User login
Radical vs. Sensible
Hospital medicine had grown rapidly and provided the platform for change in our nation’s hospitals long before there was any meaningful healthcare legislation in Washington. With President Obama’s appointment of an innovator—Don Berwick—to head the Centers for Medicare and Medicaid Services (CMS), there is increased opportunity to ramp up revisions, large and small, to provide the incentives and the impetus to create a healthcare system for the 21st century.
With that in mind, I thought I’d offer a few ideas that Don could institute on Day 1, which could start us in the right direction, or throw us all into chaos, depending on how it plays out. While most of my attention is directed to the Medicare population, all of these ideas would be equally applicable to the commercially insured population.
Advanced Directives
We all know that too few people in this country have taken the opportunity to discuss with their families and their personal physicians how they want their care managed at critical junctures, whether it comes on suddenly with an accident or with aging. The suggestion that Medicare would pay for an office visit with your doctor to discuss this imploded with the news media’s fanning of the “death panel” flames first stoked by Sarah Palin, which sidetracked all rational discussion.
Besides setting up people for unwarranted and unwanted assaults and protracted misery, mismanaging the end stages of life leads to an enormous misallocation of physicians’ focus at a time when we all need to be mindful stewards of our limited healthcare resources.
It is acceptable if, after careful consideration, anyone chooses to not have any advanced directive, but it should be a cognitive directed choice, not just a failure to engage.
Therefore, I am proposing that Medicare offer an incentive (e.g., waiving co-payments or deductibles) to have all Medicare beneficiaries complete an advanced directive annually, or sign a form indicating they were offered an advanced directive and declined to have one invoked. In addition, Medicare could set up a system that would allow physicians (or facilities) who would manage the patient’s healthcare to have access to the conditions of the advance directives. The forms could be attached to individual Medicare profiles, possibly on the Web, in addition to being held by a patient’s PCP or medical home, if they have one.
Personal Health Records
Most people in this country can access most of the information about their personal financial status in real time from any computer in the world. Less than 10% of Americans can retrieve meaningful personal medical information. This is in spite of the prevalence of Web-based personal health record (PHR) software from Microsoft, Revolution Health, and other software vendors, along with Kaiser Permanente and a handful of insurance companies.
PHRs allow for an initial baseline set of data to be recorded and updated as new tests, diagnoses, and medications are employed. It allows for a composite knowledge of what has been tried in the past and what is being utilized in the present. This can be under patients’ control, but it would allow for appropriate access at times of acute need (e.g., an ED visit or a hospital admission).
Too often, patients with a long-term relationship with a local PCP present to the hospital and all of the healthcare professionals are forced to make critical decisions in the first few hours with insufficient or inaccurate information. This leads to needless repetition of tests or inability to compare current data with previous data (wouldn’t it be nice to have the last EKG or labs?), or in retrying a treatment regimen that hasn’t worked in the past.
And if you are in another city or if you don’t have a local physician with all of your old records, the information gap is far worse.
Once again, we could incentivize patients to have an up-to-date PHR with reduced premiums, or lower deductibles or copayments. We could look for ways to incentivize PCPs and hospitals to help patients build and maintain their PHR. We could make it a matter of course that a patient’s PHR would be updated at each intersection with healthcare information (e.g., the pharmacy or the lab or each office visit).
Physician Accountability
Somehow, we have evolved into a fragmented health system. We need to repair the disconnect between patients and physicians. The professional pact between the patient and their primary physician needs to be in place until the patient and the “next” physician agree to the handoff of responsibility. As hospitalists, we see this at both ends of the continuum. Patients shouldn’t just be “sent” to the ED or the hospital, especially not when they are acutely ill. Their personal physician, their medical home, should “arrange” for an orderly transfer of care. This would involve a transfer of information (possibly facilitated by an updated PHR), but as much by the assurance that the accepting physician or institution is prepared for the handoff, acknowledges this to the PCP, and that the patient understands the handoff has taken place.
In the same way, patients would not be just “sent out” from the hospital. The treating physician (it could be the hospitalist, but also the surgeon or cardiologist) would remain the doctor of record—the first resource for patients’ question and issues—until another “receiving” physician has accepted the handoff, acknowledges this role, and the patient agrees.
We could rapidly shift this process by allowing the patient to decide when the hospitalization has ended. We could change the system overnight by making one of the conditions for payment for a hospitalization (to the physicians and the hospital) that the patient has signed off that indeed the hospitalization has ended.
This might include a discussion of chronic medications to continue, acute therapies to complete, understanding by the patient of where and when to receive follow-up testing and evaluation, and a clear understanding of which physician is now accountable for future issues and questions as patients travel from acute illness to normal function.
There certainly are economic and societal issues. Not everyone has a PCP or can pay for their outpatient care, and this could be a full-employment plan for liability attorneys, but in the end I am confident medical professionals would create the linkages that would minimize the deep white space patients find themselves in once they are wheeled to the front door of their hospitals.
Creatively Complete the Hospitalization
In a perfect world, everyone would have a functional, robust medical home to return to after an acute hospitalization. Unfortunately, a patient-centered medical home (PCMH) is much more of a hope than a reality for most Americans. While we are working to create a better “horizontal” hospitalization, there are clear gaps in the vertical-care world.
If we are going to be responsible for bundled care that encompasses pre-admit and post-discharge care (e.g., 30 days after discharge), then we must beef up our outpatient capabilities.
Hopefully in the long run, this can be supplied by a reinvigorated and reinvented medical home, but it is still a long way off. If payment and accountability continue to blur just when the hospitalization ends, then hospitals (and hospitalists) and Medicare and insurers will need to be creative in how and who will manage the patient. We’ll need to solve the issues around patients who are no longer sick enough to require a hospital bed but clearly are not back to their steady state.
This ties in with the accountability gap that vexes our patients every day. Very likely, hospitalists will have to assume a role in managing the patients after hospital discharge. This might take the form of a few follow-up visits and continued support systems via the Web and telephone. It will probably require a new class of hospitalist—the ambulist or the subacutist—supported by dedicated ancillary staff and systems.
Once again, Medicare and insurers can drive to a better system of post-acute care by supplying incentives: a more robust discharge payment or rewarding successful completion of a hospitalization, possibly by bundled payment incentives. In addition, there could be clear standards set that would define when this is done well with associated rewards.
I know some of these ideas are radical and make us uncomfortable. They seem to assign more responsibilities to an already overburdened profession. To be successful, these innovations require an active participation and accountability of our patients. We as the providers of healthcare cannot do this alone. It also requires the evolution of the hospital as an institution from just the healthcare provider for the acutely ill, horizontal patient, but as more a part of a continuum from acute illness to return to function. And it cries out for a robust, capable, outpatient partner in a medical home or accountable care organization (ACO) that is equally dedicated, incentivized, and accountable.
We won’t get there tomorrow, even if Dr. Berwick reads this and acts on all of the ideas on his first day at CMS.
But if we don’t get started, we know we definitely won’t get there at any time in our future. TH
Dr. Wellikson is CEO of SHM.
Hospital medicine had grown rapidly and provided the platform for change in our nation’s hospitals long before there was any meaningful healthcare legislation in Washington. With President Obama’s appointment of an innovator—Don Berwick—to head the Centers for Medicare and Medicaid Services (CMS), there is increased opportunity to ramp up revisions, large and small, to provide the incentives and the impetus to create a healthcare system for the 21st century.
With that in mind, I thought I’d offer a few ideas that Don could institute on Day 1, which could start us in the right direction, or throw us all into chaos, depending on how it plays out. While most of my attention is directed to the Medicare population, all of these ideas would be equally applicable to the commercially insured population.
Advanced Directives
We all know that too few people in this country have taken the opportunity to discuss with their families and their personal physicians how they want their care managed at critical junctures, whether it comes on suddenly with an accident or with aging. The suggestion that Medicare would pay for an office visit with your doctor to discuss this imploded with the news media’s fanning of the “death panel” flames first stoked by Sarah Palin, which sidetracked all rational discussion.
Besides setting up people for unwarranted and unwanted assaults and protracted misery, mismanaging the end stages of life leads to an enormous misallocation of physicians’ focus at a time when we all need to be mindful stewards of our limited healthcare resources.
It is acceptable if, after careful consideration, anyone chooses to not have any advanced directive, but it should be a cognitive directed choice, not just a failure to engage.
Therefore, I am proposing that Medicare offer an incentive (e.g., waiving co-payments or deductibles) to have all Medicare beneficiaries complete an advanced directive annually, or sign a form indicating they were offered an advanced directive and declined to have one invoked. In addition, Medicare could set up a system that would allow physicians (or facilities) who would manage the patient’s healthcare to have access to the conditions of the advance directives. The forms could be attached to individual Medicare profiles, possibly on the Web, in addition to being held by a patient’s PCP or medical home, if they have one.
Personal Health Records
Most people in this country can access most of the information about their personal financial status in real time from any computer in the world. Less than 10% of Americans can retrieve meaningful personal medical information. This is in spite of the prevalence of Web-based personal health record (PHR) software from Microsoft, Revolution Health, and other software vendors, along with Kaiser Permanente and a handful of insurance companies.
PHRs allow for an initial baseline set of data to be recorded and updated as new tests, diagnoses, and medications are employed. It allows for a composite knowledge of what has been tried in the past and what is being utilized in the present. This can be under patients’ control, but it would allow for appropriate access at times of acute need (e.g., an ED visit or a hospital admission).
Too often, patients with a long-term relationship with a local PCP present to the hospital and all of the healthcare professionals are forced to make critical decisions in the first few hours with insufficient or inaccurate information. This leads to needless repetition of tests or inability to compare current data with previous data (wouldn’t it be nice to have the last EKG or labs?), or in retrying a treatment regimen that hasn’t worked in the past.
And if you are in another city or if you don’t have a local physician with all of your old records, the information gap is far worse.
Once again, we could incentivize patients to have an up-to-date PHR with reduced premiums, or lower deductibles or copayments. We could look for ways to incentivize PCPs and hospitals to help patients build and maintain their PHR. We could make it a matter of course that a patient’s PHR would be updated at each intersection with healthcare information (e.g., the pharmacy or the lab or each office visit).
Physician Accountability
Somehow, we have evolved into a fragmented health system. We need to repair the disconnect between patients and physicians. The professional pact between the patient and their primary physician needs to be in place until the patient and the “next” physician agree to the handoff of responsibility. As hospitalists, we see this at both ends of the continuum. Patients shouldn’t just be “sent” to the ED or the hospital, especially not when they are acutely ill. Their personal physician, their medical home, should “arrange” for an orderly transfer of care. This would involve a transfer of information (possibly facilitated by an updated PHR), but as much by the assurance that the accepting physician or institution is prepared for the handoff, acknowledges this to the PCP, and that the patient understands the handoff has taken place.
In the same way, patients would not be just “sent out” from the hospital. The treating physician (it could be the hospitalist, but also the surgeon or cardiologist) would remain the doctor of record—the first resource for patients’ question and issues—until another “receiving” physician has accepted the handoff, acknowledges this role, and the patient agrees.
We could rapidly shift this process by allowing the patient to decide when the hospitalization has ended. We could change the system overnight by making one of the conditions for payment for a hospitalization (to the physicians and the hospital) that the patient has signed off that indeed the hospitalization has ended.
This might include a discussion of chronic medications to continue, acute therapies to complete, understanding by the patient of where and when to receive follow-up testing and evaluation, and a clear understanding of which physician is now accountable for future issues and questions as patients travel from acute illness to normal function.
There certainly are economic and societal issues. Not everyone has a PCP or can pay for their outpatient care, and this could be a full-employment plan for liability attorneys, but in the end I am confident medical professionals would create the linkages that would minimize the deep white space patients find themselves in once they are wheeled to the front door of their hospitals.
Creatively Complete the Hospitalization
In a perfect world, everyone would have a functional, robust medical home to return to after an acute hospitalization. Unfortunately, a patient-centered medical home (PCMH) is much more of a hope than a reality for most Americans. While we are working to create a better “horizontal” hospitalization, there are clear gaps in the vertical-care world.
If we are going to be responsible for bundled care that encompasses pre-admit and post-discharge care (e.g., 30 days after discharge), then we must beef up our outpatient capabilities.
Hopefully in the long run, this can be supplied by a reinvigorated and reinvented medical home, but it is still a long way off. If payment and accountability continue to blur just when the hospitalization ends, then hospitals (and hospitalists) and Medicare and insurers will need to be creative in how and who will manage the patient. We’ll need to solve the issues around patients who are no longer sick enough to require a hospital bed but clearly are not back to their steady state.
This ties in with the accountability gap that vexes our patients every day. Very likely, hospitalists will have to assume a role in managing the patients after hospital discharge. This might take the form of a few follow-up visits and continued support systems via the Web and telephone. It will probably require a new class of hospitalist—the ambulist or the subacutist—supported by dedicated ancillary staff and systems.
Once again, Medicare and insurers can drive to a better system of post-acute care by supplying incentives: a more robust discharge payment or rewarding successful completion of a hospitalization, possibly by bundled payment incentives. In addition, there could be clear standards set that would define when this is done well with associated rewards.
I know some of these ideas are radical and make us uncomfortable. They seem to assign more responsibilities to an already overburdened profession. To be successful, these innovations require an active participation and accountability of our patients. We as the providers of healthcare cannot do this alone. It also requires the evolution of the hospital as an institution from just the healthcare provider for the acutely ill, horizontal patient, but as more a part of a continuum from acute illness to return to function. And it cries out for a robust, capable, outpatient partner in a medical home or accountable care organization (ACO) that is equally dedicated, incentivized, and accountable.
We won’t get there tomorrow, even if Dr. Berwick reads this and acts on all of the ideas on his first day at CMS.
But if we don’t get started, we know we definitely won’t get there at any time in our future. TH
Dr. Wellikson is CEO of SHM.
Hospital medicine had grown rapidly and provided the platform for change in our nation’s hospitals long before there was any meaningful healthcare legislation in Washington. With President Obama’s appointment of an innovator—Don Berwick—to head the Centers for Medicare and Medicaid Services (CMS), there is increased opportunity to ramp up revisions, large and small, to provide the incentives and the impetus to create a healthcare system for the 21st century.
With that in mind, I thought I’d offer a few ideas that Don could institute on Day 1, which could start us in the right direction, or throw us all into chaos, depending on how it plays out. While most of my attention is directed to the Medicare population, all of these ideas would be equally applicable to the commercially insured population.
Advanced Directives
We all know that too few people in this country have taken the opportunity to discuss with their families and their personal physicians how they want their care managed at critical junctures, whether it comes on suddenly with an accident or with aging. The suggestion that Medicare would pay for an office visit with your doctor to discuss this imploded with the news media’s fanning of the “death panel” flames first stoked by Sarah Palin, which sidetracked all rational discussion.
Besides setting up people for unwarranted and unwanted assaults and protracted misery, mismanaging the end stages of life leads to an enormous misallocation of physicians’ focus at a time when we all need to be mindful stewards of our limited healthcare resources.
It is acceptable if, after careful consideration, anyone chooses to not have any advanced directive, but it should be a cognitive directed choice, not just a failure to engage.
Therefore, I am proposing that Medicare offer an incentive (e.g., waiving co-payments or deductibles) to have all Medicare beneficiaries complete an advanced directive annually, or sign a form indicating they were offered an advanced directive and declined to have one invoked. In addition, Medicare could set up a system that would allow physicians (or facilities) who would manage the patient’s healthcare to have access to the conditions of the advance directives. The forms could be attached to individual Medicare profiles, possibly on the Web, in addition to being held by a patient’s PCP or medical home, if they have one.
Personal Health Records
Most people in this country can access most of the information about their personal financial status in real time from any computer in the world. Less than 10% of Americans can retrieve meaningful personal medical information. This is in spite of the prevalence of Web-based personal health record (PHR) software from Microsoft, Revolution Health, and other software vendors, along with Kaiser Permanente and a handful of insurance companies.
PHRs allow for an initial baseline set of data to be recorded and updated as new tests, diagnoses, and medications are employed. It allows for a composite knowledge of what has been tried in the past and what is being utilized in the present. This can be under patients’ control, but it would allow for appropriate access at times of acute need (e.g., an ED visit or a hospital admission).
Too often, patients with a long-term relationship with a local PCP present to the hospital and all of the healthcare professionals are forced to make critical decisions in the first few hours with insufficient or inaccurate information. This leads to needless repetition of tests or inability to compare current data with previous data (wouldn’t it be nice to have the last EKG or labs?), or in retrying a treatment regimen that hasn’t worked in the past.
And if you are in another city or if you don’t have a local physician with all of your old records, the information gap is far worse.
Once again, we could incentivize patients to have an up-to-date PHR with reduced premiums, or lower deductibles or copayments. We could look for ways to incentivize PCPs and hospitals to help patients build and maintain their PHR. We could make it a matter of course that a patient’s PHR would be updated at each intersection with healthcare information (e.g., the pharmacy or the lab or each office visit).
Physician Accountability
Somehow, we have evolved into a fragmented health system. We need to repair the disconnect between patients and physicians. The professional pact between the patient and their primary physician needs to be in place until the patient and the “next” physician agree to the handoff of responsibility. As hospitalists, we see this at both ends of the continuum. Patients shouldn’t just be “sent” to the ED or the hospital, especially not when they are acutely ill. Their personal physician, their medical home, should “arrange” for an orderly transfer of care. This would involve a transfer of information (possibly facilitated by an updated PHR), but as much by the assurance that the accepting physician or institution is prepared for the handoff, acknowledges this to the PCP, and that the patient understands the handoff has taken place.
In the same way, patients would not be just “sent out” from the hospital. The treating physician (it could be the hospitalist, but also the surgeon or cardiologist) would remain the doctor of record—the first resource for patients’ question and issues—until another “receiving” physician has accepted the handoff, acknowledges this role, and the patient agrees.
We could rapidly shift this process by allowing the patient to decide when the hospitalization has ended. We could change the system overnight by making one of the conditions for payment for a hospitalization (to the physicians and the hospital) that the patient has signed off that indeed the hospitalization has ended.
This might include a discussion of chronic medications to continue, acute therapies to complete, understanding by the patient of where and when to receive follow-up testing and evaluation, and a clear understanding of which physician is now accountable for future issues and questions as patients travel from acute illness to normal function.
There certainly are economic and societal issues. Not everyone has a PCP or can pay for their outpatient care, and this could be a full-employment plan for liability attorneys, but in the end I am confident medical professionals would create the linkages that would minimize the deep white space patients find themselves in once they are wheeled to the front door of their hospitals.
Creatively Complete the Hospitalization
In a perfect world, everyone would have a functional, robust medical home to return to after an acute hospitalization. Unfortunately, a patient-centered medical home (PCMH) is much more of a hope than a reality for most Americans. While we are working to create a better “horizontal” hospitalization, there are clear gaps in the vertical-care world.
If we are going to be responsible for bundled care that encompasses pre-admit and post-discharge care (e.g., 30 days after discharge), then we must beef up our outpatient capabilities.
Hopefully in the long run, this can be supplied by a reinvigorated and reinvented medical home, but it is still a long way off. If payment and accountability continue to blur just when the hospitalization ends, then hospitals (and hospitalists) and Medicare and insurers will need to be creative in how and who will manage the patient. We’ll need to solve the issues around patients who are no longer sick enough to require a hospital bed but clearly are not back to their steady state.
This ties in with the accountability gap that vexes our patients every day. Very likely, hospitalists will have to assume a role in managing the patients after hospital discharge. This might take the form of a few follow-up visits and continued support systems via the Web and telephone. It will probably require a new class of hospitalist—the ambulist or the subacutist—supported by dedicated ancillary staff and systems.
Once again, Medicare and insurers can drive to a better system of post-acute care by supplying incentives: a more robust discharge payment or rewarding successful completion of a hospitalization, possibly by bundled payment incentives. In addition, there could be clear standards set that would define when this is done well with associated rewards.
I know some of these ideas are radical and make us uncomfortable. They seem to assign more responsibilities to an already overburdened profession. To be successful, these innovations require an active participation and accountability of our patients. We as the providers of healthcare cannot do this alone. It also requires the evolution of the hospital as an institution from just the healthcare provider for the acutely ill, horizontal patient, but as more a part of a continuum from acute illness to return to function. And it cries out for a robust, capable, outpatient partner in a medical home or accountable care organization (ACO) that is equally dedicated, incentivized, and accountable.
We won’t get there tomorrow, even if Dr. Berwick reads this and acts on all of the ideas on his first day at CMS.
But if we don’t get started, we know we definitely won’t get there at any time in our future. TH
Dr. Wellikson is CEO of SHM.
Rise of the Napturnist
The best sleep I ever got was in the winter of 1996. I remember it vividly. It was effortless, natural, blissful. I had swiftly slipped through the early phases of non-REM sleep, skillfully side-stepping alpha and theta waves, leaving myoclonic jerks in my wake. There was a brief hypnagogic dream involving sun-swept landscapes, playful butterflies, and a field of rhythmically blowing lavender that waved me further along on my slumbering voyage. And then, magically, I was basking in the pillowy splendor of stage 4 sleep, delta waves soothingly serenading me into hibernation.
I had found the celebrated state of suspended sensory and motor activity characterized by unconsciousness and loss of voluntary muscle movement.1 I was asleep.
I was an intern, had just worked 36 continuous hours, and was driving a car.
ACGME Outlines Resident Duty-Hours Changes
I was reminded of this incident on June 23, when I reviewed the freshly minted recommendations from the Accreditation Council for Graduate Medical Education (ACGME) task force regarding duty hours.2 This proposal follows on ACGME’s 2003 report, which enacted such national resident duty-hour standards as the 80-hour work week, the maximum 24-hour shift (plus six hours for administrative time), and the requirement for 10 hours off between shifts.
Like the 2003 report, which has played a large role in the rise of academic HM, the 2010 recommendations have major implications for academic hospitalists and our community brethren who will receive our residency graduates. As such, the reaction within the hospitalist community was immediate. Within minutes of ACGME’s notification, I was inundated with e-mail from colleagues both locally and nationally. Everyone was struggling with the repercussions. Was this good for resident education, a boon or bust for HM, a death knell for teaching hospitals?
So What’s in There?
This hotly anticipated report focuses its energy on four key elements of what the ACGME has morphed from “duty hours” into the resident “work environment”: resident supervision, handoffs of patient care, use of systems to enhance patient safety, and the effects of sleep on performance. Much of this is not really controversial and likely good for both residents and HM—an emphasis on systems-based practice, transitions of care, and expectations around communication.
The most discussed and controversial changes regard the move toward supervision and work hours that are customized to trainees’ levels. Unlike in the past, when the intern bore the brunt of the hours and patient duties, this proposal emphasizes graded supervision and duty-hour expectations. Practically, this means first-year residents will require closer supervision (whether by a resident or an attending has yet to be delineated) than more senior residents. Likewise, although all residents can only work a maximum of 80 hours averaged over four weeks (no change from 2003), the maximum shift length for interns will be limited to 16 hours. Upper-level residents will be limited to no more than 24 consecutive hours with an additional four hours for administrative work, but it is “strongly suggested” that residents working longer than 16 hours be provided with opportunities for “strategic napping.”
Read the Tea Leaves
I think these recommendations are rational and reasonable. To be sure, when these go into effect on July 1, 2011, they will have a tremendous impact on my residency program, my hospital, and my hospitalist faculty. My program, like most, likely will move toward a shift system of patient care, instead of overnight call. My hospital likely will have to expend millions of dollars annually to back-fill the work that residents would have done. My hospitalist group likely will have to expand our nonresident coverage both during the day and at night, inducing one of my partners to query, “Does this mean we’ll have to hire napturnists to cover the residents’ strategic nap time?”
It’s easy to deride these work environment changes, especially if we see the world through the uphill-in-the-snow-both-ways lenses most of us use to recollect (fondly?) our residency days. Yes, the field of medicine is losing a rite of passage many of us endured, and I too retrospectively feel the nostalgic tug of the long shifts, the seemingly insurmountable avalanche of admissions, and the autonomy in resident decision-making that made my training so valuable. But are we really against ensuring that residents are properly supervised, handoffs are more standardized, and systems are in place to protect the safety of our patients?
Sleep = Safety
The push behind limiting the maximum duration of work to 16 and 24 hours for interns and residents, respectively, stems from the growing body of literature regarding the detrimental effects of the fatigue and sleep deprivation that comes with long shifts. Not so shockingly, the data show that residents are sleepy. When administered the Epworth Sleepiness Score, residents appear sleepier than sleep apneic patients and nearly as sleepy as narcoleptics.3,4 In fact, they are so sleepy they often don’t even recognize when they are sleeping. One study showed that nearly half the time that anesthesia residents were asleep, they were unaware that they were actually sleeping.5
Further data suggest that residents who sleep less than five hours per night are twice as likely to be sued, and significantly more likely to report adverse events and errors in patient care.6 When comparing traditional, every-third-night call and 24- to 30-hour shifts with 16-hour shifts, the former staffing model is associated with 36% more serious errors than the latter.
Furthermore, there is a five-fold increase in the rate of serious diagnostic errors in the residents in the longer-shift group.7 And to finish where I began, residents who worked shifts that lasted more than 24 hours are more than twice as likely to crash their cars as those working less than 24-hour shifts. In fact, every additional extended-duration shift per month increases the chances of a car crash while commuting by 16%.8
So ask yourself this: If you were designing, from scratch, residency training today, would you really design a system similar to what we had 10 years ago? Like the one we have now? Would you ask residents, many just months out of medical school, to admit a dozen or more patients a day, stay awake for more than 30 hours, and care for the sickest, most frail patients without the assistance of more senior physicians?
The field of medicine is at a crossroads, and it faces many questions, not the least of which is how best to train our future physicians. The ACGME has published its proposal and given the public, including you, until Aug. 9 to voice your comments. These are big issues to ponder.
Maybe you should sleep on it. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
References
- Sleep. Wikipedia website. Available at: http://en.wikipedia.org/wiki/Sleep. Accessed July 10, 2010.
- Nasca TJ, Day SH, Amis S. The new recommendations on duty hours from the ACGME task force. New England Journal of Medicine website. Available at: content.nejm.org/cgi/content/full/NEJMsb1005800. Accessed July 2, 2010.
- Mustafa M, Erokwu N, Ebose I, Strohl K. Sleep problems and the risk for sleep disorders in an outpatient veteran population. Sleep & Breathing. 2005;9:57-63.
- Papp KK, Stoller EP, Sage P, et al. The effects of sleep loss and fatigue on resident-physicians: a multi-institutional, mixed-method study. Acad Med. 2005; 79:394-406.
- Howard SK, Gaba DM, Rosekind MR, Zaracone VP. The risks and implications of excessive daytime sleepiness in resident physicians. Acad Med. 2002; 77:1019-1025.
- Baldwin DC, Daugherty SR. Sleep deprivation and fatigue in residency training: results of a national survey of 1st- and 2nd-year residents. Sleep. 2004;27:371-372.
- Landrigan CP, Rothschild JM, Cronin JW, et al. Effect of reducing interns’ work hours on serious medical errors in intensive care units. N Engl J Med. 2004;18:1838-1848.
- Barger LK, Cade BE, Ayas NT, et al. Extended work shifts and the risk of motor vehicle crashes among interns. N Engl J Med. 2005;352:125-134.
The best sleep I ever got was in the winter of 1996. I remember it vividly. It was effortless, natural, blissful. I had swiftly slipped through the early phases of non-REM sleep, skillfully side-stepping alpha and theta waves, leaving myoclonic jerks in my wake. There was a brief hypnagogic dream involving sun-swept landscapes, playful butterflies, and a field of rhythmically blowing lavender that waved me further along on my slumbering voyage. And then, magically, I was basking in the pillowy splendor of stage 4 sleep, delta waves soothingly serenading me into hibernation.
I had found the celebrated state of suspended sensory and motor activity characterized by unconsciousness and loss of voluntary muscle movement.1 I was asleep.
I was an intern, had just worked 36 continuous hours, and was driving a car.
ACGME Outlines Resident Duty-Hours Changes
I was reminded of this incident on June 23, when I reviewed the freshly minted recommendations from the Accreditation Council for Graduate Medical Education (ACGME) task force regarding duty hours.2 This proposal follows on ACGME’s 2003 report, which enacted such national resident duty-hour standards as the 80-hour work week, the maximum 24-hour shift (plus six hours for administrative time), and the requirement for 10 hours off between shifts.
Like the 2003 report, which has played a large role in the rise of academic HM, the 2010 recommendations have major implications for academic hospitalists and our community brethren who will receive our residency graduates. As such, the reaction within the hospitalist community was immediate. Within minutes of ACGME’s notification, I was inundated with e-mail from colleagues both locally and nationally. Everyone was struggling with the repercussions. Was this good for resident education, a boon or bust for HM, a death knell for teaching hospitals?
So What’s in There?
This hotly anticipated report focuses its energy on four key elements of what the ACGME has morphed from “duty hours” into the resident “work environment”: resident supervision, handoffs of patient care, use of systems to enhance patient safety, and the effects of sleep on performance. Much of this is not really controversial and likely good for both residents and HM—an emphasis on systems-based practice, transitions of care, and expectations around communication.
The most discussed and controversial changes regard the move toward supervision and work hours that are customized to trainees’ levels. Unlike in the past, when the intern bore the brunt of the hours and patient duties, this proposal emphasizes graded supervision and duty-hour expectations. Practically, this means first-year residents will require closer supervision (whether by a resident or an attending has yet to be delineated) than more senior residents. Likewise, although all residents can only work a maximum of 80 hours averaged over four weeks (no change from 2003), the maximum shift length for interns will be limited to 16 hours. Upper-level residents will be limited to no more than 24 consecutive hours with an additional four hours for administrative work, but it is “strongly suggested” that residents working longer than 16 hours be provided with opportunities for “strategic napping.”
Read the Tea Leaves
I think these recommendations are rational and reasonable. To be sure, when these go into effect on July 1, 2011, they will have a tremendous impact on my residency program, my hospital, and my hospitalist faculty. My program, like most, likely will move toward a shift system of patient care, instead of overnight call. My hospital likely will have to expend millions of dollars annually to back-fill the work that residents would have done. My hospitalist group likely will have to expand our nonresident coverage both during the day and at night, inducing one of my partners to query, “Does this mean we’ll have to hire napturnists to cover the residents’ strategic nap time?”
It’s easy to deride these work environment changes, especially if we see the world through the uphill-in-the-snow-both-ways lenses most of us use to recollect (fondly?) our residency days. Yes, the field of medicine is losing a rite of passage many of us endured, and I too retrospectively feel the nostalgic tug of the long shifts, the seemingly insurmountable avalanche of admissions, and the autonomy in resident decision-making that made my training so valuable. But are we really against ensuring that residents are properly supervised, handoffs are more standardized, and systems are in place to protect the safety of our patients?
Sleep = Safety
The push behind limiting the maximum duration of work to 16 and 24 hours for interns and residents, respectively, stems from the growing body of literature regarding the detrimental effects of the fatigue and sleep deprivation that comes with long shifts. Not so shockingly, the data show that residents are sleepy. When administered the Epworth Sleepiness Score, residents appear sleepier than sleep apneic patients and nearly as sleepy as narcoleptics.3,4 In fact, they are so sleepy they often don’t even recognize when they are sleeping. One study showed that nearly half the time that anesthesia residents were asleep, they were unaware that they were actually sleeping.5
Further data suggest that residents who sleep less than five hours per night are twice as likely to be sued, and significantly more likely to report adverse events and errors in patient care.6 When comparing traditional, every-third-night call and 24- to 30-hour shifts with 16-hour shifts, the former staffing model is associated with 36% more serious errors than the latter.
