User login
Skin and soft-tissue infections: Classifying and treating a spectrum
Skin and soft-tissue infections (SSTIs) are a common reason for presentation to outpatient practices, emergency rooms, and hospitals.1–5 They account for more than 14 million outpatient visits in the United States each year,1 and visits to the emergency room and admissions to the hospital for them are increasing.2,3 Hospital admissions for SSTIs increased by 29% from 2000 to 2004.3
MORE MRSA NOW, BUT STREPTOCOCCI ARE STILL COMMON
The increase in hospital admissions for SSTIs has been attributed to a rising number of infections with methicillin-resistant Staphylococcus aureus (MRSA).3–5
In addition, strains once seen mostly in the community and other strains that were associated with health care are now being seen more often in both settings. Clinical characteristics do not differ between community-acquired and health-care-associated MRSA, and therefore the distinction between the two is becoming less useful in guiding empiric therapy.6,7
After steadily increasing for several years, the incidence of MRSA has recently stabilized. The US Centers for Disease Control and Prevention maintains a surveillance program and a Web site on MRSA.8
COMPLICATED OR UNCOMPLICATED
The intent of the 1998 guideline was to provide not a clinical framework but rather a guide for industry in designing trials that would include similar groups of infections and therefore be relevant when compared with each other. In 2008, the Anti-Infective Drugs Advisory Committee was convened,11 and subsequently, in August 2010, the FDA released a revision of the guide.12
The revised guidelines specifically exclude many diagnoses, such as bite wounds, bone and joint infections, necrotizing fasciitis, diabetic foot infections, decubitus ulcers, catheter site infections, myonecrosis, and ecthyma gangrenosum. Notably, the word “bacterial” in the title excludes mycobacterial and fungal infections from consideration. The diagnoses that are included include cellulitis, erysipelas, major cutaneous abscess, and burn infections. These are further specified to include 75 cm2 of redness, edema, or induration to standardize the extent of the infection—ie, the infection has to be at least this large or else it is not “complicated.”
The terms “complicated” and “uncomplicated” skin and skin structure infections persist and can be useful adjuncts in describing SSTIs.13–16 However, more specific descriptions of SSTIs based on pathogenesis are more useful to the clinician and are usually the basis for guidelines, such as for preventing surgical site infections or for reducing amputations in diabetic foot infections.
This review will focus on the general categories of SSTI and will not address surgical site infections, pressure ulcers, diabetic foot infections, perirectal wounds, or adjuvant therapies in severe SSTIs, such as negative pressure wound care (vacuum-assisted closure devices) and hyperbaric chambers.
OTHER DISEASES CAN MIMIC SSTIs
SSTIs vary broadly in their location and severity.
Although the classic presentation of erythema, warmth, edema, and tenderness often signals infection, other diseases can mimic SSTIs. Common ones that should be included in the differential diagnosis include gout, thrombophlebitis, deep vein thrombosis, contact dermatitis, carcinoma erysipeloides, drug eruption, and a foreign body reaction.17,18
CLUES FROM THE HISTORY
Wounds. Skin infections are usually precipitated by a break in the skin from a cut, laceration, excoriation, fungal infection, insect or animal bite, or puncture wound.
Impaired response. Patients with diabetes, renal failure, cirrhosis, chronic glucocorticoid use, history of organ transplantation, chronic immunosuppressive therapy, HIV infection, or malnourishment have impaired host responses to infection and are at risk for both more severe infections and recurrent infections. Immunocompromised hosts may also have atypical infections with opportunistic organisms such as Pseudomonas, Proteus, Serratia, Enterobacter, Citrobacter, and anaerobes. Close follow-up of these patients is warranted to ascertain appropriate response to therapy.19
Surgery that includes lymph node dissection or saphenous vein resection for coronary artery bypass can lead to impaired lymphatic drainage and edema, and therefore predisposes patients to SSTIs.
PHYSICAL EXAMINATION
The physical examination should include descriptions of the extent and location of erythema, edema, warmth, and tenderness so that progression or resolution with treatment can be followed in detail.
Crepitus can be felt in gas-forming infections and raises the concern for necrotizing fasciitis and infection with anaerobic organisms such as Clostridium perfringens.
Necrosis can occur in brown recluse spider bites, venous snake bites, or group A streptococcal infections.
Fluctuance indicates fluid and a likely abscess that may need incision and drainage.
Purpura may be present in patients on anticoagulation therapy, but if it is accompanying an SSTI, it also raises the concern for the possibility of sepsis and disseminated intravascular coagulation, especially from streptococcal infections.
Bullae can be seen in impetigo caused by staphylococci or in infection with Vibrio vulnificus or Streptococcus pneumoniae.19
Systemic signs, in addition to fever, can include hypotension and tachycardia, which would prompt closer monitoring and possible hospitalization.
Lymphangitic spread also indicates severe infection.
LABORATORY STUDIES
Simple, localized SSTIs usually do not require laboratory evaluation. Jenkins et al21 recently demonstrated that by using an algorithm for the management of hospitalized patients with cellulitis or cutaneous abscess, they could decrease resource utilization, including laboratory testing, without adversely affecting clinical outcome.
If patients have underlying disease or more extensive infection, then baseline chemistry values, a complete blood cell count, and the C-reactive protein level should be acquired.19 Laboratory findings that suggest more severe disease include low sodium, low bicarbonate (or an anion gap), and high creatinine levels; new anemia; a high or very low white blood cell count; and a high C-reactive protein level. A high C-reactive protein level has been associated with longer hospitalization.22
A score to estimate the risk of necrotizing fasciitis
This tool was developed retrospectively but has been validated prospectively. It has a high sensitivity and a positive predictive value of 92% in patients with a score of six points or more. Its specificity is also high, with a negative predictive value of 96%.20,24
Necrotizing fasciitis has a mortality rate of 23.5%, but this may be reduced to 10% with early detection and prompt surgical intervention.15 Since necrotizing fasciitis is very difficult to diagnose, clinicians must maintain a high level of suspicion and use the LRINEC score to trigger early surgical evaluation. Surgical exploration is the only way to definitively diagnose necrotizing fasciitis.
Blood cultures in some cases
Blood cultures have a low yield and are usually not cost-effective, but they should be obtained in patients who have lymphedema, immune deficiency, fever, pain out of proportion to the findings on examination, tachycardia, or hypotension, as blood cultures are more likely to be positive in more serious infections and can help guide antimicrobial therapy. Blood cultures are also recommended in patients with infections involving specific anatomic sites, such as the mouth and eyes.19
Aspiration, swabs, incision and drainage
Fluid aspirated from abscesses and swabs of debrided ulcerated wounds should be sent for Gram stain and culture. Gram stain and culture have widely varying yields, from less than 5% to 40%, depending on the source and technique.19 Cultures were not routinely obtained before MRSA emerged, but knowing antimicrobial susceptibility is now important to guide antibiotic therapy. Unfortunately, in cellulitis, swabs and aspirates of the leading edge have a low yield of around 10%.25 One prospective study of 25 hospitalized patients did report a higher yield of positive cultures in patients with fever or underlying disease,26 so aspirates may be used in selected cases. In small studies, the yield of punch biopsies was slightly better than that of needle aspirates and was as high as 20% to 30%.27
IMAGING STUDIES
Imaging can be helpful in determining the depth of involvement. Plain radiography can reveal gas or periosteal inflammation and is especially helpful in diabetic foot infections. Ultrasonography can detect abscesses.
Both magnetic resonance imaging (MRI) and computed tomography (CT) are useful to image fascial planes, although MRI is more sensitive. However, in cases of suspected necrotizing fasciitis, imaging should not delay surgical evaluation and debridement or be used as the definitive study. Therefore, the practicality of CT and MRI can be limited.15,16
ANTIMICROBIAL TREATMENT FOR SSTIs IN OUTPATIENTS
For minor skin infections such as impetigo and secondarily infected skin lesions such as eczema, ulcers, or lacerations, mupirocin 2% topical ointment (Bactroban) can be effective.27
For a simple abscess or boil, incision and drainage is the primary treatment, and antibiotics are not needed.
For a complicated abscess or boil. Patients should be given oral or intravenous antibiotic therapy to cover MRSA and, depending on the severity, should be considered for hospitalization if the abscess is associated with severe disease, rapid progression in the presence of associated cellulitis, septic phlebitis, constitutional symptoms, comorbidity (including immunosuppression), or an abscess or boil in an area difficult to drain, such as the face, hands, or genitalia.27
For purulent cellulitis in outpatients, empiric therapy for community-acquired MRSA is recommended, pending culture results. Empiric therapy for streptococcal infection is likely unnecessary. For empiric coverage of community-acquired MRSA in purulent cellulitis, oral antibiotic options include clindamycin (Cleocin), trimethoprim-sulfamethoxazole (Bactrim), doxycycline (Doryx), minocycline (Minocin), and linezolid (Zyvox).
For nonpurulent cellulitis in outpatients, empiric coverage for beta-hemolytic streptococci is warranted. Coverage for community-acquired MRSA should subsequently be added for patients who do not respond to beta-lactam therapy within 48 to 72 hours or who have chills, fever, a new abscess, increasing erythema, or uncontrolled pain.
Options for coverage of both beta-hemolytic streptococci and community-acquired MRSA for outpatient therapy include clindamycin on its own, trimethoprim-sulfamethoxazole or a tetracycline in combination with a beta-lactam, or linezolid on its own.
Increasing rates of resistance to clindamycin, tetracycline, and trimethoprim-sulfamethoxazole in community-acquired MRSA may limit empiric treatment. In areas where resistance is prevalent, culture with antimicrobial susceptibility testing may be required before starting one of these antibiotics.
The use of rifampin (Rifadin) as a single agent is not recommended because resistance is likely to develop. Also, rifampin is not useful as adjunctive therapy, as evidence does not support its efficacy.19,27,29
ANTIMICROBIAL TREATMENT FOR SSTIs IN HOSPITALIZED PATIENTS
For hospitalized patients with a complicated or severe SSTI, empiric therapy for MRSA should be started pending culture results. FDA-approved options are vancomycin, linezolid, daptomycin (Cubicin), tigecycline (Tygacil), and telavancin (Vibativ). Data on clindamycin are very limited in this population. A beta-lactam antibiotic such as cefazolin (Ancef) may be considered in hospitalized patients with nonpurulent cellulitis, and the regimen can be modified to MRSA-active therapy if there is no clinical response. Linezolid, daptomycin, vancomycin, and telavancin have adequate streptococcal coverage in addition to MRSA coverage.
Clindamycin is approved by the FDA for treating serious infections due to S aureus. It has excellent tissue penetration, particularly in bone and abscesses.
Clindamycin resistance in staphylococci can be either constitutive or inducible, and clinicians must be watchful for signs of resistance.
Diarrhea is the most common adverse effect and occurs in up to 20% of patients. Clostridium difficile colitis may occur more frequently with clindamycin than with other oral agents, but it has also has been reported with fluoroquinolones and can be associated with any antibiotic therapy.30
Trimethoprim-sulfamethoxazole is not FDA-approved for treating any staphylococcal infection. However, because 95% to 100% of community-acquired MRSA strains are susceptible to it in vitro, it has become an important option in the outpatient treatment of SSTIs. Caution is advised when using it in elderly patients, particularly those with chronic renal insufficiency, because of an increased risk of hyperkalemia.
Tetracyclines. Doxycycline is FDA-approved for treating SSTIs due to S aureus, although not specifically for MRSA. Minocycline may be an option even when strains are resistant to doxycycline, since it does not induce its own resistance as doxycycline does.
Tigecycline is a glycylcycline (a tetracycline derivative) and is FDA-approved in adults for complicated SSTIs and intra-abdominal infections. It has a large volume of distribution and achieves high concentrations in tissues and low concentrations in serum.
The FDA recently issued a warning to consider alternative agents in patients with serious infections because of higher rates of all-cause mortality noted in phase III and phase IV clinical trials. Due to this warning and the availability of multiple alternatives active against MRSA, tigecycline was not included in the Infectious Diseases Society of America guidelines.31
Linezolid is a synthetic oxazolidinone and is FDA-approved for treating SSTIs and nosocomial pneumonia caused by MRSA. It has 100% oral bioavailability, so parenteral therapy should only be given if there are problems with gastrointestinal absorption or if the patient is unable to take oral medications.
Long-term use of linezolid (> 2 weeks) is limited by hematologic toxicity, especially thrombocytopenia, which occurs more frequently than anemia and neutropenia. Lactic acidosis and peripheral and optic neuropathy are also limiting toxicities. Although myelosuppression is generally reversible, peripheral and optic neuropathy may not be.
Linezolid should not used in patients taking selective serotonin reuptake inhibitors if they cannot stop taking these antidepressant drugs during therapy, as the combination can lead to the serotonin syndrome.
Vancomycin is still the mainstay of parenteral therapy for MRSA infections. However, its efficacy has come into question, with concerns over its slow bactericidal activity and the emergence of resistant strains. The rate of treatment failure is high in those with infection caused by MRSA having minimum inhibitory concentrations of 1 μg/mL or greater. Vancomycin kills staphylococci more slowly than do beta-lactams in vitro and is clearly inferior to beta-lactams for methicillin-sensitive S aureus bacteremia.
Daptomycin is a lipopeptide antibiotic that is FDA-approved for adults with MRSA bacteremia, right-sided infective endocarditis, and complicated SSTI. Elevations in creatinine phosphokinase, which are rarely treatment-limiting, have occurred in patients receiving 6 mg/kg/day but not in those receiving 4 mg/kg/day. Patients should be observed for development of muscle pain or weakness and should have their creatine phosphokinase levels checked weekly, with more frequent monitoring in those with renal insufficiency or who are receiving concomitant statin therapy.
Telavancin is a parenteral lipoglycopeptide that is bactericidal against MRSA. It is FDA-approved for complicated SSTIs in adults. Creatinine levels should be monitored, and the dosage should be adjusted on the basis of creatinine clearance, because nephrotoxicity was more commonly reported among individuals treated with telavancin than among those treated with vancomycin.
Ceftaroline (Teflaro), a fifth-generation cephalosporin, was approved for SSTIs by the FDA in October 2010. It is active against MRSA and gram-negative pathogens.
Cost is a consideration
Cost is a consideration, as it may limit the availability of and access to treatment. In 2008, the expense for 10 days of treatment with generic vancomycin was $183, compared with $1,661 for daptomycin, $1,362 for tigecycline, and $1,560 for linezolid. For outpatient therapy, the contrast was even starker, as generic trimethoprim-sulfamethoxazole cost $9.40 and generic clindamycin cost $95.10.32
INDICATIONS FOR HOSPITALIZATION
Patients who have evidence of tissue necrosis, fever, hypotension, severe pain, altered mental status, an immunocompromised state, or organ failure (respiratory, renal, or hepatic) must be hospitalized.
Although therapy for MRSA is the mainstay of empiric therapy, polymicrobial infections are not uncommon, and gram-negative and anaerobic coverage should be added as appropriate. One study revealed a longer length of stay for hospitalized patients who had inadequate initial empiric coverage.33
Vigilance should be maintained for overlying cellulitis which can mask necrotizing fasciitis, septic joints, or osteomyelitis.
Perianal abscesses and infections, infected decubitus ulcers, and moderate to severe diabetic foot infections are often polymicrobial and warrant coverage for streptococci, MRSA, aerobic gram-negative bacilli, and anaerobes until culture results can guide therapy.
INDICATIONS FOR SURGICAL REFERRAL
Extensive perianal or multiple abscesses may require surgical drainage and debridement.
Surgical site infections should be referred for consideration of opening the incision for drainage.
Necrotizing infections warrant prompt aggressive surgical debridement. Strongly suggestive clinical signs include bullae, crepitus, gas on radiography, hypotension with systolic blood pressure less than 90 mm Hg, or skin necrosis. However, these are late findings, and fewer than 50% of these patients have one of these. Most cases of necrotizing fasciitis originally have an admitting diagnosis of cellulitis and cases of fasciitis are relatively rare, so the diagnosis is easy to miss.15,16 Patients with an LRINEC score of six or more should have prompt surgical evaluation.20,24,34,35
- Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med 2008; 168:1585–1591.
- Pallin DJ, Egan DJ, Pelletier AJ, Espinola JA, Hooper DC, Camargo CA. Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med 2008; 51:291–298.
- Edelsberg J, Taneja C, Zervos M, et al. Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis 2009; 15:1516–1518.
- Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med 2007; 357:380–390.
- Klevens RM, Morrison MA, Nadle J, et al; Active Bacterial Core surveillance (ABCs) MRSA Investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007; 298:1763–1771.
- Chua K, Laurent F, Coombs G, Grayson ML, Howden BP. Antimicrobial resistance: not community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA)! A clinician’s guide to community MRSA—its evolving antimicrobial resistance and implications for therapy. Clin Infect Dis 2011; 52:99–114.
- Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S. aureus infection: a prospective investigation. Clin Infect Dis 2007; 44:471–482.
- Centers for Disease Control and Prevention. MRSA Infections. http://www.cdc.gov/mrsa/statistics/MRSA-Surveillance-Summary.html. Accessed December 14, 2011.
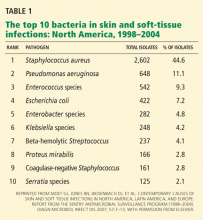
- Moet GJ, Jones RN, Biedenbach DJ, Stilwell MG, Fritsche TR. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998–2004). Diagn Microbiol Infect Dis 2007; 57:7–13.
- US Department of Health and Human Services. Guidance for Industry: Uncomplicated and Complicated Skin and Skin Structure Infections—Developing Antimicrobial Drugs for Treatment (draft guidance). July 1998. http://www.fda.gov/ohrms/dockets/98fr/2566dft.pdf. Accessed September 7, 2011.
- US Food and Drug Administration. CDER 2008 Meeting Documents. Anti-Infective Drugs Advisory Committee. http://www.fda.gov/ohrms/dockets/ac/cder08.html#AntiInfective. Accessed September 7, 2011.
- US Department of Health and Human Services. Guidance for Industry: Acute Bacterial Skin and Skin Structure Infections: Developing Drugs for Treatment (draft guidance). August 2010. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071185.pdf. Accessed December 14, 2011.
- Cornia PB, Davidson HL, Lipsky BA. The evaluation and treatment of complicated skin and skin structure infections. Expert Opin Pharmacother 2008; 9:717–730.
- Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: a review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol 2008; 19:173–184.
- May AK, Stafford RE, Bulger EM, et al; Surgical Infection Society. Treatment of complicated skin and soft tissue infections. Surg Infect (Larchmt) 2009; 10:467–499.
- Napolitano LM. Severe soft tissue infections. Infect Dis Clin North Am 2009; 23:571–591.
- Papadavid E, Dalamaga M, Stavrianeas N, Papiris SA. Subcutaneous sarcoidosis masquerading as cellulitis. Dermatology 2008; 217:212–214.
- Falagas ME, Vergidis PI. Narrative review: diseases that masquerade as infectious cellulitis. Ann Intern Med 2005; 142:47–55.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005; 41:1373–1406.
- Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med 2004; 32:1535–1541.
- Jenkins TC, Knepper BC, Sabel AL, et al. Decreased antibiotic utilization after implementation of a guideline for inpatient cellulitis and cutaneous abscess. Arch Intern Med 2011; 171:1072–1079.
- Lazzarini L, Conti E, Tositti G, de Lalla F. Erysipelas and cellulitis: clinical and microbiological spectrum in an Italian tertiary care hospital. J Infect 2005; 51:383–389.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis 2007; 44:705–710.
- Hasham S, Matteucci P, Stanley PR, Hart NB. Necrotising fasciitis. BMJ 2005; 330:830–833.
- Newell PM, Norden CW. Value of needle aspiration in bacteriologic diagnosis of cellulitis in adults. J Clin Microbiol 1988; 26:401–404.
- Sachs MK. The optimum use of needle aspiration in the bacteriologic diagnosis of cellulitis in adults. Arch Intern Med 1990; 150:1907–1912.
- Liu C, Bayer A, Cosgrove SE, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:e18–e55.
- Hammond SP, Baden LR. Clinical decisions. Management of skin and soft-tissue infection—polling results. N Engl J Med 2008; 359:e20.
- Perlroth J, Kuo M, Tan J, Bayer AS, Miller LG. Adjunctive use of rifampin for the treatment of Staphylococcus aureus infections: a systematic review of the literature. Arch Intern Med 2008; 168:805–819.
- Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect 1998; 40:1–15.
- US Food and Drug Administration. FDA Drug Safety Communication: increased risk of death with Tygacil (tigecycline) compared to other antibiotics used to treat similar infections. September 2010. http://www.fda.gov/Drugs/DrugSafety/ucm224370.htm. Accessed September 7, 2011.
- Moellering RC. A 39-year-old man with a skin infection. JAMA 2008; 299:79–87.
- Zilberberg MD, Shorr AF, Micek ST, et al. Hospitalizations with healthcare-associated complicated skin and skin structure infections: impact of inappropriate empiric therapy on outcomes. J Hosp Med 2010; 5:535–540.
- Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am 2003; 85:1454–1460.
- Hsiao CT, Weng HH, Yuan YD, Chen CT, Chen IC. Predictors of mortality in patients with necrotizing fasciitis. Am J Emerg Med 2008; 26:170–175.
Skin and soft-tissue infections (SSTIs) are a common reason for presentation to outpatient practices, emergency rooms, and hospitals.1–5 They account for more than 14 million outpatient visits in the United States each year,1 and visits to the emergency room and admissions to the hospital for them are increasing.2,3 Hospital admissions for SSTIs increased by 29% from 2000 to 2004.3
MORE MRSA NOW, BUT STREPTOCOCCI ARE STILL COMMON
The increase in hospital admissions for SSTIs has been attributed to a rising number of infections with methicillin-resistant Staphylococcus aureus (MRSA).3–5
In addition, strains once seen mostly in the community and other strains that were associated with health care are now being seen more often in both settings. Clinical characteristics do not differ between community-acquired and health-care-associated MRSA, and therefore the distinction between the two is becoming less useful in guiding empiric therapy.6,7
After steadily increasing for several years, the incidence of MRSA has recently stabilized. The US Centers for Disease Control and Prevention maintains a surveillance program and a Web site on MRSA.8
COMPLICATED OR UNCOMPLICATED
The intent of the 1998 guideline was to provide not a clinical framework but rather a guide for industry in designing trials that would include similar groups of infections and therefore be relevant when compared with each other. In 2008, the Anti-Infective Drugs Advisory Committee was convened,11 and subsequently, in August 2010, the FDA released a revision of the guide.12
The revised guidelines specifically exclude many diagnoses, such as bite wounds, bone and joint infections, necrotizing fasciitis, diabetic foot infections, decubitus ulcers, catheter site infections, myonecrosis, and ecthyma gangrenosum. Notably, the word “bacterial” in the title excludes mycobacterial and fungal infections from consideration. The diagnoses that are included include cellulitis, erysipelas, major cutaneous abscess, and burn infections. These are further specified to include 75 cm2 of redness, edema, or induration to standardize the extent of the infection—ie, the infection has to be at least this large or else it is not “complicated.”
The terms “complicated” and “uncomplicated” skin and skin structure infections persist and can be useful adjuncts in describing SSTIs.13–16 However, more specific descriptions of SSTIs based on pathogenesis are more useful to the clinician and are usually the basis for guidelines, such as for preventing surgical site infections or for reducing amputations in diabetic foot infections.
This review will focus on the general categories of SSTI and will not address surgical site infections, pressure ulcers, diabetic foot infections, perirectal wounds, or adjuvant therapies in severe SSTIs, such as negative pressure wound care (vacuum-assisted closure devices) and hyperbaric chambers.
OTHER DISEASES CAN MIMIC SSTIs
SSTIs vary broadly in their location and severity.
Although the classic presentation of erythema, warmth, edema, and tenderness often signals infection, other diseases can mimic SSTIs. Common ones that should be included in the differential diagnosis include gout, thrombophlebitis, deep vein thrombosis, contact dermatitis, carcinoma erysipeloides, drug eruption, and a foreign body reaction.17,18
CLUES FROM THE HISTORY
Wounds. Skin infections are usually precipitated by a break in the skin from a cut, laceration, excoriation, fungal infection, insect or animal bite, or puncture wound.
Impaired response. Patients with diabetes, renal failure, cirrhosis, chronic glucocorticoid use, history of organ transplantation, chronic immunosuppressive therapy, HIV infection, or malnourishment have impaired host responses to infection and are at risk for both more severe infections and recurrent infections. Immunocompromised hosts may also have atypical infections with opportunistic organisms such as Pseudomonas, Proteus, Serratia, Enterobacter, Citrobacter, and anaerobes. Close follow-up of these patients is warranted to ascertain appropriate response to therapy.19
Surgery that includes lymph node dissection or saphenous vein resection for coronary artery bypass can lead to impaired lymphatic drainage and edema, and therefore predisposes patients to SSTIs.
PHYSICAL EXAMINATION
The physical examination should include descriptions of the extent and location of erythema, edema, warmth, and tenderness so that progression or resolution with treatment can be followed in detail.
Crepitus can be felt in gas-forming infections and raises the concern for necrotizing fasciitis and infection with anaerobic organisms such as Clostridium perfringens.
Necrosis can occur in brown recluse spider bites, venous snake bites, or group A streptococcal infections.
Fluctuance indicates fluid and a likely abscess that may need incision and drainage.
Purpura may be present in patients on anticoagulation therapy, but if it is accompanying an SSTI, it also raises the concern for the possibility of sepsis and disseminated intravascular coagulation, especially from streptococcal infections.
Bullae can be seen in impetigo caused by staphylococci or in infection with Vibrio vulnificus or Streptococcus pneumoniae.19
Systemic signs, in addition to fever, can include hypotension and tachycardia, which would prompt closer monitoring and possible hospitalization.
Lymphangitic spread also indicates severe infection.
LABORATORY STUDIES
Simple, localized SSTIs usually do not require laboratory evaluation. Jenkins et al21 recently demonstrated that by using an algorithm for the management of hospitalized patients with cellulitis or cutaneous abscess, they could decrease resource utilization, including laboratory testing, without adversely affecting clinical outcome.
If patients have underlying disease or more extensive infection, then baseline chemistry values, a complete blood cell count, and the C-reactive protein level should be acquired.19 Laboratory findings that suggest more severe disease include low sodium, low bicarbonate (or an anion gap), and high creatinine levels; new anemia; a high or very low white blood cell count; and a high C-reactive protein level. A high C-reactive protein level has been associated with longer hospitalization.22
A score to estimate the risk of necrotizing fasciitis
This tool was developed retrospectively but has been validated prospectively. It has a high sensitivity and a positive predictive value of 92% in patients with a score of six points or more. Its specificity is also high, with a negative predictive value of 96%.20,24
Necrotizing fasciitis has a mortality rate of 23.5%, but this may be reduced to 10% with early detection and prompt surgical intervention.15 Since necrotizing fasciitis is very difficult to diagnose, clinicians must maintain a high level of suspicion and use the LRINEC score to trigger early surgical evaluation. Surgical exploration is the only way to definitively diagnose necrotizing fasciitis.
