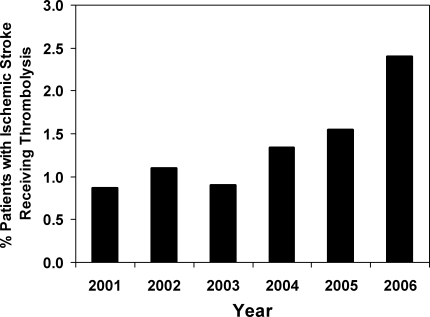
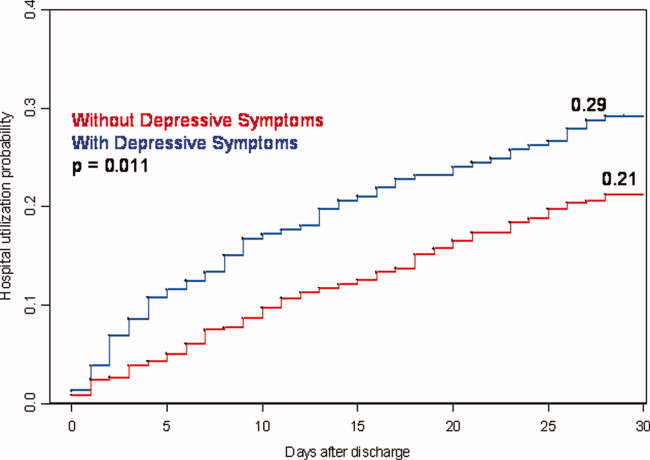
User login
The Child or Adolescent With Anxiety
Identification of children and adolescents with anxiety is important, so consider the diagnosis in your differential. Always think: Could this be anxiety?
Pediatricians are well trained to rule out medical or other causes of anxiety. Questions to ask include: Is the child hypoxic? Does the patient have hypothyroidism? Is the anxiety caused by stress or social factors, including sexual and/or physical abuse? Do the symptoms come from a general adjustment disorder from a major life change or event, such as a move or divorce?
Does the patient have a secret she is afraid to share with anyone else? A shy child, for example, may have something she is afraid to discuss that, together with stressors, can lead her into a true anxiety disorder.
Panic attacks, in particular, can be clinically challenging. Is the attack anxiety driven or caused by an underlying medical problem? We tend to minimize cardiac symptoms, for example, in some children because it is easier to say these symptoms are related only to anxiety. But we need due diligence to rule out any major cardiac or pulmonary etiologies.
When screening patients for anxiety disorders, child and adolescent psychiatrists use comprehensive instruments like the Screen for Child Anxiety-Related Emotional Disorders (SCARED). In a busy primary care setting, I would recommend that pediatricians use the SCARED tool. It is available at no cost and features separate rating scales that can be completed by the child and parent.
For a more comprehensive screening tool, use the Child Behavior Checklist (CBCL), the Child Symptom Inventory (CSI), or the Behavior Assessment Symptom for Children (BASC). Other screening instruments are available that are more disease specific, such as the Children's Yale-Brown Obsessive Compulsive Scale (CY-BOCS) for obsessive-compulsive disorder (OCD).
It is appropriate for pediatricians to manage the treatment of an anxious child or adolescent when the patient is stabilized and continues to improve with treatment. In this way, a child with anxiety is managed no differently than a patient with asthma or diabetes.
Some pediatricians refer a child with a suspected anxiety disorder for an initial evaluation by a mental health specialist such as a child and adolescent psychiatrist, followed by annual consultations. We are happy to consult with pediatricians. One challenge, however, is an overall workforce shortage of child and adolescent psychiatrists. The American Academy of Child & Adolescent Psychiatry offers an online map of the United States that shows the number of specialists per county (www.aacap.org/cs/physicians.AlliedProfessionals/workforce_issues
When is it appropriate for a pediatrician to initiate medication in this patient population? Any time it is indicated! And that really depends on the diagnosis: for OCD, yes; for PTSD, maybe; and for social phobias, probably not. Medication use also is based on symptom severity, especially in generalized anxiety disorder. If the child is not sleeping well or participating in activities of daily living, you have to get him or her stabilized first. The bulk of our treatment for anxiety disorders is psychotherapy, but the child is less likely to benefit from therapy if anxiety impedes the ability to participate in therapy.
Referral to a specialist is indicated when anxiety symptoms interfere with activities of daily living. School refusal is another scenario that warrants immediate referral. Some parents will allow anxious children to stay out of school, so try to determine the reason: Is the parent making it more comfortable for the child to stay at home? Or is the patient avoiding school because they are the target of teasing?
Copies of a recent physical examination, growth chart, and any laboratory work already ordered are helpful with a referral to a child and adolescent psychiatrist. In addition, a detailed clinical assessment facilitates management by a child and adolescent psychiatrist. In other words, it is helpful to get a note that states: “Referring Johnny to you. He was a developmentally normal 5-year-old until he nearly drowned in a pool last summer. He now refuses to sleep alone.” In contrast, a less helpful note might read: “Here is a 5-year-old named Johnny. Please assess.”
Unless you suspect a true organic etiology, such as an abnormal neurologic examination, avoid ordering routine imaging studies for a child with anxiety prior to referral. I am concerned about the risks of sedation for pediatric patients and risks associated with radiation exposure (with CT scans, for example).
Avoid excessive laboratory testing as well, unless there is a clear indication that results could rule out a suspected medical diagnosis.
Identification of children and adolescents with anxiety is important, so consider the diagnosis in your differential. Always think: Could this be anxiety?
Pediatricians are well trained to rule out medical or other causes of anxiety. Questions to ask include: Is the child hypoxic? Does the patient have hypothyroidism? Is the anxiety caused by stress or social factors, including sexual and/or physical abuse? Do the symptoms come from a general adjustment disorder from a major life change or event, such as a move or divorce?
Does the patient have a secret she is afraid to share with anyone else? A shy child, for example, may have something she is afraid to discuss that, together with stressors, can lead her into a true anxiety disorder.
Panic attacks, in particular, can be clinically challenging. Is the attack anxiety driven or caused by an underlying medical problem? We tend to minimize cardiac symptoms, for example, in some children because it is easier to say these symptoms are related only to anxiety. But we need due diligence to rule out any major cardiac or pulmonary etiologies.
When screening patients for anxiety disorders, child and adolescent psychiatrists use comprehensive instruments like the Screen for Child Anxiety-Related Emotional Disorders (SCARED). In a busy primary care setting, I would recommend that pediatricians use the SCARED tool. It is available at no cost and features separate rating scales that can be completed by the child and parent.
For a more comprehensive screening tool, use the Child Behavior Checklist (CBCL), the Child Symptom Inventory (CSI), or the Behavior Assessment Symptom for Children (BASC). Other screening instruments are available that are more disease specific, such as the Children's Yale-Brown Obsessive Compulsive Scale (CY-BOCS) for obsessive-compulsive disorder (OCD).
It is appropriate for pediatricians to manage the treatment of an anxious child or adolescent when the patient is stabilized and continues to improve with treatment. In this way, a child with anxiety is managed no differently than a patient with asthma or diabetes.
Some pediatricians refer a child with a suspected anxiety disorder for an initial evaluation by a mental health specialist such as a child and adolescent psychiatrist, followed by annual consultations. We are happy to consult with pediatricians. One challenge, however, is an overall workforce shortage of child and adolescent psychiatrists. The American Academy of Child & Adolescent Psychiatry offers an online map of the United States that shows the number of specialists per county (www.aacap.org/cs/physicians.AlliedProfessionals/workforce_issues
When is it appropriate for a pediatrician to initiate medication in this patient population? Any time it is indicated! And that really depends on the diagnosis: for OCD, yes; for PTSD, maybe; and for social phobias, probably not. Medication use also is based on symptom severity, especially in generalized anxiety disorder. If the child is not sleeping well or participating in activities of daily living, you have to get him or her stabilized first. The bulk of our treatment for anxiety disorders is psychotherapy, but the child is less likely to benefit from therapy if anxiety impedes the ability to participate in therapy.
Referral to a specialist is indicated when anxiety symptoms interfere with activities of daily living. School refusal is another scenario that warrants immediate referral. Some parents will allow anxious children to stay out of school, so try to determine the reason: Is the parent making it more comfortable for the child to stay at home? Or is the patient avoiding school because they are the target of teasing?
Copies of a recent physical examination, growth chart, and any laboratory work already ordered are helpful with a referral to a child and adolescent psychiatrist. In addition, a detailed clinical assessment facilitates management by a child and adolescent psychiatrist. In other words, it is helpful to get a note that states: “Referring Johnny to you. He was a developmentally normal 5-year-old until he nearly drowned in a pool last summer. He now refuses to sleep alone.” In contrast, a less helpful note might read: “Here is a 5-year-old named Johnny. Please assess.”
Unless you suspect a true organic etiology, such as an abnormal neurologic examination, avoid ordering routine imaging studies for a child with anxiety prior to referral. I am concerned about the risks of sedation for pediatric patients and risks associated with radiation exposure (with CT scans, for example).
Avoid excessive laboratory testing as well, unless there is a clear indication that results could rule out a suspected medical diagnosis.
Identification of children and adolescents with anxiety is important, so consider the diagnosis in your differential. Always think: Could this be anxiety?
Pediatricians are well trained to rule out medical or other causes of anxiety. Questions to ask include: Is the child hypoxic? Does the patient have hypothyroidism? Is the anxiety caused by stress or social factors, including sexual and/or physical abuse? Do the symptoms come from a general adjustment disorder from a major life change or event, such as a move or divorce?
Does the patient have a secret she is afraid to share with anyone else? A shy child, for example, may have something she is afraid to discuss that, together with stressors, can lead her into a true anxiety disorder.
Panic attacks, in particular, can be clinically challenging. Is the attack anxiety driven or caused by an underlying medical problem? We tend to minimize cardiac symptoms, for example, in some children because it is easier to say these symptoms are related only to anxiety. But we need due diligence to rule out any major cardiac or pulmonary etiologies.
When screening patients for anxiety disorders, child and adolescent psychiatrists use comprehensive instruments like the Screen for Child Anxiety-Related Emotional Disorders (SCARED). In a busy primary care setting, I would recommend that pediatricians use the SCARED tool. It is available at no cost and features separate rating scales that can be completed by the child and parent.
For a more comprehensive screening tool, use the Child Behavior Checklist (CBCL), the Child Symptom Inventory (CSI), or the Behavior Assessment Symptom for Children (BASC). Other screening instruments are available that are more disease specific, such as the Children's Yale-Brown Obsessive Compulsive Scale (CY-BOCS) for obsessive-compulsive disorder (OCD).
It is appropriate for pediatricians to manage the treatment of an anxious child or adolescent when the patient is stabilized and continues to improve with treatment. In this way, a child with anxiety is managed no differently than a patient with asthma or diabetes.
Some pediatricians refer a child with a suspected anxiety disorder for an initial evaluation by a mental health specialist such as a child and adolescent psychiatrist, followed by annual consultations. We are happy to consult with pediatricians. One challenge, however, is an overall workforce shortage of child and adolescent psychiatrists. The American Academy of Child & Adolescent Psychiatry offers an online map of the United States that shows the number of specialists per county (www.aacap.org/cs/physicians.AlliedProfessionals/workforce_issues
When is it appropriate for a pediatrician to initiate medication in this patient population? Any time it is indicated! And that really depends on the diagnosis: for OCD, yes; for PTSD, maybe; and for social phobias, probably not. Medication use also is based on symptom severity, especially in generalized anxiety disorder. If the child is not sleeping well or participating in activities of daily living, you have to get him or her stabilized first. The bulk of our treatment for anxiety disorders is psychotherapy, but the child is less likely to benefit from therapy if anxiety impedes the ability to participate in therapy.
Referral to a specialist is indicated when anxiety symptoms interfere with activities of daily living. School refusal is another scenario that warrants immediate referral. Some parents will allow anxious children to stay out of school, so try to determine the reason: Is the parent making it more comfortable for the child to stay at home? Or is the patient avoiding school because they are the target of teasing?
Copies of a recent physical examination, growth chart, and any laboratory work already ordered are helpful with a referral to a child and adolescent psychiatrist. In addition, a detailed clinical assessment facilitates management by a child and adolescent psychiatrist. In other words, it is helpful to get a note that states: “Referring Johnny to you. He was a developmentally normal 5-year-old until he nearly drowned in a pool last summer. He now refuses to sleep alone.” In contrast, a less helpful note might read: “Here is a 5-year-old named Johnny. Please assess.”
Unless you suspect a true organic etiology, such as an abnormal neurologic examination, avoid ordering routine imaging studies for a child with anxiety prior to referral. I am concerned about the risks of sedation for pediatric patients and risks associated with radiation exposure (with CT scans, for example).
Avoid excessive laboratory testing as well, unless there is a clear indication that results could rule out a suspected medical diagnosis.
Urge Parents to React Calmly to Sibling Rivalry
www.CHADIS.com[email protected]
From Cane and Abel to Linus and Lucy, Wally and the Beaver to Bart and Lisa Simpson, sibling rivalry is the stuff of legend and comedy. But when it presents as a source of serious concern for parents during pediatric office visits, it's usually no laughing matter for them.
Research suggests that 64% of school-age siblings fight “sometimes or often”—a figure likely matched in magnitude if not muscle by younger siblings as well.
Sibling rivalry is so common, in fact, that we may tend to think back to our own sibling spats, or those of our kids, roll our eyes and offer the “they'll grow out of it” platitude.
But in truth, sibling wars can have consequences. While injuries are rare in most sibling disputes, in 25% of child abuse cases a sibling has been involved in victimization (usually in concert with adults).
Serious sibling conflict tremendously compromises quality of life for children, and for their parents as well. We know that marriages suffer in households with high levels of sibling discord, with the issue a common flashpoint for disagreements between parents about how to respond. Children exposed to serious sibling conflict in middle childhood appear to suffer higher levels of anxiety, depression, and delinquent behavior in early adolescence. Down the road, people carry the grudges of sibling difficulties for decades, undermining bonds that might otherwise be a significant source of support in our increasingly fragmented society.
So sibling struggles are worthy of our time and thoughtfulness, and addressing them productively will build trust in your relationship with parents and perhaps bring some semblance of peace to their households.
The first response to a parent's frustration over sibling quarrels should be to listen with respect. Their pain is often significant as they describe the battles unfolding among children they hold precious. Patiently listening to the details of sibling encounters also can help you sort out whether the issues they're describing fall into the normal range or may signify more serious individual or relational issues that deserve attention.
Assuming it's the former, I think it helps to remind parents of how common sibling rivalry is, and more importantly, why it occurs. Annoying as they may be, fracases actually serve a number of important biological functions. Watch any nature documentary featuring lions lounging under a tree on the savannah, and what are the cubs doing? Attacking, defending, tumbling, and biting, growling all the while.
In kids, like cubs, important social skills arise from the sibling relationship, even when the dust flies. Siblings teach each other to giggle and laugh, bait and switch, sneak and chase, parry and defend. From each other, they learn which jokes fly and which land with a thud, how to toss out an insult and absorb one tossed their way.
Siblings also learn how to pull their punches, practicing evolutionarily useful conflict skills while stopping short of inflicting serious harm.
The question remains, how does a family foster productive resilience-building sibling interactions while preserving affectionate connections and at least a modicum of household calm?
Like so many things in life, household chaos is associated with unhealthy levels of sibling conflict, according to research by psychologist Judy Dunn, the author of “Sisters and Brothers” (Cambridge: Harvard University Press, 1985), “Separate Lives: Why Siblings Are So Different” (New York: Basic Books, 1992), and “From One Child to Two” (New York: Ballantine Books, 1995).
Corporal punishment in the family makes rivalry worse as well.
Individual temperaments, the presence of a child with special needs, and family structure (children of opposite sexes) also have been found to play roles in sibling relationships, but spacing of children makes less of a difference than most people think. In general, children spaced more than 4 years apart have less conflict, but they also spend less time together and have less of an integrated relationship than closely spaced siblings do.
When looking at underlying dynamics, research points to the perception of favoritism by the parents as the main contributing factor. Importantly, the children's impressions of favoritism are not always accurate, but they are such an important driver of sibling conflict that they deserve consideration.
I suggest to parents that they make a special effort to provide roughly equal “alone” time with each child. When one child's needs really do require inordinate attention—as in the case of homework time for a child with learning disabilities—they need to be up front about that reality, and say, “If you need something special, I will be there for you, too.” Remind the child who feels slighted about exceptional times when all the focus was on them: during assembly of the science fair project, or when they learned to ride a bike, for example.
Acknowledge jealousy as a real and understandable emotion, but one that must be handled within limits and household rules.
Parents will do well to practice prevention with siblings, reinforcing cooperation in general and any specific examples of good deeds performed on behalf of each other with acknowledgment or even rewards if the rivalry is serious.
Advise parents to be sensitive to situations, like boredom, that lend themselves to sibling disputes, and to intervene with distractions. Promote cooperative projects and noncompetitive games: building a fort or puzzle, playing in the sprinkler, or making breakfast as a family, instead of games with winners and losers.
When board games are necessarily competitive, make it a practice to turn the board around every fourth move to minimize age-related inequities. Even out the teams in driveway basketball as well.
Once children are old enough to participate, family meetings are an excellent forum in which to air grievances. Again, ground rules apply; everyone gets to be heard. No interrupting. Solutions can be brainstormed and tried out, to be reviewed at the next regularly scheduled session.
A stepwise approach to dealing with actual sibling disputes also helps bring order to the chaos that feeds sibling wars. Parents may want to read the popular if optimistically titled book by Adele Faber and Irene Mazlish, “Siblings Without Rivalry” (New York: HarperCollins Publishing, 2004).
Essentially, their basic plan is to teach parents to ignore whatever can be ignored, thus avoiding a self-feeding loop of inadvertent reinforcement of the conflicts.
Situations that are a bit too much to ignore should be handled dispassionately. The parent may want to ask, “Is this a real fight or a play fight?” If it's a play fight but noisy, they might want to suggest a new venue—in the basement or outdoors.
If it's a real fight, encourage parents to simply describe the situation they see. “It looks like you both want to play with the truck, and it's hard to decide how to work it out.” Follow this with an affirming statement like, “I'm sure you can figure out a solution.”
If things are even more volatile—maybe someone has hit or pinched—parents should intervene, but in an unbiased manner and with the least amount of punishment that makes sense. They need to emphasize that hitting is never acceptable, but not take sides. A useful mantra for parents: “Don't try to judge who started it. You can never tell.”
Depending on the situation, both children may need to be sent to a room away from the toy to make a plan for resolution. The toy may need to be put in time out. Both kids may need to be put in time out for the same amount of time, with duration based on the younger child's age. Each child may need to take on an individual chore card, or even chores requiring the effort of both kids.
Whatever the solution, it should be brief.
Counsel parents that rivalry is part of sibling interaction: a challenge best met through prevention, structured responses, and reliance on family rules.
Remind them of the fleeting nature of sibling spats—don't they hear the kids giggling 15 minutes later?—and the permanence of warm, mutually respectful, sibling bonds through a lifetime.
www.CHADIS.com[email protected]
From Cane and Abel to Linus and Lucy, Wally and the Beaver to Bart and Lisa Simpson, sibling rivalry is the stuff of legend and comedy. But when it presents as a source of serious concern for parents during pediatric office visits, it's usually no laughing matter for them.
Research suggests that 64% of school-age siblings fight “sometimes or often”—a figure likely matched in magnitude if not muscle by younger siblings as well.
Sibling rivalry is so common, in fact, that we may tend to think back to our own sibling spats, or those of our kids, roll our eyes and offer the “they'll grow out of it” platitude.
But in truth, sibling wars can have consequences. While injuries are rare in most sibling disputes, in 25% of child abuse cases a sibling has been involved in victimization (usually in concert with adults).
Serious sibling conflict tremendously compromises quality of life for children, and for their parents as well. We know that marriages suffer in households with high levels of sibling discord, with the issue a common flashpoint for disagreements between parents about how to respond. Children exposed to serious sibling conflict in middle childhood appear to suffer higher levels of anxiety, depression, and delinquent behavior in early adolescence. Down the road, people carry the grudges of sibling difficulties for decades, undermining bonds that might otherwise be a significant source of support in our increasingly fragmented society.
So sibling struggles are worthy of our time and thoughtfulness, and addressing them productively will build trust in your relationship with parents and perhaps bring some semblance of peace to their households.
The first response to a parent's frustration over sibling quarrels should be to listen with respect. Their pain is often significant as they describe the battles unfolding among children they hold precious. Patiently listening to the details of sibling encounters also can help you sort out whether the issues they're describing fall into the normal range or may signify more serious individual or relational issues that deserve attention.
Assuming it's the former, I think it helps to remind parents of how common sibling rivalry is, and more importantly, why it occurs. Annoying as they may be, fracases actually serve a number of important biological functions. Watch any nature documentary featuring lions lounging under a tree on the savannah, and what are the cubs doing? Attacking, defending, tumbling, and biting, growling all the while.
In kids, like cubs, important social skills arise from the sibling relationship, even when the dust flies. Siblings teach each other to giggle and laugh, bait and switch, sneak and chase, parry and defend. From each other, they learn which jokes fly and which land with a thud, how to toss out an insult and absorb one tossed their way.
Siblings also learn how to pull their punches, practicing evolutionarily useful conflict skills while stopping short of inflicting serious harm.
The question remains, how does a family foster productive resilience-building sibling interactions while preserving affectionate connections and at least a modicum of household calm?
Like so many things in life, household chaos is associated with unhealthy levels of sibling conflict, according to research by psychologist Judy Dunn, the author of “Sisters and Brothers” (Cambridge: Harvard University Press, 1985), “Separate Lives: Why Siblings Are So Different” (New York: Basic Books, 1992), and “From One Child to Two” (New York: Ballantine Books, 1995).
Corporal punishment in the family makes rivalry worse as well.
Individual temperaments, the presence of a child with special needs, and family structure (children of opposite sexes) also have been found to play roles in sibling relationships, but spacing of children makes less of a difference than most people think. In general, children spaced more than 4 years apart have less conflict, but they also spend less time together and have less of an integrated relationship than closely spaced siblings do.
When looking at underlying dynamics, research points to the perception of favoritism by the parents as the main contributing factor. Importantly, the children's impressions of favoritism are not always accurate, but they are such an important driver of sibling conflict that they deserve consideration.
I suggest to parents that they make a special effort to provide roughly equal “alone” time with each child. When one child's needs really do require inordinate attention—as in the case of homework time for a child with learning disabilities—they need to be up front about that reality, and say, “If you need something special, I will be there for you, too.” Remind the child who feels slighted about exceptional times when all the focus was on them: during assembly of the science fair project, or when they learned to ride a bike, for example.
Acknowledge jealousy as a real and understandable emotion, but one that must be handled within limits and household rules.
Parents will do well to practice prevention with siblings, reinforcing cooperation in general and any specific examples of good deeds performed on behalf of each other with acknowledgment or even rewards if the rivalry is serious.
Advise parents to be sensitive to situations, like boredom, that lend themselves to sibling disputes, and to intervene with distractions. Promote cooperative projects and noncompetitive games: building a fort or puzzle, playing in the sprinkler, or making breakfast as a family, instead of games with winners and losers.
When board games are necessarily competitive, make it a practice to turn the board around every fourth move to minimize age-related inequities. Even out the teams in driveway basketball as well.
Once children are old enough to participate, family meetings are an excellent forum in which to air grievances. Again, ground rules apply; everyone gets to be heard. No interrupting. Solutions can be brainstormed and tried out, to be reviewed at the next regularly scheduled session.
A stepwise approach to dealing with actual sibling disputes also helps bring order to the chaos that feeds sibling wars. Parents may want to read the popular if optimistically titled book by Adele Faber and Irene Mazlish, “Siblings Without Rivalry” (New York: HarperCollins Publishing, 2004).
Essentially, their basic plan is to teach parents to ignore whatever can be ignored, thus avoiding a self-feeding loop of inadvertent reinforcement of the conflicts.
Situations that are a bit too much to ignore should be handled dispassionately. The parent may want to ask, “Is this a real fight or a play fight?” If it's a play fight but noisy, they might want to suggest a new venue—in the basement or outdoors.
If it's a real fight, encourage parents to simply describe the situation they see. “It looks like you both want to play with the truck, and it's hard to decide how to work it out.” Follow this with an affirming statement like, “I'm sure you can figure out a solution.”
If things are even more volatile—maybe someone has hit or pinched—parents should intervene, but in an unbiased manner and with the least amount of punishment that makes sense. They need to emphasize that hitting is never acceptable, but not take sides. A useful mantra for parents: “Don't try to judge who started it. You can never tell.”
Depending on the situation, both children may need to be sent to a room away from the toy to make a plan for resolution. The toy may need to be put in time out. Both kids may need to be put in time out for the same amount of time, with duration based on the younger child's age. Each child may need to take on an individual chore card, or even chores requiring the effort of both kids.
Whatever the solution, it should be brief.
Counsel parents that rivalry is part of sibling interaction: a challenge best met through prevention, structured responses, and reliance on family rules.
Remind them of the fleeting nature of sibling spats—don't they hear the kids giggling 15 minutes later?—and the permanence of warm, mutually respectful, sibling bonds through a lifetime.
www.CHADIS.com[email protected]
From Cane and Abel to Linus and Lucy, Wally and the Beaver to Bart and Lisa Simpson, sibling rivalry is the stuff of legend and comedy. But when it presents as a source of serious concern for parents during pediatric office visits, it's usually no laughing matter for them.
Research suggests that 64% of school-age siblings fight “sometimes or often”—a figure likely matched in magnitude if not muscle by younger siblings as well.
Sibling rivalry is so common, in fact, that we may tend to think back to our own sibling spats, or those of our kids, roll our eyes and offer the “they'll grow out of it” platitude.
But in truth, sibling wars can have consequences. While injuries are rare in most sibling disputes, in 25% of child abuse cases a sibling has been involved in victimization (usually in concert with adults).
Serious sibling conflict tremendously compromises quality of life for children, and for their parents as well. We know that marriages suffer in households with high levels of sibling discord, with the issue a common flashpoint for disagreements between parents about how to respond. Children exposed to serious sibling conflict in middle childhood appear to suffer higher levels of anxiety, depression, and delinquent behavior in early adolescence. Down the road, people carry the grudges of sibling difficulties for decades, undermining bonds that might otherwise be a significant source of support in our increasingly fragmented society.
So sibling struggles are worthy of our time and thoughtfulness, and addressing them productively will build trust in your relationship with parents and perhaps bring some semblance of peace to their households.
The first response to a parent's frustration over sibling quarrels should be to listen with respect. Their pain is often significant as they describe the battles unfolding among children they hold precious. Patiently listening to the details of sibling encounters also can help you sort out whether the issues they're describing fall into the normal range or may signify more serious individual or relational issues that deserve attention.
Assuming it's the former, I think it helps to remind parents of how common sibling rivalry is, and more importantly, why it occurs. Annoying as they may be, fracases actually serve a number of important biological functions. Watch any nature documentary featuring lions lounging under a tree on the savannah, and what are the cubs doing? Attacking, defending, tumbling, and biting, growling all the while.
In kids, like cubs, important social skills arise from the sibling relationship, even when the dust flies. Siblings teach each other to giggle and laugh, bait and switch, sneak and chase, parry and defend. From each other, they learn which jokes fly and which land with a thud, how to toss out an insult and absorb one tossed their way.
Siblings also learn how to pull their punches, practicing evolutionarily useful conflict skills while stopping short of inflicting serious harm.
The question remains, how does a family foster productive resilience-building sibling interactions while preserving affectionate connections and at least a modicum of household calm?
Like so many things in life, household chaos is associated with unhealthy levels of sibling conflict, according to research by psychologist Judy Dunn, the author of “Sisters and Brothers” (Cambridge: Harvard University Press, 1985), “Separate Lives: Why Siblings Are So Different” (New York: Basic Books, 1992), and “From One Child to Two” (New York: Ballantine Books, 1995).
Corporal punishment in the family makes rivalry worse as well.
Individual temperaments, the presence of a child with special needs, and family structure (children of opposite sexes) also have been found to play roles in sibling relationships, but spacing of children makes less of a difference than most people think. In general, children spaced more than 4 years apart have less conflict, but they also spend less time together and have less of an integrated relationship than closely spaced siblings do.
When looking at underlying dynamics, research points to the perception of favoritism by the parents as the main contributing factor. Importantly, the children's impressions of favoritism are not always accurate, but they are such an important driver of sibling conflict that they deserve consideration.
I suggest to parents that they make a special effort to provide roughly equal “alone” time with each child. When one child's needs really do require inordinate attention—as in the case of homework time for a child with learning disabilities—they need to be up front about that reality, and say, “If you need something special, I will be there for you, too.” Remind the child who feels slighted about exceptional times when all the focus was on them: during assembly of the science fair project, or when they learned to ride a bike, for example.
Acknowledge jealousy as a real and understandable emotion, but one that must be handled within limits and household rules.
Parents will do well to practice prevention with siblings, reinforcing cooperation in general and any specific examples of good deeds performed on behalf of each other with acknowledgment or even rewards if the rivalry is serious.
Advise parents to be sensitive to situations, like boredom, that lend themselves to sibling disputes, and to intervene with distractions. Promote cooperative projects and noncompetitive games: building a fort or puzzle, playing in the sprinkler, or making breakfast as a family, instead of games with winners and losers.
When board games are necessarily competitive, make it a practice to turn the board around every fourth move to minimize age-related inequities. Even out the teams in driveway basketball as well.
Once children are old enough to participate, family meetings are an excellent forum in which to air grievances. Again, ground rules apply; everyone gets to be heard. No interrupting. Solutions can be brainstormed and tried out, to be reviewed at the next regularly scheduled session.
A stepwise approach to dealing with actual sibling disputes also helps bring order to the chaos that feeds sibling wars. Parents may want to read the popular if optimistically titled book by Adele Faber and Irene Mazlish, “Siblings Without Rivalry” (New York: HarperCollins Publishing, 2004).
Essentially, their basic plan is to teach parents to ignore whatever can be ignored, thus avoiding a self-feeding loop of inadvertent reinforcement of the conflicts.
Situations that are a bit too much to ignore should be handled dispassionately. The parent may want to ask, “Is this a real fight or a play fight?” If it's a play fight but noisy, they might want to suggest a new venue—in the basement or outdoors.
If it's a real fight, encourage parents to simply describe the situation they see. “It looks like you both want to play with the truck, and it's hard to decide how to work it out.” Follow this with an affirming statement like, “I'm sure you can figure out a solution.”
If things are even more volatile—maybe someone has hit or pinched—parents should intervene, but in an unbiased manner and with the least amount of punishment that makes sense. They need to emphasize that hitting is never acceptable, but not take sides. A useful mantra for parents: “Don't try to judge who started it. You can never tell.”
Depending on the situation, both children may need to be sent to a room away from the toy to make a plan for resolution. The toy may need to be put in time out. Both kids may need to be put in time out for the same amount of time, with duration based on the younger child's age. Each child may need to take on an individual chore card, or even chores requiring the effort of both kids.
Whatever the solution, it should be brief.
Counsel parents that rivalry is part of sibling interaction: a challenge best met through prevention, structured responses, and reliance on family rules.
Remind them of the fleeting nature of sibling spats—don't they hear the kids giggling 15 minutes later?—and the permanence of warm, mutually respectful, sibling bonds through a lifetime.
MGMA Releases Compensation and Productivity Data
New hospitalist compensation and productivity information is available via the 2010 Physician Compensation and Production Survey Report, the Medical Group Management Association’s (MGMA) annual survey. However, HM leaders are urging restraint to group directors and individual hospitalists pining for the latest industry benchmarks.
“We want to be careful not to read too much into trends at this point. This is a new set of data,” says William “Tex” Landis, MD, FHM, medical director of Wellspan Hospitalists in York, Pa., and chair of SHM’s Practice Analysis Committee. “I think the trending might be beneficial, but I think it should be done with great caution.”
The report, which surveyed 4,211 hospitalists from 443 groups, shows median hospitalist compensation at $215,000 annually. That’s an increase of about $20,000 per year compared with SHM’s 2007-2008 survey data.
The report also shows the median number of work RVUs at 4,107 per hospitalist per year.
SHM, which collaborated on the survey with MGMA, will release a more detailed compensation and productivity report in September. That report replaces SHM’s biannual survey, and will break down such hospitalist-specific data as night coverage, financial support, and staffing models.
The MGMA survey adds new layers of detail, as compared with past SHM surveys. In addition to mean and median values, the MGMA report breaks down production and compensation values to 25th-, 75th-, and 90th-percentile ranges. “It provides a lot more ways to cut the data than [SHM] has traditionally done,” says Leslie Flores, SHM senior advisor of practice management.
Although he warns of taking the MGMA survey information too literally, Dr. Landis knows his peers are anxiously awaiting the new numbers. “It provides the best possible data to help with optimal decision-making, especially as it pertains to resourcing hospitalist programs,” he says. “What will be more important, however, will be what next year’s numbers show; then, we will be comparing like with like.”
New hospitalist compensation and productivity information is available via the 2010 Physician Compensation and Production Survey Report, the Medical Group Management Association’s (MGMA) annual survey. However, HM leaders are urging restraint to group directors and individual hospitalists pining for the latest industry benchmarks.
“We want to be careful not to read too much into trends at this point. This is a new set of data,” says William “Tex” Landis, MD, FHM, medical director of Wellspan Hospitalists in York, Pa., and chair of SHM’s Practice Analysis Committee. “I think the trending might be beneficial, but I think it should be done with great caution.”
The report, which surveyed 4,211 hospitalists from 443 groups, shows median hospitalist compensation at $215,000 annually. That’s an increase of about $20,000 per year compared with SHM’s 2007-2008 survey data.
The report also shows the median number of work RVUs at 4,107 per hospitalist per year.
SHM, which collaborated on the survey with MGMA, will release a more detailed compensation and productivity report in September. That report replaces SHM’s biannual survey, and will break down such hospitalist-specific data as night coverage, financial support, and staffing models.
The MGMA survey adds new layers of detail, as compared with past SHM surveys. In addition to mean and median values, the MGMA report breaks down production and compensation values to 25th-, 75th-, and 90th-percentile ranges. “It provides a lot more ways to cut the data than [SHM] has traditionally done,” says Leslie Flores, SHM senior advisor of practice management.
Although he warns of taking the MGMA survey information too literally, Dr. Landis knows his peers are anxiously awaiting the new numbers. “It provides the best possible data to help with optimal decision-making, especially as it pertains to resourcing hospitalist programs,” he says. “What will be more important, however, will be what next year’s numbers show; then, we will be comparing like with like.”
New hospitalist compensation and productivity information is available via the 2010 Physician Compensation and Production Survey Report, the Medical Group Management Association’s (MGMA) annual survey. However, HM leaders are urging restraint to group directors and individual hospitalists pining for the latest industry benchmarks.
“We want to be careful not to read too much into trends at this point. This is a new set of data,” says William “Tex” Landis, MD, FHM, medical director of Wellspan Hospitalists in York, Pa., and chair of SHM’s Practice Analysis Committee. “I think the trending might be beneficial, but I think it should be done with great caution.”
The report, which surveyed 4,211 hospitalists from 443 groups, shows median hospitalist compensation at $215,000 annually. That’s an increase of about $20,000 per year compared with SHM’s 2007-2008 survey data.
The report also shows the median number of work RVUs at 4,107 per hospitalist per year.
SHM, which collaborated on the survey with MGMA, will release a more detailed compensation and productivity report in September. That report replaces SHM’s biannual survey, and will break down such hospitalist-specific data as night coverage, financial support, and staffing models.
