workup for these women so that only appropriate patients undergo surgery?
- How often will a mass be malignant?
- How can we safely remove an adnexal mass to maximize patient safety, reduce overall recovery time, and prevent less favorable outcomes in women who are eventually found to have a malignancy?
A thorough workup and, sometimes, conservative management can prevent unnecessary surgery that may lead to early menopause or surgical complications. And maximizing the use of minimally invasive techniques in women who do require surgery can shorten hospital stay and recovery time. At the time of surgery, careful abdominal entry and meticulous surgical dissection and mass removal can limit the potential risks of laparoscopic excision in women who have an ultimate diagnosis of cancer.
In this article, we review the workup for women who have an adnexal mass, describe patient-selection criteria for laparoscopic surgery, including the risks and benefits of this approach ( TABLE 1 ), and present several techniques to safely manage a mass with potentially malignant histology via laparoscopy.
TABLE 1
There are benefits and risks to managing an adnexal mass laparoscopically
| Benefit | Risk |
|---|---|
| Shorter recovery Fewer adhesions Decreased overall cost Magnification Decreased pain and narcotic use Fewer wound complications | Expensive equipment* Loss of tactile sensation Concern for malignancy Risk of tumor dissemination/spillage/chemical peritonitis Trocar-site metastasis |
| *Though greater expense is not a risk per se, it does enter into decision making. | |
Begin with the physical
When a woman is known to have a pelvic mass, the aim of the office exam is to 1) identify characteristics that suggest malignancy and 2) rule out nongynecologic causes of the mass. Physical findings that are worrisome for a malignant process include:
- fixed or nodular pelvic mass
- bilateral masses
- nodular abdominal mass
- ascites
- pleural effusion on auscultation or percussion of the lung.
Although these findings can be present under benign conditions, they increase the risk that a malignancy will be detected at surgery.
Other causes of a pelvic mass should also be considered, including infection (pelvic abscess) and tumors of the colon, particularly when the pelvic mass occurs on the left side.
Some symptoms, though vague, are worth noting
Although ovarian cancer was once thought to be a silent disease, recent research has shown that bloating, pelvic or abdominal pain, early satiety, and urinary frequency and urgency are more common among women with ovarian cancer than among healthy controls and patients in high-risk screening clinics.2-4 Although these symptoms are generally nonspecific, they merit attention if they occur more than 12 times a month and have been present for less than 1 year. When they meet these criteria, the symptoms have a sensitivity for diagnosing early- and late-stage ovarian cancer of 56.7% and 79.5%, respectively.4
Sensitivity for the diagnosis of early-stage ovarian cancer may be as high as 80% when the symptom index score is combined with an elevated level of the tumor marker CA 125.3
Transvaginal US is crucial
Transvaginal US is now standard practice to obtain high-resolution images of an adnexal mass. Grayscale US has traditionally been used alone for evaluation.
Specificity is typically lower in women who are premenopausal because many benign lesions, such as endometrioma, have a similar sonographic appearance to cancer.
A number of US scoring algorithms have now been proposed to aid in the triage of women who have an adnexal mass. Sensitivity of these algorithms ranges from 65% to 100%; specificity, from 77% to 95%.5
CA 125 is the standard tumor marker
For the past two decades, CA 125 has been the standard serum marker in the screening of high-risk women for ovarian cancer and the triage of women who have an adnexal mass.
This blood test has been studied widely since its introduction in 1983. It typically has sensitivity of 75% to 85% and specificity of 85% to 95% in identifying women who have ovarian cancer. However, it is elevated in only 50% to 60% of women who have stage I ovarian cancer. Its lack of specificity and poor positive predictive value have kept researchers busy trying to identify other serum markers, for both ovarian cancer and identification of high-risk pelvic masses.
Our recommended workup and management of adnexal masses In postmenopausal women who had a pelvic mass, one study found that a CA 125 level above 65 IU/mL had sensitivity of 71% and specificity of 92.5% in the identification of ovarian cancer.6 Another group found that CA 125 levels above 65 IU/mL were more than 95% sensitive in the diagnosis of ovarian cancer in postmenopausal women.7
Several studies have combined CA 125 with other markers or with US to screen high-risk women or triage those who have an adnexal mass. These studies have shown modest improvements in sensitivity but usually lower specificity than with CA 125 testing alone.
Markers that may be used for suspected sex cord stromas and germ-cell tumors are:
- lactate dehydrogenase (LDH) for dysgerminomas
- alpha-fetoprotein (AFP) and human chorionic gonadotropin (hCG) for yolk sac tumors
- testosterone for Sertoli-Leydig cell tumors
- inhibin A and B for granulosa cell tumors.
An algorithm for working up and managing adnexal masses appears above.
How to gain abdominal access
In the opening case, the patient clearly has a benign mass. The treatment? Safe entry into the peritoneal cavity to remove the cyst and as little normal tissue as possible. This is critical in this patient because she has only one ovary.
Peritoneal access for abdominal and pelvic laparoscopy has been studied widely. Options include:
- direct insertion using a Veress needle
- open laparoscopy
- direct trocar insertion.
The technique usually depends on the preference of the surgeon. The primary goal of abdominal entry is to minimize the risk of injury, particularly unrecognized injury.
Data on complication rates show no definite benefit for open versus closed techniques in the prevention of injury to underlying viscera. However, evidence does suggest that the open technique may lower the risk of major vascular injury.8
We employ direct trocar insertion using radially expanding or optical trocars.
The Veress needle option
When the Veress needle is used to gain intraperitoneal access, data indicate that initial intraperitoneal pressure below 10 mm Hg is a reliable marker for peritoneal entry, even in obese patients.9 Insufflation pressure as high as 25 to 30 mm Hg prior to placement of the initial trocar is safe from a cardiopulmonary standpoint and may allow easier entry with a nonbladed trocar.10
Tests to confirm intraperitoneal placement of the Veress needle, such as the hanging-drop test or saline flush, do not appear to offer any additional useful information.11
Open laparoscopy is suitable when adhesions are unlikely
Open laparoscopy is typically performed by making a minilaparotomy incision at the umbilicus and then dissecting and entering the peritoneal cavity. A blunt-tip trocar is inserted.
The disadvantage of this approach is that there may be extensive adhesions under the umbilicus, and it is difficult to dissect such adhesions sufficiently to introduce a cannula and laparoscope. Adhesions left behind often obscure the field of view after introduction of the trocar.
Our preference? Left upper-quadrant insertion
In Case 1, the previous midline incision mandates an alternative approach. When abdominal entry at the umbilicus is unsuccessful or potentially difficult because of an earlier midline incision, umbilical hernia repair, or history of multiple lower abdominal or pelvic surgeries, a left upper-quadrant insertion is useful. It is, in fact, our preferred technique, and involves a small incision at the midclavicular line 5 cm below the left costal margin, at a site called Palmer’s point.
The direction of insertion usually ranges from 45° to 90°, depending on the patient’s body weight. If the trocar is placed properly, the closest organs are the stomach and the left lobe of the liver (4 to 6 cm).12 Given the stomach’s close proximity, it should be decompressed with an orogastric tube prior to trocar insertion.
Several studies have demonstrated the safety and efficacy of this entry technique.12-14 It can be helpful in cases that involve difficult access. We usually use a 5-mm primary trocar site for a 5-mm laparoscope. Modern optics allow for a pristine view with these small scopes, eliminating the need to close fascia and perhaps causing less pain at the incision.
Accessory trocar sites facilitate complex technique
We usually use three accessory sites. Two of them are lower-quadrant ports that are placed 2 cm medial and 2 cm cephalad to the anterior superior iliac spine. This area generally lies well away from the inferior epigastric vessels and remains above the area of the ilioinguinal and iliohypogastric nerves, making it a safer point of insertion.15 One trocar is 5 mm in size and the other is 10 mm. The larger one is used to extract the specimen.
We place an additional 5-mm port lateral to the rectus muscle at the level of the umbilicus. This allows the principal surgeon to use two instruments (a toothed forceps and scissors) comfortably while the assistant holds the laparoscope and assists with a grasper.
Does the type of trocar matter?
No randomized studies have directly compared all types of trocars. Options include:
- a pyramidal tip (as in reusable trocars) or shielded tip
- radial expansion
- visible entry
- blunt (Hasson-type) trocar.
Safety data on direct comparison of trocars are limited, but it appears that a radially expanding trocar may offer less port-site pain and potentially less bleeding than a traditional cutting trocar.16 Moreover, the rate of hernia at the port site appears to be relatively low with a radially expanding trocar, even when fascia is left unclosed at a 10-mm site.17
None of these trocars appears to be clearly superior at avoiding visceral or vascular injury.
Technique of laparoscopic cyst removal
A video clip of the surgery is linked to this article in the Video Library at www.obgmanagement.com. In this case, a trocar was inserted in the left upper quadrant, and a laparoscopic cystectomy was initiated using the trocars already specified.
The peritoneal cavity and adnexa were inspected, followed by pelvic washings, as detailed in TABLE 2 . Next, the ovarian cortex was incised ( FIGURE 1A ) with scissors using bipolar or unipolar energy, typically at a low power setting, such as 12 to 15 watts.
It was relatively easy to develop a tissue plane between the cortex and underlying dermoid cyst using simple or aqua-dissection ( FIGURE 1B and C ). The cyst was enucleated without rupture and inserted into a specimen bag through a 10-mm port ( FIGURE 1D ). (The specimen bags generally are available in 10-mm and 15-mm sizes.) Once the trocar was removed, the entire specimen was brought out through the incision ( FIGURE 1E ).
Cystic masses can generally be carefully aspirated using a laparoscopic needle or angiocath, or they can be incised and drained using a standard suction device. Manufacturers of most specimen bags do not recommend the morcellation of tissue within the bag because of the potential for rupture of the bag with tumor spillage or injury to underlying structures.
Occasionally, the mass remains too large to remove after drainage of the cyst fluid. Remedies include enlarging the port site with a scalpel or using a gallbladder speculum to increase the diameter of the port site ( FIGURE 1E ). If possible, the incision should be large enough to deliver the entire bag intact. Use of excessive force will rupture the bag and may cause the specimen to be lost or malignant cells to be inadvertently spilled (in the case of a cancer diagnosis).
TABLE 2
Take these 10 steps to safe laparoscopic surgery
| Examine the anesthetized patient |
| Enter the abdomen |
| Inspect the mass and peritoneal surfaces, including the diaphragm. Biopsy sites suspicious for metastasis and obtain frozen section |
| Perform pelvic and abdominal washings |
Closely inspect adnexa. If findings are not suggestive of malignancy, proceed with laparoscopy. If findings indicate obvious malignancy
|
Perform cystectomy or oophorectomy
|
| Inspect for hemostasis |
| Place cyst/ovary in endopouch |
| Open bag at abdominal wall and remove for frozen section |
| Reinspect and close |
Avoid:
|
FIGURE 1 Laparoscopic cyst removal
Begin by incising the cortex using scissors with or without an energy source.
Dissect the cyst free from the cortex using sharp dissection.
Remove the cyst from the ovary.
Place the cyst in a specimen bag, and …
… bring it to the surface for extraction. The abdominal incision may have to be enlarged to accommodate the specimen.
CASE 1: OUTCOME
The patient’s cyst is removed intact and she is discharged home. Rupture of a dermoid cyst is not associated with any problems as long as copious irrigation is used to aspirate the cyst content.18 Other cysts, such as endometriomas, may not be as easily dissected, and rupture is inevitable.
CASE 2: Symptoms suggestive of cancer
Mrs. B is a 47-year-old woman who reports abdominal bloating for the past 3 weeks. She also complains of early satiety and occasional constipation. She has no history of cancer, but her sister was given a diagnosis of breast cancer at 41 years of age, and her maternal aunt had breast cancer at 55 years.
Mrs. B is moderately obese, with a nontender abdomen and no palpable mass. Her pelvic exam also is negative for a mass or nodularity, but the extent of the exam is limited by body habitus. Her physician orders a transvaginal US, which reveals a 6-cm complex mass with thin septation and a 1-cm solid nodule, with no definite blood flow. The patient’s CA 125 level is 80 IU/mL, which we consider to be within the low-risk range for a premenopausal woman.
The patient is counseled about the need to have the mass removed and is scheduled for laparoscopic right salpingooophorectomy. Given the family history of breast cancer, the physician also requests consultation with a gynecologic oncologist, who agrees to assist with surgery and perform a laparotomy and staging in the event that a malignancy is diagnosed.
Is the mass likely to be malignant?
Given the patient’s family history of breast cancer, the recent onset of symptoms associated with ovarian cancer,2 and the characteristics of the mass (complex, with a nodule), malignancy is possible. This patient has an intermediate risk of cancer and requires additional counseling and planning.
However, most women who undergo laparoscopy for removal of an adnexal mass have benign pathologic findings.
What is the real risk of ovarian cancer?
The lifetime risk of developing ovarian cancer in the general population remains stable at approximately 1 in 70 women, with a mean age at diagnosis of 63 years.19 Ninety percent of ovarian cancer cases are sporadic, and less than 10% can be linked to genetic syndromes.
Women who have mutations in the BRCA1 gene carry a lifetime risk of ovarian cancer of up to 50%, and women who have mutations in BRCA2 have a lifetime risk of up to 25%.20,21 Women who have mutations associated with Lynch II syndrome or Hereditary Nonpolyposis Colorectal Cancer syndrome may have a lifetime risk of ovarian cancer of 12%.22,23
Some women who have a strong family history of breast and ovarian cancer do not carry a known mutation, but are likely to be at increased risk.
Additional risk factors known to be associated with ovarian cancer are nulliparity and infertility. However, the single most important risk factor for epithelial ovarian cancer is age.
Risk-reducing strategies include:
In the case of PBSO, it is imperative to ensure that all ovarian surface epithelium is removed. This means excising the infundibulopelvic ligament at least 1.5 cm above the proximal end of the ovary and excising any adjacent tissue to which the ovary is adherent (including pelvic sidewall peritoneum). Both requirements are easily achieved using the techniques outlined here.
