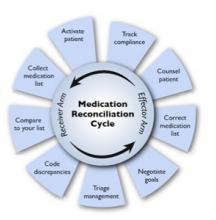
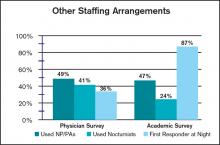
User login
Pomalidomide and loDex achieve impressive results in MM

Convention Center, site of
the 53rd ASH Annual Meeting
Photo courtesy of ASH
SAN DIEGO—Impressive results have been achieved with pomalidomide and low-dose dexamethasone (loDex) in relapsed and refractory patients with multiple myeloma (MM), according to study investigators.
The combination achieved a 34% response rate, including a complete response of 1% and very good partial response of 10% in this population of MM patients refractory to lenalidomide, bortezomib, or both.
Paul Richardson, MD, of Dana-Farber Cancer Institute, reported the results of the phase 1/2 study, known as MM-002, at the 53rd ASH Annual Meeting (abstract 634).
The phase 1 portion of the trial had been reported previously. It established a maximum-tolerated dose of 4 mg daily of pomalidomide.
Dr Richardson called pomalidomide “arguably the most potent of all the IMiDs.” It has significant antimyeloma activity in vitro, promising activity as a single agent in patients with relapsed/refractory MM and efficacy when combined with loDex in patients previously treated with lenalidomide and/or bortezomib.
Dr Richardson pointed out that the investigators defined relapsed and refractory disease rigorously.
“Patients had to have progressed during or 60 days from last treatment, and this had to be confirmed with source documentation prior to study entry,” he said.
In addition, patients had to have measurable levels of M-paraprotein in serum or urine. They must have had 2 or more prior therapies that included 2 or more cycles of lenalidomide and 2 or more cycles of bortezomib, either separately or in combination.
Two hundred twenty-one patients were randomized, 108 to pomalidomide alone and 113 to pomalidomide plus loDex. The pomalidomide regimen was 4 mg daily for 3 weeks with 1 week off. The loDex consisted of 40 mg per week.
Patients were a median age of 63 years. Seventy-eight percent were refractory to lenalidomide, 71% to bortezomib, and 60% to both agents. They had a median of 5 prior therapies, range 2-13.
One hundred ninety-one patients were evaluable for response. They had a median number of 5 cycles of therapy (range, 1-17) and a median duration of 5 months of treatment.
The combination arm had a 34% overall response rate, including partial response or better, compared to 13% in the pomalidomide-alone arm.
Forty-five percent in the combination arm achieved a minor response, compared to 29% in the pomalidomide-alone arm.
And 81% of the patients overall achieved stable disease or better. Responses were rapid and appeared durable, Dr Richardson said.
“What was particularly reassuring and encouraging as well,” he said, “is to see a very similar rate of response for those patients who were refractory to lenalidomide and bortezomib together.” The overall response rate in those patients was 30%.
The median progression-free survival for patients on the combination was 4.7 months. For those on pomalidomide alone, it was 2.7 months.
And the median overall survival on the combination arm was 16.9 months. For those on pomalidomide alone, it was 14 months.
Patients who were refractory to both lenalidomide and bortezomib achieved progression-free survival approaching 4 months with the drug combination and 2 months for pomalidomide alone.
Neutropenia was the dominant grade 3-4 hematologic side effect, followed by thrombocytopenia and anemia. Dose reduction was required in a minority of patients.
The nonhematologic side effects were quite uncommon and included pneumonia and fatigue. No grade 3-4 peripheral neuropathy was reported.
The rate of thromboembolism was less than 5% in either arm. Anticoagulation with low-dose aspirin was required, however.
The investigators concluded that pomalidomide and loDex are synergistic in terms of response rate, and they were impressed that the consistent and durable responses occurred regardless of prior therapy or refractoriness.
On this basis, pomalidomide plus loDex is being investigated in phase 3 trials and as part of combination treatments, including bortezomib. ![]()

Convention Center, site of
the 53rd ASH Annual Meeting
Photo courtesy of ASH
SAN DIEGO—Impressive results have been achieved with pomalidomide and low-dose dexamethasone (loDex) in relapsed and refractory patients with multiple myeloma (MM), according to study investigators.
The combination achieved a 34% response rate, including a complete response of 1% and very good partial response of 10% in this population of MM patients refractory to lenalidomide, bortezomib, or both.
Paul Richardson, MD, of Dana-Farber Cancer Institute, reported the results of the phase 1/2 study, known as MM-002, at the 53rd ASH Annual Meeting (abstract 634).
The phase 1 portion of the trial had been reported previously. It established a maximum-tolerated dose of 4 mg daily of pomalidomide.
Dr Richardson called pomalidomide “arguably the most potent of all the IMiDs.” It has significant antimyeloma activity in vitro, promising activity as a single agent in patients with relapsed/refractory MM and efficacy when combined with loDex in patients previously treated with lenalidomide and/or bortezomib.
Dr Richardson pointed out that the investigators defined relapsed and refractory disease rigorously.
“Patients had to have progressed during or 60 days from last treatment, and this had to be confirmed with source documentation prior to study entry,” he said.
In addition, patients had to have measurable levels of M-paraprotein in serum or urine. They must have had 2 or more prior therapies that included 2 or more cycles of lenalidomide and 2 or more cycles of bortezomib, either separately or in combination.
Two hundred twenty-one patients were randomized, 108 to pomalidomide alone and 113 to pomalidomide plus loDex. The pomalidomide regimen was 4 mg daily for 3 weeks with 1 week off. The loDex consisted of 40 mg per week.
Patients were a median age of 63 years. Seventy-eight percent were refractory to lenalidomide, 71% to bortezomib, and 60% to both agents. They had a median of 5 prior therapies, range 2-13.
One hundred ninety-one patients were evaluable for response. They had a median number of 5 cycles of therapy (range, 1-17) and a median duration of 5 months of treatment.
The combination arm had a 34% overall response rate, including partial response or better, compared to 13% in the pomalidomide-alone arm.
Forty-five percent in the combination arm achieved a minor response, compared to 29% in the pomalidomide-alone arm.
And 81% of the patients overall achieved stable disease or better. Responses were rapid and appeared durable, Dr Richardson said.
“What was particularly reassuring and encouraging as well,” he said, “is to see a very similar rate of response for those patients who were refractory to lenalidomide and bortezomib together.” The overall response rate in those patients was 30%.
The median progression-free survival for patients on the combination was 4.7 months. For those on pomalidomide alone, it was 2.7 months.
And the median overall survival on the combination arm was 16.9 months. For those on pomalidomide alone, it was 14 months.
Patients who were refractory to both lenalidomide and bortezomib achieved progression-free survival approaching 4 months with the drug combination and 2 months for pomalidomide alone.
Neutropenia was the dominant grade 3-4 hematologic side effect, followed by thrombocytopenia and anemia. Dose reduction was required in a minority of patients.
The nonhematologic side effects were quite uncommon and included pneumonia and fatigue. No grade 3-4 peripheral neuropathy was reported.
The rate of thromboembolism was less than 5% in either arm. Anticoagulation with low-dose aspirin was required, however.
The investigators concluded that pomalidomide and loDex are synergistic in terms of response rate, and they were impressed that the consistent and durable responses occurred regardless of prior therapy or refractoriness.
On this basis, pomalidomide plus loDex is being investigated in phase 3 trials and as part of combination treatments, including bortezomib. ![]()

Convention Center, site of
the 53rd ASH Annual Meeting
Photo courtesy of ASH
SAN DIEGO—Impressive results have been achieved with pomalidomide and low-dose dexamethasone (loDex) in relapsed and refractory patients with multiple myeloma (MM), according to study investigators.
The combination achieved a 34% response rate, including a complete response of 1% and very good partial response of 10% in this population of MM patients refractory to lenalidomide, bortezomib, or both.
Paul Richardson, MD, of Dana-Farber Cancer Institute, reported the results of the phase 1/2 study, known as MM-002, at the 53rd ASH Annual Meeting (abstract 634).
The phase 1 portion of the trial had been reported previously. It established a maximum-tolerated dose of 4 mg daily of pomalidomide.
Dr Richardson called pomalidomide “arguably the most potent of all the IMiDs.” It has significant antimyeloma activity in vitro, promising activity as a single agent in patients with relapsed/refractory MM and efficacy when combined with loDex in patients previously treated with lenalidomide and/or bortezomib.
Dr Richardson pointed out that the investigators defined relapsed and refractory disease rigorously.
“Patients had to have progressed during or 60 days from last treatment, and this had to be confirmed with source documentation prior to study entry,” he said.
In addition, patients had to have measurable levels of M-paraprotein in serum or urine. They must have had 2 or more prior therapies that included 2 or more cycles of lenalidomide and 2 or more cycles of bortezomib, either separately or in combination.
Two hundred twenty-one patients were randomized, 108 to pomalidomide alone and 113 to pomalidomide plus loDex. The pomalidomide regimen was 4 mg daily for 3 weeks with 1 week off. The loDex consisted of 40 mg per week.
Patients were a median age of 63 years. Seventy-eight percent were refractory to lenalidomide, 71% to bortezomib, and 60% to both agents. They had a median of 5 prior therapies, range 2-13.
One hundred ninety-one patients were evaluable for response. They had a median number of 5 cycles of therapy (range, 1-17) and a median duration of 5 months of treatment.
The combination arm had a 34% overall response rate, including partial response or better, compared to 13% in the pomalidomide-alone arm.
Forty-five percent in the combination arm achieved a minor response, compared to 29% in the pomalidomide-alone arm.
And 81% of the patients overall achieved stable disease or better. Responses were rapid and appeared durable, Dr Richardson said.
“What was particularly reassuring and encouraging as well,” he said, “is to see a very similar rate of response for those patients who were refractory to lenalidomide and bortezomib together.” The overall response rate in those patients was 30%.
The median progression-free survival for patients on the combination was 4.7 months. For those on pomalidomide alone, it was 2.7 months.
And the median overall survival on the combination arm was 16.9 months. For those on pomalidomide alone, it was 14 months.
Patients who were refractory to both lenalidomide and bortezomib achieved progression-free survival approaching 4 months with the drug combination and 2 months for pomalidomide alone.
Neutropenia was the dominant grade 3-4 hematologic side effect, followed by thrombocytopenia and anemia. Dose reduction was required in a minority of patients.
The nonhematologic side effects were quite uncommon and included pneumonia and fatigue. No grade 3-4 peripheral neuropathy was reported.
The rate of thromboembolism was less than 5% in either arm. Anticoagulation with low-dose aspirin was required, however.
The investigators concluded that pomalidomide and loDex are synergistic in terms of response rate, and they were impressed that the consistent and durable responses occurred regardless of prior therapy or refractoriness.
On this basis, pomalidomide plus loDex is being investigated in phase 3 trials and as part of combination treatments, including bortezomib. ![]()
The Age of Statins
It has been 17 years since the first publication of the Scandinavian Simvastatin Survival Study (Lancet 1994;344;1383-9) describing the benefit of coenzyme A reductase inhibitors (statins) on cardiovascular events in patients with coronary heart disease.
During this period, statins have become a major part of our strategy for the treatment and prevention of acute and chronic coronary heart disease. A number of statins have been introduced in a variety of clinical settings, and all have shown a consistent benefit. Although associated with some side effects, they have been relatively well tolerated by patients requiring either dose adjustment or switching to other drugs within the class.
Along the way, questions have been raised about the long-term safety of statins, and their potential oncogenic potential. The impact of statins directly on the cardiovascular intima has been elucidated using angiographic and intravascular imaging techniques. These studies have shown that statins mitigate to some degree the progression of localized intravascular abnormalities. Two recent studies provide important information both about the safety and long-term benefit of statins, and their remarkable effect on remodeling of the coronary arteries and regression of vascular lesions.
A recent intracoronary ultrasound study that compared high-dose rosuvastatin with atorvastatin in 1,039 patients with coronary disease provides striking evidence for the benefit of both of these drugs on regression of coronary plaque. In patients with residual lesions of 20%-50% by angiography, treatment with these drugs demonstrated a decrease in percent of atherosclerotic volume by 1.22% with rosuvastatin and by 0.99% with atorvastatin, and a decrease in total atheroma volume of 6.39 mm3 and 4.42 mm3, respectively. With doses of 40 mg atorvastatin and 80 mg rosuvastatin, low-density lipoprotein (LDL) cholesterol was decreased to 62.6 mg/dL and 70.2 mg/dL, respectively, and HDL cholesterol increased to 50.4 mg/dL and 48.6 mg/dL in the rosuvastatin and atorvastatin patients, respectively (N. Engl. J. Med. 2011;365;2078-87).
Regression of coronary lesions was observed in approximately two-thirds of the patients. Because of the short duration of the study, there were very few ischemic events and there was no difference between the two drug strategies. Since remodeling of the coronary artery is a surrogate indicator for clinical disease prevention, we cannot be certain that these ultrasound changes in plaques are directly related to the prevention of coronary events.
The second study of interest is the extended follow-up of the Heart Protection Study, which included more than 20,536 patients treated with 40 mg simvastatin or placebo for 5.3 years in-trial and a post-trial follow-up for a total 11 years (Lancet 2011:378;2013-20). There was an overall 23% decrease in major vascular events in the simvastatin-treated patients in the first year of the trial that continued to decrease annually during the trial phase. After study conclusion, when all patients were placed on therapy, there was a legacy benefit that persisted during the remaining 6 years of the study for the patients who were initially in the simvastatin arm of the study. The study confirms the long-term benefit of simvastatin and its safety without any evidence for an increase in cancer or other adverse events during the 11 years of follow-up.
The demonstration that statins can remodel the coronary artery provides pathologic support for their clinical benefit. Questions still remain in regard to mechanisms of action. The changes in the plaque volume theoretically could explain the therapeutic benefit of statins, but they must still be considered as surrogates for clinical benefit. Newer intravascular imaging technology using near-infrared spectroscopy is now able to characterize and measure the lipid core of the coronary plaque (JACC Cardiovasc. Imaging 2008;1:638-48), and may provide a better understanding of the mechanism by which statins modify plaque architecture.
Challenges still remain in regard to how the one-third of patients who did not experience any plaque regression should be approached. A treatment strategy for these presumably high-risk patients remains elusive.
Much has been learned from statin therapy, but many questions still remain in regard to the pathologic process by which the plaque progresses to cause an acute event, and how to prevent it.
This column, "Heart of the Matter," appears regularly in Cardiology News, a publication of Elsevier. Dr. Goldstein, medical editor of Cardiology News, is a professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
It has been 17 years since the first publication of the Scandinavian Simvastatin Survival Study (Lancet 1994;344;1383-9) describing the benefit of coenzyme A reductase inhibitors (statins) on cardiovascular events in patients with coronary heart disease.
During this period, statins have become a major part of our strategy for the treatment and prevention of acute and chronic coronary heart disease. A number of statins have been introduced in a variety of clinical settings, and all have shown a consistent benefit. Although associated with some side effects, they have been relatively well tolerated by patients requiring either dose adjustment or switching to other drugs within the class.
Along the way, questions have been raised about the long-term safety of statins, and their potential oncogenic potential. The impact of statins directly on the cardiovascular intima has been elucidated using angiographic and intravascular imaging techniques. These studies have shown that statins mitigate to some degree the progression of localized intravascular abnormalities. Two recent studies provide important information both about the safety and long-term benefit of statins, and their remarkable effect on remodeling of the coronary arteries and regression of vascular lesions.
A recent intracoronary ultrasound study that compared high-dose rosuvastatin with atorvastatin in 1,039 patients with coronary disease provides striking evidence for the benefit of both of these drugs on regression of coronary plaque. In patients with residual lesions of 20%-50% by angiography, treatment with these drugs demonstrated a decrease in percent of atherosclerotic volume by 1.22% with rosuvastatin and by 0.99% with atorvastatin, and a decrease in total atheroma volume of 6.39 mm3 and 4.42 mm3, respectively. With doses of 40 mg atorvastatin and 80 mg rosuvastatin, low-density lipoprotein (LDL) cholesterol was decreased to 62.6 mg/dL and 70.2 mg/dL, respectively, and HDL cholesterol increased to 50.4 mg/dL and 48.6 mg/dL in the rosuvastatin and atorvastatin patients, respectively (N. Engl. J. Med. 2011;365;2078-87).
Regression of coronary lesions was observed in approximately two-thirds of the patients. Because of the short duration of the study, there were very few ischemic events and there was no difference between the two drug strategies. Since remodeling of the coronary artery is a surrogate indicator for clinical disease prevention, we cannot be certain that these ultrasound changes in plaques are directly related to the prevention of coronary events.
The second study of interest is the extended follow-up of the Heart Protection Study, which included more than 20,536 patients treated with 40 mg simvastatin or placebo for 5.3 years in-trial and a post-trial follow-up for a total 11 years (Lancet 2011:378;2013-20). There was an overall 23% decrease in major vascular events in the simvastatin-treated patients in the first year of the trial that continued to decrease annually during the trial phase. After study conclusion, when all patients were placed on therapy, there was a legacy benefit that persisted during the remaining 6 years of the study for the patients who were initially in the simvastatin arm of the study. The study confirms the long-term benefit of simvastatin and its safety without any evidence for an increase in cancer or other adverse events during the 11 years of follow-up.
The demonstration that statins can remodel the coronary artery provides pathologic support for their clinical benefit. Questions still remain in regard to mechanisms of action. The changes in the plaque volume theoretically could explain the therapeutic benefit of statins, but they must still be considered as surrogates for clinical benefit. Newer intravascular imaging technology using near-infrared spectroscopy is now able to characterize and measure the lipid core of the coronary plaque (JACC Cardiovasc. Imaging 2008;1:638-48), and may provide a better understanding of the mechanism by which statins modify plaque architecture.
Challenges still remain in regard to how the one-third of patients who did not experience any plaque regression should be approached. A treatment strategy for these presumably high-risk patients remains elusive.
Much has been learned from statin therapy, but many questions still remain in regard to the pathologic process by which the plaque progresses to cause an acute event, and how to prevent it.
This column, "Heart of the Matter," appears regularly in Cardiology News, a publication of Elsevier. Dr. Goldstein, medical editor of Cardiology News, is a professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
It has been 17 years since the first publication of the Scandinavian Simvastatin Survival Study (Lancet 1994;344;1383-9) describing the benefit of coenzyme A reductase inhibitors (statins) on cardiovascular events in patients with coronary heart disease.
During this period, statins have become a major part of our strategy for the treatment and prevention of acute and chronic coronary heart disease. A number of statins have been introduced in a variety of clinical settings, and all have shown a consistent benefit. Although associated with some side effects, they have been relatively well tolerated by patients requiring either dose adjustment or switching to other drugs within the class.
Along the way, questions have been raised about the long-term safety of statins, and their potential oncogenic potential. The impact of statins directly on the cardiovascular intima has been elucidated using angiographic and intravascular imaging techniques. These studies have shown that statins mitigate to some degree the progression of localized intravascular abnormalities. Two recent studies provide important information both about the safety and long-term benefit of statins, and their remarkable effect on remodeling of the coronary arteries and regression of vascular lesions.
A recent intracoronary ultrasound study that compared high-dose rosuvastatin with atorvastatin in 1,039 patients with coronary disease provides striking evidence for the benefit of both of these drugs on regression of coronary plaque. In patients with residual lesions of 20%-50% by angiography, treatment with these drugs demonstrated a decrease in percent of atherosclerotic volume by 1.22% with rosuvastatin and by 0.99% with atorvastatin, and a decrease in total atheroma volume of 6.39 mm3 and 4.42 mm3, respectively. With doses of 40 mg atorvastatin and 80 mg rosuvastatin, low-density lipoprotein (LDL) cholesterol was decreased to 62.6 mg/dL and 70.2 mg/dL, respectively, and HDL cholesterol increased to 50.4 mg/dL and 48.6 mg/dL in the rosuvastatin and atorvastatin patients, respectively (N. Engl. J. Med. 2011;365;2078-87).
Regression of coronary lesions was observed in approximately two-thirds of the patients. Because of the short duration of the study, there were very few ischemic events and there was no difference between the two drug strategies. Since remodeling of the coronary artery is a surrogate indicator for clinical disease prevention, we cannot be certain that these ultrasound changes in plaques are directly related to the prevention of coronary events.
The second study of interest is the extended follow-up of the Heart Protection Study, which included more than 20,536 patients treated with 40 mg simvastatin or placebo for 5.3 years in-trial and a post-trial follow-up for a total 11 years (Lancet 2011:378;2013-20). There was an overall 23% decrease in major vascular events in the simvastatin-treated patients in the first year of the trial that continued to decrease annually during the trial phase. After study conclusion, when all patients were placed on therapy, there was a legacy benefit that persisted during the remaining 6 years of the study for the patients who were initially in the simvastatin arm of the study. The study confirms the long-term benefit of simvastatin and its safety without any evidence for an increase in cancer or other adverse events during the 11 years of follow-up.
The demonstration that statins can remodel the coronary artery provides pathologic support for their clinical benefit. Questions still remain in regard to mechanisms of action. The changes in the plaque volume theoretically could explain the therapeutic benefit of statins, but they must still be considered as surrogates for clinical benefit. Newer intravascular imaging technology using near-infrared spectroscopy is now able to characterize and measure the lipid core of the coronary plaque (JACC Cardiovasc. Imaging 2008;1:638-48), and may provide a better understanding of the mechanism by which statins modify plaque architecture.
Challenges still remain in regard to how the one-third of patients who did not experience any plaque regression should be approached. A treatment strategy for these presumably high-risk patients remains elusive.
Much has been learned from statin therapy, but many questions still remain in regard to the pathologic process by which the plaque progresses to cause an acute event, and how to prevent it.
This column, "Heart of the Matter," appears regularly in Cardiology News, a publication of Elsevier. Dr. Goldstein, medical editor of Cardiology News, is a professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Pediatric Hodgkin's Regimens Suit Adolescents and Young Adults
SAN DIEGO – Adolescents and young adults with Hodgkin’s lymphoma can have high response rates and durable remissions under protocols developed for children, with potentially lower long-term toxicities than those commonly seen with adult-oriented regimens, said investigators at the annual meeting of the American Society of Hematology.
The 15- to 20-year-olds with Hodgkin’s lymphoma who were treated under two similar Children’s Oncology Group protocols had a 5-year event-free survival rate of 85.9%, compared with 87.0% for patients younger than 15 years. The 10-year rate was 77.3% in the older patients and 83.8% in the younger group (P = .515), reported Dr. Karen S. Fernandez of the pediatrics department at the University of Illinois, Peoria.
These 5-year event-free survival results are comparable to outcomes of other studies in which adolescents and young adults were treated with adult cooperative-group protocols. Moreover, the cumulative doses of alkylators, anthracyclines, and etoposide used are below thresholds usually associated with significant long-term toxicities, Dr. Fernandez noted.
