User login
Research Agenda for Older Patient Care
Older adults with high levels of medical complexity occupy an increasing fraction of beds in acute‐care hospitals in the United States.[1, 2] By 2007, patients age 65 years and older accounted for nearly half of adult inpatient days of care.[1] These patients are commonly cared for by hospitalists who number more than 40,000.[3] Although hospitalists are most often trained in internal medicine, they have typically received limited formal geriatrics training. Increasingly, access to experts in geriatric medicine is limited.[4] Further, hospitalists and others who practice in acute care are limited by the lack of research to address the needs of the older adult population, specifically in the diagnosis and management of conditions encountered during acute illness.
To better support hospitalists in providing acute inpatient geriatric care, the Society of Hospital Medicine (SHM) partnered with the Association of Specialty Professors to develop a research agenda to bridge this gap. Using methodology from the James Lind Alliance (JLA) and the Patient Centered Outcomes Research Institute (PCORI), the SHM joined with older adult advocacy groups, professional societies of providers, and funders to create a geriatric‐focused acute‐care research agenda, highlighting 10 key research questions.[5, 6, 7] The goal of this approach was to produce and promote high integrity, evidence‐based information that comes from research guided by patients, caregivers, and the broader healthcare community.[8] In this article, we describe the methodology and results of this agenda‐setting process, referred to as the Acute Care of Older Patients (ACOP) Priority Setting Partnership.
METHODS
Overview
This project focused on topic generation, the first step in the PCORI framework for identification and prioritization of research areas.[5] We employed a specific and defined methodology to elicit and prioritize potential research topics incorporating input from representatives of older patients, family caregivers, and healthcare providers.[6]
To elicit this input, we chose a collaborative and consultative approach to stakeholder engagement, drawing heavily from the published work of the JLA, an initiative promoting patient‐clinician partnerships in health research developed in the United Kingdom.[6] We previously described the approach elsewhere.[7]
The ACOP process for determining the research agenda consisted of 4 steps: (1) convene, (2) consult, (3) collate, and (4) prioritize.[6] Through these steps, detailed below, we were able to obtain input from a broad group of stakeholders and engage the stakeholders in a process of reducing and refining our research questions.
Convene
The steering committee (the article's authors) convened a stakeholder partnership group that included stakeholders representing patients and caregivers, advocacy organizations for the elderly, organizations that address diseases and conditions common among hospitalized older patients, provider professional societies (eg, hospitalists, subspecialists, and nurses and social workers), payers, and funders. Patient, caregiver, and advocacy organizations were identified based on their engagement in aging and health policy advocacy by SHM staff and 1 author who had completed a Health and Aging Policy Fellowship (H.L.W.).
The steering committee issued e‐mail invitations to stakeholder organizations, making initial inquiries through professional staff and relevant committee chairs. Second inquiries were made via e‐mail to each organization's volunteer leadership. We developed a webinar that outlined the overall research agenda setting process and distributed the webinar to all stakeholders. The stakeholder organizations were asked to commit to (1) surveying their memberships and (2) participating actively in prioritization by e‐mail and at a 1‐day meeting in Washington DC.
Consult
Each stakeholder organization conducted a survey of its membership via an Internet‐based survey in the summer of 2013 (see Supporting Information, Appendix A, in the online version of this article). Stakeholder organizations were asked to provide up to 75 survey responses each. Though a standard survey was used, the steering committee was not prescriptive in the methodology of survey distribution to accommodate the structure and communication methods of the individual stakeholder organizations. Survey respondents were asked to identify up to 5 unanswered questions relevant to the acute care of older persons and also provide demographic information.
Collate
In the collating process, we clarified and categorized the unanswered questions submitted in the individual surveys. Each question was initially reviewed by a member of the steering committee, using explicit criteria (see Supporting Information, Appendix B, in the online version of this article). Questions that did not meet all 4 criteria were removed. For questions that met all criteria, we clarified language, combined similar questions, and categorized each question. Categories were created in a grounded process, in which individual reviewers assigned categories based on the content of the questions. Each question could be assigned to up to 2 categories. Each question was then reviewed by a second member of the steering committee using the same 4 criteria. As part of this review, similar questions were consolidated, and when possible, questions were rewritten in a standard format.[6]
Finally, the steering committee reviewed previously published research agendas looking for additional relevant unanswered questions, specifically the New Frontiers Research Agenda created by the American Geriatrics Society in conjunction with participating subspecialty societies,[9] the Cochrane Library, and other systematic reviews identified in the literature via PubMed search.[10, 11, 12, 13, 14, 15]
Prioritize
The resulting list of unanswered questions was prioritized in 2 phases. First, the list was e‐mailed to all stakeholder organizations. The organizations were asked to vote on their top 10 priorities from this list using an online ballot, assigning 10 points to their highest priority down to 1 point for their lowest priority. In so doing, they were asked to consider explicit criteria (see Supporting Information, Appendix B, in the online version of this article). Each organization had only 1 ballot and could arrive at their top 10 list in any manner they wished. The balloting from this phase was used to develop a list of unanswered questions for the second round of in‐person prioritization. Each priority's scores were totaled across all voting organizations. The 29 priorities with the highest point totals were brought to the final prioritization round because of a natural cut point at priority number 29, rather than number 30.
For the final prioritization round, the steering committee facilitated an in‐person meeting in Washington, DC in October 2013 using nominal group technique (NGT) methodologies to arrive at consensus.[16] During this process stakeholders were asked to consider additional criteria (see Supporting Information, Appendix B, in the online version of this article).
RESULTS
Table 1 lists the organizations who engaged in 1 or more parts of the topic generation process. Eighteen stakeholder organizations agreed to participate in the convening process. Ten organizations did not respond to our solicitation and 1 declined to participate.
| Organization (N=18) | Consultation % of Survey Responses (N=580) | Prioritization Round 1 | Prioritization Round 2 |
|---|---|---|---|
| Alzheimer's Association | 7.0% | Yes | Yes |
| American Academy of Neurology | 3.4% | Yes | Yes |
| American Association of Retired Persons | 0.8% | No | No |
| American College of Cardiology | 11.4% | Yes | Yes |
| American College of Emergency Physicians | 1.3% | No | No |
| American College of Surgeons | 1.0% | Yes | Yes |
| American Geriatrics Society | 7.6% | Yes | Yes |
| American Hospital Association | 1.7% | Yes | No |
| Centers for Medicare & Medicaid Services | 0.8% | Yes | Yes |
| Gerontological Society of America | 18.9% | Yes | Yes |
| National Alliance for Caregiving | 1.0% | Yes | Yes |
| National Association of Social Workers | 5.9% | Yes | Yes |
| National Coalition for Healthcare | 0.6% | No | No |
| National Institute on Aging | 2.1% | Yes | Yes |
| National Partnership for Women and Families | 0.0% | Yes | Yes |
| Nursing Improving Care for Healthsystem Elders | 28.6% | Yes | No |
| Society of Critical Care Medicine | 12.0% | Yes | Yes |
| Society of Hospital Medicine | 4.6% | Yes | Yes |
Seventeen stakeholder organizations obtained survey responses from a total of 580 individuals (range, 3150 per organization), who were asked to identify important unanswered questions in the acute care of older persons. Survey respondents were typically female (77%), white (85%), aged 45 to 65 years (65%), and identified themselves as health professionals (90%). Twenty‐six percent of respondents also identified as patients or family caregivers. Their surveys included 1299 individual questions.
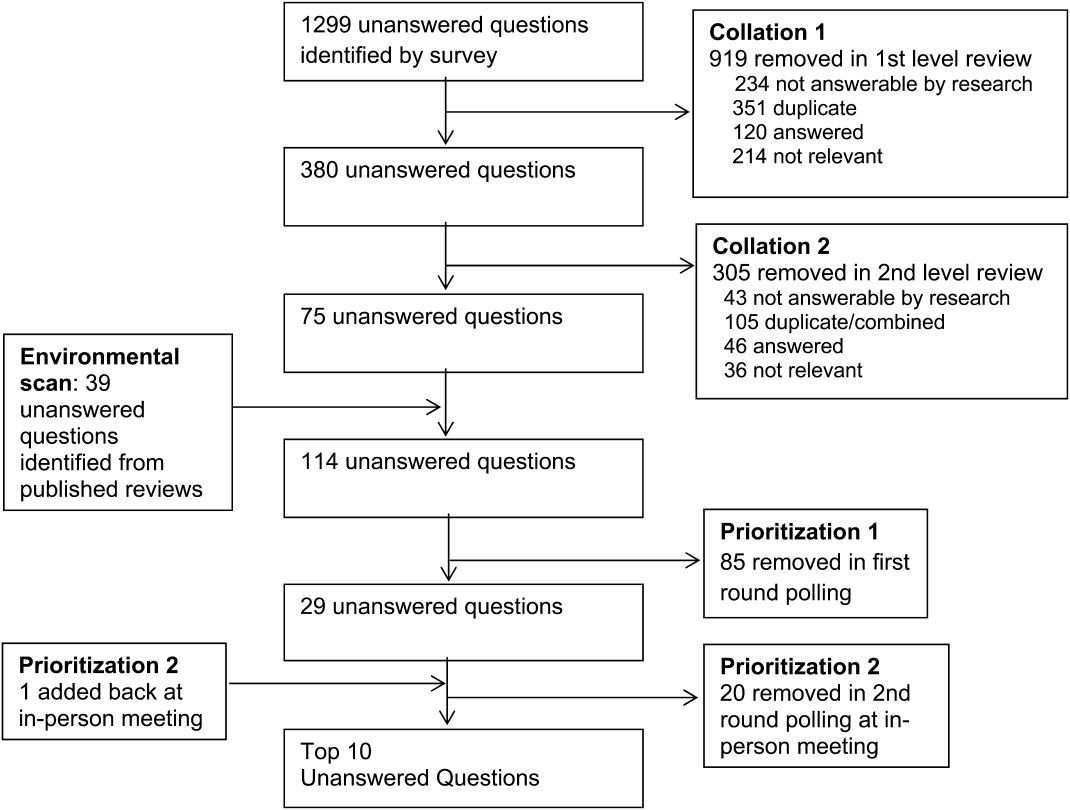
Figure 1 summarizes our collation and prioritization process and reports the numbers of questions resulting at each stage. Nine hundred nineteen questions were removed during the first review conducted by steering committee members, and 31 question categories were identified. An additional 305 questions were removed in the second review, with 75 questions remaining. As the final step of the collating process, literature review identified 39 relevant questions not already suggested or moved forward through our consultation and collation process. These questions were added to the list of unanswered questions.
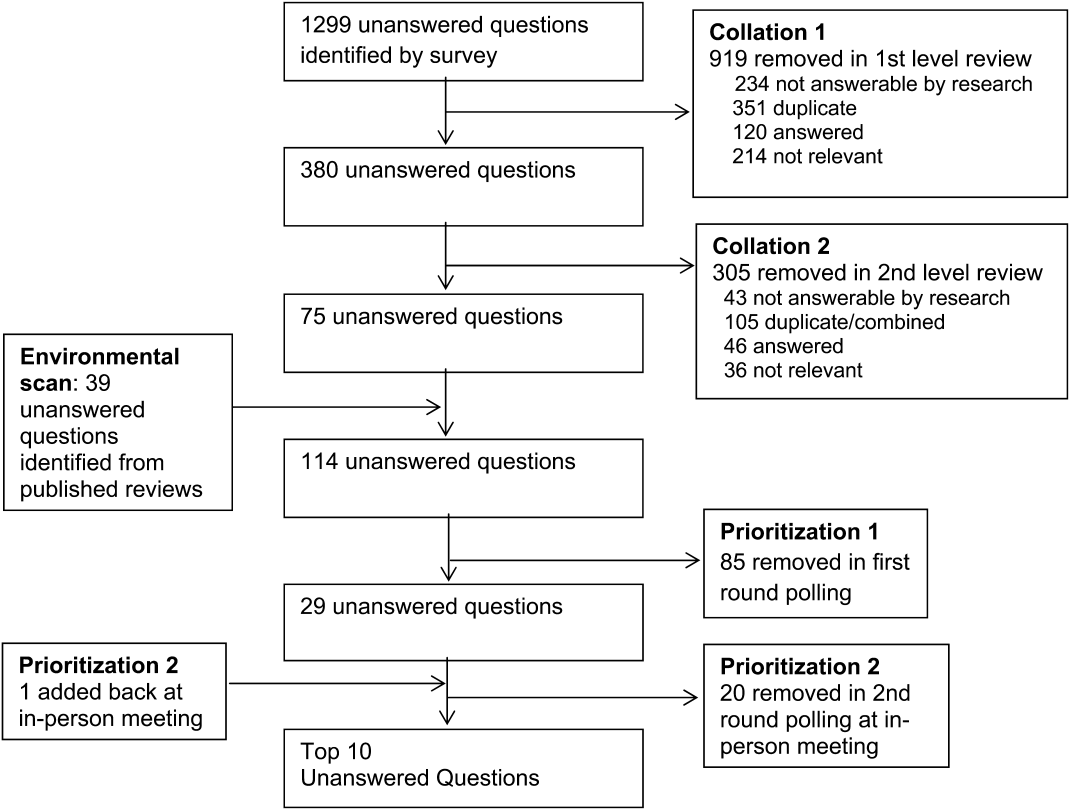
In the first round of prioritization, this list of 114 questions was emailed to each stakeholder organization (Table 1). After the stakeholder voting process was completed, 29 unanswered questions remained (see Supporting Information, Appendix C, in the online version of this article). These questions were refined and prioritized in the in‐person meeting to create the final list of 10 questions. The stakeholders present in the meeting represented 13 organizations (Table 1). Using the NGT with several rounds of small group breakouts and large group deliberation, 9 of the top 10 questions were selected from the list of 29. One additional highly relevant question that had been removed earlier in the collation process regarding workforce was added back by the stakeholder group.
This prioritized research agenda appears in Table 2 and below, organized alphabetically by topic.
- Advanced care planning: What approaches for determining and communicating goals of care across and within healthcare settings are most effective in promoting goal‐concordant care for hospitalized older patients?
- Care transitions: What is the comparative effectiveness of transitional care models on patient‐centered outcomes for hospitalized older adults?
- Delirium: What practices are most effective for consistent recognition, prevention, and treatment of delirium subtypes among hospitalized older adults?
- Dementia: Does universal assessment of hospitalized older adults for cognitive impairment (eg, at presentation and/or discharge) lead to more appropriate application of geriatric care principles and improve patient‐centered outcomes?
- Depression: Does identifying depressive symptoms during a hospital stay and initiating a therapeutic plan prior to discharge improve patient‐centered and/or disease‐specific outcomes?
- Medications: What systems interventions improve medication management for older adults (ie, appropriateness of medication choices and dosing, compliance, cost) in the hospital and postacute care?
- Models of care: For which populations of hospitalized older adults does systematic implementation of geriatric care principles/processes improve patient‐centered outcomes?
- Physical function: What is the comparative effectiveness of interventions that promote in‐hospital mobility, improve and preserve physical function, and reduce falls among older hospitalized patients?
- Surgery: What perioperative strategies can be used to optimize care processes and improve outcomes in older surgical patients?
- Training: What is the most effective approach to training hospital‐based providers in geriatric and palliative care competencies?
| Topic | Scope of Problem | What Is known | Unanswered Question | Proposed Dimensions |
|---|---|---|---|---|
| ||||
| Advanced‐care planning | Older persons who lack decision‐making capacity often do not have surrogates or clear goals of care documented.[19] Advanced‐care directives are associated with an increase in patient autonomy and empowerment, and although 15% to 25% of adults completed the documentation in 2004,[20] a recent study found completion rates have increased to 72%.[21] | Nursing home residents with advanced directives are less likely to be hospitalized.[22, 23] Advanced directive tools, such as POLST, work to translate patient preferences to medical order.[24] standardized patient transfer tools may help to improve transitions between nursing homes and hospitals.[25] However, advanced care planning fails to integrate into courses of care if providers are unwilling or unskilled in using advanced care documentation.[26] | What approaches for determining and communicating goals of care across and within healthcare settings are most effective in promoting goal‐concordant care for hospitalized older patients? | Potential interventions: |
| Decision aids | ||||
| Standard interdisciplinary advanced care planning approach | ||||
| Patient advocates | ||||
| Potential outcomes might include: | ||||
| Completion of advanced directives and healthcare power of attorney | ||||
| Patient‐centered outcomesa | ||||
| Care transitions | Hospital readmission from home and skilled nursing facilities occurs within 30 days in up to a quarter of patients.[27, 28] The discharge of complex older hospitalized patients is fraught with challenges. The quality of the hospital discharge process can influence outcomes for vulnerable older patients.[29, 30, 31, 32] Studies measuring the quality of hospital discharge frequently find deficits in documentation of assessment of geriatric syndromes,[33] poor patient/caregiver understanding,[34, 35] and poor communication and follow‐up with postacute providers.[35, 36, 37, 38] | As many as 10 separate domains may influence the success of a discharge.[39] There is limited evidence, regarding quality‐of‐care transitions for hospitalized older patients. The Coordinated‐Transitional Care Program found that follow‐up with telecommunication decreased readmission rates and improved transitional care for a high‐risk condition veteran population.[40] There is modest evidence for single interventions,[41] whereas the most effective hospital‐to‐community care interventions address multiple processes in nongeriatric populations.[39, 42, 43] | What is the comparative effectiveness of the transitional care models on patient‐centered outcomes for hospitalized older adults? | Possible models: |
| Established vs novel care‐transition models | ||||
| Disease‐specific vs general approaches | ||||
| Accountable care models | ||||
| Caregiver and family engagement | ||||
| Community engagement | ||||
| Populations of interest: | ||||
| Patients with dementia | ||||
| Patients with multimorbidity | ||||
| Patients with geriatric syndromes | ||||
| Patients with psychiatric disease | ||||
| Racially and ethnically diverse patients | ||||
| Outcomes: | ||||
| Readmission | ||||
| Other adverse events | ||||
| Cost and healthcare utilization | ||||
| Patient‐centered outcomesa | ||||
| Delirium | Among older inpatients, the prevalence of delirium varies with severity of illness. Among general medical patients, in‐hospital prevalence ranges from 10% to 25 %.[44, 45] In the ICU, prevalence estimates are higher, ranging from 25% to as high as 80%.[46, 47] Delirium independently predicts increased length of stay,[48, 49] long‐term cognitive impairment,[50, 51] functional decline,[51] institutionalization,[52] and short‐ and long‐term mortality.[52, 53, 54] | Multicomponent strategies have been shown to be effective in preventing delirium. A systematic review of 19 such interventions identified the most commonly included such as[55]: early mobilization, nutrition supplements, medication review, pain management, sleep enhancement, vision/hearing protocols, and specialized geriatric care. Studies have included general medical patients, postoperative patients, and patients in the ICU. The majority of these studies found reductions in either delirium incidence (including postoperative), delirium prevalence, or delirium duration. Although medications have not been effective in treating delirium in general medical patients,[48] the choice and dose of sedative agents has been shown to impact delirium in the ICU.[56, 57, 58] | What practices are most effective for consistent recognition, prevention, and treatment of delirium subtypes (hypoactive, hyperactive, and mixed) among hospitalized older adults? | Outcomes to examine: |
| Delirium incidence (including postoperative) | ||||
| Delirium duration | ||||
| Delirium‐/coma‐free days | ||||
| Delirium prevalence at discharge | ||||
| Subsyndromal delirium | ||||
| Potential prevention and treatment modalities: | ||||
| Family education or psychosocial interventions | ||||
| Pharmacologic interventions | ||||
| Environmental modifications | ||||
| Possible areas of focus: | ||||
| Special populations | ||||
| Patients with varying stages of dementia | ||||
| Patients with multimorbidity | ||||
| Patients with geriatric syndromes | ||||
| Observation patients | ||||
| Diverse settings | ||||
| Emergency department | ||||
| Perioperative | ||||
| Skilled nursing/rehab/long‐term acute‐care facilities | ||||
| Dementia | 13% to 63% of older persons in the hospital have dementia.[59] Dementia is often unrecognized among hospitalized patients.[60] The presence of dementia is associated with a more rapid functional decline during admission and delayed hospital discharge.[59] Patients with dementia require more nursing hours, and are more likely to have complications[61] or die in care homes rather than in their preferred site.[59] | Several tools have been validated to screen for dementia in the hospital setting.[62] Studies have assessed approaches to diagnosing delirium in hospitalized patients with dementia.[63] Cognitive and functional stimulation interventions may have a positive impact on reducing behavioral issues.[64, 65] | Does universal assessment of hospitalized older adults for cognitive impairment (eg, at presentation and/or discharge) lead to more appropriate application of geriatric care principles and improve patient centered outcomes? | Potential interventions: |
| Dementia or delirium care | ||||
| Patient/family communication and engagement strategies | ||||
| Maintenance/recovery of independent functional status | ||||
| Potential outcomes: | ||||
| Patient‐centered outcomesa | ||||
| Length of stay, cost, and healthcare utilization (including palliative care) | ||||
| Immediate invasive vs early conservative treatments pursued | ||||
| Depression | Depression is a common geriatric syndrome among acutely ill older patients, occurring in up to 45% of patients.[66, 67] Rates of depression are similar among patients discharged following a critical illness, with somatic, rather than cognitive‐affective complaints being the most prevalent.[68] Depression among inpatients or immediately following hospitalization independently predicts worse functional outcomes,[69] cognitive decline,[70] hospital readmission,[71, 72] and long‐term mortality.[69, 73] Finally, geriatric patients are known to respond differently to medical treatment.[74, 75] | Although highly prevalent, depression is poorly recognized and managed in the inpatient setting. Depression is recognized in only 50% of patients, with previously undiagnosed or untreated depression being at highest risk for being missed.[76] The role of treatment of depression in the inpatient setting is poorly understood, particularly for those with newly recognized depression or depressive symptoms. Some novel collaborative care and telephone outreach programs have led to increases in depression treatment in patients with specific medical and surgical conditions, resulting in early promising mental health and comorbid outcomes.[77, 78] The efficacy of such programs for older patients is unknown. | Does identifying depressive symptoms during a hospital stay and initiating a therapeutic plan prior to discharge improve patient‐centered and/or disease‐specific outcomes? | Possible areas of focus: |
| Comprehensive geriatric and psychosocial assessment; | ||||
| Inpatient vs outpatient initiation of pharmacological therapy | ||||
| Integration of confusion assessment method into therapeutic approaches | ||||
| Linkages with outpatient mental health resources | ||||
| Medications | Medication exposure, particularly potentially inappropriate medications, is common in hospitalized elders.[79] Medication errorsof dosage, type, and discrepancy between what a patient takes at home and what is known to his/her prescribing physicianare common and adversely affects patient safety.[80] Geriatric populations are disproportionately affected, especially those taking more than 5 prescription medications per day.[81] | Numerous strategies including electronic alerts, screening protocols, and potentially inappropriate medication lists (Beers list, STOPP) exist, though the optimal strategies to limit the use of potentially inappropriate medications is not yet known.[82, 83, 84] | What systems interventions improve medication management for older adults (ie, appropriateness of medication choices and dosing, compliance, cost) in hospital and post‐acute care? | Possible areas of focus: |
| Use of healthcare information technology | ||||
| Communication across sites of care | ||||
| Reducing medication‐related adverse events | ||||
| Engagement of family caregivers | ||||
| Patient‐centered strategies to simplify regimens | ||||
| Models of care | Hospitalization marks a time of high risk for older patients. Up to half die during hospitalization or within the year following the hospitalization. There is high risk of nosocomial events, and more than a third experience a decline in health resulting in longer hospitalizations and/or placement in extended‐care facilities.[73, 85, 86] | Comprehensive inpatient care for older adults (acute care for elders units, geriatric evaluation and management units, geriatric consultation services) were studied in 2 meta‐analyses, 5 RCTs, and 1 quasiexperimental study and summarized in a systematic review.[87] The studies reported improved quality of care (1 of 1 article), quality of life (3 of 4), functional autonomy (5 of 6), survival (3 of 6), and equal or lower healthcare utilization (7 of 8). | For which populations of hospitalized older adults does systematic implementation of geriatric care principles/processes improve patient‐centered outcomes? | Potential populations: |
| Patients of the emergency department, critical care, perioperative, and targeted medical/surgical units | ||||
| Examples of care principles: | ||||
| Geriatric assessment, early mobility, medication management, delirium prevention, advanced‐care planning, risk‐factor modification, caregiver engagement | ||||
| Potential outcomes: | ||||
| Patient‐centered outcomesa | ||||
| Cost | ||||
| Physical function | Half of older patients will lose functional capacity during hospitalization.[88] Loss of physical function, particularly of lower extremities, is a risk factor for nursing home placement.[89, 90] Older hospitalized patients spend the majority (up to 80%) of their time lying in bed, even when they are capable of walking independently.[91] | Loss of independences with ADL capabilities is associated with longer hospital stays, higher readmission rates, and higher mortality risk.[92] Excessive time in bed during a hospital stay is also associated with falls.[93] Often, hospital nursing protocols and physician orders increase in‐hospital immobility in patients.[91, 94] However, nursing‐driven mobility protocols can improve functional outcomes of older hospitalized patients.[95, 96] | What is the comparative effectiveness of interventions that promote in‐hospital mobility, improve and preserve physical function, and reduce falls among older hospitalized patients? | Potential interventions: |
| Intensive physical therapy | ||||
| Incidental functional training | ||||
| Restraint reduction | ||||
| Medication management | ||||
| Potential outcomes: | ||||
| Discharge location | ||||
| Delirium, pressure ulcers, and falls | ||||
| Surgery | An increasing number of persons over age 65 years are undergoing surgical procedures.[97] These persons are at increased risk for developing delirium/cogitative dysfunction,[98] loss of functional status,[99] and exacerbations of chronic illness.[97] Additionally, pain management may be harder to address in this population.[100] Current outcomes may not reflect the clinical needs of elder surgical patients.[101] | Tailored drug selection and nursing protocols may prevent delirium.[98] Postoperative cognitive dysfunction may require weeks for resolution. Identifying frail patients preoperatively may lead to more appropriate risk stratification and improved surgical outcomes.[99] Pain management strategies focused on mitigating cognitive impact and other effects may also be beneficial.[100] Development of risk‐adjustment tools specific to older populations, as well as measures of frailty and patient‐centered care, have been proposed.[101] | What perioperative strategies can be used to optimize care processes and improve outcomes in older surgical patients? | Potential strategies: |
| Preoperative risk assessment and optimization for frail or multimorbid older patients | ||||
| Perioperative management protocols for frail or multimorbid older patients | ||||
| Potential outcomes: | ||||
| Postoperative patient centered outcomesa | ||||
| Perioperative cost, healthcare utilization | ||||
| Training | Adults over age 65 years comprise 13.2 % of the US population, but account for >30% of hospital discharges and 50% of hospital days.[86, 102, 103] By 2030, there will only be 1 geriatrician for every 3798 Americans >75 years.[4] Between 1997 and 2006, the odds that a hospitalist would treat a hospitalized Medicare patient rose 29% per year.[3] | Train the trainer programs for physicians include the CHAMP, the AGESP, and the PAGE. Education for nurses include the NICHE. Outcomes include improved self‐confidence, attitudes, teaching skills, and geriatric care environment.[104, 105, 106] | What is the most effective approach to training hospital‐based providers in geriatric and palliative care competencies? | Potential interventions: |
| Mentored implementation | ||||
| Train the trainer | ||||
| Technical support | ||||
Table 2 also contains a capsule summary of the scope of the problem addressed by each research priority, a capsule summary of related work in the content area (what is known) not intended as a systematic review, and proposed dimensions or subquestions suggested by the stakeholders at the final prioritization meeting
DISCUSSION
Older hospitalized patients account for an increasing number and proportion of hospitalized patients,[1, 2] and hospitalists increasingly are responsible for inpatient care for this population.[3] The knowledge required for hospitalists to deliver optimal care and improve outcomes has not kept pace with the rapid growth of either hospitalists or hospitalized elders. Through a rigorous prioritization process, we identified 10 areas that deserve the highest priority in directing future research efforts to improve care for the older hospitalized patient. Assessment, prevention, and treatment of geriatric syndromes in the hospital account for almost half of the priority areas. Additional research is needed to improve advanced care planning, develop new care models, and develop training models for future hospitalists competent in geriatric and palliative care competencies.
A decade ago, the American Geriatric Society and the John A. Hartford Foundation embarked upon a research agenda aimed at improving the care of hospitalized elders cared for by specialists (ie, New Frontiers in Geriatrics Research: An Agenda for Surgical and Related Medical Specialties).[9] This effort differed in many important ways from the current priortization process. First, the New Frontiers agenda focused upon specific diseases, whereas the ACOP agenda addresses geriatric syndromes that cut across multiple diseases. Second, the New Frontiers agenda was made by researchers and based upon published literature, whereas the ACOP agenda involved the input of multiple stakeholders. Finally, the New Frontiers prioritized a research agenda across a number of surgical specialties, emergency medicine, and geriatric rehabilitation. Hospital medicine, however, was still early in its development and was not considered a unique specialty. Since that time, hospital medicine has matured into a unique specialty, with increased numbers of hospitalists,[3] increased research in hospital medicine,[17] and a separate recertification pathway for internal medicine licensure.[18] To date, there has not been a similar effort performed to direct geriatric research efforts for hospital medicine.
For researchers working in the field of hospital medicine, this list of topics has several implications. First, as hospitalists are commonly generalists, hospitalist researchers may be particularly well‐suited to study syndromes that cut across specialties. However, this does raise concerns about funding sources, as most National Institutes of Health institutes are disease‐focused. Funders that are not disease‐focused such as PCORI, National Institute on Aging, National Institute of Nursing Research, and Agency for Healthcare Research and Quality, and private foundations (Hartford, Robert Wood Johnson, and Commonwealth) may be more fruitful sources of funding for this work, but funding may be challenging. Nonetheless, the increased focus on patient‐centered work may increase funders' interest in such work. Second, the topics on this list would suggest that interventions will not be pharmacologic, but will focus on nonpharmacologic, behavioral, and social interventions. Similarly, outcomes of interest must expand beyond utilization metrics such as length of stay and mortality, to include functional status and symptom management, and goal‐concordant care. Therefore, research in geriatric acute care will necessarily be multidisciplinary.
Although these 10 high‐priority areas have been selected, this prioritized list is inherently limited by our methodology. First, our survey question was not focused on a disease state, and this wording may have resulted in the list favoring geriatric syndromes rather than common disease processes. Additionally, the resulting questions encompass large research areas and not specific questions about discrete interventions. Our results may also have been skewed by the types of engaged respondents who participated in the consultation, collating, and prioritization phases. In particular, we had a large response from geriatric medicine nurses, whereas some stakeholder groups provided no survey responses. Thus, these respondents were not representative of all possible stakeholders, nor were the survey respondents necessarily representative of each of their organizations. Nonetheless, the participants self‐identified as representative of diverse viewpoints that included patients, caregivers, and advocacy groups, with the majority of stakeholder organizations remaining engaged through the completion of the process. Thus, the general nature of this agenda helps us focus upon larger areas of importance, leaving researchers the flexibility to choose to narrow the focus on a specific research question that may include potential interventions and unique outcomes. Finally, our methodology may have inadvertently limited the number of patient and family caregiver voices in the process given our approach to large advocacy groups, our desire to be inclusive of healthcare professional organizations, and our survey methodology. Other methodologies may have reached more patients and caregivers, yet many healthcare professionals have served as family caregivers to frail elders requiring hospitalization and may have been in an ideal position to answer the survey.
In conclusion, several forces are shaping the future of acute inpatient care. These include the changing demographics of the hospitalized patient population, a rapid increase in the proportion of multimorbid hospitalized older adults, an inpatient workforce (hospitalists, generalists, and subspecialists) with potentially limited geriatrics training, and gaps in evidence‐based guidance to inform diagnostic and therapeutic decision making for acutely ill older patients. Training programs in hospital medicine should be aware of and could benefit from the resulting list of unanswered questions. Our findings also have implications for training to enrich education in geriatrics. Moreover, there is growing recognition that patients and other stakeholders deserve a greater voice in determining the direction of research. In addition to efforts to improve patient‐centeredness of research, these areas have been uniquely identified by stakeholders as important, and therefore are in line with newer priorities of PCORI. This project followed a road map resulting in a patient‐centered research agenda at the intersection of hospital medicine and geriatric medicine.[7] In creating this agenda, we relied heavily on the framework proposed by PCORI. We propose to pursue a dissemination and evaluation strategy for this research agenda as well as additional prioritization steps. We believe the adoption of this methodology will create a knowledge base that is rigorously derived and most relevant to the care of hospitalized older adults and their families. Its application will ultimately result in improved outcomes for hospitalized older adults.
Acknowledgements
The authors acknowledge Claudia Stahl, Society of Hospital Medicine; Cynthia Drake, University of Colorado; and the ACOP stakeholder organizations.
Disclosures: This work was supported by the Association of Specialty Professors/American Society of Internal Medicine and the John A. Hartford Foundation. Dr. Vasilevskis was supported by the National Institute on Aging of the National Institutes of Health under award number K23AG040157 and the Veterans Affairs Clinical Research Center of Excellence, and the Geriatric Research, Education and Clinical Center (GRECC). Dr. Vasilevskis' institution receives grant funding for an aspect of submitted work. Dr. Meltzer is a PCORI Methodology Committee member. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans' Affairs. The authors report no conflicts of interest.
- , , , , . National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report. 2010(29):1–20, 24.
- Centers for Medicare 2012. Available at: http://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/Chronic‐Conditions/Downloads/2012Chartbook.pdf. Accessed December 12, 2014.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , . A revelation of numbers: will America's eldercare workforce be ready to care for an aging America? Generations. 2010;34(4):11–19.
- Patient‐Centered Outcomes Research Institute Methodology Committee. The PCORI methodology report. Available at: http://www.pcori.org/assets/2013/11/PCORI‐Methodology‐Report.pdf. Published November 2013. Accessed December 19, 2013.
- The James Lind Alliance. JLA method. Available at: http://www.lindalliance.org/JLA_Method.asp. Accessed December 19, 2013.
- , , , , . Road map to a patient‐centered research agenda at the intersection of hospital medicine and geriatric medicine. J Gen Intern Med. 2014;29(6):926–931.
- Patient‐Centered Outcomes Research Institute. About us. Available at: http://www.pcori.org/about‐us. Accessed February 23, 2015.
- , , . New Frontiers of Geriatrics Research: An Agenda for Surgical and Related Medical Specialties. New York, NY: American Geriatrics Society; 2004.
- , , , . Linking the NIH strategic plan to the research agenda for social workers in health and aging. J Gerontol Soc Work. 2010;53(1):77–93.
- , . Assessing the capacity to make everyday decisions: a guide for clinicians and an agenda for future research. Am J Geriatr Psychiatry. 2007;15(2):101–111.
- , , , et al. Practitioners' views on elder mistreatment research priorities: recommendations from a Research‐to‐Practice Consensus conference. J Elder Abuse Negl. 2011;23(2):115–126.
- , . The intersection between geriatrics and palliative care: a call for a new research agenda. J Am Geriatr Soc. 2005;53(9):1593–1598.
- . The cancer aging interface: a research agenda. J Clin Oncol. 2007;25(14):1945–1948.
- , , , . Clinical care of persons with dementia in the emergency department: a review of the literature and agenda for research. J Am Geriatr Soc. 2012;60(9):1742–1748.
- , . A group process model for problem identification and program planning. J Appl Behav Sci. 1971;7(4):466–492.
- , , , , . Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148–154.
- . ABFM and ABIM to jointly participate in recognition of focused practice (rfp) in hospital medicine pilot approved by abms. Ann Fam Med. 2010;8(1):87.
- , , , . Medical decision‐making for older adults without family. J Am Geriatr Soc. 2012;60(11):2144–2150.
- , , , , , . Promoting advance directives among elderly primary care patients. J Gen Intern Med. 2004;19(9):944–951.
- , , . Advance directive completion by elderly Americans: a decade of change. J Am Geriatr Soc. 2014;62(4):706–710.
- , . Care transitions by older adults from nursing homes to hospitals: implications for long‐term care practice, geriatrics education, and research. J Am Med Dir Assoc. 2010;11(4):231–238.
- , , , . Decisions to hospitalize nursing home residents dying with advanced dementia. J Am Geriatr Soc. 2005;53(8):1396–1401.
- , , , , , . A comparison of methods to communicate treatment preferences in nursing facilities: traditional practices versus the physician orders for life‐sustaining treatment program. J Am Geriatr Soc. 2010;58(7):1241–1248.
- , , , , . Interventions to improve transitional care between nursing homes and hospitals: a systematic review. J Am Geriatr Soc. 2010;58(4):777–782.
- , , . Opening end‐of‐life discussions: how to introduce Voicing My CHOiCES, an advance care planning guide for adolescents and young adults [published online ahead of print March 13, 2014]. Palliat Support Care. doi: 10.1017/S1478951514000054.
- , , , . The revolving door of rehospitalization from skilled nursing facilities. Health Aff (Millwood). 2010;29(1):57–64.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Predictors of rehospitalization among elderly patients admitted to a rehabilitation hospital: the role of polypharmacy, functional status, and length of stay. J Am Med Dir Assoc. 2013;14(10):761–767.
- , , , et al. Mobility after hospital discharge as a marker for 30‐day readmission. J Gerontol A Biol Sci Med Sci. 2013;68(7):805–810.
- , , , , . The association between the quality of inpatient care and early readmission: a meta‐analysis of the evidence. Med Care. 1997;35(10):1044–1059.
- , , , , , . Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. May 2014;9(5):277–282.
- , , , , , . A prospective cohort study of geriatric syndromes among older medical patients admitted to acute care hospitals. J Am Geriatr Soc. 2011;59(11):2001–2008.
- , , , et al. Hospital discharge instructions: comprehension and compliance among older adults. J Gen Intern Med. 2014;29(11):1491–1498.
- , , , et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385–391.
- , , , et al. Communication and information deficits in patients discharged to rehabilitation facilities: an evaluation of five acute care hospitals. J Hosp Med. 2009;4(8):E28–E33.
- , , , , , . The consequences of poor communication during transitions from hospital to skilled nursing facility: a qualitative study. J Am Geriatr Soc. 2013;61(7):1095–1102.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102–109.
- , , , et al. Low‐cost transitional care with nurse managers making mostly phone contact with patients cut rehospitalization at a VA hospital. Health Aff (Millwood). 2012;31(12):2659–2668.
- , , , et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160(11):774–784.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , , , . Hospital‐initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):433–440.
- , , , . Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26(3):277–287.
- , , , , . Does delirium contribute to poor hospital outcomes? A three‐site epidemiologic study. J Gen Intern Med. 1998;13(4):234–242.
- , , , . Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33(1):66–73.
- , , , et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM‐ICU). JAMA. 2001;286(21):2703–2710.
- , , , et al. Impact and recognition of cognitive impairment among hospitalized elders. J Hosp Med. 2010;5(2):69–75.
- , , , et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900.
- , , . Long‐term cognitive impairment after critical illness. N Engl J Med. 2014;370(2):185–186.
- , , , et al. Delirium in the ICU and subsequent long‐term disability among survivors of mechanical ventilation. Crit Care Med. 2014;42(2):369–377.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304(4):443–451.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762.
- , , , , , . Days of delirium are associated with 1‐year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180(11):1092–1097.
- , . In‐facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):375–380.
- , , , et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489–499.
- , , , et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653.
- , , , , , . Protocolized intensive care unit management of analgesia, sedation, and delirium improves analgesia and subsyndromal delirium rates. Anesth Analg. 2010;111(2):451–463.
- , . A systematic review of the prevalence, associations and outcomes of dementia in older general hospital inpatients. Int Psychogeriatr. 2011;23(3):344–355.
- , , , . Cognitive impairment is undetected in medical inpatients: a study of mortality and recognition amongst healthcare professionals. BMC Geriatr. 2012;12:47.
- , , . How can we keep patients with dementia safe in our acute hospitals? A review of challenges and solutions. J R Soc Med. 2013;106(9):355–361.
- , , . Screening for dementia in general hospital inpatients: a systematic review and meta‐analysis of available instruments. Age Ageing. 2013;42(6):689–695.
- , , , et al. Tools to detect delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2012;60(11):2005–2013.
- , , , , , . Functional analysis‐based interventions for challenging behaviour in dementia. Cochrane Database Syst Rev. 2012;2:CD006929.
- , , , . Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012;2:CD005562.
- , , , et al. The prevalence and correlates of major and minor depression in older medical inpatients. J Am Geriatr Soc. 2005;53(8):1344–1353.
- , , , , , . Major depressive disorder in hospitalized medically ill patients: an examination of young and elderly male veterans. J Am Geriatr Soc. 1991;39(9):881–890.
- , , , et al. Depression, post‐traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN‐ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2(5):369–379.
- , , , et al. Depressive symptoms after hospitalization in older adults: function and mortality outcomes. J Am Geriatr Soc. 2012;60(12):2254–2262.
- , , , , . 12‐month cognitive outcomes of major and minor depression in older medical patients. Am J Geriatr Psychiatry. 2008;16(9):742–751.
- , , , et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256–262.
- , , , , , . Dose‐response relationship between depressive symptoms and hospital readmission. J Hosp Med. 2014;9(6):358–364.
- , , , , , . Depressive symptoms and 3‐year mortality in older hospitalized medical patients. Ann Intern Med. 1999;130(7):563–569.
- , , , et al. Support for the vascular depression hypothesis in late‐life depression: results of a 2‐site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67(3):277–285.
- , , , , , . Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58(3):204–210.
- , , , , . Recognition of depression in older medical inpatients. J Gen Intern Med. 2007;22(5):559–564.
- , , , , , . A collaborative care depression management program for cardiac inpatients: depression characteristics and in‐hospital outcomes. Psychosomatics. 2011;52(1):26–33.
- , , , , , . Impact of a depression care management program for hospitalized cardiac patients. Circ Cardiovasc Qual Outcomes. 2011;4(2):198–205.
- , , , et al. Potentially inappropriate medication use in hospitalized elders. J Hosp Med. 2008;3(2):91–102.
- , , , , , . Prevalence, incidence and nature of prescribing errors in hospital inpatients: a systematic review. Drug Saf. 2009;32(5):379–389.
- , . Minimizing adverse drug events in older patients. Am Fam Physician. 2007;76(12):1837–1844.
- , , , , . STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72–83.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616–631.
- , , , . Preventing potentially inappropriate medication use in hospitalized older patients with a computerized provider order entry warning system. Arch Intern Med. 2010;170(15):1331–1336.
- . Hazards of Hospitalization of the Elderly. Ann Intern Med. 1993;118(3):219–223.
- . Improving health care for older persons. Ann Intern Med. 2003;139(5 part 2):421–424.
- , , , , , . Successful models of comprehensive care for older adults with chronic conditions: evidence for the Institute of Medicine's "retooling for an aging America" report. J Am Geriatr Soc. 2009;57(12):2328–2337.
- , , , et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56(12):2171–2179.
- , , , . Changes in functional status and the risks of subsequent nursing home placement and death. J Gerontol. 1993;48(3):S94–S101.
- , , . Risk factors for nursing home placement in a population‐based dementia cohort. J Am Geriatr Soc. 2000;48(5):519–525.
- , , , . The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660–1665.
- , , , , . A systematic review of predictors and screening instruments to identify older hospitalized patients at risk for functional decline. J Clin Nurs. 2007;16(1):46–57.
- . Immobility and falls. Clin Geriatr Med. 1998;14(4):699–726.
- , , . Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52(8):1263–1270.
- , , . Impact of a nurse‐driven mobility protocol on functional decline in hospitalized older adults. J Nurs Care Qual. 2009;24(4):325–331.
- , . Impact of early mobilization protocol on the medical‐surgical inpatient population: an integrated review of literature. Clin Nurse Spec. 2012;26(2):87–94.
- , , , . The aging population and its impact on the surgery workforce. Ann Surg. 2003;238(2):170–177.