Blood cultures in some cases
Blood cultures have a low yield and are usually not cost-effective, but they should be obtained in patients who have lymphedema, immune deficiency, fever, pain out of proportion to the findings on examination, tachycardia, or hypotension, as blood cultures are more likely to be positive in more serious infections and can help guide antimicrobial therapy. Blood cultures are also recommended in patients with infections involving specific anatomic sites, such as the mouth and eyes.19
Aspiration, swabs, incision and drainage
Fluid aspirated from abscesses and swabs of debrided ulcerated wounds should be sent for Gram stain and culture. Gram stain and culture have widely varying yields, from less than 5% to 40%, depending on the source and technique.19 Cultures were not routinely obtained before MRSA emerged, but knowing antimicrobial susceptibility is now important to guide antibiotic therapy. Unfortunately, in cellulitis, swabs and aspirates of the leading edge have a low yield of around 10%.25 One prospective study of 25 hospitalized patients did report a higher yield of positive cultures in patients with fever or underlying disease,26 so aspirates may be used in selected cases. In small studies, the yield of punch biopsies was slightly better than that of needle aspirates and was as high as 20% to 30%.27
IMAGING STUDIES
Imaging can be helpful in determining the depth of involvement. Plain radiography can reveal gas or periosteal inflammation and is especially helpful in diabetic foot infections. Ultrasonography can detect abscesses.
Both magnetic resonance imaging (MRI) and computed tomography (CT) are useful to image fascial planes, although MRI is more sensitive. However, in cases of suspected necrotizing fasciitis, imaging should not delay surgical evaluation and debridement or be used as the definitive study. Therefore, the practicality of CT and MRI can be limited.15,16
ANTIMICROBIAL TREATMENT FOR SSTIs IN OUTPATIENTS
For minor skin infections such as impetigo and secondarily infected skin lesions such as eczema, ulcers, or lacerations, mupirocin 2% topical ointment (Bactroban) can be effective.27
For a simple abscess or boil, incision and drainage is the primary treatment, and antibiotics are not needed.
For a complicated abscess or boil. Patients should be given oral or intravenous antibiotic therapy to cover MRSA and, depending on the severity, should be considered for hospitalization if the abscess is associated with severe disease, rapid progression in the presence of associated cellulitis, septic phlebitis, constitutional symptoms, comorbidity (including immunosuppression), or an abscess or boil in an area difficult to drain, such as the face, hands, or genitalia.27
For purulent cellulitis in outpatients, empiric therapy for community-acquired MRSA is recommended, pending culture results. Empiric therapy for streptococcal infection is likely unnecessary. For empiric coverage of community-acquired MRSA in purulent cellulitis, oral antibiotic options include clindamycin (Cleocin), trimethoprim-sulfamethoxazole (Bactrim), doxycycline (Doryx), minocycline (Minocin), and linezolid (Zyvox).
For nonpurulent cellulitis in outpatients, empiric coverage for beta-hemolytic streptococci is warranted. Coverage for community-acquired MRSA should subsequently be added for patients who do not respond to beta-lactam therapy within 48 to 72 hours or who have chills, fever, a new abscess, increasing erythema, or uncontrolled pain.
Options for coverage of both beta-hemolytic streptococci and community-acquired MRSA for outpatient therapy include clindamycin on its own, trimethoprim-sulfamethoxazole or a tetracycline in combination with a beta-lactam, or linezolid on its own.
Increasing rates of resistance to clindamycin, tetracycline, and trimethoprim-sulfamethoxazole in community-acquired MRSA may limit empiric treatment. In areas where resistance is prevalent, culture with antimicrobial susceptibility testing may be required before starting one of these antibiotics.
The use of rifampin (Rifadin) as a single agent is not recommended because resistance is likely to develop. Also, rifampin is not useful as adjunctive therapy, as evidence does not support its efficacy.19,27,29
ANTIMICROBIAL TREATMENT FOR SSTIs IN HOSPITALIZED PATIENTS
For hospitalized patients with a complicated or severe SSTI, empiric therapy for MRSA should be started pending culture results. FDA-approved options are vancomycin, linezolid, daptomycin (Cubicin), tigecycline (Tygacil), and telavancin (Vibativ). Data on clindamycin are very limited in this population. A beta-lactam antibiotic such as cefazolin (Ancef) may be considered in hospitalized patients with nonpurulent cellulitis, and the regimen can be modified to MRSA-active therapy if there is no clinical response. Linezolid, daptomycin, vancomycin, and telavancin have adequate streptococcal coverage in addition to MRSA coverage.
Clindamycin is approved by the FDA for treating serious infections due to S aureus. It has excellent tissue penetration, particularly in bone and abscesses.
Clindamycin resistance in staphylococci can be either constitutive or inducible, and clinicians must be watchful for signs of resistance.
Diarrhea is the most common adverse effect and occurs in up to 20% of patients. Clostridium difficile colitis may occur more frequently with clindamycin than with other oral agents, but it has also has been reported with fluoroquinolones and can be associated with any antibiotic therapy.30
Trimethoprim-sulfamethoxazole is not FDA-approved for treating any staphylococcal infection. However, because 95% to 100% of community-acquired MRSA strains are susceptible to it in vitro, it has become an important option in the outpatient treatment of SSTIs. Caution is advised when using it in elderly patients, particularly those with chronic renal insufficiency, because of an increased risk of hyperkalemia.
Tetracyclines. Doxycycline is FDA-approved for treating SSTIs due to S aureus, although not specifically for MRSA. Minocycline may be an option even when strains are resistant to doxycycline, since it does not induce its own resistance as doxycycline does.
Tigecycline is a glycylcycline (a tetracycline derivative) and is FDA-approved in adults for complicated SSTIs and intra-abdominal infections. It has a large volume of distribution and achieves high concentrations in tissues and low concentrations in serum.
The FDA recently issued a warning to consider alternative agents in patients with serious infections because of higher rates of all-cause mortality noted in phase III and phase IV clinical trials. Due to this warning and the availability of multiple alternatives active against MRSA, tigecycline was not included in the Infectious Diseases Society of America guidelines.31
Linezolid is a synthetic oxazolidinone and is FDA-approved for treating SSTIs and nosocomial pneumonia caused by MRSA. It has 100% oral bioavailability, so parenteral therapy should only be given if there are problems with gastrointestinal absorption or if the patient is unable to take oral medications.
Long-term use of linezolid (> 2 weeks) is limited by hematologic toxicity, especially thrombocytopenia, which occurs more frequently than anemia and neutropenia. Lactic acidosis and peripheral and optic neuropathy are also limiting toxicities. Although myelosuppression is generally reversible, peripheral and optic neuropathy may not be.
Linezolid should not used in patients taking selective serotonin reuptake inhibitors if they cannot stop taking these antidepressant drugs during therapy, as the combination can lead to the serotonin syndrome.
Vancomycin is still the mainstay of parenteral therapy for MRSA infections. However, its efficacy has come into question, with concerns over its slow bactericidal activity and the emergence of resistant strains. The rate of treatment failure is high in those with infection caused by MRSA having minimum inhibitory concentrations of 1 μg/mL or greater. Vancomycin kills staphylococci more slowly than do beta-lactams in vitro and is clearly inferior to beta-lactams for methicillin-sensitive S aureus bacteremia.
Daptomycin is a lipopeptide antibiotic that is FDA-approved for adults with MRSA bacteremia, right-sided infective endocarditis, and complicated SSTI. Elevations in creatinine phosphokinase, which are rarely treatment-limiting, have occurred in patients receiving 6 mg/kg/day but not in those receiving 4 mg/kg/day. Patients should be observed for development of muscle pain or weakness and should have their creatine phosphokinase levels checked weekly, with more frequent monitoring in those with renal insufficiency or who are receiving concomitant statin therapy.
Telavancin is a parenteral lipoglycopeptide that is bactericidal against MRSA. It is FDA-approved for complicated SSTIs in adults. Creatinine levels should be monitored, and the dosage should be adjusted on the basis of creatinine clearance, because nephrotoxicity was more commonly reported among individuals treated with telavancin than among those treated with vancomycin.
Ceftaroline (Teflaro), a fifth-generation cephalosporin, was approved for SSTIs by the FDA in October 2010. It is active against MRSA and gram-negative pathogens.
Cost is a consideration
Cost is a consideration, as it may limit the availability of and access to treatment. In 2008, the expense for 10 days of treatment with generic vancomycin was $183, compared with $1,661 for daptomycin, $1,362 for tigecycline, and $1,560 for linezolid. For outpatient therapy, the contrast was even starker, as generic trimethoprim-sulfamethoxazole cost $9.40 and generic clindamycin cost $95.10.32
INDICATIONS FOR HOSPITALIZATION
Patients who have evidence of tissue necrosis, fever, hypotension, severe pain, altered mental status, an immunocompromised state, or organ failure (respiratory, renal, or hepatic) must be hospitalized.
Although therapy for MRSA is the mainstay of empiric therapy, polymicrobial infections are not uncommon, and gram-negative and anaerobic coverage should be added as appropriate. One study revealed a longer length of stay for hospitalized patients who had inadequate initial empiric coverage.33
Vigilance should be maintained for overlying cellulitis which can mask necrotizing fasciitis, septic joints, or osteomyelitis.
Perianal abscesses and infections, infected decubitus ulcers, and moderate to severe diabetic foot infections are often polymicrobial and warrant coverage for streptococci, MRSA, aerobic gram-negative bacilli, and anaerobes until culture results can guide therapy.
INDICATIONS FOR SURGICAL REFERRAL
Extensive perianal or multiple abscesses may require surgical drainage and debridement.
Surgical site infections should be referred for consideration of opening the incision for drainage.
Necrotizing infections warrant prompt aggressive surgical debridement. Strongly suggestive clinical signs include bullae, crepitus, gas on radiography, hypotension with systolic blood pressure less than 90 mm Hg, or skin necrosis. However, these are late findings, and fewer than 50% of these patients have one of these. Most cases of necrotizing fasciitis originally have an admitting diagnosis of cellulitis and cases of fasciitis are relatively rare, so the diagnosis is easy to miss.15,16 Patients with an LRINEC score of six or more should have prompt surgical evaluation.20,24,34,35
Skin and soft-tissue infections (SSTIs) are a common reason for presentation to outpatient practices, emergency rooms, and hospitals.1–5 They account for more than 14 million outpatient visits in the United States each year,1 and visits to the emergency room and admissions to the hospital for them are increasing.2,3 Hospital admissions for SSTIs increased by 29% from 2000 to 2004.3
MORE MRSA NOW, BUT STREPTOCOCCI ARE STILL COMMON
The increase in hospital admissions for SSTIs has been attributed to a rising number of infections with methicillin-resistant Staphylococcus aureus (MRSA).3–5
In addition, strains once seen mostly in the community and other strains that were associated with health care are now being seen more often in both settings. Clinical characteristics do not differ between community-acquired and health-care-associated MRSA, and therefore the distinction between the two is becoming less useful in guiding empiric therapy.6,7
After steadily increasing for several years, the incidence of MRSA has recently stabilized. The US Centers for Disease Control and Prevention maintains a surveillance program and a Web site on MRSA.8
COMPLICATED OR UNCOMPLICATED
The intent of the 1998 guideline was to provide not a clinical framework but rather a guide for industry in designing trials that would include similar groups of infections and therefore be relevant when compared with each other. In 2008, the Anti-Infective Drugs Advisory Committee was convened,11 and subsequently, in August 2010, the FDA released a revision of the guide.12
The revised guidelines specifically exclude many diagnoses, such as bite wounds, bone and joint infections, necrotizing fasciitis, diabetic foot infections, decubitus ulcers, catheter site infections, myonecrosis, and ecthyma gangrenosum. Notably, the word “bacterial” in the title excludes mycobacterial and fungal infections from consideration. The diagnoses that are included include cellulitis, erysipelas, major cutaneous abscess, and burn infections. These are further specified to include 75 cm2 of redness, edema, or induration to standardize the extent of the infection—ie, the infection has to be at least this large or else it is not “complicated.”
The terms “complicated” and “uncomplicated” skin and skin structure infections persist and can be useful adjuncts in describing SSTIs.13–16 However, more specific descriptions of SSTIs based on pathogenesis are more useful to the clinician and are usually the basis for guidelines, such as for preventing surgical site infections or for reducing amputations in diabetic foot infections.
This review will focus on the general categories of SSTI and will not address surgical site infections, pressure ulcers, diabetic foot infections, perirectal wounds, or adjuvant therapies in severe SSTIs, such as negative pressure wound care (vacuum-assisted closure devices) and hyperbaric chambers.
OTHER DISEASES CAN MIMIC SSTIs
SSTIs vary broadly in their location and severity.
Although the classic presentation of erythema, warmth, edema, and tenderness often signals infection, other diseases can mimic SSTIs. Common ones that should be included in the differential diagnosis include gout, thrombophlebitis, deep vein thrombosis, contact dermatitis, carcinoma erysipeloides, drug eruption, and a foreign body reaction.17,18
CLUES FROM THE HISTORY
Wounds. Skin infections are usually precipitated by a break in the skin from a cut, laceration, excoriation, fungal infection, insect or animal bite, or puncture wound.
Impaired response. Patients with diabetes, renal failure, cirrhosis, chronic glucocorticoid use, history of organ transplantation, chronic immunosuppressive therapy, HIV infection, or malnourishment have impaired host responses to infection and are at risk for both more severe infections and recurrent infections. Immunocompromised hosts may also have atypical infections with opportunistic organisms such as Pseudomonas, Proteus, Serratia, Enterobacter, Citrobacter, and anaerobes. Close follow-up of these patients is warranted to ascertain appropriate response to therapy.19
Surgery that includes lymph node dissection or saphenous vein resection for coronary artery bypass can lead to impaired lymphatic drainage and edema, and therefore predisposes patients to SSTIs.
PHYSICAL EXAMINATION
The physical examination should include descriptions of the extent and location of erythema, edema, warmth, and tenderness so that progression or resolution with treatment can be followed in detail.
Crepitus can be felt in gas-forming infections and raises the concern for necrotizing fasciitis and infection with anaerobic organisms such as Clostridium perfringens.
Necrosis can occur in brown recluse spider bites, venous snake bites, or group A streptococcal infections.
Fluctuance indicates fluid and a likely abscess that may need incision and drainage.
Purpura may be present in patients on anticoagulation therapy, but if it is accompanying an SSTI, it also raises the concern for the possibility of sepsis and disseminated intravascular coagulation, especially from streptococcal infections.
Bullae can be seen in impetigo caused by staphylococci or in infection with Vibrio vulnificus or Streptococcus pneumoniae.19
Systemic signs, in addition to fever, can include hypotension and tachycardia, which would prompt closer monitoring and possible hospitalization.
Lymphangitic spread also indicates severe infection.
LABORATORY STUDIES
Simple, localized SSTIs usually do not require laboratory evaluation. Jenkins et al21 recently demonstrated that by using an algorithm for the management of hospitalized patients with cellulitis or cutaneous abscess, they could decrease resource utilization, including laboratory testing, without adversely affecting clinical outcome.
If patients have underlying disease or more extensive infection, then baseline chemistry values, a complete blood cell count, and the C-reactive protein level should be acquired.19 Laboratory findings that suggest more severe disease include low sodium, low bicarbonate (or an anion gap), and high creatinine levels; new anemia; a high or very low white blood cell count; and a high C-reactive protein level. A high C-reactive protein level has been associated with longer hospitalization.22
A score to estimate the risk of necrotizing fasciitis
This tool was developed retrospectively but has been validated prospectively. It has a high sensitivity and a positive predictive value of 92% in patients with a score of six points or more. Its specificity is also high, with a negative predictive value of 96%.20,24
Necrotizing fasciitis has a mortality rate of 23.5%, but this may be reduced to 10% with early detection and prompt surgical intervention.15 Since necrotizing fasciitis is very difficult to diagnose, clinicians must maintain a high level of suspicion and use the LRINEC score to trigger early surgical evaluation. Surgical exploration is the only way to definitively diagnose necrotizing fasciitis.
Blood cultures in some cases
Blood cultures have a low yield and are usually not cost-effective, but they should be obtained in patients who have lymphedema, immune deficiency, fever, pain out of proportion to the findings on examination, tachycardia, or hypotension, as blood cultures are more likely to be positive in more serious infections and can help guide antimicrobial therapy. Blood cultures are also recommended in patients with infections involving specific anatomic sites, such as the mouth and eyes.19
Aspiration, swabs, incision and drainage
Fluid aspirated from abscesses and swabs of debrided ulcerated wounds should be sent for Gram stain and culture. Gram stain and culture have widely varying yields, from less than 5% to 40%, depending on the source and technique.19 Cultures were not routinely obtained before MRSA emerged, but knowing antimicrobial susceptibility is now important to guide antibiotic therapy. Unfortunately, in cellulitis, swabs and aspirates of the leading edge have a low yield of around 10%.25 One prospective study of 25 hospitalized patients did report a higher yield of positive cultures in patients with fever or underlying disease,26 so aspirates may be used in selected cases. In small studies, the yield of punch biopsies was slightly better than that of needle aspirates and was as high as 20% to 30%.27
IMAGING STUDIES
Imaging can be helpful in determining the depth of involvement. Plain radiography can reveal gas or periosteal inflammation and is especially helpful in diabetic foot infections. Ultrasonography can detect abscesses.
Both magnetic resonance imaging (MRI) and computed tomography (CT) are useful to image fascial planes, although MRI is more sensitive. However, in cases of suspected necrotizing fasciitis, imaging should not delay surgical evaluation and debridement or be used as the definitive study. Therefore, the practicality of CT and MRI can be limited.15,16
ANTIMICROBIAL TREATMENT FOR SSTIs IN OUTPATIENTS
For minor skin infections such as impetigo and secondarily infected skin lesions such as eczema, ulcers, or lacerations, mupirocin 2% topical ointment (Bactroban) can be effective.27
For a simple abscess or boil, incision and drainage is the primary treatment, and antibiotics are not needed.
For a complicated abscess or boil. Patients should be given oral or intravenous antibiotic therapy to cover MRSA and, depending on the severity, should be considered for hospitalization if the abscess is associated with severe disease, rapid progression in the presence of associated cellulitis, septic phlebitis, constitutional symptoms, comorbidity (including immunosuppression), or an abscess or boil in an area difficult to drain, such as the face, hands, or genitalia.27
For purulent cellulitis in outpatients, empiric therapy for community-acquired MRSA is recommended, pending culture results. Empiric therapy for streptococcal infection is likely unnecessary. For empiric coverage of community-acquired MRSA in purulent cellulitis, oral antibiotic options include clindamycin (Cleocin), trimethoprim-sulfamethoxazole (Bactrim), doxycycline (Doryx), minocycline (Minocin), and linezolid (Zyvox).
For nonpurulent cellulitis in outpatients, empiric coverage for beta-hemolytic streptococci is warranted. Coverage for community-acquired MRSA should subsequently be added for patients who do not respond to beta-lactam therapy within 48 to 72 hours or who have chills, fever, a new abscess, increasing erythema, or uncontrolled pain.
Options for coverage of both beta-hemolytic streptococci and community-acquired MRSA for outpatient therapy include clindamycin on its own, trimethoprim-sulfamethoxazole or a tetracycline in combination with a beta-lactam, or linezolid on its own.
Increasing rates of resistance to clindamycin, tetracycline, and trimethoprim-sulfamethoxazole in community-acquired MRSA may limit empiric treatment. In areas where resistance is prevalent, culture with antimicrobial susceptibility testing may be required before starting one of these antibiotics.
The use of rifampin (Rifadin) as a single agent is not recommended because resistance is likely to develop. Also, rifampin is not useful as adjunctive therapy, as evidence does not support its efficacy.19,27,29
ANTIMICROBIAL TREATMENT FOR SSTIs IN HOSPITALIZED PATIENTS
For hospitalized patients with a complicated or severe SSTI, empiric therapy for MRSA should be started pending culture results. FDA-approved options are vancomycin, linezolid, daptomycin (Cubicin), tigecycline (Tygacil), and telavancin (Vibativ). Data on clindamycin are very limited in this population. A beta-lactam antibiotic such as cefazolin (Ancef) may be considered in hospitalized patients with nonpurulent cellulitis, and the regimen can be modified to MRSA-active therapy if there is no clinical response. Linezolid, daptomycin, vancomycin, and telavancin have adequate streptococcal coverage in addition to MRSA coverage.
Clindamycin is approved by the FDA for treating serious infections due to S aureus. It has excellent tissue penetration, particularly in bone and abscesses.
Clindamycin resistance in staphylococci can be either constitutive or inducible, and clinicians must be watchful for signs of resistance.
Diarrhea is the most common adverse effect and occurs in up to 20% of patients. Clostridium difficile colitis may occur more frequently with clindamycin than with other oral agents, but it has also has been reported with fluoroquinolones and can be associated with any antibiotic therapy.30
Trimethoprim-sulfamethoxazole is not FDA-approved for treating any staphylococcal infection. However, because 95% to 100% of community-acquired MRSA strains are susceptible to it in vitro, it has become an important option in the outpatient treatment of SSTIs. Caution is advised when using it in elderly patients, particularly those with chronic renal insufficiency, because of an increased risk of hyperkalemia.
Tetracyclines. Doxycycline is FDA-approved for treating SSTIs due to S aureus, although not specifically for MRSA. Minocycline may be an option even when strains are resistant to doxycycline, since it does not induce its own resistance as doxycycline does.
Tigecycline is a glycylcycline (a tetracycline derivative) and is FDA-approved in adults for complicated SSTIs and intra-abdominal infections. It has a large volume of distribution and achieves high concentrations in tissues and low concentrations in serum.
The FDA recently issued a warning to consider alternative agents in patients with serious infections because of higher rates of all-cause mortality noted in phase III and phase IV clinical trials. Due to this warning and the availability of multiple alternatives active against MRSA, tigecycline was not included in the Infectious Diseases Society of America guidelines.31
Linezolid is a synthetic oxazolidinone and is FDA-approved for treating SSTIs and nosocomial pneumonia caused by MRSA. It has 100% oral bioavailability, so parenteral therapy should only be given if there are problems with gastrointestinal absorption or if the patient is unable to take oral medications.
Long-term use of linezolid (> 2 weeks) is limited by hematologic toxicity, especially thrombocytopenia, which occurs more frequently than anemia and neutropenia. Lactic acidosis and peripheral and optic neuropathy are also limiting toxicities. Although myelosuppression is generally reversible, peripheral and optic neuropathy may not be.
Linezolid should not used in patients taking selective serotonin reuptake inhibitors if they cannot stop taking these antidepressant drugs during therapy, as the combination can lead to the serotonin syndrome.
Vancomycin is still the mainstay of parenteral therapy for MRSA infections. However, its efficacy has come into question, with concerns over its slow bactericidal activity and the emergence of resistant strains. The rate of treatment failure is high in those with infection caused by MRSA having minimum inhibitory concentrations of 1 μg/mL or greater. Vancomycin kills staphylococci more slowly than do beta-lactams in vitro and is clearly inferior to beta-lactams for methicillin-sensitive S aureus bacteremia.
Daptomycin is a lipopeptide antibiotic that is FDA-approved for adults with MRSA bacteremia, right-sided infective endocarditis, and complicated SSTI. Elevations in creatinine phosphokinase, which are rarely treatment-limiting, have occurred in patients receiving 6 mg/kg/day but not in those receiving 4 mg/kg/day. Patients should be observed for development of muscle pain or weakness and should have their creatine phosphokinase levels checked weekly, with more frequent monitoring in those with renal insufficiency or who are receiving concomitant statin therapy.
Telavancin is a parenteral lipoglycopeptide that is bactericidal against MRSA. It is FDA-approved for complicated SSTIs in adults. Creatinine levels should be monitored, and the dosage should be adjusted on the basis of creatinine clearance, because nephrotoxicity was more commonly reported among individuals treated with telavancin than among those treated with vancomycin.
Ceftaroline (Teflaro), a fifth-generation cephalosporin, was approved for SSTIs by the FDA in October 2010. It is active against MRSA and gram-negative pathogens.
Cost is a consideration
Cost is a consideration, as it may limit the availability of and access to treatment. In 2008, the expense for 10 days of treatment with generic vancomycin was $183, compared with $1,661 for daptomycin, $1,362 for tigecycline, and $1,560 for linezolid. For outpatient therapy, the contrast was even starker, as generic trimethoprim-sulfamethoxazole cost $9.40 and generic clindamycin cost $95.10.32
INDICATIONS FOR HOSPITALIZATION
Patients who have evidence of tissue necrosis, fever, hypotension, severe pain, altered mental status, an immunocompromised state, or organ failure (respiratory, renal, or hepatic) must be hospitalized.
Although therapy for MRSA is the mainstay of empiric therapy, polymicrobial infections are not uncommon, and gram-negative and anaerobic coverage should be added as appropriate. One study revealed a longer length of stay for hospitalized patients who had inadequate initial empiric coverage.33
Vigilance should be maintained for overlying cellulitis which can mask necrotizing fasciitis, septic joints, or osteomyelitis.
Perianal abscesses and infections, infected decubitus ulcers, and moderate to severe diabetic foot infections are often polymicrobial and warrant coverage for streptococci, MRSA, aerobic gram-negative bacilli, and anaerobes until culture results can guide therapy.
INDICATIONS FOR SURGICAL REFERRAL
Extensive perianal or multiple abscesses may require surgical drainage and debridement.
Surgical site infections should be referred for consideration of opening the incision for drainage.
Necrotizing infections warrant prompt aggressive surgical debridement. Strongly suggestive clinical signs include bullae, crepitus, gas on radiography, hypotension with systolic blood pressure less than 90 mm Hg, or skin necrosis. However, these are late findings, and fewer than 50% of these patients have one of these. Most cases of necrotizing fasciitis originally have an admitting diagnosis of cellulitis and cases of fasciitis are relatively rare, so the diagnosis is easy to miss.15,16 Patients with an LRINEC score of six or more should have prompt surgical evaluation.20,24,34,35
- Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med 2008; 168:1585–1591.
- Pallin DJ, Egan DJ, Pelletier AJ, Espinola JA, Hooper DC, Camargo CA. Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med 2008; 51:291–298.
- Edelsberg J, Taneja C, Zervos M, et al. Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis 2009; 15:1516–1518.
- Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med 2007; 357:380–390.
- Klevens RM, Morrison MA, Nadle J, et al; Active Bacterial Core surveillance (ABCs) MRSA Investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007; 298:1763–1771.
- Chua K, Laurent F, Coombs G, Grayson ML, Howden BP. Antimicrobial resistance: not community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA)! A clinician’s guide to community MRSA—its evolving antimicrobial resistance and implications for therapy. Clin Infect Dis 2011; 52:99–114.
- Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S. aureus infection: a prospective investigation. Clin Infect Dis 2007; 44:471–482.
- Centers for Disease Control and Prevention. MRSA Infections. http://www.cdc.gov/mrsa/statistics/MRSA-Surveillance-Summary.html. Accessed December 14, 2011.
- Moet GJ, Jones RN, Biedenbach DJ, Stilwell MG, Fritsche TR. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998–2004). Diagn Microbiol Infect Dis 2007; 57:7–13.
- US Department of Health and Human Services. Guidance for Industry: Uncomplicated and Complicated Skin and Skin Structure Infections—Developing Antimicrobial Drugs for Treatment (draft guidance). July 1998. http://www.fda.gov/ohrms/dockets/98fr/2566dft.pdf. Accessed September 7, 2011.
- US Food and Drug Administration. CDER 2008 Meeting Documents. Anti-Infective Drugs Advisory Committee. http://www.fda.gov/ohrms/dockets/ac/cder08.html#AntiInfective. Accessed September 7, 2011.
- US Department of Health and Human Services. Guidance for Industry: Acute Bacterial Skin and Skin Structure Infections: Developing Drugs for Treatment (draft guidance). August 2010. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071185.pdf. Accessed December 14, 2011.