Who should perform surgery?
The American College of Obstetricians and Gynecologists (ACOG) and the Society of Gynecologic Oncologists (SGO) have published guidelines for referral to a gynecologic oncologist ( TABLE 3 ). When Im and colleagues reviewed the records of more than 1,000 women who underwent surgery for a pelvic mass at six institutions over 12 months, they found that 70% of premenopausal women and 94% of postmenopausal women who were diagnosed with ovarian cancer were properly referred to a qualified subspecialist.27 “Over”-referral occurred in 30% to 40% of women who had a benign mass, but may be preferable given the importance of proper staging and debulking to survival.
ACOG and the SGO recommend referral for women who have:
- elevated tumor markers
- ascites
- a fixed or nodular mass
- a strong family history of breast or ovarian cancer.
Consider preoperative referral of all high-risk and, probably, intermediate-risk women, depending on the availability of qualified specialists for complete surgical staging.
In addition, women need to be counseled thoroughly about the possibility that a malignancy will be diagnosed by frozen section, necessitating additional surgical procedures.
TABLE 3
Your patient has a newly diagnosed pelvic mass. Should you refer her?
| Is she premenopausal? Then refer her when… | Is she postmenopausal? Then refer her when… |
|---|---|
| CA125 >200 IU/mL Ascites is present Evidence of abdominal or distant metastasis on exam or imaging Family history of breast or ovarian cancer in a first-degree relative | CA125 >35 IU/mL Ascites is present Nodular or fixed pelvic mass Evidence of abdominal or distant metastasis on exam or imaging Family history of breast or ovarian cancer in a first-degree relative |
Technique of laparoscopic adnexectomy
In Case 2, an umbilical site was chosen for the primary cannula. In general, the direction of insertion depends on the patient’s body habitus. Heavier patients have a thicker abdominal wall and an umbilicus below the level of the aortic bifurcation. In these women, the angle of insertion should be adjusted from the usual 45° (for patients of normal weight) to an angle nearer to 90°. Lateral ports are typical, as in Case 1.
Treat every mass like cancer
Laparoscopic excision of an adnexal mass can be safe and effective, with better outcomes and recovery than with laparotomy, provided the surgeon adheres to basic principles ( TABLE 2 ). This means treating every mass as though it is potentially malignant, and thoroughly inspecting the abdominal cavity before and after excision of the mass.
Know the retroperitoneal space
As with Case 1, successful laparoscopic excision of an adnexal mass begins with inspection of the peritoneal cavity, abdominopelvic washings, and identification of both the infundibulopelvic ligament and ureter. Knowledge of the retroperitoneal space can be of great value in difficult cases that involve significant pelvic adhesions or sidewall fibrosis. We generally use a retroperitoneal approach for laparoscopic adnexectomy ( FIGURE 2A ).
In our typical approach, we incise the peritoneum lateral to the uteroovarian ligament and continue the incision up the pelvic sidewall lateral to the infundibulopelvic ligament and up along the paracolic gutter, if needed ( FIGURE 2A ). We then mobilize the medial leaf of the broad ligament from the sidewall using blunt dissection between the external iliac vessels laterally and the ureter medially ( FIGURE 2B ).
Once we have identified the ureter, we use scissors to create a window in the medial leaf of the broad ligament just beneath the gonadal vessels. We then use an energy source to occlude and transect the pedicle. Using this technique, we secure the infundibulopelvic ligaments and safely mobilize the ureter before initiating more aggressive sidewall dissection distally for adhesions or fibrosis.
Once the ureter is mobilized, we excise the involved peritoneum along with the mass. We then seal and transect the uteroovarian ligament.
FIGURE 2 The retroperitoneal approach
Grasp and incise the peritoneum just lateral to the adnexal mass and enter the retroperitoneal space, where loose areolar tissue is visible, with the ureter seen on the medial leaf of the broad ligament.
After dissecting this tissue, identify the ureter and internal iliac vessels.
Remove the mass in a bag
Once the mass is excised, we place it in a laparoscopic bag, as described for Case 1. Solid adnexal masses are problematic because they are not amenable to drainage, and morcellation is usually discouraged. Laparoscopic excision can still be carried out, and a minilaparotomy or posterior colpotomy can be used to extract the mass in a bag. Patients managed in this way still have a complication rate similar to or lower than that of patients undergoing laparotomy for oophorectomy; they also recover faster.
Risks in cases of malignancy
The risk of tumor spillage in laparoscopic surgery can be lowered using laparoscopically guided minilaparotomy techniques. Preoperative rupture appears to be more predictive of outcome.28
The concern about metastatic implants in a laparoscopic port site in patients who have gynecologic cancer is real, with an incidence ranging from 0.97% to 1.1%. The phenomenon usually affects women who had ovarian cancer and is most common after laparoscopy with findings of ascites, carcinomatosis, or persistent disease (in the case of second-look laparoscopy performed after completion of primary therapy for ovarian cancer).
These data suggest that port-site implantation is not a concern that should deter clinicians from laparoscopic evaluation of a suspected ovarian neoplasm unless a patient presents with ascites or carcinomatosis suggestive of advanced disease.29
Data are limited regarding delays in definitive therapy. One study suggests that definitive staging on the day of rupture does not influence overall outcome, but delay by more than 2 weeks may lead to poorer prognosis.30
CASE 2: OUTCOME
Laparoscopy is performed. On initial inspection, the peritoneal cavity is unremarkable. The right ovary is multicystic with normal surface anatomy. The mass is somewhat adherent to the pelvic sidewall. Adnexectomy is performed, and frozen section reveals the mass to be a serous cystadenoma.
1. Curtin JP. Management of the adnexal mass. Gynecol Oncol. 1994;55:S42-S46.
2. Goff BA, Mandel LS, Melancon CH, Muntz HG. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291:2705-2712.
3. Andersen MR, Goff BA, Lowe KA, et al. Combining a symptoms index with CA 125 to improve detection of ovarian cancer. Cancer. 2008;113:484-489.
4. Goff BA, Mandel LS, Drescher CW, et al. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer. 2007;109:221-227.
5. Alcázar JL, Mercé LT, Laparte C, et al. A new scoring system to differentiate benign from malignant adnexal masses. Am J Obstet Gynecol. 2003;188:685-692.
6. Maggino T, Gadducci A, D’Addario V, et al. Prospective multicenter study on CA 125 in postmenopausal pelvic masses. Gynecol Oncol. 1994;54:117-123.
7. Malkasian GD, Jr, Knapp RC, Lavin PT, et al. Preoperative evaluation of serum CA 125 levels in premenopausal and postmenopausal patients with pelvic masses: discrimination of benign from malignant disease. Am J Obstet Gynecol. 1988;159:341-346.
8. Larobina M, Nottle P. Complete evidence regarding major vascular injuries during laparoscopic access. Surg Laparosc Endosc Percutan Tech. 2005;15:119-123.
9. Vilos GA, Vilos AG. Safe laparoscopic entry guided by Veress needle CO2 insufflation pressure. J Am Assoc Gynecol Laparosc. 2003;10:415-420.
10. Vilos GA, Vilos AG, Abu-Rafea B, Hollett-Caines J, Nikkhah-Abyaneh Z, Edris F. Three simple steps during closed laparoscopic entry may minimize major injuries. Surg Endosc. 2008 July 15. [Epub ahead of print]
11. Vilos GA, Ternamian A, Dempster J, Laberge PY. The Society of Obstetricians and Gynaecologists of Canada. Laparoscopic entry: a review of techniques, technologies, and complications. J Obstet Gynaecol Can. 2007;29:433-465.
12. Tulikangas PK, Nicklas A, Falcone T, Price LL. Anatomy of the left upper quadrant for cannula insertion. J Am Assoc Gynecol Laparosc. 2000;7:211-214.
13. Stepp KJ, Tulikangas PK, Goldberg JM, Attaran M, Falcone T. Laparoscopy for adnexal masses in the second trimester of pregnancy. J Am Assoc Gynecol Laparosc. 2003;10:55-59.
14. Tulikangas PK, Robinson DS, Falcone T. Left upper quadrant cannula insertion. Fertil Steril. 2003;79:411-412.
15. Whiteside JL, Barber MD, Walters MD, Falcone T. Anatomy of ilioinguinal and iliohypogastric nerves in relation to trocar placement and low transverse incisions. Am J Obstet Gynecol. 2003;189:1574-1578.
16. Yim SF, Yuen PM. Randomized double-masked comparison of radially expanding access device and conventional cutting tip trocar in laparoscopy. Obstet Gynecol. 2001;97:435-438.
17. Johnson WH, Fecher AM, McMahon RL, et al. VersaStep trocar hernia rate in unclosed fascial defects in bariatric patients. Surg Endosc. 2006;20:1584-1586.
18. Lin P, Falcone T, Tulandi T. Excision of ovarian dermoid cyst by laparoscopy and by laparotomy. Am J Obstet Gynecol. 1995;173:769-771.
19. Jemal A, Murray T, Samuels A, et al. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5-26.
20. Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingooophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609-1615.
21. King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643-646.
22. Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214-218.
23. Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105-110.
24. Dowdy SC, Stefanek M, Hartmann LC. Surgical risk reduction: prophylactic salpingooophorectomy and prophylactic mastectomy. Am J Obstet Gynecol. 2004;191:1113-1123.
25. Narod SA, Risch H, Moslehi R, et al. Oral contraceptives and the risk of hereditary ovarian cancer. Hereditary Ovarian Cancer Clinical Study Group. N Engl J Med. 1998;339:424-428.
26. Whittemore AS, Balise RR, Pharoah PD, et al. Oral contraceptive use and ovarian cancer risk among carriers of BRCA1 or BRCA2 mutations. Br J Cancer. 2004;91:1911-1915.
27. Im SS, Gordon AN, Buttin BM, et al. Validation of referral guidelines for women with pelvic masses. Obstet Gynecol. 2005;105:35-41.
28. Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357:176-182.
29. Ramirez PT, Wolf JK, Levenback C. Laparoscopic port-site metastases: etiology and prevention. Gynecol Oncol. 2003;91:179-189.
30. Lehner R, Wenzl R, Heinzl H, Husslein P, Sevelda P. Influence of delayed staging laparotomy after laparoscopic removal of ovarian masses later found malignant. Obstet Gynecol. 1998;92:967-971.
1. Curtin JP. Management of the adnexal mass. Gynecol Oncol. 1994;55:S42-S46.
2. Goff BA, Mandel LS, Melancon CH, Muntz HG. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291:2705-2712.
3. Andersen MR, Goff BA, Lowe KA, et al. Combining a symptoms index with CA 125 to improve detection of ovarian cancer. Cancer. 2008;113:484-489.
4. Goff BA, Mandel LS, Drescher CW, et al. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer. 2007;109:221-227.
5. Alcázar JL, Mercé LT, Laparte C, et al. A new scoring system to differentiate benign from malignant adnexal masses. Am J Obstet Gynecol. 2003;188:685-692.
6. Maggino T, Gadducci A, D’Addario V, et al. Prospective multicenter study on CA 125 in postmenopausal pelvic masses. Gynecol Oncol. 1994;54:117-123.
7. Malkasian GD, Jr, Knapp RC, Lavin PT, et al. Preoperative evaluation of serum CA 125 levels in premenopausal and postmenopausal patients with pelvic masses: discrimination of benign from malignant disease. Am J Obstet Gynecol. 1988;159:341-346.
8. Larobina M, Nottle P. Complete evidence regarding major vascular injuries during laparoscopic access. Surg Laparosc Endosc Percutan Tech. 2005;15:119-123.
9. Vilos GA, Vilos AG. Safe laparoscopic entry guided by Veress needle CO2 insufflation pressure. J Am Assoc Gynecol Laparosc. 2003;10:415-420.
10. Vilos GA, Vilos AG, Abu-Rafea B, Hollett-Caines J, Nikkhah-Abyaneh Z, Edris F. Three simple steps during closed laparoscopic entry may minimize major injuries. Surg Endosc. 2008 July 15. [Epub ahead of print]
11. Vilos GA, Ternamian A, Dempster J, Laberge PY. The Society of Obstetricians and Gynaecologists of Canada. Laparoscopic entry: a review of techniques, technologies, and complications. J Obstet Gynaecol Can. 2007;29:433-465.
12. Tulikangas PK, Nicklas A, Falcone T, Price LL. Anatomy of the left upper quadrant for cannula insertion. J Am Assoc Gynecol Laparosc. 2000;7:211-214.
13. Stepp KJ, Tulikangas PK, Goldberg JM, Attaran M, Falcone T. Laparoscopy for adnexal masses in the second trimester of pregnancy. J Am Assoc Gynecol Laparosc. 2003;10:55-59.
14. Tulikangas PK, Robinson DS, Falcone T. Left upper quadrant cannula insertion. Fertil Steril. 2003;79:411-412.
15. Whiteside JL, Barber MD, Walters MD, Falcone T. Anatomy of ilioinguinal and iliohypogastric nerves in relation to trocar placement and low transverse incisions. Am J Obstet Gynecol. 2003;189:1574-1578.
16. Yim SF, Yuen PM. Randomized double-masked comparison of radially expanding access device and conventional cutting tip trocar in laparoscopy. Obstet Gynecol. 2001;97:435-438.
17. Johnson WH, Fecher AM, McMahon RL, et al. VersaStep trocar hernia rate in unclosed fascial defects in bariatric patients. Surg Endosc. 2006;20:1584-1586.
18. Lin P, Falcone T, Tulandi T. Excision of ovarian dermoid cyst by laparoscopy and by laparotomy. Am J Obstet Gynecol. 1995;173:769-771.
19. Jemal A, Murray T, Samuels A, et al. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5-26.
20. Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingooophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609-1615.
21. King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643-646.
22. Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214-218.
23. Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105-110.