- . Perioperative care of the elderly patient: an update. Cleve Clin J Med. 2009;76(suppl 4):S16–S21.
- , , . Frailty in the older surgical patient: a review. Age Ageing. 2012;41(2):142–147.
- . The assessment and management of peri‐operative pain in older adults. Anaesthesia. 2014;69(suppl 1):54–60.
- , . National Research Strategies: what outcomes are important in peri‐operative elderly care? Anaesthesia. 2014;69(suppl 1):61–69.
- , . 2002 National Hospital Discharge Survey. Adv Data. 2004;342:1–30.
- , . Hospitalization in the United States, 2002. Rockville, MD: Agency for Healthcare Research and Quality; 2005.
- , , , et al. The Curriculum for the Hospitalized Aging Medical Patient program: a collaborative faculty development program for hospitalists, general internists, and geriatricians. J Hosp Med. 2008;3(5):384–393.
- , . Advancement of geriatrics education. J Hosp Med. 2011;6(6):370.
- , , , , , . Advancing geriatrics education: an efficient faculty development program for academic hospitalists increases geriatric teaching. J Hosp Med. 2010;5(9):541–546.
Older adults with high levels of medical complexity occupy an increasing fraction of beds in acute‐care hospitals in the United States.[1, 2] By 2007, patients age 65 years and older accounted for nearly half of adult inpatient days of care.[1] These patients are commonly cared for by hospitalists who number more than 40,000.[3] Although hospitalists are most often trained in internal medicine, they have typically received limited formal geriatrics training. Increasingly, access to experts in geriatric medicine is limited.[4] Further, hospitalists and others who practice in acute care are limited by the lack of research to address the needs of the older adult population, specifically in the diagnosis and management of conditions encountered during acute illness.
To better support hospitalists in providing acute inpatient geriatric care, the Society of Hospital Medicine (SHM) partnered with the Association of Specialty Professors to develop a research agenda to bridge this gap. Using methodology from the James Lind Alliance (JLA) and the Patient Centered Outcomes Research Institute (PCORI), the SHM joined with older adult advocacy groups, professional societies of providers, and funders to create a geriatric‐focused acute‐care research agenda, highlighting 10 key research questions.[5, 6, 7] The goal of this approach was to produce and promote high integrity, evidence‐based information that comes from research guided by patients, caregivers, and the broader healthcare community.[8] In this article, we describe the methodology and results of this agenda‐setting process, referred to as the Acute Care of Older Patients (ACOP) Priority Setting Partnership.
METHODS
Overview
This project focused on topic generation, the first step in the PCORI framework for identification and prioritization of research areas.[5] We employed a specific and defined methodology to elicit and prioritize potential research topics incorporating input from representatives of older patients, family caregivers, and healthcare providers.[6]
To elicit this input, we chose a collaborative and consultative approach to stakeholder engagement, drawing heavily from the published work of the JLA, an initiative promoting patient‐clinician partnerships in health research developed in the United Kingdom.[6] We previously described the approach elsewhere.[7]
The ACOP process for determining the research agenda consisted of 4 steps: (1) convene, (2) consult, (3) collate, and (4) prioritize.[6] Through these steps, detailed below, we were able to obtain input from a broad group of stakeholders and engage the stakeholders in a process of reducing and refining our research questions.
Convene
The steering committee (the article's authors) convened a stakeholder partnership group that included stakeholders representing patients and caregivers, advocacy organizations for the elderly, organizations that address diseases and conditions common among hospitalized older patients, provider professional societies (eg, hospitalists, subspecialists, and nurses and social workers), payers, and funders. Patient, caregiver, and advocacy organizations were identified based on their engagement in aging and health policy advocacy by SHM staff and 1 author who had completed a Health and Aging Policy Fellowship (H.L.W.).
The steering committee issued e‐mail invitations to stakeholder organizations, making initial inquiries through professional staff and relevant committee chairs. Second inquiries were made via e‐mail to each organization's volunteer leadership. We developed a webinar that outlined the overall research agenda setting process and distributed the webinar to all stakeholders. The stakeholder organizations were asked to commit to (1) surveying their memberships and (2) participating actively in prioritization by e‐mail and at a 1‐day meeting in Washington DC.
Consult
Each stakeholder organization conducted a survey of its membership via an Internet‐based survey in the summer of 2013 (see Supporting Information, Appendix A, in the online version of this article). Stakeholder organizations were asked to provide up to 75 survey responses each. Though a standard survey was used, the steering committee was not prescriptive in the methodology of survey distribution to accommodate the structure and communication methods of the individual stakeholder organizations. Survey respondents were asked to identify up to 5 unanswered questions relevant to the acute care of older persons and also provide demographic information.
Collate
In the collating process, we clarified and categorized the unanswered questions submitted in the individual surveys. Each question was initially reviewed by a member of the steering committee, using explicit criteria (see Supporting Information, Appendix B, in the online version of this article). Questions that did not meet all 4 criteria were removed. For questions that met all criteria, we clarified language, combined similar questions, and categorized each question. Categories were created in a grounded process, in which individual reviewers assigned categories based on the content of the questions. Each question could be assigned to up to 2 categories. Each question was then reviewed by a second member of the steering committee using the same 4 criteria. As part of this review, similar questions were consolidated, and when possible, questions were rewritten in a standard format.[6]
Finally, the steering committee reviewed previously published research agendas looking for additional relevant unanswered questions, specifically the New Frontiers Research Agenda created by the American Geriatrics Society in conjunction with participating subspecialty societies,[9] the Cochrane Library, and other systematic reviews identified in the literature via PubMed search.[10, 11, 12, 13, 14, 15]
Prioritize
The resulting list of unanswered questions was prioritized in 2 phases. First, the list was e‐mailed to all stakeholder organizations. The organizations were asked to vote on their top 10 priorities from this list using an online ballot, assigning 10 points to their highest priority down to 1 point for their lowest priority. In so doing, they were asked to consider explicit criteria (see Supporting Information, Appendix B, in the online version of this article). Each organization had only 1 ballot and could arrive at their top 10 list in any manner they wished. The balloting from this phase was used to develop a list of unanswered questions for the second round of in‐person prioritization. Each priority's scores were totaled across all voting organizations. The 29 priorities with the highest point totals were brought to the final prioritization round because of a natural cut point at priority number 29, rather than number 30.
For the final prioritization round, the steering committee facilitated an in‐person meeting in Washington, DC in October 2013 using nominal group technique (NGT) methodologies to arrive at consensus.[16] During this process stakeholders were asked to consider additional criteria (see Supporting Information, Appendix B, in the online version of this article).
RESULTS
Table 1 lists the organizations who engaged in 1 or more parts of the topic generation process. Eighteen stakeholder organizations agreed to participate in the convening process. Ten organizations did not respond to our solicitation and 1 declined to participate.
| Organization (N=18) | Consultation % of Survey Responses (N=580) | Prioritization Round 1 | Prioritization Round 2 |
|---|---|---|---|
| Alzheimer's Association | 7.0% | Yes | Yes |
| American Academy of Neurology | 3.4% | Yes | Yes |
| American Association of Retired Persons | 0.8% | No | No |
| American College of Cardiology | 11.4% | Yes | Yes |
| American College of Emergency Physicians | 1.3% | No | No |
| American College of Surgeons | 1.0% | Yes | Yes |
| American Geriatrics Society | 7.6% | Yes | Yes |
| American Hospital Association | 1.7% | Yes | No |
| Centers for Medicare & Medicaid Services | 0.8% | Yes | Yes |
| Gerontological Society of America | 18.9% | Yes | Yes |
| National Alliance for Caregiving | 1.0% | Yes | Yes |
| National Association of Social Workers | 5.9% | Yes | Yes |
| National Coalition for Healthcare | 0.6% | No | No |
| National Institute on Aging | 2.1% | Yes | Yes |
| National Partnership for Women and Families | 0.0% | Yes | Yes |
| Nursing Improving Care for Healthsystem Elders | 28.6% | Yes | No |
| Society of Critical Care Medicine | 12.0% | Yes | Yes |
| Society of Hospital Medicine | 4.6% | Yes | Yes |
Seventeen stakeholder organizations obtained survey responses from a total of 580 individuals (range, 3150 per organization), who were asked to identify important unanswered questions in the acute care of older persons. Survey respondents were typically female (77%), white (85%), aged 45 to 65 years (65%), and identified themselves as health professionals (90%). Twenty‐six percent of respondents also identified as patients or family caregivers. Their surveys included 1299 individual questions.
Figure 1 summarizes our collation and prioritization process and reports the numbers of questions resulting at each stage. Nine hundred nineteen questions were removed during the first review conducted by steering committee members, and 31 question categories were identified. An additional 305 questions were removed in the second review, with 75 questions remaining. As the final step of the collating process, literature review identified 39 relevant questions not already suggested or moved forward through our consultation and collation process. These questions were added to the list of unanswered questions.

In the first round of prioritization, this list of 114 questions was emailed to each stakeholder organization (Table 1). After the stakeholder voting process was completed, 29 unanswered questions remained (see Supporting Information, Appendix C, in the online version of this article). These questions were refined and prioritized in the in‐person meeting to create the final list of 10 questions. The stakeholders present in the meeting represented 13 organizations (Table 1). Using the NGT with several rounds of small group breakouts and large group deliberation, 9 of the top 10 questions were selected from the list of 29. One additional highly relevant question that had been removed earlier in the collation process regarding workforce was added back by the stakeholder group.
This prioritized research agenda appears in Table 2 and below, organized alphabetically by topic.
- Advanced care planning: What approaches for determining and communicating goals of care across and within healthcare settings are most effective in promoting goal‐concordant care for hospitalized older patients?
- Care transitions: What is the comparative effectiveness of transitional care models on patient‐centered outcomes for hospitalized older adults?
- Delirium: What practices are most effective for consistent recognition, prevention, and treatment of delirium subtypes among hospitalized older adults?
- Dementia: Does universal assessment of hospitalized older adults for cognitive impairment (eg, at presentation and/or discharge) lead to more appropriate application of geriatric care principles and improve patient‐centered outcomes?
- Depression: Does identifying depressive symptoms during a hospital stay and initiating a therapeutic plan prior to discharge improve patient‐centered and/or disease‐specific outcomes?
- Medications: What systems interventions improve medication management for older adults (ie, appropriateness of medication choices and dosing, compliance, cost) in the hospital and postacute care?
- Models of care: For which populations of hospitalized older adults does systematic implementation of geriatric care principles/processes improve patient‐centered outcomes?
- Physical function: What is the comparative effectiveness of interventions that promote in‐hospital mobility, improve and preserve physical function, and reduce falls among older hospitalized patients?
- Surgery: What perioperative strategies can be used to optimize care processes and improve outcomes in older surgical patients?
- Training: What is the most effective approach to training hospital‐based providers in geriatric and palliative care competencies?
| Topic | Scope of Problem | What Is known | Unanswered Question | Proposed Dimensions |
|---|---|---|---|---|
| ||||
| Advanced‐care planning | Older persons who lack decision‐making capacity often do not have surrogates or clear goals of care documented.[19] Advanced‐care directives are associated with an increase in patient autonomy and empowerment, and although 15% to 25% of adults completed the documentation in 2004,[20] a recent study found completion rates have increased to 72%.[21] | Nursing home residents with advanced directives are less likely to be hospitalized.[22, 23] Advanced directive tools, such as POLST, work to translate patient preferences to medical order.[24] standardized patient transfer tools may help to improve transitions between nursing homes and hospitals.[25] However, advanced care planning fails to integrate into courses of care if providers are unwilling or unskilled in using advanced care documentation.[26] | What approaches for determining and communicating goals of care across and within healthcare settings are most effective in promoting goal‐concordant care for hospitalized older patients? | Potential interventions: |
| Decision aids | ||||
| Standard interdisciplinary advanced care planning approach | ||||
| Patient advocates | ||||
| Potential outcomes might include: | ||||
| Completion of advanced directives and healthcare power of attorney | ||||
| Patient‐centered outcomesa | ||||
| Care transitions | Hospital readmission from home and skilled nursing facilities occurs within 30 days in up to a quarter of patients.[27, 28] The discharge of complex older hospitalized patients is fraught with challenges. The quality of the hospital discharge process can influence outcomes for vulnerable older patients.[29, 30, 31, 32] Studies measuring the quality of hospital discharge frequently find deficits in documentation of assessment of geriatric syndromes,[33] poor patient/caregiver understanding,[34, 35] and poor communication and follow‐up with postacute providers.[35, 36, 37, 38] | As many as 10 separate domains may influence the success of a discharge.[39] There is limited evidence, regarding quality‐of‐care transitions for hospitalized older patients. The Coordinated‐Transitional Care Program found that follow‐up with telecommunication decreased readmission rates and improved transitional care for a high‐risk condition veteran population.[40] There is modest evidence for single interventions,[41] whereas the most effective hospital‐to‐community care interventions address multiple processes in nongeriatric populations.[39, 42, 43] | What is the comparative effectiveness of the transitional care models on patient‐centered outcomes for hospitalized older adults? | Possible models: |
| Established vs novel care‐transition models | ||||
| Disease‐specific vs general approaches | ||||
| Accountable care models | ||||
| Caregiver and family engagement | ||||
| Community engagement | ||||
| Populations of interest: | ||||
| Patients with dementia | ||||
| Patients with multimorbidity | ||||
| Patients with geriatric syndromes | ||||
| Patients with psychiatric disease | ||||
| Racially and ethnically diverse patients | ||||
| Outcomes: | ||||
| Readmission | ||||
| Other adverse events | ||||
| Cost and healthcare utilization | ||||
| Patient‐centered outcomesa | ||||
| Delirium | Among older inpatients, the prevalence of delirium varies with severity of illness. Among general medical patients, in‐hospital prevalence ranges from 10% to 25 %.[44, 45] In the ICU, prevalence estimates are higher, ranging from 25% to as high as 80%.[46, 47] Delirium independently predicts increased length of stay,[48, 49] long‐term cognitive impairment,[50, 51] functional decline,[51] institutionalization,[52] and short‐ and long‐term mortality.[52, 53, 54] | Multicomponent strategies have been shown to be effective in preventing delirium. A systematic review of 19 such interventions identified the most commonly included such as[55]: early mobilization, nutrition supplements, medication review, pain management, sleep enhancement, vision/hearing protocols, and specialized geriatric care. Studies have included general medical patients, postoperative patients, and patients in the ICU. The majority of these studies found reductions in either delirium incidence (including postoperative), delirium prevalence, or delirium duration. Although medications have not been effective in treating delirium in general medical patients,[48] the choice and dose of sedative agents has been shown to impact delirium in the ICU.[56, 57, 58] | What practices are most effective for consistent recognition, prevention, and treatment of delirium subtypes (hypoactive, hyperactive, and mixed) among hospitalized older adults? | Outcomes to examine: |
| Delirium incidence (including postoperative) | ||||
| Delirium duration | ||||
| Delirium‐/coma‐free days | ||||
| Delirium prevalence at discharge | ||||
| Subsyndromal delirium | ||||
| Potential prevention and treatment modalities: | ||||
| Family education or psychosocial interventions | ||||
| Pharmacologic interventions | ||||
| Environmental modifications | ||||
| Possible areas of focus: | ||||
| Special populations | ||||
| Patients with varying stages of dementia | ||||
| Patients with multimorbidity | ||||
| Patients with geriatric syndromes | ||||
| Observation patients | ||||
| Diverse settings | ||||
| Emergency department | ||||
| Perioperative | ||||
| Skilled nursing/rehab/long‐term acute‐care facilities | ||||
| Dementia | 13% to 63% of older persons in the hospital have dementia.[59] Dementia is often unrecognized among hospitalized patients.[60] The presence of dementia is associated with a more rapid functional decline during admission and delayed hospital discharge.[59] Patients with dementia require more nursing hours, and are more likely to have complications[61] or die in care homes rather than in their preferred site.[59] | Several tools have been validated to screen for dementia in the hospital setting.[62] Studies have assessed approaches to diagnosing delirium in hospitalized patients with dementia.[63] Cognitive and functional stimulation interventions may have a positive impact on reducing behavioral issues.[64, 65] | Does universal assessment of hospitalized older adults for cognitive impairment (eg, at presentation and/or discharge) lead to more appropriate application of geriatric care principles and improve patient centered outcomes? | Potential interventions: |
| Dementia or delirium care | ||||
| Patient/family communication and engagement strategies | ||||
| Maintenance/recovery of independent functional status | ||||
| Potential outcomes: | ||||
| Patient‐centered outcomesa | ||||
| Length of stay, cost, and healthcare utilization (including palliative care) | ||||
| Immediate invasive vs early conservative treatments pursued | ||||
| Depression | Depression is a common geriatric syndrome among acutely ill older patients, occurring in up to 45% of patients.[66, 67] Rates of depression are similar among patients discharged following a critical illness, with somatic, rather than cognitive‐affective complaints being the most prevalent.[68] Depression among inpatients or immediately following hospitalization independently predicts worse functional outcomes,[69] cognitive decline,[70] hospital readmission,[71, 72] and long‐term mortality.[69, 73] Finally, geriatric patients are known to respond differently to medical treatment.[74, 75] | Although highly prevalent, depression is poorly recognized and managed in the inpatient setting. Depression is recognized in only 50% of patients, with previously undiagnosed or untreated depression being at highest risk for being missed.[76] The role of treatment of depression in the inpatient setting is poorly understood, particularly for those with newly recognized depression or depressive symptoms. Some novel collaborative care and telephone outreach programs have led to increases in depression treatment in patients with specific medical and surgical conditions, resulting in early promising mental health and comorbid outcomes.[77, 78] The efficacy of such programs for older patients is unknown. | Does identifying depressive symptoms during a hospital stay and initiating a therapeutic plan prior to discharge improve patient‐centered and/or disease‐specific outcomes? | Possible areas of focus: |
| Comprehensive geriatric and psychosocial assessment; | ||||
| Inpatient vs outpatient initiation of pharmacological therapy | ||||
| Integration of confusion assessment method into therapeutic approaches | ||||
| Linkages with outpatient mental health resources | ||||
| Medications | Medication exposure, particularly potentially inappropriate medications, is common in hospitalized elders.[79] Medication errorsof dosage, type, and discrepancy between what a patient takes at home and what is known to his/her prescribing physicianare common and adversely affects patient safety.[80] Geriatric populations are disproportionately affected, especially those taking more than 5 prescription medications per day.[81] | Numerous strategies including electronic alerts, screening protocols, and potentially inappropriate medication lists (Beers list, STOPP) exist, though the optimal strategies to limit the use of potentially inappropriate medications is not yet known.[82, 83, 84] | What systems interventions improve medication management for older adults (ie, appropriateness of medication choices and dosing, compliance, cost) in hospital and post‐acute care? | Possible areas of focus: |
| Use of healthcare information technology | ||||
| Communication across sites of care | ||||
| Reducing medication‐related adverse events | ||||
| Engagement of family caregivers | ||||
| Patient‐centered strategies to simplify regimens | ||||
| Models of care | Hospitalization marks a time of high risk for older patients. Up to half die during hospitalization or within the year following the hospitalization. There is high risk of nosocomial events, and more than a third experience a decline in health resulting in longer hospitalizations and/or placement in extended‐care facilities.[73, 85, 86] | Comprehensive inpatient care for older adults (acute care for elders units, geriatric evaluation and management units, geriatric consultation services) were studied in 2 meta‐analyses, 5 RCTs, and 1 quasiexperimental study and summarized in a systematic review.[87] The studies reported improved quality of care (1 of 1 article), quality of life (3 of 4), functional autonomy (5 of 6), survival (3 of 6), and equal or lower healthcare utilization (7 of 8). | For which populations of hospitalized older adults does systematic implementation of geriatric care principles/processes improve patient‐centered outcomes? | Potential populations: |
| Patients of the emergency department, critical care, perioperative, and targeted medical/surgical units | ||||
| Examples of care principles: | ||||
| Geriatric assessment, early mobility, medication management, delirium prevention, advanced‐care planning, risk‐factor modification, caregiver engagement | ||||
| Potential outcomes: | ||||
| Patient‐centered outcomesa | ||||
| Cost | ||||
| Physical function | Half of older patients will lose functional capacity during hospitalization.[88] Loss of physical function, particularly of lower extremities, is a risk factor for nursing home placement.[89, 90] Older hospitalized patients spend the majority (up to 80%) of their time lying in bed, even when they are capable of walking independently.[91] | Loss of independences with ADL capabilities is associated with longer hospital stays, higher readmission rates, and higher mortality risk.[92] Excessive time in bed during a hospital stay is also associated with falls.[93] Often, hospital nursing protocols and physician orders increase in‐hospital immobility in patients.[91, 94] However, nursing‐driven mobility protocols can improve functional outcomes of older hospitalized patients.[95, 96] | What is the comparative effectiveness of interventions that promote in‐hospital mobility, improve and preserve physical function, and reduce falls among older hospitalized patients? | Potential interventions: |
| Intensive physical therapy | ||||
| Incidental functional training | ||||
| Restraint reduction | ||||
| Medication management | ||||
| Potential outcomes: | ||||
| Discharge location | ||||
| Delirium, pressure ulcers, and falls | ||||
| Surgery | An increasing number of persons over age 65 years are undergoing surgical procedures.[97] These persons are at increased risk for developing delirium/cogitative dysfunction,[98] loss of functional status,[99] and exacerbations of chronic illness.[97] Additionally, pain management may be harder to address in this population.[100] Current outcomes may not reflect the clinical needs of elder surgical patients.[101] | Tailored drug selection and nursing protocols may prevent delirium.[98] Postoperative cognitive dysfunction may require weeks for resolution. Identifying frail patients preoperatively may lead to more appropriate risk stratification and improved surgical outcomes.[99] Pain management strategies focused on mitigating cognitive impact and other effects may also be beneficial.[100] Development of risk‐adjustment tools specific to older populations, as well as measures of frailty and patient‐centered care, have been proposed.[101] | What perioperative strategies can be used to optimize care processes and improve outcomes in older surgical patients? | Potential strategies: |
| Preoperative risk assessment and optimization for frail or multimorbid older patients | ||||
| Perioperative management protocols for frail or multimorbid older patients | ||||
| Potential outcomes: | ||||
| Postoperative patient centered outcomesa | ||||
| Perioperative cost, healthcare utilization | ||||
| Training | Adults over age 65 years comprise 13.2 % of the US population, but account for >30% of hospital discharges and 50% of hospital days.[86, 102, 103] By 2030, there will only be 1 geriatrician for every 3798 Americans >75 years.[4] Between 1997 and 2006, the odds that a hospitalist would treat a hospitalized Medicare patient rose 29% per year.[3] | Train the trainer programs for physicians include the CHAMP, the AGESP, and the PAGE. Education for nurses include the NICHE. Outcomes include improved self‐confidence, attitudes, teaching skills, and geriatric care environment.[104, 105, 106] | What is the most effective approach to training hospital‐based providers in geriatric and palliative care competencies? | Potential interventions: |
| Mentored implementation | ||||
| Train the trainer | ||||
| Technical support | ||||
Table 2 also contains a capsule summary of the scope of the problem addressed by each research priority, a capsule summary of related work in the content area (what is known) not intended as a systematic review, and proposed dimensions or subquestions suggested by the stakeholders at the final prioritization meeting
DISCUSSION
Older hospitalized patients account for an increasing number and proportion of hospitalized patients,[1, 2] and hospitalists increasingly are responsible for inpatient care for this population.[3] The knowledge required for hospitalists to deliver optimal care and improve outcomes has not kept pace with the rapid growth of either hospitalists or hospitalized elders. Through a rigorous prioritization process, we identified 10 areas that deserve the highest priority in directing future research efforts to improve care for the older hospitalized patient. Assessment, prevention, and treatment of geriatric syndromes in the hospital account for almost half of the priority areas. Additional research is needed to improve advanced care planning, develop new care models, and develop training models for future hospitalists competent in geriatric and palliative care competencies.
A decade ago, the American Geriatric Society and the John A. Hartford Foundation embarked upon a research agenda aimed at improving the care of hospitalized elders cared for by specialists (ie, New Frontiers in Geriatrics Research: An Agenda for Surgical and Related Medical Specialties).[9] This effort differed in many important ways from the current priortization process. First, the New Frontiers agenda focused upon specific diseases, whereas the ACOP agenda addresses geriatric syndromes that cut across multiple diseases. Second, the New Frontiers agenda was made by researchers and based upon published literature, whereas the ACOP agenda involved the input of multiple stakeholders. Finally, the New Frontiers prioritized a research agenda across a number of surgical specialties, emergency medicine, and geriatric rehabilitation. Hospital medicine, however, was still early in its development and was not considered a unique specialty. Since that time, hospital medicine has matured into a unique specialty, with increased numbers of hospitalists,[3] increased research in hospital medicine,[17] and a separate recertification pathway for internal medicine licensure.[18] To date, there has not been a similar effort performed to direct geriatric research efforts for hospital medicine.
For researchers working in the field of hospital medicine, this list of topics has several implications. First, as hospitalists are commonly generalists, hospitalist researchers may be particularly well‐suited to study syndromes that cut across specialties. However, this does raise concerns about funding sources, as most National Institutes of Health institutes are disease‐focused. Funders that are not disease‐focused such as PCORI, National Institute on Aging, National Institute of Nursing Research, and Agency for Healthcare Research and Quality, and private foundations (Hartford, Robert Wood Johnson, and Commonwealth) may be more fruitful sources of funding for this work, but funding may be challenging. Nonetheless, the increased focus on patient‐centered work may increase funders' interest in such work. Second, the topics on this list would suggest that interventions will not be pharmacologic, but will focus on nonpharmacologic, behavioral, and social interventions. Similarly, outcomes of interest must expand beyond utilization metrics such as length of stay and mortality, to include functional status and symptom management, and goal‐concordant care. Therefore, research in geriatric acute care will necessarily be multidisciplinary.
Although these 10 high‐priority areas have been selected, this prioritized list is inherently limited by our methodology. First, our survey question was not focused on a disease state, and this wording may have resulted in the list favoring geriatric syndromes rather than common disease processes. Additionally, the resulting questions encompass large research areas and not specific questions about discrete interventions. Our results may also have been skewed by the types of engaged respondents who participated in the consultation, collating, and prioritization phases. In particular, we had a large response from geriatric medicine nurses, whereas some stakeholder groups provided no survey responses. Thus, these respondents were not representative of all possible stakeholders, nor were the survey respondents necessarily representative of each of their organizations. Nonetheless, the participants self‐identified as representative of diverse viewpoints that included patients, caregivers, and advocacy groups, with the majority of stakeholder organizations remaining engaged through the completion of the process. Thus, the general nature of this agenda helps us focus upon larger areas of importance, leaving researchers the flexibility to choose to narrow the focus on a specific research question that may include potential interventions and unique outcomes. Finally, our methodology may have inadvertently limited the number of patient and family caregiver voices in the process given our approach to large advocacy groups, our desire to be inclusive of healthcare professional organizations, and our survey methodology. Other methodologies may have reached more patients and caregivers, yet many healthcare professionals have served as family caregivers to frail elders requiring hospitalization and may have been in an ideal position to answer the survey.
In conclusion, several forces are shaping the future of acute inpatient care. These include the changing demographics of the hospitalized patient population, a rapid increase in the proportion of multimorbid hospitalized older adults, an inpatient workforce (hospitalists, generalists, and subspecialists) with potentially limited geriatrics training, and gaps in evidence‐based guidance to inform diagnostic and therapeutic decision making for acutely ill older patients. Training programs in hospital medicine should be aware of and could benefit from the resulting list of unanswered questions. Our findings also have implications for training to enrich education in geriatrics. Moreover, there is growing recognition that patients and other stakeholders deserve a greater voice in determining the direction of research. In addition to efforts to improve patient‐centeredness of research, these areas have been uniquely identified by stakeholders as important, and therefore are in line with newer priorities of PCORI. This project followed a road map resulting in a patient‐centered research agenda at the intersection of hospital medicine and geriatric medicine.[7] In creating this agenda, we relied heavily on the framework proposed by PCORI. We propose to pursue a dissemination and evaluation strategy for this research agenda as well as additional prioritization steps. We believe the adoption of this methodology will create a knowledge base that is rigorously derived and most relevant to the care of hospitalized older adults and their families. Its application will ultimately result in improved outcomes for hospitalized older adults.
Acknowledgements
The authors acknowledge Claudia Stahl, Society of Hospital Medicine; Cynthia Drake, University of Colorado; and the ACOP stakeholder organizations.
Disclosures: This work was supported by the Association of Specialty Professors/American Society of Internal Medicine and the John A. Hartford Foundation. Dr. Vasilevskis was supported by the National Institute on Aging of the National Institutes of Health under award number K23AG040157 and the Veterans Affairs Clinical Research Center of Excellence, and the Geriatric Research, Education and Clinical Center (GRECC). Dr. Vasilevskis' institution receives grant funding for an aspect of submitted work. Dr. Meltzer is a PCORI Methodology Committee member. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans' Affairs. The authors report no conflicts of interest.
Older adults with high levels of medical complexity occupy an increasing fraction of beds in acute‐care hospitals in the United States.[1, 2] By 2007, patients age 65 years and older accounted for nearly half of adult inpatient days of care.[1] These patients are commonly cared for by hospitalists who number more than 40,000.[3] Although hospitalists are most often trained in internal medicine, they have typically received limited formal geriatrics training. Increasingly, access to experts in geriatric medicine is limited.[4] Further, hospitalists and others who practice in acute care are limited by the lack of research to address the needs of the older adult population, specifically in the diagnosis and management of conditions encountered during acute illness.
To better support hospitalists in providing acute inpatient geriatric care, the Society of Hospital Medicine (SHM) partnered with the Association of Specialty Professors to develop a research agenda to bridge this gap. Using methodology from the James Lind Alliance (JLA) and the Patient Centered Outcomes Research Institute (PCORI), the SHM joined with older adult advocacy groups, professional societies of providers, and funders to create a geriatric‐focused acute‐care research agenda, highlighting 10 key research questions.[5, 6, 7] The goal of this approach was to produce and promote high integrity, evidence‐based information that comes from research guided by patients, caregivers, and the broader healthcare community.[8] In this article, we describe the methodology and results of this agenda‐setting process, referred to as the Acute Care of Older Patients (ACOP) Priority Setting Partnership.
METHODS
Overview
This project focused on topic generation, the first step in the PCORI framework for identification and prioritization of research areas.[5] We employed a specific and defined methodology to elicit and prioritize potential research topics incorporating input from representatives of older patients, family caregivers, and healthcare providers.[6]
To elicit this input, we chose a collaborative and consultative approach to stakeholder engagement, drawing heavily from the published work of the JLA, an initiative promoting patient‐clinician partnerships in health research developed in the United Kingdom.[6] We previously described the approach elsewhere.[7]
The ACOP process for determining the research agenda consisted of 4 steps: (1) convene, (2) consult, (3) collate, and (4) prioritize.[6] Through these steps, detailed below, we were able to obtain input from a broad group of stakeholders and engage the stakeholders in a process of reducing and refining our research questions.
Convene
The steering committee (the article's authors) convened a stakeholder partnership group that included stakeholders representing patients and caregivers, advocacy organizations for the elderly, organizations that address diseases and conditions common among hospitalized older patients, provider professional societies (eg, hospitalists, subspecialists, and nurses and social workers), payers, and funders. Patient, caregiver, and advocacy organizations were identified based on their engagement in aging and health policy advocacy by SHM staff and 1 author who had completed a Health and Aging Policy Fellowship (H.L.W.).
The steering committee issued e‐mail invitations to stakeholder organizations, making initial inquiries through professional staff and relevant committee chairs. Second inquiries were made via e‐mail to each organization's volunteer leadership. We developed a webinar that outlined the overall research agenda setting process and distributed the webinar to all stakeholders. The stakeholder organizations were asked to commit to (1) surveying their memberships and (2) participating actively in prioritization by e‐mail and at a 1‐day meeting in Washington DC.
Consult
Each stakeholder organization conducted a survey of its membership via an Internet‐based survey in the summer of 2013 (see Supporting Information, Appendix A, in the online version of this article). Stakeholder organizations were asked to provide up to 75 survey responses each. Though a standard survey was used, the steering committee was not prescriptive in the methodology of survey distribution to accommodate the structure and communication methods of the individual stakeholder organizations. Survey respondents were asked to identify up to 5 unanswered questions relevant to the acute care of older persons and also provide demographic information.
Collate
In the collating process, we clarified and categorized the unanswered questions submitted in the individual surveys. Each question was initially reviewed by a member of the steering committee, using explicit criteria (see Supporting Information, Appendix B, in the online version of this article). Questions that did not meet all 4 criteria were removed. For questions that met all criteria, we clarified language, combined similar questions, and categorized each question. Categories were created in a grounded process, in which individual reviewers assigned categories based on the content of the questions. Each question could be assigned to up to 2 categories. Each question was then reviewed by a second member of the steering committee using the same 4 criteria. As part of this review, similar questions were consolidated, and when possible, questions were rewritten in a standard format.[6]
Finally, the steering committee reviewed previously published research agendas looking for additional relevant unanswered questions, specifically the New Frontiers Research Agenda created by the American Geriatrics Society in conjunction with participating subspecialty societies,[9] the Cochrane Library, and other systematic reviews identified in the literature via PubMed search.[10, 11, 12, 13, 14, 15]
Prioritize
The resulting list of unanswered questions was prioritized in 2 phases. First, the list was e‐mailed to all stakeholder organizations. The organizations were asked to vote on their top 10 priorities from this list using an online ballot, assigning 10 points to their highest priority down to 1 point for their lowest priority. In so doing, they were asked to consider explicit criteria (see Supporting Information, Appendix B, in the online version of this article). Each organization had only 1 ballot and could arrive at their top 10 list in any manner they wished. The balloting from this phase was used to develop a list of unanswered questions for the second round of in‐person prioritization. Each priority's scores were totaled across all voting organizations. The 29 priorities with the highest point totals were brought to the final prioritization round because of a natural cut point at priority number 29, rather than number 30.
For the final prioritization round, the steering committee facilitated an in‐person meeting in Washington, DC in October 2013 using nominal group technique (NGT) methodologies to arrive at consensus.[16] During this process stakeholders were asked to consider additional criteria (see Supporting Information, Appendix B, in the online version of this article).
RESULTS
Table 1 lists the organizations who engaged in 1 or more parts of the topic generation process. Eighteen stakeholder organizations agreed to participate in the convening process. Ten organizations did not respond to our solicitation and 1 declined to participate.
| Organization (N=18) | Consultation % of Survey Responses (N=580) | Prioritization Round 1 | Prioritization Round 2 |
|---|---|---|---|
| Alzheimer's Association | 7.0% | Yes | Yes |
| American Academy of Neurology | 3.4% | Yes | Yes |
| American Association of Retired Persons | 0.8% | No | No |
| American College of Cardiology | 11.4% | Yes | Yes |
| American College of Emergency Physicians | 1.3% | No | No |
| American College of Surgeons | 1.0% | Yes | Yes |
| American Geriatrics Society | 7.6% | Yes | Yes |
| American Hospital Association | 1.7% | Yes | No |
| Centers for Medicare & Medicaid Services | 0.8% | Yes | Yes |
| Gerontological Society of America | 18.9% | Yes | Yes |
| National Alliance for Caregiving | 1.0% | Yes | Yes |
| National Association of Social Workers | 5.9% | Yes | Yes |
| National Coalition for Healthcare | 0.6% | No | No |
| National Institute on Aging | 2.1% | Yes | Yes |
| National Partnership for Women and Families | 0.0% | Yes | Yes |
| Nursing Improving Care for Healthsystem Elders | 28.6% | Yes | No |
| Society of Critical Care Medicine | 12.0% | Yes | Yes |
| Society of Hospital Medicine | 4.6% | Yes | Yes |
Seventeen stakeholder organizations obtained survey responses from a total of 580 individuals (range, 3150 per organization), who were asked to identify important unanswered questions in the acute care of older persons. Survey respondents were typically female (77%), white (85%), aged 45 to 65 years (65%), and identified themselves as health professionals (90%). Twenty‐six percent of respondents also identified as patients or family caregivers. Their surveys included 1299 individual questions.
Figure 1 summarizes our collation and prioritization process and reports the numbers of questions resulting at each stage. Nine hundred nineteen questions were removed during the first review conducted by steering committee members, and 31 question categories were identified. An additional 305 questions were removed in the second review, with 75 questions remaining. As the final step of the collating process, literature review identified 39 relevant questions not already suggested or moved forward through our consultation and collation process. These questions were added to the list of unanswered questions.

In the first round of prioritization, this list of 114 questions was emailed to each stakeholder organization (Table 1). After the stakeholder voting process was completed, 29 unanswered questions remained (see Supporting Information, Appendix C, in the online version of this article). These questions were refined and prioritized in the in‐person meeting to create the final list of 10 questions. The stakeholders present in the meeting represented 13 organizations (Table 1). Using the NGT with several rounds of small group breakouts and large group deliberation, 9 of the top 10 questions were selected from the list of 29. One additional highly relevant question that had been removed earlier in the collation process regarding workforce was added back by the stakeholder group.
This prioritized research agenda appears in Table 2 and below, organized alphabetically by topic.
- Advanced care planning: What approaches for determining and communicating goals of care across and within healthcare settings are most effective in promoting goal‐concordant care for hospitalized older patients?
- Care transitions: What is the comparative effectiveness of transitional care models on patient‐centered outcomes for hospitalized older adults?
- Delirium: What practices are most effective for consistent recognition, prevention, and treatment of delirium subtypes among hospitalized older adults?
- Dementia: Does universal assessment of hospitalized older adults for cognitive impairment (eg, at presentation and/or discharge) lead to more appropriate application of geriatric care principles and improve patient‐centered outcomes?
- Depression: Does identifying depressive symptoms during a hospital stay and initiating a therapeutic plan prior to discharge improve patient‐centered and/or disease‐specific outcomes?
- Medications: What systems interventions improve medication management for older adults (ie, appropriateness of medication choices and dosing, compliance, cost) in the hospital and postacute care?
- Models of care: For which populations of hospitalized older adults does systematic implementation of geriatric care principles/processes improve patient‐centered outcomes?
- Physical function: What is the comparative effectiveness of interventions that promote in‐hospital mobility, improve and preserve physical function, and reduce falls among older hospitalized patients?
- Surgery: What perioperative strategies can be used to optimize care processes and improve outcomes in older surgical patients?