Furthermore, there is a five-fold increase in the rate of serious diagnostic errors in the residents in the longer-shift group.7 And to finish where I began, residents who worked shifts that lasted more than 24 hours are more than twice as likely to crash their cars as those working less than 24-hour shifts. In fact, every additional extended-duration shift per month increases the chances of a car crash while commuting by 16%.8
So ask yourself this: If you were designing, from scratch, residency training today, would you really design a system similar to what we had 10 years ago? Like the one we have now? Would you ask residents, many just months out of medical school, to admit a dozen or more patients a day, stay awake for more than 30 hours, and care for the sickest, most frail patients without the assistance of more senior physicians?
The field of medicine is at a crossroads, and it faces many questions, not the least of which is how best to train our future physicians. The ACGME has published its proposal and given the public, including you, until Aug. 9 to voice your comments. These are big issues to ponder.
Maybe you should sleep on it. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
References
- Sleep. Wikipedia website. Available at: http://en.wikipedia.org/wiki/Sleep. Accessed July 10, 2010.
- Nasca TJ, Day SH, Amis S. The new recommendations on duty hours from the ACGME task force. New England Journal of Medicine website. Available at: content.nejm.org/cgi/content/full/NEJMsb1005800. Accessed July 2, 2010.
- Mustafa M, Erokwu N, Ebose I, Strohl K. Sleep problems and the risk for sleep disorders in an outpatient veteran population. Sleep & Breathing. 2005;9:57-63.
- Papp KK, Stoller EP, Sage P, et al. The effects of sleep loss and fatigue on resident-physicians: a multi-institutional, mixed-method study. Acad Med. 2005; 79:394-406.
- Howard SK, Gaba DM, Rosekind MR, Zaracone VP. The risks and implications of excessive daytime sleepiness in resident physicians. Acad Med. 2002; 77:1019-1025.
- Baldwin DC, Daugherty SR. Sleep deprivation and fatigue in residency training: results of a national survey of 1st- and 2nd-year residents. Sleep. 2004;27:371-372.
- Landrigan CP, Rothschild JM, Cronin JW, et al. Effect of reducing interns’ work hours on serious medical errors in intensive care units. N Engl J Med. 2004;18:1838-1848.
- Barger LK, Cade BE, Ayas NT, et al. Extended work shifts and the risk of motor vehicle crashes among interns. N Engl J Med. 2005;352:125-134.
The best sleep I ever got was in the winter of 1996. I remember it vividly. It was effortless, natural, blissful. I had swiftly slipped through the early phases of non-REM sleep, skillfully side-stepping alpha and theta waves, leaving myoclonic jerks in my wake. There was a brief hypnagogic dream involving sun-swept landscapes, playful butterflies, and a field of rhythmically blowing lavender that waved me further along on my slumbering voyage. And then, magically, I was basking in the pillowy splendor of stage 4 sleep, delta waves soothingly serenading me into hibernation.
I had found the celebrated state of suspended sensory and motor activity characterized by unconsciousness and loss of voluntary muscle movement.1 I was asleep.
I was an intern, had just worked 36 continuous hours, and was driving a car.
ACGME Outlines Resident Duty-Hours Changes
I was reminded of this incident on June 23, when I reviewed the freshly minted recommendations from the Accreditation Council for Graduate Medical Education (ACGME) task force regarding duty hours.2 This proposal follows on ACGME’s 2003 report, which enacted such national resident duty-hour standards as the 80-hour work week, the maximum 24-hour shift (plus six hours for administrative time), and the requirement for 10 hours off between shifts.
Like the 2003 report, which has played a large role in the rise of academic HM, the 2010 recommendations have major implications for academic hospitalists and our community brethren who will receive our residency graduates. As such, the reaction within the hospitalist community was immediate. Within minutes of ACGME’s notification, I was inundated with e-mail from colleagues both locally and nationally. Everyone was struggling with the repercussions. Was this good for resident education, a boon or bust for HM, a death knell for teaching hospitals?
So What’s in There?
This hotly anticipated report focuses its energy on four key elements of what the ACGME has morphed from “duty hours” into the resident “work environment”: resident supervision, handoffs of patient care, use of systems to enhance patient safety, and the effects of sleep on performance. Much of this is not really controversial and likely good for both residents and HM—an emphasis on systems-based practice, transitions of care, and expectations around communication.
The most discussed and controversial changes regard the move toward supervision and work hours that are customized to trainees’ levels. Unlike in the past, when the intern bore the brunt of the hours and patient duties, this proposal emphasizes graded supervision and duty-hour expectations. Practically, this means first-year residents will require closer supervision (whether by a resident or an attending has yet to be delineated) than more senior residents. Likewise, although all residents can only work a maximum of 80 hours averaged over four weeks (no change from 2003), the maximum shift length for interns will be limited to 16 hours. Upper-level residents will be limited to no more than 24 consecutive hours with an additional four hours for administrative work, but it is “strongly suggested” that residents working longer than 16 hours be provided with opportunities for “strategic napping.”
Read the Tea Leaves
I think these recommendations are rational and reasonable. To be sure, when these go into effect on July 1, 2011, they will have a tremendous impact on my residency program, my hospital, and my hospitalist faculty. My program, like most, likely will move toward a shift system of patient care, instead of overnight call. My hospital likely will have to expend millions of dollars annually to back-fill the work that residents would have done. My hospitalist group likely will have to expand our nonresident coverage both during the day and at night, inducing one of my partners to query, “Does this mean we’ll have to hire napturnists to cover the residents’ strategic nap time?”
It’s easy to deride these work environment changes, especially if we see the world through the uphill-in-the-snow-both-ways lenses most of us use to recollect (fondly?) our residency days. Yes, the field of medicine is losing a rite of passage many of us endured, and I too retrospectively feel the nostalgic tug of the long shifts, the seemingly insurmountable avalanche of admissions, and the autonomy in resident decision-making that made my training so valuable. But are we really against ensuring that residents are properly supervised, handoffs are more standardized, and systems are in place to protect the safety of our patients?
Sleep = Safety
The push behind limiting the maximum duration of work to 16 and 24 hours for interns and residents, respectively, stems from the growing body of literature regarding the detrimental effects of the fatigue and sleep deprivation that comes with long shifts. Not so shockingly, the data show that residents are sleepy. When administered the Epworth Sleepiness Score, residents appear sleepier than sleep apneic patients and nearly as sleepy as narcoleptics.3,4 In fact, they are so sleepy they often don’t even recognize when they are sleeping. One study showed that nearly half the time that anesthesia residents were asleep, they were unaware that they were actually sleeping.5
Further data suggest that residents who sleep less than five hours per night are twice as likely to be sued, and significantly more likely to report adverse events and errors in patient care.6 When comparing traditional, every-third-night call and 24- to 30-hour shifts with 16-hour shifts, the former staffing model is associated with 36% more serious errors than the latter.
Furthermore, there is a five-fold increase in the rate of serious diagnostic errors in the residents in the longer-shift group.7 And to finish where I began, residents who worked shifts that lasted more than 24 hours are more than twice as likely to crash their cars as those working less than 24-hour shifts. In fact, every additional extended-duration shift per month increases the chances of a car crash while commuting by 16%.8
So ask yourself this: If you were designing, from scratch, residency training today, would you really design a system similar to what we had 10 years ago? Like the one we have now? Would you ask residents, many just months out of medical school, to admit a dozen or more patients a day, stay awake for more than 30 hours, and care for the sickest, most frail patients without the assistance of more senior physicians?
The field of medicine is at a crossroads, and it faces many questions, not the least of which is how best to train our future physicians. The ACGME has published its proposal and given the public, including you, until Aug. 9 to voice your comments. These are big issues to ponder.
Maybe you should sleep on it. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
References
- Sleep. Wikipedia website. Available at: http://en.wikipedia.org/wiki/Sleep. Accessed July 10, 2010.
- Nasca TJ, Day SH, Amis S. The new recommendations on duty hours from the ACGME task force. New England Journal of Medicine website. Available at: content.nejm.org/cgi/content/full/NEJMsb1005800. Accessed July 2, 2010.
- Mustafa M, Erokwu N, Ebose I, Strohl K. Sleep problems and the risk for sleep disorders in an outpatient veteran population. Sleep & Breathing. 2005;9:57-63.
- Papp KK, Stoller EP, Sage P, et al. The effects of sleep loss and fatigue on resident-physicians: a multi-institutional, mixed-method study. Acad Med. 2005; 79:394-406.
- Howard SK, Gaba DM, Rosekind MR, Zaracone VP. The risks and implications of excessive daytime sleepiness in resident physicians. Acad Med. 2002; 77:1019-1025.
- Baldwin DC, Daugherty SR. Sleep deprivation and fatigue in residency training: results of a national survey of 1st- and 2nd-year residents. Sleep. 2004;27:371-372.
- Landrigan CP, Rothschild JM, Cronin JW, et al. Effect of reducing interns’ work hours on serious medical errors in intensive care units. N Engl J Med. 2004;18:1838-1848.
- Barger LK, Cade BE, Ayas NT, et al. Extended work shifts and the risk of motor vehicle crashes among interns. N Engl J Med. 2005;352:125-134.
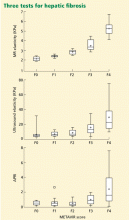
ONLINE EXCLUSIVE: Palliative Care Documentation Key to Core Measures
A growing number of publicly reported hospital quality initiatives include severity-adjusted hospital mortality rates. Although individual hospitalists are unlikely to be rated based on their patients’ mortality, this is an important component of how hospitals are evaluated—and thus a natural target for the hospital’s quality-improvement (QI) efforts and for hospitalists’ participation in them.
The challenge is that some hospital-connected deaths are unavoidable, predictable, and even appropriate when care plans focused on maximizing comfort and quality of life replace medical efforts to stave off death. Referring seriously ill patients to the hospital’s palliative-care service or to a community hospice can influence a hospital’s mortality rate, but not always in the same ways.
Where hospice care and palliative care fit in hospital mortality rates, how they are defined and counted, and how predictable deaths are either included or excluded from hospitals’ risk-adjusted mortality tallies vary between the reporting programs, according to J. Brian Cassel, PhD, senior analyst at Virginia Commonwealth University (VCU), a presenter at the National Hospice and Palliative Care Organization’s Management and Leadership Conference in April 2010 in Washington, D.C.
“Hospitals are naturally concerned about mortality rates because they want to be seen as quality health providers,” Dr. Cassel says. “How hospital mortality rates are determined can be quite complex,” with varied data sources and various methods of adjusting for severity and balancing mortality with other quality metrics. Dr. Cassel says he began digging into mortality data when concerns were raised that VCU’s acute-palliative-care unit might be causing the medical center’s overall mortality rates to spike. His research found that the unit’s operation was probably neutral relative to VCU’s overall mortality rates.
Typically, the risk-adjusted mortality rate is for selected diagnoses but counts deaths from all causes, either during the index hospitalization or within 30 days of that admission, Dr. Cassel says. Three examples of QI programs that use mortality data: CMS’ Hospital Compare, which publicly reports data on patient satisfaction and hospital processes and outcomes, including mortality; U.S. News & World Report’s “Best Hospitals” list, for which one-third of total scores are derived from its mortality index; and HealthGrades, a Golden, Colo.-based company that ranks hospitals and other health providers within a region, one condition or procedure at a time.
An ICD-9 billing code, V66.7 for “palliative care encounter,” can flag the involvement of palliative-care consultants on a hospital case, although this code often goes unused and should be among the top nine listed diagnoses in order to turn up in most quality calculations. Palliative-care consultants can help promote the use and higher positioning of this code in hospital billing, along with more complete documentation of comorbidities and symptoms. It also is possible that involving hospice and palliative-care teams with seriously ill patients earlier in their disease progression could help manage their care in community settings, avoiding hospitalizations when death is likely in the next few months.
Some hospitals might choose to refer patients thought to be close to death to contracted hospice programs—and some hospice and palliative-care advocates are using the rates as conversation starters with hospital administrators. Dr. Cassel’s advice for those advocates: Know which quality-measurement systems the hospital’s leadership follows, where adjusted mortality rates fit in those systems, and how hospice and palliative care affect them.
Regardless of mortality metrics, Dr. Cassell says, a clinician’s primary responsibility is to provide the best possible care to patients and families, reflecting their values, hopes, and treatment goals.
A growing number of publicly reported hospital quality initiatives include severity-adjusted hospital mortality rates. Although individual hospitalists are unlikely to be rated based on their patients’ mortality, this is an important component of how hospitals are evaluated—and thus a natural target for the hospital’s quality-improvement (QI) efforts and for hospitalists’ participation in them.
The challenge is that some hospital-connected deaths are unavoidable, predictable, and even appropriate when care plans focused on maximizing comfort and quality of life replace medical efforts to stave off death. Referring seriously ill patients to the hospital’s palliative-care service or to a community hospice can influence a hospital’s mortality rate, but not always in the same ways.
Where hospice care and palliative care fit in hospital mortality rates, how they are defined and counted, and how predictable deaths are either included or excluded from hospitals’ risk-adjusted mortality tallies vary between the reporting programs, according to J. Brian Cassel, PhD, senior analyst at Virginia Commonwealth University (VCU), a presenter at the National Hospice and Palliative Care Organization’s Management and Leadership Conference in April 2010 in Washington, D.C.
“Hospitals are naturally concerned about mortality rates because they want to be seen as quality health providers,” Dr. Cassel says. “How hospital mortality rates are determined can be quite complex,” with varied data sources and various methods of adjusting for severity and balancing mortality with other quality metrics. Dr. Cassel says he began digging into mortality data when concerns were raised that VCU’s acute-palliative-care unit might be causing the medical center’s overall mortality rates to spike. His research found that the unit’s operation was probably neutral relative to VCU’s overall mortality rates.
Typically, the risk-adjusted mortality rate is for selected diagnoses but counts deaths from all causes, either during the index hospitalization or within 30 days of that admission, Dr. Cassel says. Three examples of QI programs that use mortality data: CMS’ Hospital Compare, which publicly reports data on patient satisfaction and hospital processes and outcomes, including mortality; U.S. News & World Report’s “Best Hospitals” list, for which one-third of total scores are derived from its mortality index; and HealthGrades, a Golden, Colo.-based company that ranks hospitals and other health providers within a region, one condition or procedure at a time.
An ICD-9 billing code, V66.7 for “palliative care encounter,” can flag the involvement of palliative-care consultants on a hospital case, although this code often goes unused and should be among the top nine listed diagnoses in order to turn up in most quality calculations. Palliative-care consultants can help promote the use and higher positioning of this code in hospital billing, along with more complete documentation of comorbidities and symptoms. It also is possible that involving hospice and palliative-care teams with seriously ill patients earlier in their disease progression could help manage their care in community settings, avoiding hospitalizations when death is likely in the next few months.
Some hospitals might choose to refer patients thought to be close to death to contracted hospice programs—and some hospice and palliative-care advocates are using the rates as conversation starters with hospital administrators. Dr. Cassel’s advice for those advocates: Know which quality-measurement systems the hospital’s leadership follows, where adjusted mortality rates fit in those systems, and how hospice and palliative care affect them.
Regardless of mortality metrics, Dr. Cassell says, a clinician’s primary responsibility is to provide the best possible care to patients and families, reflecting their values, hopes, and treatment goals.
A growing number of publicly reported hospital quality initiatives include severity-adjusted hospital mortality rates. Although individual hospitalists are unlikely to be rated based on their patients’ mortality, this is an important component of how hospitals are evaluated—and thus a natural target for the hospital’s quality-improvement (QI) efforts and for hospitalists’ participation in them.
The challenge is that some hospital-connected deaths are unavoidable, predictable, and even appropriate when care plans focused on maximizing comfort and quality of life replace medical efforts to stave off death. Referring seriously ill patients to the hospital’s palliative-care service or to a community hospice can influence a hospital’s mortality rate, but not always in the same ways.
Where hospice care and palliative care fit in hospital mortality rates, how they are defined and counted, and how predictable deaths are either included or excluded from hospitals’ risk-adjusted mortality tallies vary between the reporting programs, according to J. Brian Cassel, PhD, senior analyst at Virginia Commonwealth University (VCU), a presenter at the National Hospice and Palliative Care Organization’s Management and Leadership Conference in April 2010 in Washington, D.C.
“Hospitals are naturally concerned about mortality rates because they want to be seen as quality health providers,” Dr. Cassel says. “How hospital mortality rates are determined can be quite complex,” with varied data sources and various methods of adjusting for severity and balancing mortality with other quality metrics. Dr. Cassel says he began digging into mortality data when concerns were raised that VCU’s acute-palliative-care unit might be causing the medical center’s overall mortality rates to spike. His research found that the unit’s operation was probably neutral relative to VCU’s overall mortality rates.
Typically, the risk-adjusted mortality rate is for selected diagnoses but counts deaths from all causes, either during the index hospitalization or within 30 days of that admission, Dr. Cassel says. Three examples of QI programs that use mortality data: CMS’ Hospital Compare, which publicly reports data on patient satisfaction and hospital processes and outcomes, including mortality; U.S. News & World Report’s “Best Hospitals” list, for which one-third of total scores are derived from its mortality index; and HealthGrades, a Golden, Colo.-based company that ranks hospitals and other health providers within a region, one condition or procedure at a time.
An ICD-9 billing code, V66.7 for “palliative care encounter,” can flag the involvement of palliative-care consultants on a hospital case, although this code often goes unused and should be among the top nine listed diagnoses in order to turn up in most quality calculations. Palliative-care consultants can help promote the use and higher positioning of this code in hospital billing, along with more complete documentation of comorbidities and symptoms. It also is possible that involving hospice and palliative-care teams with seriously ill patients earlier in their disease progression could help manage their care in community settings, avoiding hospitalizations when death is likely in the next few months.
Some hospitals might choose to refer patients thought to be close to death to contracted hospice programs—and some hospice and palliative-care advocates are using the rates as conversation starters with hospital administrators. Dr. Cassel’s advice for those advocates: Know which quality-measurement systems the hospital’s leadership follows, where adjusted mortality rates fit in those systems, and how hospice and palliative care affect them.
Regardless of mortality metrics, Dr. Cassell says, a clinician’s primary responsibility is to provide the best possible care to patients and families, reflecting their values, hopes, and treatment goals.
ONLINE EXCLUSIVE: Audio interview with a pediatric hospitalist who is starting a palliative care team
Financial Risk
When I started writing this, Congress hadn’t settled the issue of the 21% cut in Medicare reimbursement for services called for by the sustainable growth rate (SGR) formula. Fortunately, Congress stepped up and passed another extension with a 2.2% pay increase; however, the quick fix only lasts until November.
The process is all too routine: The deadline for these reimbursement cuts looms, Medicare instructs its fiscal intermediaries (the organizations that actually write the checks to providers) to hold claims rather than pay at the lower rate, and, within a few days of the deadline passing, Congress decides to pass an extension, which allows Medicare to continue paying the historical (higher) rate for the time being.
Imagine Medicare reimbursement rates dropping 21% overnight. I suspect it would be cataclysmic. But I hear remarkably little chatter about this possibility. In fact, while with 2,500 other hospitalists for several days at HM10 in April, I didn’t hear a single person bring up the SGR issue.
One reason there isn’t more handwringing about the looming, draconian cuts is that we’ve been there before. In fact, reimbursement cuts required by the SGR have come up every year since 2001. Each time, Congress has chosen not to implement the cuts; and in some years it has approved reimbursement increases instead. So most in healthcare circles basically have come to expect Congress to pass last-minute legislation to avoid the drastic cuts. (SHM and most other medical societies want a repeal of the flawed SGR formula. Visit SHM’s Legislative Action Center, http://capwiz.com/hospitalmedicine/home/, to write your legislators and urge repeal of the SGR. It only takes about two minutes, and you don’t even need to remember who your representatives are; you just need to know your ZIP code.)
Don’t Be Too Smug
There is another reason many hospitalists, and other doctors who are employed and salaried by a large entity like a hospital, might not be more concerned about proposed cuts: They probably think their own salaries will be unaffected by decreases in reimbursement from Medicare and other payors. My experience is that a lot of hospitalists are so unconcerned about payor reimbursement rates that they aren’t even aware of the threatened Medicare cuts.
Their thinking goes something like this: “I’m paid mostly via a fixed annual salary with a small productivity and quality incentive. None of this is connected to the payor mix or collection rates from the patients I see. So if the portion of uninsured patients I see goes up, my compensation is unaffected. Or if payors decrease their rates, my compensation is unaffected. So I don’t need to sweat the possibility of a 21% decrease in Medicare rates. The hospital will have to make up the difference, so my salary is unaffected, and it will be up to bean counters at the hospital to get the numbers to work out.”
In fact, this is true, in theory, for the majority of hospitalists. But I think it is a mistake to assume your salary is untouchable. If Medicare were to cut rates by 21%, you’d better run to your hospital CEO’s office right away, because a long line will form immediately. Every doctor who sees patients at your hospital will be in that line asking the CEO to provide some money to offset the Medicare cuts, and I doubt any hospital will be able to satisfy their doctors without spending so much money that the hospital goes bankrupt or out of business.
Even if you have a valid contract that calls for your compensation to be paid independent of the amount of professional fee collections, a dire shortage of money could lead a hospital to lay off hospitalists or cancel the contract (most contracts would allow the hospital to do this simply by giving a 90-day notice).
I suggest that no hospitalist feel too smug about how well their employment contract protects the group from broader market forces like reimbursement rates. I doubt we’ll ever see an overnight 21% reduction in Medicare rates, but over time, we could see ever-increasing pressure to limit the growth in our incomes.
I believe every hospitalist should spend at least a little time following broader financial issues like this one, and get involved in the political process to let your legislators know your thoughts. For the record, I think the financial underpinnings of our healthcare system are disastrously messed up and something has to be done. And I don’t think anyone’s salary, including mine, is untouchable. But I also believe the SGR is an ineffective way to make the system more financially sound. That said, you don’t need to agree with me; I only recommend that you have a reasonably informed opinion.
One approach might be for your HM group to appoint a “political” or “marketplace” watchdog. This person could be charged with following issues closely and reporting back to the whole group during regular meetings.
“Marketplace” Risk
Medicare rates are only one part of the complex financial ecosystem on which we depend. It is awfully common, and I think pretty reasonable, for hospitalists to have a contractual arrangement with hospitals. The majority of the time, the hospital has most—or all—of the risk for the financial performance of the practice. In fact, most prospective hospitalists, especially those seeking their first jobs out after residency, say one of the most attractive reasons for choosing work as a hospitalist is that many practices provide a salary that is nearly fixed. Any variable components to the salary, such as those based on production or quality, are typically very small.
A hospitalist might think, “I want a practice that pays a fixed salary so I don’t have to worry about any business and financial issues other than when to show up to work.” In fact, a lot of recruitment ads trumpet this very idea (i.e., “you handle the doctoring and get to enjoy the wonderful recreational opportunities and schools our locale provides, and we’ll worry about all the business issues”). That may sound nice, but I worry it is a little short-sighted.
Here is another point of view, which is only slightly more complicated. In most cases, you should try to negotiate a contract that insulates you from “payor risk” (e.g., changes in payor mix and rates paid by payors don’t flow through to your compensation). But you should think twice before asking your employer to assume all the risk for staffing and scheduling decisions, such as whether you get the work done with 10 hospitalists or 11, or whether you have an evening admitter (“swing”) shift. If the employer holds all the risk, then the hospitalists give up nearly all their autonomy to decide how hard they want to work and how they want to schedule themselves. This causes problems for many practices, and is the No. 1 reason I’m called in as a consultant. Contrary to being very risky and stressful, many hospitalists find it liberating to assume financial risk for their staffing and workload decisions.
You should realize that if your employer pays you a fixed compensation, then someone has to ensure that you do enough work to justify that compensation. This can mean that the employer “issues decrees” (i.e., “we won’t add another provide to the practice until we’ve averaged ‘X’ encounters per month for 6 months”). A hospitalist might see this as unreasonable, yet the group has limited recourse since the employer has already guaranteed the compensation.
If you’d rather have more autonomy in your staffing and workload, then you will need to connect your paycheck to these decisions. Although it might sound terribly risky, those who make the switch often say they wouldn’t have it any other way. Most importantly, it ensures hospitalists have much more say in big decisions. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
When I started writing this, Congress hadn’t settled the issue of the 21% cut in Medicare reimbursement for services called for by the sustainable growth rate (SGR) formula. Fortunately, Congress stepped up and passed another extension with a 2.2% pay increase; however, the quick fix only lasts until November.
The process is all too routine: The deadline for these reimbursement cuts looms, Medicare instructs its fiscal intermediaries (the organizations that actually write the checks to providers) to hold claims rather than pay at the lower rate, and, within a few days of the deadline passing, Congress decides to pass an extension, which allows Medicare to continue paying the historical (higher) rate for the time being.
Imagine Medicare reimbursement rates dropping 21% overnight. I suspect it would be cataclysmic. But I hear remarkably little chatter about this possibility. In fact, while with 2,500 other hospitalists for several days at HM10 in April, I didn’t hear a single person bring up the SGR issue.
One reason there isn’t more handwringing about the looming, draconian cuts is that we’ve been there before. In fact, reimbursement cuts required by the SGR have come up every year since 2001. Each time, Congress has chosen not to implement the cuts; and in some years it has approved reimbursement increases instead. So most in healthcare circles basically have come to expect Congress to pass last-minute legislation to avoid the drastic cuts. (SHM and most other medical societies want a repeal of the flawed SGR formula. Visit SHM’s Legislative Action Center, http://capwiz.com/hospitalmedicine/home/, to write your legislators and urge repeal of the SGR. It only takes about two minutes, and you don’t even need to remember who your representatives are; you just need to know your ZIP code.)
Don’t Be Too Smug
There is another reason many hospitalists, and other doctors who are employed and salaried by a large entity like a hospital, might not be more concerned about proposed cuts: They probably think their own salaries will be unaffected by decreases in reimbursement from Medicare and other payors. My experience is that a lot of hospitalists are so unconcerned about payor reimbursement rates that they aren’t even aware of the threatened Medicare cuts.
Their thinking goes something like this: “I’m paid mostly via a fixed annual salary with a small productivity and quality incentive. None of this is connected to the payor mix or collection rates from the patients I see. So if the portion of uninsured patients I see goes up, my compensation is unaffected. Or if payors decrease their rates, my compensation is unaffected. So I don’t need to sweat the possibility of a 21% decrease in Medicare rates. The hospital will have to make up the difference, so my salary is unaffected, and it will be up to bean counters at the hospital to get the numbers to work out.”
In fact, this is true, in theory, for the majority of hospitalists. But I think it is a mistake to assume your salary is untouchable. If Medicare were to cut rates by 21%, you’d better run to your hospital CEO’s office right away, because a long line will form immediately. Every doctor who sees patients at your hospital will be in that line asking the CEO to provide some money to offset the Medicare cuts, and I doubt any hospital will be able to satisfy their doctors without spending so much money that the hospital goes bankrupt or out of business.
Even if you have a valid contract that calls for your compensation to be paid independent of the amount of professional fee collections, a dire shortage of money could lead a hospital to lay off hospitalists or cancel the contract (most contracts would allow the hospital to do this simply by giving a 90-day notice).
I suggest that no hospitalist feel too smug about how well their employment contract protects the group from broader market forces like reimbursement rates. I doubt we’ll ever see an overnight 21% reduction in Medicare rates, but over time, we could see ever-increasing pressure to limit the growth in our incomes.
I believe every hospitalist should spend at least a little time following broader financial issues like this one, and get involved in the political process to let your legislators know your thoughts. For the record, I think the financial underpinnings of our healthcare system are disastrously messed up and something has to be done. And I don’t think anyone’s salary, including mine, is untouchable. But I also believe the SGR is an ineffective way to make the system more financially sound. That said, you don’t need to agree with me; I only recommend that you have a reasonably informed opinion.
One approach might be for your HM group to appoint a “political” or “marketplace” watchdog. This person could be charged with following issues closely and reporting back to the whole group during regular meetings.
“Marketplace” Risk
Medicare rates are only one part of the complex financial ecosystem on which we depend. It is awfully common, and I think pretty reasonable, for hospitalists to have a contractual arrangement with hospitals. The majority of the time, the hospital has most—or all—of the risk for the financial performance of the practice. In fact, most prospective hospitalists, especially those seeking their first jobs out after residency, say one of the most attractive reasons for choosing work as a hospitalist is that many practices provide a salary that is nearly fixed. Any variable components to the salary, such as those based on production or quality, are typically very small.
A hospitalist might think, “I want a practice that pays a fixed salary so I don’t have to worry about any business and financial issues other than when to show up to work.” In fact, a lot of recruitment ads trumpet this very idea (i.e., “you handle the doctoring and get to enjoy the wonderful recreational opportunities and schools our locale provides, and we’ll worry about all the business issues”). That may sound nice, but I worry it is a little short-sighted.
Here is another point of view, which is only slightly more complicated. In most cases, you should try to negotiate a contract that insulates you from “payor risk” (e.g., changes in payor mix and rates paid by payors don’t flow through to your compensation). But you should think twice before asking your employer to assume all the risk for staffing and scheduling decisions, such as whether you get the work done with 10 hospitalists or 11, or whether you have an evening admitter (“swing”) shift. If the employer holds all the risk, then the hospitalists give up nearly all their autonomy to decide how hard they want to work and how they want to schedule themselves. This causes problems for many practices, and is the No. 1 reason I’m called in as a consultant. Contrary to being very risky and stressful, many hospitalists find it liberating to assume financial risk for their staffing and workload decisions.
You should realize that if your employer pays you a fixed compensation, then someone has to ensure that you do enough work to justify that compensation. This can mean that the employer “issues decrees” (i.e., “we won’t add another provide to the practice until we’ve averaged ‘X’ encounters per month for 6 months”). A hospitalist might see this as unreasonable, yet the group has limited recourse since the employer has already guaranteed the compensation.
If you’d rather have more autonomy in your staffing and workload, then you will need to connect your paycheck to these decisions. Although it might sound terribly risky, those who make the switch often say they wouldn’t have it any other way. Most importantly, it ensures hospitalists have much more say in big decisions. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
When I started writing this, Congress hadn’t settled the issue of the 21% cut in Medicare reimbursement for services called for by the sustainable growth rate (SGR) formula. Fortunately, Congress stepped up and passed another extension with a 2.2% pay increase; however, the quick fix only lasts until November.
The process is all too routine: The deadline for these reimbursement cuts looms, Medicare instructs its fiscal intermediaries (the organizations that actually write the checks to providers) to hold claims rather than pay at the lower rate, and, within a few days of the deadline passing, Congress decides to pass an extension, which allows Medicare to continue paying the historical (higher) rate for the time being.
Imagine Medicare reimbursement rates dropping 21% overnight. I suspect it would be cataclysmic. But I hear remarkably little chatter about this possibility. In fact, while with 2,500 other hospitalists for several days at HM10 in April, I didn’t hear a single person bring up the SGR issue.
One reason there isn’t more handwringing about the looming, draconian cuts is that we’ve been there before. In fact, reimbursement cuts required by the SGR have come up every year since 2001. Each time, Congress has chosen not to implement the cuts; and in some years it has approved reimbursement increases instead. So most in healthcare circles basically have come to expect Congress to pass last-minute legislation to avoid the drastic cuts. (SHM and most other medical societies want a repeal of the flawed SGR formula. Visit SHM’s Legislative Action Center, http://capwiz.com/hospitalmedicine/home/, to write your legislators and urge repeal of the SGR. It only takes about two minutes, and you don’t even need to remember who your representatives are; you just need to know your ZIP code.)
