User login
Pharmacogenomics for the primary care provider: Why should we care?
Since the human genome was sequenced in 2000, the American public has continued to hold hope that our growing understanding of genetics will revolutionize the practice of medicine.
One way genetics promises to improve the quality and value of health care is in personalized medicine, by helping us tailor treatment to a person’s individual genetic makeup. One such approach is called pharmacogenomics.
Pharmacogenomics uses knowledge of a person’s genetics to understand how a particular drug will work, or not work, in his or her body. For instance, some people might carry genes that make them more sensitive than average to a drug, and therefore they would require a lower dose. Others might have genes that make them resistant to the drug, meaning the drug is ineffective unless they receive a higher dose.
Adverse drug reactions are a leading cause of death in hospitalized patients in the United States and are responsible for billions of dollars in health care costs.1,2 Our current practice of prescribing for adult patients is largely trial-and-error, with the same dose given to all patients, in many cases with little regard even to sex, height, or weight.
Pharmacogenomics promises to change this way of prescribing to a customized approach that uses genetic information to predict an individual’s response to medications. It is one piece of an overall initiative to personalize patient care based on the patient’s individual characteristics and preferences.
OVERCOMING BARRIERS TO USING PHARMACOGENOMICS IN PRACTICE
If personalized medicine has promised to improve the quality and value of health care for our patients, why have we been so slow to adopt this information in clinical practice?
The usual barriers to clinical adoption certainly exist. We need further studies to determine whether genetic-based prescribing is truly valid, and for which patient populations. We need to determine whether this approach is cost-effective and better than the current standard of care. We need to work on payment options.
However, one of the largest barriers for busy primary care physicians is the lack of time to keep up with new information. Many practicing physicians were taught little about formal genetics in medical school. The body of scientific literature on pharmacogenomics is fragmented, and it crosses disease states and specialties, making it difficult to unite. Given the breadth of diseases treated and drugs prescribed by primary care physicians, it is unrealistic for most to keep track of the vast body of literature of pharmacogenomic testing and to decipher how to apply this to clinical practice.
In this issue of the Journal, Kitzmiller et al3 provide one solution to this problem, giving an overview of pharmacogenomic applications that might be pertinent to practicing physicians. However, as we try to make pharmacogenomics accessible to busy physicians, we need other solutions to integrate pharmacogenomic information efficiently into the clinical work flow. One approach might be to build pharmacogenomics into the electronic medical record. We can also store the integrated information in research databases and provide clinical recommendations on Internet sites such as www.pharmgkb.org, and we can develop applications to run on cell phones and iPads.
QUESTIONS REMAIN
Kitzmiller et al discuss an important step in this process, highlighting several key questions:
Should we seek genetics-based information to personalize drug selection? Based on the information presented in the literature and in the Kitzmiller paper, there may be circumstances when it is appropriate to consider doing so. While the evidence is not yet compelling to order these tests on a regular basis in clinical practice, this information might be helpful in some situations, such as for patients who have had adverse effects from minimal doses of antidepressants.
For now, clinicians should not abandon their current practice of personalizing patient care on the basis of personal, cultural, and economic preferences. Rather, they should consider pharmacogenomic information an additional piece of information when selecting drug therapy. We should also encourage health care systems and interested providers to be early adopters and to study how their outcomes compare with the standard of care.
Once we have this information, what is our obligation to use it? An increasing number of patients already have genetic information in their health record, either ordered by or provided to their physicians. However, there is little in the scientific literature to guide us in this arena.
Yet most of us would agree that if we have information (genetic or otherwise) that can help to select a drug type or dose or reduce adverse events or costs, we should consider this information in our decision-making. Several circumstances are documented in this paper and in the literature in which prior knowledge about drug metabolism can help in selecting a dose of medication. One example would be the 50% recommended reduction in tricyclic antidepressant dose if the patient is a CYP2D6 poor metabolizer.4
MOVING FORWARD AS A TEAM
In summary, Kitzmiller et al bring to light the promise and the uncertainties that currently exist in the field of pharmacogenomics. While it is unclear if we should incorporate pharmacogenomic tests into standard medical practice at this time, it is clear that this information is becoming more readily available, whether or not we have requested it. Some would argue that, once we have the information, we have an obligation to use it, just as we use other information in our clinical decision-making. This means we need to develop tools and resources to help practitioners evaluate pharmacogenomic data and incorporate it into clinical care in an efficient manner.
The authors also highlight the need for more education about drug metabolism in general, and they cite several instances in which knowledge of drug interactions and metabolism can clearly influence decision-making. An example is paroxetine (Paxil) inhibition of tamoxifen (Nolvadex).5
Lastly, regardless of our personal feelings about the clinical usefulness of genetic testing in large populations, we need to work together to determine clinical utility and validity and to develop efficient ways to put into practice findings that could affect patient care. As we move forward, we need to work as a team, utilizing our clinical partners—pharmacists, pharmacologists, metabolism and health information technology experts, and medical geneticists. Working as a team, pooling our resources and tools, we move closer to providing world-class personalized health care.
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998; 279:1200–1205.
- Field TS, Gilman BH, Subramanian S, Fuller JC, Bates DW, Gurwitz JH. The costs associated with adverse drug events among older adults in the ambulatory setting. Med Care 2005; 43:1171–1176.
- Kitzmiller JP, Groen DK, Phelps MA, Sadee W. Pharmacogenomic testing: relevance in medical practice. Why drugs work in some patients but not others. Cleve Clin J Med 2011; 78:243–257.
- Thuerauf N, Lunkenheimer J. The impact of the CYP2D6-polymorphism on dose recommendations for current antidepressants. Eur Arch Psychiatry Clin Neurosci 2006; 256:287–293.
- Schwarz EB, McNamara M, Miller RG, Walsh JM. Update in women’s health for the general internist. J Gen Intern Med201; 26:207–213.
Since the human genome was sequenced in 2000, the American public has continued to hold hope that our growing understanding of genetics will revolutionize the practice of medicine.
One way genetics promises to improve the quality and value of health care is in personalized medicine, by helping us tailor treatment to a person’s individual genetic makeup. One such approach is called pharmacogenomics.
Pharmacogenomics uses knowledge of a person’s genetics to understand how a particular drug will work, or not work, in his or her body. For instance, some people might carry genes that make them more sensitive than average to a drug, and therefore they would require a lower dose. Others might have genes that make them resistant to the drug, meaning the drug is ineffective unless they receive a higher dose.
Adverse drug reactions are a leading cause of death in hospitalized patients in the United States and are responsible for billions of dollars in health care costs.1,2 Our current practice of prescribing for adult patients is largely trial-and-error, with the same dose given to all patients, in many cases with little regard even to sex, height, or weight.
Pharmacogenomics promises to change this way of prescribing to a customized approach that uses genetic information to predict an individual’s response to medications. It is one piece of an overall initiative to personalize patient care based on the patient’s individual characteristics and preferences.
OVERCOMING BARRIERS TO USING PHARMACOGENOMICS IN PRACTICE
If personalized medicine has promised to improve the quality and value of health care for our patients, why have we been so slow to adopt this information in clinical practice?
The usual barriers to clinical adoption certainly exist. We need further studies to determine whether genetic-based prescribing is truly valid, and for which patient populations. We need to determine whether this approach is cost-effective and better than the current standard of care. We need to work on payment options.
However, one of the largest barriers for busy primary care physicians is the lack of time to keep up with new information. Many practicing physicians were taught little about formal genetics in medical school. The body of scientific literature on pharmacogenomics is fragmented, and it crosses disease states and specialties, making it difficult to unite. Given the breadth of diseases treated and drugs prescribed by primary care physicians, it is unrealistic for most to keep track of the vast body of literature of pharmacogenomic testing and to decipher how to apply this to clinical practice.
In this issue of the Journal, Kitzmiller et al3 provide one solution to this problem, giving an overview of pharmacogenomic applications that might be pertinent to practicing physicians. However, as we try to make pharmacogenomics accessible to busy physicians, we need other solutions to integrate pharmacogenomic information efficiently into the clinical work flow. One approach might be to build pharmacogenomics into the electronic medical record. We can also store the integrated information in research databases and provide clinical recommendations on Internet sites such as www.pharmgkb.org, and we can develop applications to run on cell phones and iPads.
QUESTIONS REMAIN
Kitzmiller et al discuss an important step in this process, highlighting several key questions:
Should we seek genetics-based information to personalize drug selection? Based on the information presented in the literature and in the Kitzmiller paper, there may be circumstances when it is appropriate to consider doing so. While the evidence is not yet compelling to order these tests on a regular basis in clinical practice, this information might be helpful in some situations, such as for patients who have had adverse effects from minimal doses of antidepressants.
For now, clinicians should not abandon their current practice of personalizing patient care on the basis of personal, cultural, and economic preferences. Rather, they should consider pharmacogenomic information an additional piece of information when selecting drug therapy. We should also encourage health care systems and interested providers to be early adopters and to study how their outcomes compare with the standard of care.
Once we have this information, what is our obligation to use it? An increasing number of patients already have genetic information in their health record, either ordered by or provided to their physicians. However, there is little in the scientific literature to guide us in this arena.
Yet most of us would agree that if we have information (genetic or otherwise) that can help to select a drug type or dose or reduce adverse events or costs, we should consider this information in our decision-making. Several circumstances are documented in this paper and in the literature in which prior knowledge about drug metabolism can help in selecting a dose of medication. One example would be the 50% recommended reduction in tricyclic antidepressant dose if the patient is a CYP2D6 poor metabolizer.4
MOVING FORWARD AS A TEAM
In summary, Kitzmiller et al bring to light the promise and the uncertainties that currently exist in the field of pharmacogenomics. While it is unclear if we should incorporate pharmacogenomic tests into standard medical practice at this time, it is clear that this information is becoming more readily available, whether or not we have requested it. Some would argue that, once we have the information, we have an obligation to use it, just as we use other information in our clinical decision-making. This means we need to develop tools and resources to help practitioners evaluate pharmacogenomic data and incorporate it into clinical care in an efficient manner.
The authors also highlight the need for more education about drug metabolism in general, and they cite several instances in which knowledge of drug interactions and metabolism can clearly influence decision-making. An example is paroxetine (Paxil) inhibition of tamoxifen (Nolvadex).5
Lastly, regardless of our personal feelings about the clinical usefulness of genetic testing in large populations, we need to work together to determine clinical utility and validity and to develop efficient ways to put into practice findings that could affect patient care. As we move forward, we need to work as a team, utilizing our clinical partners—pharmacists, pharmacologists, metabolism and health information technology experts, and medical geneticists. Working as a team, pooling our resources and tools, we move closer to providing world-class personalized health care.
Since the human genome was sequenced in 2000, the American public has continued to hold hope that our growing understanding of genetics will revolutionize the practice of medicine.
One way genetics promises to improve the quality and value of health care is in personalized medicine, by helping us tailor treatment to a person’s individual genetic makeup. One such approach is called pharmacogenomics.
Pharmacogenomics uses knowledge of a person’s genetics to understand how a particular drug will work, or not work, in his or her body. For instance, some people might carry genes that make them more sensitive than average to a drug, and therefore they would require a lower dose. Others might have genes that make them resistant to the drug, meaning the drug is ineffective unless they receive a higher dose.
Adverse drug reactions are a leading cause of death in hospitalized patients in the United States and are responsible for billions of dollars in health care costs.1,2 Our current practice of prescribing for adult patients is largely trial-and-error, with the same dose given to all patients, in many cases with little regard even to sex, height, or weight.
Pharmacogenomics promises to change this way of prescribing to a customized approach that uses genetic information to predict an individual’s response to medications. It is one piece of an overall initiative to personalize patient care based on the patient’s individual characteristics and preferences.
OVERCOMING BARRIERS TO USING PHARMACOGENOMICS IN PRACTICE
If personalized medicine has promised to improve the quality and value of health care for our patients, why have we been so slow to adopt this information in clinical practice?
The usual barriers to clinical adoption certainly exist. We need further studies to determine whether genetic-based prescribing is truly valid, and for which patient populations. We need to determine whether this approach is cost-effective and better than the current standard of care. We need to work on payment options.
However, one of the largest barriers for busy primary care physicians is the lack of time to keep up with new information. Many practicing physicians were taught little about formal genetics in medical school. The body of scientific literature on pharmacogenomics is fragmented, and it crosses disease states and specialties, making it difficult to unite. Given the breadth of diseases treated and drugs prescribed by primary care physicians, it is unrealistic for most to keep track of the vast body of literature of pharmacogenomic testing and to decipher how to apply this to clinical practice.
In this issue of the Journal, Kitzmiller et al3 provide one solution to this problem, giving an overview of pharmacogenomic applications that might be pertinent to practicing physicians. However, as we try to make pharmacogenomics accessible to busy physicians, we need other solutions to integrate pharmacogenomic information efficiently into the clinical work flow. One approach might be to build pharmacogenomics into the electronic medical record. We can also store the integrated information in research databases and provide clinical recommendations on Internet sites such as www.pharmgkb.org, and we can develop applications to run on cell phones and iPads.
QUESTIONS REMAIN
Kitzmiller et al discuss an important step in this process, highlighting several key questions:
Should we seek genetics-based information to personalize drug selection? Based on the information presented in the literature and in the Kitzmiller paper, there may be circumstances when it is appropriate to consider doing so. While the evidence is not yet compelling to order these tests on a regular basis in clinical practice, this information might be helpful in some situations, such as for patients who have had adverse effects from minimal doses of antidepressants.
For now, clinicians should not abandon their current practice of personalizing patient care on the basis of personal, cultural, and economic preferences. Rather, they should consider pharmacogenomic information an additional piece of information when selecting drug therapy. We should also encourage health care systems and interested providers to be early adopters and to study how their outcomes compare with the standard of care.
Once we have this information, what is our obligation to use it? An increasing number of patients already have genetic information in their health record, either ordered by or provided to their physicians. However, there is little in the scientific literature to guide us in this arena.
Yet most of us would agree that if we have information (genetic or otherwise) that can help to select a drug type or dose or reduce adverse events or costs, we should consider this information in our decision-making. Several circumstances are documented in this paper and in the literature in which prior knowledge about drug metabolism can help in selecting a dose of medication. One example would be the 50% recommended reduction in tricyclic antidepressant dose if the patient is a CYP2D6 poor metabolizer.4
MOVING FORWARD AS A TEAM
In summary, Kitzmiller et al bring to light the promise and the uncertainties that currently exist in the field of pharmacogenomics. While it is unclear if we should incorporate pharmacogenomic tests into standard medical practice at this time, it is clear that this information is becoming more readily available, whether or not we have requested it. Some would argue that, once we have the information, we have an obligation to use it, just as we use other information in our clinical decision-making. This means we need to develop tools and resources to help practitioners evaluate pharmacogenomic data and incorporate it into clinical care in an efficient manner.
The authors also highlight the need for more education about drug metabolism in general, and they cite several instances in which knowledge of drug interactions and metabolism can clearly influence decision-making. An example is paroxetine (Paxil) inhibition of tamoxifen (Nolvadex).5
Lastly, regardless of our personal feelings about the clinical usefulness of genetic testing in large populations, we need to work together to determine clinical utility and validity and to develop efficient ways to put into practice findings that could affect patient care. As we move forward, we need to work as a team, utilizing our clinical partners—pharmacists, pharmacologists, metabolism and health information technology experts, and medical geneticists. Working as a team, pooling our resources and tools, we move closer to providing world-class personalized health care.
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998; 279:1200–1205.
- Field TS, Gilman BH, Subramanian S, Fuller JC, Bates DW, Gurwitz JH. The costs associated with adverse drug events among older adults in the ambulatory setting. Med Care 2005; 43:1171–1176.
- Kitzmiller JP, Groen DK, Phelps MA, Sadee W. Pharmacogenomic testing: relevance in medical practice. Why drugs work in some patients but not others. Cleve Clin J Med 2011; 78:243–257.
- Thuerauf N, Lunkenheimer J. The impact of the CYP2D6-polymorphism on dose recommendations for current antidepressants. Eur Arch Psychiatry Clin Neurosci 2006; 256:287–293.
- Schwarz EB, McNamara M, Miller RG, Walsh JM. Update in women’s health for the general internist. J Gen Intern Med201; 26:207–213.
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998; 279:1200–1205.
- Field TS, Gilman BH, Subramanian S, Fuller JC, Bates DW, Gurwitz JH. The costs associated with adverse drug events among older adults in the ambulatory setting. Med Care 2005; 43:1171–1176.
- Kitzmiller JP, Groen DK, Phelps MA, Sadee W. Pharmacogenomic testing: relevance in medical practice. Why drugs work in some patients but not others. Cleve Clin J Med 2011; 78:243–257.
- Thuerauf N, Lunkenheimer J. The impact of the CYP2D6-polymorphism on dose recommendations for current antidepressants. Eur Arch Psychiatry Clin Neurosci 2006; 256:287–293.
- Schwarz EB, McNamara M, Miller RG, Walsh JM. Update in women’s health for the general internist. J Gen Intern Med201; 26:207–213.
Pharmacogenomic testing: Relevance in medical practice
In many patients, certain drugs do not work as well as expected, whereas in other patients they cause toxic effects, even at lower doses. For some patients, the reason may be genetic.
Sizeable minorities of the population carry genetic variants—polymorphisms— that affect their response to various drugs. Thanks to genetic research, our understanding of the variability of drug response has advanced markedly in the last decade. Many relevant polymorphisms have been identified, and tests for some of them are available.
Armed with the knowledge of their patients’ genetic status, physicians could predict their response to certain drugs, leading to better efficacy, fewer adverse drug reactions, and a better cost-benefit ratio.
The possible impact is substantial, since more than half of the drugs most commonly involved in adverse drug reactions are metabolized by polymorphic enzymes.1 Adverse drug reactions remain a significant detriment to public health, having a substantial impact on rates of morbidity and death and on healthcare costs.2–8 In the United States, adverse drug reactions are a leading cause of death in hospitalized patients4 and are annually responsible for hundreds of thousands of deaths and hundreds of billions of dollars in added costs.2,4,6–8
In the meantime, physicians can educate their patients and promote efforts to incorporate genomic information into standard clinical decision-making.
This article offers an overview of pharmacogenomic testing, discussing implications and limitations of a few validated tests. Specifically, we will discuss testing that is relevant when using warfarin (Coumadin), clopidogrel (Plavix), statins, tamoxifen (Nolvadex), codeine, and psychotropic medications, as well as the future role of pharmacogenomic testing in medicine.
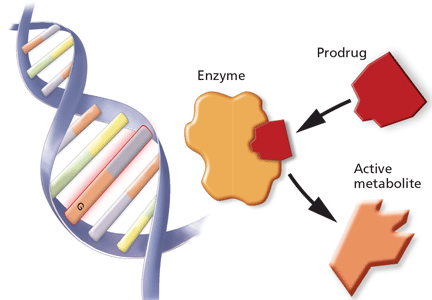
WHAT IS PHARMACOGENOMICS?
Pharmacogenomics is the study of how genetic factors relate to interindividual variability of drug response.
Many clinicians may not be familiar with the background and terminology used in the pharmacogenomic literature. Below, a brief review of the terminology is followed by a schematic describing the various stages of research involved in pharmacogenomics and the advancement of a test into standard practice.
The review and schematic may be helpful for evaluating the clinical significance of pharmacogenomics-related articles.
From genotype to phenotype
Genotype refers to the coding sequence of DNA base pairs for a particular gene, and phenotype (eg, disease or drug response) refers to a trait resulting from the protein product encoded by the gene. The name of a gene often refers to its protein product and is italicized (eg, the CYP3A4 gene encodes for the CYP3A4 enzyme).
Two alleles per autosomal gene (one paternal and one maternal) form the genotype. Heterozygotes possess two different alleles, and homozygotes possess two of the same alleles. The most common allele in a population is referred to as the wild type, and allele frequencies can vary greatly in different populations.9
Most sequence variations are single nucleotide polymorphisms (SNPs, pronounced “snips”), a single DNA base pair substitution that may result in a different gene product. SNPs can be classified as structural RNA polymorphisms (srSNPs), regulatory polymorphisms (rSNPs), or polymorphisms in coding regions (cSNPs)10: srSNPs alter mRNA processing and translation, rSNPs alter transcription, and cSNPs alter protein sequence and function.
Recently, genetic associations with a phenotype have been done on a large scale, with millions of SNPs measured in each of many subjects. This approach, called a genomewide association study or GWAS, has revealed countless candidate genes for clinical traits, but only a few have resulted in a practical clinical application. SNPs may by themselves exert a pharmacokinetic effect (ie, how the body processes the drug), a pharmacodynamic effect (ie, how the drug affects the body), or both, or they may act in concert with other genetic factors. Pharmacodynamic effects can result from a pharmacokinetic effect or can result from variations in a pharmacologic target.
Establishing a genotype-phenotype association can involve clinical studies, animal transgenic studies, or molecular and cellular functional assays.
Clinical applications are emerging
WARFARIN: IMPORTANCE OF CYP2C9, VKORC1
Warfarin is used for the long-term treatment and prevention of thromboembolic events.
This drug has a narrow therapeutic window and shows substantial interpatient dose variability. The start of warfarin therapy is associated with one of the highest rates of adverse events and emergency room visits of any single drug.12 More than 2 million patients start warfarin each year in the United States alone,13 and about 20% of them are hospitalized within the first 6 months because of bleeding due to overanticoagulation.14
The findings from a recent study suggest that pharmacogenomic testing may eventually allow more patients to safely benefit from warfarin therapy. In this large, nationwide, prospective study, hospitalization rates were 30% lower when pharmacogenomic testing was used.14 However, no reduction was seen in the time needed to reach the target international normalized ratio (INR) or in the need for INR checks at 6 months. Furthermore, this study used historical control data, and some or all of the reduction in hospitalization rates may be attributed to more frequent INR checks in the patients who underwent testing than in the historical control group.
A relationship between warfarin dose requirements and the genetic status of CYP2C9, which encodes a major drug-metabolizing enzyme, has been demonstrated in retrospective and prospective studies.15–17
S-warfarin is metabolized by CYP2C9, which is polymorphic
Warfarin contains equal amounts of two isomers, designated S and R. S-warfarin, which is more potent, is metabolized principally by CYP2C9, while R-warfarin is metabolized by CYP1A2, CYP2C19, and CYP3A4.
People who possess two copies of the wild type CYP2C9 gene CYP2C9*1 metabolize warfarin very well and so are called “extensive warfarin metabolizers.” Carriers of the allelic variants CYP2C9*2 and CYP2C9*3 (which have point mutations in exons 3 and 7 of CYP2C9, respectively), have less capacity. Compared with those who are homozygous for the wild-type gene, homozygous carriers of CYP2C9* 3 clear S-warfarin at a rate that is 90% lower, and those with the CYP2C9*1/*3, CYP2C9* 1/*2, CYP2C9*2/*2, or CYP2C9*2/*3 genotypes clear it at a rate 50% to 75% lower. A meta-analysis of 12 studies found that the CYP2C9 genotype accounted for 12% of the interindividual variability of warfarin dose requirements.18
About 8% of whites carry at least one copy of CYP2C9*2, as do 1% of African Americans; the allele is rare in Asian populations. The frequency of CYP2C9*3 is 6% in whites, 1% in African Americans, and 3% in Asians.19,20 People with CYP2C9*4 or CYP2C9*5 have a diminished capacity to clear warfarin; however, these variants occur so infrequently that their clinical relevance may be minimal.
Warfarin’s target, VKOR, is also polymorphic
Genetic variation in warfarin’s pharmacologic target, vitamin K 2,3-epoxide reductase (VKOR), also influences dose requirements. Warfarin decreases the synthesis of vitamin-K-dependent clotting factors by inhibiting VKOR. This inhibition depends on the patient’s C1 subunit gene, VKORC1. Patients with a guanine-to-adenine SNP 1,639 bases upstream of VKORC1 (−1639G>A) need lower warfarin doses—an average of 25% lower in those with the GA genotype (ie, one allele has guanine in the −1639 position and the other allele has adenine in that position) and 50% lower in those with the AA genotype compared with the wild-type genotype GG.21 This promoter SNP, positioned upstream (ie, before the gene-coding region), greatly influences VKORC1 expression.
A meta-analysis of 10 studies found that the VKORC1 polymorphism accounts for 25% of the interindividual variation in warfarin dose.18 In one study, the frequency of the AA genotype in a white population was 14%, AG 47%, and GG 39%; in a Chinese population the frequency of AA was 82%, AG 18%, and GG 0.35%.22
CYP4F2 and GGCX also affect warfarin’s dose requirements
Genetic variations in the enzymes CYP4F2 and gamma-glutamyl carboxylase (GGCX) also influence warfarin dose requirements. Although the data are limited and the effects are smaller than those of CYP2C9 and VKORC1, people with a SNP in CYP4F2 need 8% higher doses of warfarin, while those with a SNP in GGCX need 6% lower doses.23
CYP2C9 and VKORC1 testing is available
Currently, the clinical pharmacogenetic tests relevant for warfarin use are for CYP2C9 and VKORC1.10
The FDA has approved four warfarin pharmacogenetic test kits, but most third-party payers are reluctant to reimburse for testing because it is not currently considered a standard of care. Testing typically costs a few hundred dollars, but it should become less expensive as it becomes more commonplace. The current FDA-approved product label for warfarin does not recommend routine pharmacogenomic testing for determining initial or maintenance doses, but it does acknowledge that dose requirements are influenced by CYP2C9 and VKORC1 and states that genotype information, when available, can assist in selecting the starting dose.24
Clinical trials of warfarin pharmacogenomic testing are under way
Although genetic status can greatly influence an individual patient’s warfarin dosing requirement, routine prospective pharmacogenomic testing is not endorsed by the FDA or by other expert panels26 because there is currently insufficient evidence to recommend for or against it.
Several large prospective trials are under way. For example, the National Heart, Lung, and Blood Institute began a prospective trial in about 1,200 patients to evaluate the use of clinical plus genetic information to guide the initiation of warfarin therapy and to improve anticoagulation control for patients.27 The results, expected in September 2011, and those of other large prospective trials should provide adequate evidence for making recommendations about the clinical utility of routine pharmacogenetic testing for guiding warfarin therapy.
Several recent cost-utility and cost-effectiveness studies have attempted to quantify the value of pharmacogenomic testing for warfarin therapy,28–30 but their analyses are largely limited because the benefit (clinical utility) is yet to be sufficiently characterized.
The relevance of such analyses may soon be drastically diminished, as several non-vitamin-K-dependent blood thinners such as rivaroxaban (Xarelto), dabigatran (Pradaxa), and apixaban are poised to enter clinical practice.31
CLOPIDOGREL IS ACTIVATED BY CYP2C19
Clopidogrel, taken by about 40 million patients worldwide, is used to prevent atherothrombotic events and cardiac stent thrombosis when given along with aspirin.
Studies of clopidogrel pharmacogenomics
A recent genome-wide association study conducted in a cohort of 429 healthy Amish persons revealed a SNP in CYP2C19 to be associated with a diminished response to clopidogrel and to account for 12% of the variation in drug response.33 Traditional factors (the patient’s age, body-mass index, and cholesterol level) combined accounted for less than 10% of the variation.
Findings were similar in a subsequent investigation in 227 cardiac patients receiving clopidogrel: 21% of those with the variant had a cardiovascular ischemic event or died during a 1-year follow-up period compared with 10% of those without the variant (hazard ratio 2.42, P = .02).33
A 12-year prospective study investigating clopidogrel efficacy in 300 cardiac patients under the age of 45 used cardiovascular death, nonfatal myocardial infarction, and urgent coronary revascularization as end points. It concluded that the only independent predictor of these events was the patient’s CYP2C19 status.34
A study in 2,200 patients with recent myocardial infarction examined whether any of the known allelic variations that modulate clopidogrel’s absorption (ABCB1), metabolic activation (CYP3A4/5 and CYP2C19), or biologic activity (P2RY12 and ITGB3) was associated with a higher rate of the combined end point of all-cause mortality, nonfatal myocardial infarction, or stroke. None of the SNPs in CYP3A4/5, P2RY12, or ITGB3 that were evaluated was associated with a higher risk at 1 year. However, the allelic variations modulating clopidogrel’s absorption (ABCB1) and metabolism (CYP2C19) were associated with higher event rates. Patients with two variant ABCB1 alleles had a higher adjusted hazard ratio (95% confidence interval [CI] 1.2–2.47) than those with the wild-type allele. Patients who had one or two CYP2C19 loss-of-function alleles had a higher event rate than those with two wild-type alleles (95% CI 1.10–3.58 and 1.71–7.51, respectively).35
Conversely, researchers from the Population Health Research Institute found no association between poor-metabolizer status and treatment outcomes when CYP2C19 analysis was retrospectively added to the findings of two large clinical trials (combined N > 5,000). However, patients with acute coronary syndrome benefited more from clopidogrel treatment if they were ultra-rapid metabolizers (possessing the gain-of-function allele CYP2C19*17).36
Current status of clopidogrel testing: Uncertain
A current FDA boxed warning states that poor CYP2C19 metabolizers may not benefit from clopidogrel and recommends that prescribers consider alternative treatment for patients in this category.37 However, routine CYP2C19 testing is not recommended, and no firm recommendations have been established regarding dose adjustments for CYP2C19 status.
In 2010, the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the American Heart Association collectively pronounced the current evidence base insufficient for recommending routine pharmacogenomic testing.39
Needed are large-scale studies examining the cost-effectiveness and clinical utility of genotype-guided clopidogrel therapy compared with other therapy options such as prasugrel (Effient), an analogue not metabolized by CYP2C19. One such study, sponsored by Medco Health Solutions, plans to enroll 14,600 cardiac patients and has an estimated completion date in June 2011.40 The expectation that clopidogrel will be available in generic form in 2012 adds to the uncertainty regarding the cost-effectiveness of CYP2C19 testing for clopidogrel therapy.
STATINS: SLC01B1*5 INCREASES MYOPATHY RISK
Statins lower the concentration of low-density lipoprotein cholesterol (LDL-C), resulting in a relative-risk reduction of about 20% for each 1 mmol/L (39 mg/dL) decrement in LDL-C.41 They are one of the most commonly prescribed classes of drugs, but their side effects can limit their appeal: statin-induced myopathy occurs in about 1:1,000 to 1:10,000 patients and is difficult to predict.
SLC01B1. The Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH), a genome-wide association study, recently found a SNP (SLCO1B1* 5) in the SLC01B1 gene to be associated with a higher risk of statin-induced myopathy in cardiac patients receiving simvastatin (Zocor) 40 or 80 mg daily.42 The SLC01B1 gene, located on chromosome 12, influences the extent of the drug’s hepatic uptake and its serum concentration. Only the SLC01B1*5 SNP emerged as a predictor of statin-induced myopathy across the entire genome.42
The authors believe the findings are likely to apply to other statins. The mechanisms leading to statin-induced myopathy and the impact of statin pharmacogenomics are still unclear.43
CYP3A4. Other genetic variants may play a vital role in determining response to statin therapy. Carriers of a newly identified CYP3A4 polymorphism (intron 6 SNP, rs35599367, C>T) required significantly lower statin doses (0.2–0.6 times less) for optimal lipid control. The analyses included atorvastatin (Lipitor), simvastatin, and lovastatin (Mevacor), and the association was robust (P = .019).44
Statin pharmacogenomic testing is not routinely recommended
Routine pharmacogenomic testing for statin therapy is not recommended. Additional studies are needed to determine the clinical utility and cost-effectiveness of pharmacogenomic testing (involving a combination of several polymorphisms) in various patient populations delineated by type of statin, dose, and concomitant use of other drugs.
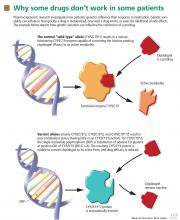
TAMOXIFEN IS ACTIVATED BY CYP2D6
Tamoxifen is prescribed to prevent the recurrence of estrogen-receptor-positive breast cancer, to treat metastatic breast cancer, to prevent cancer in high-risk populations, and to treat ductal carcinoma in situ.
Tamoxifen is metabolized to form endoxifen, which has much higher potency and higher systemic levels than tamoxifen.45 Both CYP2D6 and CYP3A4/5 are required to produce endoxifen via two intermediates, but CYP2D6 catalyzes the critical step leading to metabolic activation.
The CYP2D6 gene is highly polymorphic, with more than 75 allelic variants identified. Extensive literature is available describing the influence of CYP2D6 polymorphisms on tamoxifen metabolism and therapy outcomes.46–52 Several CYP2D6 variants result in reduced or no enzyme activity, and people who have more than two normally functioning alleles have exaggerated enzyme activity (gene amplification).
Classification of CYP2D6 status
Several systems have been developed to categorize the phenotypic activity of CYP2D6 based on genotype.
A genetic basis for the observed diversity in the metabolism of cytochrome P450 substrates was recognized more than 30 years ago. People were categorized as either extensive or poor metabolizers, reflecting normal vs impaired ability to metabolize the CYP2D6 substrates sparteine and debrisoquine. Later work expanded this system to include categories for intermediate (between poor and extensive) and ultra-rapid (better than extensive) metabolizers.
The genetic basis for these categories includes homozygosity for dysfunctional variants (the poor-metabolizer group) or extra copies of normal functioning variants (the ultra-rapid-metabolizer group).
Newer systems have been described for characterizing the CYP2D6 activity phenotype whereby CYP2D6 variants are assigned activity scores.53–56 The various scoring systems have been reviewed by Kirchheiner.57
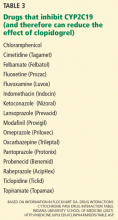
A recent version of the activity scoring system also takes into consideration the many drugs that inhibit CYP2D6, such as amiodarone (Cordarone) and fluoxetine (Prozac) that can reduce the action of tamoxifen if given with it (Table 4).58 For example, the tamoxifen exposure (as predicted by the CYP2D6-activity score) experienced by a CYP2D6 extensive metabolizer taking a CYP2D6-inhibiting drug may be similar to the exposure experienced by a CYP2D6 poor metabolizer receiving the same tamoxifen dose but not taking a CYP2D6-inhibiting drug.
Likewise, the activity score of a CYP2D6 intermediate metabolizer taking a CYP2D6-inducing drug may be similar to that of a CYP2D6 ultra-rapid metabolizer not taking a CYP2D6-inducing drug. Examples of CYP2D6 inducers are dexamethasone, rifampin, and hyperforin (St. John’s wort).
While the newer systems are reported to provide better correlations between genotype and phenotype scores, the older scoring systems and the categorical names are still widely used (eg, in the FDA-approved AmpliChip CYP450 test from Roche,59 which includes genotype data for CYP2D6 and CYP2C19).
No firm recommendations for CYP2D6 testing in tamoxifen users
The different genotypes and phenotypes vary in prevalence in different ethnic groups, and significantly different activity levels for endoxifen formation are observed. Tamoxifen lacks efficacy in those who are poor CYP2D6 metabolizers—ie, about 7% of the white population.
However, the FDA has not made firm recommendations about CYP2D6 testing for prescribing tamoxifen because the evidence of benefit, although suggestive, has been considered insufficient.
Clinicians should be aware that tamoxifen’s efficacy is greatly reduced by concomitant therapy with CYP2D6-inhibiting drugs (Table 4).
Other genes affecting tamoxifen: CYP3A4/5, SULT1A1, and UGT2B15
Some investigators propose that polymorphisms in additional genes encoding enzymes in the tamoxifen metabolic and elimination pathways (eg, CYP3A4/5, SULT1A1, and UGT2B15) also need to be considered to account adequately for interindividual variation in drug response.
For example, CYP3A4 and CYP3A5 are also polymorphic, and large interindividual variation exists in their enzyme activities. These enzymes have overlapping substrate specificities, represent the most abundant drug-metabolizing enzymes in the human liver, and are involved in the biotransformation of a broad range of endogenous substrates and most drugs.60
Clinical studies evaluating the impact of CYP3A4/5 polymorphisms have been inconsistent in their conclusions, which is generally attributed to the relatively low functional impact or the low prevalence of the SNPs evaluated. Many of the nearly 100 CYP3A4/5 polymorphisms identified have not yet been characterized regarding their functional impact on enzyme expression or activity. CYP-3A4/5 enzyme activity is highly variable between individuals and warrants further study of its role in outcomes of tamoxifen therapy. Ongoing and future prospective clinical trials evaluating CYP2D6, CYP3A4/5, and other relevant polymorphisms are necessary to define their clinical relevance before routine genetic testing for tamoxifen can be justified.
CODEINE IS ALSO ACTIVATED BY CYP2D6
Codeine also depends on the CYP2D6 gene, as it must be activated to its more potent opioid metabolites, including morphine. Poor CYP2D6 metabolizers do not benefit from codeine therapy.
The pharmacogenomics of codeine has become a hot topic, especially regarding breast-feeding mothers. The debate was ignited with the publication in 2006 of a case report of an infant’s death, apparently the result of metabolic polymorphisms.61 The evolution of this debate and the outcome of the case may be noteworthy to clinicians, as they illustrate the gravity of public and patient interest in pharmacogenomic testing. In this case, the breast-feeding mother had taken codeine regularly for about 14 days when her 13-day-old infant died from toxic levels of morphine. Unknown to her and the prescriber, both the mother and infant were ultra-rapid CYP2D6 metabolizers, resulting in a more rapid and extensive conversion of codeine to morphine.
A logical strategy for preventing similar deaths would be routine CYP2D6 genotyping when prescribing codeine to breast-feeding mothers. However, after several investigations examined the metabolic and excretion pathways of codeine in their entirety, the FDA did not recommend routine CYP2D6 testing when prescribing codeine to breastfeeding mothers because several other factors, including rare genetic variations of other enzymes, proved necessary for reaching the opioid toxicity leading to the infant’s death.62
PHARMACOGENOMICS OF PSYCHOTROPIC DRUGS
Pharmacogenomic testing has clinical utility for some psychotropic drugs.