"Based on this data, we favor the use of pediatric-focused therapy with dose-limited regimens for adolescents and young adults with Hodgkin’s lymphoma in whom decreasing long-term effects is important to improve quality of life, particularly for adolescents with advanced stages," she said.
There is no widely accepted standard of treatment for adolescents and young adults with Hodgkin’s lymphoma, in part because different centers variously treat teens with adult or pediatric protocols, and published data specifically regarding the treatment of adolescents and young adults with Hodgkin’s lymphoma are scarce, Dr. Fernandez said.
She and her colleagues in the Children’s Oncology Group tried to find a balance between maximum possible cure rates and reduced long-term effects by conducting a retrospective analysis comparing children younger than 15 years with adolescents and young adults aged 15-20 years, in the Children’s Oncology Group protocols P9425 and P9426.
The P9425 study looked at the ABVE-PC regimen (doxorubicin [Adriamycin], bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide in dose-dense application) in patients with advanced-stage Hodgkin’s lymphoma. P9426 evaluated the ABVE regimen (the same combination, also dose-dense but without prednisone or cyclophosphamide) in patients with low-stage Hodgkin’s lymphoma.
Patients with an early response in P9425 received 21 Gy of radiation after three cycles of ABVE-PC spaced 21 days apart; patients with a slow response received the same radiation dose after five cycles.
In the P9426 protocol, patients with early responses received 25 Gy after two cycles, while all others received the same radiation dose after four cycles of ABVE. Patients in both protocols received dexrazoxane at the investigators’ discretion.
The cumulative chemotherapy doses delivered in the ABVE and ABVE-PC regimens in these trials were significantly lower than those delivered in other trials of patients with standard or high- to intermediate-risk Hodgkin’s disease, including the BEACOPP regimen (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine [Oncovin], prednisone, and procarbazine), COPP-ABVD (the same drugs as BEACOPP plus vinblastine and dacarbazine), and ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine).
When Dr. Fernandez and her colleagues conducted a Cox regression analysis of patients in the combined studies, they found that neither sex, histologic subtypes, nor tumor staging was predictive of outcomes.
She noted that the study was limited by the retrospective design and the small number of adolescents in each group (104 in P9425 and 99 in P9426). Another limitation was the fact that neither study was designed to distinguish between children and adolescents/young adults, she said.
The investigators plan to collaborate with adult oncology groups to standardize treatment of adolescents and young adults, Dr. Fernandez concluded.
In the question and response session following the presentation, Dr. Jonathan Friedberg, chief of hematology/oncology at the University of Rochester (N.Y.), noted that the toxicities associated with therapy are not all drug related.
"Your pediatric group seems to be very focused on decreasing the doses of chemotherapy in an effort to mediate late toxicity, but I think most people would accept that radiation is probably the big driver of late toxicity, and it appears that in these regimens you’re giving radiation to patients with advanced-stage Hodgkin’s," commented Dr. Friedberg, who moderated the session but was not involved in the study.
Dr. Fernandez acknowledged that all patients in the P9425 and P9426 protocols received radiation, but added that the group is currently conducting trials in which radiation will be given based on patient responses determined by PET scans following the first cycle of chemotherapy.
The study was supported by the Children’s Oncology Group. Dr. Fernandez reported that she had no relevant conflicts of interest. Dr. Friedberg reported being a consultant to or receiving honoraria from Genentech, Astellas, Lilly, Trubion, Seattle Genetics, and Cephalon.
Adolescents
and young adults with Hodgkin’s lymphoma were treated on two Children’s
Oncology Group clinical trials that were designed to mitigate the late effects
of successful therapy by reducing the cumulative doses of alkylating agents,
anthracyclines, and etoposide. They experienced 5- and 10-year event-free
outcomes which were not statistically different than those experienced by
younger patients.
In most other cancers
in the pediatric age group, age at diagnosis has nearly always exerted
prognostic significance. Hodgkin’s has a peak incidence in adolescence and
young adulthood, and favorable histologic subtypes of the disease are known to
predominate in younger children. The importance of this report is that
excellent outcomes can be obtained in the older patients with a combination
chemotherapy regimen that uses lower cumulative doses of classes of drugs known
to be associated with significant potential for deleterious late effects. This
is, indeed, good news that overall drug doses can be safely reduced without
jeopardizing disease control; however, any long-term quality of life
differences between younger children and the adolescent and young adult
population as a result of reducing cumulative doses is not yet established.
Although dose
reduction of chemotherapy is one strategy to reduce the long-term and delayed
side effects of therapy for a highly curable disease, the reduction in exposure
to therapeutic irradiation or its elimination entirely remains an equally
important consideration for the optimal management of younger children as well
as adolescents and young adults with Hodgkin’s disease.
Dr. Gregory H. Reaman, an associate editor of The Oncology Report, is professor of
pediatrics at the George Washington University
School of Medicine and Health Sciences
and Children’s National Medical Center
in Washington.
Adolescents
and young adults with Hodgkin’s lymphoma were treated on two Children’s
Oncology Group clinical trials that were designed to mitigate the late effects
of successful therapy by reducing the cumulative doses of alkylating agents,
anthracyclines, and etoposide. They experienced 5- and 10-year event-free
outcomes which were not statistically different than those experienced by
younger patients.
In most other cancers
in the pediatric age group, age at diagnosis has nearly always exerted
prognostic significance. Hodgkin’s has a peak incidence in adolescence and
young adulthood, and favorable histologic subtypes of the disease are known to
predominate in younger children. The importance of this report is that
excellent outcomes can be obtained in the older patients with a combination
chemotherapy regimen that uses lower cumulative doses of classes of drugs known
to be associated with significant potential for deleterious late effects. This
is, indeed, good news that overall drug doses can be safely reduced without
jeopardizing disease control; however, any long-term quality of life
differences between younger children and the adolescent and young adult
population as a result of reducing cumulative doses is not yet established.
Although dose
reduction of chemotherapy is one strategy to reduce the long-term and delayed
side effects of therapy for a highly curable disease, the reduction in exposure
to therapeutic irradiation or its elimination entirely remains an equally
important consideration for the optimal management of younger children as well
as adolescents and young adults with Hodgkin’s disease.
Dr. Gregory H. Reaman, an associate editor of The Oncology Report, is professor of
pediatrics at the George Washington University
School of Medicine and Health Sciences
and Children’s National Medical Center
in Washington.
Adolescents
and young adults with Hodgkin’s lymphoma were treated on two Children’s
Oncology Group clinical trials that were designed to mitigate the late effects
of successful therapy by reducing the cumulative doses of alkylating agents,
anthracyclines, and etoposide. They experienced 5- and 10-year event-free
outcomes which were not statistically different than those experienced by
younger patients.
In most other cancers
in the pediatric age group, age at diagnosis has nearly always exerted
prognostic significance. Hodgkin’s has a peak incidence in adolescence and
young adulthood, and favorable histologic subtypes of the disease are known to
predominate in younger children. The importance of this report is that
excellent outcomes can be obtained in the older patients with a combination
chemotherapy regimen that uses lower cumulative doses of classes of drugs known
to be associated with significant potential for deleterious late effects. This
is, indeed, good news that overall drug doses can be safely reduced without
jeopardizing disease control; however, any long-term quality of life
differences between younger children and the adolescent and young adult
population as a result of reducing cumulative doses is not yet established.
Although dose
reduction of chemotherapy is one strategy to reduce the long-term and delayed
side effects of therapy for a highly curable disease, the reduction in exposure
to therapeutic irradiation or its elimination entirely remains an equally
important consideration for the optimal management of younger children as well
as adolescents and young adults with Hodgkin’s disease.
Dr. Gregory H. Reaman, an associate editor of The Oncology Report, is professor of
pediatrics at the George Washington University
School of Medicine and Health Sciences
and Children’s National Medical Center
in Washington.
SAN DIEGO – Adolescents and young adults with Hodgkin’s lymphoma can have high response rates and durable remissions under protocols developed for children, with potentially lower long-term toxicities than those commonly seen with adult-oriented regimens, said investigators at the annual meeting of the American Society of Hematology.
The 15- to 20-year-olds with Hodgkin’s lymphoma who were treated under two similar Children’s Oncology Group protocols had a 5-year event-free survival rate of 85.9%, compared with 87.0% for patients younger than 15 years. The 10-year rate was 77.3% in the older patients and 83.8% in the younger group (P = .515), reported Dr. Karen S. Fernandez of the pediatrics department at the University of Illinois, Peoria.
These 5-year event-free survival results are comparable to outcomes of other studies in which adolescents and young adults were treated with adult cooperative-group protocols. Moreover, the cumulative doses of alkylators, anthracyclines, and etoposide used are below thresholds usually associated with significant long-term toxicities, Dr. Fernandez noted.
"Based on this data, we favor the use of pediatric-focused therapy with dose-limited regimens for adolescents and young adults with Hodgkin’s lymphoma in whom decreasing long-term effects is important to improve quality of life, particularly for adolescents with advanced stages," she said.
There is no widely accepted standard of treatment for adolescents and young adults with Hodgkin’s lymphoma, in part because different centers variously treat teens with adult or pediatric protocols, and published data specifically regarding the treatment of adolescents and young adults with Hodgkin’s lymphoma are scarce, Dr. Fernandez said.
She and her colleagues in the Children’s Oncology Group tried to find a balance between maximum possible cure rates and reduced long-term effects by conducting a retrospective analysis comparing children younger than 15 years with adolescents and young adults aged 15-20 years, in the Children’s Oncology Group protocols P9425 and P9426.
The P9425 study looked at the ABVE-PC regimen (doxorubicin [Adriamycin], bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide in dose-dense application) in patients with advanced-stage Hodgkin’s lymphoma. P9426 evaluated the ABVE regimen (the same combination, also dose-dense but without prednisone or cyclophosphamide) in patients with low-stage Hodgkin’s lymphoma.
Patients with an early response in P9425 received 21 Gy of radiation after three cycles of ABVE-PC spaced 21 days apart; patients with a slow response received the same radiation dose after five cycles.
In the P9426 protocol, patients with early responses received 25 Gy after two cycles, while all others received the same radiation dose after four cycles of ABVE. Patients in both protocols received dexrazoxane at the investigators’ discretion.
The cumulative chemotherapy doses delivered in the ABVE and ABVE-PC regimens in these trials were significantly lower than those delivered in other trials of patients with standard or high- to intermediate-risk Hodgkin’s disease, including the BEACOPP regimen (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine [Oncovin], prednisone, and procarbazine), COPP-ABVD (the same drugs as BEACOPP plus vinblastine and dacarbazine), and ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine).
When Dr. Fernandez and her colleagues conducted a Cox regression analysis of patients in the combined studies, they found that neither sex, histologic subtypes, nor tumor staging was predictive of outcomes.
She noted that the study was limited by the retrospective design and the small number of adolescents in each group (104 in P9425 and 99 in P9426). Another limitation was the fact that neither study was designed to distinguish between children and adolescents/young adults, she said.
The investigators plan to collaborate with adult oncology groups to standardize treatment of adolescents and young adults, Dr. Fernandez concluded.
In the question and response session following the presentation, Dr. Jonathan Friedberg, chief of hematology/oncology at the University of Rochester (N.Y.), noted that the toxicities associated with therapy are not all drug related.
"Your pediatric group seems to be very focused on decreasing the doses of chemotherapy in an effort to mediate late toxicity, but I think most people would accept that radiation is probably the big driver of late toxicity, and it appears that in these regimens you’re giving radiation to patients with advanced-stage Hodgkin’s," commented Dr. Friedberg, who moderated the session but was not involved in the study.
Dr. Fernandez acknowledged that all patients in the P9425 and P9426 protocols received radiation, but added that the group is currently conducting trials in which radiation will be given based on patient responses determined by PET scans following the first cycle of chemotherapy.
The study was supported by the Children’s Oncology Group. Dr. Fernandez reported that she had no relevant conflicts of interest. Dr. Friedberg reported being a consultant to or receiving honoraria from Genentech, Astellas, Lilly, Trubion, Seattle Genetics, and Cephalon.
SAN DIEGO – Adolescents and young adults with Hodgkin’s lymphoma can have high response rates and durable remissions under protocols developed for children, with potentially lower long-term toxicities than those commonly seen with adult-oriented regimens, said investigators at the annual meeting of the American Society of Hematology.
The 15- to 20-year-olds with Hodgkin’s lymphoma who were treated under two similar Children’s Oncology Group protocols had a 5-year event-free survival rate of 85.9%, compared with 87.0% for patients younger than 15 years. The 10-year rate was 77.3% in the older patients and 83.8% in the younger group (P = .515), reported Dr. Karen S. Fernandez of the pediatrics department at the University of Illinois, Peoria.
These 5-year event-free survival results are comparable to outcomes of other studies in which adolescents and young adults were treated with adult cooperative-group protocols. Moreover, the cumulative doses of alkylators, anthracyclines, and etoposide used are below thresholds usually associated with significant long-term toxicities, Dr. Fernandez noted.
"Based on this data, we favor the use of pediatric-focused therapy with dose-limited regimens for adolescents and young adults with Hodgkin’s lymphoma in whom decreasing long-term effects is important to improve quality of life, particularly for adolescents with advanced stages," she said.
There is no widely accepted standard of treatment for adolescents and young adults with Hodgkin’s lymphoma, in part because different centers variously treat teens with adult or pediatric protocols, and published data specifically regarding the treatment of adolescents and young adults with Hodgkin’s lymphoma are scarce, Dr. Fernandez said.
She and her colleagues in the Children’s Oncology Group tried to find a balance between maximum possible cure rates and reduced long-term effects by conducting a retrospective analysis comparing children younger than 15 years with adolescents and young adults aged 15-20 years, in the Children’s Oncology Group protocols P9425 and P9426.
The P9425 study looked at the ABVE-PC regimen (doxorubicin [Adriamycin], bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide in dose-dense application) in patients with advanced-stage Hodgkin’s lymphoma. P9426 evaluated the ABVE regimen (the same combination, also dose-dense but without prednisone or cyclophosphamide) in patients with low-stage Hodgkin’s lymphoma.
Patients with an early response in P9425 received 21 Gy of radiation after three cycles of ABVE-PC spaced 21 days apart; patients with a slow response received the same radiation dose after five cycles.
In the P9426 protocol, patients with early responses received 25 Gy after two cycles, while all others received the same radiation dose after four cycles of ABVE. Patients in both protocols received dexrazoxane at the investigators’ discretion.
The cumulative chemotherapy doses delivered in the ABVE and ABVE-PC regimens in these trials were significantly lower than those delivered in other trials of patients with standard or high- to intermediate-risk Hodgkin’s disease, including the BEACOPP regimen (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine [Oncovin], prednisone, and procarbazine), COPP-ABVD (the same drugs as BEACOPP plus vinblastine and dacarbazine), and ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine).
When Dr. Fernandez and her colleagues conducted a Cox regression analysis of patients in the combined studies, they found that neither sex, histologic subtypes, nor tumor staging was predictive of outcomes.
She noted that the study was limited by the retrospective design and the small number of adolescents in each group (104 in P9425 and 99 in P9426). Another limitation was the fact that neither study was designed to distinguish between children and adolescents/young adults, she said.
The investigators plan to collaborate with adult oncology groups to standardize treatment of adolescents and young adults, Dr. Fernandez concluded.
In the question and response session following the presentation, Dr. Jonathan Friedberg, chief of hematology/oncology at the University of Rochester (N.Y.), noted that the toxicities associated with therapy are not all drug related.
"Your pediatric group seems to be very focused on decreasing the doses of chemotherapy in an effort to mediate late toxicity, but I think most people would accept that radiation is probably the big driver of late toxicity, and it appears that in these regimens you’re giving radiation to patients with advanced-stage Hodgkin’s," commented Dr. Friedberg, who moderated the session but was not involved in the study.
Dr. Fernandez acknowledged that all patients in the P9425 and P9426 protocols received radiation, but added that the group is currently conducting trials in which radiation will be given based on patient responses determined by PET scans following the first cycle of chemotherapy.
The study was supported by the Children’s Oncology Group. Dr. Fernandez reported that she had no relevant conflicts of interest. Dr. Friedberg reported being a consultant to or receiving honoraria from Genentech, Astellas, Lilly, Trubion, Seattle Genetics, and Cephalon.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF HEMATOLOGY
Major Finding: Adolescents and young adults with Hodgkin’s lymphoma who were treated under two similar Children’s Oncology Group protocols had a 5-year event-free survival (EFS) rate of 85.9%, compared with 87.0% for patients younger than 15 years, and a 10-year EFS rate of 77.3% compared with 83.8% (P = .515).
Data Source: A retrospective analysis of two published trials.
Disclosures: The study was supported by the Children’s Oncology Group. Dr. Fernandez reported that she had no relevant conflicts of interest. Dr. Friedberg reported being a consultant to or receiving honoraria from Genentech, Astellas, Lilly, Trubion, Seattle Genetics, and Cephalon.
A Winnable Battle
Redness, pain, and drainage from the skin around a catheter. Bloody, cloudy, or pungent urine and mental confusion. Severe diarrhea and a dilated colon: The potential symptoms of some of the most common hospital-acquired infections in the United States aren’t particularly pleasant.
The numbers don’t paint a pretty picture, either.
One widely cited study estimates that the nation’s hospitals report 1.7 million healthcare-associated infections (HAIs) every year, along with nearly 100,000 associated deaths.1 All told, the additional healthcare costs could tally $30 billion or more annually, according to a 2009 Centers for Disease Control and Prevention (CDC) analysis. Though some bright spots have emerged, healthcare advocates, including Kevin Kavanagh, MD, MS, FACS, director of Kentucky-based patient advocacy group Health Watch USA, contend that the overall size of the problem is likely underestimated. And unless brought under control, he says, emerging pathogens could make a bad situation worse.
The incredible variety of healthcare settings precludes any across-the-board solutions for hospitalists and other care providers. A recent survey of more than 1,200 healthcare professionals from 33 hospitals, however, revealed some common themes. According to the study authors, the data show that “hand hygiene is consistently identified as the greatest barrier to reducing HAIs, and that sustaining the necessary behavioral change to overcome this barrier is difficult.”2 In fact, the study highlighted a widespread belief that medical staff, and doctors in particular, are not fully engaged or in compliance with HAI reduction efforts. “The data suggest that physicians often do not adhere to protocols and guidelines; moreover, some respondents questioned whether many physicians are open to change,” the authors wrote.
So what can hospitalists do to help their colleagues embrace a culture of positive change and turn back a worsening healthcare epidemic? If simple answers are in short supply, studies aimed at central-line-associated bloodstream infections, catheter-associated urinary tract infections, and Clostridium difficile infections have at least highlighted some keys to a successful intervention.
In Focus: Central Lines
Central-line-associated bloodstream infections (CLABSIs) arguably have received the most attention of any HAI. Not coincidentally, most researchers point to the anti-CLABSI effort as the high point in the struggle to reduce infection rates.
In a landmark 2006 study led by hospitalist Peter Pronovost, MD, more than 100 ICUs in Michigan nearly eliminated CLABSIs over an 18-month period.3 The “remarkably successful” intervention, according to its authors, focused on changing provider behavior through education and collaboration with infection-control specialists. Nationwide, a recent CDC study estimated that absolute CLABSI numbers in ICUs dropped to 18,000 in 2009 from 43,000 in 2001, a 58% decline.4
Sheri Chernetsky Tejedor, MD, SFHM, assistant professor in the division of hospital medicine at Emory University School of Medicine in Atlanta, points out a major caveat in CLABSI prevention efforts, however: To date, she says, most have targeted insertion practices and ICU patients, which do little for the more than half of CLABSIs that occur on hospital wards. These wards thus represent “a ripe opportunity for intervention, especially the low-hanging, ‘low tech’ interventions of removing unnecessary devices,” she says.
Among a small random sample of patients with temporary central venous lines (CVCs), including peripherally inserted central catheters (PICCs), a study led by Dr. Chernetsky Tejedor found that 25% of all CVC days were “idle.”5 In other words, in 1 out of every 4 CVC days, the catheter was retained despite failing to meet standard justification criteria. In particular, the study suggested that PICCs were associated with longer catheter use and more idle days, fueling her group’s suspicion that increased PICC availability has changed CVC use patterns.
Among her group’s interventions, a patient safety checklist has been built, with questions about catheter use entered into a daily electronic progress note used by all services on the inpatient wards. One particularly successful strategy empowered nurses to determine whether continued catheter use seems justified and to ask physicians whether an “idle” CVC could be removed.
With a decline in CLABSI rates in ICUs and a shift in focus to the medical wards, Dr. Chernetsky Tejedor says, hospitalists are in a prime position to help reduce the inappropriate use of catheters and other invasive devices. More broadly, hospitalists may be well-suited to help change the culture of medicine toward a wider acceptance of proven interventions, such as central-line checklists.
Dr. Kavanagh argues that the slow and arduous process to move past what he calls “a huge resistance to checklists” and win universal adoption of such protocols “is not a good chapter in medicine.” He isn’t laying the blame at the feet of doctors alone, however. “In some institutions,” he says, “there needs to be a change from a profit-driven to a patient-centered culture.”
Greg Maynard, MD, MSc, SFHM, director of the University of California at San Diego Center for Innovation and Improvement Science and senior vice president of SHM’s Center for Hospital Innovation and Improvement, acknowledges the integral role of checklists in improvement efforts. He cautions, however, that they should be viewed as only one part of a multipronged approach.
As with hand hygiene, Dr. Maynard concedes that some facilities are still facing resistance from medical staff in integrating checklists into their routines, though he argues that an ingrained anti-checklist medical culture may not be solely at fault. “If you insert a checklist in such a way that it’s very inconvenient, that stops the flow of work,” he says, “you’re not going to have as much success as if you’ve thoughtfully designed it so that the checklist is called into play when it makes sense to bring it into play and made it easy to do, as opposed to difficult and awkward to do.”
In Focus: Catheter-Associated UTIs
Catheter-associated urinary tract infections (CAUTIs) account for roughly 1 in 3 healthcare-associated infections, according to the CDC. And yet many researchers say the “Rodney Dangerfield of HAIs” has long been overlooked.
A frequently cited study led by Sanjay Saint, MD, MPH, FHM, professor of internal medicine at the University of Michigan and the Ann Arbor VA Medical Center, found that nearly 40% of attending physicians were unaware that their patients even had an indwelling urinary catheter.6
Dr. Saint has dubbed the phenomenon “immaculate catheterization” to highlight the glaring discrepancy. “Because we also found, in a significant number of patients, there was no documentation anywhere in the medical record that the catheter existed,” he says. “It’s not in the physician’s notes, it’s not in the nursing notes, but we knew it was there because we could see it coming out of the patient.” Catheters that were inappropriately placed, he found, were more often “forgotten” than appropriate ones.
Perhaps more than anything else, the startling observation underscores the intense need for basic awareness of the two biggest UTI risk factors: whether a catheter is used, and how long it’s left in place. “You can get marked reductions by just taking care of those two factors,” Health Watch USA’s Dr. Kavanagh says.
