User login
Mood instability in ADHD
Discuss this article at www.facebook.com/CurrentPsychiatry
Dr. Goldberg makes an important point that not all mood lability indicates bipolar disorder (BD) in “Ultra-rapid cycling bipolar disorder: A critical look” (Current Psychiatry, December 2011, p. 42-52).
However, there was 1 significant diagnostic omission. Patients with adult attention-deficit/hyperactivity disorder (ADHD) can present with an unremarkable mental status exam, yet can give a history of abrupt episodes of dyscontrol, often in interpersonal situations. As opposed to children manifesting ADHD, where comorbidity with BD is substantial, adults may primarily display impulsivity rather than hyperactivity or inattention. By ignoring this diagnostic consideration, important pharmacotherapeutic options have been discarded, although cognitive-behavioral therapy and dialectical behavior therapy for “borderline” patients are always relevant. Regardless of diagnostic terms and the fate of DSM-5, our treatment approach serves to strengthen prefrontal cortex inhibitory activity and block limbic system reactivity.
Robert Barris, MD
Attending Psychiatrist
Nassau University Medical Center
East Meadow, NY
Dr. Goldberg responds
Drs. Bunt and Barris each raise the clinically and theoretically interesting observation that in patients whose childhood attention-deficit/hyperactivity disorder (ADHD) persists into adulthood, affective instability may be a prominent feature. Consequently, they advise that complaints of frequent mood swings within 1 day should alert clinicians to consider ADHD in their differential diagnosis.
Importantly, emotional dysregulation is not an established criterion for ADHD, although investigators have begun to study impaired emotional processing in adults with ADHD.1 Because observational research examining emotional dysregulation in adult ADHD is preliminary, I cannot concur with Dr. Bunt’s assertion that “an omission of this sort does a disservice to the field.”
To the contrary, it would seem premature to counsel practitioners to look for mood instability as a red flag for adult ADHD. In fact, given the nontrivial rates of comorbid mood disorders with ADHD as cited by Dr. Bunt, it’s plausible that mood instability co-occurring with ADHD simply may be the epiphenomenon of a psychiatric comorbidity such as borderline personality disorder,2 a disruptive behavior disorder,3 or substance abuse.3
Moreover, endophenotype studies suggest that emotional lability and ADHD do not cosegregate in families.3 Further research is needed to determine whether moment-to-moment mood fluctuations are an intrinsic feature of ADHD that is not better accounted for by another accompanying condition.
Dr. Bunt appears to have misconstrued my use of the term “validation” with respect to ultra-rapid cycling (URC) as if I had been referring to validation of URC as a diagnosis—which I never suggested—rather than as a putative course modifier or specifier in an otherwise-diagnosed bipolar disorder patient—as was the case when researchers empirically validated rapid cycling (RC) as a bipolar course specifier, leading to its inclusion in DSM-IV.4 To my knowledge there’s no movement to consider URC as a bipolar course specifier in DSM-5, which would be a difficult undertaking in the absence of field trials such as those conducted for bipolar RC.
Drs. Barris, Bunt, and I seem to agree that mood shifts occurring on a daily or more frequent basis constitute a non-pathognomonic phenomenon for which “careful evaluation” is necessary to discern the broader psychopathologic condition and context in which it arises.
Joseph F. Goldberg, MD
Associate Clinical Professor of Psychiatry
Mt. Sinai School of Medicine
New York, NY
References
1. Herrmann MJ, Biehl SC, Jacob C, et al. Neurobiological and psychophysiological correlates of emotional dysregulation in ADHD patients. Atten Defic Hyperact Disord. 2010;2(4):233-239.
2. Philipsen A, Feige B, Hesslinger B, et al. Borderline typical symptoms in adult patients with attention deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2009;1(1):11-18.
3. Sobanski E, Banaschewski T, Asherson P, et al. Emotional lability in children and adolescents with attention deficit/hyperactivity disorder (ADHD): clinical correlates and familial prevalence. J Child Psychol Psychiatry. 2010;51(8):915-923.
4. Bauer MS, Calabrese J, Dunner DL, et al. Multisite data reanalysis of the validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry. 1994;151(4):506-515.
Discuss this article at www.facebook.com/CurrentPsychiatry
Dr. Goldberg makes an important point that not all mood lability indicates bipolar disorder (BD) in “Ultra-rapid cycling bipolar disorder: A critical look” (Current Psychiatry, December 2011, p. 42-52).
However, there was 1 significant diagnostic omission. Patients with adult attention-deficit/hyperactivity disorder (ADHD) can present with an unremarkable mental status exam, yet can give a history of abrupt episodes of dyscontrol, often in interpersonal situations. As opposed to children manifesting ADHD, where comorbidity with BD is substantial, adults may primarily display impulsivity rather than hyperactivity or inattention. By ignoring this diagnostic consideration, important pharmacotherapeutic options have been discarded, although cognitive-behavioral therapy and dialectical behavior therapy for “borderline” patients are always relevant. Regardless of diagnostic terms and the fate of DSM-5, our treatment approach serves to strengthen prefrontal cortex inhibitory activity and block limbic system reactivity.
Robert Barris, MD
Attending Psychiatrist
Nassau University Medical Center
East Meadow, NY
Dr. Goldberg responds
Drs. Bunt and Barris each raise the clinically and theoretically interesting observation that in patients whose childhood attention-deficit/hyperactivity disorder (ADHD) persists into adulthood, affective instability may be a prominent feature. Consequently, they advise that complaints of frequent mood swings within 1 day should alert clinicians to consider ADHD in their differential diagnosis.
Importantly, emotional dysregulation is not an established criterion for ADHD, although investigators have begun to study impaired emotional processing in adults with ADHD.1 Because observational research examining emotional dysregulation in adult ADHD is preliminary, I cannot concur with Dr. Bunt’s assertion that “an omission of this sort does a disservice to the field.”
To the contrary, it would seem premature to counsel practitioners to look for mood instability as a red flag for adult ADHD. In fact, given the nontrivial rates of comorbid mood disorders with ADHD as cited by Dr. Bunt, it’s plausible that mood instability co-occurring with ADHD simply may be the epiphenomenon of a psychiatric comorbidity such as borderline personality disorder,2 a disruptive behavior disorder,3 or substance abuse.3
Moreover, endophenotype studies suggest that emotional lability and ADHD do not cosegregate in families.3 Further research is needed to determine whether moment-to-moment mood fluctuations are an intrinsic feature of ADHD that is not better accounted for by another accompanying condition.
Dr. Bunt appears to have misconstrued my use of the term “validation” with respect to ultra-rapid cycling (URC) as if I had been referring to validation of URC as a diagnosis—which I never suggested—rather than as a putative course modifier or specifier in an otherwise-diagnosed bipolar disorder patient—as was the case when researchers empirically validated rapid cycling (RC) as a bipolar course specifier, leading to its inclusion in DSM-IV.4 To my knowledge there’s no movement to consider URC as a bipolar course specifier in DSM-5, which would be a difficult undertaking in the absence of field trials such as those conducted for bipolar RC.
Drs. Barris, Bunt, and I seem to agree that mood shifts occurring on a daily or more frequent basis constitute a non-pathognomonic phenomenon for which “careful evaluation” is necessary to discern the broader psychopathologic condition and context in which it arises.
Joseph F. Goldberg, MD
Associate Clinical Professor of Psychiatry
Mt. Sinai School of Medicine
New York, NY
References
1. Herrmann MJ, Biehl SC, Jacob C, et al. Neurobiological and psychophysiological correlates of emotional dysregulation in ADHD patients. Atten Defic Hyperact Disord. 2010;2(4):233-239.
2. Philipsen A, Feige B, Hesslinger B, et al. Borderline typical symptoms in adult patients with attention deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2009;1(1):11-18.
3. Sobanski E, Banaschewski T, Asherson P, et al. Emotional lability in children and adolescents with attention deficit/hyperactivity disorder (ADHD): clinical correlates and familial prevalence. J Child Psychol Psychiatry. 2010;51(8):915-923.
4. Bauer MS, Calabrese J, Dunner DL, et al. Multisite data reanalysis of the validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry. 1994;151(4):506-515.
Discuss this article at www.facebook.com/CurrentPsychiatry
Dr. Goldberg makes an important point that not all mood lability indicates bipolar disorder (BD) in “Ultra-rapid cycling bipolar disorder: A critical look” (Current Psychiatry, December 2011, p. 42-52).
However, there was 1 significant diagnostic omission. Patients with adult attention-deficit/hyperactivity disorder (ADHD) can present with an unremarkable mental status exam, yet can give a history of abrupt episodes of dyscontrol, often in interpersonal situations. As opposed to children manifesting ADHD, where comorbidity with BD is substantial, adults may primarily display impulsivity rather than hyperactivity or inattention. By ignoring this diagnostic consideration, important pharmacotherapeutic options have been discarded, although cognitive-behavioral therapy and dialectical behavior therapy for “borderline” patients are always relevant. Regardless of diagnostic terms and the fate of DSM-5, our treatment approach serves to strengthen prefrontal cortex inhibitory activity and block limbic system reactivity.
Robert Barris, MD
Attending Psychiatrist
Nassau University Medical Center
East Meadow, NY
Dr. Goldberg responds
Drs. Bunt and Barris each raise the clinically and theoretically interesting observation that in patients whose childhood attention-deficit/hyperactivity disorder (ADHD) persists into adulthood, affective instability may be a prominent feature. Consequently, they advise that complaints of frequent mood swings within 1 day should alert clinicians to consider ADHD in their differential diagnosis.
Importantly, emotional dysregulation is not an established criterion for ADHD, although investigators have begun to study impaired emotional processing in adults with ADHD.1 Because observational research examining emotional dysregulation in adult ADHD is preliminary, I cannot concur with Dr. Bunt’s assertion that “an omission of this sort does a disservice to the field.”
To the contrary, it would seem premature to counsel practitioners to look for mood instability as a red flag for adult ADHD. In fact, given the nontrivial rates of comorbid mood disorders with ADHD as cited by Dr. Bunt, it’s plausible that mood instability co-occurring with ADHD simply may be the epiphenomenon of a psychiatric comorbidity such as borderline personality disorder,2 a disruptive behavior disorder,3 or substance abuse.3
Moreover, endophenotype studies suggest that emotional lability and ADHD do not cosegregate in families.3 Further research is needed to determine whether moment-to-moment mood fluctuations are an intrinsic feature of ADHD that is not better accounted for by another accompanying condition.
Dr. Bunt appears to have misconstrued my use of the term “validation” with respect to ultra-rapid cycling (URC) as if I had been referring to validation of URC as a diagnosis—which I never suggested—rather than as a putative course modifier or specifier in an otherwise-diagnosed bipolar disorder patient—as was the case when researchers empirically validated rapid cycling (RC) as a bipolar course specifier, leading to its inclusion in DSM-IV.4 To my knowledge there’s no movement to consider URC as a bipolar course specifier in DSM-5, which would be a difficult undertaking in the absence of field trials such as those conducted for bipolar RC.
Drs. Barris, Bunt, and I seem to agree that mood shifts occurring on a daily or more frequent basis constitute a non-pathognomonic phenomenon for which “careful evaluation” is necessary to discern the broader psychopathologic condition and context in which it arises.
Joseph F. Goldberg, MD
Associate Clinical Professor of Psychiatry
Mt. Sinai School of Medicine
New York, NY
References
1. Herrmann MJ, Biehl SC, Jacob C, et al. Neurobiological and psychophysiological correlates of emotional dysregulation in ADHD patients. Atten Defic Hyperact Disord. 2010;2(4):233-239.
2. Philipsen A, Feige B, Hesslinger B, et al. Borderline typical symptoms in adult patients with attention deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2009;1(1):11-18.
3. Sobanski E, Banaschewski T, Asherson P, et al. Emotional lability in children and adolescents with attention deficit/hyperactivity disorder (ADHD): clinical correlates and familial prevalence. J Child Psychol Psychiatry. 2010;51(8):915-923.
4. Bauer MS, Calabrese J, Dunner DL, et al. Multisite data reanalysis of the validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry. 1994;151(4):506-515.
A paranoid, violent teenager
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Sleepless and paranoid
Ms. V, age 16, is referred to our psychiatric hospital from a juvenile detention center after she is charged with killing her sister with a hammer. She reports paranoid delusions, including believing that her sister was poisoning her food. Ms. V’s troubling behavior increased in the 6 months before the murder. She began to ask her mother to smell her food for possible poison. Her school grades dropped and she experienced decreased sleep and appetite. According to her mother, Ms. V’s insomnia worsened recently because of her paranoid thinking, which was evident when she noticed that her daughter slept with a hammer. Ms. V stopped socializing with her peers and no longer went to the gym.
Ms. V’s mother describes her daughter’s negative symptoms as consisting of social isolation and a flat affect. There was no evidence of auditory or visual hallucinations. After noticing the change in her daughter’s behavior, Ms. V’s mother attempted to schedule an appointment with a mental health professional, but there was a 2-month waiting list.
Ms. V cleaned her room before the murder, which was uncharacteristic of her routine behavior. On the day of the murder, Ms. V approached her sister while she was sleeping on the sofa and struck her on the head several times with a hammer. After the sister died, neighbors spotted Ms. V washing blood off her hands in their backyard with a sprinkler. Soaked in blood, she approached one of the neighbors and said that someone had been killed in the house. The neighbors called the police and Ms. V was arrested. She did not express remorse. She did not exhibit physical aggression toward others before the murder. Ms. V’s sense of entitlement and grandiosity persisted after the murder.
The authors’ observations
Paranoid delusions are fixed false beliefs with severe fears of others that may impair functioning at school or work, in personal relationships, and in other social dimensions. Paranoid thinking can have diverse presentations, ranging from social concerns such as fear of rejection to severe threat perceptions of people trying to cause substantial physical harm.1 Paranoid thoughts can be a result of misinterpretation of language, a personality disorder, anxiety, or psychosis.
Feelings of low self-esteem2 and anger1 may develop in a patient experiencing paranoid ideations. When anger begins to escalate, it may erupt into violent behavior. In Ms. V’s case, her paranoid ideations increased until she killed her younger sister. Ms. V’s case is similar to a mass shooting near Tucson, AZ on January 8, 2011 in that it possibly could have been prevented with earlier psychiatric intervention (Box).3-6
On January 8, 2011, a mass shooting occurred near Tucson, AZ that killed 6 and wounded 13. The suspect, 22-year-old Jared Lee Loughner, refused to cooperate with authorities by invoking his right to remain silent.3 Although the motives behind this crime remain undisclosed, mental illness appears to be a contributing factor.
Reports indicate that Mr. Loughner was abusing drugs and those close to him had noticed personality changes.4,5 The college he was attending advised Mr. Loughner to undergo a mental health evaluation, but he refused and dropped out of school.4,5 While in custody after the shooting, Mr. Loughner was diagnosed with paranoid schizophrenia, deemed incompetent to stand trial, and ordered to receive psychiatric treatment.6
This tragic mass shooting and similar incidents have led to questions regarding the adequacy of the mental health care infrastructure in United States. Experts suggest that this tragedy could have been prevented with more aggressive psychiatric prevention and intervention. Critical analysis of similar recent cases and expert opinions are needed to address this problem effectively.
EVALUATION: Remorseless
At admission, Ms. V’s affect is restricted and, at times, inappropriate. She is guarded about discussing the homicide but describes paranoid thoughts about her sister poisoning her. She is eager to learn if the police had found poison in her food. Her speech is soft with good articulation. Based on her presentation, her intelligence is average. She shows no evidence of remorse and is preoccupied with her sister poisoning her.
The Rorschach Inkblot Technique reveals positive evidence for a severe thought disorder. Ms. V’s thinking seems regressed. Ms. V’s medical workup, including MRI, electroencephalogram, and laboratory tests, are all within normal limits.
In the 5th grade, Ms. V’s primary care provider prescribed amphetamine and dextroamphetamine for attention-deficit/hyperactivity disorder, but she discontinued the drug after 1 year. Ms. V has never been hospitalized for psychiatric illness. She had no chronic medical conditions and no developmental delays.
Ms. V also has a history of periodic temper problems characterized by verbal aggression such as threatening the assistant principal at her school, and throwing her cellphone at her mother a few weeks before the murder, but no other aggressive episodes. Ms. V’s history does not suggest conduct disorder. She has no history of suicidal ideation or suicide attempts. Ms. V has used alcohol since age 15, but her mother reports that she was not a heavy or frequent user. Her last reported alcohol use was 10 days before the murder. A maternal uncle had been diagnosed with schizophrenia.
Before the murder, Ms. V lived with her sister and mother. Her parents were divorced. At age 9, Ms. V was sexually abused by a soccer coach; however, she denied symptoms of posttraumatic stress disorder related to the sexual abuse. She had no criminal history before the murder.
The authors’ observations
Based on Ms. V’s presentation and history, schizophrenia, paranoid type seems to be the most likely diagnosis because of her negative symptoms, including affective flattening, positive family history for schizophrenia, and paranoid delusions leading to dysfunction (Table).7 Delusional disorder seems less likely because Ms. V is young and has negative symptoms. Because she is generally healthy and her medical workup is negative, psychotic disorder due to a general medical condition is ruled out. She does not appear to be over-reporting, malingering, or exaggerating symptoms. In the context of psychosis, adolescent psychopathy does not seem likely even though there is evidence of grandiosity and a lack of remorse.
Table
DSM-IV-TR criteria for schizophrenia
A. Characteristic symptoms: ≥2 of the following, each present for a significant portion of time during a 1-month period:
|
| B. Social/occupational dysfunction: For a significant portion of the time since the onset of the disturbance, ≥1 major areas of functioning such as work, interpersonal relations, or self-care are markedly below the level achieved prior to the onset |
| C. Duration: Continuous signs of the disturbance persist for at least 6 months. This 6-month period must include at least 1 month of symptoms that meet Criterion A and may include periods of prodromal or residual symptoms |
| D. Schizoaffective and mood disorder exclusion: Schizoaffective disorder and mood disorder with psychotic features have been ruled out because either (1) no major depressive, manic, or mixed episodes have occurred concurrently with the active-phase symptoms; or (2) if mood episodes have occurred during active-phase symptoms, their total duration has been brief relative to the duration of the active and residual periods |
| E. Substance/general medical condition exclusion: The disturbance is not due to the direct physiological effects of a substance or a general medical condition |
| F. Relationship to a pervasive developmental disorder: If there is a history of autistic disorder or another pervasive developmental disorder, the additional diagnosis of schizophrenia is made only if prominent delusions or hallucinations are also present for at least 1 month |
| Diagnostic criteria for paranoid type: A type of schizophrenia in which the following criteria are met: A. Preoccupation with ≥1 delusions or frequent auditory hallucinations B. None of the following are prominent: disorganized speech, disorganized or catatonic behavior, or flat or inappropriate affect |
| Source: Reference 7 |
The authors’ observations
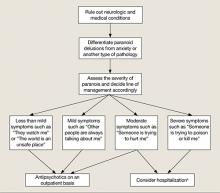
Various treatments can be used for paranoia with aggression, but the severity of the paranoia should be assessed before initiating treatment. Although categorizing paranoid ideations as mild, moderate, and severe is mainly a clinical judgment, Freeman et al1 have attempted to design a paranoia hierarchy from social concerns to severe threats. CBT8 and antipsychotic medication may help reduce mild to moderate paranoid delusions, particularly those associated with schizophrenia or mood disorders. For severe paranoia, hospitalization should carefully be considered.
When a patient exhibits moderate paranoia, the probability of progressing to severe symptoms or improving to mild symptoms depends on several variables. Pharmacologic treatment, family insight, and social support may be important variables in such circumstances. Psychoeducation for the family is vital.
In patients experiencing paranoia, violence may be prevented by proper assessment and treatment. The patient’s family should be educated about paranoid ideation and the need for treatment to improve symptoms and ensure safety. The long-term effects of untreated paranoia and types of treatment modalities available should be discussed with the family and the patient. During these teaching sessions, focus on improving the overall insight of the family and the patient about the psychotic illness to improve treatment adherence.9 This step may be challenging if the family is resistant to the patient receiving mental health treatment.
Gaining a detailed clinical history of a patient’s paranoia is important. A clinician should look for changes in behavior, such as the patient becoming quieter or more hostile, and impaired academic or social functioning. After gathering sufficient evidence contrary to the delusion, clinicians can help patients improve their reality testing.
Rule out medical and neurologic conditions that may be contributing to paranoia and aggression.
TREATMENT: Some improvement
Ms. V is started on risperidone, 1 mg/d, which leads to a partial response. She starts interacting more with staff and her peers on the unit, but her delusions of her sister poisioning her persist. Given the severity of the crime, Ms. V is sent to adult court, where she is found not guilty by reason of insanity and committed to a state hospital.
The authors’ observations
New-onset paranoia is a serious symptom that requires immediate evaluation and treatment. We recommend an approach presened in a flowchart (Figure) that highlights the importance of early intervention and aggressive treatment.
The MacArthur Violence Risk Assessment Study10 indicated that a “suspicious” attitude toward others can be a precipitating cause for increased violence in some cases. In light of ongoing controversy regarding the link between violence and mental illnesses such as schizophrenia,10-12 addressing an individual’s psychiatric illness early is preferable to prevent possible complications such as violent crimes. Because patients with paranoid ideations may have severely impaired ego control, they may be at risk for acting out aggressive and/or destructive urges. Therefore, new-onset paranoia should be thought of as a medical emergency similar to chest pain. Although accurately predicting and preventing violence may be impossible, in Ms. V’s case, earlier mental health treatment and intervention may have been able to prevent a murder.
Figure: Paranoia: A suggested approach to treatment
aBased on clinical judgment and extent of social support
Symptoms may become less severe or more severe (bidirectional). Strong social support has a positive effect on all levels and complements therapy. Regular counseling sessions and enhanced family insight about the patient’s paranoia helps strengthen social support
- Marneros A, Pillmann F, Wustmann T. Delusional disorders—are they simply paranoid schizophrenia? [published online ahead of print November 15, 2010]. Schizophr Bull. doi: 10.1093/schbul/sbq125.
Drug Brand Names
Amphetamine and dextroamphetamine • Adderall
Risperidone • Risperdal
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Personal and clinical details of this case were altered to maintain patient confidentiality.
1. Freeman D, Garety PA, Bebbington PE, et al. Psychological investigation of the structure of paranoia in a non-clinical population. Br J Psychiatry. 2005;186:427-435.
2. Kendler KS, Hays P. Paranoid psychosis (delusional disorder) and schizophrenia. A family history study. Arch Gen Psychiatry. 1981;38(5):547-551.
3. CNN Wire Staff. Police “actively pursuing” second person in Tucson shooting. CNN. http://us.cnn.com/2011/CRIME/01/08/arizona.shooting. Published January 9 2011. Accessed January 9, 2012.
4. Lipton E, Savage C, Shane S. Arizona suspect’s recent acts offer hints of alienation. The New York Times. January 8 2011:A8. http://www.nytimes.com/2011/01/09/us/politics/09shooter.html. Accessed January 10, 2012.
5. Berger J. Mental health warnings preceded rampage as Arizona gunman likely went untreated. http://www.foxnews.com/us/2011/01/10/mental-health-warnings-preceded-arizona-rampage-evidence-gunman-sought. Published January 10, 2011. Accessed January 11, 2012.
6. Lacey M. Suspect in shooting of Giffords ruled unfit for trial. The New York Times. May 25 2011:A1. http://www.nytimes.com/2011/05/26/us/26loughner.html. Accessed January 5, 2012.
7. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
8. Turkington D, Kingdon D, Weiden PJ. Cognitive behavior therapy for schizophrenia. Am J Psychiatry. 2006;163(3):365-373.
9. Smith CM, Barzman DH, Pristach CA. Effect of patient and family insight on compliance of schizophrenic patients. J Clin Pharmacol. 1997;37(2):147-154.
10. Appelbaum PS, Robbins PC, Monahan J. Violence and delusions: data from the MacArthur Violence Risk Assessment Study. Am J Psychiatry. 2000;157(4):566-572.
11. Mullen PE. A reassessment of the link between mental disorder and violent behaviour and its implications for clinical practice. Aust N Z J Psychiatry. 1997;31(1):3-11.
12. Fazel S, Gulati G, Linsell L, et al. Schizophrenia and violence: systematic review and meta-analysis. PLoS Med. 2009;6(8):e1000120.-
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Sleepless and paranoid
Ms. V, age 16, is referred to our psychiatric hospital from a juvenile detention center after she is charged with killing her sister with a hammer. She reports paranoid delusions, including believing that her sister was poisoning her food. Ms. V’s troubling behavior increased in the 6 months before the murder. She began to ask her mother to smell her food for possible poison. Her school grades dropped and she experienced decreased sleep and appetite. According to her mother, Ms. V’s insomnia worsened recently because of her paranoid thinking, which was evident when she noticed that her daughter slept with a hammer. Ms. V stopped socializing with her peers and no longer went to the gym.
Ms. V’s mother describes her daughter’s negative symptoms as consisting of social isolation and a flat affect. There was no evidence of auditory or visual hallucinations. After noticing the change in her daughter’s behavior, Ms. V’s mother attempted to schedule an appointment with a mental health professional, but there was a 2-month waiting list.
Ms. V cleaned her room before the murder, which was uncharacteristic of her routine behavior. On the day of the murder, Ms. V approached her sister while she was sleeping on the sofa and struck her on the head several times with a hammer. After the sister died, neighbors spotted Ms. V washing blood off her hands in their backyard with a sprinkler. Soaked in blood, she approached one of the neighbors and said that someone had been killed in the house. The neighbors called the police and Ms. V was arrested. She did not express remorse. She did not exhibit physical aggression toward others before the murder. Ms. V’s sense of entitlement and grandiosity persisted after the murder.
The authors’ observations
Paranoid delusions are fixed false beliefs with severe fears of others that may impair functioning at school or work, in personal relationships, and in other social dimensions. Paranoid thinking can have diverse presentations, ranging from social concerns such as fear of rejection to severe threat perceptions of people trying to cause substantial physical harm.1 Paranoid thoughts can be a result of misinterpretation of language, a personality disorder, anxiety, or psychosis.
Feelings of low self-esteem2 and anger1 may develop in a patient experiencing paranoid ideations. When anger begins to escalate, it may erupt into violent behavior. In Ms. V’s case, her paranoid ideations increased until she killed her younger sister. Ms. V’s case is similar to a mass shooting near Tucson, AZ on January 8, 2011 in that it possibly could have been prevented with earlier psychiatric intervention (Box).3-6
On January 8, 2011, a mass shooting occurred near Tucson, AZ that killed 6 and wounded 13. The suspect, 22-year-old Jared Lee Loughner, refused to cooperate with authorities by invoking his right to remain silent.3 Although the motives behind this crime remain undisclosed, mental illness appears to be a contributing factor.