Don’t Be Too Smug
There is another reason many hospitalists, and other doctors who are employed and salaried by a large entity like a hospital, might not be more concerned about proposed cuts: They probably think their own salaries will be unaffected by decreases in reimbursement from Medicare and other payors. My experience is that a lot of hospitalists are so unconcerned about payor reimbursement rates that they aren’t even aware of the threatened Medicare cuts.
Their thinking goes something like this: “I’m paid mostly via a fixed annual salary with a small productivity and quality incentive. None of this is connected to the payor mix or collection rates from the patients I see. So if the portion of uninsured patients I see goes up, my compensation is unaffected. Or if payors decrease their rates, my compensation is unaffected. So I don’t need to sweat the possibility of a 21% decrease in Medicare rates. The hospital will have to make up the difference, so my salary is unaffected, and it will be up to bean counters at the hospital to get the numbers to work out.”
In fact, this is true, in theory, for the majority of hospitalists. But I think it is a mistake to assume your salary is untouchable. If Medicare were to cut rates by 21%, you’d better run to your hospital CEO’s office right away, because a long line will form immediately. Every doctor who sees patients at your hospital will be in that line asking the CEO to provide some money to offset the Medicare cuts, and I doubt any hospital will be able to satisfy their doctors without spending so much money that the hospital goes bankrupt or out of business.
Even if you have a valid contract that calls for your compensation to be paid independent of the amount of professional fee collections, a dire shortage of money could lead a hospital to lay off hospitalists or cancel the contract (most contracts would allow the hospital to do this simply by giving a 90-day notice).
I suggest that no hospitalist feel too smug about how well their employment contract protects the group from broader market forces like reimbursement rates. I doubt we’ll ever see an overnight 21% reduction in Medicare rates, but over time, we could see ever-increasing pressure to limit the growth in our incomes.
I believe every hospitalist should spend at least a little time following broader financial issues like this one, and get involved in the political process to let your legislators know your thoughts. For the record, I think the financial underpinnings of our healthcare system are disastrously messed up and something has to be done. And I don’t think anyone’s salary, including mine, is untouchable. But I also believe the SGR is an ineffective way to make the system more financially sound. That said, you don’t need to agree with me; I only recommend that you have a reasonably informed opinion.
One approach might be for your HM group to appoint a “political” or “marketplace” watchdog. This person could be charged with following issues closely and reporting back to the whole group during regular meetings.
“Marketplace” Risk
Medicare rates are only one part of the complex financial ecosystem on which we depend. It is awfully common, and I think pretty reasonable, for hospitalists to have a contractual arrangement with hospitals. The majority of the time, the hospital has most—or all—of the risk for the financial performance of the practice. In fact, most prospective hospitalists, especially those seeking their first jobs out after residency, say one of the most attractive reasons for choosing work as a hospitalist is that many practices provide a salary that is nearly fixed. Any variable components to the salary, such as those based on production or quality, are typically very small.
A hospitalist might think, “I want a practice that pays a fixed salary so I don’t have to worry about any business and financial issues other than when to show up to work.” In fact, a lot of recruitment ads trumpet this very idea (i.e., “you handle the doctoring and get to enjoy the wonderful recreational opportunities and schools our locale provides, and we’ll worry about all the business issues”). That may sound nice, but I worry it is a little short-sighted.
Here is another point of view, which is only slightly more complicated. In most cases, you should try to negotiate a contract that insulates you from “payor risk” (e.g., changes in payor mix and rates paid by payors don’t flow through to your compensation). But you should think twice before asking your employer to assume all the risk for staffing and scheduling decisions, such as whether you get the work done with 10 hospitalists or 11, or whether you have an evening admitter (“swing”) shift. If the employer holds all the risk, then the hospitalists give up nearly all their autonomy to decide how hard they want to work and how they want to schedule themselves. This causes problems for many practices, and is the No. 1 reason I’m called in as a consultant. Contrary to being very risky and stressful, many hospitalists find it liberating to assume financial risk for their staffing and workload decisions.
You should realize that if your employer pays you a fixed compensation, then someone has to ensure that you do enough work to justify that compensation. This can mean that the employer “issues decrees” (i.e., “we won’t add another provide to the practice until we’ve averaged ‘X’ encounters per month for 6 months”). A hospitalist might see this as unreasonable, yet the group has limited recourse since the employer has already guaranteed the compensation.
If you’d rather have more autonomy in your staffing and workload, then you will need to connect your paycheck to these decisions. Although it might sound terribly risky, those who make the switch often say they wouldn’t have it any other way. Most importantly, it ensures hospitalists have much more say in big decisions. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Dr. Hospitalist
I have been asked to create a proposal for incentive-based reimbursements for our group. One of the more common areas cited in the literature is incentives for “good citizenship.” What exactly constitutes good citizenship and how is it tracked? Thanks.
Lou O’Boyle
Dr. Hospitalist responds:
Congratulations on your new responsibility! Most hospitalist programs in the U.S. have incentive-based compensation as part of their provider compensation plans. While some groups succeed with their incentive-based compensation plans, others fail at what the plan is intended to achieve. In addition to answering your question, I will discuss some keys to developing a successful plan.
From the nature of your question, it sounds as if you are a staff hospitalist or a group administrator who was tasked by the leader or the group to come up with the terms of an incentive-based plan. I am not aware of any guidelines on who is best suited to develop an incentive-based compensation plan, but, in general, I do think it is a mistake for group leaders to unilaterally mandate the terms the of the plan without input from its clinical providers. After all, it seems like common sense to speak with the people who you are trying to motivate before developing an incentive plan. Depending on the size of the group, I think most groups would do well to have a small, representative group of the frontline providers who would work with the leader to develop the plan.
First and foremost, the plan rules must be clear to all participants. Your question is an excellent example. “Good citizenship” probably means different things to different people. For some, it means attending all staff meetings, or active participation on hospital committees. For others, it represents high customer satisfaction or adherence to clinical guidelines. I am not aware of a universal definition for “good citizenship” when it comes to hospitalist incentive-based compensation plans.
After you have determined what you want your plan to motivate the staff to do, I urge you to define the plan rules as clearly as possible; write it down for all providers to see. If the plan rules are vague, opaque, or open to interpretation, participants might not be motivated to reach the goals, because they don’t really understand the plan rules. Even worse, participants might leave with the falsely held belief that someone is trying to mislead them.
Next, figure out a way to easily gather and display the data. Don’t underestimate the amount of work this involves. It is vitally important for everyone to understand who, when, where, and how the data will be gathered and displayed. Needless to say, the process of gathering and displaying the data must be done in a fashion that eliminates questions of validity.
At the core of any incentive-based compensation plan is the actual incentive. The process of determining the actual incentive can be fraught with controversy. I urge all working groups to proceed through this step with caution. What motivates people can vary widely. It is important for participants to view the incentives as sufficiently significant so that they are motivated to take the desired steps to achieve the goal. That said, if participants view the incentive as too large a component of total compensation, they might look for alternative employment with incentive plans they view as “safer” for their personal income.
Most incentive-based compensation plans are from 15% to 25% of total compensation. Again, this is not a fixed rule. Some groups choose incentives that are 5% to 10% of total compensation; others have incentives up to 40% of total compensation. The important takeaway here is to understand what is necessary to motivate your group.
Although most incentives are monetary, I encourage you to think beyond money as the only motivator in your plan. Some examples include time off from work; flat-screen televisions; or all-expenses-paid vacations.
Whether you choose money or nonmonetary items, it is important to be clear on when the payout will occur. Many groups pay the incentive annually. It might be the easy way to do it, but it also doesn’t mean a once-a-year payout is right for your group. The goal of the incentive is to change provider behavior. In order to accomplish this goal, participants must associate their behavior with the incentive-based reward. Paying the incentive-based reward at the right frequency (quarterly, every six months) might increase the chance this will occur. I don’t advise weekly incentives; not only is that process cumbersome, but the rewards also are likely to be small and potentially ineffective. The frequency of payout should be part of the planning discussions.
My last piece of advice is to take steps to help your providers succeed. In addition to telling your providers how to reach their incentives, show them how to succeed. This does not mean setting the bar low. Providers should have to work hard to reach their goals, and there is no reason why you shouldn’t give them the tools to help them succeed. TH
I have been asked to create a proposal for incentive-based reimbursements for our group. One of the more common areas cited in the literature is incentives for “good citizenship.” What exactly constitutes good citizenship and how is it tracked? Thanks.
Lou O’Boyle
Dr. Hospitalist responds:
Congratulations on your new responsibility! Most hospitalist programs in the U.S. have incentive-based compensation as part of their provider compensation plans. While some groups succeed with their incentive-based compensation plans, others fail at what the plan is intended to achieve. In addition to answering your question, I will discuss some keys to developing a successful plan.
From the nature of your question, it sounds as if you are a staff hospitalist or a group administrator who was tasked by the leader or the group to come up with the terms of an incentive-based plan. I am not aware of any guidelines on who is best suited to develop an incentive-based compensation plan, but, in general, I do think it is a mistake for group leaders to unilaterally mandate the terms the of the plan without input from its clinical providers. After all, it seems like common sense to speak with the people who you are trying to motivate before developing an incentive plan. Depending on the size of the group, I think most groups would do well to have a small, representative group of the frontline providers who would work with the leader to develop the plan.
First and foremost, the plan rules must be clear to all participants. Your question is an excellent example. “Good citizenship” probably means different things to different people. For some, it means attending all staff meetings, or active participation on hospital committees. For others, it represents high customer satisfaction or adherence to clinical guidelines. I am not aware of a universal definition for “good citizenship” when it comes to hospitalist incentive-based compensation plans.
After you have determined what you want your plan to motivate the staff to do, I urge you to define the plan rules as clearly as possible; write it down for all providers to see. If the plan rules are vague, opaque, or open to interpretation, participants might not be motivated to reach the goals, because they don’t really understand the plan rules. Even worse, participants might leave with the falsely held belief that someone is trying to mislead them.
Next, figure out a way to easily gather and display the data. Don’t underestimate the amount of work this involves. It is vitally important for everyone to understand who, when, where, and how the data will be gathered and displayed. Needless to say, the process of gathering and displaying the data must be done in a fashion that eliminates questions of validity.
At the core of any incentive-based compensation plan is the actual incentive. The process of determining the actual incentive can be fraught with controversy. I urge all working groups to proceed through this step with caution. What motivates people can vary widely. It is important for participants to view the incentives as sufficiently significant so that they are motivated to take the desired steps to achieve the goal. That said, if participants view the incentive as too large a component of total compensation, they might look for alternative employment with incentive plans they view as “safer” for their personal income.
Most incentive-based compensation plans are from 15% to 25% of total compensation. Again, this is not a fixed rule. Some groups choose incentives that are 5% to 10% of total compensation; others have incentives up to 40% of total compensation. The important takeaway here is to understand what is necessary to motivate your group.
Although most incentives are monetary, I encourage you to think beyond money as the only motivator in your plan. Some examples include time off from work; flat-screen televisions; or all-expenses-paid vacations.
Whether you choose money or nonmonetary items, it is important to be clear on when the payout will occur. Many groups pay the incentive annually. It might be the easy way to do it, but it also doesn’t mean a once-a-year payout is right for your group. The goal of the incentive is to change provider behavior. In order to accomplish this goal, participants must associate their behavior with the incentive-based reward. Paying the incentive-based reward at the right frequency (quarterly, every six months) might increase the chance this will occur. I don’t advise weekly incentives; not only is that process cumbersome, but the rewards also are likely to be small and potentially ineffective. The frequency of payout should be part of the planning discussions.
My last piece of advice is to take steps to help your providers succeed. In addition to telling your providers how to reach their incentives, show them how to succeed. This does not mean setting the bar low. Providers should have to work hard to reach their goals, and there is no reason why you shouldn’t give them the tools to help them succeed. TH
I have been asked to create a proposal for incentive-based reimbursements for our group. One of the more common areas cited in the literature is incentives for “good citizenship.” What exactly constitutes good citizenship and how is it tracked? Thanks.
Lou O’Boyle
Dr. Hospitalist responds:
Congratulations on your new responsibility! Most hospitalist programs in the U.S. have incentive-based compensation as part of their provider compensation plans. While some groups succeed with their incentive-based compensation plans, others fail at what the plan is intended to achieve. In addition to answering your question, I will discuss some keys to developing a successful plan.
From the nature of your question, it sounds as if you are a staff hospitalist or a group administrator who was tasked by the leader or the group to come up with the terms of an incentive-based plan. I am not aware of any guidelines on who is best suited to develop an incentive-based compensation plan, but, in general, I do think it is a mistake for group leaders to unilaterally mandate the terms the of the plan without input from its clinical providers. After all, it seems like common sense to speak with the people who you are trying to motivate before developing an incentive plan. Depending on the size of the group, I think most groups would do well to have a small, representative group of the frontline providers who would work with the leader to develop the plan.
First and foremost, the plan rules must be clear to all participants. Your question is an excellent example. “Good citizenship” probably means different things to different people. For some, it means attending all staff meetings, or active participation on hospital committees. For others, it represents high customer satisfaction or adherence to clinical guidelines. I am not aware of a universal definition for “good citizenship” when it comes to hospitalist incentive-based compensation plans.
After you have determined what you want your plan to motivate the staff to do, I urge you to define the plan rules as clearly as possible; write it down for all providers to see. If the plan rules are vague, opaque, or open to interpretation, participants might not be motivated to reach the goals, because they don’t really understand the plan rules. Even worse, participants might leave with the falsely held belief that someone is trying to mislead them.
Next, figure out a way to easily gather and display the data. Don’t underestimate the amount of work this involves. It is vitally important for everyone to understand who, when, where, and how the data will be gathered and displayed. Needless to say, the process of gathering and displaying the data must be done in a fashion that eliminates questions of validity.
At the core of any incentive-based compensation plan is the actual incentive. The process of determining the actual incentive can be fraught with controversy. I urge all working groups to proceed through this step with caution. What motivates people can vary widely. It is important for participants to view the incentives as sufficiently significant so that they are motivated to take the desired steps to achieve the goal. That said, if participants view the incentive as too large a component of total compensation, they might look for alternative employment with incentive plans they view as “safer” for their personal income.
Most incentive-based compensation plans are from 15% to 25% of total compensation. Again, this is not a fixed rule. Some groups choose incentives that are 5% to 10% of total compensation; others have incentives up to 40% of total compensation. The important takeaway here is to understand what is necessary to motivate your group.
Although most incentives are monetary, I encourage you to think beyond money as the only motivator in your plan. Some examples include time off from work; flat-screen televisions; or all-expenses-paid vacations.
Whether you choose money or nonmonetary items, it is important to be clear on when the payout will occur. Many groups pay the incentive annually. It might be the easy way to do it, but it also doesn’t mean a once-a-year payout is right for your group. The goal of the incentive is to change provider behavior. In order to accomplish this goal, participants must associate their behavior with the incentive-based reward. Paying the incentive-based reward at the right frequency (quarterly, every six months) might increase the chance this will occur. I don’t advise weekly incentives; not only is that process cumbersome, but the rewards also are likely to be small and potentially ineffective. The frequency of payout should be part of the planning discussions.
My last piece of advice is to take steps to help your providers succeed. In addition to telling your providers how to reach their incentives, show them how to succeed. This does not mean setting the bar low. Providers should have to work hard to reach their goals, and there is no reason why you shouldn’t give them the tools to help them succeed. TH
A young woman with a breast mass: What every internist should know
A 40-year-old premenopausal woman presents with a palpable lump in her left breast. She first noted it 2 months ago on self-examination, and it has steadily grown in size regardless of the phase of her menstrual cycle.
The patient has never undergone mammography. Her menarche was at age 12. At age 35, she had one child (whom she breastfed) after a normal first full-term pregnancy. She took oral contraceptives for 10 years before her pregnancy. She has no other medical problems. She has no family history of breast or ovarian cancer.
On examination, her breasts are slightly asymmetric, without skin discoloration, tenderness, swelling, nipple retraction, or discharge. A 1.5- to 2-cm, rubbery, mobile lump can be felt in the left breast at about the 2 o’clock position. No axillary lymph nodes can be palpated. The rest of her examination is normal.
BREAST CANCER MUST BE RULED OUT
Benign breast disease is found in approximately 90% of women 20 to 50 years of age who come to a physician with a breast problem.1
Nevertheless, breast cancer is of major concern. It is the most common type of cancer in women in the United States, responsible for an estimated 194,440 new cases and 40,610 deaths in 2009. It is also the leading cause of cancer-related death in women age 45 to 55 years in this country.2,3
Breast cancer is most common in postmenopausal women, its incidence rising sharply after the age of 45 and leveling off at age 75. The median age at diagnosis is 61 years. Still, 1.9% of breast cancers in women are diagnosed at age 20 to 34, 10.6% at age 35 to 44, and 22.4% at age 45 to 54.4
Thus, it is paramount to perform a thorough assessment and workup of women who have breast lumps, regardless of their age. Doing so allows breast cancer to be detected at an early stage. The 5-year survival rate is 98.0% for women with localized disease, 83.6% with regional disease, and 23.4% with distant disease.4
WHAT IS THE APPROPRIATE WORKUP?
1. Which of the following are appropriate in the workup of this patient?
- Mammography
- Ultrasonography
- Percutaneous needle biopsy of the lesion
- Magnetic resonance imaging (MRI) of the brain
- Computed tomography (CT) of the chest, abdomen, and pelvis
- Positron emission tomography (PET)
She should undergo mammography, ultrasonography, and percutaneous needle biopsy.
Physical findings that suggest breast cancer include a hard, isolated, sometimes nonmobile lump, serosanguinous nipple discharge, and unilateral nipple retraction. Peau d’orange skin discoloration can occur. A scaly, vesicular, or ulcerated rash with or without pruritus, burning, irritation, or pain of the nipple or skin (Paget disease of the breast) is found in 1% to 3% of breast cancers and may be initially dismissed as mastitis.5,6 Palpable enlarged axillary lymph nodes can suggest invasive breast cancer.
Mammography is recommended in all cases of suspicious breast lumps. In a patient with a palpable lump, diagnostic mammography has a positive predictive value of 21.8%, a specificity of 85.8%, and a sensitivity of 87.7%, which are higher values than in a patient without signs or symptoms.7
The BIRADS score. Mammographic findings are summarized using a scoring system devised by the American College of Radiology called BIRADS (Breast Imaging Reporting and Data System). This system is based on mass irregularity, density, spiculation, and presence or absence of microcalcifications. It standardizes the results of mammography, gives an estimate of the risk of breast cancer, and recommends the frequency of follow-up examinations.8 Scores range from 0 to 6:
- 0—Incomplete assessment warranting additional evaluation
- 1—Completely negative mammogram
- 2—Benign lesion
- 3—Requires follow-up mammogram at 6 months
- 4—Risk of cancer is 2% to 95%; core biopsy needed
- 5—Risk of cancer is more than 95%; core biopsy needed
- 6—Cases that have already been proven to be malignant.
Ultrasonography is also done if a suspicious lesion is found on mammography or physical examination. It helps differentiate between solid and cystic masses. If a mass is identified as a cyst, ultrasonography can further characterize it as simple, complicated-simple, or complex. Simple cysts and complicated-simple cysts are unlikely to be malignant.9,10 Complex cysts or cysts associated with solid tissue are evaluated by biopsy.
Percutaneous needle biopsy should be done for a definitive diagnosis of most suspicious breast masses.
MRI can sometimes provide more accurate information about the possibility of multifocal breast cancer by revealing additional lesions missed on mammography or ultrasonography. It is also useful in determining more accurately the size of the breast tumor and looking for any possible contralateral lesions. In addition, it can sometimes detect enlarged axillary lymph nodes. However, it has poor specificity for breast cancer and may lead to additional and sometimes unnecessary diagnostic tests, which can delay treatment.
MRI’s role is therefore not clearly established, but it is commonly used in clinical practice. It is argued that workup of MRI findings may help in planning more accurate surgical procedures and may prevent reoperations. Based on retrospective analyses, results of breast MRI may lead to altered surgical treatment in approximately 13% of patients.11
Interestingly, a recent randomized trial showed no difference in reoperation rates between patients who underwent MRI before surgery vs those who did not. However, diagnostic workup of new MRI findings was not mandated by the study protocol, making the results of this trial difficult to interpret.12
DIFFERENTIAL DIAGNOSIS
2. Which of the following is in the differential diagnosis of a woman presenting with a breast abnormality?
- Fibrocystic changes
- Breast cyst
- Ductal ectasia
- Simple fibroadenoma
- Intraductal papilloma
- Ductal carcinoma in situ
- Mastitis
- Infiltrating ductal carcinoma
- Phyllodes tumor
All of these choices are part of the differential diagnosis.
Benign breast lesions
Simple fibroadenoma, one of the most common proliferative lesions, is not associated with a higher risk of developing breast cancer.
Fibrocystic changes are the most common nonproliferative lesions. Occasionally breast pain, nipple discharge, or significant lumpiness that varies during the course of the menstrual cycle can occur. The nipple discharge in women with fibrocystic changes is physiologic and pale green to brown in color. It can also be yellow, whitish, clear, or bloody. Bloody nipple discharge is considered pathologic and suggests a process other than fibrocystic changes, necessitating further workup. However, bloody discharge is not always a sign of malignancy, as it can have a benign cause as well.
Ductal ectasia, another nonproliferative lesion, is a result of dilation of subareolar ducts that contain fluid with a crystalline material. It can penetrate the duct, forming a nodule, which causes pain and occasionally fever.
Precancerous and cancerous lesions
Ductal carcinoma in situ is a true neoplasm that has not yet developed the ability to invade through the basement membrane of the ducts. The likelihood of progression to invasive breast cancer depends on the histologic grade, the tumor size, and the patient’s age.
Lobular carcinoma in situ arises from lobules and terminal ducts of breast tissue. Much controversy surrounds this type of tumor, which was thought to be a marker of increased risk of developing ipsilateral and contralateral breast cancer and not to be a malignant lesion itself.15 However, there is emerging evidence to suggest that a pleomorphic variant of lobular carcinoma in situ is associated with development of breast cancer in the same site as the lesion, whereas a nonpleomorphic form is a marker of increased risk of ipsilateral and contralateral breast cancer.16
Invasive ductal and lobular carcinomas are the true invasive breast cancers, with a potential to metastasize.
Phyllodes tumors are uncommon fibroepithelial lesions that account for less than 1% of all breast neoplasms. The median age at presentation is 45 years.17 Despite the historical name “cystosarcoma phyllodes,” these lesions are not true sarcomas and have stromal and epithelial components.
These tumors display very heterogeneous behavior and, based on predefined histologic criteria, are often classified as benign, borderline, or malignant. Benign phyllodes tumors are similar to fibroadenomas in both histology and prognosis, making their diagnosis challenging. The most aggressive phyllodes tumors lose their epithelial component and have high metastatic potential. These tumors often have a biphasic growth pattern, and women may present with a smooth, round, well-defined breast lump that was stable for many years but then started to grow rapidly.17
Surgical resection with wide margins is the primary management of these tumors.18
Mastitis, ie, inflammation of the breast tissue, often presents with symptoms of breast erythema, swelling, tenderness, and nipple discharge. It may be secondary to infection (most often in lactating women) or other causes such as radiation or underlying malignancy. A complication of infectious mastitis is formation of a breast abscess. Underlying malignancy, especially inflammatory breast cancer, is a common cause of noninfectious mastitis and is very important to recognize.19
RISK FACTORS FOR BREAST CANCER
3. Which of the following are risk factors for breast cancer?
- Menarche before age 12
- Female sex
- Personal history of breast cancer
- Obesity
- Never having had children, or having given birth for the first time at an older age
- Older age
- History of hormone replacement therapy with estrogen and progesterone
- Family history of breast cancer
All of these choices are risk factors for breast cancer.
Family history
The overall relative risk of developing breast cancer in a woman with a first-degree relative with the disease is 1.7. However, the relative risk is about 3 if the first-degree relative developed breast cancer before menopause, and 9 if the first-degree relative developed bilateral breast cancer before menopause.5
Estrogen exposure
The duration and amount of estrogen exposure are also risk factors. For example, menarche before age 12 and menopause after age 55 are associated with a higher risk. Women who go through menopause after age 55 have a twofold higher risk of breast cancer compared with women who go through menopause at an early age. Pregnancy before age 30 lowers the risk of breast cancer; late first full-term pregnancy or nulliparity increases it. Lactation, on the other hand, has a protective effect.5
Oral contraceptives have traditionally been thought to increase the risk of breast cancer. In the 1990s, a meta-analysis involving 153,506 women found that those who had used oral contraceptives had a 24% higher risk of developing breast cancer.26 However, this association has come into question since newer oral contraceptive pills containing different progestins and lower amounts of estrogen have become available. In fact, recent studies showed no link between oral contraceptive use and breast cancer.27,28 Nevertheless, women at higher risk of developing breast cancer are advised not to use oral contraceptives.
Hormone replacement therapy with estrogen and progesterone was found to increase the risk of breast cancer by 26% in the Women’s Health Initiative (WHI) study, which involved 16,608 healthy women followed for a median of 5.6 years.29
In a study reported separately, the WHI investigators randomized 10,739 women who had undergone hysterectomy to receive either hormone replacement therapy with unopposed estrogen (which is feasible only in women without a uterus) or placebo. They found no increase in the risk of invasive breast cancer in women on hormone replacement therapy with estrogen alone. In fact, the study showed a trend towards a modest reduction of this risk (odds ratio 0.77; 95% confidence interval 0.59–1.01).30
After the results of the WHI were published, the use of hormone replacement therapy in postmenopausal women declined significantly. And in 2003—1 year later—the incidence of breast cancer had dropped by 6.7%.31
Most experts now recommend that estrogen-progestin combinations be used only selectively to treat the symptoms of menopause, and only for the short term.
Other risk factors
Other factors found to modestly increase the risk of breast cancer include:
- Alcohol use
- Obesity
- Radiation exposure. Patients are at higher risk of breast cancer 15 to 20 years after receiving upper-mantle radiotherapy for Hodgkin lymphoma.5
Case continues: Bad news on mammography, ultrasonography, biopsy
The patient undergoes mammography, which shows a 2.5-cm spiculated lesion with areas of calcifications (BIRADS score of 5). Subsequently, ultrasonography confirms that the suspicious mass is not a cyst. Ultrasound-guided core needle biopsy reveals that the lesion is a high-grade invasive ductal carcinoma. The tumor is positive for both estrogen and progesterone receptors and negative for HER2/neu overexpression.
STAGING EVALUATION
4. Given these findings, what is the next step to take?
- CT of the chest, abdomen, and pelvis
- MRI of the brain
- PET
- Referral to a surgeon for a possible mastectomy with sentinel lymph node dissection
- Referral to a surgeon for a possible lumpectomy with sentinel lymph node dissection
At this point, the patient should be referred to a surgeon for possible mastectomy or lumpectomy.
Women who appear clinically to have early breast cancer, such as in this case, should have a complete blood count, comprehensive metabolic panel, and chest x-ray as their initial staging evaluation. No further studies are recommended unless the findings on history, physical examination, or the above testing suggest possible metastases.
Mastectomy vs lumpectomy
Early-stage breast cancer is managed with definitive surgery. The two options are mastectomy and breast conservation therapy, the latter involving lumpectomy followed by breast radiation therapy.
Multiple randomized studies comparing mastectomy and lumpectomy showed no difference in survival rates, but patients in the lumpectomy groups had higher rates of local recurrence.32 Breast radiation therapy after lumpectomy lowered the rates of local recurrence and breast cancer death.33 Therefore, most patients can opt to undergo either lumpectomy with radiation or mastectomy, depending on personal preference.
However, mastectomy rather than breast conservation therapy is still recommended in cases of prior radiation therapy, inability to achieve negative surgical margins (as in cases of large tumors), multicentric disease (cancer in separate breast quadrants), or multiple areas of calcifications. Mastectomy is also preferred in most pregnant women unless the diagnosis of breast cancer is made in the third trimester and radiation therapy can be given after delivery. Patients who have large lesions in a small breast may also choose mastectomy with breast reconstruction rather than breast conservation therapy. Patients with a history of scleroderma are encouraged to undergo mastectomy because of increased toxicity from radiation treatment.
Sentinel vs axillary lymph node dissection
Knowledge of axillary lymph node involvement is important because it determines the stage in the tumor-node-metastasis (TNM) system, and it influences the choice of further therapy. Therefore, all patients with nonmetastatic invasive breast cancer must have their axillary lymph nodes sampled.
Conventionally, this involves axillary lymph node dissection. Unfortunately, upper extremity lymphedema develops in 6% to 30% of patients within the first 3 years, and in 49% of patients after 20 years following axillary lymph node dissection.34
Sentinel lymph node dissection was developed to minimize this complication. This procedure involves the injection of a blue dye, isosulfan blue (Lymphazurin), around the edge of the tumor or in the dermis overlying the tumor. The most proximal axillary lymph nodes that stain blue are dissected. Alternatively, a radioactive colloid (most commonly technetium sulfur colloid agents) may be injected, allowing sentinel lymph nodes to be identified by lymphoscintigraphy. If no metastases are found in the sentinel lymph nodes, axillary lymph node dissection is not performed.
A prospective study in 536 women found that at 5 years of follow-up, lymphedema developed in only 5% of patients after sentinel lymph node dissection compared with 16% of those who underwent axillary lymph node dissection (P < .001), with comparable outcomes in terms of disease recurrence.35
Case continues: Patient undergoes surgery
The patient elects to undergo lumpectomy with sentinel lymph node dissection. Pathologic review of the resection specimen reveals a 2.5-cm poorly differentiated invasive ductal carcinoma. Sentinel lymph node dissection shows metastases, and therefore axillary lymph node dissection is performed. One of eight lymph nodes removed is positive for metastases. All surgical margins are negative.
POSTOPERATIVE CARE
5. What would be the next step for our patient?
- Radiation followed by observation
- Tamoxifen (Nolvadex) for 5 years
- Observation only
- Chemotherapy followed by radiation therapy and 5 years of tamoxifen
She should receive chemotherapy, followed by radiation therapy and then tamoxifen for 5 years.
Chemotherapy. Almost all patients who have lymph-node-positive disease are advised to undergo chemotherapy.
The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) performed a metaanalysis of 194 randomized trials that compared adjuvant chemotherapy and no treatment in early-stage breast cancer. Chemotherapy led to a 10% absolute improvement in survival at 15 years for women younger than 50 years and 3% in women age 51 to 69.36
Indications for chemotherapy include axillary lymph node involvement, locally advanced disease, and other risk factors for recurrence such as young age at diagnosis, strong positive family history of breast cancer, prior history of breast cancer, or lymph-node-negative, estrogen-receptor-negative tumors that are larger than 1 cm in diameter.
The Oncotype DX assay is a new tool to help oncologists decide whether to use chemotherapy in cases of estrogen-receptor-positive breast cancer, in which the benefit of chemotherapy is uncertain. It is a polymerase chain reaction assay that measures the expression of 16 cancer-specific genes and five reference genes within the breast tumor. Based on the pattern of expression of these genes, breast cancer can be characterized as low-risk, intermediate-risk, or high-risk. Patients in the high-risk group have a high chance of cancer recurrence and benefit from chemotherapy. Patients in the low-risk group are unlikely to have a recurrence or to benefit from chemotherapy.37 It is far less clear if patients in the intermediate-risk group benefit from chemotherapy, but this assay might eventually prove useful in deciding for or against chemotherapy in this group of patients as well.38 The Oncotype DX assay is presently being studied in a clinical trial.