HLA-B and carbamazepine
Considered a standard of care, HLA-B genotyping is appropriate before prescribing carbamazepine (Tegretol, Equetro) to patients in populations in which HLAB*1502 is likely to be present, such as Asians. Carriers of HLAB* 1502 are at higher risk of life-threatening skin reactions such as Stevens-Johnson syndrome.11
Several other pharmacogenomic applications for psychotropic medications have been suggested, but routine testing has not been recommended by the FDA or endorsed by any expert panel because sufficient clinical utility and cost-effectiveness have not been demonstrated. A brief summary of study findings and a few practical suggestions follow.
Polymorphisms in metabolizing enzymes have been investigated in patients receiving psychotropic drugs.
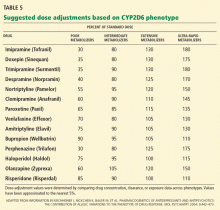
CYP2D6 and antidepressants
Many antidepressants show significant differences in plasma drug levels with CYP2D6 polymorphisms (in descending order of influence)55:
- Imipramine (Tofranil)
- Doxepin (Adapin, Silenor, Sinequan)
- Maprotiline (Deprilept, Ludiomil, Psymion)
- Trimipramine (Surmontil)
- Desipramine (Noraprim)
- Nortriptyline (Aventyl, Pamelor)
- Clomipramine (Anafranil)
- Paroxetine (Paxil)
- Venlafaxine (Effexor)
- Amitriptyline (Elavil)
- Mianserin
- Trazadone (Desyrel)
- Bupropion (Wellbutrin)
- Nefazodone (Serzone)
- Citalopram (Celexa)
- Sertraline (Zoloft).
CYP2D6 and antipsychotics
Several antipsychotics are also influenced by CYP2D6 polymorphisms (also in descending order of influence)55:
- Perphenazine (Trilafon)
- Thioridazine (Mellaril)
- Olanzapine (Zyprexa)
- Zuclopenthixol (Cisordinol, Clopixol, Acuphase)
- Aripiprazole (Abilify)
- Flupentixol (Depixol, Fluanxol)
- Haloperidol (Haldol)
- Perazine (Taxilan)
- Risperidone (Risperdal)
- Pimozide (Orap).
CYP2C19 and antidepressants
CYP2C19 polymorphisms are likewise associated with differences in drug metabolism for many antidepressants, such as (in descending order of CYP2C19-mediated influence)55:
- Trimipramine
- Doxepin
- Amitriptyline
- Imipramine
- Citalopram (Celexa)
- Clomipramine
- Moclobemide (Aurorix, Manerix)
- Sertraline
- Fluvoxamine (Luvox).
Clinical relevance of CYP2D6 and CYP2C19
Several studies have demonstrated that poor and intermediate CYP2D6 metabolizers have a higher incidence of adverse effects when taking CYP2D6-dependent antidepressants63–68; however, an almost equal number of studies did not find statistically significant associations.69–72 Likewise, several studies have found an association between ultra-rapid CYP2D6 metabolizer status and diminished response to antidepressants,65,73,74 but no association was found in a larger retrospective study.75
Routine CYP2D6 and CYP2C19 screening is not recommended when prescribing psychotropic drugs. However, reviews of the pharmacokinetic data have suggested a few practical applications when genetic status is already known. In general, clinicians can consider reducing the dose of tricyclic antidepressants by about 50% when prescribing to CYP2D6-poor-metabolizers.55,76–78
Genes that affect serotonin metabolism
Several genes in the serotonin pathway have been investigated to determine whether they influence patients’ susceptibility to depression and adverse effects and response to psychotropic medications.
SLC6A4. Polymorphisms in the promoter region of the serotonin transporter gene SLC6A4 appear to influence the treatment response and side-effect profiles of selective serotonin reuptake inhibitors (SSRIs). Carriers of the SLC6A4 5-HTTLPR L alleles have fewer side effects79 and better response to SSRI treatment, and carriers of the S allele have a higher incidence of antidepressant-induced mania80 and poorer response to SSRI treatment.81
5-HT. Polymorphisms in serotonin receptors (2A and 2C subtypes) appear to influence SSRI response and side effects. Carriers of 5-HT 2A C alleles had more severe adverse effects from paroxetine,71 but another 5-HT 2A polymorphism common to Asians is associated with better response to antidepressant therapy.82 A 5-HT 2C polymorphism was associated with a lower incidence of antipsychotic-induced weight gain.83
Although the understanding of these relationships is incomplete and routine pharmacogenomic testing is not currently recommended, reviews of the pharmacodynamic data have suggested a few practical applications when a patient’s genetic status is already known. One should consider:
- Selecting treatments other than SSRIs for depressed patients known to possess the SLC6A4 variant
- Selecting citalopram for depressed patients known to carry the 5-HT 2A polymorphism
- Avoiding treatment with antipsychotic drugs for patients known to possess the 5-HT 2C polymorphism.
THE FUTURE OF PHARMACOGENOMIC TESTING
The examples discussed in this article provide some insight about how pharmacogenomic testing is maturing and slowly being integrated into the practice of medicine. They also illustrate the complexity of the multiple stages of research that pharmacogenomic applications must go through in order to be adopted as standard practice.
In the future, pharmacogenomic data will continue to accumulate, and the clinical utility of many other pharmacogenomic tests may be uncovered. The FDA provides information on emerging pharmacogenomic tests at its Web site, www.fda.gov.11 Its up-to-date “Table of Valid Genomic Biomarkers in the Context of Approved Drug Labels” includes boxed warnings, recommendations, research outcomes, and relevant population genetics.
If the FDA continues its current policy, prospective randomized trials that show improvement in patient outcomes will remain the gold standard for determining the clinical significance of a pharmacogenomic test. Furthermore, cost-benefit analyses are likely to continue dictating policy regarding pharmacogenomic testing, and cost-benefit profiles should improve as technology advances and as information gathered from a single test becomes applicable to multiple medications and clinical scenarios.
In the meantime, physicians should become familiar with the terms used in medical genetics and pharmacogenomics and begin to understand genetic contributions to the outcomes of drug therapy. For example, understanding the consequences of metabolizer status and the frequency of variants in a given population can be tremendously helpful when advising our patients about anticipating potential problems when taking specific medications and about making informed decisions about pharmacogenomic testing.
This exchange of information alone may go a long way in improving therapy outcomes even when prospective pharmacogenomic testing is not routinely performed. Furthermore, an increasing number of patients will already have genotyping information available when they come to us, and clinicians need to be aware of the many pharmacogenomic applications recommended by the FDA when genetic status is known.10
- King HC, Sinha AA. Gene expression profile analysis by DNA micro-arrays: promise and pitfalls. JAMA 2001; 286:2280–2288.
- Nuckols TK, Paddock SM, Bower AG, et al. Costs of intravenous adverse drug events in academic and nonacademic intensive care units. Med Care 2008; 46:17–24.
- Gurwitz JH, Field TS, Judge J, et al. The incidence of adverse events in two long-term care facilities. Am J Med 2005; 118:251–258.
- Vargas E, Terleira A, Hernando F, et al. Effects of adverse drug reactions on length of stay in surgical intensive care units. Crit Care Med 2003; 31:694–698.
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998; 279:1200–1205.
- Field TS, Gilman BH, Subramanian S, Fuller JC, Bates DW, Gurwitz JH. The costs associated with adverse drug events in older adults in the ambulatory setting. Med Care 2005; 43:1171–1176.
- Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA 1997; 277:301–306.
- Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc (Wash) 2001; 41:192–199.
- Bradford LD. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics 2002; 3:229–243.
- Sadee W. Measuring cis-acting regulatory variants genome-wide: new insights into expression genetics and disease susceptibility. Genome Med 2009; 1:116.
- US Food and Drug Administration. Table of pharmacogenomic biomarkers in drug labels. http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm. Accessed 1/18/2011.
- Budnitz DS, Shehab N, Kegler SR, Richards CL. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med 2007; 147:755–765.
- Elias DJ, Topol EJ. Warfarin pharmacogenomics: a big step forward for individualized medicine: enlightened dosing of warfarin. Eur J Hum Genet 2008; 16:532–534.
- Epstein RS, Moyer TP, Aubert RE, et al. Warfarin genotyping reduces hospitalization rates results from the MM-WES (Medco-Mayo Warfarin Effectiveness study). J Am Coll Cardiol 2010; 55:2804–2812.
- Rettie AE, Wienkers LC, Gonzalez FJ, Trager WF, Korzekwa KR. Impaired (S)-warfarin metabolism catalysed by the R144C allelic variant of CYP2C9. Pharmacogenetics 1994; 4:39–42.
- Higashi MK, Veenstra DL, Kondo LM, et al. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA 2002; 287:1690–1698.
- Sanderson S, Emery J, Higgins J. CYP2C9 gene variants, drug dose, and bleeding risk in warfarin-treated patients: a HuGEnet systematic review and meta-analysis. Genet Med 2005; 7:97–104.
- Au N, Rettie AE. Pharmacogenomics of 4-hydroxycoumarin anticoagulants. Drug Metab Rev 2008; 40:355–375.
- García-Martín E, Martínez C, Ladero JM, Agúndez JA. Interethnic and intraethnic variability of CYP2C8 and CYP2C9 polymorphisms in healthy individuals. Mol Diagn Ther 2006; 10:29–40.
- Sullivan-Klose TH, Ghanayem BI, Bell DA, et al. The role of the CYP2C9-Leu359 allelic variant in the tolbutamide polymorphism. Pharmacogenetics 1996; 6:341–349.
- Wen MS, Lee M, Chen JJ, et al. Prospective study of warfarin dosage requirements based on CYP2C9 and VKORC1 genotypes. Clin Pharmacol Ther 2008; 84:83–89.
- Larramendy-Gozalo C, Yang JQ, Verstuyft C, et al. Genetic polymorphism of vitamin K epoxide reductase (VKORC1) 1173C>T in a Chinese and a Caucasian population. Basic Clin Pharmacol Toxicol 2006; 98:611–613.
- Caldwell MD, Awad T, Johnson JA, et al. CYP4F2 genetic variant alters required warfarin dose. Blood 2008; 111:4106–4112.
- Bristol-Myers Squibb. Coumadin (warfarin sodium) Prescribing Information. January 2010.
- Barnes-Jewish Hospital at Washington University Medical Center. Warfarin dosing. http://warfarindosing.orgAccessed 1/20/2011.
- Flockhart DA, O’Kane D, Williams MS, et al. Pharmacogenetic testing of CYP2C9 and VKORC1 alleles for warfarin. Genet Med 2008; 10:139–150.
- National Institutes of Health. ClinicalTrials.gov. http://clinicaltrials.gov. Accessed January 20, 2011.
- Eckman MH, Rosand J, Greenberg SM, Gage BF. Cost-effectiveness of using pharmacogenetic information in warfarin dosing for patients with nonvalvular atrial fibrillation. Ann Intern Med 2009; 150:73–83.
- Meckley LM, Gudgeon JM, Anderson JL, Williams MS, Veenstra DL. A policy model to evaluate the benefits, risks, and costs of warfarin pharmacogenomic testing. Pharmacoeconomics 2010; 28:61–74.
- Patrick AR, Avron J, Choudhry NK. Cost-effectiveness of genotype-guided warfarin dosing for patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes 2009; 2:429–436.
- Haas S. New oral Xa and IIa inhibitors: updates on clinical trial results. J Thromb Thrombolysis 2008; 25:52–60.
- Bhatt DL. Tailoring antiplatelet therapy based on pharmacogenomics: how well do the data fit? JAMA 2009; 302:896–897.
- Shuldiner AR, O’Connell JR, Bliden KP, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 2009; 302:849–857.
- Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 2009; 373:309–317.
- Simon T, Verstuyft C, Mary-Krause M, et al; French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 2009; 360:363–375.
- Paré G, Mehta SR, Yusuf S, et al. Effects of CYP2C19 genotype on outcomes of clopidogrel treatment. N Engl J Med 2010; 363:1704–1714.
- Bristol-Myers Squibb/Sanofi Pharmaceutical Partnership. Plavix (clopidogrel bisulfate) prescribing information. August 2010.
- P450 Drug Interaction Table. Indiana University School of Medicine. http://medicine.iupui.edu/clinpharm/ddis/table.asp. Accessed 1/21/2011.
- Society for Cardiovascular Angiography and Interventions; Holmes DR, Dehmer GJ, Kaul S, Leifer D, O’Gara PT, Stein CM. ACCF/AHA clopidogrel clinical alert: approaches to the FDA “boxed warning”: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the American Heart Association. Circulation 2010; 122:537–557.
- National Institutes of Health. Genotype Guided Comparison of Clopidogrel and Prasugrel Outcomes Study. http://clinicaltrialsfeeds.org/clinical-trials/show/NCT00995514. Accessed 1/20/2011.
- Amarenco P, Labreuche J. Lipid management in the prevention of stroke: Review and updated meta-analysis of statins for stroke prevention. Lancet Neurol 2009; 8:453–463.
- SEARCH Collaborative Group, Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Romaine SP, Bailey KM, Hall AS, Balmforth AJ. The influence of SLC01B1 (OATP1B1) gene polymorphisms on response to statin therapy. Pharmacogenomics J 2010; 10:1–11.
- Wang D, Guo Y, Wrighton SA, Cooke GE, Sadee W. Intronic polymorphism in CYP3A4 affects hepatic expression and response to statin drugs. Pharmacogenomics J 2010; Apr 13 [Epub ahead of print].
- Goetz MP, Rae JM, Suman VJ, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol 2005; 23:9312–9318.
- Beverage JN, Sissung TM, Sion AM, Danesi R, Figg WD. CYP2D6 polymorphisms and the impact on tamoxifen therapy. J Pharm Sci 2007; 96:2224–2231.
- Flockhart D. CYP2D6 genotyping and the pharmacogenetics of tamoxifen. Clin Adv Hematol Oncol 2008; 6:493–494.
- Goetz MP, Kamal A, Ames MM. Tamoxifen pharmacogenomics: the role of CYP2D6 as a predictor of drug response. Clin Pharmacol Ther 2008; 83:160–166.
- Stearns V, Rae JM. Pharmacogenetics and breast cancer endocrine therapy: CYP2D6 as a predictive factor for tamoxifen metabolism and drug response? Expert Rev Mol Med 2008; 10:e34.
- Dezentjé VO, Guchelaar HJ, Nortier JW, van del Velde CJ, Gelderblom H. Clinical implications of CYP2D6 genotyping in tamoxifen treatment for breast cancer. Clin Cancer Res 2009; 15:15–21.
- Higgins MJ, Rae JM, Flockhart DA, Hayes DF, Stearns V. Pharmacogenetics of tamoxifen: who should undergo CYP2D6 genetic testing? J Natl Compr Canc Netw 2009; 7:203–213.
- Hoskins JM, Carey LA, McLeod HL. CYP2D6 and tamoxifen: DNA matters in breast cancer. Nat Rev Cancer 2009; 9:576–586.
- Steimer W, Zöpf K, von Amelunxen S, et al. Allele-specific change of concentration and functional gene dose for the prediction of steady-state serum concentrations of amitriptyline and nortriptyline in CYP2C19 and CYP2D6 extensive and intermediate metabolizers. Clinical Cancer 2004; 50:1623–1633.
- Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharm Ther 2008; 83:234–242.
- Kirchheiner J, Nickchen K, Bauer M, et al. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry 2004; 9:442–473.
- Kirchheiner J, Schmidt H, Tzvetkov M, et al. Pharmacokinetics of codeine and its metabolite morphine in ultra-rapid metabolizers due to CYP2D6 duplication. Pharmacogenomics J 2007; 7:257–265.
- Kirchheiner J. CYP2D6 phenotype prediction from genotype: which system is the best? Clin Pharmacol Ther 2008; 83:225–227.
- Borges S, Desta Z, Jin Y, et al. Composite functional genetic and comedication CYP2D6 activity score in predicting tamoxifen drug exposure among breast cancer patients. J Clin Pharmacol 2010; 50:450–458.
- Hoffmann-La Roche Ltd. AmpliChip CYP450 Test. http://www.roche.com/assays/Pages/AmpliChipCYP450Test.aspx. Accessed 1/21/2011.
- Anzenbacher P, Anzenbacherová E. Cytochromes P450 and metabolism of xenobiotics. Cell Mol Life Sci 2001; 58:737–747.
- Koren G, Cairns J, Chitayat D, Gaedigk A, Leeder SJ. Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother. Lancet 2006; 368:704.
- Willmann S, Edginton AN, Coboeken K, Ahr G, Lippert J. Risk to the breast-fed neonate from codeine treatment to the mother: a quantitative mechanistic modeling study. Clin Pharm Ther 2009; 86:634–643.
- Grzesiak M, Beszłej A, Lebioda A, Jonkisz A, Dobosz T, Kienja A. [Retrospective assessment of the antidepressants tolerance in the group of patients with diagnosis of depression and different CYP2D6 genotype.] [In Polish] Psychiatr Pol 2003; 37:433–444.
- Laika B, Leucht S, Heres S, Steimer W. Intermediate metabolizer: increased side effects in psychoactive drug therapy. The key to cost-effectiveness of pretreatment CYP2D6 screening? Pharmacogenomics J 2009; 9:395–403.
- Rau T, Wohlleben G, Wuttke H, et al. CYP2D6 genotype: Impact on adverse effects and nonresponse during treatment with antidepressants—a pilot study. Clin Pharm Ther 2004; 75:386–393.
- McAlpine DE, O’Kane DJ, Black JL, Mrazek DA. Cytochrome P450 2D6 genotype variation and venlafaxine dosage. Mayo Clin Proc 2007; 82:1065–1068.
- Chen S, Chou WH, Blouin RA, et al. The cytochrome P450 2D6 (CYP2D6) enzyme polymorphism: screening costs and influence on clinical outcomes in psychiatry. Clin Pharmacol Ther 1996; 60:522–534.
- Shams ME, Arneth B, Hiemke C, et al. CYP2D6 polymorphism and clinical effect of the antidepressant venlafaxine. J Clin Pharm Ther 2006; 31:493–502.
- Whyte EM, Romkes M, Mulsant BH, et al. CYP2D6 genotype and venlafaxine-XR concentrations in depressed elderly. Int J Geriatr Psychiatry 2006; 21:542–549.
- Roberts RL, Mulder RT, Joyce PR, Luty SE, Kennedy MA. No evidence of increased adverse drug reactions in cytochrome P450 CYP2D6 poor metabolizers treated with fluoxetine or nortriptyline. Hum Psychopharmacol 2004; 19:17–23.
- Murphy GM, Kremer C, Rodrigues HE, Schatzberg AF. Pharmacogenetics of antidepressant medication intolerance. Am J Psychiatry 2003; 160:1830–1835.
- Gillman PK. Re: no evidence of increased adverse drug reactions in cytochrome P450 CYP2D6 poor metabolizers treated with fluoxetine or nortriptyline. Hum Psychopharmacol 2005; 20:61–62.
- Gex-Fabry M, Eap CB, Oneda B, et al. CYP2D6 and ABCB1 genetic variability: influence on paroxetine plasma level and therapeutic response. Ther Drug Monit 2008; 30:474–482.
- Kawanishi C, Lundgren S, Agren H, Bertilsson L. Increased incidence of CYP2D6 gene duplication in patients with persistent mood disorders: ultrarapid metabolism of antidepressants as a cause of nonresponse. A pilot study. Eur J Clin Pharmacol 2004; 59:803–807.
- Serretti A, Calati R, Massat I, et al. Cytochrome P450 CYP1A2, CYP2C9, CYP2C19 and CYP2D6 genes are not associated with response and remission in a sample of depressive patients. Int Clin Psychopharmacol 2009; 24:250–256.
- de Leon J, Armstrong SC, Cozza KL. Clinical guidelines for psychiatrists for the use of pharmacogenetic testing for CYP450 2D6 and CYP450 2C19. Psychosomatics 2006; 47:75–85.
- de Leon J, Susce MT, Johnson M, et al. DNA microarray technology in the clinical environment: the AmpliChip CYP450 test for CYP2D6 and CYP2C19 genotyping. CNS Spectr 2009; 14:19–34.
- Thuerauf N, Lunkenheimer J. The impact of the CYP2D6-polymorphism on dose recommendations for current antidepressants. Eur Arch Psychiatry Clin Neurosci 2006; 256:287–293.
- Horstmann S, Binder EB. Pharmacogenomics of antidepressant drugs. Pharmacol Ther 2009; 124:57–73.
- Ferreira Ade A, Neves FS, da Rocha FF, et al. The role of 5-HTTLPR polymorphism in antidepressant-associated mania in bipolar disorder. J Affect Disord 2009; 112:267–272.
- Serretti A, Kato M, De Ronchi D, Kinoshita T. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry 2007; 12:247–257.
- Kato M, Serretti A. Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder. Mol Psychiatry 2010; 15:473–500.
- Reynolds GP, Zhang Z, Zhang X. Polymorphism of the promoter region of the serotonin 5-HT(2C) receptor gene and clozapine-induced weight gain. Am J Psychiatry 2003; 160:677–679.
In many patients, certain drugs do not work as well as expected, whereas in other patients they cause toxic effects, even at lower doses. For some patients, the reason may be genetic.
Sizeable minorities of the population carry genetic variants—polymorphisms— that affect their response to various drugs. Thanks to genetic research, our understanding of the variability of drug response has advanced markedly in the last decade. Many relevant polymorphisms have been identified, and tests for some of them are available.
Armed with the knowledge of their patients’ genetic status, physicians could predict their response to certain drugs, leading to better efficacy, fewer adverse drug reactions, and a better cost-benefit ratio.
The possible impact is substantial, since more than half of the drugs most commonly involved in adverse drug reactions are metabolized by polymorphic enzymes.1 Adverse drug reactions remain a significant detriment to public health, having a substantial impact on rates of morbidity and death and on healthcare costs.2–8 In the United States, adverse drug reactions are a leading cause of death in hospitalized patients4 and are annually responsible for hundreds of thousands of deaths and hundreds of billions of dollars in added costs.2,4,6–8
In the meantime, physicians can educate their patients and promote efforts to incorporate genomic information into standard clinical decision-making.
This article offers an overview of pharmacogenomic testing, discussing implications and limitations of a few validated tests. Specifically, we will discuss testing that is relevant when using warfarin (Coumadin), clopidogrel (Plavix), statins, tamoxifen (Nolvadex), codeine, and psychotropic medications, as well as the future role of pharmacogenomic testing in medicine.
WHAT IS PHARMACOGENOMICS?
Pharmacogenomics is the study of how genetic factors relate to interindividual variability of drug response.
Many clinicians may not be familiar with the background and terminology used in the pharmacogenomic literature. Below, a brief review of the terminology is followed by a schematic describing the various stages of research involved in pharmacogenomics and the advancement of a test into standard practice.
The review and schematic may be helpful for evaluating the clinical significance of pharmacogenomics-related articles.
From genotype to phenotype
Genotype refers to the coding sequence of DNA base pairs for a particular gene, and phenotype (eg, disease or drug response) refers to a trait resulting from the protein product encoded by the gene. The name of a gene often refers to its protein product and is italicized (eg, the CYP3A4 gene encodes for the CYP3A4 enzyme).
Two alleles per autosomal gene (one paternal and one maternal) form the genotype. Heterozygotes possess two different alleles, and homozygotes possess two of the same alleles. The most common allele in a population is referred to as the wild type, and allele frequencies can vary greatly in different populations.9
Most sequence variations are single nucleotide polymorphisms (SNPs, pronounced “snips”), a single DNA base pair substitution that may result in a different gene product. SNPs can be classified as structural RNA polymorphisms (srSNPs), regulatory polymorphisms (rSNPs), or polymorphisms in coding regions (cSNPs)10: srSNPs alter mRNA processing and translation, rSNPs alter transcription, and cSNPs alter protein sequence and function.
Recently, genetic associations with a phenotype have been done on a large scale, with millions of SNPs measured in each of many subjects. This approach, called a genomewide association study or GWAS, has revealed countless candidate genes for clinical traits, but only a few have resulted in a practical clinical application. SNPs may by themselves exert a pharmacokinetic effect (ie, how the body processes the drug), a pharmacodynamic effect (ie, how the drug affects the body), or both, or they may act in concert with other genetic factors. Pharmacodynamic effects can result from a pharmacokinetic effect or can result from variations in a pharmacologic target.
Establishing a genotype-phenotype association can involve clinical studies, animal transgenic studies, or molecular and cellular functional assays.
Clinical applications are emerging
WARFARIN: IMPORTANCE OF CYP2C9, VKORC1
Warfarin is used for the long-term treatment and prevention of thromboembolic events.
This drug has a narrow therapeutic window and shows substantial interpatient dose variability. The start of warfarin therapy is associated with one of the highest rates of adverse events and emergency room visits of any single drug.12 More than 2 million patients start warfarin each year in the United States alone,13 and about 20% of them are hospitalized within the first 6 months because of bleeding due to overanticoagulation.14
The findings from a recent study suggest that pharmacogenomic testing may eventually allow more patients to safely benefit from warfarin therapy. In this large, nationwide, prospective study, hospitalization rates were 30% lower when pharmacogenomic testing was used.14 However, no reduction was seen in the time needed to reach the target international normalized ratio (INR) or in the need for INR checks at 6 months. Furthermore, this study used historical control data, and some or all of the reduction in hospitalization rates may be attributed to more frequent INR checks in the patients who underwent testing than in the historical control group.
A relationship between warfarin dose requirements and the genetic status of CYP2C9, which encodes a major drug-metabolizing enzyme, has been demonstrated in retrospective and prospective studies.15–17
S-warfarin is metabolized by CYP2C9, which is polymorphic
Warfarin contains equal amounts of two isomers, designated S and R. S-warfarin, which is more potent, is metabolized principally by CYP2C9, while R-warfarin is metabolized by CYP1A2, CYP2C19, and CYP3A4.
People who possess two copies of the wild type CYP2C9 gene CYP2C9*1 metabolize warfarin very well and so are called “extensive warfarin metabolizers.” Carriers of the allelic variants CYP2C9*2 and CYP2C9*3 (which have point mutations in exons 3 and 7 of CYP2C9, respectively), have less capacity. Compared with those who are homozygous for the wild-type gene, homozygous carriers of CYP2C9* 3 clear S-warfarin at a rate that is 90% lower, and those with the CYP2C9*1/*3, CYP2C9* 1/*2, CYP2C9*2/*2, or CYP2C9*2/*3 genotypes clear it at a rate 50% to 75% lower. A meta-analysis of 12 studies found that the CYP2C9 genotype accounted for 12% of the interindividual variability of warfarin dose requirements.18
About 8% of whites carry at least one copy of CYP2C9*2, as do 1% of African Americans; the allele is rare in Asian populations. The frequency of CYP2C9*3 is 6% in whites, 1% in African Americans, and 3% in Asians.19,20 People with CYP2C9*4 or CYP2C9*5 have a diminished capacity to clear warfarin; however, these variants occur so infrequently that their clinical relevance may be minimal.
Warfarin’s target, VKOR, is also polymorphic
Genetic variation in warfarin’s pharmacologic target, vitamin K 2,3-epoxide reductase (VKOR), also influences dose requirements. Warfarin decreases the synthesis of vitamin-K-dependent clotting factors by inhibiting VKOR. This inhibition depends on the patient’s C1 subunit gene, VKORC1. Patients with a guanine-to-adenine SNP 1,639 bases upstream of VKORC1 (−1639G>A) need lower warfarin doses—an average of 25% lower in those with the GA genotype (ie, one allele has guanine in the −1639 position and the other allele has adenine in that position) and 50% lower in those with the AA genotype compared with the wild-type genotype GG.21 This promoter SNP, positioned upstream (ie, before the gene-coding region), greatly influences VKORC1 expression.
A meta-analysis of 10 studies found that the VKORC1 polymorphism accounts for 25% of the interindividual variation in warfarin dose.18 In one study, the frequency of the AA genotype in a white population was 14%, AG 47%, and GG 39%; in a Chinese population the frequency of AA was 82%, AG 18%, and GG 0.35%.22
CYP4F2 and GGCX also affect warfarin’s dose requirements
Genetic variations in the enzymes CYP4F2 and gamma-glutamyl carboxylase (GGCX) also influence warfarin dose requirements. Although the data are limited and the effects are smaller than those of CYP2C9 and VKORC1, people with a SNP in CYP4F2 need 8% higher doses of warfarin, while those with a SNP in GGCX need 6% lower doses.23
CYP2C9 and VKORC1 testing is available
Currently, the clinical pharmacogenetic tests relevant for warfarin use are for CYP2C9 and VKORC1.10
The FDA has approved four warfarin pharmacogenetic test kits, but most third-party payers are reluctant to reimburse for testing because it is not currently considered a standard of care. Testing typically costs a few hundred dollars, but it should become less expensive as it becomes more commonplace. The current FDA-approved product label for warfarin does not recommend routine pharmacogenomic testing for determining initial or maintenance doses, but it does acknowledge that dose requirements are influenced by CYP2C9 and VKORC1 and states that genotype information, when available, can assist in selecting the starting dose.24
Clinical trials of warfarin pharmacogenomic testing are under way
Although genetic status can greatly influence an individual patient’s warfarin dosing requirement, routine prospective pharmacogenomic testing is not endorsed by the FDA or by other expert panels26 because there is currently insufficient evidence to recommend for or against it.
Several large prospective trials are under way. For example, the National Heart, Lung, and Blood Institute began a prospective trial in about 1,200 patients to evaluate the use of clinical plus genetic information to guide the initiation of warfarin therapy and to improve anticoagulation control for patients.27 The results, expected in September 2011, and those of other large prospective trials should provide adequate evidence for making recommendations about the clinical utility of routine pharmacogenetic testing for guiding warfarin therapy.
Several recent cost-utility and cost-effectiveness studies have attempted to quantify the value of pharmacogenomic testing for warfarin therapy,28–30 but their analyses are largely limited because the benefit (clinical utility) is yet to be sufficiently characterized.
The relevance of such analyses may soon be drastically diminished, as several non-vitamin-K-dependent blood thinners such as rivaroxaban (Xarelto), dabigatran (Pradaxa), and apixaban are poised to enter clinical practice.31
CLOPIDOGREL IS ACTIVATED BY CYP2C19
Clopidogrel, taken by about 40 million patients worldwide, is used to prevent atherothrombotic events and cardiac stent thrombosis when given along with aspirin.
Studies of clopidogrel pharmacogenomics
A recent genome-wide association study conducted in a cohort of 429 healthy Amish persons revealed a SNP in CYP2C19 to be associated with a diminished response to clopidogrel and to account for 12% of the variation in drug response.33 Traditional factors (the patient’s age, body-mass index, and cholesterol level) combined accounted for less than 10% of the variation.
Findings were similar in a subsequent investigation in 227 cardiac patients receiving clopidogrel: 21% of those with the variant had a cardiovascular ischemic event or died during a 1-year follow-up period compared with 10% of those without the variant (hazard ratio 2.42, P = .02).33
A 12-year prospective study investigating clopidogrel efficacy in 300 cardiac patients under the age of 45 used cardiovascular death, nonfatal myocardial infarction, and urgent coronary revascularization as end points. It concluded that the only independent predictor of these events was the patient’s CYP2C19 status.34
A study in 2,200 patients with recent myocardial infarction examined whether any of the known allelic variations that modulate clopidogrel’s absorption (ABCB1), metabolic activation (CYP3A4/5 and CYP2C19), or biologic activity (P2RY12 and ITGB3) was associated with a higher rate of the combined end point of all-cause mortality, nonfatal myocardial infarction, or stroke. None of the SNPs in CYP3A4/5, P2RY12, or ITGB3 that were evaluated was associated with a higher risk at 1 year. However, the allelic variations modulating clopidogrel’s absorption (ABCB1) and metabolism (CYP2C19) were associated with higher event rates. Patients with two variant ABCB1 alleles had a higher adjusted hazard ratio (95% confidence interval [CI] 1.2–2.47) than those with the wild-type allele. Patients who had one or two CYP2C19 loss-of-function alleles had a higher event rate than those with two wild-type alleles (95% CI 1.10–3.58 and 1.71–7.51, respectively).35
Conversely, researchers from the Population Health Research Institute found no association between poor-metabolizer status and treatment outcomes when CYP2C19 analysis was retrospectively added to the findings of two large clinical trials (combined N > 5,000). However, patients with acute coronary syndrome benefited more from clopidogrel treatment if they were ultra-rapid metabolizers (possessing the gain-of-function allele CYP2C19*17).36
Current status of clopidogrel testing: Uncertain
A current FDA boxed warning states that poor CYP2C19 metabolizers may not benefit from clopidogrel and recommends that prescribers consider alternative treatment for patients in this category.37 However, routine CYP2C19 testing is not recommended, and no firm recommendations have been established regarding dose adjustments for CYP2C19 status.
In 2010, the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the American Heart Association collectively pronounced the current evidence base insufficient for recommending routine pharmacogenomic testing.39
Needed are large-scale studies examining the cost-effectiveness and clinical utility of genotype-guided clopidogrel therapy compared with other therapy options such as prasugrel (Effient), an analogue not metabolized by CYP2C19. One such study, sponsored by Medco Health Solutions, plans to enroll 14,600 cardiac patients and has an estimated completion date in June 2011.40 The expectation that clopidogrel will be available in generic form in 2012 adds to the uncertainty regarding the cost-effectiveness of CYP2C19 testing for clopidogrel therapy.
STATINS: SLC01B1*5 INCREASES MYOPATHY RISK
Statins lower the concentration of low-density lipoprotein cholesterol (LDL-C), resulting in a relative-risk reduction of about 20% for each 1 mmol/L (39 mg/dL) decrement in LDL-C.41 They are one of the most commonly prescribed classes of drugs, but their side effects can limit their appeal: statin-induced myopathy occurs in about 1:1,000 to 1:10,000 patients and is difficult to predict.
SLC01B1. The Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH), a genome-wide association study, recently found a SNP (SLCO1B1* 5) in the SLC01B1 gene to be associated with a higher risk of statin-induced myopathy in cardiac patients receiving simvastatin (Zocor) 40 or 80 mg daily.42 The SLC01B1 gene, located on chromosome 12, influences the extent of the drug’s hepatic uptake and its serum concentration. Only the SLC01B1*5 SNP emerged as a predictor of statin-induced myopathy across the entire genome.42
The authors believe the findings are likely to apply to other statins. The mechanisms leading to statin-induced myopathy and the impact of statin pharmacogenomics are still unclear.43
CYP3A4. Other genetic variants may play a vital role in determining response to statin therapy. Carriers of a newly identified CYP3A4 polymorphism (intron 6 SNP, rs35599367, C>T) required significantly lower statin doses (0.2–0.6 times less) for optimal lipid control. The analyses included atorvastatin (Lipitor), simvastatin, and lovastatin (Mevacor), and the association was robust (P = .019).44
Statin pharmacogenomic testing is not routinely recommended
Routine pharmacogenomic testing for statin therapy is not recommended. Additional studies are needed to determine the clinical utility and cost-effectiveness of pharmacogenomic testing (involving a combination of several polymorphisms) in various patient populations delineated by type of statin, dose, and concomitant use of other drugs.
TAMOXIFEN IS ACTIVATED BY CYP2D6
Tamoxifen is prescribed to prevent the recurrence of estrogen-receptor-positive breast cancer, to treat metastatic breast cancer, to prevent cancer in high-risk populations, and to treat ductal carcinoma in situ.
Tamoxifen is metabolized to form endoxifen, which has much higher potency and higher systemic levels than tamoxifen.45 Both CYP2D6 and CYP3A4/5 are required to produce endoxifen via two intermediates, but CYP2D6 catalyzes the critical step leading to metabolic activation.
The CYP2D6 gene is highly polymorphic, with more than 75 allelic variants identified. Extensive literature is available describing the influence of CYP2D6 polymorphisms on tamoxifen metabolism and therapy outcomes.46–52 Several CYP2D6 variants result in reduced or no enzyme activity, and people who have more than two normally functioning alleles have exaggerated enzyme activity (gene amplification).
Classification of CYP2D6 status
Several systems have been developed to categorize the phenotypic activity of CYP2D6 based on genotype.
A genetic basis for the observed diversity in the metabolism of cytochrome P450 substrates was recognized more than 30 years ago. People were categorized as either extensive or poor metabolizers, reflecting normal vs impaired ability to metabolize the CYP2D6 substrates sparteine and debrisoquine. Later work expanded this system to include categories for intermediate (between poor and extensive) and ultra-rapid (better than extensive) metabolizers.
The genetic basis for these categories includes homozygosity for dysfunctional variants (the poor-metabolizer group) or extra copies of normal functioning variants (the ultra-rapid-metabolizer group).
Newer systems have been described for characterizing the CYP2D6 activity phenotype whereby CYP2D6 variants are assigned activity scores.53–56 The various scoring systems have been reviewed by Kirchheiner.57
A recent version of the activity scoring system also takes into consideration the many drugs that inhibit CYP2D6, such as amiodarone (Cordarone) and fluoxetine (Prozac) that can reduce the action of tamoxifen if given with it (Table 4).58 For example, the tamoxifen exposure (as predicted by the CYP2D6-activity score) experienced by a CYP2D6 extensive metabolizer taking a CYP2D6-inhibiting drug may be similar to the exposure experienced by a CYP2D6 poor metabolizer receiving the same tamoxifen dose but not taking a CYP2D6-inhibiting drug.