At the Ann Arbor VA Hospital, one unit’s presence of a “Patient Safety Professional” to help ensure better oversight virtually brought the inappropriate use of indwelling catheters to a standstill. Dr. Saint and his colleagues are now gathering data to determine whether that decrease has translated to a drop in infections.

—Greg Maynard, MD, MSc, SFHM, director, University of California at San Diego Center for Innovation and Improvement Science, senior vice president, SHM Center for Hospital Innovation and Improvement
One fundamental key, he says, is paying close attention to whether a catheter is really in the patient’s best interests. “If we ask that question—‘If this was my family member, what would I want?’—we usually do the right thing,” Dr. Saint explains. Another key is leveraging the hospitalist’s core skill in communicating often and well with nurses to ensure that they are in sync during the “team sport” of CAUTI prevention.
With pockets of success in reducing inappropriate catheterization, the larger question now is how to scale up the individual interventions to achieve nationwide reductions. “How do you take what will work in one hospital, given its culture and microculture, and then apply it to hospitals across the state, or even across the country?” Dr. Saint asks.
Karen Clarke, MD, MS, MPH, a hospitalist and assistant professor of medicine at Emory University Hospital in Atlanta, is in the midst of tackling such issues. Using a bundled approach that included four interventions, she and colleagues reduced CAUTI rates by 70% at 276-bed West Georgia Medical Center in La Grange, Ga.7
The interventions were straightforward and inexpensive, Dr. Clarke says, meaning that they could be widely applied. “The only thing is that there has to be a champion overseeing the interventions to make sure that the steps are followed through on,” she says. Even at cash-strapped facilities, then, a similar approach could prove effective as long as someone assumes responsibility—and hospitalists would be a natural choice.
Based on her study’s promising results, Dr. Clarke hopes to begin implementing the intervention in at least one other hospital starting Jan. 1. If the success can be replicated, she says, the CAUTI-reduction protocol will branch out to include more regional hospitals.
In Focus: C. Diff-Associated Disease
Even as many hospitals are improving their CLABSI and CAUTI rates, hospital-acquired Clostridium difficile infections appear to be getting worse, particularly among older patients. In some facilities, the potentially fatal, diarrhea-causing microbe is now the top pathogen (see “Gut Reaction,” December 2011).
With a timely intervention, however, Kaiser Permanente Medical Center in Santa Clara, Calif., cut its own infection rates by one-third.8 In brainstorming how to improve the medical center’s rates, Susanne Mierendorf, MD, MS, FHM, a hospitalist and associate residency program director for internal medicine, joined colleagues in thinking through the barriers for healthcare providers. “It wasn’t ‘Why don’t they follow the infection-control guidelines?’ It was, ‘Why can’t they?’” Dr. Mierendorf says.
The thought exercise led to some eye-opening observations, including the realization that disposable gowns, gloves, and other personal protective equipment weren’t in the room and were hard to find. To help establish habits, Dr. Mierendorf’s team picked a consistent drawer in each patient’s room to stow the equipment and instructed that a wall-mounted holder be filled with gloves at all times.
The researchers also realized that the rooms of patients with suspected or confirmed C. diff infections had warning signs that were too simplistic at first, then overly wordy. Both were being ignored. The solution was simple signage with yellow color-coding and easily recognizable symbols that readily conveyed the infection-control message to staff: sterile gowns, gloves, hands with soap and water, bleach. Those messages were reinforced through a brief, simple, and mandatory educational module for all hospital workers who might come into contact with the patients.
National Implications
On a national scale, hospitalists have helped compile other educational packets and toolkits to address the spectrum of HAIs, from ventilator-associated pneumonia to methicillin-resistant Staphylococcus aureus (MRSA).
More help may be forthcoming through the American Hospital Association’s Health Research and Education Trust. The broad-based effort, funded by the federal Agency for Healthcare Research and Quality (AHRQ), is partnering with SHM and other professional societies to implement small HAI-reduction successes on ever-wider scales. The University of Michigan’s Dr. Saint, for example, is a key partner in the trust’s national initiative to reduce CAUTI rates and improve patient safety.

—Sanjay Saint, MD, MPH, FHM, professor of internal medicine, University of Michigan, Ann Arbor
Medicare has rolled out its own collection of carrots and sticks to address the problem. The largest carrot, the public-private Partnership for Patients, has joined with SHM, other professional societies, and roughly 3,000 hospitals, so far, and set the ambitious goal of reducing hospital-acquired conditions by 40% by the end of 2013, compared with 2010 tallies. Although applauding the program’s overall goals, physicians, including Dr. Maynard, are taking a wait-and-see attitude, pointing out that many of the details have yet to be ironed out.
Among the sticks, Medicare has begun withholding reimbursement payments for such HAIs as CLABSIs and CAUTIs. Due to intricacies in how hospitals bill under the current DRG system, Dr. Kavanagh says Medicare’s nonpayment policy has recouped relatively little money from its first full year: about $18.8 million in all.
“The policies that cover public reporting, however, do have much more of an impact,” he says. “It’s more of a perceptual sting. Believe me, it is more concerning to have data of bad results that are available to the public than to be penalized by the current financial incentives.”
Other financial policies could carry considerably more weight. The threat of nonpayment for hospital readmissions, Dr. Maynard says, “totally changed the game” by intensifying efforts to reduce those rates, addressing contributing factors such as HAIs in the process. In combination, he says, public accountability through mandated reporting plus financial penalties could prove more powerful than either tactic alone.
Regardless of how federal policy plays out, experts say a new era of accountability and transparency is on its way. As the champions of positive change, hospitalists have a distinct opportunity to help lead the way and bring about a culture that consistently embraces effective interventions—before things get really ugly.
Bryn Nelson is a freelance medical writer based in Seattle.
References
- Klevens RM, Edwards JR, Richards CL, Horan TC, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160-166.
- Flanagan ME, Welsh CA, Kiess C, Hoke S, et al. A national collaborative for reducing health care-associated infections: current initiatives, challenges, and opportunities. Am J Infect Control. 2011;39:685-689.
- Pronovost P, Needham D, Berenholtz S, Sinopoli D, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725-2732.
- Srinivasan A, Wise M, Bell M, Cardo D, et al. Vital signs: central line-associated blood stream infections—United States, 2001, 2008, and 2009. MMWR. 2011;60(8):243-248.
- Chernetsky Tejedor S, Tong D, Stein J, Payne C, et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter.” Infect Control Hosp Epidemiol. 2012;33(1): in press.
- Saint S, Wiese J, Amory JK, Bernstein ML, et al. Are physicians aware of which of their patients have indwelling urinary catheters? Am J Med. 2000;109(6):476-480.
- Clarke K, Norrick B, Easley K, Pan Y, et al. Reduction of catheter-associated urinary tract infections through a bundled intervention in a community hospital. J Hosp Med. 2011;6(4):S22.
- Mierendorf S, Rushton M. Decreasing barriers in prevention of hospital-acquired Clostridium difficile colitis. J Hosp Med. 2011;6(4):S50-S51.
Redness, pain, and drainage from the skin around a catheter. Bloody, cloudy, or pungent urine and mental confusion. Severe diarrhea and a dilated colon: The potential symptoms of some of the most common hospital-acquired infections in the United States aren’t particularly pleasant.
The numbers don’t paint a pretty picture, either.
One widely cited study estimates that the nation’s hospitals report 1.7 million healthcare-associated infections (HAIs) every year, along with nearly 100,000 associated deaths.1 All told, the additional healthcare costs could tally $30 billion or more annually, according to a 2009 Centers for Disease Control and Prevention (CDC) analysis. Though some bright spots have emerged, healthcare advocates, including Kevin Kavanagh, MD, MS, FACS, director of Kentucky-based patient advocacy group Health Watch USA, contend that the overall size of the problem is likely underestimated. And unless brought under control, he says, emerging pathogens could make a bad situation worse.
The incredible variety of healthcare settings precludes any across-the-board solutions for hospitalists and other care providers. A recent survey of more than 1,200 healthcare professionals from 33 hospitals, however, revealed some common themes. According to the study authors, the data show that “hand hygiene is consistently identified as the greatest barrier to reducing HAIs, and that sustaining the necessary behavioral change to overcome this barrier is difficult.”2 In fact, the study highlighted a widespread belief that medical staff, and doctors in particular, are not fully engaged or in compliance with HAI reduction efforts. “The data suggest that physicians often do not adhere to protocols and guidelines; moreover, some respondents questioned whether many physicians are open to change,” the authors wrote.
So what can hospitalists do to help their colleagues embrace a culture of positive change and turn back a worsening healthcare epidemic? If simple answers are in short supply, studies aimed at central-line-associated bloodstream infections, catheter-associated urinary tract infections, and Clostridium difficile infections have at least highlighted some keys to a successful intervention.
In Focus: Central Lines
Central-line-associated bloodstream infections (CLABSIs) arguably have received the most attention of any HAI. Not coincidentally, most researchers point to the anti-CLABSI effort as the high point in the struggle to reduce infection rates.
In a landmark 2006 study led by hospitalist Peter Pronovost, MD, more than 100 ICUs in Michigan nearly eliminated CLABSIs over an 18-month period.3 The “remarkably successful” intervention, according to its authors, focused on changing provider behavior through education and collaboration with infection-control specialists. Nationwide, a recent CDC study estimated that absolute CLABSI numbers in ICUs dropped to 18,000 in 2009 from 43,000 in 2001, a 58% decline.4
Sheri Chernetsky Tejedor, MD, SFHM, assistant professor in the division of hospital medicine at Emory University School of Medicine in Atlanta, points out a major caveat in CLABSI prevention efforts, however: To date, she says, most have targeted insertion practices and ICU patients, which do little for the more than half of CLABSIs that occur on hospital wards. These wards thus represent “a ripe opportunity for intervention, especially the low-hanging, ‘low tech’ interventions of removing unnecessary devices,” she says.
Among a small random sample of patients with temporary central venous lines (CVCs), including peripherally inserted central catheters (PICCs), a study led by Dr. Chernetsky Tejedor found that 25% of all CVC days were “idle.”5 In other words, in 1 out of every 4 CVC days, the catheter was retained despite failing to meet standard justification criteria. In particular, the study suggested that PICCs were associated with longer catheter use and more idle days, fueling her group’s suspicion that increased PICC availability has changed CVC use patterns.
Among her group’s interventions, a patient safety checklist has been built, with questions about catheter use entered into a daily electronic progress note used by all services on the inpatient wards. One particularly successful strategy empowered nurses to determine whether continued catheter use seems justified and to ask physicians whether an “idle” CVC could be removed.
With a decline in CLABSI rates in ICUs and a shift in focus to the medical wards, Dr. Chernetsky Tejedor says, hospitalists are in a prime position to help reduce the inappropriate use of catheters and other invasive devices. More broadly, hospitalists may be well-suited to help change the culture of medicine toward a wider acceptance of proven interventions, such as central-line checklists.
Dr. Kavanagh argues that the slow and arduous process to move past what he calls “a huge resistance to checklists” and win universal adoption of such protocols “is not a good chapter in medicine.” He isn’t laying the blame at the feet of doctors alone, however. “In some institutions,” he says, “there needs to be a change from a profit-driven to a patient-centered culture.”
Greg Maynard, MD, MSc, SFHM, director of the University of California at San Diego Center for Innovation and Improvement Science and senior vice president of SHM’s Center for Hospital Innovation and Improvement, acknowledges the integral role of checklists in improvement efforts. He cautions, however, that they should be viewed as only one part of a multipronged approach.
As with hand hygiene, Dr. Maynard concedes that some facilities are still facing resistance from medical staff in integrating checklists into their routines, though he argues that an ingrained anti-checklist medical culture may not be solely at fault. “If you insert a checklist in such a way that it’s very inconvenient, that stops the flow of work,” he says, “you’re not going to have as much success as if you’ve thoughtfully designed it so that the checklist is called into play when it makes sense to bring it into play and made it easy to do, as opposed to difficult and awkward to do.”
In Focus: Catheter-Associated UTIs
Catheter-associated urinary tract infections (CAUTIs) account for roughly 1 in 3 healthcare-associated infections, according to the CDC. And yet many researchers say the “Rodney Dangerfield of HAIs” has long been overlooked.
A frequently cited study led by Sanjay Saint, MD, MPH, FHM, professor of internal medicine at the University of Michigan and the Ann Arbor VA Medical Center, found that nearly 40% of attending physicians were unaware that their patients even had an indwelling urinary catheter.6
Dr. Saint has dubbed the phenomenon “immaculate catheterization” to highlight the glaring discrepancy. “Because we also found, in a significant number of patients, there was no documentation anywhere in the medical record that the catheter existed,” he says. “It’s not in the physician’s notes, it’s not in the nursing notes, but we knew it was there because we could see it coming out of the patient.” Catheters that were inappropriately placed, he found, were more often “forgotten” than appropriate ones.
Perhaps more than anything else, the startling observation underscores the intense need for basic awareness of the two biggest UTI risk factors: whether a catheter is used, and how long it’s left in place. “You can get marked reductions by just taking care of those two factors,” Health Watch USA’s Dr. Kavanagh says.
At the Ann Arbor VA Hospital, one unit’s presence of a “Patient Safety Professional” to help ensure better oversight virtually brought the inappropriate use of indwelling catheters to a standstill. Dr. Saint and his colleagues are now gathering data to determine whether that decrease has translated to a drop in infections.

—Greg Maynard, MD, MSc, SFHM, director, University of California at San Diego Center for Innovation and Improvement Science, senior vice president, SHM Center for Hospital Innovation and Improvement
One fundamental key, he says, is paying close attention to whether a catheter is really in the patient’s best interests. “If we ask that question—‘If this was my family member, what would I want?’—we usually do the right thing,” Dr. Saint explains. Another key is leveraging the hospitalist’s core skill in communicating often and well with nurses to ensure that they are in sync during the “team sport” of CAUTI prevention.
With pockets of success in reducing inappropriate catheterization, the larger question now is how to scale up the individual interventions to achieve nationwide reductions. “How do you take what will work in one hospital, given its culture and microculture, and then apply it to hospitals across the state, or even across the country?” Dr. Saint asks.
Karen Clarke, MD, MS, MPH, a hospitalist and assistant professor of medicine at Emory University Hospital in Atlanta, is in the midst of tackling such issues. Using a bundled approach that included four interventions, she and colleagues reduced CAUTI rates by 70% at 276-bed West Georgia Medical Center in La Grange, Ga.7
The interventions were straightforward and inexpensive, Dr. Clarke says, meaning that they could be widely applied. “The only thing is that there has to be a champion overseeing the interventions to make sure that the steps are followed through on,” she says. Even at cash-strapped facilities, then, a similar approach could prove effective as long as someone assumes responsibility—and hospitalists would be a natural choice.
Based on her study’s promising results, Dr. Clarke hopes to begin implementing the intervention in at least one other hospital starting Jan. 1. If the success can be replicated, she says, the CAUTI-reduction protocol will branch out to include more regional hospitals.
In Focus: C. Diff-Associated Disease
Even as many hospitals are improving their CLABSI and CAUTI rates, hospital-acquired Clostridium difficile infections appear to be getting worse, particularly among older patients. In some facilities, the potentially fatal, diarrhea-causing microbe is now the top pathogen (see “Gut Reaction,” December 2011).
With a timely intervention, however, Kaiser Permanente Medical Center in Santa Clara, Calif., cut its own infection rates by one-third.8 In brainstorming how to improve the medical center’s rates, Susanne Mierendorf, MD, MS, FHM, a hospitalist and associate residency program director for internal medicine, joined colleagues in thinking through the barriers for healthcare providers. “It wasn’t ‘Why don’t they follow the infection-control guidelines?’ It was, ‘Why can’t they?’” Dr. Mierendorf says.
The thought exercise led to some eye-opening observations, including the realization that disposable gowns, gloves, and other personal protective equipment weren’t in the room and were hard to find. To help establish habits, Dr. Mierendorf’s team picked a consistent drawer in each patient’s room to stow the equipment and instructed that a wall-mounted holder be filled with gloves at all times.
The researchers also realized that the rooms of patients with suspected or confirmed C. diff infections had warning signs that were too simplistic at first, then overly wordy. Both were being ignored. The solution was simple signage with yellow color-coding and easily recognizable symbols that readily conveyed the infection-control message to staff: sterile gowns, gloves, hands with soap and water, bleach. Those messages were reinforced through a brief, simple, and mandatory educational module for all hospital workers who might come into contact with the patients.
National Implications
On a national scale, hospitalists have helped compile other educational packets and toolkits to address the spectrum of HAIs, from ventilator-associated pneumonia to methicillin-resistant Staphylococcus aureus (MRSA).
More help may be forthcoming through the American Hospital Association’s Health Research and Education Trust. The broad-based effort, funded by the federal Agency for Healthcare Research and Quality (AHRQ), is partnering with SHM and other professional societies to implement small HAI-reduction successes on ever-wider scales. The University of Michigan’s Dr. Saint, for example, is a key partner in the trust’s national initiative to reduce CAUTI rates and improve patient safety.

—Sanjay Saint, MD, MPH, FHM, professor of internal medicine, University of Michigan, Ann Arbor
Medicare has rolled out its own collection of carrots and sticks to address the problem. The largest carrot, the public-private Partnership for Patients, has joined with SHM, other professional societies, and roughly 3,000 hospitals, so far, and set the ambitious goal of reducing hospital-acquired conditions by 40% by the end of 2013, compared with 2010 tallies. Although applauding the program’s overall goals, physicians, including Dr. Maynard, are taking a wait-and-see attitude, pointing out that many of the details have yet to be ironed out.
Among the sticks, Medicare has begun withholding reimbursement payments for such HAIs as CLABSIs and CAUTIs. Due to intricacies in how hospitals bill under the current DRG system, Dr. Kavanagh says Medicare’s nonpayment policy has recouped relatively little money from its first full year: about $18.8 million in all.
“The policies that cover public reporting, however, do have much more of an impact,” he says. “It’s more of a perceptual sting. Believe me, it is more concerning to have data of bad results that are available to the public than to be penalized by the current financial incentives.”
Other financial policies could carry considerably more weight. The threat of nonpayment for hospital readmissions, Dr. Maynard says, “totally changed the game” by intensifying efforts to reduce those rates, addressing contributing factors such as HAIs in the process. In combination, he says, public accountability through mandated reporting plus financial penalties could prove more powerful than either tactic alone.
Regardless of how federal policy plays out, experts say a new era of accountability and transparency is on its way. As the champions of positive change, hospitalists have a distinct opportunity to help lead the way and bring about a culture that consistently embraces effective interventions—before things get really ugly.
Bryn Nelson is a freelance medical writer based in Seattle.
References
- Klevens RM, Edwards JR, Richards CL, Horan TC, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160-166.
- Flanagan ME, Welsh CA, Kiess C, Hoke S, et al. A national collaborative for reducing health care-associated infections: current initiatives, challenges, and opportunities. Am J Infect Control. 2011;39:685-689.
- Pronovost P, Needham D, Berenholtz S, Sinopoli D, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725-2732.
- Srinivasan A, Wise M, Bell M, Cardo D, et al. Vital signs: central line-associated blood stream infections—United States, 2001, 2008, and 2009. MMWR. 2011;60(8):243-248.
- Chernetsky Tejedor S, Tong D, Stein J, Payne C, et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter.” Infect Control Hosp Epidemiol. 2012;33(1): in press.
- Saint S, Wiese J, Amory JK, Bernstein ML, et al. Are physicians aware of which of their patients have indwelling urinary catheters? Am J Med. 2000;109(6):476-480.
- Clarke K, Norrick B, Easley K, Pan Y, et al. Reduction of catheter-associated urinary tract infections through a bundled intervention in a community hospital. J Hosp Med. 2011;6(4):S22.
- Mierendorf S, Rushton M. Decreasing barriers in prevention of hospital-acquired Clostridium difficile colitis. J Hosp Med. 2011;6(4):S50-S51.
Redness, pain, and drainage from the skin around a catheter. Bloody, cloudy, or pungent urine and mental confusion. Severe diarrhea and a dilated colon: The potential symptoms of some of the most common hospital-acquired infections in the United States aren’t particularly pleasant.
The numbers don’t paint a pretty picture, either.
One widely cited study estimates that the nation’s hospitals report 1.7 million healthcare-associated infections (HAIs) every year, along with nearly 100,000 associated deaths.1 All told, the additional healthcare costs could tally $30 billion or more annually, according to a 2009 Centers for Disease Control and Prevention (CDC) analysis. Though some bright spots have emerged, healthcare advocates, including Kevin Kavanagh, MD, MS, FACS, director of Kentucky-based patient advocacy group Health Watch USA, contend that the overall size of the problem is likely underestimated. And unless brought under control, he says, emerging pathogens could make a bad situation worse.
The incredible variety of healthcare settings precludes any across-the-board solutions for hospitalists and other care providers. A recent survey of more than 1,200 healthcare professionals from 33 hospitals, however, revealed some common themes. According to the study authors, the data show that “hand hygiene is consistently identified as the greatest barrier to reducing HAIs, and that sustaining the necessary behavioral change to overcome this barrier is difficult.”2 In fact, the study highlighted a widespread belief that medical staff, and doctors in particular, are not fully engaged or in compliance with HAI reduction efforts. “The data suggest that physicians often do not adhere to protocols and guidelines; moreover, some respondents questioned whether many physicians are open to change,” the authors wrote.
So what can hospitalists do to help their colleagues embrace a culture of positive change and turn back a worsening healthcare epidemic? If simple answers are in short supply, studies aimed at central-line-associated bloodstream infections, catheter-associated urinary tract infections, and Clostridium difficile infections have at least highlighted some keys to a successful intervention.
In Focus: Central Lines
Central-line-associated bloodstream infections (CLABSIs) arguably have received the most attention of any HAI. Not coincidentally, most researchers point to the anti-CLABSI effort as the high point in the struggle to reduce infection rates.
In a landmark 2006 study led by hospitalist Peter Pronovost, MD, more than 100 ICUs in Michigan nearly eliminated CLABSIs over an 18-month period.3 The “remarkably successful” intervention, according to its authors, focused on changing provider behavior through education and collaboration with infection-control specialists. Nationwide, a recent CDC study estimated that absolute CLABSI numbers in ICUs dropped to 18,000 in 2009 from 43,000 in 2001, a 58% decline.4
Sheri Chernetsky Tejedor, MD, SFHM, assistant professor in the division of hospital medicine at Emory University School of Medicine in Atlanta, points out a major caveat in CLABSI prevention efforts, however: To date, she says, most have targeted insertion practices and ICU patients, which do little for the more than half of CLABSIs that occur on hospital wards. These wards thus represent “a ripe opportunity for intervention, especially the low-hanging, ‘low tech’ interventions of removing unnecessary devices,” she says.
Among a small random sample of patients with temporary central venous lines (CVCs), including peripherally inserted central catheters (PICCs), a study led by Dr. Chernetsky Tejedor found that 25% of all CVC days were “idle.”5 In other words, in 1 out of every 4 CVC days, the catheter was retained despite failing to meet standard justification criteria. In particular, the study suggested that PICCs were associated with longer catheter use and more idle days, fueling her group’s suspicion that increased PICC availability has changed CVC use patterns.