Radiation therapy after mastectomy is recommended in patients who have breast tumors larger than 5 cm or metastases to more than three axillary lymph nodes.39
Antiestrogen therapy. After chemotherapy, patients with estrogen-receptor-positive cancers also receive 5 years of antiestrogen therapy. Available antiestrogen agents for such patients include tamoxifen, which is a selective estrogen receptor modulator, and drugs called aromatase inhibitors that block conversion of androgens to estrogens in peripheral tissues. Anastrozole (Arimidex), letrozole (Femara), and exemestane (Aromasin) are examples of available aromatase inhibitors. Premenopausal women are treated with tamoxifen, and postmenopausal women are offered aromatase inhibitors.
Table 4 lists the most common adverse effects of these agents. Aromatase inhibitors are associated with a higher risk of osteoporosis and arthralgia, while tamoxifen increases the risks of thromboembolism, endometrial cancer, and vaginal discharge. Both agents may produce menopausal symptoms such as hot flashes and mood swings.
Case continues: Seven years later, metastases in the spine
The patient achieves a complete remission. She is seen for a routine visit 7 years after diagnosis. She now reports mid-back pain that has worsened over the last 2 months. A bone scan reveals diffuse metastatic disease in the spine and in both humeral bones. CT of the chest, abdomen, and pelvis is negative for visceral metastases. Bone marrow aspiration and biopsy study show marrow infiltration by adenocarcinoma that stains positive for estrogen receptors and negative for HER2. The patient otherwise feels well and has no other symptoms.
WHAT TREATMENT FOR METASTATIC BREAST CANCER?
6. What should you now do for our patient?
- Discuss end-of-life care and refer her to a hospice program
- Educate the patient that no options for treatment exist and recommend enrolling in a phase I clinical trial
- Refer her to an oncologist for consideration of chemotherapy
- Refer her to an oncologist for consideration of endocrine treatment
She should be referred to an oncologist for consideration of endocrine treatment.
The most common sites of breast cancer metastases are the bones, followed by the liver and lungs. Metastatic breast cancer almost always is incurable. However, treatment can palliate symptoms.
Although a randomized trial of treatment vs best supportive care has never been done, many believe that treatment may improve survival. 40 The median survival of patients treated with standard therapy is about 3 years if the breast cancer is estrogen-receptor-positive and 2 years if it is estrogen-receptor-negative, but survival rates vary widely from patient to patient.41,42
Standard therapy or enrollment in a clinical phase II or III trial is indicated for this patient before considering enrollment in a phase I clinical trial or supportive care alone.
Endocrine therapy is the first-line therapy in women with estrogen-receptor-positive metastatic breast cancer. Postmenopausal women usually receive an aromatase inhibitor first.43,44 Response to endocrine therapy usually takes weeks to months but may last for several years.
Premenopausal women with estrogen-receptor-positive breast cancer also receive ovarian ablation therapy (oophorectomy or chemical ovarian ablation) with gonadotropin-releasing hormone agonists.
In addition, most patients with bone involvement are treated with high doses of intravenous bisphosphonates, which can reduce skeletal complications.45
Chemotherapy is reserved for patients with estrogen-receptor-negative breast cancer and those with cancer that progresses despite treatment with multiple antiestrogen agents. The time to response when chemotherapy is used is quicker, but the duration of response is usually shorter, lasting on average less than 1 year.37
Trastuzumab (Herceptin), a monoclonal humanized murine antibody to the extracellular domain of the HER2 protein, is indicated in patients with HER2-overexpressing tumors.46,47
STABLE 2 YEARS LATER
The patient was started on letrozole and a bisphosphonate, zolendronic acid (Zometa). Ovarian ablation was initiated with goserelin (Zoladex) given monthly. A bone scan performed 2 months after starting treatment showed improvement in bony metastases. She also noted significant improvement in pain. Her disease remains stable 2 years after starting endocrine therapy.
- Barton MB, Elmore JG, Fletcher SW. Breast symptoms among women enrolled in a health maintenance organization: frequency, evaluation, and outcome. Ann Intern Med 1999; 130:651–657.
- Petrelli NJ, Winer EP, Brahmer J, et al. Clinical cancer advances 2009: major research advances in cancer treatment, prevention, and screening—a report from the American Society of Clinical Oncology. J Clin Oncol 2009; 27:6052–6069.
- Jemal A, Siegel R, Ward E, et al. Cancer statistics 2008. CA Cancer J Clin 2008; 58:71–96.
- National Cancer Institute. SEER Stat Fact Sheets. www.seer.cancer.gov/statfacts/html/breast.html#ref09. Accessed June 7, 2010.
- Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ; the publishers of the journal Oncology. Cancer Management: A multidisciplinary Approach. Medical, Surgical & Radiation Oncology. 11th ed. CMP Medica; 2008.
- Kollmorgen DR, Varanasi JS, Edge SB, Carson WE. Paget’s disease of the breast: a 33-year experience. J Am Coll Surg 1998; 187:171–177.
- Barlow WE, Lehman CD, Zheng Y, et al. Performance of diagnostic mammography for women with signs or symptoms of breast cancer. J Natl Cancer Inst 2002; 94:1151–1159.
- American College of Radiology. Breast Imaging Reporting and Data System: BIRADS Atlas. 4th ed. Reston, VA: American College of Radiology; 2003.
- Hong AS, Rosen EL, Soo MS, Baker JA. BI-RADS for sonography: positive and negative predictive values of sonographic features. AJR Am J Roentgenol 2005; 184:1260–1265.
- Berg WA, Campassi CI, Ioffe OB. Cystic lesions of the breast: sonographic-pathologic correlation. Radiology 2003; 227:183–191.
- Schell AM, Rosenkranz K, Lewis PJ. Role of breast MRI in the preoperative evaluation of patients with newly diagnosed breast cancer. AJR Am J Roentgenol 2009; 192:1438–1444.
- Turnbull L, Brown S, Harvey I, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet 2010; 375:563–571.
- Worsham MJ, Abrams J, Raju U, et al. Breast cancer incidence in a cohort of women with benign breast disease from a multiethnic, primary health care population. Breast J 2007; 13:115–121.
- Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med 1985; 312:146–151.
- Page DL, Kidd TE, Dupont WD, Simpson JF, Rogers LW. Lobular neoplasia of the breast: higher risk for subsequent invasive cancer predicted by more extensive disease. Hum Pathol 1991; 22:1232–1239.
- Sneige N, Wang J, Baker BA, Krishnamurthy S, Middleton LP. Clinical, histopathologic, and biologic features of pleomorphic lobular (ductallobular) carcinoma in situ of the breast: a report of 24 cases. Mod Pathol 2002; 15:1044–1050.
- Telli ML, Horst KC, Guardino AE, Dirbas FM, Carlson RW. Phyllodes tumors of the breast: natural history, diagnosis, and treatment. J Natl Compr Canc Netw 2007; 5:324–330.
- Reinfuss M, Mitus J, Duda K, Stelmach A, Rys J, Smolak K. The treatment and prognosis of patients with phyllodes tumor of the breast: an analysis of 170 cases. Cancer 1996; 77:910–916.
- Kamal RM, Hamed ST, Salem DS. Classification of inflammatory breast disorders and step by step diagnosis. Breast J 2009; 15:367–380.
- Hartge P, Struewing JP, Wacholder S, Brody LC, Tucker MA. The prevalence of common BRCA1 and BRCA2 mutations among Ashkenazi Jews. Am J Hum Genet 1999; 64:963–970.
- Wooster R, Weber BL. Breast and ovarian cancer. N Engl J Med 2003; 348:2339–2347.
- Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med 2009; 361:170–177.
- Hisada M, Garber JE, Fung CY, Fraumeni JF, Li FP. Multiple primary cancers in families with Li-Fraumeni syndrome. J Natl Cancer Inst 1998; 90:606–611.
- Bell DW, Varley JM, Szydlo TE, et al. Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science 1999; 286:2528–2531.
- Kaurah P, MacMillan A, Boyd N, et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA 2007; 297:2360–2372.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet 1996; 347:1713–1727.
- Hankinson SE, Colditz GA, Manson JE, et al. A prospective study of oral contraceptive use and risk of breast cancer (Nurses’ Health Study, United States). Cancer Causes Control 1997; 8:65–72.
- Marchbanks PA, McDonald JA, Wilson HG, et al. Oral contraceptives and the risk of breast cancer. N Engl J Med 2002; 346:2025–2032.
- Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288:321–333.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 2004; 291:1701–1712.
- Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med 2007; 356:1670–1674.
- Fisher B, Anderson S, Redmond CK, Wolmark N, Wickerham DL, Cronin WM. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med 1995; 333:1456–1461.
- Clarke M, Collins R, Darby S, et al; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 366:2087–2106.
- Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 2001; 92:1368–1377.
- McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol 2008; 26:5213–5219.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 365:1687–1717.
- Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004; 351:2817–2826.
- Albain KS, Barlow WE, Shak S, et al; Breast Cancer Intergroup of North America. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010; 11:55–65.
- Harris JR, Halpin-Murphy P, McNeese M, Mendenhall NP, Morrow M, Robert NJ. Consensus Statement on postmastectomy radiation therapy. Int J Radiat Oncol Biol Phys 1999; 44:989–990.
- Gennari A, Conte P, Rosso R, Orlandini C, Bruzzi P. Survival of metastatic breast carcinoma patients over a 20-year period: a retrospective analysis based on individual patient data from six consecutive studies. Cancer 2005; 104:1742–1750.
- Mouridsen H, Gershanovich M, Sun Y, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol 2003; 21:2101–2109.
- Gamucci T, D’Ottavio AM, Magnolfi E, et al. Weekly epirubicin plus docetaxel as first-line treatment in metastatic breast cancer. Br J Cancer 2007; 97:1040–1045.
- Bonneterre J, Thürlimann B, Robertson JF, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol 2000; 18:3748–3757.
- Nabholtz JM, Buzdar A, Pollak M, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol 2000; 18:3758–3767.
- Hortobagyi GN, Theriault RL, Porter L, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med 1996; 335:1785–1791.
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005; 353:1673–1684.
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344:783–792.
A 40-year-old premenopausal woman presents with a palpable lump in her left breast. She first noted it 2 months ago on self-examination, and it has steadily grown in size regardless of the phase of her menstrual cycle.
The patient has never undergone mammography. Her menarche was at age 12. At age 35, she had one child (whom she breastfed) after a normal first full-term pregnancy. She took oral contraceptives for 10 years before her pregnancy. She has no other medical problems. She has no family history of breast or ovarian cancer.
On examination, her breasts are slightly asymmetric, without skin discoloration, tenderness, swelling, nipple retraction, or discharge. A 1.5- to 2-cm, rubbery, mobile lump can be felt in the left breast at about the 2 o’clock position. No axillary lymph nodes can be palpated. The rest of her examination is normal.
BREAST CANCER MUST BE RULED OUT
Benign breast disease is found in approximately 90% of women 20 to 50 years of age who come to a physician with a breast problem.1
Nevertheless, breast cancer is of major concern. It is the most common type of cancer in women in the United States, responsible for an estimated 194,440 new cases and 40,610 deaths in 2009. It is also the leading cause of cancer-related death in women age 45 to 55 years in this country.2,3
Breast cancer is most common in postmenopausal women, its incidence rising sharply after the age of 45 and leveling off at age 75. The median age at diagnosis is 61 years. Still, 1.9% of breast cancers in women are diagnosed at age 20 to 34, 10.6% at age 35 to 44, and 22.4% at age 45 to 54.4
Thus, it is paramount to perform a thorough assessment and workup of women who have breast lumps, regardless of their age. Doing so allows breast cancer to be detected at an early stage. The 5-year survival rate is 98.0% for women with localized disease, 83.6% with regional disease, and 23.4% with distant disease.4
WHAT IS THE APPROPRIATE WORKUP?
1. Which of the following are appropriate in the workup of this patient?
- Mammography
- Ultrasonography
- Percutaneous needle biopsy of the lesion
- Magnetic resonance imaging (MRI) of the brain
- Computed tomography (CT) of the chest, abdomen, and pelvis
- Positron emission tomography (PET)
She should undergo mammography, ultrasonography, and percutaneous needle biopsy.
Physical findings that suggest breast cancer include a hard, isolated, sometimes nonmobile lump, serosanguinous nipple discharge, and unilateral nipple retraction. Peau d’orange skin discoloration can occur. A scaly, vesicular, or ulcerated rash with or without pruritus, burning, irritation, or pain of the nipple or skin (Paget disease of the breast) is found in 1% to 3% of breast cancers and may be initially dismissed as mastitis.5,6 Palpable enlarged axillary lymph nodes can suggest invasive breast cancer.
Mammography is recommended in all cases of suspicious breast lumps. In a patient with a palpable lump, diagnostic mammography has a positive predictive value of 21.8%, a specificity of 85.8%, and a sensitivity of 87.7%, which are higher values than in a patient without signs or symptoms.7
The BIRADS score. Mammographic findings are summarized using a scoring system devised by the American College of Radiology called BIRADS (Breast Imaging Reporting and Data System). This system is based on mass irregularity, density, spiculation, and presence or absence of microcalcifications. It standardizes the results of mammography, gives an estimate of the risk of breast cancer, and recommends the frequency of follow-up examinations.8 Scores range from 0 to 6:
- 0—Incomplete assessment warranting additional evaluation
- 1—Completely negative mammogram
- 2—Benign lesion
- 3—Requires follow-up mammogram at 6 months
- 4—Risk of cancer is 2% to 95%; core biopsy needed
- 5—Risk of cancer is more than 95%; core biopsy needed
- 6—Cases that have already been proven to be malignant.
Ultrasonography is also done if a suspicious lesion is found on mammography or physical examination. It helps differentiate between solid and cystic masses. If a mass is identified as a cyst, ultrasonography can further characterize it as simple, complicated-simple, or complex. Simple cysts and complicated-simple cysts are unlikely to be malignant.9,10 Complex cysts or cysts associated with solid tissue are evaluated by biopsy.
Percutaneous needle biopsy should be done for a definitive diagnosis of most suspicious breast masses.
MRI can sometimes provide more accurate information about the possibility of multifocal breast cancer by revealing additional lesions missed on mammography or ultrasonography. It is also useful in determining more accurately the size of the breast tumor and looking for any possible contralateral lesions. In addition, it can sometimes detect enlarged axillary lymph nodes. However, it has poor specificity for breast cancer and may lead to additional and sometimes unnecessary diagnostic tests, which can delay treatment.
MRI’s role is therefore not clearly established, but it is commonly used in clinical practice. It is argued that workup of MRI findings may help in planning more accurate surgical procedures and may prevent reoperations. Based on retrospective analyses, results of breast MRI may lead to altered surgical treatment in approximately 13% of patients.11
Interestingly, a recent randomized trial showed no difference in reoperation rates between patients who underwent MRI before surgery vs those who did not. However, diagnostic workup of new MRI findings was not mandated by the study protocol, making the results of this trial difficult to interpret.12
DIFFERENTIAL DIAGNOSIS
2. Which of the following is in the differential diagnosis of a woman presenting with a breast abnormality?
- Fibrocystic changes
- Breast cyst
- Ductal ectasia
- Simple fibroadenoma
- Intraductal papilloma
- Ductal carcinoma in situ
- Mastitis
- Infiltrating ductal carcinoma
- Phyllodes tumor
All of these choices are part of the differential diagnosis.
Benign breast lesions
Simple fibroadenoma, one of the most common proliferative lesions, is not associated with a higher risk of developing breast cancer.
Fibrocystic changes are the most common nonproliferative lesions. Occasionally breast pain, nipple discharge, or significant lumpiness that varies during the course of the menstrual cycle can occur. The nipple discharge in women with fibrocystic changes is physiologic and pale green to brown in color. It can also be yellow, whitish, clear, or bloody. Bloody nipple discharge is considered pathologic and suggests a process other than fibrocystic changes, necessitating further workup. However, bloody discharge is not always a sign of malignancy, as it can have a benign cause as well.
Ductal ectasia, another nonproliferative lesion, is a result of dilation of subareolar ducts that contain fluid with a crystalline material. It can penetrate the duct, forming a nodule, which causes pain and occasionally fever.
Precancerous and cancerous lesions
Ductal carcinoma in situ is a true neoplasm that has not yet developed the ability to invade through the basement membrane of the ducts. The likelihood of progression to invasive breast cancer depends on the histologic grade, the tumor size, and the patient’s age.
Lobular carcinoma in situ arises from lobules and terminal ducts of breast tissue. Much controversy surrounds this type of tumor, which was thought to be a marker of increased risk of developing ipsilateral and contralateral breast cancer and not to be a malignant lesion itself.15 However, there is emerging evidence to suggest that a pleomorphic variant of lobular carcinoma in situ is associated with development of breast cancer in the same site as the lesion, whereas a nonpleomorphic form is a marker of increased risk of ipsilateral and contralateral breast cancer.16
Invasive ductal and lobular carcinomas are the true invasive breast cancers, with a potential to metastasize.
Phyllodes tumors are uncommon fibroepithelial lesions that account for less than 1% of all breast neoplasms. The median age at presentation is 45 years.17 Despite the historical name “cystosarcoma phyllodes,” these lesions are not true sarcomas and have stromal and epithelial components.
These tumors display very heterogeneous behavior and, based on predefined histologic criteria, are often classified as benign, borderline, or malignant. Benign phyllodes tumors are similar to fibroadenomas in both histology and prognosis, making their diagnosis challenging. The most aggressive phyllodes tumors lose their epithelial component and have high metastatic potential. These tumors often have a biphasic growth pattern, and women may present with a smooth, round, well-defined breast lump that was stable for many years but then started to grow rapidly.17
Surgical resection with wide margins is the primary management of these tumors.18
Mastitis, ie, inflammation of the breast tissue, often presents with symptoms of breast erythema, swelling, tenderness, and nipple discharge. It may be secondary to infection (most often in lactating women) or other causes such as radiation or underlying malignancy. A complication of infectious mastitis is formation of a breast abscess. Underlying malignancy, especially inflammatory breast cancer, is a common cause of noninfectious mastitis and is very important to recognize.19
RISK FACTORS FOR BREAST CANCER
3. Which of the following are risk factors for breast cancer?
- Menarche before age 12
- Female sex
- Personal history of breast cancer
- Obesity
- Never having had children, or having given birth for the first time at an older age
- Older age
- History of hormone replacement therapy with estrogen and progesterone
- Family history of breast cancer
All of these choices are risk factors for breast cancer.
Family history
The overall relative risk of developing breast cancer in a woman with a first-degree relative with the disease is 1.7. However, the relative risk is about 3 if the first-degree relative developed breast cancer before menopause, and 9 if the first-degree relative developed bilateral breast cancer before menopause.5
Estrogen exposure
The duration and amount of estrogen exposure are also risk factors. For example, menarche before age 12 and menopause after age 55 are associated with a higher risk. Women who go through menopause after age 55 have a twofold higher risk of breast cancer compared with women who go through menopause at an early age. Pregnancy before age 30 lowers the risk of breast cancer; late first full-term pregnancy or nulliparity increases it. Lactation, on the other hand, has a protective effect.5
Oral contraceptives have traditionally been thought to increase the risk of breast cancer. In the 1990s, a meta-analysis involving 153,506 women found that those who had used oral contraceptives had a 24% higher risk of developing breast cancer.26 However, this association has come into question since newer oral contraceptive pills containing different progestins and lower amounts of estrogen have become available. In fact, recent studies showed no link between oral contraceptive use and breast cancer.27,28 Nevertheless, women at higher risk of developing breast cancer are advised not to use oral contraceptives.
Hormone replacement therapy with estrogen and progesterone was found to increase the risk of breast cancer by 26% in the Women’s Health Initiative (WHI) study, which involved 16,608 healthy women followed for a median of 5.6 years.29
In a study reported separately, the WHI investigators randomized 10,739 women who had undergone hysterectomy to receive either hormone replacement therapy with unopposed estrogen (which is feasible only in women without a uterus) or placebo. They found no increase in the risk of invasive breast cancer in women on hormone replacement therapy with estrogen alone. In fact, the study showed a trend towards a modest reduction of this risk (odds ratio 0.77; 95% confidence interval 0.59–1.01).30
After the results of the WHI were published, the use of hormone replacement therapy in postmenopausal women declined significantly. And in 2003—1 year later—the incidence of breast cancer had dropped by 6.7%.31
Most experts now recommend that estrogen-progestin combinations be used only selectively to treat the symptoms of menopause, and only for the short term.
Other risk factors
Other factors found to modestly increase the risk of breast cancer include:
- Alcohol use
- Obesity
- Radiation exposure. Patients are at higher risk of breast cancer 15 to 20 years after receiving upper-mantle radiotherapy for Hodgkin lymphoma.5
Case continues: Bad news on mammography, ultrasonography, biopsy
The patient undergoes mammography, which shows a 2.5-cm spiculated lesion with areas of calcifications (BIRADS score of 5). Subsequently, ultrasonography confirms that the suspicious mass is not a cyst. Ultrasound-guided core needle biopsy reveals that the lesion is a high-grade invasive ductal carcinoma. The tumor is positive for both estrogen and progesterone receptors and negative for HER2/neu overexpression.
STAGING EVALUATION
4. Given these findings, what is the next step to take?
- CT of the chest, abdomen, and pelvis
- MRI of the brain
- PET
- Referral to a surgeon for a possible mastectomy with sentinel lymph node dissection
- Referral to a surgeon for a possible lumpectomy with sentinel lymph node dissection
At this point, the patient should be referred to a surgeon for possible mastectomy or lumpectomy.
Women who appear clinically to have early breast cancer, such as in this case, should have a complete blood count, comprehensive metabolic panel, and chest x-ray as their initial staging evaluation. No further studies are recommended unless the findings on history, physical examination, or the above testing suggest possible metastases.
Mastectomy vs lumpectomy
Early-stage breast cancer is managed with definitive surgery. The two options are mastectomy and breast conservation therapy, the latter involving lumpectomy followed by breast radiation therapy.
Multiple randomized studies comparing mastectomy and lumpectomy showed no difference in survival rates, but patients in the lumpectomy groups had higher rates of local recurrence.32 Breast radiation therapy after lumpectomy lowered the rates of local recurrence and breast cancer death.33 Therefore, most patients can opt to undergo either lumpectomy with radiation or mastectomy, depending on personal preference.
However, mastectomy rather than breast conservation therapy is still recommended in cases of prior radiation therapy, inability to achieve negative surgical margins (as in cases of large tumors), multicentric disease (cancer in separate breast quadrants), or multiple areas of calcifications. Mastectomy is also preferred in most pregnant women unless the diagnosis of breast cancer is made in the third trimester and radiation therapy can be given after delivery. Patients who have large lesions in a small breast may also choose mastectomy with breast reconstruction rather than breast conservation therapy. Patients with a history of scleroderma are encouraged to undergo mastectomy because of increased toxicity from radiation treatment.
Sentinel vs axillary lymph node dissection
Knowledge of axillary lymph node involvement is important because it determines the stage in the tumor-node-metastasis (TNM) system, and it influences the choice of further therapy. Therefore, all patients with nonmetastatic invasive breast cancer must have their axillary lymph nodes sampled.
Conventionally, this involves axillary lymph node dissection. Unfortunately, upper extremity lymphedema develops in 6% to 30% of patients within the first 3 years, and in 49% of patients after 20 years following axillary lymph node dissection.34
Sentinel lymph node dissection was developed to minimize this complication. This procedure involves the injection of a blue dye, isosulfan blue (Lymphazurin), around the edge of the tumor or in the dermis overlying the tumor. The most proximal axillary lymph nodes that stain blue are dissected. Alternatively, a radioactive colloid (most commonly technetium sulfur colloid agents) may be injected, allowing sentinel lymph nodes to be identified by lymphoscintigraphy. If no metastases are found in the sentinel lymph nodes, axillary lymph node dissection is not performed.
A prospective study in 536 women found that at 5 years of follow-up, lymphedema developed in only 5% of patients after sentinel lymph node dissection compared with 16% of those who underwent axillary lymph node dissection (P < .001), with comparable outcomes in terms of disease recurrence.35
Case continues: Patient undergoes surgery
The patient elects to undergo lumpectomy with sentinel lymph node dissection. Pathologic review of the resection specimen reveals a 2.5-cm poorly differentiated invasive ductal carcinoma. Sentinel lymph node dissection shows metastases, and therefore axillary lymph node dissection is performed. One of eight lymph nodes removed is positive for metastases. All surgical margins are negative.
POSTOPERATIVE CARE
5. What would be the next step for our patient?
- Radiation followed by observation
- Tamoxifen (Nolvadex) for 5 years
- Observation only
- Chemotherapy followed by radiation therapy and 5 years of tamoxifen
She should receive chemotherapy, followed by radiation therapy and then tamoxifen for 5 years.
Chemotherapy. Almost all patients who have lymph-node-positive disease are advised to undergo chemotherapy.
The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) performed a metaanalysis of 194 randomized trials that compared adjuvant chemotherapy and no treatment in early-stage breast cancer. Chemotherapy led to a 10% absolute improvement in survival at 15 years for women younger than 50 years and 3% in women age 51 to 69.36
Indications for chemotherapy include axillary lymph node involvement, locally advanced disease, and other risk factors for recurrence such as young age at diagnosis, strong positive family history of breast cancer, prior history of breast cancer, or lymph-node-negative, estrogen-receptor-negative tumors that are larger than 1 cm in diameter.
The Oncotype DX assay is a new tool to help oncologists decide whether to use chemotherapy in cases of estrogen-receptor-positive breast cancer, in which the benefit of chemotherapy is uncertain. It is a polymerase chain reaction assay that measures the expression of 16 cancer-specific genes and five reference genes within the breast tumor. Based on the pattern of expression of these genes, breast cancer can be characterized as low-risk, intermediate-risk, or high-risk. Patients in the high-risk group have a high chance of cancer recurrence and benefit from chemotherapy. Patients in the low-risk group are unlikely to have a recurrence or to benefit from chemotherapy.37 It is far less clear if patients in the intermediate-risk group benefit from chemotherapy, but this assay might eventually prove useful in deciding for or against chemotherapy in this group of patients as well.38 The Oncotype DX assay is presently being studied in a clinical trial.
Radiation therapy after mastectomy is recommended in patients who have breast tumors larger than 5 cm or metastases to more than three axillary lymph nodes.39
Antiestrogen therapy. After chemotherapy, patients with estrogen-receptor-positive cancers also receive 5 years of antiestrogen therapy. Available antiestrogen agents for such patients include tamoxifen, which is a selective estrogen receptor modulator, and drugs called aromatase inhibitors that block conversion of androgens to estrogens in peripheral tissues. Anastrozole (Arimidex), letrozole (Femara), and exemestane (Aromasin) are examples of available aromatase inhibitors. Premenopausal women are treated with tamoxifen, and postmenopausal women are offered aromatase inhibitors.
Table 4 lists the most common adverse effects of these agents. Aromatase inhibitors are associated with a higher risk of osteoporosis and arthralgia, while tamoxifen increases the risks of thromboembolism, endometrial cancer, and vaginal discharge. Both agents may produce menopausal symptoms such as hot flashes and mood swings.
Case continues: Seven years later, metastases in the spine
The patient achieves a complete remission. She is seen for a routine visit 7 years after diagnosis. She now reports mid-back pain that has worsened over the last 2 months. A bone scan reveals diffuse metastatic disease in the spine and in both humeral bones. CT of the chest, abdomen, and pelvis is negative for visceral metastases. Bone marrow aspiration and biopsy study show marrow infiltration by adenocarcinoma that stains positive for estrogen receptors and negative for HER2. The patient otherwise feels well and has no other symptoms.
WHAT TREATMENT FOR METASTATIC BREAST CANCER?
6. What should you now do for our patient?
- Discuss end-of-life care and refer her to a hospice program
- Educate the patient that no options for treatment exist and recommend enrolling in a phase I clinical trial
- Refer her to an oncologist for consideration of chemotherapy
- Refer her to an oncologist for consideration of endocrine treatment
She should be referred to an oncologist for consideration of endocrine treatment.
The most common sites of breast cancer metastases are the bones, followed by the liver and lungs. Metastatic breast cancer almost always is incurable. However, treatment can palliate symptoms.
Although a randomized trial of treatment vs best supportive care has never been done, many believe that treatment may improve survival. 40 The median survival of patients treated with standard therapy is about 3 years if the breast cancer is estrogen-receptor-positive and 2 years if it is estrogen-receptor-negative, but survival rates vary widely from patient to patient.41,42
Standard therapy or enrollment in a clinical phase II or III trial is indicated for this patient before considering enrollment in a phase I clinical trial or supportive care alone.
Endocrine therapy is the first-line therapy in women with estrogen-receptor-positive metastatic breast cancer. Postmenopausal women usually receive an aromatase inhibitor first.43,44 Response to endocrine therapy usually takes weeks to months but may last for several years.
Premenopausal women with estrogen-receptor-positive breast cancer also receive ovarian ablation therapy (oophorectomy or chemical ovarian ablation) with gonadotropin-releasing hormone agonists.
In addition, most patients with bone involvement are treated with high doses of intravenous bisphosphonates, which can reduce skeletal complications.45
Chemotherapy is reserved for patients with estrogen-receptor-negative breast cancer and those with cancer that progresses despite treatment with multiple antiestrogen agents. The time to response when chemotherapy is used is quicker, but the duration of response is usually shorter, lasting on average less than 1 year.37
Trastuzumab (Herceptin), a monoclonal humanized murine antibody to the extracellular domain of the HER2 protein, is indicated in patients with HER2-overexpressing tumors.46,47
STABLE 2 YEARS LATER
The patient was started on letrozole and a bisphosphonate, zolendronic acid (Zometa). Ovarian ablation was initiated with goserelin (Zoladex) given monthly. A bone scan performed 2 months after starting treatment showed improvement in bony metastases. She also noted significant improvement in pain. Her disease remains stable 2 years after starting endocrine therapy.
A 40-year-old premenopausal woman presents with a palpable lump in her left breast. She first noted it 2 months ago on self-examination, and it has steadily grown in size regardless of the phase of her menstrual cycle.
The patient has never undergone mammography. Her menarche was at age 12. At age 35, she had one child (whom she breastfed) after a normal first full-term pregnancy. She took oral contraceptives for 10 years before her pregnancy. She has no other medical problems. She has no family history of breast or ovarian cancer.
On examination, her breasts are slightly asymmetric, without skin discoloration, tenderness, swelling, nipple retraction, or discharge. A 1.5- to 2-cm, rubbery, mobile lump can be felt in the left breast at about the 2 o’clock position. No axillary lymph nodes can be palpated. The rest of her examination is normal.
BREAST CANCER MUST BE RULED OUT
Benign breast disease is found in approximately 90% of women 20 to 50 years of age who come to a physician with a breast problem.1
Nevertheless, breast cancer is of major concern. It is the most common type of cancer in women in the United States, responsible for an estimated 194,440 new cases and 40,610 deaths in 2009. It is also the leading cause of cancer-related death in women age 45 to 55 years in this country.2,3
Breast cancer is most common in postmenopausal women, its incidence rising sharply after the age of 45 and leveling off at age 75. The median age at diagnosis is 61 years. Still, 1.9% of breast cancers in women are diagnosed at age 20 to 34, 10.6% at age 35 to 44, and 22.4% at age 45 to 54.4
Thus, it is paramount to perform a thorough assessment and workup of women who have breast lumps, regardless of their age. Doing so allows breast cancer to be detected at an early stage. The 5-year survival rate is 98.0% for women with localized disease, 83.6% with regional disease, and 23.4% with distant disease.4
WHAT IS THE APPROPRIATE WORKUP?