Reports indicate that Mr. Loughner was abusing drugs and those close to him had noticed personality changes.4,5 The college he was attending advised Mr. Loughner to undergo a mental health evaluation, but he refused and dropped out of school.4,5 While in custody after the shooting, Mr. Loughner was diagnosed with paranoid schizophrenia, deemed incompetent to stand trial, and ordered to receive psychiatric treatment.6
This tragic mass shooting and similar incidents have led to questions regarding the adequacy of the mental health care infrastructure in United States. Experts suggest that this tragedy could have been prevented with more aggressive psychiatric prevention and intervention. Critical analysis of similar recent cases and expert opinions are needed to address this problem effectively.
EVALUATION: Remorseless
At admission, Ms. V’s affect is restricted and, at times, inappropriate. She is guarded about discussing the homicide but describes paranoid thoughts about her sister poisoning her. She is eager to learn if the police had found poison in her food. Her speech is soft with good articulation. Based on her presentation, her intelligence is average. She shows no evidence of remorse and is preoccupied with her sister poisoning her.
The Rorschach Inkblot Technique reveals positive evidence for a severe thought disorder. Ms. V’s thinking seems regressed. Ms. V’s medical workup, including MRI, electroencephalogram, and laboratory tests, are all within normal limits.
In the 5th grade, Ms. V’s primary care provider prescribed amphetamine and dextroamphetamine for attention-deficit/hyperactivity disorder, but she discontinued the drug after 1 year. Ms. V has never been hospitalized for psychiatric illness. She had no chronic medical conditions and no developmental delays.
Ms. V also has a history of periodic temper problems characterized by verbal aggression such as threatening the assistant principal at her school, and throwing her cellphone at her mother a few weeks before the murder, but no other aggressive episodes. Ms. V’s history does not suggest conduct disorder. She has no history of suicidal ideation or suicide attempts. Ms. V has used alcohol since age 15, but her mother reports that she was not a heavy or frequent user. Her last reported alcohol use was 10 days before the murder. A maternal uncle had been diagnosed with schizophrenia.
Before the murder, Ms. V lived with her sister and mother. Her parents were divorced. At age 9, Ms. V was sexually abused by a soccer coach; however, she denied symptoms of posttraumatic stress disorder related to the sexual abuse. She had no criminal history before the murder.
The authors’ observations
Based on Ms. V’s presentation and history, schizophrenia, paranoid type seems to be the most likely diagnosis because of her negative symptoms, including affective flattening, positive family history for schizophrenia, and paranoid delusions leading to dysfunction (Table).7 Delusional disorder seems less likely because Ms. V is young and has negative symptoms. Because she is generally healthy and her medical workup is negative, psychotic disorder due to a general medical condition is ruled out. She does not appear to be over-reporting, malingering, or exaggerating symptoms. In the context of psychosis, adolescent psychopathy does not seem likely even though there is evidence of grandiosity and a lack of remorse.
Table
DSM-IV-TR criteria for schizophrenia
A. Characteristic symptoms: ≥2 of the following, each present for a significant portion of time during a 1-month period:
|
| B. Social/occupational dysfunction: For a significant portion of the time since the onset of the disturbance, ≥1 major areas of functioning such as work, interpersonal relations, or self-care are markedly below the level achieved prior to the onset |
| C. Duration: Continuous signs of the disturbance persist for at least 6 months. This 6-month period must include at least 1 month of symptoms that meet Criterion A and may include periods of prodromal or residual symptoms |
| D. Schizoaffective and mood disorder exclusion: Schizoaffective disorder and mood disorder with psychotic features have been ruled out because either (1) no major depressive, manic, or mixed episodes have occurred concurrently with the active-phase symptoms; or (2) if mood episodes have occurred during active-phase symptoms, their total duration has been brief relative to the duration of the active and residual periods |
| E. Substance/general medical condition exclusion: The disturbance is not due to the direct physiological effects of a substance or a general medical condition |
| F. Relationship to a pervasive developmental disorder: If there is a history of autistic disorder or another pervasive developmental disorder, the additional diagnosis of schizophrenia is made only if prominent delusions or hallucinations are also present for at least 1 month |
| Diagnostic criteria for paranoid type: A type of schizophrenia in which the following criteria are met: A. Preoccupation with ≥1 delusions or frequent auditory hallucinations B. None of the following are prominent: disorganized speech, disorganized or catatonic behavior, or flat or inappropriate affect |
| Source: Reference 7 |
The authors’ observations
Various treatments can be used for paranoia with aggression, but the severity of the paranoia should be assessed before initiating treatment. Although categorizing paranoid ideations as mild, moderate, and severe is mainly a clinical judgment, Freeman et al1 have attempted to design a paranoia hierarchy from social concerns to severe threats. CBT8 and antipsychotic medication may help reduce mild to moderate paranoid delusions, particularly those associated with schizophrenia or mood disorders. For severe paranoia, hospitalization should carefully be considered.
When a patient exhibits moderate paranoia, the probability of progressing to severe symptoms or improving to mild symptoms depends on several variables. Pharmacologic treatment, family insight, and social support may be important variables in such circumstances. Psychoeducation for the family is vital.
In patients experiencing paranoia, violence may be prevented by proper assessment and treatment. The patient’s family should be educated about paranoid ideation and the need for treatment to improve symptoms and ensure safety. The long-term effects of untreated paranoia and types of treatment modalities available should be discussed with the family and the patient. During these teaching sessions, focus on improving the overall insight of the family and the patient about the psychotic illness to improve treatment adherence.9 This step may be challenging if the family is resistant to the patient receiving mental health treatment.
Gaining a detailed clinical history of a patient’s paranoia is important. A clinician should look for changes in behavior, such as the patient becoming quieter or more hostile, and impaired academic or social functioning. After gathering sufficient evidence contrary to the delusion, clinicians can help patients improve their reality testing.
Rule out medical and neurologic conditions that may be contributing to paranoia and aggression.
TREATMENT: Some improvement
Ms. V is started on risperidone, 1 mg/d, which leads to a partial response. She starts interacting more with staff and her peers on the unit, but her delusions of her sister poisioning her persist. Given the severity of the crime, Ms. V is sent to adult court, where she is found not guilty by reason of insanity and committed to a state hospital.
The authors’ observations
New-onset paranoia is a serious symptom that requires immediate evaluation and treatment. We recommend an approach presened in a flowchart (Figure) that highlights the importance of early intervention and aggressive treatment.
The MacArthur Violence Risk Assessment Study10 indicated that a “suspicious” attitude toward others can be a precipitating cause for increased violence in some cases. In light of ongoing controversy regarding the link between violence and mental illnesses such as schizophrenia,10-12 addressing an individual’s psychiatric illness early is preferable to prevent possible complications such as violent crimes. Because patients with paranoid ideations may have severely impaired ego control, they may be at risk for acting out aggressive and/or destructive urges. Therefore, new-onset paranoia should be thought of as a medical emergency similar to chest pain. Although accurately predicting and preventing violence may be impossible, in Ms. V’s case, earlier mental health treatment and intervention may have been able to prevent a murder.
Figure: Paranoia: A suggested approach to treatment
aBased on clinical judgment and extent of social support
Symptoms may become less severe or more severe (bidirectional). Strong social support has a positive effect on all levels and complements therapy. Regular counseling sessions and enhanced family insight about the patient’s paranoia helps strengthen social support
- Marneros A, Pillmann F, Wustmann T. Delusional disorders—are they simply paranoid schizophrenia? [published online ahead of print November 15, 2010]. Schizophr Bull. doi: 10.1093/schbul/sbq125.
Drug Brand Names
Amphetamine and dextroamphetamine • Adderall
Risperidone • Risperdal
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Personal and clinical details of this case were altered to maintain patient confidentiality.
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Sleepless and paranoid
Ms. V, age 16, is referred to our psychiatric hospital from a juvenile detention center after she is charged with killing her sister with a hammer. She reports paranoid delusions, including believing that her sister was poisoning her food. Ms. V’s troubling behavior increased in the 6 months before the murder. She began to ask her mother to smell her food for possible poison. Her school grades dropped and she experienced decreased sleep and appetite. According to her mother, Ms. V’s insomnia worsened recently because of her paranoid thinking, which was evident when she noticed that her daughter slept with a hammer. Ms. V stopped socializing with her peers and no longer went to the gym.
Ms. V’s mother describes her daughter’s negative symptoms as consisting of social isolation and a flat affect. There was no evidence of auditory or visual hallucinations. After noticing the change in her daughter’s behavior, Ms. V’s mother attempted to schedule an appointment with a mental health professional, but there was a 2-month waiting list.
Ms. V cleaned her room before the murder, which was uncharacteristic of her routine behavior. On the day of the murder, Ms. V approached her sister while she was sleeping on the sofa and struck her on the head several times with a hammer. After the sister died, neighbors spotted Ms. V washing blood off her hands in their backyard with a sprinkler. Soaked in blood, she approached one of the neighbors and said that someone had been killed in the house. The neighbors called the police and Ms. V was arrested. She did not express remorse. She did not exhibit physical aggression toward others before the murder. Ms. V’s sense of entitlement and grandiosity persisted after the murder.
The authors’ observations
Paranoid delusions are fixed false beliefs with severe fears of others that may impair functioning at school or work, in personal relationships, and in other social dimensions. Paranoid thinking can have diverse presentations, ranging from social concerns such as fear of rejection to severe threat perceptions of people trying to cause substantial physical harm.1 Paranoid thoughts can be a result of misinterpretation of language, a personality disorder, anxiety, or psychosis.
Feelings of low self-esteem2 and anger1 may develop in a patient experiencing paranoid ideations. When anger begins to escalate, it may erupt into violent behavior. In Ms. V’s case, her paranoid ideations increased until she killed her younger sister. Ms. V’s case is similar to a mass shooting near Tucson, AZ on January 8, 2011 in that it possibly could have been prevented with earlier psychiatric intervention (Box).3-6
On January 8, 2011, a mass shooting occurred near Tucson, AZ that killed 6 and wounded 13. The suspect, 22-year-old Jared Lee Loughner, refused to cooperate with authorities by invoking his right to remain silent.3 Although the motives behind this crime remain undisclosed, mental illness appears to be a contributing factor.
Reports indicate that Mr. Loughner was abusing drugs and those close to him had noticed personality changes.4,5 The college he was attending advised Mr. Loughner to undergo a mental health evaluation, but he refused and dropped out of school.4,5 While in custody after the shooting, Mr. Loughner was diagnosed with paranoid schizophrenia, deemed incompetent to stand trial, and ordered to receive psychiatric treatment.6
This tragic mass shooting and similar incidents have led to questions regarding the adequacy of the mental health care infrastructure in United States. Experts suggest that this tragedy could have been prevented with more aggressive psychiatric prevention and intervention. Critical analysis of similar recent cases and expert opinions are needed to address this problem effectively.
EVALUATION: Remorseless
At admission, Ms. V’s affect is restricted and, at times, inappropriate. She is guarded about discussing the homicide but describes paranoid thoughts about her sister poisoning her. She is eager to learn if the police had found poison in her food. Her speech is soft with good articulation. Based on her presentation, her intelligence is average. She shows no evidence of remorse and is preoccupied with her sister poisoning her.
The Rorschach Inkblot Technique reveals positive evidence for a severe thought disorder. Ms. V’s thinking seems regressed. Ms. V’s medical workup, including MRI, electroencephalogram, and laboratory tests, are all within normal limits.
In the 5th grade, Ms. V’s primary care provider prescribed amphetamine and dextroamphetamine for attention-deficit/hyperactivity disorder, but she discontinued the drug after 1 year. Ms. V has never been hospitalized for psychiatric illness. She had no chronic medical conditions and no developmental delays.
Ms. V also has a history of periodic temper problems characterized by verbal aggression such as threatening the assistant principal at her school, and throwing her cellphone at her mother a few weeks before the murder, but no other aggressive episodes. Ms. V’s history does not suggest conduct disorder. She has no history of suicidal ideation or suicide attempts. Ms. V has used alcohol since age 15, but her mother reports that she was not a heavy or frequent user. Her last reported alcohol use was 10 days before the murder. A maternal uncle had been diagnosed with schizophrenia.
Before the murder, Ms. V lived with her sister and mother. Her parents were divorced. At age 9, Ms. V was sexually abused by a soccer coach; however, she denied symptoms of posttraumatic stress disorder related to the sexual abuse. She had no criminal history before the murder.
The authors’ observations
Based on Ms. V’s presentation and history, schizophrenia, paranoid type seems to be the most likely diagnosis because of her negative symptoms, including affective flattening, positive family history for schizophrenia, and paranoid delusions leading to dysfunction (Table).7 Delusional disorder seems less likely because Ms. V is young and has negative symptoms. Because she is generally healthy and her medical workup is negative, psychotic disorder due to a general medical condition is ruled out. She does not appear to be over-reporting, malingering, or exaggerating symptoms. In the context of psychosis, adolescent psychopathy does not seem likely even though there is evidence of grandiosity and a lack of remorse.
Table
DSM-IV-TR criteria for schizophrenia
A. Characteristic symptoms: ≥2 of the following, each present for a significant portion of time during a 1-month period:
|
| B. Social/occupational dysfunction: For a significant portion of the time since the onset of the disturbance, ≥1 major areas of functioning such as work, interpersonal relations, or self-care are markedly below the level achieved prior to the onset |
| C. Duration: Continuous signs of the disturbance persist for at least 6 months. This 6-month period must include at least 1 month of symptoms that meet Criterion A and may include periods of prodromal or residual symptoms |
| D. Schizoaffective and mood disorder exclusion: Schizoaffective disorder and mood disorder with psychotic features have been ruled out because either (1) no major depressive, manic, or mixed episodes have occurred concurrently with the active-phase symptoms; or (2) if mood episodes have occurred during active-phase symptoms, their total duration has been brief relative to the duration of the active and residual periods |
| E. Substance/general medical condition exclusion: The disturbance is not due to the direct physiological effects of a substance or a general medical condition |
| F. Relationship to a pervasive developmental disorder: If there is a history of autistic disorder or another pervasive developmental disorder, the additional diagnosis of schizophrenia is made only if prominent delusions or hallucinations are also present for at least 1 month |
| Diagnostic criteria for paranoid type: A type of schizophrenia in which the following criteria are met: A. Preoccupation with ≥1 delusions or frequent auditory hallucinations B. None of the following are prominent: disorganized speech, disorganized or catatonic behavior, or flat or inappropriate affect |
| Source: Reference 7 |
The authors’ observations
Various treatments can be used for paranoia with aggression, but the severity of the paranoia should be assessed before initiating treatment. Although categorizing paranoid ideations as mild, moderate, and severe is mainly a clinical judgment, Freeman et al1 have attempted to design a paranoia hierarchy from social concerns to severe threats. CBT8 and antipsychotic medication may help reduce mild to moderate paranoid delusions, particularly those associated with schizophrenia or mood disorders. For severe paranoia, hospitalization should carefully be considered.
When a patient exhibits moderate paranoia, the probability of progressing to severe symptoms or improving to mild symptoms depends on several variables. Pharmacologic treatment, family insight, and social support may be important variables in such circumstances. Psychoeducation for the family is vital.
In patients experiencing paranoia, violence may be prevented by proper assessment and treatment. The patient’s family should be educated about paranoid ideation and the need for treatment to improve symptoms and ensure safety. The long-term effects of untreated paranoia and types of treatment modalities available should be discussed with the family and the patient. During these teaching sessions, focus on improving the overall insight of the family and the patient about the psychotic illness to improve treatment adherence.9 This step may be challenging if the family is resistant to the patient receiving mental health treatment.
Gaining a detailed clinical history of a patient’s paranoia is important. A clinician should look for changes in behavior, such as the patient becoming quieter or more hostile, and impaired academic or social functioning. After gathering sufficient evidence contrary to the delusion, clinicians can help patients improve their reality testing.
Rule out medical and neurologic conditions that may be contributing to paranoia and aggression.
TREATMENT: Some improvement
Ms. V is started on risperidone, 1 mg/d, which leads to a partial response. She starts interacting more with staff and her peers on the unit, but her delusions of her sister poisioning her persist. Given the severity of the crime, Ms. V is sent to adult court, where she is found not guilty by reason of insanity and committed to a state hospital.
The authors’ observations
New-onset paranoia is a serious symptom that requires immediate evaluation and treatment. We recommend an approach presened in a flowchart (Figure) that highlights the importance of early intervention and aggressive treatment.
The MacArthur Violence Risk Assessment Study10 indicated that a “suspicious” attitude toward others can be a precipitating cause for increased violence in some cases. In light of ongoing controversy regarding the link between violence and mental illnesses such as schizophrenia,10-12 addressing an individual’s psychiatric illness early is preferable to prevent possible complications such as violent crimes. Because patients with paranoid ideations may have severely impaired ego control, they may be at risk for acting out aggressive and/or destructive urges. Therefore, new-onset paranoia should be thought of as a medical emergency similar to chest pain. Although accurately predicting and preventing violence may be impossible, in Ms. V’s case, earlier mental health treatment and intervention may have been able to prevent a murder.
Figure: Paranoia: A suggested approach to treatment
aBased on clinical judgment and extent of social support
Symptoms may become less severe or more severe (bidirectional). Strong social support has a positive effect on all levels and complements therapy. Regular counseling sessions and enhanced family insight about the patient’s paranoia helps strengthen social support
- Marneros A, Pillmann F, Wustmann T. Delusional disorders—are they simply paranoid schizophrenia? [published online ahead of print November 15, 2010]. Schizophr Bull. doi: 10.1093/schbul/sbq125.
Drug Brand Names
Amphetamine and dextroamphetamine • Adderall
Risperidone • Risperdal
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Personal and clinical details of this case were altered to maintain patient confidentiality.
1. Freeman D, Garety PA, Bebbington PE, et al. Psychological investigation of the structure of paranoia in a non-clinical population. Br J Psychiatry. 2005;186:427-435.
2. Kendler KS, Hays P. Paranoid psychosis (delusional disorder) and schizophrenia. A family history study. Arch Gen Psychiatry. 1981;38(5):547-551.
3. CNN Wire Staff. Police “actively pursuing” second person in Tucson shooting. CNN. http://us.cnn.com/2011/CRIME/01/08/arizona.shooting. Published January 9 2011. Accessed January 9, 2012.
4. Lipton E, Savage C, Shane S. Arizona suspect’s recent acts offer hints of alienation. The New York Times. January 8 2011:A8. http://www.nytimes.com/2011/01/09/us/politics/09shooter.html. Accessed January 10, 2012.
5. Berger J. Mental health warnings preceded rampage as Arizona gunman likely went untreated. http://www.foxnews.com/us/2011/01/10/mental-health-warnings-preceded-arizona-rampage-evidence-gunman-sought. Published January 10, 2011. Accessed January 11, 2012.
6. Lacey M. Suspect in shooting of Giffords ruled unfit for trial. The New York Times. May 25 2011:A1. http://www.nytimes.com/2011/05/26/us/26loughner.html. Accessed January 5, 2012.
7. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
8. Turkington D, Kingdon D, Weiden PJ. Cognitive behavior therapy for schizophrenia. Am J Psychiatry. 2006;163(3):365-373.
9. Smith CM, Barzman DH, Pristach CA. Effect of patient and family insight on compliance of schizophrenic patients. J Clin Pharmacol. 1997;37(2):147-154.
10. Appelbaum PS, Robbins PC, Monahan J. Violence and delusions: data from the MacArthur Violence Risk Assessment Study. Am J Psychiatry. 2000;157(4):566-572.
11. Mullen PE. A reassessment of the link between mental disorder and violent behaviour and its implications for clinical practice. Aust N Z J Psychiatry. 1997;31(1):3-11.
12. Fazel S, Gulati G, Linsell L, et al. Schizophrenia and violence: systematic review and meta-analysis. PLoS Med. 2009;6(8):e1000120.-
1. Freeman D, Garety PA, Bebbington PE, et al. Psychological investigation of the structure of paranoia in a non-clinical population. Br J Psychiatry. 2005;186:427-435.
2. Kendler KS, Hays P. Paranoid psychosis (delusional disorder) and schizophrenia. A family history study. Arch Gen Psychiatry. 1981;38(5):547-551.
3. CNN Wire Staff. Police “actively pursuing” second person in Tucson shooting. CNN. http://us.cnn.com/2011/CRIME/01/08/arizona.shooting. Published January 9 2011. Accessed January 9, 2012.
4. Lipton E, Savage C, Shane S. Arizona suspect’s recent acts offer hints of alienation. The New York Times. January 8 2011:A8. http://www.nytimes.com/2011/01/09/us/politics/09shooter.html. Accessed January 10, 2012.
5. Berger J. Mental health warnings preceded rampage as Arizona gunman likely went untreated. http://www.foxnews.com/us/2011/01/10/mental-health-warnings-preceded-arizona-rampage-evidence-gunman-sought. Published January 10, 2011. Accessed January 11, 2012.
6. Lacey M. Suspect in shooting of Giffords ruled unfit for trial. The New York Times. May 25 2011:A1. http://www.nytimes.com/2011/05/26/us/26loughner.html. Accessed January 5, 2012.
7. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
8. Turkington D, Kingdon D, Weiden PJ. Cognitive behavior therapy for schizophrenia. Am J Psychiatry. 2006;163(3):365-373.
9. Smith CM, Barzman DH, Pristach CA. Effect of patient and family insight on compliance of schizophrenic patients. J Clin Pharmacol. 1997;37(2):147-154.
10. Appelbaum PS, Robbins PC, Monahan J. Violence and delusions: data from the MacArthur Violence Risk Assessment Study. Am J Psychiatry. 2000;157(4):566-572.
11. Mullen PE. A reassessment of the link between mental disorder and violent behaviour and its implications for clinical practice. Aust N Z J Psychiatry. 1997;31(1):3-11.
12. Fazel S, Gulati G, Linsell L, et al. Schizophrenia and violence: systematic review and meta-analysis. PLoS Med. 2009;6(8):e1000120.-
Not your garden variety neck pain ... Untimely death blamed on undiagnosed PE ... More
Not your garden variety neck pain
PERSISTENT BILATERAL NECK PAIN so severe that he couldn’t sit down brought a man to the emergency department (ED), where he was given ketorolac and diazepam. About an hour later, he said that the pain was better and was discharged with a diagnosis of neck strain and spasm and instructions to see his primary care physician if the pain persisted or worsened.
Four days later, the patient went to his primary care physician complaining of neck pain radiating down both arms, numbness in the right thumb, fever, chills, dysuria, and myalgia in his legs. The doctor observed decreased range of motion of the neck in all directions and diagnosed likely prostatitis. He ordered co-trimoxazole (trimethoprim and sulfamethoxazole), a nonemergent magnetic resonance imaging (MRI) scan, and physical therapy.
Fourteen hours after the doctor visit, the patient went back to the ED in a wheelchair. An emergency MRI showed epidural disease up and down the cervical spine and extending into the thoracic spine. An epidural abscess with spinal cord compression was diagnosed and decompression and evacuation surgery with spinal fusion was performed.
After several weeks in the hospital, the patient was referred to rehabilitation for partial quadriplegia. He has no use of his legs and very limited use of his hands and fingers. He’s confined to a wheelchair and needs help with most activities of daily living.
PLAINTIFF’S CLAIM When the patient visited his primary care physician, he had a classic presentation of a spinal abscess and should have undergone an emergent MRI, which would have revealed the abscess and allowed treatment with antibiotics and surgery before permanent damage occurred.
THE DEFENSE The patient’s symptoms weren’t a typical presentation of spinal abscess. There was no way the physician could have known what would happen the next day.
VERDICT $3 million Massachusetts settlement.
COMMENT Yes, there are zebras among the horses. We have to be vigilant to diagnose the rare serious cause of common problems such as neck pain. The combination of neck pain, patchy neurologic findings, signs of infection, and bladder symptoms should have raised red flags.
Untimely death blamed on undiagnosed PE
A 28-YEAR-OLD MAN went to the emergency department (ED) complaining of low-grade fever, nonproductive cough, and dizziness for 2 days. He also had tachycardia and significant hypoxia. An ED physician who saw the patient an hour after his arrival noted that he complained of weakness, shortness of breath, and light-headedness. The differential diagnosis included pneumonia, congestive heart failure, and pulmonary embolism.
After reviewing an electrocardiogram, chest radiograph, and laboratory studies, the ED doctor diagnosed pneumonia and renal insufficiency. The patient was admitted to the hospital, then transferred to another hospital about 8 hours later. He wasn’t evaluated by a physician when he was admitted to the second hospital.
About 5 hours after admission, the patient got out of bed and collapsed in the presence of his wife. A code was called, but the patient never regained consciousness and died about an hour and a half later. An autopsy established a pulmonary embolism as the cause of death.
PLAINTIFF’S CLAIM The doctors were negligent in failing to diagnose and treat the pulmonary embolism. Proper treatment would have allowed the patient to survive.
THE DEFENSE There was no negligence; heparin therapy wouldn’t have prevented the patient’s death.
VERDICT $6.1 million Maryland verdict.
COMMENT It isn’t enough to think of pulmonary embolism; a prompt definitive diagnostic work-up and timely treatment are key to preventing such a catastrophic outcome.
Delayed herpes diagnosis leads to lifelong consequences
A 10-DAY-OLD INFANT was examined by a pediatrician, who noted vesicles dotting the baby’s tongue, a possible manifestation of herpes, and observed herpes labialis on the mother’s lips. The pediatrician concluded that the vesicles didn’t indicate herpes and discharged the baby, instructing the parents to have him reexamined if he developed a fever, irritability, or lethargy. The next day the pediatrician consulted a neonatologist, who advised immediate reexamination. The baby was taken to a hospital, but then was immediately transported to another hospital.
At the second hospital, a physician examined the baby and consulted an oral surgeon. The surgeon believed that the vesicles were caused by burns from a hot baby bottle. The baby was discharged.
Six days later, the mother brought the baby to his regular pediatrician. She reported that the infant had been feverish and lethargic. The pediatrician didn’t find vesicles or other abnormalities. She ordered a complete blood count and blood culture, gave antibiotics, and told the parents to bring the baby back to see her the next day.
Very early the next day, the parents brought the baby to a hospital with a temperature of 101.2°F. The examining physician contacted the child’s pediatrician, who said she wanted to see the baby at 8:00 AM. When the pediatrician examined him, the infant’s temperature was 100.5°F. She gave antibiotics and instructed the parents to bring the baby back the next day, when his test results would be available.
The next day, the parents told the pediatrician’s assistant who examined the baby that his arms and legs had been twitching the previous evening. The infant received antibiotics but began to exhibit jerky movements. The parents were told to take him to a hospital, where he was diagnosed with herpes simplex and residual brain damage.
The child has quadriparesis and can’t talk, walk, or feed himself. He can eat only pureed food.
PLAINTIFF’S CLAIM The herpes simplex infection should have been diagnosed earlier. The pediatrician who examined the infant initially should have cultured the vesicles (and made sure that acyclovir was given) or consulted with, or referred the child to, a specialist. The physician who saw the child at the second hospital should have consulted a specialist, which would have led to the administration of acyclovir.