- Training: What is the most effective approach to training hospital‐based providers in geriatric and palliative care competencies?
| Topic | Scope of Problem | What Is known | Unanswered Question | Proposed Dimensions |
|---|---|---|---|---|
| ||||
| Advanced‐care planning | Older persons who lack decision‐making capacity often do not have surrogates or clear goals of care documented.[19] Advanced‐care directives are associated with an increase in patient autonomy and empowerment, and although 15% to 25% of adults completed the documentation in 2004,[20] a recent study found completion rates have increased to 72%.[21] | Nursing home residents with advanced directives are less likely to be hospitalized.[22, 23] Advanced directive tools, such as POLST, work to translate patient preferences to medical order.[24] standardized patient transfer tools may help to improve transitions between nursing homes and hospitals.[25] However, advanced care planning fails to integrate into courses of care if providers are unwilling or unskilled in using advanced care documentation.[26] | What approaches for determining and communicating goals of care across and within healthcare settings are most effective in promoting goal‐concordant care for hospitalized older patients? | Potential interventions: |
| Decision aids | ||||
| Standard interdisciplinary advanced care planning approach | ||||
| Patient advocates | ||||
| Potential outcomes might include: | ||||
| Completion of advanced directives and healthcare power of attorney | ||||
| Patient‐centered outcomesa | ||||
| Care transitions | Hospital readmission from home and skilled nursing facilities occurs within 30 days in up to a quarter of patients.[27, 28] The discharge of complex older hospitalized patients is fraught with challenges. The quality of the hospital discharge process can influence outcomes for vulnerable older patients.[29, 30, 31, 32] Studies measuring the quality of hospital discharge frequently find deficits in documentation of assessment of geriatric syndromes,[33] poor patient/caregiver understanding,[34, 35] and poor communication and follow‐up with postacute providers.[35, 36, 37, 38] | As many as 10 separate domains may influence the success of a discharge.[39] There is limited evidence, regarding quality‐of‐care transitions for hospitalized older patients. The Coordinated‐Transitional Care Program found that follow‐up with telecommunication decreased readmission rates and improved transitional care for a high‐risk condition veteran population.[40] There is modest evidence for single interventions,[41] whereas the most effective hospital‐to‐community care interventions address multiple processes in nongeriatric populations.[39, 42, 43] | What is the comparative effectiveness of the transitional care models on patient‐centered outcomes for hospitalized older adults? | Possible models: |
| Established vs novel care‐transition models | ||||
| Disease‐specific vs general approaches | ||||
| Accountable care models | ||||
| Caregiver and family engagement | ||||
| Community engagement | ||||
| Populations of interest: | ||||
| Patients with dementia | ||||
| Patients with multimorbidity | ||||
| Patients with geriatric syndromes | ||||
| Patients with psychiatric disease | ||||
| Racially and ethnically diverse patients | ||||
| Outcomes: | ||||
| Readmission | ||||
| Other adverse events | ||||
| Cost and healthcare utilization | ||||
| Patient‐centered outcomesa | ||||
| Delirium | Among older inpatients, the prevalence of delirium varies with severity of illness. Among general medical patients, in‐hospital prevalence ranges from 10% to 25 %.[44, 45] In the ICU, prevalence estimates are higher, ranging from 25% to as high as 80%.[46, 47] Delirium independently predicts increased length of stay,[48, 49] long‐term cognitive impairment,[50, 51] functional decline,[51] institutionalization,[52] and short‐ and long‐term mortality.[52, 53, 54] | Multicomponent strategies have been shown to be effective in preventing delirium. A systematic review of 19 such interventions identified the most commonly included such as[55]: early mobilization, nutrition supplements, medication review, pain management, sleep enhancement, vision/hearing protocols, and specialized geriatric care. Studies have included general medical patients, postoperative patients, and patients in the ICU. The majority of these studies found reductions in either delirium incidence (including postoperative), delirium prevalence, or delirium duration. Although medications have not been effective in treating delirium in general medical patients,[48] the choice and dose of sedative agents has been shown to impact delirium in the ICU.[56, 57, 58] | What practices are most effective for consistent recognition, prevention, and treatment of delirium subtypes (hypoactive, hyperactive, and mixed) among hospitalized older adults? | Outcomes to examine: |
| Delirium incidence (including postoperative) | ||||
| Delirium duration | ||||
| Delirium‐/coma‐free days | ||||
| Delirium prevalence at discharge | ||||
| Subsyndromal delirium | ||||
| Potential prevention and treatment modalities: | ||||
| Family education or psychosocial interventions | ||||
| Pharmacologic interventions | ||||
| Environmental modifications | ||||
| Possible areas of focus: | ||||
| Special populations | ||||
| Patients with varying stages of dementia | ||||
| Patients with multimorbidity | ||||
| Patients with geriatric syndromes | ||||
| Observation patients | ||||
| Diverse settings | ||||
| Emergency department | ||||
| Perioperative | ||||
| Skilled nursing/rehab/long‐term acute‐care facilities | ||||
| Dementia | 13% to 63% of older persons in the hospital have dementia.[59] Dementia is often unrecognized among hospitalized patients.[60] The presence of dementia is associated with a more rapid functional decline during admission and delayed hospital discharge.[59] Patients with dementia require more nursing hours, and are more likely to have complications[61] or die in care homes rather than in their preferred site.[59] | Several tools have been validated to screen for dementia in the hospital setting.[62] Studies have assessed approaches to diagnosing delirium in hospitalized patients with dementia.[63] Cognitive and functional stimulation interventions may have a positive impact on reducing behavioral issues.[64, 65] | Does universal assessment of hospitalized older adults for cognitive impairment (eg, at presentation and/or discharge) lead to more appropriate application of geriatric care principles and improve patient centered outcomes? | Potential interventions: |
| Dementia or delirium care | ||||
| Patient/family communication and engagement strategies | ||||
| Maintenance/recovery of independent functional status | ||||
| Potential outcomes: | ||||
| Patient‐centered outcomesa | ||||
| Length of stay, cost, and healthcare utilization (including palliative care) | ||||
| Immediate invasive vs early conservative treatments pursued | ||||
| Depression | Depression is a common geriatric syndrome among acutely ill older patients, occurring in up to 45% of patients.[66, 67] Rates of depression are similar among patients discharged following a critical illness, with somatic, rather than cognitive‐affective complaints being the most prevalent.[68] Depression among inpatients or immediately following hospitalization independently predicts worse functional outcomes,[69] cognitive decline,[70] hospital readmission,[71, 72] and long‐term mortality.[69, 73] Finally, geriatric patients are known to respond differently to medical treatment.[74, 75] | Although highly prevalent, depression is poorly recognized and managed in the inpatient setting. Depression is recognized in only 50% of patients, with previously undiagnosed or untreated depression being at highest risk for being missed.[76] The role of treatment of depression in the inpatient setting is poorly understood, particularly for those with newly recognized depression or depressive symptoms. Some novel collaborative care and telephone outreach programs have led to increases in depression treatment in patients with specific medical and surgical conditions, resulting in early promising mental health and comorbid outcomes.[77, 78] The efficacy of such programs for older patients is unknown. | Does identifying depressive symptoms during a hospital stay and initiating a therapeutic plan prior to discharge improve patient‐centered and/or disease‐specific outcomes? | Possible areas of focus: |
| Comprehensive geriatric and psychosocial assessment; | ||||
| Inpatient vs outpatient initiation of pharmacological therapy | ||||
| Integration of confusion assessment method into therapeutic approaches | ||||
| Linkages with outpatient mental health resources | ||||
| Medications | Medication exposure, particularly potentially inappropriate medications, is common in hospitalized elders.[79] Medication errorsof dosage, type, and discrepancy between what a patient takes at home and what is known to his/her prescribing physicianare common and adversely affects patient safety.[80] Geriatric populations are disproportionately affected, especially those taking more than 5 prescription medications per day.[81] | Numerous strategies including electronic alerts, screening protocols, and potentially inappropriate medication lists (Beers list, STOPP) exist, though the optimal strategies to limit the use of potentially inappropriate medications is not yet known.[82, 83, 84] | What systems interventions improve medication management for older adults (ie, appropriateness of medication choices and dosing, compliance, cost) in hospital and post‐acute care? | Possible areas of focus: |
| Use of healthcare information technology | ||||
| Communication across sites of care | ||||
| Reducing medication‐related adverse events | ||||
| Engagement of family caregivers | ||||
| Patient‐centered strategies to simplify regimens | ||||
| Models of care | Hospitalization marks a time of high risk for older patients. Up to half die during hospitalization or within the year following the hospitalization. There is high risk of nosocomial events, and more than a third experience a decline in health resulting in longer hospitalizations and/or placement in extended‐care facilities.[73, 85, 86] | Comprehensive inpatient care for older adults (acute care for elders units, geriatric evaluation and management units, geriatric consultation services) were studied in 2 meta‐analyses, 5 RCTs, and 1 quasiexperimental study and summarized in a systematic review.[87] The studies reported improved quality of care (1 of 1 article), quality of life (3 of 4), functional autonomy (5 of 6), survival (3 of 6), and equal or lower healthcare utilization (7 of 8). | For which populations of hospitalized older adults does systematic implementation of geriatric care principles/processes improve patient‐centered outcomes? | Potential populations: |
| Patients of the emergency department, critical care, perioperative, and targeted medical/surgical units | ||||
| Examples of care principles: | ||||
| Geriatric assessment, early mobility, medication management, delirium prevention, advanced‐care planning, risk‐factor modification, caregiver engagement | ||||
| Potential outcomes: | ||||
| Patient‐centered outcomesa | ||||
| Cost | ||||
| Physical function | Half of older patients will lose functional capacity during hospitalization.[88] Loss of physical function, particularly of lower extremities, is a risk factor for nursing home placement.[89, 90] Older hospitalized patients spend the majority (up to 80%) of their time lying in bed, even when they are capable of walking independently.[91] | Loss of independences with ADL capabilities is associated with longer hospital stays, higher readmission rates, and higher mortality risk.[92] Excessive time in bed during a hospital stay is also associated with falls.[93] Often, hospital nursing protocols and physician orders increase in‐hospital immobility in patients.[91, 94] However, nursing‐driven mobility protocols can improve functional outcomes of older hospitalized patients.[95, 96] | What is the comparative effectiveness of interventions that promote in‐hospital mobility, improve and preserve physical function, and reduce falls among older hospitalized patients? | Potential interventions: |
| Intensive physical therapy | ||||
| Incidental functional training | ||||
| Restraint reduction | ||||
| Medication management | ||||
| Potential outcomes: | ||||
| Discharge location | ||||
| Delirium, pressure ulcers, and falls | ||||
| Surgery | An increasing number of persons over age 65 years are undergoing surgical procedures.[97] These persons are at increased risk for developing delirium/cogitative dysfunction,[98] loss of functional status,[99] and exacerbations of chronic illness.[97] Additionally, pain management may be harder to address in this population.[100] Current outcomes may not reflect the clinical needs of elder surgical patients.[101] | Tailored drug selection and nursing protocols may prevent delirium.[98] Postoperative cognitive dysfunction may require weeks for resolution. Identifying frail patients preoperatively may lead to more appropriate risk stratification and improved surgical outcomes.[99] Pain management strategies focused on mitigating cognitive impact and other effects may also be beneficial.[100] Development of risk‐adjustment tools specific to older populations, as well as measures of frailty and patient‐centered care, have been proposed.[101] | What perioperative strategies can be used to optimize care processes and improve outcomes in older surgical patients? | Potential strategies: |
| Preoperative risk assessment and optimization for frail or multimorbid older patients | ||||
| Perioperative management protocols for frail or multimorbid older patients | ||||
| Potential outcomes: | ||||
| Postoperative patient centered outcomesa | ||||
| Perioperative cost, healthcare utilization | ||||
| Training | Adults over age 65 years comprise 13.2 % of the US population, but account for >30% of hospital discharges and 50% of hospital days.[86, 102, 103] By 2030, there will only be 1 geriatrician for every 3798 Americans >75 years.[4] Between 1997 and 2006, the odds that a hospitalist would treat a hospitalized Medicare patient rose 29% per year.[3] | Train the trainer programs for physicians include the CHAMP, the AGESP, and the PAGE. Education for nurses include the NICHE. Outcomes include improved self‐confidence, attitudes, teaching skills, and geriatric care environment.[104, 105, 106] | What is the most effective approach to training hospital‐based providers in geriatric and palliative care competencies? | Potential interventions: |
| Mentored implementation | ||||
| Train the trainer | ||||
| Technical support | ||||
Table 2 also contains a capsule summary of the scope of the problem addressed by each research priority, a capsule summary of related work in the content area (what is known) not intended as a systematic review, and proposed dimensions or subquestions suggested by the stakeholders at the final prioritization meeting
DISCUSSION
Older hospitalized patients account for an increasing number and proportion of hospitalized patients,[1, 2] and hospitalists increasingly are responsible for inpatient care for this population.[3] The knowledge required for hospitalists to deliver optimal care and improve outcomes has not kept pace with the rapid growth of either hospitalists or hospitalized elders. Through a rigorous prioritization process, we identified 10 areas that deserve the highest priority in directing future research efforts to improve care for the older hospitalized patient. Assessment, prevention, and treatment of geriatric syndromes in the hospital account for almost half of the priority areas. Additional research is needed to improve advanced care planning, develop new care models, and develop training models for future hospitalists competent in geriatric and palliative care competencies.
A decade ago, the American Geriatric Society and the John A. Hartford Foundation embarked upon a research agenda aimed at improving the care of hospitalized elders cared for by specialists (ie, New Frontiers in Geriatrics Research: An Agenda for Surgical and Related Medical Specialties).[9] This effort differed in many important ways from the current priortization process. First, the New Frontiers agenda focused upon specific diseases, whereas the ACOP agenda addresses geriatric syndromes that cut across multiple diseases. Second, the New Frontiers agenda was made by researchers and based upon published literature, whereas the ACOP agenda involved the input of multiple stakeholders. Finally, the New Frontiers prioritized a research agenda across a number of surgical specialties, emergency medicine, and geriatric rehabilitation. Hospital medicine, however, was still early in its development and was not considered a unique specialty. Since that time, hospital medicine has matured into a unique specialty, with increased numbers of hospitalists,[3] increased research in hospital medicine,[17] and a separate recertification pathway for internal medicine licensure.[18] To date, there has not been a similar effort performed to direct geriatric research efforts for hospital medicine.
For researchers working in the field of hospital medicine, this list of topics has several implications. First, as hospitalists are commonly generalists, hospitalist researchers may be particularly well‐suited to study syndromes that cut across specialties. However, this does raise concerns about funding sources, as most National Institutes of Health institutes are disease‐focused. Funders that are not disease‐focused such as PCORI, National Institute on Aging, National Institute of Nursing Research, and Agency for Healthcare Research and Quality, and private foundations (Hartford, Robert Wood Johnson, and Commonwealth) may be more fruitful sources of funding for this work, but funding may be challenging. Nonetheless, the increased focus on patient‐centered work may increase funders' interest in such work. Second, the topics on this list would suggest that interventions will not be pharmacologic, but will focus on nonpharmacologic, behavioral, and social interventions. Similarly, outcomes of interest must expand beyond utilization metrics such as length of stay and mortality, to include functional status and symptom management, and goal‐concordant care. Therefore, research in geriatric acute care will necessarily be multidisciplinary.
Although these 10 high‐priority areas have been selected, this prioritized list is inherently limited by our methodology. First, our survey question was not focused on a disease state, and this wording may have resulted in the list favoring geriatric syndromes rather than common disease processes. Additionally, the resulting questions encompass large research areas and not specific questions about discrete interventions. Our results may also have been skewed by the types of engaged respondents who participated in the consultation, collating, and prioritization phases. In particular, we had a large response from geriatric medicine nurses, whereas some stakeholder groups provided no survey responses. Thus, these respondents were not representative of all possible stakeholders, nor were the survey respondents necessarily representative of each of their organizations. Nonetheless, the participants self‐identified as representative of diverse viewpoints that included patients, caregivers, and advocacy groups, with the majority of stakeholder organizations remaining engaged through the completion of the process. Thus, the general nature of this agenda helps us focus upon larger areas of importance, leaving researchers the flexibility to choose to narrow the focus on a specific research question that may include potential interventions and unique outcomes. Finally, our methodology may have inadvertently limited the number of patient and family caregiver voices in the process given our approach to large advocacy groups, our desire to be inclusive of healthcare professional organizations, and our survey methodology. Other methodologies may have reached more patients and caregivers, yet many healthcare professionals have served as family caregivers to frail elders requiring hospitalization and may have been in an ideal position to answer the survey.
In conclusion, several forces are shaping the future of acute inpatient care. These include the changing demographics of the hospitalized patient population, a rapid increase in the proportion of multimorbid hospitalized older adults, an inpatient workforce (hospitalists, generalists, and subspecialists) with potentially limited geriatrics training, and gaps in evidence‐based guidance to inform diagnostic and therapeutic decision making for acutely ill older patients. Training programs in hospital medicine should be aware of and could benefit from the resulting list of unanswered questions. Our findings also have implications for training to enrich education in geriatrics. Moreover, there is growing recognition that patients and other stakeholders deserve a greater voice in determining the direction of research. In addition to efforts to improve patient‐centeredness of research, these areas have been uniquely identified by stakeholders as important, and therefore are in line with newer priorities of PCORI. This project followed a road map resulting in a patient‐centered research agenda at the intersection of hospital medicine and geriatric medicine.[7] In creating this agenda, we relied heavily on the framework proposed by PCORI. We propose to pursue a dissemination and evaluation strategy for this research agenda as well as additional prioritization steps. We believe the adoption of this methodology will create a knowledge base that is rigorously derived and most relevant to the care of hospitalized older adults and their families. Its application will ultimately result in improved outcomes for hospitalized older adults.
Acknowledgements
The authors acknowledge Claudia Stahl, Society of Hospital Medicine; Cynthia Drake, University of Colorado; and the ACOP stakeholder organizations.
Disclosures: This work was supported by the Association of Specialty Professors/American Society of Internal Medicine and the John A. Hartford Foundation. Dr. Vasilevskis was supported by the National Institute on Aging of the National Institutes of Health under award number K23AG040157 and the Veterans Affairs Clinical Research Center of Excellence, and the Geriatric Research, Education and Clinical Center (GRECC). Dr. Vasilevskis' institution receives grant funding for an aspect of submitted work. Dr. Meltzer is a PCORI Methodology Committee member. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans' Affairs. The authors report no conflicts of interest.
- , , , , . National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report. 2010(29):1–20, 24.
- Centers for Medicare 2012. Available at: http://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/Chronic‐Conditions/Downloads/2012Chartbook.pdf. Accessed December 12, 2014.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , . A revelation of numbers: will America's eldercare workforce be ready to care for an aging America? Generations. 2010;34(4):11–19.
- Patient‐Centered Outcomes Research Institute Methodology Committee. The PCORI methodology report. Available at: http://www.pcori.org/assets/2013/11/PCORI‐Methodology‐Report.pdf. Published November 2013. Accessed December 19, 2013.
- The James Lind Alliance. JLA method. Available at: http://www.lindalliance.org/JLA_Method.asp. Accessed December 19, 2013.
- , , , , . Road map to a patient‐centered research agenda at the intersection of hospital medicine and geriatric medicine. J Gen Intern Med. 2014;29(6):926–931.
- Patient‐Centered Outcomes Research Institute. About us. Available at: http://www.pcori.org/about‐us. Accessed February 23, 2015.
- , , . New Frontiers of Geriatrics Research: An Agenda for Surgical and Related Medical Specialties. New York, NY: American Geriatrics Society; 2004.
- , , , . Linking the NIH strategic plan to the research agenda for social workers in health and aging. J Gerontol Soc Work. 2010;53(1):77–93.
- , . Assessing the capacity to make everyday decisions: a guide for clinicians and an agenda for future research. Am J Geriatr Psychiatry. 2007;15(2):101–111.
- , , , et al. Practitioners' views on elder mistreatment research priorities: recommendations from a Research‐to‐Practice Consensus conference. J Elder Abuse Negl. 2011;23(2):115–126.
- , . The intersection between geriatrics and palliative care: a call for a new research agenda. J Am Geriatr Soc. 2005;53(9):1593–1598.
- . The cancer aging interface: a research agenda. J Clin Oncol. 2007;25(14):1945–1948.
- , , , . Clinical care of persons with dementia in the emergency department: a review of the literature and agenda for research. J Am Geriatr Soc. 2012;60(9):1742–1748.
- , . A group process model for problem identification and program planning. J Appl Behav Sci. 1971;7(4):466–492.
- , , , , . Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148–154.
- . ABFM and ABIM to jointly participate in recognition of focused practice (rfp) in hospital medicine pilot approved by abms. Ann Fam Med. 2010;8(1):87.
- , , , . Medical decision‐making for older adults without family. J Am Geriatr Soc. 2012;60(11):2144–2150.
- , , , , , . Promoting advance directives among elderly primary care patients. J Gen Intern Med. 2004;19(9):944–951.
- , , . Advance directive completion by elderly Americans: a decade of change. J Am Geriatr Soc. 2014;62(4):706–710.
- , . Care transitions by older adults from nursing homes to hospitals: implications for long‐term care practice, geriatrics education, and research. J Am Med Dir Assoc. 2010;11(4):231–238.
- , , , . Decisions to hospitalize nursing home residents dying with advanced dementia. J Am Geriatr Soc. 2005;53(8):1396–1401.
- , , , , , . A comparison of methods to communicate treatment preferences in nursing facilities: traditional practices versus the physician orders for life‐sustaining treatment program. J Am Geriatr Soc. 2010;58(7):1241–1248.
- , , , , . Interventions to improve transitional care between nursing homes and hospitals: a systematic review. J Am Geriatr Soc. 2010;58(4):777–782.
- , , . Opening end‐of‐life discussions: how to introduce Voicing My CHOiCES, an advance care planning guide for adolescents and young adults [published online ahead of print March 13, 2014]. Palliat Support Care. doi: 10.1017/S1478951514000054.
- , , , . The revolving door of rehospitalization from skilled nursing facilities. Health Aff (Millwood). 2010;29(1):57–64.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Predictors of rehospitalization among elderly patients admitted to a rehabilitation hospital: the role of polypharmacy, functional status, and length of stay. J Am Med Dir Assoc. 2013;14(10):761–767.
- , , , et al. Mobility after hospital discharge as a marker for 30‐day readmission. J Gerontol A Biol Sci Med Sci. 2013;68(7):805–810.
- , , , , . The association between the quality of inpatient care and early readmission: a meta‐analysis of the evidence. Med Care. 1997;35(10):1044–1059.
- , , , , , . Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. May 2014;9(5):277–282.
- , , , , , . A prospective cohort study of geriatric syndromes among older medical patients admitted to acute care hospitals. J Am Geriatr Soc. 2011;59(11):2001–2008.
- , , , et al. Hospital discharge instructions: comprehension and compliance among older adults. J Gen Intern Med. 2014;29(11):1491–1498.
- , , , et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385–391.
- , , , et al. Communication and information deficits in patients discharged to rehabilitation facilities: an evaluation of five acute care hospitals. J Hosp Med. 2009;4(8):E28–E33.
- , , , , , . The consequences of poor communication during transitions from hospital to skilled nursing facility: a qualitative study. J Am Geriatr Soc. 2013;61(7):1095–1102.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102–109.
- , , , et al. Low‐cost transitional care with nurse managers making mostly phone contact with patients cut rehospitalization at a VA hospital. Health Aff (Millwood). 2012;31(12):2659–2668.
- , , , et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160(11):774–784.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , , , . Hospital‐initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):433–440.
- , , , . Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26(3):277–287.
- , , , , . Does delirium contribute to poor hospital outcomes? A three‐site epidemiologic study. J Gen Intern Med. 1998;13(4):234–242.
- , , , . Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33(1):66–73.
- , , , et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM‐ICU). JAMA. 2001;286(21):2703–2710.
- , , , et al. Impact and recognition of cognitive impairment among hospitalized elders. J Hosp Med. 2010;5(2):69–75.
- , , , et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900.
- , , . Long‐term cognitive impairment after critical illness. N Engl J Med. 2014;370(2):185–186.
- , , , et al. Delirium in the ICU and subsequent long‐term disability among survivors of mechanical ventilation. Crit Care Med. 2014;42(2):369–377.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304(4):443–451.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762.
- , , , , , . Days of delirium are associated with 1‐year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180(11):1092–1097.
- , . In‐facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):375–380.
- , , , et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489–499.
- , , , et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653.
- , , , , , . Protocolized intensive care unit management of analgesia, sedation, and delirium improves analgesia and subsyndromal delirium rates. Anesth Analg. 2010;111(2):451–463.
- , . A systematic review of the prevalence, associations and outcomes of dementia in older general hospital inpatients. Int Psychogeriatr. 2011;23(3):344–355.
- , , , . Cognitive impairment is undetected in medical inpatients: a study of mortality and recognition amongst healthcare professionals. BMC Geriatr. 2012;12:47.
- , , . How can we keep patients with dementia safe in our acute hospitals? A review of challenges and solutions. J R Soc Med. 2013;106(9):355–361.
- , , . Screening for dementia in general hospital inpatients: a systematic review and meta‐analysis of available instruments. Age Ageing. 2013;42(6):689–695.
- , , , et al. Tools to detect delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2012;60(11):2005–2013.
- , , , , , . Functional analysis‐based interventions for challenging behaviour in dementia. Cochrane Database Syst Rev. 2012;2:CD006929.
- , , , . Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012;2:CD005562.
- , , , et al. The prevalence and correlates of major and minor depression in older medical inpatients. J Am Geriatr Soc. 2005;53(8):1344–1353.
- , , , , , . Major depressive disorder in hospitalized medically ill patients: an examination of young and elderly male veterans. J Am Geriatr Soc. 1991;39(9):881–890.
- , , , et al. Depression, post‐traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN‐ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2(5):369–379.
- , , , et al. Depressive symptoms after hospitalization in older adults: function and mortality outcomes. J Am Geriatr Soc. 2012;60(12):2254–2262.
- , , , , . 12‐month cognitive outcomes of major and minor depression in older medical patients. Am J Geriatr Psychiatry. 2008;16(9):742–751.
- , , , et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256–262.
- , , , , , . Dose‐response relationship between depressive symptoms and hospital readmission. J Hosp Med. 2014;9(6):358–364.
- , , , , , . Depressive symptoms and 3‐year mortality in older hospitalized medical patients. Ann Intern Med. 1999;130(7):563–569.
- , , , et al. Support for the vascular depression hypothesis in late‐life depression: results of a 2‐site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67(3):277–285.
- , , , , , . Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58(3):204–210.
- , , , , . Recognition of depression in older medical inpatients. J Gen Intern Med. 2007;22(5):559–564.
- , , , , , . A collaborative care depression management program for cardiac inpatients: depression characteristics and in‐hospital outcomes. Psychosomatics. 2011;52(1):26–33.
- , , , , , . Impact of a depression care management program for hospitalized cardiac patients. Circ Cardiovasc Qual Outcomes. 2011;4(2):198–205.
- , , , et al. Potentially inappropriate medication use in hospitalized elders. J Hosp Med. 2008;3(2):91–102.
- , , , , , . Prevalence, incidence and nature of prescribing errors in hospital inpatients: a systematic review. Drug Saf. 2009;32(5):379–389.
- , . Minimizing adverse drug events in older patients. Am Fam Physician. 2007;76(12):1837–1844.
- , , , , . STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72–83.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616–631.
- , , , . Preventing potentially inappropriate medication use in hospitalized older patients with a computerized provider order entry warning system. Arch Intern Med. 2010;170(15):1331–1336.
- . Hazards of Hospitalization of the Elderly. Ann Intern Med. 1993;118(3):219–223.
- . Improving health care for older persons. Ann Intern Med. 2003;139(5 part 2):421–424.
- , , , , , . Successful models of comprehensive care for older adults with chronic conditions: evidence for the Institute of Medicine's "retooling for an aging America" report. J Am Geriatr Soc. 2009;57(12):2328–2337.
- , , , et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56(12):2171–2179.
- , , , . Changes in functional status and the risks of subsequent nursing home placement and death. J Gerontol. 1993;48(3):S94–S101.
- , , . Risk factors for nursing home placement in a population‐based dementia cohort. J Am Geriatr Soc. 2000;48(5):519–525.
- , , , . The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660–1665.
- , , , , . A systematic review of predictors and screening instruments to identify older hospitalized patients at risk for functional decline. J Clin Nurs. 2007;16(1):46–57.
- . Immobility and falls. Clin Geriatr Med. 1998;14(4):699–726.
- , , . Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52(8):1263–1270.
- , , . Impact of a nurse‐driven mobility protocol on functional decline in hospitalized older adults. J Nurs Care Qual. 2009;24(4):325–331.
- , . Impact of early mobilization protocol on the medical‐surgical inpatient population: an integrated review of literature. Clin Nurse Spec. 2012;26(2):87–94.
- , , , . The aging population and its impact on the surgery workforce. Ann Surg. 2003;238(2):170–177.
- . Perioperative care of the elderly patient: an update. Cleve Clin J Med. 2009;76(suppl 4):S16–S21.
- , , . Frailty in the older surgical patient: a review. Age Ageing. 2012;41(2):142–147.
- . The assessment and management of peri‐operative pain in older adults. Anaesthesia. 2014;69(suppl 1):54–60.
- , . National Research Strategies: what outcomes are important in peri‐operative elderly care? Anaesthesia. 2014;69(suppl 1):61–69.
- , . 2002 National Hospital Discharge Survey. Adv Data. 2004;342:1–30.
- , . Hospitalization in the United States, 2002. Rockville, MD: Agency for Healthcare Research and Quality; 2005.
- , , , et al. The Curriculum for the Hospitalized Aging Medical Patient program: a collaborative faculty development program for hospitalists, general internists, and geriatricians. J Hosp Med. 2008;3(5):384–393.
- , . Advancement of geriatrics education. J Hosp Med. 2011;6(6):370.
- , , , , , . Advancing geriatrics education: an efficient faculty development program for academic hospitalists increases geriatric teaching. J Hosp Med. 2010;5(9):541–546.
- , , , , . National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report. 2010(29):1–20, 24.
- Centers for Medicare 2012. Available at: http://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/Chronic‐Conditions/Downloads/2012Chartbook.pdf. Accessed December 12, 2014.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , . A revelation of numbers: will America's eldercare workforce be ready to care for an aging America? Generations. 2010;34(4):11–19.
- Patient‐Centered Outcomes Research Institute Methodology Committee. The PCORI methodology report. Available at: http://www.pcori.org/assets/2013/11/PCORI‐Methodology‐Report.pdf. Published November 2013. Accessed December 19, 2013.
- The James Lind Alliance. JLA method. Available at: http://www.lindalliance.org/JLA_Method.asp. Accessed December 19, 2013.
- , , , , . Road map to a patient‐centered research agenda at the intersection of hospital medicine and geriatric medicine. J Gen Intern Med. 2014;29(6):926–931.
- Patient‐Centered Outcomes Research Institute. About us. Available at: http://www.pcori.org/about‐us. Accessed February 23, 2015.
- , , . New Frontiers of Geriatrics Research: An Agenda for Surgical and Related Medical Specialties. New York, NY: American Geriatrics Society; 2004.
- , , , . Linking the NIH strategic plan to the research agenda for social workers in health and aging. J Gerontol Soc Work. 2010;53(1):77–93.
- , . Assessing the capacity to make everyday decisions: a guide for clinicians and an agenda for future research. Am J Geriatr Psychiatry. 2007;15(2):101–111.
- , , , et al. Practitioners' views on elder mistreatment research priorities: recommendations from a Research‐to‐Practice Consensus conference. J Elder Abuse Negl. 2011;23(2):115–126.
- , . The intersection between geriatrics and palliative care: a call for a new research agenda. J Am Geriatr Soc. 2005;53(9):1593–1598.
- . The cancer aging interface: a research agenda. J Clin Oncol. 2007;25(14):1945–1948.
- , , , . Clinical care of persons with dementia in the emergency department: a review of the literature and agenda for research. J Am Geriatr Soc. 2012;60(9):1742–1748.
- , . A group process model for problem identification and program planning. J Appl Behav Sci. 1971;7(4):466–492.
- , , , , . Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148–154.
- . ABFM and ABIM to jointly participate in recognition of focused practice (rfp) in hospital medicine pilot approved by abms. Ann Fam Med. 2010;8(1):87.
- , , , . Medical decision‐making for older adults without family. J Am Geriatr Soc. 2012;60(11):2144–2150.
- , , , , , . Promoting advance directives among elderly primary care patients. J Gen Intern Med. 2004;19(9):944–951.
- , , . Advance directive completion by elderly Americans: a decade of change. J Am Geriatr Soc. 2014;62(4):706–710.
- , . Care transitions by older adults from nursing homes to hospitals: implications for long‐term care practice, geriatrics education, and research. J Am Med Dir Assoc. 2010;11(4):231–238.
- , , , . Decisions to hospitalize nursing home residents dying with advanced dementia. J Am Geriatr Soc. 2005;53(8):1396–1401.
- , , , , , . A comparison of methods to communicate treatment preferences in nursing facilities: traditional practices versus the physician orders for life‐sustaining treatment program. J Am Geriatr Soc. 2010;58(7):1241–1248.
- , , , , . Interventions to improve transitional care between nursing homes and hospitals: a systematic review. J Am Geriatr Soc. 2010;58(4):777–782.
- , , . Opening end‐of‐life discussions: how to introduce Voicing My CHOiCES, an advance care planning guide for adolescents and young adults [published online ahead of print March 13, 2014]. Palliat Support Care. doi: 10.1017/S1478951514000054.
- , , , . The revolving door of rehospitalization from skilled nursing facilities. Health Aff (Millwood). 2010;29(1):57–64.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Predictors of rehospitalization among elderly patients admitted to a rehabilitation hospital: the role of polypharmacy, functional status, and length of stay. J Am Med Dir Assoc. 2013;14(10):761–767.
- , , , et al. Mobility after hospital discharge as a marker for 30‐day readmission. J Gerontol A Biol Sci Med Sci. 2013;68(7):805–810.
- , , , , . The association between the quality of inpatient care and early readmission: a meta‐analysis of the evidence. Med Care. 1997;35(10):1044–1059.
- , , , , , . Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. May 2014;9(5):277–282.
- , , , , , . A prospective cohort study of geriatric syndromes among older medical patients admitted to acute care hospitals. J Am Geriatr Soc. 2011;59(11):2001–2008.
- , , , et al. Hospital discharge instructions: comprehension and compliance among older adults. J Gen Intern Med. 2014;29(11):1491–1498.
- , , , et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385–391.
- , , , et al. Communication and information deficits in patients discharged to rehabilitation facilities: an evaluation of five acute care hospitals. J Hosp Med. 2009;4(8):E28–E33.
- , , , , , . The consequences of poor communication during transitions from hospital to skilled nursing facility: a qualitative study. J Am Geriatr Soc. 2013;61(7):1095–1102.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102–109.
- , , , et al. Low‐cost transitional care with nurse managers making mostly phone contact with patients cut rehospitalization at a VA hospital. Health Aff (Millwood). 2012;31(12):2659–2668.
- , , , et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160(11):774–784.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , , , . Hospital‐initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):433–440.
- , , , . Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26(3):277–287.
- , , , , . Does delirium contribute to poor hospital outcomes? A three‐site epidemiologic study. J Gen Intern Med. 1998;13(4):234–242.
- , , , . Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33(1):66–73.
- , , , et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM‐ICU). JAMA. 2001;286(21):2703–2710.
- , , , et al. Impact and recognition of cognitive impairment among hospitalized elders. J Hosp Med. 2010;5(2):69–75.
- , , , et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900.
- , , . Long‐term cognitive impairment after critical illness. N Engl J Med. 2014;370(2):185–186.
- , , , et al. Delirium in the ICU and subsequent long‐term disability among survivors of mechanical ventilation. Crit Care Med. 2014;42(2):369–377.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304(4):443–451.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762.
- , , , , , . Days of delirium are associated with 1‐year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180(11):1092–1097.
- , . In‐facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):375–380.
- , , , et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489–499.
- , , , et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653.
- , , , , , . Protocolized intensive care unit management of analgesia, sedation, and delirium improves analgesia and subsyndromal delirium rates. Anesth Analg. 2010;111(2):451–463.
- , . A systematic review of the prevalence, associations and outcomes of dementia in older general hospital inpatients. Int Psychogeriatr. 2011;23(3):344–355.
- , , , . Cognitive impairment is undetected in medical inpatients: a study of mortality and recognition amongst healthcare professionals. BMC Geriatr. 2012;12:47.
- , , . How can we keep patients with dementia safe in our acute hospitals? A review of challenges and solutions. J R Soc Med. 2013;106(9):355–361.
- , , . Screening for dementia in general hospital inpatients: a systematic review and meta‐analysis of available instruments. Age Ageing. 2013;42(6):689–695.
- , , , et al. Tools to detect delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2012;60(11):2005–2013.
- , , , , , . Functional analysis‐based interventions for challenging behaviour in dementia. Cochrane Database Syst Rev. 2012;2:CD006929.
- , , , . Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012;2:CD005562.
- , , , et al. The prevalence and correlates of major and minor depression in older medical inpatients. J Am Geriatr Soc. 2005;53(8):1344–1353.
- , , , , , . Major depressive disorder in hospitalized medically ill patients: an examination of young and elderly male veterans. J Am Geriatr Soc. 1991;39(9):881–890.
- , , , et al. Depression, post‐traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN‐ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2(5):369–379.
- , , , et al. Depressive symptoms after hospitalization in older adults: function and mortality outcomes. J Am Geriatr Soc. 2012;60(12):2254–2262.
- , , , , . 12‐month cognitive outcomes of major and minor depression in older medical patients. Am J Geriatr Psychiatry. 2008;16(9):742–751.
- , , , et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256–262.
- , , , , , . Dose‐response relationship between depressive symptoms and hospital readmission. J Hosp Med. 2014;9(6):358–364.
- , , , , , . Depressive symptoms and 3‐year mortality in older hospitalized medical patients. Ann Intern Med. 1999;130(7):563–569.
- , , , et al. Support for the vascular depression hypothesis in late‐life depression: results of a 2‐site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67(3):277–285.
- , , , , , . Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58(3):204–210.
- , , , , . Recognition of depression in older medical inpatients. J Gen Intern Med. 2007;22(5):559–564.
- , , , , , . A collaborative care depression management program for cardiac inpatients: depression characteristics and in‐hospital outcomes. Psychosomatics. 2011;52(1):26–33.
- , , , , , . Impact of a depression care management program for hospitalized cardiac patients. Circ Cardiovasc Qual Outcomes. 2011;4(2):198–205.
- , , , et al. Potentially inappropriate medication use in hospitalized elders. J Hosp Med. 2008;3(2):91–102.
- , , , , , . Prevalence, incidence and nature of prescribing errors in hospital inpatients: a systematic review. Drug Saf. 2009;32(5):379–389.
- , . Minimizing adverse drug events in older patients. Am Fam Physician. 2007;76(12):1837–1844.
- , , , , . STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72–83.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616–631.
- , , , . Preventing potentially inappropriate medication use in hospitalized older patients with a computerized provider order entry warning system. Arch Intern Med. 2010;170(15):1331–1336.
- . Hazards of Hospitalization of the Elderly. Ann Intern Med. 1993;118(3):219–223.
- . Improving health care for older persons. Ann Intern Med. 2003;139(5 part 2):421–424.
- , , , , , . Successful models of comprehensive care for older adults with chronic conditions: evidence for the Institute of Medicine's "retooling for an aging America" report. J Am Geriatr Soc. 2009;57(12):2328–2337.
- , , , et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56(12):2171–2179.
- , , , . Changes in functional status and the risks of subsequent nursing home placement and death. J Gerontol. 1993;48(3):S94–S101.
- , , . Risk factors for nursing home placement in a population‐based dementia cohort. J Am Geriatr Soc. 2000;48(5):519–525.
- , , , . The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660–1665.
- , , , , . A systematic review of predictors and screening instruments to identify older hospitalized patients at risk for functional decline. J Clin Nurs. 2007;16(1):46–57.
- . Immobility and falls. Clin Geriatr Med. 1998;14(4):699–726.
- , , . Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52(8):1263–1270.
- , , . Impact of a nurse‐driven mobility protocol on functional decline in hospitalized older adults. J Nurs Care Qual. 2009;24(4):325–331.
- , . Impact of early mobilization protocol on the medical‐surgical inpatient population: an integrated review of literature. Clin Nurse Spec. 2012;26(2):87–94.
- , , , . The aging population and its impact on the surgery workforce. Ann Surg. 2003;238(2):170–177.
- . Perioperative care of the elderly patient: an update. Cleve Clin J Med. 2009;76(suppl 4):S16–S21.
- , , . Frailty in the older surgical patient: a review. Age Ageing. 2012;41(2):142–147.
- . The assessment and management of peri‐operative pain in older adults. Anaesthesia. 2014;69(suppl 1):54–60.
- , . National Research Strategies: what outcomes are important in peri‐operative elderly care? Anaesthesia. 2014;69(suppl 1):61–69.
- , . 2002 National Hospital Discharge Survey. Adv Data. 2004;342:1–30.
- , . Hospitalization in the United States, 2002. Rockville, MD: Agency for Healthcare Research and Quality; 2005.
- , , , et al. The Curriculum for the Hospitalized Aging Medical Patient program: a collaborative faculty development program for hospitalists, general internists, and geriatricians. J Hosp Med. 2008;3(5):384–393.
- , . Advancement of geriatrics education. J Hosp Med. 2011;6(6):370.
- , , , , , . Advancing geriatrics education: an efficient faculty development program for academic hospitalists increases geriatric teaching. J Hosp Med. 2010;5(9):541–546.
Hospitalist‐Run Acute Care for Elderly
For the frail older patient, hospitalization marks a period of high risk of poor outcomes and adverse events including functional decline, delirium, pressure ulcers, adverse drug events, nosocomial infections, and falls.1, 2 Physician recognition of elderly patients at risk for adverse outcomes is poor, making it difficult to intervene to prevent them.3, 4 Among frail, elderly inpatients at an urban academic medical center, doctors documented cognitive assessments in only 5% of patients. Functional assessments are appropriately documented in 40%80% of inpatients.3, 5
The Acute Care for Elders (ACE) unit is one of several models of comprehensive inpatient geriatric care that have been developed by geriatrician researchers to address the adverse events and functional decline that often accompany hospitalization.6 The ACE unit model generally incorporates: 1) a modified hospital environment, 2) early assessment and intensive management to minimize the adverse effects of hospital care, 3) early discharge planning, 4) patient centered care protocols, and 5) a consistent nursing staff.7 Two randomized, controlled trials have shown the ACE unit model to be successful in reducing functional decline among frail older inpatients during and after hospitalization.7, 8 While meta‐analyses data also suggests the ACE unit model reduces functional decline and future institutionalization, significant impact on other outcomes is not proven.9, 10
Several barriers have prevented the successful dissemination of the ACE unit model. The chief limitations are the upfront resources required to create and maintain a modified, dedicated unit, as well as the lack of a geriatrics trained workforce.7, 1113 The rapid growth of hospital medicine presents opportunities for innovation in the care of older patients. Still, a 2006 census demonstrated that few hospitalist groups had identified geriatric care as a priority.14
In response to these challenges, the University of Colorado Hospital Medicine Group created a hospitalist‐run inpatient medical service designed for the care of the frail older patient. This Hospitalist‐Acute Care for the Elderly (Hospitalist‐ACE) unit is a hybrid of a general medical service and an inpatient geriatrics unit.7 The goals of the Hospitalist‐ACE service are to provide high quality care tailored to older inpatients, thus minimizing the risks of functional decline and adverse events associate with hospitalization, and to provide a clinical geriatrics teaching experience for Hospitalist Training Track Residents within the Internal Medicine Residency Training Program and medical students at the University of Colorado Denver School of Medicine. The Hospitalist‐ACE unit is staffed with a core group of hospitalist attendings who have, at a minimum, attended an intensive mini‐course in inpatient geriatrics. The service employs interdisciplinary rounds; a brief, standardized geriatric assessment including screens of function, cognition, and mood; a clinical focus on mitigating the hazards of hospitalization, early discharge planning; and a novel geriatric educational curriculum for medicine residents and medical students.
This article will: 1) describe the creation of the Hospitalist‐ACE service at the University of Colorado Hospital; and 2) summarize the evaluation of the Hospitalist‐ACE service in a quasi‐randomized, controlled manner during its first year. We hypothesized that, when compared to patients receiving usual care, patients cared for on the Hospitalist‐ACE service would have increased recognition of abnormal functional status; recognition of abnormal cognitive status and delirium; equivalent lengths of stay and hospital charges; and decreased falls, 30‐day readmissions, and restraint use.
METHODS
Design
We performed a quasi‐randomized, controlled study of the Hospitalist‐ACE service.
Setting
The study setting was the inpatient general medical services of the Anschutz Inpatient Pavilion (AIP) of the University of Colorado Hospital (UCH). The AIP is a 425‐bed tertiary care hospital that is the major teaching affiliate of the University of Colorado School of Medicine and a regional referral center. The control services, hereafter referred to as usual care, were comprised of the four inpatient general medicine teaching services that take admissions on a four‐day rotation (in general, two were staffed by outpatient general internists and medical subspecialists, and two were staffed by academic hospitalists). The Hospitalist‐ACE service was a novel hospitalist teaching service that began in July 2007. Hospitalist‐ACE patients were admitted to a single 12‐bed medical unit (12 West) when beds were available; 12 West is similar to the other medical/surgical units at UCH and did not have any modifications to the rooms, equipment, or common areas for the intervention. The nursing staff on this unit had no formal geriatric nursing training. The Hospitalist‐ACE team admitted patients daily (between 7 AM and 3 PM MondayFriday; between 7 AM and 12 noon Saturday and Sunday). Patients assigned to the Hospitalist‐ACE service after hours were admitted by the internal medicine resident on call for the usual care services and handed off to the Hospitalist‐ACE team at 7 AM the next morning.
Study Subjects
Eligible subjects were inpatients age 70 years admitted to the usual care or Hospitalist‐ACE services at the AIP from November 2, 2007 to April 15, 2008. All patients age 70 years were randomized to the Hospitalist‐ACE service or usual care on a general internal medicine service by the last digit of the medical record number (odd numbers admitted to the Hospitalist‐ACE service and even numbers admitted to usual care). Patients followed by the Hospitalist‐ACE service but not admitted to 12 West were included in the study. To isolate the impact of the intervention, patients admitted to a medicine subspecialty service (such as cardiology, pulmonary, or oncology), or transferred to or from the Hospitalist‐ACE or control services to another service (eg, intensive care unit [ICU] or orthopedic surgery service) were excluded from the study.
Intervention
The Hospitalist‐ACE unit implemented an interdisciplinary team approach to identify and address geriatric syndromes in patients aged 70 and over. The Hospitalist‐ACE model of care consisted of clinical care provided by a hospitalist attending with additional training in geriatric medicine, administration of standardized geriatric screens assessing function, cognition, and mood, 15 minute daily (MondayFriday) interdisciplinary rounds focusing on recognition and management of geriatric syndromes and early discharge planning, and a standardized educational curriculum for medical residents and medical students addressing hazards of hospitalization.
The Hospitalist‐ACE service was a unique rotation within the Hospitalist Training Track of the Internal Medicine Residency that was developed with the support of the University of Colorado Hospital and the Internal Medicine Residency Training Program, and input from the Geriatrics Division at the University of Colorado Denver. The director received additional training from the Donald W. Reynolds FoundationUCLA Faculty Development to Advance Geriatric Education Mini‐Fellowship for hospitalist faculty. The mission of the service was to excel at educating the next generation of hospitalists while providing a model for excellence of care for hospitalized elderly patients. Important stakeholders were identified, and a leadership teamincluding representatives from nursing, physical and occupational therapy, pharmacy, social work, case management, and later, volunteer servicescreated the model daily interdisciplinary rounds. As geographic concentration was essential for the viability of interdisciplinary rounds, one unit (12 West) within the hospital was designated as the preferred location for patients admitted to the Hospitalist‐ACE service.
The Hospitalist‐ACE unit team consisted of one attending hospitalist, one resident, one intern, and medical students. The attending was one of five hospitalists, with additional training in geriatric medicine, who rotated attending responsibilities on the service. One of the hospitalists was board certified in geriatric medicine. Each of the other four hospitalists attended the Reynolds FoundationUCLA mini‐fellowship in geriatric medicine. Hospitalist‐ACE attendings rotated on a variety of other hospitalist services throughout the academic year, including the usual care services.
The brief standardized geriatric assessment consisted of six validated instruments, and was completed by house staff or medical students on admission, following instruction by the attending physician. The complete assessment tool is shown in Figure 1. The cognitive items included the Mini‐Cog,15 a two‐item depression screen,16 and the Confusion Assessment Method.17 The functional items included the Vulnerable Elders Survey (VES‐13),18 the Timed Get Up and Go test,19 and a two‐question falls screen.20 The elements of the assessment tool were selected by the Hospitalist‐ACE attendings for brevity and the potential to inform clinical management. To standardize the clinical and educational approach, the Hospitalist‐ACE attendings regularly discussed appropriate orders recommended in response to each positive screen, but no templated order sets were used during the study period.

Interdisciplinary rounds were attended by Hospitalist‐ACE physicians, nurses, case managers, social workers, physical or occupational therapists, pharmacists, and volunteers. Rounds were led by the attending or medical resident.