Among her group’s interventions, a patient safety checklist has been built, with questions about catheter use entered into a daily electronic progress note used by all services on the inpatient wards. One particularly successful strategy empowered nurses to determine whether continued catheter use seems justified and to ask physicians whether an “idle” CVC could be removed.
With a decline in CLABSI rates in ICUs and a shift in focus to the medical wards, Dr. Chernetsky Tejedor says, hospitalists are in a prime position to help reduce the inappropriate use of catheters and other invasive devices. More broadly, hospitalists may be well-suited to help change the culture of medicine toward a wider acceptance of proven interventions, such as central-line checklists.
Dr. Kavanagh argues that the slow and arduous process to move past what he calls “a huge resistance to checklists” and win universal adoption of such protocols “is not a good chapter in medicine.” He isn’t laying the blame at the feet of doctors alone, however. “In some institutions,” he says, “there needs to be a change from a profit-driven to a patient-centered culture.”
Greg Maynard, MD, MSc, SFHM, director of the University of California at San Diego Center for Innovation and Improvement Science and senior vice president of SHM’s Center for Hospital Innovation and Improvement, acknowledges the integral role of checklists in improvement efforts. He cautions, however, that they should be viewed as only one part of a multipronged approach.
As with hand hygiene, Dr. Maynard concedes that some facilities are still facing resistance from medical staff in integrating checklists into their routines, though he argues that an ingrained anti-checklist medical culture may not be solely at fault. “If you insert a checklist in such a way that it’s very inconvenient, that stops the flow of work,” he says, “you’re not going to have as much success as if you’ve thoughtfully designed it so that the checklist is called into play when it makes sense to bring it into play and made it easy to do, as opposed to difficult and awkward to do.”
In Focus: Catheter-Associated UTIs
Catheter-associated urinary tract infections (CAUTIs) account for roughly 1 in 3 healthcare-associated infections, according to the CDC. And yet many researchers say the “Rodney Dangerfield of HAIs” has long been overlooked.
A frequently cited study led by Sanjay Saint, MD, MPH, FHM, professor of internal medicine at the University of Michigan and the Ann Arbor VA Medical Center, found that nearly 40% of attending physicians were unaware that their patients even had an indwelling urinary catheter.6
Dr. Saint has dubbed the phenomenon “immaculate catheterization” to highlight the glaring discrepancy. “Because we also found, in a significant number of patients, there was no documentation anywhere in the medical record that the catheter existed,” he says. “It’s not in the physician’s notes, it’s not in the nursing notes, but we knew it was there because we could see it coming out of the patient.” Catheters that were inappropriately placed, he found, were more often “forgotten” than appropriate ones.
Perhaps more than anything else, the startling observation underscores the intense need for basic awareness of the two biggest UTI risk factors: whether a catheter is used, and how long it’s left in place. “You can get marked reductions by just taking care of those two factors,” Health Watch USA’s Dr. Kavanagh says.
At the Ann Arbor VA Hospital, one unit’s presence of a “Patient Safety Professional” to help ensure better oversight virtually brought the inappropriate use of indwelling catheters to a standstill. Dr. Saint and his colleagues are now gathering data to determine whether that decrease has translated to a drop in infections.

—Greg Maynard, MD, MSc, SFHM, director, University of California at San Diego Center for Innovation and Improvement Science, senior vice president, SHM Center for Hospital Innovation and Improvement
One fundamental key, he says, is paying close attention to whether a catheter is really in the patient’s best interests. “If we ask that question—‘If this was my family member, what would I want?’—we usually do the right thing,” Dr. Saint explains. Another key is leveraging the hospitalist’s core skill in communicating often and well with nurses to ensure that they are in sync during the “team sport” of CAUTI prevention.
With pockets of success in reducing inappropriate catheterization, the larger question now is how to scale up the individual interventions to achieve nationwide reductions. “How do you take what will work in one hospital, given its culture and microculture, and then apply it to hospitals across the state, or even across the country?” Dr. Saint asks.
Karen Clarke, MD, MS, MPH, a hospitalist and assistant professor of medicine at Emory University Hospital in Atlanta, is in the midst of tackling such issues. Using a bundled approach that included four interventions, she and colleagues reduced CAUTI rates by 70% at 276-bed West Georgia Medical Center in La Grange, Ga.7
The interventions were straightforward and inexpensive, Dr. Clarke says, meaning that they could be widely applied. “The only thing is that there has to be a champion overseeing the interventions to make sure that the steps are followed through on,” she says. Even at cash-strapped facilities, then, a similar approach could prove effective as long as someone assumes responsibility—and hospitalists would be a natural choice.
Based on her study’s promising results, Dr. Clarke hopes to begin implementing the intervention in at least one other hospital starting Jan. 1. If the success can be replicated, she says, the CAUTI-reduction protocol will branch out to include more regional hospitals.
In Focus: C. Diff-Associated Disease
Even as many hospitals are improving their CLABSI and CAUTI rates, hospital-acquired Clostridium difficile infections appear to be getting worse, particularly among older patients. In some facilities, the potentially fatal, diarrhea-causing microbe is now the top pathogen (see “Gut Reaction,” December 2011).
With a timely intervention, however, Kaiser Permanente Medical Center in Santa Clara, Calif., cut its own infection rates by one-third.8 In brainstorming how to improve the medical center’s rates, Susanne Mierendorf, MD, MS, FHM, a hospitalist and associate residency program director for internal medicine, joined colleagues in thinking through the barriers for healthcare providers. “It wasn’t ‘Why don’t they follow the infection-control guidelines?’ It was, ‘Why can’t they?’” Dr. Mierendorf says.
The thought exercise led to some eye-opening observations, including the realization that disposable gowns, gloves, and other personal protective equipment weren’t in the room and were hard to find. To help establish habits, Dr. Mierendorf’s team picked a consistent drawer in each patient’s room to stow the equipment and instructed that a wall-mounted holder be filled with gloves at all times.
The researchers also realized that the rooms of patients with suspected or confirmed C. diff infections had warning signs that were too simplistic at first, then overly wordy. Both were being ignored. The solution was simple signage with yellow color-coding and easily recognizable symbols that readily conveyed the infection-control message to staff: sterile gowns, gloves, hands with soap and water, bleach. Those messages were reinforced through a brief, simple, and mandatory educational module for all hospital workers who might come into contact with the patients.
National Implications
On a national scale, hospitalists have helped compile other educational packets and toolkits to address the spectrum of HAIs, from ventilator-associated pneumonia to methicillin-resistant Staphylococcus aureus (MRSA).
More help may be forthcoming through the American Hospital Association’s Health Research and Education Trust. The broad-based effort, funded by the federal Agency for Healthcare Research and Quality (AHRQ), is partnering with SHM and other professional societies to implement small HAI-reduction successes on ever-wider scales. The University of Michigan’s Dr. Saint, for example, is a key partner in the trust’s national initiative to reduce CAUTI rates and improve patient safety.

—Sanjay Saint, MD, MPH, FHM, professor of internal medicine, University of Michigan, Ann Arbor
Medicare has rolled out its own collection of carrots and sticks to address the problem. The largest carrot, the public-private Partnership for Patients, has joined with SHM, other professional societies, and roughly 3,000 hospitals, so far, and set the ambitious goal of reducing hospital-acquired conditions by 40% by the end of 2013, compared with 2010 tallies. Although applauding the program’s overall goals, physicians, including Dr. Maynard, are taking a wait-and-see attitude, pointing out that many of the details have yet to be ironed out.
Among the sticks, Medicare has begun withholding reimbursement payments for such HAIs as CLABSIs and CAUTIs. Due to intricacies in how hospitals bill under the current DRG system, Dr. Kavanagh says Medicare’s nonpayment policy has recouped relatively little money from its first full year: about $18.8 million in all.
“The policies that cover public reporting, however, do have much more of an impact,” he says. “It’s more of a perceptual sting. Believe me, it is more concerning to have data of bad results that are available to the public than to be penalized by the current financial incentives.”
Other financial policies could carry considerably more weight. The threat of nonpayment for hospital readmissions, Dr. Maynard says, “totally changed the game” by intensifying efforts to reduce those rates, addressing contributing factors such as HAIs in the process. In combination, he says, public accountability through mandated reporting plus financial penalties could prove more powerful than either tactic alone.
Regardless of how federal policy plays out, experts say a new era of accountability and transparency is on its way. As the champions of positive change, hospitalists have a distinct opportunity to help lead the way and bring about a culture that consistently embraces effective interventions—before things get really ugly.
Bryn Nelson is a freelance medical writer based in Seattle.
References
- Klevens RM, Edwards JR, Richards CL, Horan TC, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160-166.
- Flanagan ME, Welsh CA, Kiess C, Hoke S, et al. A national collaborative for reducing health care-associated infections: current initiatives, challenges, and opportunities. Am J Infect Control. 2011;39:685-689.
- Pronovost P, Needham D, Berenholtz S, Sinopoli D, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725-2732.
- Srinivasan A, Wise M, Bell M, Cardo D, et al. Vital signs: central line-associated blood stream infections—United States, 2001, 2008, and 2009. MMWR. 2011;60(8):243-248.
- Chernetsky Tejedor S, Tong D, Stein J, Payne C, et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter.” Infect Control Hosp Epidemiol. 2012;33(1): in press.
- Saint S, Wiese J, Amory JK, Bernstein ML, et al. Are physicians aware of which of their patients have indwelling urinary catheters? Am J Med. 2000;109(6):476-480.
- Clarke K, Norrick B, Easley K, Pan Y, et al. Reduction of catheter-associated urinary tract infections through a bundled intervention in a community hospital. J Hosp Med. 2011;6(4):S22.
- Mierendorf S, Rushton M. Decreasing barriers in prevention of hospital-acquired Clostridium difficile colitis. J Hosp Med. 2011;6(4):S50-S51.
Reconciliation Act
Pharmacist Kristine M. Gleason, RPh, got the chance to personally test her ability to help ED providers with medication reconciliation—known by most in healthcare as “med rec”—when she broke her leg a couple of years ago. No problem, she thought: “I’ve been involved in med-rec efforts for eight-plus years.”
But when asked to provide her current medications, Gleason, who is the clinical quality leader in the department of clinical quality and analytics at Northwestern Memorial Hospital in Chicago, says she was in pain and overwhelmed. “I couldn’t even remember my children’s names, let alone the names and dosages of my aspirin and my thyroid medication,” she says. Moreover, she didn’t carry a list in her wallet because “I’m a pharmacist and I do med rec,” she says.
Gleason’s experience highlights why, six years after The Joint Commission introduced medication reconciliation as National Patient Safety Goal (NPSG) No. 8, hospitals and providers still struggle with the process.1 As a younger patient, Gleason took few medications. But for the majority of elderly inpatients with comorbid conditions, just establishing the patient’s medication list can bring the whole process to a halt; without that foundational list, reconciling other medications becomes problematic.
Although the commission has taken the goals under review and has, since July 1, required compliance with the revised NPSG 03.06.01 (see “Additional Resources,”), hospitalization-associated adverse drug events continue to mount. A recent Canadian study caused a ripple this summer with its findings that patients discharged from acute-care hospitals were at higher risk for unintentional discontinuation of their medications prescribed for chronic diseases than control groups, and those who had an ICU stay are at even higher risk.2
There’s been no shortage of med-rec initiatives in recent years. Medication reconciliation was at the top of the list for ways to prevent errors when the Institute for Healthcare Improvement launched its “5 Million Lives Campaign” in December 2006. SHM weighed in on the issue in 2010 with a consensus statement on key principles and necessary first steps in med rec.3
“This isn’t a new problem,” Gleason says. “Med rec has become more heightened because we have many more medications and complex therapies, more care providers, more specialists—more players, if you will.”
The March launch of the Hospital Readmissions Reduction Program, part of the Centers for Medicaid & Medicare Services’ (CMS) Inpatient Prospective Payment System, will again shine the spotlight on med rec’s role in the prevention of 30-day readmissions. The Hospitalist talked with researchers, pharmacists, and hospitalists about the reasons behind medication discrepancies, and their strategies for addressing mismatches.
Why So Difficult?
The goal of medication reconciliation is to generate and maintain an accurate and coherent record of patients’ medications across all transitions of care, which sounds straightforward enough. But the process involves much more than just checking items off a list, says Jeffrey Schnipper, MD, MPH, FHM, currently the principal investigator for the $1.5 million study funded by the Agency for Healthcare Research and Quality (AHRQ) to research and implement best practices in med rec, dubbed MARQUIS (Multicenter Medication Reconciliation Quality Improvement Study). Those immersed in med rec know that it’s nonlinear, multilayered, and surprisingly complex, requiring partnerships among diverse providers across many domains of care.
“Medication reconciliation gets right at all the weaknesses of our healthcare system,” says Dr. Schnipper, a hospitalist and director of clinical research for the HM service at Brigham and Women’s Hospital (BWH) and assistant professor of medicine at Harvard Medical School, both in Boston. “We have an excellent healthcare system in so many ways, but what we do not do such a good job of is coordination of care across settings, easy transfer of information, and having one person who is responsible for the accuracy of a patient’s health information.”
Dr. Schnipper’s studies attest to the common occurrence of unintentional medical discrepancies, pointing to the need for accurate medication histories, identifying high-risk patients for intensive interventions, and careful med rec at time of discharge.4
Other factors might come into play, says Ted Tsomides, MD, PhD, an attending physician on the HM service at WakeMed Hospital and assistant professor of medicine at the University of North Carolina’s School of Medicine in Raleigh, N.C. For example, he surmises that a “fatigue factor” sets in for some providers. “After five years of working on any initiative, people get worn out and push it to the back burner, unless they are really incentivized to stay on it,” he says.
List Capture
Medication reconciliation is a multifaceted process, and the first step is to gather the history of medications the patient has been taking. Hospitalist Blake J. Lesselroth, MD, MBI, assistant professor of medicine and medical informatics and director of the Portland Patient Safety Center of Inquiry at the Portland VA Medical Center in Oregon, points out that “the initial exposure to the patient is like a pencil sketch. You start to realize that med rec involves iterative loops of communication between you, the patient, and other knowledge resources (see Figure 1). As you start to pull in more information, you begin to complete your narrative. At the end of hospitalization, you’ve got a vibrant portrait with much more nuance to it. So it can’t be a linear process.”
—Kristine M. Gleason, RPh, clinical quality leader, department of clinical quality and analytics, Northwestern Memorial Hospital, Chicago
The list is dynamic, especially in the ICU setting, says Gleason, where it represents only one point in time.
In a closed system, such as the Veterans Administration or Kaiser Permanente, it’s often easier to establish a patient’s ongoing medications. With an integrated electronic health record (EHR), providers can call up the patient’s list of medications during admittance to the hospital. Verifying those medications remains critical: The health record lists patients’ prescriptions, but that doesn’t always mean they have actually filled or are taking those medications.
At the Kaiser Permanente Southern California site in Santa Clarita, Calif., where hospitalist David W. Wong, MD, works, pharmacists review their medications with patients when they are admitted, provide any needed consultation, then repeat the process at discharge. “So far,” Dr. Wong says, “this has resulted in the best medication reconciliation that we’ve seen.”
Pharmacy Is Key
In 2006, Kenneth Boockvar, MD, of the James J. Peters VA Medical Center in Bronx, N.Y., found in a pre- and post-intervention study that using pharmacists to ferret out and communicate prescribing discrepancies to physicians resulted in lower risk of adverse drug events (ADEs) for patients transferred between the hospital and the nursing home.5 Likewise, Dr. Schnipper and his colleagues found that using pharmacists to conduct medication reviews, counsel patients at discharge, and make follow-up telephone calls to patients was associated with a lower rate of preventable ADEs 30 days after hospital discharge.6
At United Hospital System’s (UHS) Kenosha Medical Center campus in Kenosha, Wis., pharmacists play a key role in generating medication lists for incoming patients. Hospitalist Corey Black, MD, regional medical director for Cogent HMG, says many patients do not recall their medications or the dosages, so UHS utilizes a team approach: If patients come in during evenings or weekends, pharmacists start calling local pharmacies to track down patients’ medication lists. “We also try to have family members bring in any medication containers they can find,” he adds. Due to a Wisconsin state law mandating nursing homes to send medication lists along with patients, generating a list is much easier.
Dr. Tsomides is a physician sponsor of a new med-rec initiative at WakeMed. With a steering committee that includes representatives from stakeholder services (medicine, nursing, pharmacy, administration, etc.), the group plans to hire and train pharmacy techs who will take home medication lists in the ED, lifting that responsibility from physicians’ task lists.
Is IT the Answer?
Would many of the barriers to med rec go away with universal EHR? So far, the literature has not borne out the superiority of using EHR to facilitate better med rec.
Peter Kaboli and colleagues found that the computerized medication record reflected what patients were actually taking for only 5.3% of the 493 VA patients enrolled in a study at the Iowa City VA.7 Kenneth Boockvar and colleagues at the Bronx VA found no difference in the overall incidence of ADEs caused by medication discrepancies between VA patients with an EHR and non-VA patients without an EHR.8 A group of researchers with Partners HealthCare in Boston evaluated a secure, Web-based patient portal to produce more accurate medication lists. The patients using this system had just as many discrepancies between medication lists and self-reporting as those who did not.9
Dr. Lesselroth, who has devised a patient kiosk touch-screen tool for reconciling patients’ medication lists and has faced barriers when implementing said technology, says med rec is much more “organic” than strictly mechanical. “It invokes theories of learning from the cognitive sciences,” he says. “We haven’t actually built tools that help people with their problem representation, with understanding not just how medications reconcile with the prior setting of care, but whether they make clinical sense within the new context of care. That requires a quantum leap in thinking.”
Re-Brand the Message
Drs. Schnipper and Tsomides believe that when The Joint Committee first coined the term “medication reconciliation” and advanced it as a mandate, most providers associated it with a regulatory requirement, and understandably so. Dr. Schnipper says med rec could be improved if providers think about it in the context of accurate orders that translate to greater patient safety. “After all,” he says, “hospitalists are ultimately responsible for the medication orders written for their patients.
“This is not about regulatory requirements,” he continues. “This is about medication safety and transitions of care. You can spend an hour on deciding what dose of Lasix you want to send this patient home on, but if the patient then takes the wrong dose of Lasix because they don’t know what they were supposed to be taking, then all that good medical care is undone.”
The med rec conversation has come full circle, then, as being truly an issue of delivering patient-centered care. (For more on this topic, visit the-hospitalist.org to read “Patient Engagement Critical.”) Rather than focusing on the sometimes-befuddling term of medication reconciliation, providers should see med rec as part of an integrated medication management process that aims to take better care of patients through prevention and treatment, Gleason says.
The med rec issue is about effective communication at every transition of care. And that’s why, says Dr. Schnipper, “Hospitalists should own this process. We don’t have to do the process entirely by ourselves—and shouldn’t. But we are responsible for errors that happen during transitions in care and we should own these initiatives.”
He notes that all six hospitals enrolled in the MARQUIS study have hospitalists at the forefront of their quality-improvement (QI) efforts.
“Medication reconciliation is potentially a high-risk process, and there are no silver bullets” for globally addressing the process, says Dorothea Wild, MD, chief hospitalist at Griffin Hospital, a 160-bed acute care hospital in Derby, Conn.
—Jeffrey Schnipper, MD, MPH, FHM, hospitalist and director of clinical research, Brigham and Women’s Hospital Hospitalist Service, assistant professor of medicine, Harvard Medical School, Boston
Dr. Wild draws a parallel between med rec and blood transfusions. Just as with correct transfusing procedures, “we envision a process where at least two people independently verify what patients’ medications are,” she says. The meds list is started in the ED by nursing staff, is verified by the ED attending, verified again by the admitting team, and triple-checked by the admitting attending. Thus, says Dr. Wild, med rec becomes a shared responsibility.
Dr. Lesselroth wholeheartedly agrees with the approach.
“This is everybody’s job,” he says. “In a larger world view, med rec is all about trying to find a medication regimen that harmonizes with what the patient can do, that improves their probability of adherence, and that also helps us gather information when the patient returns and we re-embrace them in the care model. Theoretically, then, everybody [interfacing with a patient] becomes a clutch player.”
Gretchen Henkel is a freelance writer in California.
References
- Joint Commission on Accreditation of Healthcare Organizations. 2005 Hospital Accreditation Standards. JCO website. Available at: http://www.jointcommissioninternational.org/ JCI-Accredited-Organizations/. Accessed Dec. 7, 2011.
- Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306:840-847.
- Greenwald JL, Halasyamani L, Green J, et al. Making inpatient medication patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5:477-485.
- Pippins JR, Gandhi TK, Hamann C, et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23:1414-1422.
- Boockvar KS, Carlson HL, Giambanco V, et al. Medication reconciliation for reducing drug-discrepancy adverse events. Am J Geriatr Pharmacother. 2006;4:236-243.
- Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166:565-571.
- Kaboli PJ, McClimon JB, Hoth AB, et al. Assessing the accuracy of computerized medication histories. Am J Manag Care. 2004;10(11 Pt 2):872-877.
- Boockvar KS, Livote EE, Goldstein N, et al. Electronic health records and adverse drug events after patient transfer. Qual Saf Health Care. 2010;5:Epub(Aug 19).
- Staroselsky M, Volk LA, Tsurikova R, et al. An effort to improve electronic health record medication list accuracy between visits: patients’ and physicians’ responses. Int J Med Inform. 2008;77:153-160.
- Gleason KM, McDaniel MR, Feinglass J, et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25:441-447.
Pharmacist Kristine M. Gleason, RPh, got the chance to personally test her ability to help ED providers with medication reconciliation—known by most in healthcare as “med rec”—when she broke her leg a couple of years ago. No problem, she thought: “I’ve been involved in med-rec efforts for eight-plus years.”
But when asked to provide her current medications, Gleason, who is the clinical quality leader in the department of clinical quality and analytics at Northwestern Memorial Hospital in Chicago, says she was in pain and overwhelmed. “I couldn’t even remember my children’s names, let alone the names and dosages of my aspirin and my thyroid medication,” she says. Moreover, she didn’t carry a list in her wallet because “I’m a pharmacist and I do med rec,” she says.
Gleason’s experience highlights why, six years after The Joint Commission introduced medication reconciliation as National Patient Safety Goal (NPSG) No. 8, hospitals and providers still struggle with the process.1 As a younger patient, Gleason took few medications. But for the majority of elderly inpatients with comorbid conditions, just establishing the patient’s medication list can bring the whole process to a halt; without that foundational list, reconciling other medications becomes problematic.