Likewise, the activity score of a CYP2D6 intermediate metabolizer taking a CYP2D6-inducing drug may be similar to that of a CYP2D6 ultra-rapid metabolizer not taking a CYP2D6-inducing drug. Examples of CYP2D6 inducers are dexamethasone, rifampin, and hyperforin (St. John’s wort).
While the newer systems are reported to provide better correlations between genotype and phenotype scores, the older scoring systems and the categorical names are still widely used (eg, in the FDA-approved AmpliChip CYP450 test from Roche,59 which includes genotype data for CYP2D6 and CYP2C19).
No firm recommendations for CYP2D6 testing in tamoxifen users
The different genotypes and phenotypes vary in prevalence in different ethnic groups, and significantly different activity levels for endoxifen formation are observed. Tamoxifen lacks efficacy in those who are poor CYP2D6 metabolizers—ie, about 7% of the white population.
However, the FDA has not made firm recommendations about CYP2D6 testing for prescribing tamoxifen because the evidence of benefit, although suggestive, has been considered insufficient.
Clinicians should be aware that tamoxifen’s efficacy is greatly reduced by concomitant therapy with CYP2D6-inhibiting drugs (Table 4).
Other genes affecting tamoxifen: CYP3A4/5, SULT1A1, and UGT2B15
Some investigators propose that polymorphisms in additional genes encoding enzymes in the tamoxifen metabolic and elimination pathways (eg, CYP3A4/5, SULT1A1, and UGT2B15) also need to be considered to account adequately for interindividual variation in drug response.
For example, CYP3A4 and CYP3A5 are also polymorphic, and large interindividual variation exists in their enzyme activities. These enzymes have overlapping substrate specificities, represent the most abundant drug-metabolizing enzymes in the human liver, and are involved in the biotransformation of a broad range of endogenous substrates and most drugs.60
Clinical studies evaluating the impact of CYP3A4/5 polymorphisms have been inconsistent in their conclusions, which is generally attributed to the relatively low functional impact or the low prevalence of the SNPs evaluated. Many of the nearly 100 CYP3A4/5 polymorphisms identified have not yet been characterized regarding their functional impact on enzyme expression or activity. CYP-3A4/5 enzyme activity is highly variable between individuals and warrants further study of its role in outcomes of tamoxifen therapy. Ongoing and future prospective clinical trials evaluating CYP2D6, CYP3A4/5, and other relevant polymorphisms are necessary to define their clinical relevance before routine genetic testing for tamoxifen can be justified.
CODEINE IS ALSO ACTIVATED BY CYP2D6
Codeine also depends on the CYP2D6 gene, as it must be activated to its more potent opioid metabolites, including morphine. Poor CYP2D6 metabolizers do not benefit from codeine therapy.
The pharmacogenomics of codeine has become a hot topic, especially regarding breast-feeding mothers. The debate was ignited with the publication in 2006 of a case report of an infant’s death, apparently the result of metabolic polymorphisms.61 The evolution of this debate and the outcome of the case may be noteworthy to clinicians, as they illustrate the gravity of public and patient interest in pharmacogenomic testing. In this case, the breast-feeding mother had taken codeine regularly for about 14 days when her 13-day-old infant died from toxic levels of morphine. Unknown to her and the prescriber, both the mother and infant were ultra-rapid CYP2D6 metabolizers, resulting in a more rapid and extensive conversion of codeine to morphine.
A logical strategy for preventing similar deaths would be routine CYP2D6 genotyping when prescribing codeine to breast-feeding mothers. However, after several investigations examined the metabolic and excretion pathways of codeine in their entirety, the FDA did not recommend routine CYP2D6 testing when prescribing codeine to breastfeeding mothers because several other factors, including rare genetic variations of other enzymes, proved necessary for reaching the opioid toxicity leading to the infant’s death.62
PHARMACOGENOMICS OF PSYCHOTROPIC DRUGS
Pharmacogenomic testing has clinical utility for some psychotropic drugs.
HLA-B and carbamazepine
Considered a standard of care, HLA-B genotyping is appropriate before prescribing carbamazepine (Tegretol, Equetro) to patients in populations in which HLAB*1502 is likely to be present, such as Asians. Carriers of HLAB* 1502 are at higher risk of life-threatening skin reactions such as Stevens-Johnson syndrome.11
Several other pharmacogenomic applications for psychotropic medications have been suggested, but routine testing has not been recommended by the FDA or endorsed by any expert panel because sufficient clinical utility and cost-effectiveness have not been demonstrated. A brief summary of study findings and a few practical suggestions follow.
Polymorphisms in metabolizing enzymes have been investigated in patients receiving psychotropic drugs.
CYP2D6 and antidepressants
Many antidepressants show significant differences in plasma drug levels with CYP2D6 polymorphisms (in descending order of influence)55:
- Imipramine (Tofranil)
- Doxepin (Adapin, Silenor, Sinequan)
- Maprotiline (Deprilept, Ludiomil, Psymion)
- Trimipramine (Surmontil)
- Desipramine (Noraprim)
- Nortriptyline (Aventyl, Pamelor)
- Clomipramine (Anafranil)
- Paroxetine (Paxil)
- Venlafaxine (Effexor)
- Amitriptyline (Elavil)
- Mianserin
- Trazadone (Desyrel)
- Bupropion (Wellbutrin)
- Nefazodone (Serzone)
- Citalopram (Celexa)
- Sertraline (Zoloft).
CYP2D6 and antipsychotics
Several antipsychotics are also influenced by CYP2D6 polymorphisms (also in descending order of influence)55:
- Perphenazine (Trilafon)
- Thioridazine (Mellaril)
- Olanzapine (Zyprexa)
- Zuclopenthixol (Cisordinol, Clopixol, Acuphase)
- Aripiprazole (Abilify)
- Flupentixol (Depixol, Fluanxol)
- Haloperidol (Haldol)
- Perazine (Taxilan)
- Risperidone (Risperdal)
- Pimozide (Orap).
CYP2C19 and antidepressants
CYP2C19 polymorphisms are likewise associated with differences in drug metabolism for many antidepressants, such as (in descending order of CYP2C19-mediated influence)55:
- Trimipramine
- Doxepin
- Amitriptyline
- Imipramine
- Citalopram (Celexa)
- Clomipramine
- Moclobemide (Aurorix, Manerix)
- Sertraline
- Fluvoxamine (Luvox).
Clinical relevance of CYP2D6 and CYP2C19
Several studies have demonstrated that poor and intermediate CYP2D6 metabolizers have a higher incidence of adverse effects when taking CYP2D6-dependent antidepressants63–68; however, an almost equal number of studies did not find statistically significant associations.69–72 Likewise, several studies have found an association between ultra-rapid CYP2D6 metabolizer status and diminished response to antidepressants,65,73,74 but no association was found in a larger retrospective study.75
Routine CYP2D6 and CYP2C19 screening is not recommended when prescribing psychotropic drugs. However, reviews of the pharmacokinetic data have suggested a few practical applications when genetic status is already known. In general, clinicians can consider reducing the dose of tricyclic antidepressants by about 50% when prescribing to CYP2D6-poor-metabolizers.55,76–78
Genes that affect serotonin metabolism
Several genes in the serotonin pathway have been investigated to determine whether they influence patients’ susceptibility to depression and adverse effects and response to psychotropic medications.
SLC6A4. Polymorphisms in the promoter region of the serotonin transporter gene SLC6A4 appear to influence the treatment response and side-effect profiles of selective serotonin reuptake inhibitors (SSRIs). Carriers of the SLC6A4 5-HTTLPR L alleles have fewer side effects79 and better response to SSRI treatment, and carriers of the S allele have a higher incidence of antidepressant-induced mania80 and poorer response to SSRI treatment.81
5-HT. Polymorphisms in serotonin receptors (2A and 2C subtypes) appear to influence SSRI response and side effects. Carriers of 5-HT 2A C alleles had more severe adverse effects from paroxetine,71 but another 5-HT 2A polymorphism common to Asians is associated with better response to antidepressant therapy.82 A 5-HT 2C polymorphism was associated with a lower incidence of antipsychotic-induced weight gain.83
Although the understanding of these relationships is incomplete and routine pharmacogenomic testing is not currently recommended, reviews of the pharmacodynamic data have suggested a few practical applications when a patient’s genetic status is already known. One should consider:
- Selecting treatments other than SSRIs for depressed patients known to possess the SLC6A4 variant
- Selecting citalopram for depressed patients known to carry the 5-HT 2A polymorphism
- Avoiding treatment with antipsychotic drugs for patients known to possess the 5-HT 2C polymorphism.
THE FUTURE OF PHARMACOGENOMIC TESTING
The examples discussed in this article provide some insight about how pharmacogenomic testing is maturing and slowly being integrated into the practice of medicine. They also illustrate the complexity of the multiple stages of research that pharmacogenomic applications must go through in order to be adopted as standard practice.
In the future, pharmacogenomic data will continue to accumulate, and the clinical utility of many other pharmacogenomic tests may be uncovered. The FDA provides information on emerging pharmacogenomic tests at its Web site, www.fda.gov.11 Its up-to-date “Table of Valid Genomic Biomarkers in the Context of Approved Drug Labels” includes boxed warnings, recommendations, research outcomes, and relevant population genetics.
If the FDA continues its current policy, prospective randomized trials that show improvement in patient outcomes will remain the gold standard for determining the clinical significance of a pharmacogenomic test. Furthermore, cost-benefit analyses are likely to continue dictating policy regarding pharmacogenomic testing, and cost-benefit profiles should improve as technology advances and as information gathered from a single test becomes applicable to multiple medications and clinical scenarios.
In the meantime, physicians should become familiar with the terms used in medical genetics and pharmacogenomics and begin to understand genetic contributions to the outcomes of drug therapy. For example, understanding the consequences of metabolizer status and the frequency of variants in a given population can be tremendously helpful when advising our patients about anticipating potential problems when taking specific medications and about making informed decisions about pharmacogenomic testing.
This exchange of information alone may go a long way in improving therapy outcomes even when prospective pharmacogenomic testing is not routinely performed. Furthermore, an increasing number of patients will already have genotyping information available when they come to us, and clinicians need to be aware of the many pharmacogenomic applications recommended by the FDA when genetic status is known.10
In many patients, certain drugs do not work as well as expected, whereas in other patients they cause toxic effects, even at lower doses. For some patients, the reason may be genetic.
Sizeable minorities of the population carry genetic variants—polymorphisms— that affect their response to various drugs. Thanks to genetic research, our understanding of the variability of drug response has advanced markedly in the last decade. Many relevant polymorphisms have been identified, and tests for some of them are available.
Armed with the knowledge of their patients’ genetic status, physicians could predict their response to certain drugs, leading to better efficacy, fewer adverse drug reactions, and a better cost-benefit ratio.
The possible impact is substantial, since more than half of the drugs most commonly involved in adverse drug reactions are metabolized by polymorphic enzymes.1 Adverse drug reactions remain a significant detriment to public health, having a substantial impact on rates of morbidity and death and on healthcare costs.2–8 In the United States, adverse drug reactions are a leading cause of death in hospitalized patients4 and are annually responsible for hundreds of thousands of deaths and hundreds of billions of dollars in added costs.2,4,6–8
In the meantime, physicians can educate their patients and promote efforts to incorporate genomic information into standard clinical decision-making.
This article offers an overview of pharmacogenomic testing, discussing implications and limitations of a few validated tests. Specifically, we will discuss testing that is relevant when using warfarin (Coumadin), clopidogrel (Plavix), statins, tamoxifen (Nolvadex), codeine, and psychotropic medications, as well as the future role of pharmacogenomic testing in medicine.
WHAT IS PHARMACOGENOMICS?
Pharmacogenomics is the study of how genetic factors relate to interindividual variability of drug response.
Many clinicians may not be familiar with the background and terminology used in the pharmacogenomic literature. Below, a brief review of the terminology is followed by a schematic describing the various stages of research involved in pharmacogenomics and the advancement of a test into standard practice.
The review and schematic may be helpful for evaluating the clinical significance of pharmacogenomics-related articles.
From genotype to phenotype
Genotype refers to the coding sequence of DNA base pairs for a particular gene, and phenotype (eg, disease or drug response) refers to a trait resulting from the protein product encoded by the gene. The name of a gene often refers to its protein product and is italicized (eg, the CYP3A4 gene encodes for the CYP3A4 enzyme).
Two alleles per autosomal gene (one paternal and one maternal) form the genotype. Heterozygotes possess two different alleles, and homozygotes possess two of the same alleles. The most common allele in a population is referred to as the wild type, and allele frequencies can vary greatly in different populations.9
Most sequence variations are single nucleotide polymorphisms (SNPs, pronounced “snips”), a single DNA base pair substitution that may result in a different gene product. SNPs can be classified as structural RNA polymorphisms (srSNPs), regulatory polymorphisms (rSNPs), or polymorphisms in coding regions (cSNPs)10: srSNPs alter mRNA processing and translation, rSNPs alter transcription, and cSNPs alter protein sequence and function.
Recently, genetic associations with a phenotype have been done on a large scale, with millions of SNPs measured in each of many subjects. This approach, called a genomewide association study or GWAS, has revealed countless candidate genes for clinical traits, but only a few have resulted in a practical clinical application. SNPs may by themselves exert a pharmacokinetic effect (ie, how the body processes the drug), a pharmacodynamic effect (ie, how the drug affects the body), or both, or they may act in concert with other genetic factors. Pharmacodynamic effects can result from a pharmacokinetic effect or can result from variations in a pharmacologic target.
Establishing a genotype-phenotype association can involve clinical studies, animal transgenic studies, or molecular and cellular functional assays.
Clinical applications are emerging
WARFARIN: IMPORTANCE OF CYP2C9, VKORC1
Warfarin is used for the long-term treatment and prevention of thromboembolic events.
This drug has a narrow therapeutic window and shows substantial interpatient dose variability. The start of warfarin therapy is associated with one of the highest rates of adverse events and emergency room visits of any single drug.12 More than 2 million patients start warfarin each year in the United States alone,13 and about 20% of them are hospitalized within the first 6 months because of bleeding due to overanticoagulation.14
The findings from a recent study suggest that pharmacogenomic testing may eventually allow more patients to safely benefit from warfarin therapy. In this large, nationwide, prospective study, hospitalization rates were 30% lower when pharmacogenomic testing was used.14 However, no reduction was seen in the time needed to reach the target international normalized ratio (INR) or in the need for INR checks at 6 months. Furthermore, this study used historical control data, and some or all of the reduction in hospitalization rates may be attributed to more frequent INR checks in the patients who underwent testing than in the historical control group.
A relationship between warfarin dose requirements and the genetic status of CYP2C9, which encodes a major drug-metabolizing enzyme, has been demonstrated in retrospective and prospective studies.15–17
S-warfarin is metabolized by CYP2C9, which is polymorphic
Warfarin contains equal amounts of two isomers, designated S and R. S-warfarin, which is more potent, is metabolized principally by CYP2C9, while R-warfarin is metabolized by CYP1A2, CYP2C19, and CYP3A4.
People who possess two copies of the wild type CYP2C9 gene CYP2C9*1 metabolize warfarin very well and so are called “extensive warfarin metabolizers.” Carriers of the allelic variants CYP2C9*2 and CYP2C9*3 (which have point mutations in exons 3 and 7 of CYP2C9, respectively), have less capacity. Compared with those who are homozygous for the wild-type gene, homozygous carriers of CYP2C9* 3 clear S-warfarin at a rate that is 90% lower, and those with the CYP2C9*1/*3, CYP2C9* 1/*2, CYP2C9*2/*2, or CYP2C9*2/*3 genotypes clear it at a rate 50% to 75% lower. A meta-analysis of 12 studies found that the CYP2C9 genotype accounted for 12% of the interindividual variability of warfarin dose requirements.18
About 8% of whites carry at least one copy of CYP2C9*2, as do 1% of African Americans; the allele is rare in Asian populations. The frequency of CYP2C9*3 is 6% in whites, 1% in African Americans, and 3% in Asians.19,20 People with CYP2C9*4 or CYP2C9*5 have a diminished capacity to clear warfarin; however, these variants occur so infrequently that their clinical relevance may be minimal.
Warfarin’s target, VKOR, is also polymorphic
Genetic variation in warfarin’s pharmacologic target, vitamin K 2,3-epoxide reductase (VKOR), also influences dose requirements. Warfarin decreases the synthesis of vitamin-K-dependent clotting factors by inhibiting VKOR. This inhibition depends on the patient’s C1 subunit gene, VKORC1. Patients with a guanine-to-adenine SNP 1,639 bases upstream of VKORC1 (−1639G>A) need lower warfarin doses—an average of 25% lower in those with the GA genotype (ie, one allele has guanine in the −1639 position and the other allele has adenine in that position) and 50% lower in those with the AA genotype compared with the wild-type genotype GG.21 This promoter SNP, positioned upstream (ie, before the gene-coding region), greatly influences VKORC1 expression.
A meta-analysis of 10 studies found that the VKORC1 polymorphism accounts for 25% of the interindividual variation in warfarin dose.18 In one study, the frequency of the AA genotype in a white population was 14%, AG 47%, and GG 39%; in a Chinese population the frequency of AA was 82%, AG 18%, and GG 0.35%.22
CYP4F2 and GGCX also affect warfarin’s dose requirements
Genetic variations in the enzymes CYP4F2 and gamma-glutamyl carboxylase (GGCX) also influence warfarin dose requirements. Although the data are limited and the effects are smaller than those of CYP2C9 and VKORC1, people with a SNP in CYP4F2 need 8% higher doses of warfarin, while those with a SNP in GGCX need 6% lower doses.23
CYP2C9 and VKORC1 testing is available
Currently, the clinical pharmacogenetic tests relevant for warfarin use are for CYP2C9 and VKORC1.10
The FDA has approved four warfarin pharmacogenetic test kits, but most third-party payers are reluctant to reimburse for testing because it is not currently considered a standard of care. Testing typically costs a few hundred dollars, but it should become less expensive as it becomes more commonplace. The current FDA-approved product label for warfarin does not recommend routine pharmacogenomic testing for determining initial or maintenance doses, but it does acknowledge that dose requirements are influenced by CYP2C9 and VKORC1 and states that genotype information, when available, can assist in selecting the starting dose.24
Clinical trials of warfarin pharmacogenomic testing are under way
Although genetic status can greatly influence an individual patient’s warfarin dosing requirement, routine prospective pharmacogenomic testing is not endorsed by the FDA or by other expert panels26 because there is currently insufficient evidence to recommend for or against it.
Several large prospective trials are under way. For example, the National Heart, Lung, and Blood Institute began a prospective trial in about 1,200 patients to evaluate the use of clinical plus genetic information to guide the initiation of warfarin therapy and to improve anticoagulation control for patients.27 The results, expected in September 2011, and those of other large prospective trials should provide adequate evidence for making recommendations about the clinical utility of routine pharmacogenetic testing for guiding warfarin therapy.
Several recent cost-utility and cost-effectiveness studies have attempted to quantify the value of pharmacogenomic testing for warfarin therapy,28–30 but their analyses are largely limited because the benefit (clinical utility) is yet to be sufficiently characterized.
The relevance of such analyses may soon be drastically diminished, as several non-vitamin-K-dependent blood thinners such as rivaroxaban (Xarelto), dabigatran (Pradaxa), and apixaban are poised to enter clinical practice.31
CLOPIDOGREL IS ACTIVATED BY CYP2C19
Clopidogrel, taken by about 40 million patients worldwide, is used to prevent atherothrombotic events and cardiac stent thrombosis when given along with aspirin.
Studies of clopidogrel pharmacogenomics
A recent genome-wide association study conducted in a cohort of 429 healthy Amish persons revealed a SNP in CYP2C19 to be associated with a diminished response to clopidogrel and to account for 12% of the variation in drug response.33 Traditional factors (the patient’s age, body-mass index, and cholesterol level) combined accounted for less than 10% of the variation.
Findings were similar in a subsequent investigation in 227 cardiac patients receiving clopidogrel: 21% of those with the variant had a cardiovascular ischemic event or died during a 1-year follow-up period compared with 10% of those without the variant (hazard ratio 2.42, P = .02).33
A 12-year prospective study investigating clopidogrel efficacy in 300 cardiac patients under the age of 45 used cardiovascular death, nonfatal myocardial infarction, and urgent coronary revascularization as end points. It concluded that the only independent predictor of these events was the patient’s CYP2C19 status.34
A study in 2,200 patients with recent myocardial infarction examined whether any of the known allelic variations that modulate clopidogrel’s absorption (ABCB1), metabolic activation (CYP3A4/5 and CYP2C19), or biologic activity (P2RY12 and ITGB3) was associated with a higher rate of the combined end point of all-cause mortality, nonfatal myocardial infarction, or stroke. None of the SNPs in CYP3A4/5, P2RY12, or ITGB3 that were evaluated was associated with a higher risk at 1 year. However, the allelic variations modulating clopidogrel’s absorption (ABCB1) and metabolism (CYP2C19) were associated with higher event rates. Patients with two variant ABCB1 alleles had a higher adjusted hazard ratio (95% confidence interval [CI] 1.2–2.47) than those with the wild-type allele. Patients who had one or two CYP2C19 loss-of-function alleles had a higher event rate than those with two wild-type alleles (95% CI 1.10–3.58 and 1.71–7.51, respectively).35
Conversely, researchers from the Population Health Research Institute found no association between poor-metabolizer status and treatment outcomes when CYP2C19 analysis was retrospectively added to the findings of two large clinical trials (combined N > 5,000). However, patients with acute coronary syndrome benefited more from clopidogrel treatment if they were ultra-rapid metabolizers (possessing the gain-of-function allele CYP2C19*17).36
Current status of clopidogrel testing: Uncertain
A current FDA boxed warning states that poor CYP2C19 metabolizers may not benefit from clopidogrel and recommends that prescribers consider alternative treatment for patients in this category.37 However, routine CYP2C19 testing is not recommended, and no firm recommendations have been established regarding dose adjustments for CYP2C19 status.
In 2010, the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the American Heart Association collectively pronounced the current evidence base insufficient for recommending routine pharmacogenomic testing.39
Needed are large-scale studies examining the cost-effectiveness and clinical utility of genotype-guided clopidogrel therapy compared with other therapy options such as prasugrel (Effient), an analogue not metabolized by CYP2C19. One such study, sponsored by Medco Health Solutions, plans to enroll 14,600 cardiac patients and has an estimated completion date in June 2011.40 The expectation that clopidogrel will be available in generic form in 2012 adds to the uncertainty regarding the cost-effectiveness of CYP2C19 testing for clopidogrel therapy.
STATINS: SLC01B1*5 INCREASES MYOPATHY RISK
Statins lower the concentration of low-density lipoprotein cholesterol (LDL-C), resulting in a relative-risk reduction of about 20% for each 1 mmol/L (39 mg/dL) decrement in LDL-C.41 They are one of the most commonly prescribed classes of drugs, but their side effects can limit their appeal: statin-induced myopathy occurs in about 1:1,000 to 1:10,000 patients and is difficult to predict.
SLC01B1. The Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH), a genome-wide association study, recently found a SNP (SLCO1B1* 5) in the SLC01B1 gene to be associated with a higher risk of statin-induced myopathy in cardiac patients receiving simvastatin (Zocor) 40 or 80 mg daily.42 The SLC01B1 gene, located on chromosome 12, influences the extent of the drug’s hepatic uptake and its serum concentration. Only the SLC01B1*5 SNP emerged as a predictor of statin-induced myopathy across the entire genome.42
The authors believe the findings are likely to apply to other statins. The mechanisms leading to statin-induced myopathy and the impact of statin pharmacogenomics are still unclear.43
CYP3A4. Other genetic variants may play a vital role in determining response to statin therapy. Carriers of a newly identified CYP3A4 polymorphism (intron 6 SNP, rs35599367, C>T) required significantly lower statin doses (0.2–0.6 times less) for optimal lipid control. The analyses included atorvastatin (Lipitor), simvastatin, and lovastatin (Mevacor), and the association was robust (P = .019).44
Statin pharmacogenomic testing is not routinely recommended
Routine pharmacogenomic testing for statin therapy is not recommended. Additional studies are needed to determine the clinical utility and cost-effectiveness of pharmacogenomic testing (involving a combination of several polymorphisms) in various patient populations delineated by type of statin, dose, and concomitant use of other drugs.
TAMOXIFEN IS ACTIVATED BY CYP2D6
Tamoxifen is prescribed to prevent the recurrence of estrogen-receptor-positive breast cancer, to treat metastatic breast cancer, to prevent cancer in high-risk populations, and to treat ductal carcinoma in situ.
Tamoxifen is metabolized to form endoxifen, which has much higher potency and higher systemic levels than tamoxifen.45 Both CYP2D6 and CYP3A4/5 are required to produce endoxifen via two intermediates, but CYP2D6 catalyzes the critical step leading to metabolic activation.
The CYP2D6 gene is highly polymorphic, with more than 75 allelic variants identified. Extensive literature is available describing the influence of CYP2D6 polymorphisms on tamoxifen metabolism and therapy outcomes.46–52 Several CYP2D6 variants result in reduced or no enzyme activity, and people who have more than two normally functioning alleles have exaggerated enzyme activity (gene amplification).
Classification of CYP2D6 status
Several systems have been developed to categorize the phenotypic activity of CYP2D6 based on genotype.
A genetic basis for the observed diversity in the metabolism of cytochrome P450 substrates was recognized more than 30 years ago. People were categorized as either extensive or poor metabolizers, reflecting normal vs impaired ability to metabolize the CYP2D6 substrates sparteine and debrisoquine. Later work expanded this system to include categories for intermediate (between poor and extensive) and ultra-rapid (better than extensive) metabolizers.
The genetic basis for these categories includes homozygosity for dysfunctional variants (the poor-metabolizer group) or extra copies of normal functioning variants (the ultra-rapid-metabolizer group).
Newer systems have been described for characterizing the CYP2D6 activity phenotype whereby CYP2D6 variants are assigned activity scores.53–56 The various scoring systems have been reviewed by Kirchheiner.57
A recent version of the activity scoring system also takes into consideration the many drugs that inhibit CYP2D6, such as amiodarone (Cordarone) and fluoxetine (Prozac) that can reduce the action of tamoxifen if given with it (Table 4).58 For example, the tamoxifen exposure (as predicted by the CYP2D6-activity score) experienced by a CYP2D6 extensive metabolizer taking a CYP2D6-inhibiting drug may be similar to the exposure experienced by a CYP2D6 poor metabolizer receiving the same tamoxifen dose but not taking a CYP2D6-inhibiting drug.
Likewise, the activity score of a CYP2D6 intermediate metabolizer taking a CYP2D6-inducing drug may be similar to that of a CYP2D6 ultra-rapid metabolizer not taking a CYP2D6-inducing drug. Examples of CYP2D6 inducers are dexamethasone, rifampin, and hyperforin (St. John’s wort).
While the newer systems are reported to provide better correlations between genotype and phenotype scores, the older scoring systems and the categorical names are still widely used (eg, in the FDA-approved AmpliChip CYP450 test from Roche,59 which includes genotype data for CYP2D6 and CYP2C19).
No firm recommendations for CYP2D6 testing in tamoxifen users
The different genotypes and phenotypes vary in prevalence in different ethnic groups, and significantly different activity levels for endoxifen formation are observed. Tamoxifen lacks efficacy in those who are poor CYP2D6 metabolizers—ie, about 7% of the white population.
However, the FDA has not made firm recommendations about CYP2D6 testing for prescribing tamoxifen because the evidence of benefit, although suggestive, has been considered insufficient.
Clinicians should be aware that tamoxifen’s efficacy is greatly reduced by concomitant therapy with CYP2D6-inhibiting drugs (Table 4).
Other genes affecting tamoxifen: CYP3A4/5, SULT1A1, and UGT2B15
Some investigators propose that polymorphisms in additional genes encoding enzymes in the tamoxifen metabolic and elimination pathways (eg, CYP3A4/5, SULT1A1, and UGT2B15) also need to be considered to account adequately for interindividual variation in drug response.
For example, CYP3A4 and CYP3A5 are also polymorphic, and large interindividual variation exists in their enzyme activities. These enzymes have overlapping substrate specificities, represent the most abundant drug-metabolizing enzymes in the human liver, and are involved in the biotransformation of a broad range of endogenous substrates and most drugs.60
Clinical studies evaluating the impact of CYP3A4/5 polymorphisms have been inconsistent in their conclusions, which is generally attributed to the relatively low functional impact or the low prevalence of the SNPs evaluated. Many of the nearly 100 CYP3A4/5 polymorphisms identified have not yet been characterized regarding their functional impact on enzyme expression or activity. CYP-3A4/5 enzyme activity is highly variable between individuals and warrants further study of its role in outcomes of tamoxifen therapy. Ongoing and future prospective clinical trials evaluating CYP2D6, CYP3A4/5, and other relevant polymorphisms are necessary to define their clinical relevance before routine genetic testing for tamoxifen can be justified.
CODEINE IS ALSO ACTIVATED BY CYP2D6
Codeine also depends on the CYP2D6 gene, as it must be activated to its more potent opioid metabolites, including morphine. Poor CYP2D6 metabolizers do not benefit from codeine therapy.
The pharmacogenomics of codeine has become a hot topic, especially regarding breast-feeding mothers. The debate was ignited with the publication in 2006 of a case report of an infant’s death, apparently the result of metabolic polymorphisms.61 The evolution of this debate and the outcome of the case may be noteworthy to clinicians, as they illustrate the gravity of public and patient interest in pharmacogenomic testing. In this case, the breast-feeding mother had taken codeine regularly for about 14 days when her 13-day-old infant died from toxic levels of morphine. Unknown to her and the prescriber, both the mother and infant were ultra-rapid CYP2D6 metabolizers, resulting in a more rapid and extensive conversion of codeine to morphine.
A logical strategy for preventing similar deaths would be routine CYP2D6 genotyping when prescribing codeine to breast-feeding mothers. However, after several investigations examined the metabolic and excretion pathways of codeine in their entirety, the FDA did not recommend routine CYP2D6 testing when prescribing codeine to breastfeeding mothers because several other factors, including rare genetic variations of other enzymes, proved necessary for reaching the opioid toxicity leading to the infant’s death.62
PHARMACOGENOMICS OF PSYCHOTROPIC DRUGS
Pharmacogenomic testing has clinical utility for some psychotropic drugs.
HLA-B and carbamazepine
Considered a standard of care, HLA-B genotyping is appropriate before prescribing carbamazepine (Tegretol, Equetro) to patients in populations in which HLAB*1502 is likely to be present, such as Asians. Carriers of HLAB* 1502 are at higher risk of life-threatening skin reactions such as Stevens-Johnson syndrome.11
Several other pharmacogenomic applications for psychotropic medications have been suggested, but routine testing has not been recommended by the FDA or endorsed by any expert panel because sufficient clinical utility and cost-effectiveness have not been demonstrated. A brief summary of study findings and a few practical suggestions follow.
Polymorphisms in metabolizing enzymes have been investigated in patients receiving psychotropic drugs.
CYP2D6 and antidepressants
Many antidepressants show significant differences in plasma drug levels with CYP2D6 polymorphisms (in descending order of influence)55:
- Imipramine (Tofranil)
- Doxepin (Adapin, Silenor, Sinequan)
- Maprotiline (Deprilept, Ludiomil, Psymion)
- Trimipramine (Surmontil)
- Desipramine (Noraprim)
- Nortriptyline (Aventyl, Pamelor)
- Clomipramine (Anafranil)
- Paroxetine (Paxil)
- Venlafaxine (Effexor)
- Amitriptyline (Elavil)
- Mianserin
- Trazadone (Desyrel)
- Bupropion (Wellbutrin)
- Nefazodone (Serzone)
- Citalopram (Celexa)
- Sertraline (Zoloft).
CYP2D6 and antipsychotics
Several antipsychotics are also influenced by CYP2D6 polymorphisms (also in descending order of influence)55:
- Perphenazine (Trilafon)
- Thioridazine (Mellaril)
- Olanzapine (Zyprexa)
- Zuclopenthixol (Cisordinol, Clopixol, Acuphase)
- Aripiprazole (Abilify)
- Flupentixol (Depixol, Fluanxol)
- Haloperidol (Haldol)
- Perazine (Taxilan)
- Risperidone (Risperdal)
- Pimozide (Orap).
CYP2C19 and antidepressants
CYP2C19 polymorphisms are likewise associated with differences in drug metabolism for many antidepressants, such as (in descending order of CYP2C19-mediated influence)55:
- Trimipramine
- Doxepin
- Amitriptyline
- Imipramine
- Citalopram (Celexa)
- Clomipramine
- Moclobemide (Aurorix, Manerix)
- Sertraline
- Fluvoxamine (Luvox).
Clinical relevance of CYP2D6 and CYP2C19
Several studies have demonstrated that poor and intermediate CYP2D6 metabolizers have a higher incidence of adverse effects when taking CYP2D6-dependent antidepressants63–68; however, an almost equal number of studies did not find statistically significant associations.69–72 Likewise, several studies have found an association between ultra-rapid CYP2D6 metabolizer status and diminished response to antidepressants,65,73,74 but no association was found in a larger retrospective study.75
Routine CYP2D6 and CYP2C19 screening is not recommended when prescribing psychotropic drugs. However, reviews of the pharmacokinetic data have suggested a few practical applications when genetic status is already known. In general, clinicians can consider reducing the dose of tricyclic antidepressants by about 50% when prescribing to CYP2D6-poor-metabolizers.55,76–78
Genes that affect serotonin metabolism
Several genes in the serotonin pathway have been investigated to determine whether they influence patients’ susceptibility to depression and adverse effects and response to psychotropic medications.
SLC6A4. Polymorphisms in the promoter region of the serotonin transporter gene SLC6A4 appear to influence the treatment response and side-effect profiles of selective serotonin reuptake inhibitors (SSRIs). Carriers of the SLC6A4 5-HTTLPR L alleles have fewer side effects79 and better response to SSRI treatment, and carriers of the S allele have a higher incidence of antidepressant-induced mania80 and poorer response to SSRI treatment.81
5-HT. Polymorphisms in serotonin receptors (2A and 2C subtypes) appear to influence SSRI response and side effects. Carriers of 5-HT 2A C alleles had more severe adverse effects from paroxetine,71 but another 5-HT 2A polymorphism common to Asians is associated with better response to antidepressant therapy.82 A 5-HT 2C polymorphism was associated with a lower incidence of antipsychotic-induced weight gain.83
Although the understanding of these relationships is incomplete and routine pharmacogenomic testing is not currently recommended, reviews of the pharmacodynamic data have suggested a few practical applications when a patient’s genetic status is already known. One should consider:
- Selecting treatments other than SSRIs for depressed patients known to possess the SLC6A4 variant
- Selecting citalopram for depressed patients known to carry the 5-HT 2A polymorphism
- Avoiding treatment with antipsychotic drugs for patients known to possess the 5-HT 2C polymorphism.
THE FUTURE OF PHARMACOGENOMIC TESTING
The examples discussed in this article provide some insight about how pharmacogenomic testing is maturing and slowly being integrated into the practice of medicine. They also illustrate the complexity of the multiple stages of research that pharmacogenomic applications must go through in order to be adopted as standard practice.
In the future, pharmacogenomic data will continue to accumulate, and the clinical utility of many other pharmacogenomic tests may be uncovered. The FDA provides information on emerging pharmacogenomic tests at its Web site, www.fda.gov.11 Its up-to-date “Table of Valid Genomic Biomarkers in the Context of Approved Drug Labels” includes boxed warnings, recommendations, research outcomes, and relevant population genetics.
If the FDA continues its current policy, prospective randomized trials that show improvement in patient outcomes will remain the gold standard for determining the clinical significance of a pharmacogenomic test. Furthermore, cost-benefit analyses are likely to continue dictating policy regarding pharmacogenomic testing, and cost-benefit profiles should improve as technology advances and as information gathered from a single test becomes applicable to multiple medications and clinical scenarios.
In the meantime, physicians should become familiar with the terms used in medical genetics and pharmacogenomics and begin to understand genetic contributions to the outcomes of drug therapy. For example, understanding the consequences of metabolizer status and the frequency of variants in a given population can be tremendously helpful when advising our patients about anticipating potential problems when taking specific medications and about making informed decisions about pharmacogenomic testing.
This exchange of information alone may go a long way in improving therapy outcomes even when prospective pharmacogenomic testing is not routinely performed. Furthermore, an increasing number of patients will already have genotyping information available when they come to us, and clinicians need to be aware of the many pharmacogenomic applications recommended by the FDA when genetic status is known.10
- King HC, Sinha AA. Gene expression profile analysis by DNA micro-arrays: promise and pitfalls. JAMA 2001; 286:2280–2288.
- Nuckols TK, Paddock SM, Bower AG, et al. Costs of intravenous adverse drug events in academic and nonacademic intensive care units. Med Care 2008; 46:17–24.
- Gurwitz JH, Field TS, Judge J, et al. The incidence of adverse events in two long-term care facilities. Am J Med 2005; 118:251–258.
- Vargas E, Terleira A, Hernando F, et al. Effects of adverse drug reactions on length of stay in surgical intensive care units. Crit Care Med 2003; 31:694–698.
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998; 279:1200–1205.
- Field TS, Gilman BH, Subramanian S, Fuller JC, Bates DW, Gurwitz JH. The costs associated with adverse drug events in older adults in the ambulatory setting. Med Care 2005; 43:1171–1176.
- Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA 1997; 277:301–306.
- Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc (Wash) 2001; 41:192–199.
- Bradford LD. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics 2002; 3:229–243.
- Sadee W. Measuring cis-acting regulatory variants genome-wide: new insights into expression genetics and disease susceptibility. Genome Med 2009; 1:116.