The educational curriculum encompassed 13 modules created by the attending faculty that cover delirium, falls, dementia, pressure ulcers, physiology of aging, movement disorders, medication safety, end of life care, advance directives, care transitions, financing of health care for the elderly, and ethical conundrums in the care of the elderly. A full table of contents appears in online Appendix 1. Additionally, portions of the curriculum have been published online.21, 22 Topic selection was guided by the Accreditation Council for Graduate Medical Education (ACGME) core geriatrics topics determined most relevant for the inpatient setting. Formal instruction of 3045 minutes duration occurred three to four days a week and was presented in addition to routine internal medicine educational conferences. Attendings coordinated teaching to ensure that each trainee was exposed to all of the content during the course of their four‐week rotation.
In contrast to the Hospitalist‐ACE service, usual care on the control general medical services consisted of either a hospitalist, a general internist, or an internal medicine subspecialist attending physician, with one medical resident, one intern, and medical students admitting every fourth day. The general medical teams attended daily discharge planning rounds with a discharge planner and social worker focused exclusively on discharge planning. The content of teaching rounds on the general medical services was largely left to the discretion of the attending physician.
This program evaluation of the Hospitalist‐ACE service was granted a waiver of consent and Health Insurance Portability and Accountability Act (HIPAA) by the Colorado Multiple Institutional Review Board.
Measures
Primary Outcome
The primary outcome for the study was the recognition of abnormal functional status by the primary team. Recognition of abnormal functional status was determined from chart review and consisted of both the physician's detection of abnormal functional status and evidence of a corresponding treatment plan identified in the notes or orders of a physician member of the primary team (Table 1).
| Measure | Criterion | Source | Content Examples |
|---|---|---|---|
| |||
| Recognition of abnormal functional status* | 1) Detection | MD's documentation of history | Presentation with change in function (new gait instability); use of gait aides (wheelchair) |
| OR | |||
| MD's documentation of physical exam | Observation of abnormal gait (eg, unsteady, wide‐based, shuffling) and/or balance Abnormal Get Up and Go test | ||
| AND | |||
| 2) Treatment | MD's order | PT/OT consult; home safety evaluation | |
| OR | |||
| MD's documentation assessment/plan | Inclusion of functional status (rehabilitation, PT/OT needs) on the MD's problem list | ||
| Recognition of abnormal cognitive status | Any of the following: | ||
| Delirium | 1) Detection | MD's history | Presentation of confusion or altered mental status |
| OR | |||
| MD's physical exam | Abnormal confusion assessment method | ||
| AND | |||
| 2) Treatment | MD's order | Sitter, reorienting communication, new halperidol order | |
| OR | |||
| MD's documentation of assessment/plan | Inclusion of delirium on the problem list | ||
| OR | |||
| Dementia | 1) Detection | MD's history | Dementia in medical history |
| OR | OR | ||
| MD's physical exam | Abnormal Folstein Mini‐Mental Status Exam or Mini‐Cog | ||
| AND | |||
| 2) Treatment | MD's order | Cholinesterase inhibitor ordered | |
| OR | OR | ||
| MD's documentation of assessment/plan | Inclusion of dementia on the problem list | ||
| OR | |||
| Depression | 1) Detection | MD's history | Depression in medical history |
| OR | OR | ||
| MD's physical exam | Positive depression screen | ||
| AND | |||
| 2) Treatment | MD's order | New antidepressant order | |
| OR | |||
| MD's documentation of assessment/plan | Inclusion of depression on the problem list | ||
Secondary Outcomes
Recognition of abnormal cognitive status was determined from chart review and consisted of both the physician's detection of dementia, depression, or delirium, and evidence of a corresponding treatment plan for any of the documented conditions identified in the notes or orders of a physician member of the primary team (Table 1). Additionally, we measured recognition and treatment of delirium alone.
Falls were determined from mandatory event reporting collected by the hospital on the University Hospitals Consortium Patient Safety Net web‐based reporting system and based on clinical assessment as reported by the nursing staff. The reports are validated by the appropriate clinical managers within 45 days of the event according to standard procedure.
Physical restraint use (type of restraint and duration) was determined from query of mandatory clinical documentation in the electronic medical record. Use of sleep aids was determined from review of the physician's order sheets in the medical record. The chart review captured any of 39 commonly prescribed hypnotic medications ordered at hour of sleep or for insomnia. The sleep medication list was compiled with the assistance of a pharmacist for an earlier chart review and included non‐benzodiazepine hypnotics, benzodiazepines, antidepressants, antihistamines, and antipsychotics.23
Length of stay, hospital charges, 30‐day readmissions to UCH (calculated from date of discharge), and discharge location were determined from administrative data.
Additional Descriptive Variables
Name, medical record number, gender, date of birth, date of admission and discharge, and primary diagnosis were obtained from the medical record. The Case Mix Index for each group of patients was determined from the average Medicare Severity‐adjusted Diagnosis Related Group (MS‐DRG) weight obtained from administrative data.
Data Collection
A two‐step, retrospective chart abstraction was employed. A professional research assistant (P.R.A.) hand‐abstracted process measures from the paper medical chart onto a data collection form designed for this study. A physician investigator performed a secondary review (H.L.W.). Discrepancies were resolved by the physician reviewer.
Data Analysis
Descriptive statistics were performed on intervention and control subjects. Means and standard deviations (age) or frequencies (gender, primary diagnoses) were calculated as appropriate. T tests were used for continuous variables, chi‐square tests for gender, and the Wilcoxon rank sum test for categorical variables.
Outcomes were reported as means and standard deviations for continuous variables (length of stay and charges) and frequencies for categorical variables (all other outcomes). T tests were used for continuous variables, Fisher's exact test for restraint use, and chi‐square tests were used for categorical variable to compare the impact of the intervention between intervention and control patients. For falls, confidence intervals were calculated for the incidence rate differences based on Poisson approximations.
Sample Size Considerations
An a priori sample size calculation was performed. A 2001 study showed that functional status is poorly documented in at least 60% of hospital charts of elderly patients.5 Given an estimated sample size of 120 per group and a power of 80%, this study was powered to be able to detect an absolute difference in the documentation of functional status of as little as 18%.
RESULTS
Two hundred seventeen patients met the study entry criteria (Table 2): 122 were admitted to the Hospitalist‐ACE service, and 95 were admitted to usual care on the general medical services. The average age of the study patients was 80.5 years, 55.3% were female. Twenty‐eight percent of subjects were admitted for pulmonary diagnoses. The two groups of patients were similar with respect to age, gender, and distribution of primary diagnoses. The Hospitalist‐ACE patients had a mean MS‐DRG weight of 1.15, which was slightly higher than that of usual care patients at 1.05 (P = 0.06). Typically, 70% of Hospitalist‐ACE patients are admitted to the designated ACE medical unit (12 West).
| Characteristic | Hospitalist‐ACE | Usual Care | P Value |
|---|---|---|---|
| N = 122 | N = 95 | ||
| |||
| Age (years), mean (SD) | 80.5 (6.5) | 80.7 (7.0) | 0.86 |
| Gender (% female) | 52.5 | 59 | 0.34 |
| Case Mix Index (mean MS‐DRG weight [SD]) | 1.15 (0.43) | 1.05 (0.31) | 0.06 |
| Primary ICD‐9 diagnosis (%) | 0.59 | ||
| Pulmonary | 27.9 | 28.4 | |
| General medicine | 15.6 | 11.6 | |
| Surgery | 13.9 | 11.6 | |
| Cardiology | 9.8 | 6.3 | |
| Nephrology | 8.2 | 7.4 | |
Processes of Care
Processes of care for older patients are displayed in Table 3. Patients on the Hospitalist‐ACE service had recognition and treatment of abnormal functional status at a rate that was nearly double that of patients on the usual care services (68.9% vs 35.8%, P < 0.0001). In addition, patients on the Hospitalist‐ACE service were significantly more likely to have had recognition and treatment of any abnormal cognitive status (55.7% vs 40.0%, P = 0.02). When delirium was evaluated alone, the Hospitalist‐ACE patients were also more likely to have had recognition and treatment of delirium (27.1% vs 17.0%, P = 0.08), although this finding did not reach statistical significance.
| Measure | Percent of Hospitalist‐ACE Patients | Percent of Usual Care Patients | P Value |
|---|---|---|---|
| N = 122 | N = 95 | ||
| |||
| Recognition and treatment of abnormal functional status | 68.9 | 35.8 | <0.0001 |
| Recognition and treatment of abnormal cognitive status* | 55.7 | 40.0 | 0.02 |
| Recognition and treatment of delirium | 27.1 | 17.0 | 0.08 |
| Documentation of resuscitation preferences | 95.1 | 91.6 | 0.3 |
| Do Not Attempt Resuscitation orders | 39.3 | 26.3 | 0.04 |
| Use of sleep medications | 28.1 | 27.4 | 0.91 |
| Use of physical restraints | 2.5 | 0 | 0.26 |
While patients on the Hospitalist‐ACE and usual care services had similar percentages of documentation of resuscitation preferences (95.1% vs 91.6%, P = 0.3), the percentage of Hospitalist‐ACE patients who had Do Not Attempt Resuscitation (DNAR) orders was significantly greater than that of the usual care patients (39.3% vs 26.3%, P = 0.04).
There were no differences in the use of physical restraints or sleep medications for Hospitalist‐ACE patients as compared to usual care patients, although the types of sleep mediations used on each service were markedly different: trazadone was employed as the first‐line sleep agent on the Hospitalist‐ACE service (77.7%), and non‐benzodiazepine hypnotics (primarily zolpidem) were employed most commonly on the usual care services (35%). There were no differences noted in the percentage of patients with benzodiazepines prescribed as sleep aids.
Outcomes
Resource utilization outcomes are reported in Table 4. Of note, there were no significant differences between Hospitalist‐ACE discharges and usual care discharges in mean length of stay (3.4 2.7 days vs 3.1 2.7 days, P = 0.52), mean charges ($24,617 15,828 vs $21,488 13,407, P = 0.12), or 30‐day readmissions to UCH (12.3% vs 9.5%, P = 0.51). Hospitalist‐ACE discharges and usual care patients were equally likely to be discharged to home (68.6% vs 67.4%, P = 0.84), with a similar proportion of Hospitalist‐ACE discharges receiving home health care or home hospice services (14.1% vs 7.4%, P = 12).
| Measure | Hospitalist‐ACE | Usual Care | P Value |
|---|---|---|---|
| N = 122 | N = 95 | ||
| |||
| Length of stay in days (mean [SD]) | 3.4 (2.7) | 3.1 (2.7) | 0.52 |
| Charges in dollars (mean [SD]) | 24,617 (15,828) | 21,488 (13,407) | 0.12 |
| 30‐Day readmissions to UCH (%) | 12.3 | 9.5 | 0.51 |
| Discharges to home (%) | 68.8* | 67.4 | 0.84 |
| Discharges to home with services (%) | 14%* | 7.4% | 0.12 |
In addition, the fall rate for Hospitalist‐ACE patients was not significantly different from the fall rate for usual care patients (4.8 falls/1000 patient days vs 6.7 falls/1000 patient days, 95% confidence interval 9.613.3).
DISCUSSION
We report the implementation and evaluation of a medical service tailored to the care of the acutely ill older patient that draws from elements of the hospitalist model and the ACE unit model.7, 14, 24 For this Hospitalist‐ACE service, we developed a specialized hospitalist workforce, assembled a brief geriatric assessment tailored to the inpatient setting, instituted an interdisciplinary rounding model, and created a novel inpatient geriatrics curriculum.
During the study period, we improved performance of important processes of care for hospitalized elders, including recognition of abnormal cognitive and functional status; maintained comparable resource use; and implemented a novel, inpatient‐focused geriatric medicine educational experience. We were unable to demonstrate an impact on key clinical outcomes such as falls, physical restraint use, and readmissions. Nonetheless, there is evidence that the performance of selected processes of care is associated with improved three‐year survival status in the community‐dwelling vulnerable older patient, and may also be associated with a mortality benefit in the hospitalized vulnerable older patient.25, 26 Therefore, methods to improve the performance of these processes of care may be of clinical importance.
The finding of increased use of DNAR orders in the face of equivalent documentation of code status is of interest and generates hypotheses for further study. It is possible that the educational experience and use of geriatric assessment provides a more complete context for the code status discussion (one that incorporates the patient's social, physical, and cognitive function). However, we do not know if the patients on the ACE service had improved concordance between their code status and their goals of care.
We believe that there was no difference in key clinical outcomes between Hospitalist‐ACE and control patients because the population in this study was relatively low acuity and, therefore, the occurrence of falls and the use of physical restraints were quite low in the study population. In particular, the readmission rate was much lower than is typical for the Medicare population at our hospital, making it challenging to draw conclusions about the impact of the intervention on readmissions, however, we cannot rule out the possibility that our early discharge planning did not address the determinants of readmission for this population.
The ACE unit paradigmcharacterized by 1) closed, modified hospital units; 2) staffing by geriatricians and nurses with geriatrics training; 3) employing geriatric nursing care protocolsrequires significant resources and is not feasible for all settings.6 There is a need for alternative models of comprehensive care for hospitalized elders that require fewer resources in the form of dedicated units and specialist personnel, and can be more responsive to institutional needs. For example, in a 2005 report, one institution reported the creation of a geriatric medicine service that utilized a geriatrician and hospitalist co‐attending model.14 More recently, a large geriatrics program replaced its inpatient geriatrics unit with a mobile inpatient geriatrics service staffed by an attending geriatricianhospitalist, a geriatrics fellow, and a nurse practitioner.27 While these innovative models have eliminated the dedicated unit, they rely on board certified geriatricians, a group in short supply nationally.28 Hospitalists are a rapidly growing provider group that, with appropriate training and building on the work of geriatricians, is poised to provide leadership in acute geriatric care.29, 30
In contrast to the comprehensive inpatient geriatric care models described above, the Hospitalist‐ACE service uses a specialized hospitalist workforce and is not dependent on continuous staffing by geriatricians. Although geographic concentration is important for the success of interdisciplinary rounds, the Hospitalist‐ACE service does not require a closed or modified unit. The nursing staff caring for Hospitalist‐ACE patients have generalist nursing training and, at the time of the study, did not utilize geriatric‐care protocols. Our results need to be interpreted in the light of these differences from the ACE unit model which is a significantly more intensive intervention than the Hospitalist‐ACE service. In addition, the current practice environment is quite different from the mid‐1990s when ACE units were developed and studied. Development and maintenance of models of comprehensive inpatient geriatric care require demonstration of both value as well as return on investment. The alignment of financial and regulatory incentives for programs that provide comprehensive care to complex patients, such as those anticipated by the Affordable Care Act, may encourage the growth of such models.
These data represent findings from a six‐month evaluation of a novel inpatient service in the middle of its first year. There are several limitations related to our study design. First, the results of this small study at a single academic medical center may be of limited generalizability to other settings. Second, the program was evaluated only three months after its inception; we did not capture further improvements in methods, training, and outcomes expected as the program matured. Third, most of the Hospitalist‐ACE service attendings and residents rotate on the UCH general medical services throughout the year. Consequently, we were unable to eliminate the possibility of contamination of the control group, and we were unable to blind the physicians to the study. Fourth, the study population had a relatively low severity of illnessthe average MS‐DRG weight was near 1and low rates of important adverse events such falls and restraint use. This may have occurred because we excluded patients transferred from the ICUs and other services. It is possible that the Hospitalist‐ACE intervention might have demonstrated a larger benefit in a sicker population that would have presented greater opportunities for reductions in length of stay, costs, and adverse events. Fifth, given the retrospective nature of the data collection, we were not able to prospectively assess the incidence of important geriatric outcomes such as delirium and functional decline, nor can we make conclusions about changes in function during the hospitalization.
While the outcome measures we used are conceptually similar to several measures developed by RAND's Assessing Care of Vulnerable Elders (ACOVE) project, this study did not explicitly rely on those constructs.31 To do so would have required prospective screening by clinical staff independent from the care team for vulnerability that was beyond the scope of this project. In addition, the ACOVE measures of interest for functional and cognitive decline are limited to documentation of cognitive or functional assessments in the medical record. The ACE service's adoption of a brief standardized geriatric assessment was almost certain to meet that documentation requirement. While documentation is important, it is not clear that documentation, in and of itself, improves outcomes. Therefore, we expanded upon the ACOVE constructs to include the need for the additional evidence of a treatment plan when abnormal physical or cognitive function was documented. These constructs are important process of care for vulnerable elders. While we demonstrated improvements in several of these important processes of care for elderly patients, we are unable to draw conclusions about the impact of these differences in care on important clinical outcomes such as development of delirium, long‐term institutionalization, or mortality.
CONCLUSIONS
The risks of hospitalization for older persons are numerous, and present challenges and opportunities for inpatient physicians. As the hospitalized population agesmirroring national demographic trends and trends in use of acute care hospitalsthe challenge of avoiding harm in the older hospitalized patient will intensify. Innovations in care to improve the experience and outcomes of hospitalization for older patients are needed in the face of limited geriatrics‐trained workforce and few discretionary funds for unit redesign. The Hospitalist‐ACE service is a promising strategy for hospitalist programs with sufficient numbers of older patients and hospitalists with interest in improving clinical care for older adults. It provides a model for hospitalists to employ geriatrics principles targeted at reducing harm to their most vulnerable patients. Hospitalist‐run geriatric care models offer great promise for improving the care of acutely ill elderly patients. Future investigation should focus on demonstrating the impact of such care on important clinical outcomes between admission and discharge; on model refinement and adaptation, such as determining what components of comprehensive geriatric care are essential to success; and on how complementary interventions, such as the use of templated orders for the hospitalized elderly, impact outcomes. Additional research is needed, with a focus on demonstrating value with regard to an array of outcomes including cost, readmissions, and preventable harms of care.
Acknowledgements
Jean Kutner, MD, MSPH; Daniel Sandy, MPH; Shelly Limon, RN; nurses of 12 West; the UCH staff on the interdisciplinary team; and ACE patients and their families.
- ,,, et al.Functional outcomes of acute medical illness and hospitalization in older persons.Arch Intern Med.1996;156:645–652.
- ,,.Delirium: a symptom of how hospital care is failing older persons and a window to improve quality of hospital care.Am J Med.1999;106:565–573.
- ,,, et al.Using assessing care of vulnerable elders quality indicators to measure quality of hospital care for vulnerable elders.J Am Geriatr Soc.2007;55(11):1705–1711.
- ,,, et al.Impact and recognition of cognitive impairment among hospitalized elders.J Hosp Med.2010;5:69–75.
- ,,,,.What does the medical record reveal about functional status? A comparison of medical record and interview data.J Gen Intern Med.2001;16(11):728–736.
- ,,,,,.Successful models of comprehensive care for older adults with chronic conditions: evidence for the Institute of Medicine's “Retooling for an Aging America” report.J Am Geriatr Soc.2009;57(12):2328–2337.
- ,,,,.A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients.N Engl J Med.1995;332:1338–1344.
- ,,, et al.Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older adults: a randomized controlled trial of Acute Care for Elders (ACE) in a community hospital.J Am Geriatr Soc.2000;48:1572–1581.
- ,,, et al.The effectiveness of inpatient geriatric evaluation and management units: a systematic review and meta‐analysis.J Am Geriatr Soc.2010;58:83–92.
- ,,,,.Effectiveness of acute geriatric units on functional decline, living at home, and case fatality among older patients admitted to hospital for acute medical disorders: meta‐analysis.BMJ.2009;338:b50.
- ,,, et al.A randomized, controlled clinical trial of a geriatrics consultation team: compliance with recommendations.JAMA.1986;255:2617–2621.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340:669–676.
- ,,,.Dissemination and characteristics of Acute Care of Elders (ACE) units in the United States.Int J Technol Assess Health Care.2003;19:220–227.
- ,,.Is there a geriatrician in the house? Geriatric care approaches in hospitalist programs.J Hosp Med.2006;1:29–35.
- ,,,,.The Mini‐Cog: a cognitive “vital signs” measure for dementia screening in multi‐lingual elderly.Int J Geriatr Psychiatry.2000;15(11):1021–1027.
- ,,.The Patient Health Questionnaire‐2: validity of a two‐item depression screener.Med Care.2003;41:1284–1292.
- ,,,,,.Clarifying confusion: the Confusion Assessment Method.Ann Intern Med.1990;113(12):941–948.
- ,,, et al.The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community.J Am Geriatr Soc.2001;49:1691–1699.
- ,.The timed “Up and Go”: a test of basic functional mobility for frail elderly persons.J Am Geriatr Soc.1991;39:142–148.
- American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopedic Surgeons Panel on Falls Prevention.Guideline for the prevention of falls in older persons.J Am Geriatr Soc.2001;49:664–672.
- . Falls for the inpatient physician. Translating knowledge into action. The Portal of Online Geriatric Education (POGOe). 6–19‐2008. Available at: http://www.pogoe.org/productid/20212.
- ,,,. Incontinence and urinary catheters for the inpatient physician. The Portal of Online Geriatric Education (POGOe). 11–27‐0008. Available at: http://www.pogoe.org/productid/20296.
- ,,,.Use of medications for insomnia in the hospitalized geriatric population.J Am Geriatr Soc.2008;56(3):579–581.
- ,,,.Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians.Ann Intern Med.1999;130(4 pt 2):343–349.
- ,,, et al.Quality of care associated with survival in vulnerable older patients.Ann Intern Med.2005;143:274–281.
- ,,, et al.Higher quality of care for hosptialized frail older adults is associated with improved survival one year after discharge.J Hosp Med.2009;4(S1):24.
- ,,,.Operational and quality outcomes of a novel mobile acute care for the elderly service.J Am Geriatr Soc.2009;57:S1.
- Institute of Medicine (IOM).Retooling for an Aging America: Building the Health Care Workforce.Washington, DC:The National Academies Press;2008.
- ,,.Alternative solutions to the geriatric workforce deficit.Am J Med.2008;121:e23.
- ,,,,.Fulfilling the promise of hospital medicine: tailoring internal medicine training to address hospitalists' needs.J Gen Intern Med.2008;23(7):1110–1115.
- ,.Assessing care of vulnerable elders: ACOVE project overview.Ann Intern Med.2001;135(8 pt 2):642–646.
For the frail older patient, hospitalization marks a period of high risk of poor outcomes and adverse events including functional decline, delirium, pressure ulcers, adverse drug events, nosocomial infections, and falls.1, 2 Physician recognition of elderly patients at risk for adverse outcomes is poor, making it difficult to intervene to prevent them.3, 4 Among frail, elderly inpatients at an urban academic medical center, doctors documented cognitive assessments in only 5% of patients. Functional assessments are appropriately documented in 40%80% of inpatients.3, 5
The Acute Care for Elders (ACE) unit is one of several models of comprehensive inpatient geriatric care that have been developed by geriatrician researchers to address the adverse events and functional decline that often accompany hospitalization.6 The ACE unit model generally incorporates: 1) a modified hospital environment, 2) early assessment and intensive management to minimize the adverse effects of hospital care, 3) early discharge planning, 4) patient centered care protocols, and 5) a consistent nursing staff.7 Two randomized, controlled trials have shown the ACE unit model to be successful in reducing functional decline among frail older inpatients during and after hospitalization.7, 8 While meta‐analyses data also suggests the ACE unit model reduces functional decline and future institutionalization, significant impact on other outcomes is not proven.9, 10
Several barriers have prevented the successful dissemination of the ACE unit model. The chief limitations are the upfront resources required to create and maintain a modified, dedicated unit, as well as the lack of a geriatrics trained workforce.7, 1113 The rapid growth of hospital medicine presents opportunities for innovation in the care of older patients. Still, a 2006 census demonstrated that few hospitalist groups had identified geriatric care as a priority.14
In response to these challenges, the University of Colorado Hospital Medicine Group created a hospitalist‐run inpatient medical service designed for the care of the frail older patient. This Hospitalist‐Acute Care for the Elderly (Hospitalist‐ACE) unit is a hybrid of a general medical service and an inpatient geriatrics unit.7 The goals of the Hospitalist‐ACE service are to provide high quality care tailored to older inpatients, thus minimizing the risks of functional decline and adverse events associate with hospitalization, and to provide a clinical geriatrics teaching experience for Hospitalist Training Track Residents within the Internal Medicine Residency Training Program and medical students at the University of Colorado Denver School of Medicine. The Hospitalist‐ACE unit is staffed with a core group of hospitalist attendings who have, at a minimum, attended an intensive mini‐course in inpatient geriatrics. The service employs interdisciplinary rounds; a brief, standardized geriatric assessment including screens of function, cognition, and mood; a clinical focus on mitigating the hazards of hospitalization, early discharge planning; and a novel geriatric educational curriculum for medicine residents and medical students.
This article will: 1) describe the creation of the Hospitalist‐ACE service at the University of Colorado Hospital; and 2) summarize the evaluation of the Hospitalist‐ACE service in a quasi‐randomized, controlled manner during its first year. We hypothesized that, when compared to patients receiving usual care, patients cared for on the Hospitalist‐ACE service would have increased recognition of abnormal functional status; recognition of abnormal cognitive status and delirium; equivalent lengths of stay and hospital charges; and decreased falls, 30‐day readmissions, and restraint use.
METHODS
Design
We performed a quasi‐randomized, controlled study of the Hospitalist‐ACE service.
Setting
The study setting was the inpatient general medical services of the Anschutz Inpatient Pavilion (AIP) of the University of Colorado Hospital (UCH). The AIP is a 425‐bed tertiary care hospital that is the major teaching affiliate of the University of Colorado School of Medicine and a regional referral center. The control services, hereafter referred to as usual care, were comprised of the four inpatient general medicine teaching services that take admissions on a four‐day rotation (in general, two were staffed by outpatient general internists and medical subspecialists, and two were staffed by academic hospitalists). The Hospitalist‐ACE service was a novel hospitalist teaching service that began in July 2007. Hospitalist‐ACE patients were admitted to a single 12‐bed medical unit (12 West) when beds were available; 12 West is similar to the other medical/surgical units at UCH and did not have any modifications to the rooms, equipment, or common areas for the intervention. The nursing staff on this unit had no formal geriatric nursing training. The Hospitalist‐ACE team admitted patients daily (between 7 AM and 3 PM MondayFriday; between 7 AM and 12 noon Saturday and Sunday). Patients assigned to the Hospitalist‐ACE service after hours were admitted by the internal medicine resident on call for the usual care services and handed off to the Hospitalist‐ACE team at 7 AM the next morning.
Study Subjects
Eligible subjects were inpatients age 70 years admitted to the usual care or Hospitalist‐ACE services at the AIP from November 2, 2007 to April 15, 2008. All patients age 70 years were randomized to the Hospitalist‐ACE service or usual care on a general internal medicine service by the last digit of the medical record number (odd numbers admitted to the Hospitalist‐ACE service and even numbers admitted to usual care). Patients followed by the Hospitalist‐ACE service but not admitted to 12 West were included in the study. To isolate the impact of the intervention, patients admitted to a medicine subspecialty service (such as cardiology, pulmonary, or oncology), or transferred to or from the Hospitalist‐ACE or control services to another service (eg, intensive care unit [ICU] or orthopedic surgery service) were excluded from the study.
Intervention
The Hospitalist‐ACE unit implemented an interdisciplinary team approach to identify and address geriatric syndromes in patients aged 70 and over. The Hospitalist‐ACE model of care consisted of clinical care provided by a hospitalist attending with additional training in geriatric medicine, administration of standardized geriatric screens assessing function, cognition, and mood, 15 minute daily (MondayFriday) interdisciplinary rounds focusing on recognition and management of geriatric syndromes and early discharge planning, and a standardized educational curriculum for medical residents and medical students addressing hazards of hospitalization.
The Hospitalist‐ACE service was a unique rotation within the Hospitalist Training Track of the Internal Medicine Residency that was developed with the support of the University of Colorado Hospital and the Internal Medicine Residency Training Program, and input from the Geriatrics Division at the University of Colorado Denver. The director received additional training from the Donald W. Reynolds FoundationUCLA Faculty Development to Advance Geriatric Education Mini‐Fellowship for hospitalist faculty. The mission of the service was to excel at educating the next generation of hospitalists while providing a model for excellence of care for hospitalized elderly patients. Important stakeholders were identified, and a leadership teamincluding representatives from nursing, physical and occupational therapy, pharmacy, social work, case management, and later, volunteer servicescreated the model daily interdisciplinary rounds. As geographic concentration was essential for the viability of interdisciplinary rounds, one unit (12 West) within the hospital was designated as the preferred location for patients admitted to the Hospitalist‐ACE service.
The Hospitalist‐ACE unit team consisted of one attending hospitalist, one resident, one intern, and medical students. The attending was one of five hospitalists, with additional training in geriatric medicine, who rotated attending responsibilities on the service. One of the hospitalists was board certified in geriatric medicine. Each of the other four hospitalists attended the Reynolds FoundationUCLA mini‐fellowship in geriatric medicine. Hospitalist‐ACE attendings rotated on a variety of other hospitalist services throughout the academic year, including the usual care services.
The brief standardized geriatric assessment consisted of six validated instruments, and was completed by house staff or medical students on admission, following instruction by the attending physician. The complete assessment tool is shown in Figure 1. The cognitive items included the Mini‐Cog,15 a two‐item depression screen,16 and the Confusion Assessment Method.17 The functional items included the Vulnerable Elders Survey (VES‐13),18 the Timed Get Up and Go test,19 and a two‐question falls screen.20 The elements of the assessment tool were selected by the Hospitalist‐ACE attendings for brevity and the potential to inform clinical management. To standardize the clinical and educational approach, the Hospitalist‐ACE attendings regularly discussed appropriate orders recommended in response to each positive screen, but no templated order sets were used during the study period.

Interdisciplinary rounds were attended by Hospitalist‐ACE physicians, nurses, case managers, social workers, physical or occupational therapists, pharmacists, and volunteers. Rounds were led by the attending or medical resident.
The educational curriculum encompassed 13 modules created by the attending faculty that cover delirium, falls, dementia, pressure ulcers, physiology of aging, movement disorders, medication safety, end of life care, advance directives, care transitions, financing of health care for the elderly, and ethical conundrums in the care of the elderly. A full table of contents appears in online Appendix 1. Additionally, portions of the curriculum have been published online.21, 22 Topic selection was guided by the Accreditation Council for Graduate Medical Education (ACGME) core geriatrics topics determined most relevant for the inpatient setting. Formal instruction of 3045 minutes duration occurred three to four days a week and was presented in addition to routine internal medicine educational conferences. Attendings coordinated teaching to ensure that each trainee was exposed to all of the content during the course of their four‐week rotation.
In contrast to the Hospitalist‐ACE service, usual care on the control general medical services consisted of either a hospitalist, a general internist, or an internal medicine subspecialist attending physician, with one medical resident, one intern, and medical students admitting every fourth day. The general medical teams attended daily discharge planning rounds with a discharge planner and social worker focused exclusively on discharge planning. The content of teaching rounds on the general medical services was largely left to the discretion of the attending physician.
This program evaluation of the Hospitalist‐ACE service was granted a waiver of consent and Health Insurance Portability and Accountability Act (HIPAA) by the Colorado Multiple Institutional Review Board.
Measures
Primary Outcome
The primary outcome for the study was the recognition of abnormal functional status by the primary team. Recognition of abnormal functional status was determined from chart review and consisted of both the physician's detection of abnormal functional status and evidence of a corresponding treatment plan identified in the notes or orders of a physician member of the primary team (Table 1).
| Measure | Criterion | Source | Content Examples |
|---|---|---|---|
| |||
| Recognition of abnormal functional status* | 1) Detection | MD's documentation of history | Presentation with change in function (new gait instability); use of gait aides (wheelchair) |
| OR | |||
| MD's documentation of physical exam | Observation of abnormal gait (eg, unsteady, wide‐based, shuffling) and/or balance Abnormal Get Up and Go test | ||
| AND | |||
| 2) Treatment | MD's order | PT/OT consult; home safety evaluation | |
| OR | |||
| MD's documentation assessment/plan | Inclusion of functional status (rehabilitation, PT/OT needs) on the MD's problem list | ||
| Recognition of abnormal cognitive status | Any of the following: | ||
| Delirium | 1) Detection | MD's history | Presentation of confusion or altered mental status |
| OR | |||
| MD's physical exam | Abnormal confusion assessment method | ||
| AND | |||
| 2) Treatment | MD's order | Sitter, reorienting communication, new halperidol order | |
| OR | |||
| MD's documentation of assessment/plan | Inclusion of delirium on the problem list | ||
| OR | |||
| Dementia | 1) Detection | MD's history | Dementia in medical history |
| OR | OR | ||
| MD's physical exam | Abnormal Folstein Mini‐Mental Status Exam or Mini‐Cog | ||
| AND | |||
| 2) Treatment | MD's order | Cholinesterase inhibitor ordered | |
| OR | OR | ||
| MD's documentation of assessment/plan | Inclusion of dementia on the problem list | ||
| OR | |||
| Depression | 1) Detection | MD's history | Depression in medical history |
| OR | OR | ||
| MD's physical exam | Positive depression screen | ||
| AND | |||
| 2) Treatment | MD's order | New antidepressant order | |
| OR | |||
| MD's documentation of assessment/plan | Inclusion of depression on the problem list | ||
Secondary Outcomes
Recognition of abnormal cognitive status was determined from chart review and consisted of both the physician's detection of dementia, depression, or delirium, and evidence of a corresponding treatment plan for any of the documented conditions identified in the notes or orders of a physician member of the primary team (Table 1). Additionally, we measured recognition and treatment of delirium alone.
Falls were determined from mandatory event reporting collected by the hospital on the University Hospitals Consortium Patient Safety Net web‐based reporting system and based on clinical assessment as reported by the nursing staff. The reports are validated by the appropriate clinical managers within 45 days of the event according to standard procedure.
Physical restraint use (type of restraint and duration) was determined from query of mandatory clinical documentation in the electronic medical record. Use of sleep aids was determined from review of the physician's order sheets in the medical record. The chart review captured any of 39 commonly prescribed hypnotic medications ordered at hour of sleep or for insomnia. The sleep medication list was compiled with the assistance of a pharmacist for an earlier chart review and included non‐benzodiazepine hypnotics, benzodiazepines, antidepressants, antihistamines, and antipsychotics.23
Length of stay, hospital charges, 30‐day readmissions to UCH (calculated from date of discharge), and discharge location were determined from administrative data.
Additional Descriptive Variables
Name, medical record number, gender, date of birth, date of admission and discharge, and primary diagnosis were obtained from the medical record. The Case Mix Index for each group of patients was determined from the average Medicare Severity‐adjusted Diagnosis Related Group (MS‐DRG) weight obtained from administrative data.
Data Collection
A two‐step, retrospective chart abstraction was employed. A professional research assistant (P.R.A.) hand‐abstracted process measures from the paper medical chart onto a data collection form designed for this study. A physician investigator performed a secondary review (H.L.W.). Discrepancies were resolved by the physician reviewer.
Data Analysis
Descriptive statistics were performed on intervention and control subjects. Means and standard deviations (age) or frequencies (gender, primary diagnoses) were calculated as appropriate. T tests were used for continuous variables, chi‐square tests for gender, and the Wilcoxon rank sum test for categorical variables.
Outcomes were reported as means and standard deviations for continuous variables (length of stay and charges) and frequencies for categorical variables (all other outcomes). T tests were used for continuous variables, Fisher's exact test for restraint use, and chi‐square tests were used for categorical variable to compare the impact of the intervention between intervention and control patients. For falls, confidence intervals were calculated for the incidence rate differences based on Poisson approximations.
Sample Size Considerations
An a priori sample size calculation was performed. A 2001 study showed that functional status is poorly documented in at least 60% of hospital charts of elderly patients.5 Given an estimated sample size of 120 per group and a power of 80%, this study was powered to be able to detect an absolute difference in the documentation of functional status of as little as 18%.
RESULTS
Two hundred seventeen patients met the study entry criteria (Table 2): 122 were admitted to the Hospitalist‐ACE service, and 95 were admitted to usual care on the general medical services. The average age of the study patients was 80.5 years, 55.3% were female. Twenty‐eight percent of subjects were admitted for pulmonary diagnoses. The two groups of patients were similar with respect to age, gender, and distribution of primary diagnoses. The Hospitalist‐ACE patients had a mean MS‐DRG weight of 1.15, which was slightly higher than that of usual care patients at 1.05 (P = 0.06). Typically, 70% of Hospitalist‐ACE patients are admitted to the designated ACE medical unit (12 West).
| Characteristic | Hospitalist‐ACE | Usual Care | P Value |
|---|---|---|---|
| N = 122 | N = 95 | ||
| |||
| Age (years), mean (SD) | 80.5 (6.5) | 80.7 (7.0) | 0.86 |
| Gender (% female) | 52.5 | 59 | 0.34 |
| Case Mix Index (mean MS‐DRG weight [SD]) | 1.15 (0.43) | 1.05 (0.31) | 0.06 |
| Primary ICD‐9 diagnosis (%) | 0.59 | ||
| Pulmonary | 27.9 | 28.4 | |
| General medicine | 15.6 | 11.6 | |
| Surgery | 13.9 | 11.6 | |
| Cardiology | 9.8 | 6.3 | |
| Nephrology | 8.2 | 7.4 | |
Processes of Care
Processes of care for older patients are displayed in Table 3. Patients on the Hospitalist‐ACE service had recognition and treatment of abnormal functional status at a rate that was nearly double that of patients on the usual care services (68.9% vs 35.8%, P < 0.0001). In addition, patients on the Hospitalist‐ACE service were significantly more likely to have had recognition and treatment of any abnormal cognitive status (55.7% vs 40.0%, P = 0.02). When delirium was evaluated alone, the Hospitalist‐ACE patients were also more likely to have had recognition and treatment of delirium (27.1% vs 17.0%, P = 0.08), although this finding did not reach statistical significance.
| Measure | Percent of Hospitalist‐ACE Patients | Percent of Usual Care Patients | P Value |
|---|---|---|---|
| N = 122 | N = 95 | ||
| |||
| Recognition and treatment of abnormal functional status | 68.9 | 35.8 | <0.0001 |
| Recognition and treatment of abnormal cognitive status* | 55.7 | 40.0 | 0.02 |
| Recognition and treatment of delirium | 27.1 | 17.0 | 0.08 |
| Documentation of resuscitation preferences | 95.1 | 91.6 | 0.3 |
| Do Not Attempt Resuscitation orders | 39.3 | 26.3 | 0.04 |
| Use of sleep medications | 28.1 | 27.4 | 0.91 |
| Use of physical restraints | 2.5 | 0 | 0.26 |
While patients on the Hospitalist‐ACE and usual care services had similar percentages of documentation of resuscitation preferences (95.1% vs 91.6%, P = 0.3), the percentage of Hospitalist‐ACE patients who had Do Not Attempt Resuscitation (DNAR) orders was significantly greater than that of the usual care patients (39.3% vs 26.3%, P = 0.04).
There were no differences in the use of physical restraints or sleep medications for Hospitalist‐ACE patients as compared to usual care patients, although the types of sleep mediations used on each service were markedly different: trazadone was employed as the first‐line sleep agent on the Hospitalist‐ACE service (77.7%), and non‐benzodiazepine hypnotics (primarily zolpidem) were employed most commonly on the usual care services (35%). There were no differences noted in the percentage of patients with benzodiazepines prescribed as sleep aids.
Outcomes
Resource utilization outcomes are reported in Table 4. Of note, there were no significant differences between Hospitalist‐ACE discharges and usual care discharges in mean length of stay (3.4 2.7 days vs 3.1 2.7 days, P = 0.52), mean charges ($24,617 15,828 vs $21,488 13,407, P = 0.12), or 30‐day readmissions to UCH (12.3% vs 9.5%, P = 0.51). Hospitalist‐ACE discharges and usual care patients were equally likely to be discharged to home (68.6% vs 67.4%, P = 0.84), with a similar proportion of Hospitalist‐ACE discharges receiving home health care or home hospice services (14.1% vs 7.4%, P = 12).
| Measure | Hospitalist‐ACE | Usual Care | P Value |
|---|---|---|---|
| N = 122 | N = 95 | ||
| |||
| Length of stay in days (mean [SD]) | 3.4 (2.7) | 3.1 (2.7) | 0.52 |
| Charges in dollars (mean [SD]) | 24,617 (15,828) | 21,488 (13,407) | 0.12 |
| 30‐Day readmissions to UCH (%) | 12.3 | 9.5 | 0.51 |
| Discharges to home (%) | 68.8* | 67.4 | 0.84 |
| Discharges to home with services (%) | 14%* | 7.4% | 0.12 |
In addition, the fall rate for Hospitalist‐ACE patients was not significantly different from the fall rate for usual care patients (4.8 falls/1000 patient days vs 6.7 falls/1000 patient days, 95% confidence interval 9.613.3).
DISCUSSION
We report the implementation and evaluation of a medical service tailored to the care of the acutely ill older patient that draws from elements of the hospitalist model and the ACE unit model.7, 14, 24 For this Hospitalist‐ACE service, we developed a specialized hospitalist workforce, assembled a brief geriatric assessment tailored to the inpatient setting, instituted an interdisciplinary rounding model, and created a novel inpatient geriatrics curriculum.
During the study period, we improved performance of important processes of care for hospitalized elders, including recognition of abnormal cognitive and functional status; maintained comparable resource use; and implemented a novel, inpatient‐focused geriatric medicine educational experience. We were unable to demonstrate an impact on key clinical outcomes such as falls, physical restraint use, and readmissions. Nonetheless, there is evidence that the performance of selected processes of care is associated with improved three‐year survival status in the community‐dwelling vulnerable older patient, and may also be associated with a mortality benefit in the hospitalized vulnerable older patient.25, 26 Therefore, methods to improve the performance of these processes of care may be of clinical importance.
The finding of increased use of DNAR orders in the face of equivalent documentation of code status is of interest and generates hypotheses for further study. It is possible that the educational experience and use of geriatric assessment provides a more complete context for the code status discussion (one that incorporates the patient's social, physical, and cognitive function). However, we do not know if the patients on the ACE service had improved concordance between their code status and their goals of care.
We believe that there was no difference in key clinical outcomes between Hospitalist‐ACE and control patients because the population in this study was relatively low acuity and, therefore, the occurrence of falls and the use of physical restraints were quite low in the study population. In particular, the readmission rate was much lower than is typical for the Medicare population at our hospital, making it challenging to draw conclusions about the impact of the intervention on readmissions, however, we cannot rule out the possibility that our early discharge planning did not address the determinants of readmission for this population.
The ACE unit paradigmcharacterized by 1) closed, modified hospital units; 2) staffing by geriatricians and nurses with geriatrics training; 3) employing geriatric nursing care protocolsrequires significant resources and is not feasible for all settings.6 There is a need for alternative models of comprehensive care for hospitalized elders that require fewer resources in the form of dedicated units and specialist personnel, and can be more responsive to institutional needs. For example, in a 2005 report, one institution reported the creation of a geriatric medicine service that utilized a geriatrician and hospitalist co‐attending model.14 More recently, a large geriatrics program replaced its inpatient geriatrics unit with a mobile inpatient geriatrics service staffed by an attending geriatricianhospitalist, a geriatrics fellow, and a nurse practitioner.27 While these innovative models have eliminated the dedicated unit, they rely on board certified geriatricians, a group in short supply nationally.28 Hospitalists are a rapidly growing provider group that, with appropriate training and building on the work of geriatricians, is poised to provide leadership in acute geriatric care.29, 30
In contrast to the comprehensive inpatient geriatric care models described above, the Hospitalist‐ACE service uses a specialized hospitalist workforce and is not dependent on continuous staffing by geriatricians. Although geographic concentration is important for the success of interdisciplinary rounds, the Hospitalist‐ACE service does not require a closed or modified unit. The nursing staff caring for Hospitalist‐ACE patients have generalist nursing training and, at the time of the study, did not utilize geriatric‐care protocols. Our results need to be interpreted in the light of these differences from the ACE unit model which is a significantly more intensive intervention than the Hospitalist‐ACE service. In addition, the current practice environment is quite different from the mid‐1990s when ACE units were developed and studied. Development and maintenance of models of comprehensive inpatient geriatric care require demonstration of both value as well as return on investment. The alignment of financial and regulatory incentives for programs that provide comprehensive care to complex patients, such as those anticipated by the Affordable Care Act, may encourage the growth of such models.