- Cornia PB, Davidson HL, Lipsky BA. The evaluation and treatment of complicated skin and skin structure infections. Expert Opin Pharmacother 2008; 9:717–730.
- Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: a review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol 2008; 19:173–184.
- May AK, Stafford RE, Bulger EM, et al; Surgical Infection Society. Treatment of complicated skin and soft tissue infections. Surg Infect (Larchmt) 2009; 10:467–499.
- Napolitano LM. Severe soft tissue infections. Infect Dis Clin North Am 2009; 23:571–591.
- Papadavid E, Dalamaga M, Stavrianeas N, Papiris SA. Subcutaneous sarcoidosis masquerading as cellulitis. Dermatology 2008; 217:212–214.
- Falagas ME, Vergidis PI. Narrative review: diseases that masquerade as infectious cellulitis. Ann Intern Med 2005; 142:47–55.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005; 41:1373–1406.
- Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med 2004; 32:1535–1541.
- Jenkins TC, Knepper BC, Sabel AL, et al. Decreased antibiotic utilization after implementation of a guideline for inpatient cellulitis and cutaneous abscess. Arch Intern Med 2011; 171:1072–1079.
- Lazzarini L, Conti E, Tositti G, de Lalla F. Erysipelas and cellulitis: clinical and microbiological spectrum in an Italian tertiary care hospital. J Infect 2005; 51:383–389.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis 2007; 44:705–710.
- Hasham S, Matteucci P, Stanley PR, Hart NB. Necrotising fasciitis. BMJ 2005; 330:830–833.
- Newell PM, Norden CW. Value of needle aspiration in bacteriologic diagnosis of cellulitis in adults. J Clin Microbiol 1988; 26:401–404.
- Sachs MK. The optimum use of needle aspiration in the bacteriologic diagnosis of cellulitis in adults. Arch Intern Med 1990; 150:1907–1912.
- Liu C, Bayer A, Cosgrove SE, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:e18–e55.
- Hammond SP, Baden LR. Clinical decisions. Management of skin and soft-tissue infection—polling results. N Engl J Med 2008; 359:e20.
- Perlroth J, Kuo M, Tan J, Bayer AS, Miller LG. Adjunctive use of rifampin for the treatment of Staphylococcus aureus infections: a systematic review of the literature. Arch Intern Med 2008; 168:805–819.
- Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect 1998; 40:1–15.
- US Food and Drug Administration. FDA Drug Safety Communication: increased risk of death with Tygacil (tigecycline) compared to other antibiotics used to treat similar infections. September 2010. http://www.fda.gov/Drugs/DrugSafety/ucm224370.htm. Accessed September 7, 2011.
- Moellering RC. A 39-year-old man with a skin infection. JAMA 2008; 299:79–87.
- Zilberberg MD, Shorr AF, Micek ST, et al. Hospitalizations with healthcare-associated complicated skin and skin structure infections: impact of inappropriate empiric therapy on outcomes. J Hosp Med 2010; 5:535–540.
- Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am 2003; 85:1454–1460.
- Hsiao CT, Weng HH, Yuan YD, Chen CT, Chen IC. Predictors of mortality in patients with necrotizing fasciitis. Am J Emerg Med 2008; 26:170–175.
- Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med 2008; 168:1585–1591.
- Pallin DJ, Egan DJ, Pelletier AJ, Espinola JA, Hooper DC, Camargo CA. Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med 2008; 51:291–298.
- Edelsberg J, Taneja C, Zervos M, et al. Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis 2009; 15:1516–1518.
- Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med 2007; 357:380–390.
- Klevens RM, Morrison MA, Nadle J, et al; Active Bacterial Core surveillance (ABCs) MRSA Investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007; 298:1763–1771.
- Chua K, Laurent F, Coombs G, Grayson ML, Howden BP. Antimicrobial resistance: not community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA)! A clinician’s guide to community MRSA—its evolving antimicrobial resistance and implications for therapy. Clin Infect Dis 2011; 52:99–114.
- Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S. aureus infection: a prospective investigation. Clin Infect Dis 2007; 44:471–482.
- Centers for Disease Control and Prevention. MRSA Infections. http://www.cdc.gov/mrsa/statistics/MRSA-Surveillance-Summary.html. Accessed December 14, 2011.
- Moet GJ, Jones RN, Biedenbach DJ, Stilwell MG, Fritsche TR. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998–2004). Diagn Microbiol Infect Dis 2007; 57:7–13.
- US Department of Health and Human Services. Guidance for Industry: Uncomplicated and Complicated Skin and Skin Structure Infections—Developing Antimicrobial Drugs for Treatment (draft guidance). July 1998. http://www.fda.gov/ohrms/dockets/98fr/2566dft.pdf. Accessed September 7, 2011.
- US Food and Drug Administration. CDER 2008 Meeting Documents. Anti-Infective Drugs Advisory Committee. http://www.fda.gov/ohrms/dockets/ac/cder08.html#AntiInfective. Accessed September 7, 2011.
- US Department of Health and Human Services. Guidance for Industry: Acute Bacterial Skin and Skin Structure Infections: Developing Drugs for Treatment (draft guidance). August 2010. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071185.pdf. Accessed December 14, 2011.
- Cornia PB, Davidson HL, Lipsky BA. The evaluation and treatment of complicated skin and skin structure infections. Expert Opin Pharmacother 2008; 9:717–730.
- Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: a review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol 2008; 19:173–184.
- May AK, Stafford RE, Bulger EM, et al; Surgical Infection Society. Treatment of complicated skin and soft tissue infections. Surg Infect (Larchmt) 2009; 10:467–499.
- Napolitano LM. Severe soft tissue infections. Infect Dis Clin North Am 2009; 23:571–591.
- Papadavid E, Dalamaga M, Stavrianeas N, Papiris SA. Subcutaneous sarcoidosis masquerading as cellulitis. Dermatology 2008; 217:212–214.
- Falagas ME, Vergidis PI. Narrative review: diseases that masquerade as infectious cellulitis. Ann Intern Med 2005; 142:47–55.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005; 41:1373–1406.
- Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med 2004; 32:1535–1541.
- Jenkins TC, Knepper BC, Sabel AL, et al. Decreased antibiotic utilization after implementation of a guideline for inpatient cellulitis and cutaneous abscess. Arch Intern Med 2011; 171:1072–1079.
- Lazzarini L, Conti E, Tositti G, de Lalla F. Erysipelas and cellulitis: clinical and microbiological spectrum in an Italian tertiary care hospital. J Infect 2005; 51:383–389.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis 2007; 44:705–710.
- Hasham S, Matteucci P, Stanley PR, Hart NB. Necrotising fasciitis. BMJ 2005; 330:830–833.
- Newell PM, Norden CW. Value of needle aspiration in bacteriologic diagnosis of cellulitis in adults. J Clin Microbiol 1988; 26:401–404.
- Sachs MK. The optimum use of needle aspiration in the bacteriologic diagnosis of cellulitis in adults. Arch Intern Med 1990; 150:1907–1912.
- Liu C, Bayer A, Cosgrove SE, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:e18–e55.
- Hammond SP, Baden LR. Clinical decisions. Management of skin and soft-tissue infection—polling results. N Engl J Med 2008; 359:e20.
- Perlroth J, Kuo M, Tan J, Bayer AS, Miller LG. Adjunctive use of rifampin for the treatment of Staphylococcus aureus infections: a systematic review of the literature. Arch Intern Med 2008; 168:805–819.
- Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect 1998; 40:1–15.
- US Food and Drug Administration. FDA Drug Safety Communication: increased risk of death with Tygacil (tigecycline) compared to other antibiotics used to treat similar infections. September 2010. http://www.fda.gov/Drugs/DrugSafety/ucm224370.htm. Accessed September 7, 2011.
- Moellering RC. A 39-year-old man with a skin infection. JAMA 2008; 299:79–87.
- Zilberberg MD, Shorr AF, Micek ST, et al. Hospitalizations with healthcare-associated complicated skin and skin structure infections: impact of inappropriate empiric therapy on outcomes. J Hosp Med 2010; 5:535–540.
- Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am 2003; 85:1454–1460.
- Hsiao CT, Weng HH, Yuan YD, Chen CT, Chen IC. Predictors of mortality in patients with necrotizing fasciitis. Am J Emerg Med 2008; 26:170–175.
KEY POINTS
- Categories and definitions of specific subtypes of infections are evolving and have implications for treatment.
- Methicillin-resistant Staphylococcus aureus (MRSA) and streptococci continue to be the predominant organisms in SSTIs.
- A careful history and examination along with clinical attention are needed to elucidate atypical and severe infections.
- Laboratory data can help characterize the severity of disease and determine the probability of necrotizing fasciitis.
- Although cultures are unfortunately not reliably positive, their yield is higher in severe disease and they should be obtained, given the importance of antimicrobial susceptibility.
- The Infectious Diseases Society of America has recently released guidelines on MRSA, and additional guidelines addressing the spectrum of SSTIs are expected within a year.
Correction: Measles
Simple Answers to Complex Mental Health Concerns
Effects of Psychosocial Issues on Medication Adherence Among HIV/AIDS Patients
Bullous Systemic Lupus Erythematosus With Lupus Nephritis: A Rare Case of a Subepidermal Bullous Disorder in a Child
Contact Allergy to Dimethacrylate
Knee OA: Which patients are unlikely to benefit from manual PT and exercise?
Background The combination of manual physical therapy and exercise provides important benefit for more than 80% of patients with knee osteoarthritis (OA). Our objective was to determine predictor variables for patients unlikely to respond to these interventions.
Methods We used a retrospective combined cohort study design to develop a preliminary clinical prediction rule (CPR). To determine useful predictors of nonsuccess, we used an extensive set of 167 baseline variables. These variables were extracted from standardized examination forms used with 101 patients (64 women and 37 men with a mean age of 60.5±11.8 and 63.6±9.3 years, respectively) in 2 previously published clinical trials. We classified patients based on whether they achieved a clinically meaningful benefit of at least 12% improvement in Western Ontario MacMaster (WOMAC) scores after 4 weeks of treatment using the smallest and most efficient subset of predictors.
Results The variables of patellofemoral pain, anterior cruciate ligament laxity, and height >1.71 m (5’7’’) comprise the CPR. Patients with at least 2 positive tests yielded a posttest probability of 88% for nonsuccess with this treatment (positive likelihood ratio=36.7). The overall prognostic accuracy of the CPR was 96%.
Conclusion Most patients with knee OA will benefit from a low-risk, cost-effective program of manual physical therapy and supporting exercise.1,2 The few patients who may not benefit from such a program are identifiable by a simple (preliminary) CPR. After validation, this rule could improve primary patient management, allowing more appropriate referrals and choices in intervention.
Although the exact cause of knee OA is unclear, its incidence increases with age and it is particularly prevalent among women and those who are obese and have occupations requiring heavy lifting and frequent kneeling or squatting.3-6 Lifelong sport-specific activity7,8 and joint injury9 also seem to increase the risk for knee OA. Knee malalignment also may predispose people to knee OA,10 and the presence of early degenerative changes predicts progression of the disease.11 The disability and pain associated with knee OA correlate with a loss of quadriceps femoris muscle strength and limited joint range of motion.12-14
Medications and surgery carry substantial risks. Pharmacologic interventions for knee OA include nonsteroidal anti-inflammatory drugs, acetaminophen, and cyclooxygenase-2-selective inhibitors.15-17 While each of these drugs reduces pain and improves function, potential side effects include gastrointestinal, cardiovascular, renal, and hepatic complications.16,18-21
Effective surgical options—most appropriate for advanced OA—include high-tibial osteotomy and total knee arthroplasty (TKA). There is good evidence that arthroscopic surgery is not an effective intervention for knee OA, yielding results for pain and function equivalent to those seen with knee capsule injections of saline, tidal irrigation, and placebo surgery.22-25 TKA reduces pain, improves function, and decreases arthritis-related costs in older individuals with advanced knee OA.26,27 However, this procedure is not without risk.28 Total knee replacement in patients younger than 55 years is associated with increased mortality.29 Reported adverse outcomes of TKA include death, deep vein thrombosis, pulmonary embolus, deep wound infections,30,31 arterial lacerations, amputations,32 postoperative ileus,33 fractures, joint stiffness, and ligamentous instability.34 Viscosupplementation reduces pain and improves function, most evident at 5 to 13 weeks posttreatment, with few reported serious complications and moderate rates of local complications.35
Physical therapy is beneficial for mild to moderate OA and confers very low risk. Both physical therapy and exercise programs for OA have demonstrated benefit in a variety of settings.36-42 As shown in 2 independently conducted randomized controlled trials (RCTs) (one placebo controlled and one with an alternate treatment comparison), manual physical therapy applied during a small number of clinical sessions and supplemented by home exercise yields large reductions in pain and stiffness and improvements in functional ability persisting to 1 year as measured on the WOMAC Osteoarthritis Index,1,2 a validated self-report outcome instrument for OA of the hip and knee.43 In these studies, 60% of subjects receiving manual physical therapy and exercise achieved more than 50% improvement in WOMAC scores (pain, stiffness, and function) postintervention. Additionally, 83% achieved more than the minimal clinically important difference (MCID) of 12% improvement.1,2 Physical therapy and exercise combined also decreased the need for TKA and long-term medication use.1,2
For an intervention that benefits most patients, there is clearly an interest in determining predictors of treatment failure44 to expedite referral for alternative care. When the time or resources required to attend physical therapy appointments would create financial or personal hardships, more appropriate interventions may be home-based physical therapy exercise programs or medications and injections. Equally important, patients for whom knee OA rehabilitation is predicted to fail can be reprioritized for physical therapy aimed at coexisting conditions or injuries such as a functionally limiting impingement syndrome of the shoulder or chronic degenerative back or hip conditions.
Methods
Using a retrospective combined-cohort study design, we reviewed baseline patient examinations from 2 RCTs1,2 to identify variables that indicate which individuals with knee OA are unlikely to benefit from manual physical therapy and exercise, and to thereby develop a preliminary CPR. We extracted data from the research folders of all study participants. The institutional review board of Brooke Army Medical Center determined that the study was exempt from review. From April to December 2008, we prepared an extensive database of examination findings and performed analyses to determine the variables that predict likely treatment nonsuccess with manual physical therapy and exercise. Improvement of <12% in the total WOMAC score after 4 weeks of treatment defined nonsuccess.45
Data sets from the previously published trials contained 22 variables measured at baseline that were potential predictors of nonsuccess. We combined these variables with an additional 145 variables manually retrieved from standardized examination forms used for each subject, for a total of 167 potential predictors. We combined only data from treatment groups receiving manual therapy and exercise.
We limited the extent of some examination procedures in the earlier studies, due to the high level of symptoms experienced by some subjects at rest and during the initial examination. For example, if there was severe pain with active knee flexion, we did not perform passive manual overpressure to flexion; nor did we record a finding. Thus, the total number of data points for each subject varied somewhat.
Data analysis
We compared success and nonsuccess groups with 2-tailed unpaired t-tests for continuous variables, and chi-square tests for categorical variables. We additionally performed logistic regression analysis on potential predictors that yielded P values <.10, using a forward conditional stepwise procedure with probability levels set to .05 for entry and .10 for removal from the model. Predictors retained by the final logistic regression model comprised the CPR.
We coded each patient in the data set as positive or negative for each predictor in the CPR. To determine a cut score, we dichotomized the single retained continuous predictor variable using receiver-operator characteristic (ROC) curve analysis and the Youden index.46 For each CPR level (ie, increasing number of predictors positive), we constructed a 2 × 2 contingency table with numbers of patients with true-positive test results, false-positive test results, true-negative test results, and false-negative test results. We characterized prognostic performance of the CPR by calculating sensitivity, specificity, and positive likelihood ratios for each level of positive predictors. To determine overall prognostic accuracy, we added true positives and true negatives and divided by the total number of patients in the cross tabulation.
For each CPR level, we derived posttest probabilities of nonsuccess from generalized pretest probability (incidence of treatment nonsuccess in the sample) and the positive likelihood ratios.47 Finally, to determine how consistently the CPR performed with subjects in the original studies,1,2 we generated separate cross-tabulations and prognostic accuracy statistics from each RCT.
Results
Baseline patient attributes are summarized in TABLE 1. Of the 101 subjects in the combined data set, 17 (16.8%) met the definition of nonsuccess. Among 47 continuous-scale variables available, 11 predictors significantly discriminated between those in the treatment success and nonsuccess groups. Among 120 categorical-scale variables, 15 predictors significantly discriminated between groups. We identified 6 potential predictors for entry into the final logistic regression analysis: height, assistive device type, prone knee bend degrees, baseline WOMAC visual analog scale (VAS) for difficulty descending stairs, anterior cruciate ligament (ACL) laxity, and pain with passive patellofemoral glide.
TABLE 1
Baseline descriptive summaries of patients (n=101)
| Sex, n (%) Men Women | 37 (36.6) 64 (63.4) |
| Age, y Mean±SD Range | 62.5±10.4 39-85 |
| Height, m Mean±SD Range | 1.66±0.1041 1.42-1.91 |
| Side(s) involved, n (%) Unilateral Bilateral | 63 (62.4) 38 (37.6) |
| Weight, kg Mean±SD Range | 84.5±17.8 48.6-132.7 |
| Duration of symptoms, mo Mean±SD Range | 76.1±87.9 1-480 |
| WOMAC (VAS) total baseline, mm Mean±SD Range | 1059.8±447.1 193-2289 |
| 6-minute walk test baseline, m Mean±SD Range | 425.6±114.8 118.2-683.3 |
| Physical activity relative to peers (self-report), n (%) Much more active Somewhat more active About the same Somewhat less active | 26 (26) 33 (33) 20 (20) 21 (21) |
| Radiographic severity score, n (%) 0 1 2 3 4 | 6 (6.1) 25 (25.5) 33 (33.7) 25 (25.5) 9 (9.2) |
| *Baseline data were available for all 101 subjects except for duration of symptoms (n=98); physical activity (n=100); and radiographic severity (n=98). VAS, visual analog scale; WOMAC, Western Ontario MacMaster. | |
The final regression model retained 3 predictors comprising the CPR: height, ACL laxity, and pain with passive patellofemoral glides. We dichotomized height with a cut point of 1.71 m (5’7”), which corresponded with a deflection point at the upper left extent of the ROC curve (area under the curve=0.72; 95% CI, 0.57-0.87; P=.001). We thus deemed a patient 1.71 m or taller as positive for nonsuccess. We considered a patient with laxity of the ACL as positive for nonsuccess if a test result on the Lachman test (or the anterior drawer test) was positive (any grade other than 0). We regarded passive patellofemoral glide as positive for nonsuccess if a patient reported pain with any direction of passive gliding motion imposed by the therapist. The final regression model was a good fit to the data: Hosmer & Lemeshow test χ2 = 2.90 (P=.940); Nagelkerke R2=0.680.
TABLE 2 presents prognostic accuracy profiles for each predictor in the CPR; TABLE 3 summarizes the accuracy for each level of the multivariate CPR. Values in TABLE 3 reflect complete sets of data for the 3 predictors found for 50 patients. Of those 50 patients, 6 (12%) were in the nonsuccess group.
TABLE 2
Prognostic accuracy statistics for individual predictors
| Predictor | Sensitivity (95% CI) | Specificity (95% CI) | Positive likelihood ratio (95% CI) | Posttest probability of nonsuccess* |
|---|---|---|---|---|
| Height ≥1.71 m | 0.65 (0.41-0.83) | 0.77 (0.67-0.85) | 2.86 (1.69-4.86) | 37% |
| ACL laxity | 0.27 (0.10-0.57) | 0.93 (0.83-0.97) | 3.68 (0.96-14.19) | 43% |
| Pain with passive patellofemoral glide in any direction | 0.71 (0.35-0.92) | 0.61 (0.47-0.74) | 1.84 (1.03-3.31) | 27% |
| ACL, anterior cruciate ligament; CI, confidence interval. *Assumes pretest probability of nonsuccess=17% (incidence in this sample). | ||||
With any 2 of the 3 tests positive, the CPR yielded a sensitivity of 83% (95% CI, 44%-97%), specificity of 98% (95% CI, 88%-100%), and positive likelihood ratio of 36.7 (95% CI, 5.1-263.0). Only 2 patients out of 50 were misclassified (one false positive and one false negative) at this level of the CPR, yielding an overall prognostic accuracy of 96% (95% CI, 87%-99%). Application of the positive likelihood ratio for a patient with any 2 positive tests yielded a posttest probability of 88% for nonsuccess with this treatment.
TABLE 3
Prognostic accuracy statistics for 3-level clinical prediction rule
| CPR level | Sensitivity (95% CI) | Specificity (95% CI) | Positive likelihood ratio (95% CI) | Posttest probability of nonsuccess* |
|---|---|---|---|---|
| All 3 tests positive | 0.21 (0.05-0.58) | 0.99 (0.90-1.00) | 19.29 (0.87-428.09) | 80% |
| At least 2 tests positive | 0.83 (0.44-0.97) | 0.98 (0.88-1.00) | 36.67 (5.11-263.01) | 88% |
| At least 1 test positive | 0.92 (0.56-0.99) | 0.48 (0.34-0.62) | 1.78 (1.26-2.52) | 27% |
| CI, confidence interval; CPR, clinical prediction rule. *Assumes pretest probability of nonsuccess=17% (incidence in this sample). | ||||
In the sensitivity analysis, the CPR performed similarly well for patients in each of the 2 original studies when applied separately to the groups of patients. Among the 30 patients from the first trial2 who had data for all 3 predictors in the CPR, only one was misclassified (a false positive), yielding a prognostic accuracy of 97% (95% CI, 83%-99%). Among the 20 patients from the second trial1 who had data for all 3 predictors, only one was misclassified (a false negative), yielding a prognostic accuracy of 95% (95% CI, 76%-99%).
DISCUSSION
Family physicians and physical therapists should be able to discuss with confidence how any given patient with knee OA will likely respond to treatment options. Our study is a preliminary step toward defining the population of patients with knee OA who are unlikely to benefit from manual physical therapy and exercise. We found such patients to be those with height >1.71 m, ACL laxity, and pain with passive glides of the patellofemoral joint.
A limitation of our study is the retrospective nature of gathering data. However, retrospective CPR derivation studies have made valuable contributions to many areas of medical practice.48-53 Additionally, if there had been uniformly available data across all patients, there may have been other, perhaps more powerful, predictors for treatment nonsuccess.
Actual cases of knee osteoarthritis (OA) evaluated by one of the authors (GD)
A 48-year-old female elementary teacher was referred for physical therapy due to right knee pain and a diagnosis of OA that was limiting her ability to climb stairs and squat to work with children in the classroom. Her goals were to be able to perform these physical activities with less pain and to reduce her anti-inflammatory medications. However, she also worried about taking time away from her job to attend physical therapy appointments. She was 1.63 m (5’4”) tall and had a body mass index of 27.5 kg/m2. Her knee was stable to ligamentous testing, with mild limitation and pain with active and passive movement of both the tibiofemoral and the patellofemoral joints. She had weakness of the quadriceps and hip abductors, and moderate tightness of the calf muscles in both lower extremities.
Given the presence of only a single predictor for nonsuccess (pain with passive movement of her patella), the likelihood that this patient would not respond to manual physical therapy and exercise was just 27%, according to the clinical prediction rule. The impairments to movement, strength, and flexibility found during the physical examination typically can be successfully addressed with manual physical therapy. Additionally, one of the patient’s goals was to reduce her medication use—a reported outcome of the clinical trials used for deriving the rule.1,2 This patient was a good candidate for the intervention, with an acceptably small chance of not achieving a clinically meaningful benefit.
A 50-year-old male soldier 1.95 m (6’5”) tall was referred for physical therapy to ameliorate chronic pain due to tricompartmental knee OA. He exhibited anterior ligamentous laxity and felt severe pain with manually performed passive patellar glides (FIGURES 1 AND 2). He also had a rotator cuff tear and a mild traumatic brain injury from a roadside bomb blast. With 3/3 predictors for failure, the likelihood of reducing this patient’s knee symptoms with manual therapy and exercise was just 20%. The physical therapist and referring physician jointly decided to focus a small number of physical therapy visits on the patient’s shoulder, while giving rehabilitation priority to ongoing cognitive therapy appointments.
FIGURE 1
Lachman test
With the patient’s knee flexed at 30°, draw the proximal tibia anteriorly to observe movement of the tibia relative to the femur and thereby gauge anterior cruciate ligament integrity. Laxity is suggested by increased movement relative to the opposite knee.
FIGURE 2
Passive patellofemoral glide
With the patient’s knee slightly flexed, apply light pressure to the medial border of the patella, moving it laterally and taking care not to compress the patella. Repeat the procedure superiorly, inferiorly, and medially. A positive test is pain experienced with any of the glides.
Patient height >1.71 m is the least intuitive of the predictors for nonsuccess, but that underscores the value of data-driven prediction rules. Variables regarded as unimportant in a typical clinical assessment may show clinical usefulness if validated in independent studies. It may be that in taller patients with knee OA, biomechanical forces are such that a positive response to conservative therapy is less likely—particularly in the presence of ligamentous laxity or patellofemoral dysfunction.
For most patients with knee OA, the combined intervention of manual physical therapy and exercise is clinically beneficial, relatively inexpensive, and has no known adverse effects.54 However, unique circumstances may increase the importance of determining the likelihood that a patient will benefit. A validated CPR will facilitate timely decisions for those relatively few patients requiring alternative interventions. Although the rule is preliminary and needs to be validated, these results provide current best evidence to define patients with knee OA who are unlikely to respond to manual physical therapy and exercise.
1. Deyle GD, Allison SC, Matekel RL, et al. Physical therapy treatment effectiveness for osteoarthritis of the knee: a randomized comparison of supervised clinical exercise and manual therapy procedures versus a home exercise program. Phys Ther. 2005;85:1301-1317.
2. Deyle GD, Henderson NE, Matekel RL, et al. Effectiveness of manual physical therapy and exercise in osteoarthritis of the knee. A randomized, controlled trial. Ann Intern Med. 2000;132:173-181
3. Sandmark H, Hogstedt C, Vingard E. Primary osteoarthrosis of the knee in men and women as a result of lifelong physical load from work. Scand J Work Environ Health. 2000;26:20-25.
4. Felson DT, Zhang Y, Hannan MT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40:728-733.
5. Jarvholm B, From C, Lewold S, et al. Incidence of surgically treated osteoarthritis in the hip and knee in male construction workers. Occup Environ Med. 2008;65:275-278.
6. Messier SP, Loeser RF, Mitchell MN, et al. Exercise and weight loss in obese older adults with knee osteoarthritis: a preliminary study. J Am Geriatr Soc. 2000;48:1062-1072.
7. Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998;41:1343-1355.
8. Sandmark H, Vingard E. Sports and risk for severe osteoarthrosis of the knee. Scand J Med Sci Sports. 1999;9:279-284.
9. Gelber AC, Hochberg MC, Mead LA, et al. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133:321-328.
10. Cerejo R, Dunlop DD, Cahue S, et al. The influence of alignment on risk of knee osteoarthritis progression according to baseline stage of disease. Arthritis Rheum. 2002;46:2632-2636.
11. Wolfe F, Lane NE. The longterm outcome of osteoarthritis: rates and predictors of joint space narrowing in symptomatic patients with knee osteoarthritis. J Rheumatol. 2002;29:139-146.
12. Lewek MD, Rudolph KS, Snyder-Mackler L. Quadriceps femoris muscle weakness and activation failure in patients with symptomatic knee osteoarthritis. J Orthop Res. 2004;22:110-115.