- US Food and Drug Administration. Table of pharmacogenomic biomarkers in drug labels. http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm. Accessed 1/18/2011.
- Budnitz DS, Shehab N, Kegler SR, Richards CL. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med 2007; 147:755–765.
- Elias DJ, Topol EJ. Warfarin pharmacogenomics: a big step forward for individualized medicine: enlightened dosing of warfarin. Eur J Hum Genet 2008; 16:532–534.
- Epstein RS, Moyer TP, Aubert RE, et al. Warfarin genotyping reduces hospitalization rates results from the MM-WES (Medco-Mayo Warfarin Effectiveness study). J Am Coll Cardiol 2010; 55:2804–2812.
- Rettie AE, Wienkers LC, Gonzalez FJ, Trager WF, Korzekwa KR. Impaired (S)-warfarin metabolism catalysed by the R144C allelic variant of CYP2C9. Pharmacogenetics 1994; 4:39–42.
- Higashi MK, Veenstra DL, Kondo LM, et al. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA 2002; 287:1690–1698.
- Sanderson S, Emery J, Higgins J. CYP2C9 gene variants, drug dose, and bleeding risk in warfarin-treated patients: a HuGEnet systematic review and meta-analysis. Genet Med 2005; 7:97–104.
- Au N, Rettie AE. Pharmacogenomics of 4-hydroxycoumarin anticoagulants. Drug Metab Rev 2008; 40:355–375.
- García-Martín E, Martínez C, Ladero JM, Agúndez JA. Interethnic and intraethnic variability of CYP2C8 and CYP2C9 polymorphisms in healthy individuals. Mol Diagn Ther 2006; 10:29–40.
- Sullivan-Klose TH, Ghanayem BI, Bell DA, et al. The role of the CYP2C9-Leu359 allelic variant in the tolbutamide polymorphism. Pharmacogenetics 1996; 6:341–349.
- Wen MS, Lee M, Chen JJ, et al. Prospective study of warfarin dosage requirements based on CYP2C9 and VKORC1 genotypes. Clin Pharmacol Ther 2008; 84:83–89.
- Larramendy-Gozalo C, Yang JQ, Verstuyft C, et al. Genetic polymorphism of vitamin K epoxide reductase (VKORC1) 1173C>T in a Chinese and a Caucasian population. Basic Clin Pharmacol Toxicol 2006; 98:611–613.
- Caldwell MD, Awad T, Johnson JA, et al. CYP4F2 genetic variant alters required warfarin dose. Blood 2008; 111:4106–4112.
- Bristol-Myers Squibb. Coumadin (warfarin sodium) Prescribing Information. January 2010.
- Barnes-Jewish Hospital at Washington University Medical Center. Warfarin dosing. http://warfarindosing.orgAccessed 1/20/2011.
- Flockhart DA, O’Kane D, Williams MS, et al. Pharmacogenetic testing of CYP2C9 and VKORC1 alleles for warfarin. Genet Med 2008; 10:139–150.
- National Institutes of Health. ClinicalTrials.gov. http://clinicaltrials.gov. Accessed January 20, 2011.
- Eckman MH, Rosand J, Greenberg SM, Gage BF. Cost-effectiveness of using pharmacogenetic information in warfarin dosing for patients with nonvalvular atrial fibrillation. Ann Intern Med 2009; 150:73–83.
- Meckley LM, Gudgeon JM, Anderson JL, Williams MS, Veenstra DL. A policy model to evaluate the benefits, risks, and costs of warfarin pharmacogenomic testing. Pharmacoeconomics 2010; 28:61–74.
- Patrick AR, Avron J, Choudhry NK. Cost-effectiveness of genotype-guided warfarin dosing for patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes 2009; 2:429–436.
- Haas S. New oral Xa and IIa inhibitors: updates on clinical trial results. J Thromb Thrombolysis 2008; 25:52–60.
- Bhatt DL. Tailoring antiplatelet therapy based on pharmacogenomics: how well do the data fit? JAMA 2009; 302:896–897.
- Shuldiner AR, O’Connell JR, Bliden KP, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 2009; 302:849–857.
- Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 2009; 373:309–317.
- Simon T, Verstuyft C, Mary-Krause M, et al; French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 2009; 360:363–375.
- Paré G, Mehta SR, Yusuf S, et al. Effects of CYP2C19 genotype on outcomes of clopidogrel treatment. N Engl J Med 2010; 363:1704–1714.
- Bristol-Myers Squibb/Sanofi Pharmaceutical Partnership. Plavix (clopidogrel bisulfate) prescribing information. August 2010.
- P450 Drug Interaction Table. Indiana University School of Medicine. http://medicine.iupui.edu/clinpharm/ddis/table.asp. Accessed 1/21/2011.
- Society for Cardiovascular Angiography and Interventions; Holmes DR, Dehmer GJ, Kaul S, Leifer D, O’Gara PT, Stein CM. ACCF/AHA clopidogrel clinical alert: approaches to the FDA “boxed warning”: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the American Heart Association. Circulation 2010; 122:537–557.
- National Institutes of Health. Genotype Guided Comparison of Clopidogrel and Prasugrel Outcomes Study. http://clinicaltrialsfeeds.org/clinical-trials/show/NCT00995514. Accessed 1/20/2011.
- Amarenco P, Labreuche J. Lipid management in the prevention of stroke: Review and updated meta-analysis of statins for stroke prevention. Lancet Neurol 2009; 8:453–463.
- SEARCH Collaborative Group, Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Romaine SP, Bailey KM, Hall AS, Balmforth AJ. The influence of SLC01B1 (OATP1B1) gene polymorphisms on response to statin therapy. Pharmacogenomics J 2010; 10:1–11.
- Wang D, Guo Y, Wrighton SA, Cooke GE, Sadee W. Intronic polymorphism in CYP3A4 affects hepatic expression and response to statin drugs. Pharmacogenomics J 2010; Apr 13 [Epub ahead of print].
- Goetz MP, Rae JM, Suman VJ, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol 2005; 23:9312–9318.
- Beverage JN, Sissung TM, Sion AM, Danesi R, Figg WD. CYP2D6 polymorphisms and the impact on tamoxifen therapy. J Pharm Sci 2007; 96:2224–2231.
- Flockhart D. CYP2D6 genotyping and the pharmacogenetics of tamoxifen. Clin Adv Hematol Oncol 2008; 6:493–494.
- Goetz MP, Kamal A, Ames MM. Tamoxifen pharmacogenomics: the role of CYP2D6 as a predictor of drug response. Clin Pharmacol Ther 2008; 83:160–166.
- Stearns V, Rae JM. Pharmacogenetics and breast cancer endocrine therapy: CYP2D6 as a predictive factor for tamoxifen metabolism and drug response? Expert Rev Mol Med 2008; 10:e34.
- Dezentjé VO, Guchelaar HJ, Nortier JW, van del Velde CJ, Gelderblom H. Clinical implications of CYP2D6 genotyping in tamoxifen treatment for breast cancer. Clin Cancer Res 2009; 15:15–21.
- Higgins MJ, Rae JM, Flockhart DA, Hayes DF, Stearns V. Pharmacogenetics of tamoxifen: who should undergo CYP2D6 genetic testing? J Natl Compr Canc Netw 2009; 7:203–213.
- Hoskins JM, Carey LA, McLeod HL. CYP2D6 and tamoxifen: DNA matters in breast cancer. Nat Rev Cancer 2009; 9:576–586.
- Steimer W, Zöpf K, von Amelunxen S, et al. Allele-specific change of concentration and functional gene dose for the prediction of steady-state serum concentrations of amitriptyline and nortriptyline in CYP2C19 and CYP2D6 extensive and intermediate metabolizers. Clinical Cancer 2004; 50:1623–1633.
- Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharm Ther 2008; 83:234–242.
- Kirchheiner J, Nickchen K, Bauer M, et al. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry 2004; 9:442–473.
- Kirchheiner J, Schmidt H, Tzvetkov M, et al. Pharmacokinetics of codeine and its metabolite morphine in ultra-rapid metabolizers due to CYP2D6 duplication. Pharmacogenomics J 2007; 7:257–265.
- Kirchheiner J. CYP2D6 phenotype prediction from genotype: which system is the best? Clin Pharmacol Ther 2008; 83:225–227.
- Borges S, Desta Z, Jin Y, et al. Composite functional genetic and comedication CYP2D6 activity score in predicting tamoxifen drug exposure among breast cancer patients. J Clin Pharmacol 2010; 50:450–458.
- Hoffmann-La Roche Ltd. AmpliChip CYP450 Test. http://www.roche.com/assays/Pages/AmpliChipCYP450Test.aspx. Accessed 1/21/2011.
- Anzenbacher P, Anzenbacherová E. Cytochromes P450 and metabolism of xenobiotics. Cell Mol Life Sci 2001; 58:737–747.
- Koren G, Cairns J, Chitayat D, Gaedigk A, Leeder SJ. Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother. Lancet 2006; 368:704.
- Willmann S, Edginton AN, Coboeken K, Ahr G, Lippert J. Risk to the breast-fed neonate from codeine treatment to the mother: a quantitative mechanistic modeling study. Clin Pharm Ther 2009; 86:634–643.
- Grzesiak M, Beszłej A, Lebioda A, Jonkisz A, Dobosz T, Kienja A. [Retrospective assessment of the antidepressants tolerance in the group of patients with diagnosis of depression and different CYP2D6 genotype.] [In Polish] Psychiatr Pol 2003; 37:433–444.
- Laika B, Leucht S, Heres S, Steimer W. Intermediate metabolizer: increased side effects in psychoactive drug therapy. The key to cost-effectiveness of pretreatment CYP2D6 screening? Pharmacogenomics J 2009; 9:395–403.
- Rau T, Wohlleben G, Wuttke H, et al. CYP2D6 genotype: Impact on adverse effects and nonresponse during treatment with antidepressants—a pilot study. Clin Pharm Ther 2004; 75:386–393.
- McAlpine DE, O’Kane DJ, Black JL, Mrazek DA. Cytochrome P450 2D6 genotype variation and venlafaxine dosage. Mayo Clin Proc 2007; 82:1065–1068.
- Chen S, Chou WH, Blouin RA, et al. The cytochrome P450 2D6 (CYP2D6) enzyme polymorphism: screening costs and influence on clinical outcomes in psychiatry. Clin Pharmacol Ther 1996; 60:522–534.
- Shams ME, Arneth B, Hiemke C, et al. CYP2D6 polymorphism and clinical effect of the antidepressant venlafaxine. J Clin Pharm Ther 2006; 31:493–502.
- Whyte EM, Romkes M, Mulsant BH, et al. CYP2D6 genotype and venlafaxine-XR concentrations in depressed elderly. Int J Geriatr Psychiatry 2006; 21:542–549.
- Roberts RL, Mulder RT, Joyce PR, Luty SE, Kennedy MA. No evidence of increased adverse drug reactions in cytochrome P450 CYP2D6 poor metabolizers treated with fluoxetine or nortriptyline. Hum Psychopharmacol 2004; 19:17–23.
- Murphy GM, Kremer C, Rodrigues HE, Schatzberg AF. Pharmacogenetics of antidepressant medication intolerance. Am J Psychiatry 2003; 160:1830–1835.
- Gillman PK. Re: no evidence of increased adverse drug reactions in cytochrome P450 CYP2D6 poor metabolizers treated with fluoxetine or nortriptyline. Hum Psychopharmacol 2005; 20:61–62.
- Gex-Fabry M, Eap CB, Oneda B, et al. CYP2D6 and ABCB1 genetic variability: influence on paroxetine plasma level and therapeutic response. Ther Drug Monit 2008; 30:474–482.
- Kawanishi C, Lundgren S, Agren H, Bertilsson L. Increased incidence of CYP2D6 gene duplication in patients with persistent mood disorders: ultrarapid metabolism of antidepressants as a cause of nonresponse. A pilot study. Eur J Clin Pharmacol 2004; 59:803–807.
- Serretti A, Calati R, Massat I, et al. Cytochrome P450 CYP1A2, CYP2C9, CYP2C19 and CYP2D6 genes are not associated with response and remission in a sample of depressive patients. Int Clin Psychopharmacol 2009; 24:250–256.
- de Leon J, Armstrong SC, Cozza KL. Clinical guidelines for psychiatrists for the use of pharmacogenetic testing for CYP450 2D6 and CYP450 2C19. Psychosomatics 2006; 47:75–85.
- de Leon J, Susce MT, Johnson M, et al. DNA microarray technology in the clinical environment: the AmpliChip CYP450 test for CYP2D6 and CYP2C19 genotyping. CNS Spectr 2009; 14:19–34.
- Thuerauf N, Lunkenheimer J. The impact of the CYP2D6-polymorphism on dose recommendations for current antidepressants. Eur Arch Psychiatry Clin Neurosci 2006; 256:287–293.
- Horstmann S, Binder EB. Pharmacogenomics of antidepressant drugs. Pharmacol Ther 2009; 124:57–73.
- Ferreira Ade A, Neves FS, da Rocha FF, et al. The role of 5-HTTLPR polymorphism in antidepressant-associated mania in bipolar disorder. J Affect Disord 2009; 112:267–272.
- Serretti A, Kato M, De Ronchi D, Kinoshita T. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry 2007; 12:247–257.
- Kato M, Serretti A. Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder. Mol Psychiatry 2010; 15:473–500.
- Reynolds GP, Zhang Z, Zhang X. Polymorphism of the promoter region of the serotonin 5-HT(2C) receptor gene and clozapine-induced weight gain. Am J Psychiatry 2003; 160:677–679.
- King HC, Sinha AA. Gene expression profile analysis by DNA micro-arrays: promise and pitfalls. JAMA 2001; 286:2280–2288.
- Nuckols TK, Paddock SM, Bower AG, et al. Costs of intravenous adverse drug events in academic and nonacademic intensive care units. Med Care 2008; 46:17–24.
- Gurwitz JH, Field TS, Judge J, et al. The incidence of adverse events in two long-term care facilities. Am J Med 2005; 118:251–258.
- Vargas E, Terleira A, Hernando F, et al. Effects of adverse drug reactions on length of stay in surgical intensive care units. Crit Care Med 2003; 31:694–698.
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998; 279:1200–1205.
- Field TS, Gilman BH, Subramanian S, Fuller JC, Bates DW, Gurwitz JH. The costs associated with adverse drug events in older adults in the ambulatory setting. Med Care 2005; 43:1171–1176.
- Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA 1997; 277:301–306.
- Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc (Wash) 2001; 41:192–199.
- Bradford LD. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics 2002; 3:229–243.
- Sadee W. Measuring cis-acting regulatory variants genome-wide: new insights into expression genetics and disease susceptibility. Genome Med 2009; 1:116.
- US Food and Drug Administration. Table of pharmacogenomic biomarkers in drug labels. http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm. Accessed 1/18/2011.
- Budnitz DS, Shehab N, Kegler SR, Richards CL. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med 2007; 147:755–765.
- Elias DJ, Topol EJ. Warfarin pharmacogenomics: a big step forward for individualized medicine: enlightened dosing of warfarin. Eur J Hum Genet 2008; 16:532–534.
- Epstein RS, Moyer TP, Aubert RE, et al. Warfarin genotyping reduces hospitalization rates results from the MM-WES (Medco-Mayo Warfarin Effectiveness study). J Am Coll Cardiol 2010; 55:2804–2812.
- Rettie AE, Wienkers LC, Gonzalez FJ, Trager WF, Korzekwa KR. Impaired (S)-warfarin metabolism catalysed by the R144C allelic variant of CYP2C9. Pharmacogenetics 1994; 4:39–42.
- Higashi MK, Veenstra DL, Kondo LM, et al. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA 2002; 287:1690–1698.
- Sanderson S, Emery J, Higgins J. CYP2C9 gene variants, drug dose, and bleeding risk in warfarin-treated patients: a HuGEnet systematic review and meta-analysis. Genet Med 2005; 7:97–104.
- Au N, Rettie AE. Pharmacogenomics of 4-hydroxycoumarin anticoagulants. Drug Metab Rev 2008; 40:355–375.
- García-Martín E, Martínez C, Ladero JM, Agúndez JA. Interethnic and intraethnic variability of CYP2C8 and CYP2C9 polymorphisms in healthy individuals. Mol Diagn Ther 2006; 10:29–40.
- Sullivan-Klose TH, Ghanayem BI, Bell DA, et al. The role of the CYP2C9-Leu359 allelic variant in the tolbutamide polymorphism. Pharmacogenetics 1996; 6:341–349.
- Wen MS, Lee M, Chen JJ, et al. Prospective study of warfarin dosage requirements based on CYP2C9 and VKORC1 genotypes. Clin Pharmacol Ther 2008; 84:83–89.
- Larramendy-Gozalo C, Yang JQ, Verstuyft C, et al. Genetic polymorphism of vitamin K epoxide reductase (VKORC1) 1173C>T in a Chinese and a Caucasian population. Basic Clin Pharmacol Toxicol 2006; 98:611–613.
- Caldwell MD, Awad T, Johnson JA, et al. CYP4F2 genetic variant alters required warfarin dose. Blood 2008; 111:4106–4112.
- Bristol-Myers Squibb. Coumadin (warfarin sodium) Prescribing Information. January 2010.
- Barnes-Jewish Hospital at Washington University Medical Center. Warfarin dosing. http://warfarindosing.orgAccessed 1/20/2011.
- Flockhart DA, O’Kane D, Williams MS, et al. Pharmacogenetic testing of CYP2C9 and VKORC1 alleles for warfarin. Genet Med 2008; 10:139–150.
- National Institutes of Health. ClinicalTrials.gov. http://clinicaltrials.gov. Accessed January 20, 2011.
- Eckman MH, Rosand J, Greenberg SM, Gage BF. Cost-effectiveness of using pharmacogenetic information in warfarin dosing for patients with nonvalvular atrial fibrillation. Ann Intern Med 2009; 150:73–83.
- Meckley LM, Gudgeon JM, Anderson JL, Williams MS, Veenstra DL. A policy model to evaluate the benefits, risks, and costs of warfarin pharmacogenomic testing. Pharmacoeconomics 2010; 28:61–74.
- Patrick AR, Avron J, Choudhry NK. Cost-effectiveness of genotype-guided warfarin dosing for patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes 2009; 2:429–436.
- Haas S. New oral Xa and IIa inhibitors: updates on clinical trial results. J Thromb Thrombolysis 2008; 25:52–60.
- Bhatt DL. Tailoring antiplatelet therapy based on pharmacogenomics: how well do the data fit? JAMA 2009; 302:896–897.
- Shuldiner AR, O’Connell JR, Bliden KP, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 2009; 302:849–857.
- Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 2009; 373:309–317.
- Simon T, Verstuyft C, Mary-Krause M, et al; French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 2009; 360:363–375.
- Paré G, Mehta SR, Yusuf S, et al. Effects of CYP2C19 genotype on outcomes of clopidogrel treatment. N Engl J Med 2010; 363:1704–1714.
- Bristol-Myers Squibb/Sanofi Pharmaceutical Partnership. Plavix (clopidogrel bisulfate) prescribing information. August 2010.
- P450 Drug Interaction Table. Indiana University School of Medicine. http://medicine.iupui.edu/clinpharm/ddis/table.asp. Accessed 1/21/2011.
- Society for Cardiovascular Angiography and Interventions; Holmes DR, Dehmer GJ, Kaul S, Leifer D, O’Gara PT, Stein CM. ACCF/AHA clopidogrel clinical alert: approaches to the FDA “boxed warning”: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the American Heart Association. Circulation 2010; 122:537–557.
- National Institutes of Health. Genotype Guided Comparison of Clopidogrel and Prasugrel Outcomes Study. http://clinicaltrialsfeeds.org/clinical-trials/show/NCT00995514. Accessed 1/20/2011.
- Amarenco P, Labreuche J. Lipid management in the prevention of stroke: Review and updated meta-analysis of statins for stroke prevention. Lancet Neurol 2009; 8:453–463.
- SEARCH Collaborative Group, Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Romaine SP, Bailey KM, Hall AS, Balmforth AJ. The influence of SLC01B1 (OATP1B1) gene polymorphisms on response to statin therapy. Pharmacogenomics J 2010; 10:1–11.
- Wang D, Guo Y, Wrighton SA, Cooke GE, Sadee W. Intronic polymorphism in CYP3A4 affects hepatic expression and response to statin drugs. Pharmacogenomics J 2010; Apr 13 [Epub ahead of print].
- Goetz MP, Rae JM, Suman VJ, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol 2005; 23:9312–9318.
- Beverage JN, Sissung TM, Sion AM, Danesi R, Figg WD. CYP2D6 polymorphisms and the impact on tamoxifen therapy. J Pharm Sci 2007; 96:2224–2231.
- Flockhart D. CYP2D6 genotyping and the pharmacogenetics of tamoxifen. Clin Adv Hematol Oncol 2008; 6:493–494.
- Goetz MP, Kamal A, Ames MM. Tamoxifen pharmacogenomics: the role of CYP2D6 as a predictor of drug response. Clin Pharmacol Ther 2008; 83:160–166.
- Stearns V, Rae JM. Pharmacogenetics and breast cancer endocrine therapy: CYP2D6 as a predictive factor for tamoxifen metabolism and drug response? Expert Rev Mol Med 2008; 10:e34.
- Dezentjé VO, Guchelaar HJ, Nortier JW, van del Velde CJ, Gelderblom H. Clinical implications of CYP2D6 genotyping in tamoxifen treatment for breast cancer. Clin Cancer Res 2009; 15:15–21.
- Higgins MJ, Rae JM, Flockhart DA, Hayes DF, Stearns V. Pharmacogenetics of tamoxifen: who should undergo CYP2D6 genetic testing? J Natl Compr Canc Netw 2009; 7:203–213.
- Hoskins JM, Carey LA, McLeod HL. CYP2D6 and tamoxifen: DNA matters in breast cancer. Nat Rev Cancer 2009; 9:576–586.
- Steimer W, Zöpf K, von Amelunxen S, et al. Allele-specific change of concentration and functional gene dose for the prediction of steady-state serum concentrations of amitriptyline and nortriptyline in CYP2C19 and CYP2D6 extensive and intermediate metabolizers. Clinical Cancer 2004; 50:1623–1633.
- Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharm Ther 2008; 83:234–242.
- Kirchheiner J, Nickchen K, Bauer M, et al. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry 2004; 9:442–473.
- Kirchheiner J, Schmidt H, Tzvetkov M, et al. Pharmacokinetics of codeine and its metabolite morphine in ultra-rapid metabolizers due to CYP2D6 duplication. Pharmacogenomics J 2007; 7:257–265.
- Kirchheiner J. CYP2D6 phenotype prediction from genotype: which system is the best? Clin Pharmacol Ther 2008; 83:225–227.
- Borges S, Desta Z, Jin Y, et al. Composite functional genetic and comedication CYP2D6 activity score in predicting tamoxifen drug exposure among breast cancer patients. J Clin Pharmacol 2010; 50:450–458.
- Hoffmann-La Roche Ltd. AmpliChip CYP450 Test. http://www.roche.com/assays/Pages/AmpliChipCYP450Test.aspx. Accessed 1/21/2011.
- Anzenbacher P, Anzenbacherová E. Cytochromes P450 and metabolism of xenobiotics. Cell Mol Life Sci 2001; 58:737–747.
- Koren G, Cairns J, Chitayat D, Gaedigk A, Leeder SJ. Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother. Lancet 2006; 368:704.
- Willmann S, Edginton AN, Coboeken K, Ahr G, Lippert J. Risk to the breast-fed neonate from codeine treatment to the mother: a quantitative mechanistic modeling study. Clin Pharm Ther 2009; 86:634–643.
- Grzesiak M, Beszłej A, Lebioda A, Jonkisz A, Dobosz T, Kienja A. [Retrospective assessment of the antidepressants tolerance in the group of patients with diagnosis of depression and different CYP2D6 genotype.] [In Polish] Psychiatr Pol 2003; 37:433–444.
- Laika B, Leucht S, Heres S, Steimer W. Intermediate metabolizer: increased side effects in psychoactive drug therapy. The key to cost-effectiveness of pretreatment CYP2D6 screening? Pharmacogenomics J 2009; 9:395–403.
- Rau T, Wohlleben G, Wuttke H, et al. CYP2D6 genotype: Impact on adverse effects and nonresponse during treatment with antidepressants—a pilot study. Clin Pharm Ther 2004; 75:386–393.
- McAlpine DE, O’Kane DJ, Black JL, Mrazek DA. Cytochrome P450 2D6 genotype variation and venlafaxine dosage. Mayo Clin Proc 2007; 82:1065–1068.
- Chen S, Chou WH, Blouin RA, et al. The cytochrome P450 2D6 (CYP2D6) enzyme polymorphism: screening costs and influence on clinical outcomes in psychiatry. Clin Pharmacol Ther 1996; 60:522–534.
- Shams ME, Arneth B, Hiemke C, et al. CYP2D6 polymorphism and clinical effect of the antidepressant venlafaxine. J Clin Pharm Ther 2006; 31:493–502.
- Whyte EM, Romkes M, Mulsant BH, et al. CYP2D6 genotype and venlafaxine-XR concentrations in depressed elderly. Int J Geriatr Psychiatry 2006; 21:542–549.
- Roberts RL, Mulder RT, Joyce PR, Luty SE, Kennedy MA. No evidence of increased adverse drug reactions in cytochrome P450 CYP2D6 poor metabolizers treated with fluoxetine or nortriptyline. Hum Psychopharmacol 2004; 19:17–23.
- Murphy GM, Kremer C, Rodrigues HE, Schatzberg AF. Pharmacogenetics of antidepressant medication intolerance. Am J Psychiatry 2003; 160:1830–1835.
- Gillman PK. Re: no evidence of increased adverse drug reactions in cytochrome P450 CYP2D6 poor metabolizers treated with fluoxetine or nortriptyline. Hum Psychopharmacol 2005; 20:61–62.
- Gex-Fabry M, Eap CB, Oneda B, et al. CYP2D6 and ABCB1 genetic variability: influence on paroxetine plasma level and therapeutic response. Ther Drug Monit 2008; 30:474–482.
- Kawanishi C, Lundgren S, Agren H, Bertilsson L. Increased incidence of CYP2D6 gene duplication in patients with persistent mood disorders: ultrarapid metabolism of antidepressants as a cause of nonresponse. A pilot study. Eur J Clin Pharmacol 2004; 59:803–807.
- Serretti A, Calati R, Massat I, et al. Cytochrome P450 CYP1A2, CYP2C9, CYP2C19 and CYP2D6 genes are not associated with response and remission in a sample of depressive patients. Int Clin Psychopharmacol 2009; 24:250–256.
- de Leon J, Armstrong SC, Cozza KL. Clinical guidelines for psychiatrists for the use of pharmacogenetic testing for CYP450 2D6 and CYP450 2C19. Psychosomatics 2006; 47:75–85.
- de Leon J, Susce MT, Johnson M, et al. DNA microarray technology in the clinical environment: the AmpliChip CYP450 test for CYP2D6 and CYP2C19 genotyping. CNS Spectr 2009; 14:19–34.
- Thuerauf N, Lunkenheimer J. The impact of the CYP2D6-polymorphism on dose recommendations for current antidepressants. Eur Arch Psychiatry Clin Neurosci 2006; 256:287–293.
- Horstmann S, Binder EB. Pharmacogenomics of antidepressant drugs. Pharmacol Ther 2009; 124:57–73.
- Ferreira Ade A, Neves FS, da Rocha FF, et al. The role of 5-HTTLPR polymorphism in antidepressant-associated mania in bipolar disorder. J Affect Disord 2009; 112:267–272.
- Serretti A, Kato M, De Ronchi D, Kinoshita T. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry 2007; 12:247–257.
- Kato M, Serretti A. Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder. Mol Psychiatry 2010; 15:473–500.
- Reynolds GP, Zhang Z, Zhang X. Polymorphism of the promoter region of the serotonin 5-HT(2C) receptor gene and clozapine-induced weight gain. Am J Psychiatry 2003; 160:677–679.
KEY POINTS
- Polymorphisms that affect the pharmacokinetics and pharmacodynamics of specific drugs are common.
- Testing for certain polymorphisms before prescribing certain drugs could help avoid adverse drug effects and improve efficacy.
- Pharmacogenomic testing has only recently begun to enter clinical practice, and routine testing is currently limited to a few clinical scenarios. However, additional applications may be just around the corner.
- Many pharmacogenomic tests are available, but testing has not yet been recommended for most drugs. Needed are large-scale trials to show that routine testing improves patient outcomes.
Managing newly diagnosed atrial fibrillation: Rate, rhythm, and risk
Three general concerns dictate the management of atrial fibrillation:
- Controlling the heart rate
- Controlling symptoms
- Preventing thromboembolic events, including stroke.
When seeing a patient with newly diagnosed atrial fibrillation, these same three concerns should be kept in mind, but several additional issues must be addressed:
- Reversible causes of atrial fibrillation must be ruled out
- The true time of onset of the atrial fibrillation and the frequency of the episodes should be ascertained, if possible
- A careful estimation of the patient’s symptom burden should be made.
Atrial fibrillation is common and has a huge impact in terms of the morbidity, death, and costs associated with it. It affects more than 2.2 million Americans.1 Approximately 1 in 10 people over the age of 80 has atrial fibrillation, and for those over the age of 40, the lifetime risk of developing it is one in four.2 Framingham data suggest that the risk of death is approximately twice as high for patients with atrial fibrillation compared with a similar cohort without.3–5
IMPORTANT QUESTIONS DURING THE INITIAL WORKUP
Does the patient have a reversible cause of atrial fibrillation?
Atrial fibrillation is thought to be due to triggers that initiate it and to a myocardial substrate that supports it. While it may develop in the absence of other heart disease, it is often associated with hypertension, diabetes, obesity, structural heart disease (including congenital heart disease), obstructive sleep apnea, advanced age, and alcohol abuse.
Therefore, once atrial fibrillation has been diagnosed, the history, examination, and diagnostic workup should be directed toward looking for potentially reversible causes and for frequently associated comorbidities. Common reversible causes include:
Hyperthyroidism. The laboratory evaluation should include a thyrotropin (thyroid-stimulating hormone, or TSH) level.
Alcohol use, especially binge drinking.
Obstructive sleep apnea, if suspected on the basis of the history or the body habitus.
Structural heart disease such as valvular heart disease or congenital heart defects may also predispose to atrial fibrillation. Therefore, listen carefully to the heart and obtain a transthoracic echocardiogram if one has not already been done or if you suspect a change in valvular disease or systolic function since the most recent study.
How long has the patient been in atrial fibrillation?
The duration of the atrial fibrillation often affects the treatment strategy. Therefore, when the diagnosis has been made, it is important to try to estimate how long the patient has been in atrial fibrillation.
Often, we must settle for an estimate, as the patient’s recollection may be vague. However, in some cases, the symptoms are pronounced or electrocardiographic or telemetric data are available, allowing the time of onset to be clearly defined.
In addition, it is helpful to know if the patient has had prior episodes that were never brought to medical attention. To this end, elicit the patient’s spectrum of symptoms and encourage him or her to think back months or years and try to recall other times when similar symptoms might have occurred.
How do the symptoms affect the patient’s quality of life?
The clinician must also estimate the extent to which the symptoms affect the patient’s quality of life. This is best done when the heart rate is under control. If the patient still has significant symptoms despite adequate rate control, then a rhythm control strategy should probably be pursued.
MANAGING NEWLY DIAGNOSED ATRIAL FIBRILLATION
Control the heart rate with a beta-blocker, a calcium channel blocker, or digoxin
Many patients present during their first episode of atrial fibrillation with a rapid ventricular rate, especially if they are not already taking a drug to slow conduction through the atrioventricular node. If the symptoms are particularly profound, one should try to get the heart rate under control quickly.
Oral agents take time to be absorbed and are not always easy to titrate. Intravenous beta-blockers such as metoprolol (Lopressor) and labetalol (Normodyne, Trandate) or intravenous diltiazem (Cardizem) can slow the heart rate quickly and can be titrated. Once the heart rate is controlled, the oral form can be started, to allow weaning from the intravenous agent. In acute management, we seek a heart rate of less than about 100 to 110 beats per minute.
If the patient’s blood pressure is marginal, loading with intravenous digoxin may be considered. The dosage is 0.5 mg intravenously, then 0.25 mg intravenously in the first 6 hours and another 0.25 mg intravenously in another 6 hours. In patients with renal insufficiency the dosage should be less, or digoxin should be avoided altogether. Often, the blood pressure will improve once the heart rate is decreased, allowing other agents to be initiated. However, if the patient is frankly hypotensive with chest pain, shortness of breath, or a diminished level of consciousness, then emergency electrical cardioversion is indicated even if anticoagulation has not yet been started (more about anticoagulation below).
Oral forms of these same agents are the workhorses for heart rate control in the outpatient setting. Oral beta-blockers and nondihydropyridine calcium channel blockers (ie, diltiazem or verapamil [Calan, Verelan]) are the first-line agents, because when digoxin is used alone, it is relatively poor at controlling the heart rate, especially when the patient is not at rest.
The choice between these agents should be dictated by whether the patient has comorbidities such as coronary artery disease, heart failure, or reactive airway disease. Nondihydropyridine calcium channel blockers are relatively contraindicated in patients with heart failure, while beta-blockers can exacerbate reactive airway disease.6
It is also important to document that the heart rate is adequately controlled outside the hospital or outpatient clinic, where the patient is typically sitting or supine. This can be done with a 6-minute walk, exercise test, or Holter monitor once rate-controlling agents have been titrated.7
When to try to restore sinus rhythm
When atrial fibrillation is first diagnosed, it may not be possible to determine if it is paroxysmal (ie, self-terminating) or persistent. If the episode does not quickly end on its own, consideration may be given to restoring sinus rhythm.
Although experts debate the merits of a rate control approach vs a rhythm control approach for managing atrial fibrillation in the long term, many, including ourselves, recommend trying to restore sinus rhythm at least once when atrial fibrillation is first discovered. It is not always clear if atrial fibrillation is truly asymptomatic. Symptoms such as fatigue or decreased exercise tolerance can be subtle. Additionally, these symptoms may be attributed to other factors such as deconditioning, obesity, or advancing age. Thus, in many cases, only restoring normal sinus rhythm for a time allows the patient and clinician to fully assess the symptoms attributable to atrial fibrillation.
Therefore, in patients with newly diagnosed atrial fibrillation, an attempt to restore sinus rhythm is often warranted. Exceptions are in select patients who have no apparent symptoms and who are very old or are deemed too frail to tolerate cardioversion.
Direct-current cardioversion is typically the treatment of choice when attempting to restore sinus rhythm. The procedure can be done without anticoagulation within 48 hours of the onset of atrial fibrillation, if the time of onset is clear.7 However, clinicians must be careful in defining the onset of atrial fibrillation for this purpose.
Symptoms such as fatigue or shortness of breath can be vague in terms of the exact time of onset and often cannot be relied upon for the purpose of deciding whether cardioversion can be done without anticoagulation. When in doubt, it is best to err on the side of safety and assume that the atrial fibrillation has been going on for more than 48 hours.
If the time of onset is unclear or if more than 48 hours have passed, there are two general strategies for proceeding to electrical cardioversion.
One is to order transesophageal echocardiography and begin anticoagulation therapy at the same time. If there is no thrombus in the left atrium, then cardioversion can be done.8 Therapeutic anticoagulation with heparin, low-molecular-weight heparin, or warfarin (Coumadin) should be achieved within 24 to 48 hours of transesophageal echocardiography and cardioversion to minimize the risk of thromboembolic events, which can occur even after sinus rhythm has been restored.
At our institution, we typically strive to achieve therapeutic anticoagulation with either heparin or low-molecular-weight heparin before cardioversion in this scenario so as to avoid situations in which a patient may undergo cardioversion but then fail to achieve therapeutic anticoagulation for some time due to unforeseen factors.
The other approach is to start warfarin and maintain a goal international normalized ratio (INR) of 2 to 3 for 3 weeks, at which time cardioversion can be performed safely without transesophageal echocardiography.8
Regardless of which strategy is used, anticoagulation should be continued for at least 4 weeks after cardioversion,8 as atrial dysfunction and the risk of stroke may persist for days to weeks after normal sinus rhythm is restored.9
Role of antiarrhythmic drugs
Antiarrhythmic drugs can be used for chemical cardioversion or, more often, to help maintain sinus rhythm after direct-current cardioversion.
Because most of these drugs have at least a small chance of restoring normal sinus rhythm, we need to follow the same rules when starting them as when performing direct-current cardioversion. Patients should not be started on an antiarrhythmic medication until they have had adequate anticoagulation for at least 3 weeks or adequate anticoagulation and a transesophageal echocardiogram confirming that there is no thrombus in the left atrium.
Antiarrhythmic drugs should be started in select patients with newly diagnosed atrial fibrillation in whom a rhythm control strategy will be pursued. For patients whose history suggests a single episode, or episodes that previously self-terminated, an antiarrhythmic may not be necessary. For those with frequent episodes or whose history suggests ongoing atrial fibrillation for a long period, an antiarrhythmic will likely be required to provide a reasonable chance of achieving freedom from atrial fibrillation.
The choice of antiarrhythmic drug should be tailored to the specific patient.
Propafenone (Rythmol) and flecainide (Tambocor) are first-line drugs7 but are contraindicated in patients with coronary artery disease and significant structural heart disease.10
Sotalol (Betapace) and dofetilide (Tikosyn) can be used in patients with coronary artery disease. However, sotalol is contraindicated in patients with congestive heart failure, and dofetilide carries a long list of drug interactions. Both must be used with extreme caution in patients with renal insufficiency, and hospital admission is required for initiation or upward titration of the dose.
