User login
POLICY CORNER: new documentation requirement could burden hospitalists
As of April 1, physicians who order home care services for their Medicare patients are required to document that they had a face-to-face encounter with the patient prior to certifying the patient’s eligibility for home care services. The face-to-face encounter is a mandated provision of the Affordable Care Act (ACA) of 2010, which is intended to reduce fraud and abuse among home health providers.
Despite this goal, the new documentation requirement poses the threat of a significant paperwork burden on practitioners, including hospitalists.
Many providers have remained unaware of this new requirement, but those who are aware have been experiencing confusion as to what, if any, additional paperwork is required of physicians. SHM, along with the American Medical Association (AMA) and other physician groups, have requested clarification from the Centers for Medicare & Medicaid Services (CMS) regarding the documentation requirement. SHM also is advocating that CMS keep the additional paperwork burden to a minimum.
CMS denied a request to extend the implementation deadline to allow for more provider education. Despite denying the extension, CMS has committed to continue monitoring for problems and unintended consequences caused by the new requirement.
CMS also has clarified the face-to-face documentation requirements: “Physicians may attach existing documentation as long as it includes necessary information and evidences the need for home health services.”
An example would be for a physician to attach the patient’s discharge summary or relevant portion of the patient’s medical record that evidences the need for home health services. Instead of creating an entirely new document or filling out an additional form to evidence the face-to-face encounter, physicians will have some flexibility in determining the existing documentation they will use. This is an option that hopefully will reduce some of the burden.
CMS could produce further guidelines in the future. SHM intends to continue following the issue and advocating on behalf of hospitalists. For the most up-to-date information, visit http://questions.cms.hhs.gov and enter the search term “home health face-to-face.” TH
As of April 1, physicians who order home care services for their Medicare patients are required to document that they had a face-to-face encounter with the patient prior to certifying the patient’s eligibility for home care services. The face-to-face encounter is a mandated provision of the Affordable Care Act (ACA) of 2010, which is intended to reduce fraud and abuse among home health providers.
Despite this goal, the new documentation requirement poses the threat of a significant paperwork burden on practitioners, including hospitalists.
Many providers have remained unaware of this new requirement, but those who are aware have been experiencing confusion as to what, if any, additional paperwork is required of physicians. SHM, along with the American Medical Association (AMA) and other physician groups, have requested clarification from the Centers for Medicare & Medicaid Services (CMS) regarding the documentation requirement. SHM also is advocating that CMS keep the additional paperwork burden to a minimum.
CMS denied a request to extend the implementation deadline to allow for more provider education. Despite denying the extension, CMS has committed to continue monitoring for problems and unintended consequences caused by the new requirement.
CMS also has clarified the face-to-face documentation requirements: “Physicians may attach existing documentation as long as it includes necessary information and evidences the need for home health services.”
An example would be for a physician to attach the patient’s discharge summary or relevant portion of the patient’s medical record that evidences the need for home health services. Instead of creating an entirely new document or filling out an additional form to evidence the face-to-face encounter, physicians will have some flexibility in determining the existing documentation they will use. This is an option that hopefully will reduce some of the burden.
CMS could produce further guidelines in the future. SHM intends to continue following the issue and advocating on behalf of hospitalists. For the most up-to-date information, visit http://questions.cms.hhs.gov and enter the search term “home health face-to-face.” TH
As of April 1, physicians who order home care services for their Medicare patients are required to document that they had a face-to-face encounter with the patient prior to certifying the patient’s eligibility for home care services. The face-to-face encounter is a mandated provision of the Affordable Care Act (ACA) of 2010, which is intended to reduce fraud and abuse among home health providers.
Despite this goal, the new documentation requirement poses the threat of a significant paperwork burden on practitioners, including hospitalists.
Many providers have remained unaware of this new requirement, but those who are aware have been experiencing confusion as to what, if any, additional paperwork is required of physicians. SHM, along with the American Medical Association (AMA) and other physician groups, have requested clarification from the Centers for Medicare & Medicaid Services (CMS) regarding the documentation requirement. SHM also is advocating that CMS keep the additional paperwork burden to a minimum.
CMS denied a request to extend the implementation deadline to allow for more provider education. Despite denying the extension, CMS has committed to continue monitoring for problems and unintended consequences caused by the new requirement.
CMS also has clarified the face-to-face documentation requirements: “Physicians may attach existing documentation as long as it includes necessary information and evidences the need for home health services.”
An example would be for a physician to attach the patient’s discharge summary or relevant portion of the patient’s medical record that evidences the need for home health services. Instead of creating an entirely new document or filling out an additional form to evidence the face-to-face encounter, physicians will have some flexibility in determining the existing documentation they will use. This is an option that hopefully will reduce some of the burden.
CMS could produce further guidelines in the future. SHM intends to continue following the issue and advocating on behalf of hospitalists. For the most up-to-date information, visit http://questions.cms.hhs.gov and enter the search term “home health face-to-face.” TH
SHM Honors Master Hospitalists
SHM will induct its second class of Masters in Hospital Medicine (MHM) at HM11 in May, and while each of the four honorees says the title is a personal honor, they all emphasize that it is a professional point of pride to see just how far HM has come in the past 15 years.
“For the specialty, it brings identity and awareness of all that we do,” Erin Stucky Fisher, MD, MHM, a pediatric hospitalist at Rady Children’s Hospital in San Diego, wrote in an email. “We are QI in mortal form, acting and pressing on to deliver excellence in healthcare within our systems. Each of us, members of the society, those with FHM, SFHM, and MHM—we each deliver on this promise every day.”
The other MHMs spoke to The Hospitalist in the April 13 TH eWire:
Ron Greeno, MD, MHM, chief medical officer for Cogent Healthcare and a member of SHM’s Public Policy Committee, says “I’ve had the privilege of working in hospital medicine for 18 years and, along with my colleagues at Cogent, have helped shape the field.
“To be one of a handful of hospitalists to be named a Master in Hospital Medicine is truly exciting, but equally exciting is to see the growing leadership capabilities of a number of our younger colleagues who will become the future leaders of our specialty.”
Russell L. Holman, MD, MHM, Cogent’s COO and past president of SHM, says “our specialty is constantly evolving; there is no paved road before us. We are cutting the path, and are part of an historical transformation of the way care is provided in this country. Twenty years from now we will reflect on an enduring legacy of dramatically improving the quality, safety, and sustainability of care for hospitalized patients. The privilege of being part of this movement is rewarding and inspirational for me.”
Mary Jo Gorman, MD, MBA, MHM, former SHM president and CEO of St. Louis-based Advanced ICU Care, says “it is a terrific honor to be recognized by SHM in this way. The group that is included has accomplished many things and it's gratifying to be recognized with them. It’s hard to believe that SHM has come so far that we have fellows and masters in the society! Those early days seem a long way away!”
SHM has now recognized seven MHMs. The first class consisted of Winthrop F. Whitcomb, MD, MHM, Robert Wachter, MD, MHM, and John Nelson, MD, MHM.
Each Master in HM is recognized for what SHM says is the “utmost demonstration of dedication to the field of hospital medicine through significant contributions to the development and maturation of the profession.” TH
SHM will induct its second class of Masters in Hospital Medicine (MHM) at HM11 in May, and while each of the four honorees says the title is a personal honor, they all emphasize that it is a professional point of pride to see just how far HM has come in the past 15 years.
“For the specialty, it brings identity and awareness of all that we do,” Erin Stucky Fisher, MD, MHM, a pediatric hospitalist at Rady Children’s Hospital in San Diego, wrote in an email. “We are QI in mortal form, acting and pressing on to deliver excellence in healthcare within our systems. Each of us, members of the society, those with FHM, SFHM, and MHM—we each deliver on this promise every day.”
The other MHMs spoke to The Hospitalist in the April 13 TH eWire:
Ron Greeno, MD, MHM, chief medical officer for Cogent Healthcare and a member of SHM’s Public Policy Committee, says “I’ve had the privilege of working in hospital medicine for 18 years and, along with my colleagues at Cogent, have helped shape the field.
“To be one of a handful of hospitalists to be named a Master in Hospital Medicine is truly exciting, but equally exciting is to see the growing leadership capabilities of a number of our younger colleagues who will become the future leaders of our specialty.”
Russell L. Holman, MD, MHM, Cogent’s COO and past president of SHM, says “our specialty is constantly evolving; there is no paved road before us. We are cutting the path, and are part of an historical transformation of the way care is provided in this country. Twenty years from now we will reflect on an enduring legacy of dramatically improving the quality, safety, and sustainability of care for hospitalized patients. The privilege of being part of this movement is rewarding and inspirational for me.”
Mary Jo Gorman, MD, MBA, MHM, former SHM president and CEO of St. Louis-based Advanced ICU Care, says “it is a terrific honor to be recognized by SHM in this way. The group that is included has accomplished many things and it's gratifying to be recognized with them. It’s hard to believe that SHM has come so far that we have fellows and masters in the society! Those early days seem a long way away!”
SHM has now recognized seven MHMs. The first class consisted of Winthrop F. Whitcomb, MD, MHM, Robert Wachter, MD, MHM, and John Nelson, MD, MHM.
Each Master in HM is recognized for what SHM says is the “utmost demonstration of dedication to the field of hospital medicine through significant contributions to the development and maturation of the profession.” TH
SHM will induct its second class of Masters in Hospital Medicine (MHM) at HM11 in May, and while each of the four honorees says the title is a personal honor, they all emphasize that it is a professional point of pride to see just how far HM has come in the past 15 years.
“For the specialty, it brings identity and awareness of all that we do,” Erin Stucky Fisher, MD, MHM, a pediatric hospitalist at Rady Children’s Hospital in San Diego, wrote in an email. “We are QI in mortal form, acting and pressing on to deliver excellence in healthcare within our systems. Each of us, members of the society, those with FHM, SFHM, and MHM—we each deliver on this promise every day.”
The other MHMs spoke to The Hospitalist in the April 13 TH eWire:
Ron Greeno, MD, MHM, chief medical officer for Cogent Healthcare and a member of SHM’s Public Policy Committee, says “I’ve had the privilege of working in hospital medicine for 18 years and, along with my colleagues at Cogent, have helped shape the field.
“To be one of a handful of hospitalists to be named a Master in Hospital Medicine is truly exciting, but equally exciting is to see the growing leadership capabilities of a number of our younger colleagues who will become the future leaders of our specialty.”
Russell L. Holman, MD, MHM, Cogent’s COO and past president of SHM, says “our specialty is constantly evolving; there is no paved road before us. We are cutting the path, and are part of an historical transformation of the way care is provided in this country. Twenty years from now we will reflect on an enduring legacy of dramatically improving the quality, safety, and sustainability of care for hospitalized patients. The privilege of being part of this movement is rewarding and inspirational for me.”
Mary Jo Gorman, MD, MBA, MHM, former SHM president and CEO of St. Louis-based Advanced ICU Care, says “it is a terrific honor to be recognized by SHM in this way. The group that is included has accomplished many things and it's gratifying to be recognized with them. It’s hard to believe that SHM has come so far that we have fellows and masters in the society! Those early days seem a long way away!”
SHM has now recognized seven MHMs. The first class consisted of Winthrop F. Whitcomb, MD, MHM, Robert Wachter, MD, MHM, and John Nelson, MD, MHM.
Each Master in HM is recognized for what SHM says is the “utmost demonstration of dedication to the field of hospital medicine through significant contributions to the development and maturation of the profession.” TH
What Is the Best Approach to Medical Therapy for Patients with Ischemic Stroke?
Case
A 58-year-old woman with diabetes mellitus and hypertension presents with dysarthria and weakness on the right side of her body starting six hours prior to presentation. She is afebrile and has a blood pressure of 162/84 mmHg. Exam reveals the absence of a heart murmur and no lower-extremity swelling or calf tenderness. There is weakness of the right side of the body on exam with diminished proprioception. A noncontrast head CT shows no intracranial hemorrhage. She is admitted to the hospital with the diagnosis of acute ischemic stroke. What anticlotting or antiplatelet medications should she receive?
Overview
Stroke remains a significant cause of morbidity and mortality in the U.S. and around the world. The majority of strokes are ischemic in etiology. Although thrombolytic therapy is the most effective way to salvage ischemic brain tissue that has not yet infarcted, there is a narrow window for the use of thrombolytics in the treatment of acute ischemic stroke. As a result, many patients will not be eligible for thrombolysis. Outside of 4.5 hours from symptom onset, evidence suggests that the risk outweighs the benefit of using the thrombolytic alteplase. For patients ineligible for thrombolytic therapy, antiplatelet therapy remains the best choice for treatment.
Medications that prevent blood from coagulating or clotting are used to treat and prevent a recurring or second stroke. Typically, an antiplatelet agent (most often aspirin) is initiated within 48 hours of an ischemic stroke and continued in low doses as maintenance. Multiple studies suggest that antiplatelet therapy can reduce the risk for a second stroke by 25%. Specific anticlotting agents might be warranted in some patients with high-risk conditions for a stroke.
Review of Data
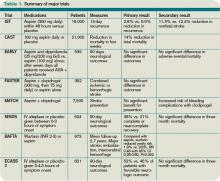
Early initiation of aspirin has shown benefit in the treatment of an acute ischemic stroke. Two major trials—the International Stroke Trial (IST) and the Chinese Acute Stroke Trial (CAST)— evaluated the role of aspirin (see Table 1, p. 15).1,2 The IST and CAST trials showed that roughly nine nonfatal strokes were avoided per every 1,000 early treatments. Taking the endpoint of death, as well as focal deficits, the two trials confirmed a rate of reduction of 13 per 1,000 patients.
Overall, the consensus was that initiating aspirin within 48 hours of a presumed ischemic cerebrovascular accident posed no major risk of hemorrhagic complication and improved the long-term outcomes.
Along with aspirin, other antiplatelet agents have been studied, most commonly dipyridamole and clopidrogel. The EARLY trial demonstrated no significant differences in the aspirin and dipyridamole groups at 90 days.3
Another large trial, which focused on clopidrogel and aspirin, looked at aspirin plus clopidrogel or aspirin alone. The FASTER trial enrolled mostly patients with mild cerebrovasular accidents (CVA) or transient ischemic attacks (TIA), and there was no difference in outcome measures between the groups.4 However, the MATCH trial found that aspirin and clopidrogel did not provide improved stroke preventions versus clopidogrel alone but had a larger risk of hemorrhagic/bleeding complications.5
Aspirin dosage is somewhat controversial. Fewer side effects occur with lower doses. Combining the trials, consensus treatment includes early aspirin dosing (325 mg initially, then 150 mg-325 mg daily) given to patients with ischemic stroke. Early aspirin should be avoided in those patients who qualify for and are receiving alteplase, heparin, or oral warfarin therapy.
There are other antiplatelet agents for long-term management of ischemic stroke. Whereas aspirin alone is used in the early management of acute ischemic stroke in those ineligible for thrombolytic therapy, many patients are transitioned to other antiplatelet strategies for secondary prevention long-term. The number needed to treat for aspirin to reduce one future stroke, myocardial infarction (MI), or vascular death when compared to placebo is quite high at 33. However, the combination of aspirin and dipyradimole does not prevent MI, vascular death, or the combined endpoint of either stroke or death.
Clopidogrel is more effective than aspirin in preventing a combined endpoint of ischemic stroke, MI, or vascular death, but it is not superior to aspirin in preventing recurrent stroke in TIA or stroke patients. The effects of clopidrogel are greater in patients with peripheral arterial disease, previous coronary artery bypass grafting, insulin-dependent diabetes, or recurrent vascular events.
There is a substantially high cost of treatment and long-term disability associated with stroke. Costs can vary from 3% to 5% of the annual healthcare budget. The newer antiplatelet agents are more expensive than aspirin, and overall cost-effectiveness is difficult to estimate. Yet, from an economic standpoint, the combination of aspirin and dipyradimole can be recommended as an alternative for secondary stroke prevention in patients without major comorbidities. In those patients with higher risk factors and/or comorbidities, clopidogrel might be more cost-effective than aspirin alone. Furthermore, in patients with aspirin intolerance, clopidogrel is a useful, but expensive, alternative.
Thrombolytic therapy. Restora-tion of blood flow with thrombolytic therapy is the most effective way of salvaging ischemic brain tissue that has not already infarcted. The window for use of the thrombolytic alteplase is narrow; studies suggest that its benefit diminishes with increasing time to treatment. Indeed, after 4.5 hours from the onset of symptoms, evidence suggests that the harm might outweigh the benefit, so the determination of who is eligible for its use has to be made quickly.
Guidelines published by the American Heart Association/American Stoke Association stroke council outline strict inclusion and exclusion criteria for the use of alteplase in the management of acute ischemic stroke.6 Obtaining informed consent and emergent neuroimaging are vital in preventing delays in alteplase administration.
Two major trials that illustrate the benefit of alteplase in the treatment of acute ischemic stroke are the NINDS trial and the ECASS 3 trial. NINDS showed that when intravenous alteplase was used within three hours of symptom onset, patients had improved functional outcome at three months.7 The ECASS 3 trial showed that intravenous alteplase has benefit when given up to 4.5 hours after symptom onset.8 Treatment with intravenous alteplase from three-4.5 hours in the ECASS 3 trial showed a modest improvement in patient outcomes at three months, with a number needed to treat of 14 for a favorable outcome.
A 2010 meta-analysis looked specifically at outcomes in stroke based on time to treat with alteplase using pooled data from the NINDS, ATLANTIS, ECASS (1, 2, and 3), and EPITHET trials.9 It showed that the number needed to treat for a favorable outcome at three months increased steadily when time to treatment was delayed. It also showed that the risk of death after alteplase administration increased significantly after 4.5 hours. Thus, after 4.5 hours, it suggests that harm might exceed the benefits of treatment.
Anticoagulant use in ischemic stroke. Clinical trials have not been effective in demonstrating the use of heparin and low-molecular-weight heparins (LMWHs). A 2008 systematic review of 24 trials (approximately 24,000 patients) demonstrated:
- Anticoagulant therapy did not reduce odds of death;
- Therapy was associated with nine fewer recurrent ischemic strokes per 1,000 patients, but also showed a similar increase in symptomatic intracranial hemorrhages; and
- Overall, researchers could not specify a particular anticoagulant mode or regimen that had an overall net patient benefit.
The use of heparin in atrial fibrillation and stroke has generated controversy in recent years. Review of the data, however, indicates that early treatment with heparin might cause more harm than benefit. A 2007 meta-analysis did not support the use of early anticoagulant therapy. Seven trials (4,200 patients) compared heparin or LMWH started within 48 hours to other treatments (aspirin, placebo). The study authors found:
- Nonsignificant reduction in recurrent ischemic stroke within seven to 14 days;
- Statistically significant increase in symptomatic intracranial hemorrhages; and
- Similar rates of death/disability at final follow-up of studies.
For those patients who continue to demonstrate neurological deterioration, heparin and LMWH use did not appear to improve outcomes. Therefore, based on a consensus of national guidelines, the use of full-dose anticoagulation with heparin or LMWH is not recommended.
The data suggest that in patients with stroke secondary to:
- Dissection of cervical or intracranial arteries;
- Intracardiac thrombus and valvular disease; and
- Mechanical heart valves, full-dose anticoagulation can be initiated. However, the benefit is unproven.
Back to the Case
Our patient with acute ischemic stroke with right-sided weakness on exam presented outside of the window within which alteplase could be administered safely. She was started on aspirin 325 mg daily. There was no indication for full anticoagulation with intravenous heparin or warfarin. Her weakness showed slight improvement on exam during the hospitalization. As an insulin-dependent diabetic, she was thought to be at high risk for recurrent stroke. As such, she was transitioned to a combination of aspirin and clopidogrel prior to her discharge to an acute inpatient rehabilitation hospital.
Bottom Line
Early aspirin therapy (within 48 hours) is recommended (initial dose 325 mg, then 150 mg-325 mg daily) for patients with ischemic stroke who are not candidates for alteplase, IV heparin, or oral anticoagulants.10 Aspirin is the only antiplatelet agent that has been shown to be effective for the early treatment of acute ischemic stroke. In patients without contraindications, aspirin, the combination of aspirin-dipyradimole, or clopidogrel is appropriate for secondary prevention.
The subset of patients at high risk of recurrent stroke should be transitioned to clopidogrel or aspirin/clopidogrel, unless otherwise contraindicated. TH
Dr. Chaturvedi is an instructor in the Division of Hospital Medicine at Northwestern University’s Feinberg School of Medicine in Chicago, and medical director of HM at Northwestern Lake Forest Hospital. Dr. Abraham is an instructor in the Division of Hospital Medicine at Northwestern University Feinberg School of Medicine.
References
- The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19,435 patients with acute ischemic stroke. International Stroke Trial Collaborative Group. Lancet. 1997;349:1569-1581.
- CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet. 1997;349:1641-1649.
- Dengler R, Diener HC, Schwartz A, et al. Early treatment with aspirin plus extended-release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:159-166.
- Kennedy J, Hill MD, Ryckborst KJ, et al. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 2007;6:961-969.
- Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
- Adams HP Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655-1711.
- Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695-1703.
- Hacke W, Kaste M, Bluhmki E, et al. Thombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329.
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581-1587.