13. Fitzgerald GK, Piva SR, Irrgang JJ. Reports of joint instability in knee osteoarthritis: its prevalence and relationship to physical function. Arthritis Rheum. 2004;51:941-946.
14. Fitzgerald GK, Piva SR, Irrgang JJ, et al. Quadriceps activation failure as a moderator of the relationship between quadriceps strength and physical function in individuals with knee osteoarthritis. Arthritis Rheum. 2004;51:40-48.
15. Scott DL, Berry H, Capell H, et al. The long-term effects of non-steroidal anti-inflammatory drugs in osteoarthritis of the knee: a randomized placebo-controlled trial. Rheumatology (Oxford). 2000;39:1095-1101.
16. Towheed TE, Maxwell L, Judd MG, et al. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;(1):CD004257.-
17. Kivitz A, Fairfax M, Sheldon EA, et al. Comparison of the effectiveness and tolerability of lidocaine patch 5% versus celecoxib for osteoarthritis-related knee pain: post hoc analysis of a 12 week, prospective, randomized, active-controlled, open-label, parallel-group trial in adults. Clin Ther. 2008;30:2366-2377.
18. Nussmeier NA, Whelton AA, Brown MT, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005;352:1081-1091.
19. Psaty BM, Furberg CD. COX-2 inhibitors—lessons in drug safety. N Engl J Med. 2005;352:1133-1135.
20. Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071-1080.
21. Wittenberg RH, Schell E, Krehan G, et al. First-dose analgesic effect of the cyclo-oxygenase-2 selective inhibitor lumiracoxib in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled comparison with celecoxib [NCT00267215]. Arthritis Res Ther. 2006;8:R35.-
22. Bradley JD, Heilman DK, Katz BP, et al. Tidal irrigation as treatment for knee osteoarthritis: a sham-controlled, randomized, double-blinded evaluation. Arthritis Rheum. 2002;46:100-108.
23. Chang RW, Falconer J, Stulberg SD, et al. A randomized, controlled trial of arthroscopic surgery versus closed-needle joint lavage for patients with osteoarthritis of the knee. Arthritis Rheum. 1993;36:289-296.
24. Moseley JB, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:81-88.
25. Laupattarakasem W, Laopaiboon M, Laupattarakasem P, et al. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst Rev. 2008;(1):CD005118.-
26. Hawker GA, Badley EM, Croxford R, et al. A population-based nested case-control study of the costs of hip and knee replacement surgery. Med Care. 2009;47:732-741.
27. Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169:1113-1121.
28. Hamel MB, Toth M, Legedza A, et al. Joint replacement surgery in elderly patients with severe osteoarthritis of the hip or knee: decision making, postoperative recovery, and clinical outcomes. Arch Intern Med. 2008;168:1430-1440.
29. Robertsson O, Stefansdottir A, Lidgren L, et al. Increased long-term mortality in patients less than 55 years old who have undergone knee replacement for osteoarthritis: results from the Swedish Knee Arthroplasty Register. J Bone Joint Surg Br. 2007;89:599-603.
30. SooHoo NF, Lieberman JR, Ko CY, et al. Factors predicting complication rates following total knee replacement. J Bone Joint Surg Am. 2006;88:480-485.
31. Solomon DH, Chibnik LB, Losina E, et al. Development of a preliminary index that predicts adverse events after total knee replacement. Arthritis Rheum. 2006;54:1536-1542.
32. Abularrage CJ, Weiswasser JM, Dezee KJ, et al. Predictors of lower extremity arterial injury after total knee or total hip arthroplasty. J Vasc Surg. 2008;47:803-807.
33. Parvizi J, Han SB, Tarity TD, et al. Postoperative ileus after total joint arthroplasty. J Arthroplasty. 2008;23:360-365.
34. Pinaroli A, Piedade SR, Servien E, et al. Intraoperative fractures and ligament tears during total knee arthroplasty. A 1795 posterostabilized TKA continuous series. Orthop Traumatol Surg Res. 2009;95:183-189
35. Bellamy N, Campbell J, Robinson V, et al. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005321.-
36. Baker K, McAlindon T. Exercise for knee osteoarthritis. Curr Opin Rheumatol. 2000;12:456-463.
37. Baker KR, Nelson ME, Felson DT, et al. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: a randomized controlled trial. J Rheumatol. 2001;28:1655-1665.
38. O’Reilly SC, Muir KR, Doherty M. Effectiveness of home exercise on pain and disability from osteoarthritis of the knee: a randomised controlled trial. Ann Rheum Dis. 1999;58:15-19.
39. Petrella RJ, Bartha C. Home based exercise therapy for older patients with knee osteoarthritis: a randomized clinical trial. J Rheumatol. 2000;27:2215-2221.
40. van Baar ME, Dekker J, Oostendorp RA, et al. Effectiveness of exercise in patients with osteoarthritis of hip or knee: nine months’ follow up. Ann Rheum Dis. 2001;60:1123-1130.
41. Silva LE, Valim V, Pessanha AP, et al. Hydrotherapy versus conventional land-based exercise for the management of patients with osteoarthritis of the knee: a randomized clinical trial. Phys Ther. 2008;88:12-21.
42. Hinman RS, Heywood SE, Day AR. Aquatic physical therapy for hip and knee osteoarthritis: results of a single-blind randomized controlled trial. Phys Ther. 2007;87:32-43.
43. Bellamy N. WOMAC: a 20-year experiential review of a patient-centered self-reported health status questionnaire. J Rheumatol. 2002;29:2473-2476.
44. Fritz JM. Clinical prediction rules in physical therapy: coming of age? J Orthop Sports Phys Ther. 2009;39:159-161
45. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45:384-391.
46. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32-35.
47. Fritz JM, Wainner RS. Examining diagnostic tests: an evidence-based perspective. Phys Ther. 2001;81:1546-1564.
48. van Walraven C, Hart RG, Wells GA, et al. A clinical prediction rule to identify patients with atrial fibrillation and a low risk for stroke while taking aspirin. Arch Intern Med. 2003;163:936-943.
49. Predictors of thromboembolism in atrial fibrillation: I. Clinical features of patients at risk. The Stroke Prevention in Atrial Fibrillation Investigators. Ann Intern Med. 1992;116:1-5
50. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041-1046.
51. Aujesky D, Obrosky DS, Stone RA, et al. A prediction rule to identify low-risk patients with pulmonary embolism. Arch Intern Med. 2006;166:169-175.
52. Espana PP, Capelastegui A, Gorordo I, et al. Development and validation of a clinical prediction rule for severe community-acquired pneumonia. Am J Respir Crit Care Med. 2006;174:1249-1256.
53. Kuijpers T, van der Heijden GJ, Vergouwe Y, et al. Good generalizability of a prediction rule for prediction of persistent shoulder pain in the short term. J Clin Epidemiol. 2007;60:947-953.
54. Ludica CA. Can a program of manual physical therapy and supervised exercise improve the symptoms of osteoarthritis of the knee? J Fam Pract. 2000;49:466-467
CORRESPONDENCE Gail D. Deyle, PT, DSc, Orthopaedic Manual Physical Therapy Fellowship, 3551 Roger Brooke Drive, Brooke Army Medical Center, Ft. Sam Houston, TX 78234; [email protected]
Background The combination of manual physical therapy and exercise provides important benefit for more than 80% of patients with knee osteoarthritis (OA). Our objective was to determine predictor variables for patients unlikely to respond to these interventions.
Methods We used a retrospective combined cohort study design to develop a preliminary clinical prediction rule (CPR). To determine useful predictors of nonsuccess, we used an extensive set of 167 baseline variables. These variables were extracted from standardized examination forms used with 101 patients (64 women and 37 men with a mean age of 60.5±11.8 and 63.6±9.3 years, respectively) in 2 previously published clinical trials. We classified patients based on whether they achieved a clinically meaningful benefit of at least 12% improvement in Western Ontario MacMaster (WOMAC) scores after 4 weeks of treatment using the smallest and most efficient subset of predictors.
Results The variables of patellofemoral pain, anterior cruciate ligament laxity, and height >1.71 m (5’7’’) comprise the CPR. Patients with at least 2 positive tests yielded a posttest probability of 88% for nonsuccess with this treatment (positive likelihood ratio=36.7). The overall prognostic accuracy of the CPR was 96%.
Conclusion Most patients with knee OA will benefit from a low-risk, cost-effective program of manual physical therapy and supporting exercise.1,2 The few patients who may not benefit from such a program are identifiable by a simple (preliminary) CPR. After validation, this rule could improve primary patient management, allowing more appropriate referrals and choices in intervention.
Although the exact cause of knee OA is unclear, its incidence increases with age and it is particularly prevalent among women and those who are obese and have occupations requiring heavy lifting and frequent kneeling or squatting.3-6 Lifelong sport-specific activity7,8 and joint injury9 also seem to increase the risk for knee OA. Knee malalignment also may predispose people to knee OA,10 and the presence of early degenerative changes predicts progression of the disease.11 The disability and pain associated with knee OA correlate with a loss of quadriceps femoris muscle strength and limited joint range of motion.12-14
Medications and surgery carry substantial risks. Pharmacologic interventions for knee OA include nonsteroidal anti-inflammatory drugs, acetaminophen, and cyclooxygenase-2-selective inhibitors.15-17 While each of these drugs reduces pain and improves function, potential side effects include gastrointestinal, cardiovascular, renal, and hepatic complications.16,18-21
Effective surgical options—most appropriate for advanced OA—include high-tibial osteotomy and total knee arthroplasty (TKA). There is good evidence that arthroscopic surgery is not an effective intervention for knee OA, yielding results for pain and function equivalent to those seen with knee capsule injections of saline, tidal irrigation, and placebo surgery.22-25 TKA reduces pain, improves function, and decreases arthritis-related costs in older individuals with advanced knee OA.26,27 However, this procedure is not without risk.28 Total knee replacement in patients younger than 55 years is associated with increased mortality.29 Reported adverse outcomes of TKA include death, deep vein thrombosis, pulmonary embolus, deep wound infections,30,31 arterial lacerations, amputations,32 postoperative ileus,33 fractures, joint stiffness, and ligamentous instability.34 Viscosupplementation reduces pain and improves function, most evident at 5 to 13 weeks posttreatment, with few reported serious complications and moderate rates of local complications.35
Physical therapy is beneficial for mild to moderate OA and confers very low risk. Both physical therapy and exercise programs for OA have demonstrated benefit in a variety of settings.36-42 As shown in 2 independently conducted randomized controlled trials (RCTs) (one placebo controlled and one with an alternate treatment comparison), manual physical therapy applied during a small number of clinical sessions and supplemented by home exercise yields large reductions in pain and stiffness and improvements in functional ability persisting to 1 year as measured on the WOMAC Osteoarthritis Index,1,2 a validated self-report outcome instrument for OA of the hip and knee.43 In these studies, 60% of subjects receiving manual physical therapy and exercise achieved more than 50% improvement in WOMAC scores (pain, stiffness, and function) postintervention. Additionally, 83% achieved more than the minimal clinically important difference (MCID) of 12% improvement.1,2 Physical therapy and exercise combined also decreased the need for TKA and long-term medication use.1,2
For an intervention that benefits most patients, there is clearly an interest in determining predictors of treatment failure44 to expedite referral for alternative care. When the time or resources required to attend physical therapy appointments would create financial or personal hardships, more appropriate interventions may be home-based physical therapy exercise programs or medications and injections. Equally important, patients for whom knee OA rehabilitation is predicted to fail can be reprioritized for physical therapy aimed at coexisting conditions or injuries such as a functionally limiting impingement syndrome of the shoulder or chronic degenerative back or hip conditions.
Methods
Using a retrospective combined-cohort study design, we reviewed baseline patient examinations from 2 RCTs1,2 to identify variables that indicate which individuals with knee OA are unlikely to benefit from manual physical therapy and exercise, and to thereby develop a preliminary CPR. We extracted data from the research folders of all study participants. The institutional review board of Brooke Army Medical Center determined that the study was exempt from review. From April to December 2008, we prepared an extensive database of examination findings and performed analyses to determine the variables that predict likely treatment nonsuccess with manual physical therapy and exercise. Improvement of <12% in the total WOMAC score after 4 weeks of treatment defined nonsuccess.45
Data sets from the previously published trials contained 22 variables measured at baseline that were potential predictors of nonsuccess. We combined these variables with an additional 145 variables manually retrieved from standardized examination forms used for each subject, for a total of 167 potential predictors. We combined only data from treatment groups receiving manual therapy and exercise.
We limited the extent of some examination procedures in the earlier studies, due to the high level of symptoms experienced by some subjects at rest and during the initial examination. For example, if there was severe pain with active knee flexion, we did not perform passive manual overpressure to flexion; nor did we record a finding. Thus, the total number of data points for each subject varied somewhat.
Data analysis
We compared success and nonsuccess groups with 2-tailed unpaired t-tests for continuous variables, and chi-square tests for categorical variables. We additionally performed logistic regression analysis on potential predictors that yielded P values <.10, using a forward conditional stepwise procedure with probability levels set to .05 for entry and .10 for removal from the model. Predictors retained by the final logistic regression model comprised the CPR.
We coded each patient in the data set as positive or negative for each predictor in the CPR. To determine a cut score, we dichotomized the single retained continuous predictor variable using receiver-operator characteristic (ROC) curve analysis and the Youden index.46 For each CPR level (ie, increasing number of predictors positive), we constructed a 2 × 2 contingency table with numbers of patients with true-positive test results, false-positive test results, true-negative test results, and false-negative test results. We characterized prognostic performance of the CPR by calculating sensitivity, specificity, and positive likelihood ratios for each level of positive predictors. To determine overall prognostic accuracy, we added true positives and true negatives and divided by the total number of patients in the cross tabulation.
For each CPR level, we derived posttest probabilities of nonsuccess from generalized pretest probability (incidence of treatment nonsuccess in the sample) and the positive likelihood ratios.47 Finally, to determine how consistently the CPR performed with subjects in the original studies,1,2 we generated separate cross-tabulations and prognostic accuracy statistics from each RCT.
Results
Baseline patient attributes are summarized in TABLE 1. Of the 101 subjects in the combined data set, 17 (16.8%) met the definition of nonsuccess. Among 47 continuous-scale variables available, 11 predictors significantly discriminated between those in the treatment success and nonsuccess groups. Among 120 categorical-scale variables, 15 predictors significantly discriminated between groups. We identified 6 potential predictors for entry into the final logistic regression analysis: height, assistive device type, prone knee bend degrees, baseline WOMAC visual analog scale (VAS) for difficulty descending stairs, anterior cruciate ligament (ACL) laxity, and pain with passive patellofemoral glide.
TABLE 1
Baseline descriptive summaries of patients (n=101)
| Sex, n (%) Men Women | 37 (36.6) 64 (63.4) |
| Age, y Mean±SD Range | 62.5±10.4 39-85 |
| Height, m Mean±SD Range | 1.66±0.1041 1.42-1.91 |
| Side(s) involved, n (%) Unilateral Bilateral | 63 (62.4) 38 (37.6) |
| Weight, kg Mean±SD Range | 84.5±17.8 48.6-132.7 |
| Duration of symptoms, mo Mean±SD Range | 76.1±87.9 1-480 |
| WOMAC (VAS) total baseline, mm Mean±SD Range | 1059.8±447.1 193-2289 |
| 6-minute walk test baseline, m Mean±SD Range | 425.6±114.8 118.2-683.3 |
| Physical activity relative to peers (self-report), n (%) Much more active Somewhat more active About the same Somewhat less active | 26 (26) 33 (33) 20 (20) 21 (21) |
| Radiographic severity score, n (%) 0 1 2 3 4 | 6 (6.1) 25 (25.5) 33 (33.7) 25 (25.5) 9 (9.2) |
| *Baseline data were available for all 101 subjects except for duration of symptoms (n=98); physical activity (n=100); and radiographic severity (n=98). VAS, visual analog scale; WOMAC, Western Ontario MacMaster. | |
The final regression model retained 3 predictors comprising the CPR: height, ACL laxity, and pain with passive patellofemoral glides. We dichotomized height with a cut point of 1.71 m (5’7”), which corresponded with a deflection point at the upper left extent of the ROC curve (area under the curve=0.72; 95% CI, 0.57-0.87; P=.001). We thus deemed a patient 1.71 m or taller as positive for nonsuccess. We considered a patient with laxity of the ACL as positive for nonsuccess if a test result on the Lachman test (or the anterior drawer test) was positive (any grade other than 0). We regarded passive patellofemoral glide as positive for nonsuccess if a patient reported pain with any direction of passive gliding motion imposed by the therapist. The final regression model was a good fit to the data: Hosmer & Lemeshow test χ2 = 2.90 (P=.940); Nagelkerke R2=0.680.
TABLE 2 presents prognostic accuracy profiles for each predictor in the CPR; TABLE 3 summarizes the accuracy for each level of the multivariate CPR. Values in TABLE 3 reflect complete sets of data for the 3 predictors found for 50 patients. Of those 50 patients, 6 (12%) were in the nonsuccess group.
TABLE 2
Prognostic accuracy statistics for individual predictors
| Predictor | Sensitivity (95% CI) | Specificity (95% CI) | Positive likelihood ratio (95% CI) | Posttest probability of nonsuccess* |
|---|---|---|---|---|
| Height ≥1.71 m | 0.65 (0.41-0.83) | 0.77 (0.67-0.85) | 2.86 (1.69-4.86) | 37% |
| ACL laxity | 0.27 (0.10-0.57) | 0.93 (0.83-0.97) | 3.68 (0.96-14.19) | 43% |
| Pain with passive patellofemoral glide in any direction | 0.71 (0.35-0.92) | 0.61 (0.47-0.74) | 1.84 (1.03-3.31) | 27% |
| ACL, anterior cruciate ligament; CI, confidence interval. *Assumes pretest probability of nonsuccess=17% (incidence in this sample). | ||||
With any 2 of the 3 tests positive, the CPR yielded a sensitivity of 83% (95% CI, 44%-97%), specificity of 98% (95% CI, 88%-100%), and positive likelihood ratio of 36.7 (95% CI, 5.1-263.0). Only 2 patients out of 50 were misclassified (one false positive and one false negative) at this level of the CPR, yielding an overall prognostic accuracy of 96% (95% CI, 87%-99%). Application of the positive likelihood ratio for a patient with any 2 positive tests yielded a posttest probability of 88% for nonsuccess with this treatment.
TABLE 3
Prognostic accuracy statistics for 3-level clinical prediction rule
| CPR level | Sensitivity (95% CI) | Specificity (95% CI) | Positive likelihood ratio (95% CI) | Posttest probability of nonsuccess* |
|---|---|---|---|---|
| All 3 tests positive | 0.21 (0.05-0.58) | 0.99 (0.90-1.00) | 19.29 (0.87-428.09) | 80% |
| At least 2 tests positive | 0.83 (0.44-0.97) | 0.98 (0.88-1.00) | 36.67 (5.11-263.01) | 88% |
| At least 1 test positive | 0.92 (0.56-0.99) | 0.48 (0.34-0.62) | 1.78 (1.26-2.52) | 27% |
| CI, confidence interval; CPR, clinical prediction rule. *Assumes pretest probability of nonsuccess=17% (incidence in this sample). | ||||
In the sensitivity analysis, the CPR performed similarly well for patients in each of the 2 original studies when applied separately to the groups of patients. Among the 30 patients from the first trial2 who had data for all 3 predictors in the CPR, only one was misclassified (a false positive), yielding a prognostic accuracy of 97% (95% CI, 83%-99%). Among the 20 patients from the second trial1 who had data for all 3 predictors, only one was misclassified (a false negative), yielding a prognostic accuracy of 95% (95% CI, 76%-99%).
DISCUSSION
Family physicians and physical therapists should be able to discuss with confidence how any given patient with knee OA will likely respond to treatment options. Our study is a preliminary step toward defining the population of patients with knee OA who are unlikely to benefit from manual physical therapy and exercise. We found such patients to be those with height >1.71 m, ACL laxity, and pain with passive glides of the patellofemoral joint.
A limitation of our study is the retrospective nature of gathering data. However, retrospective CPR derivation studies have made valuable contributions to many areas of medical practice.48-53 Additionally, if there had been uniformly available data across all patients, there may have been other, perhaps more powerful, predictors for treatment nonsuccess.
Actual cases of knee osteoarthritis (OA) evaluated by one of the authors (GD)
A 48-year-old female elementary teacher was referred for physical therapy due to right knee pain and a diagnosis of OA that was limiting her ability to climb stairs and squat to work with children in the classroom. Her goals were to be able to perform these physical activities with less pain and to reduce her anti-inflammatory medications. However, she also worried about taking time away from her job to attend physical therapy appointments. She was 1.63 m (5’4”) tall and had a body mass index of 27.5 kg/m2. Her knee was stable to ligamentous testing, with mild limitation and pain with active and passive movement of both the tibiofemoral and the patellofemoral joints. She had weakness of the quadriceps and hip abductors, and moderate tightness of the calf muscles in both lower extremities.
Given the presence of only a single predictor for nonsuccess (pain with passive movement of her patella), the likelihood that this patient would not respond to manual physical therapy and exercise was just 27%, according to the clinical prediction rule. The impairments to movement, strength, and flexibility found during the physical examination typically can be successfully addressed with manual physical therapy. Additionally, one of the patient’s goals was to reduce her medication use—a reported outcome of the clinical trials used for deriving the rule.1,2 This patient was a good candidate for the intervention, with an acceptably small chance of not achieving a clinically meaningful benefit.
A 50-year-old male soldier 1.95 m (6’5”) tall was referred for physical therapy to ameliorate chronic pain due to tricompartmental knee OA. He exhibited anterior ligamentous laxity and felt severe pain with manually performed passive patellar glides (FIGURES 1 AND 2). He also had a rotator cuff tear and a mild traumatic brain injury from a roadside bomb blast. With 3/3 predictors for failure, the likelihood of reducing this patient’s knee symptoms with manual therapy and exercise was just 20%. The physical therapist and referring physician jointly decided to focus a small number of physical therapy visits on the patient’s shoulder, while giving rehabilitation priority to ongoing cognitive therapy appointments.
FIGURE 1
Lachman test
With the patient’s knee flexed at 30°, draw the proximal tibia anteriorly to observe movement of the tibia relative to the femur and thereby gauge anterior cruciate ligament integrity. Laxity is suggested by increased movement relative to the opposite knee.
FIGURE 2
Passive patellofemoral glide
With the patient’s knee slightly flexed, apply light pressure to the medial border of the patella, moving it laterally and taking care not to compress the patella. Repeat the procedure superiorly, inferiorly, and medially. A positive test is pain experienced with any of the glides.
Patient height >1.71 m is the least intuitive of the predictors for nonsuccess, but that underscores the value of data-driven prediction rules. Variables regarded as unimportant in a typical clinical assessment may show clinical usefulness if validated in independent studies. It may be that in taller patients with knee OA, biomechanical forces are such that a positive response to conservative therapy is less likely—particularly in the presence of ligamentous laxity or patellofemoral dysfunction.
For most patients with knee OA, the combined intervention of manual physical therapy and exercise is clinically beneficial, relatively inexpensive, and has no known adverse effects.54 However, unique circumstances may increase the importance of determining the likelihood that a patient will benefit. A validated CPR will facilitate timely decisions for those relatively few patients requiring alternative interventions. Although the rule is preliminary and needs to be validated, these results provide current best evidence to define patients with knee OA who are unlikely to respond to manual physical therapy and exercise.
Background The combination of manual physical therapy and exercise provides important benefit for more than 80% of patients with knee osteoarthritis (OA). Our objective was to determine predictor variables for patients unlikely to respond to these interventions.
Methods We used a retrospective combined cohort study design to develop a preliminary clinical prediction rule (CPR). To determine useful predictors of nonsuccess, we used an extensive set of 167 baseline variables. These variables were extracted from standardized examination forms used with 101 patients (64 women and 37 men with a mean age of 60.5±11.8 and 63.6±9.3 years, respectively) in 2 previously published clinical trials. We classified patients based on whether they achieved a clinically meaningful benefit of at least 12% improvement in Western Ontario MacMaster (WOMAC) scores after 4 weeks of treatment using the smallest and most efficient subset of predictors.
Results The variables of patellofemoral pain, anterior cruciate ligament laxity, and height >1.71 m (5’7’’) comprise the CPR. Patients with at least 2 positive tests yielded a posttest probability of 88% for nonsuccess with this treatment (positive likelihood ratio=36.7). The overall prognostic accuracy of the CPR was 96%.
Conclusion Most patients with knee OA will benefit from a low-risk, cost-effective program of manual physical therapy and supporting exercise.1,2 The few patients who may not benefit from such a program are identifiable by a simple (preliminary) CPR. After validation, this rule could improve primary patient management, allowing more appropriate referrals and choices in intervention.
Although the exact cause of knee OA is unclear, its incidence increases with age and it is particularly prevalent among women and those who are obese and have occupations requiring heavy lifting and frequent kneeling or squatting.3-6 Lifelong sport-specific activity7,8 and joint injury9 also seem to increase the risk for knee OA. Knee malalignment also may predispose people to knee OA,10 and the presence of early degenerative changes predicts progression of the disease.11 The disability and pain associated with knee OA correlate with a loss of quadriceps femoris muscle strength and limited joint range of motion.12-14
Medications and surgery carry substantial risks. Pharmacologic interventions for knee OA include nonsteroidal anti-inflammatory drugs, acetaminophen, and cyclooxygenase-2-selective inhibitors.15-17 While each of these drugs reduces pain and improves function, potential side effects include gastrointestinal, cardiovascular, renal, and hepatic complications.16,18-21
Effective surgical options—most appropriate for advanced OA—include high-tibial osteotomy and total knee arthroplasty (TKA). There is good evidence that arthroscopic surgery is not an effective intervention for knee OA, yielding results for pain and function equivalent to those seen with knee capsule injections of saline, tidal irrigation, and placebo surgery.22-25 TKA reduces pain, improves function, and decreases arthritis-related costs in older individuals with advanced knee OA.26,27 However, this procedure is not without risk.28 Total knee replacement in patients younger than 55 years is associated with increased mortality.29 Reported adverse outcomes of TKA include death, deep vein thrombosis, pulmonary embolus, deep wound infections,30,31 arterial lacerations, amputations,32 postoperative ileus,33 fractures, joint stiffness, and ligamentous instability.34 Viscosupplementation reduces pain and improves function, most evident at 5 to 13 weeks posttreatment, with few reported serious complications and moderate rates of local complications.35
Physical therapy is beneficial for mild to moderate OA and confers very low risk. Both physical therapy and exercise programs for OA have demonstrated benefit in a variety of settings.36-42 As shown in 2 independently conducted randomized controlled trials (RCTs) (one placebo controlled and one with an alternate treatment comparison), manual physical therapy applied during a small number of clinical sessions and supplemented by home exercise yields large reductions in pain and stiffness and improvements in functional ability persisting to 1 year as measured on the WOMAC Osteoarthritis Index,1,2 a validated self-report outcome instrument for OA of the hip and knee.43 In these studies, 60% of subjects receiving manual physical therapy and exercise achieved more than 50% improvement in WOMAC scores (pain, stiffness, and function) postintervention. Additionally, 83% achieved more than the minimal clinically important difference (MCID) of 12% improvement.1,2 Physical therapy and exercise combined also decreased the need for TKA and long-term medication use.1,2
For an intervention that benefits most patients, there is clearly an interest in determining predictors of treatment failure44 to expedite referral for alternative care. When the time or resources required to attend physical therapy appointments would create financial or personal hardships, more appropriate interventions may be home-based physical therapy exercise programs or medications and injections. Equally important, patients for whom knee OA rehabilitation is predicted to fail can be reprioritized for physical therapy aimed at coexisting conditions or injuries such as a functionally limiting impingement syndrome of the shoulder or chronic degenerative back or hip conditions.