24. Dowdy SC, Stefanek M, Hartmann LC. Surgical risk reduction: prophylactic salpingooophorectomy and prophylactic mastectomy. Am J Obstet Gynecol. 2004;191:1113-1123.
25. Narod SA, Risch H, Moslehi R, et al. Oral contraceptives and the risk of hereditary ovarian cancer. Hereditary Ovarian Cancer Clinical Study Group. N Engl J Med. 1998;339:424-428.
26. Whittemore AS, Balise RR, Pharoah PD, et al. Oral contraceptive use and ovarian cancer risk among carriers of BRCA1 or BRCA2 mutations. Br J Cancer. 2004;91:1911-1915.
27. Im SS, Gordon AN, Buttin BM, et al. Validation of referral guidelines for women with pelvic masses. Obstet Gynecol. 2005;105:35-41.
28. Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357:176-182.
29. Ramirez PT, Wolf JK, Levenback C. Laparoscopic port-site metastases: etiology and prevention. Gynecol Oncol. 2003;91:179-189.
30. Lehner R, Wenzl R, Heinzl H, Husslein P, Sevelda P. Influence of delayed staging laparotomy after laparoscopic removal of ovarian masses later found malignant. Obstet Gynecol. 1998;92:967-971.
‘Night owls’: Reset the physiologic clock in delayed sleep phase disorder
Jason, age 16, has had difficulty with sleep initiation for 2 years. He describes going to bed at 10:30 PM on school nights but falling asleep no sooner than midnight and typically after 1:30 AM. He denies contributions from an “active mind” or environmental disturbances, and his bedroom contains no TV, computer, or other media devices. He does not sleep better with a change in environment. He denies pervasive low mood symptoms and believes his mood hinges predominantly on his ability to achieve sufficient sleep.
Once asleep, Jason generally enjoys good sleep consolidation until he needs to arise at 6:30 AM. His mother awakens him with difficulty, as he often sleeps through his alarm. He sleeps approximately 5 hours nightly during the school week, endorses impaired concentration, and often dozes during his first several classes. When he returns home from school, he finds it very difficult to resist napping.
On weekends he retires at 1 AM or later and typically falls asleep within 30 minutes. He usually awakens at noon but can sleep as late as 4:30 PM. He feels slightly more refreshed on weekends and describes his mood then as improved. During a recent spring break, he felt much better when allowed to sleep as much as he wanted.
Delayed sleep phase disorder (DSPD)—characterized by a pathological “night owl” circadian preference—is seen most commonly in adolescents and is associated with psychiatric morbidity, psychosocial impairment, and poor academic performance. Proper identification of the condition can be enhanced with a variety of assessment tools, and successful treatment requires an awareness of potential endogenous and exogenous contributors.
This article describes what is known about DSPD and uses the case example to illustrate diagnostic assessment and treatment choices. Intriguing data support various pathophysiologic explanations for DSPD (Box 1).1-6 Facilitating the adjustment of patients’ physiologic clocks is the overall goal in managing DSPD.
In individuals normally entrained to the light/dark cycle, circadian rhythms are:
- delayed by evening exposure to bright light (≥ 2,500 lux) prior to the core body temperature minimum (Tmin)
- advanced by morning light exposure after the Tmin.1
These opposing effects attune most people to the light/dark cycle, with sleep and wakefulness occurring on a conventional schedule. Persons with delayed sleep phase disorder (DSPD) live at a delayed phase that resists advancement and is incompatible with their personal and social obligations.
Theories have been proposed, but DSPD’s etiology has not been fully explained. Affected adolescents may exhibit an extreme in circadian preference. Case reports also describe DSPD emerging after traumatic brain injury.2
Intriguing evidence supports various pathophysiologic explanations for DSPD. An abnormally long intrinsic circadian period (>25 hours) was recently demonstrated during temporal isolation in 1 individual with DSPD.3 Both this case report and controlled studies describe deviations from expected relationships between the sleep/wake cycle and physiologic circadian markers. Most consistently described are longer intervals from Tmin4 to sleep offset (final rise time) in DSPD patients compared with controls.
Other research suggests:
Extreme ‘eveningness’
Because of their extreme seemingly innate preference to retire and arise at relatively late clock hours (an “eveningness” trait), school-aged patients with DSPD represent a high-risk population for problematic sleepiness. In a survey of 612 high school students, the 63% who felt they needed more sleep on school nights showed a strong eveningness preference (as assessed by questionnaire), compared with students who described getting sufficient sleep.7 Other studies have revealed psychiatric morbidity (including affective and personality disorders), psychosocial impairment, and poor academic performance associated with the condition.8-10
DSPD may affect 7% to 16% of patients presenting with insomnia complaints in sleep medicine clinics.11 The condition appears most common among young cohorts and has been reported to affect up to 7% of adolescents in the United States.12 Its high frequency in this age group may be a pathologic exaggeration of the normal tendency toward delayed timing of sleep and wakefulness linked with pubertal development.13
Sleep and wakefulness regulation
Conceptually, 2 processes govern sleep and wakefulness:
- The homeostatic drive to sleep (process S) is proportional to the duration of sleep restriction and becomes maximal at about 40 hours.
- Circadian regulation (process C) creates a drive for wakefulness that variably opposes process S and depends upon intrinsic rhythms.14
Neurons of the suprachiasmatic nucleus in the hypothalamus exert master coordination of this sleep/wake rhythm, along with other behavioral and physiologic variables.15 Because the typical intrinsic period is slightly longer than 24 hours, synchronization to the 24-hour day (entrainment) is accomplished by environmental inputs (zeitgebers, or “time givers”), the most important of which is exposure to light.16
Misalignment between endogenous circadian rhythms and the light/dark cycle can result in circadian rhythm sleep disorders, such as:
- delayed sleep timing (DSPD)
- advanced sleep timing (advanced sleep phase disorder)
- erratic sleep timing (irregular sleep/wake rhythm)
- complete dissociation from the light/dark cycle (circadian rhythm sleep disorder, free-running type).
These 4 conditions are thought to involve predominantly intrinsic mechanisms, but circadian dysrhythmias also can be induced by exogenous factors. Extreme work schedules or rapid travel across time zones can challenge the circadian system’s ability to acclimate and the individual’s ability to achieve a desired sleep schedule.17
Differential diagnosis
Because DSPD relates primarily to an aberration in timing of sleep, it is characterized as a disorder only if the individual’s preferred schedule interferes substantially with social or occupational functioning. The International Classification of Sleep Disorders (ICSD) provides detailed diagnostic criteria (Table).17
Table
Diagnostic criteria for delayed sleep phase disorder
A. Delay exists in the phase of the major sleep period in relation to desired sleep time and wake-up time, as evidenced by:
|
| B. When allowed to choose a preferred schedule, patients exhibit normal sleep quality and duration for age and maintain a delayed but stable phase of entrainment to the 24-hour sleep/wake pattern. |
| C. Monitoring with a sleep log or actigraphy (including sleep diary) for at least 7 days demonstrates a stable delay in the timing of the habitual sleep period. |
| D. The sleep disturbance is not better explained by another sleep disorder, medical or neurologic disorder, mental disorder, medication use, or substance use disorder. |
| Source: Adapted and reprinted with permission from International classification of sleep disorders. Diagnostic and coding manual. 2nd ed17 |
Depression and anxiety often manifest with sleep difficulties, as do inadequate sleep hygiene and other conditions associated with prolonged sleep initiation. According to ICSD criteria, primary insomnia can be differentiated from DSPD if the patient readily initiates and maintains sleep when allowed to sleep on his/her desired sleep/wake schedule. Accumulated evidence has largely debunked this notion, however, as polysomnographic studies have demonstrated both prolonged sleep latency and impaired sleep efficiency in DSPD patients versus matched controls.3
Assessment tools can complement the clinical history in diagnosing DSPD. Either a sleep log or actigraphy is required to demonstrate a stable phase delay, but actigraphy typically generates more reliable data.18 Actigraphs are compact “motion detectors” whose output while being worn by patients allows longitudinal assessment of sleep/wake parameters.
Eveningness tendencies of presumptive DSPD patients can be further verified with the Morningness-Eveningness Questionnaire (MEQ) (Box 2).19 Low scores are associated with evening types—felt to correspond to the endogenous circadian period—and can help narrow the differential diagnosis of sleep-initiation complaints.20
The Morningness-Eveningness Questionnaire (MEQ) developed by Horne and Ostberg19 can be used to verify eveningness tendencies of patients with presumptive delayed sleep phase disorder. The MEQ is a 19-item self-assessment tool with responses that are assigned values totaling up to 86 points. Examples of the questions include:
- Considering only your own ‘feeling best’ rhythm, at what time would you get up if you were entirely free to plan your day?
- Considering only your own ‘feeling best’ rhythm, at what time would you go to bed if you were entirely free to plan your day?
- How easy do you find it to get up each day?
- When you have no commitments the next day, how much later do you go to bed compared to your usual bedtime?
- One hears about ‘morning’ and ‘evening’ types of people. Which ONE of these types do you consider yourself to be?
Lower scores are associated with evening types—felt to correspond to the endogenous circadian period—and can help in narrowing the differential diagnosis of sleep-initiation complaints.20 Scores on the MEQ are interpreted as:
- 70 to 86: definite morning type
- 59 to 69: moderately morning type
- 42 to 58: neither type
- 31 to 41: moderately evening type
- 16 to 30: definite evening type
CASE CONTINUED: ‘Definite evening type’
Jason scores 28 on the MEQ, consistent with a “definite evening type.” Actigraphic monitoring is scheduled during a school holiday, when he is instructed to sleep according to his preferred schedule with the least possible restriction.
A clearly delayed sleep phase is evident, with the habitual sleep period occurring between 5 AM and 1 PM. Even on days when he was quite sleep-restricted because of an enforced wake time, sleep onset on the ensuing evening was substantially delayed, suggesting an obligate nature for the delayed sleep/wake schedule. Overall, Jason had few complaints with respect to impaired alertness while on this unrestricted schedule and experienced a much more stable mood.
Interventions
Without physiologic assessments, understanding the patient’s “natural” sleep schedule can allow for rational recommendations about using phototherapy and oral melatonin (Figure21). However, referral to a sleep specialist is required unless the general psychiatrist has experience in treating circadian rhythm sleep disorders.
Morning phototherapy. Properly timed morning bright light therapy (≥2,500 lux) has been shown to help DSPD patients achieve physiologically measured sleep phase advances, objective improvements in daytime alertness, and earlier reported bedtimes compared with controls.22 Unfortunately, the described 2-hour treatment duration make this research protocol clinically impractical, and most clinicians commence with a 30-minute duration of therapy, as described in the seasonal affective disorder literature.
Relatively new and widely available blue light boxes have been reported to exhibit at least equivalent efficacy to bright light devices (as reported in the literature pertaining to seasonal affective disorder), but with markedly decreased light intensity and fewer associated adverse effects.23 As the research addressing their use in the treatment of circadian rhythm sleep disorders is still emerging, their future role remains uncertain.
Precautions. Most psychiatrists would not perform a physiologic determination of a patient’s circadian phase, and further undesired phase delays can occur if phototherapy is administered before the core body temperature minimum (Tmin).24 Also, use caution if prescribing phototherapy to patients taking photosensitizing drugs and/or those with ocular or retinal pathology.20
Evening light avoidance. Whether or not you prescribe morning phototherapy, recommending that DSPD patients avoid evening light is essential to avoid further induction or exacerbation of phase delays. Protective eyewear is warranted in instances where these advisory precautions are insufficient (see Related Resources). Such an intervention has been shown effective in decreasing light exposure and undesired phase advances in studies involving subjects exposed to simulated shift work.25
Oral melatonin. Abundant evidence supports melatonin use in achieving phase advances in individuals with DSPD.26,27 A synergistic effect can be obtained when melatonin is combined with phototherapy.28
Proper timing of melatonin to achieve a maximal phase advance can be estimated based on the individual’s dim light melatonin onset (DLMO), which occurs approximately 14 hours after the habitual (unrestricted) wake time.29 Maximal phase advances appear to occur when melatonin is given approximately 6 hours before the DLMO.26 Thus, a rational practice is to recommend that patients take melatonin 8 hours after their natural wake time. Doses of ≤0.5 mg appear to achieve the maximal chronobiotic effect while avoiding an undesired hypnotic effect.30
Precautions. Verifying the purity of over-the-counter melatonin is difficult. A review by the National Academy of Sciences states that short-term use of melatonin, ≤10 mg/d, appears to be safe in healthy adults but recommends caution in children/adolescents and women of reproductive age. Doses recommended for circadian-based interventions are typically physiologic in nature (i.e., ≤0.5 mg), which may serve to mitigate these concerns.
Adverse effects such as headaches, somnolence, hypotension, hypertension, gastrointestinal upset, and exacerbation of alopecia areata have been reported at higher melatonin doses in healthy adults and at lower doses in persons with preexisting central nervous system, cardiovascular, gastrointestinal, or dermatologic conditions.31
Figure Light and melatonin phase response curves: Normal vs. delayed
This schematic compares ‘normal sleep’ phase response curves (PRCs) to light and exogenous melatonin with postulated PRCs for an individual with delayed sleep phase disorder (DSPD), presumed to be 5 hours ‘out of phase.’ Y-axis shows the direction and relative magnitude of phase shifts produced by light or melatonin at times shown on the x-axis. X-axis covers >24 hours to better illustrate the PRCs.
Relationships between ‘normal sleepers’ and DSPD patients are depicted by:
- rectangles (sleep period)
- triangles (core body temperature minimum [Tmin])
- arrows (dim light melatonin onsets [DLMOs]).
‘Normal’ sleep is shown to occur from midnight to 8 AM, and the DSPD patient’s sleep from 5 AM to 1 PM; DLMO and Tmin are similarly delayed by 5 hours in the DSPD patient. This schematic assumes that phase relationships are maintained in DSPD patients, which is not a certainty.