1. Which of the following are appropriate in the workup of this patient?
- Mammography
- Ultrasonography
- Percutaneous needle biopsy of the lesion
- Magnetic resonance imaging (MRI) of the brain
- Computed tomography (CT) of the chest, abdomen, and pelvis
- Positron emission tomography (PET)
She should undergo mammography, ultrasonography, and percutaneous needle biopsy.
Physical findings that suggest breast cancer include a hard, isolated, sometimes nonmobile lump, serosanguinous nipple discharge, and unilateral nipple retraction. Peau d’orange skin discoloration can occur. A scaly, vesicular, or ulcerated rash with or without pruritus, burning, irritation, or pain of the nipple or skin (Paget disease of the breast) is found in 1% to 3% of breast cancers and may be initially dismissed as mastitis.5,6 Palpable enlarged axillary lymph nodes can suggest invasive breast cancer.
Mammography is recommended in all cases of suspicious breast lumps. In a patient with a palpable lump, diagnostic mammography has a positive predictive value of 21.8%, a specificity of 85.8%, and a sensitivity of 87.7%, which are higher values than in a patient without signs or symptoms.7
The BIRADS score. Mammographic findings are summarized using a scoring system devised by the American College of Radiology called BIRADS (Breast Imaging Reporting and Data System). This system is based on mass irregularity, density, spiculation, and presence or absence of microcalcifications. It standardizes the results of mammography, gives an estimate of the risk of breast cancer, and recommends the frequency of follow-up examinations.8 Scores range from 0 to 6:
- 0—Incomplete assessment warranting additional evaluation
- 1—Completely negative mammogram
- 2—Benign lesion
- 3—Requires follow-up mammogram at 6 months
- 4—Risk of cancer is 2% to 95%; core biopsy needed
- 5—Risk of cancer is more than 95%; core biopsy needed
- 6—Cases that have already been proven to be malignant.
Ultrasonography is also done if a suspicious lesion is found on mammography or physical examination. It helps differentiate between solid and cystic masses. If a mass is identified as a cyst, ultrasonography can further characterize it as simple, complicated-simple, or complex. Simple cysts and complicated-simple cysts are unlikely to be malignant.9,10 Complex cysts or cysts associated with solid tissue are evaluated by biopsy.
Percutaneous needle biopsy should be done for a definitive diagnosis of most suspicious breast masses.
MRI can sometimes provide more accurate information about the possibility of multifocal breast cancer by revealing additional lesions missed on mammography or ultrasonography. It is also useful in determining more accurately the size of the breast tumor and looking for any possible contralateral lesions. In addition, it can sometimes detect enlarged axillary lymph nodes. However, it has poor specificity for breast cancer and may lead to additional and sometimes unnecessary diagnostic tests, which can delay treatment.
MRI’s role is therefore not clearly established, but it is commonly used in clinical practice. It is argued that workup of MRI findings may help in planning more accurate surgical procedures and may prevent reoperations. Based on retrospective analyses, results of breast MRI may lead to altered surgical treatment in approximately 13% of patients.11
Interestingly, a recent randomized trial showed no difference in reoperation rates between patients who underwent MRI before surgery vs those who did not. However, diagnostic workup of new MRI findings was not mandated by the study protocol, making the results of this trial difficult to interpret.12
DIFFERENTIAL DIAGNOSIS
2. Which of the following is in the differential diagnosis of a woman presenting with a breast abnormality?
- Fibrocystic changes
- Breast cyst
- Ductal ectasia
- Simple fibroadenoma
- Intraductal papilloma
- Ductal carcinoma in situ
- Mastitis
- Infiltrating ductal carcinoma
- Phyllodes tumor
All of these choices are part of the differential diagnosis.
Benign breast lesions
Simple fibroadenoma, one of the most common proliferative lesions, is not associated with a higher risk of developing breast cancer.
Fibrocystic changes are the most common nonproliferative lesions. Occasionally breast pain, nipple discharge, or significant lumpiness that varies during the course of the menstrual cycle can occur. The nipple discharge in women with fibrocystic changes is physiologic and pale green to brown in color. It can also be yellow, whitish, clear, or bloody. Bloody nipple discharge is considered pathologic and suggests a process other than fibrocystic changes, necessitating further workup. However, bloody discharge is not always a sign of malignancy, as it can have a benign cause as well.
Ductal ectasia, another nonproliferative lesion, is a result of dilation of subareolar ducts that contain fluid with a crystalline material. It can penetrate the duct, forming a nodule, which causes pain and occasionally fever.
Precancerous and cancerous lesions
Ductal carcinoma in situ is a true neoplasm that has not yet developed the ability to invade through the basement membrane of the ducts. The likelihood of progression to invasive breast cancer depends on the histologic grade, the tumor size, and the patient’s age.
Lobular carcinoma in situ arises from lobules and terminal ducts of breast tissue. Much controversy surrounds this type of tumor, which was thought to be a marker of increased risk of developing ipsilateral and contralateral breast cancer and not to be a malignant lesion itself.15 However, there is emerging evidence to suggest that a pleomorphic variant of lobular carcinoma in situ is associated with development of breast cancer in the same site as the lesion, whereas a nonpleomorphic form is a marker of increased risk of ipsilateral and contralateral breast cancer.16
Invasive ductal and lobular carcinomas are the true invasive breast cancers, with a potential to metastasize.
Phyllodes tumors are uncommon fibroepithelial lesions that account for less than 1% of all breast neoplasms. The median age at presentation is 45 years.17 Despite the historical name “cystosarcoma phyllodes,” these lesions are not true sarcomas and have stromal and epithelial components.
These tumors display very heterogeneous behavior and, based on predefined histologic criteria, are often classified as benign, borderline, or malignant. Benign phyllodes tumors are similar to fibroadenomas in both histology and prognosis, making their diagnosis challenging. The most aggressive phyllodes tumors lose their epithelial component and have high metastatic potential. These tumors often have a biphasic growth pattern, and women may present with a smooth, round, well-defined breast lump that was stable for many years but then started to grow rapidly.17
Surgical resection with wide margins is the primary management of these tumors.18
Mastitis, ie, inflammation of the breast tissue, often presents with symptoms of breast erythema, swelling, tenderness, and nipple discharge. It may be secondary to infection (most often in lactating women) or other causes such as radiation or underlying malignancy. A complication of infectious mastitis is formation of a breast abscess. Underlying malignancy, especially inflammatory breast cancer, is a common cause of noninfectious mastitis and is very important to recognize.19
RISK FACTORS FOR BREAST CANCER
3. Which of the following are risk factors for breast cancer?
- Menarche before age 12
- Female sex
- Personal history of breast cancer
- Obesity
- Never having had children, or having given birth for the first time at an older age
- Older age
- History of hormone replacement therapy with estrogen and progesterone
- Family history of breast cancer
All of these choices are risk factors for breast cancer.
Family history
The overall relative risk of developing breast cancer in a woman with a first-degree relative with the disease is 1.7. However, the relative risk is about 3 if the first-degree relative developed breast cancer before menopause, and 9 if the first-degree relative developed bilateral breast cancer before menopause.5
Estrogen exposure
The duration and amount of estrogen exposure are also risk factors. For example, menarche before age 12 and menopause after age 55 are associated with a higher risk. Women who go through menopause after age 55 have a twofold higher risk of breast cancer compared with women who go through menopause at an early age. Pregnancy before age 30 lowers the risk of breast cancer; late first full-term pregnancy or nulliparity increases it. Lactation, on the other hand, has a protective effect.5
Oral contraceptives have traditionally been thought to increase the risk of breast cancer. In the 1990s, a meta-analysis involving 153,506 women found that those who had used oral contraceptives had a 24% higher risk of developing breast cancer.26 However, this association has come into question since newer oral contraceptive pills containing different progestins and lower amounts of estrogen have become available. In fact, recent studies showed no link between oral contraceptive use and breast cancer.27,28 Nevertheless, women at higher risk of developing breast cancer are advised not to use oral contraceptives.
Hormone replacement therapy with estrogen and progesterone was found to increase the risk of breast cancer by 26% in the Women’s Health Initiative (WHI) study, which involved 16,608 healthy women followed for a median of 5.6 years.29
In a study reported separately, the WHI investigators randomized 10,739 women who had undergone hysterectomy to receive either hormone replacement therapy with unopposed estrogen (which is feasible only in women without a uterus) or placebo. They found no increase in the risk of invasive breast cancer in women on hormone replacement therapy with estrogen alone. In fact, the study showed a trend towards a modest reduction of this risk (odds ratio 0.77; 95% confidence interval 0.59–1.01).30
After the results of the WHI were published, the use of hormone replacement therapy in postmenopausal women declined significantly. And in 2003—1 year later—the incidence of breast cancer had dropped by 6.7%.31
Most experts now recommend that estrogen-progestin combinations be used only selectively to treat the symptoms of menopause, and only for the short term.
Other risk factors
Other factors found to modestly increase the risk of breast cancer include:
- Alcohol use
- Obesity
- Radiation exposure. Patients are at higher risk of breast cancer 15 to 20 years after receiving upper-mantle radiotherapy for Hodgkin lymphoma.5
Case continues: Bad news on mammography, ultrasonography, biopsy
The patient undergoes mammography, which shows a 2.5-cm spiculated lesion with areas of calcifications (BIRADS score of 5). Subsequently, ultrasonography confirms that the suspicious mass is not a cyst. Ultrasound-guided core needle biopsy reveals that the lesion is a high-grade invasive ductal carcinoma. The tumor is positive for both estrogen and progesterone receptors and negative for HER2/neu overexpression.
STAGING EVALUATION
4. Given these findings, what is the next step to take?
- CT of the chest, abdomen, and pelvis
- MRI of the brain
- PET
- Referral to a surgeon for a possible mastectomy with sentinel lymph node dissection
- Referral to a surgeon for a possible lumpectomy with sentinel lymph node dissection
At this point, the patient should be referred to a surgeon for possible mastectomy or lumpectomy.
Women who appear clinically to have early breast cancer, such as in this case, should have a complete blood count, comprehensive metabolic panel, and chest x-ray as their initial staging evaluation. No further studies are recommended unless the findings on history, physical examination, or the above testing suggest possible metastases.
Mastectomy vs lumpectomy
Early-stage breast cancer is managed with definitive surgery. The two options are mastectomy and breast conservation therapy, the latter involving lumpectomy followed by breast radiation therapy.
Multiple randomized studies comparing mastectomy and lumpectomy showed no difference in survival rates, but patients in the lumpectomy groups had higher rates of local recurrence.32 Breast radiation therapy after lumpectomy lowered the rates of local recurrence and breast cancer death.33 Therefore, most patients can opt to undergo either lumpectomy with radiation or mastectomy, depending on personal preference.
However, mastectomy rather than breast conservation therapy is still recommended in cases of prior radiation therapy, inability to achieve negative surgical margins (as in cases of large tumors), multicentric disease (cancer in separate breast quadrants), or multiple areas of calcifications. Mastectomy is also preferred in most pregnant women unless the diagnosis of breast cancer is made in the third trimester and radiation therapy can be given after delivery. Patients who have large lesions in a small breast may also choose mastectomy with breast reconstruction rather than breast conservation therapy. Patients with a history of scleroderma are encouraged to undergo mastectomy because of increased toxicity from radiation treatment.
Sentinel vs axillary lymph node dissection
Knowledge of axillary lymph node involvement is important because it determines the stage in the tumor-node-metastasis (TNM) system, and it influences the choice of further therapy. Therefore, all patients with nonmetastatic invasive breast cancer must have their axillary lymph nodes sampled.
Conventionally, this involves axillary lymph node dissection. Unfortunately, upper extremity lymphedema develops in 6% to 30% of patients within the first 3 years, and in 49% of patients after 20 years following axillary lymph node dissection.34
Sentinel lymph node dissection was developed to minimize this complication. This procedure involves the injection of a blue dye, isosulfan blue (Lymphazurin), around the edge of the tumor or in the dermis overlying the tumor. The most proximal axillary lymph nodes that stain blue are dissected. Alternatively, a radioactive colloid (most commonly technetium sulfur colloid agents) may be injected, allowing sentinel lymph nodes to be identified by lymphoscintigraphy. If no metastases are found in the sentinel lymph nodes, axillary lymph node dissection is not performed.
A prospective study in 536 women found that at 5 years of follow-up, lymphedema developed in only 5% of patients after sentinel lymph node dissection compared with 16% of those who underwent axillary lymph node dissection (P < .001), with comparable outcomes in terms of disease recurrence.35
Case continues: Patient undergoes surgery
The patient elects to undergo lumpectomy with sentinel lymph node dissection. Pathologic review of the resection specimen reveals a 2.5-cm poorly differentiated invasive ductal carcinoma. Sentinel lymph node dissection shows metastases, and therefore axillary lymph node dissection is performed. One of eight lymph nodes removed is positive for metastases. All surgical margins are negative.
POSTOPERATIVE CARE
5. What would be the next step for our patient?
- Radiation followed by observation
- Tamoxifen (Nolvadex) for 5 years
- Observation only
- Chemotherapy followed by radiation therapy and 5 years of tamoxifen
She should receive chemotherapy, followed by radiation therapy and then tamoxifen for 5 years.
Chemotherapy. Almost all patients who have lymph-node-positive disease are advised to undergo chemotherapy.
The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) performed a metaanalysis of 194 randomized trials that compared adjuvant chemotherapy and no treatment in early-stage breast cancer. Chemotherapy led to a 10% absolute improvement in survival at 15 years for women younger than 50 years and 3% in women age 51 to 69.36
Indications for chemotherapy include axillary lymph node involvement, locally advanced disease, and other risk factors for recurrence such as young age at diagnosis, strong positive family history of breast cancer, prior history of breast cancer, or lymph-node-negative, estrogen-receptor-negative tumors that are larger than 1 cm in diameter.
The Oncotype DX assay is a new tool to help oncologists decide whether to use chemotherapy in cases of estrogen-receptor-positive breast cancer, in which the benefit of chemotherapy is uncertain. It is a polymerase chain reaction assay that measures the expression of 16 cancer-specific genes and five reference genes within the breast tumor. Based on the pattern of expression of these genes, breast cancer can be characterized as low-risk, intermediate-risk, or high-risk. Patients in the high-risk group have a high chance of cancer recurrence and benefit from chemotherapy. Patients in the low-risk group are unlikely to have a recurrence or to benefit from chemotherapy.37 It is far less clear if patients in the intermediate-risk group benefit from chemotherapy, but this assay might eventually prove useful in deciding for or against chemotherapy in this group of patients as well.38 The Oncotype DX assay is presently being studied in a clinical trial.
Radiation therapy after mastectomy is recommended in patients who have breast tumors larger than 5 cm or metastases to more than three axillary lymph nodes.39
Antiestrogen therapy. After chemotherapy, patients with estrogen-receptor-positive cancers also receive 5 years of antiestrogen therapy. Available antiestrogen agents for such patients include tamoxifen, which is a selective estrogen receptor modulator, and drugs called aromatase inhibitors that block conversion of androgens to estrogens in peripheral tissues. Anastrozole (Arimidex), letrozole (Femara), and exemestane (Aromasin) are examples of available aromatase inhibitors. Premenopausal women are treated with tamoxifen, and postmenopausal women are offered aromatase inhibitors.
Table 4 lists the most common adverse effects of these agents. Aromatase inhibitors are associated with a higher risk of osteoporosis and arthralgia, while tamoxifen increases the risks of thromboembolism, endometrial cancer, and vaginal discharge. Both agents may produce menopausal symptoms such as hot flashes and mood swings.
Case continues: Seven years later, metastases in the spine
The patient achieves a complete remission. She is seen for a routine visit 7 years after diagnosis. She now reports mid-back pain that has worsened over the last 2 months. A bone scan reveals diffuse metastatic disease in the spine and in both humeral bones. CT of the chest, abdomen, and pelvis is negative for visceral metastases. Bone marrow aspiration and biopsy study show marrow infiltration by adenocarcinoma that stains positive for estrogen receptors and negative for HER2. The patient otherwise feels well and has no other symptoms.
WHAT TREATMENT FOR METASTATIC BREAST CANCER?
6. What should you now do for our patient?
- Discuss end-of-life care and refer her to a hospice program
- Educate the patient that no options for treatment exist and recommend enrolling in a phase I clinical trial
- Refer her to an oncologist for consideration of chemotherapy
- Refer her to an oncologist for consideration of endocrine treatment
She should be referred to an oncologist for consideration of endocrine treatment.
The most common sites of breast cancer metastases are the bones, followed by the liver and lungs. Metastatic breast cancer almost always is incurable. However, treatment can palliate symptoms.
Although a randomized trial of treatment vs best supportive care has never been done, many believe that treatment may improve survival. 40 The median survival of patients treated with standard therapy is about 3 years if the breast cancer is estrogen-receptor-positive and 2 years if it is estrogen-receptor-negative, but survival rates vary widely from patient to patient.41,42
Standard therapy or enrollment in a clinical phase II or III trial is indicated for this patient before considering enrollment in a phase I clinical trial or supportive care alone.
Endocrine therapy is the first-line therapy in women with estrogen-receptor-positive metastatic breast cancer. Postmenopausal women usually receive an aromatase inhibitor first.43,44 Response to endocrine therapy usually takes weeks to months but may last for several years.
Premenopausal women with estrogen-receptor-positive breast cancer also receive ovarian ablation therapy (oophorectomy or chemical ovarian ablation) with gonadotropin-releasing hormone agonists.
In addition, most patients with bone involvement are treated with high doses of intravenous bisphosphonates, which can reduce skeletal complications.45
Chemotherapy is reserved for patients with estrogen-receptor-negative breast cancer and those with cancer that progresses despite treatment with multiple antiestrogen agents. The time to response when chemotherapy is used is quicker, but the duration of response is usually shorter, lasting on average less than 1 year.37
Trastuzumab (Herceptin), a monoclonal humanized murine antibody to the extracellular domain of the HER2 protein, is indicated in patients with HER2-overexpressing tumors.46,47
STABLE 2 YEARS LATER
The patient was started on letrozole and a bisphosphonate, zolendronic acid (Zometa). Ovarian ablation was initiated with goserelin (Zoladex) given monthly. A bone scan performed 2 months after starting treatment showed improvement in bony metastases. She also noted significant improvement in pain. Her disease remains stable 2 years after starting endocrine therapy.
- Barton MB, Elmore JG, Fletcher SW. Breast symptoms among women enrolled in a health maintenance organization: frequency, evaluation, and outcome. Ann Intern Med 1999; 130:651–657.
- Petrelli NJ, Winer EP, Brahmer J, et al. Clinical cancer advances 2009: major research advances in cancer treatment, prevention, and screening—a report from the American Society of Clinical Oncology. J Clin Oncol 2009; 27:6052–6069.
- Jemal A, Siegel R, Ward E, et al. Cancer statistics 2008. CA Cancer J Clin 2008; 58:71–96.
- National Cancer Institute. SEER Stat Fact Sheets. www.seer.cancer.gov/statfacts/html/breast.html#ref09. Accessed June 7, 2010.
- Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ; the publishers of the journal Oncology. Cancer Management: A multidisciplinary Approach. Medical, Surgical & Radiation Oncology. 11th ed. CMP Medica; 2008.
- Kollmorgen DR, Varanasi JS, Edge SB, Carson WE. Paget’s disease of the breast: a 33-year experience. J Am Coll Surg 1998; 187:171–177.
- Barlow WE, Lehman CD, Zheng Y, et al. Performance of diagnostic mammography for women with signs or symptoms of breast cancer. J Natl Cancer Inst 2002; 94:1151–1159.
- American College of Radiology. Breast Imaging Reporting and Data System: BIRADS Atlas. 4th ed. Reston, VA: American College of Radiology; 2003.
- Hong AS, Rosen EL, Soo MS, Baker JA. BI-RADS for sonography: positive and negative predictive values of sonographic features. AJR Am J Roentgenol 2005; 184:1260–1265.
- Berg WA, Campassi CI, Ioffe OB. Cystic lesions of the breast: sonographic-pathologic correlation. Radiology 2003; 227:183–191.
- Schell AM, Rosenkranz K, Lewis PJ. Role of breast MRI in the preoperative evaluation of patients with newly diagnosed breast cancer. AJR Am J Roentgenol 2009; 192:1438–1444.
- Turnbull L, Brown S, Harvey I, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet 2010; 375:563–571.
- Worsham MJ, Abrams J, Raju U, et al. Breast cancer incidence in a cohort of women with benign breast disease from a multiethnic, primary health care population. Breast J 2007; 13:115–121.
- Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med 1985; 312:146–151.
- Page DL, Kidd TE, Dupont WD, Simpson JF, Rogers LW. Lobular neoplasia of the breast: higher risk for subsequent invasive cancer predicted by more extensive disease. Hum Pathol 1991; 22:1232–1239.
- Sneige N, Wang J, Baker BA, Krishnamurthy S, Middleton LP. Clinical, histopathologic, and biologic features of pleomorphic lobular (ductallobular) carcinoma in situ of the breast: a report of 24 cases. Mod Pathol 2002; 15:1044–1050.
- Telli ML, Horst KC, Guardino AE, Dirbas FM, Carlson RW. Phyllodes tumors of the breast: natural history, diagnosis, and treatment. J Natl Compr Canc Netw 2007; 5:324–330.
- Reinfuss M, Mitus J, Duda K, Stelmach A, Rys J, Smolak K. The treatment and prognosis of patients with phyllodes tumor of the breast: an analysis of 170 cases. Cancer 1996; 77:910–916.
- Kamal RM, Hamed ST, Salem DS. Classification of inflammatory breast disorders and step by step diagnosis. Breast J 2009; 15:367–380.
- Hartge P, Struewing JP, Wacholder S, Brody LC, Tucker MA. The prevalence of common BRCA1 and BRCA2 mutations among Ashkenazi Jews. Am J Hum Genet 1999; 64:963–970.
- Wooster R, Weber BL. Breast and ovarian cancer. N Engl J Med 2003; 348:2339–2347.
- Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med 2009; 361:170–177.
- Hisada M, Garber JE, Fung CY, Fraumeni JF, Li FP. Multiple primary cancers in families with Li-Fraumeni syndrome. J Natl Cancer Inst 1998; 90:606–611.
- Bell DW, Varley JM, Szydlo TE, et al. Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science 1999; 286:2528–2531.
- Kaurah P, MacMillan A, Boyd N, et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA 2007; 297:2360–2372.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet 1996; 347:1713–1727.
- Hankinson SE, Colditz GA, Manson JE, et al. A prospective study of oral contraceptive use and risk of breast cancer (Nurses’ Health Study, United States). Cancer Causes Control 1997; 8:65–72.
- Marchbanks PA, McDonald JA, Wilson HG, et al. Oral contraceptives and the risk of breast cancer. N Engl J Med 2002; 346:2025–2032.
- Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288:321–333.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 2004; 291:1701–1712.
- Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med 2007; 356:1670–1674.
- Fisher B, Anderson S, Redmond CK, Wolmark N, Wickerham DL, Cronin WM. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med 1995; 333:1456–1461.
- Clarke M, Collins R, Darby S, et al; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 366:2087–2106.
- Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 2001; 92:1368–1377.
- McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol 2008; 26:5213–5219.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 365:1687–1717.
- Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004; 351:2817–2826.
- Albain KS, Barlow WE, Shak S, et al; Breast Cancer Intergroup of North America. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010; 11:55–65.
- Harris JR, Halpin-Murphy P, McNeese M, Mendenhall NP, Morrow M, Robert NJ. Consensus Statement on postmastectomy radiation therapy. Int J Radiat Oncol Biol Phys 1999; 44:989–990.
- Gennari A, Conte P, Rosso R, Orlandini C, Bruzzi P. Survival of metastatic breast carcinoma patients over a 20-year period: a retrospective analysis based on individual patient data from six consecutive studies. Cancer 2005; 104:1742–1750.
- Mouridsen H, Gershanovich M, Sun Y, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol 2003; 21:2101–2109.
- Gamucci T, D’Ottavio AM, Magnolfi E, et al. Weekly epirubicin plus docetaxel as first-line treatment in metastatic breast cancer. Br J Cancer 2007; 97:1040–1045.
- Bonneterre J, Thürlimann B, Robertson JF, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol 2000; 18:3748–3757.
- Nabholtz JM, Buzdar A, Pollak M, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol 2000; 18:3758–3767.
- Hortobagyi GN, Theriault RL, Porter L, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med 1996; 335:1785–1791.
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005; 353:1673–1684.
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344:783–792.
- Barton MB, Elmore JG, Fletcher SW. Breast symptoms among women enrolled in a health maintenance organization: frequency, evaluation, and outcome. Ann Intern Med 1999; 130:651–657.
- Petrelli NJ, Winer EP, Brahmer J, et al. Clinical cancer advances 2009: major research advances in cancer treatment, prevention, and screening—a report from the American Society of Clinical Oncology. J Clin Oncol 2009; 27:6052–6069.
- Jemal A, Siegel R, Ward E, et al. Cancer statistics 2008. CA Cancer J Clin 2008; 58:71–96.
- National Cancer Institute. SEER Stat Fact Sheets. www.seer.cancer.gov/statfacts/html/breast.html#ref09. Accessed June 7, 2010.
- Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ; the publishers of the journal Oncology. Cancer Management: A multidisciplinary Approach. Medical, Surgical & Radiation Oncology. 11th ed. CMP Medica; 2008.
- Kollmorgen DR, Varanasi JS, Edge SB, Carson WE. Paget’s disease of the breast: a 33-year experience. J Am Coll Surg 1998; 187:171–177.
- Barlow WE, Lehman CD, Zheng Y, et al. Performance of diagnostic mammography for women with signs or symptoms of breast cancer. J Natl Cancer Inst 2002; 94:1151–1159.
- American College of Radiology. Breast Imaging Reporting and Data System: BIRADS Atlas. 4th ed. Reston, VA: American College of Radiology; 2003.
- Hong AS, Rosen EL, Soo MS, Baker JA. BI-RADS for sonography: positive and negative predictive values of sonographic features. AJR Am J Roentgenol 2005; 184:1260–1265.
- Berg WA, Campassi CI, Ioffe OB. Cystic lesions of the breast: sonographic-pathologic correlation. Radiology 2003; 227:183–191.
- Schell AM, Rosenkranz K, Lewis PJ. Role of breast MRI in the preoperative evaluation of patients with newly diagnosed breast cancer. AJR Am J Roentgenol 2009; 192:1438–1444.
- Turnbull L, Brown S, Harvey I, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet 2010; 375:563–571.
- Worsham MJ, Abrams J, Raju U, et al. Breast cancer incidence in a cohort of women with benign breast disease from a multiethnic, primary health care population. Breast J 2007; 13:115–121.
- Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med 1985; 312:146–151.
- Page DL, Kidd TE, Dupont WD, Simpson JF, Rogers LW. Lobular neoplasia of the breast: higher risk for subsequent invasive cancer predicted by more extensive disease. Hum Pathol 1991; 22:1232–1239.
- Sneige N, Wang J, Baker BA, Krishnamurthy S, Middleton LP. Clinical, histopathologic, and biologic features of pleomorphic lobular (ductallobular) carcinoma in situ of the breast: a report of 24 cases. Mod Pathol 2002; 15:1044–1050.
- Telli ML, Horst KC, Guardino AE, Dirbas FM, Carlson RW. Phyllodes tumors of the breast: natural history, diagnosis, and treatment. J Natl Compr Canc Netw 2007; 5:324–330.
- Reinfuss M, Mitus J, Duda K, Stelmach A, Rys J, Smolak K. The treatment and prognosis of patients with phyllodes tumor of the breast: an analysis of 170 cases. Cancer 1996; 77:910–916.
- Kamal RM, Hamed ST, Salem DS. Classification of inflammatory breast disorders and step by step diagnosis. Breast J 2009; 15:367–380.
- Hartge P, Struewing JP, Wacholder S, Brody LC, Tucker MA. The prevalence of common BRCA1 and BRCA2 mutations among Ashkenazi Jews. Am J Hum Genet 1999; 64:963–970.
- Wooster R, Weber BL. Breast and ovarian cancer. N Engl J Med 2003; 348:2339–2347.
- Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med 2009; 361:170–177.
- Hisada M, Garber JE, Fung CY, Fraumeni JF, Li FP. Multiple primary cancers in families with Li-Fraumeni syndrome. J Natl Cancer Inst 1998; 90:606–611.
- Bell DW, Varley JM, Szydlo TE, et al. Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science 1999; 286:2528–2531.
- Kaurah P, MacMillan A, Boyd N, et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA 2007; 297:2360–2372.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet 1996; 347:1713–1727.
- Hankinson SE, Colditz GA, Manson JE, et al. A prospective study of oral contraceptive use and risk of breast cancer (Nurses’ Health Study, United States). Cancer Causes Control 1997; 8:65–72.
- Marchbanks PA, McDonald JA, Wilson HG, et al. Oral contraceptives and the risk of breast cancer. N Engl J Med 2002; 346:2025–2032.
- Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288:321–333.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 2004; 291:1701–1712.
- Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med 2007; 356:1670–1674.
- Fisher B, Anderson S, Redmond CK, Wolmark N, Wickerham DL, Cronin WM. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med 1995; 333:1456–1461.
- Clarke M, Collins R, Darby S, et al; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 366:2087–2106.
- Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 2001; 92:1368–1377.
- McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol 2008; 26:5213–5219.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 365:1687–1717.
- Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004; 351:2817–2826.
- Albain KS, Barlow WE, Shak S, et al; Breast Cancer Intergroup of North America. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010; 11:55–65.
- Harris JR, Halpin-Murphy P, McNeese M, Mendenhall NP, Morrow M, Robert NJ. Consensus Statement on postmastectomy radiation therapy. Int J Radiat Oncol Biol Phys 1999; 44:989–990.
- Gennari A, Conte P, Rosso R, Orlandini C, Bruzzi P. Survival of metastatic breast carcinoma patients over a 20-year period: a retrospective analysis based on individual patient data from six consecutive studies. Cancer 2005; 104:1742–1750.
- Mouridsen H, Gershanovich M, Sun Y, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol 2003; 21:2101–2109.
- Gamucci T, D’Ottavio AM, Magnolfi E, et al. Weekly epirubicin plus docetaxel as first-line treatment in metastatic breast cancer. Br J Cancer 2007; 97:1040–1045.
- Bonneterre J, Thürlimann B, Robertson JF, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol 2000; 18:3748–3757.
- Nabholtz JM, Buzdar A, Pollak M, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol 2000; 18:3758–3767.
- Hortobagyi GN, Theriault RL, Porter L, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med 1996; 335:1785–1791.
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005; 353:1673–1684.
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344:783–792.
Noninvasive tests for liver disease, fibrosis, and cirrhosis: Is liver biopsy obsolete?
Primary care physicians and specialists alike often encounter patients with chronic liver disease. Fortunately, these days we need to resort to liver biopsy less often than in the past.
The purpose of this review is to provide a critical assessment of the growing number of noninvasive tests available for diagnosing liver disease and assessing hepatic fibrosis, and to discuss the implications of these advances related to the indications for needle liver biopsy.
WHEN IS LIVER BIOPSY USEFUL?
In diagnosis
Needle liver biopsy for diagnosis remains important in cases of:
Diagnostic uncertainty (eg, in patients with atypical features)
Coexisting disorders (eg, human immunodeficiency virus [HIV] and hepatitis C virus infection, or alcoholic liver disease and hepatitis C)
An overlapping syndrome (eg, primary biliary cirrhosis with autoimmune hepatitis).
Fatty liver. Needle liver biopsy can distinguish between benign steatosis and progressive steatohepatitis in a patient with a fatty liver found on imaging, subject to the limitations of sampling error.