Methods
Using a retrospective combined-cohort study design, we reviewed baseline patient examinations from 2 RCTs1,2 to identify variables that indicate which individuals with knee OA are unlikely to benefit from manual physical therapy and exercise, and to thereby develop a preliminary CPR. We extracted data from the research folders of all study participants. The institutional review board of Brooke Army Medical Center determined that the study was exempt from review. From April to December 2008, we prepared an extensive database of examination findings and performed analyses to determine the variables that predict likely treatment nonsuccess with manual physical therapy and exercise. Improvement of <12% in the total WOMAC score after 4 weeks of treatment defined nonsuccess.45
Data sets from the previously published trials contained 22 variables measured at baseline that were potential predictors of nonsuccess. We combined these variables with an additional 145 variables manually retrieved from standardized examination forms used for each subject, for a total of 167 potential predictors. We combined only data from treatment groups receiving manual therapy and exercise.
We limited the extent of some examination procedures in the earlier studies, due to the high level of symptoms experienced by some subjects at rest and during the initial examination. For example, if there was severe pain with active knee flexion, we did not perform passive manual overpressure to flexion; nor did we record a finding. Thus, the total number of data points for each subject varied somewhat.
Data analysis
We compared success and nonsuccess groups with 2-tailed unpaired t-tests for continuous variables, and chi-square tests for categorical variables. We additionally performed logistic regression analysis on potential predictors that yielded P values <.10, using a forward conditional stepwise procedure with probability levels set to .05 for entry and .10 for removal from the model. Predictors retained by the final logistic regression model comprised the CPR.
We coded each patient in the data set as positive or negative for each predictor in the CPR. To determine a cut score, we dichotomized the single retained continuous predictor variable using receiver-operator characteristic (ROC) curve analysis and the Youden index.46 For each CPR level (ie, increasing number of predictors positive), we constructed a 2 × 2 contingency table with numbers of patients with true-positive test results, false-positive test results, true-negative test results, and false-negative test results. We characterized prognostic performance of the CPR by calculating sensitivity, specificity, and positive likelihood ratios for each level of positive predictors. To determine overall prognostic accuracy, we added true positives and true negatives and divided by the total number of patients in the cross tabulation.
For each CPR level, we derived posttest probabilities of nonsuccess from generalized pretest probability (incidence of treatment nonsuccess in the sample) and the positive likelihood ratios.47 Finally, to determine how consistently the CPR performed with subjects in the original studies,1,2 we generated separate cross-tabulations and prognostic accuracy statistics from each RCT.
Results
Baseline patient attributes are summarized in TABLE 1. Of the 101 subjects in the combined data set, 17 (16.8%) met the definition of nonsuccess. Among 47 continuous-scale variables available, 11 predictors significantly discriminated between those in the treatment success and nonsuccess groups. Among 120 categorical-scale variables, 15 predictors significantly discriminated between groups. We identified 6 potential predictors for entry into the final logistic regression analysis: height, assistive device type, prone knee bend degrees, baseline WOMAC visual analog scale (VAS) for difficulty descending stairs, anterior cruciate ligament (ACL) laxity, and pain with passive patellofemoral glide.
TABLE 1
Baseline descriptive summaries of patients (n=101)
| Sex, n (%) Men Women | 37 (36.6) 64 (63.4) |
| Age, y Mean±SD Range | 62.5±10.4 39-85 |
| Height, m Mean±SD Range | 1.66±0.1041 1.42-1.91 |
| Side(s) involved, n (%) Unilateral Bilateral | 63 (62.4) 38 (37.6) |
| Weight, kg Mean±SD Range | 84.5±17.8 48.6-132.7 |
| Duration of symptoms, mo Mean±SD Range | 76.1±87.9 1-480 |
| WOMAC (VAS) total baseline, mm Mean±SD Range | 1059.8±447.1 193-2289 |
| 6-minute walk test baseline, m Mean±SD Range | 425.6±114.8 118.2-683.3 |
| Physical activity relative to peers (self-report), n (%) Much more active Somewhat more active About the same Somewhat less active | 26 (26) 33 (33) 20 (20) 21 (21) |
| Radiographic severity score, n (%) 0 1 2 3 4 | 6 (6.1) 25 (25.5) 33 (33.7) 25 (25.5) 9 (9.2) |
| *Baseline data were available for all 101 subjects except for duration of symptoms (n=98); physical activity (n=100); and radiographic severity (n=98). VAS, visual analog scale; WOMAC, Western Ontario MacMaster. | |
The final regression model retained 3 predictors comprising the CPR: height, ACL laxity, and pain with passive patellofemoral glides. We dichotomized height with a cut point of 1.71 m (5’7”), which corresponded with a deflection point at the upper left extent of the ROC curve (area under the curve=0.72; 95% CI, 0.57-0.87; P=.001). We thus deemed a patient 1.71 m or taller as positive for nonsuccess. We considered a patient with laxity of the ACL as positive for nonsuccess if a test result on the Lachman test (or the anterior drawer test) was positive (any grade other than 0). We regarded passive patellofemoral glide as positive for nonsuccess if a patient reported pain with any direction of passive gliding motion imposed by the therapist. The final regression model was a good fit to the data: Hosmer & Lemeshow test χ2 = 2.90 (P=.940); Nagelkerke R2=0.680.
TABLE 2 presents prognostic accuracy profiles for each predictor in the CPR; TABLE 3 summarizes the accuracy for each level of the multivariate CPR. Values in TABLE 3 reflect complete sets of data for the 3 predictors found for 50 patients. Of those 50 patients, 6 (12%) were in the nonsuccess group.
TABLE 2
Prognostic accuracy statistics for individual predictors
| Predictor | Sensitivity (95% CI) | Specificity (95% CI) | Positive likelihood ratio (95% CI) | Posttest probability of nonsuccess* |
|---|---|---|---|---|
| Height ≥1.71 m | 0.65 (0.41-0.83) | 0.77 (0.67-0.85) | 2.86 (1.69-4.86) | 37% |
| ACL laxity | 0.27 (0.10-0.57) | 0.93 (0.83-0.97) | 3.68 (0.96-14.19) | 43% |
| Pain with passive patellofemoral glide in any direction | 0.71 (0.35-0.92) | 0.61 (0.47-0.74) | 1.84 (1.03-3.31) | 27% |
| ACL, anterior cruciate ligament; CI, confidence interval. *Assumes pretest probability of nonsuccess=17% (incidence in this sample). | ||||
With any 2 of the 3 tests positive, the CPR yielded a sensitivity of 83% (95% CI, 44%-97%), specificity of 98% (95% CI, 88%-100%), and positive likelihood ratio of 36.7 (95% CI, 5.1-263.0). Only 2 patients out of 50 were misclassified (one false positive and one false negative) at this level of the CPR, yielding an overall prognostic accuracy of 96% (95% CI, 87%-99%). Application of the positive likelihood ratio for a patient with any 2 positive tests yielded a posttest probability of 88% for nonsuccess with this treatment.
TABLE 3
Prognostic accuracy statistics for 3-level clinical prediction rule
| CPR level | Sensitivity (95% CI) | Specificity (95% CI) | Positive likelihood ratio (95% CI) | Posttest probability of nonsuccess* |
|---|---|---|---|---|
| All 3 tests positive | 0.21 (0.05-0.58) | 0.99 (0.90-1.00) | 19.29 (0.87-428.09) | 80% |
| At least 2 tests positive | 0.83 (0.44-0.97) | 0.98 (0.88-1.00) | 36.67 (5.11-263.01) | 88% |
| At least 1 test positive | 0.92 (0.56-0.99) | 0.48 (0.34-0.62) | 1.78 (1.26-2.52) | 27% |
| CI, confidence interval; CPR, clinical prediction rule. *Assumes pretest probability of nonsuccess=17% (incidence in this sample). | ||||
In the sensitivity analysis, the CPR performed similarly well for patients in each of the 2 original studies when applied separately to the groups of patients. Among the 30 patients from the first trial2 who had data for all 3 predictors in the CPR, only one was misclassified (a false positive), yielding a prognostic accuracy of 97% (95% CI, 83%-99%). Among the 20 patients from the second trial1 who had data for all 3 predictors, only one was misclassified (a false negative), yielding a prognostic accuracy of 95% (95% CI, 76%-99%).
DISCUSSION
Family physicians and physical therapists should be able to discuss with confidence how any given patient with knee OA will likely respond to treatment options. Our study is a preliminary step toward defining the population of patients with knee OA who are unlikely to benefit from manual physical therapy and exercise. We found such patients to be those with height >1.71 m, ACL laxity, and pain with passive glides of the patellofemoral joint.
A limitation of our study is the retrospective nature of gathering data. However, retrospective CPR derivation studies have made valuable contributions to many areas of medical practice.48-53 Additionally, if there had been uniformly available data across all patients, there may have been other, perhaps more powerful, predictors for treatment nonsuccess.
Actual cases of knee osteoarthritis (OA) evaluated by one of the authors (GD)
A 48-year-old female elementary teacher was referred for physical therapy due to right knee pain and a diagnosis of OA that was limiting her ability to climb stairs and squat to work with children in the classroom. Her goals were to be able to perform these physical activities with less pain and to reduce her anti-inflammatory medications. However, she also worried about taking time away from her job to attend physical therapy appointments. She was 1.63 m (5’4”) tall and had a body mass index of 27.5 kg/m2. Her knee was stable to ligamentous testing, with mild limitation and pain with active and passive movement of both the tibiofemoral and the patellofemoral joints. She had weakness of the quadriceps and hip abductors, and moderate tightness of the calf muscles in both lower extremities.
Given the presence of only a single predictor for nonsuccess (pain with passive movement of her patella), the likelihood that this patient would not respond to manual physical therapy and exercise was just 27%, according to the clinical prediction rule. The impairments to movement, strength, and flexibility found during the physical examination typically can be successfully addressed with manual physical therapy. Additionally, one of the patient’s goals was to reduce her medication use—a reported outcome of the clinical trials used for deriving the rule.1,2 This patient was a good candidate for the intervention, with an acceptably small chance of not achieving a clinically meaningful benefit.
A 50-year-old male soldier 1.95 m (6’5”) tall was referred for physical therapy to ameliorate chronic pain due to tricompartmental knee OA. He exhibited anterior ligamentous laxity and felt severe pain with manually performed passive patellar glides (FIGURES 1 AND 2). He also had a rotator cuff tear and a mild traumatic brain injury from a roadside bomb blast. With 3/3 predictors for failure, the likelihood of reducing this patient’s knee symptoms with manual therapy and exercise was just 20%. The physical therapist and referring physician jointly decided to focus a small number of physical therapy visits on the patient’s shoulder, while giving rehabilitation priority to ongoing cognitive therapy appointments.
FIGURE 1
Lachman test
With the patient’s knee flexed at 30°, draw the proximal tibia anteriorly to observe movement of the tibia relative to the femur and thereby gauge anterior cruciate ligament integrity. Laxity is suggested by increased movement relative to the opposite knee.
FIGURE 2
Passive patellofemoral glide
With the patient’s knee slightly flexed, apply light pressure to the medial border of the patella, moving it laterally and taking care not to compress the patella. Repeat the procedure superiorly, inferiorly, and medially. A positive test is pain experienced with any of the glides.
Patient height >1.71 m is the least intuitive of the predictors for nonsuccess, but that underscores the value of data-driven prediction rules. Variables regarded as unimportant in a typical clinical assessment may show clinical usefulness if validated in independent studies. It may be that in taller patients with knee OA, biomechanical forces are such that a positive response to conservative therapy is less likely—particularly in the presence of ligamentous laxity or patellofemoral dysfunction.
For most patients with knee OA, the combined intervention of manual physical therapy and exercise is clinically beneficial, relatively inexpensive, and has no known adverse effects.54 However, unique circumstances may increase the importance of determining the likelihood that a patient will benefit. A validated CPR will facilitate timely decisions for those relatively few patients requiring alternative interventions. Although the rule is preliminary and needs to be validated, these results provide current best evidence to define patients with knee OA who are unlikely to respond to manual physical therapy and exercise.
1. Deyle GD, Allison SC, Matekel RL, et al. Physical therapy treatment effectiveness for osteoarthritis of the knee: a randomized comparison of supervised clinical exercise and manual therapy procedures versus a home exercise program. Phys Ther. 2005;85:1301-1317.
2. Deyle GD, Henderson NE, Matekel RL, et al. Effectiveness of manual physical therapy and exercise in osteoarthritis of the knee. A randomized, controlled trial. Ann Intern Med. 2000;132:173-181
3. Sandmark H, Hogstedt C, Vingard E. Primary osteoarthrosis of the knee in men and women as a result of lifelong physical load from work. Scand J Work Environ Health. 2000;26:20-25.
4. Felson DT, Zhang Y, Hannan MT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40:728-733.
5. Jarvholm B, From C, Lewold S, et al. Incidence of surgically treated osteoarthritis in the hip and knee in male construction workers. Occup Environ Med. 2008;65:275-278.
6. Messier SP, Loeser RF, Mitchell MN, et al. Exercise and weight loss in obese older adults with knee osteoarthritis: a preliminary study. J Am Geriatr Soc. 2000;48:1062-1072.
7. Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998;41:1343-1355.
8. Sandmark H, Vingard E. Sports and risk for severe osteoarthrosis of the knee. Scand J Med Sci Sports. 1999;9:279-284.
9. Gelber AC, Hochberg MC, Mead LA, et al. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133:321-328.
10. Cerejo R, Dunlop DD, Cahue S, et al. The influence of alignment on risk of knee osteoarthritis progression according to baseline stage of disease. Arthritis Rheum. 2002;46:2632-2636.
11. Wolfe F, Lane NE. The longterm outcome of osteoarthritis: rates and predictors of joint space narrowing in symptomatic patients with knee osteoarthritis. J Rheumatol. 2002;29:139-146.
12. Lewek MD, Rudolph KS, Snyder-Mackler L. Quadriceps femoris muscle weakness and activation failure in patients with symptomatic knee osteoarthritis. J Orthop Res. 2004;22:110-115.
13. Fitzgerald GK, Piva SR, Irrgang JJ. Reports of joint instability in knee osteoarthritis: its prevalence and relationship to physical function. Arthritis Rheum. 2004;51:941-946.
14. Fitzgerald GK, Piva SR, Irrgang JJ, et al. Quadriceps activation failure as a moderator of the relationship between quadriceps strength and physical function in individuals with knee osteoarthritis. Arthritis Rheum. 2004;51:40-48.
15. Scott DL, Berry H, Capell H, et al. The long-term effects of non-steroidal anti-inflammatory drugs in osteoarthritis of the knee: a randomized placebo-controlled trial. Rheumatology (Oxford). 2000;39:1095-1101.
16. Towheed TE, Maxwell L, Judd MG, et al. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;(1):CD004257.-
17. Kivitz A, Fairfax M, Sheldon EA, et al. Comparison of the effectiveness and tolerability of lidocaine patch 5% versus celecoxib for osteoarthritis-related knee pain: post hoc analysis of a 12 week, prospective, randomized, active-controlled, open-label, parallel-group trial in adults. Clin Ther. 2008;30:2366-2377.
18. Nussmeier NA, Whelton AA, Brown MT, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005;352:1081-1091.
19. Psaty BM, Furberg CD. COX-2 inhibitors—lessons in drug safety. N Engl J Med. 2005;352:1133-1135.
20. Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071-1080.
21. Wittenberg RH, Schell E, Krehan G, et al. First-dose analgesic effect of the cyclo-oxygenase-2 selective inhibitor lumiracoxib in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled comparison with celecoxib [NCT00267215]. Arthritis Res Ther. 2006;8:R35.-
22. Bradley JD, Heilman DK, Katz BP, et al. Tidal irrigation as treatment for knee osteoarthritis: a sham-controlled, randomized, double-blinded evaluation. Arthritis Rheum. 2002;46:100-108.
23. Chang RW, Falconer J, Stulberg SD, et al. A randomized, controlled trial of arthroscopic surgery versus closed-needle joint lavage for patients with osteoarthritis of the knee. Arthritis Rheum. 1993;36:289-296.
24. Moseley JB, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:81-88.
25. Laupattarakasem W, Laopaiboon M, Laupattarakasem P, et al. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst Rev. 2008;(1):CD005118.-
26. Hawker GA, Badley EM, Croxford R, et al. A population-based nested case-control study of the costs of hip and knee replacement surgery. Med Care. 2009;47:732-741.
27. Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169:1113-1121.
28. Hamel MB, Toth M, Legedza A, et al. Joint replacement surgery in elderly patients with severe osteoarthritis of the hip or knee: decision making, postoperative recovery, and clinical outcomes. Arch Intern Med. 2008;168:1430-1440.
29. Robertsson O, Stefansdottir A, Lidgren L, et al. Increased long-term mortality in patients less than 55 years old who have undergone knee replacement for osteoarthritis: results from the Swedish Knee Arthroplasty Register. J Bone Joint Surg Br. 2007;89:599-603.
30. SooHoo NF, Lieberman JR, Ko CY, et al. Factors predicting complication rates following total knee replacement. J Bone Joint Surg Am. 2006;88:480-485.
31. Solomon DH, Chibnik LB, Losina E, et al. Development of a preliminary index that predicts adverse events after total knee replacement. Arthritis Rheum. 2006;54:1536-1542.
32. Abularrage CJ, Weiswasser JM, Dezee KJ, et al. Predictors of lower extremity arterial injury after total knee or total hip arthroplasty. J Vasc Surg. 2008;47:803-807.
33. Parvizi J, Han SB, Tarity TD, et al. Postoperative ileus after total joint arthroplasty. J Arthroplasty. 2008;23:360-365.
34. Pinaroli A, Piedade SR, Servien E, et al. Intraoperative fractures and ligament tears during total knee arthroplasty. A 1795 posterostabilized TKA continuous series. Orthop Traumatol Surg Res. 2009;95:183-189
35. Bellamy N, Campbell J, Robinson V, et al. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005321.-
36. Baker K, McAlindon T. Exercise for knee osteoarthritis. Curr Opin Rheumatol. 2000;12:456-463.
37. Baker KR, Nelson ME, Felson DT, et al. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: a randomized controlled trial. J Rheumatol. 2001;28:1655-1665.
38. O’Reilly SC, Muir KR, Doherty M. Effectiveness of home exercise on pain and disability from osteoarthritis of the knee: a randomised controlled trial. Ann Rheum Dis. 1999;58:15-19.
39. Petrella RJ, Bartha C. Home based exercise therapy for older patients with knee osteoarthritis: a randomized clinical trial. J Rheumatol. 2000;27:2215-2221.
40. van Baar ME, Dekker J, Oostendorp RA, et al. Effectiveness of exercise in patients with osteoarthritis of hip or knee: nine months’ follow up. Ann Rheum Dis. 2001;60:1123-1130.
41. Silva LE, Valim V, Pessanha AP, et al. Hydrotherapy versus conventional land-based exercise for the management of patients with osteoarthritis of the knee: a randomized clinical trial. Phys Ther. 2008;88:12-21.
42. Hinman RS, Heywood SE, Day AR. Aquatic physical therapy for hip and knee osteoarthritis: results of a single-blind randomized controlled trial. Phys Ther. 2007;87:32-43.
43. Bellamy N. WOMAC: a 20-year experiential review of a patient-centered self-reported health status questionnaire. J Rheumatol. 2002;29:2473-2476.
44. Fritz JM. Clinical prediction rules in physical therapy: coming of age? J Orthop Sports Phys Ther. 2009;39:159-161
45. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45:384-391.
46. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32-35.
47. Fritz JM, Wainner RS. Examining diagnostic tests: an evidence-based perspective. Phys Ther. 2001;81:1546-1564.
48. van Walraven C, Hart RG, Wells GA, et al. A clinical prediction rule to identify patients with atrial fibrillation and a low risk for stroke while taking aspirin. Arch Intern Med. 2003;163:936-943.
49. Predictors of thromboembolism in atrial fibrillation: I. Clinical features of patients at risk. The Stroke Prevention in Atrial Fibrillation Investigators. Ann Intern Med. 1992;116:1-5
50. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041-1046.
51. Aujesky D, Obrosky DS, Stone RA, et al. A prediction rule to identify low-risk patients with pulmonary embolism. Arch Intern Med. 2006;166:169-175.
52. Espana PP, Capelastegui A, Gorordo I, et al. Development and validation of a clinical prediction rule for severe community-acquired pneumonia. Am J Respir Crit Care Med. 2006;174:1249-1256.
53. Kuijpers T, van der Heijden GJ, Vergouwe Y, et al. Good generalizability of a prediction rule for prediction of persistent shoulder pain in the short term. J Clin Epidemiol. 2007;60:947-953.
54. Ludica CA. Can a program of manual physical therapy and supervised exercise improve the symptoms of osteoarthritis of the knee? J Fam Pract. 2000;49:466-467
CORRESPONDENCE Gail D. Deyle, PT, DSc, Orthopaedic Manual Physical Therapy Fellowship, 3551 Roger Brooke Drive, Brooke Army Medical Center, Ft. Sam Houston, TX 78234; [email protected]
1. Deyle GD, Allison SC, Matekel RL, et al. Physical therapy treatment effectiveness for osteoarthritis of the knee: a randomized comparison of supervised clinical exercise and manual therapy procedures versus a home exercise program. Phys Ther. 2005;85:1301-1317.
2. Deyle GD, Henderson NE, Matekel RL, et al. Effectiveness of manual physical therapy and exercise in osteoarthritis of the knee. A randomized, controlled trial. Ann Intern Med. 2000;132:173-181
3. Sandmark H, Hogstedt C, Vingard E. Primary osteoarthrosis of the knee in men and women as a result of lifelong physical load from work. Scand J Work Environ Health. 2000;26:20-25.
4. Felson DT, Zhang Y, Hannan MT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40:728-733.
5. Jarvholm B, From C, Lewold S, et al. Incidence of surgically treated osteoarthritis in the hip and knee in male construction workers. Occup Environ Med. 2008;65:275-278.
6. Messier SP, Loeser RF, Mitchell MN, et al. Exercise and weight loss in obese older adults with knee osteoarthritis: a preliminary study. J Am Geriatr Soc. 2000;48:1062-1072.
7. Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998;41:1343-1355.
8. Sandmark H, Vingard E. Sports and risk for severe osteoarthrosis of the knee. Scand J Med Sci Sports. 1999;9:279-284.
9. Gelber AC, Hochberg MC, Mead LA, et al. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133:321-328.
10. Cerejo R, Dunlop DD, Cahue S, et al. The influence of alignment on risk of knee osteoarthritis progression according to baseline stage of disease. Arthritis Rheum. 2002;46:2632-2636.
11. Wolfe F, Lane NE. The longterm outcome of osteoarthritis: rates and predictors of joint space narrowing in symptomatic patients with knee osteoarthritis. J Rheumatol. 2002;29:139-146.
12. Lewek MD, Rudolph KS, Snyder-Mackler L. Quadriceps femoris muscle weakness and activation failure in patients with symptomatic knee osteoarthritis. J Orthop Res. 2004;22:110-115.
13. Fitzgerald GK, Piva SR, Irrgang JJ. Reports of joint instability in knee osteoarthritis: its prevalence and relationship to physical function. Arthritis Rheum. 2004;51:941-946.
14. Fitzgerald GK, Piva SR, Irrgang JJ, et al. Quadriceps activation failure as a moderator of the relationship between quadriceps strength and physical function in individuals with knee osteoarthritis. Arthritis Rheum. 2004;51:40-48.
15. Scott DL, Berry H, Capell H, et al. The long-term effects of non-steroidal anti-inflammatory drugs in osteoarthritis of the knee: a randomized placebo-controlled trial. Rheumatology (Oxford). 2000;39:1095-1101.
16. Towheed TE, Maxwell L, Judd MG, et al. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;(1):CD004257.-
17. Kivitz A, Fairfax M, Sheldon EA, et al. Comparison of the effectiveness and tolerability of lidocaine patch 5% versus celecoxib for osteoarthritis-related knee pain: post hoc analysis of a 12 week, prospective, randomized, active-controlled, open-label, parallel-group trial in adults. Clin Ther. 2008;30:2366-2377.
18. Nussmeier NA, Whelton AA, Brown MT, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005;352:1081-1091.
19. Psaty BM, Furberg CD. COX-2 inhibitors—lessons in drug safety. N Engl J Med. 2005;352:1133-1135.
20. Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071-1080.
21. Wittenberg RH, Schell E, Krehan G, et al. First-dose analgesic effect of the cyclo-oxygenase-2 selective inhibitor lumiracoxib in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled comparison with celecoxib [NCT00267215]. Arthritis Res Ther. 2006;8:R35.-
22. Bradley JD, Heilman DK, Katz BP, et al. Tidal irrigation as treatment for knee osteoarthritis: a sham-controlled, randomized, double-blinded evaluation. Arthritis Rheum. 2002;46:100-108.
23. Chang RW, Falconer J, Stulberg SD, et al. A randomized, controlled trial of arthroscopic surgery versus closed-needle joint lavage for patients with osteoarthritis of the knee. Arthritis Rheum. 1993;36:289-296.
24. Moseley JB, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:81-88.
25. Laupattarakasem W, Laopaiboon M, Laupattarakasem P, et al. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst Rev. 2008;(1):CD005118.-
26. Hawker GA, Badley EM, Croxford R, et al. A population-based nested case-control study of the costs of hip and knee replacement surgery. Med Care. 2009;47:732-741.
27. Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169:1113-1121.
28. Hamel MB, Toth M, Legedza A, et al. Joint replacement surgery in elderly patients with severe osteoarthritis of the hip or knee: decision making, postoperative recovery, and clinical outcomes. Arch Intern Med. 2008;168:1430-1440.
29. Robertsson O, Stefansdottir A, Lidgren L, et al. Increased long-term mortality in patients less than 55 years old who have undergone knee replacement for osteoarthritis: results from the Swedish Knee Arthroplasty Register. J Bone Joint Surg Br. 2007;89:599-603.
30. SooHoo NF, Lieberman JR, Ko CY, et al. Factors predicting complication rates following total knee replacement. J Bone Joint Surg Am. 2006;88:480-485.
31. Solomon DH, Chibnik LB, Losina E, et al. Development of a preliminary index that predicts adverse events after total knee replacement. Arthritis Rheum. 2006;54:1536-1542.
32. Abularrage CJ, Weiswasser JM, Dezee KJ, et al. Predictors of lower extremity arterial injury after total knee or total hip arthroplasty. J Vasc Surg. 2008;47:803-807.
33. Parvizi J, Han SB, Tarity TD, et al. Postoperative ileus after total joint arthroplasty. J Arthroplasty. 2008;23:360-365.
34. Pinaroli A, Piedade SR, Servien E, et al. Intraoperative fractures and ligament tears during total knee arthroplasty. A 1795 posterostabilized TKA continuous series. Orthop Traumatol Surg Res. 2009;95:183-189
35. Bellamy N, Campbell J, Robinson V, et al. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005321.-
36. Baker K, McAlindon T. Exercise for knee osteoarthritis. Curr Opin Rheumatol. 2000;12:456-463.
37. Baker KR, Nelson ME, Felson DT, et al. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: a randomized controlled trial. J Rheumatol. 2001;28:1655-1665.