These data represent findings from a six‐month evaluation of a novel inpatient service in the middle of its first year. There are several limitations related to our study design. First, the results of this small study at a single academic medical center may be of limited generalizability to other settings. Second, the program was evaluated only three months after its inception; we did not capture further improvements in methods, training, and outcomes expected as the program matured. Third, most of the Hospitalist‐ACE service attendings and residents rotate on the UCH general medical services throughout the year. Consequently, we were unable to eliminate the possibility of contamination of the control group, and we were unable to blind the physicians to the study. Fourth, the study population had a relatively low severity of illnessthe average MS‐DRG weight was near 1and low rates of important adverse events such falls and restraint use. This may have occurred because we excluded patients transferred from the ICUs and other services. It is possible that the Hospitalist‐ACE intervention might have demonstrated a larger benefit in a sicker population that would have presented greater opportunities for reductions in length of stay, costs, and adverse events. Fifth, given the retrospective nature of the data collection, we were not able to prospectively assess the incidence of important geriatric outcomes such as delirium and functional decline, nor can we make conclusions about changes in function during the hospitalization.
While the outcome measures we used are conceptually similar to several measures developed by RAND's Assessing Care of Vulnerable Elders (ACOVE) project, this study did not explicitly rely on those constructs.31 To do so would have required prospective screening by clinical staff independent from the care team for vulnerability that was beyond the scope of this project. In addition, the ACOVE measures of interest for functional and cognitive decline are limited to documentation of cognitive or functional assessments in the medical record. The ACE service's adoption of a brief standardized geriatric assessment was almost certain to meet that documentation requirement. While documentation is important, it is not clear that documentation, in and of itself, improves outcomes. Therefore, we expanded upon the ACOVE constructs to include the need for the additional evidence of a treatment plan when abnormal physical or cognitive function was documented. These constructs are important process of care for vulnerable elders. While we demonstrated improvements in several of these important processes of care for elderly patients, we are unable to draw conclusions about the impact of these differences in care on important clinical outcomes such as development of delirium, long‐term institutionalization, or mortality.
CONCLUSIONS
The risks of hospitalization for older persons are numerous, and present challenges and opportunities for inpatient physicians. As the hospitalized population agesmirroring national demographic trends and trends in use of acute care hospitalsthe challenge of avoiding harm in the older hospitalized patient will intensify. Innovations in care to improve the experience and outcomes of hospitalization for older patients are needed in the face of limited geriatrics‐trained workforce and few discretionary funds for unit redesign. The Hospitalist‐ACE service is a promising strategy for hospitalist programs with sufficient numbers of older patients and hospitalists with interest in improving clinical care for older adults. It provides a model for hospitalists to employ geriatrics principles targeted at reducing harm to their most vulnerable patients. Hospitalist‐run geriatric care models offer great promise for improving the care of acutely ill elderly patients. Future investigation should focus on demonstrating the impact of such care on important clinical outcomes between admission and discharge; on model refinement and adaptation, such as determining what components of comprehensive geriatric care are essential to success; and on how complementary interventions, such as the use of templated orders for the hospitalized elderly, impact outcomes. Additional research is needed, with a focus on demonstrating value with regard to an array of outcomes including cost, readmissions, and preventable harms of care.
Acknowledgements
Jean Kutner, MD, MSPH; Daniel Sandy, MPH; Shelly Limon, RN; nurses of 12 West; the UCH staff on the interdisciplinary team; and ACE patients and their families.
For the frail older patient, hospitalization marks a period of high risk of poor outcomes and adverse events including functional decline, delirium, pressure ulcers, adverse drug events, nosocomial infections, and falls.1, 2 Physician recognition of elderly patients at risk for adverse outcomes is poor, making it difficult to intervene to prevent them.3, 4 Among frail, elderly inpatients at an urban academic medical center, doctors documented cognitive assessments in only 5% of patients. Functional assessments are appropriately documented in 40%80% of inpatients.3, 5
The Acute Care for Elders (ACE) unit is one of several models of comprehensive inpatient geriatric care that have been developed by geriatrician researchers to address the adverse events and functional decline that often accompany hospitalization.6 The ACE unit model generally incorporates: 1) a modified hospital environment, 2) early assessment and intensive management to minimize the adverse effects of hospital care, 3) early discharge planning, 4) patient centered care protocols, and 5) a consistent nursing staff.7 Two randomized, controlled trials have shown the ACE unit model to be successful in reducing functional decline among frail older inpatients during and after hospitalization.7, 8 While meta‐analyses data also suggests the ACE unit model reduces functional decline and future institutionalization, significant impact on other outcomes is not proven.9, 10
Several barriers have prevented the successful dissemination of the ACE unit model. The chief limitations are the upfront resources required to create and maintain a modified, dedicated unit, as well as the lack of a geriatrics trained workforce.7, 1113 The rapid growth of hospital medicine presents opportunities for innovation in the care of older patients. Still, a 2006 census demonstrated that few hospitalist groups had identified geriatric care as a priority.14
In response to these challenges, the University of Colorado Hospital Medicine Group created a hospitalist‐run inpatient medical service designed for the care of the frail older patient. This Hospitalist‐Acute Care for the Elderly (Hospitalist‐ACE) unit is a hybrid of a general medical service and an inpatient geriatrics unit.7 The goals of the Hospitalist‐ACE service are to provide high quality care tailored to older inpatients, thus minimizing the risks of functional decline and adverse events associate with hospitalization, and to provide a clinical geriatrics teaching experience for Hospitalist Training Track Residents within the Internal Medicine Residency Training Program and medical students at the University of Colorado Denver School of Medicine. The Hospitalist‐ACE unit is staffed with a core group of hospitalist attendings who have, at a minimum, attended an intensive mini‐course in inpatient geriatrics. The service employs interdisciplinary rounds; a brief, standardized geriatric assessment including screens of function, cognition, and mood; a clinical focus on mitigating the hazards of hospitalization, early discharge planning; and a novel geriatric educational curriculum for medicine residents and medical students.
This article will: 1) describe the creation of the Hospitalist‐ACE service at the University of Colorado Hospital; and 2) summarize the evaluation of the Hospitalist‐ACE service in a quasi‐randomized, controlled manner during its first year. We hypothesized that, when compared to patients receiving usual care, patients cared for on the Hospitalist‐ACE service would have increased recognition of abnormal functional status; recognition of abnormal cognitive status and delirium; equivalent lengths of stay and hospital charges; and decreased falls, 30‐day readmissions, and restraint use.
METHODS
Design
We performed a quasi‐randomized, controlled study of the Hospitalist‐ACE service.
Setting
The study setting was the inpatient general medical services of the Anschutz Inpatient Pavilion (AIP) of the University of Colorado Hospital (UCH). The AIP is a 425‐bed tertiary care hospital that is the major teaching affiliate of the University of Colorado School of Medicine and a regional referral center. The control services, hereafter referred to as usual care, were comprised of the four inpatient general medicine teaching services that take admissions on a four‐day rotation (in general, two were staffed by outpatient general internists and medical subspecialists, and two were staffed by academic hospitalists). The Hospitalist‐ACE service was a novel hospitalist teaching service that began in July 2007. Hospitalist‐ACE patients were admitted to a single 12‐bed medical unit (12 West) when beds were available; 12 West is similar to the other medical/surgical units at UCH and did not have any modifications to the rooms, equipment, or common areas for the intervention. The nursing staff on this unit had no formal geriatric nursing training. The Hospitalist‐ACE team admitted patients daily (between 7 AM and 3 PM MondayFriday; between 7 AM and 12 noon Saturday and Sunday). Patients assigned to the Hospitalist‐ACE service after hours were admitted by the internal medicine resident on call for the usual care services and handed off to the Hospitalist‐ACE team at 7 AM the next morning.
Study Subjects
Eligible subjects were inpatients age 70 years admitted to the usual care or Hospitalist‐ACE services at the AIP from November 2, 2007 to April 15, 2008. All patients age 70 years were randomized to the Hospitalist‐ACE service or usual care on a general internal medicine service by the last digit of the medical record number (odd numbers admitted to the Hospitalist‐ACE service and even numbers admitted to usual care). Patients followed by the Hospitalist‐ACE service but not admitted to 12 West were included in the study. To isolate the impact of the intervention, patients admitted to a medicine subspecialty service (such as cardiology, pulmonary, or oncology), or transferred to or from the Hospitalist‐ACE or control services to another service (eg, intensive care unit [ICU] or orthopedic surgery service) were excluded from the study.
Intervention
The Hospitalist‐ACE unit implemented an interdisciplinary team approach to identify and address geriatric syndromes in patients aged 70 and over. The Hospitalist‐ACE model of care consisted of clinical care provided by a hospitalist attending with additional training in geriatric medicine, administration of standardized geriatric screens assessing function, cognition, and mood, 15 minute daily (MondayFriday) interdisciplinary rounds focusing on recognition and management of geriatric syndromes and early discharge planning, and a standardized educational curriculum for medical residents and medical students addressing hazards of hospitalization.
The Hospitalist‐ACE service was a unique rotation within the Hospitalist Training Track of the Internal Medicine Residency that was developed with the support of the University of Colorado Hospital and the Internal Medicine Residency Training Program, and input from the Geriatrics Division at the University of Colorado Denver. The director received additional training from the Donald W. Reynolds FoundationUCLA Faculty Development to Advance Geriatric Education Mini‐Fellowship for hospitalist faculty. The mission of the service was to excel at educating the next generation of hospitalists while providing a model for excellence of care for hospitalized elderly patients. Important stakeholders were identified, and a leadership teamincluding representatives from nursing, physical and occupational therapy, pharmacy, social work, case management, and later, volunteer servicescreated the model daily interdisciplinary rounds. As geographic concentration was essential for the viability of interdisciplinary rounds, one unit (12 West) within the hospital was designated as the preferred location for patients admitted to the Hospitalist‐ACE service.
The Hospitalist‐ACE unit team consisted of one attending hospitalist, one resident, one intern, and medical students. The attending was one of five hospitalists, with additional training in geriatric medicine, who rotated attending responsibilities on the service. One of the hospitalists was board certified in geriatric medicine. Each of the other four hospitalists attended the Reynolds FoundationUCLA mini‐fellowship in geriatric medicine. Hospitalist‐ACE attendings rotated on a variety of other hospitalist services throughout the academic year, including the usual care services.
The brief standardized geriatric assessment consisted of six validated instruments, and was completed by house staff or medical students on admission, following instruction by the attending physician. The complete assessment tool is shown in Figure 1. The cognitive items included the Mini‐Cog,15 a two‐item depression screen,16 and the Confusion Assessment Method.17 The functional items included the Vulnerable Elders Survey (VES‐13),18 the Timed Get Up and Go test,19 and a two‐question falls screen.20 The elements of the assessment tool were selected by the Hospitalist‐ACE attendings for brevity and the potential to inform clinical management. To standardize the clinical and educational approach, the Hospitalist‐ACE attendings regularly discussed appropriate orders recommended in response to each positive screen, but no templated order sets were used during the study period.

Interdisciplinary rounds were attended by Hospitalist‐ACE physicians, nurses, case managers, social workers, physical or occupational therapists, pharmacists, and volunteers. Rounds were led by the attending or medical resident.
The educational curriculum encompassed 13 modules created by the attending faculty that cover delirium, falls, dementia, pressure ulcers, physiology of aging, movement disorders, medication safety, end of life care, advance directives, care transitions, financing of health care for the elderly, and ethical conundrums in the care of the elderly. A full table of contents appears in online Appendix 1. Additionally, portions of the curriculum have been published online.21, 22 Topic selection was guided by the Accreditation Council for Graduate Medical Education (ACGME) core geriatrics topics determined most relevant for the inpatient setting. Formal instruction of 3045 minutes duration occurred three to four days a week and was presented in addition to routine internal medicine educational conferences. Attendings coordinated teaching to ensure that each trainee was exposed to all of the content during the course of their four‐week rotation.
In contrast to the Hospitalist‐ACE service, usual care on the control general medical services consisted of either a hospitalist, a general internist, or an internal medicine subspecialist attending physician, with one medical resident, one intern, and medical students admitting every fourth day. The general medical teams attended daily discharge planning rounds with a discharge planner and social worker focused exclusively on discharge planning. The content of teaching rounds on the general medical services was largely left to the discretion of the attending physician.
This program evaluation of the Hospitalist‐ACE service was granted a waiver of consent and Health Insurance Portability and Accountability Act (HIPAA) by the Colorado Multiple Institutional Review Board.
Measures
Primary Outcome
The primary outcome for the study was the recognition of abnormal functional status by the primary team. Recognition of abnormal functional status was determined from chart review and consisted of both the physician's detection of abnormal functional status and evidence of a corresponding treatment plan identified in the notes or orders of a physician member of the primary team (Table 1).
| Measure | Criterion | Source | Content Examples |
|---|---|---|---|
| |||
| Recognition of abnormal functional status* | 1) Detection | MD's documentation of history | Presentation with change in function (new gait instability); use of gait aides (wheelchair) |
| OR | |||
| MD's documentation of physical exam | Observation of abnormal gait (eg, unsteady, wide‐based, shuffling) and/or balance Abnormal Get Up and Go test | ||
| AND | |||
| 2) Treatment | MD's order | PT/OT consult; home safety evaluation | |
| OR | |||
| MD's documentation assessment/plan | Inclusion of functional status (rehabilitation, PT/OT needs) on the MD's problem list | ||
| Recognition of abnormal cognitive status | Any of the following: | ||
| Delirium | 1) Detection | MD's history | Presentation of confusion or altered mental status |
| OR | |||
| MD's physical exam | Abnormal confusion assessment method | ||
| AND | |||
| 2) Treatment | MD's order | Sitter, reorienting communication, new halperidol order | |
| OR | |||
| MD's documentation of assessment/plan | Inclusion of delirium on the problem list | ||
| OR | |||
| Dementia | 1) Detection | MD's history | Dementia in medical history |
| OR | OR | ||
| MD's physical exam | Abnormal Folstein Mini‐Mental Status Exam or Mini‐Cog | ||
| AND | |||
| 2) Treatment | MD's order | Cholinesterase inhibitor ordered | |
| OR | OR | ||
| MD's documentation of assessment/plan | Inclusion of dementia on the problem list | ||
| OR | |||
| Depression | 1) Detection | MD's history | Depression in medical history |
| OR | OR | ||
| MD's physical exam | Positive depression screen | ||
| AND | |||
| 2) Treatment | MD's order | New antidepressant order | |
| OR | |||
| MD's documentation of assessment/plan | Inclusion of depression on the problem list | ||
Secondary Outcomes
Recognition of abnormal cognitive status was determined from chart review and consisted of both the physician's detection of dementia, depression, or delirium, and evidence of a corresponding treatment plan for any of the documented conditions identified in the notes or orders of a physician member of the primary team (Table 1). Additionally, we measured recognition and treatment of delirium alone.
Falls were determined from mandatory event reporting collected by the hospital on the University Hospitals Consortium Patient Safety Net web‐based reporting system and based on clinical assessment as reported by the nursing staff. The reports are validated by the appropriate clinical managers within 45 days of the event according to standard procedure.
Physical restraint use (type of restraint and duration) was determined from query of mandatory clinical documentation in the electronic medical record. Use of sleep aids was determined from review of the physician's order sheets in the medical record. The chart review captured any of 39 commonly prescribed hypnotic medications ordered at hour of sleep or for insomnia. The sleep medication list was compiled with the assistance of a pharmacist for an earlier chart review and included non‐benzodiazepine hypnotics, benzodiazepines, antidepressants, antihistamines, and antipsychotics.23
Length of stay, hospital charges, 30‐day readmissions to UCH (calculated from date of discharge), and discharge location were determined from administrative data.
Additional Descriptive Variables
Name, medical record number, gender, date of birth, date of admission and discharge, and primary diagnosis were obtained from the medical record. The Case Mix Index for each group of patients was determined from the average Medicare Severity‐adjusted Diagnosis Related Group (MS‐DRG) weight obtained from administrative data.
Data Collection
A two‐step, retrospective chart abstraction was employed. A professional research assistant (P.R.A.) hand‐abstracted process measures from the paper medical chart onto a data collection form designed for this study. A physician investigator performed a secondary review (H.L.W.). Discrepancies were resolved by the physician reviewer.
Data Analysis
Descriptive statistics were performed on intervention and control subjects. Means and standard deviations (age) or frequencies (gender, primary diagnoses) were calculated as appropriate. T tests were used for continuous variables, chi‐square tests for gender, and the Wilcoxon rank sum test for categorical variables.
Outcomes were reported as means and standard deviations for continuous variables (length of stay and charges) and frequencies for categorical variables (all other outcomes). T tests were used for continuous variables, Fisher's exact test for restraint use, and chi‐square tests were used for categorical variable to compare the impact of the intervention between intervention and control patients. For falls, confidence intervals were calculated for the incidence rate differences based on Poisson approximations.
Sample Size Considerations
An a priori sample size calculation was performed. A 2001 study showed that functional status is poorly documented in at least 60% of hospital charts of elderly patients.5 Given an estimated sample size of 120 per group and a power of 80%, this study was powered to be able to detect an absolute difference in the documentation of functional status of as little as 18%.
RESULTS
Two hundred seventeen patients met the study entry criteria (Table 2): 122 were admitted to the Hospitalist‐ACE service, and 95 were admitted to usual care on the general medical services. The average age of the study patients was 80.5 years, 55.3% were female. Twenty‐eight percent of subjects were admitted for pulmonary diagnoses. The two groups of patients were similar with respect to age, gender, and distribution of primary diagnoses. The Hospitalist‐ACE patients had a mean MS‐DRG weight of 1.15, which was slightly higher than that of usual care patients at 1.05 (P = 0.06). Typically, 70% of Hospitalist‐ACE patients are admitted to the designated ACE medical unit (12 West).
| Characteristic | Hospitalist‐ACE | Usual Care | P Value |
|---|---|---|---|
| N = 122 | N = 95 | ||
| |||
| Age (years), mean (SD) | 80.5 (6.5) | 80.7 (7.0) | 0.86 |
| Gender (% female) | 52.5 | 59 | 0.34 |
| Case Mix Index (mean MS‐DRG weight [SD]) | 1.15 (0.43) | 1.05 (0.31) | 0.06 |
| Primary ICD‐9 diagnosis (%) | 0.59 | ||
| Pulmonary | 27.9 | 28.4 | |
| General medicine | 15.6 | 11.6 | |
| Surgery | 13.9 | 11.6 | |
| Cardiology | 9.8 | 6.3 | |
| Nephrology | 8.2 | 7.4 | |
Processes of Care
Processes of care for older patients are displayed in Table 3. Patients on the Hospitalist‐ACE service had recognition and treatment of abnormal functional status at a rate that was nearly double that of patients on the usual care services (68.9% vs 35.8%, P < 0.0001). In addition, patients on the Hospitalist‐ACE service were significantly more likely to have had recognition and treatment of any abnormal cognitive status (55.7% vs 40.0%, P = 0.02). When delirium was evaluated alone, the Hospitalist‐ACE patients were also more likely to have had recognition and treatment of delirium (27.1% vs 17.0%, P = 0.08), although this finding did not reach statistical significance.
| Measure | Percent of Hospitalist‐ACE Patients | Percent of Usual Care Patients | P Value |
|---|---|---|---|
| N = 122 | N = 95 | ||
| |||
| Recognition and treatment of abnormal functional status | 68.9 | 35.8 | <0.0001 |
| Recognition and treatment of abnormal cognitive status* | 55.7 | 40.0 | 0.02 |
| Recognition and treatment of delirium | 27.1 | 17.0 | 0.08 |
| Documentation of resuscitation preferences | 95.1 | 91.6 | 0.3 |
| Do Not Attempt Resuscitation orders | 39.3 | 26.3 | 0.04 |
| Use of sleep medications | 28.1 | 27.4 | 0.91 |
| Use of physical restraints | 2.5 | 0 | 0.26 |
While patients on the Hospitalist‐ACE and usual care services had similar percentages of documentation of resuscitation preferences (95.1% vs 91.6%, P = 0.3), the percentage of Hospitalist‐ACE patients who had Do Not Attempt Resuscitation (DNAR) orders was significantly greater than that of the usual care patients (39.3% vs 26.3%, P = 0.04).
There were no differences in the use of physical restraints or sleep medications for Hospitalist‐ACE patients as compared to usual care patients, although the types of sleep mediations used on each service were markedly different: trazadone was employed as the first‐line sleep agent on the Hospitalist‐ACE service (77.7%), and non‐benzodiazepine hypnotics (primarily zolpidem) were employed most commonly on the usual care services (35%). There were no differences noted in the percentage of patients with benzodiazepines prescribed as sleep aids.
Outcomes
Resource utilization outcomes are reported in Table 4. Of note, there were no significant differences between Hospitalist‐ACE discharges and usual care discharges in mean length of stay (3.4 2.7 days vs 3.1 2.7 days, P = 0.52), mean charges ($24,617 15,828 vs $21,488 13,407, P = 0.12), or 30‐day readmissions to UCH (12.3% vs 9.5%, P = 0.51). Hospitalist‐ACE discharges and usual care patients were equally likely to be discharged to home (68.6% vs 67.4%, P = 0.84), with a similar proportion of Hospitalist‐ACE discharges receiving home health care or home hospice services (14.1% vs 7.4%, P = 12).
| Measure | Hospitalist‐ACE | Usual Care | P Value |
|---|---|---|---|
| N = 122 | N = 95 | ||
| |||
| Length of stay in days (mean [SD]) | 3.4 (2.7) | 3.1 (2.7) | 0.52 |
| Charges in dollars (mean [SD]) | 24,617 (15,828) | 21,488 (13,407) | 0.12 |
| 30‐Day readmissions to UCH (%) | 12.3 | 9.5 | 0.51 |
| Discharges to home (%) | 68.8* | 67.4 | 0.84 |
| Discharges to home with services (%) | 14%* | 7.4% | 0.12 |
In addition, the fall rate for Hospitalist‐ACE patients was not significantly different from the fall rate for usual care patients (4.8 falls/1000 patient days vs 6.7 falls/1000 patient days, 95% confidence interval 9.613.3).
DISCUSSION
We report the implementation and evaluation of a medical service tailored to the care of the acutely ill older patient that draws from elements of the hospitalist model and the ACE unit model.7, 14, 24 For this Hospitalist‐ACE service, we developed a specialized hospitalist workforce, assembled a brief geriatric assessment tailored to the inpatient setting, instituted an interdisciplinary rounding model, and created a novel inpatient geriatrics curriculum.
During the study period, we improved performance of important processes of care for hospitalized elders, including recognition of abnormal cognitive and functional status; maintained comparable resource use; and implemented a novel, inpatient‐focused geriatric medicine educational experience. We were unable to demonstrate an impact on key clinical outcomes such as falls, physical restraint use, and readmissions. Nonetheless, there is evidence that the performance of selected processes of care is associated with improved three‐year survival status in the community‐dwelling vulnerable older patient, and may also be associated with a mortality benefit in the hospitalized vulnerable older patient.25, 26 Therefore, methods to improve the performance of these processes of care may be of clinical importance.
The finding of increased use of DNAR orders in the face of equivalent documentation of code status is of interest and generates hypotheses for further study. It is possible that the educational experience and use of geriatric assessment provides a more complete context for the code status discussion (one that incorporates the patient's social, physical, and cognitive function). However, we do not know if the patients on the ACE service had improved concordance between their code status and their goals of care.
We believe that there was no difference in key clinical outcomes between Hospitalist‐ACE and control patients because the population in this study was relatively low acuity and, therefore, the occurrence of falls and the use of physical restraints were quite low in the study population. In particular, the readmission rate was much lower than is typical for the Medicare population at our hospital, making it challenging to draw conclusions about the impact of the intervention on readmissions, however, we cannot rule out the possibility that our early discharge planning did not address the determinants of readmission for this population.
The ACE unit paradigmcharacterized by 1) closed, modified hospital units; 2) staffing by geriatricians and nurses with geriatrics training; 3) employing geriatric nursing care protocolsrequires significant resources and is not feasible for all settings.6 There is a need for alternative models of comprehensive care for hospitalized elders that require fewer resources in the form of dedicated units and specialist personnel, and can be more responsive to institutional needs. For example, in a 2005 report, one institution reported the creation of a geriatric medicine service that utilized a geriatrician and hospitalist co‐attending model.14 More recently, a large geriatrics program replaced its inpatient geriatrics unit with a mobile inpatient geriatrics service staffed by an attending geriatricianhospitalist, a geriatrics fellow, and a nurse practitioner.27 While these innovative models have eliminated the dedicated unit, they rely on board certified geriatricians, a group in short supply nationally.28 Hospitalists are a rapidly growing provider group that, with appropriate training and building on the work of geriatricians, is poised to provide leadership in acute geriatric care.29, 30
In contrast to the comprehensive inpatient geriatric care models described above, the Hospitalist‐ACE service uses a specialized hospitalist workforce and is not dependent on continuous staffing by geriatricians. Although geographic concentration is important for the success of interdisciplinary rounds, the Hospitalist‐ACE service does not require a closed or modified unit. The nursing staff caring for Hospitalist‐ACE patients have generalist nursing training and, at the time of the study, did not utilize geriatric‐care protocols. Our results need to be interpreted in the light of these differences from the ACE unit model which is a significantly more intensive intervention than the Hospitalist‐ACE service. In addition, the current practice environment is quite different from the mid‐1990s when ACE units were developed and studied. Development and maintenance of models of comprehensive inpatient geriatric care require demonstration of both value as well as return on investment. The alignment of financial and regulatory incentives for programs that provide comprehensive care to complex patients, such as those anticipated by the Affordable Care Act, may encourage the growth of such models.
These data represent findings from a six‐month evaluation of a novel inpatient service in the middle of its first year. There are several limitations related to our study design. First, the results of this small study at a single academic medical center may be of limited generalizability to other settings. Second, the program was evaluated only three months after its inception; we did not capture further improvements in methods, training, and outcomes expected as the program matured. Third, most of the Hospitalist‐ACE service attendings and residents rotate on the UCH general medical services throughout the year. Consequently, we were unable to eliminate the possibility of contamination of the control group, and we were unable to blind the physicians to the study. Fourth, the study population had a relatively low severity of illnessthe average MS‐DRG weight was near 1and low rates of important adverse events such falls and restraint use. This may have occurred because we excluded patients transferred from the ICUs and other services. It is possible that the Hospitalist‐ACE intervention might have demonstrated a larger benefit in a sicker population that would have presented greater opportunities for reductions in length of stay, costs, and adverse events. Fifth, given the retrospective nature of the data collection, we were not able to prospectively assess the incidence of important geriatric outcomes such as delirium and functional decline, nor can we make conclusions about changes in function during the hospitalization.
While the outcome measures we used are conceptually similar to several measures developed by RAND's Assessing Care of Vulnerable Elders (ACOVE) project, this study did not explicitly rely on those constructs.31 To do so would have required prospective screening by clinical staff independent from the care team for vulnerability that was beyond the scope of this project. In addition, the ACOVE measures of interest for functional and cognitive decline are limited to documentation of cognitive or functional assessments in the medical record. The ACE service's adoption of a brief standardized geriatric assessment was almost certain to meet that documentation requirement. While documentation is important, it is not clear that documentation, in and of itself, improves outcomes. Therefore, we expanded upon the ACOVE constructs to include the need for the additional evidence of a treatment plan when abnormal physical or cognitive function was documented. These constructs are important process of care for vulnerable elders. While we demonstrated improvements in several of these important processes of care for elderly patients, we are unable to draw conclusions about the impact of these differences in care on important clinical outcomes such as development of delirium, long‐term institutionalization, or mortality.
CONCLUSIONS
The risks of hospitalization for older persons are numerous, and present challenges and opportunities for inpatient physicians. As the hospitalized population agesmirroring national demographic trends and trends in use of acute care hospitalsthe challenge of avoiding harm in the older hospitalized patient will intensify. Innovations in care to improve the experience and outcomes of hospitalization for older patients are needed in the face of limited geriatrics‐trained workforce and few discretionary funds for unit redesign. The Hospitalist‐ACE service is a promising strategy for hospitalist programs with sufficient numbers of older patients and hospitalists with interest in improving clinical care for older adults. It provides a model for hospitalists to employ geriatrics principles targeted at reducing harm to their most vulnerable patients. Hospitalist‐run geriatric care models offer great promise for improving the care of acutely ill elderly patients. Future investigation should focus on demonstrating the impact of such care on important clinical outcomes between admission and discharge; on model refinement and adaptation, such as determining what components of comprehensive geriatric care are essential to success; and on how complementary interventions, such as the use of templated orders for the hospitalized elderly, impact outcomes. Additional research is needed, with a focus on demonstrating value with regard to an array of outcomes including cost, readmissions, and preventable harms of care.
Acknowledgements
Jean Kutner, MD, MSPH; Daniel Sandy, MPH; Shelly Limon, RN; nurses of 12 West; the UCH staff on the interdisciplinary team; and ACE patients and their families.
- ,,, et al.Functional outcomes of acute medical illness and hospitalization in older persons.Arch Intern Med.1996;156:645–652.
- ,,.Delirium: a symptom of how hospital care is failing older persons and a window to improve quality of hospital care.Am J Med.1999;106:565–573.
- ,,, et al.Using assessing care of vulnerable elders quality indicators to measure quality of hospital care for vulnerable elders.J Am Geriatr Soc.2007;55(11):1705–1711.
- ,,, et al.Impact and recognition of cognitive impairment among hospitalized elders.J Hosp Med.2010;5:69–75.
- ,,,,.What does the medical record reveal about functional status? A comparison of medical record and interview data.J Gen Intern Med.2001;16(11):728–736.
- ,,,,,.Successful models of comprehensive care for older adults with chronic conditions: evidence for the Institute of Medicine's “Retooling for an Aging America” report.J Am Geriatr Soc.2009;57(12):2328–2337.
- ,,,,.A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients.N Engl J Med.1995;332:1338–1344.
- ,,, et al.Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older adults: a randomized controlled trial of Acute Care for Elders (ACE) in a community hospital.J Am Geriatr Soc.2000;48:1572–1581.
- ,,, et al.The effectiveness of inpatient geriatric evaluation and management units: a systematic review and meta‐analysis.J Am Geriatr Soc.2010;58:83–92.
- ,,,,.Effectiveness of acute geriatric units on functional decline, living at home, and case fatality among older patients admitted to hospital for acute medical disorders: meta‐analysis.BMJ.2009;338:b50.
- ,,, et al.A randomized, controlled clinical trial of a geriatrics consultation team: compliance with recommendations.JAMA.1986;255:2617–2621.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340:669–676.
- ,,,.Dissemination and characteristics of Acute Care of Elders (ACE) units in the United States.Int J Technol Assess Health Care.2003;19:220–227.
- ,,.Is there a geriatrician in the house? Geriatric care approaches in hospitalist programs.J Hosp Med.2006;1:29–35.
- ,,,,.The Mini‐Cog: a cognitive “vital signs” measure for dementia screening in multi‐lingual elderly.Int J Geriatr Psychiatry.2000;15(11):1021–1027.
- ,,.The Patient Health Questionnaire‐2: validity of a two‐item depression screener.Med Care.2003;41:1284–1292.
- ,,,,,.Clarifying confusion: the Confusion Assessment Method.Ann Intern Med.1990;113(12):941–948.
- ,,, et al.The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community.J Am Geriatr Soc.2001;49:1691–1699.
- ,.The timed “Up and Go”: a test of basic functional mobility for frail elderly persons.J Am Geriatr Soc.1991;39:142–148.
- American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopedic Surgeons Panel on Falls Prevention.Guideline for the prevention of falls in older persons.J Am Geriatr Soc.2001;49:664–672.
- . Falls for the inpatient physician. Translating knowledge into action. The Portal of Online Geriatric Education (POGOe). 6–19‐2008. Available at: http://www.pogoe.org/productid/20212.
- ,,,. Incontinence and urinary catheters for the inpatient physician. The Portal of Online Geriatric Education (POGOe). 11–27‐0008. Available at: http://www.pogoe.org/productid/20296.
- ,,,.Use of medications for insomnia in the hospitalized geriatric population.J Am Geriatr Soc.2008;56(3):579–581.
- ,,,.Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians.Ann Intern Med.1999;130(4 pt 2):343–349.
- ,,, et al.Quality of care associated with survival in vulnerable older patients.Ann Intern Med.2005;143:274–281.
- ,,, et al.Higher quality of care for hosptialized frail older adults is associated with improved survival one year after discharge.J Hosp Med.2009;4(S1):24.
- ,,,.Operational and quality outcomes of a novel mobile acute care for the elderly service.J Am Geriatr Soc.2009;57:S1.
- Institute of Medicine (IOM).Retooling for an Aging America: Building the Health Care Workforce.Washington, DC:The National Academies Press;2008.
- ,,.Alternative solutions to the geriatric workforce deficit.Am J Med.2008;121:e23.
- ,,,,.Fulfilling the promise of hospital medicine: tailoring internal medicine training to address hospitalists' needs.J Gen Intern Med.2008;23(7):1110–1115.
- ,.Assessing care of vulnerable elders: ACOVE project overview.Ann Intern Med.2001;135(8 pt 2):642–646.
- ,,, et al.Functional outcomes of acute medical illness and hospitalization in older persons.Arch Intern Med.1996;156:645–652.
- ,,.Delirium: a symptom of how hospital care is failing older persons and a window to improve quality of hospital care.Am J Med.1999;106:565–573.
- ,,, et al.Using assessing care of vulnerable elders quality indicators to measure quality of hospital care for vulnerable elders.J Am Geriatr Soc.2007;55(11):1705–1711.
- ,,, et al.Impact and recognition of cognitive impairment among hospitalized elders.J Hosp Med.2010;5:69–75.
- ,,,,.What does the medical record reveal about functional status? A comparison of medical record and interview data.J Gen Intern Med.2001;16(11):728–736.
- ,,,,,.Successful models of comprehensive care for older adults with chronic conditions: evidence for the Institute of Medicine's “Retooling for an Aging America” report.J Am Geriatr Soc.2009;57(12):2328–2337.
- ,,,,.A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients.N Engl J Med.1995;332:1338–1344.
- ,,, et al.Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older adults: a randomized controlled trial of Acute Care for Elders (ACE) in a community hospital.J Am Geriatr Soc.2000;48:1572–1581.
- ,,, et al.The effectiveness of inpatient geriatric evaluation and management units: a systematic review and meta‐analysis.J Am Geriatr Soc.2010;58:83–92.
- ,,,,.Effectiveness of acute geriatric units on functional decline, living at home, and case fatality among older patients admitted to hospital for acute medical disorders: meta‐analysis.BMJ.2009;338:b50.
- ,,, et al.A randomized, controlled clinical trial of a geriatrics consultation team: compliance with recommendations.JAMA.1986;255:2617–2621.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340:669–676.
- ,,,.Dissemination and characteristics of Acute Care of Elders (ACE) units in the United States.Int J Technol Assess Health Care.2003;19:220–227.
- ,,.Is there a geriatrician in the house? Geriatric care approaches in hospitalist programs.J Hosp Med.2006;1:29–35.
- ,,,,.The Mini‐Cog: a cognitive “vital signs” measure for dementia screening in multi‐lingual elderly.Int J Geriatr Psychiatry.2000;15(11):1021–1027.
- ,,.The Patient Health Questionnaire‐2: validity of a two‐item depression screener.Med Care.2003;41:1284–1292.
- ,,,,,.Clarifying confusion: the Confusion Assessment Method.Ann Intern Med.1990;113(12):941–948.
- ,,, et al.The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community.J Am Geriatr Soc.2001;49:1691–1699.
- ,.The timed “Up and Go”: a test of basic functional mobility for frail elderly persons.J Am Geriatr Soc.1991;39:142–148.
- American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopedic Surgeons Panel on Falls Prevention.Guideline for the prevention of falls in older persons.J Am Geriatr Soc.2001;49:664–672.
- . Falls for the inpatient physician. Translating knowledge into action. The Portal of Online Geriatric Education (POGOe). 6–19‐2008. Available at: http://www.pogoe.org/productid/20212.
- ,,,. Incontinence and urinary catheters for the inpatient physician. The Portal of Online Geriatric Education (POGOe). 11–27‐0008. Available at: http://www.pogoe.org/productid/20296.
- ,,,.Use of medications for insomnia in the hospitalized geriatric population.J Am Geriatr Soc.2008;56(3):579–581.
- ,,,.Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians.Ann Intern Med.1999;130(4 pt 2):343–349.
- ,,, et al.Quality of care associated with survival in vulnerable older patients.Ann Intern Med.2005;143:274–281.
- ,,, et al.Higher quality of care for hosptialized frail older adults is associated with improved survival one year after discharge.J Hosp Med.2009;4(S1):24.
- ,,,.Operational and quality outcomes of a novel mobile acute care for the elderly service.J Am Geriatr Soc.2009;57:S1.
- Institute of Medicine (IOM).Retooling for an Aging America: Building the Health Care Workforce.Washington, DC:The National Academies Press;2008.
- ,,.Alternative solutions to the geriatric workforce deficit.Am J Med.2008;121:e23.
- ,,,,.Fulfilling the promise of hospital medicine: tailoring internal medicine training to address hospitalists' needs.J Gen Intern Med.2008;23(7):1110–1115.
- ,.Assessing care of vulnerable elders: ACOVE project overview.Ann Intern Med.2001;135(8 pt 2):642–646.
Copyright © 2011 Society of Hospital Medicine
Audit and Feedback Urinary Catheter Duration
The ubiquitous urinary catheter is associated with 80% of hospital‐acquired urinary tract infections (UTIs)1estimated to number one million annuallyaccounting for 40% of all nosocomial infections.2, 3 The clinical consequences of these catheter‐associated urinary tract infections (CAUTIs) are substantial and include prolonged hospital stay, bacteremia, and death.4 Despite the known risks of CAUTIs, it is estimated that 25% of hospitalized patients receive urinary catheters and that inappropriate urinary catheter use is widespread.5 Among catheterized patients, catheter duration is the most important modifiable risk factor for CAUTI. The excess risk of any bacteriuria accrues at a rate of 5% per catheter‐day beyond the first 48 hours of catheterization.6, 7 The reduction of catheter‐days for a given patient is an important component of quality improvement efforts to reduce CAUTIs. Unfortunately, as of a 2005 survey, most hospitals do not systematically track urinary catheter insertions and removals.8
The above concerns are highlighted for surgical patients among whom indwelling urinary catheter use is particularly high. In a 2001 sample of Medicare beneficiaries, 85% of major surgical patients had perioperative urinary catheters.9 In this population, postoperative catheter duration exceeded 48 hours in nearly 50%, despite concern that the risks of infection offset the benefits of continued catheterization after 24 hours to 48 hours postoperatively.4, 7, 10 Patients with catheters greater than 2 postoperative days had a 21% increased likelihood of in‐hospital UTI, increased 30‐day mortality, and decreased odds of discharge to home.9
To address the risk of CAUTI associated with excess urinary catheter days, The Centers for Medicare and Medicaid Services' (CMS) Surgical Care Improvement Project (SCIP) added catheter removal on postoperative day 1 or 2 to its process measure set beginning in October 2009. SCIP is 1 of several high‐profile surgical quality improvement programs that employs performance audit and feedback of patient‐level process or outcome measures to address deficiencies in surgical care.11, 12 In addition, audit and feedback of CAUTI rates has been used to successfully reduce CAUTIs in medical‐surgical patients.13 The goal of our study was to audit patient‐level postoperative urinary catheter duration and measure the impact of its feedback to nursing staff on postoperative catheter duration, CAUTI rates, and nurse's attitudes about CAUTI prevention.
Methods
Study Setting
The study was conducted within the orthopedic and general surgery units at the University of Colorado Hospital (UCH) Anschutz Inpatient Pavilion (AIP) in Aurora, CO. The AIP is a 425‐bed tertiary care hospital which is the major teaching affiliate of the University of Colorado Medical School. The orthopedic surgery unit has 22 beds. The general surgery unit has 18 beds.
Study Population
All postoperative patients 18 years of age admitted to the general surgery unit and orthopedic surgery units who had perioperative placement of an indwelling urinary catheter were eligible for study inclusion. Exclusion criteria included: evidence of a chronic indwelling catheter or chronic intermittent catheterization, a urologic or gynecologic surgery. For patients undergoing more than one operation in the same hospitalization, only the final operation was included in the study. For patients who were recatheterized after initial catheter removal, only the first catheterization and removal were included in the study. The registered nurses (RNs) on the study floors (n = 29 orthopedic surgery and 31 general surgery nurses) were the targets of the audit and feedback intervention with education. The baseline period was September 1, 2007 through January 31, 2008 and, the follow‐up period was April 1 through July 31, 2008.
Measures
The primary study outcome was postoperative urinary catheter duration measured in 2 ways:
Postoperative catheter duration in days defined as: the date of surgery subtracted from the date of catheter removal.
Postoperative catheter duration performance measure defined as: the number of patients with catheter removal before postoperative day three divided by the number of study eligible patients.
Both measures were calculated for each of the surgical units using data from all eligible study patients on the unit during the study period. For patients who were recatheterized in the same hospitalization, only the days to the first removal were counted. If the catheter was removed on the day of surgery, the postoperative catheter duration was zero days.
Total device days were calculated as the sum of the postoperative catheter duration for every eligible patient for each unit. Total device days/hospital days was calculated as the total device days divided by the sum of the lengths of stay for every eligible patient for each unit.
Secondary Outcome
CAUTI was the secondary outcome. CAUTI was defined as a positive urine culture (105 organisms/cc of no more than 2 microorganisms) sent 3 or more days following admission and 7 days following catheter removal. The definition for CAUTI was based on that used by the National Healthcare Safety Network for infection control surveillance purposes at the time of the study and included both symptomatic CAUTI and asymptomatic bacteriuria. CAUTI was reported as the number of infections per 1000 catheter‐days for eligible patients on each surgical unit for the baseline and follow‐up data collection periods.
Additional Descriptive Variables
Descriptive variables included the patient's name, surgical procedure, surgeon, presence of a chronic indwelling catheter, date of admission to the floor, date of surgery, date of birth, and length of stay in days.
Data Collection
A professional research assistant (PRA) identified eligible patients on the 2 surgical units of interest and collected the number of postoperative urinary catheter days per patient using daily and weekly automated electronic queries of an EHR containing all nursing documentation on medical and surgical floors at UCH. These queries identified all patients on the floors of interest with urinary bladder elimination management documentation. Those with documented indwelling catheters were included in the study unless exclusion criteria were met. During the study period, the EHR was configured to provide the following documentation of urinary output management: date and location of catheter insertion, routine assessment of urinary output and devices, and date and time of catheter removal. At UCH, catheter insertion and removal are documented in the EHR for 93% and 88% of surgical patients, respectively. When documentation of catheter insertion was missing from the EMR, the operative note was reviewed electronically for documentation of insertion. If the operative note did not reference the insertion, it was presumed to have occurred perioperatively. Likewise, if there was no documentation of catheter removal, it was presumed to have occurred prior to documentation of urinary continence. The PRA abstracted additional information (surgical procedure and date) from the discharge abstracts and operative notes using a standardized data collection sheet.
Laboratory‐based surveillance was used to determine the incidence of CAUTIs in a manner similar to that employed by infection preventionists at UCH. The microbiology laboratory provided a monthly summary of all positive urine cultures for both study units. Positive culture results were cross‐referenced with the catheter removal dates for all eligible study patients.
Validation
Validation of catheter documentation in the EHR was carried out on each of the 2 surgical units for a 2‐week period at the outset of the data collection. During each day of the validation period, the PRA compared the EHR report with the charge nurse report on each floor. Any discrepancies regarding the presence or absence of the indwelling catheter were resolved by querying the patient's nurse directly. All patients with catheters during their inpatient stay were captured by EHR documentation. The daily EHR reports had a 91% agreement with a daily nursing query (reference standard) and a Kappa (percent agreement adjusted for chance agreement) of 0.77. Instances of disagreement were generally due to a lag in EHR documentation on the part of the nursing staff.