- Albers GW, Amarenco P, Easton JD, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:630S-669S.
Case
A 58-year-old woman with diabetes mellitus and hypertension presents with dysarthria and weakness on the right side of her body starting six hours prior to presentation. She is afebrile and has a blood pressure of 162/84 mmHg. Exam reveals the absence of a heart murmur and no lower-extremity swelling or calf tenderness. There is weakness of the right side of the body on exam with diminished proprioception. A noncontrast head CT shows no intracranial hemorrhage. She is admitted to the hospital with the diagnosis of acute ischemic stroke. What anticlotting or antiplatelet medications should she receive?
Overview
Stroke remains a significant cause of morbidity and mortality in the U.S. and around the world. The majority of strokes are ischemic in etiology. Although thrombolytic therapy is the most effective way to salvage ischemic brain tissue that has not yet infarcted, there is a narrow window for the use of thrombolytics in the treatment of acute ischemic stroke. As a result, many patients will not be eligible for thrombolysis. Outside of 4.5 hours from symptom onset, evidence suggests that the risk outweighs the benefit of using the thrombolytic alteplase. For patients ineligible for thrombolytic therapy, antiplatelet therapy remains the best choice for treatment.
Medications that prevent blood from coagulating or clotting are used to treat and prevent a recurring or second stroke. Typically, an antiplatelet agent (most often aspirin) is initiated within 48 hours of an ischemic stroke and continued in low doses as maintenance. Multiple studies suggest that antiplatelet therapy can reduce the risk for a second stroke by 25%. Specific anticlotting agents might be warranted in some patients with high-risk conditions for a stroke.
Review of Data
Early initiation of aspirin has shown benefit in the treatment of an acute ischemic stroke. Two major trials—the International Stroke Trial (IST) and the Chinese Acute Stroke Trial (CAST)— evaluated the role of aspirin (see Table 1, p. 15).1,2 The IST and CAST trials showed that roughly nine nonfatal strokes were avoided per every 1,000 early treatments. Taking the endpoint of death, as well as focal deficits, the two trials confirmed a rate of reduction of 13 per 1,000 patients.
Overall, the consensus was that initiating aspirin within 48 hours of a presumed ischemic cerebrovascular accident posed no major risk of hemorrhagic complication and improved the long-term outcomes.
Along with aspirin, other antiplatelet agents have been studied, most commonly dipyridamole and clopidrogel. The EARLY trial demonstrated no significant differences in the aspirin and dipyridamole groups at 90 days.3
Another large trial, which focused on clopidrogel and aspirin, looked at aspirin plus clopidrogel or aspirin alone. The FASTER trial enrolled mostly patients with mild cerebrovasular accidents (CVA) or transient ischemic attacks (TIA), and there was no difference in outcome measures between the groups.4 However, the MATCH trial found that aspirin and clopidrogel did not provide improved stroke preventions versus clopidogrel alone but had a larger risk of hemorrhagic/bleeding complications.5
Aspirin dosage is somewhat controversial. Fewer side effects occur with lower doses. Combining the trials, consensus treatment includes early aspirin dosing (325 mg initially, then 150 mg-325 mg daily) given to patients with ischemic stroke. Early aspirin should be avoided in those patients who qualify for and are receiving alteplase, heparin, or oral warfarin therapy.
There are other antiplatelet agents for long-term management of ischemic stroke. Whereas aspirin alone is used in the early management of acute ischemic stroke in those ineligible for thrombolytic therapy, many patients are transitioned to other antiplatelet strategies for secondary prevention long-term. The number needed to treat for aspirin to reduce one future stroke, myocardial infarction (MI), or vascular death when compared to placebo is quite high at 33. However, the combination of aspirin and dipyradimole does not prevent MI, vascular death, or the combined endpoint of either stroke or death.
Clopidogrel is more effective than aspirin in preventing a combined endpoint of ischemic stroke, MI, or vascular death, but it is not superior to aspirin in preventing recurrent stroke in TIA or stroke patients. The effects of clopidrogel are greater in patients with peripheral arterial disease, previous coronary artery bypass grafting, insulin-dependent diabetes, or recurrent vascular events.
There is a substantially high cost of treatment and long-term disability associated with stroke. Costs can vary from 3% to 5% of the annual healthcare budget. The newer antiplatelet agents are more expensive than aspirin, and overall cost-effectiveness is difficult to estimate. Yet, from an economic standpoint, the combination of aspirin and dipyradimole can be recommended as an alternative for secondary stroke prevention in patients without major comorbidities. In those patients with higher risk factors and/or comorbidities, clopidogrel might be more cost-effective than aspirin alone. Furthermore, in patients with aspirin intolerance, clopidogrel is a useful, but expensive, alternative.
Thrombolytic therapy. Restora-tion of blood flow with thrombolytic therapy is the most effective way of salvaging ischemic brain tissue that has not already infarcted. The window for use of the thrombolytic alteplase is narrow; studies suggest that its benefit diminishes with increasing time to treatment. Indeed, after 4.5 hours from the onset of symptoms, evidence suggests that the harm might outweigh the benefit, so the determination of who is eligible for its use has to be made quickly.
Guidelines published by the American Heart Association/American Stoke Association stroke council outline strict inclusion and exclusion criteria for the use of alteplase in the management of acute ischemic stroke.6 Obtaining informed consent and emergent neuroimaging are vital in preventing delays in alteplase administration.
Two major trials that illustrate the benefit of alteplase in the treatment of acute ischemic stroke are the NINDS trial and the ECASS 3 trial. NINDS showed that when intravenous alteplase was used within three hours of symptom onset, patients had improved functional outcome at three months.7 The ECASS 3 trial showed that intravenous alteplase has benefit when given up to 4.5 hours after symptom onset.8 Treatment with intravenous alteplase from three-4.5 hours in the ECASS 3 trial showed a modest improvement in patient outcomes at three months, with a number needed to treat of 14 for a favorable outcome.
A 2010 meta-analysis looked specifically at outcomes in stroke based on time to treat with alteplase using pooled data from the NINDS, ATLANTIS, ECASS (1, 2, and 3), and EPITHET trials.9 It showed that the number needed to treat for a favorable outcome at three months increased steadily when time to treatment was delayed. It also showed that the risk of death after alteplase administration increased significantly after 4.5 hours. Thus, after 4.5 hours, it suggests that harm might exceed the benefits of treatment.
Anticoagulant use in ischemic stroke. Clinical trials have not been effective in demonstrating the use of heparin and low-molecular-weight heparins (LMWHs). A 2008 systematic review of 24 trials (approximately 24,000 patients) demonstrated:
- Anticoagulant therapy did not reduce odds of death;
- Therapy was associated with nine fewer recurrent ischemic strokes per 1,000 patients, but also showed a similar increase in symptomatic intracranial hemorrhages; and
- Overall, researchers could not specify a particular anticoagulant mode or regimen that had an overall net patient benefit.
The use of heparin in atrial fibrillation and stroke has generated controversy in recent years. Review of the data, however, indicates that early treatment with heparin might cause more harm than benefit. A 2007 meta-analysis did not support the use of early anticoagulant therapy. Seven trials (4,200 patients) compared heparin or LMWH started within 48 hours to other treatments (aspirin, placebo). The study authors found:
- Nonsignificant reduction in recurrent ischemic stroke within seven to 14 days;
- Statistically significant increase in symptomatic intracranial hemorrhages; and
- Similar rates of death/disability at final follow-up of studies.
For those patients who continue to demonstrate neurological deterioration, heparin and LMWH use did not appear to improve outcomes. Therefore, based on a consensus of national guidelines, the use of full-dose anticoagulation with heparin or LMWH is not recommended.
The data suggest that in patients with stroke secondary to:
- Dissection of cervical or intracranial arteries;
- Intracardiac thrombus and valvular disease; and
- Mechanical heart valves, full-dose anticoagulation can be initiated. However, the benefit is unproven.
Back to the Case
Our patient with acute ischemic stroke with right-sided weakness on exam presented outside of the window within which alteplase could be administered safely. She was started on aspirin 325 mg daily. There was no indication for full anticoagulation with intravenous heparin or warfarin. Her weakness showed slight improvement on exam during the hospitalization. As an insulin-dependent diabetic, she was thought to be at high risk for recurrent stroke. As such, she was transitioned to a combination of aspirin and clopidogrel prior to her discharge to an acute inpatient rehabilitation hospital.
Bottom Line
Early aspirin therapy (within 48 hours) is recommended (initial dose 325 mg, then 150 mg-325 mg daily) for patients with ischemic stroke who are not candidates for alteplase, IV heparin, or oral anticoagulants.10 Aspirin is the only antiplatelet agent that has been shown to be effective for the early treatment of acute ischemic stroke. In patients without contraindications, aspirin, the combination of aspirin-dipyradimole, or clopidogrel is appropriate for secondary prevention.
The subset of patients at high risk of recurrent stroke should be transitioned to clopidogrel or aspirin/clopidogrel, unless otherwise contraindicated. TH
Dr. Chaturvedi is an instructor in the Division of Hospital Medicine at Northwestern University’s Feinberg School of Medicine in Chicago, and medical director of HM at Northwestern Lake Forest Hospital. Dr. Abraham is an instructor in the Division of Hospital Medicine at Northwestern University Feinberg School of Medicine.
References
- The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19,435 patients with acute ischemic stroke. International Stroke Trial Collaborative Group. Lancet. 1997;349:1569-1581.
- CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet. 1997;349:1641-1649.
- Dengler R, Diener HC, Schwartz A, et al. Early treatment with aspirin plus extended-release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:159-166.
- Kennedy J, Hill MD, Ryckborst KJ, et al. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 2007;6:961-969.
- Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
- Adams HP Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655-1711.
- Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695-1703.
- Hacke W, Kaste M, Bluhmki E, et al. Thombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329.
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581-1587.
- Albers GW, Amarenco P, Easton JD, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:630S-669S.
Case
A 58-year-old woman with diabetes mellitus and hypertension presents with dysarthria and weakness on the right side of her body starting six hours prior to presentation. She is afebrile and has a blood pressure of 162/84 mmHg. Exam reveals the absence of a heart murmur and no lower-extremity swelling or calf tenderness. There is weakness of the right side of the body on exam with diminished proprioception. A noncontrast head CT shows no intracranial hemorrhage. She is admitted to the hospital with the diagnosis of acute ischemic stroke. What anticlotting or antiplatelet medications should she receive?
Overview
Stroke remains a significant cause of morbidity and mortality in the U.S. and around the world. The majority of strokes are ischemic in etiology. Although thrombolytic therapy is the most effective way to salvage ischemic brain tissue that has not yet infarcted, there is a narrow window for the use of thrombolytics in the treatment of acute ischemic stroke. As a result, many patients will not be eligible for thrombolysis. Outside of 4.5 hours from symptom onset, evidence suggests that the risk outweighs the benefit of using the thrombolytic alteplase. For patients ineligible for thrombolytic therapy, antiplatelet therapy remains the best choice for treatment.
Medications that prevent blood from coagulating or clotting are used to treat and prevent a recurring or second stroke. Typically, an antiplatelet agent (most often aspirin) is initiated within 48 hours of an ischemic stroke and continued in low doses as maintenance. Multiple studies suggest that antiplatelet therapy can reduce the risk for a second stroke by 25%. Specific anticlotting agents might be warranted in some patients with high-risk conditions for a stroke.
Review of Data
Early initiation of aspirin has shown benefit in the treatment of an acute ischemic stroke. Two major trials—the International Stroke Trial (IST) and the Chinese Acute Stroke Trial (CAST)— evaluated the role of aspirin (see Table 1, p. 15).1,2 The IST and CAST trials showed that roughly nine nonfatal strokes were avoided per every 1,000 early treatments. Taking the endpoint of death, as well as focal deficits, the two trials confirmed a rate of reduction of 13 per 1,000 patients.
Overall, the consensus was that initiating aspirin within 48 hours of a presumed ischemic cerebrovascular accident posed no major risk of hemorrhagic complication and improved the long-term outcomes.
Along with aspirin, other antiplatelet agents have been studied, most commonly dipyridamole and clopidrogel. The EARLY trial demonstrated no significant differences in the aspirin and dipyridamole groups at 90 days.3
Another large trial, which focused on clopidrogel and aspirin, looked at aspirin plus clopidrogel or aspirin alone. The FASTER trial enrolled mostly patients with mild cerebrovasular accidents (CVA) or transient ischemic attacks (TIA), and there was no difference in outcome measures between the groups.4 However, the MATCH trial found that aspirin and clopidrogel did not provide improved stroke preventions versus clopidogrel alone but had a larger risk of hemorrhagic/bleeding complications.5
Aspirin dosage is somewhat controversial. Fewer side effects occur with lower doses. Combining the trials, consensus treatment includes early aspirin dosing (325 mg initially, then 150 mg-325 mg daily) given to patients with ischemic stroke. Early aspirin should be avoided in those patients who qualify for and are receiving alteplase, heparin, or oral warfarin therapy.
There are other antiplatelet agents for long-term management of ischemic stroke. Whereas aspirin alone is used in the early management of acute ischemic stroke in those ineligible for thrombolytic therapy, many patients are transitioned to other antiplatelet strategies for secondary prevention long-term. The number needed to treat for aspirin to reduce one future stroke, myocardial infarction (MI), or vascular death when compared to placebo is quite high at 33. However, the combination of aspirin and dipyradimole does not prevent MI, vascular death, or the combined endpoint of either stroke or death.
Clopidogrel is more effective than aspirin in preventing a combined endpoint of ischemic stroke, MI, or vascular death, but it is not superior to aspirin in preventing recurrent stroke in TIA or stroke patients. The effects of clopidrogel are greater in patients with peripheral arterial disease, previous coronary artery bypass grafting, insulin-dependent diabetes, or recurrent vascular events.
There is a substantially high cost of treatment and long-term disability associated with stroke. Costs can vary from 3% to 5% of the annual healthcare budget. The newer antiplatelet agents are more expensive than aspirin, and overall cost-effectiveness is difficult to estimate. Yet, from an economic standpoint, the combination of aspirin and dipyradimole can be recommended as an alternative for secondary stroke prevention in patients without major comorbidities. In those patients with higher risk factors and/or comorbidities, clopidogrel might be more cost-effective than aspirin alone. Furthermore, in patients with aspirin intolerance, clopidogrel is a useful, but expensive, alternative.
Thrombolytic therapy. Restora-tion of blood flow with thrombolytic therapy is the most effective way of salvaging ischemic brain tissue that has not already infarcted. The window for use of the thrombolytic alteplase is narrow; studies suggest that its benefit diminishes with increasing time to treatment. Indeed, after 4.5 hours from the onset of symptoms, evidence suggests that the harm might outweigh the benefit, so the determination of who is eligible for its use has to be made quickly.
Guidelines published by the American Heart Association/American Stoke Association stroke council outline strict inclusion and exclusion criteria for the use of alteplase in the management of acute ischemic stroke.6 Obtaining informed consent and emergent neuroimaging are vital in preventing delays in alteplase administration.
Two major trials that illustrate the benefit of alteplase in the treatment of acute ischemic stroke are the NINDS trial and the ECASS 3 trial. NINDS showed that when intravenous alteplase was used within three hours of symptom onset, patients had improved functional outcome at three months.7 The ECASS 3 trial showed that intravenous alteplase has benefit when given up to 4.5 hours after symptom onset.8 Treatment with intravenous alteplase from three-4.5 hours in the ECASS 3 trial showed a modest improvement in patient outcomes at three months, with a number needed to treat of 14 for a favorable outcome.
A 2010 meta-analysis looked specifically at outcomes in stroke based on time to treat with alteplase using pooled data from the NINDS, ATLANTIS, ECASS (1, 2, and 3), and EPITHET trials.9 It showed that the number needed to treat for a favorable outcome at three months increased steadily when time to treatment was delayed. It also showed that the risk of death after alteplase administration increased significantly after 4.5 hours. Thus, after 4.5 hours, it suggests that harm might exceed the benefits of treatment.
Anticoagulant use in ischemic stroke. Clinical trials have not been effective in demonstrating the use of heparin and low-molecular-weight heparins (LMWHs). A 2008 systematic review of 24 trials (approximately 24,000 patients) demonstrated:
- Anticoagulant therapy did not reduce odds of death;
- Therapy was associated with nine fewer recurrent ischemic strokes per 1,000 patients, but also showed a similar increase in symptomatic intracranial hemorrhages; and
- Overall, researchers could not specify a particular anticoagulant mode or regimen that had an overall net patient benefit.
The use of heparin in atrial fibrillation and stroke has generated controversy in recent years. Review of the data, however, indicates that early treatment with heparin might cause more harm than benefit. A 2007 meta-analysis did not support the use of early anticoagulant therapy. Seven trials (4,200 patients) compared heparin or LMWH started within 48 hours to other treatments (aspirin, placebo). The study authors found:
- Nonsignificant reduction in recurrent ischemic stroke within seven to 14 days;
- Statistically significant increase in symptomatic intracranial hemorrhages; and
- Similar rates of death/disability at final follow-up of studies.
For those patients who continue to demonstrate neurological deterioration, heparin and LMWH use did not appear to improve outcomes. Therefore, based on a consensus of national guidelines, the use of full-dose anticoagulation with heparin or LMWH is not recommended.
The data suggest that in patients with stroke secondary to:
- Dissection of cervical or intracranial arteries;
- Intracardiac thrombus and valvular disease; and
- Mechanical heart valves, full-dose anticoagulation can be initiated. However, the benefit is unproven.
Back to the Case
Our patient with acute ischemic stroke with right-sided weakness on exam presented outside of the window within which alteplase could be administered safely. She was started on aspirin 325 mg daily. There was no indication for full anticoagulation with intravenous heparin or warfarin. Her weakness showed slight improvement on exam during the hospitalization. As an insulin-dependent diabetic, she was thought to be at high risk for recurrent stroke. As such, she was transitioned to a combination of aspirin and clopidogrel prior to her discharge to an acute inpatient rehabilitation hospital.
Bottom Line
Early aspirin therapy (within 48 hours) is recommended (initial dose 325 mg, then 150 mg-325 mg daily) for patients with ischemic stroke who are not candidates for alteplase, IV heparin, or oral anticoagulants.10 Aspirin is the only antiplatelet agent that has been shown to be effective for the early treatment of acute ischemic stroke. In patients without contraindications, aspirin, the combination of aspirin-dipyradimole, or clopidogrel is appropriate for secondary prevention.
The subset of patients at high risk of recurrent stroke should be transitioned to clopidogrel or aspirin/clopidogrel, unless otherwise contraindicated. TH
Dr. Chaturvedi is an instructor in the Division of Hospital Medicine at Northwestern University’s Feinberg School of Medicine in Chicago, and medical director of HM at Northwestern Lake Forest Hospital. Dr. Abraham is an instructor in the Division of Hospital Medicine at Northwestern University Feinberg School of Medicine.
References
- The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19,435 patients with acute ischemic stroke. International Stroke Trial Collaborative Group. Lancet. 1997;349:1569-1581.
- CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet. 1997;349:1641-1649.
- Dengler R, Diener HC, Schwartz A, et al. Early treatment with aspirin plus extended-release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:159-166.
- Kennedy J, Hill MD, Ryckborst KJ, et al. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 2007;6:961-969.
- Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
- Adams HP Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655-1711.
- Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695-1703.
- Hacke W, Kaste M, Bluhmki E, et al. Thombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329.
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581-1587.
- Albers GW, Amarenco P, Easton JD, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:630S-669S.
Risk factors associated with nephrotoxicity in children receiving vancomycin?
Clinical question: What are the risk factors associated with nephrotoxicity in children receiving vancomycin?
Background: As rates of antimicrobial resistance increase for such common bacteria as Streptococcus pneumoniae and Staphylococcus aureus, vancomycin increasingly has been used in children. Notably, rates of serious methicillin-resistant Staphylococcus aureus (MRSA) infection have increased significantly, and aggressive vancomycin-dosing regimens have been recommended in these situations. Rates and risk factors associated with nephrotoxicity in children receiving vancomycin are not well-established.
Study design: Retrospective cohort study.
Setting: Tertiary-care children’s hospital.
Synopsis: Using a pharmacy database, which included comprehensive clinical and pharmacokinetic data, the records of 167 children from one week to 18 years of age were reviewed if they received at least 48 hours of vancomycin from December 2007 to April 2009. Nephrotoxicity was defined as an increase in the serum creatinine (SCr) of at least 0.5 mg/dL or a 50% increase in baseline SCr on at least two consecutive days. Average trough levels were calculated and categorized as high (≥15 mg/dL) or low (<15 mg/dL).
Significantly more patients in the high-trough group developed nephrotoxicity (28%) compared with the low-trough group (7%). After multivariable logistic regression analysis, patients with high trough concentrations, ICU stays, and furosemide administration were more likely to have nephrotoxicity.
This study replicates findings from the adult literature demonstrating an association between high vancomycin troughs and nephrotoxicity. It remains difficult to demonstrate causality given the use of indirect markers of vancomycin-induced renal injury, as well as the lack of a control group (particularly a group of similarly ill ICU patients). Nevertheless, the authors provide useful and detailed pharmacologic observations for patients who receive aggressive vancomycin dosing.