The MGMA survey adds new layers of detail, as compared with past SHM surveys. In addition to mean and median values, the MGMA report breaks down production and compensation values to 25th-, 75th-, and 90th-percentile ranges. “It provides a lot more ways to cut the data than [SHM] has traditionally done,” says Leslie Flores, SHM senior advisor of practice management.
Although he warns of taking the MGMA survey information too literally, Dr. Landis knows his peers are anxiously awaiting the new numbers. “It provides the best possible data to help with optimal decision-making, especially as it pertains to resourcing hospitalist programs,” he says. “What will be more important, however, will be what next year’s numbers show; then, we will be comparing like with like.”
Belt Tightening
Let the debate formally begin.
Proposed regulations (PDF) from the Accreditation Council for Graduate Medical Education (ACGME) that limit first-year residents to 16 hours of duty will be seen either as an awakening or an abomination to educational leaders, according to the incoming president of the Association of Program Directors in Internal Medicine (APDIM).
“The draft ... will be welcomed by programs wishing to manage fatigue and will be seen as a threat by programs who have not yet accepted the need to reform graduate medical education,” says Ethan Fried, who takes over as APDIM president July 1.
The new changes come as no shock to academic hospitalists who have been waiting for the prescribed five-year update to the landmark 2003 duty-hour standards, especially after the recommendations published in the Institute of Medicine’s 2008 report “Resident Duty Hours: Enhancing Sleep, Supervision and Safety.” If approved, the new regulations will likely take effect in July 2011.
The data points of the rules will be debated thoroughly between now and then, but Dr. Fried views the recommendations as more than just tweaks to the existing infrastructure governing residency programs. He sees the suggestions as a sea change, particularly allowances for added duty time for second- and third-year residents, as well as situational exceptions that allow residents to work longer to ensure continuity of care.
“The draft turns the old concept of professionalism 180 degrees by telling residents that sleep deprivation is no longer a lifestyle choice,” adds Dr. Fried, MD, MS, FACP, assistant professor of clinical medicine at Columbia University, vice chair for education in the Department of Medicine and director of Graduate Medical Education at St. Luke's-Roosevelt in New York City. “Residents must explicitly believe that it is their personal responsibility to work rested and free of fatigue in most cases. Furthermore, the draft makes explicit the rare but real situation in which the care of an individual patient supersedes the duty hour restrictions.”
In an editorial, members of the ACGME Duty Hour Task Force also argue that their recommendations should be viewed as more than a singular recommendation on how many hours young doctors can work (10.1056/NEJMsb1005800).
“Although much of the debate has focused on establishing appropriate limits on resident hours,” the authors wrote, “the task force recognized that ensuring patient safety and providing an excellent teaching environment entail more than setting these limits.”
Let the debate formally begin.
Proposed regulations (PDF) from the Accreditation Council for Graduate Medical Education (ACGME) that limit first-year residents to 16 hours of duty will be seen either as an awakening or an abomination to educational leaders, according to the incoming president of the Association of Program Directors in Internal Medicine (APDIM).
“The draft ... will be welcomed by programs wishing to manage fatigue and will be seen as a threat by programs who have not yet accepted the need to reform graduate medical education,” says Ethan Fried, who takes over as APDIM president July 1.
The new changes come as no shock to academic hospitalists who have been waiting for the prescribed five-year update to the landmark 2003 duty-hour standards, especially after the recommendations published in the Institute of Medicine’s 2008 report “Resident Duty Hours: Enhancing Sleep, Supervision and Safety.” If approved, the new regulations will likely take effect in July 2011.
The data points of the rules will be debated thoroughly between now and then, but Dr. Fried views the recommendations as more than just tweaks to the existing infrastructure governing residency programs. He sees the suggestions as a sea change, particularly allowances for added duty time for second- and third-year residents, as well as situational exceptions that allow residents to work longer to ensure continuity of care.
“The draft turns the old concept of professionalism 180 degrees by telling residents that sleep deprivation is no longer a lifestyle choice,” adds Dr. Fried, MD, MS, FACP, assistant professor of clinical medicine at Columbia University, vice chair for education in the Department of Medicine and director of Graduate Medical Education at St. Luke's-Roosevelt in New York City. “Residents must explicitly believe that it is their personal responsibility to work rested and free of fatigue in most cases. Furthermore, the draft makes explicit the rare but real situation in which the care of an individual patient supersedes the duty hour restrictions.”
In an editorial, members of the ACGME Duty Hour Task Force also argue that their recommendations should be viewed as more than a singular recommendation on how many hours young doctors can work (10.1056/NEJMsb1005800).
“Although much of the debate has focused on establishing appropriate limits on resident hours,” the authors wrote, “the task force recognized that ensuring patient safety and providing an excellent teaching environment entail more than setting these limits.”
Let the debate formally begin.
Proposed regulations (PDF) from the Accreditation Council for Graduate Medical Education (ACGME) that limit first-year residents to 16 hours of duty will be seen either as an awakening or an abomination to educational leaders, according to the incoming president of the Association of Program Directors in Internal Medicine (APDIM).
“The draft ... will be welcomed by programs wishing to manage fatigue and will be seen as a threat by programs who have not yet accepted the need to reform graduate medical education,” says Ethan Fried, who takes over as APDIM president July 1.
The new changes come as no shock to academic hospitalists who have been waiting for the prescribed five-year update to the landmark 2003 duty-hour standards, especially after the recommendations published in the Institute of Medicine’s 2008 report “Resident Duty Hours: Enhancing Sleep, Supervision and Safety.” If approved, the new regulations will likely take effect in July 2011.
The data points of the rules will be debated thoroughly between now and then, but Dr. Fried views the recommendations as more than just tweaks to the existing infrastructure governing residency programs. He sees the suggestions as a sea change, particularly allowances for added duty time for second- and third-year residents, as well as situational exceptions that allow residents to work longer to ensure continuity of care.
“The draft turns the old concept of professionalism 180 degrees by telling residents that sleep deprivation is no longer a lifestyle choice,” adds Dr. Fried, MD, MS, FACP, assistant professor of clinical medicine at Columbia University, vice chair for education in the Department of Medicine and director of Graduate Medical Education at St. Luke's-Roosevelt in New York City. “Residents must explicitly believe that it is their personal responsibility to work rested and free of fatigue in most cases. Furthermore, the draft makes explicit the rare but real situation in which the care of an individual patient supersedes the duty hour restrictions.”
In an editorial, members of the ACGME Duty Hour Task Force also argue that their recommendations should be viewed as more than a singular recommendation on how many hours young doctors can work (10.1056/NEJMsb1005800).
“Although much of the debate has focused on establishing appropriate limits on resident hours,” the authors wrote, “the task force recognized that ensuring patient safety and providing an excellent teaching environment entail more than setting these limits.”
Save Time, Save Money
A new study that shows time and money could be saved by standardizing billing practices will likely find a supportive audience from HM groups and their business staffs, one hospitalist program executive says.
James Kodjababian, chief revenue officer and vice president of management services for Sound Physicians in Tacoma, Wash., says his firm is large enough that it has put in place systems and information technology (IT) to navigate the complex billing infrastructure that varies from carrier to carrier. But he thinks smaller HM groups likely struggle to deal with the labyrinthine codes and regulations that different insurance companies use.
“It’s like translating 43 different languages to consolidate it and manage it,” he says.
His sentiment is buttressed by “Saving Billions of Dollars—and Physician’s Time—By Streamlining Billing Practices,” which reported that standardized payment rules and claim forms “would translate into $7 million of savings annually in physician and clinical services” (doi: 10.1377/hlthaff.2009.0075). The study in Health Affairs also reported that four hours of physician time and five of support staff time could be saved each week.
Kodjababian acknowledges that to achieve such industrywide standardization, insurance companies would have to invest funding and man-hours. However, he says, the data that could be culled from an improved system would prove beneficial both to carriers and physicians.
“It’s time and money, but at the end of the day, you create a much better information set for people to benefit from,” Kodjababian says. “Right now, it’s very difficult to compare notes. If everybody is processing the same way, you can start to run statistics. You can start to see in a more macro perspective what things we should be doing.”
A new study that shows time and money could be saved by standardizing billing practices will likely find a supportive audience from HM groups and their business staffs, one hospitalist program executive says.
James Kodjababian, chief revenue officer and vice president of management services for Sound Physicians in Tacoma, Wash., says his firm is large enough that it has put in place systems and information technology (IT) to navigate the complex billing infrastructure that varies from carrier to carrier. But he thinks smaller HM groups likely struggle to deal with the labyrinthine codes and regulations that different insurance companies use.
“It’s like translating 43 different languages to consolidate it and manage it,” he says.
His sentiment is buttressed by “Saving Billions of Dollars—and Physician’s Time—By Streamlining Billing Practices,” which reported that standardized payment rules and claim forms “would translate into $7 million of savings annually in physician and clinical services” (doi: 10.1377/hlthaff.2009.0075). The study in Health Affairs also reported that four hours of physician time and five of support staff time could be saved each week.
Kodjababian acknowledges that to achieve such industrywide standardization, insurance companies would have to invest funding and man-hours. However, he says, the data that could be culled from an improved system would prove beneficial both to carriers and physicians.
“It’s time and money, but at the end of the day, you create a much better information set for people to benefit from,” Kodjababian says. “Right now, it’s very difficult to compare notes. If everybody is processing the same way, you can start to run statistics. You can start to see in a more macro perspective what things we should be doing.”
A new study that shows time and money could be saved by standardizing billing practices will likely find a supportive audience from HM groups and their business staffs, one hospitalist program executive says.
James Kodjababian, chief revenue officer and vice president of management services for Sound Physicians in Tacoma, Wash., says his firm is large enough that it has put in place systems and information technology (IT) to navigate the complex billing infrastructure that varies from carrier to carrier. But he thinks smaller HM groups likely struggle to deal with the labyrinthine codes and regulations that different insurance companies use.
“It’s like translating 43 different languages to consolidate it and manage it,” he says.
His sentiment is buttressed by “Saving Billions of Dollars—and Physician’s Time—By Streamlining Billing Practices,” which reported that standardized payment rules and claim forms “would translate into $7 million of savings annually in physician and clinical services” (doi: 10.1377/hlthaff.2009.0075). The study in Health Affairs also reported that four hours of physician time and five of support staff time could be saved each week.
Kodjababian acknowledges that to achieve such industrywide standardization, insurance companies would have to invest funding and man-hours. However, he says, the data that could be culled from an improved system would prove beneficial both to carriers and physicians.
“It’s time and money, but at the end of the day, you create a much better information set for people to benefit from,” Kodjababian says. “Right now, it’s very difficult to compare notes. If everybody is processing the same way, you can start to run statistics. You can start to see in a more macro perspective what things we should be doing.”
In the Literature: Research You Need to Know
Clinical question: Does a resident’s ability to make decisions in the management of critically ill patients deteriorate with longer periods of wakefulness?
Background: Residents work long shifts, particularly in the ICU. Their cognitive performance on standardized tests and clinical performance in surgical simulators deteriorates with sleep deprivation. The effect of prolonged wakefulness on resident management of critically ill patients is not known.
Study design: Experimental within-subjects comparison.
Setting: Simulator at the Centre of Excellence for Surgical Education and Innovation, Vancouver General Hospital, Canada.
Synopsis: Twelve internal medicine residents at various levels of training from the University of British Columbia were studied. The residents provided simulated care for critically ill patients at four time points over 26 hours of wakefulness. At each time point, the residents first managed a cardiac dysrhythmia, then a complex patient scenario that would require ICU-level care. They were then scored for errors and given a global score by two of the investigators.
Resident errors in the management of dysrhythmias decreased at the first time point, and remained stable through the next two time points. The mean error rate for the complex patient scenarios increased from 0.92, with a steady rate of rise to 1.58 at the last session. The mean global score for the complex patient scenario showed a trend toward decline as well. Despite this being a small study with relatively subjective outcomes, the results are consistent with previous studies and raise concern for increasing the risk for error in the care of highly vulnerable critically ill patients by residents working long hours.
Bottom line: There is a progressive decrement in resident performance with increasing periods of wakefulness when delivering ICU-level patient-management decisions in a simulator environment.
Citation: Sharpe R, Koval V, Ronco JJ, et al. The impact of prolonged continuous wakefulness on resident clinical performance in the intensive care unit: A patient simulator study. Crit Care Med. 2010;38:766-770.
Reviewed for TH eWire by Dimitriy Levin, MD, Jeffrey Carter, MD, Erin Egan, MD, JD, Jonathan Pell, MD, Laura Rosenthal, MSN, ACNP, Nichole Zehnder, MD, Hospital Medicine Group, University of Colorado Denver
For more physician reviews of HM-related research, visit our website.
Clinical question: Does a resident’s ability to make decisions in the management of critically ill patients deteriorate with longer periods of wakefulness?
Background: Residents work long shifts, particularly in the ICU. Their cognitive performance on standardized tests and clinical performance in surgical simulators deteriorates with sleep deprivation. The effect of prolonged wakefulness on resident management of critically ill patients is not known.
Study design: Experimental within-subjects comparison.
Setting: Simulator at the Centre of Excellence for Surgical Education and Innovation, Vancouver General Hospital, Canada.
Synopsis: Twelve internal medicine residents at various levels of training from the University of British Columbia were studied. The residents provided simulated care for critically ill patients at four time points over 26 hours of wakefulness. At each time point, the residents first managed a cardiac dysrhythmia, then a complex patient scenario that would require ICU-level care. They were then scored for errors and given a global score by two of the investigators.
Resident errors in the management of dysrhythmias decreased at the first time point, and remained stable through the next two time points. The mean error rate for the complex patient scenarios increased from 0.92, with a steady rate of rise to 1.58 at the last session. The mean global score for the complex patient scenario showed a trend toward decline as well. Despite this being a small study with relatively subjective outcomes, the results are consistent with previous studies and raise concern for increasing the risk for error in the care of highly vulnerable critically ill patients by residents working long hours.
Bottom line: There is a progressive decrement in resident performance with increasing periods of wakefulness when delivering ICU-level patient-management decisions in a simulator environment.
Citation: Sharpe R, Koval V, Ronco JJ, et al. The impact of prolonged continuous wakefulness on resident clinical performance in the intensive care unit: A patient simulator study. Crit Care Med. 2010;38:766-770.
Reviewed for TH eWire by Dimitriy Levin, MD, Jeffrey Carter, MD, Erin Egan, MD, JD, Jonathan Pell, MD, Laura Rosenthal, MSN, ACNP, Nichole Zehnder, MD, Hospital Medicine Group, University of Colorado Denver
For more physician reviews of HM-related research, visit our website.
Clinical question: Does a resident’s ability to make decisions in the management of critically ill patients deteriorate with longer periods of wakefulness?
Background: Residents work long shifts, particularly in the ICU. Their cognitive performance on standardized tests and clinical performance in surgical simulators deteriorates with sleep deprivation. The effect of prolonged wakefulness on resident management of critically ill patients is not known.
Study design: Experimental within-subjects comparison.
Setting: Simulator at the Centre of Excellence for Surgical Education and Innovation, Vancouver General Hospital, Canada.
Synopsis: Twelve internal medicine residents at various levels of training from the University of British Columbia were studied. The residents provided simulated care for critically ill patients at four time points over 26 hours of wakefulness. At each time point, the residents first managed a cardiac dysrhythmia, then a complex patient scenario that would require ICU-level care. They were then scored for errors and given a global score by two of the investigators.
Resident errors in the management of dysrhythmias decreased at the first time point, and remained stable through the next two time points. The mean error rate for the complex patient scenarios increased from 0.92, with a steady rate of rise to 1.58 at the last session. The mean global score for the complex patient scenario showed a trend toward decline as well. Despite this being a small study with relatively subjective outcomes, the results are consistent with previous studies and raise concern for increasing the risk for error in the care of highly vulnerable critically ill patients by residents working long hours.
Bottom line: There is a progressive decrement in resident performance with increasing periods of wakefulness when delivering ICU-level patient-management decisions in a simulator environment.
Citation: Sharpe R, Koval V, Ronco JJ, et al. The impact of prolonged continuous wakefulness on resident clinical performance in the intensive care unit: A patient simulator study. Crit Care Med. 2010;38:766-770.
Reviewed for TH eWire by Dimitriy Levin, MD, Jeffrey Carter, MD, Erin Egan, MD, JD, Jonathan Pell, MD, Laura Rosenthal, MSN, ACNP, Nichole Zehnder, MD, Hospital Medicine Group, University of Colorado Denver
For more physician reviews of HM-related research, visit our website.
Short‐term Femoral Vein Catheterization
Central venous catheters (CVC) are routinely used to deliver medications and monitor intravascular pressures of critically ill patients. Experts and national regulatory bodies have questioned the safety of femoral vein catheterization (FVC), and currently recommend against venous access at this site whenever possible. 13 However, a large prospective nonrandomized study has suggested that rates of FVC infections are not higher than jugular or subclavian sites. 4 Some authors have suggested that increased risk of deep vein thrombosis (DVT) also relatively contraindicates the femoral site. 5 No study has prospectively examined rates of DVT in patients receiving FVC for short durations (<72 hours). In this brief report, we prospectively examined the rates of catheter‐related bloodstream infections (CRBI) and DVT in critically ill patients receiving CVC.
Methods
This prospective observational cohort study was conducted in the medical intensive care unit (MICU) of Bridgeport Hospital, a 350‐bed community teaching hospital. The hospital's Institutional Review Board approved the study and waived the informed consent requirement because it has been the practice for the past decade to favor use of the femoral site for initial resuscitations with very low complication rates. All patients admitted to the MICU between September 1, 2008 and March 31, 2009 were eligible. VC were defined as catheters placed in the jugular, subclavian or femoral veins or peripherally inserted and guided to a central intrathoracic vein (PICC). CVC refers to catheters placed directly into central veins. In early 2008, a hospital‐wide initiative was introduced to insert all CVC using the Pronovost check‐list. 1 VC sites were chosen at the discretion of caregivers in the emergency department and MICU. The policy of our intensive care units is to use only saline flushes of VCs.
Demographic data including age, gender, and body mass index, were collected on all patients. In addition the following parameters were monitored for the duration of ICU stay for the purpose of this study: (1) site and duration of installation of all intravascular catheters, (2) level of training of clinician inserting CVC, (3) catheter/blood culture results. For the purposes of this study, bilateral femoral Doppler compression ultrasound studies were expected to be performed by radiology house officers within 24 hours of removing and again 5 to 7 days following removal of FVC. Local VC complications, methods of thromboprophylaxis and risk factors for venous thromboembolism (VTE) were recorded. Patient outcomes and disposition destinations were also recorded.
CRBI were defined using the Centers for Disease Control definitions. 2 CRBI were identified by daily review of all positive blood cultures and review of patients' medical records. In addition, Infection Control Committee data were reviewed to corroborate contemporaneously determined CRBI during the study period and for 1 year prior to the study period. Patients with FVC were examined each day for signs or symptoms of thrombosis (tenderness along the vein, leg swelling, pitting edema or visible collateral superficial veins). Patients were followed up until death or hospital discharge for clinical signs, symptoms or diagnosis of thromboembolic disease.
Bedside Duplex ultrasounds of bilateral common femoral and superficial femoral veins were performed using graded compression and color Doppler techniques. The leg without FVC served as the control. Evaluations were conducted by senior radiology residents (>100 hours training) utilizing a high‐resolution (>7.5 MHz) linear array transducer. Frame capture images were digitally stored and subsequently reviewed by a Board‐certified radiologist, who was blinded to side of insertion and clinical outcomes, and rendered a final interpretation.
Values are listed as means standard deviations. Comparisons of group means were performed using nonpaired Student's t tests. A P value of <0.05 signified statistical significance.
Results
During the study period, 675 patients were admitted to the MICU. VCs were inserted in 238 (35% of) patients. During their MICU stay, 182 (77% of) patients had 1 VC, 48 (20%) had 2 VC, and 8 (3%) had 3 VC. On admission, 38 patients (6%) had preexisting VC (tunneled catheter 58%, PICC 32%, and dialysis catheters 10%). Additional VCs were placed in 10 of these patients (26%).
Of the 302 VC, 85 (28%) were PICCs and 217 were CVC (107, 49% FVC; 82, 38% internal jugular; 28, 13% subclavian). A total of 151 (28%) patients had radial arterial catheters placed around the time of admission. The types of CVC included triple lumen in 164 (75%), dialysis catheters in 29 (13%), single‐lumen large bore catheters in 17 (8%), and tunneled catheters in 4 patients (2%). The average duration (standard deviation [SD]) of CVC was 2.7 2.2 days for FVC, 5.7 9.6 days for internal jugular and 3.6 3.1 days for subclavian vein catheters.
During these seven months, including 1200 catheter‐days, only 1 CRBI was identified in a patient who only had a PICC, yielding an infection rate of 0.83 CRBI per 1000 catheter‐days. No femoral, subclavian or internal jugular catheter infections were detected. Hospital epidemiologic data confirmed this finding, and demonstrated only 1 other CRBI during 3721 line‐days, in the 7 months of this study and 12 months before, yielding an average of 0.40 CRBI/1000 catheter‐days.
Of 107 FVC, 101 were placed during initial resuscitations and 6 as second‐access sites, (2 for dialysis, 4 triple lumen catheters). Thromboprophylaxis was administered to 104 (97% of) patients with FVC. Thromboprophylaxis was pharmacological (heparins) in 63 (59% of) patients and mechanical (pneumatic compression) in 46 (43%). Five patients had both mechanical and pharmacological prophylaxis. Catheters were placed by a critical care or emergency department attending in 11%, critical care fellows in 11%, and residents in 78%. Ultrasound studies of the legs were performed in 57 patients; 56 had studies within 24 hours of removing FVC. Of these 56 patients, 53 studies were interpreted as negative and 3 were considered incomplete. The 3 initially incomplete studies were repeated, and found to be negative. Six patients were discharged from the hospital before the post‐FVC‐removal ultrasound could be performed. Of the 50 patients who had both ultrasounds (initial and follow up 57 days after removal of FVC), none had a DVT on the side of the catheter or in the control leg. Of the 50 patients with no ultrasound follow‐up, no patient developed clinically detected VTE; these patients had FVC for shorter duration (2.4 2.4 vs. 3.4 1.9 days for those with 2 Duplex; P = 0.02) and their ICU length of stay was shorter (3.8 4.6 vs. 6.6 5.6 days for those with 2 Duplex; P = 0.01).
Since no VTE or CRBI were detected further analyses regarding risks for these complications was not possible.
Discussion
Contrary to regulatory guidelines suggesting a poor safety profile, we found that short‐term FVC was associated with no episode of DVT or CRBI. While the incidence of complications is lower in more experienced operators, 6 most FVC in our hospital were placed by resident‐trainees (78%) with or without supervision from an attending physician. There were no immediate or subacute (ie, thrombosis, infection) major complications. There are a number of features that favor short‐term FVC for initial resuscitation of critically ill patients. Subclavian and intrajugular CVC require prolonged Trendelenburg position, which may not be well tolerated by some patients. FVC does not require Trendelenburg position. Major bleeding1.0% to 1.5% for all the CVCis minimized because direct compression of femoral vessels is possible. Compression of subclavian hemorrhage is impossible while compression of the jugular vessels is uncomfortable. Pneumothorax, while uncommon in the subclavian and intrajugular approaches, 7 has serious consequences for an unstable patient, whereas FVC obviates the risk. Some might argue that FVC cannot accurately reflect cardiovascular filling thereby defeating 1 of the important purposes of the catheter. While this is certainly true in patients with raised intraabdominal pressures, a small case series suggests that (longer‐than‐normal) FVC can accurately measure central filling pressures. 8 Another potential shortcoming of FVC is that if used only for short durations during initial resuscitationsas in this studysome patients will require a second CVC or PICC with incumbent risks.
Our study differs from previous studies that have shown infection rates ranging from 1.5/1000 to 20/1000 catheter‐days 4, 9, 10 and thrombosis rates of 6.6% to 25%. 5, 1013 Some previous studies have suggested higher rates of infection of FVC relative to internal jugular or subclavian sites (3.7/1000 vs. 20/1000 catheter‐days) 9 while others found similar infection or colonization rates between femoral and nonfemoral sites. 4, 10 Our 0.83 CRBSI per 1000 catheter‐day rate is similar to that of Pronovost et al. 1 who avoided FVC, whereas it was the preferred site (nearly half of all CVC) in our MICU. The incidence of VTE in critically ill patients ranges from 9% to 33 %, 14, 15 and CVC are a well recognized risk factor of VTE. 5 The reported incidence of DVT in patients with CVC varies widely from 3% to 10% in subclavian catheters 9 to 6.6% to 25% in FVC. 11, 12 We attribute the remarkable difference in our results to the fact that FVC was used for brief durations (mean 2.7 days, range 116 days) for the primary purpose of resuscitating critically ill patients. Also, techniques introduced by Pronovost et al. 1 to reduce CRBI had permeated our institutional practices by the time of this study; our results match his, of very low rates of CRBI when checklists are employed. In previous studies, FVC was used for extended durations similar to other CVC sites (ranging from 4 to 9.6 days). 5, 9, 12, 13, 16 Additionally, almost all of our patients received VTE prophylaxis whereas rates were variable in previous studies.
This study has several limitations. First, catheter insertion sites were not randomly assigned. This can introduce selection bias. For example, often femoral access is used in more unstable patients 4 who are less tolerant of Trendelenberg position whereas it is often avoided in obese patients. Another important limitation is that ultrasound studies were not performed in 47% of patients who had FVC. While these missed cases were not advertent (eg, CVC on weekends when no study personnel available), we cannot exclude the possibility of bias. However, no FVC patients who did not have ultrasounds developed clinically detected VTE. It is also possible that DVT could have appeared >5 to 7 days after our follow‐up ultrasound, though later development might favor spontaneous DVT unrelated to CVC. Finally, this was a relatively small study, but it appears that the rate of DVT from FVC, if placed for short durations and accompanied by thromboprophylaxis, is very low.
In conclusion, short‐term FVC was used safelywith no major complicationsin our MICU. Our data support that short‐term FVC (with thromboprophylaxis) has a reasonable safety profile for initial resuscitation of critically ill patients. Notwithstanding the limitations of our study, we suggest that it may be premature to abandon entirely 3, 17 the use of FVC for resuscitation of critically ill patients. We propose that our data suggest the need for a larger study to examine more definitively the safety profile of short‐term FVC.
- , , , et al. An intervention to decrease catheter related bloodstream infections in the ICU. N Engl J Med. 2006; 355: 2725– 2732.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infection. MMWR Website. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5110a1.htm. Accessed February 2010.
- Joint Commission. National Accreditation: Hospital Patient Safety Goals. Available at: http://www.jointcommission.org/NR/rdonlyres/31666E86‐E7F4‐423E‐9BE8‐F05BD1CB0AA8/0/HAP_NPSG.pdf. Accessed February 2010.
- , , , et al. The incidence of infectious complications of central venous catheters at the subclavian, internal jugular and femoral sites in an intensive care unit population. Crit Care Med. 2005; 33: 13– 20.
- , , , . Femoral deep vein thrombosis associated with central venous catheterization: Results from a prospective, randomized trial. Crit Care Med. 1995; 23: 52– 59.
- , , , , . Central vein catheterization. Failure and complicagtion rates by three percutaneous approaches. Arch Intern Med. 1986; 146: 259– 261.
- , , . Complications of central venous catheters: internal jugular versus subclavian access—a systematic review. Crit Care Med. 2002; 30: 454– 460.
- , , , , , . Comparison of intrathoracic and intra‐abdominal measurements of central venous pressure. Lancet. 1996; 347: 1155– 1157.
- , , , et al. Complications of femoral and subclavian venous catheterization in critically ill patients. A randomized controlled trial. JAMA. 2001; 286: 700– 707.
- , , , et al. Femoral vs jugular venous catheterization and risk of nosocomial events in adults requiring acute renal replacement therapy. A randomized trial. JAMA. 2008; 299: 2413– 2422.
- , , , , , . A prospective evaluation of the use of femoral venous catheters in critically ill adults. Crit Care Med. 1997; 25: 1986– 1989.
- , , , , . Lower extremity deep vein thrombosis: a prospective, randomized, controlled trial in comatose or sedated patients undergoing femoral vein catheterization. Crit Care Med. 1997; 25: 1982– 1985.
- , , , , . Deep venous thrombosis caused by femoral venous catheters in critically ill adult patients. Chest. 2000; 117: 178– 183.
- , , . The incidence of deep venous thrombosis in ICU patients. Chest. 1997; 111: 661– 664.
- , , , et al. Deep venous thrombosis in medical‐surgical critically il patients: prevalence, incidence and risk factors. Crit Care Med. 2005; 33: 1565– 1571.
- , , , et al. Central vein catheter related thrombosis in intensive care patients: incidence, risk factors and relationship with catheter related sepsis. Chest. 1998; 114: 207– 213.
- Institute for Healthcare Improvement. Optimal catheter site selection, with avoidance of the femoral vein for central venous access in adults. Available at: http://www.ihi.org/IHI/Topics/CriticalCare/IntensiveCare/Changes/IndividualChanges/OptimalCatheterSiteSelectionwithAvoidanceofFemoralVeinforCentralVenousAccessinAdultPatients.htm. Accessed February 2010.
Central venous catheters (CVC) are routinely used to deliver medications and monitor intravascular pressures of critically ill patients. Experts and national regulatory bodies have questioned the safety of femoral vein catheterization (FVC), and currently recommend against venous access at this site whenever possible. 13 However, a large prospective nonrandomized study has suggested that rates of FVC infections are not higher than jugular or subclavian sites. 4 Some authors have suggested that increased risk of deep vein thrombosis (DVT) also relatively contraindicates the femoral site. 5 No study has prospectively examined rates of DVT in patients receiving FVC for short durations (<72 hours). In this brief report, we prospectively examined the rates of catheter‐related bloodstream infections (CRBI) and DVT in critically ill patients receiving CVC.
Methods
This prospective observational cohort study was conducted in the medical intensive care unit (MICU) of Bridgeport Hospital, a 350‐bed community teaching hospital. The hospital's Institutional Review Board approved the study and waived the informed consent requirement because it has been the practice for the past decade to favor use of the femoral site for initial resuscitations with very low complication rates. All patients admitted to the MICU between September 1, 2008 and March 31, 2009 were eligible. VC were defined as catheters placed in the jugular, subclavian or femoral veins or peripherally inserted and guided to a central intrathoracic vein (PICC). CVC refers to catheters placed directly into central veins. In early 2008, a hospital‐wide initiative was introduced to insert all CVC using the Pronovost check‐list. 1 VC sites were chosen at the discretion of caregivers in the emergency department and MICU. The policy of our intensive care units is to use only saline flushes of VCs.
Demographic data including age, gender, and body mass index, were collected on all patients. In addition the following parameters were monitored for the duration of ICU stay for the purpose of this study: (1) site and duration of installation of all intravascular catheters, (2) level of training of clinician inserting CVC, (3) catheter/blood culture results. For the purposes of this study, bilateral femoral Doppler compression ultrasound studies were expected to be performed by radiology house officers within 24 hours of removing and again 5 to 7 days following removal of FVC. Local VC complications, methods of thromboprophylaxis and risk factors for venous thromboembolism (VTE) were recorded. Patient outcomes and disposition destinations were also recorded.
CRBI were defined using the Centers for Disease Control definitions. 2 CRBI were identified by daily review of all positive blood cultures and review of patients' medical records. In addition, Infection Control Committee data were reviewed to corroborate contemporaneously determined CRBI during the study period and for 1 year prior to the study period. Patients with FVC were examined each day for signs or symptoms of thrombosis (tenderness along the vein, leg swelling, pitting edema or visible collateral superficial veins). Patients were followed up until death or hospital discharge for clinical signs, symptoms or diagnosis of thromboembolic disease.
Bedside Duplex ultrasounds of bilateral common femoral and superficial femoral veins were performed using graded compression and color Doppler techniques. The leg without FVC served as the control. Evaluations were conducted by senior radiology residents (>100 hours training) utilizing a high‐resolution (>7.5 MHz) linear array transducer. Frame capture images were digitally stored and subsequently reviewed by a Board‐certified radiologist, who was blinded to side of insertion and clinical outcomes, and rendered a final interpretation.
Values are listed as means standard deviations. Comparisons of group means were performed using nonpaired Student's t tests. A P value of <0.05 signified statistical significance.
Results
During the study period, 675 patients were admitted to the MICU. VCs were inserted in 238 (35% of) patients. During their MICU stay, 182 (77% of) patients had 1 VC, 48 (20%) had 2 VC, and 8 (3%) had 3 VC. On admission, 38 patients (6%) had preexisting VC (tunneled catheter 58%, PICC 32%, and dialysis catheters 10%). Additional VCs were placed in 10 of these patients (26%).
Of the 302 VC, 85 (28%) were PICCs and 217 were CVC (107, 49% FVC; 82, 38% internal jugular; 28, 13% subclavian). A total of 151 (28%) patients had radial arterial catheters placed around the time of admission. The types of CVC included triple lumen in 164 (75%), dialysis catheters in 29 (13%), single‐lumen large bore catheters in 17 (8%), and tunneled catheters in 4 patients (2%). The average duration (standard deviation [SD]) of CVC was 2.7 2.2 days for FVC, 5.7 9.6 days for internal jugular and 3.6 3.1 days for subclavian vein catheters.
During these seven months, including 1200 catheter‐days, only 1 CRBI was identified in a patient who only had a PICC, yielding an infection rate of 0.83 CRBI per 1000 catheter‐days. No femoral, subclavian or internal jugular catheter infections were detected. Hospital epidemiologic data confirmed this finding, and demonstrated only 1 other CRBI during 3721 line‐days, in the 7 months of this study and 12 months before, yielding an average of 0.40 CRBI/1000 catheter‐days.
Of 107 FVC, 101 were placed during initial resuscitations and 6 as second‐access sites, (2 for dialysis, 4 triple lumen catheters). Thromboprophylaxis was administered to 104 (97% of) patients with FVC. Thromboprophylaxis was pharmacological (heparins) in 63 (59% of) patients and mechanical (pneumatic compression) in 46 (43%). Five patients had both mechanical and pharmacological prophylaxis. Catheters were placed by a critical care or emergency department attending in 11%, critical care fellows in 11%, and residents in 78%. Ultrasound studies of the legs were performed in 57 patients; 56 had studies within 24 hours of removing FVC. Of these 56 patients, 53 studies were interpreted as negative and 3 were considered incomplete. The 3 initially incomplete studies were repeated, and found to be negative. Six patients were discharged from the hospital before the post‐FVC‐removal ultrasound could be performed. Of the 50 patients who had both ultrasounds (initial and follow up 57 days after removal of FVC), none had a DVT on the side of the catheter or in the control leg. Of the 50 patients with no ultrasound follow‐up, no patient developed clinically detected VTE; these patients had FVC for shorter duration (2.4 2.4 vs. 3.4 1.9 days for those with 2 Duplex; P = 0.02) and their ICU length of stay was shorter (3.8 4.6 vs. 6.6 5.6 days for those with 2 Duplex; P = 0.01).
Since no VTE or CRBI were detected further analyses regarding risks for these complications was not possible.
Discussion
Contrary to regulatory guidelines suggesting a poor safety profile, we found that short‐term FVC was associated with no episode of DVT or CRBI. While the incidence of complications is lower in more experienced operators, 6 most FVC in our hospital were placed by resident‐trainees (78%) with or without supervision from an attending physician. There were no immediate or subacute (ie, thrombosis, infection) major complications. There are a number of features that favor short‐term FVC for initial resuscitation of critically ill patients. Subclavian and intrajugular CVC require prolonged Trendelenburg position, which may not be well tolerated by some patients. FVC does not require Trendelenburg position. Major bleeding1.0% to 1.5% for all the CVCis minimized because direct compression of femoral vessels is possible. Compression of subclavian hemorrhage is impossible while compression of the jugular vessels is uncomfortable. Pneumothorax, while uncommon in the subclavian and intrajugular approaches, 7 has serious consequences for an unstable patient, whereas FVC obviates the risk. Some might argue that FVC cannot accurately reflect cardiovascular filling thereby defeating 1 of the important purposes of the catheter. While this is certainly true in patients with raised intraabdominal pressures, a small case series suggests that (longer‐than‐normal) FVC can accurately measure central filling pressures. 8 Another potential shortcoming of FVC is that if used only for short durations during initial resuscitationsas in this studysome patients will require a second CVC or PICC with incumbent risks.