THE DEFENSE Hospitalization wasn’t necessary and a culture wasn’t appropriate. The appearance of the vesicles when the baby was examined at the second hospital didn’t suggest herpes.
VERDICT Multiple New York settlements totaling $10.2 million.
COMMENT As with many malpractice cases, there were many opportunities to prevent an egregious outcome. I wonder whether anyone involved stopped to entertain a differential diagnosis and note the urgent conditions the presentation clearly suggested.
Not your garden variety neck pain
PERSISTENT BILATERAL NECK PAIN so severe that he couldn’t sit down brought a man to the emergency department (ED), where he was given ketorolac and diazepam. About an hour later, he said that the pain was better and was discharged with a diagnosis of neck strain and spasm and instructions to see his primary care physician if the pain persisted or worsened.
Four days later, the patient went to his primary care physician complaining of neck pain radiating down both arms, numbness in the right thumb, fever, chills, dysuria, and myalgia in his legs. The doctor observed decreased range of motion of the neck in all directions and diagnosed likely prostatitis. He ordered co-trimoxazole (trimethoprim and sulfamethoxazole), a nonemergent magnetic resonance imaging (MRI) scan, and physical therapy.
Fourteen hours after the doctor visit, the patient went back to the ED in a wheelchair. An emergency MRI showed epidural disease up and down the cervical spine and extending into the thoracic spine. An epidural abscess with spinal cord compression was diagnosed and decompression and evacuation surgery with spinal fusion was performed.
After several weeks in the hospital, the patient was referred to rehabilitation for partial quadriplegia. He has no use of his legs and very limited use of his hands and fingers. He’s confined to a wheelchair and needs help with most activities of daily living.
PLAINTIFF’S CLAIM When the patient visited his primary care physician, he had a classic presentation of a spinal abscess and should have undergone an emergent MRI, which would have revealed the abscess and allowed treatment with antibiotics and surgery before permanent damage occurred.
THE DEFENSE The patient’s symptoms weren’t a typical presentation of spinal abscess. There was no way the physician could have known what would happen the next day.
VERDICT $3 million Massachusetts settlement.
COMMENT Yes, there are zebras among the horses. We have to be vigilant to diagnose the rare serious cause of common problems such as neck pain. The combination of neck pain, patchy neurologic findings, signs of infection, and bladder symptoms should have raised red flags.
Untimely death blamed on undiagnosed PE
A 28-YEAR-OLD MAN went to the emergency department (ED) complaining of low-grade fever, nonproductive cough, and dizziness for 2 days. He also had tachycardia and significant hypoxia. An ED physician who saw the patient an hour after his arrival noted that he complained of weakness, shortness of breath, and light-headedness. The differential diagnosis included pneumonia, congestive heart failure, and pulmonary embolism.
After reviewing an electrocardiogram, chest radiograph, and laboratory studies, the ED doctor diagnosed pneumonia and renal insufficiency. The patient was admitted to the hospital, then transferred to another hospital about 8 hours later. He wasn’t evaluated by a physician when he was admitted to the second hospital.
About 5 hours after admission, the patient got out of bed and collapsed in the presence of his wife. A code was called, but the patient never regained consciousness and died about an hour and a half later. An autopsy established a pulmonary embolism as the cause of death.
PLAINTIFF’S CLAIM The doctors were negligent in failing to diagnose and treat the pulmonary embolism. Proper treatment would have allowed the patient to survive.
THE DEFENSE There was no negligence; heparin therapy wouldn’t have prevented the patient’s death.
VERDICT $6.1 million Maryland verdict.
COMMENT It isn’t enough to think of pulmonary embolism; a prompt definitive diagnostic work-up and timely treatment are key to preventing such a catastrophic outcome.
Delayed herpes diagnosis leads to lifelong consequences
A 10-DAY-OLD INFANT was examined by a pediatrician, who noted vesicles dotting the baby’s tongue, a possible manifestation of herpes, and observed herpes labialis on the mother’s lips. The pediatrician concluded that the vesicles didn’t indicate herpes and discharged the baby, instructing the parents to have him reexamined if he developed a fever, irritability, or lethargy. The next day the pediatrician consulted a neonatologist, who advised immediate reexamination. The baby was taken to a hospital, but then was immediately transported to another hospital.
At the second hospital, a physician examined the baby and consulted an oral surgeon. The surgeon believed that the vesicles were caused by burns from a hot baby bottle. The baby was discharged.
Six days later, the mother brought the baby to his regular pediatrician. She reported that the infant had been feverish and lethargic. The pediatrician didn’t find vesicles or other abnormalities. She ordered a complete blood count and blood culture, gave antibiotics, and told the parents to bring the baby back to see her the next day.
Very early the next day, the parents brought the baby to a hospital with a temperature of 101.2°F. The examining physician contacted the child’s pediatrician, who said she wanted to see the baby at 8:00 AM. When the pediatrician examined him, the infant’s temperature was 100.5°F. She gave antibiotics and instructed the parents to bring the baby back the next day, when his test results would be available.
The next day, the parents told the pediatrician’s assistant who examined the baby that his arms and legs had been twitching the previous evening. The infant received antibiotics but began to exhibit jerky movements. The parents were told to take him to a hospital, where he was diagnosed with herpes simplex and residual brain damage.
The child has quadriparesis and can’t talk, walk, or feed himself. He can eat only pureed food.
PLAINTIFF’S CLAIM The herpes simplex infection should have been diagnosed earlier. The pediatrician who examined the infant initially should have cultured the vesicles (and made sure that acyclovir was given) or consulted with, or referred the child to, a specialist. The physician who saw the child at the second hospital should have consulted a specialist, which would have led to the administration of acyclovir.
THE DEFENSE Hospitalization wasn’t necessary and a culture wasn’t appropriate. The appearance of the vesicles when the baby was examined at the second hospital didn’t suggest herpes.
VERDICT Multiple New York settlements totaling $10.2 million.
COMMENT As with many malpractice cases, there were many opportunities to prevent an egregious outcome. I wonder whether anyone involved stopped to entertain a differential diagnosis and note the urgent conditions the presentation clearly suggested.
Not your garden variety neck pain
PERSISTENT BILATERAL NECK PAIN so severe that he couldn’t sit down brought a man to the emergency department (ED), where he was given ketorolac and diazepam. About an hour later, he said that the pain was better and was discharged with a diagnosis of neck strain and spasm and instructions to see his primary care physician if the pain persisted or worsened.
Four days later, the patient went to his primary care physician complaining of neck pain radiating down both arms, numbness in the right thumb, fever, chills, dysuria, and myalgia in his legs. The doctor observed decreased range of motion of the neck in all directions and diagnosed likely prostatitis. He ordered co-trimoxazole (trimethoprim and sulfamethoxazole), a nonemergent magnetic resonance imaging (MRI) scan, and physical therapy.
Fourteen hours after the doctor visit, the patient went back to the ED in a wheelchair. An emergency MRI showed epidural disease up and down the cervical spine and extending into the thoracic spine. An epidural abscess with spinal cord compression was diagnosed and decompression and evacuation surgery with spinal fusion was performed.
After several weeks in the hospital, the patient was referred to rehabilitation for partial quadriplegia. He has no use of his legs and very limited use of his hands and fingers. He’s confined to a wheelchair and needs help with most activities of daily living.
PLAINTIFF’S CLAIM When the patient visited his primary care physician, he had a classic presentation of a spinal abscess and should have undergone an emergent MRI, which would have revealed the abscess and allowed treatment with antibiotics and surgery before permanent damage occurred.
THE DEFENSE The patient’s symptoms weren’t a typical presentation of spinal abscess. There was no way the physician could have known what would happen the next day.
VERDICT $3 million Massachusetts settlement.
COMMENT Yes, there are zebras among the horses. We have to be vigilant to diagnose the rare serious cause of common problems such as neck pain. The combination of neck pain, patchy neurologic findings, signs of infection, and bladder symptoms should have raised red flags.
Untimely death blamed on undiagnosed PE
A 28-YEAR-OLD MAN went to the emergency department (ED) complaining of low-grade fever, nonproductive cough, and dizziness for 2 days. He also had tachycardia and significant hypoxia. An ED physician who saw the patient an hour after his arrival noted that he complained of weakness, shortness of breath, and light-headedness. The differential diagnosis included pneumonia, congestive heart failure, and pulmonary embolism.
After reviewing an electrocardiogram, chest radiograph, and laboratory studies, the ED doctor diagnosed pneumonia and renal insufficiency. The patient was admitted to the hospital, then transferred to another hospital about 8 hours later. He wasn’t evaluated by a physician when he was admitted to the second hospital.
About 5 hours after admission, the patient got out of bed and collapsed in the presence of his wife. A code was called, but the patient never regained consciousness and died about an hour and a half later. An autopsy established a pulmonary embolism as the cause of death.
PLAINTIFF’S CLAIM The doctors were negligent in failing to diagnose and treat the pulmonary embolism. Proper treatment would have allowed the patient to survive.
THE DEFENSE There was no negligence; heparin therapy wouldn’t have prevented the patient’s death.
VERDICT $6.1 million Maryland verdict.
COMMENT It isn’t enough to think of pulmonary embolism; a prompt definitive diagnostic work-up and timely treatment are key to preventing such a catastrophic outcome.
Delayed herpes diagnosis leads to lifelong consequences
A 10-DAY-OLD INFANT was examined by a pediatrician, who noted vesicles dotting the baby’s tongue, a possible manifestation of herpes, and observed herpes labialis on the mother’s lips. The pediatrician concluded that the vesicles didn’t indicate herpes and discharged the baby, instructing the parents to have him reexamined if he developed a fever, irritability, or lethargy. The next day the pediatrician consulted a neonatologist, who advised immediate reexamination. The baby was taken to a hospital, but then was immediately transported to another hospital.
At the second hospital, a physician examined the baby and consulted an oral surgeon. The surgeon believed that the vesicles were caused by burns from a hot baby bottle. The baby was discharged.
Six days later, the mother brought the baby to his regular pediatrician. She reported that the infant had been feverish and lethargic. The pediatrician didn’t find vesicles or other abnormalities. She ordered a complete blood count and blood culture, gave antibiotics, and told the parents to bring the baby back to see her the next day.
Very early the next day, the parents brought the baby to a hospital with a temperature of 101.2°F. The examining physician contacted the child’s pediatrician, who said she wanted to see the baby at 8:00 AM. When the pediatrician examined him, the infant’s temperature was 100.5°F. She gave antibiotics and instructed the parents to bring the baby back the next day, when his test results would be available.
The next day, the parents told the pediatrician’s assistant who examined the baby that his arms and legs had been twitching the previous evening. The infant received antibiotics but began to exhibit jerky movements. The parents were told to take him to a hospital, where he was diagnosed with herpes simplex and residual brain damage.
The child has quadriparesis and can’t talk, walk, or feed himself. He can eat only pureed food.
PLAINTIFF’S CLAIM The herpes simplex infection should have been diagnosed earlier. The pediatrician who examined the infant initially should have cultured the vesicles (and made sure that acyclovir was given) or consulted with, or referred the child to, a specialist. The physician who saw the child at the second hospital should have consulted a specialist, which would have led to the administration of acyclovir.
THE DEFENSE Hospitalization wasn’t necessary and a culture wasn’t appropriate. The appearance of the vesicles when the baby was examined at the second hospital didn’t suggest herpes.
VERDICT Multiple New York settlements totaling $10.2 million.
COMMENT As with many malpractice cases, there were many opportunities to prevent an egregious outcome. I wonder whether anyone involved stopped to entertain a differential diagnosis and note the urgent conditions the presentation clearly suggested.
Which drugs work best for early Parkinson’s disease?
LEVODOPA/CARBIDOPA is the most effective medical therapy for Parkinson’s disease, but it’s associated with dyskinesia (strength of recommendation [SOR]: A, Cochrane reviews and randomized controlled trials [RCTs]). Treating early Parkinson’s disease with dopamine agonists such as bromocriptine can improve symptoms (SOR: B, Cochrane reviews, RCTs with heterogeneity).
Evidence summary
Levodopa/carbidopa is the most commonly prescribed medication for Parkinson’s disease. Although its efficacy is established, it can cause dyskinesia and dystonia.1 Recent studies (TABLE) have evaluated the use of other medications early in the course of Parkinson’s disease in hopes of delaying the waning effectiveness of levodopa over time.
TABLE
Medications commonly used to treat Parkinson’s disease
| Medication class brand (generic)9 | Advantages | Disadvantages | Approximate monthly cost at usual dosage (in US $) for generic (brand name prices cited if no generic available)10 |
|---|---|---|---|
| Carbidopa/levodopa Sinemet (carbidopa/ levodopa) Sinemet CR (carbidopa/levodopa controlled-release) | First-line therapy; most effective at improving motor disability1 | Dyskinesia, dystonia, hallucinations No documented benefit of long-acting form1,8 | $34.99-$101.98 $80.99-$295.97 (Highly variable due to dose range) |
| COMT inhibitor Comtan (entacapone) Stalevo (carbidopa/levodopa/entacapone) | Augments levodopa, may improve activities of daily living6 | Same side effects as above plus possible increased nausea, vomiting, diarrhea6 Possible increased cardiovascular risk and prostate cancer | $310.97-$414.62 $318.00-$1043.97 |
| Dopamine agonist Mirapex (pramipexole) Requip (ropinirole) Parlodel (bromocriptine) | Reduced dyskinesias, dystonia, and motor complications2 | Nausea, dizziness, constipation, somnolence, hallucinations, edema2 | $239.99 $71.99-$143.98 $385.97-$1133.92 |
| MAO-B inhibitor Eldepryl (selegiline) | Mild improved motor symptoms of disease, decreased motor fluctuations of treatment, possible “levosparing effect”5 | Limited efficacy and multiple adverse effects leading to high dropout rate; not recommended by Cochrane review5 | $101.99 |
| Anticholinergic Cogentin (benztropine mesylate) | Improved symptoms, mostly tremor7 | Confusion, memory loss, hallucinations, restlessness; contraindicated in dementia7 | $13.99-$22.99 |
| Other Symmetrel (amantadine) | No good updated studies, unproven long-term benefit, nausea, dizziness, insomnia, can cause psychosis9 | $43.17 | |
| COMT, catechol-O-methyltransferase; MAO-B, monoamine oxidase type B. | |||
Dopamine agonists: Dyskinesia reduction, but at a price
A Cochrane review of 29 trials with 5247 patients compared dopamine agonists with levodopa.2 Levodopa controlled symptoms better than dopamine agonists, but inconsistent data reporting prevented quantifying this result.
Compared with the group taking levodopa, patients taking dopamine agonists demonstrated a significant reduction in dyskinesia (odds ratio [OR]=0.45; 95% CI, 0.37-0.54), dystonia (OR=0.64; 95% CI, 0.51-0.81), and motor fluctuations (OR= 0.71; 95% CI, 0.58-0.87).
However, patients taking dopamine agonists with or without levodopa experienced significantly more adverse effects than patients taking levodopa alone. Side effects included increased edema (OR=3.68; 95% CI, 2.62-5.18), somnolence (OR=1.49; 95% CI, 1.12-2.00), constipation (OR=1.59; 95% CI, 1.11-2.28), dizziness (OR=1.45; 95% CI, 1.09-1.92), hallucinations (OR=1.69; 95% CI, 1.13-2.52), and nausea (OR=1.32; 95% CI, 1.05-1.66). Patients treated with dopamine agonists were also significantly more likely to discontinue treatment because of adverse events (OR=2.49; 95% CI, 2.08-2.98; P<.00001).
Bromocriptine studies hampered by poor quality
Two Cochrane reviews specifically evaluated the dopamine agonist bromocriptine.3,4 The first focused on 6 head-to-head trials with levodopa that enrolled 850 patients.3 The studies were of poor quality, marred by methodological flaws and clinical heterogeneity. Problems included inadequate power, high variability in study duration (23 weeks to 5 years), differences in reporting, and lack of description of the randomization method in 3 of the 6 trials. Although bromocriptine showed a trend toward lower incidence of motor complications, many patients dropped out of the studies because of increased non-motor adverse effects and inadequate response to treatment.
The second review, of 7 trials with a total of 1100 patients, compared bromocriptine plus levodopa with levodopa alone.4 The studies were of poor quality for reasons similar to the studies in the first review. Researchers found no statistically significant or consistent evidence to determine whether bromocriptine plus levodopa prevents or delays motor complications.
MAO-B inhibitors: Minimally effective with troubling side effects
A Cochrane review of monoamine oxidase type B (MAO-B) inhibitors included 10 trials with 2422 participants.5 The review found statistically, but not clinically, significant improvements in scores on 2 sections of the United Parkinson Disease Rating Scale (UPDRS), a standardized assessment tool that facilitates accurate documentation of disease progression and treatment response.
Compared with the control groups (either placebo or levodopa at study onset), the MAO-B group (either alone or with levodopa) showed significant improvement on the motor section (weighted mean difference [WMD]=–3.81 on a 108-point scale; 95% CI, –5.36 to –2.27) and activities of daily living section (WMD=–1.50 on a 52-point scale; 95% CI, –2.53 to –0.48). Fewer motor complications occurred in the MAO-B group (OR=0.75; 95% CI, 0.59-0.94) than the control group. Lower doses and shorter treatment with levodopa were necessary to control symptoms in the MAO-B group.
The clinical impact of MAO-B inhibitors on Parkinson’s symptoms was small, and almost all patients required the addition of levodopa to the treatment regimen after 3 or 4 years. Withdrawals because of medication side effects were significantly higher in the MAO-B inhibitor group than controls (OR=2.36; 95% CI, 1.32-4.20). Side effects included nausea, confusion, hallucinations, and postural hypotension. Concerns about cardiovascular adverse effects raised in previous studies, especially with selegiline, weren’t found to be significant (OR=1.15; 95% CI, 0.92-1.44). Because of their minimal effectiveness and worrisome adverse effects, MAO-B inhibitors aren’t recommended for routine use in early Parkinson’s disease.
COMT inhibitors may boost levodopa/carbidopa’s effects
A randomized double-blinded trial followed 423 patients for 39 weeks to compare the combination of the catechol-O-methyltransferase (COMT) inhibitor entacapone and levodopa/carbidopa (LCE) with levodopa/carbidopa alone (LC).6 The researchers found statistically significant improvements with LCE in UPDRS scores for activities of daily living (mean change from baseline=3.0 for LCE vs 2.3 for LC on a 52-point scale; P=.025) but not mentation or motor symptoms.
Dyskinesia and wearing-off symptoms (motor fluctuations) didn’t differ significantly between the 2 groups. LCE was associated with a higher incidence of adverse effects than LC, and involved mostly nausea (26.6% vs 13.5%) and diarrhea (8.7% vs 2.8%).
Anticholinergics may help, but cause adverse mental effects
Another Cochrane review compared anticholinergic agents with placebo or no treatment in 9 studies that included 221 patients.7 Meta-analysis wasn’t possible because of heterogeneity in patient populations, outcomes, and measurements and incomplete reporting. Compared with placebo, anticholinergic agents may improve Parkinson’s-related motor symptoms but have significant mental adverse effects, including confusion, memory problems, restlessness, and hallucinations.
Recommendations
The most recent guidelines (2002) from the American Academy of Neurology recommend levodopa and dopamine agonists as first-line therapies.8 Levodopa is more effective at improving the motor symptoms of Parkinson’s disease but is associated with a higher risk of dyskinesia than dopamine agonists. No compelling evidence suggests a difference in efficacy between long- and short-acting levodopa.
1. Hauser RA. Levodopa: past, present, and future. Eur Neurol. 2009;62:1-8.
2. Stowe RL, Ives NJ, Clarke C, et al. Dopamine agonist therapy in early Parkinson’s disease. Cochrane Database Syst Rev. 2008;(2):CD006564.-
3. van Hilten JJ, Ramaker CC, Stowe R, et al. Bromocriptine versus levodopa in early Parkinson’s disease. Cochrane Database Syst Rev. 2007;(4):CD002258.-
4. van Hilten JJ, Ramaker CC, Stowe R, et al. Bromocriptine/levodopa combined versus levodopa alone for early Parkinson’s disease. Cochrane Database Syst Rev. 2007;(4):CD003634.-
5. Macleod AD, Counsell CE, Ives N, et al. Monoamine oxidase B inhibitors for early Parkinson’s disease. Cochrane Database Syst Rev. 2005;(3):CD004898.-
6. Hauser RA, Panisset M, Abbruzzese G, et al. Double-blind trial of levodopa/carbidopa/entacapone versus levodopa/ carbidopa in early Parkinson’s disease. Mov Disord. 2009;24:541-550.
7. Katzenschlager R, Sampaio C, Costa J, et al. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst Rev. 2003;(2):CD003735.-
8. Miyasaki JM, Martin W, Suchowersky O, et al. Practice parameter: initiation of treatment for Parkinson’s disease: an evidence-based review: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2002;58:11-17.
9. Drugs for Parkinson’s disease Treat Guidl Med Lett. 2011;9:1-6
10. Drugstore.com Online Pharmacy. Pharmacy drug costs. Available at http://www.drugstore.com. Accessed August 30, 2011.
LEVODOPA/CARBIDOPA is the most effective medical therapy for Parkinson’s disease, but it’s associated with dyskinesia (strength of recommendation [SOR]: A, Cochrane reviews and randomized controlled trials [RCTs]). Treating early Parkinson’s disease with dopamine agonists such as bromocriptine can improve symptoms (SOR: B, Cochrane reviews, RCTs with heterogeneity).
Evidence summary
Levodopa/carbidopa is the most commonly prescribed medication for Parkinson’s disease. Although its efficacy is established, it can cause dyskinesia and dystonia.1 Recent studies (TABLE) have evaluated the use of other medications early in the course of Parkinson’s disease in hopes of delaying the waning effectiveness of levodopa over time.
TABLE
Medications commonly used to treat Parkinson’s disease
| Medication class brand (generic)9 | Advantages | Disadvantages | Approximate monthly cost at usual dosage (in US $) for generic (brand name prices cited if no generic available)10 |
|---|---|---|---|
| Carbidopa/levodopa Sinemet (carbidopa/ levodopa) Sinemet CR (carbidopa/levodopa controlled-release) | First-line therapy; most effective at improving motor disability1 | Dyskinesia, dystonia, hallucinations No documented benefit of long-acting form1,8 | $34.99-$101.98 $80.99-$295.97 (Highly variable due to dose range) |
| COMT inhibitor Comtan (entacapone) Stalevo (carbidopa/levodopa/entacapone) | Augments levodopa, may improve activities of daily living6 | Same side effects as above plus possible increased nausea, vomiting, diarrhea6 Possible increased cardiovascular risk and prostate cancer | $310.97-$414.62 $318.00-$1043.97 |
| Dopamine agonist Mirapex (pramipexole) Requip (ropinirole) Parlodel (bromocriptine) | Reduced dyskinesias, dystonia, and motor complications2 | Nausea, dizziness, constipation, somnolence, hallucinations, edema2 | $239.99 $71.99-$143.98 $385.97-$1133.92 |
| MAO-B inhibitor Eldepryl (selegiline) | Mild improved motor symptoms of disease, decreased motor fluctuations of treatment, possible “levosparing effect”5 | Limited efficacy and multiple adverse effects leading to high dropout rate; not recommended by Cochrane review5 | $101.99 |
| Anticholinergic Cogentin (benztropine mesylate) | Improved symptoms, mostly tremor7 | Confusion, memory loss, hallucinations, restlessness; contraindicated in dementia7 | $13.99-$22.99 |
| Other Symmetrel (amantadine) | No good updated studies, unproven long-term benefit, nausea, dizziness, insomnia, can cause psychosis9 | $43.17 | |
| COMT, catechol-O-methyltransferase; MAO-B, monoamine oxidase type B. | |||
Dopamine agonists: Dyskinesia reduction, but at a price
A Cochrane review of 29 trials with 5247 patients compared dopamine agonists with levodopa.2 Levodopa controlled symptoms better than dopamine agonists, but inconsistent data reporting prevented quantifying this result.
Compared with the group taking levodopa, patients taking dopamine agonists demonstrated a significant reduction in dyskinesia (odds ratio [OR]=0.45; 95% CI, 0.37-0.54), dystonia (OR=0.64; 95% CI, 0.51-0.81), and motor fluctuations (OR= 0.71; 95% CI, 0.58-0.87).
However, patients taking dopamine agonists with or without levodopa experienced significantly more adverse effects than patients taking levodopa alone. Side effects included increased edema (OR=3.68; 95% CI, 2.62-5.18), somnolence (OR=1.49; 95% CI, 1.12-2.00), constipation (OR=1.59; 95% CI, 1.11-2.28), dizziness (OR=1.45; 95% CI, 1.09-1.92), hallucinations (OR=1.69; 95% CI, 1.13-2.52), and nausea (OR=1.32; 95% CI, 1.05-1.66). Patients treated with dopamine agonists were also significantly more likely to discontinue treatment because of adverse events (OR=2.49; 95% CI, 2.08-2.98; P<.00001).
Bromocriptine studies hampered by poor quality
Two Cochrane reviews specifically evaluated the dopamine agonist bromocriptine.3,4 The first focused on 6 head-to-head trials with levodopa that enrolled 850 patients.3 The studies were of poor quality, marred by methodological flaws and clinical heterogeneity. Problems included inadequate power, high variability in study duration (23 weeks to 5 years), differences in reporting, and lack of description of the randomization method in 3 of the 6 trials. Although bromocriptine showed a trend toward lower incidence of motor complications, many patients dropped out of the studies because of increased non-motor adverse effects and inadequate response to treatment.
The second review, of 7 trials with a total of 1100 patients, compared bromocriptine plus levodopa with levodopa alone.4 The studies were of poor quality for reasons similar to the studies in the first review. Researchers found no statistically significant or consistent evidence to determine whether bromocriptine plus levodopa prevents or delays motor complications.
MAO-B inhibitors: Minimally effective with troubling side effects
A Cochrane review of monoamine oxidase type B (MAO-B) inhibitors included 10 trials with 2422 participants.5 The review found statistically, but not clinically, significant improvements in scores on 2 sections of the United Parkinson Disease Rating Scale (UPDRS), a standardized assessment tool that facilitates accurate documentation of disease progression and treatment response.
Compared with the control groups (either placebo or levodopa at study onset), the MAO-B group (either alone or with levodopa) showed significant improvement on the motor section (weighted mean difference [WMD]=–3.81 on a 108-point scale; 95% CI, –5.36 to –2.27) and activities of daily living section (WMD=–1.50 on a 52-point scale; 95% CI, –2.53 to –0.48). Fewer motor complications occurred in the MAO-B group (OR=0.75; 95% CI, 0.59-0.94) than the control group. Lower doses and shorter treatment with levodopa were necessary to control symptoms in the MAO-B group.