Audit and Feedback Intervention
An educational presentation was developed to cover the following topics: the definition and epidemiology of CAUTI, harms associated with CAUTI, risk factors for CAUTI, commonly accepted indications for indwelling catheters, and alternatives to catheters. In addition, the catheter duration performance measure was defined, followed by the feedback of unit‐specific performance from the baseline data collection period. The presentation was made by the principal investigator to nursing staff on each of the 2 surgical units on 3 occasions per unit with days and times selected so as to reach as many unit nurses as possible. At the conclusion of each session, nurses were asked to brainstorm barriers to evidence‐based management of indwelling catheters. Light refreshments and a hour continuing education credit were provided regardless of participation in the brainstorming session. Additionally, participants completed brief evaluations of the sessions.
Analyses
Descriptive data are reported as means and standard deviations for continuous variables and percentages for categorical variables. Outcome measures were calculated as defined above. For these comparisons, we used t‐tests for continuous variables and chi‐square tests for dichotomous variables. We used Cochran‐Mantel‐Haenszel to test for trend for categorical variables. Confidence intervals were calculated for the incidence rate differences based on Poisson approximations. Analyses were completed using SAS Statistical Software Version 9.2.
This study was approved by the Colorado Multiple Institutional Review Board. Waivers of Health Insurance Portability and Accountability Act (HIPAA) and informed consent were obtained for study patients. Nurses participating in the educational sessions provided informed consent.
Results
During the study period there were a total of 1657 surgeries on the 2 study units during the baseline and follow‐up periods. After exclusions for urologic or gynecologic surgery (271), no indwelling catheter for surgery (505), or first surgery of 2 or more during the hospitalization (31), there were 846 eligible surgeries (51%).
Table 1 describes the population for the baseline and follow‐up periods for orthopedic and general surgery patients. Within each unit, the surgical populations were comparable during the baseline and follow‐up periods with the exception that the mean length of stay for eligible general surgery patients was significantly shorter in the baseline period as compared to the follow‐up period (6.6 vs. 8.5 days, P = 0.02). Cases on the orthopedic surgery unit were predominantly knee, hip and spine surgeries (85.9%), while those on the general surgery unit were predominantly gut and other gastrointestinal (GI) procedures (80.3%).
| Characteristic | Orthopedic Surgery | General Surgery | |||||
|---|---|---|---|---|---|---|---|
| Baseline, n = 206 | Follow‐Up, n = 290 | P Value | Baseline, n = 167 | Follow‐Up, n = 183 | P Value | ||
| |||||||
| Age in years, mean (SD) | 58.3 (15.6) | 58.1 (14.7) | 0.87 | 53.8 (16.1) | 52.7 (15.7) | 0.54 | |
| Male gender (%) | 47.1 | 45.2 | 0.67 | 43.1 | 48.6 | 0.30 | |
| Length of stay in days, mean (SD) | 4.0 (3.5) | 3.7 (2.8) | 0.22 | 6.6 (5.5) | 8.5 (8.6) | 0.02 | |
| Type of surgery (%) | |||||||
| Knee | 24.8 | 27.9 | 0.91 | Gut | 54.5 | 50.3 | 0.09 |
| Hip | 37.4 | 35.5 | Other GI | 22.8 | 32.8 | ||
| Spine | 22.8 | 23.1 | Non‐GI | 22.8 | 16.9 | ||
| Other Ortho | 5.3 | 4.1 | |||||
| Non‐Ortho | 9.7 | 9.3 | |||||
The Intervention
The educational intervention and feedback was received by two‐thirds of registered nurses on each unit and was rated highly by participants. A total of 79% of nurses agreed or strongly agreed that the information provided was relevant to their daily practice and 42% strongly agreed that they would change their practice based on the presentation. Barriers to evidence‐based use of urinary catheters identified by surgical nurses on each unit are shown in Table 2. They included the following domains: communication, patient concerns, clinical concerns, equipment, policies and procedures, and skills. General surgery and orthopedic surgery nurses identified different concerns arising from the different patient populations and surgeries cared for on each unit.
| Domain | Orthopedic Surgery | General Surgery |
|---|---|---|
| ||
| Communication | Communication among teams | Occasional need to call MD for order |
| Patient Comfort | Discomfort first overnight postop without catheter; discomfort of straight cathethers | Discomfort and embarrassment associated with straight catheters; patient request for indwelling catheter |
| Clinical concerns | Removal POD 1 too soon | Need to monitor I/Os in patients with low output |
| Equipment | Portable ultrasound on a different floor | |
| Policies and Procedures | ||
| 1. Epidural anesthesia | Duration of epidural/delay post epidural removal | |
| 2. Straight catheters | Risk of infection | Risk of trauma; infection |
| 3. Management of Urinary Retention | No standardized protocol for urinary retention. | Need for traumatic reinsertion of catheter. |
| Skills | Perineal care; catheter care | Perineal care |
BaselineFollow‐Up Comparison
Table 3 describes the measures of urinary catheter use for each surgical population for both data collection periods. On both units, measures of catheter duration were improved following the education and feedback intervention. For the orthopedic unit, mean postoperative catheter duration was reduced from 1.7 to 1.4 days (P = 0.01) and the proportion of patients with catheter removal before day 3 was increased from 86% to 92% (P = 0.04). For the general surgery unit, mean postoperative catheter duration was reduced from 2.6 to 2.2 days (P = 0.01) and the proportion of patients with catheter removal before day 3 was increased from 56% to 63% (P = 0.14). When the general surgery measures were adjusted to account for the difference in length of stay between the 2 time periods, the odds of meeting the performance measure at follow‐up compared to baseline increased from unadjusted odds of 1.38 (P = 0.14) to adjusted odds of 1.69 (P = 0.02).
| Measure | Orthopedic Surgery | General Surgery | ||||
|---|---|---|---|---|---|---|
| Baseline, n = 206 | Follow‐Up, n = 290 | P Value | Baseline, n = 167 | Follow‐Up, n = 183 | P Value | |
| ||||||
| Postoperative catheter duration in days (mean, SD) | 1.70 (1.24) | 1.44 (0.85) | 0.01 | 2.64 (1.85) | 2.19 (1.40) | 0.01 |
| Postoperative catheter duration performance measure (%) | 86 | 92 | 0.04 | 56 | 63 | 0.14 |
| Total catheter days | 350 | 418 | 441 | 401 | ||
| Catheter days/1000 hospital days | 423 | 394 | ns* | 398 | 259 | s* |
| Catheter‐associated UTIs | 3 | 0 | 3 | 3 | ||
| Catheter‐associated UTI rate (infections/1000 device days) | 8.6 | 0 | ns* | 6.8 | 7.5 | ns* |
Figure 1a and b are histograms of the frequency of cases having a given postoperative catheter duration in days. The dark bars show the baseline distribution and the light bars show the follow‐up distribution. Although the number of patients in the follow‐up period is greater than for the baseline period for the orthopedic surgery cohort, the images are instructive. In both groups of patients, but most notably in the general surgery population, the reduction in catheter measures resulted from a left shift in the frequency distribution, both for the longer duration outliers (removing the tail of each plot) and for the shorter duration catheters (3 days), increasing the proportion of catheters removed on postoperative day 1.
CAUTIs
The CAUTI rate on the orthopedic surgery unit demonstrated a nonsignificant decline from 8.9 to 0 infections per 1000 device days, and on the general surgery unit the rate was constant at approximately 7 infections per 1000 device days.
Discussion
This preobservational and postobservational study found that audit and feedback of patient‐level postoperative urinary catheter duration delivered in the context of an educational intervention and brainstorming session was temporally associated with clinically meaningful reductions in urinary catheter duration. In so doing, we demonstrated the feasibility of collecting patient level urinary catheter duration, and delivering it in a manner that had utility for frontline staff. Our results are consistent with the quality improvement literature which demonstrates that audit and feedback is a successful quality improvement strategy in many contexts and may be as good as more complex interventions at increasing adherence to the performance of process measures for surgical infection prevention.14 Two large national programs, the VA National Surgical Quality Improvement Program (NSQIP) and SCIP, use audit and feedback as the backbone of their large‐scale quality improvement strategies with promising results.11, 12
Given that the 2 study units were so different in practice patterns regarding urinary catheter management and nursing‐identified barriers to evidence‐based care, this work suggests that urinary catheter management may pose unique challenges for different clinical areas and provides a caution that one‐size‐fits‐all interventions for the rationalization of urinary catheter management and reduction of CAUTIs may be of limited effectiveness in the absence of local tailoring. As such, audit and feedback is well‐suited to this purpose as more proscriptive quality improvement strategies may meet with a variety of implementation challenges.
The impact of our intervention on CAUTI rates was not significant. There are several possible explanations for this finding. First, the study was not powered to detect a difference in CAUTI rates given the low infection rate at our institution. However, we cannot exclude the possibility that a reduction in mean catheter duration of one‐third to one‐half of a day is insufficient to impact CAUTI rates in postoperative patients, particularly when many of the follow‐up patients still had postoperative catheter duration 2 daysthe timeframe beyond which bacterial colonization of the catheter begins. While both study units had similar increases in postoperative catheter duration, the UTI rate was only decreased in the orthopedic surgery group which had much higher rates of postoperative catheter duration 2 days at baseline.
In recent years, there has been a renewed focus on the eradication of hospital‐acquired infections prompted by intense interest from the public, federal and state legislators, and others.15, 16 The CMS has recently used the revamping of the Inpatient Prospective Payment System (IPPS) as an opportunity to align financial incentives so that reimbursements for claims with certain hospital‐acquired conditions, including CAUTIs, will be reduced to that of the reimbursement of the same claim without the presence of the complication.17 This move is just one of several strategies to motivate hospitals and clinicians to address the pervasive problem of hospital‐acquired infection.
As urinary catheters are intimately linked to hospital‐acquired UTI, a focus of reduction efforts on catheter use is appropriate. The National Quality Forum (NQF) endorsed a postoperative catheter duration quality measure which was incorporated by the CMS's SCIP in late 2009.18 As a result, every hospital in the country that performs surgery and participates in the Medicare program is now tasked with determining patient‐level urinary catheter duration for selected surgical patients. This move represents a departure from current recommendations from the Centers for Disease Control and Prevention (CDC) and its National Healthcare Safety Network19, 20 which endorse the measurement of a catheter utilization ratio (urinary catheter days/patient days) for patient care units, but does not endorse any patient‐level utilization measures. In this instance, the use of patient‐level data may be better suited to quality improvement interventions such as audit and feedback because of its clinical relevance to frontline providers. However, it may also increase the data collection burden on hospitals. Notably, the measurement of postoperative catheter duration in this study was semiautomated using queries of an EHR. Such an approach can significantly reduce the data collection burden for this process measure and is consistent with national initiatives to integrate EHRs with quality improvement initiatives going forward.21
Our study has several limitations. This study took place in the year following the announcement of a high profile Medicare rule change regarding payment for hospital‐acquired harms. Certainly, the uncontrolled prestudy and poststudy design does not allow for the assessment of the impact of our intervention independent of this context. We are unable, therefore, to attribute the observed reduction solely to the intervention. Additionally, we did not follow postoperative urinary catheter duration beyond the immediate follow‐up period. It is anticipated that the impact of an audit and feedback intervention may diminish over time without a mechanism for repeated feedback. Certainly the sustainability of such repeated feedback in a single institution would be improved with an appropriately configured EHR.
In addition, we have reliable data on catheter reinsertions only from the follow‐up period. While the rates of reinsertions we recorded (0.7% on orthopedic surgery and 2.7% on general surgery) were lower than expected based on the literature,22 we are unable to determine if our intervention led to increases in postoperative urinary retention.
This study was limited to 2 surgical units of a single academic medical center and therefore the urinary catheter utilization patterns may not be representative of other patient populations at other institutions. However, the urinary catheter patterns were comparable to those identified in our prior work in a national sample of Medicare patients undergoing elective surgery.9
Finally, the field of CAUTI prevention has evolved rapidly since this study was performed. In particular, the surveillance definition of CAUTI was altered twice by the CDC in December of 2008 and March of 2009. In addition, the Infectious Diseases Society of America issued a new definition of CAUTI in February of 2010.23 All of these changes highlight the difference between asymptomatic bateriuria (ASB) and symptomatic CAUTI. However, the surveillance definition in use at the time of this study did not make this distinction. Therefore, we are unable to comment on the relative occurrence of ASB versus symptomatic CAUTI under the new definitions.
Rational urinary catheter use is a central component of CAUTI prevention efforts.24 We describe the use of patient‐level urinary catheter use in an audit and feedback intervention to frontline staff that was associated with reductions in urinary catheter duration. To do so, we employed a methodology for tracking urinary catheter use patterns that can provide important data for infection preventionists and frontline providers in efforts to improve urinary output management. This promising approach merits further study as an adjunct to current efforts to rationalize urinary catheter utilization and reduce CAUTIs. In the current environment, having the right data is a powerful aide for ongoing performance improvement.
Acknowledgements
The authors acknowledge the contributions of Daniel Sandy, BA, MPN, Vivienne Smith, RN, UCH; and the insights of Michelle Barron, MD, Linda Burton, RN, Teri Hulett, RN, UCH, and Jean Kutner, MD, MSPH.
- ,,.Urinary tract etiology of bloodstream infections in hospitalized patients.J Infect Dis.1983;148:57–62.
- ,,, et al.Nosocomial infections in US hospitals, 1975–1976: estimated frequency by selected characteristics of patients.Am J Med.1981;70:947–959.
- ,,,,.The nationwide nosocomial infection rate. a new need for vital statistics.Am J Epidemiol.1985;121:159–167.
- .Clinical and economic consequences of nosocomial catheter‐related bacteriuria.Am J Infect Control.2000;28:68–75.
- ,,,.Overuse of the indwelling urinary tract catheter in hospitalized medical patients.Arch Intern Med.1995;155:1425–1429.
- .Catheter‐associated bacteriuria.Urol Clin North Am.1986;13:735.
- .Guidelines for prevention of catheter‐associated urinary tract infections.Ann Intern Med.1975;82:386.
- ,,, et al.Preventing hospital‐acquired urinary tract infection in the united states: a national study.Clin Infect Dis.2008;46:243–250.
- ,,,.Indwelling urinary catheter use in the postoperative period: analysis of the national surgical infection prevention project data.Arch Surg.2008;143:551–557.
- ,,,,.Management of urinary retention after surgical repair of hip fracture.Can Med Assoc J.1992;146:1185–1188.
- ,,.The comparative assessment and improvement of quality of surgical care in the department of veterans affairs.Arch Surg.2002;137:20–27.
- .The surgical infection prevention and surgical care improvement projects: promises and pitfalls.Am Surg.2006;72:1010–1016.
- ,,,.Feedback to nursing staff as an intervention to reduce catheter‐associated urinary tract infections.Am J Infect Control.1999;27:402–424.
- ,,, et al.The effect of a quality improvement collaborative to improve antimicrobial prophylaxis in surgical patients.Ann Intern Med.2008;149:480.
- ,.Nonpayment for harms resulting from medical care: catheter‐associated urinary tract infections.JAMA.2007;289:2782–2784.
- Kaiser Family Foundation. Hospital‐based infections reporting requirements,2008. Available at: www.Kaiser Family Foundation State Health Facts.org. Accessed August 25, 2010.
- Centers for Medicare and Medicaid Services, Department of Health and Human Services.Medicare Program: Changes to the Hospital Inpatient Prospective Payment Systems and Fiscal Year 2008 Rates. CMS‐1390‐F. 8–1‐2007.
- Centers for Medicare and Medicaid Services, Department of Health and Human Services and The Joint Commission.Specifications Manual for National Hospital Inpatient Quality Measures, Discharges 10/1/09 (4Q09) through 3/31/10 (1Q10). 5–4‐2009.
- ,,,,, andthe Healthcare Infection Control Practices Advisory Committee.Guideline for the Prevention of Catheter‐associated Urinary Tract Infections,2009. Centers for Disease Control and Prevention. 1–22‐2010.
- Centers for Disease Control and Prevention.NHSN Patient Safety Component Key Terms. 1–22‐2010.
- .Stimulating the adoption of health information technology.N Engl J Med.2010;360:1477–1479.
- ,.Management of postoperative urinary retention: a randomized trial of in‐out versus overnight catheterization.ANZ J Surg.2004;2004:658–661.
- ,,, et al.Diagnosis, prevention, and treatment of catheter‐associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America.Clin Infect Dis.2010;50:625–663.
- ,,, et al.Strategies to prevent catheter‐associated urinary tract infections in acute care hospitals.Infect Control Hosp Epidemiol.2008;29:S41–S50.
The ubiquitous urinary catheter is associated with 80% of hospital‐acquired urinary tract infections (UTIs)1estimated to number one million annuallyaccounting for 40% of all nosocomial infections.2, 3 The clinical consequences of these catheter‐associated urinary tract infections (CAUTIs) are substantial and include prolonged hospital stay, bacteremia, and death.4 Despite the known risks of CAUTIs, it is estimated that 25% of hospitalized patients receive urinary catheters and that inappropriate urinary catheter use is widespread.5 Among catheterized patients, catheter duration is the most important modifiable risk factor for CAUTI. The excess risk of any bacteriuria accrues at a rate of 5% per catheter‐day beyond the first 48 hours of catheterization.6, 7 The reduction of catheter‐days for a given patient is an important component of quality improvement efforts to reduce CAUTIs. Unfortunately, as of a 2005 survey, most hospitals do not systematically track urinary catheter insertions and removals.8
The above concerns are highlighted for surgical patients among whom indwelling urinary catheter use is particularly high. In a 2001 sample of Medicare beneficiaries, 85% of major surgical patients had perioperative urinary catheters.9 In this population, postoperative catheter duration exceeded 48 hours in nearly 50%, despite concern that the risks of infection offset the benefits of continued catheterization after 24 hours to 48 hours postoperatively.4, 7, 10 Patients with catheters greater than 2 postoperative days had a 21% increased likelihood of in‐hospital UTI, increased 30‐day mortality, and decreased odds of discharge to home.9
To address the risk of CAUTI associated with excess urinary catheter days, The Centers for Medicare and Medicaid Services' (CMS) Surgical Care Improvement Project (SCIP) added catheter removal on postoperative day 1 or 2 to its process measure set beginning in October 2009. SCIP is 1 of several high‐profile surgical quality improvement programs that employs performance audit and feedback of patient‐level process or outcome measures to address deficiencies in surgical care.11, 12 In addition, audit and feedback of CAUTI rates has been used to successfully reduce CAUTIs in medical‐surgical patients.13 The goal of our study was to audit patient‐level postoperative urinary catheter duration and measure the impact of its feedback to nursing staff on postoperative catheter duration, CAUTI rates, and nurse's attitudes about CAUTI prevention.
Methods
Study Setting
The study was conducted within the orthopedic and general surgery units at the University of Colorado Hospital (UCH) Anschutz Inpatient Pavilion (AIP) in Aurora, CO. The AIP is a 425‐bed tertiary care hospital which is the major teaching affiliate of the University of Colorado Medical School. The orthopedic surgery unit has 22 beds. The general surgery unit has 18 beds.
Study Population
All postoperative patients 18 years of age admitted to the general surgery unit and orthopedic surgery units who had perioperative placement of an indwelling urinary catheter were eligible for study inclusion. Exclusion criteria included: evidence of a chronic indwelling catheter or chronic intermittent catheterization, a urologic or gynecologic surgery. For patients undergoing more than one operation in the same hospitalization, only the final operation was included in the study. For patients who were recatheterized after initial catheter removal, only the first catheterization and removal were included in the study. The registered nurses (RNs) on the study floors (n = 29 orthopedic surgery and 31 general surgery nurses) were the targets of the audit and feedback intervention with education. The baseline period was September 1, 2007 through January 31, 2008 and, the follow‐up period was April 1 through July 31, 2008.
Measures
The primary study outcome was postoperative urinary catheter duration measured in 2 ways:
Postoperative catheter duration in days defined as: the date of surgery subtracted from the date of catheter removal.
Postoperative catheter duration performance measure defined as: the number of patients with catheter removal before postoperative day three divided by the number of study eligible patients.
Both measures were calculated for each of the surgical units using data from all eligible study patients on the unit during the study period. For patients who were recatheterized in the same hospitalization, only the days to the first removal were counted. If the catheter was removed on the day of surgery, the postoperative catheter duration was zero days.
Total device days were calculated as the sum of the postoperative catheter duration for every eligible patient for each unit. Total device days/hospital days was calculated as the total device days divided by the sum of the lengths of stay for every eligible patient for each unit.
Secondary Outcome
CAUTI was the secondary outcome. CAUTI was defined as a positive urine culture (105 organisms/cc of no more than 2 microorganisms) sent 3 or more days following admission and 7 days following catheter removal. The definition for CAUTI was based on that used by the National Healthcare Safety Network for infection control surveillance purposes at the time of the study and included both symptomatic CAUTI and asymptomatic bacteriuria. CAUTI was reported as the number of infections per 1000 catheter‐days for eligible patients on each surgical unit for the baseline and follow‐up data collection periods.
Additional Descriptive Variables
Descriptive variables included the patient's name, surgical procedure, surgeon, presence of a chronic indwelling catheter, date of admission to the floor, date of surgery, date of birth, and length of stay in days.
Data Collection
A professional research assistant (PRA) identified eligible patients on the 2 surgical units of interest and collected the number of postoperative urinary catheter days per patient using daily and weekly automated electronic queries of an EHR containing all nursing documentation on medical and surgical floors at UCH. These queries identified all patients on the floors of interest with urinary bladder elimination management documentation. Those with documented indwelling catheters were included in the study unless exclusion criteria were met. During the study period, the EHR was configured to provide the following documentation of urinary output management: date and location of catheter insertion, routine assessment of urinary output and devices, and date and time of catheter removal. At UCH, catheter insertion and removal are documented in the EHR for 93% and 88% of surgical patients, respectively. When documentation of catheter insertion was missing from the EMR, the operative note was reviewed electronically for documentation of insertion. If the operative note did not reference the insertion, it was presumed to have occurred perioperatively. Likewise, if there was no documentation of catheter removal, it was presumed to have occurred prior to documentation of urinary continence. The PRA abstracted additional information (surgical procedure and date) from the discharge abstracts and operative notes using a standardized data collection sheet.
Laboratory‐based surveillance was used to determine the incidence of CAUTIs in a manner similar to that employed by infection preventionists at UCH. The microbiology laboratory provided a monthly summary of all positive urine cultures for both study units. Positive culture results were cross‐referenced with the catheter removal dates for all eligible study patients.
Validation
Validation of catheter documentation in the EHR was carried out on each of the 2 surgical units for a 2‐week period at the outset of the data collection. During each day of the validation period, the PRA compared the EHR report with the charge nurse report on each floor. Any discrepancies regarding the presence or absence of the indwelling catheter were resolved by querying the patient's nurse directly. All patients with catheters during their inpatient stay were captured by EHR documentation. The daily EHR reports had a 91% agreement with a daily nursing query (reference standard) and a Kappa (percent agreement adjusted for chance agreement) of 0.77. Instances of disagreement were generally due to a lag in EHR documentation on the part of the nursing staff.
Audit and Feedback Intervention
An educational presentation was developed to cover the following topics: the definition and epidemiology of CAUTI, harms associated with CAUTI, risk factors for CAUTI, commonly accepted indications for indwelling catheters, and alternatives to catheters. In addition, the catheter duration performance measure was defined, followed by the feedback of unit‐specific performance from the baseline data collection period. The presentation was made by the principal investigator to nursing staff on each of the 2 surgical units on 3 occasions per unit with days and times selected so as to reach as many unit nurses as possible. At the conclusion of each session, nurses were asked to brainstorm barriers to evidence‐based management of indwelling catheters. Light refreshments and a hour continuing education credit were provided regardless of participation in the brainstorming session. Additionally, participants completed brief evaluations of the sessions.
Analyses
Descriptive data are reported as means and standard deviations for continuous variables and percentages for categorical variables. Outcome measures were calculated as defined above. For these comparisons, we used t‐tests for continuous variables and chi‐square tests for dichotomous variables. We used Cochran‐Mantel‐Haenszel to test for trend for categorical variables. Confidence intervals were calculated for the incidence rate differences based on Poisson approximations. Analyses were completed using SAS Statistical Software Version 9.2.
This study was approved by the Colorado Multiple Institutional Review Board. Waivers of Health Insurance Portability and Accountability Act (HIPAA) and informed consent were obtained for study patients. Nurses participating in the educational sessions provided informed consent.
Results
During the study period there were a total of 1657 surgeries on the 2 study units during the baseline and follow‐up periods. After exclusions for urologic or gynecologic surgery (271), no indwelling catheter for surgery (505), or first surgery of 2 or more during the hospitalization (31), there were 846 eligible surgeries (51%).
Table 1 describes the population for the baseline and follow‐up periods for orthopedic and general surgery patients. Within each unit, the surgical populations were comparable during the baseline and follow‐up periods with the exception that the mean length of stay for eligible general surgery patients was significantly shorter in the baseline period as compared to the follow‐up period (6.6 vs. 8.5 days, P = 0.02). Cases on the orthopedic surgery unit were predominantly knee, hip and spine surgeries (85.9%), while those on the general surgery unit were predominantly gut and other gastrointestinal (GI) procedures (80.3%).
| Characteristic | Orthopedic Surgery | General Surgery | |||||
|---|---|---|---|---|---|---|---|
| Baseline, n = 206 | Follow‐Up, n = 290 | P Value | Baseline, n = 167 | Follow‐Up, n = 183 | P Value | ||
| |||||||
| Age in years, mean (SD) | 58.3 (15.6) | 58.1 (14.7) | 0.87 | 53.8 (16.1) | 52.7 (15.7) | 0.54 | |
| Male gender (%) | 47.1 | 45.2 | 0.67 | 43.1 | 48.6 | 0.30 | |
| Length of stay in days, mean (SD) | 4.0 (3.5) | 3.7 (2.8) | 0.22 | 6.6 (5.5) | 8.5 (8.6) | 0.02 | |
| Type of surgery (%) | |||||||
| Knee | 24.8 | 27.9 | 0.91 | Gut | 54.5 | 50.3 | 0.09 |
| Hip | 37.4 | 35.5 | Other GI | 22.8 | 32.8 | ||
| Spine | 22.8 | 23.1 | Non‐GI | 22.8 | 16.9 | ||
| Other Ortho | 5.3 | 4.1 | |||||
| Non‐Ortho | 9.7 | 9.3 | |||||
The Intervention
The educational intervention and feedback was received by two‐thirds of registered nurses on each unit and was rated highly by participants. A total of 79% of nurses agreed or strongly agreed that the information provided was relevant to their daily practice and 42% strongly agreed that they would change their practice based on the presentation. Barriers to evidence‐based use of urinary catheters identified by surgical nurses on each unit are shown in Table 2. They included the following domains: communication, patient concerns, clinical concerns, equipment, policies and procedures, and skills. General surgery and orthopedic surgery nurses identified different concerns arising from the different patient populations and surgeries cared for on each unit.
| Domain | Orthopedic Surgery | General Surgery |
|---|---|---|
| ||
| Communication | Communication among teams | Occasional need to call MD for order |
| Patient Comfort | Discomfort first overnight postop without catheter; discomfort of straight cathethers | Discomfort and embarrassment associated with straight catheters; patient request for indwelling catheter |
| Clinical concerns | Removal POD 1 too soon | Need to monitor I/Os in patients with low output |
| Equipment | Portable ultrasound on a different floor | |
| Policies and Procedures | ||
| 1. Epidural anesthesia | Duration of epidural/delay post epidural removal | |
| 2. Straight catheters | Risk of infection | Risk of trauma; infection |
| 3. Management of Urinary Retention | No standardized protocol for urinary retention. | Need for traumatic reinsertion of catheter. |
| Skills | Perineal care; catheter care | Perineal care |
BaselineFollow‐Up Comparison
Table 3 describes the measures of urinary catheter use for each surgical population for both data collection periods. On both units, measures of catheter duration were improved following the education and feedback intervention. For the orthopedic unit, mean postoperative catheter duration was reduced from 1.7 to 1.4 days (P = 0.01) and the proportion of patients with catheter removal before day 3 was increased from 86% to 92% (P = 0.04). For the general surgery unit, mean postoperative catheter duration was reduced from 2.6 to 2.2 days (P = 0.01) and the proportion of patients with catheter removal before day 3 was increased from 56% to 63% (P = 0.14). When the general surgery measures were adjusted to account for the difference in length of stay between the 2 time periods, the odds of meeting the performance measure at follow‐up compared to baseline increased from unadjusted odds of 1.38 (P = 0.14) to adjusted odds of 1.69 (P = 0.02).
| Measure | Orthopedic Surgery | General Surgery | ||||
|---|---|---|---|---|---|---|
| Baseline, n = 206 | Follow‐Up, n = 290 | P Value | Baseline, n = 167 | Follow‐Up, n = 183 | P Value | |
| ||||||
| Postoperative catheter duration in days (mean, SD) | 1.70 (1.24) | 1.44 (0.85) | 0.01 | 2.64 (1.85) | 2.19 (1.40) | 0.01 |
| Postoperative catheter duration performance measure (%) | 86 | 92 | 0.04 | 56 | 63 | 0.14 |
| Total catheter days | 350 | 418 | 441 | 401 | ||
| Catheter days/1000 hospital days | 423 | 394 | ns* | 398 | 259 | s* |
| Catheter‐associated UTIs | 3 | 0 | 3 | 3 | ||
| Catheter‐associated UTI rate (infections/1000 device days) | 8.6 | 0 | ns* | 6.8 | 7.5 | ns* |
Figure 1a and b are histograms of the frequency of cases having a given postoperative catheter duration in days. The dark bars show the baseline distribution and the light bars show the follow‐up distribution. Although the number of patients in the follow‐up period is greater than for the baseline period for the orthopedic surgery cohort, the images are instructive. In both groups of patients, but most notably in the general surgery population, the reduction in catheter measures resulted from a left shift in the frequency distribution, both for the longer duration outliers (removing the tail of each plot) and for the shorter duration catheters (3 days), increasing the proportion of catheters removed on postoperative day 1.

CAUTIs
The CAUTI rate on the orthopedic surgery unit demonstrated a nonsignificant decline from 8.9 to 0 infections per 1000 device days, and on the general surgery unit the rate was constant at approximately 7 infections per 1000 device days.
Discussion
This preobservational and postobservational study found that audit and feedback of patient‐level postoperative urinary catheter duration delivered in the context of an educational intervention and brainstorming session was temporally associated with clinically meaningful reductions in urinary catheter duration. In so doing, we demonstrated the feasibility of collecting patient level urinary catheter duration, and delivering it in a manner that had utility for frontline staff. Our results are consistent with the quality improvement literature which demonstrates that audit and feedback is a successful quality improvement strategy in many contexts and may be as good as more complex interventions at increasing adherence to the performance of process measures for surgical infection prevention.14 Two large national programs, the VA National Surgical Quality Improvement Program (NSQIP) and SCIP, use audit and feedback as the backbone of their large‐scale quality improvement strategies with promising results.11, 12
Given that the 2 study units were so different in practice patterns regarding urinary catheter management and nursing‐identified barriers to evidence‐based care, this work suggests that urinary catheter management may pose unique challenges for different clinical areas and provides a caution that one‐size‐fits‐all interventions for the rationalization of urinary catheter management and reduction of CAUTIs may be of limited effectiveness in the absence of local tailoring. As such, audit and feedback is well‐suited to this purpose as more proscriptive quality improvement strategies may meet with a variety of implementation challenges.
The impact of our intervention on CAUTI rates was not significant. There are several possible explanations for this finding. First, the study was not powered to detect a difference in CAUTI rates given the low infection rate at our institution. However, we cannot exclude the possibility that a reduction in mean catheter duration of one‐third to one‐half of a day is insufficient to impact CAUTI rates in postoperative patients, particularly when many of the follow‐up patients still had postoperative catheter duration 2 daysthe timeframe beyond which bacterial colonization of the catheter begins. While both study units had similar increases in postoperative catheter duration, the UTI rate was only decreased in the orthopedic surgery group which had much higher rates of postoperative catheter duration 2 days at baseline.
In recent years, there has been a renewed focus on the eradication of hospital‐acquired infections prompted by intense interest from the public, federal and state legislators, and others.15, 16 The CMS has recently used the revamping of the Inpatient Prospective Payment System (IPPS) as an opportunity to align financial incentives so that reimbursements for claims with certain hospital‐acquired conditions, including CAUTIs, will be reduced to that of the reimbursement of the same claim without the presence of the complication.17 This move is just one of several strategies to motivate hospitals and clinicians to address the pervasive problem of hospital‐acquired infection.
As urinary catheters are intimately linked to hospital‐acquired UTI, a focus of reduction efforts on catheter use is appropriate. The National Quality Forum (NQF) endorsed a postoperative catheter duration quality measure which was incorporated by the CMS's SCIP in late 2009.18 As a result, every hospital in the country that performs surgery and participates in the Medicare program is now tasked with determining patient‐level urinary catheter duration for selected surgical patients. This move represents a departure from current recommendations from the Centers for Disease Control and Prevention (CDC) and its National Healthcare Safety Network19, 20 which endorse the measurement of a catheter utilization ratio (urinary catheter days/patient days) for patient care units, but does not endorse any patient‐level utilization measures. In this instance, the use of patient‐level data may be better suited to quality improvement interventions such as audit and feedback because of its clinical relevance to frontline providers. However, it may also increase the data collection burden on hospitals. Notably, the measurement of postoperative catheter duration in this study was semiautomated using queries of an EHR. Such an approach can significantly reduce the data collection burden for this process measure and is consistent with national initiatives to integrate EHRs with quality improvement initiatives going forward.21
Our study has several limitations. This study took place in the year following the announcement of a high profile Medicare rule change regarding payment for hospital‐acquired harms. Certainly, the uncontrolled prestudy and poststudy design does not allow for the assessment of the impact of our intervention independent of this context. We are unable, therefore, to attribute the observed reduction solely to the intervention. Additionally, we did not follow postoperative urinary catheter duration beyond the immediate follow‐up period. It is anticipated that the impact of an audit and feedback intervention may diminish over time without a mechanism for repeated feedback. Certainly the sustainability of such repeated feedback in a single institution would be improved with an appropriately configured EHR.
In addition, we have reliable data on catheter reinsertions only from the follow‐up period. While the rates of reinsertions we recorded (0.7% on orthopedic surgery and 2.7% on general surgery) were lower than expected based on the literature,22 we are unable to determine if our intervention led to increases in postoperative urinary retention.
This study was limited to 2 surgical units of a single academic medical center and therefore the urinary catheter utilization patterns may not be representative of other patient populations at other institutions. However, the urinary catheter patterns were comparable to those identified in our prior work in a national sample of Medicare patients undergoing elective surgery.9
Finally, the field of CAUTI prevention has evolved rapidly since this study was performed. In particular, the surveillance definition of CAUTI was altered twice by the CDC in December of 2008 and March of 2009. In addition, the Infectious Diseases Society of America issued a new definition of CAUTI in February of 2010.23 All of these changes highlight the difference between asymptomatic bateriuria (ASB) and symptomatic CAUTI. However, the surveillance definition in use at the time of this study did not make this distinction. Therefore, we are unable to comment on the relative occurrence of ASB versus symptomatic CAUTI under the new definitions.
Rational urinary catheter use is a central component of CAUTI prevention efforts.24 We describe the use of patient‐level urinary catheter use in an audit and feedback intervention to frontline staff that was associated with reductions in urinary catheter duration. To do so, we employed a methodology for tracking urinary catheter use patterns that can provide important data for infection preventionists and frontline providers in efforts to improve urinary output management. This promising approach merits further study as an adjunct to current efforts to rationalize urinary catheter utilization and reduce CAUTIs. In the current environment, having the right data is a powerful aide for ongoing performance improvement.
Acknowledgements
The authors acknowledge the contributions of Daniel Sandy, BA, MPN, Vivienne Smith, RN, UCH; and the insights of Michelle Barron, MD, Linda Burton, RN, Teri Hulett, RN, UCH, and Jean Kutner, MD, MSPH.
The ubiquitous urinary catheter is associated with 80% of hospital‐acquired urinary tract infections (UTIs)1estimated to number one million annuallyaccounting for 40% of all nosocomial infections.2, 3 The clinical consequences of these catheter‐associated urinary tract infections (CAUTIs) are substantial and include prolonged hospital stay, bacteremia, and death.4 Despite the known risks of CAUTIs, it is estimated that 25% of hospitalized patients receive urinary catheters and that inappropriate urinary catheter use is widespread.5 Among catheterized patients, catheter duration is the most important modifiable risk factor for CAUTI. The excess risk of any bacteriuria accrues at a rate of 5% per catheter‐day beyond the first 48 hours of catheterization.6, 7 The reduction of catheter‐days for a given patient is an important component of quality improvement efforts to reduce CAUTIs. Unfortunately, as of a 2005 survey, most hospitals do not systematically track urinary catheter insertions and removals.8
The above concerns are highlighted for surgical patients among whom indwelling urinary catheter use is particularly high. In a 2001 sample of Medicare beneficiaries, 85% of major surgical patients had perioperative urinary catheters.9 In this population, postoperative catheter duration exceeded 48 hours in nearly 50%, despite concern that the risks of infection offset the benefits of continued catheterization after 24 hours to 48 hours postoperatively.4, 7, 10 Patients with catheters greater than 2 postoperative days had a 21% increased likelihood of in‐hospital UTI, increased 30‐day mortality, and decreased odds of discharge to home.9
To address the risk of CAUTI associated with excess urinary catheter days, The Centers for Medicare and Medicaid Services' (CMS) Surgical Care Improvement Project (SCIP) added catheter removal on postoperative day 1 or 2 to its process measure set beginning in October 2009. SCIP is 1 of several high‐profile surgical quality improvement programs that employs performance audit and feedback of patient‐level process or outcome measures to address deficiencies in surgical care.11, 12 In addition, audit and feedback of CAUTI rates has been used to successfully reduce CAUTIs in medical‐surgical patients.13 The goal of our study was to audit patient‐level postoperative urinary catheter duration and measure the impact of its feedback to nursing staff on postoperative catheter duration, CAUTI rates, and nurse's attitudes about CAUTI prevention.
Methods
Study Setting
The study was conducted within the orthopedic and general surgery units at the University of Colorado Hospital (UCH) Anschutz Inpatient Pavilion (AIP) in Aurora, CO. The AIP is a 425‐bed tertiary care hospital which is the major teaching affiliate of the University of Colorado Medical School. The orthopedic surgery unit has 22 beds. The general surgery unit has 18 beds.
Study Population
All postoperative patients 18 years of age admitted to the general surgery unit and orthopedic surgery units who had perioperative placement of an indwelling urinary catheter were eligible for study inclusion. Exclusion criteria included: evidence of a chronic indwelling catheter or chronic intermittent catheterization, a urologic or gynecologic surgery. For patients undergoing more than one operation in the same hospitalization, only the final operation was included in the study. For patients who were recatheterized after initial catheter removal, only the first catheterization and removal were included in the study. The registered nurses (RNs) on the study floors (n = 29 orthopedic surgery and 31 general surgery nurses) were the targets of the audit and feedback intervention with education. The baseline period was September 1, 2007 through January 31, 2008 and, the follow‐up period was April 1 through July 31, 2008.
Measures
The primary study outcome was postoperative urinary catheter duration measured in 2 ways:
Postoperative catheter duration in days defined as: the date of surgery subtracted from the date of catheter removal.
Postoperative catheter duration performance measure defined as: the number of patients with catheter removal before postoperative day three divided by the number of study eligible patients.
Both measures were calculated for each of the surgical units using data from all eligible study patients on the unit during the study period. For patients who were recatheterized in the same hospitalization, only the days to the first removal were counted. If the catheter was removed on the day of surgery, the postoperative catheter duration was zero days.
Total device days were calculated as the sum of the postoperative catheter duration for every eligible patient for each unit. Total device days/hospital days was calculated as the total device days divided by the sum of the lengths of stay for every eligible patient for each unit.
Secondary Outcome
CAUTI was the secondary outcome. CAUTI was defined as a positive urine culture (105 organisms/cc of no more than 2 microorganisms) sent 3 or more days following admission and 7 days following catheter removal. The definition for CAUTI was based on that used by the National Healthcare Safety Network for infection control surveillance purposes at the time of the study and included both symptomatic CAUTI and asymptomatic bacteriuria. CAUTI was reported as the number of infections per 1000 catheter‐days for eligible patients on each surgical unit for the baseline and follow‐up data collection periods.
Additional Descriptive Variables
Descriptive variables included the patient's name, surgical procedure, surgeon, presence of a chronic indwelling catheter, date of admission to the floor, date of surgery, date of birth, and length of stay in days.
Data Collection
A professional research assistant (PRA) identified eligible patients on the 2 surgical units of interest and collected the number of postoperative urinary catheter days per patient using daily and weekly automated electronic queries of an EHR containing all nursing documentation on medical and surgical floors at UCH. These queries identified all patients on the floors of interest with urinary bladder elimination management documentation. Those with documented indwelling catheters were included in the study unless exclusion criteria were met. During the study period, the EHR was configured to provide the following documentation of urinary output management: date and location of catheter insertion, routine assessment of urinary output and devices, and date and time of catheter removal. At UCH, catheter insertion and removal are documented in the EHR for 93% and 88% of surgical patients, respectively. When documentation of catheter insertion was missing from the EMR, the operative note was reviewed electronically for documentation of insertion. If the operative note did not reference the insertion, it was presumed to have occurred perioperatively. Likewise, if there was no documentation of catheter removal, it was presumed to have occurred prior to documentation of urinary continence. The PRA abstracted additional information (surgical procedure and date) from the discharge abstracts and operative notes using a standardized data collection sheet.
Laboratory‐based surveillance was used to determine the incidence of CAUTIs in a manner similar to that employed by infection preventionists at UCH. The microbiology laboratory provided a monthly summary of all positive urine cultures for both study units. Positive culture results were cross‐referenced with the catheter removal dates for all eligible study patients.
Validation
Validation of catheter documentation in the EHR was carried out on each of the 2 surgical units for a 2‐week period at the outset of the data collection. During each day of the validation period, the PRA compared the EHR report with the charge nurse report on each floor. Any discrepancies regarding the presence or absence of the indwelling catheter were resolved by querying the patient's nurse directly. All patients with catheters during their inpatient stay were captured by EHR documentation. The daily EHR reports had a 91% agreement with a daily nursing query (reference standard) and a Kappa (percent agreement adjusted for chance agreement) of 0.77. Instances of disagreement were generally due to a lag in EHR documentation on the part of the nursing staff.
Audit and Feedback Intervention
An educational presentation was developed to cover the following topics: the definition and epidemiology of CAUTI, harms associated with CAUTI, risk factors for CAUTI, commonly accepted indications for indwelling catheters, and alternatives to catheters. In addition, the catheter duration performance measure was defined, followed by the feedback of unit‐specific performance from the baseline data collection period. The presentation was made by the principal investigator to nursing staff on each of the 2 surgical units on 3 occasions per unit with days and times selected so as to reach as many unit nurses as possible. At the conclusion of each session, nurses were asked to brainstorm barriers to evidence‐based management of indwelling catheters. Light refreshments and a hour continuing education credit were provided regardless of participation in the brainstorming session. Additionally, participants completed brief evaluations of the sessions.
Analyses
Descriptive data are reported as means and standard deviations for continuous variables and percentages for categorical variables. Outcome measures were calculated as defined above. For these comparisons, we used t‐tests for continuous variables and chi‐square tests for dichotomous variables. We used Cochran‐Mantel‐Haenszel to test for trend for categorical variables. Confidence intervals were calculated for the incidence rate differences based on Poisson approximations. Analyses were completed using SAS Statistical Software Version 9.2.