Our study differs from previous studies that have shown infection rates ranging from 1.5/1000 to 20/1000 catheter‐days 4, 9, 10 and thrombosis rates of 6.6% to 25%. 5, 1013 Some previous studies have suggested higher rates of infection of FVC relative to internal jugular or subclavian sites (3.7/1000 vs. 20/1000 catheter‐days) 9 while others found similar infection or colonization rates between femoral and nonfemoral sites. 4, 10 Our 0.83 CRBSI per 1000 catheter‐day rate is similar to that of Pronovost et al. 1 who avoided FVC, whereas it was the preferred site (nearly half of all CVC) in our MICU. The incidence of VTE in critically ill patients ranges from 9% to 33 %, 14, 15 and CVC are a well recognized risk factor of VTE. 5 The reported incidence of DVT in patients with CVC varies widely from 3% to 10% in subclavian catheters 9 to 6.6% to 25% in FVC. 11, 12 We attribute the remarkable difference in our results to the fact that FVC was used for brief durations (mean 2.7 days, range 116 days) for the primary purpose of resuscitating critically ill patients. Also, techniques introduced by Pronovost et al. 1 to reduce CRBI had permeated our institutional practices by the time of this study; our results match his, of very low rates of CRBI when checklists are employed. In previous studies, FVC was used for extended durations similar to other CVC sites (ranging from 4 to 9.6 days). 5, 9, 12, 13, 16 Additionally, almost all of our patients received VTE prophylaxis whereas rates were variable in previous studies.
This study has several limitations. First, catheter insertion sites were not randomly assigned. This can introduce selection bias. For example, often femoral access is used in more unstable patients 4 who are less tolerant of Trendelenberg position whereas it is often avoided in obese patients. Another important limitation is that ultrasound studies were not performed in 47% of patients who had FVC. While these missed cases were not advertent (eg, CVC on weekends when no study personnel available), we cannot exclude the possibility of bias. However, no FVC patients who did not have ultrasounds developed clinically detected VTE. It is also possible that DVT could have appeared >5 to 7 days after our follow‐up ultrasound, though later development might favor spontaneous DVT unrelated to CVC. Finally, this was a relatively small study, but it appears that the rate of DVT from FVC, if placed for short durations and accompanied by thromboprophylaxis, is very low.
In conclusion, short‐term FVC was used safelywith no major complicationsin our MICU. Our data support that short‐term FVC (with thromboprophylaxis) has a reasonable safety profile for initial resuscitation of critically ill patients. Notwithstanding the limitations of our study, we suggest that it may be premature to abandon entirely 3, 17 the use of FVC for resuscitation of critically ill patients. We propose that our data suggest the need for a larger study to examine more definitively the safety profile of short‐term FVC.
Central venous catheters (CVC) are routinely used to deliver medications and monitor intravascular pressures of critically ill patients. Experts and national regulatory bodies have questioned the safety of femoral vein catheterization (FVC), and currently recommend against venous access at this site whenever possible. 13 However, a large prospective nonrandomized study has suggested that rates of FVC infections are not higher than jugular or subclavian sites. 4 Some authors have suggested that increased risk of deep vein thrombosis (DVT) also relatively contraindicates the femoral site. 5 No study has prospectively examined rates of DVT in patients receiving FVC for short durations (<72 hours). In this brief report, we prospectively examined the rates of catheter‐related bloodstream infections (CRBI) and DVT in critically ill patients receiving CVC.
Methods
This prospective observational cohort study was conducted in the medical intensive care unit (MICU) of Bridgeport Hospital, a 350‐bed community teaching hospital. The hospital's Institutional Review Board approved the study and waived the informed consent requirement because it has been the practice for the past decade to favor use of the femoral site for initial resuscitations with very low complication rates. All patients admitted to the MICU between September 1, 2008 and March 31, 2009 were eligible. VC were defined as catheters placed in the jugular, subclavian or femoral veins or peripherally inserted and guided to a central intrathoracic vein (PICC). CVC refers to catheters placed directly into central veins. In early 2008, a hospital‐wide initiative was introduced to insert all CVC using the Pronovost check‐list. 1 VC sites were chosen at the discretion of caregivers in the emergency department and MICU. The policy of our intensive care units is to use only saline flushes of VCs.
Demographic data including age, gender, and body mass index, were collected on all patients. In addition the following parameters were monitored for the duration of ICU stay for the purpose of this study: (1) site and duration of installation of all intravascular catheters, (2) level of training of clinician inserting CVC, (3) catheter/blood culture results. For the purposes of this study, bilateral femoral Doppler compression ultrasound studies were expected to be performed by radiology house officers within 24 hours of removing and again 5 to 7 days following removal of FVC. Local VC complications, methods of thromboprophylaxis and risk factors for venous thromboembolism (VTE) were recorded. Patient outcomes and disposition destinations were also recorded.
CRBI were defined using the Centers for Disease Control definitions. 2 CRBI were identified by daily review of all positive blood cultures and review of patients' medical records. In addition, Infection Control Committee data were reviewed to corroborate contemporaneously determined CRBI during the study period and for 1 year prior to the study period. Patients with FVC were examined each day for signs or symptoms of thrombosis (tenderness along the vein, leg swelling, pitting edema or visible collateral superficial veins). Patients were followed up until death or hospital discharge for clinical signs, symptoms or diagnosis of thromboembolic disease.
Bedside Duplex ultrasounds of bilateral common femoral and superficial femoral veins were performed using graded compression and color Doppler techniques. The leg without FVC served as the control. Evaluations were conducted by senior radiology residents (>100 hours training) utilizing a high‐resolution (>7.5 MHz) linear array transducer. Frame capture images were digitally stored and subsequently reviewed by a Board‐certified radiologist, who was blinded to side of insertion and clinical outcomes, and rendered a final interpretation.
Values are listed as means standard deviations. Comparisons of group means were performed using nonpaired Student's t tests. A P value of <0.05 signified statistical significance.
Results
During the study period, 675 patients were admitted to the MICU. VCs were inserted in 238 (35% of) patients. During their MICU stay, 182 (77% of) patients had 1 VC, 48 (20%) had 2 VC, and 8 (3%) had 3 VC. On admission, 38 patients (6%) had preexisting VC (tunneled catheter 58%, PICC 32%, and dialysis catheters 10%). Additional VCs were placed in 10 of these patients (26%).
Of the 302 VC, 85 (28%) were PICCs and 217 were CVC (107, 49% FVC; 82, 38% internal jugular; 28, 13% subclavian). A total of 151 (28%) patients had radial arterial catheters placed around the time of admission. The types of CVC included triple lumen in 164 (75%), dialysis catheters in 29 (13%), single‐lumen large bore catheters in 17 (8%), and tunneled catheters in 4 patients (2%). The average duration (standard deviation [SD]) of CVC was 2.7 2.2 days for FVC, 5.7 9.6 days for internal jugular and 3.6 3.1 days for subclavian vein catheters.
During these seven months, including 1200 catheter‐days, only 1 CRBI was identified in a patient who only had a PICC, yielding an infection rate of 0.83 CRBI per 1000 catheter‐days. No femoral, subclavian or internal jugular catheter infections were detected. Hospital epidemiologic data confirmed this finding, and demonstrated only 1 other CRBI during 3721 line‐days, in the 7 months of this study and 12 months before, yielding an average of 0.40 CRBI/1000 catheter‐days.
Of 107 FVC, 101 were placed during initial resuscitations and 6 as second‐access sites, (2 for dialysis, 4 triple lumen catheters). Thromboprophylaxis was administered to 104 (97% of) patients with FVC. Thromboprophylaxis was pharmacological (heparins) in 63 (59% of) patients and mechanical (pneumatic compression) in 46 (43%). Five patients had both mechanical and pharmacological prophylaxis. Catheters were placed by a critical care or emergency department attending in 11%, critical care fellows in 11%, and residents in 78%. Ultrasound studies of the legs were performed in 57 patients; 56 had studies within 24 hours of removing FVC. Of these 56 patients, 53 studies were interpreted as negative and 3 were considered incomplete. The 3 initially incomplete studies were repeated, and found to be negative. Six patients were discharged from the hospital before the post‐FVC‐removal ultrasound could be performed. Of the 50 patients who had both ultrasounds (initial and follow up 57 days after removal of FVC), none had a DVT on the side of the catheter or in the control leg. Of the 50 patients with no ultrasound follow‐up, no patient developed clinically detected VTE; these patients had FVC for shorter duration (2.4 2.4 vs. 3.4 1.9 days for those with 2 Duplex; P = 0.02) and their ICU length of stay was shorter (3.8 4.6 vs. 6.6 5.6 days for those with 2 Duplex; P = 0.01).
Since no VTE or CRBI were detected further analyses regarding risks for these complications was not possible.
Discussion
Contrary to regulatory guidelines suggesting a poor safety profile, we found that short‐term FVC was associated with no episode of DVT or CRBI. While the incidence of complications is lower in more experienced operators, 6 most FVC in our hospital were placed by resident‐trainees (78%) with or without supervision from an attending physician. There were no immediate or subacute (ie, thrombosis, infection) major complications. There are a number of features that favor short‐term FVC for initial resuscitation of critically ill patients. Subclavian and intrajugular CVC require prolonged Trendelenburg position, which may not be well tolerated by some patients. FVC does not require Trendelenburg position. Major bleeding1.0% to 1.5% for all the CVCis minimized because direct compression of femoral vessels is possible. Compression of subclavian hemorrhage is impossible while compression of the jugular vessels is uncomfortable. Pneumothorax, while uncommon in the subclavian and intrajugular approaches, 7 has serious consequences for an unstable patient, whereas FVC obviates the risk. Some might argue that FVC cannot accurately reflect cardiovascular filling thereby defeating 1 of the important purposes of the catheter. While this is certainly true in patients with raised intraabdominal pressures, a small case series suggests that (longer‐than‐normal) FVC can accurately measure central filling pressures. 8 Another potential shortcoming of FVC is that if used only for short durations during initial resuscitationsas in this studysome patients will require a second CVC or PICC with incumbent risks.
Our study differs from previous studies that have shown infection rates ranging from 1.5/1000 to 20/1000 catheter‐days 4, 9, 10 and thrombosis rates of 6.6% to 25%. 5, 1013 Some previous studies have suggested higher rates of infection of FVC relative to internal jugular or subclavian sites (3.7/1000 vs. 20/1000 catheter‐days) 9 while others found similar infection or colonization rates between femoral and nonfemoral sites. 4, 10 Our 0.83 CRBSI per 1000 catheter‐day rate is similar to that of Pronovost et al. 1 who avoided FVC, whereas it was the preferred site (nearly half of all CVC) in our MICU. The incidence of VTE in critically ill patients ranges from 9% to 33 %, 14, 15 and CVC are a well recognized risk factor of VTE. 5 The reported incidence of DVT in patients with CVC varies widely from 3% to 10% in subclavian catheters 9 to 6.6% to 25% in FVC. 11, 12 We attribute the remarkable difference in our results to the fact that FVC was used for brief durations (mean 2.7 days, range 116 days) for the primary purpose of resuscitating critically ill patients. Also, techniques introduced by Pronovost et al. 1 to reduce CRBI had permeated our institutional practices by the time of this study; our results match his, of very low rates of CRBI when checklists are employed. In previous studies, FVC was used for extended durations similar to other CVC sites (ranging from 4 to 9.6 days). 5, 9, 12, 13, 16 Additionally, almost all of our patients received VTE prophylaxis whereas rates were variable in previous studies.
This study has several limitations. First, catheter insertion sites were not randomly assigned. This can introduce selection bias. For example, often femoral access is used in more unstable patients 4 who are less tolerant of Trendelenberg position whereas it is often avoided in obese patients. Another important limitation is that ultrasound studies were not performed in 47% of patients who had FVC. While these missed cases were not advertent (eg, CVC on weekends when no study personnel available), we cannot exclude the possibility of bias. However, no FVC patients who did not have ultrasounds developed clinically detected VTE. It is also possible that DVT could have appeared >5 to 7 days after our follow‐up ultrasound, though later development might favor spontaneous DVT unrelated to CVC. Finally, this was a relatively small study, but it appears that the rate of DVT from FVC, if placed for short durations and accompanied by thromboprophylaxis, is very low.
In conclusion, short‐term FVC was used safelywith no major complicationsin our MICU. Our data support that short‐term FVC (with thromboprophylaxis) has a reasonable safety profile for initial resuscitation of critically ill patients. Notwithstanding the limitations of our study, we suggest that it may be premature to abandon entirely 3, 17 the use of FVC for resuscitation of critically ill patients. We propose that our data suggest the need for a larger study to examine more definitively the safety profile of short‐term FVC.
- , , , et al. An intervention to decrease catheter related bloodstream infections in the ICU. N Engl J Med. 2006; 355: 2725– 2732.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infection. MMWR Website. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5110a1.htm. Accessed February 2010.
- Joint Commission. National Accreditation: Hospital Patient Safety Goals. Available at: http://www.jointcommission.org/NR/rdonlyres/31666E86‐E7F4‐423E‐9BE8‐F05BD1CB0AA8/0/HAP_NPSG.pdf. Accessed February 2010.
- , , , et al. The incidence of infectious complications of central venous catheters at the subclavian, internal jugular and femoral sites in an intensive care unit population. Crit Care Med. 2005; 33: 13– 20.
- , , , . Femoral deep vein thrombosis associated with central venous catheterization: Results from a prospective, randomized trial. Crit Care Med. 1995; 23: 52– 59.
- , , , , . Central vein catheterization. Failure and complicagtion rates by three percutaneous approaches. Arch Intern Med. 1986; 146: 259– 261.
- , , . Complications of central venous catheters: internal jugular versus subclavian access—a systematic review. Crit Care Med. 2002; 30: 454– 460.
- , , , , , . Comparison of intrathoracic and intra‐abdominal measurements of central venous pressure. Lancet. 1996; 347: 1155– 1157.
- , , , et al. Complications of femoral and subclavian venous catheterization in critically ill patients. A randomized controlled trial. JAMA. 2001; 286: 700– 707.
- , , , et al. Femoral vs jugular venous catheterization and risk of nosocomial events in adults requiring acute renal replacement therapy. A randomized trial. JAMA. 2008; 299: 2413– 2422.
- , , , , , . A prospective evaluation of the use of femoral venous catheters in critically ill adults. Crit Care Med. 1997; 25: 1986– 1989.
- , , , , . Lower extremity deep vein thrombosis: a prospective, randomized, controlled trial in comatose or sedated patients undergoing femoral vein catheterization. Crit Care Med. 1997; 25: 1982– 1985.
- , , , , . Deep venous thrombosis caused by femoral venous catheters in critically ill adult patients. Chest. 2000; 117: 178– 183.
- , , . The incidence of deep venous thrombosis in ICU patients. Chest. 1997; 111: 661– 664.
- , , , et al. Deep venous thrombosis in medical‐surgical critically il patients: prevalence, incidence and risk factors. Crit Care Med. 2005; 33: 1565– 1571.
- , , , et al. Central vein catheter related thrombosis in intensive care patients: incidence, risk factors and relationship with catheter related sepsis. Chest. 1998; 114: 207– 213.
- Institute for Healthcare Improvement. Optimal catheter site selection, with avoidance of the femoral vein for central venous access in adults. Available at: http://www.ihi.org/IHI/Topics/CriticalCare/IntensiveCare/Changes/IndividualChanges/OptimalCatheterSiteSelectionwithAvoidanceofFemoralVeinforCentralVenousAccessinAdultPatients.htm. Accessed February 2010.
- , , , et al. An intervention to decrease catheter related bloodstream infections in the ICU. N Engl J Med. 2006; 355: 2725– 2732.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infection. MMWR Website. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5110a1.htm. Accessed February 2010.
- Joint Commission. National Accreditation: Hospital Patient Safety Goals. Available at: http://www.jointcommission.org/NR/rdonlyres/31666E86‐E7F4‐423E‐9BE8‐F05BD1CB0AA8/0/HAP_NPSG.pdf. Accessed February 2010.
- , , , et al. The incidence of infectious complications of central venous catheters at the subclavian, internal jugular and femoral sites in an intensive care unit population. Crit Care Med. 2005; 33: 13– 20.
- , , , . Femoral deep vein thrombosis associated with central venous catheterization: Results from a prospective, randomized trial. Crit Care Med. 1995; 23: 52– 59.
- , , , , . Central vein catheterization. Failure and complicagtion rates by three percutaneous approaches. Arch Intern Med. 1986; 146: 259– 261.
- , , . Complications of central venous catheters: internal jugular versus subclavian access—a systematic review. Crit Care Med. 2002; 30: 454– 460.
- , , , , , . Comparison of intrathoracic and intra‐abdominal measurements of central venous pressure. Lancet. 1996; 347: 1155– 1157.
- , , , et al. Complications of femoral and subclavian venous catheterization in critically ill patients. A randomized controlled trial. JAMA. 2001; 286: 700– 707.
- , , , et al. Femoral vs jugular venous catheterization and risk of nosocomial events in adults requiring acute renal replacement therapy. A randomized trial. JAMA. 2008; 299: 2413– 2422.
- , , , , , . A prospective evaluation of the use of femoral venous catheters in critically ill adults. Crit Care Med. 1997; 25: 1986– 1989.
- , , , , . Lower extremity deep vein thrombosis: a prospective, randomized, controlled trial in comatose or sedated patients undergoing femoral vein catheterization. Crit Care Med. 1997; 25: 1982– 1985.
- , , , , . Deep venous thrombosis caused by femoral venous catheters in critically ill adult patients. Chest. 2000; 117: 178– 183.
- , , . The incidence of deep venous thrombosis in ICU patients. Chest. 1997; 111: 661– 664.
- , , , et al. Deep venous thrombosis in medical‐surgical critically il patients: prevalence, incidence and risk factors. Crit Care Med. 2005; 33: 1565– 1571.
- , , , et al. Central vein catheter related thrombosis in intensive care patients: incidence, risk factors and relationship with catheter related sepsis. Chest. 1998; 114: 207– 213.
- Institute for Healthcare Improvement. Optimal catheter site selection, with avoidance of the femoral vein for central venous access in adults. Available at: http://www.ihi.org/IHI/Topics/CriticalCare/IntensiveCare/Changes/IndividualChanges/OptimalCatheterSiteSelectionwithAvoidanceofFemoralVeinforCentralVenousAccessinAdultPatients.htm. Accessed February 2010.
Trends in Thrombolytic Use for Stroke
Recombinant tissue plasminogen activator (tPA), approved for use in the United States for the treatment for acute ischemic stroke since 1996, improves overall outcomes from ischemic stroke when administered to selected patients.14 Several prominent guidelines, including the Brain Attack Coalition and the American Stroke Association, have recommended increasing the use of tPA for acute ischemic stroke.57 In addition, in 2003 the Joint Commission on Accreditation of Healthcare Organizations developed a disease‐specific certification program to designate certain institutions Primary Stroke Centers, with one of the performance measures being the availability of thrombolysis.8
Despite guidelines and regulatory agencies promoting the use of thrombolysis for ischemic stroke, previous studies have shown disappointingly low rates of use.912 The goals of this study were to assess whether national trends in the use of thrombolysis for acute ischemic stroke have increased in light of increased regulatory activity as well as to identify patient characteristics associated with thrombolytic administration.
Materials and Methods
Data for this study were obtained from the 2001 through 2006 National Hospital Discharge Survey (NHDS), a nationally representative sample of inpatient hospitalizations conducted annually by the National Center for Health Statistics.13 The NHDS collects data on approximately 300,000 patients from about 500 short‐stay nonfederal hospitals in the United States and uses a 3‐stage sampling strategy that allows for extrapolation to national level estimates. Response rates typically exceed 90% from participating hospitals. The survey collects demographic data, including age, sex, race, hospital geographic region, hospital bedsize and patient insurance status. In addition, up to 7 diagnostic and 4 procedural codes from the hospitalization are available, as is hospitalization length of stay and patient discharge disposition. No information on timing of symptoms, degree of neurologic compromise, or results of imaging tests were available in the NHDS.
We searched for all patients age 18 years or older with a primary diagnostic code of ischemic stroke using the International Classification of Diseases, 9th Edition, Clinical Modification (ICD‐9‐CM) codes 433, 434, 436, 437.0, and 437.1, excluding codes with a fifth digit of 0 (which indicated arterial occlusion without mention of cerebral infarction). We then searched for the presence of an ICD‐9‐CM procedure code for injection or infusion of thrombolytic agent (code 99.10). Specific comorbid conditions associated with ischemic stroke were identified by searching for specific ICD‐9‐CM codes, including for heart failure, coronary artery disease, hypertension, diabetes mellitus, and atrial fibrillation. To provide a general assessment of the severity of illness of the patients, we calculated an adapted Charlson comorbidity score for each patient using available secondary discharge diagnosis codes.14 We also searched for codes corresponding to intracranial hemorrhage, a complication associated with tPA administration.
Statistical Analysis
We defined thrombolytic utilization rates as the number of patients hospitalized with a primary diagnosis of ischemic stroke who had a procedure code for thrombolysis divided by the total number of patients hospitalized with ischemic stroke. To calculate nationally representative prevalence rates, we used the sample weights provided by the NHDS to account for the complex sampling design of the survey. Differences in thrombolytic administration rates by patient and hospital characteristics were tested using chi‐squared tests for categorical variables and t‐tests for continuous variables. Variables underwent a backwards selection process with a significance level of 0.05 to develop the final multivariable model of predictors of thrombolytic administration. Length of stay and hospital discharge status were not included in the variable selection process, as the focus was on predictors of initial administration of thrombolytics. All analyses were conducted using SAS Version 9.1 (SAS Institute Inc., Cary, NC).
Results
From years 2001 through 2006, we identified 22,842 patients with a primary diagnosis of ischemic stroke. Of these, 313 (1.37%, 95% confidence interval [CI], 1.22‐1.53%) had a procedure code for injection or infusion of thrombolysis. Using NHDS sample weights, these numbers corresponded to an estimated 2.55 million hospitalizations for ischemic stroke in the United States during the time period and to 35,082 patients receiving intravenous thrombolytics. Although the overall rate of thrombolysis administration was quite low overall, the administration rate increased over time, from 0.87% [95% CI, 0.61‐1.22%] of stroke patients in year 2001 to 2.40% [95% CI, 1.95‐2.93%] in year 2006 and with a particular increase especially noted after year 2003 (P <0.001 for trend, Figure 1).
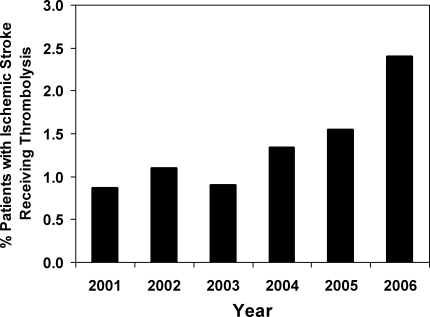
On bivariate analysis, a lower proportion of African‐American patients received tPA compared to white patients (0.8% vs. 1.5%, P = 0.003), while a higher proportion of patients with atrial fibrillation received tPA (2.3% vs. 1.2%, P < 0.001). Older patients were less likely than younger patients to receive tPA (Table 1). The rate of intracranial hemorrhage was significantly higher in patients who received tPA (5.4% vs. 0.6%, P < 0.001) and the overall inpatient mortality in patients who received tPA was 9.0%. Mortality in patients receiving tPA continued to be higher than in patients who did not receive tPA even when patients with intracranial hemorrhage were excluded (8.1% vs. 5.3%, P < 0.001). Larger hospitals were more likely to administer tPA to patients with ischemic stroke, with a 1.79% administration rate in hospitals with 300 beds compared to 0.90% in hospitals with 100 to 199 beds and 0.52% in hospitals with 6 to 99 beds (P < 0.001).
| Thrombolysis, n (%) | No Thrombolysis, n (%) | P Value | |
|---|---|---|---|
| |||
| Age | 0.001 | ||
| <60 | 76 (24.3) | 4478 (19.9) | |
| 60‐69 | 73 (23.3) | 3942 (17.5) | |
| 70‐79 | 82 (26.2) | 6265 (27.8) | |
| 80+ | 82 (26.2) | 7844 (34.8) | |
| Female | 155 (49.5) | 12625 (56.0) | 0.02 |
| Race | 0.003 | ||
| White | 173 (55.3) | 11542 (51.2) | |
| African American | 29 (9.3) | 3774 (16.8) | |
| Other | 16 (5.1) | 814 (3.6) | |
| Not stated | 95 (30.4) | 6399 (28.4) | |
| Region | 0.05 | ||
| Northeast | 78 (24.9) | 4570 (20.3) | |
| Midwest | 84 (26.8) | 6924 (30.7) | |
| South | 104 (33.2) | 8284 (36.8) | |
| West | 47 (15.0) | 2751 (12.2) | |
| Type of admission | <0.001 | ||
| Emergent | 247 (78.9) | 14233 (63.2) | |
| Urgent | 33 (10.5) | 3703 (16.4) | |
| Elective | 5 (1.6) | 1346 (6.0) | |
| Unknown | 28 (9.0) | 3247 (14.4) | |
| Length of stay, days [95% CI] | 7.2 [6.6‐7.8] | 6.0 [5.9‐6.1] | <0.001 |
| Hospital bedsize | <0.001 | ||
| 6‐99 | 16 (5.1) | 3065 (13.6) | |
| 100‐199 | 48 (15.3) | 5289 (23.5) | |
| 200‐299 | 86 (27.5) | 5212 (23.1) | |
| 300+ | 163 (52.1) | 8963 (39.8) | |
| Payment type | <0.001 | ||
| Medicare | 176 (56.2) | 15197 (67.5) | |
| Medicaid | 21 (6.7) | 1245 (5.5) | |
| Private | 45 (14.4) | 2483 (11.0) | |
| HMO/PPO | 39 (12.5) | 2224 (9.9) | |
| Other/unknown | 32 (10.2) | 1380 (6.1) | |
| Discharge status | <0.001 | ||
| Home | 98 (31.3) | 9507 (42.2) | |
| Short term care facility | 25 (8.0) | 1271 (5.6) | |
| Long term care facility | 62 (19.8) | 5400 (24.0) | |
| Alive, status unknown | 89 (28.4) | 4514 (20.0) | |
| Death | 28 (9.0) | 1218 (5.4) | |
| Unknown | 11 (3.5) | 619 (2.8) | |
| Comorbid conditions | |||
| Congestive heart failure | 48 (15.3) | 2769 (12.3) | 0.10 |
| Coronary artery disease | 49 (15.7) | 4082 (18.1) | 0.26 |
| Hypertension | 164 (52.4) | 12480 (55.4) | 0.29 |
| Diabetes mellitus | 42 (13.4) | 4965 (22.0) | <0.001 |
| Atrial fibrillation | 96 (30.7) | 4096 (18.2) | <0.001 |
| Intracranial hemorrhage | 17 (5.4) | 139 (0.6) | <0.001 |
| Charlson score14 (mean) | 2.48 [2.32‐2.64] | 2.38 [2.36‐2.40] | 0.23 |
After adjusting for patient and hospital characteristics, the absolute rate of thrombolysis administration increased by an average of 0.19% per year (95% CI, 0.12‐0.26%). Factors that were significantly associated with administration of thrombolytics included being hospitalized in a larger hospital, having a history of atrial fibrillation, and a higher Charlson comorbidity index (Table 2). Patients aged 80 years or older, African American patients, and those with diabetes mellitus were significantly less likely to receive thrombolysis.
| Characteristic | Adjusted OR (95% CI) |
|---|---|
| |
| Year (per year, from 2001 to 2006) | 1.2 (1.1‐1.3) |
| Age, years | |
| <60 | Referent |
| 60‐69 | 1.0 (0.7‐1.4) |
| 70‐79 | 0.6 (0.5‐0.9) |
| 80+ | 0.4 (0.3‐0.6) |
| Race | |
| Not African American | Referent |
| African‐American | 0.4 (0.3‐0.7) |
| Unknown | 1.0 (0.8‐1.2) |
| Hospital bedsize | |
| 6‐99 | Referent |
| 100‐199 | 1.7 (1.0‐3.1) |
| 200‐299 | 3.2 (1.8‐5.4) |
| 300+ | 3.3 (2.0‐5.6) |
| Diabetes mellitus | 0.5 (0.3‐0.6) |
| Atrial fibrillation | 2.2 (1.7‐2.9) |
| Charlson comorbidity score14 (per point increase) | 1.1 (1.1‐1.2) |
Discussion
Despite strong recommendations from guidelines and regulatory agencies, national rates of intravenous thrombolysis for ischemic stroke continue to be quite low overall. However, tPA administration appears to have increased from previous years and particularly increased in years after the Joint Commission began to accredit institutions as Primary Stroke Centers.11 The oldest patients and African Americans were less likely to receive thrombolytics, while patients with atrial fibrillation were more likely to receive thrombolysis, potentially related to atrial fibrillation causing more severe strokes.15 A total of 5.4% of patients who received tPA were diagnosed with intracranial hemorrhage, and the inpatient mortality rate of patients with tPA was 9.0%.
The exact optimal rate of thrombolysis administration for the patients in our study is unknown, as the NHDS database lacked detailed information on factors that would preclude tPA administration such as late timing of presentation and mild stroke symptoms.3 Studies conducted in stroke registries and regional settings have found that only approximately 15% to 32% of patients presenting with ischemic stroke arrive within 3 hours of symptom onset, and of these, only about 40% to 50% are eligible for tPA clinically.9, 10, 1619 However, even among presumed eligible patients, tPA administration rates only range between 25% and 43%,17, 19, 20 and the ideal rate is likely to be higher than the very low rates we observed in our study. Newer evidence that extending the time window where tPA may be given safely may increase the number of eligible patients.21
Patients who received thrombolysis had higher mortality rates than patients who did not. Although we were unable to determine a causal association, prior observational studies of tPA administration for acute stroke have found that patients with more severe neurologic deficits were more likely to receive thrombolysis.17, 18 The 9.0% inpatient case‐fatality rate observed in our study compares favorably to the 13.4% mortality rate after tPA reported in a post‐approval meta‐analysis of safety outcomes22 and the rate of intracranial hemorrhage in our analysis was similar to those observed in other settings.9, 2225 We were unable to determine whether intracranial hemorrhages in our study were as a result of tPA administration or whether patients who received tPA were more likely to have intracranial hemorrhages detected, such as may be due to increased frequency of head imaging.
Larger hospitals were more likely to administer tPA. This may reflect regionalization of stroke care, particularly in those designated as stroke centers of excellence. As well, there is some evidence that there is a learning curve with thrombolysis administration, where guideline‐recommended practice and use of tPA increases with additional experience with the drug.9, 26 Promoting systems that allow for rapid triage and diagnosis of acute stroke should be encouraged and hospital leaders should develop strategies that allow for early recognition of potential tPA candidates.
There are several limitations to our analysis. The NHDS does not collect detailed data on clinical or presenting features of stroke, and so we lacked information on stroke severity and eligibility for administration of thrombolysis. Our study may have underestimated the overall rates of thrombolysis, as it was dependent on diagnostic codes. A previous study of 34 patients who received tPA found that although the 99.10 code was 100% specific, the code identified only 17 patients who actually received tPA (sensitivity of 50%).20 Another study comparing Medicare administrative claims data to actual pharmacy billing charges for tPA found that administrative data underestimated the rate of tPA administration by approximately 25% to 30%.12 If a diagnostic code sensitivity of 50% was assumed, rates of tPA administration in our study may have been as high as 4.8% (95% CI, 4.1‐5.5%) by year 2006.
Conclusion
In conclusion, the use of intravenous thrombolysis in patients admitted with acute ischemic stroke in the United States has risen over time, but overall use remains very low. Further efforts to improve appropriate administration rates should be encouraged, particularly as the acceptable time‐window for using tPA widens.
Acknowledgements
The authors thank Mr. Loren Yglecias for his assistance with manuscript text and references.
- The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group.Tissue plasminogen activator for acute ischemic stroke.N Engl J Med.1995;333:1581–1587.
- ,,, et al.Effects of tissue plasminogen activator for acute ischemic stroke at one year. National Institute of Neurological Disorders and Stroke Recombinant Tissue Plasminogen Activator Stroke Study Group.N Engl J Med.1999;340:1781–1787.
- ,,, et al.Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS, and NINDS rT‐PA stroke trials.Lancet.2004;363:768–774.
- ,,, et al.Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke‐monitoring study (SITS‐MOST): An observational study.Lancet.2007;369:275–282.
- ,,, et al.Recommendations for the establishment of primary stroke centers.JAMA.2000;283:3102–3109.
- ,.Management of acute ischaemic stroke: new guidelines from the American Stroke Association and European Stroke Initiative.Lancet Neurol.2003;2:698–701.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists.Circulation.2007;115:e478–534.
- The Joint Commission Primary Stroke Center Certification. Available at: http://www.jointcommission.org/CertificationPrograms/PrimaryStrokeCenters. Accessed February 2010.
- ,,, et al.Use of tissue‐type plasminogen activator for acute ischemic stroke: The Cleveland area experience.JAMA.2000;283:1151–1158.
- California Acute Stroke Pilot Registry (CASPR) Investigators.Prioritizing interventions to improve rates of thrombolysis for ischemic stroke.Neurology.2005;64:654–659.
- ,,, et al.Thrombolysis for ischemic stroke in the united states: Data from National Hospital Discharge Survey 1999–2001.Neurosurgery.2005;57:647–654; discussion647–654.
- ,,,,.National US estimates of recombinant tissue plasminogen activator use: ICD‐9 codes substantially underestimate.Stroke.2008;39:924–928.
- US Department of Health and Human Services Public Health Service and National Center for Health Statistics.National Hospital Discharge Durvey 1991–2006. Multi‐year public‐use data file documentation.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- ,,, et al.Stroke severity in atrial fibrillation. The Framingham study.Stroke.1996;27:1760–1764.
- ,,,,.Why are stroke patients excluded from tPA therapy? An analysis of patient eligibility.Neurology.2001;57:1739–1740.
- ,,,,,.Utilization of intravenous tissue plasminogen activator for acute ischemic stroke.Arch Neurol.2004;61:346–350.
- ,,, et al.Eligibility for recombinant tissue plasminogen activator in acute ischemic stroke: a population‐based study.Stroke.2004;35:27–29.
- ,,, et al.Tpa use for stroke in the registry of the Canadian stroke network.Can J Neurol Sci.2005;32:433–439.
- ,,, et al.Utilization of intravenous tissue‐type plasminogen activator for ischemic stroke at academic medical centers: the influence of ethnicity.Stroke.2001;32:1061–1068.
- ,,, et al.Number needed to treat to benefit and to harm for intravenous tissue plasminogen activator therapy in the 3‐ to 4.5‐hour window: Joint outcome table analysis of the ECASS 3 trial.Stroke.2009;40:2433–2437.
- .Tissue plasminogen activator for acute ischemic stroke in clinical practice: a meta‐analysis of safety data.Stroke.2003;34:2847–2850.
- ,,, et al.Early intravenous thrombolysis for acute ischemic stroke in a community‐based approach.Stroke.1998;29:1544–1549.
- ,,, et al.Factors associated with in‐hospital mortality after administration of thrombolysis in acute ischemic stroke patients: an analysis of the Nationwide Inpatient Sample 1999 to 2002.Stroke.2006;37:440–446.