Source: Adapted from reference 21
CASE CONTINUED: Under the bright lights
Jason starts phototherapy treatment during his winter break, administering bright light daily upon natural awakening using a 10,000 lux light box for at least 30 minutes. As instructed, he gradually advances the time of administration by approximately 30 minutes every other day, striving for a nocturnal sleep period of 11 PM to 7 AM. He also wears protective eyewear to reduce light exposure during evening hours to avoid further delays in sleep phase. To further promote a phase advance, he takes oral melatonin, 0.5 mg/d at approximately 8 PM, as determined by his self-report and results of actigraphic recording.
Other options
Hypnotics. Little evidence supports the use of hypnotics in DSPD,32 and patients may show resistance to these drugs.33 Nevertheless, hypnotics can heighten confidence in the ability to initiate sleep in individuals with a concomitant conditioned insomnia.
With chronotherapy, patients are prescribed a sleep schedule that is delayed several hours incrementally until sleep is aligned to a target bedtime. The individual then is advised to rigorously maintain a regular sleep/wake schedule, repeating the process as necessary.
Although case reports have shown positive results with chronotherapy for DSPD,34 no controlled trials have demonstrated its efficacy or safety. One study reported high relapse rates,31 and 1 patient with DSPD developed free-running circadian rhythms.35 Clinical experience suggests chronotherapy is impractical for patients who must adhere to a fixed schedule.
Behavioral approaches
For an adolescent with DSPD, consider asking the school district to allow him or her a later school start-time. This alone often can substantially increase total sleep time and mitigate associated impairments.36 In all instances pursue and address external contributors to DSPD, such as poor sleep hygiene (including excessive caffeine use) and substance misuse.
Emphasize regular wake times, as arising later on weekends can cause phase delays.37 DSPD patients may have a concomitant conditioned insomnia that responds to evidence-based behavioral treatments.38
Whatever intervention you choose, schedule a follow-up appointment in approximately 2 months to evaluate patients’ progress and compliance. Encourage them to contact you with questions or concerns in the interim. Review sleep logs or actigraphy during this visit, and adjust the timing and/or nature of interventions as needed. Adolescents can be particularly noncompliant with clinical interventions, and therapeutic goals cannot be reached without their full investment.
Because no guidelines exist on how long to treat DSPD, stop on a “trial-and-error” basis when symptoms are controlled, and resume if they recur. Another approach is to maintain a desired sleep/wake schedule with bedtime melatonin and encourage continued adherence to other measures.
CASE CONTINUED: Maintenance therapy
Jason returns to the clinic approximately 10 weeks later. After an obviously concerted effort to adhere to treatment, his progress is quite remarkable. He rarely falls asleep later than 11 PM, so he is obtaining 2.5 hours more sleep each night before arising for school at 6:30 AM. Sleepiness at school is rarely problematic, and his mood is more stable.
He nevertheless describes a persistent tendency to retire and arise later and asks to continue melatonin and phototherapy. Because no guidelines exist for long-term therapy of DSPD, he is advised to switch melatonin to bedtime dosing (with a presumed phase-neutral “maintenance” effect), and to continue phototherapy as prescribed.
- Wyatt JK. Delayed sleep phase syndrome: pathophysiology and treatment options. Sleep. 2004;27(6):1195-1203.
- Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed sleep phase in adolescence. Sleep Med. 2007;8(6):602-612.
- National Sleep Foundation. Adolescent sleep needs and patterns: research report and resource guide. Washington, DC; 2000:1-30.
- Products designed to assist in the avoidance of light at improper times. www.lowbluelights.com.
Disclosure
Dr. Auger reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Khalsa SB, Jewett ME, Cajochen C, et al. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549(Pt 3):945-952.
2. Quinto C, Gellido C, Chokroverty S, et al. Posttraumatic delayed sleep phase syndrome. Neurology. 2000;54(1):250-252.
3. Campbell SS, Murphy PJ. Delayed sleep phase disorder in temporal isolation. Sleep. 2007;30(9):1225-1228.
4. Uchiyama M, Okawa M, Shibui K, et al. Altered phase relation between sleep timing and core body temperature rhythm in delayed sleep phase syndrome and non-24-hour sleep-wake syndrome in humans. Neurosci Lett. 2000;294(2):101-104.
5. Aoki H, Ozeki Y, Yamada N. Hypersensitivity of melatonin suppression in response to light in patients with delayed sleep phase syndrome. Chronobiol Int. 2001;18(2):263-271.
6. Uchiyama M, Okawa M, Shibui K, et al. Poor compensatory function for sleep loss as a pathogenic factor in patients with delayed sleep phase syndrome. Sleep. 2000;23(4):553-558.
7. Mercer PW, Merritt SL, Cowell JM. Differences in reported sleep need among adolescents. J Adolesc Health. 1998;23(5):259-263.
8. Krahn LE, Pankratz VS, Harris AM, et al. Long-term outcome of adolescents with delayed sleep phase disorder [abstract]. Sleep. 2003;26:A115.-
9. Dagan Y, Stein D, Steinbock M, et al. Frequency of delayed sleep phase syndrome among hospitalized adolescent psychiatric patients. J Psychosom Res. 1998;45(1):15-20.
10. Thorpy MJ, Korman E, Spielman AJ, et al. Delayed sleep phase syndrome in adolescents. J Adolesc Health Care. 1998;9(1):22-27.
11. Regestein QR, Monk TH. Delayed sleep phase syndrome: a review of the clinical aspects. Am J Psychiatry. 1995;152(4):602-608.
12. Pelayo RP, Thorpy MJ, Glovinsky P. Prevalence of delayed sleep phase syndrome among adolescents [abstract]. Sleep Res. 1988;17:391.-
13. Gau SF, Soong WT. The transition of sleep-wake patterns in early adolescence. Sleep. 2003;26(4):449-454.
14. Beersma DG, Gordijn MC. Circadian control of the sleep-wake cycle. Physiol Behav. 2007;90(2-3):190-195.
15. Ralph MR, Foster RG, Davis FC, et al. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247(4945):975-978.
16. Waterhouse J, DeCoursey PJ. Chronobiology: biological timekeeping. Sunderland, MA: Sinauer Associates, Inc. Publishers; 2004:291-323.
17. American Academy of Sleep Medicine. International classification of sleep disorders. Diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005.
18. Bradshaw DA, Yanagi MA, Pak ES, et al. Nightly sleep duration in the 2-week period preceding multiple sleep latency testing. J Clin Sleep Med. 2007;3(6):613-619.
19. Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97-110.
20. Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: Part I. basic principles, shift work and jet lag disorders. Sleep. 2007;30(11):1460-1483.
21. Burgess HJ, Sharkey KM, Eastman CI. Bright light, dark and melatonin can promote circadian adaptation in night shift workers. Sleep Med Rev. 2002;6(5):407-420.
22. Rosenthal NE, Joseph-Vanderpool JR, Levendosky AA, et al. Phase-shifting effects of bright morning light as treatment for delayed sleep phase syndrome. Sleep. 1990;13(4):354-361.
23. Glickman G, Byrne B, Pineda C, et al. Light therapy for seasonal affective disorder with blue narrow-band light-emitting diodes (LEDs). Biol Psychiatry. 2006;59:502-507.
24. Czeisler C, Wright K, Jr. Influence of light on circadian rhythmicity in humans. New York, NY: Marcel Dekker; 1999:149-180.
25. Crowley SJ, Lee C, Tseng CY, et al. Combinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift work. J Biol Rhythms. 2003;18(6):513-523.
26. Mundey K, Benloucif S, Harsanyi K, et al. Phase-dependent treatment of delayed sleep phase syndrome with melatonin. Sleep. 2005;28(10):1271-1278.
27. Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: Part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. Sleep. 2007;30(11):1484-1501.
28. Revell VL, Burgess HJ, Gazda CJ, et al. Advancing human circadian rhythms with afternoon melatonin and morning intermittent bright light. J Clin Endocrinol Metab. 2006;91(1):54-59.
29. Burgess HJ, Eastman CI. The dim light melatonin onset following fixed and free sleep schedules. J Sleep Res. 2005;14(3):229-237.
30. Lewy AJ. Clinical applications of melatonin in circadian disorders. Dialog Clin Neurosci. 2003;5:399-413.
31. Committee on the Framework for Evaluating the Safety of Dietary Supplements FaNB, Board on Life Sciences, Institute of Medicine and National Research Council of the National Academies. Dietary supplements: a framework for evaluating safety. Washington, DC: The National Academies Press; 2005.
32. Ito A, Ando K, Hayakawa T, et al. Long-term course of adult patients with delayed sleep phase syndrome. Jpn J Psychiatry Neurol. 1993;47(3):563-567.
33. Auger RR. Circadian rhythm sleep disorder, delayed sleep phase type (pediatric case). In: Winkelman JW (chair), Henderson JH, Kotagal S, et al, eds. Case book of sleep medicine. Westchester, IL: American Academy of Sleep Medicine; 2008:195-199.
34. Czeisler C, Weitzman E, Moore, et al. Chronotherapy: resetting the circadian clocks of patients with delayed sleep phase insomnia. Sleep. 1981;4:1-21.
35. Oren DA, Wehr TA. Hypernyctohemeral syndrome after chronotherapy for delayed sleep phase syndrome. N Engl J Med. 1992;327(24):1762.-
36. Wahlstrom K. Changing times: findings from the first longitudinal study of later high school start times. NASSP Bulletin. 2002;86(633):3-21.
37. Burgess HJ, Eastman CI. A late wake time phase delays the human dim light melatonin rhythm. Neurosci Lett. 2006;395(3):191-195.
38. Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep. 2006;29(11):1415-1419.
Jason, age 16, has had difficulty with sleep initiation for 2 years. He describes going to bed at 10:30 PM on school nights but falling asleep no sooner than midnight and typically after 1:30 AM. He denies contributions from an “active mind” or environmental disturbances, and his bedroom contains no TV, computer, or other media devices. He does not sleep better with a change in environment. He denies pervasive low mood symptoms and believes his mood hinges predominantly on his ability to achieve sufficient sleep.
Once asleep, Jason generally enjoys good sleep consolidation until he needs to arise at 6:30 AM. His mother awakens him with difficulty, as he often sleeps through his alarm. He sleeps approximately 5 hours nightly during the school week, endorses impaired concentration, and often dozes during his first several classes. When he returns home from school, he finds it very difficult to resist napping.
On weekends he retires at 1 AM or later and typically falls asleep within 30 minutes. He usually awakens at noon but can sleep as late as 4:30 PM. He feels slightly more refreshed on weekends and describes his mood then as improved. During a recent spring break, he felt much better when allowed to sleep as much as he wanted.
Delayed sleep phase disorder (DSPD)—characterized by a pathological “night owl” circadian preference—is seen most commonly in adolescents and is associated with psychiatric morbidity, psychosocial impairment, and poor academic performance. Proper identification of the condition can be enhanced with a variety of assessment tools, and successful treatment requires an awareness of potential endogenous and exogenous contributors.
This article describes what is known about DSPD and uses the case example to illustrate diagnostic assessment and treatment choices. Intriguing data support various pathophysiologic explanations for DSPD (Box 1).1-6 Facilitating the adjustment of patients’ physiologic clocks is the overall goal in managing DSPD.
In individuals normally entrained to the light/dark cycle, circadian rhythms are:
- delayed by evening exposure to bright light (≥ 2,500 lux) prior to the core body temperature minimum (Tmin)
- advanced by morning light exposure after the Tmin.1
These opposing effects attune most people to the light/dark cycle, with sleep and wakefulness occurring on a conventional schedule. Persons with delayed sleep phase disorder (DSPD) live at a delayed phase that resists advancement and is incompatible with their personal and social obligations.
Theories have been proposed, but DSPD’s etiology has not been fully explained. Affected adolescents may exhibit an extreme in circadian preference. Case reports also describe DSPD emerging after traumatic brain injury.2
Intriguing evidence supports various pathophysiologic explanations for DSPD. An abnormally long intrinsic circadian period (>25 hours) was recently demonstrated during temporal isolation in 1 individual with DSPD.3 Both this case report and controlled studies describe deviations from expected relationships between the sleep/wake cycle and physiologic circadian markers. Most consistently described are longer intervals from Tmin4 to sleep offset (final rise time) in DSPD patients compared with controls.
Other research suggests:
Extreme ‘eveningness’
Because of their extreme seemingly innate preference to retire and arise at relatively late clock hours (an “eveningness” trait), school-aged patients with DSPD represent a high-risk population for problematic sleepiness. In a survey of 612 high school students, the 63% who felt they needed more sleep on school nights showed a strong eveningness preference (as assessed by questionnaire), compared with students who described getting sufficient sleep.7 Other studies have revealed psychiatric morbidity (including affective and personality disorders), psychosocial impairment, and poor academic performance associated with the condition.8-10
DSPD may affect 7% to 16% of patients presenting with insomnia complaints in sleep medicine clinics.11 The condition appears most common among young cohorts and has been reported to affect up to 7% of adolescents in the United States.12 Its high frequency in this age group may be a pathologic exaggeration of the normal tendency toward delayed timing of sleep and wakefulness linked with pubertal development.13
Sleep and wakefulness regulation
Conceptually, 2 processes govern sleep and wakefulness:
- The homeostatic drive to sleep (process S) is proportional to the duration of sleep restriction and becomes maximal at about 40 hours.
- Circadian regulation (process C) creates a drive for wakefulness that variably opposes process S and depends upon intrinsic rhythms.14
Neurons of the suprachiasmatic nucleus in the hypothalamus exert master coordination of this sleep/wake rhythm, along with other behavioral and physiologic variables.15 Because the typical intrinsic period is slightly longer than 24 hours, synchronization to the 24-hour day (entrainment) is accomplished by environmental inputs (zeitgebers, or “time givers”), the most important of which is exposure to light.16
Misalignment between endogenous circadian rhythms and the light/dark cycle can result in circadian rhythm sleep disorders, such as:
- delayed sleep timing (DSPD)
- advanced sleep timing (advanced sleep phase disorder)
- erratic sleep timing (irregular sleep/wake rhythm)
- complete dissociation from the light/dark cycle (circadian rhythm sleep disorder, free-running type).