38. O’Reilly SC, Muir KR, Doherty M. Effectiveness of home exercise on pain and disability from osteoarthritis of the knee: a randomised controlled trial. Ann Rheum Dis. 1999;58:15-19.
39. Petrella RJ, Bartha C. Home based exercise therapy for older patients with knee osteoarthritis: a randomized clinical trial. J Rheumatol. 2000;27:2215-2221.
40. van Baar ME, Dekker J, Oostendorp RA, et al. Effectiveness of exercise in patients with osteoarthritis of hip or knee: nine months’ follow up. Ann Rheum Dis. 2001;60:1123-1130.
41. Silva LE, Valim V, Pessanha AP, et al. Hydrotherapy versus conventional land-based exercise for the management of patients with osteoarthritis of the knee: a randomized clinical trial. Phys Ther. 2008;88:12-21.
42. Hinman RS, Heywood SE, Day AR. Aquatic physical therapy for hip and knee osteoarthritis: results of a single-blind randomized controlled trial. Phys Ther. 2007;87:32-43.
43. Bellamy N. WOMAC: a 20-year experiential review of a patient-centered self-reported health status questionnaire. J Rheumatol. 2002;29:2473-2476.
44. Fritz JM. Clinical prediction rules in physical therapy: coming of age? J Orthop Sports Phys Ther. 2009;39:159-161
45. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45:384-391.
46. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32-35.
47. Fritz JM, Wainner RS. Examining diagnostic tests: an evidence-based perspective. Phys Ther. 2001;81:1546-1564.
48. van Walraven C, Hart RG, Wells GA, et al. A clinical prediction rule to identify patients with atrial fibrillation and a low risk for stroke while taking aspirin. Arch Intern Med. 2003;163:936-943.
49. Predictors of thromboembolism in atrial fibrillation: I. Clinical features of patients at risk. The Stroke Prevention in Atrial Fibrillation Investigators. Ann Intern Med. 1992;116:1-5
50. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041-1046.
51. Aujesky D, Obrosky DS, Stone RA, et al. A prediction rule to identify low-risk patients with pulmonary embolism. Arch Intern Med. 2006;166:169-175.
52. Espana PP, Capelastegui A, Gorordo I, et al. Development and validation of a clinical prediction rule for severe community-acquired pneumonia. Am J Respir Crit Care Med. 2006;174:1249-1256.
53. Kuijpers T, van der Heijden GJ, Vergouwe Y, et al. Good generalizability of a prediction rule for prediction of persistent shoulder pain in the short term. J Clin Epidemiol. 2007;60:947-953.
54. Ludica CA. Can a program of manual physical therapy and supervised exercise improve the symptoms of osteoarthritis of the knee? J Fam Pract. 2000;49:466-467
CORRESPONDENCE Gail D. Deyle, PT, DSc, Orthopaedic Manual Physical Therapy Fellowship, 3551 Roger Brooke Drive, Brooke Army Medical Center, Ft. Sam Houston, TX 78234; [email protected]
Does ultrasound screening for abdominal aortic aneurysm reduce mortality?
YES, screening reduces mortality in men, although it’s unclear whether it has the same effect in women. Screening for aortic abdominal aneurysm (AAA) with ultrasound in men 65 to 79 years of age reduces AAA-specific mortality (number needed to screen [NNS] to prevent one death from AAA=769 men over 3 years). However, a trend toward reduced all-cause mortality doesn’t reach significance, possibly because of the low incidence of AAA (strength of recommendation [SOR]: A, systematic review of 4 population-based randomized controlled trials [RCTs]).
Evidence is inadequate to demonstrate benefits of screening in women.
Evidence summary
AAAs occur in 5% to 10% of men and 0.5% to 1.5% of women between 65 and 79 years of age.1,2 Risk factors include age, smoking, male sex, and family history.2 AAAs are 3 to 5 times more likely in patients with a smoking history.2 Approximately 9000 deaths annually are linked to AAAs in the United States, mostly in men older than 65 years.2 Mortality after rupture approaches 80% for patients who reach a hospital and 50% for patients who undergo emergent surgery.2
Screening reduces AAA deaths in men, but not all-cause mortality
A Cochrane review assessing the use of ultrasound to screen for AAA analyzed 4 population-based RCTs involving 127,891 men and 9342 women.1 Participants in each trial were randomly assigned to screening with ultrasound or no intervention.
The reviewers reported that screening significantly reduced mortality from AAA in men 65 to 79 years of age (odds ratio [OR]=0.60; 95% confidence interval [CI], 0.47-0.78). They found no support for decreased mortality in women (OR=1.99; 95% CI, 0.36-10.88).
The study also found no significant reduction in all-cause mortality 3 to 5 years after screening in men 65 to 79 years of age (OR=0.95; 95% CI, 0.85-1.07) or women (OR=1.06; 95% CI, 0.93-1.21), probably because of the low overall incidence of AAA.1 For men 65 to 79 years of age, the NNS is 769 over 3 years to prevent one death.1
Limitations of the study include disproportionate male representation because only 1 of the 4 trials in the Cochrane review enrolled women. Moreover, the analysis didn’t include smoking, although smoking increases the risk of AAA 3- to 5-fold. The NNS may be significantly different for smokers than nonsmokers.1,2
Recommendations
The US Preventive Services Task Force (USPSTF) recommends a one-time ultrasound screening for AAA in men between 65 and 74 years of age who have ever smoked.2 The USPSTF advises against routine screening in women and concludes that insufficient evidence exists to advocate for or against routine screening in men 65 to 74 years who have never smoked.2
The Canadian Society for Vascular Surgery recommends a population-based screening program for men 65 to 75 years of age who are candidates for surgery and are willing to participate.3
Acknowledgements
The opinions and assertions contained herein are the private views of the authors and not to be construed as official nor as reflecting the views of the United States Air Force Medical Service or the US Air Force at large.
1. Cosford PA, Leng GC. Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev. 2007;(2):CD002945.-
2. Fleming C, Whitlock EP, Beil TL, et al. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med. 2005;142:203-211.
3. Mastracci TM, Cina CS. Screening for abdominal aortic aneurysm in Canada: review and position statement of the Canadian Society for Vascular Surgery. J Vasc Surg. 2007;45:1268-1276.
YES, screening reduces mortality in men, although it’s unclear whether it has the same effect in women. Screening for aortic abdominal aneurysm (AAA) with ultrasound in men 65 to 79 years of age reduces AAA-specific mortality (number needed to screen [NNS] to prevent one death from AAA=769 men over 3 years). However, a trend toward reduced all-cause mortality doesn’t reach significance, possibly because of the low incidence of AAA (strength of recommendation [SOR]: A, systematic review of 4 population-based randomized controlled trials [RCTs]).
Evidence is inadequate to demonstrate benefits of screening in women.
Evidence summary
AAAs occur in 5% to 10% of men and 0.5% to 1.5% of women between 65 and 79 years of age.1,2 Risk factors include age, smoking, male sex, and family history.2 AAAs are 3 to 5 times more likely in patients with a smoking history.2 Approximately 9000 deaths annually are linked to AAAs in the United States, mostly in men older than 65 years.2 Mortality after rupture approaches 80% for patients who reach a hospital and 50% for patients who undergo emergent surgery.2
Screening reduces AAA deaths in men, but not all-cause mortality
A Cochrane review assessing the use of ultrasound to screen for AAA analyzed 4 population-based RCTs involving 127,891 men and 9342 women.1 Participants in each trial were randomly assigned to screening with ultrasound or no intervention.
The reviewers reported that screening significantly reduced mortality from AAA in men 65 to 79 years of age (odds ratio [OR]=0.60; 95% confidence interval [CI], 0.47-0.78). They found no support for decreased mortality in women (OR=1.99; 95% CI, 0.36-10.88).
The study also found no significant reduction in all-cause mortality 3 to 5 years after screening in men 65 to 79 years of age (OR=0.95; 95% CI, 0.85-1.07) or women (OR=1.06; 95% CI, 0.93-1.21), probably because of the low overall incidence of AAA.1 For men 65 to 79 years of age, the NNS is 769 over 3 years to prevent one death.1
Limitations of the study include disproportionate male representation because only 1 of the 4 trials in the Cochrane review enrolled women. Moreover, the analysis didn’t include smoking, although smoking increases the risk of AAA 3- to 5-fold. The NNS may be significantly different for smokers than nonsmokers.1,2
Recommendations
The US Preventive Services Task Force (USPSTF) recommends a one-time ultrasound screening for AAA in men between 65 and 74 years of age who have ever smoked.2 The USPSTF advises against routine screening in women and concludes that insufficient evidence exists to advocate for or against routine screening in men 65 to 74 years who have never smoked.2
The Canadian Society for Vascular Surgery recommends a population-based screening program for men 65 to 75 years of age who are candidates for surgery and are willing to participate.3
Acknowledgements
The opinions and assertions contained herein are the private views of the authors and not to be construed as official nor as reflecting the views of the United States Air Force Medical Service or the US Air Force at large.
YES, screening reduces mortality in men, although it’s unclear whether it has the same effect in women. Screening for aortic abdominal aneurysm (AAA) with ultrasound in men 65 to 79 years of age reduces AAA-specific mortality (number needed to screen [NNS] to prevent one death from AAA=769 men over 3 years). However, a trend toward reduced all-cause mortality doesn’t reach significance, possibly because of the low incidence of AAA (strength of recommendation [SOR]: A, systematic review of 4 population-based randomized controlled trials [RCTs]).
Evidence is inadequate to demonstrate benefits of screening in women.
Evidence summary
AAAs occur in 5% to 10% of men and 0.5% to 1.5% of women between 65 and 79 years of age.1,2 Risk factors include age, smoking, male sex, and family history.2 AAAs are 3 to 5 times more likely in patients with a smoking history.2 Approximately 9000 deaths annually are linked to AAAs in the United States, mostly in men older than 65 years.2 Mortality after rupture approaches 80% for patients who reach a hospital and 50% for patients who undergo emergent surgery.2
Screening reduces AAA deaths in men, but not all-cause mortality
A Cochrane review assessing the use of ultrasound to screen for AAA analyzed 4 population-based RCTs involving 127,891 men and 9342 women.1 Participants in each trial were randomly assigned to screening with ultrasound or no intervention.
The reviewers reported that screening significantly reduced mortality from AAA in men 65 to 79 years of age (odds ratio [OR]=0.60; 95% confidence interval [CI], 0.47-0.78). They found no support for decreased mortality in women (OR=1.99; 95% CI, 0.36-10.88).
The study also found no significant reduction in all-cause mortality 3 to 5 years after screening in men 65 to 79 years of age (OR=0.95; 95% CI, 0.85-1.07) or women (OR=1.06; 95% CI, 0.93-1.21), probably because of the low overall incidence of AAA.1 For men 65 to 79 years of age, the NNS is 769 over 3 years to prevent one death.1
Limitations of the study include disproportionate male representation because only 1 of the 4 trials in the Cochrane review enrolled women. Moreover, the analysis didn’t include smoking, although smoking increases the risk of AAA 3- to 5-fold. The NNS may be significantly different for smokers than nonsmokers.1,2
Recommendations
The US Preventive Services Task Force (USPSTF) recommends a one-time ultrasound screening for AAA in men between 65 and 74 years of age who have ever smoked.2 The USPSTF advises against routine screening in women and concludes that insufficient evidence exists to advocate for or against routine screening in men 65 to 74 years who have never smoked.2
The Canadian Society for Vascular Surgery recommends a population-based screening program for men 65 to 75 years of age who are candidates for surgery and are willing to participate.3
Acknowledgements
The opinions and assertions contained herein are the private views of the authors and not to be construed as official nor as reflecting the views of the United States Air Force Medical Service or the US Air Force at large.
1. Cosford PA, Leng GC. Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev. 2007;(2):CD002945.-
2. Fleming C, Whitlock EP, Beil TL, et al. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med. 2005;142:203-211.
3. Mastracci TM, Cina CS. Screening for abdominal aortic aneurysm in Canada: review and position statement of the Canadian Society for Vascular Surgery. J Vasc Surg. 2007;45:1268-1276.
1. Cosford PA, Leng GC. Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev. 2007;(2):CD002945.-
2. Fleming C, Whitlock EP, Beil TL, et al. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med. 2005;142:203-211.
3. Mastracci TM, Cina CS. Screening for abdominal aortic aneurysm in Canada: review and position statement of the Canadian Society for Vascular Surgery. J Vasc Surg. 2007;45:1268-1276.
Evidence-based answers from the Family Physicians Inquiries Network
HPV vaccine is now routinely indicated for males
At its October 2011 meeting, the Advisory Committee on Immunization Practices (ACIP) recommended to the CDC that quadrivalent human papilloma virus vaccine (HPV4, Gardasil) be routinely given to all males ages 11 to 21 and to men ages 22 to 26 who have sex with men or who are HIV positive, if they have not been previously vaccinated. This replaces a 2009 recommendation that stated HPV4 vaccine could be used in males to prevent genital warts, but stopped short of advocating routine use for all males.1
There were 3 reasons the previous recommendation did not include HPV4 for routine vaccination of males:
- The vaccine had been shown to be effective only for prevention of genital warts.
- The cost effectiveness of the vaccine for use in boys was poor and, in modeling, it yielded less benefit as more females were vaccinated.
- It was thought that a more effective approach to preventing HPV disease would be to emphasize high rates of vaccination of females.
The new recommendation takes into account recent evidence demonstrating that the vaccine prevents anal intraepithelial neoplasia (AIN) in males, in addition to genital warts. Moreover, vaccination rates in females remain low, which makes vaccinating males more cost effective and additionally protective for females.
Female vaccination rates lower than expected
Despite its effectiveness and safety record, HPV vaccination has had a slow rate of acceptance among females ages 13 to 17 years. Coverage for this group documented in the last national vaccine survey was 48.7% for one dose and 32% for the recommended 3 doses.2
The vaccine is effective in preventing cervical intraepithelial neoplasia (TABLE 1),3 condyloma, and vaginal intraepithelial neoplasia in women ~15 to 26 years of age. Large studies of vaccine safety have documented no serious adverse reactions, other than syncope, which could occur as frequently as 17.9/10,000 females and 12.5/10,000 males.4 Another study that involved post-licensure safety data from >600,000 HPV4 doses found no increased risk for a variety of outcomes, including Guillain-Barré syndrome, stroke, venous thromboembolism, appendicitis, seizures, syncope, allergic reactions, and anaphylaxis.5,6
TABLE 1
HPV vaccine efficacy against HPV type-related CIN2+ in females ages ~15 to 26 years3
| Vaccine/HPV type | Vaccine | Placebo | Efficacy | |||
|---|---|---|---|---|---|---|
| N | CIN cases | N | CIN cases | % | CI* | |
| Bivalent HPV 16/18 HPV 16 HPV 18 | 7344 6303 6794 | 4 2 2 | 7312 6165 6746 | 56 46 15 | 93 96 87 | 80-98 83-100 40-99 |
| Quadrivalent HPV 16/18 HPV 16 HPV 18 | 7738 6647 7382 | 2 2 0 | 7714 6455 7316 | 100 81 29 | 98 98 100 | 93-100 91-100 87-100 |
| CI, confidence interval; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus. *Confidence interval for bivalent results was 96.1%, and for quadrivalent results was 95%. | ||||||
HPV-associated disease in males
HPV causes anal, penile, and oropharyngeal cancers in males, with about 7500 cancers occurring each year in the United States.3 In addition, about 1% of sexually active males in America have genital warts at any one time.7 HPV types 6 and 11 cause about 90% of cases.1
The HPV4 vaccine—when all 3 doses are given—is 89.3% effective in preventing genital warts related to HPV types 6 and 11. Even a single dose is 68.1% effective (95% CI, 48.8–80.7).1 New evidence shows that HPV4 prevents AIN, which can lead to anal cancer.8 Effectiveness in preventing AIN 2/3 is 74.9% (95% CI, 8.8–95.4) in those completing 3 doses before onset of infection with one of the HPV types contained in vaccine. Notably, these results were obtained in a subgroup analysis of men who have sex with men. And although the reduction in AIN is expected to lower the incidence of anal cancer, ongoing studies require time to confirm this. If such a reduction is confirmed (and vaccination is started at age 12 in the general male population), the number-needed-to-vaccinate to prevent one case of genital warts would be 18, and to prevent one case of anal cancer, 1581.6
No studies have evaluated efficacy of HPV4 in preventing penile or oropharyngeal cancers.
Men who have sex with men at high risk
Men who have sex with men have higher rates of AIN, anal cancers, and genital warts than the general male population.3 Those who are additionally HIV positive have higher rates of genital warts, which are also more difficult to treat.3 AIN is also more common in HIV-infected males.3 The HPV4 vaccine is immunogenic in those who are HIV infected, although the resulting antibody titers are lower than in other populations.
A look at the 2 HPV vaccines
Two HPV vaccines are available (TABLE 2).3 HPV4 vaccine protects against HPV 6, 11, 16, and 18. Bivalent (HPV2, Cervarix) vaccine contains antigens from HPV 16 and 18. Both vaccines are approved for use in females for the prevention of cervical cancer; HPV4 is preferred if protection against genital warts is also desired. Only HPV4 has been licensed for use in males.
TABLE 2
A look at the human papillomavirus vaccines3
| Quadrivalent (Gardasil) | Bivalent (Cervarix) | |
|---|---|---|
| Manufacturer/VLP types | Merck/6, 11, 16, 18 | GlaxoSmithKline/16, 18 |
| Date of US licensure | 2006, females 2009, males | 2009, females |
| Dose of protein | 20/40/40/20 μg | 20/20 μg |
| Producer cells | Saccharomyces cerevisiae (yeast) | Baculovirus-infected Trichoplusia ni (insect cell line) |
| Adjuvant | AAHS: 225 μg amorphous aluminum hydroxyphosphate sulfate | AS04: 500 μg aluminum hydroxide; 50 μg 3-O-desacyl-4’-monophosphoryl lipid A |
| Schedule (IM) | 3-dose series | 3-dose series |
| VLP, virus-like particle; IM, intramuscular. | ||
HPV vaccine is effective, but costly
A major consideration with HPV vaccines is their cost. With 3 doses required and each dose costing about $130,9 cost effectiveness is poor when preventing uncommon diseases such as cervical and anal cancer, and a relatively benign disease such as genital warts. Male vaccination at age 12 years, when added to a female vaccination program, costs about $20,000 to $40,000 per quality-adjusted life year (QALY) if all potential HPV morbidity is included, not just that which has been proven to be prevented by the vaccine (assuming oral and penile cancer will also be prevented). Counting only HPV disease demonstrated to be prevented by the vaccine, the result is $75,000 to $250,000+ per QALY.6 Vaccinating males older than 21 years results in a cost per QALY 2 to 4 times that of vaccinating males younger than 18 years.10
A final decision. After considering these factors, ACIP approved a set of recommendations at its October 2011 meeting that will become official once they are published in the Morbidity and Mortality Weekly Report. (See “ACIP recommendations for HPV vaccine use in males”.)
- Routinely vaccinate males ages 11 to 12 years with 3 doses of HPV4. The vaccination series can be started at 9 years of age. (A recommendation)
- Vaccinate males, ages 13 to 21 years, who have not been vaccinated previously or who have not completed the 3-dose series. (A recommendation)
- Consider vaccinating males ages 22 to 26 years. (B recommendation)
- Vaccinate men ages 22 to 26 years of age who have sex with men and those in this age group who are HIV positive, if they have not been previously vaccinated. (A recommendation)
Levels of recommendation
A: Applies to all individuals in an age- or risk factor-based group.
B: Defers to clinician judgment in determining benefit for individuals.
Source: ACIP meeting; October 25, 2011; Atlanta, Ga.
1. CDC. FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2010;59:630-632.
2. CDC. National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60:1117-1123.
3. Markowitz L. HPV vaccine for males: background and review of data. Presented at: ACIP meeting; October 25, 2011; Atlanta, GA. http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-oct11/04-HPV-Markowitz.pdf. Accessed December 2, 2011.
4. Gee J. Safety of quadrivalent human papilloma virus (HPV4) vaccine. Presented at: ACIP meeting; October 25, 2011; Atlanta, GA. http://www.cdc.gov/vaccines/recs/acip/downloads/ mtg-slides-oct11/02-HPV-Gee.pdf. Accessed December 2, 2011.
5. Gee J, Naleway A, Shui I, et al. Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the Vaccine Safety Datalink. Vaccine. 2011;29:8279-8284.
6. Dunne EF. HPV vaccine considerations for males. Presented at: ACIP meeting; October 25, 2011; Atlanta, GA. http://www.cdc. gov/vaccines/recs/acip/downloads/mtg-slides-oct11/05-HPVDunne.pdf. Accessed December 2, 2011.
7. CDC. HPV and men—fact sheet. http://www.cdc.gov/std/hpv/std/hpv/stdfact-hpv-and-men.htm. Accessed December 19, 2011.
8. Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
9. CDC. Sexually transmitted diseases (STDs): HPV vaccine information for young women—fact sheet. http://www.cdc.gov/std/hpv/stdfact-hpv-vaccine-young-women.htm. Accessed December 2, 2011.
10. Chesson HW. HPV vaccine cost-effectiveness: updates and review. Presented at: ACIP meeting; June 22, 2011; Atlanta, GA. http://www.cdc.gov/vaccines/recs/acip/down-loads/mtg-slides-jun11/07-5-hpv-cost-effect.pdf. Accessed December 2, 2011.
At its October 2011 meeting, the Advisory Committee on Immunization Practices (ACIP) recommended to the CDC that quadrivalent human papilloma virus vaccine (HPV4, Gardasil) be routinely given to all males ages 11 to 21 and to men ages 22 to 26 who have sex with men or who are HIV positive, if they have not been previously vaccinated. This replaces a 2009 recommendation that stated HPV4 vaccine could be used in males to prevent genital warts, but stopped short of advocating routine use for all males.1
There were 3 reasons the previous recommendation did not include HPV4 for routine vaccination of males:
- The vaccine had been shown to be effective only for prevention of genital warts.
- The cost effectiveness of the vaccine for use in boys was poor and, in modeling, it yielded less benefit as more females were vaccinated.
- It was thought that a more effective approach to preventing HPV disease would be to emphasize high rates of vaccination of females.
The new recommendation takes into account recent evidence demonstrating that the vaccine prevents anal intraepithelial neoplasia (AIN) in males, in addition to genital warts. Moreover, vaccination rates in females remain low, which makes vaccinating males more cost effective and additionally protective for females.
Female vaccination rates lower than expected
Despite its effectiveness and safety record, HPV vaccination has had a slow rate of acceptance among females ages 13 to 17 years. Coverage for this group documented in the last national vaccine survey was 48.7% for one dose and 32% for the recommended 3 doses.2
The vaccine is effective in preventing cervical intraepithelial neoplasia (TABLE 1),3 condyloma, and vaginal intraepithelial neoplasia in women ~15 to 26 years of age. Large studies of vaccine safety have documented no serious adverse reactions, other than syncope, which could occur as frequently as 17.9/10,000 females and 12.5/10,000 males.4 Another study that involved post-licensure safety data from >600,000 HPV4 doses found no increased risk for a variety of outcomes, including Guillain-Barré syndrome, stroke, venous thromboembolism, appendicitis, seizures, syncope, allergic reactions, and anaphylaxis.5,6
TABLE 1
HPV vaccine efficacy against HPV type-related CIN2+ in females ages ~15 to 26 years3
| Vaccine/HPV type | Vaccine | Placebo | Efficacy | |||
|---|---|---|---|---|---|---|
| N | CIN cases | N | CIN cases | % | CI* | |
| Bivalent HPV 16/18 HPV 16 HPV 18 | 7344 6303 6794 | 4 2 2 | 7312 6165 6746 | 56 46 15 | 93 96 87 | 80-98 83-100 40-99 |
| Quadrivalent HPV 16/18 HPV 16 HPV 18 | 7738 6647 7382 | 2 2 0 | 7714 6455 7316 | 100 81 29 | 98 98 100 | 93-100 91-100 87-100 |
| CI, confidence interval; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus. *Confidence interval for bivalent results was 96.1%, and for quadrivalent results was 95%. | ||||||
HPV-associated disease in males
HPV causes anal, penile, and oropharyngeal cancers in males, with about 7500 cancers occurring each year in the United States.3 In addition, about 1% of sexually active males in America have genital warts at any one time.7 HPV types 6 and 11 cause about 90% of cases.1
The HPV4 vaccine—when all 3 doses are given—is 89.3% effective in preventing genital warts related to HPV types 6 and 11. Even a single dose is 68.1% effective (95% CI, 48.8–80.7).1 New evidence shows that HPV4 prevents AIN, which can lead to anal cancer.8 Effectiveness in preventing AIN 2/3 is 74.9% (95% CI, 8.8–95.4) in those completing 3 doses before onset of infection with one of the HPV types contained in vaccine. Notably, these results were obtained in a subgroup analysis of men who have sex with men. And although the reduction in AIN is expected to lower the incidence of anal cancer, ongoing studies require time to confirm this. If such a reduction is confirmed (and vaccination is started at age 12 in the general male population), the number-needed-to-vaccinate to prevent one case of genital warts would be 18, and to prevent one case of anal cancer, 1581.6
No studies have evaluated efficacy of HPV4 in preventing penile or oropharyngeal cancers.
Men who have sex with men at high risk
Men who have sex with men have higher rates of AIN, anal cancers, and genital warts than the general male population.3 Those who are additionally HIV positive have higher rates of genital warts, which are also more difficult to treat.3 AIN is also more common in HIV-infected males.3 The HPV4 vaccine is immunogenic in those who are HIV infected, although the resulting antibody titers are lower than in other populations.
A look at the 2 HPV vaccines
Two HPV vaccines are available (TABLE 2).3 HPV4 vaccine protects against HPV 6, 11, 16, and 18. Bivalent (HPV2, Cervarix) vaccine contains antigens from HPV 16 and 18. Both vaccines are approved for use in females for the prevention of cervical cancer; HPV4 is preferred if protection against genital warts is also desired. Only HPV4 has been licensed for use in males.
TABLE 2
A look at the human papillomavirus vaccines3
| Quadrivalent (Gardasil) | Bivalent (Cervarix) | |
|---|---|---|
| Manufacturer/VLP types | Merck/6, 11, 16, 18 | GlaxoSmithKline/16, 18 |
| Date of US licensure | 2006, females 2009, males | 2009, females |
| Dose of protein | 20/40/40/20 μg | 20/20 μg |
| Producer cells | Saccharomyces cerevisiae (yeast) | Baculovirus-infected Trichoplusia ni (insect cell line) |
| Adjuvant | AAHS: 225 μg amorphous aluminum hydroxyphosphate sulfate | AS04: 500 μg aluminum hydroxide; 50 μg 3-O-desacyl-4’-monophosphoryl lipid A |
| Schedule (IM) | 3-dose series | 3-dose series |
| VLP, virus-like particle; IM, intramuscular. | ||
HPV vaccine is effective, but costly
A major consideration with HPV vaccines is their cost. With 3 doses required and each dose costing about $130,9 cost effectiveness is poor when preventing uncommon diseases such as cervical and anal cancer, and a relatively benign disease such as genital warts. Male vaccination at age 12 years, when added to a female vaccination program, costs about $20,000 to $40,000 per quality-adjusted life year (QALY) if all potential HPV morbidity is included, not just that which has been proven to be prevented by the vaccine (assuming oral and penile cancer will also be prevented). Counting only HPV disease demonstrated to be prevented by the vaccine, the result is $75,000 to $250,000+ per QALY.6 Vaccinating males older than 21 years results in a cost per QALY 2 to 4 times that of vaccinating males younger than 18 years.10
A final decision. After considering these factors, ACIP approved a set of recommendations at its October 2011 meeting that will become official once they are published in the Morbidity and Mortality Weekly Report. (See “ACIP recommendations for HPV vaccine use in males”.)