- ,,, et al.Intravenous t‐PA for acute ischemic stroke: therapeutic yield of a stroke code system.Neurology.1998;50:501–503.
- ,,, et al.Intravenous tissue‐type plasminogen activator therapy for ischemic stroke: Houston experience 1996 to 2000.Arch Neurol.2001;58:2009–2013.
Recombinant tissue plasminogen activator (tPA), approved for use in the United States for the treatment for acute ischemic stroke since 1996, improves overall outcomes from ischemic stroke when administered to selected patients.14 Several prominent guidelines, including the Brain Attack Coalition and the American Stroke Association, have recommended increasing the use of tPA for acute ischemic stroke.57 In addition, in 2003 the Joint Commission on Accreditation of Healthcare Organizations developed a disease‐specific certification program to designate certain institutions Primary Stroke Centers, with one of the performance measures being the availability of thrombolysis.8
Despite guidelines and regulatory agencies promoting the use of thrombolysis for ischemic stroke, previous studies have shown disappointingly low rates of use.912 The goals of this study were to assess whether national trends in the use of thrombolysis for acute ischemic stroke have increased in light of increased regulatory activity as well as to identify patient characteristics associated with thrombolytic administration.
Materials and Methods
Data for this study were obtained from the 2001 through 2006 National Hospital Discharge Survey (NHDS), a nationally representative sample of inpatient hospitalizations conducted annually by the National Center for Health Statistics.13 The NHDS collects data on approximately 300,000 patients from about 500 short‐stay nonfederal hospitals in the United States and uses a 3‐stage sampling strategy that allows for extrapolation to national level estimates. Response rates typically exceed 90% from participating hospitals. The survey collects demographic data, including age, sex, race, hospital geographic region, hospital bedsize and patient insurance status. In addition, up to 7 diagnostic and 4 procedural codes from the hospitalization are available, as is hospitalization length of stay and patient discharge disposition. No information on timing of symptoms, degree of neurologic compromise, or results of imaging tests were available in the NHDS.
We searched for all patients age 18 years or older with a primary diagnostic code of ischemic stroke using the International Classification of Diseases, 9th Edition, Clinical Modification (ICD‐9‐CM) codes 433, 434, 436, 437.0, and 437.1, excluding codes with a fifth digit of 0 (which indicated arterial occlusion without mention of cerebral infarction). We then searched for the presence of an ICD‐9‐CM procedure code for injection or infusion of thrombolytic agent (code 99.10). Specific comorbid conditions associated with ischemic stroke were identified by searching for specific ICD‐9‐CM codes, including for heart failure, coronary artery disease, hypertension, diabetes mellitus, and atrial fibrillation. To provide a general assessment of the severity of illness of the patients, we calculated an adapted Charlson comorbidity score for each patient using available secondary discharge diagnosis codes.14 We also searched for codes corresponding to intracranial hemorrhage, a complication associated with tPA administration.
Statistical Analysis
We defined thrombolytic utilization rates as the number of patients hospitalized with a primary diagnosis of ischemic stroke who had a procedure code for thrombolysis divided by the total number of patients hospitalized with ischemic stroke. To calculate nationally representative prevalence rates, we used the sample weights provided by the NHDS to account for the complex sampling design of the survey. Differences in thrombolytic administration rates by patient and hospital characteristics were tested using chi‐squared tests for categorical variables and t‐tests for continuous variables. Variables underwent a backwards selection process with a significance level of 0.05 to develop the final multivariable model of predictors of thrombolytic administration. Length of stay and hospital discharge status were not included in the variable selection process, as the focus was on predictors of initial administration of thrombolytics. All analyses were conducted using SAS Version 9.1 (SAS Institute Inc., Cary, NC).
Results
From years 2001 through 2006, we identified 22,842 patients with a primary diagnosis of ischemic stroke. Of these, 313 (1.37%, 95% confidence interval [CI], 1.22‐1.53%) had a procedure code for injection or infusion of thrombolysis. Using NHDS sample weights, these numbers corresponded to an estimated 2.55 million hospitalizations for ischemic stroke in the United States during the time period and to 35,082 patients receiving intravenous thrombolytics. Although the overall rate of thrombolysis administration was quite low overall, the administration rate increased over time, from 0.87% [95% CI, 0.61‐1.22%] of stroke patients in year 2001 to 2.40% [95% CI, 1.95‐2.93%] in year 2006 and with a particular increase especially noted after year 2003 (P <0.001 for trend, Figure 1).

On bivariate analysis, a lower proportion of African‐American patients received tPA compared to white patients (0.8% vs. 1.5%, P = 0.003), while a higher proportion of patients with atrial fibrillation received tPA (2.3% vs. 1.2%, P < 0.001). Older patients were less likely than younger patients to receive tPA (Table 1). The rate of intracranial hemorrhage was significantly higher in patients who received tPA (5.4% vs. 0.6%, P < 0.001) and the overall inpatient mortality in patients who received tPA was 9.0%. Mortality in patients receiving tPA continued to be higher than in patients who did not receive tPA even when patients with intracranial hemorrhage were excluded (8.1% vs. 5.3%, P < 0.001). Larger hospitals were more likely to administer tPA to patients with ischemic stroke, with a 1.79% administration rate in hospitals with 300 beds compared to 0.90% in hospitals with 100 to 199 beds and 0.52% in hospitals with 6 to 99 beds (P < 0.001).
| Thrombolysis, n (%) | No Thrombolysis, n (%) | P Value | |
|---|---|---|---|
| |||
| Age | 0.001 | ||
| <60 | 76 (24.3) | 4478 (19.9) | |
| 60‐69 | 73 (23.3) | 3942 (17.5) | |
| 70‐79 | 82 (26.2) | 6265 (27.8) | |
| 80+ | 82 (26.2) | 7844 (34.8) | |
| Female | 155 (49.5) | 12625 (56.0) | 0.02 |
| Race | 0.003 | ||
| White | 173 (55.3) | 11542 (51.2) | |
| African American | 29 (9.3) | 3774 (16.8) | |
| Other | 16 (5.1) | 814 (3.6) | |
| Not stated | 95 (30.4) | 6399 (28.4) | |
| Region | 0.05 | ||
| Northeast | 78 (24.9) | 4570 (20.3) | |
| Midwest | 84 (26.8) | 6924 (30.7) | |
| South | 104 (33.2) | 8284 (36.8) | |
| West | 47 (15.0) | 2751 (12.2) | |
| Type of admission | <0.001 | ||
| Emergent | 247 (78.9) | 14233 (63.2) | |
| Urgent | 33 (10.5) | 3703 (16.4) | |
| Elective | 5 (1.6) | 1346 (6.0) | |
| Unknown | 28 (9.0) | 3247 (14.4) | |
| Length of stay, days [95% CI] | 7.2 [6.6‐7.8] | 6.0 [5.9‐6.1] | <0.001 |
| Hospital bedsize | <0.001 | ||
| 6‐99 | 16 (5.1) | 3065 (13.6) | |
| 100‐199 | 48 (15.3) | 5289 (23.5) | |
| 200‐299 | 86 (27.5) | 5212 (23.1) | |
| 300+ | 163 (52.1) | 8963 (39.8) | |
| Payment type | <0.001 | ||
| Medicare | 176 (56.2) | 15197 (67.5) | |
| Medicaid | 21 (6.7) | 1245 (5.5) | |
| Private | 45 (14.4) | 2483 (11.0) | |
| HMO/PPO | 39 (12.5) | 2224 (9.9) | |
| Other/unknown | 32 (10.2) | 1380 (6.1) | |
| Discharge status | <0.001 | ||
| Home | 98 (31.3) | 9507 (42.2) | |
| Short term care facility | 25 (8.0) | 1271 (5.6) | |
| Long term care facility | 62 (19.8) | 5400 (24.0) | |
| Alive, status unknown | 89 (28.4) | 4514 (20.0) | |
| Death | 28 (9.0) | 1218 (5.4) | |
| Unknown | 11 (3.5) | 619 (2.8) | |
| Comorbid conditions | |||
| Congestive heart failure | 48 (15.3) | 2769 (12.3) | 0.10 |
| Coronary artery disease | 49 (15.7) | 4082 (18.1) | 0.26 |
| Hypertension | 164 (52.4) | 12480 (55.4) | 0.29 |
| Diabetes mellitus | 42 (13.4) | 4965 (22.0) | <0.001 |
| Atrial fibrillation | 96 (30.7) | 4096 (18.2) | <0.001 |
| Intracranial hemorrhage | 17 (5.4) | 139 (0.6) | <0.001 |
| Charlson score14 (mean) | 2.48 [2.32‐2.64] | 2.38 [2.36‐2.40] | 0.23 |
After adjusting for patient and hospital characteristics, the absolute rate of thrombolysis administration increased by an average of 0.19% per year (95% CI, 0.12‐0.26%). Factors that were significantly associated with administration of thrombolytics included being hospitalized in a larger hospital, having a history of atrial fibrillation, and a higher Charlson comorbidity index (Table 2). Patients aged 80 years or older, African American patients, and those with diabetes mellitus were significantly less likely to receive thrombolysis.
| Characteristic | Adjusted OR (95% CI) |
|---|---|
| |
| Year (per year, from 2001 to 2006) | 1.2 (1.1‐1.3) |
| Age, years | |
| <60 | Referent |
| 60‐69 | 1.0 (0.7‐1.4) |
| 70‐79 | 0.6 (0.5‐0.9) |
| 80+ | 0.4 (0.3‐0.6) |
| Race | |
| Not African American | Referent |
| African‐American | 0.4 (0.3‐0.7) |
| Unknown | 1.0 (0.8‐1.2) |
| Hospital bedsize | |
| 6‐99 | Referent |
| 100‐199 | 1.7 (1.0‐3.1) |
| 200‐299 | 3.2 (1.8‐5.4) |
| 300+ | 3.3 (2.0‐5.6) |
| Diabetes mellitus | 0.5 (0.3‐0.6) |
| Atrial fibrillation | 2.2 (1.7‐2.9) |
| Charlson comorbidity score14 (per point increase) | 1.1 (1.1‐1.2) |
Discussion
Despite strong recommendations from guidelines and regulatory agencies, national rates of intravenous thrombolysis for ischemic stroke continue to be quite low overall. However, tPA administration appears to have increased from previous years and particularly increased in years after the Joint Commission began to accredit institutions as Primary Stroke Centers.11 The oldest patients and African Americans were less likely to receive thrombolytics, while patients with atrial fibrillation were more likely to receive thrombolysis, potentially related to atrial fibrillation causing more severe strokes.15 A total of 5.4% of patients who received tPA were diagnosed with intracranial hemorrhage, and the inpatient mortality rate of patients with tPA was 9.0%.
The exact optimal rate of thrombolysis administration for the patients in our study is unknown, as the NHDS database lacked detailed information on factors that would preclude tPA administration such as late timing of presentation and mild stroke symptoms.3 Studies conducted in stroke registries and regional settings have found that only approximately 15% to 32% of patients presenting with ischemic stroke arrive within 3 hours of symptom onset, and of these, only about 40% to 50% are eligible for tPA clinically.9, 10, 1619 However, even among presumed eligible patients, tPA administration rates only range between 25% and 43%,17, 19, 20 and the ideal rate is likely to be higher than the very low rates we observed in our study. Newer evidence that extending the time window where tPA may be given safely may increase the number of eligible patients.21
Patients who received thrombolysis had higher mortality rates than patients who did not. Although we were unable to determine a causal association, prior observational studies of tPA administration for acute stroke have found that patients with more severe neurologic deficits were more likely to receive thrombolysis.17, 18 The 9.0% inpatient case‐fatality rate observed in our study compares favorably to the 13.4% mortality rate after tPA reported in a post‐approval meta‐analysis of safety outcomes22 and the rate of intracranial hemorrhage in our analysis was similar to those observed in other settings.9, 2225 We were unable to determine whether intracranial hemorrhages in our study were as a result of tPA administration or whether patients who received tPA were more likely to have intracranial hemorrhages detected, such as may be due to increased frequency of head imaging.
Larger hospitals were more likely to administer tPA. This may reflect regionalization of stroke care, particularly in those designated as stroke centers of excellence. As well, there is some evidence that there is a learning curve with thrombolysis administration, where guideline‐recommended practice and use of tPA increases with additional experience with the drug.9, 26 Promoting systems that allow for rapid triage and diagnosis of acute stroke should be encouraged and hospital leaders should develop strategies that allow for early recognition of potential tPA candidates.
There are several limitations to our analysis. The NHDS does not collect detailed data on clinical or presenting features of stroke, and so we lacked information on stroke severity and eligibility for administration of thrombolysis. Our study may have underestimated the overall rates of thrombolysis, as it was dependent on diagnostic codes. A previous study of 34 patients who received tPA found that although the 99.10 code was 100% specific, the code identified only 17 patients who actually received tPA (sensitivity of 50%).20 Another study comparing Medicare administrative claims data to actual pharmacy billing charges for tPA found that administrative data underestimated the rate of tPA administration by approximately 25% to 30%.12 If a diagnostic code sensitivity of 50% was assumed, rates of tPA administration in our study may have been as high as 4.8% (95% CI, 4.1‐5.5%) by year 2006.
Conclusion
In conclusion, the use of intravenous thrombolysis in patients admitted with acute ischemic stroke in the United States has risen over time, but overall use remains very low. Further efforts to improve appropriate administration rates should be encouraged, particularly as the acceptable time‐window for using tPA widens.
Acknowledgements
The authors thank Mr. Loren Yglecias for his assistance with manuscript text and references.
Recombinant tissue plasminogen activator (tPA), approved for use in the United States for the treatment for acute ischemic stroke since 1996, improves overall outcomes from ischemic stroke when administered to selected patients.14 Several prominent guidelines, including the Brain Attack Coalition and the American Stroke Association, have recommended increasing the use of tPA for acute ischemic stroke.57 In addition, in 2003 the Joint Commission on Accreditation of Healthcare Organizations developed a disease‐specific certification program to designate certain institutions Primary Stroke Centers, with one of the performance measures being the availability of thrombolysis.8
Despite guidelines and regulatory agencies promoting the use of thrombolysis for ischemic stroke, previous studies have shown disappointingly low rates of use.912 The goals of this study were to assess whether national trends in the use of thrombolysis for acute ischemic stroke have increased in light of increased regulatory activity as well as to identify patient characteristics associated with thrombolytic administration.
Materials and Methods
Data for this study were obtained from the 2001 through 2006 National Hospital Discharge Survey (NHDS), a nationally representative sample of inpatient hospitalizations conducted annually by the National Center for Health Statistics.13 The NHDS collects data on approximately 300,000 patients from about 500 short‐stay nonfederal hospitals in the United States and uses a 3‐stage sampling strategy that allows for extrapolation to national level estimates. Response rates typically exceed 90% from participating hospitals. The survey collects demographic data, including age, sex, race, hospital geographic region, hospital bedsize and patient insurance status. In addition, up to 7 diagnostic and 4 procedural codes from the hospitalization are available, as is hospitalization length of stay and patient discharge disposition. No information on timing of symptoms, degree of neurologic compromise, or results of imaging tests were available in the NHDS.
We searched for all patients age 18 years or older with a primary diagnostic code of ischemic stroke using the International Classification of Diseases, 9th Edition, Clinical Modification (ICD‐9‐CM) codes 433, 434, 436, 437.0, and 437.1, excluding codes with a fifth digit of 0 (which indicated arterial occlusion without mention of cerebral infarction). We then searched for the presence of an ICD‐9‐CM procedure code for injection or infusion of thrombolytic agent (code 99.10). Specific comorbid conditions associated with ischemic stroke were identified by searching for specific ICD‐9‐CM codes, including for heart failure, coronary artery disease, hypertension, diabetes mellitus, and atrial fibrillation. To provide a general assessment of the severity of illness of the patients, we calculated an adapted Charlson comorbidity score for each patient using available secondary discharge diagnosis codes.14 We also searched for codes corresponding to intracranial hemorrhage, a complication associated with tPA administration.
Statistical Analysis
We defined thrombolytic utilization rates as the number of patients hospitalized with a primary diagnosis of ischemic stroke who had a procedure code for thrombolysis divided by the total number of patients hospitalized with ischemic stroke. To calculate nationally representative prevalence rates, we used the sample weights provided by the NHDS to account for the complex sampling design of the survey. Differences in thrombolytic administration rates by patient and hospital characteristics were tested using chi‐squared tests for categorical variables and t‐tests for continuous variables. Variables underwent a backwards selection process with a significance level of 0.05 to develop the final multivariable model of predictors of thrombolytic administration. Length of stay and hospital discharge status were not included in the variable selection process, as the focus was on predictors of initial administration of thrombolytics. All analyses were conducted using SAS Version 9.1 (SAS Institute Inc., Cary, NC).
Results
From years 2001 through 2006, we identified 22,842 patients with a primary diagnosis of ischemic stroke. Of these, 313 (1.37%, 95% confidence interval [CI], 1.22‐1.53%) had a procedure code for injection or infusion of thrombolysis. Using NHDS sample weights, these numbers corresponded to an estimated 2.55 million hospitalizations for ischemic stroke in the United States during the time period and to 35,082 patients receiving intravenous thrombolytics. Although the overall rate of thrombolysis administration was quite low overall, the administration rate increased over time, from 0.87% [95% CI, 0.61‐1.22%] of stroke patients in year 2001 to 2.40% [95% CI, 1.95‐2.93%] in year 2006 and with a particular increase especially noted after year 2003 (P <0.001 for trend, Figure 1).

On bivariate analysis, a lower proportion of African‐American patients received tPA compared to white patients (0.8% vs. 1.5%, P = 0.003), while a higher proportion of patients with atrial fibrillation received tPA (2.3% vs. 1.2%, P < 0.001). Older patients were less likely than younger patients to receive tPA (Table 1). The rate of intracranial hemorrhage was significantly higher in patients who received tPA (5.4% vs. 0.6%, P < 0.001) and the overall inpatient mortality in patients who received tPA was 9.0%. Mortality in patients receiving tPA continued to be higher than in patients who did not receive tPA even when patients with intracranial hemorrhage were excluded (8.1% vs. 5.3%, P < 0.001). Larger hospitals were more likely to administer tPA to patients with ischemic stroke, with a 1.79% administration rate in hospitals with 300 beds compared to 0.90% in hospitals with 100 to 199 beds and 0.52% in hospitals with 6 to 99 beds (P < 0.001).
| Thrombolysis, n (%) | No Thrombolysis, n (%) | P Value | |
|---|---|---|---|
| |||
| Age | 0.001 | ||
| <60 | 76 (24.3) | 4478 (19.9) | |
| 60‐69 | 73 (23.3) | 3942 (17.5) | |
| 70‐79 | 82 (26.2) | 6265 (27.8) | |
| 80+ | 82 (26.2) | 7844 (34.8) | |
| Female | 155 (49.5) | 12625 (56.0) | 0.02 |
| Race | 0.003 | ||
| White | 173 (55.3) | 11542 (51.2) | |
| African American | 29 (9.3) | 3774 (16.8) | |
| Other | 16 (5.1) | 814 (3.6) | |
| Not stated | 95 (30.4) | 6399 (28.4) | |
| Region | 0.05 | ||
| Northeast | 78 (24.9) | 4570 (20.3) | |
| Midwest | 84 (26.8) | 6924 (30.7) | |
| South | 104 (33.2) | 8284 (36.8) | |
| West | 47 (15.0) | 2751 (12.2) | |
| Type of admission | <0.001 | ||
| Emergent | 247 (78.9) | 14233 (63.2) | |
| Urgent | 33 (10.5) | 3703 (16.4) | |
| Elective | 5 (1.6) | 1346 (6.0) | |
| Unknown | 28 (9.0) | 3247 (14.4) | |
| Length of stay, days [95% CI] | 7.2 [6.6‐7.8] | 6.0 [5.9‐6.1] | <0.001 |
| Hospital bedsize | <0.001 | ||
| 6‐99 | 16 (5.1) | 3065 (13.6) | |
| 100‐199 | 48 (15.3) | 5289 (23.5) | |
| 200‐299 | 86 (27.5) | 5212 (23.1) | |
| 300+ | 163 (52.1) | 8963 (39.8) | |
| Payment type | <0.001 | ||
| Medicare | 176 (56.2) | 15197 (67.5) | |
| Medicaid | 21 (6.7) | 1245 (5.5) | |
| Private | 45 (14.4) | 2483 (11.0) | |
| HMO/PPO | 39 (12.5) | 2224 (9.9) | |
| Other/unknown | 32 (10.2) | 1380 (6.1) | |
| Discharge status | <0.001 | ||
| Home | 98 (31.3) | 9507 (42.2) | |
| Short term care facility | 25 (8.0) | 1271 (5.6) | |
| Long term care facility | 62 (19.8) | 5400 (24.0) | |
| Alive, status unknown | 89 (28.4) | 4514 (20.0) | |
| Death | 28 (9.0) | 1218 (5.4) | |
| Unknown | 11 (3.5) | 619 (2.8) | |
| Comorbid conditions | |||
| Congestive heart failure | 48 (15.3) | 2769 (12.3) | 0.10 |
| Coronary artery disease | 49 (15.7) | 4082 (18.1) | 0.26 |
| Hypertension | 164 (52.4) | 12480 (55.4) | 0.29 |
| Diabetes mellitus | 42 (13.4) | 4965 (22.0) | <0.001 |
| Atrial fibrillation | 96 (30.7) | 4096 (18.2) | <0.001 |
| Intracranial hemorrhage | 17 (5.4) | 139 (0.6) | <0.001 |
| Charlson score14 (mean) | 2.48 [2.32‐2.64] | 2.38 [2.36‐2.40] | 0.23 |
After adjusting for patient and hospital characteristics, the absolute rate of thrombolysis administration increased by an average of 0.19% per year (95% CI, 0.12‐0.26%). Factors that were significantly associated with administration of thrombolytics included being hospitalized in a larger hospital, having a history of atrial fibrillation, and a higher Charlson comorbidity index (Table 2). Patients aged 80 years or older, African American patients, and those with diabetes mellitus were significantly less likely to receive thrombolysis.
| Characteristic | Adjusted OR (95% CI) |
|---|---|
| |
| Year (per year, from 2001 to 2006) | 1.2 (1.1‐1.3) |
| Age, years | |
| <60 | Referent |
| 60‐69 | 1.0 (0.7‐1.4) |
| 70‐79 | 0.6 (0.5‐0.9) |
| 80+ | 0.4 (0.3‐0.6) |
| Race | |
| Not African American | Referent |
| African‐American | 0.4 (0.3‐0.7) |
| Unknown | 1.0 (0.8‐1.2) |
| Hospital bedsize | |
| 6‐99 | Referent |
| 100‐199 | 1.7 (1.0‐3.1) |
| 200‐299 | 3.2 (1.8‐5.4) |
| 300+ | 3.3 (2.0‐5.6) |
| Diabetes mellitus | 0.5 (0.3‐0.6) |
| Atrial fibrillation | 2.2 (1.7‐2.9) |
| Charlson comorbidity score14 (per point increase) | 1.1 (1.1‐1.2) |
Discussion
Despite strong recommendations from guidelines and regulatory agencies, national rates of intravenous thrombolysis for ischemic stroke continue to be quite low overall. However, tPA administration appears to have increased from previous years and particularly increased in years after the Joint Commission began to accredit institutions as Primary Stroke Centers.11 The oldest patients and African Americans were less likely to receive thrombolytics, while patients with atrial fibrillation were more likely to receive thrombolysis, potentially related to atrial fibrillation causing more severe strokes.15 A total of 5.4% of patients who received tPA were diagnosed with intracranial hemorrhage, and the inpatient mortality rate of patients with tPA was 9.0%.
The exact optimal rate of thrombolysis administration for the patients in our study is unknown, as the NHDS database lacked detailed information on factors that would preclude tPA administration such as late timing of presentation and mild stroke symptoms.3 Studies conducted in stroke registries and regional settings have found that only approximately 15% to 32% of patients presenting with ischemic stroke arrive within 3 hours of symptom onset, and of these, only about 40% to 50% are eligible for tPA clinically.9, 10, 1619 However, even among presumed eligible patients, tPA administration rates only range between 25% and 43%,17, 19, 20 and the ideal rate is likely to be higher than the very low rates we observed in our study. Newer evidence that extending the time window where tPA may be given safely may increase the number of eligible patients.21
Patients who received thrombolysis had higher mortality rates than patients who did not. Although we were unable to determine a causal association, prior observational studies of tPA administration for acute stroke have found that patients with more severe neurologic deficits were more likely to receive thrombolysis.17, 18 The 9.0% inpatient case‐fatality rate observed in our study compares favorably to the 13.4% mortality rate after tPA reported in a post‐approval meta‐analysis of safety outcomes22 and the rate of intracranial hemorrhage in our analysis was similar to those observed in other settings.9, 2225 We were unable to determine whether intracranial hemorrhages in our study were as a result of tPA administration or whether patients who received tPA were more likely to have intracranial hemorrhages detected, such as may be due to increased frequency of head imaging.
Larger hospitals were more likely to administer tPA. This may reflect regionalization of stroke care, particularly in those designated as stroke centers of excellence. As well, there is some evidence that there is a learning curve with thrombolysis administration, where guideline‐recommended practice and use of tPA increases with additional experience with the drug.9, 26 Promoting systems that allow for rapid triage and diagnosis of acute stroke should be encouraged and hospital leaders should develop strategies that allow for early recognition of potential tPA candidates.
There are several limitations to our analysis. The NHDS does not collect detailed data on clinical or presenting features of stroke, and so we lacked information on stroke severity and eligibility for administration of thrombolysis. Our study may have underestimated the overall rates of thrombolysis, as it was dependent on diagnostic codes. A previous study of 34 patients who received tPA found that although the 99.10 code was 100% specific, the code identified only 17 patients who actually received tPA (sensitivity of 50%).20 Another study comparing Medicare administrative claims data to actual pharmacy billing charges for tPA found that administrative data underestimated the rate of tPA administration by approximately 25% to 30%.12 If a diagnostic code sensitivity of 50% was assumed, rates of tPA administration in our study may have been as high as 4.8% (95% CI, 4.1‐5.5%) by year 2006.
Conclusion
In conclusion, the use of intravenous thrombolysis in patients admitted with acute ischemic stroke in the United States has risen over time, but overall use remains very low. Further efforts to improve appropriate administration rates should be encouraged, particularly as the acceptable time‐window for using tPA widens.
Acknowledgements
The authors thank Mr. Loren Yglecias for his assistance with manuscript text and references.
- The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group.Tissue plasminogen activator for acute ischemic stroke.N Engl J Med.1995;333:1581–1587.
- ,,, et al.Effects of tissue plasminogen activator for acute ischemic stroke at one year. National Institute of Neurological Disorders and Stroke Recombinant Tissue Plasminogen Activator Stroke Study Group.N Engl J Med.1999;340:1781–1787.
- ,,, et al.Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS, and NINDS rT‐PA stroke trials.Lancet.2004;363:768–774.
- ,,, et al.Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke‐monitoring study (SITS‐MOST): An observational study.Lancet.2007;369:275–282.
- ,,, et al.Recommendations for the establishment of primary stroke centers.JAMA.2000;283:3102–3109.
- ,.Management of acute ischaemic stroke: new guidelines from the American Stroke Association and European Stroke Initiative.Lancet Neurol.2003;2:698–701.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists.Circulation.2007;115:e478–534.
- The Joint Commission Primary Stroke Center Certification. Available at: http://www.jointcommission.org/CertificationPrograms/PrimaryStrokeCenters. Accessed February 2010.
- ,,, et al.Use of tissue‐type plasminogen activator for acute ischemic stroke: The Cleveland area experience.JAMA.2000;283:1151–1158.
- California Acute Stroke Pilot Registry (CASPR) Investigators.Prioritizing interventions to improve rates of thrombolysis for ischemic stroke.Neurology.2005;64:654–659.
- ,,, et al.Thrombolysis for ischemic stroke in the united states: Data from National Hospital Discharge Survey 1999–2001.Neurosurgery.2005;57:647–654; discussion647–654.
- ,,,,.National US estimates of recombinant tissue plasminogen activator use: ICD‐9 codes substantially underestimate.Stroke.2008;39:924–928.
- US Department of Health and Human Services Public Health Service and National Center for Health Statistics.National Hospital Discharge Durvey 1991–2006. Multi‐year public‐use data file documentation.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- ,,, et al.Stroke severity in atrial fibrillation. The Framingham study.Stroke.1996;27:1760–1764.
- ,,,,.Why are stroke patients excluded from tPA therapy? An analysis of patient eligibility.Neurology.2001;57:1739–1740.
- ,,,,,.Utilization of intravenous tissue plasminogen activator for acute ischemic stroke.Arch Neurol.2004;61:346–350.
- ,,, et al.Eligibility for recombinant tissue plasminogen activator in acute ischemic stroke: a population‐based study.Stroke.2004;35:27–29.
- ,,, et al.Tpa use for stroke in the registry of the Canadian stroke network.Can J Neurol Sci.2005;32:433–439.
- ,,, et al.Utilization of intravenous tissue‐type plasminogen activator for ischemic stroke at academic medical centers: the influence of ethnicity.Stroke.2001;32:1061–1068.
- ,,, et al.Number needed to treat to benefit and to harm for intravenous tissue plasminogen activator therapy in the 3‐ to 4.5‐hour window: Joint outcome table analysis of the ECASS 3 trial.Stroke.2009;40:2433–2437.
- .Tissue plasminogen activator for acute ischemic stroke in clinical practice: a meta‐analysis of safety data.Stroke.2003;34:2847–2850.
- ,,, et al.Early intravenous thrombolysis for acute ischemic stroke in a community‐based approach.Stroke.1998;29:1544–1549.
- ,,, et al.Factors associated with in‐hospital mortality after administration of thrombolysis in acute ischemic stroke patients: an analysis of the Nationwide Inpatient Sample 1999 to 2002.Stroke.2006;37:440–446.
- ,,, et al.Intravenous t‐PA for acute ischemic stroke: therapeutic yield of a stroke code system.Neurology.1998;50:501–503.
- ,,, et al.Intravenous tissue‐type plasminogen activator therapy for ischemic stroke: Houston experience 1996 to 2000.Arch Neurol.2001;58:2009–2013.
- The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group.Tissue plasminogen activator for acute ischemic stroke.N Engl J Med.1995;333:1581–1587.
- ,,, et al.Effects of tissue plasminogen activator for acute ischemic stroke at one year. National Institute of Neurological Disorders and Stroke Recombinant Tissue Plasminogen Activator Stroke Study Group.N Engl J Med.1999;340:1781–1787.
- ,,, et al.Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS, and NINDS rT‐PA stroke trials.Lancet.2004;363:768–774.
- ,,, et al.Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke‐monitoring study (SITS‐MOST): An observational study.Lancet.2007;369:275–282.
- ,,, et al.Recommendations for the establishment of primary stroke centers.JAMA.2000;283:3102–3109.
- ,.Management of acute ischaemic stroke: new guidelines from the American Stroke Association and European Stroke Initiative.Lancet Neurol.2003;2:698–701.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists.Circulation.2007;115:e478–534.
- The Joint Commission Primary Stroke Center Certification. Available at: http://www.jointcommission.org/CertificationPrograms/PrimaryStrokeCenters. Accessed February 2010.
- ,,, et al.Use of tissue‐type plasminogen activator for acute ischemic stroke: The Cleveland area experience.JAMA.2000;283:1151–1158.
- California Acute Stroke Pilot Registry (CASPR) Investigators.Prioritizing interventions to improve rates of thrombolysis for ischemic stroke.Neurology.2005;64:654–659.
- ,,, et al.Thrombolysis for ischemic stroke in the united states: Data from National Hospital Discharge Survey 1999–2001.Neurosurgery.2005;57:647–654; discussion647–654.
- ,,,,.National US estimates of recombinant tissue plasminogen activator use: ICD‐9 codes substantially underestimate.Stroke.2008;39:924–928.
- US Department of Health and Human Services Public Health Service and National Center for Health Statistics.National Hospital Discharge Durvey 1991–2006. Multi‐year public‐use data file documentation.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- ,,, et al.Stroke severity in atrial fibrillation. The Framingham study.Stroke.1996;27:1760–1764.
- ,,,,.Why are stroke patients excluded from tPA therapy? An analysis of patient eligibility.Neurology.2001;57:1739–1740.
- ,,,,,.Utilization of intravenous tissue plasminogen activator for acute ischemic stroke.Arch Neurol.2004;61:346–350.
- ,,, et al.Eligibility for recombinant tissue plasminogen activator in acute ischemic stroke: a population‐based study.Stroke.2004;35:27–29.
- ,,, et al.Tpa use for stroke in the registry of the Canadian stroke network.Can J Neurol Sci.2005;32:433–439.
- ,,, et al.Utilization of intravenous tissue‐type plasminogen activator for ischemic stroke at academic medical centers: the influence of ethnicity.Stroke.2001;32:1061–1068.
- ,,, et al.Number needed to treat to benefit and to harm for intravenous tissue plasminogen activator therapy in the 3‐ to 4.5‐hour window: Joint outcome table analysis of the ECASS 3 trial.Stroke.2009;40:2433–2437.
- .Tissue plasminogen activator for acute ischemic stroke in clinical practice: a meta‐analysis of safety data.Stroke.2003;34:2847–2850.
- ,,, et al.Early intravenous thrombolysis for acute ischemic stroke in a community‐based approach.Stroke.1998;29:1544–1549.
- ,,, et al.Factors associated with in‐hospital mortality after administration of thrombolysis in acute ischemic stroke patients: an analysis of the Nationwide Inpatient Sample 1999 to 2002.Stroke.2006;37:440–446.
- ,,, et al.Intravenous t‐PA for acute ischemic stroke: therapeutic yield of a stroke code system.Neurology.1998;50:501–503.
- ,,, et al.Intravenous tissue‐type plasminogen activator therapy for ischemic stroke: Houston experience 1996 to 2000.Arch Neurol.2001;58:2009–2013.
Copyright © 2010 Society of Hospital Medicine
Lack of Timely PCP Follow‐Up
Care transitions between the inpatient and outpatient settings are a known period of risk in a patient's care. For instance, 1 in 5 medical patients suffers an adverse event during the first several weeks after hospital discharge, with half of these requiring the use of additional healthcare resources.1 Additionally, medication and lab monitoring errors occur in up to half of all discharged patients.2 Nearly 1 in 5 hospitalized patients, admitted with 1 of 16 different conditions including asthma, diabetes, congestive heart failure and urinary tract infection is readmitted to the hospital within six months. Up to 60% of resources are used in rehospitalized patients.3, 4 In Medicare beneficiaries, the readmission rate is as high as 20% at 30 days. The same study suggests that up to half of Medicare patients readmitted within 30 days are not seen in the outpatient setting following discharge.5 Such statistics underscore the need for seamless post‐discharge care.
Studies of post‐discharge primary care provider (PCP) follow‐up highlight the gaps in current practice within the transition from the hospital to PCP follow‐up. For instance, while more than 1 in 4 discharged patients (27.6%) at one large teaching hospital had outpatient work‐ups recommended by their hospital physicians, more than a third (35.9%) of these recommendations were ultimately not completed. Furthermore, at this same center, an increased time interval between hospital discharge and PCP follow‐up decreased the likelihood that a work‐up recommended by a hospital physician was completed.6 In patients who do have a PCP, post‐hospitalization follow‐up is frequently impacted by a variety of factors, including co‐payment requirements, transportation issues, lack of health insurance, as well as scheduling a follow‐up appointment while in the hospital.710 Uninsured patients are at particular risk for failures in transitions, have poorer health outcomes and higher mortality than insured counterparts, and are nearly 3 times more likely to make an ED visit following hospital discharge.1113
In order to better understand the role of post‐discharge PCP follow‐up, we sought to identify: (1) the percentage of general medical inpatients lacking timely PCP follow‐up after discharge from the hospital, and (2) the impact of patients lacking timely PCP follow‐up on 30‐day readmission rate and hospital length of stay (LOS). For the purposes of this study, we have defined timely PCP follow up as occurring within 4 weeks of hospital discharge.