Although the commission has taken the goals under review and has, since July 1, required compliance with the revised NPSG 03.06.01 (see “Additional Resources,”), hospitalization-associated adverse drug events continue to mount. A recent Canadian study caused a ripple this summer with its findings that patients discharged from acute-care hospitals were at higher risk for unintentional discontinuation of their medications prescribed for chronic diseases than control groups, and those who had an ICU stay are at even higher risk.2
There’s been no shortage of med-rec initiatives in recent years. Medication reconciliation was at the top of the list for ways to prevent errors when the Institute for Healthcare Improvement launched its “5 Million Lives Campaign” in December 2006. SHM weighed in on the issue in 2010 with a consensus statement on key principles and necessary first steps in med rec.3
“This isn’t a new problem,” Gleason says. “Med rec has become more heightened because we have many more medications and complex therapies, more care providers, more specialists—more players, if you will.”
The March launch of the Hospital Readmissions Reduction Program, part of the Centers for Medicaid & Medicare Services’ (CMS) Inpatient Prospective Payment System, will again shine the spotlight on med rec’s role in the prevention of 30-day readmissions. The Hospitalist talked with researchers, pharmacists, and hospitalists about the reasons behind medication discrepancies, and their strategies for addressing mismatches.
Why So Difficult?
The goal of medication reconciliation is to generate and maintain an accurate and coherent record of patients’ medications across all transitions of care, which sounds straightforward enough. But the process involves much more than just checking items off a list, says Jeffrey Schnipper, MD, MPH, FHM, currently the principal investigator for the $1.5 million study funded by the Agency for Healthcare Research and Quality (AHRQ) to research and implement best practices in med rec, dubbed MARQUIS (Multicenter Medication Reconciliation Quality Improvement Study). Those immersed in med rec know that it’s nonlinear, multilayered, and surprisingly complex, requiring partnerships among diverse providers across many domains of care.
“Medication reconciliation gets right at all the weaknesses of our healthcare system,” says Dr. Schnipper, a hospitalist and director of clinical research for the HM service at Brigham and Women’s Hospital (BWH) and assistant professor of medicine at Harvard Medical School, both in Boston. “We have an excellent healthcare system in so many ways, but what we do not do such a good job of is coordination of care across settings, easy transfer of information, and having one person who is responsible for the accuracy of a patient’s health information.”
Dr. Schnipper’s studies attest to the common occurrence of unintentional medical discrepancies, pointing to the need for accurate medication histories, identifying high-risk patients for intensive interventions, and careful med rec at time of discharge.4
Other factors might come into play, says Ted Tsomides, MD, PhD, an attending physician on the HM service at WakeMed Hospital and assistant professor of medicine at the University of North Carolina’s School of Medicine in Raleigh, N.C. For example, he surmises that a “fatigue factor” sets in for some providers. “After five years of working on any initiative, people get worn out and push it to the back burner, unless they are really incentivized to stay on it,” he says.
List Capture
Medication reconciliation is a multifaceted process, and the first step is to gather the history of medications the patient has been taking. Hospitalist Blake J. Lesselroth, MD, MBI, assistant professor of medicine and medical informatics and director of the Portland Patient Safety Center of Inquiry at the Portland VA Medical Center in Oregon, points out that “the initial exposure to the patient is like a pencil sketch. You start to realize that med rec involves iterative loops of communication between you, the patient, and other knowledge resources (see Figure 1). As you start to pull in more information, you begin to complete your narrative. At the end of hospitalization, you’ve got a vibrant portrait with much more nuance to it. So it can’t be a linear process.”
—Kristine M. Gleason, RPh, clinical quality leader, department of clinical quality and analytics, Northwestern Memorial Hospital, Chicago
The list is dynamic, especially in the ICU setting, says Gleason, where it represents only one point in time.
In a closed system, such as the Veterans Administration or Kaiser Permanente, it’s often easier to establish a patient’s ongoing medications. With an integrated electronic health record (EHR), providers can call up the patient’s list of medications during admittance to the hospital. Verifying those medications remains critical: The health record lists patients’ prescriptions, but that doesn’t always mean they have actually filled or are taking those medications.
At the Kaiser Permanente Southern California site in Santa Clarita, Calif., where hospitalist David W. Wong, MD, works, pharmacists review their medications with patients when they are admitted, provide any needed consultation, then repeat the process at discharge. “So far,” Dr. Wong says, “this has resulted in the best medication reconciliation that we’ve seen.”
Pharmacy Is Key
In 2006, Kenneth Boockvar, MD, of the James J. Peters VA Medical Center in Bronx, N.Y., found in a pre- and post-intervention study that using pharmacists to ferret out and communicate prescribing discrepancies to physicians resulted in lower risk of adverse drug events (ADEs) for patients transferred between the hospital and the nursing home.5 Likewise, Dr. Schnipper and his colleagues found that using pharmacists to conduct medication reviews, counsel patients at discharge, and make follow-up telephone calls to patients was associated with a lower rate of preventable ADEs 30 days after hospital discharge.6
At United Hospital System’s (UHS) Kenosha Medical Center campus in Kenosha, Wis., pharmacists play a key role in generating medication lists for incoming patients. Hospitalist Corey Black, MD, regional medical director for Cogent HMG, says many patients do not recall their medications or the dosages, so UHS utilizes a team approach: If patients come in during evenings or weekends, pharmacists start calling local pharmacies to track down patients’ medication lists. “We also try to have family members bring in any medication containers they can find,” he adds. Due to a Wisconsin state law mandating nursing homes to send medication lists along with patients, generating a list is much easier.
Dr. Tsomides is a physician sponsor of a new med-rec initiative at WakeMed. With a steering committee that includes representatives from stakeholder services (medicine, nursing, pharmacy, administration, etc.), the group plans to hire and train pharmacy techs who will take home medication lists in the ED, lifting that responsibility from physicians’ task lists.
Is IT the Answer?
Would many of the barriers to med rec go away with universal EHR? So far, the literature has not borne out the superiority of using EHR to facilitate better med rec.
Peter Kaboli and colleagues found that the computerized medication record reflected what patients were actually taking for only 5.3% of the 493 VA patients enrolled in a study at the Iowa City VA.7 Kenneth Boockvar and colleagues at the Bronx VA found no difference in the overall incidence of ADEs caused by medication discrepancies between VA patients with an EHR and non-VA patients without an EHR.8 A group of researchers with Partners HealthCare in Boston evaluated a secure, Web-based patient portal to produce more accurate medication lists. The patients using this system had just as many discrepancies between medication lists and self-reporting as those who did not.9
Dr. Lesselroth, who has devised a patient kiosk touch-screen tool for reconciling patients’ medication lists and has faced barriers when implementing said technology, says med rec is much more “organic” than strictly mechanical. “It invokes theories of learning from the cognitive sciences,” he says. “We haven’t actually built tools that help people with their problem representation, with understanding not just how medications reconcile with the prior setting of care, but whether they make clinical sense within the new context of care. That requires a quantum leap in thinking.”
Re-Brand the Message
Drs. Schnipper and Tsomides believe that when The Joint Committee first coined the term “medication reconciliation” and advanced it as a mandate, most providers associated it with a regulatory requirement, and understandably so. Dr. Schnipper says med rec could be improved if providers think about it in the context of accurate orders that translate to greater patient safety. “After all,” he says, “hospitalists are ultimately responsible for the medication orders written for their patients.
“This is not about regulatory requirements,” he continues. “This is about medication safety and transitions of care. You can spend an hour on deciding what dose of Lasix you want to send this patient home on, but if the patient then takes the wrong dose of Lasix because they don’t know what they were supposed to be taking, then all that good medical care is undone.”
The med rec conversation has come full circle, then, as being truly an issue of delivering patient-centered care. (For more on this topic, visit the-hospitalist.org to read “Patient Engagement Critical.”) Rather than focusing on the sometimes-befuddling term of medication reconciliation, providers should see med rec as part of an integrated medication management process that aims to take better care of patients through prevention and treatment, Gleason says.
The med rec issue is about effective communication at every transition of care. And that’s why, says Dr. Schnipper, “Hospitalists should own this process. We don’t have to do the process entirely by ourselves—and shouldn’t. But we are responsible for errors that happen during transitions in care and we should own these initiatives.”
He notes that all six hospitals enrolled in the MARQUIS study have hospitalists at the forefront of their quality-improvement (QI) efforts.
“Medication reconciliation is potentially a high-risk process, and there are no silver bullets” for globally addressing the process, says Dorothea Wild, MD, chief hospitalist at Griffin Hospital, a 160-bed acute care hospital in Derby, Conn.
—Jeffrey Schnipper, MD, MPH, FHM, hospitalist and director of clinical research, Brigham and Women’s Hospital Hospitalist Service, assistant professor of medicine, Harvard Medical School, Boston
Dr. Wild draws a parallel between med rec and blood transfusions. Just as with correct transfusing procedures, “we envision a process where at least two people independently verify what patients’ medications are,” she says. The meds list is started in the ED by nursing staff, is verified by the ED attending, verified again by the admitting team, and triple-checked by the admitting attending. Thus, says Dr. Wild, med rec becomes a shared responsibility.
Dr. Lesselroth wholeheartedly agrees with the approach.
“This is everybody’s job,” he says. “In a larger world view, med rec is all about trying to find a medication regimen that harmonizes with what the patient can do, that improves their probability of adherence, and that also helps us gather information when the patient returns and we re-embrace them in the care model. Theoretically, then, everybody [interfacing with a patient] becomes a clutch player.”
Gretchen Henkel is a freelance writer in California.
References
- Joint Commission on Accreditation of Healthcare Organizations. 2005 Hospital Accreditation Standards. JCO website. Available at: http://www.jointcommissioninternational.org/ JCI-Accredited-Organizations/. Accessed Dec. 7, 2011.
- Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306:840-847.
- Greenwald JL, Halasyamani L, Green J, et al. Making inpatient medication patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5:477-485.
- Pippins JR, Gandhi TK, Hamann C, et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23:1414-1422.
- Boockvar KS, Carlson HL, Giambanco V, et al. Medication reconciliation for reducing drug-discrepancy adverse events. Am J Geriatr Pharmacother. 2006;4:236-243.
- Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166:565-571.
- Kaboli PJ, McClimon JB, Hoth AB, et al. Assessing the accuracy of computerized medication histories. Am J Manag Care. 2004;10(11 Pt 2):872-877.
- Boockvar KS, Livote EE, Goldstein N, et al. Electronic health records and adverse drug events after patient transfer. Qual Saf Health Care. 2010;5:Epub(Aug 19).
- Staroselsky M, Volk LA, Tsurikova R, et al. An effort to improve electronic health record medication list accuracy between visits: patients’ and physicians’ responses. Int J Med Inform. 2008;77:153-160.
- Gleason KM, McDaniel MR, Feinglass J, et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25:441-447.
Pharmacist Kristine M. Gleason, RPh, got the chance to personally test her ability to help ED providers with medication reconciliation—known by most in healthcare as “med rec”—when she broke her leg a couple of years ago. No problem, she thought: “I’ve been involved in med-rec efforts for eight-plus years.”
But when asked to provide her current medications, Gleason, who is the clinical quality leader in the department of clinical quality and analytics at Northwestern Memorial Hospital in Chicago, says she was in pain and overwhelmed. “I couldn’t even remember my children’s names, let alone the names and dosages of my aspirin and my thyroid medication,” she says. Moreover, she didn’t carry a list in her wallet because “I’m a pharmacist and I do med rec,” she says.
Gleason’s experience highlights why, six years after The Joint Commission introduced medication reconciliation as National Patient Safety Goal (NPSG) No. 8, hospitals and providers still struggle with the process.1 As a younger patient, Gleason took few medications. But for the majority of elderly inpatients with comorbid conditions, just establishing the patient’s medication list can bring the whole process to a halt; without that foundational list, reconciling other medications becomes problematic.
Although the commission has taken the goals under review and has, since July 1, required compliance with the revised NPSG 03.06.01 (see “Additional Resources,”), hospitalization-associated adverse drug events continue to mount. A recent Canadian study caused a ripple this summer with its findings that patients discharged from acute-care hospitals were at higher risk for unintentional discontinuation of their medications prescribed for chronic diseases than control groups, and those who had an ICU stay are at even higher risk.2
There’s been no shortage of med-rec initiatives in recent years. Medication reconciliation was at the top of the list for ways to prevent errors when the Institute for Healthcare Improvement launched its “5 Million Lives Campaign” in December 2006. SHM weighed in on the issue in 2010 with a consensus statement on key principles and necessary first steps in med rec.3
“This isn’t a new problem,” Gleason says. “Med rec has become more heightened because we have many more medications and complex therapies, more care providers, more specialists—more players, if you will.”
The March launch of the Hospital Readmissions Reduction Program, part of the Centers for Medicaid & Medicare Services’ (CMS) Inpatient Prospective Payment System, will again shine the spotlight on med rec’s role in the prevention of 30-day readmissions. The Hospitalist talked with researchers, pharmacists, and hospitalists about the reasons behind medication discrepancies, and their strategies for addressing mismatches.
Why So Difficult?
The goal of medication reconciliation is to generate and maintain an accurate and coherent record of patients’ medications across all transitions of care, which sounds straightforward enough. But the process involves much more than just checking items off a list, says Jeffrey Schnipper, MD, MPH, FHM, currently the principal investigator for the $1.5 million study funded by the Agency for Healthcare Research and Quality (AHRQ) to research and implement best practices in med rec, dubbed MARQUIS (Multicenter Medication Reconciliation Quality Improvement Study). Those immersed in med rec know that it’s nonlinear, multilayered, and surprisingly complex, requiring partnerships among diverse providers across many domains of care.
“Medication reconciliation gets right at all the weaknesses of our healthcare system,” says Dr. Schnipper, a hospitalist and director of clinical research for the HM service at Brigham and Women’s Hospital (BWH) and assistant professor of medicine at Harvard Medical School, both in Boston. “We have an excellent healthcare system in so many ways, but what we do not do such a good job of is coordination of care across settings, easy transfer of information, and having one person who is responsible for the accuracy of a patient’s health information.”
Dr. Schnipper’s studies attest to the common occurrence of unintentional medical discrepancies, pointing to the need for accurate medication histories, identifying high-risk patients for intensive interventions, and careful med rec at time of discharge.4
Other factors might come into play, says Ted Tsomides, MD, PhD, an attending physician on the HM service at WakeMed Hospital and assistant professor of medicine at the University of North Carolina’s School of Medicine in Raleigh, N.C. For example, he surmises that a “fatigue factor” sets in for some providers. “After five years of working on any initiative, people get worn out and push it to the back burner, unless they are really incentivized to stay on it,” he says.
List Capture
Medication reconciliation is a multifaceted process, and the first step is to gather the history of medications the patient has been taking. Hospitalist Blake J. Lesselroth, MD, MBI, assistant professor of medicine and medical informatics and director of the Portland Patient Safety Center of Inquiry at the Portland VA Medical Center in Oregon, points out that “the initial exposure to the patient is like a pencil sketch. You start to realize that med rec involves iterative loops of communication between you, the patient, and other knowledge resources (see Figure 1). As you start to pull in more information, you begin to complete your narrative. At the end of hospitalization, you’ve got a vibrant portrait with much more nuance to it. So it can’t be a linear process.”
—Kristine M. Gleason, RPh, clinical quality leader, department of clinical quality and analytics, Northwestern Memorial Hospital, Chicago
The list is dynamic, especially in the ICU setting, says Gleason, where it represents only one point in time.
In a closed system, such as the Veterans Administration or Kaiser Permanente, it’s often easier to establish a patient’s ongoing medications. With an integrated electronic health record (EHR), providers can call up the patient’s list of medications during admittance to the hospital. Verifying those medications remains critical: The health record lists patients’ prescriptions, but that doesn’t always mean they have actually filled or are taking those medications.
At the Kaiser Permanente Southern California site in Santa Clarita, Calif., where hospitalist David W. Wong, MD, works, pharmacists review their medications with patients when they are admitted, provide any needed consultation, then repeat the process at discharge. “So far,” Dr. Wong says, “this has resulted in the best medication reconciliation that we’ve seen.”
Pharmacy Is Key
In 2006, Kenneth Boockvar, MD, of the James J. Peters VA Medical Center in Bronx, N.Y., found in a pre- and post-intervention study that using pharmacists to ferret out and communicate prescribing discrepancies to physicians resulted in lower risk of adverse drug events (ADEs) for patients transferred between the hospital and the nursing home.5 Likewise, Dr. Schnipper and his colleagues found that using pharmacists to conduct medication reviews, counsel patients at discharge, and make follow-up telephone calls to patients was associated with a lower rate of preventable ADEs 30 days after hospital discharge.6
At United Hospital System’s (UHS) Kenosha Medical Center campus in Kenosha, Wis., pharmacists play a key role in generating medication lists for incoming patients. Hospitalist Corey Black, MD, regional medical director for Cogent HMG, says many patients do not recall their medications or the dosages, so UHS utilizes a team approach: If patients come in during evenings or weekends, pharmacists start calling local pharmacies to track down patients’ medication lists. “We also try to have family members bring in any medication containers they can find,” he adds. Due to a Wisconsin state law mandating nursing homes to send medication lists along with patients, generating a list is much easier.
Dr. Tsomides is a physician sponsor of a new med-rec initiative at WakeMed. With a steering committee that includes representatives from stakeholder services (medicine, nursing, pharmacy, administration, etc.), the group plans to hire and train pharmacy techs who will take home medication lists in the ED, lifting that responsibility from physicians’ task lists.
Is IT the Answer?
Would many of the barriers to med rec go away with universal EHR? So far, the literature has not borne out the superiority of using EHR to facilitate better med rec.
Peter Kaboli and colleagues found that the computerized medication record reflected what patients were actually taking for only 5.3% of the 493 VA patients enrolled in a study at the Iowa City VA.7 Kenneth Boockvar and colleagues at the Bronx VA found no difference in the overall incidence of ADEs caused by medication discrepancies between VA patients with an EHR and non-VA patients without an EHR.8 A group of researchers with Partners HealthCare in Boston evaluated a secure, Web-based patient portal to produce more accurate medication lists. The patients using this system had just as many discrepancies between medication lists and self-reporting as those who did not.9
Dr. Lesselroth, who has devised a patient kiosk touch-screen tool for reconciling patients’ medication lists and has faced barriers when implementing said technology, says med rec is much more “organic” than strictly mechanical. “It invokes theories of learning from the cognitive sciences,” he says. “We haven’t actually built tools that help people with their problem representation, with understanding not just how medications reconcile with the prior setting of care, but whether they make clinical sense within the new context of care. That requires a quantum leap in thinking.”
Re-Brand the Message
Drs. Schnipper and Tsomides believe that when The Joint Committee first coined the term “medication reconciliation” and advanced it as a mandate, most providers associated it with a regulatory requirement, and understandably so. Dr. Schnipper says med rec could be improved if providers think about it in the context of accurate orders that translate to greater patient safety. “After all,” he says, “hospitalists are ultimately responsible for the medication orders written for their patients.
“This is not about regulatory requirements,” he continues. “This is about medication safety and transitions of care. You can spend an hour on deciding what dose of Lasix you want to send this patient home on, but if the patient then takes the wrong dose of Lasix because they don’t know what they were supposed to be taking, then all that good medical care is undone.”
The med rec conversation has come full circle, then, as being truly an issue of delivering patient-centered care. (For more on this topic, visit the-hospitalist.org to read “Patient Engagement Critical.”) Rather than focusing on the sometimes-befuddling term of medication reconciliation, providers should see med rec as part of an integrated medication management process that aims to take better care of patients through prevention and treatment, Gleason says.
The med rec issue is about effective communication at every transition of care. And that’s why, says Dr. Schnipper, “Hospitalists should own this process. We don’t have to do the process entirely by ourselves—and shouldn’t. But we are responsible for errors that happen during transitions in care and we should own these initiatives.”
He notes that all six hospitals enrolled in the MARQUIS study have hospitalists at the forefront of their quality-improvement (QI) efforts.
“Medication reconciliation is potentially a high-risk process, and there are no silver bullets” for globally addressing the process, says Dorothea Wild, MD, chief hospitalist at Griffin Hospital, a 160-bed acute care hospital in Derby, Conn.
—Jeffrey Schnipper, MD, MPH, FHM, hospitalist and director of clinical research, Brigham and Women’s Hospital Hospitalist Service, assistant professor of medicine, Harvard Medical School, Boston
Dr. Wild draws a parallel between med rec and blood transfusions. Just as with correct transfusing procedures, “we envision a process where at least two people independently verify what patients’ medications are,” she says. The meds list is started in the ED by nursing staff, is verified by the ED attending, verified again by the admitting team, and triple-checked by the admitting attending. Thus, says Dr. Wild, med rec becomes a shared responsibility.
Dr. Lesselroth wholeheartedly agrees with the approach.
“This is everybody’s job,” he says. “In a larger world view, med rec is all about trying to find a medication regimen that harmonizes with what the patient can do, that improves their probability of adherence, and that also helps us gather information when the patient returns and we re-embrace them in the care model. Theoretically, then, everybody [interfacing with a patient] becomes a clutch player.”
Gretchen Henkel is a freelance writer in California.
References
- Joint Commission on Accreditation of Healthcare Organizations. 2005 Hospital Accreditation Standards. JCO website. Available at: http://www.jointcommissioninternational.org/ JCI-Accredited-Organizations/. Accessed Dec. 7, 2011.
- Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306:840-847.
- Greenwald JL, Halasyamani L, Green J, et al. Making inpatient medication patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5:477-485.
- Pippins JR, Gandhi TK, Hamann C, et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23:1414-1422.
- Boockvar KS, Carlson HL, Giambanco V, et al. Medication reconciliation for reducing drug-discrepancy adverse events. Am J Geriatr Pharmacother. 2006;4:236-243.
- Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166:565-571.
- Kaboli PJ, McClimon JB, Hoth AB, et al. Assessing the accuracy of computerized medication histories. Am J Manag Care. 2004;10(11 Pt 2):872-877.
- Boockvar KS, Livote EE, Goldstein N, et al. Electronic health records and adverse drug events after patient transfer. Qual Saf Health Care. 2010;5:Epub(Aug 19).
- Staroselsky M, Volk LA, Tsurikova R, et al. An effort to improve electronic health record medication list accuracy between visits: patients’ and physicians’ responses. Int J Med Inform. 2008;77:153-160.
- Gleason KM, McDaniel MR, Feinglass J, et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25:441-447.
In the Literature: The latest research you need to know
In This Edition
Literature At A Glance
A guide to this month’s studies
- Procalcitonin to guide antimicrobial use in ICU patients
- Platelet reactivity and event rates after PCI
- Conservative treatment of necrotizing pancreatitis
- Use of MRI to diagnose Takotsubo cardiomyopathy
- Rates of inadvertent medication discontinuation after hospitalization
- Role of history and physical exam in diagnosing medical illness
- Red-cell distribution width and rates of mortality
Procalcitonin-Guided Therapy Decreases Antimicrobial Duration in ICU Patients
Clinical question: Can utilization of serum procalcitonin (PCT) levels safely reduce antimicrobial exposure in ICU patients?