These 4 conditions are thought to involve predominantly intrinsic mechanisms, but circadian dysrhythmias also can be induced by exogenous factors. Extreme work schedules or rapid travel across time zones can challenge the circadian system’s ability to acclimate and the individual’s ability to achieve a desired sleep schedule.17
Differential diagnosis
Because DSPD relates primarily to an aberration in timing of sleep, it is characterized as a disorder only if the individual’s preferred schedule interferes substantially with social or occupational functioning. The International Classification of Sleep Disorders (ICSD) provides detailed diagnostic criteria (Table).17
Table
Diagnostic criteria for delayed sleep phase disorder
A. Delay exists in the phase of the major sleep period in relation to desired sleep time and wake-up time, as evidenced by:
|
| B. When allowed to choose a preferred schedule, patients exhibit normal sleep quality and duration for age and maintain a delayed but stable phase of entrainment to the 24-hour sleep/wake pattern. |
| C. Monitoring with a sleep log or actigraphy (including sleep diary) for at least 7 days demonstrates a stable delay in the timing of the habitual sleep period. |
| D. The sleep disturbance is not better explained by another sleep disorder, medical or neurologic disorder, mental disorder, medication use, or substance use disorder. |
| Source: Adapted and reprinted with permission from International classification of sleep disorders. Diagnostic and coding manual. 2nd ed17 |
Depression and anxiety often manifest with sleep difficulties, as do inadequate sleep hygiene and other conditions associated with prolonged sleep initiation. According to ICSD criteria, primary insomnia can be differentiated from DSPD if the patient readily initiates and maintains sleep when allowed to sleep on his/her desired sleep/wake schedule. Accumulated evidence has largely debunked this notion, however, as polysomnographic studies have demonstrated both prolonged sleep latency and impaired sleep efficiency in DSPD patients versus matched controls.3
Assessment tools can complement the clinical history in diagnosing DSPD. Either a sleep log or actigraphy is required to demonstrate a stable phase delay, but actigraphy typically generates more reliable data.18 Actigraphs are compact “motion detectors” whose output while being worn by patients allows longitudinal assessment of sleep/wake parameters.
Eveningness tendencies of presumptive DSPD patients can be further verified with the Morningness-Eveningness Questionnaire (MEQ) (Box 2).19 Low scores are associated with evening types—felt to correspond to the endogenous circadian period—and can help narrow the differential diagnosis of sleep-initiation complaints.20
The Morningness-Eveningness Questionnaire (MEQ) developed by Horne and Ostberg19 can be used to verify eveningness tendencies of patients with presumptive delayed sleep phase disorder. The MEQ is a 19-item self-assessment tool with responses that are assigned values totaling up to 86 points. Examples of the questions include:
- Considering only your own ‘feeling best’ rhythm, at what time would you get up if you were entirely free to plan your day?
- Considering only your own ‘feeling best’ rhythm, at what time would you go to bed if you were entirely free to plan your day?
- How easy do you find it to get up each day?
- When you have no commitments the next day, how much later do you go to bed compared to your usual bedtime?
- One hears about ‘morning’ and ‘evening’ types of people. Which ONE of these types do you consider yourself to be?
Lower scores are associated with evening types—felt to correspond to the endogenous circadian period—and can help in narrowing the differential diagnosis of sleep-initiation complaints.20 Scores on the MEQ are interpreted as:
- 70 to 86: definite morning type
- 59 to 69: moderately morning type
- 42 to 58: neither type
- 31 to 41: moderately evening type
- 16 to 30: definite evening type
CASE CONTINUED: ‘Definite evening type’
Jason scores 28 on the MEQ, consistent with a “definite evening type.” Actigraphic monitoring is scheduled during a school holiday, when he is instructed to sleep according to his preferred schedule with the least possible restriction.
A clearly delayed sleep phase is evident, with the habitual sleep period occurring between 5 AM and 1 PM. Even on days when he was quite sleep-restricted because of an enforced wake time, sleep onset on the ensuing evening was substantially delayed, suggesting an obligate nature for the delayed sleep/wake schedule. Overall, Jason had few complaints with respect to impaired alertness while on this unrestricted schedule and experienced a much more stable mood.
Interventions
Without physiologic assessments, understanding the patient’s “natural” sleep schedule can allow for rational recommendations about using phototherapy and oral melatonin (Figure21). However, referral to a sleep specialist is required unless the general psychiatrist has experience in treating circadian rhythm sleep disorders.
Morning phototherapy. Properly timed morning bright light therapy (≥2,500 lux) has been shown to help DSPD patients achieve physiologically measured sleep phase advances, objective improvements in daytime alertness, and earlier reported bedtimes compared with controls.22 Unfortunately, the described 2-hour treatment duration make this research protocol clinically impractical, and most clinicians commence with a 30-minute duration of therapy, as described in the seasonal affective disorder literature.
Relatively new and widely available blue light boxes have been reported to exhibit at least equivalent efficacy to bright light devices (as reported in the literature pertaining to seasonal affective disorder), but with markedly decreased light intensity and fewer associated adverse effects.23 As the research addressing their use in the treatment of circadian rhythm sleep disorders is still emerging, their future role remains uncertain.
Precautions. Most psychiatrists would not perform a physiologic determination of a patient’s circadian phase, and further undesired phase delays can occur if phototherapy is administered before the core body temperature minimum (Tmin).24 Also, use caution if prescribing phototherapy to patients taking photosensitizing drugs and/or those with ocular or retinal pathology.20
Evening light avoidance. Whether or not you prescribe morning phototherapy, recommending that DSPD patients avoid evening light is essential to avoid further induction or exacerbation of phase delays. Protective eyewear is warranted in instances where these advisory precautions are insufficient (see Related Resources). Such an intervention has been shown effective in decreasing light exposure and undesired phase advances in studies involving subjects exposed to simulated shift work.25
Oral melatonin. Abundant evidence supports melatonin use in achieving phase advances in individuals with DSPD.26,27 A synergistic effect can be obtained when melatonin is combined with phototherapy.28
Proper timing of melatonin to achieve a maximal phase advance can be estimated based on the individual’s dim light melatonin onset (DLMO), which occurs approximately 14 hours after the habitual (unrestricted) wake time.29 Maximal phase advances appear to occur when melatonin is given approximately 6 hours before the DLMO.26 Thus, a rational practice is to recommend that patients take melatonin 8 hours after their natural wake time. Doses of ≤0.5 mg appear to achieve the maximal chronobiotic effect while avoiding an undesired hypnotic effect.30
Precautions. Verifying the purity of over-the-counter melatonin is difficult. A review by the National Academy of Sciences states that short-term use of melatonin, ≤10 mg/d, appears to be safe in healthy adults but recommends caution in children/adolescents and women of reproductive age. Doses recommended for circadian-based interventions are typically physiologic in nature (i.e., ≤0.5 mg), which may serve to mitigate these concerns.
Adverse effects such as headaches, somnolence, hypotension, hypertension, gastrointestinal upset, and exacerbation of alopecia areata have been reported at higher melatonin doses in healthy adults and at lower doses in persons with preexisting central nervous system, cardiovascular, gastrointestinal, or dermatologic conditions.31

Figure Light and melatonin phase response curves: Normal vs. delayed
This schematic compares ‘normal sleep’ phase response curves (PRCs) to light and exogenous melatonin with postulated PRCs for an individual with delayed sleep phase disorder (DSPD), presumed to be 5 hours ‘out of phase.’ Y-axis shows the direction and relative magnitude of phase shifts produced by light or melatonin at times shown on the x-axis. X-axis covers >24 hours to better illustrate the PRCs.
Relationships between ‘normal sleepers’ and DSPD patients are depicted by:
- rectangles (sleep period)
- triangles (core body temperature minimum [Tmin])
- arrows (dim light melatonin onsets [DLMOs]).
‘Normal’ sleep is shown to occur from midnight to 8 AM, and the DSPD patient’s sleep from 5 AM to 1 PM; DLMO and Tmin are similarly delayed by 5 hours in the DSPD patient. This schematic assumes that phase relationships are maintained in DSPD patients, which is not a certainty.
Source: Adapted from reference 21
CASE CONTINUED: Under the bright lights
Jason starts phototherapy treatment during his winter break, administering bright light daily upon natural awakening using a 10,000 lux light box for at least 30 minutes. As instructed, he gradually advances the time of administration by approximately 30 minutes every other day, striving for a nocturnal sleep period of 11 PM to 7 AM. He also wears protective eyewear to reduce light exposure during evening hours to avoid further delays in sleep phase. To further promote a phase advance, he takes oral melatonin, 0.5 mg/d at approximately 8 PM, as determined by his self-report and results of actigraphic recording.
Other options
Hypnotics. Little evidence supports the use of hypnotics in DSPD,32 and patients may show resistance to these drugs.33 Nevertheless, hypnotics can heighten confidence in the ability to initiate sleep in individuals with a concomitant conditioned insomnia.
With chronotherapy, patients are prescribed a sleep schedule that is delayed several hours incrementally until sleep is aligned to a target bedtime. The individual then is advised to rigorously maintain a regular sleep/wake schedule, repeating the process as necessary.
Although case reports have shown positive results with chronotherapy for DSPD,34 no controlled trials have demonstrated its efficacy or safety. One study reported high relapse rates,31 and 1 patient with DSPD developed free-running circadian rhythms.35 Clinical experience suggests chronotherapy is impractical for patients who must adhere to a fixed schedule.
Behavioral approaches
For an adolescent with DSPD, consider asking the school district to allow him or her a later school start-time. This alone often can substantially increase total sleep time and mitigate associated impairments.36 In all instances pursue and address external contributors to DSPD, such as poor sleep hygiene (including excessive caffeine use) and substance misuse.
Emphasize regular wake times, as arising later on weekends can cause phase delays.37 DSPD patients may have a concomitant conditioned insomnia that responds to evidence-based behavioral treatments.38
Whatever intervention you choose, schedule a follow-up appointment in approximately 2 months to evaluate patients’ progress and compliance. Encourage them to contact you with questions or concerns in the interim. Review sleep logs or actigraphy during this visit, and adjust the timing and/or nature of interventions as needed. Adolescents can be particularly noncompliant with clinical interventions, and therapeutic goals cannot be reached without their full investment.
Because no guidelines exist on how long to treat DSPD, stop on a “trial-and-error” basis when symptoms are controlled, and resume if they recur. Another approach is to maintain a desired sleep/wake schedule with bedtime melatonin and encourage continued adherence to other measures.
CASE CONTINUED: Maintenance therapy
Jason returns to the clinic approximately 10 weeks later. After an obviously concerted effort to adhere to treatment, his progress is quite remarkable. He rarely falls asleep later than 11 PM, so he is obtaining 2.5 hours more sleep each night before arising for school at 6:30 AM. Sleepiness at school is rarely problematic, and his mood is more stable.
He nevertheless describes a persistent tendency to retire and arise later and asks to continue melatonin and phototherapy. Because no guidelines exist for long-term therapy of DSPD, he is advised to switch melatonin to bedtime dosing (with a presumed phase-neutral “maintenance” effect), and to continue phototherapy as prescribed.
- Wyatt JK. Delayed sleep phase syndrome: pathophysiology and treatment options. Sleep. 2004;27(6):1195-1203.
- Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed sleep phase in adolescence. Sleep Med. 2007;8(6):602-612.
- National Sleep Foundation. Adolescent sleep needs and patterns: research report and resource guide. Washington, DC; 2000:1-30.
- Products designed to assist in the avoidance of light at improper times. www.lowbluelights.com.
Disclosure
Dr. Auger reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Jason, age 16, has had difficulty with sleep initiation for 2 years. He describes going to bed at 10:30 PM on school nights but falling asleep no sooner than midnight and typically after 1:30 AM. He denies contributions from an “active mind” or environmental disturbances, and his bedroom contains no TV, computer, or other media devices. He does not sleep better with a change in environment. He denies pervasive low mood symptoms and believes his mood hinges predominantly on his ability to achieve sufficient sleep.
Once asleep, Jason generally enjoys good sleep consolidation until he needs to arise at 6:30 AM. His mother awakens him with difficulty, as he often sleeps through his alarm. He sleeps approximately 5 hours nightly during the school week, endorses impaired concentration, and often dozes during his first several classes. When he returns home from school, he finds it very difficult to resist napping.
On weekends he retires at 1 AM or later and typically falls asleep within 30 minutes. He usually awakens at noon but can sleep as late as 4:30 PM. He feels slightly more refreshed on weekends and describes his mood then as improved. During a recent spring break, he felt much better when allowed to sleep as much as he wanted.
Delayed sleep phase disorder (DSPD)—characterized by a pathological “night owl” circadian preference—is seen most commonly in adolescents and is associated with psychiatric morbidity, psychosocial impairment, and poor academic performance. Proper identification of the condition can be enhanced with a variety of assessment tools, and successful treatment requires an awareness of potential endogenous and exogenous contributors.
This article describes what is known about DSPD and uses the case example to illustrate diagnostic assessment and treatment choices. Intriguing data support various pathophysiologic explanations for DSPD (Box 1).1-6 Facilitating the adjustment of patients’ physiologic clocks is the overall goal in managing DSPD.
In individuals normally entrained to the light/dark cycle, circadian rhythms are:
- delayed by evening exposure to bright light (≥ 2,500 lux) prior to the core body temperature minimum (Tmin)
- advanced by morning light exposure after the Tmin.1
These opposing effects attune most people to the light/dark cycle, with sleep and wakefulness occurring on a conventional schedule. Persons with delayed sleep phase disorder (DSPD) live at a delayed phase that resists advancement and is incompatible with their personal and social obligations.