Methods
Study Setting and Population
This prospective cohort enrolled a convenience sample of patients admitted to Internal Medicine ward teams at the University of Colorado Hospital Anschutz Inpatient Pavilion between December 2007 and March 2008. Up to 2 patients were enrolled on weekdays on the morning following admission (ie, Sunday night through Thursday night admissions). Patients were screened for study entry if they were able to participate in an interview as identified by their medical team and available in their room. Of a total of 121 patients screened for study entry by a professional research assistant (PRA), 75 ultimately provided HIPAA authorization, informed consent, and completed the in‐hospital interview. The most common reasons for screened patients refusing study enrollment included being not interested (26) and too ill (10). Ten subjects were lost to follow‐up after hospital discharge, including one subject who was deceased. Therefore, 65 patients successfully completed the follow‐up phone interview and were included in the analyses. Characteristics of the 121 screened patients and the 75 study patients were similar with respect to sex, age, race, and payer mix, and representative of the demographics of the patient population at large. Case mix indices (mean) were similar among the 121 screened (1.23), 75 enrolled (1.27), and final 65 study patients (1.25).
Exclusion Criteria
Patients admitted to the medical observation unit; patients admitted at night who are ultimately reassigned to specialty services (Oncology, Cardiology, Hepatology and Acute Care for the Elderly) were excluded. Human immunodeficiency virus (HIV) patients were excluded because of routine outpatient ID follow‐up; patients <18 years of age; patients lacking a telephone; patients admitted on Friday and Saturday nights; and outside hospital transfers.
Measures
The primary study outcome was the rate of timely PCP follow‐up defined as that occurring within 4 weeks of hospital discharge. PCP was defined in this study as either a patient's known PCP (or another provider in the same clinic), or a nurse practitioner/physician assistant. Patients seen in follow‐up by a specialist related to the discharge diagnosis, eg, an Endocrinologist in a patient hospitalized for Diabetic complications; a Rheumatologist following up an SLE patient, etc., were also counted as having PCP follow‐up as defined in this study.
Additional outcomes included three measures of hospital readmission: hospital readmission for same condition; hospital readmission or other care sought (ie, ED, Urgent Care) for same condition; and hospital readmission for any condition, and index hospital LOS. The distinction between same condition and any condition was made in an attempt to delineate a potentially preventable readmission (as an example, one study patient was subsequently readmitted with a gunshot injury that clearly would not have been affected by the presence of any PCP follow‐up). Determination of same vs. any condition was made by the investigators through information obtained from patients on follow‐up phone interviews: Have you been readmitted to the University Hospital or another hospital since your discharge last month from the University Hospital? If yes: where, when, and why? The investigators determined same vs. any through comparing this information to the primary diagnosis from the index hospitalization obtained from the final discharge documentation. A condition was considered same if the readmission was for the same condition or for treatment/complications related to the index hospitalized condition.
Descriptive data collected included patient demographics, diagnoses, insurance status, presence of an identified, established PCP, time to PCP follow‐up in weeks, effects of payer source, admitting service (hospitalist vs. General Internal Medicine (GIM) attending), and nature of presenting illness (acute vs. acute on chronic condition). Categories of insurance obtained from chart review included commercial, self‐pay (uninsured), Medicare, Medicaid and Veterans.
Data Collection
A PRA screened and obtained informed consent and a Health Insurance Portability and Accountability Act (HIPAA) waiver from patients the day following admission. At that time, the PRA obtained the patients' vital information from chart review and a scripted patient interview: age, sex, PCP, categories of insurance, contact phone numbers, and admitting date and diagnoses. The in‐house interview included eight questions examining a patient's experiences of and attitudes toward PCPs. Four weeks after discharge, patients were contacted by the PRA via telephone. Scripted telephone interviews were used to determine occurrence and timing of PCP follow‐up and hospital readmission status (to any hospital) per patient self‐report. Potential barriers to PCP follow‐up were assessed. Up to 3 attempts were made to contact study subjects out to 4 weeks from the initial call (8 weeks total). If an appointment for an enrolled patient had been made, but had not yet occurred, an additional phone call was made 2 weeks later to determine whether, and when, the appointment was kept. Review of discharge summaries determined a patient's hospital LOS.
Data Analysis
Descriptive statistics were calculated for the study population. Univariate comparisons were completed for patient characteristics and study outcomes for patients with and without PCP follow‐up. We used t‐tests for continuous variables (age and LOS) and chi‐square or Fisher's exact tests when necessary for dichotomous variables (gender, uninsured vs. insured, and all hospital readmission outcomes). Comparisons according to PCP follow‐up for the categorical variables were tested with the Cochran‐Mantel‐Haenszel statistic for general association (race and insurance category) or for trends in the ordinal variable (education).
Patient characteristics and study outcomes with univariate P value < 0.1 were assessed for inclusion in the multivariate logistic regression models. Separate logistic regression models were examined with PCP follow‐up (yes/no) as the explanatory variable and the 3 hospital readmission rates as the outcomes. Final logistic regression models included the primary predictor, PCP follow‐up, along with potential predictor variables with P value < 0.05. Statistical analyses were carried out using SAS version 9.2 (SAS Institute, Cary, NC).
This protocol was approved by the Colorado Multiple Institutional Review Board (COMIRB) prior to the implemented study.
Results
Sixty‐five patients completed this study. The mean age of the study population was 55.3 years and approximately half (52.3%) of the study participants were female. Fifty‐two subjects reported having an established PCP on admission to the hospital (80%). The rate of timely PCP follow‐up overall was 49.2%. Table 1 shows the study population characteristics stratified by presence of timely PCP follow‐up. Patients lacking timely PCP follow‐up were much younger (48.4 vs. 62.4 years; P < 0.001) than those with timely PCP follow‐up; there were also non‐significant trends toward patients lacking timely PCP follow‐up being non‐white: (33.3% vs. 25%, P = 0.23) and having lower education level (72.7% with high school or lower education vs. 56.2% for those with PCP follow‐up, P = 0.15) than those with timely PCP follow‐up. Of the 32 patients having timely PCP follow‐up, 15.6% were uninsured. In comparison, among the 33 patients lacking timely PCP follow‐up after hospital discharge, over a third (36%) were uninsured (P = 0.06). Among the uninsured, a large majority (70.5%) lacked timely PCP follow‐up (P = 0.06). In contrast, only 11 of the 26 Medicare patients (42.3%) lacked timely PCP follow‐up (P = 0.13).
| Study Demographics | Timely PCP Follow‐Up (n = 32) | No PCP Follow‐Up (n = 33) | P Value |
|---|---|---|---|
| |||
| Female, n (%) | 17 (53.1) | 17 (51.5) | 0.90 |
| Age, years, mean (SD) | 62.4 | 48.4 | <0.001 |
| Race, n (%) | |||
| Caucasian | 24 (75.0) | 23 (69.7) | 0.23 |
| African American | 7 (21.9) | 5 (15.2) | |
| Hispanic/Latino | 1 (3.1) | 5 (15.2) | |
| Highest grade completed, n (%) | |||
| Grammar school | 2 (6.3) | 3 (9.1) | 0.15 |
| High school | 16 (50.0) | 21 (63.6) | |
| College | 13 (40.6) | 9 (27.3) | |
| Postgraduate | 1 (3.1) | 0 (0) | |
| Insurance*, n (%) | |||
| Medicare | 15 (46.9) | 11 (33.3) | 0.13 |
| Medicaid | 1 (3.1) | 3 (9.1) | |
| Commercial/private | 6 (18.8) | 6 (18.2) | |
| VA/Tri‐Care | 5 (15.6) | 1 (3.0) | |
| Self‐pay/uninsured | 5 (15.6) | 12 (36.4) | 0.06 |
| Case mix index, median | 1.15 | 1.11 | |
Readmissions
The 30‐day readmission rates for all study subjects were 12.3% for a patient's same medical condition, 17.2% for readmission or other care sought for the same condition, and 21.5% for any condition. Table 2 contains univariate comparisons for the patient outcomes of readmission and LOS stratified by timely PCP follow‐up. Hospital readmission for the same medical condition was significantly higher in patients lacking timely PCP follow‐up compared to those with timely PCP follow‐up (21.2% vs. 3.1%, P = 0.05). The composite outcome of hospital readmission and/or other care sought (emergency department or urgent care) for a patient's same condition was also significantly higher in patients lacking timely PCP follow‐up (28.1% vs. 6.3%; P = 0.02). However, hospital readmission for any condition did not differ with absence of timely PCP follow‐up.
| Outcome | Timely PCP Follow‐Up (n = 32) | No PCP Follow‐Up (n = 33) | P Value |
|---|---|---|---|
| |||
| Length of stay (days), mean (SD) | 4.4 (3.7) | 6.3 (5.2) | 0.11 |
| Hospital readmission for same condition within 30‐days of discharge, n (%) | 1 (3.1) | 7 (21.2) | 0.05 |
| Hospital readmission or other care sought (ie, ED, urgent care) for same condition within 30‐days of discharge, n (%) | 2 (6.3) | 9 (28.1)* | 0.02 |
| Hospital readmission for any condition within 30‐days of discharge, n (%) | 5 (15.6) | 9 (27.3) | 0.25 |
Multiple logistic regression revealed that patients lacking timely PCP follow‐up were 10 times more likely to be readmitted for the same condition within 30 days of hospital discharge (odds ratio [OR] = 9.9; P = 0.04) and nearly seven times as likely to be readmitted for the same condition or receive other care (OR = 6.8, P = 0.02) (Table 3).
| Outcome | Odds Ratio (CI) | P Value |
|---|---|---|
| ||
| Hospital readmission for same condition | 9.9 (1.2‐84.7) | 0.04 |
| Hospital readmission or other care for same condition | 6.8 (1.4‐34.3) | 0.02 |
| Hospital readmission for any condition | 2.3 (0.7‐7.9) | 0.17 |
LOS
Overall hospital LOS in all patients was 5.4 4.6 days. In patients lacking timely PCP follow‐up, there was a trend toward longer hospital LOS: 6.3 days vs. 4.4 days, P = 0.11. For all uninsured study patients (17), the mean LOS was 6.4 days vs. 5.0 days for all other insurance categories, P = 0.31.
Insurance Status
Being uninsured was associated with a patient lacking timely PCP follow‐up (P = 0.06), but was not directly associated with higher readmission or longer hospital LOS (OR = 1.0, P = 0.96). The lack of insurance was not a significant predictor of hospital readmission in the multiple logistic regression models.
Timing of PCP Follow‐Up
In evaluating timing of any PCP follow‐up after hospital discharge and clinical outcomes, most PCP follow‐up (90.6%) occurred within the first 2 weeks following hospital discharge. However, we found no statistical difference between timing of post‐discharge PCP follow‐up and hospital readmission outcomes (hospital readmission for same reason, P = 0.51; hospital readmission or other care sought for same reason, P = 0.89), or in hospital LOS (P = 0.87). Timing of PCP follow‐upwhen comparing post‐hospitalization follow‐up <1 week, 1 to 2 weeks, and 2 to 4 weekswas not predictive of readmission rates or LOS.
Established PCP
When significance of having an established PCP prior to hospital admission was evaluated, 52 patients reported having an established PCP on hospital admission (80%), half of whom were Medicare patients. Of the 13 patients with no PCP on admission, the majority (10) were self‐pay (77%, P < 0.0001). Interestingly, only 29 (55.8%) of the patients who reported a PCP on admission to the hospital saw their PCP within 4 weeks of hospital discharge. Of 13 patients without a PCP on admission, only 3 obtained 4‐week PCP follow‐up. When we examined our study outcomes for subjects stratified by the presence of an established PCP prior to hospitalization, we found univariate association with timely post‐discharge PCP follow‐up (56% of those with established PCP vs. 23% of those without, P = 0.04), but no difference in readmission rates or hospital LOS.
Severity of patient illnessmeasured using hospital data and the case mix index (CMI)of the 3 patient populations (screened, enrolled, final) was quite similar. The CMI (mean) for the 121 screened patients was 1.23. The CMI for the 75 enrolled patients was 1.27. And the CMI in the 65 final study patients was 1.25. When evaluating illness severity (CMI) of patients in relation to hospital LOS between the 2 final study populations, the CMI (median) was also similar: 1.15 for the 32 patients with timely PCP follow‐up vs. 1.11 for the 33 patients without timely PCP follow‐up.
We found no association when looking at the rate of timely PCP follow‐up based on admitting service attending, or acute vs. acute on chronic diagnosis.
Barriers to PCP follow‐up most frequently cited by study patients were: lacking a PCP (no established PCP prior to hospital, no insurance, out of town, recently changed insurance), could not get an appointment, discharged to a half‐way house, and saw another doctor (specialist unrelated to discharge diagnosis).
Discussion
A growing body of work highlights the role of multiple, varied interventions at, or following discharge, in improving outcomes during the transition from inpatient to outpatient care. Examples include care coordination by advanced nurse practitioners, follow‐up pharmacist phone calls, and involvement of a transition coach encouraging active patient involvementall are known to improve patient outcomes following a hospitalization.1418 The active involvement of a PCP is central to a number of these proven interventions to ensure effective completion of ongoing patient care. And while some previous studies suggest increased overall resource utilization when PCP follow‐up occurs after hospitalization,19 the level of fragmented care that occurs in today's hospitalized patient, as well as the fact many patients lack PCP care at all, raises questions about clinical outcomes after hospitalization related to timely PCP follow‐up. The issue of appropriateness of resources utilized has also not been adequately explored.
Within this context, this study examines the role that PCP follow‐up might play in such interventions and its' effects on patient outcomes. Notably, in this urban academic medical center, we found that timely PCP follow‐up after hospital discharge occurred in fewer than half of general medical inpatients. Lack of timely PCP follow‐up was associated with increased hospital readmission for the same condition and a trend toward a longer index hospital LOS.
While this small study cannot fully elucidate the impact of lack of timely PCP follow‐up on post‐discharge care, our findings suggest some mechanisms by which lack of timely PCP follow‐up might result in poor outcomes. For instance, patients lacking a PCP visit after discharge may not obtain needed follow‐up care in the post‐discharge period, leading to clinical deterioration and hospital readmission. Uninsured patients may be at particular risk for failed transition because they are less likely to have consistent PCP access, whether as an already established patient or one newly assigned.20, 21 Perhaps a larger study would better demonstrate statistical significance in reflecting the association between uninsured patients, lack of a PCP, and post‐discharge follow‐up deficiencies. There may, in fact, be issues related to patient attitudes and beliefs, such as subjectively feeling better or even an implicit distrust of the healthcare system among the uninsured, that exist as well. Even among patients with a PCP prior to hospitalization, PCP follow‐up after hospital discharge may be lacking due to modifiable factors such as patient attitudes and beliefs and logistical barriers in arranging follow‐up.
Patients without potential for timely PCP follow‐up might be kept in the hospital longer to ensure they are well enough medically to sufficiently meet their own follow‐up needs. Hospital LOS might be increased by providers to compensate for the lack of PCP follow‐up. Alternatively, these patients may be sicker with their index hospitalization.
It is not surprising that payer source appears to influence a patients' ability to obtain timely PCP follow‐up. It is well documented that uninsured patients have higher healthcare resource utilization.2224 Lack of access to primary care in such patients contributes to a cycle of using the most expensive sites of care. In our study, we found many of the patients lacking timely PCP follow‐up were younger, perhaps reflecting the same patient population who have higher rates of being uninsured. Conversely, older patients are more likely to have PCP access, in large part due to having Medicare benefits (although this dynamic has shown a shift in recent years). The uninsured may present sicker as a result of lacking pre‐hospital PCP access or transportation to a PCP visit.
Limitations
This study was performed at a single, academic institution limiting its' generalizability. In addition, this small cohort study, which took place over four winter months, may have implicit biases toward certain disease entities and follow‐up issues unique to study size and season. The small study size was dictated by a finite amount of available resources, potentially contributing to minor inconsistencies with some of the results. While statistical significance was still seen with many of our results, a much larger study may better enhance the study outcomes.
It also remains unclear why the effects of PCP follow‐up were evident for a patient's same condition, but not for any condition. The distinction between designations is potentially subjective and may be difficult to accurately determine. Most existing readmission studies in the literature assign readmission for any condition. A future, larger study may be able to examine whether this difference exists between same vs. any condition.
As an academic medical center, access to specialty clinics may be facilitated, thus increasing PCP follow‐up in patients who might otherwise not have it available to them. Additionally, our subjects were limited to a convenience sample of the population of the general medicine wards and may not be representative of all medical inpatients. Patients lacking a telephone were missed. We relied on patient recollection and self‐report of PCP follow‐up visits and re‐hospitalizations. While we acknowledge limitations of patient self‐report, both in communication and comprehension, we believe patients are reasonably able to report on whether or not they were readmitted to the hospital, the cause of their readmission and whether/when they had PCP follow‐up. Patient self‐report could be collected systematically and without long time lags. Finally, the research team did not have reliable access to readmission data for hospitals other than the facility in which the study was conducted.
It is possible patients readmitted early after discharge may have been counted as lacking PCP follow‐up simply because the readmission occurred so soon after discharge precluding the opportunity for PCP follow‐up to occur. The effects of patients having non‐PCP (home health nurse, pharmacist, phone advice) follow‐up after hospital discharge were not examined.
Also, LOS and readmission to a hospital may be more a reflection of disease severity than the absence of PCP follow‐up, ie, patients ultimately readmitted after hospital discharge may have been a sicker subset of patients upon index hospitalization.
In this urban academic medical center, discharged medicine patients commonly lack timely PCP follow‐up. The lack of timely PCP follow‐up after hospital discharge was associated with higher rates of readmission and a non‐significant trend toward longer hospital lengths of stay. Hospital discharge represents a period of significant risk in patient care necessitating the effective continuation of treatment plans including follow‐up of laboratory, radiology or other testing, and management by a variety of providers. PCPs may play a crucial role in care coordination during this period. Structured intervention performed at the time of discharge might increase post‐hospital PCP access and facilitate timely PCP follow‐up to ensure continuity of needed care after hospital discharge in the most vulnerable patients. Such interventions might include systems improvements, such as increasing PCP access in the post‐hospital period, to increase the likelihood that complex needs are met at a vulnerable period in patient care.
A more effective handoff between inpatient and outpatient settings may ultimately improve clinical outcomes, diminish resource utilization, and decrease overall healthcare costs.
Acknowledgements
The authors thank Traci Yamashita and Karen Mellis, Professional Research Assistants.
- , , , .The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;13:161–167.
- , , , .Medical errors related to discontinuity of care from an inpatient to outpatient setting.J Gen Intern Med.200318:646–651.
- , .The high cost users of medical care.N Engl J Med.1980;302:996–1002.
- , .The rate and cost of hospital readmissions for preventable conditions.Med Care Res Rev.2004;61:225–240.
- , , .Rehospitalizations among patients in the medicare fee‐for‐service program.N Engl J Med.2009;360;14:1418–1428.
- , , .Tying up loose ends. Discharging patients with unresolved medical issues.Arch Intern Med.2007;167:1305–1311.
- , .Post‐hospitalization followup appointment‐keeping among the medically indigent.J Community Health.1993;18(5):271–282.
- , , .Factors related to the keeping of appointments by indigent clients.J Health Care Poor Underserved.1993;4(1):21–39.
- , , , , , .Inpatient to outpatient transfer of diabetes care: perceptions of barriers to postdischarge followup in urban African American patients.Ethn Dis.2007;17(2):238–243.
- , , .Effect of a nurse case manager on postdischarge follow‐up.J Gen Intern Med.1996v;11(11):684–688.
- , , .Emergency department visits by persons recently discharges from U.S. hospitals.Natl Health Stat Report.2008;(6):1–9.
- , , .Comparing uninsured and privately insured hospital patients: admission severity, health outcomes and resource use.Health Serv Manage Res.2001;14(3):203–210.
- , , .Comparison of uninsured and privately insured hospital patients. Condition on admission, resource use, and outcome.JAMA.1991;265(3):374–379.
- , , .Effect of a nurse case manager on postdischarge follow‐up.J Gen Intern Med.1996;11:684–688.
- , , , .The care transitions intervention: results of a randomized controlled trial.Arch Intern Med.2006;166(17):1822–1828.
- , , , , , .A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333:1190–1195.
- , , , .Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631.
- , , , et al.A reengineered hospital discharge program to decrease rehospitalization.Ann Intern Med.2009;150:178–187.
- , , .Does increased access to primary care reduce hospital readmissions?N Engl J Med.1996;334:1441–1447.
- , .Health insurance and access to health care in the united states.Ann NY Acad Sci.1008;1136:149–160.
- , .2006.Summary health statistics for U.S. adults: National Health Interview Survey. 2005, NCHS/CDC/USDHHS, Vital Health Statistics, Series 10.
- , , .Emergency department visits by persons recently discharges from U.S. hospitals.Natl Health Stat Report.2008;(6):1–9.
- , , .Comparison of uninsured and privately insured hospital patients. Condition on admission, resource use, and outcome.JAMA.1991;265(3):374–379.
- , , .Comparing uninsured and privately insured hospital patients: admission severity, health outcomes and resource use.Health Serv Manage Res.2001;14(3):203–210.
Care transitions between the inpatient and outpatient settings are a known period of risk in a patient's care. For instance, 1 in 5 medical patients suffers an adverse event during the first several weeks after hospital discharge, with half of these requiring the use of additional healthcare resources.1 Additionally, medication and lab monitoring errors occur in up to half of all discharged patients.2 Nearly 1 in 5 hospitalized patients, admitted with 1 of 16 different conditions including asthma, diabetes, congestive heart failure and urinary tract infection is readmitted to the hospital within six months. Up to 60% of resources are used in rehospitalized patients.3, 4 In Medicare beneficiaries, the readmission rate is as high as 20% at 30 days. The same study suggests that up to half of Medicare patients readmitted within 30 days are not seen in the outpatient setting following discharge.5 Such statistics underscore the need for seamless post‐discharge care.
Studies of post‐discharge primary care provider (PCP) follow‐up highlight the gaps in current practice within the transition from the hospital to PCP follow‐up. For instance, while more than 1 in 4 discharged patients (27.6%) at one large teaching hospital had outpatient work‐ups recommended by their hospital physicians, more than a third (35.9%) of these recommendations were ultimately not completed. Furthermore, at this same center, an increased time interval between hospital discharge and PCP follow‐up decreased the likelihood that a work‐up recommended by a hospital physician was completed.6 In patients who do have a PCP, post‐hospitalization follow‐up is frequently impacted by a variety of factors, including co‐payment requirements, transportation issues, lack of health insurance, as well as scheduling a follow‐up appointment while in the hospital.710 Uninsured patients are at particular risk for failures in transitions, have poorer health outcomes and higher mortality than insured counterparts, and are nearly 3 times more likely to make an ED visit following hospital discharge.1113
In order to better understand the role of post‐discharge PCP follow‐up, we sought to identify: (1) the percentage of general medical inpatients lacking timely PCP follow‐up after discharge from the hospital, and (2) the impact of patients lacking timely PCP follow‐up on 30‐day readmission rate and hospital length of stay (LOS). For the purposes of this study, we have defined timely PCP follow up as occurring within 4 weeks of hospital discharge.
Methods
Study Setting and Population
This prospective cohort enrolled a convenience sample of patients admitted to Internal Medicine ward teams at the University of Colorado Hospital Anschutz Inpatient Pavilion between December 2007 and March 2008. Up to 2 patients were enrolled on weekdays on the morning following admission (ie, Sunday night through Thursday night admissions). Patients were screened for study entry if they were able to participate in an interview as identified by their medical team and available in their room. Of a total of 121 patients screened for study entry by a professional research assistant (PRA), 75 ultimately provided HIPAA authorization, informed consent, and completed the in‐hospital interview. The most common reasons for screened patients refusing study enrollment included being not interested (26) and too ill (10). Ten subjects were lost to follow‐up after hospital discharge, including one subject who was deceased. Therefore, 65 patients successfully completed the follow‐up phone interview and were included in the analyses. Characteristics of the 121 screened patients and the 75 study patients were similar with respect to sex, age, race, and payer mix, and representative of the demographics of the patient population at large. Case mix indices (mean) were similar among the 121 screened (1.23), 75 enrolled (1.27), and final 65 study patients (1.25).
Exclusion Criteria
Patients admitted to the medical observation unit; patients admitted at night who are ultimately reassigned to specialty services (Oncology, Cardiology, Hepatology and Acute Care for the Elderly) were excluded. Human immunodeficiency virus (HIV) patients were excluded because of routine outpatient ID follow‐up; patients <18 years of age; patients lacking a telephone; patients admitted on Friday and Saturday nights; and outside hospital transfers.
Measures
The primary study outcome was the rate of timely PCP follow‐up defined as that occurring within 4 weeks of hospital discharge. PCP was defined in this study as either a patient's known PCP (or another provider in the same clinic), or a nurse practitioner/physician assistant. Patients seen in follow‐up by a specialist related to the discharge diagnosis, eg, an Endocrinologist in a patient hospitalized for Diabetic complications; a Rheumatologist following up an SLE patient, etc., were also counted as having PCP follow‐up as defined in this study.
Additional outcomes included three measures of hospital readmission: hospital readmission for same condition; hospital readmission or other care sought (ie, ED, Urgent Care) for same condition; and hospital readmission for any condition, and index hospital LOS. The distinction between same condition and any condition was made in an attempt to delineate a potentially preventable readmission (as an example, one study patient was subsequently readmitted with a gunshot injury that clearly would not have been affected by the presence of any PCP follow‐up). Determination of same vs. any condition was made by the investigators through information obtained from patients on follow‐up phone interviews: Have you been readmitted to the University Hospital or another hospital since your discharge last month from the University Hospital? If yes: where, when, and why? The investigators determined same vs. any through comparing this information to the primary diagnosis from the index hospitalization obtained from the final discharge documentation. A condition was considered same if the readmission was for the same condition or for treatment/complications related to the index hospitalized condition.
Descriptive data collected included patient demographics, diagnoses, insurance status, presence of an identified, established PCP, time to PCP follow‐up in weeks, effects of payer source, admitting service (hospitalist vs. General Internal Medicine (GIM) attending), and nature of presenting illness (acute vs. acute on chronic condition). Categories of insurance obtained from chart review included commercial, self‐pay (uninsured), Medicare, Medicaid and Veterans.
Data Collection
A PRA screened and obtained informed consent and a Health Insurance Portability and Accountability Act (HIPAA) waiver from patients the day following admission. At that time, the PRA obtained the patients' vital information from chart review and a scripted patient interview: age, sex, PCP, categories of insurance, contact phone numbers, and admitting date and diagnoses. The in‐house interview included eight questions examining a patient's experiences of and attitudes toward PCPs. Four weeks after discharge, patients were contacted by the PRA via telephone. Scripted telephone interviews were used to determine occurrence and timing of PCP follow‐up and hospital readmission status (to any hospital) per patient self‐report. Potential barriers to PCP follow‐up were assessed. Up to 3 attempts were made to contact study subjects out to 4 weeks from the initial call (8 weeks total). If an appointment for an enrolled patient had been made, but had not yet occurred, an additional phone call was made 2 weeks later to determine whether, and when, the appointment was kept. Review of discharge summaries determined a patient's hospital LOS.
Data Analysis
Descriptive statistics were calculated for the study population. Univariate comparisons were completed for patient characteristics and study outcomes for patients with and without PCP follow‐up. We used t‐tests for continuous variables (age and LOS) and chi‐square or Fisher's exact tests when necessary for dichotomous variables (gender, uninsured vs. insured, and all hospital readmission outcomes). Comparisons according to PCP follow‐up for the categorical variables were tested with the Cochran‐Mantel‐Haenszel statistic for general association (race and insurance category) or for trends in the ordinal variable (education).
Patient characteristics and study outcomes with univariate P value < 0.1 were assessed for inclusion in the multivariate logistic regression models. Separate logistic regression models were examined with PCP follow‐up (yes/no) as the explanatory variable and the 3 hospital readmission rates as the outcomes. Final logistic regression models included the primary predictor, PCP follow‐up, along with potential predictor variables with P value < 0.05. Statistical analyses were carried out using SAS version 9.2 (SAS Institute, Cary, NC).
This protocol was approved by the Colorado Multiple Institutional Review Board (COMIRB) prior to the implemented study.
Results
Sixty‐five patients completed this study. The mean age of the study population was 55.3 years and approximately half (52.3%) of the study participants were female. Fifty‐two subjects reported having an established PCP on admission to the hospital (80%). The rate of timely PCP follow‐up overall was 49.2%. Table 1 shows the study population characteristics stratified by presence of timely PCP follow‐up. Patients lacking timely PCP follow‐up were much younger (48.4 vs. 62.4 years; P < 0.001) than those with timely PCP follow‐up; there were also non‐significant trends toward patients lacking timely PCP follow‐up being non‐white: (33.3% vs. 25%, P = 0.23) and having lower education level (72.7% with high school or lower education vs. 56.2% for those with PCP follow‐up, P = 0.15) than those with timely PCP follow‐up. Of the 32 patients having timely PCP follow‐up, 15.6% were uninsured. In comparison, among the 33 patients lacking timely PCP follow‐up after hospital discharge, over a third (36%) were uninsured (P = 0.06). Among the uninsured, a large majority (70.5%) lacked timely PCP follow‐up (P = 0.06). In contrast, only 11 of the 26 Medicare patients (42.3%) lacked timely PCP follow‐up (P = 0.13).
| Study Demographics | Timely PCP Follow‐Up (n = 32) | No PCP Follow‐Up (n = 33) | P Value |
|---|---|---|---|
| |||
| Female, n (%) | 17 (53.1) | 17 (51.5) | 0.90 |
| Age, years, mean (SD) | 62.4 | 48.4 | <0.001 |
| Race, n (%) | |||
| Caucasian | 24 (75.0) | 23 (69.7) | 0.23 |
| African American | 7 (21.9) | 5 (15.2) | |
| Hispanic/Latino | 1 (3.1) | 5 (15.2) | |
| Highest grade completed, n (%) | |||
| Grammar school | 2 (6.3) | 3 (9.1) | 0.15 |
| High school | 16 (50.0) | 21 (63.6) | |
| College | 13 (40.6) | 9 (27.3) | |
| Postgraduate | 1 (3.1) | 0 (0) | |
| Insurance*, n (%) | |||
| Medicare | 15 (46.9) | 11 (33.3) | 0.13 |
| Medicaid | 1 (3.1) | 3 (9.1) | |
| Commercial/private | 6 (18.8) | 6 (18.2) | |
| VA/Tri‐Care | 5 (15.6) | 1 (3.0) | |
| Self‐pay/uninsured | 5 (15.6) | 12 (36.4) | 0.06 |
| Case mix index, median | 1.15 | 1.11 | |
Readmissions
The 30‐day readmission rates for all study subjects were 12.3% for a patient's same medical condition, 17.2% for readmission or other care sought for the same condition, and 21.5% for any condition. Table 2 contains univariate comparisons for the patient outcomes of readmission and LOS stratified by timely PCP follow‐up. Hospital readmission for the same medical condition was significantly higher in patients lacking timely PCP follow‐up compared to those with timely PCP follow‐up (21.2% vs. 3.1%, P = 0.05). The composite outcome of hospital readmission and/or other care sought (emergency department or urgent care) for a patient's same condition was also significantly higher in patients lacking timely PCP follow‐up (28.1% vs. 6.3%; P = 0.02). However, hospital readmission for any condition did not differ with absence of timely PCP follow‐up.
| Outcome | Timely PCP Follow‐Up (n = 32) | No PCP Follow‐Up (n = 33) | P Value |
|---|---|---|---|
| |||
| Length of stay (days), mean (SD) | 4.4 (3.7) | 6.3 (5.2) | 0.11 |
| Hospital readmission for same condition within 30‐days of discharge, n (%) | 1 (3.1) | 7 (21.2) | 0.05 |
| Hospital readmission or other care sought (ie, ED, urgent care) for same condition within 30‐days of discharge, n (%) | 2 (6.3) | 9 (28.1)* | 0.02 |
| Hospital readmission for any condition within 30‐days of discharge, n (%) | 5 (15.6) | 9 (27.3) | 0.25 |
Multiple logistic regression revealed that patients lacking timely PCP follow‐up were 10 times more likely to be readmitted for the same condition within 30 days of hospital discharge (odds ratio [OR] = 9.9; P = 0.04) and nearly seven times as likely to be readmitted for the same condition or receive other care (OR = 6.8, P = 0.02) (Table 3).
| Outcome | Odds Ratio (CI) | P Value |
|---|---|---|
| ||
| Hospital readmission for same condition | 9.9 (1.2‐84.7) | 0.04 |
| Hospital readmission or other care for same condition | 6.8 (1.4‐34.3) | 0.02 |
| Hospital readmission for any condition | 2.3 (0.7‐7.9) | 0.17 |
LOS
Overall hospital LOS in all patients was 5.4 4.6 days. In patients lacking timely PCP follow‐up, there was a trend toward longer hospital LOS: 6.3 days vs. 4.4 days, P = 0.11. For all uninsured study patients (17), the mean LOS was 6.4 days vs. 5.0 days for all other insurance categories, P = 0.31.
Insurance Status
Being uninsured was associated with a patient lacking timely PCP follow‐up (P = 0.06), but was not directly associated with higher readmission or longer hospital LOS (OR = 1.0, P = 0.96). The lack of insurance was not a significant predictor of hospital readmission in the multiple logistic regression models.
Timing of PCP Follow‐Up
In evaluating timing of any PCP follow‐up after hospital discharge and clinical outcomes, most PCP follow‐up (90.6%) occurred within the first 2 weeks following hospital discharge. However, we found no statistical difference between timing of post‐discharge PCP follow‐up and hospital readmission outcomes (hospital readmission for same reason, P = 0.51; hospital readmission or other care sought for same reason, P = 0.89), or in hospital LOS (P = 0.87). Timing of PCP follow‐upwhen comparing post‐hospitalization follow‐up <1 week, 1 to 2 weeks, and 2 to 4 weekswas not predictive of readmission rates or LOS.
Established PCP
When significance of having an established PCP prior to hospital admission was evaluated, 52 patients reported having an established PCP on hospital admission (80%), half of whom were Medicare patients. Of the 13 patients with no PCP on admission, the majority (10) were self‐pay (77%, P < 0.0001). Interestingly, only 29 (55.8%) of the patients who reported a PCP on admission to the hospital saw their PCP within 4 weeks of hospital discharge. Of 13 patients without a PCP on admission, only 3 obtained 4‐week PCP follow‐up. When we examined our study outcomes for subjects stratified by the presence of an established PCP prior to hospitalization, we found univariate association with timely post‐discharge PCP follow‐up (56% of those with established PCP vs. 23% of those without, P = 0.04), but no difference in readmission rates or hospital LOS.
Severity of patient illnessmeasured using hospital data and the case mix index (CMI)of the 3 patient populations (screened, enrolled, final) was quite similar. The CMI (mean) for the 121 screened patients was 1.23. The CMI for the 75 enrolled patients was 1.27. And the CMI in the 65 final study patients was 1.25. When evaluating illness severity (CMI) of patients in relation to hospital LOS between the 2 final study populations, the CMI (median) was also similar: 1.15 for the 32 patients with timely PCP follow‐up vs. 1.11 for the 33 patients without timely PCP follow‐up.
We found no association when looking at the rate of timely PCP follow‐up based on admitting service attending, or acute vs. acute on chronic diagnosis.