Amiodarone (Cordarone) is effective, and in the short term it is typically very well tolerated. However, it has a long half-life, and its potential for long-term toxicity often makes it a poor choice for long-term treatment. The toxicity of amiodarone increases with the cumulative dose. Therefore, this drug should be avoided for long-term therapy of atrial fibrillation in younger patients.
The ‘pill-in-the-pocket’ strategy
The “pill-in-the-pocket” strategy, in which patients are instructed to take their medication only when they have a bout of atrial fibrillation, is a reasonable option for patients with symptomatic recurrences of paroxysmal atrial fibrillation. This strategy is mainly reserved for patients who have relatively infrequent recurrences. Those who have frequent recurrences are usually more effectively treated with daily dosing of an antiarrhythmic. Flecainide and propafenone are the agents of choice for this approach because of their safety profile and efficacy in chemical cardioversion.
While this strategy may be started on an outpatient basis in patients with lone, paroxysmal atrial fibrillation, those with structural heart disease or conduction abnormalities should be observed in the hospital during initiation of therapy to observe for excessive PR prolongation or development of dangerous or worrisome arrhythmias.11–13
Additionally, these agents can decrease the refractory period of the atrioventricular node, thereby increasing the ventricular rate. In the case of atrial flutter, patients may be converted from variable or 2:1 response to a 1:1 conduction. Thus, one should consider also using a beta-blocker with this strategy.
Since the goal of this approach is to convert the patient to sinus rhythm within a few hours of the onset of atrial fibrillation, it may be implemented without the use of warfarin. Patients are instructed that if they do not convert to normal sinus rhythm within a few hours, they should notify the physician so they can undergo electrical cardioversion within the 48-hour window from the onset of atrial fibrillation.
Dronedarone, a new antiarrhythmic drug
Dronedarone (Multaq) is now available and has been shown to be effective in treating atrial fibrillation.14 It has a long half-life and a mechanism of action similar to that of amiodarone. However, it may be inferior to amiodarone in terms of efficacy.15 It is metabolized by CYP3A4. No dosage adjustment is needed for patients with renal insufficiency.
Because dronedarone lacks the iodine moiety found in amiodarone, it should not carry the same toxicity profile. No pulmonary or thyroid toxicity was reported in early trials.16
Nevertheless, dronedarone has several important limitations. It carries a black box warning stating it is contraindicated in patients with severe or recently decompensated heart failure, as the mortality rate was twice as high when this drug was used in such patients in initial studies.17 Additionally, there have been reports of hepatotoxicity requiring liver transplantation in a small number of patients. The extent of this problem and strategies for avoiding it are not yet defined as of the writing of this paper. As with any new medication, patients who are started on dronedarone should be observed closely for any side effects, and these should be reported to assist in the development of the drug’s safety profile.
Pulmonary vein isolation
In a procedure that can potentially cure atrial fibrillation, catheters are inserted into the left atrium and rings of scar tissue are created around the ostia of the pulmonary veins using radiofrequency energy, electrically isolating them from the rest of the left atrium.
Some debate exists as to whether this procedure may be reasonable as a first-line therapy for some patients with atrial fibrillation.18,19 It may be considered as an early treatment strategy in a small subset of patients, specifically young patients with symptomatic, recurrent atrial fibrillation, especially if they are averse to long-term antiarrhythmic therapy.
Because patients may still be more prone to atrial arrhythmias for several weeks to months after the procedure, they must be able to tolerate anticoagulation with warfarin for at least several months.
Rate control vs rhythm control
The choice between a rate control strategy or a rhythm control strategy in the long term is not always straightforward. While atrial fibrillation is clearly associated with higher morbidity and mortality rates, there are few data to date showing that restoring and maintaining sinus rhythm in patients with atrial fibrillation reduce the incidence of morbid complications or the likelihood of death.
Thus, current guidelines recommend a rate control strategy in patients who have no symptoms, and a rhythm control strategy if rate control cannot be achieved or if symptoms persist despite adequate control of the heart rate.7 The circumstances and preferences of the individual patient should carry weight in this decision.
Trials are under way that may shed more light on the relative benefits of rhythm control with ablation or antiarrhythmics and rate control.
PREVENTING THROMBOEMBOLIC EVENTS
Warfarin
In the short term, warfarin therapy may be dictated by plans to restore sinus rhythm. Patients need warfarin for at least 4 weeks after cardioversion unless it is performed within 48 hours of the onset of atrial fibrillation.
The CHADS2score (1 point each for congestive heart failure, hypertension, age 75 or older, and diabetes mellitus; 2 points for prior stroke or transient ischemic attack) is useful when deciding whether to give long-term anticoagulation.
For patients with a score of 0, the risk of stroke is lower than the risk of a major bleeding complication while on therapeutic warfarin.20,21 For these patients, aspirin 81 to 325 mg daily is recommended for stroke prophylaxis.
For those with a score of 2 or greater, the risk of stroke without warfarin is greater than the risk of a major bleeding complication with warfarin. These patients should receive warfarin with a goal INR of 2.0 to 3.0.7
Patients with a CHADS2 score of 1 present a dilemma, as their risk of stroke without warfarin is about the same as their risk of a major bleeding complication with warfarin. They can be managed with either warfarin or aspirin, according to the physician’s judgment.7 In these cases, factors such as hobbies or professions that might increase the risk of bleeding, perceived frequency of atrial fibrillation episodes, and even patient preconceptions about warfarin are often used when deciding between aspirin and warfarin.
Patients with a CHADS2 score of 2 or greater with a single episode of atrial fibrillation and a likely reversible cause may also pose a dilemma when deciding whether to start warfarin. These patients have demonstrated they at least have the substrate to maintain atrial fibrillation. This situation again calls for physician judgment. Bear in mind that asymptomatic recurrences are common in patients with atrial fibrillation.22,23 A higher CHADS2 score denotes a greater risk of stroke and may influence this decision. It is usually beneficial to enlist the patient in this decision-making process, as patients often have very strong opinions about tolerance of the risk of stroke or of warfarin therapy itself.
Another strategy is to start anticoagulation with warfarin and aggressively monitor for recurrences. If the patient has no recurrences of atrial fibrillation after 6 to 12 months and the reversible cause is resolved, one can then revisit the need for warfarin.
Role of aspirin and clopidogrel
Aspirin, alone or in conjunction with clopidogrel (Plavix), may provide an alternative for stroke prophylaxis in patients in whom warfarin is contraindicated. While inferior to warfarin, the combination of aspirin and clopidogrel has been shown to decrease the incidence of major thromboembolic events, especially stroke.24 However, the risk of a major bleeding complication was also significantly increased.
This combination may be a reasonable strategy in select patients with a CHADS2 score of 2 or greater in whom warfarin cannot be used for reasons such as personal aversion to the medication, side effects, or nonbleeding complications or in patients whose INR is exceedingly difficult to keep within the therapeutic range.
Dabigatran, a new anticoagulant
The newest option for anticoagulation in patients with atrial fibrillation is a direct thrombin inhibitor, dabigatran (Pradaxa).
In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial,25 dabigatran was studied head-to-head with warfarin. The doses of dabigatran studied were 110 mg and 150 mg twice a day. At 150 mg twice a day, patients on dabigatran had a lower rate of stroke than with warfarin (1.11% vs 1.69%, P < .001), as well as a lower rate of central nervous system bleeding (0.10% vs 0.38% with warfarin, P < .001). The rates of major bleeding were comparable in the patients receiving warfarin or dabigatran 150 mg twice a day, but the rate of gastrointestinal bleeding was higher in the dabigatran group (1.51% vs 1.02% with warfarin, P < .001).25
Dabigatran was recently approved by the US Food and Drug Administration for use in patients with atrial fibrillation. Doses of 150 mg and 75 mg are available.
Dabigatran is renally excreted, and the 150 mg twice-a-day dosing is intended for patients with a creatinine clearance greater than 30 mL/min. The 75-mg twice-a-day dosing is intended for patients with a creatinine clearance of 15 to 30 mL/min. However, it should be noted that currently there are no data to support the 75-mg twice-a-day dosing.
Dabigatran does have several advantages over warfarin. Patients do not need to avoid foods containing vitamin K, and routine serial monitoring does not appear to be needed. As with any new medication, patients who are started on dabigatran should be observed closely for any side effects, and these should be reported to assist in the development of the drug’s safety profile.
SPECIAL CIRCUMSTANCES
After cardiac or noncardiac surgery
Atrial fibrillation is common after open heart surgery, occurring in approximately 25% to 50% of patients.26–28
When this happens, at least one or two attempts are made to restore sinus rhythm. Especially in the early postoperative period, anticoagulation with heparin or warfarin may be contraindicated, so careful attention must be paid to the patient’s heart rhythm so that atrial fibrillation can be recognized quickly and cardioversion performed within a 48-hour window of onset. Beta-blockers, diltiazem, and verapamil are typically used for rate control.
When atrial fibrillation recurs in patients who have undergone open heart surgery, antiarrhythmics are started early to help prevent further recurrences. At our institution, we usually use amiodarone, as it is highly effective and well tolerated in the short term. When started on amiodarone for postoperative atrial fibrillation, patients are informed that the drug will be stopped after about 2 to 3 months. For patients who continue to have bouts of atrial fibrillation, the need for antiarrhythmic medications can be reassessed, and, if needed, the optimal antiarrhythmic medication for long-term therapy for the patient can be chosen.
Atrial fibrillation in severe, acute illness
Atrial fibrillation is common in the setting of extreme systemic stressors such as shock and sepsis and when the patient is being supported with inotropic agents. In this setting, patients may be in a high-catecholamine state, and both the heart rate and the heart rhythm may be very difficult to control.
Beta-blockers and nondihydropyridine calcium channel blockers should not be used when patients are on medications to support blood pressure, and in this setting, when the patient’s hemodynamic status permits the use of these agents, their effect may be minimal.
Amiodarone or perhaps digoxin may slow the heart rate somewhat without too much effect on the blood pressure. However, with amiodarone, one may have to accept a risk of chemical cardioversion.
Electrical cardioversion with or without the assistance of an antiarrhythmic drug may control the heart rate by restoring sinus rhythm. However, atrial fibrillation often recurs, and if it recurs quickly one may have to accept elevated heart rates until the underlying process is addressed.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285:2370–2375.
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004; 110:1042–1046.
- Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998; 98:946–952.
- Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 1998; 82( 8A):2N–9N.
- Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med 1982; 306:1018–1022.
- The Multicenter Diltiazem Postinfarction Trial Research Group. The effect of diltiazem on mortality and reinfarction after myocardial infarction. N Engl J Med 1988; 319:385–392.
- European heart Rhythm Association; Heart Rhythm society, Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J Am Coll Cardiol 2006; 48:854–906.
- Klein AL, Grimm RA, Murray RD, et al; Assessment of Cardioversion Using Transesophageal Echocardiography Investigators. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med 2001; 344:1411–1120.
- Grimm RA, Leung DY, Black IW, Stewart WJ, Thomas JD, Klein AL. Left atrial appendage “stunning” after spontaneous conversion of atrial fibrillation demonstrated by transesophageal Doppler echocardiography. Am Heart J 1995; 130:174–176.
- The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med 1989; 321:406–412.
- Alboni P, Tomasi C, Menozzi C, et al. Efficacy and safety of out-of-hospital self-administered single-dose oral drug treatment in the management of infrequent, well-tolerated paroxysmal supraventricular tachycardia. J Am Coll Cardiol 2001; 37:548–553.
- Capucci A, Villani GQ, Piepoli MF. Reproducible efficacy of loading oral propafenone in restoring sinus rhythm in patients with paroxysmal atrial fibrillation. Am J Cardiol 2003; 92:1345–1347.
- Khan IA. Single oral loading dose of propafenone for pharmacological cardioversion of recent-onset atrial fibrillation. J Am Coll Cardiol 2001; 37:542–547.
- Singh BN, Connolly SJ, Crijns HJ, et al; EURIDIS and ADONIS Investigators. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med 2007; 357:987–999.
- Le Heuzey J, De Ferrari GM, Radzik D, Santini M, Zhu J, Davy JM. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS study. J Cardiovasc Electrophysiol 2010; 21:597–605.
- Hohnloser SH, Crijns HJ, van Eickels M, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009; 360:668–678.
- Køber L, Torp-Pedersen C, McMurray JJ, et al; Dronedarone Study Group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med 2008; 358:2678–2687.
- Pappone C, Rosanio S, Augello G, et al. Mortality, morbidity, and quality of life after circumferential pulmonary vein ablation for atrial fibrillation: outcomes from a controlled nonrandomized long-term study. J Am Coll Cardiol 2003; 42:185–197.
- Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005; 293:2634–2640.
- van Walraven C, Hart RG, Singer DE, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient metaanalysis. JAMA 2002; 288:2441–2448.
- Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a metaanalysis. Ann Intern Med 1999; 131:492–501.
- Page RL, Wilkinson WE, Clair WK, McCarthy EA, Pritchett EL. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation 1994; 89:224–227.
- Savelieva I, Camm AJ. Clinical relevance of silent atrial fibrillation: prevalence, prognosis, quality of life, and management. J Intervent Card Electrophysiol 2000; 4:369–382.
- ACTIVE Investigators, Connolly SJ, Pogue J, Hart RG, et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med 2009; 360:2066–2078.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151. Erratum in: N Engl J Med 2010; 363:1877.
- Almassi GH, Schowalter T, Nicolosi AC, et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg 1997; 226:501–511.
- Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg 1993; 56:539–549.
- Mathew JP, Fontes ML, Tudor IC, et al; Investigators of the Ischemia Research and Education Foundation; Multicenter Study of Perioperative Ischemia Research Group. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA 2004; 291:1720–1729.
Three general concerns dictate the management of atrial fibrillation:
- Controlling the heart rate
- Controlling symptoms
- Preventing thromboembolic events, including stroke.
When seeing a patient with newly diagnosed atrial fibrillation, these same three concerns should be kept in mind, but several additional issues must be addressed:
- Reversible causes of atrial fibrillation must be ruled out
- The true time of onset of the atrial fibrillation and the frequency of the episodes should be ascertained, if possible
- A careful estimation of the patient’s symptom burden should be made.
Atrial fibrillation is common and has a huge impact in terms of the morbidity, death, and costs associated with it. It affects more than 2.2 million Americans.1 Approximately 1 in 10 people over the age of 80 has atrial fibrillation, and for those over the age of 40, the lifetime risk of developing it is one in four.2 Framingham data suggest that the risk of death is approximately twice as high for patients with atrial fibrillation compared with a similar cohort without.3–5
IMPORTANT QUESTIONS DURING THE INITIAL WORKUP
Does the patient have a reversible cause of atrial fibrillation?
Atrial fibrillation is thought to be due to triggers that initiate it and to a myocardial substrate that supports it. While it may develop in the absence of other heart disease, it is often associated with hypertension, diabetes, obesity, structural heart disease (including congenital heart disease), obstructive sleep apnea, advanced age, and alcohol abuse.
Therefore, once atrial fibrillation has been diagnosed, the history, examination, and diagnostic workup should be directed toward looking for potentially reversible causes and for frequently associated comorbidities. Common reversible causes include:
Hyperthyroidism. The laboratory evaluation should include a thyrotropin (thyroid-stimulating hormone, or TSH) level.
Alcohol use, especially binge drinking.
Obstructive sleep apnea, if suspected on the basis of the history or the body habitus.
Structural heart disease such as valvular heart disease or congenital heart defects may also predispose to atrial fibrillation. Therefore, listen carefully to the heart and obtain a transthoracic echocardiogram if one has not already been done or if you suspect a change in valvular disease or systolic function since the most recent study.
How long has the patient been in atrial fibrillation?
The duration of the atrial fibrillation often affects the treatment strategy. Therefore, when the diagnosis has been made, it is important to try to estimate how long the patient has been in atrial fibrillation.
Often, we must settle for an estimate, as the patient’s recollection may be vague. However, in some cases, the symptoms are pronounced or electrocardiographic or telemetric data are available, allowing the time of onset to be clearly defined.
In addition, it is helpful to know if the patient has had prior episodes that were never brought to medical attention. To this end, elicit the patient’s spectrum of symptoms and encourage him or her to think back months or years and try to recall other times when similar symptoms might have occurred.
How do the symptoms affect the patient’s quality of life?
The clinician must also estimate the extent to which the symptoms affect the patient’s quality of life. This is best done when the heart rate is under control. If the patient still has significant symptoms despite adequate rate control, then a rhythm control strategy should probably be pursued.
MANAGING NEWLY DIAGNOSED ATRIAL FIBRILLATION
Control the heart rate with a beta-blocker, a calcium channel blocker, or digoxin
Many patients present during their first episode of atrial fibrillation with a rapid ventricular rate, especially if they are not already taking a drug to slow conduction through the atrioventricular node. If the symptoms are particularly profound, one should try to get the heart rate under control quickly.
Oral agents take time to be absorbed and are not always easy to titrate. Intravenous beta-blockers such as metoprolol (Lopressor) and labetalol (Normodyne, Trandate) or intravenous diltiazem (Cardizem) can slow the heart rate quickly and can be titrated. Once the heart rate is controlled, the oral form can be started, to allow weaning from the intravenous agent. In acute management, we seek a heart rate of less than about 100 to 110 beats per minute.
If the patient’s blood pressure is marginal, loading with intravenous digoxin may be considered. The dosage is 0.5 mg intravenously, then 0.25 mg intravenously in the first 6 hours and another 0.25 mg intravenously in another 6 hours. In patients with renal insufficiency the dosage should be less, or digoxin should be avoided altogether. Often, the blood pressure will improve once the heart rate is decreased, allowing other agents to be initiated. However, if the patient is frankly hypotensive with chest pain, shortness of breath, or a diminished level of consciousness, then emergency electrical cardioversion is indicated even if anticoagulation has not yet been started (more about anticoagulation below).
Oral forms of these same agents are the workhorses for heart rate control in the outpatient setting. Oral beta-blockers and nondihydropyridine calcium channel blockers (ie, diltiazem or verapamil [Calan, Verelan]) are the first-line agents, because when digoxin is used alone, it is relatively poor at controlling the heart rate, especially when the patient is not at rest.
The choice between these agents should be dictated by whether the patient has comorbidities such as coronary artery disease, heart failure, or reactive airway disease. Nondihydropyridine calcium channel blockers are relatively contraindicated in patients with heart failure, while beta-blockers can exacerbate reactive airway disease.6
It is also important to document that the heart rate is adequately controlled outside the hospital or outpatient clinic, where the patient is typically sitting or supine. This can be done with a 6-minute walk, exercise test, or Holter monitor once rate-controlling agents have been titrated.7
When to try to restore sinus rhythm
When atrial fibrillation is first diagnosed, it may not be possible to determine if it is paroxysmal (ie, self-terminating) or persistent. If the episode does not quickly end on its own, consideration may be given to restoring sinus rhythm.
Although experts debate the merits of a rate control approach vs a rhythm control approach for managing atrial fibrillation in the long term, many, including ourselves, recommend trying to restore sinus rhythm at least once when atrial fibrillation is first discovered. It is not always clear if atrial fibrillation is truly asymptomatic. Symptoms such as fatigue or decreased exercise tolerance can be subtle. Additionally, these symptoms may be attributed to other factors such as deconditioning, obesity, or advancing age. Thus, in many cases, only restoring normal sinus rhythm for a time allows the patient and clinician to fully assess the symptoms attributable to atrial fibrillation.
Therefore, in patients with newly diagnosed atrial fibrillation, an attempt to restore sinus rhythm is often warranted. Exceptions are in select patients who have no apparent symptoms and who are very old or are deemed too frail to tolerate cardioversion.
Direct-current cardioversion is typically the treatment of choice when attempting to restore sinus rhythm. The procedure can be done without anticoagulation within 48 hours of the onset of atrial fibrillation, if the time of onset is clear.7 However, clinicians must be careful in defining the onset of atrial fibrillation for this purpose.
Symptoms such as fatigue or shortness of breath can be vague in terms of the exact time of onset and often cannot be relied upon for the purpose of deciding whether cardioversion can be done without anticoagulation. When in doubt, it is best to err on the side of safety and assume that the atrial fibrillation has been going on for more than 48 hours.
If the time of onset is unclear or if more than 48 hours have passed, there are two general strategies for proceeding to electrical cardioversion.
One is to order transesophageal echocardiography and begin anticoagulation therapy at the same time. If there is no thrombus in the left atrium, then cardioversion can be done.8 Therapeutic anticoagulation with heparin, low-molecular-weight heparin, or warfarin (Coumadin) should be achieved within 24 to 48 hours of transesophageal echocardiography and cardioversion to minimize the risk of thromboembolic events, which can occur even after sinus rhythm has been restored.
At our institution, we typically strive to achieve therapeutic anticoagulation with either heparin or low-molecular-weight heparin before cardioversion in this scenario so as to avoid situations in which a patient may undergo cardioversion but then fail to achieve therapeutic anticoagulation for some time due to unforeseen factors.
The other approach is to start warfarin and maintain a goal international normalized ratio (INR) of 2 to 3 for 3 weeks, at which time cardioversion can be performed safely without transesophageal echocardiography.8
Regardless of which strategy is used, anticoagulation should be continued for at least 4 weeks after cardioversion,8 as atrial dysfunction and the risk of stroke may persist for days to weeks after normal sinus rhythm is restored.9
Role of antiarrhythmic drugs
Antiarrhythmic drugs can be used for chemical cardioversion or, more often, to help maintain sinus rhythm after direct-current cardioversion.
Because most of these drugs have at least a small chance of restoring normal sinus rhythm, we need to follow the same rules when starting them as when performing direct-current cardioversion. Patients should not be started on an antiarrhythmic medication until they have had adequate anticoagulation for at least 3 weeks or adequate anticoagulation and a transesophageal echocardiogram confirming that there is no thrombus in the left atrium.
Antiarrhythmic drugs should be started in select patients with newly diagnosed atrial fibrillation in whom a rhythm control strategy will be pursued. For patients whose history suggests a single episode, or episodes that previously self-terminated, an antiarrhythmic may not be necessary. For those with frequent episodes or whose history suggests ongoing atrial fibrillation for a long period, an antiarrhythmic will likely be required to provide a reasonable chance of achieving freedom from atrial fibrillation.
The choice of antiarrhythmic drug should be tailored to the specific patient.
Propafenone (Rythmol) and flecainide (Tambocor) are first-line drugs7 but are contraindicated in patients with coronary artery disease and significant structural heart disease.10
Sotalol (Betapace) and dofetilide (Tikosyn) can be used in patients with coronary artery disease. However, sotalol is contraindicated in patients with congestive heart failure, and dofetilide carries a long list of drug interactions. Both must be used with extreme caution in patients with renal insufficiency, and hospital admission is required for initiation or upward titration of the dose.
Amiodarone (Cordarone) is effective, and in the short term it is typically very well tolerated. However, it has a long half-life, and its potential for long-term toxicity often makes it a poor choice for long-term treatment. The toxicity of amiodarone increases with the cumulative dose. Therefore, this drug should be avoided for long-term therapy of atrial fibrillation in younger patients.
The ‘pill-in-the-pocket’ strategy
The “pill-in-the-pocket” strategy, in which patients are instructed to take their medication only when they have a bout of atrial fibrillation, is a reasonable option for patients with symptomatic recurrences of paroxysmal atrial fibrillation. This strategy is mainly reserved for patients who have relatively infrequent recurrences. Those who have frequent recurrences are usually more effectively treated with daily dosing of an antiarrhythmic. Flecainide and propafenone are the agents of choice for this approach because of their safety profile and efficacy in chemical cardioversion.
While this strategy may be started on an outpatient basis in patients with lone, paroxysmal atrial fibrillation, those with structural heart disease or conduction abnormalities should be observed in the hospital during initiation of therapy to observe for excessive PR prolongation or development of dangerous or worrisome arrhythmias.11–13
Additionally, these agents can decrease the refractory period of the atrioventricular node, thereby increasing the ventricular rate. In the case of atrial flutter, patients may be converted from variable or 2:1 response to a 1:1 conduction. Thus, one should consider also using a beta-blocker with this strategy.
Since the goal of this approach is to convert the patient to sinus rhythm within a few hours of the onset of atrial fibrillation, it may be implemented without the use of warfarin. Patients are instructed that if they do not convert to normal sinus rhythm within a few hours, they should notify the physician so they can undergo electrical cardioversion within the 48-hour window from the onset of atrial fibrillation.
Dronedarone, a new antiarrhythmic drug
Dronedarone (Multaq) is now available and has been shown to be effective in treating atrial fibrillation.14 It has a long half-life and a mechanism of action similar to that of amiodarone. However, it may be inferior to amiodarone in terms of efficacy.15 It is metabolized by CYP3A4. No dosage adjustment is needed for patients with renal insufficiency.
Because dronedarone lacks the iodine moiety found in amiodarone, it should not carry the same toxicity profile. No pulmonary or thyroid toxicity was reported in early trials.16
Nevertheless, dronedarone has several important limitations. It carries a black box warning stating it is contraindicated in patients with severe or recently decompensated heart failure, as the mortality rate was twice as high when this drug was used in such patients in initial studies.17 Additionally, there have been reports of hepatotoxicity requiring liver transplantation in a small number of patients. The extent of this problem and strategies for avoiding it are not yet defined as of the writing of this paper. As with any new medication, patients who are started on dronedarone should be observed closely for any side effects, and these should be reported to assist in the development of the drug’s safety profile.
Pulmonary vein isolation
In a procedure that can potentially cure atrial fibrillation, catheters are inserted into the left atrium and rings of scar tissue are created around the ostia of the pulmonary veins using radiofrequency energy, electrically isolating them from the rest of the left atrium.
Some debate exists as to whether this procedure may be reasonable as a first-line therapy for some patients with atrial fibrillation.18,19 It may be considered as an early treatment strategy in a small subset of patients, specifically young patients with symptomatic, recurrent atrial fibrillation, especially if they are averse to long-term antiarrhythmic therapy.
Because patients may still be more prone to atrial arrhythmias for several weeks to months after the procedure, they must be able to tolerate anticoagulation with warfarin for at least several months.
Rate control vs rhythm control
The choice between a rate control strategy or a rhythm control strategy in the long term is not always straightforward. While atrial fibrillation is clearly associated with higher morbidity and mortality rates, there are few data to date showing that restoring and maintaining sinus rhythm in patients with atrial fibrillation reduce the incidence of morbid complications or the likelihood of death.
Thus, current guidelines recommend a rate control strategy in patients who have no symptoms, and a rhythm control strategy if rate control cannot be achieved or if symptoms persist despite adequate control of the heart rate.7 The circumstances and preferences of the individual patient should carry weight in this decision.
Trials are under way that may shed more light on the relative benefits of rhythm control with ablation or antiarrhythmics and rate control.
PREVENTING THROMBOEMBOLIC EVENTS
Warfarin
In the short term, warfarin therapy may be dictated by plans to restore sinus rhythm. Patients need warfarin for at least 4 weeks after cardioversion unless it is performed within 48 hours of the onset of atrial fibrillation.
The CHADS2score (1 point each for congestive heart failure, hypertension, age 75 or older, and diabetes mellitus; 2 points for prior stroke or transient ischemic attack) is useful when deciding whether to give long-term anticoagulation.
For patients with a score of 0, the risk of stroke is lower than the risk of a major bleeding complication while on therapeutic warfarin.20,21 For these patients, aspirin 81 to 325 mg daily is recommended for stroke prophylaxis.
For those with a score of 2 or greater, the risk of stroke without warfarin is greater than the risk of a major bleeding complication with warfarin. These patients should receive warfarin with a goal INR of 2.0 to 3.0.7
Patients with a CHADS2 score of 1 present a dilemma, as their risk of stroke without warfarin is about the same as their risk of a major bleeding complication with warfarin. They can be managed with either warfarin or aspirin, according to the physician’s judgment.7 In these cases, factors such as hobbies or professions that might increase the risk of bleeding, perceived frequency of atrial fibrillation episodes, and even patient preconceptions about warfarin are often used when deciding between aspirin and warfarin.
Patients with a CHADS2 score of 2 or greater with a single episode of atrial fibrillation and a likely reversible cause may also pose a dilemma when deciding whether to start warfarin. These patients have demonstrated they at least have the substrate to maintain atrial fibrillation. This situation again calls for physician judgment. Bear in mind that asymptomatic recurrences are common in patients with atrial fibrillation.22,23 A higher CHADS2 score denotes a greater risk of stroke and may influence this decision. It is usually beneficial to enlist the patient in this decision-making process, as patients often have very strong opinions about tolerance of the risk of stroke or of warfarin therapy itself.
Another strategy is to start anticoagulation with warfarin and aggressively monitor for recurrences. If the patient has no recurrences of atrial fibrillation after 6 to 12 months and the reversible cause is resolved, one can then revisit the need for warfarin.
Role of aspirin and clopidogrel
Aspirin, alone or in conjunction with clopidogrel (Plavix), may provide an alternative for stroke prophylaxis in patients in whom warfarin is contraindicated. While inferior to warfarin, the combination of aspirin and clopidogrel has been shown to decrease the incidence of major thromboembolic events, especially stroke.24 However, the risk of a major bleeding complication was also significantly increased.
This combination may be a reasonable strategy in select patients with a CHADS2 score of 2 or greater in whom warfarin cannot be used for reasons such as personal aversion to the medication, side effects, or nonbleeding complications or in patients whose INR is exceedingly difficult to keep within the therapeutic range.
Dabigatran, a new anticoagulant
The newest option for anticoagulation in patients with atrial fibrillation is a direct thrombin inhibitor, dabigatran (Pradaxa).
In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial,25 dabigatran was studied head-to-head with warfarin. The doses of dabigatran studied were 110 mg and 150 mg twice a day. At 150 mg twice a day, patients on dabigatran had a lower rate of stroke than with warfarin (1.11% vs 1.69%, P < .001), as well as a lower rate of central nervous system bleeding (0.10% vs 0.38% with warfarin, P < .001). The rates of major bleeding were comparable in the patients receiving warfarin or dabigatran 150 mg twice a day, but the rate of gastrointestinal bleeding was higher in the dabigatran group (1.51% vs 1.02% with warfarin, P < .001).25
Dabigatran was recently approved by the US Food and Drug Administration for use in patients with atrial fibrillation. Doses of 150 mg and 75 mg are available.
Dabigatran is renally excreted, and the 150 mg twice-a-day dosing is intended for patients with a creatinine clearance greater than 30 mL/min. The 75-mg twice-a-day dosing is intended for patients with a creatinine clearance of 15 to 30 mL/min. However, it should be noted that currently there are no data to support the 75-mg twice-a-day dosing.
Dabigatran does have several advantages over warfarin. Patients do not need to avoid foods containing vitamin K, and routine serial monitoring does not appear to be needed. As with any new medication, patients who are started on dabigatran should be observed closely for any side effects, and these should be reported to assist in the development of the drug’s safety profile.
SPECIAL CIRCUMSTANCES
After cardiac or noncardiac surgery
Atrial fibrillation is common after open heart surgery, occurring in approximately 25% to 50% of patients.26–28
When this happens, at least one or two attempts are made to restore sinus rhythm. Especially in the early postoperative period, anticoagulation with heparin or warfarin may be contraindicated, so careful attention must be paid to the patient’s heart rhythm so that atrial fibrillation can be recognized quickly and cardioversion performed within a 48-hour window of onset. Beta-blockers, diltiazem, and verapamil are typically used for rate control.
When atrial fibrillation recurs in patients who have undergone open heart surgery, antiarrhythmics are started early to help prevent further recurrences. At our institution, we usually use amiodarone, as it is highly effective and well tolerated in the short term. When started on amiodarone for postoperative atrial fibrillation, patients are informed that the drug will be stopped after about 2 to 3 months. For patients who continue to have bouts of atrial fibrillation, the need for antiarrhythmic medications can be reassessed, and, if needed, the optimal antiarrhythmic medication for long-term therapy for the patient can be chosen.
Atrial fibrillation in severe, acute illness
Atrial fibrillation is common in the setting of extreme systemic stressors such as shock and sepsis and when the patient is being supported with inotropic agents. In this setting, patients may be in a high-catecholamine state, and both the heart rate and the heart rhythm may be very difficult to control.
Beta-blockers and nondihydropyridine calcium channel blockers should not be used when patients are on medications to support blood pressure, and in this setting, when the patient’s hemodynamic status permits the use of these agents, their effect may be minimal.
Amiodarone or perhaps digoxin may slow the heart rate somewhat without too much effect on the blood pressure. However, with amiodarone, one may have to accept a risk of chemical cardioversion.
Electrical cardioversion with or without the assistance of an antiarrhythmic drug may control the heart rate by restoring sinus rhythm. However, atrial fibrillation often recurs, and if it recurs quickly one may have to accept elevated heart rates until the underlying process is addressed.
Three general concerns dictate the management of atrial fibrillation:
- Controlling the heart rate
- Controlling symptoms
- Preventing thromboembolic events, including stroke.
When seeing a patient with newly diagnosed atrial fibrillation, these same three concerns should be kept in mind, but several additional issues must be addressed:
- Reversible causes of atrial fibrillation must be ruled out
- The true time of onset of the atrial fibrillation and the frequency of the episodes should be ascertained, if possible
- A careful estimation of the patient’s symptom burden should be made.
Atrial fibrillation is common and has a huge impact in terms of the morbidity, death, and costs associated with it. It affects more than 2.2 million Americans.1 Approximately 1 in 10 people over the age of 80 has atrial fibrillation, and for those over the age of 40, the lifetime risk of developing it is one in four.2 Framingham data suggest that the risk of death is approximately twice as high for patients with atrial fibrillation compared with a similar cohort without.3–5
IMPORTANT QUESTIONS DURING THE INITIAL WORKUP
Does the patient have a reversible cause of atrial fibrillation?
Atrial fibrillation is thought to be due to triggers that initiate it and to a myocardial substrate that supports it. While it may develop in the absence of other heart disease, it is often associated with hypertension, diabetes, obesity, structural heart disease (including congenital heart disease), obstructive sleep apnea, advanced age, and alcohol abuse.
Therefore, once atrial fibrillation has been diagnosed, the history, examination, and diagnostic workup should be directed toward looking for potentially reversible causes and for frequently associated comorbidities. Common reversible causes include:
Hyperthyroidism. The laboratory evaluation should include a thyrotropin (thyroid-stimulating hormone, or TSH) level.
Alcohol use, especially binge drinking.
Obstructive sleep apnea, if suspected on the basis of the history or the body habitus.
Structural heart disease such as valvular heart disease or congenital heart defects may also predispose to atrial fibrillation. Therefore, listen carefully to the heart and obtain a transthoracic echocardiogram if one has not already been done or if you suspect a change in valvular disease or systolic function since the most recent study.
How long has the patient been in atrial fibrillation?
The duration of the atrial fibrillation often affects the treatment strategy. Therefore, when the diagnosis has been made, it is important to try to estimate how long the patient has been in atrial fibrillation.
Often, we must settle for an estimate, as the patient’s recollection may be vague. However, in some cases, the symptoms are pronounced or electrocardiographic or telemetric data are available, allowing the time of onset to be clearly defined.
In addition, it is helpful to know if the patient has had prior episodes that were never brought to medical attention. To this end, elicit the patient’s spectrum of symptoms and encourage him or her to think back months or years and try to recall other times when similar symptoms might have occurred.
How do the symptoms affect the patient’s quality of life?
The clinician must also estimate the extent to which the symptoms affect the patient’s quality of life. This is best done when the heart rate is under control. If the patient still has significant symptoms despite adequate rate control, then a rhythm control strategy should probably be pursued.
MANAGING NEWLY DIAGNOSED ATRIAL FIBRILLATION
Control the heart rate with a beta-blocker, a calcium channel blocker, or digoxin
Many patients present during their first episode of atrial fibrillation with a rapid ventricular rate, especially if they are not already taking a drug to slow conduction through the atrioventricular node. If the symptoms are particularly profound, one should try to get the heart rate under control quickly.
Oral agents take time to be absorbed and are not always easy to titrate. Intravenous beta-blockers such as metoprolol (Lopressor) and labetalol (Normodyne, Trandate) or intravenous diltiazem (Cardizem) can slow the heart rate quickly and can be titrated. Once the heart rate is controlled, the oral form can be started, to allow weaning from the intravenous agent. In acute management, we seek a heart rate of less than about 100 to 110 beats per minute.
If the patient’s blood pressure is marginal, loading with intravenous digoxin may be considered. The dosage is 0.5 mg intravenously, then 0.25 mg intravenously in the first 6 hours and another 0.25 mg intravenously in another 6 hours. In patients with renal insufficiency the dosage should be less, or digoxin should be avoided altogether. Often, the blood pressure will improve once the heart rate is decreased, allowing other agents to be initiated. However, if the patient is frankly hypotensive with chest pain, shortness of breath, or a diminished level of consciousness, then emergency electrical cardioversion is indicated even if anticoagulation has not yet been started (more about anticoagulation below).
Oral forms of these same agents are the workhorses for heart rate control in the outpatient setting. Oral beta-blockers and nondihydropyridine calcium channel blockers (ie, diltiazem or verapamil [Calan, Verelan]) are the first-line agents, because when digoxin is used alone, it is relatively poor at controlling the heart rate, especially when the patient is not at rest.