Theories have been proposed, but DSPD’s etiology has not been fully explained. Affected adolescents may exhibit an extreme in circadian preference. Case reports also describe DSPD emerging after traumatic brain injury.2
Intriguing evidence supports various pathophysiologic explanations for DSPD. An abnormally long intrinsic circadian period (>25 hours) was recently demonstrated during temporal isolation in 1 individual with DSPD.3 Both this case report and controlled studies describe deviations from expected relationships between the sleep/wake cycle and physiologic circadian markers. Most consistently described are longer intervals from Tmin4 to sleep offset (final rise time) in DSPD patients compared with controls.
Other research suggests:
Extreme ‘eveningness’
Because of their extreme seemingly innate preference to retire and arise at relatively late clock hours (an “eveningness” trait), school-aged patients with DSPD represent a high-risk population for problematic sleepiness. In a survey of 612 high school students, the 63% who felt they needed more sleep on school nights showed a strong eveningness preference (as assessed by questionnaire), compared with students who described getting sufficient sleep.7 Other studies have revealed psychiatric morbidity (including affective and personality disorders), psychosocial impairment, and poor academic performance associated with the condition.8-10
DSPD may affect 7% to 16% of patients presenting with insomnia complaints in sleep medicine clinics.11 The condition appears most common among young cohorts and has been reported to affect up to 7% of adolescents in the United States.12 Its high frequency in this age group may be a pathologic exaggeration of the normal tendency toward delayed timing of sleep and wakefulness linked with pubertal development.13
Sleep and wakefulness regulation
Conceptually, 2 processes govern sleep and wakefulness:
- The homeostatic drive to sleep (process S) is proportional to the duration of sleep restriction and becomes maximal at about 40 hours.
- Circadian regulation (process C) creates a drive for wakefulness that variably opposes process S and depends upon intrinsic rhythms.14
Neurons of the suprachiasmatic nucleus in the hypothalamus exert master coordination of this sleep/wake rhythm, along with other behavioral and physiologic variables.15 Because the typical intrinsic period is slightly longer than 24 hours, synchronization to the 24-hour day (entrainment) is accomplished by environmental inputs (zeitgebers, or “time givers”), the most important of which is exposure to light.16
Misalignment between endogenous circadian rhythms and the light/dark cycle can result in circadian rhythm sleep disorders, such as:
- delayed sleep timing (DSPD)
- advanced sleep timing (advanced sleep phase disorder)
- erratic sleep timing (irregular sleep/wake rhythm)
- complete dissociation from the light/dark cycle (circadian rhythm sleep disorder, free-running type).
These 4 conditions are thought to involve predominantly intrinsic mechanisms, but circadian dysrhythmias also can be induced by exogenous factors. Extreme work schedules or rapid travel across time zones can challenge the circadian system’s ability to acclimate and the individual’s ability to achieve a desired sleep schedule.17
Differential diagnosis
Because DSPD relates primarily to an aberration in timing of sleep, it is characterized as a disorder only if the individual’s preferred schedule interferes substantially with social or occupational functioning. The International Classification of Sleep Disorders (ICSD) provides detailed diagnostic criteria (Table).17
Table
Diagnostic criteria for delayed sleep phase disorder
A. Delay exists in the phase of the major sleep period in relation to desired sleep time and wake-up time, as evidenced by:
|
| B. When allowed to choose a preferred schedule, patients exhibit normal sleep quality and duration for age and maintain a delayed but stable phase of entrainment to the 24-hour sleep/wake pattern. |
| C. Monitoring with a sleep log or actigraphy (including sleep diary) for at least 7 days demonstrates a stable delay in the timing of the habitual sleep period. |
| D. The sleep disturbance is not better explained by another sleep disorder, medical or neurologic disorder, mental disorder, medication use, or substance use disorder. |
| Source: Adapted and reprinted with permission from International classification of sleep disorders. Diagnostic and coding manual. 2nd ed17 |
Depression and anxiety often manifest with sleep difficulties, as do inadequate sleep hygiene and other conditions associated with prolonged sleep initiation. According to ICSD criteria, primary insomnia can be differentiated from DSPD if the patient readily initiates and maintains sleep when allowed to sleep on his/her desired sleep/wake schedule. Accumulated evidence has largely debunked this notion, however, as polysomnographic studies have demonstrated both prolonged sleep latency and impaired sleep efficiency in DSPD patients versus matched controls.3
Assessment tools can complement the clinical history in diagnosing DSPD. Either a sleep log or actigraphy is required to demonstrate a stable phase delay, but actigraphy typically generates more reliable data.18 Actigraphs are compact “motion detectors” whose output while being worn by patients allows longitudinal assessment of sleep/wake parameters.
Eveningness tendencies of presumptive DSPD patients can be further verified with the Morningness-Eveningness Questionnaire (MEQ) (Box 2).19 Low scores are associated with evening types—felt to correspond to the endogenous circadian period—and can help narrow the differential diagnosis of sleep-initiation complaints.20
The Morningness-Eveningness Questionnaire (MEQ) developed by Horne and Ostberg19 can be used to verify eveningness tendencies of patients with presumptive delayed sleep phase disorder. The MEQ is a 19-item self-assessment tool with responses that are assigned values totaling up to 86 points. Examples of the questions include:
- Considering only your own ‘feeling best’ rhythm, at what time would you get up if you were entirely free to plan your day?
- Considering only your own ‘feeling best’ rhythm, at what time would you go to bed if you were entirely free to plan your day?
- How easy do you find it to get up each day?
- When you have no commitments the next day, how much later do you go to bed compared to your usual bedtime?
- One hears about ‘morning’ and ‘evening’ types of people. Which ONE of these types do you consider yourself to be?
Lower scores are associated with evening types—felt to correspond to the endogenous circadian period—and can help in narrowing the differential diagnosis of sleep-initiation complaints.20 Scores on the MEQ are interpreted as:
- 70 to 86: definite morning type
- 59 to 69: moderately morning type
- 42 to 58: neither type
- 31 to 41: moderately evening type
- 16 to 30: definite evening type
CASE CONTINUED: ‘Definite evening type’
Jason scores 28 on the MEQ, consistent with a “definite evening type.” Actigraphic monitoring is scheduled during a school holiday, when he is instructed to sleep according to his preferred schedule with the least possible restriction.
A clearly delayed sleep phase is evident, with the habitual sleep period occurring between 5 AM and 1 PM. Even on days when he was quite sleep-restricted because of an enforced wake time, sleep onset on the ensuing evening was substantially delayed, suggesting an obligate nature for the delayed sleep/wake schedule. Overall, Jason had few complaints with respect to impaired alertness while on this unrestricted schedule and experienced a much more stable mood.
Interventions
Without physiologic assessments, understanding the patient’s “natural” sleep schedule can allow for rational recommendations about using phototherapy and oral melatonin (Figure21). However, referral to a sleep specialist is required unless the general psychiatrist has experience in treating circadian rhythm sleep disorders.
Morning phototherapy. Properly timed morning bright light therapy (≥2,500 lux) has been shown to help DSPD patients achieve physiologically measured sleep phase advances, objective improvements in daytime alertness, and earlier reported bedtimes compared with controls.22 Unfortunately, the described 2-hour treatment duration make this research protocol clinically impractical, and most clinicians commence with a 30-minute duration of therapy, as described in the seasonal affective disorder literature.
Relatively new and widely available blue light boxes have been reported to exhibit at least equivalent efficacy to bright light devices (as reported in the literature pertaining to seasonal affective disorder), but with markedly decreased light intensity and fewer associated adverse effects.23 As the research addressing their use in the treatment of circadian rhythm sleep disorders is still emerging, their future role remains uncertain.
Precautions. Most psychiatrists would not perform a physiologic determination of a patient’s circadian phase, and further undesired phase delays can occur if phototherapy is administered before the core body temperature minimum (Tmin).24 Also, use caution if prescribing phototherapy to patients taking photosensitizing drugs and/or those with ocular or retinal pathology.20
Evening light avoidance. Whether or not you prescribe morning phototherapy, recommending that DSPD patients avoid evening light is essential to avoid further induction or exacerbation of phase delays. Protective eyewear is warranted in instances where these advisory precautions are insufficient (see Related Resources). Such an intervention has been shown effective in decreasing light exposure and undesired phase advances in studies involving subjects exposed to simulated shift work.25
Oral melatonin. Abundant evidence supports melatonin use in achieving phase advances in individuals with DSPD.26,27 A synergistic effect can be obtained when melatonin is combined with phototherapy.28
Proper timing of melatonin to achieve a maximal phase advance can be estimated based on the individual’s dim light melatonin onset (DLMO), which occurs approximately 14 hours after the habitual (unrestricted) wake time.29 Maximal phase advances appear to occur when melatonin is given approximately 6 hours before the DLMO.26 Thus, a rational practice is to recommend that patients take melatonin 8 hours after their natural wake time. Doses of ≤0.5 mg appear to achieve the maximal chronobiotic effect while avoiding an undesired hypnotic effect.30
Precautions. Verifying the purity of over-the-counter melatonin is difficult. A review by the National Academy of Sciences states that short-term use of melatonin, ≤10 mg/d, appears to be safe in healthy adults but recommends caution in children/adolescents and women of reproductive age. Doses recommended for circadian-based interventions are typically physiologic in nature (i.e., ≤0.5 mg), which may serve to mitigate these concerns.
Adverse effects such as headaches, somnolence, hypotension, hypertension, gastrointestinal upset, and exacerbation of alopecia areata have been reported at higher melatonin doses in healthy adults and at lower doses in persons with preexisting central nervous system, cardiovascular, gastrointestinal, or dermatologic conditions.31

Figure Light and melatonin phase response curves: Normal vs. delayed
This schematic compares ‘normal sleep’ phase response curves (PRCs) to light and exogenous melatonin with postulated PRCs for an individual with delayed sleep phase disorder (DSPD), presumed to be 5 hours ‘out of phase.’ Y-axis shows the direction and relative magnitude of phase shifts produced by light or melatonin at times shown on the x-axis. X-axis covers >24 hours to better illustrate the PRCs.
Relationships between ‘normal sleepers’ and DSPD patients are depicted by:
- rectangles (sleep period)
- triangles (core body temperature minimum [Tmin])
- arrows (dim light melatonin onsets [DLMOs]).
‘Normal’ sleep is shown to occur from midnight to 8 AM, and the DSPD patient’s sleep from 5 AM to 1 PM; DLMO and Tmin are similarly delayed by 5 hours in the DSPD patient. This schematic assumes that phase relationships are maintained in DSPD patients, which is not a certainty.
Source: Adapted from reference 21
CASE CONTINUED: Under the bright lights
Jason starts phototherapy treatment during his winter break, administering bright light daily upon natural awakening using a 10,000 lux light box for at least 30 minutes. As instructed, he gradually advances the time of administration by approximately 30 minutes every other day, striving for a nocturnal sleep period of 11 PM to 7 AM. He also wears protective eyewear to reduce light exposure during evening hours to avoid further delays in sleep phase. To further promote a phase advance, he takes oral melatonin, 0.5 mg/d at approximately 8 PM, as determined by his self-report and results of actigraphic recording.
Other options
Hypnotics. Little evidence supports the use of hypnotics in DSPD,32 and patients may show resistance to these drugs.33 Nevertheless, hypnotics can heighten confidence in the ability to initiate sleep in individuals with a concomitant conditioned insomnia.
With chronotherapy, patients are prescribed a sleep schedule that is delayed several hours incrementally until sleep is aligned to a target bedtime. The individual then is advised to rigorously maintain a regular sleep/wake schedule, repeating the process as necessary.
Although case reports have shown positive results with chronotherapy for DSPD,34 no controlled trials have demonstrated its efficacy or safety. One study reported high relapse rates,31 and 1 patient with DSPD developed free-running circadian rhythms.35 Clinical experience suggests chronotherapy is impractical for patients who must adhere to a fixed schedule.
Behavioral approaches
For an adolescent with DSPD, consider asking the school district to allow him or her a later school start-time. This alone often can substantially increase total sleep time and mitigate associated impairments.36 In all instances pursue and address external contributors to DSPD, such as poor sleep hygiene (including excessive caffeine use) and substance misuse.
Emphasize regular wake times, as arising later on weekends can cause phase delays.37 DSPD patients may have a concomitant conditioned insomnia that responds to evidence-based behavioral treatments.38
Whatever intervention you choose, schedule a follow-up appointment in approximately 2 months to evaluate patients’ progress and compliance. Encourage them to contact you with questions or concerns in the interim. Review sleep logs or actigraphy during this visit, and adjust the timing and/or nature of interventions as needed. Adolescents can be particularly noncompliant with clinical interventions, and therapeutic goals cannot be reached without their full investment.
Because no guidelines exist on how long to treat DSPD, stop on a “trial-and-error” basis when symptoms are controlled, and resume if they recur. Another approach is to maintain a desired sleep/wake schedule with bedtime melatonin and encourage continued adherence to other measures.
CASE CONTINUED: Maintenance therapy
Jason returns to the clinic approximately 10 weeks later. After an obviously concerted effort to adhere to treatment, his progress is quite remarkable. He rarely falls asleep later than 11 PM, so he is obtaining 2.5 hours more sleep each night before arising for school at 6:30 AM. Sleepiness at school is rarely problematic, and his mood is more stable.
He nevertheless describes a persistent tendency to retire and arise later and asks to continue melatonin and phototherapy. Because no guidelines exist for long-term therapy of DSPD, he is advised to switch melatonin to bedtime dosing (with a presumed phase-neutral “maintenance” effect), and to continue phototherapy as prescribed.
- Wyatt JK. Delayed sleep phase syndrome: pathophysiology and treatment options. Sleep. 2004;27(6):1195-1203.
- Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed sleep phase in adolescence. Sleep Med. 2007;8(6):602-612.
- National Sleep Foundation. Adolescent sleep needs and patterns: research report and resource guide. Washington, DC; 2000:1-30.
- Products designed to assist in the avoidance of light at improper times. www.lowbluelights.com.
Disclosure
Dr. Auger reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Khalsa SB, Jewett ME, Cajochen C, et al. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549(Pt 3):945-952.