Background: Serum PCT levels are elevated in bacterial infections and sepsis and have been used in some settings to guide antimicrobial therapy. Randomized controlled trials have demonstrated reduction of antibiotic use with PCT measurement. This systematic review assessed the safety and effectiveness of PCT measurements in reducing antimicrobial exposure in ICU patients.
Study design: Systematic review.
Setting: Adult medical and surgical ICUs.
Synopsis: A search of MEDLINE and EMBASE yielded 1,018 publications related to PCT, critically ill patients, and antimicrobial therapy. Six randomized controlled trials involving 1,476 patients were reviewed. The duration of antimicrobial use was significantly decreased in all five studies that evaluated treatment duration. The remaining study only assessed the impact of PCT on initiation of antimicrobial therapy and did not demonstrate decreased antimicrobial exposure. Compared with the control group, patients randomized to PCT-guided therapy had relative reductions in duration of first antibiotic course by 21%, down to 38%, and decreases in days of antimicrobial therapy per 1,000 ICU patient days by 20%, down to 23%. PCT intervention also was associated with a 23% to 37% increase in days alive without antimicrobial therapy during the first 28 days. The length of ICU stay was significantly decreased in two studies but was not significantly different in the other studies. There were no significant differences in rates of mortality, infection relapse, or days free of mechanical ventilation.
Bottom line: PCT guidance reduced antimicrobial exposure of ICU patients without increasing rates of mortality or infection relapse.
Citation: Agarwal R, Schwartz DN. Procalcitonin to guide duration of antimicrobial therapy in intensive care units: a systematic review. Clin Infect Dis. 2011;53:379-387.
High Residual Platelet Reactivity Increases Risk of Cardiovascular Events among Patients with Acute Coronary Syndromes Undergoing PCI
Clinical question: Is high residual platelet reactivity (HRPR) in patients receiving clopidogrel associated with increased risk of ischemic events after percutaneous coronary intervention (PCI)?
Background: Studies have demonstrated an increased risk of cardiovascular events associated with HRPR in patients receiving clopidogrel while undergoing PCI. However, these studies have used a variety of platelet function tests, and thresholds for positive tests have not been established. In addition, these studies have enrolled heterogeneous populations with short-term follow-up and few have included patients with acute coronary syndromes (ACSs).
Study design: Prospective cohort study.
Setting: Cardiology service of a referral center in Italy.
Synopsis: This study included 1,789 consecutive patients with ACS undergoing PCI. Patients were given 325 mg aspirin and a 600-mg loading dose of clopidogrel on admission, followed by a daily maintenance dose of aspirin 325 mg and clopidogrel 75 mg for at least six months. Platelet reactivity was measured using adenosine diphosphate (ADP) testing, and those with HRPR (≥70% platelet aggregation) were given increased dosing of clopidogrel or switched to ticlopidine using ADP test guidance. At two-year follow-up, patients with HRPR experienced an increased composite endpoint of cardiac death, myocardial infarction, urgent coronary revascularization, and stroke with a combined event rate of 14.6% in patients with HRPR and 8.7% in patients with low residual platelet reactivity (95% CI, 1.6%-11.1%; P=0.003). Stent thrombosis was also higher in HRPR patients (absolute risk increase 3.2%; 95% CI, 0.4%-6.7%; P=0.01).
This study is nonrandomized, and residual unmeasured confounding cannot be excluded. In addition, use of non-antiplatelet drugs and adherence to recommended drugs might have influenced outcomes.
Bottom line: HRPR is associated with increased risk of ischemic events in patients with ACS receiving antiplatelet agents after PCI.
Citation: Parodi G, Marcucci R, Valenti R, et al. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA. 2011;306:1215-1223.
Conservative Treatment of Necrotizing Pancreatitis Is Associated with Improved Outcomes
Clinical question: What are the outcomes of conservative and interventional management of necrotizing pancreatitis?
Background: Open necrosectomy was historically the treatment of choice for necrotizing pancreatitis, but, currently, pancreatic necrosis is only managed invasively when complicated by infection. Other changes in management over time have included a shift in the timing of intervention and the use of minimally invasive techniques. Existing studies do not reflect these changes in practice patterns and have been limited by small sample sizes or the exclusion of important subgroups of patients.
Study design: Prospective cohort study.
Setting: Twenty-one Dutch hospitals.
Synopsis: This study included 639 patients with acute necrotizing pancreatitis confirmed by imaging. Overall mortality was 15%. Conservative treatment was performed in 62% of the patients with a mortality of 7%; however, patients with organ failure (pulmonary, circulatory, and/or renal) who received conservative therapy had a mortality rate of 37%. Intervention (percutaneous drainage, video-assisted retroperitoneal debridement, endoscopic transluminal necrosectomy, laparotomy) in patients with suspected or confirmed infected pancreatic necrosis was performed on 38% of the patients with associated mortality of 27%. Interventions performed within the first 14 days of hospitalization resulted in a 56% mortality rate, whereas interventions performed after Day 29 resulted in a 15% mortality rate (P<0.001). Patients with organ failure experienced significantly greater mortality compared with patients with no organ failure (35% vs. 2%; P<0.001). Primary percutaneous drainage was associated with fewer complications than was primary necrosectomy (42% vs. 64%; P=0.003).
This study was nonrandomized, and final decisions regarding management were left to the treating physician. Notably, while there was no significant difference in APACHE II scores between the conservative and intervention groups, intervention patients had more severe pancreatic disease and scored higher on other measures of disease severity.
Bottom line: Patients with necrotizing pancreatitis can frequently be managed conservatively, though the presence of organ failure and parenchymal necrosis confer poorer prognosis. When intervention is indicated, postponing intervention and utilizing minimally invasive techniques decrease morbidity and mortality.
Citation: Van Santvoort HC, Bakker OJ, Bollen TL, et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254-1263.
Cardiac MRI Complements Clinical Findings in Diagnosis of Stress Cardiomyopathy
Clinical question: What are the clinical features and cardiovascular MRI findings in patients with stress (Takotsubo) cardiomyopathy?
Background: Stress cardiomyopathy (SC) is characterized by acute and profound reversible left ventricular dysfunction that is thought to result from increased sympathetic activity related to emotional and/or physical stress. Prior studies evaluating the clinical features of SC were limited by small sample sizes and single-center enrollment, and cardiac MRI use in SC has not been well studied.
Study design: Prospective cohort study.
Setting: Seven North American and European tertiary-care centers.
Synopsis: This study enrolled 256 patients who met diagnostic criteria for SC according to Mayo criteria. Postmenopausal women were most commonly affected; only 11% of participants were men and 8% were women younger than 50 years old. An identifiable stressor was found in 71% of the patients. Clinical presentation was notable for symptoms of acute coronary syndrome (ACS) in 88% of patients, abnormal electrocardiogram in 87%, and elevated troponin T in 90%. Coronary angiography was normal in three-fourths of patients, and no patients had features of acute plaque rupture. Typical apical ballooning was seen on left ventriculography in 82% of patients.
Cardiac MRI findings included severe left ventricular dysfunction in a noncoronary distribution, myocardial edema in areas of regional wall abnormalities, absence of high signal areas in late gadolinium enhancement (e.g., absence of necrosis/fibrosis), and increased early gadolinium uptake (i.e. early inflammation). Repeat cardiac MRI four weeks after initial diagnosis showed near or complete resolution of imaging findings.
Bottom line: Stress cardiomyopathy typically presents like ACS, usually affects postmenopausal women, is often preceded by a stressful event, and is characterized by cardiac MRI findings of regional wall motion abnormalities, reversible myocardial injury, and the absence of fibrosis. Cardiac MRI may be valuable in diagnosing SC in patients who present without the classic clinical features.
Citation: Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (Takotsubo) cardiomyopathy. JAMA. 2011;306:277-286.
Increased Risk of Potentially Inadvertent Medication Discontinuation Following Acute-Care Hospitalization
Clinical question: Are medications for chronic diseases inadvertently discontinued after acute-care hospitalization, and is this risk increased in patients who had an ICU stay?
Background: Transitions of care are associated with medical errors. Two such transitions are a shift from the ICU to floor setting and from the inpatient to outpatient setting. Medications for chronic diseases might be held during hospitalization for a variety of reasons, and medication errors may occur if these drugs are not restarted when the acute problem resolves or the patient is discharged from the hospital.
Study design: Population-based cohort study.
Setting: Ontario, Canada.
Synopsis: Using four separate databases, administrative records were reviewed for 396,380 patients aged >65 years who were continuous users of at least one of five evidence-based medication groups for common chronic diseases. Medications included statins, antiplatelet/anticoagulant agents, levothyroxine, respiratory inhalers, and gastric acid suppressants. The primary outcome was potentially unintentional medication discontinuation (measured by failure to renew the prescription at 90 days) for hospitalized versus nonhospitalized patients. All medication groups had statistically significant adjusted odds ratios ranging from 1.18 (95% CI, 1.14-1.23) for discontinuation of levothyroxine to 1.86 (95% CI, 1.77-1.97) for discontinuation of antiplatelet/anticoagulant medications. Treatment in an ICU further increased this risk compared with nonhospitalized patients, and increased the risk for medication discontinuation in four of the five medication groups when compared with patients hospitalized without ICU treatment.
Important study limitations include the lack of appropriate clinical details to classify medication discontinuation as unintentional and the inability of administrative data to prove causality. This study highlights the importance of medication reconciliation and calls attention to inadvertent medication discontinuation during care transitions (see “Reconciliation Act,”).
Bottom line: Patients discharged from the hospital, particularly after ICU treatment, have a higher risk of discontinuation of long-term medications for chronic medical problems when compared with nonhospitalized patients.
Citation: Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306:840-847.
Clinical Exam Remains Valuable in the Diagnosis of Patients Admitted to a Medicine Service
Clinical question: Is a clinical exam useful in the diagnosis of newly admitted patients to a general medicine service?
Background: The clinical exam, which comprises the history and physical examination, has long been described as essential to the diagnosis of illness. However, the literature supporting this claim is limited to the ambulatory setting. There has not been evaluation of the clinical exam as a diagnostic tool in the hospital setting, where more advanced testing is readily available.
Study design: Retrospective chart review.
Setting: Urban academic medical center.
Synopsis: The study included 442 adult patients consecutively admitted from the emergency department to the general medicine service who were separately assessed by one senior resident (SR) and one experienced hospital physician (HP) not involved with the case. The SR and HP each made an initial diagnosis and documented the most helpful component(s) in arriving at that diagnosis. Outcomes included comparison of the SR and HP’s admission diagnosis with the discharge diagnosis, and the diagnostic value of the various components of the clinical exam and initial studies.
Compared with the discharge diagnosis, the SR’s initial diagnosis was correct in 80.1% of cases, while the HP was correct in 84.4%. The patient’s history was the most important element in the initial assessment, independently influencing approximately 20% of the correct diagnoses for both physicians. Approximately 60% of correct diagnoses were established using the history and/or physical, and more than 90% were made using a combination of history, physical exam, and/or basic tests (admission labs, electrocardiogram, chest X-ray).
The generalizability of these results is limited by the retrospective, single-center study design, involvement of only one resident physician, and the lack of information regarding number of experienced clinicians and types of diagnoses.
Bottom line: Among patients admitted to a general medicine service, the most powerful tool in obtaining an accurate diagnosis was the combination of a patient’s history and a physical exam.
Citation: Paley L, Zornitzki T, Cohen J, et al. Utility of clinical examination in the diagnosis of emergency department patients admitted to the department of medicine of an academic hospital. Arch Intern Med. 2011;171:1394-1396.
RDW Predicts All-Cause Mortality and Bloodstream Infection in ICU Patients
Clinical question: Among patients admitted to the ICU, is red blood cell distribution width (RDW) a reliable indicator of mortality?
Background: The RDW is an inexpensive test that is commonly included in routine laboratory studies. It has been associated with multiple disease processes and found to be a strong predictor of mortality in the general adult population. However, there has been limited study of the association between RDW and outcomes in critically ill patients.
Study design: Observational cohort study.
Setting: Urban tertiary-care academic medical center.
Synopsis: Data from 51,413 adult patients who received critical care between 1997 and 2007 were obtained from a computerized registry and evaluated for the primary outcome of 30-day mortality after critical-care initiation. Secondary outcomes included 90-day, 365-day, and in-hospital mortality, as well as bloodstream infection. Logistic regression examined both primary and secondary outcomes in association with pre-established RDW quintiles. After multivariable adjustment, RDW was found to be associated with mortality at 30, 90, and 365 days, in addition to in-hospital mortality. The highest RDW quintile (RDW >15.8%) had an adjusted OR of 2.61 (95% CI, 2.37-2.86; P<0.001) for the primary outcome, with similar risk for secondary outcomes of mortality. A subgroup of 18,525 patients with blood culture data was analyzed and an adjusted OR of 1.44 was found in the highest RDW quintile for the secondary outcome of bloodstream infection.
Bottom line: Red blood cell distribution width is a strong independent predictor of all-cause mortality and bloodstream infection in patients receiving intensive care.
Citation: Bazick HS, Chang D, Mahadevappa K, Gibbons FK, Christopher KB. Red cell distribution width and all-cause mortality in critically ill patients. Crit Care Med. 2011;39:1913-1921.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Procalcitonin to guide antimicrobial use in ICU patients
- Platelet reactivity and event rates after PCI
- Conservative treatment of necrotizing pancreatitis
- Use of MRI to diagnose Takotsubo cardiomyopathy
- Rates of inadvertent medication discontinuation after hospitalization
- Role of history and physical exam in diagnosing medical illness
- Red-cell distribution width and rates of mortality
Procalcitonin-Guided Therapy Decreases Antimicrobial Duration in ICU Patients
Clinical question: Can utilization of serum procalcitonin (PCT) levels safely reduce antimicrobial exposure in ICU patients?
Background: Serum PCT levels are elevated in bacterial infections and sepsis and have been used in some settings to guide antimicrobial therapy. Randomized controlled trials have demonstrated reduction of antibiotic use with PCT measurement. This systematic review assessed the safety and effectiveness of PCT measurements in reducing antimicrobial exposure in ICU patients.
Study design: Systematic review.
Setting: Adult medical and surgical ICUs.
Synopsis: A search of MEDLINE and EMBASE yielded 1,018 publications related to PCT, critically ill patients, and antimicrobial therapy. Six randomized controlled trials involving 1,476 patients were reviewed. The duration of antimicrobial use was significantly decreased in all five studies that evaluated treatment duration. The remaining study only assessed the impact of PCT on initiation of antimicrobial therapy and did not demonstrate decreased antimicrobial exposure. Compared with the control group, patients randomized to PCT-guided therapy had relative reductions in duration of first antibiotic course by 21%, down to 38%, and decreases in days of antimicrobial therapy per 1,000 ICU patient days by 20%, down to 23%. PCT intervention also was associated with a 23% to 37% increase in days alive without antimicrobial therapy during the first 28 days. The length of ICU stay was significantly decreased in two studies but was not significantly different in the other studies. There were no significant differences in rates of mortality, infection relapse, or days free of mechanical ventilation.
Bottom line: PCT guidance reduced antimicrobial exposure of ICU patients without increasing rates of mortality or infection relapse.
Citation: Agarwal R, Schwartz DN. Procalcitonin to guide duration of antimicrobial therapy in intensive care units: a systematic review. Clin Infect Dis. 2011;53:379-387.
High Residual Platelet Reactivity Increases Risk of Cardiovascular Events among Patients with Acute Coronary Syndromes Undergoing PCI
Clinical question: Is high residual platelet reactivity (HRPR) in patients receiving clopidogrel associated with increased risk of ischemic events after percutaneous coronary intervention (PCI)?
Background: Studies have demonstrated an increased risk of cardiovascular events associated with HRPR in patients receiving clopidogrel while undergoing PCI. However, these studies have used a variety of platelet function tests, and thresholds for positive tests have not been established. In addition, these studies have enrolled heterogeneous populations with short-term follow-up and few have included patients with acute coronary syndromes (ACSs).
Study design: Prospective cohort study.
Setting: Cardiology service of a referral center in Italy.
Synopsis: This study included 1,789 consecutive patients with ACS undergoing PCI. Patients were given 325 mg aspirin and a 600-mg loading dose of clopidogrel on admission, followed by a daily maintenance dose of aspirin 325 mg and clopidogrel 75 mg for at least six months. Platelet reactivity was measured using adenosine diphosphate (ADP) testing, and those with HRPR (≥70% platelet aggregation) were given increased dosing of clopidogrel or switched to ticlopidine using ADP test guidance. At two-year follow-up, patients with HRPR experienced an increased composite endpoint of cardiac death, myocardial infarction, urgent coronary revascularization, and stroke with a combined event rate of 14.6% in patients with HRPR and 8.7% in patients with low residual platelet reactivity (95% CI, 1.6%-11.1%; P=0.003). Stent thrombosis was also higher in HRPR patients (absolute risk increase 3.2%; 95% CI, 0.4%-6.7%; P=0.01).
This study is nonrandomized, and residual unmeasured confounding cannot be excluded. In addition, use of non-antiplatelet drugs and adherence to recommended drugs might have influenced outcomes.
Bottom line: HRPR is associated with increased risk of ischemic events in patients with ACS receiving antiplatelet agents after PCI.
Citation: Parodi G, Marcucci R, Valenti R, et al. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA. 2011;306:1215-1223.
Conservative Treatment of Necrotizing Pancreatitis Is Associated with Improved Outcomes
Clinical question: What are the outcomes of conservative and interventional management of necrotizing pancreatitis?
Background: Open necrosectomy was historically the treatment of choice for necrotizing pancreatitis, but, currently, pancreatic necrosis is only managed invasively when complicated by infection. Other changes in management over time have included a shift in the timing of intervention and the use of minimally invasive techniques. Existing studies do not reflect these changes in practice patterns and have been limited by small sample sizes or the exclusion of important subgroups of patients.
Study design: Prospective cohort study.
Setting: Twenty-one Dutch hospitals.
Synopsis: This study included 639 patients with acute necrotizing pancreatitis confirmed by imaging. Overall mortality was 15%. Conservative treatment was performed in 62% of the patients with a mortality of 7%; however, patients with organ failure (pulmonary, circulatory, and/or renal) who received conservative therapy had a mortality rate of 37%. Intervention (percutaneous drainage, video-assisted retroperitoneal debridement, endoscopic transluminal necrosectomy, laparotomy) in patients with suspected or confirmed infected pancreatic necrosis was performed on 38% of the patients with associated mortality of 27%. Interventions performed within the first 14 days of hospitalization resulted in a 56% mortality rate, whereas interventions performed after Day 29 resulted in a 15% mortality rate (P<0.001). Patients with organ failure experienced significantly greater mortality compared with patients with no organ failure (35% vs. 2%; P<0.001). Primary percutaneous drainage was associated with fewer complications than was primary necrosectomy (42% vs. 64%; P=0.003).
This study was nonrandomized, and final decisions regarding management were left to the treating physician. Notably, while there was no significant difference in APACHE II scores between the conservative and intervention groups, intervention patients had more severe pancreatic disease and scored higher on other measures of disease severity.
Bottom line: Patients with necrotizing pancreatitis can frequently be managed conservatively, though the presence of organ failure and parenchymal necrosis confer poorer prognosis. When intervention is indicated, postponing intervention and utilizing minimally invasive techniques decrease morbidity and mortality.
Citation: Van Santvoort HC, Bakker OJ, Bollen TL, et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254-1263.
Cardiac MRI Complements Clinical Findings in Diagnosis of Stress Cardiomyopathy
Clinical question: What are the clinical features and cardiovascular MRI findings in patients with stress (Takotsubo) cardiomyopathy?
Background: Stress cardiomyopathy (SC) is characterized by acute and profound reversible left ventricular dysfunction that is thought to result from increased sympathetic activity related to emotional and/or physical stress. Prior studies evaluating the clinical features of SC were limited by small sample sizes and single-center enrollment, and cardiac MRI use in SC has not been well studied.
Study design: Prospective cohort study.
Setting: Seven North American and European tertiary-care centers.
Synopsis: This study enrolled 256 patients who met diagnostic criteria for SC according to Mayo criteria. Postmenopausal women were most commonly affected; only 11% of participants were men and 8% were women younger than 50 years old. An identifiable stressor was found in 71% of the patients. Clinical presentation was notable for symptoms of acute coronary syndrome (ACS) in 88% of patients, abnormal electrocardiogram in 87%, and elevated troponin T in 90%. Coronary angiography was normal in three-fourths of patients, and no patients had features of acute plaque rupture. Typical apical ballooning was seen on left ventriculography in 82% of patients.
Cardiac MRI findings included severe left ventricular dysfunction in a noncoronary distribution, myocardial edema in areas of regional wall abnormalities, absence of high signal areas in late gadolinium enhancement (e.g., absence of necrosis/fibrosis), and increased early gadolinium uptake (i.e. early inflammation). Repeat cardiac MRI four weeks after initial diagnosis showed near or complete resolution of imaging findings.
Bottom line: Stress cardiomyopathy typically presents like ACS, usually affects postmenopausal women, is often preceded by a stressful event, and is characterized by cardiac MRI findings of regional wall motion abnormalities, reversible myocardial injury, and the absence of fibrosis. Cardiac MRI may be valuable in diagnosing SC in patients who present without the classic clinical features.
Citation: Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (Takotsubo) cardiomyopathy. JAMA. 2011;306:277-286.
Increased Risk of Potentially Inadvertent Medication Discontinuation Following Acute-Care Hospitalization
Clinical question: Are medications for chronic diseases inadvertently discontinued after acute-care hospitalization, and is this risk increased in patients who had an ICU stay?
Background: Transitions of care are associated with medical errors. Two such transitions are a shift from the ICU to floor setting and from the inpatient to outpatient setting. Medications for chronic diseases might be held during hospitalization for a variety of reasons, and medication errors may occur if these drugs are not restarted when the acute problem resolves or the patient is discharged from the hospital.
Study design: Population-based cohort study.
Setting: Ontario, Canada.
Synopsis: Using four separate databases, administrative records were reviewed for 396,380 patients aged >65 years who were continuous users of at least one of five evidence-based medication groups for common chronic diseases. Medications included statins, antiplatelet/anticoagulant agents, levothyroxine, respiratory inhalers, and gastric acid suppressants. The primary outcome was potentially unintentional medication discontinuation (measured by failure to renew the prescription at 90 days) for hospitalized versus nonhospitalized patients. All medication groups had statistically significant adjusted odds ratios ranging from 1.18 (95% CI, 1.14-1.23) for discontinuation of levothyroxine to 1.86 (95% CI, 1.77-1.97) for discontinuation of antiplatelet/anticoagulant medications. Treatment in an ICU further increased this risk compared with nonhospitalized patients, and increased the risk for medication discontinuation in four of the five medication groups when compared with patients hospitalized without ICU treatment.
Important study limitations include the lack of appropriate clinical details to classify medication discontinuation as unintentional and the inability of administrative data to prove causality. This study highlights the importance of medication reconciliation and calls attention to inadvertent medication discontinuation during care transitions (see “Reconciliation Act,”).