Bottom line: High vancomycin troughs are associated with nephrotoxicity.
Citation: McKamy S, Hernandez E, Jahng M, Moriwaki T, Deveikis A, Le J. Incidence and risk factors influencing the development of vancomycin nephrotoxicity in children. J Pediatr. 2011;158:422-426.
Reviewed by Pediatric Editor Mark Shen, MD, medical director of hospital medicine at Dell Children’s Medical Center, Austin, Texas.
Clinical question: What are the risk factors associated with nephrotoxicity in children receiving vancomycin?
Background: As rates of antimicrobial resistance increase for such common bacteria as Streptococcus pneumoniae and Staphylococcus aureus, vancomycin increasingly has been used in children. Notably, rates of serious methicillin-resistant Staphylococcus aureus (MRSA) infection have increased significantly, and aggressive vancomycin-dosing regimens have been recommended in these situations. Rates and risk factors associated with nephrotoxicity in children receiving vancomycin are not well-established.
Study design: Retrospective cohort study.
Setting: Tertiary-care children’s hospital.
Synopsis: Using a pharmacy database, which included comprehensive clinical and pharmacokinetic data, the records of 167 children from one week to 18 years of age were reviewed if they received at least 48 hours of vancomycin from December 2007 to April 2009. Nephrotoxicity was defined as an increase in the serum creatinine (SCr) of at least 0.5 mg/dL or a 50% increase in baseline SCr on at least two consecutive days. Average trough levels were calculated and categorized as high (≥15 mg/dL) or low (<15 mg/dL).
Significantly more patients in the high-trough group developed nephrotoxicity (28%) compared with the low-trough group (7%). After multivariable logistic regression analysis, patients with high trough concentrations, ICU stays, and furosemide administration were more likely to have nephrotoxicity.
This study replicates findings from the adult literature demonstrating an association between high vancomycin troughs and nephrotoxicity. It remains difficult to demonstrate causality given the use of indirect markers of vancomycin-induced renal injury, as well as the lack of a control group (particularly a group of similarly ill ICU patients). Nevertheless, the authors provide useful and detailed pharmacologic observations for patients who receive aggressive vancomycin dosing.
Bottom line: High vancomycin troughs are associated with nephrotoxicity.
Citation: McKamy S, Hernandez E, Jahng M, Moriwaki T, Deveikis A, Le J. Incidence and risk factors influencing the development of vancomycin nephrotoxicity in children. J Pediatr. 2011;158:422-426.
Reviewed by Pediatric Editor Mark Shen, MD, medical director of hospital medicine at Dell Children’s Medical Center, Austin, Texas.
Clinical question: What are the risk factors associated with nephrotoxicity in children receiving vancomycin?
Background: As rates of antimicrobial resistance increase for such common bacteria as Streptococcus pneumoniae and Staphylococcus aureus, vancomycin increasingly has been used in children. Notably, rates of serious methicillin-resistant Staphylococcus aureus (MRSA) infection have increased significantly, and aggressive vancomycin-dosing regimens have been recommended in these situations. Rates and risk factors associated with nephrotoxicity in children receiving vancomycin are not well-established.
Study design: Retrospective cohort study.
Setting: Tertiary-care children’s hospital.
Synopsis: Using a pharmacy database, which included comprehensive clinical and pharmacokinetic data, the records of 167 children from one week to 18 years of age were reviewed if they received at least 48 hours of vancomycin from December 2007 to April 2009. Nephrotoxicity was defined as an increase in the serum creatinine (SCr) of at least 0.5 mg/dL or a 50% increase in baseline SCr on at least two consecutive days. Average trough levels were calculated and categorized as high (≥15 mg/dL) or low (<15 mg/dL).
Significantly more patients in the high-trough group developed nephrotoxicity (28%) compared with the low-trough group (7%). After multivariable logistic regression analysis, patients with high trough concentrations, ICU stays, and furosemide administration were more likely to have nephrotoxicity.
This study replicates findings from the adult literature demonstrating an association between high vancomycin troughs and nephrotoxicity. It remains difficult to demonstrate causality given the use of indirect markers of vancomycin-induced renal injury, as well as the lack of a control group (particularly a group of similarly ill ICU patients). Nevertheless, the authors provide useful and detailed pharmacologic observations for patients who receive aggressive vancomycin dosing.
Bottom line: High vancomycin troughs are associated with nephrotoxicity.
Citation: McKamy S, Hernandez E, Jahng M, Moriwaki T, Deveikis A, Le J. Incidence and risk factors influencing the development of vancomycin nephrotoxicity in children. J Pediatr. 2011;158:422-426.
Reviewed by Pediatric Editor Mark Shen, MD, medical director of hospital medicine at Dell Children’s Medical Center, Austin, Texas.
In the Literature: HM-Related Research You Need to Know
Literature at a Glance
A guide to this month’s studies
- Rivaroxaban for VTE
- Cost-effectiveness of dabigatran in atrial fibrillation
- Effect of new resident duty-hour limits
- Outcomes of care at acute-stroke centers
- Effect on MIC in patients with MRSA pneumonia
- Optimal hemodialysis frequency
- Effect of BNP testing on hospital length of stay
- Impact of herpes zoster vaccination
- 30-day readmission rates in for-profit hospitals
Oral Rivaroxaban Could Play a Role in VTE Treatment
Clinical question: Is oral rivaroxaban an acceptable treatment option for acute symptomatic deep-vein thrombosis (DVT) and venous thromboembolism (VTE)?
Background: Treatment of acute DVT requires frequent laboratory monitoring, which may be obviated by the use of fixed-dose oral rivaroxaban.
Study designs: Parallel randomized, open-label, event-driven, noninferiority study (the acute DVT study) and randomized, double-blind, placebo-controlled, event-driven superiority trial (continued treatment study).
Setting: Multicenter study.
Synopsis: The acute DVT study randomly assigned 3,449 patients with acute DVT to oral rivaroxaban 15 mg twice daily for three weeks followed by 20 mg daily for three, six, or 12 months or enoxaparin 1 mg/kg subcutaneously twice daily and daily warfarin until a therapeutic INR was achieved, at which time the enoxaparin was discontinued. Rivaroxaban was not inferior in terms of preventing recurrent VTE (2.1% vs. 3.0%; P<0.001). Major or clinically relevant nonmajor bleeding occurred equally in both groups (8.1%).
The continued treatment study randomly assigned 1,196 patients with six to 12 months of prior VTE treatment to rivaroxaban 20 mg daily versus placebo for six or 12 months. Rivaroxaban was superior in preventing recurrent VTE (1.3% vs. 7.1%; P<0.001). A statistically nonsignificant increase in major bleeding was reported with rivaroxaban (0.7% vs. 0.0%). The open-label design and pharmaceutical support create potential for bias.
Bottom line: Oral rivaroxaban might offer a simplified, effective, and safe alternative to enoxaparin and warfarin for short- and long-term VTE treatment.
Citation: The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363 (26):2499-2510.
Dabigatran Might Be a Cost-Effective Alternative to Warfarin in Atrial Fibrillation
Clinical question: Is dabigatran cost-effective compared to warfarin for prevention of stroke in atrial fibrillation?
Background: Dabigatran, a direct thrombin inhibitor, is FDA-approved for the prevention of stroke and systemic embolism in atrial fibrillation. In the 2009 RE-LY trial, dabigatran 150 mg twice daily was associated with fewer embolic strokes than warfarin with similar episodes of major hemorrhage. Dabigatran costs more than warfarin; its cost-effectiveness is unknown.
Study design: Markov decision model.
Setting: Data from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY), a multinational randomized trial, and other anticoagulation studies.
Synopsis: This model simulated costs and outcomes for a theoretical cohort of patients >65 with atrial fibrillation and CHADS2 score ≥1 taking either lifelong warfarin or dabigatran. The model included assumptions about costs and quality-of-life effects of INR monitoring, stroke, hemorrhage, and myocardial infarction. Because U.S. pricing for dabigatran was pending, the authors assumed $13 per day.
Both life expectancy in quality-adjusted life years (QALYs) and lifetime costs were higher for dabigatran than for warfarin (10.84 vs. 10.28 QALYs and $168,398 vs. $143,193, respectively). The incremental cost per QALY for dabigatran was $45,372. Limitations include dependence on data from a single-manufacturer-sponsored trial with limited follow-up.
Retail costs for dabigatran are now known to be about $8 per day. When the model is adjusted to that price, an additional QALY would cost $12,000, well below the commonly accepted threshold of $50,000.
Bottom line: Dabigatran is likely a cost-effective alternative to warfarin in nonvalvular atrial fibrillation.
Citation: Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154(1):1-11.
Effects of New ACGME Mandates on Patients and Residents Unclear
Clinical question: How will new intern duty-hour standards impact patient care, residents’ health, and education?
Background: The Accreditation Council for Graduate Medical Education (ACGME) has mandated new duty-hour standards that limit interns’ shifts to 16 hours and night float to six consecutive nights. They also strongly recommend a nighttime nap.
Study design: Systematic review of English-language, original research studies addressing shift length, night float, or protected sleep time, published from 1989 to 2010.
Synopsis: Sixty-four out of 5,345 articles met eligibility criteria, including four randomized controlled trials and five multi-institutional studies. Although 73% of studies examining shift length supported reducing hours, optimal shift duration was not determined. All studies addressing night float examined five to seven consecutive nights of work; data were too heterogeneous for generalization. Data on protected sleep time were too limited to determine effect on residents and patients.
The majority of studies were conducted at single institutions and study designs carried high risk for interpretation bias. Additionally, publication bias might have influenced the results of this review of English-language-only studies.
Bottom line: The available studies that attempt to elucidate the effects of major changes in residency training have significant limitations, and the potential impact of the new standards on patients and residents remains uncertain.
Citation: Reed DA, Fletcher KE, Arora VM. Systematic review: association of shift length, protected sleep time, and night float with patient care, residents’ health, and education. Ann Intern Med. 2010;153:829-842.
Admission to Stroke Centers for Acute Ischemic Stroke Might Improve Mortality
Clinical question: Does admission to a certified stroke center improve survival in patients with acute ischemic stroke?
Background: Since 2003, the Joint Commission has designated fewer than 700 acute-care hospitals as certified stroke centers. However, no large studies have examined whether patients with acute stroke admitted to stroke centers have lower mortality than those admitted to noncertified acute-care hospitals.
Study design: Observational cohort study.
Setting: All acute-care hospitals in New York state.
Synopsis: Data from the New York Statewide Planning and Research Cooperative System identified 30,947 adult patients who were hospitalized with acute stroke over a two-year period. Mean age of patients was 73. Thirty-day all-cause mortality was compared between stroke centers and all other acute-care hospitals. Secondary outcomes were one-day, seven-day, and one-year all-cause mortality. To adjust for unmeasured confounders, the analyses accounted for distance to the nearest stroke center relative to the distance to the nearest acute-care hospital.
Almost half the patients in this study were admitted to stroke centers, where they had an adjusted absolute risk reduction in 30-day mortality of 2.5%. Seven-day mortality was reduced 1.3% and one-year mortality was reduced 3.0%. These findings were statistically significant.
There were no differences in one-day mortality, 30-day readmission rates, or rates of discharge to skilled nursing facilities between hospital designation.
The study was not designed to identify which elements of a certified stroke center contribute to the mortality benefit and did not account for stroke severity. Results may not be generalizable beyond New York state.
Bottom line: Admission to an acute-stroke center is associated with a modest reduction in mortality.
Citation: Xian Y, Holloway RG, Chan PS, et al. Association between stroke center hospitalization for acute ischemic stroke and mortality. JAMA. 2011;305(4):373-380.
Mortality from MRSA Pneumonia Increases with Higher Vancomycin Minimum Inhibitory Concentration
Clinical question: Does vancomycin minimum inhibitory concentration (MIC) affect mortality due to healthcare-associated pneumonia (HCAP), ventilator-associated pneumonia (VAP), and hospital-acquired pneumonia (HAP) from methicillin-resistant Staphylococcus aureus (MRSA)?
Background: S. aureus is considered vancomycin-susceptible if the MIC is ≤2 mg/mL. Mortality from MRSA bacteremia increases as vancomycin MIC rises. The effect of higher vancomycin MICs on outcomes in MRSA pneumonia is not known.
Study design: Prospective cohort study.
Setting: Four academic centers in Kentucky, Ohio, Michigan, and Florida.
Synopsis: One hundred fifty-eight patients with HCAP, VAP, or HAP based on American Thoracic Society/Infectious Disease Society of American (ATS/IDSA) definitions and ≥1 MRSA-positive blood or respiratory culture were identified from the prospectively collected Improving Medicine through Pathway Assessment of Critical Therapy in Hospital-Acquired Pneumonia (IMPACT-HAP) database. All were treated with a regimen including vancomycin based on 2005 ATS/IDSA guidelines.
Vancomycin MIC was ≤1 mg/mL in 27% of MRSA isolates; 1.5 mg/mL in 55%; and ≥2mg/mL in 18%. Overall, all-cause 28-day mortality was 32%. After correcting for confounding factors, such as age and comorbid illnesses, all-cause 28-day mortality was higher in patients with higher vancomycin MICs (adjusted odds ratio of death 2.97 per 1 mg/mL increase in vancomycin MIC). Heteroresistance to vancomycin was present in 21% of MRSA isolates but was not associated with an increase in mortality.
Bottom line: Death due to MRSA HCAP, VAP, and HAP increases as the vancomycin MIC increases, even with MICs within the susceptible range.
Citation: Haque NZ, Zuniga LC, Peyrani P, et al. Relationship of vancomycin minimum inhibitory concentration to mortality in patients with methicillin-resistant Staphylococcus aureus hospital-acquired, ventilator-associated, or health-care-associated pneumonia. Chest. 2010;138(6): 1356-1362.
More Frequent In-Center Hemodialysis Improves Outcomes
Clinical question: Does more frequent hemodialysis reduce mortality, improve cardiovascular outcomes, and improve quality of life in patients undergoing maintenance hemodialysis?
Background: Despite technological improvements over the last 40 years, hemodialysis is still associated with significant morbidity, mortality, and decreased quality of life. The optimal frequency of hemodialysis remains uncertain.
Study design: Randomized clinical trial with blinded analysis.
Setting: Eleven university-based and 54 community-based hemodialysis facilities in North America.
Synopsis: Researchers randomized 245 patients with end-stage renal disease to receive hemodialysis either three times per week or six times per week. Composite of death or one-year increase in left ventricular mass as assessed by cardiac MR was one primary outcome; composite outcome of death or one-year decrease in self-reported physical health was a co-primary outcome.
Frequent hemodialysis was associated with benefits in both composite primary outcomes (hazard ratio [HR] 0.61 for death/increase in left ventricular mass; HR 0.70 for death/decreased physical health). Notably, patients with frequent dialysis were more likely to undergo interventions related to vascular access than with conventional dialysis (HR 1.71). Blood pressure control (P<0.001) and hyperphosphotemia (P=0.002) also were improved with frequent dialysis.
Depression, cognitive performance, albumin, and anemia did not improve. Direct impact on mortality and hospital admission could not be assessed. Results might not be generalizable.
Bottom line: More frequent hemodialysis was associated with a significant reduction in left ventricular mass, improvement in self-reported physical health, and a reduction in mortality using combined composite outcomes. Further cost-benefit and quality-of-life analyses are needed to determine optimal dosing of hemodialysis.
Citation: FHN Trial Group. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287-2300.
BNP Testing in the Emergency Department Might Decrease Hospital Length Of Stay
Clinical question: Does BNP testing of patients presenting to the ED with acute dyspnea reduce admissions, shorten length of stay (LOS), or improve short-term survival?
Background: B-type natriuretic peptide (BNP) and the N-terminal peptide of its precursor, pro-BNP, are widely used to evaluate patients with acute dyspnea to distinguish cardiac from noncardiac causes. However, clinical outcomes related to this commonly used test are not clearly understood.
Study design: Systematic review and meta-analysis of randomized trials.
Setting: Five randomized controlled trials in EDs in five hospitals (Switzerland, Canada, the Netherlands, United States, and Australia) involving 2,513 patients.
Synopsis: Studies compared BNP testing with routine testing and clinical assessment and described >1 of three clinical outcomes: hospital admission rate, LOS, and mortality. Nonrandomized and retrospective studies and subgroup analyses of larger studies were excluded.
Testing with BNP decreased LOS by a mean of 1.22 days and critical-care-unit stay was modestly reduced (-0.56 days). This change was attributed to improved acute management and more rapid discharge with knowledge of BNP values. There was a nonsignificant trend toward decreased hospital admission from the ED in the BNP group (odds ratio 0.82). The effect of BNP testing on mortality was inconclusive.
Bottom line: BNP testing in the ED is associated with decreased hospital LOS, as well as a trend toward decreased admission rates from the ED. There is no conclusive effect on mortality.
Citation: Lam LL, Cameron PA, Schneider HG, Abramson MJ, Müller C, Krum H. Meta-analysis: effect of B-type natriuretic peptide testing on clinical outcome in patients with acute dyspnea in the emergency setting. Ann Intern Med. 2010;153:728-735.
Vaccination Reduces Incidence of Herpes Zoster in Community-Dwelling Adults Age 60 and Older
Clinical question: What is the impact of herpes zoster vaccination on the incidence of disease in older community-dwelling adults with and without chronic medical conditions?
Background: Live-attenuated vaccination was recently approved in older adults to reduce the incidence of herpes zoster and postherpetic neuralgia. Vaccination practices and efficacy in a clinical setting among patients with varying comorbidities are unknown.
Study design: Retrospective cohort.
Setting: Single health plan in California.
Synopsis: Data were collected from 2007 to 2009 on 75,761 health-plan members who received the vaccine. The data were compared with unvaccinated, age-matched controls. Vaccine recipients were more likely to be white and female, with more outpatient visits and fewer chronic diseases.
A 55% percent reduction in the incidence of herpes zoster was found among recipients. Benefit was seen across all age groups and comorbidities. Incidence of herpes zoster increased as age increased, but the relative rate reduction with vaccination remained nearly constant, including among those older than 80. Patients with chronic diseases also had an increased baseline incidence of herpes zoster but a similar relative reduction with vaccination. The study was not designed to look at post-herpetic neuralgia or to assess severity or duration of symptoms in herpes zoster cases.
Bottom line: Vaccination for herpes zoster is indicated for all adults age 60 and older, including the oldest and most medically complicated, in whom vaccination is not contraindicated.
Citation: Tseng HF, Smith N, Harpaz R, Bialek SR, Sy LS, Jacobsen SJ. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA. 2011; 305(2):160-166.
For-Profit Hospital Status Might Increase Risk of 30-Day Readmission to Different Hospitals
Clinical question: Are patients admitted to a for-profit hospital more likely to be readmitted to a different hospital if rehospitalized within 30 days?
Background: Thirty-day readmission occurs in 20% of hospitalized Medicare patients, with at least a quarter of rehospitalized patients admitted to a different hospital. Recent healthcare legislation proposes penalties to reduce readmission rates. This could provide unintended incentives for hospitals to divert patients at high risk for readmission to other hospitals.
Study design: Observational cohort study.
Setting: Hospitalized Medicare patients.
Synopsis: Analysis of a 5% sample of Medicare patients readmitted within 30 days of discharge over a 22-month period identified 74,564 patients who were rehospitalized in a facility different from their initial admission. For-profit status of the initial and subsequent hospital was identified. Twenty-eight percent of patients initially admitted to a for-profit hospital were readmitted to a different hospital within 30 days. By comparison, only 21% of patients initially admitted to a nonprofit hospital were readmitted to a different hospital (P<.001).
The most significant risk factors for readmission to a different hospital were admission to a lower-volume hospital (221% increased risk), disability (21% increased risk), admission to an academic hospital (18% increased risk), and admission to a for-profit hospital (17% increased risk). Thirty-day mortality did not differ between patients readmitted to the same or different hospital, regardless of for-profit status. Admission to a different hospital was associated with increased cost.
This study was not designed to look at why patients were rehospitalized at different hospitals, and findings cannot be generalized beyond Medicare patients.
Bottom line: Discharge from a for-profit hospital is one of several risk factors for 30-day readmission to a different hospital.
Citation: Kind AJ, Bartels C, Mell MW, Mullahy J, Smith M. For-profit hospital status and rehospitalizations at different hospitals: an analysis of Medicare data. Ann Intern Med. 2010;153(11):718-727. TH
Literature at a Glance
A guide to this month’s studies
- Rivaroxaban for VTE
- Cost-effectiveness of dabigatran in atrial fibrillation
- Effect of new resident duty-hour limits
- Outcomes of care at acute-stroke centers
- Effect on MIC in patients with MRSA pneumonia
- Optimal hemodialysis frequency
- Effect of BNP testing on hospital length of stay
- Impact of herpes zoster vaccination
- 30-day readmission rates in for-profit hospitals
Oral Rivaroxaban Could Play a Role in VTE Treatment
Clinical question: Is oral rivaroxaban an acceptable treatment option for acute symptomatic deep-vein thrombosis (DVT) and venous thromboembolism (VTE)?
Background: Treatment of acute DVT requires frequent laboratory monitoring, which may be obviated by the use of fixed-dose oral rivaroxaban.