The clinical impact of MAO-B inhibitors on Parkinson’s symptoms was small, and almost all patients required the addition of levodopa to the treatment regimen after 3 or 4 years. Withdrawals because of medication side effects were significantly higher in the MAO-B inhibitor group than controls (OR=2.36; 95% CI, 1.32-4.20). Side effects included nausea, confusion, hallucinations, and postural hypotension. Concerns about cardiovascular adverse effects raised in previous studies, especially with selegiline, weren’t found to be significant (OR=1.15; 95% CI, 0.92-1.44). Because of their minimal effectiveness and worrisome adverse effects, MAO-B inhibitors aren’t recommended for routine use in early Parkinson’s disease.
COMT inhibitors may boost levodopa/carbidopa’s effects
A randomized double-blinded trial followed 423 patients for 39 weeks to compare the combination of the catechol-O-methyltransferase (COMT) inhibitor entacapone and levodopa/carbidopa (LCE) with levodopa/carbidopa alone (LC).6 The researchers found statistically significant improvements with LCE in UPDRS scores for activities of daily living (mean change from baseline=3.0 for LCE vs 2.3 for LC on a 52-point scale; P=.025) but not mentation or motor symptoms.
Dyskinesia and wearing-off symptoms (motor fluctuations) didn’t differ significantly between the 2 groups. LCE was associated with a higher incidence of adverse effects than LC, and involved mostly nausea (26.6% vs 13.5%) and diarrhea (8.7% vs 2.8%).
Anticholinergics may help, but cause adverse mental effects
Another Cochrane review compared anticholinergic agents with placebo or no treatment in 9 studies that included 221 patients.7 Meta-analysis wasn’t possible because of heterogeneity in patient populations, outcomes, and measurements and incomplete reporting. Compared with placebo, anticholinergic agents may improve Parkinson’s-related motor symptoms but have significant mental adverse effects, including confusion, memory problems, restlessness, and hallucinations.
Recommendations
The most recent guidelines (2002) from the American Academy of Neurology recommend levodopa and dopamine agonists as first-line therapies.8 Levodopa is more effective at improving the motor symptoms of Parkinson’s disease but is associated with a higher risk of dyskinesia than dopamine agonists. No compelling evidence suggests a difference in efficacy between long- and short-acting levodopa.
LEVODOPA/CARBIDOPA is the most effective medical therapy for Parkinson’s disease, but it’s associated with dyskinesia (strength of recommendation [SOR]: A, Cochrane reviews and randomized controlled trials [RCTs]). Treating early Parkinson’s disease with dopamine agonists such as bromocriptine can improve symptoms (SOR: B, Cochrane reviews, RCTs with heterogeneity).
Evidence summary
Levodopa/carbidopa is the most commonly prescribed medication for Parkinson’s disease. Although its efficacy is established, it can cause dyskinesia and dystonia.1 Recent studies (TABLE) have evaluated the use of other medications early in the course of Parkinson’s disease in hopes of delaying the waning effectiveness of levodopa over time.
TABLE
Medications commonly used to treat Parkinson’s disease
| Medication class brand (generic)9 | Advantages | Disadvantages | Approximate monthly cost at usual dosage (in US $) for generic (brand name prices cited if no generic available)10 |
|---|---|---|---|
| Carbidopa/levodopa Sinemet (carbidopa/ levodopa) Sinemet CR (carbidopa/levodopa controlled-release) | First-line therapy; most effective at improving motor disability1 | Dyskinesia, dystonia, hallucinations No documented benefit of long-acting form1,8 | $34.99-$101.98 $80.99-$295.97 (Highly variable due to dose range) |
| COMT inhibitor Comtan (entacapone) Stalevo (carbidopa/levodopa/entacapone) | Augments levodopa, may improve activities of daily living6 | Same side effects as above plus possible increased nausea, vomiting, diarrhea6 Possible increased cardiovascular risk and prostate cancer | $310.97-$414.62 $318.00-$1043.97 |
| Dopamine agonist Mirapex (pramipexole) Requip (ropinirole) Parlodel (bromocriptine) | Reduced dyskinesias, dystonia, and motor complications2 | Nausea, dizziness, constipation, somnolence, hallucinations, edema2 | $239.99 $71.99-$143.98 $385.97-$1133.92 |
| MAO-B inhibitor Eldepryl (selegiline) | Mild improved motor symptoms of disease, decreased motor fluctuations of treatment, possible “levosparing effect”5 | Limited efficacy and multiple adverse effects leading to high dropout rate; not recommended by Cochrane review5 | $101.99 |
| Anticholinergic Cogentin (benztropine mesylate) | Improved symptoms, mostly tremor7 | Confusion, memory loss, hallucinations, restlessness; contraindicated in dementia7 | $13.99-$22.99 |
| Other Symmetrel (amantadine) | No good updated studies, unproven long-term benefit, nausea, dizziness, insomnia, can cause psychosis9 | $43.17 | |
| COMT, catechol-O-methyltransferase; MAO-B, monoamine oxidase type B. | |||
Dopamine agonists: Dyskinesia reduction, but at a price
A Cochrane review of 29 trials with 5247 patients compared dopamine agonists with levodopa.2 Levodopa controlled symptoms better than dopamine agonists, but inconsistent data reporting prevented quantifying this result.
Compared with the group taking levodopa, patients taking dopamine agonists demonstrated a significant reduction in dyskinesia (odds ratio [OR]=0.45; 95% CI, 0.37-0.54), dystonia (OR=0.64; 95% CI, 0.51-0.81), and motor fluctuations (OR= 0.71; 95% CI, 0.58-0.87).
However, patients taking dopamine agonists with or without levodopa experienced significantly more adverse effects than patients taking levodopa alone. Side effects included increased edema (OR=3.68; 95% CI, 2.62-5.18), somnolence (OR=1.49; 95% CI, 1.12-2.00), constipation (OR=1.59; 95% CI, 1.11-2.28), dizziness (OR=1.45; 95% CI, 1.09-1.92), hallucinations (OR=1.69; 95% CI, 1.13-2.52), and nausea (OR=1.32; 95% CI, 1.05-1.66). Patients treated with dopamine agonists were also significantly more likely to discontinue treatment because of adverse events (OR=2.49; 95% CI, 2.08-2.98; P<.00001).
Bromocriptine studies hampered by poor quality
Two Cochrane reviews specifically evaluated the dopamine agonist bromocriptine.3,4 The first focused on 6 head-to-head trials with levodopa that enrolled 850 patients.3 The studies were of poor quality, marred by methodological flaws and clinical heterogeneity. Problems included inadequate power, high variability in study duration (23 weeks to 5 years), differences in reporting, and lack of description of the randomization method in 3 of the 6 trials. Although bromocriptine showed a trend toward lower incidence of motor complications, many patients dropped out of the studies because of increased non-motor adverse effects and inadequate response to treatment.
The second review, of 7 trials with a total of 1100 patients, compared bromocriptine plus levodopa with levodopa alone.4 The studies were of poor quality for reasons similar to the studies in the first review. Researchers found no statistically significant or consistent evidence to determine whether bromocriptine plus levodopa prevents or delays motor complications.
MAO-B inhibitors: Minimally effective with troubling side effects
A Cochrane review of monoamine oxidase type B (MAO-B) inhibitors included 10 trials with 2422 participants.5 The review found statistically, but not clinically, significant improvements in scores on 2 sections of the United Parkinson Disease Rating Scale (UPDRS), a standardized assessment tool that facilitates accurate documentation of disease progression and treatment response.
Compared with the control groups (either placebo or levodopa at study onset), the MAO-B group (either alone or with levodopa) showed significant improvement on the motor section (weighted mean difference [WMD]=–3.81 on a 108-point scale; 95% CI, –5.36 to –2.27) and activities of daily living section (WMD=–1.50 on a 52-point scale; 95% CI, –2.53 to –0.48). Fewer motor complications occurred in the MAO-B group (OR=0.75; 95% CI, 0.59-0.94) than the control group. Lower doses and shorter treatment with levodopa were necessary to control symptoms in the MAO-B group.
The clinical impact of MAO-B inhibitors on Parkinson’s symptoms was small, and almost all patients required the addition of levodopa to the treatment regimen after 3 or 4 years. Withdrawals because of medication side effects were significantly higher in the MAO-B inhibitor group than controls (OR=2.36; 95% CI, 1.32-4.20). Side effects included nausea, confusion, hallucinations, and postural hypotension. Concerns about cardiovascular adverse effects raised in previous studies, especially with selegiline, weren’t found to be significant (OR=1.15; 95% CI, 0.92-1.44). Because of their minimal effectiveness and worrisome adverse effects, MAO-B inhibitors aren’t recommended for routine use in early Parkinson’s disease.
COMT inhibitors may boost levodopa/carbidopa’s effects
A randomized double-blinded trial followed 423 patients for 39 weeks to compare the combination of the catechol-O-methyltransferase (COMT) inhibitor entacapone and levodopa/carbidopa (LCE) with levodopa/carbidopa alone (LC).6 The researchers found statistically significant improvements with LCE in UPDRS scores for activities of daily living (mean change from baseline=3.0 for LCE vs 2.3 for LC on a 52-point scale; P=.025) but not mentation or motor symptoms.
Dyskinesia and wearing-off symptoms (motor fluctuations) didn’t differ significantly between the 2 groups. LCE was associated with a higher incidence of adverse effects than LC, and involved mostly nausea (26.6% vs 13.5%) and diarrhea (8.7% vs 2.8%).
Anticholinergics may help, but cause adverse mental effects
Another Cochrane review compared anticholinergic agents with placebo or no treatment in 9 studies that included 221 patients.7 Meta-analysis wasn’t possible because of heterogeneity in patient populations, outcomes, and measurements and incomplete reporting. Compared with placebo, anticholinergic agents may improve Parkinson’s-related motor symptoms but have significant mental adverse effects, including confusion, memory problems, restlessness, and hallucinations.
Recommendations
The most recent guidelines (2002) from the American Academy of Neurology recommend levodopa and dopamine agonists as first-line therapies.8 Levodopa is more effective at improving the motor symptoms of Parkinson’s disease but is associated with a higher risk of dyskinesia than dopamine agonists. No compelling evidence suggests a difference in efficacy between long- and short-acting levodopa.
1. Hauser RA. Levodopa: past, present, and future. Eur Neurol. 2009;62:1-8.
2. Stowe RL, Ives NJ, Clarke C, et al. Dopamine agonist therapy in early Parkinson’s disease. Cochrane Database Syst Rev. 2008;(2):CD006564.-
3. van Hilten JJ, Ramaker CC, Stowe R, et al. Bromocriptine versus levodopa in early Parkinson’s disease. Cochrane Database Syst Rev. 2007;(4):CD002258.-
4. van Hilten JJ, Ramaker CC, Stowe R, et al. Bromocriptine/levodopa combined versus levodopa alone for early Parkinson’s disease. Cochrane Database Syst Rev. 2007;(4):CD003634.-
5. Macleod AD, Counsell CE, Ives N, et al. Monoamine oxidase B inhibitors for early Parkinson’s disease. Cochrane Database Syst Rev. 2005;(3):CD004898.-
6. Hauser RA, Panisset M, Abbruzzese G, et al. Double-blind trial of levodopa/carbidopa/entacapone versus levodopa/ carbidopa in early Parkinson’s disease. Mov Disord. 2009;24:541-550.
7. Katzenschlager R, Sampaio C, Costa J, et al. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst Rev. 2003;(2):CD003735.-
8. Miyasaki JM, Martin W, Suchowersky O, et al. Practice parameter: initiation of treatment for Parkinson’s disease: an evidence-based review: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2002;58:11-17.
9. Drugs for Parkinson’s disease Treat Guidl Med Lett. 2011;9:1-6
10. Drugstore.com Online Pharmacy. Pharmacy drug costs. Available at http://www.drugstore.com. Accessed August 30, 2011.
1. Hauser RA. Levodopa: past, present, and future. Eur Neurol. 2009;62:1-8.
2. Stowe RL, Ives NJ, Clarke C, et al. Dopamine agonist therapy in early Parkinson’s disease. Cochrane Database Syst Rev. 2008;(2):CD006564.-
3. van Hilten JJ, Ramaker CC, Stowe R, et al. Bromocriptine versus levodopa in early Parkinson’s disease. Cochrane Database Syst Rev. 2007;(4):CD002258.-
4. van Hilten JJ, Ramaker CC, Stowe R, et al. Bromocriptine/levodopa combined versus levodopa alone for early Parkinson’s disease. Cochrane Database Syst Rev. 2007;(4):CD003634.-
5. Macleod AD, Counsell CE, Ives N, et al. Monoamine oxidase B inhibitors for early Parkinson’s disease. Cochrane Database Syst Rev. 2005;(3):CD004898.-
6. Hauser RA, Panisset M, Abbruzzese G, et al. Double-blind trial of levodopa/carbidopa/entacapone versus levodopa/ carbidopa in early Parkinson’s disease. Mov Disord. 2009;24:541-550.
7. Katzenschlager R, Sampaio C, Costa J, et al. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst Rev. 2003;(2):CD003735.-
8. Miyasaki JM, Martin W, Suchowersky O, et al. Practice parameter: initiation of treatment for Parkinson’s disease: an evidence-based review: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2002;58:11-17.
9. Drugs for Parkinson’s disease Treat Guidl Med Lett. 2011;9:1-6
10. Drugstore.com Online Pharmacy. Pharmacy drug costs. Available at http://www.drugstore.com. Accessed August 30, 2011.
Evidence-based answers from the Family Physicians Inquiries Network
Combatting the cough that won’t quit
• Always include postnasal drip, asthma, and gastroesophageal reflux disease in the differential diagnosis for persistent cough, regardless of clinical signs and symptoms. B
• Do not rely on a patient’s description of the character and timing of the cough or the absence (or presence) of sputum to narrow down the differential diagnosis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Margaret M, a 52-year-old nonsmoker, came to our clinic because of a persistent cough that had started about 4 weeks earlier. She had tried multiple over-the-counter cough suppressants, including dextromethorphan and guaifenesin, as well as cough drops, but none had been effective.
Margaret denied having had a cold or respiratory infection in the past few months or being in close contact with anyone with a chronic cough, and she had never had an asthma diagnosis. In response to a question about previous coughing episodes, the patient recalled having had several bouts of chronic cough in the past, including one about a year ago.
While Margaret had no known allergies, she did have occasional heartburn, which an antacid—or, at times, a drink of water—always relieved. Thyroid medication and calcium were the only things she took on a regular basis, separated by several hours to avoid problems with absorption.
Patients like Margaret, who seek help from their primary care physician only after attempting to combat a persistent cough on their own, may be quite frustrated by the time they arrive in your office. They’re counting on you to provide a cure. Fortunately, you’re likely to find it, as the differential diagnosis for subacute cough (a cough of 3-8 weeks’ duration) is limited.
Nonetheless, finding the cause of a subacute or chronic cough (lasting >8 weeks) is sometimes a matter of trial and error. Postnasal drip (also known as upper airway cough syndrome, or UACS), asthma, and gastroesophageal reflux disease (GERD) are the most common causes,1,2 followed by postinfectious cough, nonasthmatic eosinophilic bronchitis (NAEB), and pertussis.3 Although these conditions are all relatively well known, they are not always easy to detect: Some disorders, including UACS, asthma, and GERD, may be “silent,” with persistent cough the only presenting sign or symptom.4 In other cases, more than one condition may be contributing to the cough.

Starting with trials of empiric therapy for the most common causes of persistent cough—with sequential therapy and diagnostic tests, as needed—is far more effective than searching for relatively uncommon or obscure conditions. Following such a protocol, as detailed in the algorithm (FIGURE)4-7 we’ve developed and in the text that follows, can help you combat subacute and chronic cough in a cost-effective, timely way.
FIGURE
Dx and treatment when persistent cough is the only symptom4-7
*May include CXR, PPD, B pertussis IgG or IgA, spirometry with methacholine inhalation challenge, barium swallow, prolonged pH monitoring, sinus CT, and sputum eosinophil count, excluding any tests that have already been performed.
ACEI, angiotensin-converting enzyme inhibitor; CT, computed tomography; COPD, chronic obstructive pulmonary disease; CXR, chest x-ray; GERD, gastroesophageal reflux disease; IgA, immunoglobulin A; IgG, immunoglobulin G; PND, postnasal drip; PPD, purified protein derivative; PPI, proton pump inhibitor.
Treat all patients for upper airway cough syndrome
Postnasal drip—renamed UACS by the guideline committee of the American Association of Chest Physicians because it isn’t clear whether the cough is caused by irritation from direct contact with postnasal drip or by inflammation of cough receptors in the upper airway—is the most common cause of chronic cough.6
The differential diagnosis for UACS, which is implicated in about 34% of cases of persistent cough, includes allergic, postinfectious, and occupational rhinitis; rhinitis due to anatomic abnormalities or physical or chemical irritants, rhinitis medicamentosa, and rhinitis of pregnancy; bacterial sinusitis; and allergic fungal sinusitis.8
The signs and symptoms of UACS are nonspecific, and a definitive diagnosis typically cannot be made from the medical history and physical examination alone. What’s more, the absence of any of the usual clinical findings—eg, rhinorrhea and excess sputum production—should not preclude an empiric trial with a first-generation antihistamine-decongestant combination such as brompheniramine/sustained-release pseudoephedrine. Second-and third-generation combination products, such as fexofenadine/pseudoephedrine, should not be used, as they are not effective in treating UACS.4
CASE Margaret’s physical exam was unremarkable. Her vital signs were stable, she had no cervical lymphadenopathy, and her chest was clear on auscultation. She had a dry cough that occurred twice during the exam, but not on inspiration.
The patient’s work-up included office spirometry, which was normal; a nasopharyngeal culture for Bordetella pertussis was negative. We prescribed a 2-week course of therapy with brompheniramine/sustained-release pseudoephedrine and scheduled a return visit shortly after it was completed.
There is no gold standard diagnostic test to confirm or rule out postnasal drip as the cause of cough. CT scanning of sinuses has a poor positive predictive value and is no longer recommended as part of an initial work-up,9 but may be useful for patients whose symptoms persist longer than 3 weeks.
Consider bronchodilator Tx when asthma is suspected
Cough-variant asthma is the second most common cause of persistent cough, and is responsible for an estimated 28% of cases.6 Asthma is the easiest of the conditions included in the differential diagnosis for persistent cough to establish in an office setting. The challenge is to remember to consider it in patients who present with cough but no sign of the classic expiratory wheezing. When you suspect that a patient has asthma, consider empiric bronchodilator therapy—or conduct spirometry testing.
Spirometric values of forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) <70% and a positive bronchodilator response (≥12%) are consistent with an asthma diagnosis. Management of asthma depends on severity, and patients should be evaluated based on the National Heart, Lung, and Blood Institute’s National Asthma Education and Prevention Program Guidelines for the Diagnosis and Management of Asthma.10
It is crucial to ask patients with asthma (and, indeed, to ask all patients with a persistent cough) about exposure to secondhand smoke, and to stress the importance of avoiding smoking and secondary exposure. Individuals who are regularly exposed to secondhand smoke report more nasal symptoms and greater use of nasal decongestants compared with people with no exposure to smoke;11 they also have poor control of asthma.12-14
Cough unresolved? Add therapy for GERD
Although GERD is primarily associated with heartburn and gastrointestinal distress, it is not unusual for cough to be its only sign or symptom.15 In fact, GERD is the third most common cause of subacute cough—affecting about 21% of patients who seek help for cough at primary care practices.3
CASE Margaret returned to the clinic shortly after completion of a 2-week course of brompheniramine/sustained-release pseudoephedrine, and reported that she was still coughing frequently—and that the medication had brought little improvement. Because of her history of heartburn, we added a 2-week trial with a proton pump inhibitor (PPI)—omeprazole 20 mg/d.
While there are diagnostic tests for GERD, including a pH probe of the esophagus, a barium esophagogram, and manometry testing, empiric therapy with a PPI—starting with a trial of at least 2 weeks—often eliminates the troublesome cough.16 If the patient responds to treatment, the medication can be continued. Risks associated with long-term PPI therapy include osteoporosis and interference with calcium and magnesium absorption,17 so it is important to monitor patients taking them and to discontinue treatment as soon as the cough symptoms resolve.
Have you ruled out postinfectious cough?
If a patient has a cough that has lingered for 3 to 8 weeks after his or her recovery from an acute upper respiratory infection (URI), postinfectious cough may be the reason.18,19 Such a cough is subacute and self-limiting. (If the cough lasts >8 weeks after an acute illness, other diagnoses, such as chronic infection, are more likely.)
The pathogenesis for postinfectious cough may be related to postviral airway inflammation or bronchial hyperresponsiveness, and antibiotics are not indicated.4 Patients may be treated with a bronchodilator such as ipratropium rather than a beta-agonist or inhaled corticosteroids; oral tapered prednisone can be prescribed, if needed, for severe paroxysms, although there is limited evidence of its efficacy.20 Central antitussive agents such as codeine and dextromethorphan can be used when other measures fail to bring relief.
Nonasthmatic eosinophilic bronchitis does not impede airflow
NAEB is less well known than the conditions discussed thus far, but it is a relatively common cause of persistent cough.21-23 In some studies, up to 13% of patients with subacute cough were diagnosed with NAEB.6
Unlike asthma, NAEB is not associated with abnormalities in airway function; patients have no dyspnea and no wheezing, and no obstruction of airflow.24 Patients will have FEV1 >80% and FEV1/FVC >75% on spirometric examination, a negative response to bronchoprovocation, and, typically, an elevated sputum eosinophil count of >3%. Because induced sputum or bronchoscopic washings are difficult, exhaled nitric oxide testing is another option. If these tests are not available, a trial of inhaled steroids is indicated, even if neither spirometry nor bronchoprovocation testing was abnormal.9
Patients with NAEB respond well to inhaled corticosteroids, and budesonide 400 mcg twice a day or prednisolone 30 mg daily may be prescribed. It is also important to remove airway irritants. Long-term follow-up studies of patients with NAEB have had conflicting results. One study found that most cases resolve completely;23 another showed a need for long-term treatment, and suggested that patients with NAEB may be at increased risk for asthma and chronic obstructive pulmonary disease. 25
Paroxysmal cough, whoops point to pertussis
When a patient has paroxysms of cough, posttussive vomiting, and/or an inspiratory whooping sound, B pertussis infection is the likely culprit.26-28 A definitive diagnosis of pertussis, or whooping cough, may be based on a positive culture from a nasopharyngeal aspirate swab.29 Suspected cases can be confirmed with a polymerase chain reaction test, and a presumptive diagnosis may be made as a result of a 4-fold increase in immunoglobulin G or immunoglobulin A antibodies for B pertussis.4
A macrolide antibiotic, usually azithromycin, is the standard treatment for pertussis.30-32 Patients should be isolated for 5 days from the start of treatment. Antibiotic therapy will reduce the risk of transmission, but will not affect the duration of the cough, which may be 6 to 8 weeks. Long-acting beta-agonists, antihistamines, and corticosteroids should not be used to treat pertussis.4
CASE After a 2-week course of omeprazole 20 mg daily, Margaret was coughing much less. We extended the prescription, and by the end of the next 4 weeks, she was no longer coughing. After 2 months, both the PPI and the antihistamine/decongestant were discontinued. We advised her to institute antireflux measures, such as elevating her head at night and not eating after 6 pm, and she has not had a relapse.
CORRESPONDENCE Rebecca H. Gladu, MD, FAAFP, San Jacinto Methodist Hospital, 4401 Garth Road, Baytown, TX 77521; [email protected]
1. Corrao WM. Chronic persistent cough: diagnosis and treatment update. Pediatr Ann. 1996;25:162-168.
2. Holmes RL, Fadden CT. Evaluation of the patient with chronic cough. Am Fam Physician. 2004;69:2159-2166.
3. Irwin RS, Curley FJ, French CL. Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Am Rev Respir Dis. 1990;141:640-647.
4. Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough. Executive summary: ACCP evidence-based practice guideline. Chest. 2006;129(1 suppl):1S-23S.
5. Pratter MR, Bartter T, Akers S, et al. An algorithmic approach to chronic cough. Ann Intern Med. 1993;119:977-983.
6. Pratter MR, Brightling CE, Boulet LP, et al. An empiric integrative approach to the management of cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129 (1 suppl):222S-231S.
7. Irwin RS, Madison JM. Anatomical diagnostic protocol in evaluating chronic cough with specific reference to gastroesophageal reflux disease. Am J Med. 2000;108(suppl 4a):126S-130S.
8. Irwin RS, Corrao WM, Pratter MR. Chronic persistent cough in the adult: the spectrum and frequency of causes and successful outcome of specific therapy. Am Rev Respir Dis. 1981;123 (4 Pt 1):413-417.
9. Birring SS. Controversies in the evaluation and management of chronic cough. Am J Respir Crit Care Med. 2011;183:708-715.
10. National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
11. Reh DD, Lin SY, Clipp SL, et al. Secondhand tobacco smoke exposure and chronic rhinosinusitis: a population-based case-control study. Am J Rhinol Allergy. 2009;23:562-567.
12. Stapleton M, Howard-Thompson A, George C, et al. Smoking and asthma. J Am Board Fam Med. 2011;24:313-322.
13. Hersoug LG, Husemoen LL, Sigsgaard T, et al. Indoor exposure to environmental cigarette smoke, but not other inhaled particulates associates with respiratory symptoms and diminished lung function in adults. Respirology. 2010;15:993-1000.
14. Self TH, Wallace JL, Gray LA, et al. Are we failing to document adequate smoking histories? A brief review 1999-2009. Curr Med Res Opin. 2010;26:1691-1696.
15. Sontag SJ. The spectrum of pulmonary symptoms due to gastroesophageal reflux. Thorac Surg Clin. 2005;15:353-368.
16. Irwin RS. Chronic cough due to gastroesophageal reflux. ACCP evidence-based clinical practice guidelines. Chest. 2006;129(suppl 1):80S-94S.
17. Chen J, Yuan YC, Leontiadis GI, et al. Recent safety concerns with proton pump inhibitors. J Clin Gastroenterol. 2012;46:93-114.
18. Braman SS. Postinfectious cough: ACCP evidence-based practice guidelines. Chest. 2006;129(suppl 1):138S-146S.
19. Pratter MR. Cough and the common cold: ACCP evidence-based practice guidelines. Chest. 2006;129(suppl 1):72S-74S.
20. Chang AB, McKean M, Morris P. Inhaled anticholinergics for prolonged non-specific cough in children. Cochrane Database Syst Rev. 2004;(1):CD004358.-
21. Brightling CE, Ward R, Goh KL, et al. Eosinophilic bronchitis is an important cause of chronic cough. Am J Respir Crit Care Med. 1999;160:406-410.
22. Gonlugur U, Gonlugur TE. Eosinophilic bronchitis without asthma. Int Arch Allergy Immunol. 2008;147:1-5.
23. Brightling CE. Cough due to asthma and nonasthmatic eosinophilic bronchitis. Lung. 2010;188 (suppl 1):S13-S17.
24. Gibson PG, Hargreave FE, Girgis-Gabardo, et al. Chronic cough with eosinophilic bronchitis: examination for variable airflow obstruction and response to corticosteroid. Clin Exp Allergy. 1995;25:127-132.