Bottom line: Patients discharged from the hospital, particularly after ICU treatment, have a higher risk of discontinuation of long-term medications for chronic medical problems when compared with nonhospitalized patients.
Citation: Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306:840-847.
Clinical Exam Remains Valuable in the Diagnosis of Patients Admitted to a Medicine Service
Clinical question: Is a clinical exam useful in the diagnosis of newly admitted patients to a general medicine service?
Background: The clinical exam, which comprises the history and physical examination, has long been described as essential to the diagnosis of illness. However, the literature supporting this claim is limited to the ambulatory setting. There has not been evaluation of the clinical exam as a diagnostic tool in the hospital setting, where more advanced testing is readily available.
Study design: Retrospective chart review.
Setting: Urban academic medical center.
Synopsis: The study included 442 adult patients consecutively admitted from the emergency department to the general medicine service who were separately assessed by one senior resident (SR) and one experienced hospital physician (HP) not involved with the case. The SR and HP each made an initial diagnosis and documented the most helpful component(s) in arriving at that diagnosis. Outcomes included comparison of the SR and HP’s admission diagnosis with the discharge diagnosis, and the diagnostic value of the various components of the clinical exam and initial studies.
Compared with the discharge diagnosis, the SR’s initial diagnosis was correct in 80.1% of cases, while the HP was correct in 84.4%. The patient’s history was the most important element in the initial assessment, independently influencing approximately 20% of the correct diagnoses for both physicians. Approximately 60% of correct diagnoses were established using the history and/or physical, and more than 90% were made using a combination of history, physical exam, and/or basic tests (admission labs, electrocardiogram, chest X-ray).
The generalizability of these results is limited by the retrospective, single-center study design, involvement of only one resident physician, and the lack of information regarding number of experienced clinicians and types of diagnoses.
Bottom line: Among patients admitted to a general medicine service, the most powerful tool in obtaining an accurate diagnosis was the combination of a patient’s history and a physical exam.
Citation: Paley L, Zornitzki T, Cohen J, et al. Utility of clinical examination in the diagnosis of emergency department patients admitted to the department of medicine of an academic hospital. Arch Intern Med. 2011;171:1394-1396.
RDW Predicts All-Cause Mortality and Bloodstream Infection in ICU Patients
Clinical question: Among patients admitted to the ICU, is red blood cell distribution width (RDW) a reliable indicator of mortality?
Background: The RDW is an inexpensive test that is commonly included in routine laboratory studies. It has been associated with multiple disease processes and found to be a strong predictor of mortality in the general adult population. However, there has been limited study of the association between RDW and outcomes in critically ill patients.
Study design: Observational cohort study.
Setting: Urban tertiary-care academic medical center.
Synopsis: Data from 51,413 adult patients who received critical care between 1997 and 2007 were obtained from a computerized registry and evaluated for the primary outcome of 30-day mortality after critical-care initiation. Secondary outcomes included 90-day, 365-day, and in-hospital mortality, as well as bloodstream infection. Logistic regression examined both primary and secondary outcomes in association with pre-established RDW quintiles. After multivariable adjustment, RDW was found to be associated with mortality at 30, 90, and 365 days, in addition to in-hospital mortality. The highest RDW quintile (RDW >15.8%) had an adjusted OR of 2.61 (95% CI, 2.37-2.86; P<0.001) for the primary outcome, with similar risk for secondary outcomes of mortality. A subgroup of 18,525 patients with blood culture data was analyzed and an adjusted OR of 1.44 was found in the highest RDW quintile for the secondary outcome of bloodstream infection.
Bottom line: Red blood cell distribution width is a strong independent predictor of all-cause mortality and bloodstream infection in patients receiving intensive care.
Citation: Bazick HS, Chang D, Mahadevappa K, Gibbons FK, Christopher KB. Red cell distribution width and all-cause mortality in critically ill patients. Crit Care Med. 2011;39:1913-1921.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Procalcitonin to guide antimicrobial use in ICU patients
- Platelet reactivity and event rates after PCI
- Conservative treatment of necrotizing pancreatitis
- Use of MRI to diagnose Takotsubo cardiomyopathy
- Rates of inadvertent medication discontinuation after hospitalization
- Role of history and physical exam in diagnosing medical illness
- Red-cell distribution width and rates of mortality
Procalcitonin-Guided Therapy Decreases Antimicrobial Duration in ICU Patients
Clinical question: Can utilization of serum procalcitonin (PCT) levels safely reduce antimicrobial exposure in ICU patients?
Background: Serum PCT levels are elevated in bacterial infections and sepsis and have been used in some settings to guide antimicrobial therapy. Randomized controlled trials have demonstrated reduction of antibiotic use with PCT measurement. This systematic review assessed the safety and effectiveness of PCT measurements in reducing antimicrobial exposure in ICU patients.
Study design: Systematic review.
Setting: Adult medical and surgical ICUs.
Synopsis: A search of MEDLINE and EMBASE yielded 1,018 publications related to PCT, critically ill patients, and antimicrobial therapy. Six randomized controlled trials involving 1,476 patients were reviewed. The duration of antimicrobial use was significantly decreased in all five studies that evaluated treatment duration. The remaining study only assessed the impact of PCT on initiation of antimicrobial therapy and did not demonstrate decreased antimicrobial exposure. Compared with the control group, patients randomized to PCT-guided therapy had relative reductions in duration of first antibiotic course by 21%, down to 38%, and decreases in days of antimicrobial therapy per 1,000 ICU patient days by 20%, down to 23%. PCT intervention also was associated with a 23% to 37% increase in days alive without antimicrobial therapy during the first 28 days. The length of ICU stay was significantly decreased in two studies but was not significantly different in the other studies. There were no significant differences in rates of mortality, infection relapse, or days free of mechanical ventilation.
Bottom line: PCT guidance reduced antimicrobial exposure of ICU patients without increasing rates of mortality or infection relapse.
Citation: Agarwal R, Schwartz DN. Procalcitonin to guide duration of antimicrobial therapy in intensive care units: a systematic review. Clin Infect Dis. 2011;53:379-387.
High Residual Platelet Reactivity Increases Risk of Cardiovascular Events among Patients with Acute Coronary Syndromes Undergoing PCI
Clinical question: Is high residual platelet reactivity (HRPR) in patients receiving clopidogrel associated with increased risk of ischemic events after percutaneous coronary intervention (PCI)?
Background: Studies have demonstrated an increased risk of cardiovascular events associated with HRPR in patients receiving clopidogrel while undergoing PCI. However, these studies have used a variety of platelet function tests, and thresholds for positive tests have not been established. In addition, these studies have enrolled heterogeneous populations with short-term follow-up and few have included patients with acute coronary syndromes (ACSs).
Study design: Prospective cohort study.
Setting: Cardiology service of a referral center in Italy.
Synopsis: This study included 1,789 consecutive patients with ACS undergoing PCI. Patients were given 325 mg aspirin and a 600-mg loading dose of clopidogrel on admission, followed by a daily maintenance dose of aspirin 325 mg and clopidogrel 75 mg for at least six months. Platelet reactivity was measured using adenosine diphosphate (ADP) testing, and those with HRPR (≥70% platelet aggregation) were given increased dosing of clopidogrel or switched to ticlopidine using ADP test guidance. At two-year follow-up, patients with HRPR experienced an increased composite endpoint of cardiac death, myocardial infarction, urgent coronary revascularization, and stroke with a combined event rate of 14.6% in patients with HRPR and 8.7% in patients with low residual platelet reactivity (95% CI, 1.6%-11.1%; P=0.003). Stent thrombosis was also higher in HRPR patients (absolute risk increase 3.2%; 95% CI, 0.4%-6.7%; P=0.01).
This study is nonrandomized, and residual unmeasured confounding cannot be excluded. In addition, use of non-antiplatelet drugs and adherence to recommended drugs might have influenced outcomes.
Bottom line: HRPR is associated with increased risk of ischemic events in patients with ACS receiving antiplatelet agents after PCI.
Citation: Parodi G, Marcucci R, Valenti R, et al. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA. 2011;306:1215-1223.
Conservative Treatment of Necrotizing Pancreatitis Is Associated with Improved Outcomes
Clinical question: What are the outcomes of conservative and interventional management of necrotizing pancreatitis?
Background: Open necrosectomy was historically the treatment of choice for necrotizing pancreatitis, but, currently, pancreatic necrosis is only managed invasively when complicated by infection. Other changes in management over time have included a shift in the timing of intervention and the use of minimally invasive techniques. Existing studies do not reflect these changes in practice patterns and have been limited by small sample sizes or the exclusion of important subgroups of patients.
Study design: Prospective cohort study.
Setting: Twenty-one Dutch hospitals.
Synopsis: This study included 639 patients with acute necrotizing pancreatitis confirmed by imaging. Overall mortality was 15%. Conservative treatment was performed in 62% of the patients with a mortality of 7%; however, patients with organ failure (pulmonary, circulatory, and/or renal) who received conservative therapy had a mortality rate of 37%. Intervention (percutaneous drainage, video-assisted retroperitoneal debridement, endoscopic transluminal necrosectomy, laparotomy) in patients with suspected or confirmed infected pancreatic necrosis was performed on 38% of the patients with associated mortality of 27%. Interventions performed within the first 14 days of hospitalization resulted in a 56% mortality rate, whereas interventions performed after Day 29 resulted in a 15% mortality rate (P<0.001). Patients with organ failure experienced significantly greater mortality compared with patients with no organ failure (35% vs. 2%; P<0.001). Primary percutaneous drainage was associated with fewer complications than was primary necrosectomy (42% vs. 64%; P=0.003).
This study was nonrandomized, and final decisions regarding management were left to the treating physician. Notably, while there was no significant difference in APACHE II scores between the conservative and intervention groups, intervention patients had more severe pancreatic disease and scored higher on other measures of disease severity.
Bottom line: Patients with necrotizing pancreatitis can frequently be managed conservatively, though the presence of organ failure and parenchymal necrosis confer poorer prognosis. When intervention is indicated, postponing intervention and utilizing minimally invasive techniques decrease morbidity and mortality.
Citation: Van Santvoort HC, Bakker OJ, Bollen TL, et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254-1263.
Cardiac MRI Complements Clinical Findings in Diagnosis of Stress Cardiomyopathy
Clinical question: What are the clinical features and cardiovascular MRI findings in patients with stress (Takotsubo) cardiomyopathy?
Background: Stress cardiomyopathy (SC) is characterized by acute and profound reversible left ventricular dysfunction that is thought to result from increased sympathetic activity related to emotional and/or physical stress. Prior studies evaluating the clinical features of SC were limited by small sample sizes and single-center enrollment, and cardiac MRI use in SC has not been well studied.
Study design: Prospective cohort study.
Setting: Seven North American and European tertiary-care centers.
Synopsis: This study enrolled 256 patients who met diagnostic criteria for SC according to Mayo criteria. Postmenopausal women were most commonly affected; only 11% of participants were men and 8% were women younger than 50 years old. An identifiable stressor was found in 71% of the patients. Clinical presentation was notable for symptoms of acute coronary syndrome (ACS) in 88% of patients, abnormal electrocardiogram in 87%, and elevated troponin T in 90%. Coronary angiography was normal in three-fourths of patients, and no patients had features of acute plaque rupture. Typical apical ballooning was seen on left ventriculography in 82% of patients.
Cardiac MRI findings included severe left ventricular dysfunction in a noncoronary distribution, myocardial edema in areas of regional wall abnormalities, absence of high signal areas in late gadolinium enhancement (e.g., absence of necrosis/fibrosis), and increased early gadolinium uptake (i.e. early inflammation). Repeat cardiac MRI four weeks after initial diagnosis showed near or complete resolution of imaging findings.
Bottom line: Stress cardiomyopathy typically presents like ACS, usually affects postmenopausal women, is often preceded by a stressful event, and is characterized by cardiac MRI findings of regional wall motion abnormalities, reversible myocardial injury, and the absence of fibrosis. Cardiac MRI may be valuable in diagnosing SC in patients who present without the classic clinical features.
Citation: Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (Takotsubo) cardiomyopathy. JAMA. 2011;306:277-286.
Increased Risk of Potentially Inadvertent Medication Discontinuation Following Acute-Care Hospitalization
Clinical question: Are medications for chronic diseases inadvertently discontinued after acute-care hospitalization, and is this risk increased in patients who had an ICU stay?
Background: Transitions of care are associated with medical errors. Two such transitions are a shift from the ICU to floor setting and from the inpatient to outpatient setting. Medications for chronic diseases might be held during hospitalization for a variety of reasons, and medication errors may occur if these drugs are not restarted when the acute problem resolves or the patient is discharged from the hospital.
Study design: Population-based cohort study.
Setting: Ontario, Canada.
Synopsis: Using four separate databases, administrative records were reviewed for 396,380 patients aged >65 years who were continuous users of at least one of five evidence-based medication groups for common chronic diseases. Medications included statins, antiplatelet/anticoagulant agents, levothyroxine, respiratory inhalers, and gastric acid suppressants. The primary outcome was potentially unintentional medication discontinuation (measured by failure to renew the prescription at 90 days) for hospitalized versus nonhospitalized patients. All medication groups had statistically significant adjusted odds ratios ranging from 1.18 (95% CI, 1.14-1.23) for discontinuation of levothyroxine to 1.86 (95% CI, 1.77-1.97) for discontinuation of antiplatelet/anticoagulant medications. Treatment in an ICU further increased this risk compared with nonhospitalized patients, and increased the risk for medication discontinuation in four of the five medication groups when compared with patients hospitalized without ICU treatment.
Important study limitations include the lack of appropriate clinical details to classify medication discontinuation as unintentional and the inability of administrative data to prove causality. This study highlights the importance of medication reconciliation and calls attention to inadvertent medication discontinuation during care transitions (see “Reconciliation Act,”).
Bottom line: Patients discharged from the hospital, particularly after ICU treatment, have a higher risk of discontinuation of long-term medications for chronic medical problems when compared with nonhospitalized patients.
Citation: Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306:840-847.
Clinical Exam Remains Valuable in the Diagnosis of Patients Admitted to a Medicine Service
Clinical question: Is a clinical exam useful in the diagnosis of newly admitted patients to a general medicine service?
Background: The clinical exam, which comprises the history and physical examination, has long been described as essential to the diagnosis of illness. However, the literature supporting this claim is limited to the ambulatory setting. There has not been evaluation of the clinical exam as a diagnostic tool in the hospital setting, where more advanced testing is readily available.
Study design: Retrospective chart review.
Setting: Urban academic medical center.
Synopsis: The study included 442 adult patients consecutively admitted from the emergency department to the general medicine service who were separately assessed by one senior resident (SR) and one experienced hospital physician (HP) not involved with the case. The SR and HP each made an initial diagnosis and documented the most helpful component(s) in arriving at that diagnosis. Outcomes included comparison of the SR and HP’s admission diagnosis with the discharge diagnosis, and the diagnostic value of the various components of the clinical exam and initial studies.
Compared with the discharge diagnosis, the SR’s initial diagnosis was correct in 80.1% of cases, while the HP was correct in 84.4%. The patient’s history was the most important element in the initial assessment, independently influencing approximately 20% of the correct diagnoses for both physicians. Approximately 60% of correct diagnoses were established using the history and/or physical, and more than 90% were made using a combination of history, physical exam, and/or basic tests (admission labs, electrocardiogram, chest X-ray).
The generalizability of these results is limited by the retrospective, single-center study design, involvement of only one resident physician, and the lack of information regarding number of experienced clinicians and types of diagnoses.
Bottom line: Among patients admitted to a general medicine service, the most powerful tool in obtaining an accurate diagnosis was the combination of a patient’s history and a physical exam.
Citation: Paley L, Zornitzki T, Cohen J, et al. Utility of clinical examination in the diagnosis of emergency department patients admitted to the department of medicine of an academic hospital. Arch Intern Med. 2011;171:1394-1396.
RDW Predicts All-Cause Mortality and Bloodstream Infection in ICU Patients
Clinical question: Among patients admitted to the ICU, is red blood cell distribution width (RDW) a reliable indicator of mortality?
Background: The RDW is an inexpensive test that is commonly included in routine laboratory studies. It has been associated with multiple disease processes and found to be a strong predictor of mortality in the general adult population. However, there has been limited study of the association between RDW and outcomes in critically ill patients.
Study design: Observational cohort study.
Setting: Urban tertiary-care academic medical center.
Synopsis: Data from 51,413 adult patients who received critical care between 1997 and 2007 were obtained from a computerized registry and evaluated for the primary outcome of 30-day mortality after critical-care initiation. Secondary outcomes included 90-day, 365-day, and in-hospital mortality, as well as bloodstream infection. Logistic regression examined both primary and secondary outcomes in association with pre-established RDW quintiles. After multivariable adjustment, RDW was found to be associated with mortality at 30, 90, and 365 days, in addition to in-hospital mortality. The highest RDW quintile (RDW >15.8%) had an adjusted OR of 2.61 (95% CI, 2.37-2.86; P<0.001) for the primary outcome, with similar risk for secondary outcomes of mortality. A subgroup of 18,525 patients with blood culture data was analyzed and an adjusted OR of 1.44 was found in the highest RDW quintile for the secondary outcome of bloodstream infection.
Bottom line: Red blood cell distribution width is a strong independent predictor of all-cause mortality and bloodstream infection in patients receiving intensive care.
Citation: Bazick HS, Chang D, Mahadevappa K, Gibbons FK, Christopher KB. Red cell distribution width and all-cause mortality in critically ill patients. Crit Care Med. 2011;39:1913-1921.
Network Connection
Education and training always will be a major draw for hospitalists attending SHM’s annual meetings, but hospitalists who let their day end after the last educational session are missing some of the meeting’s biggest opportunities for professional growth, according to conference veterans.
Networking, whether in between sessions, on the exhibit floor, at receptions, or after hours, can be a powerful career development tool, according to Jeff Glasheen, MD, SFHM, HM12 course director, and Michael Pistoria, DO, FACP, SFHM, course director for HM13.
“Hospitalists who haven’t attended an SHM annual meeting before are going to be pleasantly surprised by the accessibility and friendliness of faculty and other leaders in the field,” Dr. Pistoria says. “Networking opportunities will be everywhere at HM12. Smart hospitalists will take advantage of them to make connections, find resources, and advance their careers.”
Dr. Pistoria also points to SHM’s special-interest groups as easy ways to quickly find hospitalists with similar interests and establish professional networks.
The impact of networking at the annual meeting extends beyond individual interests, Dr. Glasheen says. Savvy networkers can impact the entire specialty, but they need to show up.
“This is our chance to influence the society—both big ‘S’ and small ‘s.’ And you can’t do that on your couch at home,” he says. “If you care about your field, be at the meeting learning with others, meeting colleagues, and influencing the direction of the field. This field is still new enough, small enough, and undifferentiated enough that you can influence its direction. Your odds of doing that are enhanced with the more people you meet.”
—Michael Pistoria, DO, FACP, SFHM, HM13 course director
Staying true to the “connect” and “collaborate” themes, HM12 will provide unprecedented opportunities to network with other hospitalists from across the country, including new breaks, receptions, and the special-interest groups.
As in years past, the most resourceful hospitalists will find even more informal opportunities, such as time between sessions and unwinding after a full day of meetings. Dr. Pistoria says the after-hours sessions pay off in the form of a support structure that he can rely on for professional advice.
“I remember the relationships that are developed and nurtured during time networking,” he says. “Those same relationships then help me when I am faced with an issue at my institution. I’ve now got built-in ‘consultants’—friends at other institutions that I can email and ask, ‘What are you doing about …’”
He also finds the networking personally rewarding, bridging the gap from professional colleagues to personal friends. “Those hours are where I’ve developed the friendships that I have with other SHM members,” he says.
Brendon Shank is associate vice president of communications for SHM.
Education and training always will be a major draw for hospitalists attending SHM’s annual meetings, but hospitalists who let their day end after the last educational session are missing some of the meeting’s biggest opportunities for professional growth, according to conference veterans.
Networking, whether in between sessions, on the exhibit floor, at receptions, or after hours, can be a powerful career development tool, according to Jeff Glasheen, MD, SFHM, HM12 course director, and Michael Pistoria, DO, FACP, SFHM, course director for HM13.
“Hospitalists who haven’t attended an SHM annual meeting before are going to be pleasantly surprised by the accessibility and friendliness of faculty and other leaders in the field,” Dr. Pistoria says. “Networking opportunities will be everywhere at HM12. Smart hospitalists will take advantage of them to make connections, find resources, and advance their careers.”
Dr. Pistoria also points to SHM’s special-interest groups as easy ways to quickly find hospitalists with similar interests and establish professional networks.
The impact of networking at the annual meeting extends beyond individual interests, Dr. Glasheen says. Savvy networkers can impact the entire specialty, but they need to show up.
“This is our chance to influence the society—both big ‘S’ and small ‘s.’ And you can’t do that on your couch at home,” he says. “If you care about your field, be at the meeting learning with others, meeting colleagues, and influencing the direction of the field. This field is still new enough, small enough, and undifferentiated enough that you can influence its direction. Your odds of doing that are enhanced with the more people you meet.”
—Michael Pistoria, DO, FACP, SFHM, HM13 course director
Staying true to the “connect” and “collaborate” themes, HM12 will provide unprecedented opportunities to network with other hospitalists from across the country, including new breaks, receptions, and the special-interest groups.
As in years past, the most resourceful hospitalists will find even more informal opportunities, such as time between sessions and unwinding after a full day of meetings. Dr. Pistoria says the after-hours sessions pay off in the form of a support structure that he can rely on for professional advice.
“I remember the relationships that are developed and nurtured during time networking,” he says. “Those same relationships then help me when I am faced with an issue at my institution. I’ve now got built-in ‘consultants’—friends at other institutions that I can email and ask, ‘What are you doing about …’”
He also finds the networking personally rewarding, bridging the gap from professional colleagues to personal friends. “Those hours are where I’ve developed the friendships that I have with other SHM members,” he says.
Brendon Shank is associate vice president of communications for SHM.
Education and training always will be a major draw for hospitalists attending SHM’s annual meetings, but hospitalists who let their day end after the last educational session are missing some of the meeting’s biggest opportunities for professional growth, according to conference veterans.
Networking, whether in between sessions, on the exhibit floor, at receptions, or after hours, can be a powerful career development tool, according to Jeff Glasheen, MD, SFHM, HM12 course director, and Michael Pistoria, DO, FACP, SFHM, course director for HM13.
“Hospitalists who haven’t attended an SHM annual meeting before are going to be pleasantly surprised by the accessibility and friendliness of faculty and other leaders in the field,” Dr. Pistoria says. “Networking opportunities will be everywhere at HM12. Smart hospitalists will take advantage of them to make connections, find resources, and advance their careers.”
Dr. Pistoria also points to SHM’s special-interest groups as easy ways to quickly find hospitalists with similar interests and establish professional networks.
The impact of networking at the annual meeting extends beyond individual interests, Dr. Glasheen says. Savvy networkers can impact the entire specialty, but they need to show up.