Study designs: Parallel randomized, open-label, event-driven, noninferiority study (the acute DVT study) and randomized, double-blind, placebo-controlled, event-driven superiority trial (continued treatment study).
Setting: Multicenter study.
Synopsis: The acute DVT study randomly assigned 3,449 patients with acute DVT to oral rivaroxaban 15 mg twice daily for three weeks followed by 20 mg daily for three, six, or 12 months or enoxaparin 1 mg/kg subcutaneously twice daily and daily warfarin until a therapeutic INR was achieved, at which time the enoxaparin was discontinued. Rivaroxaban was not inferior in terms of preventing recurrent VTE (2.1% vs. 3.0%; P<0.001). Major or clinically relevant nonmajor bleeding occurred equally in both groups (8.1%).
The continued treatment study randomly assigned 1,196 patients with six to 12 months of prior VTE treatment to rivaroxaban 20 mg daily versus placebo for six or 12 months. Rivaroxaban was superior in preventing recurrent VTE (1.3% vs. 7.1%; P<0.001). A statistically nonsignificant increase in major bleeding was reported with rivaroxaban (0.7% vs. 0.0%). The open-label design and pharmaceutical support create potential for bias.
Bottom line: Oral rivaroxaban might offer a simplified, effective, and safe alternative to enoxaparin and warfarin for short- and long-term VTE treatment.
Citation: The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363 (26):2499-2510.
Dabigatran Might Be a Cost-Effective Alternative to Warfarin in Atrial Fibrillation
Clinical question: Is dabigatran cost-effective compared to warfarin for prevention of stroke in atrial fibrillation?
Background: Dabigatran, a direct thrombin inhibitor, is FDA-approved for the prevention of stroke and systemic embolism in atrial fibrillation. In the 2009 RE-LY trial, dabigatran 150 mg twice daily was associated with fewer embolic strokes than warfarin with similar episodes of major hemorrhage. Dabigatran costs more than warfarin; its cost-effectiveness is unknown.
Study design: Markov decision model.
Setting: Data from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY), a multinational randomized trial, and other anticoagulation studies.
Synopsis: This model simulated costs and outcomes for a theoretical cohort of patients >65 with atrial fibrillation and CHADS2 score ≥1 taking either lifelong warfarin or dabigatran. The model included assumptions about costs and quality-of-life effects of INR monitoring, stroke, hemorrhage, and myocardial infarction. Because U.S. pricing for dabigatran was pending, the authors assumed $13 per day.
Both life expectancy in quality-adjusted life years (QALYs) and lifetime costs were higher for dabigatran than for warfarin (10.84 vs. 10.28 QALYs and $168,398 vs. $143,193, respectively). The incremental cost per QALY for dabigatran was $45,372. Limitations include dependence on data from a single-manufacturer-sponsored trial with limited follow-up.
Retail costs for dabigatran are now known to be about $8 per day. When the model is adjusted to that price, an additional QALY would cost $12,000, well below the commonly accepted threshold of $50,000.
Bottom line: Dabigatran is likely a cost-effective alternative to warfarin in nonvalvular atrial fibrillation.
Citation: Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154(1):1-11.
Effects of New ACGME Mandates on Patients and Residents Unclear
Clinical question: How will new intern duty-hour standards impact patient care, residents’ health, and education?
Background: The Accreditation Council for Graduate Medical Education (ACGME) has mandated new duty-hour standards that limit interns’ shifts to 16 hours and night float to six consecutive nights. They also strongly recommend a nighttime nap.
Study design: Systematic review of English-language, original research studies addressing shift length, night float, or protected sleep time, published from 1989 to 2010.
Synopsis: Sixty-four out of 5,345 articles met eligibility criteria, including four randomized controlled trials and five multi-institutional studies. Although 73% of studies examining shift length supported reducing hours, optimal shift duration was not determined. All studies addressing night float examined five to seven consecutive nights of work; data were too heterogeneous for generalization. Data on protected sleep time were too limited to determine effect on residents and patients.
The majority of studies were conducted at single institutions and study designs carried high risk for interpretation bias. Additionally, publication bias might have influenced the results of this review of English-language-only studies.
Bottom line: The available studies that attempt to elucidate the effects of major changes in residency training have significant limitations, and the potential impact of the new standards on patients and residents remains uncertain.
Citation: Reed DA, Fletcher KE, Arora VM. Systematic review: association of shift length, protected sleep time, and night float with patient care, residents’ health, and education. Ann Intern Med. 2010;153:829-842.
Admission to Stroke Centers for Acute Ischemic Stroke Might Improve Mortality
Clinical question: Does admission to a certified stroke center improve survival in patients with acute ischemic stroke?
Background: Since 2003, the Joint Commission has designated fewer than 700 acute-care hospitals as certified stroke centers. However, no large studies have examined whether patients with acute stroke admitted to stroke centers have lower mortality than those admitted to noncertified acute-care hospitals.
Study design: Observational cohort study.
Setting: All acute-care hospitals in New York state.
Synopsis: Data from the New York Statewide Planning and Research Cooperative System identified 30,947 adult patients who were hospitalized with acute stroke over a two-year period. Mean age of patients was 73. Thirty-day all-cause mortality was compared between stroke centers and all other acute-care hospitals. Secondary outcomes were one-day, seven-day, and one-year all-cause mortality. To adjust for unmeasured confounders, the analyses accounted for distance to the nearest stroke center relative to the distance to the nearest acute-care hospital.
Almost half the patients in this study were admitted to stroke centers, where they had an adjusted absolute risk reduction in 30-day mortality of 2.5%. Seven-day mortality was reduced 1.3% and one-year mortality was reduced 3.0%. These findings were statistically significant.
There were no differences in one-day mortality, 30-day readmission rates, or rates of discharge to skilled nursing facilities between hospital designation.
The study was not designed to identify which elements of a certified stroke center contribute to the mortality benefit and did not account for stroke severity. Results may not be generalizable beyond New York state.
Bottom line: Admission to an acute-stroke center is associated with a modest reduction in mortality.
Citation: Xian Y, Holloway RG, Chan PS, et al. Association between stroke center hospitalization for acute ischemic stroke and mortality. JAMA. 2011;305(4):373-380.
Mortality from MRSA Pneumonia Increases with Higher Vancomycin Minimum Inhibitory Concentration
Clinical question: Does vancomycin minimum inhibitory concentration (MIC) affect mortality due to healthcare-associated pneumonia (HCAP), ventilator-associated pneumonia (VAP), and hospital-acquired pneumonia (HAP) from methicillin-resistant Staphylococcus aureus (MRSA)?
Background: S. aureus is considered vancomycin-susceptible if the MIC is ≤2 mg/mL. Mortality from MRSA bacteremia increases as vancomycin MIC rises. The effect of higher vancomycin MICs on outcomes in MRSA pneumonia is not known.
Study design: Prospective cohort study.
Setting: Four academic centers in Kentucky, Ohio, Michigan, and Florida.
Synopsis: One hundred fifty-eight patients with HCAP, VAP, or HAP based on American Thoracic Society/Infectious Disease Society of American (ATS/IDSA) definitions and ≥1 MRSA-positive blood or respiratory culture were identified from the prospectively collected Improving Medicine through Pathway Assessment of Critical Therapy in Hospital-Acquired Pneumonia (IMPACT-HAP) database. All were treated with a regimen including vancomycin based on 2005 ATS/IDSA guidelines.
Vancomycin MIC was ≤1 mg/mL in 27% of MRSA isolates; 1.5 mg/mL in 55%; and ≥2mg/mL in 18%. Overall, all-cause 28-day mortality was 32%. After correcting for confounding factors, such as age and comorbid illnesses, all-cause 28-day mortality was higher in patients with higher vancomycin MICs (adjusted odds ratio of death 2.97 per 1 mg/mL increase in vancomycin MIC). Heteroresistance to vancomycin was present in 21% of MRSA isolates but was not associated with an increase in mortality.
Bottom line: Death due to MRSA HCAP, VAP, and HAP increases as the vancomycin MIC increases, even with MICs within the susceptible range.
Citation: Haque NZ, Zuniga LC, Peyrani P, et al. Relationship of vancomycin minimum inhibitory concentration to mortality in patients with methicillin-resistant Staphylococcus aureus hospital-acquired, ventilator-associated, or health-care-associated pneumonia. Chest. 2010;138(6): 1356-1362.
More Frequent In-Center Hemodialysis Improves Outcomes
Clinical question: Does more frequent hemodialysis reduce mortality, improve cardiovascular outcomes, and improve quality of life in patients undergoing maintenance hemodialysis?
Background: Despite technological improvements over the last 40 years, hemodialysis is still associated with significant morbidity, mortality, and decreased quality of life. The optimal frequency of hemodialysis remains uncertain.
Study design: Randomized clinical trial with blinded analysis.
Setting: Eleven university-based and 54 community-based hemodialysis facilities in North America.
Synopsis: Researchers randomized 245 patients with end-stage renal disease to receive hemodialysis either three times per week or six times per week. Composite of death or one-year increase in left ventricular mass as assessed by cardiac MR was one primary outcome; composite outcome of death or one-year decrease in self-reported physical health was a co-primary outcome.
Frequent hemodialysis was associated with benefits in both composite primary outcomes (hazard ratio [HR] 0.61 for death/increase in left ventricular mass; HR 0.70 for death/decreased physical health). Notably, patients with frequent dialysis were more likely to undergo interventions related to vascular access than with conventional dialysis (HR 1.71). Blood pressure control (P<0.001) and hyperphosphotemia (P=0.002) also were improved with frequent dialysis.
Depression, cognitive performance, albumin, and anemia did not improve. Direct impact on mortality and hospital admission could not be assessed. Results might not be generalizable.
Bottom line: More frequent hemodialysis was associated with a significant reduction in left ventricular mass, improvement in self-reported physical health, and a reduction in mortality using combined composite outcomes. Further cost-benefit and quality-of-life analyses are needed to determine optimal dosing of hemodialysis.
Citation: FHN Trial Group. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287-2300.
BNP Testing in the Emergency Department Might Decrease Hospital Length Of Stay
Clinical question: Does BNP testing of patients presenting to the ED with acute dyspnea reduce admissions, shorten length of stay (LOS), or improve short-term survival?
Background: B-type natriuretic peptide (BNP) and the N-terminal peptide of its precursor, pro-BNP, are widely used to evaluate patients with acute dyspnea to distinguish cardiac from noncardiac causes. However, clinical outcomes related to this commonly used test are not clearly understood.
Study design: Systematic review and meta-analysis of randomized trials.
Setting: Five randomized controlled trials in EDs in five hospitals (Switzerland, Canada, the Netherlands, United States, and Australia) involving 2,513 patients.
Synopsis: Studies compared BNP testing with routine testing and clinical assessment and described >1 of three clinical outcomes: hospital admission rate, LOS, and mortality. Nonrandomized and retrospective studies and subgroup analyses of larger studies were excluded.
Testing with BNP decreased LOS by a mean of 1.22 days and critical-care-unit stay was modestly reduced (-0.56 days). This change was attributed to improved acute management and more rapid discharge with knowledge of BNP values. There was a nonsignificant trend toward decreased hospital admission from the ED in the BNP group (odds ratio 0.82). The effect of BNP testing on mortality was inconclusive.
Bottom line: BNP testing in the ED is associated with decreased hospital LOS, as well as a trend toward decreased admission rates from the ED. There is no conclusive effect on mortality.
Citation: Lam LL, Cameron PA, Schneider HG, Abramson MJ, Müller C, Krum H. Meta-analysis: effect of B-type natriuretic peptide testing on clinical outcome in patients with acute dyspnea in the emergency setting. Ann Intern Med. 2010;153:728-735.
Vaccination Reduces Incidence of Herpes Zoster in Community-Dwelling Adults Age 60 and Older
Clinical question: What is the impact of herpes zoster vaccination on the incidence of disease in older community-dwelling adults with and without chronic medical conditions?
Background: Live-attenuated vaccination was recently approved in older adults to reduce the incidence of herpes zoster and postherpetic neuralgia. Vaccination practices and efficacy in a clinical setting among patients with varying comorbidities are unknown.
Study design: Retrospective cohort.
Setting: Single health plan in California.
Synopsis: Data were collected from 2007 to 2009 on 75,761 health-plan members who received the vaccine. The data were compared with unvaccinated, age-matched controls. Vaccine recipients were more likely to be white and female, with more outpatient visits and fewer chronic diseases.
A 55% percent reduction in the incidence of herpes zoster was found among recipients. Benefit was seen across all age groups and comorbidities. Incidence of herpes zoster increased as age increased, but the relative rate reduction with vaccination remained nearly constant, including among those older than 80. Patients with chronic diseases also had an increased baseline incidence of herpes zoster but a similar relative reduction with vaccination. The study was not designed to look at post-herpetic neuralgia or to assess severity or duration of symptoms in herpes zoster cases.
Bottom line: Vaccination for herpes zoster is indicated for all adults age 60 and older, including the oldest and most medically complicated, in whom vaccination is not contraindicated.
Citation: Tseng HF, Smith N, Harpaz R, Bialek SR, Sy LS, Jacobsen SJ. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA. 2011; 305(2):160-166.
For-Profit Hospital Status Might Increase Risk of 30-Day Readmission to Different Hospitals
Clinical question: Are patients admitted to a for-profit hospital more likely to be readmitted to a different hospital if rehospitalized within 30 days?
Background: Thirty-day readmission occurs in 20% of hospitalized Medicare patients, with at least a quarter of rehospitalized patients admitted to a different hospital. Recent healthcare legislation proposes penalties to reduce readmission rates. This could provide unintended incentives for hospitals to divert patients at high risk for readmission to other hospitals.
Study design: Observational cohort study.
Setting: Hospitalized Medicare patients.
Synopsis: Analysis of a 5% sample of Medicare patients readmitted within 30 days of discharge over a 22-month period identified 74,564 patients who were rehospitalized in a facility different from their initial admission. For-profit status of the initial and subsequent hospital was identified. Twenty-eight percent of patients initially admitted to a for-profit hospital were readmitted to a different hospital within 30 days. By comparison, only 21% of patients initially admitted to a nonprofit hospital were readmitted to a different hospital (P<.001).
The most significant risk factors for readmission to a different hospital were admission to a lower-volume hospital (221% increased risk), disability (21% increased risk), admission to an academic hospital (18% increased risk), and admission to a for-profit hospital (17% increased risk). Thirty-day mortality did not differ between patients readmitted to the same or different hospital, regardless of for-profit status. Admission to a different hospital was associated with increased cost.
This study was not designed to look at why patients were rehospitalized at different hospitals, and findings cannot be generalized beyond Medicare patients.
Bottom line: Discharge from a for-profit hospital is one of several risk factors for 30-day readmission to a different hospital.
Citation: Kind AJ, Bartels C, Mell MW, Mullahy J, Smith M. For-profit hospital status and rehospitalizations at different hospitals: an analysis of Medicare data. Ann Intern Med. 2010;153(11):718-727. TH
Literature at a Glance
A guide to this month’s studies
- Rivaroxaban for VTE
- Cost-effectiveness of dabigatran in atrial fibrillation
- Effect of new resident duty-hour limits
- Outcomes of care at acute-stroke centers
- Effect on MIC in patients with MRSA pneumonia
- Optimal hemodialysis frequency
- Effect of BNP testing on hospital length of stay
- Impact of herpes zoster vaccination
- 30-day readmission rates in for-profit hospitals
Oral Rivaroxaban Could Play a Role in VTE Treatment
Clinical question: Is oral rivaroxaban an acceptable treatment option for acute symptomatic deep-vein thrombosis (DVT) and venous thromboembolism (VTE)?
Background: Treatment of acute DVT requires frequent laboratory monitoring, which may be obviated by the use of fixed-dose oral rivaroxaban.
Study designs: Parallel randomized, open-label, event-driven, noninferiority study (the acute DVT study) and randomized, double-blind, placebo-controlled, event-driven superiority trial (continued treatment study).
Setting: Multicenter study.
Synopsis: The acute DVT study randomly assigned 3,449 patients with acute DVT to oral rivaroxaban 15 mg twice daily for three weeks followed by 20 mg daily for three, six, or 12 months or enoxaparin 1 mg/kg subcutaneously twice daily and daily warfarin until a therapeutic INR was achieved, at which time the enoxaparin was discontinued. Rivaroxaban was not inferior in terms of preventing recurrent VTE (2.1% vs. 3.0%; P<0.001). Major or clinically relevant nonmajor bleeding occurred equally in both groups (8.1%).
The continued treatment study randomly assigned 1,196 patients with six to 12 months of prior VTE treatment to rivaroxaban 20 mg daily versus placebo for six or 12 months. Rivaroxaban was superior in preventing recurrent VTE (1.3% vs. 7.1%; P<0.001). A statistically nonsignificant increase in major bleeding was reported with rivaroxaban (0.7% vs. 0.0%). The open-label design and pharmaceutical support create potential for bias.
Bottom line: Oral rivaroxaban might offer a simplified, effective, and safe alternative to enoxaparin and warfarin for short- and long-term VTE treatment.
Citation: The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363 (26):2499-2510.
Dabigatran Might Be a Cost-Effective Alternative to Warfarin in Atrial Fibrillation
Clinical question: Is dabigatran cost-effective compared to warfarin for prevention of stroke in atrial fibrillation?
Background: Dabigatran, a direct thrombin inhibitor, is FDA-approved for the prevention of stroke and systemic embolism in atrial fibrillation. In the 2009 RE-LY trial, dabigatran 150 mg twice daily was associated with fewer embolic strokes than warfarin with similar episodes of major hemorrhage. Dabigatran costs more than warfarin; its cost-effectiveness is unknown.
Study design: Markov decision model.
Setting: Data from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY), a multinational randomized trial, and other anticoagulation studies.
Synopsis: This model simulated costs and outcomes for a theoretical cohort of patients >65 with atrial fibrillation and CHADS2 score ≥1 taking either lifelong warfarin or dabigatran. The model included assumptions about costs and quality-of-life effects of INR monitoring, stroke, hemorrhage, and myocardial infarction. Because U.S. pricing for dabigatran was pending, the authors assumed $13 per day.
Both life expectancy in quality-adjusted life years (QALYs) and lifetime costs were higher for dabigatran than for warfarin (10.84 vs. 10.28 QALYs and $168,398 vs. $143,193, respectively). The incremental cost per QALY for dabigatran was $45,372. Limitations include dependence on data from a single-manufacturer-sponsored trial with limited follow-up.
Retail costs for dabigatran are now known to be about $8 per day. When the model is adjusted to that price, an additional QALY would cost $12,000, well below the commonly accepted threshold of $50,000.
Bottom line: Dabigatran is likely a cost-effective alternative to warfarin in nonvalvular atrial fibrillation.
Citation: Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154(1):1-11.
Effects of New ACGME Mandates on Patients and Residents Unclear
Clinical question: How will new intern duty-hour standards impact patient care, residents’ health, and education?
Background: The Accreditation Council for Graduate Medical Education (ACGME) has mandated new duty-hour standards that limit interns’ shifts to 16 hours and night float to six consecutive nights. They also strongly recommend a nighttime nap.
Study design: Systematic review of English-language, original research studies addressing shift length, night float, or protected sleep time, published from 1989 to 2010.
Synopsis: Sixty-four out of 5,345 articles met eligibility criteria, including four randomized controlled trials and five multi-institutional studies. Although 73% of studies examining shift length supported reducing hours, optimal shift duration was not determined. All studies addressing night float examined five to seven consecutive nights of work; data were too heterogeneous for generalization. Data on protected sleep time were too limited to determine effect on residents and patients.
The majority of studies were conducted at single institutions and study designs carried high risk for interpretation bias. Additionally, publication bias might have influenced the results of this review of English-language-only studies.
Bottom line: The available studies that attempt to elucidate the effects of major changes in residency training have significant limitations, and the potential impact of the new standards on patients and residents remains uncertain.
Citation: Reed DA, Fletcher KE, Arora VM. Systematic review: association of shift length, protected sleep time, and night float with patient care, residents’ health, and education. Ann Intern Med. 2010;153:829-842.
Admission to Stroke Centers for Acute Ischemic Stroke Might Improve Mortality
Clinical question: Does admission to a certified stroke center improve survival in patients with acute ischemic stroke?
Background: Since 2003, the Joint Commission has designated fewer than 700 acute-care hospitals as certified stroke centers. However, no large studies have examined whether patients with acute stroke admitted to stroke centers have lower mortality than those admitted to noncertified acute-care hospitals.
Study design: Observational cohort study.
Setting: All acute-care hospitals in New York state.
Synopsis: Data from the New York Statewide Planning and Research Cooperative System identified 30,947 adult patients who were hospitalized with acute stroke over a two-year period. Mean age of patients was 73. Thirty-day all-cause mortality was compared between stroke centers and all other acute-care hospitals. Secondary outcomes were one-day, seven-day, and one-year all-cause mortality. To adjust for unmeasured confounders, the analyses accounted for distance to the nearest stroke center relative to the distance to the nearest acute-care hospital.
Almost half the patients in this study were admitted to stroke centers, where they had an adjusted absolute risk reduction in 30-day mortality of 2.5%. Seven-day mortality was reduced 1.3% and one-year mortality was reduced 3.0%. These findings were statistically significant.