Because fatty liver disease is common and proven treatments are few, no consensus has emerged about which patients with suspected fatty liver disease should undergo needle biopsy. Many specialists eschew needle biopsy and treat the underlying risk factors of metabolic syndrome, reserving biopsy for patients with findings that raise the concern of cirrhosis.
Hereditary disorders, eg, hemochromatosis, alpha-1 antitrypsin deficiency, and Wilson disease.
In management
Periodic needle biopsy is also valuable in the management of a few diseases.
In autoimmune hepatitis, monitoring the plasma cell score on liver biopsy may help predict relapse when a physician is considering reducing or discontinuing immunosuppressive therapy.1
After liver transplantation, a liver biopsy is highly valuable to assess for rejection and the presence and intensity of disease recurrence.
PROBLEMS WITH LIVER BIOPSY
Liver biopsy is invasive and can cause significant complications. Nearly 30% of patients report having substantial pain after liver biopsy, and some experience serious complications such as pneumothorax, bleeding, or puncture of the biliary tree. In rare cases, patients die of bleeding.2
Furthermore, hepatic pathology, particularly fibrosis, is not always uniformly distributed. Surgical wedge biopsy provides adequate tissue volume to overcome this problem. Needle biopsy, on the other hand, provides a much smaller volume of tissue (1/50,000 of the total mass of the liver).3
As examples of the resulting sampling errors that can occur, consider the two most common chronic liver diseases: hepatitis C and fatty liver disease.
Regev et al4 performed laparoscopically guided biopsy of the right and left hepatic lobes in a series of 124 patients with chronic hepatitis C. Biopsy samples from the right and left lobes differed in the intensity of inflammation in 24.2% of cases, and in the intensity of fibrosis in 33.1%. Differences of more than one grade of inflammation or stage of fibrosis were uncommon. However, in 14.5%, cirrhosis was diagnosed in one lobe but not the other.
In a study in patients with nonalcoholic fatty liver disease, Ratziu et al5 found that none of the features characteristic of nonalcoholic steatohepatitis were highly concordant in paired liver biopsies. Clearly, needle liver biopsy is far from an ideal test.
Increasingly, liver diseases can be diagnosed precisely with laboratory tests, imaging studies, or both. Thus, needle liver biopsy is playing a lesser role in diagnosis.
ADVANCES IN NONINVASIVE DIAGNOSIS OF LIVER DISEASE
Over the past 30 years, substantial strides have been made in our ability to make certain diagnoses through noninvasive means.
Blood tests can be used to diagnose viral hepatitis A, B, and C and many cases of hemochromatosis and primary biliary cirrhosis. For a detailed discussion of how blood tests are used in diagnosing liver diseases, see www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/hepatology/guide-to-common-liver-tests/.
Imaging studies. Primary sclerosing cholangitis can be diagnosed with an imaging study, ie, magnetic resonance cholangiopancreatography (MRCP) or endoscopic retrograde cholangiopancreatography (ERCP). The value of needle biopsy in these patients is limited to assessing the degree of fibrosis to help with management of the disease and, less often, to discovering other liver pathologies.6
Most benign space-occupying liver lesions, both cystic and solid, can be fully characterized by imaging, especially in patients who have no underlying chronic liver disease, and no biopsy is needed. Whether biopsy should be performed to investigate liver lesions depends on the clinical scenario; the topic is beyond the scope of this paper but has been reviewed in detail by Rockey et al.2
CAN NONINVASIVE TESTS DETECT HEPATIC FIBROSIS?
Cirrhosis (stage 4 fibrosis) results in nodular transformation of the liver and impedance of portal blood flow, setting the stage for portal hypertension and its sequelae. Knowing whether cirrhosis is present is important in subsequent management.
In advanced cases, cirrhosis is associated with typical clinical manifestations and laboratory and radiographic findings. In such cases, needle biopsy will add little. However, in most cases, particularly early in the course, clinical, laboratory, and radiologic correlates of cirrhosis are absent. In one study of patients with hepatitis C, 27% had cirrhosis, but in only a small number would cirrhosis have been apparent from clinical signs and laboratory and imaging studies.6
Since a major contemporary role for liver biopsy is in assessing the degree of fibrosis, it is reasonable to ask if newer noninvasive means are available to estimate hepatic fibrosis. The remainder of this review focuses on assessing our increasing ability to stage the degree of fibrosis (including the presence or absence of cirrhosis) by noninvasive means.
Clinical features point to cirrhosis, but not earlier fibrosis
Clinical manifestations help point to the diagnosis of cirrhosis but not to earlier stages of fibrosis.
For example, if a patient is known to have liver disease, the findings of ascites, splenomegaly, or asterixis mean that cirrhosis is highly probable. Similarly, hypersplenism (splenomegaly with a decrease in circulating blood cells but a normal to hyperactive bone marrow) in a patient with liver test abnormalities almost always represents portal hypertension due to cirrhosis, although other, nonhepatic causes are possible, such as congestive heart failure and constrictive pericarditis.
These features generally emerge late in the course of cirrhosis. The absence of such stigmata certainly does not preclude the presence of cirrhosis. Thus, these clinical signs have a high positive predictive value but a low negative predictive value, making them insufficient by themselves to diagnose or stage liver disease.
Laboratory tests are of limited value in assessing the degree of fibrosis
Standard liver tests are of limited value in assessing the degree of fibrosis.
Usual laboratory tests. At one end of the spectrum, anemia, thrombocytopenia, and leukopenia in the presence of liver disease correlate with cirrhosis. At the other end, a serum ferritin concentration of less than 1,000 mg/mL in a patient with hemochromatosis and no confounding features such as hepatitis C, HIV infection, or heavy alcohol use strongly predicts that the patient does not have significant hepatic fibrosis.8
Bilirubin elevation is a late finding in cirrhosis, but in cholestatic diseases bilirubin may be elevated before cirrhosis occurs.
Albumin is made exclusively in the liver, and its concentration falls as liver function worsens with progressive cirrhosis.
The prothrombin time increases as the liver loses its ability to synthesize clotting factors in cirrhosis. Coagulopathy correlates with the degree of liver disease.
Hyponatremia due to impaired ability to excrete free water is seen in patients with cirrhosis and ascites.
In summary, the usual laboratory tests related to liver disease are imprecise and, when abnormal, often indicate not just the presence of cirrhosis, but impending or actual decompensation.
Newer serologic markers, alone or in combination, have been proposed as aids in determining the degree of fibrosis or cirrhosis in the liver. Direct markers of fibrosis measure the turnover or metabolism of extracellular matrix. Indirect markers of fibrosis reflect alterations in hepatic function (see below).
Parkes et al9 reviewed 10 different panels of serum markers of hepatic fibrosis in chronic hepatitis C. Only 35% of patients had fibrosis adequately ruled in or ruled out by these panels, and the stage of fibrosis could not be adequately determined.
These serologic markers have not been validated in other chronic liver diseases or in liver disease due to multiple causes. Thus, although they show promise for use by the general internist, they need to be validated in patients with disease and in normal reference populations before they are ready for “prime time.”
Direct serologic markers of fibrosis
Direct serologic markers of fibrosis include those associated with matrix deposition—eg, procollagen type III amino-terminal peptide (P3NP), type I and IV collagens, laminin, hyaluronic acid, and chondrex.
P3NP is the most widely studied marker of hepatic fibrosis. It is elevated in both acute and chronic liver diseases; serum levels reflect the histologic stage of hepatic fibrosis in various chronic liver diseases, including alcoholic, viral, and primary biliary cirrhosis.10–12 Successful treatment of autoimmune hepatitis has been shown to lead to reductions of P3NP levels.13
Other direct markers of fibrosis are those associated with matrix degradation, ie, matrix metalloproteinases 2 and 3 (MMP-2, MMP-3) and tissue inhibitors of metalloproteinases 1 and 2 (TIMP-1, TIMP-2). Levels of MMP-2 proenzymes and active enzymes are increased in liver disease, but studies are inconsistent in correlating serum levels of MMP-2 to the degree of hepatic fibrosis.14,15 These tests are not commercially available, and the components are not readily available in most clinical laboratories.
Indirect serologic markers of fibrosis
Some indirect markers are readily available:
The AST:ALT ratio. The normal ratio of aspartate aminotransferase (AST) to alanine aminotransferase (ALT) is approximately 0.8. A ratio greater than 1.0 provides evidence of cirrhosis. However, findings have been inconsistent.
The AST:platelet ratio index (APRI), a commonly used index, is calculated by the following formula:
In studies of hepatitis C and hepatitis C-HIV, the APRI has shown a sensitivity of 37% to 80% and a specificity of 45% to 98%, depending on the cutoff value and whether a diagnosis of severe fibrosis or cirrhosis was being tested.16–19 These sensitivities and specificities are disappointing and do not provide information equal to that provided by needle liver biopsy in most patients with chronic liver disease.
The combination of prothrombin, gamma glutamyl, and apolipoprotein AI levels (PGA index) has been validated in patients with many types of chronic liver disease, and its accuracy for detecting cirrhosis is highest (66%–72%) in patients with alcoholic liver disease.20,21
FibroIndex uses the platelet count, AST level, and gamma globulin level to detect significant fibrosis in chronic hepatitis C, but its accuracy has yet to be validated.22
The FIB-4 index is based on four independent predictors of fibrosis, ie, age, the platelet count, AST level, and ALT level. It has shown good accuracy for detecting advanced fibrosis in two studies in patients with hepatitis C.23,24
Fibrometer (based on the platelet count; the prothrombin index; the levels of AST, alfa-2 macroglobulin, hyaluronate, and blood urea nitrogen; and age) predicted fibrosis well in chronic viral hepatitis.25,26
Fibrotest and Fibrosure are proprietary commercial tests available in many laboratories. They employ a mathematical formula to predict fibrosis (characterized as mild, significant, or indeterminate) using the levels of alpha-2 macroglobulin, alpha-2 globulin, gamma globulin, apolipoprotein A1, gamma glutamyl transferase, and total bilirubin. For detecting significant fibrosis, these tests are reported to have a sensitivity of about 75% and a specificity of 85%.27–29
ActiTest incorporates the ALT level into the Fibrotest to reflect liver fibrosis and necro-inflammatory activity.
A meta-analysis showed that Fibrotest and ActiTest could be reliable alternatives to liver biopsy in patients with chronic hepatitis C.30 The area under the receiver operator characteristic curve for the diagnosis of significant fibrosis ranged from 0.73 to 0.87; for the diagnosis of significant histologic activity it ranged from 0.75 to 0.86. Fibrotest had a negative predictive value for excluding significant fibrosis of 91% with a cutoff of 0.31. ActiTest’s negative predictive value for excluding significant necrosis was 85% with a cutoff of 0.36. None of these serum tests have become part of standard of practice for diagnosing fibrosis or cirrhosis.
The Sequential Algorithm for Fibrosis Evaluation (SAFE) combines the APRI and Fibrotest-Fibrosure tests in a sequential fashion to test for fibrosis and cirrhosis. In a large multicenter study31 validating this algorithm to detect significant fibrosis (stage F2 or greater by the F0–F4 METAVIR scoring system32), its accuracy was 90.1%, the area under the receiver operating characteristic curve was 0.89 (95% CI 0.87–0.90), and it reduced the number of liver biopsies needed by 46.5%. When the algorithm was used to detect cirrhosis, its accuracy was 92.5%, the area under the curve was 0.92 (95% CI 0.89–0.94), and it reduced the number of liver biopsies needed by 81.5%.
Another algorithm was developed to simultaneously detect significant fibrosis and cirrhosis. It had a 97.4% accuracy, but 64% of patients still required a liver biopsy.31
SAFE algorithms have the potential to reduce the number of needle biopsies needed to assess the degree of hepatic fibrosis.
CONVENTIONAL IMAGING STUDIES ARE NOT SENSITIVE FOR FIBROSIS
Standard imaging studies often show findings of cirrhosis but are not particularly sensitive, with a low negative predictive value.
Ultrasonography can show a small, nodular liver in advanced cirrhosis, but surface nodularity or increased echogenicity can be seen in hepatic steatosis as well as in cirrhosis. In one study,33 ultrasonography identified diffuse parenchymal disease but could not reliably distinguish fat from fibrosis or diagnose cirrhosis.
Often, in cirrhosis, the right lobe of the liver is atrophied and the caudate or left lobes are hypertrophied. Efforts to use the ratio of the widths of the lobes to diagnose cirrhosis have shown varying performance characterstics.34,35
One study of the splenic artery pulsatility index has shown this to be an accurate predictor of cirrhosis.36
Computed tomography provides information similar to that of ultrasonography, and it can identify complications of cirrhosis, including portal hypertension and ascites. On the other hand, it costs more and it exposes the patient to radiation and contrast media.
ELASTOGRAPHY, A PROMISING TEST
Hepatic elastography, a method for estimating liver stiffness, is an exciting recent development in the noninvasive measurement of hepatic fibrosis. Currently, elastography can be accomplished by ultrasound or magnetic resonance.
Ultrasound elastography
The FibroScan device (EchoSens, Paris, France) uses a mild-amplitude, low-frequency (50-Hz) vibration transmitted through the liver.37 It induces an elastic shear wave that is detected by pulse-echo ultrasonography as the wave propagates through the organ.
The velocity of the wave correlates with tissue stiffness: the wave travels faster through denser, fibrotic tissue.38,39
Ultrasound elastography (also called transient elastography) can sample a much larger area than liver biopsy can, providing a better understanding of the entire hepatic parenchyma. 40 Moreover, it can be repeated often without risk. This device is in widespread use in many parts of the world, but it is not yet approved in the United States.
A meta-analysis of 50 studies assessed the overall performance of ultrasound elastography for diagnosing liver fibrosis.41 The areas under the receiver operating characteristic curve were as follows:
- For significant fibrosis: 0.84 (95% CI 0.82–0.86)
- For severe fibrosis: 0.89 (95% CI 0.88–0.91)
- For cirrhosis: 0.94 (95% CI 0.93–0.95).
The type of underlying liver disease influenced the diagnosis of significant fibrosis, which was diagnosed most consistently in patients with hepatitis C. The authors concluded that ultrasound elastography had excellent diagnostic accuracy for diagnosing cirrhosis irrespective of the underlying liver disease, while the diagnosis of significant fibrosis had higher variation, which was dependent on the underlying liver disease.
A meta-analysis of nine studies42 showed ultrasound elastography to have a sensitivity of 87% (95% CI 84%–90%) and a specificity of 91% (95% CI 89%–92%) for the diagnosis of cirrhosis. In seven of the nine studies, it diagnosed stage II to IV fibrosis with 70% sensitivity (95% CI 67%–73%) and 84% specificity (95% CI 80%–88%).
Limitations. Ultrasound elastography is less effective in obese patients, as the adipose tissue attenuates the elastic wave, and it has not been reliable in patients with acute viral hepatitis.43 Male sex, body mass index greater than 30, and metabolic syndrome seem to increase liver stiffness, thus limiting the use of this test.44
Until more data are available, the ultimate value of ultrasound elastography in reducing the number of liver biopsies needed remains unknown. However, this test shows potential as a reliable and noninvasive way to assess the degree of fibrosis in patients with liver disease.
Magnetic resonance elastography
Studies have shown a magnetic resonance scoring system that distinguishes Child-Pugh grade A cirrhosis from other grades to be 93% sensitive and 82% specific.45
Cost may limit the use of magnetic resonance elastography, and some patients may be unable to tolerate the procedure because of claustrophobia. It seems clear, though, that this test currently has the most promise in reducing the need for liver biopsy for grading the severity of hepatic fibrosis.
WHERE ARE WE NOW?
The importance of liver biopsy in arriving at a diagnosis of diffuse parenchymal liver disease is being diminished by accurate blood testing strategies for chronic viral hepatitis, autoimmune hepatitis, and primary biliary cirrhosis. Further, imaging tests are superior to liver biopsy in the diagnosis of primary sclerosing cholangitis.
However, many cases remain in which diagnostic confusion exists even after suitable laboratory testing and imaging studies. Diagnosing infiltrative disease (eg, amyloidosis, sarcoidosis), separating benign fatty liver disease from steatohepatitis, and evaluating liver parenchyma after liver transplantation are best accomplished by liver biopsy.
While needle biopsy is still the mainstay in diagnosing hepatic fibrosis, its days of dominance seem limited as technology improves. When physical examination or standard laboratory tests reveal clear-cut signs of portal hypertension, liver biopsy will seldom add useful information. Similarly, when imaging studies provide compelling evidence of cirrhosis and portal hypertension, needle biopsy is not warranted.
The SAFE algorithms warrant further evaluation in all chronic liver diseases, as they may help decrease the number of liver biopsies required. And we believe elastography will play an ever-increasing role in the assessment of hepatic fibrosis and will significantly reduce the need for biopsy in patients with liver disease.
- Verma S, Gunuwan B, Mendler M, Govindrajan S, Redeker A. Factors predicting relapse and poor outcome in type I autoimmune hepatitis: role of cirrhosis development, patterns of transaminases during remission and plasma cell activity in the liver biopsy. Am J Gastroenterol 2004; 99:1510–1516.
- Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology 2009; 49:1017–1044.
- Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001; 344:495–500.
- Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002; 97:2614–2618.
- Ratziu V, Charlotte F, Heurtier A, et al; LIDO Study Group Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005; 128:1898–1906.
- Saadeh S, Cammell G, Carey WD, Younossi Z, Barnes D, Easley K. The role of liver biopsy in chronic hepatitis C. Hepatology 2001; 33:196–200.
- Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol 1995; 19:1409–1417.
- Morrison ED, Brandhagen DJ, Phatak PD, et al. Serum ferritin level predicts advanced hepatic fibrosis among U.S. patients with phenotypic hemochromatosis. Ann Intern Med 2003; 138:627–633.
- Parkes J, Guha IN, Roderick P, Rosenberg W. Performance of serum marker panels for liver fibrosis in chronic hepatitis C. J Hepatol 2006; 44:462–474.
- Montalto G, Soresi M, Aragona F, et al. Procollagen III and laminin in chronic viral hepatopathies. Presse Med 1996; 25:59–62.
- Teare JP, Sherman D, Greenfield SM, et al. Comparison of serum procollagen III peptide concentrations and PGA index for assessment of hepatic fibrosis. Lancet 1993; 342:895–898.
- Trinchet JC, Hartmann DJ, Pateron D, et al. Serum type I collagen and N-terminal peptide of type III procollagen in chronic hepatitis. Relationship to liver histology and conventional liver tests. J Hepatol 1991; 12:139–144.
- McCullough AJ, Stassen WN, Wiesner RH, Czaja AJ. Serial determinations of the amino-terminal peptide of type III procollagen in severe chronic active hepatitis. J Lab Clin Med 1987; 109:55–61.
- Takahara T, Furui K, Funaki J, et al. Increased expression of matrix metalloproteinase-II in experimental liver fibrosis in rats. Hepatology 1995; 21:787–795.
- Takahara T, Furui K, Yata Y, et al. Dual expression of matrix metalloproteinase-2 and membrane-type 1-matrix metalloproteinase in fibrotic human livers. Hepatology 1997; 26:1521–1529.
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38:518–526.
- Kelleher TB, Mehta SH, Bhaskar R, et al. Prediction of hepatic fibrosis in HIV/HCV co-infected patients using serum fibrosis markers: the SHASTA index. J Hepatol 2005; 43:78–84.
- Islam S, Antonsson L, Westin J, Lagging M. Cirrhosis in hepatitis C virus-infected patients can be excluded using an index of standard biochemical serum markers. Scand J Gastroenterol 2005; 40:867–872.
- Lackner C, Struber G, Liegl B, et al. Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis C. Hepatology 2005; 41:1376–1382.
- Poynard T, Aubert A, Bedossa P, et al. A simple biological index for detection of alcoholic liver disease in drinkers. Gastroenterology 1991; 100:1397–1402.
- Oberti F, Valsesia E, Pilette C, et al. Noninvasive diagnosis of hepatic fibrosis or cirrhosis. Gastroenterology 1997; 113:1609–1616.
- Koda M, Matunaga Y, Kawakami M, Kishimoto Y, Suou T, Murawaki Y. FibroIndex, a practical index for predicting significant fibrosis in patients with chronic hepatitis C. Hepatology 2007; 45:297–306.
- Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 2007; 46:32–36.
- Sterling RK, Lissen E, Clumeck N, et al; APRI COT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43:1317–1325.
- Calès P, Oberti F, Michalak S, et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology 2005; 42:1373–1381.
- Leroy V, Hilleret MN, Sturm N, et al. Prospective comparison of six non-invasive scores for the diagnosis of liver fibrosis in chronic hepatitis C. J Hepatol 2007; 46:775–782.
- Myers RP, De Torres M, Imbert-Bismut F, Ratziu V, Charlotte F, Poynard T; MULTIVIRC Group. Biochemical markers of fibrosis in patients with chronic hepatitis C: a comparison with prothrombin time, platelet count, and age-platelet index. Dig Dis Sci 2003; 48:146–153.
- Rossi E, Adams L, Prins A, et al. Validation of the FibroTest biochemical markers score in assessing liver fibrosis in hepatitis C patients. Clin Chem 2003; 49:450–454.
- Halfon P, Bourliere M, Deydier R, et al. Independent prospective multicenter validation of biochemical markers (fibrotest-actitest) for the prediction of liver fibrosis and activity in patients with chronic hepatitis C: the fibropaca study. Am J Gastroenterol 2006; 101:547–555.
- Poynard T, Imbert-Bismut F, Munteanu M, et al. Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C. Comp Hepatol 2004; 3:8.
- Sebastiani G, Halfon P, Castera L, et al. SAFE biopsy: a validated method for large-scale staging of liver fibrosis in chronic hepatitis C. Hepatology 2009; 49:1821–1827.
- The French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretations in patients with chronic hepatitis C. Hepatology 1994; 20:15–20.
- Sanford NL, Walsh P, Matis C, Baddeley H, Powell LW. Is ultrasonography useful in the assessment of diffuse parenchymal liver disease? Gastroenterology 1985; 89:186–191.
- Harbin WP, Robert NJ, Ferrucci JT. Diagnosis of cirrhosis based on regional changes in hepatic morphology: a radiological and pathological analysis. Radiology 1980; 135:273–283.
- Giorgio A, Amoroso P, Lettieri G, et al. Cirrhosis: value of caudate to right lobe ratio in diagnosis with US. Radiology 1986; 161:443–445.
- Liu CH, Hsu SJ, Lin JW, et al. Noninvasive diagnosis of hepatic fibrosis in patients with chronic hepatitis C by splenic Doppler impedance index. Clin Gastroenterol Hepatol 2007; 5:1199–1206.
- Talawalkar JA. Elastography for detecting hepatic fibrosis: options and considerations. Gastroenterology 2008; 135:299–302.
- Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 2003; 29:1705–1713.
- Kettaneh A, Marcellin P, Douvin C, et al. Features associated with success rate and performance of FibroScan measurements for the diagnosis of cirrhosis in HCV patients: a prospective study of 935 patients. J Hepatol 2007; 46:628–634.
- Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005; 41:48–54.
- Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 2008; 134:960–974.
- Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2007; 5:1214–1220.
- Arena U, Vizzutti F, Corti G, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology 2008; 47:380–384.
- Roulot D, Czernichow S, Le Clésiau H, Costes JL, Vergnaud AC, Beaugrand M. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol 2008; 48:606–613.
- Ito K, Mitchell DG, Hann HW, et al. Viral-induced cirrhosis: grading of severity using MR imaging. AJR Am J Roentgenol 1999; 173:591–596.
- Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 2008; 135:32–40.
Primary care physicians and specialists alike often encounter patients with chronic liver disease. Fortunately, these days we need to resort to liver biopsy less often than in the past.
The purpose of this review is to provide a critical assessment of the growing number of noninvasive tests available for diagnosing liver disease and assessing hepatic fibrosis, and to discuss the implications of these advances related to the indications for needle liver biopsy.
WHEN IS LIVER BIOPSY USEFUL?
In diagnosis
Needle liver biopsy for diagnosis remains important in cases of:
Diagnostic uncertainty (eg, in patients with atypical features)
Coexisting disorders (eg, human immunodeficiency virus [HIV] and hepatitis C virus infection, or alcoholic liver disease and hepatitis C)
An overlapping syndrome (eg, primary biliary cirrhosis with autoimmune hepatitis).
Fatty liver. Needle liver biopsy can distinguish between benign steatosis and progressive steatohepatitis in a patient with a fatty liver found on imaging, subject to the limitations of sampling error.
Because fatty liver disease is common and proven treatments are few, no consensus has emerged about which patients with suspected fatty liver disease should undergo needle biopsy. Many specialists eschew needle biopsy and treat the underlying risk factors of metabolic syndrome, reserving biopsy for patients with findings that raise the concern of cirrhosis.
Hereditary disorders, eg, hemochromatosis, alpha-1 antitrypsin deficiency, and Wilson disease.
In management
Periodic needle biopsy is also valuable in the management of a few diseases.
In autoimmune hepatitis, monitoring the plasma cell score on liver biopsy may help predict relapse when a physician is considering reducing or discontinuing immunosuppressive therapy.1
After liver transplantation, a liver biopsy is highly valuable to assess for rejection and the presence and intensity of disease recurrence.
PROBLEMS WITH LIVER BIOPSY
Liver biopsy is invasive and can cause significant complications. Nearly 30% of patients report having substantial pain after liver biopsy, and some experience serious complications such as pneumothorax, bleeding, or puncture of the biliary tree. In rare cases, patients die of bleeding.2
Furthermore, hepatic pathology, particularly fibrosis, is not always uniformly distributed. Surgical wedge biopsy provides adequate tissue volume to overcome this problem. Needle biopsy, on the other hand, provides a much smaller volume of tissue (1/50,000 of the total mass of the liver).3
As examples of the resulting sampling errors that can occur, consider the two most common chronic liver diseases: hepatitis C and fatty liver disease.
Regev et al4 performed laparoscopically guided biopsy of the right and left hepatic lobes in a series of 124 patients with chronic hepatitis C. Biopsy samples from the right and left lobes differed in the intensity of inflammation in 24.2% of cases, and in the intensity of fibrosis in 33.1%. Differences of more than one grade of inflammation or stage of fibrosis were uncommon. However, in 14.5%, cirrhosis was diagnosed in one lobe but not the other.
In a study in patients with nonalcoholic fatty liver disease, Ratziu et al5 found that none of the features characteristic of nonalcoholic steatohepatitis were highly concordant in paired liver biopsies. Clearly, needle liver biopsy is far from an ideal test.
Increasingly, liver diseases can be diagnosed precisely with laboratory tests, imaging studies, or both. Thus, needle liver biopsy is playing a lesser role in diagnosis.
ADVANCES IN NONINVASIVE DIAGNOSIS OF LIVER DISEASE
Over the past 30 years, substantial strides have been made in our ability to make certain diagnoses through noninvasive means.
Blood tests can be used to diagnose viral hepatitis A, B, and C and many cases of hemochromatosis and primary biliary cirrhosis. For a detailed discussion of how blood tests are used in diagnosing liver diseases, see www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/hepatology/guide-to-common-liver-tests/.
Imaging studies. Primary sclerosing cholangitis can be diagnosed with an imaging study, ie, magnetic resonance cholangiopancreatography (MRCP) or endoscopic retrograde cholangiopancreatography (ERCP). The value of needle biopsy in these patients is limited to assessing the degree of fibrosis to help with management of the disease and, less often, to discovering other liver pathologies.6
Most benign space-occupying liver lesions, both cystic and solid, can be fully characterized by imaging, especially in patients who have no underlying chronic liver disease, and no biopsy is needed. Whether biopsy should be performed to investigate liver lesions depends on the clinical scenario; the topic is beyond the scope of this paper but has been reviewed in detail by Rockey et al.2
CAN NONINVASIVE TESTS DETECT HEPATIC FIBROSIS?
Cirrhosis (stage 4 fibrosis) results in nodular transformation of the liver and impedance of portal blood flow, setting the stage for portal hypertension and its sequelae. Knowing whether cirrhosis is present is important in subsequent management.
In advanced cases, cirrhosis is associated with typical clinical manifestations and laboratory and radiographic findings. In such cases, needle biopsy will add little. However, in most cases, particularly early in the course, clinical, laboratory, and radiologic correlates of cirrhosis are absent. In one study of patients with hepatitis C, 27% had cirrhosis, but in only a small number would cirrhosis have been apparent from clinical signs and laboratory and imaging studies.6
Since a major contemporary role for liver biopsy is in assessing the degree of fibrosis, it is reasonable to ask if newer noninvasive means are available to estimate hepatic fibrosis. The remainder of this review focuses on assessing our increasing ability to stage the degree of fibrosis (including the presence or absence of cirrhosis) by noninvasive means.
Clinical features point to cirrhosis, but not earlier fibrosis
Clinical manifestations help point to the diagnosis of cirrhosis but not to earlier stages of fibrosis.
For example, if a patient is known to have liver disease, the findings of ascites, splenomegaly, or asterixis mean that cirrhosis is highly probable. Similarly, hypersplenism (splenomegaly with a decrease in circulating blood cells but a normal to hyperactive bone marrow) in a patient with liver test abnormalities almost always represents portal hypertension due to cirrhosis, although other, nonhepatic causes are possible, such as congestive heart failure and constrictive pericarditis.
These features generally emerge late in the course of cirrhosis. The absence of such stigmata certainly does not preclude the presence of cirrhosis. Thus, these clinical signs have a high positive predictive value but a low negative predictive value, making them insufficient by themselves to diagnose or stage liver disease.
Laboratory tests are of limited value in assessing the degree of fibrosis
Standard liver tests are of limited value in assessing the degree of fibrosis.
Usual laboratory tests. At one end of the spectrum, anemia, thrombocytopenia, and leukopenia in the presence of liver disease correlate with cirrhosis. At the other end, a serum ferritin concentration of less than 1,000 mg/mL in a patient with hemochromatosis and no confounding features such as hepatitis C, HIV infection, or heavy alcohol use strongly predicts that the patient does not have significant hepatic fibrosis.8
Bilirubin elevation is a late finding in cirrhosis, but in cholestatic diseases bilirubin may be elevated before cirrhosis occurs.
Albumin is made exclusively in the liver, and its concentration falls as liver function worsens with progressive cirrhosis.
The prothrombin time increases as the liver loses its ability to synthesize clotting factors in cirrhosis. Coagulopathy correlates with the degree of liver disease.
Hyponatremia due to impaired ability to excrete free water is seen in patients with cirrhosis and ascites.
In summary, the usual laboratory tests related to liver disease are imprecise and, when abnormal, often indicate not just the presence of cirrhosis, but impending or actual decompensation.
Newer serologic markers, alone or in combination, have been proposed as aids in determining the degree of fibrosis or cirrhosis in the liver. Direct markers of fibrosis measure the turnover or metabolism of extracellular matrix. Indirect markers of fibrosis reflect alterations in hepatic function (see below).
Parkes et al9 reviewed 10 different panels of serum markers of hepatic fibrosis in chronic hepatitis C. Only 35% of patients had fibrosis adequately ruled in or ruled out by these panels, and the stage of fibrosis could not be adequately determined.
These serologic markers have not been validated in other chronic liver diseases or in liver disease due to multiple causes. Thus, although they show promise for use by the general internist, they need to be validated in patients with disease and in normal reference populations before they are ready for “prime time.”
Direct serologic markers of fibrosis
Direct serologic markers of fibrosis include those associated with matrix deposition—eg, procollagen type III amino-terminal peptide (P3NP), type I and IV collagens, laminin, hyaluronic acid, and chondrex.