25. Berry MA, Hargadon B, McKenna S, et al. Observational study of the natural history of eosinophilic bronchitis. Clin Exp Allergy. 2005;35:598-601.
26. Antico A, Fabozzi F, Scipiotti C. Pertussis in adults. A study in an Italian population with chronic cough. Monaldi Arch Chest Dis. 2002;57:247-252.
27. Birkebaek NH, Kristiansen M, Seefeldt T, et al. Bordetella pertussis and chronic cough in adults. Clin Infect Dis. 1999;29:1239-1242.
28. Kapaskelis AM, Vouloumanou EK, Rafailidis PI, et al. High prevalence of antibody titers against Bordetella pertussis in an adult population with prolonged cough. Respir Med. 2008;102:1586-1591.
29. Cornia PB, Hersh AL, Lipsky BA, et al. Does this coughing adolescent or adult patient have pertussis? JAMA. 2010;304:890-896.
30. Devasia RA, Jones TF, Collier B, et al. Compliance with azithromycin versus erythromycin in the setting of a pertussis outbreak. Am J Med Sci. 2009;337:176-178.
31. Poe RH, Harder RV, Israel RH, et al. Chronic persistent cough. Experience in diagnosis and outcome using an anatomic diagnostic protocol. Chest. 1989;95:723-728.
32. Altunaji SM, Kukuruzovic RH, Curtis NC, et al. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev. 2007;(3):CD004404.-
• Always include postnasal drip, asthma, and gastroesophageal reflux disease in the differential diagnosis for persistent cough, regardless of clinical signs and symptoms. B
• Do not rely on a patient’s description of the character and timing of the cough or the absence (or presence) of sputum to narrow down the differential diagnosis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Margaret M, a 52-year-old nonsmoker, came to our clinic because of a persistent cough that had started about 4 weeks earlier. She had tried multiple over-the-counter cough suppressants, including dextromethorphan and guaifenesin, as well as cough drops, but none had been effective.
Margaret denied having had a cold or respiratory infection in the past few months or being in close contact with anyone with a chronic cough, and she had never had an asthma diagnosis. In response to a question about previous coughing episodes, the patient recalled having had several bouts of chronic cough in the past, including one about a year ago.
While Margaret had no known allergies, she did have occasional heartburn, which an antacid—or, at times, a drink of water—always relieved. Thyroid medication and calcium were the only things she took on a regular basis, separated by several hours to avoid problems with absorption.
Patients like Margaret, who seek help from their primary care physician only after attempting to combat a persistent cough on their own, may be quite frustrated by the time they arrive in your office. They’re counting on you to provide a cure. Fortunately, you’re likely to find it, as the differential diagnosis for subacute cough (a cough of 3-8 weeks’ duration) is limited.
Nonetheless, finding the cause of a subacute or chronic cough (lasting >8 weeks) is sometimes a matter of trial and error. Postnasal drip (also known as upper airway cough syndrome, or UACS), asthma, and gastroesophageal reflux disease (GERD) are the most common causes,1,2 followed by postinfectious cough, nonasthmatic eosinophilic bronchitis (NAEB), and pertussis.3 Although these conditions are all relatively well known, they are not always easy to detect: Some disorders, including UACS, asthma, and GERD, may be “silent,” with persistent cough the only presenting sign or symptom.4 In other cases, more than one condition may be contributing to the cough.

Starting with trials of empiric therapy for the most common causes of persistent cough—with sequential therapy and diagnostic tests, as needed—is far more effective than searching for relatively uncommon or obscure conditions. Following such a protocol, as detailed in the algorithm (FIGURE)4-7 we’ve developed and in the text that follows, can help you combat subacute and chronic cough in a cost-effective, timely way.
FIGURE
Dx and treatment when persistent cough is the only symptom4-7
*May include CXR, PPD, B pertussis IgG or IgA, spirometry with methacholine inhalation challenge, barium swallow, prolonged pH monitoring, sinus CT, and sputum eosinophil count, excluding any tests that have already been performed.
ACEI, angiotensin-converting enzyme inhibitor; CT, computed tomography; COPD, chronic obstructive pulmonary disease; CXR, chest x-ray; GERD, gastroesophageal reflux disease; IgA, immunoglobulin A; IgG, immunoglobulin G; PND, postnasal drip; PPD, purified protein derivative; PPI, proton pump inhibitor.
Treat all patients for upper airway cough syndrome
Postnasal drip—renamed UACS by the guideline committee of the American Association of Chest Physicians because it isn’t clear whether the cough is caused by irritation from direct contact with postnasal drip or by inflammation of cough receptors in the upper airway—is the most common cause of chronic cough.6
The differential diagnosis for UACS, which is implicated in about 34% of cases of persistent cough, includes allergic, postinfectious, and occupational rhinitis; rhinitis due to anatomic abnormalities or physical or chemical irritants, rhinitis medicamentosa, and rhinitis of pregnancy; bacterial sinusitis; and allergic fungal sinusitis.8
The signs and symptoms of UACS are nonspecific, and a definitive diagnosis typically cannot be made from the medical history and physical examination alone. What’s more, the absence of any of the usual clinical findings—eg, rhinorrhea and excess sputum production—should not preclude an empiric trial with a first-generation antihistamine-decongestant combination such as brompheniramine/sustained-release pseudoephedrine. Second-and third-generation combination products, such as fexofenadine/pseudoephedrine, should not be used, as they are not effective in treating UACS.4
CASE Margaret’s physical exam was unremarkable. Her vital signs were stable, she had no cervical lymphadenopathy, and her chest was clear on auscultation. She had a dry cough that occurred twice during the exam, but not on inspiration.
The patient’s work-up included office spirometry, which was normal; a nasopharyngeal culture for Bordetella pertussis was negative. We prescribed a 2-week course of therapy with brompheniramine/sustained-release pseudoephedrine and scheduled a return visit shortly after it was completed.
There is no gold standard diagnostic test to confirm or rule out postnasal drip as the cause of cough. CT scanning of sinuses has a poor positive predictive value and is no longer recommended as part of an initial work-up,9 but may be useful for patients whose symptoms persist longer than 3 weeks.
Consider bronchodilator Tx when asthma is suspected
Cough-variant asthma is the second most common cause of persistent cough, and is responsible for an estimated 28% of cases.6 Asthma is the easiest of the conditions included in the differential diagnosis for persistent cough to establish in an office setting. The challenge is to remember to consider it in patients who present with cough but no sign of the classic expiratory wheezing. When you suspect that a patient has asthma, consider empiric bronchodilator therapy—or conduct spirometry testing.
Spirometric values of forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) <70% and a positive bronchodilator response (≥12%) are consistent with an asthma diagnosis. Management of asthma depends on severity, and patients should be evaluated based on the National Heart, Lung, and Blood Institute’s National Asthma Education and Prevention Program Guidelines for the Diagnosis and Management of Asthma.10
It is crucial to ask patients with asthma (and, indeed, to ask all patients with a persistent cough) about exposure to secondhand smoke, and to stress the importance of avoiding smoking and secondary exposure. Individuals who are regularly exposed to secondhand smoke report more nasal symptoms and greater use of nasal decongestants compared with people with no exposure to smoke;11 they also have poor control of asthma.12-14
Cough unresolved? Add therapy for GERD
Although GERD is primarily associated with heartburn and gastrointestinal distress, it is not unusual for cough to be its only sign or symptom.15 In fact, GERD is the third most common cause of subacute cough—affecting about 21% of patients who seek help for cough at primary care practices.3
CASE Margaret returned to the clinic shortly after completion of a 2-week course of brompheniramine/sustained-release pseudoephedrine, and reported that she was still coughing frequently—and that the medication had brought little improvement. Because of her history of heartburn, we added a 2-week trial with a proton pump inhibitor (PPI)—omeprazole 20 mg/d.
While there are diagnostic tests for GERD, including a pH probe of the esophagus, a barium esophagogram, and manometry testing, empiric therapy with a PPI—starting with a trial of at least 2 weeks—often eliminates the troublesome cough.16 If the patient responds to treatment, the medication can be continued. Risks associated with long-term PPI therapy include osteoporosis and interference with calcium and magnesium absorption,17 so it is important to monitor patients taking them and to discontinue treatment as soon as the cough symptoms resolve.
Have you ruled out postinfectious cough?
If a patient has a cough that has lingered for 3 to 8 weeks after his or her recovery from an acute upper respiratory infection (URI), postinfectious cough may be the reason.18,19 Such a cough is subacute and self-limiting. (If the cough lasts >8 weeks after an acute illness, other diagnoses, such as chronic infection, are more likely.)
The pathogenesis for postinfectious cough may be related to postviral airway inflammation or bronchial hyperresponsiveness, and antibiotics are not indicated.4 Patients may be treated with a bronchodilator such as ipratropium rather than a beta-agonist or inhaled corticosteroids; oral tapered prednisone can be prescribed, if needed, for severe paroxysms, although there is limited evidence of its efficacy.20 Central antitussive agents such as codeine and dextromethorphan can be used when other measures fail to bring relief.
Nonasthmatic eosinophilic bronchitis does not impede airflow
NAEB is less well known than the conditions discussed thus far, but it is a relatively common cause of persistent cough.21-23 In some studies, up to 13% of patients with subacute cough were diagnosed with NAEB.6
Unlike asthma, NAEB is not associated with abnormalities in airway function; patients have no dyspnea and no wheezing, and no obstruction of airflow.24 Patients will have FEV1 >80% and FEV1/FVC >75% on spirometric examination, a negative response to bronchoprovocation, and, typically, an elevated sputum eosinophil count of >3%. Because induced sputum or bronchoscopic washings are difficult, exhaled nitric oxide testing is another option. If these tests are not available, a trial of inhaled steroids is indicated, even if neither spirometry nor bronchoprovocation testing was abnormal.9
Patients with NAEB respond well to inhaled corticosteroids, and budesonide 400 mcg twice a day or prednisolone 30 mg daily may be prescribed. It is also important to remove airway irritants. Long-term follow-up studies of patients with NAEB have had conflicting results. One study found that most cases resolve completely;23 another showed a need for long-term treatment, and suggested that patients with NAEB may be at increased risk for asthma and chronic obstructive pulmonary disease. 25
Paroxysmal cough, whoops point to pertussis
When a patient has paroxysms of cough, posttussive vomiting, and/or an inspiratory whooping sound, B pertussis infection is the likely culprit.26-28 A definitive diagnosis of pertussis, or whooping cough, may be based on a positive culture from a nasopharyngeal aspirate swab.29 Suspected cases can be confirmed with a polymerase chain reaction test, and a presumptive diagnosis may be made as a result of a 4-fold increase in immunoglobulin G or immunoglobulin A antibodies for B pertussis.4
A macrolide antibiotic, usually azithromycin, is the standard treatment for pertussis.30-32 Patients should be isolated for 5 days from the start of treatment. Antibiotic therapy will reduce the risk of transmission, but will not affect the duration of the cough, which may be 6 to 8 weeks. Long-acting beta-agonists, antihistamines, and corticosteroids should not be used to treat pertussis.4
CASE After a 2-week course of omeprazole 20 mg daily, Margaret was coughing much less. We extended the prescription, and by the end of the next 4 weeks, she was no longer coughing. After 2 months, both the PPI and the antihistamine/decongestant were discontinued. We advised her to institute antireflux measures, such as elevating her head at night and not eating after 6 pm, and she has not had a relapse.
CORRESPONDENCE Rebecca H. Gladu, MD, FAAFP, San Jacinto Methodist Hospital, 4401 Garth Road, Baytown, TX 77521; [email protected]
• Always include postnasal drip, asthma, and gastroesophageal reflux disease in the differential diagnosis for persistent cough, regardless of clinical signs and symptoms. B
• Do not rely on a patient’s description of the character and timing of the cough or the absence (or presence) of sputum to narrow down the differential diagnosis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Margaret M, a 52-year-old nonsmoker, came to our clinic because of a persistent cough that had started about 4 weeks earlier. She had tried multiple over-the-counter cough suppressants, including dextromethorphan and guaifenesin, as well as cough drops, but none had been effective.
Margaret denied having had a cold or respiratory infection in the past few months or being in close contact with anyone with a chronic cough, and she had never had an asthma diagnosis. In response to a question about previous coughing episodes, the patient recalled having had several bouts of chronic cough in the past, including one about a year ago.
While Margaret had no known allergies, she did have occasional heartburn, which an antacid—or, at times, a drink of water—always relieved. Thyroid medication and calcium were the only things she took on a regular basis, separated by several hours to avoid problems with absorption.
Patients like Margaret, who seek help from their primary care physician only after attempting to combat a persistent cough on their own, may be quite frustrated by the time they arrive in your office. They’re counting on you to provide a cure. Fortunately, you’re likely to find it, as the differential diagnosis for subacute cough (a cough of 3-8 weeks’ duration) is limited.
Nonetheless, finding the cause of a subacute or chronic cough (lasting >8 weeks) is sometimes a matter of trial and error. Postnasal drip (also known as upper airway cough syndrome, or UACS), asthma, and gastroesophageal reflux disease (GERD) are the most common causes,1,2 followed by postinfectious cough, nonasthmatic eosinophilic bronchitis (NAEB), and pertussis.3 Although these conditions are all relatively well known, they are not always easy to detect: Some disorders, including UACS, asthma, and GERD, may be “silent,” with persistent cough the only presenting sign or symptom.4 In other cases, more than one condition may be contributing to the cough.

Starting with trials of empiric therapy for the most common causes of persistent cough—with sequential therapy and diagnostic tests, as needed—is far more effective than searching for relatively uncommon or obscure conditions. Following such a protocol, as detailed in the algorithm (FIGURE)4-7 we’ve developed and in the text that follows, can help you combat subacute and chronic cough in a cost-effective, timely way.
FIGURE
Dx and treatment when persistent cough is the only symptom4-7
*May include CXR, PPD, B pertussis IgG or IgA, spirometry with methacholine inhalation challenge, barium swallow, prolonged pH monitoring, sinus CT, and sputum eosinophil count, excluding any tests that have already been performed.
ACEI, angiotensin-converting enzyme inhibitor; CT, computed tomography; COPD, chronic obstructive pulmonary disease; CXR, chest x-ray; GERD, gastroesophageal reflux disease; IgA, immunoglobulin A; IgG, immunoglobulin G; PND, postnasal drip; PPD, purified protein derivative; PPI, proton pump inhibitor.
Treat all patients for upper airway cough syndrome
Postnasal drip—renamed UACS by the guideline committee of the American Association of Chest Physicians because it isn’t clear whether the cough is caused by irritation from direct contact with postnasal drip or by inflammation of cough receptors in the upper airway—is the most common cause of chronic cough.6
The differential diagnosis for UACS, which is implicated in about 34% of cases of persistent cough, includes allergic, postinfectious, and occupational rhinitis; rhinitis due to anatomic abnormalities or physical or chemical irritants, rhinitis medicamentosa, and rhinitis of pregnancy; bacterial sinusitis; and allergic fungal sinusitis.8
The signs and symptoms of UACS are nonspecific, and a definitive diagnosis typically cannot be made from the medical history and physical examination alone. What’s more, the absence of any of the usual clinical findings—eg, rhinorrhea and excess sputum production—should not preclude an empiric trial with a first-generation antihistamine-decongestant combination such as brompheniramine/sustained-release pseudoephedrine. Second-and third-generation combination products, such as fexofenadine/pseudoephedrine, should not be used, as they are not effective in treating UACS.4
CASE Margaret’s physical exam was unremarkable. Her vital signs were stable, she had no cervical lymphadenopathy, and her chest was clear on auscultation. She had a dry cough that occurred twice during the exam, but not on inspiration.
The patient’s work-up included office spirometry, which was normal; a nasopharyngeal culture for Bordetella pertussis was negative. We prescribed a 2-week course of therapy with brompheniramine/sustained-release pseudoephedrine and scheduled a return visit shortly after it was completed.
There is no gold standard diagnostic test to confirm or rule out postnasal drip as the cause of cough. CT scanning of sinuses has a poor positive predictive value and is no longer recommended as part of an initial work-up,9 but may be useful for patients whose symptoms persist longer than 3 weeks.
Consider bronchodilator Tx when asthma is suspected
Cough-variant asthma is the second most common cause of persistent cough, and is responsible for an estimated 28% of cases.6 Asthma is the easiest of the conditions included in the differential diagnosis for persistent cough to establish in an office setting. The challenge is to remember to consider it in patients who present with cough but no sign of the classic expiratory wheezing. When you suspect that a patient has asthma, consider empiric bronchodilator therapy—or conduct spirometry testing.
Spirometric values of forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) <70% and a positive bronchodilator response (≥12%) are consistent with an asthma diagnosis. Management of asthma depends on severity, and patients should be evaluated based on the National Heart, Lung, and Blood Institute’s National Asthma Education and Prevention Program Guidelines for the Diagnosis and Management of Asthma.10
It is crucial to ask patients with asthma (and, indeed, to ask all patients with a persistent cough) about exposure to secondhand smoke, and to stress the importance of avoiding smoking and secondary exposure. Individuals who are regularly exposed to secondhand smoke report more nasal symptoms and greater use of nasal decongestants compared with people with no exposure to smoke;11 they also have poor control of asthma.12-14
Cough unresolved? Add therapy for GERD
Although GERD is primarily associated with heartburn and gastrointestinal distress, it is not unusual for cough to be its only sign or symptom.15 In fact, GERD is the third most common cause of subacute cough—affecting about 21% of patients who seek help for cough at primary care practices.3
CASE Margaret returned to the clinic shortly after completion of a 2-week course of brompheniramine/sustained-release pseudoephedrine, and reported that she was still coughing frequently—and that the medication had brought little improvement. Because of her history of heartburn, we added a 2-week trial with a proton pump inhibitor (PPI)—omeprazole 20 mg/d.
While there are diagnostic tests for GERD, including a pH probe of the esophagus, a barium esophagogram, and manometry testing, empiric therapy with a PPI—starting with a trial of at least 2 weeks—often eliminates the troublesome cough.16 If the patient responds to treatment, the medication can be continued. Risks associated with long-term PPI therapy include osteoporosis and interference with calcium and magnesium absorption,17 so it is important to monitor patients taking them and to discontinue treatment as soon as the cough symptoms resolve.
Have you ruled out postinfectious cough?
If a patient has a cough that has lingered for 3 to 8 weeks after his or her recovery from an acute upper respiratory infection (URI), postinfectious cough may be the reason.18,19 Such a cough is subacute and self-limiting. (If the cough lasts >8 weeks after an acute illness, other diagnoses, such as chronic infection, are more likely.)
The pathogenesis for postinfectious cough may be related to postviral airway inflammation or bronchial hyperresponsiveness, and antibiotics are not indicated.4 Patients may be treated with a bronchodilator such as ipratropium rather than a beta-agonist or inhaled corticosteroids; oral tapered prednisone can be prescribed, if needed, for severe paroxysms, although there is limited evidence of its efficacy.20 Central antitussive agents such as codeine and dextromethorphan can be used when other measures fail to bring relief.
Nonasthmatic eosinophilic bronchitis does not impede airflow
NAEB is less well known than the conditions discussed thus far, but it is a relatively common cause of persistent cough.21-23 In some studies, up to 13% of patients with subacute cough were diagnosed with NAEB.6
Unlike asthma, NAEB is not associated with abnormalities in airway function; patients have no dyspnea and no wheezing, and no obstruction of airflow.24 Patients will have FEV1 >80% and FEV1/FVC >75% on spirometric examination, a negative response to bronchoprovocation, and, typically, an elevated sputum eosinophil count of >3%. Because induced sputum or bronchoscopic washings are difficult, exhaled nitric oxide testing is another option. If these tests are not available, a trial of inhaled steroids is indicated, even if neither spirometry nor bronchoprovocation testing was abnormal.9
Patients with NAEB respond well to inhaled corticosteroids, and budesonide 400 mcg twice a day or prednisolone 30 mg daily may be prescribed. It is also important to remove airway irritants. Long-term follow-up studies of patients with NAEB have had conflicting results. One study found that most cases resolve completely;23 another showed a need for long-term treatment, and suggested that patients with NAEB may be at increased risk for asthma and chronic obstructive pulmonary disease. 25
Paroxysmal cough, whoops point to pertussis
When a patient has paroxysms of cough, posttussive vomiting, and/or an inspiratory whooping sound, B pertussis infection is the likely culprit.26-28 A definitive diagnosis of pertussis, or whooping cough, may be based on a positive culture from a nasopharyngeal aspirate swab.29 Suspected cases can be confirmed with a polymerase chain reaction test, and a presumptive diagnosis may be made as a result of a 4-fold increase in immunoglobulin G or immunoglobulin A antibodies for B pertussis.4
A macrolide antibiotic, usually azithromycin, is the standard treatment for pertussis.30-32 Patients should be isolated for 5 days from the start of treatment. Antibiotic therapy will reduce the risk of transmission, but will not affect the duration of the cough, which may be 6 to 8 weeks. Long-acting beta-agonists, antihistamines, and corticosteroids should not be used to treat pertussis.4
CASE After a 2-week course of omeprazole 20 mg daily, Margaret was coughing much less. We extended the prescription, and by the end of the next 4 weeks, she was no longer coughing. After 2 months, both the PPI and the antihistamine/decongestant were discontinued. We advised her to institute antireflux measures, such as elevating her head at night and not eating after 6 pm, and she has not had a relapse.
CORRESPONDENCE Rebecca H. Gladu, MD, FAAFP, San Jacinto Methodist Hospital, 4401 Garth Road, Baytown, TX 77521; [email protected]
1. Corrao WM. Chronic persistent cough: diagnosis and treatment update. Pediatr Ann. 1996;25:162-168.
2. Holmes RL, Fadden CT. Evaluation of the patient with chronic cough. Am Fam Physician. 2004;69:2159-2166.
3. Irwin RS, Curley FJ, French CL. Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Am Rev Respir Dis. 1990;141:640-647.
4. Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough. Executive summary: ACCP evidence-based practice guideline. Chest. 2006;129(1 suppl):1S-23S.
5. Pratter MR, Bartter T, Akers S, et al. An algorithmic approach to chronic cough. Ann Intern Med. 1993;119:977-983.
6. Pratter MR, Brightling CE, Boulet LP, et al. An empiric integrative approach to the management of cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129 (1 suppl):222S-231S.
7. Irwin RS, Madison JM. Anatomical diagnostic protocol in evaluating chronic cough with specific reference to gastroesophageal reflux disease. Am J Med. 2000;108(suppl 4a):126S-130S.
8. Irwin RS, Corrao WM, Pratter MR. Chronic persistent cough in the adult: the spectrum and frequency of causes and successful outcome of specific therapy. Am Rev Respir Dis. 1981;123 (4 Pt 1):413-417.
9. Birring SS. Controversies in the evaluation and management of chronic cough. Am J Respir Crit Care Med. 2011;183:708-715.
10. National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
11. Reh DD, Lin SY, Clipp SL, et al. Secondhand tobacco smoke exposure and chronic rhinosinusitis: a population-based case-control study. Am J Rhinol Allergy. 2009;23:562-567.
12. Stapleton M, Howard-Thompson A, George C, et al. Smoking and asthma. J Am Board Fam Med. 2011;24:313-322.
13. Hersoug LG, Husemoen LL, Sigsgaard T, et al. Indoor exposure to environmental cigarette smoke, but not other inhaled particulates associates with respiratory symptoms and diminished lung function in adults. Respirology. 2010;15:993-1000.
14. Self TH, Wallace JL, Gray LA, et al. Are we failing to document adequate smoking histories? A brief review 1999-2009. Curr Med Res Opin. 2010;26:1691-1696.
15. Sontag SJ. The spectrum of pulmonary symptoms due to gastroesophageal reflux. Thorac Surg Clin. 2005;15:353-368.
16. Irwin RS. Chronic cough due to gastroesophageal reflux. ACCP evidence-based clinical practice guidelines. Chest. 2006;129(suppl 1):80S-94S.
17. Chen J, Yuan YC, Leontiadis GI, et al. Recent safety concerns with proton pump inhibitors. J Clin Gastroenterol. 2012;46:93-114.
18. Braman SS. Postinfectious cough: ACCP evidence-based practice guidelines. Chest. 2006;129(suppl 1):138S-146S.
19. Pratter MR. Cough and the common cold: ACCP evidence-based practice guidelines. Chest. 2006;129(suppl 1):72S-74S.
20. Chang AB, McKean M, Morris P. Inhaled anticholinergics for prolonged non-specific cough in children. Cochrane Database Syst Rev. 2004;(1):CD004358.-
21. Brightling CE, Ward R, Goh KL, et al. Eosinophilic bronchitis is an important cause of chronic cough. Am J Respir Crit Care Med. 1999;160:406-410.
22. Gonlugur U, Gonlugur TE. Eosinophilic bronchitis without asthma. Int Arch Allergy Immunol. 2008;147:1-5.
23. Brightling CE. Cough due to asthma and nonasthmatic eosinophilic bronchitis. Lung. 2010;188 (suppl 1):S13-S17.
24. Gibson PG, Hargreave FE, Girgis-Gabardo, et al. Chronic cough with eosinophilic bronchitis: examination for variable airflow obstruction and response to corticosteroid. Clin Exp Allergy. 1995;25:127-132.
25. Berry MA, Hargadon B, McKenna S, et al. Observational study of the natural history of eosinophilic bronchitis. Clin Exp Allergy. 2005;35:598-601.
26. Antico A, Fabozzi F, Scipiotti C. Pertussis in adults. A study in an Italian population with chronic cough. Monaldi Arch Chest Dis. 2002;57:247-252.
27. Birkebaek NH, Kristiansen M, Seefeldt T, et al. Bordetella pertussis and chronic cough in adults. Clin Infect Dis. 1999;29:1239-1242.
28. Kapaskelis AM, Vouloumanou EK, Rafailidis PI, et al. High prevalence of antibody titers against Bordetella pertussis in an adult population with prolonged cough. Respir Med. 2008;102:1586-1591.
29. Cornia PB, Hersh AL, Lipsky BA, et al. Does this coughing adolescent or adult patient have pertussis? JAMA. 2010;304:890-896.
30. Devasia RA, Jones TF, Collier B, et al. Compliance with azithromycin versus erythromycin in the setting of a pertussis outbreak. Am J Med Sci. 2009;337:176-178.
31. Poe RH, Harder RV, Israel RH, et al. Chronic persistent cough. Experience in diagnosis and outcome using an anatomic diagnostic protocol. Chest. 1989;95:723-728.
32. Altunaji SM, Kukuruzovic RH, Curtis NC, et al. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev. 2007;(3):CD004404.-
1. Corrao WM. Chronic persistent cough: diagnosis and treatment update. Pediatr Ann. 1996;25:162-168.
2. Holmes RL, Fadden CT. Evaluation of the patient with chronic cough. Am Fam Physician. 2004;69:2159-2166.
3. Irwin RS, Curley FJ, French CL. Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Am Rev Respir Dis. 1990;141:640-647.
4. Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough. Executive summary: ACCP evidence-based practice guideline. Chest. 2006;129(1 suppl):1S-23S.
5. Pratter MR, Bartter T, Akers S, et al. An algorithmic approach to chronic cough. Ann Intern Med. 1993;119:977-983.
6. Pratter MR, Brightling CE, Boulet LP, et al. An empiric integrative approach to the management of cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129 (1 suppl):222S-231S.
7. Irwin RS, Madison JM. Anatomical diagnostic protocol in evaluating chronic cough with specific reference to gastroesophageal reflux disease. Am J Med. 2000;108(suppl 4a):126S-130S.
8. Irwin RS, Corrao WM, Pratter MR. Chronic persistent cough in the adult: the spectrum and frequency of causes and successful outcome of specific therapy. Am Rev Respir Dis. 1981;123 (4 Pt 1):413-417.
9. Birring SS. Controversies in the evaluation and management of chronic cough. Am J Respir Crit Care Med. 2011;183:708-715.
10. National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
11. Reh DD, Lin SY, Clipp SL, et al. Secondhand tobacco smoke exposure and chronic rhinosinusitis: a population-based case-control study. Am J Rhinol Allergy. 2009;23:562-567.
12. Stapleton M, Howard-Thompson A, George C, et al. Smoking and asthma. J Am Board Fam Med. 2011;24:313-322.
13. Hersoug LG, Husemoen LL, Sigsgaard T, et al. Indoor exposure to environmental cigarette smoke, but not other inhaled particulates associates with respiratory symptoms and diminished lung function in adults. Respirology. 2010;15:993-1000.
14. Self TH, Wallace JL, Gray LA, et al. Are we failing to document adequate smoking histories? A brief review 1999-2009. Curr Med Res Opin. 2010;26:1691-1696.
15. Sontag SJ. The spectrum of pulmonary symptoms due to gastroesophageal reflux. Thorac Surg Clin. 2005;15:353-368.
16. Irwin RS. Chronic cough due to gastroesophageal reflux. ACCP evidence-based clinical practice guidelines. Chest. 2006;129(suppl 1):80S-94S.
17. Chen J, Yuan YC, Leontiadis GI, et al. Recent safety concerns with proton pump inhibitors. J Clin Gastroenterol. 2012;46:93-114.
18. Braman SS. Postinfectious cough: ACCP evidence-based practice guidelines. Chest. 2006;129(suppl 1):138S-146S.
19. Pratter MR. Cough and the common cold: ACCP evidence-based practice guidelines. Chest. 2006;129(suppl 1):72S-74S.
20. Chang AB, McKean M, Morris P. Inhaled anticholinergics for prolonged non-specific cough in children. Cochrane Database Syst Rev. 2004;(1):CD004358.-
21. Brightling CE, Ward R, Goh KL, et al. Eosinophilic bronchitis is an important cause of chronic cough. Am J Respir Crit Care Med. 1999;160:406-410.
22. Gonlugur U, Gonlugur TE. Eosinophilic bronchitis without asthma. Int Arch Allergy Immunol. 2008;147:1-5.
23. Brightling CE. Cough due to asthma and nonasthmatic eosinophilic bronchitis. Lung. 2010;188 (suppl 1):S13-S17.
24. Gibson PG, Hargreave FE, Girgis-Gabardo, et al. Chronic cough with eosinophilic bronchitis: examination for variable airflow obstruction and response to corticosteroid. Clin Exp Allergy. 1995;25:127-132.
25. Berry MA, Hargadon B, McKenna S, et al. Observational study of the natural history of eosinophilic bronchitis. Clin Exp Allergy. 2005;35:598-601.
26. Antico A, Fabozzi F, Scipiotti C. Pertussis in adults. A study in an Italian population with chronic cough. Monaldi Arch Chest Dis. 2002;57:247-252.
27. Birkebaek NH, Kristiansen M, Seefeldt T, et al. Bordetella pertussis and chronic cough in adults. Clin Infect Dis. 1999;29:1239-1242.
28. Kapaskelis AM, Vouloumanou EK, Rafailidis PI, et al. High prevalence of antibody titers against Bordetella pertussis in an adult population with prolonged cough. Respir Med. 2008;102:1586-1591.
29. Cornia PB, Hersh AL, Lipsky BA, et al. Does this coughing adolescent or adult patient have pertussis? JAMA. 2010;304:890-896.
30. Devasia RA, Jones TF, Collier B, et al. Compliance with azithromycin versus erythromycin in the setting of a pertussis outbreak. Am J Med Sci. 2009;337:176-178.
31. Poe RH, Harder RV, Israel RH, et al. Chronic persistent cough. Experience in diagnosis and outcome using an anatomic diagnostic protocol. Chest. 1989;95:723-728.
32. Altunaji SM, Kukuruzovic RH, Curtis NC, et al. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev. 2007;(3):CD004404.-
Step by step: Obliterating the vaginal canal to correct pelvic organ prolapse
- LeFort partial colpocleisis
- Colpectomy and colpocleisis
- Colpectomy and colpocleisis after two previously failed obliterative procedures
These videos were selected by Mickey Karram, MD, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS)
This article, with accompanying video footage, is presented with the support of the International Academy of Pelvic Surgery.
As women live longer, on average, pelvic floor disorders are, as a whole, becoming more prevalent and a greater health and social problem. Many women entering the eighth and ninth decades of life display symptomatic pelvic organ prolapse (POP)—often after an unsuccessful trial of a pessary or even surgery.
These elderly patients often have other concomitant medical issues and are not sexually active, making extensive surgery for them less than an ideal solution. Instead, surgical procedures that obliterate the vaginal canal can alleviate their symptoms of POP.
In this article, we provide a step-by-step description of:
- LeFort partial colpocleisis in a woman who still has her uterus in place
- partial or complete colpectomy and colpocleisis in a woman who has post-hysterectomy prolapse
- levator plication and perineorrhaphy, as essential concluding steps in these procedures.
LeFort partial colpocleisis
An obliterative procedure in the form of a LeFort partial colpocleisis is an option when a patient 1) has her uterus and 2) is no longer sexually active. Because the uterus is retained in this procedure, however, keep in mind that it will be difficult to evaluate any uterine bleeding or cervical pathology in the future. Endovaginal ultrasonography or an endometrial biopsy, and a Pap smear, must be done before LeFort surgery.
The ideal candidate for LeFort partial colpocleisis is a woman who has complete uterine prolapse, or procidentia (FIGURE 1), which is characterized by symmetric eversion of the anterior and posterior vaginal walls.

FIGURE 1 Pelvic organ prolapse, preoperatively
Top: Uterine procidentia. A patient who has this condition is an ideal candidate for LeFort partial colpocleisis. Bottom: Asymmetric anterior vaginal prolapse.
LeFort partial colpocleisis: Key step by key step
![]()
![]()
![]()
![]()
![]()
![]()
![]()
FIGURE 2 shows key steps in performing LeFort partial colpocleisis. See Video #1 at www.obgmanagement.com for demonstrations of how to perform LeFort partial colpocleisis.
FIGURE 2 Steps: LeFort partial colpocleisis
A. Denude the anterior vaginal epithelium. B. Plicate the neck of the bladder. C. Next, denude the posterior vaginal epithelium. D. Approximate most proximal surfaces. E. Place lateral sutures to allow for drainage canals. F. The uterus has been replaced and most of the distal incisions closed.
Total colpectomy and colpocleisis: Key step by key step
In a patient who has post-hysterectomy prolapse and is not interested in continued sexual function, total colpectomy and colpocleisis provide a highly minimally invasive, durable option to correct her prolapse.
If there is complete eversion of the vagina then, truly, total colpectomy and colpocleisis is the procedure of choice. If there is significant prolapse of only one segment of the pelvic floor, however—for example, the anterior vaginal wall (FIGURE 1)—then aggressive repair of this variant with a narrowing down of the genital hiatus accomplishes the same result without requiring complete removal of what appears to be fairly well supported vaginal mucosa.
Here are key steps for performing partial or complete colpectomy and colpocleisis.
![]()
![]()
Completely remove the vaginal epithelium (FIGURES 3A and 3B); your goal is to leave most of the muscularis of the vaginal wall on the prolapse.
Avoid the peritoneal cavity if at all possible; when the main portion of the prolapse is secondary to an enterocele and the vaginal epithelium is very thin, however, formal excision of the enterocele sac, with closing of the defect, may be required.
![]()
If at all possible, avoid the peritoneum and the wall of the viscera, whether bladder or bowel. Invert the apex of the soft tissue, using the tip of forceps, as each purse-string suture is tied.
There is a variation of this procedure: Perform a separate anterior and posterior colporrhaphy, with two purse-string sutures used to approximate the anterior and posterior segments, thus obliterating any dead space.
![]()
![]()
See Video #2 and Video #3 for a demonstration of how to perform a complete colpectomy and colpocleisis. FIGURE 3D shows the completed colpocleisis.
FIGURE 3 Steps: Total colpectomy and colpocleisis
Denude the anterior vaginal epithelium (A) and then the posterior epithelium (B). C. Place sequential purse-string sutures. D. The completed colpocleisis, in cross-section.
Distal levatoroplasty with high perineorrhaphy: Key step by key step
![]()
![]()
![]()
![]()
FIGURE 4 Steps: Distal levatoroplasty with high perineorrhaphy
A. Lateral dissection to the levator ani muscles. Inset: levator ani plicated with sequential sutures. B. Place three sutures to plicate the levator ani. C. Secure the plication sutures. Inset C, and D: Completed levatoroplasty.
Our experience
We are often asked questions about the procedures that we’ve just described, including patients’ satisfaction with the outcome, complications, and the risk that prolapse will recur. In the accompanying box, “Questions we’re asked (and answers we give) about obliterative surgery,” opposite, we give our responses to eight common inquiries.
about obliterative surgery
Q1 How satisfied are women with the outcome of these procedures—do many regret having their vaginal canal obliterated?
A Overall, studies indicate that 85% to 100% of patients are “satisfied” or “very satisfied” with the outcomes of obliterative procedures.1 There are rare reports of regret after colpocleisis over loss of coital ability; in one study of a series of procedures,2 5% of subjects expressed regret postoperatively.
Q2 Why is levatoroplasty and perineorrhaphy such an important part of both the LeFort partial colpocleisis and colpectomy and colpocleisis?
A The aim of both these procedures is to reduce prolapsed tissue. The true durability of repair comes from significantly decreasing the caliber of the genital hiatus, with the hope of closing off the bulk of the distal vaginal canal. This can really only be accomplished by utilizing an aggressive levatoroplasty and perineorrhaphy, described in the text.
Q3 How often do patients develop de novo stress incontinence or significant voiding dysfunction, or both, after an obliterative procedure?
A The risk of developing urinary incontinence after an obliterative procedure is difficult to ascertain. In general, patients who had retention or a high postvoid residual volume preoperatively have a good outcome in regard to correcting their voiding dysfunction. This is because, in most cases, the voiding dysfunction is directly related to the anatomic distortion created by the prolapse.
Q4 What is the rate of prolapse recurrence after these procedures, and how is a recurrence managed?
A Multiple studies have documented an excellent anatomic outcome after these procedures, with a prolapse recurrence rate of only 1% to 8%.3 Very little has been written about how to best manage recurrent prolapse after an obliterative procedure. Most surgeons would, most likely, recommend repeat colpocleisis or aggressive levatoroplasty and perineorrhaphy. (Note: The patient whose colpectomy and colpocleisis is shown in Video #3 failed two previous colpectomy and colpocleisis procedures.)
Q5 Can these procedures be performed under local anesthesia, with some intravenous sedation, or under regional anesthesia—thereby avoiding intubation?
A Yes. We have utilized IV sedation and bilateral block successfully to perform these procedures. (Note: Video #3 of LeFort partial colpocleisis shows the procedure performed under local anesthesia.)
Q6 What does the literature say about common complications after these procedures?
A Postoperative morbidity and mortality in the elderly surgical population is a considerable concern. Significant postoperative complications occur in approximately 5% of patients in modern series4—often attributed to the effects of age and to the frail condition of patients who are commonly selected for colpocleisis.
Specifically, approximately 5% of patients experience a postoperative cardiac, thromboembolic, pulmonary, or cerebrovascular event. Transfusion is the most commonly reported major complication related to the procedure itself. Other complications include:
- fever and its associated morbidity
- pneumonia
- ongoing vaginal bleeding
- pyelonephritis
- hematoma
- cystotomy
- ureteral occlusion.
Minor surgical complications occur at a rate of approximately 15%. Surgical mortality is about 1 in 400 cases.
Q7 Do you routinely undertake urodynamic study of patients who are scheduled to undergo an obliterative procedure?
A At minimum, a lower urinary tract evaluation should include a postvoid residual volume study and, we believe, some kind of a filling study and stress test, with reduction of the prolapse. Beyond that, we recommend that you conduct more detailed urodynamic tests on a patient-by-patient basis, when you think that the findings will add to the clinical picture.
Q8 Would you ever perform a vaginal hysterectomy and then proceed with a colpectomy and colpocleisis?
A The principal rationale for performing hysterectomy at the time of colpocleisis is to eliminate the risk of endometrial or cervical carcinoma. Hysterectomy also eliminates the risk of pyometra, a rare but serious complication that can occur when the lateral canals become obstructed after a LeFort procedure.
A recent study5 looked at 1) concomitant hysterectomy in conjunction with colpectomy and colpocleisis and 2) traditional LeFort partial colpocleisis. In this retrospective review, objective and subjective success rates were high, but patients who underwent hysterectomy had a statistically significantly greater decline in postoperative hematocrit and a significant increase in the need for transfusion, compared with patients who did not undergo hysterectomy (35% vs. 13%).
References
1. Fitzgerald MP, Richter HE, Bradley CS, et al. Pelvic support, pelvic symptoms and patient satisfaction after colpocleisis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(12):1603-1609.
2. Hullfish K, Bobbjerg B, Steers W. Colpocleisis for pelvic organ prolapse Patient Goals Quality of life and Satisfaction. Obstet Gynecol. 2007;110(2 Pt 1):341-345.
3. Fitzgerald MP, Brubaker L. Colpocleisis and urinary incontinence. Am J Obstet Gynecol. 2003;189(5):1241-1244.
4. von Pechmann WS, Muton M, Fyffe J, Hale DS. Total colpocleisis with high levator placation for the treatment of advanced organ prolapse. Am J Obstet Gynecol. 2003;189(1):121-126.
5. Kohli NE, Sze E, Karram M. Pyometra following LeFort colpocleisis. Int Urogyn J. 1996;7(5):264-266.
We want to hear from you! Tell us what you think.
- LeFort partial colpocleisis
- Colpectomy and colpocleisis
- Colpectomy and colpocleisis after two previously failed obliterative procedures
These videos were selected by Mickey Karram, MD, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS)
This article, with accompanying video footage, is presented with the support of the International Academy of Pelvic Surgery.
As women live longer, on average, pelvic floor disorders are, as a whole, becoming more prevalent and a greater health and social problem. Many women entering the eighth and ninth decades of life display symptomatic pelvic organ prolapse (POP)—often after an unsuccessful trial of a pessary or even surgery.
These elderly patients often have other concomitant medical issues and are not sexually active, making extensive surgery for them less than an ideal solution. Instead, surgical procedures that obliterate the vaginal canal can alleviate their symptoms of POP.
In this article, we provide a step-by-step description of:
- LeFort partial colpocleisis in a woman who still has her uterus in place
- partial or complete colpectomy and colpocleisis in a woman who has post-hysterectomy prolapse
- levator plication and perineorrhaphy, as essential concluding steps in these procedures.
LeFort partial colpocleisis
An obliterative procedure in the form of a LeFort partial colpocleisis is an option when a patient 1) has her uterus and 2) is no longer sexually active. Because the uterus is retained in this procedure, however, keep in mind that it will be difficult to evaluate any uterine bleeding or cervical pathology in the future. Endovaginal ultrasonography or an endometrial biopsy, and a Pap smear, must be done before LeFort surgery.
The ideal candidate for LeFort partial colpocleisis is a woman who has complete uterine prolapse, or procidentia (FIGURE 1), which is characterized by symmetric eversion of the anterior and posterior vaginal walls.

FIGURE 1 Pelvic organ prolapse, preoperatively
Top: Uterine procidentia. A patient who has this condition is an ideal candidate for LeFort partial colpocleisis. Bottom: Asymmetric anterior vaginal prolapse.
LeFort partial colpocleisis: Key step by key step
![]()
![]()
![]()
![]()
![]()
![]()
![]()
FIGURE 2 shows key steps in performing LeFort partial colpocleisis. See Video #1 at www.obgmanagement.com for demonstrations of how to perform LeFort partial colpocleisis.

FIGURE 2 Steps: LeFort partial colpocleisis
A. Denude the anterior vaginal epithelium. B. Plicate the neck of the bladder. C. Next, denude the posterior vaginal epithelium. D. Approximate most proximal surfaces. E. Place lateral sutures to allow for drainage canals. F. The uterus has been replaced and most of the distal incisions closed.
Total colpectomy and colpocleisis: Key step by key step
In a patient who has post-hysterectomy prolapse and is not interested in continued sexual function, total colpectomy and colpocleisis provide a highly minimally invasive, durable option to correct her prolapse.
If there is complete eversion of the vagina then, truly, total colpectomy and colpocleisis is the procedure of choice. If there is significant prolapse of only one segment of the pelvic floor, however—for example, the anterior vaginal wall (FIGURE 1)—then aggressive repair of this variant with a narrowing down of the genital hiatus accomplishes the same result without requiring complete removal of what appears to be fairly well supported vaginal mucosa.
Here are key steps for performing partial or complete colpectomy and colpocleisis.
![]()
![]()
Completely remove the vaginal epithelium (FIGURES 3A and 3B); your goal is to leave most of the muscularis of the vaginal wall on the prolapse.
Avoid the peritoneal cavity if at all possible; when the main portion of the prolapse is secondary to an enterocele and the vaginal epithelium is very thin, however, formal excision of the enterocele sac, with closing of the defect, may be required.
![]()
If at all possible, avoid the peritoneum and the wall of the viscera, whether bladder or bowel. Invert the apex of the soft tissue, using the tip of forceps, as each purse-string suture is tied.
There is a variation of this procedure: Perform a separate anterior and posterior colporrhaphy, with two purse-string sutures used to approximate the anterior and posterior segments, thus obliterating any dead space.
![]()
![]()
See Video #2 and Video #3 for a demonstration of how to perform a complete colpectomy and colpocleisis. FIGURE 3D shows the completed colpocleisis.

FIGURE 3 Steps: Total colpectomy and colpocleisis
Denude the anterior vaginal epithelium (A) and then the posterior epithelium (B). C. Place sequential purse-string sutures. D. The completed colpocleisis, in cross-section.
Distal levatoroplasty with high perineorrhaphy: Key step by key step
![]()
![]()
![]()
![]()

FIGURE 4 Steps: Distal levatoroplasty with high perineorrhaphy
A. Lateral dissection to the levator ani muscles. Inset: levator ani plicated with sequential sutures. B. Place three sutures to plicate the levator ani. C. Secure the plication sutures. Inset C, and D: Completed levatoroplasty.
Our experience
We are often asked questions about the procedures that we’ve just described, including patients’ satisfaction with the outcome, complications, and the risk that prolapse will recur. In the accompanying box, “Questions we’re asked (and answers we give) about obliterative surgery,” opposite, we give our responses to eight common inquiries.
about obliterative surgery
Q1 How satisfied are women with the outcome of these procedures—do many regret having their vaginal canal obliterated?
A Overall, studies indicate that 85% to 100% of patients are “satisfied” or “very satisfied” with the outcomes of obliterative procedures.1 There are rare reports of regret after colpocleisis over loss of coital ability; in one study of a series of procedures,2 5% of subjects expressed regret postoperatively.
Q2 Why is levatoroplasty and perineorrhaphy such an important part of both the LeFort partial colpocleisis and colpectomy and colpocleisis?
A The aim of both these procedures is to reduce prolapsed tissue. The true durability of repair comes from significantly decreasing the caliber of the genital hiatus, with the hope of closing off the bulk of the distal vaginal canal. This can really only be accomplished by utilizing an aggressive levatoroplasty and perineorrhaphy, described in the text.
Q3 How often do patients develop de novo stress incontinence or significant voiding dysfunction, or both, after an obliterative procedure?
A The risk of developing urinary incontinence after an obliterative procedure is difficult to ascertain. In general, patients who had retention or a high postvoid residual volume preoperatively have a good outcome in regard to correcting their voiding dysfunction. This is because, in most cases, the voiding dysfunction is directly related to the anatomic distortion created by the prolapse.
Q4 What is the rate of prolapse recurrence after these procedures, and how is a recurrence managed?
A Multiple studies have documented an excellent anatomic outcome after these procedures, with a prolapse recurrence rate of only 1% to 8%.3 Very little has been written about how to best manage recurrent prolapse after an obliterative procedure. Most surgeons would, most likely, recommend repeat colpocleisis or aggressive levatoroplasty and perineorrhaphy. (Note: The patient whose colpectomy and colpocleisis is shown in Video #3 failed two previous colpectomy and colpocleisis procedures.)
Q5 Can these procedures be performed under local anesthesia, with some intravenous sedation, or under regional anesthesia—thereby avoiding intubation?
A Yes. We have utilized IV sedation and bilateral block successfully to perform these procedures. (Note: Video #3 of LeFort partial colpocleisis shows the procedure performed under local anesthesia.)
Q6 What does the literature say about common complications after these procedures?
A Postoperative morbidity and mortality in the elderly surgical population is a considerable concern. Significant postoperative complications occur in approximately 5% of patients in modern series4—often attributed to the effects of age and to the frail condition of patients who are commonly selected for colpocleisis.
Specifically, approximately 5% of patients experience a postoperative cardiac, thromboembolic, pulmonary, or cerebrovascular event. Transfusion is the most commonly reported major complication related to the procedure itself. Other complications include:
- fever and its associated morbidity
- pneumonia
- ongoing vaginal bleeding
- pyelonephritis
- hematoma
- cystotomy
- ureteral occlusion.
Minor surgical complications occur at a rate of approximately 15%. Surgical mortality is about 1 in 400 cases.
Q7 Do you routinely undertake urodynamic study of patients who are scheduled to undergo an obliterative procedure?
A At minimum, a lower urinary tract evaluation should include a postvoid residual volume study and, we believe, some kind of a filling study and stress test, with reduction of the prolapse. Beyond that, we recommend that you conduct more detailed urodynamic tests on a patient-by-patient basis, when you think that the findings will add to the clinical picture.
Q8 Would you ever perform a vaginal hysterectomy and then proceed with a colpectomy and colpocleisis?
A The principal rationale for performing hysterectomy at the time of colpocleisis is to eliminate the risk of endometrial or cervical carcinoma. Hysterectomy also eliminates the risk of pyometra, a rare but serious complication that can occur when the lateral canals become obstructed after a LeFort procedure.
A recent study5 looked at 1) concomitant hysterectomy in conjunction with colpectomy and colpocleisis and 2) traditional LeFort partial colpocleisis. In this retrospective review, objective and subjective success rates were high, but patients who underwent hysterectomy had a statistically significantly greater decline in postoperative hematocrit and a significant increase in the need for transfusion, compared with patients who did not undergo hysterectomy (35% vs. 13%).
References
1. Fitzgerald MP, Richter HE, Bradley CS, et al. Pelvic support, pelvic symptoms and patient satisfaction after colpocleisis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(12):1603-1609.
2. Hullfish K, Bobbjerg B, Steers W. Colpocleisis for pelvic organ prolapse Patient Goals Quality of life and Satisfaction. Obstet Gynecol. 2007;110(2 Pt 1):341-345.
3. Fitzgerald MP, Brubaker L. Colpocleisis and urinary incontinence. Am J Obstet Gynecol. 2003;189(5):1241-1244.
4. von Pechmann WS, Muton M, Fyffe J, Hale DS. Total colpocleisis with high levator placation for the treatment of advanced organ prolapse. Am J Obstet Gynecol. 2003;189(1):121-126.
5. Kohli NE, Sze E, Karram M. Pyometra following LeFort colpocleisis. Int Urogyn J. 1996;7(5):264-266.
We want to hear from you! Tell us what you think.
- LeFort partial colpocleisis
- Colpectomy and colpocleisis
- Colpectomy and colpocleisis after two previously failed obliterative procedures
These videos were selected by Mickey Karram, MD, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS)
This article, with accompanying video footage, is presented with the support of the International Academy of Pelvic Surgery.
As women live longer, on average, pelvic floor disorders are, as a whole, becoming more prevalent and a greater health and social problem. Many women entering the eighth and ninth decades of life display symptomatic pelvic organ prolapse (POP)—often after an unsuccessful trial of a pessary or even surgery.
These elderly patients often have other concomitant medical issues and are not sexually active, making extensive surgery for them less than an ideal solution. Instead, surgical procedures that obliterate the vaginal canal can alleviate their symptoms of POP.
In this article, we provide a step-by-step description of:
- LeFort partial colpocleisis in a woman who still has her uterus in place
- partial or complete colpectomy and colpocleisis in a woman who has post-hysterectomy prolapse
- levator plication and perineorrhaphy, as essential concluding steps in these procedures.
LeFort partial colpocleisis
An obliterative procedure in the form of a LeFort partial colpocleisis is an option when a patient 1) has her uterus and 2) is no longer sexually active. Because the uterus is retained in this procedure, however, keep in mind that it will be difficult to evaluate any uterine bleeding or cervical pathology in the future. Endovaginal ultrasonography or an endometrial biopsy, and a Pap smear, must be done before LeFort surgery.
The ideal candidate for LeFort partial colpocleisis is a woman who has complete uterine prolapse, or procidentia (FIGURE 1), which is characterized by symmetric eversion of the anterior and posterior vaginal walls.

FIGURE 1 Pelvic organ prolapse, preoperatively
Top: Uterine procidentia. A patient who has this condition is an ideal candidate for LeFort partial colpocleisis. Bottom: Asymmetric anterior vaginal prolapse.
LeFort partial colpocleisis: Key step by key step
![]()
![]()
![]()
![]()
![]()
![]()
![]()
FIGURE 2 shows key steps in performing LeFort partial colpocleisis. See Video #1 at www.obgmanagement.com for demonstrations of how to perform LeFort partial colpocleisis.

FIGURE 2 Steps: LeFort partial colpocleisis
A. Denude the anterior vaginal epithelium. B. Plicate the neck of the bladder. C. Next, denude the posterior vaginal epithelium. D. Approximate most proximal surfaces. E. Place lateral sutures to allow for drainage canals. F. The uterus has been replaced and most of the distal incisions closed.
Total colpectomy and colpocleisis: Key step by key step
In a patient who has post-hysterectomy prolapse and is not interested in continued sexual function, total colpectomy and colpocleisis provide a highly minimally invasive, durable option to correct her prolapse.