“This is our chance to influence the society—both big ‘S’ and small ‘s.’ And you can’t do that on your couch at home,” he says. “If you care about your field, be at the meeting learning with others, meeting colleagues, and influencing the direction of the field. This field is still new enough, small enough, and undifferentiated enough that you can influence its direction. Your odds of doing that are enhanced with the more people you meet.”
—Michael Pistoria, DO, FACP, SFHM, HM13 course director
Staying true to the “connect” and “collaborate” themes, HM12 will provide unprecedented opportunities to network with other hospitalists from across the country, including new breaks, receptions, and the special-interest groups.
As in years past, the most resourceful hospitalists will find even more informal opportunities, such as time between sessions and unwinding after a full day of meetings. Dr. Pistoria says the after-hours sessions pay off in the form of a support structure that he can rely on for professional advice.
“I remember the relationships that are developed and nurtured during time networking,” he says. “Those same relationships then help me when I am faced with an issue at my institution. I’ve now got built-in ‘consultants’—friends at other institutions that I can email and ask, ‘What are you doing about …’”
He also finds the networking personally rewarding, bridging the gap from professional colleagues to personal friends. “Those hours are where I’ve developed the friendships that I have with other SHM members,” he says.
Brendon Shank is associate vice president of communications for SHM.
Policy Corner: An Inside Look at the Most Pressing Policy Issues
In early November, the Institute of Medicine (IOM) released a report on the current status of health information technology (HIT). Although the report was developed at the request of the Office of the National Coordinator (ONC), the arm within the Department of Health and Human Services (HHS) responsible for promoting the use of HIT, not everything in the report was positive—and could leave the impression that HIT is not quite as successful as some think.
The report recommends that the ONC should work with the private and public sectors to make comparative user experiences across vendors publicly available.
Many hospitalists have developed significant expertise with HIT, played significant roles in its effective implementation and use, and are acutely aware of implementation pitfalls. This practical experience could be very helpful in working with the ONC to develop solutions. It is for this reason that hospitalists should reach out to the ONC and offer their expertise instead of waiting for the ONC to act.
The report, “Patient Safety and Health IT: Building Safer Systems for Better Care,” did praise HIT’s potential for eventual cost savings and increased patient safety but stopped short of being a ringing endorsement of the pace HM is taking toward implementation initiatives, such as meaningful use. An overall theme of the report is that greater oversight of HIT is needed to protect patients from potential medical errors associated with its use.
A few of the recommendations given by the IOM to achieve a greater level of safety range from the establishment of a mechanism for vendors and users to report health IT-related deaths, injuries, or unsafe conditions to possible FDA regulation of the systems themselves.
Information-sharing and reporting in a nonpunitive environment, as recommended by the IOM, would go a long way when it comes to remedying or avoiding IT-related problems, and hospitalists probably have some ideas about how this could be done.
Unfortunately, IT vendor contracts often prevent the open sharing of information, so working toward doing away with such contract terms might be a worthy step before making a push toward overall FDA regulation and the unintended consequences that may come with it.
At first glance, FDA regulation seems like the easiest solution because the FDA can theoretically control every aspect of what might go wrong with HIT, but at what cost would such regulation come? FDA approval can be long, complicated and expensive. The whole process could result in cutting-edge technology becoming outdated by the time approval is granted or innovations being overlooked entirely because of a negative cost-benefit analysis. Furthermore, the expense associated with FDA approval could in turn increase the cost of already costly electronic health records (EHR).
Despite the myriad problems that can arise if implementation moves too fast, HIT holds promise and has shown success when done well.
SHM is currently working to position hospitalists as a resource for the ONC, so hospitalists with expertise in this area should not hesitate to come forward with ideas on how to make HIT work better and more safely. HIT is not going to go away, so the best option is to help make it better.
In early November, the Institute of Medicine (IOM) released a report on the current status of health information technology (HIT). Although the report was developed at the request of the Office of the National Coordinator (ONC), the arm within the Department of Health and Human Services (HHS) responsible for promoting the use of HIT, not everything in the report was positive—and could leave the impression that HIT is not quite as successful as some think.
The report recommends that the ONC should work with the private and public sectors to make comparative user experiences across vendors publicly available.
Many hospitalists have developed significant expertise with HIT, played significant roles in its effective implementation and use, and are acutely aware of implementation pitfalls. This practical experience could be very helpful in working with the ONC to develop solutions. It is for this reason that hospitalists should reach out to the ONC and offer their expertise instead of waiting for the ONC to act.
The report, “Patient Safety and Health IT: Building Safer Systems for Better Care,” did praise HIT’s potential for eventual cost savings and increased patient safety but stopped short of being a ringing endorsement of the pace HM is taking toward implementation initiatives, such as meaningful use. An overall theme of the report is that greater oversight of HIT is needed to protect patients from potential medical errors associated with its use.
A few of the recommendations given by the IOM to achieve a greater level of safety range from the establishment of a mechanism for vendors and users to report health IT-related deaths, injuries, or unsafe conditions to possible FDA regulation of the systems themselves.
Information-sharing and reporting in a nonpunitive environment, as recommended by the IOM, would go a long way when it comes to remedying or avoiding IT-related problems, and hospitalists probably have some ideas about how this could be done.
Unfortunately, IT vendor contracts often prevent the open sharing of information, so working toward doing away with such contract terms might be a worthy step before making a push toward overall FDA regulation and the unintended consequences that may come with it.
At first glance, FDA regulation seems like the easiest solution because the FDA can theoretically control every aspect of what might go wrong with HIT, but at what cost would such regulation come? FDA approval can be long, complicated and expensive. The whole process could result in cutting-edge technology becoming outdated by the time approval is granted or innovations being overlooked entirely because of a negative cost-benefit analysis. Furthermore, the expense associated with FDA approval could in turn increase the cost of already costly electronic health records (EHR).
Despite the myriad problems that can arise if implementation moves too fast, HIT holds promise and has shown success when done well.
SHM is currently working to position hospitalists as a resource for the ONC, so hospitalists with expertise in this area should not hesitate to come forward with ideas on how to make HIT work better and more safely. HIT is not going to go away, so the best option is to help make it better.
In early November, the Institute of Medicine (IOM) released a report on the current status of health information technology (HIT). Although the report was developed at the request of the Office of the National Coordinator (ONC), the arm within the Department of Health and Human Services (HHS) responsible for promoting the use of HIT, not everything in the report was positive—and could leave the impression that HIT is not quite as successful as some think.
The report recommends that the ONC should work with the private and public sectors to make comparative user experiences across vendors publicly available.
Many hospitalists have developed significant expertise with HIT, played significant roles in its effective implementation and use, and are acutely aware of implementation pitfalls. This practical experience could be very helpful in working with the ONC to develop solutions. It is for this reason that hospitalists should reach out to the ONC and offer their expertise instead of waiting for the ONC to act.
The report, “Patient Safety and Health IT: Building Safer Systems for Better Care,” did praise HIT’s potential for eventual cost savings and increased patient safety but stopped short of being a ringing endorsement of the pace HM is taking toward implementation initiatives, such as meaningful use. An overall theme of the report is that greater oversight of HIT is needed to protect patients from potential medical errors associated with its use.
A few of the recommendations given by the IOM to achieve a greater level of safety range from the establishment of a mechanism for vendors and users to report health IT-related deaths, injuries, or unsafe conditions to possible FDA regulation of the systems themselves.
Information-sharing and reporting in a nonpunitive environment, as recommended by the IOM, would go a long way when it comes to remedying or avoiding IT-related problems, and hospitalists probably have some ideas about how this could be done.
Unfortunately, IT vendor contracts often prevent the open sharing of information, so working toward doing away with such contract terms might be a worthy step before making a push toward overall FDA regulation and the unintended consequences that may come with it.
At first glance, FDA regulation seems like the easiest solution because the FDA can theoretically control every aspect of what might go wrong with HIT, but at what cost would such regulation come? FDA approval can be long, complicated and expensive. The whole process could result in cutting-edge technology becoming outdated by the time approval is granted or innovations being overlooked entirely because of a negative cost-benefit analysis. Furthermore, the expense associated with FDA approval could in turn increase the cost of already costly electronic health records (EHR).
Despite the myriad problems that can arise if implementation moves too fast, HIT holds promise and has shown success when done well.
SHM is currently working to position hospitalists as a resource for the ONC, so hospitalists with expertise in this area should not hesitate to come forward with ideas on how to make HIT work better and more safely. HIT is not going to go away, so the best option is to help make it better.
CODE-H: Optimize Revenue with Improved Coding Education
Documentation and coding are facts of life for every HM group. Yet almost every group knows that it could be doing better, according to Barb Pierce, CCS-P, ACS-EM, of Barb Pierce Coding and Consulting Inc.
“Despite being a highly structured system, effective coding still depends on the acumen and experience of the people doing the coding,” she says. “No hospitalist will get it perfect every time, but everyone can improve through training. And that training can improve the practice’s bottom line.”
The challenges of coding optimization are getting more and more difficult as changes in government reimbursements impact both documentation and coding requirements. The financial and compliance imperatives to code accurately—even as these requirements shift—are the major driver behind SHM’s new remote learning series, CODE-H.
Short for “Coding Optimally by Documenting Effectively for Hospitalists,” CODE-H can improve the confidence that practice leaders and administrators have in their documentation and coding efforts through a comprehensive, eight-month program that includes webinars and a variety of other support resources. Six expert-led webinars will cover basic and more nuanced issues of documentation, coding, and compliance, including:
- Basics of E&M Coding for Hospitalists, Part 1 and Part 2
- Coding for Hospitalists’ Expanding Scope of Services
- Staying Out of Trouble
- Integrating Physician Billing & Hospital DRG Assurance
- Optimizing Performance and Compliance
Subscribers also receive exclusive access to an online learning community, pre- and post-webinar tests to evaluate learning, a library of additional resources, and CME or CEU credits, pending approval.
The program kicks off Feb. 1 with the first webinar, led by Pierce, the series course director. She is a veteran faculty member for SHM’s one-day coding pre-course. The remote learning series, which runs through Aug. 29, is offered as a site-based subscription for up to 10 individuals from the same HM group for $1,200. Additional participants from the same practice can be registered for a modest additional fee.
“Practice groups continue to lose tens of thousands of dollars through inappropriate documentation and coding,” says Leslie Flores, MHA, a partner in Nelson Flores Hospital Medicine Consultants and SHM’s senior advisor for practice management. “Investing in CODE-H is a way to recoup some of those losses and is a great value. A practice can provide eight months of coding education and support for 10 people for about the same cost as sending a single doctor to SHM’s all-day coding course.”
Visit www.hospitalmedicine.org/codeh to subscribe.
Documentation and coding are facts of life for every HM group. Yet almost every group knows that it could be doing better, according to Barb Pierce, CCS-P, ACS-EM, of Barb Pierce Coding and Consulting Inc.
“Despite being a highly structured system, effective coding still depends on the acumen and experience of the people doing the coding,” she says. “No hospitalist will get it perfect every time, but everyone can improve through training. And that training can improve the practice’s bottom line.”
The challenges of coding optimization are getting more and more difficult as changes in government reimbursements impact both documentation and coding requirements. The financial and compliance imperatives to code accurately—even as these requirements shift—are the major driver behind SHM’s new remote learning series, CODE-H.
Short for “Coding Optimally by Documenting Effectively for Hospitalists,” CODE-H can improve the confidence that practice leaders and administrators have in their documentation and coding efforts through a comprehensive, eight-month program that includes webinars and a variety of other support resources. Six expert-led webinars will cover basic and more nuanced issues of documentation, coding, and compliance, including:
- Basics of E&M Coding for Hospitalists, Part 1 and Part 2
- Coding for Hospitalists’ Expanding Scope of Services
- Staying Out of Trouble
- Integrating Physician Billing & Hospital DRG Assurance
- Optimizing Performance and Compliance
Subscribers also receive exclusive access to an online learning community, pre- and post-webinar tests to evaluate learning, a library of additional resources, and CME or CEU credits, pending approval.
The program kicks off Feb. 1 with the first webinar, led by Pierce, the series course director. She is a veteran faculty member for SHM’s one-day coding pre-course. The remote learning series, which runs through Aug. 29, is offered as a site-based subscription for up to 10 individuals from the same HM group for $1,200. Additional participants from the same practice can be registered for a modest additional fee.
“Practice groups continue to lose tens of thousands of dollars through inappropriate documentation and coding,” says Leslie Flores, MHA, a partner in Nelson Flores Hospital Medicine Consultants and SHM’s senior advisor for practice management. “Investing in CODE-H is a way to recoup some of those losses and is a great value. A practice can provide eight months of coding education and support for 10 people for about the same cost as sending a single doctor to SHM’s all-day coding course.”
Visit www.hospitalmedicine.org/codeh to subscribe.
Documentation and coding are facts of life for every HM group. Yet almost every group knows that it could be doing better, according to Barb Pierce, CCS-P, ACS-EM, of Barb Pierce Coding and Consulting Inc.
“Despite being a highly structured system, effective coding still depends on the acumen and experience of the people doing the coding,” she says. “No hospitalist will get it perfect every time, but everyone can improve through training. And that training can improve the practice’s bottom line.”
The challenges of coding optimization are getting more and more difficult as changes in government reimbursements impact both documentation and coding requirements. The financial and compliance imperatives to code accurately—even as these requirements shift—are the major driver behind SHM’s new remote learning series, CODE-H.
Short for “Coding Optimally by Documenting Effectively for Hospitalists,” CODE-H can improve the confidence that practice leaders and administrators have in their documentation and coding efforts through a comprehensive, eight-month program that includes webinars and a variety of other support resources. Six expert-led webinars will cover basic and more nuanced issues of documentation, coding, and compliance, including:
- Basics of E&M Coding for Hospitalists, Part 1 and Part 2
- Coding for Hospitalists’ Expanding Scope of Services
- Staying Out of Trouble
- Integrating Physician Billing & Hospital DRG Assurance
- Optimizing Performance and Compliance
Subscribers also receive exclusive access to an online learning community, pre- and post-webinar tests to evaluate learning, a library of additional resources, and CME or CEU credits, pending approval.
The program kicks off Feb. 1 with the first webinar, led by Pierce, the series course director. She is a veteran faculty member for SHM’s one-day coding pre-course. The remote learning series, which runs through Aug. 29, is offered as a site-based subscription for up to 10 individuals from the same HM group for $1,200. Additional participants from the same practice can be registered for a modest additional fee.
“Practice groups continue to lose tens of thousands of dollars through inappropriate documentation and coding,” says Leslie Flores, MHA, a partner in Nelson Flores Hospital Medicine Consultants and SHM’s senior advisor for practice management. “Investing in CODE-H is a way to recoup some of those losses and is a great value. A practice can provide eight months of coding education and support for 10 people for about the same cost as sending a single doctor to SHM’s all-day coding course.”
Visit www.hospitalmedicine.org/codeh to subscribe.
Survey Insights: Peeking under the Hood of Academic HM
The 2011 State of Hospital Medicine report offers some tantalizing insights into the operation of academic hospital medicine practices and how they compare with their nonacademic peers. Some results are not surprising, such as the fact that academic hospital medicine groups tend to be larger than nonacademic groups, and that compensation and clinical-FTE-adjusted productivity both tend to be lower for academic hospitalists. Interestingly, turnover rates were about the same in academic and nonacademic practices.
Among the more unexpected findings, however, is that academic HM practices tend to employ a higher proportion of women (44%) than nonacademic practices (35%). In addition, academic practices employed a wider range of staffing models, with only 43% of practices using shift-based staffing, compared with 78% of nonacademic respondents. Similarly, only 47% of academic groups provided on-site coverage at night, compared with 81% of nonacademic groups.
Additional differences between the way academic and nonacademic HM groups staff their programs are shown in the table, “Other Staffing Arrangements.” While the use of nurse practitioners and physician assistants (PA) was similar for academic and nonacademic practices, academic groups were much less likely to utilize nocturnists, and far more likely to have a nonphysician first responder at night (resident, nonphysician provider/PA, or other) than nonacademic groups.
It will be interesting to follow these trends over time. Because of new resident work-hour limits that went into effect in July, SHM Practice Analysis Committee (PAC) member Andrew White, MD, expects that there will be very few places that continue to use residents to cross-cover at night. “I suspect most academic centers have or will hire nocturnists,” he says, “but we’ll see.”
On the other hand, PAC member Scarlett Blue, RN, believes that continued growth in HM, coupled with a competitive job market, could result in increased use of nonphysician first responders at night—and in general. “Hospital medicine group leaders who are looking for alternative ways to meet the supply-demand conundrum may find a blended physician-NP/PA team to be one such answer,” she says.
Finally, the clinical services provided by academic HM groups vary from their nonacademic counterparts in some other important ways. Only 25% of academic practices provide care for ICU patients, compared with 78% of nonacademic practices, while 75% of academic groups perform procedures, compared with only 52% of nonacademic groups. And while the overwhelming majority of both academic and nonacademic practices provide surgical comanagement, academic practices were more than twice as likely to provide comanagement for medical subspecialty patients (45%, compared with 20% for nonacademic practices).
PAC member Troy Ahlstrom, MD, explains, tongue-in-cheek, that “academic hospitalists don’t do procedures because they have oodles of residents, fellows, and interventional radiologists to do them instead, and academics do more medical comanagement because the subspecialist who only does Waldenstrom’s macroglobulinemia probably doesn’t do diabetes.”
Whatever the reason, there are meaningful differences between academic and nonacademic HM practices that bear watching over time. You can help us identify and track these differences by ensuring that your group participates in SHM’s annual State of Hospital Medicine survey, launching this month.
The 2011 State of Hospital Medicine report offers some tantalizing insights into the operation of academic hospital medicine practices and how they compare with their nonacademic peers. Some results are not surprising, such as the fact that academic hospital medicine groups tend to be larger than nonacademic groups, and that compensation and clinical-FTE-adjusted productivity both tend to be lower for academic hospitalists. Interestingly, turnover rates were about the same in academic and nonacademic practices.
Among the more unexpected findings, however, is that academic HM practices tend to employ a higher proportion of women (44%) than nonacademic practices (35%). In addition, academic practices employed a wider range of staffing models, with only 43% of practices using shift-based staffing, compared with 78% of nonacademic respondents. Similarly, only 47% of academic groups provided on-site coverage at night, compared with 81% of nonacademic groups.
Additional differences between the way academic and nonacademic HM groups staff their programs are shown in the table, “Other Staffing Arrangements.” While the use of nurse practitioners and physician assistants (PA) was similar for academic and nonacademic practices, academic groups were much less likely to utilize nocturnists, and far more likely to have a nonphysician first responder at night (resident, nonphysician provider/PA, or other) than nonacademic groups.
It will be interesting to follow these trends over time. Because of new resident work-hour limits that went into effect in July, SHM Practice Analysis Committee (PAC) member Andrew White, MD, expects that there will be very few places that continue to use residents to cross-cover at night. “I suspect most academic centers have or will hire nocturnists,” he says, “but we’ll see.”
On the other hand, PAC member Scarlett Blue, RN, believes that continued growth in HM, coupled with a competitive job market, could result in increased use of nonphysician first responders at night—and in general. “Hospital medicine group leaders who are looking for alternative ways to meet the supply-demand conundrum may find a blended physician-NP/PA team to be one such answer,” she says.
Finally, the clinical services provided by academic HM groups vary from their nonacademic counterparts in some other important ways. Only 25% of academic practices provide care for ICU patients, compared with 78% of nonacademic practices, while 75% of academic groups perform procedures, compared with only 52% of nonacademic groups. And while the overwhelming majority of both academic and nonacademic practices provide surgical comanagement, academic practices were more than twice as likely to provide comanagement for medical subspecialty patients (45%, compared with 20% for nonacademic practices).
PAC member Troy Ahlstrom, MD, explains, tongue-in-cheek, that “academic hospitalists don’t do procedures because they have oodles of residents, fellows, and interventional radiologists to do them instead, and academics do more medical comanagement because the subspecialist who only does Waldenstrom’s macroglobulinemia probably doesn’t do diabetes.”
Whatever the reason, there are meaningful differences between academic and nonacademic HM practices that bear watching over time. You can help us identify and track these differences by ensuring that your group participates in SHM’s annual State of Hospital Medicine survey, launching this month.
The 2011 State of Hospital Medicine report offers some tantalizing insights into the operation of academic hospital medicine practices and how they compare with their nonacademic peers. Some results are not surprising, such as the fact that academic hospital medicine groups tend to be larger than nonacademic groups, and that compensation and clinical-FTE-adjusted productivity both tend to be lower for academic hospitalists. Interestingly, turnover rates were about the same in academic and nonacademic practices.
Among the more unexpected findings, however, is that academic HM practices tend to employ a higher proportion of women (44%) than nonacademic practices (35%). In addition, academic practices employed a wider range of staffing models, with only 43% of practices using shift-based staffing, compared with 78% of nonacademic respondents. Similarly, only 47% of academic groups provided on-site coverage at night, compared with 81% of nonacademic groups.
Additional differences between the way academic and nonacademic HM groups staff their programs are shown in the table, “Other Staffing Arrangements.” While the use of nurse practitioners and physician assistants (PA) was similar for academic and nonacademic practices, academic groups were much less likely to utilize nocturnists, and far more likely to have a nonphysician first responder at night (resident, nonphysician provider/PA, or other) than nonacademic groups.
It will be interesting to follow these trends over time. Because of new resident work-hour limits that went into effect in July, SHM Practice Analysis Committee (PAC) member Andrew White, MD, expects that there will be very few places that continue to use residents to cross-cover at night. “I suspect most academic centers have or will hire nocturnists,” he says, “but we’ll see.”
On the other hand, PAC member Scarlett Blue, RN, believes that continued growth in HM, coupled with a competitive job market, could result in increased use of nonphysician first responders at night—and in general. “Hospital medicine group leaders who are looking for alternative ways to meet the supply-demand conundrum may find a blended physician-NP/PA team to be one such answer,” she says.
Finally, the clinical services provided by academic HM groups vary from their nonacademic counterparts in some other important ways. Only 25% of academic practices provide care for ICU patients, compared with 78% of nonacademic practices, while 75% of academic groups perform procedures, compared with only 52% of nonacademic groups. And while the overwhelming majority of both academic and nonacademic practices provide surgical comanagement, academic practices were more than twice as likely to provide comanagement for medical subspecialty patients (45%, compared with 20% for nonacademic practices).
PAC member Troy Ahlstrom, MD, explains, tongue-in-cheek, that “academic hospitalists don’t do procedures because they have oodles of residents, fellows, and interventional radiologists to do them instead, and academics do more medical comanagement because the subspecialist who only does Waldenstrom’s macroglobulinemia probably doesn’t do diabetes.”
Whatever the reason, there are meaningful differences between academic and nonacademic HM practices that bear watching over time. You can help us identify and track these differences by ensuring that your group participates in SHM’s annual State of Hospital Medicine survey, launching this month.