There were no differences in one-day mortality, 30-day readmission rates, or rates of discharge to skilled nursing facilities between hospital designation.
The study was not designed to identify which elements of a certified stroke center contribute to the mortality benefit and did not account for stroke severity. Results may not be generalizable beyond New York state.
Bottom line: Admission to an acute-stroke center is associated with a modest reduction in mortality.
Citation: Xian Y, Holloway RG, Chan PS, et al. Association between stroke center hospitalization for acute ischemic stroke and mortality. JAMA. 2011;305(4):373-380.
Mortality from MRSA Pneumonia Increases with Higher Vancomycin Minimum Inhibitory Concentration
Clinical question: Does vancomycin minimum inhibitory concentration (MIC) affect mortality due to healthcare-associated pneumonia (HCAP), ventilator-associated pneumonia (VAP), and hospital-acquired pneumonia (HAP) from methicillin-resistant Staphylococcus aureus (MRSA)?
Background: S. aureus is considered vancomycin-susceptible if the MIC is ≤2 mg/mL. Mortality from MRSA bacteremia increases as vancomycin MIC rises. The effect of higher vancomycin MICs on outcomes in MRSA pneumonia is not known.
Study design: Prospective cohort study.
Setting: Four academic centers in Kentucky, Ohio, Michigan, and Florida.
Synopsis: One hundred fifty-eight patients with HCAP, VAP, or HAP based on American Thoracic Society/Infectious Disease Society of American (ATS/IDSA) definitions and ≥1 MRSA-positive blood or respiratory culture were identified from the prospectively collected Improving Medicine through Pathway Assessment of Critical Therapy in Hospital-Acquired Pneumonia (IMPACT-HAP) database. All were treated with a regimen including vancomycin based on 2005 ATS/IDSA guidelines.
Vancomycin MIC was ≤1 mg/mL in 27% of MRSA isolates; 1.5 mg/mL in 55%; and ≥2mg/mL in 18%. Overall, all-cause 28-day mortality was 32%. After correcting for confounding factors, such as age and comorbid illnesses, all-cause 28-day mortality was higher in patients with higher vancomycin MICs (adjusted odds ratio of death 2.97 per 1 mg/mL increase in vancomycin MIC). Heteroresistance to vancomycin was present in 21% of MRSA isolates but was not associated with an increase in mortality.
Bottom line: Death due to MRSA HCAP, VAP, and HAP increases as the vancomycin MIC increases, even with MICs within the susceptible range.
Citation: Haque NZ, Zuniga LC, Peyrani P, et al. Relationship of vancomycin minimum inhibitory concentration to mortality in patients with methicillin-resistant Staphylococcus aureus hospital-acquired, ventilator-associated, or health-care-associated pneumonia. Chest. 2010;138(6): 1356-1362.
More Frequent In-Center Hemodialysis Improves Outcomes
Clinical question: Does more frequent hemodialysis reduce mortality, improve cardiovascular outcomes, and improve quality of life in patients undergoing maintenance hemodialysis?
Background: Despite technological improvements over the last 40 years, hemodialysis is still associated with significant morbidity, mortality, and decreased quality of life. The optimal frequency of hemodialysis remains uncertain.
Study design: Randomized clinical trial with blinded analysis.
Setting: Eleven university-based and 54 community-based hemodialysis facilities in North America.
Synopsis: Researchers randomized 245 patients with end-stage renal disease to receive hemodialysis either three times per week or six times per week. Composite of death or one-year increase in left ventricular mass as assessed by cardiac MR was one primary outcome; composite outcome of death or one-year decrease in self-reported physical health was a co-primary outcome.
Frequent hemodialysis was associated with benefits in both composite primary outcomes (hazard ratio [HR] 0.61 for death/increase in left ventricular mass; HR 0.70 for death/decreased physical health). Notably, patients with frequent dialysis were more likely to undergo interventions related to vascular access than with conventional dialysis (HR 1.71). Blood pressure control (P<0.001) and hyperphosphotemia (P=0.002) also were improved with frequent dialysis.
Depression, cognitive performance, albumin, and anemia did not improve. Direct impact on mortality and hospital admission could not be assessed. Results might not be generalizable.
Bottom line: More frequent hemodialysis was associated with a significant reduction in left ventricular mass, improvement in self-reported physical health, and a reduction in mortality using combined composite outcomes. Further cost-benefit and quality-of-life analyses are needed to determine optimal dosing of hemodialysis.
Citation: FHN Trial Group. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287-2300.
BNP Testing in the Emergency Department Might Decrease Hospital Length Of Stay
Clinical question: Does BNP testing of patients presenting to the ED with acute dyspnea reduce admissions, shorten length of stay (LOS), or improve short-term survival?
Background: B-type natriuretic peptide (BNP) and the N-terminal peptide of its precursor, pro-BNP, are widely used to evaluate patients with acute dyspnea to distinguish cardiac from noncardiac causes. However, clinical outcomes related to this commonly used test are not clearly understood.
Study design: Systematic review and meta-analysis of randomized trials.
Setting: Five randomized controlled trials in EDs in five hospitals (Switzerland, Canada, the Netherlands, United States, and Australia) involving 2,513 patients.
Synopsis: Studies compared BNP testing with routine testing and clinical assessment and described >1 of three clinical outcomes: hospital admission rate, LOS, and mortality. Nonrandomized and retrospective studies and subgroup analyses of larger studies were excluded.
Testing with BNP decreased LOS by a mean of 1.22 days and critical-care-unit stay was modestly reduced (-0.56 days). This change was attributed to improved acute management and more rapid discharge with knowledge of BNP values. There was a nonsignificant trend toward decreased hospital admission from the ED in the BNP group (odds ratio 0.82). The effect of BNP testing on mortality was inconclusive.
Bottom line: BNP testing in the ED is associated with decreased hospital LOS, as well as a trend toward decreased admission rates from the ED. There is no conclusive effect on mortality.
Citation: Lam LL, Cameron PA, Schneider HG, Abramson MJ, Müller C, Krum H. Meta-analysis: effect of B-type natriuretic peptide testing on clinical outcome in patients with acute dyspnea in the emergency setting. Ann Intern Med. 2010;153:728-735.
Vaccination Reduces Incidence of Herpes Zoster in Community-Dwelling Adults Age 60 and Older
Clinical question: What is the impact of herpes zoster vaccination on the incidence of disease in older community-dwelling adults with and without chronic medical conditions?
Background: Live-attenuated vaccination was recently approved in older adults to reduce the incidence of herpes zoster and postherpetic neuralgia. Vaccination practices and efficacy in a clinical setting among patients with varying comorbidities are unknown.
Study design: Retrospective cohort.
Setting: Single health plan in California.
Synopsis: Data were collected from 2007 to 2009 on 75,761 health-plan members who received the vaccine. The data were compared with unvaccinated, age-matched controls. Vaccine recipients were more likely to be white and female, with more outpatient visits and fewer chronic diseases.
A 55% percent reduction in the incidence of herpes zoster was found among recipients. Benefit was seen across all age groups and comorbidities. Incidence of herpes zoster increased as age increased, but the relative rate reduction with vaccination remained nearly constant, including among those older than 80. Patients with chronic diseases also had an increased baseline incidence of herpes zoster but a similar relative reduction with vaccination. The study was not designed to look at post-herpetic neuralgia or to assess severity or duration of symptoms in herpes zoster cases.
Bottom line: Vaccination for herpes zoster is indicated for all adults age 60 and older, including the oldest and most medically complicated, in whom vaccination is not contraindicated.
Citation: Tseng HF, Smith N, Harpaz R, Bialek SR, Sy LS, Jacobsen SJ. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA. 2011; 305(2):160-166.
For-Profit Hospital Status Might Increase Risk of 30-Day Readmission to Different Hospitals
Clinical question: Are patients admitted to a for-profit hospital more likely to be readmitted to a different hospital if rehospitalized within 30 days?
Background: Thirty-day readmission occurs in 20% of hospitalized Medicare patients, with at least a quarter of rehospitalized patients admitted to a different hospital. Recent healthcare legislation proposes penalties to reduce readmission rates. This could provide unintended incentives for hospitals to divert patients at high risk for readmission to other hospitals.
Study design: Observational cohort study.
Setting: Hospitalized Medicare patients.
Synopsis: Analysis of a 5% sample of Medicare patients readmitted within 30 days of discharge over a 22-month period identified 74,564 patients who were rehospitalized in a facility different from their initial admission. For-profit status of the initial and subsequent hospital was identified. Twenty-eight percent of patients initially admitted to a for-profit hospital were readmitted to a different hospital within 30 days. By comparison, only 21% of patients initially admitted to a nonprofit hospital were readmitted to a different hospital (P<.001).
The most significant risk factors for readmission to a different hospital were admission to a lower-volume hospital (221% increased risk), disability (21% increased risk), admission to an academic hospital (18% increased risk), and admission to a for-profit hospital (17% increased risk). Thirty-day mortality did not differ between patients readmitted to the same or different hospital, regardless of for-profit status. Admission to a different hospital was associated with increased cost.
This study was not designed to look at why patients were rehospitalized at different hospitals, and findings cannot be generalized beyond Medicare patients.
Bottom line: Discharge from a for-profit hospital is one of several risk factors for 30-day readmission to a different hospital.
Citation: Kind AJ, Bartels C, Mell MW, Mullahy J, Smith M. For-profit hospital status and rehospitalizations at different hospitals: an analysis of Medicare data. Ann Intern Med. 2010;153(11):718-727. TH
Due Diligence: Denials
Before submitting a claim, hospitalists should ensure that the service is rendered, that it is completely and accurately documented in the medical record, that the correct information is entered on the claim form, and that it is a covered benefit and eligible for payment.
Although the latter two elements typically are delegated to the billing team, hospitalists should encourage or request feedback regarding payment and denials. The ensuing open dialogue between physicians and billers might prove helpful in understanding and resolving future billing issues. Less-experienced billers first respond to claim denials by submitting documentation (i.e. “appeal with paper”) despite the inappropriateness of this action. If the denial is upheld, this attempt is viewed as unsuccessful and, without further consideration, “written off.” However, careful examination of the payor’s initial claim determination could elicit a more suitable response.
Service Provider
Provider enrollment issues can sidetrack claim submissions. Physicians must register their NPI (national provider identifier) with the correct practice location and group assignment, particularly when previously practicing physicians join a new group practice. Failure to do so is an infrequent, yet valid, cause for denial.
Alternatively, enrollment issues play a greater role when services involve nurse practitioners (NPs) and physician assistants (PAs) who are enrolled with Medicare but might be prohibited from enrolling with other payors. For example, an NP independently provides subsequent hospital care (e.g. 99232) to a Medicare beneficiary. The claim is submitted in the NP’s name and reimbursed at the correct amount by Medicare as the primary insurer. The remaining balance is submitted to the secondary insurer, who does not enroll NPPs. The claim is rejected. If the physician group has a contractual agreement to recognize NPP services by reporting them under the collaborating physician’s name, the claim can be resubmitted in the physician’s name. In absence of such an agreement, the claim should be written off.
Location
The place of service (POS) must match the reported service/procedure code. For example, a hospitalist is asked to see a patient in the ED. The patient requires further testing but does not meet the criterion for an inpatient stay. The hospitalist admits the patient to observation, treats him, and discharges him to home.
Hospitalists need to avoid the common mistake of mismatching the service code with the location/POS. Observation services performed by the “physician of record” should be reported with the corresponding codes: initial observation care (99218-99220), subsequent observation care (99224-99226), or observation discharge (99217), as appropriate.1 The correct POS should be reported as outpatient hospital (POS 22), not inpatient hospital (POS 21). Trying to report outpatient codes with an inpatient POS will result in claim denial.
A similar denial occurs when trying to report inpatient codes (99231-99233) in an outpatient location (e.g. 23-ED). These denials require claim resubmission with the correct POS and/or service/procedure code. A complete list of POS codes and corresponding definitions can be obtained from Chapter 26, Section 10.5 of the Medicare Claims Processing Manual, available at www.cms.hhs.gov/manuals/downloads/clm104c26.pdf.
Diagnosis
Denials involving diagnoses produce issues of “medical necessity.”1 Examine these denials carefully. Consider the service/procedure code when trying to formulate a response to the denial. The diagnosis code represents the reason for the service or procedure and might be a sign, symptom, or condition with which the patient presents. Medicare reimburses for procedures and services that are deemed “reasonable and necessary.”
In an effort to unify standards, Medicare has developed national coverage determinations (NCDs) to identify coverage requirements for frequent or problematic procedures or services. These coverage requirements can identify specific conditions (i.e. ICD-9-CM codes) for which the services or procedures are considered medically necessary. In the absence of a national coverage policy, an item or service could be covered at the discretion of Medicare contractors based on a local coverage determination (LCD), which varies by contractor.
Medical necessity denials often involve a mismatched or missing diagnosis. For example, a payor might deny a claim for cardiopulmonary resuscitation (92950) that is associated with a diagnosis code of congestive heart failure (428.0), despite this being the underlying condition that prompted the decline in the patient’s condition. The payor might only accept “cardiac arrest” (427.5) as the “medically necessary” diagnosis for cardiopulmonary resuscitation, as this is the direct reason necessitating the procedure. After reviewing the documentation to ensure that the documentation supports the diagnosis, the claim can be resubmitted with a confirmed and corrected diagnosis code.
Initial-Request Response
While diagnoses can lead to medical necessity issues, not all medical necessity denials are due to incorrect diagnoses. Some “medical necessity” denials result from a failure to respond to a payor request. More specifically, if the “medical necessity” denial involves a covered evaluation and management visit, the denial is more likely the result of a failure to respond to a prepayment request for documentation.
Medicare typically issues prepayment requests for documentation for the following inpatient CPT codes: 99223, 99233, 99232, 99239, and 99292.1 If the documentation is not provided to the Medicare review department within a designated time frame (e.g. 30-45 days), the claim is automatically denied. The reason for denial is cited as being “not deemed a medical necessity.” These claims do not require electronic resubmission, and instead require submission of documentation to the Medicare appeals department. Once the supporting documentation is reviewed, reimbursement is issued.
Supportive Documentation
There are times when payor requests for additional information or documentation is handled in a timely fashion. However, the paper submission might have been incomplete, as the encounter note itself might not contain the cumulative information representing the reported service.
For example, other pieces of pertinent information may be obtained from the data or order section of the chart. If the individual responsible for gathering the requested documentation does not review it before submission, important or referenced entries may be missed, and the complexity of the billed service might be sacrificed. The provider should submit any entry with the same date as the requested documentation in support: labs, diagnostic testing, physician orders, patient instructions, nursing notes, resident notes, notes by other physicians in the same group, discharge summaries, etc.
Legibility of the encounter note is crucial when the documentation is sent for review. Most reviewers will seek another reviewer’s assistance in translating, but they are not obligated to do this. If the note is deemed incomprehensible, the service is denied, resulting in a nonpayment or a refund. Electronic medical records (EMRs) are assisting physicians and other providers with legibility issues and improving review findings. If a physician is still writing notes by hand, a transcription might be sent along with the documentation to prevent unnecessary denials. Only consider this for requests involving providers with problematic handwriting. A legible signature is required. If a denial ensues in absence of a signature, the provider can submit an appeal with an acceptable attestation.
Modifier Considerations
Some services are denied for being “incidental/integral” to another reimbursed service (i.e. bundled). Payors implement electronic payment edits that disallow separate payment for “related” services. The industry standard, known as the National Correct Coding Initiative (NCCI), identifies code pairs that should not be reported together on the same date by either a single physician or physicians of the same specialty within a provider group.
When a claim is denied for this reason, billers tend to automatically and erroneously resubmit the claim with a modifier appended to the disallowed or “bundled” procedure code. Documentation should be reviewed to determine if the denied service is separately reportable from the paid service. The biller might append the appropriate modifier and resubmit the claim only when well supported by documentation.
For example, the hospitalist evaluated a patient with congestive heart failure and pleural effusions. The hospitalist determined that the patient requires placement of a central venous catheter (36556). Because the patient’s underlying condition was evaluated, and resulted in the decision to place a catheter, both the visit (99233) and the procedure (36556) can be reported. If submitted without modifiers, some payors will deny payment for the visit for being integral to the catheter placement. In this case, the claim should be resubmitted with modifier 25 appended to the visit. Payors might still require documentation review to ensure legitimacy of this modifier before the claim is paid. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
Reference
- Abraham M, Ahlman J, Boudreau A, Connelly J, Evans D. Current Procedural Terminology Professional Edition. Chicago: AMA Press; 2011.
Before submitting a claim, hospitalists should ensure that the service is rendered, that it is completely and accurately documented in the medical record, that the correct information is entered on the claim form, and that it is a covered benefit and eligible for payment.
Although the latter two elements typically are delegated to the billing team, hospitalists should encourage or request feedback regarding payment and denials. The ensuing open dialogue between physicians and billers might prove helpful in understanding and resolving future billing issues. Less-experienced billers first respond to claim denials by submitting documentation (i.e. “appeal with paper”) despite the inappropriateness of this action. If the denial is upheld, this attempt is viewed as unsuccessful and, without further consideration, “written off.” However, careful examination of the payor’s initial claim determination could elicit a more suitable response.
Service Provider
Provider enrollment issues can sidetrack claim submissions. Physicians must register their NPI (national provider identifier) with the correct practice location and group assignment, particularly when previously practicing physicians join a new group practice. Failure to do so is an infrequent, yet valid, cause for denial.
Alternatively, enrollment issues play a greater role when services involve nurse practitioners (NPs) and physician assistants (PAs) who are enrolled with Medicare but might be prohibited from enrolling with other payors. For example, an NP independently provides subsequent hospital care (e.g. 99232) to a Medicare beneficiary. The claim is submitted in the NP’s name and reimbursed at the correct amount by Medicare as the primary insurer. The remaining balance is submitted to the secondary insurer, who does not enroll NPPs. The claim is rejected. If the physician group has a contractual agreement to recognize NPP services by reporting them under the collaborating physician’s name, the claim can be resubmitted in the physician’s name. In absence of such an agreement, the claim should be written off.
Location
The place of service (POS) must match the reported service/procedure code. For example, a hospitalist is asked to see a patient in the ED. The patient requires further testing but does not meet the criterion for an inpatient stay. The hospitalist admits the patient to observation, treats him, and discharges him to home.
Hospitalists need to avoid the common mistake of mismatching the service code with the location/POS. Observation services performed by the “physician of record” should be reported with the corresponding codes: initial observation care (99218-99220), subsequent observation care (99224-99226), or observation discharge (99217), as appropriate.1 The correct POS should be reported as outpatient hospital (POS 22), not inpatient hospital (POS 21). Trying to report outpatient codes with an inpatient POS will result in claim denial.
A similar denial occurs when trying to report inpatient codes (99231-99233) in an outpatient location (e.g. 23-ED). These denials require claim resubmission with the correct POS and/or service/procedure code. A complete list of POS codes and corresponding definitions can be obtained from Chapter 26, Section 10.5 of the Medicare Claims Processing Manual, available at www.cms.hhs.gov/manuals/downloads/clm104c26.pdf.
Diagnosis
Denials involving diagnoses produce issues of “medical necessity.”1 Examine these denials carefully. Consider the service/procedure code when trying to formulate a response to the denial. The diagnosis code represents the reason for the service or procedure and might be a sign, symptom, or condition with which the patient presents. Medicare reimburses for procedures and services that are deemed “reasonable and necessary.”
In an effort to unify standards, Medicare has developed national coverage determinations (NCDs) to identify coverage requirements for frequent or problematic procedures or services. These coverage requirements can identify specific conditions (i.e. ICD-9-CM codes) for which the services or procedures are considered medically necessary. In the absence of a national coverage policy, an item or service could be covered at the discretion of Medicare contractors based on a local coverage determination (LCD), which varies by contractor.
Medical necessity denials often involve a mismatched or missing diagnosis. For example, a payor might deny a claim for cardiopulmonary resuscitation (92950) that is associated with a diagnosis code of congestive heart failure (428.0), despite this being the underlying condition that prompted the decline in the patient’s condition. The payor might only accept “cardiac arrest” (427.5) as the “medically necessary” diagnosis for cardiopulmonary resuscitation, as this is the direct reason necessitating the procedure. After reviewing the documentation to ensure that the documentation supports the diagnosis, the claim can be resubmitted with a confirmed and corrected diagnosis code.
Initial-Request Response
While diagnoses can lead to medical necessity issues, not all medical necessity denials are due to incorrect diagnoses. Some “medical necessity” denials result from a failure to respond to a payor request. More specifically, if the “medical necessity” denial involves a covered evaluation and management visit, the denial is more likely the result of a failure to respond to a prepayment request for documentation.
Medicare typically issues prepayment requests for documentation for the following inpatient CPT codes: 99223, 99233, 99232, 99239, and 99292.1 If the documentation is not provided to the Medicare review department within a designated time frame (e.g. 30-45 days), the claim is automatically denied. The reason for denial is cited as being “not deemed a medical necessity.” These claims do not require electronic resubmission, and instead require submission of documentation to the Medicare appeals department. Once the supporting documentation is reviewed, reimbursement is issued.