The choice between these agents should be dictated by whether the patient has comorbidities such as coronary artery disease, heart failure, or reactive airway disease. Nondihydropyridine calcium channel blockers are relatively contraindicated in patients with heart failure, while beta-blockers can exacerbate reactive airway disease.6
It is also important to document that the heart rate is adequately controlled outside the hospital or outpatient clinic, where the patient is typically sitting or supine. This can be done with a 6-minute walk, exercise test, or Holter monitor once rate-controlling agents have been titrated.7
When to try to restore sinus rhythm
When atrial fibrillation is first diagnosed, it may not be possible to determine if it is paroxysmal (ie, self-terminating) or persistent. If the episode does not quickly end on its own, consideration may be given to restoring sinus rhythm.
Although experts debate the merits of a rate control approach vs a rhythm control approach for managing atrial fibrillation in the long term, many, including ourselves, recommend trying to restore sinus rhythm at least once when atrial fibrillation is first discovered. It is not always clear if atrial fibrillation is truly asymptomatic. Symptoms such as fatigue or decreased exercise tolerance can be subtle. Additionally, these symptoms may be attributed to other factors such as deconditioning, obesity, or advancing age. Thus, in many cases, only restoring normal sinus rhythm for a time allows the patient and clinician to fully assess the symptoms attributable to atrial fibrillation.
Therefore, in patients with newly diagnosed atrial fibrillation, an attempt to restore sinus rhythm is often warranted. Exceptions are in select patients who have no apparent symptoms and who are very old or are deemed too frail to tolerate cardioversion.
Direct-current cardioversion is typically the treatment of choice when attempting to restore sinus rhythm. The procedure can be done without anticoagulation within 48 hours of the onset of atrial fibrillation, if the time of onset is clear.7 However, clinicians must be careful in defining the onset of atrial fibrillation for this purpose.
Symptoms such as fatigue or shortness of breath can be vague in terms of the exact time of onset and often cannot be relied upon for the purpose of deciding whether cardioversion can be done without anticoagulation. When in doubt, it is best to err on the side of safety and assume that the atrial fibrillation has been going on for more than 48 hours.
If the time of onset is unclear or if more than 48 hours have passed, there are two general strategies for proceeding to electrical cardioversion.
One is to order transesophageal echocardiography and begin anticoagulation therapy at the same time. If there is no thrombus in the left atrium, then cardioversion can be done.8 Therapeutic anticoagulation with heparin, low-molecular-weight heparin, or warfarin (Coumadin) should be achieved within 24 to 48 hours of transesophageal echocardiography and cardioversion to minimize the risk of thromboembolic events, which can occur even after sinus rhythm has been restored.
At our institution, we typically strive to achieve therapeutic anticoagulation with either heparin or low-molecular-weight heparin before cardioversion in this scenario so as to avoid situations in which a patient may undergo cardioversion but then fail to achieve therapeutic anticoagulation for some time due to unforeseen factors.
The other approach is to start warfarin and maintain a goal international normalized ratio (INR) of 2 to 3 for 3 weeks, at which time cardioversion can be performed safely without transesophageal echocardiography.8
Regardless of which strategy is used, anticoagulation should be continued for at least 4 weeks after cardioversion,8 as atrial dysfunction and the risk of stroke may persist for days to weeks after normal sinus rhythm is restored.9
Role of antiarrhythmic drugs
Antiarrhythmic drugs can be used for chemical cardioversion or, more often, to help maintain sinus rhythm after direct-current cardioversion.
Because most of these drugs have at least a small chance of restoring normal sinus rhythm, we need to follow the same rules when starting them as when performing direct-current cardioversion. Patients should not be started on an antiarrhythmic medication until they have had adequate anticoagulation for at least 3 weeks or adequate anticoagulation and a transesophageal echocardiogram confirming that there is no thrombus in the left atrium.
Antiarrhythmic drugs should be started in select patients with newly diagnosed atrial fibrillation in whom a rhythm control strategy will be pursued. For patients whose history suggests a single episode, or episodes that previously self-terminated, an antiarrhythmic may not be necessary. For those with frequent episodes or whose history suggests ongoing atrial fibrillation for a long period, an antiarrhythmic will likely be required to provide a reasonable chance of achieving freedom from atrial fibrillation.
The choice of antiarrhythmic drug should be tailored to the specific patient.
Propafenone (Rythmol) and flecainide (Tambocor) are first-line drugs7 but are contraindicated in patients with coronary artery disease and significant structural heart disease.10
Sotalol (Betapace) and dofetilide (Tikosyn) can be used in patients with coronary artery disease. However, sotalol is contraindicated in patients with congestive heart failure, and dofetilide carries a long list of drug interactions. Both must be used with extreme caution in patients with renal insufficiency, and hospital admission is required for initiation or upward titration of the dose.
Amiodarone (Cordarone) is effective, and in the short term it is typically very well tolerated. However, it has a long half-life, and its potential for long-term toxicity often makes it a poor choice for long-term treatment. The toxicity of amiodarone increases with the cumulative dose. Therefore, this drug should be avoided for long-term therapy of atrial fibrillation in younger patients.
The ‘pill-in-the-pocket’ strategy
The “pill-in-the-pocket” strategy, in which patients are instructed to take their medication only when they have a bout of atrial fibrillation, is a reasonable option for patients with symptomatic recurrences of paroxysmal atrial fibrillation. This strategy is mainly reserved for patients who have relatively infrequent recurrences. Those who have frequent recurrences are usually more effectively treated with daily dosing of an antiarrhythmic. Flecainide and propafenone are the agents of choice for this approach because of their safety profile and efficacy in chemical cardioversion.
While this strategy may be started on an outpatient basis in patients with lone, paroxysmal atrial fibrillation, those with structural heart disease or conduction abnormalities should be observed in the hospital during initiation of therapy to observe for excessive PR prolongation or development of dangerous or worrisome arrhythmias.11–13
Additionally, these agents can decrease the refractory period of the atrioventricular node, thereby increasing the ventricular rate. In the case of atrial flutter, patients may be converted from variable or 2:1 response to a 1:1 conduction. Thus, one should consider also using a beta-blocker with this strategy.
Since the goal of this approach is to convert the patient to sinus rhythm within a few hours of the onset of atrial fibrillation, it may be implemented without the use of warfarin. Patients are instructed that if they do not convert to normal sinus rhythm within a few hours, they should notify the physician so they can undergo electrical cardioversion within the 48-hour window from the onset of atrial fibrillation.
Dronedarone, a new antiarrhythmic drug
Dronedarone (Multaq) is now available and has been shown to be effective in treating atrial fibrillation.14 It has a long half-life and a mechanism of action similar to that of amiodarone. However, it may be inferior to amiodarone in terms of efficacy.15 It is metabolized by CYP3A4. No dosage adjustment is needed for patients with renal insufficiency.
Because dronedarone lacks the iodine moiety found in amiodarone, it should not carry the same toxicity profile. No pulmonary or thyroid toxicity was reported in early trials.16
Nevertheless, dronedarone has several important limitations. It carries a black box warning stating it is contraindicated in patients with severe or recently decompensated heart failure, as the mortality rate was twice as high when this drug was used in such patients in initial studies.17 Additionally, there have been reports of hepatotoxicity requiring liver transplantation in a small number of patients. The extent of this problem and strategies for avoiding it are not yet defined as of the writing of this paper. As with any new medication, patients who are started on dronedarone should be observed closely for any side effects, and these should be reported to assist in the development of the drug’s safety profile.
Pulmonary vein isolation
In a procedure that can potentially cure atrial fibrillation, catheters are inserted into the left atrium and rings of scar tissue are created around the ostia of the pulmonary veins using radiofrequency energy, electrically isolating them from the rest of the left atrium.
Some debate exists as to whether this procedure may be reasonable as a first-line therapy for some patients with atrial fibrillation.18,19 It may be considered as an early treatment strategy in a small subset of patients, specifically young patients with symptomatic, recurrent atrial fibrillation, especially if they are averse to long-term antiarrhythmic therapy.
Because patients may still be more prone to atrial arrhythmias for several weeks to months after the procedure, they must be able to tolerate anticoagulation with warfarin for at least several months.
Rate control vs rhythm control
The choice between a rate control strategy or a rhythm control strategy in the long term is not always straightforward. While atrial fibrillation is clearly associated with higher morbidity and mortality rates, there are few data to date showing that restoring and maintaining sinus rhythm in patients with atrial fibrillation reduce the incidence of morbid complications or the likelihood of death.
Thus, current guidelines recommend a rate control strategy in patients who have no symptoms, and a rhythm control strategy if rate control cannot be achieved or if symptoms persist despite adequate control of the heart rate.7 The circumstances and preferences of the individual patient should carry weight in this decision.
Trials are under way that may shed more light on the relative benefits of rhythm control with ablation or antiarrhythmics and rate control.
PREVENTING THROMBOEMBOLIC EVENTS
Warfarin
In the short term, warfarin therapy may be dictated by plans to restore sinus rhythm. Patients need warfarin for at least 4 weeks after cardioversion unless it is performed within 48 hours of the onset of atrial fibrillation.
The CHADS2score (1 point each for congestive heart failure, hypertension, age 75 or older, and diabetes mellitus; 2 points for prior stroke or transient ischemic attack) is useful when deciding whether to give long-term anticoagulation.
For patients with a score of 0, the risk of stroke is lower than the risk of a major bleeding complication while on therapeutic warfarin.20,21 For these patients, aspirin 81 to 325 mg daily is recommended for stroke prophylaxis.
For those with a score of 2 or greater, the risk of stroke without warfarin is greater than the risk of a major bleeding complication with warfarin. These patients should receive warfarin with a goal INR of 2.0 to 3.0.7
Patients with a CHADS2 score of 1 present a dilemma, as their risk of stroke without warfarin is about the same as their risk of a major bleeding complication with warfarin. They can be managed with either warfarin or aspirin, according to the physician’s judgment.7 In these cases, factors such as hobbies or professions that might increase the risk of bleeding, perceived frequency of atrial fibrillation episodes, and even patient preconceptions about warfarin are often used when deciding between aspirin and warfarin.
Patients with a CHADS2 score of 2 or greater with a single episode of atrial fibrillation and a likely reversible cause may also pose a dilemma when deciding whether to start warfarin. These patients have demonstrated they at least have the substrate to maintain atrial fibrillation. This situation again calls for physician judgment. Bear in mind that asymptomatic recurrences are common in patients with atrial fibrillation.22,23 A higher CHADS2 score denotes a greater risk of stroke and may influence this decision. It is usually beneficial to enlist the patient in this decision-making process, as patients often have very strong opinions about tolerance of the risk of stroke or of warfarin therapy itself.
Another strategy is to start anticoagulation with warfarin and aggressively monitor for recurrences. If the patient has no recurrences of atrial fibrillation after 6 to 12 months and the reversible cause is resolved, one can then revisit the need for warfarin.
Role of aspirin and clopidogrel
Aspirin, alone or in conjunction with clopidogrel (Plavix), may provide an alternative for stroke prophylaxis in patients in whom warfarin is contraindicated. While inferior to warfarin, the combination of aspirin and clopidogrel has been shown to decrease the incidence of major thromboembolic events, especially stroke.24 However, the risk of a major bleeding complication was also significantly increased.
This combination may be a reasonable strategy in select patients with a CHADS2 score of 2 or greater in whom warfarin cannot be used for reasons such as personal aversion to the medication, side effects, or nonbleeding complications or in patients whose INR is exceedingly difficult to keep within the therapeutic range.
Dabigatran, a new anticoagulant
The newest option for anticoagulation in patients with atrial fibrillation is a direct thrombin inhibitor, dabigatran (Pradaxa).
In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial,25 dabigatran was studied head-to-head with warfarin. The doses of dabigatran studied were 110 mg and 150 mg twice a day. At 150 mg twice a day, patients on dabigatran had a lower rate of stroke than with warfarin (1.11% vs 1.69%, P < .001), as well as a lower rate of central nervous system bleeding (0.10% vs 0.38% with warfarin, P < .001). The rates of major bleeding were comparable in the patients receiving warfarin or dabigatran 150 mg twice a day, but the rate of gastrointestinal bleeding was higher in the dabigatran group (1.51% vs 1.02% with warfarin, P < .001).25
Dabigatran was recently approved by the US Food and Drug Administration for use in patients with atrial fibrillation. Doses of 150 mg and 75 mg are available.
Dabigatran is renally excreted, and the 150 mg twice-a-day dosing is intended for patients with a creatinine clearance greater than 30 mL/min. The 75-mg twice-a-day dosing is intended for patients with a creatinine clearance of 15 to 30 mL/min. However, it should be noted that currently there are no data to support the 75-mg twice-a-day dosing.
Dabigatran does have several advantages over warfarin. Patients do not need to avoid foods containing vitamin K, and routine serial monitoring does not appear to be needed. As with any new medication, patients who are started on dabigatran should be observed closely for any side effects, and these should be reported to assist in the development of the drug’s safety profile.
SPECIAL CIRCUMSTANCES
After cardiac or noncardiac surgery
Atrial fibrillation is common after open heart surgery, occurring in approximately 25% to 50% of patients.26–28
When this happens, at least one or two attempts are made to restore sinus rhythm. Especially in the early postoperative period, anticoagulation with heparin or warfarin may be contraindicated, so careful attention must be paid to the patient’s heart rhythm so that atrial fibrillation can be recognized quickly and cardioversion performed within a 48-hour window of onset. Beta-blockers, diltiazem, and verapamil are typically used for rate control.
When atrial fibrillation recurs in patients who have undergone open heart surgery, antiarrhythmics are started early to help prevent further recurrences. At our institution, we usually use amiodarone, as it is highly effective and well tolerated in the short term. When started on amiodarone for postoperative atrial fibrillation, patients are informed that the drug will be stopped after about 2 to 3 months. For patients who continue to have bouts of atrial fibrillation, the need for antiarrhythmic medications can be reassessed, and, if needed, the optimal antiarrhythmic medication for long-term therapy for the patient can be chosen.
Atrial fibrillation in severe, acute illness
Atrial fibrillation is common in the setting of extreme systemic stressors such as shock and sepsis and when the patient is being supported with inotropic agents. In this setting, patients may be in a high-catecholamine state, and both the heart rate and the heart rhythm may be very difficult to control.
Beta-blockers and nondihydropyridine calcium channel blockers should not be used when patients are on medications to support blood pressure, and in this setting, when the patient’s hemodynamic status permits the use of these agents, their effect may be minimal.
Amiodarone or perhaps digoxin may slow the heart rate somewhat without too much effect on the blood pressure. However, with amiodarone, one may have to accept a risk of chemical cardioversion.
Electrical cardioversion with or without the assistance of an antiarrhythmic drug may control the heart rate by restoring sinus rhythm. However, atrial fibrillation often recurs, and if it recurs quickly one may have to accept elevated heart rates until the underlying process is addressed.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285:2370–2375.
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004; 110:1042–1046.
- Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998; 98:946–952.
- Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 1998; 82( 8A):2N–9N.
- Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med 1982; 306:1018–1022.
- The Multicenter Diltiazem Postinfarction Trial Research Group. The effect of diltiazem on mortality and reinfarction after myocardial infarction. N Engl J Med 1988; 319:385–392.
- European heart Rhythm Association; Heart Rhythm society, Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J Am Coll Cardiol 2006; 48:854–906.
- Klein AL, Grimm RA, Murray RD, et al; Assessment of Cardioversion Using Transesophageal Echocardiography Investigators. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med 2001; 344:1411–1120.
- Grimm RA, Leung DY, Black IW, Stewart WJ, Thomas JD, Klein AL. Left atrial appendage “stunning” after spontaneous conversion of atrial fibrillation demonstrated by transesophageal Doppler echocardiography. Am Heart J 1995; 130:174–176.
- The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med 1989; 321:406–412.
- Alboni P, Tomasi C, Menozzi C, et al. Efficacy and safety of out-of-hospital self-administered single-dose oral drug treatment in the management of infrequent, well-tolerated paroxysmal supraventricular tachycardia. J Am Coll Cardiol 2001; 37:548–553.
- Capucci A, Villani GQ, Piepoli MF. Reproducible efficacy of loading oral propafenone in restoring sinus rhythm in patients with paroxysmal atrial fibrillation. Am J Cardiol 2003; 92:1345–1347.
- Khan IA. Single oral loading dose of propafenone for pharmacological cardioversion of recent-onset atrial fibrillation. J Am Coll Cardiol 2001; 37:542–547.
- Singh BN, Connolly SJ, Crijns HJ, et al; EURIDIS and ADONIS Investigators. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med 2007; 357:987–999.
- Le Heuzey J, De Ferrari GM, Radzik D, Santini M, Zhu J, Davy JM. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS study. J Cardiovasc Electrophysiol 2010; 21:597–605.
- Hohnloser SH, Crijns HJ, van Eickels M, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009; 360:668–678.
- Køber L, Torp-Pedersen C, McMurray JJ, et al; Dronedarone Study Group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med 2008; 358:2678–2687.
- Pappone C, Rosanio S, Augello G, et al. Mortality, morbidity, and quality of life after circumferential pulmonary vein ablation for atrial fibrillation: outcomes from a controlled nonrandomized long-term study. J Am Coll Cardiol 2003; 42:185–197.
- Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005; 293:2634–2640.
- van Walraven C, Hart RG, Singer DE, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient metaanalysis. JAMA 2002; 288:2441–2448.
- Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a metaanalysis. Ann Intern Med 1999; 131:492–501.
- Page RL, Wilkinson WE, Clair WK, McCarthy EA, Pritchett EL. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation 1994; 89:224–227.
- Savelieva I, Camm AJ. Clinical relevance of silent atrial fibrillation: prevalence, prognosis, quality of life, and management. J Intervent Card Electrophysiol 2000; 4:369–382.
- ACTIVE Investigators, Connolly SJ, Pogue J, Hart RG, et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med 2009; 360:2066–2078.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151. Erratum in: N Engl J Med 2010; 363:1877.
- Almassi GH, Schowalter T, Nicolosi AC, et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg 1997; 226:501–511.
- Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg 1993; 56:539–549.
- Mathew JP, Fontes ML, Tudor IC, et al; Investigators of the Ischemia Research and Education Foundation; Multicenter Study of Perioperative Ischemia Research Group. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA 2004; 291:1720–1729.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285:2370–2375.
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004; 110:1042–1046.
- Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998; 98:946–952.
- Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 1998; 82( 8A):2N–9N.
- Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med 1982; 306:1018–1022.
- The Multicenter Diltiazem Postinfarction Trial Research Group. The effect of diltiazem on mortality and reinfarction after myocardial infarction. N Engl J Med 1988; 319:385–392.
- European heart Rhythm Association; Heart Rhythm society, Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J Am Coll Cardiol 2006; 48:854–906.
- Klein AL, Grimm RA, Murray RD, et al; Assessment of Cardioversion Using Transesophageal Echocardiography Investigators. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med 2001; 344:1411–1120.
- Grimm RA, Leung DY, Black IW, Stewart WJ, Thomas JD, Klein AL. Left atrial appendage “stunning” after spontaneous conversion of atrial fibrillation demonstrated by transesophageal Doppler echocardiography. Am Heart J 1995; 130:174–176.
- The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med 1989; 321:406–412.
- Alboni P, Tomasi C, Menozzi C, et al. Efficacy and safety of out-of-hospital self-administered single-dose oral drug treatment in the management of infrequent, well-tolerated paroxysmal supraventricular tachycardia. J Am Coll Cardiol 2001; 37:548–553.
- Capucci A, Villani GQ, Piepoli MF. Reproducible efficacy of loading oral propafenone in restoring sinus rhythm in patients with paroxysmal atrial fibrillation. Am J Cardiol 2003; 92:1345–1347.
- Khan IA. Single oral loading dose of propafenone for pharmacological cardioversion of recent-onset atrial fibrillation. J Am Coll Cardiol 2001; 37:542–547.
- Singh BN, Connolly SJ, Crijns HJ, et al; EURIDIS and ADONIS Investigators. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med 2007; 357:987–999.
- Le Heuzey J, De Ferrari GM, Radzik D, Santini M, Zhu J, Davy JM. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS study. J Cardiovasc Electrophysiol 2010; 21:597–605.
- Hohnloser SH, Crijns HJ, van Eickels M, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009; 360:668–678.
- Køber L, Torp-Pedersen C, McMurray JJ, et al; Dronedarone Study Group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med 2008; 358:2678–2687.
- Pappone C, Rosanio S, Augello G, et al. Mortality, morbidity, and quality of life after circumferential pulmonary vein ablation for atrial fibrillation: outcomes from a controlled nonrandomized long-term study. J Am Coll Cardiol 2003; 42:185–197.
- Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005; 293:2634–2640.
- van Walraven C, Hart RG, Singer DE, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient metaanalysis. JAMA 2002; 288:2441–2448.
- Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a metaanalysis. Ann Intern Med 1999; 131:492–501.
- Page RL, Wilkinson WE, Clair WK, McCarthy EA, Pritchett EL. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation 1994; 89:224–227.
- Savelieva I, Camm AJ. Clinical relevance of silent atrial fibrillation: prevalence, prognosis, quality of life, and management. J Intervent Card Electrophysiol 2000; 4:369–382.
- ACTIVE Investigators, Connolly SJ, Pogue J, Hart RG, et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med 2009; 360:2066–2078.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151. Erratum in: N Engl J Med 2010; 363:1877.
- Almassi GH, Schowalter T, Nicolosi AC, et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg 1997; 226:501–511.
- Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg 1993; 56:539–549.
- Mathew JP, Fontes ML, Tudor IC, et al; Investigators of the Ischemia Research and Education Foundation; Multicenter Study of Perioperative Ischemia Research Group. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA 2004; 291:1720–1729.
KEY POINTS
- When atrial fibrillation is newly diagnosed, reversible causes and commonly associated processes should be sought.
- Agents to control the heart rate, eg, beta-blockers or nondihydropyridine calcium channel blockers, are often started and titrated intravenously and then changed to oral dosing.
- The benefit of rhythm control has not been firmly established. Although we try cardioversion at least once when atrial fibrillation is first diagnosed, rhythm control is generally reserved for patients whose symptoms persist despite rate control, or for patients in whom the heart rate cannot be controlled.
- The need for short-term or long-term anticoagulation must be estimated.
Endovascular Repair Can Treat Mesenteric Insufficiency
PALM BEACH, FLA. - Many patients with chronic mesenteric vascular insufficiency can be treated with an endovascular approach - either angioplasty or stenting with bare metal or covered devices.
Unlike open procedures, the endovascular approach carries a low risk of postoperative morbidity and mortality, Dr. Phillip Burns said at the annual meeting of the Southern Surgical Association. However, he noted, the recurrence rate for stenosis or occlusion was high in his retrospective series, with about one-third of patients requiring a second, or even third, intervention within the first 2 years after surgery.
Thus, an intensive follow-up is necessary for patients who undergo endovascular reconstruction. Under his protocol, patients return every 6 months for a clinical exam and duplex ultrasound, said Dr. Burns of the University of Tennessee, Chattanooga.
"Secondary procedures are often indicated for recurrent stenosis, and you have to look for this carefully," he said. Signs and symptoms might not be observed initially, "but if you continue to search for them, you will find them and be able to perform the reinterventions."
Dr. Burns and his colleagues presented a retrospective study of 107 patients with 127 vessels treated from 2004 to June 2010. All of the procedures were performed by a vascular specialist in an endovascular operating suite.
Patients were usually referred for gastrointestinal symptoms and already had undergone a GI workup. Most (88%) complained of abdominal pain; 55% had experienced weight loss and 29% reported nausea. All underwent an abdominal ultrasound that showed mesenteric vascular abnormalities.
The patients' average age was 59 years (range 18-90 years). Most (70%) were women. As a group, they displayed several important comorbidities, including hypertension, diabetes, coronary artery disease, and smoking.
Of the 127 vessels treated, 68 were superior mesenteric arteries, 52 were celiac arteries, and 7 were inferior mesenteric arteries. A balloon-expandable bare metal stent was most commonly used (66%; 87 patients) followed by balloon angioplasty (22%; 29), covered stent (10%; 14), and one self-expanding stent (1%; 1). All patients were put on either aspirin or clopidogrel therapy for at least 30 days after surgery. Patients also received continuing treatment for the hyperlipidemia that caused their atherosclerotic disease.
At 1 year, primary patency was seen in 67% of those with angioplasty, 54% of those with bare metal stents, and 100% of those with covered stents. The patency rates for the two stent types were significantly different.
More than half of the patients (57%; 55) experienced complete resolution of their symptoms, but 38% of these (21) required a second intervention. Of the 43% (41) who reported partial symptom relief, more than half (59%; 24) required a reintervention.
"That led to 45 patients going back for at least one other intervention," Dr. Burns said. "Five were not deemed treatable endovascularly and were done as open procedures. All others were done endovascularly, with 25 requiring a second reintervention and 8, a third reintervention."
In the reinterventions, 1-year patency was 86% with angioplasty, 97% with bare metal stents, and 100% with covered stents. There were 11 deaths in the cohort, 2 of which were due to chronic mesenteric vascular disease.
"In our opinion, both of those were in patients who did not follow up appropriately," said Dr. Christopher J. LeSar, also of the University of Tennessee, who closed the paper. "It's our belief that in treating this disease, the endovascular approach is reasonable if it's married to a very stringent follow-up protocol. Patients were counseled to be very wary of any recurrent symptoms and to act on them immediately if they occurred."
During the discussion, Dr. Eugene M. Langan III "facs" of the Greenville (S.C.) Hospital System University Medical Center, asked if the good outcomes associated with the covered stent are enough to recommend its use as the primary reconstruction method.
It's too early to make the assumption, said Dr. LeSar. The covered stents were only used in only 25 patients with an average follow-up of just 6 months. "This was related to the fact that we discovered [the covered stent success] later in our clinical experience," said Dr. LeSar. "In this population, one of the main problems was recurrent disease, and we found that only four patients with a covered stent required additional reinterventions, which led us to consider placing this type of stent as the primary answer for prevention of stenosis."
The crux of the issue is how to predict who can reap the biggest benefit from endovascular repair, leaving open surgery only to those who really require it, said Dr. Marc Mitchell of the University of Mississippi, Jackson. That seems to remain an unknown, said Dr. LeSar.
"We don't know why many develop hyperplasia, but some tend to and it seems to be random," he said.
Neither Dr. Burns nor Dr. LeSar reported any financial conflicts.
PALM BEACH, FLA. - Many patients with chronic mesenteric vascular insufficiency can be treated with an endovascular approach - either angioplasty or stenting with bare metal or covered devices.
Unlike open procedures, the endovascular approach carries a low risk of postoperative morbidity and mortality, Dr. Phillip Burns said at the annual meeting of the Southern Surgical Association. However, he noted, the recurrence rate for stenosis or occlusion was high in his retrospective series, with about one-third of patients requiring a second, or even third, intervention within the first 2 years after surgery.
Thus, an intensive follow-up is necessary for patients who undergo endovascular reconstruction. Under his protocol, patients return every 6 months for a clinical exam and duplex ultrasound, said Dr. Burns of the University of Tennessee, Chattanooga.
"Secondary procedures are often indicated for recurrent stenosis, and you have to look for this carefully," he said. Signs and symptoms might not be observed initially, "but if you continue to search for them, you will find them and be able to perform the reinterventions."
Dr. Burns and his colleagues presented a retrospective study of 107 patients with 127 vessels treated from 2004 to June 2010. All of the procedures were performed by a vascular specialist in an endovascular operating suite.
Patients were usually referred for gastrointestinal symptoms and already had undergone a GI workup. Most (88%) complained of abdominal pain; 55% had experienced weight loss and 29% reported nausea. All underwent an abdominal ultrasound that showed mesenteric vascular abnormalities.
The patients' average age was 59 years (range 18-90 years). Most (70%) were women. As a group, they displayed several important comorbidities, including hypertension, diabetes, coronary artery disease, and smoking.
Of the 127 vessels treated, 68 were superior mesenteric arteries, 52 were celiac arteries, and 7 were inferior mesenteric arteries. A balloon-expandable bare metal stent was most commonly used (66%; 87 patients) followed by balloon angioplasty (22%; 29), covered stent (10%; 14), and one self-expanding stent (1%; 1). All patients were put on either aspirin or clopidogrel therapy for at least 30 days after surgery. Patients also received continuing treatment for the hyperlipidemia that caused their atherosclerotic disease.
At 1 year, primary patency was seen in 67% of those with angioplasty, 54% of those with bare metal stents, and 100% of those with covered stents. The patency rates for the two stent types were significantly different.
More than half of the patients (57%; 55) experienced complete resolution of their symptoms, but 38% of these (21) required a second intervention. Of the 43% (41) who reported partial symptom relief, more than half (59%; 24) required a reintervention.
"That led to 45 patients going back for at least one other intervention," Dr. Burns said. "Five were not deemed treatable endovascularly and were done as open procedures. All others were done endovascularly, with 25 requiring a second reintervention and 8, a third reintervention."
In the reinterventions, 1-year patency was 86% with angioplasty, 97% with bare metal stents, and 100% with covered stents. There were 11 deaths in the cohort, 2 of which were due to chronic mesenteric vascular disease.
"In our opinion, both of those were in patients who did not follow up appropriately," said Dr. Christopher J. LeSar, also of the University of Tennessee, who closed the paper. "It's our belief that in treating this disease, the endovascular approach is reasonable if it's married to a very stringent follow-up protocol. Patients were counseled to be very wary of any recurrent symptoms and to act on them immediately if they occurred."
During the discussion, Dr. Eugene M. Langan III "facs" of the Greenville (S.C.) Hospital System University Medical Center, asked if the good outcomes associated with the covered stent are enough to recommend its use as the primary reconstruction method.
It's too early to make the assumption, said Dr. LeSar. The covered stents were only used in only 25 patients with an average follow-up of just 6 months. "This was related to the fact that we discovered [the covered stent success] later in our clinical experience," said Dr. LeSar. "In this population, one of the main problems was recurrent disease, and we found that only four patients with a covered stent required additional reinterventions, which led us to consider placing this type of stent as the primary answer for prevention of stenosis."
The crux of the issue is how to predict who can reap the biggest benefit from endovascular repair, leaving open surgery only to those who really require it, said Dr. Marc Mitchell of the University of Mississippi, Jackson. That seems to remain an unknown, said Dr. LeSar.
"We don't know why many develop hyperplasia, but some tend to and it seems to be random," he said.
Neither Dr. Burns nor Dr. LeSar reported any financial conflicts.
PALM BEACH, FLA. - Many patients with chronic mesenteric vascular insufficiency can be treated with an endovascular approach - either angioplasty or stenting with bare metal or covered devices.
Unlike open procedures, the endovascular approach carries a low risk of postoperative morbidity and mortality, Dr. Phillip Burns said at the annual meeting of the Southern Surgical Association. However, he noted, the recurrence rate for stenosis or occlusion was high in his retrospective series, with about one-third of patients requiring a second, or even third, intervention within the first 2 years after surgery.
Thus, an intensive follow-up is necessary for patients who undergo endovascular reconstruction. Under his protocol, patients return every 6 months for a clinical exam and duplex ultrasound, said Dr. Burns of the University of Tennessee, Chattanooga.
"Secondary procedures are often indicated for recurrent stenosis, and you have to look for this carefully," he said. Signs and symptoms might not be observed initially, "but if you continue to search for them, you will find them and be able to perform the reinterventions."
Dr. Burns and his colleagues presented a retrospective study of 107 patients with 127 vessels treated from 2004 to June 2010. All of the procedures were performed by a vascular specialist in an endovascular operating suite.
Patients were usually referred for gastrointestinal symptoms and already had undergone a GI workup. Most (88%) complained of abdominal pain; 55% had experienced weight loss and 29% reported nausea. All underwent an abdominal ultrasound that showed mesenteric vascular abnormalities.
The patients' average age was 59 years (range 18-90 years). Most (70%) were women. As a group, they displayed several important comorbidities, including hypertension, diabetes, coronary artery disease, and smoking.
Of the 127 vessels treated, 68 were superior mesenteric arteries, 52 were celiac arteries, and 7 were inferior mesenteric arteries. A balloon-expandable bare metal stent was most commonly used (66%; 87 patients) followed by balloon angioplasty (22%; 29), covered stent (10%; 14), and one self-expanding stent (1%; 1). All patients were put on either aspirin or clopidogrel therapy for at least 30 days after surgery. Patients also received continuing treatment for the hyperlipidemia that caused their atherosclerotic disease.
At 1 year, primary patency was seen in 67% of those with angioplasty, 54% of those with bare metal stents, and 100% of those with covered stents. The patency rates for the two stent types were significantly different.
More than half of the patients (57%; 55) experienced complete resolution of their symptoms, but 38% of these (21) required a second intervention. Of the 43% (41) who reported partial symptom relief, more than half (59%; 24) required a reintervention.
"That led to 45 patients going back for at least one other intervention," Dr. Burns said. "Five were not deemed treatable endovascularly and were done as open procedures. All others were done endovascularly, with 25 requiring a second reintervention and 8, a third reintervention."
In the reinterventions, 1-year patency was 86% with angioplasty, 97% with bare metal stents, and 100% with covered stents. There were 11 deaths in the cohort, 2 of which were due to chronic mesenteric vascular disease.
"In our opinion, both of those were in patients who did not follow up appropriately," said Dr. Christopher J. LeSar, also of the University of Tennessee, who closed the paper. "It's our belief that in treating this disease, the endovascular approach is reasonable if it's married to a very stringent follow-up protocol. Patients were counseled to be very wary of any recurrent symptoms and to act on them immediately if they occurred."
During the discussion, Dr. Eugene M. Langan III "facs" of the Greenville (S.C.) Hospital System University Medical Center, asked if the good outcomes associated with the covered stent are enough to recommend its use as the primary reconstruction method.
It's too early to make the assumption, said Dr. LeSar. The covered stents were only used in only 25 patients with an average follow-up of just 6 months. "This was related to the fact that we discovered [the covered stent success] later in our clinical experience," said Dr. LeSar. "In this population, one of the main problems was recurrent disease, and we found that only four patients with a covered stent required additional reinterventions, which led us to consider placing this type of stent as the primary answer for prevention of stenosis."
The crux of the issue is how to predict who can reap the biggest benefit from endovascular repair, leaving open surgery only to those who really require it, said Dr. Marc Mitchell of the University of Mississippi, Jackson. That seems to remain an unknown, said Dr. LeSar.
"We don't know why many develop hyperplasia, but some tend to and it seems to be random," he said.
Neither Dr. Burns nor Dr. LeSar reported any financial conflicts.
Man, 54, With Delusions and Seizures
A 54-year-old African-American man was brought by police officers to the emergency department (ED) after he called 911 several times to report seeing a Rottweiler looking into his second-story window. At the scene, the police were unable to confirm his story, thought the man seemed intoxicated, and brought him to the ED for evaluation.
The patient reported that he had been drinking the previous evening but denied current intoxication or illicit drug use. He denied experiencing symptoms of alcohol withdrawal.
Regarding his medical history, the patient admitted to having had seizures, including two episodes that he said required hospitalization. He described these episodes as right-hand “tingling” (paresthesias), accompanied by right-facial numbness and aphasia. The patient said his physician had instructed him to take “a few phenytoin pills” whenever these episodes occurred. He reported that the medication usually helped resolve his symptoms. He said he had taken phenytoin shortly before his current presentation.
According to friends of the patient who were questioned, he had had noticeable memory problems during the previous six to eight months. They said that he often told the same joke, day after day. His speech had become increasingly slurred, even when he was not drinking.
Once the patient’s medical records were retrieved, it was revealed that he had been hospitalized twice for witnessed grand mal seizures about six months before his current admission; he had been drinking alcohol prior to both episodes. He underwent electroencephalography (EEG) during one of these hospitalizations, with results reported as normal. On both occasions, the patient was discharged with phenytoin and was instructed to follow up with his primary care provider and neurologist.
The patient, who reported working in customer service, had no known allergies. He claimed to drink one or two 40-ounce beers twice per week and admitted to occasional cocaine use. Of significance in his family history was a fatal MI in his mother. Although the patient denied any history of rashes or lesions, his current delirium made it impossible to obtain a reliable sexual history; a friend who was questioned, however, described the patient as promiscuous.
On initial physical examination, the man was afebrile, tachycardic, and somewhat combative with the ED staff. He was fully oriented to self but only partially to place and time.
His right pupil was 3+ and his left pupil was 2+, with neither reactive to light. He spoke with tangential speech and his gait was unsteady, but no other significant abnormalities were noted. A full assessment revealed no rashes or other lesions.
Significant laboratory findings included a low level of phenytoin, a negative blood alcohol level, presence of cocaine on urine drug screening, and normal levels of thyroid-stimulating hormone (TSH), vitamin B12, and folate. The patient’s serum VDRL (venereal disease research laboratory) titer was positive at 1:256.
Electroencephalography showed diffuse slowing, and brain CT performed in the ED showed atrophy that was mild but appropriate for a person of the patient’s age, with no evidence of a cerebrovascular accident (CVA). Aneurysm was ruled out by CT angiography of the brain. MRI revealed persistent increased signal in the subarachnoid space.
The patient was admitted with an initial diagnosis of paranoid delusional psychosis and monitored for alcohol withdrawal. He was given lorazepam as needed for agitation. Consultations were arranged with the psychiatry service regarding his delusions, and with neurology to determine whether to continue phenytoin.
The patient showed little response during the next several days. Based on positive results on serum VDRL with high titer, the presence of Argyll-Robertson pupils on exam, and his history of dementia-like symptoms, a lumbar puncture was performed to rule out neurosyphilis. In the patient’s cerebral spinal fluid (CSF) analysis, the first tube was clear and colorless, with 72 cells (28% neutrophils, 59% lymphocytes); glucose, 64 mg/dL; and total protein, 117 mg/dL. The fourth tube had 34 cells (17% neutrophils, 65% lymphocytes) and a positive VDRL titer at 1:128. Results from a serum syphilis immunoglobulin G (IgG) test were positive, and HIV antibody testing was nonreactive, confirming the diagnosis of neurosyphilis.