2. Quinto C, Gellido C, Chokroverty S, et al. Posttraumatic delayed sleep phase syndrome. Neurology. 2000;54(1):250-252.
3. Campbell SS, Murphy PJ. Delayed sleep phase disorder in temporal isolation. Sleep. 2007;30(9):1225-1228.
4. Uchiyama M, Okawa M, Shibui K, et al. Altered phase relation between sleep timing and core body temperature rhythm in delayed sleep phase syndrome and non-24-hour sleep-wake syndrome in humans. Neurosci Lett. 2000;294(2):101-104.
5. Aoki H, Ozeki Y, Yamada N. Hypersensitivity of melatonin suppression in response to light in patients with delayed sleep phase syndrome. Chronobiol Int. 2001;18(2):263-271.
6. Uchiyama M, Okawa M, Shibui K, et al. Poor compensatory function for sleep loss as a pathogenic factor in patients with delayed sleep phase syndrome. Sleep. 2000;23(4):553-558.
7. Mercer PW, Merritt SL, Cowell JM. Differences in reported sleep need among adolescents. J Adolesc Health. 1998;23(5):259-263.
8. Krahn LE, Pankratz VS, Harris AM, et al. Long-term outcome of adolescents with delayed sleep phase disorder [abstract]. Sleep. 2003;26:A115.-
9. Dagan Y, Stein D, Steinbock M, et al. Frequency of delayed sleep phase syndrome among hospitalized adolescent psychiatric patients. J Psychosom Res. 1998;45(1):15-20.
10. Thorpy MJ, Korman E, Spielman AJ, et al. Delayed sleep phase syndrome in adolescents. J Adolesc Health Care. 1998;9(1):22-27.
11. Regestein QR, Monk TH. Delayed sleep phase syndrome: a review of the clinical aspects. Am J Psychiatry. 1995;152(4):602-608.
12. Pelayo RP, Thorpy MJ, Glovinsky P. Prevalence of delayed sleep phase syndrome among adolescents [abstract]. Sleep Res. 1988;17:391.-
13. Gau SF, Soong WT. The transition of sleep-wake patterns in early adolescence. Sleep. 2003;26(4):449-454.
14. Beersma DG, Gordijn MC. Circadian control of the sleep-wake cycle. Physiol Behav. 2007;90(2-3):190-195.
15. Ralph MR, Foster RG, Davis FC, et al. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247(4945):975-978.
16. Waterhouse J, DeCoursey PJ. Chronobiology: biological timekeeping. Sunderland, MA: Sinauer Associates, Inc. Publishers; 2004:291-323.
17. American Academy of Sleep Medicine. International classification of sleep disorders. Diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005.
18. Bradshaw DA, Yanagi MA, Pak ES, et al. Nightly sleep duration in the 2-week period preceding multiple sleep latency testing. J Clin Sleep Med. 2007;3(6):613-619.
19. Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97-110.
20. Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: Part I. basic principles, shift work and jet lag disorders. Sleep. 2007;30(11):1460-1483.
21. Burgess HJ, Sharkey KM, Eastman CI. Bright light, dark and melatonin can promote circadian adaptation in night shift workers. Sleep Med Rev. 2002;6(5):407-420.
22. Rosenthal NE, Joseph-Vanderpool JR, Levendosky AA, et al. Phase-shifting effects of bright morning light as treatment for delayed sleep phase syndrome. Sleep. 1990;13(4):354-361.
23. Glickman G, Byrne B, Pineda C, et al. Light therapy for seasonal affective disorder with blue narrow-band light-emitting diodes (LEDs). Biol Psychiatry. 2006;59:502-507.
24. Czeisler C, Wright K, Jr. Influence of light on circadian rhythmicity in humans. New York, NY: Marcel Dekker; 1999:149-180.
25. Crowley SJ, Lee C, Tseng CY, et al. Combinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift work. J Biol Rhythms. 2003;18(6):513-523.
26. Mundey K, Benloucif S, Harsanyi K, et al. Phase-dependent treatment of delayed sleep phase syndrome with melatonin. Sleep. 2005;28(10):1271-1278.
27. Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: Part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. Sleep. 2007;30(11):1484-1501.
28. Revell VL, Burgess HJ, Gazda CJ, et al. Advancing human circadian rhythms with afternoon melatonin and morning intermittent bright light. J Clin Endocrinol Metab. 2006;91(1):54-59.
29. Burgess HJ, Eastman CI. The dim light melatonin onset following fixed and free sleep schedules. J Sleep Res. 2005;14(3):229-237.
30. Lewy AJ. Clinical applications of melatonin in circadian disorders. Dialog Clin Neurosci. 2003;5:399-413.
31. Committee on the Framework for Evaluating the Safety of Dietary Supplements FaNB, Board on Life Sciences, Institute of Medicine and National Research Council of the National Academies. Dietary supplements: a framework for evaluating safety. Washington, DC: The National Academies Press; 2005.
32. Ito A, Ando K, Hayakawa T, et al. Long-term course of adult patients with delayed sleep phase syndrome. Jpn J Psychiatry Neurol. 1993;47(3):563-567.
33. Auger RR. Circadian rhythm sleep disorder, delayed sleep phase type (pediatric case). In: Winkelman JW (chair), Henderson JH, Kotagal S, et al, eds. Case book of sleep medicine. Westchester, IL: American Academy of Sleep Medicine; 2008:195-199.
34. Czeisler C, Weitzman E, Moore, et al. Chronotherapy: resetting the circadian clocks of patients with delayed sleep phase insomnia. Sleep. 1981;4:1-21.
35. Oren DA, Wehr TA. Hypernyctohemeral syndrome after chronotherapy for delayed sleep phase syndrome. N Engl J Med. 1992;327(24):1762.-
36. Wahlstrom K. Changing times: findings from the first longitudinal study of later high school start times. NASSP Bulletin. 2002;86(633):3-21.
37. Burgess HJ, Eastman CI. A late wake time phase delays the human dim light melatonin rhythm. Neurosci Lett. 2006;395(3):191-195.
38. Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep. 2006;29(11):1415-1419.
1. Khalsa SB, Jewett ME, Cajochen C, et al. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549(Pt 3):945-952.
2. Quinto C, Gellido C, Chokroverty S, et al. Posttraumatic delayed sleep phase syndrome. Neurology. 2000;54(1):250-252.
3. Campbell SS, Murphy PJ. Delayed sleep phase disorder in temporal isolation. Sleep. 2007;30(9):1225-1228.
4. Uchiyama M, Okawa M, Shibui K, et al. Altered phase relation between sleep timing and core body temperature rhythm in delayed sleep phase syndrome and non-24-hour sleep-wake syndrome in humans. Neurosci Lett. 2000;294(2):101-104.
5. Aoki H, Ozeki Y, Yamada N. Hypersensitivity of melatonin suppression in response to light in patients with delayed sleep phase syndrome. Chronobiol Int. 2001;18(2):263-271.
6. Uchiyama M, Okawa M, Shibui K, et al. Poor compensatory function for sleep loss as a pathogenic factor in patients with delayed sleep phase syndrome. Sleep. 2000;23(4):553-558.
7. Mercer PW, Merritt SL, Cowell JM. Differences in reported sleep need among adolescents. J Adolesc Health. 1998;23(5):259-263.
8. Krahn LE, Pankratz VS, Harris AM, et al. Long-term outcome of adolescents with delayed sleep phase disorder [abstract]. Sleep. 2003;26:A115.-
9. Dagan Y, Stein D, Steinbock M, et al. Frequency of delayed sleep phase syndrome among hospitalized adolescent psychiatric patients. J Psychosom Res. 1998;45(1):15-20.
10. Thorpy MJ, Korman E, Spielman AJ, et al. Delayed sleep phase syndrome in adolescents. J Adolesc Health Care. 1998;9(1):22-27.
11. Regestein QR, Monk TH. Delayed sleep phase syndrome: a review of the clinical aspects. Am J Psychiatry. 1995;152(4):602-608.
12. Pelayo RP, Thorpy MJ, Glovinsky P. Prevalence of delayed sleep phase syndrome among adolescents [abstract]. Sleep Res. 1988;17:391.-
13. Gau SF, Soong WT. The transition of sleep-wake patterns in early adolescence. Sleep. 2003;26(4):449-454.
14. Beersma DG, Gordijn MC. Circadian control of the sleep-wake cycle. Physiol Behav. 2007;90(2-3):190-195.
15. Ralph MR, Foster RG, Davis FC, et al. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247(4945):975-978.
16. Waterhouse J, DeCoursey PJ. Chronobiology: biological timekeeping. Sunderland, MA: Sinauer Associates, Inc. Publishers; 2004:291-323.
17. American Academy of Sleep Medicine. International classification of sleep disorders. Diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005.
18. Bradshaw DA, Yanagi MA, Pak ES, et al. Nightly sleep duration in the 2-week period preceding multiple sleep latency testing. J Clin Sleep Med. 2007;3(6):613-619.
19. Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97-110.
20. Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: Part I. basic principles, shift work and jet lag disorders. Sleep. 2007;30(11):1460-1483.
21. Burgess HJ, Sharkey KM, Eastman CI. Bright light, dark and melatonin can promote circadian adaptation in night shift workers. Sleep Med Rev. 2002;6(5):407-420.
22. Rosenthal NE, Joseph-Vanderpool JR, Levendosky AA, et al. Phase-shifting effects of bright morning light as treatment for delayed sleep phase syndrome. Sleep. 1990;13(4):354-361.
23. Glickman G, Byrne B, Pineda C, et al. Light therapy for seasonal affective disorder with blue narrow-band light-emitting diodes (LEDs). Biol Psychiatry. 2006;59:502-507.
24. Czeisler C, Wright K, Jr. Influence of light on circadian rhythmicity in humans. New York, NY: Marcel Dekker; 1999:149-180.
25. Crowley SJ, Lee C, Tseng CY, et al. Combinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift work. J Biol Rhythms. 2003;18(6):513-523.
26. Mundey K, Benloucif S, Harsanyi K, et al. Phase-dependent treatment of delayed sleep phase syndrome with melatonin. Sleep. 2005;28(10):1271-1278.
27. Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: Part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. Sleep. 2007;30(11):1484-1501.
28. Revell VL, Burgess HJ, Gazda CJ, et al. Advancing human circadian rhythms with afternoon melatonin and morning intermittent bright light. J Clin Endocrinol Metab. 2006;91(1):54-59.
29. Burgess HJ, Eastman CI. The dim light melatonin onset following fixed and free sleep schedules. J Sleep Res. 2005;14(3):229-237.
30. Lewy AJ. Clinical applications of melatonin in circadian disorders. Dialog Clin Neurosci. 2003;5:399-413.
31. Committee on the Framework for Evaluating the Safety of Dietary Supplements FaNB, Board on Life Sciences, Institute of Medicine and National Research Council of the National Academies. Dietary supplements: a framework for evaluating safety. Washington, DC: The National Academies Press; 2005.
32. Ito A, Ando K, Hayakawa T, et al. Long-term course of adult patients with delayed sleep phase syndrome. Jpn J Psychiatry Neurol. 1993;47(3):563-567.
33. Auger RR. Circadian rhythm sleep disorder, delayed sleep phase type (pediatric case). In: Winkelman JW (chair), Henderson JH, Kotagal S, et al, eds. Case book of sleep medicine. Westchester, IL: American Academy of Sleep Medicine; 2008:195-199.
34. Czeisler C, Weitzman E, Moore, et al. Chronotherapy: resetting the circadian clocks of patients with delayed sleep phase insomnia. Sleep. 1981;4:1-21.
35. Oren DA, Wehr TA. Hypernyctohemeral syndrome after chronotherapy for delayed sleep phase syndrome. N Engl J Med. 1992;327(24):1762.-
36. Wahlstrom K. Changing times: findings from the first longitudinal study of later high school start times. NASSP Bulletin. 2002;86(633):3-21.
37. Burgess HJ, Eastman CI. A late wake time phase delays the human dim light melatonin rhythm. Neurosci Lett. 2006;395(3):191-195.
38. Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep. 2006;29(11):1415-1419.
Stimulus Offers Cash for Quality
The economic stimulus bill that became law last week includes several items on SHM's healthcare policy wish list.
"It clearly hits some key issues," Eric Siegal, MD, chair of SHM's Public Policy Committee, says in regard to the American Recovery and Reinvestment Act. "This is a step in the right direction."
The $787 billion stimulus package includes:
- $1.1 billion for comparative effectiveness research (CER). Funding for CER is one of SHM's top policy priorities, says Laura Allendorf, SHM's senior advisor for advocacy and government affairs. CER examines the effectiveness of multiple therapies for specific medical conditions, or for a specific set of patients, to determine the best care options. "Funding for this is long overdue and key to healthcare reform," Dr. Siegal says. CER money will be split among the major players in this research, including the National Institutes of Health (NIH) and the Agency for Healthcare Research and Quality (AHRQ).
- A 34% increase in NIH funding. This includes $8.5 billion for research grants and programs that will allow for as many as 15,000 additional grants. "We know that an investment in biomedical research is an investment in the future of healthcare," says David Meltzer, MD, PhD, chair of SHM's Research Committee.
- $19 billion for health information technology. Incentives—and penalties—will target adoption of electronic health records by hospitals and office-based practices. "This will help improve patient safety, as well as care coordination," Allendorf says.
Other healthcare provisions in the package include an additional $86.6 billion in federal Medicaid funds, as well as temporary Medicaid coverage for the recently unemployed.
To keep up with public policy initiatives, check out SHM's advocacy portal.
The economic stimulus bill that became law last week includes several items on SHM's healthcare policy wish list.
"It clearly hits some key issues," Eric Siegal, MD, chair of SHM's Public Policy Committee, says in regard to the American Recovery and Reinvestment Act. "This is a step in the right direction."