Supportive Documentation
There are times when payor requests for additional information or documentation is handled in a timely fashion. However, the paper submission might have been incomplete, as the encounter note itself might not contain the cumulative information representing the reported service.
For example, other pieces of pertinent information may be obtained from the data or order section of the chart. If the individual responsible for gathering the requested documentation does not review it before submission, important or referenced entries may be missed, and the complexity of the billed service might be sacrificed. The provider should submit any entry with the same date as the requested documentation in support: labs, diagnostic testing, physician orders, patient instructions, nursing notes, resident notes, notes by other physicians in the same group, discharge summaries, etc.
Legibility of the encounter note is crucial when the documentation is sent for review. Most reviewers will seek another reviewer’s assistance in translating, but they are not obligated to do this. If the note is deemed incomprehensible, the service is denied, resulting in a nonpayment or a refund. Electronic medical records (EMRs) are assisting physicians and other providers with legibility issues and improving review findings. If a physician is still writing notes by hand, a transcription might be sent along with the documentation to prevent unnecessary denials. Only consider this for requests involving providers with problematic handwriting. A legible signature is required. If a denial ensues in absence of a signature, the provider can submit an appeal with an acceptable attestation.
Modifier Considerations
Some services are denied for being “incidental/integral” to another reimbursed service (i.e. bundled). Payors implement electronic payment edits that disallow separate payment for “related” services. The industry standard, known as the National Correct Coding Initiative (NCCI), identifies code pairs that should not be reported together on the same date by either a single physician or physicians of the same specialty within a provider group.
When a claim is denied for this reason, billers tend to automatically and erroneously resubmit the claim with a modifier appended to the disallowed or “bundled” procedure code. Documentation should be reviewed to determine if the denied service is separately reportable from the paid service. The biller might append the appropriate modifier and resubmit the claim only when well supported by documentation.
For example, the hospitalist evaluated a patient with congestive heart failure and pleural effusions. The hospitalist determined that the patient requires placement of a central venous catheter (36556). Because the patient’s underlying condition was evaluated, and resulted in the decision to place a catheter, both the visit (99233) and the procedure (36556) can be reported. If submitted without modifiers, some payors will deny payment for the visit for being integral to the catheter placement. In this case, the claim should be resubmitted with modifier 25 appended to the visit. Payors might still require documentation review to ensure legitimacy of this modifier before the claim is paid. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
Reference
- Abraham M, Ahlman J, Boudreau A, Connelly J, Evans D. Current Procedural Terminology Professional Edition. Chicago: AMA Press; 2011.
Before submitting a claim, hospitalists should ensure that the service is rendered, that it is completely and accurately documented in the medical record, that the correct information is entered on the claim form, and that it is a covered benefit and eligible for payment.
Although the latter two elements typically are delegated to the billing team, hospitalists should encourage or request feedback regarding payment and denials. The ensuing open dialogue between physicians and billers might prove helpful in understanding and resolving future billing issues. Less-experienced billers first respond to claim denials by submitting documentation (i.e. “appeal with paper”) despite the inappropriateness of this action. If the denial is upheld, this attempt is viewed as unsuccessful and, without further consideration, “written off.” However, careful examination of the payor’s initial claim determination could elicit a more suitable response.
Service Provider
Provider enrollment issues can sidetrack claim submissions. Physicians must register their NPI (national provider identifier) with the correct practice location and group assignment, particularly when previously practicing physicians join a new group practice. Failure to do so is an infrequent, yet valid, cause for denial.
Alternatively, enrollment issues play a greater role when services involve nurse practitioners (NPs) and physician assistants (PAs) who are enrolled with Medicare but might be prohibited from enrolling with other payors. For example, an NP independently provides subsequent hospital care (e.g. 99232) to a Medicare beneficiary. The claim is submitted in the NP’s name and reimbursed at the correct amount by Medicare as the primary insurer. The remaining balance is submitted to the secondary insurer, who does not enroll NPPs. The claim is rejected. If the physician group has a contractual agreement to recognize NPP services by reporting them under the collaborating physician’s name, the claim can be resubmitted in the physician’s name. In absence of such an agreement, the claim should be written off.
Location
The place of service (POS) must match the reported service/procedure code. For example, a hospitalist is asked to see a patient in the ED. The patient requires further testing but does not meet the criterion for an inpatient stay. The hospitalist admits the patient to observation, treats him, and discharges him to home.
Hospitalists need to avoid the common mistake of mismatching the service code with the location/POS. Observation services performed by the “physician of record” should be reported with the corresponding codes: initial observation care (99218-99220), subsequent observation care (99224-99226), or observation discharge (99217), as appropriate.1 The correct POS should be reported as outpatient hospital (POS 22), not inpatient hospital (POS 21). Trying to report outpatient codes with an inpatient POS will result in claim denial.
A similar denial occurs when trying to report inpatient codes (99231-99233) in an outpatient location (e.g. 23-ED). These denials require claim resubmission with the correct POS and/or service/procedure code. A complete list of POS codes and corresponding definitions can be obtained from Chapter 26, Section 10.5 of the Medicare Claims Processing Manual, available at www.cms.hhs.gov/manuals/downloads/clm104c26.pdf.
Diagnosis
Denials involving diagnoses produce issues of “medical necessity.”1 Examine these denials carefully. Consider the service/procedure code when trying to formulate a response to the denial. The diagnosis code represents the reason for the service or procedure and might be a sign, symptom, or condition with which the patient presents. Medicare reimburses for procedures and services that are deemed “reasonable and necessary.”
In an effort to unify standards, Medicare has developed national coverage determinations (NCDs) to identify coverage requirements for frequent or problematic procedures or services. These coverage requirements can identify specific conditions (i.e. ICD-9-CM codes) for which the services or procedures are considered medically necessary. In the absence of a national coverage policy, an item or service could be covered at the discretion of Medicare contractors based on a local coverage determination (LCD), which varies by contractor.
Medical necessity denials often involve a mismatched or missing diagnosis. For example, a payor might deny a claim for cardiopulmonary resuscitation (92950) that is associated with a diagnosis code of congestive heart failure (428.0), despite this being the underlying condition that prompted the decline in the patient’s condition. The payor might only accept “cardiac arrest” (427.5) as the “medically necessary” diagnosis for cardiopulmonary resuscitation, as this is the direct reason necessitating the procedure. After reviewing the documentation to ensure that the documentation supports the diagnosis, the claim can be resubmitted with a confirmed and corrected diagnosis code.
Initial-Request Response
While diagnoses can lead to medical necessity issues, not all medical necessity denials are due to incorrect diagnoses. Some “medical necessity” denials result from a failure to respond to a payor request. More specifically, if the “medical necessity” denial involves a covered evaluation and management visit, the denial is more likely the result of a failure to respond to a prepayment request for documentation.
Medicare typically issues prepayment requests for documentation for the following inpatient CPT codes: 99223, 99233, 99232, 99239, and 99292.1 If the documentation is not provided to the Medicare review department within a designated time frame (e.g. 30-45 days), the claim is automatically denied. The reason for denial is cited as being “not deemed a medical necessity.” These claims do not require electronic resubmission, and instead require submission of documentation to the Medicare appeals department. Once the supporting documentation is reviewed, reimbursement is issued.
Supportive Documentation
There are times when payor requests for additional information or documentation is handled in a timely fashion. However, the paper submission might have been incomplete, as the encounter note itself might not contain the cumulative information representing the reported service.
For example, other pieces of pertinent information may be obtained from the data or order section of the chart. If the individual responsible for gathering the requested documentation does not review it before submission, important or referenced entries may be missed, and the complexity of the billed service might be sacrificed. The provider should submit any entry with the same date as the requested documentation in support: labs, diagnostic testing, physician orders, patient instructions, nursing notes, resident notes, notes by other physicians in the same group, discharge summaries, etc.
Legibility of the encounter note is crucial when the documentation is sent for review. Most reviewers will seek another reviewer’s assistance in translating, but they are not obligated to do this. If the note is deemed incomprehensible, the service is denied, resulting in a nonpayment or a refund. Electronic medical records (EMRs) are assisting physicians and other providers with legibility issues and improving review findings. If a physician is still writing notes by hand, a transcription might be sent along with the documentation to prevent unnecessary denials. Only consider this for requests involving providers with problematic handwriting. A legible signature is required. If a denial ensues in absence of a signature, the provider can submit an appeal with an acceptable attestation.
Modifier Considerations
Some services are denied for being “incidental/integral” to another reimbursed service (i.e. bundled). Payors implement electronic payment edits that disallow separate payment for “related” services. The industry standard, known as the National Correct Coding Initiative (NCCI), identifies code pairs that should not be reported together on the same date by either a single physician or physicians of the same specialty within a provider group.
When a claim is denied for this reason, billers tend to automatically and erroneously resubmit the claim with a modifier appended to the disallowed or “bundled” procedure code. Documentation should be reviewed to determine if the denied service is separately reportable from the paid service. The biller might append the appropriate modifier and resubmit the claim only when well supported by documentation.
For example, the hospitalist evaluated a patient with congestive heart failure and pleural effusions. The hospitalist determined that the patient requires placement of a central venous catheter (36556). Because the patient’s underlying condition was evaluated, and resulted in the decision to place a catheter, both the visit (99233) and the procedure (36556) can be reported. If submitted without modifiers, some payors will deny payment for the visit for being integral to the catheter placement. In this case, the claim should be resubmitted with modifier 25 appended to the visit. Payors might still require documentation review to ensure legitimacy of this modifier before the claim is paid. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
Reference
- Abraham M, Ahlman J, Boudreau A, Connelly J, Evans D. Current Procedural Terminology Professional Edition. Chicago: AMA Press; 2011.
Accurate Measures?
Everyone’s talking about quality. Encouraging high-value care is one of the stated objectives of the value-based purchasing program being rolled out by the Centers for Medicare & Medicaid Services (CMS). It’s also the subject of a new report to Congress from the Department of Health and Human Services (HHS), “National Strategy for Quality Improvement in Health Care” (www.healthcare.gov/center/reports/quality03212011a.html). For its part, SHM is placing added emphasis on a range of mentored quality-improvement (QI) initiatives for hospitalists.
Amid the flurry of activity, researchers are still attempting to address a central question that could determine the success or failure of many such efforts: How do you accurately measure what constitutes high-quality care?
Chris Murray, MD, DPhil, director of the Seattle-based Institute for Health Metrics and Evaluation, says the healthcare field traditionally has tried to assess quality in three main ways. One is to ask patients about their own experience: Were they satisfied with the level of care they received? Another is to assess what are known as process of care measures: Did the providers follow guidelines in providing patients with appropriate care? The third is to look at risk-adjusted outcomes: How did the patients ultimately fare?
Focused on Facts
CMS’s value-based purchasing program, at least initially, is focusing on the first two types of metrics. Process measures, Dr. Murray says, are popular in part because they’re relatively easy to gauge. For many of them, however, “the connection to improved health is a bit weak,” he says. Whether heart patients get a prescription for a beta-blocker drug, for example, doesn’t address the outcome. “The problem there is that we don’t know if they ever filled the prescription or if the patient takes the beta-blocker,” Dr. Murray says.
That uncertainty feeds into the larger question of how broadly to consider the accountability of providers when measuring quality. “Should we be thinking that quality means putting in place the supports required for a patient to actually achieve a good outcome, or just offering them?” Dr. Murray asks. The debate might be far from settled, but a growing number of tools and studies are at least helping researchers to connect the dots on how care is delivered, on what kind of practices might affect outcomes the most, and how a community’s underlying risks could influence both considerations.
A recent Annals of Internal Medicine study that scrutinized 30-day mortality rates for heart-attack patients found few quantitative differences between the top 5% and bottom 5% of hospitals, based on rates published on the CMS Hospital Compare website.1 Site visits and in-depth interviews with nearly 160 medical staff members, however, uncovered some telling distinctions.
The study found that following evidence-based protocols and processes, while important, likely is not sufficient to attain a high performance level in caring for heart-attack patients. Instead, “high-performing hospitals were characterized by an organizational culture that supported efforts to improve AMI [acute myocardial infarction] care across the hospital.” In other words, everyone from management to the medical staff was fully invested in QI efforts. Notably, the staff “reported the presence of physician champions and empowered nursing staff, pharmacist involvement in patient care, and high qualification standards for all staff.”
For its 13th annual HealthGrades Quality in America study, the Denver-based ratings organization HealthGrades tried to look more quantitatively at the link between top hospitals and patient outcomes. Its study coauthors culled data from roughly 40 million Medicare discharges from 2007 through 2009 for most of the nation’s 5,000 hospitals, and assigned ratings based on 26 measures related to mortality and complication rates (www.healthgrades.com/business/news/press-releases/hospital-quality-2010.aspx).
If all hospitals were performing on par with what HealthGrades terms a five-star hospital, the study suggests the U.S. healthcare system could have saved the lives of more than 230,000 Medicare beneficiaries over the three-year period. More than half of the preventable deaths were associated with sepsis, pneumonia, respiratory failure, and heart failure.
Although the high number raises the question of whether some preventable deaths might exist only on paper, the study does raise other eye-popping calculations. Typical patients who went to a five-star hospital instead of a one-star hospital had a 72% lower risk of dying and reduced their risk by 53% compared with U.S. hospitals overall. The survival advantage persisted after hospitalization, too: Patients discharged from five-star-rated hospitals were 57% less likely to die within 30 days than all patients.
Ali Mokdad, PhD, professor of global health at the Institute for Health Metrics and Evaluation, says one big caveat to such rankings is the matter of adjusted risk. What kind of patient populations are these hospitals treating? Are people in the area inherently less healthy? Are significant barriers to healthcare blocking access to preventive medicine?
Dr. Murray says measuring quality with risk-adjusted outcomes has periodically fallen in and out of favor, due in part to concerns over how the risk is calculated and whether the assessments could be biased against providers that see more difficult patients. Nonetheless, he believes the metric is underused in the U.S. “I think the pendulum went way away from risk-adjusted outcomes to process measures too much, and we need to have a mixed combination,” he says.
With improvements to the methodology, he sees a wealth of potential in picking out risk predictors from large data sets. “The world is getting better at predicting rehospitalization, predicting death from attributes of the patient,” he says. “If you can do a better job at risk adjustment, you can do a better job on identifying quality.”
Risk Adjustment
One area in which the U.S. has lagged is in integrating the risk of death due to chronic conditions into broader measures of healthcare. At the recent Global Health Metrics & Evaluation Conference in Seattle, Dr. Mokdad pointed out the stringent oversight applied to commercial airliners. An avoidable crash and loss of life would quickly lead to a full-scale investigation. Why, he wondered, can’t the same scrutiny be brought to bear on preventable deaths due to chronic conditions such as diabetes and heart disease?
An ambitious new surveillance project, in fact, is trying to do exactly that. Known as the Monitoring Disparities in Chronic Conditions (MDCC) Study, the effort will use Washington state’s King County as a test case to hone the necessary data collection techniques. If it pans out, the study could become a national model for how to assess a population’s health status. “You know how a physician takes your pulse?” Dr. Mokdad says. “We’re doing that for the community.”
The research team, which includes Dr. Mokdad, Dr. Murray, and collaborators from Dartmouth and Harvard universities, will administer in-depth, culturally sensitive surveys to more than 3,000 county residents. A subset of 750 participants also will receive physical exams that measure markers of health and activity.
One goal is to work out how to efficiently integrate data from multiple sources so researchers can apply their risk adjustment strategies. For example, can they get enough information to ask how many heart attack patients are on beta-blockers one year after a hospital discharge? “There is also this big question of community background health risk,” Dr. Murray says. “Is this a community where people are just sicker, and how do you factor that in addition to taking into account the comorbidities that individuals have when they show up in the hospital?”
Researchers are close to obtaining enough information on such key factors as blood pressure, cholesterol, tobacco use, and obesity to actually rate communities according to risk, he says.2 “That’s never been done at the local level, and I think it’s where we need to go to truly put things on a level playing field when you’re assessing quality.” TH
Bryn Nelson is a freelance medical writer based in Seattle.
References
- Curry LA, Spatz E, Cherlin E, et al. What distinguishes top-performing hospitals in acute myocardial infarction mortality rates? Ann Intern Med. 2001;154(6):384-390.
- Murray CJ, Kulkarni SC, Michaud C, et al. Eight Americas: investigating mortality disparities across races, counties, and race-counties in the United States. PLoS Med. 2006;3(9):e260.
Everyone’s talking about quality. Encouraging high-value care is one of the stated objectives of the value-based purchasing program being rolled out by the Centers for Medicare & Medicaid Services (CMS). It’s also the subject of a new report to Congress from the Department of Health and Human Services (HHS), “National Strategy for Quality Improvement in Health Care” (www.healthcare.gov/center/reports/quality03212011a.html). For its part, SHM is placing added emphasis on a range of mentored quality-improvement (QI) initiatives for hospitalists.
Amid the flurry of activity, researchers are still attempting to address a central question that could determine the success or failure of many such efforts: How do you accurately measure what constitutes high-quality care?
Chris Murray, MD, DPhil, director of the Seattle-based Institute for Health Metrics and Evaluation, says the healthcare field traditionally has tried to assess quality in three main ways. One is to ask patients about their own experience: Were they satisfied with the level of care they received? Another is to assess what are known as process of care measures: Did the providers follow guidelines in providing patients with appropriate care? The third is to look at risk-adjusted outcomes: How did the patients ultimately fare?
Focused on Facts
CMS’s value-based purchasing program, at least initially, is focusing on the first two types of metrics. Process measures, Dr. Murray says, are popular in part because they’re relatively easy to gauge. For many of them, however, “the connection to improved health is a bit weak,” he says. Whether heart patients get a prescription for a beta-blocker drug, for example, doesn’t address the outcome. “The problem there is that we don’t know if they ever filled the prescription or if the patient takes the beta-blocker,” Dr. Murray says.
That uncertainty feeds into the larger question of how broadly to consider the accountability of providers when measuring quality. “Should we be thinking that quality means putting in place the supports required for a patient to actually achieve a good outcome, or just offering them?” Dr. Murray asks. The debate might be far from settled, but a growing number of tools and studies are at least helping researchers to connect the dots on how care is delivered, on what kind of practices might affect outcomes the most, and how a community’s underlying risks could influence both considerations.
A recent Annals of Internal Medicine study that scrutinized 30-day mortality rates for heart-attack patients found few quantitative differences between the top 5% and bottom 5% of hospitals, based on rates published on the CMS Hospital Compare website.1 Site visits and in-depth interviews with nearly 160 medical staff members, however, uncovered some telling distinctions.
The study found that following evidence-based protocols and processes, while important, likely is not sufficient to attain a high performance level in caring for heart-attack patients. Instead, “high-performing hospitals were characterized by an organizational culture that supported efforts to improve AMI [acute myocardial infarction] care across the hospital.” In other words, everyone from management to the medical staff was fully invested in QI efforts. Notably, the staff “reported the presence of physician champions and empowered nursing staff, pharmacist involvement in patient care, and high qualification standards for all staff.”
For its 13th annual HealthGrades Quality in America study, the Denver-based ratings organization HealthGrades tried to look more quantitatively at the link between top hospitals and patient outcomes. Its study coauthors culled data from roughly 40 million Medicare discharges from 2007 through 2009 for most of the nation’s 5,000 hospitals, and assigned ratings based on 26 measures related to mortality and complication rates (www.healthgrades.com/business/news/press-releases/hospital-quality-2010.aspx).
If all hospitals were performing on par with what HealthGrades terms a five-star hospital, the study suggests the U.S. healthcare system could have saved the lives of more than 230,000 Medicare beneficiaries over the three-year period. More than half of the preventable deaths were associated with sepsis, pneumonia, respiratory failure, and heart failure.
Although the high number raises the question of whether some preventable deaths might exist only on paper, the study does raise other eye-popping calculations. Typical patients who went to a five-star hospital instead of a one-star hospital had a 72% lower risk of dying and reduced their risk by 53% compared with U.S. hospitals overall. The survival advantage persisted after hospitalization, too: Patients discharged from five-star-rated hospitals were 57% less likely to die within 30 days than all patients.
Ali Mokdad, PhD, professor of global health at the Institute for Health Metrics and Evaluation, says one big caveat to such rankings is the matter of adjusted risk. What kind of patient populations are these hospitals treating? Are people in the area inherently less healthy? Are significant barriers to healthcare blocking access to preventive medicine?
Dr. Murray says measuring quality with risk-adjusted outcomes has periodically fallen in and out of favor, due in part to concerns over how the risk is calculated and whether the assessments could be biased against providers that see more difficult patients. Nonetheless, he believes the metric is underused in the U.S. “I think the pendulum went way away from risk-adjusted outcomes to process measures too much, and we need to have a mixed combination,” he says.
With improvements to the methodology, he sees a wealth of potential in picking out risk predictors from large data sets. “The world is getting better at predicting rehospitalization, predicting death from attributes of the patient,” he says. “If you can do a better job at risk adjustment, you can do a better job on identifying quality.”