This study was approved by the Colorado Multiple Institutional Review Board. Waivers of Health Insurance Portability and Accountability Act (HIPAA) and informed consent were obtained for study patients. Nurses participating in the educational sessions provided informed consent.
Results
During the study period there were a total of 1657 surgeries on the 2 study units during the baseline and follow‐up periods. After exclusions for urologic or gynecologic surgery (271), no indwelling catheter for surgery (505), or first surgery of 2 or more during the hospitalization (31), there were 846 eligible surgeries (51%).
Table 1 describes the population for the baseline and follow‐up periods for orthopedic and general surgery patients. Within each unit, the surgical populations were comparable during the baseline and follow‐up periods with the exception that the mean length of stay for eligible general surgery patients was significantly shorter in the baseline period as compared to the follow‐up period (6.6 vs. 8.5 days, P = 0.02). Cases on the orthopedic surgery unit were predominantly knee, hip and spine surgeries (85.9%), while those on the general surgery unit were predominantly gut and other gastrointestinal (GI) procedures (80.3%).
| Characteristic | Orthopedic Surgery | General Surgery | |||||
|---|---|---|---|---|---|---|---|
| Baseline, n = 206 | Follow‐Up, n = 290 | P Value | Baseline, n = 167 | Follow‐Up, n = 183 | P Value | ||
| |||||||
| Age in years, mean (SD) | 58.3 (15.6) | 58.1 (14.7) | 0.87 | 53.8 (16.1) | 52.7 (15.7) | 0.54 | |
| Male gender (%) | 47.1 | 45.2 | 0.67 | 43.1 | 48.6 | 0.30 | |
| Length of stay in days, mean (SD) | 4.0 (3.5) | 3.7 (2.8) | 0.22 | 6.6 (5.5) | 8.5 (8.6) | 0.02 | |
| Type of surgery (%) | |||||||
| Knee | 24.8 | 27.9 | 0.91 | Gut | 54.5 | 50.3 | 0.09 |
| Hip | 37.4 | 35.5 | Other GI | 22.8 | 32.8 | ||
| Spine | 22.8 | 23.1 | Non‐GI | 22.8 | 16.9 | ||
| Other Ortho | 5.3 | 4.1 | |||||
| Non‐Ortho | 9.7 | 9.3 | |||||
The Intervention
The educational intervention and feedback was received by two‐thirds of registered nurses on each unit and was rated highly by participants. A total of 79% of nurses agreed or strongly agreed that the information provided was relevant to their daily practice and 42% strongly agreed that they would change their practice based on the presentation. Barriers to evidence‐based use of urinary catheters identified by surgical nurses on each unit are shown in Table 2. They included the following domains: communication, patient concerns, clinical concerns, equipment, policies and procedures, and skills. General surgery and orthopedic surgery nurses identified different concerns arising from the different patient populations and surgeries cared for on each unit.
| Domain | Orthopedic Surgery | General Surgery |
|---|---|---|
| ||
| Communication | Communication among teams | Occasional need to call MD for order |
| Patient Comfort | Discomfort first overnight postop without catheter; discomfort of straight cathethers | Discomfort and embarrassment associated with straight catheters; patient request for indwelling catheter |
| Clinical concerns | Removal POD 1 too soon | Need to monitor I/Os in patients with low output |
| Equipment | Portable ultrasound on a different floor | |
| Policies and Procedures | ||
| 1. Epidural anesthesia | Duration of epidural/delay post epidural removal | |
| 2. Straight catheters | Risk of infection | Risk of trauma; infection |
| 3. Management of Urinary Retention | No standardized protocol for urinary retention. | Need for traumatic reinsertion of catheter. |
| Skills | Perineal care; catheter care | Perineal care |
BaselineFollow‐Up Comparison
Table 3 describes the measures of urinary catheter use for each surgical population for both data collection periods. On both units, measures of catheter duration were improved following the education and feedback intervention. For the orthopedic unit, mean postoperative catheter duration was reduced from 1.7 to 1.4 days (P = 0.01) and the proportion of patients with catheter removal before day 3 was increased from 86% to 92% (P = 0.04). For the general surgery unit, mean postoperative catheter duration was reduced from 2.6 to 2.2 days (P = 0.01) and the proportion of patients with catheter removal before day 3 was increased from 56% to 63% (P = 0.14). When the general surgery measures were adjusted to account for the difference in length of stay between the 2 time periods, the odds of meeting the performance measure at follow‐up compared to baseline increased from unadjusted odds of 1.38 (P = 0.14) to adjusted odds of 1.69 (P = 0.02).
| Measure | Orthopedic Surgery | General Surgery | ||||
|---|---|---|---|---|---|---|
| Baseline, n = 206 | Follow‐Up, n = 290 | P Value | Baseline, n = 167 | Follow‐Up, n = 183 | P Value | |
| ||||||
| Postoperative catheter duration in days (mean, SD) | 1.70 (1.24) | 1.44 (0.85) | 0.01 | 2.64 (1.85) | 2.19 (1.40) | 0.01 |
| Postoperative catheter duration performance measure (%) | 86 | 92 | 0.04 | 56 | 63 | 0.14 |
| Total catheter days | 350 | 418 | 441 | 401 | ||
| Catheter days/1000 hospital days | 423 | 394 | ns* | 398 | 259 | s* |
| Catheter‐associated UTIs | 3 | 0 | 3 | 3 | ||
| Catheter‐associated UTI rate (infections/1000 device days) | 8.6 | 0 | ns* | 6.8 | 7.5 | ns* |
Figure 1a and b are histograms of the frequency of cases having a given postoperative catheter duration in days. The dark bars show the baseline distribution and the light bars show the follow‐up distribution. Although the number of patients in the follow‐up period is greater than for the baseline period for the orthopedic surgery cohort, the images are instructive. In both groups of patients, but most notably in the general surgery population, the reduction in catheter measures resulted from a left shift in the frequency distribution, both for the longer duration outliers (removing the tail of each plot) and for the shorter duration catheters (3 days), increasing the proportion of catheters removed on postoperative day 1.

CAUTIs
The CAUTI rate on the orthopedic surgery unit demonstrated a nonsignificant decline from 8.9 to 0 infections per 1000 device days, and on the general surgery unit the rate was constant at approximately 7 infections per 1000 device days.
Discussion
This preobservational and postobservational study found that audit and feedback of patient‐level postoperative urinary catheter duration delivered in the context of an educational intervention and brainstorming session was temporally associated with clinically meaningful reductions in urinary catheter duration. In so doing, we demonstrated the feasibility of collecting patient level urinary catheter duration, and delivering it in a manner that had utility for frontline staff. Our results are consistent with the quality improvement literature which demonstrates that audit and feedback is a successful quality improvement strategy in many contexts and may be as good as more complex interventions at increasing adherence to the performance of process measures for surgical infection prevention.14 Two large national programs, the VA National Surgical Quality Improvement Program (NSQIP) and SCIP, use audit and feedback as the backbone of their large‐scale quality improvement strategies with promising results.11, 12
Given that the 2 study units were so different in practice patterns regarding urinary catheter management and nursing‐identified barriers to evidence‐based care, this work suggests that urinary catheter management may pose unique challenges for different clinical areas and provides a caution that one‐size‐fits‐all interventions for the rationalization of urinary catheter management and reduction of CAUTIs may be of limited effectiveness in the absence of local tailoring. As such, audit and feedback is well‐suited to this purpose as more proscriptive quality improvement strategies may meet with a variety of implementation challenges.
The impact of our intervention on CAUTI rates was not significant. There are several possible explanations for this finding. First, the study was not powered to detect a difference in CAUTI rates given the low infection rate at our institution. However, we cannot exclude the possibility that a reduction in mean catheter duration of one‐third to one‐half of a day is insufficient to impact CAUTI rates in postoperative patients, particularly when many of the follow‐up patients still had postoperative catheter duration 2 daysthe timeframe beyond which bacterial colonization of the catheter begins. While both study units had similar increases in postoperative catheter duration, the UTI rate was only decreased in the orthopedic surgery group which had much higher rates of postoperative catheter duration 2 days at baseline.
In recent years, there has been a renewed focus on the eradication of hospital‐acquired infections prompted by intense interest from the public, federal and state legislators, and others.15, 16 The CMS has recently used the revamping of the Inpatient Prospective Payment System (IPPS) as an opportunity to align financial incentives so that reimbursements for claims with certain hospital‐acquired conditions, including CAUTIs, will be reduced to that of the reimbursement of the same claim without the presence of the complication.17 This move is just one of several strategies to motivate hospitals and clinicians to address the pervasive problem of hospital‐acquired infection.
As urinary catheters are intimately linked to hospital‐acquired UTI, a focus of reduction efforts on catheter use is appropriate. The National Quality Forum (NQF) endorsed a postoperative catheter duration quality measure which was incorporated by the CMS's SCIP in late 2009.18 As a result, every hospital in the country that performs surgery and participates in the Medicare program is now tasked with determining patient‐level urinary catheter duration for selected surgical patients. This move represents a departure from current recommendations from the Centers for Disease Control and Prevention (CDC) and its National Healthcare Safety Network19, 20 which endorse the measurement of a catheter utilization ratio (urinary catheter days/patient days) for patient care units, but does not endorse any patient‐level utilization measures. In this instance, the use of patient‐level data may be better suited to quality improvement interventions such as audit and feedback because of its clinical relevance to frontline providers. However, it may also increase the data collection burden on hospitals. Notably, the measurement of postoperative catheter duration in this study was semiautomated using queries of an EHR. Such an approach can significantly reduce the data collection burden for this process measure and is consistent with national initiatives to integrate EHRs with quality improvement initiatives going forward.21
Our study has several limitations. This study took place in the year following the announcement of a high profile Medicare rule change regarding payment for hospital‐acquired harms. Certainly, the uncontrolled prestudy and poststudy design does not allow for the assessment of the impact of our intervention independent of this context. We are unable, therefore, to attribute the observed reduction solely to the intervention. Additionally, we did not follow postoperative urinary catheter duration beyond the immediate follow‐up period. It is anticipated that the impact of an audit and feedback intervention may diminish over time without a mechanism for repeated feedback. Certainly the sustainability of such repeated feedback in a single institution would be improved with an appropriately configured EHR.
In addition, we have reliable data on catheter reinsertions only from the follow‐up period. While the rates of reinsertions we recorded (0.7% on orthopedic surgery and 2.7% on general surgery) were lower than expected based on the literature,22 we are unable to determine if our intervention led to increases in postoperative urinary retention.
This study was limited to 2 surgical units of a single academic medical center and therefore the urinary catheter utilization patterns may not be representative of other patient populations at other institutions. However, the urinary catheter patterns were comparable to those identified in our prior work in a national sample of Medicare patients undergoing elective surgery.9
Finally, the field of CAUTI prevention has evolved rapidly since this study was performed. In particular, the surveillance definition of CAUTI was altered twice by the CDC in December of 2008 and March of 2009. In addition, the Infectious Diseases Society of America issued a new definition of CAUTI in February of 2010.23 All of these changes highlight the difference between asymptomatic bateriuria (ASB) and symptomatic CAUTI. However, the surveillance definition in use at the time of this study did not make this distinction. Therefore, we are unable to comment on the relative occurrence of ASB versus symptomatic CAUTI under the new definitions.
Rational urinary catheter use is a central component of CAUTI prevention efforts.24 We describe the use of patient‐level urinary catheter use in an audit and feedback intervention to frontline staff that was associated with reductions in urinary catheter duration. To do so, we employed a methodology for tracking urinary catheter use patterns that can provide important data for infection preventionists and frontline providers in efforts to improve urinary output management. This promising approach merits further study as an adjunct to current efforts to rationalize urinary catheter utilization and reduce CAUTIs. In the current environment, having the right data is a powerful aide for ongoing performance improvement.
Acknowledgements
The authors acknowledge the contributions of Daniel Sandy, BA, MPN, Vivienne Smith, RN, UCH; and the insights of Michelle Barron, MD, Linda Burton, RN, Teri Hulett, RN, UCH, and Jean Kutner, MD, MSPH.
- ,,.Urinary tract etiology of bloodstream infections in hospitalized patients.J Infect Dis.1983;148:57–62.
- ,,, et al.Nosocomial infections in US hospitals, 1975–1976: estimated frequency by selected characteristics of patients.Am J Med.1981;70:947–959.
- ,,,,.The nationwide nosocomial infection rate. a new need for vital statistics.Am J Epidemiol.1985;121:159–167.
- .Clinical and economic consequences of nosocomial catheter‐related bacteriuria.Am J Infect Control.2000;28:68–75.
- ,,,.Overuse of the indwelling urinary tract catheter in hospitalized medical patients.Arch Intern Med.1995;155:1425–1429.
- .Catheter‐associated bacteriuria.Urol Clin North Am.1986;13:735.
- .Guidelines for prevention of catheter‐associated urinary tract infections.Ann Intern Med.1975;82:386.
- ,,, et al.Preventing hospital‐acquired urinary tract infection in the united states: a national study.Clin Infect Dis.2008;46:243–250.
- ,,,.Indwelling urinary catheter use in the postoperative period: analysis of the national surgical infection prevention project data.Arch Surg.2008;143:551–557.
- ,,,,.Management of urinary retention after surgical repair of hip fracture.Can Med Assoc J.1992;146:1185–1188.
- ,,.The comparative assessment and improvement of quality of surgical care in the department of veterans affairs.Arch Surg.2002;137:20–27.
- .The surgical infection prevention and surgical care improvement projects: promises and pitfalls.Am Surg.2006;72:1010–1016.
- ,,,.Feedback to nursing staff as an intervention to reduce catheter‐associated urinary tract infections.Am J Infect Control.1999;27:402–424.
- ,,, et al.The effect of a quality improvement collaborative to improve antimicrobial prophylaxis in surgical patients.Ann Intern Med.2008;149:480.
- ,.Nonpayment for harms resulting from medical care: catheter‐associated urinary tract infections.JAMA.2007;289:2782–2784.
- Kaiser Family Foundation. Hospital‐based infections reporting requirements,2008. Available at: www.Kaiser Family Foundation State Health Facts.org. Accessed August 25, 2010.
- Centers for Medicare and Medicaid Services, Department of Health and Human Services.Medicare Program: Changes to the Hospital Inpatient Prospective Payment Systems and Fiscal Year 2008 Rates. CMS‐1390‐F. 8–1‐2007.
- Centers for Medicare and Medicaid Services, Department of Health and Human Services and The Joint Commission.Specifications Manual for National Hospital Inpatient Quality Measures, Discharges 10/1/09 (4Q09) through 3/31/10 (1Q10). 5–4‐2009.
- ,,,,, andthe Healthcare Infection Control Practices Advisory Committee.Guideline for the Prevention of Catheter‐associated Urinary Tract Infections,2009. Centers for Disease Control and Prevention. 1–22‐2010.
- Centers for Disease Control and Prevention.NHSN Patient Safety Component Key Terms. 1–22‐2010.
- .Stimulating the adoption of health information technology.N Engl J Med.2010;360:1477–1479.
- ,.Management of postoperative urinary retention: a randomized trial of in‐out versus overnight catheterization.ANZ J Surg.2004;2004:658–661.
- ,,, et al.Diagnosis, prevention, and treatment of catheter‐associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America.Clin Infect Dis.2010;50:625–663.
- ,,, et al.Strategies to prevent catheter‐associated urinary tract infections in acute care hospitals.Infect Control Hosp Epidemiol.2008;29:S41–S50.
- ,,.Urinary tract etiology of bloodstream infections in hospitalized patients.J Infect Dis.1983;148:57–62.
- ,,, et al.Nosocomial infections in US hospitals, 1975–1976: estimated frequency by selected characteristics of patients.Am J Med.1981;70:947–959.
- ,,,,.The nationwide nosocomial infection rate. a new need for vital statistics.Am J Epidemiol.1985;121:159–167.
- .Clinical and economic consequences of nosocomial catheter‐related bacteriuria.Am J Infect Control.2000;28:68–75.
- ,,,.Overuse of the indwelling urinary tract catheter in hospitalized medical patients.Arch Intern Med.1995;155:1425–1429.
- .Catheter‐associated bacteriuria.Urol Clin North Am.1986;13:735.
- .Guidelines for prevention of catheter‐associated urinary tract infections.Ann Intern Med.1975;82:386.
- ,,, et al.Preventing hospital‐acquired urinary tract infection in the united states: a national study.Clin Infect Dis.2008;46:243–250.
- ,,,.Indwelling urinary catheter use in the postoperative period: analysis of the national surgical infection prevention project data.Arch Surg.2008;143:551–557.
- ,,,,.Management of urinary retention after surgical repair of hip fracture.Can Med Assoc J.1992;146:1185–1188.
- ,,.The comparative assessment and improvement of quality of surgical care in the department of veterans affairs.Arch Surg.2002;137:20–27.
- .The surgical infection prevention and surgical care improvement projects: promises and pitfalls.Am Surg.2006;72:1010–1016.
- ,,,.Feedback to nursing staff as an intervention to reduce catheter‐associated urinary tract infections.Am J Infect Control.1999;27:402–424.
- ,,, et al.The effect of a quality improvement collaborative to improve antimicrobial prophylaxis in surgical patients.Ann Intern Med.2008;149:480.
- ,.Nonpayment for harms resulting from medical care: catheter‐associated urinary tract infections.JAMA.2007;289:2782–2784.
- Kaiser Family Foundation. Hospital‐based infections reporting requirements,2008. Available at: www.Kaiser Family Foundation State Health Facts.org. Accessed August 25, 2010.
- Centers for Medicare and Medicaid Services, Department of Health and Human Services.Medicare Program: Changes to the Hospital Inpatient Prospective Payment Systems and Fiscal Year 2008 Rates. CMS‐1390‐F. 8–1‐2007.
- Centers for Medicare and Medicaid Services, Department of Health and Human Services and The Joint Commission.Specifications Manual for National Hospital Inpatient Quality Measures, Discharges 10/1/09 (4Q09) through 3/31/10 (1Q10). 5–4‐2009.
- ,,,,, andthe Healthcare Infection Control Practices Advisory Committee.Guideline for the Prevention of Catheter‐associated Urinary Tract Infections,2009. Centers for Disease Control and Prevention. 1–22‐2010.
- Centers for Disease Control and Prevention.NHSN Patient Safety Component Key Terms. 1–22‐2010.
- .Stimulating the adoption of health information technology.N Engl J Med.2010;360:1477–1479.
- ,.Management of postoperative urinary retention: a randomized trial of in‐out versus overnight catheterization.ANZ J Surg.2004;2004:658–661.
- ,,, et al.Diagnosis, prevention, and treatment of catheter‐associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America.Clin Infect Dis.2010;50:625–663.
- ,,, et al.Strategies to prevent catheter‐associated urinary tract infections in acute care hospitals.Infect Control Hosp Epidemiol.2008;29:S41–S50.
Copyright © 2010 Society of Hospital Medicine
Lack of Timely PCP Follow‐Up
Care transitions between the inpatient and outpatient settings are a known period of risk in a patient's care. For instance, 1 in 5 medical patients suffers an adverse event during the first several weeks after hospital discharge, with half of these requiring the use of additional healthcare resources.1 Additionally, medication and lab monitoring errors occur in up to half of all discharged patients.2 Nearly 1 in 5 hospitalized patients, admitted with 1 of 16 different conditions including asthma, diabetes, congestive heart failure and urinary tract infection is readmitted to the hospital within six months. Up to 60% of resources are used in rehospitalized patients.3, 4 In Medicare beneficiaries, the readmission rate is as high as 20% at 30 days. The same study suggests that up to half of Medicare patients readmitted within 30 days are not seen in the outpatient setting following discharge.5 Such statistics underscore the need for seamless post‐discharge care.
Studies of post‐discharge primary care provider (PCP) follow‐up highlight the gaps in current practice within the transition from the hospital to PCP follow‐up. For instance, while more than 1 in 4 discharged patients (27.6%) at one large teaching hospital had outpatient work‐ups recommended by their hospital physicians, more than a third (35.9%) of these recommendations were ultimately not completed. Furthermore, at this same center, an increased time interval between hospital discharge and PCP follow‐up decreased the likelihood that a work‐up recommended by a hospital physician was completed.6 In patients who do have a PCP, post‐hospitalization follow‐up is frequently impacted by a variety of factors, including co‐payment requirements, transportation issues, lack of health insurance, as well as scheduling a follow‐up appointment while in the hospital.710 Uninsured patients are at particular risk for failures in transitions, have poorer health outcomes and higher mortality than insured counterparts, and are nearly 3 times more likely to make an ED visit following hospital discharge.1113
In order to better understand the role of post‐discharge PCP follow‐up, we sought to identify: (1) the percentage of general medical inpatients lacking timely PCP follow‐up after discharge from the hospital, and (2) the impact of patients lacking timely PCP follow‐up on 30‐day readmission rate and hospital length of stay (LOS). For the purposes of this study, we have defined timely PCP follow up as occurring within 4 weeks of hospital discharge.
Methods
Study Setting and Population
This prospective cohort enrolled a convenience sample of patients admitted to Internal Medicine ward teams at the University of Colorado Hospital Anschutz Inpatient Pavilion between December 2007 and March 2008. Up to 2 patients were enrolled on weekdays on the morning following admission (ie, Sunday night through Thursday night admissions). Patients were screened for study entry if they were able to participate in an interview as identified by their medical team and available in their room. Of a total of 121 patients screened for study entry by a professional research assistant (PRA), 75 ultimately provided HIPAA authorization, informed consent, and completed the in‐hospital interview. The most common reasons for screened patients refusing study enrollment included being not interested (26) and too ill (10). Ten subjects were lost to follow‐up after hospital discharge, including one subject who was deceased. Therefore, 65 patients successfully completed the follow‐up phone interview and were included in the analyses. Characteristics of the 121 screened patients and the 75 study patients were similar with respect to sex, age, race, and payer mix, and representative of the demographics of the patient population at large. Case mix indices (mean) were similar among the 121 screened (1.23), 75 enrolled (1.27), and final 65 study patients (1.25).
Exclusion Criteria
Patients admitted to the medical observation unit; patients admitted at night who are ultimately reassigned to specialty services (Oncology, Cardiology, Hepatology and Acute Care for the Elderly) were excluded. Human immunodeficiency virus (HIV) patients were excluded because of routine outpatient ID follow‐up; patients <18 years of age; patients lacking a telephone; patients admitted on Friday and Saturday nights; and outside hospital transfers.
Measures
The primary study outcome was the rate of timely PCP follow‐up defined as that occurring within 4 weeks of hospital discharge. PCP was defined in this study as either a patient's known PCP (or another provider in the same clinic), or a nurse practitioner/physician assistant. Patients seen in follow‐up by a specialist related to the discharge diagnosis, eg, an Endocrinologist in a patient hospitalized for Diabetic complications; a Rheumatologist following up an SLE patient, etc., were also counted as having PCP follow‐up as defined in this study.
Additional outcomes included three measures of hospital readmission: hospital readmission for same condition; hospital readmission or other care sought (ie, ED, Urgent Care) for same condition; and hospital readmission for any condition, and index hospital LOS. The distinction between same condition and any condition was made in an attempt to delineate a potentially preventable readmission (as an example, one study patient was subsequently readmitted with a gunshot injury that clearly would not have been affected by the presence of any PCP follow‐up). Determination of same vs. any condition was made by the investigators through information obtained from patients on follow‐up phone interviews: Have you been readmitted to the University Hospital or another hospital since your discharge last month from the University Hospital? If yes: where, when, and why? The investigators determined same vs. any through comparing this information to the primary diagnosis from the index hospitalization obtained from the final discharge documentation. A condition was considered same if the readmission was for the same condition or for treatment/complications related to the index hospitalized condition.
Descriptive data collected included patient demographics, diagnoses, insurance status, presence of an identified, established PCP, time to PCP follow‐up in weeks, effects of payer source, admitting service (hospitalist vs. General Internal Medicine (GIM) attending), and nature of presenting illness (acute vs. acute on chronic condition). Categories of insurance obtained from chart review included commercial, self‐pay (uninsured), Medicare, Medicaid and Veterans.
Data Collection
A PRA screened and obtained informed consent and a Health Insurance Portability and Accountability Act (HIPAA) waiver from patients the day following admission. At that time, the PRA obtained the patients' vital information from chart review and a scripted patient interview: age, sex, PCP, categories of insurance, contact phone numbers, and admitting date and diagnoses. The in‐house interview included eight questions examining a patient's experiences of and attitudes toward PCPs. Four weeks after discharge, patients were contacted by the PRA via telephone. Scripted telephone interviews were used to determine occurrence and timing of PCP follow‐up and hospital readmission status (to any hospital) per patient self‐report. Potential barriers to PCP follow‐up were assessed. Up to 3 attempts were made to contact study subjects out to 4 weeks from the initial call (8 weeks total). If an appointment for an enrolled patient had been made, but had not yet occurred, an additional phone call was made 2 weeks later to determine whether, and when, the appointment was kept. Review of discharge summaries determined a patient's hospital LOS.
Data Analysis
Descriptive statistics were calculated for the study population. Univariate comparisons were completed for patient characteristics and study outcomes for patients with and without PCP follow‐up. We used t‐tests for continuous variables (age and LOS) and chi‐square or Fisher's exact tests when necessary for dichotomous variables (gender, uninsured vs. insured, and all hospital readmission outcomes). Comparisons according to PCP follow‐up for the categorical variables were tested with the Cochran‐Mantel‐Haenszel statistic for general association (race and insurance category) or for trends in the ordinal variable (education).
Patient characteristics and study outcomes with univariate P value < 0.1 were assessed for inclusion in the multivariate logistic regression models. Separate logistic regression models were examined with PCP follow‐up (yes/no) as the explanatory variable and the 3 hospital readmission rates as the outcomes. Final logistic regression models included the primary predictor, PCP follow‐up, along with potential predictor variables with P value < 0.05. Statistical analyses were carried out using SAS version 9.2 (SAS Institute, Cary, NC).
This protocol was approved by the Colorado Multiple Institutional Review Board (COMIRB) prior to the implemented study.
Results
Sixty‐five patients completed this study. The mean age of the study population was 55.3 years and approximately half (52.3%) of the study participants were female. Fifty‐two subjects reported having an established PCP on admission to the hospital (80%). The rate of timely PCP follow‐up overall was 49.2%. Table 1 shows the study population characteristics stratified by presence of timely PCP follow‐up. Patients lacking timely PCP follow‐up were much younger (48.4 vs. 62.4 years; P < 0.001) than those with timely PCP follow‐up; there were also non‐significant trends toward patients lacking timely PCP follow‐up being non‐white: (33.3% vs. 25%, P = 0.23) and having lower education level (72.7% with high school or lower education vs. 56.2% for those with PCP follow‐up, P = 0.15) than those with timely PCP follow‐up. Of the 32 patients having timely PCP follow‐up, 15.6% were uninsured. In comparison, among the 33 patients lacking timely PCP follow‐up after hospital discharge, over a third (36%) were uninsured (P = 0.06). Among the uninsured, a large majority (70.5%) lacked timely PCP follow‐up (P = 0.06). In contrast, only 11 of the 26 Medicare patients (42.3%) lacked timely PCP follow‐up (P = 0.13).
| Study Demographics | Timely PCP Follow‐Up (n = 32) | No PCP Follow‐Up (n = 33) | P Value |
|---|---|---|---|
| |||
| Female, n (%) | 17 (53.1) | 17 (51.5) | 0.90 |
| Age, years, mean (SD) | 62.4 | 48.4 | <0.001 |
| Race, n (%) | |||
| Caucasian | 24 (75.0) | 23 (69.7) | 0.23 |
| African American | 7 (21.9) | 5 (15.2) | |
| Hispanic/Latino | 1 (3.1) | 5 (15.2) | |
| Highest grade completed, n (%) | |||
| Grammar school | 2 (6.3) | 3 (9.1) | 0.15 |
| High school | 16 (50.0) | 21 (63.6) | |
| College | 13 (40.6) | 9 (27.3) | |
| Postgraduate | 1 (3.1) | 0 (0) | |
| Insurance*, n (%) | |||
| Medicare | 15 (46.9) | 11 (33.3) | 0.13 |
| Medicaid | 1 (3.1) | 3 (9.1) | |
| Commercial/private | 6 (18.8) | 6 (18.2) | |
| VA/Tri‐Care | 5 (15.6) | 1 (3.0) | |
| Self‐pay/uninsured | 5 (15.6) | 12 (36.4) | 0.06 |
| Case mix index, median | 1.15 | 1.11 | |
Readmissions
The 30‐day readmission rates for all study subjects were 12.3% for a patient's same medical condition, 17.2% for readmission or other care sought for the same condition, and 21.5% for any condition. Table 2 contains univariate comparisons for the patient outcomes of readmission and LOS stratified by timely PCP follow‐up. Hospital readmission for the same medical condition was significantly higher in patients lacking timely PCP follow‐up compared to those with timely PCP follow‐up (21.2% vs. 3.1%, P = 0.05). The composite outcome of hospital readmission and/or other care sought (emergency department or urgent care) for a patient's same condition was also significantly higher in patients lacking timely PCP follow‐up (28.1% vs. 6.3%; P = 0.02). However, hospital readmission for any condition did not differ with absence of timely PCP follow‐up.
| Outcome | Timely PCP Follow‐Up (n = 32) | No PCP Follow‐Up (n = 33) | P Value |
|---|---|---|---|
| |||
| Length of stay (days), mean (SD) | 4.4 (3.7) | 6.3 (5.2) | 0.11 |
| Hospital readmission for same condition within 30‐days of discharge, n (%) | 1 (3.1) | 7 (21.2) | 0.05 |
| Hospital readmission or other care sought (ie, ED, urgent care) for same condition within 30‐days of discharge, n (%) | 2 (6.3) | 9 (28.1)* | 0.02 |
| Hospital readmission for any condition within 30‐days of discharge, n (%) | 5 (15.6) | 9 (27.3) | 0.25 |
Multiple logistic regression revealed that patients lacking timely PCP follow‐up were 10 times more likely to be readmitted for the same condition within 30 days of hospital discharge (odds ratio [OR] = 9.9; P = 0.04) and nearly seven times as likely to be readmitted for the same condition or receive other care (OR = 6.8, P = 0.02) (Table 3).
| Outcome | Odds Ratio (CI) | P Value |
|---|---|---|
| ||
| Hospital readmission for same condition | 9.9 (1.2‐84.7) | 0.04 |
| Hospital readmission or other care for same condition | 6.8 (1.4‐34.3) | 0.02 |
| Hospital readmission for any condition | 2.3 (0.7‐7.9) | 0.17 |
LOS
Overall hospital LOS in all patients was 5.4 4.6 days. In patients lacking timely PCP follow‐up, there was a trend toward longer hospital LOS: 6.3 days vs. 4.4 days, P = 0.11. For all uninsured study patients (17), the mean LOS was 6.4 days vs. 5.0 days for all other insurance categories, P = 0.31.
Insurance Status
Being uninsured was associated with a patient lacking timely PCP follow‐up (P = 0.06), but was not directly associated with higher readmission or longer hospital LOS (OR = 1.0, P = 0.96). The lack of insurance was not a significant predictor of hospital readmission in the multiple logistic regression models.
Timing of PCP Follow‐Up
In evaluating timing of any PCP follow‐up after hospital discharge and clinical outcomes, most PCP follow‐up (90.6%) occurred within the first 2 weeks following hospital discharge. However, we found no statistical difference between timing of post‐discharge PCP follow‐up and hospital readmission outcomes (hospital readmission for same reason, P = 0.51; hospital readmission or other care sought for same reason, P = 0.89), or in hospital LOS (P = 0.87). Timing of PCP follow‐upwhen comparing post‐hospitalization follow‐up <1 week, 1 to 2 weeks, and 2 to 4 weekswas not predictive of readmission rates or LOS.
Established PCP
When significance of having an established PCP prior to hospital admission was evaluated, 52 patients reported having an established PCP on hospital admission (80%), half of whom were Medicare patients. Of the 13 patients with no PCP on admission, the majority (10) were self‐pay (77%, P < 0.0001). Interestingly, only 29 (55.8%) of the patients who reported a PCP on admission to the hospital saw their PCP within 4 weeks of hospital discharge. Of 13 patients without a PCP on admission, only 3 obtained 4‐week PCP follow‐up. When we examined our study outcomes for subjects stratified by the presence of an established PCP prior to hospitalization, we found univariate association with timely post‐discharge PCP follow‐up (56% of those with established PCP vs. 23% of those without, P = 0.04), but no difference in readmission rates or hospital LOS.
Severity of patient illnessmeasured using hospital data and the case mix index (CMI)of the 3 patient populations (screened, enrolled, final) was quite similar. The CMI (mean) for the 121 screened patients was 1.23. The CMI for the 75 enrolled patients was 1.27. And the CMI in the 65 final study patients was 1.25. When evaluating illness severity (CMI) of patients in relation to hospital LOS between the 2 final study populations, the CMI (median) was also similar: 1.15 for the 32 patients with timely PCP follow‐up vs. 1.11 for the 33 patients without timely PCP follow‐up.
We found no association when looking at the rate of timely PCP follow‐up based on admitting service attending, or acute vs. acute on chronic diagnosis.
Barriers to PCP follow‐up most frequently cited by study patients were: lacking a PCP (no established PCP prior to hospital, no insurance, out of town, recently changed insurance), could not get an appointment, discharged to a half‐way house, and saw another doctor (specialist unrelated to discharge diagnosis).
Discussion
A growing body of work highlights the role of multiple, varied interventions at, or following discharge, in improving outcomes during the transition from inpatient to outpatient care. Examples include care coordination by advanced nurse practitioners, follow‐up pharmacist phone calls, and involvement of a transition coach encouraging active patient involvementall are known to improve patient outcomes following a hospitalization.1418 The active involvement of a PCP is central to a number of these proven interventions to ensure effective completion of ongoing patient care. And while some previous studies suggest increased overall resource utilization when PCP follow‐up occurs after hospitalization,19 the level of fragmented care that occurs in today's hospitalized patient, as well as the fact many patients lack PCP care at all, raises questions about clinical outcomes after hospitalization related to timely PCP follow‐up. The issue of appropriateness of resources utilized has also not been adequately explored.
Within this context, this study examines the role that PCP follow‐up might play in such interventions and its' effects on patient outcomes. Notably, in this urban academic medical center, we found that timely PCP follow‐up after hospital discharge occurred in fewer than half of general medical inpatients. Lack of timely PCP follow‐up was associated with increased hospital readmission for the same condition and a trend toward a longer index hospital LOS.
While this small study cannot fully elucidate the impact of lack of timely PCP follow‐up on post‐discharge care, our findings suggest some mechanisms by which lack of timely PCP follow‐up might result in poor outcomes. For instance, patients lacking a PCP visit after discharge may not obtain needed follow‐up care in the post‐discharge period, leading to clinical deterioration and hospital readmission. Uninsured patients may be at particular risk for failed transition because they are less likely to have consistent PCP access, whether as an already established patient or one newly assigned.20, 21 Perhaps a larger study would better demonstrate statistical significance in reflecting the association between uninsured patients, lack of a PCP, and post‐discharge follow‐up deficiencies. There may, in fact, be issues related to patient attitudes and beliefs, such as subjectively feeling better or even an implicit distrust of the healthcare system among the uninsured, that exist as well. Even among patients with a PCP prior to hospitalization, PCP follow‐up after hospital discharge may be lacking due to modifiable factors such as patient attitudes and beliefs and logistical barriers in arranging follow‐up.
Patients without potential for timely PCP follow‐up might be kept in the hospital longer to ensure they are well enough medically to sufficiently meet their own follow‐up needs. Hospital LOS might be increased by providers to compensate for the lack of PCP follow‐up. Alternatively, these patients may be sicker with their index hospitalization.
It is not surprising that payer source appears to influence a patients' ability to obtain timely PCP follow‐up. It is well documented that uninsured patients have higher healthcare resource utilization.2224 Lack of access to primary care in such patients contributes to a cycle of using the most expensive sites of care. In our study, we found many of the patients lacking timely PCP follow‐up were younger, perhaps reflecting the same patient population who have higher rates of being uninsured. Conversely, older patients are more likely to have PCP access, in large part due to having Medicare benefits (although this dynamic has shown a shift in recent years). The uninsured may present sicker as a result of lacking pre‐hospital PCP access or transportation to a PCP visit.
Limitations
This study was performed at a single, academic institution limiting its' generalizability. In addition, this small cohort study, which took place over four winter months, may have implicit biases toward certain disease entities and follow‐up issues unique to study size and season. The small study size was dictated by a finite amount of available resources, potentially contributing to minor inconsistencies with some of the results. While statistical significance was still seen with many of our results, a much larger study may better enhance the study outcomes.
It also remains unclear why the effects of PCP follow‐up were evident for a patient's same condition, but not for any condition. The distinction between designations is potentially subjective and may be difficult to accurately determine. Most existing readmission studies in the literature assign readmission for any condition. A future, larger study may be able to examine whether this difference exists between same vs. any condition.
As an academic medical center, access to specialty clinics may be facilitated, thus increasing PCP follow‐up in patients who might otherwise not have it available to them. Additionally, our subjects were limited to a convenience sample of the population of the general medicine wards and may not be representative of all medical inpatients. Patients lacking a telephone were missed. We relied on patient recollection and self‐report of PCP follow‐up visits and re‐hospitalizations. While we acknowledge limitations of patient self‐report, both in communication and comprehension, we believe patients are reasonably able to report on whether or not they were readmitted to the hospital, the cause of their readmission and whether/when they had PCP follow‐up. Patient self‐report could be collected systematically and without long time lags. Finally, the research team did not have reliable access to readmission data for hospitals other than the facility in which the study was conducted.
It is possible patients readmitted early after discharge may have been counted as lacking PCP follow‐up simply because the readmission occurred so soon after discharge precluding the opportunity for PCP follow‐up to occur. The effects of patients having non‐PCP (home health nurse, pharmacist, phone advice) follow‐up after hospital discharge were not examined.
Also, LOS and readmission to a hospital may be more a reflection of disease severity than the absence of PCP follow‐up, ie, patients ultimately readmitted after hospital discharge may have been a sicker subset of patients upon index hospitalization.
In this urban academic medical center, discharged medicine patients commonly lack timely PCP follow‐up. The lack of timely PCP follow‐up after hospital discharge was associated with higher rates of readmission and a non‐significant trend toward longer hospital lengths of stay. Hospital discharge represents a period of significant risk in patient care necessitating the effective continuation of treatment plans including follow‐up of laboratory, radiology or other testing, and management by a variety of providers. PCPs may play a crucial role in care coordination during this period. Structured intervention performed at the time of discharge might increase post‐hospital PCP access and facilitate timely PCP follow‐up to ensure continuity of needed care after hospital discharge in the most vulnerable patients. Such interventions might include systems improvements, such as increasing PCP access in the post‐hospital period, to increase the likelihood that complex needs are met at a vulnerable period in patient care.
A more effective handoff between inpatient and outpatient settings may ultimately improve clinical outcomes, diminish resource utilization, and decrease overall healthcare costs.
Acknowledgements
The authors thank Traci Yamashita and Karen Mellis, Professional Research Assistants.
- , , , .The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;13:161–167.
- , , , .Medical errors related to discontinuity of care from an inpatient to outpatient setting.J Gen Intern Med.200318:646–651.
- , .The high cost users of medical care.N Engl J Med.1980;302:996–1002.
- , .The rate and cost of hospital readmissions for preventable conditions.Med Care Res Rev.2004;61:225–240.
- , , .Rehospitalizations among patients in the medicare fee‐for‐service program.N Engl J Med.2009;360;14:1418–1428.
- , , .Tying up loose ends. Discharging patients with unresolved medical issues.Arch Intern Med.2007;167:1305–1311.
- , .Post‐hospitalization followup appointment‐keeping among the medically indigent.J Community Health.1993;18(5):271–282.
- , , .Factors related to the keeping of appointments by indigent clients.J Health Care Poor Underserved.1993;4(1):21–39.
- , , , , , .Inpatient to outpatient transfer of diabetes care: perceptions of barriers to postdischarge followup in urban African American patients.Ethn Dis.2007;17(2):238–243.
- , , .Effect of a nurse case manager on postdischarge follow‐up.J Gen Intern Med.1996v;11(11):684–688.
- , , .Emergency department visits by persons recently discharges from U.S. hospitals.Natl Health Stat Report.2008;(6):1–9.
- , , .Comparing uninsured and privately insured hospital patients: admission severity, health outcomes and resource use.Health Serv Manage Res.2001;14(3):203–210.
- , , .Comparison of uninsured and privately insured hospital patients. Condition on admission, resource use, and outcome.JAMA.1991;265(3):374–379.
- , , .Effect of a nurse case manager on postdischarge follow‐up.J Gen Intern Med.1996;11:684–688.
- , , , .The care transitions intervention: results of a randomized controlled trial.Arch Intern Med.2006;166(17):1822–1828.
- , , , , , .A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333:1190–1195.
- , , , .Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631.
- , , , et al.A reengineered hospital discharge program to decrease rehospitalization.Ann Intern Med.2009;150:178–187.
- , , .Does increased access to primary care reduce hospital readmissions?N Engl J Med.1996;334:1441–1447.
- , .Health insurance and access to health care in the united states.Ann NY Acad Sci.1008;1136:149–160.
- , .2006.Summary health statistics for U.S. adults: National Health Interview Survey. 2005, NCHS/CDC/USDHHS, Vital Health Statistics, Series 10.
- , , .Emergency department visits by persons recently discharges from U.S. hospitals.Natl Health Stat Report.2008;(6):1–9.
- , , .Comparison of uninsured and privately insured hospital patients. Condition on admission, resource use, and outcome.JAMA.1991;265(3):374–379.
- , , .Comparing uninsured and privately insured hospital patients: admission severity, health outcomes and resource use.Health Serv Manage Res.2001;14(3):203–210.
Care transitions between the inpatient and outpatient settings are a known period of risk in a patient's care. For instance, 1 in 5 medical patients suffers an adverse event during the first several weeks after hospital discharge, with half of these requiring the use of additional healthcare resources.1 Additionally, medication and lab monitoring errors occur in up to half of all discharged patients.2 Nearly 1 in 5 hospitalized patients, admitted with 1 of 16 different conditions including asthma, diabetes, congestive heart failure and urinary tract infection is readmitted to the hospital within six months. Up to 60% of resources are used in rehospitalized patients.3, 4 In Medicare beneficiaries, the readmission rate is as high as 20% at 30 days. The same study suggests that up to half of Medicare patients readmitted within 30 days are not seen in the outpatient setting following discharge.5 Such statistics underscore the need for seamless post‐discharge care.
Studies of post‐discharge primary care provider (PCP) follow‐up highlight the gaps in current practice within the transition from the hospital to PCP follow‐up. For instance, while more than 1 in 4 discharged patients (27.6%) at one large teaching hospital had outpatient work‐ups recommended by their hospital physicians, more than a third (35.9%) of these recommendations were ultimately not completed. Furthermore, at this same center, an increased time interval between hospital discharge and PCP follow‐up decreased the likelihood that a work‐up recommended by a hospital physician was completed.6 In patients who do have a PCP, post‐hospitalization follow‐up is frequently impacted by a variety of factors, including co‐payment requirements, transportation issues, lack of health insurance, as well as scheduling a follow‐up appointment while in the hospital.710 Uninsured patients are at particular risk for failures in transitions, have poorer health outcomes and higher mortality than insured counterparts, and are nearly 3 times more likely to make an ED visit following hospital discharge.1113
In order to better understand the role of post‐discharge PCP follow‐up, we sought to identify: (1) the percentage of general medical inpatients lacking timely PCP follow‐up after discharge from the hospital, and (2) the impact of patients lacking timely PCP follow‐up on 30‐day readmission rate and hospital length of stay (LOS). For the purposes of this study, we have defined timely PCP follow up as occurring within 4 weeks of hospital discharge.