- Routinely vaccinate males ages 11 to 12 years with 3 doses of HPV4. The vaccination series can be started at 9 years of age. (A recommendation)
- Vaccinate males, ages 13 to 21 years, who have not been vaccinated previously or who have not completed the 3-dose series. (A recommendation)
- Consider vaccinating males ages 22 to 26 years. (B recommendation)
- Vaccinate men ages 22 to 26 years of age who have sex with men and those in this age group who are HIV positive, if they have not been previously vaccinated. (A recommendation)
Levels of recommendation
A: Applies to all individuals in an age- or risk factor-based group.
B: Defers to clinician judgment in determining benefit for individuals.
Source: ACIP meeting; October 25, 2011; Atlanta, Ga.
At its October 2011 meeting, the Advisory Committee on Immunization Practices (ACIP) recommended to the CDC that quadrivalent human papilloma virus vaccine (HPV4, Gardasil) be routinely given to all males ages 11 to 21 and to men ages 22 to 26 who have sex with men or who are HIV positive, if they have not been previously vaccinated. This replaces a 2009 recommendation that stated HPV4 vaccine could be used in males to prevent genital warts, but stopped short of advocating routine use for all males.1
There were 3 reasons the previous recommendation did not include HPV4 for routine vaccination of males:
- The vaccine had been shown to be effective only for prevention of genital warts.
- The cost effectiveness of the vaccine for use in boys was poor and, in modeling, it yielded less benefit as more females were vaccinated.
- It was thought that a more effective approach to preventing HPV disease would be to emphasize high rates of vaccination of females.
The new recommendation takes into account recent evidence demonstrating that the vaccine prevents anal intraepithelial neoplasia (AIN) in males, in addition to genital warts. Moreover, vaccination rates in females remain low, which makes vaccinating males more cost effective and additionally protective for females.
Female vaccination rates lower than expected
Despite its effectiveness and safety record, HPV vaccination has had a slow rate of acceptance among females ages 13 to 17 years. Coverage for this group documented in the last national vaccine survey was 48.7% for one dose and 32% for the recommended 3 doses.2
The vaccine is effective in preventing cervical intraepithelial neoplasia (TABLE 1),3 condyloma, and vaginal intraepithelial neoplasia in women ~15 to 26 years of age. Large studies of vaccine safety have documented no serious adverse reactions, other than syncope, which could occur as frequently as 17.9/10,000 females and 12.5/10,000 males.4 Another study that involved post-licensure safety data from >600,000 HPV4 doses found no increased risk for a variety of outcomes, including Guillain-Barré syndrome, stroke, venous thromboembolism, appendicitis, seizures, syncope, allergic reactions, and anaphylaxis.5,6
TABLE 1
HPV vaccine efficacy against HPV type-related CIN2+ in females ages ~15 to 26 years3
| Vaccine/HPV type | Vaccine | Placebo | Efficacy | |||
|---|---|---|---|---|---|---|
| N | CIN cases | N | CIN cases | % | CI* | |
| Bivalent HPV 16/18 HPV 16 HPV 18 | 7344 6303 6794 | 4 2 2 | 7312 6165 6746 | 56 46 15 | 93 96 87 | 80-98 83-100 40-99 |
| Quadrivalent HPV 16/18 HPV 16 HPV 18 | 7738 6647 7382 | 2 2 0 | 7714 6455 7316 | 100 81 29 | 98 98 100 | 93-100 91-100 87-100 |
| CI, confidence interval; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus. *Confidence interval for bivalent results was 96.1%, and for quadrivalent results was 95%. | ||||||
HPV-associated disease in males
HPV causes anal, penile, and oropharyngeal cancers in males, with about 7500 cancers occurring each year in the United States.3 In addition, about 1% of sexually active males in America have genital warts at any one time.7 HPV types 6 and 11 cause about 90% of cases.1
The HPV4 vaccine—when all 3 doses are given—is 89.3% effective in preventing genital warts related to HPV types 6 and 11. Even a single dose is 68.1% effective (95% CI, 48.8–80.7).1 New evidence shows that HPV4 prevents AIN, which can lead to anal cancer.8 Effectiveness in preventing AIN 2/3 is 74.9% (95% CI, 8.8–95.4) in those completing 3 doses before onset of infection with one of the HPV types contained in vaccine. Notably, these results were obtained in a subgroup analysis of men who have sex with men. And although the reduction in AIN is expected to lower the incidence of anal cancer, ongoing studies require time to confirm this. If such a reduction is confirmed (and vaccination is started at age 12 in the general male population), the number-needed-to-vaccinate to prevent one case of genital warts would be 18, and to prevent one case of anal cancer, 1581.6
No studies have evaluated efficacy of HPV4 in preventing penile or oropharyngeal cancers.
Men who have sex with men at high risk
Men who have sex with men have higher rates of AIN, anal cancers, and genital warts than the general male population.3 Those who are additionally HIV positive have higher rates of genital warts, which are also more difficult to treat.3 AIN is also more common in HIV-infected males.3 The HPV4 vaccine is immunogenic in those who are HIV infected, although the resulting antibody titers are lower than in other populations.
A look at the 2 HPV vaccines
Two HPV vaccines are available (TABLE 2).3 HPV4 vaccine protects against HPV 6, 11, 16, and 18. Bivalent (HPV2, Cervarix) vaccine contains antigens from HPV 16 and 18. Both vaccines are approved for use in females for the prevention of cervical cancer; HPV4 is preferred if protection against genital warts is also desired. Only HPV4 has been licensed for use in males.
TABLE 2
A look at the human papillomavirus vaccines3
| Quadrivalent (Gardasil) | Bivalent (Cervarix) | |
|---|---|---|
| Manufacturer/VLP types | Merck/6, 11, 16, 18 | GlaxoSmithKline/16, 18 |
| Date of US licensure | 2006, females 2009, males | 2009, females |
| Dose of protein | 20/40/40/20 μg | 20/20 μg |
| Producer cells | Saccharomyces cerevisiae (yeast) | Baculovirus-infected Trichoplusia ni (insect cell line) |
| Adjuvant | AAHS: 225 μg amorphous aluminum hydroxyphosphate sulfate | AS04: 500 μg aluminum hydroxide; 50 μg 3-O-desacyl-4’-monophosphoryl lipid A |
| Schedule (IM) | 3-dose series | 3-dose series |
| VLP, virus-like particle; IM, intramuscular. | ||
HPV vaccine is effective, but costly
A major consideration with HPV vaccines is their cost. With 3 doses required and each dose costing about $130,9 cost effectiveness is poor when preventing uncommon diseases such as cervical and anal cancer, and a relatively benign disease such as genital warts. Male vaccination at age 12 years, when added to a female vaccination program, costs about $20,000 to $40,000 per quality-adjusted life year (QALY) if all potential HPV morbidity is included, not just that which has been proven to be prevented by the vaccine (assuming oral and penile cancer will also be prevented). Counting only HPV disease demonstrated to be prevented by the vaccine, the result is $75,000 to $250,000+ per QALY.6 Vaccinating males older than 21 years results in a cost per QALY 2 to 4 times that of vaccinating males younger than 18 years.10
A final decision. After considering these factors, ACIP approved a set of recommendations at its October 2011 meeting that will become official once they are published in the Morbidity and Mortality Weekly Report. (See “ACIP recommendations for HPV vaccine use in males”.)
- Routinely vaccinate males ages 11 to 12 years with 3 doses of HPV4. The vaccination series can be started at 9 years of age. (A recommendation)
- Vaccinate males, ages 13 to 21 years, who have not been vaccinated previously or who have not completed the 3-dose series. (A recommendation)
- Consider vaccinating males ages 22 to 26 years. (B recommendation)
- Vaccinate men ages 22 to 26 years of age who have sex with men and those in this age group who are HIV positive, if they have not been previously vaccinated. (A recommendation)
Levels of recommendation
A: Applies to all individuals in an age- or risk factor-based group.
B: Defers to clinician judgment in determining benefit for individuals.
Source: ACIP meeting; October 25, 2011; Atlanta, Ga.
1. CDC. FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2010;59:630-632.
2. CDC. National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60:1117-1123.
3. Markowitz L. HPV vaccine for males: background and review of data. Presented at: ACIP meeting; October 25, 2011; Atlanta, GA. http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-oct11/04-HPV-Markowitz.pdf. Accessed December 2, 2011.
4. Gee J. Safety of quadrivalent human papilloma virus (HPV4) vaccine. Presented at: ACIP meeting; October 25, 2011; Atlanta, GA. http://www.cdc.gov/vaccines/recs/acip/downloads/ mtg-slides-oct11/02-HPV-Gee.pdf. Accessed December 2, 2011.
5. Gee J, Naleway A, Shui I, et al. Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the Vaccine Safety Datalink. Vaccine. 2011;29:8279-8284.
6. Dunne EF. HPV vaccine considerations for males. Presented at: ACIP meeting; October 25, 2011; Atlanta, GA. http://www.cdc. gov/vaccines/recs/acip/downloads/mtg-slides-oct11/05-HPVDunne.pdf. Accessed December 2, 2011.
7. CDC. HPV and men—fact sheet. http://www.cdc.gov/std/hpv/std/hpv/stdfact-hpv-and-men.htm. Accessed December 19, 2011.
8. Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
9. CDC. Sexually transmitted diseases (STDs): HPV vaccine information for young women—fact sheet. http://www.cdc.gov/std/hpv/stdfact-hpv-vaccine-young-women.htm. Accessed December 2, 2011.
10. Chesson HW. HPV vaccine cost-effectiveness: updates and review. Presented at: ACIP meeting; June 22, 2011; Atlanta, GA. http://www.cdc.gov/vaccines/recs/acip/down-loads/mtg-slides-jun11/07-5-hpv-cost-effect.pdf. Accessed December 2, 2011.
1. CDC. FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2010;59:630-632.
2. CDC. National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60:1117-1123.
3. Markowitz L. HPV vaccine for males: background and review of data. Presented at: ACIP meeting; October 25, 2011; Atlanta, GA. http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-oct11/04-HPV-Markowitz.pdf. Accessed December 2, 2011.
4. Gee J. Safety of quadrivalent human papilloma virus (HPV4) vaccine. Presented at: ACIP meeting; October 25, 2011; Atlanta, GA. http://www.cdc.gov/vaccines/recs/acip/downloads/ mtg-slides-oct11/02-HPV-Gee.pdf. Accessed December 2, 2011.
5. Gee J, Naleway A, Shui I, et al. Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the Vaccine Safety Datalink. Vaccine. 2011;29:8279-8284.
6. Dunne EF. HPV vaccine considerations for males. Presented at: ACIP meeting; October 25, 2011; Atlanta, GA. http://www.cdc. gov/vaccines/recs/acip/downloads/mtg-slides-oct11/05-HPVDunne.pdf. Accessed December 2, 2011.
7. CDC. HPV and men—fact sheet. http://www.cdc.gov/std/hpv/std/hpv/stdfact-hpv-and-men.htm. Accessed December 19, 2011.
8. Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
9. CDC. Sexually transmitted diseases (STDs): HPV vaccine information for young women—fact sheet. http://www.cdc.gov/std/hpv/stdfact-hpv-vaccine-young-women.htm. Accessed December 2, 2011.
10. Chesson HW. HPV vaccine cost-effectiveness: updates and review. Presented at: ACIP meeting; June 22, 2011; Atlanta, GA. http://www.cdc.gov/vaccines/recs/acip/down-loads/mtg-slides-jun11/07-5-hpv-cost-effect.pdf. Accessed December 2, 2011.
Exercise-induced proteinuria?
• Rely on a spot urine microalbumin-to-creatinine or protein-to-creatinine ratio to accurately assess proteinuria. B
• Repeat testing if routine urinalysis detects proteinuria—especially if the patient reports having exercised in the previous 24 hours. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE As part of a routine physical examination, urinalysis reveals that a patient new to your practice is excreting an excessive level of protein. The patient is physically fit and shared during the history taking that he is an avid runner. The physical examination and other laboratory values were unremarkable. How concerned should you be about the finding of proteinuria?
Exercise-induced proteinuria is generally benign and a function of the intensity—rather than the duration—of exercise.1 It occurs most often among athletes participating in such sports as running, swimming, rowing, football, or boxing.2 It’s also transient—lasting 24 to 48 hours.1 Recognizing exercise-induced proteinuria is fairly straightforward—once you know what to look for.
But first, a word about the processes at work.
Diverse processes that work alone—or together
The normal range of protein excretion in healthy individuals is 150 to 200 mg of protein per day, of which albumin constitutes 10 to 20 mg.3 Individuals with proteinuria persistently higher than this level need further evaluation.
Diverse processes leading to proteinuria—working alone or concomitantly—occur at the level of the nephron.3
Glomerular proteinuria results from increased filtration of macromolecules such as albumin across the glomerular capillary barrier. This type of proteinuria can occur with different glomerulopathies, upright posture, or exercise.4
Researchers have not identified the mechanisms leading to postexercise proteinuria, but there are several theories. (For more on this, see “Why does exercise increase protein excretion?”.)
Tubular proteinuria is due to a deranged tubular apparatus with an intact glomerulus. This results in the escape of β2-microglobulin and immunoglobulin light chains from proximal tubular reabsorption. It is often missed on dipstick testing, which detects only albumin. This type of proteinuria is usually seen in tubulointerstitial diseases or in patients with idiopathic nephrotic syndrome.5
Overflow proteinuria occurs when small molecular light chains escape the glomerular filtration barrier and overwhelm the tubular reabsorptive capacity. This type of proteinuria can be seen in multiple myeloma, and is detectable by protein-to-creatinine ratio or urine protein electrophoresis.
The root cause of exercise-induced proteinuria is unclear, but the renin-angiotensin system (RAS) and prostaglandins are thought to play a major role. The plasma concentration of angiotensin II increases during exercise, leading to filtration of protein through the glomerular membrane.30 And angiotensin-converting enzyme (ACE) inhibitors have been shown to significantly diminish exercise-induced proteinuria, thus supporting this theory.31,32
Also, strenuous exercise increases sympathetic nervous system activity as well as blood levels of catecholamines, thereby increasing permeability of the glomerular capillary membrane.33 Furthermore, lactate increases with strenuous exercise and causes conformational changes in serum proteins that, when coupled with glomerular barrier changes, can lead to increased permeability and protein excretion.
The surest means of detecting proteinuria
Albumin excretion >300 mg/d is called macroalbuminuria, overt proteinuria, or dipstick-positive proteinuria. Albumin persistently excreted in the urine between 30 and 300 mg/d is referred to as microalbuminuria.
Because microalbuminuria is not detectable by a standard urine dipstick test, some providers routinely screen for protein with the microalbumin-to-creatinine ratio. A first-voided morning urine specimen is recommended, but random urine samples are an acceptable alternative.6 The microalbumin-to-creatinine ratio is recommended as a screen for early diabetic nephropathy and other kidney diseases. And a positive test result may also suggest increased risk of cardiovascular disease.6 Microalbuminuria is defined as persistent albumin excretion between 30 and 300 mg/d.7
When exercise is a factor, here’s what to look for
As noted earlier, exercise-induced proteinuria is a function of the intensity of the exercise. Moderate and strenuous (vigorous) exercise are the 2 types of exercise that come into play when discussing proteinuria. Differentiating them is not precise, but is often defined by maximal oxygen consumption (vigorous=60% of VO2max; moderate <60% VO2max); metabolic equivalents (vigorous=6 METS; moderate <6 METS); walking/running speeds (various); and heart rate reserve (vigorous=60% HRR; moderate <60% HRR).8
Moderate exercise produces glomerular proteinuria, with an increase in macromolecular (albumin) filtration across the glomerular barrier. Strenuous exercise increases glomerular filtration of low-molecular-weight proteins (β2-microglobulin), which overwhelm the reabsorbing capacity of the tubular apparatus, causing temporary dysfunction and tubular proteinuria.9 Thus, the pathophysiology is mixed, with a major contribution from glomerular proteinuria.10
Strenuous exercise can cause protein excretion to exceed 1.5 mg/min.11 However, it seldom rises beyond 1 to 2 g/d,4 and this increase usually reverts to normal physiologic levels within 24 to 48 hours after exercise.12
Exercise-induced proteinuria is biphasic.13 Increased protein excretion occurs 30 minutes after exercise and is related to changes in intraglomerular hemodynamics and the resulting saturation of the renal tubules. Around 24 hours after exercise, oxidative stress on the glomeruli causes another slight elevation in albumin excretion without changes in β2-microglobulin, thereby indicating glomerular proteinuria exclusively.
Even the pros aren’t exempt. Exercise-induced proteinuria does not decrease with regular physical training. This was demonstrated in a study of 10 well-trained professional cyclists for whom strenuous exercise increased overnight protein excretion of both tubular and glomerular origin despite ongoing regular physical training.14
Creatine supplements do not increase proteinuria. A study of creatine supplementation in animal models noted no changes in 24-hour proteinuria or albumin excretion in both normal and two-thirds-nephrectomized animals.15 Another study compared creatine use with nonuse in athletes who had been training regularly and strenuously (12- 18 h/wk) for 5 to 10 years. They were evaluated for 10 months to 5 years. The groups exhibited equivalent urine excretion rates for albumin and creatinine, with no deleterious effect on kidney function.16
What happens when chronic disease is factored into the exercise equation?
Patients with a 2- to 20-year history of insulin-dependent diabetes without chronic kidney disease (CKD) who exhibited normal albumin excretion at baseline were more likely to develop proteinuria after exercise than healthy controls.17,18 The postulated cause was undetected glomerular changes due to diabetes. An exercise-provocation test may one day be useful in predicting future development of nephropathy, but further studies are needed.19-21
Exercise increases proteinuria immediately in individuals with metabolic disorders like obesity, through a mechanism different from diabetes mellitus. Proteinuria in the obese population is thought to be glomerular in origin, as opposed to both tubular and glomerular proteinuria in diabetic nephropathy.22,23
In CKD, low-intensity exercise long term does not promote proteinuria or lead to rapid progression of CKD. In one study, obese patients (body mass index >30 kg/m2) with diabetes and CKD stage II to IV who exercised 3 times weekly (aerobic training for 6 weeks, followed by 18 weeks of supervised home exercise) increased their stamina and exhibited slight, statistically insignificant decreases in resting systolic blood pressure and 24-hour proteinuria.24 A 12-week low-intensity aquatic exercise program for 26 patients with mild to moderate CKD decreased blood pressure and proteinuria and slightly improved glomerular filtration rate (GFR).25 These results for proteinuria and GFR were shown previously in rats with subtotal nephrectomy.26
Elevated urinary albumin excretion with exercise is significantly higher in patients with acromegaly when compared with normal healthy subjects. The underlying pathology is thought to occur at the glomerular filtration barrier with intact tubular function. Somatostatin analog treatment for acromegaly leads to reductions in postexercise albuminuria.27,28
How to manage suspected exercise-induced proteinuria
When interpreting the meaning of proteinuria detected on routine urinalysis, keep in mind the temporal relevance between exercise and urine collection. If urine is found to have been collected within 24 hours of intense exercise, repeat testing in the absence of prior exercise on at least one other occasion to differentiate between transient and persistent proteinuria. In confirming transient proteinuria after exercise, reassure the patient that it is a benign condition. This holds true as well for routine microalbumin-to-creatinine urine testing in patients with diabetes who exercise. If the result of a repeat test is high, turn your attention to another possible cause of proteinuria, such as diabetic nephropathy.
Screening for proteinuria during sports preparticipation examinations is not recommended because the diagnostic utility is low.29 Researchers performed urine dipstick testing for protein, blood, and glucose in preparticipation assessments of 701 students.29 They detected proteinuria in 40 students and glucosuria in one. Follow-up testing with first-voided morning urine specimens and glucose tolerance testing was normal in all students.
CORRESPONDENCE Fahad Saeed, MD, 313 Brook Hollow, Hanover, NH 03755; [email protected]
1. Poortmans JR. Exercise and renal function. Sports Med. 1984;1:125-153.
2. Gebke KB. Genitourinary system. In: McKeag DB, Moeller JL, eds. ACSM’s Primary Care Sports Medicine. 2nd ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2007;234.-
3. Venkat KK. Proteinuria and microalbuminuria in adults: significance, evaluation, and treatment. South Med J. 2004;97:969-979.
4. Rose BD. Pathophysiology of Renal Disease. 2nd ed. New York, NY: McGraw-Hill; 1987;11-16.
5. Sesso R, Santos AP, Nishida SK, et al. Prediction of steroid responsiveness in the idiopathic nephrotic syndrome using urinary retinol-binding protein and beta-2-microglobulin. Ann Intern Med. 1992;116:905-909.
6. Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137-147.
7. Family Practice Notebook Urine protein to creatinine ratio. Available at: http://www.fpnotebook.com/urology/lab/urnprtntcrtnrt.htm. Accessed August 9, 2011.
8. Swain DP, Franklin BA. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol. 2006;97:141-147.
9. Poortmans JR, Labilloy D. The influence of work intensity on postexercise proteinuria. Eur J Appl Physiol Occup Physiol. 1988;57:260-263.
10. Estivi P, Urbino R, Tetta C, et al. Urinary protein excretion induced by exercise: effect of a mountain agonistic footrace in healthy subjects. Renal function and mountain footrace. J Sports Med Phys Fitness. 1992;32:196-200.
11. Poortmans JR, Brauman H, Staroukine M, et al. Indirect evidence of glomerular/tubular mixed-type postexercise proteinuria in healthy humans. Am J Physiol. 1988;254:F277-F283.
12. Heathcote KL, Wilson MP, Quest DW, et al. Prevalence and duration of exercise induced albuminuria in healthy people. Clin Invest Med. 2009;32:E261-E265.
13. Sentürk UK, Kuru O, Koçer G, et al. Biphasic pattern of exercise-induced proteinuria in sedentary and trained men. Nephron Physiol. 2007;105:22-32.
14. Clerico A, Giammattei C, Cecchini L, et al. Exercise-induced proteinuria in well-trained athletes. Clin Chem. 1990;36:562-564.
15. Taes YE, Delanghe JR, Wuyts B, et al. Creatine supplementation does not affect kidney function in an animal model with pre-existing renal failure. Nephrol Dial Transplant. 2003;18:258-264.
16. Poortmans JR, Francaux M. Long-term oral creatine supplementation does not impair renal function in healthy athletes. Med Sci Sports Exerc. 1999;31:1108-1110.
17. Mogensen CE, Vittinghus E, Sølling K. Abnormal albumin excretion after two provocative renal tests in diabetes: physical exercise and lysine injection. Kidney Int. 1979;16:385-393.
18. Vittinghus E, Mogensen CE. Albumin excretion during physical exercise in diabetes. Studies on the effect of insulin treatment and of the renal haemodynamic response. Acta Endocrinol Suppl (Copenh). 1981;242:61-62.
19. Watts GF, Williams I, Morris RW, et al. An acceptable exercise test to study microalbuminuria in type 1 diabetes. Diabet Med. 1989;6:787-792.
20. Pan X, Wang P, Hu N, et al. A physiologically based pharmacokinetic model characterizing mechanism-based inhibition of CYP1A2 for predicting theophylline/antofloxacin interaction in both rats and humans. Drug Metab Pharmacokinet. 2011;26:387-398.
21. O’Brien SF, Watts GF, Powrie JK, et al. Exercise testing as a long-term predictor of the development of microalbuminuria in normoalbuminuric IDDM patients. Diabetes Care. 1995;18:1602-1605.
22. Hidaka S, Kaneko O, Shirai M, et al. Do obesity and non-insulin dependent diabetes mellitus aggravate exercise-induced microproteinuria? Clin Chim Acta. 1998;275:115-126.
23. Hidaka S, Kakuta S, Okada H, et al. Exercise-induced proteinuria in diseases with metabolic disorders. Contrib Nephrol. 1990;83:136-143.
24. Leehey DJ, Moinuddin I, Bast JP, et al. Aerobic exercise in obese diabetic patients with chronic kidney disease: a randomized and controlled pilot study. Cardiovasc Diabetol. 2009;8:62.-
25. Pechter U, Ots M, Mesikepp S, et al. Beneficial effects of water-based exercise in patients with chronic kidney disease. Int J Rehabil Res. 2003;26:153-156.
26. Heifets M, Davis TA, Tegtmeyer E, et al. Exercise training ameliorates progressive renal disease in rats with subtotal nephrectomy. Kidney Int. 1987;32:815-820.
27. Manelli F, Bossoni S, Burattin A, et al. Exercise-induced microalbuminuria in patients with active acromegaly: acute effects of slow-release lanreotide, a long-acting somatostatin analog. Metabolism. 2000;49:634-639.
28. Hoogenberg K, Sluiter WJ, Dullaart RP. Effect of growth hormone and insulin-like growth factor I on urinary albumin excretion: studies in acromegaly and growth hormone deficiency. Acta Endocrinol (Copenh). 1993;129:151-157.
29. Goldberg B, Saraniti A, Witman P, et al. Pre-participation sports assessment—an objective evaluation. Pediatrics. 1980;66:736-745.
30. Garrett WE, Kirkendall DT, Squire DL. eds. Principles and Practice of Primary Care Sports Medicine. Philadelphia, Pa: Lippincott Williams & Wilkins; 2001;299-310.
31. Cosenzi A, Carraro M, Sacerdote A, et al. Involvement of the renin angiotensin system in the pathogenesis of postexercise proteinuria. Scand J Urol Nephrol. 1993;27:301-304.
32. Székács B, Vajo Z, Dachman W. Effect of ACE inhibition by benazepril, enalapril and captopril on chronic and post exercise proteinuria. Acta Physiol Hung. 1996;84:361-367.
33. Poortmans JR, Haggenmacher C, Vanderstraeten J. Postexercise proteinuria in humans and its adrenergic component. J Sports Med Phys Fitness. 2001;41:95-100.
• Rely on a spot urine microalbumin-to-creatinine or protein-to-creatinine ratio to accurately assess proteinuria. B
• Repeat testing if routine urinalysis detects proteinuria—especially if the patient reports having exercised in the previous 24 hours. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE As part of a routine physical examination, urinalysis reveals that a patient new to your practice is excreting an excessive level of protein. The patient is physically fit and shared during the history taking that he is an avid runner. The physical examination and other laboratory values were unremarkable. How concerned should you be about the finding of proteinuria?
Exercise-induced proteinuria is generally benign and a function of the intensity—rather than the duration—of exercise.1 It occurs most often among athletes participating in such sports as running, swimming, rowing, football, or boxing.2 It’s also transient—lasting 24 to 48 hours.1 Recognizing exercise-induced proteinuria is fairly straightforward—once you know what to look for.
But first, a word about the processes at work.
Diverse processes that work alone—or together
The normal range of protein excretion in healthy individuals is 150 to 200 mg of protein per day, of which albumin constitutes 10 to 20 mg.3 Individuals with proteinuria persistently higher than this level need further evaluation.