Risk Adjustment
One area in which the U.S. has lagged is in integrating the risk of death due to chronic conditions into broader measures of healthcare. At the recent Global Health Metrics & Evaluation Conference in Seattle, Dr. Mokdad pointed out the stringent oversight applied to commercial airliners. An avoidable crash and loss of life would quickly lead to a full-scale investigation. Why, he wondered, can’t the same scrutiny be brought to bear on preventable deaths due to chronic conditions such as diabetes and heart disease?
An ambitious new surveillance project, in fact, is trying to do exactly that. Known as the Monitoring Disparities in Chronic Conditions (MDCC) Study, the effort will use Washington state’s King County as a test case to hone the necessary data collection techniques. If it pans out, the study could become a national model for how to assess a population’s health status. “You know how a physician takes your pulse?” Dr. Mokdad says. “We’re doing that for the community.”
The research team, which includes Dr. Mokdad, Dr. Murray, and collaborators from Dartmouth and Harvard universities, will administer in-depth, culturally sensitive surveys to more than 3,000 county residents. A subset of 750 participants also will receive physical exams that measure markers of health and activity.
One goal is to work out how to efficiently integrate data from multiple sources so researchers can apply their risk adjustment strategies. For example, can they get enough information to ask how many heart attack patients are on beta-blockers one year after a hospital discharge? “There is also this big question of community background health risk,” Dr. Murray says. “Is this a community where people are just sicker, and how do you factor that in addition to taking into account the comorbidities that individuals have when they show up in the hospital?”
Researchers are close to obtaining enough information on such key factors as blood pressure, cholesterol, tobacco use, and obesity to actually rate communities according to risk, he says.2 “That’s never been done at the local level, and I think it’s where we need to go to truly put things on a level playing field when you’re assessing quality.” TH
Bryn Nelson is a freelance medical writer based in Seattle.
References
- Curry LA, Spatz E, Cherlin E, et al. What distinguishes top-performing hospitals in acute myocardial infarction mortality rates? Ann Intern Med. 2001;154(6):384-390.
- Murray CJ, Kulkarni SC, Michaud C, et al. Eight Americas: investigating mortality disparities across races, counties, and race-counties in the United States. PLoS Med. 2006;3(9):e260.
Everyone’s talking about quality. Encouraging high-value care is one of the stated objectives of the value-based purchasing program being rolled out by the Centers for Medicare & Medicaid Services (CMS). It’s also the subject of a new report to Congress from the Department of Health and Human Services (HHS), “National Strategy for Quality Improvement in Health Care” (www.healthcare.gov/center/reports/quality03212011a.html). For its part, SHM is placing added emphasis on a range of mentored quality-improvement (QI) initiatives for hospitalists.
Amid the flurry of activity, researchers are still attempting to address a central question that could determine the success or failure of many such efforts: How do you accurately measure what constitutes high-quality care?
Chris Murray, MD, DPhil, director of the Seattle-based Institute for Health Metrics and Evaluation, says the healthcare field traditionally has tried to assess quality in three main ways. One is to ask patients about their own experience: Were they satisfied with the level of care they received? Another is to assess what are known as process of care measures: Did the providers follow guidelines in providing patients with appropriate care? The third is to look at risk-adjusted outcomes: How did the patients ultimately fare?
Focused on Facts
CMS’s value-based purchasing program, at least initially, is focusing on the first two types of metrics. Process measures, Dr. Murray says, are popular in part because they’re relatively easy to gauge. For many of them, however, “the connection to improved health is a bit weak,” he says. Whether heart patients get a prescription for a beta-blocker drug, for example, doesn’t address the outcome. “The problem there is that we don’t know if they ever filled the prescription or if the patient takes the beta-blocker,” Dr. Murray says.
That uncertainty feeds into the larger question of how broadly to consider the accountability of providers when measuring quality. “Should we be thinking that quality means putting in place the supports required for a patient to actually achieve a good outcome, or just offering them?” Dr. Murray asks. The debate might be far from settled, but a growing number of tools and studies are at least helping researchers to connect the dots on how care is delivered, on what kind of practices might affect outcomes the most, and how a community’s underlying risks could influence both considerations.
A recent Annals of Internal Medicine study that scrutinized 30-day mortality rates for heart-attack patients found few quantitative differences between the top 5% and bottom 5% of hospitals, based on rates published on the CMS Hospital Compare website.1 Site visits and in-depth interviews with nearly 160 medical staff members, however, uncovered some telling distinctions.
The study found that following evidence-based protocols and processes, while important, likely is not sufficient to attain a high performance level in caring for heart-attack patients. Instead, “high-performing hospitals were characterized by an organizational culture that supported efforts to improve AMI [acute myocardial infarction] care across the hospital.” In other words, everyone from management to the medical staff was fully invested in QI efforts. Notably, the staff “reported the presence of physician champions and empowered nursing staff, pharmacist involvement in patient care, and high qualification standards for all staff.”
For its 13th annual HealthGrades Quality in America study, the Denver-based ratings organization HealthGrades tried to look more quantitatively at the link between top hospitals and patient outcomes. Its study coauthors culled data from roughly 40 million Medicare discharges from 2007 through 2009 for most of the nation’s 5,000 hospitals, and assigned ratings based on 26 measures related to mortality and complication rates (www.healthgrades.com/business/news/press-releases/hospital-quality-2010.aspx).
If all hospitals were performing on par with what HealthGrades terms a five-star hospital, the study suggests the U.S. healthcare system could have saved the lives of more than 230,000 Medicare beneficiaries over the three-year period. More than half of the preventable deaths were associated with sepsis, pneumonia, respiratory failure, and heart failure.
Although the high number raises the question of whether some preventable deaths might exist only on paper, the study does raise other eye-popping calculations. Typical patients who went to a five-star hospital instead of a one-star hospital had a 72% lower risk of dying and reduced their risk by 53% compared with U.S. hospitals overall. The survival advantage persisted after hospitalization, too: Patients discharged from five-star-rated hospitals were 57% less likely to die within 30 days than all patients.
Ali Mokdad, PhD, professor of global health at the Institute for Health Metrics and Evaluation, says one big caveat to such rankings is the matter of adjusted risk. What kind of patient populations are these hospitals treating? Are people in the area inherently less healthy? Are significant barriers to healthcare blocking access to preventive medicine?
Dr. Murray says measuring quality with risk-adjusted outcomes has periodically fallen in and out of favor, due in part to concerns over how the risk is calculated and whether the assessments could be biased against providers that see more difficult patients. Nonetheless, he believes the metric is underused in the U.S. “I think the pendulum went way away from risk-adjusted outcomes to process measures too much, and we need to have a mixed combination,” he says.
With improvements to the methodology, he sees a wealth of potential in picking out risk predictors from large data sets. “The world is getting better at predicting rehospitalization, predicting death from attributes of the patient,” he says. “If you can do a better job at risk adjustment, you can do a better job on identifying quality.”
Risk Adjustment
One area in which the U.S. has lagged is in integrating the risk of death due to chronic conditions into broader measures of healthcare. At the recent Global Health Metrics & Evaluation Conference in Seattle, Dr. Mokdad pointed out the stringent oversight applied to commercial airliners. An avoidable crash and loss of life would quickly lead to a full-scale investigation. Why, he wondered, can’t the same scrutiny be brought to bear on preventable deaths due to chronic conditions such as diabetes and heart disease?
An ambitious new surveillance project, in fact, is trying to do exactly that. Known as the Monitoring Disparities in Chronic Conditions (MDCC) Study, the effort will use Washington state’s King County as a test case to hone the necessary data collection techniques. If it pans out, the study could become a national model for how to assess a population’s health status. “You know how a physician takes your pulse?” Dr. Mokdad says. “We’re doing that for the community.”
The research team, which includes Dr. Mokdad, Dr. Murray, and collaborators from Dartmouth and Harvard universities, will administer in-depth, culturally sensitive surveys to more than 3,000 county residents. A subset of 750 participants also will receive physical exams that measure markers of health and activity.
One goal is to work out how to efficiently integrate data from multiple sources so researchers can apply their risk adjustment strategies. For example, can they get enough information to ask how many heart attack patients are on beta-blockers one year after a hospital discharge? “There is also this big question of community background health risk,” Dr. Murray says. “Is this a community where people are just sicker, and how do you factor that in addition to taking into account the comorbidities that individuals have when they show up in the hospital?”
Researchers are close to obtaining enough information on such key factors as blood pressure, cholesterol, tobacco use, and obesity to actually rate communities according to risk, he says.2 “That’s never been done at the local level, and I think it’s where we need to go to truly put things on a level playing field when you’re assessing quality.” TH
Bryn Nelson is a freelance medical writer based in Seattle.
References
- Curry LA, Spatz E, Cherlin E, et al. What distinguishes top-performing hospitals in acute myocardial infarction mortality rates? Ann Intern Med. 2001;154(6):384-390.
- Murray CJ, Kulkarni SC, Michaud C, et al. Eight Americas: investigating mortality disparities across races, counties, and race-counties in the United States. PLoS Med. 2006;3(9):e260.
Multiple Choices
Seven on, seven off. That’s what you can expect as a hospitalist, right? Maybe. As you consider an HM career, your first thought should be just that—is this a career or a job? Is this a year between residency and fellowship, clinical shifts that allow you to have 26 weeks off every year, or is it something else?
Either way, HM offers abundant opportunities conducive to work-life balance and career satisfaction. HM careers reside in clinical, academic, and administrative settings.
Clinical
As a physician entering the workplace, clinical practice often is the most familiar, but it does not have to be an extension of your residency floor rotation. Your individual schedule and employer will play a role in determining when you provide clinical service. Some employers, whether hospital-based, a private physician practice, or a national hospitalist company, utilize fixed schedules, which might include night shifts. The popular seven-on, seven-off model allows you to provide direct patient care every other week, creating personal or administrative time in between. You can opt to become a nocturnist and limit yourself to clinical service at night. Some hospital-based programs implement individualized schedules to meet the nonclinical demands of academic or administrative hospitalists. These schedules might combine Monday-through-Friday weeks, weekends, and nights to create flexibility within your group.
Clinical service can be carved out to fit both your interests and the setting in which you provide care. If placing central lines is your thing, a career as a proceduralist might be for you. Other hospitalists find themselves in clinical niches in specialty collaboration and specific care settings, including surgical comanagement, intensive care, emergency, clinical decision or observation units, or pre-admission and post-hospitalization clinics.
Hospitalists can improve quality, patient safety, and efficiency when working in specialized areas like a clinical decision or observation unit. In these settings, hospitalists often collaborate with midlevel providers, such as nurse practitioners or physician assistants, to provide observation or outpatient care to patients with medical conditions that require more than an ED visit. For example, many patients who present to the ED with chest pain are ideal patients for these settings to evaluate their symptoms and provide an optimal care transition out of the hospital or to an inpatient unit, if needed.
Perhaps you enjoy patient care, but just not all the time involved: You might find a fulfilling career that blends clinical service with research, teaching, or administrative work.
Academic
As an academic hospitalist, you have various options. Hospitalists provide education and oversight to trainees, both medical students and residents, in academic medical centers and community teaching hospitals. You might join an academic center and receive a faculty appointment, either as clinical instructor or assistant professor for first-time candidates.
Clinician educators generally serve as internal-medicine-ward attendings, teaching inpatient care to house staff and students in a traditional sense. Studies have demonstrated that students and house staff are more satisfied and feel they learn more when their ward attending is a hospitalist.1 Academic hospitalists foster career development by serving as mentors or residency program directors. Academic hospitalists also educate fellow physicians through faculty development series and programs.
Hospitalists can have roles in academics as clinician-researchers, usually following formal research training. Hospitalist researchers focus on numerous areas, including basic science, specific disease states, and hospital outcomes. A focus on hospital outcomes allows clinician-researchers to link “evidence-based medicine with quality improvement by systematically studying hospital care. The outcomes are used to optimize healthcare delivery at the level of both the individual patient and the hospital.”2
Administrative
Hospitalists can pursue leadership opportunities in academic, hospital-based, or community-based settings. Administrative hospitalists develop and guide programs: hospitalist, hospital-based, and multidisciplinary. Hospitalist-leaders serve as program managers, division heads, and medical directors in operational leadership. Aside from running the day-to-day operations of physician groups and hospital units, hospitalists lead in other arenas, such as utilization management, QI and patient safety, medical informatics, and hospital operations.
When serving as physician advisor or utilization management director, it is an opportunity for the hospitalist to lead care coordination within an organization and identify where opportunities related to hospital utilization exist. Many hospitalists lead multidisciplinary hospital committees in QI and patient safety. Hospitalists are perfectly positioned to identify areas within patient care where existing practices need improvement. As quality leaders, hospitalists facilitate the process changes necessary to implement evidence-based care. Some of the hospitalist-led QI areas include care transitions (patients moving from one setting to another; for example, inpatient to outpatient), VTE prophylaxis, inpatient glycemic control, and reduction of hospital-associated conditions.
Directing or guiding medical informatics as health systems across the country implement electronic health records (EHR) is an area where hospitalists can impact both quality and efficiency of care. Medical informaticists can guide clinical-decision support systems within an EHR, easing evidence-based, disease-specific care for other clinicians. As EHR becomes more available, this opportunity for hospitalists will grow.
In addition to these areas, hospitalists can manage or direct a hospital’s patient flow or throughput. Considering that more EDs and hospitals are overcrowded, improving patient flow is an area where hospitalists can join or lead a hospital’s throughput initiative. Evidence has shown that hospitalist-driven active bed management can improve ED crowding and overall hospital flow.3
So now that you know there is more to an HM career than the seven-on, seven-off job that you get between residency and fellowship, determining how and where to find that just-right combination is up to you—with a little help from your local hospitalist mentor. TH
Dr. McAllister is assistant professor in the division of hospital medicine at Cooper University Hospital/UMDNJ-Robert Wood Johnson Medical School in Camden, N.J. Dr. Kupersmith is assistant professor of medicine, UMDNJ-Robert Wood Johnson Medical School, division head, hospital medicine, medical director throughput, Cooper Health System.
References
- Hauer KE, Wachter RM, McCulloch CE, Woo GA, Auerbach AD. Effects of hospitalist attending physicians on trainee satisfaction with teaching and with internal medicine rotations. Arch Intern Med. 2004;164(17):1866-1871.
- Career options. SHM website. Available at: www.hospitalmedicine.org/AM/Template.cfm?Section=Young_Physicians&Template=/CM/HTMLDisplay.cfm&ContentID=22474. Accessed Dec. 28, 2010.
- Howell E, Bessman E, Kravet S, Kolodner K, Marshall R, Wright S. Active bed management by hospitalists and emergency department throughput. Ann Intern Med. 2008;149(11):804-811.
Seven on, seven off. That’s what you can expect as a hospitalist, right? Maybe. As you consider an HM career, your first thought should be just that—is this a career or a job? Is this a year between residency and fellowship, clinical shifts that allow you to have 26 weeks off every year, or is it something else?
Either way, HM offers abundant opportunities conducive to work-life balance and career satisfaction. HM careers reside in clinical, academic, and administrative settings.
Clinical
As a physician entering the workplace, clinical practice often is the most familiar, but it does not have to be an extension of your residency floor rotation. Your individual schedule and employer will play a role in determining when you provide clinical service. Some employers, whether hospital-based, a private physician practice, or a national hospitalist company, utilize fixed schedules, which might include night shifts. The popular seven-on, seven-off model allows you to provide direct patient care every other week, creating personal or administrative time in between. You can opt to become a nocturnist and limit yourself to clinical service at night. Some hospital-based programs implement individualized schedules to meet the nonclinical demands of academic or administrative hospitalists. These schedules might combine Monday-through-Friday weeks, weekends, and nights to create flexibility within your group.
Clinical service can be carved out to fit both your interests and the setting in which you provide care. If placing central lines is your thing, a career as a proceduralist might be for you. Other hospitalists find themselves in clinical niches in specialty collaboration and specific care settings, including surgical comanagement, intensive care, emergency, clinical decision or observation units, or pre-admission and post-hospitalization clinics.
Hospitalists can improve quality, patient safety, and efficiency when working in specialized areas like a clinical decision or observation unit. In these settings, hospitalists often collaborate with midlevel providers, such as nurse practitioners or physician assistants, to provide observation or outpatient care to patients with medical conditions that require more than an ED visit. For example, many patients who present to the ED with chest pain are ideal patients for these settings to evaluate their symptoms and provide an optimal care transition out of the hospital or to an inpatient unit, if needed.
Perhaps you enjoy patient care, but just not all the time involved: You might find a fulfilling career that blends clinical service with research, teaching, or administrative work.
Academic
As an academic hospitalist, you have various options. Hospitalists provide education and oversight to trainees, both medical students and residents, in academic medical centers and community teaching hospitals. You might join an academic center and receive a faculty appointment, either as clinical instructor or assistant professor for first-time candidates.
Clinician educators generally serve as internal-medicine-ward attendings, teaching inpatient care to house staff and students in a traditional sense. Studies have demonstrated that students and house staff are more satisfied and feel they learn more when their ward attending is a hospitalist.1 Academic hospitalists foster career development by serving as mentors or residency program directors. Academic hospitalists also educate fellow physicians through faculty development series and programs.
Hospitalists can have roles in academics as clinician-researchers, usually following formal research training. Hospitalist researchers focus on numerous areas, including basic science, specific disease states, and hospital outcomes. A focus on hospital outcomes allows clinician-researchers to link “evidence-based medicine with quality improvement by systematically studying hospital care. The outcomes are used to optimize healthcare delivery at the level of both the individual patient and the hospital.”2
Administrative
Hospitalists can pursue leadership opportunities in academic, hospital-based, or community-based settings. Administrative hospitalists develop and guide programs: hospitalist, hospital-based, and multidisciplinary. Hospitalist-leaders serve as program managers, division heads, and medical directors in operational leadership. Aside from running the day-to-day operations of physician groups and hospital units, hospitalists lead in other arenas, such as utilization management, QI and patient safety, medical informatics, and hospital operations.
When serving as physician advisor or utilization management director, it is an opportunity for the hospitalist to lead care coordination within an organization and identify where opportunities related to hospital utilization exist. Many hospitalists lead multidisciplinary hospital committees in QI and patient safety. Hospitalists are perfectly positioned to identify areas within patient care where existing practices need improvement. As quality leaders, hospitalists facilitate the process changes necessary to implement evidence-based care. Some of the hospitalist-led QI areas include care transitions (patients moving from one setting to another; for example, inpatient to outpatient), VTE prophylaxis, inpatient glycemic control, and reduction of hospital-associated conditions.
Directing or guiding medical informatics as health systems across the country implement electronic health records (EHR) is an area where hospitalists can impact both quality and efficiency of care. Medical informaticists can guide clinical-decision support systems within an EHR, easing evidence-based, disease-specific care for other clinicians. As EHR becomes more available, this opportunity for hospitalists will grow.
In addition to these areas, hospitalists can manage or direct a hospital’s patient flow or throughput. Considering that more EDs and hospitals are overcrowded, improving patient flow is an area where hospitalists can join or lead a hospital’s throughput initiative. Evidence has shown that hospitalist-driven active bed management can improve ED crowding and overall hospital flow.3
So now that you know there is more to an HM career than the seven-on, seven-off job that you get between residency and fellowship, determining how and where to find that just-right combination is up to you—with a little help from your local hospitalist mentor. TH
Dr. McAllister is assistant professor in the division of hospital medicine at Cooper University Hospital/UMDNJ-Robert Wood Johnson Medical School in Camden, N.J. Dr. Kupersmith is assistant professor of medicine, UMDNJ-Robert Wood Johnson Medical School, division head, hospital medicine, medical director throughput, Cooper Health System.
References
- Hauer KE, Wachter RM, McCulloch CE, Woo GA, Auerbach AD. Effects of hospitalist attending physicians on trainee satisfaction with teaching and with internal medicine rotations. Arch Intern Med. 2004;164(17):1866-1871.
- Career options. SHM website. Available at: www.hospitalmedicine.org/AM/Template.cfm?Section=Young_Physicians&Template=/CM/HTMLDisplay.cfm&ContentID=22474. Accessed Dec. 28, 2010.
- Howell E, Bessman E, Kravet S, Kolodner K, Marshall R, Wright S. Active bed management by hospitalists and emergency department throughput. Ann Intern Med. 2008;149(11):804-811.
Seven on, seven off. That’s what you can expect as a hospitalist, right? Maybe. As you consider an HM career, your first thought should be just that—is this a career or a job? Is this a year between residency and fellowship, clinical shifts that allow you to have 26 weeks off every year, or is it something else?
Either way, HM offers abundant opportunities conducive to work-life balance and career satisfaction. HM careers reside in clinical, academic, and administrative settings.
Clinical
As a physician entering the workplace, clinical practice often is the most familiar, but it does not have to be an extension of your residency floor rotation. Your individual schedule and employer will play a role in determining when you provide clinical service. Some employers, whether hospital-based, a private physician practice, or a national hospitalist company, utilize fixed schedules, which might include night shifts. The popular seven-on, seven-off model allows you to provide direct patient care every other week, creating personal or administrative time in between. You can opt to become a nocturnist and limit yourself to clinical service at night. Some hospital-based programs implement individualized schedules to meet the nonclinical demands of academic or administrative hospitalists. These schedules might combine Monday-through-Friday weeks, weekends, and nights to create flexibility within your group.