If there is complete eversion of the vagina then, truly, total colpectomy and colpocleisis is the procedure of choice. If there is significant prolapse of only one segment of the pelvic floor, however—for example, the anterior vaginal wall (FIGURE 1)—then aggressive repair of this variant with a narrowing down of the genital hiatus accomplishes the same result without requiring complete removal of what appears to be fairly well supported vaginal mucosa.
Here are key steps for performing partial or complete colpectomy and colpocleisis.
![]()
![]()
Completely remove the vaginal epithelium (FIGURES 3A and 3B); your goal is to leave most of the muscularis of the vaginal wall on the prolapse.
Avoid the peritoneal cavity if at all possible; when the main portion of the prolapse is secondary to an enterocele and the vaginal epithelium is very thin, however, formal excision of the enterocele sac, with closing of the defect, may be required.
![]()
If at all possible, avoid the peritoneum and the wall of the viscera, whether bladder or bowel. Invert the apex of the soft tissue, using the tip of forceps, as each purse-string suture is tied.
There is a variation of this procedure: Perform a separate anterior and posterior colporrhaphy, with two purse-string sutures used to approximate the anterior and posterior segments, thus obliterating any dead space.
![]()
![]()
See Video #2 and Video #3 for a demonstration of how to perform a complete colpectomy and colpocleisis. FIGURE 3D shows the completed colpocleisis.

FIGURE 3 Steps: Total colpectomy and colpocleisis
Denude the anterior vaginal epithelium (A) and then the posterior epithelium (B). C. Place sequential purse-string sutures. D. The completed colpocleisis, in cross-section.
Distal levatoroplasty with high perineorrhaphy: Key step by key step
![]()
![]()
![]()
![]()

FIGURE 4 Steps: Distal levatoroplasty with high perineorrhaphy
A. Lateral dissection to the levator ani muscles. Inset: levator ani plicated with sequential sutures. B. Place three sutures to plicate the levator ani. C. Secure the plication sutures. Inset C, and D: Completed levatoroplasty.
Our experience
We are often asked questions about the procedures that we’ve just described, including patients’ satisfaction with the outcome, complications, and the risk that prolapse will recur. In the accompanying box, “Questions we’re asked (and answers we give) about obliterative surgery,” opposite, we give our responses to eight common inquiries.
about obliterative surgery
Q1 How satisfied are women with the outcome of these procedures—do many regret having their vaginal canal obliterated?
A Overall, studies indicate that 85% to 100% of patients are “satisfied” or “very satisfied” with the outcomes of obliterative procedures.1 There are rare reports of regret after colpocleisis over loss of coital ability; in one study of a series of procedures,2 5% of subjects expressed regret postoperatively.
Q2 Why is levatoroplasty and perineorrhaphy such an important part of both the LeFort partial colpocleisis and colpectomy and colpocleisis?
A The aim of both these procedures is to reduce prolapsed tissue. The true durability of repair comes from significantly decreasing the caliber of the genital hiatus, with the hope of closing off the bulk of the distal vaginal canal. This can really only be accomplished by utilizing an aggressive levatoroplasty and perineorrhaphy, described in the text.
Q3 How often do patients develop de novo stress incontinence or significant voiding dysfunction, or both, after an obliterative procedure?
A The risk of developing urinary incontinence after an obliterative procedure is difficult to ascertain. In general, patients who had retention or a high postvoid residual volume preoperatively have a good outcome in regard to correcting their voiding dysfunction. This is because, in most cases, the voiding dysfunction is directly related to the anatomic distortion created by the prolapse.
Q4 What is the rate of prolapse recurrence after these procedures, and how is a recurrence managed?
A Multiple studies have documented an excellent anatomic outcome after these procedures, with a prolapse recurrence rate of only 1% to 8%.3 Very little has been written about how to best manage recurrent prolapse after an obliterative procedure. Most surgeons would, most likely, recommend repeat colpocleisis or aggressive levatoroplasty and perineorrhaphy. (Note: The patient whose colpectomy and colpocleisis is shown in Video #3 failed two previous colpectomy and colpocleisis procedures.)
Q5 Can these procedures be performed under local anesthesia, with some intravenous sedation, or under regional anesthesia—thereby avoiding intubation?
A Yes. We have utilized IV sedation and bilateral block successfully to perform these procedures. (Note: Video #3 of LeFort partial colpocleisis shows the procedure performed under local anesthesia.)
Q6 What does the literature say about common complications after these procedures?
A Postoperative morbidity and mortality in the elderly surgical population is a considerable concern. Significant postoperative complications occur in approximately 5% of patients in modern series4—often attributed to the effects of age and to the frail condition of patients who are commonly selected for colpocleisis.
Specifically, approximately 5% of patients experience a postoperative cardiac, thromboembolic, pulmonary, or cerebrovascular event. Transfusion is the most commonly reported major complication related to the procedure itself. Other complications include:
- fever and its associated morbidity
- pneumonia
- ongoing vaginal bleeding
- pyelonephritis
- hematoma
- cystotomy
- ureteral occlusion.
Minor surgical complications occur at a rate of approximately 15%. Surgical mortality is about 1 in 400 cases.
Q7 Do you routinely undertake urodynamic study of patients who are scheduled to undergo an obliterative procedure?
A At minimum, a lower urinary tract evaluation should include a postvoid residual volume study and, we believe, some kind of a filling study and stress test, with reduction of the prolapse. Beyond that, we recommend that you conduct more detailed urodynamic tests on a patient-by-patient basis, when you think that the findings will add to the clinical picture.
Q8 Would you ever perform a vaginal hysterectomy and then proceed with a colpectomy and colpocleisis?
A The principal rationale for performing hysterectomy at the time of colpocleisis is to eliminate the risk of endometrial or cervical carcinoma. Hysterectomy also eliminates the risk of pyometra, a rare but serious complication that can occur when the lateral canals become obstructed after a LeFort procedure.
A recent study5 looked at 1) concomitant hysterectomy in conjunction with colpectomy and colpocleisis and 2) traditional LeFort partial colpocleisis. In this retrospective review, objective and subjective success rates were high, but patients who underwent hysterectomy had a statistically significantly greater decline in postoperative hematocrit and a significant increase in the need for transfusion, compared with patients who did not undergo hysterectomy (35% vs. 13%).
References
1. Fitzgerald MP, Richter HE, Bradley CS, et al. Pelvic support, pelvic symptoms and patient satisfaction after colpocleisis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(12):1603-1609.
2. Hullfish K, Bobbjerg B, Steers W. Colpocleisis for pelvic organ prolapse Patient Goals Quality of life and Satisfaction. Obstet Gynecol. 2007;110(2 Pt 1):341-345.
3. Fitzgerald MP, Brubaker L. Colpocleisis and urinary incontinence. Am J Obstet Gynecol. 2003;189(5):1241-1244.
4. von Pechmann WS, Muton M, Fyffe J, Hale DS. Total colpocleisis with high levator placation for the treatment of advanced organ prolapse. Am J Obstet Gynecol. 2003;189(1):121-126.
5. Kohli NE, Sze E, Karram M. Pyometra following LeFort colpocleisis. Int Urogyn J. 1996;7(5):264-266.
We want to hear from you! Tell us what you think.
Have you tried a progestin for your patient’s pelvic pain?
The date of the changeover to the 10th revision of International Classification of Diseases (ICD-10-CM) codes is incorrectly stated in the November 2011 Reimbursement Adviser, page 51. The date should be October 1, 2013.
To read the corrected version of this article, Click here
—The Editors
CASE
Your patient is a 26-year-old G0 woman who has a long history of progressively worsening dysmenorrhea, pelvic pain, and dyspareunia. In the recent past, she was treated with nonsteroidal anti-inflammatory drugs, a cyclic estrogen-progestin contraceptive, and a continuous estrogen-progestin contraceptive—in that order, and without appreciable relief of the pain.
Recently, the woman underwent laparoscopy, which demonstrated Stage-II endometriosis, which was ablated.
What would you prescribe for her postoperatively to alleviate symptoms?
Endometriosis will be diagnosed in approximately 8% of women of reproductive age.1 Pelvic pain, dysmenorrhea, and deep dyspareunia are common symptoms of endometriosis that interfere with quality of life.
Endometriosis is a chronic disease best managed by developing a life-long treatment plan. Following laparoscopic diagnosis and treatment, many experts strongly recommend postoperative hormone-suppressive therapy to reduce the risk that severe pelvic pain will recur, requiring re-operation.
Options for postoperative hormonal treatment of endometriosis include:
- an estrogen–progestin contraceptive
- a progestin (norethindrone acetate [NEA]; depot medroxyprogesterone acetate [DMPA]; oral medroxyprogesterone acetate; the levonorgestrel-releasing intrauterine system [LNG-IUS; Mirena]; and the progestin-releasing implant [Implanon])
- a gonadotropin-releasing hormone (GnRH) agonist (depot leuprolide [Depot Lupron]; nafarelin nasal spray [Synarel]).
CASE Continued
Considering that both cyclic and continuous estrogen-progestin contraceptives have already failed to provide adequate pain relief for your patient, you know that you should offer an alternative to her. Taking into account that progestins are significantly less costly than a GnRH agonist, a progestin formulation might, for her, be considered a first-line postoperative treatment of symptoms of endometriosis.
Options when considering a progestin
Norethindrone acetate
This agent is available in a single formulation: a 5-mg tablet; however, dosages ranging from 2.5 mg/d (half of a tablet) to 15 mg/d have been reported to be effective for relieving pain caused by endometriosis.
What is it? NEA is an androgenic progestin that suppresses luteinizing hormone and follicle-stimulating hormone, thus reducing production of ovarian estrogen. In the absence of ovarian estrogen, endometriosis lesions atrophy. In addition, NEA binds to, and stimulates, endometrial progestin and androgen receptors, resulting in decidualization and atrophy of both eutopic and ectopic endometrial tissue.
Importantly, NEA does not appear to cause bone loss, a phenomenon that is common with agents such as the GnRH agonists or DPMA.2-4
The research record. One randomized study, two pilot studies, and one large observational study have reported that NEA is effective for pelvic pain caused by endometriosis.
In the randomized trial, 90 women who had moderate or severe pelvic pain and rectovaginal endometriosis, and who remained symptomatic after conservative surgery, were randomized to receive NEA, 2.5 mg/d, or a low-dose estrogen-progestin contraceptive (ethinyl estradiol, 10 μg, plus cyproterone acetate, 3 mg) daily for 12 months.5 Both treatment groups reported significant and similar decreases in dysmenorrhea, deep dyspareunia, non-menstrual pain and dyschezia.
In a small pilot study, 40 women who had pelvic pain and colorectal endometriosis were treated with NEA 2.5 mg/d for 12 months. The drug produced significant improvement in dysmenorrhea, pelvic pain, deep dyspareunia, dyschezia, and cyclic rectal bleeding.6
In another pilot study, women who had pelvic pain and rectovaginal endometriosis were treated with either an aromatase inhibitor (letrozole, 2.5 mg/d) plus NEA (2.5 mg/d) or NEA (2.5 mg/d) alone for 6 months. Both treatments resulted in a significant improvement in pelvic pain and deep dyspareunia. Improvement in pain scores was greater with letrozole plus NEA; patients were more satisfied with NEA monotherapy than with the combined letrozole-NEA treatment, however, because the former was associated with fewer side effects.7
In a large (n=194) observational study of the postoperative use of NEA in young women with pelvic pain and endometriosis, NEA at dosages as high as 15 mg/d significantly diminished pelvic pain and self-reported menstrual bleeding. All subjects were started on a dosage of 5 mg/d, which was increased in 2.5-mg increments every 2 weeks to achieve the goals of amenorrhea and a lessening of pelvic pain; the maximum dosage administered was 15 mg/d. Mean duration of NEA use was 13 months; 75% of subjects took the maximum prescribed dosage of 15 mg at some point during treatment. The most commonly reported side effects were weight gain (16% of women); acne (10%); mood lability (9%); and vasomotor symptoms (8%).8
In summary. NEA is effective for treating pelvic pain caused by endometriosis at dosages from 2.5 mg/d to 15 mg/d. An important goal of treatment is a decrease in pain symptoms and amenorrhea; a dosage of 2.5 mg is often insufficient to reliably achieve both of those objectives.
In my practice I begin therapy at a dosage of 5 mg/d; the drug is effective for most patients at that dosage. If 5 mg/d does not reduce pain, I increase the dosage by 2.5 mg (half of a tablet) daily every 4 weeks, to a maximum dosage of 10 mg/d (two tablets). If that dosage is ineffective, I usually discontinue NEA and switch to a GnRH agonist.
Depot medroxyprogesterone acetate; oral medroxy-progesterone acetate
DMPA is available in two FDA-approved formulations:
- a 150-mg dose given by intramuscular injection every 3 months
- a 104-mg dose given by subcutaneous injection every 3 months.
Research. The results of two large clinical trials, comprising a total of more than 550 subjects, showed that DMPA (104 mg, SC, every 3 months) and depot leuprolide (11.25 mg, IM, every 3 months or 3.75 mg, monthly) were each equally effective in relieving dysmenorrhea, dyspareunia, pelvic pain, pelvic tenderness, and pelvic induration in women who had endometriosis.9,10
DMPA was associated with a greater rate of episodes of irregular bleeding than depot leuprolide; conversely, depot leuprolide was associated with greater loss of bone density and a higher incidence of vasomotor symptoms. Weight gain was in the range of 0.6 kg in both groups.
Of note, DPMA is much less expensive than depot leuprolide.
Another study showed that increasing the dosage of DMPA did not improve efficacy over the standard dosage11: DMPA, 150 mg IM, monthly, and DMPA, 150 mg IM, every 3 months produced similar relief of pelvic pain.
Oral medroxyprogesterone acetate, prescribed at high dosages, is also effective for pelvic pain caused by endometriosis. In a pilot study (n=21), oral MPA, 50 mg/d for 4 months, alleviated dysmenorrhea, dyspareunia, pelvic pain, dyschezia, and pelvic tenderness and decreased pelvic nodularity. Sixty percent of subjects reported weight gain— 1.5 kg, on average.12
Progestin-releasing devices: Mirena and Implanon
Many pilot studies have reported that the levonorgestrel-releasing intrauterine system (LNG-IUS) is effective for pelvic pain caused by endometriosis.13-17 For example:
Research. In a small clinical trial, 30 women who had pelvic pain and endometriosis were randomized to receive an LNG-IUS (Mirena) or DMPA, 150 mg IM, every 3 months for 3 years.13 Both therapies were effective at reducing pelvic pain.
At the conclusion of the study, more women opted to retain the LNG-IUS (87%) than to continue DMPA injection (47%). Bone density was maintained in women who had the LNG-IUS placed but slightly diminished in women receiving DMPA.
In a pilot study of an etonogestrel releasing implant (Implanon), 41 women who had pelvic pain and endometriosis were randomized to receive the implant or DMPA, 150 mg IM, every 3 months for 1 year.18 Both therapies were similarly effective at reducing pelvic pain.
Notably, irregular uterine bleeding is a common problem when the etonogestrel-releasing implant is used to treat endometriosis. Achieving amenorrhea or oligomenorrhea is an important goal for women who suffer from pelvic pain caused by endometriosis.
My recommendation
Most ObGyns see patients who are suffering from difficult-to-treat pelvic pain caused by endometriosis. Many of these patients have not had a trial of a progestin, such as NEA, DMPA, or the LNG-IUS that I use in my practice.
Progestins are, as I’ve described, effective for pelvic pain. They are also relatively inexpensive and have a side-effect profile that most patients find acceptable. I recommend that you try a progestin for your patients who have refractory pelvic pain.
What is your preferred hormone treatment for women with unrelieved pelvic pain from endometriosis?
1. Missmer SA, Hankinson S, Spiegelman D, et al. The incidence of laparoscopically confirmed endometriosis by demographic, anthropomorphic and lifestyle factors. Am J Epidemiol. 2004;160(8):784-796.
2. Abdalla HI, Hart DM, Lindsay R, Leggate I, Hooke A. Prevention of bone mineral loss in postmenopausal women by norethisterone. Obstet Gynecol. 1985;66(6):789-792.
3. Riss BJ, Lehmann HJ, Christiansen C. Norethisterone acetate in combination with estrogen: effects on the skeleton and other organs. Am J Obstet Gynecol. 2002;187(4):1101-1116.
4. Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-back Study Group. Obstet Gynecol. 1998;91(1):16-24.
5. Vercellini P, Pietropauolo G, De Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84(5):1375-1387.
6. Ferrero S, Camerini G, Ragni N, Venturini PL, Biscaldi E, Remorgida V. Norethisterone acetate in the treatment of colorectal endometriosis: a pilot study. Hum Reprod. 2010;25(1):94-100.
7. Ferrero S, Camerini G, Seracchioli R, Ragni N, Venturini PL, Remorgida V. Letrozole combined with norethisterone acetate compared with norethisterone acetate alone in the treatment of pain symptoms caused by endometriosis. Hum Reprod. 2009;24(12):3033-3341.
8. Kaser DJ, Missmer SA, Berry KF, Laufer MR. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms [published online ahead of print December 9 2011]. J Pediatr Adolesc Gynecol. doi:10.1016/j.jpag.2011.09.013.
9. Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. Subcutaneous injection of depot medroxyprogesterone acetate compared with leuprolide acetate in the treatment of endometriosis associated pain. Fertil Steril. 2006;85(2):314-325.
10. Crosignani PG, Luciano A, Ray A, Bergqvist A. Subcutaneous depot medroxyprogesterone acetate versus leuprolide acetate in the treatment of endometriosis-associated pain. Hum Reprod. 2006;21(1):248-256.
11. Cheewadhanaraks S, Peeyananjarassri K, Choksuchat C, Dhanaworavibul K, Choobun T, Bunyapipat S. Interval of injections of intramuscular depot medroxyprogesterone acetate in the long-term treatment of endometriosis-associated pain: a randomized clinical trial. Gynecol Obstet Invest. 2009;68(2):116-121.
12. Luciano AA, Turksoy RN, Carleo J. Evaluation of oral medroxyprogesterone acetate in the treatment of endometriosis. Obstet Gynecol. 1988;72(3 Pt 1):323-327.
13. Wong AY, Tang LC, Chin RK. Levonorgestrel-releasing intrauterine system (Mirena) and depot medroxyprogesterone acetate (Depoprovera) as long-term maintenance therapy for patients with moderate and severe endometriosis: a randomized controlled trial. Aust N Z J Obstet Gynaecol. 2010;50(3):273-279.
14. Lockhat FB, Emembolu JO, Konje JC. The efficacy side-effects and continuation rates in women with symptomatic endometriosis undergoing treatment with an intrauterine administered progestogen (levonorgestrel): a 3 year follow-up. Hum Reprod. 2005;20(3):789-793.
15. Petta CA, Ferriani RA, Abrao MS, et al. Randomized clinical trial of a levonorgestrel-releasing intrauterine system and a depot GnRH analogue for the treatment of chronic pelvic pain in women with endometriosis. Hum Reprod. 2005;20(7):1993-1998.
16. Vercellini P, Aimi G, Panazza S, De Giorgi O, Pesole A, Crosignani PG. A levonorgestrel-releasing intrauterine system for the treatment of dysmenorrhea associated with endometriosis: a pilot study. Fertil Steril. 1999;72(3):505-508.
17. Vercellini P, Frontino G, De Giorgi O, Aimi G, Zaina B, Crosignani PG. Comparison of a levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: a pilot study. Fertil Steril. 2003;80(2):305-309.
18. Walch K, Unfried G, Huber J, Kurz C, van Trotsenburg M, Pernicka E, Wenzl R. Implanon versus medroxyprogesterone acetate: effects on pain scores in patients with symptomatic endometriosis—a pilot study. Contraception. 2009;79(1):29-34.
The date of the changeover to the 10th revision of International Classification of Diseases (ICD-10-CM) codes is incorrectly stated in the November 2011 Reimbursement Adviser, page 51. The date should be October 1, 2013.
To read the corrected version of this article, Click here
—The Editors
CASE
Your patient is a 26-year-old G0 woman who has a long history of progressively worsening dysmenorrhea, pelvic pain, and dyspareunia. In the recent past, she was treated with nonsteroidal anti-inflammatory drugs, a cyclic estrogen-progestin contraceptive, and a continuous estrogen-progestin contraceptive—in that order, and without appreciable relief of the pain.
Recently, the woman underwent laparoscopy, which demonstrated Stage-II endometriosis, which was ablated.
What would you prescribe for her postoperatively to alleviate symptoms?
Endometriosis will be diagnosed in approximately 8% of women of reproductive age.1 Pelvic pain, dysmenorrhea, and deep dyspareunia are common symptoms of endometriosis that interfere with quality of life.
Endometriosis is a chronic disease best managed by developing a life-long treatment plan. Following laparoscopic diagnosis and treatment, many experts strongly recommend postoperative hormone-suppressive therapy to reduce the risk that severe pelvic pain will recur, requiring re-operation.
Options for postoperative hormonal treatment of endometriosis include:
- an estrogen–progestin contraceptive
- a progestin (norethindrone acetate [NEA]; depot medroxyprogesterone acetate [DMPA]; oral medroxyprogesterone acetate; the levonorgestrel-releasing intrauterine system [LNG-IUS; Mirena]; and the progestin-releasing implant [Implanon])
- a gonadotropin-releasing hormone (GnRH) agonist (depot leuprolide [Depot Lupron]; nafarelin nasal spray [Synarel]).
CASE Continued
Considering that both cyclic and continuous estrogen-progestin contraceptives have already failed to provide adequate pain relief for your patient, you know that you should offer an alternative to her. Taking into account that progestins are significantly less costly than a GnRH agonist, a progestin formulation might, for her, be considered a first-line postoperative treatment of symptoms of endometriosis.
Options when considering a progestin
Norethindrone acetate
This agent is available in a single formulation: a 5-mg tablet; however, dosages ranging from 2.5 mg/d (half of a tablet) to 15 mg/d have been reported to be effective for relieving pain caused by endometriosis.
What is it? NEA is an androgenic progestin that suppresses luteinizing hormone and follicle-stimulating hormone, thus reducing production of ovarian estrogen. In the absence of ovarian estrogen, endometriosis lesions atrophy. In addition, NEA binds to, and stimulates, endometrial progestin and androgen receptors, resulting in decidualization and atrophy of both eutopic and ectopic endometrial tissue.
Importantly, NEA does not appear to cause bone loss, a phenomenon that is common with agents such as the GnRH agonists or DPMA.2-4
The research record. One randomized study, two pilot studies, and one large observational study have reported that NEA is effective for pelvic pain caused by endometriosis.
In the randomized trial, 90 women who had moderate or severe pelvic pain and rectovaginal endometriosis, and who remained symptomatic after conservative surgery, were randomized to receive NEA, 2.5 mg/d, or a low-dose estrogen-progestin contraceptive (ethinyl estradiol, 10 μg, plus cyproterone acetate, 3 mg) daily for 12 months.5 Both treatment groups reported significant and similar decreases in dysmenorrhea, deep dyspareunia, non-menstrual pain and dyschezia.
In a small pilot study, 40 women who had pelvic pain and colorectal endometriosis were treated with NEA 2.5 mg/d for 12 months. The drug produced significant improvement in dysmenorrhea, pelvic pain, deep dyspareunia, dyschezia, and cyclic rectal bleeding.6
In another pilot study, women who had pelvic pain and rectovaginal endometriosis were treated with either an aromatase inhibitor (letrozole, 2.5 mg/d) plus NEA (2.5 mg/d) or NEA (2.5 mg/d) alone for 6 months. Both treatments resulted in a significant improvement in pelvic pain and deep dyspareunia. Improvement in pain scores was greater with letrozole plus NEA; patients were more satisfied with NEA monotherapy than with the combined letrozole-NEA treatment, however, because the former was associated with fewer side effects.7
In a large (n=194) observational study of the postoperative use of NEA in young women with pelvic pain and endometriosis, NEA at dosages as high as 15 mg/d significantly diminished pelvic pain and self-reported menstrual bleeding. All subjects were started on a dosage of 5 mg/d, which was increased in 2.5-mg increments every 2 weeks to achieve the goals of amenorrhea and a lessening of pelvic pain; the maximum dosage administered was 15 mg/d. Mean duration of NEA use was 13 months; 75% of subjects took the maximum prescribed dosage of 15 mg at some point during treatment. The most commonly reported side effects were weight gain (16% of women); acne (10%); mood lability (9%); and vasomotor symptoms (8%).8
In summary. NEA is effective for treating pelvic pain caused by endometriosis at dosages from 2.5 mg/d to 15 mg/d. An important goal of treatment is a decrease in pain symptoms and amenorrhea; a dosage of 2.5 mg is often insufficient to reliably achieve both of those objectives.
In my practice I begin therapy at a dosage of 5 mg/d; the drug is effective for most patients at that dosage. If 5 mg/d does not reduce pain, I increase the dosage by 2.5 mg (half of a tablet) daily every 4 weeks, to a maximum dosage of 10 mg/d (two tablets). If that dosage is ineffective, I usually discontinue NEA and switch to a GnRH agonist.
Depot medroxyprogesterone acetate; oral medroxy-progesterone acetate
DMPA is available in two FDA-approved formulations:
- a 150-mg dose given by intramuscular injection every 3 months
- a 104-mg dose given by subcutaneous injection every 3 months.
Research. The results of two large clinical trials, comprising a total of more than 550 subjects, showed that DMPA (104 mg, SC, every 3 months) and depot leuprolide (11.25 mg, IM, every 3 months or 3.75 mg, monthly) were each equally effective in relieving dysmenorrhea, dyspareunia, pelvic pain, pelvic tenderness, and pelvic induration in women who had endometriosis.9,10
DMPA was associated with a greater rate of episodes of irregular bleeding than depot leuprolide; conversely, depot leuprolide was associated with greater loss of bone density and a higher incidence of vasomotor symptoms. Weight gain was in the range of 0.6 kg in both groups.
Of note, DPMA is much less expensive than depot leuprolide.
Another study showed that increasing the dosage of DMPA did not improve efficacy over the standard dosage11: DMPA, 150 mg IM, monthly, and DMPA, 150 mg IM, every 3 months produced similar relief of pelvic pain.
Oral medroxyprogesterone acetate, prescribed at high dosages, is also effective for pelvic pain caused by endometriosis. In a pilot study (n=21), oral MPA, 50 mg/d for 4 months, alleviated dysmenorrhea, dyspareunia, pelvic pain, dyschezia, and pelvic tenderness and decreased pelvic nodularity. Sixty percent of subjects reported weight gain— 1.5 kg, on average.12
Progestin-releasing devices: Mirena and Implanon
Many pilot studies have reported that the levonorgestrel-releasing intrauterine system (LNG-IUS) is effective for pelvic pain caused by endometriosis.13-17 For example:
Research. In a small clinical trial, 30 women who had pelvic pain and endometriosis were randomized to receive an LNG-IUS (Mirena) or DMPA, 150 mg IM, every 3 months for 3 years.13 Both therapies were effective at reducing pelvic pain.