Methods
Study Setting and Population
This prospective cohort enrolled a convenience sample of patients admitted to Internal Medicine ward teams at the University of Colorado Hospital Anschutz Inpatient Pavilion between December 2007 and March 2008. Up to 2 patients were enrolled on weekdays on the morning following admission (ie, Sunday night through Thursday night admissions). Patients were screened for study entry if they were able to participate in an interview as identified by their medical team and available in their room. Of a total of 121 patients screened for study entry by a professional research assistant (PRA), 75 ultimately provided HIPAA authorization, informed consent, and completed the in‐hospital interview. The most common reasons for screened patients refusing study enrollment included being not interested (26) and too ill (10). Ten subjects were lost to follow‐up after hospital discharge, including one subject who was deceased. Therefore, 65 patients successfully completed the follow‐up phone interview and were included in the analyses. Characteristics of the 121 screened patients and the 75 study patients were similar with respect to sex, age, race, and payer mix, and representative of the demographics of the patient population at large. Case mix indices (mean) were similar among the 121 screened (1.23), 75 enrolled (1.27), and final 65 study patients (1.25).
Exclusion Criteria
Patients admitted to the medical observation unit; patients admitted at night who are ultimately reassigned to specialty services (Oncology, Cardiology, Hepatology and Acute Care for the Elderly) were excluded. Human immunodeficiency virus (HIV) patients were excluded because of routine outpatient ID follow‐up; patients <18 years of age; patients lacking a telephone; patients admitted on Friday and Saturday nights; and outside hospital transfers.
Measures
The primary study outcome was the rate of timely PCP follow‐up defined as that occurring within 4 weeks of hospital discharge. PCP was defined in this study as either a patient's known PCP (or another provider in the same clinic), or a nurse practitioner/physician assistant. Patients seen in follow‐up by a specialist related to the discharge diagnosis, eg, an Endocrinologist in a patient hospitalized for Diabetic complications; a Rheumatologist following up an SLE patient, etc., were also counted as having PCP follow‐up as defined in this study.
Additional outcomes included three measures of hospital readmission: hospital readmission for same condition; hospital readmission or other care sought (ie, ED, Urgent Care) for same condition; and hospital readmission for any condition, and index hospital LOS. The distinction between same condition and any condition was made in an attempt to delineate a potentially preventable readmission (as an example, one study patient was subsequently readmitted with a gunshot injury that clearly would not have been affected by the presence of any PCP follow‐up). Determination of same vs. any condition was made by the investigators through information obtained from patients on follow‐up phone interviews: Have you been readmitted to the University Hospital or another hospital since your discharge last month from the University Hospital? If yes: where, when, and why? The investigators determined same vs. any through comparing this information to the primary diagnosis from the index hospitalization obtained from the final discharge documentation. A condition was considered same if the readmission was for the same condition or for treatment/complications related to the index hospitalized condition.
Descriptive data collected included patient demographics, diagnoses, insurance status, presence of an identified, established PCP, time to PCP follow‐up in weeks, effects of payer source, admitting service (hospitalist vs. General Internal Medicine (GIM) attending), and nature of presenting illness (acute vs. acute on chronic condition). Categories of insurance obtained from chart review included commercial, self‐pay (uninsured), Medicare, Medicaid and Veterans.
Data Collection
A PRA screened and obtained informed consent and a Health Insurance Portability and Accountability Act (HIPAA) waiver from patients the day following admission. At that time, the PRA obtained the patients' vital information from chart review and a scripted patient interview: age, sex, PCP, categories of insurance, contact phone numbers, and admitting date and diagnoses. The in‐house interview included eight questions examining a patient's experiences of and attitudes toward PCPs. Four weeks after discharge, patients were contacted by the PRA via telephone. Scripted telephone interviews were used to determine occurrence and timing of PCP follow‐up and hospital readmission status (to any hospital) per patient self‐report. Potential barriers to PCP follow‐up were assessed. Up to 3 attempts were made to contact study subjects out to 4 weeks from the initial call (8 weeks total). If an appointment for an enrolled patient had been made, but had not yet occurred, an additional phone call was made 2 weeks later to determine whether, and when, the appointment was kept. Review of discharge summaries determined a patient's hospital LOS.
Data Analysis
Descriptive statistics were calculated for the study population. Univariate comparisons were completed for patient characteristics and study outcomes for patients with and without PCP follow‐up. We used t‐tests for continuous variables (age and LOS) and chi‐square or Fisher's exact tests when necessary for dichotomous variables (gender, uninsured vs. insured, and all hospital readmission outcomes). Comparisons according to PCP follow‐up for the categorical variables were tested with the Cochran‐Mantel‐Haenszel statistic for general association (race and insurance category) or for trends in the ordinal variable (education).
Patient characteristics and study outcomes with univariate P value < 0.1 were assessed for inclusion in the multivariate logistic regression models. Separate logistic regression models were examined with PCP follow‐up (yes/no) as the explanatory variable and the 3 hospital readmission rates as the outcomes. Final logistic regression models included the primary predictor, PCP follow‐up, along with potential predictor variables with P value < 0.05. Statistical analyses were carried out using SAS version 9.2 (SAS Institute, Cary, NC).
This protocol was approved by the Colorado Multiple Institutional Review Board (COMIRB) prior to the implemented study.
Results
Sixty‐five patients completed this study. The mean age of the study population was 55.3 years and approximately half (52.3%) of the study participants were female. Fifty‐two subjects reported having an established PCP on admission to the hospital (80%). The rate of timely PCP follow‐up overall was 49.2%. Table 1 shows the study population characteristics stratified by presence of timely PCP follow‐up. Patients lacking timely PCP follow‐up were much younger (48.4 vs. 62.4 years; P < 0.001) than those with timely PCP follow‐up; there were also non‐significant trends toward patients lacking timely PCP follow‐up being non‐white: (33.3% vs. 25%, P = 0.23) and having lower education level (72.7% with high school or lower education vs. 56.2% for those with PCP follow‐up, P = 0.15) than those with timely PCP follow‐up. Of the 32 patients having timely PCP follow‐up, 15.6% were uninsured. In comparison, among the 33 patients lacking timely PCP follow‐up after hospital discharge, over a third (36%) were uninsured (P = 0.06). Among the uninsured, a large majority (70.5%) lacked timely PCP follow‐up (P = 0.06). In contrast, only 11 of the 26 Medicare patients (42.3%) lacked timely PCP follow‐up (P = 0.13).
| Study Demographics | Timely PCP Follow‐Up (n = 32) | No PCP Follow‐Up (n = 33) | P Value |
|---|---|---|---|
| |||
| Female, n (%) | 17 (53.1) | 17 (51.5) | 0.90 |
| Age, years, mean (SD) | 62.4 | 48.4 | <0.001 |
| Race, n (%) | |||
| Caucasian | 24 (75.0) | 23 (69.7) | 0.23 |
| African American | 7 (21.9) | 5 (15.2) | |
| Hispanic/Latino | 1 (3.1) | 5 (15.2) | |
| Highest grade completed, n (%) | |||
| Grammar school | 2 (6.3) | 3 (9.1) | 0.15 |
| High school | 16 (50.0) | 21 (63.6) | |
| College | 13 (40.6) | 9 (27.3) | |
| Postgraduate | 1 (3.1) | 0 (0) | |
| Insurance*, n (%) | |||
| Medicare | 15 (46.9) | 11 (33.3) | 0.13 |
| Medicaid | 1 (3.1) | 3 (9.1) | |
| Commercial/private | 6 (18.8) | 6 (18.2) | |
| VA/Tri‐Care | 5 (15.6) | 1 (3.0) | |
| Self‐pay/uninsured | 5 (15.6) | 12 (36.4) | 0.06 |
| Case mix index, median | 1.15 | 1.11 | |
Readmissions
The 30‐day readmission rates for all study subjects were 12.3% for a patient's same medical condition, 17.2% for readmission or other care sought for the same condition, and 21.5% for any condition. Table 2 contains univariate comparisons for the patient outcomes of readmission and LOS stratified by timely PCP follow‐up. Hospital readmission for the same medical condition was significantly higher in patients lacking timely PCP follow‐up compared to those with timely PCP follow‐up (21.2% vs. 3.1%, P = 0.05). The composite outcome of hospital readmission and/or other care sought (emergency department or urgent care) for a patient's same condition was also significantly higher in patients lacking timely PCP follow‐up (28.1% vs. 6.3%; P = 0.02). However, hospital readmission for any condition did not differ with absence of timely PCP follow‐up.
| Outcome | Timely PCP Follow‐Up (n = 32) | No PCP Follow‐Up (n = 33) | P Value |
|---|---|---|---|
| |||
| Length of stay (days), mean (SD) | 4.4 (3.7) | 6.3 (5.2) | 0.11 |
| Hospital readmission for same condition within 30‐days of discharge, n (%) | 1 (3.1) | 7 (21.2) | 0.05 |
| Hospital readmission or other care sought (ie, ED, urgent care) for same condition within 30‐days of discharge, n (%) | 2 (6.3) | 9 (28.1)* | 0.02 |
| Hospital readmission for any condition within 30‐days of discharge, n (%) | 5 (15.6) | 9 (27.3) | 0.25 |
Multiple logistic regression revealed that patients lacking timely PCP follow‐up were 10 times more likely to be readmitted for the same condition within 30 days of hospital discharge (odds ratio [OR] = 9.9; P = 0.04) and nearly seven times as likely to be readmitted for the same condition or receive other care (OR = 6.8, P = 0.02) (Table 3).
| Outcome | Odds Ratio (CI) | P Value |
|---|---|---|
| ||
| Hospital readmission for same condition | 9.9 (1.2‐84.7) | 0.04 |
| Hospital readmission or other care for same condition | 6.8 (1.4‐34.3) | 0.02 |
| Hospital readmission for any condition | 2.3 (0.7‐7.9) | 0.17 |
LOS
Overall hospital LOS in all patients was 5.4 4.6 days. In patients lacking timely PCP follow‐up, there was a trend toward longer hospital LOS: 6.3 days vs. 4.4 days, P = 0.11. For all uninsured study patients (17), the mean LOS was 6.4 days vs. 5.0 days for all other insurance categories, P = 0.31.
Insurance Status
Being uninsured was associated with a patient lacking timely PCP follow‐up (P = 0.06), but was not directly associated with higher readmission or longer hospital LOS (OR = 1.0, P = 0.96). The lack of insurance was not a significant predictor of hospital readmission in the multiple logistic regression models.
Timing of PCP Follow‐Up
In evaluating timing of any PCP follow‐up after hospital discharge and clinical outcomes, most PCP follow‐up (90.6%) occurred within the first 2 weeks following hospital discharge. However, we found no statistical difference between timing of post‐discharge PCP follow‐up and hospital readmission outcomes (hospital readmission for same reason, P = 0.51; hospital readmission or other care sought for same reason, P = 0.89), or in hospital LOS (P = 0.87). Timing of PCP follow‐upwhen comparing post‐hospitalization follow‐up <1 week, 1 to 2 weeks, and 2 to 4 weekswas not predictive of readmission rates or LOS.
Established PCP
When significance of having an established PCP prior to hospital admission was evaluated, 52 patients reported having an established PCP on hospital admission (80%), half of whom were Medicare patients. Of the 13 patients with no PCP on admission, the majority (10) were self‐pay (77%, P < 0.0001). Interestingly, only 29 (55.8%) of the patients who reported a PCP on admission to the hospital saw their PCP within 4 weeks of hospital discharge. Of 13 patients without a PCP on admission, only 3 obtained 4‐week PCP follow‐up. When we examined our study outcomes for subjects stratified by the presence of an established PCP prior to hospitalization, we found univariate association with timely post‐discharge PCP follow‐up (56% of those with established PCP vs. 23% of those without, P = 0.04), but no difference in readmission rates or hospital LOS.
Severity of patient illnessmeasured using hospital data and the case mix index (CMI)of the 3 patient populations (screened, enrolled, final) was quite similar. The CMI (mean) for the 121 screened patients was 1.23. The CMI for the 75 enrolled patients was 1.27. And the CMI in the 65 final study patients was 1.25. When evaluating illness severity (CMI) of patients in relation to hospital LOS between the 2 final study populations, the CMI (median) was also similar: 1.15 for the 32 patients with timely PCP follow‐up vs. 1.11 for the 33 patients without timely PCP follow‐up.
We found no association when looking at the rate of timely PCP follow‐up based on admitting service attending, or acute vs. acute on chronic diagnosis.
Barriers to PCP follow‐up most frequently cited by study patients were: lacking a PCP (no established PCP prior to hospital, no insurance, out of town, recently changed insurance), could not get an appointment, discharged to a half‐way house, and saw another doctor (specialist unrelated to discharge diagnosis).
Discussion
A growing body of work highlights the role of multiple, varied interventions at, or following discharge, in improving outcomes during the transition from inpatient to outpatient care. Examples include care coordination by advanced nurse practitioners, follow‐up pharmacist phone calls, and involvement of a transition coach encouraging active patient involvementall are known to improve patient outcomes following a hospitalization.1418 The active involvement of a PCP is central to a number of these proven interventions to ensure effective completion of ongoing patient care. And while some previous studies suggest increased overall resource utilization when PCP follow‐up occurs after hospitalization,19 the level of fragmented care that occurs in today's hospitalized patient, as well as the fact many patients lack PCP care at all, raises questions about clinical outcomes after hospitalization related to timely PCP follow‐up. The issue of appropriateness of resources utilized has also not been adequately explored.
Within this context, this study examines the role that PCP follow‐up might play in such interventions and its' effects on patient outcomes. Notably, in this urban academic medical center, we found that timely PCP follow‐up after hospital discharge occurred in fewer than half of general medical inpatients. Lack of timely PCP follow‐up was associated with increased hospital readmission for the same condition and a trend toward a longer index hospital LOS.
While this small study cannot fully elucidate the impact of lack of timely PCP follow‐up on post‐discharge care, our findings suggest some mechanisms by which lack of timely PCP follow‐up might result in poor outcomes. For instance, patients lacking a PCP visit after discharge may not obtain needed follow‐up care in the post‐discharge period, leading to clinical deterioration and hospital readmission. Uninsured patients may be at particular risk for failed transition because they are less likely to have consistent PCP access, whether as an already established patient or one newly assigned.20, 21 Perhaps a larger study would better demonstrate statistical significance in reflecting the association between uninsured patients, lack of a PCP, and post‐discharge follow‐up deficiencies. There may, in fact, be issues related to patient attitudes and beliefs, such as subjectively feeling better or even an implicit distrust of the healthcare system among the uninsured, that exist as well. Even among patients with a PCP prior to hospitalization, PCP follow‐up after hospital discharge may be lacking due to modifiable factors such as patient attitudes and beliefs and logistical barriers in arranging follow‐up.
Patients without potential for timely PCP follow‐up might be kept in the hospital longer to ensure they are well enough medically to sufficiently meet their own follow‐up needs. Hospital LOS might be increased by providers to compensate for the lack of PCP follow‐up. Alternatively, these patients may be sicker with their index hospitalization.
It is not surprising that payer source appears to influence a patients' ability to obtain timely PCP follow‐up. It is well documented that uninsured patients have higher healthcare resource utilization.2224 Lack of access to primary care in such patients contributes to a cycle of using the most expensive sites of care. In our study, we found many of the patients lacking timely PCP follow‐up were younger, perhaps reflecting the same patient population who have higher rates of being uninsured. Conversely, older patients are more likely to have PCP access, in large part due to having Medicare benefits (although this dynamic has shown a shift in recent years). The uninsured may present sicker as a result of lacking pre‐hospital PCP access or transportation to a PCP visit.
Limitations
This study was performed at a single, academic institution limiting its' generalizability. In addition, this small cohort study, which took place over four winter months, may have implicit biases toward certain disease entities and follow‐up issues unique to study size and season. The small study size was dictated by a finite amount of available resources, potentially contributing to minor inconsistencies with some of the results. While statistical significance was still seen with many of our results, a much larger study may better enhance the study outcomes.
It also remains unclear why the effects of PCP follow‐up were evident for a patient's same condition, but not for any condition. The distinction between designations is potentially subjective and may be difficult to accurately determine. Most existing readmission studies in the literature assign readmission for any condition. A future, larger study may be able to examine whether this difference exists between same vs. any condition.
As an academic medical center, access to specialty clinics may be facilitated, thus increasing PCP follow‐up in patients who might otherwise not have it available to them. Additionally, our subjects were limited to a convenience sample of the population of the general medicine wards and may not be representative of all medical inpatients. Patients lacking a telephone were missed. We relied on patient recollection and self‐report of PCP follow‐up visits and re‐hospitalizations. While we acknowledge limitations of patient self‐report, both in communication and comprehension, we believe patients are reasonably able to report on whether or not they were readmitted to the hospital, the cause of their readmission and whether/when they had PCP follow‐up. Patient self‐report could be collected systematically and without long time lags. Finally, the research team did not have reliable access to readmission data for hospitals other than the facility in which the study was conducted.
It is possible patients readmitted early after discharge may have been counted as lacking PCP follow‐up simply because the readmission occurred so soon after discharge precluding the opportunity for PCP follow‐up to occur. The effects of patients having non‐PCP (home health nurse, pharmacist, phone advice) follow‐up after hospital discharge were not examined.
Also, LOS and readmission to a hospital may be more a reflection of disease severity than the absence of PCP follow‐up, ie, patients ultimately readmitted after hospital discharge may have been a sicker subset of patients upon index hospitalization.
In this urban academic medical center, discharged medicine patients commonly lack timely PCP follow‐up. The lack of timely PCP follow‐up after hospital discharge was associated with higher rates of readmission and a non‐significant trend toward longer hospital lengths of stay. Hospital discharge represents a period of significant risk in patient care necessitating the effective continuation of treatment plans including follow‐up of laboratory, radiology or other testing, and management by a variety of providers. PCPs may play a crucial role in care coordination during this period. Structured intervention performed at the time of discharge might increase post‐hospital PCP access and facilitate timely PCP follow‐up to ensure continuity of needed care after hospital discharge in the most vulnerable patients. Such interventions might include systems improvements, such as increasing PCP access in the post‐hospital period, to increase the likelihood that complex needs are met at a vulnerable period in patient care.
A more effective handoff between inpatient and outpatient settings may ultimately improve clinical outcomes, diminish resource utilization, and decrease overall healthcare costs.
Acknowledgements
The authors thank Traci Yamashita and Karen Mellis, Professional Research Assistants.
Care transitions between the inpatient and outpatient settings are a known period of risk in a patient's care. For instance, 1 in 5 medical patients suffers an adverse event during the first several weeks after hospital discharge, with half of these requiring the use of additional healthcare resources.1 Additionally, medication and lab monitoring errors occur in up to half of all discharged patients.2 Nearly 1 in 5 hospitalized patients, admitted with 1 of 16 different conditions including asthma, diabetes, congestive heart failure and urinary tract infection is readmitted to the hospital within six months. Up to 60% of resources are used in rehospitalized patients.3, 4 In Medicare beneficiaries, the readmission rate is as high as 20% at 30 days. The same study suggests that up to half of Medicare patients readmitted within 30 days are not seen in the outpatient setting following discharge.5 Such statistics underscore the need for seamless post‐discharge care.
Studies of post‐discharge primary care provider (PCP) follow‐up highlight the gaps in current practice within the transition from the hospital to PCP follow‐up. For instance, while more than 1 in 4 discharged patients (27.6%) at one large teaching hospital had outpatient work‐ups recommended by their hospital physicians, more than a third (35.9%) of these recommendations were ultimately not completed. Furthermore, at this same center, an increased time interval between hospital discharge and PCP follow‐up decreased the likelihood that a work‐up recommended by a hospital physician was completed.6 In patients who do have a PCP, post‐hospitalization follow‐up is frequently impacted by a variety of factors, including co‐payment requirements, transportation issues, lack of health insurance, as well as scheduling a follow‐up appointment while in the hospital.710 Uninsured patients are at particular risk for failures in transitions, have poorer health outcomes and higher mortality than insured counterparts, and are nearly 3 times more likely to make an ED visit following hospital discharge.1113
In order to better understand the role of post‐discharge PCP follow‐up, we sought to identify: (1) the percentage of general medical inpatients lacking timely PCP follow‐up after discharge from the hospital, and (2) the impact of patients lacking timely PCP follow‐up on 30‐day readmission rate and hospital length of stay (LOS). For the purposes of this study, we have defined timely PCP follow up as occurring within 4 weeks of hospital discharge.
Methods
Study Setting and Population
This prospective cohort enrolled a convenience sample of patients admitted to Internal Medicine ward teams at the University of Colorado Hospital Anschutz Inpatient Pavilion between December 2007 and March 2008. Up to 2 patients were enrolled on weekdays on the morning following admission (ie, Sunday night through Thursday night admissions). Patients were screened for study entry if they were able to participate in an interview as identified by their medical team and available in their room. Of a total of 121 patients screened for study entry by a professional research assistant (PRA), 75 ultimately provided HIPAA authorization, informed consent, and completed the in‐hospital interview. The most common reasons for screened patients refusing study enrollment included being not interested (26) and too ill (10). Ten subjects were lost to follow‐up after hospital discharge, including one subject who was deceased. Therefore, 65 patients successfully completed the follow‐up phone interview and were included in the analyses. Characteristics of the 121 screened patients and the 75 study patients were similar with respect to sex, age, race, and payer mix, and representative of the demographics of the patient population at large. Case mix indices (mean) were similar among the 121 screened (1.23), 75 enrolled (1.27), and final 65 study patients (1.25).
Exclusion Criteria
Patients admitted to the medical observation unit; patients admitted at night who are ultimately reassigned to specialty services (Oncology, Cardiology, Hepatology and Acute Care for the Elderly) were excluded. Human immunodeficiency virus (HIV) patients were excluded because of routine outpatient ID follow‐up; patients <18 years of age; patients lacking a telephone; patients admitted on Friday and Saturday nights; and outside hospital transfers.
Measures
The primary study outcome was the rate of timely PCP follow‐up defined as that occurring within 4 weeks of hospital discharge. PCP was defined in this study as either a patient's known PCP (or another provider in the same clinic), or a nurse practitioner/physician assistant. Patients seen in follow‐up by a specialist related to the discharge diagnosis, eg, an Endocrinologist in a patient hospitalized for Diabetic complications; a Rheumatologist following up an SLE patient, etc., were also counted as having PCP follow‐up as defined in this study.
Additional outcomes included three measures of hospital readmission: hospital readmission for same condition; hospital readmission or other care sought (ie, ED, Urgent Care) for same condition; and hospital readmission for any condition, and index hospital LOS. The distinction between same condition and any condition was made in an attempt to delineate a potentially preventable readmission (as an example, one study patient was subsequently readmitted with a gunshot injury that clearly would not have been affected by the presence of any PCP follow‐up). Determination of same vs. any condition was made by the investigators through information obtained from patients on follow‐up phone interviews: Have you been readmitted to the University Hospital or another hospital since your discharge last month from the University Hospital? If yes: where, when, and why? The investigators determined same vs. any through comparing this information to the primary diagnosis from the index hospitalization obtained from the final discharge documentation. A condition was considered same if the readmission was for the same condition or for treatment/complications related to the index hospitalized condition.
Descriptive data collected included patient demographics, diagnoses, insurance status, presence of an identified, established PCP, time to PCP follow‐up in weeks, effects of payer source, admitting service (hospitalist vs. General Internal Medicine (GIM) attending), and nature of presenting illness (acute vs. acute on chronic condition). Categories of insurance obtained from chart review included commercial, self‐pay (uninsured), Medicare, Medicaid and Veterans.
Data Collection
A PRA screened and obtained informed consent and a Health Insurance Portability and Accountability Act (HIPAA) waiver from patients the day following admission. At that time, the PRA obtained the patients' vital information from chart review and a scripted patient interview: age, sex, PCP, categories of insurance, contact phone numbers, and admitting date and diagnoses. The in‐house interview included eight questions examining a patient's experiences of and attitudes toward PCPs. Four weeks after discharge, patients were contacted by the PRA via telephone. Scripted telephone interviews were used to determine occurrence and timing of PCP follow‐up and hospital readmission status (to any hospital) per patient self‐report. Potential barriers to PCP follow‐up were assessed. Up to 3 attempts were made to contact study subjects out to 4 weeks from the initial call (8 weeks total). If an appointment for an enrolled patient had been made, but had not yet occurred, an additional phone call was made 2 weeks later to determine whether, and when, the appointment was kept. Review of discharge summaries determined a patient's hospital LOS.
Data Analysis
Descriptive statistics were calculated for the study population. Univariate comparisons were completed for patient characteristics and study outcomes for patients with and without PCP follow‐up. We used t‐tests for continuous variables (age and LOS) and chi‐square or Fisher's exact tests when necessary for dichotomous variables (gender, uninsured vs. insured, and all hospital readmission outcomes). Comparisons according to PCP follow‐up for the categorical variables were tested with the Cochran‐Mantel‐Haenszel statistic for general association (race and insurance category) or for trends in the ordinal variable (education).
Patient characteristics and study outcomes with univariate P value < 0.1 were assessed for inclusion in the multivariate logistic regression models. Separate logistic regression models were examined with PCP follow‐up (yes/no) as the explanatory variable and the 3 hospital readmission rates as the outcomes. Final logistic regression models included the primary predictor, PCP follow‐up, along with potential predictor variables with P value < 0.05. Statistical analyses were carried out using SAS version 9.2 (SAS Institute, Cary, NC).
This protocol was approved by the Colorado Multiple Institutional Review Board (COMIRB) prior to the implemented study.
Results
Sixty‐five patients completed this study. The mean age of the study population was 55.3 years and approximately half (52.3%) of the study participants were female. Fifty‐two subjects reported having an established PCP on admission to the hospital (80%). The rate of timely PCP follow‐up overall was 49.2%. Table 1 shows the study population characteristics stratified by presence of timely PCP follow‐up. Patients lacking timely PCP follow‐up were much younger (48.4 vs. 62.4 years; P < 0.001) than those with timely PCP follow‐up; there were also non‐significant trends toward patients lacking timely PCP follow‐up being non‐white: (33.3% vs. 25%, P = 0.23) and having lower education level (72.7% with high school or lower education vs. 56.2% for those with PCP follow‐up, P = 0.15) than those with timely PCP follow‐up. Of the 32 patients having timely PCP follow‐up, 15.6% were uninsured. In comparison, among the 33 patients lacking timely PCP follow‐up after hospital discharge, over a third (36%) were uninsured (P = 0.06). Among the uninsured, a large majority (70.5%) lacked timely PCP follow‐up (P = 0.06). In contrast, only 11 of the 26 Medicare patients (42.3%) lacked timely PCP follow‐up (P = 0.13).
| Study Demographics | Timely PCP Follow‐Up (n = 32) | No PCP Follow‐Up (n = 33) | P Value |
|---|---|---|---|
| |||
| Female, n (%) | 17 (53.1) | 17 (51.5) | 0.90 |
| Age, years, mean (SD) | 62.4 | 48.4 | <0.001 |
| Race, n (%) | |||
| Caucasian | 24 (75.0) | 23 (69.7) | 0.23 |
| African American | 7 (21.9) | 5 (15.2) | |
| Hispanic/Latino | 1 (3.1) | 5 (15.2) | |
| Highest grade completed, n (%) | |||
| Grammar school | 2 (6.3) | 3 (9.1) | 0.15 |
| High school | 16 (50.0) | 21 (63.6) | |
| College | 13 (40.6) | 9 (27.3) | |
| Postgraduate | 1 (3.1) | 0 (0) | |
| Insurance*, n (%) | |||
| Medicare | 15 (46.9) | 11 (33.3) | 0.13 |
| Medicaid | 1 (3.1) | 3 (9.1) | |
| Commercial/private | 6 (18.8) | 6 (18.2) | |
| VA/Tri‐Care | 5 (15.6) | 1 (3.0) | |
| Self‐pay/uninsured | 5 (15.6) | 12 (36.4) | 0.06 |
| Case mix index, median | 1.15 | 1.11 | |
Readmissions
The 30‐day readmission rates for all study subjects were 12.3% for a patient's same medical condition, 17.2% for readmission or other care sought for the same condition, and 21.5% for any condition. Table 2 contains univariate comparisons for the patient outcomes of readmission and LOS stratified by timely PCP follow‐up. Hospital readmission for the same medical condition was significantly higher in patients lacking timely PCP follow‐up compared to those with timely PCP follow‐up (21.2% vs. 3.1%, P = 0.05). The composite outcome of hospital readmission and/or other care sought (emergency department or urgent care) for a patient's same condition was also significantly higher in patients lacking timely PCP follow‐up (28.1% vs. 6.3%; P = 0.02). However, hospital readmission for any condition did not differ with absence of timely PCP follow‐up.
| Outcome | Timely PCP Follow‐Up (n = 32) | No PCP Follow‐Up (n = 33) | P Value |
|---|---|---|---|
| |||
| Length of stay (days), mean (SD) | 4.4 (3.7) | 6.3 (5.2) | 0.11 |
| Hospital readmission for same condition within 30‐days of discharge, n (%) | 1 (3.1) | 7 (21.2) | 0.05 |
| Hospital readmission or other care sought (ie, ED, urgent care) for same condition within 30‐days of discharge, n (%) | 2 (6.3) | 9 (28.1)* | 0.02 |
| Hospital readmission for any condition within 30‐days of discharge, n (%) | 5 (15.6) | 9 (27.3) | 0.25 |
Multiple logistic regression revealed that patients lacking timely PCP follow‐up were 10 times more likely to be readmitted for the same condition within 30 days of hospital discharge (odds ratio [OR] = 9.9; P = 0.04) and nearly seven times as likely to be readmitted for the same condition or receive other care (OR = 6.8, P = 0.02) (Table 3).
| Outcome | Odds Ratio (CI) | P Value |
|---|---|---|
| ||
| Hospital readmission for same condition | 9.9 (1.2‐84.7) | 0.04 |
| Hospital readmission or other care for same condition | 6.8 (1.4‐34.3) | 0.02 |
| Hospital readmission for any condition | 2.3 (0.7‐7.9) | 0.17 |
LOS
Overall hospital LOS in all patients was 5.4 4.6 days. In patients lacking timely PCP follow‐up, there was a trend toward longer hospital LOS: 6.3 days vs. 4.4 days, P = 0.11. For all uninsured study patients (17), the mean LOS was 6.4 days vs. 5.0 days for all other insurance categories, P = 0.31.
Insurance Status
Being uninsured was associated with a patient lacking timely PCP follow‐up (P = 0.06), but was not directly associated with higher readmission or longer hospital LOS (OR = 1.0, P = 0.96). The lack of insurance was not a significant predictor of hospital readmission in the multiple logistic regression models.
Timing of PCP Follow‐Up
In evaluating timing of any PCP follow‐up after hospital discharge and clinical outcomes, most PCP follow‐up (90.6%) occurred within the first 2 weeks following hospital discharge. However, we found no statistical difference between timing of post‐discharge PCP follow‐up and hospital readmission outcomes (hospital readmission for same reason, P = 0.51; hospital readmission or other care sought for same reason, P = 0.89), or in hospital LOS (P = 0.87). Timing of PCP follow‐upwhen comparing post‐hospitalization follow‐up <1 week, 1 to 2 weeks, and 2 to 4 weekswas not predictive of readmission rates or LOS.
Established PCP
When significance of having an established PCP prior to hospital admission was evaluated, 52 patients reported having an established PCP on hospital admission (80%), half of whom were Medicare patients. Of the 13 patients with no PCP on admission, the majority (10) were self‐pay (77%, P < 0.0001). Interestingly, only 29 (55.8%) of the patients who reported a PCP on admission to the hospital saw their PCP within 4 weeks of hospital discharge. Of 13 patients without a PCP on admission, only 3 obtained 4‐week PCP follow‐up. When we examined our study outcomes for subjects stratified by the presence of an established PCP prior to hospitalization, we found univariate association with timely post‐discharge PCP follow‐up (56% of those with established PCP vs. 23% of those without, P = 0.04), but no difference in readmission rates or hospital LOS.
Severity of patient illnessmeasured using hospital data and the case mix index (CMI)of the 3 patient populations (screened, enrolled, final) was quite similar. The CMI (mean) for the 121 screened patients was 1.23. The CMI for the 75 enrolled patients was 1.27. And the CMI in the 65 final study patients was 1.25. When evaluating illness severity (CMI) of patients in relation to hospital LOS between the 2 final study populations, the CMI (median) was also similar: 1.15 for the 32 patients with timely PCP follow‐up vs. 1.11 for the 33 patients without timely PCP follow‐up.
We found no association when looking at the rate of timely PCP follow‐up based on admitting service attending, or acute vs. acute on chronic diagnosis.
Barriers to PCP follow‐up most frequently cited by study patients were: lacking a PCP (no established PCP prior to hospital, no insurance, out of town, recently changed insurance), could not get an appointment, discharged to a half‐way house, and saw another doctor (specialist unrelated to discharge diagnosis).
Discussion
A growing body of work highlights the role of multiple, varied interventions at, or following discharge, in improving outcomes during the transition from inpatient to outpatient care. Examples include care coordination by advanced nurse practitioners, follow‐up pharmacist phone calls, and involvement of a transition coach encouraging active patient involvementall are known to improve patient outcomes following a hospitalization.1418 The active involvement of a PCP is central to a number of these proven interventions to ensure effective completion of ongoing patient care. And while some previous studies suggest increased overall resource utilization when PCP follow‐up occurs after hospitalization,19 the level of fragmented care that occurs in today's hospitalized patient, as well as the fact many patients lack PCP care at all, raises questions about clinical outcomes after hospitalization related to timely PCP follow‐up. The issue of appropriateness of resources utilized has also not been adequately explored.
Within this context, this study examines the role that PCP follow‐up might play in such interventions and its' effects on patient outcomes. Notably, in this urban academic medical center, we found that timely PCP follow‐up after hospital discharge occurred in fewer than half of general medical inpatients. Lack of timely PCP follow‐up was associated with increased hospital readmission for the same condition and a trend toward a longer index hospital LOS.
While this small study cannot fully elucidate the impact of lack of timely PCP follow‐up on post‐discharge care, our findings suggest some mechanisms by which lack of timely PCP follow‐up might result in poor outcomes. For instance, patients lacking a PCP visit after discharge may not obtain needed follow‐up care in the post‐discharge period, leading to clinical deterioration and hospital readmission. Uninsured patients may be at particular risk for failed transition because they are less likely to have consistent PCP access, whether as an already established patient or one newly assigned.20, 21 Perhaps a larger study would better demonstrate statistical significance in reflecting the association between uninsured patients, lack of a PCP, and post‐discharge follow‐up deficiencies. There may, in fact, be issues related to patient attitudes and beliefs, such as subjectively feeling better or even an implicit distrust of the healthcare system among the uninsured, that exist as well. Even among patients with a PCP prior to hospitalization, PCP follow‐up after hospital discharge may be lacking due to modifiable factors such as patient attitudes and beliefs and logistical barriers in arranging follow‐up.
Patients without potential for timely PCP follow‐up might be kept in the hospital longer to ensure they are well enough medically to sufficiently meet their own follow‐up needs. Hospital LOS might be increased by providers to compensate for the lack of PCP follow‐up. Alternatively, these patients may be sicker with their index hospitalization.
It is not surprising that payer source appears to influence a patients' ability to obtain timely PCP follow‐up. It is well documented that uninsured patients have higher healthcare resource utilization.2224 Lack of access to primary care in such patients contributes to a cycle of using the most expensive sites of care. In our study, we found many of the patients lacking timely PCP follow‐up were younger, perhaps reflecting the same patient population who have higher rates of being uninsured. Conversely, older patients are more likely to have PCP access, in large part due to having Medicare benefits (although this dynamic has shown a shift in recent years). The uninsured may present sicker as a result of lacking pre‐hospital PCP access or transportation to a PCP visit.
Limitations
This study was performed at a single, academic institution limiting its' generalizability. In addition, this small cohort study, which took place over four winter months, may have implicit biases toward certain disease entities and follow‐up issues unique to study size and season. The small study size was dictated by a finite amount of available resources, potentially contributing to minor inconsistencies with some of the results. While statistical significance was still seen with many of our results, a much larger study may better enhance the study outcomes.
It also remains unclear why the effects of PCP follow‐up were evident for a patient's same condition, but not for any condition. The distinction between designations is potentially subjective and may be difficult to accurately determine. Most existing readmission studies in the literature assign readmission for any condition. A future, larger study may be able to examine whether this difference exists between same vs. any condition.
As an academic medical center, access to specialty clinics may be facilitated, thus increasing PCP follow‐up in patients who might otherwise not have it available to them. Additionally, our subjects were limited to a convenience sample of the population of the general medicine wards and may not be representative of all medical inpatients. Patients lacking a telephone were missed. We relied on patient recollection and self‐report of PCP follow‐up visits and re‐hospitalizations. While we acknowledge limitations of patient self‐report, both in communication and comprehension, we believe patients are reasonably able to report on whether or not they were readmitted to the hospital, the cause of their readmission and whether/when they had PCP follow‐up. Patient self‐report could be collected systematically and without long time lags. Finally, the research team did not have reliable access to readmission data for hospitals other than the facility in which the study was conducted.
It is possible patients readmitted early after discharge may have been counted as lacking PCP follow‐up simply because the readmission occurred so soon after discharge precluding the opportunity for PCP follow‐up to occur. The effects of patients having non‐PCP (home health nurse, pharmacist, phone advice) follow‐up after hospital discharge were not examined.
Also, LOS and readmission to a hospital may be more a reflection of disease severity than the absence of PCP follow‐up, ie, patients ultimately readmitted after hospital discharge may have been a sicker subset of patients upon index hospitalization.
In this urban academic medical center, discharged medicine patients commonly lack timely PCP follow‐up. The lack of timely PCP follow‐up after hospital discharge was associated with higher rates of readmission and a non‐significant trend toward longer hospital lengths of stay. Hospital discharge represents a period of significant risk in patient care necessitating the effective continuation of treatment plans including follow‐up of laboratory, radiology or other testing, and management by a variety of providers. PCPs may play a crucial role in care coordination during this period. Structured intervention performed at the time of discharge might increase post‐hospital PCP access and facilitate timely PCP follow‐up to ensure continuity of needed care after hospital discharge in the most vulnerable patients. Such interventions might include systems improvements, such as increasing PCP access in the post‐hospital period, to increase the likelihood that complex needs are met at a vulnerable period in patient care.
A more effective handoff between inpatient and outpatient settings may ultimately improve clinical outcomes, diminish resource utilization, and decrease overall healthcare costs.
Acknowledgements
The authors thank Traci Yamashita and Karen Mellis, Professional Research Assistants.
- , , , .The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;13:161–167.
- , , , .Medical errors related to discontinuity of care from an inpatient to outpatient setting.J Gen Intern Med.200318:646–651.
- , .The high cost users of medical care.N Engl J Med.1980;302:996–1002.
- , .The rate and cost of hospital readmissions for preventable conditions.Med Care Res Rev.2004;61:225–240.
- , , .Rehospitalizations among patients in the medicare fee‐for‐service program.N Engl J Med.2009;360;14:1418–1428.
- , , .Tying up loose ends. Discharging patients with unresolved medical issues.Arch Intern Med.2007;167:1305–1311.
- , .Post‐hospitalization followup appointment‐keeping among the medically indigent.J Community Health.1993;18(5):271–282.
- , , .Factors related to the keeping of appointments by indigent clients.J Health Care Poor Underserved.1993;4(1):21–39.
- , , , , , .Inpatient to outpatient transfer of diabetes care: perceptions of barriers to postdischarge followup in urban African American patients.Ethn Dis.2007;17(2):238–243.
- , , .Effect of a nurse case manager on postdischarge follow‐up.J Gen Intern Med.1996v;11(11):684–688.
- , , .Emergency department visits by persons recently discharges from U.S. hospitals.Natl Health Stat Report.2008;(6):1–9.
- , , .Comparing uninsured and privately insured hospital patients: admission severity, health outcomes and resource use.Health Serv Manage Res.2001;14(3):203–210.
- , , .Comparison of uninsured and privately insured hospital patients. Condition on admission, resource use, and outcome.JAMA.1991;265(3):374–379.
- , , .Effect of a nurse case manager on postdischarge follow‐up.J Gen Intern Med.1996;11:684–688.
- , , , .The care transitions intervention: results of a randomized controlled trial.Arch Intern Med.2006;166(17):1822–1828.
- , , , , , .A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333:1190–1195.
- , , , .Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631.
- , , , et al.A reengineered hospital discharge program to decrease rehospitalization.Ann Intern Med.2009;150:178–187.
- , , .Does increased access to primary care reduce hospital readmissions?N Engl J Med.1996;334:1441–1447.
- , .Health insurance and access to health care in the united states.Ann NY Acad Sci.1008;1136:149–160.
- , .2006.Summary health statistics for U.S. adults: National Health Interview Survey. 2005, NCHS/CDC/USDHHS, Vital Health Statistics, Series 10.
- , , .Emergency department visits by persons recently discharges from U.S. hospitals.Natl Health Stat Report.2008;(6):1–9.
- , , .Comparison of uninsured and privately insured hospital patients. Condition on admission, resource use, and outcome.JAMA.1991;265(3):374–379.
- , , .Comparing uninsured and privately insured hospital patients: admission severity, health outcomes and resource use.Health Serv Manage Res.2001;14(3):203–210.
- , , , .The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;13:161–167.
- , , , .Medical errors related to discontinuity of care from an inpatient to outpatient setting.J Gen Intern Med.200318:646–651.
- , .The high cost users of medical care.N Engl J Med.1980;302:996–1002.
- , .The rate and cost of hospital readmissions for preventable conditions.Med Care Res Rev.2004;61:225–240.
- , , .Rehospitalizations among patients in the medicare fee‐for‐service program.N Engl J Med.2009;360;14:1418–1428.
- , , .Tying up loose ends. Discharging patients with unresolved medical issues.Arch Intern Med.2007;167:1305–1311.
- , .Post‐hospitalization followup appointment‐keeping among the medically indigent.J Community Health.1993;18(5):271–282.
- , , .Factors related to the keeping of appointments by indigent clients.J Health Care Poor Underserved.1993;4(1):21–39.
- , , , , , .Inpatient to outpatient transfer of diabetes care: perceptions of barriers to postdischarge followup in urban African American patients.Ethn Dis.2007;17(2):238–243.
- , , .Effect of a nurse case manager on postdischarge follow‐up.J Gen Intern Med.1996v;11(11):684–688.
- , , .Emergency department visits by persons recently discharges from U.S. hospitals.Natl Health Stat Report.2008;(6):1–9.
- , , .Comparing uninsured and privately insured hospital patients: admission severity, health outcomes and resource use.Health Serv Manage Res.2001;14(3):203–210.
- , , .Comparison of uninsured and privately insured hospital patients. Condition on admission, resource use, and outcome.JAMA.1991;265(3):374–379.
- , , .Effect of a nurse case manager on postdischarge follow‐up.J Gen Intern Med.1996;11:684–688.
- , , , .The care transitions intervention: results of a randomized controlled trial.Arch Intern Med.2006;166(17):1822–1828.
- , , , , , .A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333:1190–1195.
- , , , .Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631.
- , , , et al.A reengineered hospital discharge program to decrease rehospitalization.Ann Intern Med.2009;150:178–187.
- , , .Does increased access to primary care reduce hospital readmissions?N Engl J Med.1996;334:1441–1447.
- , .Health insurance and access to health care in the united states.Ann NY Acad Sci.1008;1136:149–160.
- , .2006.Summary health statistics for U.S. adults: National Health Interview Survey. 2005, NCHS/CDC/USDHHS, Vital Health Statistics, Series 10.
- , , .Emergency department visits by persons recently discharges from U.S. hospitals.Natl Health Stat Report.2008;(6):1–9.
- , , .Comparison of uninsured and privately insured hospital patients. Condition on admission, resource use, and outcome.JAMA.1991;265(3):374–379.
- , , .Comparing uninsured and privately insured hospital patients: admission severity, health outcomes and resource use.Health Serv Manage Res.2001;14(3):203–210.
Copyright © 2010 Society of Hospital Medicine