Clinical service can be carved out to fit both your interests and the setting in which you provide care. If placing central lines is your thing, a career as a proceduralist might be for you. Other hospitalists find themselves in clinical niches in specialty collaboration and specific care settings, including surgical comanagement, intensive care, emergency, clinical decision or observation units, or pre-admission and post-hospitalization clinics.
Hospitalists can improve quality, patient safety, and efficiency when working in specialized areas like a clinical decision or observation unit. In these settings, hospitalists often collaborate with midlevel providers, such as nurse practitioners or physician assistants, to provide observation or outpatient care to patients with medical conditions that require more than an ED visit. For example, many patients who present to the ED with chest pain are ideal patients for these settings to evaluate their symptoms and provide an optimal care transition out of the hospital or to an inpatient unit, if needed.
Perhaps you enjoy patient care, but just not all the time involved: You might find a fulfilling career that blends clinical service with research, teaching, or administrative work.
Academic
As an academic hospitalist, you have various options. Hospitalists provide education and oversight to trainees, both medical students and residents, in academic medical centers and community teaching hospitals. You might join an academic center and receive a faculty appointment, either as clinical instructor or assistant professor for first-time candidates.
Clinician educators generally serve as internal-medicine-ward attendings, teaching inpatient care to house staff and students in a traditional sense. Studies have demonstrated that students and house staff are more satisfied and feel they learn more when their ward attending is a hospitalist.1 Academic hospitalists foster career development by serving as mentors or residency program directors. Academic hospitalists also educate fellow physicians through faculty development series and programs.
Hospitalists can have roles in academics as clinician-researchers, usually following formal research training. Hospitalist researchers focus on numerous areas, including basic science, specific disease states, and hospital outcomes. A focus on hospital outcomes allows clinician-researchers to link “evidence-based medicine with quality improvement by systematically studying hospital care. The outcomes are used to optimize healthcare delivery at the level of both the individual patient and the hospital.”2
Administrative
Hospitalists can pursue leadership opportunities in academic, hospital-based, or community-based settings. Administrative hospitalists develop and guide programs: hospitalist, hospital-based, and multidisciplinary. Hospitalist-leaders serve as program managers, division heads, and medical directors in operational leadership. Aside from running the day-to-day operations of physician groups and hospital units, hospitalists lead in other arenas, such as utilization management, QI and patient safety, medical informatics, and hospital operations.
When serving as physician advisor or utilization management director, it is an opportunity for the hospitalist to lead care coordination within an organization and identify where opportunities related to hospital utilization exist. Many hospitalists lead multidisciplinary hospital committees in QI and patient safety. Hospitalists are perfectly positioned to identify areas within patient care where existing practices need improvement. As quality leaders, hospitalists facilitate the process changes necessary to implement evidence-based care. Some of the hospitalist-led QI areas include care transitions (patients moving from one setting to another; for example, inpatient to outpatient), VTE prophylaxis, inpatient glycemic control, and reduction of hospital-associated conditions.
Directing or guiding medical informatics as health systems across the country implement electronic health records (EHR) is an area where hospitalists can impact both quality and efficiency of care. Medical informaticists can guide clinical-decision support systems within an EHR, easing evidence-based, disease-specific care for other clinicians. As EHR becomes more available, this opportunity for hospitalists will grow.
In addition to these areas, hospitalists can manage or direct a hospital’s patient flow or throughput. Considering that more EDs and hospitals are overcrowded, improving patient flow is an area where hospitalists can join or lead a hospital’s throughput initiative. Evidence has shown that hospitalist-driven active bed management can improve ED crowding and overall hospital flow.3
So now that you know there is more to an HM career than the seven-on, seven-off job that you get between residency and fellowship, determining how and where to find that just-right combination is up to you—with a little help from your local hospitalist mentor. TH
Dr. McAllister is assistant professor in the division of hospital medicine at Cooper University Hospital/UMDNJ-Robert Wood Johnson Medical School in Camden, N.J. Dr. Kupersmith is assistant professor of medicine, UMDNJ-Robert Wood Johnson Medical School, division head, hospital medicine, medical director throughput, Cooper Health System.
References
- Hauer KE, Wachter RM, McCulloch CE, Woo GA, Auerbach AD. Effects of hospitalist attending physicians on trainee satisfaction with teaching and with internal medicine rotations. Arch Intern Med. 2004;164(17):1866-1871.
- Career options. SHM website. Available at: www.hospitalmedicine.org/AM/Template.cfm?Section=Young_Physicians&Template=/CM/HTMLDisplay.cfm&ContentID=22474. Accessed Dec. 28, 2010.
- Howell E, Bessman E, Kravet S, Kolodner K, Marshall R, Wright S. Active bed management by hospitalists and emergency department throughput. Ann Intern Med. 2008;149(11):804-811.
CON: Should Hospitals Get Reimbursements Based on Quality Performance?
If the road to hell is paved with good intentions, then hell is full of unintended consequences.
The debate about whether hospitals’ reimbursements should be based on quality performance is not a unique concept. Similar systems have been implemented in other fields (e.g. education), and we in the medical field can learn from their experiences. In education, testing students is the driving force in measuring a “quality outcome.”
A growing number of educators now believe that the focus on testing to measure quality has actually reduced the quality of education; they cite the bureaucratic, inflexible, and cumbersome requirements placed on the educators, and the diversion of precious resources to focus on standardized test scores. The actual education of the students becomes secondary, and there are allegations of school systems manipulating their data to ensure maximum funding.
With the drive to pay-for-performance in the medical field, will the actual medical care of the patient become secondary to hitting the “quality” metrics set by the government? Add in a volatile mix of money, and this becomes a recipe for disaster.
Questions are many:
- What standards of quality are we going to use?
- Do these metrics truly translate into “quality”?
- Will the goal of reaching these metrics become the main focus of the hospitals instead of actual patient care?
- Is the goal to really improve the quality of healthcare, or is it just another vehicle for government and private third parties to come up with another excuse to reduce reimbursement in the name of quality?
Even now, ED physicians are giving antibiotics liberally for fear that they will be admonished for “missing” the pneumonia core measures. Whether this is appropriate care for the patient is irrelevant to hitting the statistical goal. Where is the incentive to deliver appropriate care? Are we even asking the right questions? Do bureaucrats know that even if appropriate, timely, quality care is given that a positive outcome is not guaranteed?
The field of medicine has made incredible advances in patient care, but the fact remains that a certain percentage of the sick and elderly become sicker and eventually die, especially in hospitals.
Potential Problems are Many
Unintended consequences pose a real danger to a system that rewards and penalizes. One potential issue is the “rich getting richer and the poor getting poorer,” as more provider focus is placed on buffering statistics by keeping healthy people healthier to achieve better outcomes, meanwhile shunning or diverting the seriously sick patients in order to keep quality metrics within goal.
How will the government and insurance providers guarantee the accuracy of each hospital’s statistics?
Think of the money and resources that will be diverted away from the clinical arena and into the bureaucratic nightmare of record-keeping needed to implement this pay-for-performance system. How many billions of dollars will be needed to fund this new bureaucracy? Do we need another bureaucratic, punitive layer in our already cumbersome medical system?
My answer is clear: No! TH
Dr. Yu is medical director of adult hospitalist services at Presbyterian Medical Group in Albuquerque, N.M., and a Team Hospitalist member.
If the road to hell is paved with good intentions, then hell is full of unintended consequences.
The debate about whether hospitals’ reimbursements should be based on quality performance is not a unique concept. Similar systems have been implemented in other fields (e.g. education), and we in the medical field can learn from their experiences. In education, testing students is the driving force in measuring a “quality outcome.”
A growing number of educators now believe that the focus on testing to measure quality has actually reduced the quality of education; they cite the bureaucratic, inflexible, and cumbersome requirements placed on the educators, and the diversion of precious resources to focus on standardized test scores. The actual education of the students becomes secondary, and there are allegations of school systems manipulating their data to ensure maximum funding.
With the drive to pay-for-performance in the medical field, will the actual medical care of the patient become secondary to hitting the “quality” metrics set by the government? Add in a volatile mix of money, and this becomes a recipe for disaster.
Questions are many:
- What standards of quality are we going to use?
- Do these metrics truly translate into “quality”?
- Will the goal of reaching these metrics become the main focus of the hospitals instead of actual patient care?
- Is the goal to really improve the quality of healthcare, or is it just another vehicle for government and private third parties to come up with another excuse to reduce reimbursement in the name of quality?
Even now, ED physicians are giving antibiotics liberally for fear that they will be admonished for “missing” the pneumonia core measures. Whether this is appropriate care for the patient is irrelevant to hitting the statistical goal. Where is the incentive to deliver appropriate care? Are we even asking the right questions? Do bureaucrats know that even if appropriate, timely, quality care is given that a positive outcome is not guaranteed?
The field of medicine has made incredible advances in patient care, but the fact remains that a certain percentage of the sick and elderly become sicker and eventually die, especially in hospitals.
Potential Problems are Many
Unintended consequences pose a real danger to a system that rewards and penalizes. One potential issue is the “rich getting richer and the poor getting poorer,” as more provider focus is placed on buffering statistics by keeping healthy people healthier to achieve better outcomes, meanwhile shunning or diverting the seriously sick patients in order to keep quality metrics within goal.
How will the government and insurance providers guarantee the accuracy of each hospital’s statistics?
Think of the money and resources that will be diverted away from the clinical arena and into the bureaucratic nightmare of record-keeping needed to implement this pay-for-performance system. How many billions of dollars will be needed to fund this new bureaucracy? Do we need another bureaucratic, punitive layer in our already cumbersome medical system?
My answer is clear: No! TH
Dr. Yu is medical director of adult hospitalist services at Presbyterian Medical Group in Albuquerque, N.M., and a Team Hospitalist member.
If the road to hell is paved with good intentions, then hell is full of unintended consequences.
The debate about whether hospitals’ reimbursements should be based on quality performance is not a unique concept. Similar systems have been implemented in other fields (e.g. education), and we in the medical field can learn from their experiences. In education, testing students is the driving force in measuring a “quality outcome.”
A growing number of educators now believe that the focus on testing to measure quality has actually reduced the quality of education; they cite the bureaucratic, inflexible, and cumbersome requirements placed on the educators, and the diversion of precious resources to focus on standardized test scores. The actual education of the students becomes secondary, and there are allegations of school systems manipulating their data to ensure maximum funding.
With the drive to pay-for-performance in the medical field, will the actual medical care of the patient become secondary to hitting the “quality” metrics set by the government? Add in a volatile mix of money, and this becomes a recipe for disaster.
Questions are many:
- What standards of quality are we going to use?
- Do these metrics truly translate into “quality”?
- Will the goal of reaching these metrics become the main focus of the hospitals instead of actual patient care?
- Is the goal to really improve the quality of healthcare, or is it just another vehicle for government and private third parties to come up with another excuse to reduce reimbursement in the name of quality?
Even now, ED physicians are giving antibiotics liberally for fear that they will be admonished for “missing” the pneumonia core measures. Whether this is appropriate care for the patient is irrelevant to hitting the statistical goal. Where is the incentive to deliver appropriate care? Are we even asking the right questions? Do bureaucrats know that even if appropriate, timely, quality care is given that a positive outcome is not guaranteed?
The field of medicine has made incredible advances in patient care, but the fact remains that a certain percentage of the sick and elderly become sicker and eventually die, especially in hospitals.
Potential Problems are Many
Unintended consequences pose a real danger to a system that rewards and penalizes. One potential issue is the “rich getting richer and the poor getting poorer,” as more provider focus is placed on buffering statistics by keeping healthy people healthier to achieve better outcomes, meanwhile shunning or diverting the seriously sick patients in order to keep quality metrics within goal.
How will the government and insurance providers guarantee the accuracy of each hospital’s statistics?
Think of the money and resources that will be diverted away from the clinical arena and into the bureaucratic nightmare of record-keeping needed to implement this pay-for-performance system. How many billions of dollars will be needed to fund this new bureaucracy? Do we need another bureaucratic, punitive layer in our already cumbersome medical system?
My answer is clear: No! TH
Dr. Yu is medical director of adult hospitalist services at Presbyterian Medical Group in Albuquerque, N.M., and a Team Hospitalist member.
PRO: Should Hospitals Get Reimbursements Based on Quality Performance?
Ask a hospitalist “Is quality important?” and most will answer “Yes.” Now ask the hospital’s CEO/CFO that same question, and you’ll get a resounding “Yes.”
Quality, as the primary determinant of value, has become priority No. 1 for hospitals.1 And with the Centers for Medicare & Medicaid Services’ (CMS) proposed rules for value-based purchasing (VBP), starting with a 1% withholding of Medicare reimbursement for demonstration of quality-measure performance, big dollars are at risk for hospitals.2
As the key providers of inpatient care, hospitalists will share in this financial accountability. The next-generation HM program must show value not only through efficiency and cost reduction, but also expanded services and quality.
Quality is a means of defining good care. Historically, the medical profession has escaped external accountability for quality as part of practitioner autonomy. Today, more than ever, consumer groups, payors, and regulatory bodies are demanding demonstration of quality outcomes, which impacts reimbursement and market share.
Is this demand for quality performance negative? Misused, it can be a mechanism for cost control through seemingly arbitrary indicators. Considered more broadly, it can be positive: We will be able to evaluate our practices to improve care.
Either way, the quality ship has sailed. Accepting this change, we see that the direction and execution are largely left open-ended, which brings another positive: HM has an opportunity to charter the course.
Hospitalists are inpatient care experts; we understand and improve health systems to provide excellent care. Above all else, quality is what we stand for. As a field, we are at the leading edge of change. Getting ahead of quality at each of our institutions is a great opportunity, and helping hospitals implement and deliver on quality initiatives is job security. Being held to what we value, hospitalists should be incentivized by quality performance.
Quality and Compensation
Why tie compensation to quality outcomes? First, hospitals are financially accountable for performance, and HM is financially accountable to hospitals. Second, we incent important objectives, in addition to other mechanisms (e.g. transparent reporting), to drive performance.
The majority of HM programs have an incentive component to their compensation structure, and quality is the leading performance incentive (hospitalists in these programs also have higher incomes).3 We can expect to see HM compensation structures evolve toward pay-for-performance or gainsharing models. HM groups should turn their focus to using incentives or bonuses. Here are some tips:
- Lead quality initiatives. Participate in hospital-based patient safety and satisfaction projects. Communicate the importance to your group to achieve buy-in.
- Define mutual goals. Choose two or three measurable areas that are the top priority items for the hospital and your group, and put them on your scorecard. Consider measuring team performance.
- Make it count. Make the amount of financial incentive a portion of compensation that is meaningful. Share data—and the effect on compensation—regularly to drive performance.
Quality is ours to lead. Define and deliver it, and you’ll find your group to be indispensable to the hospital, with dollars to gain—for all the right reasons. TH
Dr. Wright is senior medical officer at Hospitalists Management Company in Wisconsin and a Team Hospitalist member.
References
- Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287:487-494.
- Centers for Medicare & Medicaid Services. Medicare program: hospital patient value-based purchasing program. Federal Register. 2011;76(9).
- State of Hospital Medicine: 2010 Report Based on 2009 Data. SHM website. Available at: www.hospitalmedicine.org/survey. Accessed April 2, 2011.
Ask a hospitalist “Is quality important?” and most will answer “Yes.” Now ask the hospital’s CEO/CFO that same question, and you’ll get a resounding “Yes.”
Quality, as the primary determinant of value, has become priority No. 1 for hospitals.1 And with the Centers for Medicare & Medicaid Services’ (CMS) proposed rules for value-based purchasing (VBP), starting with a 1% withholding of Medicare reimbursement for demonstration of quality-measure performance, big dollars are at risk for hospitals.2
As the key providers of inpatient care, hospitalists will share in this financial accountability. The next-generation HM program must show value not only through efficiency and cost reduction, but also expanded services and quality.
Quality is a means of defining good care. Historically, the medical profession has escaped external accountability for quality as part of practitioner autonomy. Today, more than ever, consumer groups, payors, and regulatory bodies are demanding demonstration of quality outcomes, which impacts reimbursement and market share.
Is this demand for quality performance negative? Misused, it can be a mechanism for cost control through seemingly arbitrary indicators. Considered more broadly, it can be positive: We will be able to evaluate our practices to improve care.
Either way, the quality ship has sailed. Accepting this change, we see that the direction and execution are largely left open-ended, which brings another positive: HM has an opportunity to charter the course.
Hospitalists are inpatient care experts; we understand and improve health systems to provide excellent care. Above all else, quality is what we stand for. As a field, we are at the leading edge of change. Getting ahead of quality at each of our institutions is a great opportunity, and helping hospitals implement and deliver on quality initiatives is job security. Being held to what we value, hospitalists should be incentivized by quality performance.
Quality and Compensation
Why tie compensation to quality outcomes? First, hospitals are financially accountable for performance, and HM is financially accountable to hospitals. Second, we incent important objectives, in addition to other mechanisms (e.g. transparent reporting), to drive performance.
The majority of HM programs have an incentive component to their compensation structure, and quality is the leading performance incentive (hospitalists in these programs also have higher incomes).3 We can expect to see HM compensation structures evolve toward pay-for-performance or gainsharing models. HM groups should turn their focus to using incentives or bonuses. Here are some tips:
- Lead quality initiatives. Participate in hospital-based patient safety and satisfaction projects. Communicate the importance to your group to achieve buy-in.
- Define mutual goals. Choose two or three measurable areas that are the top priority items for the hospital and your group, and put them on your scorecard. Consider measuring team performance.
- Make it count. Make the amount of financial incentive a portion of compensation that is meaningful. Share data—and the effect on compensation—regularly to drive performance.
Quality is ours to lead. Define and deliver it, and you’ll find your group to be indispensable to the hospital, with dollars to gain—for all the right reasons. TH
Dr. Wright is senior medical officer at Hospitalists Management Company in Wisconsin and a Team Hospitalist member.
References
- Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287:487-494.
- Centers for Medicare & Medicaid Services. Medicare program: hospital patient value-based purchasing program. Federal Register. 2011;76(9).
- State of Hospital Medicine: 2010 Report Based on 2009 Data. SHM website. Available at: www.hospitalmedicine.org/survey. Accessed April 2, 2011.
Ask a hospitalist “Is quality important?” and most will answer “Yes.” Now ask the hospital’s CEO/CFO that same question, and you’ll get a resounding “Yes.”
Quality, as the primary determinant of value, has become priority No. 1 for hospitals.1 And with the Centers for Medicare & Medicaid Services’ (CMS) proposed rules for value-based purchasing (VBP), starting with a 1% withholding of Medicare reimbursement for demonstration of quality-measure performance, big dollars are at risk for hospitals.2
As the key providers of inpatient care, hospitalists will share in this financial accountability. The next-generation HM program must show value not only through efficiency and cost reduction, but also expanded services and quality.
Quality is a means of defining good care. Historically, the medical profession has escaped external accountability for quality as part of practitioner autonomy. Today, more than ever, consumer groups, payors, and regulatory bodies are demanding demonstration of quality outcomes, which impacts reimbursement and market share.
Is this demand for quality performance negative? Misused, it can be a mechanism for cost control through seemingly arbitrary indicators. Considered more broadly, it can be positive: We will be able to evaluate our practices to improve care.
Either way, the quality ship has sailed. Accepting this change, we see that the direction and execution are largely left open-ended, which brings another positive: HM has an opportunity to charter the course.
Hospitalists are inpatient care experts; we understand and improve health systems to provide excellent care. Above all else, quality is what we stand for. As a field, we are at the leading edge of change. Getting ahead of quality at each of our institutions is a great opportunity, and helping hospitals implement and deliver on quality initiatives is job security. Being held to what we value, hospitalists should be incentivized by quality performance.
Quality and Compensation
Why tie compensation to quality outcomes? First, hospitals are financially accountable for performance, and HM is financially accountable to hospitals. Second, we incent important objectives, in addition to other mechanisms (e.g. transparent reporting), to drive performance.
The majority of HM programs have an incentive component to their compensation structure, and quality is the leading performance incentive (hospitalists in these programs also have higher incomes).3 We can expect to see HM compensation structures evolve toward pay-for-performance or gainsharing models. HM groups should turn their focus to using incentives or bonuses. Here are some tips:
- Lead quality initiatives. Participate in hospital-based patient safety and satisfaction projects. Communicate the importance to your group to achieve buy-in.
- Define mutual goals. Choose two or three measurable areas that are the top priority items for the hospital and your group, and put them on your scorecard. Consider measuring team performance.
- Make it count. Make the amount of financial incentive a portion of compensation that is meaningful. Share data—and the effect on compensation—regularly to drive performance.
Quality is ours to lead. Define and deliver it, and you’ll find your group to be indispensable to the hospital, with dollars to gain—for all the right reasons. TH
Dr. Wright is senior medical officer at Hospitalists Management Company in Wisconsin and a Team Hospitalist member.
References
- Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287:487-494.
- Centers for Medicare & Medicaid Services. Medicare program: hospital patient value-based purchasing program. Federal Register. 2011;76(9).
- State of Hospital Medicine: 2010 Report Based on 2009 Data. SHM website. Available at: www.hospitalmedicine.org/survey. Accessed April 2, 2011.