At the conclusion of the study, more women opted to retain the LNG-IUS (87%) than to continue DMPA injection (47%). Bone density was maintained in women who had the LNG-IUS placed but slightly diminished in women receiving DMPA.
In a pilot study of an etonogestrel releasing implant (Implanon), 41 women who had pelvic pain and endometriosis were randomized to receive the implant or DMPA, 150 mg IM, every 3 months for 1 year.18 Both therapies were similarly effective at reducing pelvic pain.
Notably, irregular uterine bleeding is a common problem when the etonogestrel-releasing implant is used to treat endometriosis. Achieving amenorrhea or oligomenorrhea is an important goal for women who suffer from pelvic pain caused by endometriosis.
My recommendation
Most ObGyns see patients who are suffering from difficult-to-treat pelvic pain caused by endometriosis. Many of these patients have not had a trial of a progestin, such as NEA, DMPA, or the LNG-IUS that I use in my practice.
Progestins are, as I’ve described, effective for pelvic pain. They are also relatively inexpensive and have a side-effect profile that most patients find acceptable. I recommend that you try a progestin for your patients who have refractory pelvic pain.
What is your preferred hormone treatment for women with unrelieved pelvic pain from endometriosis?
The date of the changeover to the 10th revision of International Classification of Diseases (ICD-10-CM) codes is incorrectly stated in the November 2011 Reimbursement Adviser, page 51. The date should be October 1, 2013.
To read the corrected version of this article, Click here
—The Editors
CASE
Your patient is a 26-year-old G0 woman who has a long history of progressively worsening dysmenorrhea, pelvic pain, and dyspareunia. In the recent past, she was treated with nonsteroidal anti-inflammatory drugs, a cyclic estrogen-progestin contraceptive, and a continuous estrogen-progestin contraceptive—in that order, and without appreciable relief of the pain.
Recently, the woman underwent laparoscopy, which demonstrated Stage-II endometriosis, which was ablated.
What would you prescribe for her postoperatively to alleviate symptoms?
Endometriosis will be diagnosed in approximately 8% of women of reproductive age.1 Pelvic pain, dysmenorrhea, and deep dyspareunia are common symptoms of endometriosis that interfere with quality of life.
Endometriosis is a chronic disease best managed by developing a life-long treatment plan. Following laparoscopic diagnosis and treatment, many experts strongly recommend postoperative hormone-suppressive therapy to reduce the risk that severe pelvic pain will recur, requiring re-operation.
Options for postoperative hormonal treatment of endometriosis include:
- an estrogen–progestin contraceptive
- a progestin (norethindrone acetate [NEA]; depot medroxyprogesterone acetate [DMPA]; oral medroxyprogesterone acetate; the levonorgestrel-releasing intrauterine system [LNG-IUS; Mirena]; and the progestin-releasing implant [Implanon])
- a gonadotropin-releasing hormone (GnRH) agonist (depot leuprolide [Depot Lupron]; nafarelin nasal spray [Synarel]).
CASE Continued
Considering that both cyclic and continuous estrogen-progestin contraceptives have already failed to provide adequate pain relief for your patient, you know that you should offer an alternative to her. Taking into account that progestins are significantly less costly than a GnRH agonist, a progestin formulation might, for her, be considered a first-line postoperative treatment of symptoms of endometriosis.
Options when considering a progestin
Norethindrone acetate
This agent is available in a single formulation: a 5-mg tablet; however, dosages ranging from 2.5 mg/d (half of a tablet) to 15 mg/d have been reported to be effective for relieving pain caused by endometriosis.
What is it? NEA is an androgenic progestin that suppresses luteinizing hormone and follicle-stimulating hormone, thus reducing production of ovarian estrogen. In the absence of ovarian estrogen, endometriosis lesions atrophy. In addition, NEA binds to, and stimulates, endometrial progestin and androgen receptors, resulting in decidualization and atrophy of both eutopic and ectopic endometrial tissue.
Importantly, NEA does not appear to cause bone loss, a phenomenon that is common with agents such as the GnRH agonists or DPMA.2-4
The research record. One randomized study, two pilot studies, and one large observational study have reported that NEA is effective for pelvic pain caused by endometriosis.
In the randomized trial, 90 women who had moderate or severe pelvic pain and rectovaginal endometriosis, and who remained symptomatic after conservative surgery, were randomized to receive NEA, 2.5 mg/d, or a low-dose estrogen-progestin contraceptive (ethinyl estradiol, 10 μg, plus cyproterone acetate, 3 mg) daily for 12 months.5 Both treatment groups reported significant and similar decreases in dysmenorrhea, deep dyspareunia, non-menstrual pain and dyschezia.
In a small pilot study, 40 women who had pelvic pain and colorectal endometriosis were treated with NEA 2.5 mg/d for 12 months. The drug produced significant improvement in dysmenorrhea, pelvic pain, deep dyspareunia, dyschezia, and cyclic rectal bleeding.6
In another pilot study, women who had pelvic pain and rectovaginal endometriosis were treated with either an aromatase inhibitor (letrozole, 2.5 mg/d) plus NEA (2.5 mg/d) or NEA (2.5 mg/d) alone for 6 months. Both treatments resulted in a significant improvement in pelvic pain and deep dyspareunia. Improvement in pain scores was greater with letrozole plus NEA; patients were more satisfied with NEA monotherapy than with the combined letrozole-NEA treatment, however, because the former was associated with fewer side effects.7
In a large (n=194) observational study of the postoperative use of NEA in young women with pelvic pain and endometriosis, NEA at dosages as high as 15 mg/d significantly diminished pelvic pain and self-reported menstrual bleeding. All subjects were started on a dosage of 5 mg/d, which was increased in 2.5-mg increments every 2 weeks to achieve the goals of amenorrhea and a lessening of pelvic pain; the maximum dosage administered was 15 mg/d. Mean duration of NEA use was 13 months; 75% of subjects took the maximum prescribed dosage of 15 mg at some point during treatment. The most commonly reported side effects were weight gain (16% of women); acne (10%); mood lability (9%); and vasomotor symptoms (8%).8
In summary. NEA is effective for treating pelvic pain caused by endometriosis at dosages from 2.5 mg/d to 15 mg/d. An important goal of treatment is a decrease in pain symptoms and amenorrhea; a dosage of 2.5 mg is often insufficient to reliably achieve both of those objectives.
In my practice I begin therapy at a dosage of 5 mg/d; the drug is effective for most patients at that dosage. If 5 mg/d does not reduce pain, I increase the dosage by 2.5 mg (half of a tablet) daily every 4 weeks, to a maximum dosage of 10 mg/d (two tablets). If that dosage is ineffective, I usually discontinue NEA and switch to a GnRH agonist.
Depot medroxyprogesterone acetate; oral medroxy-progesterone acetate
DMPA is available in two FDA-approved formulations:
- a 150-mg dose given by intramuscular injection every 3 months
- a 104-mg dose given by subcutaneous injection every 3 months.
Research. The results of two large clinical trials, comprising a total of more than 550 subjects, showed that DMPA (104 mg, SC, every 3 months) and depot leuprolide (11.25 mg, IM, every 3 months or 3.75 mg, monthly) were each equally effective in relieving dysmenorrhea, dyspareunia, pelvic pain, pelvic tenderness, and pelvic induration in women who had endometriosis.9,10
DMPA was associated with a greater rate of episodes of irregular bleeding than depot leuprolide; conversely, depot leuprolide was associated with greater loss of bone density and a higher incidence of vasomotor symptoms. Weight gain was in the range of 0.6 kg in both groups.
Of note, DPMA is much less expensive than depot leuprolide.
Another study showed that increasing the dosage of DMPA did not improve efficacy over the standard dosage11: DMPA, 150 mg IM, monthly, and DMPA, 150 mg IM, every 3 months produced similar relief of pelvic pain.
Oral medroxyprogesterone acetate, prescribed at high dosages, is also effective for pelvic pain caused by endometriosis. In a pilot study (n=21), oral MPA, 50 mg/d for 4 months, alleviated dysmenorrhea, dyspareunia, pelvic pain, dyschezia, and pelvic tenderness and decreased pelvic nodularity. Sixty percent of subjects reported weight gain— 1.5 kg, on average.12
Progestin-releasing devices: Mirena and Implanon
Many pilot studies have reported that the levonorgestrel-releasing intrauterine system (LNG-IUS) is effective for pelvic pain caused by endometriosis.13-17 For example:
Research. In a small clinical trial, 30 women who had pelvic pain and endometriosis were randomized to receive an LNG-IUS (Mirena) or DMPA, 150 mg IM, every 3 months for 3 years.13 Both therapies were effective at reducing pelvic pain.
At the conclusion of the study, more women opted to retain the LNG-IUS (87%) than to continue DMPA injection (47%). Bone density was maintained in women who had the LNG-IUS placed but slightly diminished in women receiving DMPA.
In a pilot study of an etonogestrel releasing implant (Implanon), 41 women who had pelvic pain and endometriosis were randomized to receive the implant or DMPA, 150 mg IM, every 3 months for 1 year.18 Both therapies were similarly effective at reducing pelvic pain.
Notably, irregular uterine bleeding is a common problem when the etonogestrel-releasing implant is used to treat endometriosis. Achieving amenorrhea or oligomenorrhea is an important goal for women who suffer from pelvic pain caused by endometriosis.
My recommendation
Most ObGyns see patients who are suffering from difficult-to-treat pelvic pain caused by endometriosis. Many of these patients have not had a trial of a progestin, such as NEA, DMPA, or the LNG-IUS that I use in my practice.
Progestins are, as I’ve described, effective for pelvic pain. They are also relatively inexpensive and have a side-effect profile that most patients find acceptable. I recommend that you try a progestin for your patients who have refractory pelvic pain.
What is your preferred hormone treatment for women with unrelieved pelvic pain from endometriosis?
1. Missmer SA, Hankinson S, Spiegelman D, et al. The incidence of laparoscopically confirmed endometriosis by demographic, anthropomorphic and lifestyle factors. Am J Epidemiol. 2004;160(8):784-796.
2. Abdalla HI, Hart DM, Lindsay R, Leggate I, Hooke A. Prevention of bone mineral loss in postmenopausal women by norethisterone. Obstet Gynecol. 1985;66(6):789-792.
3. Riss BJ, Lehmann HJ, Christiansen C. Norethisterone acetate in combination with estrogen: effects on the skeleton and other organs. Am J Obstet Gynecol. 2002;187(4):1101-1116.
4. Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-back Study Group. Obstet Gynecol. 1998;91(1):16-24.
5. Vercellini P, Pietropauolo G, De Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84(5):1375-1387.
6. Ferrero S, Camerini G, Ragni N, Venturini PL, Biscaldi E, Remorgida V. Norethisterone acetate in the treatment of colorectal endometriosis: a pilot study. Hum Reprod. 2010;25(1):94-100.
7. Ferrero S, Camerini G, Seracchioli R, Ragni N, Venturini PL, Remorgida V. Letrozole combined with norethisterone acetate compared with norethisterone acetate alone in the treatment of pain symptoms caused by endometriosis. Hum Reprod. 2009;24(12):3033-3341.
8. Kaser DJ, Missmer SA, Berry KF, Laufer MR. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms [published online ahead of print December 9 2011]. J Pediatr Adolesc Gynecol. doi:10.1016/j.jpag.2011.09.013.
9. Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. Subcutaneous injection of depot medroxyprogesterone acetate compared with leuprolide acetate in the treatment of endometriosis associated pain. Fertil Steril. 2006;85(2):314-325.
10. Crosignani PG, Luciano A, Ray A, Bergqvist A. Subcutaneous depot medroxyprogesterone acetate versus leuprolide acetate in the treatment of endometriosis-associated pain. Hum Reprod. 2006;21(1):248-256.
11. Cheewadhanaraks S, Peeyananjarassri K, Choksuchat C, Dhanaworavibul K, Choobun T, Bunyapipat S. Interval of injections of intramuscular depot medroxyprogesterone acetate in the long-term treatment of endometriosis-associated pain: a randomized clinical trial. Gynecol Obstet Invest. 2009;68(2):116-121.
12. Luciano AA, Turksoy RN, Carleo J. Evaluation of oral medroxyprogesterone acetate in the treatment of endometriosis. Obstet Gynecol. 1988;72(3 Pt 1):323-327.
13. Wong AY, Tang LC, Chin RK. Levonorgestrel-releasing intrauterine system (Mirena) and depot medroxyprogesterone acetate (Depoprovera) as long-term maintenance therapy for patients with moderate and severe endometriosis: a randomized controlled trial. Aust N Z J Obstet Gynaecol. 2010;50(3):273-279.
14. Lockhat FB, Emembolu JO, Konje JC. The efficacy side-effects and continuation rates in women with symptomatic endometriosis undergoing treatment with an intrauterine administered progestogen (levonorgestrel): a 3 year follow-up. Hum Reprod. 2005;20(3):789-793.
15. Petta CA, Ferriani RA, Abrao MS, et al. Randomized clinical trial of a levonorgestrel-releasing intrauterine system and a depot GnRH analogue for the treatment of chronic pelvic pain in women with endometriosis. Hum Reprod. 2005;20(7):1993-1998.
16. Vercellini P, Aimi G, Panazza S, De Giorgi O, Pesole A, Crosignani PG. A levonorgestrel-releasing intrauterine system for the treatment of dysmenorrhea associated with endometriosis: a pilot study. Fertil Steril. 1999;72(3):505-508.
17. Vercellini P, Frontino G, De Giorgi O, Aimi G, Zaina B, Crosignani PG. Comparison of a levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: a pilot study. Fertil Steril. 2003;80(2):305-309.
18. Walch K, Unfried G, Huber J, Kurz C, van Trotsenburg M, Pernicka E, Wenzl R. Implanon versus medroxyprogesterone acetate: effects on pain scores in patients with symptomatic endometriosis—a pilot study. Contraception. 2009;79(1):29-34.
1. Missmer SA, Hankinson S, Spiegelman D, et al. The incidence of laparoscopically confirmed endometriosis by demographic, anthropomorphic and lifestyle factors. Am J Epidemiol. 2004;160(8):784-796.
2. Abdalla HI, Hart DM, Lindsay R, Leggate I, Hooke A. Prevention of bone mineral loss in postmenopausal women by norethisterone. Obstet Gynecol. 1985;66(6):789-792.
3. Riss BJ, Lehmann HJ, Christiansen C. Norethisterone acetate in combination with estrogen: effects on the skeleton and other organs. Am J Obstet Gynecol. 2002;187(4):1101-1116.
4. Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-back Study Group. Obstet Gynecol. 1998;91(1):16-24.
5. Vercellini P, Pietropauolo G, De Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84(5):1375-1387.
6. Ferrero S, Camerini G, Ragni N, Venturini PL, Biscaldi E, Remorgida V. Norethisterone acetate in the treatment of colorectal endometriosis: a pilot study. Hum Reprod. 2010;25(1):94-100.
7. Ferrero S, Camerini G, Seracchioli R, Ragni N, Venturini PL, Remorgida V. Letrozole combined with norethisterone acetate compared with norethisterone acetate alone in the treatment of pain symptoms caused by endometriosis. Hum Reprod. 2009;24(12):3033-3341.
8. Kaser DJ, Missmer SA, Berry KF, Laufer MR. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms [published online ahead of print December 9 2011]. J Pediatr Adolesc Gynecol. doi:10.1016/j.jpag.2011.09.013.
9. Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. Subcutaneous injection of depot medroxyprogesterone acetate compared with leuprolide acetate in the treatment of endometriosis associated pain. Fertil Steril. 2006;85(2):314-325.
10. Crosignani PG, Luciano A, Ray A, Bergqvist A. Subcutaneous depot medroxyprogesterone acetate versus leuprolide acetate in the treatment of endometriosis-associated pain. Hum Reprod. 2006;21(1):248-256.
11. Cheewadhanaraks S, Peeyananjarassri K, Choksuchat C, Dhanaworavibul K, Choobun T, Bunyapipat S. Interval of injections of intramuscular depot medroxyprogesterone acetate in the long-term treatment of endometriosis-associated pain: a randomized clinical trial. Gynecol Obstet Invest. 2009;68(2):116-121.
12. Luciano AA, Turksoy RN, Carleo J. Evaluation of oral medroxyprogesterone acetate in the treatment of endometriosis. Obstet Gynecol. 1988;72(3 Pt 1):323-327.
13. Wong AY, Tang LC, Chin RK. Levonorgestrel-releasing intrauterine system (Mirena) and depot medroxyprogesterone acetate (Depoprovera) as long-term maintenance therapy for patients with moderate and severe endometriosis: a randomized controlled trial. Aust N Z J Obstet Gynaecol. 2010;50(3):273-279.
14. Lockhat FB, Emembolu JO, Konje JC. The efficacy side-effects and continuation rates in women with symptomatic endometriosis undergoing treatment with an intrauterine administered progestogen (levonorgestrel): a 3 year follow-up. Hum Reprod. 2005;20(3):789-793.
15. Petta CA, Ferriani RA, Abrao MS, et al. Randomized clinical trial of a levonorgestrel-releasing intrauterine system and a depot GnRH analogue for the treatment of chronic pelvic pain in women with endometriosis. Hum Reprod. 2005;20(7):1993-1998.
16. Vercellini P, Aimi G, Panazza S, De Giorgi O, Pesole A, Crosignani PG. A levonorgestrel-releasing intrauterine system for the treatment of dysmenorrhea associated with endometriosis: a pilot study. Fertil Steril. 1999;72(3):505-508.
17. Vercellini P, Frontino G, De Giorgi O, Aimi G, Zaina B, Crosignani PG. Comparison of a levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: a pilot study. Fertil Steril. 2003;80(2):305-309.
18. Walch K, Unfried G, Huber J, Kurz C, van Trotsenburg M, Pernicka E, Wenzl R. Implanon versus medroxyprogesterone acetate: effects on pain scores in patients with symptomatic endometriosis—a pilot study. Contraception. 2009;79(1):29-34.
Survivorship: Evaluating needs and the integrated model
Who is a survivor? Am I a survivor? Are you a survivor? What does a survivor need? How can community oncologists help? These are among the many questions a community oncologist can expect to hear during the course of treating a cancer patient, from the diagnosis, through the decisions about therapy, at all stages of treatment, and well into aftercare and follow-up. A “patient” used to be someone in active treatment, and a “survivor” was someone who had been free of disease for 5 years. More recently, Ellen Stoval of the National Coalition for Cancer Survivorship noted that the term “cancer survivor” can be used “to describe anyone who has been diagnosed with cancer as well as caregivers and loved ones of those diagnosed with the disease.”1 Her broader perspective more accurately captures the multifaceted nature of survivorship...
*For a PDF of the full article, click on the link to the left of this introduction.
Who is a survivor? Am I a survivor? Are you a survivor? What does a survivor need? How can community oncologists help? These are among the many questions a community oncologist can expect to hear during the course of treating a cancer patient, from the diagnosis, through the decisions about therapy, at all stages of treatment, and well into aftercare and follow-up. A “patient” used to be someone in active treatment, and a “survivor” was someone who had been free of disease for 5 years. More recently, Ellen Stoval of the National Coalition for Cancer Survivorship noted that the term “cancer survivor” can be used “to describe anyone who has been diagnosed with cancer as well as caregivers and loved ones of those diagnosed with the disease.”1 Her broader perspective more accurately captures the multifaceted nature of survivorship...
*For a PDF of the full article, click on the link to the left of this introduction.
Who is a survivor? Am I a survivor? Are you a survivor? What does a survivor need? How can community oncologists help? These are among the many questions a community oncologist can expect to hear during the course of treating a cancer patient, from the diagnosis, through the decisions about therapy, at all stages of treatment, and well into aftercare and follow-up. A “patient” used to be someone in active treatment, and a “survivor” was someone who had been free of disease for 5 years. More recently, Ellen Stoval of the National Coalition for Cancer Survivorship noted that the term “cancer survivor” can be used “to describe anyone who has been diagnosed with cancer as well as caregivers and loved ones of those diagnosed with the disease.”1 Her broader perspective more accurately captures the multifaceted nature of survivorship...
*For a PDF of the full article, click on the link to the left of this introduction.
Implications of Improved Survival in Patients With Chronic Myeloid Leukemia: A Nursing Perspective
With the introduction of tyrosine kinase inhibitor (TKI) therapy and the development of more sensitive monitoring techniques, the management of patients with chronic myeloid leukemia (CML) has evolved considerably over the last decade. In this review, we summarize the available literature evaluating the safety and efficacy of the TKIs imatinib, dasatinib, and nilotinib for information relevant to patient management to provide insight into long-term management of CML patients who receive TKI therapy. We suggest that these developments in treatment have expanded the role of oncology nurses, who can help address new issues that have arisen for patients learning to adapt to a chronic condition. The essential practice of monitoring, the critical importance of medication adherence, the safety profile of the three available TKIs, strategies for supportive care related to adverse events, drugdrug and drug-food interactions, and family planning are important aspects of long-term patient management...
*For a PDF of the full article, click on the link to the left of this introduction.
With the introduction of tyrosine kinase inhibitor (TKI) therapy and the development of more sensitive monitoring techniques, the management of patients with chronic myeloid leukemia (CML) has evolved considerably over the last decade. In this review, we summarize the available literature evaluating the safety and efficacy of the TKIs imatinib, dasatinib, and nilotinib for information relevant to patient management to provide insight into long-term management of CML patients who receive TKI therapy. We suggest that these developments in treatment have expanded the role of oncology nurses, who can help address new issues that have arisen for patients learning to adapt to a chronic condition. The essential practice of monitoring, the critical importance of medication adherence, the safety profile of the three available TKIs, strategies for supportive care related to adverse events, drugdrug and drug-food interactions, and family planning are important aspects of long-term patient management...
*For a PDF of the full article, click on the link to the left of this introduction.
With the introduction of tyrosine kinase inhibitor (TKI) therapy and the development of more sensitive monitoring techniques, the management of patients with chronic myeloid leukemia (CML) has evolved considerably over the last decade. In this review, we summarize the available literature evaluating the safety and efficacy of the TKIs imatinib, dasatinib, and nilotinib for information relevant to patient management to provide insight into long-term management of CML patients who receive TKI therapy. We suggest that these developments in treatment have expanded the role of oncology nurses, who can help address new issues that have arisen for patients learning to adapt to a chronic condition. The essential practice of monitoring, the critical importance of medication adherence, the safety profile of the three available TKIs, strategies for supportive care related to adverse events, drugdrug and drug-food interactions, and family planning are important aspects of long-term patient management...
*For a PDF of the full article, click on the link to the left of this introduction.
Maximizing clinical outcomes with axitinib therapy in advanced renal cell carcinoma through proactive side-effect management
Renal cell carcinoma (RCC) continues to exert a substantial disease burden. Increasing knowledge of the molecular signaling pathways associated with renal cancer has led to the development of targeted therapies for advanced RCC, including several antiangiogenic agents designed to inhibit development of abnormal blood vessels that sustain tumor growth. Axitinib is an investigational antiangiogenic agent that targets vascular endothelial growth factor receptors 1, 2, and 3. In phase II studies, axitinib elicited significant response rates in patients with advanced RCC refractory to cytokines or sorafenib. In a phase III study of axitinib versus sorafenib in patients with metastatic RCC, axitinib demonstrated clinically significant improvement in progression-free survival compared with sorafenib. As with other targeted agents, side effects associated with axitinib, such as hypertension, fatigue, and diarrhea, can negatively affect the patient’s physical and emotional states and quality of life, thus jeopardizing adherence to and the effectiveness of the treatment plan. Clinicians should be aware of side effects that may occur during treatment and manage them proactively. Nurses should educate patients about possible side effects and their management before axitinib treatment is initiated. Management strategies include early reporting of the symptoms, regular clinic visits and laboratory tests, ongoing review of concomitant medications, and prompt treatment of side effects and follow-up to assess the effectiveness of interventions, which could include treatment interruption and/or dose reduction. These approaches would help maximize the patient adherence to therapy, quality of life, and clinical outcomes.
*For a PDF of the full article, click on the link to the left of this introduction.
Renal cell carcinoma (RCC) continues to exert a substantial disease burden. Increasing knowledge of the molecular signaling pathways associated with renal cancer has led to the development of targeted therapies for advanced RCC, including several antiangiogenic agents designed to inhibit development of abnormal blood vessels that sustain tumor growth. Axitinib is an investigational antiangiogenic agent that targets vascular endothelial growth factor receptors 1, 2, and 3. In phase II studies, axitinib elicited significant response rates in patients with advanced RCC refractory to cytokines or sorafenib. In a phase III study of axitinib versus sorafenib in patients with metastatic RCC, axitinib demonstrated clinically significant improvement in progression-free survival compared with sorafenib. As with other targeted agents, side effects associated with axitinib, such as hypertension, fatigue, and diarrhea, can negatively affect the patient’s physical and emotional states and quality of life, thus jeopardizing adherence to and the effectiveness of the treatment plan. Clinicians should be aware of side effects that may occur during treatment and manage them proactively. Nurses should educate patients about possible side effects and their management before axitinib treatment is initiated. Management strategies include early reporting of the symptoms, regular clinic visits and laboratory tests, ongoing review of concomitant medications, and prompt treatment of side effects and follow-up to assess the effectiveness of interventions, which could include treatment interruption and/or dose reduction. These approaches would help maximize the patient adherence to therapy, quality of life, and clinical outcomes.
*For a PDF of the full article, click on the link to the left of this introduction.
Renal cell carcinoma (RCC) continues to exert a substantial disease burden. Increasing knowledge of the molecular signaling pathways associated with renal cancer has led to the development of targeted therapies for advanced RCC, including several antiangiogenic agents designed to inhibit development of abnormal blood vessels that sustain tumor growth. Axitinib is an investigational antiangiogenic agent that targets vascular endothelial growth factor receptors 1, 2, and 3. In phase II studies, axitinib elicited significant response rates in patients with advanced RCC refractory to cytokines or sorafenib. In a phase III study of axitinib versus sorafenib in patients with metastatic RCC, axitinib demonstrated clinically significant improvement in progression-free survival compared with sorafenib. As with other targeted agents, side effects associated with axitinib, such as hypertension, fatigue, and diarrhea, can negatively affect the patient’s physical and emotional states and quality of life, thus jeopardizing adherence to and the effectiveness of the treatment plan. Clinicians should be aware of side effects that may occur during treatment and manage them proactively. Nurses should educate patients about possible side effects and their management before axitinib treatment is initiated. Management strategies include early reporting of the symptoms, regular clinic visits and laboratory tests, ongoing review of concomitant medications, and prompt treatment of side effects and follow-up to assess the effectiveness of interventions, which could include treatment interruption and/or dose reduction. These approaches would help maximize the patient adherence to therapy, quality of life, and clinical outcomes.
*For a PDF of the full article, click on the link to the left of this introduction.