Diverse processes leading to proteinuria—working alone or concomitantly—occur at the level of the nephron.3
Glomerular proteinuria results from increased filtration of macromolecules such as albumin across the glomerular capillary barrier. This type of proteinuria can occur with different glomerulopathies, upright posture, or exercise.4
Researchers have not identified the mechanisms leading to postexercise proteinuria, but there are several theories. (For more on this, see “Why does exercise increase protein excretion?”.)
Tubular proteinuria is due to a deranged tubular apparatus with an intact glomerulus. This results in the escape of β2-microglobulin and immunoglobulin light chains from proximal tubular reabsorption. It is often missed on dipstick testing, which detects only albumin. This type of proteinuria is usually seen in tubulointerstitial diseases or in patients with idiopathic nephrotic syndrome.5
Overflow proteinuria occurs when small molecular light chains escape the glomerular filtration barrier and overwhelm the tubular reabsorptive capacity. This type of proteinuria can be seen in multiple myeloma, and is detectable by protein-to-creatinine ratio or urine protein electrophoresis.
The root cause of exercise-induced proteinuria is unclear, but the renin-angiotensin system (RAS) and prostaglandins are thought to play a major role. The plasma concentration of angiotensin II increases during exercise, leading to filtration of protein through the glomerular membrane.30 And angiotensin-converting enzyme (ACE) inhibitors have been shown to significantly diminish exercise-induced proteinuria, thus supporting this theory.31,32
Also, strenuous exercise increases sympathetic nervous system activity as well as blood levels of catecholamines, thereby increasing permeability of the glomerular capillary membrane.33 Furthermore, lactate increases with strenuous exercise and causes conformational changes in serum proteins that, when coupled with glomerular barrier changes, can lead to increased permeability and protein excretion.
The surest means of detecting proteinuria
Albumin excretion >300 mg/d is called macroalbuminuria, overt proteinuria, or dipstick-positive proteinuria. Albumin persistently excreted in the urine between 30 and 300 mg/d is referred to as microalbuminuria.
Because microalbuminuria is not detectable by a standard urine dipstick test, some providers routinely screen for protein with the microalbumin-to-creatinine ratio. A first-voided morning urine specimen is recommended, but random urine samples are an acceptable alternative.6 The microalbumin-to-creatinine ratio is recommended as a screen for early diabetic nephropathy and other kidney diseases. And a positive test result may also suggest increased risk of cardiovascular disease.6 Microalbuminuria is defined as persistent albumin excretion between 30 and 300 mg/d.7
When exercise is a factor, here’s what to look for
As noted earlier, exercise-induced proteinuria is a function of the intensity of the exercise. Moderate and strenuous (vigorous) exercise are the 2 types of exercise that come into play when discussing proteinuria. Differentiating them is not precise, but is often defined by maximal oxygen consumption (vigorous=60% of VO2max; moderate <60% VO2max); metabolic equivalents (vigorous=6 METS; moderate <6 METS); walking/running speeds (various); and heart rate reserve (vigorous=60% HRR; moderate <60% HRR).8
Moderate exercise produces glomerular proteinuria, with an increase in macromolecular (albumin) filtration across the glomerular barrier. Strenuous exercise increases glomerular filtration of low-molecular-weight proteins (β2-microglobulin), which overwhelm the reabsorbing capacity of the tubular apparatus, causing temporary dysfunction and tubular proteinuria.9 Thus, the pathophysiology is mixed, with a major contribution from glomerular proteinuria.10
Strenuous exercise can cause protein excretion to exceed 1.5 mg/min.11 However, it seldom rises beyond 1 to 2 g/d,4 and this increase usually reverts to normal physiologic levels within 24 to 48 hours after exercise.12
Exercise-induced proteinuria is biphasic.13 Increased protein excretion occurs 30 minutes after exercise and is related to changes in intraglomerular hemodynamics and the resulting saturation of the renal tubules. Around 24 hours after exercise, oxidative stress on the glomeruli causes another slight elevation in albumin excretion without changes in β2-microglobulin, thereby indicating glomerular proteinuria exclusively.
Even the pros aren’t exempt. Exercise-induced proteinuria does not decrease with regular physical training. This was demonstrated in a study of 10 well-trained professional cyclists for whom strenuous exercise increased overnight protein excretion of both tubular and glomerular origin despite ongoing regular physical training.14
Creatine supplements do not increase proteinuria. A study of creatine supplementation in animal models noted no changes in 24-hour proteinuria or albumin excretion in both normal and two-thirds-nephrectomized animals.15 Another study compared creatine use with nonuse in athletes who had been training regularly and strenuously (12- 18 h/wk) for 5 to 10 years. They were evaluated for 10 months to 5 years. The groups exhibited equivalent urine excretion rates for albumin and creatinine, with no deleterious effect on kidney function.16
What happens when chronic disease is factored into the exercise equation?
Patients with a 2- to 20-year history of insulin-dependent diabetes without chronic kidney disease (CKD) who exhibited normal albumin excretion at baseline were more likely to develop proteinuria after exercise than healthy controls.17,18 The postulated cause was undetected glomerular changes due to diabetes. An exercise-provocation test may one day be useful in predicting future development of nephropathy, but further studies are needed.19-21
Exercise increases proteinuria immediately in individuals with metabolic disorders like obesity, through a mechanism different from diabetes mellitus. Proteinuria in the obese population is thought to be glomerular in origin, as opposed to both tubular and glomerular proteinuria in diabetic nephropathy.22,23
In CKD, low-intensity exercise long term does not promote proteinuria or lead to rapid progression of CKD. In one study, obese patients (body mass index >30 kg/m2) with diabetes and CKD stage II to IV who exercised 3 times weekly (aerobic training for 6 weeks, followed by 18 weeks of supervised home exercise) increased their stamina and exhibited slight, statistically insignificant decreases in resting systolic blood pressure and 24-hour proteinuria.24 A 12-week low-intensity aquatic exercise program for 26 patients with mild to moderate CKD decreased blood pressure and proteinuria and slightly improved glomerular filtration rate (GFR).25 These results for proteinuria and GFR were shown previously in rats with subtotal nephrectomy.26
Elevated urinary albumin excretion with exercise is significantly higher in patients with acromegaly when compared with normal healthy subjects. The underlying pathology is thought to occur at the glomerular filtration barrier with intact tubular function. Somatostatin analog treatment for acromegaly leads to reductions in postexercise albuminuria.27,28
How to manage suspected exercise-induced proteinuria
When interpreting the meaning of proteinuria detected on routine urinalysis, keep in mind the temporal relevance between exercise and urine collection. If urine is found to have been collected within 24 hours of intense exercise, repeat testing in the absence of prior exercise on at least one other occasion to differentiate between transient and persistent proteinuria. In confirming transient proteinuria after exercise, reassure the patient that it is a benign condition. This holds true as well for routine microalbumin-to-creatinine urine testing in patients with diabetes who exercise. If the result of a repeat test is high, turn your attention to another possible cause of proteinuria, such as diabetic nephropathy.
Screening for proteinuria during sports preparticipation examinations is not recommended because the diagnostic utility is low.29 Researchers performed urine dipstick testing for protein, blood, and glucose in preparticipation assessments of 701 students.29 They detected proteinuria in 40 students and glucosuria in one. Follow-up testing with first-voided morning urine specimens and glucose tolerance testing was normal in all students.
CORRESPONDENCE Fahad Saeed, MD, 313 Brook Hollow, Hanover, NH 03755; [email protected]
• Rely on a spot urine microalbumin-to-creatinine or protein-to-creatinine ratio to accurately assess proteinuria. B
• Repeat testing if routine urinalysis detects proteinuria—especially if the patient reports having exercised in the previous 24 hours. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE As part of a routine physical examination, urinalysis reveals that a patient new to your practice is excreting an excessive level of protein. The patient is physically fit and shared during the history taking that he is an avid runner. The physical examination and other laboratory values were unremarkable. How concerned should you be about the finding of proteinuria?
Exercise-induced proteinuria is generally benign and a function of the intensity—rather than the duration—of exercise.1 It occurs most often among athletes participating in such sports as running, swimming, rowing, football, or boxing.2 It’s also transient—lasting 24 to 48 hours.1 Recognizing exercise-induced proteinuria is fairly straightforward—once you know what to look for.
But first, a word about the processes at work.
Diverse processes that work alone—or together
The normal range of protein excretion in healthy individuals is 150 to 200 mg of protein per day, of which albumin constitutes 10 to 20 mg.3 Individuals with proteinuria persistently higher than this level need further evaluation.
Diverse processes leading to proteinuria—working alone or concomitantly—occur at the level of the nephron.3
Glomerular proteinuria results from increased filtration of macromolecules such as albumin across the glomerular capillary barrier. This type of proteinuria can occur with different glomerulopathies, upright posture, or exercise.4
Researchers have not identified the mechanisms leading to postexercise proteinuria, but there are several theories. (For more on this, see “Why does exercise increase protein excretion?”.)
Tubular proteinuria is due to a deranged tubular apparatus with an intact glomerulus. This results in the escape of β2-microglobulin and immunoglobulin light chains from proximal tubular reabsorption. It is often missed on dipstick testing, which detects only albumin. This type of proteinuria is usually seen in tubulointerstitial diseases or in patients with idiopathic nephrotic syndrome.5
Overflow proteinuria occurs when small molecular light chains escape the glomerular filtration barrier and overwhelm the tubular reabsorptive capacity. This type of proteinuria can be seen in multiple myeloma, and is detectable by protein-to-creatinine ratio or urine protein electrophoresis.
The root cause of exercise-induced proteinuria is unclear, but the renin-angiotensin system (RAS) and prostaglandins are thought to play a major role. The plasma concentration of angiotensin II increases during exercise, leading to filtration of protein through the glomerular membrane.30 And angiotensin-converting enzyme (ACE) inhibitors have been shown to significantly diminish exercise-induced proteinuria, thus supporting this theory.31,32
Also, strenuous exercise increases sympathetic nervous system activity as well as blood levels of catecholamines, thereby increasing permeability of the glomerular capillary membrane.33 Furthermore, lactate increases with strenuous exercise and causes conformational changes in serum proteins that, when coupled with glomerular barrier changes, can lead to increased permeability and protein excretion.
The surest means of detecting proteinuria
Albumin excretion >300 mg/d is called macroalbuminuria, overt proteinuria, or dipstick-positive proteinuria. Albumin persistently excreted in the urine between 30 and 300 mg/d is referred to as microalbuminuria.
Because microalbuminuria is not detectable by a standard urine dipstick test, some providers routinely screen for protein with the microalbumin-to-creatinine ratio. A first-voided morning urine specimen is recommended, but random urine samples are an acceptable alternative.6 The microalbumin-to-creatinine ratio is recommended as a screen for early diabetic nephropathy and other kidney diseases. And a positive test result may also suggest increased risk of cardiovascular disease.6 Microalbuminuria is defined as persistent albumin excretion between 30 and 300 mg/d.7
When exercise is a factor, here’s what to look for
As noted earlier, exercise-induced proteinuria is a function of the intensity of the exercise. Moderate and strenuous (vigorous) exercise are the 2 types of exercise that come into play when discussing proteinuria. Differentiating them is not precise, but is often defined by maximal oxygen consumption (vigorous=60% of VO2max; moderate <60% VO2max); metabolic equivalents (vigorous=6 METS; moderate <6 METS); walking/running speeds (various); and heart rate reserve (vigorous=60% HRR; moderate <60% HRR).8
Moderate exercise produces glomerular proteinuria, with an increase in macromolecular (albumin) filtration across the glomerular barrier. Strenuous exercise increases glomerular filtration of low-molecular-weight proteins (β2-microglobulin), which overwhelm the reabsorbing capacity of the tubular apparatus, causing temporary dysfunction and tubular proteinuria.9 Thus, the pathophysiology is mixed, with a major contribution from glomerular proteinuria.10
Strenuous exercise can cause protein excretion to exceed 1.5 mg/min.11 However, it seldom rises beyond 1 to 2 g/d,4 and this increase usually reverts to normal physiologic levels within 24 to 48 hours after exercise.12
Exercise-induced proteinuria is biphasic.13 Increased protein excretion occurs 30 minutes after exercise and is related to changes in intraglomerular hemodynamics and the resulting saturation of the renal tubules. Around 24 hours after exercise, oxidative stress on the glomeruli causes another slight elevation in albumin excretion without changes in β2-microglobulin, thereby indicating glomerular proteinuria exclusively.
Even the pros aren’t exempt. Exercise-induced proteinuria does not decrease with regular physical training. This was demonstrated in a study of 10 well-trained professional cyclists for whom strenuous exercise increased overnight protein excretion of both tubular and glomerular origin despite ongoing regular physical training.14
Creatine supplements do not increase proteinuria. A study of creatine supplementation in animal models noted no changes in 24-hour proteinuria or albumin excretion in both normal and two-thirds-nephrectomized animals.15 Another study compared creatine use with nonuse in athletes who had been training regularly and strenuously (12- 18 h/wk) for 5 to 10 years. They were evaluated for 10 months to 5 years. The groups exhibited equivalent urine excretion rates for albumin and creatinine, with no deleterious effect on kidney function.16
What happens when chronic disease is factored into the exercise equation?
Patients with a 2- to 20-year history of insulin-dependent diabetes without chronic kidney disease (CKD) who exhibited normal albumin excretion at baseline were more likely to develop proteinuria after exercise than healthy controls.17,18 The postulated cause was undetected glomerular changes due to diabetes. An exercise-provocation test may one day be useful in predicting future development of nephropathy, but further studies are needed.19-21
Exercise increases proteinuria immediately in individuals with metabolic disorders like obesity, through a mechanism different from diabetes mellitus. Proteinuria in the obese population is thought to be glomerular in origin, as opposed to both tubular and glomerular proteinuria in diabetic nephropathy.22,23
In CKD, low-intensity exercise long term does not promote proteinuria or lead to rapid progression of CKD. In one study, obese patients (body mass index >30 kg/m2) with diabetes and CKD stage II to IV who exercised 3 times weekly (aerobic training for 6 weeks, followed by 18 weeks of supervised home exercise) increased their stamina and exhibited slight, statistically insignificant decreases in resting systolic blood pressure and 24-hour proteinuria.24 A 12-week low-intensity aquatic exercise program for 26 patients with mild to moderate CKD decreased blood pressure and proteinuria and slightly improved glomerular filtration rate (GFR).25 These results for proteinuria and GFR were shown previously in rats with subtotal nephrectomy.26
Elevated urinary albumin excretion with exercise is significantly higher in patients with acromegaly when compared with normal healthy subjects. The underlying pathology is thought to occur at the glomerular filtration barrier with intact tubular function. Somatostatin analog treatment for acromegaly leads to reductions in postexercise albuminuria.27,28
How to manage suspected exercise-induced proteinuria
When interpreting the meaning of proteinuria detected on routine urinalysis, keep in mind the temporal relevance between exercise and urine collection. If urine is found to have been collected within 24 hours of intense exercise, repeat testing in the absence of prior exercise on at least one other occasion to differentiate between transient and persistent proteinuria. In confirming transient proteinuria after exercise, reassure the patient that it is a benign condition. This holds true as well for routine microalbumin-to-creatinine urine testing in patients with diabetes who exercise. If the result of a repeat test is high, turn your attention to another possible cause of proteinuria, such as diabetic nephropathy.
Screening for proteinuria during sports preparticipation examinations is not recommended because the diagnostic utility is low.29 Researchers performed urine dipstick testing for protein, blood, and glucose in preparticipation assessments of 701 students.29 They detected proteinuria in 40 students and glucosuria in one. Follow-up testing with first-voided morning urine specimens and glucose tolerance testing was normal in all students.
CORRESPONDENCE Fahad Saeed, MD, 313 Brook Hollow, Hanover, NH 03755; [email protected]
1. Poortmans JR. Exercise and renal function. Sports Med. 1984;1:125-153.
2. Gebke KB. Genitourinary system. In: McKeag DB, Moeller JL, eds. ACSM’s Primary Care Sports Medicine. 2nd ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2007;234.-
3. Venkat KK. Proteinuria and microalbuminuria in adults: significance, evaluation, and treatment. South Med J. 2004;97:969-979.
4. Rose BD. Pathophysiology of Renal Disease. 2nd ed. New York, NY: McGraw-Hill; 1987;11-16.
5. Sesso R, Santos AP, Nishida SK, et al. Prediction of steroid responsiveness in the idiopathic nephrotic syndrome using urinary retinol-binding protein and beta-2-microglobulin. Ann Intern Med. 1992;116:905-909.
6. Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137-147.
7. Family Practice Notebook Urine protein to creatinine ratio. Available at: http://www.fpnotebook.com/urology/lab/urnprtntcrtnrt.htm. Accessed August 9, 2011.
8. Swain DP, Franklin BA. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol. 2006;97:141-147.
9. Poortmans JR, Labilloy D. The influence of work intensity on postexercise proteinuria. Eur J Appl Physiol Occup Physiol. 1988;57:260-263.
10. Estivi P, Urbino R, Tetta C, et al. Urinary protein excretion induced by exercise: effect of a mountain agonistic footrace in healthy subjects. Renal function and mountain footrace. J Sports Med Phys Fitness. 1992;32:196-200.
11. Poortmans JR, Brauman H, Staroukine M, et al. Indirect evidence of glomerular/tubular mixed-type postexercise proteinuria in healthy humans. Am J Physiol. 1988;254:F277-F283.
12. Heathcote KL, Wilson MP, Quest DW, et al. Prevalence and duration of exercise induced albuminuria in healthy people. Clin Invest Med. 2009;32:E261-E265.
13. Sentürk UK, Kuru O, Koçer G, et al. Biphasic pattern of exercise-induced proteinuria in sedentary and trained men. Nephron Physiol. 2007;105:22-32.
14. Clerico A, Giammattei C, Cecchini L, et al. Exercise-induced proteinuria in well-trained athletes. Clin Chem. 1990;36:562-564.
15. Taes YE, Delanghe JR, Wuyts B, et al. Creatine supplementation does not affect kidney function in an animal model with pre-existing renal failure. Nephrol Dial Transplant. 2003;18:258-264.
16. Poortmans JR, Francaux M. Long-term oral creatine supplementation does not impair renal function in healthy athletes. Med Sci Sports Exerc. 1999;31:1108-1110.
17. Mogensen CE, Vittinghus E, Sølling K. Abnormal albumin excretion after two provocative renal tests in diabetes: physical exercise and lysine injection. Kidney Int. 1979;16:385-393.
18. Vittinghus E, Mogensen CE. Albumin excretion during physical exercise in diabetes. Studies on the effect of insulin treatment and of the renal haemodynamic response. Acta Endocrinol Suppl (Copenh). 1981;242:61-62.
19. Watts GF, Williams I, Morris RW, et al. An acceptable exercise test to study microalbuminuria in type 1 diabetes. Diabet Med. 1989;6:787-792.
20. Pan X, Wang P, Hu N, et al. A physiologically based pharmacokinetic model characterizing mechanism-based inhibition of CYP1A2 for predicting theophylline/antofloxacin interaction in both rats and humans. Drug Metab Pharmacokinet. 2011;26:387-398.
21. O’Brien SF, Watts GF, Powrie JK, et al. Exercise testing as a long-term predictor of the development of microalbuminuria in normoalbuminuric IDDM patients. Diabetes Care. 1995;18:1602-1605.
22. Hidaka S, Kaneko O, Shirai M, et al. Do obesity and non-insulin dependent diabetes mellitus aggravate exercise-induced microproteinuria? Clin Chim Acta. 1998;275:115-126.
23. Hidaka S, Kakuta S, Okada H, et al. Exercise-induced proteinuria in diseases with metabolic disorders. Contrib Nephrol. 1990;83:136-143.
24. Leehey DJ, Moinuddin I, Bast JP, et al. Aerobic exercise in obese diabetic patients with chronic kidney disease: a randomized and controlled pilot study. Cardiovasc Diabetol. 2009;8:62.-
25. Pechter U, Ots M, Mesikepp S, et al. Beneficial effects of water-based exercise in patients with chronic kidney disease. Int J Rehabil Res. 2003;26:153-156.
26. Heifets M, Davis TA, Tegtmeyer E, et al. Exercise training ameliorates progressive renal disease in rats with subtotal nephrectomy. Kidney Int. 1987;32:815-820.
27. Manelli F, Bossoni S, Burattin A, et al. Exercise-induced microalbuminuria in patients with active acromegaly: acute effects of slow-release lanreotide, a long-acting somatostatin analog. Metabolism. 2000;49:634-639.
28. Hoogenberg K, Sluiter WJ, Dullaart RP. Effect of growth hormone and insulin-like growth factor I on urinary albumin excretion: studies in acromegaly and growth hormone deficiency. Acta Endocrinol (Copenh). 1993;129:151-157.
29. Goldberg B, Saraniti A, Witman P, et al. Pre-participation sports assessment—an objective evaluation. Pediatrics. 1980;66:736-745.
30. Garrett WE, Kirkendall DT, Squire DL. eds. Principles and Practice of Primary Care Sports Medicine. Philadelphia, Pa: Lippincott Williams & Wilkins; 2001;299-310.
31. Cosenzi A, Carraro M, Sacerdote A, et al. Involvement of the renin angiotensin system in the pathogenesis of postexercise proteinuria. Scand J Urol Nephrol. 1993;27:301-304.
32. Székács B, Vajo Z, Dachman W. Effect of ACE inhibition by benazepril, enalapril and captopril on chronic and post exercise proteinuria. Acta Physiol Hung. 1996;84:361-367.
33. Poortmans JR, Haggenmacher C, Vanderstraeten J. Postexercise proteinuria in humans and its adrenergic component. J Sports Med Phys Fitness. 2001;41:95-100.
1. Poortmans JR. Exercise and renal function. Sports Med. 1984;1:125-153.
2. Gebke KB. Genitourinary system. In: McKeag DB, Moeller JL, eds. ACSM’s Primary Care Sports Medicine. 2nd ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2007;234.-
3. Venkat KK. Proteinuria and microalbuminuria in adults: significance, evaluation, and treatment. South Med J. 2004;97:969-979.
4. Rose BD. Pathophysiology of Renal Disease. 2nd ed. New York, NY: McGraw-Hill; 1987;11-16.
5. Sesso R, Santos AP, Nishida SK, et al. Prediction of steroid responsiveness in the idiopathic nephrotic syndrome using urinary retinol-binding protein and beta-2-microglobulin. Ann Intern Med. 1992;116:905-909.
6. Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137-147.
7. Family Practice Notebook Urine protein to creatinine ratio. Available at: http://www.fpnotebook.com/urology/lab/urnprtntcrtnrt.htm. Accessed August 9, 2011.
8. Swain DP, Franklin BA. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol. 2006;97:141-147.
9. Poortmans JR, Labilloy D. The influence of work intensity on postexercise proteinuria. Eur J Appl Physiol Occup Physiol. 1988;57:260-263.
10. Estivi P, Urbino R, Tetta C, et al. Urinary protein excretion induced by exercise: effect of a mountain agonistic footrace in healthy subjects. Renal function and mountain footrace. J Sports Med Phys Fitness. 1992;32:196-200.
11. Poortmans JR, Brauman H, Staroukine M, et al. Indirect evidence of glomerular/tubular mixed-type postexercise proteinuria in healthy humans. Am J Physiol. 1988;254:F277-F283.
12. Heathcote KL, Wilson MP, Quest DW, et al. Prevalence and duration of exercise induced albuminuria in healthy people. Clin Invest Med. 2009;32:E261-E265.
13. Sentürk UK, Kuru O, Koçer G, et al. Biphasic pattern of exercise-induced proteinuria in sedentary and trained men. Nephron Physiol. 2007;105:22-32.
14. Clerico A, Giammattei C, Cecchini L, et al. Exercise-induced proteinuria in well-trained athletes. Clin Chem. 1990;36:562-564.
15. Taes YE, Delanghe JR, Wuyts B, et al. Creatine supplementation does not affect kidney function in an animal model with pre-existing renal failure. Nephrol Dial Transplant. 2003;18:258-264.
16. Poortmans JR, Francaux M. Long-term oral creatine supplementation does not impair renal function in healthy athletes. Med Sci Sports Exerc. 1999;31:1108-1110.
17. Mogensen CE, Vittinghus E, Sølling K. Abnormal albumin excretion after two provocative renal tests in diabetes: physical exercise and lysine injection. Kidney Int. 1979;16:385-393.
18. Vittinghus E, Mogensen CE. Albumin excretion during physical exercise in diabetes. Studies on the effect of insulin treatment and of the renal haemodynamic response. Acta Endocrinol Suppl (Copenh). 1981;242:61-62.
19. Watts GF, Williams I, Morris RW, et al. An acceptable exercise test to study microalbuminuria in type 1 diabetes. Diabet Med. 1989;6:787-792.
20. Pan X, Wang P, Hu N, et al. A physiologically based pharmacokinetic model characterizing mechanism-based inhibition of CYP1A2 for predicting theophylline/antofloxacin interaction in both rats and humans. Drug Metab Pharmacokinet. 2011;26:387-398.
21. O’Brien SF, Watts GF, Powrie JK, et al. Exercise testing as a long-term predictor of the development of microalbuminuria in normoalbuminuric IDDM patients. Diabetes Care. 1995;18:1602-1605.
22. Hidaka S, Kaneko O, Shirai M, et al. Do obesity and non-insulin dependent diabetes mellitus aggravate exercise-induced microproteinuria? Clin Chim Acta. 1998;275:115-126.
23. Hidaka S, Kakuta S, Okada H, et al. Exercise-induced proteinuria in diseases with metabolic disorders. Contrib Nephrol. 1990;83:136-143.
24. Leehey DJ, Moinuddin I, Bast JP, et al. Aerobic exercise in obese diabetic patients with chronic kidney disease: a randomized and controlled pilot study. Cardiovasc Diabetol. 2009;8:62.-
25. Pechter U, Ots M, Mesikepp S, et al. Beneficial effects of water-based exercise in patients with chronic kidney disease. Int J Rehabil Res. 2003;26:153-156.
26. Heifets M, Davis TA, Tegtmeyer E, et al. Exercise training ameliorates progressive renal disease in rats with subtotal nephrectomy. Kidney Int. 1987;32:815-820.
27. Manelli F, Bossoni S, Burattin A, et al. Exercise-induced microalbuminuria in patients with active acromegaly: acute effects of slow-release lanreotide, a long-acting somatostatin analog. Metabolism. 2000;49:634-639.
28. Hoogenberg K, Sluiter WJ, Dullaart RP. Effect of growth hormone and insulin-like growth factor I on urinary albumin excretion: studies in acromegaly and growth hormone deficiency. Acta Endocrinol (Copenh). 1993;129:151-157.
29. Goldberg B, Saraniti A, Witman P, et al. Pre-participation sports assessment—an objective evaluation. Pediatrics. 1980;66:736-745.
30. Garrett WE, Kirkendall DT, Squire DL. eds. Principles and Practice of Primary Care Sports Medicine. Philadelphia, Pa: Lippincott Williams & Wilkins; 2001;299-310.
31. Cosenzi A, Carraro M, Sacerdote A, et al. Involvement of the renin angiotensin system in the pathogenesis of postexercise proteinuria. Scand J Urol Nephrol. 1993;27:301-304.
32. Székács B, Vajo Z, Dachman W. Effect of ACE inhibition by benazepril, enalapril and captopril on chronic and post exercise proteinuria. Acta Physiol Hung. 1996;84:361-367.
33. Poortmans JR, Haggenmacher C, Vanderstraeten J. Postexercise proteinuria in humans and its adrenergic component. J Sports Med Phys Fitness. 2001;41:95-100.