The hospital’s infectious disease (ID) team recommended treatment with IV penicillin for 14 days. Once this was completed, the patient was discharged with instructions to follow up at the ID clinic in three months for a repeat CSF VDRL titer to monitor for resolution of the disease. His prescription for phenytoin was discontinued.
At the time of discharge, it was noted that the patient showed no evidence of having regained cognitive function. He was deemed by the psychiatry service to lack decision-making capacity—a likely sequelae of untreated neurosyphilis of unknown duration.
He did return to the ID clinic six months after his discharge. At that visit, a VDRL serum titer was drawn with a result of 1:64, a decrease from 1:128. His syphilis IgG remained positive, however.
Discussion
Definition and Epidemiology
Syphilis is commonly known as a sexually transmitted disease with primary, secondary, and tertiary (early and late latent) stages.1 Neurosyphilis is defined as a manifestation of the inflammatory response to invasion over decades by the Treponema pallidum spirochete in the CSF as a result of untreated primary and/or secondary syphilis.2 About one in 10 patients with untreated syphilis will experience neurologic involvement.3,4 Before 2005, neurosyphilis was required to be reported as a specific stage of syphilis (ie, a manifestation of tertiary syphilis4), but now should be reported as syphilis with neurologic manifestations.5
A reportable infectious disease, syphilis was widespread until the advent of penicillin. According to CDC statistics,6 the number of reported cases of primary and secondary syphilis has declined steadily since 1943. In the late 1970s and early 1980s, the number of tertiary cases also began to plateau, likely as a result of earlier diagnosis and more widespread use of penicillin. Recent case reports suggest greater prevalence of syphilis among men than women and increased incidence among men who have sex with men.7
Pathogenesis
Syphilis is most commonly spread by sexual contact or contact with an infected primary lesion (chancre). Less likely routes of transmission are placental passage or blood transfusion. Infectivity is greatest in the early disease stages.8
Primary syphilis is marked by transmission of the spirochete, ending with development of secondary syphilis (usually two to 12 weeks after transmission). A chancre commonly develops but is often missed by patients because it is painless and can heal spontaneously.7 The chancre is also often confused with two other sources of genital lesions, herpes simplex (genital herpes) and Haemophilus ducreyi (chancroid). In two-thirds of cases of untreated primary syphilis, the infection clears spontaneously, but in the remaining one-third, the disease progresses.8
Secondary syphilis, with or without presence of a chancre, manifests with constitutional symptoms, including lymphadenopathy, fever, headache, and malaise. Patients in this disease phase may also present with a generalized, nonpruritic, macular to maculopapular or pustular rash. The rash can affect the skin of the trunk, the proximal extremities, and the palms and soles. Ocular involvement may occur, especially in patients who are coinfected with HIV.8 In either primary or secondary syphilis, infection can invade the central nervous system.1
During latent syphilis, patients show serologic conversion without overt symptoms. Early latent syphilis is defined as infection within the previous year, as demonstrated by conversion from negative to positive testing, or an increase in titers within the previous year. Any case occurring after one year is defined as late or unknown latent syphilis.8
Tertiary syphilis is marked by complications resulting from untreated syphilis; affected patients commonly experience central nervous system and cardiovascular involvement. Gummatous disease is seen in 15% of patients.1
The early stages of neurosyphilis may be asymptomatic, acute meningeal, and meningovascular.1,4,8,9 Only 5% of patients with early neurosyphilis are symptomatic, with the added potential for cranial neuritis or ocular involvement.1 The late stages of neurosyphilis are detailed in the table.1,4,8
Diagnosis
A diagnosis of syphilis is made by testing blood samples or scrapings from a lesion. In patients with suspected syphilis, rapid plasma reagin (RPR) testing or a VDRL titer is commonly ordered. When results are positive, a serum treponemal test is recommended to confirm a diagnosis of syphilis. Options include the fluorescent treponemal antibody absorption test (FTA-ABS) and the microhemagglutinin assay for antibody to T pallidum (MHA-TP).5
If neurologic symptoms are present, a CSF sample should be obtained, followed by the same testing. A confirmed diagnosis of neurosyphilis is defined by the CDC as syphilis at any stage that meets laboratory criteria for neurosyphilis5; these include increased CSF protein or an elevated CSF leukocyte count with no other known cause, and clinical signs or symptoms without other known causes.7
Treatment
Treatment of syphilis generally consists of penicillin, administered intramuscularly (IM) or IV, depending on the stage. According to 2006 guidelines from the CDC,10,11 treatment for adults with primary and secondary syphilis is a single dose of IM penicillin G, 2.4 million units. If neurosyphilis is suspected, recommended treatment is IV penicillin G, 18 to 24 million units per day divided into six doses (ie, 3 to 4 million units every four hours) or continuous pump infusion for 10 to 14 days.10-12 Follow-up is recommended by monitoring CSF titers to ensure clearance of infection; retreatment may be required if CSF abnormalities persist after two years.11
Patients with a penicillin allergy should undergo desensitization, as penicillin is the preferred agent; the potential exists for cross-reactivity with ceftriaxone, a possible alternative for patients with neurosyphilis.11 All patients diagnosed with syphilis should also be tested for HIV and other sexually transmitted diseases.10-12
The prognosis of patients treated for neurosyphilis is generally good if the condition is diagnosed and treated early. In patients with cerebral atrophy, frontal lesions, dementia, or tabes dorsalis, the potential for recovery decreases.2,13,14
Teaching Points
There are several teaching points to take away from this case:
• Remember to rule out a CVA in any patient who presents with numbness, paresthesias, or slurred speech. In this case, a brain CT and CT angiography of the brain were both obtained in the ED before the patient was admitted. They both yielded negative results; because the patient’s history was consistent with alcohol and drug use and he had a history of seizures, he was monitored closely for signs of withdrawal or further seizure.
• Phenytoin is an antiepileptic agent whose use requires proper patient education and drug level monitoring. Appropriate follow-up must be ensured before phenytoin therapy is begun, as toxicity can result in nystagmus, ataxia, slurred speech, decreased coordination, mental confusion, and possibly death.15,16
• For patients with a suspected acute change in mental status, a workup is required and should be tailored appropriately, based on findings. This should include, but not be limited to, a thorough history and physical exam, CT of the brain (to rule out an acute brain injury17), and, if warranted, MRI of the brain. Also, a urine drug screen and alcohol level, a complete blood count, a TSH level (to evaluate for altered thyroid function that may explain mental status changes), comprehensive panel, RPR testing and/or a VDRL titer should be obtained, depending on the facility’s protocol18,19; at some facilities, a treponemal test, rather than VDRL, is being obtained at the outset.20 Levels of vitamin B12 (as part of the dementia workup), folate, thiamine, and ammonia (in patients with suspected liver disease) can also be obtained in patients with change in mental status.18,19 Urinalysis should not be overlooked to check for a urinary tract infection, especially in elderly patients.21
• If primary syphilis is suspected, treatment must be undertaken.20
Conclusion
Despite the decline seen since the 1940s in cases of primary and secondary syphilis, and the effectiveness of penicillin in treating the infection early, patients with late-stage syphilis, including those with neurosyphilis, may still present to the emergency care, urgent care, or primary care setting. Immediate treatment with penicillin is recommended to achieve an optimal prognosis for the affected patient.
1. Golden MR, Marra CM, Holmes KK. Update on syphilis: resurgence of an old problem. JAMA. 2003;290(11):1510-1514.
2. Simon RP. Chapter 20. Neurosyphilis. In: Klausner JD, Hook EW III, eds. Current Diagnosis & Treatment of Sexually Transmitted Diseases. USA: The McGraw-Hill Companies; 2007:130-137.
3. Sanchez FM, Zisselman MH. Treatment of psychiatric symptoms associated with neurosyphilis. Psychosomatics. 2007;48:440-445.
4. Marra CM. Neurosyphilis. Curr Neurol Neurosci Rep. 2004;4(6):435-440.
5. CDC. Sexually transmitted diseases surveillance, 2007: STD surveillance case definitions. www.cdc.gov/std/stats07/app-casedef.htm. Accessed March 23, 2011.
6. CDC. 2008 Sexually Transmitted Diseases Surveillance: Table 1. Cases of sexually transmitted diseases reported by state health departments and rates per 100,000 population: United States, 1941-2008. www.cdc.gov/std/stats08/tables/1.htm. Accessed March 23, 2011.
7. CDC. Sexually transmitted diseases (STDs): Syphilis: CDC fact sheet. www.cdc.gov/std/syphilis/STDfact-syphilis.htm. Accessed March 23, 2011.
8. Tramont EC. Chapter 238. Treponema pallidum (syphilis). In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia: Elsevier Churchill Livingstone; 2009.
9. Ghanem KG. Neurosyphilis: a historical perspective and review. CNS Neurosci Ther. 2010; 16(5):e157-e168.
10. Workowski KA, Berman SM; CDC. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
11. CDC. Sexually transmitted diseases: treatment guidelines 2006. www.cdc.gov/std/treatment/2006/genital-ulcers.htm#genulc6. Accessed March 29, 2011.
12. Drugs for sexually transmitted infections. Treatment Guidelines from the Medical Letter. 2010;95:95a. http://secure.medicalletter.org. Accessed March 23, 2011.
13. Russouw HG, Roberts MC, Emsley RA, et al. Psychiatric manifestations and magnetic resonance imaging in HIV-negative neurosyphilis. Biol Psychiatry. 1997;41(4):467-473.
14. Hooshmand H, Escobar MR, Kopf SW. Neurosyphylis: a study of 241 patients. JAMA. 1972;219 (6):726-729.
15. Miller CA, Joyce DM. Toxicity, phenytoin. http://emedicine.medscape.com/article/816447-overview. Accessed March 23, 2011.
16. Earnest MP, Marx JA, Drury LR. Complications of intravenous phenytoin for acute treatment of seizures: recommendations for usage. JAMA. 1983; 246(6):762-765.
17. Geschwind MD, Shu H, Haman A, et al. Rapidly progressive dementia. Ann Neurol. 2008;64(1): 97-108.
18. Mechem CC. Chapter 143. Altered mental status and coma. In: Ma J, Cline DM, Tintinalli JE, et al, eds. Emergency Medicine Manual, 6e. www.access emergencymedicine.com/content.aspx?aID=2020. Accessed March 23, 2011.
19. Knopman DS, DeKosky ST, Cummings JL, et al; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: diagnosis of dementia (an evidence-based review). Neurology. 2001;56(9):1143-1153.
20. CDC. Syphilis testing algorithms using treponemal tests for initial screening—four laboratories, New York City, 2005-2006. MMWR Morb Mortal Wkly Rep. 2008;57(32):872-875.
21. Anderson CA, Filley CM. Chapter 33. Behavioral presentations of medical and neurologic disorders. In: Jacobson JL, Jacobson AM, eds. Psychiatric Secrets. 2nd ed. St. Louis, MO: Hanley & Belfus; 2001.
A 54-year-old African-American man was brought by police officers to the emergency department (ED) after he called 911 several times to report seeing a Rottweiler looking into his second-story window. At the scene, the police were unable to confirm his story, thought the man seemed intoxicated, and brought him to the ED for evaluation.
The patient reported that he had been drinking the previous evening but denied current intoxication or illicit drug use. He denied experiencing symptoms of alcohol withdrawal.
Regarding his medical history, the patient admitted to having had seizures, including two episodes that he said required hospitalization. He described these episodes as right-hand “tingling” (paresthesias), accompanied by right-facial numbness and aphasia. The patient said his physician had instructed him to take “a few phenytoin pills” whenever these episodes occurred. He reported that the medication usually helped resolve his symptoms. He said he had taken phenytoin shortly before his current presentation.
According to friends of the patient who were questioned, he had had noticeable memory problems during the previous six to eight months. They said that he often told the same joke, day after day. His speech had become increasingly slurred, even when he was not drinking.
Once the patient’s medical records were retrieved, it was revealed that he had been hospitalized twice for witnessed grand mal seizures about six months before his current admission; he had been drinking alcohol prior to both episodes. He underwent electroencephalography (EEG) during one of these hospitalizations, with results reported as normal. On both occasions, the patient was discharged with phenytoin and was instructed to follow up with his primary care provider and neurologist.
The patient, who reported working in customer service, had no known allergies. He claimed to drink one or two 40-ounce beers twice per week and admitted to occasional cocaine use. Of significance in his family history was a fatal MI in his mother. Although the patient denied any history of rashes or lesions, his current delirium made it impossible to obtain a reliable sexual history; a friend who was questioned, however, described the patient as promiscuous.
On initial physical examination, the man was afebrile, tachycardic, and somewhat combative with the ED staff. He was fully oriented to self but only partially to place and time.
His right pupil was 3+ and his left pupil was 2+, with neither reactive to light. He spoke with tangential speech and his gait was unsteady, but no other significant abnormalities were noted. A full assessment revealed no rashes or other lesions.
Significant laboratory findings included a low level of phenytoin, a negative blood alcohol level, presence of cocaine on urine drug screening, and normal levels of thyroid-stimulating hormone (TSH), vitamin B12, and folate. The patient’s serum VDRL (venereal disease research laboratory) titer was positive at 1:256.
Electroencephalography showed diffuse slowing, and brain CT performed in the ED showed atrophy that was mild but appropriate for a person of the patient’s age, with no evidence of a cerebrovascular accident (CVA). Aneurysm was ruled out by CT angiography of the brain. MRI revealed persistent increased signal in the subarachnoid space.
The patient was admitted with an initial diagnosis of paranoid delusional psychosis and monitored for alcohol withdrawal. He was given lorazepam as needed for agitation. Consultations were arranged with the psychiatry service regarding his delusions, and with neurology to determine whether to continue phenytoin.
The patient showed little response during the next several days. Based on positive results on serum VDRL with high titer, the presence of Argyll-Robertson pupils on exam, and his history of dementia-like symptoms, a lumbar puncture was performed to rule out neurosyphilis. In the patient’s cerebral spinal fluid (CSF) analysis, the first tube was clear and colorless, with 72 cells (28% neutrophils, 59% lymphocytes); glucose, 64 mg/dL; and total protein, 117 mg/dL. The fourth tube had 34 cells (17% neutrophils, 65% lymphocytes) and a positive VDRL titer at 1:128. Results from a serum syphilis immunoglobulin G (IgG) test were positive, and HIV antibody testing was nonreactive, confirming the diagnosis of neurosyphilis.
The hospital’s infectious disease (ID) team recommended treatment with IV penicillin for 14 days. Once this was completed, the patient was discharged with instructions to follow up at the ID clinic in three months for a repeat CSF VDRL titer to monitor for resolution of the disease. His prescription for phenytoin was discontinued.
At the time of discharge, it was noted that the patient showed no evidence of having regained cognitive function. He was deemed by the psychiatry service to lack decision-making capacity—a likely sequelae of untreated neurosyphilis of unknown duration.
He did return to the ID clinic six months after his discharge. At that visit, a VDRL serum titer was drawn with a result of 1:64, a decrease from 1:128. His syphilis IgG remained positive, however.
Discussion
Definition and Epidemiology
Syphilis is commonly known as a sexually transmitted disease with primary, secondary, and tertiary (early and late latent) stages.1 Neurosyphilis is defined as a manifestation of the inflammatory response to invasion over decades by the Treponema pallidum spirochete in the CSF as a result of untreated primary and/or secondary syphilis.2 About one in 10 patients with untreated syphilis will experience neurologic involvement.3,4 Before 2005, neurosyphilis was required to be reported as a specific stage of syphilis (ie, a manifestation of tertiary syphilis4), but now should be reported as syphilis with neurologic manifestations.5
A reportable infectious disease, syphilis was widespread until the advent of penicillin. According to CDC statistics,6 the number of reported cases of primary and secondary syphilis has declined steadily since 1943. In the late 1970s and early 1980s, the number of tertiary cases also began to plateau, likely as a result of earlier diagnosis and more widespread use of penicillin. Recent case reports suggest greater prevalence of syphilis among men than women and increased incidence among men who have sex with men.7
Pathogenesis
Syphilis is most commonly spread by sexual contact or contact with an infected primary lesion (chancre). Less likely routes of transmission are placental passage or blood transfusion. Infectivity is greatest in the early disease stages.8
Primary syphilis is marked by transmission of the spirochete, ending with development of secondary syphilis (usually two to 12 weeks after transmission). A chancre commonly develops but is often missed by patients because it is painless and can heal spontaneously.7 The chancre is also often confused with two other sources of genital lesions, herpes simplex (genital herpes) and Haemophilus ducreyi (chancroid). In two-thirds of cases of untreated primary syphilis, the infection clears spontaneously, but in the remaining one-third, the disease progresses.8
Secondary syphilis, with or without presence of a chancre, manifests with constitutional symptoms, including lymphadenopathy, fever, headache, and malaise. Patients in this disease phase may also present with a generalized, nonpruritic, macular to maculopapular or pustular rash. The rash can affect the skin of the trunk, the proximal extremities, and the palms and soles. Ocular involvement may occur, especially in patients who are coinfected with HIV.8 In either primary or secondary syphilis, infection can invade the central nervous system.1
During latent syphilis, patients show serologic conversion without overt symptoms. Early latent syphilis is defined as infection within the previous year, as demonstrated by conversion from negative to positive testing, or an increase in titers within the previous year. Any case occurring after one year is defined as late or unknown latent syphilis.8
Tertiary syphilis is marked by complications resulting from untreated syphilis; affected patients commonly experience central nervous system and cardiovascular involvement. Gummatous disease is seen in 15% of patients.1
The early stages of neurosyphilis may be asymptomatic, acute meningeal, and meningovascular.1,4,8,9 Only 5% of patients with early neurosyphilis are symptomatic, with the added potential for cranial neuritis or ocular involvement.1 The late stages of neurosyphilis are detailed in the table.1,4,8
Diagnosis
A diagnosis of syphilis is made by testing blood samples or scrapings from a lesion. In patients with suspected syphilis, rapid plasma reagin (RPR) testing or a VDRL titer is commonly ordered. When results are positive, a serum treponemal test is recommended to confirm a diagnosis of syphilis. Options include the fluorescent treponemal antibody absorption test (FTA-ABS) and the microhemagglutinin assay for antibody to T pallidum (MHA-TP).5
If neurologic symptoms are present, a CSF sample should be obtained, followed by the same testing. A confirmed diagnosis of neurosyphilis is defined by the CDC as syphilis at any stage that meets laboratory criteria for neurosyphilis5; these include increased CSF protein or an elevated CSF leukocyte count with no other known cause, and clinical signs or symptoms without other known causes.7
Treatment
Treatment of syphilis generally consists of penicillin, administered intramuscularly (IM) or IV, depending on the stage. According to 2006 guidelines from the CDC,10,11 treatment for adults with primary and secondary syphilis is a single dose of IM penicillin G, 2.4 million units. If neurosyphilis is suspected, recommended treatment is IV penicillin G, 18 to 24 million units per day divided into six doses (ie, 3 to 4 million units every four hours) or continuous pump infusion for 10 to 14 days.10-12 Follow-up is recommended by monitoring CSF titers to ensure clearance of infection; retreatment may be required if CSF abnormalities persist after two years.11
Patients with a penicillin allergy should undergo desensitization, as penicillin is the preferred agent; the potential exists for cross-reactivity with ceftriaxone, a possible alternative for patients with neurosyphilis.11 All patients diagnosed with syphilis should also be tested for HIV and other sexually transmitted diseases.10-12
The prognosis of patients treated for neurosyphilis is generally good if the condition is diagnosed and treated early. In patients with cerebral atrophy, frontal lesions, dementia, or tabes dorsalis, the potential for recovery decreases.2,13,14
Teaching Points
There are several teaching points to take away from this case:
• Remember to rule out a CVA in any patient who presents with numbness, paresthesias, or slurred speech. In this case, a brain CT and CT angiography of the brain were both obtained in the ED before the patient was admitted. They both yielded negative results; because the patient’s history was consistent with alcohol and drug use and he had a history of seizures, he was monitored closely for signs of withdrawal or further seizure.
• Phenytoin is an antiepileptic agent whose use requires proper patient education and drug level monitoring. Appropriate follow-up must be ensured before phenytoin therapy is begun, as toxicity can result in nystagmus, ataxia, slurred speech, decreased coordination, mental confusion, and possibly death.15,16
• For patients with a suspected acute change in mental status, a workup is required and should be tailored appropriately, based on findings. This should include, but not be limited to, a thorough history and physical exam, CT of the brain (to rule out an acute brain injury17), and, if warranted, MRI of the brain. Also, a urine drug screen and alcohol level, a complete blood count, a TSH level (to evaluate for altered thyroid function that may explain mental status changes), comprehensive panel, RPR testing and/or a VDRL titer should be obtained, depending on the facility’s protocol18,19; at some facilities, a treponemal test, rather than VDRL, is being obtained at the outset.20 Levels of vitamin B12 (as part of the dementia workup), folate, thiamine, and ammonia (in patients with suspected liver disease) can also be obtained in patients with change in mental status.18,19 Urinalysis should not be overlooked to check for a urinary tract infection, especially in elderly patients.21
• If primary syphilis is suspected, treatment must be undertaken.20
Conclusion
Despite the decline seen since the 1940s in cases of primary and secondary syphilis, and the effectiveness of penicillin in treating the infection early, patients with late-stage syphilis, including those with neurosyphilis, may still present to the emergency care, urgent care, or primary care setting. Immediate treatment with penicillin is recommended to achieve an optimal prognosis for the affected patient.
A 54-year-old African-American man was brought by police officers to the emergency department (ED) after he called 911 several times to report seeing a Rottweiler looking into his second-story window. At the scene, the police were unable to confirm his story, thought the man seemed intoxicated, and brought him to the ED for evaluation.
The patient reported that he had been drinking the previous evening but denied current intoxication or illicit drug use. He denied experiencing symptoms of alcohol withdrawal.
Regarding his medical history, the patient admitted to having had seizures, including two episodes that he said required hospitalization. He described these episodes as right-hand “tingling” (paresthesias), accompanied by right-facial numbness and aphasia. The patient said his physician had instructed him to take “a few phenytoin pills” whenever these episodes occurred. He reported that the medication usually helped resolve his symptoms. He said he had taken phenytoin shortly before his current presentation.
According to friends of the patient who were questioned, he had had noticeable memory problems during the previous six to eight months. They said that he often told the same joke, day after day. His speech had become increasingly slurred, even when he was not drinking.
Once the patient’s medical records were retrieved, it was revealed that he had been hospitalized twice for witnessed grand mal seizures about six months before his current admission; he had been drinking alcohol prior to both episodes. He underwent electroencephalography (EEG) during one of these hospitalizations, with results reported as normal. On both occasions, the patient was discharged with phenytoin and was instructed to follow up with his primary care provider and neurologist.
The patient, who reported working in customer service, had no known allergies. He claimed to drink one or two 40-ounce beers twice per week and admitted to occasional cocaine use. Of significance in his family history was a fatal MI in his mother. Although the patient denied any history of rashes or lesions, his current delirium made it impossible to obtain a reliable sexual history; a friend who was questioned, however, described the patient as promiscuous.
On initial physical examination, the man was afebrile, tachycardic, and somewhat combative with the ED staff. He was fully oriented to self but only partially to place and time.
His right pupil was 3+ and his left pupil was 2+, with neither reactive to light. He spoke with tangential speech and his gait was unsteady, but no other significant abnormalities were noted. A full assessment revealed no rashes or other lesions.
Significant laboratory findings included a low level of phenytoin, a negative blood alcohol level, presence of cocaine on urine drug screening, and normal levels of thyroid-stimulating hormone (TSH), vitamin B12, and folate. The patient’s serum VDRL (venereal disease research laboratory) titer was positive at 1:256.
Electroencephalography showed diffuse slowing, and brain CT performed in the ED showed atrophy that was mild but appropriate for a person of the patient’s age, with no evidence of a cerebrovascular accident (CVA). Aneurysm was ruled out by CT angiography of the brain. MRI revealed persistent increased signal in the subarachnoid space.
The patient was admitted with an initial diagnosis of paranoid delusional psychosis and monitored for alcohol withdrawal. He was given lorazepam as needed for agitation. Consultations were arranged with the psychiatry service regarding his delusions, and with neurology to determine whether to continue phenytoin.
The patient showed little response during the next several days. Based on positive results on serum VDRL with high titer, the presence of Argyll-Robertson pupils on exam, and his history of dementia-like symptoms, a lumbar puncture was performed to rule out neurosyphilis. In the patient’s cerebral spinal fluid (CSF) analysis, the first tube was clear and colorless, with 72 cells (28% neutrophils, 59% lymphocytes); glucose, 64 mg/dL; and total protein, 117 mg/dL. The fourth tube had 34 cells (17% neutrophils, 65% lymphocytes) and a positive VDRL titer at 1:128. Results from a serum syphilis immunoglobulin G (IgG) test were positive, and HIV antibody testing was nonreactive, confirming the diagnosis of neurosyphilis.
The hospital’s infectious disease (ID) team recommended treatment with IV penicillin for 14 days. Once this was completed, the patient was discharged with instructions to follow up at the ID clinic in three months for a repeat CSF VDRL titer to monitor for resolution of the disease. His prescription for phenytoin was discontinued.
At the time of discharge, it was noted that the patient showed no evidence of having regained cognitive function. He was deemed by the psychiatry service to lack decision-making capacity—a likely sequelae of untreated neurosyphilis of unknown duration.
He did return to the ID clinic six months after his discharge. At that visit, a VDRL serum titer was drawn with a result of 1:64, a decrease from 1:128. His syphilis IgG remained positive, however.
Discussion
Definition and Epidemiology
Syphilis is commonly known as a sexually transmitted disease with primary, secondary, and tertiary (early and late latent) stages.1 Neurosyphilis is defined as a manifestation of the inflammatory response to invasion over decades by the Treponema pallidum spirochete in the CSF as a result of untreated primary and/or secondary syphilis.2 About one in 10 patients with untreated syphilis will experience neurologic involvement.3,4 Before 2005, neurosyphilis was required to be reported as a specific stage of syphilis (ie, a manifestation of tertiary syphilis4), but now should be reported as syphilis with neurologic manifestations.5
A reportable infectious disease, syphilis was widespread until the advent of penicillin. According to CDC statistics,6 the number of reported cases of primary and secondary syphilis has declined steadily since 1943. In the late 1970s and early 1980s, the number of tertiary cases also began to plateau, likely as a result of earlier diagnosis and more widespread use of penicillin. Recent case reports suggest greater prevalence of syphilis among men than women and increased incidence among men who have sex with men.7
Pathogenesis
Syphilis is most commonly spread by sexual contact or contact with an infected primary lesion (chancre). Less likely routes of transmission are placental passage or blood transfusion. Infectivity is greatest in the early disease stages.8
Primary syphilis is marked by transmission of the spirochete, ending with development of secondary syphilis (usually two to 12 weeks after transmission). A chancre commonly develops but is often missed by patients because it is painless and can heal spontaneously.7 The chancre is also often confused with two other sources of genital lesions, herpes simplex (genital herpes) and Haemophilus ducreyi (chancroid). In two-thirds of cases of untreated primary syphilis, the infection clears spontaneously, but in the remaining one-third, the disease progresses.8
Secondary syphilis, with or without presence of a chancre, manifests with constitutional symptoms, including lymphadenopathy, fever, headache, and malaise. Patients in this disease phase may also present with a generalized, nonpruritic, macular to maculopapular or pustular rash. The rash can affect the skin of the trunk, the proximal extremities, and the palms and soles. Ocular involvement may occur, especially in patients who are coinfected with HIV.8 In either primary or secondary syphilis, infection can invade the central nervous system.1
During latent syphilis, patients show serologic conversion without overt symptoms. Early latent syphilis is defined as infection within the previous year, as demonstrated by conversion from negative to positive testing, or an increase in titers within the previous year. Any case occurring after one year is defined as late or unknown latent syphilis.8
Tertiary syphilis is marked by complications resulting from untreated syphilis; affected patients commonly experience central nervous system and cardiovascular involvement. Gummatous disease is seen in 15% of patients.1
The early stages of neurosyphilis may be asymptomatic, acute meningeal, and meningovascular.1,4,8,9 Only 5% of patients with early neurosyphilis are symptomatic, with the added potential for cranial neuritis or ocular involvement.1 The late stages of neurosyphilis are detailed in the table.1,4,8
Diagnosis
A diagnosis of syphilis is made by testing blood samples or scrapings from a lesion. In patients with suspected syphilis, rapid plasma reagin (RPR) testing or a VDRL titer is commonly ordered. When results are positive, a serum treponemal test is recommended to confirm a diagnosis of syphilis. Options include the fluorescent treponemal antibody absorption test (FTA-ABS) and the microhemagglutinin assay for antibody to T pallidum (MHA-TP).5
If neurologic symptoms are present, a CSF sample should be obtained, followed by the same testing. A confirmed diagnosis of neurosyphilis is defined by the CDC as syphilis at any stage that meets laboratory criteria for neurosyphilis5; these include increased CSF protein or an elevated CSF leukocyte count with no other known cause, and clinical signs or symptoms without other known causes.7
Treatment
Treatment of syphilis generally consists of penicillin, administered intramuscularly (IM) or IV, depending on the stage. According to 2006 guidelines from the CDC,10,11 treatment for adults with primary and secondary syphilis is a single dose of IM penicillin G, 2.4 million units. If neurosyphilis is suspected, recommended treatment is IV penicillin G, 18 to 24 million units per day divided into six doses (ie, 3 to 4 million units every four hours) or continuous pump infusion for 10 to 14 days.10-12 Follow-up is recommended by monitoring CSF titers to ensure clearance of infection; retreatment may be required if CSF abnormalities persist after two years.11
Patients with a penicillin allergy should undergo desensitization, as penicillin is the preferred agent; the potential exists for cross-reactivity with ceftriaxone, a possible alternative for patients with neurosyphilis.11 All patients diagnosed with syphilis should also be tested for HIV and other sexually transmitted diseases.10-12
The prognosis of patients treated for neurosyphilis is generally good if the condition is diagnosed and treated early. In patients with cerebral atrophy, frontal lesions, dementia, or tabes dorsalis, the potential for recovery decreases.2,13,14
Teaching Points
There are several teaching points to take away from this case:
• Remember to rule out a CVA in any patient who presents with numbness, paresthesias, or slurred speech. In this case, a brain CT and CT angiography of the brain were both obtained in the ED before the patient was admitted. They both yielded negative results; because the patient’s history was consistent with alcohol and drug use and he had a history of seizures, he was monitored closely for signs of withdrawal or further seizure.
• Phenytoin is an antiepileptic agent whose use requires proper patient education and drug level monitoring. Appropriate follow-up must be ensured before phenytoin therapy is begun, as toxicity can result in nystagmus, ataxia, slurred speech, decreased coordination, mental confusion, and possibly death.15,16
• For patients with a suspected acute change in mental status, a workup is required and should be tailored appropriately, based on findings. This should include, but not be limited to, a thorough history and physical exam, CT of the brain (to rule out an acute brain injury17), and, if warranted, MRI of the brain. Also, a urine drug screen and alcohol level, a complete blood count, a TSH level (to evaluate for altered thyroid function that may explain mental status changes), comprehensive panel, RPR testing and/or a VDRL titer should be obtained, depending on the facility’s protocol18,19; at some facilities, a treponemal test, rather than VDRL, is being obtained at the outset.20 Levels of vitamin B12 (as part of the dementia workup), folate, thiamine, and ammonia (in patients with suspected liver disease) can also be obtained in patients with change in mental status.18,19 Urinalysis should not be overlooked to check for a urinary tract infection, especially in elderly patients.21
• If primary syphilis is suspected, treatment must be undertaken.20
Conclusion
Despite the decline seen since the 1940s in cases of primary and secondary syphilis, and the effectiveness of penicillin in treating the infection early, patients with late-stage syphilis, including those with neurosyphilis, may still present to the emergency care, urgent care, or primary care setting. Immediate treatment with penicillin is recommended to achieve an optimal prognosis for the affected patient.
1. Golden MR, Marra CM, Holmes KK. Update on syphilis: resurgence of an old problem. JAMA. 2003;290(11):1510-1514.
2. Simon RP. Chapter 20. Neurosyphilis. In: Klausner JD, Hook EW III, eds. Current Diagnosis & Treatment of Sexually Transmitted Diseases. USA: The McGraw-Hill Companies; 2007:130-137.
3. Sanchez FM, Zisselman MH. Treatment of psychiatric symptoms associated with neurosyphilis. Psychosomatics. 2007;48:440-445.
4. Marra CM. Neurosyphilis. Curr Neurol Neurosci Rep. 2004;4(6):435-440.
5. CDC. Sexually transmitted diseases surveillance, 2007: STD surveillance case definitions. www.cdc.gov/std/stats07/app-casedef.htm. Accessed March 23, 2011.
6. CDC. 2008 Sexually Transmitted Diseases Surveillance: Table 1. Cases of sexually transmitted diseases reported by state health departments and rates per 100,000 population: United States, 1941-2008. www.cdc.gov/std/stats08/tables/1.htm. Accessed March 23, 2011.
7. CDC. Sexually transmitted diseases (STDs): Syphilis: CDC fact sheet. www.cdc.gov/std/syphilis/STDfact-syphilis.htm. Accessed March 23, 2011.
8. Tramont EC. Chapter 238. Treponema pallidum (syphilis). In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia: Elsevier Churchill Livingstone; 2009.
9. Ghanem KG. Neurosyphilis: a historical perspective and review. CNS Neurosci Ther. 2010; 16(5):e157-e168.
10. Workowski KA, Berman SM; CDC. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
11. CDC. Sexually transmitted diseases: treatment guidelines 2006. www.cdc.gov/std/treatment/2006/genital-ulcers.htm#genulc6. Accessed March 29, 2011.
12. Drugs for sexually transmitted infections. Treatment Guidelines from the Medical Letter. 2010;95:95a. http://secure.medicalletter.org. Accessed March 23, 2011.
13. Russouw HG, Roberts MC, Emsley RA, et al. Psychiatric manifestations and magnetic resonance imaging in HIV-negative neurosyphilis. Biol Psychiatry. 1997;41(4):467-473.
14. Hooshmand H, Escobar MR, Kopf SW. Neurosyphylis: a study of 241 patients. JAMA. 1972;219 (6):726-729.
15. Miller CA, Joyce DM. Toxicity, phenytoin. http://emedicine.medscape.com/article/816447-overview. Accessed March 23, 2011.
16. Earnest MP, Marx JA, Drury LR. Complications of intravenous phenytoin for acute treatment of seizures: recommendations for usage. JAMA. 1983; 246(6):762-765.
17. Geschwind MD, Shu H, Haman A, et al. Rapidly progressive dementia. Ann Neurol. 2008;64(1): 97-108.
18. Mechem CC. Chapter 143. Altered mental status and coma. In: Ma J, Cline DM, Tintinalli JE, et al, eds. Emergency Medicine Manual, 6e. www.access emergencymedicine.com/content.aspx?aID=2020. Accessed March 23, 2011.
19. Knopman DS, DeKosky ST, Cummings JL, et al; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: diagnosis of dementia (an evidence-based review). Neurology. 2001;56(9):1143-1153.
20. CDC. Syphilis testing algorithms using treponemal tests for initial screening—four laboratories, New York City, 2005-2006. MMWR Morb Mortal Wkly Rep. 2008;57(32):872-875.
21. Anderson CA, Filley CM. Chapter 33. Behavioral presentations of medical and neurologic disorders. In: Jacobson JL, Jacobson AM, eds. Psychiatric Secrets. 2nd ed. St. Louis, MO: Hanley & Belfus; 2001.
1. Golden MR, Marra CM, Holmes KK. Update on syphilis: resurgence of an old problem. JAMA. 2003;290(11):1510-1514.
2. Simon RP. Chapter 20. Neurosyphilis. In: Klausner JD, Hook EW III, eds. Current Diagnosis & Treatment of Sexually Transmitted Diseases. USA: The McGraw-Hill Companies; 2007:130-137.
3. Sanchez FM, Zisselman MH. Treatment of psychiatric symptoms associated with neurosyphilis. Psychosomatics. 2007;48:440-445.
4. Marra CM. Neurosyphilis. Curr Neurol Neurosci Rep. 2004;4(6):435-440.
5. CDC. Sexually transmitted diseases surveillance, 2007: STD surveillance case definitions. www.cdc.gov/std/stats07/app-casedef.htm. Accessed March 23, 2011.
6. CDC. 2008 Sexually Transmitted Diseases Surveillance: Table 1. Cases of sexually transmitted diseases reported by state health departments and rates per 100,000 population: United States, 1941-2008. www.cdc.gov/std/stats08/tables/1.htm. Accessed March 23, 2011.
7. CDC. Sexually transmitted diseases (STDs): Syphilis: CDC fact sheet. www.cdc.gov/std/syphilis/STDfact-syphilis.htm. Accessed March 23, 2011.
8. Tramont EC. Chapter 238. Treponema pallidum (syphilis). In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia: Elsevier Churchill Livingstone; 2009.
9. Ghanem KG. Neurosyphilis: a historical perspective and review. CNS Neurosci Ther. 2010; 16(5):e157-e168.
10. Workowski KA, Berman SM; CDC. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
11. CDC. Sexually transmitted diseases: treatment guidelines 2006. www.cdc.gov/std/treatment/2006/genital-ulcers.htm#genulc6. Accessed March 29, 2011.
12. Drugs for sexually transmitted infections. Treatment Guidelines from the Medical Letter. 2010;95:95a. http://secure.medicalletter.org. Accessed March 23, 2011.