Barriers to PCP follow‐up most frequently cited by study patients were: lacking a PCP (no established PCP prior to hospital, no insurance, out of town, recently changed insurance), could not get an appointment, discharged to a half‐way house, and saw another doctor (specialist unrelated to discharge diagnosis).
Discussion
A growing body of work highlights the role of multiple, varied interventions at, or following discharge, in improving outcomes during the transition from inpatient to outpatient care. Examples include care coordination by advanced nurse practitioners, follow‐up pharmacist phone calls, and involvement of a transition coach encouraging active patient involvementall are known to improve patient outcomes following a hospitalization.1418 The active involvement of a PCP is central to a number of these proven interventions to ensure effective completion of ongoing patient care. And while some previous studies suggest increased overall resource utilization when PCP follow‐up occurs after hospitalization,19 the level of fragmented care that occurs in today's hospitalized patient, as well as the fact many patients lack PCP care at all, raises questions about clinical outcomes after hospitalization related to timely PCP follow‐up. The issue of appropriateness of resources utilized has also not been adequately explored.
Within this context, this study examines the role that PCP follow‐up might play in such interventions and its' effects on patient outcomes. Notably, in this urban academic medical center, we found that timely PCP follow‐up after hospital discharge occurred in fewer than half of general medical inpatients. Lack of timely PCP follow‐up was associated with increased hospital readmission for the same condition and a trend toward a longer index hospital LOS.
While this small study cannot fully elucidate the impact of lack of timely PCP follow‐up on post‐discharge care, our findings suggest some mechanisms by which lack of timely PCP follow‐up might result in poor outcomes. For instance, patients lacking a PCP visit after discharge may not obtain needed follow‐up care in the post‐discharge period, leading to clinical deterioration and hospital readmission. Uninsured patients may be at particular risk for failed transition because they are less likely to have consistent PCP access, whether as an already established patient or one newly assigned.20, 21 Perhaps a larger study would better demonstrate statistical significance in reflecting the association between uninsured patients, lack of a PCP, and post‐discharge follow‐up deficiencies. There may, in fact, be issues related to patient attitudes and beliefs, such as subjectively feeling better or even an implicit distrust of the healthcare system among the uninsured, that exist as well. Even among patients with a PCP prior to hospitalization, PCP follow‐up after hospital discharge may be lacking due to modifiable factors such as patient attitudes and beliefs and logistical barriers in arranging follow‐up.
Patients without potential for timely PCP follow‐up might be kept in the hospital longer to ensure they are well enough medically to sufficiently meet their own follow‐up needs. Hospital LOS might be increased by providers to compensate for the lack of PCP follow‐up. Alternatively, these patients may be sicker with their index hospitalization.
It is not surprising that payer source appears to influence a patients' ability to obtain timely PCP follow‐up. It is well documented that uninsured patients have higher healthcare resource utilization.2224 Lack of access to primary care in such patients contributes to a cycle of using the most expensive sites of care. In our study, we found many of the patients lacking timely PCP follow‐up were younger, perhaps reflecting the same patient population who have higher rates of being uninsured. Conversely, older patients are more likely to have PCP access, in large part due to having Medicare benefits (although this dynamic has shown a shift in recent years). The uninsured may present sicker as a result of lacking pre‐hospital PCP access or transportation to a PCP visit.
Limitations
This study was performed at a single, academic institution limiting its' generalizability. In addition, this small cohort study, which took place over four winter months, may have implicit biases toward certain disease entities and follow‐up issues unique to study size and season. The small study size was dictated by a finite amount of available resources, potentially contributing to minor inconsistencies with some of the results. While statistical significance was still seen with many of our results, a much larger study may better enhance the study outcomes.
It also remains unclear why the effects of PCP follow‐up were evident for a patient's same condition, but not for any condition. The distinction between designations is potentially subjective and may be difficult to accurately determine. Most existing readmission studies in the literature assign readmission for any condition. A future, larger study may be able to examine whether this difference exists between same vs. any condition.
As an academic medical center, access to specialty clinics may be facilitated, thus increasing PCP follow‐up in patients who might otherwise not have it available to them. Additionally, our subjects were limited to a convenience sample of the population of the general medicine wards and may not be representative of all medical inpatients. Patients lacking a telephone were missed. We relied on patient recollection and self‐report of PCP follow‐up visits and re‐hospitalizations. While we acknowledge limitations of patient self‐report, both in communication and comprehension, we believe patients are reasonably able to report on whether or not they were readmitted to the hospital, the cause of their readmission and whether/when they had PCP follow‐up. Patient self‐report could be collected systematically and without long time lags. Finally, the research team did not have reliable access to readmission data for hospitals other than the facility in which the study was conducted.
It is possible patients readmitted early after discharge may have been counted as lacking PCP follow‐up simply because the readmission occurred so soon after discharge precluding the opportunity for PCP follow‐up to occur. The effects of patients having non‐PCP (home health nurse, pharmacist, phone advice) follow‐up after hospital discharge were not examined.
Also, LOS and readmission to a hospital may be more a reflection of disease severity than the absence of PCP follow‐up, ie, patients ultimately readmitted after hospital discharge may have been a sicker subset of patients upon index hospitalization.
In this urban academic medical center, discharged medicine patients commonly lack timely PCP follow‐up. The lack of timely PCP follow‐up after hospital discharge was associated with higher rates of readmission and a non‐significant trend toward longer hospital lengths of stay. Hospital discharge represents a period of significant risk in patient care necessitating the effective continuation of treatment plans including follow‐up of laboratory, radiology or other testing, and management by a variety of providers. PCPs may play a crucial role in care coordination during this period. Structured intervention performed at the time of discharge might increase post‐hospital PCP access and facilitate timely PCP follow‐up to ensure continuity of needed care after hospital discharge in the most vulnerable patients. Such interventions might include systems improvements, such as increasing PCP access in the post‐hospital period, to increase the likelihood that complex needs are met at a vulnerable period in patient care.
A more effective handoff between inpatient and outpatient settings may ultimately improve clinical outcomes, diminish resource utilization, and decrease overall healthcare costs.
Acknowledgements
The authors thank Traci Yamashita and Karen Mellis, Professional Research Assistants.
Care transitions between the inpatient and outpatient settings are a known period of risk in a patient's care. For instance, 1 in 5 medical patients suffers an adverse event during the first several weeks after hospital discharge, with half of these requiring the use of additional healthcare resources.1 Additionally, medication and lab monitoring errors occur in up to half of all discharged patients.2 Nearly 1 in 5 hospitalized patients, admitted with 1 of 16 different conditions including asthma, diabetes, congestive heart failure and urinary tract infection is readmitted to the hospital within six months. Up to 60% of resources are used in rehospitalized patients.3, 4 In Medicare beneficiaries, the readmission rate is as high as 20% at 30 days. The same study suggests that up to half of Medicare patients readmitted within 30 days are not seen in the outpatient setting following discharge.5 Such statistics underscore the need for seamless post‐discharge care.
Studies of post‐discharge primary care provider (PCP) follow‐up highlight the gaps in current practice within the transition from the hospital to PCP follow‐up. For instance, while more than 1 in 4 discharged patients (27.6%) at one large teaching hospital had outpatient work‐ups recommended by their hospital physicians, more than a third (35.9%) of these recommendations were ultimately not completed. Furthermore, at this same center, an increased time interval between hospital discharge and PCP follow‐up decreased the likelihood that a work‐up recommended by a hospital physician was completed.6 In patients who do have a PCP, post‐hospitalization follow‐up is frequently impacted by a variety of factors, including co‐payment requirements, transportation issues, lack of health insurance, as well as scheduling a follow‐up appointment while in the hospital.710 Uninsured patients are at particular risk for failures in transitions, have poorer health outcomes and higher mortality than insured counterparts, and are nearly 3 times more likely to make an ED visit following hospital discharge.1113
In order to better understand the role of post‐discharge PCP follow‐up, we sought to identify: (1) the percentage of general medical inpatients lacking timely PCP follow‐up after discharge from the hospital, and (2) the impact of patients lacking timely PCP follow‐up on 30‐day readmission rate and hospital length of stay (LOS). For the purposes of this study, we have defined timely PCP follow up as occurring within 4 weeks of hospital discharge.
Methods
Study Setting and Population
This prospective cohort enrolled a convenience sample of patients admitted to Internal Medicine ward teams at the University of Colorado Hospital Anschutz Inpatient Pavilion between December 2007 and March 2008. Up to 2 patients were enrolled on weekdays on the morning following admission (ie, Sunday night through Thursday night admissions). Patients were screened for study entry if they were able to participate in an interview as identified by their medical team and available in their room. Of a total of 121 patients screened for study entry by a professional research assistant (PRA), 75 ultimately provided HIPAA authorization, informed consent, and completed the in‐hospital interview. The most common reasons for screened patients refusing study enrollment included being not interested (26) and too ill (10). Ten subjects were lost to follow‐up after hospital discharge, including one subject who was deceased. Therefore, 65 patients successfully completed the follow‐up phone interview and were included in the analyses. Characteristics of the 121 screened patients and the 75 study patients were similar with respect to sex, age, race, and payer mix, and representative of the demographics of the patient population at large. Case mix indices (mean) were similar among the 121 screened (1.23), 75 enrolled (1.27), and final 65 study patients (1.25).
Exclusion Criteria
Patients admitted to the medical observation unit; patients admitted at night who are ultimately reassigned to specialty services (Oncology, Cardiology, Hepatology and Acute Care for the Elderly) were excluded. Human immunodeficiency virus (HIV) patients were excluded because of routine outpatient ID follow‐up; patients <18 years of age; patients lacking a telephone; patients admitted on Friday and Saturday nights; and outside hospital transfers.
Measures
The primary study outcome was the rate of timely PCP follow‐up defined as that occurring within 4 weeks of hospital discharge. PCP was defined in this study as either a patient's known PCP (or another provider in the same clinic), or a nurse practitioner/physician assistant. Patients seen in follow‐up by a specialist related to the discharge diagnosis, eg, an Endocrinologist in a patient hospitalized for Diabetic complications; a Rheumatologist following up an SLE patient, etc., were also counted as having PCP follow‐up as defined in this study.
Additional outcomes included three measures of hospital readmission: hospital readmission for same condition; hospital readmission or other care sought (ie, ED, Urgent Care) for same condition; and hospital readmission for any condition, and index hospital LOS. The distinction between same condition and any condition was made in an attempt to delineate a potentially preventable readmission (as an example, one study patient was subsequently readmitted with a gunshot injury that clearly would not have been affected by the presence of any PCP follow‐up). Determination of same vs. any condition was made by the investigators through information obtained from patients on follow‐up phone interviews: Have you been readmitted to the University Hospital or another hospital since your discharge last month from the University Hospital? If yes: where, when, and why? The investigators determined same vs. any through comparing this information to the primary diagnosis from the index hospitalization obtained from the final discharge documentation. A condition was considered same if the readmission was for the same condition or for treatment/complications related to the index hospitalized condition.
Descriptive data collected included patient demographics, diagnoses, insurance status, presence of an identified, established PCP, time to PCP follow‐up in weeks, effects of payer source, admitting service (hospitalist vs. General Internal Medicine (GIM) attending), and nature of presenting illness (acute vs. acute on chronic condition). Categories of insurance obtained from chart review included commercial, self‐pay (uninsured), Medicare, Medicaid and Veterans.
Data Collection
A PRA screened and obtained informed consent and a Health Insurance Portability and Accountability Act (HIPAA) waiver from patients the day following admission. At that time, the PRA obtained the patients' vital information from chart review and a scripted patient interview: age, sex, PCP, categories of insurance, contact phone numbers, and admitting date and diagnoses. The in‐house interview included eight questions examining a patient's experiences of and attitudes toward PCPs. Four weeks after discharge, patients were contacted by the PRA via telephone. Scripted telephone interviews were used to determine occurrence and timing of PCP follow‐up and hospital readmission status (to any hospital) per patient self‐report. Potential barriers to PCP follow‐up were assessed. Up to 3 attempts were made to contact study subjects out to 4 weeks from the initial call (8 weeks total). If an appointment for an enrolled patient had been made, but had not yet occurred, an additional phone call was made 2 weeks later to determine whether, and when, the appointment was kept. Review of discharge summaries determined a patient's hospital LOS.
Data Analysis
Descriptive statistics were calculated for the study population. Univariate comparisons were completed for patient characteristics and study outcomes for patients with and without PCP follow‐up. We used t‐tests for continuous variables (age and LOS) and chi‐square or Fisher's exact tests when necessary for dichotomous variables (gender, uninsured vs. insured, and all hospital readmission outcomes). Comparisons according to PCP follow‐up for the categorical variables were tested with the Cochran‐Mantel‐Haenszel statistic for general association (race and insurance category) or for trends in the ordinal variable (education).
Patient characteristics and study outcomes with univariate P value < 0.1 were assessed for inclusion in the multivariate logistic regression models. Separate logistic regression models were examined with PCP follow‐up (yes/no) as the explanatory variable and the 3 hospital readmission rates as the outcomes. Final logistic regression models included the primary predictor, PCP follow‐up, along with potential predictor variables with P value < 0.05. Statistical analyses were carried out using SAS version 9.2 (SAS Institute, Cary, NC).
This protocol was approved by the Colorado Multiple Institutional Review Board (COMIRB) prior to the implemented study.
Results
Sixty‐five patients completed this study. The mean age of the study population was 55.3 years and approximately half (52.3%) of the study participants were female. Fifty‐two subjects reported having an established PCP on admission to the hospital (80%). The rate of timely PCP follow‐up overall was 49.2%. Table 1 shows the study population characteristics stratified by presence of timely PCP follow‐up. Patients lacking timely PCP follow‐up were much younger (48.4 vs. 62.4 years; P < 0.001) than those with timely PCP follow‐up; there were also non‐significant trends toward patients lacking timely PCP follow‐up being non‐white: (33.3% vs. 25%, P = 0.23) and having lower education level (72.7% with high school or lower education vs. 56.2% for those with PCP follow‐up, P = 0.15) than those with timely PCP follow‐up. Of the 32 patients having timely PCP follow‐up, 15.6% were uninsured. In comparison, among the 33 patients lacking timely PCP follow‐up after hospital discharge, over a third (36%) were uninsured (P = 0.06). Among the uninsured, a large majority (70.5%) lacked timely PCP follow‐up (P = 0.06). In contrast, only 11 of the 26 Medicare patients (42.3%) lacked timely PCP follow‐up (P = 0.13).
| Study Demographics | Timely PCP Follow‐Up (n = 32) | No PCP Follow‐Up (n = 33) | P Value |
|---|---|---|---|
| |||
| Female, n (%) | 17 (53.1) | 17 (51.5) | 0.90 |
| Age, years, mean (SD) | 62.4 | 48.4 | <0.001 |
| Race, n (%) | |||
| Caucasian | 24 (75.0) | 23 (69.7) | 0.23 |
| African American | 7 (21.9) | 5 (15.2) | |
| Hispanic/Latino | 1 (3.1) | 5 (15.2) | |
| Highest grade completed, n (%) | |||
| Grammar school | 2 (6.3) | 3 (9.1) | 0.15 |
| High school | 16 (50.0) | 21 (63.6) | |
| College | 13 (40.6) | 9 (27.3) | |
| Postgraduate | 1 (3.1) | 0 (0) | |
| Insurance*, n (%) | |||
| Medicare | 15 (46.9) | 11 (33.3) | 0.13 |
| Medicaid | 1 (3.1) | 3 (9.1) | |
| Commercial/private | 6 (18.8) | 6 (18.2) | |
| VA/Tri‐Care | 5 (15.6) | 1 (3.0) | |
| Self‐pay/uninsured | 5 (15.6) | 12 (36.4) | 0.06 |
| Case mix index, median | 1.15 | 1.11 | |
Readmissions
The 30‐day readmission rates for all study subjects were 12.3% for a patient's same medical condition, 17.2% for readmission or other care sought for the same condition, and 21.5% for any condition. Table 2 contains univariate comparisons for the patient outcomes of readmission and LOS stratified by timely PCP follow‐up. Hospital readmission for the same medical condition was significantly higher in patients lacking timely PCP follow‐up compared to those with timely PCP follow‐up (21.2% vs. 3.1%, P = 0.05). The composite outcome of hospital readmission and/or other care sought (emergency department or urgent care) for a patient's same condition was also significantly higher in patients lacking timely PCP follow‐up (28.1% vs. 6.3%; P = 0.02). However, hospital readmission for any condition did not differ with absence of timely PCP follow‐up.
| Outcome | Timely PCP Follow‐Up (n = 32) | No PCP Follow‐Up (n = 33) | P Value |
|---|---|---|---|
| |||
| Length of stay (days), mean (SD) | 4.4 (3.7) | 6.3 (5.2) | 0.11 |
| Hospital readmission for same condition within 30‐days of discharge, n (%) | 1 (3.1) | 7 (21.2) | 0.05 |
| Hospital readmission or other care sought (ie, ED, urgent care) for same condition within 30‐days of discharge, n (%) | 2 (6.3) | 9 (28.1)* | 0.02 |
| Hospital readmission for any condition within 30‐days of discharge, n (%) | 5 (15.6) | 9 (27.3) | 0.25 |
Multiple logistic regression revealed that patients lacking timely PCP follow‐up were 10 times more likely to be readmitted for the same condition within 30 days of hospital discharge (odds ratio [OR] = 9.9; P = 0.04) and nearly seven times as likely to be readmitted for the same condition or receive other care (OR = 6.8, P = 0.02) (Table 3).
| Outcome | Odds Ratio (CI) | P Value |
|---|---|---|
| ||
| Hospital readmission for same condition | 9.9 (1.2‐84.7) | 0.04 |
| Hospital readmission or other care for same condition | 6.8 (1.4‐34.3) | 0.02 |
| Hospital readmission for any condition | 2.3 (0.7‐7.9) | 0.17 |
LOS
Overall hospital LOS in all patients was 5.4 4.6 days. In patients lacking timely PCP follow‐up, there was a trend toward longer hospital LOS: 6.3 days vs. 4.4 days, P = 0.11. For all uninsured study patients (17), the mean LOS was 6.4 days vs. 5.0 days for all other insurance categories, P = 0.31.
Insurance Status
Being uninsured was associated with a patient lacking timely PCP follow‐up (P = 0.06), but was not directly associated with higher readmission or longer hospital LOS (OR = 1.0, P = 0.96). The lack of insurance was not a significant predictor of hospital readmission in the multiple logistic regression models.
Timing of PCP Follow‐Up
In evaluating timing of any PCP follow‐up after hospital discharge and clinical outcomes, most PCP follow‐up (90.6%) occurred within the first 2 weeks following hospital discharge. However, we found no statistical difference between timing of post‐discharge PCP follow‐up and hospital readmission outcomes (hospital readmission for same reason, P = 0.51; hospital readmission or other care sought for same reason, P = 0.89), or in hospital LOS (P = 0.87). Timing of PCP follow‐upwhen comparing post‐hospitalization follow‐up <1 week, 1 to 2 weeks, and 2 to 4 weekswas not predictive of readmission rates or LOS.
Established PCP
When significance of having an established PCP prior to hospital admission was evaluated, 52 patients reported having an established PCP on hospital admission (80%), half of whom were Medicare patients. Of the 13 patients with no PCP on admission, the majority (10) were self‐pay (77%, P < 0.0001). Interestingly, only 29 (55.8%) of the patients who reported a PCP on admission to the hospital saw their PCP within 4 weeks of hospital discharge. Of 13 patients without a PCP on admission, only 3 obtained 4‐week PCP follow‐up. When we examined our study outcomes for subjects stratified by the presence of an established PCP prior to hospitalization, we found univariate association with timely post‐discharge PCP follow‐up (56% of those with established PCP vs. 23% of those without, P = 0.04), but no difference in readmission rates or hospital LOS.
Severity of patient illnessmeasured using hospital data and the case mix index (CMI)of the 3 patient populations (screened, enrolled, final) was quite similar. The CMI (mean) for the 121 screened patients was 1.23. The CMI for the 75 enrolled patients was 1.27. And the CMI in the 65 final study patients was 1.25. When evaluating illness severity (CMI) of patients in relation to hospital LOS between the 2 final study populations, the CMI (median) was also similar: 1.15 for the 32 patients with timely PCP follow‐up vs. 1.11 for the 33 patients without timely PCP follow‐up.
We found no association when looking at the rate of timely PCP follow‐up based on admitting service attending, or acute vs. acute on chronic diagnosis.
Barriers to PCP follow‐up most frequently cited by study patients were: lacking a PCP (no established PCP prior to hospital, no insurance, out of town, recently changed insurance), could not get an appointment, discharged to a half‐way house, and saw another doctor (specialist unrelated to discharge diagnosis).
Discussion
A growing body of work highlights the role of multiple, varied interventions at, or following discharge, in improving outcomes during the transition from inpatient to outpatient care. Examples include care coordination by advanced nurse practitioners, follow‐up pharmacist phone calls, and involvement of a transition coach encouraging active patient involvementall are known to improve patient outcomes following a hospitalization.1418 The active involvement of a PCP is central to a number of these proven interventions to ensure effective completion of ongoing patient care. And while some previous studies suggest increased overall resource utilization when PCP follow‐up occurs after hospitalization,19 the level of fragmented care that occurs in today's hospitalized patient, as well as the fact many patients lack PCP care at all, raises questions about clinical outcomes after hospitalization related to timely PCP follow‐up. The issue of appropriateness of resources utilized has also not been adequately explored.
Within this context, this study examines the role that PCP follow‐up might play in such interventions and its' effects on patient outcomes. Notably, in this urban academic medical center, we found that timely PCP follow‐up after hospital discharge occurred in fewer than half of general medical inpatients. Lack of timely PCP follow‐up was associated with increased hospital readmission for the same condition and a trend toward a longer index hospital LOS.
While this small study cannot fully elucidate the impact of lack of timely PCP follow‐up on post‐discharge care, our findings suggest some mechanisms by which lack of timely PCP follow‐up might result in poor outcomes. For instance, patients lacking a PCP visit after discharge may not obtain needed follow‐up care in the post‐discharge period, leading to clinical deterioration and hospital readmission. Uninsured patients may be at particular risk for failed transition because they are less likely to have consistent PCP access, whether as an already established patient or one newly assigned.20, 21 Perhaps a larger study would better demonstrate statistical significance in reflecting the association between uninsured patients, lack of a PCP, and post‐discharge follow‐up deficiencies. There may, in fact, be issues related to patient attitudes and beliefs, such as subjectively feeling better or even an implicit distrust of the healthcare system among the uninsured, that exist as well. Even among patients with a PCP prior to hospitalization, PCP follow‐up after hospital discharge may be lacking due to modifiable factors such as patient attitudes and beliefs and logistical barriers in arranging follow‐up.
Patients without potential for timely PCP follow‐up might be kept in the hospital longer to ensure they are well enough medically to sufficiently meet their own follow‐up needs. Hospital LOS might be increased by providers to compensate for the lack of PCP follow‐up. Alternatively, these patients may be sicker with their index hospitalization.
It is not surprising that payer source appears to influence a patients' ability to obtain timely PCP follow‐up. It is well documented that uninsured patients have higher healthcare resource utilization.2224 Lack of access to primary care in such patients contributes to a cycle of using the most expensive sites of care. In our study, we found many of the patients lacking timely PCP follow‐up were younger, perhaps reflecting the same patient population who have higher rates of being uninsured. Conversely, older patients are more likely to have PCP access, in large part due to having Medicare benefits (although this dynamic has shown a shift in recent years). The uninsured may present sicker as a result of lacking pre‐hospital PCP access or transportation to a PCP visit.
Limitations
This study was performed at a single, academic institution limiting its' generalizability. In addition, this small cohort study, which took place over four winter months, may have implicit biases toward certain disease entities and follow‐up issues unique to study size and season. The small study size was dictated by a finite amount of available resources, potentially contributing to minor inconsistencies with some of the results. While statistical significance was still seen with many of our results, a much larger study may better enhance the study outcomes.
It also remains unclear why the effects of PCP follow‐up were evident for a patient's same condition, but not for any condition. The distinction between designations is potentially subjective and may be difficult to accurately determine. Most existing readmission studies in the literature assign readmission for any condition. A future, larger study may be able to examine whether this difference exists between same vs. any condition.
As an academic medical center, access to specialty clinics may be facilitated, thus increasing PCP follow‐up in patients who might otherwise not have it available to them. Additionally, our subjects were limited to a convenience sample of the population of the general medicine wards and may not be representative of all medical inpatients. Patients lacking a telephone were missed. We relied on patient recollection and self‐report of PCP follow‐up visits and re‐hospitalizations. While we acknowledge limitations of patient self‐report, both in communication and comprehension, we believe patients are reasonably able to report on whether or not they were readmitted to the hospital, the cause of their readmission and whether/when they had PCP follow‐up. Patient self‐report could be collected systematically and without long time lags. Finally, the research team did not have reliable access to readmission data for hospitals other than the facility in which the study was conducted.
It is possible patients readmitted early after discharge may have been counted as lacking PCP follow‐up simply because the readmission occurred so soon after discharge precluding the opportunity for PCP follow‐up to occur. The effects of patients having non‐PCP (home health nurse, pharmacist, phone advice) follow‐up after hospital discharge were not examined.
Also, LOS and readmission to a hospital may be more a reflection of disease severity than the absence of PCP follow‐up, ie, patients ultimately readmitted after hospital discharge may have been a sicker subset of patients upon index hospitalization.
In this urban academic medical center, discharged medicine patients commonly lack timely PCP follow‐up. The lack of timely PCP follow‐up after hospital discharge was associated with higher rates of readmission and a non‐significant trend toward longer hospital lengths of stay. Hospital discharge represents a period of significant risk in patient care necessitating the effective continuation of treatment plans including follow‐up of laboratory, radiology or other testing, and management by a variety of providers. PCPs may play a crucial role in care coordination during this period. Structured intervention performed at the time of discharge might increase post‐hospital PCP access and facilitate timely PCP follow‐up to ensure continuity of needed care after hospital discharge in the most vulnerable patients. Such interventions might include systems improvements, such as increasing PCP access in the post‐hospital period, to increase the likelihood that complex needs are met at a vulnerable period in patient care.
A more effective handoff between inpatient and outpatient settings may ultimately improve clinical outcomes, diminish resource utilization, and decrease overall healthcare costs.
Acknowledgements
The authors thank Traci Yamashita and Karen Mellis, Professional Research Assistants.
- , , , .The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;13:161–167.
- , , , .Medical errors related to discontinuity of care from an inpatient to outpatient setting.J Gen Intern Med.200318:646–651.
- , .The high cost users of medical care.N Engl J Med.1980;302:996–1002.
- , .The rate and cost of hospital readmissions for preventable conditions.Med Care Res Rev.2004;61:225–240.
- , , .Rehospitalizations among patients in the medicare fee‐for‐service program.N Engl J Med.2009;360;14:1418–1428.
- , , .Tying up loose ends. Discharging patients with unresolved medical issues.Arch Intern Med.2007;167:1305–1311.
- , .Post‐hospitalization followup appointment‐keeping among the medically indigent.J Community Health.1993;18(5):271–282.
- , , .Factors related to the keeping of appointments by indigent clients.J Health Care Poor Underserved.1993;4(1):21–39.
- , , , , , .Inpatient to outpatient transfer of diabetes care: perceptions of barriers to postdischarge followup in urban African American patients.Ethn Dis.2007;17(2):238–243.
- , , .Effect of a nurse case manager on postdischarge follow‐up.J Gen Intern Med.1996v;11(11):684–688.
- , , .Emergency department visits by persons recently discharges from U.S. hospitals.Natl Health Stat Report.2008;(6):1–9.
- , , .Comparing uninsured and privately insured hospital patients: admission severity, health outcomes and resource use.Health Serv Manage Res.2001;14(3):203–210.
- , , .Comparison of uninsured and privately insured hospital patients. Condition on admission, resource use, and outcome.JAMA.1991;265(3):374–379.
- , , .Effect of a nurse case manager on postdischarge follow‐up.J Gen Intern Med.1996;11:684–688.
- , , , .The care transitions intervention: results of a randomized controlled trial.Arch Intern Med.2006;166(17):1822–1828.
- , , , , , .A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333:1190–1195.
- , , , .Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631.
- , , , et al.A reengineered hospital discharge program to decrease rehospitalization.Ann Intern Med.2009;150:178–187.
- , , .Does increased access to primary care reduce hospital readmissions?N Engl J Med.1996;334:1441–1447.
- , .Health insurance and access to health care in the united states.Ann NY Acad Sci.1008;1136:149–160.
- , .2006.Summary health statistics for U.S. adults: National Health Interview Survey. 2005, NCHS/CDC/USDHHS, Vital Health Statistics, Series 10.
- , , .Emergency department visits by persons recently discharges from U.S. hospitals.Natl Health Stat Report.2008;(6):1–9.
- , , .Comparison of uninsured and privately insured hospital patients. Condition on admission, resource use, and outcome.JAMA.1991;265(3):374–379.
- , , .Comparing uninsured and privately insured hospital patients: admission severity, health outcomes and resource use.Health Serv Manage Res.2001;14(3):203–210.
- , , , .The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;13:161–167.
- , , , .Medical errors related to discontinuity of care from an inpatient to outpatient setting.J Gen Intern Med.200318:646–651.
- , .The high cost users of medical care.N Engl J Med.1980;302:996–1002.
- , .The rate and cost of hospital readmissions for preventable conditions.Med Care Res Rev.2004;61:225–240.
- , , .Rehospitalizations among patients in the medicare fee‐for‐service program.N Engl J Med.2009;360;14:1418–1428.
- , , .Tying up loose ends. Discharging patients with unresolved medical issues.Arch Intern Med.2007;167:1305–1311.
- , .Post‐hospitalization followup appointment‐keeping among the medically indigent.J Community Health.1993;18(5):271–282.
- , , .Factors related to the keeping of appointments by indigent clients.J Health Care Poor Underserved.1993;4(1):21–39.
- , , , , , .Inpatient to outpatient transfer of diabetes care: perceptions of barriers to postdischarge followup in urban African American patients.Ethn Dis.2007;17(2):238–243.
- , , .Effect of a nurse case manager on postdischarge follow‐up.J Gen Intern Med.1996v;11(11):684–688.
- , , .Emergency department visits by persons recently discharges from U.S. hospitals.Natl Health Stat Report.2008;(6):1–9.
- , , .Comparing uninsured and privately insured hospital patients: admission severity, health outcomes and resource use.Health Serv Manage Res.2001;14(3):203–210.
- , , .Comparison of uninsured and privately insured hospital patients. Condition on admission, resource use, and outcome.JAMA.1991;265(3):374–379.
- , , .Effect of a nurse case manager on postdischarge follow‐up.J Gen Intern Med.1996;11:684–688.
- , , , .The care transitions intervention: results of a randomized controlled trial.Arch Intern Med.2006;166(17):1822–1828.
- , , , , , .A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333:1190–1195.
- , , , .Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631.
- , , , et al.A reengineered hospital discharge program to decrease rehospitalization.Ann Intern Med.2009;150:178–187.
- , , .Does increased access to primary care reduce hospital readmissions?N Engl J Med.1996;334:1441–1447.
- , .Health insurance and access to health care in the united states.Ann NY Acad Sci.1008;1136:149–160.
- , .2006.Summary health statistics for U.S. adults: National Health Interview Survey. 2005, NCHS/CDC/USDHHS, Vital Health Statistics, Series 10.
- , , .Emergency department visits by persons recently discharges from U.S. hospitals.Natl Health Stat Report.2008;(6):1–9.
- , , .Comparison of uninsured and privately insured hospital patients. Condition on admission, resource use, and outcome.JAMA.1991;265(3):374–379.
- , , .Comparing uninsured and privately insured hospital patients: admission severity, health outcomes and resource use.Health Serv Manage Res.2001;14(3):203–210.
Copyright © 2010 Society of Hospital Medicine
Post‐Discharge Inpatients With Depressive Symptoms
Fully 19% of Medicare patients are readmitted to the hospital within 30 days of discharge.1 This represents a large amount of potentially avoidable morbidity and cost. Indeed, projects to improve the discharge process and post‐hospital care have shown that as much as one‐third of hospital utilization in the month after discharge can be avoided.2 Consequently, the rate of early, unplanned hospital utilization after discharge has emerged as an important indicator of hospital quality and the Centers for Medicare and Medicaid Services (CMS) has proposed a policy to decrease payments to hospitals with high rates of early unplanned hospital utilization. Thus, there is great interest in identifying modifiable risk factors for rehospitalization that could be used to refine intervention models and lead to improvements in quality of care, patient outcomes, and cost savings.
To date, known predictors of readmission include: lower socioeconomic status,3 history of prior hospitalization4 and advanced age,5 length of stay greater than 7 days,6 a high burden of comorbid illnesses (based on Charlson score),7 poor social support,8 and specific diagnoses (eg, congestive heart failure, chronic obstructive pulmonary disease [COPD] and myocardial infarction).5, 9, 10 In addition, unplanned readmissions and emergency department (ED) visits have been linked to polypharmacy and adverse drug events related to treatment with medications such as warfarin, digoxin and narcotics.11, 12 Another characteristic that has also been linked to readmission is depression;13 however5 reports supporting this association are from studies of elderly patients or with patients who have specific diagnoses (eg, congestive heart failure [CHF], COPD, myocardial infarction).1416
Depression is common, affecting 13% to 16% of people in the US, and is recognized as an important risk factor for poor outcomes among patients with various chronic illnesses.1719 The mechanisms by which depression can be linked to health outcomes and health service utilization have been studied in age‐specific or disease‐specific cohorts such as cardiac patients or frail elders and include both physiologic factors such as hypercoagulability and hyperinflammatory conditions, as well as behavioral factors such as poor self‐care behaviors and heightened sensitivity to somatic symptoms. How these mechanisms link depression to health outcomes and hospital utilization in a general medical population is not clearly understood. Kartha et al.13 reported findings indicating that depression is a risk factor for rehospitalization in general medical inpatients, but the study sample was relatively small and the study design methodology significantly limited its generalizability.12 It would be useful to provide supporting evidence showing depression as an important risk factor for readmission in the general medical in‐patient population using more rigorous study methods and a larger cohort.
We hypothesized that depressive symptoms would be an independent risk factor for early unplanned hospital utilization after discharge for all medical patients. Therefore, we conducted a secondary analysis of the Project RED clinical trial dataset to assess the association between a positive depression screen during inpatient hospitalization and the rate of subsequent hospital utilization.
Methods
Data from the Project RED clinical trial were reviewed for inclusion in a secondary analysis. Complete data were available for 738 of the 749 subjects recruited for Project RED.
Project RED Setting and Participants
Project RED was a two‐armed randomized controlled trial of English‐speaking adult patients, 18 years or older, admitted to the teaching service of Boston Medical Center, a large urban safety‐net hospital with an ethnically diverse patient population. A total of 749 subjects were enrolled and randomized between January 3, 2006 and October 18, 2007. Patients were required to have a telephone, be able to comprehend study details and the consent process in English, and have plans to be discharged to a US community. Patients were not enrolled if they were admitted from a skilled nursing facility or other hospital, transferred to a different hospital service prior to enrollment, admitted for a planned hospitalization, on hospital precautions, on suicide watch, deaf or blind. The Institutional Review Board of Boston University approved all study activities. A full description of the methods for the Project RED trial has been described previously.2
Outcome Variable
The primary endpoint was rate of hospital utilization within 30 days of discharge from the index admission, defined as the total number of ED visits and readmissions per subject within 30 days of the index discharge. Hospital utilization rates within 60 and 90 days of the index hospitalization discharge were also analyzed as secondary outcomes. Any ED visit in which a subject was subsequently admitted to the hospital was only counted as a readmission. Outcome data were collected by reviewing the hospital's electronic medical records (EMRs) and by contacting subjects by telephone 30 days after discharge. Dates of hospital utilization occurring at Boston Medical Center were obtained from the EMR, while those at other hospitals were collected through subject report. Subjects who could not be reached within 60 days of discharge were assumed alive.