P3NP is the most widely studied marker of hepatic fibrosis. It is elevated in both acute and chronic liver diseases; serum levels reflect the histologic stage of hepatic fibrosis in various chronic liver diseases, including alcoholic, viral, and primary biliary cirrhosis.10–12 Successful treatment of autoimmune hepatitis has been shown to lead to reductions of P3NP levels.13
Other direct markers of fibrosis are those associated with matrix degradation, ie, matrix metalloproteinases 2 and 3 (MMP-2, MMP-3) and tissue inhibitors of metalloproteinases 1 and 2 (TIMP-1, TIMP-2). Levels of MMP-2 proenzymes and active enzymes are increased in liver disease, but studies are inconsistent in correlating serum levels of MMP-2 to the degree of hepatic fibrosis.14,15 These tests are not commercially available, and the components are not readily available in most clinical laboratories.
Indirect serologic markers of fibrosis
Some indirect markers are readily available:
The AST:ALT ratio. The normal ratio of aspartate aminotransferase (AST) to alanine aminotransferase (ALT) is approximately 0.8. A ratio greater than 1.0 provides evidence of cirrhosis. However, findings have been inconsistent.
The AST:platelet ratio index (APRI), a commonly used index, is calculated by the following formula:
In studies of hepatitis C and hepatitis C-HIV, the APRI has shown a sensitivity of 37% to 80% and a specificity of 45% to 98%, depending on the cutoff value and whether a diagnosis of severe fibrosis or cirrhosis was being tested.16–19 These sensitivities and specificities are disappointing and do not provide information equal to that provided by needle liver biopsy in most patients with chronic liver disease.
The combination of prothrombin, gamma glutamyl, and apolipoprotein AI levels (PGA index) has been validated in patients with many types of chronic liver disease, and its accuracy for detecting cirrhosis is highest (66%–72%) in patients with alcoholic liver disease.20,21
FibroIndex uses the platelet count, AST level, and gamma globulin level to detect significant fibrosis in chronic hepatitis C, but its accuracy has yet to be validated.22
The FIB-4 index is based on four independent predictors of fibrosis, ie, age, the platelet count, AST level, and ALT level. It has shown good accuracy for detecting advanced fibrosis in two studies in patients with hepatitis C.23,24
Fibrometer (based on the platelet count; the prothrombin index; the levels of AST, alfa-2 macroglobulin, hyaluronate, and blood urea nitrogen; and age) predicted fibrosis well in chronic viral hepatitis.25,26
Fibrotest and Fibrosure are proprietary commercial tests available in many laboratories. They employ a mathematical formula to predict fibrosis (characterized as mild, significant, or indeterminate) using the levels of alpha-2 macroglobulin, alpha-2 globulin, gamma globulin, apolipoprotein A1, gamma glutamyl transferase, and total bilirubin. For detecting significant fibrosis, these tests are reported to have a sensitivity of about 75% and a specificity of 85%.27–29
ActiTest incorporates the ALT level into the Fibrotest to reflect liver fibrosis and necro-inflammatory activity.
A meta-analysis showed that Fibrotest and ActiTest could be reliable alternatives to liver biopsy in patients with chronic hepatitis C.30 The area under the receiver operator characteristic curve for the diagnosis of significant fibrosis ranged from 0.73 to 0.87; for the diagnosis of significant histologic activity it ranged from 0.75 to 0.86. Fibrotest had a negative predictive value for excluding significant fibrosis of 91% with a cutoff of 0.31. ActiTest’s negative predictive value for excluding significant necrosis was 85% with a cutoff of 0.36. None of these serum tests have become part of standard of practice for diagnosing fibrosis or cirrhosis.
The Sequential Algorithm for Fibrosis Evaluation (SAFE) combines the APRI and Fibrotest-Fibrosure tests in a sequential fashion to test for fibrosis and cirrhosis. In a large multicenter study31 validating this algorithm to detect significant fibrosis (stage F2 or greater by the F0–F4 METAVIR scoring system32), its accuracy was 90.1%, the area under the receiver operating characteristic curve was 0.89 (95% CI 0.87–0.90), and it reduced the number of liver biopsies needed by 46.5%. When the algorithm was used to detect cirrhosis, its accuracy was 92.5%, the area under the curve was 0.92 (95% CI 0.89–0.94), and it reduced the number of liver biopsies needed by 81.5%.
Another algorithm was developed to simultaneously detect significant fibrosis and cirrhosis. It had a 97.4% accuracy, but 64% of patients still required a liver biopsy.31
SAFE algorithms have the potential to reduce the number of needle biopsies needed to assess the degree of hepatic fibrosis.
CONVENTIONAL IMAGING STUDIES ARE NOT SENSITIVE FOR FIBROSIS
Standard imaging studies often show findings of cirrhosis but are not particularly sensitive, with a low negative predictive value.
Ultrasonography can show a small, nodular liver in advanced cirrhosis, but surface nodularity or increased echogenicity can be seen in hepatic steatosis as well as in cirrhosis. In one study,33 ultrasonography identified diffuse parenchymal disease but could not reliably distinguish fat from fibrosis or diagnose cirrhosis.
Often, in cirrhosis, the right lobe of the liver is atrophied and the caudate or left lobes are hypertrophied. Efforts to use the ratio of the widths of the lobes to diagnose cirrhosis have shown varying performance characterstics.34,35
One study of the splenic artery pulsatility index has shown this to be an accurate predictor of cirrhosis.36
Computed tomography provides information similar to that of ultrasonography, and it can identify complications of cirrhosis, including portal hypertension and ascites. On the other hand, it costs more and it exposes the patient to radiation and contrast media.
ELASTOGRAPHY, A PROMISING TEST
Hepatic elastography, a method for estimating liver stiffness, is an exciting recent development in the noninvasive measurement of hepatic fibrosis. Currently, elastography can be accomplished by ultrasound or magnetic resonance.
Ultrasound elastography
The FibroScan device (EchoSens, Paris, France) uses a mild-amplitude, low-frequency (50-Hz) vibration transmitted through the liver.37 It induces an elastic shear wave that is detected by pulse-echo ultrasonography as the wave propagates through the organ.
The velocity of the wave correlates with tissue stiffness: the wave travels faster through denser, fibrotic tissue.38,39
Ultrasound elastography (also called transient elastography) can sample a much larger area than liver biopsy can, providing a better understanding of the entire hepatic parenchyma. 40 Moreover, it can be repeated often without risk. This device is in widespread use in many parts of the world, but it is not yet approved in the United States.
A meta-analysis of 50 studies assessed the overall performance of ultrasound elastography for diagnosing liver fibrosis.41 The areas under the receiver operating characteristic curve were as follows:
- For significant fibrosis: 0.84 (95% CI 0.82–0.86)
- For severe fibrosis: 0.89 (95% CI 0.88–0.91)
- For cirrhosis: 0.94 (95% CI 0.93–0.95).
The type of underlying liver disease influenced the diagnosis of significant fibrosis, which was diagnosed most consistently in patients with hepatitis C. The authors concluded that ultrasound elastography had excellent diagnostic accuracy for diagnosing cirrhosis irrespective of the underlying liver disease, while the diagnosis of significant fibrosis had higher variation, which was dependent on the underlying liver disease.
A meta-analysis of nine studies42 showed ultrasound elastography to have a sensitivity of 87% (95% CI 84%–90%) and a specificity of 91% (95% CI 89%–92%) for the diagnosis of cirrhosis. In seven of the nine studies, it diagnosed stage II to IV fibrosis with 70% sensitivity (95% CI 67%–73%) and 84% specificity (95% CI 80%–88%).
Limitations. Ultrasound elastography is less effective in obese patients, as the adipose tissue attenuates the elastic wave, and it has not been reliable in patients with acute viral hepatitis.43 Male sex, body mass index greater than 30, and metabolic syndrome seem to increase liver stiffness, thus limiting the use of this test.44
Until more data are available, the ultimate value of ultrasound elastography in reducing the number of liver biopsies needed remains unknown. However, this test shows potential as a reliable and noninvasive way to assess the degree of fibrosis in patients with liver disease.
Magnetic resonance elastography
Studies have shown a magnetic resonance scoring system that distinguishes Child-Pugh grade A cirrhosis from other grades to be 93% sensitive and 82% specific.45
Cost may limit the use of magnetic resonance elastography, and some patients may be unable to tolerate the procedure because of claustrophobia. It seems clear, though, that this test currently has the most promise in reducing the need for liver biopsy for grading the severity of hepatic fibrosis.
WHERE ARE WE NOW?
The importance of liver biopsy in arriving at a diagnosis of diffuse parenchymal liver disease is being diminished by accurate blood testing strategies for chronic viral hepatitis, autoimmune hepatitis, and primary biliary cirrhosis. Further, imaging tests are superior to liver biopsy in the diagnosis of primary sclerosing cholangitis.
However, many cases remain in which diagnostic confusion exists even after suitable laboratory testing and imaging studies. Diagnosing infiltrative disease (eg, amyloidosis, sarcoidosis), separating benign fatty liver disease from steatohepatitis, and evaluating liver parenchyma after liver transplantation are best accomplished by liver biopsy.
While needle biopsy is still the mainstay in diagnosing hepatic fibrosis, its days of dominance seem limited as technology improves. When physical examination or standard laboratory tests reveal clear-cut signs of portal hypertension, liver biopsy will seldom add useful information. Similarly, when imaging studies provide compelling evidence of cirrhosis and portal hypertension, needle biopsy is not warranted.
The SAFE algorithms warrant further evaluation in all chronic liver diseases, as they may help decrease the number of liver biopsies required. And we believe elastography will play an ever-increasing role in the assessment of hepatic fibrosis and will significantly reduce the need for biopsy in patients with liver disease.
Primary care physicians and specialists alike often encounter patients with chronic liver disease. Fortunately, these days we need to resort to liver biopsy less often than in the past.
The purpose of this review is to provide a critical assessment of the growing number of noninvasive tests available for diagnosing liver disease and assessing hepatic fibrosis, and to discuss the implications of these advances related to the indications for needle liver biopsy.
WHEN IS LIVER BIOPSY USEFUL?
In diagnosis
Needle liver biopsy for diagnosis remains important in cases of:
Diagnostic uncertainty (eg, in patients with atypical features)
Coexisting disorders (eg, human immunodeficiency virus [HIV] and hepatitis C virus infection, or alcoholic liver disease and hepatitis C)
An overlapping syndrome (eg, primary biliary cirrhosis with autoimmune hepatitis).
Fatty liver. Needle liver biopsy can distinguish between benign steatosis and progressive steatohepatitis in a patient with a fatty liver found on imaging, subject to the limitations of sampling error.
Because fatty liver disease is common and proven treatments are few, no consensus has emerged about which patients with suspected fatty liver disease should undergo needle biopsy. Many specialists eschew needle biopsy and treat the underlying risk factors of metabolic syndrome, reserving biopsy for patients with findings that raise the concern of cirrhosis.
Hereditary disorders, eg, hemochromatosis, alpha-1 antitrypsin deficiency, and Wilson disease.
In management
Periodic needle biopsy is also valuable in the management of a few diseases.
In autoimmune hepatitis, monitoring the plasma cell score on liver biopsy may help predict relapse when a physician is considering reducing or discontinuing immunosuppressive therapy.1
After liver transplantation, a liver biopsy is highly valuable to assess for rejection and the presence and intensity of disease recurrence.
PROBLEMS WITH LIVER BIOPSY
Liver biopsy is invasive and can cause significant complications. Nearly 30% of patients report having substantial pain after liver biopsy, and some experience serious complications such as pneumothorax, bleeding, or puncture of the biliary tree. In rare cases, patients die of bleeding.2
Furthermore, hepatic pathology, particularly fibrosis, is not always uniformly distributed. Surgical wedge biopsy provides adequate tissue volume to overcome this problem. Needle biopsy, on the other hand, provides a much smaller volume of tissue (1/50,000 of the total mass of the liver).3
As examples of the resulting sampling errors that can occur, consider the two most common chronic liver diseases: hepatitis C and fatty liver disease.
Regev et al4 performed laparoscopically guided biopsy of the right and left hepatic lobes in a series of 124 patients with chronic hepatitis C. Biopsy samples from the right and left lobes differed in the intensity of inflammation in 24.2% of cases, and in the intensity of fibrosis in 33.1%. Differences of more than one grade of inflammation or stage of fibrosis were uncommon. However, in 14.5%, cirrhosis was diagnosed in one lobe but not the other.
In a study in patients with nonalcoholic fatty liver disease, Ratziu et al5 found that none of the features characteristic of nonalcoholic steatohepatitis were highly concordant in paired liver biopsies. Clearly, needle liver biopsy is far from an ideal test.
Increasingly, liver diseases can be diagnosed precisely with laboratory tests, imaging studies, or both. Thus, needle liver biopsy is playing a lesser role in diagnosis.
ADVANCES IN NONINVASIVE DIAGNOSIS OF LIVER DISEASE
Over the past 30 years, substantial strides have been made in our ability to make certain diagnoses through noninvasive means.
Blood tests can be used to diagnose viral hepatitis A, B, and C and many cases of hemochromatosis and primary biliary cirrhosis. For a detailed discussion of how blood tests are used in diagnosing liver diseases, see www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/hepatology/guide-to-common-liver-tests/.
Imaging studies. Primary sclerosing cholangitis can be diagnosed with an imaging study, ie, magnetic resonance cholangiopancreatography (MRCP) or endoscopic retrograde cholangiopancreatography (ERCP). The value of needle biopsy in these patients is limited to assessing the degree of fibrosis to help with management of the disease and, less often, to discovering other liver pathologies.6
Most benign space-occupying liver lesions, both cystic and solid, can be fully characterized by imaging, especially in patients who have no underlying chronic liver disease, and no biopsy is needed. Whether biopsy should be performed to investigate liver lesions depends on the clinical scenario; the topic is beyond the scope of this paper but has been reviewed in detail by Rockey et al.2
CAN NONINVASIVE TESTS DETECT HEPATIC FIBROSIS?
Cirrhosis (stage 4 fibrosis) results in nodular transformation of the liver and impedance of portal blood flow, setting the stage for portal hypertension and its sequelae. Knowing whether cirrhosis is present is important in subsequent management.
In advanced cases, cirrhosis is associated with typical clinical manifestations and laboratory and radiographic findings. In such cases, needle biopsy will add little. However, in most cases, particularly early in the course, clinical, laboratory, and radiologic correlates of cirrhosis are absent. In one study of patients with hepatitis C, 27% had cirrhosis, but in only a small number would cirrhosis have been apparent from clinical signs and laboratory and imaging studies.6
Since a major contemporary role for liver biopsy is in assessing the degree of fibrosis, it is reasonable to ask if newer noninvasive means are available to estimate hepatic fibrosis. The remainder of this review focuses on assessing our increasing ability to stage the degree of fibrosis (including the presence or absence of cirrhosis) by noninvasive means.
Clinical features point to cirrhosis, but not earlier fibrosis
Clinical manifestations help point to the diagnosis of cirrhosis but not to earlier stages of fibrosis.
For example, if a patient is known to have liver disease, the findings of ascites, splenomegaly, or asterixis mean that cirrhosis is highly probable. Similarly, hypersplenism (splenomegaly with a decrease in circulating blood cells but a normal to hyperactive bone marrow) in a patient with liver test abnormalities almost always represents portal hypertension due to cirrhosis, although other, nonhepatic causes are possible, such as congestive heart failure and constrictive pericarditis.
These features generally emerge late in the course of cirrhosis. The absence of such stigmata certainly does not preclude the presence of cirrhosis. Thus, these clinical signs have a high positive predictive value but a low negative predictive value, making them insufficient by themselves to diagnose or stage liver disease.
Laboratory tests are of limited value in assessing the degree of fibrosis
Standard liver tests are of limited value in assessing the degree of fibrosis.
Usual laboratory tests. At one end of the spectrum, anemia, thrombocytopenia, and leukopenia in the presence of liver disease correlate with cirrhosis. At the other end, a serum ferritin concentration of less than 1,000 mg/mL in a patient with hemochromatosis and no confounding features such as hepatitis C, HIV infection, or heavy alcohol use strongly predicts that the patient does not have significant hepatic fibrosis.8
Bilirubin elevation is a late finding in cirrhosis, but in cholestatic diseases bilirubin may be elevated before cirrhosis occurs.
Albumin is made exclusively in the liver, and its concentration falls as liver function worsens with progressive cirrhosis.
The prothrombin time increases as the liver loses its ability to synthesize clotting factors in cirrhosis. Coagulopathy correlates with the degree of liver disease.
Hyponatremia due to impaired ability to excrete free water is seen in patients with cirrhosis and ascites.
In summary, the usual laboratory tests related to liver disease are imprecise and, when abnormal, often indicate not just the presence of cirrhosis, but impending or actual decompensation.
Newer serologic markers, alone or in combination, have been proposed as aids in determining the degree of fibrosis or cirrhosis in the liver. Direct markers of fibrosis measure the turnover or metabolism of extracellular matrix. Indirect markers of fibrosis reflect alterations in hepatic function (see below).
Parkes et al9 reviewed 10 different panels of serum markers of hepatic fibrosis in chronic hepatitis C. Only 35% of patients had fibrosis adequately ruled in or ruled out by these panels, and the stage of fibrosis could not be adequately determined.
These serologic markers have not been validated in other chronic liver diseases or in liver disease due to multiple causes. Thus, although they show promise for use by the general internist, they need to be validated in patients with disease and in normal reference populations before they are ready for “prime time.”
Direct serologic markers of fibrosis
Direct serologic markers of fibrosis include those associated with matrix deposition—eg, procollagen type III amino-terminal peptide (P3NP), type I and IV collagens, laminin, hyaluronic acid, and chondrex.
P3NP is the most widely studied marker of hepatic fibrosis. It is elevated in both acute and chronic liver diseases; serum levels reflect the histologic stage of hepatic fibrosis in various chronic liver diseases, including alcoholic, viral, and primary biliary cirrhosis.10–12 Successful treatment of autoimmune hepatitis has been shown to lead to reductions of P3NP levels.13
Other direct markers of fibrosis are those associated with matrix degradation, ie, matrix metalloproteinases 2 and 3 (MMP-2, MMP-3) and tissue inhibitors of metalloproteinases 1 and 2 (TIMP-1, TIMP-2). Levels of MMP-2 proenzymes and active enzymes are increased in liver disease, but studies are inconsistent in correlating serum levels of MMP-2 to the degree of hepatic fibrosis.14,15 These tests are not commercially available, and the components are not readily available in most clinical laboratories.
Indirect serologic markers of fibrosis
Some indirect markers are readily available:
The AST:ALT ratio. The normal ratio of aspartate aminotransferase (AST) to alanine aminotransferase (ALT) is approximately 0.8. A ratio greater than 1.0 provides evidence of cirrhosis. However, findings have been inconsistent.
The AST:platelet ratio index (APRI), a commonly used index, is calculated by the following formula:
In studies of hepatitis C and hepatitis C-HIV, the APRI has shown a sensitivity of 37% to 80% and a specificity of 45% to 98%, depending on the cutoff value and whether a diagnosis of severe fibrosis or cirrhosis was being tested.16–19 These sensitivities and specificities are disappointing and do not provide information equal to that provided by needle liver biopsy in most patients with chronic liver disease.
The combination of prothrombin, gamma glutamyl, and apolipoprotein AI levels (PGA index) has been validated in patients with many types of chronic liver disease, and its accuracy for detecting cirrhosis is highest (66%–72%) in patients with alcoholic liver disease.20,21
FibroIndex uses the platelet count, AST level, and gamma globulin level to detect significant fibrosis in chronic hepatitis C, but its accuracy has yet to be validated.22
The FIB-4 index is based on four independent predictors of fibrosis, ie, age, the platelet count, AST level, and ALT level. It has shown good accuracy for detecting advanced fibrosis in two studies in patients with hepatitis C.23,24
Fibrometer (based on the platelet count; the prothrombin index; the levels of AST, alfa-2 macroglobulin, hyaluronate, and blood urea nitrogen; and age) predicted fibrosis well in chronic viral hepatitis.25,26
Fibrotest and Fibrosure are proprietary commercial tests available in many laboratories. They employ a mathematical formula to predict fibrosis (characterized as mild, significant, or indeterminate) using the levels of alpha-2 macroglobulin, alpha-2 globulin, gamma globulin, apolipoprotein A1, gamma glutamyl transferase, and total bilirubin. For detecting significant fibrosis, these tests are reported to have a sensitivity of about 75% and a specificity of 85%.27–29
ActiTest incorporates the ALT level into the Fibrotest to reflect liver fibrosis and necro-inflammatory activity.
A meta-analysis showed that Fibrotest and ActiTest could be reliable alternatives to liver biopsy in patients with chronic hepatitis C.30 The area under the receiver operator characteristic curve for the diagnosis of significant fibrosis ranged from 0.73 to 0.87; for the diagnosis of significant histologic activity it ranged from 0.75 to 0.86. Fibrotest had a negative predictive value for excluding significant fibrosis of 91% with a cutoff of 0.31. ActiTest’s negative predictive value for excluding significant necrosis was 85% with a cutoff of 0.36. None of these serum tests have become part of standard of practice for diagnosing fibrosis or cirrhosis.
The Sequential Algorithm for Fibrosis Evaluation (SAFE) combines the APRI and Fibrotest-Fibrosure tests in a sequential fashion to test for fibrosis and cirrhosis. In a large multicenter study31 validating this algorithm to detect significant fibrosis (stage F2 or greater by the F0–F4 METAVIR scoring system32), its accuracy was 90.1%, the area under the receiver operating characteristic curve was 0.89 (95% CI 0.87–0.90), and it reduced the number of liver biopsies needed by 46.5%. When the algorithm was used to detect cirrhosis, its accuracy was 92.5%, the area under the curve was 0.92 (95% CI 0.89–0.94), and it reduced the number of liver biopsies needed by 81.5%.
Another algorithm was developed to simultaneously detect significant fibrosis and cirrhosis. It had a 97.4% accuracy, but 64% of patients still required a liver biopsy.31
SAFE algorithms have the potential to reduce the number of needle biopsies needed to assess the degree of hepatic fibrosis.
CONVENTIONAL IMAGING STUDIES ARE NOT SENSITIVE FOR FIBROSIS
Standard imaging studies often show findings of cirrhosis but are not particularly sensitive, with a low negative predictive value.
Ultrasonography can show a small, nodular liver in advanced cirrhosis, but surface nodularity or increased echogenicity can be seen in hepatic steatosis as well as in cirrhosis. In one study,33 ultrasonography identified diffuse parenchymal disease but could not reliably distinguish fat from fibrosis or diagnose cirrhosis.
Often, in cirrhosis, the right lobe of the liver is atrophied and the caudate or left lobes are hypertrophied. Efforts to use the ratio of the widths of the lobes to diagnose cirrhosis have shown varying performance characterstics.34,35
One study of the splenic artery pulsatility index has shown this to be an accurate predictor of cirrhosis.36
Computed tomography provides information similar to that of ultrasonography, and it can identify complications of cirrhosis, including portal hypertension and ascites. On the other hand, it costs more and it exposes the patient to radiation and contrast media.
ELASTOGRAPHY, A PROMISING TEST
Hepatic elastography, a method for estimating liver stiffness, is an exciting recent development in the noninvasive measurement of hepatic fibrosis. Currently, elastography can be accomplished by ultrasound or magnetic resonance.
Ultrasound elastography
The FibroScan device (EchoSens, Paris, France) uses a mild-amplitude, low-frequency (50-Hz) vibration transmitted through the liver.37 It induces an elastic shear wave that is detected by pulse-echo ultrasonography as the wave propagates through the organ.
The velocity of the wave correlates with tissue stiffness: the wave travels faster through denser, fibrotic tissue.38,39
Ultrasound elastography (also called transient elastography) can sample a much larger area than liver biopsy can, providing a better understanding of the entire hepatic parenchyma. 40 Moreover, it can be repeated often without risk. This device is in widespread use in many parts of the world, but it is not yet approved in the United States.
A meta-analysis of 50 studies assessed the overall performance of ultrasound elastography for diagnosing liver fibrosis.41 The areas under the receiver operating characteristic curve were as follows:
- For significant fibrosis: 0.84 (95% CI 0.82–0.86)
- For severe fibrosis: 0.89 (95% CI 0.88–0.91)
- For cirrhosis: 0.94 (95% CI 0.93–0.95).
The type of underlying liver disease influenced the diagnosis of significant fibrosis, which was diagnosed most consistently in patients with hepatitis C. The authors concluded that ultrasound elastography had excellent diagnostic accuracy for diagnosing cirrhosis irrespective of the underlying liver disease, while the diagnosis of significant fibrosis had higher variation, which was dependent on the underlying liver disease.
A meta-analysis of nine studies42 showed ultrasound elastography to have a sensitivity of 87% (95% CI 84%–90%) and a specificity of 91% (95% CI 89%–92%) for the diagnosis of cirrhosis. In seven of the nine studies, it diagnosed stage II to IV fibrosis with 70% sensitivity (95% CI 67%–73%) and 84% specificity (95% CI 80%–88%).
Limitations. Ultrasound elastography is less effective in obese patients, as the adipose tissue attenuates the elastic wave, and it has not been reliable in patients with acute viral hepatitis.43 Male sex, body mass index greater than 30, and metabolic syndrome seem to increase liver stiffness, thus limiting the use of this test.44
Until more data are available, the ultimate value of ultrasound elastography in reducing the number of liver biopsies needed remains unknown. However, this test shows potential as a reliable and noninvasive way to assess the degree of fibrosis in patients with liver disease.
Magnetic resonance elastography
Studies have shown a magnetic resonance scoring system that distinguishes Child-Pugh grade A cirrhosis from other grades to be 93% sensitive and 82% specific.45
Cost may limit the use of magnetic resonance elastography, and some patients may be unable to tolerate the procedure because of claustrophobia. It seems clear, though, that this test currently has the most promise in reducing the need for liver biopsy for grading the severity of hepatic fibrosis.
WHERE ARE WE NOW?
The importance of liver biopsy in arriving at a diagnosis of diffuse parenchymal liver disease is being diminished by accurate blood testing strategies for chronic viral hepatitis, autoimmune hepatitis, and primary biliary cirrhosis. Further, imaging tests are superior to liver biopsy in the diagnosis of primary sclerosing cholangitis.
However, many cases remain in which diagnostic confusion exists even after suitable laboratory testing and imaging studies. Diagnosing infiltrative disease (eg, amyloidosis, sarcoidosis), separating benign fatty liver disease from steatohepatitis, and evaluating liver parenchyma after liver transplantation are best accomplished by liver biopsy.
While needle biopsy is still the mainstay in diagnosing hepatic fibrosis, its days of dominance seem limited as technology improves. When physical examination or standard laboratory tests reveal clear-cut signs of portal hypertension, liver biopsy will seldom add useful information. Similarly, when imaging studies provide compelling evidence of cirrhosis and portal hypertension, needle biopsy is not warranted.
The SAFE algorithms warrant further evaluation in all chronic liver diseases, as they may help decrease the number of liver biopsies required. And we believe elastography will play an ever-increasing role in the assessment of hepatic fibrosis and will significantly reduce the need for biopsy in patients with liver disease.
- Verma S, Gunuwan B, Mendler M, Govindrajan S, Redeker A. Factors predicting relapse and poor outcome in type I autoimmune hepatitis: role of cirrhosis development, patterns of transaminases during remission and plasma cell activity in the liver biopsy. Am J Gastroenterol 2004; 99:1510–1516.
- Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology 2009; 49:1017–1044.
- Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001; 344:495–500.
- Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002; 97:2614–2618.
- Ratziu V, Charlotte F, Heurtier A, et al; LIDO Study Group Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005; 128:1898–1906.
- Saadeh S, Cammell G, Carey WD, Younossi Z, Barnes D, Easley K. The role of liver biopsy in chronic hepatitis C. Hepatology 2001; 33:196–200.
- Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol 1995; 19:1409–1417.
- Morrison ED, Brandhagen DJ, Phatak PD, et al. Serum ferritin level predicts advanced hepatic fibrosis among U.S. patients with phenotypic hemochromatosis. Ann Intern Med 2003; 138:627–633.
- Parkes J, Guha IN, Roderick P, Rosenberg W. Performance of serum marker panels for liver fibrosis in chronic hepatitis C. J Hepatol 2006; 44:462–474.
- Montalto G, Soresi M, Aragona F, et al. Procollagen III and laminin in chronic viral hepatopathies. Presse Med 1996; 25:59–62.
- Teare JP, Sherman D, Greenfield SM, et al. Comparison of serum procollagen III peptide concentrations and PGA index for assessment of hepatic fibrosis. Lancet 1993; 342:895–898.
- Trinchet JC, Hartmann DJ, Pateron D, et al. Serum type I collagen and N-terminal peptide of type III procollagen in chronic hepatitis. Relationship to liver histology and conventional liver tests. J Hepatol 1991; 12:139–144.
- McCullough AJ, Stassen WN, Wiesner RH, Czaja AJ. Serial determinations of the amino-terminal peptide of type III procollagen in severe chronic active hepatitis. J Lab Clin Med 1987; 109:55–61.
- Takahara T, Furui K, Funaki J, et al. Increased expression of matrix metalloproteinase-II in experimental liver fibrosis in rats. Hepatology 1995; 21:787–795.
- Takahara T, Furui K, Yata Y, et al. Dual expression of matrix metalloproteinase-2 and membrane-type 1-matrix metalloproteinase in fibrotic human livers. Hepatology 1997; 26:1521–1529.
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38:518–526.
- Kelleher TB, Mehta SH, Bhaskar R, et al. Prediction of hepatic fibrosis in HIV/HCV co-infected patients using serum fibrosis markers: the SHASTA index. J Hepatol 2005; 43:78–84.
- Islam S, Antonsson L, Westin J, Lagging M. Cirrhosis in hepatitis C virus-infected patients can be excluded using an index of standard biochemical serum markers. Scand J Gastroenterol 2005; 40:867–872.
- Lackner C, Struber G, Liegl B, et al. Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis C. Hepatology 2005; 41:1376–1382.
- Poynard T, Aubert A, Bedossa P, et al. A simple biological index for detection of alcoholic liver disease in drinkers. Gastroenterology 1991; 100:1397–1402.
- Oberti F, Valsesia E, Pilette C, et al. Noninvasive diagnosis of hepatic fibrosis or cirrhosis. Gastroenterology 1997; 113:1609–1616.
- Koda M, Matunaga Y, Kawakami M, Kishimoto Y, Suou T, Murawaki Y. FibroIndex, a practical index for predicting significant fibrosis in patients with chronic hepatitis C. Hepatology 2007; 45:297–306.
- Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 2007; 46:32–36.
- Sterling RK, Lissen E, Clumeck N, et al; APRI COT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43:1317–1325.
- Calès P, Oberti F, Michalak S, et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology 2005; 42:1373–1381.
- Leroy V, Hilleret MN, Sturm N, et al. Prospective comparison of six non-invasive scores for the diagnosis of liver fibrosis in chronic hepatitis C. J Hepatol 2007; 46:775–782.
- Myers RP, De Torres M, Imbert-Bismut F, Ratziu V, Charlotte F, Poynard T; MULTIVIRC Group. Biochemical markers of fibrosis in patients with chronic hepatitis C: a comparison with prothrombin time, platelet count, and age-platelet index. Dig Dis Sci 2003; 48:146–153.
- Rossi E, Adams L, Prins A, et al. Validation of the FibroTest biochemical markers score in assessing liver fibrosis in hepatitis C patients. Clin Chem 2003; 49:450–454.
- Halfon P, Bourliere M, Deydier R, et al. Independent prospective multicenter validation of biochemical markers (fibrotest-actitest) for the prediction of liver fibrosis and activity in patients with chronic hepatitis C: the fibropaca study. Am J Gastroenterol 2006; 101:547–555.