The $787 billion stimulus package includes:
- $1.1 billion for comparative effectiveness research (CER). Funding for CER is one of SHM's top policy priorities, says Laura Allendorf, SHM's senior advisor for advocacy and government affairs. CER examines the effectiveness of multiple therapies for specific medical conditions, or for a specific set of patients, to determine the best care options. "Funding for this is long overdue and key to healthcare reform," Dr. Siegal says. CER money will be split among the major players in this research, including the National Institutes of Health (NIH) and the Agency for Healthcare Research and Quality (AHRQ).
- A 34% increase in NIH funding. This includes $8.5 billion for research grants and programs that will allow for as many as 15,000 additional grants. "We know that an investment in biomedical research is an investment in the future of healthcare," says David Meltzer, MD, PhD, chair of SHM's Research Committee.
- $19 billion for health information technology. Incentives—and penalties—will target adoption of electronic health records by hospitals and office-based practices. "This will help improve patient safety, as well as care coordination," Allendorf says.
Other healthcare provisions in the package include an additional $86.6 billion in federal Medicaid funds, as well as temporary Medicaid coverage for the recently unemployed.
To keep up with public policy initiatives, check out SHM's advocacy portal.
The economic stimulus bill that became law last week includes several items on SHM's healthcare policy wish list.
"It clearly hits some key issues," Eric Siegal, MD, chair of SHM's Public Policy Committee, says in regard to the American Recovery and Reinvestment Act. "This is a step in the right direction."
The $787 billion stimulus package includes:
- $1.1 billion for comparative effectiveness research (CER). Funding for CER is one of SHM's top policy priorities, says Laura Allendorf, SHM's senior advisor for advocacy and government affairs. CER examines the effectiveness of multiple therapies for specific medical conditions, or for a specific set of patients, to determine the best care options. "Funding for this is long overdue and key to healthcare reform," Dr. Siegal says. CER money will be split among the major players in this research, including the National Institutes of Health (NIH) and the Agency for Healthcare Research and Quality (AHRQ).
- A 34% increase in NIH funding. This includes $8.5 billion for research grants and programs that will allow for as many as 15,000 additional grants. "We know that an investment in biomedical research is an investment in the future of healthcare," says David Meltzer, MD, PhD, chair of SHM's Research Committee.
- $19 billion for health information technology. Incentives—and penalties—will target adoption of electronic health records by hospitals and office-based practices. "This will help improve patient safety, as well as care coordination," Allendorf says.
Other healthcare provisions in the package include an additional $86.6 billion in federal Medicaid funds, as well as temporary Medicaid coverage for the recently unemployed.
To keep up with public policy initiatives, check out SHM's advocacy portal.
Model's Death Sheds Spotlight on Sepsis Treatment
The recent death of an up-and-coming Brazilian model due to sepsis has again drawn attention to the common disease and how hospitalists should be wary not to miss its diagnosis in hospitalized patients.
Mariana Bridi da Costa, a 20-year-old beauty queen who participated in international competitions, died Jan. 24 from septicemia that began with a urinary tract infection. The original diagnosis did not include the urinary tract infection, leading to severe sepsis. Septicemia kills about 1,400 people a day worldwide, according to the Society of Critical Care Medicine (SCCM). The sepsis mortality rate is on a similar scale to lung, breast, and colon cancer, SCCM data shows.
"When someone comes in with high fever, high heart rate, high respiratory rate, and low blood pressure, you have to begin thinking about how well their organs are working," says Jeffrey Dichter, MD, medical director of cardiovascular intensive care at Regions Hospital in St. Paul, Minn., and former HM program director. "How sick are they? ... Hospitalists should look for the source of infection and evidence of organ failure."
According to the Mayo Clinic, about 750,000 people in the U.S. contract severe sepsis each year, and more than 200,000 people die of it.
Dr. Dichter notes that hospitalists in any setting—from acute-care hospitals to off-site clinics—can run blood tests for kidney and liver functions, as well as electrolyte levels, that serve as quick windows into the presence of sepsis. He also promotes www.survivingsepsis.org, the SCCM's program that aims to reduce sepsis mortality by 25% in five years by defining the disease more clearly and improving its diagnosis.
"Sometimes the symptoms patients come in with seem subtle," Dr. Dichter says. "Sometimes they may come in with evidence of an infection or low blood pressure, but they're awake and alert and talking and seem very normal. … Hospitalists need to be attune."
The recent death of an up-and-coming Brazilian model due to sepsis has again drawn attention to the common disease and how hospitalists should be wary not to miss its diagnosis in hospitalized patients.
Mariana Bridi da Costa, a 20-year-old beauty queen who participated in international competitions, died Jan. 24 from septicemia that began with a urinary tract infection. The original diagnosis did not include the urinary tract infection, leading to severe sepsis. Septicemia kills about 1,400 people a day worldwide, according to the Society of Critical Care Medicine (SCCM). The sepsis mortality rate is on a similar scale to lung, breast, and colon cancer, SCCM data shows.
"When someone comes in with high fever, high heart rate, high respiratory rate, and low blood pressure, you have to begin thinking about how well their organs are working," says Jeffrey Dichter, MD, medical director of cardiovascular intensive care at Regions Hospital in St. Paul, Minn., and former HM program director. "How sick are they? ... Hospitalists should look for the source of infection and evidence of organ failure."
According to the Mayo Clinic, about 750,000 people in the U.S. contract severe sepsis each year, and more than 200,000 people die of it.
Dr. Dichter notes that hospitalists in any setting—from acute-care hospitals to off-site clinics—can run blood tests for kidney and liver functions, as well as electrolyte levels, that serve as quick windows into the presence of sepsis. He also promotes www.survivingsepsis.org, the SCCM's program that aims to reduce sepsis mortality by 25% in five years by defining the disease more clearly and improving its diagnosis.
"Sometimes the symptoms patients come in with seem subtle," Dr. Dichter says. "Sometimes they may come in with evidence of an infection or low blood pressure, but they're awake and alert and talking and seem very normal. … Hospitalists need to be attune."
The recent death of an up-and-coming Brazilian model due to sepsis has again drawn attention to the common disease and how hospitalists should be wary not to miss its diagnosis in hospitalized patients.
Mariana Bridi da Costa, a 20-year-old beauty queen who participated in international competitions, died Jan. 24 from septicemia that began with a urinary tract infection. The original diagnosis did not include the urinary tract infection, leading to severe sepsis. Septicemia kills about 1,400 people a day worldwide, according to the Society of Critical Care Medicine (SCCM). The sepsis mortality rate is on a similar scale to lung, breast, and colon cancer, SCCM data shows.
"When someone comes in with high fever, high heart rate, high respiratory rate, and low blood pressure, you have to begin thinking about how well their organs are working," says Jeffrey Dichter, MD, medical director of cardiovascular intensive care at Regions Hospital in St. Paul, Minn., and former HM program director. "How sick are they? ... Hospitalists should look for the source of infection and evidence of organ failure."
According to the Mayo Clinic, about 750,000 people in the U.S. contract severe sepsis each year, and more than 200,000 people die of it.
Dr. Dichter notes that hospitalists in any setting—from acute-care hospitals to off-site clinics—can run blood tests for kidney and liver functions, as well as electrolyte levels, that serve as quick windows into the presence of sepsis. He also promotes www.survivingsepsis.org, the SCCM's program that aims to reduce sepsis mortality by 25% in five years by defining the disease more clearly and improving its diagnosis.
"Sometimes the symptoms patients come in with seem subtle," Dr. Dichter says. "Sometimes they may come in with evidence of an infection or low blood pressure, but they're awake and alert and talking and seem very normal. … Hospitalists need to be attune."
Research Roundup
Question: Do blood transfusions in hospitalized cancer patients with anemia or thrombocytopenia affect thrombotic event and in-hospital mortality rates?
Background: Erythropoiesis-stimulating agents have recently been shown to increase thrombotic risk and decrease survival in cancer patients. Blood transfusions are a common alternative for anemic patients. However, there are no randomized trials demonstrating improved outcomes in cancer patients receiving transfusions. Furthermore, the safety of transfusions has not been clearly defined.
Study design: Retrospective, cohort study.
Setting: 60 U.S. medical centers.
Synopsis: Using discharge data from the University Health System Consortium, 504,208 hospitalizations of cancer patients revealed that 14% of patients received at least one RBC transfusion and 3% of patients received a platelet transfusion. RBC and platelet transfusions were associated with increased risk of arterial thrombosis (RBCs: OR 1.53; 95% CI 1.46-1.61; platelets: OR 1.55; 1.4-1.71, P<0.001) and venous thrombotic events (RBCs: OR 1.34; 1.29-1.38; platelets: OR 1.2; 1.11-1.29, P<0.001). Additionally, transfusions were associated with increased in-hospital mortality (RBCs: OR 1.34; 1.29-1.38; platelets: OR 2.4; 2.27-2.52, P<0.001). Study results are limited by several factors, including the observational nature and the use of administrative coding data. Information on venous thromboembolism prophylaxis was not available, and the timing of transfusions in relation to the diagnosis of thrombotic events is unknown. Finally, anemia-necessitating transfusions may be a surrogate for "sicker" patients, explaining the increased in-hospital mortality. Blood transfusions in hospitalized patients require further study to determine whether there is a causal relationship between transfusions and increased thrombotic events and mortality.
Bottom line: Blood transfusions in hospitalized cancer patients should be used cautiously, as they are associated with increased thrombotic events and in-hospital mortality.
Citation: Arch Int Med. 2008;168:2377-2381
—Reviewed for the eWire by Kerry Will, MD, Jayne Barr, MD, Kim Tartaglia, MD, Aaron Wenger, MD, Jonathan Wynbrandt, MD, Nathan J. O’Dorisio, MD, The Ohio State University Medical Center, Columbus, OH.
Question: Do blood transfusions in hospitalized cancer patients with anemia or thrombocytopenia affect thrombotic event and in-hospital mortality rates?
Background: Erythropoiesis-stimulating agents have recently been shown to increase thrombotic risk and decrease survival in cancer patients. Blood transfusions are a common alternative for anemic patients. However, there are no randomized trials demonstrating improved outcomes in cancer patients receiving transfusions. Furthermore, the safety of transfusions has not been clearly defined.
Study design: Retrospective, cohort study.
Setting: 60 U.S. medical centers.
Synopsis: Using discharge data from the University Health System Consortium, 504,208 hospitalizations of cancer patients revealed that 14% of patients received at least one RBC transfusion and 3% of patients received a platelet transfusion. RBC and platelet transfusions were associated with increased risk of arterial thrombosis (RBCs: OR 1.53; 95% CI 1.46-1.61; platelets: OR 1.55; 1.4-1.71, P<0.001) and venous thrombotic events (RBCs: OR 1.34; 1.29-1.38; platelets: OR 1.2; 1.11-1.29, P<0.001). Additionally, transfusions were associated with increased in-hospital mortality (RBCs: OR 1.34; 1.29-1.38; platelets: OR 2.4; 2.27-2.52, P<0.001). Study results are limited by several factors, including the observational nature and the use of administrative coding data. Information on venous thromboembolism prophylaxis was not available, and the timing of transfusions in relation to the diagnosis of thrombotic events is unknown. Finally, anemia-necessitating transfusions may be a surrogate for "sicker" patients, explaining the increased in-hospital mortality. Blood transfusions in hospitalized patients require further study to determine whether there is a causal relationship between transfusions and increased thrombotic events and mortality.
Bottom line: Blood transfusions in hospitalized cancer patients should be used cautiously, as they are associated with increased thrombotic events and in-hospital mortality.
Citation: Arch Int Med. 2008;168:2377-2381
—Reviewed for the eWire by Kerry Will, MD, Jayne Barr, MD, Kim Tartaglia, MD, Aaron Wenger, MD, Jonathan Wynbrandt, MD, Nathan J. O’Dorisio, MD, The Ohio State University Medical Center, Columbus, OH.
Question: Do blood transfusions in hospitalized cancer patients with anemia or thrombocytopenia affect thrombotic event and in-hospital mortality rates?
Background: Erythropoiesis-stimulating agents have recently been shown to increase thrombotic risk and decrease survival in cancer patients. Blood transfusions are a common alternative for anemic patients. However, there are no randomized trials demonstrating improved outcomes in cancer patients receiving transfusions. Furthermore, the safety of transfusions has not been clearly defined.
Study design: Retrospective, cohort study.
Setting: 60 U.S. medical centers.
Synopsis: Using discharge data from the University Health System Consortium, 504,208 hospitalizations of cancer patients revealed that 14% of patients received at least one RBC transfusion and 3% of patients received a platelet transfusion. RBC and platelet transfusions were associated with increased risk of arterial thrombosis (RBCs: OR 1.53; 95% CI 1.46-1.61; platelets: OR 1.55; 1.4-1.71, P<0.001) and venous thrombotic events (RBCs: OR 1.34; 1.29-1.38; platelets: OR 1.2; 1.11-1.29, P<0.001). Additionally, transfusions were associated with increased in-hospital mortality (RBCs: OR 1.34; 1.29-1.38; platelets: OR 2.4; 2.27-2.52, P<0.001). Study results are limited by several factors, including the observational nature and the use of administrative coding data. Information on venous thromboembolism prophylaxis was not available, and the timing of transfusions in relation to the diagnosis of thrombotic events is unknown. Finally, anemia-necessitating transfusions may be a surrogate for "sicker" patients, explaining the increased in-hospital mortality. Blood transfusions in hospitalized patients require further study to determine whether there is a causal relationship between transfusions and increased thrombotic events and mortality.
Bottom line: Blood transfusions in hospitalized cancer patients should be used cautiously, as they are associated with increased thrombotic events and in-hospital mortality.
Citation: Arch Int Med. 2008;168:2377-2381
—Reviewed for the eWire by Kerry Will, MD, Jayne Barr, MD, Kim Tartaglia, MD, Aaron Wenger, MD, Jonathan Wynbrandt, MD, Nathan J. O’Dorisio, MD, The Ohio State University Medical Center, Columbus, OH.