Primary Independent Variable
The primary independent variable of interest was depressive symptoms defined as a positive score for minor or major depression on the nine‐item Patient Health Questionnaire (PHQ‐9) depression screening tool.20 A dichotomized variable was created using a standardized scoring system to determine the screening cut‐off for major or minor depressive symptoms.19
Statistical Analysis
Demographic and other characteristics of the subjects were compared by depression status (Table 1). Potential confounders were identified a priori from the available literature on factors associated with rehospitalization. These included age, gender, marital status, health literacy score (rapid estimate of health literacy in adult medicine tool [REALM]),21 Charlson score,22 insurance type, employment status, income level, homelessness status within past three months, hospital utilization within the 6 months prior to the index hospitalization, educational attainment, length of hospital stay and Project RED study group assignment. Bivariate analyses were conducted to determine which covariates were significant confounders of the relationship between depression and hospital utilization within 30 days of discharge. Chi‐square tests were used for categorical variables and t‐tests for continuous variables.
| Characteristic | Depression Screen* | ||
|---|---|---|---|
| Negative (n = 500) | Positive (n = 238) | P Value | |
| |||
| Race, No. (%) | |||
| White | 140 (30) | 66 (30) | |
| Black | 268 (58) | 117 (54) | |
| Hispanic | 47 (10) | 29 (13) | 0.760 |
| Insurance, No. (%) | |||
| Private | 95 (19) | 22 (9) | |
| Medicare | 69 (14) | 30 (13) | |
| Medicaid | 214 (43) | 143 (61) | |
| Free care | 118 (24) | 40 (17) | <0.001 |
| Education, No. (%) | |||
| <8th grade | 33 (7) | 21 (9) | |
| Some high school | 82 (17) | 52 (22) | |
| High school grad | 192 (38) | 90 (38) | |
| Some college | 126 (25) | 51 (22) | |
| College grad | 67 (13) | 22 (9) | 0.135 |
| Health Literacy | |||
| Grade 3 and below | 64 (13) | 44 (19) | |
| Grade 46 | 54 (11) | 22 (10) | |
| Grade 78 | 156 (32) | 73 (32) | |
| Grade 9 and above | 213 (44) | 89 (39) | 0.170 |
| Income, $, No. (%) | |||
| No income | 61 (12) | 37 (16) | |
| <10K | 77 (15) | 61 (26) | |
| 1020K | 96 (19) | 35 (15) | |
| 2050K | 97 (19) | 34 (14) | |
| 50100K | 35 (8) | 7 (2) | |
| No answer | 132 (27) | 64 (27) | 0.002 |
| Employment status, No. (%) | |||
| Full time | 142 (28) | 34 (14) | |
| Part time | 57 (11) | 30 (13) | |
| Not Working | 297 (59) | 171 (72) | <0.001 |
| Age, mean (SD), years | 49.9 (16.0) | 49.6 (13.3) | 0.802 |
| Gender: No. (%) Female | 239 (48) | 133 (56) | 0.040 |
| Have PCP, No. (%) Yes | 399 (80) | 197 (83) | 0.340 |
| Marital status,∥ No. (%) unmarried | 365 (73) | 201 (85) | <0.001 |
| Charlson score, mean (SD) | 1.058 (1.6) | 1.56 (2.39) | 0.001 |
| RED study group,# No. (%) | |||
| Intervention | 243 (49) | 127 (53) | 0.22 |
| Length of stay, days, mean (SD) | 2.5 (2.8) | 3.1 (3.8) | 0.016 |
| Homeless in last 3 months, No. (%) | 45 (9) | 30 (13) | 0.130 |
| Frequent utilizer,** No. (%) | 159 (32) | 104 (44) | 0.002 |
Age, length of stay, and Charlson score were used as continuous variables. Gender, marital status, frequent prior utilization (01 vs. 2 or more), and homelessness were treated as dichotomous variables. Categorical variables were created for, educational attainment (less than eighth grade, some high school, high school graduate, some college, college graduate), insurance type (Medicare, Medicaid, private insurance or free care), income level (no income, less than $10,000 per year, $10,00020,000, $20,00050,000, $50,000100,000, no answer), level of health literacy (grade 3 and below, grade 46, grade 78, grade 9 or above) and employment status(working full‐time, working part‐time, not working, no answer).
The 30‐day hospital utilization rate reflects the number of hospital utilization events within 30 days of discharge per subject. The same method was used to calculate hospital utilization rates within 60 and 90 days of discharge respectively. The unadjusted incident rate ratio (IRR) was calculated as the ratio of the rate of hospital utilizations among patients with depressive symptoms versus patients without depressive symptoms. Data for hospital utilization at 30, 60, and 90 days are cumulative.
Poisson models were used to test for significant differences between the predicted and observed number of hospitalization events at 30 days. A backward stepwise regression was conducted to identify and control for relevant confounders and construct the final, best‐fit model for the association between depression and hospital reutilization. A statistical significance level of P = 0.10 was used for the stepwise regression. To evaluate potential interactions between depression and the Project RED intervention, interaction terms were included. Two‐sided significance tests were used. P values of less than 0.05 were considered to indicate statistical significance. All data were analyzed with S‐Plus 8.0 (Seattle, WA).
In addition, a Kaplan‐Meier hazard curve was generated for the first hospital utilization event, ED visit or readmission, for the 30‐day period following discharge and compared with a log‐rank test.
Results
A total of 28% of subjects were categorized as having a positive depression screen. More women (36%) had positive depression screens than men (28%). Among patients with a positive depression screen, 58% had a history of depression and 53% were currently taking medications at the time of enrollment, compared with 25% and 22% respectively for subjects with a negative depression screen. Table 1 presents the means or percentages for baseline characteristics by depression status in the analytic cohort. Subjects with Medicaid for insurance had a higher rate of depression (61%) than subjects with Medicare (13%), private insurance (9%), or those who qualified for the Free Care pool (17%) which is the Massachusetts state funding for healthcare to uninsured persons. Subjects who were unemployed, unmarried, or who reported earnings less than $10,000 per year were also more likely to screen positive for depression. In addition, depressed subjects had a higher severity of co‐morbid disease and longer length of stay for the index hospitalization. Patients categorized as frequent utilizers (2 or more prior admissions) for the 6 months prior to the index hospitalization were also more likely to be depressed. Of further note, is the relatively younger average age among both depressive patients (49.6 years) and non‐depressive patients (49.9) of these study subjects.
The unadjusted hospital utilization rate at 30, 60, and 90 days post‐discharge by depression status is shown in Table 2. At 30 days post‐discharge, those with depressive symptoms had a higher rate of hospital utilization than those without depressive symptoms (0.563 vs. 0.296). In other words, 56 utilization events occurred per 100 patients with depressive symptoms, compared with 30 utilization events per 100 patients without depressive symptoms. The unadjusted 30‐day post‐discharge hospital utilization rate among those with depressive symptoms was higher compared with those without symptoms (IRR, 1.90, 95% confidence interval [CI], 1.242.71). A similar trend was found among subjects at 60 and 90 days post‐discharge.
| Hospital Utilization | Depression Screen* | P Value | IRR (CI) | |
|---|---|---|---|---|
| Negative, n = 500 (68%) | Positive, n = 238 (32%) | |||
| ||||
| No. of hospital utilizations | 140 | 134 | 1.90 (1.51,2.40) | |
| 30‐day hospital utilization rate | 0.296 | 0.563 | <0.001 | |
| No. of hospital utilizations | 231 | 205 | 1.87 (1.55,2.26) | |
| 60‐day hospital utilization rate | 0.463 | 0.868 | <0.001 | |
| No. of hospital utilizations | 324 | 275 | 1.79 (1.53,2.10) | |
| 90‐day hospital utilization rate | 0.648 | 1.165 | <0.001 | |
Poisson regression analyses were conducted to control for potential confounding in the relationship between depressive symptoms and hospital utilization rate within 30 days after discharge (Table 3). After controlling for relevant confounders, including age, gender, employment status, frequent prior hospitalization status, marital status, Charlson score, Project RED study group assignment and the interaction variable for RED study group assignment and depression, the association between symptoms of depression, and hospital utilization rate remained significant (IRR, 1.73; 95% CI, 1.272.36).
| Characteristics | IRR | CI | P Value |
|---|---|---|---|
| |||
| Depression symptoms* | <0.001 | ||
| Positive | 1.73 | 1.272.36 | |
| Negative | REF | 1.0 | |
| Gender | <0.001 | ||
| Male | 1.87 | 1.472.40 | |
| Female | REF | 1.0 | |
| Marital status | 0.005 | ||
| Married | 0.625 | 0.440.89 | |
| Unmarried | 1.0 | REF | |
| Frequent utilizer | <0.001 | ||
| 2+ prior visits | 2.45 | 1.923.15 | |
| <2 prior visits | 1.0 | REF | |
| Study group | 0.054 | ||
| Intervention | 0.76 | 0.551.06 | |
| Control | 1.0 | REF | |
| Employment | |||
| Part time | 1.40 | 0.852.30 | 0.095 |
| Not working | 1.67 | 1.152.44 | 0.003 |
| Other | 0.52 | 0.073.85 | 0.262 |
| Full time | 1.0 | REF | |
| Charlson Score∥ | 0.98 | 0.921.04 | 0.250 |
| Group* depression | 0.84 | 0.521.36 | 0.236 |
| Age | 1.00 | 0.991.01 | 0.375 |
Figure 1 depicts the Kaplan‐Meier hazard curve generated for time to first hospital utilization, stratified by depression status. While 21% of participants without symptoms of depression had a hospital utilization within 30 days, fully 29% of participants with symptoms of depression had a hospital utilization within 30 days (P = 0.011).
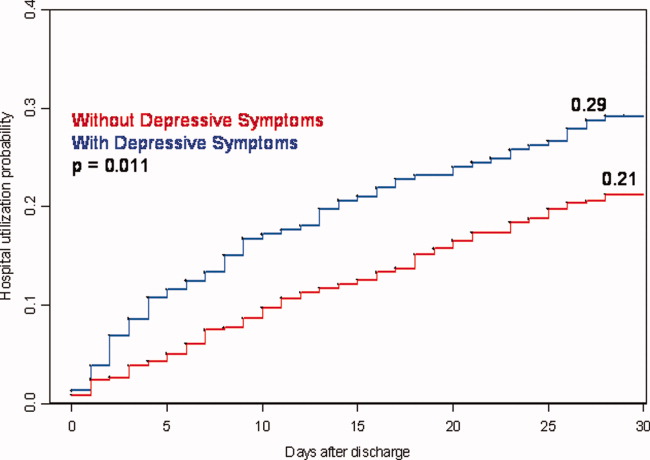
Discussion
Our study shows hospitalized patients who screen positive for depressive symptoms are significantly more likely to have a hospital visit (emergency room or rehospitalization) within 30 days of discharge than those who do not screen positive for depressive symptoms among medical patients admitted to an urban, academic, safety‐net hospital. These findings are consistent with, and extend, prior reports regarding depression and rehospitalization in specific populations (ie, geriatrics) and specific diagnoses (ie, cardiovascular disease [CVD] and COPD).1012 We observed a 73% higher incidence rate for hospital utilization within 30 days of discharge for those with symptoms of depression. This puts symptoms of depression on par with frequent prior rehospitalization, advanced age and low social support, as known risk factors for rehospitalization.4, 5, 23
Also of significance is the relatively young age of this study population (49.9 years non‐depressive patients and 49.6 years for depressive patients) compared with the study cohorts used for research in the majority of the existing literature. The chief reason for the young age of our cohort is that potential subjects were excluded if they came from a skilled nursing facility or other hospital. This may limit the generalizability of our findings; however, it seems likely that interventions relating to depression and transitions of care will need to be quite different for patients that reside in long‐term care facilities vs. patients that live in the community. For example, patients living in the community may have significant barriers to access post‐discharge services due to insurance status and are more likely to be sensitive to variations in social support.
Early rehospitalization is associated with significant morbidity, mortality, and expense. It is also a potential marker for poor quality of care.24 Concerns for patient safety, escalating healthcare costs, and possible change in hospital reimbursement mechanisms are fueling the search for modifiable risk factors associated with early rehospitalization. Our data provide evidence that symptoms of depression may be an important focus of attention. We do not know, however whether treating hospitalized patients who screen positive for depression will decrease early rehospitalization and emergency room utilization rates.
Various physiologic and behavioral mechanisms may link symptoms of depression to hospital utilization after discharge. For example, depressed patients with features of somatization may be more likely to experience worrisome physical symptoms after discharge and present prematurely for reevaluation. Patients who are sicker in some fashion not captured by our measured confounders may have symptoms of depression related to chronic, debilitating disease warranting early return to the hospital. Depression may also yield nonadherence to aspects of the discharge treatment plan leading to rehospitalization as a result of poor post‐discharge disease management. For example, research shows that patients with depression following coronary artery bypass surgery are less likely to adhere with cardiac rehabilitation programs.25 Likewise, depression among chronically ill patients such as diabetics, asthmatics, or human immunodeficiency virus (HIV)‐positive patients impairs medication adherence and self‐care behavior which may lead to disease relapse or recurrence.2628 One study examining depression effects on hypertensive medicine adherence in African Americans identified self‐efficacy as a mediating factor between depression and nonadherence.29 This implies that interventions such as self‐management education, a program through which chronically‐ill patients learn to better manage their illnesses through enhanced self‐confidence and problem‐solving strategies (including mood disorder challenges) may reduce early rehospitalization among depressed patients.30
There is also evidence that depression may have direct physiologic consequences. In patients with CVD, depression is associated with poor outcomes possibly related to decreased heart rate variability, hypercoagulability, high burdens of inflammatory markers, and severity of left ventricular dysfunction.3134 Similarly, depression among HIV/acquired immune deficiency syndrome (AIDS), diabetics and multiple sclerosis (MS) patients is linked to heightened levels of proinflammatory markers and less favorable outcomes that may signal a more severe form of the disease or an impaired response to treatment.3538 Indeed, MS investigators now hypothesize that the proinflammatory environment associated with the neurologic manifestations of MS are also causing depression symptoms among MS patients.34 This theory contrasts the common belief that depression in the chronically ill manifests independent of the chronic illness or in response to living with chronic disease.
A major strength of the current study is the large dataset and the broad range of covariates available for analyses. However, several limitations should be noted. First, data on hospital utilization outside Boston Medical Center were determined by patient self‐report and were not confirmed by document review. Second, we do not know the direction of the associations we report. If symptoms of depression are merely the consequence of having a higher disease burden, treatment of the underlying disease may be the most important response. While this is possible, our model does include several variables (eg, Charlson score and length of stay) that are likely to adjust for disease severity, pointing to the likelihood that symptoms of depression truly predict hospital utilization in a fashion that is independent of disease severity. Third, our results may not be generalizable to populations other than those served by urban safety‐net hospitals or other populations excluded from the Project RED trial (eg, non‐English speaking patients and patients from nursing homes). Finally, social factors such as substance use and social support system variables may residually confound the relationship between depression and hospital reutilization demonstrated in this study. While this dataset does not include a measure of social support other than marital status and housing status, data is available on substance use. Analyses conducted by our colleagues using Project RED data found that in this study population depression was significantly more prevalent among substance users (29% vs. 14%) compared with non‐users and that substance use is an independent risk factor for hospital reutilization (unpublished data).
Our findings linking depression to increased hospital utilization also warrant further consideration from healthcare policymakers. Central to the Obama Administration's February 2009 healthcare reform proposal is the pursuit of cost savings through reductions in unplanned hospital readmissions.39 Thus, identifying potentially modifiable risk factors for readmission, such as depression, is of great concern to healthcare providers and policymakers across the nation. If, through testing of interventions, depression proves to be a modifiable risk for readmission, policymakers, while negotiating healthcare reform measures, must provide for the services required to address this comorbidity at the time of discharge. For example, if a patient screens positive for depressive symptoms during a hospitalization for COPD exacerbation, will the proposed payment reforms allow for mental health services during the immediate post‐discharge period in order to reduce the likelihood of hospital readmission? Will those mental health services be readily available? Payment reforms that account for all necessary transitional care services will indeed help reduce readmission costs with less risk for untoward consequences.
In conclusion, our results indicate that a positive depression screen is a significant risk factor for early post‐discharge hospital utilization among hospitalized adults on a general medical service, even after controlling for relevant confounders. Screening for depression during acute hospitalizations may be an important step in identifying patients at increased risk for readmission. Future research should focus on further characterizing and stratifying populations at highest risk for depression. Efforts should also include developing and evaluating targeted interventions for patients with symptoms of depression among hospitalized patients as part of discharge planning. Timely depression therapy during the hospitalization or following hospital discharge might reduce costly readmissions and enhance patient safety.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360(14):1457–1459.
- ,,, et al.The reengineered hospital discharge program to decrease rehospitalization.Ann Intern Med.2009;150(3):178–187.
- ,,.The impact of patient socioeconomic status and other social factors on readmission: a prospective study in four Massachusetts hospitals.Inquiry.1994;31(2):163–172.
- ,,,.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631. [PMID: 15209600]
- ,,,,,.Factors associated with unplanned hospital readmission among patients 65 years of age and older in a Medicare managed care plan.Am J Med.1999;107(1):13–17.
- ,,, et al.Readmission after hospitalization for congestive heart failure among Medicare beneficiaries.Arch Intern Med.1997;157(1):99–104.
- ,,.Chronic comorbidity and outcomes of hospital care: length of stay, mortality and readmission at 30 and 365 days.J Clin Epidemiol.1999;52(3):171–179.
- ,,, et al.Social network as a predictor of hospital readmission and mortality among older patients with heart failure.J Card Fail.2006;12:621–627.
- ,,,,.Acute exacerbation of chronic obstructive pulmonary disease: influence of social factors in determining length of stay and readmission rates.Can Respir J.2008;15(7):361–364.
- ,,, et al.Time course of depression and outcome of myocardial infarction.Arch Intern Med.2006;166(18):2035–2043.
- ,,, et al.Medication use leading to emergency department visits for adverse drug events in older adults.Ann Intern Med.2007;147(11):755–765.
- ,,.A systematic literature review of factors affecting outcomes in older medical patients admitted to hospital.Age Ageing.2004;33(2):110–115.
- ,,, et al.Depression is a risk factor for rehospitalization in medical inpatients.Prim Care Companion J Clin Psychiatry.2007;9(4):256–262.
- ,,, et al.Risk factors for hospital readmission in patients with chronic obstructive pulmonary disease.Respiration.2006;73:311–317.
- ,,, et al.Depression and healthcare costs during the first year following myocardial infarction.J Psychosom Res.2000;48(4–5):471–478.
- ,,, et al.Relationship of depression to increased risk of mortality and rehospitalization.Arch Intern Med.2001;161(15):1849–1856.
- ,,, et al.Time course of depression and outcome of myocardial infarction.Arch Intern Med.2006;166:2035–2043.
- ,.Single item on positive affect is associated with 1‐year survival in consecutive medical inpatients.J Gen Hosp Psych.2009;31:8–13.
- ,,,.Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions.Arch Gen Psychiatry.2005;62(10):1097–106.
- ,,.The PHQ‐9: Validity of a brief depression severity measure.J Gen Intern Med.2001;16:606–613. [PMID:11556941]
- ,,, et al.Rapid estimate of adult literacy in medicine: a shortened screening instrument.Fam Med.1993;25:391–395. [PMID:8349060]
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40:373–383. [PMID: 3558716]
- ,,, et al.Social network as a predictor of hospital readmission and mortality among older patients with heart failure.J Card Fail.2006;12(8):621–627.
- ,,,,.The association between the quality of inpatient care and early readmission: a meta‐analysis of the evidence.Med Care.1997;35(10):1044–1059.
- ,,, et al.Persistent depression affects adherence to secondary prevention behaviors after acute coronary syndromes.J Gen Intern Med.2006;21(11):1178–1183.
- ,,,,.Depression is an important contributor to low medication adherence in hemodialyzed patients and transplant recipients.Kidney Int.2009;75(11):1223–1229.
- ,,, et al.Symptoms of depression prospectively predict poorer self‐care in patients with Type 2 diabetes.Diabet Med.2008;25(9):1102–1107.
- ,,, et al.The effect of adherence on the association between depressive symptoms and mortality among HIV‐infected individuals first initiating HAART.AIDS.2007;21(9):1175–1183.
- ,,.Self‐efficacy mediates the relationship between depressive symptoms and medication adherence.Health Educ Behav.2009;36(1):127–137.
- ,,,.Patient self‐management of chronic disease in primary care.JAMA.2002;288(19):2469–2475.
- .Effects of sertraline on the recovery rate of cardiac autonomic function in depressed patients after acute myocardial infarction.Am Heart J.2001;142:617–623.
- ,,, et al.Relationship between left ventricular dysfunction and depression following myocardial infarction: data from the MIND‐IT.Eur Heart J.2005;26:2650–2656.
- ,,, et al.Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acugte coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet SubStudy.Circulation.2003;108:939–944.
- ,.Inflammation in acute coronary syndromes.Cleve Clin J Med.2002;69(Suppl2):SII130–SII142.
- ,.Depression and immunity: inflammation and depressive symptoms in multiple sclerosis.Neurol Clin.2006;24(3):507–519.
- ,,, et al.Synergistic effects of psychological and immune stressors on inflammatory cytokines and sickness responses in humans.Brain Behav Immun.2009;23(2):217–224.
- ,,,,,.Psychological distress, killer lymphocytes and disease severity in HIV/AIDS.Brain Behav Immun.2008;22(6):901–911.
- ,,,.Analysis of potential predictors of depression among coronary heart disease risk factors including heart rate variability, markers of inflammation, and endothelial function.Eur Heart J.2008;29(9):1110–1117.
- .Obama proposes $634 billion fund for health care.Washington Post. February 26,2009:A1.
Fully 19% of Medicare patients are readmitted to the hospital within 30 days of discharge.1 This represents a large amount of potentially avoidable morbidity and cost. Indeed, projects to improve the discharge process and post‐hospital care have shown that as much as one‐third of hospital utilization in the month after discharge can be avoided.2 Consequently, the rate of early, unplanned hospital utilization after discharge has emerged as an important indicator of hospital quality and the Centers for Medicare and Medicaid Services (CMS) has proposed a policy to decrease payments to hospitals with high rates of early unplanned hospital utilization. Thus, there is great interest in identifying modifiable risk factors for rehospitalization that could be used to refine intervention models and lead to improvements in quality of care, patient outcomes, and cost savings.
To date, known predictors of readmission include: lower socioeconomic status,3 history of prior hospitalization4 and advanced age,5 length of stay greater than 7 days,6 a high burden of comorbid illnesses (based on Charlson score),7 poor social support,8 and specific diagnoses (eg, congestive heart failure, chronic obstructive pulmonary disease [COPD] and myocardial infarction).5, 9, 10 In addition, unplanned readmissions and emergency department (ED) visits have been linked to polypharmacy and adverse drug events related to treatment with medications such as warfarin, digoxin and narcotics.11, 12 Another characteristic that has also been linked to readmission is depression;13 however5 reports supporting this association are from studies of elderly patients or with patients who have specific diagnoses (eg, congestive heart failure [CHF], COPD, myocardial infarction).1416
Depression is common, affecting 13% to 16% of people in the US, and is recognized as an important risk factor for poor outcomes among patients with various chronic illnesses.1719 The mechanisms by which depression can be linked to health outcomes and health service utilization have been studied in age‐specific or disease‐specific cohorts such as cardiac patients or frail elders and include both physiologic factors such as hypercoagulability and hyperinflammatory conditions, as well as behavioral factors such as poor self‐care behaviors and heightened sensitivity to somatic symptoms. How these mechanisms link depression to health outcomes and hospital utilization in a general medical population is not clearly understood. Kartha et al.13 reported findings indicating that depression is a risk factor for rehospitalization in general medical inpatients, but the study sample was relatively small and the study design methodology significantly limited its generalizability.12 It would be useful to provide supporting evidence showing depression as an important risk factor for readmission in the general medical in‐patient population using more rigorous study methods and a larger cohort.
We hypothesized that depressive symptoms would be an independent risk factor for early unplanned hospital utilization after discharge for all medical patients. Therefore, we conducted a secondary analysis of the Project RED clinical trial dataset to assess the association between a positive depression screen during inpatient hospitalization and the rate of subsequent hospital utilization.
Methods
Data from the Project RED clinical trial were reviewed for inclusion in a secondary analysis. Complete data were available for 738 of the 749 subjects recruited for Project RED.
Project RED Setting and Participants
Project RED was a two‐armed randomized controlled trial of English‐speaking adult patients, 18 years or older, admitted to the teaching service of Boston Medical Center, a large urban safety‐net hospital with an ethnically diverse patient population. A total of 749 subjects were enrolled and randomized between January 3, 2006 and October 18, 2007. Patients were required to have a telephone, be able to comprehend study details and the consent process in English, and have plans to be discharged to a US community. Patients were not enrolled if they were admitted from a skilled nursing facility or other hospital, transferred to a different hospital service prior to enrollment, admitted for a planned hospitalization, on hospital precautions, on suicide watch, deaf or blind. The Institutional Review Board of Boston University approved all study activities. A full description of the methods for the Project RED trial has been described previously.2
Outcome Variable
The primary endpoint was rate of hospital utilization within 30 days of discharge from the index admission, defined as the total number of ED visits and readmissions per subject within 30 days of the index discharge. Hospital utilization rates within 60 and 90 days of the index hospitalization discharge were also analyzed as secondary outcomes. Any ED visit in which a subject was subsequently admitted to the hospital was only counted as a readmission. Outcome data were collected by reviewing the hospital's electronic medical records (EMRs) and by contacting subjects by telephone 30 days after discharge. Dates of hospital utilization occurring at Boston Medical Center were obtained from the EMR, while those at other hospitals were collected through subject report. Subjects who could not be reached within 60 days of discharge were assumed alive.
Primary Independent Variable
The primary independent variable of interest was depressive symptoms defined as a positive score for minor or major depression on the nine‐item Patient Health Questionnaire (PHQ‐9) depression screening tool.20 A dichotomized variable was created using a standardized scoring system to determine the screening cut‐off for major or minor depressive symptoms.19
Statistical Analysis
Demographic and other characteristics of the subjects were compared by depression status (Table 1). Potential confounders were identified a priori from the available literature on factors associated with rehospitalization. These included age, gender, marital status, health literacy score (rapid estimate of health literacy in adult medicine tool [REALM]),21 Charlson score,22 insurance type, employment status, income level, homelessness status within past three months, hospital utilization within the 6 months prior to the index hospitalization, educational attainment, length of hospital stay and Project RED study group assignment. Bivariate analyses were conducted to determine which covariates were significant confounders of the relationship between depression and hospital utilization within 30 days of discharge. Chi‐square tests were used for categorical variables and t‐tests for continuous variables.
| Characteristic | Depression Screen* | ||
|---|---|---|---|
| Negative (n = 500) | Positive (n = 238) | P Value | |
| |||
| Race, No. (%) | |||
| White | 140 (30) | 66 (30) | |
| Black | 268 (58) | 117 (54) | |
| Hispanic | 47 (10) | 29 (13) | 0.760 |
| Insurance, No. (%) | |||
| Private | 95 (19) | 22 (9) | |
| Medicare | 69 (14) | 30 (13) | |
| Medicaid | 214 (43) | 143 (61) | |
| Free care | 118 (24) | 40 (17) | <0.001 |
| Education, No. (%) | |||
| <8th grade | 33 (7) | 21 (9) | |
| Some high school | 82 (17) | 52 (22) | |
| High school grad | 192 (38) | 90 (38) | |
| Some college | 126 (25) | 51 (22) | |
| College grad | 67 (13) | 22 (9) | 0.135 |
| Health Literacy | |||
| Grade 3 and below | 64 (13) | 44 (19) | |
| Grade 46 | 54 (11) | 22 (10) | |
| Grade 78 | 156 (32) | 73 (32) | |
| Grade 9 and above | 213 (44) | 89 (39) | 0.170 |
| Income, $, No. (%) | |||
| No income | 61 (12) | 37 (16) | |
| <10K | 77 (15) | 61 (26) | |
| 1020K | 96 (19) | 35 (15) | |
| 2050K | 97 (19) | 34 (14) | |
| 50100K | 35 (8) | 7 (2) | |
| No answer | 132 (27) | 64 (27) | 0.002 |
| Employment status, No. (%) | |||
| Full time | 142 (28) | 34 (14) | |
| Part time | 57 (11) | 30 (13) | |
| Not Working | 297 (59) | 171 (72) | <0.001 |
| Age, mean (SD), years | 49.9 (16.0) | 49.6 (13.3) | 0.802 |
| Gender: No. (%) Female | 239 (48) | 133 (56) | 0.040 |
| Have PCP, No. (%) Yes | 399 (80) | 197 (83) | 0.340 |
| Marital status,∥ No. (%) unmarried | 365 (73) | 201 (85) | <0.001 |
| Charlson score, mean (SD) | 1.058 (1.6) | 1.56 (2.39) | 0.001 |
| RED study group,# No. (%) | |||
| Intervention | 243 (49) | 127 (53) | 0.22 |
| Length of stay, days, mean (SD) | 2.5 (2.8) | 3.1 (3.8) | 0.016 |
| Homeless in last 3 months, No. (%) | 45 (9) | 30 (13) | 0.130 |
| Frequent utilizer,** No. (%) | 159 (32) | 104 (44) | 0.002 |
Age, length of stay, and Charlson score were used as continuous variables. Gender, marital status, frequent prior utilization (01 vs. 2 or more), and homelessness were treated as dichotomous variables. Categorical variables were created for, educational attainment (less than eighth grade, some high school, high school graduate, some college, college graduate), insurance type (Medicare, Medicaid, private insurance or free care), income level (no income, less than $10,000 per year, $10,00020,000, $20,00050,000, $50,000100,000, no answer), level of health literacy (grade 3 and below, grade 46, grade 78, grade 9 or above) and employment status(working full‐time, working part‐time, not working, no answer).
The 30‐day hospital utilization rate reflects the number of hospital utilization events within 30 days of discharge per subject. The same method was used to calculate hospital utilization rates within 60 and 90 days of discharge respectively. The unadjusted incident rate ratio (IRR) was calculated as the ratio of the rate of hospital utilizations among patients with depressive symptoms versus patients without depressive symptoms. Data for hospital utilization at 30, 60, and 90 days are cumulative.
Poisson models were used to test for significant differences between the predicted and observed number of hospitalization events at 30 days. A backward stepwise regression was conducted to identify and control for relevant confounders and construct the final, best‐fit model for the association between depression and hospital reutilization. A statistical significance level of P = 0.10 was used for the stepwise regression. To evaluate potential interactions between depression and the Project RED intervention, interaction terms were included. Two‐sided significance tests were used. P values of less than 0.05 were considered to indicate statistical significance. All data were analyzed with S‐Plus 8.0 (Seattle, WA).
In addition, a Kaplan‐Meier hazard curve was generated for the first hospital utilization event, ED visit or readmission, for the 30‐day period following discharge and compared with a log‐rank test.
Results
A total of 28% of subjects were categorized as having a positive depression screen. More women (36%) had positive depression screens than men (28%). Among patients with a positive depression screen, 58% had a history of depression and 53% were currently taking medications at the time of enrollment, compared with 25% and 22% respectively for subjects with a negative depression screen. Table 1 presents the means or percentages for baseline characteristics by depression status in the analytic cohort. Subjects with Medicaid for insurance had a higher rate of depression (61%) than subjects with Medicare (13%), private insurance (9%), or those who qualified for the Free Care pool (17%) which is the Massachusetts state funding for healthcare to uninsured persons. Subjects who were unemployed, unmarried, or who reported earnings less than $10,000 per year were also more likely to screen positive for depression. In addition, depressed subjects had a higher severity of co‐morbid disease and longer length of stay for the index hospitalization. Patients categorized as frequent utilizers (2 or more prior admissions) for the 6 months prior to the index hospitalization were also more likely to be depressed. Of further note, is the relatively younger average age among both depressive patients (49.6 years) and non‐depressive patients (49.9) of these study subjects.
The unadjusted hospital utilization rate at 30, 60, and 90 days post‐discharge by depression status is shown in Table 2. At 30 days post‐discharge, those with depressive symptoms had a higher rate of hospital utilization than those without depressive symptoms (0.563 vs. 0.296). In other words, 56 utilization events occurred per 100 patients with depressive symptoms, compared with 30 utilization events per 100 patients without depressive symptoms. The unadjusted 30‐day post‐discharge hospital utilization rate among those with depressive symptoms was higher compared with those without symptoms (IRR, 1.90, 95% confidence interval [CI], 1.242.71). A similar trend was found among subjects at 60 and 90 days post‐discharge.
| Hospital Utilization | Depression Screen* | P Value | IRR (CI) | |
|---|---|---|---|---|
| Negative, n = 500 (68%) | Positive, n = 238 (32%) | |||
| ||||
| No. of hospital utilizations | 140 | 134 | 1.90 (1.51,2.40) | |
| 30‐day hospital utilization rate | 0.296 | 0.563 | <0.001 | |
| No. of hospital utilizations | 231 | 205 | 1.87 (1.55,2.26) | |
| 60‐day hospital utilization rate | 0.463 | 0.868 | <0.001 | |
| No. of hospital utilizations | 324 | 275 | 1.79 (1.53,2.10) | |
| 90‐day hospital utilization rate | 0.648 | 1.165 | <0.001 | |
Poisson regression analyses were conducted to control for potential confounding in the relationship between depressive symptoms and hospital utilization rate within 30 days after discharge (Table 3). After controlling for relevant confounders, including age, gender, employment status, frequent prior hospitalization status, marital status, Charlson score, Project RED study group assignment and the interaction variable for RED study group assignment and depression, the association between symptoms of depression, and hospital utilization rate remained significant (IRR, 1.73; 95% CI, 1.272.36).
| Characteristics | IRR | CI | P Value |
|---|---|---|---|
| |||
| Depression symptoms* | <0.001 | ||
| Positive | 1.73 | 1.272.36 | |
| Negative | REF | 1.0 | |
| Gender | <0.001 | ||
| Male | 1.87 | 1.472.40 | |
| Female | REF | 1.0 | |
| Marital status | 0.005 | ||
| Married | 0.625 | 0.440.89 | |
| Unmarried | 1.0 | REF | |
| Frequent utilizer | <0.001 | ||
| 2+ prior visits | 2.45 | 1.923.15 | |
| <2 prior visits | 1.0 | REF | |
| Study group | 0.054 | ||
| Intervention | 0.76 | 0.551.06 | |
| Control | 1.0 | REF | |
| Employment | |||
| Part time | 1.40 | 0.852.30 | 0.095 |
| Not working | 1.67 | 1.152.44 | 0.003 |
| Other | 0.52 | 0.073.85 | 0.262 |
| Full time | 1.0 | REF | |
| Charlson Score∥ | 0.98 | 0.921.04 | 0.250 |
| Group* depression | 0.84 | 0.521.36 | 0.236 |
| Age | 1.00 | 0.991.01 | 0.375 |
Figure 1 depicts the Kaplan‐Meier hazard curve generated for time to first hospital utilization, stratified by depression status. While 21% of participants without symptoms of depression had a hospital utilization within 30 days, fully 29% of participants with symptoms of depression had a hospital utilization within 30 days (P = 0.011).