13. Russouw HG, Roberts MC, Emsley RA, et al. Psychiatric manifestations and magnetic resonance imaging in HIV-negative neurosyphilis. Biol Psychiatry. 1997;41(4):467-473.
14. Hooshmand H, Escobar MR, Kopf SW. Neurosyphylis: a study of 241 patients. JAMA. 1972;219 (6):726-729.
15. Miller CA, Joyce DM. Toxicity, phenytoin. http://emedicine.medscape.com/article/816447-overview. Accessed March 23, 2011.
16. Earnest MP, Marx JA, Drury LR. Complications of intravenous phenytoin for acute treatment of seizures: recommendations for usage. JAMA. 1983; 246(6):762-765.
17. Geschwind MD, Shu H, Haman A, et al. Rapidly progressive dementia. Ann Neurol. 2008;64(1): 97-108.
18. Mechem CC. Chapter 143. Altered mental status and coma. In: Ma J, Cline DM, Tintinalli JE, et al, eds. Emergency Medicine Manual, 6e. www.access emergencymedicine.com/content.aspx?aID=2020. Accessed March 23, 2011.
19. Knopman DS, DeKosky ST, Cummings JL, et al; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: diagnosis of dementia (an evidence-based review). Neurology. 2001;56(9):1143-1153.
20. CDC. Syphilis testing algorithms using treponemal tests for initial screening—four laboratories, New York City, 2005-2006. MMWR Morb Mortal Wkly Rep. 2008;57(32):872-875.
21. Anderson CA, Filley CM. Chapter 33. Behavioral presentations of medical and neurologic disorders. In: Jacobson JL, Jacobson AM, eds. Psychiatric Secrets. 2nd ed. St. Louis, MO: Hanley & Belfus; 2001.
Modified Technique for Unipolar Allograft Ankle Replacement: Midterm Follow-up. A Case Report
Erratum (2011;87:81-84)
Hodgkin Lymphoma Presenting as Generalized Pruritus in an Adolescent
What's Eating You? Hyalomma Ticks
Applying single-incision laparoscopic surgery to gyn practice: What’s involved
- Update on minimally invasive surgery
Amy Garcia, MD (April 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
The benefits of minimally invasive surgery—including less pain, faster recovery, and improved cosmesis—are well known.1,2 Standard laparotomy has been replaced by multiple-port operative laparoscopy for a great array of procedures, and advances in medical technology allow for a minimally invasive surgical approach even when a surgeon is faced with complex pathology.
Single-port laparoscopic surgery (SPLS) represents the latest advance in minimally invasive surgery. Using flexible endoscopes and articulating instruments, the surgeon can complete complex procedures through a single 2-cm incision in the abdomen. The incision is usually placed in the umbilicus, where it is easily hidden.3-8
Since the first laparoscopic hysterectomy through a single incision was performed 20 years ago, SPLS has been used successfully to perform nephrectomy, prostatectomy, hemicolectomy, cholecystectomy, splenectomy, intussusception reduction, gastrostomy tube placement, thoracoscopic lung biopsy, thoracoscopic decortication, and appendectomy.4-7
In gynecology, SPLS has been used to perform oophorectomy, salpingectomy, bilateral tubal ligation, ovarian cystectomy, surgical treatment of ectopic pregnancy, and both total and partial hysterectomy.7-11 At least two recent studies have concluded that SPLS is an acceptable way to treat many benign and malignant gynecologic conditions that are currently treated using multiport laparoscopy.3,11
This article outlines our approach to SPLS in the gynecologic patient and provides an overview of instrumentation, with the aim of allowing you to consider whether this approach might be feasible in your surgical practice, at your institution.
Unique setup required
When SPLS is performed through the umbilicus, the instruments must be held closer to the midline and more cephalad than during conventional laparoscopy to permit adequate visualization and manipulation. For this reason, the surgeon needs to assume a position higher over the torso and thorax of the patient, and both of the patient’s arms need to be tucked. Place the patient in a dorsal lithotomy position with a uterine manipulator in place to facilitate surgery—even when the uterus will be preserved and surgery involves only the adnexae.
With appropriate equipment and positioning, visualization and manipulation of anatomy are comparable to those of standard multiport laparoscopy.
New instruments simplify SPLS
Innovative surgical instruments allow for appropriate hand positioning outside the abdomen and minimize the internal collision of instruments brought through a single midline incision (FIGURE 1). A variety of single-port options are available, each with a unique patented design and method of insertion. In fact, the development of ports with multiple instrument channels has revolutionized SPLS.
FIGURE 1 Setup
Desired triangulation of instruments in SPLS setup.Before true single ports became available, it was necessary to place three 5-mm low-profile trocars in the fascia at three separate sites through a single skin incision. Pneumoperitoneum was established with a Veress needle, but the fascial incisions gradually merged with repeated cannula manipulation, producing air leaks.
Today, multiple-channel ports are placed using an open technique into a single skin and fascial incision. Trocars and instruments of varying size can be exchanged with ease without jeopardizing pneumoperitoneum.
Among the options:
- the SILS Port (Covidien) – a soft, flexible, three-channel port that allows for placement of blunt trocars ranging in size from 5 mm to 12 mm (FIGURE 2)
- the TriPort (Olympus America) – two flexible rings joined by a sleeve and multiple-channel port (FIGURE 3)
- GelPOINT Advanced Access Platform (Applied Medical) – a system constructed of synthetic gel material and consisting of a “GelSeal” cap, cannulas, and seals to accommodate 5-mm to 10-mm instrumentation (FIGURE 4).
We have found that all three devices allow for good range of motion while maintaining pneumoperitoneum.
(A recent article from Korea reports an inventive technique to perform single-incision laparoscopy using standard instrumentation: The authors fitted a self-retaining ring retractor with a surgical glove that had three of the fingers cut off and replaced by trocars.12)
FIGURE 2 SILS Port
The SILS Port is a soft, flexible, three-channel port that allows for placement of blunt trocars ranging in size from 5 mm to 12 mm.
FIGURE 3 TriPort
The TriPort system comprises two flexible rings joined by a sleeve and multiple-channel port.
FIGURE 4 GelPOINT
The GelPOINT Advanced Access Platform is constructed of synthetic gel material and accommodates 5-mm to 10-mm instrumentation.
A flexible laparoscope improves visualization
The ability to visualize the operative field is vital to any surgery, including SPLS. Use of a flexible laparoscope facilitates uncompromised visualization of the entire pelvis (FIGURE 5). Outside the abdomen, the flexible camera can be held laterally and away from the midline to help reduce the clashing of instruments and hands.
FIGURE 5 Flexible-tip laparoscope
A flexible-tip laparoscope ensures good visualization of the surgical field.If a flexible laparoscope is not available, a rigid 30-degree or 45-degree angled scope can be used, although visualization may be limited and adequate triangulation of instruments may be difficult to achieve.
When using a rigid laparoscope, a light cord that inserts into the back of the camera is necessary; otherwise, a 90-degree light cord adapter can be purchased.
Design enhancements facilitate coordination of instruments
One of the disadvantages of SPLS has been the restriction of movement that arises because of the close proximity of instruments and instrument handles. The latest designs have made articulation possible for tissue graspers, scissors, vessel sealers, and scopes.9,10 The value of articulation is apparent inside the abdomen, where it allows perfect positioning of the area of dissection. Outside the abdomen, the handles can be arranged in an angled pattern to allow the surgeon and assistant to operate comfortably (FIGURE 1).
In a four-handed procedure, one hand is on the camera, one on the uterine manipulator, and the remaining two hands operate the articulating grasper, vessel sealer, or needle driver, depending on the task.
Standard straight instruments can also be used for portions of the procedure.
Dissection and hemostasis are achieved in a manner similar to that of conventional laparoscopy. Our instrument of choice is an enhanced bipolar instrument, although harmonic and traditional biopolar energy can be used as well, depending on the preference of the surgeon.
The latest instruments are designed to dissect, cauterize, and cut, thereby decreasing the number of instrument exchanges necessary.
With the right aids, suturing can be simplified
Suturing through a single port can be a challenge. When possible, closure of the vaginal cuff following a total laparoscopic or laparoscopic-assisted vaginal hysterectomy should be performed from below. When endoscopic suturing is required, standard suturing using both intracorporeal and extracorporeal methods is possible.
Suturing aids such as the Endo Stitch (Covidien) or Lapra-Ty (Ethicon) are helpful. One author recommends Quill bidirectional, self-retaining suture with barbs (Angiotech) to avoid the need for knot-tying.6 (For more on self-retaining suture, see “Barbed suture, now in the toolbox of minimally invasive gyn surgery,” by Jon I. Einarsson, MD, MPH, and James A. Greenberg, MD, in the September 2009 issue at obgmanagement.com.)
The MiniLap (Stryker) is a 2.3-mm grasper that is inserted percutaneously directly through the abdominal wall without an incision. It can be used to set the needle on the needle driver or manipulate tissue while suturing. The resulting skin incision is barely visible and does not require closure.
Options are varied for specimen removal
Small specimens can be removed directly through a single-port system that has been opened, or they can be extracted after the system is removed, with rapid desufflation (FIGURE 6).
FIGURE 6 Single-incision oophorectomy
An ovary and tube removed through a single incision using the A) TriPort and B) SILS Port systems.Compared with conventional laparoscopy, the larger incision associated with single-port surgery facilitates specimen removal. Larger or potentially malignant specimens can be placed into an EndoCatch bag (Covidien) inserted through the single-incision 10-mm cannula (FIGURE 7).
FIGURE 7 Specimen removal
In this case, the specimen was placed in a 10-mm EndoCatch bag and removed through the 10-mm cannula of the SILS Port.In total laparoscopic hysterectomy or laparoscopic-assisted vaginal hysterectomy, the uterus is removed through the vagina. In supracervical hysterectomy, a small uterus can be removed through the cul-de-sac or directly through the single incision after placement in a bag.
When morcellation is required, the instrument can be placed through the cul-desac, cervix, or a single port. The morcellator can be placed directly through the SPLS port while utilizing the flexible scope in an angled direction (looking back toward the morcellator) for complete visualization (note: Covidien does not recommend this usage).
Transcervical tissue morcellation has also been described. In this approach, the cervix is dilated once the uterine body has been amputated, and the tissue morcellator is inserted through the cervix while the surgeon maintains visualization from above.6,13
How to master the technique
Many patients desire SPLS for its superior cosmetic outcome, but the approach may not always be appropriate. Depending on the procedure and characteristics of the patient (TABLE), multiple-port laparoscopy may be a better option. When a surgeon first attempts SPLS, we recommend that it be limited to the treatment of adnexal pathology only.
In your early SPlS cases, look for these patient characteristics
|
By using the techniques described in this article and selecting patients carefully, the surgeon can develop expertise in SPLS.11,14 During the learning process, the use of additional ports or Mini-Lap instruments (Stryker) can reduce the challenges of more difficult procedures and should be considered without reservation, as should the use of articulating accessory instruments and flexible or angled laparoscopes.
Although the clinical benefits of SPLS have yet to be determined, the cosmetic advantage of a single, hidden, umbilical incision likely increases patient satisfaction.
Clearly, the goal of SPLS is to use technology in a way that offers all of the benefits of traditional multiport laparoscopy without any of the limitations. Further study is required to determine whether SPLS meets this standard and, more important, whether it has any advantages over conventional techniques.
We want to hear from you! Tell us what you think.
1. Medeiros LR, Rosa DD, Bozzerri MC, et al. Laparoscopy versus laparotomy for benign ovarian tumour. Cochrane Database Syst Rev. 2009;(2):CD004751.-
2. Chapron C, Fauconnier A, Goffinet F, Breart G, Dubuisson JB. Laparoscopic surgery is not inherently dangerous for patients presenting with benign gynecological pathology. Results of a metaanalysis. Hum Reprod. 2002;17(5):1334-1342.
3. Fader AN, Escobar PF. Laparoendoscopic single-site surgery (LESS) in gynecologic oncology: technique and initial report. Gynecol Oncol. 2009;114(2):157-161.
4. Pelosi MA, Pelosi MA 3rd. Laparoscopic hysterectomy with bilateral salpingo-oophorectomy using a single umbilical puncture. N J Med. 1991;88(1):721-726.
5. Curcillo PG, King SA, Podolsky ER, Rottman SJ. Single port access (SPA) minimal access surgery through a single incision. Surg Technol Int. 2009;18:19-25.
6. Romanelli JR, Earle DB. Single-port laparoscopic surgery: an overview. Surg Endosc. 2009;23(7):1419-1427.
7. Ponsky TA. Single port laparoscopic cholecystectomy in adults and children: tools and techniques. J Am Coll Surg. 2009;209(5):e1-6.
8. Kim YW. Single port transumbilical myomectomy and ovarian cystectomy. J Minim Invasive Gynecol. 2009;16(6 suppl):S74.-
9. Ghezzi F, Cromi A, Fasola M, Bolis P. One-trocar salpingectomy for the treatment of tubal pregnancy: a “marionette-like” technique. BJOG. 2005;112(10):1417-1419.
10. Lim MC, Kim TJ, Kang S, Bae DS, Park SY, Seo SS. Embryonic natural orifice transumbilical endoscopic surgery (E-NOTES) for adnexal tumors. Surg Endosc. 2009;23(11):2445-2449.
11. Escobar PF, Starks DC, Fader AN, et al. Single-port risk-reducing salpingo-oophorectomy with and without hysterectomy: surgical outcomes and learning curve analysis. Gynecol Oncol. 2010;119(1):43-47.
12. Jeon HG, Jeong W, Oh CK. Initial experience with 50 laparoendoscopic single site surgeries using a homemade, single port device at a single center. J Urol. 2010;183(5):1866-1871.
13. Yoon G, Kim TJ, Lee YY, et al. Single port access subtotal hysterectomy with transcervical morcellation: a pilot study. J Minim Invasive Gynecol. 2010;17(1):78-81.
14. Ghomi A, Littman P, Prasad A, et al. Assessing the learning curve for laparoscopic supracervical hysterectomy. JSLS. 2007;11(2):190-194.
- Update on minimally invasive surgery
Amy Garcia, MD (April 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
The benefits of minimally invasive surgery—including less pain, faster recovery, and improved cosmesis—are well known.1,2 Standard laparotomy has been replaced by multiple-port operative laparoscopy for a great array of procedures, and advances in medical technology allow for a minimally invasive surgical approach even when a surgeon is faced with complex pathology.
Single-port laparoscopic surgery (SPLS) represents the latest advance in minimally invasive surgery. Using flexible endoscopes and articulating instruments, the surgeon can complete complex procedures through a single 2-cm incision in the abdomen. The incision is usually placed in the umbilicus, where it is easily hidden.3-8
Since the first laparoscopic hysterectomy through a single incision was performed 20 years ago, SPLS has been used successfully to perform nephrectomy, prostatectomy, hemicolectomy, cholecystectomy, splenectomy, intussusception reduction, gastrostomy tube placement, thoracoscopic lung biopsy, thoracoscopic decortication, and appendectomy.4-7
In gynecology, SPLS has been used to perform oophorectomy, salpingectomy, bilateral tubal ligation, ovarian cystectomy, surgical treatment of ectopic pregnancy, and both total and partial hysterectomy.7-11 At least two recent studies have concluded that SPLS is an acceptable way to treat many benign and malignant gynecologic conditions that are currently treated using multiport laparoscopy.3,11
This article outlines our approach to SPLS in the gynecologic patient and provides an overview of instrumentation, with the aim of allowing you to consider whether this approach might be feasible in your surgical practice, at your institution.
Unique setup required
When SPLS is performed through the umbilicus, the instruments must be held closer to the midline and more cephalad than during conventional laparoscopy to permit adequate visualization and manipulation. For this reason, the surgeon needs to assume a position higher over the torso and thorax of the patient, and both of the patient’s arms need to be tucked. Place the patient in a dorsal lithotomy position with a uterine manipulator in place to facilitate surgery—even when the uterus will be preserved and surgery involves only the adnexae.
With appropriate equipment and positioning, visualization and manipulation of anatomy are comparable to those of standard multiport laparoscopy.
New instruments simplify SPLS
Innovative surgical instruments allow for appropriate hand positioning outside the abdomen and minimize the internal collision of instruments brought through a single midline incision (FIGURE 1). A variety of single-port options are available, each with a unique patented design and method of insertion. In fact, the development of ports with multiple instrument channels has revolutionized SPLS.
FIGURE 1 Setup
Desired triangulation of instruments in SPLS setup.Before true single ports became available, it was necessary to place three 5-mm low-profile trocars in the fascia at three separate sites through a single skin incision. Pneumoperitoneum was established with a Veress needle, but the fascial incisions gradually merged with repeated cannula manipulation, producing air leaks.
Today, multiple-channel ports are placed using an open technique into a single skin and fascial incision. Trocars and instruments of varying size can be exchanged with ease without jeopardizing pneumoperitoneum.
Among the options:
- the SILS Port (Covidien) – a soft, flexible, three-channel port that allows for placement of blunt trocars ranging in size from 5 mm to 12 mm (FIGURE 2)
- the TriPort (Olympus America) – two flexible rings joined by a sleeve and multiple-channel port (FIGURE 3)
- GelPOINT Advanced Access Platform (Applied Medical) – a system constructed of synthetic gel material and consisting of a “GelSeal” cap, cannulas, and seals to accommodate 5-mm to 10-mm instrumentation (FIGURE 4).
We have found that all three devices allow for good range of motion while maintaining pneumoperitoneum.
(A recent article from Korea reports an inventive technique to perform single-incision laparoscopy using standard instrumentation: The authors fitted a self-retaining ring retractor with a surgical glove that had three of the fingers cut off and replaced by trocars.12)
FIGURE 2 SILS Port
The SILS Port is a soft, flexible, three-channel port that allows for placement of blunt trocars ranging in size from 5 mm to 12 mm.
FIGURE 3 TriPort
The TriPort system comprises two flexible rings joined by a sleeve and multiple-channel port.
FIGURE 4 GelPOINT
The GelPOINT Advanced Access Platform is constructed of synthetic gel material and accommodates 5-mm to 10-mm instrumentation.
A flexible laparoscope improves visualization
The ability to visualize the operative field is vital to any surgery, including SPLS. Use of a flexible laparoscope facilitates uncompromised visualization of the entire pelvis (FIGURE 5). Outside the abdomen, the flexible camera can be held laterally and away from the midline to help reduce the clashing of instruments and hands.
FIGURE 5 Flexible-tip laparoscope
A flexible-tip laparoscope ensures good visualization of the surgical field.If a flexible laparoscope is not available, a rigid 30-degree or 45-degree angled scope can be used, although visualization may be limited and adequate triangulation of instruments may be difficult to achieve.
When using a rigid laparoscope, a light cord that inserts into the back of the camera is necessary; otherwise, a 90-degree light cord adapter can be purchased.
Design enhancements facilitate coordination of instruments
One of the disadvantages of SPLS has been the restriction of movement that arises because of the close proximity of instruments and instrument handles. The latest designs have made articulation possible for tissue graspers, scissors, vessel sealers, and scopes.9,10 The value of articulation is apparent inside the abdomen, where it allows perfect positioning of the area of dissection. Outside the abdomen, the handles can be arranged in an angled pattern to allow the surgeon and assistant to operate comfortably (FIGURE 1).
In a four-handed procedure, one hand is on the camera, one on the uterine manipulator, and the remaining two hands operate the articulating grasper, vessel sealer, or needle driver, depending on the task.
Standard straight instruments can also be used for portions of the procedure.
Dissection and hemostasis are achieved in a manner similar to that of conventional laparoscopy. Our instrument of choice is an enhanced bipolar instrument, although harmonic and traditional biopolar energy can be used as well, depending on the preference of the surgeon.
The latest instruments are designed to dissect, cauterize, and cut, thereby decreasing the number of instrument exchanges necessary.
With the right aids, suturing can be simplified
Suturing through a single port can be a challenge. When possible, closure of the vaginal cuff following a total laparoscopic or laparoscopic-assisted vaginal hysterectomy should be performed from below. When endoscopic suturing is required, standard suturing using both intracorporeal and extracorporeal methods is possible.
Suturing aids such as the Endo Stitch (Covidien) or Lapra-Ty (Ethicon) are helpful. One author recommends Quill bidirectional, self-retaining suture with barbs (Angiotech) to avoid the need for knot-tying.6 (For more on self-retaining suture, see “Barbed suture, now in the toolbox of minimally invasive gyn surgery,” by Jon I. Einarsson, MD, MPH, and James A. Greenberg, MD, in the September 2009 issue at obgmanagement.com.)
The MiniLap (Stryker) is a 2.3-mm grasper that is inserted percutaneously directly through the abdominal wall without an incision. It can be used to set the needle on the needle driver or manipulate tissue while suturing. The resulting skin incision is barely visible and does not require closure.
Options are varied for specimen removal
Small specimens can be removed directly through a single-port system that has been opened, or they can be extracted after the system is removed, with rapid desufflation (FIGURE 6).
FIGURE 6 Single-incision oophorectomy
An ovary and tube removed through a single incision using the A) TriPort and B) SILS Port systems.Compared with conventional laparoscopy, the larger incision associated with single-port surgery facilitates specimen removal. Larger or potentially malignant specimens can be placed into an EndoCatch bag (Covidien) inserted through the single-incision 10-mm cannula (FIGURE 7).
FIGURE 7 Specimen removal
In this case, the specimen was placed in a 10-mm EndoCatch bag and removed through the 10-mm cannula of the SILS Port.In total laparoscopic hysterectomy or laparoscopic-assisted vaginal hysterectomy, the uterus is removed through the vagina. In supracervical hysterectomy, a small uterus can be removed through the cul-de-sac or directly through the single incision after placement in a bag.
When morcellation is required, the instrument can be placed through the cul-desac, cervix, or a single port. The morcellator can be placed directly through the SPLS port while utilizing the flexible scope in an angled direction (looking back toward the morcellator) for complete visualization (note: Covidien does not recommend this usage).
Transcervical tissue morcellation has also been described. In this approach, the cervix is dilated once the uterine body has been amputated, and the tissue morcellator is inserted through the cervix while the surgeon maintains visualization from above.6,13
How to master the technique
Many patients desire SPLS for its superior cosmetic outcome, but the approach may not always be appropriate. Depending on the procedure and characteristics of the patient (TABLE), multiple-port laparoscopy may be a better option. When a surgeon first attempts SPLS, we recommend that it be limited to the treatment of adnexal pathology only.
In your early SPlS cases, look for these patient characteristics
|
By using the techniques described in this article and selecting patients carefully, the surgeon can develop expertise in SPLS.11,14 During the learning process, the use of additional ports or Mini-Lap instruments (Stryker) can reduce the challenges of more difficult procedures and should be considered without reservation, as should the use of articulating accessory instruments and flexible or angled laparoscopes.
Although the clinical benefits of SPLS have yet to be determined, the cosmetic advantage of a single, hidden, umbilical incision likely increases patient satisfaction.
Clearly, the goal of SPLS is to use technology in a way that offers all of the benefits of traditional multiport laparoscopy without any of the limitations. Further study is required to determine whether SPLS meets this standard and, more important, whether it has any advantages over conventional techniques.
We want to hear from you! Tell us what you think.
- Update on minimally invasive surgery
Amy Garcia, MD (April 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
The benefits of minimally invasive surgery—including less pain, faster recovery, and improved cosmesis—are well known.1,2 Standard laparotomy has been replaced by multiple-port operative laparoscopy for a great array of procedures, and advances in medical technology allow for a minimally invasive surgical approach even when a surgeon is faced with complex pathology.
Single-port laparoscopic surgery (SPLS) represents the latest advance in minimally invasive surgery. Using flexible endoscopes and articulating instruments, the surgeon can complete complex procedures through a single 2-cm incision in the abdomen. The incision is usually placed in the umbilicus, where it is easily hidden.3-8
Since the first laparoscopic hysterectomy through a single incision was performed 20 years ago, SPLS has been used successfully to perform nephrectomy, prostatectomy, hemicolectomy, cholecystectomy, splenectomy, intussusception reduction, gastrostomy tube placement, thoracoscopic lung biopsy, thoracoscopic decortication, and appendectomy.4-7
In gynecology, SPLS has been used to perform oophorectomy, salpingectomy, bilateral tubal ligation, ovarian cystectomy, surgical treatment of ectopic pregnancy, and both total and partial hysterectomy.7-11 At least two recent studies have concluded that SPLS is an acceptable way to treat many benign and malignant gynecologic conditions that are currently treated using multiport laparoscopy.3,11
This article outlines our approach to SPLS in the gynecologic patient and provides an overview of instrumentation, with the aim of allowing you to consider whether this approach might be feasible in your surgical practice, at your institution.
Unique setup required
When SPLS is performed through the umbilicus, the instruments must be held closer to the midline and more cephalad than during conventional laparoscopy to permit adequate visualization and manipulation. For this reason, the surgeon needs to assume a position higher over the torso and thorax of the patient, and both of the patient’s arms need to be tucked. Place the patient in a dorsal lithotomy position with a uterine manipulator in place to facilitate surgery—even when the uterus will be preserved and surgery involves only the adnexae.
With appropriate equipment and positioning, visualization and manipulation of anatomy are comparable to those of standard multiport laparoscopy.
New instruments simplify SPLS
Innovative surgical instruments allow for appropriate hand positioning outside the abdomen and minimize the internal collision of instruments brought through a single midline incision (FIGURE 1). A variety of single-port options are available, each with a unique patented design and method of insertion. In fact, the development of ports with multiple instrument channels has revolutionized SPLS.
FIGURE 1 Setup
Desired triangulation of instruments in SPLS setup.Before true single ports became available, it was necessary to place three 5-mm low-profile trocars in the fascia at three separate sites through a single skin incision. Pneumoperitoneum was established with a Veress needle, but the fascial incisions gradually merged with repeated cannula manipulation, producing air leaks.
Today, multiple-channel ports are placed using an open technique into a single skin and fascial incision. Trocars and instruments of varying size can be exchanged with ease without jeopardizing pneumoperitoneum.
Among the options:
- the SILS Port (Covidien) – a soft, flexible, three-channel port that allows for placement of blunt trocars ranging in size from 5 mm to 12 mm (FIGURE 2)
- the TriPort (Olympus America) – two flexible rings joined by a sleeve and multiple-channel port (FIGURE 3)
- GelPOINT Advanced Access Platform (Applied Medical) – a system constructed of synthetic gel material and consisting of a “GelSeal” cap, cannulas, and seals to accommodate 5-mm to 10-mm instrumentation (FIGURE 4).
We have found that all three devices allow for good range of motion while maintaining pneumoperitoneum.
(A recent article from Korea reports an inventive technique to perform single-incision laparoscopy using standard instrumentation: The authors fitted a self-retaining ring retractor with a surgical glove that had three of the fingers cut off and replaced by trocars.12)
FIGURE 2 SILS Port
The SILS Port is a soft, flexible, three-channel port that allows for placement of blunt trocars ranging in size from 5 mm to 12 mm.
FIGURE 3 TriPort
The TriPort system comprises two flexible rings joined by a sleeve and multiple-channel port.
FIGURE 4 GelPOINT
The GelPOINT Advanced Access Platform is constructed of synthetic gel material and accommodates 5-mm to 10-mm instrumentation.
A flexible laparoscope improves visualization
The ability to visualize the operative field is vital to any surgery, including SPLS. Use of a flexible laparoscope facilitates uncompromised visualization of the entire pelvis (FIGURE 5). Outside the abdomen, the flexible camera can be held laterally and away from the midline to help reduce the clashing of instruments and hands.
FIGURE 5 Flexible-tip laparoscope
A flexible-tip laparoscope ensures good visualization of the surgical field.If a flexible laparoscope is not available, a rigid 30-degree or 45-degree angled scope can be used, although visualization may be limited and adequate triangulation of instruments may be difficult to achieve.
When using a rigid laparoscope, a light cord that inserts into the back of the camera is necessary; otherwise, a 90-degree light cord adapter can be purchased.
Design enhancements facilitate coordination of instruments
One of the disadvantages of SPLS has been the restriction of movement that arises because of the close proximity of instruments and instrument handles. The latest designs have made articulation possible for tissue graspers, scissors, vessel sealers, and scopes.9,10 The value of articulation is apparent inside the abdomen, where it allows perfect positioning of the area of dissection. Outside the abdomen, the handles can be arranged in an angled pattern to allow the surgeon and assistant to operate comfortably (FIGURE 1).
In a four-handed procedure, one hand is on the camera, one on the uterine manipulator, and the remaining two hands operate the articulating grasper, vessel sealer, or needle driver, depending on the task.
Standard straight instruments can also be used for portions of the procedure.
Dissection and hemostasis are achieved in a manner similar to that of conventional laparoscopy. Our instrument of choice is an enhanced bipolar instrument, although harmonic and traditional biopolar energy can be used as well, depending on the preference of the surgeon.
The latest instruments are designed to dissect, cauterize, and cut, thereby decreasing the number of instrument exchanges necessary.
With the right aids, suturing can be simplified
Suturing through a single port can be a challenge. When possible, closure of the vaginal cuff following a total laparoscopic or laparoscopic-assisted vaginal hysterectomy should be performed from below. When endoscopic suturing is required, standard suturing using both intracorporeal and extracorporeal methods is possible.
Suturing aids such as the Endo Stitch (Covidien) or Lapra-Ty (Ethicon) are helpful. One author recommends Quill bidirectional, self-retaining suture with barbs (Angiotech) to avoid the need for knot-tying.6 (For more on self-retaining suture, see “Barbed suture, now in the toolbox of minimally invasive gyn surgery,” by Jon I. Einarsson, MD, MPH, and James A. Greenberg, MD, in the September 2009 issue at obgmanagement.com.)
The MiniLap (Stryker) is a 2.3-mm grasper that is inserted percutaneously directly through the abdominal wall without an incision. It can be used to set the needle on the needle driver or manipulate tissue while suturing. The resulting skin incision is barely visible and does not require closure.
Options are varied for specimen removal
Small specimens can be removed directly through a single-port system that has been opened, or they can be extracted after the system is removed, with rapid desufflation (FIGURE 6).
FIGURE 6 Single-incision oophorectomy
An ovary and tube removed through a single incision using the A) TriPort and B) SILS Port systems.Compared with conventional laparoscopy, the larger incision associated with single-port surgery facilitates specimen removal. Larger or potentially malignant specimens can be placed into an EndoCatch bag (Covidien) inserted through the single-incision 10-mm cannula (FIGURE 7).
FIGURE 7 Specimen removal
In this case, the specimen was placed in a 10-mm EndoCatch bag and removed through the 10-mm cannula of the SILS Port.In total laparoscopic hysterectomy or laparoscopic-assisted vaginal hysterectomy, the uterus is removed through the vagina. In supracervical hysterectomy, a small uterus can be removed through the cul-de-sac or directly through the single incision after placement in a bag.
When morcellation is required, the instrument can be placed through the cul-desac, cervix, or a single port. The morcellator can be placed directly through the SPLS port while utilizing the flexible scope in an angled direction (looking back toward the morcellator) for complete visualization (note: Covidien does not recommend this usage).
Transcervical tissue morcellation has also been described. In this approach, the cervix is dilated once the uterine body has been amputated, and the tissue morcellator is inserted through the cervix while the surgeon maintains visualization from above.6,13
How to master the technique
Many patients desire SPLS for its superior cosmetic outcome, but the approach may not always be appropriate. Depending on the procedure and characteristics of the patient (TABLE), multiple-port laparoscopy may be a better option. When a surgeon first attempts SPLS, we recommend that it be limited to the treatment of adnexal pathology only.
In your early SPlS cases, look for these patient characteristics
|
By using the techniques described in this article and selecting patients carefully, the surgeon can develop expertise in SPLS.11,14 During the learning process, the use of additional ports or Mini-Lap instruments (Stryker) can reduce the challenges of more difficult procedures and should be considered without reservation, as should the use of articulating accessory instruments and flexible or angled laparoscopes.
Although the clinical benefits of SPLS have yet to be determined, the cosmetic advantage of a single, hidden, umbilical incision likely increases patient satisfaction.
Clearly, the goal of SPLS is to use technology in a way that offers all of the benefits of traditional multiport laparoscopy without any of the limitations. Further study is required to determine whether SPLS meets this standard and, more important, whether it has any advantages over conventional techniques.
We want to hear from you! Tell us what you think.
1. Medeiros LR, Rosa DD, Bozzerri MC, et al. Laparoscopy versus laparotomy for benign ovarian tumour. Cochrane Database Syst Rev. 2009;(2):CD004751.-
2. Chapron C, Fauconnier A, Goffinet F, Breart G, Dubuisson JB. Laparoscopic surgery is not inherently dangerous for patients presenting with benign gynecological pathology. Results of a metaanalysis. Hum Reprod. 2002;17(5):1334-1342.
3. Fader AN, Escobar PF. Laparoendoscopic single-site surgery (LESS) in gynecologic oncology: technique and initial report. Gynecol Oncol. 2009;114(2):157-161.
4. Pelosi MA, Pelosi MA 3rd. Laparoscopic hysterectomy with bilateral salpingo-oophorectomy using a single umbilical puncture. N J Med. 1991;88(1):721-726.
5. Curcillo PG, King SA, Podolsky ER, Rottman SJ. Single port access (SPA) minimal access surgery through a single incision. Surg Technol Int. 2009;18:19-25.
6. Romanelli JR, Earle DB. Single-port laparoscopic surgery: an overview. Surg Endosc. 2009;23(7):1419-1427.
7. Ponsky TA. Single port laparoscopic cholecystectomy in adults and children: tools and techniques. J Am Coll Surg. 2009;209(5):e1-6.
8. Kim YW. Single port transumbilical myomectomy and ovarian cystectomy. J Minim Invasive Gynecol. 2009;16(6 suppl):S74.-
9. Ghezzi F, Cromi A, Fasola M, Bolis P. One-trocar salpingectomy for the treatment of tubal pregnancy: a “marionette-like” technique. BJOG. 2005;112(10):1417-1419.
10. Lim MC, Kim TJ, Kang S, Bae DS, Park SY, Seo SS. Embryonic natural orifice transumbilical endoscopic surgery (E-NOTES) for adnexal tumors. Surg Endosc. 2009;23(11):2445-2449.
11. Escobar PF, Starks DC, Fader AN, et al. Single-port risk-reducing salpingo-oophorectomy with and without hysterectomy: surgical outcomes and learning curve analysis. Gynecol Oncol. 2010;119(1):43-47.
12. Jeon HG, Jeong W, Oh CK. Initial experience with 50 laparoendoscopic single site surgeries using a homemade, single port device at a single center. J Urol. 2010;183(5):1866-1871.
13. Yoon G, Kim TJ, Lee YY, et al. Single port access subtotal hysterectomy with transcervical morcellation: a pilot study. J Minim Invasive Gynecol. 2010;17(1):78-81.
14. Ghomi A, Littman P, Prasad A, et al. Assessing the learning curve for laparoscopic supracervical hysterectomy. JSLS. 2007;11(2):190-194.
1. Medeiros LR, Rosa DD, Bozzerri MC, et al. Laparoscopy versus laparotomy for benign ovarian tumour. Cochrane Database Syst Rev. 2009;(2):CD004751.-
2. Chapron C, Fauconnier A, Goffinet F, Breart G, Dubuisson JB. Laparoscopic surgery is not inherently dangerous for patients presenting with benign gynecological pathology. Results of a metaanalysis. Hum Reprod. 2002;17(5):1334-1342.
3. Fader AN, Escobar PF. Laparoendoscopic single-site surgery (LESS) in gynecologic oncology: technique and initial report. Gynecol Oncol. 2009;114(2):157-161.
4. Pelosi MA, Pelosi MA 3rd. Laparoscopic hysterectomy with bilateral salpingo-oophorectomy using a single umbilical puncture. N J Med. 1991;88(1):721-726.
5. Curcillo PG, King SA, Podolsky ER, Rottman SJ. Single port access (SPA) minimal access surgery through a single incision. Surg Technol Int. 2009;18:19-25.
6. Romanelli JR, Earle DB. Single-port laparoscopic surgery: an overview. Surg Endosc. 2009;23(7):1419-1427.
7. Ponsky TA. Single port laparoscopic cholecystectomy in adults and children: tools and techniques. J Am Coll Surg. 2009;209(5):e1-6.
8. Kim YW. Single port transumbilical myomectomy and ovarian cystectomy. J Minim Invasive Gynecol. 2009;16(6 suppl):S74.-
9. Ghezzi F, Cromi A, Fasola M, Bolis P. One-trocar salpingectomy for the treatment of tubal pregnancy: a “marionette-like” technique. BJOG. 2005;112(10):1417-1419.
10. Lim MC, Kim TJ, Kang S, Bae DS, Park SY, Seo SS. Embryonic natural orifice transumbilical endoscopic surgery (E-NOTES) for adnexal tumors. Surg Endosc. 2009;23(11):2445-2449.
11. Escobar PF, Starks DC, Fader AN, et al. Single-port risk-reducing salpingo-oophorectomy with and without hysterectomy: surgical outcomes and learning curve analysis. Gynecol Oncol. 2010;119(1):43-47.
12. Jeon HG, Jeong W, Oh CK. Initial experience with 50 laparoendoscopic single site surgeries using a homemade, single port device at a single center. J Urol. 2010;183(5):1866-1871.
13. Yoon G, Kim TJ, Lee YY, et al. Single port access subtotal hysterectomy with transcervical morcellation: a pilot study. J Minim Invasive Gynecol. 2010;17(1):78-81.
14. Ghomi A, Littman P, Prasad A, et al. Assessing the learning curve for laparoscopic supracervical hysterectomy. JSLS. 2007;11(2):190-194.