- Poynard T, Imbert-Bismut F, Munteanu M, et al. Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C. Comp Hepatol 2004; 3:8.
- Sebastiani G, Halfon P, Castera L, et al. SAFE biopsy: a validated method for large-scale staging of liver fibrosis in chronic hepatitis C. Hepatology 2009; 49:1821–1827.
- The French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretations in patients with chronic hepatitis C. Hepatology 1994; 20:15–20.
- Sanford NL, Walsh P, Matis C, Baddeley H, Powell LW. Is ultrasonography useful in the assessment of diffuse parenchymal liver disease? Gastroenterology 1985; 89:186–191.
- Harbin WP, Robert NJ, Ferrucci JT. Diagnosis of cirrhosis based on regional changes in hepatic morphology: a radiological and pathological analysis. Radiology 1980; 135:273–283.
- Giorgio A, Amoroso P, Lettieri G, et al. Cirrhosis: value of caudate to right lobe ratio in diagnosis with US. Radiology 1986; 161:443–445.
- Liu CH, Hsu SJ, Lin JW, et al. Noninvasive diagnosis of hepatic fibrosis in patients with chronic hepatitis C by splenic Doppler impedance index. Clin Gastroenterol Hepatol 2007; 5:1199–1206.
- Talawalkar JA. Elastography for detecting hepatic fibrosis: options and considerations. Gastroenterology 2008; 135:299–302.
- Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 2003; 29:1705–1713.
- Kettaneh A, Marcellin P, Douvin C, et al. Features associated with success rate and performance of FibroScan measurements for the diagnosis of cirrhosis in HCV patients: a prospective study of 935 patients. J Hepatol 2007; 46:628–634.
- Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005; 41:48–54.
- Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 2008; 134:960–974.
- Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2007; 5:1214–1220.
- Arena U, Vizzutti F, Corti G, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology 2008; 47:380–384.
- Roulot D, Czernichow S, Le Clésiau H, Costes JL, Vergnaud AC, Beaugrand M. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol 2008; 48:606–613.
- Ito K, Mitchell DG, Hann HW, et al. Viral-induced cirrhosis: grading of severity using MR imaging. AJR Am J Roentgenol 1999; 173:591–596.
- Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 2008; 135:32–40.
- Verma S, Gunuwan B, Mendler M, Govindrajan S, Redeker A. Factors predicting relapse and poor outcome in type I autoimmune hepatitis: role of cirrhosis development, patterns of transaminases during remission and plasma cell activity in the liver biopsy. Am J Gastroenterol 2004; 99:1510–1516.
- Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology 2009; 49:1017–1044.
- Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001; 344:495–500.
- Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002; 97:2614–2618.
- Ratziu V, Charlotte F, Heurtier A, et al; LIDO Study Group Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005; 128:1898–1906.
- Saadeh S, Cammell G, Carey WD, Younossi Z, Barnes D, Easley K. The role of liver biopsy in chronic hepatitis C. Hepatology 2001; 33:196–200.
- Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol 1995; 19:1409–1417.
- Morrison ED, Brandhagen DJ, Phatak PD, et al. Serum ferritin level predicts advanced hepatic fibrosis among U.S. patients with phenotypic hemochromatosis. Ann Intern Med 2003; 138:627–633.
- Parkes J, Guha IN, Roderick P, Rosenberg W. Performance of serum marker panels for liver fibrosis in chronic hepatitis C. J Hepatol 2006; 44:462–474.
- Montalto G, Soresi M, Aragona F, et al. Procollagen III and laminin in chronic viral hepatopathies. Presse Med 1996; 25:59–62.
- Teare JP, Sherman D, Greenfield SM, et al. Comparison of serum procollagen III peptide concentrations and PGA index for assessment of hepatic fibrosis. Lancet 1993; 342:895–898.
- Trinchet JC, Hartmann DJ, Pateron D, et al. Serum type I collagen and N-terminal peptide of type III procollagen in chronic hepatitis. Relationship to liver histology and conventional liver tests. J Hepatol 1991; 12:139–144.
- McCullough AJ, Stassen WN, Wiesner RH, Czaja AJ. Serial determinations of the amino-terminal peptide of type III procollagen in severe chronic active hepatitis. J Lab Clin Med 1987; 109:55–61.
- Takahara T, Furui K, Funaki J, et al. Increased expression of matrix metalloproteinase-II in experimental liver fibrosis in rats. Hepatology 1995; 21:787–795.
- Takahara T, Furui K, Yata Y, et al. Dual expression of matrix metalloproteinase-2 and membrane-type 1-matrix metalloproteinase in fibrotic human livers. Hepatology 1997; 26:1521–1529.
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38:518–526.
- Kelleher TB, Mehta SH, Bhaskar R, et al. Prediction of hepatic fibrosis in HIV/HCV co-infected patients using serum fibrosis markers: the SHASTA index. J Hepatol 2005; 43:78–84.
- Islam S, Antonsson L, Westin J, Lagging M. Cirrhosis in hepatitis C virus-infected patients can be excluded using an index of standard biochemical serum markers. Scand J Gastroenterol 2005; 40:867–872.
- Lackner C, Struber G, Liegl B, et al. Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis C. Hepatology 2005; 41:1376–1382.
- Poynard T, Aubert A, Bedossa P, et al. A simple biological index for detection of alcoholic liver disease in drinkers. Gastroenterology 1991; 100:1397–1402.
- Oberti F, Valsesia E, Pilette C, et al. Noninvasive diagnosis of hepatic fibrosis or cirrhosis. Gastroenterology 1997; 113:1609–1616.
- Koda M, Matunaga Y, Kawakami M, Kishimoto Y, Suou T, Murawaki Y. FibroIndex, a practical index for predicting significant fibrosis in patients with chronic hepatitis C. Hepatology 2007; 45:297–306.
- Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 2007; 46:32–36.
- Sterling RK, Lissen E, Clumeck N, et al; APRI COT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43:1317–1325.
- Calès P, Oberti F, Michalak S, et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology 2005; 42:1373–1381.
- Leroy V, Hilleret MN, Sturm N, et al. Prospective comparison of six non-invasive scores for the diagnosis of liver fibrosis in chronic hepatitis C. J Hepatol 2007; 46:775–782.
- Myers RP, De Torres M, Imbert-Bismut F, Ratziu V, Charlotte F, Poynard T; MULTIVIRC Group. Biochemical markers of fibrosis in patients with chronic hepatitis C: a comparison with prothrombin time, platelet count, and age-platelet index. Dig Dis Sci 2003; 48:146–153.
- Rossi E, Adams L, Prins A, et al. Validation of the FibroTest biochemical markers score in assessing liver fibrosis in hepatitis C patients. Clin Chem 2003; 49:450–454.
- Halfon P, Bourliere M, Deydier R, et al. Independent prospective multicenter validation of biochemical markers (fibrotest-actitest) for the prediction of liver fibrosis and activity in patients with chronic hepatitis C: the fibropaca study. Am J Gastroenterol 2006; 101:547–555.
- Poynard T, Imbert-Bismut F, Munteanu M, et al. Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C. Comp Hepatol 2004; 3:8.
- Sebastiani G, Halfon P, Castera L, et al. SAFE biopsy: a validated method for large-scale staging of liver fibrosis in chronic hepatitis C. Hepatology 2009; 49:1821–1827.
- The French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretations in patients with chronic hepatitis C. Hepatology 1994; 20:15–20.
- Sanford NL, Walsh P, Matis C, Baddeley H, Powell LW. Is ultrasonography useful in the assessment of diffuse parenchymal liver disease? Gastroenterology 1985; 89:186–191.
- Harbin WP, Robert NJ, Ferrucci JT. Diagnosis of cirrhosis based on regional changes in hepatic morphology: a radiological and pathological analysis. Radiology 1980; 135:273–283.
- Giorgio A, Amoroso P, Lettieri G, et al. Cirrhosis: value of caudate to right lobe ratio in diagnosis with US. Radiology 1986; 161:443–445.
- Liu CH, Hsu SJ, Lin JW, et al. Noninvasive diagnosis of hepatic fibrosis in patients with chronic hepatitis C by splenic Doppler impedance index. Clin Gastroenterol Hepatol 2007; 5:1199–1206.
- Talawalkar JA. Elastography for detecting hepatic fibrosis: options and considerations. Gastroenterology 2008; 135:299–302.
- Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 2003; 29:1705–1713.
- Kettaneh A, Marcellin P, Douvin C, et al. Features associated with success rate and performance of FibroScan measurements for the diagnosis of cirrhosis in HCV patients: a prospective study of 935 patients. J Hepatol 2007; 46:628–634.
- Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005; 41:48–54.
- Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 2008; 134:960–974.
- Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2007; 5:1214–1220.
- Arena U, Vizzutti F, Corti G, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology 2008; 47:380–384.
- Roulot D, Czernichow S, Le Clésiau H, Costes JL, Vergnaud AC, Beaugrand M. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol 2008; 48:606–613.
- Ito K, Mitchell DG, Hann HW, et al. Viral-induced cirrhosis: grading of severity using MR imaging. AJR Am J Roentgenol 1999; 173:591–596.
- Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 2008; 135:32–40.
KEY POINTS
- Liver biopsy remains an important tool in the evaluation and management of liver disease.
- The role of liver biopsy for diagnosis of chronic liver disease has diminished, owing to accurate blood tests and imaging studies.
- Noninvasive tests for assessing the degree of hepatic fibrosis are showing more promise and may further reduce the need for liver biopsy. Elastography, in particular, shows promise in measuring hepatic fibrosis.
- Liver biopsy is still needed if laboratory testing and imaging studies are inconclusive.
Can patients with COPD or asthma take a beta-blocker?
Yes. Treatment with beta-adrenergic receptor blockers decreases the mortality rate in patients with coronary artery disease or heart failure, as well as during the perioperative period in selected patients (eg, those with a history of myocardial infarction, a positive stress test, or current chest pain due to myocardial ischemia). The current evidence supports giving beta-blockers to patients with coronary artery disease and chronic obstructive pulmonary disease (COPD) or asthma, which lowers the 1-year mortality rate to a degree similar to that in patients without COPD or asthma, and without worsening respiratory function.1 However, many clinicians still hesitate to start patients with COPD or asthma on a beta-blocker due to the fear of bronchoconstriction.2
THE RISKS
In patients with reversible airway disease, beta-blockers may increase airway reactivity and bronchospasm, as well as decrease the response to inhaled or oral beta-receptor agonists.3 Even topical ophthalmic nonselective beta-blockers for glaucoma can cause a worsening of pulmonary function.4 However, these data are from small trials in the 1970s and 1980s.
On the other hand, not giving beta-blockers can pose a risk of death. In a retrospective study of more than 200,000 patients with myocardial infarction, Gottlieb et al5 found that beta-blockers were associated with a 40% reduction in mortality rates in patients with conditions often considered a contraindication to beta-blocker therapy, such as congestive heart failure, pulmonary disease, and older age.5
CARDIOSELECTIVE BETA-BLOCKERS
Cardioselective beta-blockers with an affinity for the beta-1 receptor theoretically result in fewer adverse effects on the lungs. They competitively block the response to beta-adrenergic stimulation and selectively block beta-1 receptors with little or no effect on beta-2 receptors, except perhaps at high doses. However, this possible high-dose effect requires further study.
The effect of cardioselective beta-blockers on respiratory function was evaluated in two meta-analyses,6,7 one in patients with mild to moderate reactive airway disease, the other in patients with mild to severe COPD. Patients with reactive airway disease who received a single dose of a beta-blocker had a 7.46% reduction in forced expiratory volume in the first second of expiration (FEV1), an effect that was completely reversed by treatment with a beta-agonist inhaler. The FEV1 increased by a statistically significantly greater amount in response to beta-agonists in patients who received beta-blockers (a single dose or continuous therapy) than in those who did not receive beta-blockers. Patients who received continuous cardioselective beta-blockers experienced no significant drop in FEV1, and no new symptoms developed. These results led the authors to conclude that cardioselective beta-blockers do not cause a significant reduction in pulmonary function in patients with mild to moderate reactive airway disease and COPD and are therefore safe to use. A single dose of a cardioselective beta-blocker may produce a small decrease in FEV1, especially in patients with reactive airway disease, but as therapy is continued over days to weeks, there is no significant change in symptoms or FEV1 and no increase in the need for beta-agonist inhalers.
A major limitation of the two meta-analyses was that the patients were younger than most patients who require beta-blockers: the average age was 40 in patients with reactive airway disease, and 54 in patients with COPD. Also important to consider is that only patients with mild to moderate reactive airway disease were included. Patients with severe asthma, especially those with active bronchospasm, may react differently to even cardioselective beta-blockers.
NONSELECTIVE BETA-BLOCKERS
Recent studies suggest that nonselective beta-blockers can affect respiratory function in patients with COPD, but they have failed to show any harm. For example, propranolol (Inderal) was shown to worsen pulmonary function and to decrease the sensitivity of the airway to the effects of long-acting beta-2-agonists, but the 15 patients included in this study had no increase in respiratory symptoms.8
It has also been suggested that combined nonselective beta- and alpha-receptor blockade—eg, with labetalol (Trandate) or carvedilol (Coreg)—might be better tolerated than nonselective beta-blockers in patients with COPD.9 However, from limited data, Kotlyar et al10 suggested that carvedilol may be less well tolerated in patients with asthma than with COPD. All current evidence on combined nonselective beta-and alpha-blockade is observational, and it is not yet clear whether this class of beta-blockers is better tolerated due to alpha-blockade or merely because nonselective beta-blockers themselves are well tolerated.
OUR RECOMMENDATIONS
- Chen J, Radford MJ, Wang Y, Marciniak TA, Krumholz HM. Effectiveness of beta-blocker therapy after acute myocardial infarction in elderly patients with chronic obstructive pulmonary disease or asthma. J Am Coll Cardiol 2001; 37:1950–1956.
- The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med 1997; 157:2413–2446.
- Benson MK, Berrill WT, Cruickshank JM, Sterling GS. A comparison of four beta-adrenoceptor antagonists in patients with asthma. Br J Clin Pharmacol 1978; 5:415–419.
- Fraunfelder FT, Barker AF. Respiratory effects of timolol. N Engl J Med 1984; 311:1441.
- Gottlieb SS, McCarter RJ, Vogel RA. Effect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarction. N Engl J Med 1998; 339:489–497.
- Salpeter SR, Ormiston TM, Salpeter EE, Poole PJ, Cates CJ. Cardioselective beta-blockers for chronic obstructive pulmonary disease: a meta-analysis. Respir Med 2003; 97:1094–1101.
- Salpeter SR, Ormiston TM, Salpeter EE. Cardioselective beta-blockers in patients with reactive airway disease: a meta-analysis. Ann Intern Med 2002; 137:715–725.
- van der Woude HJ, Zaagsma J, Postma DS, Winter TH, van Hulst M, Aalbers R. Detrimental effects of beta-blockers in COPD: a concern for nonselective beta-blockers. Chest 2005; 127:818–824.
- Sirak TE, Jelic S, Le Jemtel TH. Therapeutic update: non-selective beta- and alpha-adrenergic blockade in patients with coexistent chronic obstructive pulmonary disease and chronic heart failure. J Am Coll Cardiol 2004; 44:497–502.
- Kotlyar E, Keogh AM, Macdonald PS, Arnold RH, McCaffrey DJ, Glanville AR. Tolerability of carvedilol in patients with heart failure and concomitant chronic obstructive pulmonary disease or asthma. J Heart Lung Transplant 2002; 21:1290–1295.
Yes. Treatment with beta-adrenergic receptor blockers decreases the mortality rate in patients with coronary artery disease or heart failure, as well as during the perioperative period in selected patients (eg, those with a history of myocardial infarction, a positive stress test, or current chest pain due to myocardial ischemia). The current evidence supports giving beta-blockers to patients with coronary artery disease and chronic obstructive pulmonary disease (COPD) or asthma, which lowers the 1-year mortality rate to a degree similar to that in patients without COPD or asthma, and without worsening respiratory function.1 However, many clinicians still hesitate to start patients with COPD or asthma on a beta-blocker due to the fear of bronchoconstriction.2
THE RISKS
In patients with reversible airway disease, beta-blockers may increase airway reactivity and bronchospasm, as well as decrease the response to inhaled or oral beta-receptor agonists.3 Even topical ophthalmic nonselective beta-blockers for glaucoma can cause a worsening of pulmonary function.4 However, these data are from small trials in the 1970s and 1980s.
On the other hand, not giving beta-blockers can pose a risk of death. In a retrospective study of more than 200,000 patients with myocardial infarction, Gottlieb et al5 found that beta-blockers were associated with a 40% reduction in mortality rates in patients with conditions often considered a contraindication to beta-blocker therapy, such as congestive heart failure, pulmonary disease, and older age.5
CARDIOSELECTIVE BETA-BLOCKERS
Cardioselective beta-blockers with an affinity for the beta-1 receptor theoretically result in fewer adverse effects on the lungs. They competitively block the response to beta-adrenergic stimulation and selectively block beta-1 receptors with little or no effect on beta-2 receptors, except perhaps at high doses. However, this possible high-dose effect requires further study.
The effect of cardioselective beta-blockers on respiratory function was evaluated in two meta-analyses,6,7 one in patients with mild to moderate reactive airway disease, the other in patients with mild to severe COPD. Patients with reactive airway disease who received a single dose of a beta-blocker had a 7.46% reduction in forced expiratory volume in the first second of expiration (FEV1), an effect that was completely reversed by treatment with a beta-agonist inhaler. The FEV1 increased by a statistically significantly greater amount in response to beta-agonists in patients who received beta-blockers (a single dose or continuous therapy) than in those who did not receive beta-blockers. Patients who received continuous cardioselective beta-blockers experienced no significant drop in FEV1, and no new symptoms developed. These results led the authors to conclude that cardioselective beta-blockers do not cause a significant reduction in pulmonary function in patients with mild to moderate reactive airway disease and COPD and are therefore safe to use. A single dose of a cardioselective beta-blocker may produce a small decrease in FEV1, especially in patients with reactive airway disease, but as therapy is continued over days to weeks, there is no significant change in symptoms or FEV1 and no increase in the need for beta-agonist inhalers.
A major limitation of the two meta-analyses was that the patients were younger than most patients who require beta-blockers: the average age was 40 in patients with reactive airway disease, and 54 in patients with COPD. Also important to consider is that only patients with mild to moderate reactive airway disease were included. Patients with severe asthma, especially those with active bronchospasm, may react differently to even cardioselective beta-blockers.
NONSELECTIVE BETA-BLOCKERS
Recent studies suggest that nonselective beta-blockers can affect respiratory function in patients with COPD, but they have failed to show any harm. For example, propranolol (Inderal) was shown to worsen pulmonary function and to decrease the sensitivity of the airway to the effects of long-acting beta-2-agonists, but the 15 patients included in this study had no increase in respiratory symptoms.8
It has also been suggested that combined nonselective beta- and alpha-receptor blockade—eg, with labetalol (Trandate) or carvedilol (Coreg)—might be better tolerated than nonselective beta-blockers in patients with COPD.9 However, from limited data, Kotlyar et al10 suggested that carvedilol may be less well tolerated in patients with asthma than with COPD. All current evidence on combined nonselective beta-and alpha-blockade is observational, and it is not yet clear whether this class of beta-blockers is better tolerated due to alpha-blockade or merely because nonselective beta-blockers themselves are well tolerated.
OUR RECOMMENDATIONS
Yes. Treatment with beta-adrenergic receptor blockers decreases the mortality rate in patients with coronary artery disease or heart failure, as well as during the perioperative period in selected patients (eg, those with a history of myocardial infarction, a positive stress test, or current chest pain due to myocardial ischemia). The current evidence supports giving beta-blockers to patients with coronary artery disease and chronic obstructive pulmonary disease (COPD) or asthma, which lowers the 1-year mortality rate to a degree similar to that in patients without COPD or asthma, and without worsening respiratory function.1 However, many clinicians still hesitate to start patients with COPD or asthma on a beta-blocker due to the fear of bronchoconstriction.2
THE RISKS
In patients with reversible airway disease, beta-blockers may increase airway reactivity and bronchospasm, as well as decrease the response to inhaled or oral beta-receptor agonists.3 Even topical ophthalmic nonselective beta-blockers for glaucoma can cause a worsening of pulmonary function.4 However, these data are from small trials in the 1970s and 1980s.
On the other hand, not giving beta-blockers can pose a risk of death. In a retrospective study of more than 200,000 patients with myocardial infarction, Gottlieb et al5 found that beta-blockers were associated with a 40% reduction in mortality rates in patients with conditions often considered a contraindication to beta-blocker therapy, such as congestive heart failure, pulmonary disease, and older age.5
CARDIOSELECTIVE BETA-BLOCKERS
Cardioselective beta-blockers with an affinity for the beta-1 receptor theoretically result in fewer adverse effects on the lungs. They competitively block the response to beta-adrenergic stimulation and selectively block beta-1 receptors with little or no effect on beta-2 receptors, except perhaps at high doses. However, this possible high-dose effect requires further study.
The effect of cardioselective beta-blockers on respiratory function was evaluated in two meta-analyses,6,7 one in patients with mild to moderate reactive airway disease, the other in patients with mild to severe COPD. Patients with reactive airway disease who received a single dose of a beta-blocker had a 7.46% reduction in forced expiratory volume in the first second of expiration (FEV1), an effect that was completely reversed by treatment with a beta-agonist inhaler. The FEV1 increased by a statistically significantly greater amount in response to beta-agonists in patients who received beta-blockers (a single dose or continuous therapy) than in those who did not receive beta-blockers. Patients who received continuous cardioselective beta-blockers experienced no significant drop in FEV1, and no new symptoms developed. These results led the authors to conclude that cardioselective beta-blockers do not cause a significant reduction in pulmonary function in patients with mild to moderate reactive airway disease and COPD and are therefore safe to use. A single dose of a cardioselective beta-blocker may produce a small decrease in FEV1, especially in patients with reactive airway disease, but as therapy is continued over days to weeks, there is no significant change in symptoms or FEV1 and no increase in the need for beta-agonist inhalers.
A major limitation of the two meta-analyses was that the patients were younger than most patients who require beta-blockers: the average age was 40 in patients with reactive airway disease, and 54 in patients with COPD. Also important to consider is that only patients with mild to moderate reactive airway disease were included. Patients with severe asthma, especially those with active bronchospasm, may react differently to even cardioselective beta-blockers.
NONSELECTIVE BETA-BLOCKERS
Recent studies suggest that nonselective beta-blockers can affect respiratory function in patients with COPD, but they have failed to show any harm. For example, propranolol (Inderal) was shown to worsen pulmonary function and to decrease the sensitivity of the airway to the effects of long-acting beta-2-agonists, but the 15 patients included in this study had no increase in respiratory symptoms.8
It has also been suggested that combined nonselective beta- and alpha-receptor blockade—eg, with labetalol (Trandate) or carvedilol (Coreg)—might be better tolerated than nonselective beta-blockers in patients with COPD.9 However, from limited data, Kotlyar et al10 suggested that carvedilol may be less well tolerated in patients with asthma than with COPD. All current evidence on combined nonselective beta-and alpha-blockade is observational, and it is not yet clear whether this class of beta-blockers is better tolerated due to alpha-blockade or merely because nonselective beta-blockers themselves are well tolerated.
OUR RECOMMENDATIONS
- Chen J, Radford MJ, Wang Y, Marciniak TA, Krumholz HM. Effectiveness of beta-blocker therapy after acute myocardial infarction in elderly patients with chronic obstructive pulmonary disease or asthma. J Am Coll Cardiol 2001; 37:1950–1956.
- The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med 1997; 157:2413–2446.
- Benson MK, Berrill WT, Cruickshank JM, Sterling GS. A comparison of four beta-adrenoceptor antagonists in patients with asthma. Br J Clin Pharmacol 1978; 5:415–419.
- Fraunfelder FT, Barker AF. Respiratory effects of timolol. N Engl J Med 1984; 311:1441.
- Gottlieb SS, McCarter RJ, Vogel RA. Effect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarction. N Engl J Med 1998; 339:489–497.
- Salpeter SR, Ormiston TM, Salpeter EE, Poole PJ, Cates CJ. Cardioselective beta-blockers for chronic obstructive pulmonary disease: a meta-analysis. Respir Med 2003; 97:1094–1101.
- Salpeter SR, Ormiston TM, Salpeter EE. Cardioselective beta-blockers in patients with reactive airway disease: a meta-analysis. Ann Intern Med 2002; 137:715–725.
- van der Woude HJ, Zaagsma J, Postma DS, Winter TH, van Hulst M, Aalbers R. Detrimental effects of beta-blockers in COPD: a concern for nonselective beta-blockers. Chest 2005; 127:818–824.
- Sirak TE, Jelic S, Le Jemtel TH. Therapeutic update: non-selective beta- and alpha-adrenergic blockade in patients with coexistent chronic obstructive pulmonary disease and chronic heart failure. J Am Coll Cardiol 2004; 44:497–502.
- Kotlyar E, Keogh AM, Macdonald PS, Arnold RH, McCaffrey DJ, Glanville AR. Tolerability of carvedilol in patients with heart failure and concomitant chronic obstructive pulmonary disease or asthma. J Heart Lung Transplant 2002; 21:1290–1295.
- Chen J, Radford MJ, Wang Y, Marciniak TA, Krumholz HM. Effectiveness of beta-blocker therapy after acute myocardial infarction in elderly patients with chronic obstructive pulmonary disease or asthma. J Am Coll Cardiol 2001; 37:1950–1956.
- The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med 1997; 157:2413–2446.
- Benson MK, Berrill WT, Cruickshank JM, Sterling GS. A comparison of four beta-adrenoceptor antagonists in patients with asthma. Br J Clin Pharmacol 1978; 5:415–419.
- Fraunfelder FT, Barker AF. Respiratory effects of timolol. N Engl J Med 1984; 311:1441.
- Gottlieb SS, McCarter RJ, Vogel RA. Effect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarction. N Engl J Med 1998; 339:489–497.
- Salpeter SR, Ormiston TM, Salpeter EE, Poole PJ, Cates CJ. Cardioselective beta-blockers for chronic obstructive pulmonary disease: a meta-analysis. Respir Med 2003; 97:1094–1101.
- Salpeter SR, Ormiston TM, Salpeter EE. Cardioselective beta-blockers in patients with reactive airway disease: a meta-analysis. Ann Intern Med 2002; 137:715–725.
- van der Woude HJ, Zaagsma J, Postma DS, Winter TH, van Hulst M, Aalbers R. Detrimental effects of beta-blockers in COPD: a concern for nonselective beta-blockers. Chest 2005; 127:818–824.
- Sirak TE, Jelic S, Le Jemtel TH. Therapeutic update: non-selective beta- and alpha-adrenergic blockade in patients with coexistent chronic obstructive pulmonary disease and chronic heart failure. J Am Coll Cardiol 2004; 44:497–502.
- Kotlyar E, Keogh AM, Macdonald PS, Arnold RH, McCaffrey DJ, Glanville AR. Tolerability of carvedilol in patients with heart failure and concomitant chronic obstructive pulmonary disease or asthma. J Heart Lung Transplant 2002; 21:1290–1295.
HIV: Just another chronic disease
In subsequent years we learned about HIV—the retrovirus, and the immune system that it cleverly and efficiently disables. For the most part, we matured professionally and moved past the social stigmas of the disease, although that was painful. We developed systems to keep acutely ill patients out of the hospital while providing them with “long-term” (weeks or months of) intravenous antibiotics and humane palliative care.
We learned about AZT and argued about when to use it. But mainly, we watched many, many young men (and some women) die in corner hospital rooms. For me, from the ′80s, there remain heartrending personal images, notes, and cassette tapes voicing thanks for my concern and time spent, but no notes of thanks like those I’ve received from my patients with chronic rheumatoid arthritis who, after years of care, are able to hold their nieces or grandchildren.
A few long-term survivors have raised the hope that immune systems could recover and exist in symbiosis with the virus, and that maybe a drug cocktail or vaccine could provide a cure or remission. Magic Johnson, known to be infected since at least 1991, is likely the most public example of a long-term survivor on highly active antiviral therapy—a hope in the flesh.
But did we ever expect a time when HIV would be viewed as a chronic disease, with patients warranting screening for coronary artery disease in order to decrease long-term coronary complications? Did we ever expect a time that we would be offering organ transplants to HIV-infected patients?
In this issue of the Journal, Drs. Malvestutto and Aberg discuss coronary issues that need to be recognized and managed in HIV-infected patients. This further complicates the management of these patients, and draws cardiologists and primary care providers back into management plans.
I can’t think of a management “complication” of a chronic illness that is more welcome—or more surprising.
In subsequent years we learned about HIV—the retrovirus, and the immune system that it cleverly and efficiently disables. For the most part, we matured professionally and moved past the social stigmas of the disease, although that was painful. We developed systems to keep acutely ill patients out of the hospital while providing them with “long-term” (weeks or months of) intravenous antibiotics and humane palliative care.
We learned about AZT and argued about when to use it. But mainly, we watched many, many young men (and some women) die in corner hospital rooms. For me, from the ′80s, there remain heartrending personal images, notes, and cassette tapes voicing thanks for my concern and time spent, but no notes of thanks like those I’ve received from my patients with chronic rheumatoid arthritis who, after years of care, are able to hold their nieces or grandchildren.
A few long-term survivors have raised the hope that immune systems could recover and exist in symbiosis with the virus, and that maybe a drug cocktail or vaccine could provide a cure or remission. Magic Johnson, known to be infected since at least 1991, is likely the most public example of a long-term survivor on highly active antiviral therapy—a hope in the flesh.
But did we ever expect a time when HIV would be viewed as a chronic disease, with patients warranting screening for coronary artery disease in order to decrease long-term coronary complications? Did we ever expect a time that we would be offering organ transplants to HIV-infected patients?
In this issue of the Journal, Drs. Malvestutto and Aberg discuss coronary issues that need to be recognized and managed in HIV-infected patients. This further complicates the management of these patients, and draws cardiologists and primary care providers back into management plans.
I can’t think of a management “complication” of a chronic illness that is more welcome—or more surprising.
In subsequent years we learned about HIV—the retrovirus, and the immune system that it cleverly and efficiently disables. For the most part, we matured professionally and moved past the social stigmas of the disease, although that was painful. We developed systems to keep acutely ill patients out of the hospital while providing them with “long-term” (weeks or months of) intravenous antibiotics and humane palliative care.
We learned about AZT and argued about when to use it. But mainly, we watched many, many young men (and some women) die in corner hospital rooms. For me, from the ′80s, there remain heartrending personal images, notes, and cassette tapes voicing thanks for my concern and time spent, but no notes of thanks like those I’ve received from my patients with chronic rheumatoid arthritis who, after years of care, are able to hold their nieces or grandchildren.
A few long-term survivors have raised the hope that immune systems could recover and exist in symbiosis with the virus, and that maybe a drug cocktail or vaccine could provide a cure or remission. Magic Johnson, known to be infected since at least 1991, is likely the most public example of a long-term survivor on highly active antiviral therapy—a hope in the flesh.
But did we ever expect a time when HIV would be viewed as a chronic disease, with patients warranting screening for coronary artery disease in order to decrease long-term coronary complications? Did we ever expect a time that we would be offering organ transplants to HIV-infected patients?
In this issue of the Journal, Drs. Malvestutto and Aberg discuss coronary issues that need to be recognized and managed in HIV-infected patients. This further complicates the management of these patients, and draws cardiologists and primary care providers back into management plans.
I can’t think of a management “complication” of a chronic illness that is more welcome—or more surprising.