Discussion
Our study shows hospitalized patients who screen positive for depressive symptoms are significantly more likely to have a hospital visit (emergency room or rehospitalization) within 30 days of discharge than those who do not screen positive for depressive symptoms among medical patients admitted to an urban, academic, safety‐net hospital. These findings are consistent with, and extend, prior reports regarding depression and rehospitalization in specific populations (ie, geriatrics) and specific diagnoses (ie, cardiovascular disease [CVD] and COPD).1012 We observed a 73% higher incidence rate for hospital utilization within 30 days of discharge for those with symptoms of depression. This puts symptoms of depression on par with frequent prior rehospitalization, advanced age and low social support, as known risk factors for rehospitalization.4, 5, 23
Also of significance is the relatively young age of this study population (49.9 years non‐depressive patients and 49.6 years for depressive patients) compared with the study cohorts used for research in the majority of the existing literature. The chief reason for the young age of our cohort is that potential subjects were excluded if they came from a skilled nursing facility or other hospital. This may limit the generalizability of our findings; however, it seems likely that interventions relating to depression and transitions of care will need to be quite different for patients that reside in long‐term care facilities vs. patients that live in the community. For example, patients living in the community may have significant barriers to access post‐discharge services due to insurance status and are more likely to be sensitive to variations in social support.
Early rehospitalization is associated with significant morbidity, mortality, and expense. It is also a potential marker for poor quality of care.24 Concerns for patient safety, escalating healthcare costs, and possible change in hospital reimbursement mechanisms are fueling the search for modifiable risk factors associated with early rehospitalization. Our data provide evidence that symptoms of depression may be an important focus of attention. We do not know, however whether treating hospitalized patients who screen positive for depression will decrease early rehospitalization and emergency room utilization rates.
Various physiologic and behavioral mechanisms may link symptoms of depression to hospital utilization after discharge. For example, depressed patients with features of somatization may be more likely to experience worrisome physical symptoms after discharge and present prematurely for reevaluation. Patients who are sicker in some fashion not captured by our measured confounders may have symptoms of depression related to chronic, debilitating disease warranting early return to the hospital. Depression may also yield nonadherence to aspects of the discharge treatment plan leading to rehospitalization as a result of poor post‐discharge disease management. For example, research shows that patients with depression following coronary artery bypass surgery are less likely to adhere with cardiac rehabilitation programs.25 Likewise, depression among chronically ill patients such as diabetics, asthmatics, or human immunodeficiency virus (HIV)‐positive patients impairs medication adherence and self‐care behavior which may lead to disease relapse or recurrence.2628 One study examining depression effects on hypertensive medicine adherence in African Americans identified self‐efficacy as a mediating factor between depression and nonadherence.29 This implies that interventions such as self‐management education, a program through which chronically‐ill patients learn to better manage their illnesses through enhanced self‐confidence and problem‐solving strategies (including mood disorder challenges) may reduce early rehospitalization among depressed patients.30
There is also evidence that depression may have direct physiologic consequences. In patients with CVD, depression is associated with poor outcomes possibly related to decreased heart rate variability, hypercoagulability, high burdens of inflammatory markers, and severity of left ventricular dysfunction.3134 Similarly, depression among HIV/acquired immune deficiency syndrome (AIDS), diabetics and multiple sclerosis (MS) patients is linked to heightened levels of proinflammatory markers and less favorable outcomes that may signal a more severe form of the disease or an impaired response to treatment.3538 Indeed, MS investigators now hypothesize that the proinflammatory environment associated with the neurologic manifestations of MS are also causing depression symptoms among MS patients.34 This theory contrasts the common belief that depression in the chronically ill manifests independent of the chronic illness or in response to living with chronic disease.
A major strength of the current study is the large dataset and the broad range of covariates available for analyses. However, several limitations should be noted. First, data on hospital utilization outside Boston Medical Center were determined by patient self‐report and were not confirmed by document review. Second, we do not know the direction of the associations we report. If symptoms of depression are merely the consequence of having a higher disease burden, treatment of the underlying disease may be the most important response. While this is possible, our model does include several variables (eg, Charlson score and length of stay) that are likely to adjust for disease severity, pointing to the likelihood that symptoms of depression truly predict hospital utilization in a fashion that is independent of disease severity. Third, our results may not be generalizable to populations other than those served by urban safety‐net hospitals or other populations excluded from the Project RED trial (eg, non‐English speaking patients and patients from nursing homes). Finally, social factors such as substance use and social support system variables may residually confound the relationship between depression and hospital reutilization demonstrated in this study. While this dataset does not include a measure of social support other than marital status and housing status, data is available on substance use. Analyses conducted by our colleagues using Project RED data found that in this study population depression was significantly more prevalent among substance users (29% vs. 14%) compared with non‐users and that substance use is an independent risk factor for hospital reutilization (unpublished data).
Our findings linking depression to increased hospital utilization also warrant further consideration from healthcare policymakers. Central to the Obama Administration's February 2009 healthcare reform proposal is the pursuit of cost savings through reductions in unplanned hospital readmissions.39 Thus, identifying potentially modifiable risk factors for readmission, such as depression, is of great concern to healthcare providers and policymakers across the nation. If, through testing of interventions, depression proves to be a modifiable risk for readmission, policymakers, while negotiating healthcare reform measures, must provide for the services required to address this comorbidity at the time of discharge. For example, if a patient screens positive for depressive symptoms during a hospitalization for COPD exacerbation, will the proposed payment reforms allow for mental health services during the immediate post‐discharge period in order to reduce the likelihood of hospital readmission? Will those mental health services be readily available? Payment reforms that account for all necessary transitional care services will indeed help reduce readmission costs with less risk for untoward consequences.
In conclusion, our results indicate that a positive depression screen is a significant risk factor for early post‐discharge hospital utilization among hospitalized adults on a general medical service, even after controlling for relevant confounders. Screening for depression during acute hospitalizations may be an important step in identifying patients at increased risk for readmission. Future research should focus on further characterizing and stratifying populations at highest risk for depression. Efforts should also include developing and evaluating targeted interventions for patients with symptoms of depression among hospitalized patients as part of discharge planning. Timely depression therapy during the hospitalization or following hospital discharge might reduce costly readmissions and enhance patient safety.
Fully 19% of Medicare patients are readmitted to the hospital within 30 days of discharge.1 This represents a large amount of potentially avoidable morbidity and cost. Indeed, projects to improve the discharge process and post‐hospital care have shown that as much as one‐third of hospital utilization in the month after discharge can be avoided.2 Consequently, the rate of early, unplanned hospital utilization after discharge has emerged as an important indicator of hospital quality and the Centers for Medicare and Medicaid Services (CMS) has proposed a policy to decrease payments to hospitals with high rates of early unplanned hospital utilization. Thus, there is great interest in identifying modifiable risk factors for rehospitalization that could be used to refine intervention models and lead to improvements in quality of care, patient outcomes, and cost savings.
To date, known predictors of readmission include: lower socioeconomic status,3 history of prior hospitalization4 and advanced age,5 length of stay greater than 7 days,6 a high burden of comorbid illnesses (based on Charlson score),7 poor social support,8 and specific diagnoses (eg, congestive heart failure, chronic obstructive pulmonary disease [COPD] and myocardial infarction).5, 9, 10 In addition, unplanned readmissions and emergency department (ED) visits have been linked to polypharmacy and adverse drug events related to treatment with medications such as warfarin, digoxin and narcotics.11, 12 Another characteristic that has also been linked to readmission is depression;13 however5 reports supporting this association are from studies of elderly patients or with patients who have specific diagnoses (eg, congestive heart failure [CHF], COPD, myocardial infarction).1416
Depression is common, affecting 13% to 16% of people in the US, and is recognized as an important risk factor for poor outcomes among patients with various chronic illnesses.1719 The mechanisms by which depression can be linked to health outcomes and health service utilization have been studied in age‐specific or disease‐specific cohorts such as cardiac patients or frail elders and include both physiologic factors such as hypercoagulability and hyperinflammatory conditions, as well as behavioral factors such as poor self‐care behaviors and heightened sensitivity to somatic symptoms. How these mechanisms link depression to health outcomes and hospital utilization in a general medical population is not clearly understood. Kartha et al.13 reported findings indicating that depression is a risk factor for rehospitalization in general medical inpatients, but the study sample was relatively small and the study design methodology significantly limited its generalizability.12 It would be useful to provide supporting evidence showing depression as an important risk factor for readmission in the general medical in‐patient population using more rigorous study methods and a larger cohort.
We hypothesized that depressive symptoms would be an independent risk factor for early unplanned hospital utilization after discharge for all medical patients. Therefore, we conducted a secondary analysis of the Project RED clinical trial dataset to assess the association between a positive depression screen during inpatient hospitalization and the rate of subsequent hospital utilization.
Methods
Data from the Project RED clinical trial were reviewed for inclusion in a secondary analysis. Complete data were available for 738 of the 749 subjects recruited for Project RED.
Project RED Setting and Participants
Project RED was a two‐armed randomized controlled trial of English‐speaking adult patients, 18 years or older, admitted to the teaching service of Boston Medical Center, a large urban safety‐net hospital with an ethnically diverse patient population. A total of 749 subjects were enrolled and randomized between January 3, 2006 and October 18, 2007. Patients were required to have a telephone, be able to comprehend study details and the consent process in English, and have plans to be discharged to a US community. Patients were not enrolled if they were admitted from a skilled nursing facility or other hospital, transferred to a different hospital service prior to enrollment, admitted for a planned hospitalization, on hospital precautions, on suicide watch, deaf or blind. The Institutional Review Board of Boston University approved all study activities. A full description of the methods for the Project RED trial has been described previously.2
Outcome Variable
The primary endpoint was rate of hospital utilization within 30 days of discharge from the index admission, defined as the total number of ED visits and readmissions per subject within 30 days of the index discharge. Hospital utilization rates within 60 and 90 days of the index hospitalization discharge were also analyzed as secondary outcomes. Any ED visit in which a subject was subsequently admitted to the hospital was only counted as a readmission. Outcome data were collected by reviewing the hospital's electronic medical records (EMRs) and by contacting subjects by telephone 30 days after discharge. Dates of hospital utilization occurring at Boston Medical Center were obtained from the EMR, while those at other hospitals were collected through subject report. Subjects who could not be reached within 60 days of discharge were assumed alive.
Primary Independent Variable
The primary independent variable of interest was depressive symptoms defined as a positive score for minor or major depression on the nine‐item Patient Health Questionnaire (PHQ‐9) depression screening tool.20 A dichotomized variable was created using a standardized scoring system to determine the screening cut‐off for major or minor depressive symptoms.19
Statistical Analysis
Demographic and other characteristics of the subjects were compared by depression status (Table 1). Potential confounders were identified a priori from the available literature on factors associated with rehospitalization. These included age, gender, marital status, health literacy score (rapid estimate of health literacy in adult medicine tool [REALM]),21 Charlson score,22 insurance type, employment status, income level, homelessness status within past three months, hospital utilization within the 6 months prior to the index hospitalization, educational attainment, length of hospital stay and Project RED study group assignment. Bivariate analyses were conducted to determine which covariates were significant confounders of the relationship between depression and hospital utilization within 30 days of discharge. Chi‐square tests were used for categorical variables and t‐tests for continuous variables.
| Characteristic | Depression Screen* | ||
|---|---|---|---|
| Negative (n = 500) | Positive (n = 238) | P Value | |
| |||
| Race, No. (%) | |||
| White | 140 (30) | 66 (30) | |
| Black | 268 (58) | 117 (54) | |
| Hispanic | 47 (10) | 29 (13) | 0.760 |
| Insurance, No. (%) | |||
| Private | 95 (19) | 22 (9) | |
| Medicare | 69 (14) | 30 (13) | |
| Medicaid | 214 (43) | 143 (61) | |
| Free care | 118 (24) | 40 (17) | <0.001 |
| Education, No. (%) | |||
| <8th grade | 33 (7) | 21 (9) | |
| Some high school | 82 (17) | 52 (22) | |
| High school grad | 192 (38) | 90 (38) | |
| Some college | 126 (25) | 51 (22) | |
| College grad | 67 (13) | 22 (9) | 0.135 |
| Health Literacy | |||
| Grade 3 and below | 64 (13) | 44 (19) | |
| Grade 46 | 54 (11) | 22 (10) | |
| Grade 78 | 156 (32) | 73 (32) | |
| Grade 9 and above | 213 (44) | 89 (39) | 0.170 |
| Income, $, No. (%) | |||
| No income | 61 (12) | 37 (16) | |
| <10K | 77 (15) | 61 (26) | |
| 1020K | 96 (19) | 35 (15) | |
| 2050K | 97 (19) | 34 (14) | |
| 50100K | 35 (8) | 7 (2) | |
| No answer | 132 (27) | 64 (27) | 0.002 |
| Employment status, No. (%) | |||
| Full time | 142 (28) | 34 (14) | |
| Part time | 57 (11) | 30 (13) | |
| Not Working | 297 (59) | 171 (72) | <0.001 |
| Age, mean (SD), years | 49.9 (16.0) | 49.6 (13.3) | 0.802 |
| Gender: No. (%) Female | 239 (48) | 133 (56) | 0.040 |
| Have PCP, No. (%) Yes | 399 (80) | 197 (83) | 0.340 |
| Marital status,∥ No. (%) unmarried | 365 (73) | 201 (85) | <0.001 |
| Charlson score, mean (SD) | 1.058 (1.6) | 1.56 (2.39) | 0.001 |
| RED study group,# No. (%) | |||
| Intervention | 243 (49) | 127 (53) | 0.22 |
| Length of stay, days, mean (SD) | 2.5 (2.8) | 3.1 (3.8) | 0.016 |
| Homeless in last 3 months, No. (%) | 45 (9) | 30 (13) | 0.130 |
| Frequent utilizer,** No. (%) | 159 (32) | 104 (44) | 0.002 |
Age, length of stay, and Charlson score were used as continuous variables. Gender, marital status, frequent prior utilization (01 vs. 2 or more), and homelessness were treated as dichotomous variables. Categorical variables were created for, educational attainment (less than eighth grade, some high school, high school graduate, some college, college graduate), insurance type (Medicare, Medicaid, private insurance or free care), income level (no income, less than $10,000 per year, $10,00020,000, $20,00050,000, $50,000100,000, no answer), level of health literacy (grade 3 and below, grade 46, grade 78, grade 9 or above) and employment status(working full‐time, working part‐time, not working, no answer).
The 30‐day hospital utilization rate reflects the number of hospital utilization events within 30 days of discharge per subject. The same method was used to calculate hospital utilization rates within 60 and 90 days of discharge respectively. The unadjusted incident rate ratio (IRR) was calculated as the ratio of the rate of hospital utilizations among patients with depressive symptoms versus patients without depressive symptoms. Data for hospital utilization at 30, 60, and 90 days are cumulative.
Poisson models were used to test for significant differences between the predicted and observed number of hospitalization events at 30 days. A backward stepwise regression was conducted to identify and control for relevant confounders and construct the final, best‐fit model for the association between depression and hospital reutilization. A statistical significance level of P = 0.10 was used for the stepwise regression. To evaluate potential interactions between depression and the Project RED intervention, interaction terms were included. Two‐sided significance tests were used. P values of less than 0.05 were considered to indicate statistical significance. All data were analyzed with S‐Plus 8.0 (Seattle, WA).
In addition, a Kaplan‐Meier hazard curve was generated for the first hospital utilization event, ED visit or readmission, for the 30‐day period following discharge and compared with a log‐rank test.
Results
A total of 28% of subjects were categorized as having a positive depression screen. More women (36%) had positive depression screens than men (28%). Among patients with a positive depression screen, 58% had a history of depression and 53% were currently taking medications at the time of enrollment, compared with 25% and 22% respectively for subjects with a negative depression screen. Table 1 presents the means or percentages for baseline characteristics by depression status in the analytic cohort. Subjects with Medicaid for insurance had a higher rate of depression (61%) than subjects with Medicare (13%), private insurance (9%), or those who qualified for the Free Care pool (17%) which is the Massachusetts state funding for healthcare to uninsured persons. Subjects who were unemployed, unmarried, or who reported earnings less than $10,000 per year were also more likely to screen positive for depression. In addition, depressed subjects had a higher severity of co‐morbid disease and longer length of stay for the index hospitalization. Patients categorized as frequent utilizers (2 or more prior admissions) for the 6 months prior to the index hospitalization were also more likely to be depressed. Of further note, is the relatively younger average age among both depressive patients (49.6 years) and non‐depressive patients (49.9) of these study subjects.
The unadjusted hospital utilization rate at 30, 60, and 90 days post‐discharge by depression status is shown in Table 2. At 30 days post‐discharge, those with depressive symptoms had a higher rate of hospital utilization than those without depressive symptoms (0.563 vs. 0.296). In other words, 56 utilization events occurred per 100 patients with depressive symptoms, compared with 30 utilization events per 100 patients without depressive symptoms. The unadjusted 30‐day post‐discharge hospital utilization rate among those with depressive symptoms was higher compared with those without symptoms (IRR, 1.90, 95% confidence interval [CI], 1.242.71). A similar trend was found among subjects at 60 and 90 days post‐discharge.
| Hospital Utilization | Depression Screen* | P Value | IRR (CI) | |
|---|---|---|---|---|
| Negative, n = 500 (68%) | Positive, n = 238 (32%) | |||
| ||||
| No. of hospital utilizations | 140 | 134 | 1.90 (1.51,2.40) | |
| 30‐day hospital utilization rate | 0.296 | 0.563 | <0.001 | |
| No. of hospital utilizations | 231 | 205 | 1.87 (1.55,2.26) | |
| 60‐day hospital utilization rate | 0.463 | 0.868 | <0.001 | |
| No. of hospital utilizations | 324 | 275 | 1.79 (1.53,2.10) | |
| 90‐day hospital utilization rate | 0.648 | 1.165 | <0.001 | |
Poisson regression analyses were conducted to control for potential confounding in the relationship between depressive symptoms and hospital utilization rate within 30 days after discharge (Table 3). After controlling for relevant confounders, including age, gender, employment status, frequent prior hospitalization status, marital status, Charlson score, Project RED study group assignment and the interaction variable for RED study group assignment and depression, the association between symptoms of depression, and hospital utilization rate remained significant (IRR, 1.73; 95% CI, 1.272.36).
| Characteristics | IRR | CI | P Value |
|---|---|---|---|
| |||
| Depression symptoms* | <0.001 | ||
| Positive | 1.73 | 1.272.36 | |
| Negative | REF | 1.0 | |
| Gender | <0.001 | ||
| Male | 1.87 | 1.472.40 | |
| Female | REF | 1.0 | |
| Marital status | 0.005 | ||
| Married | 0.625 | 0.440.89 | |
| Unmarried | 1.0 | REF | |
| Frequent utilizer | <0.001 | ||
| 2+ prior visits | 2.45 | 1.923.15 | |
| <2 prior visits | 1.0 | REF | |
| Study group | 0.054 | ||
| Intervention | 0.76 | 0.551.06 | |
| Control | 1.0 | REF | |
| Employment | |||
| Part time | 1.40 | 0.852.30 | 0.095 |
| Not working | 1.67 | 1.152.44 | 0.003 |
| Other | 0.52 | 0.073.85 | 0.262 |
| Full time | 1.0 | REF | |
| Charlson Score∥ | 0.98 | 0.921.04 | 0.250 |
| Group* depression | 0.84 | 0.521.36 | 0.236 |
| Age | 1.00 | 0.991.01 | 0.375 |
Figure 1 depicts the Kaplan‐Meier hazard curve generated for time to first hospital utilization, stratified by depression status. While 21% of participants without symptoms of depression had a hospital utilization within 30 days, fully 29% of participants with symptoms of depression had a hospital utilization within 30 days (P = 0.011).

Discussion
Our study shows hospitalized patients who screen positive for depressive symptoms are significantly more likely to have a hospital visit (emergency room or rehospitalization) within 30 days of discharge than those who do not screen positive for depressive symptoms among medical patients admitted to an urban, academic, safety‐net hospital. These findings are consistent with, and extend, prior reports regarding depression and rehospitalization in specific populations (ie, geriatrics) and specific diagnoses (ie, cardiovascular disease [CVD] and COPD).1012 We observed a 73% higher incidence rate for hospital utilization within 30 days of discharge for those with symptoms of depression. This puts symptoms of depression on par with frequent prior rehospitalization, advanced age and low social support, as known risk factors for rehospitalization.4, 5, 23
Also of significance is the relatively young age of this study population (49.9 years non‐depressive patients and 49.6 years for depressive patients) compared with the study cohorts used for research in the majority of the existing literature. The chief reason for the young age of our cohort is that potential subjects were excluded if they came from a skilled nursing facility or other hospital. This may limit the generalizability of our findings; however, it seems likely that interventions relating to depression and transitions of care will need to be quite different for patients that reside in long‐term care facilities vs. patients that live in the community. For example, patients living in the community may have significant barriers to access post‐discharge services due to insurance status and are more likely to be sensitive to variations in social support.
Early rehospitalization is associated with significant morbidity, mortality, and expense. It is also a potential marker for poor quality of care.24 Concerns for patient safety, escalating healthcare costs, and possible change in hospital reimbursement mechanisms are fueling the search for modifiable risk factors associated with early rehospitalization. Our data provide evidence that symptoms of depression may be an important focus of attention. We do not know, however whether treating hospitalized patients who screen positive for depression will decrease early rehospitalization and emergency room utilization rates.
Various physiologic and behavioral mechanisms may link symptoms of depression to hospital utilization after discharge. For example, depressed patients with features of somatization may be more likely to experience worrisome physical symptoms after discharge and present prematurely for reevaluation. Patients who are sicker in some fashion not captured by our measured confounders may have symptoms of depression related to chronic, debilitating disease warranting early return to the hospital. Depression may also yield nonadherence to aspects of the discharge treatment plan leading to rehospitalization as a result of poor post‐discharge disease management. For example, research shows that patients with depression following coronary artery bypass surgery are less likely to adhere with cardiac rehabilitation programs.25 Likewise, depression among chronically ill patients such as diabetics, asthmatics, or human immunodeficiency virus (HIV)‐positive patients impairs medication adherence and self‐care behavior which may lead to disease relapse or recurrence.2628 One study examining depression effects on hypertensive medicine adherence in African Americans identified self‐efficacy as a mediating factor between depression and nonadherence.29 This implies that interventions such as self‐management education, a program through which chronically‐ill patients learn to better manage their illnesses through enhanced self‐confidence and problem‐solving strategies (including mood disorder challenges) may reduce early rehospitalization among depressed patients.30
There is also evidence that depression may have direct physiologic consequences. In patients with CVD, depression is associated with poor outcomes possibly related to decreased heart rate variability, hypercoagulability, high burdens of inflammatory markers, and severity of left ventricular dysfunction.3134 Similarly, depression among HIV/acquired immune deficiency syndrome (AIDS), diabetics and multiple sclerosis (MS) patients is linked to heightened levels of proinflammatory markers and less favorable outcomes that may signal a more severe form of the disease or an impaired response to treatment.3538 Indeed, MS investigators now hypothesize that the proinflammatory environment associated with the neurologic manifestations of MS are also causing depression symptoms among MS patients.34 This theory contrasts the common belief that depression in the chronically ill manifests independent of the chronic illness or in response to living with chronic disease.
A major strength of the current study is the large dataset and the broad range of covariates available for analyses. However, several limitations should be noted. First, data on hospital utilization outside Boston Medical Center were determined by patient self‐report and were not confirmed by document review. Second, we do not know the direction of the associations we report. If symptoms of depression are merely the consequence of having a higher disease burden, treatment of the underlying disease may be the most important response. While this is possible, our model does include several variables (eg, Charlson score and length of stay) that are likely to adjust for disease severity, pointing to the likelihood that symptoms of depression truly predict hospital utilization in a fashion that is independent of disease severity. Third, our results may not be generalizable to populations other than those served by urban safety‐net hospitals or other populations excluded from the Project RED trial (eg, non‐English speaking patients and patients from nursing homes). Finally, social factors such as substance use and social support system variables may residually confound the relationship between depression and hospital reutilization demonstrated in this study. While this dataset does not include a measure of social support other than marital status and housing status, data is available on substance use. Analyses conducted by our colleagues using Project RED data found that in this study population depression was significantly more prevalent among substance users (29% vs. 14%) compared with non‐users and that substance use is an independent risk factor for hospital reutilization (unpublished data).
Our findings linking depression to increased hospital utilization also warrant further consideration from healthcare policymakers. Central to the Obama Administration's February 2009 healthcare reform proposal is the pursuit of cost savings through reductions in unplanned hospital readmissions.39 Thus, identifying potentially modifiable risk factors for readmission, such as depression, is of great concern to healthcare providers and policymakers across the nation. If, through testing of interventions, depression proves to be a modifiable risk for readmission, policymakers, while negotiating healthcare reform measures, must provide for the services required to address this comorbidity at the time of discharge. For example, if a patient screens positive for depressive symptoms during a hospitalization for COPD exacerbation, will the proposed payment reforms allow for mental health services during the immediate post‐discharge period in order to reduce the likelihood of hospital readmission? Will those mental health services be readily available? Payment reforms that account for all necessary transitional care services will indeed help reduce readmission costs with less risk for untoward consequences.
In conclusion, our results indicate that a positive depression screen is a significant risk factor for early post‐discharge hospital utilization among hospitalized adults on a general medical service, even after controlling for relevant confounders. Screening for depression during acute hospitalizations may be an important step in identifying patients at increased risk for readmission. Future research should focus on further characterizing and stratifying populations at highest risk for depression. Efforts should also include developing and evaluating targeted interventions for patients with symptoms of depression among hospitalized patients as part of discharge planning. Timely depression therapy during the hospitalization or following hospital discharge might reduce costly readmissions and enhance patient safety.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360(14):1457–1459.
- ,,, et al.The reengineered hospital discharge program to decrease rehospitalization.Ann Intern Med.2009;150(3):178–187.
- ,,.The impact of patient socioeconomic status and other social factors on readmission: a prospective study in four Massachusetts hospitals.Inquiry.1994;31(2):163–172.
- ,,,.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631. [PMID: 15209600]
- ,,,,,.Factors associated with unplanned hospital readmission among patients 65 years of age and older in a Medicare managed care plan.Am J Med.1999;107(1):13–17.
- ,,, et al.Readmission after hospitalization for congestive heart failure among Medicare beneficiaries.Arch Intern Med.1997;157(1):99–104.
- ,,.Chronic comorbidity and outcomes of hospital care: length of stay, mortality and readmission at 30 and 365 days.J Clin Epidemiol.1999;52(3):171–179.
- ,,, et al.Social network as a predictor of hospital readmission and mortality among older patients with heart failure.J Card Fail.2006;12:621–627.
- ,,,,.Acute exacerbation of chronic obstructive pulmonary disease: influence of social factors in determining length of stay and readmission rates.Can Respir J.2008;15(7):361–364.
- ,,, et al.Time course of depression and outcome of myocardial infarction.Arch Intern Med.2006;166(18):2035–2043.
- ,,, et al.Medication use leading to emergency department visits for adverse drug events in older adults.Ann Intern Med.2007;147(11):755–765.
- ,,.A systematic literature review of factors affecting outcomes in older medical patients admitted to hospital.Age Ageing.2004;33(2):110–115.
- ,,, et al.Depression is a risk factor for rehospitalization in medical inpatients.Prim Care Companion J Clin Psychiatry.2007;9(4):256–262.
- ,,, et al.Risk factors for hospital readmission in patients with chronic obstructive pulmonary disease.Respiration.2006;73:311–317.
- ,,, et al.Depression and healthcare costs during the first year following myocardial infarction.J Psychosom Res.2000;48(4–5):471–478.
- ,,, et al.Relationship of depression to increased risk of mortality and rehospitalization.Arch Intern Med.2001;161(15):1849–1856.
- ,,, et al.Time course of depression and outcome of myocardial infarction.Arch Intern Med.2006;166:2035–2043.
- ,.Single item on positive affect is associated with 1‐year survival in consecutive medical inpatients.J Gen Hosp Psych.2009;31:8–13.
- ,,,.Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions.Arch Gen Psychiatry.2005;62(10):1097–106.
- ,,.The PHQ‐9: Validity of a brief depression severity measure.J Gen Intern Med.2001;16:606–613. [PMID:11556941]
- ,,, et al.Rapid estimate of adult literacy in medicine: a shortened screening instrument.Fam Med.1993;25:391–395. [PMID:8349060]
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40:373–383. [PMID: 3558716]
- ,,, et al.Social network as a predictor of hospital readmission and mortality among older patients with heart failure.J Card Fail.2006;12(8):621–627.
- ,,,,.The association between the quality of inpatient care and early readmission: a meta‐analysis of the evidence.Med Care.1997;35(10):1044–1059.
- ,,, et al.Persistent depression affects adherence to secondary prevention behaviors after acute coronary syndromes.J Gen Intern Med.2006;21(11):1178–1183.
- ,,,,.Depression is an important contributor to low medication adherence in hemodialyzed patients and transplant recipients.Kidney Int.2009;75(11):1223–1229.
- ,,, et al.Symptoms of depression prospectively predict poorer self‐care in patients with Type 2 diabetes.Diabet Med.2008;25(9):1102–1107.
- ,,, et al.The effect of adherence on the association between depressive symptoms and mortality among HIV‐infected individuals first initiating HAART.AIDS.2007;21(9):1175–1183.
- ,,.Self‐efficacy mediates the relationship between depressive symptoms and medication adherence.Health Educ Behav.2009;36(1):127–137.
- ,,,.Patient self‐management of chronic disease in primary care.JAMA.2002;288(19):2469–2475.
- .Effects of sertraline on the recovery rate of cardiac autonomic function in depressed patients after acute myocardial infarction.Am Heart J.2001;142:617–623.
- ,,, et al.Relationship between left ventricular dysfunction and depression following myocardial infarction: data from the MIND‐IT.Eur Heart J.2005;26:2650–2656.
- ,,, et al.Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acugte coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet SubStudy.Circulation.2003;108:939–944.
- ,.Inflammation in acute coronary syndromes.Cleve Clin J Med.2002;69(Suppl2):SII130–SII142.
- ,.Depression and immunity: inflammation and depressive symptoms in multiple sclerosis.Neurol Clin.2006;24(3):507–519.
- ,,, et al.Synergistic effects of psychological and immune stressors on inflammatory cytokines and sickness responses in humans.Brain Behav Immun.2009;23(2):217–224.
- ,,,,,.Psychological distress, killer lymphocytes and disease severity in HIV/AIDS.Brain Behav Immun.2008;22(6):901–911.
- ,,,.Analysis of potential predictors of depression among coronary heart disease risk factors including heart rate variability, markers of inflammation, and endothelial function.Eur Heart J.2008;29(9):1110–1117.
- .Obama proposes $634 billion fund for health care.Washington Post. February 26,2009:A1.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360(14):1457–1459.
- ,,, et al.The reengineered hospital discharge program to decrease rehospitalization.Ann Intern Med.2009;150(3):178–187.
- ,,.The impact of patient socioeconomic status and other social factors on readmission: a prospective study in four Massachusetts hospitals.Inquiry.1994;31(2):163–172.
- ,,,.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631. [PMID: 15209600]
- ,,,,,.Factors associated with unplanned hospital readmission among patients 65 years of age and older in a Medicare managed care plan.Am J Med.1999;107(1):13–17.
- ,,, et al.Readmission after hospitalization for congestive heart failure among Medicare beneficiaries.Arch Intern Med.1997;157(1):99–104.
- ,,.Chronic comorbidity and outcomes of hospital care: length of stay, mortality and readmission at 30 and 365 days.J Clin Epidemiol.1999;52(3):171–179.
- ,,, et al.Social network as a predictor of hospital readmission and mortality among older patients with heart failure.J Card Fail.2006;12:621–627.
- ,,,,.Acute exacerbation of chronic obstructive pulmonary disease: influence of social factors in determining length of stay and readmission rates.Can Respir J.2008;15(7):361–364.
- ,,, et al.Time course of depression and outcome of myocardial infarction.Arch Intern Med.2006;166(18):2035–2043.
- ,,, et al.Medication use leading to emergency department visits for adverse drug events in older adults.Ann Intern Med.2007;147(11):755–765.
- ,,.A systematic literature review of factors affecting outcomes in older medical patients admitted to hospital.Age Ageing.2004;33(2):110–115.
- ,,, et al.Depression is a risk factor for rehospitalization in medical inpatients.Prim Care Companion J Clin Psychiatry.2007;9(4):256–262.
- ,,, et al.Risk factors for hospital readmission in patients with chronic obstructive pulmonary disease.Respiration.2006;73:311–317.
- ,,, et al.Depression and healthcare costs during the first year following myocardial infarction.J Psychosom Res.2000;48(4–5):471–478.
- ,,, et al.Relationship of depression to increased risk of mortality and rehospitalization.Arch Intern Med.2001;161(15):1849–1856.
- ,,, et al.Time course of depression and outcome of myocardial infarction.Arch Intern Med.2006;166:2035–2043.
- ,.Single item on positive affect is associated with 1‐year survival in consecutive medical inpatients.J Gen Hosp Psych.2009;31:8–13.
- ,,,.Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions.Arch Gen Psychiatry.2005;62(10):1097–106.
- ,,.The PHQ‐9: Validity of a brief depression severity measure.J Gen Intern Med.2001;16:606–613. [PMID:11556941]
- ,,, et al.Rapid estimate of adult literacy in medicine: a shortened screening instrument.Fam Med.1993;25:391–395. [PMID:8349060]
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40:373–383. [PMID: 3558716]
- ,,, et al.Social network as a predictor of hospital readmission and mortality among older patients with heart failure.J Card Fail.2006;12(8):621–627.
- ,,,,.The association between the quality of inpatient care and early readmission: a meta‐analysis of the evidence.Med Care.1997;35(10):1044–1059.
- ,,, et al.Persistent depression affects adherence to secondary prevention behaviors after acute coronary syndromes.J Gen Intern Med.2006;21(11):1178–1183.
- ,,,,.Depression is an important contributor to low medication adherence in hemodialyzed patients and transplant recipients.Kidney Int.2009;75(11):1223–1229.
- ,,, et al.Symptoms of depression prospectively predict poorer self‐care in patients with Type 2 diabetes.Diabet Med.2008;25(9):1102–1107.
- ,,, et al.The effect of adherence on the association between depressive symptoms and mortality among HIV‐infected individuals first initiating HAART.AIDS.2007;21(9):1175–1183.
- ,,.Self‐efficacy mediates the relationship between depressive symptoms and medication adherence.Health Educ Behav.2009;36(1):127–137.
- ,,,.Patient self‐management of chronic disease in primary care.JAMA.2002;288(19):2469–2475.
- .Effects of sertraline on the recovery rate of cardiac autonomic function in depressed patients after acute myocardial infarction.Am Heart J.2001;142:617–623.
- ,,, et al.Relationship between left ventricular dysfunction and depression following myocardial infarction: data from the MIND‐IT.Eur Heart J.2005;26:2650–2656.
- ,,, et al.Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acugte coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet SubStudy.Circulation.2003;108:939–944.
- ,.Inflammation in acute coronary syndromes.Cleve Clin J Med.2002;69(Suppl2):SII130–SII142.
- ,.Depression and immunity: inflammation and depressive symptoms in multiple sclerosis.Neurol Clin.2006;24(3):507–519.
- ,,, et al.Synergistic effects of psychological and immune stressors on inflammatory cytokines and sickness responses in humans.Brain Behav Immun.2009;23(2):217–224.
- ,,,,,.Psychological distress, killer lymphocytes and disease severity in HIV/AIDS.Brain Behav Immun.2008;22(6):901–911.
- ,,,.Analysis of potential predictors of depression among coronary heart disease risk factors including heart rate variability, markers of inflammation, and endothelial function.Eur Heart J.2008;29(9):1110–1117.
- .Obama proposes $634 billion fund for health care.Washington Post. February 26,2009:A1.
Copyright © 2010 Society of Hospital Medicine